
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 91
ALDRIN AND DIELDRIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1989
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Aldrin and Dieldrin.
(Environmental health criteria ; 91)
1.Aldrin 2.Dieldrin I.Series
ISBN 92 4 154291 8 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1989
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ALDRIN AND DIELDRIN
1. SUMMARY
1.1. General
1.2. Environmental transport, distribution, and transformation
1.3. Environmental levels and human exposure
1.4. Kinetics and metabolism
1.5. Effects on organisms in the environment
1.5.1. Accumulation
1.5.2. Toxicity for microorganisms
1.5.3. Toxicity for aquatic organisms
1.5.4. Toxicity for terrestrial organisms
1.5.5. Population and ecosystem effects
1.6. Effects on experimental animals and in vitro test systems
1.7. Effects on man
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Primary constituent: aldrin
2.1.2. Primary constituent: dieldrin
2.2. Physical and chemical properties
2.2.1. Aldrin
2.2.2. Dieldrin
2.3. Analytical methods
2.3.1. Sampling methods
2.3.2. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Man-made sources
3.2.1. Production levels and processes; uses
3.2.1.1 World production figures
3.2.1.2 Manufacturing processes
3.2.1.3 Release into the environment during
normal production
3.2.2. Uses
3.2.2.1 Aldrin
3.2.2.2 Dieldrin
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Leaching of aldrin and dieldrin
4.1.2. Surface run-off
4.1.3. Loss of aldrin and dieldrin from soils -
volatilization
4.1.3.1 Movement within the soil profile - mass
flow
4.1.3.2 Movement within the soil profile -
diffusion
4.1.3.3 Actual volatilization losses - laboratory
studies
4.1.3.4 Actual volatilization losses - field
studies
4.1.4. Losses of residues following treatment of soil
with aldrin
4.1.5. Losses of residues from water
4.1.6. Aldrin and dieldrin in the atmosphere
4.1.7. Aldrin and dieldrin in water
4.2. Translocation from soil into plants
4.3. Models of the behaviour of water and chemicals in soil
4.4. Biodegradation of aldrin and dieldrin
4.4.1. Epoxidation of aldrin
4.4.2. Other metabolic pathways of aldrin
4.4.3. Biotransformation of dieldrin
4.4.4. Conclusions
4.5. Abiotic degradation
4.5.1. Photochemistry
4.5.1.1 Photochemistry of aldrin and dieldrin in
water
4.5.1.2 Photochemistry of aldrin and dieldrin in
air
4.5.1.3 Photochemistry of aldrin and dieldrin on
plant surfaces
4.5.1.4 Photochemistry of aldrin and dieldrin in
soils
4.5.1.5 Conclusions
4.5.2. Other abiotic processes
4.5.2.1 Reaction with ozone
4.5.2.2 Clay-catalysed decomposition
4.6. Bioaccumulation
4.7. The fate of aldrin and dieldrin in the environment
4.7.1. Aldrin and dieldrin in soils
4.7.2. Aldrin and dieldrin in the atmosphere
4.7.3. Conclusion
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air and rainwater
5.1.1.1 Aldrin
5.1.1.2 Dieldrin
5.1.2. Concentrations in houses
5.1.2.1 Aldrin used for subterranean termite
control
5.1.2.2 Aldrin and dieldrin used for remedial
treatment of wood
5.1.3. Aquatic environment
5.1.4. Soil
5.1.5. Drinking-water
5.1.6. Food and feed
5.1.6.1 Joint FAO/WHO food contamination
monitoring programme
5.1.6.2 Information summarized by GIFAP (1984)
5.1.6.3 United Kingdom (UK MAFF, 1983-1985)
5.1.6.4 USA
5.1.6.5 Appraisal of intake studies
5.1.7. Concentrations of dieldrin in non-target species
5.1.7.1 Occurrence of dieldrin in birds of prey
and fish-eating birds
5.2. General population exposure
5.2.1. Adults
5.2.1.1 Aldrin
5.2.1.2 Concentrations of dieldrin in adipose
tissue
5.2.1.3 Concentrations of dieldrin in blood
5.2.1.4 Concentrations of dieldrin in other
tissues
5.2.2. Babies, infants, and mother's milk
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Aldrin
6.1.1.1 Ingestion
6.1.1.2 Inhalation
6.1.2. Dieldrin
6.1.3. Photodieldrin (and other metabolites of dieldrin)
6.2. Distribution
6.2.1. Aldrin
6.2.1.1 Mouse
6.2.1.2 Rat
6.2.1.3 Dog
6.2.1.4 Human studies
6.2.2. Dieldrin
6.2.2.1 Laboratory animals
6.2.2.2 Transplacental transport
6.2.2.3 Domestic animals
6.2.2.4 Human volunteers
6.2.2.5 General population
6.2.3. Photodieldrin (and major metabolites of dieldrin)
6.2.3.1 Laboratory animals
6.2.3.2 Human beings
6.3. Metabolic transformation
6.3.1. Aldrin and dieldrin
6.3.1.1 Laboratory animals
6.3.1.2 Human studies
6.3.1.3 Non-domestic organisms
6.3.2. Photodieldrin (and major metabolites of dieldrin)
6.3.2.1 Rat
6.3.2.2 Monkey
6.4. Elimination and excretion
6.4.1. Aldrin
6.4.1.1 Rat
6.4.2. Dieldrin
6.4.2.1 Laboratory animals
6.4.2.2 Human studies
6.4.3. Photodieldrin (and major metabolites of dieldrin)
6.4.3.1 Rat
6.4.3.2 Monkey
6.5. Retention and turnover
6.5.1. Non-domestic organisms
6.5.2. Biological half-life in human beings
6.5.3. Body burden and (critical) organ burden; indicator
media
6.6. Appraisal
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
7.2. Aquatic organisms
7.2.1. Aquatic invertebrates
7.2.1.1 Acute toxicity
7.2.1.2 Short-term toxicity, reproduction, and
behaviour
7.2.2. Fish
7.2.2.1 Acute toxicity
7.2.2.2 Long-term toxicity
7.2.2.3 Reproduction
7.2.3. Amphibia and reptiles
7.3. Terrestrial organisms
7.3.1. Higher plants
7.3.2. Earthworms
7.3.3. Bees and other beneficial insects
7.3.4. Birds
7.3.4.1 Acute toxicity
7.3.4.2 Short- and long-term toxicity
7.3.4.3 Reproductive studies
7.3.4.4 Eggshell thinning
7.3.4.5 Concentrations of dieldrin in tissues of
experimentally poisoned birds
7.3.5. Mammals
7.4. Effect on populations and ecosystems
7.4.1. Exposure to dieldrin
7.4.2. Effects on populations of birds
7.4.3. Effects on populations of mammals
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.1.1. Aldrin and dieldrin
8.1.1.1 Oral
8.1.1.2 Dermal
8.1.1.3 Inhalation
8.1.1.4 Parenteral
8.1.2. Formulated materials
8.1.2.1 Oral and dermal
8.1.2.2 Inhalation
8.2. Short-term exposures
8.2.1. Oral
8.2.1.1 Rat
8.2.1.2 Dog
8.2.1.3 Domestic animals
8.2.2. Dermal
8.2.3. Inhalation
8.3. Skin and eye irritation; sensitization
8.3.1. Skin and eye irritation
8.3.2. Sensitization
8.4. Long-term toxicity and carcinogenicity
8.4.1. Mouse
8.4.1.1 Appraisal
8.4.2. Rat
8.4.2.1 Appraisal
8.4.3. Hamster
8.4.4. Monkey
8.4.5. Mode of action
8.5. Reproduction, embryotoxicity, and teratogenicity
8.5.1. Reproduction
8.5.1.1 Mouse
8.5.1.2 Rat
8.5.1.3 Dog
8.5.1.4 Appraisal
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Mouse
8.5.2.2 Rat
8.5.2.3 Hamster
8.5.2.4 Rabbit
8.5.2.5 Appraisal
8.6. Mutagenicity and related end-points
8.6.1. Microorganisms
8.6.2. Mammalian cell point mutations
8.6.3. Dominant lethal assays and heritable translocation
assays in mice
8.6.4. Micronucleus test
8.6.5. Chromosome and cytogenicity studies
8.6.6. Host-mediated assays
8.6.7. Cell transformation in mammalian cell systems
8.6.8. Drosophila melanogaster and other insect systems
8.6.9. Effects on DNA
8.6.10. Cell to cell communication
8.6.11. Appraisal
8.7. Special studies
8.7.1. Liver enzyme induction
8.7.2. Nervous system
8.7.2.1 Rat
8.7.2.2 Dog
8.7.2.3 Monkey
8.7.3. Weight loss and stress
8.7.3.1 Rat
8.7.4. Immunosuppressive action
8.8. Toxicity of photodieldrin and major metabolites
8.8.1. Photodieldrin
8.8.1.1 Acute toxicity
8.8.1.2 Short-term toxicity
8.8.1.3 Long-term toxicity
8.8.1.4 Reproduction, embryotoxicity, and
teratogenicity
8.8.1.5 Appraisal
8.8.2. Major metabolites of dieldrin
8.8.2.1 Acute toxicity
8.8.2.2 Short-term toxicity
8.9. Mechanisms of toxicity; mode of action
8.9.1. Central nervous system
8.9.2. Liver
9. EFFECTS ON HUMAN BEINGS
9.1. General population exposure
9.1.1. Acute toxicity - poisoning incidents
9.1.2. Effects of short- and long-term exposure -
controlled human studies
9.1.2.1 Accidental poisoning
9.1.2.2 Controlled human studies
9.1.3. Tissue concentrations of dieldrin in hospitalized
people
9.1.3.1 Pathological findings
9.1.3.2 Influence of weight loss and stress on
dieldrin concentrations in tissues
9.1.4. Exposure in treated homes
9.2. Occupational exposure
9.2.1. Acute toxicity - poisoning incidents
9.2.1.1 Blood levels diagnostic of
aldrin/dieldrin poisoning
9.2.1.2 Electroencephalography
9.2.2. Effects of short- and long-term exposure
9.2.3. Epidemiological studies
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
10.3. Conclusions
11. RECOMMENDATIONS
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX I. NOMENCLATURE
FRENCH TRANSLATION OF SUMMARY, EVALUATION, AND RECOMMENDATIONS
WHO TASK GROUP ON ALDRIN AND DIELDRIN
Members
Dr G. Burin, Office of Pesticide Programs, US Environmental
Protection Agency, Washington DC, USA
Dr I. Desi, Department of Hygiene and Epidemiology, University
Medical School, Szeged, Hungary (Vice-Chairman)
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Abbots Ripton, Huntingdon, United Kingdom
Dr R. Goulding, Guy's Hospital, London, United Kingdom (Chairman)
Dr A. Furtado Rahde, Ministry of Public Health, Porto Alegre,
Brazil
Dr S.K. Kashyap, National Institute of Occupational Health,
Ahmedabad, India
Dr M. Takeda, Division of Environmental Chemistry, National
Institute of Hygienic Sciences, Tokyo, Japan
Dr H.G.S. Van Raalte, The Hague, Netherlands
Observers
Dr R. Rimpau, European Chemical Industry, Ecology and Toxicology
Centre, Brussels, Belgium
Dr R.C. Tincknell, International Group of National Associations of
Agrochemical Manufacturers, Brussels, Belgium
Dr H.G.S. Van Raalte, International Commission on Occupational
Health, Geneva
Secretariat
Dr J.R.P. Cabral, International Agency for Research on Cancer,
Lyons, France
Dr J. Copplestone, Pesticide Development and Safe Use Unit, World
Health Organization, Geneva, Switzerland
Dr M. Gilbert, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Ms B. Goelzer, Office of Occupational Health, World Health
Organization, Geneva, Switzerland
Dr H. Galal Gorchev, Food Safety Unit, World Health Organization,
Geneva, Switzerland
Secretariat (contd.)
Dr K.W. Jager, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland (Secretary)
Dr G.J. van Esch, Bilthoven, Netherlands (Rapporteur)
Dr N. Watfa, Safety and Health Branch, International Labour Office,
Geneva, Switzerland
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 7988400 -
7985850).
* * *
The proprietary information contained in this document cannot
replace documentation for registration purposes because the latter
has to be closely linked to the source, the manufacturing route,
and the purity/impurities of the substance to be registered. The
data should be used in accordance with paragraphs 82 - 84 and
recommendations paragraph 90 of the Second FAO Government
Consultation (FAO, 1982).
ENVIRONMENTAL HEALTH CRITERIA FOR ALDRIN AND DIELDRIN
A WHO Task Group on Environmental Health Criteria for Aldrin
and Dieldrin met in Geneva from 13 to 17 July 1987. Dr K.W. Jager,
IPCS, opened the meeting and welcomed the participants on behalf
of the heads of the three IPCS cooperating organizations
(UNEP/ILO/WHO). The group reviewed and revised the draft criteria
document and made an evaluation of the risks for human health and
the environment from exposure to aldrin and dieldrin.
The first draft of this document was prepared by Dr G.J. VAN
ESCH of the Netherlands on the basis of a review of all studies on
aldrin and dieldrin including the proprietary information, made
available to the IPCS by Shell International Chemical Company
Limited, London, United Kingdom.
The second draft was also prepared by Dr van Esch,
incorporating comments received following the circulation of the
first draft to the IPCS contact points for Environmental Health
Criteria documents.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the technical development and
editing, respectively, of this monograph.
The assistance of Shell in making available to the IPCS and the
Task Group its toxicological proprietary information on aldrin and
dieldrin is gratefully acknowledged. This allowed the Task Group
to make its evaluation on a more complete data base.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the cost of printing.
INTRODUCTION
Aldrin and dieldrin are the common names of insecticides
containing 95% HHDN and 85% HEOD, respectively.
Throughout this monograph the names aldrin and dieldrin are
used, although concentrations determined in the different matrices
are actually those of the active molecules HHDN and HEOD.
Aldrin is readily metabolized to dieldrin (HEOD) in plants and
animals. Only rarely are aldrin residues present in food or in the
great majority of animals, and then only in very small amounts.
Therefore, national and international regulatory bodies have
considered these two closely related insecticides together. The
practicality of considering them jointly is further emphasized by
the lack of significant difference in their acute and chronic
toxicity and by their common mode of action.
1. SUMMARY
1.1. General
Aldrin and dieldrin, both organochlorine pesticides and
manufactured commercially since 1950, were used throughout the
world up to the early 1970s. Both compounds were used as
insecticides in agriculture for the control of many soil pests and
in the treatment of seed. Insects controlled by these compounds
include termites, grasshoppers, wood borers, beetles, and textile
pests. Dieldrin has also been used in public health for the
control of tsetse flies and other vectors of debilitating tropical
diseases. Both aldrin and dieldrin act as a contact and stomach
poison for insects.
Since the early 1970s, both compounds have been severely
restricted or banned, in a number of countries, from use,
especially in agriculture. Nevertheless, the use for termite
control continues in other countries. Global production, which was
estimated to be 13 000 tonnes/year in 1972, decreased to less than
2500 tonnes/year in 1984.
The purity of technical grade aldrin and dieldrin is 90%
and > 95%, respectively. Impurities for aldrin include
octachlorocyclopentene, hexachlorobutadiene, and polymerization
products, and for dieldrin polychloroepoxyoctahydrodimethano-
naphthalenes.
Both compounds are practically insoluble in water and
moderately to highly soluble in most paraffinic, aromatic, and
halogenated hydrocarbons, and in esters, ketones, and alcohols.
The vapour pressure of aldrin is 6.5 x 10-5 mmHg at 25 °C and that
of dieldrin is 3.2 x 10-6 mmHg at 25 °C.
Analytical methods for the determination of aldrin and dieldrin
in food, feed, and the environment are described in section 2.
1.2. Environmental Transport, Distribution, and Transformation
A major use of aldrin is as a soil insecticide. Hence, aldrin-
treated soil is an important source of aldrin and its reaction
product dieldrin in the environment.
Aldrin has a low propensity for movement away from treated
areas, either through volatilization or by leaching. It is mainly
and rapidly adsorbed on soils with a high organic matter content,
but only moderately adsorbed by clay soils. Aldrin and dieldrin
rarely penetrate more than 20 cm beneath the top treated layer of
soil. Aldrin adheres to soil particles to such an extent that only
traces can be removed by water. For this reason, contamination of
ground water does not generally occur.
The disappearance of aldrin from soil resembles a first-order
reaction. Immediately after application, there is a short period
of rapid loss due to volatilization and thereafter a second longer
exponential period of decline, mainly due to conversion to
dieldrin, which is slower to dissipate. However, there is the
possibility of migration by way of soil erosion, as wind drift,
sediment transport, and surface run-off. From data on residues of
aldrin in the environment, it appears that it is mainly retained in
the soil and that 97% of the primary residue is not the parent
compound but its epoxide, dieldrin.
Photodieldrin is a photodegradation product of dieldrin and
does not occur widely in the environment.
Aldrin applied to soils is lost slowly in temperate areas,
three-quarters of the applied aldrin being lost during the first
year in a typical case. The rate of loss slows later as aldrin is
converted to dieldrin. There is some evidence that the rate of loss
is greater under the anaerobic conditions of rice paddies than under
aerobic conditions. Dieldrin is lost from the soil very rapidly in
tropical areas, up to 90% disappearing within 1 month, whereas the
half-life of dieldrin in temperate soils is approximately 5 years.
Volatilization appears to be the principal route of loss from the
soil, though atmospheric levels of dieldrin and aldrin are generally
low. Some dieldrin is washed from the atmosphere by rain, but
levels in ground water are very low because of strong adsorption to
soil particles. Dieldrin has been detected, in small amounts, in
surface water contaminated by run-off from agricultural land.
1.3. Environmental Levels and Human Exposure
Aldrin and dieldrin have been found in the atmosphere, in the
vapour phase, adsorbed on dust particles, or in rainwater at
variable levels according to the situation. They have been
detected mainly in agricultural areas, where the mean level in the
air has been of the order of 1 - 2 ng/m3, with maximum levels of
about 40 ng/m3. In rainwater, concentrations of the order of
10 - 20 ng/litre, or occasionally higher, have been found.
Concentrations found in the air in houses treated for the
control of termites were much higher, ranging from 0.04 to 7 µg/m3,
depending on the time of sampling (i.e., the number of days of
after application) and the type of house. Within 8 weeks, the
concentrations decreased rapidly. Treatment of internal wood in
houses resulted in dieldrin concentrations in the air ranging from
0.01 to 0.5 µg/m3. Aldrin and dieldrin migrated into food from
treated laminated timber and plywood, and by direct contact and/or
sorption from the atmosphere.
The occurrence of dieldrin in the aquatic environment has been
reported. However, the concentrations were very low, mainly less
than 5 ng/litre. Higher levels have been generally attributed to
industrial effluents or soil erosion during agricultural usage.
River sediments may contain much higher concentrations (up to 1 mg/kg).
Aldrin is found only rarely in food, but dieldrin is more
common, especially in dairy products, meat products, fish, oils and
fats, potatoes, and certain other vegetables (especially the root
vegetables). Maximum residue limits (MRLs) in the range of 0.02 to
0.2 mg/kg product have been recommended over the years by the
FAO/WHO Joint Meetings on Pesticide Residues. Recent studies in
different countries have shown that the actual concentrations of
dieldrin in these food commodities are generally lower. Studies
from the United Kingdom indicate this decrease clearly. In
1966 - 67, the mean level of dieldrin residues in a total diet
study was 0.004 mg/kg food, whereas in the period 1975 - 77 it was
0.0015 mg/kg, and in 1981, 0.0005 mg/kg. This downward trend has
been confirmed in other countries, for instance in the USA. This
may be due to the restriction or banning of the use of these
compounds.
A large number of investigations has been reported in which the
adipose tissue, organs, blood, or other tissues of the general
population have been examined for the presence of dieldrin. Over
the last 25 years, surveys have been carried out in many countries
all over the world. Most of the mean values for adipose tissue
have been in the range of 0.1 - 0.4 mg/kg. Surveys in the
Netherlands, the United Kingdom, and the USA have indicated a
decline in concentrations in adipose tissue, since the mid-1970s.
Blood concentrations range from 1 to 2 µg/litre. Levels in the
liver are below 0.4 mg/kg, while those in other tissues, including
the kidneys, brain, and gonads, are below 0.1 mg/kg tissue.
As a result of transplacental exposure, dieldrin is present in
the blood, adipose tissue, and other tissues of the fetus and
newborn infants. The concentrations are one tenth to one half of
those of their mothers. There is no difference between infants and
adults in the brain/liver/fat ratio of dieldrin concentrations.
Dieldrin is also excreted in mother's milk. Over the last 15
years, samples of mother's milk have been analysed for the presence
of organochlorine pesticides, including dieldrin, in various
countries. In most countries, the dieldrin concentration in milk
amounts to 6 µg/litre, though higher levels have occasionally been
found.
1.4. Kinetics and Metabolism
In both animals and human beings, aldrin and dieldrin are
readily absorbed into the circulating blood from the
gastrointestinal tract, through the skin, or through the lungs
following inhalation of the vapour. A study on human volunteers
showed that absorption through the intact skin amounts to 7 - 8% of
the applied dose. Inhalation studies with human volunteers
suggested that up to 50% of inhaled aldrin vapour is absorbed and
retained in the human body. After absorption, it is rapidly
distributed throughout the organs and tissues of the body and a
continuous exchange between the blood and other tissues takes
place. In the meantime, aldrin is readily converted to dieldrin,
mainly in the liver but also to a much lesser extent in some other
tissues, such as the lungs. This conversion proceeds very rapidly.
When 1-day-old rats were given oral doses of 10 mg aldrin/kg
body weight, their livers contained dieldrin 2 h after treatment.
Over the course of the next few hours, dieldrin concentrated to a
greater extent in the lipid tissues.
Numerous studies carried out with 14C-labelled aldrin and
dieldrin have shown that part of the ingested material is passed
unabsorbed through the intestinal tract and eliminated from the
body, part is excreted unchanged from the liver into the bile, part
is stored in the various organs and tissues particularly in the
adipose tissue, and part is metabolized in the liver to more polar
and hydrophilic metabolites. In human beings and most animals, the
metabolites are excreted primarily via the bile in the faeces. It
has also been shown that both aldrin and dieldrin are biodegraded
into the same metabolites.
Most of the currently available information on the
biodegradation metabolism in mammals is based on studies on
dieldrin in the mouse, rat, rabbit, sheep, dog, monkey, chimpanzee,
and in human beings. The overall picture shows only quantitative
variations between species, and the mechanisms in rats seem to be
similar to those in primates.
The major metabolite, except in the case of the rabbit, is the
9-hydroxy derivative. This metabolite is found in the faeces and
in a free or conjugated form in the urine. Small amounts of three
other metabolites have been found and identified in experimental
animals. These are the trans-6,7-dihydroxy derivative,
dicarboxylic acid derived from the dihydroxy compound, and the
bridged pentachloroketone.
Only the 9-hydroxy compound has been demonstrated in the faeces
of human beings and neither this nor the other metabolites have
been found in human blood or other tissues. Dieldrin was found to
be present in the faeces of occupationally exposed workers, whereas
the concentrations in the samples from the general population were
below the limits of detection. Examination of the urine of five
workers indicated that urinary excretion of dieldrin and its four
metabolites was minor compared to the elimination of the 9-hydroxy
metabolite via the faeces.
The conversion of aldrin to dieldrin by mixed-function
monooxygenases (aldrin-epoxidase) in the liver and the distribution
and the subsequent deposition of dieldrin (mainly in lipid-
containing tissues, such as adipose tissue, liver, kidneys, heart,
and brain) proceed much more rapidly than the biodegradation and
ultimate elimination of unchanged dieldrin and its metabolites from
the body. Thus, at a given average daily intake of aldrin and/or
dieldrin, dieldrin slowly accumulates in the body. However, this
accumulation does not continue indefinitely. As dosing continues,
a "steady state" is eventually reached at which the rates of
excretion and intake are equal. The upper limit of storage is
related to the daily intake. This has been demonstrated in rats,
dogs, and human beings.
When the intake of aldrin/dieldrin ceases or decreases, the
body burden decreases. The biological half-life in man is
approximately 9 - 12 months. Significant relationships have been
found between the concentrations of dieldrin in the blood and those
in other tissues in rats, dogs, and human beings.
Numerous investigations of the concentrations of dieldrin in
the blood, adipose tissue, and other tissues of members of the
general population and from special groups, carried out in several
different countries, have shown that at equilibrium the ratio of
dieldrin concentrations in the adipose tissue, liver, brain, and
blood is about 150:15:3:1.
Dieldrin is transported via the placenta and reaches the fetus.
Accumulation takes place in the same organs and tissues as in the
adult, but to a much lower level. There seems to be an equilibrium
between the levels in the mother and the fetus.
Photodieldrin is also metabolized into bridged pentachloroketone
in the rat and dog. Both compounds were found in the adipose
tissue, liver, and kidneys when animals were administered high
levels of photodieldrin. No residues of these compounds could be
detected in human adipose tissue, kidneys, or breast milk. The
accumulation of photodieldrin in the adipose tissue of experimental
animals was much less than that of dieldrin.
1.5. Effects on Organisms in the Environment
1.5.1. Accumulation
Most residues in organisms are of dieldrin, since aldrin is
readily converted to dieldrin in all organisms.
The uptake of dieldrin from medium into fungi, streptomycetes,
and bacteria over 4 h has yielded concentration factors ranging
from 0.3 to >100. Protozoa take up more dieldrin than algae.
Algae take up dieldrin from the culture medium very rapidly, maxima
often being reached within a few hours.
Many species of aquatic invertebrates concentrate dieldrin from
very low water concentrations, yielding high concentration factors.
A steady state is reached within a few days. On transfer to clean
water, the loss of dieldrin is rapid, the half-life being 60 - 120 h.
Bioconcentration factors for whole fish are greater than
10 000. The half-life for loss of accumulated dieldrin was found
to be 16 days for one species of fish.
The bioconcentration of dieldrin in aquatic organisms is
principally from the water rather than by ingestion of food.
Earthworms take up dieldrin from the soil and concentrate it to
a maximum of about 170 times. There is little correlation between
levels in earthworms and levels in most types of soil.
Many investigations have been carried out to estimate the
occurrence of dieldrin in the tissues or eggs of non-target
species. The concentrations found cover a wide range from 0.001
mg/kg up to 100 mg/kg tissue, but most are below 1 mg/kg tissue.
Both the body tissues and eggs of birds accumulate dieldrin
readily. Similarly, various mammal species have been shown to
accumulate dieldrin, particularly in the fatty tissues.
1.5.2. Toxicity for microorganisms
The effects of dieldrin on unicellular algae are very variable,
some species being markedly affected by 10 µg/litre and others
unaffected even by 1000 µg/litre. Aldrin and dieldrin have only
minor effects on soil bacteria, even at levels far exceeding those
normally encountered. Most studies have shown no effects at
exposure levels of 2000 mg/kg soil. Effects on photosynthesis have
been reported in several different species of algae, with aldrin
showing a more marked effect than dieldrin at the same
concentration. However, these slight effects on the biochemical
processes of soil algae were only transitory.
1.5.3. Toxicity for aquatic organisms
Aldrin and dieldrin are highly toxic for aquatic crustaceans,
most 96-h LC50 values being below 50 µg/litre. However, a few
reported results of up to 4300 µg/litre illustrate species
variability. Daphnids are less sensitive to dieldrin than aldrin,
with 48-h tests yielding LC50 values of 23 - 32 µg/litre for aldrin
and 190 - 330 µg/litre for dieldrin. Molluscs are significantly
more resistant, with 48 h values ranging up to >10 000 µg/litre.
The results of studies over several weeks have confirmed the
relative resistance of daphnids and molluscs. The most susceptible
aquatic invertebrates are the larval stages of insects with 96-h
values of 0.5 - 39 µg/litre for dieldrin and 1.3 - 180 µg/litre for
aldrin.
Both aldrin and dieldrin were highly toxic in acute tests on
fish. Values for 96-h LC50s in various fish species varied from
2.2 to 53 µg/litre for aldrin, and from 1.1 to 41 µg/litre for
dieldrin. Several studies have revealed that toxicity increases
with increasing temperature. In a long-term study on Poecilia
latipinna, there was 100% mortality at dieldrin concentrations of
3 µg/litre or more. Dieldrin administered in the food of rainbow
trout at up to 430 µg/kg body weight per day did not have any
effects on mortality, but enzymic changes were reported.
Morphological changes in liver mitochondria were seen using the
electron microscope. The ammonia-detoxifying mechanism of fish is
sensitive to dieldrin, the no-observed-adverse-effect level being
less than 14 µg/kg body weight per day. Different life stages of
fish have been found to have different susceptibilities to
dieldrin. Eggs were resistant and juvenile stages were less
susceptible than adults.
The acute toxicity of both aldrin and dieldrin is high for
larval amphibia with 96-h LC50s of the order of 100 µg/litre.
1.5.4. Toxicity for terrestrial organisms
The toxicity of dieldrin for higher plants is low, crops only
being affected at application rates greater than 22 kg/ha. Aldrin
is more phytotoxic, to tomatoes and cucumbers particularly, but
only at application rates many times greater than those
recommended. Cabbage is the most sensitive crop to aldrin.
Oral LD50s for honey bees ranging from 0.24 to 0.45 µg/bee for
aldrin and from 0.15 to 0.32 µg/bee for dieldrin have been reported.
Contact toxicity ranged from 0.15 to 0.80 µg/bee for aldrin and from
0.15 to 0.41 µg/bee for dieldrin. Two studies have indicated that
dieldrin is relatively non-toxic for predatory insects eating pest
species.
In laboratory studies, earthworms tolerated aldrin at a level
of 13 mg/kg of artificial soil with <1% mortality. The 6-week
LC50 was 60 mg aldrin/kg soil.
The acute toxicities of aldrin and dieldrin have been found to
vary by more than an order of magnitude for 13 species of birds,
ranging from 6.6 to 520 mg/kg body weight for aldrin and from 6.9
and 381 mg/kg body weight for dieldrin. In four bird species,
subacute oral toxicity varied between 34 and 155 mg/kg for aldrin
and 37 and 169 mg/kg for dieldrin. Repeated testing over a period
of time did not indicate the development of resistance in these
species. Reproductive studies on several species of domestic birds
have indicated that levels of dieldrin in the diet of more than
10 mg/kg cause some adult mortality. There are no reproductive
effects on egg production, fertility, hatchability, or chick
survival at levels of dietary dieldrin not causing maternal
toxicity. Eggshell thickness is not directly affected by dieldrin.
However, reduced food consumption is a symptom of dieldrin
poisoning, and eggshell thickness can be reduced by decreased food
intake.
Among non-laboratory mammals, the response to dieldrin varies
from species to species. Four vole species showed acute LD50s
ranging from 100 to 210 mg/kg body weight, making them less
susceptible to dieldrin than laboratory species. Shrews survived a
diet containing 50 mg dieldrin/kg but died with a dietary level of
200 mg/kg. Blesbuck (antelope) survived for 90 days at 5 and 15
mg/kg diet but all died within 24 days at levels of 25 mg/kg or
more. All blesbuck in an area sprayed with dieldrin at 0.16 kg/ha
died, the calculated dietary intake being 1.82 mg/kg per day. Thirty
percent of springbok survived the spray with no after-effects.
Toxicological signs of dieldrin poisoning were similar to those of
laboratory mammals.
1.5.5. Population and ecosystem effects
It has been suggested that some mammal populations have been
affected by dieldrin. Small mammals were probably killed by eating
dieldrin-dressed seed, but populations were replenished by
immigration. Bats have been killed by dieldrin in wood preservatives.
Residues of dieldrin have been reported in many species of
birds. Throughout the world, the highest residues have been found
in birds of prey at the top of foodchains. The dieldrin content of
bird tissues and eggs has paralleled usage patterns and decreased
with restrictions in the use of aldrin and dieldrin. It is not
easy to identify the effects of dieldrin, because residues occur
together with residues of other organochlorines. Dieldrin is more
toxic to birds than DDT and probably has been responsible for more
adult deaths that DDT. However, the reproductive effects of
dieldrin in the field are more difficult to prove. There are
seasonal changes in the contents of dieldrin in bird tissues.
Furthermore, effects can occur long after exposure to the source of
the pollutant.
1.6. Effects on Experimental Animals and In Vitro Test Systems
Aldrin and dieldrin are of a high order of toxicity; the oral
LD50s for both compounds in the mouse and rat range from 40 to 70
mg/kg body weight. The dermal toxicity is in the range of 40 - 150
mg/kg body weight, depending on the animal species and the solvent
used. Technical aldrin and dieldrin were found to produce slight
to severe irritation in the rabbit skin, but this effect was mainly
caused by the solvent. In the Magnusson & Kligman guinea-pig
maximization test, aldrin produced a sensitization effect.
However, during 20 years of manufacture and formulation, no cases
of skin sensitization occurred in a group of over 1000 workers.
The vapour pressures of both aldrin and dieldrin are low and
acute inhalation effects do not normally arise. The effects
observed in acute toxicity studies by all routes involve the
central nervous system and include hyperexcitability, tremors, and
convulsions.
Short- and long-term oral studies have been carried out with
aldrin and dieldrin on the mouse, rat, dog, hamster, and monkey.
The liver is the major target organ in the rat and mouse, with an
increased liver/body weight ratio and hypertrophy of the
centrilobular hepatocytes occurring, which in the early stages may
be reversible. Microscopically these changes include increased
cytoplasmatic oxyphilia and peripheral migration of basophilic
granules. These changes were not found in the liver of the hamster
and the monkey. In the dog, mild liver changes (fatty changes and
slight hepatic cell atrophy) were accompanied by kidney changes
consisting of vacuolization in the epithelia of distal renal
tubules and tubular degeneration. In the rat, the overall no-
observed-adverse-effect level from the available short-term and
long-term studies is 0.5 mg/kg diet, equivalent to 0.025 mg/kg body
weight. With feeding levels equivalent to 0.05 mg/kg body weight
or more, an increasing dose-related hepatomegaly and histological
changes occurred. In the dog, no-effect levels of 0.04 - 0.2 mg/kg
body weight were found.
A number of long-term carcinogenicity studies on mice of
different strains were carried out with aldrin or dieldrin. In all
studies, benign and/or malignant liver cell tumours were found.
Females seemed to be less sensitive than males. No other types of
tumours were induced.
Long-term studies on the other animal species (rat, hamster)
did not show any increase in tumour incidence. Photodieldrin, fed
at concentrations up to 7.5 mg/kg diet, did not induce tumours.
In addition, a number of special studies have been published
that have so far failed to elucidate the mechanism of the induction
of the liver tumours in mice.
In most of the reproduction studies (over 1 - 6 generations)
carried out with aldrin or dieldrin on mice and rats, the major
effect was an increased mortality rate in pre-weaning pups.
Reproductive performance was only affected at doses causing
maternal intoxication. Studies on dogs were too limited to draw
firm conclusions, apart from a consistent increase in pre-weaning
pup mortality.
It can be concluded from the results of these reproduction
studies that 2 mg dieldrin/kg in the rat diet and 3 mg dieldrin/kg
in the mouse diet, equivalent to 0.1 and 0.4 mg/kg body weight per
day, respectively, are no-observed-adverse-effect levels for
reproduction.
No evidence of teratogenic potential was found in studies on
the mouse, rat, or rabbit using oral doses of aldrin and dieldrin
of up to 6 mg/kg body weight. Single doses of aldrin and dieldrin,
equal to about half the LD50, caused severe fetotoxicity and an
increased incidence of teratogenic abnormalities in the mouse and
hamster. The significance of these findings in the presence of
likely maternal toxicity is doubtful.
Many in vivo and in vitro mutagenicity studies have been
carried out, but the results of nearly all these studies were
negative.
The acute oral toxicity of photodieldrin is higher than that of
dieldrin in the mouse, rat, and guinea-pig. In acute and short-
term toxicity studies, the symptoms of intoxication and the effects
on target organs are quantitatively and qualitatively similar to
those of dieldrin. Photodieldrin did not induce tumours in mice
and rats.
Like most other chemical substances, aldrin and dieldrin do not
have a single mechanism of toxicity. The target organs are the
central nervous system and the liver. In human beings and other
vertebrates, intoxication following acute or long-term overexposure
is characterized by involuntary muscle movements and epileptiform
convulsions. Survivors recover completely after a short period of
time of residual signs and symptoms. In the liver there is an
increased activity of microsomal biotransformation enzymes,
particularly of the monooxygenase system with cytochrome P-450.
This induction of the microsomal enzymes is reversible and, if it
exceeds a certain level, it appears to be linked to cytoplasmic
changes and hepatomegaly in the liver of rodents.
All the available information on aldrin and dieldrin taken
together, including studies on human beings, supports the view that
for practical purposes these chemicals make very little
contribution, if any, to the incidence of cancer in man.
1.7. Effects on Man
Aldrin and dieldrin are highly toxic for human beings. Severe
cases of both accidental and occupational poisoning have occurred
but only rarely have fatalities been reported. The lowest dose
with a fatal outcome has been estimated to be 10 mg/kg body weight.
Survivors of acute or subacute intoxications recovered completely.
Irreversible effects or residual pathology have not been reported.
Adverse effects from aldrin and dieldrin are related to the
level of dieldrin in the blood. Determination of the level of
dieldrin in the blood provides a specific diagnostic test of
aldrin/dieldrin exposure. The level of dieldrin in the blood of
male workers below which adverse effects do not occur, (the
threshold no-observed-adverse-effect level) is 105 µg/litre blood.
This corresponds to a daily intake of 0.02 mg dieldrin/kg body
weight per day.
Environmental exposure (mainly dietary though also, to a small
extent, respiratory) leads to the presence of dieldrin at very low
levels in organs, adipose tissue, blood, and mother's milk. As far
as can be judged from the extensive clinical and epidemiological
studies, there is no reason to believe that these prevailing body
burdens constitute a health hazard for the general population. In
a continuing study lasting more than 20 years, involving more than
1000 industrial workers in an aldrin/dieldrin insecticide-
manufacturing plant, no increase in cancer incidence occurred among
workers who had been exposed to high levels of aldrin and dieldrin.
More significantly, there were no signs of any premonitory change
in liver function in these workers.
An epidemiological mortality study was carried out at a
manufacturing plant in the USA on a cohort of 870 workers exposed
to aldrin, dieldrin, and endrin. With almost 25 000 man-years of
observation, no specific cancer risk associated with employment at
this plant could be identified.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.1.1. Primary constituent: aldrina
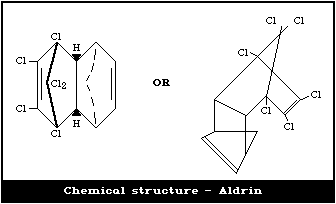 Chemical formula: C12H8Cl6
Relative molecular mass: 364.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 S,8 R,8 R,a R)-1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-1,
4:5,8-dimethanonaphthalene or 1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-
exo-1,4- endo-5,8-dimethanonaphthalene
Common synonyms
and trade names: ENT 15 949 (compound 118), HHDN,
Octalene, OMS 194
CAS registry number: 309-00-2
RTECS registry number: I02100000
Technical product
Common trade name: Aldrin. This is the common name of an
insecticide containing 95% of HHDN.
Purity: The minimum content of aldrin (as defined
above) in technical aldrin is 90%.
Impurities: octachlorocyclopentene (0.4%),
hexachlorobutadiene (0.5%), toluene (0.6%),
a complex mixture of compounds formed by
polymerization during the aldrin reaction
(3.7%) and carbonyl compounds (2%)
(FAO/WHO, 1968b)
-------------------------------------------------------------------
a From: Worthing & Walker (1983).
b Other chemical names are given in Appendix I.
2.1.2. Primary constituent: dieldrina
Chemical formula: C12H8Cl6
Relative molecular mass: 364.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 S,8 R,8 R,a R)-1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-1,
4:5,8-dimethanonaphthalene or 1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-
exo-1,4- endo-5,8-dimethanonaphthalene
Common synonyms
and trade names: ENT 15 949 (compound 118), HHDN,
Octalene, OMS 194
CAS registry number: 309-00-2
RTECS registry number: I02100000
Technical product
Common trade name: Aldrin. This is the common name of an
insecticide containing 95% of HHDN.
Purity: The minimum content of aldrin (as defined
above) in technical aldrin is 90%.
Impurities: octachlorocyclopentene (0.4%),
hexachlorobutadiene (0.5%), toluene (0.6%),
a complex mixture of compounds formed by
polymerization during the aldrin reaction
(3.7%) and carbonyl compounds (2%)
(FAO/WHO, 1968b)
-------------------------------------------------------------------
a From: Worthing & Walker (1983).
b Other chemical names are given in Appendix I.
2.1.2. Primary constituent: dieldrina
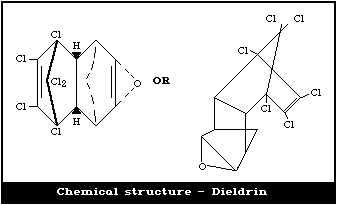 Chemical formula: C12H8OCl6
Relative molecular mass: 380.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 R,6 R,7 S,8 S,8a R)-1,2,3,
4,10,10-hexachloro-1,4,4a,5,6,7,8,8a-
octahydro-6,7-epoxy-1,4:5,8-
dimethanonaphthalene or 1,2,3,4,10,10-
hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-
octahydro- endo-1,4- exo-5,8,-
dimethanonaphthalene
Common synonyms ENT 16 225 (compound 497), HEOD, Alvit,
and trade names: Octalox, OMS 18, Quintox
CAS registry number: 60-57-1
RTECS registry number: I01750000
Technical product
Common trade name Dieldrin. This is the common name of an
insecticide containing 85% of HEOD.
Purity: Technical dieldrin contains not less than
95% of dieldrin, as defined above.
Impurities: other polychloroepoxyoctahydrodimethano-
naphthalenes, endrin 3.5% (FAO/WHO,
1968b)
-------------------------------------------------------------------
a From: Worthing & Walker (1983).
b Other chemical names are given in Annex I.
2.2. Physical and Chemical Properties
2.2.1. Aldrin
Pure aldrin is a colourless crystalline solid. It has a
melting point of 104 - 104.5 °C.
Technical aldrin (90%) is a tan to dark brown solid with a
melting point of 49 - 60 °C. Its vapour pressure is 8.6 mPa at
20 °C (6.5 x 10-5 mmHg at 25 °C). Its density is 1.54 g/ml at
20 °C. Its solubility in water is 27 µg/litre at 27 °C
(practically insoluble), and in acetone, benzene, and xylene
is > 600 g/litre. Aldrin is stable at < 200 °C and at pH 4 - 8,
but oxidizing agents and concentrated acids attack the
unchlorinated ring. Aldrin is non-corrosive or slightly corrosive
to metals because of the slow formation of hydrogen chloride on
storage (Shell, 1976, 1984; Worthing & Walker, 1983).
2.2.2. Dieldrin
Technical dieldrin (95%) consists of buff to light tan flakes
(setting point > 95 °C) with a mild odour. Its melting point is
175 - 176 °C. Its vapour pressure is 0.4 mPa at 20 °C (3.2 x 10-6
mmHg at 25 °C). Its density is 1.62 g/ml at 20 °C. Its solubility
in water is 186 µg/litre at 20 °C (practically insoluble), but it
is moderately soluble in most paraffinic and aromatic hydrocarbons,
halogenated hydrocarbons, ethers, esters, ketones, and alcohols.
Dieldrin is stable to alkali, mild acids, and to light. It reacts
with concentrated mineral acids, acid catalysts, acid oxidizing
agents, and active metals (iron, copper). It is non-corrosive or
slightly corrosive to metals in the same way as aldrin (Shell,
1976; Worthing & Walker, 1983).
2.3. Analytical Methods
2.3.1. Sampling methods
Methods of sampling and storage have been reviewed by Beynon &
Elgar (1966). Sample collection is broadly divisible into two types:
adventitious sampling (particularly of wildlife) and systematic
sampling (soil, total diet surveys) in which samples are collected
in accordance with the principles of statistical design. Surveys
of dieldrin in human blood and adipose tissue are a partial
combination of these two classes of sample collection. The
sampling methods for total diet surveys were reviewed by Cummings
(1966), and the sampling of air for pesticide residues has been
discussed in detail by Lewis (1976).
2.3.2. Analytical methods
Since the introduction of the method of gas-liquid
chromatography with electron capture detection (GLC/EC) (Goodwin et
al., 1961), old methods, based on, for instance, total organic
chlorine or the colorimetric phenyl azide procedure, have been
abandoned. The great majority of analytical data relating to the
occurrence of residues of aldrin or dieldrin since that time have
been based on GLC/EC procedures. There has been considerable
evolution of various aspects (especially extraction and clean up
procedures) of the methodology. The many publications on specific
procedures are reviewed in the Codex Publication "Recommendations
for methods of analysis of pesticide residues", CAC/PR 8-1986,
(FAO/WHO, 1986b). This review lists 22 individual publications,
four of which refer to simplified methods. It also lists the
following compendia of methods which may also be consulted.
- Official methods of analysis of the Association of Official
Analytical Chemists, 14th Edition 1984.
- Pesticide analytical manual, Food & Drug Administration,
Washington DC, USA.
- Manual on Analytical methods for pesticide residues in foods,
Health Protection Branch, Health and Welfare, Ottawa, Canada,
1985.
- Methodensammlung zur Rueckstandsanalytik von
Pflanzenschutzmitteln (Methods for analysing residues of plant
protective agents) 1984 Verlag Chemie GmbH, Weinheim, Federal
Republic of Germany.
- Chemistry Laboratory Guidebook, USDA.
Whatever procedure is adopted should be carried out within the
requirements of the CAC publication "Codex Guidelines on Good
Laboratory Practice in Pesticide Residue Analysis", CAC/PR 7-1984,
(FAO/WHO, 1984).
It is important to recognize that the electron capture detector
is not specific for aldrin and dieldrin and in the analysis of
samples without a precise history of treatment, confirmation of the
identity of the residue is an essential part of the analysis.
Reports of the occurrence of aldrin in environmental samples in the
past, are now thought, in many cases, to have been instances of
misidentification. The occurrence of PCBs in the same sample has
been a particularly troublesome source of interference. Many
procedures for the confirmation of identity are available and
include comparison of the position of the peak on different
chromatographic columns, thin-layer chromatography, and
derivatization. The most definitive method, however, involves the
uses of mass spectrography as the detector. With this procedure,
much of the uncertainty with regard to the identification of the
residue has been eliminated. The mass spectrography procedure
described by Hargesheimer (1984) is effective for the determination
of chlorinated hydrocarbon residues in the presence of PCBs. The
limit of determination of individual methods depends to a
considerable extent on the amount of effort the analyst devotes to
extraction and clean-up procedures. With samples of food and
feeds, for example, a limit of determination of 0.01 mg/kg is
normally regarded as acceptable, but in water and air far lower
levels are achievable, depending on the care and effort taken.
It should be recognized that there is considerable variation in
the results that can be obtained on the same sample by different
analysts and in different laboratories and variations of 100% are
by no means uncommon at the lower end of the scale. A valuable
account of the variation found among 120 laboratories for a sample
of butterfat containing known amounts of 11 different chlorinated
hydrocarbon insecticides was given by Elgar (1979).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Aldrin and dieldrin are not known to occur as natural products.
3.2. Man-Made Sources
3.2.1. Production levels and processes; uses
3.2.1.1 World production figures
The first laboratory synthesis of aldrin and dieldrin was in
1948 by J. Hyman & Co. (Thompson, 1976). The method was licensed
to Shell and manufacture began in 1950, first in the USA and later
on in the Netherlands (IARC, 1974).
Production has decreased since the early 1960s. The production
capacity was 20 000 tonnes in 1971, and the estimated 1972
production was 13 000 tonnes. In 1984, less than 2500 tonnes of
aldrin and dieldrin were manufactured, approximately one third of
which was used in Australia, the United Kingdom, and the USA (Van
Duursen, 1985).
Up to the late 1960s and early 1970s, aldrin and dieldrin were
used throughout the world. Since then, many countries have
severely restricted or banned their use, especially in agriculture,
because of their persistent character in the environment (IARC,
1974). The main remaining uses are in the control of disease
vectors and termites and industrial applications.
3.2.1.2 Manufacturing processes
Aldrin is synthesized by the Diels-Alder reaction of
hexachlorocyclopentadiene with an excess of bicycloheptadiene at
100 °C. The yield is more than 80%, calculated on the
hexachlorocyclopentadiene (Melnikov, 1971).
Commercial production of dieldrin is believed to be through
epoxidation of aldrin with a peracid (e.g., peracetic or perbenzoic
acid), but an alternate synthetic route involves the condensation
of hexachlorocyclopentadiene with the epoxide of bicycloheptadiene
(Galley, 1970).
3.2.1.3 Release into the environment during normal production
Loss of aldrin and dieldrin, together with isobenzan, in waste
water from a manufacturing plant in the Botlek area of the
Netherlands caused deaths among sandwich terns (Sterna
sandvicentis), eider ducks (Somateria mollissima), and, to a lesser
extent, some other bird species, feeding on marine organisms
containing high levels of these insecticides in the Wadden Sea
during 1962 - 65. Following improvement of the waste-water
purification of the plant, the residue levels in the marine
organisms decreased during subsequent years (Koeman, 1971).
3.2.2. Uses
3.2.2.1 Aldrin
Aldrin is a highly effective broad-spectrum soil insecticide.
It kills insects by contact and ingestion, and possesses slight
fumigant action within the soil, which ensures distribution in the
top soil where the pests are found.
It is used to control soil insects, including termites, corn
rootworms, seed corn beetle, seed corn maggot, wireworms, rice
water weevil, grasshoppers, and Japanese beetles, etc. Crops
protected by aldrin soil treatment include corn and potatoes; it is
used as a seed dressing on rice. Aldrin is also used for the
protection of wooden structures against termite attack. It is
supplied mainly as an emulsifiable concentrate or wettable powder.
3.2.2.2 Dieldrin
Dieldrin is used mainly for the protection of wood and
structures against attack by insects and termites and in industry
against termites, wood borers, and textile pests (moth-proofing).
It acts as a contact and stomach poison.
Dieldrin is no longer used in agriculture. It has been used as
a residual spray and as a larvacide for the control of several
insect vectors of disease. Such uses are no longer permitted in a
number of countries.
It is available as an emulsifiable concentrate or wettable
powder.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
4.1.1. Leaching of aldrin and dieldrin
As would be expected from their very low water solubility,
hydrophobic character, and strong adsorption by soil, aldrin and
dieldrin are very resistant to downward leaching through the soil
profile.
Since one of the major uses of aldrin is as a soil insecticide,
aldrin-treated soil is an important source of aldrin in the
environment.
Bowman et al. (1965) studied the leaching of aldrin through six
different types of soil, by passing water through them. In five
out of six soil types, only traces were recovered in the leachates.
However, 16% of applied aldrin was found in the leachate from a
sandy soil type. Other studies indicate that leaching of aldrin
through soil is minimal (Harris, 1969; Herzel, 1971; El Beit et
al., 1981a,b).
A study was carried out to determine the possible involvement
of aldrin applied for the control of termites around house
foundations. Seven types of soil collected from different
geographical areas in the USA were investigated by placing the
soils (adjusted to 0, 5, 10, or 15% water content) in glass
columns. The soil columns were separated into five layers of 5 cm
by filter paper support cloth. An emulsion of aldrin was placed on
the top of the column, equivalent to 0.365 kg aldrin/m2. The
layers of soil were removed approximately 24 h after application of
the emulsion and the concentration of aldrin determined.
Penetration below 20 cm did not occur in any soil at any of the
water contents. In certain soils, penetration only took place in
the first 5 cm and, in others, in the third layer (10 - 15 cm).
Water content also plays a role in the penetration. In another
study, layers of 4 cm were used, with comparable results (Carter &
Stringer, 1970).
Several field studies on the leaching of aldrin through
different types of soil have been carried out. In these studies,
aldrin was applied to the surface or tilled to a depth of about 15
cm at dose levels of 1.8 - 20.7 kg/ha. From the results, it is
clear that, even up to 5 years after application, aldrin and
dieldrin were still present in the treated layer, with little
penetration to layers immediately below the treated layer. From
these studies, it appears that there is little movement
(Lichtenstein et al., 1962; Daniels, 1966; Park & McKone, 1966).
However, Wiese & Basson (1966) found some movement, even in clay
soil.
In studies by Powell et al. (1979), sandy soil in which tomato
plants were growing was sprayed with an aldrin emulsion (2.2 kg/ha)
on six occasions at intervals of 1 - 2 weeks. Approximately one
year after the final treatment, soil core samples were taken and
the concentrations of aldrin and dieldrin in the 0 - 5, 5 - 10,
10 - 15, 15 - 22.5 cm layers were determined. About 73% of the
total residue in the 0 - 22.5 cm layer was in the 0 - 15 cm layer.
The ratio of aldrin to dieldrin in the four strata was similar.
The remark should be made that in this study there were a number of
confounding factors (e.g., the field was ploughed).
Stewart & Fox (1971) applied aldrin as a spray to four turf
plots at doses of 3.3, 4.4, or 6.6 kg/ha. Loam and silt soil core
samples were taken to a depth of 30 cm 9 - 13 years after
treatment. Aldrin was not detected; 93 - 100% of the total
dieldrin in the 30 cm core was in the top 15 cm layer of soil.
In studies by Lichtenstein et al. (1971), aldrin was applied to
a silt loam at a rate of 4.4 kg/ha and rototilled to a depth of
10 - 12.5 cm. After 10 years, the percentage of the applied aldrin
in the 0 - 22.5 cm layer was 0.18% as aldrin and 5.2% as dieldrin.
The ratios of concentrations in the 0 - 15 cm layer relative to the
15 - 22.5 cm layer were: aldrin, 2.5; dieldrin, 4.9.
14C-Aldrin was incorporated to a depth of 15 cm in experimental
plots in which potatoes were grown in the Federal Republic of
Germany (sandy loam; equivalent to 2.9 kg/ha) and England (sandy
clay loam; equivalent to 3.2 kg/ha). After 6 months, the
concentrations of aldrin in both cases were as follows: at 0 - 10
cm, 0.58 and 0.59 mg/kg; at 10 - 20 cm, 0.23 mg/kg and < 0.01
mg/kg; at 20 - 40 cm 0.02 and < 0.01 mg/kg and at 40 - 60 cm, < 0.01
mg/kg (in both locations) (Klein et al., 1973). In a parallel
study, the 14C activity in leach water collected at a depth of 60
cm was determined over a 3-year period; the cumulative rainfall
during this period was 160 cm. About 10% of the 14C activity,
applied initially to a depth of 15 cm, was found in the leachate
over a period of 3 years. Almost all the 14C activity was present
as dihydrochlordene dicarboxylic acid (Moza et al., 1972).
In studies by Stewart & Gaul (1977), aldrin (5.6 and 11.2
kg/ha) was incorporated to a depth of 15 cm into a sandy loam soil
for three successive years. Various crops were grown and soil
samples were collected for 14 years. Residues of aldrin and
dieldrin below 15 cm were negligible in the tenth year after the
initial application, whereas the residues of aldrin plus dieldrin
in the 0 - 15 cm layer were 0.2 and 1.7 mg/kg, respectively, at the
two different treatments levels.
The results of these leaching studies indicate the almost
quantitative adsorption of aldrin by organic matter and clay
minerals. Water molecules compete with aldrin for the adsorption
sites in clay minerals, and it has been found that aldrin is bound
to a greater extent in dry soil (Baluja et al., 1975; Kushwaha et
al., 1978b). The adsorption and desorption of aldrin has been
studied by Tejedor et al. (1974) in whole soil and in the clay and
organic (humic) fractions. It was concluded that the organic
fraction was mainly involved in the adsorptive uptake of aldrin and
that the clay fraction was the major factor affecting the retention
of aldrin. There does not appear to be a simple relationship
between water solubility and leaching, presumably because of the
variations in the adsorptive capacity of clay minerals in various
types of soil (Yaron et al., 1967). A chromatographic model of the
movement of pesticides through soils has been proposed (King &
McCarty, 1968; Oddson et al., 1970).
In the laboratory, the investigations by Eye (1968) and Harris
(1969) of the transport of dieldrin by water through soil are
particularly relevant and are consistent with the chromatographic
model for chemicals in soil of King & McCarty (1968). The elution
of dieldrin from soil by 1600 ml water was investigated in a study
of six types of soil placed in chromatographic columns. The
dieldrin content of the total eluate, as a proportion of the
applied dieldrin, varied from 1% (loam soil) to 65% (soil
containing 93% sand) (Bowman et al., 1965).
The leaching of dieldrin through soil columns (30 cm diameter)
was studied by Thompson et al. (1970). A dieldrin emulsion was
applied to the surface (equivalent to 31 kg dieldrin/ha) of soil
columns 35 cm deep, and water was added to the surface until about
30 litres (equivalent to about 6 months rainfall) had passed down
the columns in 120 h. It was concluded that dieldrin did not
readily leach from the three types of soil investigated into
drainage water, and that cracks and crevices caused by drying or by
earthworms and other animals favour the leaching of dieldrin. The
results of an investigation using sloping troughs gave results
consistent with the soil column study.
4.1.2. Surface run-off
Run-off from treated land caused by soil erosion is a potential
source of dieldrin residues in surface waters in areas where
erosion is not controlled by good farming practice. Sediments
bearing aldrin and dieldrin can result in low concentrations in
aqueous solution, although these are limited due to adsorption onto
the sediments. Thus, rain-water run-off (without sediment) does
not appear to be a major contributor.
Richard et al. (1975) and Sparr et al. (1966) sampled various
surface waters in the USA and reported levels of dieldrin ranging
from < 1 to 42 ng/litre and of aldrin in the region of 0.05
µg/litre.
To gain data on the erosion of treated land, Caro & Taylor
(1971) and Caro et al. (1976) incorporated dieldrin into the soils
of two small watersheds in Ohio, USA, and studied run-off losses
over a three-year period. In the first case, there was practically
no surface soil erosion and the total loss of dieldrin was confined
to run-off water. The area was 1.07 ha and the loss over the
period was less than 0.5 g dieldrin, the highest level in the water
being 4 µg/litre. In the second study, there was a substantial
loss of soil by erosion and the amount of dieldrin lost in the
solid sediment was 77 g in only 8 months. The loss in the water
itself was just under 2.5 g and the highest water concentration was
20 µg/litre. It should, however, be borne in mind that in this
case the soil had been mechanically compacted to aggravate the
effects of erosion, so that it is questionable whether the results
bear much relation to normal agricultural practice. The authors
commented that there was only a poor correlation between rainfall
events and the amounts of dieldrin lost.
Sediment-bearing residues of aldrin or dieldrin will yield some
of their burden to true solution in the water which they enters.
Sharom et al. (1980) showed that the ratio of dieldrin
concentration in soil to that in water (in equilibrium with the
soil) was between 100 and 500 for mineral soils, whilst that same
ratio for aldrin was likely to be around 5 - 6 times higher. Thus,
with 1 mg dieldrin/kg sediment, one could expect a water
concentration of about 10 µg/litre.
The movement of aldrin and dieldrin by run-off and soil erosion
was studied by Haan (1971). Each pesticide was applied at 1.65
kg/ha to the surface of small plots, mainly consisting of silt loam
(slope, 1 - 2%), in a greenhouse. Water was applied and the run-
off water, sediment, and surface soil (0.6 cm deep) were analysed.
It was estimated that 94.8% and 95.4%, respectively, of the applied
aldrin and dieldrin remained in the surface soil (0.6 cm depth).
It was concluded that there was no difference in the potential for
loss from soil by rainfall, whether the rainfall occurred shortly
after aldrin application or several days later.
4.1.3. Loss of aldrin and dieldrin from soils - volatilization
Most authors consider that the principal loss of aldrin and
dieldrin from soils is by volatilization. There is widespread
evidence for this, although other mechanisms (sections 4.4.1 and
4.4.2) may also play an important role.
Volatilization from soils was first demonstrated when it was
shown that mosquitoes were killed by vapour emanating from treated
soil blocks (Barlow & Hadaway, 1955, 1956; Gerolt, 1961).
When aldrin is incorporated into the soil, it is most readily
lost from the surface layer. Subsequently, material from deeper
layers has to rise to the surface to replenish what was lost. The
position is somewhat complicated by its gradual conversion to the
less volatile dieldrin, although this, too, behaves in a
qualitatively similar manner.
There are two routes to the surface: transport in ascending
capillary water - analogous to the process of salinization - and
vapour diffusion through the soil pores. Both of these processes
are strongly affected by hydrophobic adsorption, a phenomenon
common to many hydrophobic pesticides of low water solubility.
Adsorption by the soil has the effect, at practical rates of
application, of reducing the vapour pressure and hence the
saturation vapour density in the soil atmosphere. It also reduces
the maximum concentration in the soil solution.
There is a very extensive literature on soil adsorption,
especially of dieldrin and the following general situation is now
well established.
Adsorption, as measured by reduced vapour density, takes place
in all soils but is greatest at low moisture levels; that is to say
soils in equilibrium with air of relative humidity below around
95%. (Barlow & Hadaway, 1955, 1956; Gerolt, 1961; Harris, 1964,
1972; Igue et al., 1972).
In dry soils, mineral components play the most important part,
whereas in moist soils it is organic matter that dominates (Harris
& Lichtenstein, 1961; Harris et al., 1966; Harris & Sans, 1967;
Harris, 1972). In fact, Harris demonstrated a linear relation
between organic matter and adsorption in moist soils. On the other
hand, in a dry mineral soil with predominantly montmorillonitic
clay and very low organic matter, practically no dieldrin
volatilized until the relative humidity of the air in equilibrium
with soil reached saturation. At this point volatilization readily
resumed.
In moist soils, Spencer et al. (1969) found that adsorption,
expressed as a reduction in vapour density, became less marked as
the dieldrin level increased. At 20 °C, 10% moisture in the soil,
and 1 mg dieldrin/kg soil, the dieldrin vapour density was only 2
ng/litre, compared with 52 ng/litre when the dieldrin level in the
soil was increased to 25 mg/kg. This level is close to the figure
for free dieldrin. Similar results were reported at 30 °C and
40 °C by Spencer & Cliath (1973).
In dry soils, however, adsorption is far stronger. At 100 mg
dieldrin/kg moist soil (Spencer et al., 1969), the depression in
vapour pressure was negligible. However, as the moisture content
of the soil fell to a critical level of 2.1%, there was a dramatic
decrease in vapour density, so that below 2% moisture the vapour
density was practically zero. The same authors showed that the
level of water in their soil needed to provide a monomolecular
layer was 2.8%. They concluded that the critical point at which
adsorption increased was when the monomolecular layer started to be
lost, leaving adsorption sites available for occupation by
dieldrin. Restoration of the moisture status of the soil, however,
restored the vapour density to its original level.
Whilst most of these studies were carried out on one soil, Gila
silt loam, and whilst the figures would be different for other
soils, the qualitative conclusions are largely valid for all soils.
Adsorption is expected to be least on sandy soils of low organic
matter content.
Adsorption by soils can also be determined by measuring the
reduction in the saturation concentration of the soil solution
(Eye, 1968; Tejedor et al., 1974; Baluja et al., 1975). As in the
case of reduced vapour pressure caused by adsorption by moist
soils, the organic matter content of the soil was the principal
soil characteristic affecting adsorption from solution. Eye (1968)
also demonstrated the dominating influence of organic matter,
whereas clay content, surface area, and cationic exchange capacity
showed very little correlation. These findings are compatible with
those of Yaron et al. (1967).
In studies involving the percolation of dieldrin, dissolved in
water, through columns of soils with differing contents of organic
matter, Sharom et al. (1980) also showed that the soil capacity for
adsorption was largely determined by its content of organic matter.
Moreover, adsorption followed the Freundlich adsorption equation.
They reported Freundlich adsorption constants for a range of soils
and pesticides, including dieldrin, and showed that, for a given
pesticide, adsorption was strongly dependent on the organic matter
content of the soil. Moreover, the strength of adsorption by a
given soil depended mainly on the water solubility of the
pesticide, so that dieldrin, with its low water solubility, was
more strongly adsorbed than, for instance, the much more water-
soluble lindane. Although aldrin was not studied, it may be
inferred from these data that aldrin would be adsorbed
correspondingly more strongly, owing to a much lower water
solubility than that of dieldrin.
4.1.3.1 Movement within the soil profile - mass flow
Spencer & Cliath (1973) concluded from laboratory studies that
dieldrin could ascend the soil profile by mass flow in capillary
water moving up to the surface through a moisture gradient, and
that this mechanism could account for 3 - 30% of the total upward
movement. However, with low solubility products such as dieldrin,
Jury et al. (1983) pointed out that volatilization decreases with
time, because ascent to the surface is rate limiting. With high
solubility compounds, however, the reverse is true as more material
reaches the surface, dissolved in capillary water, to become
available for evaporation. However, it is not only water
solubility that determines the behaviour, but the value of Henry's
constant for the partition of the compound between air and water.
These authors considered the critical value to be 2.7 x 10-5; above
this value mass flow is progressively less important. The value of
Henry's constant for dieldrin (6.7 x 10-4) is substantially higher
(Jury et al., 1983) and that for aldrin higher still, so that on
this basis it is doubtful whether mass flow ever does play a
significant role in the transport of aldrin or dieldrin up the soil
profile.
In support of the view that transport by mass flow is not
appreciable, the mathematical models that have been proposed to
describe the loss of aldrin and dieldrin from soils (Farmer &
Letey, 1974; Mayer et al., 1974; Jury et al., 1983) tend to
demonstrate, in comparisons with laboratory data, that ascent to
the surface is predominantly by vapour diffusion rather than mass
flow.
4.1.3.2 Movement within the soil profile - diffusion
Diffusion is regarded as the main route by which aldrin and
dieldrin ascend the soil profile to reach the surface. Diffusion
increases with soil temperature, concentration, decreasing
adsorption capacity (usually the same as decreasing organic
matter), maintenance of moisture content above the wilting point,
and the "tortuosity" of the soil pore system (a measure of the
openness of the soil). With regard to moisture content, Farmer &
Jensen (1970) found that diffusion coefficients of dieldrin in
three soils in equilibrium with air of 94% relative humidity were
9.7, 4.4, and 3.8, but at 75% relative humidity the values were
0.6, 0.4, and 0.4, respectively. According to Farmer & Letey
(1974), the critical moisture level is probably the "fifteen
atmosphere percentage", usually considered to be a reasonable
measure of the water content at the wilting point.
Tortuosity increases as soils are compacted. Working with
moist soils of differing bulk densities, Farmer et al. (1973),
showed that diffusion of dieldrin was about twice as fast in a soil
with a density of 0.75 g/cm3 as when it was compressed to a bulk
density of 1.5 g/cm3.
4.1.3.3 Actual volatilization losses - laboratory studies
Lichtenstein & Schulz (1970) reported that aldrin was lost by
volatilization from a silt loam soil about 20 times faster than
dieldrin. Helene et al. (1981) reported a 31% loss of aldrin from
a highly humic soil after 120 days but 62% from a soil of low
organic matter content.
In studies of moist soils in volatilization chambers, Farmer et
al. (1972) and Igue et al. (1972) found that the rate of loss by
volatilization gradually decreased with time. However, if
translated into terms of the open field, this could still represent
a loss of between 0.2 and 1.4 kg/ha per year, depending on the
depth of incorporation.
With a surface application of dieldrin in a microagroecosystem
chamber, Nash (1983) reported loss of dieldrin at the rate of 1 - 4
g/day, but this rate fell to about a half of its initial value
within 6 - 7 h. Incorporation of the dieldrin had the effect of
greatly slowing this loss rate (Nash, 1983).
4.1.3.4 Actual volatilization losses - field studies
The data on volatilization losses in the field are limited and
refer only to dieldrin. Caro & Taylor (1971) reported loss by
volatilization from an incorporated dieldrin application (5.6
kg/ha) of 2.8% of that applied (after 18 weeks). Spencer et al.
(1973) cited unpublished studies by Caro & Taylor (1971) where a
surface application was lost at the rate of 3% per hour. In a
later study, Caro & Taylor (1976) found that 4.5% of a dieldrin
application was lost by volatilization in the first year after
treatment. By the autumn, the loss rate was only 0.2 g/ha per day,
although this increased to 0.9 g/ha per day immediately after the
land was cultivated, due, presumably, to the exposure of fresh
soil.
Taylor et al. (1972, 1976) estimated a loss of dieldrin of 0.2
kg/ha from an incorporated application of dieldrin. However, only
6% remained from a surface application after 16 weeks, although in
this case a small amount was recovered as photodieldrin (Turner et
al., 1977).
Willis et al. (1972) demonstrated an 18% loss from a very high
application (22 kg/ha) of dieldrin after 5 months where the soil
was kept moist by irrigation. However, losses were substantially
less when the soil was not irrigated or when maintained under flood
conditions. The maximum rate of loss by volatilization was 0.2
kg/ha per day.
4.1.4 Losses of residues following treatment of soil with aldrin
One of the earliest systematic studies of the decline of aldrin
and dieldrin residues in soils, arising from the application of
aldrin to the soil, was by Decker et al. (1965), who sampled a wide
range of soils of known treatment history from Illinois, USA. They
demonstrated the transformation of aldrin to dieldrin and
considered that the loss of residues was a two-stage process.
There was a comparatively rapid loss in the first year after
treatment, a typical loss being 75% of the applied dose.
Thereafter, residues declined with a half-life of 2 - 4 years, the
reduced rate being apparently due to the greater proportion of
dieldrin in the residues. Elgar (1966) incorporated 2.2 kg
aldrin/ha into soils in the United Kingdom and reported somewhat
similar results for the decline of residues, although there were
indications that the rate of decline slowed in later years as the
level in the soil fell to around 0.3 mg/kg. Further studies of
this kind have been reported by Lichtenstein et al. (1970), Onsager
et al. (1970), and Korschgen (1971). Although the rates of decline
were very variable, they were not inconsistent with the data of
Decker et al. (1965), bearing in mind the inherent variability of
soil data.
There are indications that loss rates are higher in tropical
soils than in temperate climates. Whilst Agnihotri et al. (1977)
found that epoxidation was faster in tropical than temperate soils,
leading to the possibility of slower decline because of higher
dieldrin levels, Gupta & Kavadia (1979) found in India that
declines were often much faster. In one case, half of the aldrin
applied had been lost in only 38 days. Wiese & Basson (1966) also
reported comparatively high loss rates in South Africa. Using
three rates of treatment and three soils, they found that half of
the original application was lost between 1 and 2 months.
Elgar (1975) conducted a series of studies in temperate, warm
temperate, and tropical soils and reported rates of decline that
were compatible with those of Decker et al. (1965). Again, losses
from the tropical sites occurred more rapidly than from the
temperate sites. He deduced the following empirical expression to
describe loss rates, expressed as the sum of aldrin and dieldrin
residues surviving n years after a single application.
C(n) = fC(o)(1-p)n-1
In this expression, C(o) is the initial residue level, C(n) is the
level after n years, f is the proportion remaining after the first
year, and p is the proportion lost in each of the succeeding years.
In Elgar's studies, the mean estimate of these latter two
parameters was f = 0.25 and p = 0.44, but in the Decker work, the
value of p was somewhat less. It is also possible to derive an
equation that describes the accumulation of residues in a soil
subject to a regular routine of annual applications. The
implications of this equation are that residue levels do not
continue to increase indefinitely, but reach a plateau. In the
case of Elgar's data, the plateau level, one year after the last of
n applications, would be around 60% of the level observed
immediately after the first application. This prediction is well
borne out by the soil monitoring data presented in Table 1.
Studies of the decline of residues arising from aldrin applied
for the control of termites (Bess & Hylin 1970; Carter & Stringer,
1970) reveal slower rates of decline than would be expected,
considering the deep application.
Separate studies have been carried out on dieldrin residue
losses. These show considerably slower rates of decline than in
the case of aldrin, but there is a very wide range in the data
reported. Thus, Edwards (1966) reported that the average time for
the disappearance of 95% of the residues was 8 years, but Wiese &
Basson (1966) found much faster rates. Intermediate rates were
reported by Stewart & Fox (1971) and Beyer & Gish (1980). It seems
probable that the rate of decline of dieldrin in the soil is
reasonably well reflected by Elgar's equation for the years that
succeed the first year of aldrin application.
4.1.5. Losses of residues from water
The partition of dieldrin between the vapour phase and water
was determined by a dynamic gas-flow method using 14C-dieldrin
(Atkins & Eggleton, 1970). The partition coefficient at 20 °C
(expressed on a weight/volume basis for air and water) was constant
at 540, up to a concentration of 0.033 mg dieldrin/litre water. At
higher concentrations, there was a rapid increase in the partition
coefficient, which was attributed to the aqueous solution becoming
saturated at 0.033 mg/litre. Using the values for vapour pressure
(3.47 x 10-4 Pa) and water solubility found in this study, the
wash-out ratio for the removal of dieldrin vapour from atmospheric
air by rain was 0.65. It was suggested that the concentration of
dieldrin in the rainfall in London (Abbott et al., 1965) (Table 6)
may indicate the presence of dieldrin in particulate matter in the
atmosphere rather than in the vapour phase.
Table 1. Concentrations of aldrin and dieldrin in soila
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
United aldrin: potatoes 21 0.02 0.09 LD < 0.03 mg/kg Wheatley et
Kingdom (0.12) (0.41) al. (1962)
1965 aldrin: potatoes; 10 0.15 0.48 LD not reported; apparently Davis (1968)
dieldrin: seed-dressing, (0.7) (0.7) < 0.02 mg/kg; various soil
carrots, and wheat; types; residues in soil
cumulative applications microfauna also determined
during 5 years prior to
sampling (0.14-3.4
kg/ha)
Canada
S.W. Ontario 1964-65 aldrin: various crops; 13 0.19 0.57 LD < 0.1 mg/kg; soil of Harris et al.
known usage (0.8) (1.3) various types (sandmuck); (1966)
aldrin used to a
considerable extent
(1954-60) on 27 sites
no reported use 1961-64 14 0.18 0.25
(2.1) (1.6)
none used 1954-64 5 LD LD
Atlantic 1965 aldrin: 1-5 applications LD 0.01 mg/kg; no detectable Duffy & Wong
provinces during 15 years prior to residues of aldrin or (1967)
sampling; cumulative dieldrin in orchard soils to
application 0.5-45 kg/ha; which aldrin/dieldrin had
not been applied
root crops 18 0.46 0.41
(1.5) (1.45)
vegetables 17 0.66 0.36
(2.5) (1.35)
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
Southern 1971 aldrin: tobacco 4 (50 ND 0.16 LD 0.001 mg/kg; woodlots Frank et al.
Ontario samples) (0.19) were adjacent to treated (1974)
areas, but not directly
sprayed
cereals 4 (60 ND 0.16
samples) (0.19)
woodlots 12 ND trace
samples
Saskatchewan 1970 soil from 21 vegetable 41 0.03 0.06 LD 0.005 mg/kg; aldrin found Saha & Sumner
farms samples (0.28) (0.77) in 25% of samples; dieldrin (1971)
found in 55% of samples
Southern 1972-75 soil samples from LD < 0.0004 mg/kg; dieldrin Frank et al.
Ontario orchards had been used (1955-65) (1976)
at recommended rates of
0.8-1.3 kg/ha
apple: 0-15 cm 31 ND 0.03
(0.38)
15-30 cm ND 0.001
(0.03)
Southern 1972-75 sweet cherry: 16 Frank et al.
Ontario 0-15 cm ND 0.001 (1976)
(0.01)
15-30 cm ND LD
sour cherry: 12
0-15 cm ND 0.005
(0.04)
15-30 cm ND 0.003
(0.02)
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
Southern peach: 11
Ontario 0-15 cm ND 0.04
(contd.) (0.11)
15-30 cm ND 0.02
(0.07)
vineyards: 16
0-15 cm ND 0.009
(0.035)
15-30 cm ND 0.004
(0.023)
USA
Seven eastern 1965 aldrin and dieldrin in 3 LD 0.05 mg/kg; proportions Seal et al.
states crops: of soil samples with (1967)
measurable residues:
peanuts: 5 ND 0.15 potatoes, 76%; carrots,
(0.20) 21%; peanuts, 100%
carrots: 19 ND 0.19
(0.26)
potatoes: 25 ND 0.10
(0.20)
1965-67 aldrin and dieldrin used 17 (278 0.02 0.21 LD 0.01 mg/kg; aldrin Stevens et al.
regularly samples) (0.47) (2.84) detected in 15% of samples (1970)
and dieldrin in 67% of
samples from areas of
regular use
limited use 16 LD 0.001
(0.001)
no known use 18 LD LD
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
Colorado 1967 aldrin: various soil 11 0.16 0.19 LD < 0.02 mg/kg; some Mullins et al.
types (1-4.3% organic (0.61) (0.44) fields had been treated (1971)
matter); nominal annually for 9 years; time
concentrations in soil at of last treatment prior to
time of application: sampling varied from 0-9
0.06-6.75 mg/kg years
dieldrin: nominal 9 ND 0.05
concentrations in soil at (0.30)
time of application:
0.13-0.63 mg/kg
Arizona 1968 3 types of soil (organic 13 LD 0.0003 LD not defined; appears to Laubscher et
matter 0.5-6.6%) from (0.0013) be about 0.0001 mg/kg; no al. (1971)
area downwind of relationship between
an area of insecticide concentration of dieldrin
use and distance from area of
application
10 major 1969 samples of soil 71 0.02 0.79 LD 0.01 mg/kg; aldrin in Wiersma et al.
areas of (0.96) (16.72) 4.2% of samples and (1972)
onion growing dieldrin in 73% of samples
9 areas 1969 samples of soil 92 0.01 0.17 LD 0.01 mg/kg; aldrin in Sand et al.
growing sweet (0.11) (2.18) 3.3% and dieldrin in 60.9% (1972)
potatoes of samples
Rice-growing 1972 samples of soil 99 0.01 0.04 LD 0.01 mg/kg; aldrin in Carey et al.
areas (0.25) (0.27) 39% and dieldrin in 85% of (1980)
samples
USA National 1970 samples of soil 1506 0.02 0.04 LD 0.01 mg/kg; aldrin in Crockett et al.
Monitoring (4.25) (1.85) 13% and dieldrin in 31% of (1974)
Program samples
(35 states)
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
12 states in 1970 average application of 12 (389 0.05 0.07 LD <0.01 mg/kg; dieldrin Carey et al.
the cornbelt dieldrin was 1.3 kg/ha samples) (2.98) (2.04) residues attributed (1973)
region primarily to the use of
aldrin; aldrin had been
used in one or more years
from 1954
14 cities 1970 soil from urban areas 356 LD 0.1 LD < 0.03 mg/kg; aldrin Carey et al.
sampled to a depth of (12.8) not detected in any (1976)
7.6 cm samples; dieldrin in
samples from 22 sites
(6.5%) in 6 cities
Japan, S.W.
Kyushu 99 0.07 0.29 LD 0.001 mg/kg Suzuki et al.
district samples (1.01) (1.73) (1973)
------------------------------------------------------------------------------------------------------------------------------
a LD = limit of detection; ND = not determined.
The rate of dry deposition of dieldrin (vapour phase) on grass,
calculated from the results of wind tunnel studies, was 4 x 10-2
cm/second. The average lifetime of dieldrin in the atmosphere,
assuming loss by wash-out and dry deposition only, was estimated to
be 28 weeks (Atkins & Eggleton, 1970).
The rate of transfer of dieldrin from water to air and vice
versa has been determined (Slater & Spedding, 1981). The transfer
velocity from water, measured in a wind tunnel, increased as the
air speed (measured at 6 cm above the water surface) increased.
When there was no air movement, the transfer velocity was 2.6 x
10-5 cm/second compared to 15 x 10-5 cm/second at an air velocity
of 31.1 km/h. The transfer velocity from air to water was measured
by passing air through a column of downward-flowing water, and was
found to increase as the interfacial velocity increased from 0.9 x
10-2 cm/second (at 10 km/h) to 5.2 x 10-2 cm/second (at 34.2 km/h).
It was suggested that the exchange of dieldrin between water and
air was controlled by diffusive processes either in the air
boundary or water boundary layers. The Henry's law constant (ratio
of the concentrations in air and aqueous phases at equilibrium) for
dieldrin was 1.3 x 10-3 at 20 °C. It was concluded that the
resistances to transfer of dieldrin from water to air and vice
versa were similar.
The physical and thermodynamic principles of exchanges of
chemicals between water and air have been discussed (Mackay &
Wolkoff, 1973; Liss & Slater, 1974; Mackay & Leinonen, 1975; Mackay
et al., 1979; Smith et al., 1981). An estimate of the half-life of
the evaporation of dieldrin at 25 °C from a column of water of 1 m
depth was derived by Mackay & Leinonen (1975). Although this
estimate (539 days) is not based on the most recent and reliable
values for the vapour pressure and water solubility of dieldrin, it
is probably of the right order.
4.1.6. Aldrin and dieldrin in the atmosphere
Small amounts of dieldrin have been detected in the atmosphere
(Table 6). Baldwin et al. (1977) conducted a study at Bantry Bay
on the west coast of Ireland, well away from point sources of
emission. They found concentrations of dieldrin between 0.06 and
1.6 ng/kg, with an average of 0.36 ng/kg, but no aldrin,
photodieldrin, or photoaldrin. No dieldrin was detected on solid
matter trapped on filter pads; the limit of determination ranged
from 1.1 to 7.2 pg/kg (parts per thousand trillion of air).
The reason for the very low level of occurrence of dieldrin in
the global atmosphere, if, as seems probable, a major part of the
aldrin used in agriculture escapes from the soil by evaporation,
has been the subject of considerable speculation. It appears
unlikely that direct photochemical reactions are involved, since
there have been no reports of photodieldrin being detected.
Washout by rain may be an important factor. Indeed, Baldwin et al.
(1977) cited literature figures for Hawaii of 1 - 97 ng/litre, and
Abbott et al. (1965) reported 1 - 95 ng/litre in rainfall in London
and other locations in the United Kingdom. MacCuaig (1975), on the
other hand, working in the vicinity of a dieldrin application in
Ethiopia, reported 100 µg/litre in rainwater. These results
support the suggestion of Atkins & Eggleton (1970) that, though
washout of the atmosphere by rain would be inefficient in the case
of dieldrin, it could lead to substantial losses. If this were so,
dieldrin deposits would be expected on soil adjacent to treated
areas, but the fact that large areas of soil in the cornbelt of the
USA (Carey et al., 1973) have no detectable levels of aldrin or
dieldrin seems to cast doubt on the extent to which rain acts to
disperse aldrin and dieldrin onto untreated land near to treated
areas.
It would appear possible, therefore, that there are losses of
aldrin and dieldrin in the atmosphere. Glotfelty (1978) mentioned
the high reactivity of free radical species in the atmosphere, in
particular hydroxyl radicals. These could presumably play an
important role in the degradation of molecules occurring as vapour.
4.1.7. Aldrin and dieldrin in water
The data regarding the occurrence of aldrin and dieldrin in
both ground and surface waters are summarized in Table 7 (section
5.1.3). As would be expected from the extreme resistance of
dieldrin and, especially, aldrin to leaching from soil, the
occurrence of either compound in groundwater is rare. Spalding et
al. (1980) took a series of groundwater samples in Nebraska, USA,
where aldrin had been used extensively for the control of corn
rootworm and could not detect it in any of the samples. Their
limit of determination was between 5 and 10 ng/litre. Junk et al.
(1980) reported somewhat similar results from Nebraska. Richard et
al. (1975), in a wide-ranging study, examined the water supplied to
a series of cities in Iowa, USA, from boreholes. Again, no aldrin
or dieldrin was reported; their limit of determination appears to
have been 0.5 ng/litre.
Surface waters, by contrast, have often been reported to
contain small amounts of dieldrin. In a programme of sampling
various surface waters in Iowa, Richard et al. (1975) reported
levels of dieldrin ranging from 3 to 75 ng/litre in rivers and
streams and levels in reservoirs from 3 to 18 ng/litre. In rivers
in Iowa and Louisiana, levels ranged from < 1 to 42 ng/litre.
During the period 1976 - 80, dieldrin was found in 2.4% of samples
from national surface waters in the USA, (maximum concentration of
0.61 µg/litre) and in 21.7% of national surface water sediments
(maximum concentration of 5300 µg/kg) (Carey & Kutz, 1985).
The dieldrin in surface water probably comes from run-off from
treated land. Sparr et al. (1966) sampled drainage ditches and a
river in a maize growing area in northwest Indiana, USA. Levels
reached 0.6 µg/litre in the river but, in the ditches from fields
treated with aldrin at up to 5.6 kg/ha, levels seldom exceeded the
limit of determination (0.05 µg/litre). Water draining from rice
paddies that had been planted with aldrin-treated seed also
contained small amounts of dieldrin (1 µg/litre after seeding and
falling by the 14th week to 0.07 µg/litre). The authors calculated
that about 1 g of aldrin had been lost from the rice paddy surface
water during the whole 14-week period.
Hindin et al. (1964) reported aldrin in irrigation water up to
2.3 µg/litre, but no dieldrin. However, in view of the readiness
with which aldrin is epoxidized to dieldrin in surface waters,
there must be some doubt as to the identity of the residue they
actually measured.
It does appear that dieldrin can occur in surface waters
draining from agricultural areas, but the amounts are usually so
small that they could not be expected to represent a major
proportion of the product applied to the soil. The ultimate fate
of these small levels of dieldrin in water is not known. It is
probably that adsorption onto particulate matter, volatilization,
and various degradation mechanisms all play a role.
4.2 Translocation From Soil Into Plants
The uptake of aldrin and dieldrin by plants is much higher in
root crops than in grain crops. It is influenced by the levels in
soils, the strength of adsorption, and the depth of application.
In grain crops, it is rare for residues to reach detectable
levels in the grain (FAO/WHO, 1970a; Gupta & Kavadia, 1979). Root
crops are much more prone to take up residues from treated soils,
as observed by Harris & Sans (1967) who found that carrots,
radishes, and turnips had the highest residues. Onions, lettuce,
and celery were intermediate and cole crops showed no detectable
uptake at all (Lichtenstein, 1959).
The level of aldrin and dieldrin in the soil influences the
degree of uptake as shown by Lichtenstein et al. (1970) and Edwards
(1973a,b), who both reported on ratios of the concentrations in
plants to those in the soil. Further work by Onsager et al.
(1970), Voerman & Besemer (1975), Bruce & Decker (1966), and Saha
et al. (1971) provided compatible results.
The availability of aldrin and dieldrin for uptake by plants
depends on the strength of adsorption by the soil and especially
the organic matter fraction. Harris & Sans (1967), Beall & Nash
(1969), Beestman et al. (1969), and Nash et al. (1970) demonstrated
that crops tend to take up more residues from soil of low than of
high organic matter. Adding activated charcoal to soil reduced
dieldrin uptake by 70% or more in carrots and potatoes
(Lichtenstein et al., 1971).
Deep application of dieldrin greatly reduces the uptake (Beall
& Nash, 1972). Residues in the plants from a deep (31 - 32 cm)
application were only 1% of those from superficial application.
The authors commented that a possible treatment for reducing the
uptake of old soil residues by crops would be simply to plough them
under.
The mechanism of uptake by crops is not entirely clear and
appears to vary considerably from species to species. Beall & Nash
(1971), in work with soyabeans grown on soil treated with 14C-
labelled dieldrin, found that residues were taken up both by
absorption through the roots and by absorption of vapour through
the leaves. In the case of cereals, it seems unlikely that root
uptake occurs to any great extent (Powell et al., 1970; Gutenmann
et al., 1972; Gupta et al., 1979). This probably accounts for the
very low levels found in cereal grains from treated crops. On the
other hand, it would seem almost certain that it is root uptake
which accounts for the residues found in root crops.
4.3. Models of the Behaviour of Water and Chemicals in Soil
Various models for the movement of water and chemicals in
porous media have been developed, based on physical variables such
as vapour pressure, diffusibility, and adsorption, etc. (Keller &
Alfaro, 1966; Bresler & Hanks, 1969; Lindstrom et al., 1971;
Davidson & McDougal, 1973; Pionke & Chester, 1973; Van Genuchten et
al., 1974). Models for run-off from soil have also been proposed
(Crawford & Donigian, 1973; Bailey et al., 1974; Bruce et al.,
1975). These models may be useful as a means of defining more
precisely the behaviour of aldrin and dieldrin in soil.
4.4. Biodegradation of Aldrin and Dieldrin
When used to protect crops from soil insects, aldrin is usually
incorporated into the soil in which the plants are grown. For this
reason, most of the work on the biodegradation of aldrin in
agriculture has been concerned with the soil system.
4.4.1. Epoxidation of aldrin
The most important transformation of aldrin in the soil is its
conversion by epoxidation to dieldrin (Fig. 2, section 6.3.1.1).
Epoxidation, essentially biological in nature (Lichtenstein &
Schulz, 1960), occurs in all aerobic and biologically active soils,
and about 50 - 70% of the residues remaining in a soil at the end
of the season in which the application was made consist of
dieldrin. Lichtenstein & Schulz (1959) reported that epoxidation
was slower on peat than on mineral soils and was inhibited at low
soil temperatures; very little conversion occurred at 7 °C.
Subsequently, many authors have demonstrated that a large number of
microorganisms are capable of promoting epoxidation, and these were
reviewed by Tu & Miles (1976).
Aldrin is also epoxidized by plants, as demonstrated by Gannon
& Decker (1958), while Yu et al. (1971) have showed that root
homogenates are very effective promoters of aldrin-to-dieldrin
epoxidation.
Aldrin is not epoxidized under anaerobic conditions. In their
studies on the degradation of aldrin in anaerobic cultures of
sewage sludge, Hill & McCarty (1967) found no dieldrin, although
aldrin was completely decomposed within 60 days. Sethunathan (1973)
reported that epoxidation of aldrin was arrested in flooded soils.
4.4.2. Other metabolic pathways of aldrin
The transformation of aldrin in the soil to aldrin dicarboxylic
acid (V, Fig. 2) appears to be well established (Klein et al.,
1973; Kohli et al., 1973b; Weisgerber et al., 1974). The
occurrence of photodieldrin (III, Fig. 2) as a metabolite derived
from aldrin soil treatment is less well established either in soil
(Lichtenstein et al., 1970) or in the leaves of wheat grown in
aldrin-treated soils (Weisgerber et al., 1974).
4.4.3. Biotransformation of dieldrin
Dieldrin is much more resistant to biodegradation than aldrin,
and microbial degradation is probably a minor route of loss from
soils, even under anaerobic conditions (Sethunathan, 1973; El Beit,
1981). Kohli et al. (1973b) added 14C-labelled dieldrin to a soil
and detected very little degradation, though he did report trace
quantities of photodieldrin. Similarly, small amounts of
photodieldrin were detected after dieldrin had been applied to
onion seed (Kohli et al., 1972).
In the search for organisms that would degrade dieldrin,
Matsumura & Boush (1967) found that only a few soil samples
produced detectable transformation of dieldrin, although, in some,
up to 6% of the dieldrin added was transformed to water-soluble
metabolites. Separation of the organisms responsible revealed that
Pseudomonas, Bacillus, and Trichoderma species were able to attack
the dieldrin molecule. Tu & Miles (1976) list organisms that have
been reported to attack dieldrin; these include bacteria, fungi,
and one actinomycete.
In spite of the large number of studies on this topic, it is
difficult to estimate the extent to which photodieldrin is evolved
in soils treated with aldrin. It is perhaps significant that the
microorganisms capable of producing photodieldrin in the laboratory
have been isolated mainly from anaerobic environments, so that
their activity would be very limited in a well-managed agricultural
soil. This is borne out by the study of Suzuki et al. (1974) who
sampled 52 soils with a history of aldrin treatment in Japan.
Photodieldrin levels were very low compared with dieldrin levels
and ranged from < 0.001 to 0.035 mg/kg soil (section 4.4.2.1).
The further fate of photodieldrin in soils has received little
attention, but Weisgerber et al. (1975) considered it to be less
persistent in the soil than dieldrin itself. They also identified
two breakdown products, the bridged equivalent of aldrin
dihydrochlordene dicarboxylic acid (XII, Fig. 2) and the bridged
equivalent of the transdiol (XI, Fig. 2), though these were only
present in very small amounts.
4.4.4. Conclusions
Although many studies have been carried out on the
biodegradation of aldrin and dieldrin, it seems improbable that
this is a major source of loss from soil. On the other hand, it
does seem as if transformation of aldrin to aldrin acid in aldrin-
treated soils can be a significant pathway, although there is
little evidence in the literature that aldrin acid occurs widely as
an environmental residue.
4.5. Abiotic Degradation
Abiotic processes play a limited role in the degradation of
aldrin and dieldrin in the environment. Of these abiotic processes,
the greatest amount of research has been carried out on photochemically
induced changes.
4.5.1. Photochemistry
Aldrin and dieldrin are susceptible to chemical change as a
result of irradiation. Robinson et al. (1966b) assigned structure
III (Fig. 2) to the transformation product generally referred to as
"photodieldrin".
Rosen & Carey (1968) demonstrated the formation of the
unepoxidized analogue from aldrin (photoaldrin) (XIII, Fig. 2) when
aldrin was irradiated by sunlight or UV light in abiotic conditions,
but the major reaction product under these conditions was an
unbridged product where a single chlorine atom had been lost at the
3 position. The addition of benzophenone greatly enhanced yields of
photoaldrin from aldrin and also photodieldrin from dieldrin.
Fischler & Korte (1969) showed that other ketones also increased the
formation of photodieldrin.
4.5.1.1 Photochemistry of aldrin and dieldrin in water
Henderson & Crosby (1968) demonstrated that saturated aqueous
solutions of dieldrin exposed outdoors to sunlight produced
photodieldrin. However, Ross & Crosby (1974, 1975) found that when
oxygenated aqueous solutions of aldrin were irradiated with UV
light there was little effect in the absence of sensitizers. The
addition of acetone or acetaldehyde led to epoxidation; no caged
products were formed. Aldrin in rice paddy water was epoxidized
but not in the absence of irradiation. Ross & Crosby (1985) showed
that a series of amino acids present in natural waters and even
humic acids were capable of initiating photooxidation of aldrin to
dieldrin in natural sunlight.
Further evidence for the role of oxidants in the photo-
transformation of aldrin was reported by Draper & Crosby (1984).
4.5.1.2 Photochemistry of aldrin and dieldrin in air
As noted by Miller & Zepp (1983), data on the atmospheric
photodegradation of aldrin and dieldrin are sparse. Turner et al.
(1977) reported small levels of photodieldrin above a field of
grass that had been treated with dieldrin, but considered that it
had arisen from volatilization of the photodieldrin from the
foliage rather than from formation in the air itself.
In their studies on the occurrence of dieldrin and its
photoisomer in the atmosphere on a global scale, Baldwin et al.
(1977) reported detectable levels of dieldrin (0.35 ng/m3) but were
unable to detect any photodieldrin (limit of determination of
approximately 0.1 ng/m3) and considered, therefore, that
photodieldrin does not accumulate in the atmosphere.
4.5.1.3 Photochemistry of aldrin and dieldrin on plant surfaces
MacCuaig (1975) reported substantial conversion of dieldrin to
photodieldrin on the leaves of plants growing in areas of Africa
sprayed for locust control. In a more detailed study, Turner et
al. (1977) reported the formation of photodieldrin on grass that
had been sprayed with dieldrin. They also found that it was lost
fairly readily from the foliage but were uncertain whether
evaporation was the sole cause.
Harrison et al. (1967) demonstrated the rapid epoxidation of
aldrin to dieldrin on apple leaves. Ivie & Casida (1970) showed
that rotenone had a very marked effect on the rate of
transformation of leaf deposits to photodieldrin and found its
activity as a sensitizer to be some 100 times that of benzophenone.
4.5.1.4 Photochemistry of aldrin and dieldrin in soils
Lotz et al. (1983) studied the irradiation of aldrin on a
series of mineral substrates. The substrate had a marked influence
on the rate of aldrin loss, river sand showing the greatest effect.
El Beit et al. (1983) irradiated dieldrin in contact with various
substrates and found that degradation was less in the case of a
clay soil than a glass surface. However, the relevance of some of
the laboratory studies to the practical situation is questionable
because of the frequent use of very hard UV as the radiation
source.
It appears that photodieldrin does not occur in large amounts
in aldrin-treated soil. Lichtenstein et al. (1970) treated a field
soil with very high levels of aldrin and found that 98 - 99% of the
surviving residues 6 or 10 years after the last treatment were in
the form of dieldrin. Photodieldrin formed 1.6% of the dieldrin
residue. Suzuki et al. (1974) sampled 52 soils with a history of
aldrin treatment in Japan and measured dieldrin levels ranging from
0.002 to 1.73 mg/kg and photodieldrin levels ranging from < 0.001
to 0.035 mg/kg.
4.5.1.5 Conclusions
The photochemistry of aldrin and dieldrin has been intensively
studied and it seems that the use of dieldrin for certain disease
vector control operations could lead to photodieldrin formation,
although its persistence seems uncertain. Current uses would seem
unlikely to represent a significant source, and it is doubtful
whether photodieldrin occurs widely in the environment.
4.5.2. Other abiotic processes
4.5.2.1 Reaction with ozone
Ross et al. (1976) reported that ozonization of water
contaminated with dieldrin led to substantial reductions in
dieldrin levels and suggested that this process could be used
commercially to help clean-up contaminated water.
4.5.2.2 Clay-catalysed decomposition
Fowkes et al. (1960) showed that clay diluents in the dust
formulations of many pesticides caused decomposition. In the case
of aldrin and dieldrin, the most pronounced reactions occurred with
kaolinite and attapulgite, especially when they were acidic. In
the case of kaolinite at 65 °C, the half-life of dieldrin was 400
min, which was reduced to only 30 min when the kaolinite had been
acidified. Although, these effects were observed at relatively
high temperatures, it is possible that this type of decomposition
could be significant in the soil environment, though evidence for
this has not been reported.
4.6. Bioaccumulation
The relationship between the bioaccumulation factor and the
partition coefficient (Kow) of a chemical between octanol and water
has been investigated intensively for a number of compounds. The
partition coefficient has been shown to be a useful preliminary
indicator of the tendency for a chemical to accumulate in
organisms, particularly aquatic ones. The partition coefficient of
hydrophobic compounds is usually given as its logarithm (log10
Kow). The values reported for aldrin and dieldrin (Briggs, 1981)
are 7.4 and 6.2, respectively.
Estimates of the bioaccumulation factors for aquatic organisms,
determined under controlled laboratory conditions, are given in
Table 2.
Aldrin bioaccumulates and biomagnifies mainly in the form of
its conversion products. In one model ecosystem study (Metcalf et
al., 1973), conversion to dieldrin occurred rapidly and nearly
quantitatively. Only 0.5% of the original radioactive aldrin was
stored as aldrin in the mosquitofish (Gambusia affinis), which was
the organism at the top of this model food chain.
The uptake of dieldrin from water (0.1 - 1 mg/litre), after
4 h, by three species each of fungi, streptomycetes, and bacteria
gave ratios for the concentration of dieldrin in cells or mycelia
to that in the supernatant ranging from 0.3 to more than 100. The
rate of uptake of dieldrin by mycelia of Streptomyces venezuelae
and Trichoderma viride was very rapid, reaching equilibrium after
about 15 min (Chacko & Lockwood, 1967).
Table 2. Bioaccumulation of dieldrin
-----------------------------------------------------------------------------------------
Species Concentration in Duration Bioaccumulation Reference
water (µg/litre) of factor
or food (mg/kg) exposure
-----------------------------------------------------------------------------------------
Guppy 0.8, 2.3, or 4.2 32 days whole fish: 12 500 Reinert (1972)
(Poecilia reticulata)
Sailfin molly 0.075 34 weeks muscle: 3900 Lane &
(Poecilia latipinna) gill: 50 100 Livingston
(1970)
1.5 34 weeks muscle: 4900 Lane &
gill: 36 400 Livingston
(1970)
Channel catfish 0.013 70 days dorsal muscle: 2400 Shannon
(Ictalurus punctatus) 0.027 70 days 1800 (1977a)
0.049 70 days 3300
small 0.075 28 days dorsal muscle: 2300 Shannon
large 0.075 28 days 3600 (1977b)
small 2 mg/kg food 28 days 0.27
large 2 mg/kg food 28 days 0.62
Sculpins 0.017, 0.17, or 32 days whole fish: 13 300 Chadwick &
(Cottus perplexus) 0.86 Brocksen
(1969)
Alga 1, 5, or 20 14 days 1300 (based on dry Reinert (1972)
(Scenedesmus obliquus) weight of alga)
Waterflea 2.1, 4.5, or 6 days 14 000 (dry weight) Reinert (1972)
(Daphnia magna) 12.8
Common frog
(Rana temporaria) 0.8 2 days whole body 387.5 Cooke (1972)
Common toad 20 2 days whole body 280 Cooke (1972)
(Bufo bufo)
Barn owl 0.5 mg/kg food 2 years carcass: 18.8 Mendenhall et
(Tyto alba) al. (1983)
Short-tailed shrew 50 mg/kg food 17 days carcass: 1.6 Blus (1978)
(Blerina brevicauda)
Mink 2.5 mg/kg food 4-10 weeks fat: 8.4 Aulerich et
(Mustela vision) al. (1972)
-----------------------------------------------------------------------------------------
The uptake of 14C-dieldrin by Chlorella pyrenoidosa or by six
species of marine algae (Skeletonema costatum, Tetraselmis chuii,
Isochrysis galbana, Olisthodiscus luteus, cyclotella nana,
Amphidinium carteri) has been studied. In Chlorella pyrenoidosa,
rapid penetration of algal cells occurred and a maximum
radioactivity was reached after 6 - 24 h, whereas in the six marine
algae, it was reached within 1 h. From the study on Chlorella, it
was concluded that the movement of dieldrin into subcellular
organelles occurs within 72 h, and that algae are scavengers of
dieldrin. The study on the six marine algae showed that there was
no correlation between the dieldrin accumulation in the different
algae and the number of cells per ml culture. However, the amount
accumulated was related to the concentration of dieldrin in the
culture (range, 1 - 1000 µg/litre), and, for each algal species, to
the number of cells per culture. No metabolites were detected
(Wheeler, 1970; Rice & Sikka, 1973).
In studies by Jefferies & Davis (1968), medium size worms
(Lumbricus terrestris) were placed in containers, and water and
dieldrin-treated compost were added to give a final concentration
of 25 mg dieldrin (nominal)/kg moist compost. The containers were
kept at 10 °C for 20 days, and the worms were then collected. The
average concentration of dieldrin in six batches of worms ranged
from 18.4 - 24.9 mg/kg live weight of worms.
When two species of earthworms (Lumbricus terrestris and
Allolobophora caliginosa) were placed in containers with compost
containing 17 mg dieldrin/kg for 4 weeks at 10 °C, the mean
concentration of dieldrin in Lumbricus terrestris (two studies) was
13.3 mg/kg live weight. The gut content of L. terrestris was
determined using worms kept in compost (32 mg dieldrin/kg) for 20
days. The mean concentration of dieldrin in whole worms was 13.8
mg/kg live weight, the air-dried gut contents constituted 11.3% of
the total live weight, and the mean dieldrin concentration in the
tissues of the dissected worms was 10.8 mg/kg tissue. The uptake
of dieldrin by L. terrestris was compared with that by A.
caliginosa; after 4 weeks, the concentration of dieldrin in A.
caliginosa (27.3 mg/kg) was more than twice that in L. terrestris.
The concentrations of dieldrin in A. caliginosa placed in five
different soils for 4 weeks are given in Table 3 (Davis, 1971).
Table 3. The concentration of dieldrin in A. caliginosa placed in five different
soils for 4 weeksa
-------------------------------------------------------------------------------------
Soil type Estimated concentration Organic matter Mean concentration of dieldrin
of dieldrin (mg/kg air- (% w/v) in A. caliginosa (mg/kg)
dried soil)
-------------------------------------------------------------------------------------
Peaty loam 3.1 30.1 0.23
Organic loam 2.7 6.6 0.78
Loamy sand 1.7 1.3 2.99
Silty loam 2.2 2.8 3.56
Clay loam 2.0 1.7 4.55
-------------------------------------------------------------------------------------
a From: Davis (1971).
A number of field studies have been carried out in which the
concentrations of aldrin and dieldrin in earthworms from fields
treated with aldrin were determined. Six species of earthworms
were collected from a field to which excessive applications of
aldrin had been made for 8 years, and two species from experimental
plots to which dieldrin had been applied (single treatment).
Samples of soil and earthworms from the aldrin-treated fields were
analysed for aldrin and dieldrin, and the mean concentrations in
the worms are given in Table 4. The overall mean geometric
concentrations in soil (dry weight) were 0.72 mg/kg (aldrin) and
0.64 mg/kg (dieldrin). It was suggested that residual soil in the
gut may have contributed appreciably to the residues of aldrin in
the earthworms. The low residues in L. terrestris, relative to the
other species, were attributed to the deeper burrowing behaviour of
this species, which enable it to live in non-treated layers of soil
for part of its life. The concentrations of dieldrin in the soil
and earthworms from the experimental plots 6 months after treatment
with dieldrin are given in Table 5. The relationship between the
concentration of dieldrin in the two species of earthworms and the
concentration in the soil was thought to be given by the function,
W = aSb, where W is the concentration of dieldrin in the earthworm
and S the concentration in the soil. The fact that the estimated
value of b (0.794) was significantly less than unity indicates that
residues tend to be relatively greater in worms when the
concentrations in the soil are low than when higher concentrations
are present (Wheatley & Hardman, 1968).
Table 4. Mean concentrations of aldrin and
dieldrin in six species of earthworms from
aldrin-treated fields
--------------------------------------------
Species Geometric mean concentration
(mg/kg wet weight)
Aldrin Dieldrin
--------------------------------------------
L. terrestris 0.053 1.6
A. longa 0.28 2.2
A. caliginosa 0.52 3.8
A. chlorotica 0.98 4.6
A. rosea 0.64 3.9
O. cyaneuma 0.84 2.4
--------------------------------------------
a One sample only.
Beyer & Gish (1980) measured the concentrations of dieldrin in
four species of earthworms collected from a depth of 0 - 50 cm in
plots that had received a single surface application of a dieldrin
wettable powder (0.6, 2.2, or 9 kg dieldrin/ha). Samples of
earthworms were collected over a period of 11 years, and the
following relationship was derived between the concentration of
dieldrin in the worms and the time interval between dieldrin
application and worm collection:
C(n) = aEbn
where C(n) is the concentration in the earthworms n years after
soil treatment, and a and b are constants calculated from the data.
The mean values of a and b were as follows:
Application rate a b
(kg dieldrin/ha)
0.6 7.8 -0.41
2.2 21 -0.32
9.0 53.5 -0.16
The average time required for the initial residues of dieldrin in
soil to be reduced by 50% was 5.1 years, and the corresponding time
for dieldrin in earthworms was 2.6 years (Beyer & Gish, 1980).
Table 5. Concentrations of dieldrin in the soil and earthworms
from experimental plots 6 months after treatment with aldrin
---------------------------------------------------------------
Applied dieldrin Concentrations of dieldrina (mg/kg)
(kg/ha) (nominal) Soilb A. longac A. chloroticac
(dry weight)
---------------------------------------------------------------
0 0.003 0.033 0.028
0.50 0.50 0.70 1.8
0.75 0.85 1.0 2.0
1.0 1.1 1.3 2.9
1.25 1.2 1.3 2.1
---------------------------------------------------------------
a Geometric means.
b Soil samples taken 6 weeks before earthworm samples.
c Wet weight.
In studies by Gish & Hughes (1982), small experimental pasture
plots were sprayed with a suspension of a dieldrin wettable powder
at application rates of 0.56, 2.24, or 8.97 kg dieldrin/ha.
Samples of soil and earthworms were collected on 12 occasions over
a period of 2 years, the soil being sampled to a depth of 2.5 cm.
The concentration of dieldrin in the soil did not decline during
the 2-year period, but that in the earthworms from the two plots
treated at the two lower rates declined significantly. The maximum
concentration of dieldrin in the earthworms occurred 4 months after
treatment. The ratios of dieldrin concentration in earthworms to
that in the soil were examined. Residues in earthworms averaged
166 times those in soil in the sampling period 4 months after
application when earthworm residues reached a maximum. The effects
of several variables on the concentration of dieldrin in earthworms
was investigated, and a multiple regression relationship,
incorporating five variables, accounted for about 77.2% of the
variability of the residues in earthworms.
The accumulation of dieldrin in live fish-food organisms,
tubificid worms, and midge larvae (Chironomidae) was investigated
by Chadwick & Brocksen (1969), in Daphnia magna by Johnston et al.
(1971) and Reinert (1972), in crab (Leptodius floridanus) and
Artemia salina nauplii by Epifanio (1973), in mollusc (Rangia
cuneata) and blue crab (Callinectes sapidus) by Petrocelli et al.
(1973, 1975), in oyster (Crassostrea virginica) by Mason & Rowe
(1976) and Emanuelsen et al. (1978), and in an ostracod
(Chlamydotheca arcuata) by Kawatski & Schmulbach (1972). These
studies were carried out at concentrations (in fresh water or sea
water) of 0.5 - 100 µg/litre or by feeding feed or organism
containing aldrin or dieldrin. The duration of the studies was a
few days up to 43 days. In all organisms, there was a rapid
increase of dieldrin concentration in organs and tissues. A steady
state was reached after 3 - 4 and 2 days, respectively, in Daphnia
magna and Cassostrea virginica. In all organisms tested, the
elimination was slow and the half-life of dieldrin for tubificed
worms and Crassostrea virginica was approximately 16 days and 75 h,
respectively.
The rate of insecticide accumulation is partly dependent on the
concentration in the water, the duration of exposure, and the
activity of the animals. The concurrent feeding of aldrin- or
dieldrin-containing feed did not have a significant effect on
dieldrin accumulation. It can be concluded that water is the
principle source of dieldrin accumulation (Kawatski & Schmulbach
1972; Reinert, 1972; Epifanio, 1973).
A number of studies on different species have been carried out
by Gakstatter (1968) (Carassius auratus), Chadwick & Brocksen
(1969) (Cottus perplexus), Lane & Livingston (1970) (Poecilia
latipinna), Hogan & Roelofs (1971) (Lepomis cyanellus), Ludke et
al. (1972) (Notemigonus chrysoleucas, Gambusia affinis, Lepomis
cyanellus, L. macrochirus, Ictalurus natalis), Reinert (1972)
(Poecilia reticulata), Wells et al. (1973) (Gambusia affinis),
Wells & Yarbrough (1973) (Gambusia affinis), Addision et al.
(1976), (Salmo salar), and Shannon (1977a,b) (Ictalurus
punctatus). In these studies, dieldrin was added to the water at
different concentrations, and in a few of the studies the dieldrin
was radiolabelled. Distribution and accumulation were examined in
various organs and tissues (section 6.3.1.3).
Chadwick & Brocksen (1969) found that the accumulation of
dieldrin in whole fish (sculpins) was related to the concentration
in the water (0.017 - 8.6 µg/litre) and appeared to reach a steady
state by day 32. Reinert (1972) found such a state after only 17
days in Poecilia reticulata. Shannon (1977a) studied this aspect
in channel catfish (Ictalurus punctatus) (length 15 cm) exposed
continuously to 0.013, 0.027, or 0.049 µg dieldrin/litre. The
concentration of dieldrin in dorsal muscle increased in a
curvilinear fashion. Little change occurred within 56 days in the
two lower exposure groups, but a significant increase occurred in
the 0.049 µg/litre group. Steady-state concentrations appear to
have been established in the dorsal muscle of the fish exposed to
the two lower concentrations (but not in those exposed to 0.049
µg/litre) after 56 - 70 days.
Feeding studies using dieldrin-contaminated tubificid worms
(25 - 350 mg/kg) as food source showed that the retention of
dieldrin by sculpins was inversely related to the amount of
dieldrin they consumed. However, sculpins fed worms containing
0.4 - 26 mg dieldrin/kg did not show this relationship. It was
suggested that the metabolism and excretion of dieldrin was
stimulated at the higher concentrations. The findings showed that
a maximum of 16% of the dieldrin accumulated would have come from
the contaminated food. Thus dieldrin is accumulated in fish far
more readily from water than from food (Chadwick & Brocksen, 1969;
Reinert, 1972).
In studies on sailfin molly (Poecilia latipinna), exposed to
concentrations of 0.75 and 1.5 µg/litre for 34 weeks (flow-through
system), Lane & Livingston (1970) found that the ratio of the
concentration of dieldrin in the tissues to that in water in the
steady state was about 10 000.
From a study on green sunfish (Lepomis cyanelles) that were
exposed to dieldrin at 6 µg/litre for 124 - 139 h, it was concluded
that the lethal concentrations of dieldrin in blood and brain were
approximately 6 and 9 mg/kg tissue, respectively (Hogan & Roelofs,
1971).
Shannon (1977b) exposed channel catfish to 0.075 µg
dieldrin/litre water and/or 2 mg dieldrin/kg food for 28 days.
Small (15 - 22.5 cm) and larger fish (3 - 40 cm) were used and
dorsal muscle of the fish was analysed. After 28 days, fish
exposed to 0.075 µg/litre had a mean concentration of dieldrin in
muscle of 0.175 (small fish) and 0.274 (large fish) mg/kg tissue,
fish fed 2 mg dieldrin/kg contained 0.544 (small fish) and 1.243
(large fish) mg/kg tissue, and those given the combined treatment
contained 0.898 (small fish) and 2.418 (large fish) mg/kg tissue.
The elimination of dieldrin from the dorsal muscle in clean water
showed that when fish were exposed to dieldrin in water only, a 50%
decrease took place in 8 days. For fish exposed to dietary or
combined exposure, it required 20 days.
In a study with different early-life stages of rainbow trout,
the bioconcentration factor in the different stages was determined
using 14C-dieldrin. It increased during embryonic development from
120, reached a maximum at the sac fry stage of 12 000 and fell
again at the early fry stage to 1500. The clearance rate constant
sharply increased at the early fry stage. Almost all the dieldrin
was recovered from the yolk (Van Leeuwen, 1986).
The yolks of eggs from chickens fed aldrin or dieldrin
(1 mg/kg) or 10 mg dieldrin/kg for 2 years contained dieldrin
concentrations of 6 - 25 mg/kg (Brown et al., 1965). Several other
studies on the accumulation of dieldrin into avian eggs have been
made, details of these being given in Tables 17 and 18 (section
5.1.6).
Clark (1975) fed red-winged blackbirds (Agelaius phoeniceus) a
diet containing 10 mg aldrin/kg, some of the birds being
artificially stressed. The mean number of days that the birds
survived was 29.9 for unstressed and 22 for stressed birds. The
mean values of brain residue levels at death were 19.8 mg
dieldrin/kg for unstressed birds and 22.2 mg dieldrin/kg for
stressed birds. Three unstressed birds, sacrificed after 76 days,
had dieldrin levels of 6.7, 7.28, and 7.4 mg/kg. The carcass
levels of dieldrin increased linearly with time and showed no
tendency to level off, as occurred in the brains of unstressed
birds. The three unstressed birds sacrificed had the highest
carcass dieldrin levels (70.3, 82.8, and 147 mg/kg).
Stickel et al. (1969) fed Japanese quail (Coturnix coturnix
japonica) diets containing 2, 10, 50, or 250 mg/kg dieldrin for up
to 158 days. The mean dieldrin levels in the brain of dead and
sacrificed birds were 18.25 mg/kg and 3.35 mg/kg (wet weight),
respectively, while the mean liver residues were 19.7 mg/kg (wet
weight) and 28.8 mg/kg (wet weight), respectively.
Mendenhall et al. (1983) fed captive barn owls (Tyto alba)
with diets containing 0.5 mg/kg dieldrin for 2 years. The mean
carcass residues were 9.4 mg/kg (wet weight) after 2 years, and the
mean dieldrin levels in eggs were 3.6 mg/kg in the first year and
8.1 mg/kg in the second.
Enderson & Berger (1970) fed each of three captive female
prairie falcons (Falco mexicanus) with 11 starlings, one per day.
The starlings had been treated for 14 days with 10 mg/kg dieldrin
in their diet. One bird died and showed levels of dieldrin in
brain, liver, and muscle of 11, 29, and 4.6 mg/kg (wet weight),
respectively. The other two were sampled and found to have mean
adipose tissue and brain levels of 532 and 5.84 mg/kg,
respectively. The authors also fed wild falcons for 6 weeks prior
to egg laying. The analysis of one egg from each clutch showed a
mean egg dieldrin content of 41.5 mg/kg, and the mean adipose
tissue level of dieldrin in dosed adult falcon, was 83 mg/kg
dieldrin.
Turtles (Pseudemys scripta elegans) were given intraperitoneal
injections of dieldrin (20 mg/kg body weight) and the accumulation
in organs and tissues was determined over a period of 70 days. The
turtles were fasted during the study. The rate of absorption of
dieldrin into the tissues was slow, and there were no clear
indications of an approach to steady-state concentrations by day
70. The highest levels of dieldrin were found in body fat and
liver, and the levels in plasma and brain were also high (Pearson
et al., 1973).
Cooke (1972) studied the effect of dieldrin at nominal
concentrations of 0.0008, 0.02, or 0.5 mg/litre on groups of 40
common frog (Rana temporaria) tadpoles with hindlimb paddles or
hind legs. The exposure lasted 24 or 48 h in amphibian saline. At
the highest dose level the mean dieldrin content after 48 h
exposure was 42.9 mg/kg tissue. At the dose levels of 0.0008 and
0.02 mg/litre, there were 0.31 and 6.1 mg/kg dieldrin in tissues,
respectively. Toad (Bufo bufo) tadpoles exposed to 0.02 or 0.5
mg/litre contained 138 mg dieldrin/kg tissue at the higher dose
level, after 48 h, and 5.6 mg/kg at the lower.
A laboratory study was undertaken concerning the lethal brain
levels for dieldrin in adult and juvenile brown bats (Myotis
lucifugus), using 47 female bats collected from a church attic in
Maryland, USA. Meal worms containing an average of 0.38 mg
dieldrin/kg (wet weight) were fed to the bats for 52 days, and then
untreated worms were administered for another 22 days. The amount
of dieldrin in bats increased during dosing and decreased
afterwards. These changes did not appear as changes in average
dieldrin concentrations in the fat because the amounts were highly
variable. During the exposure period a continuous build up of the
concentration in fat was seen, but an equilibrium was not reached.
The initial half-life for dieldrin loss was estimated to be 24
days. Measurable dieldrin was found in the brains of only 6 out of
47 bats. The levels measured (0.5 to 0.9 mg/kg tissue) were all
far below lethal levels. The highest dieldrin level determined in
the carcass of 37 bats was 110 mg/kg tissue (lipid weight) (Clark &
Prouty, 1984).
Short-tailed shrews (Blerina brevicauda) were fed diets
containing dieldrin (nominal concentrations of 50, 100, or 200
mg/kg diet) for up to 14 days. All of the animals fed 50 mg/kg
survived, but all those fed 200 mg/kg died. The mean dieldrin
concentration in the brains of 14 shrews that died was 6.8 mg/kg
(range, 3.7 - 12.6). Some animals sacrificed after 17 days of
feeding the 50 mg/kg diet contained mean residues in the brain of
1.8 mg/kg and in the carcass of 58 mg/kg. After 14 days on an
untreated diet, the concentrations in the carcass declined by 76%
in both sexes, and in the brain by 59% and 84% in males and
females, respectively. The half-life of dieldrin was estimated to
be less than 14 days (Blus, 1978).
Male mink (3 months old) were fed a diet containing dieldrin
(nominal concentrations of 0 and 2.5 mg/kg diet) for 10 weeks, and
samples of abdominal fat were taken by biopsy at two-weekly
intervals. The mean concentration of dieldrin after 2 weeks was
12.5 mg/kg body fat. For weeks 4 - 10, an average concentration of
21 mg was found, a steady state being reached after approximately 4
weeks (Aulerich et al., 1972).
4.7. The Fate of Aldrin and Dieldrin in the Environment
On the basis of the current uses of aldrin in agriculture, the
first point at which aldrin and dieldrin enter the environment is
the soil, dieldrin being derived from aldrin by biological
epoxidation. Understanding the fate of aldrin and dieldrin in the
environment, therefore, depends firstly on an understanding of its
behaviour in the soil.
4.7.1. Aldrin and dieldrin in soils
It was concluded in section 4.1.4 that the regular application
of aldrin to soils for the control of soil pests does not lead to
an indefinite accumulation in the soil. The results of a
considerable number of soil monitoring studies, summarized in
Table 1, support this conclusion. Some of the individual
monitoring studies are discussed at greater length in this section.
Carey et al. (1973) monitored residues of aldrin and dieldrin
over a very wide area of the corn belt in the USA in 1970, when the
use of aldrin on maize was probably close to its maximum and the
levels were representative of residues in a situation of continuing
use. Average values for aldrin plus dieldrin, recalculated for
samples that contained positive residues, ranged from 0.05 to 0.87
mg/kg for each of the twelve states. The maximum levels for the
whole study were 2.98 mg aldrin/kg and 2.04 mg dieldrin/kg (these
values were not both derived from the same sample). In many cases
the residues had come from relatively recent applications, as may
be judged from the comparatively high proportion of aldrin still
remaining. The average was greater than 50% of the combined
residues in four of the twelve states, so that many of the samples
were probably taken from soils in the same year in which they were
treated.
Carey et al. (1980) carried out a further study on rice soils
in the USA during the year 1972. At that time, aldrin was used
extensively as a rice seed dressing and, according to the data
presented by Sparr et al. (1966), overall application rates of
aldrin would have been between 0.2 and 0.4 kg/ha. Between 50% and
100% (depending on the state) of the land sampled had received
aldrin-dressed seed. As in the case of the maize data, figures for
aldrin and dieldrin were presented separately and not paired, so
that total residue levels are difficult to deduce. The average
level for aldrin was only about 0.02 mg/kg soil and for dieldrin
was 0.05 mg/kg, although there were occasional samples that reached
0.25 mg/kg for either aldrin or dieldrin. According to the
information presented earlier, degradation of aldrin occurs more
readily in the anaerobic conditions of a rice paddy than in fully
aerobic soils, and this may have contributed to the much lower
level of residues surviving in rice compared with maize. However,
it should also be remembered that the initial rates of application
were substantially lower in rice than in maize.
In Canada, Harris et al. (1966) reported a series of data for
soils in S.W. Ontario and there was limited information on the
treatment history of the soils sampled. About a half of the soils
showed residues, and these ranged from < 0.01 to 1.5 mg/kg for
aldrin plus dieldrin residues. One high figure of 3.5 mg/kg seems
anomalous in that it was reported from land that had no treatment
history with aldrin or dieldrin. With the exception of this
anomalous sample, there was no evidence for accumulation. Fairly
similar results were reported by Duffy & Wong (1967), who sampled a
series of vegetable-growing areas in Canada in 1965. In cases
where it was reported that aldrin or dieldrin had been used
(sometimes over a period of several years) residues were mostly
below 2 mg/kg.
None of these studies mentioned the occurrence of any dieldrin
degradation products, in particular photodieldrin, and yet this
would presumably have been detected had it been present. This,
taken in conjunction with the work of Suzuki et al. (1974) (section
4.4.2) would seem to be useful evidence that photodieldrin does
not, to any appreciable extent, represent a terminal metabolite of
aldrin in the soil.
4.7.2. Aldrin and dieldrin in the atmosphere
The relative contributions of the various mechanisms for the
loss of aldrin and dieldrin from the soil have not been estimated
(as far as can be judged from the literature) but, as mentioned in
section 4.1.3, volatilization is usually considered to be the major
loss route. Consequently, the occurrence of aldrin or dieldrin
vapour in the atmosphere has been the subject of considerable
study.
Spencer & Cliath (1975) considered that many pesticides enter
the atmosphere after application. This occurs by volatilization
during spraying, from treated crops or soils, or from dust from
treated soil surfaces blown up by the wind. These routes are
difficult to quantify, and only sparse data are available, though a
few relating to dieldrin have been cited in section 4.1.3.
Small amounts of dieldrin have been detected in the atmosphere,
particularly in agricultural areas and, in one case, close to a
formulating plant (section 5.1.1.2). Aldrin has also been
detected, though relatively less often (section 5.1.1.1). There is
further information on the levels in the atmosphere of aldrin and
dieldrin in section 4.1.6.
4.7.3. Conclusion
In spite of the slow rate at which aldrin and dieldrin are lost
from soils when applied to them for insect control, there is no
evidence for their indefinite accumulation in the environment,
either in the soil itself, in water, or in the atmosphere. The
evidence suggests that photodegradation products do not accumulate
either.
Although there is evidence that a considerable proportion of
the aldrin and dieldrin used in agriculture reaches the atmosphere,
it seems probable that the degradation processes in the atmosphere
described by Glotfelty (1978) for pesticides in general operate to
prevent accumulation of aldrin and dieldrin.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1 Air and rainwater
5.1.1.1 Aldrin
Residues of aldrin in the general atmosphere, either in the
vapour phase, adsorbed by dust particles, or in rainwater, have
been reported less frequently than for other organochlorine
insecticides. Concentrations in the range 0.1 - 4 ng/m3 have been
found in the air of agricultural communities (Tabor, 1966). In a
pilot survey in 1967 - 1968, out of nine localities in the USA,
only one sample from a total of 880 contained aldrin (8 ng/m3)
(Stanley et al., 1971). Aldrin was detected in 13.5% of 2479 air
samples collected from 16 states in the USA in the 3-year period
1970 - 1972. The mean value in these positive samples was 1.6
ng/m3 and the maximum was 24.6 ng/m3 (Kutz et al., 1976).
Aldrin was found at a level of 0.9 ng/m3 in 16 out of 66 air
samples taken within 800 m of two formulation plants in 1970 (Lewis
& Lee, 1976). One year later, 6 out of 60 samples were found to
contain a level of 1.5 ng/m3, and in 1972 no aldrin could be
detected.
5.1.1.2 Dieldrin
In a pilot survey in 1967 - 1968 in the USA, dieldrin was found
in 6% of the 880 samples analysed. The maximum level was 29.7
ng/m3 (Stanley et al., 1971).
In a survey mentioned above (Kutz et al., 1976), dieldrin was
detected in 94% of the 2479 samples. The mean value in these
positive samples was 1.7 ng/m3 and the maximum was 23.9 ng/m3.
A summary of the concentrations of dieldrin in air and
rainwater (washout from the air) is given in Table 6.
5.1.2. Concentrations in houses
5.1.2.1 Aldrin used for subterranean termite control
Aldrin EC (480 g/litre) was applied in a 0.5% solution as a
termiticide to typical slab and crawl-space type houses in
California, 1982. Samples of air were taken in the kitchen,
bedroom, and crawl-space at intervals up to 1 year after
application. Kitchen wipe samples were taken at the same time.
Air concentrations of aldrin in kitchen and bedrooms of the treated
houses showed transient peaks 24 h after treatment. In the slab-
type houses, concentrations were 0.04 - 0.27 µg/m3 and in the
crawl-space houses 0.09 - 7 µg/m3. These concentrations fell
rapidly. The air concentrations in slab houses were < 0.04 - 0.09
µg/m3 at day 28 and were < 0.05 µg/m3 at day 56, whereas those in
crawl-space houses were 0.06 - 1.5 µg/m3 at day 7, 0.04 - 0.36
µg/m3 at day 28, and 0.06 - 0.55 µg/m3 at day 56. One year after
application the air concentration of aldrin was 0.08 µg/m3 or less,
whereas dieldrin was not detected in any of the samples of air.
The concentrations of aldrin in surface wipes from kitchens showed
transient peaks 7 days after treatment, the concentrations being
higher in wipes from crawl-space houses (0.09 µg/m2) than from slab
houses (0.012 µg/m2). Between day 28 and day 56 the concentrations
remained at about 0.04 µg/m2 in crawl-space houses and 0.002 µg/m2
in slab houses. One year after application, aldrin and dieldrin
were not detectable in the kitchen wipe samples from the slab
houses, while in the samples from the crawl-space houses the
concentration of aldrin amounted to 0.04 µg/m2 and that of dieldrin
to 0.018 µg/m2 (Marlow et al., 1982; Marlow & Wallace, 1983).
5.1.2.2 Aldrin and dieldrin used for remedial treatment of wood
One to ten years after the remedial treatment of inside wood in
houses with dieldrin, its concentration in the air was measured.
Forty samples from 16 houses in the United Kingdom, covering a wide
range of construction type, size, and occupational pattern, were
analysed. The concentrations of dieldrin in the air in all
interior areas other than roof-voids were between 0.01 and 0.51
µg/m3, and in roof-voids, they were between 0.03 and 2.7 µg/m3
(Dobbs & Williams, 1983).
Paton et al. (1984) observed that aldrin and dieldrin migrated
from treated laminated timber and plywood, used as structural
components of commercial containers, into flour in polyethylene
bags or metal tubes that were stored in the containers for up to 40
days. The migration was thought to occur through contact with the
floor or sorption from the atmosphere. The residues in the flour
varied widely depending on temperature, type of packaging material,
and location of the flour in the container.
5.1.3. Aquatic environment
Dieldrin occurs more commonly in the aquatic environment than
does aldrin, albeit at very low concentrations. The major sources
of contamination of rivers, etc., by aldrin and dieldrin are
industrial effluents (manufacturing, formulation, and moth-proofing
in the textile industry) and soil erosion during agricultural usage
(Lichtenstein et al., 1962; Park & McKone, 1966; Epstein & Grant,
1968; Eye, 1968; Croll, 1969; Lowden et al., 1969; Rowe et al.,
1971; Burns et al., 1975; Brown et al., 1979). Local use appears
to contribute to the presence of dieldrin in sediments in urban
areas (Mattraw, 1975). In the USA, the Environmental Protection
Agency found that between 118 kg and 14.2 tonnes of dieldrin was
carried in the Mississippi River past St. Louis each year.
Although this is certainly not the case today, it does illustrate
the contribution of run-off to the pesticide load in river systems.
This is of special concern with dieldrin because of its stability
in water (Eichelberger & Lichtenberg, 1971).
Table 6. Concentration of dieldrin in air, rainwater, and dust
----------------------------------------------------------------------------------------------------------------
Location Year Number Medium Analyticala Mean Comments Reference
of procedure concentration
samples (range)
----------------------------------------------------------------------------------------------------------------
Netherlands
Delft 1979-81 55 air glass fibre 0.073 ng/m3 24-h samples Guicherit
filter; GC/EC (maximum, 370 & Schulting
ng/m3) aldrin: (1985)
0.039 ng/m3
(maximum, 640
ng/m3)
United Kingdom
Wellesbourne 1964-65 11 rain- TLC followed by 24 ng/litre samples of monthly Wheatley
water GLC/EC (10-36) rainfall & Hardman
(1965)
1965 5 9 ng/litre samples collected
(3-16) during periods of
prolonged rainfall
London 1965 11 rain- GLC/EC 42 ng/litre samples of monthly Abbott et
water (10-95) rainfall; 2 sampling al. (1965)
sites; limit of
determination: 10
ng/litre
London 1965 1 air TLC followed by 20 ng/kg Abbott et
GLC/EC al. (1966)
United 1966-67 28 rain- alumina column 8 ng/litre samples from 7 Tarrant
Kingdom water chromatography (1-35) locations during 12 & Tatton
and TLC followed months; limit of (1968)
by GLC/EC determination:
1 ng/litre
----------------------------------------------------------------------------------------------------------------
Table 6. (contd.)
----------------------------------------------------------------------------------------------------------------
Location Year Number Medium Analyticala Mean Comments Reference
of procedure concentration
samples (range)
----------------------------------------------------------------------------------------------------------------
USA
Cincinnati 1965 1 dust florisil column 3 ng/g dust source of the dust was Cohen &
washed chromatography the Southern High Pinkerton
out by followed by Plains, approximately (1966)
gentle GLC/EC 1500 km southwest of
rain Cincinnati
16 states 1970-72 2479 air florisil column 1.7 ng/m3 limit of determination: Kutz et al.
chromatography (1-23.9) 1-10 ng/m3 (1976)
followed by
GLC/EC
Hawaii 1970-71 5 rain- GLC/EC 5 ng/litre Bevenue et
water (1-27) al. (1972a)
1971-72 14 12 ng/litre Bevenue et
(1-97) al. (1972b)
West Indies
Barbados 1965-66 15 dust TLC followed by 2.2 ng/g dust samples of dust Risebrough
GLC/EC (1-8.1) collected in nylon et al. (1968)
screens; limit of
determination:
1 ng/g (?)
Barbados July 12 air- silicic acid 49 ng/m3 air source of dust in air Prospero &
1970 borne column (1-190) probably North Africa Seba (1972)
dust chromatography
followed by
GLC/EC
----------------------------------------------------------------------------------------------------------------
Table 6. (contd.)
----------------------------------------------------------------------------------------------------------------
Location Year Number Medium Analyticala Mean Comments Reference
of procedure concentration
samples (range)
----------------------------------------------------------------------------------------------------------------
Ireland
Bantry Bay 1973 17 air florisil column 0.36 ng/m3 aldrin and photo- Baldwin et
chromatography (0.06-1.6) dieldrin less than al. (1977)
followed by limits of detection
GLC/EC (0.1 ng/kg); origin
of air samples either
westerly from Atlantic
Ocean (13 occasions)
or easterly from
continental Europe
(4 occasions)
----------------------------------------------------------------------------------------------------------------
a TLC = thin-layer chromatography; GC/EC = gas chromatography/electron capture detection; GLC/EC = gas-liquid
chromatography/electron capture detection.
Dieldrin has been detected in the northern Atlantic Ocean at a
mean concentration of 5.8 ng/litre (Jonas & Pfaender, 1976), and it
is of interest that the dieldrin concentration was apparently
unrelated to depth or distance from shore. It was suggested that
this may be the result of adsorption to particulate matter. The
identification of the component measured as dieldrin was based on
gas-liquid chromatographic behaviour (three different stationary
phases), but was not rigorously confirmed. Other investigators
(Harvey et al., 1973, 1974; Bidleman & Olney, 1974) did not report
the presence of dieldrin in the northern Atlantic Ocean, although
the analytical methods were appropriate for the detection of aldrin
and dieldrin.
Dieldrin residues (0.01 - 0.3 ng/litre) have also been reported
off the coast of Ireland, in the English Channel, and in the North
Sea (Dawson & Riley, 1977).
Low levels of dieldrin have been reported in surface waters
from several countries. The results of several surveys are
summarized in Table 7.
5.1.4. Soil
Aldrin is applied more frequently to the soil (directly or
indirectly) than dieldrin. However, as a result of the relatively
rapid conversion of aldrin to dieldrin, residues of dieldrin are
usually found more frequently in soil and at higher concentrations,
except shortly after the application of aldrin to soil. Sediment
residue levels tend to lie between those of soil and water, with
values up to 140 µg/kg) (Dawson & Riley, 1977). Dieldrin has been
reported in the sediments of Lake Ontario and Lake Superior (Frank
et al., 1974; Frank et al., 1981), rivers of the USA (Ryckman et
al., 1972), bays (Sheets et al., 1970), and off the coasts of
Ireland (Dawson & Riley, 1977). A summary of some of the results
of monitoring surveys was given in Table 1 (section 4.1.4). This
is not a comprehensive review of residues, but it indicates the
variations of the concentrations that occur in practice. These
results also illustrate the potential for absorption into root
crops, and for uptake by soil organisms.
5.1.5. Drinking-water
Studies on drinking-water in the USA have indicated dieldrin
residue values up to 8 µg/litre (Kraybill, 1977; Sandhu et al.,
1978). In a comprehensive study in the USA, dieldrin residues were
found in less than 17% of the samples with 78% of these positive
results lying within the range 4 - 10 ng/litre. The highest
residue level found in this study was 110 ng/litre. Dieldrin has
also been found in drinking-water in Canada (0.1 - 4 ng/litre)
(Williams et al., 1978) and in the Virgin Islands (average
concentration 0.19 µg/litre (Lenon et al., 1972).
Table 7. Concentrations of aldrin and dieldrin in the aquatic environment
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Argentina
Santa Fe 1981 surface water 4 4 (29) LD LD not defined; samples Lenardon et
and Parana (20 cm depth) collected twice monthly al. (1984)
(March-December)
suspended 4 150 ng/g LD occasional high residues of
solids (1625) aldrin attributable to local
source of application
(1966-68)
Canada
Ontario 1971 agricultural 2 1 (7) 11 (41) LD less than 1 ng/litre Miles & Harris
and urban (1973)
rivers
resort rivers 1 1 4 (11)
bottom mud 2 LD 0.9 LD less than 1 ng/g mud
(dry weight)
(4.5)
1 LD 0.9
(dry weight)
(1.4)
Nova Scotia 1972-73 river 7 (23 77 (670) 979 LD not defined; water, Burns et al.
samples) (11 800) probably less than 10 (1975)
ng/litre; sediment, probably
less than 1 ng/g
artesian wells 4 LD 10 (10)
holding pond 3 37 (40) 100 (200)
and reservoirs
natural 2 (4 90 (330) 17.5 (50) national drainage ditch in a
drainage samples) tobacco-growing area
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Canada (contd.)
Nova Scotia 1972-73 sediment from 25 31 (368) 4.9 (86) high residues in water and
stream bed sediments attributable to
soil erosion, particularly
during and after prolonged
heavy showers
sediment from 4 1088 670
natural (2220) (13 750)
drainage ditch
Lake 1974 filtered lake 34 LD 0.5 (0.5) LD: filtered water, less than Glooschenko
Superior water 0.5 ng/litre; sediment, less et al. (1976)
and Lake than 1 ng/g; dieldrin detected
Huron sediment 34 LD 0.1 mg/kg (0.5 ng/litre) in one sample
dry weight of filtered water and in 5
(0.1) samples of sediment (0.1 ng/g)
Greece
Northern 1981-82 coastal water 10 ND 0.55 (1.1) Fytianos et
Greece al. (1985)
United Kingdom
Kent and 1965-66 rivers 9 (224 LD (4) LD (59) LD less than 3 ng/litre; Croll (1969)
Essex samples aldrin found in one sample
collected only
at 2-weekly
intervals)
Great 1965-66 rivers 11 (75 LD 24.3 (423) high residues of dieldrin Croll (1969)
Britain samples attributable to effluent from
collected moth-proofing plants using
at 2-monthly dieldrin
intervals)
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Great 1965-67 rivers 15 LD 292 (2840) high residues of dieldrin Croll (1969)
Britain attributable to effluent from
moth-proofing plants using
dieldrin
Kent 1965-66 underground 11 LD LD Croll (1969)
water
Hereford- 1966 River Lee 6 LD LD LD less than 2 ng/litre Lowden et al.
shire (1969)
Yorkshire 1966-68 rivers 14 (30 LD 114 (650)
samples)
Birmingham 1966 sewage 24 LD 132 (1900) high concentrations of Lowden et al.
and effluent dieldrin attributable to (1969)
Hertford- industrial effluent from
shire moth-proofing plants
Yorkshire 1976-77 rivers 7 (18 LD 902 (4900) LD less than 1 ng/litre; high Brown et al.
samples) concentrations of dieldrin in (1979)
individual rivers (and sewage
sewage 1 sample LD 6240 effluent) attributable to the
effluent use of dieldrin for moth-
river 10 LD 124 ng/g proofing of wool
sediments (dry weight)
(432)
Netherlands 1969-75 raw water 11 (120 LD LD (50) unfiltered water to be used Greve (1972);
samples) for drinking-water preparation Wegman & Greve
(1978)
surface water 26 (1246 LD 10 (140) aldrin was detected Wegman & Greve
(depth about samples) occasionally at low (1978)
1 m) concentrations; limit of Greve (1972)
detection about 10 ng/litre
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Federal 1970-71 unfiltered 28 (119 LD LD (45) dieldrin reported in only one Herzel (1972)
Republic of surface water samples) sample of water
Germany
suspended 26 LD LD LD not given (appears to be
solids approximately 10 ng/litre)
USA
Major river 1965 surface water 99 LD 1 (100) LD 1 ng/litre; aldrin not Breidenbach et
basins detected in any sample; al. (1967)
dieldrin detected in 42% of
samples
Western USA 1965, rivers 11 0.2 (5) 2.3 (15) lower LD 5 ng/litre Brown &
1966 Nishioka
(1967)
1966-68 rivers 20 LD (40) LD (70) LD not defined; presumed to be Manigold &
about 10 ng/litre Schulze (1969)
Major river 1964-68 surface water about 100 LD range: 4-407 LD 2 ng/litre; in 37% of the Lichtenberg et
basins stations samples dieldrin was present al. (1970)
Iowa 1968 rivers 6 LD 2 (10) LD about 1 ng/litre; dieldrin Johnson &
1969 10 LD 8.5 (63) found in 40% of samples (179) Morris (1971)
1970 10 LD 9 (65) analysed
Western USA 1968-71 rivers 20 LD (10) LD (30) LD 5 ng/litre; aldrin detected Schulze et al.
in only 1 sample (total 716); (1973)
dieldrin detected in 5% of
samples
Hawaii 1970-71 non-potable 10 LD 4.8 (18.6) LD about 0.2 ng/litre Bevenue et al.
(1972a)
canals 3 LD 11.9 (18.6)
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Hawaii sewage 1 LD 198
(contd.) discharge
Gulf coast 1971 canal water 1 90 (440) drainage water from a rice Ginn & Fisher
of Texas field-marshland ecosystem; (1974)
samples (7) collected for 15
weeks after aldrin-dressed
rice seed had been planted;
one sample out of 7 contained
aldrin (270 ng/litre)
Lower surface water 1 LD 5 (10) LD not defined Brodtmann
Mississippi (samples (1976)
River taken
monthly)
Iowa 1974 surface water, 18 (104 not 12 (76) LD less than 0.5 ng/litre Richard et al.
rivers, and samples) reported (1975)
reservoirs
Western 1972 ocean surface 10 0.2 6.4 (19.4) lower LD: aldrin, less than Jonas &
North (0.2) 0.2 ng/litre; dieldrin, less Pfaender
Atlantic than 0.4 ng/litre; (1976)
50 m depth 7 LD 6.2 (9.8) concentrations of aldrin
below LD in 29 samples;
500 m depth 7 LD 3.7 (11.9) one surface sample contained
a component with the same
1000 m depth 6 LD 7 (18.3) retention time of aldrin,
corresponding to 0.2 ng/litre
USA 1976-80 rivers LD 610 occurrence of dieldrin in 2.4% Carey & Kutz
of samples (1985)
--------------------------------------------------------------------------------------------------------------------------
a Concentrations in bottom mud, sediments, and suspended solids (ng/g); LD = limit of detection; ND = not determined.
Maximum concentration indicated in parentheses.
5.1.6. Food and feed
Aldrin is rarely found in plants and animals, since it is
readily converted to dieldrin (IARC, 1974). A total-diet study of
Balby pensioners in Sweden did not detect any aldrin (Abdulla et
al., 1979). Similarly, a market-basket study in the USA in
1974 - 1975 (Johnson & Manske, 1977) found aldrin in only one
composite out of 240, with a value of 7 µg/kg. Traces or low
levels of aldrin have been found in vegetable products and meat
products (Balayannis, 1974; Saschenbrecker, 1976; Chaudry et al.,
1978; Wessels, 1978). In all cases, dieldrin residues were greater
than those of aldrin, even when aldrin was the only compound
applied. Aldrin is rarely found in milk or milk fat or in the body
fat of cows fed aldrin (Frank et al., 1985; Vreman & Poortvliet,
1982). In one study where aldrin was found in dairy products, milk
samples contained 0.04 mg/litre, butter samples 0.02 mg/kg, and
cheese samples 0.02 mg/kg (Heeschen, 1972). For the occurrence of
residues in breastmilk, see section 5.2.2.
The analytical procedures used in well conducted dietary
surveys are capable of detecting all of the commonly used
organochlorine pesticides, so that if aldrin did occur in a dietary
sample it would be detected. The lack of mention of aldrin,
therefore, can usually be taken as an indication that it was not
detected.
Dieldrin residues in food and feed, resulting from the
application of aldrin and dieldrin in normal use as well as from
field studies, have been reviewed by the FAO/WHO Joint Meeting on
Pesticide Residues (JMPR) at its meetings in 1963, 1965, 1966,
1967, 1968, 1969, 1970, 1974, 1975, and 1977 (FAO/WHO, 1964,
1965a,b, 1967a,b, 1968a,b, 1969a,b, 1970a,b, 1971a,b, 1975a,b,
1976a,b, 1978a,b).
Australia, Canada, Japan, the Netherlands, the United Kingdom,
and the USA have all reported daily intakes below the ADI (Duggan &
Lipscomb, 1969; Uyeta et al., 1971; Duggan & Corneliussen, 1972;
IARC, 1974; Smith, 1978; de Vos et al., 1984).
In Australia, Canada, Italy, Japan, the United Kingdom, and the
USA, analyses of total diets revealed dieldrin residues (Cummings,
1966; Duggan et al., 1967; Abbott et al., 1969; Corneliussen, 1972;
Duggan & Corneliussen, 1972; Johnson & Manske, 1977; Dick et al.,
1978; Smith, 1978) ranging from 0.06 mg/kg (Cummings, 1966;
Corneliussen, 1972) to 0.2 mg/kg (Duggan et al., 1967;
Corneliussen, 1970). In 1982 - 1983, dieldrin was determined in 73
typically composed, prepared daily meals in Switzerland, and was
detected in 46 of the meals. It was calculated from these results
that the average daily intake of the Swiss consumer was 0.9 ng/day.
The levels in 1971 - 1972 were 3.4 ng/day (Wüthrich et al., 1985).
Residue levels of up to 0.125 mg/kg in Canadian pork
(Saschenbrecker, 1976) and of 0.03 - 0.1 mg/kg in herring oil
(Addison et al., 1972) have been reported.
In a market-basket survey in 1974 - 1975, dieldrin was present
in only three food groups, with maximum residues of 5 µg/kg in
dairy products, 15 µg/kg in meat, fish, and poultry, and 8 µg/kg in
potatoes (Johnson & Manske, 1977).
The JMPR meeting in 1970 summarized data concerning dieldrin
residues from the feeding of dieldrin to cattle and poultry. The
average ratio of dieldrin levels in fat to levels in feed was
2.43:1 in milking cows and 3.95:1 in steers (Gannon et al., 1959a).
At intake rates of less than 1 mg/kg, the average ratio of dieldrin
levels in milk to levels in feed was about 0.1:1 after 12 weeks
(Gannon et al., 1959b; Williams et al., 1964). In Denmark, the
average concentration of dieldrin in butter fat declined from 0.05
mg/kg in 1964 to 0.03 mg/kg in 1966 and to 0.02 mg/kg in 1968
(Bro-Rasmussen et al., 1968). Similar residue levels and decreases
were found in Australia, Ireland, New Zealand, Norway, and the
United Kingdom. In Canada and the USA, residues in milk fat of
0.011 - 0.09 mg dieldrin/kg have been measured (Duggan et al.,
1967; Wedberg et al., 1978; Frank et al., 1985).
Dieldrin losses resulting from cooking or processing food can
be quite substantial, as demonstrated with trout and soybean
(Chaudry et al., 1978; Zabik et al., 1979).
More recent information on the occurrence of dieldrin residues
in foods is relatively scarce. However, a number of reviews exist.
5.1.6.1 Joint FAO/WHO Food Contamination Monitoring Programme
Information on dietary intakes of aldrin and dieldrin were
collected from seven collaborating centres participating in the
Joint FAO/WHO Food Contamination Monitoring Programme. The data
cover the period from 1971 - 1983, and the countries involved were
Australia, Canada, Guatemala, Japan, New Zealand, the United
Kingdom, and the USA. The mean daily intake during this period
varied from 0.007 to 0.056 µg/kg body weight (the 90th percentile
varied from 0.016 to 0.105 µg/kg body weight). During the later
years of this period, the mean values ranged from 7% to 56% of the
acceptable daily intake (ADI). A decrease in the dietary intake of
aldrin and dieldrin residues was noted during this period in some
of the countries. Possibly this decrease was the result of
restricting or banning the use of aldrin and dieldrin (Gorchev &
Jelinek, 1985).
5.1.6.2 Information summarized by GIFAP (1984)
Australia, 1980: Twenty-four samples of each of 50 different
foods were analysed for a range of organochlorine pesticides.
In the case of dieldrin, the limit of determination was 0.01
mg/kg. Dieldrin occurred above this level in only 0.04% of the
samples, and the maximum level was 0.05 mg/kg.
Canada, 1976 - 1978: The results of analyses of food
commodities were expressed in terms of estimated intakes for
the population. The average daily dietary intake of dieldrin
for this period was 0.002 µg/kg body weight.
Italy, 1982: Apples were sampled from a variety of locations
representing 70% of the country's apple production. There were
300 samples and 80% or 90% were below the limit of determination
for aldrin and dieldrin, respectively.
Netherlands, 1977 - 1978: During the period 1977 - 1978,
residues of organochlorine pesticides were determined in a wide
range of market baskets composed of items considered to be
representative of the diet of 16 - 18-year-old boys. Although
dieldrin was not specifically mentioned, the studies revealed
that none of the organochlorine pesticides contributed
residues in excess of the Maximum Residue Limit (MRLs).
5.1.6.3 United Kingdom (UK MAFF, 1982-1985)
Residues of dieldrin found in a range of dietary components in
1981 are listed in Table 8.
Table 8. Dieldrin residues in individual food groups of
the total-diet study (24 sets of total diet samples,
January - December 1981)a
----------------------------------------------------------
Food group Range of residues Average residues
(µg/kg) (µg/kg)
----------------------------------------------------------
Bread ND ND
Other cereal products ND ND
Carcass meat ND - 40 3.5
Offals ND - 5 0.5
Meat products ND ND
Poultry ND - 4 1
Fish ND - 8 2
Oils and fats ND - 15 1
Eggs ND - 4 0.5
Green vegetables ND ND
Potatoes ND - 1 < 0.5
Other vegetables ND - 10 1
Fresh fruit ND ND
Milk ND - 2 0.5
Dairy products ND - 150 4
----------------------------------------------------------
a The limit of detection in these studies varied with the
food group but was sometimes as low as 1 µg/kg.
ND = not detectable.
On the basis of these data, it was estimated that the mean
level of dieldrin residues in the total diet in 1981 in the United
Kingdom was 0.5 µg/kg. This figure compares with 1.5 µg/kg for the
period 1975 - 1977 and 4 µg/kg for the period 1966 - 1967. The
computed daily intake derived from the 1981 figure was < 0.8
µg/person or < 0.01 µg/kg body weight.
Further data on certain individual products were also reported.
Maize: Two samples of imported maize, representing 3% of the
total number of samples taken in a survey conducted in 1981,
contained detectable levels of dieldrin, the highest
concentration being 0.04 mg/kg. The rest of the samples did
not contain dieldrin residues above the limit of determination
of 0.01 mg/kg.
Pulses: Separate surveys of residues in pulses obtained from
retail outlets were carried out in 1982 and 1983. In 1982, 42
samples involving 12 different kinds of pulses were analysed.
In this case, aldrin was found in one sample of haricot beans
(0.04 mg/kg) but was below the limit of determination (< 0.01
mg/kg) in all the others. Dieldrin was found in a limited
number of mung beans (0.05 mg/kg) but was below the limit of
determination (< 0.01 mg/kg) in all of the others. Thus,
neither aldrin nor dieldrin were detected in the majority of
pulses sampled in 1982.
In 1983, 40 samples were analysed and there were no residues
reported for aldrin or dieldrin above the level of
determination, with the exception of limited samples of mung
beans containing dieldrin (maximum, 0.04 mg/kg; mean, < 0.01
mg/kg).
Fruit and vegetables, 1981 - 1984: A large-scale monitoring
project of fruit and vegetables was undertaken in the United
Kingdom during the period 1981 - 1984. Some 40 commodities
were sampled during that period, 1649 samples being obtained
from retail outlets and analysed. The data were not
individually reported but, although most of the commodities
were analysed for organochlorine pesticide residues, there were
no reports of any sample containing residues of dieldrin
exceeding either Codex or EEC maximum residue limits.
Information on the incidence of detectable residues of dieldrin
was not presented in this case. It is of interest to note that
648 of the samples were grown in the United Kingdom and 1001
were imported.
Lamb meat: Sampling of kidney fat from home-grown lamb
destined for export began in October 1984 and data for 988
samples were reported. None contained dieldrin residues above
the limit of determination of 0.02 mg/kg.
Fish: The information in Table 9 was obtained from fish caught
in areas around the English and Welsh coast where the levels of
chemical contamination were known to be high.
Residues of dieldrin in processed fish imported into the United
Kingdom were determined in 155 samples of different products
obtained from retail outlets in 1983. The data are summarized
in Table 10. In a further study, dieldrin residues were
determined in a limited number of fish oil products obtained
through retail outlets (Table 11).
Table 9. Mean residue levels of dieldrin (mg/kg)
in the liver and muscle of marine fish from
England and Wales, 1982
------------------------------------------------
Fish Muscle Number Liver Number
of samples of samples
------------------------------------------------
Cod 0.003 43 0.26 73
Dab 0.003 50 0.13 50
Flounder 0.003 49 0.027 49
Mackerel 0.007 29 0.053 23
Plaice 0.004 43 0.040 68
Sole 0.003 50 0.031 50
Whiting 0.009 62 0.26 62
------------------------------------------------
Table 10. Residues of dieldrin (mg/kg) in
processed imported fish and shellfish in 1983
---------------------------------------------
Fish Rangea Average Number of
samples
---------------------------------------------
Pilchards < 0.009 0.001 21
Plaice < 0.006 0.001 19
Salmon < 0.02 0.002 36
Sardines < 0.004 0.001 11
Tuna ND ND 15
Cockles and < 0.01 0.004 5
mussels
Crab ND ND 15
Prawns < 0.001 < 0.001 17
Shrimps < 0.004 0.001 16
---------------------------------------------
a ND = not detectable.
Table 11. Residues of dieldrin (mg/kg) in fish
oil products, 1984
-----------------------------------------------
Product Range Mean Number of
samples
-----------------------------------------------
Cod liver oil
Mixtures 0.01-0.21 0.08 8
Capsules 0.06-0.20 0.12 3
Halibut liver oil
Capsules 0.01-0.1 0.04 5
"Fish lipid" oil
Capsules 0.01 0.01 1
-----------------------------------------------
5.1.6.4 USA
Surveys were carried out in the USA, during the period
1980 - 1982, covering the diets of infants (aged 6 months),
toddlers (aged 2 years) (Gartrell et al., 1986a), and adults
(youths aged 16 - 19 years) (Gartrell et al., 1986b). In each
case, the samples were taken from a number of locations (13 in the
case of infants and toddlers and 27 in the case of youths). They
were selected as being representative of the composition of diets
for the three population groups studied. Individual foods were
bulked together in food groups and the bulked samples analysed.
The lower level of determination was not precisely stated, since it
varied according to the food group concerned, but from the data
presented it would appear to have been either 1 or 2 µg/kg food
item. Results that were below these limits (and hence
unquantifiable), but where the identity of the residue could be
confirmed, were reported as "T". The analysts' estimate of the
value of "T" was used to estimate the average level of residues in
the whole food group. Data for dieldrin residues are given in
Tables 12, 13, and 14.
Table 12. Dieldrin residues (lg/kg) in infant dietary
componentsa
---------------------------------------------------------
Food group Range of residues Average level
---------------------------------------------------------
Drinking-water 0 0
Whole milk T 0.1
Other dairy products T - 1 0.3
Meat, fish, poultry T - 2 0.5
Grain and cereals 0 0
Potatoes T - 2 0.2
Vegetables T - 1 0.1
Fruit and fruit juices 0 0
Oils and fats 0 0
Sugar and adjuncts 0 0
Beverages 0 0
---------------------------------------------------------
a For breast milk, see section 5.2.2.
5.1.6.5 Appraisal of intake studies
The above data demonstrate that in the United Kingdom and the
USA the intake of dieldrin residues in food is well below the ADI
of 0.1 µg/kg body weight. Moreover, taking into account the rather
high dietary intake estimated for adults in the USA, the agreement
between estimates for the United Kingdom and the USA is striking,
notwithstanding the widely differing origins of the basic food
commodities, especially the relatively high proportion of imports
in the case of the United Kingdom. The estimated levels of intake
in Canada were even lower. The residues in Australia, though very
low, were not expressed in terms of intakes.
Table 13. Dieldrin residues (µg/kg) in toddler dietary
components
--------------------------------------------------------
Food group Range of residues Average level
--------------------------------------------------------
Drinking-water 0 0
Whole milk T 0.1
Other dairy products T - 3 1.2
Meat, fish, poultry T - 3 0.8
Grain and cereals 0 0
Potatoes T - 3 0.3
Vegetables T - 2 0.5
Fruit and fruit juices 0 0
Oils and fats 2 0.3
Sugar and adjuncts 0 0
Beverages 0 0
--------------------------------------------------------
Table 14. Dieldrin residues (µg/kg) in adult dietary
components
------------------------------------------------------
Food group Range of residues Average level
------------------------------------------------------
Dairy products T - 3 0.6
Meat, fish, poultry T - 4 1.2
Grain and cereals 4 0.1
Potatoes T - 2 0.4
Leafy vegetables T - 2 0.2
Legume vegetables 0 0
Root vegetables T - 5 0.4
Garden fruits T - 11 2.1
Fruits 1 0.1
Oils and fats T - 2 0.3
Sugar and adjuncts 0 0
Beverages 0 0
------------------------------------------------------
Dietary levels of dieldrin residues in both the United Kingdom
and the USA appear still to be decreasing, though less so than in
previous years.
In 1966, the JMPR established an acceptable daily intake (ADI)
of 0 - 0.1 µg/kg body weight (combined total for aldrin + dieldrin).
5.1.7. Concentrations of dieldrin in non-target species
There have been many investigations of the occurrence of
dieldrin in the body tissues or eggs of non-target species. The
residues range from less than 0.001 mg/kg to about 100 mg/kg, but
most reported residues are less than 1 mg/kg. The wide range of
concentrations is partly a reflection of the extreme sensitivity of
modern analytical techniques, but there are a number of other
factors involved, e.g., the source and magnitude of the exposure;
the component analysed (brain, adipose tissue, eggs, etc.), and
whether the samples are representative of living, apparently
healthy populations (specimens collected by capture, shooting,
etc., during systemic monitoring surveys) or consist of animals
found dead or dying. Interspecies differences in rates of
metabolism also contribute to the variability of residues. The
highest residues are found in two main groups of organisms. The
first group consists of organisms living near the source of release
into the environment; thus, high residues may be found in aquatic
organisms near the point of release of an industrial effluent, or
in seed-eating birds in areas where seed dressed with aldrin or
dieldrin is used in agriculture. The second group of organisms
consists of predators, particularly those feeding on aquatic
organisms or seed-eating birds or mammals.
The results of some analyses of various species from different
geographical areas are summarized in Tables 15 and 16.
There have been very extensive surveys of dieldrin residues in
biota that are not directly associated with a particular use of
aldrin/dieldrin or their waste disposal.
Soil and earthworms (four genera) were collected from 67 fields
from eight states in the USA. The geometric mean concentrations of
aldrin and dieldrin in soil were 0.014 and 0.023 and, in
earthworms, 0.088 and 0.19 mg/kg dry weight, respectively.
Correlation coefficients between the concentrations of dieldrin in
earthworms and soil were derived for six types of crops, but none
were significant. They were also derived from four different soil
types; only the concentrations in the earthworms from silt loam
soils were significantly related to the concentration in the soil
(Gish, 1970).
Henderson et al. (1969, 1971) studied the occurrence during the
period 1967 - 1969, of dieldrin in various species of fish from 50
monitoring stations located in the Great Lakes and in major river
basins in the USA. The mean concentrations of dieldrin in whole
fish lay in the range 0.01 - 0.28 mg/kg, and the maximum value
found was 1.94 mg/kg. The concentrations above 1 mg/kg were found
in fish from the Atlantic coast streams, Gulf coast streams, and
Great Lake drainage.
Koeman et al. (1967, 1971) and Koeman (1971) studied the
presence of dieldrin in fish, mussels, zooplankton, and birds in
the Wadden area of the Netherlands during the period 1965 - 1971.
The mean concentrations in mussels, marine fish, freshwater fish,
and zooplankton were below 0.1 mg/kg (maximum concentration, 0.23
mg/kg), except in three species of marine fish. In these, the mean
concentration was 0.27 mg/kg (maximum concentration, 0.42 mg/kg).
The levels in the liver and/or eggs of the sandwich tern (Sterna
sandwincensis) and grey heron (Ardea cinerea) were up to 5.1 mg/kg
(maximum concentration, 12 mg/kg). Mortality among sandwich terns
(Sterna sandvicensis), eider duck (Somateria mollissima), and a few
other bird species was reported.
Table 15. Residues of dieldrin in non-target species and their environment
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Antarctica
Signy Island 1966 Chinstrap penguin liver 11 0.002 0.001-0.006 LD not defined; Tatton &
(Pygoscelis antarctica) presumably < 0.001 Ruzicka (1967)
mg/kg
Fish liver 4 0.003 0.001-0.009
(Notothenia neglecta)
Skuas and shags liver 4 0.001 LD - 0.002
Sheathbills 3 0.009 LD - 0.015 sudden deaths of
(Chionis alba) sheathbills of
unknown causes had
occurred
Canada
Southwestern Fish (two species) river water 52 0.000005 LD - 0.00011 LD: water, Miles & Harris
Ontario 1970 bottom mud 14 0.002 LD - 0.01 < 0.000001 mg/litre; (1971)
30/4a 0.071 0.023-0.189 bottom mud, < 0.001
mg/kg
Province of Fish (various species) composites of 62 0.10 LD - 0.56 LD < 0.005 mg/kg Reinke et al.
Ontario headless dressed (1972)
specimens
Four other Fish (various species) composites of 119 0.01 LD - 0.08 76 composites from Reinke et al.
provinces headless dressed these four provinces (1972)
specimen contained residues;
LD < 0.005 mg/kg
Eastern Canada Leach's storm egg 18 0.05 0.03-0.13 Pearce et al.
1970-1976 petrel (Oceanodroma (1979)
leucorhoa)
Double-crested cormorant egg 90 0.13 0.01-0.68
(Phalacrocorax auritus)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Eastern Canada Common eider egg 25 0.02 0.01-0.04
1970-1976 (contd.) (Somerteria mollissima)
Common tern egg 50 0.04 0.01-0.13
(Sterna hirundo)
Razorbill egg 13 0.12 0.01-0.52
(Alca torda)
Common guillemot egg 4 0.02 0.02-0.03
(Uria aalge)
Black guillemot egg 3 0.02 0.01-0.05
(Cepphus grylle)
Atlantic puffin egg 48 0.06 0.03-0.13
(Fratercula arctica)
Falkland Islands Marine, coastal, and egg 46 0.002 LD - 0.011 LD not defined, but Hoerschelmann
1977 freshwater birds < 0.002 mg/kg et al. (1979)
Greece
Saronikos Gulf Striped mullet muscle 74 0.004 0.0001-0.050 residues attributed Voutsinou-
1975 (Mullus barbatus) to the discharge of Taliadouri &
domestic waste and Satsmadjis
industrial effluents (1982)
Iraq
Shatt al-Arab Cyprinid muscle 2 0.003 ND - 0.008 Douabul et al.
river (Barbus xanthopetrus) (1987)
Indian shad muscle 2 0.028 0.016-0.041 Douabul et al.
(Tenualosa ilistra) (1987)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Kenya
Lake Nakuru 1975 water 10 < 0.0001 - Greichus et
al. (1978b)
bottom sediment 10 < 0.001b -
Plankton composite 1 0.03b - Greichus et
al. (1978b)
Chironomids composite 1 < 0.01b -
Water boatmen composite 1 < 0.01b -
(Coroxidae)
Fish composite 100/10a 0.02b -
(Tilapia grahami)
Netherlands 1965 Mussel 22 0.033 0.014-0.084 Koeman (1971)
(Mytilus edulis)
1965 marine fish (3 species) whole body 103 0.27 0.16-0.42 Koeman et al.
(1967)
1966 marine fish (2 species) whole body 37 0.07 0.01-0.23 fish species on Koeman et al.
which sandwich terns (1967)
feed
1965 Sandwich tern liver 19 5.1 1.9-12 found dead or dying Koeman et al.
(Sterna sandvincensis) (1967)
1965-1966 Sandwich tern liver 14 0.6 0.2-2 killed, shot, or Koeman et al.
found dead after a (1967)
storm
1967 freshwater fish 28 0.02 LD - 0.05 LD < 0.01 mg/kg Koeman (1971)
(3 species)
1969 Mussel 10/2a 0.012 0.007-0.016 Koeman et al.
(Mytilus edulis) 199/8a 0.013 0.007-0.023 (1971)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Netherlands (contd.)
1970 Pike whole body 10 0.01 LD - 0.022 LD < 0.003 mg/kg Koeman et al.
(Esox lucius) (1971)
1971 Roach whole body 81/6a 0.004 LD - 0.013 LD < 0.005 mg/kg Koeman et al.
(Rutilus rutilus) (1971)
1971 zooplankton composite - 0.005 - Koeman et al.
(1971)
1970 Shrimp 50/1a 0.009 - Koeman et al.
(Crangon vulgaris) (1971)
1969-1970 marine fish (5 species) 37/5a 0.022 0.008-0.043 Koeman et al.
(1971)
1970 Sandwich tern egg 10 0.082 0.054-0.099 Koeman et al.
(1971)
1971 Grey heron egg 27/4a 1.25 0.5-1.9 Koeman et al.
(Ardea cinerea) (1971)
New Zealand and Marine birds egg, 7 0.01 LD - 0.05 LD not defined, but Bennington et
sub-Antarctic (various spp.) breast muscle 7 0.1 0.03-0.28 < 0.02 mg/kg al. (1975)
islands 1970-1971
Norway
Four regions 1983 Shags (Phalacrocorax egg approx- 0.126-0.286 Barrett et al.
aristotelis) imately in 7.1% of (1985)
Herring gull (Larus 10 samples
argentatus)
Kittwakes (Rissa
tridactyla)
Common guillemots
(Uria aalge)
Razorbills (Alca torda)
Puffins (Fratercula
arctica)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
United Kingdom
England, Brown shrimp homogenates of 12 0.0055 0.0012-0.020 Van Den Broek
Medway estuary (Crangon vulgaris) 50 specimens (1979)
1974-1975
Sand goby homogenates of 9 0.047 0.024-0.077 Van Den Broek
(Pomatoschistus minutus) 50 specimens (1979)
Sprat homogenates of 13 0.084 0.030-0.142 Van Den Broek
(Sprattus sprattus) 50 specimens (1979)
Eel liver 16 0.051 0.0085-0.090 Van Den Broek
(Anguilla anguilla) (1979)
Whiting liver 9 0.57 0.25-1.10 Van Den Broek
(Merlangius merlangus) (1979)
Flounder liver 16 0.21 0.043-0.39 Van Den Broek
(Platichthys flesus) (1979)
Plaice liver 12 0.12 0.015-0.23 Van Den Broek
(Pleuronectes platessa) (1979)
Scotland, Plankton 12 0.072 0.019-0.230 Williams &
Firth of Clyde (various estuarine Holden (1973)
1971-1972 and marine species)
North Atlantic, Plankton 14 0.003 LD - 0.015 LD < 0.001 mg/kg Williams &
northeast transect (various estuarine Holden (1973)
from Mull of and marine species)
Kintyre 1971-1972
Firth of Clyde Mussel 25 0.178 0.012-2.43 Cowan (1981)
(coastal waters) (Mytilus edulis) homogenates of 80 0.022 0.006-0.216
1977 50-100 specimens
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Shetland Isles Mussel 12 0.013 0.006-0.029 Cowan (1981)
(8 other coastal (Mytilus edulis)
sites) 1977
Irish Sea and Seabirds liver 21 1.23 0.07-5 heavy mortality of Lloyd et al.
Firth of Clyde (various species) seabirds in Irish (1974)
1974 Sea; continuous
winter storms may
have been cause of
mortality
Irish Sea 1969 Guillemot liver high mortality of Parslow &
(Uria aalge) guillemots in 1969; Jefferies
shot birds 9 0.09 0.01-0.41 primary cause of (1973)
dead birds 8 0.48 0.10-0.80 death was probably
malnutrition: mean
body weights for
shot and dead birds,
963 g and 580 g,
respectively; mean
liver weights,
43.8 g and 12.5 g,
respectively; PCBs
may also have been
responsible for the
death of guillemots
Great Britain Grey heron egg
1964-1977 (Ardea cinerea)
March 135 0.75 0.65-0.86b concentration Cooke et al.
April 103 1.19 1.05-1.35b increased (1982)
May 45 3.20 2.64-3.88b significantly
between March and
May
Shetland Isles, Great skua egg 12 0.091 0.022-0.15 Furness &
Foula 1976 (Catharacta skua) Hutton (1979)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
USA 15 states:
1965-1972 Estuarine molluscs composites of LD 0.005 mg/kg Butler (1973)
(10 species) meat from 15
or more mature
molliscs
North Carolina, 71 0.01 LD - 0.019 concentrations in 69
Point of Marsh samples below 0.005
mg/kg
Mississippi, 78 0.01 LD - 0.019 concentrations
Biloxi Bay in 70 samples below
0.005 mg/kg
Texas, 48 0.021 LD - 0.046
Arroyo Colorado
New York, 74 0.024 LD - 0.132
Hempstead Harbor
Georgia, 64 0.028 LD - 0.230
Lazaretta Creek
Major river fish composites of LD < 0.001 mg/kg Henderson et
basins in the (various species) whole fish al. (1969,
USA: 1971)
Atlantic coast 741/141a,d 0.14 LD - 1.94
streams 157/36a,e 0.13 LD - 0.55
Gulf coast 204/48a,d 0.12 LD - 1.26
streams 59/12a,e 0.28 LD - 1.59
Great Lakes 378/63a,d 0.05 LD - 0.50
drainage 81/18a,e 0.06 LD - 0.37
Mississippi River 657/139a,d 0.06 LD - 0.52
system 153/34a,e 0.06 0.01-0.49
Hudson Bay 51/13a,d 0.12 0.03-0.37
drainage 5/2a,e 0.01 0.01
Colorado River 112/24a,d 0.02 LD - 0.10
system 24/6a,e 0.01 0.01
Interior basins 120/25a,d 0.01 LD - 0.06
30/6a,e 0.02 LD - 0.03
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
California 90/24a,d 0.06 LD - 0.31
streams 28/6a,e 0.10 0.01-0.36
Columbia River 246/64a,d 0.02 LD - 0.10
system 70/16a,e 0.03 LD - 0.09
Pacific Coast 83/20a,d 0.06 LD - 0.52
streams 29/6a,e 0.01 LD - 0.02
Alaskan streams 105/24a,d 0.003 LD - 0.01
30/6a,e 0.006 LD - 0.01
Upper continental Bathyl-demersal liver 4 0.017 0.011-0.026 fish caught by trawl Meith-Avcin et
rise (southeast fish (Antimora at a depth of 2500 m al. (1973)
of (Cape rostrata)
Hatteras)
California 1970 Common egret brain 5 4.36 0.60-6.76 birds found dead or Faber et al.
(Casmerodius albus) moribund; dieldrin (1972)
considered to be a
contributory cause
of death of 4 birds
South Dakota Pheasant adipose tissue 48 0.08 LD - 1.07 LD < 0.01 mg/kg; 13 Greichus et
1965-1967 (Phasianus colchicus) samples of fat al. (1968)
contained < 0.01
mg/kg
Sharp-tailed grouse living birds 46 0.17 LD - 1.71 13 samples of fat
(Pedioecetes phasianellus contained < 0.01
campestris) mg/kg
South Dakota 1967 Pheasant egg 67 0.02 LD - 0.12 LD < 0.01 mg/kg; 13 Linder &
eggs contained 0.01 Dahlgren
mg/kg (1970)
Maine and Common eider and herring egg 88 LD LD LD < 0.1 mg/kg Szaro et al.
Virginia 1977 gull (1979)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
Maine and Great black-backed egg 28 0.12 LD - 0.55 24 of the eggs
Virginia (contd.) gull contained < 0.1 mg/kg
Texas, Corpus Wintering shorebirds carcass 56 0.11 LD - 1 LD < 0.1 mg/kg; 2 White et al.
Christi Bay (7 species) (shot birds) carcasses contained (1980)
1976-1977 < 0.1 mg/kg
Lake Michigan Red-breasted merganser egg 206 0.77 0.2-2.3 LD < 0.1 mg/kg Haseltine et
1977-1978 (Mergus serrator) al. (1981)
Mallard egg 27 0.07 LD - 0.53 22 of the eggs
(Anas platyrhynchos) contained < 0.1
mg/kg
Gadwall egg 9 0.1 LD - 0.56 5 of the eggs
(Anas strepera) contained < 0.1
mg/kg
Florida Brown pelican LD < 0.05 mg/kg Blus et al.
(Pelecanus occidentalis) egg (1974b)
Atlantic coast egg 22 0.36 LD - 1.52
Gulf coast egg 27 0.10 trace - 0.40
Florida carcass 16 0.65 LD - 1.60 Blus et al.
1969 (shot birds) (1974b)
South Carolina carcass 5 0.51 LD - 1.50
(shot birds)
1969 Brown pelican egg 11 0.94 0.60-1.62 Blus et al.
1970 egg 10 0.62 0.20-1.30 (1974b, 1977,
1971 egg 65 0.46 0.20-1.02 1979b)
1972 egg 72 0.45 LD - 1.76
1973 egg 104 0.45 0.16-1.65
1974 egg 116 0.54 0.17-2.89
1975 egg 102 0.36 LD - 1.04
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Louisiana
1971 Brown pelican egg 3 0.33 0.24-0.54 Blus et al.
1972 egg 12 0.45 0.30-0.79 (1979a)
1973 egg 21 0.64 0.30-1.12
1974 egg 25 0.84 0.49-1.61
1975 egg 30 1.08 0.64-2.25
1976 egg 25 0.94 0.44-3.03
Zimbabwe
Lake McIlwaine water 10 < 0.0001 Greichus et
1974 al. (1978a)
bottom sediment 10 0.004
Plankton composite 1 < 0.01b
Oligochaete composite 1 0.08b
(Branchiura sowerbyi)
Fish (3 spp.) composite 200/15a 0.04b 0.03-0.07
Cormorant (2 spp.) brain 10 1.4b
----------------------------------------------------------------------------------------------------------------------------------------
a N1/N2: N1 is the number of individuals incorporated into N2 composites; the range corresponds to the composites.
b Concentration expressed on dry weight basis.
c LD = limit of detection, ND = not detectable.
d Samples taken in 1967-1968.
e Samples taken in 1969.
Table 16. Concentrations of dieldrin in non-target organisms
------------------------------------------------------------------------------------------
Species Geographical Year Concentration of dieldrin Reference
(component area (mg/kg wet weight)
analysed) Geometric Arithmetic Range
mean mean (N)c
------------------------------------------------------------------------------------------
Fish (3 spp.) Great Britain 1977-79 - 0.00042a <0.00035- Rickard &
(muscle) R. Thames 0.0020 (83) Dulley (1983)
(tidal)
Oysters USA: 1968-69 - 0.0014a <0.001- Rowe et al.
(flesh) Louisiana 0.0034 (113) (1971)
Penguin Antarctic 1966-67 - 0.008a <0.006-0.010 Tatton &
(abdominal (5) Ruzicka
fat) (1967)
Fish, USA: Virgin 1972-74 - 0.005a <0.005-0.021 Reimold
invertebrates Islands, (141) (1975)
(various spp.) Puerto Rico
(whole body)
Fish (various USA: Western 1967-69 - 0.01a <0.01-0.08 Klaassen &
spp.) (whole Kansas (393) Kadoum (1973)
body/tissues)
Northern fur USA: Alaska 1968-69 - 0.05a <0.01-0.091 Anas & Wilson
seals (liver) (23) (1970a,b)
Birds (various Zimbabwe 1973-76 - 0.004b <0.01-0.67 Tannock et
spp. including (34) dry al. (1983)
birds of prey) weight
(eggs)
Woodcock USA: eastern, 1970-71 - 0.018a <0.01-0.55 Clark & McLane
(breast mid-western (129) (1974)
muscle)
Starlings USA 1967-68 - 0.139a <0.005-1.18 White (1976)
(carcass) (360)
1970 - 0.117a <0.005-3.59 White (1976)
(125)
Starlings USA 1972 - 0.098a <0.005-1.56 White (1976)
(carcass) (130)
1974 - 0.057a <0.005-1.01 White (1976)
(126)
Migratory USA: Florida 1964-73 - 0.2a <0.01-1.10 Johnston
birds (various (829) (1975)
spp.) (breast
muscle)
------------------------------------------------------------------------------------------
Table 16. (contd.)
------------------------------------------------------------------------------------------
Species Geographical Year Concentration of dieldrin Reference
(component area (mg/kg wet weight)
analysed) Geometric Arithmetic Range
mean mean (N)c
------------------------------------------------------------------------------------------
Bats (3 spp.) USA: 1973 0.2a - <0.1-3.2 Clark &
(carcass) Maryland, (110) Prouty (1976)
West Virginia
Golden eagle USA: western, 1964-70 - 0.1 <0.1-12 Reidinger &
(fat) mid-western (69) Crabtree
states (1974)
Golden eagle Scotland 1964-74 0.12 - <0.05-6.9 Cooke et al.
(eggs) (100) (1982)
Tawny owl Great Britain 1963-65 0.15 - <0.05-12.7 Cooke et al.
(liver) (55) (1982)
Peregrine Great Britain 1964-77 0.20 - <0.05-7.6 Cooke et al.
falcon (eggs) (145) (1982)
Bald eagle USA 1971-72 0.6b - <0.05-7.8 Cromartie et
(brain) (37) al. (1975)
Barn owl Great Britain 1963-75 1.21b - <0.05-70.2 Cooke et al.
(liver) (251) (1982)
Hawks, Netherlands 1968-69 - 10.8 0.45-31 Koeman et al.
falcons, owls (19) (1969)
(liver)
------------------------------------------------------------------------------------------
a Indicates living organisms collected by capture, shooting, etc.
b Indicates organisms found dead or dying.
c Number in parentheses is the number of specimens.
Butler (1973) found mean dieldrin concentrations of 0.01 -
0.028 mg/kg (maximum concentration, 0.23 mg/kg) in estuarine
molluscs collected from 15 coastal states in the USA during the
period 1965 - 1972.
Fish sampled in Canada, in 1970, were found to have a mean
concentration of 0.071 mg dieldrin/kg (maximum concentration, 0.189
mg/kg). The concentrations in the water and bottom mud were of the
order of 0.005 µg/litre and 0.002 mg/kg, respectively (Miles &
Harris, 1971). In another study, fish from five provinces (78
locations) in Canada showed mean concentrations of 0.1 - 1 mg/kg
(maximum concentration, 0.56 mg/kg) (Reinke et al., 1972).
In coastal waters around England, Scotland, and Ireland, a
number of studies were carried out to determine dieldrin levels in
plankton, mussels, shrimp, and various other marine species
(1971 - 1975). The mean concentrations ranged from 0.003 to 0.178
mg/kg (maximum level, 2.43 mg/kg). Mussels showed the highest
levels (Williams & Holden, 1973; Lloyd et al., 1974; Van Den Broek,
1979; Cowan, 1981).
The presence of dieldrin in water, bottom sediment, and living
organisms has been studied in Africa (Kenya, Zimbabwe), Signy
Island (Antartica), New Zealand, and sub-antartic islands. The
concentrations of dieldrin in water were very low (< 0.01
µg/litre), those in bottom sediment were up to 0.004 mg/kg, and
those in water organisms (mainly plankton and invertebrates) were
0.01 - 0.03 mg/kg (dry weight basis). Penguin abdominal fat
contained 0.008 mg/kg and liver 0.002 mg/kg. The levels in fish
were < 0.1 mg/kg (dry weight) (Tatton & Ruzicka, 1967; Bennington
et al., 1975; Greichus et al., 1978a,b).
In the different areas where water, invertebrates, and fish
were analysed, birds and eggs were also studied for the presence of
dieldrin. In the eggs of a number of bird species from the
Falkland Islands, Hoerschelmann et al. (1979) found an average of
about 0.005 mg/kg dieldrin in 17 of the 46 eggs. In eggs of
coastal birds in the Federal Republic of Germany, the average
concentration (in 27 eggs) was higher (average, 0.031 mg/kg; range,
0.004 - 0.187 mg/kg).
Parslow & Jefferies (1973) found mean concentrations of up to
0.48 mg/kg in the liver of guillemots (Uria aalge) in the Irish
Sea. In eggs of the great skua (Catharacta skua), collected on the
Shetland Islands, Furness & Hutton (1979) measured a concentration
of 0.091 mg/kg (maximum concentration, 0.15 mg/kg). In South
Dakota, USA (1965 - 1967), Greichus et al. (1968) and Linder &
Dahlgren (1970) determined concentrations of up to 0.08 mg/kg in
the adipose tissue of pheasants and 0.02 mg/kg in eggs. In adipose
tissue of grouse, a mean concentration of 0.17 mg/kg was found.
When a number of eggs of several bird species was analysed in
eastern Canada (1970 - 1976), the mean concentrations were 0.06
mg/kg (maximum level, 0.68 mg/kg) (Szaro et al., 1979). In
different species of birds (and eggs) in the north and south of the
USA, White et al. (1980) found average concentrations in the
carcass of 0.13 - 0.47 mg/kg and Haseltine et al. (1981) found in
eggs of mergansers (Mergus serrator) a geometric mean concentration
of 0.78 mg/kg.
It is of interest that very low residues are found in the great
majority of eggs from areas remote from the regions of major
aldrin/dieldrin use. This is true of samples from the Falkland
Islands and Antartica and it is also true of a survey of 440 eggs
from 19 species of seabirds collected in 1973 - 1976 in Alaska
showing that residues in 410 eggs were less than 0.05 mg/kg (wet
weight). The highest residue found was 0.6 mg/kg (Ohlendorf et
al., 1982).
In Florida, Louisiana, and South Carolina, Blus et al. (1974b,
1977, 1979a,b) studied the dieldrin concentrations in the carcass
and eggs of the brown pelican (Pelecanus occidentalis) during the
period 1969 - 1976. The mean concentration in the carcass was
about 0.6 mg/kg (maximum concentration, 1.6 mg/kg) and, in the
eggs, about 0.6 mg/kg (maximum concentration, 2.89 mg/kg).
Jefferies (1972) carried out a survey of the residue levels in
bats from the East Anglian area, United Kingdom, to provide more
information on the situation concerning the British bat population.
Four species of bats were studied, Pipistrellus pipistrellus,
Plecotus auritus, Myotis nattereri, and Myotis daubentoni.
Thirty specimens were collected during the period 1963 - 1970.
Dieldrin was found in eight liver specimens (range 0.04 to 3.3 mg/kg
tissue), in two adipose tissue samples (4.0 and 7.9 mg/kg), and in
six total body samples (0.07 to 0.50 mg/kg tissue).
Clark et al (1978) estimated the dieldrin levels in 28 juvenile
grey bats (Myotis grisesceus) taken from three caves in Missouri,
USA. The concentrations varied between the individual animals and
between the caves. Dieldrin was detected in the brain of 18/28
bats, the range being 0.4 - 10 mg/kg tissue (wet weight basis), and
in the carcass of 22/28 bats (range 1.7 - 1379 mg/kg carcass; lipid
weight). The authors believed that there was a direct link between
the field mortality of bats and dieldrin residues acquired through
the food chain.
Clark et al. (1980, 1983b) detected dieldrin in the brain and
carcass of grey bats found dead in a Missouri cave in 1976 and
1977. In 1976, the geometric mean was 7.5 and 650 mg/kg tissue
(respectively for brain on wet weight basis and for carcass on
lipid weight basis) and in 1977, 8.6 and 867 mg/kg tissue,
respectively. Other chlorinated hydrocarbons were also present
such as heptachlor epoxide, DDE, and PCBs.
Clark (1981) studied the brain to carcass lipid relationship
for dieldrin and estimated a minimum lethal level for brain tissue
of 4.6 mg dieldrin/kg (wet weight) and for carcass of 390 (210 -
800) mg dieldrin/kg tissue (lipid weight).
In two other caves in Missouri, dead grey bats were found in
1980, and dieldrin and other halogenated insecticides were found in
the brain and carcass. Seven animals were studied and dieldrin
concentrations ranging from not detectable to 21 mg/kg tissue (wet
weight) were found in the brain and 4.1 - 970 mg/kg in the carcass
(lipid weight). The concentrations in brain were of the same order
as those found in the other caves in Missouri. Bat mortality in
July 1981 occurred simultaneously, in one case, with the death of
macroinvertebrates in the outlet stream of the cave (Clarke et al.,
1983a).
Dieldrin residues ranging from trace to 3.3 mg/kg have been
detected in marine mammals, including whales and seals (Holden,
1975; Rosewell et al., 1979). Other mammals in which dieldrin has
been found include the fisher, fox, marten, mink, raccoon, and
skunk (Frank et al., 1979), the highest concentrations being found
in the predators at the top of the food chain, i.e., mink and
marten (9.7 µg/kg wet tissue).
Other studies on the presence of dieldrin in non-target species
and their environment are summarized in Tables 15 and 16 (Bugg et
al., 1967; Koeman et al., 1967; Rowe et al., 1971; Faber et al.,
1972; Meith-Avcin et al., 1973; Voutsinou-Talia-Douri & Satsmadjis,
1982). Most of these results are an indication of adventitious
contamination, i.e., there is no close relationship to a particular
use of aldrin or dieldrin.
The use of aldrin and dieldrin as seed-dressing agents has
undoubtedly resulted in high concentrations of dieldrin in the body
tissues of animals found dead. An association between the use of
aldrin and dieldrin seed dressings and the deaths of wood-pigeons
(Columba palumbus) was first noted by Carnaghan & Blaxland (1957)
and Turtle et al. (1963, 1965). In wood-pigeon, pheasant,
partridge, and corvids found dead, Turtle et al. (1965) found mean
concentrations of 10, 2.3, 7.3, and 2 mg/kg liver, respectively,
(maximum concentrations of 59.2, 28.8, 46.3, and 14 mg/kg liver).
The concentrations in birds that had been shot were much lower.
Table 17 summarizes the residue levels found following the use
of dieldrin for the control of the tsetse fly and arising from
other uses of aldrin and dieldrin, e.g., as seed-dressing agents.
Several other reports on seed-dressing incidents in the United
Kingdom have been published, e.g., Murton & Vizoso (1963) and
Jefferies et al. (1973).
5.1.7.1 Occurrence of dieldrin in birds of prey and fish-eating
birds
Changes in the populations of hawks, falcons, and other raptors
have prompted extensive studies of the concentrations of dieldrin
in the tissues of birds and eggs. These data are summarized in
Table 18.
The concentrations of dieldrin in the tissues of bald eagles
(Haliacetus leucocephalus) that were found dead during the period
1967 - 1977 were estimated by Mulhern et al. (1970), Belisle et al.
(1972), Cromartie et al. (1975), Prouty et al. (1977), and Kaiser
et al. (1980). The concentrations (geometric mean) in the brain
were 0.1 - 2.0 mg/kg tissue, with a maximum of 11 mg/kg. Because
the population declines of some birds of prey and some fish-eating
birds have been associated with the use of aldrin and dieldrin, the
residues in some of these species will be discussed in more detail.
Table 17. Residues in non-target species - concentrations related to particular uses or discharges of
aldrin/dieldrin
---------------------------------------------------------------------------------------------------------
Species Component Geographical Year No. of Concentration of Comments Reference
analysed area speci- dieldrin (mg/kg)
mensa Meanb Range
---------------------------------------------------------------------------------------------------------
Woodpigeon liver Netherlands 1966 20 2.8b 0.05-27.1 seed dressing Fuchs
(Columba 4 79c 44.2-136 (1967)
palumbus)
Pink-footed liver United 1972 6 31c 15-48 seed dressing Stanley &
goose (Anser Kingdom -73 Bunyan
branchyrhynchus) (1979)
Pheasant egg USA: 1966 120 0.3 0.02-2.82 soil Greenberg &
(Phasianus Illinois insecticide Edwards
colchicus) (1970)
Birds (various liver Kenya 1968 12 28.2c 18-57 tsetse fly Koeman &
spp.) 21 1.7b 0.16-6 control (dead Pennings
brain 10 14.3c 6-22 birds found (1970)
11 0.2b 0.06-0.68 during 10 days
after spray
application;
live birds
collected 2
months later)
Insects (various whole Cameroon 1979 227 0.2c NDe - 13.2 tsetse fly Mueller et.
spp.) body control al. (1981)
Fish whole 124 0.09 ND - 214.3
(Aphyosemion body
bualanum)
Birds (various liver 40 1.51b ND - 7.24
spp.)
Fruit bat liver 20 79.2b ND - 174.81
(2 spp.)
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Species Component Geographical Year No. of Concentration of Comments Reference
analysed area speci- dieldrin (mg/kg)
mensa Meanb Range
---------------------------------------------------------------------------------------------------------
Rat (Praomys liver 13 0.37 ND - 1.20 Mueller et
tullbergi) al. (1981)
Common gallinule egg USA: 1965 4/23d 9.6 2.23-13.17 rice fields Causey et
(Gallinula Louisiana sown with al. (1968)
chloropus) 1966 14 9.4 1.13-22.12 aldrin-treated
seed
Purple gallinule egg 1965 2/16d 9.7 6.47-12.94
(Porphyrula 1966 56 6.5 0.49-15.35
martinica)
Common gallinule egg USA: 1968 6 17.5 4.69-28.07 rice fields Fowler et
Louisiana sown with al.(1971)
1969 12 4.8 1.16-10.7 aldrin-treated
seed
Purple gallinule egg 1968 26 9.4 3.23-16.43
1969 33 3.8 1.56-13.62
Invertebrates composites USA: Texas 1967 1208/ 1.1b LDf - 3.2 aldrin-treated Flickinger
of whole Gulf coast -71 16 (3.1) (LD - 16.3) seed & King
body (1972)
Crayfish whole 105/8 6.3c LD - 17
(2 spp.) body (2.1) (LD - 9)
Cricket frog whole 18/3 0.1b LD - 0.1
(Acris crepitans body
blanchardi)
Fish (4 spp.) whole 592/4 1.2b 0.4-2.8
body
Turtles (2 spp.) whole 5/2 0.9b 0.6-1.2
body (2.4) (LD - 4.8)
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Species Component Geographical Year No. of Concentration of Comments Reference
analysed area speci- dieldrin (mg/kg)
mensa Meanb Range
---------------------------------------------------------------------------------------------------------
Snakes (3 spp.) whole USA: Texas 1967 3/3 2.4b 0.1-5.7 Flickinger
body Gulf coast -71 & King
(1972)
Great horned owl brain 1 6.3c -
Birds (various brain 27 8.5c LD - 22 192 dead birds
spp.) (0.1) (LD - 0.2) collected from
1967-71
Fulvous tree egg 69/14 2.5 <0.1-9.5
duck
(Dendrocygna
bicolor)
Owls (various liver United 1974 22 24c 1.7-46 death of many Jones et
spp.) Kingdom -76 owls due to al. (1978)
(London Zoo) dieldrin
poisoning;
sawdust from
dieldrin-
treated wood
the probable
source of
contamination
---------------------------------------------------------------------------------------------------------
a N1/N2: N1 is the number of incorporated into N2 composites; the range corresponds to the composites.
b Indicates living organisms collected by capture, shooting, etc. Values in parentheses are the
concentrations of aldrin.
c Indicates organisms found dead or dying. Values in parentheses are the concentrations of aldrin.
d Clutches/eggs.
e ND = not determined.
f LD = limit of detection.
Table 18. Concentrations of dieldrin in tissues and eggs of birds of prey and fish-eating
birds found dead
-------------------------------------------------------------------------------------------
Species Type of Geographical Year No. of Concentration of Reference
sample area speci- dieldrin (mg/kg)
mens Meana Rangeb
-------------------------------------------------------------------------------------------
Kestrel (Falco liver Netherlands 1968-69 7 - 1.1-24 Koeman et
tinnunculus) al. (1969)
Kestrel (Falco liver United 1963-65 74 1.09 0.94-1.27 Cooke et
tinnunculus) Kindgom 1966-71 144 1.06 0.91-1.24 al. (1982)
1972-75 125 1.43 1.19-1.72
1977 31 0.31 0.21-0.45
Sparrow-hawk liver Netherlands 1969 3 - 0.9-19 Koeman et
(Accipiter nisus) al. (1969)
Sparrow-hawk liver United 1963-65 30 1.20 0.97-1.49 Cooke et
(Accipiter nisus) Kingdom 1966-71 82 0.29 0.22-0.38 al. (1982)
1972-75 83 0.61 0.48-0.78
1977 26 0.22 0.15-0.32
Sparrow-hawk egg United 1963-65 24 2.09 1.63-2.67 Cooke et
(Accipiter nisus) Kingdom 1966-71 154 0.69 0.60-0.79 al. (1982)
Buzzard liver Netherlands 1968-69 5 - 0.45-31 Koeman et
(Buteo buteo) al. (1969)
Grey heron liver United 1963-65 26 1.13 0.66-1.94 Cooke et
(Ardea cinerea) Kingdom 1966-67 69 0.92 0.67-1.26 al. (1982)
1972-75 57 0.74 0.57-0.96
1977 12 0.17 0.08-0.37
Kingfisher liver United 1964-65 4 6.83 3.95-11.8 Cooke et
(Alcedo atthis) Kingdom 1966-71 37 1.56 1.23-1.98 al. (1982)
1972-75 22 1.16 0.89-1.53
Peregrine falcon liver United 1963-77 15 1.91 1.35-2.71 Cooke et
(Falco peregrinus) Kingdom al. (1982)
Barn owl liver United 1963-65 48 1.31 1.08-1.60 Cooke et
(Tyto alba) Kingdom 1966-71 94 1.42 1.19-1.69 al. (1982)
1972-75 114 1.07 0.90-1.28
1977 29 0.26 0.18-0.37
Long-eared owl liver United 1963-77 30 1.75 1.14-2.70 Cooke et
(Asio otus) Kingdom al. (1982)
Bald eagle liver USA 1964-65 44 0.28 LD - 11.9 Reichel et
(Haliacetus (LD <0.05 al. (1969)
leucocephalus) mg/kg)
-------------------------------------------------------------------------------------------
Table 18. (contd.)
-------------------------------------------------------------------------------------------
Species Type of Geographical Year No. of Concentration of Reference
sample area speci- dieldrin (mg/kg)
mens Meana Rangeb
-------------------------------------------------------------------------------------------
Peregrine falcon egg United 1963-65 23 0.59 0.49-0.71 Cooke et
(Falco peregrinus) Kingdom 1966-71 76 0.14 0.11-0.17 al. (1982)
1972-75 34 0.18 0.11-0.28
1977 12 0.34 0.25-0.46
Bald eagle egg USA 1969-70 12 0.08c LD - 0.3 Wiemeyer et
(Haliacetus (LD <0.05 al. (1972)
leucocephalus) mg/kg)
USA: 11 0.83c 0.15-2.3
Alaska, 4
other states
-------------------------------------------------------------------------------------------
a Geometric mean, except for footnote c which is arithmetic mean.
b Range of value within 1 standard error.
c Arithmetic mean.
(a) Grey heron (Ardea cinerea)
This is one of the highly contaminated species in the United
Kingdom. Relatively high levels of dieldrin have been measured in
the livers of herons found dead, together with high levels of DDT-
type compounds and polychlorinated biphenyls (Cooke et al., 1982).
The geometric mean concentrations of dieldrin for various periods
within the range 1963 - 1977 are given in Table 18. The geometric
mean concentration of dieldrin in the livers of 143 samples over
the period 1963 - 1975 was 0.9 mg/kg. Of the herons found dead,
50% contained less than 1 mg dieldrin/kg liver, whereas 14%
contained 10 mg/kg or more.
(b) Kestrel (Falco tinnunculus)
The geometric mean concentration of dieldrin in the livers of
374 kestrels found dead in the United Kingdom during the period
1963 - 1977 was 1.2 mg/kg (Cooke et al., 1982). Some 50% of the
kestrels found dead contained less than 1 mg dieldrin/kg liver, 18%
contained more than 10 mg/kg, and 8% more than 20 mg/kg. Higher
levels were found in the Netherlands (Fuchs, 1967; Koeman et al.,
1969).
Sierra et al. (1987) studied the presence of residues of aldrin
and dieldrin in the liver, muscle, fat, kidneys, and brain of four
kestrels from the province of Leon, Spain. The concentrations of
aldrin ranged from 0.003 to 0.65 mg/kg tissue (highest in fat and
kidneys), whereas those of dieldrin ranged from 0.005 to 0.151
mg/kg tissue (highest in liver; fat not estimated). All values
were based on wet weight.
(c) Sparrow-hawk (Accipiter nisus)
The geometric mean concentration of dieldrin in the liver of
195 sparrow-hawks found dead in the United Kingdom over the period
1963 - 1977 was 0.5 mg/kg (Cooke et al., 1982). About 62% of the
dead sparrow-hawks contained less than 1 mg dieldrin/kg liver, and
about 7% contained more than 10 mg dieldrin/kg liver.
Three sparrow-hawks found dead or dying in the Netherlands in
1969 contained 0.89, 1.1, and 19 mg dieldrin/kg liver, respectively
(Koeman et al., 1969). One dead sparrow-hawk (1966) contained 18.4
mg dieldrin/kg liver (Fuchs, 1967).
Sierra et al. (1987) studied the presence of residues of aldrin
and dieldrin in three sparrow-hawks in Leon, Spain. The average
concentrations of dieldrin ranged from 0.1 to 0.45 mg dieldrin/kg
tissue (liver, kidneys, brain) but in fat an average level of 17.3
mg/kg was found (all values were based on wet weight). Only low
levels (< 0.01 mg/kg tissue) of aldrin were found in fat.
(d) Barn owl (Tyto alba)
The geometric mean concentration of dieldrin in the liver of
251 barn owls found dead in the United Kingdom (1963 - 1977) was
1.2 mg/kg (Cooke et al., 1982). About 49% of the barn owls
contained less than 1 mg dieldrin/kg liver, while about 15%
contained at least 10 mg dieldrin/kg liver.
The concentration of aldrin and dieldrin in the muscle, liver,
fat, brain, and kidneys of 23 barn owls, collected in the province
of Leon, Spain, was determined (91 samples in total). The
incidence of aldrin in the tissues ranged from 76 to 83%, and of
dieldrin from 4 to 27%. The average concentration in these organs
and tissues was 0.03 - 0.11 mg aldrin/kg and 0.009 - 0.2 mg
dieldrin/kg tissue (wet weight). The highest concentration was in
the kidneys for aldrin and in the brain for dieldrin (Sierra &
Santiago, 1987).
The concentrations in all four of the above species in the
United Kingdom showed seasonal, annual, and regional trends.
Residue levels in herons decreased progressively after 1963 - 1965
until 1977, whereas the main decrease in levels in sparrow-hawks
occurred between 1963 - 1965 and 1966 - 1971, there being little
subsequent change. In kestrels and barn owls, there was no overall
trend between 1963 and 1974 - 1975, but significant declines in
levels had occurred by 1977. The residues in the livers of herons,
kestrels, and barn owls were significantly higher in areas of
eastern England (the main wheat bulb fly infestation areas) than in
other regions of the United Kingdom. These differences are
probably indicative of the use of aldrin- or dieldrin-dressed grain
in eastern England. Few samples of sparrow-hawk's livers were
available from eastern England, but the residues showed a similar
regional difference.
(e) Bald eagle (Haliacetus leucocephalus)
A survey of the residues of dieldrin in the carcass, liver, and
brain of bald eagles was initiated in 1960 by the Patuxent Wildlife
Research Centre, USA. The median concentration (1964 - 1965) was
0.1 mg dieldrin/kg brain and about 0.3 mg dieldrin/kg liver
(Reichel et al., 1969). During the period 1966 - 1977, mean
concentrations ranged from 0.1 to 2 mg dieldrin/kg brain (Mulhern
et al., 1970; Belisle et al., 1972; Cromartie et al., 1975; Prouty
et al., 1977; Kaiser et al., 1980).
(f) Other birds of prey and fish-eating birds
The surveys of the residues of dieldrin in other raptors have
been less extensive than those for the five species discussed
above. The geometric mean concentrations in the livers of 12 other
species (283 birds) in the United Kingdom (Cooke et al., 1982) in
the period 1963 - 1977 were between 0.02 and 2.35 mg/kg. Those for
the golden eagle (14 birds) in the USA during the period 1964 -
1965 were between trace levels and 0.4 mg/kg (Reichel et al.,
1969).
5.2. General Population Exposure
5.2.1. Adults
5.2.1.1 Aldrin
In the great majority of investigations into the presence of
organochlorine compounds in human blood and other tissues, the
level of aldrin was below the limits of detection. However, there
are a few reports of aldrin being present in human blood, placenta,
adipose tissue, and other tissues (Radomski & Fiserova-Bergerova,
1965; Kanitz & Castello, 1966; Selby et al., 1969a,b; Herrera
Marteache et al., 1978; Fernicola & Azevedo, 1982; Mossing et al.,
1985). These findings are unusual. The report that aldrin was
present in eight samples of blood, when none was found in the
matched adipose tissue samples, also seems anomalous (Selby et al.,
1969b). Fernicola & Azevedo (1982) suggested that some other
compounds with the same retention time as aldrin had perhaps led to
false results. None of these investigators established the
identity of the component, reported as "aldrin".
5.2.1.2 Concentrations of dieldrin in adipose tissue
Following the introduction of gas-liquid chromatography, there
have been numerous investigations of the concentration of dieldrin
in the adipose tissue of members of the general population who have
had no know occupational exposure to aldrin or dieldrin. Surveys
have been made in more than 20 countries, but in some surveys the
number of samples of fat analysed was small. In the USA and the
United Kingdom, there have been several surveys during the period
1961 - 1977. The results are summarized in Table 19, using two
statistics to define the samples: arithmetic mean (or geometric
mean in some American surveys) and maximum value as an indication
of the upper limit of variability (upper confidence limit in a few
surveys). The distribution tends to be skewed to the right, i.e.,
there is a greater number of high values than would be expected if
the samples had a normal distribution (Hunter et al., 1963; Morgan
& Roan, 1970). The maximum values in some surveys are so large
that they may correspond to individuals with an occupational
exposure. The results for stillborns and young babies and children
are discussed in section 5.2.2.
Most of the mean values are in the range 0.1 - 0.3 mg dieldrin
kg body fat and are usually smaller than those of total DDT by at
least a factor of 10. Surveys in the USA, United Kingdom, and
Netherlands indicate that there has been a decline of about 50% in
the concentration of dieldrin in the body fat since the mid 1970s
(Abbott et al., 1981; Ministry of Welfare, Health and Culture, The
Netherlands, 1983).
Table 19. Concentrations of dieldrin in the body fat of the general population
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
North America
Canada 1966 47 (N) I 0.22 0.53 Brown (1967)
1967-68 51 (N) II 0.12 0.83 Kadis et al. (1970)
1969 221 (N) II 0.12 0.46 Ritcey et al. (1973)
1969 5 (-) - 0.08 - Mastromatteo (1971)
1970 3 (-) - 0.22 - Mastromatteo (1971)
1972 168 (N) II 0.069 0.35 Mes et al. (1977)
1969-74 448 (N) - 0.12 0.88 Holdrinet et al.
(1977)
1976 99 (N) 0.049 0.211 Mes et al. (1982)
1979-81 175 (N) II 0.04 0.13 Williams et al.
(1984)
1980 29 (N) 0.046 Mes et al. (1985)
USA 1961-62 28 (B) II 0.15 0.36 Dale & Quinby (1963)
1962-66 221 (N) II 0.14 1.39 Hoffman et al. (1967)
1964 25 (N) II 0.29 1.15 Hayes et al. (1965)
1964 64 (N) - 0.31 2.82 Zavon et al. (1965)
1964-67 42 (N) none 0.21 0.70 Radomski et al.
(1968)
1965-67 146 (N) none 0.22 0.77 Edmundson et al.
(1968)
1966-68 70 (N) II 0.14 - Morgan & Roan (1970)
1967 30 (N) II 0.03f - Casarett et al.
(1968)
1968 48 (N) II 0.20 - Warnick (1972)
1969 15 (N) II 0.15 - Warnick (1972)
1969 26 (B) II 0.33f 0.80c Burns (1974)
1970 40 (N) II 0.15 - Warnick (1972)
1970 68 (B) II 0.29f 0.73c Burns (1974)
1970 202 (B) II 0.2 1.0 Wyllie et al. (1972)
1970 1412 (N/B) II 0.18f 15.20 Kutz et al. (1979)
1971 88 (B) II 0.36f 0.78c Burns (1974)
1971 1615 (N/B) II 0.22f 2.91 Kutz et al. (1979)
1972 39 (B) II 0.43f 1.00c Burns (1974)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
USA (contd.)
1972 1913 (N/B) II 0.18f 2.91 Kutz et al. (1979)
1973 1094 (N/B) II 0.18f 5.64 Kutz et al. (1979)
1974 898 (N/B) II 0.15f 2.21 Kutz et al. (1979)
Louisiana 1980 8 (B) II 0.15f 0.34 Holt et al. (1986)
1984 10 (B) II 0.10f 0.19 Holt et al. (1986)
Central and South America
Mexico 1975 19 (N) II 0.06f 0.24 Albert et al. (1980)
1975 9 (B) II 0.18f 0.49 Albert et al. (1980)
1975 9 (N) II 0.05f 0.12 Albert et al. (1980)
Argentina - 47 (N) IV 0.38 0.66c Wassermann et al.
(1969)
Brazil 1969-70 17 (N/B) III 0.02e 0.12 Wassermann et al.
(1972a)
1969-70 69 (N/B) III 0.12e 1.62 Wassermann et al.
(1972a)
Europe
Belgium 1968-69 37 (N) II 0.13 0.50 Wit (1971)
1975 60 (N) II 0.26 1.16 Dejonckheere et al.
(1977)
1977 58 (N) II 0.12 0.69 Van Haver et al.
(1978)
Denmark 1965 18 (N) - 0.20 0.34 Weihe (1966)
1972-73 70 (N) II 0.16f 0.53 Kraul & Karlog (1976)
France 1971 100 (N) II 0.45 1.45 Fournier et al.
(1972)
Germany, 1967 15 (B) I 0.18f 0.36 Wuenscher & Acker
Federal (1969)
Republic of 1973 50 (N) - 0.14 0.23 Acker & Schulte
(1974)
Greece - 50 (N/B) II 0.23 0.87 Panetsos et al.
(1975)
Italy 1965 9 (N) II 0.59 2.77 Kanitz & Castello
(1966)
1966 22 (N/B) II 0.68f 1.55 Del Vecchio & Leoni
(1967)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
Italy 1965-68 33 (B) - 0.32 3.15 Paccagnella et al.
(contd.) (1971)
1965-68 11 (B) - 1.95 5.70 Paccagnella et al.
(1971)
1965-68 52 (N) - 0.91 3.55 Paccagnella et al.
(1971)
Netherlands 1964 34 (N) II 0.31f - Wit (1971)
1966 11 (N) II 0.20 0.50 De Vlieger et al.
(1968)
1968-69 34 (N) II 0.27f 1.5 Wit (1971)
1973-74 102 (N) - 0.2 - Greve & Wegman (1985)
1975 25 (N) - 0.11 - Greve & Wegman (1985)
1976 74 (N) - 0.09 - Greve & Wegman (1985)
1977-78 78 (N) - 0.11 - Greve & Wegman (1985)
1979 25 (B) - 0.09 - Greve & Wegman (1985)
1980 24 (N) - 0.10 - Greve & Wegman (1985)
1981 53 (N) - 0.07 - Greve & Wegman (1985)
1982 54 (N) - 0.07 - Greve & Wegman (1985)
1983 78 (N) - 0.06 - Greve & Wegman (1985)
Spain - 40 (B) III 0.15 0.49 Herrera Marteache et
al. (1978)
Switzerland 1972 13 (B) II 0.29 0.57 Zimmerli & Marek
(1973)
United 1961 131 (N) II 0.21 1.29 Hunter et al. (1963)
Kingdom 1963-64 66 (N) II 0.26 0.9 Egan et al. (1965)
1964 50 (N) II 0.27 0.85 Robinson et al.
(1965)
1964 50 (B) II 0.25 0.65 Robinson et al.
(1965)
1965 101 (N) II 0.34 1.80 Cassidy et al. (1967)
1966 53 (B) II 0.21 0.60 Hunter et al. (1967)
1965-67 248 (N) II 0.21 1.0 Abbott et al. (1968)
1967 18 (B) II 0.27 0.68 Hunter et al. (1967)
1969-71 201 (N) II 0.16 0.68 Abbott et al. (1972)
1976-77 236 (N) II 0.11 0.49 Abbott et al. (1981)
1982-83 187 (N) - 0.074 0.27 UK-HMSO (1986)
Africa
Kenya 1969-70 32 (N) III 0.030d 0.18 Wassermann et al.
(1972b)
1969-70 51 (N) III 0.064e 0.28 Wassermann et al.
(1972b)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
Africa (contd.)
Nigeria 1969 46 (N) III 0.059d 0.73 Wassermann et al.
(1972c)
1969 90 (N) III 0.13e 0.98 Wassermann et al.
(1972c)
South Africa 1969 114 (N/B) IV 0.039 - Wassermann et al.
(1970)
Uganda 1969-70 16 (N) III 0.023d 0.058 Wassermann et al.
(1974a)
1969-70 39 (N) III 0.031e 0.59 Wassermann et al.
(1974a)
Asia
India 1964 35 (N) II 0.04 0.36 Dale et al. (1965)
Iran 1974-76 170 II 0.049 0.75 Hashemy-Tonkabony &
Soleimani-Amiri
(1978)
Israel 1967-69 61 (N) III 0.10d 0.315 Wassermann et al.
(1974b)
1967-69 162 (N) III 0.14e 3.96 Wassermann et al.
(1974b)
Japan Prior to 1973 241 (N) II 0.13 0.98 Curley et al. (1973)
1974-75 59 (N) II 0.09f 0.51 Yoshimura et al.
(1979)
Thailand 1969-70 8 (N) III 0.077d 0.459 Wassermann et al.
(1972d)
1969-70 27 (N) III 0.10e 1.20 Wassermann et al.
(1972d)
1975-76 9 II 0.322 - Department of
Agriculture Thailand
(1976)g
Oceania
Australia 1965 53 (N) II 0.046 0.43 Bick (1967)
1965-66 12 (N) IV 0.67 0.99 Wassermann et al.
(1968)
1969-70 75 (N) II 0.21 2.60 Brady & Siyali (1972)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
Oceania (contd.)
New Zealand Prior to 1967 45 (N) II 0.28 0.77 Brewerton & McGrath
(1967)
1965 43 (B) II 0.41 - Copplestone et al.
(1973)
1966 54 (B) II 0.30 - Copplestone et al.
(1973)
1967 68 (B) II 0.43 - Copplestone et al.
(1973)
1968 64 (B) II 0.33 - Copplestone et al.
(1973)
1969 25 (B) II 0.27 - Copplestone et al.
(1973)
Papua New 1969-70 38 (N) II 0.17 0.72 Brady & Siyali (1972)
Guinea
------------------------------------------------------------------------------------------
a Samples taken at necropsy (N) or during elective surgery (B).
b Method of clean-up:
I Removal of neutral lipids at -70 °C.
II Separation into two or more fractions by eluting from a Florisil column (with prior
liquid/liquid partition to reduce neutral lipid content, in most investigations using
this clean-up procedure).
III Florisil column clean-up without separation into two or more fractions.
IV Kontes co-distillation.
- Method not reported.
c Upper confidence limit ( P = 0.025) for the set of samples.
d Age group 5-24 years.
e Age group 25 years and older.
f Results expressed in terms of extractable lipid content.
g Personal communication to IPCS in 1987.
5.2.1.3 Concentrations of dieldrin in blood
The concentrations of dieldrin in whole blood or serum of
members of the general population have been determined in a few
countries and are summarized in Table 20. The concentrations are
very low (µg/litre) and it is essential that the sensitivity of the
analytical method is at least 0.1 µg/litre. Two analytical
procedures have been used (Dale et al., 1966; Richardson et al.,
1967a), which give significant different results: the acetone
extraction procedure (method II in Table 20) gives results that are
about 50% higher than the hexane extraction procedure (method I in
Table 20) and showed a better reproducibility (Robinson et al.,
1967a). An interlaboratory comparison of the hexane extraction
method showed that large variations in results may occur (Thompson,
1976).
Table 20. Concentration of dieldrin in the blood of the general population
------------------------------------------------------------------------------------------
Country Year Number of Analytical Dieldrin Reference
samplesa methodb Mean Maximum
(µg/litre)
------------------------------------------------------------------------------------------
USA 1965 10 (B) I 1.4 2.8 Dale et al. (1966)
1967-68 1000 (S) I 0.5 25 Watson et al. (1970)
1967-71 970 (S) I 0.9 - Warnick (1972)
1967-68 37 (H) III 4 - Morgan & Roan (1970)
1970 202 (S) I 0.9 10 Wyllie et al. (1972)
Prior to 1981 59 (S) I 0.6 10.1 Barquet et al.
(1981)
1976-80 6078 (S) ? ~1.4c 16 Murphy & Harvey
(1985)
Hawaii 1968-70 1107 (S) I 1.46 11 Klemmer et al.
(1973)
Lanai Island 1968-70 484 (S) I 1.3 26 Klemmer et al.
(1973)
Europe
Netherlands 1978 70 (B) - < 0.5 - Greve & Wegman
(1985)
1980 48 (B) - < 0.4 - Greve & Wegman
(1985)
1981 127 (B) - < 0.4 - Greve & Wegman
(1985)
1982 54 (B) - < 0.5 - Greve & Wegman
(1985)
Switzerland 1972 ~100 (S) I 1.1 - Zimmerli & Marek
(1973)
United Kingdom 1962 20 (B) II 1.6 10.0 Hunter et al. (1967)
1964 61 (B) II 1.4 5.0 Hunter et al. (1967)
1965 25 (B) II 1.7 8.7 Hunter et al. (1967)
1966 55 (B) II 1.8 4.3 Hunter et al. (1967)
1968 18 (B) II 0.9 1.1 Robinson & Roberts
(1969)
Oceania
Australia - 52 (B) Iust 2.3 13 Siyali (1972)
- 47 (B) Iust none - Siyali (1973)
------------------------------------------------------------------------------------------
a Samples of whole blood (B), serum (S), whole blood from heart chamber (during autopsy)
(H).
b Analytical methods (all use gas-liquid chromatography with an electron-capture detector):
I Hexane extraction.
Iust Hexane extraction combined with ultrasonic treatment.
II Acetone extract on silica gel column.
III Solvent extraction and Florisil column clean-up.
c In 260 positive samples.
5.2.1.4 Concentration of dieldrin in other tissues
A few investigations of the concentrations of dieldrin in other
body tissues have been made and some of the results are summarized
in Table 21.
Table 21. Concentration of dieldrin in various tissues from members of the general
population
------------------------------------------------------------------------------------------
Tissue Country Year No. of Dieldrin Reference
samples Mean Maximum
(mg/kg)
------------------------------------------------------------------------------------------
Liver Canada 1967-68 50 0.25a 3.0a Kadis et al. (1970)
USA 1967 42 0.009 - Casarett et al. (1968)
USA 1966 42 0.035 0.22 Fiserova-Bergerova et al. (1967)
USA 1966-68 35 0.047 - Morgan & Roan (1970)
Denmark 1972-73 18 0.29a - Kraul & Karlog (1976)
Netherlands 1966 11 0.034 0.081 De Vlieger et al. (1968)
Japan 1974-75 30 0.39a 1.73a Yoshimura et al. (1979)
Thailand 1975-76 16 0.010 - Dept. of Agriculture, Thailand
(1976)b
Kidneys Canada 1967-68 47 0.10a 1.35a Kadis et al. (1970)
USA 1967 38 0.021 - Casarett et al. (1968)
USA 1966 42 0.013 0.04 Fiserova-Bergerova et al. (1967)
USA 1966-68 35 0.014 - Morgan & Roan (1970)
USA 1973 12 0.006 0.009 Anon (1974c)
Thailand 1975-76 16 0.010 - Dept. of Agriculture, Thailand
(1976)b
Brain Canada 1967-68 30 0.002a - Kadis et al. (1970)
USA 1967 32 0.003 - Casarett et al. (1968)
USA 1966 42 0.035 0.10 Fiserova-Bergerova et al. (1967)
USA 1966-68 35 0.007 - Morgan & Roan (1970)
Denmark 1972-73 21 0.057a - Kraul & Karlog (1976)
Netherlands 1966 28 0.0075 0.021 De Vlieger et al. (1968)
------------------------------------------------------------------------------------------
Table 21. (contd.)
------------------------------------------------------------------------------------------
Tissue Country Year No. of Dieldrin Reference
samples Mean Maximum
(mg/kg)
------------------------------------------------------------------------------------------
Brain Thailand 1975-76 16 0.010 - Dept. of Agriculture, Thailand
(contd.) (1976)b
Gonads Canada 1967-68 39 0.06a 0.86a Kadis et al. (1970)
USA 1967 36 0.008 - Casarett et al. (1968)
USA 1966 42 0.035 0.20 Fiserova-Bergerova et al. (1967)
------------------------------------------------------------------------------------------
a Results expressed in terms of extractable lipid content.
b Personal communication to IPCS in 1987.
5.2.2. Babies, infants, and mother's milk
Dieldrin penetrates the placenta and, as a result of
transplacental exposure, may occur in the blood, adipose tissue,
and other tissues of the fetus and newborn baby (Table 22). The
concentrations are lower by a factor of 2 - 10 than those of their
mothers or other adults (Table 19). There is no difference between
infants and adults in the brain/liver/fat ratio of dieldrin
concentrations (Fiserova-Bergerova et al., 1967; Casarett et al.,
1968). A similar situation exists in animals, e.g., pigs (Uzoukwu
& Sleight, 1972).
Dieldrin is also excreted in the milk of human beings and
various animal species. Table 23 summarizes the concentrations of
dieldrin found in human milk over the last 15 years in various
countries, mean concentrations up to 6 µg/litre having been
reported. Higher values, occurring occasionally in a few regions,
have been associated with house and garden use of aldrin/dieldrin.
Thus, in the first several months, a breast-fed infant drinking
approximately 150 ml milk/kg body weight per day has a daily intake
of 0.15 - 0.9 µg dieldrin/kg body weight.
Acker et al. (1984) studied the problem of residues in human
milk and the importance of breast-feeding for the newborn baby.
They concluded that, at least in the early months, the value of
breast-feeding outweighed the possible risks from residues of
dieldrin, in this case, in human milk. They calculated that the
average daily intake of dieldrin by newborn babies was
approximately 0.7, 0.75, 0.65, and 0.65 µg/day, respectively, for
the 1st, 2nd, 3rd, and 4th months of breast-feeding.
Aldrin has rarely been detected in human milk. It was not
detectable in 202 samples of Dutch human milk (Wegman & Greve,
1974; Greve & Wegman, 1985), and in only one (21.8 µg/litre) of 50
Norwegian samples (Bakken & Seip, 1976).
Table 22. Concentration of dieldrin in blood and fat of fetus, newborns, infants, and adults
---------------------------------------------------------------------------------------------------------
Country Year Age Number Dieldrin in Number Dieldrin in Reference
of blood of fat
samples Mean Maximum samples Mean Maximum
(µg/litre) (mg/kg fat)
---------------------------------------------------------------------------------------------------------
North America
Canada 1982 mothers during 16 0.1 - Mes et al.
lactation (1984)
USA 1966 fetus, stillborn 6 0.17 0.38 Fiserova-
0-5 years 12 0.14 0.34 Bergerova et
6-10 years 6 0.07 0.26 al. (1967)
31-83 years 12 0.34 0.7
USA 1968 newborn 26 0.7 1.5 3a 0.24 0.35 Curley et al.
stillborn 4 NDb 7 ND (1969)
South America
Argentina 1969 mothers 13 1.63 Radomski et al.
-70 newborn 13 0.59 (1971)
1-5 years 19 0.54
5-10 years 18 0.94
adults 20 1.43
1970 newborn 3 0.12 0.13 Astolfi et al.
0-4 months 6 0.02 0.07 (1973)
4-12 months 4 0.05 0.07
1-4 years 14 0.06 0.13
over 4 years 20 0.07 0.25
Brazil 1969 stillborn 28 0.011 0.174 Wassermann et
-70 5-24 years 17 0.023 0.122 al. (1972a)
Europe
Netherlands 1979 newborn 87 0.3 4.6 Eckenhausen et
2 weeks 22 0.5 - al. (1981)
2 months 17 0.4 -
3 months 8 0.5 -
---------------------------------------------------------------------------------------------------------
Table 22. (contd.)
---------------------------------------------------------------------------------------------------------
Country Year Age Number Dieldrin in Number Dieldrin in Reference
of blood of fat
samples Mean Maximum samples Mean Maximum
(µg/litre) (mg/kg fat)
---------------------------------------------------------------------------------------------------------
Netherlands mothers, pre-natal 48 0.8 3.5
(contd.) mothers, post-natal 73 0.4 4.1
Spain 1982 mothers 10 6 23 Gonzalez-
(Cordoba) babies 10 8 50 Rodriquez
Cordoba et al.
(1983)
United 1969 1 newborn, stillborn 3 0.01 0.02 Abbott et al.
Kingdom -71 1 day-3 months 8 0.03 0.07 (1972)
3 months-4 years 9 0.05 0.10
over 4 years 201 0.16 0.68
1976 newborn 1 0.03 - Abbott et al.
-77 2 months 1 0.02 - (1981)
3 months 1 0.09 -
over 4 years 236 0.11 0.49
Africa
Nigeria 1969 stillborn 31 0.002 0.014 Wassermann et
0-11 months 47 0.019 0.087 al. (1972c)
1-4 years 54 0.023 0.083
adults 90 0.13 0.98
Asia
Israel 1968 fetus 23 1.3 - - - - Polishuk et al.
-69 pregnant woman 24 1.6 - 16 0.084 - (1970)
non-pregnant woman - - - 33 0.172 -
Israel 1967 stillborn 44 0.019 0.118 Wassermann et
-69 0-11 months 40 0.021 0.125 al. (1974b)
5-24 years 61 0.101 0.315
adults 162 0.136 3.96
---------------------------------------------------------------------------------------------------------
a Stillborn.
b ND = not determined.
Table 23. Concentration of dieldrin in mother's whole milk
-------------------------------------------------------------------
Country Year No. of Dieldrin Reference
samples Mean Maximum
(µg/litre)
-------------------------------------------------------------------
North America
Canada 1969-70 48 0.09g 0.25g Holdrinet et al.
(Ontario) 1971-72 34 0.04g 0.17g (1977)
1973-74 24 0.04g 0.08g
1978-79 154 1a 26b Dillon et al.
(1981)
1982 ~128c ~1.3 1.8 Mes et al. (1984)
USA 1972-73 57 < 10 50 Kutz et al. (1979)
1973-74 57 4 50 Strassman & Kutz
(1977)
1973-75 40 6 42b Barnett et al.
(1979)
1972-75 1436 ~5 15 Savage et al.
(1981)
Hawaii 1979-80 54 0.04g 0.09g Takei et al.
(1983)
Central America
El Salvador 1973-74 40 5 15 De Campos &
Olszyna-Marzys
(1979)
Guatemala 1971 46 2 10 De Campos &
Olszyna-Marzys
(1979)
Europe
Belgium 1968 20 3.4 8 Heyndrickx & Maes
(1969)
Denmark 1982 57 0.04g 0.47g Anderson & Orbaek
(1984)
Germany, 1981 91 0.05g 0.44g Rohwer (1983b)
Federal
Republic of 1982 132 0.01g 0.3g Cetinkaya et al.
(1984)
Netherlands 1969 48 3 11 Tuinstra (1971)
1972 202 5 - Wegman & Greve
(1974)
1983 278 0.03g 0.22g Greve & Wegman
(1985)
-------------------------------------------------------------------
Table 23. (contd.)
-------------------------------------------------------------------
Country Year No. of Dieldrin Reference
samples Mean Maximum
(µg/litre)
-------------------------------------------------------------------
Europe (contd.)
Netherlands 1979 69 2.3 - Eckenhausen et al.
(1981)
Norway 1975 50 2.75 3.6 Bakken & Seip
(1976)
Portugal 1972 164 11 21 Graca et al.
(1974)
Spain 1981 20 3 14 Baluja et al.
(1982)
Sweden 1978 51d 22g 54g Noren (1983a,b)
(Stockholm) 1979 54d 20g 31g
1980 36d 18g 23g
Switzerland 1983 6 0.5 1 Disler et al.
(1984)
United 1963-64 19 6 13 Egan et al. (1965)
Kingdom 1979-80 102 2 12 Collins et al.
(1982)
1983-84 40 5 32 UK-HMSO (1986)
Africa
Kenya 1983-85 292 range: 2.3-98 Kanja et al.
(1986)
Asia
Israel 1975 29 7 - Polishuk et al.
(1977)
Japan 1973-77 116 2.3 - Yakushiji et al.
(1979)
Oceania
Australia 1970-71 23 5 11 Stacey & Thomas
(1975)
1971-72 40 25 68 Miller & Fox
(1973)
-------------------------------------------------------------------
Table 23. (contd.)
-------------------------------------------------------------------
Country Year No. of Dieldrin Reference
samples Mean Maximum
(µg/litre)
-------------------------------------------------------------------
Oceania (contd.)
Australia 1973 45 5 13 Siyali (1973)
1979-80 267c ~8.5 31 Stacey et al.
(1985)
1981 74e 13 35 Stacey & Tatum
(1985)
New Guinea 1972 74 0.7 13.2 Hornabrook et al.
(1972)
-------------------------------------------------------------------
a The authors stated that they found aldrin. However, they
probably meant dieldrin, since, in mother's milk, the presence of
aldrin without dieldrin is highly unlikely, whereas the reverse
is the rule.
b In an area of high pesticide use.
c 128 samples from 16 women.
d Number of samples included 745, 805, and 973, respectively.
e 74 samples from 14 women.
f Many of the houses had been treated against termites, but the
pesticides used were unknown.
g On lipid basis in mg/kg.
During the first trimester, and usually during the first year,
of a baby's life, the concentration of dieldrin in the blood and
adipose tissue does not increase and, in most cases, decreases
(Astolfi et al., 1974) (Table 22).
The concentration of dieldrin in the blood of breast-fed babies
is not higher that that in bottle-fed babies (Eckenhausen et al.,
1981), and it is lower than it is in adults.
A study on organochlorine insecticides in the blood of mothers
and newborn babies was carried out in an agricultural rural area in
the Mississippi Delta (USA). In total, 209 black and 130 white
mother-newborn pairs participated. Dieldrin was detected in the
blood of 43.5% of black and 51.5% of white mothers and in the blood
of 19.1% of black babies and 10% of white babies. The blood
concentrations of both mothers and babies were less than 1
µg/litre. Maternal age and birth weight of the baby did not
correlate significantly with the prevalence, or with the mean
level, of maternal and infant insecticide residues in the blood
(d'Ercole et al., 1976).
Data on the occurrence of aldrin and dieldrin in human milk
have been submitted by Australia, Guatemala, Japan, and
Switzerland, Japan reporting a decline of the concentrations in
human milk during the period 1971 - 1979. Data on dieldrin in
human milk have been reported by Canada, the Federal Republic of
Germany, Mexico, the Netherlands, Sweden, Switzerland, and the USA,
none of the median levels exceeding 3 µg/kg milk. Levels in the
USA were below 10 µg/kg milk (limit of detection) (National Food
Administration, Uppsala, 1982).
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Aldrin
6.1.1.1 Ingestion
Aldrin is readily absorbed from the gastrointestinal tract and
through the skin; it is stored as dieldrin, mainly in adipose
tissue (section 6.2.1). Aldrin is readily metabolized to dieldrin
in plants and animals and is rarely found as such in food or in the
great majority of animals.
6.1.1.2 Inhalation
Inhalation studies by Beyermann & Eckrich (1973) on human
volunteers suggested that about 50% of inhaled aldrin vapour was
absorbed and retained in the human body. However, a study on 10
male volunteers exposed to actual aldrin vapour concentrations of
1.31 µg/m3 and some weeks later to 15.5 µg/m3 air for a period of
60 min suggested an actual retention in man of 20%.
Physical exertion did not have any significant effect on the
retention. Dieldrin could not be detected in the exhaled air. The
concentration of dieldrin in the blood of the volunteers was lower
than 1 µg/litre before and after exposure (Bragt et al., 1984).
6.1.2. Dieldrin
Studies on rabbits, dogs, monkeys, and human beings have shown
that dieldrin is absorbed through the intact skin (Shah & Guthrie,
1976; Sundaram et al., 1978; Fisher et al., 1985). There have been
many studies demonstrating the absorption of dieldrin through the
gastrointestinal tract (section 6.2).
6.1.3. Photodieldrin (and other metabolites of dieldrin)
Studies demonstrating the absorption of photodieldrin through
the gastrointestinal tract are summarized in section 6.2.3.
6.2. Distribution
6.2.1. Aldrin
6.2.1.1 Mouse
In studies by Deichmann et al. (1975), Swiss-Webster mice were
fed diets containing 0, 5, or 10 mg aldrin/kg, over seven
generations. The retention of dieldrin following the feeding of
aldrin over four generations significantly increased the
concentration of dieldrin in abdominal fat and in the lipids of the
total carcass. There was also a significantly increased retention
of dieldrin in the carcass in the F1 generation, with some further
(but not statistically significant) increase in concentration and
total retention of dieldrin in the F2 and F3 generation. The
dieldrin concentration in the total lipids of mouse carcasses were:
for the F0 generation, 60 mg/kg; for the males in the F1, F2, and
F3 generations, a mean of 100 mg/kg; and for the females in the F1,
F2, and F3 generations, a mean of 132 mg/kg. The dieldrin
concentration was below 1 mg/kg in pups from the F4 generation,
born of parents that carried a considerable load of aldrin or
dieldrin (thus exposed in utero and via lactation) and fed the
control diet from weaning to the age of 260 days. The
concentrations of dieldrin in the F5 and F6 generations were
similar to those in the 2nd - 4th generations.
6.2.1.2 Rat
When single oral doses of 10 mg aldrin/kg body weight were
given to neonate Sprague Dawley rats, aldrin was detectable up to 6
days after dosing in the stomach and small intestine, but only for
72 h in the kidneys. In the liver, the aldrin concentration
increased during the first 6 h, and then declined during the
following days. Dieldrin was detected as early as 2 h after dosing
and had reached a maximum after 24 h. It then declined. The only
metabolic conversion product detected in the liver was dieldrin.
The concentration of aldrin was very low relative to that of
dieldrin, except in the case of studies in which tissues were
analysed within a few hours of dosing with aldrin (Farb et al.,
1973).
In studies by Ludwig et al. (1964), two male Wistar rats were
given daily oral doses of 4.3 µg 14C-aldrin by stomach tube for 3
months and were killed 24 h after the final dose. The total
radioactivity in the body as a proportion of the total cumulative
dose was 3.6%, but, after 82 days, the value had fallen to 0.21%.
The ratio of dieldrin to aldrin in the carcass was approximately
15:1; in abdominal fat, it was about 18:1.
6.2.1.3 Dog
Deichmann et al. (1969, 1971), gave beagle dogs oral doses of
aldrin in capsules. Three males were given 0.3 mg aldrin/kg body
weight and 4 females were given 0.15 or 0.3 mg aldrin/kg body
weight, 5 days per week, for 14 months. During the last 10 months
of the dosing period, the concentration of dieldrin in the blood of
dogs given 0.3 mg aldrin/kg body weight was in the range 42 - 183
µg/litre, while the concentration in the subcutaneous fat was
37 - 208 mg/kg. The levels in the animals receiving 0.15 mg
aldrin/kg body weight were 40 - 130 µg/litre and 12 - 67 mg/kg in
blood and subcutaneous fat, respectively. The apparent partition
ratio, subcutaneous fat/blood, was about 1000.
6.2.1.4 Human studies
Little is known about the distribution of aldrin in the human
body after transfer from the gastrointestinal tract or skin into
the circulating blood. As a result of its relatively rapid
conversion to dieldrin, aldrin is rarely detected in human tissues.
6.2.2. Dieldrin
6.2.2.1 Laboratory animals
(a) Mouse
Following a preliminary comparison of the distribution of
dieldrin and three known animal metabolites in CFE rats and CFI
mice (Baldwin et al., 1972), a more detailed comparison was made of
male CFE rats and two strains of male mice (CFI and LACG) (Hutson,
1976). The latter study also included a comparison of the effects
of a pretreatment with diets containing dieldrin at 20 mg/kg diet
(rats) or 10 mg/kg diet (mice) for 4 weeks. 14C-Dieldrin was
administered orally as a single dose of about 3 mg/kg body weight
to both the pretreated and non-pretreated groups, and the animals
were killed 8 days after dosing. The concentrations of the
6,7-dihydroxy metabolite were below the limits of detection (less
than 0.02 mg/kg) in the fat, liver, and kidneys of all the animals.
The concentrations of the 9-hydroxy metabolite were very small or
below the limits of detection (less than 0.03 mg/kg) in the fat and
kidneys; small concentrations (about 0.4 mg/kg) were found in the
livers of the two strains of mice. The bridged pentachloroketone
(PCK) was present in the liver of CFE rats in small amounts (about
0.04 mg/kg), but quite large concentrations were found in the
kidneys: 2.48 (no pretreatment) and 6.11 mg/kg (4-week
pretreatment). The concentrations in the fat in both groups were
small (mean, 0.17 mg/kg). In the two strains of mice, the
concentrations of PCK in the liver were very small (about 0.5
mg/kg) except in the pretreated animals. Concentrations in the
kidneys of the two strains of mice were below the limits of
detection (less than 0.02 mg/kg) in the absence of pretreatment or
small (about 0.15 mg/kg) in pretreated mice. In the fat of the
mice (no pretreatment), the PCK concentrations were below the
limits of detection (less than 0.04 mg/kg), but, in the pretreated
mice, the concentrations were about 1.3 mg/kg. The concentrations
of dieldrin in the fat were much higher than in the other tissues,
and those in the mice were about twice those in the rat.
(b) Rat
Heath & Vandekar (1964) studied the transport of 36Cl-dieldrin
from the gastrointestinal tract by cannulation of the thoracic
lymph duct in rats. They found that only one-seventh of the
absorbed dieldrin was recovered from the lymph and most of the
dieldrin was absorbed via the portal vein.
Iatropoulos et al. (1975) indicated that the transport of
dieldrin from the gastrointestinal tract to the liver of Sprague-
Dawley rats is mainly through the portal venous system. However,
during the subsequent redistribution of dieldrin, the lymphatic
system seemed to be a major route.
When female Osborne-Mendel rats were fed a diet containing 50
mg technical dieldrin (87%)/kg for 6 months, the concentrations of
dieldrin in the blood, liver, and fat increased rapidly during the
first 2 weeks. During the next 26 weeks, the concentrations
fluctuated but did not appear to increase significantly. The mean
concentrations for the final 4 months were (groups of four to six
animals): in blood, 240 µg/litre; in liver, 6.8 mg/kg; and in fat,
159.5 mg/kg tissue. The distribution ratios (blood = 1) for this
period were: liver, 28 and fat, 666 (Deichmann et al., 1968).
In the studies by Walker et al. (1969b), groups of 25 male and
25 female Carworth Farm E rats were fed diets containing 0.1, 1, or
10 mg dieldrin (99%)/kg diet. The control group consisted of 45
animals of each sex. Small groups of rats were killed after 26,
52, and 78 weeks and the remaining animals after 104 weeks. The
concentration of dieldrin in blood, brain, liver, and fat was
estimated. An approximate plateau level was reached during the
first 26 weeks. The tissue uptake ratios (concentration of
dieldrin in tissues/concentration in diet) for female rats in the
three test groups were: in blood, 0.056; in brain, 0.19; in liver,
0.35; and in fat, 8.8. The uptake ratios for male rats were
significantly lower than those for females. The partition ratios
(concentration in tissues relative to that in blood) for
males/females, respectively, were: in brain, 3.3/2.6; in liver,
7.8/5.9; and in fat, 104/137. It was considered that the results
were consistent with the use of a compartmental model.
Osborne-Mendel rats (6 male and 6 female) were orally
administered approximately 50 µg 14C-dieldrin/kg body weight,
dissolved in corn oil, 5 days/week, for 9 weeks. The animals were
killed 24 h after the last dose, and the radioactivity in nine
tissues was measured. More radioactivity was retained in the
tissues by females than by males, except in the case of kidneys
(where the female:male ratio was about 0.3:1). Adipose tissue was
the main storage site for dieldrin. The lowest levels were present
in spleen, brain, and heart, while higher levels were found in
liver, lung, adrenals, and especially in the kidneys (Dailey et
al., 1970).
In a study on Charles River rats, administered 14C-dieldrin in
the diet for 8 h, Matthews et al. (1971) found a high level of
radioactivity in the kidneys. The same was found in the kidneys of
male rats in the study by Iatropoulos et al. (1975).
In studies by Baron & Walton (1971), male Osborne-Mendel rats
were fed diets containing 25 mg dieldrin/kg diet for 8 weeks. On
the first 4 days of the 9th week, oral doses of 14C-dieldrin were
administered, together with sufficient non-radioactive dieldrin to
maintain a 24-h intake equivalent to 25 mg/kg diet. Groups of five
rats were killed on days 1 - 4 of the 9th week. The remaining rats
were divided into two groups, one group being fed the diet
containing 25 mg dieldrin/kg and the other being given the control
diet. An equilibrium level of 50 mg dieldrin/kg adipose tissue was
reached by the 8th week. The concentration of dieldrin in the
adipose tissue of the animals given the control diet in the 9th
week declined rapidly during the subsequent 18 days. The rate of
decline corresponded to a half-life of about 4 - 5 days. It was
postulated that an active transport of dieldrin into and out of
fat, differing from the mechanism for lipids, may have occurred
(Baron & Walton, 1971).
Groups of two male and two female Sprague-Dawley rats were
administered dietary concentrations of 0.04 mg 14C-dieldrin/kg,
0.04 mg 14C-dieldrin/kg plus 0.16 mg dieldrin/kg, or 0.04 mg
14C-dieldrin/kg plus 1.96 mg dieldrin (99%)/kg, for 39 weeks, and
the animals were then killed. The daily intake of food was
restricted to 12 and 15 g for female and male animals,
respectively. In all three groups, the recovery of 14C activity in
whole carcasses, as a proportion of the total administered dose,
was significantly higher in female rats (mean 6.9%) than in male
rats (mean 2.1%) (Davison, 1973).
When single doses of 10 mg dieldrin/kg body weight (in corn
oil) were administered orally to male Sprague-Dawley rats, the
concentration of dieldrin in the plasma attained a maximum value
(500 µg/litre) after about 2 h. Up to 48 h after dosing, it
fluctuated between 200 and 500 µg/litre, but then declined quite
rapidly to about 10 µg/litre during the next 8 days. In the brain,
the highest concentration (about 1 mg/kg) was attained after about
4 h; it remained essentially steady for a further 44 h, and then
declined in a similar manner to that in the plasma. The
concentration/time relationships for muscle, kidneys, and liver
were similar to those for the brain. A slower approach to a
maximum value was observed in retroperitoneal fat, the 4 h and 24 h
concentrations being about 10 and 40 mg dieldrin/kg fat,
respectively. After 48 h, the concentration in fat declined in a
similar manner as did those in the plasma and brain (Hayes, 1974).
Moss & Hathway (1964) administered 14C-dieldrin
intraperitoneally to rats, and determined the partition of
radioactivity between plasma and erythrocytes. The ratio
(plasma:erythrocytes) 2 h after dosing was 2.1:1; 4 days after
dosing, it was 1.6:1, though the activities had declined by 49% and
32%, respectively, in plasma and erythrocytes.
(c) Rat and rabbit in vitro
The partition of 14C-dieldrin-related activity between the
soluble proteins of blood and the cellular components has been
studied in vitro. The radioactivity was located mainly in the
erythrocytes and plasma of rats and rabbits, whereas that in
leukocytes, platelets, and erythrocyte membranes was much lower.
The activity in the erythrocytes was associated with haemoglobin
and an unknown constituent. The radioactivity in the serum of rats
(electrophoresis at pH 8.6) was associated with pre- and post-
albumin, whereas that in rabbit serum was associated with albumin
and alpha-globulin. Electrophoresis at pH 4.5 gave a pattern which
was similar in rats and rabbits but the patterns at pH 4.5 were
different from those at pH 8.6; there were four incompletely
separated peaks of radioactivity (Moss & Hathway, 1964).
It has been demonstrated in vitro that the transport of
dieldrin between rat hepatocytes and the extracellular medium is a
much faster process than the metabolic transformation reaction in
hepatocytes (Ichinose & Kurihara, 1985).
(d) Dog
In studies by Richardson et al. (1967b), three beagle dogs were
fed a diet containing dieldrin (equivalent to 0.1 mg/kg body
weight) for 128 days, and two animals were used as controls. The
concentration of dieldrin in the blood increased in an
approximately curvilinear manner up to day 93. There were
fluctuations during the next 5 weeks, but any increase was small
relative to that during the first 5 weeks of the study (a mean
plateau concentration of about 130 µg/litre blood appears to be
consistent with the data). One week after the dieldrin diet was
discontinued, the dogs were killed and samples of blood, fat,
heart, liver, kidneys, pancreas, spleen, lung, and muscle were
taken for analysis. The mean concentrations of dieldrin in the
organs and tissues were 150 µg/litre in blood, 1090 µg/kg in the
heart, 4420 µg/kg in liver, 2330 µg/kg in kidneys, 14 030 µg/kg in
pancreas, 710 µg/kg in spleen, 1227 µg/kg in lungs, 25 333 µg/kg in
fat, and 566 µg/kg in muscle. The mean partition ratio fat/blood
was 161. There was a highly significant linear relationship
between the logarithm (log10) of the concentration of dieldrin in
the blood and the logarithm (log10) of the length of the dosing
period.
Six mongrel dogs (four males, two females) were orally dosed
daily with dieldrin dissolved in corn oil for 5 days (1 mg
dieldrin/kg body weight) and thereafter at doses of 0.2 mg/kg body
weight for a further 54 days. Six control animals were used.
Samples of blood were taken twice weekly from day 7 onwards and
analysed for dieldrin content. The concentration of dieldrin in
the blood of all the animals showed a small but significant
increase from day 7 to day 59. Biopsy samples of subcutaneous fat
were obtained on days 16 and 50. The fat/blood partition ratio on
day 16 was 216 and that on day 50 was 117 (Keane & Zavon, 1969b).
In studies by Walker et al. (1969b), groups of five male and
five female beagle dogs were given daily oral doses (by capsule in
olive oil) of dieldrin (99%) at 0, 0.005, or 0.05 mg/kg body
weight, for 2 years. The concentration of dieldrin in the blood
increased in all animals during the first 12 weeks of the study and
reached an approximately steady state value from week 18 to about
week 76. During the last 6 months, there were significant
deviations from the apparent asymptotic value for weeks 18 - 76.
The reasons for this are not understood, but there was also an
upward tendency in the concentration of dieldrin in the control
animals. There were statistically significant relationships
between the concentrations of dieldrin in the diet (calculated
from the daily oral dose) and those in the blood, brain, liver, and
adipose tissue. The tissue uptake ratios were similar in both
males and females, those for males being (concentration of dieldrin
in diet = 1): blood, 0.06; brain, 0.22; liver, 4.4; and adipose
tissue, 10.0. There were also statistically significant
relationships between the concentrations of dieldrin in the blood
and those in the other three tissues. The partition ratios
(concentration of dieldrin in blood = 1) for the male dogs were:
brain, 3.7; liver, 10; and adipose tissue, 169.
(e) Monkey
Two female rhesus monkeys were given an intravenous injection
of 14C-dieldrin (2.5 mg/kg body weight) in 1,2-propylene glycol and
two male rhesus monkeys received, respectively, a single oral dose
of 14C-dieldrin at 0.5 or 0.36 mg/kg body weight. The females were
killed 75 days after dosing and the males 10 days after dosing.
With both routes of administration, the highest radioactivity was
found in the adipose tissue, bone marrow, and liver. The activity
in the brain was relatively low (about 2% of that in the adipose
tissue). Metabolites were not found in the organs, but they were
present in the bile (Mueller et al., 1975b).
In studies by Mueller et al. (1979), groups of 1 - 5 male
rhesus monkeys were fed diets containing 0, 0.01, 0.1, 0.5, or 1 mg
dieldrin/kg diet for 70 - 74 months. Two other rhesus monkeys were
fed 5 mg dieldrin/kg diet for 4 months, 2.5 mg/kg for the next 5
months, and 1.75 mg/kg for a further 64 months. One rhesus monkey
was fed 5 mg/kg for 4 months, 2.5 mg/kg for the next 5 months, and
then 1.75 mg/kg diet, this dietary concentration gradually
increasing until after 23 months from the onset of the trial it had
reached 5 mg/kg (this feeding level being continued for a further
46 months). The mean concentrations of dieldrin in the livers of
these monkeys were: in the 0.01 mg/kg group, 1.2 mg/kg; in the 0.1
mg/kg group, 1.3 mg/kg; in the 0.5 mg/kg group, 4.1 mg/kg; in the 1
mg/kg group, 5.5 mg/kg; in the 5.0/2.5/1.75 mg/kg group, 13.6
mg/kg; and in the one animal fed 5, 2.5, 1.75, and 5 mg/kg diet,
23.3 mg/kg. The distribution of dieldrin in liver subcellular
fractions was determined by isotope dilution. The highest
proportion of dieldrin was present in the microsomal fraction, with
about 60% of the total in the subcellular fractions, and about
12.5% of the total in the soluble fraction. The remaining 3
fractions (nuclear, mitochondrial, and lysosomal) contained similar
proportions, about 9% in each fraction (Wright et al., 1978). The
modes of distribution of dieldrin (and metabolites) in rhesus
monkeys were similar to those in rats.
6.2.2.2 Transplacental transport
(a) Mice
Pregnant mice were each given 0.4 mg 14C-dieldrin
intramuscularly and its distribution was studied by means of whole-
body autoradiography. The highest values for 14C activity were
found in the fat, liver, intestines, and mammary glands, while
moderate activity was found in the ovaries and brain. Moderate
levels were also found in fetal liver, fat, and intestines,
indicating transfer across the placenta (Baeckstroem et al., 1965).
(b) Rat
Transplacental transfer of 14C-dieldrin was found in Sprague
Dawley rats that were administered the compound intravenously (tail
vein) on days 13, 16, or 21 of gestation. Relatively high levels
were present in the fetus 5 min after injection, and they continued
to increase for 40 - 60 min after which they declined by about 60%
in 2 - 3 days. The transfer of 14C activity was greater during
late gestation. Phenobarbital pretreatment decreased the amount of
radioactivity in the fetus (Eliason & Posner, 1971).
(c) Rabbit
The transport of 14C activity from mother to blastocyst and
from mother to fetus was demonstrated in pregnant New Zealand white
rabbits following intravenous injection of 14C-dieldrin into the
ear vein (0.14 mg dieldrin/kg body weight). The 14C activity in
blastocysts of rabbits injected on the 6th day of pregnancy was
generally low compared with the activity in maternal blood.
However, 40 - 60 min after dosing, the activities were very
similar. After 60 min, the 14C activity in blastocysts declined
rapidly, relative to that in maternal blood. In rabbits dosed
intravenously on the 16th day of pregnancy, the transfer of 14C
activity was transplacental, no activity being detected in
allantoic or amnionic fluids. The ratio of 14C activity in the
whole fetus to that in the maternal blood remained fairly constant
up to 100 min after dosing, suggesting an equilibrium between the
mother and the fetus. The results for rabbits injected on the 24th
day of pregnancy indicated that two-way placental transport of 14C
activity was occurring (Hathway et al., 1967).
6.2.2.3 Domestic animals
Studies on domestic animals, in which body tissues, milk, or
eggs were analysed, indicate that the pharmacokinetics of aldrin
and dieldrin in these species are broadly similar to those in
laboratory animals (Gannon et al., 1959a,b; Ivey et al., 1961;
Williams et al., 1964; Cummings et al., 1966; Davison, 1970, 1973;
Brown et al., 1974). None of the known metabolites of dieldrin
were detected in the body tissues or milk of cows fed 14C-dieldrin
in their diet for 41 days (Baldwin, 1972; Potter et al., 1972).
Dieldrin accumulation ratios (concentration in tissues, milk,
or eggs relative to the concentration in the diet) are given in
Table 24.
Table 24. Accumulation ratios for dieldrin in domestic animals
-----------------------------------------------------------------------------
Animal Sample Feeding period Accumulation Reference
analysed (months) ratio
-----------------------------------------------------------------------------
Cow renal body fat 3 2.43 Gannon et al. (1959a)
whole milk 3 0.18 Gannon et al. (1959b)
milk fat 12 6 Vreman et al. (1980)
Hen renal body fat 3 43.1 Gannon et al. (1959a)
body fat 13 10-24 Brown et al. (1974)
egg 7 1.5 Cummings et al. (1966)
Hog renal body fat 3 2.9 Gannon et al. (1959a)
Hog body fat 2 1.14 Dobson & Baugh (1976)
(young) (body weight
increase, 290%)
Lamb renal body fat 3 1.05 Gannon et al. (1959a)
Steer renal body fat 3 3.95 Gannon et al. (1959a)
-----------------------------------------------------------------------------
6.2.2.4 Human volunteers
A study on volunteers was carried out in which daily oral doses
of 0, 10, 50, or 211 µg dieldrin/man (three men per dose group)
were given in gelatine capsules for 18 months (Hunter & Robinson,
1967; Hunter et al., 1969). The control group comprised four men.
From the 18th month to the 24th month, the volunteers given 50 µg
continued to receive dieldrin at this level, whereas all other
volunteers, including those in the control group, received 211
µg/day. The concentrations of dieldrin in the blood of the
volunteers given 211 µg dieldrin daily throughout the study had
increased 10-fold by the end of 18 months to 15 µg/litre, while
that of the group given 50 µg/day had increased 4-fold to 5
µg/litre. The increase in the case of the group given 10 µg/day
was slight; after 5 months, a 2-fold increase had occurred to 3
µg/litre, and there was little change during the subsequent 13
months. From 21 - 24 months, the concentrations of dieldrin in the
blood of the groups given 50 or 211 µg/day fluctuated, but there
was no indication of a significant continuing increase in either
set of samples. The concentrations of dieldrin in adipose tissue
after 15 months had increased approximately 3-fold in the group
given 10 µg/day (mean: 0.4 mg/kg tissue), approximately 4-fold in
the group given 50 µg/day (mean, 0.7 mg/kg tissue), and
approximately 11-fold in the group given 211 µg/day (mean, 2 mg/kg
tissue). The concentrations of dieldrin in the adipose tissue
showed an apparent increase at 24 months relative to those at 18
months, but this may be partly related to the fact that the samples
were taken by needle biopsy at 24 months. Overall, it was
concluded that the results for the groups given 50 or 211 µg/day
indicated an approach to an upper limit (asymptote), the
relationship being of the form:
concentration of dieldrin in tissues = A - Be-kt
where A is the asymptotic value attained as time (t) approaches
infinity, and B and k are empirical constants (k corresponds to the
first-order rate constant for the elimination of dieldrin). The
mean values of the asymptote (A) for blood were 5.9 µg/litre in the
group given 50 µg/day and 20.2 µg/litre in the group given 211
µg/day. Relationships were also derived between the daily intake
of dieldrin and the steady-state (asymptotic) values for blood and
adipose tissue, respectively:
concentration of dieldrin in
blood (µg/litre)
amount of dieldrin ingested = -------------------------------------
(µg/day) 0.086
concentration of dieldrin in
adipose tissue (mg/kg)
= -------------------------------------
0.0185
It is emphasized that these relationships correspond to the
condition of a steady state between intake, storage, and
elimination of this compound. The distribution ratio
(concentration of dieldrin in adipose tissue/concentration in
blood) was 136 (Hunter & Robinson, 1967; Hunter et al., 1969).
6.2.2.5 General population
De Vlieger et al. (1968) collected samples of brain tissue,
liver, and adipose tissue from 11 routine autopsies in the
Netherlands, and found a significant relationship between the
dieldrin concentrations in the various tissues. They suggested a
tentative scheme for the distribution of dieldrin between the
various tissues. This scheme is reproduced in Fig. 1, but the
figures have been updated by recalculation conforming to the latest
empirical formula of Hunter et al. (1969) (Jager, 1970).
Chemical formula: C12H8OCl6
Relative molecular mass: 380.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 R,6 R,7 S,8 S,8a R)-1,2,3,
4,10,10-hexachloro-1,4,4a,5,6,7,8,8a-
octahydro-6,7-epoxy-1,4:5,8-
dimethanonaphthalene or 1,2,3,4,10,10-
hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-
octahydro- endo-1,4- exo-5,8,-
dimethanonaphthalene
Common synonyms ENT 16 225 (compound 497), HEOD, Alvit,
and trade names: Octalox, OMS 18, Quintox
CAS registry number: 60-57-1
RTECS registry number: I01750000
Technical product
Common trade name Dieldrin. This is the common name of an
insecticide containing 85% of HEOD.
Purity: Technical dieldrin contains not less than
95% of dieldrin, as defined above.
Impurities: other polychloroepoxyoctahydrodimethano-
naphthalenes, endrin 3.5% (FAO/WHO,
1968b)
-------------------------------------------------------------------
a From: Worthing & Walker (1983).
b Other chemical names are given in Annex I.
2.2. Physical and Chemical Properties
2.2.1. Aldrin
Pure aldrin is a colourless crystalline solid. It has a
melting point of 104 - 104.5 °C.
Technical aldrin (90%) is a tan to dark brown solid with a
melting point of 49 - 60 °C. Its vapour pressure is 8.6 mPa at
20 °C (6.5 x 10-5 mmHg at 25 °C). Its density is 1.54 g/ml at
20 °C. Its solubility in water is 27 µg/litre at 27 °C
(practically insoluble), and in acetone, benzene, and xylene
is > 600 g/litre. Aldrin is stable at < 200 °C and at pH 4 - 8,
but oxidizing agents and concentrated acids attack the
unchlorinated ring. Aldrin is non-corrosive or slightly corrosive
to metals because of the slow formation of hydrogen chloride on
storage (Shell, 1976, 1984; Worthing & Walker, 1983).
2.2.2. Dieldrin
Technical dieldrin (95%) consists of buff to light tan flakes
(setting point > 95 °C) with a mild odour. Its melting point is
175 - 176 °C. Its vapour pressure is 0.4 mPa at 20 °C (3.2 x 10-6
mmHg at 25 °C). Its density is 1.62 g/ml at 20 °C. Its solubility
in water is 186 µg/litre at 20 °C (practically insoluble), but it
is moderately soluble in most paraffinic and aromatic hydrocarbons,
halogenated hydrocarbons, ethers, esters, ketones, and alcohols.
Dieldrin is stable to alkali, mild acids, and to light. It reacts
with concentrated mineral acids, acid catalysts, acid oxidizing
agents, and active metals (iron, copper). It is non-corrosive or
slightly corrosive to metals in the same way as aldrin (Shell,
1976; Worthing & Walker, 1983).
2.3. Analytical Methods
2.3.1. Sampling methods
Methods of sampling and storage have been reviewed by Beynon &
Elgar (1966). Sample collection is broadly divisible into two types:
adventitious sampling (particularly of wildlife) and systematic
sampling (soil, total diet surveys) in which samples are collected
in accordance with the principles of statistical design. Surveys
of dieldrin in human blood and adipose tissue are a partial
combination of these two classes of sample collection. The
sampling methods for total diet surveys were reviewed by Cummings
(1966), and the sampling of air for pesticide residues has been
discussed in detail by Lewis (1976).
2.3.2. Analytical methods
Since the introduction of the method of gas-liquid
chromatography with electron capture detection (GLC/EC) (Goodwin et
al., 1961), old methods, based on, for instance, total organic
chlorine or the colorimetric phenyl azide procedure, have been
abandoned. The great majority of analytical data relating to the
occurrence of residues of aldrin or dieldrin since that time have
been based on GLC/EC procedures. There has been considerable
evolution of various aspects (especially extraction and clean up
procedures) of the methodology. The many publications on specific
procedures are reviewed in the Codex Publication "Recommendations
for methods of analysis of pesticide residues", CAC/PR 8-1986,
(FAO/WHO, 1986b). This review lists 22 individual publications,
four of which refer to simplified methods. It also lists the
following compendia of methods which may also be consulted.
- Official methods of analysis of the Association of Official
Analytical Chemists, 14th Edition 1984.
- Pesticide analytical manual, Food & Drug Administration,
Washington DC, USA.
- Manual on Analytical methods for pesticide residues in foods,
Health Protection Branch, Health and Welfare, Ottawa, Canada,
1985.
- Methodensammlung zur Rueckstandsanalytik von
Pflanzenschutzmitteln (Methods for analysing residues of plant
protective agents) 1984 Verlag Chemie GmbH, Weinheim, Federal
Republic of Germany.
- Chemistry Laboratory Guidebook, USDA.
Whatever procedure is adopted should be carried out within the
requirements of the CAC publication "Codex Guidelines on Good
Laboratory Practice in Pesticide Residue Analysis", CAC/PR 7-1984,
(FAO/WHO, 1984).
It is important to recognize that the electron capture detector
is not specific for aldrin and dieldrin and in the analysis of
samples without a precise history of treatment, confirmation of the
identity of the residue is an essential part of the analysis.
Reports of the occurrence of aldrin in environmental samples in the
past, are now thought, in many cases, to have been instances of
misidentification. The occurrence of PCBs in the same sample has
been a particularly troublesome source of interference. Many
procedures for the confirmation of identity are available and
include comparison of the position of the peak on different
chromatographic columns, thin-layer chromatography, and
derivatization. The most definitive method, however, involves the
uses of mass spectrography as the detector. With this procedure,
much of the uncertainty with regard to the identification of the
residue has been eliminated. The mass spectrography procedure
described by Hargesheimer (1984) is effective for the determination
of chlorinated hydrocarbon residues in the presence of PCBs. The
limit of determination of individual methods depends to a
considerable extent on the amount of effort the analyst devotes to
extraction and clean-up procedures. With samples of food and
feeds, for example, a limit of determination of 0.01 mg/kg is
normally regarded as acceptable, but in water and air far lower
levels are achievable, depending on the care and effort taken.
It should be recognized that there is considerable variation in
the results that can be obtained on the same sample by different
analysts and in different laboratories and variations of 100% are
by no means uncommon at the lower end of the scale. A valuable
account of the variation found among 120 laboratories for a sample
of butterfat containing known amounts of 11 different chlorinated
hydrocarbon insecticides was given by Elgar (1979).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural Occurrence
Aldrin and dieldrin are not known to occur as natural products.
3.2. Man-Made Sources
3.2.1. Production levels and processes; uses
3.2.1.1 World production figures
The first laboratory synthesis of aldrin and dieldrin was in
1948 by J. Hyman & Co. (Thompson, 1976). The method was licensed
to Shell and manufacture began in 1950, first in the USA and later
on in the Netherlands (IARC, 1974).
Production has decreased since the early 1960s. The production
capacity was 20 000 tonnes in 1971, and the estimated 1972
production was 13 000 tonnes. In 1984, less than 2500 tonnes of
aldrin and dieldrin were manufactured, approximately one third of
which was used in Australia, the United Kingdom, and the USA (Van
Duursen, 1985).
Up to the late 1960s and early 1970s, aldrin and dieldrin were
used throughout the world. Since then, many countries have
severely restricted or banned their use, especially in agriculture,
because of their persistent character in the environment (IARC,
1974). The main remaining uses are in the control of disease
vectors and termites and industrial applications.
3.2.1.2 Manufacturing processes
Aldrin is synthesized by the Diels-Alder reaction of
hexachlorocyclopentadiene with an excess of bicycloheptadiene at
100 °C. The yield is more than 80%, calculated on the
hexachlorocyclopentadiene (Melnikov, 1971).
Commercial production of dieldrin is believed to be through
epoxidation of aldrin with a peracid (e.g., peracetic or perbenzoic
acid), but an alternate synthetic route involves the condensation
of hexachlorocyclopentadiene with the epoxide of bicycloheptadiene
(Galley, 1970).
3.2.1.3 Release into the environment during normal production
Loss of aldrin and dieldrin, together with isobenzan, in waste
water from a manufacturing plant in the Botlek area of the
Netherlands caused deaths among sandwich terns (Sterna
sandvicentis), eider ducks (Somateria mollissima), and, to a lesser
extent, some other bird species, feeding on marine organisms
containing high levels of these insecticides in the Wadden Sea
during 1962 - 65. Following improvement of the waste-water
purification of the plant, the residue levels in the marine
organisms decreased during subsequent years (Koeman, 1971).
3.2.2. Uses
3.2.2.1 Aldrin
Aldrin is a highly effective broad-spectrum soil insecticide.
It kills insects by contact and ingestion, and possesses slight
fumigant action within the soil, which ensures distribution in the
top soil where the pests are found.
It is used to control soil insects, including termites, corn
rootworms, seed corn beetle, seed corn maggot, wireworms, rice
water weevil, grasshoppers, and Japanese beetles, etc. Crops
protected by aldrin soil treatment include corn and potatoes; it is
used as a seed dressing on rice. Aldrin is also used for the
protection of wooden structures against termite attack. It is
supplied mainly as an emulsifiable concentrate or wettable powder.
3.2.2.2 Dieldrin
Dieldrin is used mainly for the protection of wood and
structures against attack by insects and termites and in industry
against termites, wood borers, and textile pests (moth-proofing).
It acts as a contact and stomach poison.
Dieldrin is no longer used in agriculture. It has been used as
a residual spray and as a larvacide for the control of several
insect vectors of disease. Such uses are no longer permitted in a
number of countries.
It is available as an emulsifiable concentrate or wettable
powder.
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution Between Media
4.1.1. Leaching of aldrin and dieldrin
As would be expected from their very low water solubility,
hydrophobic character, and strong adsorption by soil, aldrin and
dieldrin are very resistant to downward leaching through the soil
profile.
Since one of the major uses of aldrin is as a soil insecticide,
aldrin-treated soil is an important source of aldrin in the
environment.
Bowman et al. (1965) studied the leaching of aldrin through six
different types of soil, by passing water through them. In five
out of six soil types, only traces were recovered in the leachates.
However, 16% of applied aldrin was found in the leachate from a
sandy soil type. Other studies indicate that leaching of aldrin
through soil is minimal (Harris, 1969; Herzel, 1971; El Beit et
al., 1981a,b).
A study was carried out to determine the possible involvement
of aldrin applied for the control of termites around house
foundations. Seven types of soil collected from different
geographical areas in the USA were investigated by placing the
soils (adjusted to 0, 5, 10, or 15% water content) in glass
columns. The soil columns were separated into five layers of 5 cm
by filter paper support cloth. An emulsion of aldrin was placed on
the top of the column, equivalent to 0.365 kg aldrin/m2. The
layers of soil were removed approximately 24 h after application of
the emulsion and the concentration of aldrin determined.
Penetration below 20 cm did not occur in any soil at any of the
water contents. In certain soils, penetration only took place in
the first 5 cm and, in others, in the third layer (10 - 15 cm).
Water content also plays a role in the penetration. In another
study, layers of 4 cm were used, with comparable results (Carter &
Stringer, 1970).
Several field studies on the leaching of aldrin through
different types of soil have been carried out. In these studies,
aldrin was applied to the surface or tilled to a depth of about 15
cm at dose levels of 1.8 - 20.7 kg/ha. From the results, it is
clear that, even up to 5 years after application, aldrin and
dieldrin were still present in the treated layer, with little
penetration to layers immediately below the treated layer. From
these studies, it appears that there is little movement
(Lichtenstein et al., 1962; Daniels, 1966; Park & McKone, 1966).
However, Wiese & Basson (1966) found some movement, even in clay
soil.
In studies by Powell et al. (1979), sandy soil in which tomato
plants were growing was sprayed with an aldrin emulsion (2.2 kg/ha)
on six occasions at intervals of 1 - 2 weeks. Approximately one
year after the final treatment, soil core samples were taken and
the concentrations of aldrin and dieldrin in the 0 - 5, 5 - 10,
10 - 15, 15 - 22.5 cm layers were determined. About 73% of the
total residue in the 0 - 22.5 cm layer was in the 0 - 15 cm layer.
The ratio of aldrin to dieldrin in the four strata was similar.
The remark should be made that in this study there were a number of
confounding factors (e.g., the field was ploughed).
Stewart & Fox (1971) applied aldrin as a spray to four turf
plots at doses of 3.3, 4.4, or 6.6 kg/ha. Loam and silt soil core
samples were taken to a depth of 30 cm 9 - 13 years after
treatment. Aldrin was not detected; 93 - 100% of the total
dieldrin in the 30 cm core was in the top 15 cm layer of soil.
In studies by Lichtenstein et al. (1971), aldrin was applied to
a silt loam at a rate of 4.4 kg/ha and rototilled to a depth of
10 - 12.5 cm. After 10 years, the percentage of the applied aldrin
in the 0 - 22.5 cm layer was 0.18% as aldrin and 5.2% as dieldrin.
The ratios of concentrations in the 0 - 15 cm layer relative to the
15 - 22.5 cm layer were: aldrin, 2.5; dieldrin, 4.9.
14C-Aldrin was incorporated to a depth of 15 cm in experimental
plots in which potatoes were grown in the Federal Republic of
Germany (sandy loam; equivalent to 2.9 kg/ha) and England (sandy
clay loam; equivalent to 3.2 kg/ha). After 6 months, the
concentrations of aldrin in both cases were as follows: at 0 - 10
cm, 0.58 and 0.59 mg/kg; at 10 - 20 cm, 0.23 mg/kg and < 0.01
mg/kg; at 20 - 40 cm 0.02 and < 0.01 mg/kg and at 40 - 60 cm, < 0.01
mg/kg (in both locations) (Klein et al., 1973). In a parallel
study, the 14C activity in leach water collected at a depth of 60
cm was determined over a 3-year period; the cumulative rainfall
during this period was 160 cm. About 10% of the 14C activity,
applied initially to a depth of 15 cm, was found in the leachate
over a period of 3 years. Almost all the 14C activity was present
as dihydrochlordene dicarboxylic acid (Moza et al., 1972).
In studies by Stewart & Gaul (1977), aldrin (5.6 and 11.2
kg/ha) was incorporated to a depth of 15 cm into a sandy loam soil
for three successive years. Various crops were grown and soil
samples were collected for 14 years. Residues of aldrin and
dieldrin below 15 cm were negligible in the tenth year after the
initial application, whereas the residues of aldrin plus dieldrin
in the 0 - 15 cm layer were 0.2 and 1.7 mg/kg, respectively, at the
two different treatments levels.
The results of these leaching studies indicate the almost
quantitative adsorption of aldrin by organic matter and clay
minerals. Water molecules compete with aldrin for the adsorption
sites in clay minerals, and it has been found that aldrin is bound
to a greater extent in dry soil (Baluja et al., 1975; Kushwaha et
al., 1978b). The adsorption and desorption of aldrin has been
studied by Tejedor et al. (1974) in whole soil and in the clay and
organic (humic) fractions. It was concluded that the organic
fraction was mainly involved in the adsorptive uptake of aldrin and
that the clay fraction was the major factor affecting the retention
of aldrin. There does not appear to be a simple relationship
between water solubility and leaching, presumably because of the
variations in the adsorptive capacity of clay minerals in various
types of soil (Yaron et al., 1967). A chromatographic model of the
movement of pesticides through soils has been proposed (King &
McCarty, 1968; Oddson et al., 1970).
In the laboratory, the investigations by Eye (1968) and Harris
(1969) of the transport of dieldrin by water through soil are
particularly relevant and are consistent with the chromatographic
model for chemicals in soil of King & McCarty (1968). The elution
of dieldrin from soil by 1600 ml water was investigated in a study
of six types of soil placed in chromatographic columns. The
dieldrin content of the total eluate, as a proportion of the
applied dieldrin, varied from 1% (loam soil) to 65% (soil
containing 93% sand) (Bowman et al., 1965).
The leaching of dieldrin through soil columns (30 cm diameter)
was studied by Thompson et al. (1970). A dieldrin emulsion was
applied to the surface (equivalent to 31 kg dieldrin/ha) of soil
columns 35 cm deep, and water was added to the surface until about
30 litres (equivalent to about 6 months rainfall) had passed down
the columns in 120 h. It was concluded that dieldrin did not
readily leach from the three types of soil investigated into
drainage water, and that cracks and crevices caused by drying or by
earthworms and other animals favour the leaching of dieldrin. The
results of an investigation using sloping troughs gave results
consistent with the soil column study.
4.1.2. Surface run-off
Run-off from treated land caused by soil erosion is a potential
source of dieldrin residues in surface waters in areas where
erosion is not controlled by good farming practice. Sediments
bearing aldrin and dieldrin can result in low concentrations in
aqueous solution, although these are limited due to adsorption onto
the sediments. Thus, rain-water run-off (without sediment) does
not appear to be a major contributor.
Richard et al. (1975) and Sparr et al. (1966) sampled various
surface waters in the USA and reported levels of dieldrin ranging
from < 1 to 42 ng/litre and of aldrin in the region of 0.05
µg/litre.
To gain data on the erosion of treated land, Caro & Taylor
(1971) and Caro et al. (1976) incorporated dieldrin into the soils
of two small watersheds in Ohio, USA, and studied run-off losses
over a three-year period. In the first case, there was practically
no surface soil erosion and the total loss of dieldrin was confined
to run-off water. The area was 1.07 ha and the loss over the
period was less than 0.5 g dieldrin, the highest level in the water
being 4 µg/litre. In the second study, there was a substantial
loss of soil by erosion and the amount of dieldrin lost in the
solid sediment was 77 g in only 8 months. The loss in the water
itself was just under 2.5 g and the highest water concentration was
20 µg/litre. It should, however, be borne in mind that in this
case the soil had been mechanically compacted to aggravate the
effects of erosion, so that it is questionable whether the results
bear much relation to normal agricultural practice. The authors
commented that there was only a poor correlation between rainfall
events and the amounts of dieldrin lost.
Sediment-bearing residues of aldrin or dieldrin will yield some
of their burden to true solution in the water which they enters.
Sharom et al. (1980) showed that the ratio of dieldrin
concentration in soil to that in water (in equilibrium with the
soil) was between 100 and 500 for mineral soils, whilst that same
ratio for aldrin was likely to be around 5 - 6 times higher. Thus,
with 1 mg dieldrin/kg sediment, one could expect a water
concentration of about 10 µg/litre.
The movement of aldrin and dieldrin by run-off and soil erosion
was studied by Haan (1971). Each pesticide was applied at 1.65
kg/ha to the surface of small plots, mainly consisting of silt loam
(slope, 1 - 2%), in a greenhouse. Water was applied and the run-
off water, sediment, and surface soil (0.6 cm deep) were analysed.
It was estimated that 94.8% and 95.4%, respectively, of the applied
aldrin and dieldrin remained in the surface soil (0.6 cm depth).
It was concluded that there was no difference in the potential for
loss from soil by rainfall, whether the rainfall occurred shortly
after aldrin application or several days later.
4.1.3. Loss of aldrin and dieldrin from soils - volatilization
Most authors consider that the principal loss of aldrin and
dieldrin from soils is by volatilization. There is widespread
evidence for this, although other mechanisms (sections 4.4.1 and
4.4.2) may also play an important role.
Volatilization from soils was first demonstrated when it was
shown that mosquitoes were killed by vapour emanating from treated
soil blocks (Barlow & Hadaway, 1955, 1956; Gerolt, 1961).
When aldrin is incorporated into the soil, it is most readily
lost from the surface layer. Subsequently, material from deeper
layers has to rise to the surface to replenish what was lost. The
position is somewhat complicated by its gradual conversion to the
less volatile dieldrin, although this, too, behaves in a
qualitatively similar manner.
There are two routes to the surface: transport in ascending
capillary water - analogous to the process of salinization - and
vapour diffusion through the soil pores. Both of these processes
are strongly affected by hydrophobic adsorption, a phenomenon
common to many hydrophobic pesticides of low water solubility.
Adsorption by the soil has the effect, at practical rates of
application, of reducing the vapour pressure and hence the
saturation vapour density in the soil atmosphere. It also reduces
the maximum concentration in the soil solution.
There is a very extensive literature on soil adsorption,
especially of dieldrin and the following general situation is now
well established.
Adsorption, as measured by reduced vapour density, takes place
in all soils but is greatest at low moisture levels; that is to say
soils in equilibrium with air of relative humidity below around
95%. (Barlow & Hadaway, 1955, 1956; Gerolt, 1961; Harris, 1964,
1972; Igue et al., 1972).
In dry soils, mineral components play the most important part,
whereas in moist soils it is organic matter that dominates (Harris
& Lichtenstein, 1961; Harris et al., 1966; Harris & Sans, 1967;
Harris, 1972). In fact, Harris demonstrated a linear relation
between organic matter and adsorption in moist soils. On the other
hand, in a dry mineral soil with predominantly montmorillonitic
clay and very low organic matter, practically no dieldrin
volatilized until the relative humidity of the air in equilibrium
with soil reached saturation. At this point volatilization readily
resumed.
In moist soils, Spencer et al. (1969) found that adsorption,
expressed as a reduction in vapour density, became less marked as
the dieldrin level increased. At 20 °C, 10% moisture in the soil,
and 1 mg dieldrin/kg soil, the dieldrin vapour density was only 2
ng/litre, compared with 52 ng/litre when the dieldrin level in the
soil was increased to 25 mg/kg. This level is close to the figure
for free dieldrin. Similar results were reported at 30 °C and
40 °C by Spencer & Cliath (1973).
In dry soils, however, adsorption is far stronger. At 100 mg
dieldrin/kg moist soil (Spencer et al., 1969), the depression in
vapour pressure was negligible. However, as the moisture content
of the soil fell to a critical level of 2.1%, there was a dramatic
decrease in vapour density, so that below 2% moisture the vapour
density was practically zero. The same authors showed that the
level of water in their soil needed to provide a monomolecular
layer was 2.8%. They concluded that the critical point at which
adsorption increased was when the monomolecular layer started to be
lost, leaving adsorption sites available for occupation by
dieldrin. Restoration of the moisture status of the soil, however,
restored the vapour density to its original level.
Whilst most of these studies were carried out on one soil, Gila
silt loam, and whilst the figures would be different for other
soils, the qualitative conclusions are largely valid for all soils.
Adsorption is expected to be least on sandy soils of low organic
matter content.
Adsorption by soils can also be determined by measuring the
reduction in the saturation concentration of the soil solution
(Eye, 1968; Tejedor et al., 1974; Baluja et al., 1975). As in the
case of reduced vapour pressure caused by adsorption by moist
soils, the organic matter content of the soil was the principal
soil characteristic affecting adsorption from solution. Eye (1968)
also demonstrated the dominating influence of organic matter,
whereas clay content, surface area, and cationic exchange capacity
showed very little correlation. These findings are compatible with
those of Yaron et al. (1967).
In studies involving the percolation of dieldrin, dissolved in
water, through columns of soils with differing contents of organic
matter, Sharom et al. (1980) also showed that the soil capacity for
adsorption was largely determined by its content of organic matter.
Moreover, adsorption followed the Freundlich adsorption equation.
They reported Freundlich adsorption constants for a range of soils
and pesticides, including dieldrin, and showed that, for a given
pesticide, adsorption was strongly dependent on the organic matter
content of the soil. Moreover, the strength of adsorption by a
given soil depended mainly on the water solubility of the
pesticide, so that dieldrin, with its low water solubility, was
more strongly adsorbed than, for instance, the much more water-
soluble lindane. Although aldrin was not studied, it may be
inferred from these data that aldrin would be adsorbed
correspondingly more strongly, owing to a much lower water
solubility than that of dieldrin.
4.1.3.1 Movement within the soil profile - mass flow
Spencer & Cliath (1973) concluded from laboratory studies that
dieldrin could ascend the soil profile by mass flow in capillary
water moving up to the surface through a moisture gradient, and
that this mechanism could account for 3 - 30% of the total upward
movement. However, with low solubility products such as dieldrin,
Jury et al. (1983) pointed out that volatilization decreases with
time, because ascent to the surface is rate limiting. With high
solubility compounds, however, the reverse is true as more material
reaches the surface, dissolved in capillary water, to become
available for evaporation. However, it is not only water
solubility that determines the behaviour, but the value of Henry's
constant for the partition of the compound between air and water.
These authors considered the critical value to be 2.7 x 10-5; above
this value mass flow is progressively less important. The value of
Henry's constant for dieldrin (6.7 x 10-4) is substantially higher
(Jury et al., 1983) and that for aldrin higher still, so that on
this basis it is doubtful whether mass flow ever does play a
significant role in the transport of aldrin or dieldrin up the soil
profile.
In support of the view that transport by mass flow is not
appreciable, the mathematical models that have been proposed to
describe the loss of aldrin and dieldrin from soils (Farmer &
Letey, 1974; Mayer et al., 1974; Jury et al., 1983) tend to
demonstrate, in comparisons with laboratory data, that ascent to
the surface is predominantly by vapour diffusion rather than mass
flow.
4.1.3.2 Movement within the soil profile - diffusion
Diffusion is regarded as the main route by which aldrin and
dieldrin ascend the soil profile to reach the surface. Diffusion
increases with soil temperature, concentration, decreasing
adsorption capacity (usually the same as decreasing organic
matter), maintenance of moisture content above the wilting point,
and the "tortuosity" of the soil pore system (a measure of the
openness of the soil). With regard to moisture content, Farmer &
Jensen (1970) found that diffusion coefficients of dieldrin in
three soils in equilibrium with air of 94% relative humidity were
9.7, 4.4, and 3.8, but at 75% relative humidity the values were
0.6, 0.4, and 0.4, respectively. According to Farmer & Letey
(1974), the critical moisture level is probably the "fifteen
atmosphere percentage", usually considered to be a reasonable
measure of the water content at the wilting point.
Tortuosity increases as soils are compacted. Working with
moist soils of differing bulk densities, Farmer et al. (1973),
showed that diffusion of dieldrin was about twice as fast in a soil
with a density of 0.75 g/cm3 as when it was compressed to a bulk
density of 1.5 g/cm3.
4.1.3.3 Actual volatilization losses - laboratory studies
Lichtenstein & Schulz (1970) reported that aldrin was lost by
volatilization from a silt loam soil about 20 times faster than
dieldrin. Helene et al. (1981) reported a 31% loss of aldrin from
a highly humic soil after 120 days but 62% from a soil of low
organic matter content.
In studies of moist soils in volatilization chambers, Farmer et
al. (1972) and Igue et al. (1972) found that the rate of loss by
volatilization gradually decreased with time. However, if
translated into terms of the open field, this could still represent
a loss of between 0.2 and 1.4 kg/ha per year, depending on the
depth of incorporation.
With a surface application of dieldrin in a microagroecosystem
chamber, Nash (1983) reported loss of dieldrin at the rate of 1 - 4
g/day, but this rate fell to about a half of its initial value
within 6 - 7 h. Incorporation of the dieldrin had the effect of
greatly slowing this loss rate (Nash, 1983).
4.1.3.4 Actual volatilization losses - field studies
The data on volatilization losses in the field are limited and
refer only to dieldrin. Caro & Taylor (1971) reported loss by
volatilization from an incorporated dieldrin application (5.6
kg/ha) of 2.8% of that applied (after 18 weeks). Spencer et al.
(1973) cited unpublished studies by Caro & Taylor (1971) where a
surface application was lost at the rate of 3% per hour. In a
later study, Caro & Taylor (1976) found that 4.5% of a dieldrin
application was lost by volatilization in the first year after
treatment. By the autumn, the loss rate was only 0.2 g/ha per day,
although this increased to 0.9 g/ha per day immediately after the
land was cultivated, due, presumably, to the exposure of fresh
soil.
Taylor et al. (1972, 1976) estimated a loss of dieldrin of 0.2
kg/ha from an incorporated application of dieldrin. However, only
6% remained from a surface application after 16 weeks, although in
this case a small amount was recovered as photodieldrin (Turner et
al., 1977).
Willis et al. (1972) demonstrated an 18% loss from a very high
application (22 kg/ha) of dieldrin after 5 months where the soil
was kept moist by irrigation. However, losses were substantially
less when the soil was not irrigated or when maintained under flood
conditions. The maximum rate of loss by volatilization was 0.2
kg/ha per day.
4.1.4 Losses of residues following treatment of soil with aldrin
One of the earliest systematic studies of the decline of aldrin
and dieldrin residues in soils, arising from the application of
aldrin to the soil, was by Decker et al. (1965), who sampled a wide
range of soils of known treatment history from Illinois, USA. They
demonstrated the transformation of aldrin to dieldrin and
considered that the loss of residues was a two-stage process.
There was a comparatively rapid loss in the first year after
treatment, a typical loss being 75% of the applied dose.
Thereafter, residues declined with a half-life of 2 - 4 years, the
reduced rate being apparently due to the greater proportion of
dieldrin in the residues. Elgar (1966) incorporated 2.2 kg
aldrin/ha into soils in the United Kingdom and reported somewhat
similar results for the decline of residues, although there were
indications that the rate of decline slowed in later years as the
level in the soil fell to around 0.3 mg/kg. Further studies of
this kind have been reported by Lichtenstein et al. (1970), Onsager
et al. (1970), and Korschgen (1971). Although the rates of decline
were very variable, they were not inconsistent with the data of
Decker et al. (1965), bearing in mind the inherent variability of
soil data.
There are indications that loss rates are higher in tropical
soils than in temperate climates. Whilst Agnihotri et al. (1977)
found that epoxidation was faster in tropical than temperate soils,
leading to the possibility of slower decline because of higher
dieldrin levels, Gupta & Kavadia (1979) found in India that
declines were often much faster. In one case, half of the aldrin
applied had been lost in only 38 days. Wiese & Basson (1966) also
reported comparatively high loss rates in South Africa. Using
three rates of treatment and three soils, they found that half of
the original application was lost between 1 and 2 months.
Elgar (1975) conducted a series of studies in temperate, warm
temperate, and tropical soils and reported rates of decline that
were compatible with those of Decker et al. (1965). Again, losses
from the tropical sites occurred more rapidly than from the
temperate sites. He deduced the following empirical expression to
describe loss rates, expressed as the sum of aldrin and dieldrin
residues surviving n years after a single application.
C(n) = fC(o)(1-p)n-1
In this expression, C(o) is the initial residue level, C(n) is the
level after n years, f is the proportion remaining after the first
year, and p is the proportion lost in each of the succeeding years.
In Elgar's studies, the mean estimate of these latter two
parameters was f = 0.25 and p = 0.44, but in the Decker work, the
value of p was somewhat less. It is also possible to derive an
equation that describes the accumulation of residues in a soil
subject to a regular routine of annual applications. The
implications of this equation are that residue levels do not
continue to increase indefinitely, but reach a plateau. In the
case of Elgar's data, the plateau level, one year after the last of
n applications, would be around 60% of the level observed
immediately after the first application. This prediction is well
borne out by the soil monitoring data presented in Table 1.
Studies of the decline of residues arising from aldrin applied
for the control of termites (Bess & Hylin 1970; Carter & Stringer,
1970) reveal slower rates of decline than would be expected,
considering the deep application.
Separate studies have been carried out on dieldrin residue
losses. These show considerably slower rates of decline than in
the case of aldrin, but there is a very wide range in the data
reported. Thus, Edwards (1966) reported that the average time for
the disappearance of 95% of the residues was 8 years, but Wiese &
Basson (1966) found much faster rates. Intermediate rates were
reported by Stewart & Fox (1971) and Beyer & Gish (1980). It seems
probable that the rate of decline of dieldrin in the soil is
reasonably well reflected by Elgar's equation for the years that
succeed the first year of aldrin application.
4.1.5. Losses of residues from water
The partition of dieldrin between the vapour phase and water
was determined by a dynamic gas-flow method using 14C-dieldrin
(Atkins & Eggleton, 1970). The partition coefficient at 20 °C
(expressed on a weight/volume basis for air and water) was constant
at 540, up to a concentration of 0.033 mg dieldrin/litre water. At
higher concentrations, there was a rapid increase in the partition
coefficient, which was attributed to the aqueous solution becoming
saturated at 0.033 mg/litre. Using the values for vapour pressure
(3.47 x 10-4 Pa) and water solubility found in this study, the
wash-out ratio for the removal of dieldrin vapour from atmospheric
air by rain was 0.65. It was suggested that the concentration of
dieldrin in the rainfall in London (Abbott et al., 1965) (Table 6)
may indicate the presence of dieldrin in particulate matter in the
atmosphere rather than in the vapour phase.
Table 1. Concentrations of aldrin and dieldrin in soila
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
United aldrin: potatoes 21 0.02 0.09 LD < 0.03 mg/kg Wheatley et
Kingdom (0.12) (0.41) al. (1962)
1965 aldrin: potatoes; 10 0.15 0.48 LD not reported; apparently Davis (1968)
dieldrin: seed-dressing, (0.7) (0.7) < 0.02 mg/kg; various soil
carrots, and wheat; types; residues in soil
cumulative applications microfauna also determined
during 5 years prior to
sampling (0.14-3.4
kg/ha)
Canada
S.W. Ontario 1964-65 aldrin: various crops; 13 0.19 0.57 LD < 0.1 mg/kg; soil of Harris et al.
known usage (0.8) (1.3) various types (sandmuck); (1966)
aldrin used to a
considerable extent
(1954-60) on 27 sites
no reported use 1961-64 14 0.18 0.25
(2.1) (1.6)
none used 1954-64 5 LD LD
Atlantic 1965 aldrin: 1-5 applications LD 0.01 mg/kg; no detectable Duffy & Wong
provinces during 15 years prior to residues of aldrin or (1967)
sampling; cumulative dieldrin in orchard soils to
application 0.5-45 kg/ha; which aldrin/dieldrin had
not been applied
root crops 18 0.46 0.41
(1.5) (1.45)
vegetables 17 0.66 0.36
(2.5) (1.35)
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
Southern 1971 aldrin: tobacco 4 (50 ND 0.16 LD 0.001 mg/kg; woodlots Frank et al.
Ontario samples) (0.19) were adjacent to treated (1974)
areas, but not directly
sprayed
cereals 4 (60 ND 0.16
samples) (0.19)
woodlots 12 ND trace
samples
Saskatchewan 1970 soil from 21 vegetable 41 0.03 0.06 LD 0.005 mg/kg; aldrin found Saha & Sumner
farms samples (0.28) (0.77) in 25% of samples; dieldrin (1971)
found in 55% of samples
Southern 1972-75 soil samples from LD < 0.0004 mg/kg; dieldrin Frank et al.
Ontario orchards had been used (1955-65) (1976)
at recommended rates of
0.8-1.3 kg/ha
apple: 0-15 cm 31 ND 0.03
(0.38)
15-30 cm ND 0.001
(0.03)
Southern 1972-75 sweet cherry: 16 Frank et al.
Ontario 0-15 cm ND 0.001 (1976)
(0.01)
15-30 cm ND LD
sour cherry: 12
0-15 cm ND 0.005
(0.04)
15-30 cm ND 0.003
(0.02)
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
Southern peach: 11
Ontario 0-15 cm ND 0.04
(contd.) (0.11)
15-30 cm ND 0.02
(0.07)
vineyards: 16
0-15 cm ND 0.009
(0.035)
15-30 cm ND 0.004
(0.023)
USA
Seven eastern 1965 aldrin and dieldrin in 3 LD 0.05 mg/kg; proportions Seal et al.
states crops: of soil samples with (1967)
measurable residues:
peanuts: 5 ND 0.15 potatoes, 76%; carrots,
(0.20) 21%; peanuts, 100%
carrots: 19 ND 0.19
(0.26)
potatoes: 25 ND 0.10
(0.20)
1965-67 aldrin and dieldrin used 17 (278 0.02 0.21 LD 0.01 mg/kg; aldrin Stevens et al.
regularly samples) (0.47) (2.84) detected in 15% of samples (1970)
and dieldrin in 67% of
samples from areas of
regular use
limited use 16 LD 0.001
(0.001)
no known use 18 LD LD
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
Colorado 1967 aldrin: various soil 11 0.16 0.19 LD < 0.02 mg/kg; some Mullins et al.
types (1-4.3% organic (0.61) (0.44) fields had been treated (1971)
matter); nominal annually for 9 years; time
concentrations in soil at of last treatment prior to
time of application: sampling varied from 0-9
0.06-6.75 mg/kg years
dieldrin: nominal 9 ND 0.05
concentrations in soil at (0.30)
time of application:
0.13-0.63 mg/kg
Arizona 1968 3 types of soil (organic 13 LD 0.0003 LD not defined; appears to Laubscher et
matter 0.5-6.6%) from (0.0013) be about 0.0001 mg/kg; no al. (1971)
area downwind of relationship between
an area of insecticide concentration of dieldrin
use and distance from area of
application
10 major 1969 samples of soil 71 0.02 0.79 LD 0.01 mg/kg; aldrin in Wiersma et al.
areas of (0.96) (16.72) 4.2% of samples and (1972)
onion growing dieldrin in 73% of samples
9 areas 1969 samples of soil 92 0.01 0.17 LD 0.01 mg/kg; aldrin in Sand et al.
growing sweet (0.11) (2.18) 3.3% and dieldrin in 60.9% (1972)
potatoes of samples
Rice-growing 1972 samples of soil 99 0.01 0.04 LD 0.01 mg/kg; aldrin in Carey et al.
areas (0.25) (0.27) 39% and dieldrin in 85% of (1980)
samples
USA National 1970 samples of soil 1506 0.02 0.04 LD 0.01 mg/kg; aldrin in Crockett et al.
Monitoring (4.25) (1.85) 13% and dieldrin in 31% of (1974)
Program samples
(35 states)
------------------------------------------------------------------------------------------------------------------------------
Table 1. (contd.)
------------------------------------------------------------------------------------------------------------------------------
Location Year Use Number Mean concentration Comments Reference
of in mg/kg (maximum
sites value in brackets)
aldrin dieldrin
------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
12 states in 1970 average application of 12 (389 0.05 0.07 LD <0.01 mg/kg; dieldrin Carey et al.
the cornbelt dieldrin was 1.3 kg/ha samples) (2.98) (2.04) residues attributed (1973)
region primarily to the use of
aldrin; aldrin had been
used in one or more years
from 1954
14 cities 1970 soil from urban areas 356 LD 0.1 LD < 0.03 mg/kg; aldrin Carey et al.
sampled to a depth of (12.8) not detected in any (1976)
7.6 cm samples; dieldrin in
samples from 22 sites
(6.5%) in 6 cities
Japan, S.W.
Kyushu 99 0.07 0.29 LD 0.001 mg/kg Suzuki et al.
district samples (1.01) (1.73) (1973)
------------------------------------------------------------------------------------------------------------------------------
a LD = limit of detection; ND = not determined.
The rate of dry deposition of dieldrin (vapour phase) on grass,
calculated from the results of wind tunnel studies, was 4 x 10-2
cm/second. The average lifetime of dieldrin in the atmosphere,
assuming loss by wash-out and dry deposition only, was estimated to
be 28 weeks (Atkins & Eggleton, 1970).
The rate of transfer of dieldrin from water to air and vice
versa has been determined (Slater & Spedding, 1981). The transfer
velocity from water, measured in a wind tunnel, increased as the
air speed (measured at 6 cm above the water surface) increased.
When there was no air movement, the transfer velocity was 2.6 x
10-5 cm/second compared to 15 x 10-5 cm/second at an air velocity
of 31.1 km/h. The transfer velocity from air to water was measured
by passing air through a column of downward-flowing water, and was
found to increase as the interfacial velocity increased from 0.9 x
10-2 cm/second (at 10 km/h) to 5.2 x 10-2 cm/second (at 34.2 km/h).
It was suggested that the exchange of dieldrin between water and
air was controlled by diffusive processes either in the air
boundary or water boundary layers. The Henry's law constant (ratio
of the concentrations in air and aqueous phases at equilibrium) for
dieldrin was 1.3 x 10-3 at 20 °C. It was concluded that the
resistances to transfer of dieldrin from water to air and vice
versa were similar.
The physical and thermodynamic principles of exchanges of
chemicals between water and air have been discussed (Mackay &
Wolkoff, 1973; Liss & Slater, 1974; Mackay & Leinonen, 1975; Mackay
et al., 1979; Smith et al., 1981). An estimate of the half-life of
the evaporation of dieldrin at 25 °C from a column of water of 1 m
depth was derived by Mackay & Leinonen (1975). Although this
estimate (539 days) is not based on the most recent and reliable
values for the vapour pressure and water solubility of dieldrin, it
is probably of the right order.
4.1.6. Aldrin and dieldrin in the atmosphere
Small amounts of dieldrin have been detected in the atmosphere
(Table 6). Baldwin et al. (1977) conducted a study at Bantry Bay
on the west coast of Ireland, well away from point sources of
emission. They found concentrations of dieldrin between 0.06 and
1.6 ng/kg, with an average of 0.36 ng/kg, but no aldrin,
photodieldrin, or photoaldrin. No dieldrin was detected on solid
matter trapped on filter pads; the limit of determination ranged
from 1.1 to 7.2 pg/kg (parts per thousand trillion of air).
The reason for the very low level of occurrence of dieldrin in
the global atmosphere, if, as seems probable, a major part of the
aldrin used in agriculture escapes from the soil by evaporation,
has been the subject of considerable speculation. It appears
unlikely that direct photochemical reactions are involved, since
there have been no reports of photodieldrin being detected.
Washout by rain may be an important factor. Indeed, Baldwin et al.
(1977) cited literature figures for Hawaii of 1 - 97 ng/litre, and
Abbott et al. (1965) reported 1 - 95 ng/litre in rainfall in London
and other locations in the United Kingdom. MacCuaig (1975), on the
other hand, working in the vicinity of a dieldrin application in
Ethiopia, reported 100 µg/litre in rainwater. These results
support the suggestion of Atkins & Eggleton (1970) that, though
washout of the atmosphere by rain would be inefficient in the case
of dieldrin, it could lead to substantial losses. If this were so,
dieldrin deposits would be expected on soil adjacent to treated
areas, but the fact that large areas of soil in the cornbelt of the
USA (Carey et al., 1973) have no detectable levels of aldrin or
dieldrin seems to cast doubt on the extent to which rain acts to
disperse aldrin and dieldrin onto untreated land near to treated
areas.
It would appear possible, therefore, that there are losses of
aldrin and dieldrin in the atmosphere. Glotfelty (1978) mentioned
the high reactivity of free radical species in the atmosphere, in
particular hydroxyl radicals. These could presumably play an
important role in the degradation of molecules occurring as vapour.
4.1.7. Aldrin and dieldrin in water
The data regarding the occurrence of aldrin and dieldrin in
both ground and surface waters are summarized in Table 7 (section
5.1.3). As would be expected from the extreme resistance of
dieldrin and, especially, aldrin to leaching from soil, the
occurrence of either compound in groundwater is rare. Spalding et
al. (1980) took a series of groundwater samples in Nebraska, USA,
where aldrin had been used extensively for the control of corn
rootworm and could not detect it in any of the samples. Their
limit of determination was between 5 and 10 ng/litre. Junk et al.
(1980) reported somewhat similar results from Nebraska. Richard et
al. (1975), in a wide-ranging study, examined the water supplied to
a series of cities in Iowa, USA, from boreholes. Again, no aldrin
or dieldrin was reported; their limit of determination appears to
have been 0.5 ng/litre.
Surface waters, by contrast, have often been reported to
contain small amounts of dieldrin. In a programme of sampling
various surface waters in Iowa, Richard et al. (1975) reported
levels of dieldrin ranging from 3 to 75 ng/litre in rivers and
streams and levels in reservoirs from 3 to 18 ng/litre. In rivers
in Iowa and Louisiana, levels ranged from < 1 to 42 ng/litre.
During the period 1976 - 80, dieldrin was found in 2.4% of samples
from national surface waters in the USA, (maximum concentration of
0.61 µg/litre) and in 21.7% of national surface water sediments
(maximum concentration of 5300 µg/kg) (Carey & Kutz, 1985).
The dieldrin in surface water probably comes from run-off from
treated land. Sparr et al. (1966) sampled drainage ditches and a
river in a maize growing area in northwest Indiana, USA. Levels
reached 0.6 µg/litre in the river but, in the ditches from fields
treated with aldrin at up to 5.6 kg/ha, levels seldom exceeded the
limit of determination (0.05 µg/litre). Water draining from rice
paddies that had been planted with aldrin-treated seed also
contained small amounts of dieldrin (1 µg/litre after seeding and
falling by the 14th week to 0.07 µg/litre). The authors calculated
that about 1 g of aldrin had been lost from the rice paddy surface
water during the whole 14-week period.
Hindin et al. (1964) reported aldrin in irrigation water up to
2.3 µg/litre, but no dieldrin. However, in view of the readiness
with which aldrin is epoxidized to dieldrin in surface waters,
there must be some doubt as to the identity of the residue they
actually measured.
It does appear that dieldrin can occur in surface waters
draining from agricultural areas, but the amounts are usually so
small that they could not be expected to represent a major
proportion of the product applied to the soil. The ultimate fate
of these small levels of dieldrin in water is not known. It is
probably that adsorption onto particulate matter, volatilization,
and various degradation mechanisms all play a role.
4.2 Translocation From Soil Into Plants
The uptake of aldrin and dieldrin by plants is much higher in
root crops than in grain crops. It is influenced by the levels in
soils, the strength of adsorption, and the depth of application.
In grain crops, it is rare for residues to reach detectable
levels in the grain (FAO/WHO, 1970a; Gupta & Kavadia, 1979). Root
crops are much more prone to take up residues from treated soils,
as observed by Harris & Sans (1967) who found that carrots,
radishes, and turnips had the highest residues. Onions, lettuce,
and celery were intermediate and cole crops showed no detectable
uptake at all (Lichtenstein, 1959).
The level of aldrin and dieldrin in the soil influences the
degree of uptake as shown by Lichtenstein et al. (1970) and Edwards
(1973a,b), who both reported on ratios of the concentrations in
plants to those in the soil. Further work by Onsager et al.
(1970), Voerman & Besemer (1975), Bruce & Decker (1966), and Saha
et al. (1971) provided compatible results.
The availability of aldrin and dieldrin for uptake by plants
depends on the strength of adsorption by the soil and especially
the organic matter fraction. Harris & Sans (1967), Beall & Nash
(1969), Beestman et al. (1969), and Nash et al. (1970) demonstrated
that crops tend to take up more residues from soil of low than of
high organic matter. Adding activated charcoal to soil reduced
dieldrin uptake by 70% or more in carrots and potatoes
(Lichtenstein et al., 1971).
Deep application of dieldrin greatly reduces the uptake (Beall
& Nash, 1972). Residues in the plants from a deep (31 - 32 cm)
application were only 1% of those from superficial application.
The authors commented that a possible treatment for reducing the
uptake of old soil residues by crops would be simply to plough them
under.
The mechanism of uptake by crops is not entirely clear and
appears to vary considerably from species to species. Beall & Nash
(1971), in work with soyabeans grown on soil treated with 14C-
labelled dieldrin, found that residues were taken up both by
absorption through the roots and by absorption of vapour through
the leaves. In the case of cereals, it seems unlikely that root
uptake occurs to any great extent (Powell et al., 1970; Gutenmann
et al., 1972; Gupta et al., 1979). This probably accounts for the
very low levels found in cereal grains from treated crops. On the
other hand, it would seem almost certain that it is root uptake
which accounts for the residues found in root crops.
4.3. Models of the Behaviour of Water and Chemicals in Soil
Various models for the movement of water and chemicals in
porous media have been developed, based on physical variables such
as vapour pressure, diffusibility, and adsorption, etc. (Keller &
Alfaro, 1966; Bresler & Hanks, 1969; Lindstrom et al., 1971;
Davidson & McDougal, 1973; Pionke & Chester, 1973; Van Genuchten et
al., 1974). Models for run-off from soil have also been proposed
(Crawford & Donigian, 1973; Bailey et al., 1974; Bruce et al.,
1975). These models may be useful as a means of defining more
precisely the behaviour of aldrin and dieldrin in soil.
4.4. Biodegradation of Aldrin and Dieldrin
When used to protect crops from soil insects, aldrin is usually
incorporated into the soil in which the plants are grown. For this
reason, most of the work on the biodegradation of aldrin in
agriculture has been concerned with the soil system.
4.4.1. Epoxidation of aldrin
The most important transformation of aldrin in the soil is its
conversion by epoxidation to dieldrin (Fig. 2, section 6.3.1.1).
Epoxidation, essentially biological in nature (Lichtenstein &
Schulz, 1960), occurs in all aerobic and biologically active soils,
and about 50 - 70% of the residues remaining in a soil at the end
of the season in which the application was made consist of
dieldrin. Lichtenstein & Schulz (1959) reported that epoxidation
was slower on peat than on mineral soils and was inhibited at low
soil temperatures; very little conversion occurred at 7 °C.
Subsequently, many authors have demonstrated that a large number of
microorganisms are capable of promoting epoxidation, and these were
reviewed by Tu & Miles (1976).
Aldrin is also epoxidized by plants, as demonstrated by Gannon
& Decker (1958), while Yu et al. (1971) have showed that root
homogenates are very effective promoters of aldrin-to-dieldrin
epoxidation.
Aldrin is not epoxidized under anaerobic conditions. In their
studies on the degradation of aldrin in anaerobic cultures of
sewage sludge, Hill & McCarty (1967) found no dieldrin, although
aldrin was completely decomposed within 60 days. Sethunathan (1973)
reported that epoxidation of aldrin was arrested in flooded soils.
4.4.2. Other metabolic pathways of aldrin
The transformation of aldrin in the soil to aldrin dicarboxylic
acid (V, Fig. 2) appears to be well established (Klein et al.,
1973; Kohli et al., 1973b; Weisgerber et al., 1974). The
occurrence of photodieldrin (III, Fig. 2) as a metabolite derived
from aldrin soil treatment is less well established either in soil
(Lichtenstein et al., 1970) or in the leaves of wheat grown in
aldrin-treated soils (Weisgerber et al., 1974).
4.4.3. Biotransformation of dieldrin
Dieldrin is much more resistant to biodegradation than aldrin,
and microbial degradation is probably a minor route of loss from
soils, even under anaerobic conditions (Sethunathan, 1973; El Beit,
1981). Kohli et al. (1973b) added 14C-labelled dieldrin to a soil
and detected very little degradation, though he did report trace
quantities of photodieldrin. Similarly, small amounts of
photodieldrin were detected after dieldrin had been applied to
onion seed (Kohli et al., 1972).
In the search for organisms that would degrade dieldrin,
Matsumura & Boush (1967) found that only a few soil samples
produced detectable transformation of dieldrin, although, in some,
up to 6% of the dieldrin added was transformed to water-soluble
metabolites. Separation of the organisms responsible revealed that
Pseudomonas, Bacillus, and Trichoderma species were able to attack
the dieldrin molecule. Tu & Miles (1976) list organisms that have
been reported to attack dieldrin; these include bacteria, fungi,
and one actinomycete.
In spite of the large number of studies on this topic, it is
difficult to estimate the extent to which photodieldrin is evolved
in soils treated with aldrin. It is perhaps significant that the
microorganisms capable of producing photodieldrin in the laboratory
have been isolated mainly from anaerobic environments, so that
their activity would be very limited in a well-managed agricultural
soil. This is borne out by the study of Suzuki et al. (1974) who
sampled 52 soils with a history of aldrin treatment in Japan.
Photodieldrin levels were very low compared with dieldrin levels
and ranged from < 0.001 to 0.035 mg/kg soil (section 4.4.2.1).
The further fate of photodieldrin in soils has received little
attention, but Weisgerber et al. (1975) considered it to be less
persistent in the soil than dieldrin itself. They also identified
two breakdown products, the bridged equivalent of aldrin
dihydrochlordene dicarboxylic acid (XII, Fig. 2) and the bridged
equivalent of the transdiol (XI, Fig. 2), though these were only
present in very small amounts.
4.4.4. Conclusions
Although many studies have been carried out on the
biodegradation of aldrin and dieldrin, it seems improbable that
this is a major source of loss from soil. On the other hand, it
does seem as if transformation of aldrin to aldrin acid in aldrin-
treated soils can be a significant pathway, although there is
little evidence in the literature that aldrin acid occurs widely as
an environmental residue.
4.5. Abiotic Degradation
Abiotic processes play a limited role in the degradation of
aldrin and dieldrin in the environment. Of these abiotic processes,
the greatest amount of research has been carried out on photochemically
induced changes.
4.5.1. Photochemistry
Aldrin and dieldrin are susceptible to chemical change as a
result of irradiation. Robinson et al. (1966b) assigned structure
III (Fig. 2) to the transformation product generally referred to as
"photodieldrin".
Rosen & Carey (1968) demonstrated the formation of the
unepoxidized analogue from aldrin (photoaldrin) (XIII, Fig. 2) when
aldrin was irradiated by sunlight or UV light in abiotic conditions,
but the major reaction product under these conditions was an
unbridged product where a single chlorine atom had been lost at the
3 position. The addition of benzophenone greatly enhanced yields of
photoaldrin from aldrin and also photodieldrin from dieldrin.
Fischler & Korte (1969) showed that other ketones also increased the
formation of photodieldrin.
4.5.1.1 Photochemistry of aldrin and dieldrin in water
Henderson & Crosby (1968) demonstrated that saturated aqueous
solutions of dieldrin exposed outdoors to sunlight produced
photodieldrin. However, Ross & Crosby (1974, 1975) found that when
oxygenated aqueous solutions of aldrin were irradiated with UV
light there was little effect in the absence of sensitizers. The
addition of acetone or acetaldehyde led to epoxidation; no caged
products were formed. Aldrin in rice paddy water was epoxidized
but not in the absence of irradiation. Ross & Crosby (1985) showed
that a series of amino acids present in natural waters and even
humic acids were capable of initiating photooxidation of aldrin to
dieldrin in natural sunlight.
Further evidence for the role of oxidants in the photo-
transformation of aldrin was reported by Draper & Crosby (1984).
4.5.1.2 Photochemistry of aldrin and dieldrin in air
As noted by Miller & Zepp (1983), data on the atmospheric
photodegradation of aldrin and dieldrin are sparse. Turner et al.
(1977) reported small levels of photodieldrin above a field of
grass that had been treated with dieldrin, but considered that it
had arisen from volatilization of the photodieldrin from the
foliage rather than from formation in the air itself.
In their studies on the occurrence of dieldrin and its
photoisomer in the atmosphere on a global scale, Baldwin et al.
(1977) reported detectable levels of dieldrin (0.35 ng/m3) but were
unable to detect any photodieldrin (limit of determination of
approximately 0.1 ng/m3) and considered, therefore, that
photodieldrin does not accumulate in the atmosphere.
4.5.1.3 Photochemistry of aldrin and dieldrin on plant surfaces
MacCuaig (1975) reported substantial conversion of dieldrin to
photodieldrin on the leaves of plants growing in areas of Africa
sprayed for locust control. In a more detailed study, Turner et
al. (1977) reported the formation of photodieldrin on grass that
had been sprayed with dieldrin. They also found that it was lost
fairly readily from the foliage but were uncertain whether
evaporation was the sole cause.
Harrison et al. (1967) demonstrated the rapid epoxidation of
aldrin to dieldrin on apple leaves. Ivie & Casida (1970) showed
that rotenone had a very marked effect on the rate of
transformation of leaf deposits to photodieldrin and found its
activity as a sensitizer to be some 100 times that of benzophenone.
4.5.1.4 Photochemistry of aldrin and dieldrin in soils
Lotz et al. (1983) studied the irradiation of aldrin on a
series of mineral substrates. The substrate had a marked influence
on the rate of aldrin loss, river sand showing the greatest effect.
El Beit et al. (1983) irradiated dieldrin in contact with various
substrates and found that degradation was less in the case of a
clay soil than a glass surface. However, the relevance of some of
the laboratory studies to the practical situation is questionable
because of the frequent use of very hard UV as the radiation
source.
It appears that photodieldrin does not occur in large amounts
in aldrin-treated soil. Lichtenstein et al. (1970) treated a field
soil with very high levels of aldrin and found that 98 - 99% of the
surviving residues 6 or 10 years after the last treatment were in
the form of dieldrin. Photodieldrin formed 1.6% of the dieldrin
residue. Suzuki et al. (1974) sampled 52 soils with a history of
aldrin treatment in Japan and measured dieldrin levels ranging from
0.002 to 1.73 mg/kg and photodieldrin levels ranging from < 0.001
to 0.035 mg/kg.
4.5.1.5 Conclusions
The photochemistry of aldrin and dieldrin has been intensively
studied and it seems that the use of dieldrin for certain disease
vector control operations could lead to photodieldrin formation,
although its persistence seems uncertain. Current uses would seem
unlikely to represent a significant source, and it is doubtful
whether photodieldrin occurs widely in the environment.
4.5.2. Other abiotic processes
4.5.2.1 Reaction with ozone
Ross et al. (1976) reported that ozonization of water
contaminated with dieldrin led to substantial reductions in
dieldrin levels and suggested that this process could be used
commercially to help clean-up contaminated water.
4.5.2.2 Clay-catalysed decomposition
Fowkes et al. (1960) showed that clay diluents in the dust
formulations of many pesticides caused decomposition. In the case
of aldrin and dieldrin, the most pronounced reactions occurred with
kaolinite and attapulgite, especially when they were acidic. In
the case of kaolinite at 65 °C, the half-life of dieldrin was 400
min, which was reduced to only 30 min when the kaolinite had been
acidified. Although, these effects were observed at relatively
high temperatures, it is possible that this type of decomposition
could be significant in the soil environment, though evidence for
this has not been reported.
4.6. Bioaccumulation
The relationship between the bioaccumulation factor and the
partition coefficient (Kow) of a chemical between octanol and water
has been investigated intensively for a number of compounds. The
partition coefficient has been shown to be a useful preliminary
indicator of the tendency for a chemical to accumulate in
organisms, particularly aquatic ones. The partition coefficient of
hydrophobic compounds is usually given as its logarithm (log10
Kow). The values reported for aldrin and dieldrin (Briggs, 1981)
are 7.4 and 6.2, respectively.
Estimates of the bioaccumulation factors for aquatic organisms,
determined under controlled laboratory conditions, are given in
Table 2.
Aldrin bioaccumulates and biomagnifies mainly in the form of
its conversion products. In one model ecosystem study (Metcalf et
al., 1973), conversion to dieldrin occurred rapidly and nearly
quantitatively. Only 0.5% of the original radioactive aldrin was
stored as aldrin in the mosquitofish (Gambusia affinis), which was
the organism at the top of this model food chain.
The uptake of dieldrin from water (0.1 - 1 mg/litre), after
4 h, by three species each of fungi, streptomycetes, and bacteria
gave ratios for the concentration of dieldrin in cells or mycelia
to that in the supernatant ranging from 0.3 to more than 100. The
rate of uptake of dieldrin by mycelia of Streptomyces venezuelae
and Trichoderma viride was very rapid, reaching equilibrium after
about 15 min (Chacko & Lockwood, 1967).
Table 2. Bioaccumulation of dieldrin
-----------------------------------------------------------------------------------------
Species Concentration in Duration Bioaccumulation Reference
water (µg/litre) of factor
or food (mg/kg) exposure
-----------------------------------------------------------------------------------------
Guppy 0.8, 2.3, or 4.2 32 days whole fish: 12 500 Reinert (1972)
(Poecilia reticulata)
Sailfin molly 0.075 34 weeks muscle: 3900 Lane &
(Poecilia latipinna) gill: 50 100 Livingston
(1970)
1.5 34 weeks muscle: 4900 Lane &
gill: 36 400 Livingston
(1970)
Channel catfish 0.013 70 days dorsal muscle: 2400 Shannon
(Ictalurus punctatus) 0.027 70 days 1800 (1977a)
0.049 70 days 3300
small 0.075 28 days dorsal muscle: 2300 Shannon
large 0.075 28 days 3600 (1977b)
small 2 mg/kg food 28 days 0.27
large 2 mg/kg food 28 days 0.62
Sculpins 0.017, 0.17, or 32 days whole fish: 13 300 Chadwick &
(Cottus perplexus) 0.86 Brocksen
(1969)
Alga 1, 5, or 20 14 days 1300 (based on dry Reinert (1972)
(Scenedesmus obliquus) weight of alga)
Waterflea 2.1, 4.5, or 6 days 14 000 (dry weight) Reinert (1972)
(Daphnia magna) 12.8
Common frog
(Rana temporaria) 0.8 2 days whole body 387.5 Cooke (1972)
Common toad 20 2 days whole body 280 Cooke (1972)
(Bufo bufo)
Barn owl 0.5 mg/kg food 2 years carcass: 18.8 Mendenhall et
(Tyto alba) al. (1983)
Short-tailed shrew 50 mg/kg food 17 days carcass: 1.6 Blus (1978)
(Blerina brevicauda)
Mink 2.5 mg/kg food 4-10 weeks fat: 8.4 Aulerich et
(Mustela vision) al. (1972)
-----------------------------------------------------------------------------------------
The uptake of 14C-dieldrin by Chlorella pyrenoidosa or by six
species of marine algae (Skeletonema costatum, Tetraselmis chuii,
Isochrysis galbana, Olisthodiscus luteus, cyclotella nana,
Amphidinium carteri) has been studied. In Chlorella pyrenoidosa,
rapid penetration of algal cells occurred and a maximum
radioactivity was reached after 6 - 24 h, whereas in the six marine
algae, it was reached within 1 h. From the study on Chlorella, it
was concluded that the movement of dieldrin into subcellular
organelles occurs within 72 h, and that algae are scavengers of
dieldrin. The study on the six marine algae showed that there was
no correlation between the dieldrin accumulation in the different
algae and the number of cells per ml culture. However, the amount
accumulated was related to the concentration of dieldrin in the
culture (range, 1 - 1000 µg/litre), and, for each algal species, to
the number of cells per culture. No metabolites were detected
(Wheeler, 1970; Rice & Sikka, 1973).
In studies by Jefferies & Davis (1968), medium size worms
(Lumbricus terrestris) were placed in containers, and water and
dieldrin-treated compost were added to give a final concentration
of 25 mg dieldrin (nominal)/kg moist compost. The containers were
kept at 10 °C for 20 days, and the worms were then collected. The
average concentration of dieldrin in six batches of worms ranged
from 18.4 - 24.9 mg/kg live weight of worms.
When two species of earthworms (Lumbricus terrestris and
Allolobophora caliginosa) were placed in containers with compost
containing 17 mg dieldrin/kg for 4 weeks at 10 °C, the mean
concentration of dieldrin in Lumbricus terrestris (two studies) was
13.3 mg/kg live weight. The gut content of L. terrestris was
determined using worms kept in compost (32 mg dieldrin/kg) for 20
days. The mean concentration of dieldrin in whole worms was 13.8
mg/kg live weight, the air-dried gut contents constituted 11.3% of
the total live weight, and the mean dieldrin concentration in the
tissues of the dissected worms was 10.8 mg/kg tissue. The uptake
of dieldrin by L. terrestris was compared with that by A.
caliginosa; after 4 weeks, the concentration of dieldrin in A.
caliginosa (27.3 mg/kg) was more than twice that in L. terrestris.
The concentrations of dieldrin in A. caliginosa placed in five
different soils for 4 weeks are given in Table 3 (Davis, 1971).
Table 3. The concentration of dieldrin in A. caliginosa placed in five different
soils for 4 weeksa
-------------------------------------------------------------------------------------
Soil type Estimated concentration Organic matter Mean concentration of dieldrin
of dieldrin (mg/kg air- (% w/v) in A. caliginosa (mg/kg)
dried soil)
-------------------------------------------------------------------------------------
Peaty loam 3.1 30.1 0.23
Organic loam 2.7 6.6 0.78
Loamy sand 1.7 1.3 2.99
Silty loam 2.2 2.8 3.56
Clay loam 2.0 1.7 4.55
-------------------------------------------------------------------------------------
a From: Davis (1971).
A number of field studies have been carried out in which the
concentrations of aldrin and dieldrin in earthworms from fields
treated with aldrin were determined. Six species of earthworms
were collected from a field to which excessive applications of
aldrin had been made for 8 years, and two species from experimental
plots to which dieldrin had been applied (single treatment).
Samples of soil and earthworms from the aldrin-treated fields were
analysed for aldrin and dieldrin, and the mean concentrations in
the worms are given in Table 4. The overall mean geometric
concentrations in soil (dry weight) were 0.72 mg/kg (aldrin) and
0.64 mg/kg (dieldrin). It was suggested that residual soil in the
gut may have contributed appreciably to the residues of aldrin in
the earthworms. The low residues in L. terrestris, relative to the
other species, were attributed to the deeper burrowing behaviour of
this species, which enable it to live in non-treated layers of soil
for part of its life. The concentrations of dieldrin in the soil
and earthworms from the experimental plots 6 months after treatment
with dieldrin are given in Table 5. The relationship between the
concentration of dieldrin in the two species of earthworms and the
concentration in the soil was thought to be given by the function,
W = aSb, where W is the concentration of dieldrin in the earthworm
and S the concentration in the soil. The fact that the estimated
value of b (0.794) was significantly less than unity indicates that
residues tend to be relatively greater in worms when the
concentrations in the soil are low than when higher concentrations
are present (Wheatley & Hardman, 1968).
Table 4. Mean concentrations of aldrin and
dieldrin in six species of earthworms from
aldrin-treated fields
--------------------------------------------
Species Geometric mean concentration
(mg/kg wet weight)
Aldrin Dieldrin
--------------------------------------------
L. terrestris 0.053 1.6
A. longa 0.28 2.2
A. caliginosa 0.52 3.8
A. chlorotica 0.98 4.6
A. rosea 0.64 3.9
O. cyaneuma 0.84 2.4
--------------------------------------------
a One sample only.
Beyer & Gish (1980) measured the concentrations of dieldrin in
four species of earthworms collected from a depth of 0 - 50 cm in
plots that had received a single surface application of a dieldrin
wettable powder (0.6, 2.2, or 9 kg dieldrin/ha). Samples of
earthworms were collected over a period of 11 years, and the
following relationship was derived between the concentration of
dieldrin in the worms and the time interval between dieldrin
application and worm collection:
C(n) = aEbn
where C(n) is the concentration in the earthworms n years after
soil treatment, and a and b are constants calculated from the data.
The mean values of a and b were as follows:
Application rate a b
(kg dieldrin/ha)
0.6 7.8 -0.41
2.2 21 -0.32
9.0 53.5 -0.16
The average time required for the initial residues of dieldrin in
soil to be reduced by 50% was 5.1 years, and the corresponding time
for dieldrin in earthworms was 2.6 years (Beyer & Gish, 1980).
Table 5. Concentrations of dieldrin in the soil and earthworms
from experimental plots 6 months after treatment with aldrin
---------------------------------------------------------------
Applied dieldrin Concentrations of dieldrina (mg/kg)
(kg/ha) (nominal) Soilb A. longac A. chloroticac
(dry weight)
---------------------------------------------------------------
0 0.003 0.033 0.028
0.50 0.50 0.70 1.8
0.75 0.85 1.0 2.0
1.0 1.1 1.3 2.9
1.25 1.2 1.3 2.1
---------------------------------------------------------------
a Geometric means.
b Soil samples taken 6 weeks before earthworm samples.
c Wet weight.
In studies by Gish & Hughes (1982), small experimental pasture
plots were sprayed with a suspension of a dieldrin wettable powder
at application rates of 0.56, 2.24, or 8.97 kg dieldrin/ha.
Samples of soil and earthworms were collected on 12 occasions over
a period of 2 years, the soil being sampled to a depth of 2.5 cm.
The concentration of dieldrin in the soil did not decline during
the 2-year period, but that in the earthworms from the two plots
treated at the two lower rates declined significantly. The maximum
concentration of dieldrin in the earthworms occurred 4 months after
treatment. The ratios of dieldrin concentration in earthworms to
that in the soil were examined. Residues in earthworms averaged
166 times those in soil in the sampling period 4 months after
application when earthworm residues reached a maximum. The effects
of several variables on the concentration of dieldrin in earthworms
was investigated, and a multiple regression relationship,
incorporating five variables, accounted for about 77.2% of the
variability of the residues in earthworms.
The accumulation of dieldrin in live fish-food organisms,
tubificid worms, and midge larvae (Chironomidae) was investigated
by Chadwick & Brocksen (1969), in Daphnia magna by Johnston et al.
(1971) and Reinert (1972), in crab (Leptodius floridanus) and
Artemia salina nauplii by Epifanio (1973), in mollusc (Rangia
cuneata) and blue crab (Callinectes sapidus) by Petrocelli et al.
(1973, 1975), in oyster (Crassostrea virginica) by Mason & Rowe
(1976) and Emanuelsen et al. (1978), and in an ostracod
(Chlamydotheca arcuata) by Kawatski & Schmulbach (1972). These
studies were carried out at concentrations (in fresh water or sea
water) of 0.5 - 100 µg/litre or by feeding feed or organism
containing aldrin or dieldrin. The duration of the studies was a
few days up to 43 days. In all organisms, there was a rapid
increase of dieldrin concentration in organs and tissues. A steady
state was reached after 3 - 4 and 2 days, respectively, in Daphnia
magna and Cassostrea virginica. In all organisms tested, the
elimination was slow and the half-life of dieldrin for tubificed
worms and Crassostrea virginica was approximately 16 days and 75 h,
respectively.
The rate of insecticide accumulation is partly dependent on the
concentration in the water, the duration of exposure, and the
activity of the animals. The concurrent feeding of aldrin- or
dieldrin-containing feed did not have a significant effect on
dieldrin accumulation. It can be concluded that water is the
principle source of dieldrin accumulation (Kawatski & Schmulbach
1972; Reinert, 1972; Epifanio, 1973).
A number of studies on different species have been carried out
by Gakstatter (1968) (Carassius auratus), Chadwick & Brocksen
(1969) (Cottus perplexus), Lane & Livingston (1970) (Poecilia
latipinna), Hogan & Roelofs (1971) (Lepomis cyanellus), Ludke et
al. (1972) (Notemigonus chrysoleucas, Gambusia affinis, Lepomis
cyanellus, L. macrochirus, Ictalurus natalis), Reinert (1972)
(Poecilia reticulata), Wells et al. (1973) (Gambusia affinis),
Wells & Yarbrough (1973) (Gambusia affinis), Addision et al.
(1976), (Salmo salar), and Shannon (1977a,b) (Ictalurus
punctatus). In these studies, dieldrin was added to the water at
different concentrations, and in a few of the studies the dieldrin
was radiolabelled. Distribution and accumulation were examined in
various organs and tissues (section 6.3.1.3).
Chadwick & Brocksen (1969) found that the accumulation of
dieldrin in whole fish (sculpins) was related to the concentration
in the water (0.017 - 8.6 µg/litre) and appeared to reach a steady
state by day 32. Reinert (1972) found such a state after only 17
days in Poecilia reticulata. Shannon (1977a) studied this aspect
in channel catfish (Ictalurus punctatus) (length 15 cm) exposed
continuously to 0.013, 0.027, or 0.049 µg dieldrin/litre. The
concentration of dieldrin in dorsal muscle increased in a
curvilinear fashion. Little change occurred within 56 days in the
two lower exposure groups, but a significant increase occurred in
the 0.049 µg/litre group. Steady-state concentrations appear to
have been established in the dorsal muscle of the fish exposed to
the two lower concentrations (but not in those exposed to 0.049
µg/litre) after 56 - 70 days.
Feeding studies using dieldrin-contaminated tubificid worms
(25 - 350 mg/kg) as food source showed that the retention of
dieldrin by sculpins was inversely related to the amount of
dieldrin they consumed. However, sculpins fed worms containing
0.4 - 26 mg dieldrin/kg did not show this relationship. It was
suggested that the metabolism and excretion of dieldrin was
stimulated at the higher concentrations. The findings showed that
a maximum of 16% of the dieldrin accumulated would have come from
the contaminated food. Thus dieldrin is accumulated in fish far
more readily from water than from food (Chadwick & Brocksen, 1969;
Reinert, 1972).
In studies on sailfin molly (Poecilia latipinna), exposed to
concentrations of 0.75 and 1.5 µg/litre for 34 weeks (flow-through
system), Lane & Livingston (1970) found that the ratio of the
concentration of dieldrin in the tissues to that in water in the
steady state was about 10 000.
From a study on green sunfish (Lepomis cyanelles) that were
exposed to dieldrin at 6 µg/litre for 124 - 139 h, it was concluded
that the lethal concentrations of dieldrin in blood and brain were
approximately 6 and 9 mg/kg tissue, respectively (Hogan & Roelofs,
1971).
Shannon (1977b) exposed channel catfish to 0.075 µg
dieldrin/litre water and/or 2 mg dieldrin/kg food for 28 days.
Small (15 - 22.5 cm) and larger fish (3 - 40 cm) were used and
dorsal muscle of the fish was analysed. After 28 days, fish
exposed to 0.075 µg/litre had a mean concentration of dieldrin in
muscle of 0.175 (small fish) and 0.274 (large fish) mg/kg tissue,
fish fed 2 mg dieldrin/kg contained 0.544 (small fish) and 1.243
(large fish) mg/kg tissue, and those given the combined treatment
contained 0.898 (small fish) and 2.418 (large fish) mg/kg tissue.
The elimination of dieldrin from the dorsal muscle in clean water
showed that when fish were exposed to dieldrin in water only, a 50%
decrease took place in 8 days. For fish exposed to dietary or
combined exposure, it required 20 days.
In a study with different early-life stages of rainbow trout,
the bioconcentration factor in the different stages was determined
using 14C-dieldrin. It increased during embryonic development from
120, reached a maximum at the sac fry stage of 12 000 and fell
again at the early fry stage to 1500. The clearance rate constant
sharply increased at the early fry stage. Almost all the dieldrin
was recovered from the yolk (Van Leeuwen, 1986).
The yolks of eggs from chickens fed aldrin or dieldrin
(1 mg/kg) or 10 mg dieldrin/kg for 2 years contained dieldrin
concentrations of 6 - 25 mg/kg (Brown et al., 1965). Several other
studies on the accumulation of dieldrin into avian eggs have been
made, details of these being given in Tables 17 and 18 (section
5.1.6).
Clark (1975) fed red-winged blackbirds (Agelaius phoeniceus) a
diet containing 10 mg aldrin/kg, some of the birds being
artificially stressed. The mean number of days that the birds
survived was 29.9 for unstressed and 22 for stressed birds. The
mean values of brain residue levels at death were 19.8 mg
dieldrin/kg for unstressed birds and 22.2 mg dieldrin/kg for
stressed birds. Three unstressed birds, sacrificed after 76 days,
had dieldrin levels of 6.7, 7.28, and 7.4 mg/kg. The carcass
levels of dieldrin increased linearly with time and showed no
tendency to level off, as occurred in the brains of unstressed
birds. The three unstressed birds sacrificed had the highest
carcass dieldrin levels (70.3, 82.8, and 147 mg/kg).
Stickel et al. (1969) fed Japanese quail (Coturnix coturnix
japonica) diets containing 2, 10, 50, or 250 mg/kg dieldrin for up
to 158 days. The mean dieldrin levels in the brain of dead and
sacrificed birds were 18.25 mg/kg and 3.35 mg/kg (wet weight),
respectively, while the mean liver residues were 19.7 mg/kg (wet
weight) and 28.8 mg/kg (wet weight), respectively.
Mendenhall et al. (1983) fed captive barn owls (Tyto alba)
with diets containing 0.5 mg/kg dieldrin for 2 years. The mean
carcass residues were 9.4 mg/kg (wet weight) after 2 years, and the
mean dieldrin levels in eggs were 3.6 mg/kg in the first year and
8.1 mg/kg in the second.
Enderson & Berger (1970) fed each of three captive female
prairie falcons (Falco mexicanus) with 11 starlings, one per day.
The starlings had been treated for 14 days with 10 mg/kg dieldrin
in their diet. One bird died and showed levels of dieldrin in
brain, liver, and muscle of 11, 29, and 4.6 mg/kg (wet weight),
respectively. The other two were sampled and found to have mean
adipose tissue and brain levels of 532 and 5.84 mg/kg,
respectively. The authors also fed wild falcons for 6 weeks prior
to egg laying. The analysis of one egg from each clutch showed a
mean egg dieldrin content of 41.5 mg/kg, and the mean adipose
tissue level of dieldrin in dosed adult falcon, was 83 mg/kg
dieldrin.
Turtles (Pseudemys scripta elegans) were given intraperitoneal
injections of dieldrin (20 mg/kg body weight) and the accumulation
in organs and tissues was determined over a period of 70 days. The
turtles were fasted during the study. The rate of absorption of
dieldrin into the tissues was slow, and there were no clear
indications of an approach to steady-state concentrations by day
70. The highest levels of dieldrin were found in body fat and
liver, and the levels in plasma and brain were also high (Pearson
et al., 1973).
Cooke (1972) studied the effect of dieldrin at nominal
concentrations of 0.0008, 0.02, or 0.5 mg/litre on groups of 40
common frog (Rana temporaria) tadpoles with hindlimb paddles or
hind legs. The exposure lasted 24 or 48 h in amphibian saline. At
the highest dose level the mean dieldrin content after 48 h
exposure was 42.9 mg/kg tissue. At the dose levels of 0.0008 and
0.02 mg/litre, there were 0.31 and 6.1 mg/kg dieldrin in tissues,
respectively. Toad (Bufo bufo) tadpoles exposed to 0.02 or 0.5
mg/litre contained 138 mg dieldrin/kg tissue at the higher dose
level, after 48 h, and 5.6 mg/kg at the lower.
A laboratory study was undertaken concerning the lethal brain
levels for dieldrin in adult and juvenile brown bats (Myotis
lucifugus), using 47 female bats collected from a church attic in
Maryland, USA. Meal worms containing an average of 0.38 mg
dieldrin/kg (wet weight) were fed to the bats for 52 days, and then
untreated worms were administered for another 22 days. The amount
of dieldrin in bats increased during dosing and decreased
afterwards. These changes did not appear as changes in average
dieldrin concentrations in the fat because the amounts were highly
variable. During the exposure period a continuous build up of the
concentration in fat was seen, but an equilibrium was not reached.
The initial half-life for dieldrin loss was estimated to be 24
days. Measurable dieldrin was found in the brains of only 6 out of
47 bats. The levels measured (0.5 to 0.9 mg/kg tissue) were all
far below lethal levels. The highest dieldrin level determined in
the carcass of 37 bats was 110 mg/kg tissue (lipid weight) (Clark &
Prouty, 1984).
Short-tailed shrews (Blerina brevicauda) were fed diets
containing dieldrin (nominal concentrations of 50, 100, or 200
mg/kg diet) for up to 14 days. All of the animals fed 50 mg/kg
survived, but all those fed 200 mg/kg died. The mean dieldrin
concentration in the brains of 14 shrews that died was 6.8 mg/kg
(range, 3.7 - 12.6). Some animals sacrificed after 17 days of
feeding the 50 mg/kg diet contained mean residues in the brain of
1.8 mg/kg and in the carcass of 58 mg/kg. After 14 days on an
untreated diet, the concentrations in the carcass declined by 76%
in both sexes, and in the brain by 59% and 84% in males and
females, respectively. The half-life of dieldrin was estimated to
be less than 14 days (Blus, 1978).
Male mink (3 months old) were fed a diet containing dieldrin
(nominal concentrations of 0 and 2.5 mg/kg diet) for 10 weeks, and
samples of abdominal fat were taken by biopsy at two-weekly
intervals. The mean concentration of dieldrin after 2 weeks was
12.5 mg/kg body fat. For weeks 4 - 10, an average concentration of
21 mg was found, a steady state being reached after approximately 4
weeks (Aulerich et al., 1972).
4.7. The Fate of Aldrin and Dieldrin in the Environment
On the basis of the current uses of aldrin in agriculture, the
first point at which aldrin and dieldrin enter the environment is
the soil, dieldrin being derived from aldrin by biological
epoxidation. Understanding the fate of aldrin and dieldrin in the
environment, therefore, depends firstly on an understanding of its
behaviour in the soil.
4.7.1. Aldrin and dieldrin in soils
It was concluded in section 4.1.4 that the regular application
of aldrin to soils for the control of soil pests does not lead to
an indefinite accumulation in the soil. The results of a
considerable number of soil monitoring studies, summarized in
Table 1, support this conclusion. Some of the individual
monitoring studies are discussed at greater length in this section.
Carey et al. (1973) monitored residues of aldrin and dieldrin
over a very wide area of the corn belt in the USA in 1970, when the
use of aldrin on maize was probably close to its maximum and the
levels were representative of residues in a situation of continuing
use. Average values for aldrin plus dieldrin, recalculated for
samples that contained positive residues, ranged from 0.05 to 0.87
mg/kg for each of the twelve states. The maximum levels for the
whole study were 2.98 mg aldrin/kg and 2.04 mg dieldrin/kg (these
values were not both derived from the same sample). In many cases
the residues had come from relatively recent applications, as may
be judged from the comparatively high proportion of aldrin still
remaining. The average was greater than 50% of the combined
residues in four of the twelve states, so that many of the samples
were probably taken from soils in the same year in which they were
treated.
Carey et al. (1980) carried out a further study on rice soils
in the USA during the year 1972. At that time, aldrin was used
extensively as a rice seed dressing and, according to the data
presented by Sparr et al. (1966), overall application rates of
aldrin would have been between 0.2 and 0.4 kg/ha. Between 50% and
100% (depending on the state) of the land sampled had received
aldrin-dressed seed. As in the case of the maize data, figures for
aldrin and dieldrin were presented separately and not paired, so
that total residue levels are difficult to deduce. The average
level for aldrin was only about 0.02 mg/kg soil and for dieldrin
was 0.05 mg/kg, although there were occasional samples that reached
0.25 mg/kg for either aldrin or dieldrin. According to the
information presented earlier, degradation of aldrin occurs more
readily in the anaerobic conditions of a rice paddy than in fully
aerobic soils, and this may have contributed to the much lower
level of residues surviving in rice compared with maize. However,
it should also be remembered that the initial rates of application
were substantially lower in rice than in maize.
In Canada, Harris et al. (1966) reported a series of data for
soils in S.W. Ontario and there was limited information on the
treatment history of the soils sampled. About a half of the soils
showed residues, and these ranged from < 0.01 to 1.5 mg/kg for
aldrin plus dieldrin residues. One high figure of 3.5 mg/kg seems
anomalous in that it was reported from land that had no treatment
history with aldrin or dieldrin. With the exception of this
anomalous sample, there was no evidence for accumulation. Fairly
similar results were reported by Duffy & Wong (1967), who sampled a
series of vegetable-growing areas in Canada in 1965. In cases
where it was reported that aldrin or dieldrin had been used
(sometimes over a period of several years) residues were mostly
below 2 mg/kg.
None of these studies mentioned the occurrence of any dieldrin
degradation products, in particular photodieldrin, and yet this
would presumably have been detected had it been present. This,
taken in conjunction with the work of Suzuki et al. (1974) (section
4.4.2) would seem to be useful evidence that photodieldrin does
not, to any appreciable extent, represent a terminal metabolite of
aldrin in the soil.
4.7.2. Aldrin and dieldrin in the atmosphere
The relative contributions of the various mechanisms for the
loss of aldrin and dieldrin from the soil have not been estimated
(as far as can be judged from the literature) but, as mentioned in
section 4.1.3, volatilization is usually considered to be the major
loss route. Consequently, the occurrence of aldrin or dieldrin
vapour in the atmosphere has been the subject of considerable
study.
Spencer & Cliath (1975) considered that many pesticides enter
the atmosphere after application. This occurs by volatilization
during spraying, from treated crops or soils, or from dust from
treated soil surfaces blown up by the wind. These routes are
difficult to quantify, and only sparse data are available, though a
few relating to dieldrin have been cited in section 4.1.3.
Small amounts of dieldrin have been detected in the atmosphere,
particularly in agricultural areas and, in one case, close to a
formulating plant (section 5.1.1.2). Aldrin has also been
detected, though relatively less often (section 5.1.1.1). There is
further information on the levels in the atmosphere of aldrin and
dieldrin in section 4.1.6.
4.7.3. Conclusion
In spite of the slow rate at which aldrin and dieldrin are lost
from soils when applied to them for insect control, there is no
evidence for their indefinite accumulation in the environment,
either in the soil itself, in water, or in the atmosphere. The
evidence suggests that photodegradation products do not accumulate
either.
Although there is evidence that a considerable proportion of
the aldrin and dieldrin used in agriculture reaches the atmosphere,
it seems probable that the degradation processes in the atmosphere
described by Glotfelty (1978) for pesticides in general operate to
prevent accumulation of aldrin and dieldrin.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental Levels
5.1.1 Air and rainwater
5.1.1.1 Aldrin
Residues of aldrin in the general atmosphere, either in the
vapour phase, adsorbed by dust particles, or in rainwater, have
been reported less frequently than for other organochlorine
insecticides. Concentrations in the range 0.1 - 4 ng/m3 have been
found in the air of agricultural communities (Tabor, 1966). In a
pilot survey in 1967 - 1968, out of nine localities in the USA,
only one sample from a total of 880 contained aldrin (8 ng/m3)
(Stanley et al., 1971). Aldrin was detected in 13.5% of 2479 air
samples collected from 16 states in the USA in the 3-year period
1970 - 1972. The mean value in these positive samples was 1.6
ng/m3 and the maximum was 24.6 ng/m3 (Kutz et al., 1976).
Aldrin was found at a level of 0.9 ng/m3 in 16 out of 66 air
samples taken within 800 m of two formulation plants in 1970 (Lewis
& Lee, 1976). One year later, 6 out of 60 samples were found to
contain a level of 1.5 ng/m3, and in 1972 no aldrin could be
detected.
5.1.1.2 Dieldrin
In a pilot survey in 1967 - 1968 in the USA, dieldrin was found
in 6% of the 880 samples analysed. The maximum level was 29.7
ng/m3 (Stanley et al., 1971).
In a survey mentioned above (Kutz et al., 1976), dieldrin was
detected in 94% of the 2479 samples. The mean value in these
positive samples was 1.7 ng/m3 and the maximum was 23.9 ng/m3.
A summary of the concentrations of dieldrin in air and
rainwater (washout from the air) is given in Table 6.
5.1.2. Concentrations in houses
5.1.2.1 Aldrin used for subterranean termite control
Aldrin EC (480 g/litre) was applied in a 0.5% solution as a
termiticide to typical slab and crawl-space type houses in
California, 1982. Samples of air were taken in the kitchen,
bedroom, and crawl-space at intervals up to 1 year after
application. Kitchen wipe samples were taken at the same time.
Air concentrations of aldrin in kitchen and bedrooms of the treated
houses showed transient peaks 24 h after treatment. In the slab-
type houses, concentrations were 0.04 - 0.27 µg/m3 and in the
crawl-space houses 0.09 - 7 µg/m3. These concentrations fell
rapidly. The air concentrations in slab houses were < 0.04 - 0.09
µg/m3 at day 28 and were < 0.05 µg/m3 at day 56, whereas those in
crawl-space houses were 0.06 - 1.5 µg/m3 at day 7, 0.04 - 0.36
µg/m3 at day 28, and 0.06 - 0.55 µg/m3 at day 56. One year after
application the air concentration of aldrin was 0.08 µg/m3 or less,
whereas dieldrin was not detected in any of the samples of air.
The concentrations of aldrin in surface wipes from kitchens showed
transient peaks 7 days after treatment, the concentrations being
higher in wipes from crawl-space houses (0.09 µg/m2) than from slab
houses (0.012 µg/m2). Between day 28 and day 56 the concentrations
remained at about 0.04 µg/m2 in crawl-space houses and 0.002 µg/m2
in slab houses. One year after application, aldrin and dieldrin
were not detectable in the kitchen wipe samples from the slab
houses, while in the samples from the crawl-space houses the
concentration of aldrin amounted to 0.04 µg/m2 and that of dieldrin
to 0.018 µg/m2 (Marlow et al., 1982; Marlow & Wallace, 1983).
5.1.2.2 Aldrin and dieldrin used for remedial treatment of wood
One to ten years after the remedial treatment of inside wood in
houses with dieldrin, its concentration in the air was measured.
Forty samples from 16 houses in the United Kingdom, covering a wide
range of construction type, size, and occupational pattern, were
analysed. The concentrations of dieldrin in the air in all
interior areas other than roof-voids were between 0.01 and 0.51
µg/m3, and in roof-voids, they were between 0.03 and 2.7 µg/m3
(Dobbs & Williams, 1983).
Paton et al. (1984) observed that aldrin and dieldrin migrated
from treated laminated timber and plywood, used as structural
components of commercial containers, into flour in polyethylene
bags or metal tubes that were stored in the containers for up to 40
days. The migration was thought to occur through contact with the
floor or sorption from the atmosphere. The residues in the flour
varied widely depending on temperature, type of packaging material,
and location of the flour in the container.
5.1.3. Aquatic environment
Dieldrin occurs more commonly in the aquatic environment than
does aldrin, albeit at very low concentrations. The major sources
of contamination of rivers, etc., by aldrin and dieldrin are
industrial effluents (manufacturing, formulation, and moth-proofing
in the textile industry) and soil erosion during agricultural usage
(Lichtenstein et al., 1962; Park & McKone, 1966; Epstein & Grant,
1968; Eye, 1968; Croll, 1969; Lowden et al., 1969; Rowe et al.,
1971; Burns et al., 1975; Brown et al., 1979). Local use appears
to contribute to the presence of dieldrin in sediments in urban
areas (Mattraw, 1975). In the USA, the Environmental Protection
Agency found that between 118 kg and 14.2 tonnes of dieldrin was
carried in the Mississippi River past St. Louis each year.
Although this is certainly not the case today, it does illustrate
the contribution of run-off to the pesticide load in river systems.
This is of special concern with dieldrin because of its stability
in water (Eichelberger & Lichtenberg, 1971).
Table 6. Concentration of dieldrin in air, rainwater, and dust
----------------------------------------------------------------------------------------------------------------
Location Year Number Medium Analyticala Mean Comments Reference
of procedure concentration
samples (range)
----------------------------------------------------------------------------------------------------------------
Netherlands
Delft 1979-81 55 air glass fibre 0.073 ng/m3 24-h samples Guicherit
filter; GC/EC (maximum, 370 & Schulting
ng/m3) aldrin: (1985)
0.039 ng/m3
(maximum, 640
ng/m3)
United Kingdom
Wellesbourne 1964-65 11 rain- TLC followed by 24 ng/litre samples of monthly Wheatley
water GLC/EC (10-36) rainfall & Hardman
(1965)
1965 5 9 ng/litre samples collected
(3-16) during periods of
prolonged rainfall
London 1965 11 rain- GLC/EC 42 ng/litre samples of monthly Abbott et
water (10-95) rainfall; 2 sampling al. (1965)
sites; limit of
determination: 10
ng/litre
London 1965 1 air TLC followed by 20 ng/kg Abbott et
GLC/EC al. (1966)
United 1966-67 28 rain- alumina column 8 ng/litre samples from 7 Tarrant
Kingdom water chromatography (1-35) locations during 12 & Tatton
and TLC followed months; limit of (1968)
by GLC/EC determination:
1 ng/litre
----------------------------------------------------------------------------------------------------------------
Table 6. (contd.)
----------------------------------------------------------------------------------------------------------------
Location Year Number Medium Analyticala Mean Comments Reference
of procedure concentration
samples (range)
----------------------------------------------------------------------------------------------------------------
USA
Cincinnati 1965 1 dust florisil column 3 ng/g dust source of the dust was Cohen &
washed chromatography the Southern High Pinkerton
out by followed by Plains, approximately (1966)
gentle GLC/EC 1500 km southwest of
rain Cincinnati
16 states 1970-72 2479 air florisil column 1.7 ng/m3 limit of determination: Kutz et al.
chromatography (1-23.9) 1-10 ng/m3 (1976)
followed by
GLC/EC
Hawaii 1970-71 5 rain- GLC/EC 5 ng/litre Bevenue et
water (1-27) al. (1972a)
1971-72 14 12 ng/litre Bevenue et
(1-97) al. (1972b)
West Indies
Barbados 1965-66 15 dust TLC followed by 2.2 ng/g dust samples of dust Risebrough
GLC/EC (1-8.1) collected in nylon et al. (1968)
screens; limit of
determination:
1 ng/g (?)
Barbados July 12 air- silicic acid 49 ng/m3 air source of dust in air Prospero &
1970 borne column (1-190) probably North Africa Seba (1972)
dust chromatography
followed by
GLC/EC
----------------------------------------------------------------------------------------------------------------
Table 6. (contd.)
----------------------------------------------------------------------------------------------------------------
Location Year Number Medium Analyticala Mean Comments Reference
of procedure concentration
samples (range)
----------------------------------------------------------------------------------------------------------------
Ireland
Bantry Bay 1973 17 air florisil column 0.36 ng/m3 aldrin and photo- Baldwin et
chromatography (0.06-1.6) dieldrin less than al. (1977)
followed by limits of detection
GLC/EC (0.1 ng/kg); origin
of air samples either
westerly from Atlantic
Ocean (13 occasions)
or easterly from
continental Europe
(4 occasions)
----------------------------------------------------------------------------------------------------------------
a TLC = thin-layer chromatography; GC/EC = gas chromatography/electron capture detection; GLC/EC = gas-liquid
chromatography/electron capture detection.
Dieldrin has been detected in the northern Atlantic Ocean at a
mean concentration of 5.8 ng/litre (Jonas & Pfaender, 1976), and it
is of interest that the dieldrin concentration was apparently
unrelated to depth or distance from shore. It was suggested that
this may be the result of adsorption to particulate matter. The
identification of the component measured as dieldrin was based on
gas-liquid chromatographic behaviour (three different stationary
phases), but was not rigorously confirmed. Other investigators
(Harvey et al., 1973, 1974; Bidleman & Olney, 1974) did not report
the presence of dieldrin in the northern Atlantic Ocean, although
the analytical methods were appropriate for the detection of aldrin
and dieldrin.
Dieldrin residues (0.01 - 0.3 ng/litre) have also been reported
off the coast of Ireland, in the English Channel, and in the North
Sea (Dawson & Riley, 1977).
Low levels of dieldrin have been reported in surface waters
from several countries. The results of several surveys are
summarized in Table 7.
5.1.4. Soil
Aldrin is applied more frequently to the soil (directly or
indirectly) than dieldrin. However, as a result of the relatively
rapid conversion of aldrin to dieldrin, residues of dieldrin are
usually found more frequently in soil and at higher concentrations,
except shortly after the application of aldrin to soil. Sediment
residue levels tend to lie between those of soil and water, with
values up to 140 µg/kg) (Dawson & Riley, 1977). Dieldrin has been
reported in the sediments of Lake Ontario and Lake Superior (Frank
et al., 1974; Frank et al., 1981), rivers of the USA (Ryckman et
al., 1972), bays (Sheets et al., 1970), and off the coasts of
Ireland (Dawson & Riley, 1977). A summary of some of the results
of monitoring surveys was given in Table 1 (section 4.1.4). This
is not a comprehensive review of residues, but it indicates the
variations of the concentrations that occur in practice. These
results also illustrate the potential for absorption into root
crops, and for uptake by soil organisms.
5.1.5. Drinking-water
Studies on drinking-water in the USA have indicated dieldrin
residue values up to 8 µg/litre (Kraybill, 1977; Sandhu et al.,
1978). In a comprehensive study in the USA, dieldrin residues were
found in less than 17% of the samples with 78% of these positive
results lying within the range 4 - 10 ng/litre. The highest
residue level found in this study was 110 ng/litre. Dieldrin has
also been found in drinking-water in Canada (0.1 - 4 ng/litre)
(Williams et al., 1978) and in the Virgin Islands (average
concentration 0.19 µg/litre (Lenon et al., 1972).
Table 7. Concentrations of aldrin and dieldrin in the aquatic environment
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Argentina
Santa Fe 1981 surface water 4 4 (29) LD LD not defined; samples Lenardon et
and Parana (20 cm depth) collected twice monthly al. (1984)
(March-December)
suspended 4 150 ng/g LD occasional high residues of
solids (1625) aldrin attributable to local
source of application
(1966-68)
Canada
Ontario 1971 agricultural 2 1 (7) 11 (41) LD less than 1 ng/litre Miles & Harris
and urban (1973)
rivers
resort rivers 1 1 4 (11)
bottom mud 2 LD 0.9 LD less than 1 ng/g mud
(dry weight)
(4.5)
1 LD 0.9
(dry weight)
(1.4)
Nova Scotia 1972-73 river 7 (23 77 (670) 979 LD not defined; water, Burns et al.
samples) (11 800) probably less than 10 (1975)
ng/litre; sediment, probably
less than 1 ng/g
artesian wells 4 LD 10 (10)
holding pond 3 37 (40) 100 (200)
and reservoirs
natural 2 (4 90 (330) 17.5 (50) national drainage ditch in a
drainage samples) tobacco-growing area
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Canada (contd.)
Nova Scotia 1972-73 sediment from 25 31 (368) 4.9 (86) high residues in water and
stream bed sediments attributable to
soil erosion, particularly
during and after prolonged
heavy showers
sediment from 4 1088 670
natural (2220) (13 750)
drainage ditch
Lake 1974 filtered lake 34 LD 0.5 (0.5) LD: filtered water, less than Glooschenko
Superior water 0.5 ng/litre; sediment, less et al. (1976)
and Lake than 1 ng/g; dieldrin detected
Huron sediment 34 LD 0.1 mg/kg (0.5 ng/litre) in one sample
dry weight of filtered water and in 5
(0.1) samples of sediment (0.1 ng/g)
Greece
Northern 1981-82 coastal water 10 ND 0.55 (1.1) Fytianos et
Greece al. (1985)
United Kingdom
Kent and 1965-66 rivers 9 (224 LD (4) LD (59) LD less than 3 ng/litre; Croll (1969)
Essex samples aldrin found in one sample
collected only
at 2-weekly
intervals)
Great 1965-66 rivers 11 (75 LD 24.3 (423) high residues of dieldrin Croll (1969)
Britain samples attributable to effluent from
collected moth-proofing plants using
at 2-monthly dieldrin
intervals)
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Great 1965-67 rivers 15 LD 292 (2840) high residues of dieldrin Croll (1969)
Britain attributable to effluent from
moth-proofing plants using
dieldrin
Kent 1965-66 underground 11 LD LD Croll (1969)
water
Hereford- 1966 River Lee 6 LD LD LD less than 2 ng/litre Lowden et al.
shire (1969)
Yorkshire 1966-68 rivers 14 (30 LD 114 (650)
samples)
Birmingham 1966 sewage 24 LD 132 (1900) high concentrations of Lowden et al.
and effluent dieldrin attributable to (1969)
Hertford- industrial effluent from
shire moth-proofing plants
Yorkshire 1976-77 rivers 7 (18 LD 902 (4900) LD less than 1 ng/litre; high Brown et al.
samples) concentrations of dieldrin in (1979)
individual rivers (and sewage
sewage 1 sample LD 6240 effluent) attributable to the
effluent use of dieldrin for moth-
river 10 LD 124 ng/g proofing of wool
sediments (dry weight)
(432)
Netherlands 1969-75 raw water 11 (120 LD LD (50) unfiltered water to be used Greve (1972);
samples) for drinking-water preparation Wegman & Greve
(1978)
surface water 26 (1246 LD 10 (140) aldrin was detected Wegman & Greve
(depth about samples) occasionally at low (1978)
1 m) concentrations; limit of Greve (1972)
detection about 10 ng/litre
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Federal 1970-71 unfiltered 28 (119 LD LD (45) dieldrin reported in only one Herzel (1972)
Republic of surface water samples) sample of water
Germany
suspended 26 LD LD LD not given (appears to be
solids approximately 10 ng/litre)
USA
Major river 1965 surface water 99 LD 1 (100) LD 1 ng/litre; aldrin not Breidenbach et
basins detected in any sample; al. (1967)
dieldrin detected in 42% of
samples
Western USA 1965, rivers 11 0.2 (5) 2.3 (15) lower LD 5 ng/litre Brown &
1966 Nishioka
(1967)
1966-68 rivers 20 LD (40) LD (70) LD not defined; presumed to be Manigold &
about 10 ng/litre Schulze (1969)
Major river 1964-68 surface water about 100 LD range: 4-407 LD 2 ng/litre; in 37% of the Lichtenberg et
basins stations samples dieldrin was present al. (1970)
Iowa 1968 rivers 6 LD 2 (10) LD about 1 ng/litre; dieldrin Johnson &
1969 10 LD 8.5 (63) found in 40% of samples (179) Morris (1971)
1970 10 LD 9 (65) analysed
Western USA 1968-71 rivers 20 LD (10) LD (30) LD 5 ng/litre; aldrin detected Schulze et al.
in only 1 sample (total 716); (1973)
dieldrin detected in 5% of
samples
Hawaii 1970-71 non-potable 10 LD 4.8 (18.6) LD about 0.2 ng/litre Bevenue et al.
(1972a)
canals 3 LD 11.9 (18.6)
--------------------------------------------------------------------------------------------------------------------------
Table 7. (contd.)
--------------------------------------------------------------------------------------------------------------------------
Location Year Type Number Mean concentration Comments Reference
of of (ng/litre) (maximum)a
water sites aldrin dieldrin
--------------------------------------------------------------------------------------------------------------------------
Hawaii sewage 1 LD 198
(contd.) discharge
Gulf coast 1971 canal water 1 90 (440) drainage water from a rice Ginn & Fisher
of Texas field-marshland ecosystem; (1974)
samples (7) collected for 15
weeks after aldrin-dressed
rice seed had been planted;
one sample out of 7 contained
aldrin (270 ng/litre)
Lower surface water 1 LD 5 (10) LD not defined Brodtmann
Mississippi (samples (1976)
River taken
monthly)
Iowa 1974 surface water, 18 (104 not 12 (76) LD less than 0.5 ng/litre Richard et al.
rivers, and samples) reported (1975)
reservoirs
Western 1972 ocean surface 10 0.2 6.4 (19.4) lower LD: aldrin, less than Jonas &
North (0.2) 0.2 ng/litre; dieldrin, less Pfaender
Atlantic than 0.4 ng/litre; (1976)
50 m depth 7 LD 6.2 (9.8) concentrations of aldrin
below LD in 29 samples;
500 m depth 7 LD 3.7 (11.9) one surface sample contained
a component with the same
1000 m depth 6 LD 7 (18.3) retention time of aldrin,
corresponding to 0.2 ng/litre
USA 1976-80 rivers LD 610 occurrence of dieldrin in 2.4% Carey & Kutz
of samples (1985)
--------------------------------------------------------------------------------------------------------------------------
a Concentrations in bottom mud, sediments, and suspended solids (ng/g); LD = limit of detection; ND = not determined.
Maximum concentration indicated in parentheses.
5.1.6. Food and feed
Aldrin is rarely found in plants and animals, since it is
readily converted to dieldrin (IARC, 1974). A total-diet study of
Balby pensioners in Sweden did not detect any aldrin (Abdulla et
al., 1979). Similarly, a market-basket study in the USA in
1974 - 1975 (Johnson & Manske, 1977) found aldrin in only one
composite out of 240, with a value of 7 µg/kg. Traces or low
levels of aldrin have been found in vegetable products and meat
products (Balayannis, 1974; Saschenbrecker, 1976; Chaudry et al.,
1978; Wessels, 1978). In all cases, dieldrin residues were greater
than those of aldrin, even when aldrin was the only compound
applied. Aldrin is rarely found in milk or milk fat or in the body
fat of cows fed aldrin (Frank et al., 1985; Vreman & Poortvliet,
1982). In one study where aldrin was found in dairy products, milk
samples contained 0.04 mg/litre, butter samples 0.02 mg/kg, and
cheese samples 0.02 mg/kg (Heeschen, 1972). For the occurrence of
residues in breastmilk, see section 5.2.2.
The analytical procedures used in well conducted dietary
surveys are capable of detecting all of the commonly used
organochlorine pesticides, so that if aldrin did occur in a dietary
sample it would be detected. The lack of mention of aldrin,
therefore, can usually be taken as an indication that it was not
detected.
Dieldrin residues in food and feed, resulting from the
application of aldrin and dieldrin in normal use as well as from
field studies, have been reviewed by the FAO/WHO Joint Meeting on
Pesticide Residues (JMPR) at its meetings in 1963, 1965, 1966,
1967, 1968, 1969, 1970, 1974, 1975, and 1977 (FAO/WHO, 1964,
1965a,b, 1967a,b, 1968a,b, 1969a,b, 1970a,b, 1971a,b, 1975a,b,
1976a,b, 1978a,b).
Australia, Canada, Japan, the Netherlands, the United Kingdom,
and the USA have all reported daily intakes below the ADI (Duggan &
Lipscomb, 1969; Uyeta et al., 1971; Duggan & Corneliussen, 1972;
IARC, 1974; Smith, 1978; de Vos et al., 1984).
In Australia, Canada, Italy, Japan, the United Kingdom, and the
USA, analyses of total diets revealed dieldrin residues (Cummings,
1966; Duggan et al., 1967; Abbott et al., 1969; Corneliussen, 1972;
Duggan & Corneliussen, 1972; Johnson & Manske, 1977; Dick et al.,
1978; Smith, 1978) ranging from 0.06 mg/kg (Cummings, 1966;
Corneliussen, 1972) to 0.2 mg/kg (Duggan et al., 1967;
Corneliussen, 1970). In 1982 - 1983, dieldrin was determined in 73
typically composed, prepared daily meals in Switzerland, and was
detected in 46 of the meals. It was calculated from these results
that the average daily intake of the Swiss consumer was 0.9 ng/day.
The levels in 1971 - 1972 were 3.4 ng/day (Wüthrich et al., 1985).
Residue levels of up to 0.125 mg/kg in Canadian pork
(Saschenbrecker, 1976) and of 0.03 - 0.1 mg/kg in herring oil
(Addison et al., 1972) have been reported.
In a market-basket survey in 1974 - 1975, dieldrin was present
in only three food groups, with maximum residues of 5 µg/kg in
dairy products, 15 µg/kg in meat, fish, and poultry, and 8 µg/kg in
potatoes (Johnson & Manske, 1977).
The JMPR meeting in 1970 summarized data concerning dieldrin
residues from the feeding of dieldrin to cattle and poultry. The
average ratio of dieldrin levels in fat to levels in feed was
2.43:1 in milking cows and 3.95:1 in steers (Gannon et al., 1959a).
At intake rates of less than 1 mg/kg, the average ratio of dieldrin
levels in milk to levels in feed was about 0.1:1 after 12 weeks
(Gannon et al., 1959b; Williams et al., 1964). In Denmark, the
average concentration of dieldrin in butter fat declined from 0.05
mg/kg in 1964 to 0.03 mg/kg in 1966 and to 0.02 mg/kg in 1968
(Bro-Rasmussen et al., 1968). Similar residue levels and decreases
were found in Australia, Ireland, New Zealand, Norway, and the
United Kingdom. In Canada and the USA, residues in milk fat of
0.011 - 0.09 mg dieldrin/kg have been measured (Duggan et al.,
1967; Wedberg et al., 1978; Frank et al., 1985).
Dieldrin losses resulting from cooking or processing food can
be quite substantial, as demonstrated with trout and soybean
(Chaudry et al., 1978; Zabik et al., 1979).
More recent information on the occurrence of dieldrin residues
in foods is relatively scarce. However, a number of reviews exist.
5.1.6.1 Joint FAO/WHO Food Contamination Monitoring Programme
Information on dietary intakes of aldrin and dieldrin were
collected from seven collaborating centres participating in the
Joint FAO/WHO Food Contamination Monitoring Programme. The data
cover the period from 1971 - 1983, and the countries involved were
Australia, Canada, Guatemala, Japan, New Zealand, the United
Kingdom, and the USA. The mean daily intake during this period
varied from 0.007 to 0.056 µg/kg body weight (the 90th percentile
varied from 0.016 to 0.105 µg/kg body weight). During the later
years of this period, the mean values ranged from 7% to 56% of the
acceptable daily intake (ADI). A decrease in the dietary intake of
aldrin and dieldrin residues was noted during this period in some
of the countries. Possibly this decrease was the result of
restricting or banning the use of aldrin and dieldrin (Gorchev &
Jelinek, 1985).
5.1.6.2 Information summarized by GIFAP (1984)
Australia, 1980: Twenty-four samples of each of 50 different
foods were analysed for a range of organochlorine pesticides.
In the case of dieldrin, the limit of determination was 0.01
mg/kg. Dieldrin occurred above this level in only 0.04% of the
samples, and the maximum level was 0.05 mg/kg.
Canada, 1976 - 1978: The results of analyses of food
commodities were expressed in terms of estimated intakes for
the population. The average daily dietary intake of dieldrin
for this period was 0.002 µg/kg body weight.
Italy, 1982: Apples were sampled from a variety of locations
representing 70% of the country's apple production. There were
300 samples and 80% or 90% were below the limit of determination
for aldrin and dieldrin, respectively.
Netherlands, 1977 - 1978: During the period 1977 - 1978,
residues of organochlorine pesticides were determined in a wide
range of market baskets composed of items considered to be
representative of the diet of 16 - 18-year-old boys. Although
dieldrin was not specifically mentioned, the studies revealed
that none of the organochlorine pesticides contributed
residues in excess of the Maximum Residue Limit (MRLs).
5.1.6.3 United Kingdom (UK MAFF, 1982-1985)
Residues of dieldrin found in a range of dietary components in
1981 are listed in Table 8.
Table 8. Dieldrin residues in individual food groups of
the total-diet study (24 sets of total diet samples,
January - December 1981)a
----------------------------------------------------------
Food group Range of residues Average residues
(µg/kg) (µg/kg)
----------------------------------------------------------
Bread ND ND
Other cereal products ND ND
Carcass meat ND - 40 3.5
Offals ND - 5 0.5
Meat products ND ND
Poultry ND - 4 1
Fish ND - 8 2
Oils and fats ND - 15 1
Eggs ND - 4 0.5
Green vegetables ND ND
Potatoes ND - 1 < 0.5
Other vegetables ND - 10 1
Fresh fruit ND ND
Milk ND - 2 0.5
Dairy products ND - 150 4
----------------------------------------------------------
a The limit of detection in these studies varied with the
food group but was sometimes as low as 1 µg/kg.
ND = not detectable.
On the basis of these data, it was estimated that the mean
level of dieldrin residues in the total diet in 1981 in the United
Kingdom was 0.5 µg/kg. This figure compares with 1.5 µg/kg for the
period 1975 - 1977 and 4 µg/kg for the period 1966 - 1967. The
computed daily intake derived from the 1981 figure was < 0.8
µg/person or < 0.01 µg/kg body weight.
Further data on certain individual products were also reported.
Maize: Two samples of imported maize, representing 3% of the
total number of samples taken in a survey conducted in 1981,
contained detectable levels of dieldrin, the highest
concentration being 0.04 mg/kg. The rest of the samples did
not contain dieldrin residues above the limit of determination
of 0.01 mg/kg.
Pulses: Separate surveys of residues in pulses obtained from
retail outlets were carried out in 1982 and 1983. In 1982, 42
samples involving 12 different kinds of pulses were analysed.
In this case, aldrin was found in one sample of haricot beans
(0.04 mg/kg) but was below the limit of determination (< 0.01
mg/kg) in all the others. Dieldrin was found in a limited
number of mung beans (0.05 mg/kg) but was below the limit of
determination (< 0.01 mg/kg) in all of the others. Thus,
neither aldrin nor dieldrin were detected in the majority of
pulses sampled in 1982.
In 1983, 40 samples were analysed and there were no residues
reported for aldrin or dieldrin above the level of
determination, with the exception of limited samples of mung
beans containing dieldrin (maximum, 0.04 mg/kg; mean, < 0.01
mg/kg).
Fruit and vegetables, 1981 - 1984: A large-scale monitoring
project of fruit and vegetables was undertaken in the United
Kingdom during the period 1981 - 1984. Some 40 commodities
were sampled during that period, 1649 samples being obtained
from retail outlets and analysed. The data were not
individually reported but, although most of the commodities
were analysed for organochlorine pesticide residues, there were
no reports of any sample containing residues of dieldrin
exceeding either Codex or EEC maximum residue limits.
Information on the incidence of detectable residues of dieldrin
was not presented in this case. It is of interest to note that
648 of the samples were grown in the United Kingdom and 1001
were imported.
Lamb meat: Sampling of kidney fat from home-grown lamb
destined for export began in October 1984 and data for 988
samples were reported. None contained dieldrin residues above
the limit of determination of 0.02 mg/kg.
Fish: The information in Table 9 was obtained from fish caught
in areas around the English and Welsh coast where the levels of
chemical contamination were known to be high.
Residues of dieldrin in processed fish imported into the United
Kingdom were determined in 155 samples of different products
obtained from retail outlets in 1983. The data are summarized
in Table 10. In a further study, dieldrin residues were
determined in a limited number of fish oil products obtained
through retail outlets (Table 11).
Table 9. Mean residue levels of dieldrin (mg/kg)
in the liver and muscle of marine fish from
England and Wales, 1982
------------------------------------------------
Fish Muscle Number Liver Number
of samples of samples
------------------------------------------------
Cod 0.003 43 0.26 73
Dab 0.003 50 0.13 50
Flounder 0.003 49 0.027 49
Mackerel 0.007 29 0.053 23
Plaice 0.004 43 0.040 68
Sole 0.003 50 0.031 50
Whiting 0.009 62 0.26 62
------------------------------------------------
Table 10. Residues of dieldrin (mg/kg) in
processed imported fish and shellfish in 1983
---------------------------------------------
Fish Rangea Average Number of
samples
---------------------------------------------
Pilchards < 0.009 0.001 21
Plaice < 0.006 0.001 19
Salmon < 0.02 0.002 36
Sardines < 0.004 0.001 11
Tuna ND ND 15
Cockles and < 0.01 0.004 5
mussels
Crab ND ND 15
Prawns < 0.001 < 0.001 17
Shrimps < 0.004 0.001 16
---------------------------------------------
a ND = not detectable.
Table 11. Residues of dieldrin (mg/kg) in fish
oil products, 1984
-----------------------------------------------
Product Range Mean Number of
samples
-----------------------------------------------
Cod liver oil
Mixtures 0.01-0.21 0.08 8
Capsules 0.06-0.20 0.12 3
Halibut liver oil
Capsules 0.01-0.1 0.04 5
"Fish lipid" oil
Capsules 0.01 0.01 1
-----------------------------------------------
5.1.6.4 USA
Surveys were carried out in the USA, during the period
1980 - 1982, covering the diets of infants (aged 6 months),
toddlers (aged 2 years) (Gartrell et al., 1986a), and adults
(youths aged 16 - 19 years) (Gartrell et al., 1986b). In each
case, the samples were taken from a number of locations (13 in the
case of infants and toddlers and 27 in the case of youths). They
were selected as being representative of the composition of diets
for the three population groups studied. Individual foods were
bulked together in food groups and the bulked samples analysed.
The lower level of determination was not precisely stated, since it
varied according to the food group concerned, but from the data
presented it would appear to have been either 1 or 2 µg/kg food
item. Results that were below these limits (and hence
unquantifiable), but where the identity of the residue could be
confirmed, were reported as "T". The analysts' estimate of the
value of "T" was used to estimate the average level of residues in
the whole food group. Data for dieldrin residues are given in
Tables 12, 13, and 14.
Table 12. Dieldrin residues (lg/kg) in infant dietary
componentsa
---------------------------------------------------------
Food group Range of residues Average level
---------------------------------------------------------
Drinking-water 0 0
Whole milk T 0.1
Other dairy products T - 1 0.3
Meat, fish, poultry T - 2 0.5
Grain and cereals 0 0
Potatoes T - 2 0.2
Vegetables T - 1 0.1
Fruit and fruit juices 0 0
Oils and fats 0 0
Sugar and adjuncts 0 0
Beverages 0 0
---------------------------------------------------------
a For breast milk, see section 5.2.2.
5.1.6.5 Appraisal of intake studies
The above data demonstrate that in the United Kingdom and the
USA the intake of dieldrin residues in food is well below the ADI
of 0.1 µg/kg body weight. Moreover, taking into account the rather
high dietary intake estimated for adults in the USA, the agreement
between estimates for the United Kingdom and the USA is striking,
notwithstanding the widely differing origins of the basic food
commodities, especially the relatively high proportion of imports
in the case of the United Kingdom. The estimated levels of intake
in Canada were even lower. The residues in Australia, though very
low, were not expressed in terms of intakes.
Table 13. Dieldrin residues (µg/kg) in toddler dietary
components
--------------------------------------------------------
Food group Range of residues Average level
--------------------------------------------------------
Drinking-water 0 0
Whole milk T 0.1
Other dairy products T - 3 1.2
Meat, fish, poultry T - 3 0.8
Grain and cereals 0 0
Potatoes T - 3 0.3
Vegetables T - 2 0.5
Fruit and fruit juices 0 0
Oils and fats 2 0.3
Sugar and adjuncts 0 0
Beverages 0 0
--------------------------------------------------------
Table 14. Dieldrin residues (µg/kg) in adult dietary
components
------------------------------------------------------
Food group Range of residues Average level
------------------------------------------------------
Dairy products T - 3 0.6
Meat, fish, poultry T - 4 1.2
Grain and cereals 4 0.1
Potatoes T - 2 0.4
Leafy vegetables T - 2 0.2
Legume vegetables 0 0
Root vegetables T - 5 0.4
Garden fruits T - 11 2.1
Fruits 1 0.1
Oils and fats T - 2 0.3
Sugar and adjuncts 0 0
Beverages 0 0
------------------------------------------------------
Dietary levels of dieldrin residues in both the United Kingdom
and the USA appear still to be decreasing, though less so than in
previous years.
In 1966, the JMPR established an acceptable daily intake (ADI)
of 0 - 0.1 µg/kg body weight (combined total for aldrin + dieldrin).
5.1.7. Concentrations of dieldrin in non-target species
There have been many investigations of the occurrence of
dieldrin in the body tissues or eggs of non-target species. The
residues range from less than 0.001 mg/kg to about 100 mg/kg, but
most reported residues are less than 1 mg/kg. The wide range of
concentrations is partly a reflection of the extreme sensitivity of
modern analytical techniques, but there are a number of other
factors involved, e.g., the source and magnitude of the exposure;
the component analysed (brain, adipose tissue, eggs, etc.), and
whether the samples are representative of living, apparently
healthy populations (specimens collected by capture, shooting,
etc., during systemic monitoring surveys) or consist of animals
found dead or dying. Interspecies differences in rates of
metabolism also contribute to the variability of residues. The
highest residues are found in two main groups of organisms. The
first group consists of organisms living near the source of release
into the environment; thus, high residues may be found in aquatic
organisms near the point of release of an industrial effluent, or
in seed-eating birds in areas where seed dressed with aldrin or
dieldrin is used in agriculture. The second group of organisms
consists of predators, particularly those feeding on aquatic
organisms or seed-eating birds or mammals.
The results of some analyses of various species from different
geographical areas are summarized in Tables 15 and 16.
There have been very extensive surveys of dieldrin residues in
biota that are not directly associated with a particular use of
aldrin/dieldrin or their waste disposal.
Soil and earthworms (four genera) were collected from 67 fields
from eight states in the USA. The geometric mean concentrations of
aldrin and dieldrin in soil were 0.014 and 0.023 and, in
earthworms, 0.088 and 0.19 mg/kg dry weight, respectively.
Correlation coefficients between the concentrations of dieldrin in
earthworms and soil were derived for six types of crops, but none
were significant. They were also derived from four different soil
types; only the concentrations in the earthworms from silt loam
soils were significantly related to the concentration in the soil
(Gish, 1970).
Henderson et al. (1969, 1971) studied the occurrence during the
period 1967 - 1969, of dieldrin in various species of fish from 50
monitoring stations located in the Great Lakes and in major river
basins in the USA. The mean concentrations of dieldrin in whole
fish lay in the range 0.01 - 0.28 mg/kg, and the maximum value
found was 1.94 mg/kg. The concentrations above 1 mg/kg were found
in fish from the Atlantic coast streams, Gulf coast streams, and
Great Lake drainage.
Koeman et al. (1967, 1971) and Koeman (1971) studied the
presence of dieldrin in fish, mussels, zooplankton, and birds in
the Wadden area of the Netherlands during the period 1965 - 1971.
The mean concentrations in mussels, marine fish, freshwater fish,
and zooplankton were below 0.1 mg/kg (maximum concentration, 0.23
mg/kg), except in three species of marine fish. In these, the mean
concentration was 0.27 mg/kg (maximum concentration, 0.42 mg/kg).
The levels in the liver and/or eggs of the sandwich tern (Sterna
sandwincensis) and grey heron (Ardea cinerea) were up to 5.1 mg/kg
(maximum concentration, 12 mg/kg). Mortality among sandwich terns
(Sterna sandvicensis), eider duck (Somateria mollissima), and a few
other bird species was reported.
Table 15. Residues of dieldrin in non-target species and their environment
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Antarctica
Signy Island 1966 Chinstrap penguin liver 11 0.002 0.001-0.006 LD not defined; Tatton &
(Pygoscelis antarctica) presumably < 0.001 Ruzicka (1967)
mg/kg
Fish liver 4 0.003 0.001-0.009
(Notothenia neglecta)
Skuas and shags liver 4 0.001 LD - 0.002
Sheathbills 3 0.009 LD - 0.015 sudden deaths of
(Chionis alba) sheathbills of
unknown causes had
occurred
Canada
Southwestern Fish (two species) river water 52 0.000005 LD - 0.00011 LD: water, Miles & Harris
Ontario 1970 bottom mud 14 0.002 LD - 0.01 < 0.000001 mg/litre; (1971)
30/4a 0.071 0.023-0.189 bottom mud, < 0.001
mg/kg
Province of Fish (various species) composites of 62 0.10 LD - 0.56 LD < 0.005 mg/kg Reinke et al.
Ontario headless dressed (1972)
specimens
Four other Fish (various species) composites of 119 0.01 LD - 0.08 76 composites from Reinke et al.
provinces headless dressed these four provinces (1972)
specimen contained residues;
LD < 0.005 mg/kg
Eastern Canada Leach's storm egg 18 0.05 0.03-0.13 Pearce et al.
1970-1976 petrel (Oceanodroma (1979)
leucorhoa)
Double-crested cormorant egg 90 0.13 0.01-0.68
(Phalacrocorax auritus)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Eastern Canada Common eider egg 25 0.02 0.01-0.04
1970-1976 (contd.) (Somerteria mollissima)
Common tern egg 50 0.04 0.01-0.13
(Sterna hirundo)
Razorbill egg 13 0.12 0.01-0.52
(Alca torda)
Common guillemot egg 4 0.02 0.02-0.03
(Uria aalge)
Black guillemot egg 3 0.02 0.01-0.05
(Cepphus grylle)
Atlantic puffin egg 48 0.06 0.03-0.13
(Fratercula arctica)
Falkland Islands Marine, coastal, and egg 46 0.002 LD - 0.011 LD not defined, but Hoerschelmann
1977 freshwater birds < 0.002 mg/kg et al. (1979)
Greece
Saronikos Gulf Striped mullet muscle 74 0.004 0.0001-0.050 residues attributed Voutsinou-
1975 (Mullus barbatus) to the discharge of Taliadouri &
domestic waste and Satsmadjis
industrial effluents (1982)
Iraq
Shatt al-Arab Cyprinid muscle 2 0.003 ND - 0.008 Douabul et al.
river (Barbus xanthopetrus) (1987)
Indian shad muscle 2 0.028 0.016-0.041 Douabul et al.
(Tenualosa ilistra) (1987)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Kenya
Lake Nakuru 1975 water 10 < 0.0001 - Greichus et
al. (1978b)
bottom sediment 10 < 0.001b -
Plankton composite 1 0.03b - Greichus et
al. (1978b)
Chironomids composite 1 < 0.01b -
Water boatmen composite 1 < 0.01b -
(Coroxidae)
Fish composite 100/10a 0.02b -
(Tilapia grahami)
Netherlands 1965 Mussel 22 0.033 0.014-0.084 Koeman (1971)
(Mytilus edulis)
1965 marine fish (3 species) whole body 103 0.27 0.16-0.42 Koeman et al.
(1967)
1966 marine fish (2 species) whole body 37 0.07 0.01-0.23 fish species on Koeman et al.
which sandwich terns (1967)
feed
1965 Sandwich tern liver 19 5.1 1.9-12 found dead or dying Koeman et al.
(Sterna sandvincensis) (1967)
1965-1966 Sandwich tern liver 14 0.6 0.2-2 killed, shot, or Koeman et al.
found dead after a (1967)
storm
1967 freshwater fish 28 0.02 LD - 0.05 LD < 0.01 mg/kg Koeman (1971)
(3 species)
1969 Mussel 10/2a 0.012 0.007-0.016 Koeman et al.
(Mytilus edulis) 199/8a 0.013 0.007-0.023 (1971)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Netherlands (contd.)
1970 Pike whole body 10 0.01 LD - 0.022 LD < 0.003 mg/kg Koeman et al.
(Esox lucius) (1971)
1971 Roach whole body 81/6a 0.004 LD - 0.013 LD < 0.005 mg/kg Koeman et al.
(Rutilus rutilus) (1971)
1971 zooplankton composite - 0.005 - Koeman et al.
(1971)
1970 Shrimp 50/1a 0.009 - Koeman et al.
(Crangon vulgaris) (1971)
1969-1970 marine fish (5 species) 37/5a 0.022 0.008-0.043 Koeman et al.
(1971)
1970 Sandwich tern egg 10 0.082 0.054-0.099 Koeman et al.
(1971)
1971 Grey heron egg 27/4a 1.25 0.5-1.9 Koeman et al.
(Ardea cinerea) (1971)
New Zealand and Marine birds egg, 7 0.01 LD - 0.05 LD not defined, but Bennington et
sub-Antarctic (various spp.) breast muscle 7 0.1 0.03-0.28 < 0.02 mg/kg al. (1975)
islands 1970-1971
Norway
Four regions 1983 Shags (Phalacrocorax egg approx- 0.126-0.286 Barrett et al.
aristotelis) imately in 7.1% of (1985)
Herring gull (Larus 10 samples
argentatus)
Kittwakes (Rissa
tridactyla)
Common guillemots
(Uria aalge)
Razorbills (Alca torda)
Puffins (Fratercula
arctica)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
United Kingdom
England, Brown shrimp homogenates of 12 0.0055 0.0012-0.020 Van Den Broek
Medway estuary (Crangon vulgaris) 50 specimens (1979)
1974-1975
Sand goby homogenates of 9 0.047 0.024-0.077 Van Den Broek
(Pomatoschistus minutus) 50 specimens (1979)
Sprat homogenates of 13 0.084 0.030-0.142 Van Den Broek
(Sprattus sprattus) 50 specimens (1979)
Eel liver 16 0.051 0.0085-0.090 Van Den Broek
(Anguilla anguilla) (1979)
Whiting liver 9 0.57 0.25-1.10 Van Den Broek
(Merlangius merlangus) (1979)
Flounder liver 16 0.21 0.043-0.39 Van Den Broek
(Platichthys flesus) (1979)
Plaice liver 12 0.12 0.015-0.23 Van Den Broek
(Pleuronectes platessa) (1979)
Scotland, Plankton 12 0.072 0.019-0.230 Williams &
Firth of Clyde (various estuarine Holden (1973)
1971-1972 and marine species)
North Atlantic, Plankton 14 0.003 LD - 0.015 LD < 0.001 mg/kg Williams &
northeast transect (various estuarine Holden (1973)
from Mull of and marine species)
Kintyre 1971-1972
Firth of Clyde Mussel 25 0.178 0.012-2.43 Cowan (1981)
(coastal waters) (Mytilus edulis) homogenates of 80 0.022 0.006-0.216
1977 50-100 specimens
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Shetland Isles Mussel 12 0.013 0.006-0.029 Cowan (1981)
(8 other coastal (Mytilus edulis)
sites) 1977
Irish Sea and Seabirds liver 21 1.23 0.07-5 heavy mortality of Lloyd et al.
Firth of Clyde (various species) seabirds in Irish (1974)
1974 Sea; continuous
winter storms may
have been cause of
mortality
Irish Sea 1969 Guillemot liver high mortality of Parslow &
(Uria aalge) guillemots in 1969; Jefferies
shot birds 9 0.09 0.01-0.41 primary cause of (1973)
dead birds 8 0.48 0.10-0.80 death was probably
malnutrition: mean
body weights for
shot and dead birds,
963 g and 580 g,
respectively; mean
liver weights,
43.8 g and 12.5 g,
respectively; PCBs
may also have been
responsible for the
death of guillemots
Great Britain Grey heron egg
1964-1977 (Ardea cinerea)
March 135 0.75 0.65-0.86b concentration Cooke et al.
April 103 1.19 1.05-1.35b increased (1982)
May 45 3.20 2.64-3.88b significantly
between March and
May
Shetland Isles, Great skua egg 12 0.091 0.022-0.15 Furness &
Foula 1976 (Catharacta skua) Hutton (1979)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
USA 15 states:
1965-1972 Estuarine molluscs composites of LD 0.005 mg/kg Butler (1973)
(10 species) meat from 15
or more mature
molliscs
North Carolina, 71 0.01 LD - 0.019 concentrations in 69
Point of Marsh samples below 0.005
mg/kg
Mississippi, 78 0.01 LD - 0.019 concentrations
Biloxi Bay in 70 samples below
0.005 mg/kg
Texas, 48 0.021 LD - 0.046
Arroyo Colorado
New York, 74 0.024 LD - 0.132
Hempstead Harbor
Georgia, 64 0.028 LD - 0.230
Lazaretta Creek
Major river fish composites of LD < 0.001 mg/kg Henderson et
basins in the (various species) whole fish al. (1969,
USA: 1971)
Atlantic coast 741/141a,d 0.14 LD - 1.94
streams 157/36a,e 0.13 LD - 0.55
Gulf coast 204/48a,d 0.12 LD - 1.26
streams 59/12a,e 0.28 LD - 1.59
Great Lakes 378/63a,d 0.05 LD - 0.50
drainage 81/18a,e 0.06 LD - 0.37
Mississippi River 657/139a,d 0.06 LD - 0.52
system 153/34a,e 0.06 0.01-0.49
Hudson Bay 51/13a,d 0.12 0.03-0.37
drainage 5/2a,e 0.01 0.01
Colorado River 112/24a,d 0.02 LD - 0.10
system 24/6a,e 0.01 0.01
Interior basins 120/25a,d 0.01 LD - 0.06
30/6a,e 0.02 LD - 0.03
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
California 90/24a,d 0.06 LD - 0.31
streams 28/6a,e 0.10 0.01-0.36
Columbia River 246/64a,d 0.02 LD - 0.10
system 70/16a,e 0.03 LD - 0.09
Pacific Coast 83/20a,d 0.06 LD - 0.52
streams 29/6a,e 0.01 LD - 0.02
Alaskan streams 105/24a,d 0.003 LD - 0.01
30/6a,e 0.006 LD - 0.01
Upper continental Bathyl-demersal liver 4 0.017 0.011-0.026 fish caught by trawl Meith-Avcin et
rise (southeast fish (Antimora at a depth of 2500 m al. (1973)
of (Cape rostrata)
Hatteras)
California 1970 Common egret brain 5 4.36 0.60-6.76 birds found dead or Faber et al.
(Casmerodius albus) moribund; dieldrin (1972)
considered to be a
contributory cause
of death of 4 birds
South Dakota Pheasant adipose tissue 48 0.08 LD - 1.07 LD < 0.01 mg/kg; 13 Greichus et
1965-1967 (Phasianus colchicus) samples of fat al. (1968)
contained < 0.01
mg/kg
Sharp-tailed grouse living birds 46 0.17 LD - 1.71 13 samples of fat
(Pedioecetes phasianellus contained < 0.01
campestris) mg/kg
South Dakota 1967 Pheasant egg 67 0.02 LD - 0.12 LD < 0.01 mg/kg; 13 Linder &
eggs contained 0.01 Dahlgren
mg/kg (1970)
Maine and Common eider and herring egg 88 LD LD LD < 0.1 mg/kg Szaro et al.
Virginia 1977 gull (1979)
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
USA (contd.)
Maine and Great black-backed egg 28 0.12 LD - 0.55 24 of the eggs
Virginia (contd.) gull contained < 0.1 mg/kg
Texas, Corpus Wintering shorebirds carcass 56 0.11 LD - 1 LD < 0.1 mg/kg; 2 White et al.
Christi Bay (7 species) (shot birds) carcasses contained (1980)
1976-1977 < 0.1 mg/kg
Lake Michigan Red-breasted merganser egg 206 0.77 0.2-2.3 LD < 0.1 mg/kg Haseltine et
1977-1978 (Mergus serrator) al. (1981)
Mallard egg 27 0.07 LD - 0.53 22 of the eggs
(Anas platyrhynchos) contained < 0.1
mg/kg
Gadwall egg 9 0.1 LD - 0.56 5 of the eggs
(Anas strepera) contained < 0.1
mg/kg
Florida Brown pelican LD < 0.05 mg/kg Blus et al.
(Pelecanus occidentalis) egg (1974b)
Atlantic coast egg 22 0.36 LD - 1.52
Gulf coast egg 27 0.10 trace - 0.40
Florida carcass 16 0.65 LD - 1.60 Blus et al.
1969 (shot birds) (1974b)
South Carolina carcass 5 0.51 LD - 1.50
(shot birds)
1969 Brown pelican egg 11 0.94 0.60-1.62 Blus et al.
1970 egg 10 0.62 0.20-1.30 (1974b, 1977,
1971 egg 65 0.46 0.20-1.02 1979b)
1972 egg 72 0.45 LD - 1.76
1973 egg 104 0.45 0.16-1.65
1974 egg 116 0.54 0.17-2.89
1975 egg 102 0.36 LD - 1.04
----------------------------------------------------------------------------------------------------------------------------------------
Table 15. (contd.)
----------------------------------------------------------------------------------------------------------------------------------------
Geographical Species Type of Number of Mean Rangec Commentsc Reference
area/Year sample samples (mg/kg) (mg/kg)
----------------------------------------------------------------------------------------------------------------------------------------
Louisiana
1971 Brown pelican egg 3 0.33 0.24-0.54 Blus et al.
1972 egg 12 0.45 0.30-0.79 (1979a)
1973 egg 21 0.64 0.30-1.12
1974 egg 25 0.84 0.49-1.61
1975 egg 30 1.08 0.64-2.25
1976 egg 25 0.94 0.44-3.03
Zimbabwe
Lake McIlwaine water 10 < 0.0001 Greichus et
1974 al. (1978a)
bottom sediment 10 0.004
Plankton composite 1 < 0.01b
Oligochaete composite 1 0.08b
(Branchiura sowerbyi)
Fish (3 spp.) composite 200/15a 0.04b 0.03-0.07
Cormorant (2 spp.) brain 10 1.4b
----------------------------------------------------------------------------------------------------------------------------------------
a N1/N2: N1 is the number of individuals incorporated into N2 composites; the range corresponds to the composites.
b Concentration expressed on dry weight basis.
c LD = limit of detection, ND = not detectable.
d Samples taken in 1967-1968.
e Samples taken in 1969.
Table 16. Concentrations of dieldrin in non-target organisms
------------------------------------------------------------------------------------------
Species Geographical Year Concentration of dieldrin Reference
(component area (mg/kg wet weight)
analysed) Geometric Arithmetic Range
mean mean (N)c
------------------------------------------------------------------------------------------
Fish (3 spp.) Great Britain 1977-79 - 0.00042a <0.00035- Rickard &
(muscle) R. Thames 0.0020 (83) Dulley (1983)
(tidal)
Oysters USA: 1968-69 - 0.0014a <0.001- Rowe et al.
(flesh) Louisiana 0.0034 (113) (1971)
Penguin Antarctic 1966-67 - 0.008a <0.006-0.010 Tatton &
(abdominal (5) Ruzicka
fat) (1967)
Fish, USA: Virgin 1972-74 - 0.005a <0.005-0.021 Reimold
invertebrates Islands, (141) (1975)
(various spp.) Puerto Rico
(whole body)
Fish (various USA: Western 1967-69 - 0.01a <0.01-0.08 Klaassen &
spp.) (whole Kansas (393) Kadoum (1973)
body/tissues)
Northern fur USA: Alaska 1968-69 - 0.05a <0.01-0.091 Anas & Wilson
seals (liver) (23) (1970a,b)
Birds (various Zimbabwe 1973-76 - 0.004b <0.01-0.67 Tannock et
spp. including (34) dry al. (1983)
birds of prey) weight
(eggs)
Woodcock USA: eastern, 1970-71 - 0.018a <0.01-0.55 Clark & McLane
(breast mid-western (129) (1974)
muscle)
Starlings USA 1967-68 - 0.139a <0.005-1.18 White (1976)
(carcass) (360)
1970 - 0.117a <0.005-3.59 White (1976)
(125)
Starlings USA 1972 - 0.098a <0.005-1.56 White (1976)
(carcass) (130)
1974 - 0.057a <0.005-1.01 White (1976)
(126)
Migratory USA: Florida 1964-73 - 0.2a <0.01-1.10 Johnston
birds (various (829) (1975)
spp.) (breast
muscle)
------------------------------------------------------------------------------------------
Table 16. (contd.)
------------------------------------------------------------------------------------------
Species Geographical Year Concentration of dieldrin Reference
(component area (mg/kg wet weight)
analysed) Geometric Arithmetic Range
mean mean (N)c
------------------------------------------------------------------------------------------
Bats (3 spp.) USA: 1973 0.2a - <0.1-3.2 Clark &
(carcass) Maryland, (110) Prouty (1976)
West Virginia
Golden eagle USA: western, 1964-70 - 0.1 <0.1-12 Reidinger &
(fat) mid-western (69) Crabtree
states (1974)
Golden eagle Scotland 1964-74 0.12 - <0.05-6.9 Cooke et al.
(eggs) (100) (1982)
Tawny owl Great Britain 1963-65 0.15 - <0.05-12.7 Cooke et al.
(liver) (55) (1982)
Peregrine Great Britain 1964-77 0.20 - <0.05-7.6 Cooke et al.
falcon (eggs) (145) (1982)
Bald eagle USA 1971-72 0.6b - <0.05-7.8 Cromartie et
(brain) (37) al. (1975)
Barn owl Great Britain 1963-75 1.21b - <0.05-70.2 Cooke et al.
(liver) (251) (1982)
Hawks, Netherlands 1968-69 - 10.8 0.45-31 Koeman et al.
falcons, owls (19) (1969)
(liver)
------------------------------------------------------------------------------------------
a Indicates living organisms collected by capture, shooting, etc.
b Indicates organisms found dead or dying.
c Number in parentheses is the number of specimens.
Butler (1973) found mean dieldrin concentrations of 0.01 -
0.028 mg/kg (maximum concentration, 0.23 mg/kg) in estuarine
molluscs collected from 15 coastal states in the USA during the
period 1965 - 1972.
Fish sampled in Canada, in 1970, were found to have a mean
concentration of 0.071 mg dieldrin/kg (maximum concentration, 0.189
mg/kg). The concentrations in the water and bottom mud were of the
order of 0.005 µg/litre and 0.002 mg/kg, respectively (Miles &
Harris, 1971). In another study, fish from five provinces (78
locations) in Canada showed mean concentrations of 0.1 - 1 mg/kg
(maximum concentration, 0.56 mg/kg) (Reinke et al., 1972).
In coastal waters around England, Scotland, and Ireland, a
number of studies were carried out to determine dieldrin levels in
plankton, mussels, shrimp, and various other marine species
(1971 - 1975). The mean concentrations ranged from 0.003 to 0.178
mg/kg (maximum level, 2.43 mg/kg). Mussels showed the highest
levels (Williams & Holden, 1973; Lloyd et al., 1974; Van Den Broek,
1979; Cowan, 1981).
The presence of dieldrin in water, bottom sediment, and living
organisms has been studied in Africa (Kenya, Zimbabwe), Signy
Island (Antartica), New Zealand, and sub-antartic islands. The
concentrations of dieldrin in water were very low (< 0.01
µg/litre), those in bottom sediment were up to 0.004 mg/kg, and
those in water organisms (mainly plankton and invertebrates) were
0.01 - 0.03 mg/kg (dry weight basis). Penguin abdominal fat
contained 0.008 mg/kg and liver 0.002 mg/kg. The levels in fish
were < 0.1 mg/kg (dry weight) (Tatton & Ruzicka, 1967; Bennington
et al., 1975; Greichus et al., 1978a,b).
In the different areas where water, invertebrates, and fish
were analysed, birds and eggs were also studied for the presence of
dieldrin. In the eggs of a number of bird species from the
Falkland Islands, Hoerschelmann et al. (1979) found an average of
about 0.005 mg/kg dieldrin in 17 of the 46 eggs. In eggs of
coastal birds in the Federal Republic of Germany, the average
concentration (in 27 eggs) was higher (average, 0.031 mg/kg; range,
0.004 - 0.187 mg/kg).
Parslow & Jefferies (1973) found mean concentrations of up to
0.48 mg/kg in the liver of guillemots (Uria aalge) in the Irish
Sea. In eggs of the great skua (Catharacta skua), collected on the
Shetland Islands, Furness & Hutton (1979) measured a concentration
of 0.091 mg/kg (maximum concentration, 0.15 mg/kg). In South
Dakota, USA (1965 - 1967), Greichus et al. (1968) and Linder &
Dahlgren (1970) determined concentrations of up to 0.08 mg/kg in
the adipose tissue of pheasants and 0.02 mg/kg in eggs. In adipose
tissue of grouse, a mean concentration of 0.17 mg/kg was found.
When a number of eggs of several bird species was analysed in
eastern Canada (1970 - 1976), the mean concentrations were 0.06
mg/kg (maximum level, 0.68 mg/kg) (Szaro et al., 1979). In
different species of birds (and eggs) in the north and south of the
USA, White et al. (1980) found average concentrations in the
carcass of 0.13 - 0.47 mg/kg and Haseltine et al. (1981) found in
eggs of mergansers (Mergus serrator) a geometric mean concentration
of 0.78 mg/kg.
It is of interest that very low residues are found in the great
majority of eggs from areas remote from the regions of major
aldrin/dieldrin use. This is true of samples from the Falkland
Islands and Antartica and it is also true of a survey of 440 eggs
from 19 species of seabirds collected in 1973 - 1976 in Alaska
showing that residues in 410 eggs were less than 0.05 mg/kg (wet
weight). The highest residue found was 0.6 mg/kg (Ohlendorf et
al., 1982).
In Florida, Louisiana, and South Carolina, Blus et al. (1974b,
1977, 1979a,b) studied the dieldrin concentrations in the carcass
and eggs of the brown pelican (Pelecanus occidentalis) during the
period 1969 - 1976. The mean concentration in the carcass was
about 0.6 mg/kg (maximum concentration, 1.6 mg/kg) and, in the
eggs, about 0.6 mg/kg (maximum concentration, 2.89 mg/kg).
Jefferies (1972) carried out a survey of the residue levels in
bats from the East Anglian area, United Kingdom, to provide more
information on the situation concerning the British bat population.
Four species of bats were studied, Pipistrellus pipistrellus,
Plecotus auritus, Myotis nattereri, and Myotis daubentoni.
Thirty specimens were collected during the period 1963 - 1970.
Dieldrin was found in eight liver specimens (range 0.04 to 3.3 mg/kg
tissue), in two adipose tissue samples (4.0 and 7.9 mg/kg), and in
six total body samples (0.07 to 0.50 mg/kg tissue).
Clark et al (1978) estimated the dieldrin levels in 28 juvenile
grey bats (Myotis grisesceus) taken from three caves in Missouri,
USA. The concentrations varied between the individual animals and
between the caves. Dieldrin was detected in the brain of 18/28
bats, the range being 0.4 - 10 mg/kg tissue (wet weight basis), and
in the carcass of 22/28 bats (range 1.7 - 1379 mg/kg carcass; lipid
weight). The authors believed that there was a direct link between
the field mortality of bats and dieldrin residues acquired through
the food chain.
Clark et al. (1980, 1983b) detected dieldrin in the brain and
carcass of grey bats found dead in a Missouri cave in 1976 and
1977. In 1976, the geometric mean was 7.5 and 650 mg/kg tissue
(respectively for brain on wet weight basis and for carcass on
lipid weight basis) and in 1977, 8.6 and 867 mg/kg tissue,
respectively. Other chlorinated hydrocarbons were also present
such as heptachlor epoxide, DDE, and PCBs.
Clark (1981) studied the brain to carcass lipid relationship
for dieldrin and estimated a minimum lethal level for brain tissue
of 4.6 mg dieldrin/kg (wet weight) and for carcass of 390 (210 -
800) mg dieldrin/kg tissue (lipid weight).
In two other caves in Missouri, dead grey bats were found in
1980, and dieldrin and other halogenated insecticides were found in
the brain and carcass. Seven animals were studied and dieldrin
concentrations ranging from not detectable to 21 mg/kg tissue (wet
weight) were found in the brain and 4.1 - 970 mg/kg in the carcass
(lipid weight). The concentrations in brain were of the same order
as those found in the other caves in Missouri. Bat mortality in
July 1981 occurred simultaneously, in one case, with the death of
macroinvertebrates in the outlet stream of the cave (Clarke et al.,
1983a).
Dieldrin residues ranging from trace to 3.3 mg/kg have been
detected in marine mammals, including whales and seals (Holden,
1975; Rosewell et al., 1979). Other mammals in which dieldrin has
been found include the fisher, fox, marten, mink, raccoon, and
skunk (Frank et al., 1979), the highest concentrations being found
in the predators at the top of the food chain, i.e., mink and
marten (9.7 µg/kg wet tissue).
Other studies on the presence of dieldrin in non-target species
and their environment are summarized in Tables 15 and 16 (Bugg et
al., 1967; Koeman et al., 1967; Rowe et al., 1971; Faber et al.,
1972; Meith-Avcin et al., 1973; Voutsinou-Talia-Douri & Satsmadjis,
1982). Most of these results are an indication of adventitious
contamination, i.e., there is no close relationship to a particular
use of aldrin or dieldrin.
The use of aldrin and dieldrin as seed-dressing agents has
undoubtedly resulted in high concentrations of dieldrin in the body
tissues of animals found dead. An association between the use of
aldrin and dieldrin seed dressings and the deaths of wood-pigeons
(Columba palumbus) was first noted by Carnaghan & Blaxland (1957)
and Turtle et al. (1963, 1965). In wood-pigeon, pheasant,
partridge, and corvids found dead, Turtle et al. (1965) found mean
concentrations of 10, 2.3, 7.3, and 2 mg/kg liver, respectively,
(maximum concentrations of 59.2, 28.8, 46.3, and 14 mg/kg liver).
The concentrations in birds that had been shot were much lower.
Table 17 summarizes the residue levels found following the use
of dieldrin for the control of the tsetse fly and arising from
other uses of aldrin and dieldrin, e.g., as seed-dressing agents.
Several other reports on seed-dressing incidents in the United
Kingdom have been published, e.g., Murton & Vizoso (1963) and
Jefferies et al. (1973).
5.1.7.1 Occurrence of dieldrin in birds of prey and fish-eating
birds
Changes in the populations of hawks, falcons, and other raptors
have prompted extensive studies of the concentrations of dieldrin
in the tissues of birds and eggs. These data are summarized in
Table 18.
The concentrations of dieldrin in the tissues of bald eagles
(Haliacetus leucocephalus) that were found dead during the period
1967 - 1977 were estimated by Mulhern et al. (1970), Belisle et al.
(1972), Cromartie et al. (1975), Prouty et al. (1977), and Kaiser
et al. (1980). The concentrations (geometric mean) in the brain
were 0.1 - 2.0 mg/kg tissue, with a maximum of 11 mg/kg. Because
the population declines of some birds of prey and some fish-eating
birds have been associated with the use of aldrin and dieldrin, the
residues in some of these species will be discussed in more detail.
Table 17. Residues in non-target species - concentrations related to particular uses or discharges of
aldrin/dieldrin
---------------------------------------------------------------------------------------------------------
Species Component Geographical Year No. of Concentration of Comments Reference
analysed area speci- dieldrin (mg/kg)
mensa Meanb Range
---------------------------------------------------------------------------------------------------------
Woodpigeon liver Netherlands 1966 20 2.8b 0.05-27.1 seed dressing Fuchs
(Columba 4 79c 44.2-136 (1967)
palumbus)
Pink-footed liver United 1972 6 31c 15-48 seed dressing Stanley &
goose (Anser Kingdom -73 Bunyan
branchyrhynchus) (1979)
Pheasant egg USA: 1966 120 0.3 0.02-2.82 soil Greenberg &
(Phasianus Illinois insecticide Edwards
colchicus) (1970)
Birds (various liver Kenya 1968 12 28.2c 18-57 tsetse fly Koeman &
spp.) 21 1.7b 0.16-6 control (dead Pennings
brain 10 14.3c 6-22 birds found (1970)
11 0.2b 0.06-0.68 during 10 days
after spray
application;
live birds
collected 2
months later)
Insects (various whole Cameroon 1979 227 0.2c NDe - 13.2 tsetse fly Mueller et.
spp.) body control al. (1981)
Fish whole 124 0.09 ND - 214.3
(Aphyosemion body
bualanum)
Birds (various liver 40 1.51b ND - 7.24
spp.)
Fruit bat liver 20 79.2b ND - 174.81
(2 spp.)
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Species Component Geographical Year No. of Concentration of Comments Reference
analysed area speci- dieldrin (mg/kg)
mensa Meanb Range
---------------------------------------------------------------------------------------------------------
Rat (Praomys liver 13 0.37 ND - 1.20 Mueller et
tullbergi) al. (1981)
Common gallinule egg USA: 1965 4/23d 9.6 2.23-13.17 rice fields Causey et
(Gallinula Louisiana sown with al. (1968)
chloropus) 1966 14 9.4 1.13-22.12 aldrin-treated
seed
Purple gallinule egg 1965 2/16d 9.7 6.47-12.94
(Porphyrula 1966 56 6.5 0.49-15.35
martinica)
Common gallinule egg USA: 1968 6 17.5 4.69-28.07 rice fields Fowler et
Louisiana sown with al.(1971)
1969 12 4.8 1.16-10.7 aldrin-treated
seed
Purple gallinule egg 1968 26 9.4 3.23-16.43
1969 33 3.8 1.56-13.62
Invertebrates composites USA: Texas 1967 1208/ 1.1b LDf - 3.2 aldrin-treated Flickinger
of whole Gulf coast -71 16 (3.1) (LD - 16.3) seed & King
body (1972)
Crayfish whole 105/8 6.3c LD - 17
(2 spp.) body (2.1) (LD - 9)
Cricket frog whole 18/3 0.1b LD - 0.1
(Acris crepitans body
blanchardi)
Fish (4 spp.) whole 592/4 1.2b 0.4-2.8
body
Turtles (2 spp.) whole 5/2 0.9b 0.6-1.2
body (2.4) (LD - 4.8)
---------------------------------------------------------------------------------------------------------
Table 17. (contd.)
---------------------------------------------------------------------------------------------------------
Species Component Geographical Year No. of Concentration of Comments Reference
analysed area speci- dieldrin (mg/kg)
mensa Meanb Range
---------------------------------------------------------------------------------------------------------
Snakes (3 spp.) whole USA: Texas 1967 3/3 2.4b 0.1-5.7 Flickinger
body Gulf coast -71 & King
(1972)
Great horned owl brain 1 6.3c -
Birds (various brain 27 8.5c LD - 22 192 dead birds
spp.) (0.1) (LD - 0.2) collected from
1967-71
Fulvous tree egg 69/14 2.5 <0.1-9.5
duck
(Dendrocygna
bicolor)
Owls (various liver United 1974 22 24c 1.7-46 death of many Jones et
spp.) Kingdom -76 owls due to al. (1978)
(London Zoo) dieldrin
poisoning;
sawdust from
dieldrin-
treated wood
the probable
source of
contamination
---------------------------------------------------------------------------------------------------------
a N1/N2: N1 is the number of incorporated into N2 composites; the range corresponds to the composites.
b Indicates living organisms collected by capture, shooting, etc. Values in parentheses are the
concentrations of aldrin.
c Indicates organisms found dead or dying. Values in parentheses are the concentrations of aldrin.
d Clutches/eggs.
e ND = not determined.
f LD = limit of detection.
Table 18. Concentrations of dieldrin in tissues and eggs of birds of prey and fish-eating
birds found dead
-------------------------------------------------------------------------------------------
Species Type of Geographical Year No. of Concentration of Reference
sample area speci- dieldrin (mg/kg)
mens Meana Rangeb
-------------------------------------------------------------------------------------------
Kestrel (Falco liver Netherlands 1968-69 7 - 1.1-24 Koeman et
tinnunculus) al. (1969)
Kestrel (Falco liver United 1963-65 74 1.09 0.94-1.27 Cooke et
tinnunculus) Kindgom 1966-71 144 1.06 0.91-1.24 al. (1982)
1972-75 125 1.43 1.19-1.72
1977 31 0.31 0.21-0.45
Sparrow-hawk liver Netherlands 1969 3 - 0.9-19 Koeman et
(Accipiter nisus) al. (1969)
Sparrow-hawk liver United 1963-65 30 1.20 0.97-1.49 Cooke et
(Accipiter nisus) Kingdom 1966-71 82 0.29 0.22-0.38 al. (1982)
1972-75 83 0.61 0.48-0.78
1977 26 0.22 0.15-0.32
Sparrow-hawk egg United 1963-65 24 2.09 1.63-2.67 Cooke et
(Accipiter nisus) Kingdom 1966-71 154 0.69 0.60-0.79 al. (1982)
Buzzard liver Netherlands 1968-69 5 - 0.45-31 Koeman et
(Buteo buteo) al. (1969)
Grey heron liver United 1963-65 26 1.13 0.66-1.94 Cooke et
(Ardea cinerea) Kingdom 1966-67 69 0.92 0.67-1.26 al. (1982)
1972-75 57 0.74 0.57-0.96
1977 12 0.17 0.08-0.37
Kingfisher liver United 1964-65 4 6.83 3.95-11.8 Cooke et
(Alcedo atthis) Kingdom 1966-71 37 1.56 1.23-1.98 al. (1982)
1972-75 22 1.16 0.89-1.53
Peregrine falcon liver United 1963-77 15 1.91 1.35-2.71 Cooke et
(Falco peregrinus) Kingdom al. (1982)
Barn owl liver United 1963-65 48 1.31 1.08-1.60 Cooke et
(Tyto alba) Kingdom 1966-71 94 1.42 1.19-1.69 al. (1982)
1972-75 114 1.07 0.90-1.28
1977 29 0.26 0.18-0.37
Long-eared owl liver United 1963-77 30 1.75 1.14-2.70 Cooke et
(Asio otus) Kingdom al. (1982)
Bald eagle liver USA 1964-65 44 0.28 LD - 11.9 Reichel et
(Haliacetus (LD <0.05 al. (1969)
leucocephalus) mg/kg)
-------------------------------------------------------------------------------------------
Table 18. (contd.)
-------------------------------------------------------------------------------------------
Species Type of Geographical Year No. of Concentration of Reference
sample area speci- dieldrin (mg/kg)
mens Meana Rangeb
-------------------------------------------------------------------------------------------
Peregrine falcon egg United 1963-65 23 0.59 0.49-0.71 Cooke et
(Falco peregrinus) Kingdom 1966-71 76 0.14 0.11-0.17 al. (1982)
1972-75 34 0.18 0.11-0.28
1977 12 0.34 0.25-0.46
Bald eagle egg USA 1969-70 12 0.08c LD - 0.3 Wiemeyer et
(Haliacetus (LD <0.05 al. (1972)
leucocephalus) mg/kg)
USA: 11 0.83c 0.15-2.3
Alaska, 4
other states
-------------------------------------------------------------------------------------------
a Geometric mean, except for footnote c which is arithmetic mean.
b Range of value within 1 standard error.
c Arithmetic mean.
(a) Grey heron (Ardea cinerea)
This is one of the highly contaminated species in the United
Kingdom. Relatively high levels of dieldrin have been measured in
the livers of herons found dead, together with high levels of DDT-
type compounds and polychlorinated biphenyls (Cooke et al., 1982).
The geometric mean concentrations of dieldrin for various periods
within the range 1963 - 1977 are given in Table 18. The geometric
mean concentration of dieldrin in the livers of 143 samples over
the period 1963 - 1975 was 0.9 mg/kg. Of the herons found dead,
50% contained less than 1 mg dieldrin/kg liver, whereas 14%
contained 10 mg/kg or more.
(b) Kestrel (Falco tinnunculus)
The geometric mean concentration of dieldrin in the livers of
374 kestrels found dead in the United Kingdom during the period
1963 - 1977 was 1.2 mg/kg (Cooke et al., 1982). Some 50% of the
kestrels found dead contained less than 1 mg dieldrin/kg liver, 18%
contained more than 10 mg/kg, and 8% more than 20 mg/kg. Higher
levels were found in the Netherlands (Fuchs, 1967; Koeman et al.,
1969).
Sierra et al. (1987) studied the presence of residues of aldrin
and dieldrin in the liver, muscle, fat, kidneys, and brain of four
kestrels from the province of Leon, Spain. The concentrations of
aldrin ranged from 0.003 to 0.65 mg/kg tissue (highest in fat and
kidneys), whereas those of dieldrin ranged from 0.005 to 0.151
mg/kg tissue (highest in liver; fat not estimated). All values
were based on wet weight.
(c) Sparrow-hawk (Accipiter nisus)
The geometric mean concentration of dieldrin in the liver of
195 sparrow-hawks found dead in the United Kingdom over the period
1963 - 1977 was 0.5 mg/kg (Cooke et al., 1982). About 62% of the
dead sparrow-hawks contained less than 1 mg dieldrin/kg liver, and
about 7% contained more than 10 mg dieldrin/kg liver.
Three sparrow-hawks found dead or dying in the Netherlands in
1969 contained 0.89, 1.1, and 19 mg dieldrin/kg liver, respectively
(Koeman et al., 1969). One dead sparrow-hawk (1966) contained 18.4
mg dieldrin/kg liver (Fuchs, 1967).
Sierra et al. (1987) studied the presence of residues of aldrin
and dieldrin in three sparrow-hawks in Leon, Spain. The average
concentrations of dieldrin ranged from 0.1 to 0.45 mg dieldrin/kg
tissue (liver, kidneys, brain) but in fat an average level of 17.3
mg/kg was found (all values were based on wet weight). Only low
levels (< 0.01 mg/kg tissue) of aldrin were found in fat.
(d) Barn owl (Tyto alba)
The geometric mean concentration of dieldrin in the liver of
251 barn owls found dead in the United Kingdom (1963 - 1977) was
1.2 mg/kg (Cooke et al., 1982). About 49% of the barn owls
contained less than 1 mg dieldrin/kg liver, while about 15%
contained at least 10 mg dieldrin/kg liver.
The concentration of aldrin and dieldrin in the muscle, liver,
fat, brain, and kidneys of 23 barn owls, collected in the province
of Leon, Spain, was determined (91 samples in total). The
incidence of aldrin in the tissues ranged from 76 to 83%, and of
dieldrin from 4 to 27%. The average concentration in these organs
and tissues was 0.03 - 0.11 mg aldrin/kg and 0.009 - 0.2 mg
dieldrin/kg tissue (wet weight). The highest concentration was in
the kidneys for aldrin and in the brain for dieldrin (Sierra &
Santiago, 1987).
The concentrations in all four of the above species in the
United Kingdom showed seasonal, annual, and regional trends.
Residue levels in herons decreased progressively after 1963 - 1965
until 1977, whereas the main decrease in levels in sparrow-hawks
occurred between 1963 - 1965 and 1966 - 1971, there being little
subsequent change. In kestrels and barn owls, there was no overall
trend between 1963 and 1974 - 1975, but significant declines in
levels had occurred by 1977. The residues in the livers of herons,
kestrels, and barn owls were significantly higher in areas of
eastern England (the main wheat bulb fly infestation areas) than in
other regions of the United Kingdom. These differences are
probably indicative of the use of aldrin- or dieldrin-dressed grain
in eastern England. Few samples of sparrow-hawk's livers were
available from eastern England, but the residues showed a similar
regional difference.
(e) Bald eagle (Haliacetus leucocephalus)
A survey of the residues of dieldrin in the carcass, liver, and
brain of bald eagles was initiated in 1960 by the Patuxent Wildlife
Research Centre, USA. The median concentration (1964 - 1965) was
0.1 mg dieldrin/kg brain and about 0.3 mg dieldrin/kg liver
(Reichel et al., 1969). During the period 1966 - 1977, mean
concentrations ranged from 0.1 to 2 mg dieldrin/kg brain (Mulhern
et al., 1970; Belisle et al., 1972; Cromartie et al., 1975; Prouty
et al., 1977; Kaiser et al., 1980).
(f) Other birds of prey and fish-eating birds
The surveys of the residues of dieldrin in other raptors have
been less extensive than those for the five species discussed
above. The geometric mean concentrations in the livers of 12 other
species (283 birds) in the United Kingdom (Cooke et al., 1982) in
the period 1963 - 1977 were between 0.02 and 2.35 mg/kg. Those for
the golden eagle (14 birds) in the USA during the period 1964 -
1965 were between trace levels and 0.4 mg/kg (Reichel et al.,
1969).
5.2. General Population Exposure
5.2.1. Adults
5.2.1.1 Aldrin
In the great majority of investigations into the presence of
organochlorine compounds in human blood and other tissues, the
level of aldrin was below the limits of detection. However, there
are a few reports of aldrin being present in human blood, placenta,
adipose tissue, and other tissues (Radomski & Fiserova-Bergerova,
1965; Kanitz & Castello, 1966; Selby et al., 1969a,b; Herrera
Marteache et al., 1978; Fernicola & Azevedo, 1982; Mossing et al.,
1985). These findings are unusual. The report that aldrin was
present in eight samples of blood, when none was found in the
matched adipose tissue samples, also seems anomalous (Selby et al.,
1969b). Fernicola & Azevedo (1982) suggested that some other
compounds with the same retention time as aldrin had perhaps led to
false results. None of these investigators established the
identity of the component, reported as "aldrin".
5.2.1.2 Concentrations of dieldrin in adipose tissue
Following the introduction of gas-liquid chromatography, there
have been numerous investigations of the concentration of dieldrin
in the adipose tissue of members of the general population who have
had no know occupational exposure to aldrin or dieldrin. Surveys
have been made in more than 20 countries, but in some surveys the
number of samples of fat analysed was small. In the USA and the
United Kingdom, there have been several surveys during the period
1961 - 1977. The results are summarized in Table 19, using two
statistics to define the samples: arithmetic mean (or geometric
mean in some American surveys) and maximum value as an indication
of the upper limit of variability (upper confidence limit in a few
surveys). The distribution tends to be skewed to the right, i.e.,
there is a greater number of high values than would be expected if
the samples had a normal distribution (Hunter et al., 1963; Morgan
& Roan, 1970). The maximum values in some surveys are so large
that they may correspond to individuals with an occupational
exposure. The results for stillborns and young babies and children
are discussed in section 5.2.2.
Most of the mean values are in the range 0.1 - 0.3 mg dieldrin
kg body fat and are usually smaller than those of total DDT by at
least a factor of 10. Surveys in the USA, United Kingdom, and
Netherlands indicate that there has been a decline of about 50% in
the concentration of dieldrin in the body fat since the mid 1970s
(Abbott et al., 1981; Ministry of Welfare, Health and Culture, The
Netherlands, 1983).
Table 19. Concentrations of dieldrin in the body fat of the general population
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
North America
Canada 1966 47 (N) I 0.22 0.53 Brown (1967)
1967-68 51 (N) II 0.12 0.83 Kadis et al. (1970)
1969 221 (N) II 0.12 0.46 Ritcey et al. (1973)
1969 5 (-) - 0.08 - Mastromatteo (1971)
1970 3 (-) - 0.22 - Mastromatteo (1971)
1972 168 (N) II 0.069 0.35 Mes et al. (1977)
1969-74 448 (N) - 0.12 0.88 Holdrinet et al.
(1977)
1976 99 (N) 0.049 0.211 Mes et al. (1982)
1979-81 175 (N) II 0.04 0.13 Williams et al.
(1984)
1980 29 (N) 0.046 Mes et al. (1985)
USA 1961-62 28 (B) II 0.15 0.36 Dale & Quinby (1963)
1962-66 221 (N) II 0.14 1.39 Hoffman et al. (1967)
1964 25 (N) II 0.29 1.15 Hayes et al. (1965)
1964 64 (N) - 0.31 2.82 Zavon et al. (1965)
1964-67 42 (N) none 0.21 0.70 Radomski et al.
(1968)
1965-67 146 (N) none 0.22 0.77 Edmundson et al.
(1968)
1966-68 70 (N) II 0.14 - Morgan & Roan (1970)
1967 30 (N) II 0.03f - Casarett et al.
(1968)
1968 48 (N) II 0.20 - Warnick (1972)
1969 15 (N) II 0.15 - Warnick (1972)
1969 26 (B) II 0.33f 0.80c Burns (1974)
1970 40 (N) II 0.15 - Warnick (1972)
1970 68 (B) II 0.29f 0.73c Burns (1974)
1970 202 (B) II 0.2 1.0 Wyllie et al. (1972)
1970 1412 (N/B) II 0.18f 15.20 Kutz et al. (1979)
1971 88 (B) II 0.36f 0.78c Burns (1974)
1971 1615 (N/B) II 0.22f 2.91 Kutz et al. (1979)
1972 39 (B) II 0.43f 1.00c Burns (1974)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
USA (contd.)
1972 1913 (N/B) II 0.18f 2.91 Kutz et al. (1979)
1973 1094 (N/B) II 0.18f 5.64 Kutz et al. (1979)
1974 898 (N/B) II 0.15f 2.21 Kutz et al. (1979)
Louisiana 1980 8 (B) II 0.15f 0.34 Holt et al. (1986)
1984 10 (B) II 0.10f 0.19 Holt et al. (1986)
Central and South America
Mexico 1975 19 (N) II 0.06f 0.24 Albert et al. (1980)
1975 9 (B) II 0.18f 0.49 Albert et al. (1980)
1975 9 (N) II 0.05f 0.12 Albert et al. (1980)
Argentina - 47 (N) IV 0.38 0.66c Wassermann et al.
(1969)
Brazil 1969-70 17 (N/B) III 0.02e 0.12 Wassermann et al.
(1972a)
1969-70 69 (N/B) III 0.12e 1.62 Wassermann et al.
(1972a)
Europe
Belgium 1968-69 37 (N) II 0.13 0.50 Wit (1971)
1975 60 (N) II 0.26 1.16 Dejonckheere et al.
(1977)
1977 58 (N) II 0.12 0.69 Van Haver et al.
(1978)
Denmark 1965 18 (N) - 0.20 0.34 Weihe (1966)
1972-73 70 (N) II 0.16f 0.53 Kraul & Karlog (1976)
France 1971 100 (N) II 0.45 1.45 Fournier et al.
(1972)
Germany, 1967 15 (B) I 0.18f 0.36 Wuenscher & Acker
Federal (1969)
Republic of 1973 50 (N) - 0.14 0.23 Acker & Schulte
(1974)
Greece - 50 (N/B) II 0.23 0.87 Panetsos et al.
(1975)
Italy 1965 9 (N) II 0.59 2.77 Kanitz & Castello
(1966)
1966 22 (N/B) II 0.68f 1.55 Del Vecchio & Leoni
(1967)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
Italy 1965-68 33 (B) - 0.32 3.15 Paccagnella et al.
(contd.) (1971)
1965-68 11 (B) - 1.95 5.70 Paccagnella et al.
(1971)
1965-68 52 (N) - 0.91 3.55 Paccagnella et al.
(1971)
Netherlands 1964 34 (N) II 0.31f - Wit (1971)
1966 11 (N) II 0.20 0.50 De Vlieger et al.
(1968)
1968-69 34 (N) II 0.27f 1.5 Wit (1971)
1973-74 102 (N) - 0.2 - Greve & Wegman (1985)
1975 25 (N) - 0.11 - Greve & Wegman (1985)
1976 74 (N) - 0.09 - Greve & Wegman (1985)
1977-78 78 (N) - 0.11 - Greve & Wegman (1985)
1979 25 (B) - 0.09 - Greve & Wegman (1985)
1980 24 (N) - 0.10 - Greve & Wegman (1985)
1981 53 (N) - 0.07 - Greve & Wegman (1985)
1982 54 (N) - 0.07 - Greve & Wegman (1985)
1983 78 (N) - 0.06 - Greve & Wegman (1985)
Spain - 40 (B) III 0.15 0.49 Herrera Marteache et
al. (1978)
Switzerland 1972 13 (B) II 0.29 0.57 Zimmerli & Marek
(1973)
United 1961 131 (N) II 0.21 1.29 Hunter et al. (1963)
Kingdom 1963-64 66 (N) II 0.26 0.9 Egan et al. (1965)
1964 50 (N) II 0.27 0.85 Robinson et al.
(1965)
1964 50 (B) II 0.25 0.65 Robinson et al.
(1965)
1965 101 (N) II 0.34 1.80 Cassidy et al. (1967)
1966 53 (B) II 0.21 0.60 Hunter et al. (1967)
1965-67 248 (N) II 0.21 1.0 Abbott et al. (1968)
1967 18 (B) II 0.27 0.68 Hunter et al. (1967)
1969-71 201 (N) II 0.16 0.68 Abbott et al. (1972)
1976-77 236 (N) II 0.11 0.49 Abbott et al. (1981)
1982-83 187 (N) - 0.074 0.27 UK-HMSO (1986)
Africa
Kenya 1969-70 32 (N) III 0.030d 0.18 Wassermann et al.
(1972b)
1969-70 51 (N) III 0.064e 0.28 Wassermann et al.
(1972b)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
Africa (contd.)
Nigeria 1969 46 (N) III 0.059d 0.73 Wassermann et al.
(1972c)
1969 90 (N) III 0.13e 0.98 Wassermann et al.
(1972c)
South Africa 1969 114 (N/B) IV 0.039 - Wassermann et al.
(1970)
Uganda 1969-70 16 (N) III 0.023d 0.058 Wassermann et al.
(1974a)
1969-70 39 (N) III 0.031e 0.59 Wassermann et al.
(1974a)
Asia
India 1964 35 (N) II 0.04 0.36 Dale et al. (1965)
Iran 1974-76 170 II 0.049 0.75 Hashemy-Tonkabony &
Soleimani-Amiri
(1978)
Israel 1967-69 61 (N) III 0.10d 0.315 Wassermann et al.
(1974b)
1967-69 162 (N) III 0.14e 3.96 Wassermann et al.
(1974b)
Japan Prior to 1973 241 (N) II 0.13 0.98 Curley et al. (1973)
1974-75 59 (N) II 0.09f 0.51 Yoshimura et al.
(1979)
Thailand 1969-70 8 (N) III 0.077d 0.459 Wassermann et al.
(1972d)
1969-70 27 (N) III 0.10e 1.20 Wassermann et al.
(1972d)
1975-76 9 II 0.322 - Department of
Agriculture Thailand
(1976)g
Oceania
Australia 1965 53 (N) II 0.046 0.43 Bick (1967)
1965-66 12 (N) IV 0.67 0.99 Wassermann et al.
(1968)
1969-70 75 (N) II 0.21 2.60 Brady & Siyali (1972)
------------------------------------------------------------------------------------------
Table 19. (contd.)
------------------------------------------------------------------------------------------
Country Year No. of Method of Dieldrin Reference
samplesa clean-upb Mean Maximum
(mg/kg fat)
------------------------------------------------------------------------------------------
Oceania (contd.)
New Zealand Prior to 1967 45 (N) II 0.28 0.77 Brewerton & McGrath
(1967)
1965 43 (B) II 0.41 - Copplestone et al.
(1973)
1966 54 (B) II 0.30 - Copplestone et al.
(1973)
1967 68 (B) II 0.43 - Copplestone et al.
(1973)
1968 64 (B) II 0.33 - Copplestone et al.
(1973)
1969 25 (B) II 0.27 - Copplestone et al.
(1973)
Papua New 1969-70 38 (N) II 0.17 0.72 Brady & Siyali (1972)
Guinea
------------------------------------------------------------------------------------------
a Samples taken at necropsy (N) or during elective surgery (B).
b Method of clean-up:
I Removal of neutral lipids at -70 °C.
II Separation into two or more fractions by eluting from a Florisil column (with prior
liquid/liquid partition to reduce neutral lipid content, in most investigations using
this clean-up procedure).
III Florisil column clean-up without separation into two or more fractions.
IV Kontes co-distillation.
- Method not reported.
c Upper confidence limit ( P = 0.025) for the set of samples.
d Age group 5-24 years.
e Age group 25 years and older.
f Results expressed in terms of extractable lipid content.
g Personal communication to IPCS in 1987.
5.2.1.3 Concentrations of dieldrin in blood
The concentrations of dieldrin in whole blood or serum of
members of the general population have been determined in a few
countries and are summarized in Table 20. The concentrations are
very low (µg/litre) and it is essential that the sensitivity of the
analytical method is at least 0.1 µg/litre. Two analytical
procedures have been used (Dale et al., 1966; Richardson et al.,
1967a), which give significant different results: the acetone
extraction procedure (method II in Table 20) gives results that are
about 50% higher than the hexane extraction procedure (method I in
Table 20) and showed a better reproducibility (Robinson et al.,
1967a). An interlaboratory comparison of the hexane extraction
method showed that large variations in results may occur (Thompson,
1976).
Table 20. Concentration of dieldrin in the blood of the general population
------------------------------------------------------------------------------------------
Country Year Number of Analytical Dieldrin Reference
samplesa methodb Mean Maximum
(µg/litre)
------------------------------------------------------------------------------------------
USA 1965 10 (B) I 1.4 2.8 Dale et al. (1966)
1967-68 1000 (S) I 0.5 25 Watson et al. (1970)
1967-71 970 (S) I 0.9 - Warnick (1972)
1967-68 37 (H) III 4 - Morgan & Roan (1970)
1970 202 (S) I 0.9 10 Wyllie et al. (1972)
Prior to 1981 59 (S) I 0.6 10.1 Barquet et al.
(1981)
1976-80 6078 (S) ? ~1.4c 16 Murphy & Harvey
(1985)
Hawaii 1968-70 1107 (S) I 1.46 11 Klemmer et al.
(1973)
Lanai Island 1968-70 484 (S) I 1.3 26 Klemmer et al.
(1973)
Europe
Netherlands 1978 70 (B) - < 0.5 - Greve & Wegman
(1985)
1980 48 (B) - < 0.4 - Greve & Wegman
(1985)
1981 127 (B) - < 0.4 - Greve & Wegman
(1985)
1982 54 (B) - < 0.5 - Greve & Wegman
(1985)
Switzerland 1972 ~100 (S) I 1.1 - Zimmerli & Marek
(1973)
United Kingdom 1962 20 (B) II 1.6 10.0 Hunter et al. (1967)
1964 61 (B) II 1.4 5.0 Hunter et al. (1967)
1965 25 (B) II 1.7 8.7 Hunter et al. (1967)
1966 55 (B) II 1.8 4.3 Hunter et al. (1967)
1968 18 (B) II 0.9 1.1 Robinson & Roberts
(1969)
Oceania
Australia - 52 (B) Iust 2.3 13 Siyali (1972)
- 47 (B) Iust none - Siyali (1973)
------------------------------------------------------------------------------------------
a Samples of whole blood (B), serum (S), whole blood from heart chamber (during autopsy)
(H).
b Analytical methods (all use gas-liquid chromatography with an electron-capture detector):
I Hexane extraction.
Iust Hexane extraction combined with ultrasonic treatment.
II Acetone extract on silica gel column.
III Solvent extraction and Florisil column clean-up.
c In 260 positive samples.
5.2.1.4 Concentration of dieldrin in other tissues
A few investigations of the concentrations of dieldrin in other
body tissues have been made and some of the results are summarized
in Table 21.
Table 21. Concentration of dieldrin in various tissues from members of the general
population
------------------------------------------------------------------------------------------
Tissue Country Year No. of Dieldrin Reference
samples Mean Maximum
(mg/kg)
------------------------------------------------------------------------------------------
Liver Canada 1967-68 50 0.25a 3.0a Kadis et al. (1970)
USA 1967 42 0.009 - Casarett et al. (1968)
USA 1966 42 0.035 0.22 Fiserova-Bergerova et al. (1967)
USA 1966-68 35 0.047 - Morgan & Roan (1970)
Denmark 1972-73 18 0.29a - Kraul & Karlog (1976)
Netherlands 1966 11 0.034 0.081 De Vlieger et al. (1968)
Japan 1974-75 30 0.39a 1.73a Yoshimura et al. (1979)
Thailand 1975-76 16 0.010 - Dept. of Agriculture, Thailand
(1976)b
Kidneys Canada 1967-68 47 0.10a 1.35a Kadis et al. (1970)
USA 1967 38 0.021 - Casarett et al. (1968)
USA 1966 42 0.013 0.04 Fiserova-Bergerova et al. (1967)
USA 1966-68 35 0.014 - Morgan & Roan (1970)
USA 1973 12 0.006 0.009 Anon (1974c)
Thailand 1975-76 16 0.010 - Dept. of Agriculture, Thailand
(1976)b
Brain Canada 1967-68 30 0.002a - Kadis et al. (1970)
USA 1967 32 0.003 - Casarett et al. (1968)
USA 1966 42 0.035 0.10 Fiserova-Bergerova et al. (1967)
USA 1966-68 35 0.007 - Morgan & Roan (1970)
Denmark 1972-73 21 0.057a - Kraul & Karlog (1976)
Netherlands 1966 28 0.0075 0.021 De Vlieger et al. (1968)
------------------------------------------------------------------------------------------
Table 21. (contd.)
------------------------------------------------------------------------------------------
Tissue Country Year No. of Dieldrin Reference
samples Mean Maximum
(mg/kg)
------------------------------------------------------------------------------------------
Brain Thailand 1975-76 16 0.010 - Dept. of Agriculture, Thailand
(contd.) (1976)b
Gonads Canada 1967-68 39 0.06a 0.86a Kadis et al. (1970)
USA 1967 36 0.008 - Casarett et al. (1968)
USA 1966 42 0.035 0.20 Fiserova-Bergerova et al. (1967)
------------------------------------------------------------------------------------------
a Results expressed in terms of extractable lipid content.
b Personal communication to IPCS in 1987.
5.2.2. Babies, infants, and mother's milk
Dieldrin penetrates the placenta and, as a result of
transplacental exposure, may occur in the blood, adipose tissue,
and other tissues of the fetus and newborn baby (Table 22). The
concentrations are lower by a factor of 2 - 10 than those of their
mothers or other adults (Table 19). There is no difference between
infants and adults in the brain/liver/fat ratio of dieldrin
concentrations (Fiserova-Bergerova et al., 1967; Casarett et al.,
1968). A similar situation exists in animals, e.g., pigs (Uzoukwu
& Sleight, 1972).
Dieldrin is also excreted in the milk of human beings and
various animal species. Table 23 summarizes the concentrations of
dieldrin found in human milk over the last 15 years in various
countries, mean concentrations up to 6 µg/litre having been
reported. Higher values, occurring occasionally in a few regions,
have been associated with house and garden use of aldrin/dieldrin.
Thus, in the first several months, a breast-fed infant drinking
approximately 150 ml milk/kg body weight per day has a daily intake
of 0.15 - 0.9 µg dieldrin/kg body weight.
Acker et al. (1984) studied the problem of residues in human
milk and the importance of breast-feeding for the newborn baby.
They concluded that, at least in the early months, the value of
breast-feeding outweighed the possible risks from residues of
dieldrin, in this case, in human milk. They calculated that the
average daily intake of dieldrin by newborn babies was
approximately 0.7, 0.75, 0.65, and 0.65 µg/day, respectively, for
the 1st, 2nd, 3rd, and 4th months of breast-feeding.
Aldrin has rarely been detected in human milk. It was not
detectable in 202 samples of Dutch human milk (Wegman & Greve,
1974; Greve & Wegman, 1985), and in only one (21.8 µg/litre) of 50
Norwegian samples (Bakken & Seip, 1976).
Table 22. Concentration of dieldrin in blood and fat of fetus, newborns, infants, and adults
---------------------------------------------------------------------------------------------------------
Country Year Age Number Dieldrin in Number Dieldrin in Reference
of blood of fat
samples Mean Maximum samples Mean Maximum
(µg/litre) (mg/kg fat)
---------------------------------------------------------------------------------------------------------
North America
Canada 1982 mothers during 16 0.1 - Mes et al.
lactation (1984)
USA 1966 fetus, stillborn 6 0.17 0.38 Fiserova-
0-5 years 12 0.14 0.34 Bergerova et
6-10 years 6 0.07 0.26 al. (1967)
31-83 years 12 0.34 0.7
USA 1968 newborn 26 0.7 1.5 3a 0.24 0.35 Curley et al.
stillborn 4 NDb 7 ND (1969)
South America
Argentina 1969 mothers 13 1.63 Radomski et al.
-70 newborn 13 0.59 (1971)
1-5 years 19 0.54
5-10 years 18 0.94
adults 20 1.43
1970 newborn 3 0.12 0.13 Astolfi et al.
0-4 months 6 0.02 0.07 (1973)
4-12 months 4 0.05 0.07
1-4 years 14 0.06 0.13
over 4 years 20 0.07 0.25
Brazil 1969 stillborn 28 0.011 0.174 Wassermann et
-70 5-24 years 17 0.023 0.122 al. (1972a)
Europe
Netherlands 1979 newborn 87 0.3 4.6 Eckenhausen et
2 weeks 22 0.5 - al. (1981)
2 months 17 0.4 -
3 months 8 0.5 -
---------------------------------------------------------------------------------------------------------
Table 22. (contd.)
---------------------------------------------------------------------------------------------------------
Country Year Age Number Dieldrin in Number Dieldrin in Reference
of blood of fat
samples Mean Maximum samples Mean Maximum
(µg/litre) (mg/kg fat)
---------------------------------------------------------------------------------------------------------
Netherlands mothers, pre-natal 48 0.8 3.5
(contd.) mothers, post-natal 73 0.4 4.1
Spain 1982 mothers 10 6 23 Gonzalez-
(Cordoba) babies 10 8 50 Rodriquez
Cordoba et al.
(1983)
United 1969 1 newborn, stillborn 3 0.01 0.02 Abbott et al.
Kingdom -71 1 day-3 months 8 0.03 0.07 (1972)
3 months-4 years 9 0.05 0.10
over 4 years 201 0.16 0.68
1976 newborn 1 0.03 - Abbott et al.
-77 2 months 1 0.02 - (1981)
3 months 1 0.09 -
over 4 years 236 0.11 0.49
Africa
Nigeria 1969 stillborn 31 0.002 0.014 Wassermann et
0-11 months 47 0.019 0.087 al. (1972c)
1-4 years 54 0.023 0.083
adults 90 0.13 0.98
Asia
Israel 1968 fetus 23 1.3 - - - - Polishuk et al.
-69 pregnant woman 24 1.6 - 16 0.084 - (1970)
non-pregnant woman - - - 33 0.172 -
Israel 1967 stillborn 44 0.019 0.118 Wassermann et
-69 0-11 months 40 0.021 0.125 al. (1974b)
5-24 years 61 0.101 0.315
adults 162 0.136 3.96
---------------------------------------------------------------------------------------------------------
a Stillborn.
b ND = not determined.
Table 23. Concentration of dieldrin in mother's whole milk
-------------------------------------------------------------------
Country Year No. of Dieldrin Reference
samples Mean Maximum
(µg/litre)
-------------------------------------------------------------------
North America
Canada 1969-70 48 0.09g 0.25g Holdrinet et al.
(Ontario) 1971-72 34 0.04g 0.17g (1977)
1973-74 24 0.04g 0.08g
1978-79 154 1a 26b Dillon et al.
(1981)
1982 ~128c ~1.3 1.8 Mes et al. (1984)
USA 1972-73 57 < 10 50 Kutz et al. (1979)
1973-74 57 4 50 Strassman & Kutz
(1977)
1973-75 40 6 42b Barnett et al.
(1979)
1972-75 1436 ~5 15 Savage et al.
(1981)
Hawaii 1979-80 54 0.04g 0.09g Takei et al.
(1983)
Central America
El Salvador 1973-74 40 5 15 De Campos &
Olszyna-Marzys
(1979)
Guatemala 1971 46 2 10 De Campos &
Olszyna-Marzys
(1979)
Europe
Belgium 1968 20 3.4 8 Heyndrickx & Maes
(1969)
Denmark 1982 57 0.04g 0.47g Anderson & Orbaek
(1984)
Germany, 1981 91 0.05g 0.44g Rohwer (1983b)
Federal
Republic of 1982 132 0.01g 0.3g Cetinkaya et al.
(1984)
Netherlands 1969 48 3 11 Tuinstra (1971)
1972 202 5 - Wegman & Greve
(1974)
1983 278 0.03g 0.22g Greve & Wegman
(1985)
-------------------------------------------------------------------
Table 23. (contd.)
-------------------------------------------------------------------
Country Year No. of Dieldrin Reference
samples Mean Maximum
(µg/litre)
-------------------------------------------------------------------
Europe (contd.)
Netherlands 1979 69 2.3 - Eckenhausen et al.
(1981)
Norway 1975 50 2.75 3.6 Bakken & Seip
(1976)
Portugal 1972 164 11 21 Graca et al.
(1974)
Spain 1981 20 3 14 Baluja et al.
(1982)
Sweden 1978 51d 22g 54g Noren (1983a,b)
(Stockholm) 1979 54d 20g 31g
1980 36d 18g 23g
Switzerland 1983 6 0.5 1 Disler et al.
(1984)
United 1963-64 19 6 13 Egan et al. (1965)
Kingdom 1979-80 102 2 12 Collins et al.
(1982)
1983-84 40 5 32 UK-HMSO (1986)
Africa
Kenya 1983-85 292 range: 2.3-98 Kanja et al.
(1986)
Asia
Israel 1975 29 7 - Polishuk et al.
(1977)
Japan 1973-77 116 2.3 - Yakushiji et al.
(1979)
Oceania
Australia 1970-71 23 5 11 Stacey & Thomas
(1975)
1971-72 40 25 68 Miller & Fox
(1973)
-------------------------------------------------------------------
Table 23. (contd.)
-------------------------------------------------------------------
Country Year No. of Dieldrin Reference
samples Mean Maximum
(µg/litre)
-------------------------------------------------------------------
Oceania (contd.)
Australia 1973 45 5 13 Siyali (1973)
1979-80 267c ~8.5 31 Stacey et al.
(1985)
1981 74e 13 35 Stacey & Tatum
(1985)
New Guinea 1972 74 0.7 13.2 Hornabrook et al.
(1972)
-------------------------------------------------------------------
a The authors stated that they found aldrin. However, they
probably meant dieldrin, since, in mother's milk, the presence of
aldrin without dieldrin is highly unlikely, whereas the reverse
is the rule.
b In an area of high pesticide use.
c 128 samples from 16 women.
d Number of samples included 745, 805, and 973, respectively.
e 74 samples from 14 women.
f Many of the houses had been treated against termites, but the
pesticides used were unknown.
g On lipid basis in mg/kg.
During the first trimester, and usually during the first year,
of a baby's life, the concentration of dieldrin in the blood and
adipose tissue does not increase and, in most cases, decreases
(Astolfi et al., 1974) (Table 22).
The concentration of dieldrin in the blood of breast-fed babies
is not higher that that in bottle-fed babies (Eckenhausen et al.,
1981), and it is lower than it is in adults.
A study on organochlorine insecticides in the blood of mothers
and newborn babies was carried out in an agricultural rural area in
the Mississippi Delta (USA). In total, 209 black and 130 white
mother-newborn pairs participated. Dieldrin was detected in the
blood of 43.5% of black and 51.5% of white mothers and in the blood
of 19.1% of black babies and 10% of white babies. The blood
concentrations of both mothers and babies were less than 1
µg/litre. Maternal age and birth weight of the baby did not
correlate significantly with the prevalence, or with the mean
level, of maternal and infant insecticide residues in the blood
(d'Ercole et al., 1976).
Data on the occurrence of aldrin and dieldrin in human milk
have been submitted by Australia, Guatemala, Japan, and
Switzerland, Japan reporting a decline of the concentrations in
human milk during the period 1971 - 1979. Data on dieldrin in
human milk have been reported by Canada, the Federal Republic of
Germany, Mexico, the Netherlands, Sweden, Switzerland, and the USA,
none of the median levels exceeding 3 µg/kg milk. Levels in the
USA were below 10 µg/kg milk (limit of detection) (National Food
Administration, Uppsala, 1982).
6. KINETICS AND METABOLISM
6.1. Absorption
6.1.1. Aldrin
6.1.1.1 Ingestion
Aldrin is readily absorbed from the gastrointestinal tract and
through the skin; it is stored as dieldrin, mainly in adipose
tissue (section 6.2.1). Aldrin is readily metabolized to dieldrin
in plants and animals and is rarely found as such in food or in the
great majority of animals.
6.1.1.2 Inhalation
Inhalation studies by Beyermann & Eckrich (1973) on human
volunteers suggested that about 50% of inhaled aldrin vapour was
absorbed and retained in the human body. However, a study on 10
male volunteers exposed to actual aldrin vapour concentrations of
1.31 µg/m3 and some weeks later to 15.5 µg/m3 air for a period of
60 min suggested an actual retention in man of 20%.
Physical exertion did not have any significant effect on the
retention. Dieldrin could not be detected in the exhaled air. The
concentration of dieldrin in the blood of the volunteers was lower
than 1 µg/litre before and after exposure (Bragt et al., 1984).
6.1.2. Dieldrin
Studies on rabbits, dogs, monkeys, and human beings have shown
that dieldrin is absorbed through the intact skin (Shah & Guthrie,
1976; Sundaram et al., 1978; Fisher et al., 1985). There have been
many studies demonstrating the absorption of dieldrin through the
gastrointestinal tract (section 6.2).
6.1.3. Photodieldrin (and other metabolites of dieldrin)
Studies demonstrating the absorption of photodieldrin through
the gastrointestinal tract are summarized in section 6.2.3.
6.2. Distribution
6.2.1. Aldrin
6.2.1.1 Mouse
In studies by Deichmann et al. (1975), Swiss-Webster mice were
fed diets containing 0, 5, or 10 mg aldrin/kg, over seven
generations. The retention of dieldrin following the feeding of
aldrin over four generations significantly increased the
concentration of dieldrin in abdominal fat and in the lipids of the
total carcass. There was also a significantly increased retention
of dieldrin in the carcass in the F1 generation, with some further
(but not statistically significant) increase in concentration and
total retention of dieldrin in the F2 and F3 generation. The
dieldrin concentration in the total lipids of mouse carcasses were:
for the F0 generation, 60 mg/kg; for the males in the F1, F2, and
F3 generations, a mean of 100 mg/kg; and for the females in the F1,
F2, and F3 generations, a mean of 132 mg/kg. The dieldrin
concentration was below 1 mg/kg in pups from the F4 generation,
born of parents that carried a considerable load of aldrin or
dieldrin (thus exposed in utero and via lactation) and fed the
control diet from weaning to the age of 260 days. The
concentrations of dieldrin in the F5 and F6 generations were
similar to those in the 2nd - 4th generations.
6.2.1.2 Rat
When single oral doses of 10 mg aldrin/kg body weight were
given to neonate Sprague Dawley rats, aldrin was detectable up to 6
days after dosing in the stomach and small intestine, but only for
72 h in the kidneys. In the liver, the aldrin concentration
increased during the first 6 h, and then declined during the
following days. Dieldrin was detected as early as 2 h after dosing
and had reached a maximum after 24 h. It then declined. The only
metabolic conversion product detected in the liver was dieldrin.
The concentration of aldrin was very low relative to that of
dieldrin, except in the case of studies in which tissues were
analysed within a few hours of dosing with aldrin (Farb et al.,
1973).
In studies by Ludwig et al. (1964), two male Wistar rats were
given daily oral doses of 4.3 µg 14C-aldrin by stomach tube for 3
months and were killed 24 h after the final dose. The total
radioactivity in the body as a proportion of the total cumulative
dose was 3.6%, but, after 82 days, the value had fallen to 0.21%.
The ratio of dieldrin to aldrin in the carcass was approximately
15:1; in abdominal fat, it was about 18:1.
6.2.1.3 Dog
Deichmann et al. (1969, 1971), gave beagle dogs oral doses of
aldrin in capsules. Three males were given 0.3 mg aldrin/kg body
weight and 4 females were given 0.15 or 0.3 mg aldrin/kg body
weight, 5 days per week, for 14 months. During the last 10 months
of the dosing period, the concentration of dieldrin in the blood of
dogs given 0.3 mg aldrin/kg body weight was in the range 42 - 183
µg/litre, while the concentration in the subcutaneous fat was
37 - 208 mg/kg. The levels in the animals receiving 0.15 mg
aldrin/kg body weight were 40 - 130 µg/litre and 12 - 67 mg/kg in
blood and subcutaneous fat, respectively. The apparent partition
ratio, subcutaneous fat/blood, was about 1000.
6.2.1.4 Human studies
Little is known about the distribution of aldrin in the human
body after transfer from the gastrointestinal tract or skin into
the circulating blood. As a result of its relatively rapid
conversion to dieldrin, aldrin is rarely detected in human tissues.
6.2.2. Dieldrin
6.2.2.1 Laboratory animals
(a) Mouse
Following a preliminary comparison of the distribution of
dieldrin and three known animal metabolites in CFE rats and CFI
mice (Baldwin et al., 1972), a more detailed comparison was made of
male CFE rats and two strains of male mice (CFI and LACG) (Hutson,
1976). The latter study also included a comparison of the effects
of a pretreatment with diets containing dieldrin at 20 mg/kg diet
(rats) or 10 mg/kg diet (mice) for 4 weeks. 14C-Dieldrin was
administered orally as a single dose of about 3 mg/kg body weight
to both the pretreated and non-pretreated groups, and the animals
were killed 8 days after dosing. The concentrations of the
6,7-dihydroxy metabolite were below the limits of detection (less
than 0.02 mg/kg) in the fat, liver, and kidneys of all the animals.
The concentrations of the 9-hydroxy metabolite were very small or
below the limits of detection (less than 0.03 mg/kg) in the fat and
kidneys; small concentrations (about 0.4 mg/kg) were found in the
livers of the two strains of mice. The bridged pentachloroketone
(PCK) was present in the liver of CFE rats in small amounts (about
0.04 mg/kg), but quite large concentrations were found in the
kidneys: 2.48 (no pretreatment) and 6.11 mg/kg (4-week
pretreatment). The concentrations in the fat in both groups were
small (mean, 0.17 mg/kg). In the two strains of mice, the
concentrations of PCK in the liver were very small (about 0.5
mg/kg) except in the pretreated animals. Concentrations in the
kidneys of the two strains of mice were below the limits of
detection (less than 0.02 mg/kg) in the absence of pretreatment or
small (about 0.15 mg/kg) in pretreated mice. In the fat of the
mice (no pretreatment), the PCK concentrations were below the
limits of detection (less than 0.04 mg/kg), but, in the pretreated
mice, the concentrations were about 1.3 mg/kg. The concentrations
of dieldrin in the fat were much higher than in the other tissues,
and those in the mice were about twice those in the rat.
(b) Rat
Heath & Vandekar (1964) studied the transport of 36Cl-dieldrin
from the gastrointestinal tract by cannulation of the thoracic
lymph duct in rats. They found that only one-seventh of the
absorbed dieldrin was recovered from the lymph and most of the
dieldrin was absorbed via the portal vein.
Iatropoulos et al. (1975) indicated that the transport of
dieldrin from the gastrointestinal tract to the liver of Sprague-
Dawley rats is mainly through the portal venous system. However,
during the subsequent redistribution of dieldrin, the lymphatic
system seemed to be a major route.
When female Osborne-Mendel rats were fed a diet containing 50
mg technical dieldrin (87%)/kg for 6 months, the concentrations of
dieldrin in the blood, liver, and fat increased rapidly during the
first 2 weeks. During the next 26 weeks, the concentrations
fluctuated but did not appear to increase significantly. The mean
concentrations for the final 4 months were (groups of four to six
animals): in blood, 240 µg/litre; in liver, 6.8 mg/kg; and in fat,
159.5 mg/kg tissue. The distribution ratios (blood = 1) for this
period were: liver, 28 and fat, 666 (Deichmann et al., 1968).
In the studies by Walker et al. (1969b), groups of 25 male and
25 female Carworth Farm E rats were fed diets containing 0.1, 1, or
10 mg dieldrin (99%)/kg diet. The control group consisted of 45
animals of each sex. Small groups of rats were killed after 26,
52, and 78 weeks and the remaining animals after 104 weeks. The
concentration of dieldrin in blood, brain, liver, and fat was
estimated. An approximate plateau level was reached during the
first 26 weeks. The tissue uptake ratios (concentration of
dieldrin in tissues/concentration in diet) for female rats in the
three test groups were: in blood, 0.056; in brain, 0.19; in liver,
0.35; and in fat, 8.8. The uptake ratios for male rats were
significantly lower than those for females. The partition ratios
(concentration in tissues relative to that in blood) for
males/females, respectively, were: in brain, 3.3/2.6; in liver,
7.8/5.9; and in fat, 104/137. It was considered that the results
were consistent with the use of a compartmental model.
Osborne-Mendel rats (6 male and 6 female) were orally
administered approximately 50 µg 14C-dieldrin/kg body weight,
dissolved in corn oil, 5 days/week, for 9 weeks. The animals were
killed 24 h after the last dose, and the radioactivity in nine
tissues was measured. More radioactivity was retained in the
tissues by females than by males, except in the case of kidneys
(where the female:male ratio was about 0.3:1). Adipose tissue was
the main storage site for dieldrin. The lowest levels were present
in spleen, brain, and heart, while higher levels were found in
liver, lung, adrenals, and especially in the kidneys (Dailey et
al., 1970).
In a study on Charles River rats, administered 14C-dieldrin in
the diet for 8 h, Matthews et al. (1971) found a high level of
radioactivity in the kidneys. The same was found in the kidneys of
male rats in the study by Iatropoulos et al. (1975).
In studies by Baron & Walton (1971), male Osborne-Mendel rats
were fed diets containing 25 mg dieldrin/kg diet for 8 weeks. On
the first 4 days of the 9th week, oral doses of 14C-dieldrin were
administered, together with sufficient non-radioactive dieldrin to
maintain a 24-h intake equivalent to 25 mg/kg diet. Groups of five
rats were killed on days 1 - 4 of the 9th week. The remaining rats
were divided into two groups, one group being fed the diet
containing 25 mg dieldrin/kg and the other being given the control
diet. An equilibrium level of 50 mg dieldrin/kg adipose tissue was
reached by the 8th week. The concentration of dieldrin in the
adipose tissue of the animals given the control diet in the 9th
week declined rapidly during the subsequent 18 days. The rate of
decline corresponded to a half-life of about 4 - 5 days. It was
postulated that an active transport of dieldrin into and out of
fat, differing from the mechanism for lipids, may have occurred
(Baron & Walton, 1971).
Groups of two male and two female Sprague-Dawley rats were
administered dietary concentrations of 0.04 mg 14C-dieldrin/kg,
0.04 mg 14C-dieldrin/kg plus 0.16 mg dieldrin/kg, or 0.04 mg
14C-dieldrin/kg plus 1.96 mg dieldrin (99%)/kg, for 39 weeks, and
the animals were then killed. The daily intake of food was
restricted to 12 and 15 g for female and male animals,
respectively. In all three groups, the recovery of 14C activity in
whole carcasses, as a proportion of the total administered dose,
was significantly higher in female rats (mean 6.9%) than in male
rats (mean 2.1%) (Davison, 1973).
When single doses of 10 mg dieldrin/kg body weight (in corn
oil) were administered orally to male Sprague-Dawley rats, the
concentration of dieldrin in the plasma attained a maximum value
(500 µg/litre) after about 2 h. Up to 48 h after dosing, it
fluctuated between 200 and 500 µg/litre, but then declined quite
rapidly to about 10 µg/litre during the next 8 days. In the brain,
the highest concentration (about 1 mg/kg) was attained after about
4 h; it remained essentially steady for a further 44 h, and then
declined in a similar manner to that in the plasma. The
concentration/time relationships for muscle, kidneys, and liver
were similar to those for the brain. A slower approach to a
maximum value was observed in retroperitoneal fat, the 4 h and 24 h
concentrations being about 10 and 40 mg dieldrin/kg fat,
respectively. After 48 h, the concentration in fat declined in a
similar manner as did those in the plasma and brain (Hayes, 1974).
Moss & Hathway (1964) administered 14C-dieldrin
intraperitoneally to rats, and determined the partition of
radioactivity between plasma and erythrocytes. The ratio
(plasma:erythrocytes) 2 h after dosing was 2.1:1; 4 days after
dosing, it was 1.6:1, though the activities had declined by 49% and
32%, respectively, in plasma and erythrocytes.
(c) Rat and rabbit in vitro
The partition of 14C-dieldrin-related activity between the
soluble proteins of blood and the cellular components has been
studied in vitro. The radioactivity was located mainly in the
erythrocytes and plasma of rats and rabbits, whereas that in
leukocytes, platelets, and erythrocyte membranes was much lower.
The activity in the erythrocytes was associated with haemoglobin
and an unknown constituent. The radioactivity in the serum of rats
(electrophoresis at pH 8.6) was associated with pre- and post-
albumin, whereas that in rabbit serum was associated with albumin
and alpha-globulin. Electrophoresis at pH 4.5 gave a pattern which
was similar in rats and rabbits but the patterns at pH 4.5 were
different from those at pH 8.6; there were four incompletely
separated peaks of radioactivity (Moss & Hathway, 1964).
It has been demonstrated in vitro that the transport of
dieldrin between rat hepatocytes and the extracellular medium is a
much faster process than the metabolic transformation reaction in
hepatocytes (Ichinose & Kurihara, 1985).
(d) Dog
In studies by Richardson et al. (1967b), three beagle dogs were
fed a diet containing dieldrin (equivalent to 0.1 mg/kg body
weight) for 128 days, and two animals were used as controls. The
concentration of dieldrin in the blood increased in an
approximately curvilinear manner up to day 93. There were
fluctuations during the next 5 weeks, but any increase was small
relative to that during the first 5 weeks of the study (a mean
plateau concentration of about 130 µg/litre blood appears to be
consistent with the data). One week after the dieldrin diet was
discontinued, the dogs were killed and samples of blood, fat,
heart, liver, kidneys, pancreas, spleen, lung, and muscle were
taken for analysis. The mean concentrations of dieldrin in the
organs and tissues were 150 µg/litre in blood, 1090 µg/kg in the
heart, 4420 µg/kg in liver, 2330 µg/kg in kidneys, 14 030 µg/kg in
pancreas, 710 µg/kg in spleen, 1227 µg/kg in lungs, 25 333 µg/kg in
fat, and 566 µg/kg in muscle. The mean partition ratio fat/blood
was 161. There was a highly significant linear relationship
between the logarithm (log10) of the concentration of dieldrin in
the blood and the logarithm (log10) of the length of the dosing
period.
Six mongrel dogs (four males, two females) were orally dosed
daily with dieldrin dissolved in corn oil for 5 days (1 mg
dieldrin/kg body weight) and thereafter at doses of 0.2 mg/kg body
weight for a further 54 days. Six control animals were used.
Samples of blood were taken twice weekly from day 7 onwards and
analysed for dieldrin content. The concentration of dieldrin in
the blood of all the animals showed a small but significant
increase from day 7 to day 59. Biopsy samples of subcutaneous fat
were obtained on days 16 and 50. The fat/blood partition ratio on
day 16 was 216 and that on day 50 was 117 (Keane & Zavon, 1969b).
In studies by Walker et al. (1969b), groups of five male and
five female beagle dogs were given daily oral doses (by capsule in
olive oil) of dieldrin (99%) at 0, 0.005, or 0.05 mg/kg body
weight, for 2 years. The concentration of dieldrin in the blood
increased in all animals during the first 12 weeks of the study and
reached an approximately steady state value from week 18 to about
week 76. During the last 6 months, there were significant
deviations from the apparent asymptotic value for weeks 18 - 76.
The reasons for this are not understood, but there was also an
upward tendency in the concentration of dieldrin in the control
animals. There were statistically significant relationships
between the concentrations of dieldrin in the diet (calculated
from the daily oral dose) and those in the blood, brain, liver, and
adipose tissue. The tissue uptake ratios were similar in both
males and females, those for males being (concentration of dieldrin
in diet = 1): blood, 0.06; brain, 0.22; liver, 4.4; and adipose
tissue, 10.0. There were also statistically significant
relationships between the concentrations of dieldrin in the blood
and those in the other three tissues. The partition ratios
(concentration of dieldrin in blood = 1) for the male dogs were:
brain, 3.7; liver, 10; and adipose tissue, 169.
(e) Monkey
Two female rhesus monkeys were given an intravenous injection
of 14C-dieldrin (2.5 mg/kg body weight) in 1,2-propylene glycol and
two male rhesus monkeys received, respectively, a single oral dose
of 14C-dieldrin at 0.5 or 0.36 mg/kg body weight. The females were
killed 75 days after dosing and the males 10 days after dosing.
With both routes of administration, the highest radioactivity was
found in the adipose tissue, bone marrow, and liver. The activity
in the brain was relatively low (about 2% of that in the adipose
tissue). Metabolites were not found in the organs, but they were
present in the bile (Mueller et al., 1975b).
In studies by Mueller et al. (1979), groups of 1 - 5 male
rhesus monkeys were fed diets containing 0, 0.01, 0.1, 0.5, or 1 mg
dieldrin/kg diet for 70 - 74 months. Two other rhesus monkeys were
fed 5 mg dieldrin/kg diet for 4 months, 2.5 mg/kg for the next 5
months, and 1.75 mg/kg for a further 64 months. One rhesus monkey
was fed 5 mg/kg for 4 months, 2.5 mg/kg for the next 5 months, and
then 1.75 mg/kg diet, this dietary concentration gradually
increasing until after 23 months from the onset of the trial it had
reached 5 mg/kg (this feeding level being continued for a further
46 months). The mean concentrations of dieldrin in the livers of
these monkeys were: in the 0.01 mg/kg group, 1.2 mg/kg; in the 0.1
mg/kg group, 1.3 mg/kg; in the 0.5 mg/kg group, 4.1 mg/kg; in the 1
mg/kg group, 5.5 mg/kg; in the 5.0/2.5/1.75 mg/kg group, 13.6
mg/kg; and in the one animal fed 5, 2.5, 1.75, and 5 mg/kg diet,
23.3 mg/kg. The distribution of dieldrin in liver subcellular
fractions was determined by isotope dilution. The highest
proportion of dieldrin was present in the microsomal fraction, with
about 60% of the total in the subcellular fractions, and about
12.5% of the total in the soluble fraction. The remaining 3
fractions (nuclear, mitochondrial, and lysosomal) contained similar
proportions, about 9% in each fraction (Wright et al., 1978). The
modes of distribution of dieldrin (and metabolites) in rhesus
monkeys were similar to those in rats.
6.2.2.2 Transplacental transport
(a) Mice
Pregnant mice were each given 0.4 mg 14C-dieldrin
intramuscularly and its distribution was studied by means of whole-
body autoradiography. The highest values for 14C activity were
found in the fat, liver, intestines, and mammary glands, while
moderate activity was found in the ovaries and brain. Moderate
levels were also found in fetal liver, fat, and intestines,
indicating transfer across the placenta (Baeckstroem et al., 1965).
(b) Rat
Transplacental transfer of 14C-dieldrin was found in Sprague
Dawley rats that were administered the compound intravenously (tail
vein) on days 13, 16, or 21 of gestation. Relatively high levels
were present in the fetus 5 min after injection, and they continued
to increase for 40 - 60 min after which they declined by about 60%
in 2 - 3 days. The transfer of 14C activity was greater during
late gestation. Phenobarbital pretreatment decreased the amount of
radioactivity in the fetus (Eliason & Posner, 1971).
(c) Rabbit
The transport of 14C activity from mother to blastocyst and
from mother to fetus was demonstrated in pregnant New Zealand white
rabbits following intravenous injection of 14C-dieldrin into the
ear vein (0.14 mg dieldrin/kg body weight). The 14C activity in
blastocysts of rabbits injected on the 6th day of pregnancy was
generally low compared with the activity in maternal blood.
However, 40 - 60 min after dosing, the activities were very
similar. After 60 min, the 14C activity in blastocysts declined
rapidly, relative to that in maternal blood. In rabbits dosed
intravenously on the 16th day of pregnancy, the transfer of 14C
activity was transplacental, no activity being detected in
allantoic or amnionic fluids. The ratio of 14C activity in the
whole fetus to that in the maternal blood remained fairly constant
up to 100 min after dosing, suggesting an equilibrium between the
mother and the fetus. The results for rabbits injected on the 24th
day of pregnancy indicated that two-way placental transport of 14C
activity was occurring (Hathway et al., 1967).
6.2.2.3 Domestic animals
Studies on domestic animals, in which body tissues, milk, or
eggs were analysed, indicate that the pharmacokinetics of aldrin
and dieldrin in these species are broadly similar to those in
laboratory animals (Gannon et al., 1959a,b; Ivey et al., 1961;
Williams et al., 1964; Cummings et al., 1966; Davison, 1970, 1973;
Brown et al., 1974). None of the known metabolites of dieldrin
were detected in the body tissues or milk of cows fed 14C-dieldrin
in their diet for 41 days (Baldwin, 1972; Potter et al., 1972).
Dieldrin accumulation ratios (concentration in tissues, milk,
or eggs relative to the concentration in the diet) are given in
Table 24.
Table 24. Accumulation ratios for dieldrin in domestic animals
-----------------------------------------------------------------------------
Animal Sample Feeding period Accumulation Reference
analysed (months) ratio
-----------------------------------------------------------------------------
Cow renal body fat 3 2.43 Gannon et al. (1959a)
whole milk 3 0.18 Gannon et al. (1959b)
milk fat 12 6 Vreman et al. (1980)
Hen renal body fat 3 43.1 Gannon et al. (1959a)
body fat 13 10-24 Brown et al. (1974)
egg 7 1.5 Cummings et al. (1966)
Hog renal body fat 3 2.9 Gannon et al. (1959a)
Hog body fat 2 1.14 Dobson & Baugh (1976)
(young) (body weight
increase, 290%)
Lamb renal body fat 3 1.05 Gannon et al. (1959a)
Steer renal body fat 3 3.95 Gannon et al. (1959a)
-----------------------------------------------------------------------------
6.2.2.4 Human volunteers
A study on volunteers was carried out in which daily oral doses
of 0, 10, 50, or 211 µg dieldrin/man (three men per dose group)
were given in gelatine capsules for 18 months (Hunter & Robinson,
1967; Hunter et al., 1969). The control group comprised four men.
From the 18th month to the 24th month, the volunteers given 50 µg
continued to receive dieldrin at this level, whereas all other
volunteers, including those in the control group, received 211
µg/day. The concentrations of dieldrin in the blood of the
volunteers given 211 µg dieldrin daily throughout the study had
increased 10-fold by the end of 18 months to 15 µg/litre, while
that of the group given 50 µg/day had increased 4-fold to 5
µg/litre. The increase in the case of the group given 10 µg/day
was slight; after 5 months, a 2-fold increase had occurred to 3
µg/litre, and there was little change during the subsequent 13
months. From 21 - 24 months, the concentrations of dieldrin in the
blood of the groups given 50 or 211 µg/day fluctuated, but there
was no indication of a significant continuing increase in either
set of samples. The concentrations of dieldrin in adipose tissue
after 15 months had increased approximately 3-fold in the group
given 10 µg/day (mean: 0.4 mg/kg tissue), approximately 4-fold in
the group given 50 µg/day (mean, 0.7 mg/kg tissue), and
approximately 11-fold in the group given 211 µg/day (mean, 2 mg/kg
tissue). The concentrations of dieldrin in the adipose tissue
showed an apparent increase at 24 months relative to those at 18
months, but this may be partly related to the fact that the samples
were taken by needle biopsy at 24 months. Overall, it was
concluded that the results for the groups given 50 or 211 µg/day
indicated an approach to an upper limit (asymptote), the
relationship being of the form:
concentration of dieldrin in tissues = A - Be-kt
where A is the asymptotic value attained as time (t) approaches
infinity, and B and k are empirical constants (k corresponds to the
first-order rate constant for the elimination of dieldrin). The
mean values of the asymptote (A) for blood were 5.9 µg/litre in the
group given 50 µg/day and 20.2 µg/litre in the group given 211
µg/day. Relationships were also derived between the daily intake
of dieldrin and the steady-state (asymptotic) values for blood and
adipose tissue, respectively:
concentration of dieldrin in
blood (µg/litre)
amount of dieldrin ingested = -------------------------------------
(µg/day) 0.086
concentration of dieldrin in
adipose tissue (mg/kg)
= -------------------------------------
0.0185
It is emphasized that these relationships correspond to the
condition of a steady state between intake, storage, and
elimination of this compound. The distribution ratio
(concentration of dieldrin in adipose tissue/concentration in
blood) was 136 (Hunter & Robinson, 1967; Hunter et al., 1969).
6.2.2.5 General population
De Vlieger et al. (1968) collected samples of brain tissue,
liver, and adipose tissue from 11 routine autopsies in the
Netherlands, and found a significant relationship between the
dieldrin concentrations in the various tissues. They suggested a
tentative scheme for the distribution of dieldrin between the
various tissues. This scheme is reproduced in Fig. 1, but the
figures have been updated by recalculation conforming to the latest
empirical formula of Hunter et al. (1969) (Jager, 1970).
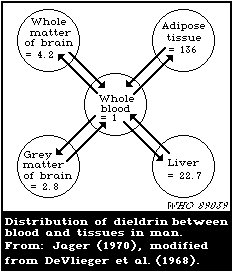 6.2.3. Photodieldrin (and major metabolites of dieldrin)
6.2.3.1 Laboratory animals
(a) Rat
Brown et al. (1967) fed rats diets containing 3 or 10 mg
photodieldrin/kg diet for 26 days, the 10 mg/kg-group then being
fed a control diet for a further 2 or 8 days. The half-life of
photodieldrin in adipose tissue was calculated to be 1.7 days in
male rats and 2.6 days in female rats. The storage ratio in
adipose tissue was considerably higher in females (1.3) than in
males (0.5).
In studies by Dailey et al. (1970), young rats were given daily
oral doses of 5 µg 14C-photodieldrin per rat, orally or
intraperitoneally, for 12 weeks. Although there was considerable
variation, the radioactivity in the tissues of female rats was
3 - 10 times greater than in male rats, except in the kidneys,
where the 14C activity in males was about 13 times that in females,
regardless of the route of administration.
When rats were fed diets containing 0, 0.1, 1, 10, or 30 mg
photodieldrin/kg diet for 13 weeks, the concentrations of
photodieldrin in the body tissues of female rats receiving up to 10
mg/kg diet were 2 - 15 times greater than those in males. High
concentrations of pentachloroketone (PCK) were found in the kidneys
of male rats receiving 30 mg/kg (276 mg PCK/kg kidney weight
compared with 29 mg photodieldrin/kg). The corresponding
concentrations for females were lower: 13.55 mg PCK and 1.85 mg
photodieldrin per kg kidneys (Walker et al., 1971).
In studies by Walton et al. (1971), groups of weanling rats
(Charles River strain) were fed photodieldrin at concentrations of
0, 1, 5, or 25 (decreased to 12.5 mg) mg/kg diet for 90 days, while
other groups of rats were fed dieldrin at the same concentrations.
The concentrations of both photodieldrin and dieldrin in the
adipose tissue of female rats were higher than in male rats.
(b) Dog
Following the administration of a single oral dose of
photodieldrin to one male and one female dog (160 and 120 mg/kg
body weight, respectively), the concentrations of photodieldrin in
the female dog's tissues, with the exception of the liver, were
much higher than those in the male (Brown et al., 1967).
The concentrations of photodieldrin in the liver and adipose
tissue of dogs fed photodieldrin at 0, 0.005, 0.05, or 0.2 mg/kg
body weight for 3 months were related to the dose rate and similar
in males and females. In the kidneys, the concentrations of
photodieldrin and its metabolite (PCK) were similar in male and
female dogs and much lower (of the order of 0.1 - 0.2 mg/kg
kidneys) than in rats (Walker et al., 1971).
6.2.3.2 Human beings
In samples of human adipose tissue, kidneys, and breast milk,
no residues of photodieldrin or the pentachloroketone metabolite
were detected (Robinson et al., 1966b; Anon., 1973, 1974a,c).
6.3. Metabolic Transformation
6.3.1. Aldrin and dieldrin
The initial and major step in the biotransformation of aldrin
is the formation of the corresponding epoxide dieldrin. There is
considerable evidence that this transformation is mediated by
mixed-function monooxygenases, sometimes called aldrin-epoxidase,
which have been found in a wide variety of organisms, e.g., plant
roots (Mehendale et al., 1972), insects (Krieger & Wilkinson, 1969;
Terriere & Yu, 1976), fish (Burns, 1976), and various mammals,
including man. The endoplasmic reticulum of the liver of
vertebrates is an important site of these enzymes.
6.3.1.1 Laboratory animals
(a) In vitro
The in vitro metabolism of 14C-dieldrin by unwashed microsomes
from a male rat pretreated with phenobarbital has been investigated
by Hutson (1976). The addition of uridine 5'-diphosphoglucuronic
acid (UDPGA) increased the yield of a polar metabolite. The
9-hydroxy derivative was not detected either in the presence or
absence of UDPGA, and investigation of the polar metabolite
indicated that it was the glucuronide of the 9-hydroxy derivate.
The rate of conversion of dieldrin to the glucuronide of 9-hydroxy
dieldrin, measured after 30 min incubation, was 0.0028 nmols/min
per mg protein. In the absence of UDPGA, the conversion of
dieldrin to 9-hydroxy dieldrin could not be detected, and the rate
was estimated to be less than 0.0002 nmols/min per mg protein.
A rat hepatocyte culture suspension effectively epoxidized
aldrin to dieldrin (Kurihara et al., 1984).
(b) In vivo
From the results of a comparative metabolic study on rat and
mouse (section 6.2.2.1), it appears that the main differences
between the species are a more rapid metabolism of dieldrin in
rats, a much greater production of the pentachloroketone by rats,
and the production of small amounts of polar urinary metabolites by
mice. The two strains of mice (CF1 and LACG) were similar to one
another in most, but not all, parameters measured. Thus, the
distinguishing features of the metabolism of dieldrin in CF1 mice,
unique to this strain and which could account for tumour initiation
in mice, have not been found. The hydroxylation of dieldrin in
mice is less efficient than in rats, and the formation of the
glucuronide of 9-hydroxy dieldrin is the result of the consecutive
action of hepatic microsomal monooxygenase and uridine
diphosphoglucuronyl transferase. The 9-hydroxy dieldrin formed
initially is probably bound to the microsomal membrane, and the
availability of UDPGA may be rate-limiting in the overall formation
of the glucuronide. The binding of 9-hydroxy dieldrin to the
microsomal membrane may inhibit the first oxidative step, unless
the concentration of bound metabolite is reduced by conversion to
the water-soluble glucuronide (Hutson, 1976).
Of the species studied, rats, mice, rabbits, sheep, rhesus
monkey, and chimpanzee (Feil et al., 1970; Mueller et al., 1975a),
the major metabolite, except in the case of the rabbit, is the
9-hydroxy derivative (Fig. 2, compound VI). This derivative is
found in the faeces and free or conjugated in the urine. Excretion
of the glucuronide occurs via the bile duct into the lower
intestines, where it is converted to the free 9-hydroxy compound.
The initial chemical identification of this metabolite was based on
a combination of physical and chemical methods (Richardson et al.,
1968; Baldwin et al., 1970; Feil et al., 1970), but it was
subsequently synthesized and the structure confirmed (Bedford &
Harrod, 1972a). The stereochemical configuration of the 9-hydroxy
group has been shown to be syn oriented with respect to the
6,7-epoxy group (Baldwin et al., 1973).
6.2.3. Photodieldrin (and major metabolites of dieldrin)
6.2.3.1 Laboratory animals
(a) Rat
Brown et al. (1967) fed rats diets containing 3 or 10 mg
photodieldrin/kg diet for 26 days, the 10 mg/kg-group then being
fed a control diet for a further 2 or 8 days. The half-life of
photodieldrin in adipose tissue was calculated to be 1.7 days in
male rats and 2.6 days in female rats. The storage ratio in
adipose tissue was considerably higher in females (1.3) than in
males (0.5).
In studies by Dailey et al. (1970), young rats were given daily
oral doses of 5 µg 14C-photodieldrin per rat, orally or
intraperitoneally, for 12 weeks. Although there was considerable
variation, the radioactivity in the tissues of female rats was
3 - 10 times greater than in male rats, except in the kidneys,
where the 14C activity in males was about 13 times that in females,
regardless of the route of administration.
When rats were fed diets containing 0, 0.1, 1, 10, or 30 mg
photodieldrin/kg diet for 13 weeks, the concentrations of
photodieldrin in the body tissues of female rats receiving up to 10
mg/kg diet were 2 - 15 times greater than those in males. High
concentrations of pentachloroketone (PCK) were found in the kidneys
of male rats receiving 30 mg/kg (276 mg PCK/kg kidney weight
compared with 29 mg photodieldrin/kg). The corresponding
concentrations for females were lower: 13.55 mg PCK and 1.85 mg
photodieldrin per kg kidneys (Walker et al., 1971).
In studies by Walton et al. (1971), groups of weanling rats
(Charles River strain) were fed photodieldrin at concentrations of
0, 1, 5, or 25 (decreased to 12.5 mg) mg/kg diet for 90 days, while
other groups of rats were fed dieldrin at the same concentrations.
The concentrations of both photodieldrin and dieldrin in the
adipose tissue of female rats were higher than in male rats.
(b) Dog
Following the administration of a single oral dose of
photodieldrin to one male and one female dog (160 and 120 mg/kg
body weight, respectively), the concentrations of photodieldrin in
the female dog's tissues, with the exception of the liver, were
much higher than those in the male (Brown et al., 1967).
The concentrations of photodieldrin in the liver and adipose
tissue of dogs fed photodieldrin at 0, 0.005, 0.05, or 0.2 mg/kg
body weight for 3 months were related to the dose rate and similar
in males and females. In the kidneys, the concentrations of
photodieldrin and its metabolite (PCK) were similar in male and
female dogs and much lower (of the order of 0.1 - 0.2 mg/kg
kidneys) than in rats (Walker et al., 1971).
6.2.3.2 Human beings
In samples of human adipose tissue, kidneys, and breast milk,
no residues of photodieldrin or the pentachloroketone metabolite
were detected (Robinson et al., 1966b; Anon., 1973, 1974a,c).
6.3. Metabolic Transformation
6.3.1. Aldrin and dieldrin
The initial and major step in the biotransformation of aldrin
is the formation of the corresponding epoxide dieldrin. There is
considerable evidence that this transformation is mediated by
mixed-function monooxygenases, sometimes called aldrin-epoxidase,
which have been found in a wide variety of organisms, e.g., plant
roots (Mehendale et al., 1972), insects (Krieger & Wilkinson, 1969;
Terriere & Yu, 1976), fish (Burns, 1976), and various mammals,
including man. The endoplasmic reticulum of the liver of
vertebrates is an important site of these enzymes.
6.3.1.1 Laboratory animals
(a) In vitro
The in vitro metabolism of 14C-dieldrin by unwashed microsomes
from a male rat pretreated with phenobarbital has been investigated
by Hutson (1976). The addition of uridine 5'-diphosphoglucuronic
acid (UDPGA) increased the yield of a polar metabolite. The
9-hydroxy derivative was not detected either in the presence or
absence of UDPGA, and investigation of the polar metabolite
indicated that it was the glucuronide of the 9-hydroxy derivate.
The rate of conversion of dieldrin to the glucuronide of 9-hydroxy
dieldrin, measured after 30 min incubation, was 0.0028 nmols/min
per mg protein. In the absence of UDPGA, the conversion of
dieldrin to 9-hydroxy dieldrin could not be detected, and the rate
was estimated to be less than 0.0002 nmols/min per mg protein.
A rat hepatocyte culture suspension effectively epoxidized
aldrin to dieldrin (Kurihara et al., 1984).
(b) In vivo
From the results of a comparative metabolic study on rat and
mouse (section 6.2.2.1), it appears that the main differences
between the species are a more rapid metabolism of dieldrin in
rats, a much greater production of the pentachloroketone by rats,
and the production of small amounts of polar urinary metabolites by
mice. The two strains of mice (CF1 and LACG) were similar to one
another in most, but not all, parameters measured. Thus, the
distinguishing features of the metabolism of dieldrin in CF1 mice,
unique to this strain and which could account for tumour initiation
in mice, have not been found. The hydroxylation of dieldrin in
mice is less efficient than in rats, and the formation of the
glucuronide of 9-hydroxy dieldrin is the result of the consecutive
action of hepatic microsomal monooxygenase and uridine
diphosphoglucuronyl transferase. The 9-hydroxy dieldrin formed
initially is probably bound to the microsomal membrane, and the
availability of UDPGA may be rate-limiting in the overall formation
of the glucuronide. The binding of 9-hydroxy dieldrin to the
microsomal membrane may inhibit the first oxidative step, unless
the concentration of bound metabolite is reduced by conversion to
the water-soluble glucuronide (Hutson, 1976).
Of the species studied, rats, mice, rabbits, sheep, rhesus
monkey, and chimpanzee (Feil et al., 1970; Mueller et al., 1975a),
the major metabolite, except in the case of the rabbit, is the
9-hydroxy derivative (Fig. 2, compound VI). This derivative is
found in the faeces and free or conjugated in the urine. Excretion
of the glucuronide occurs via the bile duct into the lower
intestines, where it is converted to the free 9-hydroxy compound.
The initial chemical identification of this metabolite was based on
a combination of physical and chemical methods (Richardson et al.,
1968; Baldwin et al., 1970; Feil et al., 1970), but it was
subsequently synthesized and the structure confirmed (Bedford &
Harrod, 1972a). The stereochemical configuration of the 9-hydroxy
group has been shown to be syn oriented with respect to the
6,7-epoxy group (Baldwin et al., 1973).
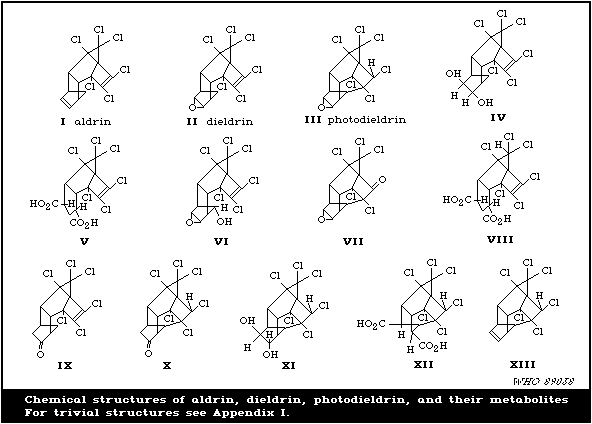 The other metabolites, the chemical identities of which have
been rigorously established, are detailed below.
(a) The trans-6,7-dihydroxy compound (Fig. 2, compound IV) is
formed by the hydration (formal) of the epoxide ring of dieldrin
(Korte & Arent, 1965). This compound is a major metabolite in
rabbit urine, but of relatively minor importance in other species.
The formation of the cis-diol by rat microsomes has been
demonstrated, together with its epimerization to the trans-diol
(McKinney et al., 1973). Both the cis- and trans-diols have been
synthesized (Korte & Arent, 1965; Chau & Cochrane, 1970b; Bedford &
Harrod, 1972b).
(b) The dicarboxylic acid (Fig. 2, compound V) is derived from
the dihydroxy metabolite (Baldwin et al., 1972; Oda & Mueller,
1972). This compound has also been synthesized (Buechel et al.,
1966), and has been shown to undergo further degradation (formation
of two isomers of a monodechlorinated derivative) after intravenous
injection into male and female rats (Lay et al., 1975).
(c) The bridged pentachloroketone (PCK) (Fig. 2, compound VII)
is mainly found in the urine and kidneys of male rats, but, even in
rats, it is a minor metabolite (Damico et al., 1968; Klein et al.,
1968; Richardson et al., 1968). In other species, it is a very
minor metabolite of dieldrin. It is also a metabolite of
photodieldrin (Klein et al., 1970), and has been synthesized
(Bedford & Smith, 1978).
The Chemical Abstract or Von Baeyer AG/IUPAC names of aldrin,
dieldrin, photodieldrin, and metabolites are given in Appendix I.
Methods for the quantitative determination of the four
metabolites are available. They depend on the availability of
authenticated analytical standards (Ludwig & Korte, 1965;
Richardson, 1971; Baldwin et al., 1972).
6.3.1.2 Human studies
A metabolite of dieldrin detected in human faeces has been
shown to be the 9-hydroxy derivative (Richardson & Robinson, 1971).
6.3.1.3 Non-domestic organisms
The conversion of aldrin to dieldrin was studied in algae
( Chlorella and diatoms) and protozoa (Dinoflagellates and mixed
protozoa) after exposure for 24 h to 0.1 mg aldrin/litre. The
amount of dieldrin present in the cultures was of the order of
0.06 - 0.2 µg/litre). The amounts of dieldrin were greater in the
protozoa than in the algae; it was concluded that these planktonic
species have enzyme systems that epoxidize aldrin (Khan et al.,
1972b).
The conversion of aldrin to dieldrin in 12 species of fresh-
water invertebrates has been compared. Ten species were exposed
for 2 h to concentrations of 0.1 or 0.25 mg aldrin/litre, and two
species of molluscs were exposed to 0.25 mg aldrin/litre for 4 h.
The concentrations of dieldrin relative to aldrin in the whole
bodies of eight species from four phyla (Coelenterata,
Platyhelminthes, Annelida, and Arthropoda) were in the range
1.03 - 8.48%. In two species of Insecta (dragon fly nymphs and
Aedes larvae), the values were 24.9% and 42.4%, respectively. The
two species of molluscs had dieldrin concentrations (relative to
aldrin) of 17 - 19% (Khan et al., 1972b).
In a study on an ostracod (Chlamydotheca arcuata) exposed to
14C-labelled aldrin (5.5 - 11.2 µg/litre), aldrin was readily
converted to dieldrin, 83% conversion occurring within 24 h. The
elimination of aldrin and dieldrin appeared to involve both passive
and active processes, and it was concluded that dieldrin was
eliminated more rapidly after dieldrin exposure than after aldrin
exposure (Kawatski & Schmulbach, 1972).
A number of in vitro studies have been carried out concerning
the influence of these insecticides on mixed-function oxidase
activity. The epoxidation of aldrin to dieldrin by this enzyme
system has been demonstrated in crayfish (Cambarus) (Khan et al.,
1972a,b), in snail (Lymnea palustris) and clam (Khan et al.,
1972b), and in midge larvae (Chironomus riparius) (Estenik &
Collins, 1979).
The conversion of aldrin to dieldrin by lobsters (Homarus
americanus) was reported by Carlson (1974).
The mixed-function oxidase activities in five species of fresh
water fish, as measured by the conversion of aldrin to dieldrin,
were investigated by Ludke et al. (1972). The fish were exposed to
aldrin (50 µg/litre) for 4 h, and the concentrations of aldrin and
dieldrin in the liver were determined. Contrary to earlier
reports, the conversion by epoxidation of aldrin to dieldrin in
fish may be the rule rather than an exception.
The epoxidation of 14C-aldrin to dieldrin in susceptible and
resistant mosquitofish (Gambusia affinis) has been investigated.
The fish were exposed to 5 µg 14C-aldrin/litre for 4 or 8 h and the
concentration of aldrin and dieldrin in liver and brain were
determined. The concentration of dieldrin (expressed in terms of
protein content) was significantly higher in the livers of
resistant fish than in susceptible fish). It was concluded that
resistant mosquitofish convert aldrin to dieldrin and/or water-
soluble compounds at a greater rate than susceptible mosquitofish
(Wells et al., 1973).
In studies by Addison et al. (1976), Atlantic salmon fry (Salmo
salar) were injected intramuscularly with 14C-aldrin, to initial
whole-body concentration of 5 mg/kg. The fish were maintained in
flowing fresh water, and were removed at five intervals up to 56
days for the measurement of whole-body residues. The time required
for 50% epoxidation of aldrin was between 1 and 2 days. Less than
10% of the radioactivity remained in the fish at the end of the
exposure. It was concluded that there was rapid elimination either
of unchanged aldrin or its epoxide, dieldrin, from the fish.
6.3.2. Photodieldrin (and major metabolites of dieldrin)
6.3.2.1 Rat
Besides unchanged photodieldrin, bridged pentachloroketone
(PCK) (Fig. 2, compound VII), a metabolite of photodieldrin, was
isolated from the brain, liver, adipose tissue, and blood of rats
(Carworth Farm, type E) fed diets containing 10 or 30 mg
photodieldrin/kg for 13 weeks (Baldwin & Robinson, 1969).
In studies by Klein et al. (1970), Osborne-Mendel rats were
given 14C-photodieldrin, orally or intraperitoneally, 5 days/week,
for 12 weeks, and urine was collected quantitatively every day. A
metabolite was found in the urine of male rats and shown to be PCK.
Small amounts of other (unidentified) more polar urinary
metabolites were also present.
6.3.2.2 Monkey
Metabolites were detected in the urine and faeces of a female
rhesus monkey given daily oral doses of 0.8 mg 14C-photodieldrin/kg
body weight for 175 days. Two metabolites were identified in the
urine: the trans-diol (Fig. 2, compound XI) and its glucuronide
conjugate. A faecal metabolite was tentatively identified as the
diol. A third metabolite was present in both urine and faeces, and
it was suggested that this might be a monohydroxy derivative of
photodieldrin (Nohynek et al., 1979).
6.4. Elimination and Excretion
6.4.1. Aldrin
6.4.1.1 Rat
When male rats were given daily oral doses of 4.3 µg 14C-aldrin
(equivalent to about 0.2 mg aldrin/kg diet) for 3 months, the
radioactivity in the urine increased from about 2% of the dose of
aldrin during the first week to about 10% during the 12th week. In
the faeces, the excreted radioactivity increased from about 48%
during the first week to about 93% during the 12th week. After
about 8 weeks, a saturation level was reached (i.e., there was a
balance between the rates of intake of aldrin and excretion of
aldrin plus aldrin-related materials). Extracts of urine and
faeces were examined by paper chromatography. Because the urine
was probably contaminated by faeces in the metabolism cages, only
the trend is given. In both faeces and urine, the aldrin content
decreased during the 12 weeks. The hydrophilic metabolites
increased, reaching 75% (faeces) and 95% (urine) of total
radioactivity after 12 weeks. The level of dieldrin was more or
less constant (Ludwig et al., 1964).
6.4.2. Dieldrin
6.4.2.1 Laboratory animals
As described in section 6.2.2.1, Hutson (1976) studied the
comparative metabolism of dieldrin in CFE rats and two strains of
mice after a single oral dose of 3 mg/kg body weight 14C-dieldrin.
The excretion of 14C activity in the faeces of the rats was 62.4%
of the administered dose in the non-pretreated group and 69% in the
dieldrin-pretreated group. In the case of the CF1 mice, the
pretreatment period did not have any effect on the faecal excretion
(51.5%), whereas, in the LACG mice, the faecal excretion of 14C
activity increased from 27.2% for the non-pretreated group to 48.8%
for the 4-week pretreated group. The total 14C activity excreted in
the urine of the two strains of mice was low (0.42 - 2.6% of dose)
compared with that in the urine of male rats (5.5 - 6.6%). In both
species of rodents, the faeces was the major route of excretion of
14C activity. In the urine of both the male CFE rat and the male
CF1 mice, the amount of the dicarboxylic acid metabolite in the
urine was small compared with that of pentachloroketone plus
dieldrin, while in the male LACG mice, the amount of the acidic
metabolite was twice that of pentachloroketone plus dieldrin. Both
strains of mice excreted, proportionally, much larger amounts of a
polar (unidentified metabolite) in the urine than did the CFE rats.
In the faeces of the male CFE rats (no pretreatment), the major
component was the 9-hydroxy derivative. This was also found by
Matthews et al. (1971). However, in both mouse strains (no
pretreatment), this compound was a minor metabolite, but it became
the major product in the dieldrin-pretreated group. In isolated
liver microsomes, most of the 14C activity appeared to be present
as dieldrin, and the 9-hydroxy metabolite was not detected.
A number of other studies on the excretion of dieldrin via
urine and/or faeces have been carried out. Dailey et al. (1970)
found that male rats excreted higher levels of 14C radioactivity
via urine and faeces than females. Davison (1973) confirmed this
in a study lasting 39 weeks. Maximal excretion of 14C activity
occurred in the 6th week in both sexes, regardless of the amount of
dieldrin given. A steady state was reached and maintained from the
6th to the 39th week.
In studies by Robinson et al. (1969), rats were fed a diet
containing 10 mg dieldrin/kg diet for 8 weeks. The decline in the
concentration of dieldrin in blood, brain, liver, and adipose
tissue was studied during the subsequent 12 weeks when a control
diet was fed. There was an initial rapid decline in the dieldrin
concentration during the first 10 days of the post-exposure period
in the blood, liver, and brain, followed by a slower decline. The
changes in the concentration of dieldrin in the brain, adipose
tissue, blood, and liver corresponded to biological half-lives of 3
to about 10 days.
When male and female rats were administered 3 g of diet
containing 10 mg 14C-dieldrin/kg diet, followed by a control diet
ad libitum, 14C activity in the kidneys of male rats was 10-fold
higher than in the female rats (the animals were killed 9 days
after administration of 14C-dieldrin). Most of the activity in the
male kidneys was due to pentachloroketone, whereas, in the female
kidneys, only dieldrin was detected (Matthews et al., 1971).
The excretion of 36Cl activity by female rats dosed
intravenously (680 µg/h for 2.5-5 h; total doses of 8 - 16 mg/kg
body weight) with 36Cl-dieldrin has been studied. The 36Cl
activity detected in the faeces was about 7 times that found in the
urine, indicating excretion via the bile (Heath & Vandekar, 1964).
Comparable results were found by Cole et al. (1970), who gave
male rats a single intravenous dose of 0.25 mg 14C dieldrin/kg body
weight. Similar doses of 14C-dieldrin were administered
intravenously to male rats with bile fistulas. About 30% of the
administered 14C activity was excreted via the bile during the
first 24 h after dosing, and after 4 days a total excretion of
about 60% had occurred. Isolated perfused rat liver preparations
were also investigated; some 20% of the original perfusate dose was
collected in the bile over a period of 8 h.
Rapid excretion of 14C-dieldrin (or its metabolites) from
isolated perfused rat livers via the bile of rats has also been
reported by Klevay (1970), the rate of excretion by male rats being
about 3 times as rapid as that by female rats.
In studies by Mueller et al. (1975a), mice, rats, rabbits,
rhesus monkeys, and one chimpanzee were given a single oral dose of
0.5 mg/kg body weight 14C-dieldrin, and urine and faeces were
collected for 10 days. For all species except the rabbit, the main
route of excretion was the faeces. The faecal excretion of
unchanged dieldrin was high in the first 48 h and then declined
rapidly. The urine samples contained only metabolites of dieldrin.
The mean total amount of radioactive material excreted (males
and/or females) in faeces and urine within 10 days after dosing
(expressed as percentage of administered dose) was 37% in mice, 11%
in rats, 2% in rabbits, 20% in rhesus monkeys, and 6% in the
chimpanzee. In all five species, 9-hydroxy-dieldrin and
4,5-aldrin- trans-dihydrodiol were the major metabolites. The
metabolism in the rat seems to be comparable to that of primates;
however, mice and rabbits showed the opening of the epoxide to diol
as the predominant reaction.
6.4.2.2 Human studies
The occurrence of a neutral metabolite of dieldrin in human
urine in amounts indicative of exposure to aldrin/dieldrin was
reported by Cueto & Hayes (1962) and Cueto & Biros (1967).
Quantitative estimates of the amounts of a metabolite of
dieldrin, 9-hydroxy-dieldrin, in the faeces of seven workmen
occupationally exposed to aldrin/dieldrin and five male members of
the general population have been made. The average concentration
of the 9-hydroxy derivative in 24-h collections of faeces of the
seven workmen was 1.74 mg/kg (range, 0.95 - 2.80 mg/kg), whereas
the average concentration in faeces of the five members of the
general population was 0.058 mg/kg (range, 0.033 - 0.12 mg/kg).
Dieldrin was present in the faeces of the workmen (average
concentration, 0.18 mg/kg), but, in samples from the general
population, it was below the limit of detection. Examination of
the urine of five of the workmen indicated that this route of
elimination of dieldrin and four known metabolites was minor. It
was concluded that the 9-hydroxy-dieldrin in the faeces represented
the major excretory pathway of dieldrin from male human beings. It
should be noted, however, that the urine was not examined for
glucuronide or other conjugates of the hydroxy metabolites). There
was good correlation between the estimated daily intake of dieldrin
(calculated from the concentrations of dieldrin in the blood) and
excretion in faeces of total equivalent dieldrin (Richardson,
1971). This relationship is based on a number of assumptions, and
it is probably more relevant that the concentration of the
9-hydroxy-dieldrin in the faeces (produced by the metabolism of
absorbed dieldrin) is significantly related to the concentration of
dieldrin in the blood, which is a measure of the body burden
arising from absorption of aldrin plus dieldrin.
When 14C-Dieldrin was applied in acetone (4 µg/cm2) once to the
forearm of volunteers, 7.7% of the applied 14C activity was
excreted in the urine over a 5-day period. A single intravenous
injection of 14C-dieldrin resulted in 3.3% being excreted in the
urine over a 5-day period (Feldman & Maibach, 1974).
6.4.3. Photodieldrin (and major metabolites of dieldrin)
6.4.3.1 Rat
In studies by Dailey et al. (1970), young rats were given daily
doses of 5 µg 14C-photodieldrin, orally or intraperitoneally, for
12 weeks. Urine and faeces were collected daily and pooled in
weekly groups. The excretion of 14C activity via the urine of
females was considerably less than that by males, by either method
of dosing. The 14C activity in urine after oral and ip
administration increased slowly during the 12 weeks (males about
10% and females 5%), the highest levels in urine (up to 33%) being
found in males dosed intraperitoneally. Faecal excretion of 14C
activity was initially lower in females, but greater during the
latter half of the study (of the order of 20 - 40%). In males,
during the whole study, it was about 30%.
6.4.3.2 Monkey
A juvenile female rhesus monkey was given daily oral doses of
2 mg 14C-photodieldrin (equivalent to 0.8 mg/kg body weight), and
the treatment was continued until, between days 70 and 76, the
daily excretion of 14C activity was in balance with the daily
intake. When dosing ceased, the animals had retained about 50% of
the cumulative dose of photodieldrin. Collection of excreta was
continued for a further 100 days, during which a further 30.1% of
the dose, administered during the 76-day period, was excreted.
During the period of dosing, a major part of the faecal 14C
excretion consisted of photodieldrin (probably indicating
incomplete absorption in the gastrointestinal tract), while
20 - 50% of the excreted activity was in the urine. After dosing
ceased, 60% of the excreted 14C activity appeared in the urine
(Nohynek et al., 1979).
In studies by Nohynek et al. (1979), one male and one female
juvenile rhesus monkey were given single intravenous doses of
4.5 mg 14C-photodieldrin (2 mg/kg body weight). Urine and faeces
were collected separately every 24 h, and the animals were killed
after 21 days. Excretion of 14C activity was high during the first
7 days (male, 39%; female, 27.3%, of the given dose). It then
decreased rapidly and reached a nearly constant value of 0.2% of
the administered dose. Approximately 45% (male) and 34% (female)
of the dose had been excreted by day 21.
6.5. Retention and Turnover
6.5.1. Non-domestic organisms
A few studies have been carried out on the uptake and
elimination of aldrin and/or dieldrin in invertebrates: marine
clams (Mya arenaria and Mercenaria mercenaria) (Butler, 1971);
naiad mollusc (Amblema plicata) (Fikes & Tubb, 1972); mussel
(Lampsilis siliquiodea) (Bedford & Zabik, 1973); crab (Leptodius
floridanus) (Epifanio, 1973); and ostracod (Chlamydotheca arcuata)
(Kawatski & Schmulbach, 1972). The concentration of aldrin or
dieldrin in organs and tissues increased rapidly during the first
1 - 2 weeks of exposure, but remained virtually constant
thereafter. When the organisms were placed in clean water, the
concentration declined in a (semi)-logarithmic manner in relation
to time. The estimated half-life for the tested organisms varied,
e.g., for Lampsilis siliquiodea, it was 4.7 days, whereas for
Amblema plicata, it was about 3 - 4 weeks.
The elimination of 14C-dieldrin from bluegills (Lepomis
macrochirus) and goldfish (Carassius auratus) was studied by
Gakstatter & Weiss (1967). The fish were exposed to 30 µg
14C-dieldrin/litre (initial concentration) until toxic symptoms
appeared (5 - 8 h), and were then placed in recovery aquaria
together with unexposed fish. The water in the recovery aquaria
was continuously renewed. Samples of five fish were taken on 10
different occasions during the recovery period. The 14C activity
in whole fish of both species, expressed as equivalent dieldrin,
declined by about 90% within 16 days, the half-time for elimination
being about 4 days. The control bluegills and goldfish accumulated
a maximum equivalent dieldrin concentration of 0.29 and 0.22 mg/kg,
respectively, on day 4 of the period in the recovery aquaria,
indicating transfer of dieldrin or derived material from
contaminated to uncontaminated fish.
In another study, the distribution of aldrin and dieldrin in
the tissues of Carassius auratus was determined following an 8-h
exposure to 14C-aldrin (50 µg/litre) in a static study. After the
exposure, fish were placed in a continuously flushed aquarium for
32 days. Dieldrin was found in all tissues examined immediately
after the exposure. The percentage of dieldrin in the total
residues in the tissue increased with time, reaching about 95% on
day 32 (except in visceral fat). During the recovery period, the
total concentration of aldrin plus dieldrin in the blood declined
from 2.1 mg/litre (as aldrin) to 0.4 mg/litre. The corresponding
changes in the brain concentrations were 5.45 mg/kg to 2.3 mg/kg.
Total residues in the nerve cord did not show a consistent decline
and varied from 4.56 to 21.6 mg/kg throughout the 32-day period;
however, these residues were determined by thin-layer
chromatography, not by gas-liquid chromatography (Gakstatter,
1968).
The partitioning of 14C activity into particulate fractions of
the brain and liver of resistant and susceptible mosquitofish has
been studied after exposure of the fish to 14C-aldrin or 14C-
dieldrin. The 14C activities in total brain, cell membrane, and
five cellular fractions were significantly higher in susceptible
fish than in resistant fish for both aldrin and dieldrin. However,
this difference was much less marked in the case of the liver. It
was suggested that a basic structural change in polarity exists in
the myelin of resistant fish, which could provide a membrane
barrier (Wells & Yarbrough, 1973).
The fate of dieldrin in the digestive tract of juvenile lake
trout (Salvelinus namaycush) has been studied. Macerated trout
flesh containing an average of 1.05 mg/kg was injected in the
stomach. The decline in the dieldrin content of the stomach was
parallel to that of the food from the stomach. Little or no
dieldrin was found in the intestines (Stewart & Stein, 1974).
In studies by Chadwick & Brocksen (1969), groups of sculpins
(Cottus perplexus) were exposed to 1.3 µg dieldrin/litre for 12
days, followed by removal to uncontaminated continuously renewed
water. The concentration in whole fish declined in a curvilinear
fashion from about 2.5 mg/kg fish to about 1 mg/kg fish in 60 days
and to about 0.5 mg/kg fish in 90 days.
Sailfin molly (Poecilia latipinna) were exposed to 12 µg
dieldrin/litre for up to 6 h by Lane et al. (1970). Two products,
thought to be metabolites of dieldrin, were detected in the liver
and other organs, and it was suggested that they were partially
dechlorinated derivatives of dieldrin.
6.5.2. Biological half-life in human beings
The concentration of dieldrin in the blood of volunteers given
oral daily doses for 2 years (section 6.2.2.4) was determined over
a period of 8 months after termination of the deliberate exposure
(Hunter et al., 1969). A small, but statistically significant,
decline occurred, corresponding to a mean value of 369 days for the
half-life of dieldrin in blood. However, there were significant
differences between the rates of decline of the individual
volunteers.
The concentration of dieldrin in the blood of 15 workmen was
determined for a period of 3 years following termination of
occupational exposure to aldrin/dieldrin (Jager, 1970). The mean
half-life was 266 days.
When a state of equilibrium has not yet been reached, the
apparent half-life will be much shorter, due mainly to a
redistribution of dieldrin between compartments in the body.
6.5.3. Body burden and (critical) organ burden; indicator media
Whatever the route of exposure, the effect, if any, will be
determined by the concentration of the chemical in the target organ
or tissue. It has been shown that the distribution between the
various tissues of mammals is fairly constant within and between
species (Robinson & Hunter, 1966; Hunter & Robinson, 1967; Hunter
et al., 1967; Robinson & Roberts, 1969; Walker et al., 1969b).
Thus, at a state of equilibrium, the dieldrin level in the blood
reflects the concentration of the active compound in the target
tissues and therefore represents the best practical parameter for
the internal exposure that is associated with a biochemical,
clinical, or pathological effect. Since the biological half-life
of dieldrin in human blood is known (266 days) (Jager, 1970),
a reliable estimation of the blood level at the time of
discontinuance of the exposure can be made. This, in turn enables,
better than anything else, the evaluation of the likelihood of an
observed symptom of disease or indisposition being associated with
exposure to dieldrin. Also, the established mathematical
relationship between the dieldrin level in the blood and the total
daily equivalent oral intake thus enables, on the basis of the
concentration of dieldrin in the blood, the evaluation of a current
exposure or an exposure of a short time ago vis-à-vis the
acceptable daily intake established by the FAO/WHO Joint Meeting on
Pesticide Residues.
Determination of the dieldrin concentration in blood is the
method of choice in monitoring exposed workers or the general
population (section 9.2.1.1).
6.6. Appraisal
Aldrin is readily absorbed through the skin, by inhalation of
the vapour, or into the circulating blood from the gastrointestinal
tract. It has not been possible to determine the percentage of an
ingested dose of aldrin or dieldrin that is actually absorbed into
the body because of the intestinal hepatic biliary cycle. Work
with human volunteers (Feldmann & Maibach, 1974) showed that
absorption through the skin amounted to 7 - 8% of the applied dose.
Inhalation studies with human volunteers (Beyermann & Eckrich,
1973; Bragt et al., 1984) suggested that about 50% of inhaled
aldrin vapour is absorbed and retained in the human body. After
absorption, it is rapidly distributed to the organs and tissues of
the body, and a continuous exchange between the blood and other
tissues takes place. In the meantime, aldrin is readily converted
to dieldrin, mainly in the liver but, to a much lesser extent, in
some other tissues, e.g., the lungs (Mehendale & El-Bassiouni,
1975).
This conversion proceeds very rapidly. The livers of even
24-h-old rats, given oral doses of 10 mg aldrin/kg body weight,
contained dieldrin 2 h after treatment (Farb et al., 1973). In the
course of the next few hours, dieldrin and what little is left of
the aldrin in blood and other tissues, concentrates more in the
lipid tissues (Heath & Vandekar, 1964; Hayes, 1974). In human
beings, aldrin is found rarely, if at all, in human blood or other
tissues, except in cases with acute poisoning by accidental or
intentional ingestion of massive doses.
Studies carried out with 14C-labelled aldrin and dieldrin have
shown that part of the ingested material is passed unabsorbed
through the intestinal tract and eliminated from the body, part is
excreted unchanged from the liver into the bile, part is stored
unchanged in the various organs and tissues (particularly the
adipose tissue), and part is metabolized in the liver to more polar
and hydrophilic metabolites. These metabolites, in human beings
and most animals, are excreted primarily via the bile in the
faeces. It had also been shown that aldrin and dieldrin are both
biodegraded into the same metabolites (Damico et al., 1968; Klein
et al., 1968). The biodegradation products have been identified in
the rat within 15 min after an intravenous injection (Moersdorf et
al., 1963). Most of the currently available information on the
biodegradation metabolism in mammals is based on studies with
dieldrin on the mouse, rat, rabbit, sheep, dog, monkey, chimpanzee,
and human beings (Ludwig et al., 1964; Datta et al., 1965; Korte,
1965; Korte & Arent, 1965; Richardson et al., 1967b, 1968; Klein et
al., 1968; Matthews & Matsumura, 1969; Baldwin et al., 1970, 1972;
Feil et al., 1970; Richardson & Robinson, 1971; Mueller et al.,
1975a,b). Although there appear to be differences between species
and, in the rat, differences between the sexes, the overall picture
shows only quantitative variations between species.
In the species studied (with the exception of the rabbit) the
major metabolite is the 9-hydroxy derivative. This is found in the
faeces and free or conjugated in the urine. Three other
metabolites have been found and identified in experimental animals:
(a) trans-6,7-dihydroxy derivative;
(b) dicarboxylic acid derived from the dihydroxy compound; and
(c) the bridged pentachloroketone (PCK).
The latter is also a metabolite of photodieldrin (Klein et al.,
1970).
Only the 9-hydroxy compound was found in the faeces of seven
occupationally exposed industrial workers (1.74 mg/kg) and five
male members of the general population (0.058 mg/kg). Neither the
9-hydroxy compound nor the other metabolites have been found in
human blood or other tissues. Dieldrin was present in the faeces
of the workmen (average 0.18 mg/kg), whereas the concentrations in
the samples from the general population were below the limits of
detection. Examination of the urine of five workmen indicated that
urinary excretion of dieldrin and its four metabolites is minor
relative to elimination of the 9-hydroxy metabolite via the faeces
(Richardson, 1971).
The conversion of aldrin to dieldrin and the distribution and
the subsequent deposition of dieldrin (mainly in lipid tissues)
proceed much faster than the biodegradation and ultimate
elimination of unchanged dieldrin and its metabolites from the
body. At a given average intake of aldrin and/or dieldrin,
dieldrin slowly accumulates in the body. This accumulation or
"storage", however, does not increase indefinitely. As the
concentration of dieldrin in the liver cells increases, the
metabolizing enzyme activity in the microsomes increases, and so
the rate of biodegradation of dieldrin, and hence the elimination
from the body, is enhanced. Thus, the accumulation proceeds at an
ever slower rate until the concentrations of dieldrin in blood and
tissues approach upper limits of storage and an amount of dieldrin
equal to the average daily intake is eliminated each day. These
upper limits of storage are related to the daily intake. This has
been demonstrated in rats and dogs (Walker et al., 1969b) and in
human beings (Hunter & Robinson, 1967; Hunter et al., 1969). When
the intake of aldrin/dieldrin ceases or decreases, the body burden
decreases. The biological half-life in human beings is 9 - 12
months (Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
Significant relationships exist between the concentrations of
dieldrin in the blood and those in other tissues of rats, dogs, and
human beings (Hunter & Robinson, 1967; Deichmann et al., 1968;
Keane & Zavon, 1969b; Hunter et al., 1969; Walker et al., 1969b).
Numerous investigations of the concentrations of dieldrin in
body fat, blood, and other tissues from members of the general
population and from special groups have been carried out in several
countries. The results are summarized and discussed in section
5.2. The ratio of dieldrin concentrations in fat, liver, brain,
blood is about 150:15:3:1.
Dieldrin penetrates the placenta and is present in the blood,
fat, or other organs of the fetus, newborn babies, and infants
(Table 22). The concentrations are much lower (by 50% or more)
than those in adults. The ratio of dieldrin concentrations in
blood, brain, liver, and fat in infants is not different from that
ratio in adults (Fiserova-Bergerova et al., 1967; Casarett et al.,
1968). Dieldrin is excreted in mother's milk, average values being
about 3 - 5 µg/litre mother's milk (Table 23). The ratio of the
dieldrin concentration in mother's blood to that in mother's milk
is about 1:2 - 3.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
Neither aldrin nor dieldrin have significant effects on
populations of microorganisms in soil or fresh water at realistic
concentrations. Some physiological processes of microorganisms are
affected by low concentrations of both aldrin and dieldrin, but
these would appear to have little or no environmental significance.
Aldrin and dieldrin have only minor deleterious effects on soil
bacterial populations, even at concentrations that are much higher
than those used in agricultural practice.
The effects of insecticides on soil microbes have been reviewed
by Tu & Miles (1976). Of 15 strains tested, aldrin did not have
any effect on the growth of 11 bacterial species (single cultures)
but caused some growth inhibition in four species. Dieldrin did
not have any effect on 13 bacterial species but had some inhibitory
effect in two species. Neither aldrin nor dieldrin at 2000 mg/kg
soil had effects on bacteria in laboratory studies; soil fungi were
also little affected. In pot studies, using aldrin at 4 and 120
mg/kg soil, there were no quantitative changes in bacteria during
any part of the vegetative period. Aldrin inhibited the growth of
Rhizoctonia solani in plate cultures by 20% or more at 6.2 mg/litre
and higher concentrations. Dieldrin was less toxic, producing an
average inhibition of about 15%, which was not dose related over
the range of 1 - 100 mg/litre. The evolution of carbon dioxide
(CO2) (a measure of soil organisms respiration) was significantly
reduced by dieldrin at 1000 mg/kg soil (but not at 100 mg/kg),
whereas aldrin produced a significant reduction at concentrations
as low as 25 mg/kg soil. Slight effects on nitrification were
initially found when aldrin and dieldrin were incorporated at 2000
mg/kg in a sandy loam soil, but nitrification was normal after
about 10 weeks. Short-term inhibition of nitrification was also
produced by aldrin and dieldrin at 25 mg/kg in a sandy loam soil
(aldrin for 1 week, dieldrin for 2 weeks). Decreased sulfur
oxidation was observed in soil containing aldrin or dieldrin (2000
mg/kg), the inhibition decreasing considerably after 3 months.
Five annual applications of aldrin or dieldrin (5.5 - 22 kg/ha) to
a Ramona sandy loam had no measurable effect on the numbers of soil
bacteria or fungi, did not influence the ability of the soil
population to decompose plant residue, and did not alter soil
aggregation.
The effect of dieldrin on the activities of three soil enzymes
was determined at concentrations of 5 or 10 mg dieldrin/kg soil by
Tu (1981). The dehydrogenase activity of the dieldrin-treated soil
(10 mg/kg) did not differ from controls, whereas at 5 mg
dieldrin/kg, the activity was significantly greater than controls
after 2 weeks (50% increase). Urease activity at both treatment
levels was significantly reduced after a 1-week incubation but
significantly increased after 2 weeks. Phosphatase activity was
significantly reduced at 5 mg dieldrin/kg, but not at 10 mg/kg.
The 96-h EC50 (growth) for algae (Chlamydomonas sp.,
Phaeodactylum tricornutum, Dunaliella sp., Chlorella ovalis, and
Chlorella pyrenoidosa) was > 100 µg dieldrin/litre (Adema & Vink,
1981).
The photosynthetic activity of four species of marine
phytoplankton in the presence of dieldrin was investigated using
14C-labelled Na2CO3. A range of nominal concentrations
(0.01 - 1000 µg/litre) was used, and the plant cultures were
exposed for 24 h. The 14C uptake of Dunaliella tertiolecta during
7 days post-treatment was unaffected by up to 1000 µg
dieldrin/litre. Two other species (Skeletonema costatum and
Coccolithus huxleyi) showed significant reductions in 14C uptake at
levels of dieldrin above 10 µg/litre, and the photosynthetic
activity of Cyclotella nana was reduced at concentrations above 1
µg dieldrin/litre (Menzel et al., 1970).
In studies by Schauberger & Wildman (1977), three species of
fresh-water algae (Anabaena cylindrica, Anacystis nidulans, Nostoc
muscorum) were exposed to aldrin or dieldrin at concentrations of
0 - 1000 µg/litre. After exposure for 7 days, there was no
significant effect on the photosynthetic pigment absorption of the
three species at concentrations up to 10 µg (nominal)/litre.
However, at 1 mg/litre, aldrin almost completely suppressed the
absorption by photosynthetic pigments (chlorophyll and
phycocyanin), these being indicators of physiological health and
growth. Dieldrin (1 mg/litre) produced a reduction of about 40%.
The growth response of two cyanobacteria (blue-green algae) in
the presence of aldrin, dieldrin, or two metabolites of dieldrin,
photoaldrin, or photodieldrin was determined at nominal
concentrations of 0.2 - 950 µg/litre (Batterton et al., 1971).
None of these compounds had significant effects on the growth rate
constants at concentrations of 95 µg/litre or at lower
concentrations over periods of 26 - 30 h. The investigators
considered that dieldrin and its derivatives reduced the growth
rate constant at 475 and 950 µg/litre, Agmenellum quadriplicatum
being more sensitive than Anacystis nidulans. Aldrin did not have
a significant effect on either species, but photoaldrin affected
Agmenellum quadriplicatum at 950 µg/litre.
In studies by Powers et al. (1977), a marine dinoflagellate
(Exuviella baltica) was incubated with dieldrin (0.1, 1, or 10 µg
(nominal)/litre), and the numbers of cells were counted during a
period of 6 days. No adverse effects on optical counts were
observed at the two lower concentrations, but there was a marked
reduction in the size and number of cells at 10 µg dieldrin/litre.
7.2. Aquatic Organisms
The toxicity of aldrin and dieldrin to aquatic invertebrates is
very variable. For some species both compounds are highly toxic,
whereas for others there is no effect until the compounds are
dissolved to artificially high concentrations, many times their
solubility in water. Both aldrin and dieldrin are highly toxic to
most species of fish in laboratory tests, with acute LC50 values
well within the solubility of the compounds. It should be borne in
mind that aldrin and dieldrin are strongly bound to particulate
matter in water, which reduces their availability to aquatic
organisms and, in consequence, their potential toxicity.
7.2.1. Aquatic invertebrates
7.2.1.1 Acute toxicity
A convenient overview, in graphical format, of the toxicity of
aldrin and dieldrin to many aquatic organisms was produced by Craig
(1977). The 96-h LC50 values of aldrin and dieldrin for
crustaceans and molluscs were in the range 0.2 - 10 000 µg/litre.
Dieldrin is moderately toxic to fresh-water annelids
(4000 - 7000 µg/litre) and molluscs (> 100 - 640 µg/litre).
Insects are the most sensitive group (aldrin, 1 - 200 µg/litre;
dieldrin, 0.2 - 40 µg/litre). The values for a number of species
are given in Table 25.
7.2.1.2 Short-term toxicity, reproduction, and behaviour
(a) Short-term toxicity
When naiads of two species of stonefly were exposed for 30 days
in a continuous-flow system, the 30-day LC50s for aldrin and
dieldrin were, respectively, 2.5 and 2 µg/litre for Pteronarcys
californica, and 22 and 0.2 µg/litre for Acroneuria pacifica
(Jensen & Gaufin, 1966).
The LC50 for adult molluscs (Mytilus edulis and Dreissena
polymorpha) exposed for 3 - 4 weeks was 180 - 200 µg dieldrin/litre
(Adema & Vink, 1981).
McLeese et al. (1982) exposed polychaete worms (Nereis vireus)
to dieldrin in sea water or sediment for 12 days. The LC50 in sea
water was > 170 µg/litre (in surficial water > 20 µg/litre; in
sediment > 13 mg/kg).
Table 26 gives the LC50 values for a number of invertebrate
species.
(b) Reproduction
The effects of dieldrin on the embryonic development of the
American oyster (Crassostrea virginica) and of aldrin on that of
the hard clam (Mercenaria mercenaria) were studied by Davis & Hidu
(1969). Table 27 gives the concentrations producing approximately
50% reduction in the development of fertilized eggs during 48 h,
those producing about 50% reduction in larval survival during 12
days (clams) or 14 days (oysters), and the effects on larval growth
during 10 or 12 days of exposure (expressed as a percentage of
growth of control larvae).
Table 25. Acute toxicity of aldrin and dieldrin for aquatic invertebrates
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Daphnids
Daphnia magna (29)a 330a Anderson (1959)
Simocephalus first instar dispersed via 15 (23)a (240)a Johnson & Finley
serrulatus acetone 21 (32)a (1980)
Daphnia pulex first instar dispersed 15 (28)a (190)a Johnson & Finley
(1980)
Crustacea
Seed shrimp mature dispersed via 21 (18)a - Johnson & Finley
(Cypridopsis acetone (1980)
vidua)
Sowbug (Asellus mature dispersed via 21 - 5 Johnson & Finley
brevicaudus) acetone (1980)
Scud (Gammarus mature dispersed via 21 4300 640 Johnson & Finley
fasciatus) acetone (1980)
Sand shrimp 0.25 g, dispersed via 20 8 7 Eisler (1969)
(Crangon 2.6 cm acetone
septemspinosa)
2 g dispersed via 20 - 0.4 McLeese & Metcalfe
hexane (1980)
2 g dispersed in 10 - 4.1 McLeese & Metcalfe
sediment (1980)
Grass shrimp 0.47 g, dispersed via 20 9 50 Eisler (1969)
(Palaemonetes 3.1 cm acetone
vulgaris)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Crustacea (contd.)
Grass shrimp mature dispersed via 21 50 - Johnson & Finley
(Palaemonetes acetone (1980)
kadiakensis)
Crayfish mature dispersed via 21 - 740 Johnson & Finley
(Orconectes acetone (1980)
nais)
Hermit crab 0.28 g, dispersed via 20 33 18 Eisler (1969)
(Pagurus 0.35 cm acetone
longicarpus)
Molluscs
Mercenaria egg dispersed via 24 (> 10 000)a - Davis & Hidu (1969)
mercenaria acetone
Crassostrea egg dispersed via 24 - (640)a Davis & Hidu (1969)
virginica acetone
Slipper limpet veliger - - - > 100 Adema & Vink (1981)
(Crepidula
fornicata)
Pond snail egg - - - > 200 Adema & Vink (1981)
(Lymnaea juvenile
stagnalis)
Insects
Pteronarcys naiad, dispersed via 15.5 1.3 0.5 Sanders & Cope
californica 3-3.5 cm ethanol (1968); Johnson &
Finley (1980)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Insects (contd.)
Pteronarcella naiad, dispersed via 15.5 - 0.5 Sanders & Cope
badia 1.5-2 cm ethanol (1968); Johnson &
Finley (1980)
Claassenia naiad, dispersed 15.5 - 0.6 Sanders & Cope
sabulosa 2-2.5 cm (1968); Johnson &
Finley (1980)
Pteronarcys naiad, 2-5 cm dispersed via 12.8 180 39 Jensen & Gaufin
californica acetone (1966)
Acroneuria naiad, dispersed via 12.8 143 24 Jensen & Gaufin
pacifica 2-2.5 cm acetone (1966)
Damselfly juvenile dispersed via 24 - 12 Johnson & Finley
(Ischnura acetone (1980)
venticalis)
Other invertebrates
Bristle worm 2-3-day-old dispersed via 21 - > 100 Hooftman & Vink
(Ophryotrocha larva acetone (1980)
diadema)
4-week-old dispersed via 21 - > 100 Hooftman & Vink
adult worm acetone (1980)
---------------------------------------------------------------------------------------------------------
a Values in parentheses are the 48-h LC50.
Table 26. Short-term LC50s of dieldrin in invertebrates
-------------------------------------------------------------------
Species Stage LC50 at end of Reference
study (µg/litre)
(time of exposure)
-------------------------------------------------------------------
Ophryotrocha larva >10 Hooftman & Vink
diadema (2-3 days) (5-6 weeks) (1980)
adult 60 Hooftman & Vink
(4 weeks) (5-6 weeks) (1980)
Daphnia magna larva 100 Adema & Vink
(3 weeks) (1981)
adult 200 Adema & Vink
(0.3 cm) (7 days) (1981)
Artemia salina larva 40 Adema & Vink
(4 weeks) (1981)
adult 50 (male) Adema & Vink
(1 cm) (7 days) (1981)
110 (female)
(7 days)
Chaetogammarus larva 1.8 Adema & Vink
marinus (4 weeks) (1981)
adult 3.6 Adema & Vink
(1 cm) (14 days) (1981)
Palaemonetes adult 0.3 Adema & Vink
varians (4 cm) (7 days) (1981)
Crangon crangon adult 4 Adema & Vink
(4 cm) (14 days) (1981)
-------------------------------------------------------------------
When adult mud snails (Nassa obsoleta) were exposed to up to
10 000 µg dieldrin/litre for 96 h, and then transferred to
dieldrin-free sea water for 33 days, no mortality occurred
throughout the study and the length of the animals was normal after
33 days. There was a significant increase in total egg deposition
during the 33-day post-treatment period in the case of snails
exposed to 10 µg dieldrin/litre, but there was a significant
reduction at 100, 1000, and 10 000 µg dieldrin/litre (Eisler,
1970).
(c) Behaviour
In studies by Klein & Lincer (1974), fiddler crabs, (Uca
pugilator) were fed diets containing 0, 0.1, 1, 10, and 50 mg
dieldrin/kg diet for 14 days and observed for another 25 days.
Behaviour, measured as righting response, was modified at dose
levels of 1 mg/kg or more, and even in the group given 0.1 mg/kg,
difficulty in righting was seen after 11 days. With 10 and 50
mg/kg diet, an increase in mortality was observed, but not with 1
mg/kg diet.
Table 27. Concentrations producing about 50%
reduction in the development of fertilized eggs
during 48 h, in larval survival during 12 days
(clams) or 14 days (oysters), and effects on
larval growth during 10 or 12 days exposurea
(Davis & Hidu, 1969)
--------------------------------------------------
Organism Effect Aldrin Dieldrin
(µg/litre) (µg/litre)
--------------------------------------------------
Clam development of > 10 000 -
Oyster fertilized eggs - 640
Clam larval survival 410 -
Oyster - > 10 000
Clam larval growth 250b -
Oyster - 500c
--------------------------------------------------
a Expressed as a percentage of growth of control
larvae.
b 80% reduction.
c 50% reduction.
7.2.2. Fish
7.2.2.1 Acute toxicity
Both aldrin and dieldrin are highly toxic to fish under
laboratory conditions. A summary of reported 96-h LC50 values for
fresh water and marine species is given in Table 28. In parallel
studies, dieldrin was consistently more toxic than aldrin. The
96-h LC50s range from 2.2 to 53 µg aldrin/litre and from 1.1 to 41
µg dieldrin/litre in various fish species. It should be noted that
the range for aldrin exceeds the water solubility of the compound.
The results of studies by Macek et al. (1969) indicate that a
rise in temperature increases the toxicity of aldrin and dieldrin
for bluegills and rainbow trout. However, Johnson & Finley (1980)
stated that toxicity was not appreciably (only a factor of 2)
changed by variations in temperature or water hardness.
Macek (1975) investigated the effects of simultaneous exposure
of bluegills to DDT and dieldrin and concluded that the acute
toxicity of dieldrin in the concentration range 5.9 - 6.6 µg/litre
was not increased by the presence of DDT (concentration range,
4.5 - 5 µg/litre).
Anderson & Weber (1975) found that new-born and juvenile
guppies (Lebistes reticulatus) were more resistant to dieldrin
than adults. A relationship between the LC50 and body weight was
derived for mature, juvenile, and new-born guppies:
LC50 = aWb
where W is the body weight. The best fit value for the exponent b
was 0.81.
Table 28. Acute toxicity of aldrin and dieldrin for fish
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water
Rainbow trout 0.6 dispersed 13 2.6 - Johnson &
(Salmo via acetone Finley (1980)
gairdneri)
1.4 dispersed 13 - 1.2 Johnson &
via acetone Finley (1980)
3.2 dispersed 20 17.7 9.9 Katz (1961)
via acetone
0.6-1.5 dispersed 1.6 3.2 2.4 Macek et al.
via acetone 7.2 3.3 1.1 (1969)
12.7 2.2 1.4
Cutthroat trout 1.1 dispersed 9 - 6a Johnson &
(Salmo clarki) via acetone Finley (1980)
Chinook salmon 1.45-5 dispersed 20 7.5 6.1 Katz (1961)
(Oncorhynchus via acetone
tshawytscha)
0.8 dispersed 15 14.3 - Johnson &
via acetone Finley (1980)
Coho salmon 2.7-4.1 dispersed 20 45.9 10.8 Katz (1961)
(Oncorhynchus via acetone
kisutch)
Goldfish 1-2 dispersed 25 32 41 Henderson et al.
(Carrassius via acetone (1959)
auratus)
Goldfish 1 dispersed 18 - 1.8 Johnson &
(Carassius via acetone Finley (1980)
auratus)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water (contd.)
Carp (Cyprinus NA NA 20 4b - Rehwoldt et al.
carpio) (1977)
Fathead minnow 0.6 dispersed 18 8.2 3.8 Johnson &
(Pimephales via acetone Finley (1980)
promelas)
1-2 dispersed 25 32 18 Henderson et al.
via acetone (1959)
Guppy (Lebistes 0.1-0.2 dispersed 25 37 25 Henderson et al.
reticulatus) via acetone (1959)
NAd NA 20 20b - Rehwoldt et al.
(1977)
NA NA 24 - 3.2-7 Adema & Vink
(young) (1981)
NA NA 24 - 35c Adema & Vink
(adult) (1981)
juvenile NA 25 10.9 Anderson & Weber
(1975)
newborns NA 36.7 Anderson & Weber
(1975)
Black bullhead 1.5 dispersed 24 19 - Johnson & Finley
(Ictalurus via acetone (1980)
melas)
Channel catfish 5.2 dispersed 18 53 - Johnson & Finley
(Ictalurus via acetone (1980)
punctatus)
1.4 dispersed 18 - 4.5 Johnson & Finley
via acetone (1980)
Bluegill 0.7 dispersed 18 6.2 - Johnson & Finley
(Lepomis via acetone (1980)
macrochirus)
1.3 dispersed 18 - 3.1 Johnson & Finley
via acetone (1980)
1-2 dispersed 25 15 8.8 Henderson et al.
via acetone (1959)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Bluegill 0.6-1.5 dispersed 12.7 7.7 17 Macek et al.
(contd.) via acetone 18.3 5.8 14 (1969)
23.8 4.6 8.8
Pumpkinseed NA NA 20 20b - Rehwoldt et al.
sunfish (1977)
(Lepomis
gibbosus)
Largemouth bass 2.5 dispersed 18 5 3.5 Johnson & Finley
(Micropterus via acetone (1980)
salmoides)
Striped bass NA NA 20 10b - Rehwoldt et al.
(Marone (1977)
saxatilis)
Banded killyfish NA NA 20 21b - Rehwoldt et al.
(Fundulus (1977)
diaphanus)
White perch NA NA 20 42b - Rehwoldt et al.
(Roccus (1977)
americanus)
American eel NA NA 20 16b - Rehwoldt et al.
(Anguilla (1977)
rostrata)
Marine species
Common goby NA NA 15 - 3.5 Adema & Vink
(Gobius microps) (adult) (1981)
Plaice length: NA 15 - 1.7 Adema & Vink
(Pleuronectes 2-3 cm (1981)
platessa)
length: NA 15 - 4 Adema & Vink
10 cm (1981)
yolk-sac NA 5-10 - 30 Adema & Vink
larva (1981)
egg-metam NA 5-10 - > 32 Adema & Vink
larva (1981)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Marine species (contd.)
Threespine 0.4-0.8 dispersed 20 27.4 13.1 Katz (1961)
stickleback via acetone
(Gasterosteus
aculeatus)
------------------------------------------------------------------------------------------
a Hardness = 162 mg CaCO3/litre.
b Hardness = 50 mg CaCO3/litre.
c 48-h LC50.
d NA = not available.
7.2.2.2 Long-term toxicity
Sailfin mollies (Lebistes latipinna) were exposed in groups of
20 to 0, 0.75, 1.5, 3, 6, or 12 µg dieldrin/litre using a flow-
through system for 34 weeks. The mortality of the 0.75 µg/litre
group was similar to that of the control group. At 1.5 µg/litre,
there was an increase in mortality, and, at 3 µg/litre or more,
100% mortality occurred. The growth rates and reproduction
performances were adversely affected in the surviving fish (Lane &
Livingstone, 1970).
Rainbow trout (Salmo gairdneri) were fed food containing
dieldrin for 240 days, the nominal dietary concentrations
corresponding to 14, 43, 143, or 430 µg dieldrin/kg body weight per
day. The growth rate was not affected at any of the concentrations
throughout the 240 days, and there was no mortality or visible
adverse effects. The activities of liver glutamate-pyruvate
transaminase (GPT) and glutamate-oxaloacetate transaminase (GOT)
were not affected, except, in the case of the latter, at the
highest dose level. Liver glutamate dehydrogenase (GDH) activity
was increased at all dose levels. Electron micrographs of liver
cells demonstrated changes in mitochondrial morphology, the highest
dose causing swelling and membrane disruption. Since GDH is an
intramitochondrial enzyme, examination by electron microscopy gave
further evidence that dieldrin altered mitochondrial metabolism.
In the brain, GOT activity was significantly decreased at 43 µg/kg
and GTP was decreased at 14 µg/kg or more. At all dose levels,
brain GDH was decreased and brain glutamine transferase (GT) was
increased. Electron microscopy of the medulla and cerebral
hemispheres did not show any effects of dieldrin. The
concentrations of 16 free amino acids in the brain were determined.
The concentrations of four were not significantly changed, whereas
eight were significantly altered at 143 µg dieldrin/kg and 12 at
430 µg/kg. Serum ammonia concentrations were significantly
increased at 143 and 430 µg dieldrin/kg, but the concentration of
ammonia in the brain was not affected. The increase in brain GT
was considered to be a possible reason for this lack of effect on
brain ammonia, since it compensated for the decrease in GDH
activity. Alternatively, brain ammonia may have been transported
via the blood to the liver with consequent effects on the liver.
The ammonia-detoxifying mechanism of fish seemed to be very
sensitive to dieldrin, the no-effect dose being below 14 µg/kg body
weight per day (equivalent to 0.36 mg/kg food) (Mehrle &
Bloomfield, 1974).
Several studies have described the influence of aldrin and/or
dieldrin on enzymes such as mitochondrial succinic hydrogenase, the
epoxidative activities of liver microsomes and the ATPase activity
of microsomes of the gills or brain (Chan et al., 1967; Davis et
al., 1972; Moffet & Yarbrough, 1972; Yap et al., 1975).
Furthermore, the influence of dieldrin during thermal stress has
been studied in darters (Etheostoma nigrum) (Silbergeld, 1973).
In studies by Verma & Tonk (1984), Heteropneustes
(Saccobranchus) fossilis was exposed to aldrin for 30 days at a
concentration of 0.03 mg/litre. Respiration, haematological
parameters, and the activity of two enzymes in liver, kidneys, and
gills were determined. The respiration rate decreased and the
blood concentrations of glucose, sodium, and chloride ions showed
significant increases. The cholesterol content and clotting time
were decreased, and the ATPase activity in the three tissues was
significantly reduced.
7.2.2.3 Reproduction
Van Leeuwen (1986) carried out studies with dieldrin to study
the susceptibility of early-life stages of rainbow trout. The test
was performed with fertilized eggs before and after water
hardening, and with early eye point eggs, late eye point eggs, sac
fry, and early fry. No mortality was found in the different early-
life stages using concentrations greater than aqueous solubility
(> 10 mg/litre), except for the early fry where a very low 96-h
LC50 of 0.003 mg/litre was found.
In studies by Cairns et al. (1967), nine populations of guppies
(Lebistes reticulatus) were exposed in a semi-static system to
nominal concentrations of 0 (three populations), 1.8, 5.6, and 10
µg dieldrin/litre (two populations of each) for 14 months. During
the first 2 - 3 months, the exposed populations in five tanks
developed greater numbers of individuals (mature, immature, and
fry) than did the controls (except one population at 5.6 µg/litre,
which was similar to the controls). The difference between the
controls and five treatment groups was attributed to the higher
predation and harassment observed in the control groups. The total
numbers of individuals in control and treatment groups became
similar during the final 6 - 8 months of the study. The average
total monthly body weights of the groups treated with 1.8 µg/litre
and 5.6 µg/litre began to increase steadily after about 8 months,
whereas the total monthly body weights of the group exposed to 10
µg/litre were similar to the controls throughout the 14 months of
the study. The production of fry by one of the groups treated with
10 µg/litre declined markedly after the thirty-second week of the
study, no new broods of fry being born after the forty-second week.
No such marked decline occurred in the other five treatment groups
(including one population exposed to 10 µg/litre).
Chadwick & Shumway (1970), conducted studies lasting 130 days
on rainbow trout (Salmo gairdneri) to determine survival from time
of fertilization through to hatching in continuously cycled water.
Embryos, alevins, and fry were exposed to dieldrin concentrations
ranging from 0.012 to 52 µg/litre. Eggs (embryos) exposed to up to
52 µg dieldrin/litre from the time of fertilization survived until
hatching as well as controls, but the mean weight of newly-hatched
alevins (minus yolk material) was reduced by higher concentrations
(not specified). Alevins were more susceptible than embryos.
Their survival was reduced at all concentrations above 0.39
µg/litre. Trout fry, whose survival was unaffected at dieldrin
levels of 0.12 µg or less, quickly succumbed at concentrations of
0.39 µg/litre or more.
Smith & Cole (1973), exposed adult winter flounder (Pseudo-
pleuronectes americanus) to 2 µg/litre in aquaria continuously
supplied with filtered sea water. When fish became ripe, they were
artificially spawned, and approximately 30 000 eggs were collected
from each of the 24 spawning pairs and cultured. The remaining
eggs were analysed. The percentage fertilization of eggs
containing 0.61 mg dieldrin/kg or less was 99% (controls, 97.8%).
The percentage fertilization of eggs containing 1.21 mg dieldrin/kg
was 12%, and all the eggs containing 1.74 mg/kg were infertile.
There was no effect on egg development except in the case of the
two groups of eggs containing the higher concentrations of
dieldrin. The effects on egg mortality were not due to dieldrin in
the gametes, and the milt of exposed male flounders contained no
detectable residue of dieldrin.
7.2.3. Amphibia and reptiles
The LC50 values for tadpoles of two species of frogs (1 week
old) and toads (4 - 5 weeks old) were determined by Sanders (1970).
The 96-h LC50 (at 15.5 °C) of dieldrin for Western chorus frog
tadpoles (Pseudacris triseriata) was 100 µg/litre, whereas that of
both aldrin and dieldrin for Fowler's toad tadpoles (Bufo
woodhousii fowleri) was 150 µg/litre.
Cooke (1972) studied the effect of dieldrin at nominal
concentrations of 0.008, 0.02, or 0.5 mg/litre on groups of 40
common frog (Rana temporaria) or toad (Bufo bufo) tadpoles with
hindlimb paddles or hind legs. The exposure was for 24 or 48 h in
amphibian saline, and the observation period 5 or 15 days. At the
highest dose level, the frogs showed an increased mortality, the
mean dieldrin content being 42.9 mg/kg tissue. At the two lower
dose levels (0.008 and 0.02), there were 0.31 and 6.1 mg/kg
dieldrin in tissues, respectively. When toad tadpoles were exposed
to 0.02 or 0.5 mg/litre, the animals with the higher dose level
showed clear behavioural and structural abnormalities and a reduced
rate of development, but these changes returned to normal a few
days after exposure. The mean dieldrin content was 138 mg/kg
tissue at a dose level of 0.5 mg/litre.
The in vitro exposure of toad embryo tissue (Bufo arenarum) to
dieldrin (4 x 10-5 mol/litre) produced an inhibition of acetyl and
butyryl cholinesterase activity. In in vivo studies with open-
mouth stage embryos, dieldrin produced acetyl cholinesterase
inhibition at 0.5 x 10-6 mol/litre. Furthermore, hyperactivity in
swimming larvae was observed (de Llamas et al., 1985).
The in vitro activity of ATPase in a number of tissues of the
male turtle (Graptemys geographica) was determined by Wells et al.
(1974). There was no consistent dose relationship for either aldrin
or dieldrin, except perhaps in the case of the cloacal bladder in
the aldrin treatments. The inhibition of Na/K/Mg ATPase by aldrin
and dieldrin was in the range of 4 - 13%. It was suggested that
aldrin and dieldrin may affect the transport of metabolites across
the cellular membranes as a result of decreased energy for active
transport.
7.3. Terrestrial Organisms
7.3.1. Higher plants
Dieldrin has low phytotoxicity, tomatoes and cucumber, for
example, being affected only at application rates greater than 22
kg/ha. Aldrin affects some crops at rates greater than 22 kg/ha,
beans and cereals being most sensitive. Tomatoes and cucumbers are
sensitive to aldrin but only at unrealistically high application
rates (Edwards, 1965).
Studies in greenhouses showed that aldrin, administered weekly
as an emulsifiable concentrate at a rate of 16 kg active
ingredient/ha to 2 - 3-week-old seedlings of tomato, cauliflower,
and Chinese cabbage, inhibited root development and reduced growth
rate of cauliflower and Chinese cabbage seedlings. A ten-fold
reduction in the aldrin level failed to produce these effects
(Hagley, 1965).
Aldrin and dieldrin at 11 kg active ingredient/ha had no effect
on the emergence, growth, yield, or chemical composition of
soybeans (Probst & Everly, 1957).
7.3.2. Earthworms
In studies by Cathey (1982), earthworms (Lumbricus terrestris)
were maintained in an artificial nutritionally complete soil, based
on shredded paper containing aldrin. The LC50 value (6-week
exposure) was 60 mg aldrin/kg bedding, and the tolerance level,
producing less than 1% mortality, was 13 mg aldrin/kg bedding.
When aldrin (2.5 - 4.6 kg/ha) was applied as a spray or dust,
respectively, to the surface of soil plots and incorporated into
the soil, the numbers of earthworms in treated plots were either
similar to or greater than those in control plots (Edwards et al.,
1967; Griffiths et al., 1967; Edwards & Lofty, 1977).
7.3.3. Bees and other beneficial insects
In a review of five investigations on the toxicity of aldrin
and dieldrin to honey bees (Sanger, 1959), the oral LD50 values for
aldrin ranged from 0.24 to 0.45 µg/bee, while the values for
dieldrin were in the range 0.15 - 0.32 µg/bee. Contact LC50 values
were 0.15 - 0.8 µg/bee for aldrin and 0.15 - 0.41 µg/bee for
dieldrin.
Cowie (1967) reported an oral LD50 of 0.3 µg dieldrin/bee
(range, 0.13 - 0.54) and a contact LD50 of 0.21 µg/bee.
The toxicity of dieldrin to two important predators of cotton
pests was investigated by Burke (1959). The contact LD50 value for
Hippodamia convergens was 1.6 mg/g body weight.
In a review of the effects of pesticides on soil fauna, it was
concluded that aldrin (and, by implication, dieldrin) is relatively
non-toxic for predatory mites ( Acarina spp.), and that this may
contribute to its success as a soil insecticide (Edwards &
Thompson, 1973).
7.3.4. Birds
7.3.4.1 Acute toxicity
Estimates of the LD50 values for several species of birds are
given in Table 29. The variation in acute oral toxicity of
dieldrin among six species of birds tested by Tucker & Haegele
(1971) was more than ten fold.
Table 29. Acute oral toxicity of aldrin and dieldrin for avian
speciesa
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Fulvous whistling duck male: female: Tucker & Crabtree
(Dendocygna bicolor) 29.2 100-200 (1970)
Mallard duck female: female: Tucker & Crabtree
(Anas platyrhynchos) 520 381 (1970)
Canada goose 50-150 Tucker & Crabtree
(Branta canadensis) (1970)
-------------------------------------------------------------------
Table 29. (contd.)
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Domestic fowl 25.5 43 Sherman &
(Gallus domesticus) Rosenberg (1953)
Japanese quail male: Tucker & Crabtree
(Coturnix coturnix 69.7 (1970)
japonica)
Bobwhite quail female: Tucker & Crabtree
(Colinus virginianus) 6.6 (1970)
California quail 8.7 Hudson et al.
(Callipepla californica) (1984)
Gray partridge female: Tucker & Crabtree
(Perdix perdix) 8.8 (1970)
Chukar partridge 23.4 Tucker & Crabtree
(Alectoris graeca) (1970)
Sharp-tailed grouse male: McEwen & Brown
(Pedioecetes phasianellus) 6.9 (1966)
Ring-necked pheasant female: female: Tucker & Crabtree
(Phasianus colchicus) 16.8 79 (1970)
Pigeon 55 67 Turtle et al.
(Columba livia) (1963)
26.6 Tucker & Crabtree
(1970)
House sparrow female: Tucker & Crabtree
(Passer domesticus) 47.6 (1970)
-------------------------------------------------------------------
a Details concerning age and weight of birds are not summarized
here but can found in the original publications.
7.3.4.2 Short- and long-term toxicity
Values for the subacute LC50s of aldrin and dieldrin,
determined using the procedure developed at the Patuxent Wildlife
Centre (Hill et al., 1975), are given in Table 30. The LC50 values
of aldrin and dieldrin for each of the four species tested were of
the same order. The annual variations in the LC50 of dieldrin over
a period of up to 8 years for these four species have been
investigated by Hill et al. (1977) (18 times per species). No
time-related changes in LC50 values were found for any of the
species. However, differences were found between birds of
different ages in some species, e.g., Japanese quail and mallards
(Hudson et al., 1984). There were also differences between the
slopes of the average regression lines for the four species. These
authors emphasized the need to evaluate both the LC50 and the slope
of the regression line. Food consumption was reduced by aldrin or
dieldrin in the diet.
Table 30. Subacute dietary toxicity of aldrin and dieldrin
for avian speciesa
-----------------------------------------------------------
Species Age LC50 (95% confidence limits)
(days) ----------------------------
Aldrin Dieldrin
(mg/kg diet)
-----------------------------------------------------------
Mallard duck 5 155 153
(Anas platyrhynchos) (129-186) (123-196)
10 - 169
Japanese quail 14 34 62
(Coturnix coturnix (28-41) (53-71)
japonica)
Bobwhite quail 14 37 37
(Colinus virginianus) (33-41) (30-46)
Ring-necked pheasant 10 57 58
(Phasianus colchicus) (50-64) (51-67)
-----------------------------------------------------------
a Aldrin or dieldrin fed for 5 days followed by 3 days of
untreated diet.
Wiese et al. (1969) fed diets containing up to 500 mg technical
dieldrin (85%)/kg diet to male and female 6-month-old crowned
guinea-fowl (Numida meleagris). None of the birds fed 1.5 mg/kg
for 21 months died. The median survival time for birds fed 5 mg/kg
was 524 days; for birds fed 150 and 500 mg/kg, it was 3 and 1 days,
respectively. No differences in susceptibility between males and
females were found.
The subacute toxicities of technical dieldrin (85%) to three
species of birds are given in Table 31 (Basson, 1971).
7.3.4.3 Reproductive studies
The first experimental studies of the effects of aldrin or
dieldrin on avian reproduction showed that these compounds were
toxic for quail and pheasants. Quail fed a diet containing aldrin
or dieldrin at a toxic dose of 0.5 or 1 mg/kg diet did not show any
clear effects on egg production, percentage fertility, or
percentage hatchability (the birds had not been exposed earlier to
the compounds) (DeWitt, 1955, 1956). No significant effect was
found on the fertility or hatchability of eggs of pheasants fed
25 mg dieldrin/kg diet, but at 50 mg/kg there was a clear effect
(Genelly & Rudd, 1956).
Table 31. Subacute toxicity of technical dieldrin for three species
of birds
-------------------------------------------------------------------
Species Concentration Median time Median lethal
(mg/kg diet) till death dose (mg
(days) dieldrin/kg
body weight)
-------------------------------------------------------------------
Guinea fowl 20 72 72.4
(Numida meleagris) 150 12 11.2
Laughing dove 5 49 15.8
(Stigmatopelia 90 4.7 17.3
senegalensis)
Sparrow (Passer 5 85.1 > 41
melanurus melanurus) 45 7 43.8
-------------------------------------------------------------------
Eggs from chickens fed 1 mg aldrin or dieldrin/kg diet for
2 years showed normal fertility and hatchability, although the
concentrations of dieldrin in the yolks of the eggs were in the
range of 6 to 25 mg/kg. The fertility and hatchability slightly
decreased at 10 mg dieldrin/kg diet (Brown et al., 1965).
Other studies on avian reproduction are summarized in Table 32.
This table gives the available information on five criteria
relevant to reproduction (parental survival, production, fertility
and hatchability of eggs, and chick survival) in relation to oral
intake of dieldrin and (where reported), the residues of dieldrin
in eggs. These studies show that, depending on the duration of
exposure, dose levels of 5 - 10 mg dieldrin/kg diet reduce the
survival of the parent birds. Egg production was reported to be
significantly increased in some studies but reduced in others. In
general, egg fertility was not influenced, except in one study.
Hatchability was not affected, neither in most cases, was the
survival of chicks. There seems to be a trend that overall
reduction in reproductive success occurs only if the parent birds
are showing signs of being affected by dieldrin, e.g., reduced food
intake with consequent loss of weight and poor condition. It
should be noted that in these studies (with one exception) the eggs
were placed in incubators for hatching. Consequently, one aspect
of the reproductive process was not studied, namely, parental
behaviour. However, in the study on homing pigeons (Robinson &
Crabtree, 1969), the parents (and subsequently, their offspring)
were free-flying, they brooded their eggs, and fed their young
until they fledged.
A 3-generation study of the effects of dieldrin on pheasants
(Phasianus colchicus), has not been included in Table 32 because
of the complexity of the experimental design. The doses of
dieldrin (hens, 6 or 10 mg dieldrin/bird per week; cocks, 4 or 6 mg
dieldrin/bird per week) were sufficient to cause mortality of
breeding birds, but the production, fertility, and hatchability of
eggs and the viability of chicks at the time of hatching were not
affected in a consistent manner in relation to dose or generation.
The survival of chicks from hens given 6 or 10 mg dieldrin/week was
reduced. Residues of dieldrin in eggs or tissues were not
determined in this study (Dahlgren & Linder, 1974).
When seven-week-old Japanese quail were given diets containing
3.1 or 50 mg dieldrin/kg diet for 21 days, a significant reduction
in egg production occurred in both groups (Call & Harrell, 1974).
With the exception of the study with the barn owl (Mendenhall
et al., 1983) and the homing pigeon (Robinson & Crabtree, 1969),
the birds that were tested are precocial species which show no
parental feeding of the young. Most birds are not precocial and
reproduction involves a period of full dependency of the offspring
on parental care. Results should, therefore, be interpreted with
care; extrapolation directly from the laboratory to the field is
difficult.
7.3.4.4 Eggshell thinning
Ratcliffe (1967a) reported that the ratio of eggshell weight to
size in three species of birds of prey in the United Kingdom had
declined during the period after 1947 relative to pre-1947. This
report has stimulated considerable interest in the relationship
between egg-shell thickness (or the related eggshell index based on
the weight/size ratio) and the breeding success of birds,
particularly as eggshell thinning seems to be quite a widespread
phenomenon, particularly among birds of prey (Hickey & Anderson,
1968; Ratcliffe, 1970; Anderson & Hickey, 1972). There has been
considerable speculation on the causes and mechanism of these
changes (Cooke, 1973; Mueller & Leach, 1974). Experimental studies
on the effects of dieldrin on eggshell thickness have given
conflicting results. The results are summarized in Table 33.
Eggshell weights of crowned guinea-fowl (Numida meleagris) fed
diets containing up to 15 mg dieldrin/kg diet for 21 months were
not affected by the treatments (Wiese et al., 1969).
American sparrow hawks (Falco sparverius) were given diets
containing dieldrin and North American prairie falcons (Falco
mexicanus) were fed starlings contaminated with an average of 29 mg
dieldrin/kg body weight, along with DDT and DDE at different
levels. The purpose of these studies was to show the influence of
these pesticides on shell thickness. The results of the two
studies, in relation to the effects of dieldrin, cannot be
interpreted, in view of the possible effects of DDE on eggshell
thinning (Porter & Wiemeyer, 1969; Enderson & Berger, 1970). It
has slowly become accepted that metabolites of DDT, particularly
DDE, are the most likely cause of eggshell thinning (Cooke, 1973;
Newton, 1979; Bunyan & Stanley, 1982). It is also pertinent that
the onset of eggshell thinning in wild birds preceded the use of
aldrin/dieldrin. There is evidence that eggshell thinning after
exposure to dieldrin is related to reduced food consumption.
Untreated Coturnix and Mallard, when fasted for 36 h, laid thin-
shelled eggs for a few days during and after fasting (Haegele &
Tucker, 1974).
Table 32. Reproductive success of birds in relation to oral intake of dieldrin and the concentration of HEOD in eggs
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Mallard duck 4 90 days - - NCg NC Red.c - Muller &
(Anas platy- Lockman (1972)
rhinchos)
Japanese quail 1 16 weeks - - NC NC NC - Shellenberger
(Coturnix 10 16 weeks - - NC NC NC - & Newell
coturnix (1965)
japonica)
10 18 weeks 19 NC NC NC NC NC Walker et al.
(6.9-26.9) (1969a)
20 9 weeks 39 Red. Red. DC NC Red.
(19.8-54.1)
30 7 weeks 48 Red. Red. DC Red. Red.
(34.7-63.2)
40 6 weeks 84 Red. Red. - - -
(76.9-92.5)
0.1 multi- - NC NC NC NC NC Shellenberger
generation (1978)
P; F1,
1 F2, F3 - NC NC NC NC NC
Bobwhite quail 10 34 weeks - Red. NC - - - Fergin &
(Colinus 20 34 weeks - Red. Red. - - - Schafer (1977)
virginianus) 40 34 weeks - Red. Red. - - -
Pheasant 2 mg/hen/ 13 weeks (0.6-26.5) NC NC NCa Inc.c NC Atkins &
(Phasianus weekb (yolks) Linder (1967)
colchicus) 2 mg/hen/ 13 weeks - NC NC NC NC NC
week
4 mg/hen/ 13 weeks (5.3-40.1) DR NC NC NC NC
week (yolks)
4 mg/hen/ 13 weeks (7.5-20.6) NC NC NC NC NC
week (yolks)
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Pheasant 6 mg/hen/ 13 weeks (13.3-52.4) NC Red.a NC NC NC
(Phasianus week (yolks)
colchicus)
(contd.) (0) 6 mg/hen/ 14 weeks - Red. Inc.c Red.c NC NC Baxter et al.
weekd (1969)
(0) 8 mg/hen/ 14 weeks - Red. Inc.c NC NC NC
week
(0) 12 mg/ 14 weeks - Red. Red.c NC - -
hen/week
(4) 0 mg/hen/ 14 weeks - NC Inc.c Red.c Red. NC
week
(4) 6 mg/hen/ 14 weeks - Red. NC Red.c NC NC
week
(6) 0 mg/hen/ 14 weeks - NC Red.c NC - -
week
(6) 6 mg/hen/ 14 weeks - Red. Red.c Red.c - -
week
Gray partridge 3 1.5 - DR NC NC NC Neill et al.
(Perdix perdix) (1-2) (1971)
Domestic fowl 2 16 weeks (0.34-1.45) NC NC - NC NC Graves et al.
(Gallus 5 16 weeks (1.2-4.8) NC NC - NC NC (1969)
domesticus)
10 13 months (7.6-16) NC NC NC NC NC Brown et al.
(1974)
20 13 months (19.6-35.7) Red. Inc.a NC NC Red.
Crowned guinea- 0.5 21 months 1.11/1.17e NC Inc.a NC NC NC Wiese et al.
fowl (Numida (1969)
meleagris) 1.5 21 months 2.38/3.35e NC Inc.a NC NC NC
5 21 months 7.18/13.56e PR Inc.a NC NC NC
15 21 months 15.79e Red. Inc.a NC NC Red.
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Homing pigeon ~2 mg/kg 2 years (0.03-13.4)f NC NC NC NC NC Robinson &
(free-flying) body weight/ (4.5-16.7)f NC NC NC NC NC Crabtree
(Columba livia) week (1969)
Barn owl 0.5 2 years 3.6 (first NC NC NC NC NC Mendenhall et
(Tyto alba) year) al. (1983)
8.1 (second
year)
--------------------------------------------------------------------------------------------------------------------
a Significant at a probability of < 0.01.
b Doses of 2, 4, or 6 mg dieldrin/hen were administered once per week in capsules.
c Significant at a probability of < 0.05.
d Second-generation hens; offspring of birds used in study by Atkins & Linder (1967). The doses in parentheses
refer to the doses administered to the first-generation hens.
e Residues in eggs of successive years.
f First and second laying periods, respectively.
g The term "no change" (NC) indicates that any differences between controls and treatment groups were within the
limits of experimental variation. If any of the results for treatment groups are reported to be increased (Inc.)
or reduced (Red.), the statistical significance, if reported, is given. Equivocal results have been described
"doubtful reduction" (DR), "probably reduced" (PR), and "doubtful change" (DC).
Table 33. Effects of dieldrin on eggshell thickness
--------------------------------------------------------------------------------------------------------------
Species Dose of dieldrin Duration Difference between egg- Reference
(mg/kg diet) shell thickness of treated
and control birds (%)
--------------------------------------------------------------------------------------------------------------
Mallard duck 1.6 16 months -3.4a Lehner & Egbert (1969)
(Anas platyrhynchos)
4 16 months -2a Lehner & Egbert (1969)
10 16 months -4.3a Lehner & Egbert (1969)
4 90 days -4.2 Muller & Lockman (1972)
Japanese quail 3.1 21 days with -8a Call & Harrell (1974)
(Coturnix coturnix changes in
japonica) 50 photoperiod -8a Call & Harrell (1974)
Domestic fowl 10 12 weeks 0 Davison & Sell (1972)
(Gallus domesticus) 20 12 weeks +0.3 Davison & Sell (1972)
10 13 months 0 Brown et al. (1974)
20 13 months +9.7 Brown et al. (1974)
Pheasant 6 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(Phasianus colchicus)
10 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(6) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(6) 6 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(10) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
--------------------------------------------------------------------------------------------------------------
a Significant at or below 0.05.
b Administered in capsules once per week (see footnoteb Table 32).
c See footnoted Table 32.
7.3.4.5 Concentrations of dieldrin in tissues of experimentally
poisoned birds
Many studies have been carried out to estimate the
concentrations of dieldrin in the liver, brain, or other tissues of
birds that died following oral intake of aldrin or dieldrin. The
intakes were either single acute doses or long-term dietary
exposure. In some investigations, the concentrations of dieldrin
in the tissues of birds that survived after treatment were also
reported. The results of these studies are not comparable, because
the dose levels and duration of the studies are different. The
concentrations that were found in the different studies ranged from
a few mg/kg up to about 100 mg/kg tissue (wet weight) (Turtle et
al., 1963; Koeman et al., 1967; Robinson et al., 1967b; Robinson,
1969; Robinson & Crabtree, 1969; Stickel et al., 1969; Enderson &
Berger, 1970; Linder et al., 1970; Brown et al., 1974; Clark, 1975;
Heinz & Johnson, 1981; Mendenhall et al., 1983) (Table 34).
Attempts to define tissue concentrations that can be used as
indicators of death by dieldrin poisoning of wild birds lack
precision as a result of the overlap between the lowest
concentrations in the tissues of birds dying under experimental
conditions and the highest concentrations in survivors. Thus, it
has been proposed that concentrations of 5 or 10 mg dieldrin/kg
brain (Robinson, 1969; Stickel et al., 1969) are indicative of
death from aldrin/dieldrin poisoning. Liver concentrations of 10
or 20 mg/kg (Robinson, 1969; Cooke et al., 1982) have been proposed
as levels diagnostic of dieldrin poisoning of birds.
As a general point, interpretation of residue data must be done
with extreme caution. Brain residues of dieldrin are probably a
good indicator of lethality. However, most bird carcasses
collected in the field cannot be analysed for brain residues,
because the brain deteriorates rapidly after death. For this
reason, most residue data from the field are for levels in the
liver, which remains discrete and usable for much longer. A liver
residue level symptomatic of death from dieldrin poisoning is more
difficult to define. A large, acutely toxic, dose of dieldrin may
leave a low residual level of dieldrin in liver because the bird
dies rapidly. A smaller, less acutely toxic, dose of dieldrin
usually leads to loss of body weight before death because of a lack
of ability or desire to feed. This period of starvation prior to
death boosts liver residues considerably as dieldrin is released
from mobilized fat and concentrated in the liver as detoxification
is attempted.
7.3.5. Mammals
The acute and long-term toxicity of aldrin and dieldrin for
laboratory mammals is summarized in section 8.
Values for the acute oral LD50 and subacute oral LC50 in the
diet (30 days) of dieldrin for four species of voles ( Microtus sp.)
are given in Table 35.
Table 34. Concentrations of dieldrin in the tissues of experimentally poisoned birds and
in survivors
------------------------------------------------------------------------------------------
Species Tissue Concentration of dieldrin (mg/kg wet weight) Reference
analysed geometric mean, (range of values)
No. of Survivors No. of Dead birds
samples samples
------------------------------------------------------------------------------------------
Domestic pigeon liver 11 8 20 45.6 Robinson et
(Columba sp.) (3.1-51.2) (23-81) al. (1967b)
brain 11 3.6 19 20
(1.6-8.5) (13.5-32.5)
House sparrow liver - - 19 44.7 Robinson
(Passer domesticus) (38.4-52.3) (1969)
brain - - 19 20
(17.6-22.7)
Japanese quail liver - 36 40 Robinson et
(Coturnix coturnix (17.7-90.4) al. (1967b)
japonica) brain 12 6.9 65 17.4
(3.1-15) (8.7-34.6)
Japanese quail liver 8 28.8 9 19.7 Stickel et
(Coturnix coturnix (2.7-140.8) (5.7-51.7) al. (1969)
japonica) brain 20 3.4 17 17.3
(0.25-11.9) (6.2-32.9)
Redwinged blackbird brain 3 7.1 27 19.8 Clark
(Agelaius phoeniceus) (6.7-7.4) (1-34.5) (1975)
27 22.2
(13.5-29.5)
Prairie falcon brain 2 2.9 1 11 Enderson &
(Falco mexicanus) (2.8-3) Berger
(1970)
Barn owl brain 2 10 Mendenhall
(Tyto alba) (5-15) et al.
carcass 19 9.4 - - (1983)
------------------------------------------------------------------------------------------
The toxicological signs in these studies were similar to those
in laboratory animals, and these four microtine rodents appear to
be less susceptible than laboratory rodents to dieldrin
intoxication (Cholakis et al., 1981). When short-tailed shrews
(Blerina brevicauda) were fed diets containing 50, 100, or 200 mg
dieldrin (nominal)/kg diet for up to 14 days, all the animals fed
50 mg/kg dieldrin survived, whereas all those fed 200 mg/kg diet
died (Blus, 1978).
Luckens & Davis (1965) studied the acute oral toxicity in big
brown bats (Eptericus fuscus) caught in Kentucky, USA. Death
occurred at all dose levels above 20 mg/kg body weight. The
approximate LD50 seemed to be within the range 20 - 40 mg/kg body
weight.
Table 35. Acute and subacute toxicity of dieldrin for volesa
------------------------------------------------------------------------
Species Acute LD50 Subacute LC50 (30 days)
(mg/kg body weight) (mg/kg body weight)
Average males/females Average males/females
------------------------------------------------------------------------
Microtus orchrogaster 210 105
Microtus canicaudus 100 40
Microtus montanus 205
Microtus pennsylvanicus 175
------------------------------------------------------------------------
a From: Cholakis et al. (1981).
White-tailed deer (Odocoileus virginianus) were fed 0, 5, or
25 mg dieldrin/kg diet for up to 3 years as were their progeny. No
signs of intoxication were observed, and the survival of adults was
not affected. The growth rate of treated females was decreased.
Relative liver weights increased at 25 mg/kg. Fertility and in
utero mortality were comparable for the three groups. Fawns from
treated does were smaller at birth, and greater postpartum
mortality occurred. The weight gain of fawns was reduced during 2
of the 3 years. Whole milk from doses fed 25 mg/kg contained
residues of 17 mg/litre. Residues in the liver of surviving
animals were about 4 mg/kg in the low-dose group and 16 mg/kg (wet
weight) in the high-dose group. Highest brain residues (12 mg/kg
wet weight) occurred in fawns only a few days before death (Murphy
& Korschgen, 1970).
When blesbuck (Damaliscus dorcas phillipsi) were fed diets
containing 5, 15, 25, 35, or 50 mg dieldrin (nominal)/kg diet, none
of the animals fed 5 or 15 mg/kg diet died during the 90 days of
the study. However, all the animals given higher dose levels died
within 24 days. The concentration of dieldrin in the liver was 3.3
and 8.2 mg/kg for the two lowest dose levels, after 90 days. The
concentrations of dieldrin in the livers of the dead animals were
9.4, 15.1, and 18.4, respectively, for the three highest treatment
groups (Wiese et al., 1973).
In studies by Wiese et al. (1973), an experimental grazing site
(250 ha) with a resident population of 35 blesbuck and 20 springbok
(Antidorcas marsupialis) was aerially sprayed with dieldrin at a
rate corresponding to 0.16 kg/ha. The concentration of dieldrin on
the veld declined rapidly from 27.6 mg/kg immediately after
treatment to 5.04 mg/kg after 14 days. The decline during the
following 106 days was much slower (it was 0.75 mg/kg on the 85th
day and 0.23 on the 120th day). Behavioural changes were observed
in both antelope species after 3 days. From the 4th day, there
were further nervous symptoms, including clonic convulsive attacks,
and partial or even complete blindness was also noted. Blesbuck
died from the 4th day onwards, and the entire population of 35
animals had died by the 19th day. The median time to death was
7.08 days, and there was no significant difference between adults
(male or female) and juveniles. The calculated mean intake of
dieldrin was 1.82 mg/kg herbage per day. This estimate was much
lower than that derived from the feeding trial (the total intake of
animals that died was 9.08 mg/kg). The mean concentration in the
livers of six blesbuck that died was 8.3 mg/kg. It was inferred
that dieldrin was unlikely to be the cause of death of the blesbuck
(further investigations implicated photodieldrin). Springbok were
less adversely affected: deaths occurred from the 6th day, and 70%
had died by the 13th day. Surviving animals recovered with no
after-effects, and two ewes lambed normally in the spring following
the winter treatment. The average dieldrin concentration in the
livers of three springbok that died was 9.2 mg/kg. The
pathological findings were similar to those in the common
laboratory species.
Few other mammalian species have been investigated. Reduced
reproductive success and some mortality has been reported in
raccoons fed 2 mg dieldrin/kg diet (Stickel, 1975).
7.4. Effect on Populations and Ecosystems
In order to show that a chemical has had an effect on
populations of organisms in the environment it is necessary to
satisfy a combination of several criteria. Ideally the exposure to
the chemical in the field should be established to compare exposure
with effects produced. Population declines should correlate with
usage of the chemical and should be reversed by controls on the use
of the chemical. Although it is generally recognized that dieldrin
affected populations of some animals when its use was widespread,
there are some difficulties in establishing its precise effects on
the environment. These difficulties arise because the use of
dieldrin coincided with the use of other persistent
organochlorines, which themselves affect populations of organisms,
and also because poisoning by dieldrin was often secondary
(poisoned organisms did not take in dieldrin directly but from prey
that had concentrated the chemical from the environment).
7.4.1. Exposure to dieldrin
It is difficult to establish the exposure of wildlife to
dieldrin unless animals are directly feeding on dressed grain or
directly exposed to preserved wood. Even where this occurs, the
animal will frequently die some distance from the source of
exposure. This is particularly true for birds but less so for
small mammals. Since exposure cannot be readily monitored
directly, most investigators have estimated exposure from the
residue remaining in dead or dying animals. Monitoring programmes
in several countries sampled both dead and dying animals and
compared them with healthy animals taken from the wild. Eggs of
birds were also monitored as a measure of dieldrin contamination
of populations. These monitoring programmes had to establish
criteria by which it could be definitively stated that particular
individuals had died from dieldrin poisoning. The criteria were
based on residue levels in experimentally poisoned animals and were
set at 5 - 10 mg/kg brain tissue and 10 - 20 mg/kg liver tissue for
birds (Robinson, 1969; Stickel et al., 1969; Cooke et al., 1982).
These criteria are probably conservative; 20 - 30% of dead wood
pigeons examined in the UK during the period 1961 - 1964 were
judged, by these criteria, to have died from dieldrin poisoning.
During this period many seed-eating birds were killed directly by
eating dieldren-dressed grain (Robinson, 1969); the actual
percentage of death attributable to dieldrin should probably be
higher. A high proportion of dead birds from areas of tsetse fly
control contained residues which would be judged lethal by these
criteria. The great majority of birds sampled contained non-lethal
residues of dieldrin (Tables 15, 16, 17, 18, and 34). It should be
remembered that all of these sampled birds contained residues of
other organochlorines, in addition to dieldrin. Although there
have been reports of populations with no dieldrin contamination,
but contamination with other organochlorines, there have been no
reports of populations contaminated by dieldrin alone. Furthermore,
residues of dieldrin always correlate well with residues of other
organochlorines; birds retaining large quantities of dieldrin also
retain large quantities of DDE and often polychlorinated biphenyls
(PCB) (Newton, 1979).
The literature reporting the presence of dieldrin in birds and
mammals from the wild is very extensive and has been selectively
reviewed elsewhere (section 5.1.6). Analysis of a few dead animals
serves to indicate the presence of dieldrin in wildlife but is of
little use in establishing effects at the population level. Only
long-term monitoring programmes, measuring changes in dieldrin
residues in the population with time and correlating this to
ecological monitoring of the size and reproductive success of the
population, can approach an objective assessment of the effects of
dieldrin. Such programmes have been reviewed by Newton (1979).
7.4.2. Effects on populations of birds
Populations of birds of prey declined during the period of
large scale use of organochlorine insecticides. Major studies of
changes in bird populations concentrated on a few species mainly in
the United Kingdom and North America, though also to some extent in
areas of mainland Europe. The following references are
illustrative on this subject of the literature: general
references, Anon (1964), Prestt (1965), Prestt & Bell (1966),
Parslow (1973), Bijleveld (1974), Cooke et al., (1976, 1982),
Havera & Duzan (1986); peregrine falcon (Falco peregrinu),
Ratcliffe (1963, 1965, 1967b, 1970, 1972, 1980, 1984), Lockie &
Ratcliffe (1964), Cade et al. (1968), Enderson & Berger (1968),
Hickey (1969); heron (Ardea cinerea), Reynolds (1974); golden eagle
(Aquila chrysaetos), Brown (1969), Lockie et al. (1969); sparrow-
hawk (Accipiter nisus), Koeman et al. (1972), Newton (1973a,b,
1974, 1976, 1979), Newton & Bogan (1974, 1978); Newton et al.
(1979), Marchant (1980); kestrel (Falco finnunculus), O'Connor
(1982).
In most of these studies, population decline correlated with
organochlorines residues in adult birds and their eggs. Reduced
breeding success was associated with thinning of eggshells,
behavioural changes resulting in egg breakage, and aggressive
interaction between adults resulting in a reduction of the number
of young fledged successfully from the clutches. The death of
adult birds was reported at the same time as seed-eating species
were dying from dieldrin poisoning.
As reported earlier in this section, dieldrin cannot be held
responsible for the eggshell-thinning effect, which has been shown
to be attributable to DDE (Cooke, 1973). Embryo deaths in shell
correlate best with PCB residues in eggs (Newton, 1979). The
contribution of dieldrin to these declines is difficult to
determine because the birds were subjected to residues of all
organochlorines. It is probable that dieldrin contributed to
population declines in some areas but not others (Newton, 1979;
Newton & Haas, 1984).
The studies of Blus et al. (1974a,b, 1975, 1979a,b) and Blus
(1982) on the brown pelican (Pelicanus occidentalis) conclude that
the decline in numbers could be ascribed entirely to DDE. The
population size of birds of prey in some of the Eastern states of
the USA declined when no dieldrin residues were present, DDE alone
being a contaminant in these populations. Newton (1979) pointed
out that the decline in populations of birds of prey contaminated
by DDE is gradual, a result of progressive effects of failure in
breeding. The decline of populations of peregrine falcon and
sparrow-hawk in the United Kingdom was more sudden and was
associated with the death of breeding adults. This was attributed
to dieldrin usage, which correlated well with the decline (Newton,
1979; Newton & Haas, 1984).
7.4.3. Effects on populations of mammals
Some mammal species in addition to birds, have been affected by
the use of organochlorine pesticides. There are reports of
decreases in the number of badgers (Meles meles) in some areas of
the United Kingdom (Jefferies, 1969, 1975). Declines in the number
of bats have been reported in the United Kingdom and the
Netherlands (Jefferies, 1972); furthermore, the grey bat (Myotis
grisesceus) in the USA (Clark et al., 1978) and the otter (Lutra
lutra) have also been affected (Jefferies et al., 1974; Chanin &
Jefferies, 1978).
Declines in bat numbers have been associated with the use of
dieldrin and lindane in wood preservatives in the United Kingdom
(Jefferies, 1972). In the United States, they appear to be related
to a combination of organochlorines. Many species migrate long
distances, using fat reserves on the journey, and are susceptible
to DDE poisoning en route (Clark & Kroll, 1977). No contribution
of dieldrin to declines in bat numbers in the USA has been proven.
Other declines have been attributed to dieldrin (Chanin &
Jefferies, 1978). Jefferies & Pendlebury (1968) studied the effect
of aldrin/dieldrin seed dressings on the populations of stoats,
weasels, and hedgehogs in the United Kingdom. None of these
species showed a decline during the period 1959 to 1962, and there
was no evidence that aldrin/dieldrin had any detrimental effects.
Jefferies et al. (1973) studied the behaviour of small mammals
in and adjacent to a field sown with dieldrin-dressed wheat. The
field mouse Apodemus, which lives on the field margin and the open
field, immediately fed on dosed grain. Residues of dieldrin in
sampled mice was very high. The bank vole Clethrionomys, which
lives in field margins, did not take the dosed grain. Residues of
dieldrin in these small mammals were monitored regularly after
sowing. These very quickly dropped to very low levels. The
authors propose that those individuals eating dressed grain died
quickly or were taken by predators. Populations were quickly
replenished by immigration from surrounding areas.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Aldrin and dieldrin
8.1.1.1 Oral
The acute oral LD50 values for technical aldrin and dieldrin in
various animal species are shown in Table 36. Intoxication with
cyclodiene insecticides consists of increased irritability and
tremor, followed later by tonic-clonic convulsions. In rats,
convulsions appear within 1 h following oral dosing at high
concentrations; death follows within 6 h, or from 2 - 7 days later.
This depends on factors such as the contents of the rat's
gastrointestinal tract, the concentration of aldrin/dieldrin in the
solvent, and the type of solvent used (Borgmann et al., 1952b;
Heath & Vandekar, 1964). Fox & Virgo (1986) reported that dieldrin
induced hyperglycemia.
Table 36. Acute oral LD50 values for technical aldrin and dieldrin
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Mouse corn oil 44 38 Borgmann et al. (1952a,b)
Mouse olive oil ~75 Jolly (1954)
Rat (newborn) arachis oil 168a Lu et al. (1965)
Rat (pre-weaning) arachis oil 25 Lu et al. (1965)
Rat (adult) arachis oil 37 Lu et al. (1965)
Rat arachis oil 51-64 Heath & Vandekar (1964)
Rat various 38-67 Lehman (1951); Borgmann et al.
(1952a); Treon & Cleveland (1955);
Gaines (1960); Worthing & Walker
(1983)
Rat various 37-87 Lehman (1951); Borgmann et al.
(1952b); Treon & Cleveland (1955);
Gaines (1960); Lu et al. (1965);
Worthing & Walker (1983)
Hamster olive oil 320 330 Gak et al. (1976)
Hamster corn oil 100 Cabral et al. (1979a,b)
Guinea-pig corn oil 33 49 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
Table 36. (contd.)
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Guinea-pig olive oil between 10 Jolly (1954)
and 25
Rabbit corn oil 50-80 45-50 Borgmann et al. (1952a,b)
Dog corn oil 65-95 65-80 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
a Transcutaneous intragastric injection.
The minimum toxic and the maximum non-toxic doses of aldrin and
dieldrin, administered orally to livestock, are indicated in Table
37.
Table 37. Acute oral toxicity of aldrin and dieldrin for livestock
-------------------------------------------------------------------
Compound Species Age Maximum Minimum Reference
non-toxic toxic
dose dose
tested found
-------------------
(mg/kg body weight)
-------------------------------------------------------------------
Aldrin calf 1-2 weeks 2.5 5 Radeleff et
al. (1955)
cattle 1 year 10 25 Radeleff et
al. (1955)
sheep 1-2 years 10 15 Radeleff et
al. (1955)
Dieldrin calf 1-2 weeks 5 10 Radeleff et
al. (1960)
cattle 1 year 10 25 Radeleff et
al. (1955)
horse - - 25 Radeleff et
al. (1960)
pig 3 weeks 25 50 Radeleff et
al. (1960)
sheep 1 year 15 25 Radeleff et
al. (1960)
sheep 9-12 months - LD50 Jolly (1954)
50-75
-------------------------------------------------------------------
8.1.1.2 Dermal
The minimum lethal dose of aldrin or dieldrin when applied as a
dry powder on the intact skin of female rabbits for 24 h was
between 600 and 1250 mg aldrin/kg body weight and between 250 and
360 mg dieldrin/kg body weight. In olive oil, the range for aldrin
was the same as for dry powder, and the range for dieldrin was
between 360 and 600 mg/kg body weight (Treon et al., 1953).
The acute dermal LD50 values for technical aldrin and dieldrin
in various animal species are shown in Table 38. The signs of
intoxication are similar to those that follow oral administration.
Table 38. Dermal LD50 values for technical aldrin and dieldrin
---------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
---------------------------------------------------------------
Mouse solvent - 40-80a Jolly (1954)
naphtha
Rat xylene ~100 60-90 Gaines (1960)
Guinea-pig solvent - 120a Jolly (1954)
naphtha
Rabbit dimethyl- 150 150 Lehman (1952)
phthalate
---------------------------------------------------------------
a With complete immersion of body.
8.1.1.3 Inhalation
The vapour pressures of technical aldrin and dieldrin are
sufficiently low that an acute inhalation hazard from aldrin or
dieldrin vapour does not normally arise.
8.1.1.4 Parenteral
The acute LD50 values for technical dieldrin (in glycerol
formal) in the rat via intraperitoneal and intravenous routes are
56 and 8 - 9 mg/kg body weight, respectively (Heath & Vandekar,
1964).
8.1.2. Formulated materials
8.1.2.1 Oral and dermal
The acute toxicity of formulated products, particularly the
dermal toxicity, is a more realistic guide than that of the
technical product to the acute hazard to the user. The percentage
of aldrin or dieldrin in the formulation, the solvent used, and the
type of formulation (such as an emulsion, wettable powder, dust,
etc.) will determine the acute toxicity of the formulated product.
Depending on these factors, the oral and dermal LD50s vary from 100
to 4500 and 500 to 16 000 mg total aldrin formulation/kg body
weight, respectively. For dieldrin formulations, these figures are
100 - 400 mg/kg and 200 - 2700 mg formulation/kg body weight,
respectively (Muir, 1970; Rose, 1982, 1984a,b).
For a dieldrin formulation for termite control (680 g/litre
suspension concentrate), the dermal LD50 in the male rat was 645 mg
formulation/kg body weight and in the female rat 284 mg
formulation/kg body weight (Rose, 1984c).
8.1.2.2 Inhalation
The acute inhalation LC50 (4-h exposure) in rats for aqueous
dilutions of a 48% (w/v) emulsifiable concentrate of aldrin (high
aromatic solvent) in the form of a spray was estimated to be
equivalent to 3% (w/v) aldrin. The median droplet size was 52 µm,
and the animals were exposed "nose only". Deaths occurred up to 6
days after exposure, but most of the animals died on the 2nd day
after exposure. Signs of intoxication consisted of a subdued
and hunched appearance with piloerection, progressing to
hypersensitivity and convulsions in the more seriously affected
animals. Surviving animals recovered within 2 - 3 days after
exposure. Oral intake (due to grooming) contributed significantly
to the results (MacDonald, 1982).
8.2. Short-Term Exposures
Short-term studies on rodents have shown that aldrin and
dieldrin affect the liver. The liver/body weight ratio is
increased, and histopathological changes that have become known as
"Chlorinated Hydrocarbon Insecticide Rodent Liver" (CHIRL) are
observed. Microscopically, these CHIRLs consist of enlarged
centrilobular hepatocytes with somewhat increased cytoplasmic
oxyphilia and peripheral migration of the basophilic granules
(Treon & Cleveland, 1955; Ortega et al., 1957).
The cellular and subcellular changes in the liver of different
mammalian species have been studied by Wright et al. (1972, 1977,
1978). These studies have shown that dieldrin produces a
generalized enlargement of the liver, which, in rats and dogs, is
associated with increased size of liver parenchymal cells but, in
mice, is associated with an increase in both cell size and cell
number. The earliest ultrastructural change in the livers of mice,
rats, and dogs treated with dieldrin was the proliferation of the
smooth endoplasmic reticulum (SER). During the initial phase of
the exposure to dieldrin, and also to phenobarbital, the increases
in the SER in the liver cells of mice, rats, and dogs were of the
vesicular type. These changes were associated with an enhanced
microsomal mixed-function oxidase, and intracellular whorls of
smooth membranes appeared in the liver cells of rats and dogs but
not in those of the mouse. The changes in liver subcellular
structure and function were reversible in mouse, rat, and dog. In
contrast, no liver enlargement or other ultrastructural changes
were observed in the livers of rhesus monkeys. In vitro
determinations showed that the activity of the liver microsomal
monooxygenase system was increased after treatment, and this was
the most sensitive effect observed. In all species examined, the
biochemical and subcellular structural response of the liver to
dieldrin was shown to be similar to that found with a number of
other chemicals, such as DDT, heptachlor, and phenobarbital
(Jansen, 1979). These chemicals also induce in the mouse a type of
liver tumour identical to that found with dieldrin (Stevenson &
Walker, 1969; Thorpe & Walker, 1973).
8.2.1. Oral
8.2.1.1 Rat
A number of short-term feeding studies (3 - 9 months duration)
were carried out on rats with aldrin. Dose levels of 0.5 - 300 mg
aldrin/kg diet were tested. The results of these old studies
showed that dose levels up to 5 mg/kg diet produced no effects, but
that levels of 25 mg/kg or more gave an increased liver/body weight
ratio and reversible hypertrophy of centrilobular hepatocytes with
cytoplastic changes (CHIRL) (section 8.4.2). Dose levels of 150
mg/kg or more resulted in increased mortality (Treon et al., 1951;
Borgmann et al., 1952a).
Five further studies were carried out with dieldrin (3 - 10
months duration), using dose levels of 1 - 300 mg/kg diet. No
effects were seen up to 5 mg/kg, except that Walton et al. (1971)
found an increased liver/body weight ratio in females at 5 mg/kg
and Ortega et al. (1957) found occasional liver changes at 2.5
mg/kg diet. These changes, e.g., liver enlargement and induction
of CHIRL, were found in the other studies at 10 mg/kg diet or more.
At 150 mg/kg or more, there was increased mortality (Treon et al.,
1951; Borgmann et al., 1952b; Ortega et al., 1957; Walton et al.,
1971).
When groups of male albino rats were fed diets equivalent to
2 mg/kg body weight for 6 months, the alkaline phosphatase, SGPT,
SGOT, and LD-hydrogenase activities in the serum were increased
after 6 months. The urea content decreased after 3 months, and
some other parameters were also changed. The growth of the animals
was considerably inhibited (Shakoori et al., 1986).
8.2.1.2 Dog
Dogs appear to be more susceptible to aldrin and dieldrin than
rats. Dogs administered aldrin in the diet for 5 or 6 days at dose
levels equivalent to 0.9 - 9.1 mg/kg body weight died within 7
months. However, beagle dogs (two males and two females) survived
15.6 months when given 0.043 - 0.25 mg aldrin/kg body weight. With
dieldrin, dogs survived dose levels up to 0.23 mg/kg body weight
for 15.7 months. In aldrin- and dieldrin-treated animals, no
effects on growth and no changes in haematology were seen. The
dogs with 0.25 mg aldrin/kg showed hepatomegaly, and the females
had local hyaline (droplet) degeneration of hepatocytes and
vacuolization in the epithelia of distal renal tubules. One of the
males of this group showed hepatocyte degeneration, while the other
exhibited the renal tubular changes seen in the females. In the
group fed 0.09 mg/kg, no effects were seen in the males, while the
females (and also one female in the group fed 0.23 mg/kg) showed
vacuolization in the epithelia of the distal renal tubules. The
liver weights of the dieldrin-treated animals were increased. No
other dogs showed gross or microscopic abnormalities in the viscera
(Treon & Cleveland, 1955).
Four beagle dogs fed dieldrin at daily oral doses of 0.4 - 0.8
mg/kg body weight showed blood concentrations of 0.27 - 1.27
mg/litre blood after eight episodes of convulsions (Brown et al.,
1964). When two dogs were given 0.2 mg dieldrin/kg body weight, in
gelatin capsules daily for 8 months, no signs of intoxication were
observed. The concentration in the blood was 0.11 - 0.22 mg/litre.
In studies by Fitzhugh et al. (1964), twelve mongrel dogs of
various ages received aldrin, 6 days/week for periods up to 25
months, at doses of 0.2, 0.5, 1, 2, or 5 mg/kg body weight.
Dieldrin was tested in 14 mongrel dogs at doses of 0.2, 0.5, 1, 2,
5, or 10 mg/kg body weight. In both cases, there were two animals
per dose level (one male, one female), with the exception of four
animals in the groups given 0.5 mg/kg. In the animals tested with
aldrin, the 5 mg/kg dogs and one 2 mg/kg dog died in 3 - 4 weeks;
the remaining male dog in the 2 mg/kg group was killed at 25 weeks
because of poor condition. All four dogs showed weight loss and
fatty changes in the liver and renal tubules. The bone marrow
showed a reduced number of mature granulocytes and erythroid cells.
At 1 mg/kg, the two dogs survived for 15 and 49 weeks and, at
autopsy, showed the same lesions. In the 0.5 mg/kg group, one dog
died after 4 days. The remaining three dogs survived for 2 years,
one male among these having convulsions during the last 2 months.
At 0.2 mg/kg body weight, there were no effects. In the animals
tested with dieldrin, all six dogs on 2, 5, and 10 mg/kg died
during weeks 2 - 5. These dogs showed weight loss, fatty changes
and slight hepatic cell atrophy in the liver, and a small amount of
atypically distributed fat in the kidneys. The bone marrow showed
a reduced number of mature granulocytes and erythroid cells. The
reported bone marrow findings, which were not replicated in other
studies, cannot be interpreted, because of the inadequacy of
clinical details, and no control dogs were used in this study. The
two dogs given 1 mg/kg survived for 12 and 43 weeks and, at
autopsy, showed the same lesions. One dog given 0.5 mg/kg was
sacrificed after 2 weeks because of anorexia and marked emaciation.
Detailed histological examination, including that of the brain, did
not show any distinct organ damage. The remaining three dogs in
the 0.5 mg/kg group died with terminal convulsions or were
sacrificed in poor condition at weeks 29, 43, and 81. Two of the
dogs showed weight loss. No effects were observed in the 0.2 mg/kg
group.
Repeated daily oral administration of 0.2, 1, or 2 mg
dieldrin/kg body weight to groups of six mongrel dogs was carried
out until intoxication occurred between the 18th and 85th day. A
direct relationship was established between the dieldrin
concentration in the blood and the severity of clinical signs of
intoxication. On the first day of muscle spasms, the average
concentration of dieldrin in the blood was about 0.50 mg/litre and,
at the time of the first full-blown convulsion, about 0.90 mg/litre
(Keane & Zavon, 1969a).
In studies by Walker et al. (1969b), groups of five beagle dogs
of each sex received, by capsule, daily doses of 0.005 or 0.05 mg
dieldrin (in olive oil)/kg body weight, for 2 years. Control dogs
were given capsules containing olive oil. The health, behaviour,
and body weight were unaffected, and EEG recordings did not differ
between the dogs fed 0.05 mg/kg and the controls. In females given
0.05 mg/kg, liver/body weight ratio was increased. In both sexes,
serum alkaline phosphatase activity was increased. However, urine,
haematology, clinical chemistry, bromosulfthalein clearance, and
relative organ weight data were not affected. No gross or
histopathological anomalies were observed.
Deichmann et al. (1969) gave groups of six beagle dogs (aged
1.5 - 3.5 years) 0 or 0.6 mg aldrin/kg body weight, 5 days/week for
10 months, and then observed them for an additional 12 months. The
treated dogs showed hyperexcitability, tremors, and weight loss.
One dog died. After 14 - 18 months, the dogs with aldrin showed
cloudy swelling and fatty degeneration in the liver and hypertrophy
of hepatocytes. Renal vascular congestion and tubular degeneration
were seen in some of the animals.
8.2.1.3 Domestic animals
A dairy cow given aldrin in soybean oil daily by capsule (2.2
mg/kg body weight) exhibited hyperirritability after 27 days. The
animal was in heat and was bred the next day. She died on day 29
with convulsions. Autopsy showed a slightly discoloured, pulpy,
congestive liver and one slightly enlarged congested kidney. No
mortality occurred among cows given 0.8, 1, or 1.5 mg/kg body
weight for 48 days (Ely et al., 1954).
In studies by Gannon et al. (1959b), groups of four dairy cows
were fed rations containing 0, 0.1, 0.25, 0.75, or 2.25 mg
dieldrin/kg for 12 weeks (average total intake, 0, 0.293, 0.75,
2.17, or 6.55 mg dieldrin/kg body weight). No signs of illness and
no abnormalities were found when the cows were slaughtered at the
end of the test feeding period or after an additional 6-week period
on dieldrin-free rations.
Ivey et al. (1961) fed groups of 2 - 3 steers, sheep, and hogs
rations containing 0, 0.25, 0.75, or 10 mg aldrin/kg diet for 12
weeks. Two steers received rations with 2 mg/kg diet for the same
period. The control groups consisted of two animals each. No
evidence of illness and no postmortem pathology were found.
Goats administered 50 mg aldrin/kg body weight showed mild
degenerative changes, congestion and petechial haemorrhages, in
various organs. In the kidneys degenerative changes of the
proximal convoluted tubules were found. Clinical changes were also
found, e.g., salivation and convulsions (Singh et al., 1985).
8.2.2. Dermal
In studies by Treon et al. (1953), aldrin and dieldrin, as dry
powders or in solutions, were applied daily to the skin of groups
of three female rabbits for 2 h on each of 5 days per week over a
period of 10 weeks. A series of graded doses was used to determine
the doses resulting in no mortality. It was clear that aldrin
or dieldrin dissolved in kerosene was very toxic (LD50 of
approximately 5 mg/kg body weight). Dissolved in vegetable oil
they were about 6 times less toxic and as dry powder about 20 times
less toxic than when dissolved in kerosene.
8.2.3. Inhalation
Mice, hamsters, and guinea-pigs did not show any adverse
effects when exposed to vapourized aldrin at a concentration of 18
mg/m3 for 178 days (Baker et al., 1959).
8.3. Skin and Eye Irritation; Sensitization
8.3.1. Skin and eye irritation
Treon et al. (1953) reported that technical aldrin or dieldrin
applied on the intact rabbit skin for 24 h occasionally caused
slight erythema. Repeated application of aldrin or dieldrin for 10
weeks (2 h per day, 5 days per week) as a dry powder did not alter
the gross condition of the rabbit skin. Slight irritation and
scaliness were observed when the compounds were applied in
vegetable oil, but their application in kerosene resulted in
damage, attributable to the solvent.
An undiluted aldrin emulsifiable concentrate formulation (48%
aldrin in high aromatic hydrocarbon solvent), applied at a dose of
0.5 ml at each test site for 24 h on the intact skin and abraded
skin of rabbits under an occlusive patch, caused severe irritation
and necrosis of the skin. One male died on day 13. When applied to
the rabbit eye, this undiluted 48% emulsifiable concentrate caused
severe initial pain and mild irritation (Rose, 1982).
8.3.2. Sensitization
In the Magnusson and Kligman guinea-pig maximization test, 48%
aldrin emulsifiable concentrate caused positive responses (at 24 h
and 48 h after removal of the challenge patches) in 3 out of the 20
test animals. Rechallenge of these animals, one week later,
confirmed that they had been sensitized to the test material (Rose,
1982).
Aldrin emulsifiable concentrate (48%) is not a skin sensitizer
under the EEC Dangerous Substances Directives (EEC, 1983).
No cases of skin sensitization occurred over a period of 20
years among a group of over 1000 workers involved in the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
8.4. Long-Term Toxicity and Carcinogenicity
8.4.1. Mouse
Davis & Fitzhugh (1962) fed groups of 100 male and 100 female
C3HeB/Fe mice a diet containing aldrin or dieldrin at 0 or 10 mg/kg
diet for 2 years. The average lifespan of the treated mice was
shortened by 2 months. A significant increase in the incidence of
benign liver tumours was observed.
In a further study, 100 male and 100 female C3HeB/Fe/J mice
received aldrin or dieldrin at 0 or 10 mg/kg diet for 2 years. An
increase in the number of animals with hepatic hyperplasia and
benign liver tumours was seen in the treated groups, but no
increase in malignant liver tumours was found. The survival in the
treated groups was lower than that of controls (Davis et al., 1965).
Groups of 300, 125, 125, and 200 CF1 mice of each sex were fed
diets containing dieldrin (> 99%) at 0, 0.1, 1, or 10 mg/kg,
respectively, for 2 years. A positive control group fed 600 mg
4-amino-2,3-dimethylazobenzene for 6 months, followed by a control
diet, was used. After 9 months, the morbidity of the mice fed 10
mg/kg started to increase, but the lifespan of the mice fed 0.1 or
1 mg/kg was unaffected. The animals with 4-amino-2,3-
dimethylazobenzene died within 14 months. The frequency of liver
tumours was increased in all groups fed dieldrin. Two types of
tumours were observed, one of which was considered benign
(hyperplastic nodules) and the other malignant. The malignant
tumours were clearly hepatocarcinomas, though no fibrosis or bile
duct proliferation, as seen in the positive control group, occurred
in the dieldrin-treated groups. A reversibility study in a
separate group fed 10 mg dieldrin/kg in the diet for up to 15
months, followed by a control diet for the rest of the 2-year
study, showed that the tumours did not regress or disappear upon
discontinuation of the treatment. However, the dieldrin induced
hepatomegaly, and cytoplasmic changes were found to be reversible
(Walker et al., 1972; Hunt et al., 1975).
In a study by Thorpe & Walker (1973), a group of 30 CF1 mice of
each sex were fed a diet containing 10 mg dieldrin/kg for 2 years.
The control group consisted of 45 mice of each sex. Liver
enlargement was detected after 50 weeks in both sexes, and liver
lesions were observed, classified as hyperplastic nodules (Type a)
and hepatocellular carcinoma (Type b) (sometimes associated with
lung metastases). In a separate study, it was shown that dieldrin
Type b liver cell tumours were capable of growing as subcutaneous
transplants in mice of the same strain and sex (Thorpe, 1973).
To compare the pathological responses to dieldrin in different
mouse strains, groups of 30 mice of each sex of the CF1, LACG, and
hybrid CF1-LACG strains were fed diets containing 10 mg dieldrin/kg
for 2 years. There was also a control group of 45 animals of each
sex for each strain. The incidence of liver tumours, particularly
Type b tumours, in male CF1 and hybrid mice and in females of all
three strains was higher than in controls. In male LACG mice, the
incidence of liver tumours was low. Qualitatively, there was no
difference in tumours between strains, and there was no increased
incidence of neoplasms in other tissues, nor were unusual tumours
found. Metastases of liver carcinoma were found in the lungs in
some of the mice (Thorpe & Hunt, 1975).
In studies by Benitz et al. (1977), nine groups of 100 Charles
River CD1 mice of each sex were given dieldrin in the diet at
concentrations ranging from 0.15 to 15 mg/kg. Six hundred mice of
each sex were used as controls. Groups of animals were sacrificed
at time intervals ranging from 2 to 25 months. Initial changes in
the liver consisted of various degrees of centrilobular and
pericentral hypertrophy. These changes were later associated with
the appearance of hepatic nodules, the occurrence of which was time
and dose related. These nodules consisted of hypertrophic
hepatocytes, which, in a few instances, were mixed with
hyperplastic cells. Various degrees of loss and distortion of
lobular architecture were seen within these nodules. Metastases in
the lung were observed in three nodule-bearing animals given 15 mg
dieldrin/kg for 25 months. These metastases contained similar
hypertrophic hepatocytes as did the primary liver tumours.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
aldrin (4 or 8 mg/kg diet for males and 3 or 6 mg/kg diet for
females) or dieldrin (2.5 or 5 mg/kg for both sexes) for 80 weeks,
followed by an observation period of 10 - 13 weeks. Concurrent
controls consisted of groups of 20 male and 10 female mice. The
pooled controls, used for statistical evaluation, consisted of the
concurrent control groups combined with 92 male and 79 female mice
from similar bioassays of other chemicals. All surviving mice were
killed at 90 - 93 weeks. Body weight was not affected in the
treated animals, but there was a dose-related increase in
mortality, especially in the high-dose groups in the second half of
the study. In the dieldrin-treated mice, clinical symptoms, such
as irritability, tremors, and alopecia occurred. Hepatocellular
carcinomas were found, as indicated in Table 39. The incidence of
hepatocellular carcinomas was clearly higher in male than female
mice. There was no difference in tumour frequencies in other
tissues (NCI, 1978a).
Groups of weanling male C3H/HE mice were fed a diet containing
10 mg dieldrin/kg diet until an age of 57 weeks and then were
either administered a control diet (12 mice) or continued on the
dieldrin diet (11 mice) for another 10 weeks. A third group served
as an untreated control group (21 mice). Laparatomies were
performed and biopsy specimens taken when about 30% of the mice in
each dieldrin-treated group had tumours. Further biopsy samples
were taken approximately 10 weeks later. Tumours were observed at
the first laparotomy in 6/21 controls and 14/23 dieldrin-treated
animals. At the second laparotomy, adenomas were seen in some
animals in which there had been no tumour at the first laparotomy.
In one animal in the continuous dieldrin-treatment group, there was
histological progression from adenoma to hepatocellular carcinoma.
Additional hepatocellular carcinomas were observed in some animals
autopsied at 2 years of age. A strong tendency to tumour
progression was found in both treated and control mice (Ruebner et
al., 1984a,b).
Table 39. Incidence of hepatocellular tumours
in mice (NCI, 1978a)
----------------------------------------------
Groups Males Females
----------------------------------------------
Concurrent controls 3/20 (15%)a 0/10 (0%)a
3/18 (17%)b 0/20 (0%)b
Pooled controls 17/92 (18%) 3/78 (4%)
Aldrin (4 mg) 16/49 (33%)
(8 mg) 25/45 (56%)
Aldrin (3 mg) 5/48 (10%)
(6 mg) 2/43 (5%)
Dieldrin (2.5 mg) 12/50 (24%) 6/50 (12%)
(5 mg) 16/45 (36%) 2/49 (4%)
----------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
In a study by Meierhenry et al. (1983), groups of 50 - 70 male
mice of three strains (C57Bl6J, C3H/He, and C57Bl6J x C3H/He B6C3F1
hybrid) were administered a diet containing 10 mg dieldrin/kg diet
for 85 weeks. The control groups consisted of approximately 60
mice of the same strains. After 4 months, the livers in a small
number of animals showed swellings of hepatocytes in the central
zone with nuclear atypia, small nodules containing basophilic or
eosinophilic foci, and multiple tumours. The percentage of benign
hepatic tumours was 28, 20, and 29, respectively, and in the
control groups, 19, 18, and 4. The percentage of hepatocellular
carcinomas was 30, 38, and 42, respectively, and, in the controls,
0, 12, and 4%. Mallory bodies were seen in all the dieldrin-
treated mice that had either benign or malignant tumours, but only
rarely in mice without tumours.
It seems from the available studies that dieldrin facilitates
and exacerbates the expression of an endogenous oncogenic factor in
CF1 mice (Tennekes et al., 1981). The dose-response characteristics
of dieldrin-mediated enhancement of liver tumour formation in CF1
mice were analysed using existing tumour data from long-term
feeding studies at six levels of continuous exposure, involving a
total of more than 1500 animals. Using the Druckrey equation, the
actual contribution of dieldrin to tumour formation was considered
to be negligible (Tennekes et al., 1985).
8.4.1.1 Appraisal
A number of long-term carcinogenicity studies have been carried
out in which mice of different strains were fed aldrin and/or
dieldrin at one or more dose levels. In all these studies, there
was an increased incidence of liver changes, some of which were of
the nature of hepatocellular carcinomas while others were regarded
as non-malignant. Females seem to be less sensitive than males.
No other tumours were induced.
8.4.2. Rat
Borgmann et al. (1952a) fed six groups of 10 male and 10 female
weanling Sprague-Dawley rats a diet containing 0, 5, 10, 50, 100,
or 150 mg aldrin/kg diet over a period of 2 years. At the two
highest dose levels, an increased mortality was found at 16 months,
which was not seen in the other groups. Liver enlargement was
observed in the groups with high dose levels, but not in the groups
with 10 mg/kg diet or less.
When groups of 40 Carworth rats of each sex were given diets
containing aldrin or dieldrin at 0, 2.5, 12.5, or 25 mg/kg diet for
2 years, there was no increase in mortality and the growth rate was
comparable with that of controls. At all dose levels, the
liver/body weight ratio was increased in males and there were
histological liver cell changes characteristic of CHIRL. No
tumours were reported (Treon & Cleveland, 1955). In a review of
all the aldrin and dieldrin studies, it was reported that there was
no excess of tumours in these rats (Cleveland, 1966).
When groups of 12 Osborne-Mendel rats of each sex were given
diets containing aldrin or dieldrin (0, 0.5, 2, 10, 50, 100, or
150 mg/kg diet) for 2 years, growth was not affected. However,
survival was markedly decreased at dose levels of 50 mg or more in
a dose-related manner. The liver/body weight ratio was increased
in males fed 10 mg/kg or more, while, in females, an increase at
all dose levels was found (no dose-response relationship at the
lower doses). CHIRL was observed in all treated groups, although
at 0.5 mg/kg only a few animals showed a trace of CHIRL. Rats at
dose levels of 50 mg/kg or more showed haemorrhagic urinary
bladders and nephritis. An overall increase in tumour incidence
was noted, but this was not dose related. On the contrary, the
lowest dose levels showed the highest tumour incidence. Only one
liver tumour was found (Fitzhugh et al., 1964).
In a study at the National Institute of Public Health of the
Netherlands, no increased incidence of tumours was found in rats
fed diets containing 75 mg dieldrin/kg for 2 years (Van Genderen,
1965, 1979).
When groups of 30 Osborne-Mendel rats of each sex were fed
5 mg/kg aldrin (95%) or a control diet for 2 years, there was no
increase in mortality, liver/body weight ratios, or tumour
incidence in the aldrin-fed group (Deichmann et al., 1967).
In studies by Walker et al. (1969b), groups of 25 Carworth Farm
E rats of each sex were given dieldrin at 0.1, 1, or 10 mg/kg diet
for 2 years. A control group consisted of 45 males and 45 females.
There was no effect on body weight. After 2 - 3 months, the
animals fed 10 mg/kg exhibited irritability and, as the study
progressed, tremors and occasional convulsions, usually during
handling. Mortality, haematology, serum enzyme levels, and
urinalysis were not affected. The females fed 1 mg/kg and 10 mg/kg
had increased liver/body weight ratios. At 10 mg/kg, one male and
six females exhibited CHIRL. In two females of the group fed 10
mg/kg and in one female control rat, microscopic nodules in the
liver parenchyma were seen. There was no increase in tumour
incidence. Subsequent re-evaluation of these data (Stevenson et
al., 1976) confirmed that there was no treatment-related increase
in tumour incidence.
Nine groups of 50 Osborne-Mendel rats of each sex were fed
diets containing aldrin or dieldrin at 0, 20, 30, or 50 mg/kg for
up to 31 months. A control group consisted of 100 male and 100
female rats. Dose-related tremors and convulsions, always
associated with weight loss, occurred at all dose levels,
particularly in females. Female rats fed 50 mg aldrin/kg or 30 or
50 mg dieldrin/kg had a shortened lifespan. The liver/body weight
ratio was increased in all dieldrin-treated groups and in males fed
30 or 50 mg aldrin/kg, but was decreased in females fed 20 mg/kg.
A moderate increase (not dose related) in the incidence of hepatic
centrilobular cloudy swelling, necrosis, or, rarely, foci of acute
or chronic inflammatory cellular infiltration was observed in all
treated groups. Hyperplasia in the liver was found in two male
rats fed 30 mg aldrin/kg. There was no increase in tumour
incidence (Deichmann et al., 1970; Deichmann, 1974).
Groups of 50 Osborne-Mendel rats of each sex were given diets
containing aldrin at levels of 30 or 60 mg/kg. Treatment of male
rats lasted 74 weeks followed by 37 - 38 weeks of observation,
while that of female rats lasted 80 weeks followed by 32 - 33 weeks
of observation. In a similar study, dieldrin was fed at a level of
29 mg/kg diet (time-weighted average dose) for 80 weeks followed by
observation for 30 - 31 weeks or 65 mg/kg diet (time-weighted
average dose) for 59 weeks followed by observation for 51 - 52
weeks. Concurrent control groups consisted of 10 rats of each
sex. Pooled controls, used for statistical evaluation, consisted
of concurrent control groups combined with 58 males and 60 females
from similar bioassays with other chemicals. All surviving rats
were killed at 110 - 111 weeks. Typical signs of organochlorine
intoxication (such as hyperexcitability) were observed with
increasing frequency and severity, especially in the second year,
but mortality was not affected. No significant increase in tumour
incidence was found (NCI, 1978a) (Table 40).
Groups of 24 Fischer 344 rats of each sex were given dieldrin
at 0, 2, 10, or 50 mg/kg diet for 2 years. Typical signs of
organochlorine intoxication were observed during the second year in
the 50 mg/kg group. Body weight and survival were not adversely
affected in any of the dieldrin groups. Liver tumours were not
observed. No significant increase in tumours was found (NCI,
1978b) (Table 40).
Table 40. Incidence of hepatocellular tumours in rats
(NCI, 1978a,b)
-------------------------------------------------------
Groups Males Females
-------------------------------------------------------
Osborne-Mendel rats
Concurrent controls 1/10 (10%)a 1/10 (10%)a
1/10 (10%)b 0/9 (0%)b
Pooled controls ? 5/59 (8.5%)a
Aldrin (30 mg/kg diet) 1/47 (2%) 0/48 (0%)
(60 mg/kg diet) 1/47 (2%) 3/49 (6%)
Dieldrin (40-20 mg/kg diet)d 0/44 (0%) 1/47 (2%)
(80-40 mg/kg diet)e 1/47 (2%) 1/44 (2.3%)
Fischer F 344 rats
Concurrent controls 2/24 (8.3%) 0/24 (0%)
Pooled controls ? ?
Dieldrin ( 2 mg/kg diet) 0/23 (0%) 0/24 (0%)
(10 mg/kg diet) 0/23 (0%) 0/24 (0%)
(50 mg/kg diet) 4/23 (17%)c 0/23 (0%)
-------------------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
c Nodular hyperplasia.
d Time-weighted average dose = 29 mg/kg.
e Time-weighted average dose = 65 mg/kg.
When groups of 50 female rats of two different strains
(Osborne-Mendel and Sprague-Dawley) were fed 0, 20, or 50 mg
aldrin/kg diet, the survival rate was reduced at 50 mg/kg but not
at 20 mg/kg. There was no increase in the incidence of mammary or
liver tumours (Deichmann, 1974; Deichmann et al., 1979).
Photodieldrin, which has metabolites identical to those of
dieldrin, was fed to groups of rats for 80 weeks at concentrations
of up to 7.5 mg/kg diet NCI (1977). No increase in tumour
incidence was found (see section 8.8.1.3).
Ito et al. (1983) studied the promoting activity of dieldrin on
the induction of hyperplastic (neoplastic) liver nodules using a
short-term test system. F344 rats received a single dose (200
mg/kg body weight) of N-nitrosodiethylamine, and 2 weeks later,
were treated for 6 weeks with dieldrin in the diet at a
concentration of 100 mg/kg. Dieldrin had a weak promoting
potential in this test system.
8.4.2.1 Appraisal
A number of long-term/carcinogenicity studies have been carried
out in which rats of different strains were fed one or more dose
levels of aldrin and/or dieldrin. The overall no-effect level in
these long-term studies, both for aldrin or dieldrin, was 0.5 mg/kg
diet. At feeding levels of 1 mg/kg or more, an increasing, dose-
related hepatomegaly and histological changes in the liver
characterized as CHIRL occurred. At levels of 10 mg/kg diet or
more, typical signs of organochlorine toxicity occurred such as
irritability, tremors, and convulsions. In all these studies, no
increase in tumour incidence in liver or other organ/tissue systems
was found.
8.4.3. Hamster
When groups of 34 - 40 Syrian golden hamsters of each sex were
fed diets containing dieldrin (99%) at 0, 20, 60, or 180 mg/kg for
120 weeks, there was no significant increase in tumour incidence
(Cabral et al., 1979b).
8.4.4. Monkey
In studies on rhesus monkeys, groups of five males were given
diets containing dieldrin (88.4%) at 0, 0.01, 0.1, 0.5, 1, or 5
mg/kg (0.0002 - 0.07 mg/kg body weight) for approximately 6 years.
After two monkeys in the group fed 5 mg/kg died, the level of
exposure to the remaining three animals was reduced to 2.5 mg/kg
and, later, to 1.75 mg/kg. Subsequently, one of these animals had
his dieldrin intake progressively increased until at the end of the
second year, he was receiving dieldrin at the initial dietary
concentration of 5 mg/kg. Clinical and haematological examinations,
liver and kidney function studies, urinalysis, and pathology did not
reveal any abnormalities. The liver/body weight ratios and liver
DNA and RNA of the test animals were not different from those of
control animals. No subcellular changes were seen in the
hepatocytes. Dose-related increases in microsomal cytochrome P-450
and in the activity of the liver mono-oxygenase enzyme system were
observed at the two highest dose levels. These alterations in
cytochrome P-450 in the liver microsomes were significant in the
monkeys fed 0.1 mg/kg or more. No effect was observed at 0.01
mg/kg. The concentrations of dieldrin in the subcutaneous fat of
the monkeys fed 0.1 mg/kg were similar to those measured in human
beings receiving a daily oral intake of similar concentration. The
dieldrin concentrations in the monkey livers were approximately 200
times higher than those in male rats receiving a daily intake of
dieldrin 3 times higher than the monkeys, and they were similar to
the concentration in the livers of male mice daily ingesting
dieldrin at a level approximately 50 times higher (Zavon & Stemmer,
1975; Wright et al., 1977, 1978).
8.4.5. Mode of Action
From long-term feeding experiments, it seems that aldrin and
dieldrin may be carcinogenic to mice, but not to rats or hamsters.
Mutagenicity findings have been consistently negative (see section
8.6). There is insufficient knowledge of the mechanism by which
these chemicals might behave epigenetically.
The latest evaluation of IARC (1987) is that there is
inadequate evidence of carcinogenicity in humans and limited
evidence for carcinogenicity in experimental animals.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
8.5.1.1 Mouse
Groups of 100 pairs of male and female virgin CFW Swiss mice
were fed diets containing 5 mg dieldrin/kg for 30 days before
mating, after which time they were randomly paired and fed the same
diet for a further 90 days. Mortality in the dieldrin-treated
group was similar to that of a control group (size not specified).
No major biological effects on fertility, fecundity, gestation
period, size of the first litters, or numbers of young produced per
day were noted as a result of feeding dieldrin. A statistically
significant (6%) decrease in mean size of all litters combined was
the only difference observed between the dieldrin-treated and
control groups (Good & Ware, 1969).
In a reproductive study, groups of 4 male and 14 female Swiss
white mice (120 days old) were fed diets containing 3, 5, 10, or
25 mg aldrin/kg or 3, 10, or 25 mg dieldrin/kg. Six groups served
as controls. The study covered six generations, and two litters
per generation. For both aldrin and dieldrin, the 25 mg/kg dose
was too toxic and resulted in high litter mortality in the few dams
reaching gestation. This dose level was therefore discontinued.
Pup survival was low in mice fed 10 mg dieldrin/kg, and so
treatment was terminated after the first generation. The most
pronounced effect observed in the group fed 10 mg aldrin/kg and, to
a lesser extent, 5 mg aldrin/kg was a low pre-weaning pup survival.
No effects on fertility, viability, or gestation were observed in
six generations of mice fed 3 mg dieldrin/kg. A decrease in pre-
weaning survival was observed in the F2b litters, but a similar
decrease was also found in one of the six control groups (Keplinger
et al., 1970).
In studies by Virgo & Bellward (1975), groups of 18 - 19
uniparous female Swiss-Vancouver mice were given diets containing
0, 2.5, 5, 10, 15, 20, or 25 mg dieldrin/kg, 4 weeks prior to their
second mating, continuing until day 28 postpartum. Significant
mortality of the females occurred at 20 and 25 mg/kg, all deaths
occurring before parturition (89 and 56%, respectively). Fertility
in the groups fed 10 and 15 mg/kg was decreased, though survivors
at higher doses were fertile. Oestrus and gestation period were
not affected. Litter size was decreased only in the group
receiving 25 mg/kg. The major effect was an increase in pre-
weaning pup mortality; 47% at 2.5 mg/kg, 80% at 5 mg/kg, and 100%
at 10 mg/kg or more (31% mortality in control animals). Dams
receiving 10 mg/kg or more exhibited hyperactivity, which was a
contributory factor to the high pup mortality. No gross
abnormalities were detected in the pups, none of whom showed
tremors or convulsions. Within the litters raised at 2.5 and 5
mg/kg, pup survival was not different from controls. The only
effect on reproductive capacity or pup survival observed in female
mice fed 2.5 mg/kg was an increase in pre-weaning pup mortality.
A study was carried out on primiparous female Swiss-Vancouver
mice to investigate whether diets containing up to 15 mg
dieldrin/kg affect maternal behaviour and pup viability. Viability
was investigated in dams fed diets containing 0, 5, 10, or 15 mg
dieldrin/kg for 4 weeks prior to mating. Pups fed 10 mg/kg were
nursed by foster dams not fed dieldrin, and all died by day 4; the
foster dams' own pups showed a very low mortality and survived
until weaning. Similar results were obtained at 5 mg/kg. Dieldrin
did not have any influence on serum progesterone levels, milk
production, or the dam's tendency to retrieve pups or build nests.
However, at 5 mg/kg or more, nursing was reduced. It was concluded
that dieldrin causes irreversible congenital inviability (not
through any effect on progesterone levels) and it was suggested
that the inviability and the reduced tendency to nurse increased
the pup mortality (Virgo & Bellward, 1977).
8.5.1.2 Rat
In studies by Treon & Cleveland (1955), rats (Carworth strain)
were fed aldrin or dieldrin at dietary concentrations of 2.5, 12.5,
or 25 mg/kg for three consecutive generations, two litters being
produced for each generation. (There was no mention of a control
group, and tabulation and description of results was limited in
this report). A reduction in the number of pregnancies, which
gradually disappeared over successive generations, was initially
observed at 12.5 and 25 mg aldrin/kg and at all three doses of
dieldrin. No effects on litter size or pup weights were observed
at any dietary concentration. A marked increase in mortality in
pre-weaning pups was found at dietary concentrations of 12.5 and
25 mg/kg for both compounds. This was thought by the authors to
be due to the high concentration of dieldrin in the milk of the
mothers. Neither aldrin nor dieldrin had any effect on
reproductive capacity. No effects, except a "slight to moderate"
increased pre-weaning pup mortality, were observed in the rats fed
for three generations with aldrin or dieldrin at 2.5 mg/kg.
When groups of 10 male and 20 female Long Evans rats were fed
dietary concentrations of 0, 0.1, 1, or 2 mg dieldrin/kg over three
generations (each generation producing two litters), no effects
were observed on the general health (including weight gain),
behaviour, fertility, gestation, viability, lactation, or organ
weight ratios. No pathological changes were found in parents or
pups. Increased pre-weaning mortality (compared to that in
controls) in the F1a litter was observed in the animals fed 2
mg/kg. This effect was not found in the five subsequent litters
from this group and was not considered to be a major toxic effect.
No changes in reproductive capacity were observed over three
generations at dietary concentrations up to and including 2 mg/kg
dieldrin (Eisenlord et al., 1967).
In studies by Harr et al. (1970), groups of 20 male and 20
female 28-day-old OSU-Wistar rats were fed 0, 0.08, 0.16, 0.31,
0.63, 1.25, 2.5, 5, 10, 20, or 40 mg dieldrin/kg diet throughout
their lifespan. Ten females from each group were mated at 146 days
of age. Mortality occurred in dams at 20 and 40 mg/kg. Fertility
and litter size were decreased in several dose groups without a
clear dose relationship. The number of pups at weaning was
markedly reduced at 2.5 mg/kg or more; none survived at 20 and 40
mg/kg. The nursing pups died in convulsions or starved. No
effects were noted at 1.25 mg/kg or less. Neural lesions, such as
cerebral oedema and hydrocephalus, occurred in pups of nursing dams
at dieldrin concentration of 0.08 mg/kg. Hepatic lesions were
found in rats fed concentrations of 0.31 mg/kg or more.
When groups of 18 - 20 Long Evans pregnant rats were given 4 mg
dieldrin/kg body weight daily by gavage from day 15 of gestation to
21 days post partum, fecundity, number of stillbirths, perinatal
mortality, and total litter weights did not differ from the control
group. No malformations in pups were observed (Coulston et al.,
1980).
8.5.1.3 Dog
In study by Kitselman (1953), seven groups of three dogs, each
group having at least one member of each sex, were fed either
aldrin or dieldrin for one year at dietary concentrations
equivalent to 0, 0.2, 0.6, or 2 mg/kg body weight (in corn oil).
Out of a total of 11 bitches fed either aldrin or dieldrin, 9
conceived. All pregnant bitches produced litters of at least four
pups/litter. The survival of pups was generally lower in the
groups fed aldrin or dieldrin. Histopathological examinations of
dead pups revealed degenerative changes in the liver and mild
degenerative changes in renal tubules. Liver changes were also
observed in treated bitches. The design and size of this study was
too limited to deduce a dose-response relationship for pup
survival, but no effects were observed in dogs receiving 0.2 mg
dieldrin/kg body weight.
8.5.1.4 Appraisal
In the reproductive studies (over one to six generations)
carried out with aldrin or dieldrin on mice and rats, the major
effect observed in most of the studies was an increased mortality
rate in pre-weaning pups. Reproductive performance, per se, was
only affected at doses causing maternal intoxication. The studies
on dogs are of a too limited nature to draw firm conclusions, apart
from the consistent increase in pre-weaning pup mortality.
The results of these reproductive studies indicate that
dieldrin at levels of 2 mg/kg in the rat diet and 3 mg/kg in the
mouse diet (equivalent to 0.1 and 0.4 mg/kg body weight per day,
respectively) are no-effect levels for reproduction. It is not
possible to establish a no-effect level for aldrin for
reproduction, because no adequate data are available.
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Mouse
Ottolenghi et al. (1974) gave groups of 10 pregnant CD-1 mice
single oral doses of 25 mg aldrin/kg body weight or 15 mg
dieldrin/kg body weight (in corn oil; equivalent to half the LD50
values) on day 9 of gestation. Control groups consisted of
untreated and corn-oil-dosed mice. No effects on fetal survival or
weight were observed. Abnormalities, such as webbed feet, cleft
palate, and open eyes, were increased in both treated groups, but
they may have been related to maternal toxicity. The percentage of
the total live fetuses that were malformed was 33% for aldrin-
treated mice and 17% for dieldrin-treated ones.
In two comparable studies, pregnant CD-1 mice (6 - 16 per
group) were given daily oral doses of dieldrin in peanut oil at 0,
1.5, 3, or 6 mg/kg body weight from day 7 to day 16 of gestation.
At 6 mg/kg, reduced body weight gain and increased liver/body
weight ratio were observed. There was an increase in supernumerary
ribs and a decreased number of caudal ossification centres in the
fetuses of mice given 6 mg/kg. In one study, the number of
supernumerary ribs was increased at all three dose levels,
(significant at the two highest dose levels) (Chernoff et al.,
1975). In the other study, the increase in supernumerary ribs at
6 mg/kg was not significant. The increase in the number of
supernumerary ribs may be an expression of developmental toxicity.
Doses of dieldrin (99%) were given either in corn oil (0, 1.5,
or 4 mg/kg body weight) or in dimethylsulfoxide (DMSO) (0, 0.25,
0.5, or 1 mg/kg) daily by gavage to pregnant CF-1 mice (7 - 14
mice/group) on days 6 - 14 of gestation. No maternal or fetal
toxicity was seen in groups treated with dieldrin in corn oil or in
the corn oil control groups, but some was seen in the dieldrin/DMSO
and DMSO-control groups. No compound-related teratogenic effects
were observed (Dix et al., 1978).
8.5.2.2 Rat
In a study by Chernoff et al. (1975), pregnant CD rats (9 - 25
per group) were given daily oral doses of dieldrin in peanut oil
(0, 1.5, 3, or 6 mg/kg body weight) from days 7 to 16 of gestation.
At 6 mg/kg, increased mortality and reduced body weight gain were
observed in the dams, but no changes in the liver/body weight
ratios were found. Fetuses did not show any differences from the
controls in mortality, body weight, or occurrence of anomalies.
There were no differences in the average number of sternal or
caudal ossification centres (as were seen in mice). No evidence of
teratogenicity was observed at a dose level of 6 mg aldrin/kg body
weight per day.
No malformations were observed in fetuses or pups from 18 - 20
Long Evans rats given 0 or 4 mg dieldrin/kg, by gavage, daily from
day 15 of gestation to day 21 of lactation (Coulston et al., 1980).
8.5.2.3 Hamster
Pregnant Syrian golden hamsters (41 - 43 per group) were given
single oral doses in corn oil of either 50 mg aldrin/kg body weight
or 30 mg dieldrin/kg body weight on either day 7, 8, or 9 of
gestation. Untreated and vehicle-control groups (respectively, 57
and 41 animals) were used. The high dose levels of both aldrin and
dieldrin caused reductions in the number of live fetuses and fetal
weight and an increased incidence of abnormalities (cleft palate,
open eyes, and webbed feet). The effects were more pronounced
after treatment on days 7 and 8 of gestation than on day 9. It was
suggested that, since webbed foot and open eye were frequently
associated with low fetal weight, these effects might be simply the
expression of growth retardation (Ottolenghi et al., 1974).
8.5.2.4 Rabbit
No teratogenic effects were observed in the offspring of groups
of pregnant Banded Dutch rabbits dosed with dieldrin in
carboxymethylcellulose (2 or 6 mg/kg body weight per day) from
days 6 to 18 of gestation. The animals were killed on day 28 of
gestation and the fetuses were examined for visceral and skeletal
abnormalities (Dix & Wilson, 1971).
8.5.2.5 Appraisal
No evidence of a teratogenic potential has been found from
studies on rats, mice, or rabbits using oral doses up to 6 mg/kg
body weight. Single high oral doses, equivalent to half the LD50,
have been found to cause fetotoxicity and abnormal development of
the fetuses in hamsters and, to a lesser extent, in mice. The
significance of these abnormalities in the presence of severe
maternal toxicity is doubtful but a specific teratogenic potential
cannot be ruled out completely. No gross malformations have been
reported in reproductive studies.
8.6. Mutagenicity and Related End-Points
8.6.1. Microorganisms
Most research workers have reported that aldrin and dieldrin,
with or without microsomal activation, are not mutagenic in
bacterial or yeast test systems. In one study, it was reported
that dieldrin, without activation, was mutagenic in two out of
three strains of Salmonella typhimurium, but there was no dose-
response relationship (Majumdar et al., 1977). The results of the
other mutagenicity studies with aldrin and dieldrin in bacterial
test systems have been negative (Table 41). A critical survey of
the published reports indicates clearly that neither aldrin nor
dieldrin is mutagenic in microbial systems (Ashwood-Smith, 1981).
8.6.2. Mammalian cell point mutations
Only one study on the in vitro mutagenicity of dieldrin to
mammalian cells has been reported. Dieldrin was weakly mutagenic
when tested at a single concentration (0.01 mmol/litre) in ouabain-
resistant Chinese hamster V-79 cells. The significance of this
result is difficult to assess because of the lack of a dose-
response relationship, and a positive control group was not used
(Ahmed et al., 1977a).
8.6.3. Dominant lethal assays and heritable translocation assays
in mice
Aldrin did not show any detectable dominant lethality when
given as a single intraperitoneal dose (8 or 40 mg/kg) or in daily
oral doses (0.5 or 1 mg/kg body weight) for 5 days to male ICR/Ha
Swiss mice (Epstein et al., 1972).
Dieldrin, also, revealed no detectable dominant lethality in
four assays in male mice following a single intraperitoneal
injection (5.2 or 26 mg/kg) or daily oral doses of 2 or 3 mg/kg
body weight for 5 days (Epstein et al., 1972). Likewise, a single
oral dose of 12.5, 25, or 50 mg/kg body weight did not produce
dominant lethality in CF-1 mice (Dean et al., 1975). Bidwell et
al. (1975) carried out a dominant lethal test on B6D2F1/J mice
orally administered 0.08, 0.8, and 8 mg/kg body weight dieldrin for
5 days, but no effects were seen.
In further studies by Bidwell et al. (1975), a heritable
translocation test was performed on male mice after oral intake of
0.008, 0.08, or 0.2 mg dieldrin/kg body weight per day for a period
of 6 weeks. The cytogenetic determination of somatic cells using
the micronucleus test and the usual analysis of spermatocytes did
not reveal an increase in the rate of translocations.
8.6.4. Micronucleus test
Aldrin did not induce a significant increase in the frequency
of micronuclei in the bone marrow of mice treated orally with 13
mg/kg body weight (Usha Rani et al., 1980).
No cytogenetic abnormalities were seen in a standard metaphase
analysis and micronucleus test after oral gavage of mice with 0.8
or 8 mg dieldrin/kg body weight per day for 5 days (Bidwell et al.,
1975).
8.6.5. Chromosome and cytogenicity studies
Chinese hamsters (three groups of four males and four females)
were orally dosed with 30 and 60 mg dieldrin/kg body weight. No
chromosome abnormalities were found in femoral bone marrow cells
(Dean et al., 1975).
Table 41. Aldrin and dieldrin: mutagenicity tests in microorganisms
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
E. coli WP2 Try- none aldrin and dieldrin; negative Ashwood-Smith et
1000 µg/plate al. (1972)
E. coli WP2 hcr rat S9 up to 5000 µg/plate negative Moriya et al.
(1983)
E. coli Ga1 Rs none aldrin and dieldrin; negative Fahrig (1974)
Serratia marcescens alpha 21 and 742 dose not stated
Saccharomyces cerevisiae
Aspergillus nidulans diploid P1 and none dieldrin; 13 or 26 negative Crebelli et al.
haploid strain 35 mmol (1986)
Saccharomyces cerevisiae 632/4 none aldrin; 5 µg/ml on positive Guerzoni et al.
disc (1976)
Bacillus subtilis (Rec-assay) none aldrin and dieldrin; negative Shirasu (1975)
Salmonella typhimurium TA 1535, 1536,
1537, 1538
E. coli WP2 hcr+, hcr-
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; 10, 50, negative Bidwell et al.
1536, 1537, 1538, 100, or 500 µg/plate (1975)
G46
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; dose not negative McCann et al.
1537 stated (1975)
Salmonella typhimurium TA 1535, 1536, rat S9 dieldrin; 1000 negative Marshall et al.
1537, 1538 µg/plate (1976)
Salmonella typhimurium TA 98, 100 none dieldrin; 10, 30, negative Glatt et al.
100, 300, 1000, 3000 (1983)
µg/plate
Salmonella typhimurium TA 90, 100, 1535, rat S9 up to 5000 µg/plate negative Moriya et al.
1537, 1538 (1983)
---------------------------------------------------------------------------------------------------------
Table 41. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium TA 98, 100, 1535, mouse S9 dieldrin; 1000 negative Van Dijck & Van
1537, 1538, 1950, µg/plate de Voorde (1976)
1978
Salmonella typhimurium not specified S9 dieldrin; dose not "weak" Ercegovich &
stated response Rashid (1977)
Salmonella typhimurium TA 98, 100, 1535 mouse S9 dieldrin; 1, 25, or positive Majumdar et al.
50 µg/ml (1977)
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; up to 2500 negative Purchase et al.
1538 µg/plate (1978)
Salmonella typhimurium TA 98, 100 rat S9 dieldrin; 50 or 1000 negative Wade et al.
µg/plate (1979)
Salmonella typhimurium TA 98, 100, 1535, rat, aldrin; dose not negative Simmon et al.
1537, 1538 mouse, and stated (1977)
human S9
Salmonella typhimurium TA 98, 100 rat S9 aldrin and dieldrin; negative Nishimura et al.
364 and 380 µg/plate (1982)
Salmonella typhimurium TA 98, 100 1535, rat S9 dieldrin 2.6 x 104 negative DeFlora (1981)
1537, 1538 nmoles/plate
---------------------------------------------------------------------------------------------------------
The effect on the bone marrow cells of STS mice was examined
after applying a single dose of dieldrin intraperitoneally at 0, 1,
30, or 50 mg/kg body weight. A decrease in the mitotic index was
noted, as dieldrin concentration increased, and differences in
chromosome aberrations (a slight increase in breaks, fragments,
and interchanges) were found (Majumdar et al., 1976).
Seventy-one juvenile mallard ducks, the parents of which had
been exposed to various dietary levels of dieldrin for 6 months or
longer, were grouped and fed dieldrin at a level that corresponded
to the diet fed to the parents (0, 4, 10, or 30 mg dieldrin/kg
diet) for approximately 60 days. At the end of this period, no
chromosomal aberrations were found in femoral bone marrow cultures.
However, the mitotic index of ducks exposed to 30 mg/kg was
significantly reduced (Bunch & Low, 1973).
When lymphocytic cultures from adult mallard ducks (which had
not been exposed to dieldrin) were treated with 0, 0.1, 1, 10, 30,
or 100 mg dieldrin/kg, there was a significant increase in the
incidence of chromosome structural alterations, but only at the
highest dose level. The mitotic index was significantly reduced at
all dose levels, the greatest decreases occurring at the two
highest dose levels (Bunch & Low, 1973).
Chromosome studies in cultured lymphocytes from current
dieldrin plant workers (12) former plant workers (9), and control
(17) did not show any differences in the frequency of chromosome
aberrations (Dean et al., 1975). In one study, it was reported
that aldrin produced chromosomal aberrations in cultured human
lymphocytes at concentrations of 19 and 38 µg/ml. In mice and
rats, an intraperitoneal injection of a dose of 19 mg/kg body
weight was reported to induce chromosomal gaps, breaks, deletions,
and fragments in bone marrow cells (Georgian, 1975).
Cultured human peripheral lymphocytes from agricultural and
public health (anti-Chagas' disease) workers with at least 10 years
exposure to dieldrin were examined for structural chromosome
aberrations and sister chromatid exchange. No differences were
seen when they were compared with lymphocytes derived from a
control group (Bordon, 1980).
8.6.6. Host-mediated assays
Bidwell et al. (1975) carried out a host-mediated assay,
incorporating blood- and urine-recovery studies, for mutagenic
substances on B6D2F1/J mice. Five daily oral doses of 20 mg
dieldrin/kg body weight were given, and the mice were then injected
intraperitoneally with Salmonella tester strains. The results were
negative. In a further host-mediated assay using Saccharomyces
cerevisiae (strain D4, heteroallelic at the ade-2 and trp-5 loci)
as tester microorganism, CF-1 mice were treated with a single oral
dose of 25 or 50 mg dieldrin/kg body weight or with five daily
doses of 5 or 10 mg/kg body weight. No mutagenic activity was
found (Dean et al., 1975).
8.6.7. Cell transformation in mammalian cell systems
Dieldrin (0.08 - 250 mg/litre) proved negative in mammalian
cell transformation tests using cell lines derived from baby Syrian
hamster kidney (BHK-21 C13) and from human lung (Wl-38), either
with or without metabolic activation by rat liver S9 microsomal
fraction (Purchase et al., 1978).
In a 6-thioguanine resistance mutation assay using FM 3A mouse
cell cultures, aldrin was weakly mutagenic (Morita & Umeda, 1984).
8.6.8. Drosophila melanogaster and other insect systems
There is no evidence of a mutagenic activity of aldrin or
dieldrin in Drosophila melanogaster (Benes & Sram, 1969; Bidwell et
al., 1975). No increase in recessive or dominant lethal mutations
was found following exposure of the wasp Bracon hebetor to
sublethal doses of dieldrin (95%) (Grosch & Valcovic, 1967).
8.6.9. Effects on DNA
DNA strand breakage was not detected in Chinese hamster V-79
cells exposed to dieldrin (0.1 - 1 mmol/litre) in the presence of
rat liver S9 microsomal fraction using the alkaline elution assay
(Swenberg et al., 1976).
Aldrin (1 - 1000 µmol/litre) and dieldrin (1-100 µmol/litre)
induced unscheduled DNA synthesis in SV-40 transformed human
fibroblast cells (VA-4) both in the presence and absence of rat
liver microsomes (Ahmed et al., 1977b). This study was repeated by
Zelle & Lohman (1977). After exposure to dieldrin, the rate of DNA
synthesis in normal primary human fibroblasts (AH) decreased, but
returned to the control level in a few hours. This suggests that
dieldrin interferes with semiconservative DNA replication without
damaging DNA. The effect of dieldrin on the induction of repair
replication was studied with both AH cells and SV-40 transformed
human cells (MM-SV-40). No evidence that dieldrin could induce DNA
repair was found (Zelle & Lohman, 1977).
The DNA breakage rates in an Escherichia coli plasmid after
treatment with aldrin or dieldrin did not differ from those in
untreated plasmid DNA, suggesting that, at least in these studies,
the compounds did not interact directly with DNA (Griffin & Hill,
1978).
The effects of aldrin and dieldrin (both at 100 µg/ml) on the
uptake of tritiated thymidine by cultured rat thymocytes and human
lymphocytes were tested under different experimental conditions.
Both compounds appeared to have marginal effects on thymidine
uptake, suggesting inhibition of DNA synthesis (Rocchi et al.,
1980).
Aldrin (100 mmol/litre) and dieldrin (500 mmol/litre) did not
induce unscheduled DNA synthesis in primary cultures of Fischer 344
rat hepatocytes (Probst et al., 1981). Williams (1982) reported
the results of the hepatocyte primary culture/DNA repair test,
using freshly isolated hepatocytes of high metabolic capability to
monitor the production of DNA damage by measuring DNA repair
synthesis. Aldrin and dieldrin gave equivocal results concerning
DNA repair, but there was no damage to DNA. Aldrin (0.3 - 3
mmol/litre) induced DNA strand breaks in an alkaline elution/rat
hepatocyte assay (Sina et al., 1983).
Cultured hepatocytes from male Balb/c mice treated with
dieldrin at a concentration of 4 x 10-4 mol/litre showed no
unscheduled DNA synthesis. The results were no different with
cells from mice treated in vivo with phenobarbital (Klaunig et al.,
1984).
A DNA synthesis inhibition/damage test on HeLa cells with S9
showed inhibition to 60% of the control value within 90 min of
treatment with 4 x 10-4 mol dieldrin/litre (Painter, 1981).
8.6.10. Cell to cell communication
Both aldrin and dieldrin inhibited gap junctional intercellular
communication between 6-thioguanine-sensitive and 6-thioguanine-
resistant human teratocarcinoma cells in culture (Zhong-Xiang et
al., 1986).
The inhibition of cell to cell communication was observed in
human teratocarcinoma cells in culture in the presence of dieldrin,
using dye transport methods (Wade et al., 1986).
Metabolic cooperation between 6TG-resistant and HGPRT-deficient
Chinese hamster V79 cells was inhibited when aldrin (2.5 - 10
µg/ml) or dieldrin (2.5 - 5 µg/ml) was added to the medium (Kurata
et al., 1982).
Trosko et al. (in press) studied the inhibition of gap
junctional-mediated intercellular communication using co-cultures
of Chinese hamster cells. To do this, an in vitro assay (in which
the metabolic cooperation between V79-6-thioguanine-sensitive (6
TGs) and resistant (6 TGr) cells is studied) has been developed to
detect the ability of non-cytotoxic and non-mutagenic chemicals to
inhibit gap junctional communication. Aldrin and dieldrin
inhibited metabolic cooperation at concentration of about 4 µg/ml
or more.
8.6.11. Appraisal
Aldrin and dieldrin are not mutagenic to mammals in a variety
of mutagenicity test systems with unrelated different end-points.
8.7. Special Studies
8.7.1. Liver enzyme induction
Aldrin and dieldrin have been shown to increase the activity of
liver microsomal enzymes, generally associated with enlargement of
the liver. They have also been found to induce microsomal
dimethylaminoantipyrine-N-demethylase and aldrin-epoxidase, and
increase the cytochrome P-450 level (Campbell et al., 1983). This
enzyme induction is the earliest and most sensitive indicator of an
effect of exposure in mouse, rat, beagle dog, and rhesus monkey
(Wright et al., 1972).
Studies on rats indicated a no-effect level for enzyme
induction, by either aldrin or dieldrin, at 1 mg/kg diet (Gillett &
Chan, 1968; Kinoshita & Kempf, 1970; Den Tonkelaar & Van Esch,
1974).
In the rhesus monkey, the activity of the liver microsomal
monooxygenase system was increased by dieldrin (at daily feeding
levels of 1.75 and 5 mg/kg diet for approximately 6 years), but no
associated liver enlargement was observed. The dietary intake of
dieldrin required for the induction of this enzyme system in
monkeys was approximately 1 mg/kg diet, corresponding to an intake
of 25 - 30 µg/kg body weight per day (section 8.4.4) (Wright et
al., 1978).
In human beings, oral dosing with 211 µg dieldrin/day
(approximately 3 µg/kg body weight per day) for two years, in
addition to the daily intake of 19 µg dieldrin from the diet, did
not increase the activity of microsomal liver enzymes as measured
by the concentration of p,p'-DDE in adipose tissue and blood. No
evidence of enzyme stimulation was observed in a group of 10
workers (at the time, they were the most highly exposed workers) in
a manufacturing plant with a mean exposure equivalent to a daily
oral intake of 17 µg/kg body weight (maximum 24 µg/kg body weight)
(Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
8.7.2. Nervous system
8.7.2.1 Rat
When three groups of eight male albino rats were fed diets
containing 0, 25, or 50 mg dieldrin/kg for 60 days, there was no
effect on body weight or learning, but muscular efficiency,
measured by pulling weights of increasing magnitude in a 250-cm
runway, was decreased (Khaïry, 1960). This finding is in agreement
with the results of a study on nerve muscle (gastrocnemius)
preparations of pre-treated rats (Ibrahim, 1964).
8.7.2.2 Dog
Groups of five dogs of each sex, given daily oral doses of
dieldrin (0.05 mg/kg body weight) by capsule for 2 years, did not
show any changes in behaviour or EEG recordings compared with
controls (Walker et al., 1969b).
8.7.2.3 Monkey
When groups of three or four adult male squirrel monkeys were
given daily oral doses of 0, 0.01, or 0.1 mg dieldrin/kg body
weight for 54 days, learning ability was impaired and changes (high
amplitude slow waves) in the EEG occurred in both test groups (Van
Gelder & Cunningham, 1975).
8.7.3. Weight loss and stress
It is known that in birds, and perhaps in some small mammals,
dieldrin intoxication may be induced by starvation, weight loss,
and stress in animals having a previously harmless body burden of
dieldrin. Concern is sometimes expressed that, by analogy to these
observations, a similar course of events might occur in human
beings. Therefore, this phenomenon was studied in rats as well as
in human beings (for human beings see section 9.1.3.2).
8.7.3.1 Rat
In studies by Treon & Cleveland (1955), rats previously fed
diets containing aldrin or dieldrin at levels of 5, 10, or 15 mg/kg
diet for 7 - 18 months were starved. The complete withdrawal of
food did not result in the release of aldrin or dieldrin from the
adipose tissue stores to an extent sufficient to induce symptoms of
intoxication of any type.
When Osborne-Mendel rats fed 7.5 mg aldrin/kg diet for 4 weeks
were subsequently starved for 6 days with free access to water,
there was a marked loss of body weight and fat and a decrease in
the liver/body weight ratio. The total body burden of dieldrin
decreased during starvation regardless of age, sex, or the previous
level of exposure. The total quantity of dieldrin in the liver
decreased in all rats. In females, particularly older females, the
concentration of dieldrin in abdominal fat increased, whereas in
all males, the level in fat decreased. The concentration of
dieldrin in the blood was not increased. Young weanling rats
reacted similarly (Deichmann et al., 1972).
8.7.4. Immunosuppressive action
Loose et al. (1981) found that macrophages from mice fed 50 mg
dieldrin/kg diet had a marked impairment in antigen processing.
The effect was statistically significant in Kupffer cells at 50
mg/kg diet, in alveolar and splenic macrophages at 0.5, 5 and 50
mg/kg diet, and in peritoneal macrophages at 5 and 50 mg/kg diet.
There was an impairment of in vivo phagocytic clearance in mice
receiving 5 or 50 mg/kg diet for 8 weeks but not at 0.5 mg/kg diet.
This was related to a decrease in serum fibronectin. Tumour cell
killing after challenge with EL-4, P388, or mKSA tumour cells was
significantly impaired in mice fed either 1 or 5 mg dieldrin/kg
diet. The mean survival time after challenge with EL-4 was reduced
by 3 weeks, and with the P388 or mKSA tumour cells impairment was
observed after 3 or 18 weeks, respectively. There was no
alteration in the oxygen uptake by isolated macrophages either at
rest or during phagocytosis, and no effect on phagocytic activity
or capacity or on chemotaxis in vitro was observed.
Loose (1982) found that dieldrin caused immunosuppression in
mice. Levels of 1 or 5 mg dieldrin/kg diet were fed to BALB/c mice
for 3.5 or 10 weeks, and the mice were challenged intradermally
with Leischmania tropica. Dieldrin acted synergistically on
lethality in a dose- and time-related manner, indicating an effect
on host mechanisms. It also resulted in decreased antibody
formation to PVP, a T-independent antigen (direct splenic plague
assay). The mitogenic response of cultured T-cells to
phytohaemagglutinin (PHA) in dosed mice was depressed. Mitomycin C
and anti-Thy-1 abolished the mitogenic response. When splenic
T-cells from treated mice were mixed with T-cells from control
mice, there was inhibition of PHA mitogenesis. The data indicated
an active cell-mediated suppressor. A soluble macrophage factor
from the hepatic Kupffer cells (but not from alveolar or peritoneal
macrophages) suppressed the T-cell response to PHA. It was
concluded that administration of 5 mg dieldrin/kg diet to mice for
10 weeks caused a profound impairment of macrophage antigen
processing.
8.8. Toxicity of Photodieldrin and Major Metabolites
The relevance of photodieldrin lies in the fact that it has
metabolites identical to those of dieldrin and is quantitatively
and qualitatively similar in toxicity.
8.8.1. Photodieldrin
The photodecomposition of deposits of dieldrin on leaves and
grass has been reported (Roburn, 1963), and the physical and
chemical properties and structure of this decomposition product
have been determined (Robinson et al., 1966b; Rosen et al., 1966).
It appears to be the pentacyclo isomer of dieldrin (hexacyclo
isomer by the alternative nomenclature used by Rosen et al.
(1966)). Photodieldrin residues were less than the limits of
detection in most of the food samples analysed (Robinson et al.,
1966a).
8.8.1.1 Acute toxicity
Photodieldrin is more acutely toxic than dieldrin for mice,
rats, and guinea-pigs (Table 42). The toxicity for dogs is about
equal to that of dieldrin. Dieldrin-like convulsions have been
observed in all species given photodieldrin.
8.8.1.2 Short-term toxicity
(a) Mouse
When groups of five male and five female Carworth Farm No. 1
mice were fed 1, 3, or 10 mg photodieldrin/kg diet for 1 month, all
animals fed 10 mg/kg and two animals fed 3 mg/kg died. No changes
were observed at necropsy (Brown et al., 1967).
Table 42. Oral LD50 values for photodieldrin
-------------------------------------------------------------------
Species Vehicle LD50 Reference
Photodieldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Mouse dimethyl- 6.8 77.3 Brown et al.
sulfoxide (1967)
Rat dimethyl- 9.6 46.8 Brown et al.
sulfoxide (1967)
Guinea-pig dimethyl- 2.3-3.9 18-30 Brown et al.
sulfoxide (1967)
Dog (male) gelatin 120-160 120 Brown et al.
capsule (1967)
Dog (female) gelatin 80-120 80-100 Brown et al.
capsule (1967)
-------------------------------------------------------------------
(b) Rat
Groups of five male and five female Carworth Farm E rats were
fed 3 or 10 mg photodieldrin/kg diet for one month without apparent
ill effects (Brown et al., 1967).
In studies by Walton et al. (1971), groups of 28 male and
28 female Charles River rats were fed 0, 1, 5, or 25 mg
photodieldrin/kg diet for 3 months. A similar study was carried
out concurrently with dieldrin. The concentrations of
photodieldrin given in the diet were lowered from 25 to 12.5 mg/kg
diet within the first week of the study because of high mortality.
At the end of 3 months, no significant differences were found in
growth or food intake, and no gross evidence of toxicity was
observed. Liver/body weight ratios were increased at 12.5 mg/kg
diet. Increases in the activity or concentration of liver mixed-
function oxidase and microsomal cytochrome P-450 at 5 and 12.5
mg/kg diet indicated the occurrence of a dose-dependent enzyme
induction. The total protein content of the liver was not
affected. The short-term toxicities of photodieldrin and dieldrin
appeared to be similar.
Walker et al. (1971) fed groups of 12 Carworth Farm E rats of
each sex diets containing 0.1, 1, 10, or 30 mg photodieldrin/kg
diet for 3 months. The control group consisted of 24 male and 24
female rats. Six females given 30 mg/kg and two females given 10
mg/kg died. The animals in these groups that survived were
irritable and showed tremors when handled. Growth was reduced,
increases in serum urea and glutamic pyruvic transaminase (SGPT)
activity were seen in females fed 30 mg/kg, and the liver/body
weight ratio was increased in this group. In the groups fed 10 or
30 mg/kg, kidney/body weight ratio was increased in males. At
autopsy, no gross lesions were seen. Some of the animals fed 10 or
30 mg/kg showed CHIRL and centrilobular fatty changes in the liver.
Eosinophilic droplets were seen in the cytoplasm of the proximal
convoluted tubules and in the lumen of affected tubules in the
kidneys of males fed 10 or 30 mg/kg. No evidence of nephron damage
was found. No effects were observed in the animals dosed with 1
mg/kg.
(c) Dog
In a study by Walker et al. (1971), groups of four male and
four female beagle dogs received photodieldrin in olive oil by
capsule (daily oral doses of 0.005, 0.05, or 0.2 mg/kg body weight)
for 3 months. A control group of six males and six females
received olive oil in gelatine capsules. The health, behaviour,
body weight, and haematology were unaffected. In the 0.2 mg/kg
males, increases occurred in the plasma alkaline phosphatase and
SGPT activities, and, after 13 weeks, their serum protein levels
were slightly reduced. Increases in the liver/body weight ratios
occurred in the 0.2 mg/kg animals and the 0.05 mg/kg females. At
autopsy, no pathological changes associated with photodieldrin were
observed. No effects were observed at 0.005 mg/kg body weight.
8.8.1.3 Long-term toxicity
(a) Mouse
In a study on the long-term toxicity of photodieldrin, groups
of 50 B6C3F1 mice of each sex were fed diets containing 0.32 or
0.64 mg/kg for 80 weeks. After 80 weeks, the animals were fed a
control diet for 12 or 13 weeks. Concurrent control groups
consisted of 10 untreated mice of each sex. Pooled controls, used
for statistical evaluation, consisted of the concurrent controls
plus 60 male and female mice from similarly performed bioassays
with six other test chemicals. All surviving mice were killed at
93 weeks. Mean body weights and mortality were not affected by
treatment, but convulsions and hyperactivity were noted in treated
male mice. No statistically significant increase in tumour
incidence was found (NCI, 1977).
(b) Rat
In similar studies to those on mice, groups of 50 Osborne-
Mendel rats of each sex were given 5 or 10 mg photodieldrin/kg diet
for 80 weeks. After 80 weeks, the animals were fed a control diet
until sacrifice at 111 - 112 weeks. Because of neurotoxicity, the
doses in the females were reduced after 30 weeks, so that the time-
weighted average doses were 3.4 or 7.5 mg/kg diet for the females.
Concurrent control groups consisted of 10 rats of each sex. Pooled
controls, used for statistical evaluation, consisted of the
concurrent controls combined with 65 rats of each sex from similarly
performed bioassays with six other chemicals. All surviving animals
were killed at 111 - 112 weeks. Mean body weights and mortality
were not affected by treatment, but convulsions and hyperactivity
occurred in treated male and female rats. Photodieldrin was not
carcinogenic in this study (NCI, 1977).
8.8.1.4 Reproduction, embryotoxicity, and teratogenicity
(a) Mouse
Chernoff et al. (1975) fed groups of pregnant CD-1 mice (16 -
20 per group) photodieldrin in peanut oil (daily oral doses of 0,
0.15, 0.3, or 0.6 mg/kg body weight) from day 7 to day 16 of
gestation. At a dose of 0.6 mg/kg, one animal died. Liver/body
weight ratios were increased in a dose-related manner, but no
significant differences in fetal mortality, litter weight,
percentage of supernumerary ribs, or sternal or caudal ossification
centres were observed at any of the doses used. Photodieldrin was
not teratogenic or fetotoxic in CD-1 mice at doses up to and
including 0.6 mg/kg body weight.
(b) Rat
In a study by Chernoff et al. (1975), groups of 24 - 27
pregnant CD rats were given daily oral doses of photodieldrin in
peanut oil (0, 015, 0.3 or 0.6 mg/kg) on days 7 - 16 of gestation.
Some maternal mortality (5 out of 24 animals) occurred in the
0.6-mg/kg group. No significant differences in liver/body weight
ratios, fetal mortality, weight of the pups, or occurrence of
anomalies in litters of treated animals, compared with the
controls, were noted. No evidence of teratogenicity in CD rats was
observed at doses of photodieldrin up to and including 0.6 mg/kg
per day.
8.8.1.5 Appraisal
The acute oral toxicity of photodieldrin to rodents is greater
than that of dieldrin. In short-term toxicity and teratogenicity
studies, no major differences between the two compounds were found.
Photodieldrin did not induce tumours in mice and rats. The
accumulation of photodieldrin in the adipose tissue of experimental
animals was less than that of dieldrin (section 6.2.3).
8.8.2. Major metabolites of dieldrin
8.8.2.1 Acute toxicity
The acute oral toxicity of the major metabolites of dieldrin is
far less than that of dieldrin itself (Table 43).
8.8.2.2 Short-term toxicity
In a study by Granville et al. (1973), groups of 12 male and 12
female rats (control group of 24 males and 24 females) were fed
diets containing aldrin dicarboxylic acid (0, 0.1, 1, 10, 100, or
1000 mg/kg diet) for 13 weeks. No adverse effects attributable to
the dosing were observed in general health, behaviour, body weight,
clinical chemical and haematological values, organ weights, or on
pathological examination of the viscera.
Table 43. Oral LD50 values for metabolites of aldrin and dieldrin
in mice
-------------------------------------------------------------------
Compound LD50 Reference
(mg/kg body weight)
-------------------------------------------------------------------
trans-6,7-dihydroxy-dihydro- 1250 Korte & Arent
aldrin (1965)
9-hydroxy-dieldrin > 400 Baldwin et al.
(1970)
hexachlorohexahydromethano- > 850 Baldwin et al.
indenedicarboxylic acid (1972)
(aldrin dicarboxylic acid)
-------------------------------------------------------------------
8.9. Mechanisms of Toxicity; Mode of Action
Like most chemicals, aldrin and dieldrin do not have a single
mechanism of toxicity. The main target organs of these chemicals
are the central nervous system and the liver.
8.9.1. Central nervous system
Intoxication following acute or long-term overexposure is
characterized by involuntary muscle movements and epileptiform
convulsions. Survivors, after a short period of residual signs and
symptoms, recover completely (Hoogendam et al., 1962; Avar &
Czegledi-Janko, 1970; Jager, 1970). In rare cases, a residual
brain injury has been reported, but this has been found to be due
to the convulsive state or prolonged cerebral anaemia rather than
to the dieldrin per se. Apparently, a still unidentified receptor
site in the central nervous system is reversibly occupied, and when
this occupation exceeds a certain degree, myoclonics and
convulsions occur (Van Genderen, 1979). In vitro, the dieldrin
metabolite aldrin transdiol appears to be more potent in this
respect that is dieldrin itself (Van den Bercken, 1972; Van den
Bercken & Narahashi, 1974). However, in cats, the aldrin transdiol
appeared to be inactive (Joy, 1977). The mechanism of action seems
to be a presynaptic inhibition as well as an increased release of
an unidentified transmitter (Akkermans, 1974; Akkermans et al.,
1975; Joy, 1976).
Joy (1982) suggested that dieldrin acts by intensifying
synaptic activity through a presynaptic locus of action and
possibly a post-synaptic action as well. Neurons having a large
number of synapses will be affected most. There does not appear
to be any selective action on a particular neurotransmitter or
neurotransmitter system. The modification of behaviour is dose
dependent and performance in complex behavioural tasks is readily
disrupted.
Aldrin and dieldrin and other cyclodiens inhibit the gamma
amino butyric acid (GABA)-induced chloride ion uptake into skeletal
muscles and the binding of tritiated dihydropicrotoxinin (anion
channel probe) to the membrane. This results in central nervous
system excitation and convulsions due to the blocking of GABA
transmitters (Lawrence & Casida, 1984; Abalis et al., 1985).
8.9.2. Liver
The mode of action of aldrin and dieldrin on the liver involves
an increase in the activity of microsomal biotransformation
enzymes, particularly of the monooxygenase system with cytochrome
P-450. This induction of liver microsomal enzymes is reversible
and, if exceeding a certain degree, appears to be associated with
the occurrence of CHIRL and hepatomegaly in the liver of rodents
(sections 6.3 and 8.2) (Jager, 1970; Wright et al., 1972, 1977,
1978).
9. EFFECTS ON HUMAN BEINGS
9.1. General Population Exposure
9.1.1. Acute toxicity - poisoning incidents
When a toxic dose of aldrin or dieldrin has been ingested or
has contaminated the skin, effects appear from 20 min to 24 h
afterwards. Signs and symptoms may include headache, dizziness,
nausea, general malaise, and vomiting, followed by muscle
twitchings, myoclonic jerks, and convulsions. Death may result
from cerebral anoxaemia (Nelson, 1953; Princi, 1954; Hayes, 1957,
1963; Hoogendam et al., 1962, 1965; Kazantzis et al., 1964;
Schafer, 1968; Jager, 1970).
The duration of the interval between oral intake or skin
contact and onset of symptoms (as well as the clinical picture)
depends on the dose absorbed. With massive overexposure,
convulsions may occur even in the absence of any premonitory
symptoms.
Initially, there is no fever or change in blood count or in
blood chemistry. However, later the temperature may be elevated
and leukocytosis may occur. Terminal hyperthermia has been
reported. Abnormal EEG patterns showing spike and dome complexes
and multiple spike and wave discharges, or in less serious
intoxications, bilateral synchronous theta discharges may be seen.
The diagnosis needs to be confirmed by determining the insecticide
concentration in the blood.
The onset of clinical intoxication is practically always acute
also in those cases where the accumulation of dieldrin in the
target tissues has taken place during a much longer period. The
latter cases are, therefore, usually indistinguishable from acute
intoxication. Survivors almost always recover completely (Jager,
1970; Hayes, 1982).
Estimates of dosages in anecdotal cases suggest that fatalities
have occurred with ingestion of approximately 10 mg dieldrin/kg
body weight, (Hayes, 1982) but Hodge et al. (1967) estimate the
lethal dose of aldrin and/or dieldrin for the adult man to be about
5 g.
Cases of poisoning have occurred by ingestion of formulated
material, mostly in children by mistake (for instance when aldrin
is used in granules as bait to control ants) or by adults with
suicidal intent. Several cases of poisoning have been the result
of ingesting food contaminated with aldrin or dieldrin during
storage or transport.
Van Raalte (1965) surveyed the world literature for all cases
of fatal poisoning by aldrin and dieldrin, and found 13 cases:
four suicides, three due to accidental ingestion, five due to
accidental contamination, and only one (a spray operative) due to
occupational exposure. No cases of fatal poisoning have been
reported during the course of aldrin and dieldrin manufacture and
formulation.
A non-exhaustive overview of published poisoning cases is given
in Table 44. A more complete review is provided by Hayes (1982).
Table 44. Case reports on accidental and suicidal acute aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Causative agent Circumstances Reference
of cases
cases
------------------------------------------------------------------------------------------
1 - aldrin emulsifiable attempted suicide Spiotta (1951)
concentrate
53 - aldrin and other consumption of seed grain WHO (1958)
pesticides
13 aldrin and dieldrin review of all fatal cases from Van Raalte (1965)
literature:
- 4 suicides, 3 accidental
ingestion, 5 accidental
contaminations, 1 sprayer
2 1 5% dieldrin accidental ingestion Garrettson & Curley
(1969)
79 consumption of dieldrin- WHO (1977)
contaminated rice in Mali
1 - dieldrin (120 mg/kg) attempted suicide Black (1974)
2 - dieldrin Fry (1964)
12 - aldrin + BHC consumption of seed grain Gupta (1975)
------------------------------------------------------------------------------------------
9.1.2. Effects of short- and long-term exposure - controlled human
studies
9.1.2.1 Accidental poisoning
Twelve cases of neurotoxicity, resulting from the repeated
consumption of wheat into which aldrin dust and gammexane (BHC)
powder had been mixed accidentally, have occurred in India (Gupta,
1975). The patients consumed this wheat for 6 - 12 months before
showing typical clinical symptoms, including convulsions.
Electroencephalographic tracings were consistent with a diagnosis
of organochlorine insecticide poisoning. The patients were treated
with phenobarbital and diazepam. The latter was more effective in
controlling seizures. All patients recovered.
The threshold dieldrin concentration in the blood below which
no adverse effects have been observed (and none are to be expected)
is 105 µg/litre (see also section 9.2.1.1). The dieldrin
concentration in the blood of the general population, in the
countries where this has been investigated, is well below this
threshold level. However, there are rare cases in which it seems
that low concentrations of dieldrin have induced effects.
A rare, well investigated and well reported case of dieldrin-
induced immunohaemolytic anaemia was observed in Iowa, USA. The
patient had a haemolytic anaemia with a positive direct
antiglobulin (Coombs) test and a positive Ham test in the serum.
The serum contained anti-bodies selectively active against
erythrocytes coated with dieldrin. The patient improved following
splenectomy. Dieldrin concentrations in blood and fat were similar
to those of the general Iowa population (Hamilton et al., 1978). A
similar case was reported by Muirhead et al. (1959).
9.1.2.2 Controlled human studies
In section 6.2.2.4, reference was made to a pharmacodynamic
study in human volunteers. This study had three objectives:
(a) to establish the relationship between the daily intake of
dieldrin and its concentration in human blood and adipose
tissue;
(b) to establish the blood/fat ratio in human beings; and
(c) to establish the relationship between the concentrations
of dieldrin in blood and fat and the length of exposure
(Hunter & Robinson, 1967, 1968; Hunter et al., 1969).
In addition, the opportunity was taken to monitor the health of the
human subjects during and after the exposure by full clinical,
physiological, and laboratory examinations as well as full
electroencephalographic (EEG) studies, polygraphic recording of
cardio-respiratory function, measurement of basal metabolic rate,
and electroneuromyographic studies at frequent intervals to detect
the possible occurrence of changes in physiological function. The
study involved 13 adult male college graduates without a history of
recent occupational exposure to pesticides. The subjects received
0, 10, 50, or 211 µg dieldrin per day for 2 years. All the men
continued in excellent health. Clinical, physiological, and
laboratory findings remained essentially unchanged throughout the
whole experimental period of 24 months and the 8 months after
exposure. No departures from what is regarded as normal for the
general population were observed. The concentration of p,p'-DDE in
adipose tissue and blood did not show any significant change during
or after the study, indicating that the liver microsomal enzyme
activity had not been induced. Thus, the total daily intake of
230 µg (211 µg plus intake from food) of dieldrin per person for 2
years had no effect on health. The concentrations of dieldrin in
both adipose tissue and blood were shown to be proportional to the
daily intake (section 6.2.2.4).
9.1.3. Tissue concentrations of dieldrin in hospitalized people
9.1.3.1 Pathological findings
Specimens of human abdominal subcutaneous fat, obtained from
four hospitals in Chicago were analysed for residues of dieldrin.
Dieldrin was not present in 103 out of 221 samples analysed for
this pesticide. Positive samples contained 0.01 - 1.39 mg
dieldrin/kg fat (mean value 0.14 mg/kg). There was no correlation
between the dieldrin concentration in adipose tissue and
pathological findings (Hoffman et al., 1967).
When organochlorine pesticide concentrations were determined in
the adipose tissue and liver of 271 hospital patients in Miami,
USA, patients with typical alcoholic (Laennec's) cirrhosis of the
liver had about twice the dieldrin concentration in the liver of
that found in the normal population. In patients with post-
necrotic cirrhosis, fatty metamorphosis of the liver, metastatic
malignancy of the liver, or primary hepatocellular carcinoma,
dieldrin concentrations were "normal". Terminal cases with
carcinomas of different organs had elevated concentrations of
organochlorine pesticides in the fat, but no association with any
particular neoplastic disease was found (Radomski et al., 1968).
In Hawaii, emaciated patients who had carcinoma and/or focal or
generalized liver pathology were found to have "normal" (for the
USA) concentrations of dieldrin in the liver and body fat (Casarett
et al., 1968).
When concentrations of organochlorine pesticides were
determined in specimens of liver, brain, and adipose tissue from
autopsies of patients with cirrhotic liver disease in Vancouver
(Canada) hospitals, the concentrations of dieldrin appeared to be
no higher than in tissues from controls (Oloffs et al., 1974).
In a case-control study on 122 matched cancer patients in south
Florida, USA, a comparison was made of dieldrin residues in the
adipose tissue of cancer patients and controls. The mean dieldrin
concentration in the adipose tissue was 0.3 mg/kg fat in both
cancer patients and controls (Davies et al., 1975).
9.1.3.2 Influence of weight loss and stress on dieldrin
concentrations in tissues
It is well known that, in birds, and perhaps in some small
mammals, dieldrin intoxication may be induced by starvation, weight
loss, or stress in animals having a previously harmless body burden
of dieldrin. Concern is sometimes expressed that, by analogy to
these observations, a similar course of events might occur in human
beings.
Twenty-nine patients (14 males, 15 females) undergoing surgery
were investigated. The concentrations of dieldrin in the blood
were unaffected by the catabolic responses to surgery. In another
study, these authors determined the concentrations of dieldrin in
the blood of 4 women undergoing voluntary near-starvation for
slimming purposes, which resulted in weight losses of up to 7.5
kg/week. There was no increase in the concentration of dieldrin in
the blood (Hunter & Robinson, 1968).
No significant difference was found between the dieldrin blood
concentrations of slimming or non-slimming mothers before and after
delivery (Eckenhausen et al., 1981).
On the basis of these results and calculations, it is suggested
that significant weight loss does not result in increased
concentrations of dieldrin in human tissues (Van Raalte, 1965;
Hunter & Robinson, 1968).
9.1.4. Exposure in treated homes
From the data on aldrin concentrations in the air of houses
treated for termite control (section 5.1.2), an estimate of the
dieldrin concentration in the blood of occupants of these houses
can be made using the mathematical formulas given by Hunter et al.
(1969) for deriving blood concentrations from the average daily
intake. The average daily intake is based on an estimated average
in-house volume of air inhaled per day (15 m3). The dieldrin blood
levels of home dwellers, calculated in this way, remain far below
the blood concentration no-effect level for the general population
(secton 9.2.1.1).
The blood dieldrin concentrations of 59 female residents of
Dade County (Florida, USA), where many houses had been treated for
termite control, were of the order of 1 µg/litre (Barquet et al.,
1981).
Also relevant to the health of home dwellers is the experience
obtained in the 1950s and 1960s when tens of thousands of houses in
more than 30 countries were sprayed with dieldrin for malaria and
yellow fever eradication. Although exposures were presumed to have
been high, as a result of surface spraying inside and outside
houses, no adverse health effects were reported in home dwellers.
Neither were adverse effects observed in well-trained and
medically-supervised spray operators (Soper, 1955 (Personal
communication at the 2nd Meeting of the Industrial Council on
Tropical Health, Boston); Fletcher et al., 1959).
9.2. Occupational Exposure
9.2.1. Acute toxicity - poisoning incidents
With the exception of poisoning cases resulting from massive
acute overexposure, most reported cases of poisoning with aldrin
and dieldrin in occupationally-exposed men have been the result of
a slow build-up of the insecticide in the body, the daily intake
exceeding the daily excretion (Jager, 1970; Hayes, 1982).
Based on the experience of Jager (1970), it was suggested that
the classification of types of intoxication by Hayes (1963) be
modified as follows:
Type 1: an acute convulsive intoxication with no (or only
minor) prodomi, resulting from one or several gross overexposures.
Type 2: a greater number of smaller doses may cause an
accumulative intoxication. Clinically, this results in a syndrome
of headache, dizziness, drowsiness, hyperirritability, general
malaise, nausea, anorexia, occasional vomiting. At times muscle
twitchings, myoclonic jerks and convulsions may occur. In these
circumstances minor increases in the insecticide level in the
blood, perhaps caused by minor fluctuations in exposure, may bring
about a convulsive intoxication.
Type 3: this is actually a combination of Types 1 and 2. In
this type an overexposure, in itself not significant, causes an
acute convulsive intoxication superimposed upon a subclinical
accumulative intoxication of Type 2.
These three types of intoxication are schematically illustrated
in Fig. 3.
The other metabolites, the chemical identities of which have
been rigorously established, are detailed below.
(a) The trans-6,7-dihydroxy compound (Fig. 2, compound IV) is
formed by the hydration (formal) of the epoxide ring of dieldrin
(Korte & Arent, 1965). This compound is a major metabolite in
rabbit urine, but of relatively minor importance in other species.
The formation of the cis-diol by rat microsomes has been
demonstrated, together with its epimerization to the trans-diol
(McKinney et al., 1973). Both the cis- and trans-diols have been
synthesized (Korte & Arent, 1965; Chau & Cochrane, 1970b; Bedford &
Harrod, 1972b).
(b) The dicarboxylic acid (Fig. 2, compound V) is derived from
the dihydroxy metabolite (Baldwin et al., 1972; Oda & Mueller,
1972). This compound has also been synthesized (Buechel et al.,
1966), and has been shown to undergo further degradation (formation
of two isomers of a monodechlorinated derivative) after intravenous
injection into male and female rats (Lay et al., 1975).
(c) The bridged pentachloroketone (PCK) (Fig. 2, compound VII)
is mainly found in the urine and kidneys of male rats, but, even in
rats, it is a minor metabolite (Damico et al., 1968; Klein et al.,
1968; Richardson et al., 1968). In other species, it is a very
minor metabolite of dieldrin. It is also a metabolite of
photodieldrin (Klein et al., 1970), and has been synthesized
(Bedford & Smith, 1978).
The Chemical Abstract or Von Baeyer AG/IUPAC names of aldrin,
dieldrin, photodieldrin, and metabolites are given in Appendix I.
Methods for the quantitative determination of the four
metabolites are available. They depend on the availability of
authenticated analytical standards (Ludwig & Korte, 1965;
Richardson, 1971; Baldwin et al., 1972).
6.3.1.2 Human studies
A metabolite of dieldrin detected in human faeces has been
shown to be the 9-hydroxy derivative (Richardson & Robinson, 1971).
6.3.1.3 Non-domestic organisms
The conversion of aldrin to dieldrin was studied in algae
( Chlorella and diatoms) and protozoa (Dinoflagellates and mixed
protozoa) after exposure for 24 h to 0.1 mg aldrin/litre. The
amount of dieldrin present in the cultures was of the order of
0.06 - 0.2 µg/litre). The amounts of dieldrin were greater in the
protozoa than in the algae; it was concluded that these planktonic
species have enzyme systems that epoxidize aldrin (Khan et al.,
1972b).
The conversion of aldrin to dieldrin in 12 species of fresh-
water invertebrates has been compared. Ten species were exposed
for 2 h to concentrations of 0.1 or 0.25 mg aldrin/litre, and two
species of molluscs were exposed to 0.25 mg aldrin/litre for 4 h.
The concentrations of dieldrin relative to aldrin in the whole
bodies of eight species from four phyla (Coelenterata,
Platyhelminthes, Annelida, and Arthropoda) were in the range
1.03 - 8.48%. In two species of Insecta (dragon fly nymphs and
Aedes larvae), the values were 24.9% and 42.4%, respectively. The
two species of molluscs had dieldrin concentrations (relative to
aldrin) of 17 - 19% (Khan et al., 1972b).
In a study on an ostracod (Chlamydotheca arcuata) exposed to
14C-labelled aldrin (5.5 - 11.2 µg/litre), aldrin was readily
converted to dieldrin, 83% conversion occurring within 24 h. The
elimination of aldrin and dieldrin appeared to involve both passive
and active processes, and it was concluded that dieldrin was
eliminated more rapidly after dieldrin exposure than after aldrin
exposure (Kawatski & Schmulbach, 1972).
A number of in vitro studies have been carried out concerning
the influence of these insecticides on mixed-function oxidase
activity. The epoxidation of aldrin to dieldrin by this enzyme
system has been demonstrated in crayfish (Cambarus) (Khan et al.,
1972a,b), in snail (Lymnea palustris) and clam (Khan et al.,
1972b), and in midge larvae (Chironomus riparius) (Estenik &
Collins, 1979).
The conversion of aldrin to dieldrin by lobsters (Homarus
americanus) was reported by Carlson (1974).
The mixed-function oxidase activities in five species of fresh
water fish, as measured by the conversion of aldrin to dieldrin,
were investigated by Ludke et al. (1972). The fish were exposed to
aldrin (50 µg/litre) for 4 h, and the concentrations of aldrin and
dieldrin in the liver were determined. Contrary to earlier
reports, the conversion by epoxidation of aldrin to dieldrin in
fish may be the rule rather than an exception.
The epoxidation of 14C-aldrin to dieldrin in susceptible and
resistant mosquitofish (Gambusia affinis) has been investigated.
The fish were exposed to 5 µg 14C-aldrin/litre for 4 or 8 h and the
concentration of aldrin and dieldrin in liver and brain were
determined. The concentration of dieldrin (expressed in terms of
protein content) was significantly higher in the livers of
resistant fish than in susceptible fish). It was concluded that
resistant mosquitofish convert aldrin to dieldrin and/or water-
soluble compounds at a greater rate than susceptible mosquitofish
(Wells et al., 1973).
In studies by Addison et al. (1976), Atlantic salmon fry (Salmo
salar) were injected intramuscularly with 14C-aldrin, to initial
whole-body concentration of 5 mg/kg. The fish were maintained in
flowing fresh water, and were removed at five intervals up to 56
days for the measurement of whole-body residues. The time required
for 50% epoxidation of aldrin was between 1 and 2 days. Less than
10% of the radioactivity remained in the fish at the end of the
exposure. It was concluded that there was rapid elimination either
of unchanged aldrin or its epoxide, dieldrin, from the fish.
6.3.2. Photodieldrin (and major metabolites of dieldrin)
6.3.2.1 Rat
Besides unchanged photodieldrin, bridged pentachloroketone
(PCK) (Fig. 2, compound VII), a metabolite of photodieldrin, was
isolated from the brain, liver, adipose tissue, and blood of rats
(Carworth Farm, type E) fed diets containing 10 or 30 mg
photodieldrin/kg for 13 weeks (Baldwin & Robinson, 1969).
In studies by Klein et al. (1970), Osborne-Mendel rats were
given 14C-photodieldrin, orally or intraperitoneally, 5 days/week,
for 12 weeks, and urine was collected quantitatively every day. A
metabolite was found in the urine of male rats and shown to be PCK.
Small amounts of other (unidentified) more polar urinary
metabolites were also present.
6.3.2.2 Monkey
Metabolites were detected in the urine and faeces of a female
rhesus monkey given daily oral doses of 0.8 mg 14C-photodieldrin/kg
body weight for 175 days. Two metabolites were identified in the
urine: the trans-diol (Fig. 2, compound XI) and its glucuronide
conjugate. A faecal metabolite was tentatively identified as the
diol. A third metabolite was present in both urine and faeces, and
it was suggested that this might be a monohydroxy derivative of
photodieldrin (Nohynek et al., 1979).
6.4. Elimination and Excretion
6.4.1. Aldrin
6.4.1.1 Rat
When male rats were given daily oral doses of 4.3 µg 14C-aldrin
(equivalent to about 0.2 mg aldrin/kg diet) for 3 months, the
radioactivity in the urine increased from about 2% of the dose of
aldrin during the first week to about 10% during the 12th week. In
the faeces, the excreted radioactivity increased from about 48%
during the first week to about 93% during the 12th week. After
about 8 weeks, a saturation level was reached (i.e., there was a
balance between the rates of intake of aldrin and excretion of
aldrin plus aldrin-related materials). Extracts of urine and
faeces were examined by paper chromatography. Because the urine
was probably contaminated by faeces in the metabolism cages, only
the trend is given. In both faeces and urine, the aldrin content
decreased during the 12 weeks. The hydrophilic metabolites
increased, reaching 75% (faeces) and 95% (urine) of total
radioactivity after 12 weeks. The level of dieldrin was more or
less constant (Ludwig et al., 1964).
6.4.2. Dieldrin
6.4.2.1 Laboratory animals
As described in section 6.2.2.1, Hutson (1976) studied the
comparative metabolism of dieldrin in CFE rats and two strains of
mice after a single oral dose of 3 mg/kg body weight 14C-dieldrin.
The excretion of 14C activity in the faeces of the rats was 62.4%
of the administered dose in the non-pretreated group and 69% in the
dieldrin-pretreated group. In the case of the CF1 mice, the
pretreatment period did not have any effect on the faecal excretion
(51.5%), whereas, in the LACG mice, the faecal excretion of 14C
activity increased from 27.2% for the non-pretreated group to 48.8%
for the 4-week pretreated group. The total 14C activity excreted in
the urine of the two strains of mice was low (0.42 - 2.6% of dose)
compared with that in the urine of male rats (5.5 - 6.6%). In both
species of rodents, the faeces was the major route of excretion of
14C activity. In the urine of both the male CFE rat and the male
CF1 mice, the amount of the dicarboxylic acid metabolite in the
urine was small compared with that of pentachloroketone plus
dieldrin, while in the male LACG mice, the amount of the acidic
metabolite was twice that of pentachloroketone plus dieldrin. Both
strains of mice excreted, proportionally, much larger amounts of a
polar (unidentified metabolite) in the urine than did the CFE rats.
In the faeces of the male CFE rats (no pretreatment), the major
component was the 9-hydroxy derivative. This was also found by
Matthews et al. (1971). However, in both mouse strains (no
pretreatment), this compound was a minor metabolite, but it became
the major product in the dieldrin-pretreated group. In isolated
liver microsomes, most of the 14C activity appeared to be present
as dieldrin, and the 9-hydroxy metabolite was not detected.
A number of other studies on the excretion of dieldrin via
urine and/or faeces have been carried out. Dailey et al. (1970)
found that male rats excreted higher levels of 14C radioactivity
via urine and faeces than females. Davison (1973) confirmed this
in a study lasting 39 weeks. Maximal excretion of 14C activity
occurred in the 6th week in both sexes, regardless of the amount of
dieldrin given. A steady state was reached and maintained from the
6th to the 39th week.
In studies by Robinson et al. (1969), rats were fed a diet
containing 10 mg dieldrin/kg diet for 8 weeks. The decline in the
concentration of dieldrin in blood, brain, liver, and adipose
tissue was studied during the subsequent 12 weeks when a control
diet was fed. There was an initial rapid decline in the dieldrin
concentration during the first 10 days of the post-exposure period
in the blood, liver, and brain, followed by a slower decline. The
changes in the concentration of dieldrin in the brain, adipose
tissue, blood, and liver corresponded to biological half-lives of 3
to about 10 days.
When male and female rats were administered 3 g of diet
containing 10 mg 14C-dieldrin/kg diet, followed by a control diet
ad libitum, 14C activity in the kidneys of male rats was 10-fold
higher than in the female rats (the animals were killed 9 days
after administration of 14C-dieldrin). Most of the activity in the
male kidneys was due to pentachloroketone, whereas, in the female
kidneys, only dieldrin was detected (Matthews et al., 1971).
The excretion of 36Cl activity by female rats dosed
intravenously (680 µg/h for 2.5-5 h; total doses of 8 - 16 mg/kg
body weight) with 36Cl-dieldrin has been studied. The 36Cl
activity detected in the faeces was about 7 times that found in the
urine, indicating excretion via the bile (Heath & Vandekar, 1964).
Comparable results were found by Cole et al. (1970), who gave
male rats a single intravenous dose of 0.25 mg 14C dieldrin/kg body
weight. Similar doses of 14C-dieldrin were administered
intravenously to male rats with bile fistulas. About 30% of the
administered 14C activity was excreted via the bile during the
first 24 h after dosing, and after 4 days a total excretion of
about 60% had occurred. Isolated perfused rat liver preparations
were also investigated; some 20% of the original perfusate dose was
collected in the bile over a period of 8 h.
Rapid excretion of 14C-dieldrin (or its metabolites) from
isolated perfused rat livers via the bile of rats has also been
reported by Klevay (1970), the rate of excretion by male rats being
about 3 times as rapid as that by female rats.
In studies by Mueller et al. (1975a), mice, rats, rabbits,
rhesus monkeys, and one chimpanzee were given a single oral dose of
0.5 mg/kg body weight 14C-dieldrin, and urine and faeces were
collected for 10 days. For all species except the rabbit, the main
route of excretion was the faeces. The faecal excretion of
unchanged dieldrin was high in the first 48 h and then declined
rapidly. The urine samples contained only metabolites of dieldrin.
The mean total amount of radioactive material excreted (males
and/or females) in faeces and urine within 10 days after dosing
(expressed as percentage of administered dose) was 37% in mice, 11%
in rats, 2% in rabbits, 20% in rhesus monkeys, and 6% in the
chimpanzee. In all five species, 9-hydroxy-dieldrin and
4,5-aldrin- trans-dihydrodiol were the major metabolites. The
metabolism in the rat seems to be comparable to that of primates;
however, mice and rabbits showed the opening of the epoxide to diol
as the predominant reaction.
6.4.2.2 Human studies
The occurrence of a neutral metabolite of dieldrin in human
urine in amounts indicative of exposure to aldrin/dieldrin was
reported by Cueto & Hayes (1962) and Cueto & Biros (1967).
Quantitative estimates of the amounts of a metabolite of
dieldrin, 9-hydroxy-dieldrin, in the faeces of seven workmen
occupationally exposed to aldrin/dieldrin and five male members of
the general population have been made. The average concentration
of the 9-hydroxy derivative in 24-h collections of faeces of the
seven workmen was 1.74 mg/kg (range, 0.95 - 2.80 mg/kg), whereas
the average concentration in faeces of the five members of the
general population was 0.058 mg/kg (range, 0.033 - 0.12 mg/kg).
Dieldrin was present in the faeces of the workmen (average
concentration, 0.18 mg/kg), but, in samples from the general
population, it was below the limit of detection. Examination of
the urine of five of the workmen indicated that this route of
elimination of dieldrin and four known metabolites was minor. It
was concluded that the 9-hydroxy-dieldrin in the faeces represented
the major excretory pathway of dieldrin from male human beings. It
should be noted, however, that the urine was not examined for
glucuronide or other conjugates of the hydroxy metabolites). There
was good correlation between the estimated daily intake of dieldrin
(calculated from the concentrations of dieldrin in the blood) and
excretion in faeces of total equivalent dieldrin (Richardson,
1971). This relationship is based on a number of assumptions, and
it is probably more relevant that the concentration of the
9-hydroxy-dieldrin in the faeces (produced by the metabolism of
absorbed dieldrin) is significantly related to the concentration of
dieldrin in the blood, which is a measure of the body burden
arising from absorption of aldrin plus dieldrin.
When 14C-Dieldrin was applied in acetone (4 µg/cm2) once to the
forearm of volunteers, 7.7% of the applied 14C activity was
excreted in the urine over a 5-day period. A single intravenous
injection of 14C-dieldrin resulted in 3.3% being excreted in the
urine over a 5-day period (Feldman & Maibach, 1974).
6.4.3. Photodieldrin (and major metabolites of dieldrin)
6.4.3.1 Rat
In studies by Dailey et al. (1970), young rats were given daily
doses of 5 µg 14C-photodieldrin, orally or intraperitoneally, for
12 weeks. Urine and faeces were collected daily and pooled in
weekly groups. The excretion of 14C activity via the urine of
females was considerably less than that by males, by either method
of dosing. The 14C activity in urine after oral and ip
administration increased slowly during the 12 weeks (males about
10% and females 5%), the highest levels in urine (up to 33%) being
found in males dosed intraperitoneally. Faecal excretion of 14C
activity was initially lower in females, but greater during the
latter half of the study (of the order of 20 - 40%). In males,
during the whole study, it was about 30%.
6.4.3.2 Monkey
A juvenile female rhesus monkey was given daily oral doses of
2 mg 14C-photodieldrin (equivalent to 0.8 mg/kg body weight), and
the treatment was continued until, between days 70 and 76, the
daily excretion of 14C activity was in balance with the daily
intake. When dosing ceased, the animals had retained about 50% of
the cumulative dose of photodieldrin. Collection of excreta was
continued for a further 100 days, during which a further 30.1% of
the dose, administered during the 76-day period, was excreted.
During the period of dosing, a major part of the faecal 14C
excretion consisted of photodieldrin (probably indicating
incomplete absorption in the gastrointestinal tract), while
20 - 50% of the excreted activity was in the urine. After dosing
ceased, 60% of the excreted 14C activity appeared in the urine
(Nohynek et al., 1979).
In studies by Nohynek et al. (1979), one male and one female
juvenile rhesus monkey were given single intravenous doses of
4.5 mg 14C-photodieldrin (2 mg/kg body weight). Urine and faeces
were collected separately every 24 h, and the animals were killed
after 21 days. Excretion of 14C activity was high during the first
7 days (male, 39%; female, 27.3%, of the given dose). It then
decreased rapidly and reached a nearly constant value of 0.2% of
the administered dose. Approximately 45% (male) and 34% (female)
of the dose had been excreted by day 21.
6.5. Retention and Turnover
6.5.1. Non-domestic organisms
A few studies have been carried out on the uptake and
elimination of aldrin and/or dieldrin in invertebrates: marine
clams (Mya arenaria and Mercenaria mercenaria) (Butler, 1971);
naiad mollusc (Amblema plicata) (Fikes & Tubb, 1972); mussel
(Lampsilis siliquiodea) (Bedford & Zabik, 1973); crab (Leptodius
floridanus) (Epifanio, 1973); and ostracod (Chlamydotheca arcuata)
(Kawatski & Schmulbach, 1972). The concentration of aldrin or
dieldrin in organs and tissues increased rapidly during the first
1 - 2 weeks of exposure, but remained virtually constant
thereafter. When the organisms were placed in clean water, the
concentration declined in a (semi)-logarithmic manner in relation
to time. The estimated half-life for the tested organisms varied,
e.g., for Lampsilis siliquiodea, it was 4.7 days, whereas for
Amblema plicata, it was about 3 - 4 weeks.
The elimination of 14C-dieldrin from bluegills (Lepomis
macrochirus) and goldfish (Carassius auratus) was studied by
Gakstatter & Weiss (1967). The fish were exposed to 30 µg
14C-dieldrin/litre (initial concentration) until toxic symptoms
appeared (5 - 8 h), and were then placed in recovery aquaria
together with unexposed fish. The water in the recovery aquaria
was continuously renewed. Samples of five fish were taken on 10
different occasions during the recovery period. The 14C activity
in whole fish of both species, expressed as equivalent dieldrin,
declined by about 90% within 16 days, the half-time for elimination
being about 4 days. The control bluegills and goldfish accumulated
a maximum equivalent dieldrin concentration of 0.29 and 0.22 mg/kg,
respectively, on day 4 of the period in the recovery aquaria,
indicating transfer of dieldrin or derived material from
contaminated to uncontaminated fish.
In another study, the distribution of aldrin and dieldrin in
the tissues of Carassius auratus was determined following an 8-h
exposure to 14C-aldrin (50 µg/litre) in a static study. After the
exposure, fish were placed in a continuously flushed aquarium for
32 days. Dieldrin was found in all tissues examined immediately
after the exposure. The percentage of dieldrin in the total
residues in the tissue increased with time, reaching about 95% on
day 32 (except in visceral fat). During the recovery period, the
total concentration of aldrin plus dieldrin in the blood declined
from 2.1 mg/litre (as aldrin) to 0.4 mg/litre. The corresponding
changes in the brain concentrations were 5.45 mg/kg to 2.3 mg/kg.
Total residues in the nerve cord did not show a consistent decline
and varied from 4.56 to 21.6 mg/kg throughout the 32-day period;
however, these residues were determined by thin-layer
chromatography, not by gas-liquid chromatography (Gakstatter,
1968).
The partitioning of 14C activity into particulate fractions of
the brain and liver of resistant and susceptible mosquitofish has
been studied after exposure of the fish to 14C-aldrin or 14C-
dieldrin. The 14C activities in total brain, cell membrane, and
five cellular fractions were significantly higher in susceptible
fish than in resistant fish for both aldrin and dieldrin. However,
this difference was much less marked in the case of the liver. It
was suggested that a basic structural change in polarity exists in
the myelin of resistant fish, which could provide a membrane
barrier (Wells & Yarbrough, 1973).
The fate of dieldrin in the digestive tract of juvenile lake
trout (Salvelinus namaycush) has been studied. Macerated trout
flesh containing an average of 1.05 mg/kg was injected in the
stomach. The decline in the dieldrin content of the stomach was
parallel to that of the food from the stomach. Little or no
dieldrin was found in the intestines (Stewart & Stein, 1974).
In studies by Chadwick & Brocksen (1969), groups of sculpins
(Cottus perplexus) were exposed to 1.3 µg dieldrin/litre for 12
days, followed by removal to uncontaminated continuously renewed
water. The concentration in whole fish declined in a curvilinear
fashion from about 2.5 mg/kg fish to about 1 mg/kg fish in 60 days
and to about 0.5 mg/kg fish in 90 days.
Sailfin molly (Poecilia latipinna) were exposed to 12 µg
dieldrin/litre for up to 6 h by Lane et al. (1970). Two products,
thought to be metabolites of dieldrin, were detected in the liver
and other organs, and it was suggested that they were partially
dechlorinated derivatives of dieldrin.
6.5.2. Biological half-life in human beings
The concentration of dieldrin in the blood of volunteers given
oral daily doses for 2 years (section 6.2.2.4) was determined over
a period of 8 months after termination of the deliberate exposure
(Hunter et al., 1969). A small, but statistically significant,
decline occurred, corresponding to a mean value of 369 days for the
half-life of dieldrin in blood. However, there were significant
differences between the rates of decline of the individual
volunteers.
The concentration of dieldrin in the blood of 15 workmen was
determined for a period of 3 years following termination of
occupational exposure to aldrin/dieldrin (Jager, 1970). The mean
half-life was 266 days.
When a state of equilibrium has not yet been reached, the
apparent half-life will be much shorter, due mainly to a
redistribution of dieldrin between compartments in the body.
6.5.3. Body burden and (critical) organ burden; indicator media
Whatever the route of exposure, the effect, if any, will be
determined by the concentration of the chemical in the target organ
or tissue. It has been shown that the distribution between the
various tissues of mammals is fairly constant within and between
species (Robinson & Hunter, 1966; Hunter & Robinson, 1967; Hunter
et al., 1967; Robinson & Roberts, 1969; Walker et al., 1969b).
Thus, at a state of equilibrium, the dieldrin level in the blood
reflects the concentration of the active compound in the target
tissues and therefore represents the best practical parameter for
the internal exposure that is associated with a biochemical,
clinical, or pathological effect. Since the biological half-life
of dieldrin in human blood is known (266 days) (Jager, 1970),
a reliable estimation of the blood level at the time of
discontinuance of the exposure can be made. This, in turn enables,
better than anything else, the evaluation of the likelihood of an
observed symptom of disease or indisposition being associated with
exposure to dieldrin. Also, the established mathematical
relationship between the dieldrin level in the blood and the total
daily equivalent oral intake thus enables, on the basis of the
concentration of dieldrin in the blood, the evaluation of a current
exposure or an exposure of a short time ago vis-à-vis the
acceptable daily intake established by the FAO/WHO Joint Meeting on
Pesticide Residues.
Determination of the dieldrin concentration in blood is the
method of choice in monitoring exposed workers or the general
population (section 9.2.1.1).
6.6. Appraisal
Aldrin is readily absorbed through the skin, by inhalation of
the vapour, or into the circulating blood from the gastrointestinal
tract. It has not been possible to determine the percentage of an
ingested dose of aldrin or dieldrin that is actually absorbed into
the body because of the intestinal hepatic biliary cycle. Work
with human volunteers (Feldmann & Maibach, 1974) showed that
absorption through the skin amounted to 7 - 8% of the applied dose.
Inhalation studies with human volunteers (Beyermann & Eckrich,
1973; Bragt et al., 1984) suggested that about 50% of inhaled
aldrin vapour is absorbed and retained in the human body. After
absorption, it is rapidly distributed to the organs and tissues of
the body, and a continuous exchange between the blood and other
tissues takes place. In the meantime, aldrin is readily converted
to dieldrin, mainly in the liver but, to a much lesser extent, in
some other tissues, e.g., the lungs (Mehendale & El-Bassiouni,
1975).
This conversion proceeds very rapidly. The livers of even
24-h-old rats, given oral doses of 10 mg aldrin/kg body weight,
contained dieldrin 2 h after treatment (Farb et al., 1973). In the
course of the next few hours, dieldrin and what little is left of
the aldrin in blood and other tissues, concentrates more in the
lipid tissues (Heath & Vandekar, 1964; Hayes, 1974). In human
beings, aldrin is found rarely, if at all, in human blood or other
tissues, except in cases with acute poisoning by accidental or
intentional ingestion of massive doses.
Studies carried out with 14C-labelled aldrin and dieldrin have
shown that part of the ingested material is passed unabsorbed
through the intestinal tract and eliminated from the body, part is
excreted unchanged from the liver into the bile, part is stored
unchanged in the various organs and tissues (particularly the
adipose tissue), and part is metabolized in the liver to more polar
and hydrophilic metabolites. These metabolites, in human beings
and most animals, are excreted primarily via the bile in the
faeces. It had also been shown that aldrin and dieldrin are both
biodegraded into the same metabolites (Damico et al., 1968; Klein
et al., 1968). The biodegradation products have been identified in
the rat within 15 min after an intravenous injection (Moersdorf et
al., 1963). Most of the currently available information on the
biodegradation metabolism in mammals is based on studies with
dieldrin on the mouse, rat, rabbit, sheep, dog, monkey, chimpanzee,
and human beings (Ludwig et al., 1964; Datta et al., 1965; Korte,
1965; Korte & Arent, 1965; Richardson et al., 1967b, 1968; Klein et
al., 1968; Matthews & Matsumura, 1969; Baldwin et al., 1970, 1972;
Feil et al., 1970; Richardson & Robinson, 1971; Mueller et al.,
1975a,b). Although there appear to be differences between species
and, in the rat, differences between the sexes, the overall picture
shows only quantitative variations between species.
In the species studied (with the exception of the rabbit) the
major metabolite is the 9-hydroxy derivative. This is found in the
faeces and free or conjugated in the urine. Three other
metabolites have been found and identified in experimental animals:
(a) trans-6,7-dihydroxy derivative;
(b) dicarboxylic acid derived from the dihydroxy compound; and
(c) the bridged pentachloroketone (PCK).
The latter is also a metabolite of photodieldrin (Klein et al.,
1970).
Only the 9-hydroxy compound was found in the faeces of seven
occupationally exposed industrial workers (1.74 mg/kg) and five
male members of the general population (0.058 mg/kg). Neither the
9-hydroxy compound nor the other metabolites have been found in
human blood or other tissues. Dieldrin was present in the faeces
of the workmen (average 0.18 mg/kg), whereas the concentrations in
the samples from the general population were below the limits of
detection. Examination of the urine of five workmen indicated that
urinary excretion of dieldrin and its four metabolites is minor
relative to elimination of the 9-hydroxy metabolite via the faeces
(Richardson, 1971).
The conversion of aldrin to dieldrin and the distribution and
the subsequent deposition of dieldrin (mainly in lipid tissues)
proceed much faster than the biodegradation and ultimate
elimination of unchanged dieldrin and its metabolites from the
body. At a given average intake of aldrin and/or dieldrin,
dieldrin slowly accumulates in the body. This accumulation or
"storage", however, does not increase indefinitely. As the
concentration of dieldrin in the liver cells increases, the
metabolizing enzyme activity in the microsomes increases, and so
the rate of biodegradation of dieldrin, and hence the elimination
from the body, is enhanced. Thus, the accumulation proceeds at an
ever slower rate until the concentrations of dieldrin in blood and
tissues approach upper limits of storage and an amount of dieldrin
equal to the average daily intake is eliminated each day. These
upper limits of storage are related to the daily intake. This has
been demonstrated in rats and dogs (Walker et al., 1969b) and in
human beings (Hunter & Robinson, 1967; Hunter et al., 1969). When
the intake of aldrin/dieldrin ceases or decreases, the body burden
decreases. The biological half-life in human beings is 9 - 12
months (Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
Significant relationships exist between the concentrations of
dieldrin in the blood and those in other tissues of rats, dogs, and
human beings (Hunter & Robinson, 1967; Deichmann et al., 1968;
Keane & Zavon, 1969b; Hunter et al., 1969; Walker et al., 1969b).
Numerous investigations of the concentrations of dieldrin in
body fat, blood, and other tissues from members of the general
population and from special groups have been carried out in several
countries. The results are summarized and discussed in section
5.2. The ratio of dieldrin concentrations in fat, liver, brain,
blood is about 150:15:3:1.
Dieldrin penetrates the placenta and is present in the blood,
fat, or other organs of the fetus, newborn babies, and infants
(Table 22). The concentrations are much lower (by 50% or more)
than those in adults. The ratio of dieldrin concentrations in
blood, brain, liver, and fat in infants is not different from that
ratio in adults (Fiserova-Bergerova et al., 1967; Casarett et al.,
1968). Dieldrin is excreted in mother's milk, average values being
about 3 - 5 µg/litre mother's milk (Table 23). The ratio of the
dieldrin concentration in mother's blood to that in mother's milk
is about 1:2 - 3.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
Neither aldrin nor dieldrin have significant effects on
populations of microorganisms in soil or fresh water at realistic
concentrations. Some physiological processes of microorganisms are
affected by low concentrations of both aldrin and dieldrin, but
these would appear to have little or no environmental significance.
Aldrin and dieldrin have only minor deleterious effects on soil
bacterial populations, even at concentrations that are much higher
than those used in agricultural practice.
The effects of insecticides on soil microbes have been reviewed
by Tu & Miles (1976). Of 15 strains tested, aldrin did not have
any effect on the growth of 11 bacterial species (single cultures)
but caused some growth inhibition in four species. Dieldrin did
not have any effect on 13 bacterial species but had some inhibitory
effect in two species. Neither aldrin nor dieldrin at 2000 mg/kg
soil had effects on bacteria in laboratory studies; soil fungi were
also little affected. In pot studies, using aldrin at 4 and 120
mg/kg soil, there were no quantitative changes in bacteria during
any part of the vegetative period. Aldrin inhibited the growth of
Rhizoctonia solani in plate cultures by 20% or more at 6.2 mg/litre
and higher concentrations. Dieldrin was less toxic, producing an
average inhibition of about 15%, which was not dose related over
the range of 1 - 100 mg/litre. The evolution of carbon dioxide
(CO2) (a measure of soil organisms respiration) was significantly
reduced by dieldrin at 1000 mg/kg soil (but not at 100 mg/kg),
whereas aldrin produced a significant reduction at concentrations
as low as 25 mg/kg soil. Slight effects on nitrification were
initially found when aldrin and dieldrin were incorporated at 2000
mg/kg in a sandy loam soil, but nitrification was normal after
about 10 weeks. Short-term inhibition of nitrification was also
produced by aldrin and dieldrin at 25 mg/kg in a sandy loam soil
(aldrin for 1 week, dieldrin for 2 weeks). Decreased sulfur
oxidation was observed in soil containing aldrin or dieldrin (2000
mg/kg), the inhibition decreasing considerably after 3 months.
Five annual applications of aldrin or dieldrin (5.5 - 22 kg/ha) to
a Ramona sandy loam had no measurable effect on the numbers of soil
bacteria or fungi, did not influence the ability of the soil
population to decompose plant residue, and did not alter soil
aggregation.
The effect of dieldrin on the activities of three soil enzymes
was determined at concentrations of 5 or 10 mg dieldrin/kg soil by
Tu (1981). The dehydrogenase activity of the dieldrin-treated soil
(10 mg/kg) did not differ from controls, whereas at 5 mg
dieldrin/kg, the activity was significantly greater than controls
after 2 weeks (50% increase). Urease activity at both treatment
levels was significantly reduced after a 1-week incubation but
significantly increased after 2 weeks. Phosphatase activity was
significantly reduced at 5 mg dieldrin/kg, but not at 10 mg/kg.
The 96-h EC50 (growth) for algae (Chlamydomonas sp.,
Phaeodactylum tricornutum, Dunaliella sp., Chlorella ovalis, and
Chlorella pyrenoidosa) was > 100 µg dieldrin/litre (Adema & Vink,
1981).
The photosynthetic activity of four species of marine
phytoplankton in the presence of dieldrin was investigated using
14C-labelled Na2CO3. A range of nominal concentrations
(0.01 - 1000 µg/litre) was used, and the plant cultures were
exposed for 24 h. The 14C uptake of Dunaliella tertiolecta during
7 days post-treatment was unaffected by up to 1000 µg
dieldrin/litre. Two other species (Skeletonema costatum and
Coccolithus huxleyi) showed significant reductions in 14C uptake at
levels of dieldrin above 10 µg/litre, and the photosynthetic
activity of Cyclotella nana was reduced at concentrations above 1
µg dieldrin/litre (Menzel et al., 1970).
In studies by Schauberger & Wildman (1977), three species of
fresh-water algae (Anabaena cylindrica, Anacystis nidulans, Nostoc
muscorum) were exposed to aldrin or dieldrin at concentrations of
0 - 1000 µg/litre. After exposure for 7 days, there was no
significant effect on the photosynthetic pigment absorption of the
three species at concentrations up to 10 µg (nominal)/litre.
However, at 1 mg/litre, aldrin almost completely suppressed the
absorption by photosynthetic pigments (chlorophyll and
phycocyanin), these being indicators of physiological health and
growth. Dieldrin (1 mg/litre) produced a reduction of about 40%.
The growth response of two cyanobacteria (blue-green algae) in
the presence of aldrin, dieldrin, or two metabolites of dieldrin,
photoaldrin, or photodieldrin was determined at nominal
concentrations of 0.2 - 950 µg/litre (Batterton et al., 1971).
None of these compounds had significant effects on the growth rate
constants at concentrations of 95 µg/litre or at lower
concentrations over periods of 26 - 30 h. The investigators
considered that dieldrin and its derivatives reduced the growth
rate constant at 475 and 950 µg/litre, Agmenellum quadriplicatum
being more sensitive than Anacystis nidulans. Aldrin did not have
a significant effect on either species, but photoaldrin affected
Agmenellum quadriplicatum at 950 µg/litre.
In studies by Powers et al. (1977), a marine dinoflagellate
(Exuviella baltica) was incubated with dieldrin (0.1, 1, or 10 µg
(nominal)/litre), and the numbers of cells were counted during a
period of 6 days. No adverse effects on optical counts were
observed at the two lower concentrations, but there was a marked
reduction in the size and number of cells at 10 µg dieldrin/litre.
7.2. Aquatic Organisms
The toxicity of aldrin and dieldrin to aquatic invertebrates is
very variable. For some species both compounds are highly toxic,
whereas for others there is no effect until the compounds are
dissolved to artificially high concentrations, many times their
solubility in water. Both aldrin and dieldrin are highly toxic to
most species of fish in laboratory tests, with acute LC50 values
well within the solubility of the compounds. It should be borne in
mind that aldrin and dieldrin are strongly bound to particulate
matter in water, which reduces their availability to aquatic
organisms and, in consequence, their potential toxicity.
7.2.1. Aquatic invertebrates
7.2.1.1 Acute toxicity
A convenient overview, in graphical format, of the toxicity of
aldrin and dieldrin to many aquatic organisms was produced by Craig
(1977). The 96-h LC50 values of aldrin and dieldrin for
crustaceans and molluscs were in the range 0.2 - 10 000 µg/litre.
Dieldrin is moderately toxic to fresh-water annelids
(4000 - 7000 µg/litre) and molluscs (> 100 - 640 µg/litre).
Insects are the most sensitive group (aldrin, 1 - 200 µg/litre;
dieldrin, 0.2 - 40 µg/litre). The values for a number of species
are given in Table 25.
7.2.1.2 Short-term toxicity, reproduction, and behaviour
(a) Short-term toxicity
When naiads of two species of stonefly were exposed for 30 days
in a continuous-flow system, the 30-day LC50s for aldrin and
dieldrin were, respectively, 2.5 and 2 µg/litre for Pteronarcys
californica, and 22 and 0.2 µg/litre for Acroneuria pacifica
(Jensen & Gaufin, 1966).
The LC50 for adult molluscs (Mytilus edulis and Dreissena
polymorpha) exposed for 3 - 4 weeks was 180 - 200 µg dieldrin/litre
(Adema & Vink, 1981).
McLeese et al. (1982) exposed polychaete worms (Nereis vireus)
to dieldrin in sea water or sediment for 12 days. The LC50 in sea
water was > 170 µg/litre (in surficial water > 20 µg/litre; in
sediment > 13 mg/kg).
Table 26 gives the LC50 values for a number of invertebrate
species.
(b) Reproduction
The effects of dieldrin on the embryonic development of the
American oyster (Crassostrea virginica) and of aldrin on that of
the hard clam (Mercenaria mercenaria) were studied by Davis & Hidu
(1969). Table 27 gives the concentrations producing approximately
50% reduction in the development of fertilized eggs during 48 h,
those producing about 50% reduction in larval survival during 12
days (clams) or 14 days (oysters), and the effects on larval growth
during 10 or 12 days of exposure (expressed as a percentage of
growth of control larvae).
Table 25. Acute toxicity of aldrin and dieldrin for aquatic invertebrates
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Daphnids
Daphnia magna (29)a 330a Anderson (1959)
Simocephalus first instar dispersed via 15 (23)a (240)a Johnson & Finley
serrulatus acetone 21 (32)a (1980)
Daphnia pulex first instar dispersed 15 (28)a (190)a Johnson & Finley
(1980)
Crustacea
Seed shrimp mature dispersed via 21 (18)a - Johnson & Finley
(Cypridopsis acetone (1980)
vidua)
Sowbug (Asellus mature dispersed via 21 - 5 Johnson & Finley
brevicaudus) acetone (1980)
Scud (Gammarus mature dispersed via 21 4300 640 Johnson & Finley
fasciatus) acetone (1980)
Sand shrimp 0.25 g, dispersed via 20 8 7 Eisler (1969)
(Crangon 2.6 cm acetone
septemspinosa)
2 g dispersed via 20 - 0.4 McLeese & Metcalfe
hexane (1980)
2 g dispersed in 10 - 4.1 McLeese & Metcalfe
sediment (1980)
Grass shrimp 0.47 g, dispersed via 20 9 50 Eisler (1969)
(Palaemonetes 3.1 cm acetone
vulgaris)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Crustacea (contd.)
Grass shrimp mature dispersed via 21 50 - Johnson & Finley
(Palaemonetes acetone (1980)
kadiakensis)
Crayfish mature dispersed via 21 - 740 Johnson & Finley
(Orconectes acetone (1980)
nais)
Hermit crab 0.28 g, dispersed via 20 33 18 Eisler (1969)
(Pagurus 0.35 cm acetone
longicarpus)
Molluscs
Mercenaria egg dispersed via 24 (> 10 000)a - Davis & Hidu (1969)
mercenaria acetone
Crassostrea egg dispersed via 24 - (640)a Davis & Hidu (1969)
virginica acetone
Slipper limpet veliger - - - > 100 Adema & Vink (1981)
(Crepidula
fornicata)
Pond snail egg - - - > 200 Adema & Vink (1981)
(Lymnaea juvenile
stagnalis)
Insects
Pteronarcys naiad, dispersed via 15.5 1.3 0.5 Sanders & Cope
californica 3-3.5 cm ethanol (1968); Johnson &
Finley (1980)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Insects (contd.)
Pteronarcella naiad, dispersed via 15.5 - 0.5 Sanders & Cope
badia 1.5-2 cm ethanol (1968); Johnson &
Finley (1980)
Claassenia naiad, dispersed 15.5 - 0.6 Sanders & Cope
sabulosa 2-2.5 cm (1968); Johnson &
Finley (1980)
Pteronarcys naiad, 2-5 cm dispersed via 12.8 180 39 Jensen & Gaufin
californica acetone (1966)
Acroneuria naiad, dispersed via 12.8 143 24 Jensen & Gaufin
pacifica 2-2.5 cm acetone (1966)
Damselfly juvenile dispersed via 24 - 12 Johnson & Finley
(Ischnura acetone (1980)
venticalis)
Other invertebrates
Bristle worm 2-3-day-old dispersed via 21 - > 100 Hooftman & Vink
(Ophryotrocha larva acetone (1980)
diadema)
4-week-old dispersed via 21 - > 100 Hooftman & Vink
adult worm acetone (1980)
---------------------------------------------------------------------------------------------------------
a Values in parentheses are the 48-h LC50.
Table 26. Short-term LC50s of dieldrin in invertebrates
-------------------------------------------------------------------
Species Stage LC50 at end of Reference
study (µg/litre)
(time of exposure)
-------------------------------------------------------------------
Ophryotrocha larva >10 Hooftman & Vink
diadema (2-3 days) (5-6 weeks) (1980)
adult 60 Hooftman & Vink
(4 weeks) (5-6 weeks) (1980)
Daphnia magna larva 100 Adema & Vink
(3 weeks) (1981)
adult 200 Adema & Vink
(0.3 cm) (7 days) (1981)
Artemia salina larva 40 Adema & Vink
(4 weeks) (1981)
adult 50 (male) Adema & Vink
(1 cm) (7 days) (1981)
110 (female)
(7 days)
Chaetogammarus larva 1.8 Adema & Vink
marinus (4 weeks) (1981)
adult 3.6 Adema & Vink
(1 cm) (14 days) (1981)
Palaemonetes adult 0.3 Adema & Vink
varians (4 cm) (7 days) (1981)
Crangon crangon adult 4 Adema & Vink
(4 cm) (14 days) (1981)
-------------------------------------------------------------------
When adult mud snails (Nassa obsoleta) were exposed to up to
10 000 µg dieldrin/litre for 96 h, and then transferred to
dieldrin-free sea water for 33 days, no mortality occurred
throughout the study and the length of the animals was normal after
33 days. There was a significant increase in total egg deposition
during the 33-day post-treatment period in the case of snails
exposed to 10 µg dieldrin/litre, but there was a significant
reduction at 100, 1000, and 10 000 µg dieldrin/litre (Eisler,
1970).
(c) Behaviour
In studies by Klein & Lincer (1974), fiddler crabs, (Uca
pugilator) were fed diets containing 0, 0.1, 1, 10, and 50 mg
dieldrin/kg diet for 14 days and observed for another 25 days.
Behaviour, measured as righting response, was modified at dose
levels of 1 mg/kg or more, and even in the group given 0.1 mg/kg,
difficulty in righting was seen after 11 days. With 10 and 50
mg/kg diet, an increase in mortality was observed, but not with 1
mg/kg diet.
Table 27. Concentrations producing about 50%
reduction in the development of fertilized eggs
during 48 h, in larval survival during 12 days
(clams) or 14 days (oysters), and effects on
larval growth during 10 or 12 days exposurea
(Davis & Hidu, 1969)
--------------------------------------------------
Organism Effect Aldrin Dieldrin
(µg/litre) (µg/litre)
--------------------------------------------------
Clam development of > 10 000 -
Oyster fertilized eggs - 640
Clam larval survival 410 -
Oyster - > 10 000
Clam larval growth 250b -
Oyster - 500c
--------------------------------------------------
a Expressed as a percentage of growth of control
larvae.
b 80% reduction.
c 50% reduction.
7.2.2. Fish
7.2.2.1 Acute toxicity
Both aldrin and dieldrin are highly toxic to fish under
laboratory conditions. A summary of reported 96-h LC50 values for
fresh water and marine species is given in Table 28. In parallel
studies, dieldrin was consistently more toxic than aldrin. The
96-h LC50s range from 2.2 to 53 µg aldrin/litre and from 1.1 to 41
µg dieldrin/litre in various fish species. It should be noted that
the range for aldrin exceeds the water solubility of the compound.
The results of studies by Macek et al. (1969) indicate that a
rise in temperature increases the toxicity of aldrin and dieldrin
for bluegills and rainbow trout. However, Johnson & Finley (1980)
stated that toxicity was not appreciably (only a factor of 2)
changed by variations in temperature or water hardness.
Macek (1975) investigated the effects of simultaneous exposure
of bluegills to DDT and dieldrin and concluded that the acute
toxicity of dieldrin in the concentration range 5.9 - 6.6 µg/litre
was not increased by the presence of DDT (concentration range,
4.5 - 5 µg/litre).
Anderson & Weber (1975) found that new-born and juvenile
guppies (Lebistes reticulatus) were more resistant to dieldrin
than adults. A relationship between the LC50 and body weight was
derived for mature, juvenile, and new-born guppies:
LC50 = aWb
where W is the body weight. The best fit value for the exponent b
was 0.81.
Table 28. Acute toxicity of aldrin and dieldrin for fish
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water
Rainbow trout 0.6 dispersed 13 2.6 - Johnson &
(Salmo via acetone Finley (1980)
gairdneri)
1.4 dispersed 13 - 1.2 Johnson &
via acetone Finley (1980)
3.2 dispersed 20 17.7 9.9 Katz (1961)
via acetone
0.6-1.5 dispersed 1.6 3.2 2.4 Macek et al.
via acetone 7.2 3.3 1.1 (1969)
12.7 2.2 1.4
Cutthroat trout 1.1 dispersed 9 - 6a Johnson &
(Salmo clarki) via acetone Finley (1980)
Chinook salmon 1.45-5 dispersed 20 7.5 6.1 Katz (1961)
(Oncorhynchus via acetone
tshawytscha)
0.8 dispersed 15 14.3 - Johnson &
via acetone Finley (1980)
Coho salmon 2.7-4.1 dispersed 20 45.9 10.8 Katz (1961)
(Oncorhynchus via acetone
kisutch)
Goldfish 1-2 dispersed 25 32 41 Henderson et al.
(Carrassius via acetone (1959)
auratus)
Goldfish 1 dispersed 18 - 1.8 Johnson &
(Carassius via acetone Finley (1980)
auratus)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water (contd.)
Carp (Cyprinus NA NA 20 4b - Rehwoldt et al.
carpio) (1977)
Fathead minnow 0.6 dispersed 18 8.2 3.8 Johnson &
(Pimephales via acetone Finley (1980)
promelas)
1-2 dispersed 25 32 18 Henderson et al.
via acetone (1959)
Guppy (Lebistes 0.1-0.2 dispersed 25 37 25 Henderson et al.
reticulatus) via acetone (1959)
NAd NA 20 20b - Rehwoldt et al.
(1977)
NA NA 24 - 3.2-7 Adema & Vink
(young) (1981)
NA NA 24 - 35c Adema & Vink
(adult) (1981)
juvenile NA 25 10.9 Anderson & Weber
(1975)
newborns NA 36.7 Anderson & Weber
(1975)
Black bullhead 1.5 dispersed 24 19 - Johnson & Finley
(Ictalurus via acetone (1980)
melas)
Channel catfish 5.2 dispersed 18 53 - Johnson & Finley
(Ictalurus via acetone (1980)
punctatus)
1.4 dispersed 18 - 4.5 Johnson & Finley
via acetone (1980)
Bluegill 0.7 dispersed 18 6.2 - Johnson & Finley
(Lepomis via acetone (1980)
macrochirus)
1.3 dispersed 18 - 3.1 Johnson & Finley
via acetone (1980)
1-2 dispersed 25 15 8.8 Henderson et al.
via acetone (1959)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Bluegill 0.6-1.5 dispersed 12.7 7.7 17 Macek et al.
(contd.) via acetone 18.3 5.8 14 (1969)
23.8 4.6 8.8
Pumpkinseed NA NA 20 20b - Rehwoldt et al.
sunfish (1977)
(Lepomis
gibbosus)
Largemouth bass 2.5 dispersed 18 5 3.5 Johnson & Finley
(Micropterus via acetone (1980)
salmoides)
Striped bass NA NA 20 10b - Rehwoldt et al.
(Marone (1977)
saxatilis)
Banded killyfish NA NA 20 21b - Rehwoldt et al.
(Fundulus (1977)
diaphanus)
White perch NA NA 20 42b - Rehwoldt et al.
(Roccus (1977)
americanus)
American eel NA NA 20 16b - Rehwoldt et al.
(Anguilla (1977)
rostrata)
Marine species
Common goby NA NA 15 - 3.5 Adema & Vink
(Gobius microps) (adult) (1981)
Plaice length: NA 15 - 1.7 Adema & Vink
(Pleuronectes 2-3 cm (1981)
platessa)
length: NA 15 - 4 Adema & Vink
10 cm (1981)
yolk-sac NA 5-10 - 30 Adema & Vink
larva (1981)
egg-metam NA 5-10 - > 32 Adema & Vink
larva (1981)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Marine species (contd.)
Threespine 0.4-0.8 dispersed 20 27.4 13.1 Katz (1961)
stickleback via acetone
(Gasterosteus
aculeatus)
------------------------------------------------------------------------------------------
a Hardness = 162 mg CaCO3/litre.
b Hardness = 50 mg CaCO3/litre.
c 48-h LC50.
d NA = not available.
7.2.2.2 Long-term toxicity
Sailfin mollies (Lebistes latipinna) were exposed in groups of
20 to 0, 0.75, 1.5, 3, 6, or 12 µg dieldrin/litre using a flow-
through system for 34 weeks. The mortality of the 0.75 µg/litre
group was similar to that of the control group. At 1.5 µg/litre,
there was an increase in mortality, and, at 3 µg/litre or more,
100% mortality occurred. The growth rates and reproduction
performances were adversely affected in the surviving fish (Lane &
Livingstone, 1970).
Rainbow trout (Salmo gairdneri) were fed food containing
dieldrin for 240 days, the nominal dietary concentrations
corresponding to 14, 43, 143, or 430 µg dieldrin/kg body weight per
day. The growth rate was not affected at any of the concentrations
throughout the 240 days, and there was no mortality or visible
adverse effects. The activities of liver glutamate-pyruvate
transaminase (GPT) and glutamate-oxaloacetate transaminase (GOT)
were not affected, except, in the case of the latter, at the
highest dose level. Liver glutamate dehydrogenase (GDH) activity
was increased at all dose levels. Electron micrographs of liver
cells demonstrated changes in mitochondrial morphology, the highest
dose causing swelling and membrane disruption. Since GDH is an
intramitochondrial enzyme, examination by electron microscopy gave
further evidence that dieldrin altered mitochondrial metabolism.
In the brain, GOT activity was significantly decreased at 43 µg/kg
and GTP was decreased at 14 µg/kg or more. At all dose levels,
brain GDH was decreased and brain glutamine transferase (GT) was
increased. Electron microscopy of the medulla and cerebral
hemispheres did not show any effects of dieldrin. The
concentrations of 16 free amino acids in the brain were determined.
The concentrations of four were not significantly changed, whereas
eight were significantly altered at 143 µg dieldrin/kg and 12 at
430 µg/kg. Serum ammonia concentrations were significantly
increased at 143 and 430 µg dieldrin/kg, but the concentration of
ammonia in the brain was not affected. The increase in brain GT
was considered to be a possible reason for this lack of effect on
brain ammonia, since it compensated for the decrease in GDH
activity. Alternatively, brain ammonia may have been transported
via the blood to the liver with consequent effects on the liver.
The ammonia-detoxifying mechanism of fish seemed to be very
sensitive to dieldrin, the no-effect dose being below 14 µg/kg body
weight per day (equivalent to 0.36 mg/kg food) (Mehrle &
Bloomfield, 1974).
Several studies have described the influence of aldrin and/or
dieldrin on enzymes such as mitochondrial succinic hydrogenase, the
epoxidative activities of liver microsomes and the ATPase activity
of microsomes of the gills or brain (Chan et al., 1967; Davis et
al., 1972; Moffet & Yarbrough, 1972; Yap et al., 1975).
Furthermore, the influence of dieldrin during thermal stress has
been studied in darters (Etheostoma nigrum) (Silbergeld, 1973).
In studies by Verma & Tonk (1984), Heteropneustes
(Saccobranchus) fossilis was exposed to aldrin for 30 days at a
concentration of 0.03 mg/litre. Respiration, haematological
parameters, and the activity of two enzymes in liver, kidneys, and
gills were determined. The respiration rate decreased and the
blood concentrations of glucose, sodium, and chloride ions showed
significant increases. The cholesterol content and clotting time
were decreased, and the ATPase activity in the three tissues was
significantly reduced.
7.2.2.3 Reproduction
Van Leeuwen (1986) carried out studies with dieldrin to study
the susceptibility of early-life stages of rainbow trout. The test
was performed with fertilized eggs before and after water
hardening, and with early eye point eggs, late eye point eggs, sac
fry, and early fry. No mortality was found in the different early-
life stages using concentrations greater than aqueous solubility
(> 10 mg/litre), except for the early fry where a very low 96-h
LC50 of 0.003 mg/litre was found.
In studies by Cairns et al. (1967), nine populations of guppies
(Lebistes reticulatus) were exposed in a semi-static system to
nominal concentrations of 0 (three populations), 1.8, 5.6, and 10
µg dieldrin/litre (two populations of each) for 14 months. During
the first 2 - 3 months, the exposed populations in five tanks
developed greater numbers of individuals (mature, immature, and
fry) than did the controls (except one population at 5.6 µg/litre,
which was similar to the controls). The difference between the
controls and five treatment groups was attributed to the higher
predation and harassment observed in the control groups. The total
numbers of individuals in control and treatment groups became
similar during the final 6 - 8 months of the study. The average
total monthly body weights of the groups treated with 1.8 µg/litre
and 5.6 µg/litre began to increase steadily after about 8 months,
whereas the total monthly body weights of the group exposed to 10
µg/litre were similar to the controls throughout the 14 months of
the study. The production of fry by one of the groups treated with
10 µg/litre declined markedly after the thirty-second week of the
study, no new broods of fry being born after the forty-second week.
No such marked decline occurred in the other five treatment groups
(including one population exposed to 10 µg/litre).
Chadwick & Shumway (1970), conducted studies lasting 130 days
on rainbow trout (Salmo gairdneri) to determine survival from time
of fertilization through to hatching in continuously cycled water.
Embryos, alevins, and fry were exposed to dieldrin concentrations
ranging from 0.012 to 52 µg/litre. Eggs (embryos) exposed to up to
52 µg dieldrin/litre from the time of fertilization survived until
hatching as well as controls, but the mean weight of newly-hatched
alevins (minus yolk material) was reduced by higher concentrations
(not specified). Alevins were more susceptible than embryos.
Their survival was reduced at all concentrations above 0.39
µg/litre. Trout fry, whose survival was unaffected at dieldrin
levels of 0.12 µg or less, quickly succumbed at concentrations of
0.39 µg/litre or more.
Smith & Cole (1973), exposed adult winter flounder (Pseudo-
pleuronectes americanus) to 2 µg/litre in aquaria continuously
supplied with filtered sea water. When fish became ripe, they were
artificially spawned, and approximately 30 000 eggs were collected
from each of the 24 spawning pairs and cultured. The remaining
eggs were analysed. The percentage fertilization of eggs
containing 0.61 mg dieldrin/kg or less was 99% (controls, 97.8%).
The percentage fertilization of eggs containing 1.21 mg dieldrin/kg
was 12%, and all the eggs containing 1.74 mg/kg were infertile.
There was no effect on egg development except in the case of the
two groups of eggs containing the higher concentrations of
dieldrin. The effects on egg mortality were not due to dieldrin in
the gametes, and the milt of exposed male flounders contained no
detectable residue of dieldrin.
7.2.3. Amphibia and reptiles
The LC50 values for tadpoles of two species of frogs (1 week
old) and toads (4 - 5 weeks old) were determined by Sanders (1970).
The 96-h LC50 (at 15.5 °C) of dieldrin for Western chorus frog
tadpoles (Pseudacris triseriata) was 100 µg/litre, whereas that of
both aldrin and dieldrin for Fowler's toad tadpoles (Bufo
woodhousii fowleri) was 150 µg/litre.
Cooke (1972) studied the effect of dieldrin at nominal
concentrations of 0.008, 0.02, or 0.5 mg/litre on groups of 40
common frog (Rana temporaria) or toad (Bufo bufo) tadpoles with
hindlimb paddles or hind legs. The exposure was for 24 or 48 h in
amphibian saline, and the observation period 5 or 15 days. At the
highest dose level, the frogs showed an increased mortality, the
mean dieldrin content being 42.9 mg/kg tissue. At the two lower
dose levels (0.008 and 0.02), there were 0.31 and 6.1 mg/kg
dieldrin in tissues, respectively. When toad tadpoles were exposed
to 0.02 or 0.5 mg/litre, the animals with the higher dose level
showed clear behavioural and structural abnormalities and a reduced
rate of development, but these changes returned to normal a few
days after exposure. The mean dieldrin content was 138 mg/kg
tissue at a dose level of 0.5 mg/litre.
The in vitro exposure of toad embryo tissue (Bufo arenarum) to
dieldrin (4 x 10-5 mol/litre) produced an inhibition of acetyl and
butyryl cholinesterase activity. In in vivo studies with open-
mouth stage embryos, dieldrin produced acetyl cholinesterase
inhibition at 0.5 x 10-6 mol/litre. Furthermore, hyperactivity in
swimming larvae was observed (de Llamas et al., 1985).
The in vitro activity of ATPase in a number of tissues of the
male turtle (Graptemys geographica) was determined by Wells et al.
(1974). There was no consistent dose relationship for either aldrin
or dieldrin, except perhaps in the case of the cloacal bladder in
the aldrin treatments. The inhibition of Na/K/Mg ATPase by aldrin
and dieldrin was in the range of 4 - 13%. It was suggested that
aldrin and dieldrin may affect the transport of metabolites across
the cellular membranes as a result of decreased energy for active
transport.
7.3. Terrestrial Organisms
7.3.1. Higher plants
Dieldrin has low phytotoxicity, tomatoes and cucumber, for
example, being affected only at application rates greater than 22
kg/ha. Aldrin affects some crops at rates greater than 22 kg/ha,
beans and cereals being most sensitive. Tomatoes and cucumbers are
sensitive to aldrin but only at unrealistically high application
rates (Edwards, 1965).
Studies in greenhouses showed that aldrin, administered weekly
as an emulsifiable concentrate at a rate of 16 kg active
ingredient/ha to 2 - 3-week-old seedlings of tomato, cauliflower,
and Chinese cabbage, inhibited root development and reduced growth
rate of cauliflower and Chinese cabbage seedlings. A ten-fold
reduction in the aldrin level failed to produce these effects
(Hagley, 1965).
Aldrin and dieldrin at 11 kg active ingredient/ha had no effect
on the emergence, growth, yield, or chemical composition of
soybeans (Probst & Everly, 1957).
7.3.2. Earthworms
In studies by Cathey (1982), earthworms (Lumbricus terrestris)
were maintained in an artificial nutritionally complete soil, based
on shredded paper containing aldrin. The LC50 value (6-week
exposure) was 60 mg aldrin/kg bedding, and the tolerance level,
producing less than 1% mortality, was 13 mg aldrin/kg bedding.
When aldrin (2.5 - 4.6 kg/ha) was applied as a spray or dust,
respectively, to the surface of soil plots and incorporated into
the soil, the numbers of earthworms in treated plots were either
similar to or greater than those in control plots (Edwards et al.,
1967; Griffiths et al., 1967; Edwards & Lofty, 1977).
7.3.3. Bees and other beneficial insects
In a review of five investigations on the toxicity of aldrin
and dieldrin to honey bees (Sanger, 1959), the oral LD50 values for
aldrin ranged from 0.24 to 0.45 µg/bee, while the values for
dieldrin were in the range 0.15 - 0.32 µg/bee. Contact LC50 values
were 0.15 - 0.8 µg/bee for aldrin and 0.15 - 0.41 µg/bee for
dieldrin.
Cowie (1967) reported an oral LD50 of 0.3 µg dieldrin/bee
(range, 0.13 - 0.54) and a contact LD50 of 0.21 µg/bee.
The toxicity of dieldrin to two important predators of cotton
pests was investigated by Burke (1959). The contact LD50 value for
Hippodamia convergens was 1.6 mg/g body weight.
In a review of the effects of pesticides on soil fauna, it was
concluded that aldrin (and, by implication, dieldrin) is relatively
non-toxic for predatory mites ( Acarina spp.), and that this may
contribute to its success as a soil insecticide (Edwards &
Thompson, 1973).
7.3.4. Birds
7.3.4.1 Acute toxicity
Estimates of the LD50 values for several species of birds are
given in Table 29. The variation in acute oral toxicity of
dieldrin among six species of birds tested by Tucker & Haegele
(1971) was more than ten fold.
Table 29. Acute oral toxicity of aldrin and dieldrin for avian
speciesa
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Fulvous whistling duck male: female: Tucker & Crabtree
(Dendocygna bicolor) 29.2 100-200 (1970)
Mallard duck female: female: Tucker & Crabtree
(Anas platyrhynchos) 520 381 (1970)
Canada goose 50-150 Tucker & Crabtree
(Branta canadensis) (1970)
-------------------------------------------------------------------
Table 29. (contd.)
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Domestic fowl 25.5 43 Sherman &
(Gallus domesticus) Rosenberg (1953)
Japanese quail male: Tucker & Crabtree
(Coturnix coturnix 69.7 (1970)
japonica)
Bobwhite quail female: Tucker & Crabtree
(Colinus virginianus) 6.6 (1970)
California quail 8.7 Hudson et al.
(Callipepla californica) (1984)
Gray partridge female: Tucker & Crabtree
(Perdix perdix) 8.8 (1970)
Chukar partridge 23.4 Tucker & Crabtree
(Alectoris graeca) (1970)
Sharp-tailed grouse male: McEwen & Brown
(Pedioecetes phasianellus) 6.9 (1966)
Ring-necked pheasant female: female: Tucker & Crabtree
(Phasianus colchicus) 16.8 79 (1970)
Pigeon 55 67 Turtle et al.
(Columba livia) (1963)
26.6 Tucker & Crabtree
(1970)
House sparrow female: Tucker & Crabtree
(Passer domesticus) 47.6 (1970)
-------------------------------------------------------------------
a Details concerning age and weight of birds are not summarized
here but can found in the original publications.
7.3.4.2 Short- and long-term toxicity
Values for the subacute LC50s of aldrin and dieldrin,
determined using the procedure developed at the Patuxent Wildlife
Centre (Hill et al., 1975), are given in Table 30. The LC50 values
of aldrin and dieldrin for each of the four species tested were of
the same order. The annual variations in the LC50 of dieldrin over
a period of up to 8 years for these four species have been
investigated by Hill et al. (1977) (18 times per species). No
time-related changes in LC50 values were found for any of the
species. However, differences were found between birds of
different ages in some species, e.g., Japanese quail and mallards
(Hudson et al., 1984). There were also differences between the
slopes of the average regression lines for the four species. These
authors emphasized the need to evaluate both the LC50 and the slope
of the regression line. Food consumption was reduced by aldrin or
dieldrin in the diet.
Table 30. Subacute dietary toxicity of aldrin and dieldrin
for avian speciesa
-----------------------------------------------------------
Species Age LC50 (95% confidence limits)
(days) ----------------------------
Aldrin Dieldrin
(mg/kg diet)
-----------------------------------------------------------
Mallard duck 5 155 153
(Anas platyrhynchos) (129-186) (123-196)
10 - 169
Japanese quail 14 34 62
(Coturnix coturnix (28-41) (53-71)
japonica)
Bobwhite quail 14 37 37
(Colinus virginianus) (33-41) (30-46)
Ring-necked pheasant 10 57 58
(Phasianus colchicus) (50-64) (51-67)
-----------------------------------------------------------
a Aldrin or dieldrin fed for 5 days followed by 3 days of
untreated diet.
Wiese et al. (1969) fed diets containing up to 500 mg technical
dieldrin (85%)/kg diet to male and female 6-month-old crowned
guinea-fowl (Numida meleagris). None of the birds fed 1.5 mg/kg
for 21 months died. The median survival time for birds fed 5 mg/kg
was 524 days; for birds fed 150 and 500 mg/kg, it was 3 and 1 days,
respectively. No differences in susceptibility between males and
females were found.
The subacute toxicities of technical dieldrin (85%) to three
species of birds are given in Table 31 (Basson, 1971).
7.3.4.3 Reproductive studies
The first experimental studies of the effects of aldrin or
dieldrin on avian reproduction showed that these compounds were
toxic for quail and pheasants. Quail fed a diet containing aldrin
or dieldrin at a toxic dose of 0.5 or 1 mg/kg diet did not show any
clear effects on egg production, percentage fertility, or
percentage hatchability (the birds had not been exposed earlier to
the compounds) (DeWitt, 1955, 1956). No significant effect was
found on the fertility or hatchability of eggs of pheasants fed
25 mg dieldrin/kg diet, but at 50 mg/kg there was a clear effect
(Genelly & Rudd, 1956).
Table 31. Subacute toxicity of technical dieldrin for three species
of birds
-------------------------------------------------------------------
Species Concentration Median time Median lethal
(mg/kg diet) till death dose (mg
(days) dieldrin/kg
body weight)
-------------------------------------------------------------------
Guinea fowl 20 72 72.4
(Numida meleagris) 150 12 11.2
Laughing dove 5 49 15.8
(Stigmatopelia 90 4.7 17.3
senegalensis)
Sparrow (Passer 5 85.1 > 41
melanurus melanurus) 45 7 43.8
-------------------------------------------------------------------
Eggs from chickens fed 1 mg aldrin or dieldrin/kg diet for
2 years showed normal fertility and hatchability, although the
concentrations of dieldrin in the yolks of the eggs were in the
range of 6 to 25 mg/kg. The fertility and hatchability slightly
decreased at 10 mg dieldrin/kg diet (Brown et al., 1965).
Other studies on avian reproduction are summarized in Table 32.
This table gives the available information on five criteria
relevant to reproduction (parental survival, production, fertility
and hatchability of eggs, and chick survival) in relation to oral
intake of dieldrin and (where reported), the residues of dieldrin
in eggs. These studies show that, depending on the duration of
exposure, dose levels of 5 - 10 mg dieldrin/kg diet reduce the
survival of the parent birds. Egg production was reported to be
significantly increased in some studies but reduced in others. In
general, egg fertility was not influenced, except in one study.
Hatchability was not affected, neither in most cases, was the
survival of chicks. There seems to be a trend that overall
reduction in reproductive success occurs only if the parent birds
are showing signs of being affected by dieldrin, e.g., reduced food
intake with consequent loss of weight and poor condition. It
should be noted that in these studies (with one exception) the eggs
were placed in incubators for hatching. Consequently, one aspect
of the reproductive process was not studied, namely, parental
behaviour. However, in the study on homing pigeons (Robinson &
Crabtree, 1969), the parents (and subsequently, their offspring)
were free-flying, they brooded their eggs, and fed their young
until they fledged.
A 3-generation study of the effects of dieldrin on pheasants
(Phasianus colchicus), has not been included in Table 32 because
of the complexity of the experimental design. The doses of
dieldrin (hens, 6 or 10 mg dieldrin/bird per week; cocks, 4 or 6 mg
dieldrin/bird per week) were sufficient to cause mortality of
breeding birds, but the production, fertility, and hatchability of
eggs and the viability of chicks at the time of hatching were not
affected in a consistent manner in relation to dose or generation.
The survival of chicks from hens given 6 or 10 mg dieldrin/week was
reduced. Residues of dieldrin in eggs or tissues were not
determined in this study (Dahlgren & Linder, 1974).
When seven-week-old Japanese quail were given diets containing
3.1 or 50 mg dieldrin/kg diet for 21 days, a significant reduction
in egg production occurred in both groups (Call & Harrell, 1974).
With the exception of the study with the barn owl (Mendenhall
et al., 1983) and the homing pigeon (Robinson & Crabtree, 1969),
the birds that were tested are precocial species which show no
parental feeding of the young. Most birds are not precocial and
reproduction involves a period of full dependency of the offspring
on parental care. Results should, therefore, be interpreted with
care; extrapolation directly from the laboratory to the field is
difficult.
7.3.4.4 Eggshell thinning
Ratcliffe (1967a) reported that the ratio of eggshell weight to
size in three species of birds of prey in the United Kingdom had
declined during the period after 1947 relative to pre-1947. This
report has stimulated considerable interest in the relationship
between egg-shell thickness (or the related eggshell index based on
the weight/size ratio) and the breeding success of birds,
particularly as eggshell thinning seems to be quite a widespread
phenomenon, particularly among birds of prey (Hickey & Anderson,
1968; Ratcliffe, 1970; Anderson & Hickey, 1972). There has been
considerable speculation on the causes and mechanism of these
changes (Cooke, 1973; Mueller & Leach, 1974). Experimental studies
on the effects of dieldrin on eggshell thickness have given
conflicting results. The results are summarized in Table 33.
Eggshell weights of crowned guinea-fowl (Numida meleagris) fed
diets containing up to 15 mg dieldrin/kg diet for 21 months were
not affected by the treatments (Wiese et al., 1969).
American sparrow hawks (Falco sparverius) were given diets
containing dieldrin and North American prairie falcons (Falco
mexicanus) were fed starlings contaminated with an average of 29 mg
dieldrin/kg body weight, along with DDT and DDE at different
levels. The purpose of these studies was to show the influence of
these pesticides on shell thickness. The results of the two
studies, in relation to the effects of dieldrin, cannot be
interpreted, in view of the possible effects of DDE on eggshell
thinning (Porter & Wiemeyer, 1969; Enderson & Berger, 1970). It
has slowly become accepted that metabolites of DDT, particularly
DDE, are the most likely cause of eggshell thinning (Cooke, 1973;
Newton, 1979; Bunyan & Stanley, 1982). It is also pertinent that
the onset of eggshell thinning in wild birds preceded the use of
aldrin/dieldrin. There is evidence that eggshell thinning after
exposure to dieldrin is related to reduced food consumption.
Untreated Coturnix and Mallard, when fasted for 36 h, laid thin-
shelled eggs for a few days during and after fasting (Haegele &
Tucker, 1974).
Table 32. Reproductive success of birds in relation to oral intake of dieldrin and the concentration of HEOD in eggs
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Mallard duck 4 90 days - - NCg NC Red.c - Muller &
(Anas platy- Lockman (1972)
rhinchos)
Japanese quail 1 16 weeks - - NC NC NC - Shellenberger
(Coturnix 10 16 weeks - - NC NC NC - & Newell
coturnix (1965)
japonica)
10 18 weeks 19 NC NC NC NC NC Walker et al.
(6.9-26.9) (1969a)
20 9 weeks 39 Red. Red. DC NC Red.
(19.8-54.1)
30 7 weeks 48 Red. Red. DC Red. Red.
(34.7-63.2)
40 6 weeks 84 Red. Red. - - -
(76.9-92.5)
0.1 multi- - NC NC NC NC NC Shellenberger
generation (1978)
P; F1,
1 F2, F3 - NC NC NC NC NC
Bobwhite quail 10 34 weeks - Red. NC - - - Fergin &
(Colinus 20 34 weeks - Red. Red. - - - Schafer (1977)
virginianus) 40 34 weeks - Red. Red. - - -
Pheasant 2 mg/hen/ 13 weeks (0.6-26.5) NC NC NCa Inc.c NC Atkins &
(Phasianus weekb (yolks) Linder (1967)
colchicus) 2 mg/hen/ 13 weeks - NC NC NC NC NC
week
4 mg/hen/ 13 weeks (5.3-40.1) DR NC NC NC NC
week (yolks)
4 mg/hen/ 13 weeks (7.5-20.6) NC NC NC NC NC
week (yolks)
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Pheasant 6 mg/hen/ 13 weeks (13.3-52.4) NC Red.a NC NC NC
(Phasianus week (yolks)
colchicus)
(contd.) (0) 6 mg/hen/ 14 weeks - Red. Inc.c Red.c NC NC Baxter et al.
weekd (1969)
(0) 8 mg/hen/ 14 weeks - Red. Inc.c NC NC NC
week
(0) 12 mg/ 14 weeks - Red. Red.c NC - -
hen/week
(4) 0 mg/hen/ 14 weeks - NC Inc.c Red.c Red. NC
week
(4) 6 mg/hen/ 14 weeks - Red. NC Red.c NC NC
week
(6) 0 mg/hen/ 14 weeks - NC Red.c NC - -
week
(6) 6 mg/hen/ 14 weeks - Red. Red.c Red.c - -
week
Gray partridge 3 1.5 - DR NC NC NC Neill et al.
(Perdix perdix) (1-2) (1971)
Domestic fowl 2 16 weeks (0.34-1.45) NC NC - NC NC Graves et al.
(Gallus 5 16 weeks (1.2-4.8) NC NC - NC NC (1969)
domesticus)
10 13 months (7.6-16) NC NC NC NC NC Brown et al.
(1974)
20 13 months (19.6-35.7) Red. Inc.a NC NC Red.
Crowned guinea- 0.5 21 months 1.11/1.17e NC Inc.a NC NC NC Wiese et al.
fowl (Numida (1969)
meleagris) 1.5 21 months 2.38/3.35e NC Inc.a NC NC NC
5 21 months 7.18/13.56e PR Inc.a NC NC NC
15 21 months 15.79e Red. Inc.a NC NC Red.
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Homing pigeon ~2 mg/kg 2 years (0.03-13.4)f NC NC NC NC NC Robinson &
(free-flying) body weight/ (4.5-16.7)f NC NC NC NC NC Crabtree
(Columba livia) week (1969)
Barn owl 0.5 2 years 3.6 (first NC NC NC NC NC Mendenhall et
(Tyto alba) year) al. (1983)
8.1 (second
year)
--------------------------------------------------------------------------------------------------------------------
a Significant at a probability of < 0.01.
b Doses of 2, 4, or 6 mg dieldrin/hen were administered once per week in capsules.
c Significant at a probability of < 0.05.
d Second-generation hens; offspring of birds used in study by Atkins & Linder (1967). The doses in parentheses
refer to the doses administered to the first-generation hens.
e Residues in eggs of successive years.
f First and second laying periods, respectively.
g The term "no change" (NC) indicates that any differences between controls and treatment groups were within the
limits of experimental variation. If any of the results for treatment groups are reported to be increased (Inc.)
or reduced (Red.), the statistical significance, if reported, is given. Equivocal results have been described
"doubtful reduction" (DR), "probably reduced" (PR), and "doubtful change" (DC).
Table 33. Effects of dieldrin on eggshell thickness
--------------------------------------------------------------------------------------------------------------
Species Dose of dieldrin Duration Difference between egg- Reference
(mg/kg diet) shell thickness of treated
and control birds (%)
--------------------------------------------------------------------------------------------------------------
Mallard duck 1.6 16 months -3.4a Lehner & Egbert (1969)
(Anas platyrhynchos)
4 16 months -2a Lehner & Egbert (1969)
10 16 months -4.3a Lehner & Egbert (1969)
4 90 days -4.2 Muller & Lockman (1972)
Japanese quail 3.1 21 days with -8a Call & Harrell (1974)
(Coturnix coturnix changes in
japonica) 50 photoperiod -8a Call & Harrell (1974)
Domestic fowl 10 12 weeks 0 Davison & Sell (1972)
(Gallus domesticus) 20 12 weeks +0.3 Davison & Sell (1972)
10 13 months 0 Brown et al. (1974)
20 13 months +9.7 Brown et al. (1974)
Pheasant 6 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(Phasianus colchicus)
10 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(6) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(6) 6 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(10) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
--------------------------------------------------------------------------------------------------------------
a Significant at or below 0.05.
b Administered in capsules once per week (see footnoteb Table 32).
c See footnoted Table 32.
7.3.4.5 Concentrations of dieldrin in tissues of experimentally
poisoned birds
Many studies have been carried out to estimate the
concentrations of dieldrin in the liver, brain, or other tissues of
birds that died following oral intake of aldrin or dieldrin. The
intakes were either single acute doses or long-term dietary
exposure. In some investigations, the concentrations of dieldrin
in the tissues of birds that survived after treatment were also
reported. The results of these studies are not comparable, because
the dose levels and duration of the studies are different. The
concentrations that were found in the different studies ranged from
a few mg/kg up to about 100 mg/kg tissue (wet weight) (Turtle et
al., 1963; Koeman et al., 1967; Robinson et al., 1967b; Robinson,
1969; Robinson & Crabtree, 1969; Stickel et al., 1969; Enderson &
Berger, 1970; Linder et al., 1970; Brown et al., 1974; Clark, 1975;
Heinz & Johnson, 1981; Mendenhall et al., 1983) (Table 34).
Attempts to define tissue concentrations that can be used as
indicators of death by dieldrin poisoning of wild birds lack
precision as a result of the overlap between the lowest
concentrations in the tissues of birds dying under experimental
conditions and the highest concentrations in survivors. Thus, it
has been proposed that concentrations of 5 or 10 mg dieldrin/kg
brain (Robinson, 1969; Stickel et al., 1969) are indicative of
death from aldrin/dieldrin poisoning. Liver concentrations of 10
or 20 mg/kg (Robinson, 1969; Cooke et al., 1982) have been proposed
as levels diagnostic of dieldrin poisoning of birds.
As a general point, interpretation of residue data must be done
with extreme caution. Brain residues of dieldrin are probably a
good indicator of lethality. However, most bird carcasses
collected in the field cannot be analysed for brain residues,
because the brain deteriorates rapidly after death. For this
reason, most residue data from the field are for levels in the
liver, which remains discrete and usable for much longer. A liver
residue level symptomatic of death from dieldrin poisoning is more
difficult to define. A large, acutely toxic, dose of dieldrin may
leave a low residual level of dieldrin in liver because the bird
dies rapidly. A smaller, less acutely toxic, dose of dieldrin
usually leads to loss of body weight before death because of a lack
of ability or desire to feed. This period of starvation prior to
death boosts liver residues considerably as dieldrin is released
from mobilized fat and concentrated in the liver as detoxification
is attempted.
7.3.5. Mammals
The acute and long-term toxicity of aldrin and dieldrin for
laboratory mammals is summarized in section 8.
Values for the acute oral LD50 and subacute oral LC50 in the
diet (30 days) of dieldrin for four species of voles ( Microtus sp.)
are given in Table 35.
Table 34. Concentrations of dieldrin in the tissues of experimentally poisoned birds and
in survivors
------------------------------------------------------------------------------------------
Species Tissue Concentration of dieldrin (mg/kg wet weight) Reference
analysed geometric mean, (range of values)
No. of Survivors No. of Dead birds
samples samples
------------------------------------------------------------------------------------------
Domestic pigeon liver 11 8 20 45.6 Robinson et
(Columba sp.) (3.1-51.2) (23-81) al. (1967b)
brain 11 3.6 19 20
(1.6-8.5) (13.5-32.5)
House sparrow liver - - 19 44.7 Robinson
(Passer domesticus) (38.4-52.3) (1969)
brain - - 19 20
(17.6-22.7)
Japanese quail liver - 36 40 Robinson et
(Coturnix coturnix (17.7-90.4) al. (1967b)
japonica) brain 12 6.9 65 17.4
(3.1-15) (8.7-34.6)
Japanese quail liver 8 28.8 9 19.7 Stickel et
(Coturnix coturnix (2.7-140.8) (5.7-51.7) al. (1969)
japonica) brain 20 3.4 17 17.3
(0.25-11.9) (6.2-32.9)
Redwinged blackbird brain 3 7.1 27 19.8 Clark
(Agelaius phoeniceus) (6.7-7.4) (1-34.5) (1975)
27 22.2
(13.5-29.5)
Prairie falcon brain 2 2.9 1 11 Enderson &
(Falco mexicanus) (2.8-3) Berger
(1970)
Barn owl brain 2 10 Mendenhall
(Tyto alba) (5-15) et al.
carcass 19 9.4 - - (1983)
------------------------------------------------------------------------------------------
The toxicological signs in these studies were similar to those
in laboratory animals, and these four microtine rodents appear to
be less susceptible than laboratory rodents to dieldrin
intoxication (Cholakis et al., 1981). When short-tailed shrews
(Blerina brevicauda) were fed diets containing 50, 100, or 200 mg
dieldrin (nominal)/kg diet for up to 14 days, all the animals fed
50 mg/kg dieldrin survived, whereas all those fed 200 mg/kg diet
died (Blus, 1978).
Luckens & Davis (1965) studied the acute oral toxicity in big
brown bats (Eptericus fuscus) caught in Kentucky, USA. Death
occurred at all dose levels above 20 mg/kg body weight. The
approximate LD50 seemed to be within the range 20 - 40 mg/kg body
weight.
Table 35. Acute and subacute toxicity of dieldrin for volesa
------------------------------------------------------------------------
Species Acute LD50 Subacute LC50 (30 days)
(mg/kg body weight) (mg/kg body weight)
Average males/females Average males/females
------------------------------------------------------------------------
Microtus orchrogaster 210 105
Microtus canicaudus 100 40
Microtus montanus 205
Microtus pennsylvanicus 175
------------------------------------------------------------------------
a From: Cholakis et al. (1981).
White-tailed deer (Odocoileus virginianus) were fed 0, 5, or
25 mg dieldrin/kg diet for up to 3 years as were their progeny. No
signs of intoxication were observed, and the survival of adults was
not affected. The growth rate of treated females was decreased.
Relative liver weights increased at 25 mg/kg. Fertility and in
utero mortality were comparable for the three groups. Fawns from
treated does were smaller at birth, and greater postpartum
mortality occurred. The weight gain of fawns was reduced during 2
of the 3 years. Whole milk from doses fed 25 mg/kg contained
residues of 17 mg/litre. Residues in the liver of surviving
animals were about 4 mg/kg in the low-dose group and 16 mg/kg (wet
weight) in the high-dose group. Highest brain residues (12 mg/kg
wet weight) occurred in fawns only a few days before death (Murphy
& Korschgen, 1970).
When blesbuck (Damaliscus dorcas phillipsi) were fed diets
containing 5, 15, 25, 35, or 50 mg dieldrin (nominal)/kg diet, none
of the animals fed 5 or 15 mg/kg diet died during the 90 days of
the study. However, all the animals given higher dose levels died
within 24 days. The concentration of dieldrin in the liver was 3.3
and 8.2 mg/kg for the two lowest dose levels, after 90 days. The
concentrations of dieldrin in the livers of the dead animals were
9.4, 15.1, and 18.4, respectively, for the three highest treatment
groups (Wiese et al., 1973).
In studies by Wiese et al. (1973), an experimental grazing site
(250 ha) with a resident population of 35 blesbuck and 20 springbok
(Antidorcas marsupialis) was aerially sprayed with dieldrin at a
rate corresponding to 0.16 kg/ha. The concentration of dieldrin on
the veld declined rapidly from 27.6 mg/kg immediately after
treatment to 5.04 mg/kg after 14 days. The decline during the
following 106 days was much slower (it was 0.75 mg/kg on the 85th
day and 0.23 on the 120th day). Behavioural changes were observed
in both antelope species after 3 days. From the 4th day, there
were further nervous symptoms, including clonic convulsive attacks,
and partial or even complete blindness was also noted. Blesbuck
died from the 4th day onwards, and the entire population of 35
animals had died by the 19th day. The median time to death was
7.08 days, and there was no significant difference between adults
(male or female) and juveniles. The calculated mean intake of
dieldrin was 1.82 mg/kg herbage per day. This estimate was much
lower than that derived from the feeding trial (the total intake of
animals that died was 9.08 mg/kg). The mean concentration in the
livers of six blesbuck that died was 8.3 mg/kg. It was inferred
that dieldrin was unlikely to be the cause of death of the blesbuck
(further investigations implicated photodieldrin). Springbok were
less adversely affected: deaths occurred from the 6th day, and 70%
had died by the 13th day. Surviving animals recovered with no
after-effects, and two ewes lambed normally in the spring following
the winter treatment. The average dieldrin concentration in the
livers of three springbok that died was 9.2 mg/kg. The
pathological findings were similar to those in the common
laboratory species.
Few other mammalian species have been investigated. Reduced
reproductive success and some mortality has been reported in
raccoons fed 2 mg dieldrin/kg diet (Stickel, 1975).
7.4. Effect on Populations and Ecosystems
In order to show that a chemical has had an effect on
populations of organisms in the environment it is necessary to
satisfy a combination of several criteria. Ideally the exposure to
the chemical in the field should be established to compare exposure
with effects produced. Population declines should correlate with
usage of the chemical and should be reversed by controls on the use
of the chemical. Although it is generally recognized that dieldrin
affected populations of some animals when its use was widespread,
there are some difficulties in establishing its precise effects on
the environment. These difficulties arise because the use of
dieldrin coincided with the use of other persistent
organochlorines, which themselves affect populations of organisms,
and also because poisoning by dieldrin was often secondary
(poisoned organisms did not take in dieldrin directly but from prey
that had concentrated the chemical from the environment).
7.4.1. Exposure to dieldrin
It is difficult to establish the exposure of wildlife to
dieldrin unless animals are directly feeding on dressed grain or
directly exposed to preserved wood. Even where this occurs, the
animal will frequently die some distance from the source of
exposure. This is particularly true for birds but less so for
small mammals. Since exposure cannot be readily monitored
directly, most investigators have estimated exposure from the
residue remaining in dead or dying animals. Monitoring programmes
in several countries sampled both dead and dying animals and
compared them with healthy animals taken from the wild. Eggs of
birds were also monitored as a measure of dieldrin contamination
of populations. These monitoring programmes had to establish
criteria by which it could be definitively stated that particular
individuals had died from dieldrin poisoning. The criteria were
based on residue levels in experimentally poisoned animals and were
set at 5 - 10 mg/kg brain tissue and 10 - 20 mg/kg liver tissue for
birds (Robinson, 1969; Stickel et al., 1969; Cooke et al., 1982).
These criteria are probably conservative; 20 - 30% of dead wood
pigeons examined in the UK during the period 1961 - 1964 were
judged, by these criteria, to have died from dieldrin poisoning.
During this period many seed-eating birds were killed directly by
eating dieldren-dressed grain (Robinson, 1969); the actual
percentage of death attributable to dieldrin should probably be
higher. A high proportion of dead birds from areas of tsetse fly
control contained residues which would be judged lethal by these
criteria. The great majority of birds sampled contained non-lethal
residues of dieldrin (Tables 15, 16, 17, 18, and 34). It should be
remembered that all of these sampled birds contained residues of
other organochlorines, in addition to dieldrin. Although there
have been reports of populations with no dieldrin contamination,
but contamination with other organochlorines, there have been no
reports of populations contaminated by dieldrin alone. Furthermore,
residues of dieldrin always correlate well with residues of other
organochlorines; birds retaining large quantities of dieldrin also
retain large quantities of DDE and often polychlorinated biphenyls
(PCB) (Newton, 1979).
The literature reporting the presence of dieldrin in birds and
mammals from the wild is very extensive and has been selectively
reviewed elsewhere (section 5.1.6). Analysis of a few dead animals
serves to indicate the presence of dieldrin in wildlife but is of
little use in establishing effects at the population level. Only
long-term monitoring programmes, measuring changes in dieldrin
residues in the population with time and correlating this to
ecological monitoring of the size and reproductive success of the
population, can approach an objective assessment of the effects of
dieldrin. Such programmes have been reviewed by Newton (1979).
7.4.2. Effects on populations of birds
Populations of birds of prey declined during the period of
large scale use of organochlorine insecticides. Major studies of
changes in bird populations concentrated on a few species mainly in
the United Kingdom and North America, though also to some extent in
areas of mainland Europe. The following references are
illustrative on this subject of the literature: general
references, Anon (1964), Prestt (1965), Prestt & Bell (1966),
Parslow (1973), Bijleveld (1974), Cooke et al., (1976, 1982),
Havera & Duzan (1986); peregrine falcon (Falco peregrinu),
Ratcliffe (1963, 1965, 1967b, 1970, 1972, 1980, 1984), Lockie &
Ratcliffe (1964), Cade et al. (1968), Enderson & Berger (1968),
Hickey (1969); heron (Ardea cinerea), Reynolds (1974); golden eagle
(Aquila chrysaetos), Brown (1969), Lockie et al. (1969); sparrow-
hawk (Accipiter nisus), Koeman et al. (1972), Newton (1973a,b,
1974, 1976, 1979), Newton & Bogan (1974, 1978); Newton et al.
(1979), Marchant (1980); kestrel (Falco finnunculus), O'Connor
(1982).
In most of these studies, population decline correlated with
organochlorines residues in adult birds and their eggs. Reduced
breeding success was associated with thinning of eggshells,
behavioural changes resulting in egg breakage, and aggressive
interaction between adults resulting in a reduction of the number
of young fledged successfully from the clutches. The death of
adult birds was reported at the same time as seed-eating species
were dying from dieldrin poisoning.
As reported earlier in this section, dieldrin cannot be held
responsible for the eggshell-thinning effect, which has been shown
to be attributable to DDE (Cooke, 1973). Embryo deaths in shell
correlate best with PCB residues in eggs (Newton, 1979). The
contribution of dieldrin to these declines is difficult to
determine because the birds were subjected to residues of all
organochlorines. It is probable that dieldrin contributed to
population declines in some areas but not others (Newton, 1979;
Newton & Haas, 1984).
The studies of Blus et al. (1974a,b, 1975, 1979a,b) and Blus
(1982) on the brown pelican (Pelicanus occidentalis) conclude that
the decline in numbers could be ascribed entirely to DDE. The
population size of birds of prey in some of the Eastern states of
the USA declined when no dieldrin residues were present, DDE alone
being a contaminant in these populations. Newton (1979) pointed
out that the decline in populations of birds of prey contaminated
by DDE is gradual, a result of progressive effects of failure in
breeding. The decline of populations of peregrine falcon and
sparrow-hawk in the United Kingdom was more sudden and was
associated with the death of breeding adults. This was attributed
to dieldrin usage, which correlated well with the decline (Newton,
1979; Newton & Haas, 1984).
7.4.3. Effects on populations of mammals
Some mammal species in addition to birds, have been affected by
the use of organochlorine pesticides. There are reports of
decreases in the number of badgers (Meles meles) in some areas of
the United Kingdom (Jefferies, 1969, 1975). Declines in the number
of bats have been reported in the United Kingdom and the
Netherlands (Jefferies, 1972); furthermore, the grey bat (Myotis
grisesceus) in the USA (Clark et al., 1978) and the otter (Lutra
lutra) have also been affected (Jefferies et al., 1974; Chanin &
Jefferies, 1978).
Declines in bat numbers have been associated with the use of
dieldrin and lindane in wood preservatives in the United Kingdom
(Jefferies, 1972). In the United States, they appear to be related
to a combination of organochlorines. Many species migrate long
distances, using fat reserves on the journey, and are susceptible
to DDE poisoning en route (Clark & Kroll, 1977). No contribution
of dieldrin to declines in bat numbers in the USA has been proven.
Other declines have been attributed to dieldrin (Chanin &
Jefferies, 1978). Jefferies & Pendlebury (1968) studied the effect
of aldrin/dieldrin seed dressings on the populations of stoats,
weasels, and hedgehogs in the United Kingdom. None of these
species showed a decline during the period 1959 to 1962, and there
was no evidence that aldrin/dieldrin had any detrimental effects.
Jefferies et al. (1973) studied the behaviour of small mammals
in and adjacent to a field sown with dieldrin-dressed wheat. The
field mouse Apodemus, which lives on the field margin and the open
field, immediately fed on dosed grain. Residues of dieldrin in
sampled mice was very high. The bank vole Clethrionomys, which
lives in field margins, did not take the dosed grain. Residues of
dieldrin in these small mammals were monitored regularly after
sowing. These very quickly dropped to very low levels. The
authors propose that those individuals eating dressed grain died
quickly or were taken by predators. Populations were quickly
replenished by immigration from surrounding areas.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Aldrin and dieldrin
8.1.1.1 Oral
The acute oral LD50 values for technical aldrin and dieldrin in
various animal species are shown in Table 36. Intoxication with
cyclodiene insecticides consists of increased irritability and
tremor, followed later by tonic-clonic convulsions. In rats,
convulsions appear within 1 h following oral dosing at high
concentrations; death follows within 6 h, or from 2 - 7 days later.
This depends on factors such as the contents of the rat's
gastrointestinal tract, the concentration of aldrin/dieldrin in the
solvent, and the type of solvent used (Borgmann et al., 1952b;
Heath & Vandekar, 1964). Fox & Virgo (1986) reported that dieldrin
induced hyperglycemia.
Table 36. Acute oral LD50 values for technical aldrin and dieldrin
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Mouse corn oil 44 38 Borgmann et al. (1952a,b)
Mouse olive oil ~75 Jolly (1954)
Rat (newborn) arachis oil 168a Lu et al. (1965)
Rat (pre-weaning) arachis oil 25 Lu et al. (1965)
Rat (adult) arachis oil 37 Lu et al. (1965)
Rat arachis oil 51-64 Heath & Vandekar (1964)
Rat various 38-67 Lehman (1951); Borgmann et al.
(1952a); Treon & Cleveland (1955);
Gaines (1960); Worthing & Walker
(1983)
Rat various 37-87 Lehman (1951); Borgmann et al.
(1952b); Treon & Cleveland (1955);
Gaines (1960); Lu et al. (1965);
Worthing & Walker (1983)
Hamster olive oil 320 330 Gak et al. (1976)
Hamster corn oil 100 Cabral et al. (1979a,b)
Guinea-pig corn oil 33 49 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
Table 36. (contd.)
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Guinea-pig olive oil between 10 Jolly (1954)
and 25
Rabbit corn oil 50-80 45-50 Borgmann et al. (1952a,b)
Dog corn oil 65-95 65-80 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
a Transcutaneous intragastric injection.
The minimum toxic and the maximum non-toxic doses of aldrin and
dieldrin, administered orally to livestock, are indicated in Table
37.
Table 37. Acute oral toxicity of aldrin and dieldrin for livestock
-------------------------------------------------------------------
Compound Species Age Maximum Minimum Reference
non-toxic toxic
dose dose
tested found
-------------------
(mg/kg body weight)
-------------------------------------------------------------------
Aldrin calf 1-2 weeks 2.5 5 Radeleff et
al. (1955)
cattle 1 year 10 25 Radeleff et
al. (1955)
sheep 1-2 years 10 15 Radeleff et
al. (1955)
Dieldrin calf 1-2 weeks 5 10 Radeleff et
al. (1960)
cattle 1 year 10 25 Radeleff et
al. (1955)
horse - - 25 Radeleff et
al. (1960)
pig 3 weeks 25 50 Radeleff et
al. (1960)
sheep 1 year 15 25 Radeleff et
al. (1960)
sheep 9-12 months - LD50 Jolly (1954)
50-75
-------------------------------------------------------------------
8.1.1.2 Dermal
The minimum lethal dose of aldrin or dieldrin when applied as a
dry powder on the intact skin of female rabbits for 24 h was
between 600 and 1250 mg aldrin/kg body weight and between 250 and
360 mg dieldrin/kg body weight. In olive oil, the range for aldrin
was the same as for dry powder, and the range for dieldrin was
between 360 and 600 mg/kg body weight (Treon et al., 1953).
The acute dermal LD50 values for technical aldrin and dieldrin
in various animal species are shown in Table 38. The signs of
intoxication are similar to those that follow oral administration.
Table 38. Dermal LD50 values for technical aldrin and dieldrin
---------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
---------------------------------------------------------------
Mouse solvent - 40-80a Jolly (1954)
naphtha
Rat xylene ~100 60-90 Gaines (1960)
Guinea-pig solvent - 120a Jolly (1954)
naphtha
Rabbit dimethyl- 150 150 Lehman (1952)
phthalate
---------------------------------------------------------------
a With complete immersion of body.
8.1.1.3 Inhalation
The vapour pressures of technical aldrin and dieldrin are
sufficiently low that an acute inhalation hazard from aldrin or
dieldrin vapour does not normally arise.
8.1.1.4 Parenteral
The acute LD50 values for technical dieldrin (in glycerol
formal) in the rat via intraperitoneal and intravenous routes are
56 and 8 - 9 mg/kg body weight, respectively (Heath & Vandekar,
1964).
8.1.2. Formulated materials
8.1.2.1 Oral and dermal
The acute toxicity of formulated products, particularly the
dermal toxicity, is a more realistic guide than that of the
technical product to the acute hazard to the user. The percentage
of aldrin or dieldrin in the formulation, the solvent used, and the
type of formulation (such as an emulsion, wettable powder, dust,
etc.) will determine the acute toxicity of the formulated product.
Depending on these factors, the oral and dermal LD50s vary from 100
to 4500 and 500 to 16 000 mg total aldrin formulation/kg body
weight, respectively. For dieldrin formulations, these figures are
100 - 400 mg/kg and 200 - 2700 mg formulation/kg body weight,
respectively (Muir, 1970; Rose, 1982, 1984a,b).
For a dieldrin formulation for termite control (680 g/litre
suspension concentrate), the dermal LD50 in the male rat was 645 mg
formulation/kg body weight and in the female rat 284 mg
formulation/kg body weight (Rose, 1984c).
8.1.2.2 Inhalation
The acute inhalation LC50 (4-h exposure) in rats for aqueous
dilutions of a 48% (w/v) emulsifiable concentrate of aldrin (high
aromatic solvent) in the form of a spray was estimated to be
equivalent to 3% (w/v) aldrin. The median droplet size was 52 µm,
and the animals were exposed "nose only". Deaths occurred up to 6
days after exposure, but most of the animals died on the 2nd day
after exposure. Signs of intoxication consisted of a subdued
and hunched appearance with piloerection, progressing to
hypersensitivity and convulsions in the more seriously affected
animals. Surviving animals recovered within 2 - 3 days after
exposure. Oral intake (due to grooming) contributed significantly
to the results (MacDonald, 1982).
8.2. Short-Term Exposures
Short-term studies on rodents have shown that aldrin and
dieldrin affect the liver. The liver/body weight ratio is
increased, and histopathological changes that have become known as
"Chlorinated Hydrocarbon Insecticide Rodent Liver" (CHIRL) are
observed. Microscopically, these CHIRLs consist of enlarged
centrilobular hepatocytes with somewhat increased cytoplasmic
oxyphilia and peripheral migration of the basophilic granules
(Treon & Cleveland, 1955; Ortega et al., 1957).
The cellular and subcellular changes in the liver of different
mammalian species have been studied by Wright et al. (1972, 1977,
1978). These studies have shown that dieldrin produces a
generalized enlargement of the liver, which, in rats and dogs, is
associated with increased size of liver parenchymal cells but, in
mice, is associated with an increase in both cell size and cell
number. The earliest ultrastructural change in the livers of mice,
rats, and dogs treated with dieldrin was the proliferation of the
smooth endoplasmic reticulum (SER). During the initial phase of
the exposure to dieldrin, and also to phenobarbital, the increases
in the SER in the liver cells of mice, rats, and dogs were of the
vesicular type. These changes were associated with an enhanced
microsomal mixed-function oxidase, and intracellular whorls of
smooth membranes appeared in the liver cells of rats and dogs but
not in those of the mouse. The changes in liver subcellular
structure and function were reversible in mouse, rat, and dog. In
contrast, no liver enlargement or other ultrastructural changes
were observed in the livers of rhesus monkeys. In vitro
determinations showed that the activity of the liver microsomal
monooxygenase system was increased after treatment, and this was
the most sensitive effect observed. In all species examined, the
biochemical and subcellular structural response of the liver to
dieldrin was shown to be similar to that found with a number of
other chemicals, such as DDT, heptachlor, and phenobarbital
(Jansen, 1979). These chemicals also induce in the mouse a type of
liver tumour identical to that found with dieldrin (Stevenson &
Walker, 1969; Thorpe & Walker, 1973).
8.2.1. Oral
8.2.1.1 Rat
A number of short-term feeding studies (3 - 9 months duration)
were carried out on rats with aldrin. Dose levels of 0.5 - 300 mg
aldrin/kg diet were tested. The results of these old studies
showed that dose levels up to 5 mg/kg diet produced no effects, but
that levels of 25 mg/kg or more gave an increased liver/body weight
ratio and reversible hypertrophy of centrilobular hepatocytes with
cytoplastic changes (CHIRL) (section 8.4.2). Dose levels of 150
mg/kg or more resulted in increased mortality (Treon et al., 1951;
Borgmann et al., 1952a).
Five further studies were carried out with dieldrin (3 - 10
months duration), using dose levels of 1 - 300 mg/kg diet. No
effects were seen up to 5 mg/kg, except that Walton et al. (1971)
found an increased liver/body weight ratio in females at 5 mg/kg
and Ortega et al. (1957) found occasional liver changes at 2.5
mg/kg diet. These changes, e.g., liver enlargement and induction
of CHIRL, were found in the other studies at 10 mg/kg diet or more.
At 150 mg/kg or more, there was increased mortality (Treon et al.,
1951; Borgmann et al., 1952b; Ortega et al., 1957; Walton et al.,
1971).
When groups of male albino rats were fed diets equivalent to
2 mg/kg body weight for 6 months, the alkaline phosphatase, SGPT,
SGOT, and LD-hydrogenase activities in the serum were increased
after 6 months. The urea content decreased after 3 months, and
some other parameters were also changed. The growth of the animals
was considerably inhibited (Shakoori et al., 1986).
8.2.1.2 Dog
Dogs appear to be more susceptible to aldrin and dieldrin than
rats. Dogs administered aldrin in the diet for 5 or 6 days at dose
levels equivalent to 0.9 - 9.1 mg/kg body weight died within 7
months. However, beagle dogs (two males and two females) survived
15.6 months when given 0.043 - 0.25 mg aldrin/kg body weight. With
dieldrin, dogs survived dose levels up to 0.23 mg/kg body weight
for 15.7 months. In aldrin- and dieldrin-treated animals, no
effects on growth and no changes in haematology were seen. The
dogs with 0.25 mg aldrin/kg showed hepatomegaly, and the females
had local hyaline (droplet) degeneration of hepatocytes and
vacuolization in the epithelia of distal renal tubules. One of the
males of this group showed hepatocyte degeneration, while the other
exhibited the renal tubular changes seen in the females. In the
group fed 0.09 mg/kg, no effects were seen in the males, while the
females (and also one female in the group fed 0.23 mg/kg) showed
vacuolization in the epithelia of the distal renal tubules. The
liver weights of the dieldrin-treated animals were increased. No
other dogs showed gross or microscopic abnormalities in the viscera
(Treon & Cleveland, 1955).
Four beagle dogs fed dieldrin at daily oral doses of 0.4 - 0.8
mg/kg body weight showed blood concentrations of 0.27 - 1.27
mg/litre blood after eight episodes of convulsions (Brown et al.,
1964). When two dogs were given 0.2 mg dieldrin/kg body weight, in
gelatin capsules daily for 8 months, no signs of intoxication were
observed. The concentration in the blood was 0.11 - 0.22 mg/litre.
In studies by Fitzhugh et al. (1964), twelve mongrel dogs of
various ages received aldrin, 6 days/week for periods up to 25
months, at doses of 0.2, 0.5, 1, 2, or 5 mg/kg body weight.
Dieldrin was tested in 14 mongrel dogs at doses of 0.2, 0.5, 1, 2,
5, or 10 mg/kg body weight. In both cases, there were two animals
per dose level (one male, one female), with the exception of four
animals in the groups given 0.5 mg/kg. In the animals tested with
aldrin, the 5 mg/kg dogs and one 2 mg/kg dog died in 3 - 4 weeks;
the remaining male dog in the 2 mg/kg group was killed at 25 weeks
because of poor condition. All four dogs showed weight loss and
fatty changes in the liver and renal tubules. The bone marrow
showed a reduced number of mature granulocytes and erythroid cells.
At 1 mg/kg, the two dogs survived for 15 and 49 weeks and, at
autopsy, showed the same lesions. In the 0.5 mg/kg group, one dog
died after 4 days. The remaining three dogs survived for 2 years,
one male among these having convulsions during the last 2 months.
At 0.2 mg/kg body weight, there were no effects. In the animals
tested with dieldrin, all six dogs on 2, 5, and 10 mg/kg died
during weeks 2 - 5. These dogs showed weight loss, fatty changes
and slight hepatic cell atrophy in the liver, and a small amount of
atypically distributed fat in the kidneys. The bone marrow showed
a reduced number of mature granulocytes and erythroid cells. The
reported bone marrow findings, which were not replicated in other
studies, cannot be interpreted, because of the inadequacy of
clinical details, and no control dogs were used in this study. The
two dogs given 1 mg/kg survived for 12 and 43 weeks and, at
autopsy, showed the same lesions. One dog given 0.5 mg/kg was
sacrificed after 2 weeks because of anorexia and marked emaciation.
Detailed histological examination, including that of the brain, did
not show any distinct organ damage. The remaining three dogs in
the 0.5 mg/kg group died with terminal convulsions or were
sacrificed in poor condition at weeks 29, 43, and 81. Two of the
dogs showed weight loss. No effects were observed in the 0.2 mg/kg
group.
Repeated daily oral administration of 0.2, 1, or 2 mg
dieldrin/kg body weight to groups of six mongrel dogs was carried
out until intoxication occurred between the 18th and 85th day. A
direct relationship was established between the dieldrin
concentration in the blood and the severity of clinical signs of
intoxication. On the first day of muscle spasms, the average
concentration of dieldrin in the blood was about 0.50 mg/litre and,
at the time of the first full-blown convulsion, about 0.90 mg/litre
(Keane & Zavon, 1969a).
In studies by Walker et al. (1969b), groups of five beagle dogs
of each sex received, by capsule, daily doses of 0.005 or 0.05 mg
dieldrin (in olive oil)/kg body weight, for 2 years. Control dogs
were given capsules containing olive oil. The health, behaviour,
and body weight were unaffected, and EEG recordings did not differ
between the dogs fed 0.05 mg/kg and the controls. In females given
0.05 mg/kg, liver/body weight ratio was increased. In both sexes,
serum alkaline phosphatase activity was increased. However, urine,
haematology, clinical chemistry, bromosulfthalein clearance, and
relative organ weight data were not affected. No gross or
histopathological anomalies were observed.
Deichmann et al. (1969) gave groups of six beagle dogs (aged
1.5 - 3.5 years) 0 or 0.6 mg aldrin/kg body weight, 5 days/week for
10 months, and then observed them for an additional 12 months. The
treated dogs showed hyperexcitability, tremors, and weight loss.
One dog died. After 14 - 18 months, the dogs with aldrin showed
cloudy swelling and fatty degeneration in the liver and hypertrophy
of hepatocytes. Renal vascular congestion and tubular degeneration
were seen in some of the animals.
8.2.1.3 Domestic animals
A dairy cow given aldrin in soybean oil daily by capsule (2.2
mg/kg body weight) exhibited hyperirritability after 27 days. The
animal was in heat and was bred the next day. She died on day 29
with convulsions. Autopsy showed a slightly discoloured, pulpy,
congestive liver and one slightly enlarged congested kidney. No
mortality occurred among cows given 0.8, 1, or 1.5 mg/kg body
weight for 48 days (Ely et al., 1954).
In studies by Gannon et al. (1959b), groups of four dairy cows
were fed rations containing 0, 0.1, 0.25, 0.75, or 2.25 mg
dieldrin/kg for 12 weeks (average total intake, 0, 0.293, 0.75,
2.17, or 6.55 mg dieldrin/kg body weight). No signs of illness and
no abnormalities were found when the cows were slaughtered at the
end of the test feeding period or after an additional 6-week period
on dieldrin-free rations.
Ivey et al. (1961) fed groups of 2 - 3 steers, sheep, and hogs
rations containing 0, 0.25, 0.75, or 10 mg aldrin/kg diet for 12
weeks. Two steers received rations with 2 mg/kg diet for the same
period. The control groups consisted of two animals each. No
evidence of illness and no postmortem pathology were found.
Goats administered 50 mg aldrin/kg body weight showed mild
degenerative changes, congestion and petechial haemorrhages, in
various organs. In the kidneys degenerative changes of the
proximal convoluted tubules were found. Clinical changes were also
found, e.g., salivation and convulsions (Singh et al., 1985).
8.2.2. Dermal
In studies by Treon et al. (1953), aldrin and dieldrin, as dry
powders or in solutions, were applied daily to the skin of groups
of three female rabbits for 2 h on each of 5 days per week over a
period of 10 weeks. A series of graded doses was used to determine
the doses resulting in no mortality. It was clear that aldrin
or dieldrin dissolved in kerosene was very toxic (LD50 of
approximately 5 mg/kg body weight). Dissolved in vegetable oil
they were about 6 times less toxic and as dry powder about 20 times
less toxic than when dissolved in kerosene.
8.2.3. Inhalation
Mice, hamsters, and guinea-pigs did not show any adverse
effects when exposed to vapourized aldrin at a concentration of 18
mg/m3 for 178 days (Baker et al., 1959).
8.3. Skin and Eye Irritation; Sensitization
8.3.1. Skin and eye irritation
Treon et al. (1953) reported that technical aldrin or dieldrin
applied on the intact rabbit skin for 24 h occasionally caused
slight erythema. Repeated application of aldrin or dieldrin for 10
weeks (2 h per day, 5 days per week) as a dry powder did not alter
the gross condition of the rabbit skin. Slight irritation and
scaliness were observed when the compounds were applied in
vegetable oil, but their application in kerosene resulted in
damage, attributable to the solvent.
An undiluted aldrin emulsifiable concentrate formulation (48%
aldrin in high aromatic hydrocarbon solvent), applied at a dose of
0.5 ml at each test site for 24 h on the intact skin and abraded
skin of rabbits under an occlusive patch, caused severe irritation
and necrosis of the skin. One male died on day 13. When applied to
the rabbit eye, this undiluted 48% emulsifiable concentrate caused
severe initial pain and mild irritation (Rose, 1982).
8.3.2. Sensitization
In the Magnusson and Kligman guinea-pig maximization test, 48%
aldrin emulsifiable concentrate caused positive responses (at 24 h
and 48 h after removal of the challenge patches) in 3 out of the 20
test animals. Rechallenge of these animals, one week later,
confirmed that they had been sensitized to the test material (Rose,
1982).
Aldrin emulsifiable concentrate (48%) is not a skin sensitizer
under the EEC Dangerous Substances Directives (EEC, 1983).
No cases of skin sensitization occurred over a period of 20
years among a group of over 1000 workers involved in the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
8.4. Long-Term Toxicity and Carcinogenicity
8.4.1. Mouse
Davis & Fitzhugh (1962) fed groups of 100 male and 100 female
C3HeB/Fe mice a diet containing aldrin or dieldrin at 0 or 10 mg/kg
diet for 2 years. The average lifespan of the treated mice was
shortened by 2 months. A significant increase in the incidence of
benign liver tumours was observed.
In a further study, 100 male and 100 female C3HeB/Fe/J mice
received aldrin or dieldrin at 0 or 10 mg/kg diet for 2 years. An
increase in the number of animals with hepatic hyperplasia and
benign liver tumours was seen in the treated groups, but no
increase in malignant liver tumours was found. The survival in the
treated groups was lower than that of controls (Davis et al., 1965).
Groups of 300, 125, 125, and 200 CF1 mice of each sex were fed
diets containing dieldrin (> 99%) at 0, 0.1, 1, or 10 mg/kg,
respectively, for 2 years. A positive control group fed 600 mg
4-amino-2,3-dimethylazobenzene for 6 months, followed by a control
diet, was used. After 9 months, the morbidity of the mice fed 10
mg/kg started to increase, but the lifespan of the mice fed 0.1 or
1 mg/kg was unaffected. The animals with 4-amino-2,3-
dimethylazobenzene died within 14 months. The frequency of liver
tumours was increased in all groups fed dieldrin. Two types of
tumours were observed, one of which was considered benign
(hyperplastic nodules) and the other malignant. The malignant
tumours were clearly hepatocarcinomas, though no fibrosis or bile
duct proliferation, as seen in the positive control group, occurred
in the dieldrin-treated groups. A reversibility study in a
separate group fed 10 mg dieldrin/kg in the diet for up to 15
months, followed by a control diet for the rest of the 2-year
study, showed that the tumours did not regress or disappear upon
discontinuation of the treatment. However, the dieldrin induced
hepatomegaly, and cytoplasmic changes were found to be reversible
(Walker et al., 1972; Hunt et al., 1975).
In a study by Thorpe & Walker (1973), a group of 30 CF1 mice of
each sex were fed a diet containing 10 mg dieldrin/kg for 2 years.
The control group consisted of 45 mice of each sex. Liver
enlargement was detected after 50 weeks in both sexes, and liver
lesions were observed, classified as hyperplastic nodules (Type a)
and hepatocellular carcinoma (Type b) (sometimes associated with
lung metastases). In a separate study, it was shown that dieldrin
Type b liver cell tumours were capable of growing as subcutaneous
transplants in mice of the same strain and sex (Thorpe, 1973).
To compare the pathological responses to dieldrin in different
mouse strains, groups of 30 mice of each sex of the CF1, LACG, and
hybrid CF1-LACG strains were fed diets containing 10 mg dieldrin/kg
for 2 years. There was also a control group of 45 animals of each
sex for each strain. The incidence of liver tumours, particularly
Type b tumours, in male CF1 and hybrid mice and in females of all
three strains was higher than in controls. In male LACG mice, the
incidence of liver tumours was low. Qualitatively, there was no
difference in tumours between strains, and there was no increased
incidence of neoplasms in other tissues, nor were unusual tumours
found. Metastases of liver carcinoma were found in the lungs in
some of the mice (Thorpe & Hunt, 1975).
In studies by Benitz et al. (1977), nine groups of 100 Charles
River CD1 mice of each sex were given dieldrin in the diet at
concentrations ranging from 0.15 to 15 mg/kg. Six hundred mice of
each sex were used as controls. Groups of animals were sacrificed
at time intervals ranging from 2 to 25 months. Initial changes in
the liver consisted of various degrees of centrilobular and
pericentral hypertrophy. These changes were later associated with
the appearance of hepatic nodules, the occurrence of which was time
and dose related. These nodules consisted of hypertrophic
hepatocytes, which, in a few instances, were mixed with
hyperplastic cells. Various degrees of loss and distortion of
lobular architecture were seen within these nodules. Metastases in
the lung were observed in three nodule-bearing animals given 15 mg
dieldrin/kg for 25 months. These metastases contained similar
hypertrophic hepatocytes as did the primary liver tumours.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
aldrin (4 or 8 mg/kg diet for males and 3 or 6 mg/kg diet for
females) or dieldrin (2.5 or 5 mg/kg for both sexes) for 80 weeks,
followed by an observation period of 10 - 13 weeks. Concurrent
controls consisted of groups of 20 male and 10 female mice. The
pooled controls, used for statistical evaluation, consisted of the
concurrent control groups combined with 92 male and 79 female mice
from similar bioassays of other chemicals. All surviving mice were
killed at 90 - 93 weeks. Body weight was not affected in the
treated animals, but there was a dose-related increase in
mortality, especially in the high-dose groups in the second half of
the study. In the dieldrin-treated mice, clinical symptoms, such
as irritability, tremors, and alopecia occurred. Hepatocellular
carcinomas were found, as indicated in Table 39. The incidence of
hepatocellular carcinomas was clearly higher in male than female
mice. There was no difference in tumour frequencies in other
tissues (NCI, 1978a).
Groups of weanling male C3H/HE mice were fed a diet containing
10 mg dieldrin/kg diet until an age of 57 weeks and then were
either administered a control diet (12 mice) or continued on the
dieldrin diet (11 mice) for another 10 weeks. A third group served
as an untreated control group (21 mice). Laparatomies were
performed and biopsy specimens taken when about 30% of the mice in
each dieldrin-treated group had tumours. Further biopsy samples
were taken approximately 10 weeks later. Tumours were observed at
the first laparotomy in 6/21 controls and 14/23 dieldrin-treated
animals. At the second laparotomy, adenomas were seen in some
animals in which there had been no tumour at the first laparotomy.
In one animal in the continuous dieldrin-treatment group, there was
histological progression from adenoma to hepatocellular carcinoma.
Additional hepatocellular carcinomas were observed in some animals
autopsied at 2 years of age. A strong tendency to tumour
progression was found in both treated and control mice (Ruebner et
al., 1984a,b).
Table 39. Incidence of hepatocellular tumours
in mice (NCI, 1978a)
----------------------------------------------
Groups Males Females
----------------------------------------------
Concurrent controls 3/20 (15%)a 0/10 (0%)a
3/18 (17%)b 0/20 (0%)b
Pooled controls 17/92 (18%) 3/78 (4%)
Aldrin (4 mg) 16/49 (33%)
(8 mg) 25/45 (56%)
Aldrin (3 mg) 5/48 (10%)
(6 mg) 2/43 (5%)
Dieldrin (2.5 mg) 12/50 (24%) 6/50 (12%)
(5 mg) 16/45 (36%) 2/49 (4%)
----------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
In a study by Meierhenry et al. (1983), groups of 50 - 70 male
mice of three strains (C57Bl6J, C3H/He, and C57Bl6J x C3H/He B6C3F1
hybrid) were administered a diet containing 10 mg dieldrin/kg diet
for 85 weeks. The control groups consisted of approximately 60
mice of the same strains. After 4 months, the livers in a small
number of animals showed swellings of hepatocytes in the central
zone with nuclear atypia, small nodules containing basophilic or
eosinophilic foci, and multiple tumours. The percentage of benign
hepatic tumours was 28, 20, and 29, respectively, and in the
control groups, 19, 18, and 4. The percentage of hepatocellular
carcinomas was 30, 38, and 42, respectively, and, in the controls,
0, 12, and 4%. Mallory bodies were seen in all the dieldrin-
treated mice that had either benign or malignant tumours, but only
rarely in mice without tumours.
It seems from the available studies that dieldrin facilitates
and exacerbates the expression of an endogenous oncogenic factor in
CF1 mice (Tennekes et al., 1981). The dose-response characteristics
of dieldrin-mediated enhancement of liver tumour formation in CF1
mice were analysed using existing tumour data from long-term
feeding studies at six levels of continuous exposure, involving a
total of more than 1500 animals. Using the Druckrey equation, the
actual contribution of dieldrin to tumour formation was considered
to be negligible (Tennekes et al., 1985).
8.4.1.1 Appraisal
A number of long-term carcinogenicity studies have been carried
out in which mice of different strains were fed aldrin and/or
dieldrin at one or more dose levels. In all these studies, there
was an increased incidence of liver changes, some of which were of
the nature of hepatocellular carcinomas while others were regarded
as non-malignant. Females seem to be less sensitive than males.
No other tumours were induced.
8.4.2. Rat
Borgmann et al. (1952a) fed six groups of 10 male and 10 female
weanling Sprague-Dawley rats a diet containing 0, 5, 10, 50, 100,
or 150 mg aldrin/kg diet over a period of 2 years. At the two
highest dose levels, an increased mortality was found at 16 months,
which was not seen in the other groups. Liver enlargement was
observed in the groups with high dose levels, but not in the groups
with 10 mg/kg diet or less.
When groups of 40 Carworth rats of each sex were given diets
containing aldrin or dieldrin at 0, 2.5, 12.5, or 25 mg/kg diet for
2 years, there was no increase in mortality and the growth rate was
comparable with that of controls. At all dose levels, the
liver/body weight ratio was increased in males and there were
histological liver cell changes characteristic of CHIRL. No
tumours were reported (Treon & Cleveland, 1955). In a review of
all the aldrin and dieldrin studies, it was reported that there was
no excess of tumours in these rats (Cleveland, 1966).
When groups of 12 Osborne-Mendel rats of each sex were given
diets containing aldrin or dieldrin (0, 0.5, 2, 10, 50, 100, or
150 mg/kg diet) for 2 years, growth was not affected. However,
survival was markedly decreased at dose levels of 50 mg or more in
a dose-related manner. The liver/body weight ratio was increased
in males fed 10 mg/kg or more, while, in females, an increase at
all dose levels was found (no dose-response relationship at the
lower doses). CHIRL was observed in all treated groups, although
at 0.5 mg/kg only a few animals showed a trace of CHIRL. Rats at
dose levels of 50 mg/kg or more showed haemorrhagic urinary
bladders and nephritis. An overall increase in tumour incidence
was noted, but this was not dose related. On the contrary, the
lowest dose levels showed the highest tumour incidence. Only one
liver tumour was found (Fitzhugh et al., 1964).
In a study at the National Institute of Public Health of the
Netherlands, no increased incidence of tumours was found in rats
fed diets containing 75 mg dieldrin/kg for 2 years (Van Genderen,
1965, 1979).
When groups of 30 Osborne-Mendel rats of each sex were fed
5 mg/kg aldrin (95%) or a control diet for 2 years, there was no
increase in mortality, liver/body weight ratios, or tumour
incidence in the aldrin-fed group (Deichmann et al., 1967).
In studies by Walker et al. (1969b), groups of 25 Carworth Farm
E rats of each sex were given dieldrin at 0.1, 1, or 10 mg/kg diet
for 2 years. A control group consisted of 45 males and 45 females.
There was no effect on body weight. After 2 - 3 months, the
animals fed 10 mg/kg exhibited irritability and, as the study
progressed, tremors and occasional convulsions, usually during
handling. Mortality, haematology, serum enzyme levels, and
urinalysis were not affected. The females fed 1 mg/kg and 10 mg/kg
had increased liver/body weight ratios. At 10 mg/kg, one male and
six females exhibited CHIRL. In two females of the group fed 10
mg/kg and in one female control rat, microscopic nodules in the
liver parenchyma were seen. There was no increase in tumour
incidence. Subsequent re-evaluation of these data (Stevenson et
al., 1976) confirmed that there was no treatment-related increase
in tumour incidence.
Nine groups of 50 Osborne-Mendel rats of each sex were fed
diets containing aldrin or dieldrin at 0, 20, 30, or 50 mg/kg for
up to 31 months. A control group consisted of 100 male and 100
female rats. Dose-related tremors and convulsions, always
associated with weight loss, occurred at all dose levels,
particularly in females. Female rats fed 50 mg aldrin/kg or 30 or
50 mg dieldrin/kg had a shortened lifespan. The liver/body weight
ratio was increased in all dieldrin-treated groups and in males fed
30 or 50 mg aldrin/kg, but was decreased in females fed 20 mg/kg.
A moderate increase (not dose related) in the incidence of hepatic
centrilobular cloudy swelling, necrosis, or, rarely, foci of acute
or chronic inflammatory cellular infiltration was observed in all
treated groups. Hyperplasia in the liver was found in two male
rats fed 30 mg aldrin/kg. There was no increase in tumour
incidence (Deichmann et al., 1970; Deichmann, 1974).
Groups of 50 Osborne-Mendel rats of each sex were given diets
containing aldrin at levels of 30 or 60 mg/kg. Treatment of male
rats lasted 74 weeks followed by 37 - 38 weeks of observation,
while that of female rats lasted 80 weeks followed by 32 - 33 weeks
of observation. In a similar study, dieldrin was fed at a level of
29 mg/kg diet (time-weighted average dose) for 80 weeks followed by
observation for 30 - 31 weeks or 65 mg/kg diet (time-weighted
average dose) for 59 weeks followed by observation for 51 - 52
weeks. Concurrent control groups consisted of 10 rats of each
sex. Pooled controls, used for statistical evaluation, consisted
of concurrent control groups combined with 58 males and 60 females
from similar bioassays with other chemicals. All surviving rats
were killed at 110 - 111 weeks. Typical signs of organochlorine
intoxication (such as hyperexcitability) were observed with
increasing frequency and severity, especially in the second year,
but mortality was not affected. No significant increase in tumour
incidence was found (NCI, 1978a) (Table 40).
Groups of 24 Fischer 344 rats of each sex were given dieldrin
at 0, 2, 10, or 50 mg/kg diet for 2 years. Typical signs of
organochlorine intoxication were observed during the second year in
the 50 mg/kg group. Body weight and survival were not adversely
affected in any of the dieldrin groups. Liver tumours were not
observed. No significant increase in tumours was found (NCI,
1978b) (Table 40).
Table 40. Incidence of hepatocellular tumours in rats
(NCI, 1978a,b)
-------------------------------------------------------
Groups Males Females
-------------------------------------------------------
Osborne-Mendel rats
Concurrent controls 1/10 (10%)a 1/10 (10%)a
1/10 (10%)b 0/9 (0%)b
Pooled controls ? 5/59 (8.5%)a
Aldrin (30 mg/kg diet) 1/47 (2%) 0/48 (0%)
(60 mg/kg diet) 1/47 (2%) 3/49 (6%)
Dieldrin (40-20 mg/kg diet)d 0/44 (0%) 1/47 (2%)
(80-40 mg/kg diet)e 1/47 (2%) 1/44 (2.3%)
Fischer F 344 rats
Concurrent controls 2/24 (8.3%) 0/24 (0%)
Pooled controls ? ?
Dieldrin ( 2 mg/kg diet) 0/23 (0%) 0/24 (0%)
(10 mg/kg diet) 0/23 (0%) 0/24 (0%)
(50 mg/kg diet) 4/23 (17%)c 0/23 (0%)
-------------------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
c Nodular hyperplasia.
d Time-weighted average dose = 29 mg/kg.
e Time-weighted average dose = 65 mg/kg.
When groups of 50 female rats of two different strains
(Osborne-Mendel and Sprague-Dawley) were fed 0, 20, or 50 mg
aldrin/kg diet, the survival rate was reduced at 50 mg/kg but not
at 20 mg/kg. There was no increase in the incidence of mammary or
liver tumours (Deichmann, 1974; Deichmann et al., 1979).
Photodieldrin, which has metabolites identical to those of
dieldrin, was fed to groups of rats for 80 weeks at concentrations
of up to 7.5 mg/kg diet NCI (1977). No increase in tumour
incidence was found (see section 8.8.1.3).
Ito et al. (1983) studied the promoting activity of dieldrin on
the induction of hyperplastic (neoplastic) liver nodules using a
short-term test system. F344 rats received a single dose (200
mg/kg body weight) of N-nitrosodiethylamine, and 2 weeks later,
were treated for 6 weeks with dieldrin in the diet at a
concentration of 100 mg/kg. Dieldrin had a weak promoting
potential in this test system.
8.4.2.1 Appraisal
A number of long-term/carcinogenicity studies have been carried
out in which rats of different strains were fed one or more dose
levels of aldrin and/or dieldrin. The overall no-effect level in
these long-term studies, both for aldrin or dieldrin, was 0.5 mg/kg
diet. At feeding levels of 1 mg/kg or more, an increasing, dose-
related hepatomegaly and histological changes in the liver
characterized as CHIRL occurred. At levels of 10 mg/kg diet or
more, typical signs of organochlorine toxicity occurred such as
irritability, tremors, and convulsions. In all these studies, no
increase in tumour incidence in liver or other organ/tissue systems
was found.
8.4.3. Hamster
When groups of 34 - 40 Syrian golden hamsters of each sex were
fed diets containing dieldrin (99%) at 0, 20, 60, or 180 mg/kg for
120 weeks, there was no significant increase in tumour incidence
(Cabral et al., 1979b).
8.4.4. Monkey
In studies on rhesus monkeys, groups of five males were given
diets containing dieldrin (88.4%) at 0, 0.01, 0.1, 0.5, 1, or 5
mg/kg (0.0002 - 0.07 mg/kg body weight) for approximately 6 years.
After two monkeys in the group fed 5 mg/kg died, the level of
exposure to the remaining three animals was reduced to 2.5 mg/kg
and, later, to 1.75 mg/kg. Subsequently, one of these animals had
his dieldrin intake progressively increased until at the end of the
second year, he was receiving dieldrin at the initial dietary
concentration of 5 mg/kg. Clinical and haematological examinations,
liver and kidney function studies, urinalysis, and pathology did not
reveal any abnormalities. The liver/body weight ratios and liver
DNA and RNA of the test animals were not different from those of
control animals. No subcellular changes were seen in the
hepatocytes. Dose-related increases in microsomal cytochrome P-450
and in the activity of the liver mono-oxygenase enzyme system were
observed at the two highest dose levels. These alterations in
cytochrome P-450 in the liver microsomes were significant in the
monkeys fed 0.1 mg/kg or more. No effect was observed at 0.01
mg/kg. The concentrations of dieldrin in the subcutaneous fat of
the monkeys fed 0.1 mg/kg were similar to those measured in human
beings receiving a daily oral intake of similar concentration. The
dieldrin concentrations in the monkey livers were approximately 200
times higher than those in male rats receiving a daily intake of
dieldrin 3 times higher than the monkeys, and they were similar to
the concentration in the livers of male mice daily ingesting
dieldrin at a level approximately 50 times higher (Zavon & Stemmer,
1975; Wright et al., 1977, 1978).
8.4.5. Mode of Action
From long-term feeding experiments, it seems that aldrin and
dieldrin may be carcinogenic to mice, but not to rats or hamsters.
Mutagenicity findings have been consistently negative (see section
8.6). There is insufficient knowledge of the mechanism by which
these chemicals might behave epigenetically.
The latest evaluation of IARC (1987) is that there is
inadequate evidence of carcinogenicity in humans and limited
evidence for carcinogenicity in experimental animals.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
8.5.1.1 Mouse
Groups of 100 pairs of male and female virgin CFW Swiss mice
were fed diets containing 5 mg dieldrin/kg for 30 days before
mating, after which time they were randomly paired and fed the same
diet for a further 90 days. Mortality in the dieldrin-treated
group was similar to that of a control group (size not specified).
No major biological effects on fertility, fecundity, gestation
period, size of the first litters, or numbers of young produced per
day were noted as a result of feeding dieldrin. A statistically
significant (6%) decrease in mean size of all litters combined was
the only difference observed between the dieldrin-treated and
control groups (Good & Ware, 1969).
In a reproductive study, groups of 4 male and 14 female Swiss
white mice (120 days old) were fed diets containing 3, 5, 10, or
25 mg aldrin/kg or 3, 10, or 25 mg dieldrin/kg. Six groups served
as controls. The study covered six generations, and two litters
per generation. For both aldrin and dieldrin, the 25 mg/kg dose
was too toxic and resulted in high litter mortality in the few dams
reaching gestation. This dose level was therefore discontinued.
Pup survival was low in mice fed 10 mg dieldrin/kg, and so
treatment was terminated after the first generation. The most
pronounced effect observed in the group fed 10 mg aldrin/kg and, to
a lesser extent, 5 mg aldrin/kg was a low pre-weaning pup survival.
No effects on fertility, viability, or gestation were observed in
six generations of mice fed 3 mg dieldrin/kg. A decrease in pre-
weaning survival was observed in the F2b litters, but a similar
decrease was also found in one of the six control groups (Keplinger
et al., 1970).
In studies by Virgo & Bellward (1975), groups of 18 - 19
uniparous female Swiss-Vancouver mice were given diets containing
0, 2.5, 5, 10, 15, 20, or 25 mg dieldrin/kg, 4 weeks prior to their
second mating, continuing until day 28 postpartum. Significant
mortality of the females occurred at 20 and 25 mg/kg, all deaths
occurring before parturition (89 and 56%, respectively). Fertility
in the groups fed 10 and 15 mg/kg was decreased, though survivors
at higher doses were fertile. Oestrus and gestation period were
not affected. Litter size was decreased only in the group
receiving 25 mg/kg. The major effect was an increase in pre-
weaning pup mortality; 47% at 2.5 mg/kg, 80% at 5 mg/kg, and 100%
at 10 mg/kg or more (31% mortality in control animals). Dams
receiving 10 mg/kg or more exhibited hyperactivity, which was a
contributory factor to the high pup mortality. No gross
abnormalities were detected in the pups, none of whom showed
tremors or convulsions. Within the litters raised at 2.5 and 5
mg/kg, pup survival was not different from controls. The only
effect on reproductive capacity or pup survival observed in female
mice fed 2.5 mg/kg was an increase in pre-weaning pup mortality.
A study was carried out on primiparous female Swiss-Vancouver
mice to investigate whether diets containing up to 15 mg
dieldrin/kg affect maternal behaviour and pup viability. Viability
was investigated in dams fed diets containing 0, 5, 10, or 15 mg
dieldrin/kg for 4 weeks prior to mating. Pups fed 10 mg/kg were
nursed by foster dams not fed dieldrin, and all died by day 4; the
foster dams' own pups showed a very low mortality and survived
until weaning. Similar results were obtained at 5 mg/kg. Dieldrin
did not have any influence on serum progesterone levels, milk
production, or the dam's tendency to retrieve pups or build nests.
However, at 5 mg/kg or more, nursing was reduced. It was concluded
that dieldrin causes irreversible congenital inviability (not
through any effect on progesterone levels) and it was suggested
that the inviability and the reduced tendency to nurse increased
the pup mortality (Virgo & Bellward, 1977).
8.5.1.2 Rat
In studies by Treon & Cleveland (1955), rats (Carworth strain)
were fed aldrin or dieldrin at dietary concentrations of 2.5, 12.5,
or 25 mg/kg for three consecutive generations, two litters being
produced for each generation. (There was no mention of a control
group, and tabulation and description of results was limited in
this report). A reduction in the number of pregnancies, which
gradually disappeared over successive generations, was initially
observed at 12.5 and 25 mg aldrin/kg and at all three doses of
dieldrin. No effects on litter size or pup weights were observed
at any dietary concentration. A marked increase in mortality in
pre-weaning pups was found at dietary concentrations of 12.5 and
25 mg/kg for both compounds. This was thought by the authors to
be due to the high concentration of dieldrin in the milk of the
mothers. Neither aldrin nor dieldrin had any effect on
reproductive capacity. No effects, except a "slight to moderate"
increased pre-weaning pup mortality, were observed in the rats fed
for three generations with aldrin or dieldrin at 2.5 mg/kg.
When groups of 10 male and 20 female Long Evans rats were fed
dietary concentrations of 0, 0.1, 1, or 2 mg dieldrin/kg over three
generations (each generation producing two litters), no effects
were observed on the general health (including weight gain),
behaviour, fertility, gestation, viability, lactation, or organ
weight ratios. No pathological changes were found in parents or
pups. Increased pre-weaning mortality (compared to that in
controls) in the F1a litter was observed in the animals fed 2
mg/kg. This effect was not found in the five subsequent litters
from this group and was not considered to be a major toxic effect.
No changes in reproductive capacity were observed over three
generations at dietary concentrations up to and including 2 mg/kg
dieldrin (Eisenlord et al., 1967).
In studies by Harr et al. (1970), groups of 20 male and 20
female 28-day-old OSU-Wistar rats were fed 0, 0.08, 0.16, 0.31,
0.63, 1.25, 2.5, 5, 10, 20, or 40 mg dieldrin/kg diet throughout
their lifespan. Ten females from each group were mated at 146 days
of age. Mortality occurred in dams at 20 and 40 mg/kg. Fertility
and litter size were decreased in several dose groups without a
clear dose relationship. The number of pups at weaning was
markedly reduced at 2.5 mg/kg or more; none survived at 20 and 40
mg/kg. The nursing pups died in convulsions or starved. No
effects were noted at 1.25 mg/kg or less. Neural lesions, such as
cerebral oedema and hydrocephalus, occurred in pups of nursing dams
at dieldrin concentration of 0.08 mg/kg. Hepatic lesions were
found in rats fed concentrations of 0.31 mg/kg or more.
When groups of 18 - 20 Long Evans pregnant rats were given 4 mg
dieldrin/kg body weight daily by gavage from day 15 of gestation to
21 days post partum, fecundity, number of stillbirths, perinatal
mortality, and total litter weights did not differ from the control
group. No malformations in pups were observed (Coulston et al.,
1980).
8.5.1.3 Dog
In study by Kitselman (1953), seven groups of three dogs, each
group having at least one member of each sex, were fed either
aldrin or dieldrin for one year at dietary concentrations
equivalent to 0, 0.2, 0.6, or 2 mg/kg body weight (in corn oil).
Out of a total of 11 bitches fed either aldrin or dieldrin, 9
conceived. All pregnant bitches produced litters of at least four
pups/litter. The survival of pups was generally lower in the
groups fed aldrin or dieldrin. Histopathological examinations of
dead pups revealed degenerative changes in the liver and mild
degenerative changes in renal tubules. Liver changes were also
observed in treated bitches. The design and size of this study was
too limited to deduce a dose-response relationship for pup
survival, but no effects were observed in dogs receiving 0.2 mg
dieldrin/kg body weight.
8.5.1.4 Appraisal
In the reproductive studies (over one to six generations)
carried out with aldrin or dieldrin on mice and rats, the major
effect observed in most of the studies was an increased mortality
rate in pre-weaning pups. Reproductive performance, per se, was
only affected at doses causing maternal intoxication. The studies
on dogs are of a too limited nature to draw firm conclusions, apart
from the consistent increase in pre-weaning pup mortality.
The results of these reproductive studies indicate that
dieldrin at levels of 2 mg/kg in the rat diet and 3 mg/kg in the
mouse diet (equivalent to 0.1 and 0.4 mg/kg body weight per day,
respectively) are no-effect levels for reproduction. It is not
possible to establish a no-effect level for aldrin for
reproduction, because no adequate data are available.
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Mouse
Ottolenghi et al. (1974) gave groups of 10 pregnant CD-1 mice
single oral doses of 25 mg aldrin/kg body weight or 15 mg
dieldrin/kg body weight (in corn oil; equivalent to half the LD50
values) on day 9 of gestation. Control groups consisted of
untreated and corn-oil-dosed mice. No effects on fetal survival or
weight were observed. Abnormalities, such as webbed feet, cleft
palate, and open eyes, were increased in both treated groups, but
they may have been related to maternal toxicity. The percentage of
the total live fetuses that were malformed was 33% for aldrin-
treated mice and 17% for dieldrin-treated ones.
In two comparable studies, pregnant CD-1 mice (6 - 16 per
group) were given daily oral doses of dieldrin in peanut oil at 0,
1.5, 3, or 6 mg/kg body weight from day 7 to day 16 of gestation.
At 6 mg/kg, reduced body weight gain and increased liver/body
weight ratio were observed. There was an increase in supernumerary
ribs and a decreased number of caudal ossification centres in the
fetuses of mice given 6 mg/kg. In one study, the number of
supernumerary ribs was increased at all three dose levels,
(significant at the two highest dose levels) (Chernoff et al.,
1975). In the other study, the increase in supernumerary ribs at
6 mg/kg was not significant. The increase in the number of
supernumerary ribs may be an expression of developmental toxicity.
Doses of dieldrin (99%) were given either in corn oil (0, 1.5,
or 4 mg/kg body weight) or in dimethylsulfoxide (DMSO) (0, 0.25,
0.5, or 1 mg/kg) daily by gavage to pregnant CF-1 mice (7 - 14
mice/group) on days 6 - 14 of gestation. No maternal or fetal
toxicity was seen in groups treated with dieldrin in corn oil or in
the corn oil control groups, but some was seen in the dieldrin/DMSO
and DMSO-control groups. No compound-related teratogenic effects
were observed (Dix et al., 1978).
8.5.2.2 Rat
In a study by Chernoff et al. (1975), pregnant CD rats (9 - 25
per group) were given daily oral doses of dieldrin in peanut oil
(0, 1.5, 3, or 6 mg/kg body weight) from days 7 to 16 of gestation.
At 6 mg/kg, increased mortality and reduced body weight gain were
observed in the dams, but no changes in the liver/body weight
ratios were found. Fetuses did not show any differences from the
controls in mortality, body weight, or occurrence of anomalies.
There were no differences in the average number of sternal or
caudal ossification centres (as were seen in mice). No evidence of
teratogenicity was observed at a dose level of 6 mg aldrin/kg body
weight per day.
No malformations were observed in fetuses or pups from 18 - 20
Long Evans rats given 0 or 4 mg dieldrin/kg, by gavage, daily from
day 15 of gestation to day 21 of lactation (Coulston et al., 1980).
8.5.2.3 Hamster
Pregnant Syrian golden hamsters (41 - 43 per group) were given
single oral doses in corn oil of either 50 mg aldrin/kg body weight
or 30 mg dieldrin/kg body weight on either day 7, 8, or 9 of
gestation. Untreated and vehicle-control groups (respectively, 57
and 41 animals) were used. The high dose levels of both aldrin and
dieldrin caused reductions in the number of live fetuses and fetal
weight and an increased incidence of abnormalities (cleft palate,
open eyes, and webbed feet). The effects were more pronounced
after treatment on days 7 and 8 of gestation than on day 9. It was
suggested that, since webbed foot and open eye were frequently
associated with low fetal weight, these effects might be simply the
expression of growth retardation (Ottolenghi et al., 1974).
8.5.2.4 Rabbit
No teratogenic effects were observed in the offspring of groups
of pregnant Banded Dutch rabbits dosed with dieldrin in
carboxymethylcellulose (2 or 6 mg/kg body weight per day) from
days 6 to 18 of gestation. The animals were killed on day 28 of
gestation and the fetuses were examined for visceral and skeletal
abnormalities (Dix & Wilson, 1971).
8.5.2.5 Appraisal
No evidence of a teratogenic potential has been found from
studies on rats, mice, or rabbits using oral doses up to 6 mg/kg
body weight. Single high oral doses, equivalent to half the LD50,
have been found to cause fetotoxicity and abnormal development of
the fetuses in hamsters and, to a lesser extent, in mice. The
significance of these abnormalities in the presence of severe
maternal toxicity is doubtful but a specific teratogenic potential
cannot be ruled out completely. No gross malformations have been
reported in reproductive studies.
8.6. Mutagenicity and Related End-Points
8.6.1. Microorganisms
Most research workers have reported that aldrin and dieldrin,
with or without microsomal activation, are not mutagenic in
bacterial or yeast test systems. In one study, it was reported
that dieldrin, without activation, was mutagenic in two out of
three strains of Salmonella typhimurium, but there was no dose-
response relationship (Majumdar et al., 1977). The results of the
other mutagenicity studies with aldrin and dieldrin in bacterial
test systems have been negative (Table 41). A critical survey of
the published reports indicates clearly that neither aldrin nor
dieldrin is mutagenic in microbial systems (Ashwood-Smith, 1981).
8.6.2. Mammalian cell point mutations
Only one study on the in vitro mutagenicity of dieldrin to
mammalian cells has been reported. Dieldrin was weakly mutagenic
when tested at a single concentration (0.01 mmol/litre) in ouabain-
resistant Chinese hamster V-79 cells. The significance of this
result is difficult to assess because of the lack of a dose-
response relationship, and a positive control group was not used
(Ahmed et al., 1977a).
8.6.3. Dominant lethal assays and heritable translocation assays
in mice
Aldrin did not show any detectable dominant lethality when
given as a single intraperitoneal dose (8 or 40 mg/kg) or in daily
oral doses (0.5 or 1 mg/kg body weight) for 5 days to male ICR/Ha
Swiss mice (Epstein et al., 1972).
Dieldrin, also, revealed no detectable dominant lethality in
four assays in male mice following a single intraperitoneal
injection (5.2 or 26 mg/kg) or daily oral doses of 2 or 3 mg/kg
body weight for 5 days (Epstein et al., 1972). Likewise, a single
oral dose of 12.5, 25, or 50 mg/kg body weight did not produce
dominant lethality in CF-1 mice (Dean et al., 1975). Bidwell et
al. (1975) carried out a dominant lethal test on B6D2F1/J mice
orally administered 0.08, 0.8, and 8 mg/kg body weight dieldrin for
5 days, but no effects were seen.
In further studies by Bidwell et al. (1975), a heritable
translocation test was performed on male mice after oral intake of
0.008, 0.08, or 0.2 mg dieldrin/kg body weight per day for a period
of 6 weeks. The cytogenetic determination of somatic cells using
the micronucleus test and the usual analysis of spermatocytes did
not reveal an increase in the rate of translocations.
8.6.4. Micronucleus test
Aldrin did not induce a significant increase in the frequency
of micronuclei in the bone marrow of mice treated orally with 13
mg/kg body weight (Usha Rani et al., 1980).
No cytogenetic abnormalities were seen in a standard metaphase
analysis and micronucleus test after oral gavage of mice with 0.8
or 8 mg dieldrin/kg body weight per day for 5 days (Bidwell et al.,
1975).
8.6.5. Chromosome and cytogenicity studies
Chinese hamsters (three groups of four males and four females)
were orally dosed with 30 and 60 mg dieldrin/kg body weight. No
chromosome abnormalities were found in femoral bone marrow cells
(Dean et al., 1975).
Table 41. Aldrin and dieldrin: mutagenicity tests in microorganisms
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
E. coli WP2 Try- none aldrin and dieldrin; negative Ashwood-Smith et
1000 µg/plate al. (1972)
E. coli WP2 hcr rat S9 up to 5000 µg/plate negative Moriya et al.
(1983)
E. coli Ga1 Rs none aldrin and dieldrin; negative Fahrig (1974)
Serratia marcescens alpha 21 and 742 dose not stated
Saccharomyces cerevisiae
Aspergillus nidulans diploid P1 and none dieldrin; 13 or 26 negative Crebelli et al.
haploid strain 35 mmol (1986)
Saccharomyces cerevisiae 632/4 none aldrin; 5 µg/ml on positive Guerzoni et al.
disc (1976)
Bacillus subtilis (Rec-assay) none aldrin and dieldrin; negative Shirasu (1975)
Salmonella typhimurium TA 1535, 1536,
1537, 1538
E. coli WP2 hcr+, hcr-
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; 10, 50, negative Bidwell et al.
1536, 1537, 1538, 100, or 500 µg/plate (1975)
G46
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; dose not negative McCann et al.
1537 stated (1975)
Salmonella typhimurium TA 1535, 1536, rat S9 dieldrin; 1000 negative Marshall et al.
1537, 1538 µg/plate (1976)
Salmonella typhimurium TA 98, 100 none dieldrin; 10, 30, negative Glatt et al.
100, 300, 1000, 3000 (1983)
µg/plate
Salmonella typhimurium TA 90, 100, 1535, rat S9 up to 5000 µg/plate negative Moriya et al.
1537, 1538 (1983)
---------------------------------------------------------------------------------------------------------
Table 41. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium TA 98, 100, 1535, mouse S9 dieldrin; 1000 negative Van Dijck & Van
1537, 1538, 1950, µg/plate de Voorde (1976)
1978
Salmonella typhimurium not specified S9 dieldrin; dose not "weak" Ercegovich &
stated response Rashid (1977)
Salmonella typhimurium TA 98, 100, 1535 mouse S9 dieldrin; 1, 25, or positive Majumdar et al.
50 µg/ml (1977)
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; up to 2500 negative Purchase et al.
1538 µg/plate (1978)
Salmonella typhimurium TA 98, 100 rat S9 dieldrin; 50 or 1000 negative Wade et al.
µg/plate (1979)
Salmonella typhimurium TA 98, 100, 1535, rat, aldrin; dose not negative Simmon et al.
1537, 1538 mouse, and stated (1977)
human S9
Salmonella typhimurium TA 98, 100 rat S9 aldrin and dieldrin; negative Nishimura et al.
364 and 380 µg/plate (1982)
Salmonella typhimurium TA 98, 100 1535, rat S9 dieldrin 2.6 x 104 negative DeFlora (1981)
1537, 1538 nmoles/plate
---------------------------------------------------------------------------------------------------------
The effect on the bone marrow cells of STS mice was examined
after applying a single dose of dieldrin intraperitoneally at 0, 1,
30, or 50 mg/kg body weight. A decrease in the mitotic index was
noted, as dieldrin concentration increased, and differences in
chromosome aberrations (a slight increase in breaks, fragments,
and interchanges) were found (Majumdar et al., 1976).
Seventy-one juvenile mallard ducks, the parents of which had
been exposed to various dietary levels of dieldrin for 6 months or
longer, were grouped and fed dieldrin at a level that corresponded
to the diet fed to the parents (0, 4, 10, or 30 mg dieldrin/kg
diet) for approximately 60 days. At the end of this period, no
chromosomal aberrations were found in femoral bone marrow cultures.
However, the mitotic index of ducks exposed to 30 mg/kg was
significantly reduced (Bunch & Low, 1973).
When lymphocytic cultures from adult mallard ducks (which had
not been exposed to dieldrin) were treated with 0, 0.1, 1, 10, 30,
or 100 mg dieldrin/kg, there was a significant increase in the
incidence of chromosome structural alterations, but only at the
highest dose level. The mitotic index was significantly reduced at
all dose levels, the greatest decreases occurring at the two
highest dose levels (Bunch & Low, 1973).
Chromosome studies in cultured lymphocytes from current
dieldrin plant workers (12) former plant workers (9), and control
(17) did not show any differences in the frequency of chromosome
aberrations (Dean et al., 1975). In one study, it was reported
that aldrin produced chromosomal aberrations in cultured human
lymphocytes at concentrations of 19 and 38 µg/ml. In mice and
rats, an intraperitoneal injection of a dose of 19 mg/kg body
weight was reported to induce chromosomal gaps, breaks, deletions,
and fragments in bone marrow cells (Georgian, 1975).
Cultured human peripheral lymphocytes from agricultural and
public health (anti-Chagas' disease) workers with at least 10 years
exposure to dieldrin were examined for structural chromosome
aberrations and sister chromatid exchange. No differences were
seen when they were compared with lymphocytes derived from a
control group (Bordon, 1980).
8.6.6. Host-mediated assays
Bidwell et al. (1975) carried out a host-mediated assay,
incorporating blood- and urine-recovery studies, for mutagenic
substances on B6D2F1/J mice. Five daily oral doses of 20 mg
dieldrin/kg body weight were given, and the mice were then injected
intraperitoneally with Salmonella tester strains. The results were
negative. In a further host-mediated assay using Saccharomyces
cerevisiae (strain D4, heteroallelic at the ade-2 and trp-5 loci)
as tester microorganism, CF-1 mice were treated with a single oral
dose of 25 or 50 mg dieldrin/kg body weight or with five daily
doses of 5 or 10 mg/kg body weight. No mutagenic activity was
found (Dean et al., 1975).
8.6.7. Cell transformation in mammalian cell systems
Dieldrin (0.08 - 250 mg/litre) proved negative in mammalian
cell transformation tests using cell lines derived from baby Syrian
hamster kidney (BHK-21 C13) and from human lung (Wl-38), either
with or without metabolic activation by rat liver S9 microsomal
fraction (Purchase et al., 1978).
In a 6-thioguanine resistance mutation assay using FM 3A mouse
cell cultures, aldrin was weakly mutagenic (Morita & Umeda, 1984).
8.6.8. Drosophila melanogaster and other insect systems
There is no evidence of a mutagenic activity of aldrin or
dieldrin in Drosophila melanogaster (Benes & Sram, 1969; Bidwell et
al., 1975). No increase in recessive or dominant lethal mutations
was found following exposure of the wasp Bracon hebetor to
sublethal doses of dieldrin (95%) (Grosch & Valcovic, 1967).
8.6.9. Effects on DNA
DNA strand breakage was not detected in Chinese hamster V-79
cells exposed to dieldrin (0.1 - 1 mmol/litre) in the presence of
rat liver S9 microsomal fraction using the alkaline elution assay
(Swenberg et al., 1976).
Aldrin (1 - 1000 µmol/litre) and dieldrin (1-100 µmol/litre)
induced unscheduled DNA synthesis in SV-40 transformed human
fibroblast cells (VA-4) both in the presence and absence of rat
liver microsomes (Ahmed et al., 1977b). This study was repeated by
Zelle & Lohman (1977). After exposure to dieldrin, the rate of DNA
synthesis in normal primary human fibroblasts (AH) decreased, but
returned to the control level in a few hours. This suggests that
dieldrin interferes with semiconservative DNA replication without
damaging DNA. The effect of dieldrin on the induction of repair
replication was studied with both AH cells and SV-40 transformed
human cells (MM-SV-40). No evidence that dieldrin could induce DNA
repair was found (Zelle & Lohman, 1977).
The DNA breakage rates in an Escherichia coli plasmid after
treatment with aldrin or dieldrin did not differ from those in
untreated plasmid DNA, suggesting that, at least in these studies,
the compounds did not interact directly with DNA (Griffin & Hill,
1978).
The effects of aldrin and dieldrin (both at 100 µg/ml) on the
uptake of tritiated thymidine by cultured rat thymocytes and human
lymphocytes were tested under different experimental conditions.
Both compounds appeared to have marginal effects on thymidine
uptake, suggesting inhibition of DNA synthesis (Rocchi et al.,
1980).
Aldrin (100 mmol/litre) and dieldrin (500 mmol/litre) did not
induce unscheduled DNA synthesis in primary cultures of Fischer 344
rat hepatocytes (Probst et al., 1981). Williams (1982) reported
the results of the hepatocyte primary culture/DNA repair test,
using freshly isolated hepatocytes of high metabolic capability to
monitor the production of DNA damage by measuring DNA repair
synthesis. Aldrin and dieldrin gave equivocal results concerning
DNA repair, but there was no damage to DNA. Aldrin (0.3 - 3
mmol/litre) induced DNA strand breaks in an alkaline elution/rat
hepatocyte assay (Sina et al., 1983).
Cultured hepatocytes from male Balb/c mice treated with
dieldrin at a concentration of 4 x 10-4 mol/litre showed no
unscheduled DNA synthesis. The results were no different with
cells from mice treated in vivo with phenobarbital (Klaunig et al.,
1984).
A DNA synthesis inhibition/damage test on HeLa cells with S9
showed inhibition to 60% of the control value within 90 min of
treatment with 4 x 10-4 mol dieldrin/litre (Painter, 1981).
8.6.10. Cell to cell communication
Both aldrin and dieldrin inhibited gap junctional intercellular
communication between 6-thioguanine-sensitive and 6-thioguanine-
resistant human teratocarcinoma cells in culture (Zhong-Xiang et
al., 1986).
The inhibition of cell to cell communication was observed in
human teratocarcinoma cells in culture in the presence of dieldrin,
using dye transport methods (Wade et al., 1986).
Metabolic cooperation between 6TG-resistant and HGPRT-deficient
Chinese hamster V79 cells was inhibited when aldrin (2.5 - 10
µg/ml) or dieldrin (2.5 - 5 µg/ml) was added to the medium (Kurata
et al., 1982).
Trosko et al. (in press) studied the inhibition of gap
junctional-mediated intercellular communication using co-cultures
of Chinese hamster cells. To do this, an in vitro assay (in which
the metabolic cooperation between V79-6-thioguanine-sensitive (6
TGs) and resistant (6 TGr) cells is studied) has been developed to
detect the ability of non-cytotoxic and non-mutagenic chemicals to
inhibit gap junctional communication. Aldrin and dieldrin
inhibited metabolic cooperation at concentration of about 4 µg/ml
or more.
8.6.11. Appraisal
Aldrin and dieldrin are not mutagenic to mammals in a variety
of mutagenicity test systems with unrelated different end-points.
8.7. Special Studies
8.7.1. Liver enzyme induction
Aldrin and dieldrin have been shown to increase the activity of
liver microsomal enzymes, generally associated with enlargement of
the liver. They have also been found to induce microsomal
dimethylaminoantipyrine-N-demethylase and aldrin-epoxidase, and
increase the cytochrome P-450 level (Campbell et al., 1983). This
enzyme induction is the earliest and most sensitive indicator of an
effect of exposure in mouse, rat, beagle dog, and rhesus monkey
(Wright et al., 1972).
Studies on rats indicated a no-effect level for enzyme
induction, by either aldrin or dieldrin, at 1 mg/kg diet (Gillett &
Chan, 1968; Kinoshita & Kempf, 1970; Den Tonkelaar & Van Esch,
1974).
In the rhesus monkey, the activity of the liver microsomal
monooxygenase system was increased by dieldrin (at daily feeding
levels of 1.75 and 5 mg/kg diet for approximately 6 years), but no
associated liver enlargement was observed. The dietary intake of
dieldrin required for the induction of this enzyme system in
monkeys was approximately 1 mg/kg diet, corresponding to an intake
of 25 - 30 µg/kg body weight per day (section 8.4.4) (Wright et
al., 1978).
In human beings, oral dosing with 211 µg dieldrin/day
(approximately 3 µg/kg body weight per day) for two years, in
addition to the daily intake of 19 µg dieldrin from the diet, did
not increase the activity of microsomal liver enzymes as measured
by the concentration of p,p'-DDE in adipose tissue and blood. No
evidence of enzyme stimulation was observed in a group of 10
workers (at the time, they were the most highly exposed workers) in
a manufacturing plant with a mean exposure equivalent to a daily
oral intake of 17 µg/kg body weight (maximum 24 µg/kg body weight)
(Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
8.7.2. Nervous system
8.7.2.1 Rat
When three groups of eight male albino rats were fed diets
containing 0, 25, or 50 mg dieldrin/kg for 60 days, there was no
effect on body weight or learning, but muscular efficiency,
measured by pulling weights of increasing magnitude in a 250-cm
runway, was decreased (Khaïry, 1960). This finding is in agreement
with the results of a study on nerve muscle (gastrocnemius)
preparations of pre-treated rats (Ibrahim, 1964).
8.7.2.2 Dog
Groups of five dogs of each sex, given daily oral doses of
dieldrin (0.05 mg/kg body weight) by capsule for 2 years, did not
show any changes in behaviour or EEG recordings compared with
controls (Walker et al., 1969b).
8.7.2.3 Monkey
When groups of three or four adult male squirrel monkeys were
given daily oral doses of 0, 0.01, or 0.1 mg dieldrin/kg body
weight for 54 days, learning ability was impaired and changes (high
amplitude slow waves) in the EEG occurred in both test groups (Van
Gelder & Cunningham, 1975).
8.7.3. Weight loss and stress
It is known that in birds, and perhaps in some small mammals,
dieldrin intoxication may be induced by starvation, weight loss,
and stress in animals having a previously harmless body burden of
dieldrin. Concern is sometimes expressed that, by analogy to these
observations, a similar course of events might occur in human
beings. Therefore, this phenomenon was studied in rats as well as
in human beings (for human beings see section 9.1.3.2).
8.7.3.1 Rat
In studies by Treon & Cleveland (1955), rats previously fed
diets containing aldrin or dieldrin at levels of 5, 10, or 15 mg/kg
diet for 7 - 18 months were starved. The complete withdrawal of
food did not result in the release of aldrin or dieldrin from the
adipose tissue stores to an extent sufficient to induce symptoms of
intoxication of any type.
When Osborne-Mendel rats fed 7.5 mg aldrin/kg diet for 4 weeks
were subsequently starved for 6 days with free access to water,
there was a marked loss of body weight and fat and a decrease in
the liver/body weight ratio. The total body burden of dieldrin
decreased during starvation regardless of age, sex, or the previous
level of exposure. The total quantity of dieldrin in the liver
decreased in all rats. In females, particularly older females, the
concentration of dieldrin in abdominal fat increased, whereas in
all males, the level in fat decreased. The concentration of
dieldrin in the blood was not increased. Young weanling rats
reacted similarly (Deichmann et al., 1972).
8.7.4. Immunosuppressive action
Loose et al. (1981) found that macrophages from mice fed 50 mg
dieldrin/kg diet had a marked impairment in antigen processing.
The effect was statistically significant in Kupffer cells at 50
mg/kg diet, in alveolar and splenic macrophages at 0.5, 5 and 50
mg/kg diet, and in peritoneal macrophages at 5 and 50 mg/kg diet.
There was an impairment of in vivo phagocytic clearance in mice
receiving 5 or 50 mg/kg diet for 8 weeks but not at 0.5 mg/kg diet.
This was related to a decrease in serum fibronectin. Tumour cell
killing after challenge with EL-4, P388, or mKSA tumour cells was
significantly impaired in mice fed either 1 or 5 mg dieldrin/kg
diet. The mean survival time after challenge with EL-4 was reduced
by 3 weeks, and with the P388 or mKSA tumour cells impairment was
observed after 3 or 18 weeks, respectively. There was no
alteration in the oxygen uptake by isolated macrophages either at
rest or during phagocytosis, and no effect on phagocytic activity
or capacity or on chemotaxis in vitro was observed.
Loose (1982) found that dieldrin caused immunosuppression in
mice. Levels of 1 or 5 mg dieldrin/kg diet were fed to BALB/c mice
for 3.5 or 10 weeks, and the mice were challenged intradermally
with Leischmania tropica. Dieldrin acted synergistically on
lethality in a dose- and time-related manner, indicating an effect
on host mechanisms. It also resulted in decreased antibody
formation to PVP, a T-independent antigen (direct splenic plague
assay). The mitogenic response of cultured T-cells to
phytohaemagglutinin (PHA) in dosed mice was depressed. Mitomycin C
and anti-Thy-1 abolished the mitogenic response. When splenic
T-cells from treated mice were mixed with T-cells from control
mice, there was inhibition of PHA mitogenesis. The data indicated
an active cell-mediated suppressor. A soluble macrophage factor
from the hepatic Kupffer cells (but not from alveolar or peritoneal
macrophages) suppressed the T-cell response to PHA. It was
concluded that administration of 5 mg dieldrin/kg diet to mice for
10 weeks caused a profound impairment of macrophage antigen
processing.
8.8. Toxicity of Photodieldrin and Major Metabolites
The relevance of photodieldrin lies in the fact that it has
metabolites identical to those of dieldrin and is quantitatively
and qualitatively similar in toxicity.
8.8.1. Photodieldrin
The photodecomposition of deposits of dieldrin on leaves and
grass has been reported (Roburn, 1963), and the physical and
chemical properties and structure of this decomposition product
have been determined (Robinson et al., 1966b; Rosen et al., 1966).
It appears to be the pentacyclo isomer of dieldrin (hexacyclo
isomer by the alternative nomenclature used by Rosen et al.
(1966)). Photodieldrin residues were less than the limits of
detection in most of the food samples analysed (Robinson et al.,
1966a).
8.8.1.1 Acute toxicity
Photodieldrin is more acutely toxic than dieldrin for mice,
rats, and guinea-pigs (Table 42). The toxicity for dogs is about
equal to that of dieldrin. Dieldrin-like convulsions have been
observed in all species given photodieldrin.
8.8.1.2 Short-term toxicity
(a) Mouse
When groups of five male and five female Carworth Farm No. 1
mice were fed 1, 3, or 10 mg photodieldrin/kg diet for 1 month, all
animals fed 10 mg/kg and two animals fed 3 mg/kg died. No changes
were observed at necropsy (Brown et al., 1967).
Table 42. Oral LD50 values for photodieldrin
-------------------------------------------------------------------
Species Vehicle LD50 Reference
Photodieldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Mouse dimethyl- 6.8 77.3 Brown et al.
sulfoxide (1967)
Rat dimethyl- 9.6 46.8 Brown et al.
sulfoxide (1967)
Guinea-pig dimethyl- 2.3-3.9 18-30 Brown et al.
sulfoxide (1967)
Dog (male) gelatin 120-160 120 Brown et al.
capsule (1967)
Dog (female) gelatin 80-120 80-100 Brown et al.
capsule (1967)
-------------------------------------------------------------------
(b) Rat
Groups of five male and five female Carworth Farm E rats were
fed 3 or 10 mg photodieldrin/kg diet for one month without apparent
ill effects (Brown et al., 1967).
In studies by Walton et al. (1971), groups of 28 male and
28 female Charles River rats were fed 0, 1, 5, or 25 mg
photodieldrin/kg diet for 3 months. A similar study was carried
out concurrently with dieldrin. The concentrations of
photodieldrin given in the diet were lowered from 25 to 12.5 mg/kg
diet within the first week of the study because of high mortality.
At the end of 3 months, no significant differences were found in
growth or food intake, and no gross evidence of toxicity was
observed. Liver/body weight ratios were increased at 12.5 mg/kg
diet. Increases in the activity or concentration of liver mixed-
function oxidase and microsomal cytochrome P-450 at 5 and 12.5
mg/kg diet indicated the occurrence of a dose-dependent enzyme
induction. The total protein content of the liver was not
affected. The short-term toxicities of photodieldrin and dieldrin
appeared to be similar.
Walker et al. (1971) fed groups of 12 Carworth Farm E rats of
each sex diets containing 0.1, 1, 10, or 30 mg photodieldrin/kg
diet for 3 months. The control group consisted of 24 male and 24
female rats. Six females given 30 mg/kg and two females given 10
mg/kg died. The animals in these groups that survived were
irritable and showed tremors when handled. Growth was reduced,
increases in serum urea and glutamic pyruvic transaminase (SGPT)
activity were seen in females fed 30 mg/kg, and the liver/body
weight ratio was increased in this group. In the groups fed 10 or
30 mg/kg, kidney/body weight ratio was increased in males. At
autopsy, no gross lesions were seen. Some of the animals fed 10 or
30 mg/kg showed CHIRL and centrilobular fatty changes in the liver.
Eosinophilic droplets were seen in the cytoplasm of the proximal
convoluted tubules and in the lumen of affected tubules in the
kidneys of males fed 10 or 30 mg/kg. No evidence of nephron damage
was found. No effects were observed in the animals dosed with 1
mg/kg.
(c) Dog
In a study by Walker et al. (1971), groups of four male and
four female beagle dogs received photodieldrin in olive oil by
capsule (daily oral doses of 0.005, 0.05, or 0.2 mg/kg body weight)
for 3 months. A control group of six males and six females
received olive oil in gelatine capsules. The health, behaviour,
body weight, and haematology were unaffected. In the 0.2 mg/kg
males, increases occurred in the plasma alkaline phosphatase and
SGPT activities, and, after 13 weeks, their serum protein levels
were slightly reduced. Increases in the liver/body weight ratios
occurred in the 0.2 mg/kg animals and the 0.05 mg/kg females. At
autopsy, no pathological changes associated with photodieldrin were
observed. No effects were observed at 0.005 mg/kg body weight.
8.8.1.3 Long-term toxicity
(a) Mouse
In a study on the long-term toxicity of photodieldrin, groups
of 50 B6C3F1 mice of each sex were fed diets containing 0.32 or
0.64 mg/kg for 80 weeks. After 80 weeks, the animals were fed a
control diet for 12 or 13 weeks. Concurrent control groups
consisted of 10 untreated mice of each sex. Pooled controls, used
for statistical evaluation, consisted of the concurrent controls
plus 60 male and female mice from similarly performed bioassays
with six other test chemicals. All surviving mice were killed at
93 weeks. Mean body weights and mortality were not affected by
treatment, but convulsions and hyperactivity were noted in treated
male mice. No statistically significant increase in tumour
incidence was found (NCI, 1977).
(b) Rat
In similar studies to those on mice, groups of 50 Osborne-
Mendel rats of each sex were given 5 or 10 mg photodieldrin/kg diet
for 80 weeks. After 80 weeks, the animals were fed a control diet
until sacrifice at 111 - 112 weeks. Because of neurotoxicity, the
doses in the females were reduced after 30 weeks, so that the time-
weighted average doses were 3.4 or 7.5 mg/kg diet for the females.
Concurrent control groups consisted of 10 rats of each sex. Pooled
controls, used for statistical evaluation, consisted of the
concurrent controls combined with 65 rats of each sex from similarly
performed bioassays with six other chemicals. All surviving animals
were killed at 111 - 112 weeks. Mean body weights and mortality
were not affected by treatment, but convulsions and hyperactivity
occurred in treated male and female rats. Photodieldrin was not
carcinogenic in this study (NCI, 1977).
8.8.1.4 Reproduction, embryotoxicity, and teratogenicity
(a) Mouse
Chernoff et al. (1975) fed groups of pregnant CD-1 mice (16 -
20 per group) photodieldrin in peanut oil (daily oral doses of 0,
0.15, 0.3, or 0.6 mg/kg body weight) from day 7 to day 16 of
gestation. At a dose of 0.6 mg/kg, one animal died. Liver/body
weight ratios were increased in a dose-related manner, but no
significant differences in fetal mortality, litter weight,
percentage of supernumerary ribs, or sternal or caudal ossification
centres were observed at any of the doses used. Photodieldrin was
not teratogenic or fetotoxic in CD-1 mice at doses up to and
including 0.6 mg/kg body weight.
(b) Rat
In a study by Chernoff et al. (1975), groups of 24 - 27
pregnant CD rats were given daily oral doses of photodieldrin in
peanut oil (0, 015, 0.3 or 0.6 mg/kg) on days 7 - 16 of gestation.
Some maternal mortality (5 out of 24 animals) occurred in the
0.6-mg/kg group. No significant differences in liver/body weight
ratios, fetal mortality, weight of the pups, or occurrence of
anomalies in litters of treated animals, compared with the
controls, were noted. No evidence of teratogenicity in CD rats was
observed at doses of photodieldrin up to and including 0.6 mg/kg
per day.
8.8.1.5 Appraisal
The acute oral toxicity of photodieldrin to rodents is greater
than that of dieldrin. In short-term toxicity and teratogenicity
studies, no major differences between the two compounds were found.
Photodieldrin did not induce tumours in mice and rats. The
accumulation of photodieldrin in the adipose tissue of experimental
animals was less than that of dieldrin (section 6.2.3).
8.8.2. Major metabolites of dieldrin
8.8.2.1 Acute toxicity
The acute oral toxicity of the major metabolites of dieldrin is
far less than that of dieldrin itself (Table 43).
8.8.2.2 Short-term toxicity
In a study by Granville et al. (1973), groups of 12 male and 12
female rats (control group of 24 males and 24 females) were fed
diets containing aldrin dicarboxylic acid (0, 0.1, 1, 10, 100, or
1000 mg/kg diet) for 13 weeks. No adverse effects attributable to
the dosing were observed in general health, behaviour, body weight,
clinical chemical and haematological values, organ weights, or on
pathological examination of the viscera.
Table 43. Oral LD50 values for metabolites of aldrin and dieldrin
in mice
-------------------------------------------------------------------
Compound LD50 Reference
(mg/kg body weight)
-------------------------------------------------------------------
trans-6,7-dihydroxy-dihydro- 1250 Korte & Arent
aldrin (1965)
9-hydroxy-dieldrin > 400 Baldwin et al.
(1970)
hexachlorohexahydromethano- > 850 Baldwin et al.
indenedicarboxylic acid (1972)
(aldrin dicarboxylic acid)
-------------------------------------------------------------------
8.9. Mechanisms of Toxicity; Mode of Action
Like most chemicals, aldrin and dieldrin do not have a single
mechanism of toxicity. The main target organs of these chemicals
are the central nervous system and the liver.
8.9.1. Central nervous system
Intoxication following acute or long-term overexposure is
characterized by involuntary muscle movements and epileptiform
convulsions. Survivors, after a short period of residual signs and
symptoms, recover completely (Hoogendam et al., 1962; Avar &
Czegledi-Janko, 1970; Jager, 1970). In rare cases, a residual
brain injury has been reported, but this has been found to be due
to the convulsive state or prolonged cerebral anaemia rather than
to the dieldrin per se. Apparently, a still unidentified receptor
site in the central nervous system is reversibly occupied, and when
this occupation exceeds a certain degree, myoclonics and
convulsions occur (Van Genderen, 1979). In vitro, the dieldrin
metabolite aldrin transdiol appears to be more potent in this
respect that is dieldrin itself (Van den Bercken, 1972; Van den
Bercken & Narahashi, 1974). However, in cats, the aldrin transdiol
appeared to be inactive (Joy, 1977). The mechanism of action seems
to be a presynaptic inhibition as well as an increased release of
an unidentified transmitter (Akkermans, 1974; Akkermans et al.,
1975; Joy, 1976).
Joy (1982) suggested that dieldrin acts by intensifying
synaptic activity through a presynaptic locus of action and
possibly a post-synaptic action as well. Neurons having a large
number of synapses will be affected most. There does not appear
to be any selective action on a particular neurotransmitter or
neurotransmitter system. The modification of behaviour is dose
dependent and performance in complex behavioural tasks is readily
disrupted.
Aldrin and dieldrin and other cyclodiens inhibit the gamma
amino butyric acid (GABA)-induced chloride ion uptake into skeletal
muscles and the binding of tritiated dihydropicrotoxinin (anion
channel probe) to the membrane. This results in central nervous
system excitation and convulsions due to the blocking of GABA
transmitters (Lawrence & Casida, 1984; Abalis et al., 1985).
8.9.2. Liver
The mode of action of aldrin and dieldrin on the liver involves
an increase in the activity of microsomal biotransformation
enzymes, particularly of the monooxygenase system with cytochrome
P-450. This induction of liver microsomal enzymes is reversible
and, if exceeding a certain degree, appears to be associated with
the occurrence of CHIRL and hepatomegaly in the liver of rodents
(sections 6.3 and 8.2) (Jager, 1970; Wright et al., 1972, 1977,
1978).
9. EFFECTS ON HUMAN BEINGS
9.1. General Population Exposure
9.1.1. Acute toxicity - poisoning incidents
When a toxic dose of aldrin or dieldrin has been ingested or
has contaminated the skin, effects appear from 20 min to 24 h
afterwards. Signs and symptoms may include headache, dizziness,
nausea, general malaise, and vomiting, followed by muscle
twitchings, myoclonic jerks, and convulsions. Death may result
from cerebral anoxaemia (Nelson, 1953; Princi, 1954; Hayes, 1957,
1963; Hoogendam et al., 1962, 1965; Kazantzis et al., 1964;
Schafer, 1968; Jager, 1970).
The duration of the interval between oral intake or skin
contact and onset of symptoms (as well as the clinical picture)
depends on the dose absorbed. With massive overexposure,
convulsions may occur even in the absence of any premonitory
symptoms.
Initially, there is no fever or change in blood count or in
blood chemistry. However, later the temperature may be elevated
and leukocytosis may occur. Terminal hyperthermia has been
reported. Abnormal EEG patterns showing spike and dome complexes
and multiple spike and wave discharges, or in less serious
intoxications, bilateral synchronous theta discharges may be seen.
The diagnosis needs to be confirmed by determining the insecticide
concentration in the blood.
The onset of clinical intoxication is practically always acute
also in those cases where the accumulation of dieldrin in the
target tissues has taken place during a much longer period. The
latter cases are, therefore, usually indistinguishable from acute
intoxication. Survivors almost always recover completely (Jager,
1970; Hayes, 1982).
Estimates of dosages in anecdotal cases suggest that fatalities
have occurred with ingestion of approximately 10 mg dieldrin/kg
body weight, (Hayes, 1982) but Hodge et al. (1967) estimate the
lethal dose of aldrin and/or dieldrin for the adult man to be about
5 g.
Cases of poisoning have occurred by ingestion of formulated
material, mostly in children by mistake (for instance when aldrin
is used in granules as bait to control ants) or by adults with
suicidal intent. Several cases of poisoning have been the result
of ingesting food contaminated with aldrin or dieldrin during
storage or transport.
Van Raalte (1965) surveyed the world literature for all cases
of fatal poisoning by aldrin and dieldrin, and found 13 cases:
four suicides, three due to accidental ingestion, five due to
accidental contamination, and only one (a spray operative) due to
occupational exposure. No cases of fatal poisoning have been
reported during the course of aldrin and dieldrin manufacture and
formulation.
A non-exhaustive overview of published poisoning cases is given
in Table 44. A more complete review is provided by Hayes (1982).
Table 44. Case reports on accidental and suicidal acute aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Causative agent Circumstances Reference
of cases
cases
------------------------------------------------------------------------------------------
1 - aldrin emulsifiable attempted suicide Spiotta (1951)
concentrate
53 - aldrin and other consumption of seed grain WHO (1958)
pesticides
13 aldrin and dieldrin review of all fatal cases from Van Raalte (1965)
literature:
- 4 suicides, 3 accidental
ingestion, 5 accidental
contaminations, 1 sprayer
2 1 5% dieldrin accidental ingestion Garrettson & Curley
(1969)
79 consumption of dieldrin- WHO (1977)
contaminated rice in Mali
1 - dieldrin (120 mg/kg) attempted suicide Black (1974)
2 - dieldrin Fry (1964)
12 - aldrin + BHC consumption of seed grain Gupta (1975)
------------------------------------------------------------------------------------------
9.1.2. Effects of short- and long-term exposure - controlled human
studies
9.1.2.1 Accidental poisoning
Twelve cases of neurotoxicity, resulting from the repeated
consumption of wheat into which aldrin dust and gammexane (BHC)
powder had been mixed accidentally, have occurred in India (Gupta,
1975). The patients consumed this wheat for 6 - 12 months before
showing typical clinical symptoms, including convulsions.
Electroencephalographic tracings were consistent with a diagnosis
of organochlorine insecticide poisoning. The patients were treated
with phenobarbital and diazepam. The latter was more effective in
controlling seizures. All patients recovered.
The threshold dieldrin concentration in the blood below which
no adverse effects have been observed (and none are to be expected)
is 105 µg/litre (see also section 9.2.1.1). The dieldrin
concentration in the blood of the general population, in the
countries where this has been investigated, is well below this
threshold level. However, there are rare cases in which it seems
that low concentrations of dieldrin have induced effects.
A rare, well investigated and well reported case of dieldrin-
induced immunohaemolytic anaemia was observed in Iowa, USA. The
patient had a haemolytic anaemia with a positive direct
antiglobulin (Coombs) test and a positive Ham test in the serum.
The serum contained anti-bodies selectively active against
erythrocytes coated with dieldrin. The patient improved following
splenectomy. Dieldrin concentrations in blood and fat were similar
to those of the general Iowa population (Hamilton et al., 1978). A
similar case was reported by Muirhead et al. (1959).
9.1.2.2 Controlled human studies
In section 6.2.2.4, reference was made to a pharmacodynamic
study in human volunteers. This study had three objectives:
(a) to establish the relationship between the daily intake of
dieldrin and its concentration in human blood and adipose
tissue;
(b) to establish the blood/fat ratio in human beings; and
(c) to establish the relationship between the concentrations
of dieldrin in blood and fat and the length of exposure
(Hunter & Robinson, 1967, 1968; Hunter et al., 1969).
In addition, the opportunity was taken to monitor the health of the
human subjects during and after the exposure by full clinical,
physiological, and laboratory examinations as well as full
electroencephalographic (EEG) studies, polygraphic recording of
cardio-respiratory function, measurement of basal metabolic rate,
and electroneuromyographic studies at frequent intervals to detect
the possible occurrence of changes in physiological function. The
study involved 13 adult male college graduates without a history of
recent occupational exposure to pesticides. The subjects received
0, 10, 50, or 211 µg dieldrin per day for 2 years. All the men
continued in excellent health. Clinical, physiological, and
laboratory findings remained essentially unchanged throughout the
whole experimental period of 24 months and the 8 months after
exposure. No departures from what is regarded as normal for the
general population were observed. The concentration of p,p'-DDE in
adipose tissue and blood did not show any significant change during
or after the study, indicating that the liver microsomal enzyme
activity had not been induced. Thus, the total daily intake of
230 µg (211 µg plus intake from food) of dieldrin per person for 2
years had no effect on health. The concentrations of dieldrin in
both adipose tissue and blood were shown to be proportional to the
daily intake (section 6.2.2.4).
9.1.3. Tissue concentrations of dieldrin in hospitalized people
9.1.3.1 Pathological findings
Specimens of human abdominal subcutaneous fat, obtained from
four hospitals in Chicago were analysed for residues of dieldrin.
Dieldrin was not present in 103 out of 221 samples analysed for
this pesticide. Positive samples contained 0.01 - 1.39 mg
dieldrin/kg fat (mean value 0.14 mg/kg). There was no correlation
between the dieldrin concentration in adipose tissue and
pathological findings (Hoffman et al., 1967).
When organochlorine pesticide concentrations were determined in
the adipose tissue and liver of 271 hospital patients in Miami,
USA, patients with typical alcoholic (Laennec's) cirrhosis of the
liver had about twice the dieldrin concentration in the liver of
that found in the normal population. In patients with post-
necrotic cirrhosis, fatty metamorphosis of the liver, metastatic
malignancy of the liver, or primary hepatocellular carcinoma,
dieldrin concentrations were "normal". Terminal cases with
carcinomas of different organs had elevated concentrations of
organochlorine pesticides in the fat, but no association with any
particular neoplastic disease was found (Radomski et al., 1968).
In Hawaii, emaciated patients who had carcinoma and/or focal or
generalized liver pathology were found to have "normal" (for the
USA) concentrations of dieldrin in the liver and body fat (Casarett
et al., 1968).
When concentrations of organochlorine pesticides were
determined in specimens of liver, brain, and adipose tissue from
autopsies of patients with cirrhotic liver disease in Vancouver
(Canada) hospitals, the concentrations of dieldrin appeared to be
no higher than in tissues from controls (Oloffs et al., 1974).
In a case-control study on 122 matched cancer patients in south
Florida, USA, a comparison was made of dieldrin residues in the
adipose tissue of cancer patients and controls. The mean dieldrin
concentration in the adipose tissue was 0.3 mg/kg fat in both
cancer patients and controls (Davies et al., 1975).
9.1.3.2 Influence of weight loss and stress on dieldrin
concentrations in tissues
It is well known that, in birds, and perhaps in some small
mammals, dieldrin intoxication may be induced by starvation, weight
loss, or stress in animals having a previously harmless body burden
of dieldrin. Concern is sometimes expressed that, by analogy to
these observations, a similar course of events might occur in human
beings.
Twenty-nine patients (14 males, 15 females) undergoing surgery
were investigated. The concentrations of dieldrin in the blood
were unaffected by the catabolic responses to surgery. In another
study, these authors determined the concentrations of dieldrin in
the blood of 4 women undergoing voluntary near-starvation for
slimming purposes, which resulted in weight losses of up to 7.5
kg/week. There was no increase in the concentration of dieldrin in
the blood (Hunter & Robinson, 1968).
No significant difference was found between the dieldrin blood
concentrations of slimming or non-slimming mothers before and after
delivery (Eckenhausen et al., 1981).
On the basis of these results and calculations, it is suggested
that significant weight loss does not result in increased
concentrations of dieldrin in human tissues (Van Raalte, 1965;
Hunter & Robinson, 1968).
9.1.4. Exposure in treated homes
From the data on aldrin concentrations in the air of houses
treated for termite control (section 5.1.2), an estimate of the
dieldrin concentration in the blood of occupants of these houses
can be made using the mathematical formulas given by Hunter et al.
(1969) for deriving blood concentrations from the average daily
intake. The average daily intake is based on an estimated average
in-house volume of air inhaled per day (15 m3). The dieldrin blood
levels of home dwellers, calculated in this way, remain far below
the blood concentration no-effect level for the general population
(secton 9.2.1.1).
The blood dieldrin concentrations of 59 female residents of
Dade County (Florida, USA), where many houses had been treated for
termite control, were of the order of 1 µg/litre (Barquet et al.,
1981).
Also relevant to the health of home dwellers is the experience
obtained in the 1950s and 1960s when tens of thousands of houses in
more than 30 countries were sprayed with dieldrin for malaria and
yellow fever eradication. Although exposures were presumed to have
been high, as a result of surface spraying inside and outside
houses, no adverse health effects were reported in home dwellers.
Neither were adverse effects observed in well-trained and
medically-supervised spray operators (Soper, 1955 (Personal
communication at the 2nd Meeting of the Industrial Council on
Tropical Health, Boston); Fletcher et al., 1959).
9.2. Occupational Exposure
9.2.1. Acute toxicity - poisoning incidents
With the exception of poisoning cases resulting from massive
acute overexposure, most reported cases of poisoning with aldrin
and dieldrin in occupationally-exposed men have been the result of
a slow build-up of the insecticide in the body, the daily intake
exceeding the daily excretion (Jager, 1970; Hayes, 1982).
Based on the experience of Jager (1970), it was suggested that
the classification of types of intoxication by Hayes (1963) be
modified as follows:
Type 1: an acute convulsive intoxication with no (or only
minor) prodomi, resulting from one or several gross overexposures.
Type 2: a greater number of smaller doses may cause an
accumulative intoxication. Clinically, this results in a syndrome
of headache, dizziness, drowsiness, hyperirritability, general
malaise, nausea, anorexia, occasional vomiting. At times muscle
twitchings, myoclonic jerks and convulsions may occur. In these
circumstances minor increases in the insecticide level in the
blood, perhaps caused by minor fluctuations in exposure, may bring
about a convulsive intoxication.
Type 3: this is actually a combination of Types 1 and 2. In
this type an overexposure, in itself not significant, causes an
acute convulsive intoxication superimposed upon a subclinical
accumulative intoxication of Type 2.
These three types of intoxication are schematically illustrated
in Fig. 3.
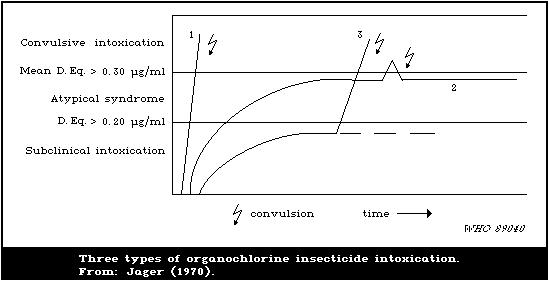 Table 45 gives a non-exhaustive overview of occupational aldrin
and dieldrin poisonings. Hayes (1982) contains further information
on this subject.
Table 45. Published case reports on occupational aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Acute Causative agent Circumstances Reference
of cases cases
cases
------------------------------------------------------------------------------------------
3 - ? 25% aldrin dust formulation with inadequate Nelson (1953)
safety precautions
~100 - ? various spraying in malaria- Hayes (1957,
eradication programmes 1959)
1 - 1 aldrin gross overexposure in aldrin Bell (1960)
packer
1 1 ? dieldrin sprayer Van Raalte
(1965)
4 - ? aldrin formulation of aldrin Kazantzis et
al. (1964)
17 - some aldrin and manufacturing and formulation Hoogendam et
dieldrin al. (1962,
1965)
32 (15 in - some aldrin, manufacturing and formulation Jager (1970)
addition dieldrin,
to endrin,
previous isobenzan
reference)
------------------------------------------------------------------------------------------
No cases of fatal poisoning have been reported during the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
However, there has been one case of a spray operator being fatally
poisoned (Van Raalte, 1965).
In developing countries it is, however, difficult to establish
the actual number of poisoning cases. Experience shows that even
when pesticides are banned, cases may occur in areas where control
is poor and where large quantities of pesticide are stocked. In
May 1987, four cases of occupational poisoning with aldrin were
reported from an area of cocoa plantations in Bahia (Brazil). One
of them was fatal, while three recovered. The fatal case had a
dieldrin level in whole blood exceeding 600 µg/litre two days after
poisoning (Rahde, personal communication).
9.2.1.1 Blood levels diagnostic of aldrin/dieldrin poisoning
Because the symptoms of aldrin/dieldrin intoxication are non-
specific, a differential diagnostic test is required to confirm
that the symptoms, signs, and clinical course of a particular case
are the result of aldrin or dieldrin intoxication.
The results of animal studies, together with those obtained
subsequently during medical surveillance of workmen employed in the
manufacture (or formulation) of aldrin/dieldrin, have shown that
adverse effects induced by aldrin/dieldrin are related to the
dieldrin blood concentration (Brown et al., 1964; Jager, 1970).
Therefore, the determination of this concentration provides a
specific differential diagnostic test.
Concentrations of dieldrin ranging from 40 to 530 µg/litre have
been reported in the blood of people who had been poisoned
relatively recently and who had recovered (Kazantzis et al., 1964;
Jager, 1970; Avar & Czegledi-Janko, 1970; Siyali & Simson, 1973).
From the limited information available, Brown et al. (1964)
concluded that the threshold concentration of dieldrin in the blood
of human beings, critical for intoxication, is approximately
150 - 200 µg/litre.
Dieldrin is present in the blood at very low concentrations in
the general population of many countries throughout the world. It
is also found at considerably higher concentrations in the blood of
healthy workers. These "healthy workmen" were men between 18 and
60 years of age who had no complaints or clinical or laboratory
signs attributable to occupational exposure, but they were, of
course, subject to the same ailments and diseases as are members of
the general population (Hayes & Curley, 1968; Jager, 1970). No
complaints of ill health and no positive results in objective
clinical or laboratory tests of ill health have ever been noted in
workers whose blood contained less than 200 µg/litre. This
concentration may, therefore, be considered to be a no-observed-
adverse-effect level in human beings. Higher concentrations may
have effects. The maximum concentration reported to be without
complaints or clinical signs or symptoms was 430 µg/litre (Jager,
1970).
Studies on animals have shown that the earliest physiological
sign of exposure to dieldrin is an increase in the activity of
certain liver microsomal enzymes. No enzyme induction has ever
been found in workers with dieldrin blood concentrations at or
below 105 µg/litre (Jager, 1970). (When liver enzyme induction
test methods became available, workers with dieldrin levels in the
blood exceeding 105 µg/litre were no longer encountered.)
Sometimes, dieldrin concentrations in plasma or serum, rather
than in whole blood, have been reported. The ratio of the dieldrin
concentration in plasma to that in erythrocytes is approximately
4:1 (Dale et al., 1965; Mick et al., 1972) (the conversion factor
to calculate the concentration of dieldrin in whole blood from the
concentration in plasma or serum is 0.66).
Great differences exist between the average concentrations of
dieldrin in the blood of the general population and of
occupationally exposed workers with or without complaints (Table
46).
Table 46. Concentrations of dieldrin in whole blood of human beings (µg/litre)
-------------------------------------------------------------------------------
No. of
Subjects persons Geometric Range Reference
involved mean
-------------------------------------------------------------------------------
General population 4592 <1 <1-16.1a US EPA (1983)
Unexposed persons 25 0.5 0-3.3 Sandifer et al. (1981)
20 2.5 0.5-10 Brown et al. (1964)
Healthy workers 35 29 <10-90 Jager (1970)
21 120e Mick et al. (1972)
37 55 -f Morgan & Roan (1974)
27 20 4.5-54 Sandifer et al. (1981)
89 38g 2-220 Brown et al. (1964)
Patients with 18 160g 8-280 Avar & Czegledi-Janko
clinical symptoms (1970)
4 40-530b Kazantzis et al. (1964)
5 130-370c Brown et al. (1964)
5 160-430d Brown et al. (1964)
Deceased (suicide) 1 850 Hayes (1982)
-------------------------------------------------------------------------------
a In serum.
b The low level of 40 µg/litre was found in a man with chronic nephritis,
complaining of headache and nausea; the occupational cause of the symptoms
is, therefore, doubtful. In one other worker, a blood level of 530 µg/litre
was found 1 month after a mild acute episode when he was exposed to aldrin
again.
c Determined some time after the acute episode.
d Estimated to be the concentration at the time of the acute episode.
e Converted from plasma figures using a factor of 0.66 (see 9.2.1.1).
f Highest value 231 kg/litre.
g Average.
Among 13 adolescent patients with colon-rectal adenocarcinomas,
who had lived in rural areas of Mississippi, USA, where pesticides
are widely sprayed, dieldrin blood levels were no higher than those
in controls (Caldwell et al., 1981).
9.2.1.2 Electroencephalography
Changes in the electroencephalogram (EEG) are sometimes of
practical importance for confirming a diagnosis of aldrin/dieldrin
intoxication (Spiotta, 1951; Winthrop & Felice, 1957; Hoogendam et
al., 1962, 1965; Kazantzis, 1964; Avar & Czegledi-Janko, 1970;
Jager, 1970). These EEG changes were first used as a practical
tool for monitoring workers and determining when they should
discontinue exposure and when they could be allowed to resume work
with aldrin/dieldrin. Characteristic changes - which, however, are
not pathognomonic for aldrin/dieldrin poisoning - include bilateral
synchronous spikes, spike and wave complexes, and slow theta waves,
thought to be possibly associated with brain stem stimulation
(Hoogendam et al., 1962, 1965). The interesting parallelism
between the rate of diminution of the EEG changes and the rate of
decrease in the dieldrin blood concentration was also reported in
the case of an accidentally poisoned child (Garrettson & Curley,
1969).
Nowadays, analysis for dieldrin in the blood has replaced EEG
examination as the method of choice for monitoring exposed workers
(Jager, 1970).
9.2.2. Effects of short- and long-term exposure
Occupational exposure occurred in the 1950s and early 1960s
among sprayers in malaria and yellow fever control programmes.
These men sprayed dieldrin inside houses day after day in prolonged
cycles without appreciable intervals of non-exposure. Quite often,
precautions and supervision were less than would be required today.
At the time, methods for the determination of blood concentrations
had not been developed. A significant percentage of these sprayers
became sick after having worked for as little as 2 days or as much
as 2 years (Hayes, 1957, 1959; Patel & Rao, 1958; Zavon & Hamman,
1961). According to communications by the Pan-American Sanitary
Bureau (Soper, 1955a), it appeared that no clinical symptoms were
observed in well-executed, well-supervised malaria and yellow fever
control programmes.
In a study carried out in East Africa, where workers were
spraying dieldrin 6 h/day for 180 days per year (with an interim of
2 months between spraying cycles) no clinical symptoms were seen.
The potential average dermal exposure of spray operators who
observed the protective measures laid down was 1.8 mg/kg body
weight per day (Fletcher et al., 1959).
Long et al. (1969) studied 159 farmers in Iowa, USA. Extensive
clinical and laboratory examinations of 33 pesticide users among
these farmers did not reveal evidence of any disease that could be
attributed to the use of pesticides, neither was the dieldrin blood
concentration correlatable with any parameter examined.
-------------------------------------------------------------------
a Personal communication at the 2nd Meeting of the Industrial
Council on Tropical Health, Boston.
The dieldrin blood concentrations in 8 locust-control workers
in Ethiopia were measured on two occasions and were found to range
between 0 and 9 µg/litre (MacCuaig, 1976).
Wolfe et al. (1963) studied the hazards from spraying orchards
with dieldrin in the US Pacific Northwest. Potential contamination
of the skin and respiratory exposure were measured. From the
results, potential skin and respiratory exposures were calculated
to amount to 14.2 and 0.25 mg/h, respectively.
Princi & Spurbeck (1951) studied a group of workers exposed
to chlordane, aldrin, and dieldrin for several years in a
manufacturing and formulating plant. The atmospheric
concentrations of aldrin were reported to be as high as 2.6 mg/m3.
Physical examinations and chest-röntgenograms did not reveal
respiratory anomalies.
A study was carried out on 71 men employed in the manufacture
and formulation of aldrin, dieldrin, endrin, and some other non-
related pesticides. Twenty-eight of these workers each contributed
a sample of blood and a sample of fat on the same day. The average
concentration of dieldrin in fat (6.12 ± 1.24 mg/kg) was 247 times
greater than the mean plasma concentration (0.025 ± 0.006
mg/litre). There was no relation between the amount of dieldrin in
the samples and the use of sick leave (Hayes & Curley, 1968).
In another study, 68 pesticide workers (including pest control
operators) and 29 unexposed controls were examined quarterly over a
period of four years. Determinations of serum pesticide
concentrations and enzyme activity, blood chemistry, haematology,
and urinalysis were carried out. The mean serum dieldrin
concentration was 3.6 ± 6.3 µg/litre (1.1 ± 1.6 µg/litre in the
controls). There was no difference between the exposed workers and
the controls in the incidence of disease or disability (Warnick &
Carter, 1972).
In a pesticide formulation plant, the blood of 21 employees was
examined at the conclusion of a 5-week period during which 900 kg
of technical aldrin was formulated. The mean dieldrin
concentration in plasma was 11 and 182.5 µg/litre for herbicide
formulators and aldrin formulators, with a maximum of 317 µg/litre
in the latter case. No mention was made of any intoxications (Mick
et al., 1972).
In a group of 42 occupationally exposed pesticide workers with
a dieldrin serum concentration 5 times as high as that in a group
of 23 controls, no indication of disturbed renal- or adrenocortical
function was found (Morgan & Roan, 1969, 1973).
A study was carried out in California, USA, where aldrin (EC as
a 0.5% solution) at 480 g/litre was applied as a termiticide to
typical slab and crawl space type houses. Personal air samples,
samples of blood, and samples of pads on clothes and gloves were
taken to monitor the exposure of the pest-control operators. The
personal air samples during application contained less than 0.3
µg/m3 aldrin for the slab houses and 30 - 75 µg/m3 for the crawl
space houses. The total work day (9 - 18 h) time-weighted average
concentration of aldrin in air was 6 - 17 µg/m3. This is far below
the threshold limit value (TLV) for aldrin established by the
American Conference of Governmental Industrial Hygienists (ACGIH,
1986) of 250 µg/m3. Data from dermal exposure samples showed large
variation. However, the maximum calculated percentage of the toxic
dose per h, based on the acute percutaneous toxicity (rat LD50) of
the formulation, was less than 0.01%. The concentration of aldrin
and dieldrin in the blood of the operators was below the limit of
detection (less than 1 µg/litre) (Marlow et al., 1982).
A case-control study, carried out on 27 pesticide workers
(4 formulators and 23 pest-control operators) with elevated blood
concentrations of dieldrin, revealed a mean blood concentration of
19.59 µg/litre (range: 4.45 - 54 µg/litre). In an extensive
clinical examination, including physical examination, comprehensive
neurological examinations, laboratory tests, and physiological and
psychomotor testing, no important differences were found compared
with results in a control group of 25 people with a mean dieldrin
blood concentration of 0.48 µg/litre (range of 0 - 3.34 µg/litre)
(Sandifer et al., 1981).
9.2.3. Epidemiological studies
An extensive study on workers in an aldrin/dieldrin
manufacturing plant has been in progress since the plant began
operations in the 1950s. The results from the first 15 years of
this epidemiological study were reported in 1970 (Jager, 1970).
From a total of more than 800 exposed workers, all those exposed
for more than 4 years (233 men) or those who had experienced an
intoxication (20 men) underwent extensive physical, neurological,
haematological, and other laboratory examinations. Clinical
chemical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein, and serum protein spectrum, were
made every 3 months and remained within normal limits. A no-effect
level in this group of workers, including those who had previously
suffered intoxications, was established at a dieldrin blood
concentration of 200 µg/litre. This level corresponds to a total
equivalent daily oral intake of 33 µg/kg body weight or a total
daily intake of 2300 µg/person per day (Hunter & Robinson, 1967).
In experimental animals, the earliest, reversible effect of
dieldrin is the induction of liver microsomal enzyme systems
(Wright et al., 1977, 1978). This finding led to an investigation
of a group of 10 workers. At the time, due to further improvements
in the industrial hygiene of the above-mentioned plant, the
geometric mean concentration of dieldrin found in the blood of
workers was 105 µg/litre. As criteria of enzyme induction
measurements were made of the blood levels of p,p'-DDE, the urinary
ratio of 6-beta-hydroxycortisole and 17-hydroxycorticosteroids, and
the urinary excretion of D-glucaric acid. No difference in these
values was found between the 10 exposed workers and a control
group. On the basis of these data, the no-effect level was 105 µg
dieldrin/litre blood, equivalent to an oral daily intake of 17.4
µg/kg body weight per day (or 1220 µg/person per day) (Jager, 1970;
Hunter et al., 1969; Hunter & Robinson, 1967; Versteeg & Jager,
1973).
Further results from this long-term survey of an industrial
population were subsequently published, based on a study of 1000
workers. Because not all of the workers had severe and/or
prolonged exposure, smaller groups with an exposure meaningful
enough for carcinogenicity evaluation were included. One group
consisted of 166 men (including workers who were still exposed and
workers who had left the company), with a mean exposure time of
16.9 years (range 4 - 19 years), who had been under observation for
more than 15 years (mean observation period 17 years; range,
15 - 20 years). A sub-group comprised 69 men with a mean exposure
time of 14.9 years (range 10 - 19 years) and a mean observation
period of 17.2 years. Among the group of 166 workers, 51 were more
than 50 years old. One man with only 5 years of comparatively mild
exposure died because of a gastric carcinoma. A lymphosarcoma
occurred in a man with 7 years of very mild exposure. Both
incidences occurred before 1964. No new cases were noted in the
final 11 years of study, and no undue mortality from other causes
that could have masked a higher cancer incidence was observed
(Versteeg & Jager, 1973; Van Raalte, 1977).
In a follow-up study on the original group of 233 men with more
than 4 years of exposure and an observation period ranging from 4
to 29 years (mean, 24 years), there were no indications of a
specific carcinogenic activity. Total observed mortality was 25
deaths versus 38 expected. Of nine cancer deaths, three were
caused by lung cancer, while the remaining six were each of a
different nature. No primary liver tumours were observed (Ribbens,
1985).
In a study by Morgan & Roan (1974), 28 pesticide formulators
and applicators, plus a separate group of 43 termite-control
workers, with occupational exposures of 5 - 22 years were examined,
together with 56 controls. The highest levels of dieldrin among
these 71 workers were found in a group of 37 men who had a mean
serum dieldrin concentration of 84 µg/litre (equivalent to about
55 µg/litre whole blood). There were no signs of liver cell injury
and the serum enzyme activities SGOT, SGPT, LDH, alkaline
phosphatase and creatine phosphokinase (CPK) were within normal
limits. There was no indication of drug-metabolizing enzyme
induction and urinary excretion of D-glucaric acid was not
different from that in a control group.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides, revealed
that, in 1962, 18 and 10 years after the introduction of DDT and
aldrin/dieldrin, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the cases of
primary liver cancer as a percentage of the total number of liver
cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) of
primary liver cancer for this period declined from 3.46 to 3.18
(Deichmann & MacDonald, 1977).
An epidemiological mortality study in a plant manufacturing
aldrin, dieldrin, and endrin was carried out on a cohort of 1155
workers who had been employed for at least 6 months between 1946
and 1976 (almost 25 000 man-years of observation). The mortality
due to all malignant neoplasms was 31, lower than expected
(standardized mortality ratio (SMR) 82). The total mortality from
all causes was 173 (SMR 84). The only disease with an SMR above
100 (SMR 212) was "non-malignant respiratory system disease",
specifically pneumonia. There was a slight excess of oesophagus
and rectum cancer (two and three cases observed with an SMR of 235
and 242, respectively), liver cancer (two cases observed versus
0.57 expected), and cancer of the lymphatic and haematopoietic
system (six cases observed versus 4.07 expected). However, there
was a deficit of cancer of other sites. The authors concluded that
"the study has not identified a specific cancer risk associated
with employment at this manufacturing plant, but several causes
should be examined further" (Ditraglia et al., 1981).
In a 1981 health survey, a total of 567 serum samples from 1811
Florida citrus workers were collected during the spraying and the
harvest season and were compared with the national ("Hanes")
sample. There were no differences in serum dieldrin levels; the
mean in both groups being 1.8 - 1.9 µg/litre serum (Griffith &
Duncan, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Aldrin and dieldrin, organochlorine pesticides, were used
throughout the world from 1950 until the early 1970s as
insecticides in agriculture and as a seed treatment, for the
control of soil pests and other types of insects (e.g., termites,
grasshoppers, and textile pests), and for the control of tsetse
flies and other disease vectors. The compounds act as contact and
stomach poisons in the insects. Since the early 1970s, both
compounds have been restricted or banned from use in several
countries, especially in agriculture. Nevertheless, use continues
in other countries for termite control.
Both compounds are practically insoluble in water and
moderately to highly soluble in many organic solvents. The vapour
pressure is low.
Dairy and meat products, fish, oils and fats, and certain
vegetables such as root vegetables often contain dieldrin. Maximum
residue limits recommended by the FAO/WHO Joint Meeting on
Pesticide Residues range from 0.02 to 0.2 mg/kg product. Recent
measurements have shown that actual levels are lower, and this has
been confirmed by total diet studies. Since the use of these two
compounds has been restricted, a steady but slow decrease in
residue levels in the different food commodities has taken place.
The intake by human beings of low concentrations in the daily
diet has resulted in dieldrin being present in adipose tissue and
in some other tissues and organs. Global surveys have shown that
mean values range from 0.1 to 0.4 mg/kg adipose tissue. Since the
early 1970s, this concentration has slowly decreased.
Transplacental exposure of the fetus occurs, with the result
that the fatty tissues of the fetus also contain dieldrin, but at
concentrations 10 - 50% of those of the mother. There seems to be
an equilibrium between levels in the fetus and those in the mother.
Dieldrin is also excreted with the milk. Inhabitants of houses
that have been treated for termite control may be exposed by
inhalation. Concentrations in the air found after indoor treatment
may range from 0.01 to 7 µg/m3, depending on the type of
applications, concentration used, type of ventilation, and time of
sampling. Under these conditions food may also be contaminated by
direct contact or by sorption from the atmosphere.
Metabolism takes place mainly in the liver where aldrin is
readily transformed to dieldrin. Dieldrin is degraded at a slower
rate to hydrophilic metabolites, which are then excreted via the
bile and urine. The structures of these metabolites have been
established. In all species examined, including human beings, it
has been shown that there is a steady state of aldrin/dieldrin
storage corresponding to the level of intake and a linear
relationship between the log of intake and storage has been
demonstrated. The concentration of dieldrin in body tissues
decreases exponentially on termination of exposure to the
compounds.
The acute oral toxicity of aldrin and dieldrin for mammals is
high, while the dermal toxicity is moderate. Dermal sensitization
has not been found. Effects observed in acute, short-term and
long-term studies involve the central nervous system. The liver is
also a target organ. In the liver of mice and rats, changes known
as "chlorinated hydrocarbon insecticide rodent liver" are found.
Aldrin and dieldrin do not appear to cause teratogenic effects
at doses below those causing maternal toxicity and fetotoxicity.
Male or female reproductive toxicity has not been reported.
Numerous in vitro and in vivo mutagenicity studies have
demonstrated that neither aldrin nor dieldrin have mutagenic
potential.
In long-term studies, aldrin and dieldrin induced benign and
malignant liver tumours in the mouse. However, no increased
incidence of liver tumours or other tumours were found in rats and
hamsters.
IARC (1987) has stated that there is inadequate evidence of
carcinogenicity in human beings and limited evidence of
carcinogenicity in experimental animals. Both aldrin and dieldrin
have been classified in Group 3: the chemicals cannot be
classified as to their carcinogenicity in human beings.
On the basis of available short-term and long-term toxicity
data, the overall no-observed-adverse-effect level in the rat is
0.5 mg dieldrin/kg diet, equivalent to 0.025 mg/kg body weight. In
the dog, the lowest no-observed-adverse-effect level found was 0.04
mg/kg body weight. The Joint Meeting on Pesticide Residues (JMPR)
established an Acceptable Daily Intake (ADI) of 0.1 µg/kg body
weight in 1966 and 1977 based on the conclusion that aldrin and
dieldrin were not human carcinogens.
Aldrin and dieldrin are highly toxic to human beings. Both
accidental and occupational cases of poisoning have occurred but
reported fatalities have been rare. Survivors of acute or subacute
intoxications recovered completely. Adverse effects are related to
the dieldrin blood concentration, the determination of which
provides a specific diagnostic test for aldrin/dieldrin exposure.
At a dieldrin blood concentration below 105 µg/litre, no adverse
effects can be expected. This level is considered a threshold no-
observed-adverse-effect level and corresponds to a daily intake of
0.02 mg dieldrin/kg body weight per day.
Environmental, mainly dietary, exposure leads to the presence
of dieldrin in low concentrations in the human body. The results
of extensive clinical and epidemiological studies indicate that
these body burdens do not present a health hazard to human beings.
No signs of any premonitory change in liver function were found
in a 20-years study, involving more than 1000 industrial workers
exposed to aldrin and dieldrin. In this study and another study in
the USA, no specific cancer risk could be identified associated
with occupational exposure to (sometimes high levels of) aldrin and
dieldrin.
All the available information on aldrin and dieldrin taken
together, including studies on human beings, supports the view that
for practical purposes, these chemicals make very little
contribution, if any, to the incidence of cancer in human beings.
Photodieldrin, the photo-decomposition product of dieldrin, is
similar to dieldrin in its short-term toxicity. It is not
teratogenic or carcinogenic in mice and rats. The accumulation of
photodieldrin in the adipose tissue of experimental animals was
less than that of dieldrin.
10.2. Evaluation of Effects on the Environment
Aldrin, used as a soil insecticide, is the major source of
dieldrin (up to 97%) in the environment. Aldrin and its reaction
product dieldrin are rapidly adsorbed on soils, especially soils
containing a high level of organic matter. Consequently there is
little penetration into the soil, and contamination of groundwater
does not generally occur. Transport of both compounds takes place
mainly through soil erosion (as wind drift) and sediment transport
(surface run-off), but not through leaching.
The use of aldrin and dieldrin in agriculture leads to residues
(mainly of dieldrin) in the soil that can persist for years; the
estimated half-life of dieldrin is between 4 and 7 years. Under
tropical conditions, the compounds are less persistent than under
temperate conditions.
Aldrin and dieldrin enter the atmosphere through volatilization
from treated crops and soil or, directly, during the application of
the pesticide. Dieldrin returns to soil and water surfaces by
washout and dry deposition. Thus, the compounds are found either
in the vapour phase (very low levels, in general 1 - 2 ng/m3),
adsorbed by dust particles, or in rainwater (of the order of
10 - 20 ng/litre).
The occurrence of dieldrin in the aquatic environment has been
reported by several authors. The concentrations in surface water
are mainly very low, less than 5 ng/litre. However, concentrations
in areas of soil erosion or agricultural use may be higher.
Sediment in rivers in these areas may contain up to 1 mg
dieldrin/kg. The high capacity for aquatic organisms to
concentrate dieldrin from very low levels in water could lead to
toxic levels in aquatic organisms. Concentration through aquatic
foodchains is of less importance than direct uptake from water.
Because of the widespread occurrence of dieldrin in the
environment and its persistence, there is a wide range of
concentrations in non-target organisms. Whereas the concentrations
previously ranged from 0.001 mg to 100 mg/kg tissue, they are now
mostly below 1 mg/kg tissue.
In terrestrial ecosystems, aldrin and dieldrin are accumulated
by a wide variety of organisms, principally as dieldrin. Dieldrin
is probably responsible for the deaths of mammals in the field and
for the decline in population size in some species, such as the
otter. Small mammals would be killed by eating dieldrin-dressed
grain, but populations of these animals are likely to have been
replenished by immigration from surrounding areas. Birds of prey
eating small mammals and small birds contaminated by dieldrin take
up and accumulate dieldrin in their own tissues and eggs.
Granivorous birds have been killed by eating dressed grain. It is
probable that the population decline in birds of prey was caused by
dieldrin residues (among other organochlorine residues) in their
tissues. The effects of dieldrin are seen some time after the
exposure, because residues are stored in fat over winter, to be
released in the spring. When dieldrin was used only at certain
times of the year, this did not prevent bird mortalities.
The widespread use of aldrin and dieldrin, in conjunction with
other organochlorine pesticides, has led to severe detrimental
effects on the environment, though with drastic curtailment of use,
particularly in seed dressings, there has been some recovery in
bird populations.
10.3. Conclusions
(a) Both aldrin and dieldrin have been subjected to intensive and
wide-ranging study, toxicologically, clinically, and
epidemiologically. The body burden is mainly the result of
the oral ingestion of residues in the diet (which seem
generally to fall within the promulgated ADIs) and, to a
lesser extent, of inhalation. Evaluation of the data suggests
strongly that the body burden resulting from the present level
of exposure constitutes no health risk to the general
population.
(b) Dieldrin occurs almost ubiquitously in human breast milk.
However, its concentration in the blood and adipose tissue of
suckling infants does not increase with age during the first
six months, nor is their blood dieldrin level higher than that
of bottle-fed babies. Under these circumstances, the benefits
of natural breast feeding still make it the preferred method
of infant feeding, in spite of the dieldrin residues.
(c) In the treatment of premises, notably for termite control, the
exposure of occupants does not appear to be increased to a
level that endangers their health, as long as the directions
for safe practice are conscientiously respected.
(d) Despite the highly toxic nature of aldrin and dieldrin, both
of these chemicals can be handled safely as long as the
recommended precautions to minimize worker exposure are always
observed. Neglect of these rules may lead to the poisoning of
operators.
(e) During the period of high aldrin and dieldrin use between 1950
and 1970, detrimental effects were undoubtedly inflicted upon
species in the environment. These effects were due partly to
dieldrin and partly to other organochlorines. Since the
drastic curtailment of the use of these materials, the
affected species have recovered in numbers.
11. RECOMMENDATIONS
1. A further, properly designed, teratogenic investigation is
required in the hamster, with dieldrin at realistic dose
levels.
2. Research into the mechanism of carcinogenesis should be
directed to explaining why the hepatic reaction in the mouse
is different from that of other species.
3. Dieldrin should be selected as an agent for further study of
neurotoxic mechanisms, both experimentally and clinically.
4. To protect the environment, large-scale use of aldrin and
dieldrin must not be resumed, and applications should be
confined to those situations in which no safer, equally
effective alternatives can be recommended.
5. For the health and welfare of workers and the general
population, the handling and application of aldrin and
dieldrin should only be entrusted to well trained competent
operators, who will follow adequate safety measures.
6. To avoid accidental poisoning from aldrin, especially among
children, the use of aldrin granules as an ant bait should be
forbidden.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Aldrin and dieldrin were evaluated by the FAO/WHO Joint
Meeting on Pesticide Residues (JMPR) in 1963, 1965, 1966, 1967,
1968, 1969, 1970, 1974, 1975, and 1977 (FAO/WHO, 1964, 1965a,b,
1967a,b, 1968a,b, 1969a,b, 1970a,b, 1971a,b, 1975a,b, 1978a,b).
From 1966 onwards, the JMPR established an acceptable daily intake
(ADI) of 0 - 0.0001 mg/kg body weight (combined total for aldrin
plus dieldrin). This was based on a level causing no toxicological
effect of:
0.5 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
rat; and
1 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
dog.
The maximum residue limits (MRLs) listed in Table 47 were
recommended by the FAO/WHO Joint Meeting on Pesticide Residues in
1970 and 1975 and are quoted as the sum of aldrin plus dieldrin.
Table 47. Maximum residue limits (MRL's) recommended by the Codex
Alimentarius Commission (FAO/WHO, 1986)
-------------------------------------------------------------------
Commodity Aldrin and dieldrin
(mg/kg)
-------------------------------------------------------------------
Potatoes 0.1
Fat of meat 0.2a
Carrots, lettuce, fat of meat 0.1a
Asparagus, aubergines, broccoli, Brussels 0.1
sprouts, cabbage, cauliflower, cucumbers,
horse radish, onions, parsnips, peppers,
pimentos, radishes, radish tops
Eggs (shell-free) 0.1a
Milk and milk products (fat basis) -
Milk 0.006a
Fruit 0.05
Rice (in husks) 0.02
Raw cereals (other than rice) 0.02a
-------------------------------------------------------------------
a Extraneous residue limit.
WHO (1984) recommended that the level of aldrin and dieldrin
in drinking-water should not exceed 0.03 µg/litre.
IARC evaluated aldrin and dieldrin on several occasions.
Aldrin and dieldrin were found to be carcinogenic in the liver in
mice, but there was no evidence for carcinogenicity in other
organs. The data available did not provide evidence of
carcinogenicity in rats. Data on dogs, monkeys, and human beings
were too limited to allow any conclusions (IARC, 1974). IARC
considered that there was inadequate evidence of carcinogenicity
in humans and limited evidence of carcinogenicity in experimental
animals. Accordingly, both chemicals were classified in Group 3
(IARC, 1987).
The Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified the acute hazard to
health for technical dieldrin as "highly hazardous" (WHO, 1988).
The same division published a data sheet on aldrin (79.41) and
dieldrin (75.17) (WHO/FAO, 1975-85).
REFERENCES
ABALIS, I.M., ELDERFRAWI, M.E., & ELDERFRAWI, A.T. (1985) High-
affinity stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic. Biochem. Physiol., 24: 95-102.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1966)
Organochlorine pesticides in the atmosphere. Nature (Lond.), 211:
259-261.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67.
Organochlorine pesticide residues in the total diet. J. Sci. Food
Agric., 20(4): 245-259.
ABBOTT, D.C., COLLINS, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71. Br.
med. J., 1972(2): 553-556.
ABBOTT, D.C., COLLINS, G.B., GOULDING, R., & HOODLESS, R.A. (1981)
Organochlorine pesticide residues in human fat in the United
Kingdom 1976-77. Br. med. J., 283: 1425-1428.
ACGIH (1986) TLVs. Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, 111 pp.
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fat.] Naturwissenschaften, 61: 32 (in German).
ACKER, L., BARKE, E., HAPKE, H.J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk.] Milchwissenschaft., 39(9): 541-544 (in German).
ADDISON, R.F., ZINCK, M.E., & ACKMAN, R.G. (1972) Residues of
organochlorine pesticides and polychlorinated biphenyls in some
commercially produced Canadian marine oils. J. Fish Res. Board
Can., 29: 349-355.
ADDISON, R.F., ZINCK, M.E., & LEAHY, J.R. (1976) Metabolism of
single and combined doses of 14C-aldrin and 3H-p,p'-DDT by
Atlantic salmon (Salmo salar) fry. J. Fish Res. Board Can., 33(9):
2073-2976.
ADEMA, D.M.M. & VINK, G.J. (1981) A comparative study of the
toxicity of 1,1,2-trichloroethane, dieldrin, pentachlorophenol, and
3,4-dichloroaniline for marine and fresh-water organisms.
Chemosphere, 10(6): 533-554.
AGNIHOTRI, N.P., PANDEY, S.Y., JAIN, H.K., & SRIVASTAVA, K.P.
(1977) Persistence of aldrin, dieldrin, lindane, heptachlor, and
p,p'-DDT in soil. J. entomol. Res., 1(1): 89-91.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977a) Pesticide induced
ouabain resistant mutants in Chinese hamster V-79 cells. Chem.-
biol. Interact., 19(3): 369-374.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977b) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res., 42:
161-174.
AKKERMANS, L.M.A. (1974) Mode of action of dieldrin. An electro-
physiological investigation, Utrecht, The Netherlands, Rijks
Universiteit (Thesis).
AKKERMANS, L.M.A., VAN DEN BERCKEN, J., & VERSLUYS-HELDER, M.
(1975) Excitatory and depressant effects of dieldrin and aldrin-
transdiol in the spinal cord of the toad (Xenopus laevis). Eur. J.
Pharmacol., 34: 133-142.
ALBERT, L., MENDEZ, F., CEBRIAN, M.E., & PORTALES, A. (1980)
Organochlorine pesticide residues in human adipose tissue in
Mexico: results of a preliminary study in three Mexican cities.
Arch. environ. Health, 35(5): 262-269.
ANAS, R.E. & WILSON, A.J. (1970a) Organochlorine pesticides in fur
seals. Pestic. monit. J., 3(4): 196-200.
ANAS, R.E. & WILSON, A.J. (1970b) Organochlorine pesticides in
nursing fur seal pups. Pestic. monit. J., 4(3): 114-116.
ANDERSON, B.G. (1960) The toxicity of organic insecticides to
Daphnia. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Proceedings of the 2nd Seminar, 1959, Cincinnati, Ohio,
Robert A. Taft Sanitary and Engineering Center, pp. 94-95
(Technical Report W60-3).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain
North American birds. In: Proceedings of the 15th International
Ornithology Congress, Leiden, Brill, pp. 514-540.
ANDERSON, P.D. & WEBER, L.J. (1975) Toxic response as a
quantitative function of body size. Toxicol. appl. Pharmacol., 33:
471-483.
ANON. (1964) Report on the Working Conference on Birds of Prey and
Owls, Caen, Normandy, 10-12 April, 1964, Washington, DC,
International Council for Bird Preservation.
ANON. (1973) Aldrin and the ultraviolet conversion products and
possible metabolites of aldrin and dieldrin in human milk from the
community studies on pesticides, Berkeley, California, Department
of Public Health, (Abstract prepared by Shell Development Company,
Modesto, California: Report TIR-24-123-73: Part II).
ANON. (1974) Data for pentachloroketone and UV-dieldrin in human
kidney and adipose tissue from Coral Gables, Florida, Modesto,
California, Shell Development Company (Report TIR-24-176-73: Part
II).
ANON. (1974b) Data for dieldrin, pentachloroketone, and UV-dieldrin
in human adipose tissue from Albany, New York, Modesto,
California, Shell Development Company (Report TIR-24-169-73: Part
II).
ANON. (1974c) Determination of dieldrin levels in human kidney and
adipose tissue, Modesto, California, Shell Development Company
(Report TIR-24-176-73).
ASHWOOD-SMITH, M.J. (1981) The genetic toxicology of aldrin and
dieldrin. Mutat. Res., 86: 137-154.
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ASTOLFI, E., GARCIA FERNANDEZ, J.C., DE JUAREZ, M.B., & PIACENTINO,
H. (1973) Chlorinated pesticides found in the fat of children in
the Argentine Republic. In: Deichmann, W.B., ed. Pesticides and the
environment, New York, Intercontinental Medical Book Company, pp.
233-243.
ASTOLFI, E., ALONSO, A.H., MENDIZABAL, A., & ZUBIZARRETTA, E.
(1974) Pesticides chlorés de l'accouchée et du cordon ombilical des
nouveaunés. J. eur. Toxicol., 7: 330-338.
ATKINS, D.H.F. & EGGLETON, A.E.J. (1970) Studies of atmospheric
washout and deposition of gamma-BHC, dieldrin, and p,p'-DDT using
radiolabelled pesticides, Harwell, United Kingdom Atomic Energy
Research Authority (Report HL 70/4532(C10)).
ATKINS, T.D. & LINDER, R.L. (1967) Effects of dieldrin on
reproduction of penned hen pheasants. J. wildl. Manage., 31(4):
746-753.
AULERICH, R.J., RINGER, R.K., & POLIN, D. (1972) Rate of
accumulation of chlorinated hydrocarbon pesticide residues in
adipose tissue of mink. Can. J. Zool., 50(9): 1167-1173.
AVAR, P. & CZEGLEDI-JANKO, G. (1970) Occupational exposure to
aldrin: clinical and laboratory findings. Br. J. ind. Med., 27(3):
279-282.
BAECKSTROEM, J., HANNSON, E., & ULLBERG, S. (1965) Distribution of
14C-DDT and 14C-dieldrin in pregnant mice determined by whole-body
autoradiography. Toxicol. appl. Pharmacol., 7: 90-96.
BAILEY, G.W., SWANK, R.R., Jr, & NICHOLSON, H.P. (1974) Predicting
pesticide runoff from agricultural land: a conceptual model. J.
environ. Qual., 3(2): 95-102.
BAKER, A.H., WHITNEY, G.F.H., & WORDEN, A.N. (1959) The toxic
hazard associated with continuous-flow heat-volatilized
insecticidal and acaracidal aerosols. Lab. Pract., 8: 3-10.
BAKKEN, A.F. & SEIP. M. (1976) Insecticides in human breast milk.
Acta paediatr. Scand., 65: 535-539.
BALAYANNIS, P.G. (1974) Organochlorine pesticide residues in
tomatoes Ann. Inst. Phytopathol. Benaki (N.S.), 11: 47-52.
BALDWIN, M.K. & ROBINSON, J. (1969) Metabolism in the rat of the
photoisomerization product of dieldrin. Nature (Lond.), 224(5216):
283-284.
BALDWIN, M.K., ROBINSON, J., & CARRINGTON, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Ind., 1970: 595-597.
BALDWIN, M.K., ROBINSON, J., & PARKE, D.V. (1972) A comparison of
the metabolism of HEOD (dieldrin) in the CF1 mouse with that in the
CFE rat. Food Cosmet. Toxicol., 10: 333-351.
BALDWIN, M.K., DAVIS, R.A., & THORBURN BURNS, D. (1973) Structural
studies and photochemical re-arrangement of an animal metabolite of
HEOD, the active component of dieldrin. Pestic. Sci., 4: 227-237.
BALDWIN, M.K., BENNETT, D., & BEYNON, K.I. (1977) The
concentrations of aldrin and dieldrin and their photoisomers in the
atmosphere. Pestic. Sci., 8: 431-445.
BALUJA, G., MURADO, M.A., & TEJEDOR, M.C. (1975) Adsorption and
desorption of lindane and aldrin by soils as affected by soil main
components. Environ. Qual. Saf., Suppl. 3: 243-249.
BALUJA, G., HERNANDEZ, L.M., GONZALEZ, J., & RICO, C. (1982)
Presence of organochlorine pesticides, polychlorinated biphenyls
and mercury in Spanish human milk samples. Bull. environ. Contam.
Toxicol., 28: 573-577.
BARLOW, F. & HADAWAY, A.B. (1955) Studies on aqueous suspensions of
insecticides V. The sorption of insecticides by soils. Bull.
entomol. Res., 46: 547-559.
BARLOW, F. & HADAWAY, A.B. (1956) Effects of changes in humidity on
the toxicity and distribution of insecticides sorbed by some dried
soils. Nature (Lond.), 178: 1299-1300.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D. (1979)
Organochlorine pesticide residues in human milk samples from women
living in northwest and northeast Mississippi, 1973-75. Pestic.
monit. J., 13(2): 47-51.
BARON, R.L. & WALTON, M.S. (1971) Dynamics of HEOD (dieldrin) in
adipose tissue of the rat. Toxicol. appl. Pharmacol., 18(4): 958-963.
BARQUET, A., MORGADE, C., & PFAFFENBERGER, C.D. (1981)
Determination of organochlorine pesticides and metabolites in
drinking-water, human blood-serum, and adipose tissue. J. Toxicol.
environ. Health, 7: 469-479.
BARRETT, R.T., SKAARE, J.U., NORHEIM, G., VADER, W., & FROSLIE, A.
(1985) Persistent organochlorines and mercury in eggs of Norwegian
seabirds 1983. Environ. Pollut. Ser. A, 39: 79-93.
BASSON, N.C.J. (1971) Effects of dieldrin and its photoisomerization
product photodieldrin on birds. Phytophylactica, 3: 115-124.
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1971) Growth
response of blue-green algae to aldrin, dieldrin, endrin, and their
metabolites. Bull. environ. Contam. Toxicol., 6(6): 589-594.
BAXTER, W.L., LINDER, R.L., & DAHLGREN, R.B. (1969) Dieldrin
effects in two generations of penned hen pheasants. J. wildl.
Manage., 33(1): 96-102.
BEALL, M.L., Jr & NASH, R.G. (1969) Crop seedling uptake of DDT,
dieldrin, endrin and heptachlor from soils. Agron. J., 61: 571-575.
BEALL, M.L., Jr & NASH, R.G. (1971) Organochlorine insecticide
residues in soybean plant tops: root vs vapour sorption. Agron. J.,
63: 460-464.
BEALL, M.L., Jr & NASH, R.G. (1972) Insecticide depth in soil -
Effect on soybean - Uptake in the greenhouse. J. environ. Qual.,
1(3): 283-288.
BEDFORD, C.T. (1974) Von Baeyer/IUPAC names and abbreviated
chemical names of metabolites and artifacts of aldrin (HHDN),
dieldrin (HEOD), and endrin. Pestic. Sci., 5: 473-489.
BEDFORD, C.T. & HARROD, R.K. (1972a) Synthesis of 9-hydroxy-HEOD,
a major mammalian metabolite of HEOD (dieldrin), Sittingbourne,
Shell Research, Tunstall Laboratory (TU/7/72).
BEDFORD, C.T. & HARROD, R.K. (1972b) An improved preparation of
trans-4,5-dihydroxy-4,5-dihydroaldrin, a metabolite of HEOD
(dieldrin) in mammals, insects, and microorganisms. Chemosphere,
1(6): 255-260.
BEDFORD, C.T. & SMITH, E.H. (1978) Synthesis of dieldrin
metabolites. III. Two-step conversion of syn-12-hydroxy dieldrin
into Klein's metabolite (3,5,6,6,7-pentachloro-11,12-exo-epoxy-
pentacyclo(6.4.0.02,10.03,7.05,9)-dodecan-4-one). J. agric. food
Chem., 26(4): 911-914.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the
aquatic environment: uptake and loss of DDT and dieldrin by fresh-
water mussels. Arch. environ. Contam. Toxicol., 1(2): 97-111.
BELISLE, A.A., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., MULHERN,
B.M., PROUTY, R.M., DEWOLF, R.B., & CROMARTIE, E. (1972) Residues
of organochlorine pesticides, polychlorinated biphenyls, and
mercury and autopsy data for bald eagles, 1969 and 1970. Pestic.
monit. J., 6(3): 133-138.
BELL, A. (1960) Aldrin poisoning: a case report. Med. J. Aust., 2:
698-700.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Ind. Med. Surg., 38(12): 50-52.
BENITZ, K.F., ROTH, R.N., & COULSTON, F. (1977) Morphologic
characteristics of hepatic nodules induced by mirex and dieldrin in
mice. Toxicol. appl. Pharmacol., 41: 154-155.
BENNINGTON, S.L., CONNORS, P.G., CONNORS, C.W., & RISEBROUGH, R.W.
(1975) Patterns of chlorinated hydrocarbon contamination in New
Zealand sub-antarctic and coastal marine birds. Environ. Pollut.,
8: 135-147.
BENSON, W.R. (1969) Note on nomenclature of dieldrin and related
compounds. J. Assoc. Off. Agric. Chem., 52: 1109-1112.
BESS, H.A. & HYLIN, J.W. (1970) Persistence of termiticides in
Hawaiian soils. J. econ. Entomol., 63(2): 633-638.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972a)
Organochlorine pesticide residues in water, sediment, algae, and
fish: Hawaii 1970-71. Pestic. monit. J., 6(1): 56-64.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972b) Organochlorine
pesticides in rainwater: Oahu, Hawaii 1971-72. Bull. environ.
Contam. Toxicol., 8(4): 238-241.
BEYER, W.N. & GISH, C.D. (1980) Persistence in earthworms and
potential hazards to birds of soil applied DDT, dieldrin, and
heptachlor. J. appl. Ecol., 17(2): 295-307.
BEYERMANN, K. & ECKRICH, W. (1973) [Gas-chromatographic
determination of insecticide traces in air.] Z. anal. Chem.,
265(1): 4-7 (in German).
BEYNON, K.I. & ELGAR, K.E. (1966) The analysis for residues of
chlorinated insecticides and acaricides. Analyst, 91(1080):
143-175.
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1127-1130.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDWELL, K., WEBER, E., NIENHOLD, I., CONNOR, T., & LEGATOR, M.S.
(1975) Comprehensive evaluation for mutagenic activity of dieldrin.
Mutat. Res., 31: 314 (Abstract).
BIJLEVELD, M. (1974) Birds of prey in Europe, London, MacMillan
Press, pp. XI and 263.
BLACK, A.M.S. (1974) Self poisoning with dieldrin: a case report
and pharmacokinetic discussion. Anaesth. intensive Care, 2: 369-374.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin, and endrin. Arch. environ. Contam.
Toxicol., 7: 83-98.
BLUS, L.J. (1982) Further interpretation of the relation of
organochlorine residues in brown pelican eggs to reproductive
success. Environ. Pollut. Ser. A, 28: 15-33.
BLUS, L.J., NEELY, B.S., Jr, BELISLE, A.A, & PROUTY, R.M. (1974a)
Organochlorine residues in brown pelican eggs: relation to
reproductive success. Environ. Pollut., 7: 81-91.
BLUS, L.J., BELISLE, A.A, & PROUTY, R.M. (1974b) Relations of the
brown pelican to certain environmental pollutants. Pestic. monit.
J., 7(3/4): 181-194.
BLUS, L.J., JOANEN, T., BELISLE, A.A., & PROUTY, R.M. (1975) The
brown pelican and certain environmental pollutants in Louisiana.
Bull. environ. Contam. Toxicol., 13(6): 646-655.
BLUS, L.J., NEELY, B.S., Jr, LAMONT, T.G., & MULHERN, B. (1977)
Residues of organochlorines and heavy metals in tissues and eggs of
brown pelicans, 1969-73. Pestic. monit. J., 11(1): 40-53.
BLUS, L.J., CROMARTIE, E., MCNEASE, L., & JOANEN, T. (1979a) Brown
pelican: population status, reproductive success, and
organochlorine residues in Louisiana, 1971-76. Bull. environ.
Contam. Toxicol., 22: 128-135.
BLUS, L.J., LAMONT, T.G., & NEELY, B.S., Jr (1979b) Effects of
organochlorine residues on eggshell thickness, reproduction, and
population status of brown pelicans (Pelicanus occidentalis) in
South Carolina and Florida, 1969-76. Pestic. monit. J., 12(4):
172-184.
BORDON, L. (1980) Possible effects of dieldrin and other pesticides
on human chromosomes in vivo and in vitro. Toxicol. Res. Proj.
Dir., 5: 11-12 (No. 11.0077).
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., & PANKASKIE, J.E.
(1952a) Toxicological studies of aldrin on small laboratory
animals, Cincinnati, Ohio, Kettering Laboratory.
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., PANKASKIE, J.E., &
DUTRA, F.R. (1952b) Toxicological studies of dieldrin on small
laboratory animals, Cincinnati, Ohio, Kettering Laboratory.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BRAGT, P.C., SCHUURBIERS, C.J., HOLLANDER, J.C.TH., SCHULTING,
F.L., & WOLTHUIS, O.L. (1984) Retention of inhaled aldrin in man,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
BRESLER, E. & HANKS, R.J. (1969) Numerical method for estimating
simultaneous flow of water and salt in unsaturated soils. Soil Sci.
Soc. Am. Proc., 33: 827-839.
BREWERTON, H.V. & MCGRATH, H.J.W. (1967) Insecticides in human fat
in New Zealand. N. Z. J. Sci., 10: 486-492.
BRIGGS, G.G. (1981) Theoretical and experimental relationships
between soil adsorption, octanol/water partition coefficients,
water solubilities, bioconcentration factors, and the parachor. J.
agric. food Chem., 29: 1050-1059.
BRODTMANN, N.V., Jr (1976) Continuous analysis of chlorinated
hydrocarbon pesticides in the Lower Mississippi River. Bull.
environ. Contam. Toxicol., 15(1): 33-39.
BROOKS, G.T. (1974) Chlorinated insecticides. I. Technology and
applications, Cleveland, Ohio, CRC Press, pp. 87-99.
BRO-RASMUSSEN, F., DALGAARD-MIKKELSEN, Sv., JAKOBSON, Th.,
KOCH, Sv.O., RODIN, F., UHL, E., & VOLDUM-CLAUSEN, K. (1968)
Examinations of Danish milk and butter for contaminating
organochlorine insecticides. Residue Rev., 23: 55-69.
BROWN, J.R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc. J., 97: 367-373.
BROWN, L., BELLINGER, E.G., & DAY, J.P. (1979) Dieldrin pollution
in the River Holme catchment, Yorkshire. Environ. Pollut., 18:
203-211.
BROWN, L.H. (1969) Status and breeding success of golden eagles in
northwest Sutherland in 1967. Br. Birds, 62(9): 345-363.
BROWN, V.K.H., HUNTER, C.G., & RICHARDSON, A. (1964) A blood test
diagnostic of exposure to aldrin and dieldrin. Br. J. ind. Med., 21:
283-286.
BROWN, V.K.H., RICHARDSON, A., ROBINSON, J., & STEVENSON, D.E.
(1965) The effects of aldrin and dieldrin on birds. Food Cosmet.
Toxicol., 3: 675-679.
BROWN, V.K.H., ROBINSON, J., & RICHARDSON, A. (1967) Preliminary
studies on the acute and subacute toxicities of a
photoisomerization product of HEOD. Food Cosmet. Toxicol., 5:
771-779.
BROWN, V.K.H., ROBINSON, J., THORPE, E., & BARRETT, J.W. (1974) The
toxicity of dieldrin (HEOD) to domestic fowl. Pestic. Sci., 5:
567-586.
BRUCE, W.N. & DECKER, G.C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin,
dieldrin, heptachlor, and heptachlor epoxide. J. agric. food Chem.,
14(4): 395-398.
BUECHEL, K.H., GINSBERG, A.E., & FISCHER, R. (1966) [Synthesis and
structure of chlordane isomers.] Chem. Ber., 99: 421-430 (in German).
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (1967) Chlorinated
pesticide levels in eastern oyster (Crassostrea virginica) from
selected areas of the South Atlantic and Gulf of Mexico. Pestic.
monit. J., 1(3): 9-12.
BUNCH, T.D. & LOW, J.B. (1973) Effects of dieldrin on chromosomes
of semi-domestic mallard ducks. J. wildl. Manage., 37(1): 51-57.
BUNYAN, P.J. & STANLEY, P.I. (1982) Toxic mechanisms in wildlife.
Regul. Toxicol. Pharmacol., 2: 106-145.
BURKE, H.R. (1959) Toxicity of several insecticides to two species
of beneficial insects on cotton. J. econ. Entomol., 52(4): 616-618.
BURNS, B.G., PEACH, M.E., & STILES, D.A. (1975) Organochlorine
pesticide residues in a farming area, Nova Scotia, 1972-73. Pestic.
monit. J., 9(1): 34-38.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue - Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURNS, K.A. (1976) Microsomal mixed-function oxidases in an
estuarine fish Fundulus heteroclitus and their induction as a
result of environmental contamination. Comp. Biochem. Physiol., 53B:
443-446.
BUTLER, P.A. (1971) Influence of pesticides on marine ecosystems.
Proc. R. Soc. Lond., B177: 321-329.
BUTLER, P.A. (1973) Organochlorine residues in estuarine mollusks,
1965-72. Pestic. monit. J., 6(4): 238-362.
CABRAL, J.R.P., RAITANO, F., MOLLNER, T., BRONCZYK, S.A., & SHUBIK,
P. (1979a) Acute toxicity of pesticides in hamsters. Toxicol. appl.
Pharmacol., 48: A192 (Abstract 384).
CABRAL, J.R.P., HALL, R.K., BRONCZYK, S.A., & SHUBIK, P. (1979b) A
carcinogenicity study of the pesticide dieldrin in hamsters. Cancer
Lett., 6(4/5): 241-246.
CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1968) Peregrines and
pesticides in Alaska. Condor, 70: 170-178.
CAIRNS, J., Jr, FOSTER, N.R., & LOOS, J.J. (1967) Effects of
sublethal concentrations of dieldrin on laboratory populations of
guppies ( Poecilia reticulata Peters). Proc. Natl Acad. Sci.
Philadelphia, 119(3): 75-91.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs on
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11(1): 70-77.
CAMPBELL, M.A., GYORKOS, J., LELCI, B., HOMONKO, K., & SAFE., S.
(1983) The effects of twenty-two organochlorine pesticides as
inducers of the hepatic drug-metabolizing enzymes. Gen. Pharmac.,
14(4): 445-454.
CAREY, A.E. & KUTZ, P.W. (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ. Monit. Assess., 5(2): 155-163.
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the Corn
Belt Region, United States, 1970. Pestic. monit. J., 6(4): 369-376.
CAREY, A.E., WIERSMA, G.B., & TAI, H. (1976) Pesticide residues in
urban soils from 14 United States cities, 1970. Pestic. monit. J.,
10(2): 54-60.
CAREY, A.E., YANG, H.S.C., WIERSMA, G.B., TAI, H., MAXEY, R.A., &
DUPUY, A.E., Jr (1980) Residual concentrations of propanil, TCAB,
and other pesticides in rice-growing areas in the United States,
1972. Pestic. monit. J., 14(1): 23-25.
CARLSON, G.P. (1974) Epoxidation of aldrin to dieldrin by lobsters.
Bull. environ. Contam. Toxicol., 11(6): 577-582.
CARNAGHAN, R.B.A. & BLAXLAND, J.D. (1957) The toxic effect of
certain seed dressings on wild and game birds. Vet. Rec., 69:
324-325.
CARO, J.H. & TAYLOR, A.W. (1971) Pathways of loss of dieldrin from
soil under field conditions. J. agric. food Chem., 19(2): 379-384.
CARO, J.H., TAYLOR, A.W., & FREEMAN, H.P. (1976) Comparative
behaviour of dieldrin and carbofuran in the field. Arch. environ.
Contam. Toxicol., 3: 437-447.
CARTER, F.L. & STRINGER, C.A. (1970) Soil moisture and soil type
influence initial penetration by organochlorine insecticides. Bull.
environ. Contam. Toxicol., 5(5): 422-428.
CASARETT, L.J., FRYER, G.C., YAUGER, W.L., Jr, & KLEMMER, H.W.
(1968) Organochlorine pesticide residues in human tissue - Hawaii.
Arch. environ. Health, 17: 306-311.
CASSIDY, W., FISHER, A.J., PEDEN, J.D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset,
London, United Kingdom, Ministry of Health, Public Health
Laboratory Services, Vol. 26, pp. 2-6 (Monthly Bulletin).
CATHEY, B. (1982) Comparative toxicities of five insecticides to
the earthworm (Lumbricus terrestris). Agric. Environ., 7: 73-81.
CAUSEY, M.K., BONNER, F.L., & GRAVES, J.B. (1968) Dieldrin residues
in the gallinules Porphyrula martinica L. and Gallinula chloropas
L. and its effect on clutch size and hatchability. Bull. environ.
Contam. Toxicol., 3(5): 274-283.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation into the connection between the diet and living
conditions of nursing mothers and the contamination of breast milk
with sparingly volatile organochloride compounds.] Act. Ernähr., 9:
157-162 (in German).
CHACKO, C.I. & LOCKWOOD, J.L. (1967) Accumulation of DDT and
dieldrin by microorganisms. Can. J. Microbiol., 13: 1123-1126.
CHADWICK, G.G. & BROCKSEN, R.W. (1969) Accumulation of dieldrin by
fish and selected fish-food organisms. J. wildl. Manage., 33(3):
693-700.
CHADWICK, G.G. & SHUMWAY, D.L. (1970) Effects of dieldrin on the
growth and development of steelhead trout. In: Gillett, J.W., ed.
The biological impact of pesticides in the environment, Corvallis,
Oregon State University, pp. 90-96 (Environmental Health Series
No. 1).
CHAN, T.M., GILLETT, J.W., & TERRIERE, L.C. (1967) Interaction
between microsomal electron transport systems of trout and male rat
in cyclodiene epoxidation. Comp. Biochem. Physiol., 20: 731-742.
CHANIN, P.R.F. & JEFFERIES, D.J. (1978) The decline of the otter
Lutra lutra L. in Britain: an analysis of hunting records and
discussion of causes. Biol. J. Linn. Soc., 10: 305-328.
CHAU, A.S.Y. & COCHRANE, W.P. (1970) Cis-opening of dieldrin
oxirane ring. Chem. Ind., 49: 1568-1569.
CHAUDRY, M.M., NELSON, A.I., & PERKINS, E.G. (1978) Distribution of
chlorinated pesticides in soybeans, soybean oil, and its by-
products during processing. J. Am. Oil Chem. Soc., 55(12): 851-853.
CHERNOFF, N., KAVLOCK, R.J., KATHREIN, J.R., DUNN, J.M., & HASEMAN,
J.K. (1975) Prenatal effects of dieldrin and photodieldrin in mice
and rats. Toxicol. appl. Pharmacol., 31: 302-308.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1981)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avian and mammalian wildlife
toxicology. Second Conference, Philadelphia, Pennsylvania,
American Society of Testing Materials, pp. 143-154 (STP757).
CLARK, D.R., Jr (1975) Effect of stress on dieldrin toxicity to
male redwinged blackbirds (Agelaius phoeniceus). Bull. environ.
Contam. Toxicol., 14(2): 250-256.
CLARK, D.R., Jr (1981) Death in bats from DDE, DDT or dieldrin:
diagnosis via residues in carcass fat. Bull. environ. Contam.
Toxicol., 26: 367-374.
CLARK, D.R., Jr & KROLL, C.J. (1977) Effects of DDE on
experimentally poisoned free-tailed bats (Tadarida brasiliensis):
lethal brain concentrations. J. Toxicol. environ. Health, 3: 893-901.
CLARK, D.R., Jr & MCLANE, M.A.R. (1974) Chlorinated hydrocarbon and
mercury residues in woodcock in the United States, 1970-71. Pestic.
monit. J., 8(1): 15-22.
CLARK, D.R., Jr & PROUTY, R.M. (1984) Disposition of dietary
dieldrin in the little brown bat and correlation of skin levels
with body burden. Bull. environ. Contam. Toxicol., 33: 177-183.
CLARK, D.R., Jr, LAVAL, R.K., & SWINEFORD, D.M. (1978) Dieldrin-
induced mortality in an endangered species, the gray bat (Myotis
grisescens). Science, 199: 1357-1359.
CLARK, D.R., Jr, LAVAL, P.K., & KRYNITSKY, A.J. (1980) Dieldrin and
heptachlor residues in dead gray bats, Franklin County, Missouri -
1976 versus 1977. Pestic. monit. J., 13(4): 137-140.
CLARK D.R., Jr, CLAWSON, R.L., & STAFFORD, C.J. (1983a) Gray bats
killed by dieldrin at two additional Missouri caves: aquatic
macroinvertebrates found dead. Bull. environ. Contam. Toxicol.,
30: 214-218.
CLARK, D.R., Jr, BUNCK, C.M., CROMARTIE, E., & LAVAL, R.K. (1983b)
Year and age effects on residues of dieldrin and heptachlor in dead
gray bats, Franklin County, Missouri - 1976, 1977, and 1978.
Environ. Toxicol. Chem., 2: 387-393.
CLEVELAND, F.P. (1966) A summary of work on aldrin and dieldrin
toxicity at the Kettering Laboratory. Arch. environ. Health, 13:
195-198.
COHEN, J.M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rain-out. In: Bould, R.F., ed.
Organic pesticides in the environment, Washington, DC, American
Chemical Society, pp. 163-176 (Advances in Chemistry Series No.
60).
COLE, J.F., KLEVAY, L.M., & ZAVON, M.R. (1970) Endrin and dieldrin:
a comparison of hepatic excretion in the rat. Toxicol. appl.
Pharmacol., 16: 547-555.
COLLINS, G.B., HOLMES, D.C., & HOODLESS, R.A. (1982) Organochlorine
pesticide residues in human milk in Great Britain, 1979-1980. Hum.
Toxicol., 1: 425-431.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Egg shell
characteristics and incidence of shell breakage for grey herons
Ardea cinerea exposed to environmental pollutants. Environ.
Pollut., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides, and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
Monks Wood Experimental Station.
COPPLESTONE, J.F., HUNNEGO, J.N., & HARRISON, D.L. (1973)
Organochlorine insecticide levels in adult New Zealanders - a five-
year study. N. Z. J. Sci., 16: 27-39.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet samples
(V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet samples
(VI). Pestic. monit. J., 5(4): 313-330.
COULSTON, F., ABRAHAM, R., & MANKES, R. (1980) Reproductive study
in female rats given dieldrin, alcohol, or aspirin orally, Albany,
New York, Albany Medical College of Union University, Institute of
Comparative and Human Toxicology.
COWAN, A.A. (1981) Organochlorine compounds in mussels from
Scottish coastal waters. Environ. Pollut. Ser. B, 2: 129-143.
CRAIG, N.C.D. (1977) A summary of the data on the toxicity of
various materials to aquatic life. III. Dieldrin, aldrin, and
endrin, Brixham United Kingdom, Imperial Chemical Industries Ltd
(Unpublished Report BL/A/1828).
CRAWFORD, N.H. & DONIGIAN, A.S., Jr (1973) Pesticide transport and
runoff model for agricultural lands, Washington, DC, US
Environmental Protection Agency (EPA-600/2-74-013).
CREBELLI, R., BELLINCAMPI, D., CONTI, G., MORPURGO, G., & CARERE,
A. (1986) A comparative study on selected chemical carcinogens for
chromosome malsegregation, mitotic crossing-over and forward
mutation induction in Aspergillus nidulans. Mutat. Res., 172:
139-149.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND, P.F.,
& CAREY, A.E. (1974) Pesticide residue levels in soils and crops:
FY-70 - National Soils Monitoring Program. II. Pestic. monit. J.,
8(2): 69-97.
CROLL, B.T. (1969) Organochlorine insecticides in water. Part I.
Proc. Soc. Water Treat. Exam., 18: 255-274.
CROMARTIE, E., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., KAISER,
T.E., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., & SWINEFORD, D.M.
(1975) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1971-72. Pestic. monit.
J., 9(1): 11-14.
CUETO, C., Jr & BIROS, F.J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol., 10:
261-269.
CUETO, C., Jr & HAYES, W.J., Jr (1962) The detection of dieldrin
metabolites in human urine. J. agric. food Chem., 10: 366-369.
CUMMINGS, J.G. (1966) Pesticides in the total diet. Residue Rev.,
16: 30-45.
CUMMINGS, J.G., ZEE, K.T., TURNER, V., & QUINN, F. (1966) Residues
in eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Assoc. Off. Agric. Chem., 49(2): 354-364.
CURLEY, A., COPELAND, M.F., & KIMBROUGH, R.D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242(5396): 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1970) Eggshell thickness in
pheasants given dieldrin. J. wildl. Manage., 34(1): 226-228.
DAHLGREN, R.B. & LINDER, R.L. (1974) Effects of dieldrin in penned
pheasants through the third generation. J. wildl. Manage., 38(2):
320-330.
DAILEY, R.E., WALTON, M.S., BECK, V., LEAVENS, C.L., & KLEIN, A.K.
(1970) Excretion, distribution, and tissue storage of a 14C-
labelled photoconversion product of 14C-dieldrin. J. agric. food
Chem., 18(3): 443-445.
DALE, W.E. & QUINBY, G.E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W.E., COPELAND, M.F., & HAYES, W.J., Jr (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World Health
Organ., 33: 471-477.
DALE, W.E., CURLEY, A., & CUETO, C., Jr (1966) Hexane extractable
chlorinated insecticides in human blood. Life Sci., 5: 47-54.
DAMICO, J.N., CHEN, J.Y.T., COSTELLO, C.E., & HAENNI, E.O. (1968)
Structure of Klein's metabolites of aldrin and of dieldrin. J.
Assoc. Off. Agric. Chem., 51(1): 48-55.
DANIELS, N.E. (1966) Soil insect control and insecticidal residue
detection. J. econ. Entomol., 59: 410-413.
DATTA, P.R., LANG, E.P., WATTS, J.O., KLEIN, A.K., & NELSON, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature (Lond.), 208: 289-290.
DAVIDSON, J.M. & MCDOUGAL, J.R. (1973) Experimental and predicted
movement of three herbicides in water-saturated soil. J. environ.
Qual., 2(4): 428-433.
DAVIES, J.E., BARQUET, A., MORGADE, C., & RAFFONELLI, A. (1975)
Epidemiological studies of DDT and dieldrin residues and their
relationship to human carcinogenesis. In: Proceedings of the
International Symposium on Recent Advances in Assessment of the
Health Effects of Environmental Pollution, Luxembourg, Commission
of the European Communities, Vol. 2, pp. 695-705.
DAVIS, B.N.K. (1968) The soil macrofauna and organochlorine
insecticide residues in twelve agricultural sites near Huntingdon.
Ann. appl. Biol., 61: 29-45.
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin
and DDT by earthworms. Soil Biol. Biochem., 3: 221-233.
DAVIS, H.C. & HIDU, H. (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish. Bull., 67(2): 393-404.
DAVIS, K.J. & FITZHUGH, O.G. (1962) Tumorigenic potential of aldrin
and dieldrin for mice. Toxicol. appl. Pharmacol., 4: 187-189.
DAVIS, K.J., HANSEN, W., & FITZHUGH, O.G. (1965) Pathology report
on mice fed aldrin, dieldrin, heptachlor, or heptachlor epoxide for
two years, Washington, DC, US Environmental Protection Agency
(Memorandum to Dr A.J. Lehman, 19th July, 1965. Publicly presented
and accepted at the US EPA Aldrin-Dieldrin Suspension Hearing,
Statement of Testimony from Dr K.J. Davis, August 1974).
DAVIS, P.W., FRIEDHOFF, J.M., & WEDEMEYER, G.A. (1972)
Organochlorine insecticide, herbicide, and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8(2): 69-72.
DAVISON, K.L. (1970) Dieldrin accumulation in tissues of the sheep.
J. agric. food Chem., 18(6): 1156-1160.
DAVISON, K.L. (1973) Dieldrin-14C balance in rats, sheep, and
chickens. Bull. environ. Contam. Toxicol., 10(1): 16-24.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p'-DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol., 7(1): 9-18.
DAWSON, R. & RILEY, J.P. (1977) Chlorine-containing pesticides and
polychlorinated biphenyls in British coastal waters. Estuarine
coastal mar. Sci., 4: 55-69.
DEAN, B.J., DOAK, S.M.A., & SOMERVILLE, H. (1975) The potential
mutagenicity of dieldrin (HEOD) in mammals. Food Cosmet. Toxicol.,
13: 317-323.
DE CAMPOS, M. & OLSZYNA-MARZYS, A.E. (1979) Contamination of human
milk with chlorinated pesticides in Guatemala and in El Salvador.
Arch. environ. Contam. Toxicol., 8: 43-58.
DECKER, G.C., BRUCE, W.N., & BIGGER, J.H. (1965) The accumulation
and dissipation of residues resulting from the use of aldrin in
soils. J. econ. Entomol., 58(2): 266-271.
DEFLORA, S. (1981) Study of 106 organic and inorganic compounds in
the Salmonella/microsome test. Carcinogenesis, 2(4): 283-298.
DEICHMANN, W.B. (1974) Certified statement of testimony before the
Environmental Protection Agency at aldrin/dieldrin suspension
hearings, Washington, DC, US Environmental Protection Agency.
DEICHMANN, W.B. & MACDONALD, W.E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-72. Ecotoxicol.
environ. Saf., 1: 89-110.
DEICHMANN, W.B., KEPLINGER, M.L., SALA, F., & GLASS, E. (1967)
Synergism among oral carcinogens. IV. The simultaneous feeding of
four tumorigens to rats. Toxicol. appl. Pharmacol., 11: 88-103.
DEICHMANN, W.B., DRESSLER, I., KEPLINGER, M., & MACDONALD, W.E.
(1968) Retention of dieldrin in blood, liver, and fat of rats fed
dieldrin for six months. Ind. Med. Surg., 37: 837-839.
DEICHMANN, W.B., KEPLINGER, M., DRESSLER, I., & SALA, F. (1969)
Retention of dieldrin and DDT in the tissues of dogs fed aldrin and
DDT individually and as a mixture. Toxicol. appl. Pharmacol., 14:
205-213.
DEICHMANN, W.B., MACDONALD, W.E., BLUM, E., BEVILACQUA, M.,
RADOMSKI, J., KEPLINGER, M., & BALKUS, M. (1970) Tumorigenicity of
aldrin, dieldrin, and endrin in the albino rat. Ind. Med., 39(10):
314, 426-434.
DEICHMANN, W.B., MACDONALD, W.E., BEASLY, A.G., & CUBIT, D.A.
(1971) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg., 40(2): 10-22.
DEICHMANN, W.B., MACDONALD, W.E., CUBIT, D.A., & BEASLY, A.G.
(1972) Effects of starvation in rats with elevated DDT and dieldrin
tissue levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEICHMANN, W.B., MACDONALD, W.E., & CUBIT, D.A. (1975) Dieldrin and
DDT in the tissues of mice fed aldrin and DDT for seven
generations. Arch. Toxicol., 34: 173-182.
DEICHMANN, W.B., MACDONALD, W.E., & LU, F.C. (1979) Effects of
chronic aldrin feeding in two strains of female rats, and a
discussion on the risks of carcinogens in man. In: Deichmann, W.B.,
ed. Toxicology and occupational medicine, Amsterdam, Elsevier/North
Holland, pp. 407-413.
DEJONCKHEERE, W., STEURBAUT, W., VERSTRAETEN, R., & KIPS, R.H.
(1977) Residues of organochlorine pesticides in human fat in
Belgium. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 42(2): 1839-1847.
DE LLAMAS, M.C., DE CASTRO, A.C., & PECHEN DE D'ANGELO, A.M. (1985)
Cholinesterase activities in developing amphibian embryos following
exposure to the insecticides dieldrin and malathion. Arch environ.
Contam. Toxicol., 14: 161-166.
DEL VECCHIO, V. & LEONI, V. (1967) [Detection and measurement of
chlorinated insecticides in biological material.] Nuovi Ann. Ig.
Microbiol., 28(2): 107-128 (in Italian).
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
D'ERCOLE, A.J., ARTHUR, R.D., CAIN, J.D., & BARRENTINE, B.F. (1976)
Insecticide exposure of mothers and newborns in a rural
agricultural area. Pediatrics, 57(6): 869-874.
DE VLIEGER, M., ROBINSON, J., BALDWIN, M.K., CRABTREE, A.N., & VAN
DIJK, M.C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRYNS, J.K., MUYS,
T., & POLL, J.M., VAN DER (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
chem. Toxicol., 22(1): 11-21.
DICK, G.L., HEENAN, M.P., LOVE, J.L., UDY, P.B., & DAVIDSON, F.
(1978) Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus pesticide
residue content. N. Z. J. Sci., 21: 71-78.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DISLER, R.L., GLATT, V., & MEIER, W. (1984) [Residues of
chlorinated insecticides and polychlorinated biphenyls in breast
milk.] Mitt. Geb. Lebensm. Hyg., 75: 205-213 (in German).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health, 4(7, suppl.):
140-146.
DIX, K.M. & WILSON, A.B. (1971) Toxicity studies with dieldrin
(HEOD). Teratogenic studies in rabbits given HEOD and thalidomide
orally, Sittingbourne, Shell Research (TLGR.0051.71).
DIX, K.M., VAN DER PAUW, C.L., & MCCARTHY, W.V. (1978) Toxicity
studies with dieldrin: teratological studies in mice dosed orally
with HEOD. Teratology, 16: 57-62.
DOBBS, A.J. & WILLIAMS, N. (1983) Indoor air pollution from
pesticides used in wood remedial treatments. Environ. Pollut.
Ser. B, 6: 271-296.
DOBSON, R.C. & BAUGH, E.R. (1976) Dieldrin residue removal from the
fat of swine. Bull. environ. Contam. Toxicol., 16(5): 567-571.
DOUABUL, A.A.Z., AL-SAAD, H.T., & AL-REKABI, H.N. (1987) Residues
of organochlorine pesticides in environmental samples from the
Shatt al-Arab river, Iraq. Environ. Pollut., 43: 175-187.
DRAPER, W.M. & CROSBY, D.G. (1984) Solar photooxidation of
pesticides in dilute hydrogen peroxide. J. agric. food Chem., 32:
231-237.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic
provinces of Canada. J. agric. food Chem., 15(3): 457-464.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States. III. June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E. & LIPSCOMB, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States. II. June 1966 - April 1968. Pestic.
monit. J., 2(4): 153-162.
DUGGAN, R.E., BARRY, H.C., & JOHNSON, L.Y. (1967) Pesticide
residues in total diet samples. II. Pestic. monit. J., 1(2): 2-12.
ECKENHAUSEN, F.W., BENNET, D., BEYNON, K.I., & ELGAR, K.E. (1981)
Organochlorine pesticide concentrations in perinatal samples from
mothers and babies. Arch. environ. Health, 36(2): 81-92.
EDMUNDSON, W.F., DAVIES, J.E., & HULL, W. (1968) Dieldrin storage
levels in necropsy adipose tissue from a south Florida population.
Pestic. monit. J., 2(2): 86-89.
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Oxford, Blackwell, pp. 239-261.
EDWARDS, C.A. (1966) Insecticide residues in soil. Residue Rev., 13:
83-132.
EDWARDS, C.A. (1973a) Persistent pesticides in the environment,
Cleveland, Ohio, CRC Press, pp. 68-74.
EDWARDS, C.A. (1973b) Persistent pesticides in soil and water. In:
Environmental pollution by pesticides, London, Plenum Press, pp.
409-458.
EDWARDS, C.A. & LOFTY, J.R. (1977) Biology of earthworms, 2nd ed.,
London, Chapman and Hall, pp. 212-213.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the soil
fauna. Residue Rev., 45: 8-15.
EDWARDS, C.A., DENNIS, E.B., & EMPSON, D.W. (1967) Pesticides and
the soil fauna: effects of aldrin and DDT in an arable field. Ann.
appl. Biol., 60: 11-22.
EEC (1983) Directives relating to the classification and labelling
of dangerous substances. Annex VI. Part II (2.4.10) to the fifth
adaptation to the Dangerous Substances Directives, Luxembourg,
European Economic Community (Commission Directive 83/467/EEC)
(Official Journal L 257/19).
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J.O'G. (1965)
Organochlorine pesticide residues in human fat and human milk. Br.
med. J., 2: 66-69.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5: 541-544.
EISENLORD, G., LOQUVAM, G.S., & NEMENZO, J. (1967) Results of
reproduction study of rats fed diets containing dieldrin over three
generations, San Francisco, California, The Hine Laboratories
(Report No. 4).
EISLER, R. (1969) Acute toxicities of insecticides to marine
decapod crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Latent effects of insecticide intoxication to
marine molluscs. Hydrobiologia, 36(3/4): 345-352.
EL BEIT, I.O.D. (1981) Pesticide microbial interactions in the
soil. Int. J. environ. Stud., 16: 171-181.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981a) Factors
involved in the dynamics of pesticides in soils: the effect of
pesticide concentration on leachability and absorption. Int. J.
environ. Stud., 16: 181-187.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981b) Factors
involved in the dynamics of pesticides in soils: the effects of
temperature and period of contact or leachability and adsorption of
pesticides by soils. Int. J. environ. Stud., 16: 189-196.
EL BEIT, I.O.D., COTTON, D.E., & WHEELOCK, J.V. (1983) Persistence
of pesticides in soil leachates: effects of pH, ultraviolet
irradiation, and temperature. Int. J. environ. Stud., 21: 251-259.
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. (1975) The dissipation and accumulation of aldrin and
dieldrin residues in soil. Environ. Qual. Saf., 3(suppl.): 250-257.
ELGAR, K.E. (1979) The variability of residue results, with
particular reference to the Codex study on organochlorines in
butterfat. In: Proceedings of the 4th IUPAC Congress on Pesticide
Chemistry, Zurich, 1978, pp. 668-672.
ELIASON, B.C. & POSNER, H.S. (1971) Reduced passage of carbon-14-
dieldrin to the fetal rat by phenobarbital but not by eight other
drugs or dieldrin. Am. J. Obstet. Gynecol., 110(7): 943-947.
ELY, R.E., MOORE, L.A., HUBANKS, P.E., CARTER, R.H., & POOS, F.W.
(1954) Studies of feeding aldrin to dairy cows. J. dairy Sci., 37(3):
294-298.
EMANUELSEN, M., LINCER, J.L., & RIFKIN, E. (1978) The residue
uptake and histology of American oysters ( Crassostrea virginica
Gmelin) exposed to dieldrin. Bull. environ. Contam. Toxicol., 19:
121-129.
ENDERSON, J.H. & BERGER, D.D. (1968) Chlorinated hydrocarbon
residues in peregrines and their prey species from northern Canada.
Condor, 70: 149-153.
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning
and lowered production of young in prairie falcons. Bioscience,
20(6): 355-356.
EPIFANIO, C.E. (1973) Dieldrin uptake by larvae of the crab
Leptodius floridanus. Mar. Biol., 19(4): 320-322.
EPSTEIN, E. & GRANT, W.J. (1968) Chlorinated insecticides in runoff
water as affected by crop rotation. Soil Sci. Soc. Am. Proc., 32:
423-426.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced in
mutant strains of Salmonella typhimurium by pesticides,
Washington, DC, American Chemical Society (Abstracts Paper 174)
(Pesticide No. 43).
ESTENIK, J.F. & COLLINS, W.J. (1979) In vivo and in vitro studies
of mixed-function oxidase in an aquatic insect Chironomus riparius.
In: Khan, M.A.Q., ed. Pesticide and xenobiotic metabolism in
aquatic organisms, Washington, DC, American Chemical Society, pp.
349-370 (ACS Symposium Series No. 99).
EYE, J.D. (1968) Aqueous transport of dieldrin residues in soil. J.
Water Pollut. Control Fed. (suppl.), 40(8): R316-332.
FABER, R.A., RISEBROUGH, R.W., & PRATT, H.M. (1972) Organochlorines
and mercury in common egrets and great blue herons. Environ.
Pollut., 3: 111-122.
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides,
Lyons, International Agency for Research on Cancer, pp. 161-181
(IARC Scientific Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a Joint Meeting of the FAO Committee on Pesticides
in Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting Report
No. PL:1965/10; WHO Food Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO Meeting Report No.
PL: 1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of the FAO
Working Party on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) 1966 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1967/M/11/1; WHO Food
Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 78;
WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1968/M/9/1; WHO Food
Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 84;
WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 87;
WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71. 42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 97;
WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Plant Production and
Protection Series No. 1; WHO Technical Report Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues and
the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 10 Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1984) Codex guidelines on good practice in pesticide
residue analysis, Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986b) Recommendations for methods of analysis of
pesticide residues, Rome, Codex Alimentarius Committee, Food and
Agriculture Organization of the United Nations (CAC/PR8-1986).
FARB, R.M., SANDERSON, T., MOORE, B.G., & HAYES, A.W. (1973)
Interaction: the effect of selected mycotoxins on the tissue
distribution and retention of aldrin and dieldrin in the neonatal
rat. In: Deichmann, W.B., ed. Pesticides in the environment, New
York, International Medical Book Corporation, pp. 179-187.
FARMER, W.J. & JENSEN, C.R. (1970) Diffusion and analysis of
carbon-14-labelled dieldrin in soils. Soil Sci. Soc. Am. Proc., 34:
28-31.
FARMER, W.J. & LETEY, J. (1974) Volatilization losses of pesticides
from soils, Washington, DC, US Environmental Protection Agency
(EPA-660/2-74-054).
FARMER, W.J., IGUE, K., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. I. Effect of
concentration, temperature, air flow rate, and vapour pressure.
Soil Sci. Soc. Am. Proc., 36: 443-447.
FARMER, W.J., IGUE, K., & SPENCER, W.F. (1973) Effect of bulk
density on the diffusion and volatilization of dieldrin from soil.
J. environ. Qual., 2(1): 107-109.
FEIL, V.J., HEDDE, R.D., ZAYLSKIE, R.G., & ZACHRISON, C.H. (1970)
Dieldrin-14C metabolism in sheep. Identification of trans-6,7-di-
hydroxydihydroaldrin and 9-(syn-epoxy)-hydroxy-1,2,3,4,10,10-hexa-
chloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-endo-5,8-exo-
dimethanonaphthalene. J. agric. food Chem., 18(1): 120-124.
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FERGIN, T.J. & SCHAFER, E.C. (1977) Toxicity of dieldrin to
bobwhite quail in relation to sex and reproductive status. Arch.
environ. Contam. Toxicol., 6: 213-219.
FERNICOLA, N.A.G.G. & AZEVEDO, F.A. (1982) Serum levels of
organochlorine insecticides in humans in Sao Paulo, Brazil. Vet.
hum. Toxicol., 24(2): 91-93.
FIKES, M.H. & TUBB, R.A. (1972) Dieldrin uptake in the three-ridge
naiad. J. wildl. Manage., 36(3): 802-809.
FISCHLER, H.M. & KORTE, F. (1969) [Sensitized and non-sensitized
photoisomerization of cyclodiene insecticides.] Tetrahedron Lett.,
32: 2793-2796 (in German).
FISEROVA-BERGEROVA, V., RADOMSKI, J.L., DAVIES, J.E., & DAVIS, J.H.
(1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHER, H.L., MOST, B., & HALL, L.L. (1985) Dermal absorption of
pesticides calculated by deconvolution. J. appl. Toxicol., 5(3):
163-177.
FITZHUGH, O.G., NELSON, A.A., & QUAIFE, M.L. (1964) Chronic oral
toxicity of aldrin and dieldrin in rats and dogs. Food Cosmet.
Toxicol., 2: 551-561.
FLETCHER, T.E., PRESS, J.M., & WILSON, D.B. (1959) Exposure of
spray-men to dieldrin in residual spraying. Bull. World Health
Organ., 20: 15-25.
FLICKINGER, E.L. & KING, K.A. (1972) Some effects of aldrin-treated
rice on Gulf coast wildlife. J. wildl. Manage., 36(3): 706-727.
FOURNIER, E., TREICH, I., CAMPAGNE, L., & CAPELLE, N. (1972)
Pesticides organo-chlorés dans le tissus adipeux d'êtres humains en
France. Eur. J. Toxicol., 5(1): 11-26.
FOWKES, F.M., BENESI, H.A., RYLAND, L.B., LOEFFLER, E.S., & SUN,
Y.P. (1960) Clay catalysed decomposition of insecticides. J. agric.
food Chem., 8: 203-210.
FOWLER, J.F., NEWSOM, L.D., GRAVES, J.B., BONNER, F.L., &
SCHILLING, P.E. (1971) Effect of dieldrin on egg hatchability,
chick survival, and eggshell thickness in purple and common
gallinules. Bull. environ. Contam. Toxicol., 6(6): 495-501.
FOX, G.R. & VIRGO B.B. (1986) Relevance of hyperglycemia to
dieldrin toxicity in suckling and adult rats. Toxicology, 38:
315-326.
FRANK, R., MONTGOMERY, K., BRAUN, H.E., BERST, A.H., & LOFTUS, K.
(1974) DDT and dieldrin in watersheds draining the tobacco belt in
southern Ontario. Pestic. monit. J., 8(3): 184-201.
FRANK, R., BRAUN, H.E., ISHIDA, K., & SUDA, P. (1976) Persistent
organic and inorganic pesticide residues in orchard soils and
vineyards of southern Ontario. Can. J. Soil Sci., 56: 463-484.
FRANK, R., HOLDRINET, M.V.H., & SUDA, P. (1979) Organochlorine and
mercury residues in wild mammals in southern Ontario, Canada 1973-
1974. Bull. environ. Contam. Toxicol., 22: 500-507.
FRANK, R., BRAUN, H.E., & HOLDRINET, M.V.H. (1981) Residues from
past uses of organochlorine insecticides and PCB in waters draining
eleven agricultural watersheds in southern Ontario, Canada, 1975-
1977. Sci. total Environ., 20: 255-276.
FRANK, R., BRAUN, H.E., SIRONS, G.H., RASPER, J., & WARD, G.G.
(1985) Organochlorine and organophosphorus insecticides and
industrial pollutants in the milk supplies of Ontario, 1983. J.
food Prot., 48(6): 499-504.
FRY, D.R. (1964) Human dieldrin poisoning. Lancet, 1: 764.
FUCHS, P. (1967) Death of birds caused by application of seed
dressings in the Netherlands. Meded. Fac. Landbouwwet. Rijksuniv.
Gent., 32(3/4): 855-859.
FURNESS, R. & HUTTON, M. (1979) Pollutant levels in the great skua
Catharacta skua. Environ. Pollut., 13: 261-268.
FYTIANOS, K., VASILIKIOTIS, G., & WEIL, L. (1985) Identification
and determination of some trace organic compounds in coastal
seawater of Northern Greece. Bull. environ. Contam. Toxicol., 34:
390-395.
GAINES, T.B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Use of the golden
hamster in toxicology. Lab. anim. Sci., 26(2): 274-280.
GAKSTATTER, J.H. (1968) Rates of accumulation of 14C-dieldrin
residues in tissues of goldfish exposed to a singe sublethal dose
of 14C-aldrin. J. Fish Res. Board Can., 25(9): 1797-1801.
GAKSTATTER, J.H. & WEISS, C.M. (1967) The elimination of DDT-14C,
dieldrin-14C, and lindane-14C from fish following a single sub-
lethal exposure in aquaria. Trans. Am. Fish. Soc., 96: 301-307.
GALLEY, R.A.E. (1970) [Chlorinated hydrocarbons. IV. Cyclodien
insecticides.] In: Wegler, R., ed. [Chemistry of crop protection
agents and pesticides,] Berlin, Heidelberg, New York, Springer-
Verlag, Vol. 1, pp. 163-192 (in German).
GANNON, N. & DECKER, G.C. (1958) The conversion of aldrin to
dieldrin on plants. J. econ. Entomol., 51: 8-11.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959a) Storage of dieldrin
in tissues of steers, hogs, lambs, and poultry fed dieldrin in
their diets. J. agric. food Chem., 7(12): 826-828.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959b) Storage of dieldrin
in tissues and its excretion in milk of dairy cows fed dieldrin in
their diets. J. agric. food Chem., 7(12): 824-826.
GARRETTSON, L.K. & CURLEY, A. (1969) Dieldrin. Studies in a
poisoned child. Arch. environ. Health, 19: 814-822.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total-diet samples, October 1980 - March 1982.
J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in adult
total-diet samples, October 1980 - March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GENELLY, R.E. & RUDD, R.L. (1956) Effects of DDT, toxaphene, and
dieldrin on pheasant reproduction. Auk, 73: 529-539.
GEORGIAN, L. (1975) The comparative cytogenetic effects of aldrin
and phosphamidon. Mutat. Res., 31: 103-108.
GEROLT, P. (1961) Investigation into the problem of insecticide
sorption by soils. Bull. World Health Organ., 24: 577-592.
GIFAP (1984) Pesticide residues in food, Brussels, Groupement
International des Associations Nationales des Fabricants de
Produits Agrochimique.
GILLETT, J.W. & CHAN, T.M. (1968) Cyclodiene insecticides as
inducers, substrates, and inhibitors of microsomal epoxidation. J.
agric. food Chem., 16(4): 590-593.
GINN, T.M. & FISHER, F.M., Jr (1974) Studies on the distribution
and flux of pesticides in waterways associated with a rice field.
Marshland ecosystems. Pestic. monit. J., 8(1): 23-32.
GISH, C.D. (1970) Organochlorine insecticide residues in soils and
soil invertebrates from agricultural lands. Pestic. monit. J., 3(4):
241-252.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 241).
GLATT, H., JUNG, R., & OESCH, F. (1983) Bacterial mutagenicity
investigation of epoxides: drugs, drug metabolites, steroids and
pesticides. Mutat. Res., 11: 99-118.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J. (1976)
Distribution of pesticides and polychlorinated biphenyls in water,
sediments, and seston of the Upper Great Lakes, 1974. Pestic.
monit. J., 10(2): 61-67.
GLOTFELTY, D.E. (1978) The atmosphere as a sink for applied
pesticides. J. Air Pollut. Control Assoc., 28: 917-921.
GONZALEZ RODRIGUEZ-CORDOBA, J.M., FERNANDES, A.L., & HENS, J.M.
(1983) [Mother neonate ratio of levels of blood contamination by
organo-chlorine insecticide residues.] Arch. Zootec., 32(122):
49-60 (in Spanish).
GOOD, E.E. & WARE, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV. Endrin and dieldrin.
Toxicol. appl. Pharmacol., 14: 201-203.
GOODWIN, E.S., GOULDEN, R., & REYNOLDS, J.G. (1961) Rapid
identification and determination of residues of chlorinated
pesticides in crops by gas-liquid chromatography. J. Soc. Anal.
Chem., 80(1028): 697-700.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ., 63(5):
945-962.
GRACA, I., SILVA FERNANDES, A.M.S., & MOURAO, H.C. (1974)
Organochlorine insecticide residues in human milk in Portugal.
Pestic. monit. J., 8(3): 148-156.
GRANVILLE, G.C., SIMPSON, B.J., & DOAK, S.M. (1973) Toxicity
studies on aldrin dicarboxylic acid: 13-week oral study in rats,
Sittingbourne, Shell Research (TLGR.0008.73) (Unpublished
proprietary report).
GRAVES, J.B., BONNER, F.L., MCKNIGHT, W.F., WATTS, A.G., & EPPS,
E.A. (1969) Residues in eggs, preening glands, liver, and muscle
from feeding dieldrin-contaminated rice bran to hens and its
effects on egg production, egg hatch, and chick survival. Bull.
environ. Contam. Toxicol., 4(6): 375-383.
GREENBERG, R.E. & EDWARDS, W.R. (1970) Insecticide residue levels
in eggs of wild pheasants in Illinois. Trans. Illinois State Acad.
Sci., 63: 136-147.
GREICHUS, Y.A., GREICHUS, A., & REIDER, E.G. (1968) Insecticide
residues in grouse and pheasant of South Dakota. Pestic. monit. J.,
2(2): 90-92.
GREICHUS, Y.A., GREICHUS, A., DRAAYER, H.A., & MARSHALL, B. (1978a)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. II. Lake McLlwaine, Rhodesia. Bull. environ. Contam.
Toxicol., 19: 444-453.
GREICHUS, Y.A., GREICHUS, A., AMMANN, B.D., & HOPCRAFT, J. (1978b)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. III. Lake Nakuru, Kenya. Bull. environ. Contam.
Toxicol., 19: 454-461.
GREVE, P.A. (1972) Potentially hazardous substances in surface
waters. Part I. Pesticides in the river Rhine. Sci. total Environ.,
1: 173-180.
GREVE, P.A. & WEGMAN, R.C.C. (1985) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands,
Copenhagen, World Health Organization, Regional Office for Europe
(ICP/CEH. 501/M05).
GRIFFIN, D.E. & HILL, W.E. (1978) In vitro breakage of plasmid DNA
by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRIFFITH, J. & DUNCAN, R.C. (1985) Serum organochlorine residues in
Florida citrus workers compared to the national health and
nutrition examination survey sample. Bull. environ. Contam.
Toxicol., 35: 411-417.
GRIFFITHS, D.C., RAW, F., & LOFTY, J.R. (1967) The effects on soil
fauna of insecticides tested against wireworms ( Agriotes ssp.) in
wheat. Ann. appl. Biol., 60: 479-490.
GROSCH, D.S. & VALCOVIC, I.R. (1967) Chlorinated hydrocarbon
insecticides are not mutagenic in Bracon hebetor tests. J. econ.
Entomol., 60(4): 1177-1179.
GUERZONI, M.E., DEL CUPOLO, L., & PONTI, I. (1976) Mutagenic
activity of pesticides. Riv. Sci. Tec. Aliment. Nutr. Um., 6:
161-165.
GUTENMANN, W.H., GREENWOOD, R.A., GYRISCO G.G., & LITTLE, R.J.
(1972) Studies of aldrin and chlordane in silt loam soils and their
possible translocation in field corn in New York. J. econ.
Entomol., 65(3): 842-844.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. Sci. total
Environ., 43: 193-219.
GUPTA, H.C.L. & KAVADIA, V.S. (1979) Dissipation of aldrin residues
in clay loam soil under the cover of root crops. Indian J. plant
Prot., 7(1): 43-49.
GUPTA, H.C.L., KUSHWAHA, K.S., KAVADIA, V.S., & SRIVASTAVA, B.P.
(1979) Aldrin residues in soils and its translocation in maize and
pearl millet. Indian J. Entomol., 41(1): 47-57.
GUPTA, P.C. (1975) Neurotoxicity of chronic chlorinated hydrocarbon
insecticide poisoning: a clinical and electroencephalographic study
in man. Indian J. med. Res., 63(4): 601-606.
HAAN, C.T. (1971) Movement of pesticides by runoff and erosion.
Trans. Am. Soc. Agric. Eng., 14: 445-447, 449.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Cournix. Bull. environ. Contam. Toxicol., 11(1): 98-102.
HAGLEY, E.A.C. (1965) Effect of insecticides on the growth of
vegetable seedlings. J. econ. Entomol., 58(4): 777-778.
HAMILTON, H.E., MORGAN, D.P., & SIMMONS, A. (1978) A pesticide
(dieldrin)-induced immunohemolytic anemia. Environ. Res., 17:
155-164.
HARGESHEIMER, E.E. (1984) Rapid determination of organochlorine
pesticides and polychlorinated biphenyls using selected ion
monitoring mass spectrometry. J. Assoc. Off. Anal. Chem., 67(6):
1067-1075.
HARR, J.R., CLAEYS, R.R., BONE, J.F., & MCCORCLE, T.W. (1970)
Dieldrin toxicosis: rat reproduction. Am. J. vet. Res., 31: 181-189.
HARRIS, C.I. (1969) Movement of pesticides in soil. J. agric. food
Chem., 17(1): 80-82.
HARRIS, C.R. (1964) Influence of soil type and soil moisture on the
toxicity of insecticides in soils to insects. Nature (Lond.), 202:
724.
HARRIS, C.R. (1972) Behaviour of dieldrin in soil. Laboratory
studies influencing biological activity. J. econ. Entomol., 65: 8-13.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J.
agric. food Chem., 15: 86-93.
HARRIS, C.R., SANS, W.W., & MILES, J.R.W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in Southwestern Ontario. J. agric. food Chem.,
14(4): 398-403.
HARRISON, R.B., HOLMES, D.C., ROBURN, J., & TATTON, J.O'G. (1967)
The fate of some organochlorine pesticides on leaves. J. Sci. Food
Agric., 18: 10.
HARVEY, G.R., STEINHAVER, W.G., & TEAL, J.M. (1973) Polychlorobiphenyls
in North Atlantic Ocean water. Science, 180: 643-644.
HARVEY, G.R., STEINHAVER, W.G., & MIKLAS, H.P. (1974) Decline of
PCB concentrations in North Atlantic surface water. Nature (Lond.),
252: 387-388.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of waterfowl nesting on
islands in Lake Michigan off Door County, Wisconsin, 1977-78.
Pestic. monit. J., 15(2): 90-97.
HASHEMY-TONKABONY, S.E. & SOLEIMANI-AMIRI, M.J. (1978) Chlorinated
pesticide residues in the body fat of people in Iran. Environ.
Res., 16: 419-422.
HATHWAY, D.E., MOSS, J.A., ROSE, J.A., & WILLIAMS, D.J.M. (1967)
Transport of dieldrin from mother to blastocyst and from mother to
foetus in pregnant rabbits. Eur. J. Pharmacol., 1: 167-175.
HAVERA, S.P. & DUZAN, R.E. (1986) Organochlorine and PCB residues
in tissues of raptors from Illinois, 1966-81. Bull. environ.
Contam. Toxicol., 36: 23-32.
HAYES, W.J. (1957) Dieldrin poisoning in man, Washington, DC, US
Department of Health, Eduction and Welfare, Public Health Service,
Vol. 72, pp. 1087-1091 (Public Health Report No. 12).
HAYES, W.J. (1959) The toxicity of dieldrin to man. Report on a
survey. Bull. World Health Organ., 20: 891-912.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons.
Emergency information for treating poisoning, Washington, DC, US
Government Printing Office (Public Health Service Publication No.
476).
HAYES, W.J. (1974) Distribution of dieldrin following a single oral
dose. Toxicol. appl. Pharmacol., 28: 485-492.
HAYES, W.J. (1982) Chlorinated hydrocarbon insecticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and
Wilkins, pp. 172-283.
HAYES, W.J. & CURLEY, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environ. Health, 16(2): 155-162.
HAYES, W.J., Jr, DALE, W.E., & BURSE, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HEATH, D.F. & VANDEKAR, M. (1964) Toxicity and metabolism of
dieldrin in rats. Br. J. ind. Med., 21: 269-279.
HEESCHEN, W. (1972) Analyses for residues in milk and milk
products. In: Coulston, F. & Korte, F., ed. Environmental quality
and safety, Stuttgart, G. Thieme Verlag, Vol. 1, p. 229.
HEINZ, G.H. & JOHNSON, R.W. (1981) Diagnostic brain residues of
dieldrin: some new insights. In: Lamb, D.W. & Kenaga, E.E., ed.
Avian and mammalian wildlife toxicology. Second Conference,
Philadelphia, Pennsylvania, American Society of Testing Materials,
pp. 72-92 (STP757).
HELENE, C.G., LORD, K.A., & RUEGG, E.F. (1981) The persistence,
leaching, and volatilization of 14C aldrin in two Brazilian soils
Cienc. Cult., 33: 101-105.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four
species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969) Organochlorine
insecticide residues in fish. Pestic. monit. J., 3(3): 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971) Organochlorine
insecticide residues in fish - fall 1969. Pestic. monit. J., 5(1):
1-11.
HENDERSON, G.L. & CROSBY, D.G. (1968) The photodecomposition of
dieldrin residues in water. Bull. environ. Contam. Toxicol., 3:
131-134.
HERRERA MARTEACHE, A., POLO VILLAR, L.M., JODRAL VILLAREJO,
M., POLO VILLAR, G., MALLOL, J., & POZO LORA, R. (1978)
[Organochlorine pesticide residues in human fat in Spain.] Rev.
Sanid. Hig. publica., 52: 1125-1144 (in Spanish).
HERZEL, F. (1971) [The behaviour of some persistent insecticides in
the soil.] Bundesgesundheitsblatt, 14(3): 23-28 (in German).
HERZEL, F. (1972) Organochlorine insecticides in surface waters in
Germany: 1970 and 1971. Pestic. monit. J., 6(3): 179-187.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg., 24:
459-463.
HICKEY, J.J., ed. (1969) Peregrine falcon populations: their
biology and decline, Madison, Wisconsin, University of Wisconsin
Press.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish-eating birds. Science, 162:
271-273.
HILL, D.W. & MCCARTY, P.L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control Fed.,
39: 1259-1277.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 191).
HILL, E.F., SPANN, J.W., & WILLIAMS, J.D. (1977) Responsiveness of
6 to 14 generations of birds to dietary dieldrin toxicity. Toxicol.
appl. Pharmacol., 42: 425-431.
HINDIN, E., MAY, D.S., & DUNSTAN, G.S. (1964) Collection and
analysis of synthetic organic pesticides from surface and ground
water. Residue Rev., 7: 130-156.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10: 613-675.
HOERSCHELMANN, H., POLZHOFER, K., FIGGE, K., & BALLSCHMITER,
K. (1979) [Organochlorine pesticides and polychlorinated biphenyls
in birds eggs from the Falkland Islands and from northern Germany.]
Environ. Pollut., 13: 247-269 (in German).
HOFFMAN, W.S., ADLER, H., FISHBEIN, W.I., & BAUER, F.C. (1967)
Relation of pesticide concentrations in fat to pathological changes
in tissues. Arch. environ. Health, 15: 758-765.
HOGAN, R.L. & ROELOFS, E.W. (1971) Concentrations of dieldrin in
the blood and brain of the green sunfish Lepomis cyanellus at
death. J. Fish Res. Board Can., 28(4): 610-612.
HOLDEN, A.V. (1975) The accumulation of oceanic contaminants in
marine mammals, Rapp. P.-V. Réun. Cons. int. Explor. Mer, 169:
353-361.
HOLDRINET, M.V.H., BRAUN, H.E., FRANK, R., STOPPS, G.E., SMOUT,
M.S., & MCWADE, J.W. (1977) Organochlorine residues in human
adipose tissue and milk from Ontario residents, 1969-1974. Can. J.
public Health, 68: 74-80.
HOLT, R.L., CRUSE, S., & GREEN, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
North East Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOOFTMAN, R.N. & VINK, G.J. (1980) The determination of toxic
effects of pollutants with the marine polychaete worm Ophryotrocha
diadema. Ecotoxicol. environ. Saf., 4: 252-262.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1962) Electro-
encephalograms in insecticide toxicity. Arch. environ. Health, 4:
86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1965) Nine
years' toxicity control in insecticide plants. Arch. environ.
Health, 10: 441-448.
HORNABROOK, R.W., DYMENT, P.G., GOMES, E.D., & WISEMAN, J.S. (1972)
DDT residues in human milk from New Guinea natives. Med. J. Aust.,
1: 1297-1300.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, 2nd ed., US Department of the
Interior, Fish and Wildlife Service, pp. 9-10 (Publication No.
153).
HUNT, P.F., STEVENSON, D.E., THORPE, E., & WALKER, A.I.T. (1975)
Mouse data. Letter to the editor. Food Cosmet. Toxicol., 13:
597-599.
HUNTER, C.G. & ROBINSON, J. (1967) Pharmacodynamics of dieldrin
(HEOD). I. Ingestion by human subjects for 18 months. Arch.
environ. Health, 15(5): 614-626.
HUNTER, C.G. & ROBINSON, J. (1968) Aldrin, dieldrin, and man. Food
Cosmet. Toxicol., 6: 253-260.
HUNTER, C.G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England. Br. med.
J., 1: 221-224.
HUNTER, C.G., ROBINSON, J., & JAGER, K.W. (1967) Aldrin and
dieldrin: the safety of present exposures of the general
populations of the United Kingdom and the United States. Food
Cosmet. Toxicol., 5: 781-787.
HUNTER, C.G., ROBINSON, J., & ROBERTS, M. (1969) Pharmaco-dynamics
of dieldrin (HEOD). II. Ingestion by human subjects for 18 to 24
months, and post-exposure for eight months. Arch. environ. Health,
18(1): 12-21.
HUTSON, D.H. (1976) Comparative metabolism of dieldrin in the rat
(CFE) and in two strains of mouse (CF1 and LACG). Food Cosmet.
Toxicol., 14: 577-591.
IARC (1974) Some organochlorine pesticides, Lyons, International
Agency for Research on Cancer, 241 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol.
5).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, Vol. 1-29, 292 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Man, Suppl. 4).
IARC (1987) Overall evaluations of carcinogenicity. An update of
IARC Monographs, Lyons, International Agency for Research on
Cancer, pp. 1-42 (Monographs on the Evaluation of Carcinogenic
Risks to Humans, Suppl. 7).
IATROPOULOS, M.J., MILLING, A., MUELLER, W.F., NOHYNEK, G., ROZMAN,
K., COULSTON, F., & KORTE, F. (1975) Absorption, transport, and
organotropism of dichlorobiphenyl (DCB), dieldrin, and
hexachlorobenzene (HCB) in rats. Environ. Res., 10: 384-389.
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, Utrecht, Rijks Universiteit (Thesis).
ICHINOSE, R. & KURIHARA, N. (1985) Uptake of dieldrin, lindane, and
DDT by isolated hepatocytes. Pestic. Biochem. Physiol., 23: 116-122.
IGUE, K., FARMER, W.J., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. II. Effect of
relative humidity and soil water content on dieldrin volatility.
Soil Sci. Soc. Am. Proc., 36: 447-450.
ITO, N., TSUDA, H., HASEGAWA, R., & IMAIDA, K. (1983) Comparison of
the promoting effects of various agents in induction of
preneoplastic lesions in rat liver. Environ. Health Perspect., 50:
131-138.
IVEY, M.C., CLABORN, H.V., MANN, H.D., RADELEFF, R.D., & WOODARD,
G.T. (1961) Aldrin and dieldrin content of body tissues of
livestock receiving aldrin in their diet. J. agric. food Chem.,
9(5): 374-376.
IVIE, G.W. & CASIDA, J.E. (1970) Enhancement of photo-alteration of
cyclodiene insecticide chemical residues by rotenone. Science, 167:
1620-1622.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Publishing Company,
234 pp.
JANSEN, J.D. (1979) The predictive value of tests for carcinogenic
or mutagenic activity. In: Deichmann, W.B., ed. Toxicology and
occupational medicine, Amsterdam, Elsevier/North Holland, pp.
71-80.
JEFFERIES, D.J. (1969) Causes of badger mortality in eastern
counties of England. J. Zool. (Lond.), 157: 429-436.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in
British bats and their significance. J. Zool. (Lond.), 166:
245-263.
JEFFERIES, D.J. (1975) Different activity patterns of male and
female badgers (Meles meles) as shown by road mortality. J. Zool.
(Lond.), 177: 504-506.
JEFFERIES, D.J. & DAVIS, B.N.K. (1968) Dynamics of dieldrin in
soil, earthworms, and song thrushes. J. wildl. Manage., 32(3):
441-456.
JEFFERIES, D.J. & PENDLEBURY, J.B. (1968) Population fluctuations
of stoats, weasels and hedgehogs in recent years. J. Zool. (Lond.),
156: 513-517.
JEFFERIES, D.J., STAINSBY, B., & FRENCH, M.C. (1973) The ecology of
small mammals in arable fields drilled with winter wheat and the
increase in their dieldrin and mercury residues. J. Zool. (Lond.),
171: 513-539.
JEFFERIES, D.J., FRENCH, M.C., & STEBBING, R.E. (1974) Pollution
and mammals: otters, Huntingdon, Natural Environment Research
Council, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, pp. 13-15 (Report for 1972-73).
JENSEN, L.D. & GAUFIN, A.R. (1966) Acute and long-term effects of
organic insecticides on two species of stonefly naiads. J. Water
Pollut. Control Fed., 38(8): 1273-1286.
JOHNSON, L.G. & MORRIS, R.L. (1971) Chlorinated hydrocarbon
pesticides in Iowa rivers. Pestic. monit. J., 4(4): 216-219.
JOHNSON, R.D. & MANSKE, D.D. (1977) Pesticide and other chemical
residues in total diet samples. XI. Pestic. monit. J., 11(3): 116-131.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 1-3, 8,
10, 29-30 (Resource Publication No. 137).
JOHNSTON, B.T., SAUNDERS C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin
by fresh-water invertebrates. J. Fish Res. Board Can., 28: 705-709.
JOHNSTON, D.W. (1975) Organochlorine pesticide residues in small
migratory birds, 1964-1973. Pestic. monit. J., 9(2): 79-88.
JOLLY, D.W. (1954) Studies on the acute toxicity of dieldrin to
sheep. Vet. Rec., 66: 444-447.
JONAS, R.B. & PFAENDER, F.K. (1976) Chlorinated hydrocarbon
pesticides in Western North Atlantic Ocean. Environ. Sci. Technol.,
10(8): 770-773.
JONES, D.M., BENNETT, D., & ELGAR, K.E. (1978) Deaths of owls
traced to insecticide-treated timber. Nature (Lond.), 272: 52.
JOY, R.M. (1976) The alteration by dieldrin of cortical
excitability conditioned by sensory stimuli. Toxicol. appl.
Pharmacol., 38(2): 357-368.
JOY, R.M. (1977) Contrasting actions of dieldrin and aldrin-
transdiol, its metabolite, on cat CNS functions. Toxicol. appl.
Pharmacol., 42: 137-148.
JOY, R.M. (1982) Mode of action of lindane, dieldrin, and related
insecticides in the central nervous system. Neurobehav. Toxicol.
Teratol., 4: 813-823.
JUNK, G.A., SPALDING, R.F., & RICHARD, J.J. (1980) Aerial,
vertical, and temporal differences in groundwater chemistry. II.
Organic constituents. J. environ. Qual., 9: 479-483.
JURY, W.A., SPENCER, W.F., & FARMER, W.J. (1983) Use of models for
assessing relative volatility, mobility, and persistence of
pesticides and other trace organics in soil systems. In: Saxena,
J., ed. Hazard assessment of chemicals, current development, Vol.
2, New York, London, Academic Press, pp 1-43.
KADIS, V.W., BREITKREITZ, W.E., & JONASSON, O.J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. public
Health, 61(5): 413-416.
KAISER, T.E., REICHEL, W.L., LOCKE, L.N., CROMARTIE, E., KRYNITSKY,
A.J., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., STAFFORD, C.J., &
SWINEFORD, D.M. (1980) Organochlorine pesticide, PCB, and PBB
residues and necropsy data for bald eagles from 29 states - 1975-
77. Pestic. monit. J., 13(4): 145-149.
KANITZ, S. & CASTELLO, G. (1966) [Presence of residues of some
disinfectants in human fatty tissue and in certain foods.] G. Ig.
Med. prev., 7: 1-19 (in Italian).
KANJA, L., SKARE, J.K., NAFSTAD, I., MAITAI, C.K., & LOKKEN, P.
(1986) Organochlorine pesticides in human milk from different areas
of Kenya, 1983-1985. J. Toxicol. environ. Health, 19: 449-464.
KATZ, M. (1961) Acute toxicity of some organic insecticides to
three species of salmonids and to the three-spine stickleback.
Trans. Am. Fish. Soc., 90(2): 264-268.
KAWATSKI, J.A. & SCHMULBACH, J.C. (1972) Uptake and elimination of
14C-aldrin and 14C-dieldrin by the ostracod Chlamydotheca arcuata
(Sars). Int. J. environ. anal. Chem., 1: 283-291.
KAZANTZIS, G., MCLAUGHLIN, A.I.G., & PRIOR, P.F. (1964) Poisoning
in industrial workers by the insecticide aldrin. Br. J. ind. Med.,
21: 46-51.
KEANE, W.T. & ZAVON, M.R. (1969a) Validity of a critical blood
level for prevention of dieldrin intoxication. Arch. environ.
Health, 19: 36-44.
KEANE, W.T. & ZAVON, M.R. (1969b) The total body burden of
dieldrin. Bull. environ. Contam. Toxicol., 4(1): 1-16.
KELLER, J. & ALFARO, J.F. (1966) Effect of water application rate
on leaching. Soil Sci., 102(2): 107-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1970) Effects of
combinations of pesticides on reproduction in mice. In: Pesticides
symposia, Miami Beach, Florida, Halos and Associates Inc., pp.
125-138.
KHAIRY, M. (1960) Effects of chronic dieldrin ingestion on the
muscular efficiency of rats. Br. J. ind. Med., 17: 146-148.
KHAN, M.A.Q., COELLO, W., KHAN, A.A., & PINTO, H. (1972a) Some
characteristics of the microsomal mixed-function oxidase in the
fresh-water crayfish Cambarus. Life Sci., 11(2): 405-415.
KHAN, M.A.Q., KAMAL, A., WOLIN, R.J., & RUNNELS, J. (1972b) In vivo
and in vitro epoxidation of aldrin by aquatic food chain organisms.
Bull. environ. Contam. Toxicol., 8(4): 219-228.
KING, P.H. & MCCARTY, P.L. (1968) A chromatographic model for
predicting pesticide migration in soils. Soil Sci., 106(4): 248-261.
KINOSHITA, F.K. & KEMPF, C.K. (1970) Quantitative measurement of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Toxicol. appl. Pharmacol.,
17(1): 288.
KITSELMAN, C.H. (1953) Long-term studies on dogs fed aldrin and
dieldrin in sublethal dosages, with reference to the
histopathological findings and reproduction. J. Am. Vet. Med.
Assoc., 123: 28-30.
KLAASSEN, H.E. & KADOUM, A.M. (1973) Pesticide residues in natural
fish populations of the Smoky Hill River of Western Kansas - 1967-
1969, Pestic. monit. J., 7(1): 53-61.
KLAUNIG, J.E., GOLDBLATT, P.J., HINTON, D.E., LIPSKY, M.M., &
TRUMP, B. (1984) Carcinogen-induced unscheduled DNA synthesis in
mouse hepatocytes. Toxicol. Pathol., 12(2): 119-125.
KLEIN, A.K., LINK, J.D., & IVES, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed
aldrin and dieldrin. J. Assoc. Off. Agric. Chem., 51: 895-898.
KLEIN, A.K., DAILEY, R.E., WALTON, M.S., BECK, V., & LINK, J.D.
(1970) Metabolites isolated from urine of rats fed 14C-
photodieldrin. J. agric. food Chem., 18(4): 705-708.
KLEIN, M.L. & LINCER, J.L. (1974) Behavioural effects of dieldrin
upon the fiddler crab Uca pugilator. In: Vernberg, F.J. &
Vernberg, W.B., ed. Pollution and physiology of marine organisms,
New York, London, Academic Press, pp. 181-196.
KLEIN, W., KOHLI, J., WEISGERBER, I., & KORTE, F. (1973) Fate of
aldrin-14C in potatoes and soil under outdoor conditions. J. agric.
food Chem., 21(2): 152-156.
KLEMMER, H.W., RASHAD, M.N., & MI, M.P. (1973) Age, sex, and race
effects on the distribution of organochlorine pesticide residues in
serum. In: Deichmann, W.B., ed. Pesticides and the environment,
New York, International Medical Book Corporation, pp. 53-61.
KLEVAY, L.M. (1970) Dieldrin excretion by the isolated perfused rat
liver: a sexual difference. Toxicol. appl. Pharmacol., 17: 813-815.
KOEMAN, J.H. (1971) [The occurrence and the toxicological
implications of some chlorinated hydrocarbons in the Dutch coastal
area in the period 1965-70], Utrecht, Rijks Universiteit (Thesis)
(in Dutch).
KOEMAN, J.H. & PENNINGS, J.H. (1970) An orientational survey on the
side effects and environmental distribution of insecticides used in
tsetse control in Africa. Bull. environ. Contam. Toxicol., 5(2):
164-170.
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROCK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis). A preliminary communication. Meded. Fac. Landouwwet.
Rijksuniv. Gent, 32: 841-854.
KOEMAN, J.H., VINK, J.A.J., & DE GOEIJ, J.J.M. (1969) Causes of
mortality in birds of prey and owls in the Netherlands in the
winter of 1968-69. Ardea, 57: 67-76.
KOEMAN, J.H., PEETERS, W.H.M., & PENNINGS, J.H. (1971) OECD
collaborative study 1969/71: pesticide residues in the environment,
Utrecht, The Netherlands, Institute of Veterinary Pharmacology and
Toxicology (Unpublished report).
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972)
Eggshell and population changes in the sparrow hawk (Accipiter
nisus). TNO Nieuws, 27: 542-550.
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1972) [Contributions to
ecological chemistry ILI(1). Transformation and residue behaviour
of dieldrin 14C in onions after seed treatment.] Chem. Mikrobiol.
Technol. Lebensm., 1: 149-150 (in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973a) [Contributions to
ecological chemistry LVIII. Transport of aldrin-14C and
transformation products in the soil.] Chemosphere, 3: 125-130
(in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973) [Contributions to
ecological chemistry LIX. Residue behaviour and transformation of
14C-dieldrin in crops, soil and seepage water after application to
the soil.] Chemosphere, 4: 153-156 (in German).
KORSCHGEN, L.J. (1971) Disappearance and persistence of aldrin
after five annual applications. J. wildl. Manage., 35(3): 494-500.
KORTE, F. (1965) Metabolism of chlorinated insecticides. In:
Radioisotopes in the detection of pesticide residues. Proceedings
of the International Atomic Energy Panel, Vienna, International
Atomic Energy Agency, p. 38.
KORTE, F. & ARENT, H. (1965) Metabolism of insecticides. IX(1).
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin-14C. Life Sci., 4:
2017-2026.
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated
compounds in human organs collected in Denmark, 1972-73. Acta
pharmacol. toxicol., 38: 38-48.
KRAYBILL, H.F. (1977) Global distribution of carcinogenic
pollutants in water. In: Proceedings of the Conference on Aquatic
Pollutants and Biological Effects with Emphasis on Neoplasia, 27-29
September, New York, New York Academy of Sciences.
KRIEGER, R.I. & WILKINSON, C.F. (1969) Microsomal mixed-function
oxidases in insects. I. Localization and properties of an enzyme
system affecting aldrin epoxidation in larvae of the southern
armyworm (Prodenia eridania). Biochem. Pharmacol., 18: 1403-1415.
KURATA, M., HIROSE, K., & UMEDA, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
KURIHARA, N., HORI, N., & ICHINOSE, R. (1984) Cytochrome P-450
content and aldrin epoxidation to dieldrin in isolated rat
hepatocytes. Pestic. Biochem. Physiol., 21: 63-73.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, N.S. (1978a) Persistence
and dissipation of aldrin and dieldrin in soil: a review.
Pesticides, 12(6): 14-18.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, V.S. (1978) Effect of
temperature on the degradation of aldrin residues in sandy loam
soil. Ann. arid Zone, 17(2): 200-206.
KUTZ, F.W., YOBS, A.R., & YANG, H.S.C. (1976) National pesticide
monitoring programs. In: Lee, R.E., ed. Air pollution from
pesticides and agricultural processes, Cleveland, Ohio, CRC Press,
pp. 95-136.
KUTZ, F.W., STRASSMAN, S., & YOBS, A.R. (1979) Survey of pesticide
residues and their metabolites in the general population of the
United States. In: Berlin, A., Wolff, A.H., & Hasegawa, Y., ed. Use
of biological specimens to assess human exposure to environmental
pollutants, The Hague, Martinus Nijhoff, pp. 267-274.
LANE, C.E. & LIVINGSTON, R.J. (1970) Some acute and chronic effects
of dieldrin on the sailfin molly Poecilia latipinna. Trans. Am.
Fish. Soc., 99(3): 489-495.
LANE, C.E., SEBA, D.B., & HEARN, W.L. (1970) Possible metabolites
of dieldrin in the sailfin molly (Poecilia latipinna). Proc. Soc.
Exp. Biol. Med., 133: 1375-1377.
LAUBSCHER, J.A., DUTT, G.R., & ROAN, C.C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of distance
from application. Pestic. monit. J., 5(3): 251-258.
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodiene with brain-specific t-butyl-
bicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAY, J.P., WEISGERBER, I., & KLEIN, W. (1975) Conversion of the
aldrin/dieldrin metabolite dihydrochlordene dicarboxylic acid-14C
in rats. Pestic. Biochem. Physiol., 5: 226-232.
LEHMAN, A.J. (1951) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Q. Bull. Assoc. Food Drug Off., 15: 122-133.
LEHMAN, A.J. (1952) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Section II. Dermal toxicity. Q. Bull. Assoc. Food Drug
Off. (USA), 16: 3-9.
LEHNER, P.N. & EGBERT, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature (Lond.), 224: 1218-1219.
LENARDON, A.M., DE HEVIA, M.I.M., FUSE, J.A., DE NOCHETTO, C.B., &
DEPETRIS, P.J. (1984) Organochlorine and organophosphorus
pesticides in the Parana river (Argentina). Sci. total Environ., 34:
289-297.
LENON, H., CURRY, L., MILLER, A., & PATULSKI, D. (1972) Insecticide
residues in water and sediment from cisterns on the US and British
Virgin Islands. Pestic. monit. J., 6: 188-193.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides.
In: Lee, R.E., Jr, ed. Air pollution from pesticides and
agricultural processes, Cleveland, Ohio, CRC Press, pp. 51-94.
LEWIS, R.G. & LEE, R.E., Jr (1976) Air pollution from pesticides:
sources, occurrence, and dispersion. In: Lee, R.E., Jr, ed. Air
pollution from pesticides and agricultural processes, Cleveland,
Ohio, CRC Press, pp. 5-50.
LICHTENBERG, J.J., EICHELBERGER, J.W., DRESSMAN, R.C., &
LONGBOTTOM, J.E. (1970) Pesticides in surface waters of the United
States: a 5-year summary, 1964-68. Pestic. monit. J., 4(2): 71-86.
LICHTENSTEIN, E.P. (1959) Absorption of some chlorinated
hydrocarbon insecticides from soils into various crops. J. agric.
food Chem., 7: 430-433.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1959) Breakdown of lindane and
aldrin in soils. J. econ. Entomol., 52(1): 118-124.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture and soil
types. J. econ. Entomol., 53(2): 192-197.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1970) Volatilization of
insecticides from various substrates. J. agric. food Chem., 18(5):
814-818.
LICHTENSTEIN, E.P., MUELLER, C.H., MYRDAL, G.R., & SCHULZ, K.R.
(1962) Vertical distribution and persistence of insecticidal
residues in soils as influenced by mode of application and cover
crop. J. econ. Entomol., 55(2): 215-219.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. agric. food Chem.,
18(1): 100-106.
LICHTENSTEIN, E.P., FUHREMANN, T.W., & SCHULZ, K.R. (1971)
Persistence and vertical distribution of DDT, lindane, and aldrin
residues, 10 and 15 years after a single soil application. J.
agric. food Chem., 19(4): 718-721.
LINDER, R.L. & DAHLGREN, R.B. (1970) Occurrence of organochlorine
insecticides in pheasants of South Dakota. Pestic. monit. J., 3(4):
227-232.
LINDER, R.L., DAHLGREN, R.B., & GREICHUS, Y.A. (1970) Residues in
the brain of adult pheasants given dieldrin. J. wildl. Manage., 34:
954-956.
LINDSTROM, F.T, BOERSMA, L., & STOCKARD, D. (1971) A theory on the
mass transport of previously distributed chemicals in a water
saturated sorbing porous medium: isothermal cases. Soil Sci., 112(5):
291-300.
LISS, P.S. & SLATER, P.G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LLOYD, C., BOGAN, J.A., BOURNE, W.R.P., DAWSON, P., & PARSLOW,
J.L.F. (1974) Seabird mortality in the North Irish Sea and Firth of
Clyde early in 1974. Mar. Pollut. Bull., 5: 136-140.
LOCKIE, J.D. & RATCLIFFE, D.A. (1964) Insecticides and Scottish
golden eagles. Br. Birds, 57(3): 89-102.
LOCKIE, J.D., RATCLIFFE, D.A., & BALHARRY, R. (1969) Breeding
success and organochlorine residues in golden eagles in west
Scotland. J. appl. Ecol., 6: 381-389.
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMAN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 298-304.
LOOSE, L.D. (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice, Envir. Health
Perspect., 43: 89-97.
LOOSE, L.D., SILKWORTH, J.B., CHARBONNEAU, T., & BLUMENSTOCK,
F. (1981) Environmental chemical-induced macrophage dysfunction.
Environ. Health Perspect., 39: 79-91.
LOTZ, F., KEMPNEY, J., & DE KEMPNEY, R.S.G. (1983) [Light-induced
breakdown of absorbed chemicals: a simple device for comparative
tests.] Chemosphere, 12: 873-878 (in German).
LOWDEN, G.F., SAUNDERS, C.L., & EDWARDS, R.W. (1969) Organochlorine
insecticides in water. Part II. Water Treat. Exam., 18: 275-287.
LU, F.C., JESSUP, D.C., & LAVALLEE, A. (1965) Toxicity of
pesticides in young versus adult rats. Food Cosmet. Toxicol., 3:
591-596.
LUCKENS, M.M. & DAVIS, W.H. (1965) Toxicity of dieldrin and endrin
to bats. Nature (Lond.), 207(4999): 879-880.
LUDKE, J.R., GIBSON, J.R., & LUSK, C.I. (1972) Mixed-function
oxidase activity in fresh-water fish: aldrin epoxidation and
parathion activation. Toxicol. appl. Pharmacol., 21: 89-97.
LUDWIG, G. & KORTE, F. (1965) Metabolism of insecticides. X.
Detection of dieldrin metabolites by GLC analysis. Life Sci., 4:
2027-2031.
LUDWIG, G., WEIS, J., & KORTE, F. (1964) Excretion and distribution
of aldrin-14C and its metabolites after oral administration for a
long period of time. Life Sci., 3: 123-130.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72(12): 5135-5139.
MACCUAIG, R.D. (1975) Occurrence and movements of pesticide
residues in Ethiopia. Environ. Qual. Saf., 3(suppl.): 850-851.
MACCUAIG, R.D. (1976) The occurrence of insecticides in the blood
of staff of a locust control organization. Bull. environ. Contam.
Toxicol., 15: 162-170.
MACDONALD, R. (1982) Toxicology of sprays: the 4-h acute inhalation
toxicity of aqueous sprays prepared from aldrin 48% (w/v) EC,
Sittingbourne, Shell Research (SBGR.82.036) (Unpublished
proprietary report).
MACEK, K.J. (1975) Acute toxicity of pesticide mixtures to
bluegills. Bull. environ. Contam. Toxicol., 14(6): 648-652.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects of
temperature on the susceptibility of bluegills and rainbow trout to
selected pesticides. Bull. environ. Contam. Toxicol., 4(3): 174-183.
MCEWEN, L.C. & BROWN, R.L. (1966) Acute toxicity of dieldrin and
malathion to wild sharp-tailed grouse. J. wildl. Manage., 30(3):
604-611.
MACKAY, D. & LEINONEN, P.J. (1975) Rate of evaporation of low-
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 9(13): 1178-1180.
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low-
solubility contaminants to atmosphere. Environ. Sci. Technol., 7(7):
611-614.
MACKAY, D., SHIU, W.Y., & SUTHERLAND, R.P. (1979) Determination of
air-water Henry's Law constants for hydrophobic pollutants.
Environ. Sci. Technol., 13(3): 333-337.
MCKINNEY, J.D., MATTHEWS, H.B., & WILSON, N.K. (1973) Determination
of optical purity and prochirality of chlorinated polycyclodiene
pesticide metabolites. Tetrahedron Lett., 21: 1895-1898.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol., 25: 921-928.
MCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982) Toxicities
of five organochlorine compounds in water and sediment to Nereis
virens. Bull. environ. Contam. Toxicol., 28: 216-220.
MAJUMDAR, S.K., KOPELMAN, H.A., & SCHNITMAN, M.J. (1976) Dieldrin-
induced chromosome damage in mouse bone-marrow and WI-38 human lung
cells. J. Hered., 67(5): 303-307.
MAJUMDAR, S.K., MAHARAM, L.G., & VIGLIANTI, G.A. (1977)
Mutagenicity of dieldrin in the Salmonella/microsome test. J.
Hered., 68: 184-185.
MANIGOLD, D.B. & SCHULZE, J.A. (1969) Pesticides in selected
western streams: a progress report. Pestic. monit. J., 3(2): 124-135.
MARCHANT, J.H. (1980) Recent trends in sparrowhawk numbers in
Britain. Bird Stud., 27: 152-154.
MARLOW, R.G. & WALLACE, B.G. (1983) Assessment of exposure
following the use of aldrin as a termiticide in homes. Part II. Air
concentrations and kitchen wipes after one year, Sittingbourne,
Shell Research (SBGR.83.285).
MARLOW, R.G., WALLACE, B.G., & MOORE, J.P. (1982) Assessment of
exposure following the use of aldrin as a termiticide in homes,
Sittingbourne, Shell Research (SBGR.82.370).
MARSHALL, T.C., DOROUGH, H.W., & SWIM, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. agric. food Chem., 24(3): 560-563.
MASON, J.W. & ROWE, D.R. (1976) The accumulation and loss of
dieldrin and endrin in the eastern oyster. Arch. environ. Contam.
Toxicol., 4: 349-360.
MASTROMATTEO, E. (1971) Pesticides and man's health: the picture in
Ontario. In: Proceedings of the Working Conference on
Epidemiological Toxicology of Pesticides, Amsterdam, 8-10 September
1971 (Unpublished paper).
MATSUMURA, F. & BOUSH, G.M. (1967) Dieldrin degradation by soil
microorganisms. Science, 156: 959-961.
MATTHEWS, H.B. & MATSUMURA, F. (1969) Metabolic fate of dieldrin in
the rat. J. agric. food Chem., 17: 845-852.
MATTHEWS, H.B., MCKINNEY, J.D., & LUCIER, G.W. (1971) Dieldrin
metabolism, excretion, and storage in male and female rats. J.
agric. food Chem., 19(6): 1244-1248.
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydrocarbon
insecticides, southern Florida, 1968-72. Pestic. monit. J., 9(2):
106-114.
MAYER, R., LETEY, J., & FARMER, W.J. (1974) Models for predicting
volatilization of soil-incorporated pesticides. Soil Sci. Soc. Am.
Proc., 38: 563-568.
MEHENDALE, H.M. & EL-BASSIOUNI, E.A. (1975) Uptake and disposition
of aldrin and dieldrin by isolated perfused rabbit lung. Drug
Metab. Disp., 3: 543.
MEHENDALE, H.M., SKRENTNY, R.F., & DOROUGH, H.W. (1972) Oxidative
metabolism of aldrin by subcellular root fractions of several plant
species. J. agric. food Chem., 20(2): 398-402.
MEHRLE, P.M. & BLOOMFIELD, R.A. (1974) Ammonia detoxifying
mechanisms of rainbow trout altered by dietary dieldrin. Toxicol.
appl. Pharmacol., 27: 355-365.
MEIERHENRY, E.F., RUEBNER, B.H., GERSHWIN, M.E., HSIEH, L.S., &
FRENCH, S.W. (1983) Dieldrin-induced mallory bodies in hepatic
tumours of mice of different strains. Hepatology, 3(1): 90-95.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 metres.
Environ. Lett., 5(4): 215-221.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
60-61.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls (Tyto alba) fed low levels of DDE and
dieldrin. Arch. environ. Contam. Toxicol., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine
phytoplankton vary in their response to chlorinated hydrocarbons.
Science, 167: 1724-1726.
MES, J., CAMPBELL, D.S., ROBINSON, R.N., & DAVIES, D.J.A. (1977)
Polychlorinated biphenyl and organochlorine pesticide residues in
adipose tissue of Canadians. Bull. environ. Contam. Toxicol., 17(2):
196-203.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 8: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Bull. environ.
Contam. Toxicol., 13: 217-233.
MES, J., DAVIES, D.J., & TURTON, D. (1985) Environmental
contaminants in human fat: a comparison between accidental and non-
accidental causes of death. Ecotoxicol. environ. Saf., 10: 70-74.
METCALFE, R.L., KAPOOR, I.P., LU, P-Y., SCHUTH, C.K., & SHERMAN, P.
(1973) Model ecosystem studies of the environment and fate of six
organochlorine pesticides. Environ. Health Perspect., 4: 35-44.
MICK, D.L., LONG, K.R., & BONDERMAN, D.P. (1972) Aldrin and
dieldrin in the blood of pesticide formulators. Am. Ind. Hyg.
Assoc. J., 33(2): 94-99.
MILES, J.R.W. & HARRIS, C.R. (1971) Insecticide residues in a
stream and a controlled drainage system in agricultural areas of
southwestern Ontario, 1970. Pestic. monit. J., 5(3): 289-294.
MILES, J.R.W. & HARRIS, C.R. (1973) Organochlorine insecticide
residues in streams draining agricultural, urban-agricultural, and
resort areas of Ontario, Canada - 1971. Pestic. monit. J., 6(4):
363-368.
MILLER, G.J. & FOX, J.A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, G.C. & ZEPP, R.G. (1983) Extrapolating photolysis rates
from the laboratory to the environment. Residue Rev., 85: 89-110.
MINISTRY OF WELFARE, HEALTH AND CULTURE (1983) Surveillance
programme: man and nutrition, The Hague, Netherlands, Ministry of
Welfare, Health and Culture, State Supervisory Public Health
Service.
MOERSDORF, K., LUDWIG, G., VOGEL, J., & KORTE, F. (1963) [Excretion
of aldrin-14C and dieldrin-14C and their metabolites through the
bile.] Med. Exp., 8: 90 (in German).
MOFFET, G.B. & YARBROUGH, J.D. (1972) The effects of DDT,
toxaphene, and dieldrin on succinic dehydrogenase activity in
insecticide-resistant and susceptible Gambusia affinis. J. agric.
food Chem., 20(3): 558-560.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health, 19:
633-636.
MORGAN, D.P. & ROAN, C.C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D.P. & ROAN, C.C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15(1): 26-28.
MORGAN, D.P. & ROAN, C.C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORITA, H. & UMEDA, M. (1984) Detection of mutagenicity of various
compounds by FM 3A cell system. Paper presented at the 12th Annual
Meeting of the Mutagenicity Society, Japan. Mutat. Res., 130(5): 371
(No. 33).
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MOSSING, M.L., REDETZKE, K.A., & APPLEGATE, H.G. (1985)
Organochlorine pesticides in blood of persons from El Paso, Texas.
J. environ. Health, 47(6): 312-313.
MOZA, P., WEISGERBER, I., & KLEIN, W. (1972) [Leaching a water-
soluble aldrin-14C breakdown product out of soil.] Chemosphere, 5:
191-195 (in German).
MUELLER, W. & LEACH, R.M., Jr (1974) Effects of chemicals on
eggshell formation. Annu. Rev. Pharmacol., 14: 289-303.
MUELLER, W., NOHYNEK, G., WOODS, G., KORTE, F., & COULSTON, F.
(1975a) Comparative metabolism of dieldrin-14C in mouse, rat,
rabbit, rhesus monkey, and chimpanzee. Chemosphere, 4(2): 89-92.
MUELLER, W., WOODS, G., KORTE, F., & COULSTON, F. (1975b)
Metabolism and organ distribution of dieldrin-14C in rhesus monkeys
after single oral and intravenous administration. Chemosphere, 2:
93-98.
MUELLER, P., NAGEL, P., & FLACKE, W. (1981) Ecological side effects
of dieldrin application against tsetse flies in Adamaoua,
Cameroon. Oecologia, 50(2): 187-194.
MUIR, C.M.C. (1970) The acute oral and percutaneous toxicities to
rats of formulations of aldrin, dieldrin, or endrin,
Sittingbourne, Shell Research (TLGR.0020.70) (Unpublished
proprietary report).
MUIRHEAD, E.E., GROVES, M., GUY, R., HALDEN, E.R., & BASS, R.K.
(1959) Acquired hemolytic anemia, exposure to insecticides and
positive Coombs test dependent on insecticide preparations. Vox
sang., 4: 277-292.
MULHERN, B.M., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., BELISLE,
A., CROMARTIE, E., BAGLEY, G.E., & PROUTY, R.M. (1970)
Organochlorine residues and autopsy data from bald eagles 1966-68.
Pestic. monit. J., 4(3): 141-144.
MULLER, H.D. & LOCKMAN, D.C. (1972) Fecundity and progeny growth
following subacute insecticide ingestion by the mallard. Poult.
Sci., 51: 239-241.
MULLINS, D.E., JOHNSEN, R.E., & STARR, R.I. (1971) Persistence of
organochlorine insecticide residues in agricultural soils of
Colorado. Pestic. monit. J., 5(3): 268-275.
MURPHY, D.A. & KORSCHGEN, L.J. (1970) Reproduction, growth, and
tissue residues of deer fed dieldrin. J. wildl. Manage., 34(4):
887-903.
MURPHY, R. & HARVEY, C. (1985) Residues and metabolites of selected
persistent halogenated hydrocarbons in blood specimens from a
general population survey. Environ. Health Perspect., 60: 115-120.
MURTON, R.K. & VIZOSO, M. (1963) Dressed cereal seed as a hazard to
woodpigeons. Ann. appl. Biol., 52: 503-517.
NASH, R.G. (1983) Comparative volatilization and dissipation rates
of several pesticides from soil. J. agric. food Chem., 31(2): 210-217.
NASH, R.G., BEALL, M.L., Jr, & WOOLSON, E.A. (1970) Plant uptake of
chlorinated insecticides from soils. Agron. J., 62: 369-372.
NATIONAL FOOD ADMINISTRATION (1982) Summary and assessment of data
received from the FAO/WHO Collaborating Centres for Food
Contamination Monitoring, Uppsala, Global Environmental Monitoring
System (GEMS), Joint FAO/WHO Food and Animal Feed Contamination
Monitoring Programme, pp. 26-29.
NCI (1977) Bioassay of photodieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-817).
NCI (1978a) Bioassay of aldrin and dieldrin for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(DHEW Publication No. (NIH) 78-821).
NCI (1978b) Bioassay of dieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-822).
NEILL, D.D., MULLER, H.D., & SHUTZE, J.V. (1971) Pesticide effects
on the fecundity of the gray partridge. Bull. environ. Contam.
Toxicol., 6(6): 546-551.
NELSON, E. (1953) Aldrin poisoning. Rocky Mt. med. J., 50: 483-486.
NEWTON, I. (1973a) Studies of sparrowhawks. Br. Birds, 66: 271-278.
NEWTON, I. (1973b) Success of sparrowhawks in an area of pesticide
usage. Bird Stud., 20: 1-8.
NEWTON, I. (1974) Changes attributed to pesticides in the nesting
success of the sparrowhawks in Britain. J. appl. Ecol., 11: 95-102.
NEWTON, I. (1976) Breeding of sparrowhawks (Accipiter nisus) in
different environments. J. anim. Ecol., 45: 831-849.
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted,
Poyser T. and A.D., Ltd.
NEWTON, I. & BOGAN, J. (1974) Organochlorine residues, eggshell
thinning, and hatching success in British sparrowhawks. Nature
(Lond.), 249: 582-583.
NEWTON, I. & BOGAN, J. (1978) The role of different organochlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol.,
15: 105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds, 77: 47-70.
NEWTON, I., MARQUISS, M., & MOSS, D. (1979) Habitat, female age,
organochlorine compounds, and breeding of European sparrowhawks. J.
appl. Ecol., 16: 777-793.
NISHIMURA, N., NISHIMURA, H., & OSHIMA, H. (1982) Survey on
mutagenicity of pesticides by the Salmonella microsome test. J.
Aichi Med. Univ. Assoc., 10(4): 305-312.
NOHYNEK, G.J., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Metabolism, excretion, and tissue distribution of 14C-photodieldrin
in non-human primates following oral administration and intravenous
injection. Ecotoxicol. environ. Saf., 3: 1-9.
O'CONNOR, R.J. (1982) Habitat occupancy and regulation of clutch
size in the European kestrel Falco tinnunculus. Bird Stud., 29:
17-26.
ODA, J. & MUELLER, W. (1972) Identification of a mammalian
breakdown product of dieldrin. Environ. Qual. Saf., 1: 248-249.
OHLENDORF, H.M., BARTONEK, J.C., DIVOKY, G.J., KLAAS, E.E., &
KRYNITSKY, A.J. (1982) Organochlorine residues in eggs of Alaskan
seabirds, Washington, DC, US Department of the Interior, Fish and
Wildlife Service (Special Scientific Report: Wildlife No. 245).
OLOFFS, P.C., HARDWICK, D.F., SZETO, S.Y., & MOERMAN, D.G. (1974)
DDT, dieldrin, and heptachlorepoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
ONSAGER, J.A., RUSK, H.W., & BUTLER, L.I. (1970) Residues of
aldrin, dieldrin, chlordane, and DDT in soil and sugar beets. J.
econ. Entomol., 63(4): 1143-1146.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic changes
in the liver of rats after feeding low levels of various
insecticides. Am. Med. Assoc. Arch. Pathol., 64(6): 614-622.
OTTOLENGHI, A.D., HASEMAN, J.K., & SUGGS, F. (1974) Teratogenic
effects of aldrin, dieldrin, and endrin in hamsters and mice.
Teratology, 9: 11-16.
PACCAGNELLA, B., GHEZZO, F., PRATI, L., FEDRAZZONI, U., & BELLONI,
G. (1971) Epidemiological study of long-term effects of pesticides
on human health. Bull. World Health Organ., 45: 181-199.
PAINTER, R.B. (1981) DNA synthesis inhibition in mammalian cells as
a test for mutagenic carcinogens. In: Stich, H.F. & San, R.H.C.,
ed. Short-term tests for chemical carcinogens, New York, Springer-
Verlag.
PARK, P.O. & MCKONE, C.E. (1966) The persistence and distribution
of soil-applied insecticides in an irrigated soil in northern
Tanzania. Trop. Agric. Trin., 43(2): 133-142.
PARSLOW, J.L.F. (1973) Breeding birds of Britain and Ireland: a
historical survey, Berkhamsted, Poyser T. and A.D., Ltd.
PARSLOW, J.L.F. & JEFFERIES, D.J. (1973) Relationship between
organochlorine residues in livers and whole bodies of guillemots.
Environ. Pollut., 5: 87-101.
PARSONS, A.M. & MOORE, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem.
Soc., C: 2026-2031.
PATEL, T.B. & RAO, V.N. (1958) Dieldrin poisoning in man. A report
of 20 cases in Bombay State. Br. med. J., 1: 919-921.
PATON, R., LUKE, B.G., & ROBERTS, G. (1984) Studies on the sorption
of organochlorine insecticides by flour stored on or near treated
laminated timber or plywood as used in freight containers. Pestic.
Sci., 15: 624-629.
PEARCE, P.A., PEAKALL, D.B., & REYNOLDS, L.M. (1979) Shell thinning
and residues of organochlorines and mercury in seabird eggs,
eastern Canada, 1970-76. Pestic. monit. J., 13(2): 61-68.
PEARSON, J.E., TINSLEY, K., & HERNANDEZ, T. (1973) Distribution of
dieldrin in the turtle. Bull. environ. Contam. Toxicol., 10(6):
360-364.
PETROCELLI, S.R., HANKS, A.R., & ANDERSON, J.W. (1973) Uptake and
accumulation of an organochlorine insecticide (dieldrin) by an
estuarine mollusc Rangia cuneata. Bull. environ. Contam. Toxicol.,
10(5): 315-320.
PETROCELLI, S.R., ANDERSON, J.W., & HANKS, A.R. (1975)
Biomagnification of dieldrin residues by food-chain transfer from
clams to blue crabs under controlled conditions. Bull. environ.
Contam. Toxicol., 13(1): 108-116.
PIONKE, H.B. & CHESTERS, G. (1973) Pesticide-sediment-water
interactions. J. environ. Qual., 2(1): 29-45.
POLISHUK, Z.W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health, 20:
215-217.
POLISHUK, Z.W., RON, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D.,
& LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. monit. J., 10(4): 121-129.
PORTER, R.D. & WIEMEYER, S.N. (1969) Dieldrin and DDT: effects on
sparrowhawk eggshells and reproduction. Science, 165: 199-200.
POTTER, J.C., DIXON, L.D., BARBER, G.F., & MARXMILLER, R.L. (1972)
Residues of 14 C-dieldrin and its metabolites in milk from cows fed
dieldrin-14 C, Modesto, California, Shell Development Company
(TIR-24-109-72) (Unpublished proprietary report).
POWELL, A.J.B., STEVENS, T., & MCCULLY, K.A. (1970) Effects of
commercial processing on residues of aldrin and dieldrin in
tomatoes and residues in subsequent crops grown on the treated
plots. J. agric. food Chem., 18(2): 224-227.
POWERS, C.D, ROWLAND, R.G., & WURSTER, C.F. (1977) Dieldrin-induced
destruction of marine algal cells with concomitant decrease in size
of survivors and their progeny. Environ. Pollut., 12: 17-25.
PRESTT, I. (1965) An enquiry into the recent breeding status of
some of the smaller birds of prey and crows in Britain. Bird Stud.,
12(3): 196-221.
PRESTT, I. & BELL, A.A. (1966) An objective method of recording
breeding distribution of common birds of prey in Britain. Bird
Stud., 13(4): 277-283.
PRINCI, F. (1954) Toxicity of the chlorinated hydrocarbon
insecticides. In: Proceedings of the XI International Congress di
Medicina del Lavoro, Pipola, Napoli, Tipografia Saverio, pp.
253-272.
PRINCI, F. & SPURBECK, G.H. (1951) A study of workers exposed to
the insecticides chlordane, aldrin, dieldrin. Arch. ind. Hyg.
occup. Med., 3: 64-72.
PROBST, A.H. & EVERLY, R.T. (1957) Effect of soil insecticides on
emergence, growth, yield, and chemical composition of soybeans.
Agron. J., 1957: 385-387.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: a comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagenesis, 3: 11-32.
PROSPERO, J.M. & SEBA, D.B. (1972) Some additional measurements of
pesticides in the lower atmosphere of the northern equatorial
Atlantic Ocean. Atmos. Environ., 6(5): 363-364.
PROUTY, R.M., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., CROMARTIE,
E., KAISER, T.E., LAMONT, T.G., MULHERN, B.M., & SWINEFORD, D.M.
(1977) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1973-74. Pestic. monit.
J., 11(3): 134-137.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON,
D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of 6
short-term tests for detecting organic chemical carcinogens. Br. J.
Cancer, 37: 873-903.
RADELEFF, R.D., WOODARD, G.T., NICKERSON, W.J., & BUSHLAND, R.C.
(1955) The acute toxicity of chlorinated hydrocarbon and organic
phosphorus insecticides to livestock, Kerville, Texas, US
Department of Agriculture, Agricultural Research Service,
(Technical Bulletin 1122).
RADELEFF, R.D., NICKERSON, W.J., & WELLS, R.W. (1960) Acute toxic
effects upon livestock and meat and milk residues of dieldrin. J.
econ. Entomol., 53(3): 425-429.
RADOMSKI, J.L. & FISEROVA-BERGEROVA, V. (1965) The determination of
pesticides in tissues with the electron-capture detector without
prior clean-up. Ind. Med. Surg., 32: 934-939.
RADOMSKI, J.L., DEICHMANN, W.B., & CLIZER, E.E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
RADOMSKI, J.L., ASTOLFI, E., DEICHMANN, W.B., & REY, A.A. (1971)
Blood levels of organochlorine pesticides in Argentina:
occupationally and non-occupationally exposed adults, children, and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RATCLIFFE, D.A. (1963) The status of the peregrine in Great
Britain. Bird Stud., 10: 56-90.
RATCLIFFE, D.A. (1965) The peregrine situation in Great Britain,
1963-64. Bird Stud., 12(2): 66-82.
RATCLIFFE, D.A. (1967a) Decrease in eggshell weight in certain
birds of prey. Nature (Lond.), 215: 208-210.
RATCLIFFE, D.A. (1967b) The peregrine situation in Great Britain,
1965-66. Bird Stud., 14(4): 238-245.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol., 7: 67-115.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Stud., 19: 117-156.
RATCLIFFE, D.A. (1980) The peregrine falcon, Calton, T. and A.D.
Poyser.
RATCLIFFE, D.A. (1984) The peregrine breeding population of the
United Kingdom in 1981. Bird Stud., 31(1): 1-18.
REICHEL, W.L., CROMARTIE, E., LAMONT, T.G., MULHERN, B.M., &
PROUTY, R.M. (1969) Pesticide residues in eagles. Pestic. monit.
J., 3(3): 142-144.
REIDINGER, R.F. & CRABTREE, D.G. (1974) Organochlorine residues in
golden eagles, United States, March 1964-July 1971. Pestic. monit.
J., 8(1): 37-43.
REINERT, R.E. (1972) Accumulation of dieldrin in an alga
(Scenedesmus obliquus), Daphnia magna, and the guppy (Poecilia
reticulata). J. Fish. Res. Board Can., 29(10): 1413-1418.
REINKE, J., UTHE, J.F., & JAMIESON, D. (1972) Organochlorine
pesticide residues in commercially caught fish in Canada, 1970.
Pestic. monit. J., 6(1): 43-49.
REIMOLD, R.J. (1975) Chlorinated hydrocarbon pesticides and mercury
in coastal biota - Puerto Rico and the US Virgin Islands 1972-1974.
Pestic. monit. J., 9(1): 39-43.
REYNOLDS, C.M. (1974) The census of heronries, 1969-73. Bird Stud.,
21: 129-134.
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin, and endrin. Ind. Arch. occup. environ. Health,
56: 75-79.
RICE, C.P. & SIKKA, H.C. (1973) Fate of dieldrin in selected
species of marine algae. Bull. environ. Contam. Toxicol., 9(2):
116-123.
RICHARD, J.J., JUNK, G.A., AVERY, M.J., NEHRING, N.L., FRITZ, J.S.,
& SVEC, H.J. (1975) Analysis of various Iowa waters for selected
pesticides: atrazine, DDE, and dieldrin - 1974. Pestic. monit. J.,
9(3): 117-123.
RICHARDSON, A. (1971) The isolation and identification of a
metabolite of HEOD (dieldrin) from human faeces, Sittingbourne,
Shell Research (TLGR.0021.71).
RICHARDSON, A. & ROBINSON, J. (1971) The identification of a major
metabolite of HEOD (dieldrin) in human faeces. Xenobiotica, 1(3):
213-219.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967a)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
RICHARDSON, L.A., LANE, J.R., GARDNER, W.S., PEELER, J.T., &
CAMPBELL, J.E. (1967b) Relationship of dietary intake to
concentration of dieldrin and endrin in dogs. Bull. environ.
Contam. Toxicol., 2(4): 207-219.
RICHARDSON, A., BALDWIN, M.K., & ROBINSON, J. (1968) Metabolites of
dieldrin (HEOD) in the urine and faeces of rats. Chem. Ind., 1968:
588-589.
RICKARD, D.G. & DULLEY, M.E.R. (1983) The levels of some heavy
metals and chlorinated hydrocarbons in fish from the tidal Thames.
Environ. Pollut. Ser. B., 5: 101-119.
RISEBROUGH, R.W., HUGGETT, R.J., GRIFFIN, J.J., & GOLDBERG, E.D.
(1968) Pesticides: transatlantic movements in the northeast trades.
Science, 159(3820): 1233-1236.
RITCEY, W.R., SAVARY, G., & MCCULLY, K.A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can. J.
public Health, 64: 380-386.
ROBINSON, J. (1969) Organochlorine insecticides and bird
populations in Britain. In: Miller, M.W. & Berg, G.G., ed. Chemical
fallout, Springfield, Illinois, C.C. Thomas, pp. 113-169.
ROBINSON, J. & CRABTREE, A.N. (1969) The effect of dieldrin on
homing pigeons ( Columba livia var.). Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 34(3): 413-427.
ROBINSON, J. & HUNTER, C.G. (1966) Organochlorine insecticides:
concentrations in human blood and adipose tissue. Arch. environ.
Health, 13: 558-563.
ROBINSON, J. & ROBERTS, M. (1969) Estimation of the exposure of the
general population to dieldrin (HEOD). Food Cosmet. Toxicol., 7:
501-514.
ROBINSON, J., RICHARDSON, A., & ELGAR, K.E. (1966a) Chemical
identity in ultramicroanalysis. In: Proceedings of the American
Chemical Society Meeting, New York, 11-16 September, 1966,
Washington, DC, American Chemical Society.
ROBINSON, J., RICHARDSON, A., BUSH, B., & ELGAR, K.E. (1966b) A
photoisomerization product of dieldrin. Bull. environ. Contam.
Toxicol., 1(4): 127-132.
ROBINSON, J., RICHARDSON, A., & DAVIES, J.M. (1967a) Comparison of
analytical methods for determination of dieldrin (HEOD) in blood.
Arch. environ. Health, 15(1): 67-69.
ROBINSON, J., BROWN, V.K.H., RICHARDSON, A., & ROBERTS, M. (1967b)
Residues of dieldrin (HEOD) in the tissues of experimentally
poisoned birds. Life Sci., 6: 1207-1220.
ROBINSON, J., ROBERTS, M., BALDWIN, M., & WALKER, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Food Cosmet.
Toxicol., 7: 317-332.
ROBURN, J. (1963) Effect of sunlight and ultraviolet radiation on
chlorinated pesticide residues. J. chem. Ind., 1: 1555-1556.
ROCCHI, P., PEROCCO, P., ALBERGHINI, W., FINI, A., & PRODI, G.
(1980) Effect of pesticides on scheduled and unscheduled DNA
synthesis of rat thymocytes and human lymphocytes. Arch. Toxicol.,
45: 101-108.
ROHWER, D. (1983a) [Pesticides in breast milk (problems with
residues of polychlorinated hydrocarbons).] Wiss. Inf., 9(3):
197-201 (in German).
ROHWER, D. (1983b) [Pesticides in breast milk.] Geburtshilfe
Frauenheilkd., 43: 160-163 (in German).
ROSE, G.P. (1982) Toxicity of insecticides: the acute oral and
percutaneous toxicity, skin and eye irritancy, and skin sensitizing
potential of a 480 g/litre emulsifiable concentrate of aldrin (EF
5159), Sittingbourne, Shell Research (SBGR.81.319) (Unpublished
proprietary report).
ROSE, G.P. (1984a) Toxicology of insecticides: the acute
percutaneous toxicity of the 480 g/litre aldrin emulsifiable
concentrate formulation CF 06323, Sittingbourne, Shell Research
(SBGR.84.047) (Unpublished proprietary report).
ROSE, G.P. (1984b) Toxicology of insecticides: the acute
percutaneous toxicity of the 200 g/litre dieldrin emulsifiable
concentrate formulation CF 06271, Sittingbourne, Shell Research
(SBGR.84.045) (Unpublished proprietary report).
ROSE, G.P. (1984c) Toxicology of insecticides (organochlorines):
the acute percutaneous toxicity of the 680 g/l dieldrin suspension
concentrate SF 06349, Sittingbourne, Shell Research (SBGR.84.210)
(Unpublished proprietary report).
ROSEN, J.D. & CAREY, W.F. (1968) Preparation of the photoisomers of
aldrin and dieldrin. J. agric. food Chem., 16: 536-537.
ROSEN, J.D., SUTHERLAND, D.J., & LIPTON, G.R. (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull.
environ. Contam. Toxicol., 1(4): 133-140.
ROSEWELL, K.T., MUIR, D.C.G., & BAKER, B.E. (1979) Organochlorine
residues in Harp Seal (Phagophilus groenlandicus) tissues, Gulf of
St. Lawrence, 1971, 1973. Pestic. monit. J., 12: 189-192.
ROSS, R.D. & CROSBY, D.G. (1974) Photosensitizers in agricultural
water samples, Washington, DC, American Chemical Society (ACS
Meeting, Abstract No. 167, Section PEST 67).
ROSS, R.D. & CROSBY, D.G. (1975) The photooxidation of aldrin in
water. Chemosphere, 4: 227.
ROSS, R.D. & CROSBY, D.G. (1985) Photooxidant activity in natural
waters. Environ. Toxicol. Chem., 4: 773-778.
ROSS, W.R., VAN LEEUWEN, J., & GRABOW, W.O.K. (1976) Studies on the
disinfection and chemical oxidation with ozone and chlorine in
water reclamation. In: Proceedings of the Second Symposium on Ozone
Technology, Montreal, International Ozone Institute, pp. 479-513.
ROWE, D.B., CANTER, L.W., SNIJDER, P.J., & MASON, J.W. (1971)
Dieldrin and endrin concentrations in a Louisiana estuary. Pestic.
monit. J., 4(4): 177-183.
RUEBNER, B.H., GERSHWIN, M.E., MEIERHENRY, E.F., HSIEH, L.S., &
DUNN, P.L. (1984a) Irreversibility of liver tumours in C3H mice. J.
Natl Cancer Inst., 73(2): 493-498.
RUEBNER, B.H., GERSHWIN, M.E., FRENCH, S.W., MEIERHENRY, E., DUNN,
P., & HSIEH, L.S. (1984b) Mouse hepatic neoplasia: Differences
among strains and carcinogens. In: Popp J.A., ed. Current
perspectives in mouse liver neoplasia, Washington, DC, Hemisphere.
SAHA, J.G. & SUMNER, A.K. (1971) Organochlorine insecticide
residues in soil from vegetable farms in Saskatchewan. Pestic.
monit. J., 5(1): 28-31.
SAHA, J.G., KARAPALLY, J.C., & JANSEN, W.K. (1971) Influence of the
type of mineral soil on the uptake of dieldrin by wheat seedlings.
J. agric. food Chem., 19: 842-845.
SAND, P.F., WIERSMA, G.B., & LANDRY, J.L. (1972) Pesticide residues
in sweet potatoes and soil - 1969. Pestic. monit. J., 5(4): 342-344.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the
western chorus frog Pseudacris triseriata and Fowler's Toad Bufo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDHU, S.-S., WARREN, W.J., & NELSON, P. (1978) Pesticidal residue
in rural potable water. Am. Water Works Assoc. J., 70(1): 41-45.
SANDIFER, S.H., CUPP, C.M., WILKINS, R.T., LOADHOLT, B., & SCHUMAN,
S.H. (1981) A case-control study of persons with elevated blood
levels of dieldrin. Arch. environ. Contam. Toxicol., 10(1): 35-45.
SANGER, A.M.H. (1959) Aldrin, dieldrin, and endrin toxicity to
bees. Span, 2(2): 59-63.
SASCHENBRECKER, P.W. (1976) Levels of terminal pesticide residues
in Canadian meat, Can. Vet. J., 17(6): 158-163.
SAVAGE, E.P., KEEFE, T.J., TESSARI, J.D., WHEELER, H.W., APPLEHAUS,
F.M., GOES, E.A., & FORD, S.A. (1981) National study of chlorinated
hydrocarbon insecticide residues in human milk, USA. I. Geographic
distribution of dieldrin, heptachlor, heptachlor epoxide,
chlordane, oxychlordane, and mirex. Am. J. Epidemiol., 113: 413-422.
SCHAFER, M.L. (1968) Pesticides in blood. Residue Rev., 24: 19-39.
SCHAUBERGER, C.W. & WILDMAN, R.B. (1977) Accumulation of aldrin and
dieldrin by blue-green algae and related effects on photosynthetic
pigments. Bull. environ. Contam. Toxicol., 17(5): 534-541.
SCHULZE, J.A., MANIGOLD, D.B., & ANDREWS, F.L. (1973) Pesticides in
selected western streams - 1968-71. Pestic. monit. J., 7(1): 73-84.
SEAL, W.L., DAWSEY, L.H., & CAVIN, G.E. (1967) Monitoring for
chlorinated hydrocarbon pesticides in soil and root crops in the
eastern states in 1965. Pestic. monit. J., 1(3): 22-25.
SELBY, L.A., NEWELL, K.W., HAUSER, G.A., & JUNKER, G. (1969a)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2(4): 247-255.
SELBY, L.A., NEWELL, K.W., WAGGENSPACK, C., HAUSER, G.A., & JUNKER,
G. (1969b) Estimating pesticide exposure in man as related to
measurable intake: environmental versus chemical index. Am. J.
Epidemiol., 89(3): 241-253.
SETHUNATHAN, N. (1973) Microbial degradation of insecticides in
flooded soil and in anaerobic cultures. Residue Rev., 47: 143-165.
SHAH, P.V. & GUTHRIE, F.E. (1976) Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Watson, D.L. &
Brown, A.W.A., ed. Pesticide management and insecticide resistance,
New York, London, Academic Press, pp. 547-554.
SHAKOORI, A.R., RASUL, Y.G., & ALI, S.S. (1986) The effect of long-
term administration of dieldrin on biochemical components in blood
serum of albino rats. Toxicol. Lett., 9(4): 35.
SHANNON, L.R. (1977a) Equilibrium between uptake and elimination of
dieldrin by channel catfish Ictalurus punctatus. Bull. environ.
Contam. Toxicol., 17(3): 278-284.
SHANNON, L.R. (1977b) Accumulation and elimination of dieldrin in
muscle tissue of channel catfish. Bull. environ. Contam. Toxicol.,
17(6): 637-644.
SHAROM, M.S., MILES, J.R.W., HARRIS, C.R., & MCEWEN, F.L. (1980)
Behaviour of 12 insecticides in soil and aqueous suspensions of
soil and sediment, Water Res., 14: 1095-1100.
SHEETS, T.J., JACKSON, M.D., & PHELPS, L.D. (1970) Water monitoring
system for pesticides in North Carolina, US Clearinghouse, 109 pp
(P.B. Report No. 189291).
SHELL (1976) Chemicals for plant protection, veterinary uses, and
public health, London, Shell International Chemical Company, Ltd,
pp. 3-4, 9-10.
SHELL (1984) Shell guide to pesticide safety, London, Shell
International Chemical Company, Ltd, Agrochemical Division, pp.
27-29.
SHELLENBERGER, T.E. (1978) A multi-generation toxicity evaluation
of p,p'-DDT and dieldrin with Japanese quail. Effects on growth and
reproduction. Drug chem. Toxicol., 1(2): 137-146.
SHELLENBERGER, T.E. & NEWELL, G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail (Coturnix coturnix
japonica). Lab. anim. Care, 15(2): 119-130.
SHERMAN, M. & ROSENBERG, M.M. (1953) Acute toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 46(6): 1067-1070.
SHIRASU, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4: 226-231.
SIERRA, M. & SANTIAGO, D. (1987) Organochlorine pesticide levels in
barn owls collected in Leon, Spain. Bull. environ. Contam.
Toxicol., 38: 261-265.
SIERRA, M., TERAN, M.T., GALLEGO, A., DIEZ, M.J., & SANTIAGO, D.
(1987) Organochlorine contamination in three species of diurnal
raptors in Leon, Spain. Bull. environ. Contam. Toxicol., 38: 254-260.
SILBERGELD, E.K. (1973) Dieldrin. Effects of chronic sublethal
exposure on adaptation to thermal stress in fresh-water fish.
Environ. Sci. Technol., 7(9): 846-849.
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.C. (1977) Mutagenic
activity of chemicals identified in drinking-water. In: Scott, D.,
Bridges, B.A., & Sobels, F.H., ed. Progress in genetic toxicology,
Amsterdam, Elsevier Science Publishers, pp. 249-258.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY, M.O.
(1983) Evaluation of the alkaline elution/rat hepatocyte assay as a
predictor of carcinogenic/mutagenic potential. Mutat. Res., 113:
357-391.
SINGH, K.K., JHA, G.J., SINGH, P.N., & CHAUHAN, H.V.S. (1985)
Pathophysiology of acute aldrin intoxication in goats. Toxicol.
Abstr., 8(7): 27 (No. 4086-x8).
SIYALI, D.S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust., 2: 1063-1066.
SIYALI, D.S. (1973) Polychlorinated biphenyls, hexachlorobenzene,
and other organochlorine pesticides in human milk. Med. J. Aust.,
2: 815-818.
SIYALI, D.S. & SIMSON, R.E. (1973) Chlorinated hydrocarbon
pesticides in human blood and fat. Med. J. Aust., 1: 212-213.
SLATER, R.M. & SPEDDING, D.J. (1981) Transport of dieldrin between
air and water. Arch. environ. Contam. Toxicol., 10: 25-33.
SMITH, J.H., BOMBERGER, D.C., Jr, & HAYNES, D.L. (1981)
Volatilization rates of intermediate and low volatility chemicals
from water. Chemosphere, 10(3): 281-289.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of
DDT and dieldrin on development in winter flounder
(Pseudopleuronectes americanus). J. Fish. Res. Board Can., 30(12):
1894-1898.
SMITH, S.W.C. (1978) Pesticide residues in the total diet. Food
Technol. Aust., 1978: 349-352.
SPALDING, R.F., JUNK, C.A., & RICHARD, J.R. (1980) Pesticides in
groundwater beneath irrigated farmland in Nebraska, August 1978.
Pestic. monit. J., 14: 70-73.
SPARR, B.I., APPLEBY, W.G., DEVRIES, D.M., OSMUN, J.V., MCBRIDE,
J.M., FOSTER, G.L., & GOULD R.F., ed. (1966) Organic pesticides in
the environment, Washington, DC, American Chemical Society, pp.
146-162 (Advances in Chemistry Series No. 60).
SPENCER, W.F. & CLIATH, M.M. (1973) Pesticide volatilization as
related to water loss from soil. J. environ. Qual., 2(2): 284-289.
SPENCER, W.F. & CLIATH, M.M. (1975) Environmental dynamics of
pesticides. In: Haque, R. & Freed, V.H. ed., New York, London,
Plenum Press, pp. 61-78.
SPENCER, W.F., CLIATH, M.M., & FARMER, W.J. (1969) Vapor density of
soil-applied dieldrin as related to soil-water content,
temperature, and dieldrin concentration. Soil Sci. Soc. Am. Proc.,
33: 509-511.
SPENCER, W.F., FARMER, W.J., & CLIATH, M.M. (1973) Pesticide
volatilization. Residue Rev., 49: 1-47.
SPIOTTA, E.J. (1951) Aldrin poisoning in man. Report of a case.
Arch. ind. Hyg. occup. Med., 4: 560-566.
STACEY, C.I. & TATUM, T. (1985) House treatment with organochlorine
pesticides and their levels in human milk - Perth, Western
Australia. Bull. environ. Contam. Toxicol., 35: 202-208.
STACEY, C.I. & THOMAS, B.W. (1975) Organochlorine pesticide
residues in human milk, Western Australia, 1970-71. Pestic. monit.
J., 9(2): 64-66.
STACEY, C.I., PERRIMAN, W.S., & WHITNEY, S. (1985) Organochlorine
pesticides residue levels in human milk: Western Australia,
1979-80. Arch. environ. Health, 40: 102-108.
STANLEY, C.W., BARNEY, J.E., HELTON, M.R., & YOBS, A.R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANLEY, P.I. & BUNYAN, P.J. (1979) Hazards to wintering geese and
other wildlife from the use of dieldrin, chlorfenvinphos, and
carbophenothion as wheat seed treatments. Proc. R. Soc. Lond. Ser
B, 205: 31-45.
STEVENS, L.J., COLLIER, C.W., & WOODHAM, D.W. (1970) Monitoring
pesticides in soils from areas of regular, limited, and no
pesticide use. Pestic. monit. J., 4(3): 145-164.
STEVENSON, D.E. & WALKER, A.I.T. (1969) Hepatic lesions produced in
mice by dieldrin and other hepatic enzyme-inducing compounds. J.
Eur. Toxicol., 2: 83-84.
STEVENSON, D.E., THORPE, E., HUNT, P.F., & WALKER, A.I.T. (1976)
The toxic effects of dieldrin in rats: a re-evaluation of data
obtained in a two-year feeding study. Toxicol. appl. Pharmacol., 36:
247-254.
STEWART, D.J. & STEIN, R.A. (1974) Short-term fate of dietary
dieldrin in the digestive tract of juvenile lake trout (Salvelinus
namaycush). Bull. environ. Contam. Toxicol., 11(6): 563-566.
STEWART, D.K.R. & FOX, C.J.S. (1971) Persistence of organochlorine
insecticides and their metabolites in Nova Scotian soils. J. econ.
Entomol., 64(2): 367-371.
STEWART, D.K.R. & GAUL, S.O. (1977) Dihydrochlordene dicarboxylic
acid residues in soil treated with high rates of aldrin. Bull.
environ. Contam. Toxicol., 17(6): 712-713.
STICKEL, W.H. (1975) Some effects of pollutants in terrestrial
ecosystems. Environ. Sci. Res., 7: 25-74.
STICKEL, W.H., STICKEL, L.F., & SPANN, J.W. (1969) Tissue residues
of dieldrin in relation to mortality in birds and mammals. In:
Miller, M.W. & Berg, G.G., ed. Chemical fallout, Springfield,
Illinois, C.C. Thomas, pp. 174-200.
SUNDARAM, K.S., DAMODARAN, V.N., & VENKITASUBRAMANIAM, T.A.
(1978) Absorption of dieldrin through monkey and dog skin. Indian
J. exp. Biol., 16: 101-103.
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1974) Photodieldrin
residues in field soils. Bull. environ. Contam. Toxicol., 12:
275-279.
SWENBERG, J.A., PETZOLD, G.L., & HARBACH, P.R. (1976) In vitro DNA
damage-alkaline elution assay of predicting carcinogenic potential.
Biochem. Biophys. Res. Commun., 72(2): 732-738.
SZARO, R.C., COON, N.C., & KOLBE, E. (1979) Pesticide and PCB of
common eider, herring gull, and great black-backed gull eggs. Bull.
environ. Contam. Toxicol., 22: 394-399.
TABOR, E.C. (1966) Contamination of urban air through the use of
insecticides. Trans. N.Y. Acad. Sci., 28: 569-578.
TAKEI, G.H., KAUAHIKAUA, S.M., & LEONG, G.H. (1983) Analyses of
human milk samples collected in Hawaii for residues of
organochlorine pesticides and polychlorobiphenyls. Bull. environ.
Contam. Toxicol., 30: 606-613.
TANNOCK, J., HOWELLS, W.W., & PHELPS, R.J. (1983) Chlorinated
hydrocarbon pesticide residues in eggs of some birds in Zimbabwe.
Environ. Pollut. Ser. B, 5: 147-155.
TARRANT, K.R. & TATTON, J.O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TATTON, J.O'G. & RUZICKA, J.H.A. (1967) Organochlorine pesticides
in Antarctica. Nature (Lond.), 215: 346-348.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., & FREEMAN, H.P. (1972)
Measurement of volatilization of dieldrin and heptachlor from a
soil in the field. In: Abstracts of the 163rd National Meeting of
the American Chemical Society, Boston, Massachusetts, Washington,
DC, American Chemical Society.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., FREEMAN, H.P., &
EDWARDS, W.M. (1976) Volatilization of dieldrin and heptachlor from
a maize field. J. agric. food Chem., 24(3): 625-631.
TEJEDOR, M.C., MURADO, M.A., & BALUJA, G. (1974) [Contamination of
the environment by organochlorine pesticides.] An. Quim., 70:
1177-1183 (in Spanish).
TENNEKES, H.A., WRIGHT, A.S., DIX, K.M., & KOEMAN, J.H. (1981)
Effects of dieldrin, diet and bedding on enzyme function and tumour
incidence in livers of male CF-1 mice. Cancer Res., 41: 3615-3620.
TENNEKES, H.A., RAVENZWAAY, B., & KUNZ, H.W. (1985) Quantitative
aspects of enhanced liver tumour formation in CF-1 mice by
dieldrin. Carcinogenesis, 6(10): 1457-1462.
TERRIERE, L.C. & YU, S.J. (1976) Microsomal oxidases in the flesh
fly ( Sarcophaga bullata Parker) and the black blow fly ( Phormia
regina Meigen). Pestic. Biochem. Physiol., 6: 223-228.
THOMPSON, A.R., EDWARDS, C.A., EDWARDS, M.J., & BEYNON, K.I. (1970)
Movement of dieldrin through soils. II. In sloping troughs and soil
columns. Pestic. Sci., 1(5): 174-178.
THOMPSON, J.F. (1976) Manual of analytical quality control for
pesticides and related compounds in human and environmental
samples, Research Triangle Park, North Carolina, US Environmental
Protection Agency, Office of Research and Development, Health
Effects Research Laboratory (EPA-600/1-76-017).
THORPE, E. (1973) The toxicology of dieldrin (HEOD):
transplantation of liver tumours in mice, Sittingbourne, Shell
Research (TLGR.0041.73) (Unpublished proprietary report).
THORPE, E. & HUNT, P.F. (1975) Toxicology of dieldrin (HEOD): study
of the pathological changes in 3 strains of mice following
prolonged ingestion of dieldrin, Sittingbourne, Shell Research
(TLGR.0012.75) (Unpublished proprietary report).
THORPE, E. & WALKER, A.I.T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, beta-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TREON, J.F. & CLEVELAND, F.P. (1955) Toxicity of certain
chlorinated hydrocarbon insecticides for laboratory animals, with
special reference to aldrin and dieldrin. J. agric. food Chem.,
3(5): 402-408.
TREON, J.F., DUTRA, F.R., SHAFFER, F.E., CLEVELAND, F.P., WAGNER,
W., & GAHEGAN, T. (1951) The toxicity of aldrin, dieldrin, and DDT
when fed to rats over the period of six months, Cincinnati, Ohio,
Kettering Laboratory.
TREON, J.F., HARTMAN, L., GAHEGAN, T., & NEDDERMANN, G. (1953) The
immediate and cumulative toxicity of aldrin, dieldrin, and DDT when
maintained in contact with the skin of rabbits, Cincinnati, Ohio,
Kettering Laboratory.
TROSKO, J.E., JONE, C., & CHANG, C.C. (in press) Inhibition of gap-
functional mediated intercellular communication in vitro by aldrin,
dieldrin and toxaphene: a possible cellular mechanism for their
tumour-promoting and neurotoxic effects. Mol. Toxicol.
TU, C.M. (1981) Effects of some pesticides on enzyme activities in
an organic soil. Bull. environ. Contam. Toxicol., 27: 109-114.
TU, C.M. & MILES, J.R.W. (1976) Interactions between insecticides
and soil microbes. Residue Rev., 64: 17-65.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington, DC, US Department of the
Interior, Bureau of Sport Fishing and Wildlife, pp. 16-17, 46-47
(Resource Publication No. 84).
TUCKER, R.K. & HAEGELE, M.A. (1971) Comparative acute oral toxicity
of pesticides to six species of birds. Toxicol. appl. Pharmacol.,
20(1): 57-65.
TUINSTRA, L.G.M.TH. (1971) Organochlorine insecticide residues in
human milk in the Leiden region. Ned. Melk. Zuiveltijdschr., 25:
24-32.
TURNER, B.C., GLOTFELTY, D.E., & TAYLOR, A. (1977) Photodieldrin
formation and volatilization from grass. J. agric. food Chem., 25:
548-550.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., THEARLE, R.J.P., EGAN, H.,
EVANS, W.H., & SOUTAR, N.M. (1963) The effects on birds of certain
chlorinated insecticides used as seed dressings. J. Sci. Food
Agric., 14(8): 567-577.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., MURTON, R.K., BRADY, J.,
THEARLE, R.J.P., JONES, F.J.S., REA, R.E., KIRBY, D.R., SMITH,
B.M., & RUTTER, M.E. (1965) Pesticides and wildlife. Report of the
Infestation Control Laboratory for 1962-64, London, Her Majesty's
Stationary Office, pp. 23-47.
UK-HMSO (1986) Food surveillance paper, London, Her Majesty's
Stationary Office, (Document No. 16).
UK-MAFF (1982-85) Report of the Working Party on Pesticide
Residues, London, Ministry of Agriculture, Fisheries and Food
(Food Surveillance Paper No. 16).
US EPA (1983) Environmental news, Washington, DC, US Environmental
Protection Agency, Office of Public Affairs.
UYETA, M., TAUE, S., CHIKAZAWA, K., & NISHIMOTO, T. (1971)
Pesticides translocated in food - organochlorine pesticides in the
total diet. J. Food Hyg. Soc. Jpn. 12: 445.
UZOUKWU, M. & SLEIGHT, S.D. (1972) Effects of dieldrin in pregnant
sows. J. Am. Vet. Med. Assoc., 160(12): 1641-1643.
VAN DEN BERCKEN, J. (1972) An electrophysiological investigation
into the action of DDT, dieldrin, and allethrin in the clawed toad
Xenopus laevis, Utrecht, Rijks Universiteit (Thesis).
VAN DEN BERCKEN, J. & NARAHASHI, T. (1974) Effects of aldrin-
transdiol, a metabolite of the insecticide dieldrin, on nerve
membrane. Eur. J. Pharmacol., 27: 255-258.
VAN DEN BROEK, W.L.F. (1979) Seasonal levels of chlorinated
hydrocarbons and heavy metals in fish and brown shrimps from the
Medway estuary, Kent. Environ. Pollut., 19: 21-38.
VAN DIJCK, P. & VAN DE VOORDE, H. (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 41(2): 1491-1498.
VAN DUURSEN, P. (1985) Open letter. Shell Post, 1985(671).
VAN GELDER, G.A. & CUNNINGHAM, W.L. (1975) The effects of low-level
dieldrin exposure on the EEG and learning ability of the squirrel
monkey. Toxicol. appl. Pharmacol., 33(1): 142 (Abstract No. 50).
VAN GENDEREN, H. (1965) The toxicology of the chlorinated
hydrocarbon insecticides. A progress report with particular
reference to the qualitative aspects of the action in warm-blooded
animals. Opzoekingsstn Staat Gent, 30(3): 1321-1335.
VAN GENDEREN, H. (1979) [Dieldrin: a troublesome insecticide.] K.
Ned. Akad. Wet. (Amsterdam) 88(3): 24-32 (in Dutch).
VAN GENUCHTEN, M.TH., DAVIDSON, J.M., & WIERENGA, P.J. (1974) An
evaluation of kinetic and equilibrium equations for the prediction
of pesticide movement through porous media. Soil Sci. Soc. Am.
Proc., 38: 29-35.
VAN HAVER, W., VANDEZANDE, A., & GORDTS, L. (1978) [Organochlorine
pesticides in human fatty tissue.] Arch. Belg. Méd. soc. Hyg. Méd.
Trav. Méd. lég., 36: 147-155 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN RAALTE, H.G.S. (1965) Aspects of pesticide toxicity. In:
Proceedings of the Conference on Occupational Health, Caracas,
Venezuela (Unpublished paper).
VAN RAALTE, H.G.S. (1977) Human experience with dieldrin in
perspective. Ecotoxicol. environ. Saf., 1: 203-210.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination
of water by a sublethal concentration of pesticides: a system
analysis approach. Acta hydrochim. hydrobiol., 12(4): 399-409.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and
telodrin. Br. J. ind. Med., 30: 201-202.
VIRGO, B.B. & BELLWARD, G.D. (1975) Effects of dietary dieldrin on
reproduction in the Swiss-Vancouver (SWV) mouse. Environ. Physiol.
Biochem., 5: 440-450.
VIRGO, B.B. & BELLWARD, G.D. (1977) Effects of dietary dieldrin on
offspring viability, maternal behaviour, and milk production in the
mouse. Res. Commun. chem. Pathol. Pharmacol., 17(3): 399-409.
VOERMAN, S. & BESEMER, A.F.H. (1975) Persistence of dieldrin,
lindane, and DDT in a light sandy soil and their uptake by grass.
Bull. environ. Contam. Toxicol., 13(4): 501-505.
VOUTSINOU-TALIADOURI, F. & SATSMADJIS, J. (1982) Influence of
metropolitan waste on the concentration of chlorinated hydrocarbons
and metals in striped mullet. Mar. Pollut. Bull., 13(8): 266-269.
VREMAN, K. & POORTVLIET, L.J. (1982) Pesticide levels in milk and
milk products in relation to their milk fat content. Neth. Milk
Dairy J., 36: 145-148.
VREMAN, K., POORTVLIET, L.J., & VAN DEN HOEK, J. (1980) Transfer of
organochlorine pesticides from feed into the milk and body fat of
cows. Long-term experiment with intake at low levels. Neth. Milk
Dairy J., 34: 87-105.
WADE, M.H., MOYER, J.W., & HINE, C.H. (1979) Mutagenic action of a
series of epoxides. Mutat. Res., 66: 367-371.
WADE, M.H., TROSKO, J.E., & SCHINDLER, M. (1986) A fluorescence
photobleaching assay of gap junction-mediated communication between
human cells. Science, 232: 525-528.
WALKER, A.I.T., NEILL, C.H., STEVENSON, D.E., & ROBINSON, J.
(1969a) The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix
coturnix japonica). Toxicol. appl. Pharmacol., 15: 69-73.
WALKER, A.I.T., STEVENSON, D.E., ROBINSON, J., THORPE, E., &
ROBERTS, M. (1969b) The toxicology and pharmacodynamics of dieldrin
(HEOD): two-year oral exposure of rats and dogs. Toxicol. appl.
Pharmacol., 15: 345-373.
WALKER, A.I.T., THORPE, E., ROBINSON, J., & BALDWIN, M.K. (1971)
Toxicity studies on the photoisomerisation product of dieldrin.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 36(1): 398-409.
WALKER, A.I.T., THORPE, E., & STEVENSON, D.E. (1972) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALTON, M.S., BECK-BASTONE, V., & BARON, R.L. (1971) Subchronic
toxicity of photodieldrin, a photodecomposition product of
dieldrin. Toxicol. appl. Pharmacol., 20(1): 82-88.
WARNICK, S.L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah, fiscal years 1967-71. Pestic. monit. J.,
6(1): 9-13.
WARNICK, S.L. & CARTER, J.E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASSERMANN, M., CURNOW, D.H., FORTE, P.N., & GRONER, Y. (1968)
Storage of organochlorine pesticides in the body fat of people in
western Australia. Int. J. ind. Med. Surg., 37(4): 295-300.
WASSERMANN, M., FRANCONE, M.P., WASSERMANN, D., MARIANI, F., &
GRONER, Y. (1969) [Organochlorine pesticide content in the fatty
tissue of the general population in the Republic of Argentina.]
Sem. med., 134: 459-462 (in Spanish).
WASSERMANN, M., WASSERMANN, D, LAZAROVICI, S., COETZEE, A.M., &
TOMATIS, L. (1970) Present state of the storage of the
organochlorine insecticides in the general population of South
Africa. South Afr. med. J., 44: 646-648.
WASSERMANN, M., NOGUEIRA, D.P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972a) Storage of organochlorine
insecticides in people of Sao Paulo, Brazil. Ind. Med. Surg., 41(3):
22-25.
WASSERMANN, M., ROGOFF, M.G., TOMATIS, L., DAY, N.E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of organochlorine
insecticides in the adipose tissue of people in Kenya. Ann. Soc.
Belg. Méd. Trop., 52(6): 509-514.
WASSERMANN, M., SOFOLUWE, G.O., TOMATIS, L., DAY, N.E., WASSERMANN,
D., & LAZAROVICI, S. (1972c) Storage of organochlorine insecticides
in people in Nigeria. Environ. Physiol. Biochem., 2: 59-67.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N.E.,
WASSERMANN, D., RUNGPITARANGSI, V., CHIAMSAKOL, V.,
DJAVAHERIAN, M., & CUCOS, S. (1972d) Storage of organochlorine
insecticides in the adipose tissue of people from Thailand.
Southeast Asian J. trop. Med. public Health, 3(2): 280-285.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol., 12(4):
501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in adipose tissue of Israelis. Pestic.
monit. J., 8(1): 1-7.
WATSON, M., BENSON, W.W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people of southern Idaho. Pestic. monit. J.,
4(2): 47-50.
WEDBERG, J.L., MOORE, S., III, AMORE, F.J., & MCAVOY, H. (1978)
Residues in food and feed. Organochlorine insecticide residues in
bovine milk and manufactured milk products in Illinois 1971-76.
Pestic. monit. J., 11: 161-164.
WEGMAN, R.C.C. & GREVE, P.A. (1974) Levels of organochlorine
pesticides and inorganic bromide in human milk. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 39: 1301-1310.
WEGMAN, R.C.C. & GREVE, P.A. (1978) Organochlorines, cholinesterase
inhibitors, and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic. monit. J., 12(3): 149-162.
WEISGERBER, I., KOHLI, J., KAUL, R., KLEIN, W., & KORTE, F. (1974)
Fate of aldrin-14C in maize, wheat, and soils under outdoor
conditions. J. agric. food Chem., 22(4): 609-612.
WEISGERBER, I., BIENIEK, D., KOHLI, J., & KLEIN, W. (1975)
Isolation and identification of three unreported photodieldrin-14C
metabolites in soil. J. agric. food Chem., 23: 873-877.
WELLS, M.R. & YARBROUGH, J.D. (1973) In vivo and in vitro retention
of 14C-aldrin and 14C-dieldrin in cellular fractions from brain and
liver tissues of insecticide-resistant and susceptible Gambusia.
Toxicol. appl. Pharmacol., 24: 190-196.
WELLS, M.R., PHILLIPS, J.B., & MURPHY, G.G. (1974) ATPase activity
in tissues of the map turtle Graptemys geographica following in
vitro treatment with aldrin and dieldrin. Bull. environ. Contam.
Toxicol., 11(6): 572-576.
WESSELS, C.L. (1978) Residues in soybean plants of aldrin and
dieldrin following soil application and of endosulphan and DDT
following foliar application. Rhodesian. J. agric. Res., 16:
205-210.
WHEATLEY, G.A. & HARDMAN, J.A. (1965) Indications of the presence
of organochlorine insecticides in rainwater in central England.
Nature (Lond.), 207: 486-487.
WHEATLEY, G.A. & HARDMAN, J.A. (1968) Organochlorine insecticide
residues in earthworms from arable soils. J. Sci. Food Agric., 19:
219-225.
WHEATLEY, G.A., HARDMAN, J.A., & STRICKLAND, A.H. (1962) Residues
of chlorinated hydrocarbon insecticides in some farm soils in
England. Plant Pathol., 11: 81-90.
WHITE, D.H. (1976) Nationwide residues of organochlorine in
starling, 1974. Pestic. monit. J., 10(1): 10-17.
WHITE, D.H., KING, K.A., & PROUTY, R.M. (1980) Significance of
organochlorine and heavy metal residues in wintering shorebirds at
Corpus Christi, Texas, 1976-77. Pestic. monit. J., 14(2): 58-63.
WHO (1958) Note by Secretariat on aldrin poisoning in Kenya,
Geneva, World Health Organization, p. 3 (Information Circular on
the Toxicity of Pesticides to Man No. 1).
WHO (1977) Outbreak of food poisoning of chemical origin, Geneva,
World Health Organization, p. 217 (Weekly Epidemiological Record No.
52).
WHO (1984) Guidelines for drinking-water quality, Geneva, World
Health Organization, Vol. 1, p. 69.
WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished document VBC/88.953).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World Health
Organization (Unpublished documents).
WIEMEYER, S.N., MULHERN, B.M., LIGAS, F.J., HENSEL, R.J., MATHISEN,
J.E., ROBARDS, F.C., & POSTUPALSKY, S. (1972) Residues of
organochlorine pesticides, polychlorinated biphenyls, and mercury
in bald eagle eggs and changes in shell thickness, 1969 and 1970.
Pestic. monit. J., 6(1): 50-55.
WIERSMA, G.B., MITCHELL, W.G., & STANFORD, C.L. (1972) Pesticide
residues in onions and soil - 1969. Pestic. monit. J., 5(4):
345-347.
WIESE, I.H. & BASSON, N.C.J. (1966) The degradation of some
persistent chlorinated hydrocarbon insecticides applied to
different soil types. South Afr. J. agric. Sci., 9: 945-969.
WIESE, I.H., BASSON, N.C.J., VAN DER VIJVER, J.H., & VAN DER MERWE,
J.H. (1969) Toxicology and dynamics of dieldrin in the crowned
guinea-fowl Numida meleagris L. Phytophylactica, 1: 161-176.
WIESE, I.H., BASSON, N.C.J., BASSON, P.A., NAUDE, T.W., & MAARTENS,
B.P. (1973) The toxicology and pathology of dieldrin and photo-
dieldrin poisoning in two antelope species. Onderstepoort J. vet.
Res., 40(1): 31-40.
WILLIAMS, D.T., BENOIT, F.M., MCNEIL, E.E., & OTSON, R. (1978)
Organochlorine pesticide levels in Ottawa drinking water, 1976.
Pestic. monit. J., 12(3): 163-166.
WILLIAMS, D.T., LEBEL, G.L., & JUNKINS, E. (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples
from two Ontario municipalities. J. Toxicol. environ. Health, 13:
19-29.
WILLIAMS, G.M. (1982) Organochlorine pesticides and inhibition of
intercellular communication as the mechanism for their liver tumor
production. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: human welfare and the environment. Oxford, New York,
Pergamon Press, Vol. 3, pp. 475-478.
WILLIAMS, R. & HOLDEN, A.V. (1973) Organochlorine residues from
plankton. Mar. Pollut. Bull., 4(7): 109-111.
WILLIAMS, S., MILLS, P.A., & MCDOWELL, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Agric. Chem.,
47(6): 1124-1128.
WILLIS, G.H., PARR, J.F., SMITH, S., & CARROLL, B.R. (1972)
Volatilization of dieldrin from fallow soil as affected by
different soil water regimes. J. environ. Qual., 1(2): 193-196.
WINTHROP, G.J. & FELICE, J.F. (1957) [A clinical toxicological
study of spraymen of a chlorinated hydrocarbon insecticide.] Bol.
Sanit. Panama, 43: 512-517 (in Spanish with English summary).
WIT, S.L. (1971) [Persistent insecticides in Dutch body fat.] Chem.
Weekbl., 67(5): 11-14 (in Dutch).
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1963) Health hazards
of the pesticides endrin and dieldrin. Arch. environ. Health, 6:
458-464.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council.
WRIGHT, A.S., POTTER, D., WOODER, M.F., DONNINGER, C., & GREENLAND,
R.D. (1972) The effects of dieldrin on the subcellular structure
and function of mammalian liver cells. Food Cosmet. Toxicol., 10:
311-332.
WRIGHT, A.S., AKINTONWA, D.A.A., & WOODER, M.F. (1977) Studies on
the interactions of dieldrin with mammalian liver cells at the
subcellular level. Ecotoxicol. environ. Saf., 1: 7-16, 427.
WRIGHT, A.S., DONNINGER, C., GREENLAND, R.D., STEMMER, K.L., &
ZAVON, M.R. (1978) The effects of prolonged ingestion of dieldrin
on the livers of male rhesus monkeys. Ecotoxicol. environ. Saf., 1:
477-502.
WUENSCHER, K. & ACKER, L. (1969) [The occurrence of chlorinated
insecticides in human fatty tissues.] Med. Ernähr., 10(4): 75-80
(in German).
WUTHRICH, C., MULLER, F., BLASER, O., & MAREK, B. (1985)
[Subjection of the population to pesticides and other foreign
substances through food.] Mitt. Geb. Lebensm. Hyg., 76: 260-276
(in German).
WYLLIE, J., GABICA, J., & BENSON, W.W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: a survey of 200 persons in southern Idaho - 1970. Pestic.
monit. J., 6(2): 84-88.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls (PCBs) and
organochlorine pesticides in human milk and blood collected in
Osaka prefecture from 1972 to 1977. Int. Arch. occup. environ.
Health, 43: 1-15.
YAP, H.H., DESAIAH, D., CUTKOMP, L.K., & KOCH, R.B. (1975) In vitro
inhibition of fish brain ATPase activity by cyclodiene insecticides
and related compounds. Bull. environ. Contam. Toxicol., 14(2):
163-167.
YARON, B., SWOBODA, A.R., & THOMAS, G.W. (1967) Aldrin adsorption
by soils and clays. J. agric. food Chem., 15(4): 671-675.
YOSHIMURA, M., YAMADA, T., SUGIYAMA, S., NODA, H., & MITSUKUNI, Y.
(1979) Organochlorinated pesticides in human organs and tissues.
Jpn. J. leg. Med., 33(2): 91-102.
YU, S.J., KIIGEMAJI, M., & TERRIERE, L.C. (1971) Oxidative
metabolism of aldrin and isodrin by bean root fractions. J. agric.
food Chem., 19: 5-9.
ZABIK, M.E., HOOJJAT, P., & WEAVER, C.M. (1979) Polychlorinated
biphenyls, dieldrin, and DDT in lake trout cooked by broiling,
roasting, or microwave. Bull. environ. Contam. Toxicol., 21:
136-143.
ZAVON, M.R. & HAMMAN, R.E. (1961) Human experience with dieldrin in
malaria control programs. Am. J. public Health, 51(7): 1026-1032.
ZAVON, M.R. & STEMMER, K.L. (1975) The effect of dieldrin ingestion
on rhesus monkeys. A six-year study, Cincinnati, Ohio, Kettering
Laboratory.
ZAVON, M.R., HINE, C.H., & PARKER, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Am. Med. Assoc., 193(10): 837-839.
ZELLE, B. & LOHMAN, P.H.M. (1977) Repair of DNA in cultured human
cells treated with dieldrin and 4-nitroquinoline-N-oxide,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
ZHONG-XIANG, L., KAVANAGH, T., TROSKO, J.E., & CHANG, C.C. (1986)
Inhibition of gap junctional intercellular communication in human
teratocarcinoma cells by organochlorine pesticides. Toxicol. appl.
Pharmacol., 83: 10-19.
ZIMMERLI, B. & MAREK, B. (1973) [Subjection of the Swiss population
to pesticides.] Mitt. Geb. Lebensm. Hyg., 64(4): 459-479
(in German).
APPENDIX I. NOMENCLATURE
Two major systems are currently used for the nomenclature of
these compounds: "polyhydroaromatic" names used by Chemical
Abstracts (American Chemical Society) and IUPAC and the von
Baeyer/IUPAC system for polycylic aliphatic compounds. That the
latter system should be used for the cyclodiene insecticides was
proposed by Benson (1969) and Bedford (1974). The "polyaromatic"
system has, unfortunately, been subject to historical variation,
and there are differences between the IUPAC, British and American
conventions for defining the 3-dimensional stereochemistry in this
system. As a consequence of the differences in the numbering of the
carbon atoms in the two major systems, and the modification of the
Chemical Abstracts "polyaromatic" name for dieldrin since 1971,
considerable confusion can occur regarding the nomenclature of
metabolites.
The various alternative names for aldrin, dieldrin, and photo-
dieldrin are summarized in Table 48. A useful discussion of
nomenclature is given by Brooks (1974).
For convenience, in view of the much more extensive usage in
the literature of the former Chemical Abstracts names for aldrin
and dieldrin, the names of their metabolites in this review are
based (if appropriate) on the former Chemical Abstracts names of
the parent compounds given in Table 48. The names of the
metabolites are given in Table 49, together with some alternative
names based on either the current Chemical Abstracts name for
dieldrin or the von Baeyer/IUPAC system.
The possible misunderstandings that may occur, particularly
for those not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that may be
given to the major faecal metabolite of dieldrin. This one
compound may be designated:
(a) 9-hydroxy dieldrin (former CA system);
(b) 8-hydroxy dieldrin (current CA system); or
(c) 12-hydroxy dieldrin (von Baeyer/IUPAC system).
Table 48. Alternative chemical names for aldrin, dieldrin, and photodieldrin
---------------------------------------------------------------------------------------------------------------
Compounda Polyhydroaromatic name Polycyclic aliphatic name
Chemical Abstracts IUPAC (von Baeyer/IUPAC)
---------------------------------------------------------------------------------------------------------------
Aldrin Formerly: Formerly: 1,8,9,10,11,11-hexachloro-2,3-7,6-
(HHDN) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- endo-2,1-7,8-exo-tetracyclo[6.2.13,6
(I) 1,4,4a,5,8,8a-hexahydro- 1,4,4a,5,8,8a-hexahydro- 02,7] dodeca-4,9-diene
endo-1,4-exo-5,8- exo-1,4-endo-5,8-
dimethanonaphthalene dimethanonaphthalene
Currently: Currently:
1,2,3,4,10,10-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1 alpha,4 alpha,4a-beta, hexachloro-1,4,4a,5,8,8a-
5 alpha,8a,8a beta-hexahydro- hexahydro-1,4:5,8-
1,4:5,8-dimethanonaphthalene dimethanonaphthalene
Dieldrin Formerly: Formerly: 1,8,9,10.11,11-hexachloro-4,5-exo-
(HEOD) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- epoxy-2,3-7,6-endo-2,1-7,8-exo-
(II) 6,7-epoxy-1,4,4a,5,6,7,8,8a- 6,7-epoxy-1,4,4a,5,6,7,8,8a- tetracyclo[6.2.1.13,6.02,7] dodec-
octahydro-endo-1,4-exo- octahydro-exo-1,4-endo-5,8- 9-ene
5,8-dimethanohaphthalene dimethanonaphthalene
Currently: Currently:
3,4,5,6,9,9-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1a alpha,2 beta,2a alpha, hexachloro-1,4,4a,5,6,7,8,8a-
3 beta,6 beta,6a alpha,7 beta, octahydro-6,7 epoxy-1,4:5,8-
7a alpha-octahydro-2,7:3,6- dimethanonaphthalene
dimethanonaphth[2,3-b]oxirene
Photo- 1,1,2,3,3a,7a-hexachloro- 3,exo-4,5,6,6,7-hexachloro-11,12-
dieldrin 6,7-epoxy-2,4,7-metheno- exo-epoxy-pentacyclo[6.4.0.02,10.
(III) decahydro-3H-cyclopenta[a]- 03,7.05,9]-dodecane
pentalene
---------------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
Table 49. Chemical nomenclature of metabolites of aldrin and dieldrin
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
9-Hydroxy dieldrin 9-hydroxy-1,2,3,4,10,10-hexa- 9-(syn-epoxy)hydroxy-1,2,3,4,10,10-hexa-
(VI) (9-Hydroxy HEOD) chloro-6,7-epoxy-1,4,4a,5,6,7, chloro-6,7,-epoxy-1,4,4a,5,6,7,8,8a-octa-
8,8a-octahydro-1,4-endo-5,8-exo- hydro-1,4-endo,exo-5,8-dimethanonaphthalene
dimethanonaphthalene
8-hydroxy-3,4,5,6,9,9-hexachloro-1a alpha,
2 beta, 2a alpha, 3 beta, 6 beta, 6a alpha,
7 beta, 7a alpha-octahydro-2,7:3,6-
dimethanonapth[2,3-b]oxirene
1,8,9,10,11,11-hexachloro-4,5-exo-epoxy-12-
(synepoxy)hydroxy-2,3-7,6-endo-2,1-7,8-exo-
tetracyclo[6.2.1.13,602,7]dodec-9-ene
Aldrin trans-diol (IV) trans-6,7-dihydroxy-1,2,3,4,10,10- 1,8,9,10,11,11-hexachloro-4,5-trans-
hexachloro-1,4,4a,6,7,5,8,8a-hexa- dihydroxy-2,3-7,6-endo-2,1-7,8-exo-
hydro-1,4-endo-5,8-exo-dimethano- tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
naphthalene
Aldrin dicarboxylic 4,5,6,7,8,8-hexachloro-4,7-methano- 1,7,8,9,10,10-hexachloro-2,3-6,5-endo-
acid (V) 3a,4,7,7a-tetrahydro-indane-1,3- tricyclo[5.2.1.02,6]dec-8-ene-3,5-exo-
dicarboxylic acid dicarboxylic acid
Bridged pentachloro- 3,5,6,6,7-pentachloro-11,12-exo-
ketone (VII) (PCK, epoxy-pentacyclo[6.4.0.02,10.03,7
Klein's metabolite) .05,9]dodecan-4-one
Dechloro-aldrin 4,5,6,7,8-pentachloro-4,7-methano-
dicarboxylic 3a,4,7,7a-tetrahydro-indane-1,3-
acid (VIII) dicarboxylic acid
Dieldrin ketone (IX) 1,2,3,4,10,10-hexachloro-1,4,4a,5, 1,8,9,10,11,11-hexachloro-2,3-7,6-endo-
6,7,8,8a-octahydro-6-keto-endo- 2,1-7,8-exo-tetracyclo[6.2.1.13,6.02,7]-
1,4-exo-5,8-dimethanonaphthalene dodec-9-en-4-one
Photodieldrin 3-exo-4,5,6,6,7-hexachloro-
ketone (X) pentacyclo[6.4.0.02,10.03,7.05,9]
dodecan-11-one
---------------------------------------------------------------------------------------------------------
Table 49. (contd.)
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
Photodieldrin trans- 3,exo-4,5,6.6,7-hexachloro-11,12
diol (XI) (caged dihydroxy-pentacyclo[6.4.0.02,10.
aldrin trans-diol) 03,7.05,9]dodecane
Photoaldrin dicarbo- 1,7,8,exo-9,10,10-hexachlorotetra-
xylic acid (XII) cyclo[5.2.1.02,6.04,8]decane-3,5-
(caged aldrin acid) exo,exo-dicarboxylic acid
Photoaldrin (XIII) 3,exo-4,5,6,6,7-hexachloropenta-
cyclo[6.4.0.02,10.03,7.05,9]dodec-
11-ene
---------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
RESUME
1. Généralités
L'aldrine et la dieldrine qui sont l'une et l'autre des
pesticides organochlorés fabriqués industriellement depuis 1950,
ont été utilisés dans le monde entier jusqu'au début des années 70
comme insecticides en agriculture, contre de nombreux ravageurs
présents dans le sol, et pour le traitement des semences. Ces
insecticides étaient actifs contre les termites, les sauterelles,
les xylophages, les coléoptères et les ravageurs des textiles. La
dieldrine a également été employée en santé publique, pour la lutte
contre la mouche tsé-tsé et d'autres vecteurs de maladies
tropicales invalidantes. L'aldrine comme la dieldrine agissent par
contact et par ingestion.
Depuis le début des années 70, ces deux composés sont
interdits ou font l'objet de limitations rigoureuses dans un
certain nombre de pays, spécialement en agriculture. Néanmoins,
ils continuent d'être employés pour la destruction des termites
dans d'autres pays. La production annuelle mondiale, estimée à
13 000 tonnes en 1972, est tombée à moins de 2500 tonnes en 1984.
L'aldrine et la dieldrine de qualité technique ont une pureté
respective de 90% et plus de 95%. Les principales impuretés sont,
pour l'aldrine, l'octachlorocyclopentène, l'hexachlorobutadiène et
des produits de polymérisation et, pour la dieldrine, des
polychloroépoxy-octahydrodiméthanonaphtalènes.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans la plupart des alcanes, des
hydrocarbures aromatiques et des hydrocarbures halogénés, ainsi que
dans les esters, les cétones et les alcools.
La tension de vapeur de l'aldrine est de 6,5 x 10-5 mmHg à
25°C et celle de la dieldrine de 3,2 x 10-6 mmHg à 25°C.
Les méthodes utilisables pour le dosage de l'aldrine et de la
dieldrine dans les aliments, les aliments pour animaux et le milieu
sont décrites à la section 2.
2. Transport, distribution et transformation dans l'environnement
L'aldrine est principalement utilisée comme insecticide épandu
au niveau du sol. Les sols ainsi traités constituent donc dans
l'environnement une source importante d'aldrine et de son produit
de réaction, la dieldrine.
L'aldrine n'a qu'une faible capacité de migration à partir des
zones traitées, par volatilisation ou lessivage. Elle s'adsorbe
préférentiellement, et rapidement, sur les sols riches en matières
organiques, mais faiblement sur les sols argileux. Il est rare que
l'aldrine et la dielrine pénètrent au-delà des 20 premiers
centimètres de la couche de sol traité. L'aldrine adhère si
solidement aux particules du sol que seules des traces peuvent être
retirées par l'eau. C'est pourquoi il n'y a généralement pas
contamination des eaux souterraines.
L'aldrine s'élimine du sol selon une cinétique qui évoque une
réaction du premier ordre. Immédiatement après épandage, on
observe une courte période d'élimination rapide par volatilisation,
suivie d'une seconde période, plus longue, de décroissance
exponentielle, principalement du fait de la transformation en
dieldrine, plus lente à se dissiper. Néanmoins, il peut y avoir
une certaine migration du fait de l'érosion du sol, sous l'action
du vent, des eaux de ruissellement de du déplacement des sédiments.
Les observations faites sur les résidus d'aldrine dans la nature
montrent que, apparemment, ce composé est essentiellement retenu
dans le sol et que pour 97%, le résidu essentiel n'est pas le
composé d'origine mais l'époxyde correspondant, la dieldrine.
La photodieldrine est un produit de photodégradation de la
dieldrine, peu répandu dans l'environnement.
Après épandage sur le sol, l'aldrine disparaît lentement dans
les régions tempérées puisque, dans le cas type, il faut un an pour
qu'elle s'élimine aux trois quarts. La vitesse d'élimination
diminue ensuite à mesure que l'aldrine se transforme en dieldrine.
Il semblerait que la vitesse d'élimination soit plus élevée en
anaérobiose, comme c'est le cas dans les rizières, qu'en aérobiose.
Dans les régions tropicales, la dieldrine s'élimine du sol très
rapidement, jusqu'à hauteur de 90% au cours du premier mois, alors
que, dans le sol des régions tempérées, la dieldrine a une demi-vie
d'environ 5 ans. La volatilisation semble être le principal
mécanisme d'élimination à partir du sol, bien que la teneur
atmosphérique de la dieldrine et de l'aldrine soit généralement
faible. Une partie de la dieldrine est éliminée de l'atmosphère
par les précipitations, mais la concentration de ce produit est
très faible dans les eaux souterraines par suite de son adsorption
énergique sur les particules telluriques. On trouve de la
dieldrine en petites quantités dans les eaux de surface qui sont
contaminées par les eaux de ruissellement provenant de terres
agricoles.
3. Concentrations environnementales et exposition humaine
On trouve de l'aldrine et de la dieldrine dans l'atmosphère en
phase vapeur, adsorbées sur des poussières ou dans les eaux de
pluie, à des teneurs variables selon la situation. Ces produits
s'observent principalement dans les régions agricoles où leur
concentration atmosphérique moyenne est de l'ordre de 1 - 2 ng/m3
avec des maximums d'environ 40 ng/m3. Dans l'eau de pluie, on
relève des concentrations de l'ordre de 10 - 20 ng/litre ou parfois
plus.
Dans les habitations traitées avec ces produits contre les
termites, on observe une concentration dans l'air allant de 0,04 à
7 µg/m3, selon le moment de l'échantillonnage (c'est-à-dire le
nombre de jours après l'épandage) et le type d'habitation. Au bout
de 8 semaines, la concentration a très nettement diminué. Lorsqu'on
traite le bois en profondeur dans ces maisons, la concentration de
la dieldrine dans l'air va de 0,01 à 0,5 µg/m3. On a constaté que
l'aldrine et la dieldrine migraient dans les produits alimentaires
à partir de panneaux lamellés et de contreplaqués traités, ainsi
que par contact direct ou sorption à partir de l'atmosphère.
On a signalé la présence de dieldrine en milieu aquatique.
Mais les concentrations étaient très faibles, le plus souvent
inférieures à 5 ng/litre. Les concentrations plus élevées ont
généralement étéattribuées au rejet d'effluents industriels ou à
l'érosion du sol à la suite de l'utilisation de ces produits en
agriculture. Dans les cours d'eau, les sédiments peuvent présenter
des teneurs beaucoup plus élevées (allant jusqu'à 1 mg/kg).
On trouve rarement de l'aldrine dans les aliments, alors que
la dieldrine est plus courante, spécialement dans les produits
laitiers, les produits carnés, le poisson, les huiles et les
graisses, les pommes de terre et certains autres légumes
(principalement des légumes-racines). Des limites maximales de
résidus (LMR) de l'ordre de 0,02 - 0,2 mg/kg de produit ont été
recommandées lors des réunions conjointes successives FAO/OMS sur
les résidus de pesticides. Les études récentes réalisées dans
différents pays montrent que la concentration effective de la
dieldrine dans les denrées alimentaires est généralement plus
faible. Le recul est net au Royaume-Uni. En 1966 - 67, la
concentration moyenne des résidus de dieldrine observée lors d'une
étude sur la ration totale était de 0,004 mg/kg d'aliments tandis
que, pendant la période 1975 - 77, elle n'était plus que de 0,0015
mg/kg pour tomber à 0,0005 mg/kg en 1981. Cette évolution en
baisse est confirmée dans d'autres pays, par exemple aux Etats-Unis
d'Amérique. Cela tient peut-être à l'interdiction ou à la
limitation d'emploi de ces composés.
Dans un grand nombre de travaux publiés, on a recherché la
présence de dieldrine dans le tissu adipeux, les organes, le sang
et d'autres tissus, chez des sujets de la population générale. Au
cours des 25 dernières années, des enquêtes ont été réalisées dans
de nombreux pays, partout dans le monde. La plupart des
concentrations moyennes observées dans le tissu adipeux se situent
entre 0,1 et 0,4 mg/kg. Aux Etats-Unis d'Amérique, aux Pays-Bas et
au Royaume-Uni, on note une diminution de la concentration dans les
tissus adipeux depuis le milieu de la décennie 70. La
concentration sanguine varie de 1 à 2 µg/litre. Dans le foie, elle
est inférieure à 0,4 mg/kg tandis que, dans les autres tissus, à
savoir les reins, l'encéphale et les gonades, elle est inférieure à
0,1 mg/kg.
A la suite d'une exposition par voie transplacentaire, on
trouve de la dieldrine dans le sang, les tissus adipeux et d'autres
tissus du foetus et du nouveau-né. Les concentrations sont 2 à 10
fois plus faibles que chez la mère. Il n'existe aucune différence
entre le nourrisson et l'adulte pour ce qui est des concentrations
relatives de la dieldrine dans le cerveau, le foie et les tissus
adipeux. La dieldrine est également excrétée dans le lait
maternel. Depuis une quinzaine d'années, on recherche dans divers
pays la présence de pesticides organochlorés dans le lait de femme.
Dans la plupart des pays, la concentration plafonne à 6 µg/litre,
sauf cas exceptionnels.
4. Cinétique et métabolisme
Chez les animaux comme chez l'homme, l'aldrine et la dieldrine
passent rapidement des voies digestives dans le courant sanguin.
L'absorption a également lieu au niveau de la peau ou des poumons
après inhalation de la vapeur. Une étude sur des volontaires a
montré que la quantité résorbée par la peau intacte représente
7 - 8% de la dose appliquée. Selon des études d'inhalation
conduites sur des volontaires, le taux d'absorption et de rétention
de l'aldrine dans l'organisme peut atteindre 50% de la vapeur
inhalée. Après absorption, le composé se répartit rapidement dans
tous les organes et tissus, et il existe un échange permanent entre
le sang et les autres tissus. Entre-temps,l'aldrine se transforme
en dieldrine, principalement au niveau du foie mais aussi, dans une
moindre proportion, dans d'autres tissus comme les poumons. Cette
conversion est très rapide.
Après administration par voie orale d'une dose de 10 mg
d'aldrine par kg de poids corporel à des rats de 1 jour, on a
retrouvé ce composé dans le foie des animaux d'expérience 2 heures
après l'administration. Au cours des quelques heures suivantes, la
dieldrine s'est concentrée dans une beaucoup plus large mesure dans
les tissus lipidiques.
Comme l'ont montré de nombreuses études effectuées avec de
l'aldrine ou de la dieldrine marquées au 14C, une partie du produit
ingéré passe telle quelle dans l'intestin d'où elle est éliminée de
l'organisme, une partie est excrétée telle quelle à partir du foie
dans la bile, une autre fraction est stockée dans les divers
organes et tissus, en particulier le tissu adipeux, et une dernière
fraction est métabolisée dans le foie en produits à caractère
hydrophile et polaire plus prononcé. Chez l'homme et la plupart des
animaux, les métabolites sont principalement éliminés dans les
excreta, par l'intermédiaire de la bile. On a établi par ailleurs
que la biodégradation de l'aldrine et de la dieldrine aboutit aux
mêmes métabolites.
La plupart des connaissances actuelles sur le catabolisme de
la dieldrine chez les mammifères proviennent d'études chez la
souris, le rat, le lapin, le mouton, le chien, les petits singes,
le chimpanzé et l'homme. Dans l'ensemble, on n'observe que des
différences quantitatives entre les diverses espèces et les
mécanismes sont apparemment semblable chez le rat et les primates.
Le principal métabolite, sauf chez le rat, est le dérivé
hydroxylé en 9. On le trouve dans les déjections ainsi que dans
l'urine, sous forme libre ou conjuguée. On a découvert et
identifié chez les animaux d'expérience trois autres métabolites,
présents en petites quantités. Il s'agit d'un dérivé 6,7-
dihydroxylé en position trans, d'un acide dicarboxylique dérivé du
composé dihydroxylé et d'une pentachlorocétone pontée.
Seul le composé hydroxylé en 9 a été mis en évidence chez
l'homme, dans les matières fécales, tandis que ni ce composé ni les
autres métabolites n'apparaissaient dans le sang ou les autres
tissus. On a observé la présence de dieldrine dans les selles
d'ouvriers professionnellement exposés, tandis que, dans la
population générale, les quantités étaient inférieures au seuil de
détection. L'examen des urines de cinq ouvriers a montré que
l'excrétion de la dieldrine et de ses quatre métabolites par voie
urinaire était minime par rapport à l'élimination du métabolite
hydroxylé en 9 par voie fécale.
La transformation de l'aldrine en dieldrine dans le foie, sous
l'action de mono-oxygénases à fonction mixte (aldrine-époxydase) et
la distribution, puis le dépôt ultérieur, de la dieldrine
(principalement dans les tissus à contenu lipidique, tels que le
tissu adipeux, le foie, les reins, le coeur et le cerveau) sont
beaucoup plus rapides que le catabolisme et l'élimination finale de
la dieldrine intacte et de ses métabolites. Dans ces conditions,
pour un apport quotidien moyen déterminé d'aldrine ou de dieldrine,
il y a accumulation lente de dieldrine dans l'organisme. Mais
cette accumulation n'est pas indéfinie. Quand l'administration se
poursuit, on finit par aboutir à un état d'équilibre dynamique, la
quantité excrétée compensantexactement l'apport. La quantité
stockée maximale dépend de l'apport quotidien, tout comme on l'a
montré chez le rat, le chien et l'homme.
Quand l'apport d'aldrine/dieldrine est réduit ou interrompu,
la charge de l'organisme diminue. Chez l'homme, la demi-vie
biologique est de l'ordre de 9 à 12 mois. Chez le rat, le chien et
l'homme, on a démontré l'existence de relations significatives
entre la concentration de la dieldrine dans le sang et sa
concentration dans d'autres tissus.
De nombreuses études sur la concentration de la dieldrine dans
divers tissus, dont le sang et les tissus adipeux, aussi bien dans
la population générale que dans des catégories particulières, ont
été réalisées dans plusieurs pays et ont montré que, à l'équilibre,
les concentrations respectives dans les tissus adipeux, le foie, le
cerveau et le sang sont sensiblement proportionnelles à 150, 15, 3
et 1.
La dieldrine est transportée par le placenta jusqu'au foetus.
Il y a accumulation dans les mêmes organes et tissus que chez
l'adulte, mais en quantités moindres. Il existe apparemment un
équilibre entre les concentrations chez la mère et chez le foetus.
Chez le rat et le chien, la photodieldrine est également
métabolisée sous forme de pentachlorocétone pontée. On a retrouvé
les deux composés dans les tissus adipeux, le foie et les reins des
animaux à qui l'on avait administré de la photodieldrine à forte
dose. Chez l'homme, aucun résidu de ces composés n'a été mis en
évidence dans le tissu adipeux, les reins ni le lait maternel.
L'accumulation de photodieldrine dans les tissus adipeux des
animaux d'expérience était beaucoup moins importante que celle de
la dieldrine.
5. Effets sur les êtres vivants dans leur milieu natural
5.1 Accumulation
La plupart des résidus présents chez les êtres vivants sont
des résidus de dieldrine, car l'aldrine se transforme facilement
chez eux en dieldrine.
Les champignons, les streptomycètes et les bactéries
concentrent la dieldrine du milieu ambiant dans une proportion qui
peut aller en 4 h de 0,3 à plus de 100. Les protozoaires absorbent
la dieldrine davantage que les algues. Celles-ci absorbent très
rapidement la dieldrine présente dans le milieu de culture, les
concentrations maximales étant souvent atteintes en quelques
heures.
De nombreuses espèces d'invertébrés aquatiques concentrent
fortement la dieldrine à partir d'une eau à très faible teneur.
L'équilibre est atteint en quelques jours. Lorsqu'on les remet en
eau pure, la dieldrine s'élimine rapidement, avec une demi-vie de
60 - 120 h.
Pour les poissons entiers, le facteur de bioconcentration
dépasse 10 000. Chez une espèce de poissons, la demi-vie
d'élimination de la dieldrine accumulée s'est établie à 16 jours.
La bioconcentration de dieldrine chez les organismes
aquatiques se fait principalement à partir de l'eau et non par
ingestion d'aliments.
Les lombrics absorbent la dieldrine présente dans le sol et
laconcentrent jusqu'à un facteur maximal d'environ 170. Pour la
plupart des types de sol, il n'existe guère de corrélation entre la
concentra-tion atteinte chez le lombric et la concentration dans le
sol.
De nombreux travaux ont été consacrés à la présence de la
dieldrine dans les tissus ou dans les oeufs d'espèces non visées.
Les concentrations observées sont extrêmement variables, allant de
0,001 mg/kg à 100 mg/kg de tissu, mais elles restent le plus
souvent inférieures à 1 mg/kg de tissu.
Chez les oiseaux, il y a accumulation rapide de dieldrine
aussi bien dans les tissus que dans les oeufs. De même, on a
montré que diverses espèces de mammifères accumulent la dieldrine,
en particulier dans les graisses.
5.2 Toxicité pour les micro-organismes
La dieldrine a des effets très variables sur les algues uni-
cellulaires, avec une action sensible sur certaines espèces dès la
concentration de 10 µg/litre tandis que d'autres espèces ne sont
pas touchées même à la concentration de 1000 µg/litre. L'aldrine
et la dieldrine n'ont que peu d'effets sur les bactéries
terricoles, même à des concentrations très supérieures aux valeurs
habituelles. Dans la plupart des études, aucun effet n'a été
constaté après exposition à une concentration de 2000 mg/kg de
terre. Des effets ont été signalés sur la photosynthèse chez
différentes espèces d'algues, avec une action plus marquée de
l'aldrine que de la dieldrine à concentrations égales. Mais ces
effets minimes sur la biochimie des algues n'étaient que
transitoires.
5.3 Toxicité pour les organismes aquatiques
L'aldrine et la dieldrine sont extrêmement toxiques pour les
crustacés aquatiques, avec des valeurs de la DL50 à 96 h
inférieures à 50 µg/litre. Cependant, les quelques résultats plus
élevés signalés (jusqu'à 4300 µg/litre) illustrent les différences
de sensibilité selon les espèces. Les daphnies sont moins
sensibles à la dieldrine qu'à l'aldrine, avec des DL50 à 48 h de
23 - 32 µg/litre dans le premier cas et de 190 - 330 µg/litre dans
le second. Les mollusques sont nettement plus résistants, les CL50
à 48 h pouvant atteindre plus de 10 000 µg/litre. Des études de
plusieurs semaines ont confirmé la résistance relative des daphnies
et des mollusques. Les invertébrés aquatiques les plus sensibles
sont les stades larvaires des insectes, avec des CL50 à 96 h de
0,5 - 39 µg/litre pour la dieldrine et de 1,3 - 180 µg/litre pour
l'aldrine.
Lors d'épreuves de toxicité aiguë, l'aldrine comme la
dieldrine se sont montrées très toxiques vis-à-vis des poissons.
Chez diverses espèces de poisson, on a relevé des valeurs de la
CL50 à 96 h allant de 2,2 à 53 µg/litre pour l'aldrine et de 1,1 à
41 µg/litre pour la dieldrine. De nombreuses études ont montré que
la toxicité augmente avec la température. Dans une étude prolongée
sur Poecilia latipinna, on a obtenu un taux de mortalité de 100% en
présence d'une concentration de dieldrine égale ou supérieure à 3
µg/litre. L'addition de dieldrine à la nourriture de truites arc-
en-ciel jusqu'à des concentrations de 430 µg/kg de poids corporel
par jour, n'a exercé aucune influence sur la mortalité mais a
entraîné des modifications enzymatiques. Des altérations
morphologiques ont été observées aumicroscope électronique dans les
mitochondries hépatiques. Le mécanisme de détoxification de
l'ammoniaque chez les poissons est sensible à la dieldrine, la dose
sans effet nocif apparent étant inférieure à 14 µg/kg de poids
corporel par jour. La sensibilité à la dieldrine s'est révélée
variable selon le stade de développement des poissons. Les oeufs
étaient résistants et les formes juvéniles moins sensibles que les
adultes.
La toxicité aiguë de l'aldrine comme de la dieldrine est
élevée pour les larves d'amphibiens, avec des CL50 à 85 h de
l'ordre de 100 µg/litre.
5.4 Toxicité pour les organismes terrestres
La dieldrine est peu toxique pour les végétaux supérieurs
puisque les cultures ne sont affectées que par des doses
supérieures à 22 kg/ha. La phytotoxicité de l'aldrine est plus
importante, notamment pour les tomates et les concombres, mais
uniquement à des doses plusieurs fois supérieures aux valeurs
recommandées. Le chou est la plante cultivée la plus sensible à
l'aldrine.
Vis-à-vis des abeilles, la DL50 par voie orale varie, selon
les observations publiées, de 0,24 à 0,45 µg/abeille pour l'aldrine
et de 0,15 à 0,32 µg/abeille pour la dieldrine. Les quantités
toxiques par contact vont de 0,15 à 0,80 µg/abeille pour l'aldrine
et de 0,15 à 0,41 µg/abeille pour la dieldrine. D'après deux
études, la dieldrine est relativement plus toxique vis-à-vis des
insectes prédateurs qui se nourrissent de ravageurs.
Selon des études effectuées en laboratoire, le lombric
supporte des doses d'aldrine de 13 mg/kg en sol artificiel, le taux
de mortalité étant inférieur à 1%. La CL50 à six semaines était de
60 mg d'aldrine par kg de sol.
Pour 13 espèces d'oiseaux, la toxicité aiguë de l'aldrine et
de la dieldrine variait de plus du simple au décuple, avec des
valeurs de 6,6 - 520 mg/kg de poids corporel pour l'aldrine et de
6,9 - 381 mg/kg de poids corporel pour la dieldrine. Chez quatre
espèces d'oiseaux, la toxicité subaiguë par voie orale
correspondait à des doses comprises entre 34 et 155 mg/kg pour
l'aldrine et 37 et 169 mg/kg pour la dieldrine. Des épreuves
répétées au cours d'une certaine période n'ont révélé aucun signe
de résistance acquise chez ces espèces. D'après des études sur la
reproduction de plusieurs espèces de volaille, une concentration de
la dieldrine dépassant 10 mg/kg dans les aliments provoque une
certaine mortalité chez les adultes. Aucun effet ne s'est fait
sentir sur la production des oeufs, la fécondité, le taux
d'éclosion ni la survie des poussins en présence de dieldrine dans
les aliments à des concentrations qui ne sont pas toxiques pour la
mère. La dieldrine n'a aucune influence directe sur l'épaisseur de
la coquille des oeufs. Cependant, la diminution de la consommation
de nourriture, qui constitue un symptôme de l'intoxication par la
dieldrine, peut entraîner une diminution de l'épaisseur de la
coquille.
Chez le mammifères non élevés au laboratoire, la réponse à la
dieldrine varie selon les espèces. Chez quatre espèces de
campagnols, on a observé des valeurs de la DL50 aiguë, allant de
100 à 210 mg/kg de poids corporel, ce qui montre que ces animaux
sont moins sensibles àla dieldrine que les animaux de laboratoire.
Des musaraignes ont survécu à la consommation d'une nourriture
contenant 50 mg de dieldrine par kg mais sont mortes quand la
concentration est passée à 200 mg/kg. Des damalisques (une espèce
d'antilope) ont survécu 90 jours à une nourriture contenant de la
dieldrine à raison de 5 ou 15 mg/kg mais sont toutes mortes dans
les 24 jours pour une concentration égale ou supérieure à 25 mg/kg.
Tous les damalisques d'une région où l'on avait pulvérisé de la
dieldrine à raison de 0,16 kg/ha sont mortes et le calcul a montré
que l'apport alimentaire était de 1,82 mg/kg par jour. Trente pour
cent des springboks ont survécu aux épandages, sans manifester
d'effets tardifs. Les signes toxicologiques de l'intoxication par
la dieldrine étaient sensiblement les mêmes que chez les mammifères
de laboratoire.
5.5 Effets sur les populations et les écosystèmes
Certaines études donnent à penser que des populations de
mammifères ont été intoxiquées par de la dieldrine. Il est
probable que de petits mammifères sont morts après avoir mangé des
semences enrobées de dieldrine mais les populations se sont
reconstituées par immigration. Des chauves-souris ont été tuées
par la dieldrine contenue dans les agents de protection du bois.
Des résidus de dieldrine ont été signalés chez de nombreuses
espèces d'oiseaux. Partout dans le monde, c'est chez les oiseaux
de proie que les résidus sont les plus abondants car les animaux se
situent en fin de chaîne alimentaire. La teneur en dieldrine des
tissus et des oeufs d'oiseau suit l'évolution de l'emploi de
l'aldrine et de la dieldrine, et elle a diminué à la suite des
restrictions imposées à leur usage. Il n'est pas facile de repérer
les effets de la dieldrine car les résidus de cet insecticide
s'accompagnent de résidus d'autres organochlorés. La dieldrine est
plus toxique que le DDT pour les oiseaux et il est probable qu'elle
a provoqué chez les adultes une plus forte mortalité que le DDT.
Il est encore plus difficile de démontrer l'existence d'effets de
la dieldrine sur la reproduction à l'état naturel. En outre, il se
peut que les effets interviennent longtemps après l'exposition.
6. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
L'aldrine et la dieldrine sont extrêmement toxiques : pour ces
deux composés, la DL50 varie, chez la souris et chez le rat, de 40
à 70 mg/kg de poids corporel. Par voie percutanée, la dose toxique
se situe entre 40 et 150 mg/kg de poids corporel selon l'espèce en
cause et le solvant utilisé. On a constaté que l'aldrine et la
dieldrine de qualité technique déterminent chez le lapin une
irritation cutanée légère à intense, mais la cause en est le
solvant. Dans l'épreuve de maximalisation de Magnusson & Kligman
chez le cobaye, l'aldrine a provoqué un effet de sensibilisation.
Pourtant, au cours de 20 années de fabrication et de préparation
des formules, aucun cas de sensibilisation cutanée n'a été observé
dans un groupe comptant plus de 1000 travailleurs.
L'aldrine, comme la dieldrine, a une faible tension de vapeur
de sorte que, en principe, il n'y a aucun effet aigu par
inhalation. Les effets observés lors des études de toxicité aiguë
après exposition par toutes les voies possibles concernent le
système nerveux central etconsistent en hyperexcitabilité,
tremblements et convulsions.
Des études d'exposition par voie orale, de courte ou longue
durée, ont été réalisées avec l'aldrine et la dieldrine, chez la
souris, le rat, le chien, le hamster et les petits singes. Chez le
rat et la souris, le foie est le principal organecible : on observe
une augmentation de son poids par rapport au poids du corps et une
hypertrophie des hépatocytes centrilobulaires, la réversibilité
étant possible à un stade précoce. Au microscope, ces altérations
se traduisent par une augmentation de l'oxyphilie cytoplasmique et
une migration périphérique des granules basophiles. Ces
altérations ne se rencontrent pas au niveau du foie chez le hamster
et le singe. Chez le chien, l'atteinte hépatique est peu prononcée
(dégénérescence graisseuse et légère atrophie des hépatocytes);
elle s'accompagne d'une atteinte rénale consistant dans une
vacuolisation de l'épithélium des tubules distaux et une
dégénérescence tubulaire. Chez le rat, la dose sans effet nocif
observable se situe dans l'ensemble, d'après les résultats dont on
dispose sur le court et le long terme, aux alentours de 0,5 mg/kg
de nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
En augmentant les quantités incorporées à la nourriture, jusqu'à
obtenir l'équivalent de 0,05 mg/kg de poids corporel ou davantage,
on observe une hépatomégalie et des altérations histologiques
d'importance proportionnée à la dose. Chez le chien une dose de
0,04 - 0,2 mg/kg de poids corporel s'est révélée sans effet.
Plusieurs études de cancérogénicité à long terme ont été
effectuées sur différentes souches de souris, avec de l'aldrine ou
de la dieldrine. Chaque fois, on a observé des tumeurs
hépatocellulaires bénignes ou malignes. Apparemment, les femelles
étaient plus sensibles que les mâles. Aucun autre type de tumeur
ne s'est manifesté dans ces études.
Des études à long terme sur d'autres espèces (rat, hamster)
n'ont révélé aucune augmentation de l'incidence tumorale.
L'administration de photodieldrine incorporée à la nourriture,
jusqu'à une concentration de 7,5 mg/kg d'aliments, ne s'est pas
révélé tumorigène.
En outre, on a publié un certain nombre d'études spéciales qui
n'ont, jusqu'ici, pas permis d'élucider le mécanisme de la
production des tumeurs hépatiques chez la souris.
Dans la plupart des études de reproduction (sur 1 à 6
générations), réalisées avec l'aldrine ou la dieldrine sur des
souris et des rats, le principal effet constaté a été
l'augmentation du taux de mortalité dans la descendance, avant
sevrage. La capacité génésique n'a été atteinte qu'à des doses
toxiques pour la mère. Les études sur le chien étaient trop
limitées pour permettre les conclusions catégoriques, si ce n'est
qu'on a noté une augmentation systématique de la mortalité des
chiots à la mamelle.
D'après les résultats de ces études sur la reproduction, on
peut conclure que, de ce point de vue, les doses sans effet nocif
décelable sont de 2 mg de dieldrine par kg de nourriture chez le
rat et de 3 mg de dieldrine par kg de nourriture chez la souris,
soit l'équivalent quotidien de 0,1 et 0,4 mg/kg de poids corporel
respectivement.
Aucun signe de tératogénicité n'a été observé chez la souris,
le rat ou le lapin, après administration par voie orale de doses
d'aldrineet de dieldrine atteignant 6 mg/kg de poids corporel.
L'administration d'une dose unique d'aldrine et de dieldrine,
représentant environ la moitié de la DL50, a provoqué des effets
toxiques intenses chez le foetus de souris et de hamster, ainsi
qu'une incidence accrue d'anomalies tératogènes. La signification
de ces observations est douteuse en présence d'effets toxiques
probables chez les femelles gravides.
Les études de mutagénicité in vivo ou in vitro ont été
nombreuses, mais elles ont presque toujours donné des résultats
négatifs.
La toxicité aiguë de la photodieldrine par voie orale est plus
élevée que celle de la dieldrine chez la souris, le rat et le
cobaye. Lors d'études de toxicité aiguë ou à long terme, on a
observé des symptômes d'intoxication et des effets sur les organes
cibles analogues à ceux de la dieldrine, tant sur le plan
quantitatif que sur le plan qualitatif. La photodieldrine ne s'est
pas montrée tumorigène chez la souris ni chez le rat.
Comme la plupart des autres substances chimiques, l'aldrine et
la dieldrine exercent leurs effets toxiques selon plusieurs
mécanismes. Les organes cibles sont le système nerveux central et
le foie. Chez l'homme et les autres vertébrés, l'intoxication
secondaire à une exposition aiguë ou chronique, se caractérise par
des mouvements musculaires involontaires et des convulsions
épileptiformes. En cas de survie, la récupération est totale après
une courte durée marquée par des symptômes résiduels. Au niveau du
foie, on observe une activité accrue des enzymes microsomales de
biotransformation, en particulier du système enzymatique cytochrome
P-450/monooxygénase. Cette induction des enzymes mircosomales est
réversible et, au-delà d'un certain niveau, elle semble liée aux
altérations cytoplasmiques au niveau du foie et à l'hépatomégalie
chez les rongeurs.
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut penser que, en pratique, ces produits ne contribuent guère à
l'incidence des cancers humains.
7. Effets chez l'homme
L'aldrine et la dieldrine sont très toxiques pour l'homme. Il
y a eu de graves cas d'intoxication accidentelle ou professionnelle
mais il est rare qu'ils aient fait des victimes. La plus faible
dose ayant provoqué une issue fatale a été estimée à 10 mg/kg de
poids corporel. Les personnes ayant survécu à une intoxication
aiguë ou subaiguë se sont complètement rétablies. Aucun effet
irréversible ni atteinte anatomopathologique résiduelle n'a été
signalée.
Les effets nocifs de l'aldrine et de la dieldrine sont
fonction de la concentration de la dieldrine dans le sang. Le
dosage de la dieldrine dans le sang permet de diagnostiquer avec
précision une exposition à l'aldrine/dieldrine. Chez les
travailleurs, le taux sanguin au-dessous duquel on n'observe aucun
effet nocif (dose limite sans effet nocif décelable) est de 105
µg/litre de sang. Cela correspond à un apport quotidien de
dieldrine de 0,02 mg/kg de poids corporel.
L'exposition environnementale (principalement par
l'intermédiairedes aliments mais aussi, dans une faible mesure, par
voie respiratoire) entraîne l'apparition de dieldrine à une très
faible concentration dans les organes, le sang et le lait maternel.
Autant qu'on puisse en juger d'après des études épidémiologiques et
cliniques poussées, il n'existe aucun raison de penser que les taux
couramment observés dans l'organisme constituent une menace pour la
santé de la population en général. Lors d'une étude poursuivie
pendant plus de 20 ans auprès de 1000 travailleurs de l'industrie,
employés dans une fabrique d'insecticides à base d'aldrine/dieldrine,
aucune augmentation de l'incidence des cancers n'a été observée chez
les sujets fortement exposés à ces deux produits. Phénomène encore
plus significatif, aucun signe avant-coureur, sous forme d'une
altération de la fonction hépatique, n'a été observé.
Une étude épidémiologique sur la mortalité a été réalisée dans
une unité de production aux Etats-Unis, sur une cohorte de 870
travailleurs exposés à l'aldrine, à la dieldrine et à l'endrine.
Malgré près de 25 000 années-homme d'observation, il n'a pas été
possible de repérer un risque particulier de cancer attributable au
travail dans cette usine.
EVALUATION DES DANGERS POUR LA SANTE DE L'HOMME ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des dangers pour la santé de l'homme
L'aldrine et la dieldrine sont des pesticides organochlorés
qui ont été utilisés partout dans le monde entre 1950 et le début
des années 70 comme insecticides en agriculture et pour le
traitement des semences, pour la destruction des ravageurs
terricoles et d'autres types d'insectes (par exemple les termites,
les sauterelles et les ravageurs des textiles) ainsi que pour la
lutte contre les glossines et autres vecteurs de maladies. Chez
les insectes, ces composés exercent leur effet toxique par contact
et par voie digestive. A partir des années 70, on en a limité ou
interdit l'emploi dans plusieurs pays, spécialement en agriculture.
Pourtant, ils continuent d'être utilisés dans d'autres pays pour la
destruction des termites.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans de nombreux solvants organiques.
Leur tension de vapeur est faible.
On trouve souvent de la dieldrine dans des produits laitiers
ou carnés, le poisson, les huiles et les graisses et certains
légumes, notamment des légumes-racines. La limite maximale de
résidus recommandée par les instances compétentes de la FAO/OMS
lors des réunions conjointes sur les résidus de pesticides varie de
0,02 à 0,2 mg/kg de produit. Des mesures récentes ont montré que
les teneurs effectives sont plus faibles, comme l'ont d'ailleurs
confirmé les études sur la ration globale. Comme l'utilisation de
ces deux composés fait maintenant l'objet de restrictions, on
observe une diminution lente mais régulière de la teneur en résidus
des différentes denrées alimentaires.
Les quantités ingérées par l'homme avec sa ration quotidienne
se traduisent, malgré la faible concentration de ces produits dans
les aliments, par la présence de dieldrine dans le tissu adipeux et
dans certains autres tissus et organes. Des enquêtes à l'échelle
mondiale montrent que les teneurs moyennes varient de 0,1 à 0,4
mg/kg de tissu adipeux. Depuis le début des années 70, cette
concentration diminue lentement.
Comme le foetus est exposé par voie transplacentaire, ses
tissus adipeux contiennent également de la dieldrine, mais à une
concentration qui n'est que de 10 à 50% de la concentration chez la
mère. Il semble exister un équilibre entre les concentrations
foetales et les concentrations maternelles. La dieldrine est
également excrétée dans le lait. Une exposition est possible par
inhalation dans les habitations où l'on utilise ce produit pour la
destruction des termites. Après traitement, on observe des
concentrations atmosphériques allant de 0,01 à 7 µg/m3, selon le
mode d'épandage, la concentration utilisée, les modalités de
l'aération et le moment où les échantillons sont prélevés. En
pareilles circonstances, les aliments peuvent aussi être
contaminés, par contact direct, ou par sorption à partir de l'air
ambiant.
Le métabolisme s'effectue principalement dans le foie où
l'aldrine se transforme rapidement en dieldrine. Le catabolisme de
la dieldrineest plus lent que celui de ses métabolites hydrophiles
qui sont excrétés dans la bile et dans les urines. La structure de
ces métabolites a été établie. Chez toutes les espèces étudiées,
notamment l'homme, on a montré que les quantités
d'aldrine/dieldrine accumulées se stabilisent à un niveau qui est
fonction de l'apport puisqu'il existe une relation linéaire entre
les quantités accumulées et le logarithme de l'apport. Quand
l'exposition prend fin, la concentration de la dieldrine dans les
tissus de l'organisme diminue selon une loi exponentielle. La
toxicité aiguë de l'aldrine et de la dieldrine est importante chez
les mammifères par voie orale, tandis que la toxicité par voie
cutanée est modérée. Aucune sensibilisation cutanée n'a été
observée. Les effets constatés à la suite d'une exposition
expérimentale aiguë ou de courte ou longue durée intéressent le
système nerveux central. Le foie est également un organe cible.
Chez les souris et les rats, on observe à ce niveau des altérations
désignées sous le nom de "foie de rongeur sous insecticide
organochloré".
Apparemment, l'aldrine et la dieldrine ne sont pas tératogènes
à des doses inférieures à celles qui sont toxiques chez la femelle
gravide et chez le foetus. On n'a pas fait état de toxicité pour
la fonction de reproduction chez le mâle ou la femelle.
De nombreuses études de mutagénicité in vitro et in vivo ont
montré que ni l'aldrine ni la dieldrine ne sont mutagènes.
Lors d'études à long terme, ces deux produits ont déterminé
chez la souris des tumeurs hépatiques, bénignes ou malignes. En
revanche, aucune augmentation de l'incidence des tumeurs hépatiques
ou autres n'a été observée chez le rat ni le hamster.
Selon le CIRC (1987), il n'existe pas de preuves suffisantes
d'un pouvoir cancérogène chez l'homme et les preuves de
cancérogénicité chez l'animal d'expérience sont limitées.
L'aldrine comme la dieldrine ont été classées dans le groupe 3, à
savoir celui des produits chimiques dont il est impossible de
préciser le pouvoir cancérogène chez l'homme.
Compte tenu des résultats obtenus lors des études de toxicité
de courte ou de longue durée, la dose globale sans effet nocif
décelable se situe chez le rat à 0,5 mg de dieldrine par kg de
nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
Chez le chien, la dose sans effet nocif décelable est de 0,04 mg/kg
de poids corporel. Lors des réunions conjointes FAO/OMS de 1966 et
1977 sur les résidus de pesticides, on a fixé la dose journalière
admissible (DJA) à 0,1 µg/kg de poids corporel, en tenant compte de
la non-cancérogénicité de ces deux substances pour l'homme.
L'aldrine et la dieldrine sont très toxiques pour l'homme. On
connaît des cas d'intoxication accidentelle ou professionnelle,
mais qui ont rarement fait des victimes. Les survivants à une
intoxication aiguë ou subaiguë se sont entièrement rétablis. Les
effets nocifs sont fonction de la concentration sanguine de la
dieldrine, dont le dosage permet de diagnostiquer avec précision
une exposition à l'aldrine/dieldrine. Pour taux sanguins inférieur
à 105 µg/litre, aucun effet indésirable n'est à craindre. Cette
concentration constitue la dose limite sans effet nocif décelable
et correspond à un apport quotidien de 0,02 mg de dieldrine par kg
de poids corporel.
L'exposition liée à l'environnement, principalement par la voie
alimentaire, entraîne la présence de faibles concentrations de
dieldrine dans l'organisme. D'après des études épidémiologiques et
cliniques poussées, ces teneurs ne constituent pas une menace pour
la santé humaine.
Aucun signe avant-coureur d'une altération de la fonction
hépatique n'a été observé lors d'une enquête de 20 ans portant sur
plus de 1000 ouvriers de l'industrie exposés à l'aldrine et à la
dieldrine. Dans cette étude ainsi que dans une autre effectuée aux
Etats-Unis d'Amérique, aucun risque particulier de cancer n'a été
repéré chez les personnes profesionnellement exposées à l'aldrine
et à la dieldrine (parfois à de fortes concentrations).
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut estimer que, en pratique, ces produits chimiques ne
contribuent que très peu, sinon pas du tout, à l'incidence des
cancers humains.
La photodieldrine, produit qui résulte de la dégradation de la
dieldrine sous l'action de la lumière, est analogue à la dieldrine
pour ce qui est de sa toxicité sur une courte durée. Elle n'est ni
tératogène ni cancérogène chez la souris et le rat. L'accumulation
de photodieldrine dans les tissus adipeux d'animaux d'expérience
s'est révélée inférieure à celle de la dieldrine.
2. Evaluation des effets sur l'environnement
La principale source de dieldrine (jusqu'à 97%) dans
l'environnement est l'aldrine, un insecticide épandu au niveau du
sol. L'aldrine et son produit de réaction, la dieldrine sont
rapidement absorbé par les sols, spécialement ceux qui sont riches
en matières organiques. De ce fait, la pénétration est limitée et
il n'y a généralement aucune contamination des eaux souterraines.
Les deux composés sont entraînés principalement du fait de
l'érosion (sous l'action du vent) et du transport des sédiments
(eaux superficielles de ruissellement), mais non par lessivage.
L'emploi d'aldrine et de dieldrine en agriculture donne lieu à
la présence de résidus (principalement de dieldrine) dans le sol où
ils peuvent persister plusieurs années; la demi-vie de la dieldrine
est estimée à 4 - 7 ans. La persistance de ces composés est
moindre dans les régions tropicales que dans les régions tempérées.
L'aldrine et la dieldrine passent, par volatilisation, des
récoltes et du sol traités à l'atmosphère; elles peuvent aussi y
pénétrer directement lors de l'épandage. La dieldrine retourne au
sol ou dans les étendues d'eau par les précipitations ou par dépôt
de particules sèches. Les composés se rencontrent donc soit en
phase vapeur (à des concentrations très faibles, en général de
l'ordre de 1 - 2 ng/m3), soit adsorbés sur des particules de
poussière, soit encore dans les eaux de pluie (à des concentrations
de l'ordre de 10 - 20 ng/litre).
Plusieurs auteurs ont signalé la présence de dieldrine en
milieu aquatique. Dans les eaux de surface, les concentrations
sont le plus souvent très faibles, inférieures à 5 ng/litre. Mais
des valeurs plus élevées s'observent dans les régions soumises à
l'érosion ou danscelles où l'on utilise ce produit en agriculture.
Dans ces régions, les sédiments des cours d'eau peuvent renfermer
jusqu'à 1 mg de dieldrine par kilogramme. La forte capacité qu'ont
les organismes aquatiques à concentrer la dieldrine à partir de
teneurs très faibles peut aboutir à l'accumulation de doses
toxiques. La concentration de ce produit tout au long de la chaîne
alimentaire aquatique est moins importante qu'une absorption
directe à partir de l'eau.
Comme la dieldrine est très répandue dans l'environnement et
qu'elle y persiste, on observe des concentrations très variées chez
les organismes non visés. Alors qu'auparavant les valeurs
observées allaient de 0,001 mg à 100 mg/kg de tissu, elles sont
aujourd'hui le plus souvent inférieures à 1 mg/kg de tissu.
Dans les écosystèmes terrestres, l'aldrine et la dieldrine
s'accumulent chez divers organismes, principalement sous forme de
dieldrine. Cette dernière est probablement responsable de la mort
de mammifères dans la nature et de la raréfaction de certaines
espèces, comme la loutre. Certains petits mammifères périssent
sans doute après avoir mangé des céréales traitées mais il est
probable que leurs populations se reconstituent par immigration à
partir des zones voisines. Les oiseaux de proie qui mangent de
petits mammifères et de petits oiseaux contaminés par la dieldrine
absorbent et concentrent cet insecticide dans leurs tissus et leurs
oeufs. Des oiseaux granivores ont été tués par la consommation de
céréales traitées. Il est probable que la raréfaction des oiseaux
de proie s'explique par la présence dans leurs tissus de résidus de
dieldrine (entre autres organochlorés). Les effets de la dieldrine
se manifestent avec un certain retard car les résidus s'accumulent
dans les graisses pendant l'hiver d'où ils ne se libèrent qu'au
printemps. Le fait de n'utiliser la dieldrine qu'à certaines
époques de l'année n'a pas réduit la mortalité des oiseaux.
La large utilisation d'aldrine et de dieldrine, parallèlement
à celle d'autres organochlorés, a exercé des effets très nocifs sur
l'environnement mais grâce à des restrictions draconiennes,
particulièrement en ce qui concerne les semences traitées, les
populations d'oiseaux commencent à se reconstituer.
3. Conclusions
a) L'aldrine et la dieldrine ont donné lieu à des études
poussées et variées sur le plan toxicologique, clinique et
épidémiologique. La charge de l'organisme résulte principalement
de l'ingestion de résidus présents dans la nourriture (les
quantités ingérées semblant diminuer de façon générale et tomber
en-dessous des DJA fixées) et, dans une moindre mesure, de
l'inhalation de ces produits. D'après l'étude des données, on a
tout lieu de penser que la charge de l'organisme résultant du
niveau actuel d'exposition ne menace en aucun cas la santé de la
population dans son ensemble.
b) La dieldrine se rencontre presque partout dans le lait
maternel. Mais, sa concentration dans le sang et dans le tissu
adipeux des nourrissons n'augmente pas avec l'âge au cours des six
premiers mois de leur vie et le taux sanguin n'est pas plus élevé
que chez un enfant nourri au biberon. Dans ces conditions,
l'allaitement au sein reste la méthode de choix pour nourir les
nourissons malgré la présence de résidus de dieldrine.
c) Lors du traitement de locaux, notamment pour la destruction
des termites, l'exposition des occupants ne semble pas présenter
des risques pour leur santé, pour autant que le traitement
s'effectue correctement.
d) Malgré leur toxicité élevée, l'aldrine et la dieldrine
peuvent être manipulées sans danger dans la mesure où l'on observe
toujours les précautions recommandées en vue de réduire au minimum
l'exposition des opérateurs. Dans le cas contraire, il y a risque
d'intoxication.
e) Pendant la période où l'on a massivement utilisé l'aldrine
et la dieldrine, c'est-à-dire de 1950 au début des années 70, il
est certain que cette pratique a eu des effets dommageables sur
diverses espèces. Ces effets sont imputables en partie à la
dieldrine à côté d'autres organochlorés. Depuis qu'on a limité de
façon draconienne l'utilisation de ces produits, les espèces
touchées se sont reconstituées.
RECOMMENDATIONS
1. Il faut effectuer des études de tératogénicité
complémentaires sur le hamster, bien conçues, avec des doses
réalistes de dieldrine.
2. Dans l'étude du mécanisme de la cancérogenèse, on
s'efforcera d'expliquer pourquoi les réactions hépatiques sont si
différentes chez la souris et chez les autres espèces.
3. On continuera d'utiliser la dieldrine pour l'étude des
mécanismes neurotoxiques, à la fois sur le plan expérimental et sur
le plan clinique.
4. Pour des raisons écologiques, toute reprise d'une
utilisation massive d'aldrine et de dieldrine est exclue, et on
n'utilisera ces produits que s'il n'existe pas de produit moins
nocif d'efficacité équivalente.
5. Afin de préserver la santé et le bien-être des travailleurs
et de la population en général, il convient de ne confier la
manipulation et l'épandage de l'aldrine et de la dieldrine qu'à des
opérateurs compétents et dûment formés, qui devront appliquer les
mesures de sécurité qui s'imposent.
6. En raison du risque d'intoxication accidentelle par
l'aldrine, spécialement chez les enfants, il faut en interdire
l'utilisation sous forme de granulés contre les fourmis.
Table 45 gives a non-exhaustive overview of occupational aldrin
and dieldrin poisonings. Hayes (1982) contains further information
on this subject.
Table 45. Published case reports on occupational aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Acute Causative agent Circumstances Reference
of cases cases
cases
------------------------------------------------------------------------------------------
3 - ? 25% aldrin dust formulation with inadequate Nelson (1953)
safety precautions
~100 - ? various spraying in malaria- Hayes (1957,
eradication programmes 1959)
1 - 1 aldrin gross overexposure in aldrin Bell (1960)
packer
1 1 ? dieldrin sprayer Van Raalte
(1965)
4 - ? aldrin formulation of aldrin Kazantzis et
al. (1964)
17 - some aldrin and manufacturing and formulation Hoogendam et
dieldrin al. (1962,
1965)
32 (15 in - some aldrin, manufacturing and formulation Jager (1970)
addition dieldrin,
to endrin,
previous isobenzan
reference)
------------------------------------------------------------------------------------------
No cases of fatal poisoning have been reported during the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
However, there has been one case of a spray operator being fatally
poisoned (Van Raalte, 1965).
In developing countries it is, however, difficult to establish
the actual number of poisoning cases. Experience shows that even
when pesticides are banned, cases may occur in areas where control
is poor and where large quantities of pesticide are stocked. In
May 1987, four cases of occupational poisoning with aldrin were
reported from an area of cocoa plantations in Bahia (Brazil). One
of them was fatal, while three recovered. The fatal case had a
dieldrin level in whole blood exceeding 600 µg/litre two days after
poisoning (Rahde, personal communication).
9.2.1.1 Blood levels diagnostic of aldrin/dieldrin poisoning
Because the symptoms of aldrin/dieldrin intoxication are non-
specific, a differential diagnostic test is required to confirm
that the symptoms, signs, and clinical course of a particular case
are the result of aldrin or dieldrin intoxication.
The results of animal studies, together with those obtained
subsequently during medical surveillance of workmen employed in the
manufacture (or formulation) of aldrin/dieldrin, have shown that
adverse effects induced by aldrin/dieldrin are related to the
dieldrin blood concentration (Brown et al., 1964; Jager, 1970).
Therefore, the determination of this concentration provides a
specific differential diagnostic test.
Concentrations of dieldrin ranging from 40 to 530 µg/litre have
been reported in the blood of people who had been poisoned
relatively recently and who had recovered (Kazantzis et al., 1964;
Jager, 1970; Avar & Czegledi-Janko, 1970; Siyali & Simson, 1973).
From the limited information available, Brown et al. (1964)
concluded that the threshold concentration of dieldrin in the blood
of human beings, critical for intoxication, is approximately
150 - 200 µg/litre.
Dieldrin is present in the blood at very low concentrations in
the general population of many countries throughout the world. It
is also found at considerably higher concentrations in the blood of
healthy workers. These "healthy workmen" were men between 18 and
60 years of age who had no complaints or clinical or laboratory
signs attributable to occupational exposure, but they were, of
course, subject to the same ailments and diseases as are members of
the general population (Hayes & Curley, 1968; Jager, 1970). No
complaints of ill health and no positive results in objective
clinical or laboratory tests of ill health have ever been noted in
workers whose blood contained less than 200 µg/litre. This
concentration may, therefore, be considered to be a no-observed-
adverse-effect level in human beings. Higher concentrations may
have effects. The maximum concentration reported to be without
complaints or clinical signs or symptoms was 430 µg/litre (Jager,
1970).
Studies on animals have shown that the earliest physiological
sign of exposure to dieldrin is an increase in the activity of
certain liver microsomal enzymes. No enzyme induction has ever
been found in workers with dieldrin blood concentrations at or
below 105 µg/litre (Jager, 1970). (When liver enzyme induction
test methods became available, workers with dieldrin levels in the
blood exceeding 105 µg/litre were no longer encountered.)
Sometimes, dieldrin concentrations in plasma or serum, rather
than in whole blood, have been reported. The ratio of the dieldrin
concentration in plasma to that in erythrocytes is approximately
4:1 (Dale et al., 1965; Mick et al., 1972) (the conversion factor
to calculate the concentration of dieldrin in whole blood from the
concentration in plasma or serum is 0.66).
Great differences exist between the average concentrations of
dieldrin in the blood of the general population and of
occupationally exposed workers with or without complaints (Table
46).
Table 46. Concentrations of dieldrin in whole blood of human beings (µg/litre)
-------------------------------------------------------------------------------
No. of
Subjects persons Geometric Range Reference
involved mean
-------------------------------------------------------------------------------
General population 4592 <1 <1-16.1a US EPA (1983)
Unexposed persons 25 0.5 0-3.3 Sandifer et al. (1981)
20 2.5 0.5-10 Brown et al. (1964)
Healthy workers 35 29 <10-90 Jager (1970)
21 120e Mick et al. (1972)
37 55 -f Morgan & Roan (1974)
27 20 4.5-54 Sandifer et al. (1981)
89 38g 2-220 Brown et al. (1964)
Patients with 18 160g 8-280 Avar & Czegledi-Janko
clinical symptoms (1970)
4 40-530b Kazantzis et al. (1964)
5 130-370c Brown et al. (1964)
5 160-430d Brown et al. (1964)
Deceased (suicide) 1 850 Hayes (1982)
-------------------------------------------------------------------------------
a In serum.
b The low level of 40 µg/litre was found in a man with chronic nephritis,
complaining of headache and nausea; the occupational cause of the symptoms
is, therefore, doubtful. In one other worker, a blood level of 530 µg/litre
was found 1 month after a mild acute episode when he was exposed to aldrin
again.
c Determined some time after the acute episode.
d Estimated to be the concentration at the time of the acute episode.
e Converted from plasma figures using a factor of 0.66 (see 9.2.1.1).
f Highest value 231 kg/litre.
g Average.
Among 13 adolescent patients with colon-rectal adenocarcinomas,
who had lived in rural areas of Mississippi, USA, where pesticides
are widely sprayed, dieldrin blood levels were no higher than those
in controls (Caldwell et al., 1981).
9.2.1.2 Electroencephalography
Changes in the electroencephalogram (EEG) are sometimes of
practical importance for confirming a diagnosis of aldrin/dieldrin
intoxication (Spiotta, 1951; Winthrop & Felice, 1957; Hoogendam et
al., 1962, 1965; Kazantzis, 1964; Avar & Czegledi-Janko, 1970;
Jager, 1970). These EEG changes were first used as a practical
tool for monitoring workers and determining when they should
discontinue exposure and when they could be allowed to resume work
with aldrin/dieldrin. Characteristic changes - which, however, are
not pathognomonic for aldrin/dieldrin poisoning - include bilateral
synchronous spikes, spike and wave complexes, and slow theta waves,
thought to be possibly associated with brain stem stimulation
(Hoogendam et al., 1962, 1965). The interesting parallelism
between the rate of diminution of the EEG changes and the rate of
decrease in the dieldrin blood concentration was also reported in
the case of an accidentally poisoned child (Garrettson & Curley,
1969).
Nowadays, analysis for dieldrin in the blood has replaced EEG
examination as the method of choice for monitoring exposed workers
(Jager, 1970).
9.2.2. Effects of short- and long-term exposure
Occupational exposure occurred in the 1950s and early 1960s
among sprayers in malaria and yellow fever control programmes.
These men sprayed dieldrin inside houses day after day in prolonged
cycles without appreciable intervals of non-exposure. Quite often,
precautions and supervision were less than would be required today.
At the time, methods for the determination of blood concentrations
had not been developed. A significant percentage of these sprayers
became sick after having worked for as little as 2 days or as much
as 2 years (Hayes, 1957, 1959; Patel & Rao, 1958; Zavon & Hamman,
1961). According to communications by the Pan-American Sanitary
Bureau (Soper, 1955a), it appeared that no clinical symptoms were
observed in well-executed, well-supervised malaria and yellow fever
control programmes.
In a study carried out in East Africa, where workers were
spraying dieldrin 6 h/day for 180 days per year (with an interim of
2 months between spraying cycles) no clinical symptoms were seen.
The potential average dermal exposure of spray operators who
observed the protective measures laid down was 1.8 mg/kg body
weight per day (Fletcher et al., 1959).
Long et al. (1969) studied 159 farmers in Iowa, USA. Extensive
clinical and laboratory examinations of 33 pesticide users among
these farmers did not reveal evidence of any disease that could be
attributed to the use of pesticides, neither was the dieldrin blood
concentration correlatable with any parameter examined.
-------------------------------------------------------------------
a Personal communication at the 2nd Meeting of the Industrial
Council on Tropical Health, Boston.
The dieldrin blood concentrations in 8 locust-control workers
in Ethiopia were measured on two occasions and were found to range
between 0 and 9 µg/litre (MacCuaig, 1976).
Wolfe et al. (1963) studied the hazards from spraying orchards
with dieldrin in the US Pacific Northwest. Potential contamination
of the skin and respiratory exposure were measured. From the
results, potential skin and respiratory exposures were calculated
to amount to 14.2 and 0.25 mg/h, respectively.
Princi & Spurbeck (1951) studied a group of workers exposed
to chlordane, aldrin, and dieldrin for several years in a
manufacturing and formulating plant. The atmospheric
concentrations of aldrin were reported to be as high as 2.6 mg/m3.
Physical examinations and chest-röntgenograms did not reveal
respiratory anomalies.
A study was carried out on 71 men employed in the manufacture
and formulation of aldrin, dieldrin, endrin, and some other non-
related pesticides. Twenty-eight of these workers each contributed
a sample of blood and a sample of fat on the same day. The average
concentration of dieldrin in fat (6.12 ± 1.24 mg/kg) was 247 times
greater than the mean plasma concentration (0.025 ± 0.006
mg/litre). There was no relation between the amount of dieldrin in
the samples and the use of sick leave (Hayes & Curley, 1968).
In another study, 68 pesticide workers (including pest control
operators) and 29 unexposed controls were examined quarterly over a
period of four years. Determinations of serum pesticide
concentrations and enzyme activity, blood chemistry, haematology,
and urinalysis were carried out. The mean serum dieldrin
concentration was 3.6 ± 6.3 µg/litre (1.1 ± 1.6 µg/litre in the
controls). There was no difference between the exposed workers and
the controls in the incidence of disease or disability (Warnick &
Carter, 1972).
In a pesticide formulation plant, the blood of 21 employees was
examined at the conclusion of a 5-week period during which 900 kg
of technical aldrin was formulated. The mean dieldrin
concentration in plasma was 11 and 182.5 µg/litre for herbicide
formulators and aldrin formulators, with a maximum of 317 µg/litre
in the latter case. No mention was made of any intoxications (Mick
et al., 1972).
In a group of 42 occupationally exposed pesticide workers with
a dieldrin serum concentration 5 times as high as that in a group
of 23 controls, no indication of disturbed renal- or adrenocortical
function was found (Morgan & Roan, 1969, 1973).
A study was carried out in California, USA, where aldrin (EC as
a 0.5% solution) at 480 g/litre was applied as a termiticide to
typical slab and crawl space type houses. Personal air samples,
samples of blood, and samples of pads on clothes and gloves were
taken to monitor the exposure of the pest-control operators. The
personal air samples during application contained less than 0.3
µg/m3 aldrin for the slab houses and 30 - 75 µg/m3 for the crawl
space houses. The total work day (9 - 18 h) time-weighted average
concentration of aldrin in air was 6 - 17 µg/m3. This is far below
the threshold limit value (TLV) for aldrin established by the
American Conference of Governmental Industrial Hygienists (ACGIH,
1986) of 250 µg/m3. Data from dermal exposure samples showed large
variation. However, the maximum calculated percentage of the toxic
dose per h, based on the acute percutaneous toxicity (rat LD50) of
the formulation, was less than 0.01%. The concentration of aldrin
and dieldrin in the blood of the operators was below the limit of
detection (less than 1 µg/litre) (Marlow et al., 1982).
A case-control study, carried out on 27 pesticide workers
(4 formulators and 23 pest-control operators) with elevated blood
concentrations of dieldrin, revealed a mean blood concentration of
19.59 µg/litre (range: 4.45 - 54 µg/litre). In an extensive
clinical examination, including physical examination, comprehensive
neurological examinations, laboratory tests, and physiological and
psychomotor testing, no important differences were found compared
with results in a control group of 25 people with a mean dieldrin
blood concentration of 0.48 µg/litre (range of 0 - 3.34 µg/litre)
(Sandifer et al., 1981).
9.2.3. Epidemiological studies
An extensive study on workers in an aldrin/dieldrin
manufacturing plant has been in progress since the plant began
operations in the 1950s. The results from the first 15 years of
this epidemiological study were reported in 1970 (Jager, 1970).
From a total of more than 800 exposed workers, all those exposed
for more than 4 years (233 men) or those who had experienced an
intoxication (20 men) underwent extensive physical, neurological,
haematological, and other laboratory examinations. Clinical
chemical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein, and serum protein spectrum, were
made every 3 months and remained within normal limits. A no-effect
level in this group of workers, including those who had previously
suffered intoxications, was established at a dieldrin blood
concentration of 200 µg/litre. This level corresponds to a total
equivalent daily oral intake of 33 µg/kg body weight or a total
daily intake of 2300 µg/person per day (Hunter & Robinson, 1967).
In experimental animals, the earliest, reversible effect of
dieldrin is the induction of liver microsomal enzyme systems
(Wright et al., 1977, 1978). This finding led to an investigation
of a group of 10 workers. At the time, due to further improvements
in the industrial hygiene of the above-mentioned plant, the
geometric mean concentration of dieldrin found in the blood of
workers was 105 µg/litre. As criteria of enzyme induction
measurements were made of the blood levels of p,p'-DDE, the urinary
ratio of 6-beta-hydroxycortisole and 17-hydroxycorticosteroids, and
the urinary excretion of D-glucaric acid. No difference in these
values was found between the 10 exposed workers and a control
group. On the basis of these data, the no-effect level was 105 µg
dieldrin/litre blood, equivalent to an oral daily intake of 17.4
µg/kg body weight per day (or 1220 µg/person per day) (Jager, 1970;
Hunter et al., 1969; Hunter & Robinson, 1967; Versteeg & Jager,
1973).
Further results from this long-term survey of an industrial
population were subsequently published, based on a study of 1000
workers. Because not all of the workers had severe and/or
prolonged exposure, smaller groups with an exposure meaningful
enough for carcinogenicity evaluation were included. One group
consisted of 166 men (including workers who were still exposed and
workers who had left the company), with a mean exposure time of
16.9 years (range 4 - 19 years), who had been under observation for
more than 15 years (mean observation period 17 years; range,
15 - 20 years). A sub-group comprised 69 men with a mean exposure
time of 14.9 years (range 10 - 19 years) and a mean observation
period of 17.2 years. Among the group of 166 workers, 51 were more
than 50 years old. One man with only 5 years of comparatively mild
exposure died because of a gastric carcinoma. A lymphosarcoma
occurred in a man with 7 years of very mild exposure. Both
incidences occurred before 1964. No new cases were noted in the
final 11 years of study, and no undue mortality from other causes
that could have masked a higher cancer incidence was observed
(Versteeg & Jager, 1973; Van Raalte, 1977).
In a follow-up study on the original group of 233 men with more
than 4 years of exposure and an observation period ranging from 4
to 29 years (mean, 24 years), there were no indications of a
specific carcinogenic activity. Total observed mortality was 25
deaths versus 38 expected. Of nine cancer deaths, three were
caused by lung cancer, while the remaining six were each of a
different nature. No primary liver tumours were observed (Ribbens,
1985).
In a study by Morgan & Roan (1974), 28 pesticide formulators
and applicators, plus a separate group of 43 termite-control
workers, with occupational exposures of 5 - 22 years were examined,
together with 56 controls. The highest levels of dieldrin among
these 71 workers were found in a group of 37 men who had a mean
serum dieldrin concentration of 84 µg/litre (equivalent to about
55 µg/litre whole blood). There were no signs of liver cell injury
and the serum enzyme activities SGOT, SGPT, LDH, alkaline
phosphatase and creatine phosphokinase (CPK) were within normal
limits. There was no indication of drug-metabolizing enzyme
induction and urinary excretion of D-glucaric acid was not
different from that in a control group.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides, revealed
that, in 1962, 18 and 10 years after the introduction of DDT and
aldrin/dieldrin, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the cases of
primary liver cancer as a percentage of the total number of liver
cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) of
primary liver cancer for this period declined from 3.46 to 3.18
(Deichmann & MacDonald, 1977).
An epidemiological mortality study in a plant manufacturing
aldrin, dieldrin, and endrin was carried out on a cohort of 1155
workers who had been employed for at least 6 months between 1946
and 1976 (almost 25 000 man-years of observation). The mortality
due to all malignant neoplasms was 31, lower than expected
(standardized mortality ratio (SMR) 82). The total mortality from
all causes was 173 (SMR 84). The only disease with an SMR above
100 (SMR 212) was "non-malignant respiratory system disease",
specifically pneumonia. There was a slight excess of oesophagus
and rectum cancer (two and three cases observed with an SMR of 235
and 242, respectively), liver cancer (two cases observed versus
0.57 expected), and cancer of the lymphatic and haematopoietic
system (six cases observed versus 4.07 expected). However, there
was a deficit of cancer of other sites. The authors concluded that
"the study has not identified a specific cancer risk associated
with employment at this manufacturing plant, but several causes
should be examined further" (Ditraglia et al., 1981).
In a 1981 health survey, a total of 567 serum samples from 1811
Florida citrus workers were collected during the spraying and the
harvest season and were compared with the national ("Hanes")
sample. There were no differences in serum dieldrin levels; the
mean in both groups being 1.8 - 1.9 µg/litre serum (Griffith &
Duncan, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Aldrin and dieldrin, organochlorine pesticides, were used
throughout the world from 1950 until the early 1970s as
insecticides in agriculture and as a seed treatment, for the
control of soil pests and other types of insects (e.g., termites,
grasshoppers, and textile pests), and for the control of tsetse
flies and other disease vectors. The compounds act as contact and
stomach poisons in the insects. Since the early 1970s, both
compounds have been restricted or banned from use in several
countries, especially in agriculture. Nevertheless, use continues
in other countries for termite control.
Both compounds are practically insoluble in water and
moderately to highly soluble in many organic solvents. The vapour
pressure is low.
Dairy and meat products, fish, oils and fats, and certain
vegetables such as root vegetables often contain dieldrin. Maximum
residue limits recommended by the FAO/WHO Joint Meeting on
Pesticide Residues range from 0.02 to 0.2 mg/kg product. Recent
measurements have shown that actual levels are lower, and this has
been confirmed by total diet studies. Since the use of these two
compounds has been restricted, a steady but slow decrease in
residue levels in the different food commodities has taken place.
The intake by human beings of low concentrations in the daily
diet has resulted in dieldrin being present in adipose tissue and
in some other tissues and organs. Global surveys have shown that
mean values range from 0.1 to 0.4 mg/kg adipose tissue. Since the
early 1970s, this concentration has slowly decreased.
Transplacental exposure of the fetus occurs, with the result
that the fatty tissues of the fetus also contain dieldrin, but at
concentrations 10 - 50% of those of the mother. There seems to be
an equilibrium between levels in the fetus and those in the mother.
Dieldrin is also excreted with the milk. Inhabitants of houses
that have been treated for termite control may be exposed by
inhalation. Concentrations in the air found after indoor treatment
may range from 0.01 to 7 µg/m3, depending on the type of
applications, concentration used, type of ventilation, and time of
sampling. Under these conditions food may also be contaminated by
direct contact or by sorption from the atmosphere.
Metabolism takes place mainly in the liver where aldrin is
readily transformed to dieldrin. Dieldrin is degraded at a slower
rate to hydrophilic metabolites, which are then excreted via the
bile and urine. The structures of these metabolites have been
established. In all species examined, including human beings, it
has been shown that there is a steady state of aldrin/dieldrin
storage corresponding to the level of intake and a linear
relationship between the log of intake and storage has been
demonstrated. The concentration of dieldrin in body tissues
decreases exponentially on termination of exposure to the
compounds.
The acute oral toxicity of aldrin and dieldrin for mammals is
high, while the dermal toxicity is moderate. Dermal sensitization
has not been found. Effects observed in acute, short-term and
long-term studies involve the central nervous system. The liver is
also a target organ. In the liver of mice and rats, changes known
as "chlorinated hydrocarbon insecticide rodent liver" are found.
Aldrin and dieldrin do not appear to cause teratogenic effects
at doses below those causing maternal toxicity and fetotoxicity.
Male or female reproductive toxicity has not been reported.
Numerous in vitro and in vivo mutagenicity studies have
demonstrated that neither aldrin nor dieldrin have mutagenic
potential.
In long-term studies, aldrin and dieldrin induced benign and
malignant liver tumours in the mouse. However, no increased
incidence of liver tumours or other tumours were found in rats and
hamsters.
IARC (1987) has stated that there is inadequate evidence of
carcinogenicity in human beings and limited evidence of
carcinogenicity in experimental animals. Both aldrin and dieldrin
have been classified in Group 3: the chemicals cannot be
classified as to their carcinogenicity in human beings.
On the basis of available short-term and long-term toxicity
data, the overall no-observed-adverse-effect level in the rat is
0.5 mg dieldrin/kg diet, equivalent to 0.025 mg/kg body weight. In
the dog, the lowest no-observed-adverse-effect level found was 0.04
mg/kg body weight. The Joint Meeting on Pesticide Residues (JMPR)
established an Acceptable Daily Intake (ADI) of 0.1 µg/kg body
weight in 1966 and 1977 based on the conclusion that aldrin and
dieldrin were not human carcinogens.
Aldrin and dieldrin are highly toxic to human beings. Both
accidental and occupational cases of poisoning have occurred but
reported fatalities have been rare. Survivors of acute or subacute
intoxications recovered completely. Adverse effects are related to
the dieldrin blood concentration, the determination of which
provides a specific diagnostic test for aldrin/dieldrin exposure.
At a dieldrin blood concentration below 105 µg/litre, no adverse
effects can be expected. This level is considered a threshold no-
observed-adverse-effect level and corresponds to a daily intake of
0.02 mg dieldrin/kg body weight per day.
Environmental, mainly dietary, exposure leads to the presence
of dieldrin in low concentrations in the human body. The results
of extensive clinical and epidemiological studies indicate that
these body burdens do not present a health hazard to human beings.
No signs of any premonitory change in liver function were found
in a 20-years study, involving more than 1000 industrial workers
exposed to aldrin and dieldrin. In this study and another study in
the USA, no specific cancer risk could be identified associated
with occupational exposure to (sometimes high levels of) aldrin and
dieldrin.
All the available information on aldrin and dieldrin taken
together, including studies on human beings, supports the view that
for practical purposes, these chemicals make very little
contribution, if any, to the incidence of cancer in human beings.
Photodieldrin, the photo-decomposition product of dieldrin, is
similar to dieldrin in its short-term toxicity. It is not
teratogenic or carcinogenic in mice and rats. The accumulation of
photodieldrin in the adipose tissue of experimental animals was
less than that of dieldrin.
10.2. Evaluation of Effects on the Environment
Aldrin, used as a soil insecticide, is the major source of
dieldrin (up to 97%) in the environment. Aldrin and its reaction
product dieldrin are rapidly adsorbed on soils, especially soils
containing a high level of organic matter. Consequently there is
little penetration into the soil, and contamination of groundwater
does not generally occur. Transport of both compounds takes place
mainly through soil erosion (as wind drift) and sediment transport
(surface run-off), but not through leaching.
The use of aldrin and dieldrin in agriculture leads to residues
(mainly of dieldrin) in the soil that can persist for years; the
estimated half-life of dieldrin is between 4 and 7 years. Under
tropical conditions, the compounds are less persistent than under
temperate conditions.
Aldrin and dieldrin enter the atmosphere through volatilization
from treated crops and soil or, directly, during the application of
the pesticide. Dieldrin returns to soil and water surfaces by
washout and dry deposition. Thus, the compounds are found either
in the vapour phase (very low levels, in general 1 - 2 ng/m3),
adsorbed by dust particles, or in rainwater (of the order of
10 - 20 ng/litre).
The occurrence of dieldrin in the aquatic environment has been
reported by several authors. The concentrations in surface water
are mainly very low, less than 5 ng/litre. However, concentrations
in areas of soil erosion or agricultural use may be higher.
Sediment in rivers in these areas may contain up to 1 mg
dieldrin/kg. The high capacity for aquatic organisms to
concentrate dieldrin from very low levels in water could lead to
toxic levels in aquatic organisms. Concentration through aquatic
foodchains is of less importance than direct uptake from water.
Because of the widespread occurrence of dieldrin in the
environment and its persistence, there is a wide range of
concentrations in non-target organisms. Whereas the concentrations
previously ranged from 0.001 mg to 100 mg/kg tissue, they are now
mostly below 1 mg/kg tissue.
In terrestrial ecosystems, aldrin and dieldrin are accumulated
by a wide variety of organisms, principally as dieldrin. Dieldrin
is probably responsible for the deaths of mammals in the field and
for the decline in population size in some species, such as the
otter. Small mammals would be killed by eating dieldrin-dressed
grain, but populations of these animals are likely to have been
replenished by immigration from surrounding areas. Birds of prey
eating small mammals and small birds contaminated by dieldrin take
up and accumulate dieldrin in their own tissues and eggs.
Granivorous birds have been killed by eating dressed grain. It is
probable that the population decline in birds of prey was caused by
dieldrin residues (among other organochlorine residues) in their
tissues. The effects of dieldrin are seen some time after the
exposure, because residues are stored in fat over winter, to be
released in the spring. When dieldrin was used only at certain
times of the year, this did not prevent bird mortalities.
The widespread use of aldrin and dieldrin, in conjunction with
other organochlorine pesticides, has led to severe detrimental
effects on the environment, though with drastic curtailment of use,
particularly in seed dressings, there has been some recovery in
bird populations.
10.3. Conclusions
(a) Both aldrin and dieldrin have been subjected to intensive and
wide-ranging study, toxicologically, clinically, and
epidemiologically. The body burden is mainly the result of
the oral ingestion of residues in the diet (which seem
generally to fall within the promulgated ADIs) and, to a
lesser extent, of inhalation. Evaluation of the data suggests
strongly that the body burden resulting from the present level
of exposure constitutes no health risk to the general
population.
(b) Dieldrin occurs almost ubiquitously in human breast milk.
However, its concentration in the blood and adipose tissue of
suckling infants does not increase with age during the first
six months, nor is their blood dieldrin level higher than that
of bottle-fed babies. Under these circumstances, the benefits
of natural breast feeding still make it the preferred method
of infant feeding, in spite of the dieldrin residues.
(c) In the treatment of premises, notably for termite control, the
exposure of occupants does not appear to be increased to a
level that endangers their health, as long as the directions
for safe practice are conscientiously respected.
(d) Despite the highly toxic nature of aldrin and dieldrin, both
of these chemicals can be handled safely as long as the
recommended precautions to minimize worker exposure are always
observed. Neglect of these rules may lead to the poisoning of
operators.
(e) During the period of high aldrin and dieldrin use between 1950
and 1970, detrimental effects were undoubtedly inflicted upon
species in the environment. These effects were due partly to
dieldrin and partly to other organochlorines. Since the
drastic curtailment of the use of these materials, the
affected species have recovered in numbers.
11. RECOMMENDATIONS
1. A further, properly designed, teratogenic investigation is
required in the hamster, with dieldrin at realistic dose
levels.
2. Research into the mechanism of carcinogenesis should be
directed to explaining why the hepatic reaction in the mouse
is different from that of other species.
3. Dieldrin should be selected as an agent for further study of
neurotoxic mechanisms, both experimentally and clinically.
4. To protect the environment, large-scale use of aldrin and
dieldrin must not be resumed, and applications should be
confined to those situations in which no safer, equally
effective alternatives can be recommended.
5. For the health and welfare of workers and the general
population, the handling and application of aldrin and
dieldrin should only be entrusted to well trained competent
operators, who will follow adequate safety measures.
6. To avoid accidental poisoning from aldrin, especially among
children, the use of aldrin granules as an ant bait should be
forbidden.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Aldrin and dieldrin were evaluated by the FAO/WHO Joint
Meeting on Pesticide Residues (JMPR) in 1963, 1965, 1966, 1967,
1968, 1969, 1970, 1974, 1975, and 1977 (FAO/WHO, 1964, 1965a,b,
1967a,b, 1968a,b, 1969a,b, 1970a,b, 1971a,b, 1975a,b, 1978a,b).
From 1966 onwards, the JMPR established an acceptable daily intake
(ADI) of 0 - 0.0001 mg/kg body weight (combined total for aldrin
plus dieldrin). This was based on a level causing no toxicological
effect of:
0.5 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
rat; and
1 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
dog.
The maximum residue limits (MRLs) listed in Table 47 were
recommended by the FAO/WHO Joint Meeting on Pesticide Residues in
1970 and 1975 and are quoted as the sum of aldrin plus dieldrin.
Table 47. Maximum residue limits (MRL's) recommended by the Codex
Alimentarius Commission (FAO/WHO, 1986)
-------------------------------------------------------------------
Commodity Aldrin and dieldrin
(mg/kg)
-------------------------------------------------------------------
Potatoes 0.1
Fat of meat 0.2a
Carrots, lettuce, fat of meat 0.1a
Asparagus, aubergines, broccoli, Brussels 0.1
sprouts, cabbage, cauliflower, cucumbers,
horse radish, onions, parsnips, peppers,
pimentos, radishes, radish tops
Eggs (shell-free) 0.1a
Milk and milk products (fat basis) -
Milk 0.006a
Fruit 0.05
Rice (in husks) 0.02
Raw cereals (other than rice) 0.02a
-------------------------------------------------------------------
a Extraneous residue limit.
WHO (1984) recommended that the level of aldrin and dieldrin
in drinking-water should not exceed 0.03 µg/litre.
IARC evaluated aldrin and dieldrin on several occasions.
Aldrin and dieldrin were found to be carcinogenic in the liver in
mice, but there was no evidence for carcinogenicity in other
organs. The data available did not provide evidence of
carcinogenicity in rats. Data on dogs, monkeys, and human beings
were too limited to allow any conclusions (IARC, 1974). IARC
considered that there was inadequate evidence of carcinogenicity
in humans and limited evidence of carcinogenicity in experimental
animals. Accordingly, both chemicals were classified in Group 3
(IARC, 1987).
The Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified the acute hazard to
health for technical dieldrin as "highly hazardous" (WHO, 1988).
The same division published a data sheet on aldrin (79.41) and
dieldrin (75.17) (WHO/FAO, 1975-85).
REFERENCES
ABALIS, I.M., ELDERFRAWI, M.E., & ELDERFRAWI, A.T. (1985) High-
affinity stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic. Biochem. Physiol., 24: 95-102.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1966)
Organochlorine pesticides in the atmosphere. Nature (Lond.), 211:
259-261.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67.
Organochlorine pesticide residues in the total diet. J. Sci. Food
Agric., 20(4): 245-259.
ABBOTT, D.C., COLLINS, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71. Br.
med. J., 1972(2): 553-556.
ABBOTT, D.C., COLLINS, G.B., GOULDING, R., & HOODLESS, R.A. (1981)
Organochlorine pesticide residues in human fat in the United
Kingdom 1976-77. Br. med. J., 283: 1425-1428.
ACGIH (1986) TLVs. Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, 111 pp.
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fat.] Naturwissenschaften, 61: 32 (in German).
ACKER, L., BARKE, E., HAPKE, H.J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk.] Milchwissenschaft., 39(9): 541-544 (in German).
ADDISON, R.F., ZINCK, M.E., & ACKMAN, R.G. (1972) Residues of
organochlorine pesticides and polychlorinated biphenyls in some
commercially produced Canadian marine oils. J. Fish Res. Board
Can., 29: 349-355.
ADDISON, R.F., ZINCK, M.E., & LEAHY, J.R. (1976) Metabolism of
single and combined doses of 14C-aldrin and 3H-p,p'-DDT by
Atlantic salmon (Salmo salar) fry. J. Fish Res. Board Can., 33(9):
2073-2976.
ADEMA, D.M.M. & VINK, G.J. (1981) A comparative study of the
toxicity of 1,1,2-trichloroethane, dieldrin, pentachlorophenol, and
3,4-dichloroaniline for marine and fresh-water organisms.
Chemosphere, 10(6): 533-554.
AGNIHOTRI, N.P., PANDEY, S.Y., JAIN, H.K., & SRIVASTAVA, K.P.
(1977) Persistence of aldrin, dieldrin, lindane, heptachlor, and
p,p'-DDT in soil. J. entomol. Res., 1(1): 89-91.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977a) Pesticide induced
ouabain resistant mutants in Chinese hamster V-79 cells. Chem.-
biol. Interact., 19(3): 369-374.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977b) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res., 42:
161-174.
AKKERMANS, L.M.A. (1974) Mode of action of dieldrin. An electro-
physiological investigation, Utrecht, The Netherlands, Rijks
Universiteit (Thesis).
AKKERMANS, L.M.A., VAN DEN BERCKEN, J., & VERSLUYS-HELDER, M.
(1975) Excitatory and depressant effects of dieldrin and aldrin-
transdiol in the spinal cord of the toad (Xenopus laevis). Eur. J.
Pharmacol., 34: 133-142.
ALBERT, L., MENDEZ, F., CEBRIAN, M.E., & PORTALES, A. (1980)
Organochlorine pesticide residues in human adipose tissue in
Mexico: results of a preliminary study in three Mexican cities.
Arch. environ. Health, 35(5): 262-269.
ANAS, R.E. & WILSON, A.J. (1970a) Organochlorine pesticides in fur
seals. Pestic. monit. J., 3(4): 196-200.
ANAS, R.E. & WILSON, A.J. (1970b) Organochlorine pesticides in
nursing fur seal pups. Pestic. monit. J., 4(3): 114-116.
ANDERSON, B.G. (1960) The toxicity of organic insecticides to
Daphnia. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Proceedings of the 2nd Seminar, 1959, Cincinnati, Ohio,
Robert A. Taft Sanitary and Engineering Center, pp. 94-95
(Technical Report W60-3).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain
North American birds. In: Proceedings of the 15th International
Ornithology Congress, Leiden, Brill, pp. 514-540.
ANDERSON, P.D. & WEBER, L.J. (1975) Toxic response as a
quantitative function of body size. Toxicol. appl. Pharmacol., 33:
471-483.
ANON. (1964) Report on the Working Conference on Birds of Prey and
Owls, Caen, Normandy, 10-12 April, 1964, Washington, DC,
International Council for Bird Preservation.
ANON. (1973) Aldrin and the ultraviolet conversion products and
possible metabolites of aldrin and dieldrin in human milk from the
community studies on pesticides, Berkeley, California, Department
of Public Health, (Abstract prepared by Shell Development Company,
Modesto, California: Report TIR-24-123-73: Part II).
ANON. (1974) Data for pentachloroketone and UV-dieldrin in human
kidney and adipose tissue from Coral Gables, Florida, Modesto,
California, Shell Development Company (Report TIR-24-176-73: Part
II).
ANON. (1974b) Data for dieldrin, pentachloroketone, and UV-dieldrin
in human adipose tissue from Albany, New York, Modesto,
California, Shell Development Company (Report TIR-24-169-73: Part
II).
ANON. (1974c) Determination of dieldrin levels in human kidney and
adipose tissue, Modesto, California, Shell Development Company
(Report TIR-24-176-73).
ASHWOOD-SMITH, M.J. (1981) The genetic toxicology of aldrin and
dieldrin. Mutat. Res., 86: 137-154.
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ASTOLFI, E., GARCIA FERNANDEZ, J.C., DE JUAREZ, M.B., & PIACENTINO,
H. (1973) Chlorinated pesticides found in the fat of children in
the Argentine Republic. In: Deichmann, W.B., ed. Pesticides and the
environment, New York, Intercontinental Medical Book Company, pp.
233-243.
ASTOLFI, E., ALONSO, A.H., MENDIZABAL, A., & ZUBIZARRETTA, E.
(1974) Pesticides chlorés de l'accouchée et du cordon ombilical des
nouveaunés. J. eur. Toxicol., 7: 330-338.
ATKINS, D.H.F. & EGGLETON, A.E.J. (1970) Studies of atmospheric
washout and deposition of gamma-BHC, dieldrin, and p,p'-DDT using
radiolabelled pesticides, Harwell, United Kingdom Atomic Energy
Research Authority (Report HL 70/4532(C10)).
ATKINS, T.D. & LINDER, R.L. (1967) Effects of dieldrin on
reproduction of penned hen pheasants. J. wildl. Manage., 31(4):
746-753.
AULERICH, R.J., RINGER, R.K., & POLIN, D. (1972) Rate of
accumulation of chlorinated hydrocarbon pesticide residues in
adipose tissue of mink. Can. J. Zool., 50(9): 1167-1173.
AVAR, P. & CZEGLEDI-JANKO, G. (1970) Occupational exposure to
aldrin: clinical and laboratory findings. Br. J. ind. Med., 27(3):
279-282.
BAECKSTROEM, J., HANNSON, E., & ULLBERG, S. (1965) Distribution of
14C-DDT and 14C-dieldrin in pregnant mice determined by whole-body
autoradiography. Toxicol. appl. Pharmacol., 7: 90-96.
BAILEY, G.W., SWANK, R.R., Jr, & NICHOLSON, H.P. (1974) Predicting
pesticide runoff from agricultural land: a conceptual model. J.
environ. Qual., 3(2): 95-102.
BAKER, A.H., WHITNEY, G.F.H., & WORDEN, A.N. (1959) The toxic
hazard associated with continuous-flow heat-volatilized
insecticidal and acaracidal aerosols. Lab. Pract., 8: 3-10.
BAKKEN, A.F. & SEIP. M. (1976) Insecticides in human breast milk.
Acta paediatr. Scand., 65: 535-539.
BALAYANNIS, P.G. (1974) Organochlorine pesticide residues in
tomatoes Ann. Inst. Phytopathol. Benaki (N.S.), 11: 47-52.
BALDWIN, M.K. & ROBINSON, J. (1969) Metabolism in the rat of the
photoisomerization product of dieldrin. Nature (Lond.), 224(5216):
283-284.
BALDWIN, M.K., ROBINSON, J., & CARRINGTON, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Ind., 1970: 595-597.
BALDWIN, M.K., ROBINSON, J., & PARKE, D.V. (1972) A comparison of
the metabolism of HEOD (dieldrin) in the CF1 mouse with that in the
CFE rat. Food Cosmet. Toxicol., 10: 333-351.
BALDWIN, M.K., DAVIS, R.A., & THORBURN BURNS, D. (1973) Structural
studies and photochemical re-arrangement of an animal metabolite of
HEOD, the active component of dieldrin. Pestic. Sci., 4: 227-237.
BALDWIN, M.K., BENNETT, D., & BEYNON, K.I. (1977) The
concentrations of aldrin and dieldrin and their photoisomers in the
atmosphere. Pestic. Sci., 8: 431-445.
BALUJA, G., MURADO, M.A., & TEJEDOR, M.C. (1975) Adsorption and
desorption of lindane and aldrin by soils as affected by soil main
components. Environ. Qual. Saf., Suppl. 3: 243-249.
BALUJA, G., HERNANDEZ, L.M., GONZALEZ, J., & RICO, C. (1982)
Presence of organochlorine pesticides, polychlorinated biphenyls
and mercury in Spanish human milk samples. Bull. environ. Contam.
Toxicol., 28: 573-577.
BARLOW, F. & HADAWAY, A.B. (1955) Studies on aqueous suspensions of
insecticides V. The sorption of insecticides by soils. Bull.
entomol. Res., 46: 547-559.
BARLOW, F. & HADAWAY, A.B. (1956) Effects of changes in humidity on
the toxicity and distribution of insecticides sorbed by some dried
soils. Nature (Lond.), 178: 1299-1300.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D. (1979)
Organochlorine pesticide residues in human milk samples from women
living in northwest and northeast Mississippi, 1973-75. Pestic.
monit. J., 13(2): 47-51.
BARON, R.L. & WALTON, M.S. (1971) Dynamics of HEOD (dieldrin) in
adipose tissue of the rat. Toxicol. appl. Pharmacol., 18(4): 958-963.
BARQUET, A., MORGADE, C., & PFAFFENBERGER, C.D. (1981)
Determination of organochlorine pesticides and metabolites in
drinking-water, human blood-serum, and adipose tissue. J. Toxicol.
environ. Health, 7: 469-479.
BARRETT, R.T., SKAARE, J.U., NORHEIM, G., VADER, W., & FROSLIE, A.
(1985) Persistent organochlorines and mercury in eggs of Norwegian
seabirds 1983. Environ. Pollut. Ser. A, 39: 79-93.
BASSON, N.C.J. (1971) Effects of dieldrin and its photoisomerization
product photodieldrin on birds. Phytophylactica, 3: 115-124.
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1971) Growth
response of blue-green algae to aldrin, dieldrin, endrin, and their
metabolites. Bull. environ. Contam. Toxicol., 6(6): 589-594.
BAXTER, W.L., LINDER, R.L., & DAHLGREN, R.B. (1969) Dieldrin
effects in two generations of penned hen pheasants. J. wildl.
Manage., 33(1): 96-102.
BEALL, M.L., Jr & NASH, R.G. (1969) Crop seedling uptake of DDT,
dieldrin, endrin and heptachlor from soils. Agron. J., 61: 571-575.
BEALL, M.L., Jr & NASH, R.G. (1971) Organochlorine insecticide
residues in soybean plant tops: root vs vapour sorption. Agron. J.,
63: 460-464.
BEALL, M.L., Jr & NASH, R.G. (1972) Insecticide depth in soil -
Effect on soybean - Uptake in the greenhouse. J. environ. Qual.,
1(3): 283-288.
BEDFORD, C.T. (1974) Von Baeyer/IUPAC names and abbreviated
chemical names of metabolites and artifacts of aldrin (HHDN),
dieldrin (HEOD), and endrin. Pestic. Sci., 5: 473-489.
BEDFORD, C.T. & HARROD, R.K. (1972a) Synthesis of 9-hydroxy-HEOD,
a major mammalian metabolite of HEOD (dieldrin), Sittingbourne,
Shell Research, Tunstall Laboratory (TU/7/72).
BEDFORD, C.T. & HARROD, R.K. (1972b) An improved preparation of
trans-4,5-dihydroxy-4,5-dihydroaldrin, a metabolite of HEOD
(dieldrin) in mammals, insects, and microorganisms. Chemosphere,
1(6): 255-260.
BEDFORD, C.T. & SMITH, E.H. (1978) Synthesis of dieldrin
metabolites. III. Two-step conversion of syn-12-hydroxy dieldrin
into Klein's metabolite (3,5,6,6,7-pentachloro-11,12-exo-epoxy-
pentacyclo(6.4.0.02,10.03,7.05,9)-dodecan-4-one). J. agric. food
Chem., 26(4): 911-914.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the
aquatic environment: uptake and loss of DDT and dieldrin by fresh-
water mussels. Arch. environ. Contam. Toxicol., 1(2): 97-111.
BELISLE, A.A., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., MULHERN,
B.M., PROUTY, R.M., DEWOLF, R.B., & CROMARTIE, E. (1972) Residues
of organochlorine pesticides, polychlorinated biphenyls, and
mercury and autopsy data for bald eagles, 1969 and 1970. Pestic.
monit. J., 6(3): 133-138.
BELL, A. (1960) Aldrin poisoning: a case report. Med. J. Aust., 2:
698-700.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Ind. Med. Surg., 38(12): 50-52.
BENITZ, K.F., ROTH, R.N., & COULSTON, F. (1977) Morphologic
characteristics of hepatic nodules induced by mirex and dieldrin in
mice. Toxicol. appl. Pharmacol., 41: 154-155.
BENNINGTON, S.L., CONNORS, P.G., CONNORS, C.W., & RISEBROUGH, R.W.
(1975) Patterns of chlorinated hydrocarbon contamination in New
Zealand sub-antarctic and coastal marine birds. Environ. Pollut.,
8: 135-147.
BENSON, W.R. (1969) Note on nomenclature of dieldrin and related
compounds. J. Assoc. Off. Agric. Chem., 52: 1109-1112.
BESS, H.A. & HYLIN, J.W. (1970) Persistence of termiticides in
Hawaiian soils. J. econ. Entomol., 63(2): 633-638.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972a)
Organochlorine pesticide residues in water, sediment, algae, and
fish: Hawaii 1970-71. Pestic. monit. J., 6(1): 56-64.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972b) Organochlorine
pesticides in rainwater: Oahu, Hawaii 1971-72. Bull. environ.
Contam. Toxicol., 8(4): 238-241.
BEYER, W.N. & GISH, C.D. (1980) Persistence in earthworms and
potential hazards to birds of soil applied DDT, dieldrin, and
heptachlor. J. appl. Ecol., 17(2): 295-307.
BEYERMANN, K. & ECKRICH, W. (1973) [Gas-chromatographic
determination of insecticide traces in air.] Z. anal. Chem.,
265(1): 4-7 (in German).
BEYNON, K.I. & ELGAR, K.E. (1966) The analysis for residues of
chlorinated insecticides and acaricides. Analyst, 91(1080):
143-175.
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1127-1130.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDWELL, K., WEBER, E., NIENHOLD, I., CONNOR, T., & LEGATOR, M.S.
(1975) Comprehensive evaluation for mutagenic activity of dieldrin.
Mutat. Res., 31: 314 (Abstract).
BIJLEVELD, M. (1974) Birds of prey in Europe, London, MacMillan
Press, pp. XI and 263.
BLACK, A.M.S. (1974) Self poisoning with dieldrin: a case report
and pharmacokinetic discussion. Anaesth. intensive Care, 2: 369-374.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin, and endrin. Arch. environ. Contam.
Toxicol., 7: 83-98.
BLUS, L.J. (1982) Further interpretation of the relation of
organochlorine residues in brown pelican eggs to reproductive
success. Environ. Pollut. Ser. A, 28: 15-33.
BLUS, L.J., NEELY, B.S., Jr, BELISLE, A.A, & PROUTY, R.M. (1974a)
Organochlorine residues in brown pelican eggs: relation to
reproductive success. Environ. Pollut., 7: 81-91.
BLUS, L.J., BELISLE, A.A, & PROUTY, R.M. (1974b) Relations of the
brown pelican to certain environmental pollutants. Pestic. monit.
J., 7(3/4): 181-194.
BLUS, L.J., JOANEN, T., BELISLE, A.A., & PROUTY, R.M. (1975) The
brown pelican and certain environmental pollutants in Louisiana.
Bull. environ. Contam. Toxicol., 13(6): 646-655.
BLUS, L.J., NEELY, B.S., Jr, LAMONT, T.G., & MULHERN, B. (1977)
Residues of organochlorines and heavy metals in tissues and eggs of
brown pelicans, 1969-73. Pestic. monit. J., 11(1): 40-53.
BLUS, L.J., CROMARTIE, E., MCNEASE, L., & JOANEN, T. (1979a) Brown
pelican: population status, reproductive success, and
organochlorine residues in Louisiana, 1971-76. Bull. environ.
Contam. Toxicol., 22: 128-135.
BLUS, L.J., LAMONT, T.G., & NEELY, B.S., Jr (1979b) Effects of
organochlorine residues on eggshell thickness, reproduction, and
population status of brown pelicans (Pelicanus occidentalis) in
South Carolina and Florida, 1969-76. Pestic. monit. J., 12(4):
172-184.
BORDON, L. (1980) Possible effects of dieldrin and other pesticides
on human chromosomes in vivo and in vitro. Toxicol. Res. Proj.
Dir., 5: 11-12 (No. 11.0077).
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., & PANKASKIE, J.E.
(1952a) Toxicological studies of aldrin on small laboratory
animals, Cincinnati, Ohio, Kettering Laboratory.
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., PANKASKIE, J.E., &
DUTRA, F.R. (1952b) Toxicological studies of dieldrin on small
laboratory animals, Cincinnati, Ohio, Kettering Laboratory.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BRAGT, P.C., SCHUURBIERS, C.J., HOLLANDER, J.C.TH., SCHULTING,
F.L., & WOLTHUIS, O.L. (1984) Retention of inhaled aldrin in man,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
BRESLER, E. & HANKS, R.J. (1969) Numerical method for estimating
simultaneous flow of water and salt in unsaturated soils. Soil Sci.
Soc. Am. Proc., 33: 827-839.
BREWERTON, H.V. & MCGRATH, H.J.W. (1967) Insecticides in human fat
in New Zealand. N. Z. J. Sci., 10: 486-492.
BRIGGS, G.G. (1981) Theoretical and experimental relationships
between soil adsorption, octanol/water partition coefficients,
water solubilities, bioconcentration factors, and the parachor. J.
agric. food Chem., 29: 1050-1059.
BRODTMANN, N.V., Jr (1976) Continuous analysis of chlorinated
hydrocarbon pesticides in the Lower Mississippi River. Bull.
environ. Contam. Toxicol., 15(1): 33-39.
BROOKS, G.T. (1974) Chlorinated insecticides. I. Technology and
applications, Cleveland, Ohio, CRC Press, pp. 87-99.
BRO-RASMUSSEN, F., DALGAARD-MIKKELSEN, Sv., JAKOBSON, Th.,
KOCH, Sv.O., RODIN, F., UHL, E., & VOLDUM-CLAUSEN, K. (1968)
Examinations of Danish milk and butter for contaminating
organochlorine insecticides. Residue Rev., 23: 55-69.
BROWN, J.R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc. J., 97: 367-373.
BROWN, L., BELLINGER, E.G., & DAY, J.P. (1979) Dieldrin pollution
in the River Holme catchment, Yorkshire. Environ. Pollut., 18:
203-211.
BROWN, L.H. (1969) Status and breeding success of golden eagles in
northwest Sutherland in 1967. Br. Birds, 62(9): 345-363.
BROWN, V.K.H., HUNTER, C.G., & RICHARDSON, A. (1964) A blood test
diagnostic of exposure to aldrin and dieldrin. Br. J. ind. Med., 21:
283-286.
BROWN, V.K.H., RICHARDSON, A., ROBINSON, J., & STEVENSON, D.E.
(1965) The effects of aldrin and dieldrin on birds. Food Cosmet.
Toxicol., 3: 675-679.
BROWN, V.K.H., ROBINSON, J., & RICHARDSON, A. (1967) Preliminary
studies on the acute and subacute toxicities of a
photoisomerization product of HEOD. Food Cosmet. Toxicol., 5:
771-779.
BROWN, V.K.H., ROBINSON, J., THORPE, E., & BARRETT, J.W. (1974) The
toxicity of dieldrin (HEOD) to domestic fowl. Pestic. Sci., 5:
567-586.
BRUCE, W.N. & DECKER, G.C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin,
dieldrin, heptachlor, and heptachlor epoxide. J. agric. food Chem.,
14(4): 395-398.
BUECHEL, K.H., GINSBERG, A.E., & FISCHER, R. (1966) [Synthesis and
structure of chlordane isomers.] Chem. Ber., 99: 421-430 (in German).
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (1967) Chlorinated
pesticide levels in eastern oyster (Crassostrea virginica) from
selected areas of the South Atlantic and Gulf of Mexico. Pestic.
monit. J., 1(3): 9-12.
BUNCH, T.D. & LOW, J.B. (1973) Effects of dieldrin on chromosomes
of semi-domestic mallard ducks. J. wildl. Manage., 37(1): 51-57.
BUNYAN, P.J. & STANLEY, P.I. (1982) Toxic mechanisms in wildlife.
Regul. Toxicol. Pharmacol., 2: 106-145.
BURKE, H.R. (1959) Toxicity of several insecticides to two species
of beneficial insects on cotton. J. econ. Entomol., 52(4): 616-618.
BURNS, B.G., PEACH, M.E., & STILES, D.A. (1975) Organochlorine
pesticide residues in a farming area, Nova Scotia, 1972-73. Pestic.
monit. J., 9(1): 34-38.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue - Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURNS, K.A. (1976) Microsomal mixed-function oxidases in an
estuarine fish Fundulus heteroclitus and their induction as a
result of environmental contamination. Comp. Biochem. Physiol., 53B:
443-446.
BUTLER, P.A. (1971) Influence of pesticides on marine ecosystems.
Proc. R. Soc. Lond., B177: 321-329.
BUTLER, P.A. (1973) Organochlorine residues in estuarine mollusks,
1965-72. Pestic. monit. J., 6(4): 238-362.
CABRAL, J.R.P., RAITANO, F., MOLLNER, T., BRONCZYK, S.A., & SHUBIK,
P. (1979a) Acute toxicity of pesticides in hamsters. Toxicol. appl.
Pharmacol., 48: A192 (Abstract 384).
CABRAL, J.R.P., HALL, R.K., BRONCZYK, S.A., & SHUBIK, P. (1979b) A
carcinogenicity study of the pesticide dieldrin in hamsters. Cancer
Lett., 6(4/5): 241-246.
CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1968) Peregrines and
pesticides in Alaska. Condor, 70: 170-178.
CAIRNS, J., Jr, FOSTER, N.R., & LOOS, J.J. (1967) Effects of
sublethal concentrations of dieldrin on laboratory populations of
guppies ( Poecilia reticulata Peters). Proc. Natl Acad. Sci.
Philadelphia, 119(3): 75-91.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs on
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11(1): 70-77.
CAMPBELL, M.A., GYORKOS, J., LELCI, B., HOMONKO, K., & SAFE., S.
(1983) The effects of twenty-two organochlorine pesticides as
inducers of the hepatic drug-metabolizing enzymes. Gen. Pharmac.,
14(4): 445-454.
CAREY, A.E. & KUTZ, P.W. (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ. Monit. Assess., 5(2): 155-163.
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the Corn
Belt Region, United States, 1970. Pestic. monit. J., 6(4): 369-376.
CAREY, A.E., WIERSMA, G.B., & TAI, H. (1976) Pesticide residues in
urban soils from 14 United States cities, 1970. Pestic. monit. J.,
10(2): 54-60.
CAREY, A.E., YANG, H.S.C., WIERSMA, G.B., TAI, H., MAXEY, R.A., &
DUPUY, A.E., Jr (1980) Residual concentrations of propanil, TCAB,
and other pesticides in rice-growing areas in the United States,
1972. Pestic. monit. J., 14(1): 23-25.
CARLSON, G.P. (1974) Epoxidation of aldrin to dieldrin by lobsters.
Bull. environ. Contam. Toxicol., 11(6): 577-582.
CARNAGHAN, R.B.A. & BLAXLAND, J.D. (1957) The toxic effect of
certain seed dressings on wild and game birds. Vet. Rec., 69:
324-325.
CARO, J.H. & TAYLOR, A.W. (1971) Pathways of loss of dieldrin from
soil under field conditions. J. agric. food Chem., 19(2): 379-384.
CARO, J.H., TAYLOR, A.W., & FREEMAN, H.P. (1976) Comparative
behaviour of dieldrin and carbofuran in the field. Arch. environ.
Contam. Toxicol., 3: 437-447.
CARTER, F.L. & STRINGER, C.A. (1970) Soil moisture and soil type
influence initial penetration by organochlorine insecticides. Bull.
environ. Contam. Toxicol., 5(5): 422-428.
CASARETT, L.J., FRYER, G.C., YAUGER, W.L., Jr, & KLEMMER, H.W.
(1968) Organochlorine pesticide residues in human tissue - Hawaii.
Arch. environ. Health, 17: 306-311.
CASSIDY, W., FISHER, A.J., PEDEN, J.D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset,
London, United Kingdom, Ministry of Health, Public Health
Laboratory Services, Vol. 26, pp. 2-6 (Monthly Bulletin).
CATHEY, B. (1982) Comparative toxicities of five insecticides to
the earthworm (Lumbricus terrestris). Agric. Environ., 7: 73-81.
CAUSEY, M.K., BONNER, F.L., & GRAVES, J.B. (1968) Dieldrin residues
in the gallinules Porphyrula martinica L. and Gallinula chloropas
L. and its effect on clutch size and hatchability. Bull. environ.
Contam. Toxicol., 3(5): 274-283.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation into the connection between the diet and living
conditions of nursing mothers and the contamination of breast milk
with sparingly volatile organochloride compounds.] Act. Ernähr., 9:
157-162 (in German).
CHACKO, C.I. & LOCKWOOD, J.L. (1967) Accumulation of DDT and
dieldrin by microorganisms. Can. J. Microbiol., 13: 1123-1126.
CHADWICK, G.G. & BROCKSEN, R.W. (1969) Accumulation of dieldrin by
fish and selected fish-food organisms. J. wildl. Manage., 33(3):
693-700.
CHADWICK, G.G. & SHUMWAY, D.L. (1970) Effects of dieldrin on the
growth and development of steelhead trout. In: Gillett, J.W., ed.
The biological impact of pesticides in the environment, Corvallis,
Oregon State University, pp. 90-96 (Environmental Health Series
No. 1).
CHAN, T.M., GILLETT, J.W., & TERRIERE, L.C. (1967) Interaction
between microsomal electron transport systems of trout and male rat
in cyclodiene epoxidation. Comp. Biochem. Physiol., 20: 731-742.
CHANIN, P.R.F. & JEFFERIES, D.J. (1978) The decline of the otter
Lutra lutra L. in Britain: an analysis of hunting records and
discussion of causes. Biol. J. Linn. Soc., 10: 305-328.
CHAU, A.S.Y. & COCHRANE, W.P. (1970) Cis-opening of dieldrin
oxirane ring. Chem. Ind., 49: 1568-1569.
CHAUDRY, M.M., NELSON, A.I., & PERKINS, E.G. (1978) Distribution of
chlorinated pesticides in soybeans, soybean oil, and its by-
products during processing. J. Am. Oil Chem. Soc., 55(12): 851-853.
CHERNOFF, N., KAVLOCK, R.J., KATHREIN, J.R., DUNN, J.M., & HASEMAN,
J.K. (1975) Prenatal effects of dieldrin and photodieldrin in mice
and rats. Toxicol. appl. Pharmacol., 31: 302-308.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1981)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avian and mammalian wildlife
toxicology. Second Conference, Philadelphia, Pennsylvania,
American Society of Testing Materials, pp. 143-154 (STP757).
CLARK, D.R., Jr (1975) Effect of stress on dieldrin toxicity to
male redwinged blackbirds (Agelaius phoeniceus). Bull. environ.
Contam. Toxicol., 14(2): 250-256.
CLARK, D.R., Jr (1981) Death in bats from DDE, DDT or dieldrin:
diagnosis via residues in carcass fat. Bull. environ. Contam.
Toxicol., 26: 367-374.
CLARK, D.R., Jr & KROLL, C.J. (1977) Effects of DDE on
experimentally poisoned free-tailed bats (Tadarida brasiliensis):
lethal brain concentrations. J. Toxicol. environ. Health, 3: 893-901.
CLARK, D.R., Jr & MCLANE, M.A.R. (1974) Chlorinated hydrocarbon and
mercury residues in woodcock in the United States, 1970-71. Pestic.
monit. J., 8(1): 15-22.
CLARK, D.R., Jr & PROUTY, R.M. (1984) Disposition of dietary
dieldrin in the little brown bat and correlation of skin levels
with body burden. Bull. environ. Contam. Toxicol., 33: 177-183.
CLARK, D.R., Jr, LAVAL, R.K., & SWINEFORD, D.M. (1978) Dieldrin-
induced mortality in an endangered species, the gray bat (Myotis
grisescens). Science, 199: 1357-1359.
CLARK, D.R., Jr, LAVAL, P.K., & KRYNITSKY, A.J. (1980) Dieldrin and
heptachlor residues in dead gray bats, Franklin County, Missouri -
1976 versus 1977. Pestic. monit. J., 13(4): 137-140.
CLARK D.R., Jr, CLAWSON, R.L., & STAFFORD, C.J. (1983a) Gray bats
killed by dieldrin at two additional Missouri caves: aquatic
macroinvertebrates found dead. Bull. environ. Contam. Toxicol.,
30: 214-218.
CLARK, D.R., Jr, BUNCK, C.M., CROMARTIE, E., & LAVAL, R.K. (1983b)
Year and age effects on residues of dieldrin and heptachlor in dead
gray bats, Franklin County, Missouri - 1976, 1977, and 1978.
Environ. Toxicol. Chem., 2: 387-393.
CLEVELAND, F.P. (1966) A summary of work on aldrin and dieldrin
toxicity at the Kettering Laboratory. Arch. environ. Health, 13:
195-198.
COHEN, J.M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rain-out. In: Bould, R.F., ed.
Organic pesticides in the environment, Washington, DC, American
Chemical Society, pp. 163-176 (Advances in Chemistry Series No.
60).
COLE, J.F., KLEVAY, L.M., & ZAVON, M.R. (1970) Endrin and dieldrin:
a comparison of hepatic excretion in the rat. Toxicol. appl.
Pharmacol., 16: 547-555.
COLLINS, G.B., HOLMES, D.C., & HOODLESS, R.A. (1982) Organochlorine
pesticide residues in human milk in Great Britain, 1979-1980. Hum.
Toxicol., 1: 425-431.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Egg shell
characteristics and incidence of shell breakage for grey herons
Ardea cinerea exposed to environmental pollutants. Environ.
Pollut., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides, and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
Monks Wood Experimental Station.
COPPLESTONE, J.F., HUNNEGO, J.N., & HARRISON, D.L. (1973)
Organochlorine insecticide levels in adult New Zealanders - a five-
year study. N. Z. J. Sci., 16: 27-39.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet samples
(V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet samples
(VI). Pestic. monit. J., 5(4): 313-330.
COULSTON, F., ABRAHAM, R., & MANKES, R. (1980) Reproductive study
in female rats given dieldrin, alcohol, or aspirin orally, Albany,
New York, Albany Medical College of Union University, Institute of
Comparative and Human Toxicology.
COWAN, A.A. (1981) Organochlorine compounds in mussels from
Scottish coastal waters. Environ. Pollut. Ser. B, 2: 129-143.
CRAIG, N.C.D. (1977) A summary of the data on the toxicity of
various materials to aquatic life. III. Dieldrin, aldrin, and
endrin, Brixham United Kingdom, Imperial Chemical Industries Ltd
(Unpublished Report BL/A/1828).
CRAWFORD, N.H. & DONIGIAN, A.S., Jr (1973) Pesticide transport and
runoff model for agricultural lands, Washington, DC, US
Environmental Protection Agency (EPA-600/2-74-013).
CREBELLI, R., BELLINCAMPI, D., CONTI, G., MORPURGO, G., & CARERE,
A. (1986) A comparative study on selected chemical carcinogens for
chromosome malsegregation, mitotic crossing-over and forward
mutation induction in Aspergillus nidulans. Mutat. Res., 172:
139-149.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND, P.F.,
& CAREY, A.E. (1974) Pesticide residue levels in soils and crops:
FY-70 - National Soils Monitoring Program. II. Pestic. monit. J.,
8(2): 69-97.
CROLL, B.T. (1969) Organochlorine insecticides in water. Part I.
Proc. Soc. Water Treat. Exam., 18: 255-274.
CROMARTIE, E., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., KAISER,
T.E., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., & SWINEFORD, D.M.
(1975) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1971-72. Pestic. monit.
J., 9(1): 11-14.
CUETO, C., Jr & BIROS, F.J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol., 10:
261-269.
CUETO, C., Jr & HAYES, W.J., Jr (1962) The detection of dieldrin
metabolites in human urine. J. agric. food Chem., 10: 366-369.
CUMMINGS, J.G. (1966) Pesticides in the total diet. Residue Rev.,
16: 30-45.
CUMMINGS, J.G., ZEE, K.T., TURNER, V., & QUINN, F. (1966) Residues
in eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Assoc. Off. Agric. Chem., 49(2): 354-364.
CURLEY, A., COPELAND, M.F., & KIMBROUGH, R.D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242(5396): 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1970) Eggshell thickness in
pheasants given dieldrin. J. wildl. Manage., 34(1): 226-228.
DAHLGREN, R.B. & LINDER, R.L. (1974) Effects of dieldrin in penned
pheasants through the third generation. J. wildl. Manage., 38(2):
320-330.
DAILEY, R.E., WALTON, M.S., BECK, V., LEAVENS, C.L., & KLEIN, A.K.
(1970) Excretion, distribution, and tissue storage of a 14C-
labelled photoconversion product of 14C-dieldrin. J. agric. food
Chem., 18(3): 443-445.
DALE, W.E. & QUINBY, G.E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W.E., COPELAND, M.F., & HAYES, W.J., Jr (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World Health
Organ., 33: 471-477.
DALE, W.E., CURLEY, A., & CUETO, C., Jr (1966) Hexane extractable
chlorinated insecticides in human blood. Life Sci., 5: 47-54.
DAMICO, J.N., CHEN, J.Y.T., COSTELLO, C.E., & HAENNI, E.O. (1968)
Structure of Klein's metabolites of aldrin and of dieldrin. J.
Assoc. Off. Agric. Chem., 51(1): 48-55.
DANIELS, N.E. (1966) Soil insect control and insecticidal residue
detection. J. econ. Entomol., 59: 410-413.
DATTA, P.R., LANG, E.P., WATTS, J.O., KLEIN, A.K., & NELSON, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature (Lond.), 208: 289-290.
DAVIDSON, J.M. & MCDOUGAL, J.R. (1973) Experimental and predicted
movement of three herbicides in water-saturated soil. J. environ.
Qual., 2(4): 428-433.
DAVIES, J.E., BARQUET, A., MORGADE, C., & RAFFONELLI, A. (1975)
Epidemiological studies of DDT and dieldrin residues and their
relationship to human carcinogenesis. In: Proceedings of the
International Symposium on Recent Advances in Assessment of the
Health Effects of Environmental Pollution, Luxembourg, Commission
of the European Communities, Vol. 2, pp. 695-705.
DAVIS, B.N.K. (1968) The soil macrofauna and organochlorine
insecticide residues in twelve agricultural sites near Huntingdon.
Ann. appl. Biol., 61: 29-45.
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin
and DDT by earthworms. Soil Biol. Biochem., 3: 221-233.
DAVIS, H.C. & HIDU, H. (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish. Bull., 67(2): 393-404.
DAVIS, K.J. & FITZHUGH, O.G. (1962) Tumorigenic potential of aldrin
and dieldrin for mice. Toxicol. appl. Pharmacol., 4: 187-189.
DAVIS, K.J., HANSEN, W., & FITZHUGH, O.G. (1965) Pathology report
on mice fed aldrin, dieldrin, heptachlor, or heptachlor epoxide for
two years, Washington, DC, US Environmental Protection Agency
(Memorandum to Dr A.J. Lehman, 19th July, 1965. Publicly presented
and accepted at the US EPA Aldrin-Dieldrin Suspension Hearing,
Statement of Testimony from Dr K.J. Davis, August 1974).
DAVIS, P.W., FRIEDHOFF, J.M., & WEDEMEYER, G.A. (1972)
Organochlorine insecticide, herbicide, and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8(2): 69-72.
DAVISON, K.L. (1970) Dieldrin accumulation in tissues of the sheep.
J. agric. food Chem., 18(6): 1156-1160.
DAVISON, K.L. (1973) Dieldrin-14C balance in rats, sheep, and
chickens. Bull. environ. Contam. Toxicol., 10(1): 16-24.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p'-DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol., 7(1): 9-18.
DAWSON, R. & RILEY, J.P. (1977) Chlorine-containing pesticides and
polychlorinated biphenyls in British coastal waters. Estuarine
coastal mar. Sci., 4: 55-69.
DEAN, B.J., DOAK, S.M.A., & SOMERVILLE, H. (1975) The potential
mutagenicity of dieldrin (HEOD) in mammals. Food Cosmet. Toxicol.,
13: 317-323.
DE CAMPOS, M. & OLSZYNA-MARZYS, A.E. (1979) Contamination of human
milk with chlorinated pesticides in Guatemala and in El Salvador.
Arch. environ. Contam. Toxicol., 8: 43-58.
DECKER, G.C., BRUCE, W.N., & BIGGER, J.H. (1965) The accumulation
and dissipation of residues resulting from the use of aldrin in
soils. J. econ. Entomol., 58(2): 266-271.
DEFLORA, S. (1981) Study of 106 organic and inorganic compounds in
the Salmonella/microsome test. Carcinogenesis, 2(4): 283-298.
DEICHMANN, W.B. (1974) Certified statement of testimony before the
Environmental Protection Agency at aldrin/dieldrin suspension
hearings, Washington, DC, US Environmental Protection Agency.
DEICHMANN, W.B. & MACDONALD, W.E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-72. Ecotoxicol.
environ. Saf., 1: 89-110.
DEICHMANN, W.B., KEPLINGER, M.L., SALA, F., & GLASS, E. (1967)
Synergism among oral carcinogens. IV. The simultaneous feeding of
four tumorigens to rats. Toxicol. appl. Pharmacol., 11: 88-103.
DEICHMANN, W.B., DRESSLER, I., KEPLINGER, M., & MACDONALD, W.E.
(1968) Retention of dieldrin in blood, liver, and fat of rats fed
dieldrin for six months. Ind. Med. Surg., 37: 837-839.
DEICHMANN, W.B., KEPLINGER, M., DRESSLER, I., & SALA, F. (1969)
Retention of dieldrin and DDT in the tissues of dogs fed aldrin and
DDT individually and as a mixture. Toxicol. appl. Pharmacol., 14:
205-213.
DEICHMANN, W.B., MACDONALD, W.E., BLUM, E., BEVILACQUA, M.,
RADOMSKI, J., KEPLINGER, M., & BALKUS, M. (1970) Tumorigenicity of
aldrin, dieldrin, and endrin in the albino rat. Ind. Med., 39(10):
314, 426-434.
DEICHMANN, W.B., MACDONALD, W.E., BEASLY, A.G., & CUBIT, D.A.
(1971) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg., 40(2): 10-22.
DEICHMANN, W.B., MACDONALD, W.E., CUBIT, D.A., & BEASLY, A.G.
(1972) Effects of starvation in rats with elevated DDT and dieldrin
tissue levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEICHMANN, W.B., MACDONALD, W.E., & CUBIT, D.A. (1975) Dieldrin and
DDT in the tissues of mice fed aldrin and DDT for seven
generations. Arch. Toxicol., 34: 173-182.
DEICHMANN, W.B., MACDONALD, W.E., & LU, F.C. (1979) Effects of
chronic aldrin feeding in two strains of female rats, and a
discussion on the risks of carcinogens in man. In: Deichmann, W.B.,
ed. Toxicology and occupational medicine, Amsterdam, Elsevier/North
Holland, pp. 407-413.
DEJONCKHEERE, W., STEURBAUT, W., VERSTRAETEN, R., & KIPS, R.H.
(1977) Residues of organochlorine pesticides in human fat in
Belgium. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 42(2): 1839-1847.
DE LLAMAS, M.C., DE CASTRO, A.C., & PECHEN DE D'ANGELO, A.M. (1985)
Cholinesterase activities in developing amphibian embryos following
exposure to the insecticides dieldrin and malathion. Arch environ.
Contam. Toxicol., 14: 161-166.
DEL VECCHIO, V. & LEONI, V. (1967) [Detection and measurement of
chlorinated insecticides in biological material.] Nuovi Ann. Ig.
Microbiol., 28(2): 107-128 (in Italian).
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
D'ERCOLE, A.J., ARTHUR, R.D., CAIN, J.D., & BARRENTINE, B.F. (1976)
Insecticide exposure of mothers and newborns in a rural
agricultural area. Pediatrics, 57(6): 869-874.
DE VLIEGER, M., ROBINSON, J., BALDWIN, M.K., CRABTREE, A.N., & VAN
DIJK, M.C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRYNS, J.K., MUYS,
T., & POLL, J.M., VAN DER (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
chem. Toxicol., 22(1): 11-21.
DICK, G.L., HEENAN, M.P., LOVE, J.L., UDY, P.B., & DAVIDSON, F.
(1978) Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus pesticide
residue content. N. Z. J. Sci., 21: 71-78.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DISLER, R.L., GLATT, V., & MEIER, W. (1984) [Residues of
chlorinated insecticides and polychlorinated biphenyls in breast
milk.] Mitt. Geb. Lebensm. Hyg., 75: 205-213 (in German).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health, 4(7, suppl.):
140-146.
DIX, K.M. & WILSON, A.B. (1971) Toxicity studies with dieldrin
(HEOD). Teratogenic studies in rabbits given HEOD and thalidomide
orally, Sittingbourne, Shell Research (TLGR.0051.71).
DIX, K.M., VAN DER PAUW, C.L., & MCCARTHY, W.V. (1978) Toxicity
studies with dieldrin: teratological studies in mice dosed orally
with HEOD. Teratology, 16: 57-62.
DOBBS, A.J. & WILLIAMS, N. (1983) Indoor air pollution from
pesticides used in wood remedial treatments. Environ. Pollut.
Ser. B, 6: 271-296.
DOBSON, R.C. & BAUGH, E.R. (1976) Dieldrin residue removal from the
fat of swine. Bull. environ. Contam. Toxicol., 16(5): 567-571.
DOUABUL, A.A.Z., AL-SAAD, H.T., & AL-REKABI, H.N. (1987) Residues
of organochlorine pesticides in environmental samples from the
Shatt al-Arab river, Iraq. Environ. Pollut., 43: 175-187.
DRAPER, W.M. & CROSBY, D.G. (1984) Solar photooxidation of
pesticides in dilute hydrogen peroxide. J. agric. food Chem., 32:
231-237.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic
provinces of Canada. J. agric. food Chem., 15(3): 457-464.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States. III. June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E. & LIPSCOMB, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States. II. June 1966 - April 1968. Pestic.
monit. J., 2(4): 153-162.
DUGGAN, R.E., BARRY, H.C., & JOHNSON, L.Y. (1967) Pesticide
residues in total diet samples. II. Pestic. monit. J., 1(2): 2-12.
ECKENHAUSEN, F.W., BENNET, D., BEYNON, K.I., & ELGAR, K.E. (1981)
Organochlorine pesticide concentrations in perinatal samples from
mothers and babies. Arch. environ. Health, 36(2): 81-92.
EDMUNDSON, W.F., DAVIES, J.E., & HULL, W. (1968) Dieldrin storage
levels in necropsy adipose tissue from a south Florida population.
Pestic. monit. J., 2(2): 86-89.
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Oxford, Blackwell, pp. 239-261.
EDWARDS, C.A. (1966) Insecticide residues in soil. Residue Rev., 13:
83-132.
EDWARDS, C.A. (1973a) Persistent pesticides in the environment,
Cleveland, Ohio, CRC Press, pp. 68-74.
EDWARDS, C.A. (1973b) Persistent pesticides in soil and water. In:
Environmental pollution by pesticides, London, Plenum Press, pp.
409-458.
EDWARDS, C.A. & LOFTY, J.R. (1977) Biology of earthworms, 2nd ed.,
London, Chapman and Hall, pp. 212-213.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the soil
fauna. Residue Rev., 45: 8-15.
EDWARDS, C.A., DENNIS, E.B., & EMPSON, D.W. (1967) Pesticides and
the soil fauna: effects of aldrin and DDT in an arable field. Ann.
appl. Biol., 60: 11-22.
EEC (1983) Directives relating to the classification and labelling
of dangerous substances. Annex VI. Part II (2.4.10) to the fifth
adaptation to the Dangerous Substances Directives, Luxembourg,
European Economic Community (Commission Directive 83/467/EEC)
(Official Journal L 257/19).
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J.O'G. (1965)
Organochlorine pesticide residues in human fat and human milk. Br.
med. J., 2: 66-69.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5: 541-544.
EISENLORD, G., LOQUVAM, G.S., & NEMENZO, J. (1967) Results of
reproduction study of rats fed diets containing dieldrin over three
generations, San Francisco, California, The Hine Laboratories
(Report No. 4).
EISLER, R. (1969) Acute toxicities of insecticides to marine
decapod crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Latent effects of insecticide intoxication to
marine molluscs. Hydrobiologia, 36(3/4): 345-352.
EL BEIT, I.O.D. (1981) Pesticide microbial interactions in the
soil. Int. J. environ. Stud., 16: 171-181.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981a) Factors
involved in the dynamics of pesticides in soils: the effect of
pesticide concentration on leachability and absorption. Int. J.
environ. Stud., 16: 181-187.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981b) Factors
involved in the dynamics of pesticides in soils: the effects of
temperature and period of contact or leachability and adsorption of
pesticides by soils. Int. J. environ. Stud., 16: 189-196.
EL BEIT, I.O.D., COTTON, D.E., & WHEELOCK, J.V. (1983) Persistence
of pesticides in soil leachates: effects of pH, ultraviolet
irradiation, and temperature. Int. J. environ. Stud., 21: 251-259.
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. (1975) The dissipation and accumulation of aldrin and
dieldrin residues in soil. Environ. Qual. Saf., 3(suppl.): 250-257.
ELGAR, K.E. (1979) The variability of residue results, with
particular reference to the Codex study on organochlorines in
butterfat. In: Proceedings of the 4th IUPAC Congress on Pesticide
Chemistry, Zurich, 1978, pp. 668-672.
ELIASON, B.C. & POSNER, H.S. (1971) Reduced passage of carbon-14-
dieldrin to the fetal rat by phenobarbital but not by eight other
drugs or dieldrin. Am. J. Obstet. Gynecol., 110(7): 943-947.
ELY, R.E., MOORE, L.A., HUBANKS, P.E., CARTER, R.H., & POOS, F.W.
(1954) Studies of feeding aldrin to dairy cows. J. dairy Sci., 37(3):
294-298.
EMANUELSEN, M., LINCER, J.L., & RIFKIN, E. (1978) The residue
uptake and histology of American oysters ( Crassostrea virginica
Gmelin) exposed to dieldrin. Bull. environ. Contam. Toxicol., 19:
121-129.
ENDERSON, J.H. & BERGER, D.D. (1968) Chlorinated hydrocarbon
residues in peregrines and their prey species from northern Canada.
Condor, 70: 149-153.
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning
and lowered production of young in prairie falcons. Bioscience,
20(6): 355-356.
EPIFANIO, C.E. (1973) Dieldrin uptake by larvae of the crab
Leptodius floridanus. Mar. Biol., 19(4): 320-322.
EPSTEIN, E. & GRANT, W.J. (1968) Chlorinated insecticides in runoff
water as affected by crop rotation. Soil Sci. Soc. Am. Proc., 32:
423-426.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced in
mutant strains of Salmonella typhimurium by pesticides,
Washington, DC, American Chemical Society (Abstracts Paper 174)
(Pesticide No. 43).
ESTENIK, J.F. & COLLINS, W.J. (1979) In vivo and in vitro studies
of mixed-function oxidase in an aquatic insect Chironomus riparius.
In: Khan, M.A.Q., ed. Pesticide and xenobiotic metabolism in
aquatic organisms, Washington, DC, American Chemical Society, pp.
349-370 (ACS Symposium Series No. 99).
EYE, J.D. (1968) Aqueous transport of dieldrin residues in soil. J.
Water Pollut. Control Fed. (suppl.), 40(8): R316-332.
FABER, R.A., RISEBROUGH, R.W., & PRATT, H.M. (1972) Organochlorines
and mercury in common egrets and great blue herons. Environ.
Pollut., 3: 111-122.
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides,
Lyons, International Agency for Research on Cancer, pp. 161-181
(IARC Scientific Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a Joint Meeting of the FAO Committee on Pesticides
in Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting Report
No. PL:1965/10; WHO Food Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO Meeting Report No.
PL: 1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of the FAO
Working Party on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) 1966 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1967/M/11/1; WHO Food
Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 78;
WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1968/M/9/1; WHO Food
Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 84;
WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 87;
WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71. 42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 97;
WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Plant Production and
Protection Series No. 1; WHO Technical Report Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues and
the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 10 Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1984) Codex guidelines on good practice in pesticide
residue analysis, Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986b) Recommendations for methods of analysis of
pesticide residues, Rome, Codex Alimentarius Committee, Food and
Agriculture Organization of the United Nations (CAC/PR8-1986).
FARB, R.M., SANDERSON, T., MOORE, B.G., & HAYES, A.W. (1973)
Interaction: the effect of selected mycotoxins on the tissue
distribution and retention of aldrin and dieldrin in the neonatal
rat. In: Deichmann, W.B., ed. Pesticides in the environment, New
York, International Medical Book Corporation, pp. 179-187.
FARMER, W.J. & JENSEN, C.R. (1970) Diffusion and analysis of
carbon-14-labelled dieldrin in soils. Soil Sci. Soc. Am. Proc., 34:
28-31.
FARMER, W.J. & LETEY, J. (1974) Volatilization losses of pesticides
from soils, Washington, DC, US Environmental Protection Agency
(EPA-660/2-74-054).
FARMER, W.J., IGUE, K., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. I. Effect of
concentration, temperature, air flow rate, and vapour pressure.
Soil Sci. Soc. Am. Proc., 36: 443-447.
FARMER, W.J., IGUE, K., & SPENCER, W.F. (1973) Effect of bulk
density on the diffusion and volatilization of dieldrin from soil.
J. environ. Qual., 2(1): 107-109.
FEIL, V.J., HEDDE, R.D., ZAYLSKIE, R.G., & ZACHRISON, C.H. (1970)
Dieldrin-14C metabolism in sheep. Identification of trans-6,7-di-
hydroxydihydroaldrin and 9-(syn-epoxy)-hydroxy-1,2,3,4,10,10-hexa-
chloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-endo-5,8-exo-
dimethanonaphthalene. J. agric. food Chem., 18(1): 120-124.
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FERGIN, T.J. & SCHAFER, E.C. (1977) Toxicity of dieldrin to
bobwhite quail in relation to sex and reproductive status. Arch.
environ. Contam. Toxicol., 6: 213-219.
FERNICOLA, N.A.G.G. & AZEVEDO, F.A. (1982) Serum levels of
organochlorine insecticides in humans in Sao Paulo, Brazil. Vet.
hum. Toxicol., 24(2): 91-93.
FIKES, M.H. & TUBB, R.A. (1972) Dieldrin uptake in the three-ridge
naiad. J. wildl. Manage., 36(3): 802-809.
FISCHLER, H.M. & KORTE, F. (1969) [Sensitized and non-sensitized
photoisomerization of cyclodiene insecticides.] Tetrahedron Lett.,
32: 2793-2796 (in German).
FISEROVA-BERGEROVA, V., RADOMSKI, J.L., DAVIES, J.E., & DAVIS, J.H.
(1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHER, H.L., MOST, B., & HALL, L.L. (1985) Dermal absorption of
pesticides calculated by deconvolution. J. appl. Toxicol., 5(3):
163-177.
FITZHUGH, O.G., NELSON, A.A., & QUAIFE, M.L. (1964) Chronic oral
toxicity of aldrin and dieldrin in rats and dogs. Food Cosmet.
Toxicol., 2: 551-561.
FLETCHER, T.E., PRESS, J.M., & WILSON, D.B. (1959) Exposure of
spray-men to dieldrin in residual spraying. Bull. World Health
Organ., 20: 15-25.
FLICKINGER, E.L. & KING, K.A. (1972) Some effects of aldrin-treated
rice on Gulf coast wildlife. J. wildl. Manage., 36(3): 706-727.
FOURNIER, E., TREICH, I., CAMPAGNE, L., & CAPELLE, N. (1972)
Pesticides organo-chlorés dans le tissus adipeux d'êtres humains en
France. Eur. J. Toxicol., 5(1): 11-26.
FOWKES, F.M., BENESI, H.A., RYLAND, L.B., LOEFFLER, E.S., & SUN,
Y.P. (1960) Clay catalysed decomposition of insecticides. J. agric.
food Chem., 8: 203-210.
FOWLER, J.F., NEWSOM, L.D., GRAVES, J.B., BONNER, F.L., &
SCHILLING, P.E. (1971) Effect of dieldrin on egg hatchability,
chick survival, and eggshell thickness in purple and common
gallinules. Bull. environ. Contam. Toxicol., 6(6): 495-501.
FOX, G.R. & VIRGO B.B. (1986) Relevance of hyperglycemia to
dieldrin toxicity in suckling and adult rats. Toxicology, 38:
315-326.
FRANK, R., MONTGOMERY, K., BRAUN, H.E., BERST, A.H., & LOFTUS, K.
(1974) DDT and dieldrin in watersheds draining the tobacco belt in
southern Ontario. Pestic. monit. J., 8(3): 184-201.
FRANK, R., BRAUN, H.E., ISHIDA, K., & SUDA, P. (1976) Persistent
organic and inorganic pesticide residues in orchard soils and
vineyards of southern Ontario. Can. J. Soil Sci., 56: 463-484.
FRANK, R., HOLDRINET, M.V.H., & SUDA, P. (1979) Organochlorine and
mercury residues in wild mammals in southern Ontario, Canada 1973-
1974. Bull. environ. Contam. Toxicol., 22: 500-507.
FRANK, R., BRAUN, H.E., & HOLDRINET, M.V.H. (1981) Residues from
past uses of organochlorine insecticides and PCB in waters draining
eleven agricultural watersheds in southern Ontario, Canada, 1975-
1977. Sci. total Environ., 20: 255-276.
FRANK, R., BRAUN, H.E., SIRONS, G.H., RASPER, J., & WARD, G.G.
(1985) Organochlorine and organophosphorus insecticides and
industrial pollutants in the milk supplies of Ontario, 1983. J.
food Prot., 48(6): 499-504.
FRY, D.R. (1964) Human dieldrin poisoning. Lancet, 1: 764.
FUCHS, P. (1967) Death of birds caused by application of seed
dressings in the Netherlands. Meded. Fac. Landbouwwet. Rijksuniv.
Gent., 32(3/4): 855-859.
FURNESS, R. & HUTTON, M. (1979) Pollutant levels in the great skua
Catharacta skua. Environ. Pollut., 13: 261-268.
FYTIANOS, K., VASILIKIOTIS, G., & WEIL, L. (1985) Identification
and determination of some trace organic compounds in coastal
seawater of Northern Greece. Bull. environ. Contam. Toxicol., 34:
390-395.
GAINES, T.B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Use of the golden
hamster in toxicology. Lab. anim. Sci., 26(2): 274-280.
GAKSTATTER, J.H. (1968) Rates of accumulation of 14C-dieldrin
residues in tissues of goldfish exposed to a singe sublethal dose
of 14C-aldrin. J. Fish Res. Board Can., 25(9): 1797-1801.
GAKSTATTER, J.H. & WEISS, C.M. (1967) The elimination of DDT-14C,
dieldrin-14C, and lindane-14C from fish following a single sub-
lethal exposure in aquaria. Trans. Am. Fish. Soc., 96: 301-307.
GALLEY, R.A.E. (1970) [Chlorinated hydrocarbons. IV. Cyclodien
insecticides.] In: Wegler, R., ed. [Chemistry of crop protection
agents and pesticides,] Berlin, Heidelberg, New York, Springer-
Verlag, Vol. 1, pp. 163-192 (in German).
GANNON, N. & DECKER, G.C. (1958) The conversion of aldrin to
dieldrin on plants. J. econ. Entomol., 51: 8-11.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959a) Storage of dieldrin
in tissues of steers, hogs, lambs, and poultry fed dieldrin in
their diets. J. agric. food Chem., 7(12): 826-828.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959b) Storage of dieldrin
in tissues and its excretion in milk of dairy cows fed dieldrin in
their diets. J. agric. food Chem., 7(12): 824-826.
GARRETTSON, L.K. & CURLEY, A. (1969) Dieldrin. Studies in a
poisoned child. Arch. environ. Health, 19: 814-822.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total-diet samples, October 1980 - March 1982.
J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in adult
total-diet samples, October 1980 - March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GENELLY, R.E. & RUDD, R.L. (1956) Effects of DDT, toxaphene, and
dieldrin on pheasant reproduction. Auk, 73: 529-539.
GEORGIAN, L. (1975) The comparative cytogenetic effects of aldrin
and phosphamidon. Mutat. Res., 31: 103-108.
GEROLT, P. (1961) Investigation into the problem of insecticide
sorption by soils. Bull. World Health Organ., 24: 577-592.
GIFAP (1984) Pesticide residues in food, Brussels, Groupement
International des Associations Nationales des Fabricants de
Produits Agrochimique.
GILLETT, J.W. & CHAN, T.M. (1968) Cyclodiene insecticides as
inducers, substrates, and inhibitors of microsomal epoxidation. J.
agric. food Chem., 16(4): 590-593.
GINN, T.M. & FISHER, F.M., Jr (1974) Studies on the distribution
and flux of pesticides in waterways associated with a rice field.
Marshland ecosystems. Pestic. monit. J., 8(1): 23-32.
GISH, C.D. (1970) Organochlorine insecticide residues in soils and
soil invertebrates from agricultural lands. Pestic. monit. J., 3(4):
241-252.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 241).
GLATT, H., JUNG, R., & OESCH, F. (1983) Bacterial mutagenicity
investigation of epoxides: drugs, drug metabolites, steroids and
pesticides. Mutat. Res., 11: 99-118.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J. (1976)
Distribution of pesticides and polychlorinated biphenyls in water,
sediments, and seston of the Upper Great Lakes, 1974. Pestic.
monit. J., 10(2): 61-67.
GLOTFELTY, D.E. (1978) The atmosphere as a sink for applied
pesticides. J. Air Pollut. Control Assoc., 28: 917-921.
GONZALEZ RODRIGUEZ-CORDOBA, J.M., FERNANDES, A.L., & HENS, J.M.
(1983) [Mother neonate ratio of levels of blood contamination by
organo-chlorine insecticide residues.] Arch. Zootec., 32(122):
49-60 (in Spanish).
GOOD, E.E. & WARE, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV. Endrin and dieldrin.
Toxicol. appl. Pharmacol., 14: 201-203.
GOODWIN, E.S., GOULDEN, R., & REYNOLDS, J.G. (1961) Rapid
identification and determination of residues of chlorinated
pesticides in crops by gas-liquid chromatography. J. Soc. Anal.
Chem., 80(1028): 697-700.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ., 63(5):
945-962.
GRACA, I., SILVA FERNANDES, A.M.S., & MOURAO, H.C. (1974)
Organochlorine insecticide residues in human milk in Portugal.
Pestic. monit. J., 8(3): 148-156.
GRANVILLE, G.C., SIMPSON, B.J., & DOAK, S.M. (1973) Toxicity
studies on aldrin dicarboxylic acid: 13-week oral study in rats,
Sittingbourne, Shell Research (TLGR.0008.73) (Unpublished
proprietary report).
GRAVES, J.B., BONNER, F.L., MCKNIGHT, W.F., WATTS, A.G., & EPPS,
E.A. (1969) Residues in eggs, preening glands, liver, and muscle
from feeding dieldrin-contaminated rice bran to hens and its
effects on egg production, egg hatch, and chick survival. Bull.
environ. Contam. Toxicol., 4(6): 375-383.
GREENBERG, R.E. & EDWARDS, W.R. (1970) Insecticide residue levels
in eggs of wild pheasants in Illinois. Trans. Illinois State Acad.
Sci., 63: 136-147.
GREICHUS, Y.A., GREICHUS, A., & REIDER, E.G. (1968) Insecticide
residues in grouse and pheasant of South Dakota. Pestic. monit. J.,
2(2): 90-92.
GREICHUS, Y.A., GREICHUS, A., DRAAYER, H.A., & MARSHALL, B. (1978a)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. II. Lake McLlwaine, Rhodesia. Bull. environ. Contam.
Toxicol., 19: 444-453.
GREICHUS, Y.A., GREICHUS, A., AMMANN, B.D., & HOPCRAFT, J. (1978b)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. III. Lake Nakuru, Kenya. Bull. environ. Contam.
Toxicol., 19: 454-461.
GREVE, P.A. (1972) Potentially hazardous substances in surface
waters. Part I. Pesticides in the river Rhine. Sci. total Environ.,
1: 173-180.
GREVE, P.A. & WEGMAN, R.C.C. (1985) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands,
Copenhagen, World Health Organization, Regional Office for Europe
(ICP/CEH. 501/M05).
GRIFFIN, D.E. & HILL, W.E. (1978) In vitro breakage of plasmid DNA
by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRIFFITH, J. & DUNCAN, R.C. (1985) Serum organochlorine residues in
Florida citrus workers compared to the national health and
nutrition examination survey sample. Bull. environ. Contam.
Toxicol., 35: 411-417.
GRIFFITHS, D.C., RAW, F., & LOFTY, J.R. (1967) The effects on soil
fauna of insecticides tested against wireworms ( Agriotes ssp.) in
wheat. Ann. appl. Biol., 60: 479-490.
GROSCH, D.S. & VALCOVIC, I.R. (1967) Chlorinated hydrocarbon
insecticides are not mutagenic in Bracon hebetor tests. J. econ.
Entomol., 60(4): 1177-1179.
GUERZONI, M.E., DEL CUPOLO, L., & PONTI, I. (1976) Mutagenic
activity of pesticides. Riv. Sci. Tec. Aliment. Nutr. Um., 6:
161-165.
GUTENMANN, W.H., GREENWOOD, R.A., GYRISCO G.G., & LITTLE, R.J.
(1972) Studies of aldrin and chlordane in silt loam soils and their
possible translocation in field corn in New York. J. econ.
Entomol., 65(3): 842-844.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. Sci. total
Environ., 43: 193-219.
GUPTA, H.C.L. & KAVADIA, V.S. (1979) Dissipation of aldrin residues
in clay loam soil under the cover of root crops. Indian J. plant
Prot., 7(1): 43-49.
GUPTA, H.C.L., KUSHWAHA, K.S., KAVADIA, V.S., & SRIVASTAVA, B.P.
(1979) Aldrin residues in soils and its translocation in maize and
pearl millet. Indian J. Entomol., 41(1): 47-57.
GUPTA, P.C. (1975) Neurotoxicity of chronic chlorinated hydrocarbon
insecticide poisoning: a clinical and electroencephalographic study
in man. Indian J. med. Res., 63(4): 601-606.
HAAN, C.T. (1971) Movement of pesticides by runoff and erosion.
Trans. Am. Soc. Agric. Eng., 14: 445-447, 449.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Cournix. Bull. environ. Contam. Toxicol., 11(1): 98-102.
HAGLEY, E.A.C. (1965) Effect of insecticides on the growth of
vegetable seedlings. J. econ. Entomol., 58(4): 777-778.
HAMILTON, H.E., MORGAN, D.P., & SIMMONS, A. (1978) A pesticide
(dieldrin)-induced immunohemolytic anemia. Environ. Res., 17:
155-164.
HARGESHEIMER, E.E. (1984) Rapid determination of organochlorine
pesticides and polychlorinated biphenyls using selected ion
monitoring mass spectrometry. J. Assoc. Off. Anal. Chem., 67(6):
1067-1075.
HARR, J.R., CLAEYS, R.R., BONE, J.F., & MCCORCLE, T.W. (1970)
Dieldrin toxicosis: rat reproduction. Am. J. vet. Res., 31: 181-189.
HARRIS, C.I. (1969) Movement of pesticides in soil. J. agric. food
Chem., 17(1): 80-82.
HARRIS, C.R. (1964) Influence of soil type and soil moisture on the
toxicity of insecticides in soils to insects. Nature (Lond.), 202:
724.
HARRIS, C.R. (1972) Behaviour of dieldrin in soil. Laboratory
studies influencing biological activity. J. econ. Entomol., 65: 8-13.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J.
agric. food Chem., 15: 86-93.
HARRIS, C.R., SANS, W.W., & MILES, J.R.W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in Southwestern Ontario. J. agric. food Chem.,
14(4): 398-403.
HARRISON, R.B., HOLMES, D.C., ROBURN, J., & TATTON, J.O'G. (1967)
The fate of some organochlorine pesticides on leaves. J. Sci. Food
Agric., 18: 10.
HARVEY, G.R., STEINHAVER, W.G., & TEAL, J.M. (1973) Polychlorobiphenyls
in North Atlantic Ocean water. Science, 180: 643-644.
HARVEY, G.R., STEINHAVER, W.G., & MIKLAS, H.P. (1974) Decline of
PCB concentrations in North Atlantic surface water. Nature (Lond.),
252: 387-388.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of waterfowl nesting on
islands in Lake Michigan off Door County, Wisconsin, 1977-78.
Pestic. monit. J., 15(2): 90-97.
HASHEMY-TONKABONY, S.E. & SOLEIMANI-AMIRI, M.J. (1978) Chlorinated
pesticide residues in the body fat of people in Iran. Environ.
Res., 16: 419-422.
HATHWAY, D.E., MOSS, J.A., ROSE, J.A., & WILLIAMS, D.J.M. (1967)
Transport of dieldrin from mother to blastocyst and from mother to
foetus in pregnant rabbits. Eur. J. Pharmacol., 1: 167-175.
HAVERA, S.P. & DUZAN, R.E. (1986) Organochlorine and PCB residues
in tissues of raptors from Illinois, 1966-81. Bull. environ.
Contam. Toxicol., 36: 23-32.
HAYES, W.J. (1957) Dieldrin poisoning in man, Washington, DC, US
Department of Health, Eduction and Welfare, Public Health Service,
Vol. 72, pp. 1087-1091 (Public Health Report No. 12).
HAYES, W.J. (1959) The toxicity of dieldrin to man. Report on a
survey. Bull. World Health Organ., 20: 891-912.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons.
Emergency information for treating poisoning, Washington, DC, US
Government Printing Office (Public Health Service Publication No.
476).
HAYES, W.J. (1974) Distribution of dieldrin following a single oral
dose. Toxicol. appl. Pharmacol., 28: 485-492.
HAYES, W.J. (1982) Chlorinated hydrocarbon insecticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and
Wilkins, pp. 172-283.
HAYES, W.J. & CURLEY, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environ. Health, 16(2): 155-162.
HAYES, W.J., Jr, DALE, W.E., & BURSE, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HEATH, D.F. & VANDEKAR, M. (1964) Toxicity and metabolism of
dieldrin in rats. Br. J. ind. Med., 21: 269-279.
HEESCHEN, W. (1972) Analyses for residues in milk and milk
products. In: Coulston, F. & Korte, F., ed. Environmental quality
and safety, Stuttgart, G. Thieme Verlag, Vol. 1, p. 229.
HEINZ, G.H. & JOHNSON, R.W. (1981) Diagnostic brain residues of
dieldrin: some new insights. In: Lamb, D.W. & Kenaga, E.E., ed.
Avian and mammalian wildlife toxicology. Second Conference,
Philadelphia, Pennsylvania, American Society of Testing Materials,
pp. 72-92 (STP757).
HELENE, C.G., LORD, K.A., & RUEGG, E.F. (1981) The persistence,
leaching, and volatilization of 14C aldrin in two Brazilian soils
Cienc. Cult., 33: 101-105.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four
species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969) Organochlorine
insecticide residues in fish. Pestic. monit. J., 3(3): 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971) Organochlorine
insecticide residues in fish - fall 1969. Pestic. monit. J., 5(1):
1-11.
HENDERSON, G.L. & CROSBY, D.G. (1968) The photodecomposition of
dieldrin residues in water. Bull. environ. Contam. Toxicol., 3:
131-134.
HERRERA MARTEACHE, A., POLO VILLAR, L.M., JODRAL VILLAREJO,
M., POLO VILLAR, G., MALLOL, J., & POZO LORA, R. (1978)
[Organochlorine pesticide residues in human fat in Spain.] Rev.
Sanid. Hig. publica., 52: 1125-1144 (in Spanish).
HERZEL, F. (1971) [The behaviour of some persistent insecticides in
the soil.] Bundesgesundheitsblatt, 14(3): 23-28 (in German).
HERZEL, F. (1972) Organochlorine insecticides in surface waters in
Germany: 1970 and 1971. Pestic. monit. J., 6(3): 179-187.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg., 24:
459-463.
HICKEY, J.J., ed. (1969) Peregrine falcon populations: their
biology and decline, Madison, Wisconsin, University of Wisconsin
Press.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish-eating birds. Science, 162:
271-273.
HILL, D.W. & MCCARTY, P.L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control Fed.,
39: 1259-1277.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 191).
HILL, E.F., SPANN, J.W., & WILLIAMS, J.D. (1977) Responsiveness of
6 to 14 generations of birds to dietary dieldrin toxicity. Toxicol.
appl. Pharmacol., 42: 425-431.
HINDIN, E., MAY, D.S., & DUNSTAN, G.S. (1964) Collection and
analysis of synthetic organic pesticides from surface and ground
water. Residue Rev., 7: 130-156.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10: 613-675.
HOERSCHELMANN, H., POLZHOFER, K., FIGGE, K., & BALLSCHMITER,
K. (1979) [Organochlorine pesticides and polychlorinated biphenyls
in birds eggs from the Falkland Islands and from northern Germany.]
Environ. Pollut., 13: 247-269 (in German).
HOFFMAN, W.S., ADLER, H., FISHBEIN, W.I., & BAUER, F.C. (1967)
Relation of pesticide concentrations in fat to pathological changes
in tissues. Arch. environ. Health, 15: 758-765.
HOGAN, R.L. & ROELOFS, E.W. (1971) Concentrations of dieldrin in
the blood and brain of the green sunfish Lepomis cyanellus at
death. J. Fish Res. Board Can., 28(4): 610-612.
HOLDEN, A.V. (1975) The accumulation of oceanic contaminants in
marine mammals, Rapp. P.-V. Réun. Cons. int. Explor. Mer, 169:
353-361.
HOLDRINET, M.V.H., BRAUN, H.E., FRANK, R., STOPPS, G.E., SMOUT,
M.S., & MCWADE, J.W. (1977) Organochlorine residues in human
adipose tissue and milk from Ontario residents, 1969-1974. Can. J.
public Health, 68: 74-80.
HOLT, R.L., CRUSE, S., & GREEN, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
North East Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOOFTMAN, R.N. & VINK, G.J. (1980) The determination of toxic
effects of pollutants with the marine polychaete worm Ophryotrocha
diadema. Ecotoxicol. environ. Saf., 4: 252-262.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1962) Electro-
encephalograms in insecticide toxicity. Arch. environ. Health, 4:
86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1965) Nine
years' toxicity control in insecticide plants. Arch. environ.
Health, 10: 441-448.
HORNABROOK, R.W., DYMENT, P.G., GOMES, E.D., & WISEMAN, J.S. (1972)
DDT residues in human milk from New Guinea natives. Med. J. Aust.,
1: 1297-1300.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, 2nd ed., US Department of the
Interior, Fish and Wildlife Service, pp. 9-10 (Publication No.
153).
HUNT, P.F., STEVENSON, D.E., THORPE, E., & WALKER, A.I.T. (1975)
Mouse data. Letter to the editor. Food Cosmet. Toxicol., 13:
597-599.
HUNTER, C.G. & ROBINSON, J. (1967) Pharmacodynamics of dieldrin
(HEOD). I. Ingestion by human subjects for 18 months. Arch.
environ. Health, 15(5): 614-626.
HUNTER, C.G. & ROBINSON, J. (1968) Aldrin, dieldrin, and man. Food
Cosmet. Toxicol., 6: 253-260.
HUNTER, C.G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England. Br. med.
J., 1: 221-224.
HUNTER, C.G., ROBINSON, J., & JAGER, K.W. (1967) Aldrin and
dieldrin: the safety of present exposures of the general
populations of the United Kingdom and the United States. Food
Cosmet. Toxicol., 5: 781-787.
HUNTER, C.G., ROBINSON, J., & ROBERTS, M. (1969) Pharmaco-dynamics
of dieldrin (HEOD). II. Ingestion by human subjects for 18 to 24
months, and post-exposure for eight months. Arch. environ. Health,
18(1): 12-21.
HUTSON, D.H. (1976) Comparative metabolism of dieldrin in the rat
(CFE) and in two strains of mouse (CF1 and LACG). Food Cosmet.
Toxicol., 14: 577-591.
IARC (1974) Some organochlorine pesticides, Lyons, International
Agency for Research on Cancer, 241 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol.
5).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, Vol. 1-29, 292 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Man, Suppl. 4).
IARC (1987) Overall evaluations of carcinogenicity. An update of
IARC Monographs, Lyons, International Agency for Research on
Cancer, pp. 1-42 (Monographs on the Evaluation of Carcinogenic
Risks to Humans, Suppl. 7).
IATROPOULOS, M.J., MILLING, A., MUELLER, W.F., NOHYNEK, G., ROZMAN,
K., COULSTON, F., & KORTE, F. (1975) Absorption, transport, and
organotropism of dichlorobiphenyl (DCB), dieldrin, and
hexachlorobenzene (HCB) in rats. Environ. Res., 10: 384-389.
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, Utrecht, Rijks Universiteit (Thesis).
ICHINOSE, R. & KURIHARA, N. (1985) Uptake of dieldrin, lindane, and
DDT by isolated hepatocytes. Pestic. Biochem. Physiol., 23: 116-122.
IGUE, K., FARMER, W.J., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. II. Effect of
relative humidity and soil water content on dieldrin volatility.
Soil Sci. Soc. Am. Proc., 36: 447-450.
ITO, N., TSUDA, H., HASEGAWA, R., & IMAIDA, K. (1983) Comparison of
the promoting effects of various agents in induction of
preneoplastic lesions in rat liver. Environ. Health Perspect., 50:
131-138.
IVEY, M.C., CLABORN, H.V., MANN, H.D., RADELEFF, R.D., & WOODARD,
G.T. (1961) Aldrin and dieldrin content of body tissues of
livestock receiving aldrin in their diet. J. agric. food Chem.,
9(5): 374-376.
IVIE, G.W. & CASIDA, J.E. (1970) Enhancement of photo-alteration of
cyclodiene insecticide chemical residues by rotenone. Science, 167:
1620-1622.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Publishing Company,
234 pp.
JANSEN, J.D. (1979) The predictive value of tests for carcinogenic
or mutagenic activity. In: Deichmann, W.B., ed. Toxicology and
occupational medicine, Amsterdam, Elsevier/North Holland, pp.
71-80.
JEFFERIES, D.J. (1969) Causes of badger mortality in eastern
counties of England. J. Zool. (Lond.), 157: 429-436.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in
British bats and their significance. J. Zool. (Lond.), 166:
245-263.
JEFFERIES, D.J. (1975) Different activity patterns of male and
female badgers (Meles meles) as shown by road mortality. J. Zool.
(Lond.), 177: 504-506.
JEFFERIES, D.J. & DAVIS, B.N.K. (1968) Dynamics of dieldrin in
soil, earthworms, and song thrushes. J. wildl. Manage., 32(3):
441-456.
JEFFERIES, D.J. & PENDLEBURY, J.B. (1968) Population fluctuations
of stoats, weasels and hedgehogs in recent years. J. Zool. (Lond.),
156: 513-517.
JEFFERIES, D.J., STAINSBY, B., & FRENCH, M.C. (1973) The ecology of
small mammals in arable fields drilled with winter wheat and the
increase in their dieldrin and mercury residues. J. Zool. (Lond.),
171: 513-539.
JEFFERIES, D.J., FRENCH, M.C., & STEBBING, R.E. (1974) Pollution
and mammals: otters, Huntingdon, Natural Environment Research
Council, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, pp. 13-15 (Report for 1972-73).
JENSEN, L.D. & GAUFIN, A.R. (1966) Acute and long-term effects of
organic insecticides on two species of stonefly naiads. J. Water
Pollut. Control Fed., 38(8): 1273-1286.
JOHNSON, L.G. & MORRIS, R.L. (1971) Chlorinated hydrocarbon
pesticides in Iowa rivers. Pestic. monit. J., 4(4): 216-219.
JOHNSON, R.D. & MANSKE, D.D. (1977) Pesticide and other chemical
residues in total diet samples. XI. Pestic. monit. J., 11(3): 116-131.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 1-3, 8,
10, 29-30 (Resource Publication No. 137).
JOHNSTON, B.T., SAUNDERS C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin
by fresh-water invertebrates. J. Fish Res. Board Can., 28: 705-709.
JOHNSTON, D.W. (1975) Organochlorine pesticide residues in small
migratory birds, 1964-1973. Pestic. monit. J., 9(2): 79-88.
JOLLY, D.W. (1954) Studies on the acute toxicity of dieldrin to
sheep. Vet. Rec., 66: 444-447.
JONAS, R.B. & PFAENDER, F.K. (1976) Chlorinated hydrocarbon
pesticides in Western North Atlantic Ocean. Environ. Sci. Technol.,
10(8): 770-773.
JONES, D.M., BENNETT, D., & ELGAR, K.E. (1978) Deaths of owls
traced to insecticide-treated timber. Nature (Lond.), 272: 52.
JOY, R.M. (1976) The alteration by dieldrin of cortical
excitability conditioned by sensory stimuli. Toxicol. appl.
Pharmacol., 38(2): 357-368.
JOY, R.M. (1977) Contrasting actions of dieldrin and aldrin-
transdiol, its metabolite, on cat CNS functions. Toxicol. appl.
Pharmacol., 42: 137-148.
JOY, R.M. (1982) Mode of action of lindane, dieldrin, and related
insecticides in the central nervous system. Neurobehav. Toxicol.
Teratol., 4: 813-823.
JUNK, G.A., SPALDING, R.F., & RICHARD, J.J. (1980) Aerial,
vertical, and temporal differences in groundwater chemistry. II.
Organic constituents. J. environ. Qual., 9: 479-483.
JURY, W.A., SPENCER, W.F., & FARMER, W.J. (1983) Use of models for
assessing relative volatility, mobility, and persistence of
pesticides and other trace organics in soil systems. In: Saxena,
J., ed. Hazard assessment of chemicals, current development, Vol.
2, New York, London, Academic Press, pp 1-43.
KADIS, V.W., BREITKREITZ, W.E., & JONASSON, O.J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. public
Health, 61(5): 413-416.
KAISER, T.E., REICHEL, W.L., LOCKE, L.N., CROMARTIE, E., KRYNITSKY,
A.J., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., STAFFORD, C.J., &
SWINEFORD, D.M. (1980) Organochlorine pesticide, PCB, and PBB
residues and necropsy data for bald eagles from 29 states - 1975-
77. Pestic. monit. J., 13(4): 145-149.
KANITZ, S. & CASTELLO, G. (1966) [Presence of residues of some
disinfectants in human fatty tissue and in certain foods.] G. Ig.
Med. prev., 7: 1-19 (in Italian).
KANJA, L., SKARE, J.K., NAFSTAD, I., MAITAI, C.K., & LOKKEN, P.
(1986) Organochlorine pesticides in human milk from different areas
of Kenya, 1983-1985. J. Toxicol. environ. Health, 19: 449-464.
KATZ, M. (1961) Acute toxicity of some organic insecticides to
three species of salmonids and to the three-spine stickleback.
Trans. Am. Fish. Soc., 90(2): 264-268.
KAWATSKI, J.A. & SCHMULBACH, J.C. (1972) Uptake and elimination of
14C-aldrin and 14C-dieldrin by the ostracod Chlamydotheca arcuata
(Sars). Int. J. environ. anal. Chem., 1: 283-291.
KAZANTZIS, G., MCLAUGHLIN, A.I.G., & PRIOR, P.F. (1964) Poisoning
in industrial workers by the insecticide aldrin. Br. J. ind. Med.,
21: 46-51.
KEANE, W.T. & ZAVON, M.R. (1969a) Validity of a critical blood
level for prevention of dieldrin intoxication. Arch. environ.
Health, 19: 36-44.
KEANE, W.T. & ZAVON, M.R. (1969b) The total body burden of
dieldrin. Bull. environ. Contam. Toxicol., 4(1): 1-16.
KELLER, J. & ALFARO, J.F. (1966) Effect of water application rate
on leaching. Soil Sci., 102(2): 107-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1970) Effects of
combinations of pesticides on reproduction in mice. In: Pesticides
symposia, Miami Beach, Florida, Halos and Associates Inc., pp.
125-138.
KHAIRY, M. (1960) Effects of chronic dieldrin ingestion on the
muscular efficiency of rats. Br. J. ind. Med., 17: 146-148.
KHAN, M.A.Q., COELLO, W., KHAN, A.A., & PINTO, H. (1972a) Some
characteristics of the microsomal mixed-function oxidase in the
fresh-water crayfish Cambarus. Life Sci., 11(2): 405-415.
KHAN, M.A.Q., KAMAL, A., WOLIN, R.J., & RUNNELS, J. (1972b) In vivo
and in vitro epoxidation of aldrin by aquatic food chain organisms.
Bull. environ. Contam. Toxicol., 8(4): 219-228.
KING, P.H. & MCCARTY, P.L. (1968) A chromatographic model for
predicting pesticide migration in soils. Soil Sci., 106(4): 248-261.
KINOSHITA, F.K. & KEMPF, C.K. (1970) Quantitative measurement of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Toxicol. appl. Pharmacol.,
17(1): 288.
KITSELMAN, C.H. (1953) Long-term studies on dogs fed aldrin and
dieldrin in sublethal dosages, with reference to the
histopathological findings and reproduction. J. Am. Vet. Med.
Assoc., 123: 28-30.
KLAASSEN, H.E. & KADOUM, A.M. (1973) Pesticide residues in natural
fish populations of the Smoky Hill River of Western Kansas - 1967-
1969, Pestic. monit. J., 7(1): 53-61.
KLAUNIG, J.E., GOLDBLATT, P.J., HINTON, D.E., LIPSKY, M.M., &
TRUMP, B. (1984) Carcinogen-induced unscheduled DNA synthesis in
mouse hepatocytes. Toxicol. Pathol., 12(2): 119-125.
KLEIN, A.K., LINK, J.D., & IVES, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed
aldrin and dieldrin. J. Assoc. Off. Agric. Chem., 51: 895-898.
KLEIN, A.K., DAILEY, R.E., WALTON, M.S., BECK, V., & LINK, J.D.
(1970) Metabolites isolated from urine of rats fed 14C-
photodieldrin. J. agric. food Chem., 18(4): 705-708.
KLEIN, M.L. & LINCER, J.L. (1974) Behavioural effects of dieldrin
upon the fiddler crab Uca pugilator. In: Vernberg, F.J. &
Vernberg, W.B., ed. Pollution and physiology of marine organisms,
New York, London, Academic Press, pp. 181-196.
KLEIN, W., KOHLI, J., WEISGERBER, I., & KORTE, F. (1973) Fate of
aldrin-14C in potatoes and soil under outdoor conditions. J. agric.
food Chem., 21(2): 152-156.
KLEMMER, H.W., RASHAD, M.N., & MI, M.P. (1973) Age, sex, and race
effects on the distribution of organochlorine pesticide residues in
serum. In: Deichmann, W.B., ed. Pesticides and the environment,
New York, International Medical Book Corporation, pp. 53-61.
KLEVAY, L.M. (1970) Dieldrin excretion by the isolated perfused rat
liver: a sexual difference. Toxicol. appl. Pharmacol., 17: 813-815.
KOEMAN, J.H. (1971) [The occurrence and the toxicological
implications of some chlorinated hydrocarbons in the Dutch coastal
area in the period 1965-70], Utrecht, Rijks Universiteit (Thesis)
(in Dutch).
KOEMAN, J.H. & PENNINGS, J.H. (1970) An orientational survey on the
side effects and environmental distribution of insecticides used in
tsetse control in Africa. Bull. environ. Contam. Toxicol., 5(2):
164-170.
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROCK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis). A preliminary communication. Meded. Fac. Landouwwet.
Rijksuniv. Gent, 32: 841-854.
KOEMAN, J.H., VINK, J.A.J., & DE GOEIJ, J.J.M. (1969) Causes of
mortality in birds of prey and owls in the Netherlands in the
winter of 1968-69. Ardea, 57: 67-76.
KOEMAN, J.H., PEETERS, W.H.M., & PENNINGS, J.H. (1971) OECD
collaborative study 1969/71: pesticide residues in the environment,
Utrecht, The Netherlands, Institute of Veterinary Pharmacology and
Toxicology (Unpublished report).
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972)
Eggshell and population changes in the sparrow hawk (Accipiter
nisus). TNO Nieuws, 27: 542-550.
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1972) [Contributions to
ecological chemistry ILI(1). Transformation and residue behaviour
of dieldrin 14C in onions after seed treatment.] Chem. Mikrobiol.
Technol. Lebensm., 1: 149-150 (in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973a) [Contributions to
ecological chemistry LVIII. Transport of aldrin-14C and
transformation products in the soil.] Chemosphere, 3: 125-130
(in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973) [Contributions to
ecological chemistry LIX. Residue behaviour and transformation of
14C-dieldrin in crops, soil and seepage water after application to
the soil.] Chemosphere, 4: 153-156 (in German).
KORSCHGEN, L.J. (1971) Disappearance and persistence of aldrin
after five annual applications. J. wildl. Manage., 35(3): 494-500.
KORTE, F. (1965) Metabolism of chlorinated insecticides. In:
Radioisotopes in the detection of pesticide residues. Proceedings
of the International Atomic Energy Panel, Vienna, International
Atomic Energy Agency, p. 38.
KORTE, F. & ARENT, H. (1965) Metabolism of insecticides. IX(1).
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin-14C. Life Sci., 4:
2017-2026.
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated
compounds in human organs collected in Denmark, 1972-73. Acta
pharmacol. toxicol., 38: 38-48.
KRAYBILL, H.F. (1977) Global distribution of carcinogenic
pollutants in water. In: Proceedings of the Conference on Aquatic
Pollutants and Biological Effects with Emphasis on Neoplasia, 27-29
September, New York, New York Academy of Sciences.
KRIEGER, R.I. & WILKINSON, C.F. (1969) Microsomal mixed-function
oxidases in insects. I. Localization and properties of an enzyme
system affecting aldrin epoxidation in larvae of the southern
armyworm (Prodenia eridania). Biochem. Pharmacol., 18: 1403-1415.
KURATA, M., HIROSE, K., & UMEDA, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
KURIHARA, N., HORI, N., & ICHINOSE, R. (1984) Cytochrome P-450
content and aldrin epoxidation to dieldrin in isolated rat
hepatocytes. Pestic. Biochem. Physiol., 21: 63-73.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, N.S. (1978a) Persistence
and dissipation of aldrin and dieldrin in soil: a review.
Pesticides, 12(6): 14-18.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, V.S. (1978) Effect of
temperature on the degradation of aldrin residues in sandy loam
soil. Ann. arid Zone, 17(2): 200-206.
KUTZ, F.W., YOBS, A.R., & YANG, H.S.C. (1976) National pesticide
monitoring programs. In: Lee, R.E., ed. Air pollution from
pesticides and agricultural processes, Cleveland, Ohio, CRC Press,
pp. 95-136.
KUTZ, F.W., STRASSMAN, S., & YOBS, A.R. (1979) Survey of pesticide
residues and their metabolites in the general population of the
United States. In: Berlin, A., Wolff, A.H., & Hasegawa, Y., ed. Use
of biological specimens to assess human exposure to environmental
pollutants, The Hague, Martinus Nijhoff, pp. 267-274.
LANE, C.E. & LIVINGSTON, R.J. (1970) Some acute and chronic effects
of dieldrin on the sailfin molly Poecilia latipinna. Trans. Am.
Fish. Soc., 99(3): 489-495.
LANE, C.E., SEBA, D.B., & HEARN, W.L. (1970) Possible metabolites
of dieldrin in the sailfin molly (Poecilia latipinna). Proc. Soc.
Exp. Biol. Med., 133: 1375-1377.
LAUBSCHER, J.A., DUTT, G.R., & ROAN, C.C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of distance
from application. Pestic. monit. J., 5(3): 251-258.
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodiene with brain-specific t-butyl-
bicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAY, J.P., WEISGERBER, I., & KLEIN, W. (1975) Conversion of the
aldrin/dieldrin metabolite dihydrochlordene dicarboxylic acid-14C
in rats. Pestic. Biochem. Physiol., 5: 226-232.
LEHMAN, A.J. (1951) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Q. Bull. Assoc. Food Drug Off., 15: 122-133.
LEHMAN, A.J. (1952) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Section II. Dermal toxicity. Q. Bull. Assoc. Food Drug
Off. (USA), 16: 3-9.
LEHNER, P.N. & EGBERT, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature (Lond.), 224: 1218-1219.
LENARDON, A.M., DE HEVIA, M.I.M., FUSE, J.A., DE NOCHETTO, C.B., &
DEPETRIS, P.J. (1984) Organochlorine and organophosphorus
pesticides in the Parana river (Argentina). Sci. total Environ., 34:
289-297.
LENON, H., CURRY, L., MILLER, A., & PATULSKI, D. (1972) Insecticide
residues in water and sediment from cisterns on the US and British
Virgin Islands. Pestic. monit. J., 6: 188-193.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides.
In: Lee, R.E., Jr, ed. Air pollution from pesticides and
agricultural processes, Cleveland, Ohio, CRC Press, pp. 51-94.
LEWIS, R.G. & LEE, R.E., Jr (1976) Air pollution from pesticides:
sources, occurrence, and dispersion. In: Lee, R.E., Jr, ed. Air
pollution from pesticides and agricultural processes, Cleveland,
Ohio, CRC Press, pp. 5-50.
LICHTENBERG, J.J., EICHELBERGER, J.W., DRESSMAN, R.C., &
LONGBOTTOM, J.E. (1970) Pesticides in surface waters of the United
States: a 5-year summary, 1964-68. Pestic. monit. J., 4(2): 71-86.
LICHTENSTEIN, E.P. (1959) Absorption of some chlorinated
hydrocarbon insecticides from soils into various crops. J. agric.
food Chem., 7: 430-433.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1959) Breakdown of lindane and
aldrin in soils. J. econ. Entomol., 52(1): 118-124.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture and soil
types. J. econ. Entomol., 53(2): 192-197.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1970) Volatilization of
insecticides from various substrates. J. agric. food Chem., 18(5):
814-818.
LICHTENSTEIN, E.P., MUELLER, C.H., MYRDAL, G.R., & SCHULZ, K.R.
(1962) Vertical distribution and persistence of insecticidal
residues in soils as influenced by mode of application and cover
crop. J. econ. Entomol., 55(2): 215-219.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. agric. food Chem.,
18(1): 100-106.
LICHTENSTEIN, E.P., FUHREMANN, T.W., & SCHULZ, K.R. (1971)
Persistence and vertical distribution of DDT, lindane, and aldrin
residues, 10 and 15 years after a single soil application. J.
agric. food Chem., 19(4): 718-721.
LINDER, R.L. & DAHLGREN, R.B. (1970) Occurrence of organochlorine
insecticides in pheasants of South Dakota. Pestic. monit. J., 3(4):
227-232.
LINDER, R.L., DAHLGREN, R.B., & GREICHUS, Y.A. (1970) Residues in
the brain of adult pheasants given dieldrin. J. wildl. Manage., 34:
954-956.
LINDSTROM, F.T, BOERSMA, L., & STOCKARD, D. (1971) A theory on the
mass transport of previously distributed chemicals in a water
saturated sorbing porous medium: isothermal cases. Soil Sci., 112(5):
291-300.
LISS, P.S. & SLATER, P.G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LLOYD, C., BOGAN, J.A., BOURNE, W.R.P., DAWSON, P., & PARSLOW,
J.L.F. (1974) Seabird mortality in the North Irish Sea and Firth of
Clyde early in 1974. Mar. Pollut. Bull., 5: 136-140.
LOCKIE, J.D. & RATCLIFFE, D.A. (1964) Insecticides and Scottish
golden eagles. Br. Birds, 57(3): 89-102.
LOCKIE, J.D., RATCLIFFE, D.A., & BALHARRY, R. (1969) Breeding
success and organochlorine residues in golden eagles in west
Scotland. J. appl. Ecol., 6: 381-389.
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMAN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 298-304.
LOOSE, L.D. (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice, Envir. Health
Perspect., 43: 89-97.
LOOSE, L.D., SILKWORTH, J.B., CHARBONNEAU, T., & BLUMENSTOCK,
F. (1981) Environmental chemical-induced macrophage dysfunction.
Environ. Health Perspect., 39: 79-91.
LOTZ, F., KEMPNEY, J., & DE KEMPNEY, R.S.G. (1983) [Light-induced
breakdown of absorbed chemicals: a simple device for comparative
tests.] Chemosphere, 12: 873-878 (in German).
LOWDEN, G.F., SAUNDERS, C.L., & EDWARDS, R.W. (1969) Organochlorine
insecticides in water. Part II. Water Treat. Exam., 18: 275-287.
LU, F.C., JESSUP, D.C., & LAVALLEE, A. (1965) Toxicity of
pesticides in young versus adult rats. Food Cosmet. Toxicol., 3:
591-596.
LUCKENS, M.M. & DAVIS, W.H. (1965) Toxicity of dieldrin and endrin
to bats. Nature (Lond.), 207(4999): 879-880.
LUDKE, J.R., GIBSON, J.R., & LUSK, C.I. (1972) Mixed-function
oxidase activity in fresh-water fish: aldrin epoxidation and
parathion activation. Toxicol. appl. Pharmacol., 21: 89-97.
LUDWIG, G. & KORTE, F. (1965) Metabolism of insecticides. X.
Detection of dieldrin metabolites by GLC analysis. Life Sci., 4:
2027-2031.
LUDWIG, G., WEIS, J., & KORTE, F. (1964) Excretion and distribution
of aldrin-14C and its metabolites after oral administration for a
long period of time. Life Sci., 3: 123-130.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72(12): 5135-5139.
MACCUAIG, R.D. (1975) Occurrence and movements of pesticide
residues in Ethiopia. Environ. Qual. Saf., 3(suppl.): 850-851.
MACCUAIG, R.D. (1976) The occurrence of insecticides in the blood
of staff of a locust control organization. Bull. environ. Contam.
Toxicol., 15: 162-170.
MACDONALD, R. (1982) Toxicology of sprays: the 4-h acute inhalation
toxicity of aqueous sprays prepared from aldrin 48% (w/v) EC,
Sittingbourne, Shell Research (SBGR.82.036) (Unpublished
proprietary report).
MACEK, K.J. (1975) Acute toxicity of pesticide mixtures to
bluegills. Bull. environ. Contam. Toxicol., 14(6): 648-652.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects of
temperature on the susceptibility of bluegills and rainbow trout to
selected pesticides. Bull. environ. Contam. Toxicol., 4(3): 174-183.
MCEWEN, L.C. & BROWN, R.L. (1966) Acute toxicity of dieldrin and
malathion to wild sharp-tailed grouse. J. wildl. Manage., 30(3):
604-611.
MACKAY, D. & LEINONEN, P.J. (1975) Rate of evaporation of low-
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 9(13): 1178-1180.
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low-
solubility contaminants to atmosphere. Environ. Sci. Technol., 7(7):
611-614.
MACKAY, D., SHIU, W.Y., & SUTHERLAND, R.P. (1979) Determination of
air-water Henry's Law constants for hydrophobic pollutants.
Environ. Sci. Technol., 13(3): 333-337.
MCKINNEY, J.D., MATTHEWS, H.B., & WILSON, N.K. (1973) Determination
of optical purity and prochirality of chlorinated polycyclodiene
pesticide metabolites. Tetrahedron Lett., 21: 1895-1898.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol., 25: 921-928.
MCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982) Toxicities
of five organochlorine compounds in water and sediment to Nereis
virens. Bull. environ. Contam. Toxicol., 28: 216-220.
MAJUMDAR, S.K., KOPELMAN, H.A., & SCHNITMAN, M.J. (1976) Dieldrin-
induced chromosome damage in mouse bone-marrow and WI-38 human lung
cells. J. Hered., 67(5): 303-307.
MAJUMDAR, S.K., MAHARAM, L.G., & VIGLIANTI, G.A. (1977)
Mutagenicity of dieldrin in the Salmonella/microsome test. J.
Hered., 68: 184-185.
MANIGOLD, D.B. & SCHULZE, J.A. (1969) Pesticides in selected
western streams: a progress report. Pestic. monit. J., 3(2): 124-135.
MARCHANT, J.H. (1980) Recent trends in sparrowhawk numbers in
Britain. Bird Stud., 27: 152-154.
MARLOW, R.G. & WALLACE, B.G. (1983) Assessment of exposure
following the use of aldrin as a termiticide in homes. Part II. Air
concentrations and kitchen wipes after one year, Sittingbourne,
Shell Research (SBGR.83.285).
MARLOW, R.G., WALLACE, B.G., & MOORE, J.P. (1982) Assessment of
exposure following the use of aldrin as a termiticide in homes,
Sittingbourne, Shell Research (SBGR.82.370).
MARSHALL, T.C., DOROUGH, H.W., & SWIM, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. agric. food Chem., 24(3): 560-563.
MASON, J.W. & ROWE, D.R. (1976) The accumulation and loss of
dieldrin and endrin in the eastern oyster. Arch. environ. Contam.
Toxicol., 4: 349-360.
MASTROMATTEO, E. (1971) Pesticides and man's health: the picture in
Ontario. In: Proceedings of the Working Conference on
Epidemiological Toxicology of Pesticides, Amsterdam, 8-10 September
1971 (Unpublished paper).
MATSUMURA, F. & BOUSH, G.M. (1967) Dieldrin degradation by soil
microorganisms. Science, 156: 959-961.
MATTHEWS, H.B. & MATSUMURA, F. (1969) Metabolic fate of dieldrin in
the rat. J. agric. food Chem., 17: 845-852.
MATTHEWS, H.B., MCKINNEY, J.D., & LUCIER, G.W. (1971) Dieldrin
metabolism, excretion, and storage in male and female rats. J.
agric. food Chem., 19(6): 1244-1248.
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydrocarbon
insecticides, southern Florida, 1968-72. Pestic. monit. J., 9(2):
106-114.
MAYER, R., LETEY, J., & FARMER, W.J. (1974) Models for predicting
volatilization of soil-incorporated pesticides. Soil Sci. Soc. Am.
Proc., 38: 563-568.
MEHENDALE, H.M. & EL-BASSIOUNI, E.A. (1975) Uptake and disposition
of aldrin and dieldrin by isolated perfused rabbit lung. Drug
Metab. Disp., 3: 543.
MEHENDALE, H.M., SKRENTNY, R.F., & DOROUGH, H.W. (1972) Oxidative
metabolism of aldrin by subcellular root fractions of several plant
species. J. agric. food Chem., 20(2): 398-402.
MEHRLE, P.M. & BLOOMFIELD, R.A. (1974) Ammonia detoxifying
mechanisms of rainbow trout altered by dietary dieldrin. Toxicol.
appl. Pharmacol., 27: 355-365.
MEIERHENRY, E.F., RUEBNER, B.H., GERSHWIN, M.E., HSIEH, L.S., &
FRENCH, S.W. (1983) Dieldrin-induced mallory bodies in hepatic
tumours of mice of different strains. Hepatology, 3(1): 90-95.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 metres.
Environ. Lett., 5(4): 215-221.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
60-61.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls (Tyto alba) fed low levels of DDE and
dieldrin. Arch. environ. Contam. Toxicol., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine
phytoplankton vary in their response to chlorinated hydrocarbons.
Science, 167: 1724-1726.
MES, J., CAMPBELL, D.S., ROBINSON, R.N., & DAVIES, D.J.A. (1977)
Polychlorinated biphenyl and organochlorine pesticide residues in
adipose tissue of Canadians. Bull. environ. Contam. Toxicol., 17(2):
196-203.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 8: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Bull. environ.
Contam. Toxicol., 13: 217-233.
MES, J., DAVIES, D.J., & TURTON, D. (1985) Environmental
contaminants in human fat: a comparison between accidental and non-
accidental causes of death. Ecotoxicol. environ. Saf., 10: 70-74.
METCALFE, R.L., KAPOOR, I.P., LU, P-Y., SCHUTH, C.K., & SHERMAN, P.
(1973) Model ecosystem studies of the environment and fate of six
organochlorine pesticides. Environ. Health Perspect., 4: 35-44.
MICK, D.L., LONG, K.R., & BONDERMAN, D.P. (1972) Aldrin and
dieldrin in the blood of pesticide formulators. Am. Ind. Hyg.
Assoc. J., 33(2): 94-99.
MILES, J.R.W. & HARRIS, C.R. (1971) Insecticide residues in a
stream and a controlled drainage system in agricultural areas of
southwestern Ontario, 1970. Pestic. monit. J., 5(3): 289-294.
MILES, J.R.W. & HARRIS, C.R. (1973) Organochlorine insecticide
residues in streams draining agricultural, urban-agricultural, and
resort areas of Ontario, Canada - 1971. Pestic. monit. J., 6(4):
363-368.
MILLER, G.J. & FOX, J.A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, G.C. & ZEPP, R.G. (1983) Extrapolating photolysis rates
from the laboratory to the environment. Residue Rev., 85: 89-110.
MINISTRY OF WELFARE, HEALTH AND CULTURE (1983) Surveillance
programme: man and nutrition, The Hague, Netherlands, Ministry of
Welfare, Health and Culture, State Supervisory Public Health
Service.
MOERSDORF, K., LUDWIG, G., VOGEL, J., & KORTE, F. (1963) [Excretion
of aldrin-14C and dieldrin-14C and their metabolites through the
bile.] Med. Exp., 8: 90 (in German).
MOFFET, G.B. & YARBROUGH, J.D. (1972) The effects of DDT,
toxaphene, and dieldrin on succinic dehydrogenase activity in
insecticide-resistant and susceptible Gambusia affinis. J. agric.
food Chem., 20(3): 558-560.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health, 19:
633-636.
MORGAN, D.P. & ROAN, C.C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D.P. & ROAN, C.C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15(1): 26-28.
MORGAN, D.P. & ROAN, C.C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORITA, H. & UMEDA, M. (1984) Detection of mutagenicity of various
compounds by FM 3A cell system. Paper presented at the 12th Annual
Meeting of the Mutagenicity Society, Japan. Mutat. Res., 130(5): 371
(No. 33).
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MOSSING, M.L., REDETZKE, K.A., & APPLEGATE, H.G. (1985)
Organochlorine pesticides in blood of persons from El Paso, Texas.
J. environ. Health, 47(6): 312-313.
MOZA, P., WEISGERBER, I., & KLEIN, W. (1972) [Leaching a water-
soluble aldrin-14C breakdown product out of soil.] Chemosphere, 5:
191-195 (in German).
MUELLER, W. & LEACH, R.M., Jr (1974) Effects of chemicals on
eggshell formation. Annu. Rev. Pharmacol., 14: 289-303.
MUELLER, W., NOHYNEK, G., WOODS, G., KORTE, F., & COULSTON, F.
(1975a) Comparative metabolism of dieldrin-14C in mouse, rat,
rabbit, rhesus monkey, and chimpanzee. Chemosphere, 4(2): 89-92.
MUELLER, W., WOODS, G., KORTE, F., & COULSTON, F. (1975b)
Metabolism and organ distribution of dieldrin-14C in rhesus monkeys
after single oral and intravenous administration. Chemosphere, 2:
93-98.
MUELLER, P., NAGEL, P., & FLACKE, W. (1981) Ecological side effects
of dieldrin application against tsetse flies in Adamaoua,
Cameroon. Oecologia, 50(2): 187-194.
MUIR, C.M.C. (1970) The acute oral and percutaneous toxicities to
rats of formulations of aldrin, dieldrin, or endrin,
Sittingbourne, Shell Research (TLGR.0020.70) (Unpublished
proprietary report).
MUIRHEAD, E.E., GROVES, M., GUY, R., HALDEN, E.R., & BASS, R.K.
(1959) Acquired hemolytic anemia, exposure to insecticides and
positive Coombs test dependent on insecticide preparations. Vox
sang., 4: 277-292.
MULHERN, B.M., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., BELISLE,
A., CROMARTIE, E., BAGLEY, G.E., & PROUTY, R.M. (1970)
Organochlorine residues and autopsy data from bald eagles 1966-68.
Pestic. monit. J., 4(3): 141-144.
MULLER, H.D. & LOCKMAN, D.C. (1972) Fecundity and progeny growth
following subacute insecticide ingestion by the mallard. Poult.
Sci., 51: 239-241.
MULLINS, D.E., JOHNSEN, R.E., & STARR, R.I. (1971) Persistence of
organochlorine insecticide residues in agricultural soils of
Colorado. Pestic. monit. J., 5(3): 268-275.
MURPHY, D.A. & KORSCHGEN, L.J. (1970) Reproduction, growth, and
tissue residues of deer fed dieldrin. J. wildl. Manage., 34(4):
887-903.
MURPHY, R. & HARVEY, C. (1985) Residues and metabolites of selected
persistent halogenated hydrocarbons in blood specimens from a
general population survey. Environ. Health Perspect., 60: 115-120.
MURTON, R.K. & VIZOSO, M. (1963) Dressed cereal seed as a hazard to
woodpigeons. Ann. appl. Biol., 52: 503-517.
NASH, R.G. (1983) Comparative volatilization and dissipation rates
of several pesticides from soil. J. agric. food Chem., 31(2): 210-217.
NASH, R.G., BEALL, M.L., Jr, & WOOLSON, E.A. (1970) Plant uptake of
chlorinated insecticides from soils. Agron. J., 62: 369-372.
NATIONAL FOOD ADMINISTRATION (1982) Summary and assessment of data
received from the FAO/WHO Collaborating Centres for Food
Contamination Monitoring, Uppsala, Global Environmental Monitoring
System (GEMS), Joint FAO/WHO Food and Animal Feed Contamination
Monitoring Programme, pp. 26-29.
NCI (1977) Bioassay of photodieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-817).
NCI (1978a) Bioassay of aldrin and dieldrin for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(DHEW Publication No. (NIH) 78-821).
NCI (1978b) Bioassay of dieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-822).
NEILL, D.D., MULLER, H.D., & SHUTZE, J.V. (1971) Pesticide effects
on the fecundity of the gray partridge. Bull. environ. Contam.
Toxicol., 6(6): 546-551.
NELSON, E. (1953) Aldrin poisoning. Rocky Mt. med. J., 50: 483-486.
NEWTON, I. (1973a) Studies of sparrowhawks. Br. Birds, 66: 271-278.
NEWTON, I. (1973b) Success of sparrowhawks in an area of pesticide
usage. Bird Stud., 20: 1-8.
NEWTON, I. (1974) Changes attributed to pesticides in the nesting
success of the sparrowhawks in Britain. J. appl. Ecol., 11: 95-102.
NEWTON, I. (1976) Breeding of sparrowhawks (Accipiter nisus) in
different environments. J. anim. Ecol., 45: 831-849.
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted,
Poyser T. and A.D., Ltd.
NEWTON, I. & BOGAN, J. (1974) Organochlorine residues, eggshell
thinning, and hatching success in British sparrowhawks. Nature
(Lond.), 249: 582-583.
NEWTON, I. & BOGAN, J. (1978) The role of different organochlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol.,
15: 105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds, 77: 47-70.
NEWTON, I., MARQUISS, M., & MOSS, D. (1979) Habitat, female age,
organochlorine compounds, and breeding of European sparrowhawks. J.
appl. Ecol., 16: 777-793.
NISHIMURA, N., NISHIMURA, H., & OSHIMA, H. (1982) Survey on
mutagenicity of pesticides by the Salmonella microsome test. J.
Aichi Med. Univ. Assoc., 10(4): 305-312.
NOHYNEK, G.J., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Metabolism, excretion, and tissue distribution of 14C-photodieldrin
in non-human primates following oral administration and intravenous
injection. Ecotoxicol. environ. Saf., 3: 1-9.
O'CONNOR, R.J. (1982) Habitat occupancy and regulation of clutch
size in the European kestrel Falco tinnunculus. Bird Stud., 29:
17-26.
ODA, J. & MUELLER, W. (1972) Identification of a mammalian
breakdown product of dieldrin. Environ. Qual. Saf., 1: 248-249.
OHLENDORF, H.M., BARTONEK, J.C., DIVOKY, G.J., KLAAS, E.E., &
KRYNITSKY, A.J. (1982) Organochlorine residues in eggs of Alaskan
seabirds, Washington, DC, US Department of the Interior, Fish and
Wildlife Service (Special Scientific Report: Wildlife No. 245).
OLOFFS, P.C., HARDWICK, D.F., SZETO, S.Y., & MOERMAN, D.G. (1974)
DDT, dieldrin, and heptachlorepoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
ONSAGER, J.A., RUSK, H.W., & BUTLER, L.I. (1970) Residues of
aldrin, dieldrin, chlordane, and DDT in soil and sugar beets. J.
econ. Entomol., 63(4): 1143-1146.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic changes
in the liver of rats after feeding low levels of various
insecticides. Am. Med. Assoc. Arch. Pathol., 64(6): 614-622.
OTTOLENGHI, A.D., HASEMAN, J.K., & SUGGS, F. (1974) Teratogenic
effects of aldrin, dieldrin, and endrin in hamsters and mice.
Teratology, 9: 11-16.
PACCAGNELLA, B., GHEZZO, F., PRATI, L., FEDRAZZONI, U., & BELLONI,
G. (1971) Epidemiological study of long-term effects of pesticides
on human health. Bull. World Health Organ., 45: 181-199.
PAINTER, R.B. (1981) DNA synthesis inhibition in mammalian cells as
a test for mutagenic carcinogens. In: Stich, H.F. & San, R.H.C.,
ed. Short-term tests for chemical carcinogens, New York, Springer-
Verlag.
PARK, P.O. & MCKONE, C.E. (1966) The persistence and distribution
of soil-applied insecticides in an irrigated soil in northern
Tanzania. Trop. Agric. Trin., 43(2): 133-142.
PARSLOW, J.L.F. (1973) Breeding birds of Britain and Ireland: a
historical survey, Berkhamsted, Poyser T. and A.D., Ltd.
PARSLOW, J.L.F. & JEFFERIES, D.J. (1973) Relationship between
organochlorine residues in livers and whole bodies of guillemots.
Environ. Pollut., 5: 87-101.
PARSONS, A.M. & MOORE, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem.
Soc., C: 2026-2031.
PATEL, T.B. & RAO, V.N. (1958) Dieldrin poisoning in man. A report
of 20 cases in Bombay State. Br. med. J., 1: 919-921.
PATON, R., LUKE, B.G., & ROBERTS, G. (1984) Studies on the sorption
of organochlorine insecticides by flour stored on or near treated
laminated timber or plywood as used in freight containers. Pestic.
Sci., 15: 624-629.
PEARCE, P.A., PEAKALL, D.B., & REYNOLDS, L.M. (1979) Shell thinning
and residues of organochlorines and mercury in seabird eggs,
eastern Canada, 1970-76. Pestic. monit. J., 13(2): 61-68.
PEARSON, J.E., TINSLEY, K., & HERNANDEZ, T. (1973) Distribution of
dieldrin in the turtle. Bull. environ. Contam. Toxicol., 10(6):
360-364.
PETROCELLI, S.R., HANKS, A.R., & ANDERSON, J.W. (1973) Uptake and
accumulation of an organochlorine insecticide (dieldrin) by an
estuarine mollusc Rangia cuneata. Bull. environ. Contam. Toxicol.,
10(5): 315-320.
PETROCELLI, S.R., ANDERSON, J.W., & HANKS, A.R. (1975)
Biomagnification of dieldrin residues by food-chain transfer from
clams to blue crabs under controlled conditions. Bull. environ.
Contam. Toxicol., 13(1): 108-116.
PIONKE, H.B. & CHESTERS, G. (1973) Pesticide-sediment-water
interactions. J. environ. Qual., 2(1): 29-45.
POLISHUK, Z.W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health, 20:
215-217.
POLISHUK, Z.W., RON, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D.,
& LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. monit. J., 10(4): 121-129.
PORTER, R.D. & WIEMEYER, S.N. (1969) Dieldrin and DDT: effects on
sparrowhawk eggshells and reproduction. Science, 165: 199-200.
POTTER, J.C., DIXON, L.D., BARBER, G.F., & MARXMILLER, R.L. (1972)
Residues of 14 C-dieldrin and its metabolites in milk from cows fed
dieldrin-14 C, Modesto, California, Shell Development Company
(TIR-24-109-72) (Unpublished proprietary report).
POWELL, A.J.B., STEVENS, T., & MCCULLY, K.A. (1970) Effects of
commercial processing on residues of aldrin and dieldrin in
tomatoes and residues in subsequent crops grown on the treated
plots. J. agric. food Chem., 18(2): 224-227.
POWERS, C.D, ROWLAND, R.G., & WURSTER, C.F. (1977) Dieldrin-induced
destruction of marine algal cells with concomitant decrease in size
of survivors and their progeny. Environ. Pollut., 12: 17-25.
PRESTT, I. (1965) An enquiry into the recent breeding status of
some of the smaller birds of prey and crows in Britain. Bird Stud.,
12(3): 196-221.
PRESTT, I. & BELL, A.A. (1966) An objective method of recording
breeding distribution of common birds of prey in Britain. Bird
Stud., 13(4): 277-283.
PRINCI, F. (1954) Toxicity of the chlorinated hydrocarbon
insecticides. In: Proceedings of the XI International Congress di
Medicina del Lavoro, Pipola, Napoli, Tipografia Saverio, pp.
253-272.
PRINCI, F. & SPURBECK, G.H. (1951) A study of workers exposed to
the insecticides chlordane, aldrin, dieldrin. Arch. ind. Hyg.
occup. Med., 3: 64-72.
PROBST, A.H. & EVERLY, R.T. (1957) Effect of soil insecticides on
emergence, growth, yield, and chemical composition of soybeans.
Agron. J., 1957: 385-387.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: a comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagenesis, 3: 11-32.
PROSPERO, J.M. & SEBA, D.B. (1972) Some additional measurements of
pesticides in the lower atmosphere of the northern equatorial
Atlantic Ocean. Atmos. Environ., 6(5): 363-364.
PROUTY, R.M., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., CROMARTIE,
E., KAISER, T.E., LAMONT, T.G., MULHERN, B.M., & SWINEFORD, D.M.
(1977) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1973-74. Pestic. monit.
J., 11(3): 134-137.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON,
D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of 6
short-term tests for detecting organic chemical carcinogens. Br. J.
Cancer, 37: 873-903.
RADELEFF, R.D., WOODARD, G.T., NICKERSON, W.J., & BUSHLAND, R.C.
(1955) The acute toxicity of chlorinated hydrocarbon and organic
phosphorus insecticides to livestock, Kerville, Texas, US
Department of Agriculture, Agricultural Research Service,
(Technical Bulletin 1122).
RADELEFF, R.D., NICKERSON, W.J., & WELLS, R.W. (1960) Acute toxic
effects upon livestock and meat and milk residues of dieldrin. J.
econ. Entomol., 53(3): 425-429.
RADOMSKI, J.L. & FISEROVA-BERGEROVA, V. (1965) The determination of
pesticides in tissues with the electron-capture detector without
prior clean-up. Ind. Med. Surg., 32: 934-939.
RADOMSKI, J.L., DEICHMANN, W.B., & CLIZER, E.E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
RADOMSKI, J.L., ASTOLFI, E., DEICHMANN, W.B., & REY, A.A. (1971)
Blood levels of organochlorine pesticides in Argentina:
occupationally and non-occupationally exposed adults, children, and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RATCLIFFE, D.A. (1963) The status of the peregrine in Great
Britain. Bird Stud., 10: 56-90.
RATCLIFFE, D.A. (1965) The peregrine situation in Great Britain,
1963-64. Bird Stud., 12(2): 66-82.
RATCLIFFE, D.A. (1967a) Decrease in eggshell weight in certain
birds of prey. Nature (Lond.), 215: 208-210.
RATCLIFFE, D.A. (1967b) The peregrine situation in Great Britain,
1965-66. Bird Stud., 14(4): 238-245.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol., 7: 67-115.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Stud., 19: 117-156.
RATCLIFFE, D.A. (1980) The peregrine falcon, Calton, T. and A.D.
Poyser.
RATCLIFFE, D.A. (1984) The peregrine breeding population of the
United Kingdom in 1981. Bird Stud., 31(1): 1-18.
REICHEL, W.L., CROMARTIE, E., LAMONT, T.G., MULHERN, B.M., &
PROUTY, R.M. (1969) Pesticide residues in eagles. Pestic. monit.
J., 3(3): 142-144.
REIDINGER, R.F. & CRABTREE, D.G. (1974) Organochlorine residues in
golden eagles, United States, March 1964-July 1971. Pestic. monit.
J., 8(1): 37-43.
REINERT, R.E. (1972) Accumulation of dieldrin in an alga
(Scenedesmus obliquus), Daphnia magna, and the guppy (Poecilia
reticulata). J. Fish. Res. Board Can., 29(10): 1413-1418.
REINKE, J., UTHE, J.F., & JAMIESON, D. (1972) Organochlorine
pesticide residues in commercially caught fish in Canada, 1970.
Pestic. monit. J., 6(1): 43-49.
REIMOLD, R.J. (1975) Chlorinated hydrocarbon pesticides and mercury
in coastal biota - Puerto Rico and the US Virgin Islands 1972-1974.
Pestic. monit. J., 9(1): 39-43.
REYNOLDS, C.M. (1974) The census of heronries, 1969-73. Bird Stud.,
21: 129-134.
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin, and endrin. Ind. Arch. occup. environ. Health,
56: 75-79.
RICE, C.P. & SIKKA, H.C. (1973) Fate of dieldrin in selected
species of marine algae. Bull. environ. Contam. Toxicol., 9(2):
116-123.
RICHARD, J.J., JUNK, G.A., AVERY, M.J., NEHRING, N.L., FRITZ, J.S.,
& SVEC, H.J. (1975) Analysis of various Iowa waters for selected
pesticides: atrazine, DDE, and dieldrin - 1974. Pestic. monit. J.,
9(3): 117-123.
RICHARDSON, A. (1971) The isolation and identification of a
metabolite of HEOD (dieldrin) from human faeces, Sittingbourne,
Shell Research (TLGR.0021.71).
RICHARDSON, A. & ROBINSON, J. (1971) The identification of a major
metabolite of HEOD (dieldrin) in human faeces. Xenobiotica, 1(3):
213-219.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967a)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
RICHARDSON, L.A., LANE, J.R., GARDNER, W.S., PEELER, J.T., &
CAMPBELL, J.E. (1967b) Relationship of dietary intake to
concentration of dieldrin and endrin in dogs. Bull. environ.
Contam. Toxicol., 2(4): 207-219.
RICHARDSON, A., BALDWIN, M.K., & ROBINSON, J. (1968) Metabolites of
dieldrin (HEOD) in the urine and faeces of rats. Chem. Ind., 1968:
588-589.
RICKARD, D.G. & DULLEY, M.E.R. (1983) The levels of some heavy
metals and chlorinated hydrocarbons in fish from the tidal Thames.
Environ. Pollut. Ser. B., 5: 101-119.
RISEBROUGH, R.W., HUGGETT, R.J., GRIFFIN, J.J., & GOLDBERG, E.D.
(1968) Pesticides: transatlantic movements in the northeast trades.
Science, 159(3820): 1233-1236.
RITCEY, W.R., SAVARY, G., & MCCULLY, K.A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can. J.
public Health, 64: 380-386.
ROBINSON, J. (1969) Organochlorine insecticides and bird
populations in Britain. In: Miller, M.W. & Berg, G.G., ed. Chemical
fallout, Springfield, Illinois, C.C. Thomas, pp. 113-169.
ROBINSON, J. & CRABTREE, A.N. (1969) The effect of dieldrin on
homing pigeons ( Columba livia var.). Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 34(3): 413-427.
ROBINSON, J. & HUNTER, C.G. (1966) Organochlorine insecticides:
concentrations in human blood and adipose tissue. Arch. environ.
Health, 13: 558-563.
ROBINSON, J. & ROBERTS, M. (1969) Estimation of the exposure of the
general population to dieldrin (HEOD). Food Cosmet. Toxicol., 7:
501-514.
ROBINSON, J., RICHARDSON, A., & ELGAR, K.E. (1966a) Chemical
identity in ultramicroanalysis. In: Proceedings of the American
Chemical Society Meeting, New York, 11-16 September, 1966,
Washington, DC, American Chemical Society.
ROBINSON, J., RICHARDSON, A., BUSH, B., & ELGAR, K.E. (1966b) A
photoisomerization product of dieldrin. Bull. environ. Contam.
Toxicol., 1(4): 127-132.
ROBINSON, J., RICHARDSON, A., & DAVIES, J.M. (1967a) Comparison of
analytical methods for determination of dieldrin (HEOD) in blood.
Arch. environ. Health, 15(1): 67-69.
ROBINSON, J., BROWN, V.K.H., RICHARDSON, A., & ROBERTS, M. (1967b)
Residues of dieldrin (HEOD) in the tissues of experimentally
poisoned birds. Life Sci., 6: 1207-1220.
ROBINSON, J., ROBERTS, M., BALDWIN, M., & WALKER, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Food Cosmet.
Toxicol., 7: 317-332.
ROBURN, J. (1963) Effect of sunlight and ultraviolet radiation on
chlorinated pesticide residues. J. chem. Ind., 1: 1555-1556.
ROCCHI, P., PEROCCO, P., ALBERGHINI, W., FINI, A., & PRODI, G.
(1980) Effect of pesticides on scheduled and unscheduled DNA
synthesis of rat thymocytes and human lymphocytes. Arch. Toxicol.,
45: 101-108.
ROHWER, D. (1983a) [Pesticides in breast milk (problems with
residues of polychlorinated hydrocarbons).] Wiss. Inf., 9(3):
197-201 (in German).
ROHWER, D. (1983b) [Pesticides in breast milk.] Geburtshilfe
Frauenheilkd., 43: 160-163 (in German).
ROSE, G.P. (1982) Toxicity of insecticides: the acute oral and
percutaneous toxicity, skin and eye irritancy, and skin sensitizing
potential of a 480 g/litre emulsifiable concentrate of aldrin (EF
5159), Sittingbourne, Shell Research (SBGR.81.319) (Unpublished
proprietary report).
ROSE, G.P. (1984a) Toxicology of insecticides: the acute
percutaneous toxicity of the 480 g/litre aldrin emulsifiable
concentrate formulation CF 06323, Sittingbourne, Shell Research
(SBGR.84.047) (Unpublished proprietary report).
ROSE, G.P. (1984b) Toxicology of insecticides: the acute
percutaneous toxicity of the 200 g/litre dieldrin emulsifiable
concentrate formulation CF 06271, Sittingbourne, Shell Research
(SBGR.84.045) (Unpublished proprietary report).
ROSE, G.P. (1984c) Toxicology of insecticides (organochlorines):
the acute percutaneous toxicity of the 680 g/l dieldrin suspension
concentrate SF 06349, Sittingbourne, Shell Research (SBGR.84.210)
(Unpublished proprietary report).
ROSEN, J.D. & CAREY, W.F. (1968) Preparation of the photoisomers of
aldrin and dieldrin. J. agric. food Chem., 16: 536-537.
ROSEN, J.D., SUTHERLAND, D.J., & LIPTON, G.R. (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull.
environ. Contam. Toxicol., 1(4): 133-140.
ROSEWELL, K.T., MUIR, D.C.G., & BAKER, B.E. (1979) Organochlorine
residues in Harp Seal (Phagophilus groenlandicus) tissues, Gulf of
St. Lawrence, 1971, 1973. Pestic. monit. J., 12: 189-192.
ROSS, R.D. & CROSBY, D.G. (1974) Photosensitizers in agricultural
water samples, Washington, DC, American Chemical Society (ACS
Meeting, Abstract No. 167, Section PEST 67).
ROSS, R.D. & CROSBY, D.G. (1975) The photooxidation of aldrin in
water. Chemosphere, 4: 227.
ROSS, R.D. & CROSBY, D.G. (1985) Photooxidant activity in natural
waters. Environ. Toxicol. Chem., 4: 773-778.
ROSS, W.R., VAN LEEUWEN, J., & GRABOW, W.O.K. (1976) Studies on the
disinfection and chemical oxidation with ozone and chlorine in
water reclamation. In: Proceedings of the Second Symposium on Ozone
Technology, Montreal, International Ozone Institute, pp. 479-513.
ROWE, D.B., CANTER, L.W., SNIJDER, P.J., & MASON, J.W. (1971)
Dieldrin and endrin concentrations in a Louisiana estuary. Pestic.
monit. J., 4(4): 177-183.
RUEBNER, B.H., GERSHWIN, M.E., MEIERHENRY, E.F., HSIEH, L.S., &
DUNN, P.L. (1984a) Irreversibility of liver tumours in C3H mice. J.
Natl Cancer Inst., 73(2): 493-498.
RUEBNER, B.H., GERSHWIN, M.E., FRENCH, S.W., MEIERHENRY, E., DUNN,
P., & HSIEH, L.S. (1984b) Mouse hepatic neoplasia: Differences
among strains and carcinogens. In: Popp J.A., ed. Current
perspectives in mouse liver neoplasia, Washington, DC, Hemisphere.
SAHA, J.G. & SUMNER, A.K. (1971) Organochlorine insecticide
residues in soil from vegetable farms in Saskatchewan. Pestic.
monit. J., 5(1): 28-31.
SAHA, J.G., KARAPALLY, J.C., & JANSEN, W.K. (1971) Influence of the
type of mineral soil on the uptake of dieldrin by wheat seedlings.
J. agric. food Chem., 19: 842-845.
SAND, P.F., WIERSMA, G.B., & LANDRY, J.L. (1972) Pesticide residues
in sweet potatoes and soil - 1969. Pestic. monit. J., 5(4): 342-344.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the
western chorus frog Pseudacris triseriata and Fowler's Toad Bufo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDHU, S.-S., WARREN, W.J., & NELSON, P. (1978) Pesticidal residue
in rural potable water. Am. Water Works Assoc. J., 70(1): 41-45.
SANDIFER, S.H., CUPP, C.M., WILKINS, R.T., LOADHOLT, B., & SCHUMAN,
S.H. (1981) A case-control study of persons with elevated blood
levels of dieldrin. Arch. environ. Contam. Toxicol., 10(1): 35-45.
SANGER, A.M.H. (1959) Aldrin, dieldrin, and endrin toxicity to
bees. Span, 2(2): 59-63.
SASCHENBRECKER, P.W. (1976) Levels of terminal pesticide residues
in Canadian meat, Can. Vet. J., 17(6): 158-163.
SAVAGE, E.P., KEEFE, T.J., TESSARI, J.D., WHEELER, H.W., APPLEHAUS,
F.M., GOES, E.A., & FORD, S.A. (1981) National study of chlorinated
hydrocarbon insecticide residues in human milk, USA. I. Geographic
distribution of dieldrin, heptachlor, heptachlor epoxide,
chlordane, oxychlordane, and mirex. Am. J. Epidemiol., 113: 413-422.
SCHAFER, M.L. (1968) Pesticides in blood. Residue Rev., 24: 19-39.
SCHAUBERGER, C.W. & WILDMAN, R.B. (1977) Accumulation of aldrin and
dieldrin by blue-green algae and related effects on photosynthetic
pigments. Bull. environ. Contam. Toxicol., 17(5): 534-541.
SCHULZE, J.A., MANIGOLD, D.B., & ANDREWS, F.L. (1973) Pesticides in
selected western streams - 1968-71. Pestic. monit. J., 7(1): 73-84.
SEAL, W.L., DAWSEY, L.H., & CAVIN, G.E. (1967) Monitoring for
chlorinated hydrocarbon pesticides in soil and root crops in the
eastern states in 1965. Pestic. monit. J., 1(3): 22-25.
SELBY, L.A., NEWELL, K.W., HAUSER, G.A., & JUNKER, G. (1969a)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2(4): 247-255.
SELBY, L.A., NEWELL, K.W., WAGGENSPACK, C., HAUSER, G.A., & JUNKER,
G. (1969b) Estimating pesticide exposure in man as related to
measurable intake: environmental versus chemical index. Am. J.
Epidemiol., 89(3): 241-253.
SETHUNATHAN, N. (1973) Microbial degradation of insecticides in
flooded soil and in anaerobic cultures. Residue Rev., 47: 143-165.
SHAH, P.V. & GUTHRIE, F.E. (1976) Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Watson, D.L. &
Brown, A.W.A., ed. Pesticide management and insecticide resistance,
New York, London, Academic Press, pp. 547-554.
SHAKOORI, A.R., RASUL, Y.G., & ALI, S.S. (1986) The effect of long-
term administration of dieldrin on biochemical components in blood
serum of albino rats. Toxicol. Lett., 9(4): 35.
SHANNON, L.R. (1977a) Equilibrium between uptake and elimination of
dieldrin by channel catfish Ictalurus punctatus. Bull. environ.
Contam. Toxicol., 17(3): 278-284.
SHANNON, L.R. (1977b) Accumulation and elimination of dieldrin in
muscle tissue of channel catfish. Bull. environ. Contam. Toxicol.,
17(6): 637-644.
SHAROM, M.S., MILES, J.R.W., HARRIS, C.R., & MCEWEN, F.L. (1980)
Behaviour of 12 insecticides in soil and aqueous suspensions of
soil and sediment, Water Res., 14: 1095-1100.
SHEETS, T.J., JACKSON, M.D., & PHELPS, L.D. (1970) Water monitoring
system for pesticides in North Carolina, US Clearinghouse, 109 pp
(P.B. Report No. 189291).
SHELL (1976) Chemicals for plant protection, veterinary uses, and
public health, London, Shell International Chemical Company, Ltd,
pp. 3-4, 9-10.
SHELL (1984) Shell guide to pesticide safety, London, Shell
International Chemical Company, Ltd, Agrochemical Division, pp.
27-29.
SHELLENBERGER, T.E. (1978) A multi-generation toxicity evaluation
of p,p'-DDT and dieldrin with Japanese quail. Effects on growth and
reproduction. Drug chem. Toxicol., 1(2): 137-146.
SHELLENBERGER, T.E. & NEWELL, G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail (Coturnix coturnix
japonica). Lab. anim. Care, 15(2): 119-130.
SHERMAN, M. & ROSENBERG, M.M. (1953) Acute toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 46(6): 1067-1070.
SHIRASU, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4: 226-231.
SIERRA, M. & SANTIAGO, D. (1987) Organochlorine pesticide levels in
barn owls collected in Leon, Spain. Bull. environ. Contam.
Toxicol., 38: 261-265.
SIERRA, M., TERAN, M.T., GALLEGO, A., DIEZ, M.J., & SANTIAGO, D.
(1987) Organochlorine contamination in three species of diurnal
raptors in Leon, Spain. Bull. environ. Contam. Toxicol., 38: 254-260.
SILBERGELD, E.K. (1973) Dieldrin. Effects of chronic sublethal
exposure on adaptation to thermal stress in fresh-water fish.
Environ. Sci. Technol., 7(9): 846-849.
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.C. (1977) Mutagenic
activity of chemicals identified in drinking-water. In: Scott, D.,
Bridges, B.A., & Sobels, F.H., ed. Progress in genetic toxicology,
Amsterdam, Elsevier Science Publishers, pp. 249-258.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY, M.O.
(1983) Evaluation of the alkaline elution/rat hepatocyte assay as a
predictor of carcinogenic/mutagenic potential. Mutat. Res., 113:
357-391.
SINGH, K.K., JHA, G.J., SINGH, P.N., & CHAUHAN, H.V.S. (1985)
Pathophysiology of acute aldrin intoxication in goats. Toxicol.
Abstr., 8(7): 27 (No. 4086-x8).
SIYALI, D.S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust., 2: 1063-1066.
SIYALI, D.S. (1973) Polychlorinated biphenyls, hexachlorobenzene,
and other organochlorine pesticides in human milk. Med. J. Aust.,
2: 815-818.
SIYALI, D.S. & SIMSON, R.E. (1973) Chlorinated hydrocarbon
pesticides in human blood and fat. Med. J. Aust., 1: 212-213.
SLATER, R.M. & SPEDDING, D.J. (1981) Transport of dieldrin between
air and water. Arch. environ. Contam. Toxicol., 10: 25-33.
SMITH, J.H., BOMBERGER, D.C., Jr, & HAYNES, D.L. (1981)
Volatilization rates of intermediate and low volatility chemicals
from water. Chemosphere, 10(3): 281-289.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of
DDT and dieldrin on development in winter flounder
(Pseudopleuronectes americanus). J. Fish. Res. Board Can., 30(12):
1894-1898.
SMITH, S.W.C. (1978) Pesticide residues in the total diet. Food
Technol. Aust., 1978: 349-352.
SPALDING, R.F., JUNK, C.A., & RICHARD, J.R. (1980) Pesticides in
groundwater beneath irrigated farmland in Nebraska, August 1978.
Pestic. monit. J., 14: 70-73.
SPARR, B.I., APPLEBY, W.G., DEVRIES, D.M., OSMUN, J.V., MCBRIDE,
J.M., FOSTER, G.L., & GOULD R.F., ed. (1966) Organic pesticides in
the environment, Washington, DC, American Chemical Society, pp.
146-162 (Advances in Chemistry Series No. 60).
SPENCER, W.F. & CLIATH, M.M. (1973) Pesticide volatilization as
related to water loss from soil. J. environ. Qual., 2(2): 284-289.
SPENCER, W.F. & CLIATH, M.M. (1975) Environmental dynamics of
pesticides. In: Haque, R. & Freed, V.H. ed., New York, London,
Plenum Press, pp. 61-78.
SPENCER, W.F., CLIATH, M.M., & FARMER, W.J. (1969) Vapor density of
soil-applied dieldrin as related to soil-water content,
temperature, and dieldrin concentration. Soil Sci. Soc. Am. Proc.,
33: 509-511.
SPENCER, W.F., FARMER, W.J., & CLIATH, M.M. (1973) Pesticide
volatilization. Residue Rev., 49: 1-47.
SPIOTTA, E.J. (1951) Aldrin poisoning in man. Report of a case.
Arch. ind. Hyg. occup. Med., 4: 560-566.
STACEY, C.I. & TATUM, T. (1985) House treatment with organochlorine
pesticides and their levels in human milk - Perth, Western
Australia. Bull. environ. Contam. Toxicol., 35: 202-208.
STACEY, C.I. & THOMAS, B.W. (1975) Organochlorine pesticide
residues in human milk, Western Australia, 1970-71. Pestic. monit.
J., 9(2): 64-66.
STACEY, C.I., PERRIMAN, W.S., & WHITNEY, S. (1985) Organochlorine
pesticides residue levels in human milk: Western Australia,
1979-80. Arch. environ. Health, 40: 102-108.
STANLEY, C.W., BARNEY, J.E., HELTON, M.R., & YOBS, A.R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANLEY, P.I. & BUNYAN, P.J. (1979) Hazards to wintering geese and
other wildlife from the use of dieldrin, chlorfenvinphos, and
carbophenothion as wheat seed treatments. Proc. R. Soc. Lond. Ser
B, 205: 31-45.
STEVENS, L.J., COLLIER, C.W., & WOODHAM, D.W. (1970) Monitoring
pesticides in soils from areas of regular, limited, and no
pesticide use. Pestic. monit. J., 4(3): 145-164.
STEVENSON, D.E. & WALKER, A.I.T. (1969) Hepatic lesions produced in
mice by dieldrin and other hepatic enzyme-inducing compounds. J.
Eur. Toxicol., 2: 83-84.
STEVENSON, D.E., THORPE, E., HUNT, P.F., & WALKER, A.I.T. (1976)
The toxic effects of dieldrin in rats: a re-evaluation of data
obtained in a two-year feeding study. Toxicol. appl. Pharmacol., 36:
247-254.
STEWART, D.J. & STEIN, R.A. (1974) Short-term fate of dietary
dieldrin in the digestive tract of juvenile lake trout (Salvelinus
namaycush). Bull. environ. Contam. Toxicol., 11(6): 563-566.
STEWART, D.K.R. & FOX, C.J.S. (1971) Persistence of organochlorine
insecticides and their metabolites in Nova Scotian soils. J. econ.
Entomol., 64(2): 367-371.
STEWART, D.K.R. & GAUL, S.O. (1977) Dihydrochlordene dicarboxylic
acid residues in soil treated with high rates of aldrin. Bull.
environ. Contam. Toxicol., 17(6): 712-713.
STICKEL, W.H. (1975) Some effects of pollutants in terrestrial
ecosystems. Environ. Sci. Res., 7: 25-74.
STICKEL, W.H., STICKEL, L.F., & SPANN, J.W. (1969) Tissue residues
of dieldrin in relation to mortality in birds and mammals. In:
Miller, M.W. & Berg, G.G., ed. Chemical fallout, Springfield,
Illinois, C.C. Thomas, pp. 174-200.
SUNDARAM, K.S., DAMODARAN, V.N., & VENKITASUBRAMANIAM, T.A.
(1978) Absorption of dieldrin through monkey and dog skin. Indian
J. exp. Biol., 16: 101-103.
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1974) Photodieldrin
residues in field soils. Bull. environ. Contam. Toxicol., 12:
275-279.
SWENBERG, J.A., PETZOLD, G.L., & HARBACH, P.R. (1976) In vitro DNA
damage-alkaline elution assay of predicting carcinogenic potential.
Biochem. Biophys. Res. Commun., 72(2): 732-738.
SZARO, R.C., COON, N.C., & KOLBE, E. (1979) Pesticide and PCB of
common eider, herring gull, and great black-backed gull eggs. Bull.
environ. Contam. Toxicol., 22: 394-399.
TABOR, E.C. (1966) Contamination of urban air through the use of
insecticides. Trans. N.Y. Acad. Sci., 28: 569-578.
TAKEI, G.H., KAUAHIKAUA, S.M., & LEONG, G.H. (1983) Analyses of
human milk samples collected in Hawaii for residues of
organochlorine pesticides and polychlorobiphenyls. Bull. environ.
Contam. Toxicol., 30: 606-613.
TANNOCK, J., HOWELLS, W.W., & PHELPS, R.J. (1983) Chlorinated
hydrocarbon pesticide residues in eggs of some birds in Zimbabwe.
Environ. Pollut. Ser. B, 5: 147-155.
TARRANT, K.R. & TATTON, J.O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TATTON, J.O'G. & RUZICKA, J.H.A. (1967) Organochlorine pesticides
in Antarctica. Nature (Lond.), 215: 346-348.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., & FREEMAN, H.P. (1972)
Measurement of volatilization of dieldrin and heptachlor from a
soil in the field. In: Abstracts of the 163rd National Meeting of
the American Chemical Society, Boston, Massachusetts, Washington,
DC, American Chemical Society.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., FREEMAN, H.P., &
EDWARDS, W.M. (1976) Volatilization of dieldrin and heptachlor from
a maize field. J. agric. food Chem., 24(3): 625-631.
TEJEDOR, M.C., MURADO, M.A., & BALUJA, G. (1974) [Contamination of
the environment by organochlorine pesticides.] An. Quim., 70:
1177-1183 (in Spanish).
TENNEKES, H.A., WRIGHT, A.S., DIX, K.M., & KOEMAN, J.H. (1981)
Effects of dieldrin, diet and bedding on enzyme function and tumour
incidence in livers of male CF-1 mice. Cancer Res., 41: 3615-3620.
TENNEKES, H.A., RAVENZWAAY, B., & KUNZ, H.W. (1985) Quantitative
aspects of enhanced liver tumour formation in CF-1 mice by
dieldrin. Carcinogenesis, 6(10): 1457-1462.
TERRIERE, L.C. & YU, S.J. (1976) Microsomal oxidases in the flesh
fly ( Sarcophaga bullata Parker) and the black blow fly ( Phormia
regina Meigen). Pestic. Biochem. Physiol., 6: 223-228.
THOMPSON, A.R., EDWARDS, C.A., EDWARDS, M.J., & BEYNON, K.I. (1970)
Movement of dieldrin through soils. II. In sloping troughs and soil
columns. Pestic. Sci., 1(5): 174-178.
THOMPSON, J.F. (1976) Manual of analytical quality control for
pesticides and related compounds in human and environmental
samples, Research Triangle Park, North Carolina, US Environmental
Protection Agency, Office of Research and Development, Health
Effects Research Laboratory (EPA-600/1-76-017).
THORPE, E. (1973) The toxicology of dieldrin (HEOD):
transplantation of liver tumours in mice, Sittingbourne, Shell
Research (TLGR.0041.73) (Unpublished proprietary report).
THORPE, E. & HUNT, P.F. (1975) Toxicology of dieldrin (HEOD): study
of the pathological changes in 3 strains of mice following
prolonged ingestion of dieldrin, Sittingbourne, Shell Research
(TLGR.0012.75) (Unpublished proprietary report).
THORPE, E. & WALKER, A.I.T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, beta-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TREON, J.F. & CLEVELAND, F.P. (1955) Toxicity of certain
chlorinated hydrocarbon insecticides for laboratory animals, with
special reference to aldrin and dieldrin. J. agric. food Chem.,
3(5): 402-408.
TREON, J.F., DUTRA, F.R., SHAFFER, F.E., CLEVELAND, F.P., WAGNER,
W., & GAHEGAN, T. (1951) The toxicity of aldrin, dieldrin, and DDT
when fed to rats over the period of six months, Cincinnati, Ohio,
Kettering Laboratory.
TREON, J.F., HARTMAN, L., GAHEGAN, T., & NEDDERMANN, G. (1953) The
immediate and cumulative toxicity of aldrin, dieldrin, and DDT when
maintained in contact with the skin of rabbits, Cincinnati, Ohio,
Kettering Laboratory.
TROSKO, J.E., JONE, C., & CHANG, C.C. (in press) Inhibition of gap-
functional mediated intercellular communication in vitro by aldrin,
dieldrin and toxaphene: a possible cellular mechanism for their
tumour-promoting and neurotoxic effects. Mol. Toxicol.
TU, C.M. (1981) Effects of some pesticides on enzyme activities in
an organic soil. Bull. environ. Contam. Toxicol., 27: 109-114.
TU, C.M. & MILES, J.R.W. (1976) Interactions between insecticides
and soil microbes. Residue Rev., 64: 17-65.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington, DC, US Department of the
Interior, Bureau of Sport Fishing and Wildlife, pp. 16-17, 46-47
(Resource Publication No. 84).
TUCKER, R.K. & HAEGELE, M.A. (1971) Comparative acute oral toxicity
of pesticides to six species of birds. Toxicol. appl. Pharmacol.,
20(1): 57-65.
TUINSTRA, L.G.M.TH. (1971) Organochlorine insecticide residues in
human milk in the Leiden region. Ned. Melk. Zuiveltijdschr., 25:
24-32.
TURNER, B.C., GLOTFELTY, D.E., & TAYLOR, A. (1977) Photodieldrin
formation and volatilization from grass. J. agric. food Chem., 25:
548-550.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., THEARLE, R.J.P., EGAN, H.,
EVANS, W.H., & SOUTAR, N.M. (1963) The effects on birds of certain
chlorinated insecticides used as seed dressings. J. Sci. Food
Agric., 14(8): 567-577.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., MURTON, R.K., BRADY, J.,
THEARLE, R.J.P., JONES, F.J.S., REA, R.E., KIRBY, D.R., SMITH,
B.M., & RUTTER, M.E. (1965) Pesticides and wildlife. Report of the
Infestation Control Laboratory for 1962-64, London, Her Majesty's
Stationary Office, pp. 23-47.
UK-HMSO (1986) Food surveillance paper, London, Her Majesty's
Stationary Office, (Document No. 16).
UK-MAFF (1982-85) Report of the Working Party on Pesticide
Residues, London, Ministry of Agriculture, Fisheries and Food
(Food Surveillance Paper No. 16).
US EPA (1983) Environmental news, Washington, DC, US Environmental
Protection Agency, Office of Public Affairs.
UYETA, M., TAUE, S., CHIKAZAWA, K., & NISHIMOTO, T. (1971)
Pesticides translocated in food - organochlorine pesticides in the
total diet. J. Food Hyg. Soc. Jpn. 12: 445.
UZOUKWU, M. & SLEIGHT, S.D. (1972) Effects of dieldrin in pregnant
sows. J. Am. Vet. Med. Assoc., 160(12): 1641-1643.
VAN DEN BERCKEN, J. (1972) An electrophysiological investigation
into the action of DDT, dieldrin, and allethrin in the clawed toad
Xenopus laevis, Utrecht, Rijks Universiteit (Thesis).
VAN DEN BERCKEN, J. & NARAHASHI, T. (1974) Effects of aldrin-
transdiol, a metabolite of the insecticide dieldrin, on nerve
membrane. Eur. J. Pharmacol., 27: 255-258.
VAN DEN BROEK, W.L.F. (1979) Seasonal levels of chlorinated
hydrocarbons and heavy metals in fish and brown shrimps from the
Medway estuary, Kent. Environ. Pollut., 19: 21-38.
VAN DIJCK, P. & VAN DE VOORDE, H. (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 41(2): 1491-1498.
VAN DUURSEN, P. (1985) Open letter. Shell Post, 1985(671).
VAN GELDER, G.A. & CUNNINGHAM, W.L. (1975) The effects of low-level
dieldrin exposure on the EEG and learning ability of the squirrel
monkey. Toxicol. appl. Pharmacol., 33(1): 142 (Abstract No. 50).
VAN GENDEREN, H. (1965) The toxicology of the chlorinated
hydrocarbon insecticides. A progress report with particular
reference to the qualitative aspects of the action in warm-blooded
animals. Opzoekingsstn Staat Gent, 30(3): 1321-1335.
VAN GENDEREN, H. (1979) [Dieldrin: a troublesome insecticide.] K.
Ned. Akad. Wet. (Amsterdam) 88(3): 24-32 (in Dutch).
VAN GENUCHTEN, M.TH., DAVIDSON, J.M., & WIERENGA, P.J. (1974) An
evaluation of kinetic and equilibrium equations for the prediction
of pesticide movement through porous media. Soil Sci. Soc. Am.
Proc., 38: 29-35.
VAN HAVER, W., VANDEZANDE, A., & GORDTS, L. (1978) [Organochlorine
pesticides in human fatty tissue.] Arch. Belg. Méd. soc. Hyg. Méd.
Trav. Méd. lég., 36: 147-155 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN RAALTE, H.G.S. (1965) Aspects of pesticide toxicity. In:
Proceedings of the Conference on Occupational Health, Caracas,
Venezuela (Unpublished paper).
VAN RAALTE, H.G.S. (1977) Human experience with dieldrin in
perspective. Ecotoxicol. environ. Saf., 1: 203-210.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination
of water by a sublethal concentration of pesticides: a system
analysis approach. Acta hydrochim. hydrobiol., 12(4): 399-409.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and
telodrin. Br. J. ind. Med., 30: 201-202.
VIRGO, B.B. & BELLWARD, G.D. (1975) Effects of dietary dieldrin on
reproduction in the Swiss-Vancouver (SWV) mouse. Environ. Physiol.
Biochem., 5: 440-450.
VIRGO, B.B. & BELLWARD, G.D. (1977) Effects of dietary dieldrin on
offspring viability, maternal behaviour, and milk production in the
mouse. Res. Commun. chem. Pathol. Pharmacol., 17(3): 399-409.
VOERMAN, S. & BESEMER, A.F.H. (1975) Persistence of dieldrin,
lindane, and DDT in a light sandy soil and their uptake by grass.
Bull. environ. Contam. Toxicol., 13(4): 501-505.
VOUTSINOU-TALIADOURI, F. & SATSMADJIS, J. (1982) Influence of
metropolitan waste on the concentration of chlorinated hydrocarbons
and metals in striped mullet. Mar. Pollut. Bull., 13(8): 266-269.
VREMAN, K. & POORTVLIET, L.J. (1982) Pesticide levels in milk and
milk products in relation to their milk fat content. Neth. Milk
Dairy J., 36: 145-148.
VREMAN, K., POORTVLIET, L.J., & VAN DEN HOEK, J. (1980) Transfer of
organochlorine pesticides from feed into the milk and body fat of
cows. Long-term experiment with intake at low levels. Neth. Milk
Dairy J., 34: 87-105.
WADE, M.H., MOYER, J.W., & HINE, C.H. (1979) Mutagenic action of a
series of epoxides. Mutat. Res., 66: 367-371.
WADE, M.H., TROSKO, J.E., & SCHINDLER, M. (1986) A fluorescence
photobleaching assay of gap junction-mediated communication between
human cells. Science, 232: 525-528.
WALKER, A.I.T., NEILL, C.H., STEVENSON, D.E., & ROBINSON, J.
(1969a) The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix
coturnix japonica). Toxicol. appl. Pharmacol., 15: 69-73.
WALKER, A.I.T., STEVENSON, D.E., ROBINSON, J., THORPE, E., &
ROBERTS, M. (1969b) The toxicology and pharmacodynamics of dieldrin
(HEOD): two-year oral exposure of rats and dogs. Toxicol. appl.
Pharmacol., 15: 345-373.
WALKER, A.I.T., THORPE, E., ROBINSON, J., & BALDWIN, M.K. (1971)
Toxicity studies on the photoisomerisation product of dieldrin.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 36(1): 398-409.
WALKER, A.I.T., THORPE, E., & STEVENSON, D.E. (1972) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALTON, M.S., BECK-BASTONE, V., & BARON, R.L. (1971) Subchronic
toxicity of photodieldrin, a photodecomposition product of
dieldrin. Toxicol. appl. Pharmacol., 20(1): 82-88.
WARNICK, S.L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah, fiscal years 1967-71. Pestic. monit. J.,
6(1): 9-13.
WARNICK, S.L. & CARTER, J.E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASSERMANN, M., CURNOW, D.H., FORTE, P.N., & GRONER, Y. (1968)
Storage of organochlorine pesticides in the body fat of people in
western Australia. Int. J. ind. Med. Surg., 37(4): 295-300.
WASSERMANN, M., FRANCONE, M.P., WASSERMANN, D., MARIANI, F., &
GRONER, Y. (1969) [Organochlorine pesticide content in the fatty
tissue of the general population in the Republic of Argentina.]
Sem. med., 134: 459-462 (in Spanish).
WASSERMANN, M., WASSERMANN, D, LAZAROVICI, S., COETZEE, A.M., &
TOMATIS, L. (1970) Present state of the storage of the
organochlorine insecticides in the general population of South
Africa. South Afr. med. J., 44: 646-648.
WASSERMANN, M., NOGUEIRA, D.P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972a) Storage of organochlorine
insecticides in people of Sao Paulo, Brazil. Ind. Med. Surg., 41(3):
22-25.
WASSERMANN, M., ROGOFF, M.G., TOMATIS, L., DAY, N.E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of organochlorine
insecticides in the adipose tissue of people in Kenya. Ann. Soc.
Belg. Méd. Trop., 52(6): 509-514.
WASSERMANN, M., SOFOLUWE, G.O., TOMATIS, L., DAY, N.E., WASSERMANN,
D., & LAZAROVICI, S. (1972c) Storage of organochlorine insecticides
in people in Nigeria. Environ. Physiol. Biochem., 2: 59-67.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N.E.,
WASSERMANN, D., RUNGPITARANGSI, V., CHIAMSAKOL, V.,
DJAVAHERIAN, M., & CUCOS, S. (1972d) Storage of organochlorine
insecticides in the adipose tissue of people from Thailand.
Southeast Asian J. trop. Med. public Health, 3(2): 280-285.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol., 12(4):
501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in adipose tissue of Israelis. Pestic.
monit. J., 8(1): 1-7.
WATSON, M., BENSON, W.W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people of southern Idaho. Pestic. monit. J.,
4(2): 47-50.
WEDBERG, J.L., MOORE, S., III, AMORE, F.J., & MCAVOY, H. (1978)
Residues in food and feed. Organochlorine insecticide residues in
bovine milk and manufactured milk products in Illinois 1971-76.
Pestic. monit. J., 11: 161-164.
WEGMAN, R.C.C. & GREVE, P.A. (1974) Levels of organochlorine
pesticides and inorganic bromide in human milk. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 39: 1301-1310.
WEGMAN, R.C.C. & GREVE, P.A. (1978) Organochlorines, cholinesterase
inhibitors, and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic. monit. J., 12(3): 149-162.
WEISGERBER, I., KOHLI, J., KAUL, R., KLEIN, W., & KORTE, F. (1974)
Fate of aldrin-14C in maize, wheat, and soils under outdoor
conditions. J. agric. food Chem., 22(4): 609-612.
WEISGERBER, I., BIENIEK, D., KOHLI, J., & KLEIN, W. (1975)
Isolation and identification of three unreported photodieldrin-14C
metabolites in soil. J. agric. food Chem., 23: 873-877.
WELLS, M.R. & YARBROUGH, J.D. (1973) In vivo and in vitro retention
of 14C-aldrin and 14C-dieldrin in cellular fractions from brain and
liver tissues of insecticide-resistant and susceptible Gambusia.
Toxicol. appl. Pharmacol., 24: 190-196.
WELLS, M.R., PHILLIPS, J.B., & MURPHY, G.G. (1974) ATPase activity
in tissues of the map turtle Graptemys geographica following in
vitro treatment with aldrin and dieldrin. Bull. environ. Contam.
Toxicol., 11(6): 572-576.
WESSELS, C.L. (1978) Residues in soybean plants of aldrin and
dieldrin following soil application and of endosulphan and DDT
following foliar application. Rhodesian. J. agric. Res., 16:
205-210.
WHEATLEY, G.A. & HARDMAN, J.A. (1965) Indications of the presence
of organochlorine insecticides in rainwater in central England.
Nature (Lond.), 207: 486-487.
WHEATLEY, G.A. & HARDMAN, J.A. (1968) Organochlorine insecticide
residues in earthworms from arable soils. J. Sci. Food Agric., 19:
219-225.
WHEATLEY, G.A., HARDMAN, J.A., & STRICKLAND, A.H. (1962) Residues
of chlorinated hydrocarbon insecticides in some farm soils in
England. Plant Pathol., 11: 81-90.
WHITE, D.H. (1976) Nationwide residues of organochlorine in
starling, 1974. Pestic. monit. J., 10(1): 10-17.
WHITE, D.H., KING, K.A., & PROUTY, R.M. (1980) Significance of
organochlorine and heavy metal residues in wintering shorebirds at
Corpus Christi, Texas, 1976-77. Pestic. monit. J., 14(2): 58-63.
WHO (1958) Note by Secretariat on aldrin poisoning in Kenya,
Geneva, World Health Organization, p. 3 (Information Circular on
the Toxicity of Pesticides to Man No. 1).
WHO (1977) Outbreak of food poisoning of chemical origin, Geneva,
World Health Organization, p. 217 (Weekly Epidemiological Record No.
52).
WHO (1984) Guidelines for drinking-water quality, Geneva, World
Health Organization, Vol. 1, p. 69.
WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished document VBC/88.953).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World Health
Organization (Unpublished documents).
WIEMEYER, S.N., MULHERN, B.M., LIGAS, F.J., HENSEL, R.J., MATHISEN,
J.E., ROBARDS, F.C., & POSTUPALSKY, S. (1972) Residues of
organochlorine pesticides, polychlorinated biphenyls, and mercury
in bald eagle eggs and changes in shell thickness, 1969 and 1970.
Pestic. monit. J., 6(1): 50-55.
WIERSMA, G.B., MITCHELL, W.G., & STANFORD, C.L. (1972) Pesticide
residues in onions and soil - 1969. Pestic. monit. J., 5(4):
345-347.
WIESE, I.H. & BASSON, N.C.J. (1966) The degradation of some
persistent chlorinated hydrocarbon insecticides applied to
different soil types. South Afr. J. agric. Sci., 9: 945-969.
WIESE, I.H., BASSON, N.C.J., VAN DER VIJVER, J.H., & VAN DER MERWE,
J.H. (1969) Toxicology and dynamics of dieldrin in the crowned
guinea-fowl Numida meleagris L. Phytophylactica, 1: 161-176.
WIESE, I.H., BASSON, N.C.J., BASSON, P.A., NAUDE, T.W., & MAARTENS,
B.P. (1973) The toxicology and pathology of dieldrin and photo-
dieldrin poisoning in two antelope species. Onderstepoort J. vet.
Res., 40(1): 31-40.
WILLIAMS, D.T., BENOIT, F.M., MCNEIL, E.E., & OTSON, R. (1978)
Organochlorine pesticide levels in Ottawa drinking water, 1976.
Pestic. monit. J., 12(3): 163-166.
WILLIAMS, D.T., LEBEL, G.L., & JUNKINS, E. (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples
from two Ontario municipalities. J. Toxicol. environ. Health, 13:
19-29.
WILLIAMS, G.M. (1982) Organochlorine pesticides and inhibition of
intercellular communication as the mechanism for their liver tumor
production. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: human welfare and the environment. Oxford, New York,
Pergamon Press, Vol. 3, pp. 475-478.
WILLIAMS, R. & HOLDEN, A.V. (1973) Organochlorine residues from
plankton. Mar. Pollut. Bull., 4(7): 109-111.
WILLIAMS, S., MILLS, P.A., & MCDOWELL, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Agric. Chem.,
47(6): 1124-1128.
WILLIS, G.H., PARR, J.F., SMITH, S., & CARROLL, B.R. (1972)
Volatilization of dieldrin from fallow soil as affected by
different soil water regimes. J. environ. Qual., 1(2): 193-196.
WINTHROP, G.J. & FELICE, J.F. (1957) [A clinical toxicological
study of spraymen of a chlorinated hydrocarbon insecticide.] Bol.
Sanit. Panama, 43: 512-517 (in Spanish with English summary).
WIT, S.L. (1971) [Persistent insecticides in Dutch body fat.] Chem.
Weekbl., 67(5): 11-14 (in Dutch).
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1963) Health hazards
of the pesticides endrin and dieldrin. Arch. environ. Health, 6:
458-464.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council.
WRIGHT, A.S., POTTER, D., WOODER, M.F., DONNINGER, C., & GREENLAND,
R.D. (1972) The effects of dieldrin on the subcellular structure
and function of mammalian liver cells. Food Cosmet. Toxicol., 10:
311-332.
WRIGHT, A.S., AKINTONWA, D.A.A., & WOODER, M.F. (1977) Studies on
the interactions of dieldrin with mammalian liver cells at the
subcellular level. Ecotoxicol. environ. Saf., 1: 7-16, 427.
WRIGHT, A.S., DONNINGER, C., GREENLAND, R.D., STEMMER, K.L., &
ZAVON, M.R. (1978) The effects of prolonged ingestion of dieldrin
on the livers of male rhesus monkeys. Ecotoxicol. environ. Saf., 1:
477-502.
WUENSCHER, K. & ACKER, L. (1969) [The occurrence of chlorinated
insecticides in human fatty tissues.] Med. Ernähr., 10(4): 75-80
(in German).
WUTHRICH, C., MULLER, F., BLASER, O., & MAREK, B. (1985)
[Subjection of the population to pesticides and other foreign
substances through food.] Mitt. Geb. Lebensm. Hyg., 76: 260-276
(in German).
WYLLIE, J., GABICA, J., & BENSON, W.W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: a survey of 200 persons in southern Idaho - 1970. Pestic.
monit. J., 6(2): 84-88.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls (PCBs) and
organochlorine pesticides in human milk and blood collected in
Osaka prefecture from 1972 to 1977. Int. Arch. occup. environ.
Health, 43: 1-15.
YAP, H.H., DESAIAH, D., CUTKOMP, L.K., & KOCH, R.B. (1975) In vitro
inhibition of fish brain ATPase activity by cyclodiene insecticides
and related compounds. Bull. environ. Contam. Toxicol., 14(2):
163-167.
YARON, B., SWOBODA, A.R., & THOMAS, G.W. (1967) Aldrin adsorption
by soils and clays. J. agric. food Chem., 15(4): 671-675.
YOSHIMURA, M., YAMADA, T., SUGIYAMA, S., NODA, H., & MITSUKUNI, Y.
(1979) Organochlorinated pesticides in human organs and tissues.
Jpn. J. leg. Med., 33(2): 91-102.
YU, S.J., KIIGEMAJI, M., & TERRIERE, L.C. (1971) Oxidative
metabolism of aldrin and isodrin by bean root fractions. J. agric.
food Chem., 19: 5-9.
ZABIK, M.E., HOOJJAT, P., & WEAVER, C.M. (1979) Polychlorinated
biphenyls, dieldrin, and DDT in lake trout cooked by broiling,
roasting, or microwave. Bull. environ. Contam. Toxicol., 21:
136-143.
ZAVON, M.R. & HAMMAN, R.E. (1961) Human experience with dieldrin in
malaria control programs. Am. J. public Health, 51(7): 1026-1032.
ZAVON, M.R. & STEMMER, K.L. (1975) The effect of dieldrin ingestion
on rhesus monkeys. A six-year study, Cincinnati, Ohio, Kettering
Laboratory.
ZAVON, M.R., HINE, C.H., & PARKER, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Am. Med. Assoc., 193(10): 837-839.
ZELLE, B. & LOHMAN, P.H.M. (1977) Repair of DNA in cultured human
cells treated with dieldrin and 4-nitroquinoline-N-oxide,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
ZHONG-XIANG, L., KAVANAGH, T., TROSKO, J.E., & CHANG, C.C. (1986)
Inhibition of gap junctional intercellular communication in human
teratocarcinoma cells by organochlorine pesticides. Toxicol. appl.
Pharmacol., 83: 10-19.
ZIMMERLI, B. & MAREK, B. (1973) [Subjection of the Swiss population
to pesticides.] Mitt. Geb. Lebensm. Hyg., 64(4): 459-479
(in German).
APPENDIX I. NOMENCLATURE
Two major systems are currently used for the nomenclature of
these compounds: "polyhydroaromatic" names used by Chemical
Abstracts (American Chemical Society) and IUPAC and the von
Baeyer/IUPAC system for polycylic aliphatic compounds. That the
latter system should be used for the cyclodiene insecticides was
proposed by Benson (1969) and Bedford (1974). The "polyaromatic"
system has, unfortunately, been subject to historical variation,
and there are differences between the IUPAC, British and American
conventions for defining the 3-dimensional stereochemistry in this
system. As a consequence of the differences in the numbering of the
carbon atoms in the two major systems, and the modification of the
Chemical Abstracts "polyaromatic" name for dieldrin since 1971,
considerable confusion can occur regarding the nomenclature of
metabolites.
The various alternative names for aldrin, dieldrin, and photo-
dieldrin are summarized in Table 48. A useful discussion of
nomenclature is given by Brooks (1974).
For convenience, in view of the much more extensive usage in
the literature of the former Chemical Abstracts names for aldrin
and dieldrin, the names of their metabolites in this review are
based (if appropriate) on the former Chemical Abstracts names of
the parent compounds given in Table 48. The names of the
metabolites are given in Table 49, together with some alternative
names based on either the current Chemical Abstracts name for
dieldrin or the von Baeyer/IUPAC system.
The possible misunderstandings that may occur, particularly
for those not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that may be
given to the major faecal metabolite of dieldrin. This one
compound may be designated:
(a) 9-hydroxy dieldrin (former CA system);
(b) 8-hydroxy dieldrin (current CA system); or
(c) 12-hydroxy dieldrin (von Baeyer/IUPAC system).
Table 48. Alternative chemical names for aldrin, dieldrin, and photodieldrin
---------------------------------------------------------------------------------------------------------------
Compounda Polyhydroaromatic name Polycyclic aliphatic name
Chemical Abstracts IUPAC (von Baeyer/IUPAC)
---------------------------------------------------------------------------------------------------------------
Aldrin Formerly: Formerly: 1,8,9,10,11,11-hexachloro-2,3-7,6-
(HHDN) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- endo-2,1-7,8-exo-tetracyclo[6.2.13,6
(I) 1,4,4a,5,8,8a-hexahydro- 1,4,4a,5,8,8a-hexahydro- 02,7] dodeca-4,9-diene
endo-1,4-exo-5,8- exo-1,4-endo-5,8-
dimethanonaphthalene dimethanonaphthalene
Currently: Currently:
1,2,3,4,10,10-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1 alpha,4 alpha,4a-beta, hexachloro-1,4,4a,5,8,8a-
5 alpha,8a,8a beta-hexahydro- hexahydro-1,4:5,8-
1,4:5,8-dimethanonaphthalene dimethanonaphthalene
Dieldrin Formerly: Formerly: 1,8,9,10.11,11-hexachloro-4,5-exo-
(HEOD) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- epoxy-2,3-7,6-endo-2,1-7,8-exo-
(II) 6,7-epoxy-1,4,4a,5,6,7,8,8a- 6,7-epoxy-1,4,4a,5,6,7,8,8a- tetracyclo[6.2.1.13,6.02,7] dodec-
octahydro-endo-1,4-exo- octahydro-exo-1,4-endo-5,8- 9-ene
5,8-dimethanohaphthalene dimethanonaphthalene
Currently: Currently:
3,4,5,6,9,9-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1a alpha,2 beta,2a alpha, hexachloro-1,4,4a,5,6,7,8,8a-
3 beta,6 beta,6a alpha,7 beta, octahydro-6,7 epoxy-1,4:5,8-
7a alpha-octahydro-2,7:3,6- dimethanonaphthalene
dimethanonaphth[2,3-b]oxirene
Photo- 1,1,2,3,3a,7a-hexachloro- 3,exo-4,5,6,6,7-hexachloro-11,12-
dieldrin 6,7-epoxy-2,4,7-metheno- exo-epoxy-pentacyclo[6.4.0.02,10.
(III) decahydro-3H-cyclopenta[a]- 03,7.05,9]-dodecane
pentalene
---------------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
Table 49. Chemical nomenclature of metabolites of aldrin and dieldrin
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
9-Hydroxy dieldrin 9-hydroxy-1,2,3,4,10,10-hexa- 9-(syn-epoxy)hydroxy-1,2,3,4,10,10-hexa-
(VI) (9-Hydroxy HEOD) chloro-6,7-epoxy-1,4,4a,5,6,7, chloro-6,7,-epoxy-1,4,4a,5,6,7,8,8a-octa-
8,8a-octahydro-1,4-endo-5,8-exo- hydro-1,4-endo,exo-5,8-dimethanonaphthalene
dimethanonaphthalene
8-hydroxy-3,4,5,6,9,9-hexachloro-1a alpha,
2 beta, 2a alpha, 3 beta, 6 beta, 6a alpha,
7 beta, 7a alpha-octahydro-2,7:3,6-
dimethanonapth[2,3-b]oxirene
1,8,9,10,11,11-hexachloro-4,5-exo-epoxy-12-
(synepoxy)hydroxy-2,3-7,6-endo-2,1-7,8-exo-
tetracyclo[6.2.1.13,602,7]dodec-9-ene
Aldrin trans-diol (IV) trans-6,7-dihydroxy-1,2,3,4,10,10- 1,8,9,10,11,11-hexachloro-4,5-trans-
hexachloro-1,4,4a,6,7,5,8,8a-hexa- dihydroxy-2,3-7,6-endo-2,1-7,8-exo-
hydro-1,4-endo-5,8-exo-dimethano- tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
naphthalene
Aldrin dicarboxylic 4,5,6,7,8,8-hexachloro-4,7-methano- 1,7,8,9,10,10-hexachloro-2,3-6,5-endo-
acid (V) 3a,4,7,7a-tetrahydro-indane-1,3- tricyclo[5.2.1.02,6]dec-8-ene-3,5-exo-
dicarboxylic acid dicarboxylic acid
Bridged pentachloro- 3,5,6,6,7-pentachloro-11,12-exo-
ketone (VII) (PCK, epoxy-pentacyclo[6.4.0.02,10.03,7
Klein's metabolite) .05,9]dodecan-4-one
Dechloro-aldrin 4,5,6,7,8-pentachloro-4,7-methano-
dicarboxylic 3a,4,7,7a-tetrahydro-indane-1,3-
acid (VIII) dicarboxylic acid
Dieldrin ketone (IX) 1,2,3,4,10,10-hexachloro-1,4,4a,5, 1,8,9,10,11,11-hexachloro-2,3-7,6-endo-
6,7,8,8a-octahydro-6-keto-endo- 2,1-7,8-exo-tetracyclo[6.2.1.13,6.02,7]-
1,4-exo-5,8-dimethanonaphthalene dodec-9-en-4-one
Photodieldrin 3-exo-4,5,6,6,7-hexachloro-
ketone (X) pentacyclo[6.4.0.02,10.03,7.05,9]
dodecan-11-one
---------------------------------------------------------------------------------------------------------
Table 49. (contd.)
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
Photodieldrin trans- 3,exo-4,5,6.6,7-hexachloro-11,12
diol (XI) (caged dihydroxy-pentacyclo[6.4.0.02,10.
aldrin trans-diol) 03,7.05,9]dodecane
Photoaldrin dicarbo- 1,7,8,exo-9,10,10-hexachlorotetra-
xylic acid (XII) cyclo[5.2.1.02,6.04,8]decane-3,5-
(caged aldrin acid) exo,exo-dicarboxylic acid
Photoaldrin (XIII) 3,exo-4,5,6,6,7-hexachloropenta-
cyclo[6.4.0.02,10.03,7.05,9]dodec-
11-ene
---------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
RESUME
1. Généralités
L'aldrine et la dieldrine qui sont l'une et l'autre des
pesticides organochlorés fabriqués industriellement depuis 1950,
ont été utilisés dans le monde entier jusqu'au début des années 70
comme insecticides en agriculture, contre de nombreux ravageurs
présents dans le sol, et pour le traitement des semences. Ces
insecticides étaient actifs contre les termites, les sauterelles,
les xylophages, les coléoptères et les ravageurs des textiles. La
dieldrine a également été employée en santé publique, pour la lutte
contre la mouche tsé-tsé et d'autres vecteurs de maladies
tropicales invalidantes. L'aldrine comme la dieldrine agissent par
contact et par ingestion.
Depuis le début des années 70, ces deux composés sont
interdits ou font l'objet de limitations rigoureuses dans un
certain nombre de pays, spécialement en agriculture. Néanmoins,
ils continuent d'être employés pour la destruction des termites
dans d'autres pays. La production annuelle mondiale, estimée à
13 000 tonnes en 1972, est tombée à moins de 2500 tonnes en 1984.
L'aldrine et la dieldrine de qualité technique ont une pureté
respective de 90% et plus de 95%. Les principales impuretés sont,
pour l'aldrine, l'octachlorocyclopentène, l'hexachlorobutadiène et
des produits de polymérisation et, pour la dieldrine, des
polychloroépoxy-octahydrodiméthanonaphtalènes.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans la plupart des alcanes, des
hydrocarbures aromatiques et des hydrocarbures halogénés, ainsi que
dans les esters, les cétones et les alcools.
La tension de vapeur de l'aldrine est de 6,5 x 10-5 mmHg à
25°C et celle de la dieldrine de 3,2 x 10-6 mmHg à 25°C.
Les méthodes utilisables pour le dosage de l'aldrine et de la
dieldrine dans les aliments, les aliments pour animaux et le milieu
sont décrites à la section 2.
2. Transport, distribution et transformation dans l'environnement
L'aldrine est principalement utilisée comme insecticide épandu
au niveau du sol. Les sols ainsi traités constituent donc dans
l'environnement une source importante d'aldrine et de son produit
de réaction, la dieldrine.
L'aldrine n'a qu'une faible capacité de migration à partir des
zones traitées, par volatilisation ou lessivage. Elle s'adsorbe
préférentiellement, et rapidement, sur les sols riches en matières
organiques, mais faiblement sur les sols argileux. Il est rare que
l'aldrine et la dielrine pénètrent au-delà des 20 premiers
centimètres de la couche de sol traité. L'aldrine adhère si
solidement aux particules du sol que seules des traces peuvent être
retirées par l'eau. C'est pourquoi il n'y a généralement pas
contamination des eaux souterraines.
L'aldrine s'élimine du sol selon une cinétique qui évoque une
réaction du premier ordre. Immédiatement après épandage, on
observe une courte période d'élimination rapide par volatilisation,
suivie d'une seconde période, plus longue, de décroissance
exponentielle, principalement du fait de la transformation en
dieldrine, plus lente à se dissiper. Néanmoins, il peut y avoir
une certaine migration du fait de l'érosion du sol, sous l'action
du vent, des eaux de ruissellement de du déplacement des sédiments.
Les observations faites sur les résidus d'aldrine dans la nature
montrent que, apparemment, ce composé est essentiellement retenu
dans le sol et que pour 97%, le résidu essentiel n'est pas le
composé d'origine mais l'époxyde correspondant, la dieldrine.
La photodieldrine est un produit de photodégradation de la
dieldrine, peu répandu dans l'environnement.
Après épandage sur le sol, l'aldrine disparaît lentement dans
les régions tempérées puisque, dans le cas type, il faut un an pour
qu'elle s'élimine aux trois quarts. La vitesse d'élimination
diminue ensuite à mesure que l'aldrine se transforme en dieldrine.
Il semblerait que la vitesse d'élimination soit plus élevée en
anaérobiose, comme c'est le cas dans les rizières, qu'en aérobiose.
Dans les régions tropicales, la dieldrine s'élimine du sol très
rapidement, jusqu'à hauteur de 90% au cours du premier mois, alors
que, dans le sol des régions tempérées, la dieldrine a une demi-vie
d'environ 5 ans. La volatilisation semble être le principal
mécanisme d'élimination à partir du sol, bien que la teneur
atmosphérique de la dieldrine et de l'aldrine soit généralement
faible. Une partie de la dieldrine est éliminée de l'atmosphère
par les précipitations, mais la concentration de ce produit est
très faible dans les eaux souterraines par suite de son adsorption
énergique sur les particules telluriques. On trouve de la
dieldrine en petites quantités dans les eaux de surface qui sont
contaminées par les eaux de ruissellement provenant de terres
agricoles.
3. Concentrations environnementales et exposition humaine
On trouve de l'aldrine et de la dieldrine dans l'atmosphère en
phase vapeur, adsorbées sur des poussières ou dans les eaux de
pluie, à des teneurs variables selon la situation. Ces produits
s'observent principalement dans les régions agricoles où leur
concentration atmosphérique moyenne est de l'ordre de 1 - 2 ng/m3
avec des maximums d'environ 40 ng/m3. Dans l'eau de pluie, on
relève des concentrations de l'ordre de 10 - 20 ng/litre ou parfois
plus.
Dans les habitations traitées avec ces produits contre les
termites, on observe une concentration dans l'air allant de 0,04 à
7 µg/m3, selon le moment de l'échantillonnage (c'est-à-dire le
nombre de jours après l'épandage) et le type d'habitation. Au bout
de 8 semaines, la concentration a très nettement diminué. Lorsqu'on
traite le bois en profondeur dans ces maisons, la concentration de
la dieldrine dans l'air va de 0,01 à 0,5 µg/m3. On a constaté que
l'aldrine et la dieldrine migraient dans les produits alimentaires
à partir de panneaux lamellés et de contreplaqués traités, ainsi
que par contact direct ou sorption à partir de l'atmosphère.
On a signalé la présence de dieldrine en milieu aquatique.
Mais les concentrations étaient très faibles, le plus souvent
inférieures à 5 ng/litre. Les concentrations plus élevées ont
généralement étéattribuées au rejet d'effluents industriels ou à
l'érosion du sol à la suite de l'utilisation de ces produits en
agriculture. Dans les cours d'eau, les sédiments peuvent présenter
des teneurs beaucoup plus élevées (allant jusqu'à 1 mg/kg).
On trouve rarement de l'aldrine dans les aliments, alors que
la dieldrine est plus courante, spécialement dans les produits
laitiers, les produits carnés, le poisson, les huiles et les
graisses, les pommes de terre et certains autres légumes
(principalement des légumes-racines). Des limites maximales de
résidus (LMR) de l'ordre de 0,02 - 0,2 mg/kg de produit ont été
recommandées lors des réunions conjointes successives FAO/OMS sur
les résidus de pesticides. Les études récentes réalisées dans
différents pays montrent que la concentration effective de la
dieldrine dans les denrées alimentaires est généralement plus
faible. Le recul est net au Royaume-Uni. En 1966 - 67, la
concentration moyenne des résidus de dieldrine observée lors d'une
étude sur la ration totale était de 0,004 mg/kg d'aliments tandis
que, pendant la période 1975 - 77, elle n'était plus que de 0,0015
mg/kg pour tomber à 0,0005 mg/kg en 1981. Cette évolution en
baisse est confirmée dans d'autres pays, par exemple aux Etats-Unis
d'Amérique. Cela tient peut-être à l'interdiction ou à la
limitation d'emploi de ces composés.
Dans un grand nombre de travaux publiés, on a recherché la
présence de dieldrine dans le tissu adipeux, les organes, le sang
et d'autres tissus, chez des sujets de la population générale. Au
cours des 25 dernières années, des enquêtes ont été réalisées dans
de nombreux pays, partout dans le monde. La plupart des
concentrations moyennes observées dans le tissu adipeux se situent
entre 0,1 et 0,4 mg/kg. Aux Etats-Unis d'Amérique, aux Pays-Bas et
au Royaume-Uni, on note une diminution de la concentration dans les
tissus adipeux depuis le milieu de la décennie 70. La
concentration sanguine varie de 1 à 2 µg/litre. Dans le foie, elle
est inférieure à 0,4 mg/kg tandis que, dans les autres tissus, à
savoir les reins, l'encéphale et les gonades, elle est inférieure à
0,1 mg/kg.
A la suite d'une exposition par voie transplacentaire, on
trouve de la dieldrine dans le sang, les tissus adipeux et d'autres
tissus du foetus et du nouveau-né. Les concentrations sont 2 à 10
fois plus faibles que chez la mère. Il n'existe aucune différence
entre le nourrisson et l'adulte pour ce qui est des concentrations
relatives de la dieldrine dans le cerveau, le foie et les tissus
adipeux. La dieldrine est également excrétée dans le lait
maternel. Depuis une quinzaine d'années, on recherche dans divers
pays la présence de pesticides organochlorés dans le lait de femme.
Dans la plupart des pays, la concentration plafonne à 6 µg/litre,
sauf cas exceptionnels.
4. Cinétique et métabolisme
Chez les animaux comme chez l'homme, l'aldrine et la dieldrine
passent rapidement des voies digestives dans le courant sanguin.
L'absorption a également lieu au niveau de la peau ou des poumons
après inhalation de la vapeur. Une étude sur des volontaires a
montré que la quantité résorbée par la peau intacte représente
7 - 8% de la dose appliquée. Selon des études d'inhalation
conduites sur des volontaires, le taux d'absorption et de rétention
de l'aldrine dans l'organisme peut atteindre 50% de la vapeur
inhalée. Après absorption, le composé se répartit rapidement dans
tous les organes et tissus, et il existe un échange permanent entre
le sang et les autres tissus. Entre-temps,l'aldrine se transforme
en dieldrine, principalement au niveau du foie mais aussi, dans une
moindre proportion, dans d'autres tissus comme les poumons. Cette
conversion est très rapide.
Après administration par voie orale d'une dose de 10 mg
d'aldrine par kg de poids corporel à des rats de 1 jour, on a
retrouvé ce composé dans le foie des animaux d'expérience 2 heures
après l'administration. Au cours des quelques heures suivantes, la
dieldrine s'est concentrée dans une beaucoup plus large mesure dans
les tissus lipidiques.
Comme l'ont montré de nombreuses études effectuées avec de
l'aldrine ou de la dieldrine marquées au 14C, une partie du produit
ingéré passe telle quelle dans l'intestin d'où elle est éliminée de
l'organisme, une partie est excrétée telle quelle à partir du foie
dans la bile, une autre fraction est stockée dans les divers
organes et tissus, en particulier le tissu adipeux, et une dernière
fraction est métabolisée dans le foie en produits à caractère
hydrophile et polaire plus prononcé. Chez l'homme et la plupart des
animaux, les métabolites sont principalement éliminés dans les
excreta, par l'intermédiaire de la bile. On a établi par ailleurs
que la biodégradation de l'aldrine et de la dieldrine aboutit aux
mêmes métabolites.
La plupart des connaissances actuelles sur le catabolisme de
la dieldrine chez les mammifères proviennent d'études chez la
souris, le rat, le lapin, le mouton, le chien, les petits singes,
le chimpanzé et l'homme. Dans l'ensemble, on n'observe que des
différences quantitatives entre les diverses espèces et les
mécanismes sont apparemment semblable chez le rat et les primates.
Le principal métabolite, sauf chez le rat, est le dérivé
hydroxylé en 9. On le trouve dans les déjections ainsi que dans
l'urine, sous forme libre ou conjuguée. On a découvert et
identifié chez les animaux d'expérience trois autres métabolites,
présents en petites quantités. Il s'agit d'un dérivé 6,7-
dihydroxylé en position trans, d'un acide dicarboxylique dérivé du
composé dihydroxylé et d'une pentachlorocétone pontée.
Seul le composé hydroxylé en 9 a été mis en évidence chez
l'homme, dans les matières fécales, tandis que ni ce composé ni les
autres métabolites n'apparaissaient dans le sang ou les autres
tissus. On a observé la présence de dieldrine dans les selles
d'ouvriers professionnellement exposés, tandis que, dans la
population générale, les quantités étaient inférieures au seuil de
détection. L'examen des urines de cinq ouvriers a montré que
l'excrétion de la dieldrine et de ses quatre métabolites par voie
urinaire était minime par rapport à l'élimination du métabolite
hydroxylé en 9 par voie fécale.
La transformation de l'aldrine en dieldrine dans le foie, sous
l'action de mono-oxygénases à fonction mixte (aldrine-époxydase) et
la distribution, puis le dépôt ultérieur, de la dieldrine
(principalement dans les tissus à contenu lipidique, tels que le
tissu adipeux, le foie, les reins, le coeur et le cerveau) sont
beaucoup plus rapides que le catabolisme et l'élimination finale de
la dieldrine intacte et de ses métabolites. Dans ces conditions,
pour un apport quotidien moyen déterminé d'aldrine ou de dieldrine,
il y a accumulation lente de dieldrine dans l'organisme. Mais
cette accumulation n'est pas indéfinie. Quand l'administration se
poursuit, on finit par aboutir à un état d'équilibre dynamique, la
quantité excrétée compensantexactement l'apport. La quantité
stockée maximale dépend de l'apport quotidien, tout comme on l'a
montré chez le rat, le chien et l'homme.
Quand l'apport d'aldrine/dieldrine est réduit ou interrompu,
la charge de l'organisme diminue. Chez l'homme, la demi-vie
biologique est de l'ordre de 9 à 12 mois. Chez le rat, le chien et
l'homme, on a démontré l'existence de relations significatives
entre la concentration de la dieldrine dans le sang et sa
concentration dans d'autres tissus.
De nombreuses études sur la concentration de la dieldrine dans
divers tissus, dont le sang et les tissus adipeux, aussi bien dans
la population générale que dans des catégories particulières, ont
été réalisées dans plusieurs pays et ont montré que, à l'équilibre,
les concentrations respectives dans les tissus adipeux, le foie, le
cerveau et le sang sont sensiblement proportionnelles à 150, 15, 3
et 1.
La dieldrine est transportée par le placenta jusqu'au foetus.
Il y a accumulation dans les mêmes organes et tissus que chez
l'adulte, mais en quantités moindres. Il existe apparemment un
équilibre entre les concentrations chez la mère et chez le foetus.
Chez le rat et le chien, la photodieldrine est également
métabolisée sous forme de pentachlorocétone pontée. On a retrouvé
les deux composés dans les tissus adipeux, le foie et les reins des
animaux à qui l'on avait administré de la photodieldrine à forte
dose. Chez l'homme, aucun résidu de ces composés n'a été mis en
évidence dans le tissu adipeux, les reins ni le lait maternel.
L'accumulation de photodieldrine dans les tissus adipeux des
animaux d'expérience était beaucoup moins importante que celle de
la dieldrine.
5. Effets sur les êtres vivants dans leur milieu natural
5.1 Accumulation
La plupart des résidus présents chez les êtres vivants sont
des résidus de dieldrine, car l'aldrine se transforme facilement
chez eux en dieldrine.
Les champignons, les streptomycètes et les bactéries
concentrent la dieldrine du milieu ambiant dans une proportion qui
peut aller en 4 h de 0,3 à plus de 100. Les protozoaires absorbent
la dieldrine davantage que les algues. Celles-ci absorbent très
rapidement la dieldrine présente dans le milieu de culture, les
concentrations maximales étant souvent atteintes en quelques
heures.
De nombreuses espèces d'invertébrés aquatiques concentrent
fortement la dieldrine à partir d'une eau à très faible teneur.
L'équilibre est atteint en quelques jours. Lorsqu'on les remet en
eau pure, la dieldrine s'élimine rapidement, avec une demi-vie de
60 - 120 h.
Pour les poissons entiers, le facteur de bioconcentration
dépasse 10 000. Chez une espèce de poissons, la demi-vie
d'élimination de la dieldrine accumulée s'est établie à 16 jours.
La bioconcentration de dieldrine chez les organismes
aquatiques se fait principalement à partir de l'eau et non par
ingestion d'aliments.
Les lombrics absorbent la dieldrine présente dans le sol et
laconcentrent jusqu'à un facteur maximal d'environ 170. Pour la
plupart des types de sol, il n'existe guère de corrélation entre la
concentra-tion atteinte chez le lombric et la concentration dans le
sol.
De nombreux travaux ont été consacrés à la présence de la
dieldrine dans les tissus ou dans les oeufs d'espèces non visées.
Les concentrations observées sont extrêmement variables, allant de
0,001 mg/kg à 100 mg/kg de tissu, mais elles restent le plus
souvent inférieures à 1 mg/kg de tissu.
Chez les oiseaux, il y a accumulation rapide de dieldrine
aussi bien dans les tissus que dans les oeufs. De même, on a
montré que diverses espèces de mammifères accumulent la dieldrine,
en particulier dans les graisses.
5.2 Toxicité pour les micro-organismes
La dieldrine a des effets très variables sur les algues uni-
cellulaires, avec une action sensible sur certaines espèces dès la
concentration de 10 µg/litre tandis que d'autres espèces ne sont
pas touchées même à la concentration de 1000 µg/litre. L'aldrine
et la dieldrine n'ont que peu d'effets sur les bactéries
terricoles, même à des concentrations très supérieures aux valeurs
habituelles. Dans la plupart des études, aucun effet n'a été
constaté après exposition à une concentration de 2000 mg/kg de
terre. Des effets ont été signalés sur la photosynthèse chez
différentes espèces d'algues, avec une action plus marquée de
l'aldrine que de la dieldrine à concentrations égales. Mais ces
effets minimes sur la biochimie des algues n'étaient que
transitoires.
5.3 Toxicité pour les organismes aquatiques
L'aldrine et la dieldrine sont extrêmement toxiques pour les
crustacés aquatiques, avec des valeurs de la DL50 à 96 h
inférieures à 50 µg/litre. Cependant, les quelques résultats plus
élevés signalés (jusqu'à 4300 µg/litre) illustrent les différences
de sensibilité selon les espèces. Les daphnies sont moins
sensibles à la dieldrine qu'à l'aldrine, avec des DL50 à 48 h de
23 - 32 µg/litre dans le premier cas et de 190 - 330 µg/litre dans
le second. Les mollusques sont nettement plus résistants, les CL50
à 48 h pouvant atteindre plus de 10 000 µg/litre. Des études de
plusieurs semaines ont confirmé la résistance relative des daphnies
et des mollusques. Les invertébrés aquatiques les plus sensibles
sont les stades larvaires des insectes, avec des CL50 à 96 h de
0,5 - 39 µg/litre pour la dieldrine et de 1,3 - 180 µg/litre pour
l'aldrine.
Lors d'épreuves de toxicité aiguë, l'aldrine comme la
dieldrine se sont montrées très toxiques vis-à-vis des poissons.
Chez diverses espèces de poisson, on a relevé des valeurs de la
CL50 à 96 h allant de 2,2 à 53 µg/litre pour l'aldrine et de 1,1 à
41 µg/litre pour la dieldrine. De nombreuses études ont montré que
la toxicité augmente avec la température. Dans une étude prolongée
sur Poecilia latipinna, on a obtenu un taux de mortalité de 100% en
présence d'une concentration de dieldrine égale ou supérieure à 3
µg/litre. L'addition de dieldrine à la nourriture de truites arc-
en-ciel jusqu'à des concentrations de 430 µg/kg de poids corporel
par jour, n'a exercé aucune influence sur la mortalité mais a
entraîné des modifications enzymatiques. Des altérations
morphologiques ont été observées aumicroscope électronique dans les
mitochondries hépatiques. Le mécanisme de détoxification de
l'ammoniaque chez les poissons est sensible à la dieldrine, la dose
sans effet nocif apparent étant inférieure à 14 µg/kg de poids
corporel par jour. La sensibilité à la dieldrine s'est révélée
variable selon le stade de développement des poissons. Les oeufs
étaient résistants et les formes juvéniles moins sensibles que les
adultes.
La toxicité aiguë de l'aldrine comme de la dieldrine est
élevée pour les larves d'amphibiens, avec des CL50 à 85 h de
l'ordre de 100 µg/litre.
5.4 Toxicité pour les organismes terrestres
La dieldrine est peu toxique pour les végétaux supérieurs
puisque les cultures ne sont affectées que par des doses
supérieures à 22 kg/ha. La phytotoxicité de l'aldrine est plus
importante, notamment pour les tomates et les concombres, mais
uniquement à des doses plusieurs fois supérieures aux valeurs
recommandées. Le chou est la plante cultivée la plus sensible à
l'aldrine.
Vis-à-vis des abeilles, la DL50 par voie orale varie, selon
les observations publiées, de 0,24 à 0,45 µg/abeille pour l'aldrine
et de 0,15 à 0,32 µg/abeille pour la dieldrine. Les quantités
toxiques par contact vont de 0,15 à 0,80 µg/abeille pour l'aldrine
et de 0,15 à 0,41 µg/abeille pour la dieldrine. D'après deux
études, la dieldrine est relativement plus toxique vis-à-vis des
insectes prédateurs qui se nourrissent de ravageurs.
Selon des études effectuées en laboratoire, le lombric
supporte des doses d'aldrine de 13 mg/kg en sol artificiel, le taux
de mortalité étant inférieur à 1%. La CL50 à six semaines était de
60 mg d'aldrine par kg de sol.
Pour 13 espèces d'oiseaux, la toxicité aiguë de l'aldrine et
de la dieldrine variait de plus du simple au décuple, avec des
valeurs de 6,6 - 520 mg/kg de poids corporel pour l'aldrine et de
6,9 - 381 mg/kg de poids corporel pour la dieldrine. Chez quatre
espèces d'oiseaux, la toxicité subaiguë par voie orale
correspondait à des doses comprises entre 34 et 155 mg/kg pour
l'aldrine et 37 et 169 mg/kg pour la dieldrine. Des épreuves
répétées au cours d'une certaine période n'ont révélé aucun signe
de résistance acquise chez ces espèces. D'après des études sur la
reproduction de plusieurs espèces de volaille, une concentration de
la dieldrine dépassant 10 mg/kg dans les aliments provoque une
certaine mortalité chez les adultes. Aucun effet ne s'est fait
sentir sur la production des oeufs, la fécondité, le taux
d'éclosion ni la survie des poussins en présence de dieldrine dans
les aliments à des concentrations qui ne sont pas toxiques pour la
mère. La dieldrine n'a aucune influence directe sur l'épaisseur de
la coquille des oeufs. Cependant, la diminution de la consommation
de nourriture, qui constitue un symptôme de l'intoxication par la
dieldrine, peut entraîner une diminution de l'épaisseur de la
coquille.
Chez le mammifères non élevés au laboratoire, la réponse à la
dieldrine varie selon les espèces. Chez quatre espèces de
campagnols, on a observé des valeurs de la DL50 aiguë, allant de
100 à 210 mg/kg de poids corporel, ce qui montre que ces animaux
sont moins sensibles àla dieldrine que les animaux de laboratoire.
Des musaraignes ont survécu à la consommation d'une nourriture
contenant 50 mg de dieldrine par kg mais sont mortes quand la
concentration est passée à 200 mg/kg. Des damalisques (une espèce
d'antilope) ont survécu 90 jours à une nourriture contenant de la
dieldrine à raison de 5 ou 15 mg/kg mais sont toutes mortes dans
les 24 jours pour une concentration égale ou supérieure à 25 mg/kg.
Tous les damalisques d'une région où l'on avait pulvérisé de la
dieldrine à raison de 0,16 kg/ha sont mortes et le calcul a montré
que l'apport alimentaire était de 1,82 mg/kg par jour. Trente pour
cent des springboks ont survécu aux épandages, sans manifester
d'effets tardifs. Les signes toxicologiques de l'intoxication par
la dieldrine étaient sensiblement les mêmes que chez les mammifères
de laboratoire.
5.5 Effets sur les populations et les écosystèmes
Certaines études donnent à penser que des populations de
mammifères ont été intoxiquées par de la dieldrine. Il est
probable que de petits mammifères sont morts après avoir mangé des
semences enrobées de dieldrine mais les populations se sont
reconstituées par immigration. Des chauves-souris ont été tuées
par la dieldrine contenue dans les agents de protection du bois.
Des résidus de dieldrine ont été signalés chez de nombreuses
espèces d'oiseaux. Partout dans le monde, c'est chez les oiseaux
de proie que les résidus sont les plus abondants car les animaux se
situent en fin de chaîne alimentaire. La teneur en dieldrine des
tissus et des oeufs d'oiseau suit l'évolution de l'emploi de
l'aldrine et de la dieldrine, et elle a diminué à la suite des
restrictions imposées à leur usage. Il n'est pas facile de repérer
les effets de la dieldrine car les résidus de cet insecticide
s'accompagnent de résidus d'autres organochlorés. La dieldrine est
plus toxique que le DDT pour les oiseaux et il est probable qu'elle
a provoqué chez les adultes une plus forte mortalité que le DDT.
Il est encore plus difficile de démontrer l'existence d'effets de
la dieldrine sur la reproduction à l'état naturel. En outre, il se
peut que les effets interviennent longtemps après l'exposition.
6. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
L'aldrine et la dieldrine sont extrêmement toxiques : pour ces
deux composés, la DL50 varie, chez la souris et chez le rat, de 40
à 70 mg/kg de poids corporel. Par voie percutanée, la dose toxique
se situe entre 40 et 150 mg/kg de poids corporel selon l'espèce en
cause et le solvant utilisé. On a constaté que l'aldrine et la
dieldrine de qualité technique déterminent chez le lapin une
irritation cutanée légère à intense, mais la cause en est le
solvant. Dans l'épreuve de maximalisation de Magnusson & Kligman
chez le cobaye, l'aldrine a provoqué un effet de sensibilisation.
Pourtant, au cours de 20 années de fabrication et de préparation
des formules, aucun cas de sensibilisation cutanée n'a été observé
dans un groupe comptant plus de 1000 travailleurs.
L'aldrine, comme la dieldrine, a une faible tension de vapeur
de sorte que, en principe, il n'y a aucun effet aigu par
inhalation. Les effets observés lors des études de toxicité aiguë
après exposition par toutes les voies possibles concernent le
système nerveux central etconsistent en hyperexcitabilité,
tremblements et convulsions.
Des études d'exposition par voie orale, de courte ou longue
durée, ont été réalisées avec l'aldrine et la dieldrine, chez la
souris, le rat, le chien, le hamster et les petits singes. Chez le
rat et la souris, le foie est le principal organecible : on observe
une augmentation de son poids par rapport au poids du corps et une
hypertrophie des hépatocytes centrilobulaires, la réversibilité
étant possible à un stade précoce. Au microscope, ces altérations
se traduisent par une augmentation de l'oxyphilie cytoplasmique et
une migration périphérique des granules basophiles. Ces
altérations ne se rencontrent pas au niveau du foie chez le hamster
et le singe. Chez le chien, l'atteinte hépatique est peu prononcée
(dégénérescence graisseuse et légère atrophie des hépatocytes);
elle s'accompagne d'une atteinte rénale consistant dans une
vacuolisation de l'épithélium des tubules distaux et une
dégénérescence tubulaire. Chez le rat, la dose sans effet nocif
observable se situe dans l'ensemble, d'après les résultats dont on
dispose sur le court et le long terme, aux alentours de 0,5 mg/kg
de nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
En augmentant les quantités incorporées à la nourriture, jusqu'à
obtenir l'équivalent de 0,05 mg/kg de poids corporel ou davantage,
on observe une hépatomégalie et des altérations histologiques
d'importance proportionnée à la dose. Chez le chien une dose de
0,04 - 0,2 mg/kg de poids corporel s'est révélée sans effet.
Plusieurs études de cancérogénicité à long terme ont été
effectuées sur différentes souches de souris, avec de l'aldrine ou
de la dieldrine. Chaque fois, on a observé des tumeurs
hépatocellulaires bénignes ou malignes. Apparemment, les femelles
étaient plus sensibles que les mâles. Aucun autre type de tumeur
ne s'est manifesté dans ces études.
Des études à long terme sur d'autres espèces (rat, hamster)
n'ont révélé aucune augmentation de l'incidence tumorale.
L'administration de photodieldrine incorporée à la nourriture,
jusqu'à une concentration de 7,5 mg/kg d'aliments, ne s'est pas
révélé tumorigène.
En outre, on a publié un certain nombre d'études spéciales qui
n'ont, jusqu'ici, pas permis d'élucider le mécanisme de la
production des tumeurs hépatiques chez la souris.
Dans la plupart des études de reproduction (sur 1 à 6
générations), réalisées avec l'aldrine ou la dieldrine sur des
souris et des rats, le principal effet constaté a été
l'augmentation du taux de mortalité dans la descendance, avant
sevrage. La capacité génésique n'a été atteinte qu'à des doses
toxiques pour la mère. Les études sur le chien étaient trop
limitées pour permettre les conclusions catégoriques, si ce n'est
qu'on a noté une augmentation systématique de la mortalité des
chiots à la mamelle.
D'après les résultats de ces études sur la reproduction, on
peut conclure que, de ce point de vue, les doses sans effet nocif
décelable sont de 2 mg de dieldrine par kg de nourriture chez le
rat et de 3 mg de dieldrine par kg de nourriture chez la souris,
soit l'équivalent quotidien de 0,1 et 0,4 mg/kg de poids corporel
respectivement.
Aucun signe de tératogénicité n'a été observé chez la souris,
le rat ou le lapin, après administration par voie orale de doses
d'aldrineet de dieldrine atteignant 6 mg/kg de poids corporel.
L'administration d'une dose unique d'aldrine et de dieldrine,
représentant environ la moitié de la DL50, a provoqué des effets
toxiques intenses chez le foetus de souris et de hamster, ainsi
qu'une incidence accrue d'anomalies tératogènes. La signification
de ces observations est douteuse en présence d'effets toxiques
probables chez les femelles gravides.
Les études de mutagénicité in vivo ou in vitro ont été
nombreuses, mais elles ont presque toujours donné des résultats
négatifs.
La toxicité aiguë de la photodieldrine par voie orale est plus
élevée que celle de la dieldrine chez la souris, le rat et le
cobaye. Lors d'études de toxicité aiguë ou à long terme, on a
observé des symptômes d'intoxication et des effets sur les organes
cibles analogues à ceux de la dieldrine, tant sur le plan
quantitatif que sur le plan qualitatif. La photodieldrine ne s'est
pas montrée tumorigène chez la souris ni chez le rat.
Comme la plupart des autres substances chimiques, l'aldrine et
la dieldrine exercent leurs effets toxiques selon plusieurs
mécanismes. Les organes cibles sont le système nerveux central et
le foie. Chez l'homme et les autres vertébrés, l'intoxication
secondaire à une exposition aiguë ou chronique, se caractérise par
des mouvements musculaires involontaires et des convulsions
épileptiformes. En cas de survie, la récupération est totale après
une courte durée marquée par des symptômes résiduels. Au niveau du
foie, on observe une activité accrue des enzymes microsomales de
biotransformation, en particulier du système enzymatique cytochrome
P-450/monooxygénase. Cette induction des enzymes mircosomales est
réversible et, au-delà d'un certain niveau, elle semble liée aux
altérations cytoplasmiques au niveau du foie et à l'hépatomégalie
chez les rongeurs.
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut penser que, en pratique, ces produits ne contribuent guère à
l'incidence des cancers humains.
7. Effets chez l'homme
L'aldrine et la dieldrine sont très toxiques pour l'homme. Il
y a eu de graves cas d'intoxication accidentelle ou professionnelle
mais il est rare qu'ils aient fait des victimes. La plus faible
dose ayant provoqué une issue fatale a été estimée à 10 mg/kg de
poids corporel. Les personnes ayant survécu à une intoxication
aiguë ou subaiguë se sont complètement rétablies. Aucun effet
irréversible ni atteinte anatomopathologique résiduelle n'a été
signalée.
Les effets nocifs de l'aldrine et de la dieldrine sont
fonction de la concentration de la dieldrine dans le sang. Le
dosage de la dieldrine dans le sang permet de diagnostiquer avec
précision une exposition à l'aldrine/dieldrine. Chez les
travailleurs, le taux sanguin au-dessous duquel on n'observe aucun
effet nocif (dose limite sans effet nocif décelable) est de 105
µg/litre de sang. Cela correspond à un apport quotidien de
dieldrine de 0,02 mg/kg de poids corporel.
L'exposition environnementale (principalement par
l'intermédiairedes aliments mais aussi, dans une faible mesure, par
voie respiratoire) entraîne l'apparition de dieldrine à une très
faible concentration dans les organes, le sang et le lait maternel.
Autant qu'on puisse en juger d'après des études épidémiologiques et
cliniques poussées, il n'existe aucun raison de penser que les taux
couramment observés dans l'organisme constituent une menace pour la
santé de la population en général. Lors d'une étude poursuivie
pendant plus de 20 ans auprès de 1000 travailleurs de l'industrie,
employés dans une fabrique d'insecticides à base d'aldrine/dieldrine,
aucune augmentation de l'incidence des cancers n'a été observée chez
les sujets fortement exposés à ces deux produits. Phénomène encore
plus significatif, aucun signe avant-coureur, sous forme d'une
altération de la fonction hépatique, n'a été observé.
Une étude épidémiologique sur la mortalité a été réalisée dans
une unité de production aux Etats-Unis, sur une cohorte de 870
travailleurs exposés à l'aldrine, à la dieldrine et à l'endrine.
Malgré près de 25 000 années-homme d'observation, il n'a pas été
possible de repérer un risque particulier de cancer attributable au
travail dans cette usine.
EVALUATION DES DANGERS POUR LA SANTE DE L'HOMME ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des dangers pour la santé de l'homme
L'aldrine et la dieldrine sont des pesticides organochlorés
qui ont été utilisés partout dans le monde entre 1950 et le début
des années 70 comme insecticides en agriculture et pour le
traitement des semences, pour la destruction des ravageurs
terricoles et d'autres types d'insectes (par exemple les termites,
les sauterelles et les ravageurs des textiles) ainsi que pour la
lutte contre les glossines et autres vecteurs de maladies. Chez
les insectes, ces composés exercent leur effet toxique par contact
et par voie digestive. A partir des années 70, on en a limité ou
interdit l'emploi dans plusieurs pays, spécialement en agriculture.
Pourtant, ils continuent d'être utilisés dans d'autres pays pour la
destruction des termites.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans de nombreux solvants organiques.
Leur tension de vapeur est faible.
On trouve souvent de la dieldrine dans des produits laitiers
ou carnés, le poisson, les huiles et les graisses et certains
légumes, notamment des légumes-racines. La limite maximale de
résidus recommandée par les instances compétentes de la FAO/OMS
lors des réunions conjointes sur les résidus de pesticides varie de
0,02 à 0,2 mg/kg de produit. Des mesures récentes ont montré que
les teneurs effectives sont plus faibles, comme l'ont d'ailleurs
confirmé les études sur la ration globale. Comme l'utilisation de
ces deux composés fait maintenant l'objet de restrictions, on
observe une diminution lente mais régulière de la teneur en résidus
des différentes denrées alimentaires.
Les quantités ingérées par l'homme avec sa ration quotidienne
se traduisent, malgré la faible concentration de ces produits dans
les aliments, par la présence de dieldrine dans le tissu adipeux et
dans certains autres tissus et organes. Des enquêtes à l'échelle
mondiale montrent que les teneurs moyennes varient de 0,1 à 0,4
mg/kg de tissu adipeux. Depuis le début des années 70, cette
concentration diminue lentement.
Comme le foetus est exposé par voie transplacentaire, ses
tissus adipeux contiennent également de la dieldrine, mais à une
concentration qui n'est que de 10 à 50% de la concentration chez la
mère. Il semble exister un équilibre entre les concentrations
foetales et les concentrations maternelles. La dieldrine est
également excrétée dans le lait. Une exposition est possible par
inhalation dans les habitations où l'on utilise ce produit pour la
destruction des termites. Après traitement, on observe des
concentrations atmosphériques allant de 0,01 à 7 µg/m3, selon le
mode d'épandage, la concentration utilisée, les modalités de
l'aération et le moment où les échantillons sont prélevés. En
pareilles circonstances, les aliments peuvent aussi être
contaminés, par contact direct, ou par sorption à partir de l'air
ambiant.
Le métabolisme s'effectue principalement dans le foie où
l'aldrine se transforme rapidement en dieldrine. Le catabolisme de
la dieldrineest plus lent que celui de ses métabolites hydrophiles
qui sont excrétés dans la bile et dans les urines. La structure de
ces métabolites a été établie. Chez toutes les espèces étudiées,
notamment l'homme, on a montré que les quantités
d'aldrine/dieldrine accumulées se stabilisent à un niveau qui est
fonction de l'apport puisqu'il existe une relation linéaire entre
les quantités accumulées et le logarithme de l'apport. Quand
l'exposition prend fin, la concentration de la dieldrine dans les
tissus de l'organisme diminue selon une loi exponentielle. La
toxicité aiguë de l'aldrine et de la dieldrine est importante chez
les mammifères par voie orale, tandis que la toxicité par voie
cutanée est modérée. Aucune sensibilisation cutanée n'a été
observée. Les effets constatés à la suite d'une exposition
expérimentale aiguë ou de courte ou longue durée intéressent le
système nerveux central. Le foie est également un organe cible.
Chez les souris et les rats, on observe à ce niveau des altérations
désignées sous le nom de "foie de rongeur sous insecticide
organochloré".
Apparemment, l'aldrine et la dieldrine ne sont pas tératogènes
à des doses inférieures à celles qui sont toxiques chez la femelle
gravide et chez le foetus. On n'a pas fait état de toxicité pour
la fonction de reproduction chez le mâle ou la femelle.
De nombreuses études de mutagénicité in vitro et in vivo ont
montré que ni l'aldrine ni la dieldrine ne sont mutagènes.
Lors d'études à long terme, ces deux produits ont déterminé
chez la souris des tumeurs hépatiques, bénignes ou malignes. En
revanche, aucune augmentation de l'incidence des tumeurs hépatiques
ou autres n'a été observée chez le rat ni le hamster.
Selon le CIRC (1987), il n'existe pas de preuves suffisantes
d'un pouvoir cancérogène chez l'homme et les preuves de
cancérogénicité chez l'animal d'expérience sont limitées.
L'aldrine comme la dieldrine ont été classées dans le groupe 3, à
savoir celui des produits chimiques dont il est impossible de
préciser le pouvoir cancérogène chez l'homme.
Compte tenu des résultats obtenus lors des études de toxicité
de courte ou de longue durée, la dose globale sans effet nocif
décelable se situe chez le rat à 0,5 mg de dieldrine par kg de
nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
Chez le chien, la dose sans effet nocif décelable est de 0,04 mg/kg
de poids corporel. Lors des réunions conjointes FAO/OMS de 1966 et
1977 sur les résidus de pesticides, on a fixé la dose journalière
admissible (DJA) à 0,1 µg/kg de poids corporel, en tenant compte de
la non-cancérogénicité de ces deux substances pour l'homme.
L'aldrine et la dieldrine sont très toxiques pour l'homme. On
connaît des cas d'intoxication accidentelle ou professionnelle,
mais qui ont rarement fait des victimes. Les survivants à une
intoxication aiguë ou subaiguë se sont entièrement rétablis. Les
effets nocifs sont fonction de la concentration sanguine de la
dieldrine, dont le dosage permet de diagnostiquer avec précision
une exposition à l'aldrine/dieldrine. Pour taux sanguins inférieur
à 105 µg/litre, aucun effet indésirable n'est à craindre. Cette
concentration constitue la dose limite sans effet nocif décelable
et correspond à un apport quotidien de 0,02 mg de dieldrine par kg
de poids corporel.
L'exposition liée à l'environnement, principalement par la voie
alimentaire, entraîne la présence de faibles concentrations de
dieldrine dans l'organisme. D'après des études épidémiologiques et
cliniques poussées, ces teneurs ne constituent pas une menace pour
la santé humaine.
Aucun signe avant-coureur d'une altération de la fonction
hépatique n'a été observé lors d'une enquête de 20 ans portant sur
plus de 1000 ouvriers de l'industrie exposés à l'aldrine et à la
dieldrine. Dans cette étude ainsi que dans une autre effectuée aux
Etats-Unis d'Amérique, aucun risque particulier de cancer n'a été
repéré chez les personnes profesionnellement exposées à l'aldrine
et à la dieldrine (parfois à de fortes concentrations).
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut estimer que, en pratique, ces produits chimiques ne
contribuent que très peu, sinon pas du tout, à l'incidence des
cancers humains.
La photodieldrine, produit qui résulte de la dégradation de la
dieldrine sous l'action de la lumière, est analogue à la dieldrine
pour ce qui est de sa toxicité sur une courte durée. Elle n'est ni
tératogène ni cancérogène chez la souris et le rat. L'accumulation
de photodieldrine dans les tissus adipeux d'animaux d'expérience
s'est révélée inférieure à celle de la dieldrine.
2. Evaluation des effets sur l'environnement
La principale source de dieldrine (jusqu'à 97%) dans
l'environnement est l'aldrine, un insecticide épandu au niveau du
sol. L'aldrine et son produit de réaction, la dieldrine sont
rapidement absorbé par les sols, spécialement ceux qui sont riches
en matières organiques. De ce fait, la pénétration est limitée et
il n'y a généralement aucune contamination des eaux souterraines.
Les deux composés sont entraînés principalement du fait de
l'érosion (sous l'action du vent) et du transport des sédiments
(eaux superficielles de ruissellement), mais non par lessivage.
L'emploi d'aldrine et de dieldrine en agriculture donne lieu à
la présence de résidus (principalement de dieldrine) dans le sol où
ils peuvent persister plusieurs années; la demi-vie de la dieldrine
est estimée à 4 - 7 ans. La persistance de ces composés est
moindre dans les régions tropicales que dans les régions tempérées.
L'aldrine et la dieldrine passent, par volatilisation, des
récoltes et du sol traités à l'atmosphère; elles peuvent aussi y
pénétrer directement lors de l'épandage. La dieldrine retourne au
sol ou dans les étendues d'eau par les précipitations ou par dépôt
de particules sèches. Les composés se rencontrent donc soit en
phase vapeur (à des concentrations très faibles, en général de
l'ordre de 1 - 2 ng/m3), soit adsorbés sur des particules de
poussière, soit encore dans les eaux de pluie (à des concentrations
de l'ordre de 10 - 20 ng/litre).
Plusieurs auteurs ont signalé la présence de dieldrine en
milieu aquatique. Dans les eaux de surface, les concentrations
sont le plus souvent très faibles, inférieures à 5 ng/litre. Mais
des valeurs plus élevées s'observent dans les régions soumises à
l'érosion ou danscelles où l'on utilise ce produit en agriculture.
Dans ces régions, les sédiments des cours d'eau peuvent renfermer
jusqu'à 1 mg de dieldrine par kilogramme. La forte capacité qu'ont
les organismes aquatiques à concentrer la dieldrine à partir de
teneurs très faibles peut aboutir à l'accumulation de doses
toxiques. La concentration de ce produit tout au long de la chaîne
alimentaire aquatique est moins importante qu'une absorption
directe à partir de l'eau.
Comme la dieldrine est très répandue dans l'environnement et
qu'elle y persiste, on observe des concentrations très variées chez
les organismes non visés. Alors qu'auparavant les valeurs
observées allaient de 0,001 mg à 100 mg/kg de tissu, elles sont
aujourd'hui le plus souvent inférieures à 1 mg/kg de tissu.
Dans les écosystèmes terrestres, l'aldrine et la dieldrine
s'accumulent chez divers organismes, principalement sous forme de
dieldrine. Cette dernière est probablement responsable de la mort
de mammifères dans la nature et de la raréfaction de certaines
espèces, comme la loutre. Certains petits mammifères périssent
sans doute après avoir mangé des céréales traitées mais il est
probable que leurs populations se reconstituent par immigration à
partir des zones voisines. Les oiseaux de proie qui mangent de
petits mammifères et de petits oiseaux contaminés par la dieldrine
absorbent et concentrent cet insecticide dans leurs tissus et leurs
oeufs. Des oiseaux granivores ont été tués par la consommation de
céréales traitées. Il est probable que la raréfaction des oiseaux
de proie s'explique par la présence dans leurs tissus de résidus de
dieldrine (entre autres organochlorés). Les effets de la dieldrine
se manifestent avec un certain retard car les résidus s'accumulent
dans les graisses pendant l'hiver d'où ils ne se libèrent qu'au
printemps. Le fait de n'utiliser la dieldrine qu'à certaines
époques de l'année n'a pas réduit la mortalité des oiseaux.
La large utilisation d'aldrine et de dieldrine, parallèlement
à celle d'autres organochlorés, a exercé des effets très nocifs sur
l'environnement mais grâce à des restrictions draconiennes,
particulièrement en ce qui concerne les semences traitées, les
populations d'oiseaux commencent à se reconstituer.
3. Conclusions
a) L'aldrine et la dieldrine ont donné lieu à des études
poussées et variées sur le plan toxicologique, clinique et
épidémiologique. La charge de l'organisme résulte principalement
de l'ingestion de résidus présents dans la nourriture (les
quantités ingérées semblant diminuer de façon générale et tomber
en-dessous des DJA fixées) et, dans une moindre mesure, de
l'inhalation de ces produits. D'après l'étude des données, on a
tout lieu de penser que la charge de l'organisme résultant du
niveau actuel d'exposition ne menace en aucun cas la santé de la
population dans son ensemble.
b) La dieldrine se rencontre presque partout dans le lait
maternel. Mais, sa concentration dans le sang et dans le tissu
adipeux des nourrissons n'augmente pas avec l'âge au cours des six
premiers mois de leur vie et le taux sanguin n'est pas plus élevé
que chez un enfant nourri au biberon. Dans ces conditions,
l'allaitement au sein reste la méthode de choix pour nourir les
nourissons malgré la présence de résidus de dieldrine.
c) Lors du traitement de locaux, notamment pour la destruction
des termites, l'exposition des occupants ne semble pas présenter
des risques pour leur santé, pour autant que le traitement
s'effectue correctement.
d) Malgré leur toxicité élevée, l'aldrine et la dieldrine
peuvent être manipulées sans danger dans la mesure où l'on observe
toujours les précautions recommandées en vue de réduire au minimum
l'exposition des opérateurs. Dans le cas contraire, il y a risque
d'intoxication.
e) Pendant la période où l'on a massivement utilisé l'aldrine
et la dieldrine, c'est-à-dire de 1950 au début des années 70, il
est certain que cette pratique a eu des effets dommageables sur
diverses espèces. Ces effets sont imputables en partie à la
dieldrine à côté d'autres organochlorés. Depuis qu'on a limité de
façon draconienne l'utilisation de ces produits, les espèces
touchées se sont reconstituées.
RECOMMENDATIONS
1. Il faut effectuer des études de tératogénicité
complémentaires sur le hamster, bien conçues, avec des doses
réalistes de dieldrine.
2. Dans l'étude du mécanisme de la cancérogenèse, on
s'efforcera d'expliquer pourquoi les réactions hépatiques sont si
différentes chez la souris et chez les autres espèces.
3. On continuera d'utiliser la dieldrine pour l'étude des
mécanismes neurotoxiques, à la fois sur le plan expérimental et sur
le plan clinique.
4. Pour des raisons écologiques, toute reprise d'une
utilisation massive d'aldrine et de dieldrine est exclue, et on
n'utilisera ces produits que s'il n'existe pas de produit moins
nocif d'efficacité équivalente.
5. Afin de préserver la santé et le bien-être des travailleurs
et de la population en général, il convient de ne confier la
manipulation et l'épandage de l'aldrine et de la dieldrine qu'à des
opérateurs compétents et dûment formés, qui devront appliquer les
mesures de sécurité qui s'imposent.
6. En raison du risque d'intoxication accidentelle par
l'aldrine, spécialement chez les enfants, il faut en interdire
l'utilisation sous forme de granulés contre les fourmis.
 Chemical formula: C12H8Cl6
Relative molecular mass: 364.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 S,8 R,8 R,a R)-1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-1,
4:5,8-dimethanonaphthalene or 1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-
exo-1,4- endo-5,8-dimethanonaphthalene
Common synonyms
and trade names: ENT 15 949 (compound 118), HHDN,
Octalene, OMS 194
CAS registry number:
Chemical formula: C12H8Cl6
Relative molecular mass: 364.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 S,8 R,8 R,a R)-1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-1,
4:5,8-dimethanonaphthalene or 1,2,3,4,10,
10-hexachloro-1,4,4a,5,8,8a-hexahydro-
exo-1,4- endo-5,8-dimethanonaphthalene
Common synonyms
and trade names: ENT 15 949 (compound 118), HHDN,
Octalene, OMS 194
CAS registry number:  Chemical formula: C12H8OCl6
Relative molecular mass: 380.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 R,6 R,7 S,8 S,8a R)-1,2,3,
4,10,10-hexachloro-1,4,4a,5,6,7,8,8a-
octahydro-6,7-epoxy-1,4:5,8-
dimethanonaphthalene or 1,2,3,4,10,10-
hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-
octahydro- endo-1,4- exo-5,8,-
dimethanonaphthalene
Common synonyms ENT 16 225 (compound 497), HEOD, Alvit,
and trade names: Octalox, OMS 18, Quintox
CAS registry number:
Chemical formula: C12H8OCl6
Relative molecular mass: 380.9
IUPAC chemical nameb: (1 R,4 S,4a S,5 R,6 R,7 S,8 S,8a R)-1,2,3,
4,10,10-hexachloro-1,4,4a,5,6,7,8,8a-
octahydro-6,7-epoxy-1,4:5,8-
dimethanonaphthalene or 1,2,3,4,10,10-
hexachloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-
octahydro- endo-1,4- exo-5,8,-
dimethanonaphthalene
Common synonyms ENT 16 225 (compound 497), HEOD, Alvit,
and trade names: Octalox, OMS 18, Quintox
CAS registry number:  6.2.3. Photodieldrin (and major metabolites of dieldrin)
6.2.3.1 Laboratory animals
(a) Rat
Brown et al. (1967) fed rats diets containing 3 or 10 mg
photodieldrin/kg diet for 26 days, the 10 mg/kg-group then being
fed a control diet for a further 2 or 8 days. The half-life of
photodieldrin in adipose tissue was calculated to be 1.7 days in
male rats and 2.6 days in female rats. The storage ratio in
adipose tissue was considerably higher in females (1.3) than in
males (0.5).
In studies by Dailey et al. (1970), young rats were given daily
oral doses of 5 µg 14C-photodieldrin per rat, orally or
intraperitoneally, for 12 weeks. Although there was considerable
variation, the radioactivity in the tissues of female rats was
3 - 10 times greater than in male rats, except in the kidneys,
where the 14C activity in males was about 13 times that in females,
regardless of the route of administration.
When rats were fed diets containing 0, 0.1, 1, 10, or 30 mg
photodieldrin/kg diet for 13 weeks, the concentrations of
photodieldrin in the body tissues of female rats receiving up to 10
mg/kg diet were 2 - 15 times greater than those in males. High
concentrations of pentachloroketone (PCK) were found in the kidneys
of male rats receiving 30 mg/kg (276 mg PCK/kg kidney weight
compared with 29 mg photodieldrin/kg). The corresponding
concentrations for females were lower: 13.55 mg PCK and 1.85 mg
photodieldrin per kg kidneys (Walker et al., 1971).
In studies by Walton et al. (1971), groups of weanling rats
(Charles River strain) were fed photodieldrin at concentrations of
0, 1, 5, or 25 (decreased to 12.5 mg) mg/kg diet for 90 days, while
other groups of rats were fed dieldrin at the same concentrations.
The concentrations of both photodieldrin and dieldrin in the
adipose tissue of female rats were higher than in male rats.
(b) Dog
Following the administration of a single oral dose of
photodieldrin to one male and one female dog (160 and 120 mg/kg
body weight, respectively), the concentrations of photodieldrin in
the female dog's tissues, with the exception of the liver, were
much higher than those in the male (Brown et al., 1967).
The concentrations of photodieldrin in the liver and adipose
tissue of dogs fed photodieldrin at 0, 0.005, 0.05, or 0.2 mg/kg
body weight for 3 months were related to the dose rate and similar
in males and females. In the kidneys, the concentrations of
photodieldrin and its metabolite (PCK) were similar in male and
female dogs and much lower (of the order of 0.1 - 0.2 mg/kg
kidneys) than in rats (Walker et al., 1971).
6.2.3.2 Human beings
In samples of human adipose tissue, kidneys, and breast milk,
no residues of photodieldrin or the pentachloroketone metabolite
were detected (Robinson et al., 1966b; Anon., 1973, 1974a,c).
6.3. Metabolic Transformation
6.3.1. Aldrin and dieldrin
The initial and major step in the biotransformation of aldrin
is the formation of the corresponding epoxide dieldrin. There is
considerable evidence that this transformation is mediated by
mixed-function monooxygenases, sometimes called aldrin-epoxidase,
which have been found in a wide variety of organisms, e.g., plant
roots (Mehendale et al., 1972), insects (Krieger & Wilkinson, 1969;
Terriere & Yu, 1976), fish (Burns, 1976), and various mammals,
including man. The endoplasmic reticulum of the liver of
vertebrates is an important site of these enzymes.
6.3.1.1 Laboratory animals
(a) In vitro
The in vitro metabolism of 14C-dieldrin by unwashed microsomes
from a male rat pretreated with phenobarbital has been investigated
by Hutson (1976). The addition of uridine 5'-diphosphoglucuronic
acid (UDPGA) increased the yield of a polar metabolite. The
9-hydroxy derivative was not detected either in the presence or
absence of UDPGA, and investigation of the polar metabolite
indicated that it was the glucuronide of the 9-hydroxy derivate.
The rate of conversion of dieldrin to the glucuronide of 9-hydroxy
dieldrin, measured after 30 min incubation, was 0.0028 nmols/min
per mg protein. In the absence of UDPGA, the conversion of
dieldrin to 9-hydroxy dieldrin could not be detected, and the rate
was estimated to be less than 0.0002 nmols/min per mg protein.
A rat hepatocyte culture suspension effectively epoxidized
aldrin to dieldrin (Kurihara et al., 1984).
(b) In vivo
From the results of a comparative metabolic study on rat and
mouse (section 6.2.2.1), it appears that the main differences
between the species are a more rapid metabolism of dieldrin in
rats, a much greater production of the pentachloroketone by rats,
and the production of small amounts of polar urinary metabolites by
mice. The two strains of mice (CF1 and LACG) were similar to one
another in most, but not all, parameters measured. Thus, the
distinguishing features of the metabolism of dieldrin in CF1 mice,
unique to this strain and which could account for tumour initiation
in mice, have not been found. The hydroxylation of dieldrin in
mice is less efficient than in rats, and the formation of the
glucuronide of 9-hydroxy dieldrin is the result of the consecutive
action of hepatic microsomal monooxygenase and uridine
diphosphoglucuronyl transferase. The 9-hydroxy dieldrin formed
initially is probably bound to the microsomal membrane, and the
availability of UDPGA may be rate-limiting in the overall formation
of the glucuronide. The binding of 9-hydroxy dieldrin to the
microsomal membrane may inhibit the first oxidative step, unless
the concentration of bound metabolite is reduced by conversion to
the water-soluble glucuronide (Hutson, 1976).
Of the species studied, rats, mice, rabbits, sheep, rhesus
monkey, and chimpanzee (Feil et al., 1970; Mueller et al., 1975a),
the major metabolite, except in the case of the rabbit, is the
9-hydroxy derivative (Fig. 2, compound VI). This derivative is
found in the faeces and free or conjugated in the urine. Excretion
of the glucuronide occurs via the bile duct into the lower
intestines, where it is converted to the free 9-hydroxy compound.
The initial chemical identification of this metabolite was based on
a combination of physical and chemical methods (Richardson et al.,
1968; Baldwin et al., 1970; Feil et al., 1970), but it was
subsequently synthesized and the structure confirmed (Bedford &
Harrod, 1972a). The stereochemical configuration of the 9-hydroxy
group has been shown to be syn oriented with respect to the
6,7-epoxy group (Baldwin et al., 1973).
6.2.3. Photodieldrin (and major metabolites of dieldrin)
6.2.3.1 Laboratory animals
(a) Rat
Brown et al. (1967) fed rats diets containing 3 or 10 mg
photodieldrin/kg diet for 26 days, the 10 mg/kg-group then being
fed a control diet for a further 2 or 8 days. The half-life of
photodieldrin in adipose tissue was calculated to be 1.7 days in
male rats and 2.6 days in female rats. The storage ratio in
adipose tissue was considerably higher in females (1.3) than in
males (0.5).
In studies by Dailey et al. (1970), young rats were given daily
oral doses of 5 µg 14C-photodieldrin per rat, orally or
intraperitoneally, for 12 weeks. Although there was considerable
variation, the radioactivity in the tissues of female rats was
3 - 10 times greater than in male rats, except in the kidneys,
where the 14C activity in males was about 13 times that in females,
regardless of the route of administration.
When rats were fed diets containing 0, 0.1, 1, 10, or 30 mg
photodieldrin/kg diet for 13 weeks, the concentrations of
photodieldrin in the body tissues of female rats receiving up to 10
mg/kg diet were 2 - 15 times greater than those in males. High
concentrations of pentachloroketone (PCK) were found in the kidneys
of male rats receiving 30 mg/kg (276 mg PCK/kg kidney weight
compared with 29 mg photodieldrin/kg). The corresponding
concentrations for females were lower: 13.55 mg PCK and 1.85 mg
photodieldrin per kg kidneys (Walker et al., 1971).
In studies by Walton et al. (1971), groups of weanling rats
(Charles River strain) were fed photodieldrin at concentrations of
0, 1, 5, or 25 (decreased to 12.5 mg) mg/kg diet for 90 days, while
other groups of rats were fed dieldrin at the same concentrations.
The concentrations of both photodieldrin and dieldrin in the
adipose tissue of female rats were higher than in male rats.
(b) Dog
Following the administration of a single oral dose of
photodieldrin to one male and one female dog (160 and 120 mg/kg
body weight, respectively), the concentrations of photodieldrin in
the female dog's tissues, with the exception of the liver, were
much higher than those in the male (Brown et al., 1967).
The concentrations of photodieldrin in the liver and adipose
tissue of dogs fed photodieldrin at 0, 0.005, 0.05, or 0.2 mg/kg
body weight for 3 months were related to the dose rate and similar
in males and females. In the kidneys, the concentrations of
photodieldrin and its metabolite (PCK) were similar in male and
female dogs and much lower (of the order of 0.1 - 0.2 mg/kg
kidneys) than in rats (Walker et al., 1971).
6.2.3.2 Human beings
In samples of human adipose tissue, kidneys, and breast milk,
no residues of photodieldrin or the pentachloroketone metabolite
were detected (Robinson et al., 1966b; Anon., 1973, 1974a,c).
6.3. Metabolic Transformation
6.3.1. Aldrin and dieldrin
The initial and major step in the biotransformation of aldrin
is the formation of the corresponding epoxide dieldrin. There is
considerable evidence that this transformation is mediated by
mixed-function monooxygenases, sometimes called aldrin-epoxidase,
which have been found in a wide variety of organisms, e.g., plant
roots (Mehendale et al., 1972), insects (Krieger & Wilkinson, 1969;
Terriere & Yu, 1976), fish (Burns, 1976), and various mammals,
including man. The endoplasmic reticulum of the liver of
vertebrates is an important site of these enzymes.
6.3.1.1 Laboratory animals
(a) In vitro
The in vitro metabolism of 14C-dieldrin by unwashed microsomes
from a male rat pretreated with phenobarbital has been investigated
by Hutson (1976). The addition of uridine 5'-diphosphoglucuronic
acid (UDPGA) increased the yield of a polar metabolite. The
9-hydroxy derivative was not detected either in the presence or
absence of UDPGA, and investigation of the polar metabolite
indicated that it was the glucuronide of the 9-hydroxy derivate.
The rate of conversion of dieldrin to the glucuronide of 9-hydroxy
dieldrin, measured after 30 min incubation, was 0.0028 nmols/min
per mg protein. In the absence of UDPGA, the conversion of
dieldrin to 9-hydroxy dieldrin could not be detected, and the rate
was estimated to be less than 0.0002 nmols/min per mg protein.
A rat hepatocyte culture suspension effectively epoxidized
aldrin to dieldrin (Kurihara et al., 1984).
(b) In vivo
From the results of a comparative metabolic study on rat and
mouse (section 6.2.2.1), it appears that the main differences
between the species are a more rapid metabolism of dieldrin in
rats, a much greater production of the pentachloroketone by rats,
and the production of small amounts of polar urinary metabolites by
mice. The two strains of mice (CF1 and LACG) were similar to one
another in most, but not all, parameters measured. Thus, the
distinguishing features of the metabolism of dieldrin in CF1 mice,
unique to this strain and which could account for tumour initiation
in mice, have not been found. The hydroxylation of dieldrin in
mice is less efficient than in rats, and the formation of the
glucuronide of 9-hydroxy dieldrin is the result of the consecutive
action of hepatic microsomal monooxygenase and uridine
diphosphoglucuronyl transferase. The 9-hydroxy dieldrin formed
initially is probably bound to the microsomal membrane, and the
availability of UDPGA may be rate-limiting in the overall formation
of the glucuronide. The binding of 9-hydroxy dieldrin to the
microsomal membrane may inhibit the first oxidative step, unless
the concentration of bound metabolite is reduced by conversion to
the water-soluble glucuronide (Hutson, 1976).
Of the species studied, rats, mice, rabbits, sheep, rhesus
monkey, and chimpanzee (Feil et al., 1970; Mueller et al., 1975a),
the major metabolite, except in the case of the rabbit, is the
9-hydroxy derivative (Fig. 2, compound VI). This derivative is
found in the faeces and free or conjugated in the urine. Excretion
of the glucuronide occurs via the bile duct into the lower
intestines, where it is converted to the free 9-hydroxy compound.
The initial chemical identification of this metabolite was based on
a combination of physical and chemical methods (Richardson et al.,
1968; Baldwin et al., 1970; Feil et al., 1970), but it was
subsequently synthesized and the structure confirmed (Bedford &
Harrod, 1972a). The stereochemical configuration of the 9-hydroxy
group has been shown to be syn oriented with respect to the
6,7-epoxy group (Baldwin et al., 1973).
 The other metabolites, the chemical identities of which have
been rigorously established, are detailed below.
(a) The trans-6,7-dihydroxy compound (Fig. 2, compound IV) is
formed by the hydration (formal) of the epoxide ring of dieldrin
(Korte & Arent, 1965). This compound is a major metabolite in
rabbit urine, but of relatively minor importance in other species.
The formation of the cis-diol by rat microsomes has been
demonstrated, together with its epimerization to the trans-diol
(McKinney et al., 1973). Both the cis- and trans-diols have been
synthesized (Korte & Arent, 1965; Chau & Cochrane, 1970b; Bedford &
Harrod, 1972b).
(b) The dicarboxylic acid (Fig. 2, compound V) is derived from
the dihydroxy metabolite (Baldwin et al., 1972; Oda & Mueller,
1972). This compound has also been synthesized (Buechel et al.,
1966), and has been shown to undergo further degradation (formation
of two isomers of a monodechlorinated derivative) after intravenous
injection into male and female rats (Lay et al., 1975).
(c) The bridged pentachloroketone (PCK) (Fig. 2, compound VII)
is mainly found in the urine and kidneys of male rats, but, even in
rats, it is a minor metabolite (Damico et al., 1968; Klein et al.,
1968; Richardson et al., 1968). In other species, it is a very
minor metabolite of dieldrin. It is also a metabolite of
photodieldrin (Klein et al., 1970), and has been synthesized
(Bedford & Smith, 1978).
The Chemical Abstract or Von Baeyer AG/IUPAC names of aldrin,
dieldrin, photodieldrin, and metabolites are given in Appendix I.
Methods for the quantitative determination of the four
metabolites are available. They depend on the availability of
authenticated analytical standards (Ludwig & Korte, 1965;
Richardson, 1971; Baldwin et al., 1972).
6.3.1.2 Human studies
A metabolite of dieldrin detected in human faeces has been
shown to be the 9-hydroxy derivative (Richardson & Robinson, 1971).
6.3.1.3 Non-domestic organisms
The conversion of aldrin to dieldrin was studied in algae
( Chlorella and diatoms) and protozoa (Dinoflagellates and mixed
protozoa) after exposure for 24 h to 0.1 mg aldrin/litre. The
amount of dieldrin present in the cultures was of the order of
0.06 - 0.2 µg/litre). The amounts of dieldrin were greater in the
protozoa than in the algae; it was concluded that these planktonic
species have enzyme systems that epoxidize aldrin (Khan et al.,
1972b).
The conversion of aldrin to dieldrin in 12 species of fresh-
water invertebrates has been compared. Ten species were exposed
for 2 h to concentrations of 0.1 or 0.25 mg aldrin/litre, and two
species of molluscs were exposed to 0.25 mg aldrin/litre for 4 h.
The concentrations of dieldrin relative to aldrin in the whole
bodies of eight species from four phyla (Coelenterata,
Platyhelminthes, Annelida, and Arthropoda) were in the range
1.03 - 8.48%. In two species of Insecta (dragon fly nymphs and
Aedes larvae), the values were 24.9% and 42.4%, respectively. The
two species of molluscs had dieldrin concentrations (relative to
aldrin) of 17 - 19% (Khan et al., 1972b).
In a study on an ostracod (Chlamydotheca arcuata) exposed to
14C-labelled aldrin (5.5 - 11.2 µg/litre), aldrin was readily
converted to dieldrin, 83% conversion occurring within 24 h. The
elimination of aldrin and dieldrin appeared to involve both passive
and active processes, and it was concluded that dieldrin was
eliminated more rapidly after dieldrin exposure than after aldrin
exposure (Kawatski & Schmulbach, 1972).
A number of in vitro studies have been carried out concerning
the influence of these insecticides on mixed-function oxidase
activity. The epoxidation of aldrin to dieldrin by this enzyme
system has been demonstrated in crayfish (Cambarus) (Khan et al.,
1972a,b), in snail (Lymnea palustris) and clam (Khan et al.,
1972b), and in midge larvae (Chironomus riparius) (Estenik &
Collins, 1979).
The conversion of aldrin to dieldrin by lobsters (Homarus
americanus) was reported by Carlson (1974).
The mixed-function oxidase activities in five species of fresh
water fish, as measured by the conversion of aldrin to dieldrin,
were investigated by Ludke et al. (1972). The fish were exposed to
aldrin (50 µg/litre) for 4 h, and the concentrations of aldrin and
dieldrin in the liver were determined. Contrary to earlier
reports, the conversion by epoxidation of aldrin to dieldrin in
fish may be the rule rather than an exception.
The epoxidation of 14C-aldrin to dieldrin in susceptible and
resistant mosquitofish (Gambusia affinis) has been investigated.
The fish were exposed to 5 µg 14C-aldrin/litre for 4 or 8 h and the
concentration of aldrin and dieldrin in liver and brain were
determined. The concentration of dieldrin (expressed in terms of
protein content) was significantly higher in the livers of
resistant fish than in susceptible fish). It was concluded that
resistant mosquitofish convert aldrin to dieldrin and/or water-
soluble compounds at a greater rate than susceptible mosquitofish
(Wells et al., 1973).
In studies by Addison et al. (1976), Atlantic salmon fry (Salmo
salar) were injected intramuscularly with 14C-aldrin, to initial
whole-body concentration of 5 mg/kg. The fish were maintained in
flowing fresh water, and were removed at five intervals up to 56
days for the measurement of whole-body residues. The time required
for 50% epoxidation of aldrin was between 1 and 2 days. Less than
10% of the radioactivity remained in the fish at the end of the
exposure. It was concluded that there was rapid elimination either
of unchanged aldrin or its epoxide, dieldrin, from the fish.
6.3.2. Photodieldrin (and major metabolites of dieldrin)
6.3.2.1 Rat
Besides unchanged photodieldrin, bridged pentachloroketone
(PCK) (Fig. 2, compound VII), a metabolite of photodieldrin, was
isolated from the brain, liver, adipose tissue, and blood of rats
(Carworth Farm, type E) fed diets containing 10 or 30 mg
photodieldrin/kg for 13 weeks (Baldwin & Robinson, 1969).
In studies by Klein et al. (1970), Osborne-Mendel rats were
given 14C-photodieldrin, orally or intraperitoneally, 5 days/week,
for 12 weeks, and urine was collected quantitatively every day. A
metabolite was found in the urine of male rats and shown to be PCK.
Small amounts of other (unidentified) more polar urinary
metabolites were also present.
6.3.2.2 Monkey
Metabolites were detected in the urine and faeces of a female
rhesus monkey given daily oral doses of 0.8 mg 14C-photodieldrin/kg
body weight for 175 days. Two metabolites were identified in the
urine: the trans-diol (Fig. 2, compound XI) and its glucuronide
conjugate. A faecal metabolite was tentatively identified as the
diol. A third metabolite was present in both urine and faeces, and
it was suggested that this might be a monohydroxy derivative of
photodieldrin (Nohynek et al., 1979).
6.4. Elimination and Excretion
6.4.1. Aldrin
6.4.1.1 Rat
When male rats were given daily oral doses of 4.3 µg 14C-aldrin
(equivalent to about 0.2 mg aldrin/kg diet) for 3 months, the
radioactivity in the urine increased from about 2% of the dose of
aldrin during the first week to about 10% during the 12th week. In
the faeces, the excreted radioactivity increased from about 48%
during the first week to about 93% during the 12th week. After
about 8 weeks, a saturation level was reached (i.e., there was a
balance between the rates of intake of aldrin and excretion of
aldrin plus aldrin-related materials). Extracts of urine and
faeces were examined by paper chromatography. Because the urine
was probably contaminated by faeces in the metabolism cages, only
the trend is given. In both faeces and urine, the aldrin content
decreased during the 12 weeks. The hydrophilic metabolites
increased, reaching 75% (faeces) and 95% (urine) of total
radioactivity after 12 weeks. The level of dieldrin was more or
less constant (Ludwig et al., 1964).
6.4.2. Dieldrin
6.4.2.1 Laboratory animals
As described in section 6.2.2.1, Hutson (1976) studied the
comparative metabolism of dieldrin in CFE rats and two strains of
mice after a single oral dose of 3 mg/kg body weight 14C-dieldrin.
The excretion of 14C activity in the faeces of the rats was 62.4%
of the administered dose in the non-pretreated group and 69% in the
dieldrin-pretreated group. In the case of the CF1 mice, the
pretreatment period did not have any effect on the faecal excretion
(51.5%), whereas, in the LACG mice, the faecal excretion of 14C
activity increased from 27.2% for the non-pretreated group to 48.8%
for the 4-week pretreated group. The total 14C activity excreted in
the urine of the two strains of mice was low (0.42 - 2.6% of dose)
compared with that in the urine of male rats (5.5 - 6.6%). In both
species of rodents, the faeces was the major route of excretion of
14C activity. In the urine of both the male CFE rat and the male
CF1 mice, the amount of the dicarboxylic acid metabolite in the
urine was small compared with that of pentachloroketone plus
dieldrin, while in the male LACG mice, the amount of the acidic
metabolite was twice that of pentachloroketone plus dieldrin. Both
strains of mice excreted, proportionally, much larger amounts of a
polar (unidentified metabolite) in the urine than did the CFE rats.
In the faeces of the male CFE rats (no pretreatment), the major
component was the 9-hydroxy derivative. This was also found by
Matthews et al. (1971). However, in both mouse strains (no
pretreatment), this compound was a minor metabolite, but it became
the major product in the dieldrin-pretreated group. In isolated
liver microsomes, most of the 14C activity appeared to be present
as dieldrin, and the 9-hydroxy metabolite was not detected.
A number of other studies on the excretion of dieldrin via
urine and/or faeces have been carried out. Dailey et al. (1970)
found that male rats excreted higher levels of 14C radioactivity
via urine and faeces than females. Davison (1973) confirmed this
in a study lasting 39 weeks. Maximal excretion of 14C activity
occurred in the 6th week in both sexes, regardless of the amount of
dieldrin given. A steady state was reached and maintained from the
6th to the 39th week.
In studies by Robinson et al. (1969), rats were fed a diet
containing 10 mg dieldrin/kg diet for 8 weeks. The decline in the
concentration of dieldrin in blood, brain, liver, and adipose
tissue was studied during the subsequent 12 weeks when a control
diet was fed. There was an initial rapid decline in the dieldrin
concentration during the first 10 days of the post-exposure period
in the blood, liver, and brain, followed by a slower decline. The
changes in the concentration of dieldrin in the brain, adipose
tissue, blood, and liver corresponded to biological half-lives of 3
to about 10 days.
When male and female rats were administered 3 g of diet
containing 10 mg 14C-dieldrin/kg diet, followed by a control diet
ad libitum, 14C activity in the kidneys of male rats was 10-fold
higher than in the female rats (the animals were killed 9 days
after administration of 14C-dieldrin). Most of the activity in the
male kidneys was due to pentachloroketone, whereas, in the female
kidneys, only dieldrin was detected (Matthews et al., 1971).
The excretion of 36Cl activity by female rats dosed
intravenously (680 µg/h for 2.5-5 h; total doses of 8 - 16 mg/kg
body weight) with 36Cl-dieldrin has been studied. The 36Cl
activity detected in the faeces was about 7 times that found in the
urine, indicating excretion via the bile (Heath & Vandekar, 1964).
Comparable results were found by Cole et al. (1970), who gave
male rats a single intravenous dose of 0.25 mg 14C dieldrin/kg body
weight. Similar doses of 14C-dieldrin were administered
intravenously to male rats with bile fistulas. About 30% of the
administered 14C activity was excreted via the bile during the
first 24 h after dosing, and after 4 days a total excretion of
about 60% had occurred. Isolated perfused rat liver preparations
were also investigated; some 20% of the original perfusate dose was
collected in the bile over a period of 8 h.
Rapid excretion of 14C-dieldrin (or its metabolites) from
isolated perfused rat livers via the bile of rats has also been
reported by Klevay (1970), the rate of excretion by male rats being
about 3 times as rapid as that by female rats.
In studies by Mueller et al. (1975a), mice, rats, rabbits,
rhesus monkeys, and one chimpanzee were given a single oral dose of
0.5 mg/kg body weight 14C-dieldrin, and urine and faeces were
collected for 10 days. For all species except the rabbit, the main
route of excretion was the faeces. The faecal excretion of
unchanged dieldrin was high in the first 48 h and then declined
rapidly. The urine samples contained only metabolites of dieldrin.
The mean total amount of radioactive material excreted (males
and/or females) in faeces and urine within 10 days after dosing
(expressed as percentage of administered dose) was 37% in mice, 11%
in rats, 2% in rabbits, 20% in rhesus monkeys, and 6% in the
chimpanzee. In all five species, 9-hydroxy-dieldrin and
4,5-aldrin- trans-dihydrodiol were the major metabolites. The
metabolism in the rat seems to be comparable to that of primates;
however, mice and rabbits showed the opening of the epoxide to diol
as the predominant reaction.
6.4.2.2 Human studies
The occurrence of a neutral metabolite of dieldrin in human
urine in amounts indicative of exposure to aldrin/dieldrin was
reported by Cueto & Hayes (1962) and Cueto & Biros (1967).
Quantitative estimates of the amounts of a metabolite of
dieldrin, 9-hydroxy-dieldrin, in the faeces of seven workmen
occupationally exposed to aldrin/dieldrin and five male members of
the general population have been made. The average concentration
of the 9-hydroxy derivative in 24-h collections of faeces of the
seven workmen was 1.74 mg/kg (range, 0.95 - 2.80 mg/kg), whereas
the average concentration in faeces of the five members of the
general population was 0.058 mg/kg (range, 0.033 - 0.12 mg/kg).
Dieldrin was present in the faeces of the workmen (average
concentration, 0.18 mg/kg), but, in samples from the general
population, it was below the limit of detection. Examination of
the urine of five of the workmen indicated that this route of
elimination of dieldrin and four known metabolites was minor. It
was concluded that the 9-hydroxy-dieldrin in the faeces represented
the major excretory pathway of dieldrin from male human beings. It
should be noted, however, that the urine was not examined for
glucuronide or other conjugates of the hydroxy metabolites). There
was good correlation between the estimated daily intake of dieldrin
(calculated from the concentrations of dieldrin in the blood) and
excretion in faeces of total equivalent dieldrin (Richardson,
1971). This relationship is based on a number of assumptions, and
it is probably more relevant that the concentration of the
9-hydroxy-dieldrin in the faeces (produced by the metabolism of
absorbed dieldrin) is significantly related to the concentration of
dieldrin in the blood, which is a measure of the body burden
arising from absorption of aldrin plus dieldrin.
When 14C-Dieldrin was applied in acetone (4 µg/cm2) once to the
forearm of volunteers, 7.7% of the applied 14C activity was
excreted in the urine over a 5-day period. A single intravenous
injection of 14C-dieldrin resulted in 3.3% being excreted in the
urine over a 5-day period (Feldman & Maibach, 1974).
6.4.3. Photodieldrin (and major metabolites of dieldrin)
6.4.3.1 Rat
In studies by Dailey et al. (1970), young rats were given daily
doses of 5 µg 14C-photodieldrin, orally or intraperitoneally, for
12 weeks. Urine and faeces were collected daily and pooled in
weekly groups. The excretion of 14C activity via the urine of
females was considerably less than that by males, by either method
of dosing. The 14C activity in urine after oral and ip
administration increased slowly during the 12 weeks (males about
10% and females 5%), the highest levels in urine (up to 33%) being
found in males dosed intraperitoneally. Faecal excretion of 14C
activity was initially lower in females, but greater during the
latter half of the study (of the order of 20 - 40%). In males,
during the whole study, it was about 30%.
6.4.3.2 Monkey
A juvenile female rhesus monkey was given daily oral doses of
2 mg 14C-photodieldrin (equivalent to 0.8 mg/kg body weight), and
the treatment was continued until, between days 70 and 76, the
daily excretion of 14C activity was in balance with the daily
intake. When dosing ceased, the animals had retained about 50% of
the cumulative dose of photodieldrin. Collection of excreta was
continued for a further 100 days, during which a further 30.1% of
the dose, administered during the 76-day period, was excreted.
During the period of dosing, a major part of the faecal 14C
excretion consisted of photodieldrin (probably indicating
incomplete absorption in the gastrointestinal tract), while
20 - 50% of the excreted activity was in the urine. After dosing
ceased, 60% of the excreted 14C activity appeared in the urine
(Nohynek et al., 1979).
In studies by Nohynek et al. (1979), one male and one female
juvenile rhesus monkey were given single intravenous doses of
4.5 mg 14C-photodieldrin (2 mg/kg body weight). Urine and faeces
were collected separately every 24 h, and the animals were killed
after 21 days. Excretion of 14C activity was high during the first
7 days (male, 39%; female, 27.3%, of the given dose). It then
decreased rapidly and reached a nearly constant value of 0.2% of
the administered dose. Approximately 45% (male) and 34% (female)
of the dose had been excreted by day 21.
6.5. Retention and Turnover
6.5.1. Non-domestic organisms
A few studies have been carried out on the uptake and
elimination of aldrin and/or dieldrin in invertebrates: marine
clams (Mya arenaria and Mercenaria mercenaria) (Butler, 1971);
naiad mollusc (Amblema plicata) (Fikes & Tubb, 1972); mussel
(Lampsilis siliquiodea) (Bedford & Zabik, 1973); crab (Leptodius
floridanus) (Epifanio, 1973); and ostracod (Chlamydotheca arcuata)
(Kawatski & Schmulbach, 1972). The concentration of aldrin or
dieldrin in organs and tissues increased rapidly during the first
1 - 2 weeks of exposure, but remained virtually constant
thereafter. When the organisms were placed in clean water, the
concentration declined in a (semi)-logarithmic manner in relation
to time. The estimated half-life for the tested organisms varied,
e.g., for Lampsilis siliquiodea, it was 4.7 days, whereas for
Amblema plicata, it was about 3 - 4 weeks.
The elimination of 14C-dieldrin from bluegills (Lepomis
macrochirus) and goldfish (Carassius auratus) was studied by
Gakstatter & Weiss (1967). The fish were exposed to 30 µg
14C-dieldrin/litre (initial concentration) until toxic symptoms
appeared (5 - 8 h), and were then placed in recovery aquaria
together with unexposed fish. The water in the recovery aquaria
was continuously renewed. Samples of five fish were taken on 10
different occasions during the recovery period. The 14C activity
in whole fish of both species, expressed as equivalent dieldrin,
declined by about 90% within 16 days, the half-time for elimination
being about 4 days. The control bluegills and goldfish accumulated
a maximum equivalent dieldrin concentration of 0.29 and 0.22 mg/kg,
respectively, on day 4 of the period in the recovery aquaria,
indicating transfer of dieldrin or derived material from
contaminated to uncontaminated fish.
In another study, the distribution of aldrin and dieldrin in
the tissues of Carassius auratus was determined following an 8-h
exposure to 14C-aldrin (50 µg/litre) in a static study. After the
exposure, fish were placed in a continuously flushed aquarium for
32 days. Dieldrin was found in all tissues examined immediately
after the exposure. The percentage of dieldrin in the total
residues in the tissue increased with time, reaching about 95% on
day 32 (except in visceral fat). During the recovery period, the
total concentration of aldrin plus dieldrin in the blood declined
from 2.1 mg/litre (as aldrin) to 0.4 mg/litre. The corresponding
changes in the brain concentrations were 5.45 mg/kg to 2.3 mg/kg.
Total residues in the nerve cord did not show a consistent decline
and varied from 4.56 to 21.6 mg/kg throughout the 32-day period;
however, these residues were determined by thin-layer
chromatography, not by gas-liquid chromatography (Gakstatter,
1968).
The partitioning of 14C activity into particulate fractions of
the brain and liver of resistant and susceptible mosquitofish has
been studied after exposure of the fish to 14C-aldrin or 14C-
dieldrin. The 14C activities in total brain, cell membrane, and
five cellular fractions were significantly higher in susceptible
fish than in resistant fish for both aldrin and dieldrin. However,
this difference was much less marked in the case of the liver. It
was suggested that a basic structural change in polarity exists in
the myelin of resistant fish, which could provide a membrane
barrier (Wells & Yarbrough, 1973).
The fate of dieldrin in the digestive tract of juvenile lake
trout (Salvelinus namaycush) has been studied. Macerated trout
flesh containing an average of 1.05 mg/kg was injected in the
stomach. The decline in the dieldrin content of the stomach was
parallel to that of the food from the stomach. Little or no
dieldrin was found in the intestines (Stewart & Stein, 1974).
In studies by Chadwick & Brocksen (1969), groups of sculpins
(Cottus perplexus) were exposed to 1.3 µg dieldrin/litre for 12
days, followed by removal to uncontaminated continuously renewed
water. The concentration in whole fish declined in a curvilinear
fashion from about 2.5 mg/kg fish to about 1 mg/kg fish in 60 days
and to about 0.5 mg/kg fish in 90 days.
Sailfin molly (Poecilia latipinna) were exposed to 12 µg
dieldrin/litre for up to 6 h by Lane et al. (1970). Two products,
thought to be metabolites of dieldrin, were detected in the liver
and other organs, and it was suggested that they were partially
dechlorinated derivatives of dieldrin.
6.5.2. Biological half-life in human beings
The concentration of dieldrin in the blood of volunteers given
oral daily doses for 2 years (section 6.2.2.4) was determined over
a period of 8 months after termination of the deliberate exposure
(Hunter et al., 1969). A small, but statistically significant,
decline occurred, corresponding to a mean value of 369 days for the
half-life of dieldrin in blood. However, there were significant
differences between the rates of decline of the individual
volunteers.
The concentration of dieldrin in the blood of 15 workmen was
determined for a period of 3 years following termination of
occupational exposure to aldrin/dieldrin (Jager, 1970). The mean
half-life was 266 days.
When a state of equilibrium has not yet been reached, the
apparent half-life will be much shorter, due mainly to a
redistribution of dieldrin between compartments in the body.
6.5.3. Body burden and (critical) organ burden; indicator media
Whatever the route of exposure, the effect, if any, will be
determined by the concentration of the chemical in the target organ
or tissue. It has been shown that the distribution between the
various tissues of mammals is fairly constant within and between
species (Robinson & Hunter, 1966; Hunter & Robinson, 1967; Hunter
et al., 1967; Robinson & Roberts, 1969; Walker et al., 1969b).
Thus, at a state of equilibrium, the dieldrin level in the blood
reflects the concentration of the active compound in the target
tissues and therefore represents the best practical parameter for
the internal exposure that is associated with a biochemical,
clinical, or pathological effect. Since the biological half-life
of dieldrin in human blood is known (266 days) (Jager, 1970),
a reliable estimation of the blood level at the time of
discontinuance of the exposure can be made. This, in turn enables,
better than anything else, the evaluation of the likelihood of an
observed symptom of disease or indisposition being associated with
exposure to dieldrin. Also, the established mathematical
relationship between the dieldrin level in the blood and the total
daily equivalent oral intake thus enables, on the basis of the
concentration of dieldrin in the blood, the evaluation of a current
exposure or an exposure of a short time ago vis-à-vis the
acceptable daily intake established by the FAO/WHO Joint Meeting on
Pesticide Residues.
Determination of the dieldrin concentration in blood is the
method of choice in monitoring exposed workers or the general
population (section 9.2.1.1).
6.6. Appraisal
Aldrin is readily absorbed through the skin, by inhalation of
the vapour, or into the circulating blood from the gastrointestinal
tract. It has not been possible to determine the percentage of an
ingested dose of aldrin or dieldrin that is actually absorbed into
the body because of the intestinal hepatic biliary cycle. Work
with human volunteers (Feldmann & Maibach, 1974) showed that
absorption through the skin amounted to 7 - 8% of the applied dose.
Inhalation studies with human volunteers (Beyermann & Eckrich,
1973; Bragt et al., 1984) suggested that about 50% of inhaled
aldrin vapour is absorbed and retained in the human body. After
absorption, it is rapidly distributed to the organs and tissues of
the body, and a continuous exchange between the blood and other
tissues takes place. In the meantime, aldrin is readily converted
to dieldrin, mainly in the liver but, to a much lesser extent, in
some other tissues, e.g., the lungs (Mehendale & El-Bassiouni,
1975).
This conversion proceeds very rapidly. The livers of even
24-h-old rats, given oral doses of 10 mg aldrin/kg body weight,
contained dieldrin 2 h after treatment (Farb et al., 1973). In the
course of the next few hours, dieldrin and what little is left of
the aldrin in blood and other tissues, concentrates more in the
lipid tissues (Heath & Vandekar, 1964; Hayes, 1974). In human
beings, aldrin is found rarely, if at all, in human blood or other
tissues, except in cases with acute poisoning by accidental or
intentional ingestion of massive doses.
Studies carried out with 14C-labelled aldrin and dieldrin have
shown that part of the ingested material is passed unabsorbed
through the intestinal tract and eliminated from the body, part is
excreted unchanged from the liver into the bile, part is stored
unchanged in the various organs and tissues (particularly the
adipose tissue), and part is metabolized in the liver to more polar
and hydrophilic metabolites. These metabolites, in human beings
and most animals, are excreted primarily via the bile in the
faeces. It had also been shown that aldrin and dieldrin are both
biodegraded into the same metabolites (Damico et al., 1968; Klein
et al., 1968). The biodegradation products have been identified in
the rat within 15 min after an intravenous injection (Moersdorf et
al., 1963). Most of the currently available information on the
biodegradation metabolism in mammals is based on studies with
dieldrin on the mouse, rat, rabbit, sheep, dog, monkey, chimpanzee,
and human beings (Ludwig et al., 1964; Datta et al., 1965; Korte,
1965; Korte & Arent, 1965; Richardson et al., 1967b, 1968; Klein et
al., 1968; Matthews & Matsumura, 1969; Baldwin et al., 1970, 1972;
Feil et al., 1970; Richardson & Robinson, 1971; Mueller et al.,
1975a,b). Although there appear to be differences between species
and, in the rat, differences between the sexes, the overall picture
shows only quantitative variations between species.
In the species studied (with the exception of the rabbit) the
major metabolite is the 9-hydroxy derivative. This is found in the
faeces and free or conjugated in the urine. Three other
metabolites have been found and identified in experimental animals:
(a) trans-6,7-dihydroxy derivative;
(b) dicarboxylic acid derived from the dihydroxy compound; and
(c) the bridged pentachloroketone (PCK).
The latter is also a metabolite of photodieldrin (Klein et al.,
1970).
Only the 9-hydroxy compound was found in the faeces of seven
occupationally exposed industrial workers (1.74 mg/kg) and five
male members of the general population (0.058 mg/kg). Neither the
9-hydroxy compound nor the other metabolites have been found in
human blood or other tissues. Dieldrin was present in the faeces
of the workmen (average 0.18 mg/kg), whereas the concentrations in
the samples from the general population were below the limits of
detection. Examination of the urine of five workmen indicated that
urinary excretion of dieldrin and its four metabolites is minor
relative to elimination of the 9-hydroxy metabolite via the faeces
(Richardson, 1971).
The conversion of aldrin to dieldrin and the distribution and
the subsequent deposition of dieldrin (mainly in lipid tissues)
proceed much faster than the biodegradation and ultimate
elimination of unchanged dieldrin and its metabolites from the
body. At a given average intake of aldrin and/or dieldrin,
dieldrin slowly accumulates in the body. This accumulation or
"storage", however, does not increase indefinitely. As the
concentration of dieldrin in the liver cells increases, the
metabolizing enzyme activity in the microsomes increases, and so
the rate of biodegradation of dieldrin, and hence the elimination
from the body, is enhanced. Thus, the accumulation proceeds at an
ever slower rate until the concentrations of dieldrin in blood and
tissues approach upper limits of storage and an amount of dieldrin
equal to the average daily intake is eliminated each day. These
upper limits of storage are related to the daily intake. This has
been demonstrated in rats and dogs (Walker et al., 1969b) and in
human beings (Hunter & Robinson, 1967; Hunter et al., 1969). When
the intake of aldrin/dieldrin ceases or decreases, the body burden
decreases. The biological half-life in human beings is 9 - 12
months (Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
Significant relationships exist between the concentrations of
dieldrin in the blood and those in other tissues of rats, dogs, and
human beings (Hunter & Robinson, 1967; Deichmann et al., 1968;
Keane & Zavon, 1969b; Hunter et al., 1969; Walker et al., 1969b).
Numerous investigations of the concentrations of dieldrin in
body fat, blood, and other tissues from members of the general
population and from special groups have been carried out in several
countries. The results are summarized and discussed in section
5.2. The ratio of dieldrin concentrations in fat, liver, brain,
blood is about 150:15:3:1.
Dieldrin penetrates the placenta and is present in the blood,
fat, or other organs of the fetus, newborn babies, and infants
(Table 22). The concentrations are much lower (by 50% or more)
than those in adults. The ratio of dieldrin concentrations in
blood, brain, liver, and fat in infants is not different from that
ratio in adults (Fiserova-Bergerova et al., 1967; Casarett et al.,
1968). Dieldrin is excreted in mother's milk, average values being
about 3 - 5 µg/litre mother's milk (Table 23). The ratio of the
dieldrin concentration in mother's blood to that in mother's milk
is about 1:2 - 3.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
Neither aldrin nor dieldrin have significant effects on
populations of microorganisms in soil or fresh water at realistic
concentrations. Some physiological processes of microorganisms are
affected by low concentrations of both aldrin and dieldrin, but
these would appear to have little or no environmental significance.
Aldrin and dieldrin have only minor deleterious effects on soil
bacterial populations, even at concentrations that are much higher
than those used in agricultural practice.
The effects of insecticides on soil microbes have been reviewed
by Tu & Miles (1976). Of 15 strains tested, aldrin did not have
any effect on the growth of 11 bacterial species (single cultures)
but caused some growth inhibition in four species. Dieldrin did
not have any effect on 13 bacterial species but had some inhibitory
effect in two species. Neither aldrin nor dieldrin at 2000 mg/kg
soil had effects on bacteria in laboratory studies; soil fungi were
also little affected. In pot studies, using aldrin at 4 and 120
mg/kg soil, there were no quantitative changes in bacteria during
any part of the vegetative period. Aldrin inhibited the growth of
Rhizoctonia solani in plate cultures by 20% or more at 6.2 mg/litre
and higher concentrations. Dieldrin was less toxic, producing an
average inhibition of about 15%, which was not dose related over
the range of 1 - 100 mg/litre. The evolution of carbon dioxide
(CO2) (a measure of soil organisms respiration) was significantly
reduced by dieldrin at 1000 mg/kg soil (but not at 100 mg/kg),
whereas aldrin produced a significant reduction at concentrations
as low as 25 mg/kg soil. Slight effects on nitrification were
initially found when aldrin and dieldrin were incorporated at 2000
mg/kg in a sandy loam soil, but nitrification was normal after
about 10 weeks. Short-term inhibition of nitrification was also
produced by aldrin and dieldrin at 25 mg/kg in a sandy loam soil
(aldrin for 1 week, dieldrin for 2 weeks). Decreased sulfur
oxidation was observed in soil containing aldrin or dieldrin (2000
mg/kg), the inhibition decreasing considerably after 3 months.
Five annual applications of aldrin or dieldrin (5.5 - 22 kg/ha) to
a Ramona sandy loam had no measurable effect on the numbers of soil
bacteria or fungi, did not influence the ability of the soil
population to decompose plant residue, and did not alter soil
aggregation.
The effect of dieldrin on the activities of three soil enzymes
was determined at concentrations of 5 or 10 mg dieldrin/kg soil by
Tu (1981). The dehydrogenase activity of the dieldrin-treated soil
(10 mg/kg) did not differ from controls, whereas at 5 mg
dieldrin/kg, the activity was significantly greater than controls
after 2 weeks (50% increase). Urease activity at both treatment
levels was significantly reduced after a 1-week incubation but
significantly increased after 2 weeks. Phosphatase activity was
significantly reduced at 5 mg dieldrin/kg, but not at 10 mg/kg.
The 96-h EC50 (growth) for algae (Chlamydomonas sp.,
Phaeodactylum tricornutum, Dunaliella sp., Chlorella ovalis, and
Chlorella pyrenoidosa) was > 100 µg dieldrin/litre (Adema & Vink,
1981).
The photosynthetic activity of four species of marine
phytoplankton in the presence of dieldrin was investigated using
14C-labelled Na2CO3. A range of nominal concentrations
(0.01 - 1000 µg/litre) was used, and the plant cultures were
exposed for 24 h. The 14C uptake of Dunaliella tertiolecta during
7 days post-treatment was unaffected by up to 1000 µg
dieldrin/litre. Two other species (Skeletonema costatum and
Coccolithus huxleyi) showed significant reductions in 14C uptake at
levels of dieldrin above 10 µg/litre, and the photosynthetic
activity of Cyclotella nana was reduced at concentrations above 1
µg dieldrin/litre (Menzel et al., 1970).
In studies by Schauberger & Wildman (1977), three species of
fresh-water algae (Anabaena cylindrica, Anacystis nidulans, Nostoc
muscorum) were exposed to aldrin or dieldrin at concentrations of
0 - 1000 µg/litre. After exposure for 7 days, there was no
significant effect on the photosynthetic pigment absorption of the
three species at concentrations up to 10 µg (nominal)/litre.
However, at 1 mg/litre, aldrin almost completely suppressed the
absorption by photosynthetic pigments (chlorophyll and
phycocyanin), these being indicators of physiological health and
growth. Dieldrin (1 mg/litre) produced a reduction of about 40%.
The growth response of two cyanobacteria (blue-green algae) in
the presence of aldrin, dieldrin, or two metabolites of dieldrin,
photoaldrin, or photodieldrin was determined at nominal
concentrations of 0.2 - 950 µg/litre (Batterton et al., 1971).
None of these compounds had significant effects on the growth rate
constants at concentrations of 95 µg/litre or at lower
concentrations over periods of 26 - 30 h. The investigators
considered that dieldrin and its derivatives reduced the growth
rate constant at 475 and 950 µg/litre, Agmenellum quadriplicatum
being more sensitive than Anacystis nidulans. Aldrin did not have
a significant effect on either species, but photoaldrin affected
Agmenellum quadriplicatum at 950 µg/litre.
In studies by Powers et al. (1977), a marine dinoflagellate
(Exuviella baltica) was incubated with dieldrin (0.1, 1, or 10 µg
(nominal)/litre), and the numbers of cells were counted during a
period of 6 days. No adverse effects on optical counts were
observed at the two lower concentrations, but there was a marked
reduction in the size and number of cells at 10 µg dieldrin/litre.
7.2. Aquatic Organisms
The toxicity of aldrin and dieldrin to aquatic invertebrates is
very variable. For some species both compounds are highly toxic,
whereas for others there is no effect until the compounds are
dissolved to artificially high concentrations, many times their
solubility in water. Both aldrin and dieldrin are highly toxic to
most species of fish in laboratory tests, with acute LC50 values
well within the solubility of the compounds. It should be borne in
mind that aldrin and dieldrin are strongly bound to particulate
matter in water, which reduces their availability to aquatic
organisms and, in consequence, their potential toxicity.
7.2.1. Aquatic invertebrates
7.2.1.1 Acute toxicity
A convenient overview, in graphical format, of the toxicity of
aldrin and dieldrin to many aquatic organisms was produced by Craig
(1977). The 96-h LC50 values of aldrin and dieldrin for
crustaceans and molluscs were in the range 0.2 - 10 000 µg/litre.
Dieldrin is moderately toxic to fresh-water annelids
(4000 - 7000 µg/litre) and molluscs (> 100 - 640 µg/litre).
Insects are the most sensitive group (aldrin, 1 - 200 µg/litre;
dieldrin, 0.2 - 40 µg/litre). The values for a number of species
are given in Table 25.
7.2.1.2 Short-term toxicity, reproduction, and behaviour
(a) Short-term toxicity
When naiads of two species of stonefly were exposed for 30 days
in a continuous-flow system, the 30-day LC50s for aldrin and
dieldrin were, respectively, 2.5 and 2 µg/litre for Pteronarcys
californica, and 22 and 0.2 µg/litre for Acroneuria pacifica
(Jensen & Gaufin, 1966).
The LC50 for adult molluscs (Mytilus edulis and Dreissena
polymorpha) exposed for 3 - 4 weeks was 180 - 200 µg dieldrin/litre
(Adema & Vink, 1981).
McLeese et al. (1982) exposed polychaete worms (Nereis vireus)
to dieldrin in sea water or sediment for 12 days. The LC50 in sea
water was > 170 µg/litre (in surficial water > 20 µg/litre; in
sediment > 13 mg/kg).
Table 26 gives the LC50 values for a number of invertebrate
species.
(b) Reproduction
The effects of dieldrin on the embryonic development of the
American oyster (Crassostrea virginica) and of aldrin on that of
the hard clam (Mercenaria mercenaria) were studied by Davis & Hidu
(1969). Table 27 gives the concentrations producing approximately
50% reduction in the development of fertilized eggs during 48 h,
those producing about 50% reduction in larval survival during 12
days (clams) or 14 days (oysters), and the effects on larval growth
during 10 or 12 days of exposure (expressed as a percentage of
growth of control larvae).
Table 25. Acute toxicity of aldrin and dieldrin for aquatic invertebrates
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Daphnids
Daphnia magna (29)a 330a Anderson (1959)
Simocephalus first instar dispersed via 15 (23)a (240)a Johnson & Finley
serrulatus acetone 21 (32)a (1980)
Daphnia pulex first instar dispersed 15 (28)a (190)a Johnson & Finley
(1980)
Crustacea
Seed shrimp mature dispersed via 21 (18)a - Johnson & Finley
(Cypridopsis acetone (1980)
vidua)
Sowbug (Asellus mature dispersed via 21 - 5 Johnson & Finley
brevicaudus) acetone (1980)
Scud (Gammarus mature dispersed via 21 4300 640 Johnson & Finley
fasciatus) acetone (1980)
Sand shrimp 0.25 g, dispersed via 20 8 7 Eisler (1969)
(Crangon 2.6 cm acetone
septemspinosa)
2 g dispersed via 20 - 0.4 McLeese & Metcalfe
hexane (1980)
2 g dispersed in 10 - 4.1 McLeese & Metcalfe
sediment (1980)
Grass shrimp 0.47 g, dispersed via 20 9 50 Eisler (1969)
(Palaemonetes 3.1 cm acetone
vulgaris)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Crustacea (contd.)
Grass shrimp mature dispersed via 21 50 - Johnson & Finley
(Palaemonetes acetone (1980)
kadiakensis)
Crayfish mature dispersed via 21 - 740 Johnson & Finley
(Orconectes acetone (1980)
nais)
Hermit crab 0.28 g, dispersed via 20 33 18 Eisler (1969)
(Pagurus 0.35 cm acetone
longicarpus)
Molluscs
Mercenaria egg dispersed via 24 (> 10 000)a - Davis & Hidu (1969)
mercenaria acetone
Crassostrea egg dispersed via 24 - (640)a Davis & Hidu (1969)
virginica acetone
Slipper limpet veliger - - - > 100 Adema & Vink (1981)
(Crepidula
fornicata)
Pond snail egg - - - > 200 Adema & Vink (1981)
(Lymnaea juvenile
stagnalis)
Insects
Pteronarcys naiad, dispersed via 15.5 1.3 0.5 Sanders & Cope
californica 3-3.5 cm ethanol (1968); Johnson &
Finley (1980)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Insects (contd.)
Pteronarcella naiad, dispersed via 15.5 - 0.5 Sanders & Cope
badia 1.5-2 cm ethanol (1968); Johnson &
Finley (1980)
Claassenia naiad, dispersed 15.5 - 0.6 Sanders & Cope
sabulosa 2-2.5 cm (1968); Johnson &
Finley (1980)
Pteronarcys naiad, 2-5 cm dispersed via 12.8 180 39 Jensen & Gaufin
californica acetone (1966)
Acroneuria naiad, dispersed via 12.8 143 24 Jensen & Gaufin
pacifica 2-2.5 cm acetone (1966)
Damselfly juvenile dispersed via 24 - 12 Johnson & Finley
(Ischnura acetone (1980)
venticalis)
Other invertebrates
Bristle worm 2-3-day-old dispersed via 21 - > 100 Hooftman & Vink
(Ophryotrocha larva acetone (1980)
diadema)
4-week-old dispersed via 21 - > 100 Hooftman & Vink
adult worm acetone (1980)
---------------------------------------------------------------------------------------------------------
a Values in parentheses are the 48-h LC50.
Table 26. Short-term LC50s of dieldrin in invertebrates
-------------------------------------------------------------------
Species Stage LC50 at end of Reference
study (µg/litre)
(time of exposure)
-------------------------------------------------------------------
Ophryotrocha larva >10 Hooftman & Vink
diadema (2-3 days) (5-6 weeks) (1980)
adult 60 Hooftman & Vink
(4 weeks) (5-6 weeks) (1980)
Daphnia magna larva 100 Adema & Vink
(3 weeks) (1981)
adult 200 Adema & Vink
(0.3 cm) (7 days) (1981)
Artemia salina larva 40 Adema & Vink
(4 weeks) (1981)
adult 50 (male) Adema & Vink
(1 cm) (7 days) (1981)
110 (female)
(7 days)
Chaetogammarus larva 1.8 Adema & Vink
marinus (4 weeks) (1981)
adult 3.6 Adema & Vink
(1 cm) (14 days) (1981)
Palaemonetes adult 0.3 Adema & Vink
varians (4 cm) (7 days) (1981)
Crangon crangon adult 4 Adema & Vink
(4 cm) (14 days) (1981)
-------------------------------------------------------------------
When adult mud snails (Nassa obsoleta) were exposed to up to
10 000 µg dieldrin/litre for 96 h, and then transferred to
dieldrin-free sea water for 33 days, no mortality occurred
throughout the study and the length of the animals was normal after
33 days. There was a significant increase in total egg deposition
during the 33-day post-treatment period in the case of snails
exposed to 10 µg dieldrin/litre, but there was a significant
reduction at 100, 1000, and 10 000 µg dieldrin/litre (Eisler,
1970).
(c) Behaviour
In studies by Klein & Lincer (1974), fiddler crabs, (Uca
pugilator) were fed diets containing 0, 0.1, 1, 10, and 50 mg
dieldrin/kg diet for 14 days and observed for another 25 days.
Behaviour, measured as righting response, was modified at dose
levels of 1 mg/kg or more, and even in the group given 0.1 mg/kg,
difficulty in righting was seen after 11 days. With 10 and 50
mg/kg diet, an increase in mortality was observed, but not with 1
mg/kg diet.
Table 27. Concentrations producing about 50%
reduction in the development of fertilized eggs
during 48 h, in larval survival during 12 days
(clams) or 14 days (oysters), and effects on
larval growth during 10 or 12 days exposurea
(Davis & Hidu, 1969)
--------------------------------------------------
Organism Effect Aldrin Dieldrin
(µg/litre) (µg/litre)
--------------------------------------------------
Clam development of > 10 000 -
Oyster fertilized eggs - 640
Clam larval survival 410 -
Oyster - > 10 000
Clam larval growth 250b -
Oyster - 500c
--------------------------------------------------
a Expressed as a percentage of growth of control
larvae.
b 80% reduction.
c 50% reduction.
7.2.2. Fish
7.2.2.1 Acute toxicity
Both aldrin and dieldrin are highly toxic to fish under
laboratory conditions. A summary of reported 96-h LC50 values for
fresh water and marine species is given in Table 28. In parallel
studies, dieldrin was consistently more toxic than aldrin. The
96-h LC50s range from 2.2 to 53 µg aldrin/litre and from 1.1 to 41
µg dieldrin/litre in various fish species. It should be noted that
the range for aldrin exceeds the water solubility of the compound.
The results of studies by Macek et al. (1969) indicate that a
rise in temperature increases the toxicity of aldrin and dieldrin
for bluegills and rainbow trout. However, Johnson & Finley (1980)
stated that toxicity was not appreciably (only a factor of 2)
changed by variations in temperature or water hardness.
Macek (1975) investigated the effects of simultaneous exposure
of bluegills to DDT and dieldrin and concluded that the acute
toxicity of dieldrin in the concentration range 5.9 - 6.6 µg/litre
was not increased by the presence of DDT (concentration range,
4.5 - 5 µg/litre).
Anderson & Weber (1975) found that new-born and juvenile
guppies (Lebistes reticulatus) were more resistant to dieldrin
than adults. A relationship between the LC50 and body weight was
derived for mature, juvenile, and new-born guppies:
LC50 = aWb
where W is the body weight. The best fit value for the exponent b
was 0.81.
Table 28. Acute toxicity of aldrin and dieldrin for fish
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water
Rainbow trout 0.6 dispersed 13 2.6 - Johnson &
(Salmo via acetone Finley (1980)
gairdneri)
1.4 dispersed 13 - 1.2 Johnson &
via acetone Finley (1980)
3.2 dispersed 20 17.7 9.9 Katz (1961)
via acetone
0.6-1.5 dispersed 1.6 3.2 2.4 Macek et al.
via acetone 7.2 3.3 1.1 (1969)
12.7 2.2 1.4
Cutthroat trout 1.1 dispersed 9 - 6a Johnson &
(Salmo clarki) via acetone Finley (1980)
Chinook salmon 1.45-5 dispersed 20 7.5 6.1 Katz (1961)
(Oncorhynchus via acetone
tshawytscha)
0.8 dispersed 15 14.3 - Johnson &
via acetone Finley (1980)
Coho salmon 2.7-4.1 dispersed 20 45.9 10.8 Katz (1961)
(Oncorhynchus via acetone
kisutch)
Goldfish 1-2 dispersed 25 32 41 Henderson et al.
(Carrassius via acetone (1959)
auratus)
Goldfish 1 dispersed 18 - 1.8 Johnson &
(Carassius via acetone Finley (1980)
auratus)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water (contd.)
Carp (Cyprinus NA NA 20 4b - Rehwoldt et al.
carpio) (1977)
Fathead minnow 0.6 dispersed 18 8.2 3.8 Johnson &
(Pimephales via acetone Finley (1980)
promelas)
1-2 dispersed 25 32 18 Henderson et al.
via acetone (1959)
Guppy (Lebistes 0.1-0.2 dispersed 25 37 25 Henderson et al.
reticulatus) via acetone (1959)
NAd NA 20 20b - Rehwoldt et al.
(1977)
NA NA 24 - 3.2-7 Adema & Vink
(young) (1981)
NA NA 24 - 35c Adema & Vink
(adult) (1981)
juvenile NA 25 10.9 Anderson & Weber
(1975)
newborns NA 36.7 Anderson & Weber
(1975)
Black bullhead 1.5 dispersed 24 19 - Johnson & Finley
(Ictalurus via acetone (1980)
melas)
Channel catfish 5.2 dispersed 18 53 - Johnson & Finley
(Ictalurus via acetone (1980)
punctatus)
1.4 dispersed 18 - 4.5 Johnson & Finley
via acetone (1980)
Bluegill 0.7 dispersed 18 6.2 - Johnson & Finley
(Lepomis via acetone (1980)
macrochirus)
1.3 dispersed 18 - 3.1 Johnson & Finley
via acetone (1980)
1-2 dispersed 25 15 8.8 Henderson et al.
via acetone (1959)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Bluegill 0.6-1.5 dispersed 12.7 7.7 17 Macek et al.
(contd.) via acetone 18.3 5.8 14 (1969)
23.8 4.6 8.8
Pumpkinseed NA NA 20 20b - Rehwoldt et al.
sunfish (1977)
(Lepomis
gibbosus)
Largemouth bass 2.5 dispersed 18 5 3.5 Johnson & Finley
(Micropterus via acetone (1980)
salmoides)
Striped bass NA NA 20 10b - Rehwoldt et al.
(Marone (1977)
saxatilis)
Banded killyfish NA NA 20 21b - Rehwoldt et al.
(Fundulus (1977)
diaphanus)
White perch NA NA 20 42b - Rehwoldt et al.
(Roccus (1977)
americanus)
American eel NA NA 20 16b - Rehwoldt et al.
(Anguilla (1977)
rostrata)
Marine species
Common goby NA NA 15 - 3.5 Adema & Vink
(Gobius microps) (adult) (1981)
Plaice length: NA 15 - 1.7 Adema & Vink
(Pleuronectes 2-3 cm (1981)
platessa)
length: NA 15 - 4 Adema & Vink
10 cm (1981)
yolk-sac NA 5-10 - 30 Adema & Vink
larva (1981)
egg-metam NA 5-10 - > 32 Adema & Vink
larva (1981)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Marine species (contd.)
Threespine 0.4-0.8 dispersed 20 27.4 13.1 Katz (1961)
stickleback via acetone
(Gasterosteus
aculeatus)
------------------------------------------------------------------------------------------
a Hardness = 162 mg CaCO3/litre.
b Hardness = 50 mg CaCO3/litre.
c 48-h LC50.
d NA = not available.
7.2.2.2 Long-term toxicity
Sailfin mollies (Lebistes latipinna) were exposed in groups of
20 to 0, 0.75, 1.5, 3, 6, or 12 µg dieldrin/litre using a flow-
through system for 34 weeks. The mortality of the 0.75 µg/litre
group was similar to that of the control group. At 1.5 µg/litre,
there was an increase in mortality, and, at 3 µg/litre or more,
100% mortality occurred. The growth rates and reproduction
performances were adversely affected in the surviving fish (Lane &
Livingstone, 1970).
Rainbow trout (Salmo gairdneri) were fed food containing
dieldrin for 240 days, the nominal dietary concentrations
corresponding to 14, 43, 143, or 430 µg dieldrin/kg body weight per
day. The growth rate was not affected at any of the concentrations
throughout the 240 days, and there was no mortality or visible
adverse effects. The activities of liver glutamate-pyruvate
transaminase (GPT) and glutamate-oxaloacetate transaminase (GOT)
were not affected, except, in the case of the latter, at the
highest dose level. Liver glutamate dehydrogenase (GDH) activity
was increased at all dose levels. Electron micrographs of liver
cells demonstrated changes in mitochondrial morphology, the highest
dose causing swelling and membrane disruption. Since GDH is an
intramitochondrial enzyme, examination by electron microscopy gave
further evidence that dieldrin altered mitochondrial metabolism.
In the brain, GOT activity was significantly decreased at 43 µg/kg
and GTP was decreased at 14 µg/kg or more. At all dose levels,
brain GDH was decreased and brain glutamine transferase (GT) was
increased. Electron microscopy of the medulla and cerebral
hemispheres did not show any effects of dieldrin. The
concentrations of 16 free amino acids in the brain were determined.
The concentrations of four were not significantly changed, whereas
eight were significantly altered at 143 µg dieldrin/kg and 12 at
430 µg/kg. Serum ammonia concentrations were significantly
increased at 143 and 430 µg dieldrin/kg, but the concentration of
ammonia in the brain was not affected. The increase in brain GT
was considered to be a possible reason for this lack of effect on
brain ammonia, since it compensated for the decrease in GDH
activity. Alternatively, brain ammonia may have been transported
via the blood to the liver with consequent effects on the liver.
The ammonia-detoxifying mechanism of fish seemed to be very
sensitive to dieldrin, the no-effect dose being below 14 µg/kg body
weight per day (equivalent to 0.36 mg/kg food) (Mehrle &
Bloomfield, 1974).
Several studies have described the influence of aldrin and/or
dieldrin on enzymes such as mitochondrial succinic hydrogenase, the
epoxidative activities of liver microsomes and the ATPase activity
of microsomes of the gills or brain (Chan et al., 1967; Davis et
al., 1972; Moffet & Yarbrough, 1972; Yap et al., 1975).
Furthermore, the influence of dieldrin during thermal stress has
been studied in darters (Etheostoma nigrum) (Silbergeld, 1973).
In studies by Verma & Tonk (1984), Heteropneustes
(Saccobranchus) fossilis was exposed to aldrin for 30 days at a
concentration of 0.03 mg/litre. Respiration, haematological
parameters, and the activity of two enzymes in liver, kidneys, and
gills were determined. The respiration rate decreased and the
blood concentrations of glucose, sodium, and chloride ions showed
significant increases. The cholesterol content and clotting time
were decreased, and the ATPase activity in the three tissues was
significantly reduced.
7.2.2.3 Reproduction
Van Leeuwen (1986) carried out studies with dieldrin to study
the susceptibility of early-life stages of rainbow trout. The test
was performed with fertilized eggs before and after water
hardening, and with early eye point eggs, late eye point eggs, sac
fry, and early fry. No mortality was found in the different early-
life stages using concentrations greater than aqueous solubility
(> 10 mg/litre), except for the early fry where a very low 96-h
LC50 of 0.003 mg/litre was found.
In studies by Cairns et al. (1967), nine populations of guppies
(Lebistes reticulatus) were exposed in a semi-static system to
nominal concentrations of 0 (three populations), 1.8, 5.6, and 10
µg dieldrin/litre (two populations of each) for 14 months. During
the first 2 - 3 months, the exposed populations in five tanks
developed greater numbers of individuals (mature, immature, and
fry) than did the controls (except one population at 5.6 µg/litre,
which was similar to the controls). The difference between the
controls and five treatment groups was attributed to the higher
predation and harassment observed in the control groups. The total
numbers of individuals in control and treatment groups became
similar during the final 6 - 8 months of the study. The average
total monthly body weights of the groups treated with 1.8 µg/litre
and 5.6 µg/litre began to increase steadily after about 8 months,
whereas the total monthly body weights of the group exposed to 10
µg/litre were similar to the controls throughout the 14 months of
the study. The production of fry by one of the groups treated with
10 µg/litre declined markedly after the thirty-second week of the
study, no new broods of fry being born after the forty-second week.
No such marked decline occurred in the other five treatment groups
(including one population exposed to 10 µg/litre).
Chadwick & Shumway (1970), conducted studies lasting 130 days
on rainbow trout (Salmo gairdneri) to determine survival from time
of fertilization through to hatching in continuously cycled water.
Embryos, alevins, and fry were exposed to dieldrin concentrations
ranging from 0.012 to 52 µg/litre. Eggs (embryos) exposed to up to
52 µg dieldrin/litre from the time of fertilization survived until
hatching as well as controls, but the mean weight of newly-hatched
alevins (minus yolk material) was reduced by higher concentrations
(not specified). Alevins were more susceptible than embryos.
Their survival was reduced at all concentrations above 0.39
µg/litre. Trout fry, whose survival was unaffected at dieldrin
levels of 0.12 µg or less, quickly succumbed at concentrations of
0.39 µg/litre or more.
Smith & Cole (1973), exposed adult winter flounder (Pseudo-
pleuronectes americanus) to 2 µg/litre in aquaria continuously
supplied with filtered sea water. When fish became ripe, they were
artificially spawned, and approximately 30 000 eggs were collected
from each of the 24 spawning pairs and cultured. The remaining
eggs were analysed. The percentage fertilization of eggs
containing 0.61 mg dieldrin/kg or less was 99% (controls, 97.8%).
The percentage fertilization of eggs containing 1.21 mg dieldrin/kg
was 12%, and all the eggs containing 1.74 mg/kg were infertile.
There was no effect on egg development except in the case of the
two groups of eggs containing the higher concentrations of
dieldrin. The effects on egg mortality were not due to dieldrin in
the gametes, and the milt of exposed male flounders contained no
detectable residue of dieldrin.
7.2.3. Amphibia and reptiles
The LC50 values for tadpoles of two species of frogs (1 week
old) and toads (4 - 5 weeks old) were determined by Sanders (1970).
The 96-h LC50 (at 15.5 °C) of dieldrin for Western chorus frog
tadpoles (Pseudacris triseriata) was 100 µg/litre, whereas that of
both aldrin and dieldrin for Fowler's toad tadpoles (Bufo
woodhousii fowleri) was 150 µg/litre.
Cooke (1972) studied the effect of dieldrin at nominal
concentrations of 0.008, 0.02, or 0.5 mg/litre on groups of 40
common frog (Rana temporaria) or toad (Bufo bufo) tadpoles with
hindlimb paddles or hind legs. The exposure was for 24 or 48 h in
amphibian saline, and the observation period 5 or 15 days. At the
highest dose level, the frogs showed an increased mortality, the
mean dieldrin content being 42.9 mg/kg tissue. At the two lower
dose levels (0.008 and 0.02), there were 0.31 and 6.1 mg/kg
dieldrin in tissues, respectively. When toad tadpoles were exposed
to 0.02 or 0.5 mg/litre, the animals with the higher dose level
showed clear behavioural and structural abnormalities and a reduced
rate of development, but these changes returned to normal a few
days after exposure. The mean dieldrin content was 138 mg/kg
tissue at a dose level of 0.5 mg/litre.
The in vitro exposure of toad embryo tissue (Bufo arenarum) to
dieldrin (4 x 10-5 mol/litre) produced an inhibition of acetyl and
butyryl cholinesterase activity. In in vivo studies with open-
mouth stage embryos, dieldrin produced acetyl cholinesterase
inhibition at 0.5 x 10-6 mol/litre. Furthermore, hyperactivity in
swimming larvae was observed (de Llamas et al., 1985).
The in vitro activity of ATPase in a number of tissues of the
male turtle (Graptemys geographica) was determined by Wells et al.
(1974). There was no consistent dose relationship for either aldrin
or dieldrin, except perhaps in the case of the cloacal bladder in
the aldrin treatments. The inhibition of Na/K/Mg ATPase by aldrin
and dieldrin was in the range of 4 - 13%. It was suggested that
aldrin and dieldrin may affect the transport of metabolites across
the cellular membranes as a result of decreased energy for active
transport.
7.3. Terrestrial Organisms
7.3.1. Higher plants
Dieldrin has low phytotoxicity, tomatoes and cucumber, for
example, being affected only at application rates greater than 22
kg/ha. Aldrin affects some crops at rates greater than 22 kg/ha,
beans and cereals being most sensitive. Tomatoes and cucumbers are
sensitive to aldrin but only at unrealistically high application
rates (Edwards, 1965).
Studies in greenhouses showed that aldrin, administered weekly
as an emulsifiable concentrate at a rate of 16 kg active
ingredient/ha to 2 - 3-week-old seedlings of tomato, cauliflower,
and Chinese cabbage, inhibited root development and reduced growth
rate of cauliflower and Chinese cabbage seedlings. A ten-fold
reduction in the aldrin level failed to produce these effects
(Hagley, 1965).
Aldrin and dieldrin at 11 kg active ingredient/ha had no effect
on the emergence, growth, yield, or chemical composition of
soybeans (Probst & Everly, 1957).
7.3.2. Earthworms
In studies by Cathey (1982), earthworms (Lumbricus terrestris)
were maintained in an artificial nutritionally complete soil, based
on shredded paper containing aldrin. The LC50 value (6-week
exposure) was 60 mg aldrin/kg bedding, and the tolerance level,
producing less than 1% mortality, was 13 mg aldrin/kg bedding.
When aldrin (2.5 - 4.6 kg/ha) was applied as a spray or dust,
respectively, to the surface of soil plots and incorporated into
the soil, the numbers of earthworms in treated plots were either
similar to or greater than those in control plots (Edwards et al.,
1967; Griffiths et al., 1967; Edwards & Lofty, 1977).
7.3.3. Bees and other beneficial insects
In a review of five investigations on the toxicity of aldrin
and dieldrin to honey bees (Sanger, 1959), the oral LD50 values for
aldrin ranged from 0.24 to 0.45 µg/bee, while the values for
dieldrin were in the range 0.15 - 0.32 µg/bee. Contact LC50 values
were 0.15 - 0.8 µg/bee for aldrin and 0.15 - 0.41 µg/bee for
dieldrin.
Cowie (1967) reported an oral LD50 of 0.3 µg dieldrin/bee
(range, 0.13 - 0.54) and a contact LD50 of 0.21 µg/bee.
The toxicity of dieldrin to two important predators of cotton
pests was investigated by Burke (1959). The contact LD50 value for
Hippodamia convergens was 1.6 mg/g body weight.
In a review of the effects of pesticides on soil fauna, it was
concluded that aldrin (and, by implication, dieldrin) is relatively
non-toxic for predatory mites ( Acarina spp.), and that this may
contribute to its success as a soil insecticide (Edwards &
Thompson, 1973).
7.3.4. Birds
7.3.4.1 Acute toxicity
Estimates of the LD50 values for several species of birds are
given in Table 29. The variation in acute oral toxicity of
dieldrin among six species of birds tested by Tucker & Haegele
(1971) was more than ten fold.
Table 29. Acute oral toxicity of aldrin and dieldrin for avian
speciesa
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Fulvous whistling duck male: female: Tucker & Crabtree
(Dendocygna bicolor) 29.2 100-200 (1970)
Mallard duck female: female: Tucker & Crabtree
(Anas platyrhynchos) 520 381 (1970)
Canada goose 50-150 Tucker & Crabtree
(Branta canadensis) (1970)
-------------------------------------------------------------------
Table 29. (contd.)
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Domestic fowl 25.5 43 Sherman &
(Gallus domesticus) Rosenberg (1953)
Japanese quail male: Tucker & Crabtree
(Coturnix coturnix 69.7 (1970)
japonica)
Bobwhite quail female: Tucker & Crabtree
(Colinus virginianus) 6.6 (1970)
California quail 8.7 Hudson et al.
(Callipepla californica) (1984)
Gray partridge female: Tucker & Crabtree
(Perdix perdix) 8.8 (1970)
Chukar partridge 23.4 Tucker & Crabtree
(Alectoris graeca) (1970)
Sharp-tailed grouse male: McEwen & Brown
(Pedioecetes phasianellus) 6.9 (1966)
Ring-necked pheasant female: female: Tucker & Crabtree
(Phasianus colchicus) 16.8 79 (1970)
Pigeon 55 67 Turtle et al.
(Columba livia) (1963)
26.6 Tucker & Crabtree
(1970)
House sparrow female: Tucker & Crabtree
(Passer domesticus) 47.6 (1970)
-------------------------------------------------------------------
a Details concerning age and weight of birds are not summarized
here but can found in the original publications.
7.3.4.2 Short- and long-term toxicity
Values for the subacute LC50s of aldrin and dieldrin,
determined using the procedure developed at the Patuxent Wildlife
Centre (Hill et al., 1975), are given in Table 30. The LC50 values
of aldrin and dieldrin for each of the four species tested were of
the same order. The annual variations in the LC50 of dieldrin over
a period of up to 8 years for these four species have been
investigated by Hill et al. (1977) (18 times per species). No
time-related changes in LC50 values were found for any of the
species. However, differences were found between birds of
different ages in some species, e.g., Japanese quail and mallards
(Hudson et al., 1984). There were also differences between the
slopes of the average regression lines for the four species. These
authors emphasized the need to evaluate both the LC50 and the slope
of the regression line. Food consumption was reduced by aldrin or
dieldrin in the diet.
Table 30. Subacute dietary toxicity of aldrin and dieldrin
for avian speciesa
-----------------------------------------------------------
Species Age LC50 (95% confidence limits)
(days) ----------------------------
Aldrin Dieldrin
(mg/kg diet)
-----------------------------------------------------------
Mallard duck 5 155 153
(Anas platyrhynchos) (129-186) (123-196)
10 - 169
Japanese quail 14 34 62
(Coturnix coturnix (28-41) (53-71)
japonica)
Bobwhite quail 14 37 37
(Colinus virginianus) (33-41) (30-46)
Ring-necked pheasant 10 57 58
(Phasianus colchicus) (50-64) (51-67)
-----------------------------------------------------------
a Aldrin or dieldrin fed for 5 days followed by 3 days of
untreated diet.
Wiese et al. (1969) fed diets containing up to 500 mg technical
dieldrin (85%)/kg diet to male and female 6-month-old crowned
guinea-fowl (Numida meleagris). None of the birds fed 1.5 mg/kg
for 21 months died. The median survival time for birds fed 5 mg/kg
was 524 days; for birds fed 150 and 500 mg/kg, it was 3 and 1 days,
respectively. No differences in susceptibility between males and
females were found.
The subacute toxicities of technical dieldrin (85%) to three
species of birds are given in Table 31 (Basson, 1971).
7.3.4.3 Reproductive studies
The first experimental studies of the effects of aldrin or
dieldrin on avian reproduction showed that these compounds were
toxic for quail and pheasants. Quail fed a diet containing aldrin
or dieldrin at a toxic dose of 0.5 or 1 mg/kg diet did not show any
clear effects on egg production, percentage fertility, or
percentage hatchability (the birds had not been exposed earlier to
the compounds) (DeWitt, 1955, 1956). No significant effect was
found on the fertility or hatchability of eggs of pheasants fed
25 mg dieldrin/kg diet, but at 50 mg/kg there was a clear effect
(Genelly & Rudd, 1956).
Table 31. Subacute toxicity of technical dieldrin for three species
of birds
-------------------------------------------------------------------
Species Concentration Median time Median lethal
(mg/kg diet) till death dose (mg
(days) dieldrin/kg
body weight)
-------------------------------------------------------------------
Guinea fowl 20 72 72.4
(Numida meleagris) 150 12 11.2
Laughing dove 5 49 15.8
(Stigmatopelia 90 4.7 17.3
senegalensis)
Sparrow (Passer 5 85.1 > 41
melanurus melanurus) 45 7 43.8
-------------------------------------------------------------------
Eggs from chickens fed 1 mg aldrin or dieldrin/kg diet for
2 years showed normal fertility and hatchability, although the
concentrations of dieldrin in the yolks of the eggs were in the
range of 6 to 25 mg/kg. The fertility and hatchability slightly
decreased at 10 mg dieldrin/kg diet (Brown et al., 1965).
Other studies on avian reproduction are summarized in Table 32.
This table gives the available information on five criteria
relevant to reproduction (parental survival, production, fertility
and hatchability of eggs, and chick survival) in relation to oral
intake of dieldrin and (where reported), the residues of dieldrin
in eggs. These studies show that, depending on the duration of
exposure, dose levels of 5 - 10 mg dieldrin/kg diet reduce the
survival of the parent birds. Egg production was reported to be
significantly increased in some studies but reduced in others. In
general, egg fertility was not influenced, except in one study.
Hatchability was not affected, neither in most cases, was the
survival of chicks. There seems to be a trend that overall
reduction in reproductive success occurs only if the parent birds
are showing signs of being affected by dieldrin, e.g., reduced food
intake with consequent loss of weight and poor condition. It
should be noted that in these studies (with one exception) the eggs
were placed in incubators for hatching. Consequently, one aspect
of the reproductive process was not studied, namely, parental
behaviour. However, in the study on homing pigeons (Robinson &
Crabtree, 1969), the parents (and subsequently, their offspring)
were free-flying, they brooded their eggs, and fed their young
until they fledged.
A 3-generation study of the effects of dieldrin on pheasants
(Phasianus colchicus), has not been included in Table 32 because
of the complexity of the experimental design. The doses of
dieldrin (hens, 6 or 10 mg dieldrin/bird per week; cocks, 4 or 6 mg
dieldrin/bird per week) were sufficient to cause mortality of
breeding birds, but the production, fertility, and hatchability of
eggs and the viability of chicks at the time of hatching were not
affected in a consistent manner in relation to dose or generation.
The survival of chicks from hens given 6 or 10 mg dieldrin/week was
reduced. Residues of dieldrin in eggs or tissues were not
determined in this study (Dahlgren & Linder, 1974).
When seven-week-old Japanese quail were given diets containing
3.1 or 50 mg dieldrin/kg diet for 21 days, a significant reduction
in egg production occurred in both groups (Call & Harrell, 1974).
With the exception of the study with the barn owl (Mendenhall
et al., 1983) and the homing pigeon (Robinson & Crabtree, 1969),
the birds that were tested are precocial species which show no
parental feeding of the young. Most birds are not precocial and
reproduction involves a period of full dependency of the offspring
on parental care. Results should, therefore, be interpreted with
care; extrapolation directly from the laboratory to the field is
difficult.
7.3.4.4 Eggshell thinning
Ratcliffe (1967a) reported that the ratio of eggshell weight to
size in three species of birds of prey in the United Kingdom had
declined during the period after 1947 relative to pre-1947. This
report has stimulated considerable interest in the relationship
between egg-shell thickness (or the related eggshell index based on
the weight/size ratio) and the breeding success of birds,
particularly as eggshell thinning seems to be quite a widespread
phenomenon, particularly among birds of prey (Hickey & Anderson,
1968; Ratcliffe, 1970; Anderson & Hickey, 1972). There has been
considerable speculation on the causes and mechanism of these
changes (Cooke, 1973; Mueller & Leach, 1974). Experimental studies
on the effects of dieldrin on eggshell thickness have given
conflicting results. The results are summarized in Table 33.
Eggshell weights of crowned guinea-fowl (Numida meleagris) fed
diets containing up to 15 mg dieldrin/kg diet for 21 months were
not affected by the treatments (Wiese et al., 1969).
American sparrow hawks (Falco sparverius) were given diets
containing dieldrin and North American prairie falcons (Falco
mexicanus) were fed starlings contaminated with an average of 29 mg
dieldrin/kg body weight, along with DDT and DDE at different
levels. The purpose of these studies was to show the influence of
these pesticides on shell thickness. The results of the two
studies, in relation to the effects of dieldrin, cannot be
interpreted, in view of the possible effects of DDE on eggshell
thinning (Porter & Wiemeyer, 1969; Enderson & Berger, 1970). It
has slowly become accepted that metabolites of DDT, particularly
DDE, are the most likely cause of eggshell thinning (Cooke, 1973;
Newton, 1979; Bunyan & Stanley, 1982). It is also pertinent that
the onset of eggshell thinning in wild birds preceded the use of
aldrin/dieldrin. There is evidence that eggshell thinning after
exposure to dieldrin is related to reduced food consumption.
Untreated Coturnix and Mallard, when fasted for 36 h, laid thin-
shelled eggs for a few days during and after fasting (Haegele &
Tucker, 1974).
Table 32. Reproductive success of birds in relation to oral intake of dieldrin and the concentration of HEOD in eggs
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Mallard duck 4 90 days - - NCg NC Red.c - Muller &
(Anas platy- Lockman (1972)
rhinchos)
Japanese quail 1 16 weeks - - NC NC NC - Shellenberger
(Coturnix 10 16 weeks - - NC NC NC - & Newell
coturnix (1965)
japonica)
10 18 weeks 19 NC NC NC NC NC Walker et al.
(6.9-26.9) (1969a)
20 9 weeks 39 Red. Red. DC NC Red.
(19.8-54.1)
30 7 weeks 48 Red. Red. DC Red. Red.
(34.7-63.2)
40 6 weeks 84 Red. Red. - - -
(76.9-92.5)
0.1 multi- - NC NC NC NC NC Shellenberger
generation (1978)
P; F1,
1 F2, F3 - NC NC NC NC NC
Bobwhite quail 10 34 weeks - Red. NC - - - Fergin &
(Colinus 20 34 weeks - Red. Red. - - - Schafer (1977)
virginianus) 40 34 weeks - Red. Red. - - -
Pheasant 2 mg/hen/ 13 weeks (0.6-26.5) NC NC NCa Inc.c NC Atkins &
(Phasianus weekb (yolks) Linder (1967)
colchicus) 2 mg/hen/ 13 weeks - NC NC NC NC NC
week
4 mg/hen/ 13 weeks (5.3-40.1) DR NC NC NC NC
week (yolks)
4 mg/hen/ 13 weeks (7.5-20.6) NC NC NC NC NC
week (yolks)
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Pheasant 6 mg/hen/ 13 weeks (13.3-52.4) NC Red.a NC NC NC
(Phasianus week (yolks)
colchicus)
(contd.) (0) 6 mg/hen/ 14 weeks - Red. Inc.c Red.c NC NC Baxter et al.
weekd (1969)
(0) 8 mg/hen/ 14 weeks - Red. Inc.c NC NC NC
week
(0) 12 mg/ 14 weeks - Red. Red.c NC - -
hen/week
(4) 0 mg/hen/ 14 weeks - NC Inc.c Red.c Red. NC
week
(4) 6 mg/hen/ 14 weeks - Red. NC Red.c NC NC
week
(6) 0 mg/hen/ 14 weeks - NC Red.c NC - -
week
(6) 6 mg/hen/ 14 weeks - Red. Red.c Red.c - -
week
Gray partridge 3 1.5 - DR NC NC NC Neill et al.
(Perdix perdix) (1-2) (1971)
Domestic fowl 2 16 weeks (0.34-1.45) NC NC - NC NC Graves et al.
(Gallus 5 16 weeks (1.2-4.8) NC NC - NC NC (1969)
domesticus)
10 13 months (7.6-16) NC NC NC NC NC Brown et al.
(1974)
20 13 months (19.6-35.7) Red. Inc.a NC NC Red.
Crowned guinea- 0.5 21 months 1.11/1.17e NC Inc.a NC NC NC Wiese et al.
fowl (Numida (1969)
meleagris) 1.5 21 months 2.38/3.35e NC Inc.a NC NC NC
5 21 months 7.18/13.56e PR Inc.a NC NC NC
15 21 months 15.79e Red. Inc.a NC NC Red.
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Homing pigeon ~2 mg/kg 2 years (0.03-13.4)f NC NC NC NC NC Robinson &
(free-flying) body weight/ (4.5-16.7)f NC NC NC NC NC Crabtree
(Columba livia) week (1969)
Barn owl 0.5 2 years 3.6 (first NC NC NC NC NC Mendenhall et
(Tyto alba) year) al. (1983)
8.1 (second
year)
--------------------------------------------------------------------------------------------------------------------
a Significant at a probability of < 0.01.
b Doses of 2, 4, or 6 mg dieldrin/hen were administered once per week in capsules.
c Significant at a probability of < 0.05.
d Second-generation hens; offspring of birds used in study by Atkins & Linder (1967). The doses in parentheses
refer to the doses administered to the first-generation hens.
e Residues in eggs of successive years.
f First and second laying periods, respectively.
g The term "no change" (NC) indicates that any differences between controls and treatment groups were within the
limits of experimental variation. If any of the results for treatment groups are reported to be increased (Inc.)
or reduced (Red.), the statistical significance, if reported, is given. Equivocal results have been described
"doubtful reduction" (DR), "probably reduced" (PR), and "doubtful change" (DC).
Table 33. Effects of dieldrin on eggshell thickness
--------------------------------------------------------------------------------------------------------------
Species Dose of dieldrin Duration Difference between egg- Reference
(mg/kg diet) shell thickness of treated
and control birds (%)
--------------------------------------------------------------------------------------------------------------
Mallard duck 1.6 16 months -3.4a Lehner & Egbert (1969)
(Anas platyrhynchos)
4 16 months -2a Lehner & Egbert (1969)
10 16 months -4.3a Lehner & Egbert (1969)
4 90 days -4.2 Muller & Lockman (1972)
Japanese quail 3.1 21 days with -8a Call & Harrell (1974)
(Coturnix coturnix changes in
japonica) 50 photoperiod -8a Call & Harrell (1974)
Domestic fowl 10 12 weeks 0 Davison & Sell (1972)
(Gallus domesticus) 20 12 weeks +0.3 Davison & Sell (1972)
10 13 months 0 Brown et al. (1974)
20 13 months +9.7 Brown et al. (1974)
Pheasant 6 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(Phasianus colchicus)
10 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(6) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(6) 6 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(10) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
--------------------------------------------------------------------------------------------------------------
a Significant at or below 0.05.
b Administered in capsules once per week (see footnoteb Table 32).
c See footnoted Table 32.
7.3.4.5 Concentrations of dieldrin in tissues of experimentally
poisoned birds
Many studies have been carried out to estimate the
concentrations of dieldrin in the liver, brain, or other tissues of
birds that died following oral intake of aldrin or dieldrin. The
intakes were either single acute doses or long-term dietary
exposure. In some investigations, the concentrations of dieldrin
in the tissues of birds that survived after treatment were also
reported. The results of these studies are not comparable, because
the dose levels and duration of the studies are different. The
concentrations that were found in the different studies ranged from
a few mg/kg up to about 100 mg/kg tissue (wet weight) (Turtle et
al., 1963; Koeman et al., 1967; Robinson et al., 1967b; Robinson,
1969; Robinson & Crabtree, 1969; Stickel et al., 1969; Enderson &
Berger, 1970; Linder et al., 1970; Brown et al., 1974; Clark, 1975;
Heinz & Johnson, 1981; Mendenhall et al., 1983) (Table 34).
Attempts to define tissue concentrations that can be used as
indicators of death by dieldrin poisoning of wild birds lack
precision as a result of the overlap between the lowest
concentrations in the tissues of birds dying under experimental
conditions and the highest concentrations in survivors. Thus, it
has been proposed that concentrations of 5 or 10 mg dieldrin/kg
brain (Robinson, 1969; Stickel et al., 1969) are indicative of
death from aldrin/dieldrin poisoning. Liver concentrations of 10
or 20 mg/kg (Robinson, 1969; Cooke et al., 1982) have been proposed
as levels diagnostic of dieldrin poisoning of birds.
As a general point, interpretation of residue data must be done
with extreme caution. Brain residues of dieldrin are probably a
good indicator of lethality. However, most bird carcasses
collected in the field cannot be analysed for brain residues,
because the brain deteriorates rapidly after death. For this
reason, most residue data from the field are for levels in the
liver, which remains discrete and usable for much longer. A liver
residue level symptomatic of death from dieldrin poisoning is more
difficult to define. A large, acutely toxic, dose of dieldrin may
leave a low residual level of dieldrin in liver because the bird
dies rapidly. A smaller, less acutely toxic, dose of dieldrin
usually leads to loss of body weight before death because of a lack
of ability or desire to feed. This period of starvation prior to
death boosts liver residues considerably as dieldrin is released
from mobilized fat and concentrated in the liver as detoxification
is attempted.
7.3.5. Mammals
The acute and long-term toxicity of aldrin and dieldrin for
laboratory mammals is summarized in section 8.
Values for the acute oral LD50 and subacute oral LC50 in the
diet (30 days) of dieldrin for four species of voles ( Microtus sp.)
are given in Table 35.
Table 34. Concentrations of dieldrin in the tissues of experimentally poisoned birds and
in survivors
------------------------------------------------------------------------------------------
Species Tissue Concentration of dieldrin (mg/kg wet weight) Reference
analysed geometric mean, (range of values)
No. of Survivors No. of Dead birds
samples samples
------------------------------------------------------------------------------------------
Domestic pigeon liver 11 8 20 45.6 Robinson et
(Columba sp.) (3.1-51.2) (23-81) al. (1967b)
brain 11 3.6 19 20
(1.6-8.5) (13.5-32.5)
House sparrow liver - - 19 44.7 Robinson
(Passer domesticus) (38.4-52.3) (1969)
brain - - 19 20
(17.6-22.7)
Japanese quail liver - 36 40 Robinson et
(Coturnix coturnix (17.7-90.4) al. (1967b)
japonica) brain 12 6.9 65 17.4
(3.1-15) (8.7-34.6)
Japanese quail liver 8 28.8 9 19.7 Stickel et
(Coturnix coturnix (2.7-140.8) (5.7-51.7) al. (1969)
japonica) brain 20 3.4 17 17.3
(0.25-11.9) (6.2-32.9)
Redwinged blackbird brain 3 7.1 27 19.8 Clark
(Agelaius phoeniceus) (6.7-7.4) (1-34.5) (1975)
27 22.2
(13.5-29.5)
Prairie falcon brain 2 2.9 1 11 Enderson &
(Falco mexicanus) (2.8-3) Berger
(1970)
Barn owl brain 2 10 Mendenhall
(Tyto alba) (5-15) et al.
carcass 19 9.4 - - (1983)
------------------------------------------------------------------------------------------
The toxicological signs in these studies were similar to those
in laboratory animals, and these four microtine rodents appear to
be less susceptible than laboratory rodents to dieldrin
intoxication (Cholakis et al., 1981). When short-tailed shrews
(Blerina brevicauda) were fed diets containing 50, 100, or 200 mg
dieldrin (nominal)/kg diet for up to 14 days, all the animals fed
50 mg/kg dieldrin survived, whereas all those fed 200 mg/kg diet
died (Blus, 1978).
Luckens & Davis (1965) studied the acute oral toxicity in big
brown bats (Eptericus fuscus) caught in Kentucky, USA. Death
occurred at all dose levels above 20 mg/kg body weight. The
approximate LD50 seemed to be within the range 20 - 40 mg/kg body
weight.
Table 35. Acute and subacute toxicity of dieldrin for volesa
------------------------------------------------------------------------
Species Acute LD50 Subacute LC50 (30 days)
(mg/kg body weight) (mg/kg body weight)
Average males/females Average males/females
------------------------------------------------------------------------
Microtus orchrogaster 210 105
Microtus canicaudus 100 40
Microtus montanus 205
Microtus pennsylvanicus 175
------------------------------------------------------------------------
a From: Cholakis et al. (1981).
White-tailed deer (Odocoileus virginianus) were fed 0, 5, or
25 mg dieldrin/kg diet for up to 3 years as were their progeny. No
signs of intoxication were observed, and the survival of adults was
not affected. The growth rate of treated females was decreased.
Relative liver weights increased at 25 mg/kg. Fertility and in
utero mortality were comparable for the three groups. Fawns from
treated does were smaller at birth, and greater postpartum
mortality occurred. The weight gain of fawns was reduced during 2
of the 3 years. Whole milk from doses fed 25 mg/kg contained
residues of 17 mg/litre. Residues in the liver of surviving
animals were about 4 mg/kg in the low-dose group and 16 mg/kg (wet
weight) in the high-dose group. Highest brain residues (12 mg/kg
wet weight) occurred in fawns only a few days before death (Murphy
& Korschgen, 1970).
When blesbuck (Damaliscus dorcas phillipsi) were fed diets
containing 5, 15, 25, 35, or 50 mg dieldrin (nominal)/kg diet, none
of the animals fed 5 or 15 mg/kg diet died during the 90 days of
the study. However, all the animals given higher dose levels died
within 24 days. The concentration of dieldrin in the liver was 3.3
and 8.2 mg/kg for the two lowest dose levels, after 90 days. The
concentrations of dieldrin in the livers of the dead animals were
9.4, 15.1, and 18.4, respectively, for the three highest treatment
groups (Wiese et al., 1973).
In studies by Wiese et al. (1973), an experimental grazing site
(250 ha) with a resident population of 35 blesbuck and 20 springbok
(Antidorcas marsupialis) was aerially sprayed with dieldrin at a
rate corresponding to 0.16 kg/ha. The concentration of dieldrin on
the veld declined rapidly from 27.6 mg/kg immediately after
treatment to 5.04 mg/kg after 14 days. The decline during the
following 106 days was much slower (it was 0.75 mg/kg on the 85th
day and 0.23 on the 120th day). Behavioural changes were observed
in both antelope species after 3 days. From the 4th day, there
were further nervous symptoms, including clonic convulsive attacks,
and partial or even complete blindness was also noted. Blesbuck
died from the 4th day onwards, and the entire population of 35
animals had died by the 19th day. The median time to death was
7.08 days, and there was no significant difference between adults
(male or female) and juveniles. The calculated mean intake of
dieldrin was 1.82 mg/kg herbage per day. This estimate was much
lower than that derived from the feeding trial (the total intake of
animals that died was 9.08 mg/kg). The mean concentration in the
livers of six blesbuck that died was 8.3 mg/kg. It was inferred
that dieldrin was unlikely to be the cause of death of the blesbuck
(further investigations implicated photodieldrin). Springbok were
less adversely affected: deaths occurred from the 6th day, and 70%
had died by the 13th day. Surviving animals recovered with no
after-effects, and two ewes lambed normally in the spring following
the winter treatment. The average dieldrin concentration in the
livers of three springbok that died was 9.2 mg/kg. The
pathological findings were similar to those in the common
laboratory species.
Few other mammalian species have been investigated. Reduced
reproductive success and some mortality has been reported in
raccoons fed 2 mg dieldrin/kg diet (Stickel, 1975).
7.4. Effect on Populations and Ecosystems
In order to show that a chemical has had an effect on
populations of organisms in the environment it is necessary to
satisfy a combination of several criteria. Ideally the exposure to
the chemical in the field should be established to compare exposure
with effects produced. Population declines should correlate with
usage of the chemical and should be reversed by controls on the use
of the chemical. Although it is generally recognized that dieldrin
affected populations of some animals when its use was widespread,
there are some difficulties in establishing its precise effects on
the environment. These difficulties arise because the use of
dieldrin coincided with the use of other persistent
organochlorines, which themselves affect populations of organisms,
and also because poisoning by dieldrin was often secondary
(poisoned organisms did not take in dieldrin directly but from prey
that had concentrated the chemical from the environment).
7.4.1. Exposure to dieldrin
It is difficult to establish the exposure of wildlife to
dieldrin unless animals are directly feeding on dressed grain or
directly exposed to preserved wood. Even where this occurs, the
animal will frequently die some distance from the source of
exposure. This is particularly true for birds but less so for
small mammals. Since exposure cannot be readily monitored
directly, most investigators have estimated exposure from the
residue remaining in dead or dying animals. Monitoring programmes
in several countries sampled both dead and dying animals and
compared them with healthy animals taken from the wild. Eggs of
birds were also monitored as a measure of dieldrin contamination
of populations. These monitoring programmes had to establish
criteria by which it could be definitively stated that particular
individuals had died from dieldrin poisoning. The criteria were
based on residue levels in experimentally poisoned animals and were
set at 5 - 10 mg/kg brain tissue and 10 - 20 mg/kg liver tissue for
birds (Robinson, 1969; Stickel et al., 1969; Cooke et al., 1982).
These criteria are probably conservative; 20 - 30% of dead wood
pigeons examined in the UK during the period 1961 - 1964 were
judged, by these criteria, to have died from dieldrin poisoning.
During this period many seed-eating birds were killed directly by
eating dieldren-dressed grain (Robinson, 1969); the actual
percentage of death attributable to dieldrin should probably be
higher. A high proportion of dead birds from areas of tsetse fly
control contained residues which would be judged lethal by these
criteria. The great majority of birds sampled contained non-lethal
residues of dieldrin (Tables 15, 16, 17, 18, and 34). It should be
remembered that all of these sampled birds contained residues of
other organochlorines, in addition to dieldrin. Although there
have been reports of populations with no dieldrin contamination,
but contamination with other organochlorines, there have been no
reports of populations contaminated by dieldrin alone. Furthermore,
residues of dieldrin always correlate well with residues of other
organochlorines; birds retaining large quantities of dieldrin also
retain large quantities of DDE and often polychlorinated biphenyls
(PCB) (Newton, 1979).
The literature reporting the presence of dieldrin in birds and
mammals from the wild is very extensive and has been selectively
reviewed elsewhere (section 5.1.6). Analysis of a few dead animals
serves to indicate the presence of dieldrin in wildlife but is of
little use in establishing effects at the population level. Only
long-term monitoring programmes, measuring changes in dieldrin
residues in the population with time and correlating this to
ecological monitoring of the size and reproductive success of the
population, can approach an objective assessment of the effects of
dieldrin. Such programmes have been reviewed by Newton (1979).
7.4.2. Effects on populations of birds
Populations of birds of prey declined during the period of
large scale use of organochlorine insecticides. Major studies of
changes in bird populations concentrated on a few species mainly in
the United Kingdom and North America, though also to some extent in
areas of mainland Europe. The following references are
illustrative on this subject of the literature: general
references, Anon (1964), Prestt (1965), Prestt & Bell (1966),
Parslow (1973), Bijleveld (1974), Cooke et al., (1976, 1982),
Havera & Duzan (1986); peregrine falcon (Falco peregrinu),
Ratcliffe (1963, 1965, 1967b, 1970, 1972, 1980, 1984), Lockie &
Ratcliffe (1964), Cade et al. (1968), Enderson & Berger (1968),
Hickey (1969); heron (Ardea cinerea), Reynolds (1974); golden eagle
(Aquila chrysaetos), Brown (1969), Lockie et al. (1969); sparrow-
hawk (Accipiter nisus), Koeman et al. (1972), Newton (1973a,b,
1974, 1976, 1979), Newton & Bogan (1974, 1978); Newton et al.
(1979), Marchant (1980); kestrel (Falco finnunculus), O'Connor
(1982).
In most of these studies, population decline correlated with
organochlorines residues in adult birds and their eggs. Reduced
breeding success was associated with thinning of eggshells,
behavioural changes resulting in egg breakage, and aggressive
interaction between adults resulting in a reduction of the number
of young fledged successfully from the clutches. The death of
adult birds was reported at the same time as seed-eating species
were dying from dieldrin poisoning.
As reported earlier in this section, dieldrin cannot be held
responsible for the eggshell-thinning effect, which has been shown
to be attributable to DDE (Cooke, 1973). Embryo deaths in shell
correlate best with PCB residues in eggs (Newton, 1979). The
contribution of dieldrin to these declines is difficult to
determine because the birds were subjected to residues of all
organochlorines. It is probable that dieldrin contributed to
population declines in some areas but not others (Newton, 1979;
Newton & Haas, 1984).
The studies of Blus et al. (1974a,b, 1975, 1979a,b) and Blus
(1982) on the brown pelican (Pelicanus occidentalis) conclude that
the decline in numbers could be ascribed entirely to DDE. The
population size of birds of prey in some of the Eastern states of
the USA declined when no dieldrin residues were present, DDE alone
being a contaminant in these populations. Newton (1979) pointed
out that the decline in populations of birds of prey contaminated
by DDE is gradual, a result of progressive effects of failure in
breeding. The decline of populations of peregrine falcon and
sparrow-hawk in the United Kingdom was more sudden and was
associated with the death of breeding adults. This was attributed
to dieldrin usage, which correlated well with the decline (Newton,
1979; Newton & Haas, 1984).
7.4.3. Effects on populations of mammals
Some mammal species in addition to birds, have been affected by
the use of organochlorine pesticides. There are reports of
decreases in the number of badgers (Meles meles) in some areas of
the United Kingdom (Jefferies, 1969, 1975). Declines in the number
of bats have been reported in the United Kingdom and the
Netherlands (Jefferies, 1972); furthermore, the grey bat (Myotis
grisesceus) in the USA (Clark et al., 1978) and the otter (Lutra
lutra) have also been affected (Jefferies et al., 1974; Chanin &
Jefferies, 1978).
Declines in bat numbers have been associated with the use of
dieldrin and lindane in wood preservatives in the United Kingdom
(Jefferies, 1972). In the United States, they appear to be related
to a combination of organochlorines. Many species migrate long
distances, using fat reserves on the journey, and are susceptible
to DDE poisoning en route (Clark & Kroll, 1977). No contribution
of dieldrin to declines in bat numbers in the USA has been proven.
Other declines have been attributed to dieldrin (Chanin &
Jefferies, 1978). Jefferies & Pendlebury (1968) studied the effect
of aldrin/dieldrin seed dressings on the populations of stoats,
weasels, and hedgehogs in the United Kingdom. None of these
species showed a decline during the period 1959 to 1962, and there
was no evidence that aldrin/dieldrin had any detrimental effects.
Jefferies et al. (1973) studied the behaviour of small mammals
in and adjacent to a field sown with dieldrin-dressed wheat. The
field mouse Apodemus, which lives on the field margin and the open
field, immediately fed on dosed grain. Residues of dieldrin in
sampled mice was very high. The bank vole Clethrionomys, which
lives in field margins, did not take the dosed grain. Residues of
dieldrin in these small mammals were monitored regularly after
sowing. These very quickly dropped to very low levels. The
authors propose that those individuals eating dressed grain died
quickly or were taken by predators. Populations were quickly
replenished by immigration from surrounding areas.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Aldrin and dieldrin
8.1.1.1 Oral
The acute oral LD50 values for technical aldrin and dieldrin in
various animal species are shown in Table 36. Intoxication with
cyclodiene insecticides consists of increased irritability and
tremor, followed later by tonic-clonic convulsions. In rats,
convulsions appear within 1 h following oral dosing at high
concentrations; death follows within 6 h, or from 2 - 7 days later.
This depends on factors such as the contents of the rat's
gastrointestinal tract, the concentration of aldrin/dieldrin in the
solvent, and the type of solvent used (Borgmann et al., 1952b;
Heath & Vandekar, 1964). Fox & Virgo (1986) reported that dieldrin
induced hyperglycemia.
Table 36. Acute oral LD50 values for technical aldrin and dieldrin
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Mouse corn oil 44 38 Borgmann et al. (1952a,b)
Mouse olive oil ~75 Jolly (1954)
Rat (newborn) arachis oil 168a Lu et al. (1965)
Rat (pre-weaning) arachis oil 25 Lu et al. (1965)
Rat (adult) arachis oil 37 Lu et al. (1965)
Rat arachis oil 51-64 Heath & Vandekar (1964)
Rat various 38-67 Lehman (1951); Borgmann et al.
(1952a); Treon & Cleveland (1955);
Gaines (1960); Worthing & Walker
(1983)
Rat various 37-87 Lehman (1951); Borgmann et al.
(1952b); Treon & Cleveland (1955);
Gaines (1960); Lu et al. (1965);
Worthing & Walker (1983)
Hamster olive oil 320 330 Gak et al. (1976)
Hamster corn oil 100 Cabral et al. (1979a,b)
Guinea-pig corn oil 33 49 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
Table 36. (contd.)
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Guinea-pig olive oil between 10 Jolly (1954)
and 25
Rabbit corn oil 50-80 45-50 Borgmann et al. (1952a,b)
Dog corn oil 65-95 65-80 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
a Transcutaneous intragastric injection.
The minimum toxic and the maximum non-toxic doses of aldrin and
dieldrin, administered orally to livestock, are indicated in Table
37.
Table 37. Acute oral toxicity of aldrin and dieldrin for livestock
-------------------------------------------------------------------
Compound Species Age Maximum Minimum Reference
non-toxic toxic
dose dose
tested found
-------------------
(mg/kg body weight)
-------------------------------------------------------------------
Aldrin calf 1-2 weeks 2.5 5 Radeleff et
al. (1955)
cattle 1 year 10 25 Radeleff et
al. (1955)
sheep 1-2 years 10 15 Radeleff et
al. (1955)
Dieldrin calf 1-2 weeks 5 10 Radeleff et
al. (1960)
cattle 1 year 10 25 Radeleff et
al. (1955)
horse - - 25 Radeleff et
al. (1960)
pig 3 weeks 25 50 Radeleff et
al. (1960)
sheep 1 year 15 25 Radeleff et
al. (1960)
sheep 9-12 months - LD50 Jolly (1954)
50-75
-------------------------------------------------------------------
8.1.1.2 Dermal
The minimum lethal dose of aldrin or dieldrin when applied as a
dry powder on the intact skin of female rabbits for 24 h was
between 600 and 1250 mg aldrin/kg body weight and between 250 and
360 mg dieldrin/kg body weight. In olive oil, the range for aldrin
was the same as for dry powder, and the range for dieldrin was
between 360 and 600 mg/kg body weight (Treon et al., 1953).
The acute dermal LD50 values for technical aldrin and dieldrin
in various animal species are shown in Table 38. The signs of
intoxication are similar to those that follow oral administration.
Table 38. Dermal LD50 values for technical aldrin and dieldrin
---------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
---------------------------------------------------------------
Mouse solvent - 40-80a Jolly (1954)
naphtha
Rat xylene ~100 60-90 Gaines (1960)
Guinea-pig solvent - 120a Jolly (1954)
naphtha
Rabbit dimethyl- 150 150 Lehman (1952)
phthalate
---------------------------------------------------------------
a With complete immersion of body.
8.1.1.3 Inhalation
The vapour pressures of technical aldrin and dieldrin are
sufficiently low that an acute inhalation hazard from aldrin or
dieldrin vapour does not normally arise.
8.1.1.4 Parenteral
The acute LD50 values for technical dieldrin (in glycerol
formal) in the rat via intraperitoneal and intravenous routes are
56 and 8 - 9 mg/kg body weight, respectively (Heath & Vandekar,
1964).
8.1.2. Formulated materials
8.1.2.1 Oral and dermal
The acute toxicity of formulated products, particularly the
dermal toxicity, is a more realistic guide than that of the
technical product to the acute hazard to the user. The percentage
of aldrin or dieldrin in the formulation, the solvent used, and the
type of formulation (such as an emulsion, wettable powder, dust,
etc.) will determine the acute toxicity of the formulated product.
Depending on these factors, the oral and dermal LD50s vary from 100
to 4500 and 500 to 16 000 mg total aldrin formulation/kg body
weight, respectively. For dieldrin formulations, these figures are
100 - 400 mg/kg and 200 - 2700 mg formulation/kg body weight,
respectively (Muir, 1970; Rose, 1982, 1984a,b).
For a dieldrin formulation for termite control (680 g/litre
suspension concentrate), the dermal LD50 in the male rat was 645 mg
formulation/kg body weight and in the female rat 284 mg
formulation/kg body weight (Rose, 1984c).
8.1.2.2 Inhalation
The acute inhalation LC50 (4-h exposure) in rats for aqueous
dilutions of a 48% (w/v) emulsifiable concentrate of aldrin (high
aromatic solvent) in the form of a spray was estimated to be
equivalent to 3% (w/v) aldrin. The median droplet size was 52 µm,
and the animals were exposed "nose only". Deaths occurred up to 6
days after exposure, but most of the animals died on the 2nd day
after exposure. Signs of intoxication consisted of a subdued
and hunched appearance with piloerection, progressing to
hypersensitivity and convulsions in the more seriously affected
animals. Surviving animals recovered within 2 - 3 days after
exposure. Oral intake (due to grooming) contributed significantly
to the results (MacDonald, 1982).
8.2. Short-Term Exposures
Short-term studies on rodents have shown that aldrin and
dieldrin affect the liver. The liver/body weight ratio is
increased, and histopathological changes that have become known as
"Chlorinated Hydrocarbon Insecticide Rodent Liver" (CHIRL) are
observed. Microscopically, these CHIRLs consist of enlarged
centrilobular hepatocytes with somewhat increased cytoplasmic
oxyphilia and peripheral migration of the basophilic granules
(Treon & Cleveland, 1955; Ortega et al., 1957).
The cellular and subcellular changes in the liver of different
mammalian species have been studied by Wright et al. (1972, 1977,
1978). These studies have shown that dieldrin produces a
generalized enlargement of the liver, which, in rats and dogs, is
associated with increased size of liver parenchymal cells but, in
mice, is associated with an increase in both cell size and cell
number. The earliest ultrastructural change in the livers of mice,
rats, and dogs treated with dieldrin was the proliferation of the
smooth endoplasmic reticulum (SER). During the initial phase of
the exposure to dieldrin, and also to phenobarbital, the increases
in the SER in the liver cells of mice, rats, and dogs were of the
vesicular type. These changes were associated with an enhanced
microsomal mixed-function oxidase, and intracellular whorls of
smooth membranes appeared in the liver cells of rats and dogs but
not in those of the mouse. The changes in liver subcellular
structure and function were reversible in mouse, rat, and dog. In
contrast, no liver enlargement or other ultrastructural changes
were observed in the livers of rhesus monkeys. In vitro
determinations showed that the activity of the liver microsomal
monooxygenase system was increased after treatment, and this was
the most sensitive effect observed. In all species examined, the
biochemical and subcellular structural response of the liver to
dieldrin was shown to be similar to that found with a number of
other chemicals, such as DDT, heptachlor, and phenobarbital
(Jansen, 1979). These chemicals also induce in the mouse a type of
liver tumour identical to that found with dieldrin (Stevenson &
Walker, 1969; Thorpe & Walker, 1973).
8.2.1. Oral
8.2.1.1 Rat
A number of short-term feeding studies (3 - 9 months duration)
were carried out on rats with aldrin. Dose levels of 0.5 - 300 mg
aldrin/kg diet were tested. The results of these old studies
showed that dose levels up to 5 mg/kg diet produced no effects, but
that levels of 25 mg/kg or more gave an increased liver/body weight
ratio and reversible hypertrophy of centrilobular hepatocytes with
cytoplastic changes (CHIRL) (section 8.4.2). Dose levels of 150
mg/kg or more resulted in increased mortality (Treon et al., 1951;
Borgmann et al., 1952a).
Five further studies were carried out with dieldrin (3 - 10
months duration), using dose levels of 1 - 300 mg/kg diet. No
effects were seen up to 5 mg/kg, except that Walton et al. (1971)
found an increased liver/body weight ratio in females at 5 mg/kg
and Ortega et al. (1957) found occasional liver changes at 2.5
mg/kg diet. These changes, e.g., liver enlargement and induction
of CHIRL, were found in the other studies at 10 mg/kg diet or more.
At 150 mg/kg or more, there was increased mortality (Treon et al.,
1951; Borgmann et al., 1952b; Ortega et al., 1957; Walton et al.,
1971).
When groups of male albino rats were fed diets equivalent to
2 mg/kg body weight for 6 months, the alkaline phosphatase, SGPT,
SGOT, and LD-hydrogenase activities in the serum were increased
after 6 months. The urea content decreased after 3 months, and
some other parameters were also changed. The growth of the animals
was considerably inhibited (Shakoori et al., 1986).
8.2.1.2 Dog
Dogs appear to be more susceptible to aldrin and dieldrin than
rats. Dogs administered aldrin in the diet for 5 or 6 days at dose
levels equivalent to 0.9 - 9.1 mg/kg body weight died within 7
months. However, beagle dogs (two males and two females) survived
15.6 months when given 0.043 - 0.25 mg aldrin/kg body weight. With
dieldrin, dogs survived dose levels up to 0.23 mg/kg body weight
for 15.7 months. In aldrin- and dieldrin-treated animals, no
effects on growth and no changes in haematology were seen. The
dogs with 0.25 mg aldrin/kg showed hepatomegaly, and the females
had local hyaline (droplet) degeneration of hepatocytes and
vacuolization in the epithelia of distal renal tubules. One of the
males of this group showed hepatocyte degeneration, while the other
exhibited the renal tubular changes seen in the females. In the
group fed 0.09 mg/kg, no effects were seen in the males, while the
females (and also one female in the group fed 0.23 mg/kg) showed
vacuolization in the epithelia of the distal renal tubules. The
liver weights of the dieldrin-treated animals were increased. No
other dogs showed gross or microscopic abnormalities in the viscera
(Treon & Cleveland, 1955).
Four beagle dogs fed dieldrin at daily oral doses of 0.4 - 0.8
mg/kg body weight showed blood concentrations of 0.27 - 1.27
mg/litre blood after eight episodes of convulsions (Brown et al.,
1964). When two dogs were given 0.2 mg dieldrin/kg body weight, in
gelatin capsules daily for 8 months, no signs of intoxication were
observed. The concentration in the blood was 0.11 - 0.22 mg/litre.
In studies by Fitzhugh et al. (1964), twelve mongrel dogs of
various ages received aldrin, 6 days/week for periods up to 25
months, at doses of 0.2, 0.5, 1, 2, or 5 mg/kg body weight.
Dieldrin was tested in 14 mongrel dogs at doses of 0.2, 0.5, 1, 2,
5, or 10 mg/kg body weight. In both cases, there were two animals
per dose level (one male, one female), with the exception of four
animals in the groups given 0.5 mg/kg. In the animals tested with
aldrin, the 5 mg/kg dogs and one 2 mg/kg dog died in 3 - 4 weeks;
the remaining male dog in the 2 mg/kg group was killed at 25 weeks
because of poor condition. All four dogs showed weight loss and
fatty changes in the liver and renal tubules. The bone marrow
showed a reduced number of mature granulocytes and erythroid cells.
At 1 mg/kg, the two dogs survived for 15 and 49 weeks and, at
autopsy, showed the same lesions. In the 0.5 mg/kg group, one dog
died after 4 days. The remaining three dogs survived for 2 years,
one male among these having convulsions during the last 2 months.
At 0.2 mg/kg body weight, there were no effects. In the animals
tested with dieldrin, all six dogs on 2, 5, and 10 mg/kg died
during weeks 2 - 5. These dogs showed weight loss, fatty changes
and slight hepatic cell atrophy in the liver, and a small amount of
atypically distributed fat in the kidneys. The bone marrow showed
a reduced number of mature granulocytes and erythroid cells. The
reported bone marrow findings, which were not replicated in other
studies, cannot be interpreted, because of the inadequacy of
clinical details, and no control dogs were used in this study. The
two dogs given 1 mg/kg survived for 12 and 43 weeks and, at
autopsy, showed the same lesions. One dog given 0.5 mg/kg was
sacrificed after 2 weeks because of anorexia and marked emaciation.
Detailed histological examination, including that of the brain, did
not show any distinct organ damage. The remaining three dogs in
the 0.5 mg/kg group died with terminal convulsions or were
sacrificed in poor condition at weeks 29, 43, and 81. Two of the
dogs showed weight loss. No effects were observed in the 0.2 mg/kg
group.
Repeated daily oral administration of 0.2, 1, or 2 mg
dieldrin/kg body weight to groups of six mongrel dogs was carried
out until intoxication occurred between the 18th and 85th day. A
direct relationship was established between the dieldrin
concentration in the blood and the severity of clinical signs of
intoxication. On the first day of muscle spasms, the average
concentration of dieldrin in the blood was about 0.50 mg/litre and,
at the time of the first full-blown convulsion, about 0.90 mg/litre
(Keane & Zavon, 1969a).
In studies by Walker et al. (1969b), groups of five beagle dogs
of each sex received, by capsule, daily doses of 0.005 or 0.05 mg
dieldrin (in olive oil)/kg body weight, for 2 years. Control dogs
were given capsules containing olive oil. The health, behaviour,
and body weight were unaffected, and EEG recordings did not differ
between the dogs fed 0.05 mg/kg and the controls. In females given
0.05 mg/kg, liver/body weight ratio was increased. In both sexes,
serum alkaline phosphatase activity was increased. However, urine,
haematology, clinical chemistry, bromosulfthalein clearance, and
relative organ weight data were not affected. No gross or
histopathological anomalies were observed.
Deichmann et al. (1969) gave groups of six beagle dogs (aged
1.5 - 3.5 years) 0 or 0.6 mg aldrin/kg body weight, 5 days/week for
10 months, and then observed them for an additional 12 months. The
treated dogs showed hyperexcitability, tremors, and weight loss.
One dog died. After 14 - 18 months, the dogs with aldrin showed
cloudy swelling and fatty degeneration in the liver and hypertrophy
of hepatocytes. Renal vascular congestion and tubular degeneration
were seen in some of the animals.
8.2.1.3 Domestic animals
A dairy cow given aldrin in soybean oil daily by capsule (2.2
mg/kg body weight) exhibited hyperirritability after 27 days. The
animal was in heat and was bred the next day. She died on day 29
with convulsions. Autopsy showed a slightly discoloured, pulpy,
congestive liver and one slightly enlarged congested kidney. No
mortality occurred among cows given 0.8, 1, or 1.5 mg/kg body
weight for 48 days (Ely et al., 1954).
In studies by Gannon et al. (1959b), groups of four dairy cows
were fed rations containing 0, 0.1, 0.25, 0.75, or 2.25 mg
dieldrin/kg for 12 weeks (average total intake, 0, 0.293, 0.75,
2.17, or 6.55 mg dieldrin/kg body weight). No signs of illness and
no abnormalities were found when the cows were slaughtered at the
end of the test feeding period or after an additional 6-week period
on dieldrin-free rations.
Ivey et al. (1961) fed groups of 2 - 3 steers, sheep, and hogs
rations containing 0, 0.25, 0.75, or 10 mg aldrin/kg diet for 12
weeks. Two steers received rations with 2 mg/kg diet for the same
period. The control groups consisted of two animals each. No
evidence of illness and no postmortem pathology were found.
Goats administered 50 mg aldrin/kg body weight showed mild
degenerative changes, congestion and petechial haemorrhages, in
various organs. In the kidneys degenerative changes of the
proximal convoluted tubules were found. Clinical changes were also
found, e.g., salivation and convulsions (Singh et al., 1985).
8.2.2. Dermal
In studies by Treon et al. (1953), aldrin and dieldrin, as dry
powders or in solutions, were applied daily to the skin of groups
of three female rabbits for 2 h on each of 5 days per week over a
period of 10 weeks. A series of graded doses was used to determine
the doses resulting in no mortality. It was clear that aldrin
or dieldrin dissolved in kerosene was very toxic (LD50 of
approximately 5 mg/kg body weight). Dissolved in vegetable oil
they were about 6 times less toxic and as dry powder about 20 times
less toxic than when dissolved in kerosene.
8.2.3. Inhalation
Mice, hamsters, and guinea-pigs did not show any adverse
effects when exposed to vapourized aldrin at a concentration of 18
mg/m3 for 178 days (Baker et al., 1959).
8.3. Skin and Eye Irritation; Sensitization
8.3.1. Skin and eye irritation
Treon et al. (1953) reported that technical aldrin or dieldrin
applied on the intact rabbit skin for 24 h occasionally caused
slight erythema. Repeated application of aldrin or dieldrin for 10
weeks (2 h per day, 5 days per week) as a dry powder did not alter
the gross condition of the rabbit skin. Slight irritation and
scaliness were observed when the compounds were applied in
vegetable oil, but their application in kerosene resulted in
damage, attributable to the solvent.
An undiluted aldrin emulsifiable concentrate formulation (48%
aldrin in high aromatic hydrocarbon solvent), applied at a dose of
0.5 ml at each test site for 24 h on the intact skin and abraded
skin of rabbits under an occlusive patch, caused severe irritation
and necrosis of the skin. One male died on day 13. When applied to
the rabbit eye, this undiluted 48% emulsifiable concentrate caused
severe initial pain and mild irritation (Rose, 1982).
8.3.2. Sensitization
In the Magnusson and Kligman guinea-pig maximization test, 48%
aldrin emulsifiable concentrate caused positive responses (at 24 h
and 48 h after removal of the challenge patches) in 3 out of the 20
test animals. Rechallenge of these animals, one week later,
confirmed that they had been sensitized to the test material (Rose,
1982).
Aldrin emulsifiable concentrate (48%) is not a skin sensitizer
under the EEC Dangerous Substances Directives (EEC, 1983).
No cases of skin sensitization occurred over a period of 20
years among a group of over 1000 workers involved in the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
8.4. Long-Term Toxicity and Carcinogenicity
8.4.1. Mouse
Davis & Fitzhugh (1962) fed groups of 100 male and 100 female
C3HeB/Fe mice a diet containing aldrin or dieldrin at 0 or 10 mg/kg
diet for 2 years. The average lifespan of the treated mice was
shortened by 2 months. A significant increase in the incidence of
benign liver tumours was observed.
In a further study, 100 male and 100 female C3HeB/Fe/J mice
received aldrin or dieldrin at 0 or 10 mg/kg diet for 2 years. An
increase in the number of animals with hepatic hyperplasia and
benign liver tumours was seen in the treated groups, but no
increase in malignant liver tumours was found. The survival in the
treated groups was lower than that of controls (Davis et al., 1965).
Groups of 300, 125, 125, and 200 CF1 mice of each sex were fed
diets containing dieldrin (> 99%) at 0, 0.1, 1, or 10 mg/kg,
respectively, for 2 years. A positive control group fed 600 mg
4-amino-2,3-dimethylazobenzene for 6 months, followed by a control
diet, was used. After 9 months, the morbidity of the mice fed 10
mg/kg started to increase, but the lifespan of the mice fed 0.1 or
1 mg/kg was unaffected. The animals with 4-amino-2,3-
dimethylazobenzene died within 14 months. The frequency of liver
tumours was increased in all groups fed dieldrin. Two types of
tumours were observed, one of which was considered benign
(hyperplastic nodules) and the other malignant. The malignant
tumours were clearly hepatocarcinomas, though no fibrosis or bile
duct proliferation, as seen in the positive control group, occurred
in the dieldrin-treated groups. A reversibility study in a
separate group fed 10 mg dieldrin/kg in the diet for up to 15
months, followed by a control diet for the rest of the 2-year
study, showed that the tumours did not regress or disappear upon
discontinuation of the treatment. However, the dieldrin induced
hepatomegaly, and cytoplasmic changes were found to be reversible
(Walker et al., 1972; Hunt et al., 1975).
In a study by Thorpe & Walker (1973), a group of 30 CF1 mice of
each sex were fed a diet containing 10 mg dieldrin/kg for 2 years.
The control group consisted of 45 mice of each sex. Liver
enlargement was detected after 50 weeks in both sexes, and liver
lesions were observed, classified as hyperplastic nodules (Type a)
and hepatocellular carcinoma (Type b) (sometimes associated with
lung metastases). In a separate study, it was shown that dieldrin
Type b liver cell tumours were capable of growing as subcutaneous
transplants in mice of the same strain and sex (Thorpe, 1973).
To compare the pathological responses to dieldrin in different
mouse strains, groups of 30 mice of each sex of the CF1, LACG, and
hybrid CF1-LACG strains were fed diets containing 10 mg dieldrin/kg
for 2 years. There was also a control group of 45 animals of each
sex for each strain. The incidence of liver tumours, particularly
Type b tumours, in male CF1 and hybrid mice and in females of all
three strains was higher than in controls. In male LACG mice, the
incidence of liver tumours was low. Qualitatively, there was no
difference in tumours between strains, and there was no increased
incidence of neoplasms in other tissues, nor were unusual tumours
found. Metastases of liver carcinoma were found in the lungs in
some of the mice (Thorpe & Hunt, 1975).
In studies by Benitz et al. (1977), nine groups of 100 Charles
River CD1 mice of each sex were given dieldrin in the diet at
concentrations ranging from 0.15 to 15 mg/kg. Six hundred mice of
each sex were used as controls. Groups of animals were sacrificed
at time intervals ranging from 2 to 25 months. Initial changes in
the liver consisted of various degrees of centrilobular and
pericentral hypertrophy. These changes were later associated with
the appearance of hepatic nodules, the occurrence of which was time
and dose related. These nodules consisted of hypertrophic
hepatocytes, which, in a few instances, were mixed with
hyperplastic cells. Various degrees of loss and distortion of
lobular architecture were seen within these nodules. Metastases in
the lung were observed in three nodule-bearing animals given 15 mg
dieldrin/kg for 25 months. These metastases contained similar
hypertrophic hepatocytes as did the primary liver tumours.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
aldrin (4 or 8 mg/kg diet for males and 3 or 6 mg/kg diet for
females) or dieldrin (2.5 or 5 mg/kg for both sexes) for 80 weeks,
followed by an observation period of 10 - 13 weeks. Concurrent
controls consisted of groups of 20 male and 10 female mice. The
pooled controls, used for statistical evaluation, consisted of the
concurrent control groups combined with 92 male and 79 female mice
from similar bioassays of other chemicals. All surviving mice were
killed at 90 - 93 weeks. Body weight was not affected in the
treated animals, but there was a dose-related increase in
mortality, especially in the high-dose groups in the second half of
the study. In the dieldrin-treated mice, clinical symptoms, such
as irritability, tremors, and alopecia occurred. Hepatocellular
carcinomas were found, as indicated in Table 39. The incidence of
hepatocellular carcinomas was clearly higher in male than female
mice. There was no difference in tumour frequencies in other
tissues (NCI, 1978a).
Groups of weanling male C3H/HE mice were fed a diet containing
10 mg dieldrin/kg diet until an age of 57 weeks and then were
either administered a control diet (12 mice) or continued on the
dieldrin diet (11 mice) for another 10 weeks. A third group served
as an untreated control group (21 mice). Laparatomies were
performed and biopsy specimens taken when about 30% of the mice in
each dieldrin-treated group had tumours. Further biopsy samples
were taken approximately 10 weeks later. Tumours were observed at
the first laparotomy in 6/21 controls and 14/23 dieldrin-treated
animals. At the second laparotomy, adenomas were seen in some
animals in which there had been no tumour at the first laparotomy.
In one animal in the continuous dieldrin-treatment group, there was
histological progression from adenoma to hepatocellular carcinoma.
Additional hepatocellular carcinomas were observed in some animals
autopsied at 2 years of age. A strong tendency to tumour
progression was found in both treated and control mice (Ruebner et
al., 1984a,b).
Table 39. Incidence of hepatocellular tumours
in mice (NCI, 1978a)
----------------------------------------------
Groups Males Females
----------------------------------------------
Concurrent controls 3/20 (15%)a 0/10 (0%)a
3/18 (17%)b 0/20 (0%)b
Pooled controls 17/92 (18%) 3/78 (4%)
Aldrin (4 mg) 16/49 (33%)
(8 mg) 25/45 (56%)
Aldrin (3 mg) 5/48 (10%)
(6 mg) 2/43 (5%)
Dieldrin (2.5 mg) 12/50 (24%) 6/50 (12%)
(5 mg) 16/45 (36%) 2/49 (4%)
----------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
In a study by Meierhenry et al. (1983), groups of 50 - 70 male
mice of three strains (C57Bl6J, C3H/He, and C57Bl6J x C3H/He B6C3F1
hybrid) were administered a diet containing 10 mg dieldrin/kg diet
for 85 weeks. The control groups consisted of approximately 60
mice of the same strains. After 4 months, the livers in a small
number of animals showed swellings of hepatocytes in the central
zone with nuclear atypia, small nodules containing basophilic or
eosinophilic foci, and multiple tumours. The percentage of benign
hepatic tumours was 28, 20, and 29, respectively, and in the
control groups, 19, 18, and 4. The percentage of hepatocellular
carcinomas was 30, 38, and 42, respectively, and, in the controls,
0, 12, and 4%. Mallory bodies were seen in all the dieldrin-
treated mice that had either benign or malignant tumours, but only
rarely in mice without tumours.
It seems from the available studies that dieldrin facilitates
and exacerbates the expression of an endogenous oncogenic factor in
CF1 mice (Tennekes et al., 1981). The dose-response characteristics
of dieldrin-mediated enhancement of liver tumour formation in CF1
mice were analysed using existing tumour data from long-term
feeding studies at six levels of continuous exposure, involving a
total of more than 1500 animals. Using the Druckrey equation, the
actual contribution of dieldrin to tumour formation was considered
to be negligible (Tennekes et al., 1985).
8.4.1.1 Appraisal
A number of long-term carcinogenicity studies have been carried
out in which mice of different strains were fed aldrin and/or
dieldrin at one or more dose levels. In all these studies, there
was an increased incidence of liver changes, some of which were of
the nature of hepatocellular carcinomas while others were regarded
as non-malignant. Females seem to be less sensitive than males.
No other tumours were induced.
8.4.2. Rat
Borgmann et al. (1952a) fed six groups of 10 male and 10 female
weanling Sprague-Dawley rats a diet containing 0, 5, 10, 50, 100,
or 150 mg aldrin/kg diet over a period of 2 years. At the two
highest dose levels, an increased mortality was found at 16 months,
which was not seen in the other groups. Liver enlargement was
observed in the groups with high dose levels, but not in the groups
with 10 mg/kg diet or less.
When groups of 40 Carworth rats of each sex were given diets
containing aldrin or dieldrin at 0, 2.5, 12.5, or 25 mg/kg diet for
2 years, there was no increase in mortality and the growth rate was
comparable with that of controls. At all dose levels, the
liver/body weight ratio was increased in males and there were
histological liver cell changes characteristic of CHIRL. No
tumours were reported (Treon & Cleveland, 1955). In a review of
all the aldrin and dieldrin studies, it was reported that there was
no excess of tumours in these rats (Cleveland, 1966).
When groups of 12 Osborne-Mendel rats of each sex were given
diets containing aldrin or dieldrin (0, 0.5, 2, 10, 50, 100, or
150 mg/kg diet) for 2 years, growth was not affected. However,
survival was markedly decreased at dose levels of 50 mg or more in
a dose-related manner. The liver/body weight ratio was increased
in males fed 10 mg/kg or more, while, in females, an increase at
all dose levels was found (no dose-response relationship at the
lower doses). CHIRL was observed in all treated groups, although
at 0.5 mg/kg only a few animals showed a trace of CHIRL. Rats at
dose levels of 50 mg/kg or more showed haemorrhagic urinary
bladders and nephritis. An overall increase in tumour incidence
was noted, but this was not dose related. On the contrary, the
lowest dose levels showed the highest tumour incidence. Only one
liver tumour was found (Fitzhugh et al., 1964).
In a study at the National Institute of Public Health of the
Netherlands, no increased incidence of tumours was found in rats
fed diets containing 75 mg dieldrin/kg for 2 years (Van Genderen,
1965, 1979).
When groups of 30 Osborne-Mendel rats of each sex were fed
5 mg/kg aldrin (95%) or a control diet for 2 years, there was no
increase in mortality, liver/body weight ratios, or tumour
incidence in the aldrin-fed group (Deichmann et al., 1967).
In studies by Walker et al. (1969b), groups of 25 Carworth Farm
E rats of each sex were given dieldrin at 0.1, 1, or 10 mg/kg diet
for 2 years. A control group consisted of 45 males and 45 females.
There was no effect on body weight. After 2 - 3 months, the
animals fed 10 mg/kg exhibited irritability and, as the study
progressed, tremors and occasional convulsions, usually during
handling. Mortality, haematology, serum enzyme levels, and
urinalysis were not affected. The females fed 1 mg/kg and 10 mg/kg
had increased liver/body weight ratios. At 10 mg/kg, one male and
six females exhibited CHIRL. In two females of the group fed 10
mg/kg and in one female control rat, microscopic nodules in the
liver parenchyma were seen. There was no increase in tumour
incidence. Subsequent re-evaluation of these data (Stevenson et
al., 1976) confirmed that there was no treatment-related increase
in tumour incidence.
Nine groups of 50 Osborne-Mendel rats of each sex were fed
diets containing aldrin or dieldrin at 0, 20, 30, or 50 mg/kg for
up to 31 months. A control group consisted of 100 male and 100
female rats. Dose-related tremors and convulsions, always
associated with weight loss, occurred at all dose levels,
particularly in females. Female rats fed 50 mg aldrin/kg or 30 or
50 mg dieldrin/kg had a shortened lifespan. The liver/body weight
ratio was increased in all dieldrin-treated groups and in males fed
30 or 50 mg aldrin/kg, but was decreased in females fed 20 mg/kg.
A moderate increase (not dose related) in the incidence of hepatic
centrilobular cloudy swelling, necrosis, or, rarely, foci of acute
or chronic inflammatory cellular infiltration was observed in all
treated groups. Hyperplasia in the liver was found in two male
rats fed 30 mg aldrin/kg. There was no increase in tumour
incidence (Deichmann et al., 1970; Deichmann, 1974).
Groups of 50 Osborne-Mendel rats of each sex were given diets
containing aldrin at levels of 30 or 60 mg/kg. Treatment of male
rats lasted 74 weeks followed by 37 - 38 weeks of observation,
while that of female rats lasted 80 weeks followed by 32 - 33 weeks
of observation. In a similar study, dieldrin was fed at a level of
29 mg/kg diet (time-weighted average dose) for 80 weeks followed by
observation for 30 - 31 weeks or 65 mg/kg diet (time-weighted
average dose) for 59 weeks followed by observation for 51 - 52
weeks. Concurrent control groups consisted of 10 rats of each
sex. Pooled controls, used for statistical evaluation, consisted
of concurrent control groups combined with 58 males and 60 females
from similar bioassays with other chemicals. All surviving rats
were killed at 110 - 111 weeks. Typical signs of organochlorine
intoxication (such as hyperexcitability) were observed with
increasing frequency and severity, especially in the second year,
but mortality was not affected. No significant increase in tumour
incidence was found (NCI, 1978a) (Table 40).
Groups of 24 Fischer 344 rats of each sex were given dieldrin
at 0, 2, 10, or 50 mg/kg diet for 2 years. Typical signs of
organochlorine intoxication were observed during the second year in
the 50 mg/kg group. Body weight and survival were not adversely
affected in any of the dieldrin groups. Liver tumours were not
observed. No significant increase in tumours was found (NCI,
1978b) (Table 40).
Table 40. Incidence of hepatocellular tumours in rats
(NCI, 1978a,b)
-------------------------------------------------------
Groups Males Females
-------------------------------------------------------
Osborne-Mendel rats
Concurrent controls 1/10 (10%)a 1/10 (10%)a
1/10 (10%)b 0/9 (0%)b
Pooled controls ? 5/59 (8.5%)a
Aldrin (30 mg/kg diet) 1/47 (2%) 0/48 (0%)
(60 mg/kg diet) 1/47 (2%) 3/49 (6%)
Dieldrin (40-20 mg/kg diet)d 0/44 (0%) 1/47 (2%)
(80-40 mg/kg diet)e 1/47 (2%) 1/44 (2.3%)
Fischer F 344 rats
Concurrent controls 2/24 (8.3%) 0/24 (0%)
Pooled controls ? ?
Dieldrin ( 2 mg/kg diet) 0/23 (0%) 0/24 (0%)
(10 mg/kg diet) 0/23 (0%) 0/24 (0%)
(50 mg/kg diet) 4/23 (17%)c 0/23 (0%)
-------------------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
c Nodular hyperplasia.
d Time-weighted average dose = 29 mg/kg.
e Time-weighted average dose = 65 mg/kg.
When groups of 50 female rats of two different strains
(Osborne-Mendel and Sprague-Dawley) were fed 0, 20, or 50 mg
aldrin/kg diet, the survival rate was reduced at 50 mg/kg but not
at 20 mg/kg. There was no increase in the incidence of mammary or
liver tumours (Deichmann, 1974; Deichmann et al., 1979).
Photodieldrin, which has metabolites identical to those of
dieldrin, was fed to groups of rats for 80 weeks at concentrations
of up to 7.5 mg/kg diet NCI (1977). No increase in tumour
incidence was found (see section 8.8.1.3).
Ito et al. (1983) studied the promoting activity of dieldrin on
the induction of hyperplastic (neoplastic) liver nodules using a
short-term test system. F344 rats received a single dose (200
mg/kg body weight) of N-nitrosodiethylamine, and 2 weeks later,
were treated for 6 weeks with dieldrin in the diet at a
concentration of 100 mg/kg. Dieldrin had a weak promoting
potential in this test system.
8.4.2.1 Appraisal
A number of long-term/carcinogenicity studies have been carried
out in which rats of different strains were fed one or more dose
levels of aldrin and/or dieldrin. The overall no-effect level in
these long-term studies, both for aldrin or dieldrin, was 0.5 mg/kg
diet. At feeding levels of 1 mg/kg or more, an increasing, dose-
related hepatomegaly and histological changes in the liver
characterized as CHIRL occurred. At levels of 10 mg/kg diet or
more, typical signs of organochlorine toxicity occurred such as
irritability, tremors, and convulsions. In all these studies, no
increase in tumour incidence in liver or other organ/tissue systems
was found.
8.4.3. Hamster
When groups of 34 - 40 Syrian golden hamsters of each sex were
fed diets containing dieldrin (99%) at 0, 20, 60, or 180 mg/kg for
120 weeks, there was no significant increase in tumour incidence
(Cabral et al., 1979b).
8.4.4. Monkey
In studies on rhesus monkeys, groups of five males were given
diets containing dieldrin (88.4%) at 0, 0.01, 0.1, 0.5, 1, or 5
mg/kg (0.0002 - 0.07 mg/kg body weight) for approximately 6 years.
After two monkeys in the group fed 5 mg/kg died, the level of
exposure to the remaining three animals was reduced to 2.5 mg/kg
and, later, to 1.75 mg/kg. Subsequently, one of these animals had
his dieldrin intake progressively increased until at the end of the
second year, he was receiving dieldrin at the initial dietary
concentration of 5 mg/kg. Clinical and haematological examinations,
liver and kidney function studies, urinalysis, and pathology did not
reveal any abnormalities. The liver/body weight ratios and liver
DNA and RNA of the test animals were not different from those of
control animals. No subcellular changes were seen in the
hepatocytes. Dose-related increases in microsomal cytochrome P-450
and in the activity of the liver mono-oxygenase enzyme system were
observed at the two highest dose levels. These alterations in
cytochrome P-450 in the liver microsomes were significant in the
monkeys fed 0.1 mg/kg or more. No effect was observed at 0.01
mg/kg. The concentrations of dieldrin in the subcutaneous fat of
the monkeys fed 0.1 mg/kg were similar to those measured in human
beings receiving a daily oral intake of similar concentration. The
dieldrin concentrations in the monkey livers were approximately 200
times higher than those in male rats receiving a daily intake of
dieldrin 3 times higher than the monkeys, and they were similar to
the concentration in the livers of male mice daily ingesting
dieldrin at a level approximately 50 times higher (Zavon & Stemmer,
1975; Wright et al., 1977, 1978).
8.4.5. Mode of Action
From long-term feeding experiments, it seems that aldrin and
dieldrin may be carcinogenic to mice, but not to rats or hamsters.
Mutagenicity findings have been consistently negative (see section
8.6). There is insufficient knowledge of the mechanism by which
these chemicals might behave epigenetically.
The latest evaluation of IARC (1987) is that there is
inadequate evidence of carcinogenicity in humans and limited
evidence for carcinogenicity in experimental animals.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
8.5.1.1 Mouse
Groups of 100 pairs of male and female virgin CFW Swiss mice
were fed diets containing 5 mg dieldrin/kg for 30 days before
mating, after which time they were randomly paired and fed the same
diet for a further 90 days. Mortality in the dieldrin-treated
group was similar to that of a control group (size not specified).
No major biological effects on fertility, fecundity, gestation
period, size of the first litters, or numbers of young produced per
day were noted as a result of feeding dieldrin. A statistically
significant (6%) decrease in mean size of all litters combined was
the only difference observed between the dieldrin-treated and
control groups (Good & Ware, 1969).
In a reproductive study, groups of 4 male and 14 female Swiss
white mice (120 days old) were fed diets containing 3, 5, 10, or
25 mg aldrin/kg or 3, 10, or 25 mg dieldrin/kg. Six groups served
as controls. The study covered six generations, and two litters
per generation. For both aldrin and dieldrin, the 25 mg/kg dose
was too toxic and resulted in high litter mortality in the few dams
reaching gestation. This dose level was therefore discontinued.
Pup survival was low in mice fed 10 mg dieldrin/kg, and so
treatment was terminated after the first generation. The most
pronounced effect observed in the group fed 10 mg aldrin/kg and, to
a lesser extent, 5 mg aldrin/kg was a low pre-weaning pup survival.
No effects on fertility, viability, or gestation were observed in
six generations of mice fed 3 mg dieldrin/kg. A decrease in pre-
weaning survival was observed in the F2b litters, but a similar
decrease was also found in one of the six control groups (Keplinger
et al., 1970).
In studies by Virgo & Bellward (1975), groups of 18 - 19
uniparous female Swiss-Vancouver mice were given diets containing
0, 2.5, 5, 10, 15, 20, or 25 mg dieldrin/kg, 4 weeks prior to their
second mating, continuing until day 28 postpartum. Significant
mortality of the females occurred at 20 and 25 mg/kg, all deaths
occurring before parturition (89 and 56%, respectively). Fertility
in the groups fed 10 and 15 mg/kg was decreased, though survivors
at higher doses were fertile. Oestrus and gestation period were
not affected. Litter size was decreased only in the group
receiving 25 mg/kg. The major effect was an increase in pre-
weaning pup mortality; 47% at 2.5 mg/kg, 80% at 5 mg/kg, and 100%
at 10 mg/kg or more (31% mortality in control animals). Dams
receiving 10 mg/kg or more exhibited hyperactivity, which was a
contributory factor to the high pup mortality. No gross
abnormalities were detected in the pups, none of whom showed
tremors or convulsions. Within the litters raised at 2.5 and 5
mg/kg, pup survival was not different from controls. The only
effect on reproductive capacity or pup survival observed in female
mice fed 2.5 mg/kg was an increase in pre-weaning pup mortality.
A study was carried out on primiparous female Swiss-Vancouver
mice to investigate whether diets containing up to 15 mg
dieldrin/kg affect maternal behaviour and pup viability. Viability
was investigated in dams fed diets containing 0, 5, 10, or 15 mg
dieldrin/kg for 4 weeks prior to mating. Pups fed 10 mg/kg were
nursed by foster dams not fed dieldrin, and all died by day 4; the
foster dams' own pups showed a very low mortality and survived
until weaning. Similar results were obtained at 5 mg/kg. Dieldrin
did not have any influence on serum progesterone levels, milk
production, or the dam's tendency to retrieve pups or build nests.
However, at 5 mg/kg or more, nursing was reduced. It was concluded
that dieldrin causes irreversible congenital inviability (not
through any effect on progesterone levels) and it was suggested
that the inviability and the reduced tendency to nurse increased
the pup mortality (Virgo & Bellward, 1977).
8.5.1.2 Rat
In studies by Treon & Cleveland (1955), rats (Carworth strain)
were fed aldrin or dieldrin at dietary concentrations of 2.5, 12.5,
or 25 mg/kg for three consecutive generations, two litters being
produced for each generation. (There was no mention of a control
group, and tabulation and description of results was limited in
this report). A reduction in the number of pregnancies, which
gradually disappeared over successive generations, was initially
observed at 12.5 and 25 mg aldrin/kg and at all three doses of
dieldrin. No effects on litter size or pup weights were observed
at any dietary concentration. A marked increase in mortality in
pre-weaning pups was found at dietary concentrations of 12.5 and
25 mg/kg for both compounds. This was thought by the authors to
be due to the high concentration of dieldrin in the milk of the
mothers. Neither aldrin nor dieldrin had any effect on
reproductive capacity. No effects, except a "slight to moderate"
increased pre-weaning pup mortality, were observed in the rats fed
for three generations with aldrin or dieldrin at 2.5 mg/kg.
When groups of 10 male and 20 female Long Evans rats were fed
dietary concentrations of 0, 0.1, 1, or 2 mg dieldrin/kg over three
generations (each generation producing two litters), no effects
were observed on the general health (including weight gain),
behaviour, fertility, gestation, viability, lactation, or organ
weight ratios. No pathological changes were found in parents or
pups. Increased pre-weaning mortality (compared to that in
controls) in the F1a litter was observed in the animals fed 2
mg/kg. This effect was not found in the five subsequent litters
from this group and was not considered to be a major toxic effect.
No changes in reproductive capacity were observed over three
generations at dietary concentrations up to and including 2 mg/kg
dieldrin (Eisenlord et al., 1967).
In studies by Harr et al. (1970), groups of 20 male and 20
female 28-day-old OSU-Wistar rats were fed 0, 0.08, 0.16, 0.31,
0.63, 1.25, 2.5, 5, 10, 20, or 40 mg dieldrin/kg diet throughout
their lifespan. Ten females from each group were mated at 146 days
of age. Mortality occurred in dams at 20 and 40 mg/kg. Fertility
and litter size were decreased in several dose groups without a
clear dose relationship. The number of pups at weaning was
markedly reduced at 2.5 mg/kg or more; none survived at 20 and 40
mg/kg. The nursing pups died in convulsions or starved. No
effects were noted at 1.25 mg/kg or less. Neural lesions, such as
cerebral oedema and hydrocephalus, occurred in pups of nursing dams
at dieldrin concentration of 0.08 mg/kg. Hepatic lesions were
found in rats fed concentrations of 0.31 mg/kg or more.
When groups of 18 - 20 Long Evans pregnant rats were given 4 mg
dieldrin/kg body weight daily by gavage from day 15 of gestation to
21 days post partum, fecundity, number of stillbirths, perinatal
mortality, and total litter weights did not differ from the control
group. No malformations in pups were observed (Coulston et al.,
1980).
8.5.1.3 Dog
In study by Kitselman (1953), seven groups of three dogs, each
group having at least one member of each sex, were fed either
aldrin or dieldrin for one year at dietary concentrations
equivalent to 0, 0.2, 0.6, or 2 mg/kg body weight (in corn oil).
Out of a total of 11 bitches fed either aldrin or dieldrin, 9
conceived. All pregnant bitches produced litters of at least four
pups/litter. The survival of pups was generally lower in the
groups fed aldrin or dieldrin. Histopathological examinations of
dead pups revealed degenerative changes in the liver and mild
degenerative changes in renal tubules. Liver changes were also
observed in treated bitches. The design and size of this study was
too limited to deduce a dose-response relationship for pup
survival, but no effects were observed in dogs receiving 0.2 mg
dieldrin/kg body weight.
8.5.1.4 Appraisal
In the reproductive studies (over one to six generations)
carried out with aldrin or dieldrin on mice and rats, the major
effect observed in most of the studies was an increased mortality
rate in pre-weaning pups. Reproductive performance, per se, was
only affected at doses causing maternal intoxication. The studies
on dogs are of a too limited nature to draw firm conclusions, apart
from the consistent increase in pre-weaning pup mortality.
The results of these reproductive studies indicate that
dieldrin at levels of 2 mg/kg in the rat diet and 3 mg/kg in the
mouse diet (equivalent to 0.1 and 0.4 mg/kg body weight per day,
respectively) are no-effect levels for reproduction. It is not
possible to establish a no-effect level for aldrin for
reproduction, because no adequate data are available.
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Mouse
Ottolenghi et al. (1974) gave groups of 10 pregnant CD-1 mice
single oral doses of 25 mg aldrin/kg body weight or 15 mg
dieldrin/kg body weight (in corn oil; equivalent to half the LD50
values) on day 9 of gestation. Control groups consisted of
untreated and corn-oil-dosed mice. No effects on fetal survival or
weight were observed. Abnormalities, such as webbed feet, cleft
palate, and open eyes, were increased in both treated groups, but
they may have been related to maternal toxicity. The percentage of
the total live fetuses that were malformed was 33% for aldrin-
treated mice and 17% for dieldrin-treated ones.
In two comparable studies, pregnant CD-1 mice (6 - 16 per
group) were given daily oral doses of dieldrin in peanut oil at 0,
1.5, 3, or 6 mg/kg body weight from day 7 to day 16 of gestation.
At 6 mg/kg, reduced body weight gain and increased liver/body
weight ratio were observed. There was an increase in supernumerary
ribs and a decreased number of caudal ossification centres in the
fetuses of mice given 6 mg/kg. In one study, the number of
supernumerary ribs was increased at all three dose levels,
(significant at the two highest dose levels) (Chernoff et al.,
1975). In the other study, the increase in supernumerary ribs at
6 mg/kg was not significant. The increase in the number of
supernumerary ribs may be an expression of developmental toxicity.
Doses of dieldrin (99%) were given either in corn oil (0, 1.5,
or 4 mg/kg body weight) or in dimethylsulfoxide (DMSO) (0, 0.25,
0.5, or 1 mg/kg) daily by gavage to pregnant CF-1 mice (7 - 14
mice/group) on days 6 - 14 of gestation. No maternal or fetal
toxicity was seen in groups treated with dieldrin in corn oil or in
the corn oil control groups, but some was seen in the dieldrin/DMSO
and DMSO-control groups. No compound-related teratogenic effects
were observed (Dix et al., 1978).
8.5.2.2 Rat
In a study by Chernoff et al. (1975), pregnant CD rats (9 - 25
per group) were given daily oral doses of dieldrin in peanut oil
(0, 1.5, 3, or 6 mg/kg body weight) from days 7 to 16 of gestation.
At 6 mg/kg, increased mortality and reduced body weight gain were
observed in the dams, but no changes in the liver/body weight
ratios were found. Fetuses did not show any differences from the
controls in mortality, body weight, or occurrence of anomalies.
There were no differences in the average number of sternal or
caudal ossification centres (as were seen in mice). No evidence of
teratogenicity was observed at a dose level of 6 mg aldrin/kg body
weight per day.
No malformations were observed in fetuses or pups from 18 - 20
Long Evans rats given 0 or 4 mg dieldrin/kg, by gavage, daily from
day 15 of gestation to day 21 of lactation (Coulston et al., 1980).
8.5.2.3 Hamster
Pregnant Syrian golden hamsters (41 - 43 per group) were given
single oral doses in corn oil of either 50 mg aldrin/kg body weight
or 30 mg dieldrin/kg body weight on either day 7, 8, or 9 of
gestation. Untreated and vehicle-control groups (respectively, 57
and 41 animals) were used. The high dose levels of both aldrin and
dieldrin caused reductions in the number of live fetuses and fetal
weight and an increased incidence of abnormalities (cleft palate,
open eyes, and webbed feet). The effects were more pronounced
after treatment on days 7 and 8 of gestation than on day 9. It was
suggested that, since webbed foot and open eye were frequently
associated with low fetal weight, these effects might be simply the
expression of growth retardation (Ottolenghi et al., 1974).
8.5.2.4 Rabbit
No teratogenic effects were observed in the offspring of groups
of pregnant Banded Dutch rabbits dosed with dieldrin in
carboxymethylcellulose (2 or 6 mg/kg body weight per day) from
days 6 to 18 of gestation. The animals were killed on day 28 of
gestation and the fetuses were examined for visceral and skeletal
abnormalities (Dix & Wilson, 1971).
8.5.2.5 Appraisal
No evidence of a teratogenic potential has been found from
studies on rats, mice, or rabbits using oral doses up to 6 mg/kg
body weight. Single high oral doses, equivalent to half the LD50,
have been found to cause fetotoxicity and abnormal development of
the fetuses in hamsters and, to a lesser extent, in mice. The
significance of these abnormalities in the presence of severe
maternal toxicity is doubtful but a specific teratogenic potential
cannot be ruled out completely. No gross malformations have been
reported in reproductive studies.
8.6. Mutagenicity and Related End-Points
8.6.1. Microorganisms
Most research workers have reported that aldrin and dieldrin,
with or without microsomal activation, are not mutagenic in
bacterial or yeast test systems. In one study, it was reported
that dieldrin, without activation, was mutagenic in two out of
three strains of Salmonella typhimurium, but there was no dose-
response relationship (Majumdar et al., 1977). The results of the
other mutagenicity studies with aldrin and dieldrin in bacterial
test systems have been negative (Table 41). A critical survey of
the published reports indicates clearly that neither aldrin nor
dieldrin is mutagenic in microbial systems (Ashwood-Smith, 1981).
8.6.2. Mammalian cell point mutations
Only one study on the in vitro mutagenicity of dieldrin to
mammalian cells has been reported. Dieldrin was weakly mutagenic
when tested at a single concentration (0.01 mmol/litre) in ouabain-
resistant Chinese hamster V-79 cells. The significance of this
result is difficult to assess because of the lack of a dose-
response relationship, and a positive control group was not used
(Ahmed et al., 1977a).
8.6.3. Dominant lethal assays and heritable translocation assays
in mice
Aldrin did not show any detectable dominant lethality when
given as a single intraperitoneal dose (8 or 40 mg/kg) or in daily
oral doses (0.5 or 1 mg/kg body weight) for 5 days to male ICR/Ha
Swiss mice (Epstein et al., 1972).
Dieldrin, also, revealed no detectable dominant lethality in
four assays in male mice following a single intraperitoneal
injection (5.2 or 26 mg/kg) or daily oral doses of 2 or 3 mg/kg
body weight for 5 days (Epstein et al., 1972). Likewise, a single
oral dose of 12.5, 25, or 50 mg/kg body weight did not produce
dominant lethality in CF-1 mice (Dean et al., 1975). Bidwell et
al. (1975) carried out a dominant lethal test on B6D2F1/J mice
orally administered 0.08, 0.8, and 8 mg/kg body weight dieldrin for
5 days, but no effects were seen.
In further studies by Bidwell et al. (1975), a heritable
translocation test was performed on male mice after oral intake of
0.008, 0.08, or 0.2 mg dieldrin/kg body weight per day for a period
of 6 weeks. The cytogenetic determination of somatic cells using
the micronucleus test and the usual analysis of spermatocytes did
not reveal an increase in the rate of translocations.
8.6.4. Micronucleus test
Aldrin did not induce a significant increase in the frequency
of micronuclei in the bone marrow of mice treated orally with 13
mg/kg body weight (Usha Rani et al., 1980).
No cytogenetic abnormalities were seen in a standard metaphase
analysis and micronucleus test after oral gavage of mice with 0.8
or 8 mg dieldrin/kg body weight per day for 5 days (Bidwell et al.,
1975).
8.6.5. Chromosome and cytogenicity studies
Chinese hamsters (three groups of four males and four females)
were orally dosed with 30 and 60 mg dieldrin/kg body weight. No
chromosome abnormalities were found in femoral bone marrow cells
(Dean et al., 1975).
Table 41. Aldrin and dieldrin: mutagenicity tests in microorganisms
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
E. coli WP2 Try- none aldrin and dieldrin; negative Ashwood-Smith et
1000 µg/plate al. (1972)
E. coli WP2 hcr rat S9 up to 5000 µg/plate negative Moriya et al.
(1983)
E. coli Ga1 Rs none aldrin and dieldrin; negative Fahrig (1974)
Serratia marcescens alpha 21 and 742 dose not stated
Saccharomyces cerevisiae
Aspergillus nidulans diploid P1 and none dieldrin; 13 or 26 negative Crebelli et al.
haploid strain 35 mmol (1986)
Saccharomyces cerevisiae 632/4 none aldrin; 5 µg/ml on positive Guerzoni et al.
disc (1976)
Bacillus subtilis (Rec-assay) none aldrin and dieldrin; negative Shirasu (1975)
Salmonella typhimurium TA 1535, 1536,
1537, 1538
E. coli WP2 hcr+, hcr-
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; 10, 50, negative Bidwell et al.
1536, 1537, 1538, 100, or 500 µg/plate (1975)
G46
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; dose not negative McCann et al.
1537 stated (1975)
Salmonella typhimurium TA 1535, 1536, rat S9 dieldrin; 1000 negative Marshall et al.
1537, 1538 µg/plate (1976)
Salmonella typhimurium TA 98, 100 none dieldrin; 10, 30, negative Glatt et al.
100, 300, 1000, 3000 (1983)
µg/plate
Salmonella typhimurium TA 90, 100, 1535, rat S9 up to 5000 µg/plate negative Moriya et al.
1537, 1538 (1983)
---------------------------------------------------------------------------------------------------------
Table 41. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium TA 98, 100, 1535, mouse S9 dieldrin; 1000 negative Van Dijck & Van
1537, 1538, 1950, µg/plate de Voorde (1976)
1978
Salmonella typhimurium not specified S9 dieldrin; dose not "weak" Ercegovich &
stated response Rashid (1977)
Salmonella typhimurium TA 98, 100, 1535 mouse S9 dieldrin; 1, 25, or positive Majumdar et al.
50 µg/ml (1977)
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; up to 2500 negative Purchase et al.
1538 µg/plate (1978)
Salmonella typhimurium TA 98, 100 rat S9 dieldrin; 50 or 1000 negative Wade et al.
µg/plate (1979)
Salmonella typhimurium TA 98, 100, 1535, rat, aldrin; dose not negative Simmon et al.
1537, 1538 mouse, and stated (1977)
human S9
Salmonella typhimurium TA 98, 100 rat S9 aldrin and dieldrin; negative Nishimura et al.
364 and 380 µg/plate (1982)
Salmonella typhimurium TA 98, 100 1535, rat S9 dieldrin 2.6 x 104 negative DeFlora (1981)
1537, 1538 nmoles/plate
---------------------------------------------------------------------------------------------------------
The effect on the bone marrow cells of STS mice was examined
after applying a single dose of dieldrin intraperitoneally at 0, 1,
30, or 50 mg/kg body weight. A decrease in the mitotic index was
noted, as dieldrin concentration increased, and differences in
chromosome aberrations (a slight increase in breaks, fragments,
and interchanges) were found (Majumdar et al., 1976).
Seventy-one juvenile mallard ducks, the parents of which had
been exposed to various dietary levels of dieldrin for 6 months or
longer, were grouped and fed dieldrin at a level that corresponded
to the diet fed to the parents (0, 4, 10, or 30 mg dieldrin/kg
diet) for approximately 60 days. At the end of this period, no
chromosomal aberrations were found in femoral bone marrow cultures.
However, the mitotic index of ducks exposed to 30 mg/kg was
significantly reduced (Bunch & Low, 1973).
When lymphocytic cultures from adult mallard ducks (which had
not been exposed to dieldrin) were treated with 0, 0.1, 1, 10, 30,
or 100 mg dieldrin/kg, there was a significant increase in the
incidence of chromosome structural alterations, but only at the
highest dose level. The mitotic index was significantly reduced at
all dose levels, the greatest decreases occurring at the two
highest dose levels (Bunch & Low, 1973).
Chromosome studies in cultured lymphocytes from current
dieldrin plant workers (12) former plant workers (9), and control
(17) did not show any differences in the frequency of chromosome
aberrations (Dean et al., 1975). In one study, it was reported
that aldrin produced chromosomal aberrations in cultured human
lymphocytes at concentrations of 19 and 38 µg/ml. In mice and
rats, an intraperitoneal injection of a dose of 19 mg/kg body
weight was reported to induce chromosomal gaps, breaks, deletions,
and fragments in bone marrow cells (Georgian, 1975).
Cultured human peripheral lymphocytes from agricultural and
public health (anti-Chagas' disease) workers with at least 10 years
exposure to dieldrin were examined for structural chromosome
aberrations and sister chromatid exchange. No differences were
seen when they were compared with lymphocytes derived from a
control group (Bordon, 1980).
8.6.6. Host-mediated assays
Bidwell et al. (1975) carried out a host-mediated assay,
incorporating blood- and urine-recovery studies, for mutagenic
substances on B6D2F1/J mice. Five daily oral doses of 20 mg
dieldrin/kg body weight were given, and the mice were then injected
intraperitoneally with Salmonella tester strains. The results were
negative. In a further host-mediated assay using Saccharomyces
cerevisiae (strain D4, heteroallelic at the ade-2 and trp-5 loci)
as tester microorganism, CF-1 mice were treated with a single oral
dose of 25 or 50 mg dieldrin/kg body weight or with five daily
doses of 5 or 10 mg/kg body weight. No mutagenic activity was
found (Dean et al., 1975).
8.6.7. Cell transformation in mammalian cell systems
Dieldrin (0.08 - 250 mg/litre) proved negative in mammalian
cell transformation tests using cell lines derived from baby Syrian
hamster kidney (BHK-21 C13) and from human lung (Wl-38), either
with or without metabolic activation by rat liver S9 microsomal
fraction (Purchase et al., 1978).
In a 6-thioguanine resistance mutation assay using FM 3A mouse
cell cultures, aldrin was weakly mutagenic (Morita & Umeda, 1984).
8.6.8. Drosophila melanogaster and other insect systems
There is no evidence of a mutagenic activity of aldrin or
dieldrin in Drosophila melanogaster (Benes & Sram, 1969; Bidwell et
al., 1975). No increase in recessive or dominant lethal mutations
was found following exposure of the wasp Bracon hebetor to
sublethal doses of dieldrin (95%) (Grosch & Valcovic, 1967).
8.6.9. Effects on DNA
DNA strand breakage was not detected in Chinese hamster V-79
cells exposed to dieldrin (0.1 - 1 mmol/litre) in the presence of
rat liver S9 microsomal fraction using the alkaline elution assay
(Swenberg et al., 1976).
Aldrin (1 - 1000 µmol/litre) and dieldrin (1-100 µmol/litre)
induced unscheduled DNA synthesis in SV-40 transformed human
fibroblast cells (VA-4) both in the presence and absence of rat
liver microsomes (Ahmed et al., 1977b). This study was repeated by
Zelle & Lohman (1977). After exposure to dieldrin, the rate of DNA
synthesis in normal primary human fibroblasts (AH) decreased, but
returned to the control level in a few hours. This suggests that
dieldrin interferes with semiconservative DNA replication without
damaging DNA. The effect of dieldrin on the induction of repair
replication was studied with both AH cells and SV-40 transformed
human cells (MM-SV-40). No evidence that dieldrin could induce DNA
repair was found (Zelle & Lohman, 1977).
The DNA breakage rates in an Escherichia coli plasmid after
treatment with aldrin or dieldrin did not differ from those in
untreated plasmid DNA, suggesting that, at least in these studies,
the compounds did not interact directly with DNA (Griffin & Hill,
1978).
The effects of aldrin and dieldrin (both at 100 µg/ml) on the
uptake of tritiated thymidine by cultured rat thymocytes and human
lymphocytes were tested under different experimental conditions.
Both compounds appeared to have marginal effects on thymidine
uptake, suggesting inhibition of DNA synthesis (Rocchi et al.,
1980).
Aldrin (100 mmol/litre) and dieldrin (500 mmol/litre) did not
induce unscheduled DNA synthesis in primary cultures of Fischer 344
rat hepatocytes (Probst et al., 1981). Williams (1982) reported
the results of the hepatocyte primary culture/DNA repair test,
using freshly isolated hepatocytes of high metabolic capability to
monitor the production of DNA damage by measuring DNA repair
synthesis. Aldrin and dieldrin gave equivocal results concerning
DNA repair, but there was no damage to DNA. Aldrin (0.3 - 3
mmol/litre) induced DNA strand breaks in an alkaline elution/rat
hepatocyte assay (Sina et al., 1983).
Cultured hepatocytes from male Balb/c mice treated with
dieldrin at a concentration of 4 x 10-4 mol/litre showed no
unscheduled DNA synthesis. The results were no different with
cells from mice treated in vivo with phenobarbital (Klaunig et al.,
1984).
A DNA synthesis inhibition/damage test on HeLa cells with S9
showed inhibition to 60% of the control value within 90 min of
treatment with 4 x 10-4 mol dieldrin/litre (Painter, 1981).
8.6.10. Cell to cell communication
Both aldrin and dieldrin inhibited gap junctional intercellular
communication between 6-thioguanine-sensitive and 6-thioguanine-
resistant human teratocarcinoma cells in culture (Zhong-Xiang et
al., 1986).
The inhibition of cell to cell communication was observed in
human teratocarcinoma cells in culture in the presence of dieldrin,
using dye transport methods (Wade et al., 1986).
Metabolic cooperation between 6TG-resistant and HGPRT-deficient
Chinese hamster V79 cells was inhibited when aldrin (2.5 - 10
µg/ml) or dieldrin (2.5 - 5 µg/ml) was added to the medium (Kurata
et al., 1982).
Trosko et al. (in press) studied the inhibition of gap
junctional-mediated intercellular communication using co-cultures
of Chinese hamster cells. To do this, an in vitro assay (in which
the metabolic cooperation between V79-6-thioguanine-sensitive (6
TGs) and resistant (6 TGr) cells is studied) has been developed to
detect the ability of non-cytotoxic and non-mutagenic chemicals to
inhibit gap junctional communication. Aldrin and dieldrin
inhibited metabolic cooperation at concentration of about 4 µg/ml
or more.
8.6.11. Appraisal
Aldrin and dieldrin are not mutagenic to mammals in a variety
of mutagenicity test systems with unrelated different end-points.
8.7. Special Studies
8.7.1. Liver enzyme induction
Aldrin and dieldrin have been shown to increase the activity of
liver microsomal enzymes, generally associated with enlargement of
the liver. They have also been found to induce microsomal
dimethylaminoantipyrine-N-demethylase and aldrin-epoxidase, and
increase the cytochrome P-450 level (Campbell et al., 1983). This
enzyme induction is the earliest and most sensitive indicator of an
effect of exposure in mouse, rat, beagle dog, and rhesus monkey
(Wright et al., 1972).
Studies on rats indicated a no-effect level for enzyme
induction, by either aldrin or dieldrin, at 1 mg/kg diet (Gillett &
Chan, 1968; Kinoshita & Kempf, 1970; Den Tonkelaar & Van Esch,
1974).
In the rhesus monkey, the activity of the liver microsomal
monooxygenase system was increased by dieldrin (at daily feeding
levels of 1.75 and 5 mg/kg diet for approximately 6 years), but no
associated liver enlargement was observed. The dietary intake of
dieldrin required for the induction of this enzyme system in
monkeys was approximately 1 mg/kg diet, corresponding to an intake
of 25 - 30 µg/kg body weight per day (section 8.4.4) (Wright et
al., 1978).
In human beings, oral dosing with 211 µg dieldrin/day
(approximately 3 µg/kg body weight per day) for two years, in
addition to the daily intake of 19 µg dieldrin from the diet, did
not increase the activity of microsomal liver enzymes as measured
by the concentration of p,p'-DDE in adipose tissue and blood. No
evidence of enzyme stimulation was observed in a group of 10
workers (at the time, they were the most highly exposed workers) in
a manufacturing plant with a mean exposure equivalent to a daily
oral intake of 17 µg/kg body weight (maximum 24 µg/kg body weight)
(Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
8.7.2. Nervous system
8.7.2.1 Rat
When three groups of eight male albino rats were fed diets
containing 0, 25, or 50 mg dieldrin/kg for 60 days, there was no
effect on body weight or learning, but muscular efficiency,
measured by pulling weights of increasing magnitude in a 250-cm
runway, was decreased (Khaïry, 1960). This finding is in agreement
with the results of a study on nerve muscle (gastrocnemius)
preparations of pre-treated rats (Ibrahim, 1964).
8.7.2.2 Dog
Groups of five dogs of each sex, given daily oral doses of
dieldrin (0.05 mg/kg body weight) by capsule for 2 years, did not
show any changes in behaviour or EEG recordings compared with
controls (Walker et al., 1969b).
8.7.2.3 Monkey
When groups of three or four adult male squirrel monkeys were
given daily oral doses of 0, 0.01, or 0.1 mg dieldrin/kg body
weight for 54 days, learning ability was impaired and changes (high
amplitude slow waves) in the EEG occurred in both test groups (Van
Gelder & Cunningham, 1975).
8.7.3. Weight loss and stress
It is known that in birds, and perhaps in some small mammals,
dieldrin intoxication may be induced by starvation, weight loss,
and stress in animals having a previously harmless body burden of
dieldrin. Concern is sometimes expressed that, by analogy to these
observations, a similar course of events might occur in human
beings. Therefore, this phenomenon was studied in rats as well as
in human beings (for human beings see section 9.1.3.2).
8.7.3.1 Rat
In studies by Treon & Cleveland (1955), rats previously fed
diets containing aldrin or dieldrin at levels of 5, 10, or 15 mg/kg
diet for 7 - 18 months were starved. The complete withdrawal of
food did not result in the release of aldrin or dieldrin from the
adipose tissue stores to an extent sufficient to induce symptoms of
intoxication of any type.
When Osborne-Mendel rats fed 7.5 mg aldrin/kg diet for 4 weeks
were subsequently starved for 6 days with free access to water,
there was a marked loss of body weight and fat and a decrease in
the liver/body weight ratio. The total body burden of dieldrin
decreased during starvation regardless of age, sex, or the previous
level of exposure. The total quantity of dieldrin in the liver
decreased in all rats. In females, particularly older females, the
concentration of dieldrin in abdominal fat increased, whereas in
all males, the level in fat decreased. The concentration of
dieldrin in the blood was not increased. Young weanling rats
reacted similarly (Deichmann et al., 1972).
8.7.4. Immunosuppressive action
Loose et al. (1981) found that macrophages from mice fed 50 mg
dieldrin/kg diet had a marked impairment in antigen processing.
The effect was statistically significant in Kupffer cells at 50
mg/kg diet, in alveolar and splenic macrophages at 0.5, 5 and 50
mg/kg diet, and in peritoneal macrophages at 5 and 50 mg/kg diet.
There was an impairment of in vivo phagocytic clearance in mice
receiving 5 or 50 mg/kg diet for 8 weeks but not at 0.5 mg/kg diet.
This was related to a decrease in serum fibronectin. Tumour cell
killing after challenge with EL-4, P388, or mKSA tumour cells was
significantly impaired in mice fed either 1 or 5 mg dieldrin/kg
diet. The mean survival time after challenge with EL-4 was reduced
by 3 weeks, and with the P388 or mKSA tumour cells impairment was
observed after 3 or 18 weeks, respectively. There was no
alteration in the oxygen uptake by isolated macrophages either at
rest or during phagocytosis, and no effect on phagocytic activity
or capacity or on chemotaxis in vitro was observed.
Loose (1982) found that dieldrin caused immunosuppression in
mice. Levels of 1 or 5 mg dieldrin/kg diet were fed to BALB/c mice
for 3.5 or 10 weeks, and the mice were challenged intradermally
with Leischmania tropica. Dieldrin acted synergistically on
lethality in a dose- and time-related manner, indicating an effect
on host mechanisms. It also resulted in decreased antibody
formation to PVP, a T-independent antigen (direct splenic plague
assay). The mitogenic response of cultured T-cells to
phytohaemagglutinin (PHA) in dosed mice was depressed. Mitomycin C
and anti-Thy-1 abolished the mitogenic response. When splenic
T-cells from treated mice were mixed with T-cells from control
mice, there was inhibition of PHA mitogenesis. The data indicated
an active cell-mediated suppressor. A soluble macrophage factor
from the hepatic Kupffer cells (but not from alveolar or peritoneal
macrophages) suppressed the T-cell response to PHA. It was
concluded that administration of 5 mg dieldrin/kg diet to mice for
10 weeks caused a profound impairment of macrophage antigen
processing.
8.8. Toxicity of Photodieldrin and Major Metabolites
The relevance of photodieldrin lies in the fact that it has
metabolites identical to those of dieldrin and is quantitatively
and qualitatively similar in toxicity.
8.8.1. Photodieldrin
The photodecomposition of deposits of dieldrin on leaves and
grass has been reported (Roburn, 1963), and the physical and
chemical properties and structure of this decomposition product
have been determined (Robinson et al., 1966b; Rosen et al., 1966).
It appears to be the pentacyclo isomer of dieldrin (hexacyclo
isomer by the alternative nomenclature used by Rosen et al.
(1966)). Photodieldrin residues were less than the limits of
detection in most of the food samples analysed (Robinson et al.,
1966a).
8.8.1.1 Acute toxicity
Photodieldrin is more acutely toxic than dieldrin for mice,
rats, and guinea-pigs (Table 42). The toxicity for dogs is about
equal to that of dieldrin. Dieldrin-like convulsions have been
observed in all species given photodieldrin.
8.8.1.2 Short-term toxicity
(a) Mouse
When groups of five male and five female Carworth Farm No. 1
mice were fed 1, 3, or 10 mg photodieldrin/kg diet for 1 month, all
animals fed 10 mg/kg and two animals fed 3 mg/kg died. No changes
were observed at necropsy (Brown et al., 1967).
Table 42. Oral LD50 values for photodieldrin
-------------------------------------------------------------------
Species Vehicle LD50 Reference
Photodieldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Mouse dimethyl- 6.8 77.3 Brown et al.
sulfoxide (1967)
Rat dimethyl- 9.6 46.8 Brown et al.
sulfoxide (1967)
Guinea-pig dimethyl- 2.3-3.9 18-30 Brown et al.
sulfoxide (1967)
Dog (male) gelatin 120-160 120 Brown et al.
capsule (1967)
Dog (female) gelatin 80-120 80-100 Brown et al.
capsule (1967)
-------------------------------------------------------------------
(b) Rat
Groups of five male and five female Carworth Farm E rats were
fed 3 or 10 mg photodieldrin/kg diet for one month without apparent
ill effects (Brown et al., 1967).
In studies by Walton et al. (1971), groups of 28 male and
28 female Charles River rats were fed 0, 1, 5, or 25 mg
photodieldrin/kg diet for 3 months. A similar study was carried
out concurrently with dieldrin. The concentrations of
photodieldrin given in the diet were lowered from 25 to 12.5 mg/kg
diet within the first week of the study because of high mortality.
At the end of 3 months, no significant differences were found in
growth or food intake, and no gross evidence of toxicity was
observed. Liver/body weight ratios were increased at 12.5 mg/kg
diet. Increases in the activity or concentration of liver mixed-
function oxidase and microsomal cytochrome P-450 at 5 and 12.5
mg/kg diet indicated the occurrence of a dose-dependent enzyme
induction. The total protein content of the liver was not
affected. The short-term toxicities of photodieldrin and dieldrin
appeared to be similar.
Walker et al. (1971) fed groups of 12 Carworth Farm E rats of
each sex diets containing 0.1, 1, 10, or 30 mg photodieldrin/kg
diet for 3 months. The control group consisted of 24 male and 24
female rats. Six females given 30 mg/kg and two females given 10
mg/kg died. The animals in these groups that survived were
irritable and showed tremors when handled. Growth was reduced,
increases in serum urea and glutamic pyruvic transaminase (SGPT)
activity were seen in females fed 30 mg/kg, and the liver/body
weight ratio was increased in this group. In the groups fed 10 or
30 mg/kg, kidney/body weight ratio was increased in males. At
autopsy, no gross lesions were seen. Some of the animals fed 10 or
30 mg/kg showed CHIRL and centrilobular fatty changes in the liver.
Eosinophilic droplets were seen in the cytoplasm of the proximal
convoluted tubules and in the lumen of affected tubules in the
kidneys of males fed 10 or 30 mg/kg. No evidence of nephron damage
was found. No effects were observed in the animals dosed with 1
mg/kg.
(c) Dog
In a study by Walker et al. (1971), groups of four male and
four female beagle dogs received photodieldrin in olive oil by
capsule (daily oral doses of 0.005, 0.05, or 0.2 mg/kg body weight)
for 3 months. A control group of six males and six females
received olive oil in gelatine capsules. The health, behaviour,
body weight, and haematology were unaffected. In the 0.2 mg/kg
males, increases occurred in the plasma alkaline phosphatase and
SGPT activities, and, after 13 weeks, their serum protein levels
were slightly reduced. Increases in the liver/body weight ratios
occurred in the 0.2 mg/kg animals and the 0.05 mg/kg females. At
autopsy, no pathological changes associated with photodieldrin were
observed. No effects were observed at 0.005 mg/kg body weight.
8.8.1.3 Long-term toxicity
(a) Mouse
In a study on the long-term toxicity of photodieldrin, groups
of 50 B6C3F1 mice of each sex were fed diets containing 0.32 or
0.64 mg/kg for 80 weeks. After 80 weeks, the animals were fed a
control diet for 12 or 13 weeks. Concurrent control groups
consisted of 10 untreated mice of each sex. Pooled controls, used
for statistical evaluation, consisted of the concurrent controls
plus 60 male and female mice from similarly performed bioassays
with six other test chemicals. All surviving mice were killed at
93 weeks. Mean body weights and mortality were not affected by
treatment, but convulsions and hyperactivity were noted in treated
male mice. No statistically significant increase in tumour
incidence was found (NCI, 1977).
(b) Rat
In similar studies to those on mice, groups of 50 Osborne-
Mendel rats of each sex were given 5 or 10 mg photodieldrin/kg diet
for 80 weeks. After 80 weeks, the animals were fed a control diet
until sacrifice at 111 - 112 weeks. Because of neurotoxicity, the
doses in the females were reduced after 30 weeks, so that the time-
weighted average doses were 3.4 or 7.5 mg/kg diet for the females.
Concurrent control groups consisted of 10 rats of each sex. Pooled
controls, used for statistical evaluation, consisted of the
concurrent controls combined with 65 rats of each sex from similarly
performed bioassays with six other chemicals. All surviving animals
were killed at 111 - 112 weeks. Mean body weights and mortality
were not affected by treatment, but convulsions and hyperactivity
occurred in treated male and female rats. Photodieldrin was not
carcinogenic in this study (NCI, 1977).
8.8.1.4 Reproduction, embryotoxicity, and teratogenicity
(a) Mouse
Chernoff et al. (1975) fed groups of pregnant CD-1 mice (16 -
20 per group) photodieldrin in peanut oil (daily oral doses of 0,
0.15, 0.3, or 0.6 mg/kg body weight) from day 7 to day 16 of
gestation. At a dose of 0.6 mg/kg, one animal died. Liver/body
weight ratios were increased in a dose-related manner, but no
significant differences in fetal mortality, litter weight,
percentage of supernumerary ribs, or sternal or caudal ossification
centres were observed at any of the doses used. Photodieldrin was
not teratogenic or fetotoxic in CD-1 mice at doses up to and
including 0.6 mg/kg body weight.
(b) Rat
In a study by Chernoff et al. (1975), groups of 24 - 27
pregnant CD rats were given daily oral doses of photodieldrin in
peanut oil (0, 015, 0.3 or 0.6 mg/kg) on days 7 - 16 of gestation.
Some maternal mortality (5 out of 24 animals) occurred in the
0.6-mg/kg group. No significant differences in liver/body weight
ratios, fetal mortality, weight of the pups, or occurrence of
anomalies in litters of treated animals, compared with the
controls, were noted. No evidence of teratogenicity in CD rats was
observed at doses of photodieldrin up to and including 0.6 mg/kg
per day.
8.8.1.5 Appraisal
The acute oral toxicity of photodieldrin to rodents is greater
than that of dieldrin. In short-term toxicity and teratogenicity
studies, no major differences between the two compounds were found.
Photodieldrin did not induce tumours in mice and rats. The
accumulation of photodieldrin in the adipose tissue of experimental
animals was less than that of dieldrin (section 6.2.3).
8.8.2. Major metabolites of dieldrin
8.8.2.1 Acute toxicity
The acute oral toxicity of the major metabolites of dieldrin is
far less than that of dieldrin itself (Table 43).
8.8.2.2 Short-term toxicity
In a study by Granville et al. (1973), groups of 12 male and 12
female rats (control group of 24 males and 24 females) were fed
diets containing aldrin dicarboxylic acid (0, 0.1, 1, 10, 100, or
1000 mg/kg diet) for 13 weeks. No adverse effects attributable to
the dosing were observed in general health, behaviour, body weight,
clinical chemical and haematological values, organ weights, or on
pathological examination of the viscera.
Table 43. Oral LD50 values for metabolites of aldrin and dieldrin
in mice
-------------------------------------------------------------------
Compound LD50 Reference
(mg/kg body weight)
-------------------------------------------------------------------
trans-6,7-dihydroxy-dihydro- 1250 Korte & Arent
aldrin (1965)
9-hydroxy-dieldrin > 400 Baldwin et al.
(1970)
hexachlorohexahydromethano- > 850 Baldwin et al.
indenedicarboxylic acid (1972)
(aldrin dicarboxylic acid)
-------------------------------------------------------------------
8.9. Mechanisms of Toxicity; Mode of Action
Like most chemicals, aldrin and dieldrin do not have a single
mechanism of toxicity. The main target organs of these chemicals
are the central nervous system and the liver.
8.9.1. Central nervous system
Intoxication following acute or long-term overexposure is
characterized by involuntary muscle movements and epileptiform
convulsions. Survivors, after a short period of residual signs and
symptoms, recover completely (Hoogendam et al., 1962; Avar &
Czegledi-Janko, 1970; Jager, 1970). In rare cases, a residual
brain injury has been reported, but this has been found to be due
to the convulsive state or prolonged cerebral anaemia rather than
to the dieldrin per se. Apparently, a still unidentified receptor
site in the central nervous system is reversibly occupied, and when
this occupation exceeds a certain degree, myoclonics and
convulsions occur (Van Genderen, 1979). In vitro, the dieldrin
metabolite aldrin transdiol appears to be more potent in this
respect that is dieldrin itself (Van den Bercken, 1972; Van den
Bercken & Narahashi, 1974). However, in cats, the aldrin transdiol
appeared to be inactive (Joy, 1977). The mechanism of action seems
to be a presynaptic inhibition as well as an increased release of
an unidentified transmitter (Akkermans, 1974; Akkermans et al.,
1975; Joy, 1976).
Joy (1982) suggested that dieldrin acts by intensifying
synaptic activity through a presynaptic locus of action and
possibly a post-synaptic action as well. Neurons having a large
number of synapses will be affected most. There does not appear
to be any selective action on a particular neurotransmitter or
neurotransmitter system. The modification of behaviour is dose
dependent and performance in complex behavioural tasks is readily
disrupted.
Aldrin and dieldrin and other cyclodiens inhibit the gamma
amino butyric acid (GABA)-induced chloride ion uptake into skeletal
muscles and the binding of tritiated dihydropicrotoxinin (anion
channel probe) to the membrane. This results in central nervous
system excitation and convulsions due to the blocking of GABA
transmitters (Lawrence & Casida, 1984; Abalis et al., 1985).
8.9.2. Liver
The mode of action of aldrin and dieldrin on the liver involves
an increase in the activity of microsomal biotransformation
enzymes, particularly of the monooxygenase system with cytochrome
P-450. This induction of liver microsomal enzymes is reversible
and, if exceeding a certain degree, appears to be associated with
the occurrence of CHIRL and hepatomegaly in the liver of rodents
(sections 6.3 and 8.2) (Jager, 1970; Wright et al., 1972, 1977,
1978).
9. EFFECTS ON HUMAN BEINGS
9.1. General Population Exposure
9.1.1. Acute toxicity - poisoning incidents
When a toxic dose of aldrin or dieldrin has been ingested or
has contaminated the skin, effects appear from 20 min to 24 h
afterwards. Signs and symptoms may include headache, dizziness,
nausea, general malaise, and vomiting, followed by muscle
twitchings, myoclonic jerks, and convulsions. Death may result
from cerebral anoxaemia (Nelson, 1953; Princi, 1954; Hayes, 1957,
1963; Hoogendam et al., 1962, 1965; Kazantzis et al., 1964;
Schafer, 1968; Jager, 1970).
The duration of the interval between oral intake or skin
contact and onset of symptoms (as well as the clinical picture)
depends on the dose absorbed. With massive overexposure,
convulsions may occur even in the absence of any premonitory
symptoms.
Initially, there is no fever or change in blood count or in
blood chemistry. However, later the temperature may be elevated
and leukocytosis may occur. Terminal hyperthermia has been
reported. Abnormal EEG patterns showing spike and dome complexes
and multiple spike and wave discharges, or in less serious
intoxications, bilateral synchronous theta discharges may be seen.
The diagnosis needs to be confirmed by determining the insecticide
concentration in the blood.
The onset of clinical intoxication is practically always acute
also in those cases where the accumulation of dieldrin in the
target tissues has taken place during a much longer period. The
latter cases are, therefore, usually indistinguishable from acute
intoxication. Survivors almost always recover completely (Jager,
1970; Hayes, 1982).
Estimates of dosages in anecdotal cases suggest that fatalities
have occurred with ingestion of approximately 10 mg dieldrin/kg
body weight, (Hayes, 1982) but Hodge et al. (1967) estimate the
lethal dose of aldrin and/or dieldrin for the adult man to be about
5 g.
Cases of poisoning have occurred by ingestion of formulated
material, mostly in children by mistake (for instance when aldrin
is used in granules as bait to control ants) or by adults with
suicidal intent. Several cases of poisoning have been the result
of ingesting food contaminated with aldrin or dieldrin during
storage or transport.
Van Raalte (1965) surveyed the world literature for all cases
of fatal poisoning by aldrin and dieldrin, and found 13 cases:
four suicides, three due to accidental ingestion, five due to
accidental contamination, and only one (a spray operative) due to
occupational exposure. No cases of fatal poisoning have been
reported during the course of aldrin and dieldrin manufacture and
formulation.
A non-exhaustive overview of published poisoning cases is given
in Table 44. A more complete review is provided by Hayes (1982).
Table 44. Case reports on accidental and suicidal acute aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Causative agent Circumstances Reference
of cases
cases
------------------------------------------------------------------------------------------
1 - aldrin emulsifiable attempted suicide Spiotta (1951)
concentrate
53 - aldrin and other consumption of seed grain WHO (1958)
pesticides
13 aldrin and dieldrin review of all fatal cases from Van Raalte (1965)
literature:
- 4 suicides, 3 accidental
ingestion, 5 accidental
contaminations, 1 sprayer
2 1 5% dieldrin accidental ingestion Garrettson & Curley
(1969)
79 consumption of dieldrin- WHO (1977)
contaminated rice in Mali
1 - dieldrin (120 mg/kg) attempted suicide Black (1974)
2 - dieldrin Fry (1964)
12 - aldrin + BHC consumption of seed grain Gupta (1975)
------------------------------------------------------------------------------------------
9.1.2. Effects of short- and long-term exposure - controlled human
studies
9.1.2.1 Accidental poisoning
Twelve cases of neurotoxicity, resulting from the repeated
consumption of wheat into which aldrin dust and gammexane (BHC)
powder had been mixed accidentally, have occurred in India (Gupta,
1975). The patients consumed this wheat for 6 - 12 months before
showing typical clinical symptoms, including convulsions.
Electroencephalographic tracings were consistent with a diagnosis
of organochlorine insecticide poisoning. The patients were treated
with phenobarbital and diazepam. The latter was more effective in
controlling seizures. All patients recovered.
The threshold dieldrin concentration in the blood below which
no adverse effects have been observed (and none are to be expected)
is 105 µg/litre (see also section 9.2.1.1). The dieldrin
concentration in the blood of the general population, in the
countries where this has been investigated, is well below this
threshold level. However, there are rare cases in which it seems
that low concentrations of dieldrin have induced effects.
A rare, well investigated and well reported case of dieldrin-
induced immunohaemolytic anaemia was observed in Iowa, USA. The
patient had a haemolytic anaemia with a positive direct
antiglobulin (Coombs) test and a positive Ham test in the serum.
The serum contained anti-bodies selectively active against
erythrocytes coated with dieldrin. The patient improved following
splenectomy. Dieldrin concentrations in blood and fat were similar
to those of the general Iowa population (Hamilton et al., 1978). A
similar case was reported by Muirhead et al. (1959).
9.1.2.2 Controlled human studies
In section 6.2.2.4, reference was made to a pharmacodynamic
study in human volunteers. This study had three objectives:
(a) to establish the relationship between the daily intake of
dieldrin and its concentration in human blood and adipose
tissue;
(b) to establish the blood/fat ratio in human beings; and
(c) to establish the relationship between the concentrations
of dieldrin in blood and fat and the length of exposure
(Hunter & Robinson, 1967, 1968; Hunter et al., 1969).
In addition, the opportunity was taken to monitor the health of the
human subjects during and after the exposure by full clinical,
physiological, and laboratory examinations as well as full
electroencephalographic (EEG) studies, polygraphic recording of
cardio-respiratory function, measurement of basal metabolic rate,
and electroneuromyographic studies at frequent intervals to detect
the possible occurrence of changes in physiological function. The
study involved 13 adult male college graduates without a history of
recent occupational exposure to pesticides. The subjects received
0, 10, 50, or 211 µg dieldrin per day for 2 years. All the men
continued in excellent health. Clinical, physiological, and
laboratory findings remained essentially unchanged throughout the
whole experimental period of 24 months and the 8 months after
exposure. No departures from what is regarded as normal for the
general population were observed. The concentration of p,p'-DDE in
adipose tissue and blood did not show any significant change during
or after the study, indicating that the liver microsomal enzyme
activity had not been induced. Thus, the total daily intake of
230 µg (211 µg plus intake from food) of dieldrin per person for 2
years had no effect on health. The concentrations of dieldrin in
both adipose tissue and blood were shown to be proportional to the
daily intake (section 6.2.2.4).
9.1.3. Tissue concentrations of dieldrin in hospitalized people
9.1.3.1 Pathological findings
Specimens of human abdominal subcutaneous fat, obtained from
four hospitals in Chicago were analysed for residues of dieldrin.
Dieldrin was not present in 103 out of 221 samples analysed for
this pesticide. Positive samples contained 0.01 - 1.39 mg
dieldrin/kg fat (mean value 0.14 mg/kg). There was no correlation
between the dieldrin concentration in adipose tissue and
pathological findings (Hoffman et al., 1967).
When organochlorine pesticide concentrations were determined in
the adipose tissue and liver of 271 hospital patients in Miami,
USA, patients with typical alcoholic (Laennec's) cirrhosis of the
liver had about twice the dieldrin concentration in the liver of
that found in the normal population. In patients with post-
necrotic cirrhosis, fatty metamorphosis of the liver, metastatic
malignancy of the liver, or primary hepatocellular carcinoma,
dieldrin concentrations were "normal". Terminal cases with
carcinomas of different organs had elevated concentrations of
organochlorine pesticides in the fat, but no association with any
particular neoplastic disease was found (Radomski et al., 1968).
In Hawaii, emaciated patients who had carcinoma and/or focal or
generalized liver pathology were found to have "normal" (for the
USA) concentrations of dieldrin in the liver and body fat (Casarett
et al., 1968).
When concentrations of organochlorine pesticides were
determined in specimens of liver, brain, and adipose tissue from
autopsies of patients with cirrhotic liver disease in Vancouver
(Canada) hospitals, the concentrations of dieldrin appeared to be
no higher than in tissues from controls (Oloffs et al., 1974).
In a case-control study on 122 matched cancer patients in south
Florida, USA, a comparison was made of dieldrin residues in the
adipose tissue of cancer patients and controls. The mean dieldrin
concentration in the adipose tissue was 0.3 mg/kg fat in both
cancer patients and controls (Davies et al., 1975).
9.1.3.2 Influence of weight loss and stress on dieldrin
concentrations in tissues
It is well known that, in birds, and perhaps in some small
mammals, dieldrin intoxication may be induced by starvation, weight
loss, or stress in animals having a previously harmless body burden
of dieldrin. Concern is sometimes expressed that, by analogy to
these observations, a similar course of events might occur in human
beings.
Twenty-nine patients (14 males, 15 females) undergoing surgery
were investigated. The concentrations of dieldrin in the blood
were unaffected by the catabolic responses to surgery. In another
study, these authors determined the concentrations of dieldrin in
the blood of 4 women undergoing voluntary near-starvation for
slimming purposes, which resulted in weight losses of up to 7.5
kg/week. There was no increase in the concentration of dieldrin in
the blood (Hunter & Robinson, 1968).
No significant difference was found between the dieldrin blood
concentrations of slimming or non-slimming mothers before and after
delivery (Eckenhausen et al., 1981).
On the basis of these results and calculations, it is suggested
that significant weight loss does not result in increased
concentrations of dieldrin in human tissues (Van Raalte, 1965;
Hunter & Robinson, 1968).
9.1.4. Exposure in treated homes
From the data on aldrin concentrations in the air of houses
treated for termite control (section 5.1.2), an estimate of the
dieldrin concentration in the blood of occupants of these houses
can be made using the mathematical formulas given by Hunter et al.
(1969) for deriving blood concentrations from the average daily
intake. The average daily intake is based on an estimated average
in-house volume of air inhaled per day (15 m3). The dieldrin blood
levels of home dwellers, calculated in this way, remain far below
the blood concentration no-effect level for the general population
(secton 9.2.1.1).
The blood dieldrin concentrations of 59 female residents of
Dade County (Florida, USA), where many houses had been treated for
termite control, were of the order of 1 µg/litre (Barquet et al.,
1981).
Also relevant to the health of home dwellers is the experience
obtained in the 1950s and 1960s when tens of thousands of houses in
more than 30 countries were sprayed with dieldrin for malaria and
yellow fever eradication. Although exposures were presumed to have
been high, as a result of surface spraying inside and outside
houses, no adverse health effects were reported in home dwellers.
Neither were adverse effects observed in well-trained and
medically-supervised spray operators (Soper, 1955 (Personal
communication at the 2nd Meeting of the Industrial Council on
Tropical Health, Boston); Fletcher et al., 1959).
9.2. Occupational Exposure
9.2.1. Acute toxicity - poisoning incidents
With the exception of poisoning cases resulting from massive
acute overexposure, most reported cases of poisoning with aldrin
and dieldrin in occupationally-exposed men have been the result of
a slow build-up of the insecticide in the body, the daily intake
exceeding the daily excretion (Jager, 1970; Hayes, 1982).
Based on the experience of Jager (1970), it was suggested that
the classification of types of intoxication by Hayes (1963) be
modified as follows:
Type 1: an acute convulsive intoxication with no (or only
minor) prodomi, resulting from one or several gross overexposures.
Type 2: a greater number of smaller doses may cause an
accumulative intoxication. Clinically, this results in a syndrome
of headache, dizziness, drowsiness, hyperirritability, general
malaise, nausea, anorexia, occasional vomiting. At times muscle
twitchings, myoclonic jerks and convulsions may occur. In these
circumstances minor increases in the insecticide level in the
blood, perhaps caused by minor fluctuations in exposure, may bring
about a convulsive intoxication.
Type 3: this is actually a combination of Types 1 and 2. In
this type an overexposure, in itself not significant, causes an
acute convulsive intoxication superimposed upon a subclinical
accumulative intoxication of Type 2.
These three types of intoxication are schematically illustrated
in Fig. 3.
The other metabolites, the chemical identities of which have
been rigorously established, are detailed below.
(a) The trans-6,7-dihydroxy compound (Fig. 2, compound IV) is
formed by the hydration (formal) of the epoxide ring of dieldrin
(Korte & Arent, 1965). This compound is a major metabolite in
rabbit urine, but of relatively minor importance in other species.
The formation of the cis-diol by rat microsomes has been
demonstrated, together with its epimerization to the trans-diol
(McKinney et al., 1973). Both the cis- and trans-diols have been
synthesized (Korte & Arent, 1965; Chau & Cochrane, 1970b; Bedford &
Harrod, 1972b).
(b) The dicarboxylic acid (Fig. 2, compound V) is derived from
the dihydroxy metabolite (Baldwin et al., 1972; Oda & Mueller,
1972). This compound has also been synthesized (Buechel et al.,
1966), and has been shown to undergo further degradation (formation
of two isomers of a monodechlorinated derivative) after intravenous
injection into male and female rats (Lay et al., 1975).
(c) The bridged pentachloroketone (PCK) (Fig. 2, compound VII)
is mainly found in the urine and kidneys of male rats, but, even in
rats, it is a minor metabolite (Damico et al., 1968; Klein et al.,
1968; Richardson et al., 1968). In other species, it is a very
minor metabolite of dieldrin. It is also a metabolite of
photodieldrin (Klein et al., 1970), and has been synthesized
(Bedford & Smith, 1978).
The Chemical Abstract or Von Baeyer AG/IUPAC names of aldrin,
dieldrin, photodieldrin, and metabolites are given in Appendix I.
Methods for the quantitative determination of the four
metabolites are available. They depend on the availability of
authenticated analytical standards (Ludwig & Korte, 1965;
Richardson, 1971; Baldwin et al., 1972).
6.3.1.2 Human studies
A metabolite of dieldrin detected in human faeces has been
shown to be the 9-hydroxy derivative (Richardson & Robinson, 1971).
6.3.1.3 Non-domestic organisms
The conversion of aldrin to dieldrin was studied in algae
( Chlorella and diatoms) and protozoa (Dinoflagellates and mixed
protozoa) after exposure for 24 h to 0.1 mg aldrin/litre. The
amount of dieldrin present in the cultures was of the order of
0.06 - 0.2 µg/litre). The amounts of dieldrin were greater in the
protozoa than in the algae; it was concluded that these planktonic
species have enzyme systems that epoxidize aldrin (Khan et al.,
1972b).
The conversion of aldrin to dieldrin in 12 species of fresh-
water invertebrates has been compared. Ten species were exposed
for 2 h to concentrations of 0.1 or 0.25 mg aldrin/litre, and two
species of molluscs were exposed to 0.25 mg aldrin/litre for 4 h.
The concentrations of dieldrin relative to aldrin in the whole
bodies of eight species from four phyla (Coelenterata,
Platyhelminthes, Annelida, and Arthropoda) were in the range
1.03 - 8.48%. In two species of Insecta (dragon fly nymphs and
Aedes larvae), the values were 24.9% and 42.4%, respectively. The
two species of molluscs had dieldrin concentrations (relative to
aldrin) of 17 - 19% (Khan et al., 1972b).
In a study on an ostracod (Chlamydotheca arcuata) exposed to
14C-labelled aldrin (5.5 - 11.2 µg/litre), aldrin was readily
converted to dieldrin, 83% conversion occurring within 24 h. The
elimination of aldrin and dieldrin appeared to involve both passive
and active processes, and it was concluded that dieldrin was
eliminated more rapidly after dieldrin exposure than after aldrin
exposure (Kawatski & Schmulbach, 1972).
A number of in vitro studies have been carried out concerning
the influence of these insecticides on mixed-function oxidase
activity. The epoxidation of aldrin to dieldrin by this enzyme
system has been demonstrated in crayfish (Cambarus) (Khan et al.,
1972a,b), in snail (Lymnea palustris) and clam (Khan et al.,
1972b), and in midge larvae (Chironomus riparius) (Estenik &
Collins, 1979).
The conversion of aldrin to dieldrin by lobsters (Homarus
americanus) was reported by Carlson (1974).
The mixed-function oxidase activities in five species of fresh
water fish, as measured by the conversion of aldrin to dieldrin,
were investigated by Ludke et al. (1972). The fish were exposed to
aldrin (50 µg/litre) for 4 h, and the concentrations of aldrin and
dieldrin in the liver were determined. Contrary to earlier
reports, the conversion by epoxidation of aldrin to dieldrin in
fish may be the rule rather than an exception.
The epoxidation of 14C-aldrin to dieldrin in susceptible and
resistant mosquitofish (Gambusia affinis) has been investigated.
The fish were exposed to 5 µg 14C-aldrin/litre for 4 or 8 h and the
concentration of aldrin and dieldrin in liver and brain were
determined. The concentration of dieldrin (expressed in terms of
protein content) was significantly higher in the livers of
resistant fish than in susceptible fish). It was concluded that
resistant mosquitofish convert aldrin to dieldrin and/or water-
soluble compounds at a greater rate than susceptible mosquitofish
(Wells et al., 1973).
In studies by Addison et al. (1976), Atlantic salmon fry (Salmo
salar) were injected intramuscularly with 14C-aldrin, to initial
whole-body concentration of 5 mg/kg. The fish were maintained in
flowing fresh water, and were removed at five intervals up to 56
days for the measurement of whole-body residues. The time required
for 50% epoxidation of aldrin was between 1 and 2 days. Less than
10% of the radioactivity remained in the fish at the end of the
exposure. It was concluded that there was rapid elimination either
of unchanged aldrin or its epoxide, dieldrin, from the fish.
6.3.2. Photodieldrin (and major metabolites of dieldrin)
6.3.2.1 Rat
Besides unchanged photodieldrin, bridged pentachloroketone
(PCK) (Fig. 2, compound VII), a metabolite of photodieldrin, was
isolated from the brain, liver, adipose tissue, and blood of rats
(Carworth Farm, type E) fed diets containing 10 or 30 mg
photodieldrin/kg for 13 weeks (Baldwin & Robinson, 1969).
In studies by Klein et al. (1970), Osborne-Mendel rats were
given 14C-photodieldrin, orally or intraperitoneally, 5 days/week,
for 12 weeks, and urine was collected quantitatively every day. A
metabolite was found in the urine of male rats and shown to be PCK.
Small amounts of other (unidentified) more polar urinary
metabolites were also present.
6.3.2.2 Monkey
Metabolites were detected in the urine and faeces of a female
rhesus monkey given daily oral doses of 0.8 mg 14C-photodieldrin/kg
body weight for 175 days. Two metabolites were identified in the
urine: the trans-diol (Fig. 2, compound XI) and its glucuronide
conjugate. A faecal metabolite was tentatively identified as the
diol. A third metabolite was present in both urine and faeces, and
it was suggested that this might be a monohydroxy derivative of
photodieldrin (Nohynek et al., 1979).
6.4. Elimination and Excretion
6.4.1. Aldrin
6.4.1.1 Rat
When male rats were given daily oral doses of 4.3 µg 14C-aldrin
(equivalent to about 0.2 mg aldrin/kg diet) for 3 months, the
radioactivity in the urine increased from about 2% of the dose of
aldrin during the first week to about 10% during the 12th week. In
the faeces, the excreted radioactivity increased from about 48%
during the first week to about 93% during the 12th week. After
about 8 weeks, a saturation level was reached (i.e., there was a
balance between the rates of intake of aldrin and excretion of
aldrin plus aldrin-related materials). Extracts of urine and
faeces were examined by paper chromatography. Because the urine
was probably contaminated by faeces in the metabolism cages, only
the trend is given. In both faeces and urine, the aldrin content
decreased during the 12 weeks. The hydrophilic metabolites
increased, reaching 75% (faeces) and 95% (urine) of total
radioactivity after 12 weeks. The level of dieldrin was more or
less constant (Ludwig et al., 1964).
6.4.2. Dieldrin
6.4.2.1 Laboratory animals
As described in section 6.2.2.1, Hutson (1976) studied the
comparative metabolism of dieldrin in CFE rats and two strains of
mice after a single oral dose of 3 mg/kg body weight 14C-dieldrin.
The excretion of 14C activity in the faeces of the rats was 62.4%
of the administered dose in the non-pretreated group and 69% in the
dieldrin-pretreated group. In the case of the CF1 mice, the
pretreatment period did not have any effect on the faecal excretion
(51.5%), whereas, in the LACG mice, the faecal excretion of 14C
activity increased from 27.2% for the non-pretreated group to 48.8%
for the 4-week pretreated group. The total 14C activity excreted in
the urine of the two strains of mice was low (0.42 - 2.6% of dose)
compared with that in the urine of male rats (5.5 - 6.6%). In both
species of rodents, the faeces was the major route of excretion of
14C activity. In the urine of both the male CFE rat and the male
CF1 mice, the amount of the dicarboxylic acid metabolite in the
urine was small compared with that of pentachloroketone plus
dieldrin, while in the male LACG mice, the amount of the acidic
metabolite was twice that of pentachloroketone plus dieldrin. Both
strains of mice excreted, proportionally, much larger amounts of a
polar (unidentified metabolite) in the urine than did the CFE rats.
In the faeces of the male CFE rats (no pretreatment), the major
component was the 9-hydroxy derivative. This was also found by
Matthews et al. (1971). However, in both mouse strains (no
pretreatment), this compound was a minor metabolite, but it became
the major product in the dieldrin-pretreated group. In isolated
liver microsomes, most of the 14C activity appeared to be present
as dieldrin, and the 9-hydroxy metabolite was not detected.
A number of other studies on the excretion of dieldrin via
urine and/or faeces have been carried out. Dailey et al. (1970)
found that male rats excreted higher levels of 14C radioactivity
via urine and faeces than females. Davison (1973) confirmed this
in a study lasting 39 weeks. Maximal excretion of 14C activity
occurred in the 6th week in both sexes, regardless of the amount of
dieldrin given. A steady state was reached and maintained from the
6th to the 39th week.
In studies by Robinson et al. (1969), rats were fed a diet
containing 10 mg dieldrin/kg diet for 8 weeks. The decline in the
concentration of dieldrin in blood, brain, liver, and adipose
tissue was studied during the subsequent 12 weeks when a control
diet was fed. There was an initial rapid decline in the dieldrin
concentration during the first 10 days of the post-exposure period
in the blood, liver, and brain, followed by a slower decline. The
changes in the concentration of dieldrin in the brain, adipose
tissue, blood, and liver corresponded to biological half-lives of 3
to about 10 days.
When male and female rats were administered 3 g of diet
containing 10 mg 14C-dieldrin/kg diet, followed by a control diet
ad libitum, 14C activity in the kidneys of male rats was 10-fold
higher than in the female rats (the animals were killed 9 days
after administration of 14C-dieldrin). Most of the activity in the
male kidneys was due to pentachloroketone, whereas, in the female
kidneys, only dieldrin was detected (Matthews et al., 1971).
The excretion of 36Cl activity by female rats dosed
intravenously (680 µg/h for 2.5-5 h; total doses of 8 - 16 mg/kg
body weight) with 36Cl-dieldrin has been studied. The 36Cl
activity detected in the faeces was about 7 times that found in the
urine, indicating excretion via the bile (Heath & Vandekar, 1964).
Comparable results were found by Cole et al. (1970), who gave
male rats a single intravenous dose of 0.25 mg 14C dieldrin/kg body
weight. Similar doses of 14C-dieldrin were administered
intravenously to male rats with bile fistulas. About 30% of the
administered 14C activity was excreted via the bile during the
first 24 h after dosing, and after 4 days a total excretion of
about 60% had occurred. Isolated perfused rat liver preparations
were also investigated; some 20% of the original perfusate dose was
collected in the bile over a period of 8 h.
Rapid excretion of 14C-dieldrin (or its metabolites) from
isolated perfused rat livers via the bile of rats has also been
reported by Klevay (1970), the rate of excretion by male rats being
about 3 times as rapid as that by female rats.
In studies by Mueller et al. (1975a), mice, rats, rabbits,
rhesus monkeys, and one chimpanzee were given a single oral dose of
0.5 mg/kg body weight 14C-dieldrin, and urine and faeces were
collected for 10 days. For all species except the rabbit, the main
route of excretion was the faeces. The faecal excretion of
unchanged dieldrin was high in the first 48 h and then declined
rapidly. The urine samples contained only metabolites of dieldrin.
The mean total amount of radioactive material excreted (males
and/or females) in faeces and urine within 10 days after dosing
(expressed as percentage of administered dose) was 37% in mice, 11%
in rats, 2% in rabbits, 20% in rhesus monkeys, and 6% in the
chimpanzee. In all five species, 9-hydroxy-dieldrin and
4,5-aldrin- trans-dihydrodiol were the major metabolites. The
metabolism in the rat seems to be comparable to that of primates;
however, mice and rabbits showed the opening of the epoxide to diol
as the predominant reaction.
6.4.2.2 Human studies
The occurrence of a neutral metabolite of dieldrin in human
urine in amounts indicative of exposure to aldrin/dieldrin was
reported by Cueto & Hayes (1962) and Cueto & Biros (1967).
Quantitative estimates of the amounts of a metabolite of
dieldrin, 9-hydroxy-dieldrin, in the faeces of seven workmen
occupationally exposed to aldrin/dieldrin and five male members of
the general population have been made. The average concentration
of the 9-hydroxy derivative in 24-h collections of faeces of the
seven workmen was 1.74 mg/kg (range, 0.95 - 2.80 mg/kg), whereas
the average concentration in faeces of the five members of the
general population was 0.058 mg/kg (range, 0.033 - 0.12 mg/kg).
Dieldrin was present in the faeces of the workmen (average
concentration, 0.18 mg/kg), but, in samples from the general
population, it was below the limit of detection. Examination of
the urine of five of the workmen indicated that this route of
elimination of dieldrin and four known metabolites was minor. It
was concluded that the 9-hydroxy-dieldrin in the faeces represented
the major excretory pathway of dieldrin from male human beings. It
should be noted, however, that the urine was not examined for
glucuronide or other conjugates of the hydroxy metabolites). There
was good correlation between the estimated daily intake of dieldrin
(calculated from the concentrations of dieldrin in the blood) and
excretion in faeces of total equivalent dieldrin (Richardson,
1971). This relationship is based on a number of assumptions, and
it is probably more relevant that the concentration of the
9-hydroxy-dieldrin in the faeces (produced by the metabolism of
absorbed dieldrin) is significantly related to the concentration of
dieldrin in the blood, which is a measure of the body burden
arising from absorption of aldrin plus dieldrin.
When 14C-Dieldrin was applied in acetone (4 µg/cm2) once to the
forearm of volunteers, 7.7% of the applied 14C activity was
excreted in the urine over a 5-day period. A single intravenous
injection of 14C-dieldrin resulted in 3.3% being excreted in the
urine over a 5-day period (Feldman & Maibach, 1974).
6.4.3. Photodieldrin (and major metabolites of dieldrin)
6.4.3.1 Rat
In studies by Dailey et al. (1970), young rats were given daily
doses of 5 µg 14C-photodieldrin, orally or intraperitoneally, for
12 weeks. Urine and faeces were collected daily and pooled in
weekly groups. The excretion of 14C activity via the urine of
females was considerably less than that by males, by either method
of dosing. The 14C activity in urine after oral and ip
administration increased slowly during the 12 weeks (males about
10% and females 5%), the highest levels in urine (up to 33%) being
found in males dosed intraperitoneally. Faecal excretion of 14C
activity was initially lower in females, but greater during the
latter half of the study (of the order of 20 - 40%). In males,
during the whole study, it was about 30%.
6.4.3.2 Monkey
A juvenile female rhesus monkey was given daily oral doses of
2 mg 14C-photodieldrin (equivalent to 0.8 mg/kg body weight), and
the treatment was continued until, between days 70 and 76, the
daily excretion of 14C activity was in balance with the daily
intake. When dosing ceased, the animals had retained about 50% of
the cumulative dose of photodieldrin. Collection of excreta was
continued for a further 100 days, during which a further 30.1% of
the dose, administered during the 76-day period, was excreted.
During the period of dosing, a major part of the faecal 14C
excretion consisted of photodieldrin (probably indicating
incomplete absorption in the gastrointestinal tract), while
20 - 50% of the excreted activity was in the urine. After dosing
ceased, 60% of the excreted 14C activity appeared in the urine
(Nohynek et al., 1979).
In studies by Nohynek et al. (1979), one male and one female
juvenile rhesus monkey were given single intravenous doses of
4.5 mg 14C-photodieldrin (2 mg/kg body weight). Urine and faeces
were collected separately every 24 h, and the animals were killed
after 21 days. Excretion of 14C activity was high during the first
7 days (male, 39%; female, 27.3%, of the given dose). It then
decreased rapidly and reached a nearly constant value of 0.2% of
the administered dose. Approximately 45% (male) and 34% (female)
of the dose had been excreted by day 21.
6.5. Retention and Turnover
6.5.1. Non-domestic organisms
A few studies have been carried out on the uptake and
elimination of aldrin and/or dieldrin in invertebrates: marine
clams (Mya arenaria and Mercenaria mercenaria) (Butler, 1971);
naiad mollusc (Amblema plicata) (Fikes & Tubb, 1972); mussel
(Lampsilis siliquiodea) (Bedford & Zabik, 1973); crab (Leptodius
floridanus) (Epifanio, 1973); and ostracod (Chlamydotheca arcuata)
(Kawatski & Schmulbach, 1972). The concentration of aldrin or
dieldrin in organs and tissues increased rapidly during the first
1 - 2 weeks of exposure, but remained virtually constant
thereafter. When the organisms were placed in clean water, the
concentration declined in a (semi)-logarithmic manner in relation
to time. The estimated half-life for the tested organisms varied,
e.g., for Lampsilis siliquiodea, it was 4.7 days, whereas for
Amblema plicata, it was about 3 - 4 weeks.
The elimination of 14C-dieldrin from bluegills (Lepomis
macrochirus) and goldfish (Carassius auratus) was studied by
Gakstatter & Weiss (1967). The fish were exposed to 30 µg
14C-dieldrin/litre (initial concentration) until toxic symptoms
appeared (5 - 8 h), and were then placed in recovery aquaria
together with unexposed fish. The water in the recovery aquaria
was continuously renewed. Samples of five fish were taken on 10
different occasions during the recovery period. The 14C activity
in whole fish of both species, expressed as equivalent dieldrin,
declined by about 90% within 16 days, the half-time for elimination
being about 4 days. The control bluegills and goldfish accumulated
a maximum equivalent dieldrin concentration of 0.29 and 0.22 mg/kg,
respectively, on day 4 of the period in the recovery aquaria,
indicating transfer of dieldrin or derived material from
contaminated to uncontaminated fish.
In another study, the distribution of aldrin and dieldrin in
the tissues of Carassius auratus was determined following an 8-h
exposure to 14C-aldrin (50 µg/litre) in a static study. After the
exposure, fish were placed in a continuously flushed aquarium for
32 days. Dieldrin was found in all tissues examined immediately
after the exposure. The percentage of dieldrin in the total
residues in the tissue increased with time, reaching about 95% on
day 32 (except in visceral fat). During the recovery period, the
total concentration of aldrin plus dieldrin in the blood declined
from 2.1 mg/litre (as aldrin) to 0.4 mg/litre. The corresponding
changes in the brain concentrations were 5.45 mg/kg to 2.3 mg/kg.
Total residues in the nerve cord did not show a consistent decline
and varied from 4.56 to 21.6 mg/kg throughout the 32-day period;
however, these residues were determined by thin-layer
chromatography, not by gas-liquid chromatography (Gakstatter,
1968).
The partitioning of 14C activity into particulate fractions of
the brain and liver of resistant and susceptible mosquitofish has
been studied after exposure of the fish to 14C-aldrin or 14C-
dieldrin. The 14C activities in total brain, cell membrane, and
five cellular fractions were significantly higher in susceptible
fish than in resistant fish for both aldrin and dieldrin. However,
this difference was much less marked in the case of the liver. It
was suggested that a basic structural change in polarity exists in
the myelin of resistant fish, which could provide a membrane
barrier (Wells & Yarbrough, 1973).
The fate of dieldrin in the digestive tract of juvenile lake
trout (Salvelinus namaycush) has been studied. Macerated trout
flesh containing an average of 1.05 mg/kg was injected in the
stomach. The decline in the dieldrin content of the stomach was
parallel to that of the food from the stomach. Little or no
dieldrin was found in the intestines (Stewart & Stein, 1974).
In studies by Chadwick & Brocksen (1969), groups of sculpins
(Cottus perplexus) were exposed to 1.3 µg dieldrin/litre for 12
days, followed by removal to uncontaminated continuously renewed
water. The concentration in whole fish declined in a curvilinear
fashion from about 2.5 mg/kg fish to about 1 mg/kg fish in 60 days
and to about 0.5 mg/kg fish in 90 days.
Sailfin molly (Poecilia latipinna) were exposed to 12 µg
dieldrin/litre for up to 6 h by Lane et al. (1970). Two products,
thought to be metabolites of dieldrin, were detected in the liver
and other organs, and it was suggested that they were partially
dechlorinated derivatives of dieldrin.
6.5.2. Biological half-life in human beings
The concentration of dieldrin in the blood of volunteers given
oral daily doses for 2 years (section 6.2.2.4) was determined over
a period of 8 months after termination of the deliberate exposure
(Hunter et al., 1969). A small, but statistically significant,
decline occurred, corresponding to a mean value of 369 days for the
half-life of dieldrin in blood. However, there were significant
differences between the rates of decline of the individual
volunteers.
The concentration of dieldrin in the blood of 15 workmen was
determined for a period of 3 years following termination of
occupational exposure to aldrin/dieldrin (Jager, 1970). The mean
half-life was 266 days.
When a state of equilibrium has not yet been reached, the
apparent half-life will be much shorter, due mainly to a
redistribution of dieldrin between compartments in the body.
6.5.3. Body burden and (critical) organ burden; indicator media
Whatever the route of exposure, the effect, if any, will be
determined by the concentration of the chemical in the target organ
or tissue. It has been shown that the distribution between the
various tissues of mammals is fairly constant within and between
species (Robinson & Hunter, 1966; Hunter & Robinson, 1967; Hunter
et al., 1967; Robinson & Roberts, 1969; Walker et al., 1969b).
Thus, at a state of equilibrium, the dieldrin level in the blood
reflects the concentration of the active compound in the target
tissues and therefore represents the best practical parameter for
the internal exposure that is associated with a biochemical,
clinical, or pathological effect. Since the biological half-life
of dieldrin in human blood is known (266 days) (Jager, 1970),
a reliable estimation of the blood level at the time of
discontinuance of the exposure can be made. This, in turn enables,
better than anything else, the evaluation of the likelihood of an
observed symptom of disease or indisposition being associated with
exposure to dieldrin. Also, the established mathematical
relationship between the dieldrin level in the blood and the total
daily equivalent oral intake thus enables, on the basis of the
concentration of dieldrin in the blood, the evaluation of a current
exposure or an exposure of a short time ago vis-à-vis the
acceptable daily intake established by the FAO/WHO Joint Meeting on
Pesticide Residues.
Determination of the dieldrin concentration in blood is the
method of choice in monitoring exposed workers or the general
population (section 9.2.1.1).
6.6. Appraisal
Aldrin is readily absorbed through the skin, by inhalation of
the vapour, or into the circulating blood from the gastrointestinal
tract. It has not been possible to determine the percentage of an
ingested dose of aldrin or dieldrin that is actually absorbed into
the body because of the intestinal hepatic biliary cycle. Work
with human volunteers (Feldmann & Maibach, 1974) showed that
absorption through the skin amounted to 7 - 8% of the applied dose.
Inhalation studies with human volunteers (Beyermann & Eckrich,
1973; Bragt et al., 1984) suggested that about 50% of inhaled
aldrin vapour is absorbed and retained in the human body. After
absorption, it is rapidly distributed to the organs and tissues of
the body, and a continuous exchange between the blood and other
tissues takes place. In the meantime, aldrin is readily converted
to dieldrin, mainly in the liver but, to a much lesser extent, in
some other tissues, e.g., the lungs (Mehendale & El-Bassiouni,
1975).
This conversion proceeds very rapidly. The livers of even
24-h-old rats, given oral doses of 10 mg aldrin/kg body weight,
contained dieldrin 2 h after treatment (Farb et al., 1973). In the
course of the next few hours, dieldrin and what little is left of
the aldrin in blood and other tissues, concentrates more in the
lipid tissues (Heath & Vandekar, 1964; Hayes, 1974). In human
beings, aldrin is found rarely, if at all, in human blood or other
tissues, except in cases with acute poisoning by accidental or
intentional ingestion of massive doses.
Studies carried out with 14C-labelled aldrin and dieldrin have
shown that part of the ingested material is passed unabsorbed
through the intestinal tract and eliminated from the body, part is
excreted unchanged from the liver into the bile, part is stored
unchanged in the various organs and tissues (particularly the
adipose tissue), and part is metabolized in the liver to more polar
and hydrophilic metabolites. These metabolites, in human beings
and most animals, are excreted primarily via the bile in the
faeces. It had also been shown that aldrin and dieldrin are both
biodegraded into the same metabolites (Damico et al., 1968; Klein
et al., 1968). The biodegradation products have been identified in
the rat within 15 min after an intravenous injection (Moersdorf et
al., 1963). Most of the currently available information on the
biodegradation metabolism in mammals is based on studies with
dieldrin on the mouse, rat, rabbit, sheep, dog, monkey, chimpanzee,
and human beings (Ludwig et al., 1964; Datta et al., 1965; Korte,
1965; Korte & Arent, 1965; Richardson et al., 1967b, 1968; Klein et
al., 1968; Matthews & Matsumura, 1969; Baldwin et al., 1970, 1972;
Feil et al., 1970; Richardson & Robinson, 1971; Mueller et al.,
1975a,b). Although there appear to be differences between species
and, in the rat, differences between the sexes, the overall picture
shows only quantitative variations between species.
In the species studied (with the exception of the rabbit) the
major metabolite is the 9-hydroxy derivative. This is found in the
faeces and free or conjugated in the urine. Three other
metabolites have been found and identified in experimental animals:
(a) trans-6,7-dihydroxy derivative;
(b) dicarboxylic acid derived from the dihydroxy compound; and
(c) the bridged pentachloroketone (PCK).
The latter is also a metabolite of photodieldrin (Klein et al.,
1970).
Only the 9-hydroxy compound was found in the faeces of seven
occupationally exposed industrial workers (1.74 mg/kg) and five
male members of the general population (0.058 mg/kg). Neither the
9-hydroxy compound nor the other metabolites have been found in
human blood or other tissues. Dieldrin was present in the faeces
of the workmen (average 0.18 mg/kg), whereas the concentrations in
the samples from the general population were below the limits of
detection. Examination of the urine of five workmen indicated that
urinary excretion of dieldrin and its four metabolites is minor
relative to elimination of the 9-hydroxy metabolite via the faeces
(Richardson, 1971).
The conversion of aldrin to dieldrin and the distribution and
the subsequent deposition of dieldrin (mainly in lipid tissues)
proceed much faster than the biodegradation and ultimate
elimination of unchanged dieldrin and its metabolites from the
body. At a given average intake of aldrin and/or dieldrin,
dieldrin slowly accumulates in the body. This accumulation or
"storage", however, does not increase indefinitely. As the
concentration of dieldrin in the liver cells increases, the
metabolizing enzyme activity in the microsomes increases, and so
the rate of biodegradation of dieldrin, and hence the elimination
from the body, is enhanced. Thus, the accumulation proceeds at an
ever slower rate until the concentrations of dieldrin in blood and
tissues approach upper limits of storage and an amount of dieldrin
equal to the average daily intake is eliminated each day. These
upper limits of storage are related to the daily intake. This has
been demonstrated in rats and dogs (Walker et al., 1969b) and in
human beings (Hunter & Robinson, 1967; Hunter et al., 1969). When
the intake of aldrin/dieldrin ceases or decreases, the body burden
decreases. The biological half-life in human beings is 9 - 12
months (Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
Significant relationships exist between the concentrations of
dieldrin in the blood and those in other tissues of rats, dogs, and
human beings (Hunter & Robinson, 1967; Deichmann et al., 1968;
Keane & Zavon, 1969b; Hunter et al., 1969; Walker et al., 1969b).
Numerous investigations of the concentrations of dieldrin in
body fat, blood, and other tissues from members of the general
population and from special groups have been carried out in several
countries. The results are summarized and discussed in section
5.2. The ratio of dieldrin concentrations in fat, liver, brain,
blood is about 150:15:3:1.
Dieldrin penetrates the placenta and is present in the blood,
fat, or other organs of the fetus, newborn babies, and infants
(Table 22). The concentrations are much lower (by 50% or more)
than those in adults. The ratio of dieldrin concentrations in
blood, brain, liver, and fat in infants is not different from that
ratio in adults (Fiserova-Bergerova et al., 1967; Casarett et al.,
1968). Dieldrin is excreted in mother's milk, average values being
about 3 - 5 µg/litre mother's milk (Table 23). The ratio of the
dieldrin concentration in mother's blood to that in mother's milk
is about 1:2 - 3.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Microorganisms
Neither aldrin nor dieldrin have significant effects on
populations of microorganisms in soil or fresh water at realistic
concentrations. Some physiological processes of microorganisms are
affected by low concentrations of both aldrin and dieldrin, but
these would appear to have little or no environmental significance.
Aldrin and dieldrin have only minor deleterious effects on soil
bacterial populations, even at concentrations that are much higher
than those used in agricultural practice.
The effects of insecticides on soil microbes have been reviewed
by Tu & Miles (1976). Of 15 strains tested, aldrin did not have
any effect on the growth of 11 bacterial species (single cultures)
but caused some growth inhibition in four species. Dieldrin did
not have any effect on 13 bacterial species but had some inhibitory
effect in two species. Neither aldrin nor dieldrin at 2000 mg/kg
soil had effects on bacteria in laboratory studies; soil fungi were
also little affected. In pot studies, using aldrin at 4 and 120
mg/kg soil, there were no quantitative changes in bacteria during
any part of the vegetative period. Aldrin inhibited the growth of
Rhizoctonia solani in plate cultures by 20% or more at 6.2 mg/litre
and higher concentrations. Dieldrin was less toxic, producing an
average inhibition of about 15%, which was not dose related over
the range of 1 - 100 mg/litre. The evolution of carbon dioxide
(CO2) (a measure of soil organisms respiration) was significantly
reduced by dieldrin at 1000 mg/kg soil (but not at 100 mg/kg),
whereas aldrin produced a significant reduction at concentrations
as low as 25 mg/kg soil. Slight effects on nitrification were
initially found when aldrin and dieldrin were incorporated at 2000
mg/kg in a sandy loam soil, but nitrification was normal after
about 10 weeks. Short-term inhibition of nitrification was also
produced by aldrin and dieldrin at 25 mg/kg in a sandy loam soil
(aldrin for 1 week, dieldrin for 2 weeks). Decreased sulfur
oxidation was observed in soil containing aldrin or dieldrin (2000
mg/kg), the inhibition decreasing considerably after 3 months.
Five annual applications of aldrin or dieldrin (5.5 - 22 kg/ha) to
a Ramona sandy loam had no measurable effect on the numbers of soil
bacteria or fungi, did not influence the ability of the soil
population to decompose plant residue, and did not alter soil
aggregation.
The effect of dieldrin on the activities of three soil enzymes
was determined at concentrations of 5 or 10 mg dieldrin/kg soil by
Tu (1981). The dehydrogenase activity of the dieldrin-treated soil
(10 mg/kg) did not differ from controls, whereas at 5 mg
dieldrin/kg, the activity was significantly greater than controls
after 2 weeks (50% increase). Urease activity at both treatment
levels was significantly reduced after a 1-week incubation but
significantly increased after 2 weeks. Phosphatase activity was
significantly reduced at 5 mg dieldrin/kg, but not at 10 mg/kg.
The 96-h EC50 (growth) for algae (Chlamydomonas sp.,
Phaeodactylum tricornutum, Dunaliella sp., Chlorella ovalis, and
Chlorella pyrenoidosa) was > 100 µg dieldrin/litre (Adema & Vink,
1981).
The photosynthetic activity of four species of marine
phytoplankton in the presence of dieldrin was investigated using
14C-labelled Na2CO3. A range of nominal concentrations
(0.01 - 1000 µg/litre) was used, and the plant cultures were
exposed for 24 h. The 14C uptake of Dunaliella tertiolecta during
7 days post-treatment was unaffected by up to 1000 µg
dieldrin/litre. Two other species (Skeletonema costatum and
Coccolithus huxleyi) showed significant reductions in 14C uptake at
levels of dieldrin above 10 µg/litre, and the photosynthetic
activity of Cyclotella nana was reduced at concentrations above 1
µg dieldrin/litre (Menzel et al., 1970).
In studies by Schauberger & Wildman (1977), three species of
fresh-water algae (Anabaena cylindrica, Anacystis nidulans, Nostoc
muscorum) were exposed to aldrin or dieldrin at concentrations of
0 - 1000 µg/litre. After exposure for 7 days, there was no
significant effect on the photosynthetic pigment absorption of the
three species at concentrations up to 10 µg (nominal)/litre.
However, at 1 mg/litre, aldrin almost completely suppressed the
absorption by photosynthetic pigments (chlorophyll and
phycocyanin), these being indicators of physiological health and
growth. Dieldrin (1 mg/litre) produced a reduction of about 40%.
The growth response of two cyanobacteria (blue-green algae) in
the presence of aldrin, dieldrin, or two metabolites of dieldrin,
photoaldrin, or photodieldrin was determined at nominal
concentrations of 0.2 - 950 µg/litre (Batterton et al., 1971).
None of these compounds had significant effects on the growth rate
constants at concentrations of 95 µg/litre or at lower
concentrations over periods of 26 - 30 h. The investigators
considered that dieldrin and its derivatives reduced the growth
rate constant at 475 and 950 µg/litre, Agmenellum quadriplicatum
being more sensitive than Anacystis nidulans. Aldrin did not have
a significant effect on either species, but photoaldrin affected
Agmenellum quadriplicatum at 950 µg/litre.
In studies by Powers et al. (1977), a marine dinoflagellate
(Exuviella baltica) was incubated with dieldrin (0.1, 1, or 10 µg
(nominal)/litre), and the numbers of cells were counted during a
period of 6 days. No adverse effects on optical counts were
observed at the two lower concentrations, but there was a marked
reduction in the size and number of cells at 10 µg dieldrin/litre.
7.2. Aquatic Organisms
The toxicity of aldrin and dieldrin to aquatic invertebrates is
very variable. For some species both compounds are highly toxic,
whereas for others there is no effect until the compounds are
dissolved to artificially high concentrations, many times their
solubility in water. Both aldrin and dieldrin are highly toxic to
most species of fish in laboratory tests, with acute LC50 values
well within the solubility of the compounds. It should be borne in
mind that aldrin and dieldrin are strongly bound to particulate
matter in water, which reduces their availability to aquatic
organisms and, in consequence, their potential toxicity.
7.2.1. Aquatic invertebrates
7.2.1.1 Acute toxicity
A convenient overview, in graphical format, of the toxicity of
aldrin and dieldrin to many aquatic organisms was produced by Craig
(1977). The 96-h LC50 values of aldrin and dieldrin for
crustaceans and molluscs were in the range 0.2 - 10 000 µg/litre.
Dieldrin is moderately toxic to fresh-water annelids
(4000 - 7000 µg/litre) and molluscs (> 100 - 640 µg/litre).
Insects are the most sensitive group (aldrin, 1 - 200 µg/litre;
dieldrin, 0.2 - 40 µg/litre). The values for a number of species
are given in Table 25.
7.2.1.2 Short-term toxicity, reproduction, and behaviour
(a) Short-term toxicity
When naiads of two species of stonefly were exposed for 30 days
in a continuous-flow system, the 30-day LC50s for aldrin and
dieldrin were, respectively, 2.5 and 2 µg/litre for Pteronarcys
californica, and 22 and 0.2 µg/litre for Acroneuria pacifica
(Jensen & Gaufin, 1966).
The LC50 for adult molluscs (Mytilus edulis and Dreissena
polymorpha) exposed for 3 - 4 weeks was 180 - 200 µg dieldrin/litre
(Adema & Vink, 1981).
McLeese et al. (1982) exposed polychaete worms (Nereis vireus)
to dieldrin in sea water or sediment for 12 days. The LC50 in sea
water was > 170 µg/litre (in surficial water > 20 µg/litre; in
sediment > 13 mg/kg).
Table 26 gives the LC50 values for a number of invertebrate
species.
(b) Reproduction
The effects of dieldrin on the embryonic development of the
American oyster (Crassostrea virginica) and of aldrin on that of
the hard clam (Mercenaria mercenaria) were studied by Davis & Hidu
(1969). Table 27 gives the concentrations producing approximately
50% reduction in the development of fertilized eggs during 48 h,
those producing about 50% reduction in larval survival during 12
days (clams) or 14 days (oysters), and the effects on larval growth
during 10 or 12 days of exposure (expressed as a percentage of
growth of control larvae).
Table 25. Acute toxicity of aldrin and dieldrin for aquatic invertebrates
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Daphnids
Daphnia magna (29)a 330a Anderson (1959)
Simocephalus first instar dispersed via 15 (23)a (240)a Johnson & Finley
serrulatus acetone 21 (32)a (1980)
Daphnia pulex first instar dispersed 15 (28)a (190)a Johnson & Finley
(1980)
Crustacea
Seed shrimp mature dispersed via 21 (18)a - Johnson & Finley
(Cypridopsis acetone (1980)
vidua)
Sowbug (Asellus mature dispersed via 21 - 5 Johnson & Finley
brevicaudus) acetone (1980)
Scud (Gammarus mature dispersed via 21 4300 640 Johnson & Finley
fasciatus) acetone (1980)
Sand shrimp 0.25 g, dispersed via 20 8 7 Eisler (1969)
(Crangon 2.6 cm acetone
septemspinosa)
2 g dispersed via 20 - 0.4 McLeese & Metcalfe
hexane (1980)
2 g dispersed in 10 - 4.1 McLeese & Metcalfe
sediment (1980)
Grass shrimp 0.47 g, dispersed via 20 9 50 Eisler (1969)
(Palaemonetes 3.1 cm acetone
vulgaris)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Crustacea (contd.)
Grass shrimp mature dispersed via 21 50 - Johnson & Finley
(Palaemonetes acetone (1980)
kadiakensis)
Crayfish mature dispersed via 21 - 740 Johnson & Finley
(Orconectes acetone (1980)
nais)
Hermit crab 0.28 g, dispersed via 20 33 18 Eisler (1969)
(Pagurus 0.35 cm acetone
longicarpus)
Molluscs
Mercenaria egg dispersed via 24 (> 10 000)a - Davis & Hidu (1969)
mercenaria acetone
Crassostrea egg dispersed via 24 - (640)a Davis & Hidu (1969)
virginica acetone
Slipper limpet veliger - - - > 100 Adema & Vink (1981)
(Crepidula
fornicata)
Pond snail egg - - - > 200 Adema & Vink (1981)
(Lymnaea juvenile
stagnalis)
Insects
Pteronarcys naiad, dispersed via 15.5 1.3 0.5 Sanders & Cope
californica 3-3.5 cm ethanol (1968); Johnson &
Finley (1980)
---------------------------------------------------------------------------------------------------------
Table 25. (contd.)
---------------------------------------------------------------------------------------------------------
Species Developmental Vehicle Temperature 96-h LC50 (static test) Reference
stage, body (°C) -----------------------
weight, or Aldrin Dieldrin
length (µg/litre)
---------------------------------------------------------------------------------------------------------
Insects (contd.)
Pteronarcella naiad, dispersed via 15.5 - 0.5 Sanders & Cope
badia 1.5-2 cm ethanol (1968); Johnson &
Finley (1980)
Claassenia naiad, dispersed 15.5 - 0.6 Sanders & Cope
sabulosa 2-2.5 cm (1968); Johnson &
Finley (1980)
Pteronarcys naiad, 2-5 cm dispersed via 12.8 180 39 Jensen & Gaufin
californica acetone (1966)
Acroneuria naiad, dispersed via 12.8 143 24 Jensen & Gaufin
pacifica 2-2.5 cm acetone (1966)
Damselfly juvenile dispersed via 24 - 12 Johnson & Finley
(Ischnura acetone (1980)
venticalis)
Other invertebrates
Bristle worm 2-3-day-old dispersed via 21 - > 100 Hooftman & Vink
(Ophryotrocha larva acetone (1980)
diadema)
4-week-old dispersed via 21 - > 100 Hooftman & Vink
adult worm acetone (1980)
---------------------------------------------------------------------------------------------------------
a Values in parentheses are the 48-h LC50.
Table 26. Short-term LC50s of dieldrin in invertebrates
-------------------------------------------------------------------
Species Stage LC50 at end of Reference
study (µg/litre)
(time of exposure)
-------------------------------------------------------------------
Ophryotrocha larva >10 Hooftman & Vink
diadema (2-3 days) (5-6 weeks) (1980)
adult 60 Hooftman & Vink
(4 weeks) (5-6 weeks) (1980)
Daphnia magna larva 100 Adema & Vink
(3 weeks) (1981)
adult 200 Adema & Vink
(0.3 cm) (7 days) (1981)
Artemia salina larva 40 Adema & Vink
(4 weeks) (1981)
adult 50 (male) Adema & Vink
(1 cm) (7 days) (1981)
110 (female)
(7 days)
Chaetogammarus larva 1.8 Adema & Vink
marinus (4 weeks) (1981)
adult 3.6 Adema & Vink
(1 cm) (14 days) (1981)
Palaemonetes adult 0.3 Adema & Vink
varians (4 cm) (7 days) (1981)
Crangon crangon adult 4 Adema & Vink
(4 cm) (14 days) (1981)
-------------------------------------------------------------------
When adult mud snails (Nassa obsoleta) were exposed to up to
10 000 µg dieldrin/litre for 96 h, and then transferred to
dieldrin-free sea water for 33 days, no mortality occurred
throughout the study and the length of the animals was normal after
33 days. There was a significant increase in total egg deposition
during the 33-day post-treatment period in the case of snails
exposed to 10 µg dieldrin/litre, but there was a significant
reduction at 100, 1000, and 10 000 µg dieldrin/litre (Eisler,
1970).
(c) Behaviour
In studies by Klein & Lincer (1974), fiddler crabs, (Uca
pugilator) were fed diets containing 0, 0.1, 1, 10, and 50 mg
dieldrin/kg diet for 14 days and observed for another 25 days.
Behaviour, measured as righting response, was modified at dose
levels of 1 mg/kg or more, and even in the group given 0.1 mg/kg,
difficulty in righting was seen after 11 days. With 10 and 50
mg/kg diet, an increase in mortality was observed, but not with 1
mg/kg diet.
Table 27. Concentrations producing about 50%
reduction in the development of fertilized eggs
during 48 h, in larval survival during 12 days
(clams) or 14 days (oysters), and effects on
larval growth during 10 or 12 days exposurea
(Davis & Hidu, 1969)
--------------------------------------------------
Organism Effect Aldrin Dieldrin
(µg/litre) (µg/litre)
--------------------------------------------------
Clam development of > 10 000 -
Oyster fertilized eggs - 640
Clam larval survival 410 -
Oyster - > 10 000
Clam larval growth 250b -
Oyster - 500c
--------------------------------------------------
a Expressed as a percentage of growth of control
larvae.
b 80% reduction.
c 50% reduction.
7.2.2. Fish
7.2.2.1 Acute toxicity
Both aldrin and dieldrin are highly toxic to fish under
laboratory conditions. A summary of reported 96-h LC50 values for
fresh water and marine species is given in Table 28. In parallel
studies, dieldrin was consistently more toxic than aldrin. The
96-h LC50s range from 2.2 to 53 µg aldrin/litre and from 1.1 to 41
µg dieldrin/litre in various fish species. It should be noted that
the range for aldrin exceeds the water solubility of the compound.
The results of studies by Macek et al. (1969) indicate that a
rise in temperature increases the toxicity of aldrin and dieldrin
for bluegills and rainbow trout. However, Johnson & Finley (1980)
stated that toxicity was not appreciably (only a factor of 2)
changed by variations in temperature or water hardness.
Macek (1975) investigated the effects of simultaneous exposure
of bluegills to DDT and dieldrin and concluded that the acute
toxicity of dieldrin in the concentration range 5.9 - 6.6 µg/litre
was not increased by the presence of DDT (concentration range,
4.5 - 5 µg/litre).
Anderson & Weber (1975) found that new-born and juvenile
guppies (Lebistes reticulatus) were more resistant to dieldrin
than adults. A relationship between the LC50 and body weight was
derived for mature, juvenile, and new-born guppies:
LC50 = aWb
where W is the body weight. The best fit value for the exponent b
was 0.81.
Table 28. Acute toxicity of aldrin and dieldrin for fish
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water
Rainbow trout 0.6 dispersed 13 2.6 - Johnson &
(Salmo via acetone Finley (1980)
gairdneri)
1.4 dispersed 13 - 1.2 Johnson &
via acetone Finley (1980)
3.2 dispersed 20 17.7 9.9 Katz (1961)
via acetone
0.6-1.5 dispersed 1.6 3.2 2.4 Macek et al.
via acetone 7.2 3.3 1.1 (1969)
12.7 2.2 1.4
Cutthroat trout 1.1 dispersed 9 - 6a Johnson &
(Salmo clarki) via acetone Finley (1980)
Chinook salmon 1.45-5 dispersed 20 7.5 6.1 Katz (1961)
(Oncorhynchus via acetone
tshawytscha)
0.8 dispersed 15 14.3 - Johnson &
via acetone Finley (1980)
Coho salmon 2.7-4.1 dispersed 20 45.9 10.8 Katz (1961)
(Oncorhynchus via acetone
kisutch)
Goldfish 1-2 dispersed 25 32 41 Henderson et al.
(Carrassius via acetone (1959)
auratus)
Goldfish 1 dispersed 18 - 1.8 Johnson &
(Carassius via acetone Finley (1980)
auratus)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Fresh-water (contd.)
Carp (Cyprinus NA NA 20 4b - Rehwoldt et al.
carpio) (1977)
Fathead minnow 0.6 dispersed 18 8.2 3.8 Johnson &
(Pimephales via acetone Finley (1980)
promelas)
1-2 dispersed 25 32 18 Henderson et al.
via acetone (1959)
Guppy (Lebistes 0.1-0.2 dispersed 25 37 25 Henderson et al.
reticulatus) via acetone (1959)
NAd NA 20 20b - Rehwoldt et al.
(1977)
NA NA 24 - 3.2-7 Adema & Vink
(young) (1981)
NA NA 24 - 35c Adema & Vink
(adult) (1981)
juvenile NA 25 10.9 Anderson & Weber
(1975)
newborns NA 36.7 Anderson & Weber
(1975)
Black bullhead 1.5 dispersed 24 19 - Johnson & Finley
(Ictalurus via acetone (1980)
melas)
Channel catfish 5.2 dispersed 18 53 - Johnson & Finley
(Ictalurus via acetone (1980)
punctatus)
1.4 dispersed 18 - 4.5 Johnson & Finley
via acetone (1980)
Bluegill 0.7 dispersed 18 6.2 - Johnson & Finley
(Lepomis via acetone (1980)
macrochirus)
1.3 dispersed 18 - 3.1 Johnson & Finley
via acetone (1980)
1-2 dispersed 25 15 8.8 Henderson et al.
via acetone (1959)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Bluegill 0.6-1.5 dispersed 12.7 7.7 17 Macek et al.
(contd.) via acetone 18.3 5.8 14 (1969)
23.8 4.6 8.8
Pumpkinseed NA NA 20 20b - Rehwoldt et al.
sunfish (1977)
(Lepomis
gibbosus)
Largemouth bass 2.5 dispersed 18 5 3.5 Johnson & Finley
(Micropterus via acetone (1980)
salmoides)
Striped bass NA NA 20 10b - Rehwoldt et al.
(Marone (1977)
saxatilis)
Banded killyfish NA NA 20 21b - Rehwoldt et al.
(Fundulus (1977)
diaphanus)
White perch NA NA 20 42b - Rehwoldt et al.
(Roccus (1977)
americanus)
American eel NA NA 20 16b - Rehwoldt et al.
(Anguilla (1977)
rostrata)
Marine species
Common goby NA NA 15 - 3.5 Adema & Vink
(Gobius microps) (adult) (1981)
Plaice length: NA 15 - 1.7 Adema & Vink
(Pleuronectes 2-3 cm (1981)
platessa)
length: NA 15 - 4 Adema & Vink
10 cm (1981)
yolk-sac NA 5-10 - 30 Adema & Vink
larva (1981)
egg-metam NA 5-10 - > 32 Adema & Vink
larva (1981)
------------------------------------------------------------------------------------------
Table 28. (contd.)
------------------------------------------------------------------------------------------
Species Weight Vehicle Temperature 96-h LC50 Reference
(g) (°C) (static test)
----------------
Aldrin Dieldrin
(µg/litre)
------------------------------------------------------------------------------------------
Marine species (contd.)
Threespine 0.4-0.8 dispersed 20 27.4 13.1 Katz (1961)
stickleback via acetone
(Gasterosteus
aculeatus)
------------------------------------------------------------------------------------------
a Hardness = 162 mg CaCO3/litre.
b Hardness = 50 mg CaCO3/litre.
c 48-h LC50.
d NA = not available.
7.2.2.2 Long-term toxicity
Sailfin mollies (Lebistes latipinna) were exposed in groups of
20 to 0, 0.75, 1.5, 3, 6, or 12 µg dieldrin/litre using a flow-
through system for 34 weeks. The mortality of the 0.75 µg/litre
group was similar to that of the control group. At 1.5 µg/litre,
there was an increase in mortality, and, at 3 µg/litre or more,
100% mortality occurred. The growth rates and reproduction
performances were adversely affected in the surviving fish (Lane &
Livingstone, 1970).
Rainbow trout (Salmo gairdneri) were fed food containing
dieldrin for 240 days, the nominal dietary concentrations
corresponding to 14, 43, 143, or 430 µg dieldrin/kg body weight per
day. The growth rate was not affected at any of the concentrations
throughout the 240 days, and there was no mortality or visible
adverse effects. The activities of liver glutamate-pyruvate
transaminase (GPT) and glutamate-oxaloacetate transaminase (GOT)
were not affected, except, in the case of the latter, at the
highest dose level. Liver glutamate dehydrogenase (GDH) activity
was increased at all dose levels. Electron micrographs of liver
cells demonstrated changes in mitochondrial morphology, the highest
dose causing swelling and membrane disruption. Since GDH is an
intramitochondrial enzyme, examination by electron microscopy gave
further evidence that dieldrin altered mitochondrial metabolism.
In the brain, GOT activity was significantly decreased at 43 µg/kg
and GTP was decreased at 14 µg/kg or more. At all dose levels,
brain GDH was decreased and brain glutamine transferase (GT) was
increased. Electron microscopy of the medulla and cerebral
hemispheres did not show any effects of dieldrin. The
concentrations of 16 free amino acids in the brain were determined.
The concentrations of four were not significantly changed, whereas
eight were significantly altered at 143 µg dieldrin/kg and 12 at
430 µg/kg. Serum ammonia concentrations were significantly
increased at 143 and 430 µg dieldrin/kg, but the concentration of
ammonia in the brain was not affected. The increase in brain GT
was considered to be a possible reason for this lack of effect on
brain ammonia, since it compensated for the decrease in GDH
activity. Alternatively, brain ammonia may have been transported
via the blood to the liver with consequent effects on the liver.
The ammonia-detoxifying mechanism of fish seemed to be very
sensitive to dieldrin, the no-effect dose being below 14 µg/kg body
weight per day (equivalent to 0.36 mg/kg food) (Mehrle &
Bloomfield, 1974).
Several studies have described the influence of aldrin and/or
dieldrin on enzymes such as mitochondrial succinic hydrogenase, the
epoxidative activities of liver microsomes and the ATPase activity
of microsomes of the gills or brain (Chan et al., 1967; Davis et
al., 1972; Moffet & Yarbrough, 1972; Yap et al., 1975).
Furthermore, the influence of dieldrin during thermal stress has
been studied in darters (Etheostoma nigrum) (Silbergeld, 1973).
In studies by Verma & Tonk (1984), Heteropneustes
(Saccobranchus) fossilis was exposed to aldrin for 30 days at a
concentration of 0.03 mg/litre. Respiration, haematological
parameters, and the activity of two enzymes in liver, kidneys, and
gills were determined. The respiration rate decreased and the
blood concentrations of glucose, sodium, and chloride ions showed
significant increases. The cholesterol content and clotting time
were decreased, and the ATPase activity in the three tissues was
significantly reduced.
7.2.2.3 Reproduction
Van Leeuwen (1986) carried out studies with dieldrin to study
the susceptibility of early-life stages of rainbow trout. The test
was performed with fertilized eggs before and after water
hardening, and with early eye point eggs, late eye point eggs, sac
fry, and early fry. No mortality was found in the different early-
life stages using concentrations greater than aqueous solubility
(> 10 mg/litre), except for the early fry where a very low 96-h
LC50 of 0.003 mg/litre was found.
In studies by Cairns et al. (1967), nine populations of guppies
(Lebistes reticulatus) were exposed in a semi-static system to
nominal concentrations of 0 (three populations), 1.8, 5.6, and 10
µg dieldrin/litre (two populations of each) for 14 months. During
the first 2 - 3 months, the exposed populations in five tanks
developed greater numbers of individuals (mature, immature, and
fry) than did the controls (except one population at 5.6 µg/litre,
which was similar to the controls). The difference between the
controls and five treatment groups was attributed to the higher
predation and harassment observed in the control groups. The total
numbers of individuals in control and treatment groups became
similar during the final 6 - 8 months of the study. The average
total monthly body weights of the groups treated with 1.8 µg/litre
and 5.6 µg/litre began to increase steadily after about 8 months,
whereas the total monthly body weights of the group exposed to 10
µg/litre were similar to the controls throughout the 14 months of
the study. The production of fry by one of the groups treated with
10 µg/litre declined markedly after the thirty-second week of the
study, no new broods of fry being born after the forty-second week.
No such marked decline occurred in the other five treatment groups
(including one population exposed to 10 µg/litre).
Chadwick & Shumway (1970), conducted studies lasting 130 days
on rainbow trout (Salmo gairdneri) to determine survival from time
of fertilization through to hatching in continuously cycled water.
Embryos, alevins, and fry were exposed to dieldrin concentrations
ranging from 0.012 to 52 µg/litre. Eggs (embryos) exposed to up to
52 µg dieldrin/litre from the time of fertilization survived until
hatching as well as controls, but the mean weight of newly-hatched
alevins (minus yolk material) was reduced by higher concentrations
(not specified). Alevins were more susceptible than embryos.
Their survival was reduced at all concentrations above 0.39
µg/litre. Trout fry, whose survival was unaffected at dieldrin
levels of 0.12 µg or less, quickly succumbed at concentrations of
0.39 µg/litre or more.
Smith & Cole (1973), exposed adult winter flounder (Pseudo-
pleuronectes americanus) to 2 µg/litre in aquaria continuously
supplied with filtered sea water. When fish became ripe, they were
artificially spawned, and approximately 30 000 eggs were collected
from each of the 24 spawning pairs and cultured. The remaining
eggs were analysed. The percentage fertilization of eggs
containing 0.61 mg dieldrin/kg or less was 99% (controls, 97.8%).
The percentage fertilization of eggs containing 1.21 mg dieldrin/kg
was 12%, and all the eggs containing 1.74 mg/kg were infertile.
There was no effect on egg development except in the case of the
two groups of eggs containing the higher concentrations of
dieldrin. The effects on egg mortality were not due to dieldrin in
the gametes, and the milt of exposed male flounders contained no
detectable residue of dieldrin.
7.2.3. Amphibia and reptiles
The LC50 values for tadpoles of two species of frogs (1 week
old) and toads (4 - 5 weeks old) were determined by Sanders (1970).
The 96-h LC50 (at 15.5 °C) of dieldrin for Western chorus frog
tadpoles (Pseudacris triseriata) was 100 µg/litre, whereas that of
both aldrin and dieldrin for Fowler's toad tadpoles (Bufo
woodhousii fowleri) was 150 µg/litre.
Cooke (1972) studied the effect of dieldrin at nominal
concentrations of 0.008, 0.02, or 0.5 mg/litre on groups of 40
common frog (Rana temporaria) or toad (Bufo bufo) tadpoles with
hindlimb paddles or hind legs. The exposure was for 24 or 48 h in
amphibian saline, and the observation period 5 or 15 days. At the
highest dose level, the frogs showed an increased mortality, the
mean dieldrin content being 42.9 mg/kg tissue. At the two lower
dose levels (0.008 and 0.02), there were 0.31 and 6.1 mg/kg
dieldrin in tissues, respectively. When toad tadpoles were exposed
to 0.02 or 0.5 mg/litre, the animals with the higher dose level
showed clear behavioural and structural abnormalities and a reduced
rate of development, but these changes returned to normal a few
days after exposure. The mean dieldrin content was 138 mg/kg
tissue at a dose level of 0.5 mg/litre.
The in vitro exposure of toad embryo tissue (Bufo arenarum) to
dieldrin (4 x 10-5 mol/litre) produced an inhibition of acetyl and
butyryl cholinesterase activity. In in vivo studies with open-
mouth stage embryos, dieldrin produced acetyl cholinesterase
inhibition at 0.5 x 10-6 mol/litre. Furthermore, hyperactivity in
swimming larvae was observed (de Llamas et al., 1985).
The in vitro activity of ATPase in a number of tissues of the
male turtle (Graptemys geographica) was determined by Wells et al.
(1974). There was no consistent dose relationship for either aldrin
or dieldrin, except perhaps in the case of the cloacal bladder in
the aldrin treatments. The inhibition of Na/K/Mg ATPase by aldrin
and dieldrin was in the range of 4 - 13%. It was suggested that
aldrin and dieldrin may affect the transport of metabolites across
the cellular membranes as a result of decreased energy for active
transport.
7.3. Terrestrial Organisms
7.3.1. Higher plants
Dieldrin has low phytotoxicity, tomatoes and cucumber, for
example, being affected only at application rates greater than 22
kg/ha. Aldrin affects some crops at rates greater than 22 kg/ha,
beans and cereals being most sensitive. Tomatoes and cucumbers are
sensitive to aldrin but only at unrealistically high application
rates (Edwards, 1965).
Studies in greenhouses showed that aldrin, administered weekly
as an emulsifiable concentrate at a rate of 16 kg active
ingredient/ha to 2 - 3-week-old seedlings of tomato, cauliflower,
and Chinese cabbage, inhibited root development and reduced growth
rate of cauliflower and Chinese cabbage seedlings. A ten-fold
reduction in the aldrin level failed to produce these effects
(Hagley, 1965).
Aldrin and dieldrin at 11 kg active ingredient/ha had no effect
on the emergence, growth, yield, or chemical composition of
soybeans (Probst & Everly, 1957).
7.3.2. Earthworms
In studies by Cathey (1982), earthworms (Lumbricus terrestris)
were maintained in an artificial nutritionally complete soil, based
on shredded paper containing aldrin. The LC50 value (6-week
exposure) was 60 mg aldrin/kg bedding, and the tolerance level,
producing less than 1% mortality, was 13 mg aldrin/kg bedding.
When aldrin (2.5 - 4.6 kg/ha) was applied as a spray or dust,
respectively, to the surface of soil plots and incorporated into
the soil, the numbers of earthworms in treated plots were either
similar to or greater than those in control plots (Edwards et al.,
1967; Griffiths et al., 1967; Edwards & Lofty, 1977).
7.3.3. Bees and other beneficial insects
In a review of five investigations on the toxicity of aldrin
and dieldrin to honey bees (Sanger, 1959), the oral LD50 values for
aldrin ranged from 0.24 to 0.45 µg/bee, while the values for
dieldrin were in the range 0.15 - 0.32 µg/bee. Contact LC50 values
were 0.15 - 0.8 µg/bee for aldrin and 0.15 - 0.41 µg/bee for
dieldrin.
Cowie (1967) reported an oral LD50 of 0.3 µg dieldrin/bee
(range, 0.13 - 0.54) and a contact LD50 of 0.21 µg/bee.
The toxicity of dieldrin to two important predators of cotton
pests was investigated by Burke (1959). The contact LD50 value for
Hippodamia convergens was 1.6 mg/g body weight.
In a review of the effects of pesticides on soil fauna, it was
concluded that aldrin (and, by implication, dieldrin) is relatively
non-toxic for predatory mites ( Acarina spp.), and that this may
contribute to its success as a soil insecticide (Edwards &
Thompson, 1973).
7.3.4. Birds
7.3.4.1 Acute toxicity
Estimates of the LD50 values for several species of birds are
given in Table 29. The variation in acute oral toxicity of
dieldrin among six species of birds tested by Tucker & Haegele
(1971) was more than ten fold.
Table 29. Acute oral toxicity of aldrin and dieldrin for avian
speciesa
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Fulvous whistling duck male: female: Tucker & Crabtree
(Dendocygna bicolor) 29.2 100-200 (1970)
Mallard duck female: female: Tucker & Crabtree
(Anas platyrhynchos) 520 381 (1970)
Canada goose 50-150 Tucker & Crabtree
(Branta canadensis) (1970)
-------------------------------------------------------------------
Table 29. (contd.)
-------------------------------------------------------------------
Species LD50 Reference
-------------------
Aldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Domestic fowl 25.5 43 Sherman &
(Gallus domesticus) Rosenberg (1953)
Japanese quail male: Tucker & Crabtree
(Coturnix coturnix 69.7 (1970)
japonica)
Bobwhite quail female: Tucker & Crabtree
(Colinus virginianus) 6.6 (1970)
California quail 8.7 Hudson et al.
(Callipepla californica) (1984)
Gray partridge female: Tucker & Crabtree
(Perdix perdix) 8.8 (1970)
Chukar partridge 23.4 Tucker & Crabtree
(Alectoris graeca) (1970)
Sharp-tailed grouse male: McEwen & Brown
(Pedioecetes phasianellus) 6.9 (1966)
Ring-necked pheasant female: female: Tucker & Crabtree
(Phasianus colchicus) 16.8 79 (1970)
Pigeon 55 67 Turtle et al.
(Columba livia) (1963)
26.6 Tucker & Crabtree
(1970)
House sparrow female: Tucker & Crabtree
(Passer domesticus) 47.6 (1970)
-------------------------------------------------------------------
a Details concerning age and weight of birds are not summarized
here but can found in the original publications.
7.3.4.2 Short- and long-term toxicity
Values for the subacute LC50s of aldrin and dieldrin,
determined using the procedure developed at the Patuxent Wildlife
Centre (Hill et al., 1975), are given in Table 30. The LC50 values
of aldrin and dieldrin for each of the four species tested were of
the same order. The annual variations in the LC50 of dieldrin over
a period of up to 8 years for these four species have been
investigated by Hill et al. (1977) (18 times per species). No
time-related changes in LC50 values were found for any of the
species. However, differences were found between birds of
different ages in some species, e.g., Japanese quail and mallards
(Hudson et al., 1984). There were also differences between the
slopes of the average regression lines for the four species. These
authors emphasized the need to evaluate both the LC50 and the slope
of the regression line. Food consumption was reduced by aldrin or
dieldrin in the diet.
Table 30. Subacute dietary toxicity of aldrin and dieldrin
for avian speciesa
-----------------------------------------------------------
Species Age LC50 (95% confidence limits)
(days) ----------------------------
Aldrin Dieldrin
(mg/kg diet)
-----------------------------------------------------------
Mallard duck 5 155 153
(Anas platyrhynchos) (129-186) (123-196)
10 - 169
Japanese quail 14 34 62
(Coturnix coturnix (28-41) (53-71)
japonica)
Bobwhite quail 14 37 37
(Colinus virginianus) (33-41) (30-46)
Ring-necked pheasant 10 57 58
(Phasianus colchicus) (50-64) (51-67)
-----------------------------------------------------------
a Aldrin or dieldrin fed for 5 days followed by 3 days of
untreated diet.
Wiese et al. (1969) fed diets containing up to 500 mg technical
dieldrin (85%)/kg diet to male and female 6-month-old crowned
guinea-fowl (Numida meleagris). None of the birds fed 1.5 mg/kg
for 21 months died. The median survival time for birds fed 5 mg/kg
was 524 days; for birds fed 150 and 500 mg/kg, it was 3 and 1 days,
respectively. No differences in susceptibility between males and
females were found.
The subacute toxicities of technical dieldrin (85%) to three
species of birds are given in Table 31 (Basson, 1971).
7.3.4.3 Reproductive studies
The first experimental studies of the effects of aldrin or
dieldrin on avian reproduction showed that these compounds were
toxic for quail and pheasants. Quail fed a diet containing aldrin
or dieldrin at a toxic dose of 0.5 or 1 mg/kg diet did not show any
clear effects on egg production, percentage fertility, or
percentage hatchability (the birds had not been exposed earlier to
the compounds) (DeWitt, 1955, 1956). No significant effect was
found on the fertility or hatchability of eggs of pheasants fed
25 mg dieldrin/kg diet, but at 50 mg/kg there was a clear effect
(Genelly & Rudd, 1956).
Table 31. Subacute toxicity of technical dieldrin for three species
of birds
-------------------------------------------------------------------
Species Concentration Median time Median lethal
(mg/kg diet) till death dose (mg
(days) dieldrin/kg
body weight)
-------------------------------------------------------------------
Guinea fowl 20 72 72.4
(Numida meleagris) 150 12 11.2
Laughing dove 5 49 15.8
(Stigmatopelia 90 4.7 17.3
senegalensis)
Sparrow (Passer 5 85.1 > 41
melanurus melanurus) 45 7 43.8
-------------------------------------------------------------------
Eggs from chickens fed 1 mg aldrin or dieldrin/kg diet for
2 years showed normal fertility and hatchability, although the
concentrations of dieldrin in the yolks of the eggs were in the
range of 6 to 25 mg/kg. The fertility and hatchability slightly
decreased at 10 mg dieldrin/kg diet (Brown et al., 1965).
Other studies on avian reproduction are summarized in Table 32.
This table gives the available information on five criteria
relevant to reproduction (parental survival, production, fertility
and hatchability of eggs, and chick survival) in relation to oral
intake of dieldrin and (where reported), the residues of dieldrin
in eggs. These studies show that, depending on the duration of
exposure, dose levels of 5 - 10 mg dieldrin/kg diet reduce the
survival of the parent birds. Egg production was reported to be
significantly increased in some studies but reduced in others. In
general, egg fertility was not influenced, except in one study.
Hatchability was not affected, neither in most cases, was the
survival of chicks. There seems to be a trend that overall
reduction in reproductive success occurs only if the parent birds
are showing signs of being affected by dieldrin, e.g., reduced food
intake with consequent loss of weight and poor condition. It
should be noted that in these studies (with one exception) the eggs
were placed in incubators for hatching. Consequently, one aspect
of the reproductive process was not studied, namely, parental
behaviour. However, in the study on homing pigeons (Robinson &
Crabtree, 1969), the parents (and subsequently, their offspring)
were free-flying, they brooded their eggs, and fed their young
until they fledged.
A 3-generation study of the effects of dieldrin on pheasants
(Phasianus colchicus), has not been included in Table 32 because
of the complexity of the experimental design. The doses of
dieldrin (hens, 6 or 10 mg dieldrin/bird per week; cocks, 4 or 6 mg
dieldrin/bird per week) were sufficient to cause mortality of
breeding birds, but the production, fertility, and hatchability of
eggs and the viability of chicks at the time of hatching were not
affected in a consistent manner in relation to dose or generation.
The survival of chicks from hens given 6 or 10 mg dieldrin/week was
reduced. Residues of dieldrin in eggs or tissues were not
determined in this study (Dahlgren & Linder, 1974).
When seven-week-old Japanese quail were given diets containing
3.1 or 50 mg dieldrin/kg diet for 21 days, a significant reduction
in egg production occurred in both groups (Call & Harrell, 1974).
With the exception of the study with the barn owl (Mendenhall
et al., 1983) and the homing pigeon (Robinson & Crabtree, 1969),
the birds that were tested are precocial species which show no
parental feeding of the young. Most birds are not precocial and
reproduction involves a period of full dependency of the offspring
on parental care. Results should, therefore, be interpreted with
care; extrapolation directly from the laboratory to the field is
difficult.
7.3.4.4 Eggshell thinning
Ratcliffe (1967a) reported that the ratio of eggshell weight to
size in three species of birds of prey in the United Kingdom had
declined during the period after 1947 relative to pre-1947. This
report has stimulated considerable interest in the relationship
between egg-shell thickness (or the related eggshell index based on
the weight/size ratio) and the breeding success of birds,
particularly as eggshell thinning seems to be quite a widespread
phenomenon, particularly among birds of prey (Hickey & Anderson,
1968; Ratcliffe, 1970; Anderson & Hickey, 1972). There has been
considerable speculation on the causes and mechanism of these
changes (Cooke, 1973; Mueller & Leach, 1974). Experimental studies
on the effects of dieldrin on eggshell thickness have given
conflicting results. The results are summarized in Table 33.
Eggshell weights of crowned guinea-fowl (Numida meleagris) fed
diets containing up to 15 mg dieldrin/kg diet for 21 months were
not affected by the treatments (Wiese et al., 1969).
American sparrow hawks (Falco sparverius) were given diets
containing dieldrin and North American prairie falcons (Falco
mexicanus) were fed starlings contaminated with an average of 29 mg
dieldrin/kg body weight, along with DDT and DDE at different
levels. The purpose of these studies was to show the influence of
these pesticides on shell thickness. The results of the two
studies, in relation to the effects of dieldrin, cannot be
interpreted, in view of the possible effects of DDE on eggshell
thinning (Porter & Wiemeyer, 1969; Enderson & Berger, 1970). It
has slowly become accepted that metabolites of DDT, particularly
DDE, are the most likely cause of eggshell thinning (Cooke, 1973;
Newton, 1979; Bunyan & Stanley, 1982). It is also pertinent that
the onset of eggshell thinning in wild birds preceded the use of
aldrin/dieldrin. There is evidence that eggshell thinning after
exposure to dieldrin is related to reduced food consumption.
Untreated Coturnix and Mallard, when fasted for 36 h, laid thin-
shelled eggs for a few days during and after fasting (Haegele &
Tucker, 1974).
Table 32. Reproductive success of birds in relation to oral intake of dieldrin and the concentration of HEOD in eggs
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Mallard duck 4 90 days - - NCg NC Red.c - Muller &
(Anas platy- Lockman (1972)
rhinchos)
Japanese quail 1 16 weeks - - NC NC NC - Shellenberger
(Coturnix 10 16 weeks - - NC NC NC - & Newell
coturnix (1965)
japonica)
10 18 weeks 19 NC NC NC NC NC Walker et al.
(6.9-26.9) (1969a)
20 9 weeks 39 Red. Red. DC NC Red.
(19.8-54.1)
30 7 weeks 48 Red. Red. DC Red. Red.
(34.7-63.2)
40 6 weeks 84 Red. Red. - - -
(76.9-92.5)
0.1 multi- - NC NC NC NC NC Shellenberger
generation (1978)
P; F1,
1 F2, F3 - NC NC NC NC NC
Bobwhite quail 10 34 weeks - Red. NC - - - Fergin &
(Colinus 20 34 weeks - Red. Red. - - - Schafer (1977)
virginianus) 40 34 weeks - Red. Red. - - -
Pheasant 2 mg/hen/ 13 weeks (0.6-26.5) NC NC NCa Inc.c NC Atkins &
(Phasianus weekb (yolks) Linder (1967)
colchicus) 2 mg/hen/ 13 weeks - NC NC NC NC NC
week
4 mg/hen/ 13 weeks (5.3-40.1) DR NC NC NC NC
week (yolks)
4 mg/hen/ 13 weeks (7.5-20.6) NC NC NC NC NC
week (yolks)
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Pheasant 6 mg/hen/ 13 weeks (13.3-52.4) NC Red.a NC NC NC
(Phasianus week (yolks)
colchicus)
(contd.) (0) 6 mg/hen/ 14 weeks - Red. Inc.c Red.c NC NC Baxter et al.
weekd (1969)
(0) 8 mg/hen/ 14 weeks - Red. Inc.c NC NC NC
week
(0) 12 mg/ 14 weeks - Red. Red.c NC - -
hen/week
(4) 0 mg/hen/ 14 weeks - NC Inc.c Red.c Red. NC
week
(4) 6 mg/hen/ 14 weeks - Red. NC Red.c NC NC
week
(6) 0 mg/hen/ 14 weeks - NC Red.c NC - -
week
(6) 6 mg/hen/ 14 weeks - Red. Red.c Red.c - -
week
Gray partridge 3 1.5 - DR NC NC NC Neill et al.
(Perdix perdix) (1-2) (1971)
Domestic fowl 2 16 weeks (0.34-1.45) NC NC - NC NC Graves et al.
(Gallus 5 16 weeks (1.2-4.8) NC NC - NC NC (1969)
domesticus)
10 13 months (7.6-16) NC NC NC NC NC Brown et al.
(1974)
20 13 months (19.6-35.7) Red. Inc.a NC NC Red.
Crowned guinea- 0.5 21 months 1.11/1.17e NC Inc.a NC NC NC Wiese et al.
fowl (Numida (1969)
meleagris) 1.5 21 months 2.38/3.35e NC Inc.a NC NC NC
5 21 months 7.18/13.56e PR Inc.a NC NC NC
15 21 months 15.79e Red. Inc.a NC NC Red.
--------------------------------------------------------------------------------------------------------------------
Table 32. (contd.)
--------------------------------------------------------------------------------------------------------------------
Species Intake of Duration Mean concen- Survival Reproductive success relative to Reference
dieldrin tration of of controls
(mg/kg diet) dieldrin in parents Eggs Fer- Hatcha- Chick
eggs (mg/kg) /hen tility bility- survival
(range) of eggs of eggs
--------------------------------------------------------------------------------------------------------------------
Homing pigeon ~2 mg/kg 2 years (0.03-13.4)f NC NC NC NC NC Robinson &
(free-flying) body weight/ (4.5-16.7)f NC NC NC NC NC Crabtree
(Columba livia) week (1969)
Barn owl 0.5 2 years 3.6 (first NC NC NC NC NC Mendenhall et
(Tyto alba) year) al. (1983)
8.1 (second
year)
--------------------------------------------------------------------------------------------------------------------
a Significant at a probability of < 0.01.
b Doses of 2, 4, or 6 mg dieldrin/hen were administered once per week in capsules.
c Significant at a probability of < 0.05.
d Second-generation hens; offspring of birds used in study by Atkins & Linder (1967). The doses in parentheses
refer to the doses administered to the first-generation hens.
e Residues in eggs of successive years.
f First and second laying periods, respectively.
g The term "no change" (NC) indicates that any differences between controls and treatment groups were within the
limits of experimental variation. If any of the results for treatment groups are reported to be increased (Inc.)
or reduced (Red.), the statistical significance, if reported, is given. Equivocal results have been described
"doubtful reduction" (DR), "probably reduced" (PR), and "doubtful change" (DC).
Table 33. Effects of dieldrin on eggshell thickness
--------------------------------------------------------------------------------------------------------------
Species Dose of dieldrin Duration Difference between egg- Reference
(mg/kg diet) shell thickness of treated
and control birds (%)
--------------------------------------------------------------------------------------------------------------
Mallard duck 1.6 16 months -3.4a Lehner & Egbert (1969)
(Anas platyrhynchos)
4 16 months -2a Lehner & Egbert (1969)
10 16 months -4.3a Lehner & Egbert (1969)
4 90 days -4.2 Muller & Lockman (1972)
Japanese quail 3.1 21 days with -8a Call & Harrell (1974)
(Coturnix coturnix changes in
japonica) 50 photoperiod -8a Call & Harrell (1974)
Domestic fowl 10 12 weeks 0 Davison & Sell (1972)
(Gallus domesticus) 20 12 weeks +0.3 Davison & Sell (1972)
10 13 months 0 Brown et al. (1974)
20 13 months +9.7 Brown et al. (1974)
Pheasant 6 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(Phasianus colchicus)
10 mg/hen/weekb - 0 Dahlgren & Linder (1970)
(6) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(6) 6 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
(10) 0 mg/hen/weekc - +4.1 Dahlgren & Linder (1970)
--------------------------------------------------------------------------------------------------------------
a Significant at or below 0.05.
b Administered in capsules once per week (see footnoteb Table 32).
c See footnoted Table 32.
7.3.4.5 Concentrations of dieldrin in tissues of experimentally
poisoned birds
Many studies have been carried out to estimate the
concentrations of dieldrin in the liver, brain, or other tissues of
birds that died following oral intake of aldrin or dieldrin. The
intakes were either single acute doses or long-term dietary
exposure. In some investigations, the concentrations of dieldrin
in the tissues of birds that survived after treatment were also
reported. The results of these studies are not comparable, because
the dose levels and duration of the studies are different. The
concentrations that were found in the different studies ranged from
a few mg/kg up to about 100 mg/kg tissue (wet weight) (Turtle et
al., 1963; Koeman et al., 1967; Robinson et al., 1967b; Robinson,
1969; Robinson & Crabtree, 1969; Stickel et al., 1969; Enderson &
Berger, 1970; Linder et al., 1970; Brown et al., 1974; Clark, 1975;
Heinz & Johnson, 1981; Mendenhall et al., 1983) (Table 34).
Attempts to define tissue concentrations that can be used as
indicators of death by dieldrin poisoning of wild birds lack
precision as a result of the overlap between the lowest
concentrations in the tissues of birds dying under experimental
conditions and the highest concentrations in survivors. Thus, it
has been proposed that concentrations of 5 or 10 mg dieldrin/kg
brain (Robinson, 1969; Stickel et al., 1969) are indicative of
death from aldrin/dieldrin poisoning. Liver concentrations of 10
or 20 mg/kg (Robinson, 1969; Cooke et al., 1982) have been proposed
as levels diagnostic of dieldrin poisoning of birds.
As a general point, interpretation of residue data must be done
with extreme caution. Brain residues of dieldrin are probably a
good indicator of lethality. However, most bird carcasses
collected in the field cannot be analysed for brain residues,
because the brain deteriorates rapidly after death. For this
reason, most residue data from the field are for levels in the
liver, which remains discrete and usable for much longer. A liver
residue level symptomatic of death from dieldrin poisoning is more
difficult to define. A large, acutely toxic, dose of dieldrin may
leave a low residual level of dieldrin in liver because the bird
dies rapidly. A smaller, less acutely toxic, dose of dieldrin
usually leads to loss of body weight before death because of a lack
of ability or desire to feed. This period of starvation prior to
death boosts liver residues considerably as dieldrin is released
from mobilized fat and concentrated in the liver as detoxification
is attempted.
7.3.5. Mammals
The acute and long-term toxicity of aldrin and dieldrin for
laboratory mammals is summarized in section 8.
Values for the acute oral LD50 and subacute oral LC50 in the
diet (30 days) of dieldrin for four species of voles ( Microtus sp.)
are given in Table 35.
Table 34. Concentrations of dieldrin in the tissues of experimentally poisoned birds and
in survivors
------------------------------------------------------------------------------------------
Species Tissue Concentration of dieldrin (mg/kg wet weight) Reference
analysed geometric mean, (range of values)
No. of Survivors No. of Dead birds
samples samples
------------------------------------------------------------------------------------------
Domestic pigeon liver 11 8 20 45.6 Robinson et
(Columba sp.) (3.1-51.2) (23-81) al. (1967b)
brain 11 3.6 19 20
(1.6-8.5) (13.5-32.5)
House sparrow liver - - 19 44.7 Robinson
(Passer domesticus) (38.4-52.3) (1969)
brain - - 19 20
(17.6-22.7)
Japanese quail liver - 36 40 Robinson et
(Coturnix coturnix (17.7-90.4) al. (1967b)
japonica) brain 12 6.9 65 17.4
(3.1-15) (8.7-34.6)
Japanese quail liver 8 28.8 9 19.7 Stickel et
(Coturnix coturnix (2.7-140.8) (5.7-51.7) al. (1969)
japonica) brain 20 3.4 17 17.3
(0.25-11.9) (6.2-32.9)
Redwinged blackbird brain 3 7.1 27 19.8 Clark
(Agelaius phoeniceus) (6.7-7.4) (1-34.5) (1975)
27 22.2
(13.5-29.5)
Prairie falcon brain 2 2.9 1 11 Enderson &
(Falco mexicanus) (2.8-3) Berger
(1970)
Barn owl brain 2 10 Mendenhall
(Tyto alba) (5-15) et al.
carcass 19 9.4 - - (1983)
------------------------------------------------------------------------------------------
The toxicological signs in these studies were similar to those
in laboratory animals, and these four microtine rodents appear to
be less susceptible than laboratory rodents to dieldrin
intoxication (Cholakis et al., 1981). When short-tailed shrews
(Blerina brevicauda) were fed diets containing 50, 100, or 200 mg
dieldrin (nominal)/kg diet for up to 14 days, all the animals fed
50 mg/kg dieldrin survived, whereas all those fed 200 mg/kg diet
died (Blus, 1978).
Luckens & Davis (1965) studied the acute oral toxicity in big
brown bats (Eptericus fuscus) caught in Kentucky, USA. Death
occurred at all dose levels above 20 mg/kg body weight. The
approximate LD50 seemed to be within the range 20 - 40 mg/kg body
weight.
Table 35. Acute and subacute toxicity of dieldrin for volesa
------------------------------------------------------------------------
Species Acute LD50 Subacute LC50 (30 days)
(mg/kg body weight) (mg/kg body weight)
Average males/females Average males/females
------------------------------------------------------------------------
Microtus orchrogaster 210 105
Microtus canicaudus 100 40
Microtus montanus 205
Microtus pennsylvanicus 175
------------------------------------------------------------------------
a From: Cholakis et al. (1981).
White-tailed deer (Odocoileus virginianus) were fed 0, 5, or
25 mg dieldrin/kg diet for up to 3 years as were their progeny. No
signs of intoxication were observed, and the survival of adults was
not affected. The growth rate of treated females was decreased.
Relative liver weights increased at 25 mg/kg. Fertility and in
utero mortality were comparable for the three groups. Fawns from
treated does were smaller at birth, and greater postpartum
mortality occurred. The weight gain of fawns was reduced during 2
of the 3 years. Whole milk from doses fed 25 mg/kg contained
residues of 17 mg/litre. Residues in the liver of surviving
animals were about 4 mg/kg in the low-dose group and 16 mg/kg (wet
weight) in the high-dose group. Highest brain residues (12 mg/kg
wet weight) occurred in fawns only a few days before death (Murphy
& Korschgen, 1970).
When blesbuck (Damaliscus dorcas phillipsi) were fed diets
containing 5, 15, 25, 35, or 50 mg dieldrin (nominal)/kg diet, none
of the animals fed 5 or 15 mg/kg diet died during the 90 days of
the study. However, all the animals given higher dose levels died
within 24 days. The concentration of dieldrin in the liver was 3.3
and 8.2 mg/kg for the two lowest dose levels, after 90 days. The
concentrations of dieldrin in the livers of the dead animals were
9.4, 15.1, and 18.4, respectively, for the three highest treatment
groups (Wiese et al., 1973).
In studies by Wiese et al. (1973), an experimental grazing site
(250 ha) with a resident population of 35 blesbuck and 20 springbok
(Antidorcas marsupialis) was aerially sprayed with dieldrin at a
rate corresponding to 0.16 kg/ha. The concentration of dieldrin on
the veld declined rapidly from 27.6 mg/kg immediately after
treatment to 5.04 mg/kg after 14 days. The decline during the
following 106 days was much slower (it was 0.75 mg/kg on the 85th
day and 0.23 on the 120th day). Behavioural changes were observed
in both antelope species after 3 days. From the 4th day, there
were further nervous symptoms, including clonic convulsive attacks,
and partial or even complete blindness was also noted. Blesbuck
died from the 4th day onwards, and the entire population of 35
animals had died by the 19th day. The median time to death was
7.08 days, and there was no significant difference between adults
(male or female) and juveniles. The calculated mean intake of
dieldrin was 1.82 mg/kg herbage per day. This estimate was much
lower than that derived from the feeding trial (the total intake of
animals that died was 9.08 mg/kg). The mean concentration in the
livers of six blesbuck that died was 8.3 mg/kg. It was inferred
that dieldrin was unlikely to be the cause of death of the blesbuck
(further investigations implicated photodieldrin). Springbok were
less adversely affected: deaths occurred from the 6th day, and 70%
had died by the 13th day. Surviving animals recovered with no
after-effects, and two ewes lambed normally in the spring following
the winter treatment. The average dieldrin concentration in the
livers of three springbok that died was 9.2 mg/kg. The
pathological findings were similar to those in the common
laboratory species.
Few other mammalian species have been investigated. Reduced
reproductive success and some mortality has been reported in
raccoons fed 2 mg dieldrin/kg diet (Stickel, 1975).
7.4. Effect on Populations and Ecosystems
In order to show that a chemical has had an effect on
populations of organisms in the environment it is necessary to
satisfy a combination of several criteria. Ideally the exposure to
the chemical in the field should be established to compare exposure
with effects produced. Population declines should correlate with
usage of the chemical and should be reversed by controls on the use
of the chemical. Although it is generally recognized that dieldrin
affected populations of some animals when its use was widespread,
there are some difficulties in establishing its precise effects on
the environment. These difficulties arise because the use of
dieldrin coincided with the use of other persistent
organochlorines, which themselves affect populations of organisms,
and also because poisoning by dieldrin was often secondary
(poisoned organisms did not take in dieldrin directly but from prey
that had concentrated the chemical from the environment).
7.4.1. Exposure to dieldrin
It is difficult to establish the exposure of wildlife to
dieldrin unless animals are directly feeding on dressed grain or
directly exposed to preserved wood. Even where this occurs, the
animal will frequently die some distance from the source of
exposure. This is particularly true for birds but less so for
small mammals. Since exposure cannot be readily monitored
directly, most investigators have estimated exposure from the
residue remaining in dead or dying animals. Monitoring programmes
in several countries sampled both dead and dying animals and
compared them with healthy animals taken from the wild. Eggs of
birds were also monitored as a measure of dieldrin contamination
of populations. These monitoring programmes had to establish
criteria by which it could be definitively stated that particular
individuals had died from dieldrin poisoning. The criteria were
based on residue levels in experimentally poisoned animals and were
set at 5 - 10 mg/kg brain tissue and 10 - 20 mg/kg liver tissue for
birds (Robinson, 1969; Stickel et al., 1969; Cooke et al., 1982).
These criteria are probably conservative; 20 - 30% of dead wood
pigeons examined in the UK during the period 1961 - 1964 were
judged, by these criteria, to have died from dieldrin poisoning.
During this period many seed-eating birds were killed directly by
eating dieldren-dressed grain (Robinson, 1969); the actual
percentage of death attributable to dieldrin should probably be
higher. A high proportion of dead birds from areas of tsetse fly
control contained residues which would be judged lethal by these
criteria. The great majority of birds sampled contained non-lethal
residues of dieldrin (Tables 15, 16, 17, 18, and 34). It should be
remembered that all of these sampled birds contained residues of
other organochlorines, in addition to dieldrin. Although there
have been reports of populations with no dieldrin contamination,
but contamination with other organochlorines, there have been no
reports of populations contaminated by dieldrin alone. Furthermore,
residues of dieldrin always correlate well with residues of other
organochlorines; birds retaining large quantities of dieldrin also
retain large quantities of DDE and often polychlorinated biphenyls
(PCB) (Newton, 1979).
The literature reporting the presence of dieldrin in birds and
mammals from the wild is very extensive and has been selectively
reviewed elsewhere (section 5.1.6). Analysis of a few dead animals
serves to indicate the presence of dieldrin in wildlife but is of
little use in establishing effects at the population level. Only
long-term monitoring programmes, measuring changes in dieldrin
residues in the population with time and correlating this to
ecological monitoring of the size and reproductive success of the
population, can approach an objective assessment of the effects of
dieldrin. Such programmes have been reviewed by Newton (1979).
7.4.2. Effects on populations of birds
Populations of birds of prey declined during the period of
large scale use of organochlorine insecticides. Major studies of
changes in bird populations concentrated on a few species mainly in
the United Kingdom and North America, though also to some extent in
areas of mainland Europe. The following references are
illustrative on this subject of the literature: general
references, Anon (1964), Prestt (1965), Prestt & Bell (1966),
Parslow (1973), Bijleveld (1974), Cooke et al., (1976, 1982),
Havera & Duzan (1986); peregrine falcon (Falco peregrinu),
Ratcliffe (1963, 1965, 1967b, 1970, 1972, 1980, 1984), Lockie &
Ratcliffe (1964), Cade et al. (1968), Enderson & Berger (1968),
Hickey (1969); heron (Ardea cinerea), Reynolds (1974); golden eagle
(Aquila chrysaetos), Brown (1969), Lockie et al. (1969); sparrow-
hawk (Accipiter nisus), Koeman et al. (1972), Newton (1973a,b,
1974, 1976, 1979), Newton & Bogan (1974, 1978); Newton et al.
(1979), Marchant (1980); kestrel (Falco finnunculus), O'Connor
(1982).
In most of these studies, population decline correlated with
organochlorines residues in adult birds and their eggs. Reduced
breeding success was associated with thinning of eggshells,
behavioural changes resulting in egg breakage, and aggressive
interaction between adults resulting in a reduction of the number
of young fledged successfully from the clutches. The death of
adult birds was reported at the same time as seed-eating species
were dying from dieldrin poisoning.
As reported earlier in this section, dieldrin cannot be held
responsible for the eggshell-thinning effect, which has been shown
to be attributable to DDE (Cooke, 1973). Embryo deaths in shell
correlate best with PCB residues in eggs (Newton, 1979). The
contribution of dieldrin to these declines is difficult to
determine because the birds were subjected to residues of all
organochlorines. It is probable that dieldrin contributed to
population declines in some areas but not others (Newton, 1979;
Newton & Haas, 1984).
The studies of Blus et al. (1974a,b, 1975, 1979a,b) and Blus
(1982) on the brown pelican (Pelicanus occidentalis) conclude that
the decline in numbers could be ascribed entirely to DDE. The
population size of birds of prey in some of the Eastern states of
the USA declined when no dieldrin residues were present, DDE alone
being a contaminant in these populations. Newton (1979) pointed
out that the decline in populations of birds of prey contaminated
by DDE is gradual, a result of progressive effects of failure in
breeding. The decline of populations of peregrine falcon and
sparrow-hawk in the United Kingdom was more sudden and was
associated with the death of breeding adults. This was attributed
to dieldrin usage, which correlated well with the decline (Newton,
1979; Newton & Haas, 1984).
7.4.3. Effects on populations of mammals
Some mammal species in addition to birds, have been affected by
the use of organochlorine pesticides. There are reports of
decreases in the number of badgers (Meles meles) in some areas of
the United Kingdom (Jefferies, 1969, 1975). Declines in the number
of bats have been reported in the United Kingdom and the
Netherlands (Jefferies, 1972); furthermore, the grey bat (Myotis
grisesceus) in the USA (Clark et al., 1978) and the otter (Lutra
lutra) have also been affected (Jefferies et al., 1974; Chanin &
Jefferies, 1978).
Declines in bat numbers have been associated with the use of
dieldrin and lindane in wood preservatives in the United Kingdom
(Jefferies, 1972). In the United States, they appear to be related
to a combination of organochlorines. Many species migrate long
distances, using fat reserves on the journey, and are susceptible
to DDE poisoning en route (Clark & Kroll, 1977). No contribution
of dieldrin to declines in bat numbers in the USA has been proven.
Other declines have been attributed to dieldrin (Chanin &
Jefferies, 1978). Jefferies & Pendlebury (1968) studied the effect
of aldrin/dieldrin seed dressings on the populations of stoats,
weasels, and hedgehogs in the United Kingdom. None of these
species showed a decline during the period 1959 to 1962, and there
was no evidence that aldrin/dieldrin had any detrimental effects.
Jefferies et al. (1973) studied the behaviour of small mammals
in and adjacent to a field sown with dieldrin-dressed wheat. The
field mouse Apodemus, which lives on the field margin and the open
field, immediately fed on dosed grain. Residues of dieldrin in
sampled mice was very high. The bank vole Clethrionomys, which
lives in field margins, did not take the dosed grain. Residues of
dieldrin in these small mammals were monitored regularly after
sowing. These very quickly dropped to very low levels. The
authors propose that those individuals eating dressed grain died
quickly or were taken by predators. Populations were quickly
replenished by immigration from surrounding areas.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
8.1.1. Aldrin and dieldrin
8.1.1.1 Oral
The acute oral LD50 values for technical aldrin and dieldrin in
various animal species are shown in Table 36. Intoxication with
cyclodiene insecticides consists of increased irritability and
tremor, followed later by tonic-clonic convulsions. In rats,
convulsions appear within 1 h following oral dosing at high
concentrations; death follows within 6 h, or from 2 - 7 days later.
This depends on factors such as the contents of the rat's
gastrointestinal tract, the concentration of aldrin/dieldrin in the
solvent, and the type of solvent used (Borgmann et al., 1952b;
Heath & Vandekar, 1964). Fox & Virgo (1986) reported that dieldrin
induced hyperglycemia.
Table 36. Acute oral LD50 values for technical aldrin and dieldrin
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Mouse corn oil 44 38 Borgmann et al. (1952a,b)
Mouse olive oil ~75 Jolly (1954)
Rat (newborn) arachis oil 168a Lu et al. (1965)
Rat (pre-weaning) arachis oil 25 Lu et al. (1965)
Rat (adult) arachis oil 37 Lu et al. (1965)
Rat arachis oil 51-64 Heath & Vandekar (1964)
Rat various 38-67 Lehman (1951); Borgmann et al.
(1952a); Treon & Cleveland (1955);
Gaines (1960); Worthing & Walker
(1983)
Rat various 37-87 Lehman (1951); Borgmann et al.
(1952b); Treon & Cleveland (1955);
Gaines (1960); Lu et al. (1965);
Worthing & Walker (1983)
Hamster olive oil 320 330 Gak et al. (1976)
Hamster corn oil 100 Cabral et al. (1979a,b)
Guinea-pig corn oil 33 49 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
Table 36. (contd.)
------------------------------------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
------------------------------------------------------------------------------------------
Guinea-pig olive oil between 10 Jolly (1954)
and 25
Rabbit corn oil 50-80 45-50 Borgmann et al. (1952a,b)
Dog corn oil 65-95 65-80 Borgmann et al. (1952a,b)
------------------------------------------------------------------------------------------
a Transcutaneous intragastric injection.
The minimum toxic and the maximum non-toxic doses of aldrin and
dieldrin, administered orally to livestock, are indicated in Table
37.
Table 37. Acute oral toxicity of aldrin and dieldrin for livestock
-------------------------------------------------------------------
Compound Species Age Maximum Minimum Reference
non-toxic toxic
dose dose
tested found
-------------------
(mg/kg body weight)
-------------------------------------------------------------------
Aldrin calf 1-2 weeks 2.5 5 Radeleff et
al. (1955)
cattle 1 year 10 25 Radeleff et
al. (1955)
sheep 1-2 years 10 15 Radeleff et
al. (1955)
Dieldrin calf 1-2 weeks 5 10 Radeleff et
al. (1960)
cattle 1 year 10 25 Radeleff et
al. (1955)
horse - - 25 Radeleff et
al. (1960)
pig 3 weeks 25 50 Radeleff et
al. (1960)
sheep 1 year 15 25 Radeleff et
al. (1960)
sheep 9-12 months - LD50 Jolly (1954)
50-75
-------------------------------------------------------------------
8.1.1.2 Dermal
The minimum lethal dose of aldrin or dieldrin when applied as a
dry powder on the intact skin of female rabbits for 24 h was
between 600 and 1250 mg aldrin/kg body weight and between 250 and
360 mg dieldrin/kg body weight. In olive oil, the range for aldrin
was the same as for dry powder, and the range for dieldrin was
between 360 and 600 mg/kg body weight (Treon et al., 1953).
The acute dermal LD50 values for technical aldrin and dieldrin
in various animal species are shown in Table 38. The signs of
intoxication are similar to those that follow oral administration.
Table 38. Dermal LD50 values for technical aldrin and dieldrin
---------------------------------------------------------------
Species Vehicle LD50 Reference
Aldrin Dieldrin
(mg/kg body weight)
---------------------------------------------------------------
Mouse solvent - 40-80a Jolly (1954)
naphtha
Rat xylene ~100 60-90 Gaines (1960)
Guinea-pig solvent - 120a Jolly (1954)
naphtha
Rabbit dimethyl- 150 150 Lehman (1952)
phthalate
---------------------------------------------------------------
a With complete immersion of body.
8.1.1.3 Inhalation
The vapour pressures of technical aldrin and dieldrin are
sufficiently low that an acute inhalation hazard from aldrin or
dieldrin vapour does not normally arise.
8.1.1.4 Parenteral
The acute LD50 values for technical dieldrin (in glycerol
formal) in the rat via intraperitoneal and intravenous routes are
56 and 8 - 9 mg/kg body weight, respectively (Heath & Vandekar,
1964).
8.1.2. Formulated materials
8.1.2.1 Oral and dermal
The acute toxicity of formulated products, particularly the
dermal toxicity, is a more realistic guide than that of the
technical product to the acute hazard to the user. The percentage
of aldrin or dieldrin in the formulation, the solvent used, and the
type of formulation (such as an emulsion, wettable powder, dust,
etc.) will determine the acute toxicity of the formulated product.
Depending on these factors, the oral and dermal LD50s vary from 100
to 4500 and 500 to 16 000 mg total aldrin formulation/kg body
weight, respectively. For dieldrin formulations, these figures are
100 - 400 mg/kg and 200 - 2700 mg formulation/kg body weight,
respectively (Muir, 1970; Rose, 1982, 1984a,b).
For a dieldrin formulation for termite control (680 g/litre
suspension concentrate), the dermal LD50 in the male rat was 645 mg
formulation/kg body weight and in the female rat 284 mg
formulation/kg body weight (Rose, 1984c).
8.1.2.2 Inhalation
The acute inhalation LC50 (4-h exposure) in rats for aqueous
dilutions of a 48% (w/v) emulsifiable concentrate of aldrin (high
aromatic solvent) in the form of a spray was estimated to be
equivalent to 3% (w/v) aldrin. The median droplet size was 52 µm,
and the animals were exposed "nose only". Deaths occurred up to 6
days after exposure, but most of the animals died on the 2nd day
after exposure. Signs of intoxication consisted of a subdued
and hunched appearance with piloerection, progressing to
hypersensitivity and convulsions in the more seriously affected
animals. Surviving animals recovered within 2 - 3 days after
exposure. Oral intake (due to grooming) contributed significantly
to the results (MacDonald, 1982).
8.2. Short-Term Exposures
Short-term studies on rodents have shown that aldrin and
dieldrin affect the liver. The liver/body weight ratio is
increased, and histopathological changes that have become known as
"Chlorinated Hydrocarbon Insecticide Rodent Liver" (CHIRL) are
observed. Microscopically, these CHIRLs consist of enlarged
centrilobular hepatocytes with somewhat increased cytoplasmic
oxyphilia and peripheral migration of the basophilic granules
(Treon & Cleveland, 1955; Ortega et al., 1957).
The cellular and subcellular changes in the liver of different
mammalian species have been studied by Wright et al. (1972, 1977,
1978). These studies have shown that dieldrin produces a
generalized enlargement of the liver, which, in rats and dogs, is
associated with increased size of liver parenchymal cells but, in
mice, is associated with an increase in both cell size and cell
number. The earliest ultrastructural change in the livers of mice,
rats, and dogs treated with dieldrin was the proliferation of the
smooth endoplasmic reticulum (SER). During the initial phase of
the exposure to dieldrin, and also to phenobarbital, the increases
in the SER in the liver cells of mice, rats, and dogs were of the
vesicular type. These changes were associated with an enhanced
microsomal mixed-function oxidase, and intracellular whorls of
smooth membranes appeared in the liver cells of rats and dogs but
not in those of the mouse. The changes in liver subcellular
structure and function were reversible in mouse, rat, and dog. In
contrast, no liver enlargement or other ultrastructural changes
were observed in the livers of rhesus monkeys. In vitro
determinations showed that the activity of the liver microsomal
monooxygenase system was increased after treatment, and this was
the most sensitive effect observed. In all species examined, the
biochemical and subcellular structural response of the liver to
dieldrin was shown to be similar to that found with a number of
other chemicals, such as DDT, heptachlor, and phenobarbital
(Jansen, 1979). These chemicals also induce in the mouse a type of
liver tumour identical to that found with dieldrin (Stevenson &
Walker, 1969; Thorpe & Walker, 1973).
8.2.1. Oral
8.2.1.1 Rat
A number of short-term feeding studies (3 - 9 months duration)
were carried out on rats with aldrin. Dose levels of 0.5 - 300 mg
aldrin/kg diet were tested. The results of these old studies
showed that dose levels up to 5 mg/kg diet produced no effects, but
that levels of 25 mg/kg or more gave an increased liver/body weight
ratio and reversible hypertrophy of centrilobular hepatocytes with
cytoplastic changes (CHIRL) (section 8.4.2). Dose levels of 150
mg/kg or more resulted in increased mortality (Treon et al., 1951;
Borgmann et al., 1952a).
Five further studies were carried out with dieldrin (3 - 10
months duration), using dose levels of 1 - 300 mg/kg diet. No
effects were seen up to 5 mg/kg, except that Walton et al. (1971)
found an increased liver/body weight ratio in females at 5 mg/kg
and Ortega et al. (1957) found occasional liver changes at 2.5
mg/kg diet. These changes, e.g., liver enlargement and induction
of CHIRL, were found in the other studies at 10 mg/kg diet or more.
At 150 mg/kg or more, there was increased mortality (Treon et al.,
1951; Borgmann et al., 1952b; Ortega et al., 1957; Walton et al.,
1971).
When groups of male albino rats were fed diets equivalent to
2 mg/kg body weight for 6 months, the alkaline phosphatase, SGPT,
SGOT, and LD-hydrogenase activities in the serum were increased
after 6 months. The urea content decreased after 3 months, and
some other parameters were also changed. The growth of the animals
was considerably inhibited (Shakoori et al., 1986).
8.2.1.2 Dog
Dogs appear to be more susceptible to aldrin and dieldrin than
rats. Dogs administered aldrin in the diet for 5 or 6 days at dose
levels equivalent to 0.9 - 9.1 mg/kg body weight died within 7
months. However, beagle dogs (two males and two females) survived
15.6 months when given 0.043 - 0.25 mg aldrin/kg body weight. With
dieldrin, dogs survived dose levels up to 0.23 mg/kg body weight
for 15.7 months. In aldrin- and dieldrin-treated animals, no
effects on growth and no changes in haematology were seen. The
dogs with 0.25 mg aldrin/kg showed hepatomegaly, and the females
had local hyaline (droplet) degeneration of hepatocytes and
vacuolization in the epithelia of distal renal tubules. One of the
males of this group showed hepatocyte degeneration, while the other
exhibited the renal tubular changes seen in the females. In the
group fed 0.09 mg/kg, no effects were seen in the males, while the
females (and also one female in the group fed 0.23 mg/kg) showed
vacuolization in the epithelia of the distal renal tubules. The
liver weights of the dieldrin-treated animals were increased. No
other dogs showed gross or microscopic abnormalities in the viscera
(Treon & Cleveland, 1955).
Four beagle dogs fed dieldrin at daily oral doses of 0.4 - 0.8
mg/kg body weight showed blood concentrations of 0.27 - 1.27
mg/litre blood after eight episodes of convulsions (Brown et al.,
1964). When two dogs were given 0.2 mg dieldrin/kg body weight, in
gelatin capsules daily for 8 months, no signs of intoxication were
observed. The concentration in the blood was 0.11 - 0.22 mg/litre.
In studies by Fitzhugh et al. (1964), twelve mongrel dogs of
various ages received aldrin, 6 days/week for periods up to 25
months, at doses of 0.2, 0.5, 1, 2, or 5 mg/kg body weight.
Dieldrin was tested in 14 mongrel dogs at doses of 0.2, 0.5, 1, 2,
5, or 10 mg/kg body weight. In both cases, there were two animals
per dose level (one male, one female), with the exception of four
animals in the groups given 0.5 mg/kg. In the animals tested with
aldrin, the 5 mg/kg dogs and one 2 mg/kg dog died in 3 - 4 weeks;
the remaining male dog in the 2 mg/kg group was killed at 25 weeks
because of poor condition. All four dogs showed weight loss and
fatty changes in the liver and renal tubules. The bone marrow
showed a reduced number of mature granulocytes and erythroid cells.
At 1 mg/kg, the two dogs survived for 15 and 49 weeks and, at
autopsy, showed the same lesions. In the 0.5 mg/kg group, one dog
died after 4 days. The remaining three dogs survived for 2 years,
one male among these having convulsions during the last 2 months.
At 0.2 mg/kg body weight, there were no effects. In the animals
tested with dieldrin, all six dogs on 2, 5, and 10 mg/kg died
during weeks 2 - 5. These dogs showed weight loss, fatty changes
and slight hepatic cell atrophy in the liver, and a small amount of
atypically distributed fat in the kidneys. The bone marrow showed
a reduced number of mature granulocytes and erythroid cells. The
reported bone marrow findings, which were not replicated in other
studies, cannot be interpreted, because of the inadequacy of
clinical details, and no control dogs were used in this study. The
two dogs given 1 mg/kg survived for 12 and 43 weeks and, at
autopsy, showed the same lesions. One dog given 0.5 mg/kg was
sacrificed after 2 weeks because of anorexia and marked emaciation.
Detailed histological examination, including that of the brain, did
not show any distinct organ damage. The remaining three dogs in
the 0.5 mg/kg group died with terminal convulsions or were
sacrificed in poor condition at weeks 29, 43, and 81. Two of the
dogs showed weight loss. No effects were observed in the 0.2 mg/kg
group.
Repeated daily oral administration of 0.2, 1, or 2 mg
dieldrin/kg body weight to groups of six mongrel dogs was carried
out until intoxication occurred between the 18th and 85th day. A
direct relationship was established between the dieldrin
concentration in the blood and the severity of clinical signs of
intoxication. On the first day of muscle spasms, the average
concentration of dieldrin in the blood was about 0.50 mg/litre and,
at the time of the first full-blown convulsion, about 0.90 mg/litre
(Keane & Zavon, 1969a).
In studies by Walker et al. (1969b), groups of five beagle dogs
of each sex received, by capsule, daily doses of 0.005 or 0.05 mg
dieldrin (in olive oil)/kg body weight, for 2 years. Control dogs
were given capsules containing olive oil. The health, behaviour,
and body weight were unaffected, and EEG recordings did not differ
between the dogs fed 0.05 mg/kg and the controls. In females given
0.05 mg/kg, liver/body weight ratio was increased. In both sexes,
serum alkaline phosphatase activity was increased. However, urine,
haematology, clinical chemistry, bromosulfthalein clearance, and
relative organ weight data were not affected. No gross or
histopathological anomalies were observed.
Deichmann et al. (1969) gave groups of six beagle dogs (aged
1.5 - 3.5 years) 0 or 0.6 mg aldrin/kg body weight, 5 days/week for
10 months, and then observed them for an additional 12 months. The
treated dogs showed hyperexcitability, tremors, and weight loss.
One dog died. After 14 - 18 months, the dogs with aldrin showed
cloudy swelling and fatty degeneration in the liver and hypertrophy
of hepatocytes. Renal vascular congestion and tubular degeneration
were seen in some of the animals.
8.2.1.3 Domestic animals
A dairy cow given aldrin in soybean oil daily by capsule (2.2
mg/kg body weight) exhibited hyperirritability after 27 days. The
animal was in heat and was bred the next day. She died on day 29
with convulsions. Autopsy showed a slightly discoloured, pulpy,
congestive liver and one slightly enlarged congested kidney. No
mortality occurred among cows given 0.8, 1, or 1.5 mg/kg body
weight for 48 days (Ely et al., 1954).
In studies by Gannon et al. (1959b), groups of four dairy cows
were fed rations containing 0, 0.1, 0.25, 0.75, or 2.25 mg
dieldrin/kg for 12 weeks (average total intake, 0, 0.293, 0.75,
2.17, or 6.55 mg dieldrin/kg body weight). No signs of illness and
no abnormalities were found when the cows were slaughtered at the
end of the test feeding period or after an additional 6-week period
on dieldrin-free rations.
Ivey et al. (1961) fed groups of 2 - 3 steers, sheep, and hogs
rations containing 0, 0.25, 0.75, or 10 mg aldrin/kg diet for 12
weeks. Two steers received rations with 2 mg/kg diet for the same
period. The control groups consisted of two animals each. No
evidence of illness and no postmortem pathology were found.
Goats administered 50 mg aldrin/kg body weight showed mild
degenerative changes, congestion and petechial haemorrhages, in
various organs. In the kidneys degenerative changes of the
proximal convoluted tubules were found. Clinical changes were also
found, e.g., salivation and convulsions (Singh et al., 1985).
8.2.2. Dermal
In studies by Treon et al. (1953), aldrin and dieldrin, as dry
powders or in solutions, were applied daily to the skin of groups
of three female rabbits for 2 h on each of 5 days per week over a
period of 10 weeks. A series of graded doses was used to determine
the doses resulting in no mortality. It was clear that aldrin
or dieldrin dissolved in kerosene was very toxic (LD50 of
approximately 5 mg/kg body weight). Dissolved in vegetable oil
they were about 6 times less toxic and as dry powder about 20 times
less toxic than when dissolved in kerosene.
8.2.3. Inhalation
Mice, hamsters, and guinea-pigs did not show any adverse
effects when exposed to vapourized aldrin at a concentration of 18
mg/m3 for 178 days (Baker et al., 1959).
8.3. Skin and Eye Irritation; Sensitization
8.3.1. Skin and eye irritation
Treon et al. (1953) reported that technical aldrin or dieldrin
applied on the intact rabbit skin for 24 h occasionally caused
slight erythema. Repeated application of aldrin or dieldrin for 10
weeks (2 h per day, 5 days per week) as a dry powder did not alter
the gross condition of the rabbit skin. Slight irritation and
scaliness were observed when the compounds were applied in
vegetable oil, but their application in kerosene resulted in
damage, attributable to the solvent.
An undiluted aldrin emulsifiable concentrate formulation (48%
aldrin in high aromatic hydrocarbon solvent), applied at a dose of
0.5 ml at each test site for 24 h on the intact skin and abraded
skin of rabbits under an occlusive patch, caused severe irritation
and necrosis of the skin. One male died on day 13. When applied to
the rabbit eye, this undiluted 48% emulsifiable concentrate caused
severe initial pain and mild irritation (Rose, 1982).
8.3.2. Sensitization
In the Magnusson and Kligman guinea-pig maximization test, 48%
aldrin emulsifiable concentrate caused positive responses (at 24 h
and 48 h after removal of the challenge patches) in 3 out of the 20
test animals. Rechallenge of these animals, one week later,
confirmed that they had been sensitized to the test material (Rose,
1982).
Aldrin emulsifiable concentrate (48%) is not a skin sensitizer
under the EEC Dangerous Substances Directives (EEC, 1983).
No cases of skin sensitization occurred over a period of 20
years among a group of over 1000 workers involved in the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
8.4. Long-Term Toxicity and Carcinogenicity
8.4.1. Mouse
Davis & Fitzhugh (1962) fed groups of 100 male and 100 female
C3HeB/Fe mice a diet containing aldrin or dieldrin at 0 or 10 mg/kg
diet for 2 years. The average lifespan of the treated mice was
shortened by 2 months. A significant increase in the incidence of
benign liver tumours was observed.
In a further study, 100 male and 100 female C3HeB/Fe/J mice
received aldrin or dieldrin at 0 or 10 mg/kg diet for 2 years. An
increase in the number of animals with hepatic hyperplasia and
benign liver tumours was seen in the treated groups, but no
increase in malignant liver tumours was found. The survival in the
treated groups was lower than that of controls (Davis et al., 1965).
Groups of 300, 125, 125, and 200 CF1 mice of each sex were fed
diets containing dieldrin (> 99%) at 0, 0.1, 1, or 10 mg/kg,
respectively, for 2 years. A positive control group fed 600 mg
4-amino-2,3-dimethylazobenzene for 6 months, followed by a control
diet, was used. After 9 months, the morbidity of the mice fed 10
mg/kg started to increase, but the lifespan of the mice fed 0.1 or
1 mg/kg was unaffected. The animals with 4-amino-2,3-
dimethylazobenzene died within 14 months. The frequency of liver
tumours was increased in all groups fed dieldrin. Two types of
tumours were observed, one of which was considered benign
(hyperplastic nodules) and the other malignant. The malignant
tumours were clearly hepatocarcinomas, though no fibrosis or bile
duct proliferation, as seen in the positive control group, occurred
in the dieldrin-treated groups. A reversibility study in a
separate group fed 10 mg dieldrin/kg in the diet for up to 15
months, followed by a control diet for the rest of the 2-year
study, showed that the tumours did not regress or disappear upon
discontinuation of the treatment. However, the dieldrin induced
hepatomegaly, and cytoplasmic changes were found to be reversible
(Walker et al., 1972; Hunt et al., 1975).
In a study by Thorpe & Walker (1973), a group of 30 CF1 mice of
each sex were fed a diet containing 10 mg dieldrin/kg for 2 years.
The control group consisted of 45 mice of each sex. Liver
enlargement was detected after 50 weeks in both sexes, and liver
lesions were observed, classified as hyperplastic nodules (Type a)
and hepatocellular carcinoma (Type b) (sometimes associated with
lung metastases). In a separate study, it was shown that dieldrin
Type b liver cell tumours were capable of growing as subcutaneous
transplants in mice of the same strain and sex (Thorpe, 1973).
To compare the pathological responses to dieldrin in different
mouse strains, groups of 30 mice of each sex of the CF1, LACG, and
hybrid CF1-LACG strains were fed diets containing 10 mg dieldrin/kg
for 2 years. There was also a control group of 45 animals of each
sex for each strain. The incidence of liver tumours, particularly
Type b tumours, in male CF1 and hybrid mice and in females of all
three strains was higher than in controls. In male LACG mice, the
incidence of liver tumours was low. Qualitatively, there was no
difference in tumours between strains, and there was no increased
incidence of neoplasms in other tissues, nor were unusual tumours
found. Metastases of liver carcinoma were found in the lungs in
some of the mice (Thorpe & Hunt, 1975).
In studies by Benitz et al. (1977), nine groups of 100 Charles
River CD1 mice of each sex were given dieldrin in the diet at
concentrations ranging from 0.15 to 15 mg/kg. Six hundred mice of
each sex were used as controls. Groups of animals were sacrificed
at time intervals ranging from 2 to 25 months. Initial changes in
the liver consisted of various degrees of centrilobular and
pericentral hypertrophy. These changes were later associated with
the appearance of hepatic nodules, the occurrence of which was time
and dose related. These nodules consisted of hypertrophic
hepatocytes, which, in a few instances, were mixed with
hyperplastic cells. Various degrees of loss and distortion of
lobular architecture were seen within these nodules. Metastases in
the lung were observed in three nodule-bearing animals given 15 mg
dieldrin/kg for 25 months. These metastases contained similar
hypertrophic hepatocytes as did the primary liver tumours.
Groups of 50 B6C3F1 mice of each sex were fed diets containing
aldrin (4 or 8 mg/kg diet for males and 3 or 6 mg/kg diet for
females) or dieldrin (2.5 or 5 mg/kg for both sexes) for 80 weeks,
followed by an observation period of 10 - 13 weeks. Concurrent
controls consisted of groups of 20 male and 10 female mice. The
pooled controls, used for statistical evaluation, consisted of the
concurrent control groups combined with 92 male and 79 female mice
from similar bioassays of other chemicals. All surviving mice were
killed at 90 - 93 weeks. Body weight was not affected in the
treated animals, but there was a dose-related increase in
mortality, especially in the high-dose groups in the second half of
the study. In the dieldrin-treated mice, clinical symptoms, such
as irritability, tremors, and alopecia occurred. Hepatocellular
carcinomas were found, as indicated in Table 39. The incidence of
hepatocellular carcinomas was clearly higher in male than female
mice. There was no difference in tumour frequencies in other
tissues (NCI, 1978a).
Groups of weanling male C3H/HE mice were fed a diet containing
10 mg dieldrin/kg diet until an age of 57 weeks and then were
either administered a control diet (12 mice) or continued on the
dieldrin diet (11 mice) for another 10 weeks. A third group served
as an untreated control group (21 mice). Laparatomies were
performed and biopsy specimens taken when about 30% of the mice in
each dieldrin-treated group had tumours. Further biopsy samples
were taken approximately 10 weeks later. Tumours were observed at
the first laparotomy in 6/21 controls and 14/23 dieldrin-treated
animals. At the second laparotomy, adenomas were seen in some
animals in which there had been no tumour at the first laparotomy.
In one animal in the continuous dieldrin-treatment group, there was
histological progression from adenoma to hepatocellular carcinoma.
Additional hepatocellular carcinomas were observed in some animals
autopsied at 2 years of age. A strong tendency to tumour
progression was found in both treated and control mice (Ruebner et
al., 1984a,b).
Table 39. Incidence of hepatocellular tumours
in mice (NCI, 1978a)
----------------------------------------------
Groups Males Females
----------------------------------------------
Concurrent controls 3/20 (15%)a 0/10 (0%)a
3/18 (17%)b 0/20 (0%)b
Pooled controls 17/92 (18%) 3/78 (4%)
Aldrin (4 mg) 16/49 (33%)
(8 mg) 25/45 (56%)
Aldrin (3 mg) 5/48 (10%)
(6 mg) 2/43 (5%)
Dieldrin (2.5 mg) 12/50 (24%) 6/50 (12%)
(5 mg) 16/45 (36%) 2/49 (4%)
----------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
In a study by Meierhenry et al. (1983), groups of 50 - 70 male
mice of three strains (C57Bl6J, C3H/He, and C57Bl6J x C3H/He B6C3F1
hybrid) were administered a diet containing 10 mg dieldrin/kg diet
for 85 weeks. The control groups consisted of approximately 60
mice of the same strains. After 4 months, the livers in a small
number of animals showed swellings of hepatocytes in the central
zone with nuclear atypia, small nodules containing basophilic or
eosinophilic foci, and multiple tumours. The percentage of benign
hepatic tumours was 28, 20, and 29, respectively, and in the
control groups, 19, 18, and 4. The percentage of hepatocellular
carcinomas was 30, 38, and 42, respectively, and, in the controls,
0, 12, and 4%. Mallory bodies were seen in all the dieldrin-
treated mice that had either benign or malignant tumours, but only
rarely in mice without tumours.
It seems from the available studies that dieldrin facilitates
and exacerbates the expression of an endogenous oncogenic factor in
CF1 mice (Tennekes et al., 1981). The dose-response characteristics
of dieldrin-mediated enhancement of liver tumour formation in CF1
mice were analysed using existing tumour data from long-term
feeding studies at six levels of continuous exposure, involving a
total of more than 1500 animals. Using the Druckrey equation, the
actual contribution of dieldrin to tumour formation was considered
to be negligible (Tennekes et al., 1985).
8.4.1.1 Appraisal
A number of long-term carcinogenicity studies have been carried
out in which mice of different strains were fed aldrin and/or
dieldrin at one or more dose levels. In all these studies, there
was an increased incidence of liver changes, some of which were of
the nature of hepatocellular carcinomas while others were regarded
as non-malignant. Females seem to be less sensitive than males.
No other tumours were induced.
8.4.2. Rat
Borgmann et al. (1952a) fed six groups of 10 male and 10 female
weanling Sprague-Dawley rats a diet containing 0, 5, 10, 50, 100,
or 150 mg aldrin/kg diet over a period of 2 years. At the two
highest dose levels, an increased mortality was found at 16 months,
which was not seen in the other groups. Liver enlargement was
observed in the groups with high dose levels, but not in the groups
with 10 mg/kg diet or less.
When groups of 40 Carworth rats of each sex were given diets
containing aldrin or dieldrin at 0, 2.5, 12.5, or 25 mg/kg diet for
2 years, there was no increase in mortality and the growth rate was
comparable with that of controls. At all dose levels, the
liver/body weight ratio was increased in males and there were
histological liver cell changes characteristic of CHIRL. No
tumours were reported (Treon & Cleveland, 1955). In a review of
all the aldrin and dieldrin studies, it was reported that there was
no excess of tumours in these rats (Cleveland, 1966).
When groups of 12 Osborne-Mendel rats of each sex were given
diets containing aldrin or dieldrin (0, 0.5, 2, 10, 50, 100, or
150 mg/kg diet) for 2 years, growth was not affected. However,
survival was markedly decreased at dose levels of 50 mg or more in
a dose-related manner. The liver/body weight ratio was increased
in males fed 10 mg/kg or more, while, in females, an increase at
all dose levels was found (no dose-response relationship at the
lower doses). CHIRL was observed in all treated groups, although
at 0.5 mg/kg only a few animals showed a trace of CHIRL. Rats at
dose levels of 50 mg/kg or more showed haemorrhagic urinary
bladders and nephritis. An overall increase in tumour incidence
was noted, but this was not dose related. On the contrary, the
lowest dose levels showed the highest tumour incidence. Only one
liver tumour was found (Fitzhugh et al., 1964).
In a study at the National Institute of Public Health of the
Netherlands, no increased incidence of tumours was found in rats
fed diets containing 75 mg dieldrin/kg for 2 years (Van Genderen,
1965, 1979).
When groups of 30 Osborne-Mendel rats of each sex were fed
5 mg/kg aldrin (95%) or a control diet for 2 years, there was no
increase in mortality, liver/body weight ratios, or tumour
incidence in the aldrin-fed group (Deichmann et al., 1967).
In studies by Walker et al. (1969b), groups of 25 Carworth Farm
E rats of each sex were given dieldrin at 0.1, 1, or 10 mg/kg diet
for 2 years. A control group consisted of 45 males and 45 females.
There was no effect on body weight. After 2 - 3 months, the
animals fed 10 mg/kg exhibited irritability and, as the study
progressed, tremors and occasional convulsions, usually during
handling. Mortality, haematology, serum enzyme levels, and
urinalysis were not affected. The females fed 1 mg/kg and 10 mg/kg
had increased liver/body weight ratios. At 10 mg/kg, one male and
six females exhibited CHIRL. In two females of the group fed 10
mg/kg and in one female control rat, microscopic nodules in the
liver parenchyma were seen. There was no increase in tumour
incidence. Subsequent re-evaluation of these data (Stevenson et
al., 1976) confirmed that there was no treatment-related increase
in tumour incidence.
Nine groups of 50 Osborne-Mendel rats of each sex were fed
diets containing aldrin or dieldrin at 0, 20, 30, or 50 mg/kg for
up to 31 months. A control group consisted of 100 male and 100
female rats. Dose-related tremors and convulsions, always
associated with weight loss, occurred at all dose levels,
particularly in females. Female rats fed 50 mg aldrin/kg or 30 or
50 mg dieldrin/kg had a shortened lifespan. The liver/body weight
ratio was increased in all dieldrin-treated groups and in males fed
30 or 50 mg aldrin/kg, but was decreased in females fed 20 mg/kg.
A moderate increase (not dose related) in the incidence of hepatic
centrilobular cloudy swelling, necrosis, or, rarely, foci of acute
or chronic inflammatory cellular infiltration was observed in all
treated groups. Hyperplasia in the liver was found in two male
rats fed 30 mg aldrin/kg. There was no increase in tumour
incidence (Deichmann et al., 1970; Deichmann, 1974).
Groups of 50 Osborne-Mendel rats of each sex were given diets
containing aldrin at levels of 30 or 60 mg/kg. Treatment of male
rats lasted 74 weeks followed by 37 - 38 weeks of observation,
while that of female rats lasted 80 weeks followed by 32 - 33 weeks
of observation. In a similar study, dieldrin was fed at a level of
29 mg/kg diet (time-weighted average dose) for 80 weeks followed by
observation for 30 - 31 weeks or 65 mg/kg diet (time-weighted
average dose) for 59 weeks followed by observation for 51 - 52
weeks. Concurrent control groups consisted of 10 rats of each
sex. Pooled controls, used for statistical evaluation, consisted
of concurrent control groups combined with 58 males and 60 females
from similar bioassays with other chemicals. All surviving rats
were killed at 110 - 111 weeks. Typical signs of organochlorine
intoxication (such as hyperexcitability) were observed with
increasing frequency and severity, especially in the second year,
but mortality was not affected. No significant increase in tumour
incidence was found (NCI, 1978a) (Table 40).
Groups of 24 Fischer 344 rats of each sex were given dieldrin
at 0, 2, 10, or 50 mg/kg diet for 2 years. Typical signs of
organochlorine intoxication were observed during the second year in
the 50 mg/kg group. Body weight and survival were not adversely
affected in any of the dieldrin groups. Liver tumours were not
observed. No significant increase in tumours was found (NCI,
1978b) (Table 40).
Table 40. Incidence of hepatocellular tumours in rats
(NCI, 1978a,b)
-------------------------------------------------------
Groups Males Females
-------------------------------------------------------
Osborne-Mendel rats
Concurrent controls 1/10 (10%)a 1/10 (10%)a
1/10 (10%)b 0/9 (0%)b
Pooled controls ? 5/59 (8.5%)a
Aldrin (30 mg/kg diet) 1/47 (2%) 0/48 (0%)
(60 mg/kg diet) 1/47 (2%) 3/49 (6%)
Dieldrin (40-20 mg/kg diet)d 0/44 (0%) 1/47 (2%)
(80-40 mg/kg diet)e 1/47 (2%) 1/44 (2.3%)
Fischer F 344 rats
Concurrent controls 2/24 (8.3%) 0/24 (0%)
Pooled controls ? ?
Dieldrin ( 2 mg/kg diet) 0/23 (0%) 0/24 (0%)
(10 mg/kg diet) 0/23 (0%) 0/24 (0%)
(50 mg/kg diet) 4/23 (17%)c 0/23 (0%)
-------------------------------------------------------
a Concurrent controls of aldrin study.
b Concurrent controls of dieldrin study.
c Nodular hyperplasia.
d Time-weighted average dose = 29 mg/kg.
e Time-weighted average dose = 65 mg/kg.
When groups of 50 female rats of two different strains
(Osborne-Mendel and Sprague-Dawley) were fed 0, 20, or 50 mg
aldrin/kg diet, the survival rate was reduced at 50 mg/kg but not
at 20 mg/kg. There was no increase in the incidence of mammary or
liver tumours (Deichmann, 1974; Deichmann et al., 1979).
Photodieldrin, which has metabolites identical to those of
dieldrin, was fed to groups of rats for 80 weeks at concentrations
of up to 7.5 mg/kg diet NCI (1977). No increase in tumour
incidence was found (see section 8.8.1.3).
Ito et al. (1983) studied the promoting activity of dieldrin on
the induction of hyperplastic (neoplastic) liver nodules using a
short-term test system. F344 rats received a single dose (200
mg/kg body weight) of N-nitrosodiethylamine, and 2 weeks later,
were treated for 6 weeks with dieldrin in the diet at a
concentration of 100 mg/kg. Dieldrin had a weak promoting
potential in this test system.
8.4.2.1 Appraisal
A number of long-term/carcinogenicity studies have been carried
out in which rats of different strains were fed one or more dose
levels of aldrin and/or dieldrin. The overall no-effect level in
these long-term studies, both for aldrin or dieldrin, was 0.5 mg/kg
diet. At feeding levels of 1 mg/kg or more, an increasing, dose-
related hepatomegaly and histological changes in the liver
characterized as CHIRL occurred. At levels of 10 mg/kg diet or
more, typical signs of organochlorine toxicity occurred such as
irritability, tremors, and convulsions. In all these studies, no
increase in tumour incidence in liver or other organ/tissue systems
was found.
8.4.3. Hamster
When groups of 34 - 40 Syrian golden hamsters of each sex were
fed diets containing dieldrin (99%) at 0, 20, 60, or 180 mg/kg for
120 weeks, there was no significant increase in tumour incidence
(Cabral et al., 1979b).
8.4.4. Monkey
In studies on rhesus monkeys, groups of five males were given
diets containing dieldrin (88.4%) at 0, 0.01, 0.1, 0.5, 1, or 5
mg/kg (0.0002 - 0.07 mg/kg body weight) for approximately 6 years.
After two monkeys in the group fed 5 mg/kg died, the level of
exposure to the remaining three animals was reduced to 2.5 mg/kg
and, later, to 1.75 mg/kg. Subsequently, one of these animals had
his dieldrin intake progressively increased until at the end of the
second year, he was receiving dieldrin at the initial dietary
concentration of 5 mg/kg. Clinical and haematological examinations,
liver and kidney function studies, urinalysis, and pathology did not
reveal any abnormalities. The liver/body weight ratios and liver
DNA and RNA of the test animals were not different from those of
control animals. No subcellular changes were seen in the
hepatocytes. Dose-related increases in microsomal cytochrome P-450
and in the activity of the liver mono-oxygenase enzyme system were
observed at the two highest dose levels. These alterations in
cytochrome P-450 in the liver microsomes were significant in the
monkeys fed 0.1 mg/kg or more. No effect was observed at 0.01
mg/kg. The concentrations of dieldrin in the subcutaneous fat of
the monkeys fed 0.1 mg/kg were similar to those measured in human
beings receiving a daily oral intake of similar concentration. The
dieldrin concentrations in the monkey livers were approximately 200
times higher than those in male rats receiving a daily intake of
dieldrin 3 times higher than the monkeys, and they were similar to
the concentration in the livers of male mice daily ingesting
dieldrin at a level approximately 50 times higher (Zavon & Stemmer,
1975; Wright et al., 1977, 1978).
8.4.5. Mode of Action
From long-term feeding experiments, it seems that aldrin and
dieldrin may be carcinogenic to mice, but not to rats or hamsters.
Mutagenicity findings have been consistently negative (see section
8.6). There is insufficient knowledge of the mechanism by which
these chemicals might behave epigenetically.
The latest evaluation of IARC (1987) is that there is
inadequate evidence of carcinogenicity in humans and limited
evidence for carcinogenicity in experimental animals.
8.5. Reproduction, Embryotoxicity, and Teratogenicity
8.5.1. Reproduction
8.5.1.1 Mouse
Groups of 100 pairs of male and female virgin CFW Swiss mice
were fed diets containing 5 mg dieldrin/kg for 30 days before
mating, after which time they were randomly paired and fed the same
diet for a further 90 days. Mortality in the dieldrin-treated
group was similar to that of a control group (size not specified).
No major biological effects on fertility, fecundity, gestation
period, size of the first litters, or numbers of young produced per
day were noted as a result of feeding dieldrin. A statistically
significant (6%) decrease in mean size of all litters combined was
the only difference observed between the dieldrin-treated and
control groups (Good & Ware, 1969).
In a reproductive study, groups of 4 male and 14 female Swiss
white mice (120 days old) were fed diets containing 3, 5, 10, or
25 mg aldrin/kg or 3, 10, or 25 mg dieldrin/kg. Six groups served
as controls. The study covered six generations, and two litters
per generation. For both aldrin and dieldrin, the 25 mg/kg dose
was too toxic and resulted in high litter mortality in the few dams
reaching gestation. This dose level was therefore discontinued.
Pup survival was low in mice fed 10 mg dieldrin/kg, and so
treatment was terminated after the first generation. The most
pronounced effect observed in the group fed 10 mg aldrin/kg and, to
a lesser extent, 5 mg aldrin/kg was a low pre-weaning pup survival.
No effects on fertility, viability, or gestation were observed in
six generations of mice fed 3 mg dieldrin/kg. A decrease in pre-
weaning survival was observed in the F2b litters, but a similar
decrease was also found in one of the six control groups (Keplinger
et al., 1970).
In studies by Virgo & Bellward (1975), groups of 18 - 19
uniparous female Swiss-Vancouver mice were given diets containing
0, 2.5, 5, 10, 15, 20, or 25 mg dieldrin/kg, 4 weeks prior to their
second mating, continuing until day 28 postpartum. Significant
mortality of the females occurred at 20 and 25 mg/kg, all deaths
occurring before parturition (89 and 56%, respectively). Fertility
in the groups fed 10 and 15 mg/kg was decreased, though survivors
at higher doses were fertile. Oestrus and gestation period were
not affected. Litter size was decreased only in the group
receiving 25 mg/kg. The major effect was an increase in pre-
weaning pup mortality; 47% at 2.5 mg/kg, 80% at 5 mg/kg, and 100%
at 10 mg/kg or more (31% mortality in control animals). Dams
receiving 10 mg/kg or more exhibited hyperactivity, which was a
contributory factor to the high pup mortality. No gross
abnormalities were detected in the pups, none of whom showed
tremors or convulsions. Within the litters raised at 2.5 and 5
mg/kg, pup survival was not different from controls. The only
effect on reproductive capacity or pup survival observed in female
mice fed 2.5 mg/kg was an increase in pre-weaning pup mortality.
A study was carried out on primiparous female Swiss-Vancouver
mice to investigate whether diets containing up to 15 mg
dieldrin/kg affect maternal behaviour and pup viability. Viability
was investigated in dams fed diets containing 0, 5, 10, or 15 mg
dieldrin/kg for 4 weeks prior to mating. Pups fed 10 mg/kg were
nursed by foster dams not fed dieldrin, and all died by day 4; the
foster dams' own pups showed a very low mortality and survived
until weaning. Similar results were obtained at 5 mg/kg. Dieldrin
did not have any influence on serum progesterone levels, milk
production, or the dam's tendency to retrieve pups or build nests.
However, at 5 mg/kg or more, nursing was reduced. It was concluded
that dieldrin causes irreversible congenital inviability (not
through any effect on progesterone levels) and it was suggested
that the inviability and the reduced tendency to nurse increased
the pup mortality (Virgo & Bellward, 1977).
8.5.1.2 Rat
In studies by Treon & Cleveland (1955), rats (Carworth strain)
were fed aldrin or dieldrin at dietary concentrations of 2.5, 12.5,
or 25 mg/kg for three consecutive generations, two litters being
produced for each generation. (There was no mention of a control
group, and tabulation and description of results was limited in
this report). A reduction in the number of pregnancies, which
gradually disappeared over successive generations, was initially
observed at 12.5 and 25 mg aldrin/kg and at all three doses of
dieldrin. No effects on litter size or pup weights were observed
at any dietary concentration. A marked increase in mortality in
pre-weaning pups was found at dietary concentrations of 12.5 and
25 mg/kg for both compounds. This was thought by the authors to
be due to the high concentration of dieldrin in the milk of the
mothers. Neither aldrin nor dieldrin had any effect on
reproductive capacity. No effects, except a "slight to moderate"
increased pre-weaning pup mortality, were observed in the rats fed
for three generations with aldrin or dieldrin at 2.5 mg/kg.
When groups of 10 male and 20 female Long Evans rats were fed
dietary concentrations of 0, 0.1, 1, or 2 mg dieldrin/kg over three
generations (each generation producing two litters), no effects
were observed on the general health (including weight gain),
behaviour, fertility, gestation, viability, lactation, or organ
weight ratios. No pathological changes were found in parents or
pups. Increased pre-weaning mortality (compared to that in
controls) in the F1a litter was observed in the animals fed 2
mg/kg. This effect was not found in the five subsequent litters
from this group and was not considered to be a major toxic effect.
No changes in reproductive capacity were observed over three
generations at dietary concentrations up to and including 2 mg/kg
dieldrin (Eisenlord et al., 1967).
In studies by Harr et al. (1970), groups of 20 male and 20
female 28-day-old OSU-Wistar rats were fed 0, 0.08, 0.16, 0.31,
0.63, 1.25, 2.5, 5, 10, 20, or 40 mg dieldrin/kg diet throughout
their lifespan. Ten females from each group were mated at 146 days
of age. Mortality occurred in dams at 20 and 40 mg/kg. Fertility
and litter size were decreased in several dose groups without a
clear dose relationship. The number of pups at weaning was
markedly reduced at 2.5 mg/kg or more; none survived at 20 and 40
mg/kg. The nursing pups died in convulsions or starved. No
effects were noted at 1.25 mg/kg or less. Neural lesions, such as
cerebral oedema and hydrocephalus, occurred in pups of nursing dams
at dieldrin concentration of 0.08 mg/kg. Hepatic lesions were
found in rats fed concentrations of 0.31 mg/kg or more.
When groups of 18 - 20 Long Evans pregnant rats were given 4 mg
dieldrin/kg body weight daily by gavage from day 15 of gestation to
21 days post partum, fecundity, number of stillbirths, perinatal
mortality, and total litter weights did not differ from the control
group. No malformations in pups were observed (Coulston et al.,
1980).
8.5.1.3 Dog
In study by Kitselman (1953), seven groups of three dogs, each
group having at least one member of each sex, were fed either
aldrin or dieldrin for one year at dietary concentrations
equivalent to 0, 0.2, 0.6, or 2 mg/kg body weight (in corn oil).
Out of a total of 11 bitches fed either aldrin or dieldrin, 9
conceived. All pregnant bitches produced litters of at least four
pups/litter. The survival of pups was generally lower in the
groups fed aldrin or dieldrin. Histopathological examinations of
dead pups revealed degenerative changes in the liver and mild
degenerative changes in renal tubules. Liver changes were also
observed in treated bitches. The design and size of this study was
too limited to deduce a dose-response relationship for pup
survival, but no effects were observed in dogs receiving 0.2 mg
dieldrin/kg body weight.
8.5.1.4 Appraisal
In the reproductive studies (over one to six generations)
carried out with aldrin or dieldrin on mice and rats, the major
effect observed in most of the studies was an increased mortality
rate in pre-weaning pups. Reproductive performance, per se, was
only affected at doses causing maternal intoxication. The studies
on dogs are of a too limited nature to draw firm conclusions, apart
from the consistent increase in pre-weaning pup mortality.
The results of these reproductive studies indicate that
dieldrin at levels of 2 mg/kg in the rat diet and 3 mg/kg in the
mouse diet (equivalent to 0.1 and 0.4 mg/kg body weight per day,
respectively) are no-effect levels for reproduction. It is not
possible to establish a no-effect level for aldrin for
reproduction, because no adequate data are available.
8.5.2. Embryotoxicity and teratogenicity
8.5.2.1 Mouse
Ottolenghi et al. (1974) gave groups of 10 pregnant CD-1 mice
single oral doses of 25 mg aldrin/kg body weight or 15 mg
dieldrin/kg body weight (in corn oil; equivalent to half the LD50
values) on day 9 of gestation. Control groups consisted of
untreated and corn-oil-dosed mice. No effects on fetal survival or
weight were observed. Abnormalities, such as webbed feet, cleft
palate, and open eyes, were increased in both treated groups, but
they may have been related to maternal toxicity. The percentage of
the total live fetuses that were malformed was 33% for aldrin-
treated mice and 17% for dieldrin-treated ones.
In two comparable studies, pregnant CD-1 mice (6 - 16 per
group) were given daily oral doses of dieldrin in peanut oil at 0,
1.5, 3, or 6 mg/kg body weight from day 7 to day 16 of gestation.
At 6 mg/kg, reduced body weight gain and increased liver/body
weight ratio were observed. There was an increase in supernumerary
ribs and a decreased number of caudal ossification centres in the
fetuses of mice given 6 mg/kg. In one study, the number of
supernumerary ribs was increased at all three dose levels,
(significant at the two highest dose levels) (Chernoff et al.,
1975). In the other study, the increase in supernumerary ribs at
6 mg/kg was not significant. The increase in the number of
supernumerary ribs may be an expression of developmental toxicity.
Doses of dieldrin (99%) were given either in corn oil (0, 1.5,
or 4 mg/kg body weight) or in dimethylsulfoxide (DMSO) (0, 0.25,
0.5, or 1 mg/kg) daily by gavage to pregnant CF-1 mice (7 - 14
mice/group) on days 6 - 14 of gestation. No maternal or fetal
toxicity was seen in groups treated with dieldrin in corn oil or in
the corn oil control groups, but some was seen in the dieldrin/DMSO
and DMSO-control groups. No compound-related teratogenic effects
were observed (Dix et al., 1978).
8.5.2.2 Rat
In a study by Chernoff et al. (1975), pregnant CD rats (9 - 25
per group) were given daily oral doses of dieldrin in peanut oil
(0, 1.5, 3, or 6 mg/kg body weight) from days 7 to 16 of gestation.
At 6 mg/kg, increased mortality and reduced body weight gain were
observed in the dams, but no changes in the liver/body weight
ratios were found. Fetuses did not show any differences from the
controls in mortality, body weight, or occurrence of anomalies.
There were no differences in the average number of sternal or
caudal ossification centres (as were seen in mice). No evidence of
teratogenicity was observed at a dose level of 6 mg aldrin/kg body
weight per day.
No malformations were observed in fetuses or pups from 18 - 20
Long Evans rats given 0 or 4 mg dieldrin/kg, by gavage, daily from
day 15 of gestation to day 21 of lactation (Coulston et al., 1980).
8.5.2.3 Hamster
Pregnant Syrian golden hamsters (41 - 43 per group) were given
single oral doses in corn oil of either 50 mg aldrin/kg body weight
or 30 mg dieldrin/kg body weight on either day 7, 8, or 9 of
gestation. Untreated and vehicle-control groups (respectively, 57
and 41 animals) were used. The high dose levels of both aldrin and
dieldrin caused reductions in the number of live fetuses and fetal
weight and an increased incidence of abnormalities (cleft palate,
open eyes, and webbed feet). The effects were more pronounced
after treatment on days 7 and 8 of gestation than on day 9. It was
suggested that, since webbed foot and open eye were frequently
associated with low fetal weight, these effects might be simply the
expression of growth retardation (Ottolenghi et al., 1974).
8.5.2.4 Rabbit
No teratogenic effects were observed in the offspring of groups
of pregnant Banded Dutch rabbits dosed with dieldrin in
carboxymethylcellulose (2 or 6 mg/kg body weight per day) from
days 6 to 18 of gestation. The animals were killed on day 28 of
gestation and the fetuses were examined for visceral and skeletal
abnormalities (Dix & Wilson, 1971).
8.5.2.5 Appraisal
No evidence of a teratogenic potential has been found from
studies on rats, mice, or rabbits using oral doses up to 6 mg/kg
body weight. Single high oral doses, equivalent to half the LD50,
have been found to cause fetotoxicity and abnormal development of
the fetuses in hamsters and, to a lesser extent, in mice. The
significance of these abnormalities in the presence of severe
maternal toxicity is doubtful but a specific teratogenic potential
cannot be ruled out completely. No gross malformations have been
reported in reproductive studies.
8.6. Mutagenicity and Related End-Points
8.6.1. Microorganisms
Most research workers have reported that aldrin and dieldrin,
with or without microsomal activation, are not mutagenic in
bacterial or yeast test systems. In one study, it was reported
that dieldrin, without activation, was mutagenic in two out of
three strains of Salmonella typhimurium, but there was no dose-
response relationship (Majumdar et al., 1977). The results of the
other mutagenicity studies with aldrin and dieldrin in bacterial
test systems have been negative (Table 41). A critical survey of
the published reports indicates clearly that neither aldrin nor
dieldrin is mutagenic in microbial systems (Ashwood-Smith, 1981).
8.6.2. Mammalian cell point mutations
Only one study on the in vitro mutagenicity of dieldrin to
mammalian cells has been reported. Dieldrin was weakly mutagenic
when tested at a single concentration (0.01 mmol/litre) in ouabain-
resistant Chinese hamster V-79 cells. The significance of this
result is difficult to assess because of the lack of a dose-
response relationship, and a positive control group was not used
(Ahmed et al., 1977a).
8.6.3. Dominant lethal assays and heritable translocation assays
in mice
Aldrin did not show any detectable dominant lethality when
given as a single intraperitoneal dose (8 or 40 mg/kg) or in daily
oral doses (0.5 or 1 mg/kg body weight) for 5 days to male ICR/Ha
Swiss mice (Epstein et al., 1972).
Dieldrin, also, revealed no detectable dominant lethality in
four assays in male mice following a single intraperitoneal
injection (5.2 or 26 mg/kg) or daily oral doses of 2 or 3 mg/kg
body weight for 5 days (Epstein et al., 1972). Likewise, a single
oral dose of 12.5, 25, or 50 mg/kg body weight did not produce
dominant lethality in CF-1 mice (Dean et al., 1975). Bidwell et
al. (1975) carried out a dominant lethal test on B6D2F1/J mice
orally administered 0.08, 0.8, and 8 mg/kg body weight dieldrin for
5 days, but no effects were seen.
In further studies by Bidwell et al. (1975), a heritable
translocation test was performed on male mice after oral intake of
0.008, 0.08, or 0.2 mg dieldrin/kg body weight per day for a period
of 6 weeks. The cytogenetic determination of somatic cells using
the micronucleus test and the usual analysis of spermatocytes did
not reveal an increase in the rate of translocations.
8.6.4. Micronucleus test
Aldrin did not induce a significant increase in the frequency
of micronuclei in the bone marrow of mice treated orally with 13
mg/kg body weight (Usha Rani et al., 1980).
No cytogenetic abnormalities were seen in a standard metaphase
analysis and micronucleus test after oral gavage of mice with 0.8
or 8 mg dieldrin/kg body weight per day for 5 days (Bidwell et al.,
1975).
8.6.5. Chromosome and cytogenicity studies
Chinese hamsters (three groups of four males and four females)
were orally dosed with 30 and 60 mg dieldrin/kg body weight. No
chromosome abnormalities were found in femoral bone marrow cells
(Dean et al., 1975).
Table 41. Aldrin and dieldrin: mutagenicity tests in microorganisms
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
E. coli WP2 Try- none aldrin and dieldrin; negative Ashwood-Smith et
1000 µg/plate al. (1972)
E. coli WP2 hcr rat S9 up to 5000 µg/plate negative Moriya et al.
(1983)
E. coli Ga1 Rs none aldrin and dieldrin; negative Fahrig (1974)
Serratia marcescens alpha 21 and 742 dose not stated
Saccharomyces cerevisiae
Aspergillus nidulans diploid P1 and none dieldrin; 13 or 26 negative Crebelli et al.
haploid strain 35 mmol (1986)
Saccharomyces cerevisiae 632/4 none aldrin; 5 µg/ml on positive Guerzoni et al.
disc (1976)
Bacillus subtilis (Rec-assay) none aldrin and dieldrin; negative Shirasu (1975)
Salmonella typhimurium TA 1535, 1536,
1537, 1538
E. coli WP2 hcr+, hcr-
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; 10, 50, negative Bidwell et al.
1536, 1537, 1538, 100, or 500 µg/plate (1975)
G46
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; dose not negative McCann et al.
1537 stated (1975)
Salmonella typhimurium TA 1535, 1536, rat S9 dieldrin; 1000 negative Marshall et al.
1537, 1538 µg/plate (1976)
Salmonella typhimurium TA 98, 100 none dieldrin; 10, 30, negative Glatt et al.
100, 300, 1000, 3000 (1983)
µg/plate
Salmonella typhimurium TA 90, 100, 1535, rat S9 up to 5000 µg/plate negative Moriya et al.
1537, 1538 (1983)
---------------------------------------------------------------------------------------------------------
Table 41. (contd.)
---------------------------------------------------------------------------------------------------------
Organism Strain Activation Compound/dose Result Reference
system
---------------------------------------------------------------------------------------------------------
Salmonella typhimurium TA 98, 100, 1535, mouse S9 dieldrin; 1000 negative Van Dijck & Van
1537, 1538, 1950, µg/plate de Voorde (1976)
1978
Salmonella typhimurium not specified S9 dieldrin; dose not "weak" Ercegovich &
stated response Rashid (1977)
Salmonella typhimurium TA 98, 100, 1535 mouse S9 dieldrin; 1, 25, or positive Majumdar et al.
50 µg/ml (1977)
Salmonella typhimurium TA 98, 100, 1535, rat S9 dieldrin; up to 2500 negative Purchase et al.
1538 µg/plate (1978)
Salmonella typhimurium TA 98, 100 rat S9 dieldrin; 50 or 1000 negative Wade et al.
µg/plate (1979)
Salmonella typhimurium TA 98, 100, 1535, rat, aldrin; dose not negative Simmon et al.
1537, 1538 mouse, and stated (1977)
human S9
Salmonella typhimurium TA 98, 100 rat S9 aldrin and dieldrin; negative Nishimura et al.
364 and 380 µg/plate (1982)
Salmonella typhimurium TA 98, 100 1535, rat S9 dieldrin 2.6 x 104 negative DeFlora (1981)
1537, 1538 nmoles/plate
---------------------------------------------------------------------------------------------------------
The effect on the bone marrow cells of STS mice was examined
after applying a single dose of dieldrin intraperitoneally at 0, 1,
30, or 50 mg/kg body weight. A decrease in the mitotic index was
noted, as dieldrin concentration increased, and differences in
chromosome aberrations (a slight increase in breaks, fragments,
and interchanges) were found (Majumdar et al., 1976).
Seventy-one juvenile mallard ducks, the parents of which had
been exposed to various dietary levels of dieldrin for 6 months or
longer, were grouped and fed dieldrin at a level that corresponded
to the diet fed to the parents (0, 4, 10, or 30 mg dieldrin/kg
diet) for approximately 60 days. At the end of this period, no
chromosomal aberrations were found in femoral bone marrow cultures.
However, the mitotic index of ducks exposed to 30 mg/kg was
significantly reduced (Bunch & Low, 1973).
When lymphocytic cultures from adult mallard ducks (which had
not been exposed to dieldrin) were treated with 0, 0.1, 1, 10, 30,
or 100 mg dieldrin/kg, there was a significant increase in the
incidence of chromosome structural alterations, but only at the
highest dose level. The mitotic index was significantly reduced at
all dose levels, the greatest decreases occurring at the two
highest dose levels (Bunch & Low, 1973).
Chromosome studies in cultured lymphocytes from current
dieldrin plant workers (12) former plant workers (9), and control
(17) did not show any differences in the frequency of chromosome
aberrations (Dean et al., 1975). In one study, it was reported
that aldrin produced chromosomal aberrations in cultured human
lymphocytes at concentrations of 19 and 38 µg/ml. In mice and
rats, an intraperitoneal injection of a dose of 19 mg/kg body
weight was reported to induce chromosomal gaps, breaks, deletions,
and fragments in bone marrow cells (Georgian, 1975).
Cultured human peripheral lymphocytes from agricultural and
public health (anti-Chagas' disease) workers with at least 10 years
exposure to dieldrin were examined for structural chromosome
aberrations and sister chromatid exchange. No differences were
seen when they were compared with lymphocytes derived from a
control group (Bordon, 1980).
8.6.6. Host-mediated assays
Bidwell et al. (1975) carried out a host-mediated assay,
incorporating blood- and urine-recovery studies, for mutagenic
substances on B6D2F1/J mice. Five daily oral doses of 20 mg
dieldrin/kg body weight were given, and the mice were then injected
intraperitoneally with Salmonella tester strains. The results were
negative. In a further host-mediated assay using Saccharomyces
cerevisiae (strain D4, heteroallelic at the ade-2 and trp-5 loci)
as tester microorganism, CF-1 mice were treated with a single oral
dose of 25 or 50 mg dieldrin/kg body weight or with five daily
doses of 5 or 10 mg/kg body weight. No mutagenic activity was
found (Dean et al., 1975).
8.6.7. Cell transformation in mammalian cell systems
Dieldrin (0.08 - 250 mg/litre) proved negative in mammalian
cell transformation tests using cell lines derived from baby Syrian
hamster kidney (BHK-21 C13) and from human lung (Wl-38), either
with or without metabolic activation by rat liver S9 microsomal
fraction (Purchase et al., 1978).
In a 6-thioguanine resistance mutation assay using FM 3A mouse
cell cultures, aldrin was weakly mutagenic (Morita & Umeda, 1984).
8.6.8. Drosophila melanogaster and other insect systems
There is no evidence of a mutagenic activity of aldrin or
dieldrin in Drosophila melanogaster (Benes & Sram, 1969; Bidwell et
al., 1975). No increase in recessive or dominant lethal mutations
was found following exposure of the wasp Bracon hebetor to
sublethal doses of dieldrin (95%) (Grosch & Valcovic, 1967).
8.6.9. Effects on DNA
DNA strand breakage was not detected in Chinese hamster V-79
cells exposed to dieldrin (0.1 - 1 mmol/litre) in the presence of
rat liver S9 microsomal fraction using the alkaline elution assay
(Swenberg et al., 1976).
Aldrin (1 - 1000 µmol/litre) and dieldrin (1-100 µmol/litre)
induced unscheduled DNA synthesis in SV-40 transformed human
fibroblast cells (VA-4) both in the presence and absence of rat
liver microsomes (Ahmed et al., 1977b). This study was repeated by
Zelle & Lohman (1977). After exposure to dieldrin, the rate of DNA
synthesis in normal primary human fibroblasts (AH) decreased, but
returned to the control level in a few hours. This suggests that
dieldrin interferes with semiconservative DNA replication without
damaging DNA. The effect of dieldrin on the induction of repair
replication was studied with both AH cells and SV-40 transformed
human cells (MM-SV-40). No evidence that dieldrin could induce DNA
repair was found (Zelle & Lohman, 1977).
The DNA breakage rates in an Escherichia coli plasmid after
treatment with aldrin or dieldrin did not differ from those in
untreated plasmid DNA, suggesting that, at least in these studies,
the compounds did not interact directly with DNA (Griffin & Hill,
1978).
The effects of aldrin and dieldrin (both at 100 µg/ml) on the
uptake of tritiated thymidine by cultured rat thymocytes and human
lymphocytes were tested under different experimental conditions.
Both compounds appeared to have marginal effects on thymidine
uptake, suggesting inhibition of DNA synthesis (Rocchi et al.,
1980).
Aldrin (100 mmol/litre) and dieldrin (500 mmol/litre) did not
induce unscheduled DNA synthesis in primary cultures of Fischer 344
rat hepatocytes (Probst et al., 1981). Williams (1982) reported
the results of the hepatocyte primary culture/DNA repair test,
using freshly isolated hepatocytes of high metabolic capability to
monitor the production of DNA damage by measuring DNA repair
synthesis. Aldrin and dieldrin gave equivocal results concerning
DNA repair, but there was no damage to DNA. Aldrin (0.3 - 3
mmol/litre) induced DNA strand breaks in an alkaline elution/rat
hepatocyte assay (Sina et al., 1983).
Cultured hepatocytes from male Balb/c mice treated with
dieldrin at a concentration of 4 x 10-4 mol/litre showed no
unscheduled DNA synthesis. The results were no different with
cells from mice treated in vivo with phenobarbital (Klaunig et al.,
1984).
A DNA synthesis inhibition/damage test on HeLa cells with S9
showed inhibition to 60% of the control value within 90 min of
treatment with 4 x 10-4 mol dieldrin/litre (Painter, 1981).
8.6.10. Cell to cell communication
Both aldrin and dieldrin inhibited gap junctional intercellular
communication between 6-thioguanine-sensitive and 6-thioguanine-
resistant human teratocarcinoma cells in culture (Zhong-Xiang et
al., 1986).
The inhibition of cell to cell communication was observed in
human teratocarcinoma cells in culture in the presence of dieldrin,
using dye transport methods (Wade et al., 1986).
Metabolic cooperation between 6TG-resistant and HGPRT-deficient
Chinese hamster V79 cells was inhibited when aldrin (2.5 - 10
µg/ml) or dieldrin (2.5 - 5 µg/ml) was added to the medium (Kurata
et al., 1982).
Trosko et al. (in press) studied the inhibition of gap
junctional-mediated intercellular communication using co-cultures
of Chinese hamster cells. To do this, an in vitro assay (in which
the metabolic cooperation between V79-6-thioguanine-sensitive (6
TGs) and resistant (6 TGr) cells is studied) has been developed to
detect the ability of non-cytotoxic and non-mutagenic chemicals to
inhibit gap junctional communication. Aldrin and dieldrin
inhibited metabolic cooperation at concentration of about 4 µg/ml
or more.
8.6.11. Appraisal
Aldrin and dieldrin are not mutagenic to mammals in a variety
of mutagenicity test systems with unrelated different end-points.
8.7. Special Studies
8.7.1. Liver enzyme induction
Aldrin and dieldrin have been shown to increase the activity of
liver microsomal enzymes, generally associated with enlargement of
the liver. They have also been found to induce microsomal
dimethylaminoantipyrine-N-demethylase and aldrin-epoxidase, and
increase the cytochrome P-450 level (Campbell et al., 1983). This
enzyme induction is the earliest and most sensitive indicator of an
effect of exposure in mouse, rat, beagle dog, and rhesus monkey
(Wright et al., 1972).
Studies on rats indicated a no-effect level for enzyme
induction, by either aldrin or dieldrin, at 1 mg/kg diet (Gillett &
Chan, 1968; Kinoshita & Kempf, 1970; Den Tonkelaar & Van Esch,
1974).
In the rhesus monkey, the activity of the liver microsomal
monooxygenase system was increased by dieldrin (at daily feeding
levels of 1.75 and 5 mg/kg diet for approximately 6 years), but no
associated liver enlargement was observed. The dietary intake of
dieldrin required for the induction of this enzyme system in
monkeys was approximately 1 mg/kg diet, corresponding to an intake
of 25 - 30 µg/kg body weight per day (section 8.4.4) (Wright et
al., 1978).
In human beings, oral dosing with 211 µg dieldrin/day
(approximately 3 µg/kg body weight per day) for two years, in
addition to the daily intake of 19 µg dieldrin from the diet, did
not increase the activity of microsomal liver enzymes as measured
by the concentration of p,p'-DDE in adipose tissue and blood. No
evidence of enzyme stimulation was observed in a group of 10
workers (at the time, they were the most highly exposed workers) in
a manufacturing plant with a mean exposure equivalent to a daily
oral intake of 17 µg/kg body weight (maximum 24 µg/kg body weight)
(Hunter & Robinson, 1967; Hunter et al., 1969; Jager, 1970).
8.7.2. Nervous system
8.7.2.1 Rat
When three groups of eight male albino rats were fed diets
containing 0, 25, or 50 mg dieldrin/kg for 60 days, there was no
effect on body weight or learning, but muscular efficiency,
measured by pulling weights of increasing magnitude in a 250-cm
runway, was decreased (Khaïry, 1960). This finding is in agreement
with the results of a study on nerve muscle (gastrocnemius)
preparations of pre-treated rats (Ibrahim, 1964).
8.7.2.2 Dog
Groups of five dogs of each sex, given daily oral doses of
dieldrin (0.05 mg/kg body weight) by capsule for 2 years, did not
show any changes in behaviour or EEG recordings compared with
controls (Walker et al., 1969b).
8.7.2.3 Monkey
When groups of three or four adult male squirrel monkeys were
given daily oral doses of 0, 0.01, or 0.1 mg dieldrin/kg body
weight for 54 days, learning ability was impaired and changes (high
amplitude slow waves) in the EEG occurred in both test groups (Van
Gelder & Cunningham, 1975).
8.7.3. Weight loss and stress
It is known that in birds, and perhaps in some small mammals,
dieldrin intoxication may be induced by starvation, weight loss,
and stress in animals having a previously harmless body burden of
dieldrin. Concern is sometimes expressed that, by analogy to these
observations, a similar course of events might occur in human
beings. Therefore, this phenomenon was studied in rats as well as
in human beings (for human beings see section 9.1.3.2).
8.7.3.1 Rat
In studies by Treon & Cleveland (1955), rats previously fed
diets containing aldrin or dieldrin at levels of 5, 10, or 15 mg/kg
diet for 7 - 18 months were starved. The complete withdrawal of
food did not result in the release of aldrin or dieldrin from the
adipose tissue stores to an extent sufficient to induce symptoms of
intoxication of any type.
When Osborne-Mendel rats fed 7.5 mg aldrin/kg diet for 4 weeks
were subsequently starved for 6 days with free access to water,
there was a marked loss of body weight and fat and a decrease in
the liver/body weight ratio. The total body burden of dieldrin
decreased during starvation regardless of age, sex, or the previous
level of exposure. The total quantity of dieldrin in the liver
decreased in all rats. In females, particularly older females, the
concentration of dieldrin in abdominal fat increased, whereas in
all males, the level in fat decreased. The concentration of
dieldrin in the blood was not increased. Young weanling rats
reacted similarly (Deichmann et al., 1972).
8.7.4. Immunosuppressive action
Loose et al. (1981) found that macrophages from mice fed 50 mg
dieldrin/kg diet had a marked impairment in antigen processing.
The effect was statistically significant in Kupffer cells at 50
mg/kg diet, in alveolar and splenic macrophages at 0.5, 5 and 50
mg/kg diet, and in peritoneal macrophages at 5 and 50 mg/kg diet.
There was an impairment of in vivo phagocytic clearance in mice
receiving 5 or 50 mg/kg diet for 8 weeks but not at 0.5 mg/kg diet.
This was related to a decrease in serum fibronectin. Tumour cell
killing after challenge with EL-4, P388, or mKSA tumour cells was
significantly impaired in mice fed either 1 or 5 mg dieldrin/kg
diet. The mean survival time after challenge with EL-4 was reduced
by 3 weeks, and with the P388 or mKSA tumour cells impairment was
observed after 3 or 18 weeks, respectively. There was no
alteration in the oxygen uptake by isolated macrophages either at
rest or during phagocytosis, and no effect on phagocytic activity
or capacity or on chemotaxis in vitro was observed.
Loose (1982) found that dieldrin caused immunosuppression in
mice. Levels of 1 or 5 mg dieldrin/kg diet were fed to BALB/c mice
for 3.5 or 10 weeks, and the mice were challenged intradermally
with Leischmania tropica. Dieldrin acted synergistically on
lethality in a dose- and time-related manner, indicating an effect
on host mechanisms. It also resulted in decreased antibody
formation to PVP, a T-independent antigen (direct splenic plague
assay). The mitogenic response of cultured T-cells to
phytohaemagglutinin (PHA) in dosed mice was depressed. Mitomycin C
and anti-Thy-1 abolished the mitogenic response. When splenic
T-cells from treated mice were mixed with T-cells from control
mice, there was inhibition of PHA mitogenesis. The data indicated
an active cell-mediated suppressor. A soluble macrophage factor
from the hepatic Kupffer cells (but not from alveolar or peritoneal
macrophages) suppressed the T-cell response to PHA. It was
concluded that administration of 5 mg dieldrin/kg diet to mice for
10 weeks caused a profound impairment of macrophage antigen
processing.
8.8. Toxicity of Photodieldrin and Major Metabolites
The relevance of photodieldrin lies in the fact that it has
metabolites identical to those of dieldrin and is quantitatively
and qualitatively similar in toxicity.
8.8.1. Photodieldrin
The photodecomposition of deposits of dieldrin on leaves and
grass has been reported (Roburn, 1963), and the physical and
chemical properties and structure of this decomposition product
have been determined (Robinson et al., 1966b; Rosen et al., 1966).
It appears to be the pentacyclo isomer of dieldrin (hexacyclo
isomer by the alternative nomenclature used by Rosen et al.
(1966)). Photodieldrin residues were less than the limits of
detection in most of the food samples analysed (Robinson et al.,
1966a).
8.8.1.1 Acute toxicity
Photodieldrin is more acutely toxic than dieldrin for mice,
rats, and guinea-pigs (Table 42). The toxicity for dogs is about
equal to that of dieldrin. Dieldrin-like convulsions have been
observed in all species given photodieldrin.
8.8.1.2 Short-term toxicity
(a) Mouse
When groups of five male and five female Carworth Farm No. 1
mice were fed 1, 3, or 10 mg photodieldrin/kg diet for 1 month, all
animals fed 10 mg/kg and two animals fed 3 mg/kg died. No changes
were observed at necropsy (Brown et al., 1967).
Table 42. Oral LD50 values for photodieldrin
-------------------------------------------------------------------
Species Vehicle LD50 Reference
Photodieldrin Dieldrin
(mg/kg body weight)
-------------------------------------------------------------------
Mouse dimethyl- 6.8 77.3 Brown et al.
sulfoxide (1967)
Rat dimethyl- 9.6 46.8 Brown et al.
sulfoxide (1967)
Guinea-pig dimethyl- 2.3-3.9 18-30 Brown et al.
sulfoxide (1967)
Dog (male) gelatin 120-160 120 Brown et al.
capsule (1967)
Dog (female) gelatin 80-120 80-100 Brown et al.
capsule (1967)
-------------------------------------------------------------------
(b) Rat
Groups of five male and five female Carworth Farm E rats were
fed 3 or 10 mg photodieldrin/kg diet for one month without apparent
ill effects (Brown et al., 1967).
In studies by Walton et al. (1971), groups of 28 male and
28 female Charles River rats were fed 0, 1, 5, or 25 mg
photodieldrin/kg diet for 3 months. A similar study was carried
out concurrently with dieldrin. The concentrations of
photodieldrin given in the diet were lowered from 25 to 12.5 mg/kg
diet within the first week of the study because of high mortality.
At the end of 3 months, no significant differences were found in
growth or food intake, and no gross evidence of toxicity was
observed. Liver/body weight ratios were increased at 12.5 mg/kg
diet. Increases in the activity or concentration of liver mixed-
function oxidase and microsomal cytochrome P-450 at 5 and 12.5
mg/kg diet indicated the occurrence of a dose-dependent enzyme
induction. The total protein content of the liver was not
affected. The short-term toxicities of photodieldrin and dieldrin
appeared to be similar.
Walker et al. (1971) fed groups of 12 Carworth Farm E rats of
each sex diets containing 0.1, 1, 10, or 30 mg photodieldrin/kg
diet for 3 months. The control group consisted of 24 male and 24
female rats. Six females given 30 mg/kg and two females given 10
mg/kg died. The animals in these groups that survived were
irritable and showed tremors when handled. Growth was reduced,
increases in serum urea and glutamic pyruvic transaminase (SGPT)
activity were seen in females fed 30 mg/kg, and the liver/body
weight ratio was increased in this group. In the groups fed 10 or
30 mg/kg, kidney/body weight ratio was increased in males. At
autopsy, no gross lesions were seen. Some of the animals fed 10 or
30 mg/kg showed CHIRL and centrilobular fatty changes in the liver.
Eosinophilic droplets were seen in the cytoplasm of the proximal
convoluted tubules and in the lumen of affected tubules in the
kidneys of males fed 10 or 30 mg/kg. No evidence of nephron damage
was found. No effects were observed in the animals dosed with 1
mg/kg.
(c) Dog
In a study by Walker et al. (1971), groups of four male and
four female beagle dogs received photodieldrin in olive oil by
capsule (daily oral doses of 0.005, 0.05, or 0.2 mg/kg body weight)
for 3 months. A control group of six males and six females
received olive oil in gelatine capsules. The health, behaviour,
body weight, and haematology were unaffected. In the 0.2 mg/kg
males, increases occurred in the plasma alkaline phosphatase and
SGPT activities, and, after 13 weeks, their serum protein levels
were slightly reduced. Increases in the liver/body weight ratios
occurred in the 0.2 mg/kg animals and the 0.05 mg/kg females. At
autopsy, no pathological changes associated with photodieldrin were
observed. No effects were observed at 0.005 mg/kg body weight.
8.8.1.3 Long-term toxicity
(a) Mouse
In a study on the long-term toxicity of photodieldrin, groups
of 50 B6C3F1 mice of each sex were fed diets containing 0.32 or
0.64 mg/kg for 80 weeks. After 80 weeks, the animals were fed a
control diet for 12 or 13 weeks. Concurrent control groups
consisted of 10 untreated mice of each sex. Pooled controls, used
for statistical evaluation, consisted of the concurrent controls
plus 60 male and female mice from similarly performed bioassays
with six other test chemicals. All surviving mice were killed at
93 weeks. Mean body weights and mortality were not affected by
treatment, but convulsions and hyperactivity were noted in treated
male mice. No statistically significant increase in tumour
incidence was found (NCI, 1977).
(b) Rat
In similar studies to those on mice, groups of 50 Osborne-
Mendel rats of each sex were given 5 or 10 mg photodieldrin/kg diet
for 80 weeks. After 80 weeks, the animals were fed a control diet
until sacrifice at 111 - 112 weeks. Because of neurotoxicity, the
doses in the females were reduced after 30 weeks, so that the time-
weighted average doses were 3.4 or 7.5 mg/kg diet for the females.
Concurrent control groups consisted of 10 rats of each sex. Pooled
controls, used for statistical evaluation, consisted of the
concurrent controls combined with 65 rats of each sex from similarly
performed bioassays with six other chemicals. All surviving animals
were killed at 111 - 112 weeks. Mean body weights and mortality
were not affected by treatment, but convulsions and hyperactivity
occurred in treated male and female rats. Photodieldrin was not
carcinogenic in this study (NCI, 1977).
8.8.1.4 Reproduction, embryotoxicity, and teratogenicity
(a) Mouse
Chernoff et al. (1975) fed groups of pregnant CD-1 mice (16 -
20 per group) photodieldrin in peanut oil (daily oral doses of 0,
0.15, 0.3, or 0.6 mg/kg body weight) from day 7 to day 16 of
gestation. At a dose of 0.6 mg/kg, one animal died. Liver/body
weight ratios were increased in a dose-related manner, but no
significant differences in fetal mortality, litter weight,
percentage of supernumerary ribs, or sternal or caudal ossification
centres were observed at any of the doses used. Photodieldrin was
not teratogenic or fetotoxic in CD-1 mice at doses up to and
including 0.6 mg/kg body weight.
(b) Rat
In a study by Chernoff et al. (1975), groups of 24 - 27
pregnant CD rats were given daily oral doses of photodieldrin in
peanut oil (0, 015, 0.3 or 0.6 mg/kg) on days 7 - 16 of gestation.
Some maternal mortality (5 out of 24 animals) occurred in the
0.6-mg/kg group. No significant differences in liver/body weight
ratios, fetal mortality, weight of the pups, or occurrence of
anomalies in litters of treated animals, compared with the
controls, were noted. No evidence of teratogenicity in CD rats was
observed at doses of photodieldrin up to and including 0.6 mg/kg
per day.
8.8.1.5 Appraisal
The acute oral toxicity of photodieldrin to rodents is greater
than that of dieldrin. In short-term toxicity and teratogenicity
studies, no major differences between the two compounds were found.
Photodieldrin did not induce tumours in mice and rats. The
accumulation of photodieldrin in the adipose tissue of experimental
animals was less than that of dieldrin (section 6.2.3).
8.8.2. Major metabolites of dieldrin
8.8.2.1 Acute toxicity
The acute oral toxicity of the major metabolites of dieldrin is
far less than that of dieldrin itself (Table 43).
8.8.2.2 Short-term toxicity
In a study by Granville et al. (1973), groups of 12 male and 12
female rats (control group of 24 males and 24 females) were fed
diets containing aldrin dicarboxylic acid (0, 0.1, 1, 10, 100, or
1000 mg/kg diet) for 13 weeks. No adverse effects attributable to
the dosing were observed in general health, behaviour, body weight,
clinical chemical and haematological values, organ weights, or on
pathological examination of the viscera.
Table 43. Oral LD50 values for metabolites of aldrin and dieldrin
in mice
-------------------------------------------------------------------
Compound LD50 Reference
(mg/kg body weight)
-------------------------------------------------------------------
trans-6,7-dihydroxy-dihydro- 1250 Korte & Arent
aldrin (1965)
9-hydroxy-dieldrin > 400 Baldwin et al.
(1970)
hexachlorohexahydromethano- > 850 Baldwin et al.
indenedicarboxylic acid (1972)
(aldrin dicarboxylic acid)
-------------------------------------------------------------------
8.9. Mechanisms of Toxicity; Mode of Action
Like most chemicals, aldrin and dieldrin do not have a single
mechanism of toxicity. The main target organs of these chemicals
are the central nervous system and the liver.
8.9.1. Central nervous system
Intoxication following acute or long-term overexposure is
characterized by involuntary muscle movements and epileptiform
convulsions. Survivors, after a short period of residual signs and
symptoms, recover completely (Hoogendam et al., 1962; Avar &
Czegledi-Janko, 1970; Jager, 1970). In rare cases, a residual
brain injury has been reported, but this has been found to be due
to the convulsive state or prolonged cerebral anaemia rather than
to the dieldrin per se. Apparently, a still unidentified receptor
site in the central nervous system is reversibly occupied, and when
this occupation exceeds a certain degree, myoclonics and
convulsions occur (Van Genderen, 1979). In vitro, the dieldrin
metabolite aldrin transdiol appears to be more potent in this
respect that is dieldrin itself (Van den Bercken, 1972; Van den
Bercken & Narahashi, 1974). However, in cats, the aldrin transdiol
appeared to be inactive (Joy, 1977). The mechanism of action seems
to be a presynaptic inhibition as well as an increased release of
an unidentified transmitter (Akkermans, 1974; Akkermans et al.,
1975; Joy, 1976).
Joy (1982) suggested that dieldrin acts by intensifying
synaptic activity through a presynaptic locus of action and
possibly a post-synaptic action as well. Neurons having a large
number of synapses will be affected most. There does not appear
to be any selective action on a particular neurotransmitter or
neurotransmitter system. The modification of behaviour is dose
dependent and performance in complex behavioural tasks is readily
disrupted.
Aldrin and dieldrin and other cyclodiens inhibit the gamma
amino butyric acid (GABA)-induced chloride ion uptake into skeletal
muscles and the binding of tritiated dihydropicrotoxinin (anion
channel probe) to the membrane. This results in central nervous
system excitation and convulsions due to the blocking of GABA
transmitters (Lawrence & Casida, 1984; Abalis et al., 1985).
8.9.2. Liver
The mode of action of aldrin and dieldrin on the liver involves
an increase in the activity of microsomal biotransformation
enzymes, particularly of the monooxygenase system with cytochrome
P-450. This induction of liver microsomal enzymes is reversible
and, if exceeding a certain degree, appears to be associated with
the occurrence of CHIRL and hepatomegaly in the liver of rodents
(sections 6.3 and 8.2) (Jager, 1970; Wright et al., 1972, 1977,
1978).
9. EFFECTS ON HUMAN BEINGS
9.1. General Population Exposure
9.1.1. Acute toxicity - poisoning incidents
When a toxic dose of aldrin or dieldrin has been ingested or
has contaminated the skin, effects appear from 20 min to 24 h
afterwards. Signs and symptoms may include headache, dizziness,
nausea, general malaise, and vomiting, followed by muscle
twitchings, myoclonic jerks, and convulsions. Death may result
from cerebral anoxaemia (Nelson, 1953; Princi, 1954; Hayes, 1957,
1963; Hoogendam et al., 1962, 1965; Kazantzis et al., 1964;
Schafer, 1968; Jager, 1970).
The duration of the interval between oral intake or skin
contact and onset of symptoms (as well as the clinical picture)
depends on the dose absorbed. With massive overexposure,
convulsions may occur even in the absence of any premonitory
symptoms.
Initially, there is no fever or change in blood count or in
blood chemistry. However, later the temperature may be elevated
and leukocytosis may occur. Terminal hyperthermia has been
reported. Abnormal EEG patterns showing spike and dome complexes
and multiple spike and wave discharges, or in less serious
intoxications, bilateral synchronous theta discharges may be seen.
The diagnosis needs to be confirmed by determining the insecticide
concentration in the blood.
The onset of clinical intoxication is practically always acute
also in those cases where the accumulation of dieldrin in the
target tissues has taken place during a much longer period. The
latter cases are, therefore, usually indistinguishable from acute
intoxication. Survivors almost always recover completely (Jager,
1970; Hayes, 1982).
Estimates of dosages in anecdotal cases suggest that fatalities
have occurred with ingestion of approximately 10 mg dieldrin/kg
body weight, (Hayes, 1982) but Hodge et al. (1967) estimate the
lethal dose of aldrin and/or dieldrin for the adult man to be about
5 g.
Cases of poisoning have occurred by ingestion of formulated
material, mostly in children by mistake (for instance when aldrin
is used in granules as bait to control ants) or by adults with
suicidal intent. Several cases of poisoning have been the result
of ingesting food contaminated with aldrin or dieldrin during
storage or transport.
Van Raalte (1965) surveyed the world literature for all cases
of fatal poisoning by aldrin and dieldrin, and found 13 cases:
four suicides, three due to accidental ingestion, five due to
accidental contamination, and only one (a spray operative) due to
occupational exposure. No cases of fatal poisoning have been
reported during the course of aldrin and dieldrin manufacture and
formulation.
A non-exhaustive overview of published poisoning cases is given
in Table 44. A more complete review is provided by Hayes (1982).
Table 44. Case reports on accidental and suicidal acute aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Causative agent Circumstances Reference
of cases
cases
------------------------------------------------------------------------------------------
1 - aldrin emulsifiable attempted suicide Spiotta (1951)
concentrate
53 - aldrin and other consumption of seed grain WHO (1958)
pesticides
13 aldrin and dieldrin review of all fatal cases from Van Raalte (1965)
literature:
- 4 suicides, 3 accidental
ingestion, 5 accidental
contaminations, 1 sprayer
2 1 5% dieldrin accidental ingestion Garrettson & Curley
(1969)
79 consumption of dieldrin- WHO (1977)
contaminated rice in Mali
1 - dieldrin (120 mg/kg) attempted suicide Black (1974)
2 - dieldrin Fry (1964)
12 - aldrin + BHC consumption of seed grain Gupta (1975)
------------------------------------------------------------------------------------------
9.1.2. Effects of short- and long-term exposure - controlled human
studies
9.1.2.1 Accidental poisoning
Twelve cases of neurotoxicity, resulting from the repeated
consumption of wheat into which aldrin dust and gammexane (BHC)
powder had been mixed accidentally, have occurred in India (Gupta,
1975). The patients consumed this wheat for 6 - 12 months before
showing typical clinical symptoms, including convulsions.
Electroencephalographic tracings were consistent with a diagnosis
of organochlorine insecticide poisoning. The patients were treated
with phenobarbital and diazepam. The latter was more effective in
controlling seizures. All patients recovered.
The threshold dieldrin concentration in the blood below which
no adverse effects have been observed (and none are to be expected)
is 105 µg/litre (see also section 9.2.1.1). The dieldrin
concentration in the blood of the general population, in the
countries where this has been investigated, is well below this
threshold level. However, there are rare cases in which it seems
that low concentrations of dieldrin have induced effects.
A rare, well investigated and well reported case of dieldrin-
induced immunohaemolytic anaemia was observed in Iowa, USA. The
patient had a haemolytic anaemia with a positive direct
antiglobulin (Coombs) test and a positive Ham test in the serum.
The serum contained anti-bodies selectively active against
erythrocytes coated with dieldrin. The patient improved following
splenectomy. Dieldrin concentrations in blood and fat were similar
to those of the general Iowa population (Hamilton et al., 1978). A
similar case was reported by Muirhead et al. (1959).
9.1.2.2 Controlled human studies
In section 6.2.2.4, reference was made to a pharmacodynamic
study in human volunteers. This study had three objectives:
(a) to establish the relationship between the daily intake of
dieldrin and its concentration in human blood and adipose
tissue;
(b) to establish the blood/fat ratio in human beings; and
(c) to establish the relationship between the concentrations
of dieldrin in blood and fat and the length of exposure
(Hunter & Robinson, 1967, 1968; Hunter et al., 1969).
In addition, the opportunity was taken to monitor the health of the
human subjects during and after the exposure by full clinical,
physiological, and laboratory examinations as well as full
electroencephalographic (EEG) studies, polygraphic recording of
cardio-respiratory function, measurement of basal metabolic rate,
and electroneuromyographic studies at frequent intervals to detect
the possible occurrence of changes in physiological function. The
study involved 13 adult male college graduates without a history of
recent occupational exposure to pesticides. The subjects received
0, 10, 50, or 211 µg dieldrin per day for 2 years. All the men
continued in excellent health. Clinical, physiological, and
laboratory findings remained essentially unchanged throughout the
whole experimental period of 24 months and the 8 months after
exposure. No departures from what is regarded as normal for the
general population were observed. The concentration of p,p'-DDE in
adipose tissue and blood did not show any significant change during
or after the study, indicating that the liver microsomal enzyme
activity had not been induced. Thus, the total daily intake of
230 µg (211 µg plus intake from food) of dieldrin per person for 2
years had no effect on health. The concentrations of dieldrin in
both adipose tissue and blood were shown to be proportional to the
daily intake (section 6.2.2.4).
9.1.3. Tissue concentrations of dieldrin in hospitalized people
9.1.3.1 Pathological findings
Specimens of human abdominal subcutaneous fat, obtained from
four hospitals in Chicago were analysed for residues of dieldrin.
Dieldrin was not present in 103 out of 221 samples analysed for
this pesticide. Positive samples contained 0.01 - 1.39 mg
dieldrin/kg fat (mean value 0.14 mg/kg). There was no correlation
between the dieldrin concentration in adipose tissue and
pathological findings (Hoffman et al., 1967).
When organochlorine pesticide concentrations were determined in
the adipose tissue and liver of 271 hospital patients in Miami,
USA, patients with typical alcoholic (Laennec's) cirrhosis of the
liver had about twice the dieldrin concentration in the liver of
that found in the normal population. In patients with post-
necrotic cirrhosis, fatty metamorphosis of the liver, metastatic
malignancy of the liver, or primary hepatocellular carcinoma,
dieldrin concentrations were "normal". Terminal cases with
carcinomas of different organs had elevated concentrations of
organochlorine pesticides in the fat, but no association with any
particular neoplastic disease was found (Radomski et al., 1968).
In Hawaii, emaciated patients who had carcinoma and/or focal or
generalized liver pathology were found to have "normal" (for the
USA) concentrations of dieldrin in the liver and body fat (Casarett
et al., 1968).
When concentrations of organochlorine pesticides were
determined in specimens of liver, brain, and adipose tissue from
autopsies of patients with cirrhotic liver disease in Vancouver
(Canada) hospitals, the concentrations of dieldrin appeared to be
no higher than in tissues from controls (Oloffs et al., 1974).
In a case-control study on 122 matched cancer patients in south
Florida, USA, a comparison was made of dieldrin residues in the
adipose tissue of cancer patients and controls. The mean dieldrin
concentration in the adipose tissue was 0.3 mg/kg fat in both
cancer patients and controls (Davies et al., 1975).
9.1.3.2 Influence of weight loss and stress on dieldrin
concentrations in tissues
It is well known that, in birds, and perhaps in some small
mammals, dieldrin intoxication may be induced by starvation, weight
loss, or stress in animals having a previously harmless body burden
of dieldrin. Concern is sometimes expressed that, by analogy to
these observations, a similar course of events might occur in human
beings.
Twenty-nine patients (14 males, 15 females) undergoing surgery
were investigated. The concentrations of dieldrin in the blood
were unaffected by the catabolic responses to surgery. In another
study, these authors determined the concentrations of dieldrin in
the blood of 4 women undergoing voluntary near-starvation for
slimming purposes, which resulted in weight losses of up to 7.5
kg/week. There was no increase in the concentration of dieldrin in
the blood (Hunter & Robinson, 1968).
No significant difference was found between the dieldrin blood
concentrations of slimming or non-slimming mothers before and after
delivery (Eckenhausen et al., 1981).
On the basis of these results and calculations, it is suggested
that significant weight loss does not result in increased
concentrations of dieldrin in human tissues (Van Raalte, 1965;
Hunter & Robinson, 1968).
9.1.4. Exposure in treated homes
From the data on aldrin concentrations in the air of houses
treated for termite control (section 5.1.2), an estimate of the
dieldrin concentration in the blood of occupants of these houses
can be made using the mathematical formulas given by Hunter et al.
(1969) for deriving blood concentrations from the average daily
intake. The average daily intake is based on an estimated average
in-house volume of air inhaled per day (15 m3). The dieldrin blood
levels of home dwellers, calculated in this way, remain far below
the blood concentration no-effect level for the general population
(secton 9.2.1.1).
The blood dieldrin concentrations of 59 female residents of
Dade County (Florida, USA), where many houses had been treated for
termite control, were of the order of 1 µg/litre (Barquet et al.,
1981).
Also relevant to the health of home dwellers is the experience
obtained in the 1950s and 1960s when tens of thousands of houses in
more than 30 countries were sprayed with dieldrin for malaria and
yellow fever eradication. Although exposures were presumed to have
been high, as a result of surface spraying inside and outside
houses, no adverse health effects were reported in home dwellers.
Neither were adverse effects observed in well-trained and
medically-supervised spray operators (Soper, 1955 (Personal
communication at the 2nd Meeting of the Industrial Council on
Tropical Health, Boston); Fletcher et al., 1959).
9.2. Occupational Exposure
9.2.1. Acute toxicity - poisoning incidents
With the exception of poisoning cases resulting from massive
acute overexposure, most reported cases of poisoning with aldrin
and dieldrin in occupationally-exposed men have been the result of
a slow build-up of the insecticide in the body, the daily intake
exceeding the daily excretion (Jager, 1970; Hayes, 1982).
Based on the experience of Jager (1970), it was suggested that
the classification of types of intoxication by Hayes (1963) be
modified as follows:
Type 1: an acute convulsive intoxication with no (or only
minor) prodomi, resulting from one or several gross overexposures.
Type 2: a greater number of smaller doses may cause an
accumulative intoxication. Clinically, this results in a syndrome
of headache, dizziness, drowsiness, hyperirritability, general
malaise, nausea, anorexia, occasional vomiting. At times muscle
twitchings, myoclonic jerks and convulsions may occur. In these
circumstances minor increases in the insecticide level in the
blood, perhaps caused by minor fluctuations in exposure, may bring
about a convulsive intoxication.
Type 3: this is actually a combination of Types 1 and 2. In
this type an overexposure, in itself not significant, causes an
acute convulsive intoxication superimposed upon a subclinical
accumulative intoxication of Type 2.
These three types of intoxication are schematically illustrated
in Fig. 3.
 Table 45 gives a non-exhaustive overview of occupational aldrin
and dieldrin poisonings. Hayes (1982) contains further information
on this subject.
Table 45. Published case reports on occupational aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Acute Causative agent Circumstances Reference
of cases cases
cases
------------------------------------------------------------------------------------------
3 - ? 25% aldrin dust formulation with inadequate Nelson (1953)
safety precautions
~100 - ? various spraying in malaria- Hayes (1957,
eradication programmes 1959)
1 - 1 aldrin gross overexposure in aldrin Bell (1960)
packer
1 1 ? dieldrin sprayer Van Raalte
(1965)
4 - ? aldrin formulation of aldrin Kazantzis et
al. (1964)
17 - some aldrin and manufacturing and formulation Hoogendam et
dieldrin al. (1962,
1965)
32 (15 in - some aldrin, manufacturing and formulation Jager (1970)
addition dieldrin,
to endrin,
previous isobenzan
reference)
------------------------------------------------------------------------------------------
No cases of fatal poisoning have been reported during the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
However, there has been one case of a spray operator being fatally
poisoned (Van Raalte, 1965).
In developing countries it is, however, difficult to establish
the actual number of poisoning cases. Experience shows that even
when pesticides are banned, cases may occur in areas where control
is poor and where large quantities of pesticide are stocked. In
May 1987, four cases of occupational poisoning with aldrin were
reported from an area of cocoa plantations in Bahia (Brazil). One
of them was fatal, while three recovered. The fatal case had a
dieldrin level in whole blood exceeding 600 µg/litre two days after
poisoning (Rahde, personal communication).
9.2.1.1 Blood levels diagnostic of aldrin/dieldrin poisoning
Because the symptoms of aldrin/dieldrin intoxication are non-
specific, a differential diagnostic test is required to confirm
that the symptoms, signs, and clinical course of a particular case
are the result of aldrin or dieldrin intoxication.
The results of animal studies, together with those obtained
subsequently during medical surveillance of workmen employed in the
manufacture (or formulation) of aldrin/dieldrin, have shown that
adverse effects induced by aldrin/dieldrin are related to the
dieldrin blood concentration (Brown et al., 1964; Jager, 1970).
Therefore, the determination of this concentration provides a
specific differential diagnostic test.
Concentrations of dieldrin ranging from 40 to 530 µg/litre have
been reported in the blood of people who had been poisoned
relatively recently and who had recovered (Kazantzis et al., 1964;
Jager, 1970; Avar & Czegledi-Janko, 1970; Siyali & Simson, 1973).
From the limited information available, Brown et al. (1964)
concluded that the threshold concentration of dieldrin in the blood
of human beings, critical for intoxication, is approximately
150 - 200 µg/litre.
Dieldrin is present in the blood at very low concentrations in
the general population of many countries throughout the world. It
is also found at considerably higher concentrations in the blood of
healthy workers. These "healthy workmen" were men between 18 and
60 years of age who had no complaints or clinical or laboratory
signs attributable to occupational exposure, but they were, of
course, subject to the same ailments and diseases as are members of
the general population (Hayes & Curley, 1968; Jager, 1970). No
complaints of ill health and no positive results in objective
clinical or laboratory tests of ill health have ever been noted in
workers whose blood contained less than 200 µg/litre. This
concentration may, therefore, be considered to be a no-observed-
adverse-effect level in human beings. Higher concentrations may
have effects. The maximum concentration reported to be without
complaints or clinical signs or symptoms was 430 µg/litre (Jager,
1970).
Studies on animals have shown that the earliest physiological
sign of exposure to dieldrin is an increase in the activity of
certain liver microsomal enzymes. No enzyme induction has ever
been found in workers with dieldrin blood concentrations at or
below 105 µg/litre (Jager, 1970). (When liver enzyme induction
test methods became available, workers with dieldrin levels in the
blood exceeding 105 µg/litre were no longer encountered.)
Sometimes, dieldrin concentrations in plasma or serum, rather
than in whole blood, have been reported. The ratio of the dieldrin
concentration in plasma to that in erythrocytes is approximately
4:1 (Dale et al., 1965; Mick et al., 1972) (the conversion factor
to calculate the concentration of dieldrin in whole blood from the
concentration in plasma or serum is 0.66).
Great differences exist between the average concentrations of
dieldrin in the blood of the general population and of
occupationally exposed workers with or without complaints (Table
46).
Table 46. Concentrations of dieldrin in whole blood of human beings (µg/litre)
-------------------------------------------------------------------------------
No. of
Subjects persons Geometric Range Reference
involved mean
-------------------------------------------------------------------------------
General population 4592 <1 <1-16.1a US EPA (1983)
Unexposed persons 25 0.5 0-3.3 Sandifer et al. (1981)
20 2.5 0.5-10 Brown et al. (1964)
Healthy workers 35 29 <10-90 Jager (1970)
21 120e Mick et al. (1972)
37 55 -f Morgan & Roan (1974)
27 20 4.5-54 Sandifer et al. (1981)
89 38g 2-220 Brown et al. (1964)
Patients with 18 160g 8-280 Avar & Czegledi-Janko
clinical symptoms (1970)
4 40-530b Kazantzis et al. (1964)
5 130-370c Brown et al. (1964)
5 160-430d Brown et al. (1964)
Deceased (suicide) 1 850 Hayes (1982)
-------------------------------------------------------------------------------
a In serum.
b The low level of 40 µg/litre was found in a man with chronic nephritis,
complaining of headache and nausea; the occupational cause of the symptoms
is, therefore, doubtful. In one other worker, a blood level of 530 µg/litre
was found 1 month after a mild acute episode when he was exposed to aldrin
again.
c Determined some time after the acute episode.
d Estimated to be the concentration at the time of the acute episode.
e Converted from plasma figures using a factor of 0.66 (see 9.2.1.1).
f Highest value 231 kg/litre.
g Average.
Among 13 adolescent patients with colon-rectal adenocarcinomas,
who had lived in rural areas of Mississippi, USA, where pesticides
are widely sprayed, dieldrin blood levels were no higher than those
in controls (Caldwell et al., 1981).
9.2.1.2 Electroencephalography
Changes in the electroencephalogram (EEG) are sometimes of
practical importance for confirming a diagnosis of aldrin/dieldrin
intoxication (Spiotta, 1951; Winthrop & Felice, 1957; Hoogendam et
al., 1962, 1965; Kazantzis, 1964; Avar & Czegledi-Janko, 1970;
Jager, 1970). These EEG changes were first used as a practical
tool for monitoring workers and determining when they should
discontinue exposure and when they could be allowed to resume work
with aldrin/dieldrin. Characteristic changes - which, however, are
not pathognomonic for aldrin/dieldrin poisoning - include bilateral
synchronous spikes, spike and wave complexes, and slow theta waves,
thought to be possibly associated with brain stem stimulation
(Hoogendam et al., 1962, 1965). The interesting parallelism
between the rate of diminution of the EEG changes and the rate of
decrease in the dieldrin blood concentration was also reported in
the case of an accidentally poisoned child (Garrettson & Curley,
1969).
Nowadays, analysis for dieldrin in the blood has replaced EEG
examination as the method of choice for monitoring exposed workers
(Jager, 1970).
9.2.2. Effects of short- and long-term exposure
Occupational exposure occurred in the 1950s and early 1960s
among sprayers in malaria and yellow fever control programmes.
These men sprayed dieldrin inside houses day after day in prolonged
cycles without appreciable intervals of non-exposure. Quite often,
precautions and supervision were less than would be required today.
At the time, methods for the determination of blood concentrations
had not been developed. A significant percentage of these sprayers
became sick after having worked for as little as 2 days or as much
as 2 years (Hayes, 1957, 1959; Patel & Rao, 1958; Zavon & Hamman,
1961). According to communications by the Pan-American Sanitary
Bureau (Soper, 1955a), it appeared that no clinical symptoms were
observed in well-executed, well-supervised malaria and yellow fever
control programmes.
In a study carried out in East Africa, where workers were
spraying dieldrin 6 h/day for 180 days per year (with an interim of
2 months between spraying cycles) no clinical symptoms were seen.
The potential average dermal exposure of spray operators who
observed the protective measures laid down was 1.8 mg/kg body
weight per day (Fletcher et al., 1959).
Long et al. (1969) studied 159 farmers in Iowa, USA. Extensive
clinical and laboratory examinations of 33 pesticide users among
these farmers did not reveal evidence of any disease that could be
attributed to the use of pesticides, neither was the dieldrin blood
concentration correlatable with any parameter examined.
-------------------------------------------------------------------
a Personal communication at the 2nd Meeting of the Industrial
Council on Tropical Health, Boston.
The dieldrin blood concentrations in 8 locust-control workers
in Ethiopia were measured on two occasions and were found to range
between 0 and 9 µg/litre (MacCuaig, 1976).
Wolfe et al. (1963) studied the hazards from spraying orchards
with dieldrin in the US Pacific Northwest. Potential contamination
of the skin and respiratory exposure were measured. From the
results, potential skin and respiratory exposures were calculated
to amount to 14.2 and 0.25 mg/h, respectively.
Princi & Spurbeck (1951) studied a group of workers exposed
to chlordane, aldrin, and dieldrin for several years in a
manufacturing and formulating plant. The atmospheric
concentrations of aldrin were reported to be as high as 2.6 mg/m3.
Physical examinations and chest-röntgenograms did not reveal
respiratory anomalies.
A study was carried out on 71 men employed in the manufacture
and formulation of aldrin, dieldrin, endrin, and some other non-
related pesticides. Twenty-eight of these workers each contributed
a sample of blood and a sample of fat on the same day. The average
concentration of dieldrin in fat (6.12 ± 1.24 mg/kg) was 247 times
greater than the mean plasma concentration (0.025 ± 0.006
mg/litre). There was no relation between the amount of dieldrin in
the samples and the use of sick leave (Hayes & Curley, 1968).
In another study, 68 pesticide workers (including pest control
operators) and 29 unexposed controls were examined quarterly over a
period of four years. Determinations of serum pesticide
concentrations and enzyme activity, blood chemistry, haematology,
and urinalysis were carried out. The mean serum dieldrin
concentration was 3.6 ± 6.3 µg/litre (1.1 ± 1.6 µg/litre in the
controls). There was no difference between the exposed workers and
the controls in the incidence of disease or disability (Warnick &
Carter, 1972).
In a pesticide formulation plant, the blood of 21 employees was
examined at the conclusion of a 5-week period during which 900 kg
of technical aldrin was formulated. The mean dieldrin
concentration in plasma was 11 and 182.5 µg/litre for herbicide
formulators and aldrin formulators, with a maximum of 317 µg/litre
in the latter case. No mention was made of any intoxications (Mick
et al., 1972).
In a group of 42 occupationally exposed pesticide workers with
a dieldrin serum concentration 5 times as high as that in a group
of 23 controls, no indication of disturbed renal- or adrenocortical
function was found (Morgan & Roan, 1969, 1973).
A study was carried out in California, USA, where aldrin (EC as
a 0.5% solution) at 480 g/litre was applied as a termiticide to
typical slab and crawl space type houses. Personal air samples,
samples of blood, and samples of pads on clothes and gloves were
taken to monitor the exposure of the pest-control operators. The
personal air samples during application contained less than 0.3
µg/m3 aldrin for the slab houses and 30 - 75 µg/m3 for the crawl
space houses. The total work day (9 - 18 h) time-weighted average
concentration of aldrin in air was 6 - 17 µg/m3. This is far below
the threshold limit value (TLV) for aldrin established by the
American Conference of Governmental Industrial Hygienists (ACGIH,
1986) of 250 µg/m3. Data from dermal exposure samples showed large
variation. However, the maximum calculated percentage of the toxic
dose per h, based on the acute percutaneous toxicity (rat LD50) of
the formulation, was less than 0.01%. The concentration of aldrin
and dieldrin in the blood of the operators was below the limit of
detection (less than 1 µg/litre) (Marlow et al., 1982).
A case-control study, carried out on 27 pesticide workers
(4 formulators and 23 pest-control operators) with elevated blood
concentrations of dieldrin, revealed a mean blood concentration of
19.59 µg/litre (range: 4.45 - 54 µg/litre). In an extensive
clinical examination, including physical examination, comprehensive
neurological examinations, laboratory tests, and physiological and
psychomotor testing, no important differences were found compared
with results in a control group of 25 people with a mean dieldrin
blood concentration of 0.48 µg/litre (range of 0 - 3.34 µg/litre)
(Sandifer et al., 1981).
9.2.3. Epidemiological studies
An extensive study on workers in an aldrin/dieldrin
manufacturing plant has been in progress since the plant began
operations in the 1950s. The results from the first 15 years of
this epidemiological study were reported in 1970 (Jager, 1970).
From a total of more than 800 exposed workers, all those exposed
for more than 4 years (233 men) or those who had experienced an
intoxication (20 men) underwent extensive physical, neurological,
haematological, and other laboratory examinations. Clinical
chemical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein, and serum protein spectrum, were
made every 3 months and remained within normal limits. A no-effect
level in this group of workers, including those who had previously
suffered intoxications, was established at a dieldrin blood
concentration of 200 µg/litre. This level corresponds to a total
equivalent daily oral intake of 33 µg/kg body weight or a total
daily intake of 2300 µg/person per day (Hunter & Robinson, 1967).
In experimental animals, the earliest, reversible effect of
dieldrin is the induction of liver microsomal enzyme systems
(Wright et al., 1977, 1978). This finding led to an investigation
of a group of 10 workers. At the time, due to further improvements
in the industrial hygiene of the above-mentioned plant, the
geometric mean concentration of dieldrin found in the blood of
workers was 105 µg/litre. As criteria of enzyme induction
measurements were made of the blood levels of p,p'-DDE, the urinary
ratio of 6-beta-hydroxycortisole and 17-hydroxycorticosteroids, and
the urinary excretion of D-glucaric acid. No difference in these
values was found between the 10 exposed workers and a control
group. On the basis of these data, the no-effect level was 105 µg
dieldrin/litre blood, equivalent to an oral daily intake of 17.4
µg/kg body weight per day (or 1220 µg/person per day) (Jager, 1970;
Hunter et al., 1969; Hunter & Robinson, 1967; Versteeg & Jager,
1973).
Further results from this long-term survey of an industrial
population were subsequently published, based on a study of 1000
workers. Because not all of the workers had severe and/or
prolonged exposure, smaller groups with an exposure meaningful
enough for carcinogenicity evaluation were included. One group
consisted of 166 men (including workers who were still exposed and
workers who had left the company), with a mean exposure time of
16.9 years (range 4 - 19 years), who had been under observation for
more than 15 years (mean observation period 17 years; range,
15 - 20 years). A sub-group comprised 69 men with a mean exposure
time of 14.9 years (range 10 - 19 years) and a mean observation
period of 17.2 years. Among the group of 166 workers, 51 were more
than 50 years old. One man with only 5 years of comparatively mild
exposure died because of a gastric carcinoma. A lymphosarcoma
occurred in a man with 7 years of very mild exposure. Both
incidences occurred before 1964. No new cases were noted in the
final 11 years of study, and no undue mortality from other causes
that could have masked a higher cancer incidence was observed
(Versteeg & Jager, 1973; Van Raalte, 1977).
In a follow-up study on the original group of 233 men with more
than 4 years of exposure and an observation period ranging from 4
to 29 years (mean, 24 years), there were no indications of a
specific carcinogenic activity. Total observed mortality was 25
deaths versus 38 expected. Of nine cancer deaths, three were
caused by lung cancer, while the remaining six were each of a
different nature. No primary liver tumours were observed (Ribbens,
1985).
In a study by Morgan & Roan (1974), 28 pesticide formulators
and applicators, plus a separate group of 43 termite-control
workers, with occupational exposures of 5 - 22 years were examined,
together with 56 controls. The highest levels of dieldrin among
these 71 workers were found in a group of 37 men who had a mean
serum dieldrin concentration of 84 µg/litre (equivalent to about
55 µg/litre whole blood). There were no signs of liver cell injury
and the serum enzyme activities SGOT, SGPT, LDH, alkaline
phosphatase and creatine phosphokinase (CPK) were within normal
limits. There was no indication of drug-metabolizing enzyme
induction and urinary excretion of D-glucaric acid was not
different from that in a control group.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides, revealed
that, in 1962, 18 and 10 years after the introduction of DDT and
aldrin/dieldrin, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the cases of
primary liver cancer as a percentage of the total number of liver
cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) of
primary liver cancer for this period declined from 3.46 to 3.18
(Deichmann & MacDonald, 1977).
An epidemiological mortality study in a plant manufacturing
aldrin, dieldrin, and endrin was carried out on a cohort of 1155
workers who had been employed for at least 6 months between 1946
and 1976 (almost 25 000 man-years of observation). The mortality
due to all malignant neoplasms was 31, lower than expected
(standardized mortality ratio (SMR) 82). The total mortality from
all causes was 173 (SMR 84). The only disease with an SMR above
100 (SMR 212) was "non-malignant respiratory system disease",
specifically pneumonia. There was a slight excess of oesophagus
and rectum cancer (two and three cases observed with an SMR of 235
and 242, respectively), liver cancer (two cases observed versus
0.57 expected), and cancer of the lymphatic and haematopoietic
system (six cases observed versus 4.07 expected). However, there
was a deficit of cancer of other sites. The authors concluded that
"the study has not identified a specific cancer risk associated
with employment at this manufacturing plant, but several causes
should be examined further" (Ditraglia et al., 1981).
In a 1981 health survey, a total of 567 serum samples from 1811
Florida citrus workers were collected during the spraying and the
harvest season and were compared with the national ("Hanes")
sample. There were no differences in serum dieldrin levels; the
mean in both groups being 1.8 - 1.9 µg/litre serum (Griffith &
Duncan, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Aldrin and dieldrin, organochlorine pesticides, were used
throughout the world from 1950 until the early 1970s as
insecticides in agriculture and as a seed treatment, for the
control of soil pests and other types of insects (e.g., termites,
grasshoppers, and textile pests), and for the control of tsetse
flies and other disease vectors. The compounds act as contact and
stomach poisons in the insects. Since the early 1970s, both
compounds have been restricted or banned from use in several
countries, especially in agriculture. Nevertheless, use continues
in other countries for termite control.
Both compounds are practically insoluble in water and
moderately to highly soluble in many organic solvents. The vapour
pressure is low.
Dairy and meat products, fish, oils and fats, and certain
vegetables such as root vegetables often contain dieldrin. Maximum
residue limits recommended by the FAO/WHO Joint Meeting on
Pesticide Residues range from 0.02 to 0.2 mg/kg product. Recent
measurements have shown that actual levels are lower, and this has
been confirmed by total diet studies. Since the use of these two
compounds has been restricted, a steady but slow decrease in
residue levels in the different food commodities has taken place.
The intake by human beings of low concentrations in the daily
diet has resulted in dieldrin being present in adipose tissue and
in some other tissues and organs. Global surveys have shown that
mean values range from 0.1 to 0.4 mg/kg adipose tissue. Since the
early 1970s, this concentration has slowly decreased.
Transplacental exposure of the fetus occurs, with the result
that the fatty tissues of the fetus also contain dieldrin, but at
concentrations 10 - 50% of those of the mother. There seems to be
an equilibrium between levels in the fetus and those in the mother.
Dieldrin is also excreted with the milk. Inhabitants of houses
that have been treated for termite control may be exposed by
inhalation. Concentrations in the air found after indoor treatment
may range from 0.01 to 7 µg/m3, depending on the type of
applications, concentration used, type of ventilation, and time of
sampling. Under these conditions food may also be contaminated by
direct contact or by sorption from the atmosphere.
Metabolism takes place mainly in the liver where aldrin is
readily transformed to dieldrin. Dieldrin is degraded at a slower
rate to hydrophilic metabolites, which are then excreted via the
bile and urine. The structures of these metabolites have been
established. In all species examined, including human beings, it
has been shown that there is a steady state of aldrin/dieldrin
storage corresponding to the level of intake and a linear
relationship between the log of intake and storage has been
demonstrated. The concentration of dieldrin in body tissues
decreases exponentially on termination of exposure to the
compounds.
The acute oral toxicity of aldrin and dieldrin for mammals is
high, while the dermal toxicity is moderate. Dermal sensitization
has not been found. Effects observed in acute, short-term and
long-term studies involve the central nervous system. The liver is
also a target organ. In the liver of mice and rats, changes known
as "chlorinated hydrocarbon insecticide rodent liver" are found.
Aldrin and dieldrin do not appear to cause teratogenic effects
at doses below those causing maternal toxicity and fetotoxicity.
Male or female reproductive toxicity has not been reported.
Numerous in vitro and in vivo mutagenicity studies have
demonstrated that neither aldrin nor dieldrin have mutagenic
potential.
In long-term studies, aldrin and dieldrin induced benign and
malignant liver tumours in the mouse. However, no increased
incidence of liver tumours or other tumours were found in rats and
hamsters.
IARC (1987) has stated that there is inadequate evidence of
carcinogenicity in human beings and limited evidence of
carcinogenicity in experimental animals. Both aldrin and dieldrin
have been classified in Group 3: the chemicals cannot be
classified as to their carcinogenicity in human beings.
On the basis of available short-term and long-term toxicity
data, the overall no-observed-adverse-effect level in the rat is
0.5 mg dieldrin/kg diet, equivalent to 0.025 mg/kg body weight. In
the dog, the lowest no-observed-adverse-effect level found was 0.04
mg/kg body weight. The Joint Meeting on Pesticide Residues (JMPR)
established an Acceptable Daily Intake (ADI) of 0.1 µg/kg body
weight in 1966 and 1977 based on the conclusion that aldrin and
dieldrin were not human carcinogens.
Aldrin and dieldrin are highly toxic to human beings. Both
accidental and occupational cases of poisoning have occurred but
reported fatalities have been rare. Survivors of acute or subacute
intoxications recovered completely. Adverse effects are related to
the dieldrin blood concentration, the determination of which
provides a specific diagnostic test for aldrin/dieldrin exposure.
At a dieldrin blood concentration below 105 µg/litre, no adverse
effects can be expected. This level is considered a threshold no-
observed-adverse-effect level and corresponds to a daily intake of
0.02 mg dieldrin/kg body weight per day.
Environmental, mainly dietary, exposure leads to the presence
of dieldrin in low concentrations in the human body. The results
of extensive clinical and epidemiological studies indicate that
these body burdens do not present a health hazard to human beings.
No signs of any premonitory change in liver function were found
in a 20-years study, involving more than 1000 industrial workers
exposed to aldrin and dieldrin. In this study and another study in
the USA, no specific cancer risk could be identified associated
with occupational exposure to (sometimes high levels of) aldrin and
dieldrin.
All the available information on aldrin and dieldrin taken
together, including studies on human beings, supports the view that
for practical purposes, these chemicals make very little
contribution, if any, to the incidence of cancer in human beings.
Photodieldrin, the photo-decomposition product of dieldrin, is
similar to dieldrin in its short-term toxicity. It is not
teratogenic or carcinogenic in mice and rats. The accumulation of
photodieldrin in the adipose tissue of experimental animals was
less than that of dieldrin.
10.2. Evaluation of Effects on the Environment
Aldrin, used as a soil insecticide, is the major source of
dieldrin (up to 97%) in the environment. Aldrin and its reaction
product dieldrin are rapidly adsorbed on soils, especially soils
containing a high level of organic matter. Consequently there is
little penetration into the soil, and contamination of groundwater
does not generally occur. Transport of both compounds takes place
mainly through soil erosion (as wind drift) and sediment transport
(surface run-off), but not through leaching.
The use of aldrin and dieldrin in agriculture leads to residues
(mainly of dieldrin) in the soil that can persist for years; the
estimated half-life of dieldrin is between 4 and 7 years. Under
tropical conditions, the compounds are less persistent than under
temperate conditions.
Aldrin and dieldrin enter the atmosphere through volatilization
from treated crops and soil or, directly, during the application of
the pesticide. Dieldrin returns to soil and water surfaces by
washout and dry deposition. Thus, the compounds are found either
in the vapour phase (very low levels, in general 1 - 2 ng/m3),
adsorbed by dust particles, or in rainwater (of the order of
10 - 20 ng/litre).
The occurrence of dieldrin in the aquatic environment has been
reported by several authors. The concentrations in surface water
are mainly very low, less than 5 ng/litre. However, concentrations
in areas of soil erosion or agricultural use may be higher.
Sediment in rivers in these areas may contain up to 1 mg
dieldrin/kg. The high capacity for aquatic organisms to
concentrate dieldrin from very low levels in water could lead to
toxic levels in aquatic organisms. Concentration through aquatic
foodchains is of less importance than direct uptake from water.
Because of the widespread occurrence of dieldrin in the
environment and its persistence, there is a wide range of
concentrations in non-target organisms. Whereas the concentrations
previously ranged from 0.001 mg to 100 mg/kg tissue, they are now
mostly below 1 mg/kg tissue.
In terrestrial ecosystems, aldrin and dieldrin are accumulated
by a wide variety of organisms, principally as dieldrin. Dieldrin
is probably responsible for the deaths of mammals in the field and
for the decline in population size in some species, such as the
otter. Small mammals would be killed by eating dieldrin-dressed
grain, but populations of these animals are likely to have been
replenished by immigration from surrounding areas. Birds of prey
eating small mammals and small birds contaminated by dieldrin take
up and accumulate dieldrin in their own tissues and eggs.
Granivorous birds have been killed by eating dressed grain. It is
probable that the population decline in birds of prey was caused by
dieldrin residues (among other organochlorine residues) in their
tissues. The effects of dieldrin are seen some time after the
exposure, because residues are stored in fat over winter, to be
released in the spring. When dieldrin was used only at certain
times of the year, this did not prevent bird mortalities.
The widespread use of aldrin and dieldrin, in conjunction with
other organochlorine pesticides, has led to severe detrimental
effects on the environment, though with drastic curtailment of use,
particularly in seed dressings, there has been some recovery in
bird populations.
10.3. Conclusions
(a) Both aldrin and dieldrin have been subjected to intensive and
wide-ranging study, toxicologically, clinically, and
epidemiologically. The body burden is mainly the result of
the oral ingestion of residues in the diet (which seem
generally to fall within the promulgated ADIs) and, to a
lesser extent, of inhalation. Evaluation of the data suggests
strongly that the body burden resulting from the present level
of exposure constitutes no health risk to the general
population.
(b) Dieldrin occurs almost ubiquitously in human breast milk.
However, its concentration in the blood and adipose tissue of
suckling infants does not increase with age during the first
six months, nor is their blood dieldrin level higher than that
of bottle-fed babies. Under these circumstances, the benefits
of natural breast feeding still make it the preferred method
of infant feeding, in spite of the dieldrin residues.
(c) In the treatment of premises, notably for termite control, the
exposure of occupants does not appear to be increased to a
level that endangers their health, as long as the directions
for safe practice are conscientiously respected.
(d) Despite the highly toxic nature of aldrin and dieldrin, both
of these chemicals can be handled safely as long as the
recommended precautions to minimize worker exposure are always
observed. Neglect of these rules may lead to the poisoning of
operators.
(e) During the period of high aldrin and dieldrin use between 1950
and 1970, detrimental effects were undoubtedly inflicted upon
species in the environment. These effects were due partly to
dieldrin and partly to other organochlorines. Since the
drastic curtailment of the use of these materials, the
affected species have recovered in numbers.
11. RECOMMENDATIONS
1. A further, properly designed, teratogenic investigation is
required in the hamster, with dieldrin at realistic dose
levels.
2. Research into the mechanism of carcinogenesis should be
directed to explaining why the hepatic reaction in the mouse
is different from that of other species.
3. Dieldrin should be selected as an agent for further study of
neurotoxic mechanisms, both experimentally and clinically.
4. To protect the environment, large-scale use of aldrin and
dieldrin must not be resumed, and applications should be
confined to those situations in which no safer, equally
effective alternatives can be recommended.
5. For the health and welfare of workers and the general
population, the handling and application of aldrin and
dieldrin should only be entrusted to well trained competent
operators, who will follow adequate safety measures.
6. To avoid accidental poisoning from aldrin, especially among
children, the use of aldrin granules as an ant bait should be
forbidden.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Aldrin and dieldrin were evaluated by the FAO/WHO Joint
Meeting on Pesticide Residues (JMPR) in 1963, 1965, 1966, 1967,
1968, 1969, 1970, 1974, 1975, and 1977 (FAO/WHO, 1964, 1965a,b,
1967a,b, 1968a,b, 1969a,b, 1970a,b, 1971a,b, 1975a,b, 1978a,b).
From 1966 onwards, the JMPR established an acceptable daily intake
(ADI) of 0 - 0.0001 mg/kg body weight (combined total for aldrin
plus dieldrin). This was based on a level causing no toxicological
effect of:
0.5 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
rat; and
1 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
dog.
The maximum residue limits (MRLs) listed in Table 47 were
recommended by the FAO/WHO Joint Meeting on Pesticide Residues in
1970 and 1975 and are quoted as the sum of aldrin plus dieldrin.
Table 47. Maximum residue limits (MRL's) recommended by the Codex
Alimentarius Commission (FAO/WHO, 1986)
-------------------------------------------------------------------
Commodity Aldrin and dieldrin
(mg/kg)
-------------------------------------------------------------------
Potatoes 0.1
Fat of meat 0.2a
Carrots, lettuce, fat of meat 0.1a
Asparagus, aubergines, broccoli, Brussels 0.1
sprouts, cabbage, cauliflower, cucumbers,
horse radish, onions, parsnips, peppers,
pimentos, radishes, radish tops
Eggs (shell-free) 0.1a
Milk and milk products (fat basis) -
Milk 0.006a
Fruit 0.05
Rice (in husks) 0.02
Raw cereals (other than rice) 0.02a
-------------------------------------------------------------------
a Extraneous residue limit.
WHO (1984) recommended that the level of aldrin and dieldrin
in drinking-water should not exceed 0.03 µg/litre.
IARC evaluated aldrin and dieldrin on several occasions.
Aldrin and dieldrin were found to be carcinogenic in the liver in
mice, but there was no evidence for carcinogenicity in other
organs. The data available did not provide evidence of
carcinogenicity in rats. Data on dogs, monkeys, and human beings
were too limited to allow any conclusions (IARC, 1974). IARC
considered that there was inadequate evidence of carcinogenicity
in humans and limited evidence of carcinogenicity in experimental
animals. Accordingly, both chemicals were classified in Group 3
(IARC, 1987).
The Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified the acute hazard to
health for technical dieldrin as "highly hazardous" (WHO, 1988).
The same division published a data sheet on aldrin (79.41) and
dieldrin (75.17) (WHO/FAO, 1975-85).
REFERENCES
ABALIS, I.M., ELDERFRAWI, M.E., & ELDERFRAWI, A.T. (1985) High-
affinity stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic. Biochem. Physiol., 24: 95-102.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1966)
Organochlorine pesticides in the atmosphere. Nature (Lond.), 211:
259-261.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67.
Organochlorine pesticide residues in the total diet. J. Sci. Food
Agric., 20(4): 245-259.
ABBOTT, D.C., COLLINS, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71. Br.
med. J., 1972(2): 553-556.
ABBOTT, D.C., COLLINS, G.B., GOULDING, R., & HOODLESS, R.A. (1981)
Organochlorine pesticide residues in human fat in the United
Kingdom 1976-77. Br. med. J., 283: 1425-1428.
ACGIH (1986) TLVs. Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, 111 pp.
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fat.] Naturwissenschaften, 61: 32 (in German).
ACKER, L., BARKE, E., HAPKE, H.J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk.] Milchwissenschaft., 39(9): 541-544 (in German).
ADDISON, R.F., ZINCK, M.E., & ACKMAN, R.G. (1972) Residues of
organochlorine pesticides and polychlorinated biphenyls in some
commercially produced Canadian marine oils. J. Fish Res. Board
Can., 29: 349-355.
ADDISON, R.F., ZINCK, M.E., & LEAHY, J.R. (1976) Metabolism of
single and combined doses of 14C-aldrin and 3H-p,p'-DDT by
Atlantic salmon (Salmo salar) fry. J. Fish Res. Board Can., 33(9):
2073-2976.
ADEMA, D.M.M. & VINK, G.J. (1981) A comparative study of the
toxicity of 1,1,2-trichloroethane, dieldrin, pentachlorophenol, and
3,4-dichloroaniline for marine and fresh-water organisms.
Chemosphere, 10(6): 533-554.
AGNIHOTRI, N.P., PANDEY, S.Y., JAIN, H.K., & SRIVASTAVA, K.P.
(1977) Persistence of aldrin, dieldrin, lindane, heptachlor, and
p,p'-DDT in soil. J. entomol. Res., 1(1): 89-91.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977a) Pesticide induced
ouabain resistant mutants in Chinese hamster V-79 cells. Chem.-
biol. Interact., 19(3): 369-374.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977b) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res., 42:
161-174.
AKKERMANS, L.M.A. (1974) Mode of action of dieldrin. An electro-
physiological investigation, Utrecht, The Netherlands, Rijks
Universiteit (Thesis).
AKKERMANS, L.M.A., VAN DEN BERCKEN, J., & VERSLUYS-HELDER, M.
(1975) Excitatory and depressant effects of dieldrin and aldrin-
transdiol in the spinal cord of the toad (Xenopus laevis). Eur. J.
Pharmacol., 34: 133-142.
ALBERT, L., MENDEZ, F., CEBRIAN, M.E., & PORTALES, A. (1980)
Organochlorine pesticide residues in human adipose tissue in
Mexico: results of a preliminary study in three Mexican cities.
Arch. environ. Health, 35(5): 262-269.
ANAS, R.E. & WILSON, A.J. (1970a) Organochlorine pesticides in fur
seals. Pestic. monit. J., 3(4): 196-200.
ANAS, R.E. & WILSON, A.J. (1970b) Organochlorine pesticides in
nursing fur seal pups. Pestic. monit. J., 4(3): 114-116.
ANDERSON, B.G. (1960) The toxicity of organic insecticides to
Daphnia. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Proceedings of the 2nd Seminar, 1959, Cincinnati, Ohio,
Robert A. Taft Sanitary and Engineering Center, pp. 94-95
(Technical Report W60-3).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain
North American birds. In: Proceedings of the 15th International
Ornithology Congress, Leiden, Brill, pp. 514-540.
ANDERSON, P.D. & WEBER, L.J. (1975) Toxic response as a
quantitative function of body size. Toxicol. appl. Pharmacol., 33:
471-483.
ANON. (1964) Report on the Working Conference on Birds of Prey and
Owls, Caen, Normandy, 10-12 April, 1964, Washington, DC,
International Council for Bird Preservation.
ANON. (1973) Aldrin and the ultraviolet conversion products and
possible metabolites of aldrin and dieldrin in human milk from the
community studies on pesticides, Berkeley, California, Department
of Public Health, (Abstract prepared by Shell Development Company,
Modesto, California: Report TIR-24-123-73: Part II).
ANON. (1974) Data for pentachloroketone and UV-dieldrin in human
kidney and adipose tissue from Coral Gables, Florida, Modesto,
California, Shell Development Company (Report TIR-24-176-73: Part
II).
ANON. (1974b) Data for dieldrin, pentachloroketone, and UV-dieldrin
in human adipose tissue from Albany, New York, Modesto,
California, Shell Development Company (Report TIR-24-169-73: Part
II).
ANON. (1974c) Determination of dieldrin levels in human kidney and
adipose tissue, Modesto, California, Shell Development Company
(Report TIR-24-176-73).
ASHWOOD-SMITH, M.J. (1981) The genetic toxicology of aldrin and
dieldrin. Mutat. Res., 86: 137-154.
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ASTOLFI, E., GARCIA FERNANDEZ, J.C., DE JUAREZ, M.B., & PIACENTINO,
H. (1973) Chlorinated pesticides found in the fat of children in
the Argentine Republic. In: Deichmann, W.B., ed. Pesticides and the
environment, New York, Intercontinental Medical Book Company, pp.
233-243.
ASTOLFI, E., ALONSO, A.H., MENDIZABAL, A., & ZUBIZARRETTA, E.
(1974) Pesticides chlorés de l'accouchée et du cordon ombilical des
nouveaunés. J. eur. Toxicol., 7: 330-338.
ATKINS, D.H.F. & EGGLETON, A.E.J. (1970) Studies of atmospheric
washout and deposition of gamma-BHC, dieldrin, and p,p'-DDT using
radiolabelled pesticides, Harwell, United Kingdom Atomic Energy
Research Authority (Report HL 70/4532(C10)).
ATKINS, T.D. & LINDER, R.L. (1967) Effects of dieldrin on
reproduction of penned hen pheasants. J. wildl. Manage., 31(4):
746-753.
AULERICH, R.J., RINGER, R.K., & POLIN, D. (1972) Rate of
accumulation of chlorinated hydrocarbon pesticide residues in
adipose tissue of mink. Can. J. Zool., 50(9): 1167-1173.
AVAR, P. & CZEGLEDI-JANKO, G. (1970) Occupational exposure to
aldrin: clinical and laboratory findings. Br. J. ind. Med., 27(3):
279-282.
BAECKSTROEM, J., HANNSON, E., & ULLBERG, S. (1965) Distribution of
14C-DDT and 14C-dieldrin in pregnant mice determined by whole-body
autoradiography. Toxicol. appl. Pharmacol., 7: 90-96.
BAILEY, G.W., SWANK, R.R., Jr, & NICHOLSON, H.P. (1974) Predicting
pesticide runoff from agricultural land: a conceptual model. J.
environ. Qual., 3(2): 95-102.
BAKER, A.H., WHITNEY, G.F.H., & WORDEN, A.N. (1959) The toxic
hazard associated with continuous-flow heat-volatilized
insecticidal and acaracidal aerosols. Lab. Pract., 8: 3-10.
BAKKEN, A.F. & SEIP. M. (1976) Insecticides in human breast milk.
Acta paediatr. Scand., 65: 535-539.
BALAYANNIS, P.G. (1974) Organochlorine pesticide residues in
tomatoes Ann. Inst. Phytopathol. Benaki (N.S.), 11: 47-52.
BALDWIN, M.K. & ROBINSON, J. (1969) Metabolism in the rat of the
photoisomerization product of dieldrin. Nature (Lond.), 224(5216):
283-284.
BALDWIN, M.K., ROBINSON, J., & CARRINGTON, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Ind., 1970: 595-597.
BALDWIN, M.K., ROBINSON, J., & PARKE, D.V. (1972) A comparison of
the metabolism of HEOD (dieldrin) in the CF1 mouse with that in the
CFE rat. Food Cosmet. Toxicol., 10: 333-351.
BALDWIN, M.K., DAVIS, R.A., & THORBURN BURNS, D. (1973) Structural
studies and photochemical re-arrangement of an animal metabolite of
HEOD, the active component of dieldrin. Pestic. Sci., 4: 227-237.
BALDWIN, M.K., BENNETT, D., & BEYNON, K.I. (1977) The
concentrations of aldrin and dieldrin and their photoisomers in the
atmosphere. Pestic. Sci., 8: 431-445.
BALUJA, G., MURADO, M.A., & TEJEDOR, M.C. (1975) Adsorption and
desorption of lindane and aldrin by soils as affected by soil main
components. Environ. Qual. Saf., Suppl. 3: 243-249.
BALUJA, G., HERNANDEZ, L.M., GONZALEZ, J., & RICO, C. (1982)
Presence of organochlorine pesticides, polychlorinated biphenyls
and mercury in Spanish human milk samples. Bull. environ. Contam.
Toxicol., 28: 573-577.
BARLOW, F. & HADAWAY, A.B. (1955) Studies on aqueous suspensions of
insecticides V. The sorption of insecticides by soils. Bull.
entomol. Res., 46: 547-559.
BARLOW, F. & HADAWAY, A.B. (1956) Effects of changes in humidity on
the toxicity and distribution of insecticides sorbed by some dried
soils. Nature (Lond.), 178: 1299-1300.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D. (1979)
Organochlorine pesticide residues in human milk samples from women
living in northwest and northeast Mississippi, 1973-75. Pestic.
monit. J., 13(2): 47-51.
BARON, R.L. & WALTON, M.S. (1971) Dynamics of HEOD (dieldrin) in
adipose tissue of the rat. Toxicol. appl. Pharmacol., 18(4): 958-963.
BARQUET, A., MORGADE, C., & PFAFFENBERGER, C.D. (1981)
Determination of organochlorine pesticides and metabolites in
drinking-water, human blood-serum, and adipose tissue. J. Toxicol.
environ. Health, 7: 469-479.
BARRETT, R.T., SKAARE, J.U., NORHEIM, G., VADER, W., & FROSLIE, A.
(1985) Persistent organochlorines and mercury in eggs of Norwegian
seabirds 1983. Environ. Pollut. Ser. A, 39: 79-93.
BASSON, N.C.J. (1971) Effects of dieldrin and its photoisomerization
product photodieldrin on birds. Phytophylactica, 3: 115-124.
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1971) Growth
response of blue-green algae to aldrin, dieldrin, endrin, and their
metabolites. Bull. environ. Contam. Toxicol., 6(6): 589-594.
BAXTER, W.L., LINDER, R.L., & DAHLGREN, R.B. (1969) Dieldrin
effects in two generations of penned hen pheasants. J. wildl.
Manage., 33(1): 96-102.
BEALL, M.L., Jr & NASH, R.G. (1969) Crop seedling uptake of DDT,
dieldrin, endrin and heptachlor from soils. Agron. J., 61: 571-575.
BEALL, M.L., Jr & NASH, R.G. (1971) Organochlorine insecticide
residues in soybean plant tops: root vs vapour sorption. Agron. J.,
63: 460-464.
BEALL, M.L., Jr & NASH, R.G. (1972) Insecticide depth in soil -
Effect on soybean - Uptake in the greenhouse. J. environ. Qual.,
1(3): 283-288.
BEDFORD, C.T. (1974) Von Baeyer/IUPAC names and abbreviated
chemical names of metabolites and artifacts of aldrin (HHDN),
dieldrin (HEOD), and endrin. Pestic. Sci., 5: 473-489.
BEDFORD, C.T. & HARROD, R.K. (1972a) Synthesis of 9-hydroxy-HEOD,
a major mammalian metabolite of HEOD (dieldrin), Sittingbourne,
Shell Research, Tunstall Laboratory (TU/7/72).
BEDFORD, C.T. & HARROD, R.K. (1972b) An improved preparation of
trans-4,5-dihydroxy-4,5-dihydroaldrin, a metabolite of HEOD
(dieldrin) in mammals, insects, and microorganisms. Chemosphere,
1(6): 255-260.
BEDFORD, C.T. & SMITH, E.H. (1978) Synthesis of dieldrin
metabolites. III. Two-step conversion of syn-12-hydroxy dieldrin
into Klein's metabolite (3,5,6,6,7-pentachloro-11,12-exo-epoxy-
pentacyclo(6.4.0.02,10.03,7.05,9)-dodecan-4-one). J. agric. food
Chem., 26(4): 911-914.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the
aquatic environment: uptake and loss of DDT and dieldrin by fresh-
water mussels. Arch. environ. Contam. Toxicol., 1(2): 97-111.
BELISLE, A.A., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., MULHERN,
B.M., PROUTY, R.M., DEWOLF, R.B., & CROMARTIE, E. (1972) Residues
of organochlorine pesticides, polychlorinated biphenyls, and
mercury and autopsy data for bald eagles, 1969 and 1970. Pestic.
monit. J., 6(3): 133-138.
BELL, A. (1960) Aldrin poisoning: a case report. Med. J. Aust., 2:
698-700.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Ind. Med. Surg., 38(12): 50-52.
BENITZ, K.F., ROTH, R.N., & COULSTON, F. (1977) Morphologic
characteristics of hepatic nodules induced by mirex and dieldrin in
mice. Toxicol. appl. Pharmacol., 41: 154-155.
BENNINGTON, S.L., CONNORS, P.G., CONNORS, C.W., & RISEBROUGH, R.W.
(1975) Patterns of chlorinated hydrocarbon contamination in New
Zealand sub-antarctic and coastal marine birds. Environ. Pollut.,
8: 135-147.
BENSON, W.R. (1969) Note on nomenclature of dieldrin and related
compounds. J. Assoc. Off. Agric. Chem., 52: 1109-1112.
BESS, H.A. & HYLIN, J.W. (1970) Persistence of termiticides in
Hawaiian soils. J. econ. Entomol., 63(2): 633-638.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972a)
Organochlorine pesticide residues in water, sediment, algae, and
fish: Hawaii 1970-71. Pestic. monit. J., 6(1): 56-64.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972b) Organochlorine
pesticides in rainwater: Oahu, Hawaii 1971-72. Bull. environ.
Contam. Toxicol., 8(4): 238-241.
BEYER, W.N. & GISH, C.D. (1980) Persistence in earthworms and
potential hazards to birds of soil applied DDT, dieldrin, and
heptachlor. J. appl. Ecol., 17(2): 295-307.
BEYERMANN, K. & ECKRICH, W. (1973) [Gas-chromatographic
determination of insecticide traces in air.] Z. anal. Chem.,
265(1): 4-7 (in German).
BEYNON, K.I. & ELGAR, K.E. (1966) The analysis for residues of
chlorinated insecticides and acaricides. Analyst, 91(1080):
143-175.
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1127-1130.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDWELL, K., WEBER, E., NIENHOLD, I., CONNOR, T., & LEGATOR, M.S.
(1975) Comprehensive evaluation for mutagenic activity of dieldrin.
Mutat. Res., 31: 314 (Abstract).
BIJLEVELD, M. (1974) Birds of prey in Europe, London, MacMillan
Press, pp. XI and 263.
BLACK, A.M.S. (1974) Self poisoning with dieldrin: a case report
and pharmacokinetic discussion. Anaesth. intensive Care, 2: 369-374.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin, and endrin. Arch. environ. Contam.
Toxicol., 7: 83-98.
BLUS, L.J. (1982) Further interpretation of the relation of
organochlorine residues in brown pelican eggs to reproductive
success. Environ. Pollut. Ser. A, 28: 15-33.
BLUS, L.J., NEELY, B.S., Jr, BELISLE, A.A, & PROUTY, R.M. (1974a)
Organochlorine residues in brown pelican eggs: relation to
reproductive success. Environ. Pollut., 7: 81-91.
BLUS, L.J., BELISLE, A.A, & PROUTY, R.M. (1974b) Relations of the
brown pelican to certain environmental pollutants. Pestic. monit.
J., 7(3/4): 181-194.
BLUS, L.J., JOANEN, T., BELISLE, A.A., & PROUTY, R.M. (1975) The
brown pelican and certain environmental pollutants in Louisiana.
Bull. environ. Contam. Toxicol., 13(6): 646-655.
BLUS, L.J., NEELY, B.S., Jr, LAMONT, T.G., & MULHERN, B. (1977)
Residues of organochlorines and heavy metals in tissues and eggs of
brown pelicans, 1969-73. Pestic. monit. J., 11(1): 40-53.
BLUS, L.J., CROMARTIE, E., MCNEASE, L., & JOANEN, T. (1979a) Brown
pelican: population status, reproductive success, and
organochlorine residues in Louisiana, 1971-76. Bull. environ.
Contam. Toxicol., 22: 128-135.
BLUS, L.J., LAMONT, T.G., & NEELY, B.S., Jr (1979b) Effects of
organochlorine residues on eggshell thickness, reproduction, and
population status of brown pelicans (Pelicanus occidentalis) in
South Carolina and Florida, 1969-76. Pestic. monit. J., 12(4):
172-184.
BORDON, L. (1980) Possible effects of dieldrin and other pesticides
on human chromosomes in vivo and in vitro. Toxicol. Res. Proj.
Dir., 5: 11-12 (No. 11.0077).
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., & PANKASKIE, J.E.
(1952a) Toxicological studies of aldrin on small laboratory
animals, Cincinnati, Ohio, Kettering Laboratory.
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., PANKASKIE, J.E., &
DUTRA, F.R. (1952b) Toxicological studies of dieldrin on small
laboratory animals, Cincinnati, Ohio, Kettering Laboratory.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BRAGT, P.C., SCHUURBIERS, C.J., HOLLANDER, J.C.TH., SCHULTING,
F.L., & WOLTHUIS, O.L. (1984) Retention of inhaled aldrin in man,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
BRESLER, E. & HANKS, R.J. (1969) Numerical method for estimating
simultaneous flow of water and salt in unsaturated soils. Soil Sci.
Soc. Am. Proc., 33: 827-839.
BREWERTON, H.V. & MCGRATH, H.J.W. (1967) Insecticides in human fat
in New Zealand. N. Z. J. Sci., 10: 486-492.
BRIGGS, G.G. (1981) Theoretical and experimental relationships
between soil adsorption, octanol/water partition coefficients,
water solubilities, bioconcentration factors, and the parachor. J.
agric. food Chem., 29: 1050-1059.
BRODTMANN, N.V., Jr (1976) Continuous analysis of chlorinated
hydrocarbon pesticides in the Lower Mississippi River. Bull.
environ. Contam. Toxicol., 15(1): 33-39.
BROOKS, G.T. (1974) Chlorinated insecticides. I. Technology and
applications, Cleveland, Ohio, CRC Press, pp. 87-99.
BRO-RASMUSSEN, F., DALGAARD-MIKKELSEN, Sv., JAKOBSON, Th.,
KOCH, Sv.O., RODIN, F., UHL, E., & VOLDUM-CLAUSEN, K. (1968)
Examinations of Danish milk and butter for contaminating
organochlorine insecticides. Residue Rev., 23: 55-69.
BROWN, J.R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc. J., 97: 367-373.
BROWN, L., BELLINGER, E.G., & DAY, J.P. (1979) Dieldrin pollution
in the River Holme catchment, Yorkshire. Environ. Pollut., 18:
203-211.
BROWN, L.H. (1969) Status and breeding success of golden eagles in
northwest Sutherland in 1967. Br. Birds, 62(9): 345-363.
BROWN, V.K.H., HUNTER, C.G., & RICHARDSON, A. (1964) A blood test
diagnostic of exposure to aldrin and dieldrin. Br. J. ind. Med., 21:
283-286.
BROWN, V.K.H., RICHARDSON, A., ROBINSON, J., & STEVENSON, D.E.
(1965) The effects of aldrin and dieldrin on birds. Food Cosmet.
Toxicol., 3: 675-679.
BROWN, V.K.H., ROBINSON, J., & RICHARDSON, A. (1967) Preliminary
studies on the acute and subacute toxicities of a
photoisomerization product of HEOD. Food Cosmet. Toxicol., 5:
771-779.
BROWN, V.K.H., ROBINSON, J., THORPE, E., & BARRETT, J.W. (1974) The
toxicity of dieldrin (HEOD) to domestic fowl. Pestic. Sci., 5:
567-586.
BRUCE, W.N. & DECKER, G.C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin,
dieldrin, heptachlor, and heptachlor epoxide. J. agric. food Chem.,
14(4): 395-398.
BUECHEL, K.H., GINSBERG, A.E., & FISCHER, R. (1966) [Synthesis and
structure of chlordane isomers.] Chem. Ber., 99: 421-430 (in German).
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (1967) Chlorinated
pesticide levels in eastern oyster (Crassostrea virginica) from
selected areas of the South Atlantic and Gulf of Mexico. Pestic.
monit. J., 1(3): 9-12.
BUNCH, T.D. & LOW, J.B. (1973) Effects of dieldrin on chromosomes
of semi-domestic mallard ducks. J. wildl. Manage., 37(1): 51-57.
BUNYAN, P.J. & STANLEY, P.I. (1982) Toxic mechanisms in wildlife.
Regul. Toxicol. Pharmacol., 2: 106-145.
BURKE, H.R. (1959) Toxicity of several insecticides to two species
of beneficial insects on cotton. J. econ. Entomol., 52(4): 616-618.
BURNS, B.G., PEACH, M.E., & STILES, D.A. (1975) Organochlorine
pesticide residues in a farming area, Nova Scotia, 1972-73. Pestic.
monit. J., 9(1): 34-38.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue - Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURNS, K.A. (1976) Microsomal mixed-function oxidases in an
estuarine fish Fundulus heteroclitus and their induction as a
result of environmental contamination. Comp. Biochem. Physiol., 53B:
443-446.
BUTLER, P.A. (1971) Influence of pesticides on marine ecosystems.
Proc. R. Soc. Lond., B177: 321-329.
BUTLER, P.A. (1973) Organochlorine residues in estuarine mollusks,
1965-72. Pestic. monit. J., 6(4): 238-362.
CABRAL, J.R.P., RAITANO, F., MOLLNER, T., BRONCZYK, S.A., & SHUBIK,
P. (1979a) Acute toxicity of pesticides in hamsters. Toxicol. appl.
Pharmacol., 48: A192 (Abstract 384).
CABRAL, J.R.P., HALL, R.K., BRONCZYK, S.A., & SHUBIK, P. (1979b) A
carcinogenicity study of the pesticide dieldrin in hamsters. Cancer
Lett., 6(4/5): 241-246.
CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1968) Peregrines and
pesticides in Alaska. Condor, 70: 170-178.
CAIRNS, J., Jr, FOSTER, N.R., & LOOS, J.J. (1967) Effects of
sublethal concentrations of dieldrin on laboratory populations of
guppies ( Poecilia reticulata Peters). Proc. Natl Acad. Sci.
Philadelphia, 119(3): 75-91.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs on
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11(1): 70-77.
CAMPBELL, M.A., GYORKOS, J., LELCI, B., HOMONKO, K., & SAFE., S.
(1983) The effects of twenty-two organochlorine pesticides as
inducers of the hepatic drug-metabolizing enzymes. Gen. Pharmac.,
14(4): 445-454.
CAREY, A.E. & KUTZ, P.W. (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ. Monit. Assess., 5(2): 155-163.
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the Corn
Belt Region, United States, 1970. Pestic. monit. J., 6(4): 369-376.
CAREY, A.E., WIERSMA, G.B., & TAI, H. (1976) Pesticide residues in
urban soils from 14 United States cities, 1970. Pestic. monit. J.,
10(2): 54-60.
CAREY, A.E., YANG, H.S.C., WIERSMA, G.B., TAI, H., MAXEY, R.A., &
DUPUY, A.E., Jr (1980) Residual concentrations of propanil, TCAB,
and other pesticides in rice-growing areas in the United States,
1972. Pestic. monit. J., 14(1): 23-25.
CARLSON, G.P. (1974) Epoxidation of aldrin to dieldrin by lobsters.
Bull. environ. Contam. Toxicol., 11(6): 577-582.
CARNAGHAN, R.B.A. & BLAXLAND, J.D. (1957) The toxic effect of
certain seed dressings on wild and game birds. Vet. Rec., 69:
324-325.
CARO, J.H. & TAYLOR, A.W. (1971) Pathways of loss of dieldrin from
soil under field conditions. J. agric. food Chem., 19(2): 379-384.
CARO, J.H., TAYLOR, A.W., & FREEMAN, H.P. (1976) Comparative
behaviour of dieldrin and carbofuran in the field. Arch. environ.
Contam. Toxicol., 3: 437-447.
CARTER, F.L. & STRINGER, C.A. (1970) Soil moisture and soil type
influence initial penetration by organochlorine insecticides. Bull.
environ. Contam. Toxicol., 5(5): 422-428.
CASARETT, L.J., FRYER, G.C., YAUGER, W.L., Jr, & KLEMMER, H.W.
(1968) Organochlorine pesticide residues in human tissue - Hawaii.
Arch. environ. Health, 17: 306-311.
CASSIDY, W., FISHER, A.J., PEDEN, J.D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset,
London, United Kingdom, Ministry of Health, Public Health
Laboratory Services, Vol. 26, pp. 2-6 (Monthly Bulletin).
CATHEY, B. (1982) Comparative toxicities of five insecticides to
the earthworm (Lumbricus terrestris). Agric. Environ., 7: 73-81.
CAUSEY, M.K., BONNER, F.L., & GRAVES, J.B. (1968) Dieldrin residues
in the gallinules Porphyrula martinica L. and Gallinula chloropas
L. and its effect on clutch size and hatchability. Bull. environ.
Contam. Toxicol., 3(5): 274-283.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation into the connection between the diet and living
conditions of nursing mothers and the contamination of breast milk
with sparingly volatile organochloride compounds.] Act. Ernähr., 9:
157-162 (in German).
CHACKO, C.I. & LOCKWOOD, J.L. (1967) Accumulation of DDT and
dieldrin by microorganisms. Can. J. Microbiol., 13: 1123-1126.
CHADWICK, G.G. & BROCKSEN, R.W. (1969) Accumulation of dieldrin by
fish and selected fish-food organisms. J. wildl. Manage., 33(3):
693-700.
CHADWICK, G.G. & SHUMWAY, D.L. (1970) Effects of dieldrin on the
growth and development of steelhead trout. In: Gillett, J.W., ed.
The biological impact of pesticides in the environment, Corvallis,
Oregon State University, pp. 90-96 (Environmental Health Series
No. 1).
CHAN, T.M., GILLETT, J.W., & TERRIERE, L.C. (1967) Interaction
between microsomal electron transport systems of trout and male rat
in cyclodiene epoxidation. Comp. Biochem. Physiol., 20: 731-742.
CHANIN, P.R.F. & JEFFERIES, D.J. (1978) The decline of the otter
Lutra lutra L. in Britain: an analysis of hunting records and
discussion of causes. Biol. J. Linn. Soc., 10: 305-328.
CHAU, A.S.Y. & COCHRANE, W.P. (1970) Cis-opening of dieldrin
oxirane ring. Chem. Ind., 49: 1568-1569.
CHAUDRY, M.M., NELSON, A.I., & PERKINS, E.G. (1978) Distribution of
chlorinated pesticides in soybeans, soybean oil, and its by-
products during processing. J. Am. Oil Chem. Soc., 55(12): 851-853.
CHERNOFF, N., KAVLOCK, R.J., KATHREIN, J.R., DUNN, J.M., & HASEMAN,
J.K. (1975) Prenatal effects of dieldrin and photodieldrin in mice
and rats. Toxicol. appl. Pharmacol., 31: 302-308.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1981)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avian and mammalian wildlife
toxicology. Second Conference, Philadelphia, Pennsylvania,
American Society of Testing Materials, pp. 143-154 (STP757).
CLARK, D.R., Jr (1975) Effect of stress on dieldrin toxicity to
male redwinged blackbirds (Agelaius phoeniceus). Bull. environ.
Contam. Toxicol., 14(2): 250-256.
CLARK, D.R., Jr (1981) Death in bats from DDE, DDT or dieldrin:
diagnosis via residues in carcass fat. Bull. environ. Contam.
Toxicol., 26: 367-374.
CLARK, D.R., Jr & KROLL, C.J. (1977) Effects of DDE on
experimentally poisoned free-tailed bats (Tadarida brasiliensis):
lethal brain concentrations. J. Toxicol. environ. Health, 3: 893-901.
CLARK, D.R., Jr & MCLANE, M.A.R. (1974) Chlorinated hydrocarbon and
mercury residues in woodcock in the United States, 1970-71. Pestic.
monit. J., 8(1): 15-22.
CLARK, D.R., Jr & PROUTY, R.M. (1984) Disposition of dietary
dieldrin in the little brown bat and correlation of skin levels
with body burden. Bull. environ. Contam. Toxicol., 33: 177-183.
CLARK, D.R., Jr, LAVAL, R.K., & SWINEFORD, D.M. (1978) Dieldrin-
induced mortality in an endangered species, the gray bat (Myotis
grisescens). Science, 199: 1357-1359.
CLARK, D.R., Jr, LAVAL, P.K., & KRYNITSKY, A.J. (1980) Dieldrin and
heptachlor residues in dead gray bats, Franklin County, Missouri -
1976 versus 1977. Pestic. monit. J., 13(4): 137-140.
CLARK D.R., Jr, CLAWSON, R.L., & STAFFORD, C.J. (1983a) Gray bats
killed by dieldrin at two additional Missouri caves: aquatic
macroinvertebrates found dead. Bull. environ. Contam. Toxicol.,
30: 214-218.
CLARK, D.R., Jr, BUNCK, C.M., CROMARTIE, E., & LAVAL, R.K. (1983b)
Year and age effects on residues of dieldrin and heptachlor in dead
gray bats, Franklin County, Missouri - 1976, 1977, and 1978.
Environ. Toxicol. Chem., 2: 387-393.
CLEVELAND, F.P. (1966) A summary of work on aldrin and dieldrin
toxicity at the Kettering Laboratory. Arch. environ. Health, 13:
195-198.
COHEN, J.M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rain-out. In: Bould, R.F., ed.
Organic pesticides in the environment, Washington, DC, American
Chemical Society, pp. 163-176 (Advances in Chemistry Series No.
60).
COLE, J.F., KLEVAY, L.M., & ZAVON, M.R. (1970) Endrin and dieldrin:
a comparison of hepatic excretion in the rat. Toxicol. appl.
Pharmacol., 16: 547-555.
COLLINS, G.B., HOLMES, D.C., & HOODLESS, R.A. (1982) Organochlorine
pesticide residues in human milk in Great Britain, 1979-1980. Hum.
Toxicol., 1: 425-431.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Egg shell
characteristics and incidence of shell breakage for grey herons
Ardea cinerea exposed to environmental pollutants. Environ.
Pollut., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides, and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
Monks Wood Experimental Station.
COPPLESTONE, J.F., HUNNEGO, J.N., & HARRISON, D.L. (1973)
Organochlorine insecticide levels in adult New Zealanders - a five-
year study. N. Z. J. Sci., 16: 27-39.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet samples
(V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet samples
(VI). Pestic. monit. J., 5(4): 313-330.
COULSTON, F., ABRAHAM, R., & MANKES, R. (1980) Reproductive study
in female rats given dieldrin, alcohol, or aspirin orally, Albany,
New York, Albany Medical College of Union University, Institute of
Comparative and Human Toxicology.
COWAN, A.A. (1981) Organochlorine compounds in mussels from
Scottish coastal waters. Environ. Pollut. Ser. B, 2: 129-143.
CRAIG, N.C.D. (1977) A summary of the data on the toxicity of
various materials to aquatic life. III. Dieldrin, aldrin, and
endrin, Brixham United Kingdom, Imperial Chemical Industries Ltd
(Unpublished Report BL/A/1828).
CRAWFORD, N.H. & DONIGIAN, A.S., Jr (1973) Pesticide transport and
runoff model for agricultural lands, Washington, DC, US
Environmental Protection Agency (EPA-600/2-74-013).
CREBELLI, R., BELLINCAMPI, D., CONTI, G., MORPURGO, G., & CARERE,
A. (1986) A comparative study on selected chemical carcinogens for
chromosome malsegregation, mitotic crossing-over and forward
mutation induction in Aspergillus nidulans. Mutat. Res., 172:
139-149.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND, P.F.,
& CAREY, A.E. (1974) Pesticide residue levels in soils and crops:
FY-70 - National Soils Monitoring Program. II. Pestic. monit. J.,
8(2): 69-97.
CROLL, B.T. (1969) Organochlorine insecticides in water. Part I.
Proc. Soc. Water Treat. Exam., 18: 255-274.
CROMARTIE, E., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., KAISER,
T.E., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., & SWINEFORD, D.M.
(1975) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1971-72. Pestic. monit.
J., 9(1): 11-14.
CUETO, C., Jr & BIROS, F.J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol., 10:
261-269.
CUETO, C., Jr & HAYES, W.J., Jr (1962) The detection of dieldrin
metabolites in human urine. J. agric. food Chem., 10: 366-369.
CUMMINGS, J.G. (1966) Pesticides in the total diet. Residue Rev.,
16: 30-45.
CUMMINGS, J.G., ZEE, K.T., TURNER, V., & QUINN, F. (1966) Residues
in eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Assoc. Off. Agric. Chem., 49(2): 354-364.
CURLEY, A., COPELAND, M.F., & KIMBROUGH, R.D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242(5396): 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1970) Eggshell thickness in
pheasants given dieldrin. J. wildl. Manage., 34(1): 226-228.
DAHLGREN, R.B. & LINDER, R.L. (1974) Effects of dieldrin in penned
pheasants through the third generation. J. wildl. Manage., 38(2):
320-330.
DAILEY, R.E., WALTON, M.S., BECK, V., LEAVENS, C.L., & KLEIN, A.K.
(1970) Excretion, distribution, and tissue storage of a 14C-
labelled photoconversion product of 14C-dieldrin. J. agric. food
Chem., 18(3): 443-445.
DALE, W.E. & QUINBY, G.E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W.E., COPELAND, M.F., & HAYES, W.J., Jr (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World Health
Organ., 33: 471-477.
DALE, W.E., CURLEY, A., & CUETO, C., Jr (1966) Hexane extractable
chlorinated insecticides in human blood. Life Sci., 5: 47-54.
DAMICO, J.N., CHEN, J.Y.T., COSTELLO, C.E., & HAENNI, E.O. (1968)
Structure of Klein's metabolites of aldrin and of dieldrin. J.
Assoc. Off. Agric. Chem., 51(1): 48-55.
DANIELS, N.E. (1966) Soil insect control and insecticidal residue
detection. J. econ. Entomol., 59: 410-413.
DATTA, P.R., LANG, E.P., WATTS, J.O., KLEIN, A.K., & NELSON, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature (Lond.), 208: 289-290.
DAVIDSON, J.M. & MCDOUGAL, J.R. (1973) Experimental and predicted
movement of three herbicides in water-saturated soil. J. environ.
Qual., 2(4): 428-433.
DAVIES, J.E., BARQUET, A., MORGADE, C., & RAFFONELLI, A. (1975)
Epidemiological studies of DDT and dieldrin residues and their
relationship to human carcinogenesis. In: Proceedings of the
International Symposium on Recent Advances in Assessment of the
Health Effects of Environmental Pollution, Luxembourg, Commission
of the European Communities, Vol. 2, pp. 695-705.
DAVIS, B.N.K. (1968) The soil macrofauna and organochlorine
insecticide residues in twelve agricultural sites near Huntingdon.
Ann. appl. Biol., 61: 29-45.
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin
and DDT by earthworms. Soil Biol. Biochem., 3: 221-233.
DAVIS, H.C. & HIDU, H. (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish. Bull., 67(2): 393-404.
DAVIS, K.J. & FITZHUGH, O.G. (1962) Tumorigenic potential of aldrin
and dieldrin for mice. Toxicol. appl. Pharmacol., 4: 187-189.
DAVIS, K.J., HANSEN, W., & FITZHUGH, O.G. (1965) Pathology report
on mice fed aldrin, dieldrin, heptachlor, or heptachlor epoxide for
two years, Washington, DC, US Environmental Protection Agency
(Memorandum to Dr A.J. Lehman, 19th July, 1965. Publicly presented
and accepted at the US EPA Aldrin-Dieldrin Suspension Hearing,
Statement of Testimony from Dr K.J. Davis, August 1974).
DAVIS, P.W., FRIEDHOFF, J.M., & WEDEMEYER, G.A. (1972)
Organochlorine insecticide, herbicide, and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8(2): 69-72.
DAVISON, K.L. (1970) Dieldrin accumulation in tissues of the sheep.
J. agric. food Chem., 18(6): 1156-1160.
DAVISON, K.L. (1973) Dieldrin-14C balance in rats, sheep, and
chickens. Bull. environ. Contam. Toxicol., 10(1): 16-24.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p'-DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol., 7(1): 9-18.
DAWSON, R. & RILEY, J.P. (1977) Chlorine-containing pesticides and
polychlorinated biphenyls in British coastal waters. Estuarine
coastal mar. Sci., 4: 55-69.
DEAN, B.J., DOAK, S.M.A., & SOMERVILLE, H. (1975) The potential
mutagenicity of dieldrin (HEOD) in mammals. Food Cosmet. Toxicol.,
13: 317-323.
DE CAMPOS, M. & OLSZYNA-MARZYS, A.E. (1979) Contamination of human
milk with chlorinated pesticides in Guatemala and in El Salvador.
Arch. environ. Contam. Toxicol., 8: 43-58.
DECKER, G.C., BRUCE, W.N., & BIGGER, J.H. (1965) The accumulation
and dissipation of residues resulting from the use of aldrin in
soils. J. econ. Entomol., 58(2): 266-271.
DEFLORA, S. (1981) Study of 106 organic and inorganic compounds in
the Salmonella/microsome test. Carcinogenesis, 2(4): 283-298.
DEICHMANN, W.B. (1974) Certified statement of testimony before the
Environmental Protection Agency at aldrin/dieldrin suspension
hearings, Washington, DC, US Environmental Protection Agency.
DEICHMANN, W.B. & MACDONALD, W.E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-72. Ecotoxicol.
environ. Saf., 1: 89-110.
DEICHMANN, W.B., KEPLINGER, M.L., SALA, F., & GLASS, E. (1967)
Synergism among oral carcinogens. IV. The simultaneous feeding of
four tumorigens to rats. Toxicol. appl. Pharmacol., 11: 88-103.
DEICHMANN, W.B., DRESSLER, I., KEPLINGER, M., & MACDONALD, W.E.
(1968) Retention of dieldrin in blood, liver, and fat of rats fed
dieldrin for six months. Ind. Med. Surg., 37: 837-839.
DEICHMANN, W.B., KEPLINGER, M., DRESSLER, I., & SALA, F. (1969)
Retention of dieldrin and DDT in the tissues of dogs fed aldrin and
DDT individually and as a mixture. Toxicol. appl. Pharmacol., 14:
205-213.
DEICHMANN, W.B., MACDONALD, W.E., BLUM, E., BEVILACQUA, M.,
RADOMSKI, J., KEPLINGER, M., & BALKUS, M. (1970) Tumorigenicity of
aldrin, dieldrin, and endrin in the albino rat. Ind. Med., 39(10):
314, 426-434.
DEICHMANN, W.B., MACDONALD, W.E., BEASLY, A.G., & CUBIT, D.A.
(1971) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg., 40(2): 10-22.
DEICHMANN, W.B., MACDONALD, W.E., CUBIT, D.A., & BEASLY, A.G.
(1972) Effects of starvation in rats with elevated DDT and dieldrin
tissue levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEICHMANN, W.B., MACDONALD, W.E., & CUBIT, D.A. (1975) Dieldrin and
DDT in the tissues of mice fed aldrin and DDT for seven
generations. Arch. Toxicol., 34: 173-182.
DEICHMANN, W.B., MACDONALD, W.E., & LU, F.C. (1979) Effects of
chronic aldrin feeding in two strains of female rats, and a
discussion on the risks of carcinogens in man. In: Deichmann, W.B.,
ed. Toxicology and occupational medicine, Amsterdam, Elsevier/North
Holland, pp. 407-413.
DEJONCKHEERE, W., STEURBAUT, W., VERSTRAETEN, R., & KIPS, R.H.
(1977) Residues of organochlorine pesticides in human fat in
Belgium. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 42(2): 1839-1847.
DE LLAMAS, M.C., DE CASTRO, A.C., & PECHEN DE D'ANGELO, A.M. (1985)
Cholinesterase activities in developing amphibian embryos following
exposure to the insecticides dieldrin and malathion. Arch environ.
Contam. Toxicol., 14: 161-166.
DEL VECCHIO, V. & LEONI, V. (1967) [Detection and measurement of
chlorinated insecticides in biological material.] Nuovi Ann. Ig.
Microbiol., 28(2): 107-128 (in Italian).
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
D'ERCOLE, A.J., ARTHUR, R.D., CAIN, J.D., & BARRENTINE, B.F. (1976)
Insecticide exposure of mothers and newborns in a rural
agricultural area. Pediatrics, 57(6): 869-874.
DE VLIEGER, M., ROBINSON, J., BALDWIN, M.K., CRABTREE, A.N., & VAN
DIJK, M.C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRYNS, J.K., MUYS,
T., & POLL, J.M., VAN DER (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
chem. Toxicol., 22(1): 11-21.
DICK, G.L., HEENAN, M.P., LOVE, J.L., UDY, P.B., & DAVIDSON, F.
(1978) Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus pesticide
residue content. N. Z. J. Sci., 21: 71-78.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DISLER, R.L., GLATT, V., & MEIER, W. (1984) [Residues of
chlorinated insecticides and polychlorinated biphenyls in breast
milk.] Mitt. Geb. Lebensm. Hyg., 75: 205-213 (in German).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health, 4(7, suppl.):
140-146.
DIX, K.M. & WILSON, A.B. (1971) Toxicity studies with dieldrin
(HEOD). Teratogenic studies in rabbits given HEOD and thalidomide
orally, Sittingbourne, Shell Research (TLGR.0051.71).
DIX, K.M., VAN DER PAUW, C.L., & MCCARTHY, W.V. (1978) Toxicity
studies with dieldrin: teratological studies in mice dosed orally
with HEOD. Teratology, 16: 57-62.
DOBBS, A.J. & WILLIAMS, N. (1983) Indoor air pollution from
pesticides used in wood remedial treatments. Environ. Pollut.
Ser. B, 6: 271-296.
DOBSON, R.C. & BAUGH, E.R. (1976) Dieldrin residue removal from the
fat of swine. Bull. environ. Contam. Toxicol., 16(5): 567-571.
DOUABUL, A.A.Z., AL-SAAD, H.T., & AL-REKABI, H.N. (1987) Residues
of organochlorine pesticides in environmental samples from the
Shatt al-Arab river, Iraq. Environ. Pollut., 43: 175-187.
DRAPER, W.M. & CROSBY, D.G. (1984) Solar photooxidation of
pesticides in dilute hydrogen peroxide. J. agric. food Chem., 32:
231-237.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic
provinces of Canada. J. agric. food Chem., 15(3): 457-464.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States. III. June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E. & LIPSCOMB, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States. II. June 1966 - April 1968. Pestic.
monit. J., 2(4): 153-162.
DUGGAN, R.E., BARRY, H.C., & JOHNSON, L.Y. (1967) Pesticide
residues in total diet samples. II. Pestic. monit. J., 1(2): 2-12.
ECKENHAUSEN, F.W., BENNET, D., BEYNON, K.I., & ELGAR, K.E. (1981)
Organochlorine pesticide concentrations in perinatal samples from
mothers and babies. Arch. environ. Health, 36(2): 81-92.
EDMUNDSON, W.F., DAVIES, J.E., & HULL, W. (1968) Dieldrin storage
levels in necropsy adipose tissue from a south Florida population.
Pestic. monit. J., 2(2): 86-89.
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Oxford, Blackwell, pp. 239-261.
EDWARDS, C.A. (1966) Insecticide residues in soil. Residue Rev., 13:
83-132.
EDWARDS, C.A. (1973a) Persistent pesticides in the environment,
Cleveland, Ohio, CRC Press, pp. 68-74.
EDWARDS, C.A. (1973b) Persistent pesticides in soil and water. In:
Environmental pollution by pesticides, London, Plenum Press, pp.
409-458.
EDWARDS, C.A. & LOFTY, J.R. (1977) Biology of earthworms, 2nd ed.,
London, Chapman and Hall, pp. 212-213.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the soil
fauna. Residue Rev., 45: 8-15.
EDWARDS, C.A., DENNIS, E.B., & EMPSON, D.W. (1967) Pesticides and
the soil fauna: effects of aldrin and DDT in an arable field. Ann.
appl. Biol., 60: 11-22.
EEC (1983) Directives relating to the classification and labelling
of dangerous substances. Annex VI. Part II (2.4.10) to the fifth
adaptation to the Dangerous Substances Directives, Luxembourg,
European Economic Community (Commission Directive 83/467/EEC)
(Official Journal L 257/19).
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J.O'G. (1965)
Organochlorine pesticide residues in human fat and human milk. Br.
med. J., 2: 66-69.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5: 541-544.
EISENLORD, G., LOQUVAM, G.S., & NEMENZO, J. (1967) Results of
reproduction study of rats fed diets containing dieldrin over three
generations, San Francisco, California, The Hine Laboratories
(Report No. 4).
EISLER, R. (1969) Acute toxicities of insecticides to marine
decapod crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Latent effects of insecticide intoxication to
marine molluscs. Hydrobiologia, 36(3/4): 345-352.
EL BEIT, I.O.D. (1981) Pesticide microbial interactions in the
soil. Int. J. environ. Stud., 16: 171-181.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981a) Factors
involved in the dynamics of pesticides in soils: the effect of
pesticide concentration on leachability and absorption. Int. J.
environ. Stud., 16: 181-187.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981b) Factors
involved in the dynamics of pesticides in soils: the effects of
temperature and period of contact or leachability and adsorption of
pesticides by soils. Int. J. environ. Stud., 16: 189-196.
EL BEIT, I.O.D., COTTON, D.E., & WHEELOCK, J.V. (1983) Persistence
of pesticides in soil leachates: effects of pH, ultraviolet
irradiation, and temperature. Int. J. environ. Stud., 21: 251-259.
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. (1975) The dissipation and accumulation of aldrin and
dieldrin residues in soil. Environ. Qual. Saf., 3(suppl.): 250-257.
ELGAR, K.E. (1979) The variability of residue results, with
particular reference to the Codex study on organochlorines in
butterfat. In: Proceedings of the 4th IUPAC Congress on Pesticide
Chemistry, Zurich, 1978, pp. 668-672.
ELIASON, B.C. & POSNER, H.S. (1971) Reduced passage of carbon-14-
dieldrin to the fetal rat by phenobarbital but not by eight other
drugs or dieldrin. Am. J. Obstet. Gynecol., 110(7): 943-947.
ELY, R.E., MOORE, L.A., HUBANKS, P.E., CARTER, R.H., & POOS, F.W.
(1954) Studies of feeding aldrin to dairy cows. J. dairy Sci., 37(3):
294-298.
EMANUELSEN, M., LINCER, J.L., & RIFKIN, E. (1978) The residue
uptake and histology of American oysters ( Crassostrea virginica
Gmelin) exposed to dieldrin. Bull. environ. Contam. Toxicol., 19:
121-129.
ENDERSON, J.H. & BERGER, D.D. (1968) Chlorinated hydrocarbon
residues in peregrines and their prey species from northern Canada.
Condor, 70: 149-153.
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning
and lowered production of young in prairie falcons. Bioscience,
20(6): 355-356.
EPIFANIO, C.E. (1973) Dieldrin uptake by larvae of the crab
Leptodius floridanus. Mar. Biol., 19(4): 320-322.
EPSTEIN, E. & GRANT, W.J. (1968) Chlorinated insecticides in runoff
water as affected by crop rotation. Soil Sci. Soc. Am. Proc., 32:
423-426.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced in
mutant strains of Salmonella typhimurium by pesticides,
Washington, DC, American Chemical Society (Abstracts Paper 174)
(Pesticide No. 43).
ESTENIK, J.F. & COLLINS, W.J. (1979) In vivo and in vitro studies
of mixed-function oxidase in an aquatic insect Chironomus riparius.
In: Khan, M.A.Q., ed. Pesticide and xenobiotic metabolism in
aquatic organisms, Washington, DC, American Chemical Society, pp.
349-370 (ACS Symposium Series No. 99).
EYE, J.D. (1968) Aqueous transport of dieldrin residues in soil. J.
Water Pollut. Control Fed. (suppl.), 40(8): R316-332.
FABER, R.A., RISEBROUGH, R.W., & PRATT, H.M. (1972) Organochlorines
and mercury in common egrets and great blue herons. Environ.
Pollut., 3: 111-122.
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides,
Lyons, International Agency for Research on Cancer, pp. 161-181
(IARC Scientific Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a Joint Meeting of the FAO Committee on Pesticides
in Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting Report
No. PL:1965/10; WHO Food Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO Meeting Report No.
PL: 1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of the FAO
Working Party on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) 1966 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1967/M/11/1; WHO Food
Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 78;
WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1968/M/9/1; WHO Food
Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 84;
WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 87;
WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71. 42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 97;
WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Plant Production and
Protection Series No. 1; WHO Technical Report Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues and
the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 10 Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1984) Codex guidelines on good practice in pesticide
residue analysis, Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986b) Recommendations for methods of analysis of
pesticide residues, Rome, Codex Alimentarius Committee, Food and
Agriculture Organization of the United Nations (CAC/PR8-1986).
FARB, R.M., SANDERSON, T., MOORE, B.G., & HAYES, A.W. (1973)
Interaction: the effect of selected mycotoxins on the tissue
distribution and retention of aldrin and dieldrin in the neonatal
rat. In: Deichmann, W.B., ed. Pesticides in the environment, New
York, International Medical Book Corporation, pp. 179-187.
FARMER, W.J. & JENSEN, C.R. (1970) Diffusion and analysis of
carbon-14-labelled dieldrin in soils. Soil Sci. Soc. Am. Proc., 34:
28-31.
FARMER, W.J. & LETEY, J. (1974) Volatilization losses of pesticides
from soils, Washington, DC, US Environmental Protection Agency
(EPA-660/2-74-054).
FARMER, W.J., IGUE, K., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. I. Effect of
concentration, temperature, air flow rate, and vapour pressure.
Soil Sci. Soc. Am. Proc., 36: 443-447.
FARMER, W.J., IGUE, K., & SPENCER, W.F. (1973) Effect of bulk
density on the diffusion and volatilization of dieldrin from soil.
J. environ. Qual., 2(1): 107-109.
FEIL, V.J., HEDDE, R.D., ZAYLSKIE, R.G., & ZACHRISON, C.H. (1970)
Dieldrin-14C metabolism in sheep. Identification of trans-6,7-di-
hydroxydihydroaldrin and 9-(syn-epoxy)-hydroxy-1,2,3,4,10,10-hexa-
chloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-endo-5,8-exo-
dimethanonaphthalene. J. agric. food Chem., 18(1): 120-124.
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FERGIN, T.J. & SCHAFER, E.C. (1977) Toxicity of dieldrin to
bobwhite quail in relation to sex and reproductive status. Arch.
environ. Contam. Toxicol., 6: 213-219.
FERNICOLA, N.A.G.G. & AZEVEDO, F.A. (1982) Serum levels of
organochlorine insecticides in humans in Sao Paulo, Brazil. Vet.
hum. Toxicol., 24(2): 91-93.
FIKES, M.H. & TUBB, R.A. (1972) Dieldrin uptake in the three-ridge
naiad. J. wildl. Manage., 36(3): 802-809.
FISCHLER, H.M. & KORTE, F. (1969) [Sensitized and non-sensitized
photoisomerization of cyclodiene insecticides.] Tetrahedron Lett.,
32: 2793-2796 (in German).
FISEROVA-BERGEROVA, V., RADOMSKI, J.L., DAVIES, J.E., & DAVIS, J.H.
(1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHER, H.L., MOST, B., & HALL, L.L. (1985) Dermal absorption of
pesticides calculated by deconvolution. J. appl. Toxicol., 5(3):
163-177.
FITZHUGH, O.G., NELSON, A.A., & QUAIFE, M.L. (1964) Chronic oral
toxicity of aldrin and dieldrin in rats and dogs. Food Cosmet.
Toxicol., 2: 551-561.
FLETCHER, T.E., PRESS, J.M., & WILSON, D.B. (1959) Exposure of
spray-men to dieldrin in residual spraying. Bull. World Health
Organ., 20: 15-25.
FLICKINGER, E.L. & KING, K.A. (1972) Some effects of aldrin-treated
rice on Gulf coast wildlife. J. wildl. Manage., 36(3): 706-727.
FOURNIER, E., TREICH, I., CAMPAGNE, L., & CAPELLE, N. (1972)
Pesticides organo-chlorés dans le tissus adipeux d'êtres humains en
France. Eur. J. Toxicol., 5(1): 11-26.
FOWKES, F.M., BENESI, H.A., RYLAND, L.B., LOEFFLER, E.S., & SUN,
Y.P. (1960) Clay catalysed decomposition of insecticides. J. agric.
food Chem., 8: 203-210.
FOWLER, J.F., NEWSOM, L.D., GRAVES, J.B., BONNER, F.L., &
SCHILLING, P.E. (1971) Effect of dieldrin on egg hatchability,
chick survival, and eggshell thickness in purple and common
gallinules. Bull. environ. Contam. Toxicol., 6(6): 495-501.
FOX, G.R. & VIRGO B.B. (1986) Relevance of hyperglycemia to
dieldrin toxicity in suckling and adult rats. Toxicology, 38:
315-326.
FRANK, R., MONTGOMERY, K., BRAUN, H.E., BERST, A.H., & LOFTUS, K.
(1974) DDT and dieldrin in watersheds draining the tobacco belt in
southern Ontario. Pestic. monit. J., 8(3): 184-201.
FRANK, R., BRAUN, H.E., ISHIDA, K., & SUDA, P. (1976) Persistent
organic and inorganic pesticide residues in orchard soils and
vineyards of southern Ontario. Can. J. Soil Sci., 56: 463-484.
FRANK, R., HOLDRINET, M.V.H., & SUDA, P. (1979) Organochlorine and
mercury residues in wild mammals in southern Ontario, Canada 1973-
1974. Bull. environ. Contam. Toxicol., 22: 500-507.
FRANK, R., BRAUN, H.E., & HOLDRINET, M.V.H. (1981) Residues from
past uses of organochlorine insecticides and PCB in waters draining
eleven agricultural watersheds in southern Ontario, Canada, 1975-
1977. Sci. total Environ., 20: 255-276.
FRANK, R., BRAUN, H.E., SIRONS, G.H., RASPER, J., & WARD, G.G.
(1985) Organochlorine and organophosphorus insecticides and
industrial pollutants in the milk supplies of Ontario, 1983. J.
food Prot., 48(6): 499-504.
FRY, D.R. (1964) Human dieldrin poisoning. Lancet, 1: 764.
FUCHS, P. (1967) Death of birds caused by application of seed
dressings in the Netherlands. Meded. Fac. Landbouwwet. Rijksuniv.
Gent., 32(3/4): 855-859.
FURNESS, R. & HUTTON, M. (1979) Pollutant levels in the great skua
Catharacta skua. Environ. Pollut., 13: 261-268.
FYTIANOS, K., VASILIKIOTIS, G., & WEIL, L. (1985) Identification
and determination of some trace organic compounds in coastal
seawater of Northern Greece. Bull. environ. Contam. Toxicol., 34:
390-395.
GAINES, T.B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Use of the golden
hamster in toxicology. Lab. anim. Sci., 26(2): 274-280.
GAKSTATTER, J.H. (1968) Rates of accumulation of 14C-dieldrin
residues in tissues of goldfish exposed to a singe sublethal dose
of 14C-aldrin. J. Fish Res. Board Can., 25(9): 1797-1801.
GAKSTATTER, J.H. & WEISS, C.M. (1967) The elimination of DDT-14C,
dieldrin-14C, and lindane-14C from fish following a single sub-
lethal exposure in aquaria. Trans. Am. Fish. Soc., 96: 301-307.
GALLEY, R.A.E. (1970) [Chlorinated hydrocarbons. IV. Cyclodien
insecticides.] In: Wegler, R., ed. [Chemistry of crop protection
agents and pesticides,] Berlin, Heidelberg, New York, Springer-
Verlag, Vol. 1, pp. 163-192 (in German).
GANNON, N. & DECKER, G.C. (1958) The conversion of aldrin to
dieldrin on plants. J. econ. Entomol., 51: 8-11.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959a) Storage of dieldrin
in tissues of steers, hogs, lambs, and poultry fed dieldrin in
their diets. J. agric. food Chem., 7(12): 826-828.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959b) Storage of dieldrin
in tissues and its excretion in milk of dairy cows fed dieldrin in
their diets. J. agric. food Chem., 7(12): 824-826.
GARRETTSON, L.K. & CURLEY, A. (1969) Dieldrin. Studies in a
poisoned child. Arch. environ. Health, 19: 814-822.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total-diet samples, October 1980 - March 1982.
J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in adult
total-diet samples, October 1980 - March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GENELLY, R.E. & RUDD, R.L. (1956) Effects of DDT, toxaphene, and
dieldrin on pheasant reproduction. Auk, 73: 529-539.
GEORGIAN, L. (1975) The comparative cytogenetic effects of aldrin
and phosphamidon. Mutat. Res., 31: 103-108.
GEROLT, P. (1961) Investigation into the problem of insecticide
sorption by soils. Bull. World Health Organ., 24: 577-592.
GIFAP (1984) Pesticide residues in food, Brussels, Groupement
International des Associations Nationales des Fabricants de
Produits Agrochimique.
GILLETT, J.W. & CHAN, T.M. (1968) Cyclodiene insecticides as
inducers, substrates, and inhibitors of microsomal epoxidation. J.
agric. food Chem., 16(4): 590-593.
GINN, T.M. & FISHER, F.M., Jr (1974) Studies on the distribution
and flux of pesticides in waterways associated with a rice field.
Marshland ecosystems. Pestic. monit. J., 8(1): 23-32.
GISH, C.D. (1970) Organochlorine insecticide residues in soils and
soil invertebrates from agricultural lands. Pestic. monit. J., 3(4):
241-252.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 241).
GLATT, H., JUNG, R., & OESCH, F. (1983) Bacterial mutagenicity
investigation of epoxides: drugs, drug metabolites, steroids and
pesticides. Mutat. Res., 11: 99-118.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J. (1976)
Distribution of pesticides and polychlorinated biphenyls in water,
sediments, and seston of the Upper Great Lakes, 1974. Pestic.
monit. J., 10(2): 61-67.
GLOTFELTY, D.E. (1978) The atmosphere as a sink for applied
pesticides. J. Air Pollut. Control Assoc., 28: 917-921.
GONZALEZ RODRIGUEZ-CORDOBA, J.M., FERNANDES, A.L., & HENS, J.M.
(1983) [Mother neonate ratio of levels of blood contamination by
organo-chlorine insecticide residues.] Arch. Zootec., 32(122):
49-60 (in Spanish).
GOOD, E.E. & WARE, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV. Endrin and dieldrin.
Toxicol. appl. Pharmacol., 14: 201-203.
GOODWIN, E.S., GOULDEN, R., & REYNOLDS, J.G. (1961) Rapid
identification and determination of residues of chlorinated
pesticides in crops by gas-liquid chromatography. J. Soc. Anal.
Chem., 80(1028): 697-700.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ., 63(5):
945-962.
GRACA, I., SILVA FERNANDES, A.M.S., & MOURAO, H.C. (1974)
Organochlorine insecticide residues in human milk in Portugal.
Pestic. monit. J., 8(3): 148-156.
GRANVILLE, G.C., SIMPSON, B.J., & DOAK, S.M. (1973) Toxicity
studies on aldrin dicarboxylic acid: 13-week oral study in rats,
Sittingbourne, Shell Research (TLGR.0008.73) (Unpublished
proprietary report).
GRAVES, J.B., BONNER, F.L., MCKNIGHT, W.F., WATTS, A.G., & EPPS,
E.A. (1969) Residues in eggs, preening glands, liver, and muscle
from feeding dieldrin-contaminated rice bran to hens and its
effects on egg production, egg hatch, and chick survival. Bull.
environ. Contam. Toxicol., 4(6): 375-383.
GREENBERG, R.E. & EDWARDS, W.R. (1970) Insecticide residue levels
in eggs of wild pheasants in Illinois. Trans. Illinois State Acad.
Sci., 63: 136-147.
GREICHUS, Y.A., GREICHUS, A., & REIDER, E.G. (1968) Insecticide
residues in grouse and pheasant of South Dakota. Pestic. monit. J.,
2(2): 90-92.
GREICHUS, Y.A., GREICHUS, A., DRAAYER, H.A., & MARSHALL, B. (1978a)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. II. Lake McLlwaine, Rhodesia. Bull. environ. Contam.
Toxicol., 19: 444-453.
GREICHUS, Y.A., GREICHUS, A., AMMANN, B.D., & HOPCRAFT, J. (1978b)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. III. Lake Nakuru, Kenya. Bull. environ. Contam.
Toxicol., 19: 454-461.
GREVE, P.A. (1972) Potentially hazardous substances in surface
waters. Part I. Pesticides in the river Rhine. Sci. total Environ.,
1: 173-180.
GREVE, P.A. & WEGMAN, R.C.C. (1985) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands,
Copenhagen, World Health Organization, Regional Office for Europe
(ICP/CEH. 501/M05).
GRIFFIN, D.E. & HILL, W.E. (1978) In vitro breakage of plasmid DNA
by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRIFFITH, J. & DUNCAN, R.C. (1985) Serum organochlorine residues in
Florida citrus workers compared to the national health and
nutrition examination survey sample. Bull. environ. Contam.
Toxicol., 35: 411-417.
GRIFFITHS, D.C., RAW, F., & LOFTY, J.R. (1967) The effects on soil
fauna of insecticides tested against wireworms ( Agriotes ssp.) in
wheat. Ann. appl. Biol., 60: 479-490.
GROSCH, D.S. & VALCOVIC, I.R. (1967) Chlorinated hydrocarbon
insecticides are not mutagenic in Bracon hebetor tests. J. econ.
Entomol., 60(4): 1177-1179.
GUERZONI, M.E., DEL CUPOLO, L., & PONTI, I. (1976) Mutagenic
activity of pesticides. Riv. Sci. Tec. Aliment. Nutr. Um., 6:
161-165.
GUTENMANN, W.H., GREENWOOD, R.A., GYRISCO G.G., & LITTLE, R.J.
(1972) Studies of aldrin and chlordane in silt loam soils and their
possible translocation in field corn in New York. J. econ.
Entomol., 65(3): 842-844.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. Sci. total
Environ., 43: 193-219.
GUPTA, H.C.L. & KAVADIA, V.S. (1979) Dissipation of aldrin residues
in clay loam soil under the cover of root crops. Indian J. plant
Prot., 7(1): 43-49.
GUPTA, H.C.L., KUSHWAHA, K.S., KAVADIA, V.S., & SRIVASTAVA, B.P.
(1979) Aldrin residues in soils and its translocation in maize and
pearl millet. Indian J. Entomol., 41(1): 47-57.
GUPTA, P.C. (1975) Neurotoxicity of chronic chlorinated hydrocarbon
insecticide poisoning: a clinical and electroencephalographic study
in man. Indian J. med. Res., 63(4): 601-606.
HAAN, C.T. (1971) Movement of pesticides by runoff and erosion.
Trans. Am. Soc. Agric. Eng., 14: 445-447, 449.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Cournix. Bull. environ. Contam. Toxicol., 11(1): 98-102.
HAGLEY, E.A.C. (1965) Effect of insecticides on the growth of
vegetable seedlings. J. econ. Entomol., 58(4): 777-778.
HAMILTON, H.E., MORGAN, D.P., & SIMMONS, A. (1978) A pesticide
(dieldrin)-induced immunohemolytic anemia. Environ. Res., 17:
155-164.
HARGESHEIMER, E.E. (1984) Rapid determination of organochlorine
pesticides and polychlorinated biphenyls using selected ion
monitoring mass spectrometry. J. Assoc. Off. Anal. Chem., 67(6):
1067-1075.
HARR, J.R., CLAEYS, R.R., BONE, J.F., & MCCORCLE, T.W. (1970)
Dieldrin toxicosis: rat reproduction. Am. J. vet. Res., 31: 181-189.
HARRIS, C.I. (1969) Movement of pesticides in soil. J. agric. food
Chem., 17(1): 80-82.
HARRIS, C.R. (1964) Influence of soil type and soil moisture on the
toxicity of insecticides in soils to insects. Nature (Lond.), 202:
724.
HARRIS, C.R. (1972) Behaviour of dieldrin in soil. Laboratory
studies influencing biological activity. J. econ. Entomol., 65: 8-13.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J.
agric. food Chem., 15: 86-93.
HARRIS, C.R., SANS, W.W., & MILES, J.R.W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in Southwestern Ontario. J. agric. food Chem.,
14(4): 398-403.
HARRISON, R.B., HOLMES, D.C., ROBURN, J., & TATTON, J.O'G. (1967)
The fate of some organochlorine pesticides on leaves. J. Sci. Food
Agric., 18: 10.
HARVEY, G.R., STEINHAVER, W.G., & TEAL, J.M. (1973) Polychlorobiphenyls
in North Atlantic Ocean water. Science, 180: 643-644.
HARVEY, G.R., STEINHAVER, W.G., & MIKLAS, H.P. (1974) Decline of
PCB concentrations in North Atlantic surface water. Nature (Lond.),
252: 387-388.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of waterfowl nesting on
islands in Lake Michigan off Door County, Wisconsin, 1977-78.
Pestic. monit. J., 15(2): 90-97.
HASHEMY-TONKABONY, S.E. & SOLEIMANI-AMIRI, M.J. (1978) Chlorinated
pesticide residues in the body fat of people in Iran. Environ.
Res., 16: 419-422.
HATHWAY, D.E., MOSS, J.A., ROSE, J.A., & WILLIAMS, D.J.M. (1967)
Transport of dieldrin from mother to blastocyst and from mother to
foetus in pregnant rabbits. Eur. J. Pharmacol., 1: 167-175.
HAVERA, S.P. & DUZAN, R.E. (1986) Organochlorine and PCB residues
in tissues of raptors from Illinois, 1966-81. Bull. environ.
Contam. Toxicol., 36: 23-32.
HAYES, W.J. (1957) Dieldrin poisoning in man, Washington, DC, US
Department of Health, Eduction and Welfare, Public Health Service,
Vol. 72, pp. 1087-1091 (Public Health Report No. 12).
HAYES, W.J. (1959) The toxicity of dieldrin to man. Report on a
survey. Bull. World Health Organ., 20: 891-912.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons.
Emergency information for treating poisoning, Washington, DC, US
Government Printing Office (Public Health Service Publication No.
476).
HAYES, W.J. (1974) Distribution of dieldrin following a single oral
dose. Toxicol. appl. Pharmacol., 28: 485-492.
HAYES, W.J. (1982) Chlorinated hydrocarbon insecticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and
Wilkins, pp. 172-283.
HAYES, W.J. & CURLEY, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environ. Health, 16(2): 155-162.
HAYES, W.J., Jr, DALE, W.E., & BURSE, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HEATH, D.F. & VANDEKAR, M. (1964) Toxicity and metabolism of
dieldrin in rats. Br. J. ind. Med., 21: 269-279.
HEESCHEN, W. (1972) Analyses for residues in milk and milk
products. In: Coulston, F. & Korte, F., ed. Environmental quality
and safety, Stuttgart, G. Thieme Verlag, Vol. 1, p. 229.
HEINZ, G.H. & JOHNSON, R.W. (1981) Diagnostic brain residues of
dieldrin: some new insights. In: Lamb, D.W. & Kenaga, E.E., ed.
Avian and mammalian wildlife toxicology. Second Conference,
Philadelphia, Pennsylvania, American Society of Testing Materials,
pp. 72-92 (STP757).
HELENE, C.G., LORD, K.A., & RUEGG, E.F. (1981) The persistence,
leaching, and volatilization of 14C aldrin in two Brazilian soils
Cienc. Cult., 33: 101-105.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four
species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969) Organochlorine
insecticide residues in fish. Pestic. monit. J., 3(3): 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971) Organochlorine
insecticide residues in fish - fall 1969. Pestic. monit. J., 5(1):
1-11.
HENDERSON, G.L. & CROSBY, D.G. (1968) The photodecomposition of
dieldrin residues in water. Bull. environ. Contam. Toxicol., 3:
131-134.
HERRERA MARTEACHE, A., POLO VILLAR, L.M., JODRAL VILLAREJO,
M., POLO VILLAR, G., MALLOL, J., & POZO LORA, R. (1978)
[Organochlorine pesticide residues in human fat in Spain.] Rev.
Sanid. Hig. publica., 52: 1125-1144 (in Spanish).
HERZEL, F. (1971) [The behaviour of some persistent insecticides in
the soil.] Bundesgesundheitsblatt, 14(3): 23-28 (in German).
HERZEL, F. (1972) Organochlorine insecticides in surface waters in
Germany: 1970 and 1971. Pestic. monit. J., 6(3): 179-187.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg., 24:
459-463.
HICKEY, J.J., ed. (1969) Peregrine falcon populations: their
biology and decline, Madison, Wisconsin, University of Wisconsin
Press.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish-eating birds. Science, 162:
271-273.
HILL, D.W. & MCCARTY, P.L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control Fed.,
39: 1259-1277.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 191).
HILL, E.F., SPANN, J.W., & WILLIAMS, J.D. (1977) Responsiveness of
6 to 14 generations of birds to dietary dieldrin toxicity. Toxicol.
appl. Pharmacol., 42: 425-431.
HINDIN, E., MAY, D.S., & DUNSTAN, G.S. (1964) Collection and
analysis of synthetic organic pesticides from surface and ground
water. Residue Rev., 7: 130-156.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10: 613-675.
HOERSCHELMANN, H., POLZHOFER, K., FIGGE, K., & BALLSCHMITER,
K. (1979) [Organochlorine pesticides and polychlorinated biphenyls
in birds eggs from the Falkland Islands and from northern Germany.]
Environ. Pollut., 13: 247-269 (in German).
HOFFMAN, W.S., ADLER, H., FISHBEIN, W.I., & BAUER, F.C. (1967)
Relation of pesticide concentrations in fat to pathological changes
in tissues. Arch. environ. Health, 15: 758-765.
HOGAN, R.L. & ROELOFS, E.W. (1971) Concentrations of dieldrin in
the blood and brain of the green sunfish Lepomis cyanellus at
death. J. Fish Res. Board Can., 28(4): 610-612.
HOLDEN, A.V. (1975) The accumulation of oceanic contaminants in
marine mammals, Rapp. P.-V. Réun. Cons. int. Explor. Mer, 169:
353-361.
HOLDRINET, M.V.H., BRAUN, H.E., FRANK, R., STOPPS, G.E., SMOUT,
M.S., & MCWADE, J.W. (1977) Organochlorine residues in human
adipose tissue and milk from Ontario residents, 1969-1974. Can. J.
public Health, 68: 74-80.
HOLT, R.L., CRUSE, S., & GREEN, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
North East Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOOFTMAN, R.N. & VINK, G.J. (1980) The determination of toxic
effects of pollutants with the marine polychaete worm Ophryotrocha
diadema. Ecotoxicol. environ. Saf., 4: 252-262.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1962) Electro-
encephalograms in insecticide toxicity. Arch. environ. Health, 4:
86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1965) Nine
years' toxicity control in insecticide plants. Arch. environ.
Health, 10: 441-448.
HORNABROOK, R.W., DYMENT, P.G., GOMES, E.D., & WISEMAN, J.S. (1972)
DDT residues in human milk from New Guinea natives. Med. J. Aust.,
1: 1297-1300.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, 2nd ed., US Department of the
Interior, Fish and Wildlife Service, pp. 9-10 (Publication No.
153).
HUNT, P.F., STEVENSON, D.E., THORPE, E., & WALKER, A.I.T. (1975)
Mouse data. Letter to the editor. Food Cosmet. Toxicol., 13:
597-599.
HUNTER, C.G. & ROBINSON, J. (1967) Pharmacodynamics of dieldrin
(HEOD). I. Ingestion by human subjects for 18 months. Arch.
environ. Health, 15(5): 614-626.
HUNTER, C.G. & ROBINSON, J. (1968) Aldrin, dieldrin, and man. Food
Cosmet. Toxicol., 6: 253-260.
HUNTER, C.G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England. Br. med.
J., 1: 221-224.
HUNTER, C.G., ROBINSON, J., & JAGER, K.W. (1967) Aldrin and
dieldrin: the safety of present exposures of the general
populations of the United Kingdom and the United States. Food
Cosmet. Toxicol., 5: 781-787.
HUNTER, C.G., ROBINSON, J., & ROBERTS, M. (1969) Pharmaco-dynamics
of dieldrin (HEOD). II. Ingestion by human subjects for 18 to 24
months, and post-exposure for eight months. Arch. environ. Health,
18(1): 12-21.
HUTSON, D.H. (1976) Comparative metabolism of dieldrin in the rat
(CFE) and in two strains of mouse (CF1 and LACG). Food Cosmet.
Toxicol., 14: 577-591.
IARC (1974) Some organochlorine pesticides, Lyons, International
Agency for Research on Cancer, 241 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol.
5).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, Vol. 1-29, 292 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Man, Suppl. 4).
IARC (1987) Overall evaluations of carcinogenicity. An update of
IARC Monographs, Lyons, International Agency for Research on
Cancer, pp. 1-42 (Monographs on the Evaluation of Carcinogenic
Risks to Humans, Suppl. 7).
IATROPOULOS, M.J., MILLING, A., MUELLER, W.F., NOHYNEK, G., ROZMAN,
K., COULSTON, F., & KORTE, F. (1975) Absorption, transport, and
organotropism of dichlorobiphenyl (DCB), dieldrin, and
hexachlorobenzene (HCB) in rats. Environ. Res., 10: 384-389.
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, Utrecht, Rijks Universiteit (Thesis).
ICHINOSE, R. & KURIHARA, N. (1985) Uptake of dieldrin, lindane, and
DDT by isolated hepatocytes. Pestic. Biochem. Physiol., 23: 116-122.
IGUE, K., FARMER, W.J., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. II. Effect of
relative humidity and soil water content on dieldrin volatility.
Soil Sci. Soc. Am. Proc., 36: 447-450.
ITO, N., TSUDA, H., HASEGAWA, R., & IMAIDA, K. (1983) Comparison of
the promoting effects of various agents in induction of
preneoplastic lesions in rat liver. Environ. Health Perspect., 50:
131-138.
IVEY, M.C., CLABORN, H.V., MANN, H.D., RADELEFF, R.D., & WOODARD,
G.T. (1961) Aldrin and dieldrin content of body tissues of
livestock receiving aldrin in their diet. J. agric. food Chem.,
9(5): 374-376.
IVIE, G.W. & CASIDA, J.E. (1970) Enhancement of photo-alteration of
cyclodiene insecticide chemical residues by rotenone. Science, 167:
1620-1622.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Publishing Company,
234 pp.
JANSEN, J.D. (1979) The predictive value of tests for carcinogenic
or mutagenic activity. In: Deichmann, W.B., ed. Toxicology and
occupational medicine, Amsterdam, Elsevier/North Holland, pp.
71-80.
JEFFERIES, D.J. (1969) Causes of badger mortality in eastern
counties of England. J. Zool. (Lond.), 157: 429-436.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in
British bats and their significance. J. Zool. (Lond.), 166:
245-263.
JEFFERIES, D.J. (1975) Different activity patterns of male and
female badgers (Meles meles) as shown by road mortality. J. Zool.
(Lond.), 177: 504-506.
JEFFERIES, D.J. & DAVIS, B.N.K. (1968) Dynamics of dieldrin in
soil, earthworms, and song thrushes. J. wildl. Manage., 32(3):
441-456.
JEFFERIES, D.J. & PENDLEBURY, J.B. (1968) Population fluctuations
of stoats, weasels and hedgehogs in recent years. J. Zool. (Lond.),
156: 513-517.
JEFFERIES, D.J., STAINSBY, B., & FRENCH, M.C. (1973) The ecology of
small mammals in arable fields drilled with winter wheat and the
increase in their dieldrin and mercury residues. J. Zool. (Lond.),
171: 513-539.
JEFFERIES, D.J., FRENCH, M.C., & STEBBING, R.E. (1974) Pollution
and mammals: otters, Huntingdon, Natural Environment Research
Council, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, pp. 13-15 (Report for 1972-73).
JENSEN, L.D. & GAUFIN, A.R. (1966) Acute and long-term effects of
organic insecticides on two species of stonefly naiads. J. Water
Pollut. Control Fed., 38(8): 1273-1286.
JOHNSON, L.G. & MORRIS, R.L. (1971) Chlorinated hydrocarbon
pesticides in Iowa rivers. Pestic. monit. J., 4(4): 216-219.
JOHNSON, R.D. & MANSKE, D.D. (1977) Pesticide and other chemical
residues in total diet samples. XI. Pestic. monit. J., 11(3): 116-131.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 1-3, 8,
10, 29-30 (Resource Publication No. 137).
JOHNSTON, B.T., SAUNDERS C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin
by fresh-water invertebrates. J. Fish Res. Board Can., 28: 705-709.
JOHNSTON, D.W. (1975) Organochlorine pesticide residues in small
migratory birds, 1964-1973. Pestic. monit. J., 9(2): 79-88.
JOLLY, D.W. (1954) Studies on the acute toxicity of dieldrin to
sheep. Vet. Rec., 66: 444-447.
JONAS, R.B. & PFAENDER, F.K. (1976) Chlorinated hydrocarbon
pesticides in Western North Atlantic Ocean. Environ. Sci. Technol.,
10(8): 770-773.
JONES, D.M., BENNETT, D., & ELGAR, K.E. (1978) Deaths of owls
traced to insecticide-treated timber. Nature (Lond.), 272: 52.
JOY, R.M. (1976) The alteration by dieldrin of cortical
excitability conditioned by sensory stimuli. Toxicol. appl.
Pharmacol., 38(2): 357-368.
JOY, R.M. (1977) Contrasting actions of dieldrin and aldrin-
transdiol, its metabolite, on cat CNS functions. Toxicol. appl.
Pharmacol., 42: 137-148.
JOY, R.M. (1982) Mode of action of lindane, dieldrin, and related
insecticides in the central nervous system. Neurobehav. Toxicol.
Teratol., 4: 813-823.
JUNK, G.A., SPALDING, R.F., & RICHARD, J.J. (1980) Aerial,
vertical, and temporal differences in groundwater chemistry. II.
Organic constituents. J. environ. Qual., 9: 479-483.
JURY, W.A., SPENCER, W.F., & FARMER, W.J. (1983) Use of models for
assessing relative volatility, mobility, and persistence of
pesticides and other trace organics in soil systems. In: Saxena,
J., ed. Hazard assessment of chemicals, current development, Vol.
2, New York, London, Academic Press, pp 1-43.
KADIS, V.W., BREITKREITZ, W.E., & JONASSON, O.J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. public
Health, 61(5): 413-416.
KAISER, T.E., REICHEL, W.L., LOCKE, L.N., CROMARTIE, E., KRYNITSKY,
A.J., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., STAFFORD, C.J., &
SWINEFORD, D.M. (1980) Organochlorine pesticide, PCB, and PBB
residues and necropsy data for bald eagles from 29 states - 1975-
77. Pestic. monit. J., 13(4): 145-149.
KANITZ, S. & CASTELLO, G. (1966) [Presence of residues of some
disinfectants in human fatty tissue and in certain foods.] G. Ig.
Med. prev., 7: 1-19 (in Italian).
KANJA, L., SKARE, J.K., NAFSTAD, I., MAITAI, C.K., & LOKKEN, P.
(1986) Organochlorine pesticides in human milk from different areas
of Kenya, 1983-1985. J. Toxicol. environ. Health, 19: 449-464.
KATZ, M. (1961) Acute toxicity of some organic insecticides to
three species of salmonids and to the three-spine stickleback.
Trans. Am. Fish. Soc., 90(2): 264-268.
KAWATSKI, J.A. & SCHMULBACH, J.C. (1972) Uptake and elimination of
14C-aldrin and 14C-dieldrin by the ostracod Chlamydotheca arcuata
(Sars). Int. J. environ. anal. Chem., 1: 283-291.
KAZANTZIS, G., MCLAUGHLIN, A.I.G., & PRIOR, P.F. (1964) Poisoning
in industrial workers by the insecticide aldrin. Br. J. ind. Med.,
21: 46-51.
KEANE, W.T. & ZAVON, M.R. (1969a) Validity of a critical blood
level for prevention of dieldrin intoxication. Arch. environ.
Health, 19: 36-44.
KEANE, W.T. & ZAVON, M.R. (1969b) The total body burden of
dieldrin. Bull. environ. Contam. Toxicol., 4(1): 1-16.
KELLER, J. & ALFARO, J.F. (1966) Effect of water application rate
on leaching. Soil Sci., 102(2): 107-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1970) Effects of
combinations of pesticides on reproduction in mice. In: Pesticides
symposia, Miami Beach, Florida, Halos and Associates Inc., pp.
125-138.
KHAIRY, M. (1960) Effects of chronic dieldrin ingestion on the
muscular efficiency of rats. Br. J. ind. Med., 17: 146-148.
KHAN, M.A.Q., COELLO, W., KHAN, A.A., & PINTO, H. (1972a) Some
characteristics of the microsomal mixed-function oxidase in the
fresh-water crayfish Cambarus. Life Sci., 11(2): 405-415.
KHAN, M.A.Q., KAMAL, A., WOLIN, R.J., & RUNNELS, J. (1972b) In vivo
and in vitro epoxidation of aldrin by aquatic food chain organisms.
Bull. environ. Contam. Toxicol., 8(4): 219-228.
KING, P.H. & MCCARTY, P.L. (1968) A chromatographic model for
predicting pesticide migration in soils. Soil Sci., 106(4): 248-261.
KINOSHITA, F.K. & KEMPF, C.K. (1970) Quantitative measurement of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Toxicol. appl. Pharmacol.,
17(1): 288.
KITSELMAN, C.H. (1953) Long-term studies on dogs fed aldrin and
dieldrin in sublethal dosages, with reference to the
histopathological findings and reproduction. J. Am. Vet. Med.
Assoc., 123: 28-30.
KLAASSEN, H.E. & KADOUM, A.M. (1973) Pesticide residues in natural
fish populations of the Smoky Hill River of Western Kansas - 1967-
1969, Pestic. monit. J., 7(1): 53-61.
KLAUNIG, J.E., GOLDBLATT, P.J., HINTON, D.E., LIPSKY, M.M., &
TRUMP, B. (1984) Carcinogen-induced unscheduled DNA synthesis in
mouse hepatocytes. Toxicol. Pathol., 12(2): 119-125.
KLEIN, A.K., LINK, J.D., & IVES, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed
aldrin and dieldrin. J. Assoc. Off. Agric. Chem., 51: 895-898.
KLEIN, A.K., DAILEY, R.E., WALTON, M.S., BECK, V., & LINK, J.D.
(1970) Metabolites isolated from urine of rats fed 14C-
photodieldrin. J. agric. food Chem., 18(4): 705-708.
KLEIN, M.L. & LINCER, J.L. (1974) Behavioural effects of dieldrin
upon the fiddler crab Uca pugilator. In: Vernberg, F.J. &
Vernberg, W.B., ed. Pollution and physiology of marine organisms,
New York, London, Academic Press, pp. 181-196.
KLEIN, W., KOHLI, J., WEISGERBER, I., & KORTE, F. (1973) Fate of
aldrin-14C in potatoes and soil under outdoor conditions. J. agric.
food Chem., 21(2): 152-156.
KLEMMER, H.W., RASHAD, M.N., & MI, M.P. (1973) Age, sex, and race
effects on the distribution of organochlorine pesticide residues in
serum. In: Deichmann, W.B., ed. Pesticides and the environment,
New York, International Medical Book Corporation, pp. 53-61.
KLEVAY, L.M. (1970) Dieldrin excretion by the isolated perfused rat
liver: a sexual difference. Toxicol. appl. Pharmacol., 17: 813-815.
KOEMAN, J.H. (1971) [The occurrence and the toxicological
implications of some chlorinated hydrocarbons in the Dutch coastal
area in the period 1965-70], Utrecht, Rijks Universiteit (Thesis)
(in Dutch).
KOEMAN, J.H. & PENNINGS, J.H. (1970) An orientational survey on the
side effects and environmental distribution of insecticides used in
tsetse control in Africa. Bull. environ. Contam. Toxicol., 5(2):
164-170.
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROCK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis). A preliminary communication. Meded. Fac. Landouwwet.
Rijksuniv. Gent, 32: 841-854.
KOEMAN, J.H., VINK, J.A.J., & DE GOEIJ, J.J.M. (1969) Causes of
mortality in birds of prey and owls in the Netherlands in the
winter of 1968-69. Ardea, 57: 67-76.
KOEMAN, J.H., PEETERS, W.H.M., & PENNINGS, J.H. (1971) OECD
collaborative study 1969/71: pesticide residues in the environment,
Utrecht, The Netherlands, Institute of Veterinary Pharmacology and
Toxicology (Unpublished report).
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972)
Eggshell and population changes in the sparrow hawk (Accipiter
nisus). TNO Nieuws, 27: 542-550.
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1972) [Contributions to
ecological chemistry ILI(1). Transformation and residue behaviour
of dieldrin 14C in onions after seed treatment.] Chem. Mikrobiol.
Technol. Lebensm., 1: 149-150 (in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973a) [Contributions to
ecological chemistry LVIII. Transport of aldrin-14C and
transformation products in the soil.] Chemosphere, 3: 125-130
(in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973) [Contributions to
ecological chemistry LIX. Residue behaviour and transformation of
14C-dieldrin in crops, soil and seepage water after application to
the soil.] Chemosphere, 4: 153-156 (in German).
KORSCHGEN, L.J. (1971) Disappearance and persistence of aldrin
after five annual applications. J. wildl. Manage., 35(3): 494-500.
KORTE, F. (1965) Metabolism of chlorinated insecticides. In:
Radioisotopes in the detection of pesticide residues. Proceedings
of the International Atomic Energy Panel, Vienna, International
Atomic Energy Agency, p. 38.
KORTE, F. & ARENT, H. (1965) Metabolism of insecticides. IX(1).
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin-14C. Life Sci., 4:
2017-2026.
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated
compounds in human organs collected in Denmark, 1972-73. Acta
pharmacol. toxicol., 38: 38-48.
KRAYBILL, H.F. (1977) Global distribution of carcinogenic
pollutants in water. In: Proceedings of the Conference on Aquatic
Pollutants and Biological Effects with Emphasis on Neoplasia, 27-29
September, New York, New York Academy of Sciences.
KRIEGER, R.I. & WILKINSON, C.F. (1969) Microsomal mixed-function
oxidases in insects. I. Localization and properties of an enzyme
system affecting aldrin epoxidation in larvae of the southern
armyworm (Prodenia eridania). Biochem. Pharmacol., 18: 1403-1415.
KURATA, M., HIROSE, K., & UMEDA, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
KURIHARA, N., HORI, N., & ICHINOSE, R. (1984) Cytochrome P-450
content and aldrin epoxidation to dieldrin in isolated rat
hepatocytes. Pestic. Biochem. Physiol., 21: 63-73.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, N.S. (1978a) Persistence
and dissipation of aldrin and dieldrin in soil: a review.
Pesticides, 12(6): 14-18.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, V.S. (1978) Effect of
temperature on the degradation of aldrin residues in sandy loam
soil. Ann. arid Zone, 17(2): 200-206.
KUTZ, F.W., YOBS, A.R., & YANG, H.S.C. (1976) National pesticide
monitoring programs. In: Lee, R.E., ed. Air pollution from
pesticides and agricultural processes, Cleveland, Ohio, CRC Press,
pp. 95-136.
KUTZ, F.W., STRASSMAN, S., & YOBS, A.R. (1979) Survey of pesticide
residues and their metabolites in the general population of the
United States. In: Berlin, A., Wolff, A.H., & Hasegawa, Y., ed. Use
of biological specimens to assess human exposure to environmental
pollutants, The Hague, Martinus Nijhoff, pp. 267-274.
LANE, C.E. & LIVINGSTON, R.J. (1970) Some acute and chronic effects
of dieldrin on the sailfin molly Poecilia latipinna. Trans. Am.
Fish. Soc., 99(3): 489-495.
LANE, C.E., SEBA, D.B., & HEARN, W.L. (1970) Possible metabolites
of dieldrin in the sailfin molly (Poecilia latipinna). Proc. Soc.
Exp. Biol. Med., 133: 1375-1377.
LAUBSCHER, J.A., DUTT, G.R., & ROAN, C.C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of distance
from application. Pestic. monit. J., 5(3): 251-258.
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodiene with brain-specific t-butyl-
bicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAY, J.P., WEISGERBER, I., & KLEIN, W. (1975) Conversion of the
aldrin/dieldrin metabolite dihydrochlordene dicarboxylic acid-14C
in rats. Pestic. Biochem. Physiol., 5: 226-232.
LEHMAN, A.J. (1951) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Q. Bull. Assoc. Food Drug Off., 15: 122-133.
LEHMAN, A.J. (1952) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Section II. Dermal toxicity. Q. Bull. Assoc. Food Drug
Off. (USA), 16: 3-9.
LEHNER, P.N. & EGBERT, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature (Lond.), 224: 1218-1219.
LENARDON, A.M., DE HEVIA, M.I.M., FUSE, J.A., DE NOCHETTO, C.B., &
DEPETRIS, P.J. (1984) Organochlorine and organophosphorus
pesticides in the Parana river (Argentina). Sci. total Environ., 34:
289-297.
LENON, H., CURRY, L., MILLER, A., & PATULSKI, D. (1972) Insecticide
residues in water and sediment from cisterns on the US and British
Virgin Islands. Pestic. monit. J., 6: 188-193.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides.
In: Lee, R.E., Jr, ed. Air pollution from pesticides and
agricultural processes, Cleveland, Ohio, CRC Press, pp. 51-94.
LEWIS, R.G. & LEE, R.E., Jr (1976) Air pollution from pesticides:
sources, occurrence, and dispersion. In: Lee, R.E., Jr, ed. Air
pollution from pesticides and agricultural processes, Cleveland,
Ohio, CRC Press, pp. 5-50.
LICHTENBERG, J.J., EICHELBERGER, J.W., DRESSMAN, R.C., &
LONGBOTTOM, J.E. (1970) Pesticides in surface waters of the United
States: a 5-year summary, 1964-68. Pestic. monit. J., 4(2): 71-86.
LICHTENSTEIN, E.P. (1959) Absorption of some chlorinated
hydrocarbon insecticides from soils into various crops. J. agric.
food Chem., 7: 430-433.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1959) Breakdown of lindane and
aldrin in soils. J. econ. Entomol., 52(1): 118-124.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture and soil
types. J. econ. Entomol., 53(2): 192-197.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1970) Volatilization of
insecticides from various substrates. J. agric. food Chem., 18(5):
814-818.
LICHTENSTEIN, E.P., MUELLER, C.H., MYRDAL, G.R., & SCHULZ, K.R.
(1962) Vertical distribution and persistence of insecticidal
residues in soils as influenced by mode of application and cover
crop. J. econ. Entomol., 55(2): 215-219.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. agric. food Chem.,
18(1): 100-106.
LICHTENSTEIN, E.P., FUHREMANN, T.W., & SCHULZ, K.R. (1971)
Persistence and vertical distribution of DDT, lindane, and aldrin
residues, 10 and 15 years after a single soil application. J.
agric. food Chem., 19(4): 718-721.
LINDER, R.L. & DAHLGREN, R.B. (1970) Occurrence of organochlorine
insecticides in pheasants of South Dakota. Pestic. monit. J., 3(4):
227-232.
LINDER, R.L., DAHLGREN, R.B., & GREICHUS, Y.A. (1970) Residues in
the brain of adult pheasants given dieldrin. J. wildl. Manage., 34:
954-956.
LINDSTROM, F.T, BOERSMA, L., & STOCKARD, D. (1971) A theory on the
mass transport of previously distributed chemicals in a water
saturated sorbing porous medium: isothermal cases. Soil Sci., 112(5):
291-300.
LISS, P.S. & SLATER, P.G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LLOYD, C., BOGAN, J.A., BOURNE, W.R.P., DAWSON, P., & PARSLOW,
J.L.F. (1974) Seabird mortality in the North Irish Sea and Firth of
Clyde early in 1974. Mar. Pollut. Bull., 5: 136-140.
LOCKIE, J.D. & RATCLIFFE, D.A. (1964) Insecticides and Scottish
golden eagles. Br. Birds, 57(3): 89-102.
LOCKIE, J.D., RATCLIFFE, D.A., & BALHARRY, R. (1969) Breeding
success and organochlorine residues in golden eagles in west
Scotland. J. appl. Ecol., 6: 381-389.
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMAN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 298-304.
LOOSE, L.D. (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice, Envir. Health
Perspect., 43: 89-97.
LOOSE, L.D., SILKWORTH, J.B., CHARBONNEAU, T., & BLUMENSTOCK,
F. (1981) Environmental chemical-induced macrophage dysfunction.
Environ. Health Perspect., 39: 79-91.
LOTZ, F., KEMPNEY, J., & DE KEMPNEY, R.S.G. (1983) [Light-induced
breakdown of absorbed chemicals: a simple device for comparative
tests.] Chemosphere, 12: 873-878 (in German).
LOWDEN, G.F., SAUNDERS, C.L., & EDWARDS, R.W. (1969) Organochlorine
insecticides in water. Part II. Water Treat. Exam., 18: 275-287.
LU, F.C., JESSUP, D.C., & LAVALLEE, A. (1965) Toxicity of
pesticides in young versus adult rats. Food Cosmet. Toxicol., 3:
591-596.
LUCKENS, M.M. & DAVIS, W.H. (1965) Toxicity of dieldrin and endrin
to bats. Nature (Lond.), 207(4999): 879-880.
LUDKE, J.R., GIBSON, J.R., & LUSK, C.I. (1972) Mixed-function
oxidase activity in fresh-water fish: aldrin epoxidation and
parathion activation. Toxicol. appl. Pharmacol., 21: 89-97.
LUDWIG, G. & KORTE, F. (1965) Metabolism of insecticides. X.
Detection of dieldrin metabolites by GLC analysis. Life Sci., 4:
2027-2031.
LUDWIG, G., WEIS, J., & KORTE, F. (1964) Excretion and distribution
of aldrin-14C and its metabolites after oral administration for a
long period of time. Life Sci., 3: 123-130.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72(12): 5135-5139.
MACCUAIG, R.D. (1975) Occurrence and movements of pesticide
residues in Ethiopia. Environ. Qual. Saf., 3(suppl.): 850-851.
MACCUAIG, R.D. (1976) The occurrence of insecticides in the blood
of staff of a locust control organization. Bull. environ. Contam.
Toxicol., 15: 162-170.
MACDONALD, R. (1982) Toxicology of sprays: the 4-h acute inhalation
toxicity of aqueous sprays prepared from aldrin 48% (w/v) EC,
Sittingbourne, Shell Research (SBGR.82.036) (Unpublished
proprietary report).
MACEK, K.J. (1975) Acute toxicity of pesticide mixtures to
bluegills. Bull. environ. Contam. Toxicol., 14(6): 648-652.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects of
temperature on the susceptibility of bluegills and rainbow trout to
selected pesticides. Bull. environ. Contam. Toxicol., 4(3): 174-183.
MCEWEN, L.C. & BROWN, R.L. (1966) Acute toxicity of dieldrin and
malathion to wild sharp-tailed grouse. J. wildl. Manage., 30(3):
604-611.
MACKAY, D. & LEINONEN, P.J. (1975) Rate of evaporation of low-
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 9(13): 1178-1180.
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low-
solubility contaminants to atmosphere. Environ. Sci. Technol., 7(7):
611-614.
MACKAY, D., SHIU, W.Y., & SUTHERLAND, R.P. (1979) Determination of
air-water Henry's Law constants for hydrophobic pollutants.
Environ. Sci. Technol., 13(3): 333-337.
MCKINNEY, J.D., MATTHEWS, H.B., & WILSON, N.K. (1973) Determination
of optical purity and prochirality of chlorinated polycyclodiene
pesticide metabolites. Tetrahedron Lett., 21: 1895-1898.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol., 25: 921-928.
MCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982) Toxicities
of five organochlorine compounds in water and sediment to Nereis
virens. Bull. environ. Contam. Toxicol., 28: 216-220.
MAJUMDAR, S.K., KOPELMAN, H.A., & SCHNITMAN, M.J. (1976) Dieldrin-
induced chromosome damage in mouse bone-marrow and WI-38 human lung
cells. J. Hered., 67(5): 303-307.
MAJUMDAR, S.K., MAHARAM, L.G., & VIGLIANTI, G.A. (1977)
Mutagenicity of dieldrin in the Salmonella/microsome test. J.
Hered., 68: 184-185.
MANIGOLD, D.B. & SCHULZE, J.A. (1969) Pesticides in selected
western streams: a progress report. Pestic. monit. J., 3(2): 124-135.
MARCHANT, J.H. (1980) Recent trends in sparrowhawk numbers in
Britain. Bird Stud., 27: 152-154.
MARLOW, R.G. & WALLACE, B.G. (1983) Assessment of exposure
following the use of aldrin as a termiticide in homes. Part II. Air
concentrations and kitchen wipes after one year, Sittingbourne,
Shell Research (SBGR.83.285).
MARLOW, R.G., WALLACE, B.G., & MOORE, J.P. (1982) Assessment of
exposure following the use of aldrin as a termiticide in homes,
Sittingbourne, Shell Research (SBGR.82.370).
MARSHALL, T.C., DOROUGH, H.W., & SWIM, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. agric. food Chem., 24(3): 560-563.
MASON, J.W. & ROWE, D.R. (1976) The accumulation and loss of
dieldrin and endrin in the eastern oyster. Arch. environ. Contam.
Toxicol., 4: 349-360.
MASTROMATTEO, E. (1971) Pesticides and man's health: the picture in
Ontario. In: Proceedings of the Working Conference on
Epidemiological Toxicology of Pesticides, Amsterdam, 8-10 September
1971 (Unpublished paper).
MATSUMURA, F. & BOUSH, G.M. (1967) Dieldrin degradation by soil
microorganisms. Science, 156: 959-961.
MATTHEWS, H.B. & MATSUMURA, F. (1969) Metabolic fate of dieldrin in
the rat. J. agric. food Chem., 17: 845-852.
MATTHEWS, H.B., MCKINNEY, J.D., & LUCIER, G.W. (1971) Dieldrin
metabolism, excretion, and storage in male and female rats. J.
agric. food Chem., 19(6): 1244-1248.
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydrocarbon
insecticides, southern Florida, 1968-72. Pestic. monit. J., 9(2):
106-114.
MAYER, R., LETEY, J., & FARMER, W.J. (1974) Models for predicting
volatilization of soil-incorporated pesticides. Soil Sci. Soc. Am.
Proc., 38: 563-568.
MEHENDALE, H.M. & EL-BASSIOUNI, E.A. (1975) Uptake and disposition
of aldrin and dieldrin by isolated perfused rabbit lung. Drug
Metab. Disp., 3: 543.
MEHENDALE, H.M., SKRENTNY, R.F., & DOROUGH, H.W. (1972) Oxidative
metabolism of aldrin by subcellular root fractions of several plant
species. J. agric. food Chem., 20(2): 398-402.
MEHRLE, P.M. & BLOOMFIELD, R.A. (1974) Ammonia detoxifying
mechanisms of rainbow trout altered by dietary dieldrin. Toxicol.
appl. Pharmacol., 27: 355-365.
MEIERHENRY, E.F., RUEBNER, B.H., GERSHWIN, M.E., HSIEH, L.S., &
FRENCH, S.W. (1983) Dieldrin-induced mallory bodies in hepatic
tumours of mice of different strains. Hepatology, 3(1): 90-95.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 metres.
Environ. Lett., 5(4): 215-221.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
60-61.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls (Tyto alba) fed low levels of DDE and
dieldrin. Arch. environ. Contam. Toxicol., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine
phytoplankton vary in their response to chlorinated hydrocarbons.
Science, 167: 1724-1726.
MES, J., CAMPBELL, D.S., ROBINSON, R.N., & DAVIES, D.J.A. (1977)
Polychlorinated biphenyl and organochlorine pesticide residues in
adipose tissue of Canadians. Bull. environ. Contam. Toxicol., 17(2):
196-203.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 8: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Bull. environ.
Contam. Toxicol., 13: 217-233.
MES, J., DAVIES, D.J., & TURTON, D. (1985) Environmental
contaminants in human fat: a comparison between accidental and non-
accidental causes of death. Ecotoxicol. environ. Saf., 10: 70-74.
METCALFE, R.L., KAPOOR, I.P., LU, P-Y., SCHUTH, C.K., & SHERMAN, P.
(1973) Model ecosystem studies of the environment and fate of six
organochlorine pesticides. Environ. Health Perspect., 4: 35-44.
MICK, D.L., LONG, K.R., & BONDERMAN, D.P. (1972) Aldrin and
dieldrin in the blood of pesticide formulators. Am. Ind. Hyg.
Assoc. J., 33(2): 94-99.
MILES, J.R.W. & HARRIS, C.R. (1971) Insecticide residues in a
stream and a controlled drainage system in agricultural areas of
southwestern Ontario, 1970. Pestic. monit. J., 5(3): 289-294.
MILES, J.R.W. & HARRIS, C.R. (1973) Organochlorine insecticide
residues in streams draining agricultural, urban-agricultural, and
resort areas of Ontario, Canada - 1971. Pestic. monit. J., 6(4):
363-368.
MILLER, G.J. & FOX, J.A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, G.C. & ZEPP, R.G. (1983) Extrapolating photolysis rates
from the laboratory to the environment. Residue Rev., 85: 89-110.
MINISTRY OF WELFARE, HEALTH AND CULTURE (1983) Surveillance
programme: man and nutrition, The Hague, Netherlands, Ministry of
Welfare, Health and Culture, State Supervisory Public Health
Service.
MOERSDORF, K., LUDWIG, G., VOGEL, J., & KORTE, F. (1963) [Excretion
of aldrin-14C and dieldrin-14C and their metabolites through the
bile.] Med. Exp., 8: 90 (in German).
MOFFET, G.B. & YARBROUGH, J.D. (1972) The effects of DDT,
toxaphene, and dieldrin on succinic dehydrogenase activity in
insecticide-resistant and susceptible Gambusia affinis. J. agric.
food Chem., 20(3): 558-560.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health, 19:
633-636.
MORGAN, D.P. & ROAN, C.C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D.P. & ROAN, C.C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15(1): 26-28.
MORGAN, D.P. & ROAN, C.C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORITA, H. & UMEDA, M. (1984) Detection of mutagenicity of various
compounds by FM 3A cell system. Paper presented at the 12th Annual
Meeting of the Mutagenicity Society, Japan. Mutat. Res., 130(5): 371
(No. 33).
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MOSSING, M.L., REDETZKE, K.A., & APPLEGATE, H.G. (1985)
Organochlorine pesticides in blood of persons from El Paso, Texas.
J. environ. Health, 47(6): 312-313.
MOZA, P., WEISGERBER, I., & KLEIN, W. (1972) [Leaching a water-
soluble aldrin-14C breakdown product out of soil.] Chemosphere, 5:
191-195 (in German).
MUELLER, W. & LEACH, R.M., Jr (1974) Effects of chemicals on
eggshell formation. Annu. Rev. Pharmacol., 14: 289-303.
MUELLER, W., NOHYNEK, G., WOODS, G., KORTE, F., & COULSTON, F.
(1975a) Comparative metabolism of dieldrin-14C in mouse, rat,
rabbit, rhesus monkey, and chimpanzee. Chemosphere, 4(2): 89-92.
MUELLER, W., WOODS, G., KORTE, F., & COULSTON, F. (1975b)
Metabolism and organ distribution of dieldrin-14C in rhesus monkeys
after single oral and intravenous administration. Chemosphere, 2:
93-98.
MUELLER, P., NAGEL, P., & FLACKE, W. (1981) Ecological side effects
of dieldrin application against tsetse flies in Adamaoua,
Cameroon. Oecologia, 50(2): 187-194.
MUIR, C.M.C. (1970) The acute oral and percutaneous toxicities to
rats of formulations of aldrin, dieldrin, or endrin,
Sittingbourne, Shell Research (TLGR.0020.70) (Unpublished
proprietary report).
MUIRHEAD, E.E., GROVES, M., GUY, R., HALDEN, E.R., & BASS, R.K.
(1959) Acquired hemolytic anemia, exposure to insecticides and
positive Coombs test dependent on insecticide preparations. Vox
sang., 4: 277-292.
MULHERN, B.M., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., BELISLE,
A., CROMARTIE, E., BAGLEY, G.E., & PROUTY, R.M. (1970)
Organochlorine residues and autopsy data from bald eagles 1966-68.
Pestic. monit. J., 4(3): 141-144.
MULLER, H.D. & LOCKMAN, D.C. (1972) Fecundity and progeny growth
following subacute insecticide ingestion by the mallard. Poult.
Sci., 51: 239-241.
MULLINS, D.E., JOHNSEN, R.E., & STARR, R.I. (1971) Persistence of
organochlorine insecticide residues in agricultural soils of
Colorado. Pestic. monit. J., 5(3): 268-275.
MURPHY, D.A. & KORSCHGEN, L.J. (1970) Reproduction, growth, and
tissue residues of deer fed dieldrin. J. wildl. Manage., 34(4):
887-903.
MURPHY, R. & HARVEY, C. (1985) Residues and metabolites of selected
persistent halogenated hydrocarbons in blood specimens from a
general population survey. Environ. Health Perspect., 60: 115-120.
MURTON, R.K. & VIZOSO, M. (1963) Dressed cereal seed as a hazard to
woodpigeons. Ann. appl. Biol., 52: 503-517.
NASH, R.G. (1983) Comparative volatilization and dissipation rates
of several pesticides from soil. J. agric. food Chem., 31(2): 210-217.
NASH, R.G., BEALL, M.L., Jr, & WOOLSON, E.A. (1970) Plant uptake of
chlorinated insecticides from soils. Agron. J., 62: 369-372.
NATIONAL FOOD ADMINISTRATION (1982) Summary and assessment of data
received from the FAO/WHO Collaborating Centres for Food
Contamination Monitoring, Uppsala, Global Environmental Monitoring
System (GEMS), Joint FAO/WHO Food and Animal Feed Contamination
Monitoring Programme, pp. 26-29.
NCI (1977) Bioassay of photodieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-817).
NCI (1978a) Bioassay of aldrin and dieldrin for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(DHEW Publication No. (NIH) 78-821).
NCI (1978b) Bioassay of dieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-822).
NEILL, D.D., MULLER, H.D., & SHUTZE, J.V. (1971) Pesticide effects
on the fecundity of the gray partridge. Bull. environ. Contam.
Toxicol., 6(6): 546-551.
NELSON, E. (1953) Aldrin poisoning. Rocky Mt. med. J., 50: 483-486.
NEWTON, I. (1973a) Studies of sparrowhawks. Br. Birds, 66: 271-278.
NEWTON, I. (1973b) Success of sparrowhawks in an area of pesticide
usage. Bird Stud., 20: 1-8.
NEWTON, I. (1974) Changes attributed to pesticides in the nesting
success of the sparrowhawks in Britain. J. appl. Ecol., 11: 95-102.
NEWTON, I. (1976) Breeding of sparrowhawks (Accipiter nisus) in
different environments. J. anim. Ecol., 45: 831-849.
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted,
Poyser T. and A.D., Ltd.
NEWTON, I. & BOGAN, J. (1974) Organochlorine residues, eggshell
thinning, and hatching success in British sparrowhawks. Nature
(Lond.), 249: 582-583.
NEWTON, I. & BOGAN, J. (1978) The role of different organochlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol.,
15: 105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds, 77: 47-70.
NEWTON, I., MARQUISS, M., & MOSS, D. (1979) Habitat, female age,
organochlorine compounds, and breeding of European sparrowhawks. J.
appl. Ecol., 16: 777-793.
NISHIMURA, N., NISHIMURA, H., & OSHIMA, H. (1982) Survey on
mutagenicity of pesticides by the Salmonella microsome test. J.
Aichi Med. Univ. Assoc., 10(4): 305-312.
NOHYNEK, G.J., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Metabolism, excretion, and tissue distribution of 14C-photodieldrin
in non-human primates following oral administration and intravenous
injection. Ecotoxicol. environ. Saf., 3: 1-9.
O'CONNOR, R.J. (1982) Habitat occupancy and regulation of clutch
size in the European kestrel Falco tinnunculus. Bird Stud., 29:
17-26.
ODA, J. & MUELLER, W. (1972) Identification of a mammalian
breakdown product of dieldrin. Environ. Qual. Saf., 1: 248-249.
OHLENDORF, H.M., BARTONEK, J.C., DIVOKY, G.J., KLAAS, E.E., &
KRYNITSKY, A.J. (1982) Organochlorine residues in eggs of Alaskan
seabirds, Washington, DC, US Department of the Interior, Fish and
Wildlife Service (Special Scientific Report: Wildlife No. 245).
OLOFFS, P.C., HARDWICK, D.F., SZETO, S.Y., & MOERMAN, D.G. (1974)
DDT, dieldrin, and heptachlorepoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
ONSAGER, J.A., RUSK, H.W., & BUTLER, L.I. (1970) Residues of
aldrin, dieldrin, chlordane, and DDT in soil and sugar beets. J.
econ. Entomol., 63(4): 1143-1146.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic changes
in the liver of rats after feeding low levels of various
insecticides. Am. Med. Assoc. Arch. Pathol., 64(6): 614-622.
OTTOLENGHI, A.D., HASEMAN, J.K., & SUGGS, F. (1974) Teratogenic
effects of aldrin, dieldrin, and endrin in hamsters and mice.
Teratology, 9: 11-16.
PACCAGNELLA, B., GHEZZO, F., PRATI, L., FEDRAZZONI, U., & BELLONI,
G. (1971) Epidemiological study of long-term effects of pesticides
on human health. Bull. World Health Organ., 45: 181-199.
PAINTER, R.B. (1981) DNA synthesis inhibition in mammalian cells as
a test for mutagenic carcinogens. In: Stich, H.F. & San, R.H.C.,
ed. Short-term tests for chemical carcinogens, New York, Springer-
Verlag.
PARK, P.O. & MCKONE, C.E. (1966) The persistence and distribution
of soil-applied insecticides in an irrigated soil in northern
Tanzania. Trop. Agric. Trin., 43(2): 133-142.
PARSLOW, J.L.F. (1973) Breeding birds of Britain and Ireland: a
historical survey, Berkhamsted, Poyser T. and A.D., Ltd.
PARSLOW, J.L.F. & JEFFERIES, D.J. (1973) Relationship between
organochlorine residues in livers and whole bodies of guillemots.
Environ. Pollut., 5: 87-101.
PARSONS, A.M. & MOORE, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem.
Soc., C: 2026-2031.
PATEL, T.B. & RAO, V.N. (1958) Dieldrin poisoning in man. A report
of 20 cases in Bombay State. Br. med. J., 1: 919-921.
PATON, R., LUKE, B.G., & ROBERTS, G. (1984) Studies on the sorption
of organochlorine insecticides by flour stored on or near treated
laminated timber or plywood as used in freight containers. Pestic.
Sci., 15: 624-629.
PEARCE, P.A., PEAKALL, D.B., & REYNOLDS, L.M. (1979) Shell thinning
and residues of organochlorines and mercury in seabird eggs,
eastern Canada, 1970-76. Pestic. monit. J., 13(2): 61-68.
PEARSON, J.E., TINSLEY, K., & HERNANDEZ, T. (1973) Distribution of
dieldrin in the turtle. Bull. environ. Contam. Toxicol., 10(6):
360-364.
PETROCELLI, S.R., HANKS, A.R., & ANDERSON, J.W. (1973) Uptake and
accumulation of an organochlorine insecticide (dieldrin) by an
estuarine mollusc Rangia cuneata. Bull. environ. Contam. Toxicol.,
10(5): 315-320.
PETROCELLI, S.R., ANDERSON, J.W., & HANKS, A.R. (1975)
Biomagnification of dieldrin residues by food-chain transfer from
clams to blue crabs under controlled conditions. Bull. environ.
Contam. Toxicol., 13(1): 108-116.
PIONKE, H.B. & CHESTERS, G. (1973) Pesticide-sediment-water
interactions. J. environ. Qual., 2(1): 29-45.
POLISHUK, Z.W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health, 20:
215-217.
POLISHUK, Z.W., RON, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D.,
& LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. monit. J., 10(4): 121-129.
PORTER, R.D. & WIEMEYER, S.N. (1969) Dieldrin and DDT: effects on
sparrowhawk eggshells and reproduction. Science, 165: 199-200.
POTTER, J.C., DIXON, L.D., BARBER, G.F., & MARXMILLER, R.L. (1972)
Residues of 14 C-dieldrin and its metabolites in milk from cows fed
dieldrin-14 C, Modesto, California, Shell Development Company
(TIR-24-109-72) (Unpublished proprietary report).
POWELL, A.J.B., STEVENS, T., & MCCULLY, K.A. (1970) Effects of
commercial processing on residues of aldrin and dieldrin in
tomatoes and residues in subsequent crops grown on the treated
plots. J. agric. food Chem., 18(2): 224-227.
POWERS, C.D, ROWLAND, R.G., & WURSTER, C.F. (1977) Dieldrin-induced
destruction of marine algal cells with concomitant decrease in size
of survivors and their progeny. Environ. Pollut., 12: 17-25.
PRESTT, I. (1965) An enquiry into the recent breeding status of
some of the smaller birds of prey and crows in Britain. Bird Stud.,
12(3): 196-221.
PRESTT, I. & BELL, A.A. (1966) An objective method of recording
breeding distribution of common birds of prey in Britain. Bird
Stud., 13(4): 277-283.
PRINCI, F. (1954) Toxicity of the chlorinated hydrocarbon
insecticides. In: Proceedings of the XI International Congress di
Medicina del Lavoro, Pipola, Napoli, Tipografia Saverio, pp.
253-272.
PRINCI, F. & SPURBECK, G.H. (1951) A study of workers exposed to
the insecticides chlordane, aldrin, dieldrin. Arch. ind. Hyg.
occup. Med., 3: 64-72.
PROBST, A.H. & EVERLY, R.T. (1957) Effect of soil insecticides on
emergence, growth, yield, and chemical composition of soybeans.
Agron. J., 1957: 385-387.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: a comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagenesis, 3: 11-32.
PROSPERO, J.M. & SEBA, D.B. (1972) Some additional measurements of
pesticides in the lower atmosphere of the northern equatorial
Atlantic Ocean. Atmos. Environ., 6(5): 363-364.
PROUTY, R.M., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., CROMARTIE,
E., KAISER, T.E., LAMONT, T.G., MULHERN, B.M., & SWINEFORD, D.M.
(1977) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1973-74. Pestic. monit.
J., 11(3): 134-137.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON,
D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of 6
short-term tests for detecting organic chemical carcinogens. Br. J.
Cancer, 37: 873-903.
RADELEFF, R.D., WOODARD, G.T., NICKERSON, W.J., & BUSHLAND, R.C.
(1955) The acute toxicity of chlorinated hydrocarbon and organic
phosphorus insecticides to livestock, Kerville, Texas, US
Department of Agriculture, Agricultural Research Service,
(Technical Bulletin 1122).
RADELEFF, R.D., NICKERSON, W.J., & WELLS, R.W. (1960) Acute toxic
effects upon livestock and meat and milk residues of dieldrin. J.
econ. Entomol., 53(3): 425-429.
RADOMSKI, J.L. & FISEROVA-BERGEROVA, V. (1965) The determination of
pesticides in tissues with the electron-capture detector without
prior clean-up. Ind. Med. Surg., 32: 934-939.
RADOMSKI, J.L., DEICHMANN, W.B., & CLIZER, E.E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
RADOMSKI, J.L., ASTOLFI, E., DEICHMANN, W.B., & REY, A.A. (1971)
Blood levels of organochlorine pesticides in Argentina:
occupationally and non-occupationally exposed adults, children, and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RATCLIFFE, D.A. (1963) The status of the peregrine in Great
Britain. Bird Stud., 10: 56-90.
RATCLIFFE, D.A. (1965) The peregrine situation in Great Britain,
1963-64. Bird Stud., 12(2): 66-82.
RATCLIFFE, D.A. (1967a) Decrease in eggshell weight in certain
birds of prey. Nature (Lond.), 215: 208-210.
RATCLIFFE, D.A. (1967b) The peregrine situation in Great Britain,
1965-66. Bird Stud., 14(4): 238-245.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol., 7: 67-115.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Stud., 19: 117-156.
RATCLIFFE, D.A. (1980) The peregrine falcon, Calton, T. and A.D.
Poyser.
RATCLIFFE, D.A. (1984) The peregrine breeding population of the
United Kingdom in 1981. Bird Stud., 31(1): 1-18.
REICHEL, W.L., CROMARTIE, E., LAMONT, T.G., MULHERN, B.M., &
PROUTY, R.M. (1969) Pesticide residues in eagles. Pestic. monit.
J., 3(3): 142-144.
REIDINGER, R.F. & CRABTREE, D.G. (1974) Organochlorine residues in
golden eagles, United States, March 1964-July 1971. Pestic. monit.
J., 8(1): 37-43.
REINERT, R.E. (1972) Accumulation of dieldrin in an alga
(Scenedesmus obliquus), Daphnia magna, and the guppy (Poecilia
reticulata). J. Fish. Res. Board Can., 29(10): 1413-1418.
REINKE, J., UTHE, J.F., & JAMIESON, D. (1972) Organochlorine
pesticide residues in commercially caught fish in Canada, 1970.
Pestic. monit. J., 6(1): 43-49.
REIMOLD, R.J. (1975) Chlorinated hydrocarbon pesticides and mercury
in coastal biota - Puerto Rico and the US Virgin Islands 1972-1974.
Pestic. monit. J., 9(1): 39-43.
REYNOLDS, C.M. (1974) The census of heronries, 1969-73. Bird Stud.,
21: 129-134.
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin, and endrin. Ind. Arch. occup. environ. Health,
56: 75-79.
RICE, C.P. & SIKKA, H.C. (1973) Fate of dieldrin in selected
species of marine algae. Bull. environ. Contam. Toxicol., 9(2):
116-123.
RICHARD, J.J., JUNK, G.A., AVERY, M.J., NEHRING, N.L., FRITZ, J.S.,
& SVEC, H.J. (1975) Analysis of various Iowa waters for selected
pesticides: atrazine, DDE, and dieldrin - 1974. Pestic. monit. J.,
9(3): 117-123.
RICHARDSON, A. (1971) The isolation and identification of a
metabolite of HEOD (dieldrin) from human faeces, Sittingbourne,
Shell Research (TLGR.0021.71).
RICHARDSON, A. & ROBINSON, J. (1971) The identification of a major
metabolite of HEOD (dieldrin) in human faeces. Xenobiotica, 1(3):
213-219.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967a)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
RICHARDSON, L.A., LANE, J.R., GARDNER, W.S., PEELER, J.T., &
CAMPBELL, J.E. (1967b) Relationship of dietary intake to
concentration of dieldrin and endrin in dogs. Bull. environ.
Contam. Toxicol., 2(4): 207-219.
RICHARDSON, A., BALDWIN, M.K., & ROBINSON, J. (1968) Metabolites of
dieldrin (HEOD) in the urine and faeces of rats. Chem. Ind., 1968:
588-589.
RICKARD, D.G. & DULLEY, M.E.R. (1983) The levels of some heavy
metals and chlorinated hydrocarbons in fish from the tidal Thames.
Environ. Pollut. Ser. B., 5: 101-119.
RISEBROUGH, R.W., HUGGETT, R.J., GRIFFIN, J.J., & GOLDBERG, E.D.
(1968) Pesticides: transatlantic movements in the northeast trades.
Science, 159(3820): 1233-1236.
RITCEY, W.R., SAVARY, G., & MCCULLY, K.A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can. J.
public Health, 64: 380-386.
ROBINSON, J. (1969) Organochlorine insecticides and bird
populations in Britain. In: Miller, M.W. & Berg, G.G., ed. Chemical
fallout, Springfield, Illinois, C.C. Thomas, pp. 113-169.
ROBINSON, J. & CRABTREE, A.N. (1969) The effect of dieldrin on
homing pigeons ( Columba livia var.). Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 34(3): 413-427.
ROBINSON, J. & HUNTER, C.G. (1966) Organochlorine insecticides:
concentrations in human blood and adipose tissue. Arch. environ.
Health, 13: 558-563.
ROBINSON, J. & ROBERTS, M. (1969) Estimation of the exposure of the
general population to dieldrin (HEOD). Food Cosmet. Toxicol., 7:
501-514.
ROBINSON, J., RICHARDSON, A., & ELGAR, K.E. (1966a) Chemical
identity in ultramicroanalysis. In: Proceedings of the American
Chemical Society Meeting, New York, 11-16 September, 1966,
Washington, DC, American Chemical Society.
ROBINSON, J., RICHARDSON, A., BUSH, B., & ELGAR, K.E. (1966b) A
photoisomerization product of dieldrin. Bull. environ. Contam.
Toxicol., 1(4): 127-132.
ROBINSON, J., RICHARDSON, A., & DAVIES, J.M. (1967a) Comparison of
analytical methods for determination of dieldrin (HEOD) in blood.
Arch. environ. Health, 15(1): 67-69.
ROBINSON, J., BROWN, V.K.H., RICHARDSON, A., & ROBERTS, M. (1967b)
Residues of dieldrin (HEOD) in the tissues of experimentally
poisoned birds. Life Sci., 6: 1207-1220.
ROBINSON, J., ROBERTS, M., BALDWIN, M., & WALKER, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Food Cosmet.
Toxicol., 7: 317-332.
ROBURN, J. (1963) Effect of sunlight and ultraviolet radiation on
chlorinated pesticide residues. J. chem. Ind., 1: 1555-1556.
ROCCHI, P., PEROCCO, P., ALBERGHINI, W., FINI, A., & PRODI, G.
(1980) Effect of pesticides on scheduled and unscheduled DNA
synthesis of rat thymocytes and human lymphocytes. Arch. Toxicol.,
45: 101-108.
ROHWER, D. (1983a) [Pesticides in breast milk (problems with
residues of polychlorinated hydrocarbons).] Wiss. Inf., 9(3):
197-201 (in German).
ROHWER, D. (1983b) [Pesticides in breast milk.] Geburtshilfe
Frauenheilkd., 43: 160-163 (in German).
ROSE, G.P. (1982) Toxicity of insecticides: the acute oral and
percutaneous toxicity, skin and eye irritancy, and skin sensitizing
potential of a 480 g/litre emulsifiable concentrate of aldrin (EF
5159), Sittingbourne, Shell Research (SBGR.81.319) (Unpublished
proprietary report).
ROSE, G.P. (1984a) Toxicology of insecticides: the acute
percutaneous toxicity of the 480 g/litre aldrin emulsifiable
concentrate formulation CF 06323, Sittingbourne, Shell Research
(SBGR.84.047) (Unpublished proprietary report).
ROSE, G.P. (1984b) Toxicology of insecticides: the acute
percutaneous toxicity of the 200 g/litre dieldrin emulsifiable
concentrate formulation CF 06271, Sittingbourne, Shell Research
(SBGR.84.045) (Unpublished proprietary report).
ROSE, G.P. (1984c) Toxicology of insecticides (organochlorines):
the acute percutaneous toxicity of the 680 g/l dieldrin suspension
concentrate SF 06349, Sittingbourne, Shell Research (SBGR.84.210)
(Unpublished proprietary report).
ROSEN, J.D. & CAREY, W.F. (1968) Preparation of the photoisomers of
aldrin and dieldrin. J. agric. food Chem., 16: 536-537.
ROSEN, J.D., SUTHERLAND, D.J., & LIPTON, G.R. (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull.
environ. Contam. Toxicol., 1(4): 133-140.
ROSEWELL, K.T., MUIR, D.C.G., & BAKER, B.E. (1979) Organochlorine
residues in Harp Seal (Phagophilus groenlandicus) tissues, Gulf of
St. Lawrence, 1971, 1973. Pestic. monit. J., 12: 189-192.
ROSS, R.D. & CROSBY, D.G. (1974) Photosensitizers in agricultural
water samples, Washington, DC, American Chemical Society (ACS
Meeting, Abstract No. 167, Section PEST 67).
ROSS, R.D. & CROSBY, D.G. (1975) The photooxidation of aldrin in
water. Chemosphere, 4: 227.
ROSS, R.D. & CROSBY, D.G. (1985) Photooxidant activity in natural
waters. Environ. Toxicol. Chem., 4: 773-778.
ROSS, W.R., VAN LEEUWEN, J., & GRABOW, W.O.K. (1976) Studies on the
disinfection and chemical oxidation with ozone and chlorine in
water reclamation. In: Proceedings of the Second Symposium on Ozone
Technology, Montreal, International Ozone Institute, pp. 479-513.
ROWE, D.B., CANTER, L.W., SNIJDER, P.J., & MASON, J.W. (1971)
Dieldrin and endrin concentrations in a Louisiana estuary. Pestic.
monit. J., 4(4): 177-183.
RUEBNER, B.H., GERSHWIN, M.E., MEIERHENRY, E.F., HSIEH, L.S., &
DUNN, P.L. (1984a) Irreversibility of liver tumours in C3H mice. J.
Natl Cancer Inst., 73(2): 493-498.
RUEBNER, B.H., GERSHWIN, M.E., FRENCH, S.W., MEIERHENRY, E., DUNN,
P., & HSIEH, L.S. (1984b) Mouse hepatic neoplasia: Differences
among strains and carcinogens. In: Popp J.A., ed. Current
perspectives in mouse liver neoplasia, Washington, DC, Hemisphere.
SAHA, J.G. & SUMNER, A.K. (1971) Organochlorine insecticide
residues in soil from vegetable farms in Saskatchewan. Pestic.
monit. J., 5(1): 28-31.
SAHA, J.G., KARAPALLY, J.C., & JANSEN, W.K. (1971) Influence of the
type of mineral soil on the uptake of dieldrin by wheat seedlings.
J. agric. food Chem., 19: 842-845.
SAND, P.F., WIERSMA, G.B., & LANDRY, J.L. (1972) Pesticide residues
in sweet potatoes and soil - 1969. Pestic. monit. J., 5(4): 342-344.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the
western chorus frog Pseudacris triseriata and Fowler's Toad Bufo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDHU, S.-S., WARREN, W.J., & NELSON, P. (1978) Pesticidal residue
in rural potable water. Am. Water Works Assoc. J., 70(1): 41-45.
SANDIFER, S.H., CUPP, C.M., WILKINS, R.T., LOADHOLT, B., & SCHUMAN,
S.H. (1981) A case-control study of persons with elevated blood
levels of dieldrin. Arch. environ. Contam. Toxicol., 10(1): 35-45.
SANGER, A.M.H. (1959) Aldrin, dieldrin, and endrin toxicity to
bees. Span, 2(2): 59-63.
SASCHENBRECKER, P.W. (1976) Levels of terminal pesticide residues
in Canadian meat, Can. Vet. J., 17(6): 158-163.
SAVAGE, E.P., KEEFE, T.J., TESSARI, J.D., WHEELER, H.W., APPLEHAUS,
F.M., GOES, E.A., & FORD, S.A. (1981) National study of chlorinated
hydrocarbon insecticide residues in human milk, USA. I. Geographic
distribution of dieldrin, heptachlor, heptachlor epoxide,
chlordane, oxychlordane, and mirex. Am. J. Epidemiol., 113: 413-422.
SCHAFER, M.L. (1968) Pesticides in blood. Residue Rev., 24: 19-39.
SCHAUBERGER, C.W. & WILDMAN, R.B. (1977) Accumulation of aldrin and
dieldrin by blue-green algae and related effects on photosynthetic
pigments. Bull. environ. Contam. Toxicol., 17(5): 534-541.
SCHULZE, J.A., MANIGOLD, D.B., & ANDREWS, F.L. (1973) Pesticides in
selected western streams - 1968-71. Pestic. monit. J., 7(1): 73-84.
SEAL, W.L., DAWSEY, L.H., & CAVIN, G.E. (1967) Monitoring for
chlorinated hydrocarbon pesticides in soil and root crops in the
eastern states in 1965. Pestic. monit. J., 1(3): 22-25.
SELBY, L.A., NEWELL, K.W., HAUSER, G.A., & JUNKER, G. (1969a)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2(4): 247-255.
SELBY, L.A., NEWELL, K.W., WAGGENSPACK, C., HAUSER, G.A., & JUNKER,
G. (1969b) Estimating pesticide exposure in man as related to
measurable intake: environmental versus chemical index. Am. J.
Epidemiol., 89(3): 241-253.
SETHUNATHAN, N. (1973) Microbial degradation of insecticides in
flooded soil and in anaerobic cultures. Residue Rev., 47: 143-165.
SHAH, P.V. & GUTHRIE, F.E. (1976) Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Watson, D.L. &
Brown, A.W.A., ed. Pesticide management and insecticide resistance,
New York, London, Academic Press, pp. 547-554.
SHAKOORI, A.R., RASUL, Y.G., & ALI, S.S. (1986) The effect of long-
term administration of dieldrin on biochemical components in blood
serum of albino rats. Toxicol. Lett., 9(4): 35.
SHANNON, L.R. (1977a) Equilibrium between uptake and elimination of
dieldrin by channel catfish Ictalurus punctatus. Bull. environ.
Contam. Toxicol., 17(3): 278-284.
SHANNON, L.R. (1977b) Accumulation and elimination of dieldrin in
muscle tissue of channel catfish. Bull. environ. Contam. Toxicol.,
17(6): 637-644.
SHAROM, M.S., MILES, J.R.W., HARRIS, C.R., & MCEWEN, F.L. (1980)
Behaviour of 12 insecticides in soil and aqueous suspensions of
soil and sediment, Water Res., 14: 1095-1100.
SHEETS, T.J., JACKSON, M.D., & PHELPS, L.D. (1970) Water monitoring
system for pesticides in North Carolina, US Clearinghouse, 109 pp
(P.B. Report No. 189291).
SHELL (1976) Chemicals for plant protection, veterinary uses, and
public health, London, Shell International Chemical Company, Ltd,
pp. 3-4, 9-10.
SHELL (1984) Shell guide to pesticide safety, London, Shell
International Chemical Company, Ltd, Agrochemical Division, pp.
27-29.
SHELLENBERGER, T.E. (1978) A multi-generation toxicity evaluation
of p,p'-DDT and dieldrin with Japanese quail. Effects on growth and
reproduction. Drug chem. Toxicol., 1(2): 137-146.
SHELLENBERGER, T.E. & NEWELL, G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail (Coturnix coturnix
japonica). Lab. anim. Care, 15(2): 119-130.
SHERMAN, M. & ROSENBERG, M.M. (1953) Acute toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 46(6): 1067-1070.
SHIRASU, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4: 226-231.
SIERRA, M. & SANTIAGO, D. (1987) Organochlorine pesticide levels in
barn owls collected in Leon, Spain. Bull. environ. Contam.
Toxicol., 38: 261-265.
SIERRA, M., TERAN, M.T., GALLEGO, A., DIEZ, M.J., & SANTIAGO, D.
(1987) Organochlorine contamination in three species of diurnal
raptors in Leon, Spain. Bull. environ. Contam. Toxicol., 38: 254-260.
SILBERGELD, E.K. (1973) Dieldrin. Effects of chronic sublethal
exposure on adaptation to thermal stress in fresh-water fish.
Environ. Sci. Technol., 7(9): 846-849.
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.C. (1977) Mutagenic
activity of chemicals identified in drinking-water. In: Scott, D.,
Bridges, B.A., & Sobels, F.H., ed. Progress in genetic toxicology,
Amsterdam, Elsevier Science Publishers, pp. 249-258.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY, M.O.
(1983) Evaluation of the alkaline elution/rat hepatocyte assay as a
predictor of carcinogenic/mutagenic potential. Mutat. Res., 113:
357-391.
SINGH, K.K., JHA, G.J., SINGH, P.N., & CHAUHAN, H.V.S. (1985)
Pathophysiology of acute aldrin intoxication in goats. Toxicol.
Abstr., 8(7): 27 (No. 4086-x8).
SIYALI, D.S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust., 2: 1063-1066.
SIYALI, D.S. (1973) Polychlorinated biphenyls, hexachlorobenzene,
and other organochlorine pesticides in human milk. Med. J. Aust.,
2: 815-818.
SIYALI, D.S. & SIMSON, R.E. (1973) Chlorinated hydrocarbon
pesticides in human blood and fat. Med. J. Aust., 1: 212-213.
SLATER, R.M. & SPEDDING, D.J. (1981) Transport of dieldrin between
air and water. Arch. environ. Contam. Toxicol., 10: 25-33.
SMITH, J.H., BOMBERGER, D.C., Jr, & HAYNES, D.L. (1981)
Volatilization rates of intermediate and low volatility chemicals
from water. Chemosphere, 10(3): 281-289.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of
DDT and dieldrin on development in winter flounder
(Pseudopleuronectes americanus). J. Fish. Res. Board Can., 30(12):
1894-1898.
SMITH, S.W.C. (1978) Pesticide residues in the total diet. Food
Technol. Aust., 1978: 349-352.
SPALDING, R.F., JUNK, C.A., & RICHARD, J.R. (1980) Pesticides in
groundwater beneath irrigated farmland in Nebraska, August 1978.
Pestic. monit. J., 14: 70-73.
SPARR, B.I., APPLEBY, W.G., DEVRIES, D.M., OSMUN, J.V., MCBRIDE,
J.M., FOSTER, G.L., & GOULD R.F., ed. (1966) Organic pesticides in
the environment, Washington, DC, American Chemical Society, pp.
146-162 (Advances in Chemistry Series No. 60).
SPENCER, W.F. & CLIATH, M.M. (1973) Pesticide volatilization as
related to water loss from soil. J. environ. Qual., 2(2): 284-289.
SPENCER, W.F. & CLIATH, M.M. (1975) Environmental dynamics of
pesticides. In: Haque, R. & Freed, V.H. ed., New York, London,
Plenum Press, pp. 61-78.
SPENCER, W.F., CLIATH, M.M., & FARMER, W.J. (1969) Vapor density of
soil-applied dieldrin as related to soil-water content,
temperature, and dieldrin concentration. Soil Sci. Soc. Am. Proc.,
33: 509-511.
SPENCER, W.F., FARMER, W.J., & CLIATH, M.M. (1973) Pesticide
volatilization. Residue Rev., 49: 1-47.
SPIOTTA, E.J. (1951) Aldrin poisoning in man. Report of a case.
Arch. ind. Hyg. occup. Med., 4: 560-566.
STACEY, C.I. & TATUM, T. (1985) House treatment with organochlorine
pesticides and their levels in human milk - Perth, Western
Australia. Bull. environ. Contam. Toxicol., 35: 202-208.
STACEY, C.I. & THOMAS, B.W. (1975) Organochlorine pesticide
residues in human milk, Western Australia, 1970-71. Pestic. monit.
J., 9(2): 64-66.
STACEY, C.I., PERRIMAN, W.S., & WHITNEY, S. (1985) Organochlorine
pesticides residue levels in human milk: Western Australia,
1979-80. Arch. environ. Health, 40: 102-108.
STANLEY, C.W., BARNEY, J.E., HELTON, M.R., & YOBS, A.R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANLEY, P.I. & BUNYAN, P.J. (1979) Hazards to wintering geese and
other wildlife from the use of dieldrin, chlorfenvinphos, and
carbophenothion as wheat seed treatments. Proc. R. Soc. Lond. Ser
B, 205: 31-45.
STEVENS, L.J., COLLIER, C.W., & WOODHAM, D.W. (1970) Monitoring
pesticides in soils from areas of regular, limited, and no
pesticide use. Pestic. monit. J., 4(3): 145-164.
STEVENSON, D.E. & WALKER, A.I.T. (1969) Hepatic lesions produced in
mice by dieldrin and other hepatic enzyme-inducing compounds. J.
Eur. Toxicol., 2: 83-84.
STEVENSON, D.E., THORPE, E., HUNT, P.F., & WALKER, A.I.T. (1976)
The toxic effects of dieldrin in rats: a re-evaluation of data
obtained in a two-year feeding study. Toxicol. appl. Pharmacol., 36:
247-254.
STEWART, D.J. & STEIN, R.A. (1974) Short-term fate of dietary
dieldrin in the digestive tract of juvenile lake trout (Salvelinus
namaycush). Bull. environ. Contam. Toxicol., 11(6): 563-566.
STEWART, D.K.R. & FOX, C.J.S. (1971) Persistence of organochlorine
insecticides and their metabolites in Nova Scotian soils. J. econ.
Entomol., 64(2): 367-371.
STEWART, D.K.R. & GAUL, S.O. (1977) Dihydrochlordene dicarboxylic
acid residues in soil treated with high rates of aldrin. Bull.
environ. Contam. Toxicol., 17(6): 712-713.
STICKEL, W.H. (1975) Some effects of pollutants in terrestrial
ecosystems. Environ. Sci. Res., 7: 25-74.
STICKEL, W.H., STICKEL, L.F., & SPANN, J.W. (1969) Tissue residues
of dieldrin in relation to mortality in birds and mammals. In:
Miller, M.W. & Berg, G.G., ed. Chemical fallout, Springfield,
Illinois, C.C. Thomas, pp. 174-200.
SUNDARAM, K.S., DAMODARAN, V.N., & VENKITASUBRAMANIAM, T.A.
(1978) Absorption of dieldrin through monkey and dog skin. Indian
J. exp. Biol., 16: 101-103.
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1974) Photodieldrin
residues in field soils. Bull. environ. Contam. Toxicol., 12:
275-279.
SWENBERG, J.A., PETZOLD, G.L., & HARBACH, P.R. (1976) In vitro DNA
damage-alkaline elution assay of predicting carcinogenic potential.
Biochem. Biophys. Res. Commun., 72(2): 732-738.
SZARO, R.C., COON, N.C., & KOLBE, E. (1979) Pesticide and PCB of
common eider, herring gull, and great black-backed gull eggs. Bull.
environ. Contam. Toxicol., 22: 394-399.
TABOR, E.C. (1966) Contamination of urban air through the use of
insecticides. Trans. N.Y. Acad. Sci., 28: 569-578.
TAKEI, G.H., KAUAHIKAUA, S.M., & LEONG, G.H. (1983) Analyses of
human milk samples collected in Hawaii for residues of
organochlorine pesticides and polychlorobiphenyls. Bull. environ.
Contam. Toxicol., 30: 606-613.
TANNOCK, J., HOWELLS, W.W., & PHELPS, R.J. (1983) Chlorinated
hydrocarbon pesticide residues in eggs of some birds in Zimbabwe.
Environ. Pollut. Ser. B, 5: 147-155.
TARRANT, K.R. & TATTON, J.O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TATTON, J.O'G. & RUZICKA, J.H.A. (1967) Organochlorine pesticides
in Antarctica. Nature (Lond.), 215: 346-348.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., & FREEMAN, H.P. (1972)
Measurement of volatilization of dieldrin and heptachlor from a
soil in the field. In: Abstracts of the 163rd National Meeting of
the American Chemical Society, Boston, Massachusetts, Washington,
DC, American Chemical Society.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., FREEMAN, H.P., &
EDWARDS, W.M. (1976) Volatilization of dieldrin and heptachlor from
a maize field. J. agric. food Chem., 24(3): 625-631.
TEJEDOR, M.C., MURADO, M.A., & BALUJA, G. (1974) [Contamination of
the environment by organochlorine pesticides.] An. Quim., 70:
1177-1183 (in Spanish).
TENNEKES, H.A., WRIGHT, A.S., DIX, K.M., & KOEMAN, J.H. (1981)
Effects of dieldrin, diet and bedding on enzyme function and tumour
incidence in livers of male CF-1 mice. Cancer Res., 41: 3615-3620.
TENNEKES, H.A., RAVENZWAAY, B., & KUNZ, H.W. (1985) Quantitative
aspects of enhanced liver tumour formation in CF-1 mice by
dieldrin. Carcinogenesis, 6(10): 1457-1462.
TERRIERE, L.C. & YU, S.J. (1976) Microsomal oxidases in the flesh
fly ( Sarcophaga bullata Parker) and the black blow fly ( Phormia
regina Meigen). Pestic. Biochem. Physiol., 6: 223-228.
THOMPSON, A.R., EDWARDS, C.A., EDWARDS, M.J., & BEYNON, K.I. (1970)
Movement of dieldrin through soils. II. In sloping troughs and soil
columns. Pestic. Sci., 1(5): 174-178.
THOMPSON, J.F. (1976) Manual of analytical quality control for
pesticides and related compounds in human and environmental
samples, Research Triangle Park, North Carolina, US Environmental
Protection Agency, Office of Research and Development, Health
Effects Research Laboratory (EPA-600/1-76-017).
THORPE, E. (1973) The toxicology of dieldrin (HEOD):
transplantation of liver tumours in mice, Sittingbourne, Shell
Research (TLGR.0041.73) (Unpublished proprietary report).
THORPE, E. & HUNT, P.F. (1975) Toxicology of dieldrin (HEOD): study
of the pathological changes in 3 strains of mice following
prolonged ingestion of dieldrin, Sittingbourne, Shell Research
(TLGR.0012.75) (Unpublished proprietary report).
THORPE, E. & WALKER, A.I.T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, beta-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TREON, J.F. & CLEVELAND, F.P. (1955) Toxicity of certain
chlorinated hydrocarbon insecticides for laboratory animals, with
special reference to aldrin and dieldrin. J. agric. food Chem.,
3(5): 402-408.
TREON, J.F., DUTRA, F.R., SHAFFER, F.E., CLEVELAND, F.P., WAGNER,
W., & GAHEGAN, T. (1951) The toxicity of aldrin, dieldrin, and DDT
when fed to rats over the period of six months, Cincinnati, Ohio,
Kettering Laboratory.
TREON, J.F., HARTMAN, L., GAHEGAN, T., & NEDDERMANN, G. (1953) The
immediate and cumulative toxicity of aldrin, dieldrin, and DDT when
maintained in contact with the skin of rabbits, Cincinnati, Ohio,
Kettering Laboratory.
TROSKO, J.E., JONE, C., & CHANG, C.C. (in press) Inhibition of gap-
functional mediated intercellular communication in vitro by aldrin,
dieldrin and toxaphene: a possible cellular mechanism for their
tumour-promoting and neurotoxic effects. Mol. Toxicol.
TU, C.M. (1981) Effects of some pesticides on enzyme activities in
an organic soil. Bull. environ. Contam. Toxicol., 27: 109-114.
TU, C.M. & MILES, J.R.W. (1976) Interactions between insecticides
and soil microbes. Residue Rev., 64: 17-65.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington, DC, US Department of the
Interior, Bureau of Sport Fishing and Wildlife, pp. 16-17, 46-47
(Resource Publication No. 84).
TUCKER, R.K. & HAEGELE, M.A. (1971) Comparative acute oral toxicity
of pesticides to six species of birds. Toxicol. appl. Pharmacol.,
20(1): 57-65.
TUINSTRA, L.G.M.TH. (1971) Organochlorine insecticide residues in
human milk in the Leiden region. Ned. Melk. Zuiveltijdschr., 25:
24-32.
TURNER, B.C., GLOTFELTY, D.E., & TAYLOR, A. (1977) Photodieldrin
formation and volatilization from grass. J. agric. food Chem., 25:
548-550.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., THEARLE, R.J.P., EGAN, H.,
EVANS, W.H., & SOUTAR, N.M. (1963) The effects on birds of certain
chlorinated insecticides used as seed dressings. J. Sci. Food
Agric., 14(8): 567-577.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., MURTON, R.K., BRADY, J.,
THEARLE, R.J.P., JONES, F.J.S., REA, R.E., KIRBY, D.R., SMITH,
B.M., & RUTTER, M.E. (1965) Pesticides and wildlife. Report of the
Infestation Control Laboratory for 1962-64, London, Her Majesty's
Stationary Office, pp. 23-47.
UK-HMSO (1986) Food surveillance paper, London, Her Majesty's
Stationary Office, (Document No. 16).
UK-MAFF (1982-85) Report of the Working Party on Pesticide
Residues, London, Ministry of Agriculture, Fisheries and Food
(Food Surveillance Paper No. 16).
US EPA (1983) Environmental news, Washington, DC, US Environmental
Protection Agency, Office of Public Affairs.
UYETA, M., TAUE, S., CHIKAZAWA, K., & NISHIMOTO, T. (1971)
Pesticides translocated in food - organochlorine pesticides in the
total diet. J. Food Hyg. Soc. Jpn. 12: 445.
UZOUKWU, M. & SLEIGHT, S.D. (1972) Effects of dieldrin in pregnant
sows. J. Am. Vet. Med. Assoc., 160(12): 1641-1643.
VAN DEN BERCKEN, J. (1972) An electrophysiological investigation
into the action of DDT, dieldrin, and allethrin in the clawed toad
Xenopus laevis, Utrecht, Rijks Universiteit (Thesis).
VAN DEN BERCKEN, J. & NARAHASHI, T. (1974) Effects of aldrin-
transdiol, a metabolite of the insecticide dieldrin, on nerve
membrane. Eur. J. Pharmacol., 27: 255-258.
VAN DEN BROEK, W.L.F. (1979) Seasonal levels of chlorinated
hydrocarbons and heavy metals in fish and brown shrimps from the
Medway estuary, Kent. Environ. Pollut., 19: 21-38.
VAN DIJCK, P. & VAN DE VOORDE, H. (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 41(2): 1491-1498.
VAN DUURSEN, P. (1985) Open letter. Shell Post, 1985(671).
VAN GELDER, G.A. & CUNNINGHAM, W.L. (1975) The effects of low-level
dieldrin exposure on the EEG and learning ability of the squirrel
monkey. Toxicol. appl. Pharmacol., 33(1): 142 (Abstract No. 50).
VAN GENDEREN, H. (1965) The toxicology of the chlorinated
hydrocarbon insecticides. A progress report with particular
reference to the qualitative aspects of the action in warm-blooded
animals. Opzoekingsstn Staat Gent, 30(3): 1321-1335.
VAN GENDEREN, H. (1979) [Dieldrin: a troublesome insecticide.] K.
Ned. Akad. Wet. (Amsterdam) 88(3): 24-32 (in Dutch).
VAN GENUCHTEN, M.TH., DAVIDSON, J.M., & WIERENGA, P.J. (1974) An
evaluation of kinetic and equilibrium equations for the prediction
of pesticide movement through porous media. Soil Sci. Soc. Am.
Proc., 38: 29-35.
VAN HAVER, W., VANDEZANDE, A., & GORDTS, L. (1978) [Organochlorine
pesticides in human fatty tissue.] Arch. Belg. Méd. soc. Hyg. Méd.
Trav. Méd. lég., 36: 147-155 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN RAALTE, H.G.S. (1965) Aspects of pesticide toxicity. In:
Proceedings of the Conference on Occupational Health, Caracas,
Venezuela (Unpublished paper).
VAN RAALTE, H.G.S. (1977) Human experience with dieldrin in
perspective. Ecotoxicol. environ. Saf., 1: 203-210.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination
of water by a sublethal concentration of pesticides: a system
analysis approach. Acta hydrochim. hydrobiol., 12(4): 399-409.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and
telodrin. Br. J. ind. Med., 30: 201-202.
VIRGO, B.B. & BELLWARD, G.D. (1975) Effects of dietary dieldrin on
reproduction in the Swiss-Vancouver (SWV) mouse. Environ. Physiol.
Biochem., 5: 440-450.
VIRGO, B.B. & BELLWARD, G.D. (1977) Effects of dietary dieldrin on
offspring viability, maternal behaviour, and milk production in the
mouse. Res. Commun. chem. Pathol. Pharmacol., 17(3): 399-409.
VOERMAN, S. & BESEMER, A.F.H. (1975) Persistence of dieldrin,
lindane, and DDT in a light sandy soil and their uptake by grass.
Bull. environ. Contam. Toxicol., 13(4): 501-505.
VOUTSINOU-TALIADOURI, F. & SATSMADJIS, J. (1982) Influence of
metropolitan waste on the concentration of chlorinated hydrocarbons
and metals in striped mullet. Mar. Pollut. Bull., 13(8): 266-269.
VREMAN, K. & POORTVLIET, L.J. (1982) Pesticide levels in milk and
milk products in relation to their milk fat content. Neth. Milk
Dairy J., 36: 145-148.
VREMAN, K., POORTVLIET, L.J., & VAN DEN HOEK, J. (1980) Transfer of
organochlorine pesticides from feed into the milk and body fat of
cows. Long-term experiment with intake at low levels. Neth. Milk
Dairy J., 34: 87-105.
WADE, M.H., MOYER, J.W., & HINE, C.H. (1979) Mutagenic action of a
series of epoxides. Mutat. Res., 66: 367-371.
WADE, M.H., TROSKO, J.E., & SCHINDLER, M. (1986) A fluorescence
photobleaching assay of gap junction-mediated communication between
human cells. Science, 232: 525-528.
WALKER, A.I.T., NEILL, C.H., STEVENSON, D.E., & ROBINSON, J.
(1969a) The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix
coturnix japonica). Toxicol. appl. Pharmacol., 15: 69-73.
WALKER, A.I.T., STEVENSON, D.E., ROBINSON, J., THORPE, E., &
ROBERTS, M. (1969b) The toxicology and pharmacodynamics of dieldrin
(HEOD): two-year oral exposure of rats and dogs. Toxicol. appl.
Pharmacol., 15: 345-373.
WALKER, A.I.T., THORPE, E., ROBINSON, J., & BALDWIN, M.K. (1971)
Toxicity studies on the photoisomerisation product of dieldrin.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 36(1): 398-409.
WALKER, A.I.T., THORPE, E., & STEVENSON, D.E. (1972) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALTON, M.S., BECK-BASTONE, V., & BARON, R.L. (1971) Subchronic
toxicity of photodieldrin, a photodecomposition product of
dieldrin. Toxicol. appl. Pharmacol., 20(1): 82-88.
WARNICK, S.L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah, fiscal years 1967-71. Pestic. monit. J.,
6(1): 9-13.
WARNICK, S.L. & CARTER, J.E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASSERMANN, M., CURNOW, D.H., FORTE, P.N., & GRONER, Y. (1968)
Storage of organochlorine pesticides in the body fat of people in
western Australia. Int. J. ind. Med. Surg., 37(4): 295-300.
WASSERMANN, M., FRANCONE, M.P., WASSERMANN, D., MARIANI, F., &
GRONER, Y. (1969) [Organochlorine pesticide content in the fatty
tissue of the general population in the Republic of Argentina.]
Sem. med., 134: 459-462 (in Spanish).
WASSERMANN, M., WASSERMANN, D, LAZAROVICI, S., COETZEE, A.M., &
TOMATIS, L. (1970) Present state of the storage of the
organochlorine insecticides in the general population of South
Africa. South Afr. med. J., 44: 646-648.
WASSERMANN, M., NOGUEIRA, D.P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972a) Storage of organochlorine
insecticides in people of Sao Paulo, Brazil. Ind. Med. Surg., 41(3):
22-25.
WASSERMANN, M., ROGOFF, M.G., TOMATIS, L., DAY, N.E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of organochlorine
insecticides in the adipose tissue of people in Kenya. Ann. Soc.
Belg. Méd. Trop., 52(6): 509-514.
WASSERMANN, M., SOFOLUWE, G.O., TOMATIS, L., DAY, N.E., WASSERMANN,
D., & LAZAROVICI, S. (1972c) Storage of organochlorine insecticides
in people in Nigeria. Environ. Physiol. Biochem., 2: 59-67.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N.E.,
WASSERMANN, D., RUNGPITARANGSI, V., CHIAMSAKOL, V.,
DJAVAHERIAN, M., & CUCOS, S. (1972d) Storage of organochlorine
insecticides in the adipose tissue of people from Thailand.
Southeast Asian J. trop. Med. public Health, 3(2): 280-285.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol., 12(4):
501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in adipose tissue of Israelis. Pestic.
monit. J., 8(1): 1-7.
WATSON, M., BENSON, W.W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people of southern Idaho. Pestic. monit. J.,
4(2): 47-50.
WEDBERG, J.L., MOORE, S., III, AMORE, F.J., & MCAVOY, H. (1978)
Residues in food and feed. Organochlorine insecticide residues in
bovine milk and manufactured milk products in Illinois 1971-76.
Pestic. monit. J., 11: 161-164.
WEGMAN, R.C.C. & GREVE, P.A. (1974) Levels of organochlorine
pesticides and inorganic bromide in human milk. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 39: 1301-1310.
WEGMAN, R.C.C. & GREVE, P.A. (1978) Organochlorines, cholinesterase
inhibitors, and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic. monit. J., 12(3): 149-162.
WEISGERBER, I., KOHLI, J., KAUL, R., KLEIN, W., & KORTE, F. (1974)
Fate of aldrin-14C in maize, wheat, and soils under outdoor
conditions. J. agric. food Chem., 22(4): 609-612.
WEISGERBER, I., BIENIEK, D., KOHLI, J., & KLEIN, W. (1975)
Isolation and identification of three unreported photodieldrin-14C
metabolites in soil. J. agric. food Chem., 23: 873-877.
WELLS, M.R. & YARBROUGH, J.D. (1973) In vivo and in vitro retention
of 14C-aldrin and 14C-dieldrin in cellular fractions from brain and
liver tissues of insecticide-resistant and susceptible Gambusia.
Toxicol. appl. Pharmacol., 24: 190-196.
WELLS, M.R., PHILLIPS, J.B., & MURPHY, G.G. (1974) ATPase activity
in tissues of the map turtle Graptemys geographica following in
vitro treatment with aldrin and dieldrin. Bull. environ. Contam.
Toxicol., 11(6): 572-576.
WESSELS, C.L. (1978) Residues in soybean plants of aldrin and
dieldrin following soil application and of endosulphan and DDT
following foliar application. Rhodesian. J. agric. Res., 16:
205-210.
WHEATLEY, G.A. & HARDMAN, J.A. (1965) Indications of the presence
of organochlorine insecticides in rainwater in central England.
Nature (Lond.), 207: 486-487.
WHEATLEY, G.A. & HARDMAN, J.A. (1968) Organochlorine insecticide
residues in earthworms from arable soils. J. Sci. Food Agric., 19:
219-225.
WHEATLEY, G.A., HARDMAN, J.A., & STRICKLAND, A.H. (1962) Residues
of chlorinated hydrocarbon insecticides in some farm soils in
England. Plant Pathol., 11: 81-90.
WHITE, D.H. (1976) Nationwide residues of organochlorine in
starling, 1974. Pestic. monit. J., 10(1): 10-17.
WHITE, D.H., KING, K.A., & PROUTY, R.M. (1980) Significance of
organochlorine and heavy metal residues in wintering shorebirds at
Corpus Christi, Texas, 1976-77. Pestic. monit. J., 14(2): 58-63.
WHO (1958) Note by Secretariat on aldrin poisoning in Kenya,
Geneva, World Health Organization, p. 3 (Information Circular on
the Toxicity of Pesticides to Man No. 1).
WHO (1977) Outbreak of food poisoning of chemical origin, Geneva,
World Health Organization, p. 217 (Weekly Epidemiological Record No.
52).
WHO (1984) Guidelines for drinking-water quality, Geneva, World
Health Organization, Vol. 1, p. 69.
WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished document VBC/88.953).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World Health
Organization (Unpublished documents).
WIEMEYER, S.N., MULHERN, B.M., LIGAS, F.J., HENSEL, R.J., MATHISEN,
J.E., ROBARDS, F.C., & POSTUPALSKY, S. (1972) Residues of
organochlorine pesticides, polychlorinated biphenyls, and mercury
in bald eagle eggs and changes in shell thickness, 1969 and 1970.
Pestic. monit. J., 6(1): 50-55.
WIERSMA, G.B., MITCHELL, W.G., & STANFORD, C.L. (1972) Pesticide
residues in onions and soil - 1969. Pestic. monit. J., 5(4):
345-347.
WIESE, I.H. & BASSON, N.C.J. (1966) The degradation of some
persistent chlorinated hydrocarbon insecticides applied to
different soil types. South Afr. J. agric. Sci., 9: 945-969.
WIESE, I.H., BASSON, N.C.J., VAN DER VIJVER, J.H., & VAN DER MERWE,
J.H. (1969) Toxicology and dynamics of dieldrin in the crowned
guinea-fowl Numida meleagris L. Phytophylactica, 1: 161-176.
WIESE, I.H., BASSON, N.C.J., BASSON, P.A., NAUDE, T.W., & MAARTENS,
B.P. (1973) The toxicology and pathology of dieldrin and photo-
dieldrin poisoning in two antelope species. Onderstepoort J. vet.
Res., 40(1): 31-40.
WILLIAMS, D.T., BENOIT, F.M., MCNEIL, E.E., & OTSON, R. (1978)
Organochlorine pesticide levels in Ottawa drinking water, 1976.
Pestic. monit. J., 12(3): 163-166.
WILLIAMS, D.T., LEBEL, G.L., & JUNKINS, E. (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples
from two Ontario municipalities. J. Toxicol. environ. Health, 13:
19-29.
WILLIAMS, G.M. (1982) Organochlorine pesticides and inhibition of
intercellular communication as the mechanism for their liver tumor
production. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: human welfare and the environment. Oxford, New York,
Pergamon Press, Vol. 3, pp. 475-478.
WILLIAMS, R. & HOLDEN, A.V. (1973) Organochlorine residues from
plankton. Mar. Pollut. Bull., 4(7): 109-111.
WILLIAMS, S., MILLS, P.A., & MCDOWELL, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Agric. Chem.,
47(6): 1124-1128.
WILLIS, G.H., PARR, J.F., SMITH, S., & CARROLL, B.R. (1972)
Volatilization of dieldrin from fallow soil as affected by
different soil water regimes. J. environ. Qual., 1(2): 193-196.
WINTHROP, G.J. & FELICE, J.F. (1957) [A clinical toxicological
study of spraymen of a chlorinated hydrocarbon insecticide.] Bol.
Sanit. Panama, 43: 512-517 (in Spanish with English summary).
WIT, S.L. (1971) [Persistent insecticides in Dutch body fat.] Chem.
Weekbl., 67(5): 11-14 (in Dutch).
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1963) Health hazards
of the pesticides endrin and dieldrin. Arch. environ. Health, 6:
458-464.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council.
WRIGHT, A.S., POTTER, D., WOODER, M.F., DONNINGER, C., & GREENLAND,
R.D. (1972) The effects of dieldrin on the subcellular structure
and function of mammalian liver cells. Food Cosmet. Toxicol., 10:
311-332.
WRIGHT, A.S., AKINTONWA, D.A.A., & WOODER, M.F. (1977) Studies on
the interactions of dieldrin with mammalian liver cells at the
subcellular level. Ecotoxicol. environ. Saf., 1: 7-16, 427.
WRIGHT, A.S., DONNINGER, C., GREENLAND, R.D., STEMMER, K.L., &
ZAVON, M.R. (1978) The effects of prolonged ingestion of dieldrin
on the livers of male rhesus monkeys. Ecotoxicol. environ. Saf., 1:
477-502.
WUENSCHER, K. & ACKER, L. (1969) [The occurrence of chlorinated
insecticides in human fatty tissues.] Med. Ernähr., 10(4): 75-80
(in German).
WUTHRICH, C., MULLER, F., BLASER, O., & MAREK, B. (1985)
[Subjection of the population to pesticides and other foreign
substances through food.] Mitt. Geb. Lebensm. Hyg., 76: 260-276
(in German).
WYLLIE, J., GABICA, J., & BENSON, W.W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: a survey of 200 persons in southern Idaho - 1970. Pestic.
monit. J., 6(2): 84-88.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls (PCBs) and
organochlorine pesticides in human milk and blood collected in
Osaka prefecture from 1972 to 1977. Int. Arch. occup. environ.
Health, 43: 1-15.
YAP, H.H., DESAIAH, D., CUTKOMP, L.K., & KOCH, R.B. (1975) In vitro
inhibition of fish brain ATPase activity by cyclodiene insecticides
and related compounds. Bull. environ. Contam. Toxicol., 14(2):
163-167.
YARON, B., SWOBODA, A.R., & THOMAS, G.W. (1967) Aldrin adsorption
by soils and clays. J. agric. food Chem., 15(4): 671-675.
YOSHIMURA, M., YAMADA, T., SUGIYAMA, S., NODA, H., & MITSUKUNI, Y.
(1979) Organochlorinated pesticides in human organs and tissues.
Jpn. J. leg. Med., 33(2): 91-102.
YU, S.J., KIIGEMAJI, M., & TERRIERE, L.C. (1971) Oxidative
metabolism of aldrin and isodrin by bean root fractions. J. agric.
food Chem., 19: 5-9.
ZABIK, M.E., HOOJJAT, P., & WEAVER, C.M. (1979) Polychlorinated
biphenyls, dieldrin, and DDT in lake trout cooked by broiling,
roasting, or microwave. Bull. environ. Contam. Toxicol., 21:
136-143.
ZAVON, M.R. & HAMMAN, R.E. (1961) Human experience with dieldrin in
malaria control programs. Am. J. public Health, 51(7): 1026-1032.
ZAVON, M.R. & STEMMER, K.L. (1975) The effect of dieldrin ingestion
on rhesus monkeys. A six-year study, Cincinnati, Ohio, Kettering
Laboratory.
ZAVON, M.R., HINE, C.H., & PARKER, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Am. Med. Assoc., 193(10): 837-839.
ZELLE, B. & LOHMAN, P.H.M. (1977) Repair of DNA in cultured human
cells treated with dieldrin and 4-nitroquinoline-N-oxide,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
ZHONG-XIANG, L., KAVANAGH, T., TROSKO, J.E., & CHANG, C.C. (1986)
Inhibition of gap junctional intercellular communication in human
teratocarcinoma cells by organochlorine pesticides. Toxicol. appl.
Pharmacol., 83: 10-19.
ZIMMERLI, B. & MAREK, B. (1973) [Subjection of the Swiss population
to pesticides.] Mitt. Geb. Lebensm. Hyg., 64(4): 459-479
(in German).
APPENDIX I. NOMENCLATURE
Two major systems are currently used for the nomenclature of
these compounds: "polyhydroaromatic" names used by Chemical
Abstracts (American Chemical Society) and IUPAC and the von
Baeyer/IUPAC system for polycylic aliphatic compounds. That the
latter system should be used for the cyclodiene insecticides was
proposed by Benson (1969) and Bedford (1974). The "polyaromatic"
system has, unfortunately, been subject to historical variation,
and there are differences between the IUPAC, British and American
conventions for defining the 3-dimensional stereochemistry in this
system. As a consequence of the differences in the numbering of the
carbon atoms in the two major systems, and the modification of the
Chemical Abstracts "polyaromatic" name for dieldrin since 1971,
considerable confusion can occur regarding the nomenclature of
metabolites.
The various alternative names for aldrin, dieldrin, and photo-
dieldrin are summarized in Table 48. A useful discussion of
nomenclature is given by Brooks (1974).
For convenience, in view of the much more extensive usage in
the literature of the former Chemical Abstracts names for aldrin
and dieldrin, the names of their metabolites in this review are
based (if appropriate) on the former Chemical Abstracts names of
the parent compounds given in Table 48. The names of the
metabolites are given in Table 49, together with some alternative
names based on either the current Chemical Abstracts name for
dieldrin or the von Baeyer/IUPAC system.
The possible misunderstandings that may occur, particularly
for those not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that may be
given to the major faecal metabolite of dieldrin. This one
compound may be designated:
(a) 9-hydroxy dieldrin (former CA system);
(b) 8-hydroxy dieldrin (current CA system); or
(c) 12-hydroxy dieldrin (von Baeyer/IUPAC system).
Table 48. Alternative chemical names for aldrin, dieldrin, and photodieldrin
---------------------------------------------------------------------------------------------------------------
Compounda Polyhydroaromatic name Polycyclic aliphatic name
Chemical Abstracts IUPAC (von Baeyer/IUPAC)
---------------------------------------------------------------------------------------------------------------
Aldrin Formerly: Formerly: 1,8,9,10,11,11-hexachloro-2,3-7,6-
(HHDN) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- endo-2,1-7,8-exo-tetracyclo[6.2.13,6
(I) 1,4,4a,5,8,8a-hexahydro- 1,4,4a,5,8,8a-hexahydro- 02,7] dodeca-4,9-diene
endo-1,4-exo-5,8- exo-1,4-endo-5,8-
dimethanonaphthalene dimethanonaphthalene
Currently: Currently:
1,2,3,4,10,10-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1 alpha,4 alpha,4a-beta, hexachloro-1,4,4a,5,8,8a-
5 alpha,8a,8a beta-hexahydro- hexahydro-1,4:5,8-
1,4:5,8-dimethanonaphthalene dimethanonaphthalene
Dieldrin Formerly: Formerly: 1,8,9,10.11,11-hexachloro-4,5-exo-
(HEOD) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- epoxy-2,3-7,6-endo-2,1-7,8-exo-
(II) 6,7-epoxy-1,4,4a,5,6,7,8,8a- 6,7-epoxy-1,4,4a,5,6,7,8,8a- tetracyclo[6.2.1.13,6.02,7] dodec-
octahydro-endo-1,4-exo- octahydro-exo-1,4-endo-5,8- 9-ene
5,8-dimethanohaphthalene dimethanonaphthalene
Currently: Currently:
3,4,5,6,9,9-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1a alpha,2 beta,2a alpha, hexachloro-1,4,4a,5,6,7,8,8a-
3 beta,6 beta,6a alpha,7 beta, octahydro-6,7 epoxy-1,4:5,8-
7a alpha-octahydro-2,7:3,6- dimethanonaphthalene
dimethanonaphth[2,3-b]oxirene
Photo- 1,1,2,3,3a,7a-hexachloro- 3,exo-4,5,6,6,7-hexachloro-11,12-
dieldrin 6,7-epoxy-2,4,7-metheno- exo-epoxy-pentacyclo[6.4.0.02,10.
(III) decahydro-3H-cyclopenta[a]- 03,7.05,9]-dodecane
pentalene
---------------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
Table 49. Chemical nomenclature of metabolites of aldrin and dieldrin
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
9-Hydroxy dieldrin 9-hydroxy-1,2,3,4,10,10-hexa- 9-(syn-epoxy)hydroxy-1,2,3,4,10,10-hexa-
(VI) (9-Hydroxy HEOD) chloro-6,7-epoxy-1,4,4a,5,6,7, chloro-6,7,-epoxy-1,4,4a,5,6,7,8,8a-octa-
8,8a-octahydro-1,4-endo-5,8-exo- hydro-1,4-endo,exo-5,8-dimethanonaphthalene
dimethanonaphthalene
8-hydroxy-3,4,5,6,9,9-hexachloro-1a alpha,
2 beta, 2a alpha, 3 beta, 6 beta, 6a alpha,
7 beta, 7a alpha-octahydro-2,7:3,6-
dimethanonapth[2,3-b]oxirene
1,8,9,10,11,11-hexachloro-4,5-exo-epoxy-12-
(synepoxy)hydroxy-2,3-7,6-endo-2,1-7,8-exo-
tetracyclo[6.2.1.13,602,7]dodec-9-ene
Aldrin trans-diol (IV) trans-6,7-dihydroxy-1,2,3,4,10,10- 1,8,9,10,11,11-hexachloro-4,5-trans-
hexachloro-1,4,4a,6,7,5,8,8a-hexa- dihydroxy-2,3-7,6-endo-2,1-7,8-exo-
hydro-1,4-endo-5,8-exo-dimethano- tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
naphthalene
Aldrin dicarboxylic 4,5,6,7,8,8-hexachloro-4,7-methano- 1,7,8,9,10,10-hexachloro-2,3-6,5-endo-
acid (V) 3a,4,7,7a-tetrahydro-indane-1,3- tricyclo[5.2.1.02,6]dec-8-ene-3,5-exo-
dicarboxylic acid dicarboxylic acid
Bridged pentachloro- 3,5,6,6,7-pentachloro-11,12-exo-
ketone (VII) (PCK, epoxy-pentacyclo[6.4.0.02,10.03,7
Klein's metabolite) .05,9]dodecan-4-one
Dechloro-aldrin 4,5,6,7,8-pentachloro-4,7-methano-
dicarboxylic 3a,4,7,7a-tetrahydro-indane-1,3-
acid (VIII) dicarboxylic acid
Dieldrin ketone (IX) 1,2,3,4,10,10-hexachloro-1,4,4a,5, 1,8,9,10,11,11-hexachloro-2,3-7,6-endo-
6,7,8,8a-octahydro-6-keto-endo- 2,1-7,8-exo-tetracyclo[6.2.1.13,6.02,7]-
1,4-exo-5,8-dimethanonaphthalene dodec-9-en-4-one
Photodieldrin 3-exo-4,5,6,6,7-hexachloro-
ketone (X) pentacyclo[6.4.0.02,10.03,7.05,9]
dodecan-11-one
---------------------------------------------------------------------------------------------------------
Table 49. (contd.)
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
Photodieldrin trans- 3,exo-4,5,6.6,7-hexachloro-11,12
diol (XI) (caged dihydroxy-pentacyclo[6.4.0.02,10.
aldrin trans-diol) 03,7.05,9]dodecane
Photoaldrin dicarbo- 1,7,8,exo-9,10,10-hexachlorotetra-
xylic acid (XII) cyclo[5.2.1.02,6.04,8]decane-3,5-
(caged aldrin acid) exo,exo-dicarboxylic acid
Photoaldrin (XIII) 3,exo-4,5,6,6,7-hexachloropenta-
cyclo[6.4.0.02,10.03,7.05,9]dodec-
11-ene
---------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
RESUME
1. Généralités
L'aldrine et la dieldrine qui sont l'une et l'autre des
pesticides organochlorés fabriqués industriellement depuis 1950,
ont été utilisés dans le monde entier jusqu'au début des années 70
comme insecticides en agriculture, contre de nombreux ravageurs
présents dans le sol, et pour le traitement des semences. Ces
insecticides étaient actifs contre les termites, les sauterelles,
les xylophages, les coléoptères et les ravageurs des textiles. La
dieldrine a également été employée en santé publique, pour la lutte
contre la mouche tsé-tsé et d'autres vecteurs de maladies
tropicales invalidantes. L'aldrine comme la dieldrine agissent par
contact et par ingestion.
Depuis le début des années 70, ces deux composés sont
interdits ou font l'objet de limitations rigoureuses dans un
certain nombre de pays, spécialement en agriculture. Néanmoins,
ils continuent d'être employés pour la destruction des termites
dans d'autres pays. La production annuelle mondiale, estimée à
13 000 tonnes en 1972, est tombée à moins de 2500 tonnes en 1984.
L'aldrine et la dieldrine de qualité technique ont une pureté
respective de 90% et plus de 95%. Les principales impuretés sont,
pour l'aldrine, l'octachlorocyclopentène, l'hexachlorobutadiène et
des produits de polymérisation et, pour la dieldrine, des
polychloroépoxy-octahydrodiméthanonaphtalènes.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans la plupart des alcanes, des
hydrocarbures aromatiques et des hydrocarbures halogénés, ainsi que
dans les esters, les cétones et les alcools.
La tension de vapeur de l'aldrine est de 6,5 x 10-5 mmHg à
25°C et celle de la dieldrine de 3,2 x 10-6 mmHg à 25°C.
Les méthodes utilisables pour le dosage de l'aldrine et de la
dieldrine dans les aliments, les aliments pour animaux et le milieu
sont décrites à la section 2.
2. Transport, distribution et transformation dans l'environnement
L'aldrine est principalement utilisée comme insecticide épandu
au niveau du sol. Les sols ainsi traités constituent donc dans
l'environnement une source importante d'aldrine et de son produit
de réaction, la dieldrine.
L'aldrine n'a qu'une faible capacité de migration à partir des
zones traitées, par volatilisation ou lessivage. Elle s'adsorbe
préférentiellement, et rapidement, sur les sols riches en matières
organiques, mais faiblement sur les sols argileux. Il est rare que
l'aldrine et la dielrine pénètrent au-delà des 20 premiers
centimètres de la couche de sol traité. L'aldrine adhère si
solidement aux particules du sol que seules des traces peuvent être
retirées par l'eau. C'est pourquoi il n'y a généralement pas
contamination des eaux souterraines.
L'aldrine s'élimine du sol selon une cinétique qui évoque une
réaction du premier ordre. Immédiatement après épandage, on
observe une courte période d'élimination rapide par volatilisation,
suivie d'une seconde période, plus longue, de décroissance
exponentielle, principalement du fait de la transformation en
dieldrine, plus lente à se dissiper. Néanmoins, il peut y avoir
une certaine migration du fait de l'érosion du sol, sous l'action
du vent, des eaux de ruissellement de du déplacement des sédiments.
Les observations faites sur les résidus d'aldrine dans la nature
montrent que, apparemment, ce composé est essentiellement retenu
dans le sol et que pour 97%, le résidu essentiel n'est pas le
composé d'origine mais l'époxyde correspondant, la dieldrine.
La photodieldrine est un produit de photodégradation de la
dieldrine, peu répandu dans l'environnement.
Après épandage sur le sol, l'aldrine disparaît lentement dans
les régions tempérées puisque, dans le cas type, il faut un an pour
qu'elle s'élimine aux trois quarts. La vitesse d'élimination
diminue ensuite à mesure que l'aldrine se transforme en dieldrine.
Il semblerait que la vitesse d'élimination soit plus élevée en
anaérobiose, comme c'est le cas dans les rizières, qu'en aérobiose.
Dans les régions tropicales, la dieldrine s'élimine du sol très
rapidement, jusqu'à hauteur de 90% au cours du premier mois, alors
que, dans le sol des régions tempérées, la dieldrine a une demi-vie
d'environ 5 ans. La volatilisation semble être le principal
mécanisme d'élimination à partir du sol, bien que la teneur
atmosphérique de la dieldrine et de l'aldrine soit généralement
faible. Une partie de la dieldrine est éliminée de l'atmosphère
par les précipitations, mais la concentration de ce produit est
très faible dans les eaux souterraines par suite de son adsorption
énergique sur les particules telluriques. On trouve de la
dieldrine en petites quantités dans les eaux de surface qui sont
contaminées par les eaux de ruissellement provenant de terres
agricoles.
3. Concentrations environnementales et exposition humaine
On trouve de l'aldrine et de la dieldrine dans l'atmosphère en
phase vapeur, adsorbées sur des poussières ou dans les eaux de
pluie, à des teneurs variables selon la situation. Ces produits
s'observent principalement dans les régions agricoles où leur
concentration atmosphérique moyenne est de l'ordre de 1 - 2 ng/m3
avec des maximums d'environ 40 ng/m3. Dans l'eau de pluie, on
relève des concentrations de l'ordre de 10 - 20 ng/litre ou parfois
plus.
Dans les habitations traitées avec ces produits contre les
termites, on observe une concentration dans l'air allant de 0,04 à
7 µg/m3, selon le moment de l'échantillonnage (c'est-à-dire le
nombre de jours après l'épandage) et le type d'habitation. Au bout
de 8 semaines, la concentration a très nettement diminué. Lorsqu'on
traite le bois en profondeur dans ces maisons, la concentration de
la dieldrine dans l'air va de 0,01 à 0,5 µg/m3. On a constaté que
l'aldrine et la dieldrine migraient dans les produits alimentaires
à partir de panneaux lamellés et de contreplaqués traités, ainsi
que par contact direct ou sorption à partir de l'atmosphère.
On a signalé la présence de dieldrine en milieu aquatique.
Mais les concentrations étaient très faibles, le plus souvent
inférieures à 5 ng/litre. Les concentrations plus élevées ont
généralement étéattribuées au rejet d'effluents industriels ou à
l'érosion du sol à la suite de l'utilisation de ces produits en
agriculture. Dans les cours d'eau, les sédiments peuvent présenter
des teneurs beaucoup plus élevées (allant jusqu'à 1 mg/kg).
On trouve rarement de l'aldrine dans les aliments, alors que
la dieldrine est plus courante, spécialement dans les produits
laitiers, les produits carnés, le poisson, les huiles et les
graisses, les pommes de terre et certains autres légumes
(principalement des légumes-racines). Des limites maximales de
résidus (LMR) de l'ordre de 0,02 - 0,2 mg/kg de produit ont été
recommandées lors des réunions conjointes successives FAO/OMS sur
les résidus de pesticides. Les études récentes réalisées dans
différents pays montrent que la concentration effective de la
dieldrine dans les denrées alimentaires est généralement plus
faible. Le recul est net au Royaume-Uni. En 1966 - 67, la
concentration moyenne des résidus de dieldrine observée lors d'une
étude sur la ration totale était de 0,004 mg/kg d'aliments tandis
que, pendant la période 1975 - 77, elle n'était plus que de 0,0015
mg/kg pour tomber à 0,0005 mg/kg en 1981. Cette évolution en
baisse est confirmée dans d'autres pays, par exemple aux Etats-Unis
d'Amérique. Cela tient peut-être à l'interdiction ou à la
limitation d'emploi de ces composés.
Dans un grand nombre de travaux publiés, on a recherché la
présence de dieldrine dans le tissu adipeux, les organes, le sang
et d'autres tissus, chez des sujets de la population générale. Au
cours des 25 dernières années, des enquêtes ont été réalisées dans
de nombreux pays, partout dans le monde. La plupart des
concentrations moyennes observées dans le tissu adipeux se situent
entre 0,1 et 0,4 mg/kg. Aux Etats-Unis d'Amérique, aux Pays-Bas et
au Royaume-Uni, on note une diminution de la concentration dans les
tissus adipeux depuis le milieu de la décennie 70. La
concentration sanguine varie de 1 à 2 µg/litre. Dans le foie, elle
est inférieure à 0,4 mg/kg tandis que, dans les autres tissus, à
savoir les reins, l'encéphale et les gonades, elle est inférieure à
0,1 mg/kg.
A la suite d'une exposition par voie transplacentaire, on
trouve de la dieldrine dans le sang, les tissus adipeux et d'autres
tissus du foetus et du nouveau-né. Les concentrations sont 2 à 10
fois plus faibles que chez la mère. Il n'existe aucune différence
entre le nourrisson et l'adulte pour ce qui est des concentrations
relatives de la dieldrine dans le cerveau, le foie et les tissus
adipeux. La dieldrine est également excrétée dans le lait
maternel. Depuis une quinzaine d'années, on recherche dans divers
pays la présence de pesticides organochlorés dans le lait de femme.
Dans la plupart des pays, la concentration plafonne à 6 µg/litre,
sauf cas exceptionnels.
4. Cinétique et métabolisme
Chez les animaux comme chez l'homme, l'aldrine et la dieldrine
passent rapidement des voies digestives dans le courant sanguin.
L'absorption a également lieu au niveau de la peau ou des poumons
après inhalation de la vapeur. Une étude sur des volontaires a
montré que la quantité résorbée par la peau intacte représente
7 - 8% de la dose appliquée. Selon des études d'inhalation
conduites sur des volontaires, le taux d'absorption et de rétention
de l'aldrine dans l'organisme peut atteindre 50% de la vapeur
inhalée. Après absorption, le composé se répartit rapidement dans
tous les organes et tissus, et il existe un échange permanent entre
le sang et les autres tissus. Entre-temps,l'aldrine se transforme
en dieldrine, principalement au niveau du foie mais aussi, dans une
moindre proportion, dans d'autres tissus comme les poumons. Cette
conversion est très rapide.
Après administration par voie orale d'une dose de 10 mg
d'aldrine par kg de poids corporel à des rats de 1 jour, on a
retrouvé ce composé dans le foie des animaux d'expérience 2 heures
après l'administration. Au cours des quelques heures suivantes, la
dieldrine s'est concentrée dans une beaucoup plus large mesure dans
les tissus lipidiques.
Comme l'ont montré de nombreuses études effectuées avec de
l'aldrine ou de la dieldrine marquées au 14C, une partie du produit
ingéré passe telle quelle dans l'intestin d'où elle est éliminée de
l'organisme, une partie est excrétée telle quelle à partir du foie
dans la bile, une autre fraction est stockée dans les divers
organes et tissus, en particulier le tissu adipeux, et une dernière
fraction est métabolisée dans le foie en produits à caractère
hydrophile et polaire plus prononcé. Chez l'homme et la plupart des
animaux, les métabolites sont principalement éliminés dans les
excreta, par l'intermédiaire de la bile. On a établi par ailleurs
que la biodégradation de l'aldrine et de la dieldrine aboutit aux
mêmes métabolites.
La plupart des connaissances actuelles sur le catabolisme de
la dieldrine chez les mammifères proviennent d'études chez la
souris, le rat, le lapin, le mouton, le chien, les petits singes,
le chimpanzé et l'homme. Dans l'ensemble, on n'observe que des
différences quantitatives entre les diverses espèces et les
mécanismes sont apparemment semblable chez le rat et les primates.
Le principal métabolite, sauf chez le rat, est le dérivé
hydroxylé en 9. On le trouve dans les déjections ainsi que dans
l'urine, sous forme libre ou conjuguée. On a découvert et
identifié chez les animaux d'expérience trois autres métabolites,
présents en petites quantités. Il s'agit d'un dérivé 6,7-
dihydroxylé en position trans, d'un acide dicarboxylique dérivé du
composé dihydroxylé et d'une pentachlorocétone pontée.
Seul le composé hydroxylé en 9 a été mis en évidence chez
l'homme, dans les matières fécales, tandis que ni ce composé ni les
autres métabolites n'apparaissaient dans le sang ou les autres
tissus. On a observé la présence de dieldrine dans les selles
d'ouvriers professionnellement exposés, tandis que, dans la
population générale, les quantités étaient inférieures au seuil de
détection. L'examen des urines de cinq ouvriers a montré que
l'excrétion de la dieldrine et de ses quatre métabolites par voie
urinaire était minime par rapport à l'élimination du métabolite
hydroxylé en 9 par voie fécale.
La transformation de l'aldrine en dieldrine dans le foie, sous
l'action de mono-oxygénases à fonction mixte (aldrine-époxydase) et
la distribution, puis le dépôt ultérieur, de la dieldrine
(principalement dans les tissus à contenu lipidique, tels que le
tissu adipeux, le foie, les reins, le coeur et le cerveau) sont
beaucoup plus rapides que le catabolisme et l'élimination finale de
la dieldrine intacte et de ses métabolites. Dans ces conditions,
pour un apport quotidien moyen déterminé d'aldrine ou de dieldrine,
il y a accumulation lente de dieldrine dans l'organisme. Mais
cette accumulation n'est pas indéfinie. Quand l'administration se
poursuit, on finit par aboutir à un état d'équilibre dynamique, la
quantité excrétée compensantexactement l'apport. La quantité
stockée maximale dépend de l'apport quotidien, tout comme on l'a
montré chez le rat, le chien et l'homme.
Quand l'apport d'aldrine/dieldrine est réduit ou interrompu,
la charge de l'organisme diminue. Chez l'homme, la demi-vie
biologique est de l'ordre de 9 à 12 mois. Chez le rat, le chien et
l'homme, on a démontré l'existence de relations significatives
entre la concentration de la dieldrine dans le sang et sa
concentration dans d'autres tissus.
De nombreuses études sur la concentration de la dieldrine dans
divers tissus, dont le sang et les tissus adipeux, aussi bien dans
la population générale que dans des catégories particulières, ont
été réalisées dans plusieurs pays et ont montré que, à l'équilibre,
les concentrations respectives dans les tissus adipeux, le foie, le
cerveau et le sang sont sensiblement proportionnelles à 150, 15, 3
et 1.
La dieldrine est transportée par le placenta jusqu'au foetus.
Il y a accumulation dans les mêmes organes et tissus que chez
l'adulte, mais en quantités moindres. Il existe apparemment un
équilibre entre les concentrations chez la mère et chez le foetus.
Chez le rat et le chien, la photodieldrine est également
métabolisée sous forme de pentachlorocétone pontée. On a retrouvé
les deux composés dans les tissus adipeux, le foie et les reins des
animaux à qui l'on avait administré de la photodieldrine à forte
dose. Chez l'homme, aucun résidu de ces composés n'a été mis en
évidence dans le tissu adipeux, les reins ni le lait maternel.
L'accumulation de photodieldrine dans les tissus adipeux des
animaux d'expérience était beaucoup moins importante que celle de
la dieldrine.
5. Effets sur les êtres vivants dans leur milieu natural
5.1 Accumulation
La plupart des résidus présents chez les êtres vivants sont
des résidus de dieldrine, car l'aldrine se transforme facilement
chez eux en dieldrine.
Les champignons, les streptomycètes et les bactéries
concentrent la dieldrine du milieu ambiant dans une proportion qui
peut aller en 4 h de 0,3 à plus de 100. Les protozoaires absorbent
la dieldrine davantage que les algues. Celles-ci absorbent très
rapidement la dieldrine présente dans le milieu de culture, les
concentrations maximales étant souvent atteintes en quelques
heures.
De nombreuses espèces d'invertébrés aquatiques concentrent
fortement la dieldrine à partir d'une eau à très faible teneur.
L'équilibre est atteint en quelques jours. Lorsqu'on les remet en
eau pure, la dieldrine s'élimine rapidement, avec une demi-vie de
60 - 120 h.
Pour les poissons entiers, le facteur de bioconcentration
dépasse 10 000. Chez une espèce de poissons, la demi-vie
d'élimination de la dieldrine accumulée s'est établie à 16 jours.
La bioconcentration de dieldrine chez les organismes
aquatiques se fait principalement à partir de l'eau et non par
ingestion d'aliments.
Les lombrics absorbent la dieldrine présente dans le sol et
laconcentrent jusqu'à un facteur maximal d'environ 170. Pour la
plupart des types de sol, il n'existe guère de corrélation entre la
concentra-tion atteinte chez le lombric et la concentration dans le
sol.
De nombreux travaux ont été consacrés à la présence de la
dieldrine dans les tissus ou dans les oeufs d'espèces non visées.
Les concentrations observées sont extrêmement variables, allant de
0,001 mg/kg à 100 mg/kg de tissu, mais elles restent le plus
souvent inférieures à 1 mg/kg de tissu.
Chez les oiseaux, il y a accumulation rapide de dieldrine
aussi bien dans les tissus que dans les oeufs. De même, on a
montré que diverses espèces de mammifères accumulent la dieldrine,
en particulier dans les graisses.
5.2 Toxicité pour les micro-organismes
La dieldrine a des effets très variables sur les algues uni-
cellulaires, avec une action sensible sur certaines espèces dès la
concentration de 10 µg/litre tandis que d'autres espèces ne sont
pas touchées même à la concentration de 1000 µg/litre. L'aldrine
et la dieldrine n'ont que peu d'effets sur les bactéries
terricoles, même à des concentrations très supérieures aux valeurs
habituelles. Dans la plupart des études, aucun effet n'a été
constaté après exposition à une concentration de 2000 mg/kg de
terre. Des effets ont été signalés sur la photosynthèse chez
différentes espèces d'algues, avec une action plus marquée de
l'aldrine que de la dieldrine à concentrations égales. Mais ces
effets minimes sur la biochimie des algues n'étaient que
transitoires.
5.3 Toxicité pour les organismes aquatiques
L'aldrine et la dieldrine sont extrêmement toxiques pour les
crustacés aquatiques, avec des valeurs de la DL50 à 96 h
inférieures à 50 µg/litre. Cependant, les quelques résultats plus
élevés signalés (jusqu'à 4300 µg/litre) illustrent les différences
de sensibilité selon les espèces. Les daphnies sont moins
sensibles à la dieldrine qu'à l'aldrine, avec des DL50 à 48 h de
23 - 32 µg/litre dans le premier cas et de 190 - 330 µg/litre dans
le second. Les mollusques sont nettement plus résistants, les CL50
à 48 h pouvant atteindre plus de 10 000 µg/litre. Des études de
plusieurs semaines ont confirmé la résistance relative des daphnies
et des mollusques. Les invertébrés aquatiques les plus sensibles
sont les stades larvaires des insectes, avec des CL50 à 96 h de
0,5 - 39 µg/litre pour la dieldrine et de 1,3 - 180 µg/litre pour
l'aldrine.
Lors d'épreuves de toxicité aiguë, l'aldrine comme la
dieldrine se sont montrées très toxiques vis-à-vis des poissons.
Chez diverses espèces de poisson, on a relevé des valeurs de la
CL50 à 96 h allant de 2,2 à 53 µg/litre pour l'aldrine et de 1,1 à
41 µg/litre pour la dieldrine. De nombreuses études ont montré que
la toxicité augmente avec la température. Dans une étude prolongée
sur Poecilia latipinna, on a obtenu un taux de mortalité de 100% en
présence d'une concentration de dieldrine égale ou supérieure à 3
µg/litre. L'addition de dieldrine à la nourriture de truites arc-
en-ciel jusqu'à des concentrations de 430 µg/kg de poids corporel
par jour, n'a exercé aucune influence sur la mortalité mais a
entraîné des modifications enzymatiques. Des altérations
morphologiques ont été observées aumicroscope électronique dans les
mitochondries hépatiques. Le mécanisme de détoxification de
l'ammoniaque chez les poissons est sensible à la dieldrine, la dose
sans effet nocif apparent étant inférieure à 14 µg/kg de poids
corporel par jour. La sensibilité à la dieldrine s'est révélée
variable selon le stade de développement des poissons. Les oeufs
étaient résistants et les formes juvéniles moins sensibles que les
adultes.
La toxicité aiguë de l'aldrine comme de la dieldrine est
élevée pour les larves d'amphibiens, avec des CL50 à 85 h de
l'ordre de 100 µg/litre.
5.4 Toxicité pour les organismes terrestres
La dieldrine est peu toxique pour les végétaux supérieurs
puisque les cultures ne sont affectées que par des doses
supérieures à 22 kg/ha. La phytotoxicité de l'aldrine est plus
importante, notamment pour les tomates et les concombres, mais
uniquement à des doses plusieurs fois supérieures aux valeurs
recommandées. Le chou est la plante cultivée la plus sensible à
l'aldrine.
Vis-à-vis des abeilles, la DL50 par voie orale varie, selon
les observations publiées, de 0,24 à 0,45 µg/abeille pour l'aldrine
et de 0,15 à 0,32 µg/abeille pour la dieldrine. Les quantités
toxiques par contact vont de 0,15 à 0,80 µg/abeille pour l'aldrine
et de 0,15 à 0,41 µg/abeille pour la dieldrine. D'après deux
études, la dieldrine est relativement plus toxique vis-à-vis des
insectes prédateurs qui se nourrissent de ravageurs.
Selon des études effectuées en laboratoire, le lombric
supporte des doses d'aldrine de 13 mg/kg en sol artificiel, le taux
de mortalité étant inférieur à 1%. La CL50 à six semaines était de
60 mg d'aldrine par kg de sol.
Pour 13 espèces d'oiseaux, la toxicité aiguë de l'aldrine et
de la dieldrine variait de plus du simple au décuple, avec des
valeurs de 6,6 - 520 mg/kg de poids corporel pour l'aldrine et de
6,9 - 381 mg/kg de poids corporel pour la dieldrine. Chez quatre
espèces d'oiseaux, la toxicité subaiguë par voie orale
correspondait à des doses comprises entre 34 et 155 mg/kg pour
l'aldrine et 37 et 169 mg/kg pour la dieldrine. Des épreuves
répétées au cours d'une certaine période n'ont révélé aucun signe
de résistance acquise chez ces espèces. D'après des études sur la
reproduction de plusieurs espèces de volaille, une concentration de
la dieldrine dépassant 10 mg/kg dans les aliments provoque une
certaine mortalité chez les adultes. Aucun effet ne s'est fait
sentir sur la production des oeufs, la fécondité, le taux
d'éclosion ni la survie des poussins en présence de dieldrine dans
les aliments à des concentrations qui ne sont pas toxiques pour la
mère. La dieldrine n'a aucune influence directe sur l'épaisseur de
la coquille des oeufs. Cependant, la diminution de la consommation
de nourriture, qui constitue un symptôme de l'intoxication par la
dieldrine, peut entraîner une diminution de l'épaisseur de la
coquille.
Chez le mammifères non élevés au laboratoire, la réponse à la
dieldrine varie selon les espèces. Chez quatre espèces de
campagnols, on a observé des valeurs de la DL50 aiguë, allant de
100 à 210 mg/kg de poids corporel, ce qui montre que ces animaux
sont moins sensibles àla dieldrine que les animaux de laboratoire.
Des musaraignes ont survécu à la consommation d'une nourriture
contenant 50 mg de dieldrine par kg mais sont mortes quand la
concentration est passée à 200 mg/kg. Des damalisques (une espèce
d'antilope) ont survécu 90 jours à une nourriture contenant de la
dieldrine à raison de 5 ou 15 mg/kg mais sont toutes mortes dans
les 24 jours pour une concentration égale ou supérieure à 25 mg/kg.
Tous les damalisques d'une région où l'on avait pulvérisé de la
dieldrine à raison de 0,16 kg/ha sont mortes et le calcul a montré
que l'apport alimentaire était de 1,82 mg/kg par jour. Trente pour
cent des springboks ont survécu aux épandages, sans manifester
d'effets tardifs. Les signes toxicologiques de l'intoxication par
la dieldrine étaient sensiblement les mêmes que chez les mammifères
de laboratoire.
5.5 Effets sur les populations et les écosystèmes
Certaines études donnent à penser que des populations de
mammifères ont été intoxiquées par de la dieldrine. Il est
probable que de petits mammifères sont morts après avoir mangé des
semences enrobées de dieldrine mais les populations se sont
reconstituées par immigration. Des chauves-souris ont été tuées
par la dieldrine contenue dans les agents de protection du bois.
Des résidus de dieldrine ont été signalés chez de nombreuses
espèces d'oiseaux. Partout dans le monde, c'est chez les oiseaux
de proie que les résidus sont les plus abondants car les animaux se
situent en fin de chaîne alimentaire. La teneur en dieldrine des
tissus et des oeufs d'oiseau suit l'évolution de l'emploi de
l'aldrine et de la dieldrine, et elle a diminué à la suite des
restrictions imposées à leur usage. Il n'est pas facile de repérer
les effets de la dieldrine car les résidus de cet insecticide
s'accompagnent de résidus d'autres organochlorés. La dieldrine est
plus toxique que le DDT pour les oiseaux et il est probable qu'elle
a provoqué chez les adultes une plus forte mortalité que le DDT.
Il est encore plus difficile de démontrer l'existence d'effets de
la dieldrine sur la reproduction à l'état naturel. En outre, il se
peut que les effets interviennent longtemps après l'exposition.
6. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
L'aldrine et la dieldrine sont extrêmement toxiques : pour ces
deux composés, la DL50 varie, chez la souris et chez le rat, de 40
à 70 mg/kg de poids corporel. Par voie percutanée, la dose toxique
se situe entre 40 et 150 mg/kg de poids corporel selon l'espèce en
cause et le solvant utilisé. On a constaté que l'aldrine et la
dieldrine de qualité technique déterminent chez le lapin une
irritation cutanée légère à intense, mais la cause en est le
solvant. Dans l'épreuve de maximalisation de Magnusson & Kligman
chez le cobaye, l'aldrine a provoqué un effet de sensibilisation.
Pourtant, au cours de 20 années de fabrication et de préparation
des formules, aucun cas de sensibilisation cutanée n'a été observé
dans un groupe comptant plus de 1000 travailleurs.
L'aldrine, comme la dieldrine, a une faible tension de vapeur
de sorte que, en principe, il n'y a aucun effet aigu par
inhalation. Les effets observés lors des études de toxicité aiguë
après exposition par toutes les voies possibles concernent le
système nerveux central etconsistent en hyperexcitabilité,
tremblements et convulsions.
Des études d'exposition par voie orale, de courte ou longue
durée, ont été réalisées avec l'aldrine et la dieldrine, chez la
souris, le rat, le chien, le hamster et les petits singes. Chez le
rat et la souris, le foie est le principal organecible : on observe
une augmentation de son poids par rapport au poids du corps et une
hypertrophie des hépatocytes centrilobulaires, la réversibilité
étant possible à un stade précoce. Au microscope, ces altérations
se traduisent par une augmentation de l'oxyphilie cytoplasmique et
une migration périphérique des granules basophiles. Ces
altérations ne se rencontrent pas au niveau du foie chez le hamster
et le singe. Chez le chien, l'atteinte hépatique est peu prononcée
(dégénérescence graisseuse et légère atrophie des hépatocytes);
elle s'accompagne d'une atteinte rénale consistant dans une
vacuolisation de l'épithélium des tubules distaux et une
dégénérescence tubulaire. Chez le rat, la dose sans effet nocif
observable se situe dans l'ensemble, d'après les résultats dont on
dispose sur le court et le long terme, aux alentours de 0,5 mg/kg
de nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
En augmentant les quantités incorporées à la nourriture, jusqu'à
obtenir l'équivalent de 0,05 mg/kg de poids corporel ou davantage,
on observe une hépatomégalie et des altérations histologiques
d'importance proportionnée à la dose. Chez le chien une dose de
0,04 - 0,2 mg/kg de poids corporel s'est révélée sans effet.
Plusieurs études de cancérogénicité à long terme ont été
effectuées sur différentes souches de souris, avec de l'aldrine ou
de la dieldrine. Chaque fois, on a observé des tumeurs
hépatocellulaires bénignes ou malignes. Apparemment, les femelles
étaient plus sensibles que les mâles. Aucun autre type de tumeur
ne s'est manifesté dans ces études.
Des études à long terme sur d'autres espèces (rat, hamster)
n'ont révélé aucune augmentation de l'incidence tumorale.
L'administration de photodieldrine incorporée à la nourriture,
jusqu'à une concentration de 7,5 mg/kg d'aliments, ne s'est pas
révélé tumorigène.
En outre, on a publié un certain nombre d'études spéciales qui
n'ont, jusqu'ici, pas permis d'élucider le mécanisme de la
production des tumeurs hépatiques chez la souris.
Dans la plupart des études de reproduction (sur 1 à 6
générations), réalisées avec l'aldrine ou la dieldrine sur des
souris et des rats, le principal effet constaté a été
l'augmentation du taux de mortalité dans la descendance, avant
sevrage. La capacité génésique n'a été atteinte qu'à des doses
toxiques pour la mère. Les études sur le chien étaient trop
limitées pour permettre les conclusions catégoriques, si ce n'est
qu'on a noté une augmentation systématique de la mortalité des
chiots à la mamelle.
D'après les résultats de ces études sur la reproduction, on
peut conclure que, de ce point de vue, les doses sans effet nocif
décelable sont de 2 mg de dieldrine par kg de nourriture chez le
rat et de 3 mg de dieldrine par kg de nourriture chez la souris,
soit l'équivalent quotidien de 0,1 et 0,4 mg/kg de poids corporel
respectivement.
Aucun signe de tératogénicité n'a été observé chez la souris,
le rat ou le lapin, après administration par voie orale de doses
d'aldrineet de dieldrine atteignant 6 mg/kg de poids corporel.
L'administration d'une dose unique d'aldrine et de dieldrine,
représentant environ la moitié de la DL50, a provoqué des effets
toxiques intenses chez le foetus de souris et de hamster, ainsi
qu'une incidence accrue d'anomalies tératogènes. La signification
de ces observations est douteuse en présence d'effets toxiques
probables chez les femelles gravides.
Les études de mutagénicité in vivo ou in vitro ont été
nombreuses, mais elles ont presque toujours donné des résultats
négatifs.
La toxicité aiguë de la photodieldrine par voie orale est plus
élevée que celle de la dieldrine chez la souris, le rat et le
cobaye. Lors d'études de toxicité aiguë ou à long terme, on a
observé des symptômes d'intoxication et des effets sur les organes
cibles analogues à ceux de la dieldrine, tant sur le plan
quantitatif que sur le plan qualitatif. La photodieldrine ne s'est
pas montrée tumorigène chez la souris ni chez le rat.
Comme la plupart des autres substances chimiques, l'aldrine et
la dieldrine exercent leurs effets toxiques selon plusieurs
mécanismes. Les organes cibles sont le système nerveux central et
le foie. Chez l'homme et les autres vertébrés, l'intoxication
secondaire à une exposition aiguë ou chronique, se caractérise par
des mouvements musculaires involontaires et des convulsions
épileptiformes. En cas de survie, la récupération est totale après
une courte durée marquée par des symptômes résiduels. Au niveau du
foie, on observe une activité accrue des enzymes microsomales de
biotransformation, en particulier du système enzymatique cytochrome
P-450/monooxygénase. Cette induction des enzymes mircosomales est
réversible et, au-delà d'un certain niveau, elle semble liée aux
altérations cytoplasmiques au niveau du foie et à l'hépatomégalie
chez les rongeurs.
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut penser que, en pratique, ces produits ne contribuent guère à
l'incidence des cancers humains.
7. Effets chez l'homme
L'aldrine et la dieldrine sont très toxiques pour l'homme. Il
y a eu de graves cas d'intoxication accidentelle ou professionnelle
mais il est rare qu'ils aient fait des victimes. La plus faible
dose ayant provoqué une issue fatale a été estimée à 10 mg/kg de
poids corporel. Les personnes ayant survécu à une intoxication
aiguë ou subaiguë se sont complètement rétablies. Aucun effet
irréversible ni atteinte anatomopathologique résiduelle n'a été
signalée.
Les effets nocifs de l'aldrine et de la dieldrine sont
fonction de la concentration de la dieldrine dans le sang. Le
dosage de la dieldrine dans le sang permet de diagnostiquer avec
précision une exposition à l'aldrine/dieldrine. Chez les
travailleurs, le taux sanguin au-dessous duquel on n'observe aucun
effet nocif (dose limite sans effet nocif décelable) est de 105
µg/litre de sang. Cela correspond à un apport quotidien de
dieldrine de 0,02 mg/kg de poids corporel.
L'exposition environnementale (principalement par
l'intermédiairedes aliments mais aussi, dans une faible mesure, par
voie respiratoire) entraîne l'apparition de dieldrine à une très
faible concentration dans les organes, le sang et le lait maternel.
Autant qu'on puisse en juger d'après des études épidémiologiques et
cliniques poussées, il n'existe aucun raison de penser que les taux
couramment observés dans l'organisme constituent une menace pour la
santé de la population en général. Lors d'une étude poursuivie
pendant plus de 20 ans auprès de 1000 travailleurs de l'industrie,
employés dans une fabrique d'insecticides à base d'aldrine/dieldrine,
aucune augmentation de l'incidence des cancers n'a été observée chez
les sujets fortement exposés à ces deux produits. Phénomène encore
plus significatif, aucun signe avant-coureur, sous forme d'une
altération de la fonction hépatique, n'a été observé.
Une étude épidémiologique sur la mortalité a été réalisée dans
une unité de production aux Etats-Unis, sur une cohorte de 870
travailleurs exposés à l'aldrine, à la dieldrine et à l'endrine.
Malgré près de 25 000 années-homme d'observation, il n'a pas été
possible de repérer un risque particulier de cancer attributable au
travail dans cette usine.
EVALUATION DES DANGERS POUR LA SANTE DE L'HOMME ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des dangers pour la santé de l'homme
L'aldrine et la dieldrine sont des pesticides organochlorés
qui ont été utilisés partout dans le monde entre 1950 et le début
des années 70 comme insecticides en agriculture et pour le
traitement des semences, pour la destruction des ravageurs
terricoles et d'autres types d'insectes (par exemple les termites,
les sauterelles et les ravageurs des textiles) ainsi que pour la
lutte contre les glossines et autres vecteurs de maladies. Chez
les insectes, ces composés exercent leur effet toxique par contact
et par voie digestive. A partir des années 70, on en a limité ou
interdit l'emploi dans plusieurs pays, spécialement en agriculture.
Pourtant, ils continuent d'être utilisés dans d'autres pays pour la
destruction des termites.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans de nombreux solvants organiques.
Leur tension de vapeur est faible.
On trouve souvent de la dieldrine dans des produits laitiers
ou carnés, le poisson, les huiles et les graisses et certains
légumes, notamment des légumes-racines. La limite maximale de
résidus recommandée par les instances compétentes de la FAO/OMS
lors des réunions conjointes sur les résidus de pesticides varie de
0,02 à 0,2 mg/kg de produit. Des mesures récentes ont montré que
les teneurs effectives sont plus faibles, comme l'ont d'ailleurs
confirmé les études sur la ration globale. Comme l'utilisation de
ces deux composés fait maintenant l'objet de restrictions, on
observe une diminution lente mais régulière de la teneur en résidus
des différentes denrées alimentaires.
Les quantités ingérées par l'homme avec sa ration quotidienne
se traduisent, malgré la faible concentration de ces produits dans
les aliments, par la présence de dieldrine dans le tissu adipeux et
dans certains autres tissus et organes. Des enquêtes à l'échelle
mondiale montrent que les teneurs moyennes varient de 0,1 à 0,4
mg/kg de tissu adipeux. Depuis le début des années 70, cette
concentration diminue lentement.
Comme le foetus est exposé par voie transplacentaire, ses
tissus adipeux contiennent également de la dieldrine, mais à une
concentration qui n'est que de 10 à 50% de la concentration chez la
mère. Il semble exister un équilibre entre les concentrations
foetales et les concentrations maternelles. La dieldrine est
également excrétée dans le lait. Une exposition est possible par
inhalation dans les habitations où l'on utilise ce produit pour la
destruction des termites. Après traitement, on observe des
concentrations atmosphériques allant de 0,01 à 7 µg/m3, selon le
mode d'épandage, la concentration utilisée, les modalités de
l'aération et le moment où les échantillons sont prélevés. En
pareilles circonstances, les aliments peuvent aussi être
contaminés, par contact direct, ou par sorption à partir de l'air
ambiant.
Le métabolisme s'effectue principalement dans le foie où
l'aldrine se transforme rapidement en dieldrine. Le catabolisme de
la dieldrineest plus lent que celui de ses métabolites hydrophiles
qui sont excrétés dans la bile et dans les urines. La structure de
ces métabolites a été établie. Chez toutes les espèces étudiées,
notamment l'homme, on a montré que les quantités
d'aldrine/dieldrine accumulées se stabilisent à un niveau qui est
fonction de l'apport puisqu'il existe une relation linéaire entre
les quantités accumulées et le logarithme de l'apport. Quand
l'exposition prend fin, la concentration de la dieldrine dans les
tissus de l'organisme diminue selon une loi exponentielle. La
toxicité aiguë de l'aldrine et de la dieldrine est importante chez
les mammifères par voie orale, tandis que la toxicité par voie
cutanée est modérée. Aucune sensibilisation cutanée n'a été
observée. Les effets constatés à la suite d'une exposition
expérimentale aiguë ou de courte ou longue durée intéressent le
système nerveux central. Le foie est également un organe cible.
Chez les souris et les rats, on observe à ce niveau des altérations
désignées sous le nom de "foie de rongeur sous insecticide
organochloré".
Apparemment, l'aldrine et la dieldrine ne sont pas tératogènes
à des doses inférieures à celles qui sont toxiques chez la femelle
gravide et chez le foetus. On n'a pas fait état de toxicité pour
la fonction de reproduction chez le mâle ou la femelle.
De nombreuses études de mutagénicité in vitro et in vivo ont
montré que ni l'aldrine ni la dieldrine ne sont mutagènes.
Lors d'études à long terme, ces deux produits ont déterminé
chez la souris des tumeurs hépatiques, bénignes ou malignes. En
revanche, aucune augmentation de l'incidence des tumeurs hépatiques
ou autres n'a été observée chez le rat ni le hamster.
Selon le CIRC (1987), il n'existe pas de preuves suffisantes
d'un pouvoir cancérogène chez l'homme et les preuves de
cancérogénicité chez l'animal d'expérience sont limitées.
L'aldrine comme la dieldrine ont été classées dans le groupe 3, à
savoir celui des produits chimiques dont il est impossible de
préciser le pouvoir cancérogène chez l'homme.
Compte tenu des résultats obtenus lors des études de toxicité
de courte ou de longue durée, la dose globale sans effet nocif
décelable se situe chez le rat à 0,5 mg de dieldrine par kg de
nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
Chez le chien, la dose sans effet nocif décelable est de 0,04 mg/kg
de poids corporel. Lors des réunions conjointes FAO/OMS de 1966 et
1977 sur les résidus de pesticides, on a fixé la dose journalière
admissible (DJA) à 0,1 µg/kg de poids corporel, en tenant compte de
la non-cancérogénicité de ces deux substances pour l'homme.
L'aldrine et la dieldrine sont très toxiques pour l'homme. On
connaît des cas d'intoxication accidentelle ou professionnelle,
mais qui ont rarement fait des victimes. Les survivants à une
intoxication aiguë ou subaiguë se sont entièrement rétablis. Les
effets nocifs sont fonction de la concentration sanguine de la
dieldrine, dont le dosage permet de diagnostiquer avec précision
une exposition à l'aldrine/dieldrine. Pour taux sanguins inférieur
à 105 µg/litre, aucun effet indésirable n'est à craindre. Cette
concentration constitue la dose limite sans effet nocif décelable
et correspond à un apport quotidien de 0,02 mg de dieldrine par kg
de poids corporel.
L'exposition liée à l'environnement, principalement par la voie
alimentaire, entraîne la présence de faibles concentrations de
dieldrine dans l'organisme. D'après des études épidémiologiques et
cliniques poussées, ces teneurs ne constituent pas une menace pour
la santé humaine.
Aucun signe avant-coureur d'une altération de la fonction
hépatique n'a été observé lors d'une enquête de 20 ans portant sur
plus de 1000 ouvriers de l'industrie exposés à l'aldrine et à la
dieldrine. Dans cette étude ainsi que dans une autre effectuée aux
Etats-Unis d'Amérique, aucun risque particulier de cancer n'a été
repéré chez les personnes profesionnellement exposées à l'aldrine
et à la dieldrine (parfois à de fortes concentrations).
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut estimer que, en pratique, ces produits chimiques ne
contribuent que très peu, sinon pas du tout, à l'incidence des
cancers humains.
La photodieldrine, produit qui résulte de la dégradation de la
dieldrine sous l'action de la lumière, est analogue à la dieldrine
pour ce qui est de sa toxicité sur une courte durée. Elle n'est ni
tératogène ni cancérogène chez la souris et le rat. L'accumulation
de photodieldrine dans les tissus adipeux d'animaux d'expérience
s'est révélée inférieure à celle de la dieldrine.
2. Evaluation des effets sur l'environnement
La principale source de dieldrine (jusqu'à 97%) dans
l'environnement est l'aldrine, un insecticide épandu au niveau du
sol. L'aldrine et son produit de réaction, la dieldrine sont
rapidement absorbé par les sols, spécialement ceux qui sont riches
en matières organiques. De ce fait, la pénétration est limitée et
il n'y a généralement aucune contamination des eaux souterraines.
Les deux composés sont entraînés principalement du fait de
l'érosion (sous l'action du vent) et du transport des sédiments
(eaux superficielles de ruissellement), mais non par lessivage.
L'emploi d'aldrine et de dieldrine en agriculture donne lieu à
la présence de résidus (principalement de dieldrine) dans le sol où
ils peuvent persister plusieurs années; la demi-vie de la dieldrine
est estimée à 4 - 7 ans. La persistance de ces composés est
moindre dans les régions tropicales que dans les régions tempérées.
L'aldrine et la dieldrine passent, par volatilisation, des
récoltes et du sol traités à l'atmosphère; elles peuvent aussi y
pénétrer directement lors de l'épandage. La dieldrine retourne au
sol ou dans les étendues d'eau par les précipitations ou par dépôt
de particules sèches. Les composés se rencontrent donc soit en
phase vapeur (à des concentrations très faibles, en général de
l'ordre de 1 - 2 ng/m3), soit adsorbés sur des particules de
poussière, soit encore dans les eaux de pluie (à des concentrations
de l'ordre de 10 - 20 ng/litre).
Plusieurs auteurs ont signalé la présence de dieldrine en
milieu aquatique. Dans les eaux de surface, les concentrations
sont le plus souvent très faibles, inférieures à 5 ng/litre. Mais
des valeurs plus élevées s'observent dans les régions soumises à
l'érosion ou danscelles où l'on utilise ce produit en agriculture.
Dans ces régions, les sédiments des cours d'eau peuvent renfermer
jusqu'à 1 mg de dieldrine par kilogramme. La forte capacité qu'ont
les organismes aquatiques à concentrer la dieldrine à partir de
teneurs très faibles peut aboutir à l'accumulation de doses
toxiques. La concentration de ce produit tout au long de la chaîne
alimentaire aquatique est moins importante qu'une absorption
directe à partir de l'eau.
Comme la dieldrine est très répandue dans l'environnement et
qu'elle y persiste, on observe des concentrations très variées chez
les organismes non visés. Alors qu'auparavant les valeurs
observées allaient de 0,001 mg à 100 mg/kg de tissu, elles sont
aujourd'hui le plus souvent inférieures à 1 mg/kg de tissu.
Dans les écosystèmes terrestres, l'aldrine et la dieldrine
s'accumulent chez divers organismes, principalement sous forme de
dieldrine. Cette dernière est probablement responsable de la mort
de mammifères dans la nature et de la raréfaction de certaines
espèces, comme la loutre. Certains petits mammifères périssent
sans doute après avoir mangé des céréales traitées mais il est
probable que leurs populations se reconstituent par immigration à
partir des zones voisines. Les oiseaux de proie qui mangent de
petits mammifères et de petits oiseaux contaminés par la dieldrine
absorbent et concentrent cet insecticide dans leurs tissus et leurs
oeufs. Des oiseaux granivores ont été tués par la consommation de
céréales traitées. Il est probable que la raréfaction des oiseaux
de proie s'explique par la présence dans leurs tissus de résidus de
dieldrine (entre autres organochlorés). Les effets de la dieldrine
se manifestent avec un certain retard car les résidus s'accumulent
dans les graisses pendant l'hiver d'où ils ne se libèrent qu'au
printemps. Le fait de n'utiliser la dieldrine qu'à certaines
époques de l'année n'a pas réduit la mortalité des oiseaux.
La large utilisation d'aldrine et de dieldrine, parallèlement
à celle d'autres organochlorés, a exercé des effets très nocifs sur
l'environnement mais grâce à des restrictions draconiennes,
particulièrement en ce qui concerne les semences traitées, les
populations d'oiseaux commencent à se reconstituer.
3. Conclusions
a) L'aldrine et la dieldrine ont donné lieu à des études
poussées et variées sur le plan toxicologique, clinique et
épidémiologique. La charge de l'organisme résulte principalement
de l'ingestion de résidus présents dans la nourriture (les
quantités ingérées semblant diminuer de façon générale et tomber
en-dessous des DJA fixées) et, dans une moindre mesure, de
l'inhalation de ces produits. D'après l'étude des données, on a
tout lieu de penser que la charge de l'organisme résultant du
niveau actuel d'exposition ne menace en aucun cas la santé de la
population dans son ensemble.
b) La dieldrine se rencontre presque partout dans le lait
maternel. Mais, sa concentration dans le sang et dans le tissu
adipeux des nourrissons n'augmente pas avec l'âge au cours des six
premiers mois de leur vie et le taux sanguin n'est pas plus élevé
que chez un enfant nourri au biberon. Dans ces conditions,
l'allaitement au sein reste la méthode de choix pour nourir les
nourissons malgré la présence de résidus de dieldrine.
c) Lors du traitement de locaux, notamment pour la destruction
des termites, l'exposition des occupants ne semble pas présenter
des risques pour leur santé, pour autant que le traitement
s'effectue correctement.
d) Malgré leur toxicité élevée, l'aldrine et la dieldrine
peuvent être manipulées sans danger dans la mesure où l'on observe
toujours les précautions recommandées en vue de réduire au minimum
l'exposition des opérateurs. Dans le cas contraire, il y a risque
d'intoxication.
e) Pendant la période où l'on a massivement utilisé l'aldrine
et la dieldrine, c'est-à-dire de 1950 au début des années 70, il
est certain que cette pratique a eu des effets dommageables sur
diverses espèces. Ces effets sont imputables en partie à la
dieldrine à côté d'autres organochlorés. Depuis qu'on a limité de
façon draconienne l'utilisation de ces produits, les espèces
touchées se sont reconstituées.
RECOMMENDATIONS
1. Il faut effectuer des études de tératogénicité
complémentaires sur le hamster, bien conçues, avec des doses
réalistes de dieldrine.
2. Dans l'étude du mécanisme de la cancérogenèse, on
s'efforcera d'expliquer pourquoi les réactions hépatiques sont si
différentes chez la souris et chez les autres espèces.
3. On continuera d'utiliser la dieldrine pour l'étude des
mécanismes neurotoxiques, à la fois sur le plan expérimental et sur
le plan clinique.
4. Pour des raisons écologiques, toute reprise d'une
utilisation massive d'aldrine et de dieldrine est exclue, et on
n'utilisera ces produits que s'il n'existe pas de produit moins
nocif d'efficacité équivalente.
5. Afin de préserver la santé et le bien-être des travailleurs
et de la population en général, il convient de ne confier la
manipulation et l'épandage de l'aldrine et de la dieldrine qu'à des
opérateurs compétents et dûment formés, qui devront appliquer les
mesures de sécurité qui s'imposent.
6. En raison du risque d'intoxication accidentelle par
l'aldrine, spécialement chez les enfants, il faut en interdire
l'utilisation sous forme de granulés contre les fourmis.
Table 45 gives a non-exhaustive overview of occupational aldrin
and dieldrin poisonings. Hayes (1982) contains further information
on this subject.
Table 45. Published case reports on occupational aldrin and dieldrin poisoning
------------------------------------------------------------------------------------------
Number Fatal Acute Causative agent Circumstances Reference
of cases cases
cases
------------------------------------------------------------------------------------------
3 - ? 25% aldrin dust formulation with inadequate Nelson (1953)
safety precautions
~100 - ? various spraying in malaria- Hayes (1957,
eradication programmes 1959)
1 - 1 aldrin gross overexposure in aldrin Bell (1960)
packer
1 1 ? dieldrin sprayer Van Raalte
(1965)
4 - ? aldrin formulation of aldrin Kazantzis et
al. (1964)
17 - some aldrin and manufacturing and formulation Hoogendam et
dieldrin al. (1962,
1965)
32 (15 in - some aldrin, manufacturing and formulation Jager (1970)
addition dieldrin,
to endrin,
previous isobenzan
reference)
------------------------------------------------------------------------------------------
No cases of fatal poisoning have been reported during the
manufacture and formulation of aldrin and dieldrin (Jager, 1970).
However, there has been one case of a spray operator being fatally
poisoned (Van Raalte, 1965).
In developing countries it is, however, difficult to establish
the actual number of poisoning cases. Experience shows that even
when pesticides are banned, cases may occur in areas where control
is poor and where large quantities of pesticide are stocked. In
May 1987, four cases of occupational poisoning with aldrin were
reported from an area of cocoa plantations in Bahia (Brazil). One
of them was fatal, while three recovered. The fatal case had a
dieldrin level in whole blood exceeding 600 µg/litre two days after
poisoning (Rahde, personal communication).
9.2.1.1 Blood levels diagnostic of aldrin/dieldrin poisoning
Because the symptoms of aldrin/dieldrin intoxication are non-
specific, a differential diagnostic test is required to confirm
that the symptoms, signs, and clinical course of a particular case
are the result of aldrin or dieldrin intoxication.
The results of animal studies, together with those obtained
subsequently during medical surveillance of workmen employed in the
manufacture (or formulation) of aldrin/dieldrin, have shown that
adverse effects induced by aldrin/dieldrin are related to the
dieldrin blood concentration (Brown et al., 1964; Jager, 1970).
Therefore, the determination of this concentration provides a
specific differential diagnostic test.
Concentrations of dieldrin ranging from 40 to 530 µg/litre have
been reported in the blood of people who had been poisoned
relatively recently and who had recovered (Kazantzis et al., 1964;
Jager, 1970; Avar & Czegledi-Janko, 1970; Siyali & Simson, 1973).
From the limited information available, Brown et al. (1964)
concluded that the threshold concentration of dieldrin in the blood
of human beings, critical for intoxication, is approximately
150 - 200 µg/litre.
Dieldrin is present in the blood at very low concentrations in
the general population of many countries throughout the world. It
is also found at considerably higher concentrations in the blood of
healthy workers. These "healthy workmen" were men between 18 and
60 years of age who had no complaints or clinical or laboratory
signs attributable to occupational exposure, but they were, of
course, subject to the same ailments and diseases as are members of
the general population (Hayes & Curley, 1968; Jager, 1970). No
complaints of ill health and no positive results in objective
clinical or laboratory tests of ill health have ever been noted in
workers whose blood contained less than 200 µg/litre. This
concentration may, therefore, be considered to be a no-observed-
adverse-effect level in human beings. Higher concentrations may
have effects. The maximum concentration reported to be without
complaints or clinical signs or symptoms was 430 µg/litre (Jager,
1970).
Studies on animals have shown that the earliest physiological
sign of exposure to dieldrin is an increase in the activity of
certain liver microsomal enzymes. No enzyme induction has ever
been found in workers with dieldrin blood concentrations at or
below 105 µg/litre (Jager, 1970). (When liver enzyme induction
test methods became available, workers with dieldrin levels in the
blood exceeding 105 µg/litre were no longer encountered.)
Sometimes, dieldrin concentrations in plasma or serum, rather
than in whole blood, have been reported. The ratio of the dieldrin
concentration in plasma to that in erythrocytes is approximately
4:1 (Dale et al., 1965; Mick et al., 1972) (the conversion factor
to calculate the concentration of dieldrin in whole blood from the
concentration in plasma or serum is 0.66).
Great differences exist between the average concentrations of
dieldrin in the blood of the general population and of
occupationally exposed workers with or without complaints (Table
46).
Table 46. Concentrations of dieldrin in whole blood of human beings (µg/litre)
-------------------------------------------------------------------------------
No. of
Subjects persons Geometric Range Reference
involved mean
-------------------------------------------------------------------------------
General population 4592 <1 <1-16.1a US EPA (1983)
Unexposed persons 25 0.5 0-3.3 Sandifer et al. (1981)
20 2.5 0.5-10 Brown et al. (1964)
Healthy workers 35 29 <10-90 Jager (1970)
21 120e Mick et al. (1972)
37 55 -f Morgan & Roan (1974)
27 20 4.5-54 Sandifer et al. (1981)
89 38g 2-220 Brown et al. (1964)
Patients with 18 160g 8-280 Avar & Czegledi-Janko
clinical symptoms (1970)
4 40-530b Kazantzis et al. (1964)
5 130-370c Brown et al. (1964)
5 160-430d Brown et al. (1964)
Deceased (suicide) 1 850 Hayes (1982)
-------------------------------------------------------------------------------
a In serum.
b The low level of 40 µg/litre was found in a man with chronic nephritis,
complaining of headache and nausea; the occupational cause of the symptoms
is, therefore, doubtful. In one other worker, a blood level of 530 µg/litre
was found 1 month after a mild acute episode when he was exposed to aldrin
again.
c Determined some time after the acute episode.
d Estimated to be the concentration at the time of the acute episode.
e Converted from plasma figures using a factor of 0.66 (see 9.2.1.1).
f Highest value 231 kg/litre.
g Average.
Among 13 adolescent patients with colon-rectal adenocarcinomas,
who had lived in rural areas of Mississippi, USA, where pesticides
are widely sprayed, dieldrin blood levels were no higher than those
in controls (Caldwell et al., 1981).
9.2.1.2 Electroencephalography
Changes in the electroencephalogram (EEG) are sometimes of
practical importance for confirming a diagnosis of aldrin/dieldrin
intoxication (Spiotta, 1951; Winthrop & Felice, 1957; Hoogendam et
al., 1962, 1965; Kazantzis, 1964; Avar & Czegledi-Janko, 1970;
Jager, 1970). These EEG changes were first used as a practical
tool for monitoring workers and determining when they should
discontinue exposure and when they could be allowed to resume work
with aldrin/dieldrin. Characteristic changes - which, however, are
not pathognomonic for aldrin/dieldrin poisoning - include bilateral
synchronous spikes, spike and wave complexes, and slow theta waves,
thought to be possibly associated with brain stem stimulation
(Hoogendam et al., 1962, 1965). The interesting parallelism
between the rate of diminution of the EEG changes and the rate of
decrease in the dieldrin blood concentration was also reported in
the case of an accidentally poisoned child (Garrettson & Curley,
1969).
Nowadays, analysis for dieldrin in the blood has replaced EEG
examination as the method of choice for monitoring exposed workers
(Jager, 1970).
9.2.2. Effects of short- and long-term exposure
Occupational exposure occurred in the 1950s and early 1960s
among sprayers in malaria and yellow fever control programmes.
These men sprayed dieldrin inside houses day after day in prolonged
cycles without appreciable intervals of non-exposure. Quite often,
precautions and supervision were less than would be required today.
At the time, methods for the determination of blood concentrations
had not been developed. A significant percentage of these sprayers
became sick after having worked for as little as 2 days or as much
as 2 years (Hayes, 1957, 1959; Patel & Rao, 1958; Zavon & Hamman,
1961). According to communications by the Pan-American Sanitary
Bureau (Soper, 1955a), it appeared that no clinical symptoms were
observed in well-executed, well-supervised malaria and yellow fever
control programmes.
In a study carried out in East Africa, where workers were
spraying dieldrin 6 h/day for 180 days per year (with an interim of
2 months between spraying cycles) no clinical symptoms were seen.
The potential average dermal exposure of spray operators who
observed the protective measures laid down was 1.8 mg/kg body
weight per day (Fletcher et al., 1959).
Long et al. (1969) studied 159 farmers in Iowa, USA. Extensive
clinical and laboratory examinations of 33 pesticide users among
these farmers did not reveal evidence of any disease that could be
attributed to the use of pesticides, neither was the dieldrin blood
concentration correlatable with any parameter examined.
-------------------------------------------------------------------
a Personal communication at the 2nd Meeting of the Industrial
Council on Tropical Health, Boston.
The dieldrin blood concentrations in 8 locust-control workers
in Ethiopia were measured on two occasions and were found to range
between 0 and 9 µg/litre (MacCuaig, 1976).
Wolfe et al. (1963) studied the hazards from spraying orchards
with dieldrin in the US Pacific Northwest. Potential contamination
of the skin and respiratory exposure were measured. From the
results, potential skin and respiratory exposures were calculated
to amount to 14.2 and 0.25 mg/h, respectively.
Princi & Spurbeck (1951) studied a group of workers exposed
to chlordane, aldrin, and dieldrin for several years in a
manufacturing and formulating plant. The atmospheric
concentrations of aldrin were reported to be as high as 2.6 mg/m3.
Physical examinations and chest-röntgenograms did not reveal
respiratory anomalies.
A study was carried out on 71 men employed in the manufacture
and formulation of aldrin, dieldrin, endrin, and some other non-
related pesticides. Twenty-eight of these workers each contributed
a sample of blood and a sample of fat on the same day. The average
concentration of dieldrin in fat (6.12 ± 1.24 mg/kg) was 247 times
greater than the mean plasma concentration (0.025 ± 0.006
mg/litre). There was no relation between the amount of dieldrin in
the samples and the use of sick leave (Hayes & Curley, 1968).
In another study, 68 pesticide workers (including pest control
operators) and 29 unexposed controls were examined quarterly over a
period of four years. Determinations of serum pesticide
concentrations and enzyme activity, blood chemistry, haematology,
and urinalysis were carried out. The mean serum dieldrin
concentration was 3.6 ± 6.3 µg/litre (1.1 ± 1.6 µg/litre in the
controls). There was no difference between the exposed workers and
the controls in the incidence of disease or disability (Warnick &
Carter, 1972).
In a pesticide formulation plant, the blood of 21 employees was
examined at the conclusion of a 5-week period during which 900 kg
of technical aldrin was formulated. The mean dieldrin
concentration in plasma was 11 and 182.5 µg/litre for herbicide
formulators and aldrin formulators, with a maximum of 317 µg/litre
in the latter case. No mention was made of any intoxications (Mick
et al., 1972).
In a group of 42 occupationally exposed pesticide workers with
a dieldrin serum concentration 5 times as high as that in a group
of 23 controls, no indication of disturbed renal- or adrenocortical
function was found (Morgan & Roan, 1969, 1973).
A study was carried out in California, USA, where aldrin (EC as
a 0.5% solution) at 480 g/litre was applied as a termiticide to
typical slab and crawl space type houses. Personal air samples,
samples of blood, and samples of pads on clothes and gloves were
taken to monitor the exposure of the pest-control operators. The
personal air samples during application contained less than 0.3
µg/m3 aldrin for the slab houses and 30 - 75 µg/m3 for the crawl
space houses. The total work day (9 - 18 h) time-weighted average
concentration of aldrin in air was 6 - 17 µg/m3. This is far below
the threshold limit value (TLV) for aldrin established by the
American Conference of Governmental Industrial Hygienists (ACGIH,
1986) of 250 µg/m3. Data from dermal exposure samples showed large
variation. However, the maximum calculated percentage of the toxic
dose per h, based on the acute percutaneous toxicity (rat LD50) of
the formulation, was less than 0.01%. The concentration of aldrin
and dieldrin in the blood of the operators was below the limit of
detection (less than 1 µg/litre) (Marlow et al., 1982).
A case-control study, carried out on 27 pesticide workers
(4 formulators and 23 pest-control operators) with elevated blood
concentrations of dieldrin, revealed a mean blood concentration of
19.59 µg/litre (range: 4.45 - 54 µg/litre). In an extensive
clinical examination, including physical examination, comprehensive
neurological examinations, laboratory tests, and physiological and
psychomotor testing, no important differences were found compared
with results in a control group of 25 people with a mean dieldrin
blood concentration of 0.48 µg/litre (range of 0 - 3.34 µg/litre)
(Sandifer et al., 1981).
9.2.3. Epidemiological studies
An extensive study on workers in an aldrin/dieldrin
manufacturing plant has been in progress since the plant began
operations in the 1950s. The results from the first 15 years of
this epidemiological study were reported in 1970 (Jager, 1970).
From a total of more than 800 exposed workers, all those exposed
for more than 4 years (233 men) or those who had experienced an
intoxication (20 men) underwent extensive physical, neurological,
haematological, and other laboratory examinations. Clinical
chemical determinations, including SGOT, SGPT, LDH, alkaline
phosphatase, total serum protein, and serum protein spectrum, were
made every 3 months and remained within normal limits. A no-effect
level in this group of workers, including those who had previously
suffered intoxications, was established at a dieldrin blood
concentration of 200 µg/litre. This level corresponds to a total
equivalent daily oral intake of 33 µg/kg body weight or a total
daily intake of 2300 µg/person per day (Hunter & Robinson, 1967).
In experimental animals, the earliest, reversible effect of
dieldrin is the induction of liver microsomal enzyme systems
(Wright et al., 1977, 1978). This finding led to an investigation
of a group of 10 workers. At the time, due to further improvements
in the industrial hygiene of the above-mentioned plant, the
geometric mean concentration of dieldrin found in the blood of
workers was 105 µg/litre. As criteria of enzyme induction
measurements were made of the blood levels of p,p'-DDE, the urinary
ratio of 6-beta-hydroxycortisole and 17-hydroxycorticosteroids, and
the urinary excretion of D-glucaric acid. No difference in these
values was found between the 10 exposed workers and a control
group. On the basis of these data, the no-effect level was 105 µg
dieldrin/litre blood, equivalent to an oral daily intake of 17.4
µg/kg body weight per day (or 1220 µg/person per day) (Jager, 1970;
Hunter et al., 1969; Hunter & Robinson, 1967; Versteeg & Jager,
1973).
Further results from this long-term survey of an industrial
population were subsequently published, based on a study of 1000
workers. Because not all of the workers had severe and/or
prolonged exposure, smaller groups with an exposure meaningful
enough for carcinogenicity evaluation were included. One group
consisted of 166 men (including workers who were still exposed and
workers who had left the company), with a mean exposure time of
16.9 years (range 4 - 19 years), who had been under observation for
more than 15 years (mean observation period 17 years; range,
15 - 20 years). A sub-group comprised 69 men with a mean exposure
time of 14.9 years (range 10 - 19 years) and a mean observation
period of 17.2 years. Among the group of 166 workers, 51 were more
than 50 years old. One man with only 5 years of comparatively mild
exposure died because of a gastric carcinoma. A lymphosarcoma
occurred in a man with 7 years of very mild exposure. Both
incidences occurred before 1964. No new cases were noted in the
final 11 years of study, and no undue mortality from other causes
that could have masked a higher cancer incidence was observed
(Versteeg & Jager, 1973; Van Raalte, 1977).
In a follow-up study on the original group of 233 men with more
than 4 years of exposure and an observation period ranging from 4
to 29 years (mean, 24 years), there were no indications of a
specific carcinogenic activity. Total observed mortality was 25
deaths versus 38 expected. Of nine cancer deaths, three were
caused by lung cancer, while the remaining six were each of a
different nature. No primary liver tumours were observed (Ribbens,
1985).
In a study by Morgan & Roan (1974), 28 pesticide formulators
and applicators, plus a separate group of 43 termite-control
workers, with occupational exposures of 5 - 22 years were examined,
together with 56 controls. The highest levels of dieldrin among
these 71 workers were found in a group of 37 men who had a mean
serum dieldrin concentration of 84 µg/litre (equivalent to about
55 µg/litre whole blood). There were no signs of liver cell injury
and the serum enzyme activities SGOT, SGPT, LDH, alkaline
phosphatase and creatine phosphokinase (CPK) were within normal
limits. There was no indication of drug-metabolizing enzyme
induction and urinary excretion of D-glucaric acid was not
different from that in a control group.
A study comparing liver cancer deaths in the USA and the
"domestic disappearance" of organochlorine pesticides, revealed
that, in 1962, 18 and 10 years after the introduction of DDT and
aldrin/dieldrin, respectively (when an increase in primary liver
cancer due to the organochlorines would be manifest), the cases of
primary liver cancer as a percentage of the total number of liver
cancer deaths began a gradual and steady decline (from 61.3% in
1962 to 56.9% in 1972). The death rate (per 100 000 per year) of
primary liver cancer for this period declined from 3.46 to 3.18
(Deichmann & MacDonald, 1977).
An epidemiological mortality study in a plant manufacturing
aldrin, dieldrin, and endrin was carried out on a cohort of 1155
workers who had been employed for at least 6 months between 1946
and 1976 (almost 25 000 man-years of observation). The mortality
due to all malignant neoplasms was 31, lower than expected
(standardized mortality ratio (SMR) 82). The total mortality from
all causes was 173 (SMR 84). The only disease with an SMR above
100 (SMR 212) was "non-malignant respiratory system disease",
specifically pneumonia. There was a slight excess of oesophagus
and rectum cancer (two and three cases observed with an SMR of 235
and 242, respectively), liver cancer (two cases observed versus
0.57 expected), and cancer of the lymphatic and haematopoietic
system (six cases observed versus 4.07 expected). However, there
was a deficit of cancer of other sites. The authors concluded that
"the study has not identified a specific cancer risk associated
with employment at this manufacturing plant, but several causes
should be examined further" (Ditraglia et al., 1981).
In a 1981 health survey, a total of 567 serum samples from 1811
Florida citrus workers were collected during the spraying and the
harvest season and were compared with the national ("Hanes")
sample. There were no differences in serum dieldrin levels; the
mean in both groups being 1.8 - 1.9 µg/litre serum (Griffith &
Duncan, 1985).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of Human Health Risks
Aldrin and dieldrin, organochlorine pesticides, were used
throughout the world from 1950 until the early 1970s as
insecticides in agriculture and as a seed treatment, for the
control of soil pests and other types of insects (e.g., termites,
grasshoppers, and textile pests), and for the control of tsetse
flies and other disease vectors. The compounds act as contact and
stomach poisons in the insects. Since the early 1970s, both
compounds have been restricted or banned from use in several
countries, especially in agriculture. Nevertheless, use continues
in other countries for termite control.
Both compounds are practically insoluble in water and
moderately to highly soluble in many organic solvents. The vapour
pressure is low.
Dairy and meat products, fish, oils and fats, and certain
vegetables such as root vegetables often contain dieldrin. Maximum
residue limits recommended by the FAO/WHO Joint Meeting on
Pesticide Residues range from 0.02 to 0.2 mg/kg product. Recent
measurements have shown that actual levels are lower, and this has
been confirmed by total diet studies. Since the use of these two
compounds has been restricted, a steady but slow decrease in
residue levels in the different food commodities has taken place.
The intake by human beings of low concentrations in the daily
diet has resulted in dieldrin being present in adipose tissue and
in some other tissues and organs. Global surveys have shown that
mean values range from 0.1 to 0.4 mg/kg adipose tissue. Since the
early 1970s, this concentration has slowly decreased.
Transplacental exposure of the fetus occurs, with the result
that the fatty tissues of the fetus also contain dieldrin, but at
concentrations 10 - 50% of those of the mother. There seems to be
an equilibrium between levels in the fetus and those in the mother.
Dieldrin is also excreted with the milk. Inhabitants of houses
that have been treated for termite control may be exposed by
inhalation. Concentrations in the air found after indoor treatment
may range from 0.01 to 7 µg/m3, depending on the type of
applications, concentration used, type of ventilation, and time of
sampling. Under these conditions food may also be contaminated by
direct contact or by sorption from the atmosphere.
Metabolism takes place mainly in the liver where aldrin is
readily transformed to dieldrin. Dieldrin is degraded at a slower
rate to hydrophilic metabolites, which are then excreted via the
bile and urine. The structures of these metabolites have been
established. In all species examined, including human beings, it
has been shown that there is a steady state of aldrin/dieldrin
storage corresponding to the level of intake and a linear
relationship between the log of intake and storage has been
demonstrated. The concentration of dieldrin in body tissues
decreases exponentially on termination of exposure to the
compounds.
The acute oral toxicity of aldrin and dieldrin for mammals is
high, while the dermal toxicity is moderate. Dermal sensitization
has not been found. Effects observed in acute, short-term and
long-term studies involve the central nervous system. The liver is
also a target organ. In the liver of mice and rats, changes known
as "chlorinated hydrocarbon insecticide rodent liver" are found.
Aldrin and dieldrin do not appear to cause teratogenic effects
at doses below those causing maternal toxicity and fetotoxicity.
Male or female reproductive toxicity has not been reported.
Numerous in vitro and in vivo mutagenicity studies have
demonstrated that neither aldrin nor dieldrin have mutagenic
potential.
In long-term studies, aldrin and dieldrin induced benign and
malignant liver tumours in the mouse. However, no increased
incidence of liver tumours or other tumours were found in rats and
hamsters.
IARC (1987) has stated that there is inadequate evidence of
carcinogenicity in human beings and limited evidence of
carcinogenicity in experimental animals. Both aldrin and dieldrin
have been classified in Group 3: the chemicals cannot be
classified as to their carcinogenicity in human beings.
On the basis of available short-term and long-term toxicity
data, the overall no-observed-adverse-effect level in the rat is
0.5 mg dieldrin/kg diet, equivalent to 0.025 mg/kg body weight. In
the dog, the lowest no-observed-adverse-effect level found was 0.04
mg/kg body weight. The Joint Meeting on Pesticide Residues (JMPR)
established an Acceptable Daily Intake (ADI) of 0.1 µg/kg body
weight in 1966 and 1977 based on the conclusion that aldrin and
dieldrin were not human carcinogens.
Aldrin and dieldrin are highly toxic to human beings. Both
accidental and occupational cases of poisoning have occurred but
reported fatalities have been rare. Survivors of acute or subacute
intoxications recovered completely. Adverse effects are related to
the dieldrin blood concentration, the determination of which
provides a specific diagnostic test for aldrin/dieldrin exposure.
At a dieldrin blood concentration below 105 µg/litre, no adverse
effects can be expected. This level is considered a threshold no-
observed-adverse-effect level and corresponds to a daily intake of
0.02 mg dieldrin/kg body weight per day.
Environmental, mainly dietary, exposure leads to the presence
of dieldrin in low concentrations in the human body. The results
of extensive clinical and epidemiological studies indicate that
these body burdens do not present a health hazard to human beings.
No signs of any premonitory change in liver function were found
in a 20-years study, involving more than 1000 industrial workers
exposed to aldrin and dieldrin. In this study and another study in
the USA, no specific cancer risk could be identified associated
with occupational exposure to (sometimes high levels of) aldrin and
dieldrin.
All the available information on aldrin and dieldrin taken
together, including studies on human beings, supports the view that
for practical purposes, these chemicals make very little
contribution, if any, to the incidence of cancer in human beings.
Photodieldrin, the photo-decomposition product of dieldrin, is
similar to dieldrin in its short-term toxicity. It is not
teratogenic or carcinogenic in mice and rats. The accumulation of
photodieldrin in the adipose tissue of experimental animals was
less than that of dieldrin.
10.2. Evaluation of Effects on the Environment
Aldrin, used as a soil insecticide, is the major source of
dieldrin (up to 97%) in the environment. Aldrin and its reaction
product dieldrin are rapidly adsorbed on soils, especially soils
containing a high level of organic matter. Consequently there is
little penetration into the soil, and contamination of groundwater
does not generally occur. Transport of both compounds takes place
mainly through soil erosion (as wind drift) and sediment transport
(surface run-off), but not through leaching.
The use of aldrin and dieldrin in agriculture leads to residues
(mainly of dieldrin) in the soil that can persist for years; the
estimated half-life of dieldrin is between 4 and 7 years. Under
tropical conditions, the compounds are less persistent than under
temperate conditions.
Aldrin and dieldrin enter the atmosphere through volatilization
from treated crops and soil or, directly, during the application of
the pesticide. Dieldrin returns to soil and water surfaces by
washout and dry deposition. Thus, the compounds are found either
in the vapour phase (very low levels, in general 1 - 2 ng/m3),
adsorbed by dust particles, or in rainwater (of the order of
10 - 20 ng/litre).
The occurrence of dieldrin in the aquatic environment has been
reported by several authors. The concentrations in surface water
are mainly very low, less than 5 ng/litre. However, concentrations
in areas of soil erosion or agricultural use may be higher.
Sediment in rivers in these areas may contain up to 1 mg
dieldrin/kg. The high capacity for aquatic organisms to
concentrate dieldrin from very low levels in water could lead to
toxic levels in aquatic organisms. Concentration through aquatic
foodchains is of less importance than direct uptake from water.
Because of the widespread occurrence of dieldrin in the
environment and its persistence, there is a wide range of
concentrations in non-target organisms. Whereas the concentrations
previously ranged from 0.001 mg to 100 mg/kg tissue, they are now
mostly below 1 mg/kg tissue.
In terrestrial ecosystems, aldrin and dieldrin are accumulated
by a wide variety of organisms, principally as dieldrin. Dieldrin
is probably responsible for the deaths of mammals in the field and
for the decline in population size in some species, such as the
otter. Small mammals would be killed by eating dieldrin-dressed
grain, but populations of these animals are likely to have been
replenished by immigration from surrounding areas. Birds of prey
eating small mammals and small birds contaminated by dieldrin take
up and accumulate dieldrin in their own tissues and eggs.
Granivorous birds have been killed by eating dressed grain. It is
probable that the population decline in birds of prey was caused by
dieldrin residues (among other organochlorine residues) in their
tissues. The effects of dieldrin are seen some time after the
exposure, because residues are stored in fat over winter, to be
released in the spring. When dieldrin was used only at certain
times of the year, this did not prevent bird mortalities.
The widespread use of aldrin and dieldrin, in conjunction with
other organochlorine pesticides, has led to severe detrimental
effects on the environment, though with drastic curtailment of use,
particularly in seed dressings, there has been some recovery in
bird populations.
10.3. Conclusions
(a) Both aldrin and dieldrin have been subjected to intensive and
wide-ranging study, toxicologically, clinically, and
epidemiologically. The body burden is mainly the result of
the oral ingestion of residues in the diet (which seem
generally to fall within the promulgated ADIs) and, to a
lesser extent, of inhalation. Evaluation of the data suggests
strongly that the body burden resulting from the present level
of exposure constitutes no health risk to the general
population.
(b) Dieldrin occurs almost ubiquitously in human breast milk.
However, its concentration in the blood and adipose tissue of
suckling infants does not increase with age during the first
six months, nor is their blood dieldrin level higher than that
of bottle-fed babies. Under these circumstances, the benefits
of natural breast feeding still make it the preferred method
of infant feeding, in spite of the dieldrin residues.
(c) In the treatment of premises, notably for termite control, the
exposure of occupants does not appear to be increased to a
level that endangers their health, as long as the directions
for safe practice are conscientiously respected.
(d) Despite the highly toxic nature of aldrin and dieldrin, both
of these chemicals can be handled safely as long as the
recommended precautions to minimize worker exposure are always
observed. Neglect of these rules may lead to the poisoning of
operators.
(e) During the period of high aldrin and dieldrin use between 1950
and 1970, detrimental effects were undoubtedly inflicted upon
species in the environment. These effects were due partly to
dieldrin and partly to other organochlorines. Since the
drastic curtailment of the use of these materials, the
affected species have recovered in numbers.
11. RECOMMENDATIONS
1. A further, properly designed, teratogenic investigation is
required in the hamster, with dieldrin at realistic dose
levels.
2. Research into the mechanism of carcinogenesis should be
directed to explaining why the hepatic reaction in the mouse
is different from that of other species.
3. Dieldrin should be selected as an agent for further study of
neurotoxic mechanisms, both experimentally and clinically.
4. To protect the environment, large-scale use of aldrin and
dieldrin must not be resumed, and applications should be
confined to those situations in which no safer, equally
effective alternatives can be recommended.
5. For the health and welfare of workers and the general
population, the handling and application of aldrin and
dieldrin should only be entrusted to well trained competent
operators, who will follow adequate safety measures.
6. To avoid accidental poisoning from aldrin, especially among
children, the use of aldrin granules as an ant bait should be
forbidden.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Aldrin and dieldrin were evaluated by the FAO/WHO Joint
Meeting on Pesticide Residues (JMPR) in 1963, 1965, 1966, 1967,
1968, 1969, 1970, 1974, 1975, and 1977 (FAO/WHO, 1964, 1965a,b,
1967a,b, 1968a,b, 1969a,b, 1970a,b, 1971a,b, 1975a,b, 1978a,b).
From 1966 onwards, the JMPR established an acceptable daily intake
(ADI) of 0 - 0.0001 mg/kg body weight (combined total for aldrin
plus dieldrin). This was based on a level causing no toxicological
effect of:
0.5 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
rat; and
1 mg/kg diet, equivalent to 0.025 mg/kg body weight, in the
dog.
The maximum residue limits (MRLs) listed in Table 47 were
recommended by the FAO/WHO Joint Meeting on Pesticide Residues in
1970 and 1975 and are quoted as the sum of aldrin plus dieldrin.
Table 47. Maximum residue limits (MRL's) recommended by the Codex
Alimentarius Commission (FAO/WHO, 1986)
-------------------------------------------------------------------
Commodity Aldrin and dieldrin
(mg/kg)
-------------------------------------------------------------------
Potatoes 0.1
Fat of meat 0.2a
Carrots, lettuce, fat of meat 0.1a
Asparagus, aubergines, broccoli, Brussels 0.1
sprouts, cabbage, cauliflower, cucumbers,
horse radish, onions, parsnips, peppers,
pimentos, radishes, radish tops
Eggs (shell-free) 0.1a
Milk and milk products (fat basis) -
Milk 0.006a
Fruit 0.05
Rice (in husks) 0.02
Raw cereals (other than rice) 0.02a
-------------------------------------------------------------------
a Extraneous residue limit.
WHO (1984) recommended that the level of aldrin and dieldrin
in drinking-water should not exceed 0.03 µg/litre.
IARC evaluated aldrin and dieldrin on several occasions.
Aldrin and dieldrin were found to be carcinogenic in the liver in
mice, but there was no evidence for carcinogenicity in other
organs. The data available did not provide evidence of
carcinogenicity in rats. Data on dogs, monkeys, and human beings
were too limited to allow any conclusions (IARC, 1974). IARC
considered that there was inadequate evidence of carcinogenicity
in humans and limited evidence of carcinogenicity in experimental
animals. Accordingly, both chemicals were classified in Group 3
(IARC, 1987).
The Pesticide Development and Safe Use Unit, Division of
Vector Biology and Control, WHO, classified the acute hazard to
health for technical dieldrin as "highly hazardous" (WHO, 1988).
The same division published a data sheet on aldrin (79.41) and
dieldrin (75.17) (WHO/FAO, 1975-85).
REFERENCES
ABALIS, I.M., ELDERFRAWI, M.E., & ELDERFRAWI, A.T. (1985) High-
affinity stereospecific binding of cyclodiene insecticides and
gamma-hexachlorocyclohexane to gamma-aminobutyric acid receptors of
rat brain. Pestic. Biochem. Physiol., 24: 95-102.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1965)
Organochlorine pesticides in the atmospheric environment. Nature
(Lond.), 208: 1317-1318.
ABBOTT, D.C., HARRISON, R.B., TATTON, J.O'G., & THOMSON, J. (1966)
Organochlorine pesticides in the atmosphere. Nature (Lond.), 211:
259-261.
ABBOTT, D.C., HOLMES, D.C., & TATTON, J.O'G. (1969) Pesticide
residues in the total diet in England and Wales, 1966-67.
Organochlorine pesticide residues in the total diet. J. Sci. Food
Agric., 20(4): 245-259.
ABBOTT, D.C., COLLINS, G.B., & GOULDING, R. (1972) Organochlorine
pesticide residues in human fat in the United Kingdom 1969-71. Br.
med. J., 1972(2): 553-556.
ABBOTT, D.C., COLLINS, G.B., GOULDING, R., & HOODLESS, R.A. (1981)
Organochlorine pesticide residues in human fat in the United
Kingdom 1976-77. Br. med. J., 283: 1425-1428.
ACGIH (1986) TLVs. Threshold limit values and biological exposure
indices for 1986-1987, Cincinnati, Ohio, American Conference of
Governmental Industrial Hygienists, 111 pp.
ACKER, L. & SCHULTE, E. (1974) [Chlorinated hydrocarbons in human
fat.] Naturwissenschaften, 61: 32 (in German).
ACKER, L., BARKE, E., HAPKE, H.J., HEESCHEN, W., KORANSKY, W.,
KUBLER, W., & REINHARDT, D. (1984) [Residues and impurities in
breast milk.] Milchwissenschaft., 39(9): 541-544 (in German).
ADDISON, R.F., ZINCK, M.E., & ACKMAN, R.G. (1972) Residues of
organochlorine pesticides and polychlorinated biphenyls in some
commercially produced Canadian marine oils. J. Fish Res. Board
Can., 29: 349-355.
ADDISON, R.F., ZINCK, M.E., & LEAHY, J.R. (1976) Metabolism of
single and combined doses of 14C-aldrin and 3H-p,p'-DDT by
Atlantic salmon (Salmo salar) fry. J. Fish Res. Board Can., 33(9):
2073-2976.
ADEMA, D.M.M. & VINK, G.J. (1981) A comparative study of the
toxicity of 1,1,2-trichloroethane, dieldrin, pentachlorophenol, and
3,4-dichloroaniline for marine and fresh-water organisms.
Chemosphere, 10(6): 533-554.
AGNIHOTRI, N.P., PANDEY, S.Y., JAIN, H.K., & SRIVASTAVA, K.P.
(1977) Persistence of aldrin, dieldrin, lindane, heptachlor, and
p,p'-DDT in soil. J. entomol. Res., 1(1): 89-91.
AHMED, F.E., LEWIS, N.J., & HART, R.W. (1977a) Pesticide induced
ouabain resistant mutants in Chinese hamster V-79 cells. Chem.-
biol. Interact., 19(3): 369-374.
AHMED, F.E., HART, R.W., & LEWIS, N.J. (1977b) Pesticide induced
DNA damage and its repair in cultured human cells. Mutat. Res., 42:
161-174.
AKKERMANS, L.M.A. (1974) Mode of action of dieldrin. An electro-
physiological investigation, Utrecht, The Netherlands, Rijks
Universiteit (Thesis).
AKKERMANS, L.M.A., VAN DEN BERCKEN, J., & VERSLUYS-HELDER, M.
(1975) Excitatory and depressant effects of dieldrin and aldrin-
transdiol in the spinal cord of the toad (Xenopus laevis). Eur. J.
Pharmacol., 34: 133-142.
ALBERT, L., MENDEZ, F., CEBRIAN, M.E., & PORTALES, A. (1980)
Organochlorine pesticide residues in human adipose tissue in
Mexico: results of a preliminary study in three Mexican cities.
Arch. environ. Health, 35(5): 262-269.
ANAS, R.E. & WILSON, A.J. (1970a) Organochlorine pesticides in fur
seals. Pestic. monit. J., 3(4): 196-200.
ANAS, R.E. & WILSON, A.J. (1970b) Organochlorine pesticides in
nursing fur seal pups. Pestic. monit. J., 4(3): 114-116.
ANDERSON, B.G. (1960) The toxicity of organic insecticides to
Daphnia. In: Tarzwell, C.M., ed. Biological problems in water
pollution. Proceedings of the 2nd Seminar, 1959, Cincinnati, Ohio,
Robert A. Taft Sanitary and Engineering Center, pp. 94-95
(Technical Report W60-3).
ANDERSON, D.W. & HICKEY, J.J. (1972) Eggshell changes in certain
North American birds. In: Proceedings of the 15th International
Ornithology Congress, Leiden, Brill, pp. 514-540.
ANDERSON, P.D. & WEBER, L.J. (1975) Toxic response as a
quantitative function of body size. Toxicol. appl. Pharmacol., 33:
471-483.
ANON. (1964) Report on the Working Conference on Birds of Prey and
Owls, Caen, Normandy, 10-12 April, 1964, Washington, DC,
International Council for Bird Preservation.
ANON. (1973) Aldrin and the ultraviolet conversion products and
possible metabolites of aldrin and dieldrin in human milk from the
community studies on pesticides, Berkeley, California, Department
of Public Health, (Abstract prepared by Shell Development Company,
Modesto, California: Report TIR-24-123-73: Part II).
ANON. (1974) Data for pentachloroketone and UV-dieldrin in human
kidney and adipose tissue from Coral Gables, Florida, Modesto,
California, Shell Development Company (Report TIR-24-176-73: Part
II).
ANON. (1974b) Data for dieldrin, pentachloroketone, and UV-dieldrin
in human adipose tissue from Albany, New York, Modesto,
California, Shell Development Company (Report TIR-24-169-73: Part
II).
ANON. (1974c) Determination of dieldrin levels in human kidney and
adipose tissue, Modesto, California, Shell Development Company
(Report TIR-24-176-73).
ASHWOOD-SMITH, M.J. (1981) The genetic toxicology of aldrin and
dieldrin. Mutat. Res., 86: 137-154.
ASHWOOD-SMITH, M.J., TREVINO, J., & RING, R. (1972) Mutagenicity of
dichlorvos. Nature (Lond.), 240: 418-420.
ASTOLFI, E., GARCIA FERNANDEZ, J.C., DE JUAREZ, M.B., & PIACENTINO,
H. (1973) Chlorinated pesticides found in the fat of children in
the Argentine Republic. In: Deichmann, W.B., ed. Pesticides and the
environment, New York, Intercontinental Medical Book Company, pp.
233-243.
ASTOLFI, E., ALONSO, A.H., MENDIZABAL, A., & ZUBIZARRETTA, E.
(1974) Pesticides chlorés de l'accouchée et du cordon ombilical des
nouveaunés. J. eur. Toxicol., 7: 330-338.
ATKINS, D.H.F. & EGGLETON, A.E.J. (1970) Studies of atmospheric
washout and deposition of gamma-BHC, dieldrin, and p,p'-DDT using
radiolabelled pesticides, Harwell, United Kingdom Atomic Energy
Research Authority (Report HL 70/4532(C10)).
ATKINS, T.D. & LINDER, R.L. (1967) Effects of dieldrin on
reproduction of penned hen pheasants. J. wildl. Manage., 31(4):
746-753.
AULERICH, R.J., RINGER, R.K., & POLIN, D. (1972) Rate of
accumulation of chlorinated hydrocarbon pesticide residues in
adipose tissue of mink. Can. J. Zool., 50(9): 1167-1173.
AVAR, P. & CZEGLEDI-JANKO, G. (1970) Occupational exposure to
aldrin: clinical and laboratory findings. Br. J. ind. Med., 27(3):
279-282.
BAECKSTROEM, J., HANNSON, E., & ULLBERG, S. (1965) Distribution of
14C-DDT and 14C-dieldrin in pregnant mice determined by whole-body
autoradiography. Toxicol. appl. Pharmacol., 7: 90-96.
BAILEY, G.W., SWANK, R.R., Jr, & NICHOLSON, H.P. (1974) Predicting
pesticide runoff from agricultural land: a conceptual model. J.
environ. Qual., 3(2): 95-102.
BAKER, A.H., WHITNEY, G.F.H., & WORDEN, A.N. (1959) The toxic
hazard associated with continuous-flow heat-volatilized
insecticidal and acaracidal aerosols. Lab. Pract., 8: 3-10.
BAKKEN, A.F. & SEIP. M. (1976) Insecticides in human breast milk.
Acta paediatr. Scand., 65: 535-539.
BALAYANNIS, P.G. (1974) Organochlorine pesticide residues in
tomatoes Ann. Inst. Phytopathol. Benaki (N.S.), 11: 47-52.
BALDWIN, M.K. & ROBINSON, J. (1969) Metabolism in the rat of the
photoisomerization product of dieldrin. Nature (Lond.), 224(5216):
283-284.
BALDWIN, M.K., ROBINSON, J., & CARRINGTON, R.A.G. (1970) Metabolism
of HEOD (dieldrin) in the rat: examination of the major faecal
metabolite. Chem. Ind., 1970: 595-597.
BALDWIN, M.K., ROBINSON, J., & PARKE, D.V. (1972) A comparison of
the metabolism of HEOD (dieldrin) in the CF1 mouse with that in the
CFE rat. Food Cosmet. Toxicol., 10: 333-351.
BALDWIN, M.K., DAVIS, R.A., & THORBURN BURNS, D. (1973) Structural
studies and photochemical re-arrangement of an animal metabolite of
HEOD, the active component of dieldrin. Pestic. Sci., 4: 227-237.
BALDWIN, M.K., BENNETT, D., & BEYNON, K.I. (1977) The
concentrations of aldrin and dieldrin and their photoisomers in the
atmosphere. Pestic. Sci., 8: 431-445.
BALUJA, G., MURADO, M.A., & TEJEDOR, M.C. (1975) Adsorption and
desorption of lindane and aldrin by soils as affected by soil main
components. Environ. Qual. Saf., Suppl. 3: 243-249.
BALUJA, G., HERNANDEZ, L.M., GONZALEZ, J., & RICO, C. (1982)
Presence of organochlorine pesticides, polychlorinated biphenyls
and mercury in Spanish human milk samples. Bull. environ. Contam.
Toxicol., 28: 573-577.
BARLOW, F. & HADAWAY, A.B. (1955) Studies on aqueous suspensions of
insecticides V. The sorption of insecticides by soils. Bull.
entomol. Res., 46: 547-559.
BARLOW, F. & HADAWAY, A.B. (1956) Effects of changes in humidity on
the toxicity and distribution of insecticides sorbed by some dried
soils. Nature (Lond.), 178: 1299-1300.
BARNETT, R.W., D'ERCOLE, A.J., CAIN, J.D., & ARTHUR, R.D. (1979)
Organochlorine pesticide residues in human milk samples from women
living in northwest and northeast Mississippi, 1973-75. Pestic.
monit. J., 13(2): 47-51.
BARON, R.L. & WALTON, M.S. (1971) Dynamics of HEOD (dieldrin) in
adipose tissue of the rat. Toxicol. appl. Pharmacol., 18(4): 958-963.
BARQUET, A., MORGADE, C., & PFAFFENBERGER, C.D. (1981)
Determination of organochlorine pesticides and metabolites in
drinking-water, human blood-serum, and adipose tissue. J. Toxicol.
environ. Health, 7: 469-479.
BARRETT, R.T., SKAARE, J.U., NORHEIM, G., VADER, W., & FROSLIE, A.
(1985) Persistent organochlorines and mercury in eggs of Norwegian
seabirds 1983. Environ. Pollut. Ser. A, 39: 79-93.
BASSON, N.C.J. (1971) Effects of dieldrin and its photoisomerization
product photodieldrin on birds. Phytophylactica, 3: 115-124.
BATTERTON, J.C., BOUSH, G.M., & MATSUMURA, F. (1971) Growth
response of blue-green algae to aldrin, dieldrin, endrin, and their
metabolites. Bull. environ. Contam. Toxicol., 6(6): 589-594.
BAXTER, W.L., LINDER, R.L., & DAHLGREN, R.B. (1969) Dieldrin
effects in two generations of penned hen pheasants. J. wildl.
Manage., 33(1): 96-102.
BEALL, M.L., Jr & NASH, R.G. (1969) Crop seedling uptake of DDT,
dieldrin, endrin and heptachlor from soils. Agron. J., 61: 571-575.
BEALL, M.L., Jr & NASH, R.G. (1971) Organochlorine insecticide
residues in soybean plant tops: root vs vapour sorption. Agron. J.,
63: 460-464.
BEALL, M.L., Jr & NASH, R.G. (1972) Insecticide depth in soil -
Effect on soybean - Uptake in the greenhouse. J. environ. Qual.,
1(3): 283-288.
BEDFORD, C.T. (1974) Von Baeyer/IUPAC names and abbreviated
chemical names of metabolites and artifacts of aldrin (HHDN),
dieldrin (HEOD), and endrin. Pestic. Sci., 5: 473-489.
BEDFORD, C.T. & HARROD, R.K. (1972a) Synthesis of 9-hydroxy-HEOD,
a major mammalian metabolite of HEOD (dieldrin), Sittingbourne,
Shell Research, Tunstall Laboratory (TU/7/72).
BEDFORD, C.T. & HARROD, R.K. (1972b) An improved preparation of
trans-4,5-dihydroxy-4,5-dihydroaldrin, a metabolite of HEOD
(dieldrin) in mammals, insects, and microorganisms. Chemosphere,
1(6): 255-260.
BEDFORD, C.T. & SMITH, E.H. (1978) Synthesis of dieldrin
metabolites. III. Two-step conversion of syn-12-hydroxy dieldrin
into Klein's metabolite (3,5,6,6,7-pentachloro-11,12-exo-epoxy-
pentacyclo(6.4.0.02,10.03,7.05,9)-dodecan-4-one). J. agric. food
Chem., 26(4): 911-914.
BEDFORD, J.W. & ZABIK, M.J. (1973) Bioactive compounds in the
aquatic environment: uptake and loss of DDT and dieldrin by fresh-
water mussels. Arch. environ. Contam. Toxicol., 1(2): 97-111.
BELISLE, A.A., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., MULHERN,
B.M., PROUTY, R.M., DEWOLF, R.B., & CROMARTIE, E. (1972) Residues
of organochlorine pesticides, polychlorinated biphenyls, and
mercury and autopsy data for bald eagles, 1969 and 1970. Pestic.
monit. J., 6(3): 133-138.
BELL, A. (1960) Aldrin poisoning: a case report. Med. J. Aust., 2:
698-700.
BENES, V. & SRAM, R. (1969) Mutagenic activity of some pesticides
in Drosophila melanogaster. Ind. Med. Surg., 38(12): 50-52.
BENITZ, K.F., ROTH, R.N., & COULSTON, F. (1977) Morphologic
characteristics of hepatic nodules induced by mirex and dieldrin in
mice. Toxicol. appl. Pharmacol., 41: 154-155.
BENNINGTON, S.L., CONNORS, P.G., CONNORS, C.W., & RISEBROUGH, R.W.
(1975) Patterns of chlorinated hydrocarbon contamination in New
Zealand sub-antarctic and coastal marine birds. Environ. Pollut.,
8: 135-147.
BENSON, W.R. (1969) Note on nomenclature of dieldrin and related
compounds. J. Assoc. Off. Agric. Chem., 52: 1109-1112.
BESS, H.A. & HYLIN, J.W. (1970) Persistence of termiticides in
Hawaiian soils. J. econ. Entomol., 63(2): 633-638.
BEVENUE, A., HYLIN, J.W., KAWANO, Y., & KELLEY, T.W. (1972a)
Organochlorine pesticide residues in water, sediment, algae, and
fish: Hawaii 1970-71. Pestic. monit. J., 6(1): 56-64.
BEVENUE, A., OGATA, J.N., & HYLIN, J.W. (1972b) Organochlorine
pesticides in rainwater: Oahu, Hawaii 1971-72. Bull. environ.
Contam. Toxicol., 8(4): 238-241.
BEYER, W.N. & GISH, C.D. (1980) Persistence in earthworms and
potential hazards to birds of soil applied DDT, dieldrin, and
heptachlor. J. appl. Ecol., 17(2): 295-307.
BEYERMANN, K. & ECKRICH, W. (1973) [Gas-chromatographic
determination of insecticide traces in air.] Z. anal. Chem.,
265(1): 4-7 (in German).
BEYNON, K.I. & ELGAR, K.E. (1966) The analysis for residues of
chlorinated insecticides and acaricides. Analyst, 91(1080):
143-175.
BICK, M. (1967) Chlorinated hydrocarbon residues in human body fat.
Med. J. Aust., 1127-1130.
BIDLEMAN, T.F. & OLNEY, C.E. (1974) Chlorinated hydrocarbons in the
Sargasso Sea atmosphere and surface water. Science, 183: 516-518.
BIDWELL, K., WEBER, E., NIENHOLD, I., CONNOR, T., & LEGATOR, M.S.
(1975) Comprehensive evaluation for mutagenic activity of dieldrin.
Mutat. Res., 31: 314 (Abstract).
BIJLEVELD, M. (1974) Birds of prey in Europe, London, MacMillan
Press, pp. XI and 263.
BLACK, A.M.S. (1974) Self poisoning with dieldrin: a case report
and pharmacokinetic discussion. Anaesth. intensive Care, 2: 369-374.
BLUS, L.J. (1978) Short-tailed shrews: toxicity and residue
relationships of DDT, dieldrin, and endrin. Arch. environ. Contam.
Toxicol., 7: 83-98.
BLUS, L.J. (1982) Further interpretation of the relation of
organochlorine residues in brown pelican eggs to reproductive
success. Environ. Pollut. Ser. A, 28: 15-33.
BLUS, L.J., NEELY, B.S., Jr, BELISLE, A.A, & PROUTY, R.M. (1974a)
Organochlorine residues in brown pelican eggs: relation to
reproductive success. Environ. Pollut., 7: 81-91.
BLUS, L.J., BELISLE, A.A, & PROUTY, R.M. (1974b) Relations of the
brown pelican to certain environmental pollutants. Pestic. monit.
J., 7(3/4): 181-194.
BLUS, L.J., JOANEN, T., BELISLE, A.A., & PROUTY, R.M. (1975) The
brown pelican and certain environmental pollutants in Louisiana.
Bull. environ. Contam. Toxicol., 13(6): 646-655.
BLUS, L.J., NEELY, B.S., Jr, LAMONT, T.G., & MULHERN, B. (1977)
Residues of organochlorines and heavy metals in tissues and eggs of
brown pelicans, 1969-73. Pestic. monit. J., 11(1): 40-53.
BLUS, L.J., CROMARTIE, E., MCNEASE, L., & JOANEN, T. (1979a) Brown
pelican: population status, reproductive success, and
organochlorine residues in Louisiana, 1971-76. Bull. environ.
Contam. Toxicol., 22: 128-135.
BLUS, L.J., LAMONT, T.G., & NEELY, B.S., Jr (1979b) Effects of
organochlorine residues on eggshell thickness, reproduction, and
population status of brown pelicans (Pelicanus occidentalis) in
South Carolina and Florida, 1969-76. Pestic. monit. J., 12(4):
172-184.
BORDON, L. (1980) Possible effects of dieldrin and other pesticides
on human chromosomes in vivo and in vitro. Toxicol. Res. Proj.
Dir., 5: 11-12 (No. 11.0077).
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., & PANKASKIE, J.E.
(1952a) Toxicological studies of aldrin on small laboratory
animals, Cincinnati, Ohio, Kettering Laboratory.
BORGMANN, A.R., KITSELMAN, C.H., DAHM, P.A., PANKASKIE, J.E., &
DUTRA, F.R. (1952b) Toxicological studies of dieldrin on small
laboratory animals, Cincinnati, Ohio, Kettering Laboratory.
BOWMAN, M.C., SCHECHTER, M.S., & CARTER, R.L. (1965) Behaviour of
chlorinated insecticides in a broad spectrum of soil types. J.
agric. food Chem., 13: 360-365.
BRAGT, P.C., SCHUURBIERS, C.J., HOLLANDER, J.C.TH., SCHULTING,
F.L., & WOLTHUIS, O.L. (1984) Retention of inhaled aldrin in man,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
BRESLER, E. & HANKS, R.J. (1969) Numerical method for estimating
simultaneous flow of water and salt in unsaturated soils. Soil Sci.
Soc. Am. Proc., 33: 827-839.
BREWERTON, H.V. & MCGRATH, H.J.W. (1967) Insecticides in human fat
in New Zealand. N. Z. J. Sci., 10: 486-492.
BRIGGS, G.G. (1981) Theoretical and experimental relationships
between soil adsorption, octanol/water partition coefficients,
water solubilities, bioconcentration factors, and the parachor. J.
agric. food Chem., 29: 1050-1059.
BRODTMANN, N.V., Jr (1976) Continuous analysis of chlorinated
hydrocarbon pesticides in the Lower Mississippi River. Bull.
environ. Contam. Toxicol., 15(1): 33-39.
BROOKS, G.T. (1974) Chlorinated insecticides. I. Technology and
applications, Cleveland, Ohio, CRC Press, pp. 87-99.
BRO-RASMUSSEN, F., DALGAARD-MIKKELSEN, Sv., JAKOBSON, Th.,
KOCH, Sv.O., RODIN, F., UHL, E., & VOLDUM-CLAUSEN, K. (1968)
Examinations of Danish milk and butter for contaminating
organochlorine insecticides. Residue Rev., 23: 55-69.
BROWN, J.R. (1967) Organochlorine pesticide residues in human depot
fat. Can. Med. Assoc. J., 97: 367-373.
BROWN, L., BELLINGER, E.G., & DAY, J.P. (1979) Dieldrin pollution
in the River Holme catchment, Yorkshire. Environ. Pollut., 18:
203-211.
BROWN, L.H. (1969) Status and breeding success of golden eagles in
northwest Sutherland in 1967. Br. Birds, 62(9): 345-363.
BROWN, V.K.H., HUNTER, C.G., & RICHARDSON, A. (1964) A blood test
diagnostic of exposure to aldrin and dieldrin. Br. J. ind. Med., 21:
283-286.
BROWN, V.K.H., RICHARDSON, A., ROBINSON, J., & STEVENSON, D.E.
(1965) The effects of aldrin and dieldrin on birds. Food Cosmet.
Toxicol., 3: 675-679.
BROWN, V.K.H., ROBINSON, J., & RICHARDSON, A. (1967) Preliminary
studies on the acute and subacute toxicities of a
photoisomerization product of HEOD. Food Cosmet. Toxicol., 5:
771-779.
BROWN, V.K.H., ROBINSON, J., THORPE, E., & BARRETT, J.W. (1974) The
toxicity of dieldrin (HEOD) to domestic fowl. Pestic. Sci., 5:
567-586.
BRUCE, W.N. & DECKER, G.C. (1966) Insecticide residues in soybeans
grown in soil containing various concentrations of aldrin,
dieldrin, heptachlor, and heptachlor epoxide. J. agric. food Chem.,
14(4): 395-398.
BUECHEL, K.H., GINSBERG, A.E., & FISCHER, R. (1966) [Synthesis and
structure of chlordane isomers.] Chem. Ber., 99: 421-430 (in German).
BUGG, J.C., HIGGINS, J.E., & ROBERTSON, E.A. (1967) Chlorinated
pesticide levels in eastern oyster (Crassostrea virginica) from
selected areas of the South Atlantic and Gulf of Mexico. Pestic.
monit. J., 1(3): 9-12.
BUNCH, T.D. & LOW, J.B. (1973) Effects of dieldrin on chromosomes
of semi-domestic mallard ducks. J. wildl. Manage., 37(1): 51-57.
BUNYAN, P.J. & STANLEY, P.I. (1982) Toxic mechanisms in wildlife.
Regul. Toxicol. Pharmacol., 2: 106-145.
BURKE, H.R. (1959) Toxicity of several insecticides to two species
of beneficial insects on cotton. J. econ. Entomol., 52(4): 616-618.
BURNS, B.G., PEACH, M.E., & STILES, D.A. (1975) Organochlorine
pesticide residues in a farming area, Nova Scotia, 1972-73. Pestic.
monit. J., 9(1): 34-38.
BURNS, J.E. (1974) Organochlorine pesticide and polychlorinated
biphenyl residues in biopsied human adipose tissue - Texas 1969-72.
Pestic. monit. J., 7(3/4): 122-126.
BURNS, K.A. (1976) Microsomal mixed-function oxidases in an
estuarine fish Fundulus heteroclitus and their induction as a
result of environmental contamination. Comp. Biochem. Physiol., 53B:
443-446.
BUTLER, P.A. (1971) Influence of pesticides on marine ecosystems.
Proc. R. Soc. Lond., B177: 321-329.
BUTLER, P.A. (1973) Organochlorine residues in estuarine mollusks,
1965-72. Pestic. monit. J., 6(4): 238-362.
CABRAL, J.R.P., RAITANO, F., MOLLNER, T., BRONCZYK, S.A., & SHUBIK,
P. (1979a) Acute toxicity of pesticides in hamsters. Toxicol. appl.
Pharmacol., 48: A192 (Abstract 384).
CABRAL, J.R.P., HALL, R.K., BRONCZYK, S.A., & SHUBIK, P. (1979b) A
carcinogenicity study of the pesticide dieldrin in hamsters. Cancer
Lett., 6(4/5): 241-246.
CADE, T.J., WHITE, C.M., & HAUGH, J.R. (1968) Peregrines and
pesticides in Alaska. Condor, 70: 170-178.
CAIRNS, J., Jr, FOSTER, N.R., & LOOS, J.J. (1967) Effects of
sublethal concentrations of dieldrin on laboratory populations of
guppies ( Poecilia reticulata Peters). Proc. Natl Acad. Sci.
Philadelphia, 119(3): 75-91.
CALL, D.J. & HARRELL, B.E. (1974) Effects of dieldrin and PCBs on
the production and morphology of Japanese quail eggs. Bull.
environ. Contam. Toxicol., 11(1): 70-77.
CAMPBELL, M.A., GYORKOS, J., LELCI, B., HOMONKO, K., & SAFE., S.
(1983) The effects of twenty-two organochlorine pesticides as
inducers of the hepatic drug-metabolizing enzymes. Gen. Pharmac.,
14(4): 445-454.
CAREY, A.E. & KUTZ, P.W. (1985) Trends in ambient concentrations of
agrochemicals in humans and the environment of the United States.
Environ. Monit. Assess., 5(2): 155-163.
CAREY, A.E., WIERSMA, G.B., TAI, H., & MITCHELL, W.G. (1973)
Organochlorine pesticide residues in soils and crops of the Corn
Belt Region, United States, 1970. Pestic. monit. J., 6(4): 369-376.
CAREY, A.E., WIERSMA, G.B., & TAI, H. (1976) Pesticide residues in
urban soils from 14 United States cities, 1970. Pestic. monit. J.,
10(2): 54-60.
CAREY, A.E., YANG, H.S.C., WIERSMA, G.B., TAI, H., MAXEY, R.A., &
DUPUY, A.E., Jr (1980) Residual concentrations of propanil, TCAB,
and other pesticides in rice-growing areas in the United States,
1972. Pestic. monit. J., 14(1): 23-25.
CARLSON, G.P. (1974) Epoxidation of aldrin to dieldrin by lobsters.
Bull. environ. Contam. Toxicol., 11(6): 577-582.
CARNAGHAN, R.B.A. & BLAXLAND, J.D. (1957) The toxic effect of
certain seed dressings on wild and game birds. Vet. Rec., 69:
324-325.
CARO, J.H. & TAYLOR, A.W. (1971) Pathways of loss of dieldrin from
soil under field conditions. J. agric. food Chem., 19(2): 379-384.
CARO, J.H., TAYLOR, A.W., & FREEMAN, H.P. (1976) Comparative
behaviour of dieldrin and carbofuran in the field. Arch. environ.
Contam. Toxicol., 3: 437-447.
CARTER, F.L. & STRINGER, C.A. (1970) Soil moisture and soil type
influence initial penetration by organochlorine insecticides. Bull.
environ. Contam. Toxicol., 5(5): 422-428.
CASARETT, L.J., FRYER, G.C., YAUGER, W.L., Jr, & KLEMMER, H.W.
(1968) Organochlorine pesticide residues in human tissue - Hawaii.
Arch. environ. Health, 17: 306-311.
CASSIDY, W., FISHER, A.J., PEDEN, J.D., & PARRY-JONES, A. (1967)
Organochlorine pesticide residues in human fats from Somerset,
London, United Kingdom, Ministry of Health, Public Health
Laboratory Services, Vol. 26, pp. 2-6 (Monthly Bulletin).
CATHEY, B. (1982) Comparative toxicities of five insecticides to
the earthworm (Lumbricus terrestris). Agric. Environ., 7: 73-81.
CAUSEY, M.K., BONNER, F.L., & GRAVES, J.B. (1968) Dieldrin residues
in the gallinules Porphyrula martinica L. and Gallinula chloropas
L. and its effect on clutch size and hatchability. Bull. environ.
Contam. Toxicol., 3(5): 274-283.
CETINKAYA, M., GABEL, B., PODBIELSKI, A., & THIEMANN, W. (1984)
[Investigation into the connection between the diet and living
conditions of nursing mothers and the contamination of breast milk
with sparingly volatile organochloride compounds.] Act. Ernähr., 9:
157-162 (in German).
CHACKO, C.I. & LOCKWOOD, J.L. (1967) Accumulation of DDT and
dieldrin by microorganisms. Can. J. Microbiol., 13: 1123-1126.
CHADWICK, G.G. & BROCKSEN, R.W. (1969) Accumulation of dieldrin by
fish and selected fish-food organisms. J. wildl. Manage., 33(3):
693-700.
CHADWICK, G.G. & SHUMWAY, D.L. (1970) Effects of dieldrin on the
growth and development of steelhead trout. In: Gillett, J.W., ed.
The biological impact of pesticides in the environment, Corvallis,
Oregon State University, pp. 90-96 (Environmental Health Series
No. 1).
CHAN, T.M., GILLETT, J.W., & TERRIERE, L.C. (1967) Interaction
between microsomal electron transport systems of trout and male rat
in cyclodiene epoxidation. Comp. Biochem. Physiol., 20: 731-742.
CHANIN, P.R.F. & JEFFERIES, D.J. (1978) The decline of the otter
Lutra lutra L. in Britain: an analysis of hunting records and
discussion of causes. Biol. J. Linn. Soc., 10: 305-328.
CHAU, A.S.Y. & COCHRANE, W.P. (1970) Cis-opening of dieldrin
oxirane ring. Chem. Ind., 49: 1568-1569.
CHAUDRY, M.M., NELSON, A.I., & PERKINS, E.G. (1978) Distribution of
chlorinated pesticides in soybeans, soybean oil, and its by-
products during processing. J. Am. Oil Chem. Soc., 55(12): 851-853.
CHERNOFF, N., KAVLOCK, R.J., KATHREIN, J.R., DUNN, J.M., & HASEMAN,
J.K. (1975) Prenatal effects of dieldrin and photodieldrin in mice
and rats. Toxicol. appl. Pharmacol., 31: 302-308.
CHOLAKIS, J.M., MCKEE, M.J., WONG, L.C.K., & GILE, J.D. (1981)
Acute and subacute toxicity of pesticides in microtine rodents. In:
Lamb, D.W. & Kenaga, E.E., ed. Avian and mammalian wildlife
toxicology. Second Conference, Philadelphia, Pennsylvania,
American Society of Testing Materials, pp. 143-154 (STP757).
CLARK, D.R., Jr (1975) Effect of stress on dieldrin toxicity to
male redwinged blackbirds (Agelaius phoeniceus). Bull. environ.
Contam. Toxicol., 14(2): 250-256.
CLARK, D.R., Jr (1981) Death in bats from DDE, DDT or dieldrin:
diagnosis via residues in carcass fat. Bull. environ. Contam.
Toxicol., 26: 367-374.
CLARK, D.R., Jr & KROLL, C.J. (1977) Effects of DDE on
experimentally poisoned free-tailed bats (Tadarida brasiliensis):
lethal brain concentrations. J. Toxicol. environ. Health, 3: 893-901.
CLARK, D.R., Jr & MCLANE, M.A.R. (1974) Chlorinated hydrocarbon and
mercury residues in woodcock in the United States, 1970-71. Pestic.
monit. J., 8(1): 15-22.
CLARK, D.R., Jr & PROUTY, R.M. (1984) Disposition of dietary
dieldrin in the little brown bat and correlation of skin levels
with body burden. Bull. environ. Contam. Toxicol., 33: 177-183.
CLARK, D.R., Jr, LAVAL, R.K., & SWINEFORD, D.M. (1978) Dieldrin-
induced mortality in an endangered species, the gray bat (Myotis
grisescens). Science, 199: 1357-1359.
CLARK, D.R., Jr, LAVAL, P.K., & KRYNITSKY, A.J. (1980) Dieldrin and
heptachlor residues in dead gray bats, Franklin County, Missouri -
1976 versus 1977. Pestic. monit. J., 13(4): 137-140.
CLARK D.R., Jr, CLAWSON, R.L., & STAFFORD, C.J. (1983a) Gray bats
killed by dieldrin at two additional Missouri caves: aquatic
macroinvertebrates found dead. Bull. environ. Contam. Toxicol.,
30: 214-218.
CLARK, D.R., Jr, BUNCK, C.M., CROMARTIE, E., & LAVAL, R.K. (1983b)
Year and age effects on residues of dieldrin and heptachlor in dead
gray bats, Franklin County, Missouri - 1976, 1977, and 1978.
Environ. Toxicol. Chem., 2: 387-393.
CLEVELAND, F.P. (1966) A summary of work on aldrin and dieldrin
toxicity at the Kettering Laboratory. Arch. environ. Health, 13:
195-198.
COHEN, J.M. & PINKERTON, C. (1966) Widespread translocation of
pesticides by air transport and rain-out. In: Bould, R.F., ed.
Organic pesticides in the environment, Washington, DC, American
Chemical Society, pp. 163-176 (Advances in Chemistry Series No.
60).
COLE, J.F., KLEVAY, L.M., & ZAVON, M.R. (1970) Endrin and dieldrin:
a comparison of hepatic excretion in the rat. Toxicol. appl.
Pharmacol., 16: 547-555.
COLLINS, G.B., HOLMES, D.C., & HOODLESS, R.A. (1982) Organochlorine
pesticide residues in human milk in Great Britain, 1979-1980. Hum.
Toxicol., 1: 425-431.
COOKE, A.S. (1972) The effects of DDT, dieldrin and 2,4-D on
amphibian spawn and tadpoles. Environ. Pollut., 3: 51-68.
COOKE, A.S. (1973) Shell thinning in avian eggs by environmental
pollutants. Environ. Pollut., 4: 85-152.
COOKE, A.S., BELL, A.A., & PRESTT, I. (1976) Egg shell
characteristics and incidence of shell breakage for grey herons
Ardea cinerea exposed to environmental pollutants. Environ.
Pollut., 11: 59-84.
COOKE, A.S., BELL, A.A., & HAAS, M.B. (1982) Predatory birds,
pesticides, and pollution, Huntingdon, United Kingdom, Natural
Environment Research Council, Institute of Terrestrial Ecology,
Monks Wood Experimental Station.
COPPLESTONE, J.F., HUNNEGO, J.N., & HARRISON, D.L. (1973)
Organochlorine insecticide levels in adult New Zealanders - a five-
year study. N. Z. J. Sci., 16: 27-39.
CORNELIUSSEN, P.E. (1970) Pesticide residues in total diet samples
(V). Pestic. monit. J., 4(3): 89-105.
CORNELIUSSEN, P.E. (1972) Pesticide residues in total diet samples
(VI). Pestic. monit. J., 5(4): 313-330.
COULSTON, F., ABRAHAM, R., & MANKES, R. (1980) Reproductive study
in female rats given dieldrin, alcohol, or aspirin orally, Albany,
New York, Albany Medical College of Union University, Institute of
Comparative and Human Toxicology.
COWAN, A.A. (1981) Organochlorine compounds in mussels from
Scottish coastal waters. Environ. Pollut. Ser. B, 2: 129-143.
CRAIG, N.C.D. (1977) A summary of the data on the toxicity of
various materials to aquatic life. III. Dieldrin, aldrin, and
endrin, Brixham United Kingdom, Imperial Chemical Industries Ltd
(Unpublished Report BL/A/1828).
CRAWFORD, N.H. & DONIGIAN, A.S., Jr (1973) Pesticide transport and
runoff model for agricultural lands, Washington, DC, US
Environmental Protection Agency (EPA-600/2-74-013).
CREBELLI, R., BELLINCAMPI, D., CONTI, G., MORPURGO, G., & CARERE,
A. (1986) A comparative study on selected chemical carcinogens for
chromosome malsegregation, mitotic crossing-over and forward
mutation induction in Aspergillus nidulans. Mutat. Res., 172:
139-149.
CROCKETT, A.B., WIERSMA, G.B., TAI, H., MITCHELL, W.G., SAND, P.F.,
& CAREY, A.E. (1974) Pesticide residue levels in soils and crops:
FY-70 - National Soils Monitoring Program. II. Pestic. monit. J.,
8(2): 69-97.
CROLL, B.T. (1969) Organochlorine insecticides in water. Part I.
Proc. Soc. Water Treat. Exam., 18: 255-274.
CROMARTIE, E., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., KAISER,
T.E., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., & SWINEFORD, D.M.
(1975) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1971-72. Pestic. monit.
J., 9(1): 11-14.
CUETO, C., Jr & BIROS, F.J. (1967) Chlorinated insecticides and
related materials in human urine. Toxicol. appl. Pharmacol., 10:
261-269.
CUETO, C., Jr & HAYES, W.J., Jr (1962) The detection of dieldrin
metabolites in human urine. J. agric. food Chem., 10: 366-369.
CUMMINGS, J.G. (1966) Pesticides in the total diet. Residue Rev.,
16: 30-45.
CUMMINGS, J.G., ZEE, K.T., TURNER, V., & QUINN, F. (1966) Residues
in eggs from low level feeding of five chlorinated hydrocarbon
insecticides to hens. J. Assoc. Off. Agric. Chem., 49(2): 354-364.
CURLEY, A., COPELAND, M.F., & KIMBROUGH, R.D. (1969) Chlorinated
hydrocarbon insecticides in organs of stillborn and blood of
newborn babies. Arch. environ. Health, 19: 628-632.
CURLEY, A., BURSE, V.W., JENNINGS, R.W., VILLANUEVA, E.C., TOMATIS,
L., & AKAZAKI, K. (1973) Chlorinated hydrocarbon pesticides and
related compounds in adipose tissue from people of Japan. Nature
(Lond.), 242(5396): 338-340.
DAHLGREN, R.B. & LINDER, R.L. (1970) Eggshell thickness in
pheasants given dieldrin. J. wildl. Manage., 34(1): 226-228.
DAHLGREN, R.B. & LINDER, R.L. (1974) Effects of dieldrin in penned
pheasants through the third generation. J. wildl. Manage., 38(2):
320-330.
DAILEY, R.E., WALTON, M.S., BECK, V., LEAVENS, C.L., & KLEIN, A.K.
(1970) Excretion, distribution, and tissue storage of a 14C-
labelled photoconversion product of 14C-dieldrin. J. agric. food
Chem., 18(3): 443-445.
DALE, W.E. & QUINBY, G.E. (1963) Chlorinated insecticides in the
body fat of people in the United States. Science, 142: 593-595.
DALE, W.E., COPELAND, M.F., & HAYES, W.J., Jr (1965) Chlorinated
insecticides in the body fat of people in India. Bull. World Health
Organ., 33: 471-477.
DALE, W.E., CURLEY, A., & CUETO, C., Jr (1966) Hexane extractable
chlorinated insecticides in human blood. Life Sci., 5: 47-54.
DAMICO, J.N., CHEN, J.Y.T., COSTELLO, C.E., & HAENNI, E.O. (1968)
Structure of Klein's metabolites of aldrin and of dieldrin. J.
Assoc. Off. Agric. Chem., 51(1): 48-55.
DANIELS, N.E. (1966) Soil insect control and insecticidal residue
detection. J. econ. Entomol., 59: 410-413.
DATTA, P.R., LANG, E.P., WATTS, J.O., KLEIN, A.K., & NELSON, M.J.
(1965) Metabolites in urine of rats on diets containing aldrin or
dieldrin. Nature (Lond.), 208: 289-290.
DAVIDSON, J.M. & MCDOUGAL, J.R. (1973) Experimental and predicted
movement of three herbicides in water-saturated soil. J. environ.
Qual., 2(4): 428-433.
DAVIES, J.E., BARQUET, A., MORGADE, C., & RAFFONELLI, A. (1975)
Epidemiological studies of DDT and dieldrin residues and their
relationship to human carcinogenesis. In: Proceedings of the
International Symposium on Recent Advances in Assessment of the
Health Effects of Environmental Pollution, Luxembourg, Commission
of the European Communities, Vol. 2, pp. 695-705.
DAVIS, B.N.K. (1968) The soil macrofauna and organochlorine
insecticide residues in twelve agricultural sites near Huntingdon.
Ann. appl. Biol., 61: 29-45.
DAVIS, B.N.K. (1971) Laboratory studies on the uptake of dieldrin
and DDT by earthworms. Soil Biol. Biochem., 3: 221-233.
DAVIS, H.C. & HIDU, H. (1969) Effects of pesticides on embryonic
development of clams and oysters and on survival and growth of the
larvae. Fish. Bull., 67(2): 393-404.
DAVIS, K.J. & FITZHUGH, O.G. (1962) Tumorigenic potential of aldrin
and dieldrin for mice. Toxicol. appl. Pharmacol., 4: 187-189.
DAVIS, K.J., HANSEN, W., & FITZHUGH, O.G. (1965) Pathology report
on mice fed aldrin, dieldrin, heptachlor, or heptachlor epoxide for
two years, Washington, DC, US Environmental Protection Agency
(Memorandum to Dr A.J. Lehman, 19th July, 1965. Publicly presented
and accepted at the US EPA Aldrin-Dieldrin Suspension Hearing,
Statement of Testimony from Dr K.J. Davis, August 1974).
DAVIS, P.W., FRIEDHOFF, J.M., & WEDEMEYER, G.A. (1972)
Organochlorine insecticide, herbicide, and polychlorinated biphenyl
(PCB) inhibition of NaK-ATPase in rainbow trout. Bull. environ.
Contam. Toxicol., 8(2): 69-72.
DAVISON, K.L. (1970) Dieldrin accumulation in tissues of the sheep.
J. agric. food Chem., 18(6): 1156-1160.
DAVISON, K.L. (1973) Dieldrin-14C balance in rats, sheep, and
chickens. Bull. environ. Contam. Toxicol., 10(1): 16-24.
DAVISON, K.L. & SELL, J.L. (1972) Dieldrin and p,p'-DDT effects on
egg production and eggshell thickness of chickens. Bull. environ.
Contam. Toxicol., 7(1): 9-18.
DAWSON, R. & RILEY, J.P. (1977) Chlorine-containing pesticides and
polychlorinated biphenyls in British coastal waters. Estuarine
coastal mar. Sci., 4: 55-69.
DEAN, B.J., DOAK, S.M.A., & SOMERVILLE, H. (1975) The potential
mutagenicity of dieldrin (HEOD) in mammals. Food Cosmet. Toxicol.,
13: 317-323.
DE CAMPOS, M. & OLSZYNA-MARZYS, A.E. (1979) Contamination of human
milk with chlorinated pesticides in Guatemala and in El Salvador.
Arch. environ. Contam. Toxicol., 8: 43-58.
DECKER, G.C., BRUCE, W.N., & BIGGER, J.H. (1965) The accumulation
and dissipation of residues resulting from the use of aldrin in
soils. J. econ. Entomol., 58(2): 266-271.
DEFLORA, S. (1981) Study of 106 organic and inorganic compounds in
the Salmonella/microsome test. Carcinogenesis, 2(4): 283-298.
DEICHMANN, W.B. (1974) Certified statement of testimony before the
Environmental Protection Agency at aldrin/dieldrin suspension
hearings, Washington, DC, US Environmental Protection Agency.
DEICHMANN, W.B. & MACDONALD, W.E. (1977) Organochlorine pesticides
and liver cancer deaths in the United States, 1930-72. Ecotoxicol.
environ. Saf., 1: 89-110.
DEICHMANN, W.B., KEPLINGER, M.L., SALA, F., & GLASS, E. (1967)
Synergism among oral carcinogens. IV. The simultaneous feeding of
four tumorigens to rats. Toxicol. appl. Pharmacol., 11: 88-103.
DEICHMANN, W.B., DRESSLER, I., KEPLINGER, M., & MACDONALD, W.E.
(1968) Retention of dieldrin in blood, liver, and fat of rats fed
dieldrin for six months. Ind. Med. Surg., 37: 837-839.
DEICHMANN, W.B., KEPLINGER, M., DRESSLER, I., & SALA, F. (1969)
Retention of dieldrin and DDT in the tissues of dogs fed aldrin and
DDT individually and as a mixture. Toxicol. appl. Pharmacol., 14:
205-213.
DEICHMANN, W.B., MACDONALD, W.E., BLUM, E., BEVILACQUA, M.,
RADOMSKI, J., KEPLINGER, M., & BALKUS, M. (1970) Tumorigenicity of
aldrin, dieldrin, and endrin in the albino rat. Ind. Med., 39(10):
314, 426-434.
DEICHMANN, W.B., MACDONALD, W.E., BEASLY, A.G., & CUBIT, D.A.
(1971) Subnormal reproduction in beagle dogs induced by DDT and
aldrin. Ind. Med. Surg., 40(2): 10-22.
DEICHMANN, W.B., MACDONALD, W.E., CUBIT, D.A., & BEASLY, A.G.
(1972) Effects of starvation in rats with elevated DDT and dieldrin
tissue levels. Int. Arch. Arbeitsmed., 29: 233-252.
DEICHMANN, W.B., MACDONALD, W.E., & CUBIT, D.A. (1975) Dieldrin and
DDT in the tissues of mice fed aldrin and DDT for seven
generations. Arch. Toxicol., 34: 173-182.
DEICHMANN, W.B., MACDONALD, W.E., & LU, F.C. (1979) Effects of
chronic aldrin feeding in two strains of female rats, and a
discussion on the risks of carcinogens in man. In: Deichmann, W.B.,
ed. Toxicology and occupational medicine, Amsterdam, Elsevier/North
Holland, pp. 407-413.
DEJONCKHEERE, W., STEURBAUT, W., VERSTRAETEN, R., & KIPS, R.H.
(1977) Residues of organochlorine pesticides in human fat in
Belgium. Meded. Fac. Landbouwwet. Rijksuniv. Gent, 42(2): 1839-1847.
DE LLAMAS, M.C., DE CASTRO, A.C., & PECHEN DE D'ANGELO, A.M. (1985)
Cholinesterase activities in developing amphibian embryos following
exposure to the insecticides dieldrin and malathion. Arch environ.
Contam. Toxicol., 14: 161-166.
DEL VECCHIO, V. & LEONI, V. (1967) [Detection and measurement of
chlorinated insecticides in biological material.] Nuovi Ann. Ig.
Microbiol., 28(2): 107-128 (in Italian).
DEN TONKELAAR, E.M. & VAN ESCH, G.J. (1974) No-effect levels of
organochlorine pesticides based on induction of microsomal liver
enzymes in short-term toxicity experiments. Toxicology, 2: 371-380.
D'ERCOLE, A.J., ARTHUR, R.D., CAIN, J.D., & BARRENTINE, B.F. (1976)
Insecticide exposure of mothers and newborns in a rural
agricultural area. Pediatrics, 57(6): 869-874.
DE VLIEGER, M., ROBINSON, J., BALDWIN, M.K., CRABTREE, A.N., & VAN
DIJK, M.C. (1968) The organochlorine insecticide content of human
tissue. Arch. environ. Health, 17: 759-767.
DE VOS, R.H., VAN DOKKUM, W., OLTHOF, P.D.A., QUIRYNS, J.K., MUYS,
T., & POLL, J.M., VAN DER (1984) Pesticides and other chemical
residues in Dutch total diet samples (June 1976-July 1978). Food
chem. Toxicol., 22(1): 11-21.
DICK, G.L., HEENAN, M.P., LOVE, J.L., UDY, P.B., & DAVIDSON, F.
(1978) Survey of trace elements and pesticide residues in the New
Zealand diet. 2. Organochlorine and organophosphorus pesticide
residue content. N. Z. J. Sci., 21: 71-78.
DILLON, J.C., MARTIN, G.B., & O'BRIEN, H.T. (1981) Pesticide
residues in human milk. Food Cosmet. Toxicol., 19: 437-442.
DISLER, R.L., GLATT, V., & MEIER, W. (1984) [Residues of
chlorinated insecticides and polychlorinated biphenyls in breast
milk.] Mitt. Geb. Lebensm. Hyg., 75: 205-213 (in German).
DITRAGLIA, D., BROWN, D.P., NAMEKATA, T., & IVERSON, N. (1981)
Mortality study of workers employed at organochlorine pesticide
manufacturing plants. Scand. J. Work Environ. Health, 4(7, suppl.):
140-146.
DIX, K.M. & WILSON, A.B. (1971) Toxicity studies with dieldrin
(HEOD). Teratogenic studies in rabbits given HEOD and thalidomide
orally, Sittingbourne, Shell Research (TLGR.0051.71).
DIX, K.M., VAN DER PAUW, C.L., & MCCARTHY, W.V. (1978) Toxicity
studies with dieldrin: teratological studies in mice dosed orally
with HEOD. Teratology, 16: 57-62.
DOBBS, A.J. & WILLIAMS, N. (1983) Indoor air pollution from
pesticides used in wood remedial treatments. Environ. Pollut.
Ser. B, 6: 271-296.
DOBSON, R.C. & BAUGH, E.R. (1976) Dieldrin residue removal from the
fat of swine. Bull. environ. Contam. Toxicol., 16(5): 567-571.
DOUABUL, A.A.Z., AL-SAAD, H.T., & AL-REKABI, H.N. (1987) Residues
of organochlorine pesticides in environmental samples from the
Shatt al-Arab river, Iraq. Environ. Pollut., 43: 175-187.
DRAPER, W.M. & CROSBY, D.G. (1984) Solar photooxidation of
pesticides in dilute hydrogen peroxide. J. agric. food Chem., 32:
231-237.
DUFFY, J.R. & WONG, N. (1967) Residues of organochlorine
insecticides and their metabolites in soils in the Atlantic
provinces of Canada. J. agric. food Chem., 15(3): 457-464.
DUGGAN, R.E. & CORNELIUSSEN, P.E. (1972) Dietary intake of
pesticide chemicals in the United States. III. June 1968 - April
1970. Pestic. monit. J., 5(4): 331-341.
DUGGAN, R.E. & LIPSCOMB, G.Q. (1969) Dietary intake of pesticide
chemicals in the United States. II. June 1966 - April 1968. Pestic.
monit. J., 2(4): 153-162.
DUGGAN, R.E., BARRY, H.C., & JOHNSON, L.Y. (1967) Pesticide
residues in total diet samples. II. Pestic. monit. J., 1(2): 2-12.
ECKENHAUSEN, F.W., BENNET, D., BEYNON, K.I., & ELGAR, K.E. (1981)
Organochlorine pesticide concentrations in perinatal samples from
mothers and babies. Arch. environ. Health, 36(2): 81-92.
EDMUNDSON, W.F., DAVIES, J.E., & HULL, W. (1968) Dieldrin storage
levels in necropsy adipose tissue from a south Florida population.
Pestic. monit. J., 2(2): 86-89.
EDWARDS, C.A. (1965) Effects of pesticide residues on soil
invertebrates and plants. In: Proceedings of the 5th Symposium of
the British Ecological Society, Oxford, Blackwell, pp. 239-261.
EDWARDS, C.A. (1966) Insecticide residues in soil. Residue Rev., 13:
83-132.
EDWARDS, C.A. (1973a) Persistent pesticides in the environment,
Cleveland, Ohio, CRC Press, pp. 68-74.
EDWARDS, C.A. (1973b) Persistent pesticides in soil and water. In:
Environmental pollution by pesticides, London, Plenum Press, pp.
409-458.
EDWARDS, C.A. & LOFTY, J.R. (1977) Biology of earthworms, 2nd ed.,
London, Chapman and Hall, pp. 212-213.
EDWARDS, C.A. & THOMPSON, A.R. (1973) Pesticides and the soil
fauna. Residue Rev., 45: 8-15.
EDWARDS, C.A., DENNIS, E.B., & EMPSON, D.W. (1967) Pesticides and
the soil fauna: effects of aldrin and DDT in an arable field. Ann.
appl. Biol., 60: 11-22.
EEC (1983) Directives relating to the classification and labelling
of dangerous substances. Annex VI. Part II (2.4.10) to the fifth
adaptation to the Dangerous Substances Directives, Luxembourg,
European Economic Community (Commission Directive 83/467/EEC)
(Official Journal L 257/19).
EGAN, H., GOULDING, R., ROBURN, J., & TATTON, J.O'G. (1965)
Organochlorine pesticide residues in human fat and human milk. Br.
med. J., 2: 66-69.
EICHELBERGER, J.W. & LICHTENBERG, J.J. (1971) Persistence of
pesticides in river water. Environ. Sci. Technol., 5: 541-544.
EISENLORD, G., LOQUVAM, G.S., & NEMENZO, J. (1967) Results of
reproduction study of rats fed diets containing dieldrin over three
generations, San Francisco, California, The Hine Laboratories
(Report No. 4).
EISLER, R. (1969) Acute toxicities of insecticides to marine
decapod crustaceans. Crustaceana, 16: 302-310.
EISLER, R. (1970) Latent effects of insecticide intoxication to
marine molluscs. Hydrobiologia, 36(3/4): 345-352.
EL BEIT, I.O.D. (1981) Pesticide microbial interactions in the
soil. Int. J. environ. Stud., 16: 171-181.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981a) Factors
involved in the dynamics of pesticides in soils: the effect of
pesticide concentration on leachability and absorption. Int. J.
environ. Stud., 16: 181-187.
EL BEIT, I.O.D., WHEELOCK, J.V., & COTTON, D.E. (1981b) Factors
involved in the dynamics of pesticides in soils: the effects of
temperature and period of contact or leachability and adsorption of
pesticides by soils. Int. J. environ. Stud., 16: 189-196.
EL BEIT, I.O.D., COTTON, D.E., & WHEELOCK, J.V. (1983) Persistence
of pesticides in soil leachates: effects of pH, ultraviolet
irradiation, and temperature. Int. J. environ. Stud., 21: 251-259.
ELGAR, K.E. (1966) Analysis of crops and soils for residues of the
soil insecticides aldrin and telodrin. J. Sci. Food Agric., 17:
541-545.
ELGAR, K.E. (1975) The dissipation and accumulation of aldrin and
dieldrin residues in soil. Environ. Qual. Saf., 3(suppl.): 250-257.
ELGAR, K.E. (1979) The variability of residue results, with
particular reference to the Codex study on organochlorines in
butterfat. In: Proceedings of the 4th IUPAC Congress on Pesticide
Chemistry, Zurich, 1978, pp. 668-672.
ELIASON, B.C. & POSNER, H.S. (1971) Reduced passage of carbon-14-
dieldrin to the fetal rat by phenobarbital but not by eight other
drugs or dieldrin. Am. J. Obstet. Gynecol., 110(7): 943-947.
ELY, R.E., MOORE, L.A., HUBANKS, P.E., CARTER, R.H., & POOS, F.W.
(1954) Studies of feeding aldrin to dairy cows. J. dairy Sci., 37(3):
294-298.
EMANUELSEN, M., LINCER, J.L., & RIFKIN, E. (1978) The residue
uptake and histology of American oysters ( Crassostrea virginica
Gmelin) exposed to dieldrin. Bull. environ. Contam. Toxicol., 19:
121-129.
ENDERSON, J.H. & BERGER, D.D. (1968) Chlorinated hydrocarbon
residues in peregrines and their prey species from northern Canada.
Condor, 70: 149-153.
ENDERSON, J.H. & BERGER, D.D. (1970) Pesticides: eggshell thinning
and lowered production of young in prairie falcons. Bioscience,
20(6): 355-356.
EPIFANIO, C.E. (1973) Dieldrin uptake by larvae of the crab
Leptodius floridanus. Mar. Biol., 19(4): 320-322.
EPSTEIN, E. & GRANT, W.J. (1968) Chlorinated insecticides in runoff
water as affected by crop rotation. Soil Sci. Soc. Am. Proc., 32:
423-426.
EPSTEIN, S.S., ARNOLD, E., ANDREA, J., BASS, W., & BISHOP, Y.
(1972) Detection of chemical mutagens by the dominant lethal assay
in the mouse. Toxicol. appl. Pharmacol., 23: 288-325.
ERCEGOVICH, C.D. & RASHID, K.A. (1977) Mutagenesis induced in
mutant strains of Salmonella typhimurium by pesticides,
Washington, DC, American Chemical Society (Abstracts Paper 174)
(Pesticide No. 43).
ESTENIK, J.F. & COLLINS, W.J. (1979) In vivo and in vitro studies
of mixed-function oxidase in an aquatic insect Chironomus riparius.
In: Khan, M.A.Q., ed. Pesticide and xenobiotic metabolism in
aquatic organisms, Washington, DC, American Chemical Society, pp.
349-370 (ACS Symposium Series No. 99).
EYE, J.D. (1968) Aqueous transport of dieldrin residues in soil. J.
Water Pollut. Control Fed. (suppl.), 40(8): R316-332.
FABER, R.A., RISEBROUGH, R.W., & PRATT, H.M. (1972) Organochlorines
and mercury in common egrets and great blue herons. Environ.
Pollut., 3: 111-122.
FAHRIG, R. (1974) Comparative mutagenicity studies with pesticides,
Lyons, International Agency for Research on Cancer, pp. 161-181
(IARC Scientific Publications No. 10).
FAO/WHO (1964) Evaluation of the toxicity of pesticide residues in
food. Report of a Joint Meeting of the FAO Committee on Pesticides
in Agriculture and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL:1963/13; WHO/Food Add./23).
FAO/WHO (1965a) Evaluation of the toxicity of pesticide residues in
food. Report of the Second Joint Meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, Geneva, World Health Organization (FAO Meeting Report
No. PL:1965/10; WHO Food Add./26.65).
FAO/WHO (1965b) Evaluation of the toxicity of pesticide residues in
food, Geneva, World Health Organization (FAO Meeting Report No.
PL: 1965/10/1; WHO Food Add./27.65).
FAO/WHO (1967a) Pesticide residues in food. Joint report of the FAO
Working Party on Pesticide Residues and the WHO Expert Committee on
Pesticide Residues, Geneva, World Health Organization (FAO
Agricultural Studies No. 73; WHO Technical Report Series No. 370).
FAO/WHO (1967b) 1966 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL/CP/15; WHO Food Add./67.
32).
FAO/WHO (1968a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Meeting Report No.
PL;1967/M/11; WHO Technical Report Series No. 391).
FAO/WHO (1968b) 1967 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1967/M/11/1; WHO Food
Add./68.30).
FAO/WHO (1969a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 78;
WHO Technical Report Series No. 417).
FAO/WHO (1969b) 1968 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1968/M/9/1; WHO Food
Add./69.35).
FAO/WHO (1970a) Pesticide residues in food. Report of the 1967
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 84;
WHO Technical Report Series No. 458).
FAO/WHO (1970b) 1969 Evaluation of some pesticide residues in food,
Geneva, World Health Organization (FAO PL: 1969/M/17/1; WHO Food
Add./70.38).
FAO/WHO (1971a) Pesticide residues in food. Report of the 1970
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 87;
WHO Technical Report Series No. 474).
FAO/WHO (1971b) 1970 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1979/M/12/1; WHO Food
Add./71. 42).
FAO/WHO (1975a) Pesticide residues in food. Report of the 1974
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Agricultural Studies No. 97;
WHO Technical Report Series No. 574).
FAO/WHO (1975b) 1974 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/11; WHO
Pesticide Residues Series No. 4).
FAO/WHO (1976a) Pesticide residues in food. Report of the 1975
Joint Meeting of the FAO Working Party of Experts on Pesticide
Residues and the WHO Expert Committee on Pesticide Residues,
Geneva, World Health Organization (FAO Plant Production and
Protection Series No. 1; WHO Technical Report Series No. 592).
FAO/WHO (1976b) 1975 Evaluations of some pesticide residues in
food, Geneva, World Health Organization (AGP 1974/M/13; WHO
Pesticide Residues Series No. 5).
FAO/WHO (1978a) Pesticide residues in food. Report of the 1977
Joint Meeting of the FAO Panel of Experts on Pesticide Residues and
the Environment and the WHO Expert Group on Pesticide Residues,
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 10 Rev.).
FAO/WHO (1978b) 1977 Evaluations of some pesticide residues in
food, Rome, Food and Agriculture Organization of the United Nations
(FAO Plant Production and Protection Paper 10 Sup.).
FAO/WHO (1984) Codex guidelines on good practice in pesticide
residue analysis, Rome, Codex Alimentarius Commission, Food and
Agriculture Organization of the United Nations (CAC/PR7-1984).
FAO/WHO (1986b) Recommendations for methods of analysis of
pesticide residues, Rome, Codex Alimentarius Committee, Food and
Agriculture Organization of the United Nations (CAC/PR8-1986).
FARB, R.M., SANDERSON, T., MOORE, B.G., & HAYES, A.W. (1973)
Interaction: the effect of selected mycotoxins on the tissue
distribution and retention of aldrin and dieldrin in the neonatal
rat. In: Deichmann, W.B., ed. Pesticides in the environment, New
York, International Medical Book Corporation, pp. 179-187.
FARMER, W.J. & JENSEN, C.R. (1970) Diffusion and analysis of
carbon-14-labelled dieldrin in soils. Soil Sci. Soc. Am. Proc., 34:
28-31.
FARMER, W.J. & LETEY, J. (1974) Volatilization losses of pesticides
from soils, Washington, DC, US Environmental Protection Agency
(EPA-660/2-74-054).
FARMER, W.J., IGUE, K., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. I. Effect of
concentration, temperature, air flow rate, and vapour pressure.
Soil Sci. Soc. Am. Proc., 36: 443-447.
FARMER, W.J., IGUE, K., & SPENCER, W.F. (1973) Effect of bulk
density on the diffusion and volatilization of dieldrin from soil.
J. environ. Qual., 2(1): 107-109.
FEIL, V.J., HEDDE, R.D., ZAYLSKIE, R.G., & ZACHRISON, C.H. (1970)
Dieldrin-14C metabolism in sheep. Identification of trans-6,7-di-
hydroxydihydroaldrin and 9-(syn-epoxy)-hydroxy-1,2,3,4,10,10-hexa-
chloro-6,7-epoxy-1,4,4a,5,6,7,8,8a-octahydro-1,4-endo-5,8-exo-
dimethanonaphthalene. J. agric. food Chem., 18(1): 120-124.
FELDMAN, R.J. & MAIBACH, H.I. (1974) Percutaneous penetration of
some pesticides and herbicides in man. Toxicol. appl. Pharmacol.,
28: 126-132.
FERGIN, T.J. & SCHAFER, E.C. (1977) Toxicity of dieldrin to
bobwhite quail in relation to sex and reproductive status. Arch.
environ. Contam. Toxicol., 6: 213-219.
FERNICOLA, N.A.G.G. & AZEVEDO, F.A. (1982) Serum levels of
organochlorine insecticides in humans in Sao Paulo, Brazil. Vet.
hum. Toxicol., 24(2): 91-93.
FIKES, M.H. & TUBB, R.A. (1972) Dieldrin uptake in the three-ridge
naiad. J. wildl. Manage., 36(3): 802-809.
FISCHLER, H.M. & KORTE, F. (1969) [Sensitized and non-sensitized
photoisomerization of cyclodiene insecticides.] Tetrahedron Lett.,
32: 2793-2796 (in German).
FISEROVA-BERGEROVA, V., RADOMSKI, J.L., DAVIES, J.E., & DAVIS, J.H.
(1967) Levels of chlorinated hydrocarbon pesticides in human
tissues. Ind. Med. Surg., 36: 65-70.
FISHER, H.L., MOST, B., & HALL, L.L. (1985) Dermal absorption of
pesticides calculated by deconvolution. J. appl. Toxicol., 5(3):
163-177.
FITZHUGH, O.G., NELSON, A.A., & QUAIFE, M.L. (1964) Chronic oral
toxicity of aldrin and dieldrin in rats and dogs. Food Cosmet.
Toxicol., 2: 551-561.
FLETCHER, T.E., PRESS, J.M., & WILSON, D.B. (1959) Exposure of
spray-men to dieldrin in residual spraying. Bull. World Health
Organ., 20: 15-25.
FLICKINGER, E.L. & KING, K.A. (1972) Some effects of aldrin-treated
rice on Gulf coast wildlife. J. wildl. Manage., 36(3): 706-727.
FOURNIER, E., TREICH, I., CAMPAGNE, L., & CAPELLE, N. (1972)
Pesticides organo-chlorés dans le tissus adipeux d'êtres humains en
France. Eur. J. Toxicol., 5(1): 11-26.
FOWKES, F.M., BENESI, H.A., RYLAND, L.B., LOEFFLER, E.S., & SUN,
Y.P. (1960) Clay catalysed decomposition of insecticides. J. agric.
food Chem., 8: 203-210.
FOWLER, J.F., NEWSOM, L.D., GRAVES, J.B., BONNER, F.L., &
SCHILLING, P.E. (1971) Effect of dieldrin on egg hatchability,
chick survival, and eggshell thickness in purple and common
gallinules. Bull. environ. Contam. Toxicol., 6(6): 495-501.
FOX, G.R. & VIRGO B.B. (1986) Relevance of hyperglycemia to
dieldrin toxicity in suckling and adult rats. Toxicology, 38:
315-326.
FRANK, R., MONTGOMERY, K., BRAUN, H.E., BERST, A.H., & LOFTUS, K.
(1974) DDT and dieldrin in watersheds draining the tobacco belt in
southern Ontario. Pestic. monit. J., 8(3): 184-201.
FRANK, R., BRAUN, H.E., ISHIDA, K., & SUDA, P. (1976) Persistent
organic and inorganic pesticide residues in orchard soils and
vineyards of southern Ontario. Can. J. Soil Sci., 56: 463-484.
FRANK, R., HOLDRINET, M.V.H., & SUDA, P. (1979) Organochlorine and
mercury residues in wild mammals in southern Ontario, Canada 1973-
1974. Bull. environ. Contam. Toxicol., 22: 500-507.
FRANK, R., BRAUN, H.E., & HOLDRINET, M.V.H. (1981) Residues from
past uses of organochlorine insecticides and PCB in waters draining
eleven agricultural watersheds in southern Ontario, Canada, 1975-
1977. Sci. total Environ., 20: 255-276.
FRANK, R., BRAUN, H.E., SIRONS, G.H., RASPER, J., & WARD, G.G.
(1985) Organochlorine and organophosphorus insecticides and
industrial pollutants in the milk supplies of Ontario, 1983. J.
food Prot., 48(6): 499-504.
FRY, D.R. (1964) Human dieldrin poisoning. Lancet, 1: 764.
FUCHS, P. (1967) Death of birds caused by application of seed
dressings in the Netherlands. Meded. Fac. Landbouwwet. Rijksuniv.
Gent., 32(3/4): 855-859.
FURNESS, R. & HUTTON, M. (1979) Pollutant levels in the great skua
Catharacta skua. Environ. Pollut., 13: 261-268.
FYTIANOS, K., VASILIKIOTIS, G., & WEIL, L. (1985) Identification
and determination of some trace organic compounds in coastal
seawater of Northern Greece. Bull. environ. Contam. Toxicol., 34:
390-395.
GAINES, T.B. (1960) The acute toxicity of pesticides to rats.
Toxicol. appl. Pharmacol., 2: 88-99.
GAK, J.C., GRAILLOT, C., & TRUHAUT, R. (1976) Use of the golden
hamster in toxicology. Lab. anim. Sci., 26(2): 274-280.
GAKSTATTER, J.H. (1968) Rates of accumulation of 14C-dieldrin
residues in tissues of goldfish exposed to a singe sublethal dose
of 14C-aldrin. J. Fish Res. Board Can., 25(9): 1797-1801.
GAKSTATTER, J.H. & WEISS, C.M. (1967) The elimination of DDT-14C,
dieldrin-14C, and lindane-14C from fish following a single sub-
lethal exposure in aquaria. Trans. Am. Fish. Soc., 96: 301-307.
GALLEY, R.A.E. (1970) [Chlorinated hydrocarbons. IV. Cyclodien
insecticides.] In: Wegler, R., ed. [Chemistry of crop protection
agents and pesticides,] Berlin, Heidelberg, New York, Springer-
Verlag, Vol. 1, pp. 163-192 (in German).
GANNON, N. & DECKER, G.C. (1958) The conversion of aldrin to
dieldrin on plants. J. econ. Entomol., 51: 8-11.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959a) Storage of dieldrin
in tissues of steers, hogs, lambs, and poultry fed dieldrin in
their diets. J. agric. food Chem., 7(12): 826-828.
GANNON, N., LINK, R.P., & DECKER, G.C. (1959b) Storage of dieldrin
in tissues and its excretion in milk of dairy cows fed dieldrin in
their diets. J. agric. food Chem., 7(12): 824-826.
GARRETTSON, L.K. & CURLEY, A. (1969) Dieldrin. Studies in a
poisoned child. Arch. environ. Health, 19: 814-822.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986a) Pesticides, selected elements, and other chemicals in
infant and toddler total-diet samples, October 1980 - March 1982.
J. Assoc. Off. Anal. Chem., 69(1): 123-145.
GARTRELL, M.J., CRAUN, J.C., PODREBARAC, D.S., & GUNDERSON, E.L.
(1986b) Pesticides, selected elements, and other chemicals in adult
total-diet samples, October 1980 - March 1982. J. Assoc. Off. Anal.
Chem., 69(1): 146-161.
GENELLY, R.E. & RUDD, R.L. (1956) Effects of DDT, toxaphene, and
dieldrin on pheasant reproduction. Auk, 73: 529-539.
GEORGIAN, L. (1975) The comparative cytogenetic effects of aldrin
and phosphamidon. Mutat. Res., 31: 103-108.
GEROLT, P. (1961) Investigation into the problem of insecticide
sorption by soils. Bull. World Health Organ., 24: 577-592.
GIFAP (1984) Pesticide residues in food, Brussels, Groupement
International des Associations Nationales des Fabricants de
Produits Agrochimique.
GILLETT, J.W. & CHAN, T.M. (1968) Cyclodiene insecticides as
inducers, substrates, and inhibitors of microsomal epoxidation. J.
agric. food Chem., 16(4): 590-593.
GINN, T.M. & FISHER, F.M., Jr (1974) Studies on the distribution
and flux of pesticides in waterways associated with a rice field.
Marshland ecosystems. Pestic. monit. J., 8(1): 23-32.
GISH, C.D. (1970) Organochlorine insecticide residues in soils and
soil invertebrates from agricultural lands. Pestic. monit. J., 3(4):
241-252.
GISH, C.D. & HUGHES, D.L. (1982) Residues of DDT, dieldrin, and
heptachlor in earthworms during two years following application,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 241).
GLATT, H., JUNG, R., & OESCH, F. (1983) Bacterial mutagenicity
investigation of epoxides: drugs, drug metabolites, steroids and
pesticides. Mutat. Res., 11: 99-118.
GLOOSCHENKO, W.A., STRACHAN, W.M.J., & SAMPSON, R.C.J. (1976)
Distribution of pesticides and polychlorinated biphenyls in water,
sediments, and seston of the Upper Great Lakes, 1974. Pestic.
monit. J., 10(2): 61-67.
GLOTFELTY, D.E. (1978) The atmosphere as a sink for applied
pesticides. J. Air Pollut. Control Assoc., 28: 917-921.
GONZALEZ RODRIGUEZ-CORDOBA, J.M., FERNANDES, A.L., & HENS, J.M.
(1983) [Mother neonate ratio of levels of blood contamination by
organo-chlorine insecticide residues.] Arch. Zootec., 32(122):
49-60 (in Spanish).
GOOD, E.E. & WARE, G.W. (1969) Effects of insecticides on
reproduction in the laboratory mouse. IV. Endrin and dieldrin.
Toxicol. appl. Pharmacol., 14: 201-203.
GOODWIN, E.S., GOULDEN, R., & REYNOLDS, J.G. (1961) Rapid
identification and determination of residues of chlorinated
pesticides in crops by gas-liquid chromatography. J. Soc. Anal.
Chem., 80(1028): 697-700.
GORCHEV, H.G. & JELINEK, C.F. (1985) A review of the dietary
intakes of chemical contaminants. Bull. World Health Organ., 63(5):
945-962.
GRACA, I., SILVA FERNANDES, A.M.S., & MOURAO, H.C. (1974)
Organochlorine insecticide residues in human milk in Portugal.
Pestic. monit. J., 8(3): 148-156.
GRANVILLE, G.C., SIMPSON, B.J., & DOAK, S.M. (1973) Toxicity
studies on aldrin dicarboxylic acid: 13-week oral study in rats,
Sittingbourne, Shell Research (TLGR.0008.73) (Unpublished
proprietary report).
GRAVES, J.B., BONNER, F.L., MCKNIGHT, W.F., WATTS, A.G., & EPPS,
E.A. (1969) Residues in eggs, preening glands, liver, and muscle
from feeding dieldrin-contaminated rice bran to hens and its
effects on egg production, egg hatch, and chick survival. Bull.
environ. Contam. Toxicol., 4(6): 375-383.
GREENBERG, R.E. & EDWARDS, W.R. (1970) Insecticide residue levels
in eggs of wild pheasants in Illinois. Trans. Illinois State Acad.
Sci., 63: 136-147.
GREICHUS, Y.A., GREICHUS, A., & REIDER, E.G. (1968) Insecticide
residues in grouse and pheasant of South Dakota. Pestic. monit. J.,
2(2): 90-92.
GREICHUS, Y.A., GREICHUS, A., DRAAYER, H.A., & MARSHALL, B. (1978a)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. II. Lake McLlwaine, Rhodesia. Bull. environ. Contam.
Toxicol., 19: 444-453.
GREICHUS, Y.A., GREICHUS, A., AMMANN, B.D., & HOPCRAFT, J. (1978b)
Insecticides, polychlorinated biphenyls, and metals in African lake
ecosystems. III. Lake Nakuru, Kenya. Bull. environ. Contam.
Toxicol., 19: 454-461.
GREVE, P.A. (1972) Potentially hazardous substances in surface
waters. Part I. Pesticides in the river Rhine. Sci. total Environ.,
1: 173-180.
GREVE, P.A. & WEGMAN, R.C.C. (1985) Organochlorine compounds in
human milk: data from a recent investigation in the Netherlands,
Copenhagen, World Health Organization, Regional Office for Europe
(ICP/CEH. 501/M05).
GRIFFIN, D.E. & HILL, W.E. (1978) In vitro breakage of plasmid DNA
by mutagens and pesticides. Mutat. Res., 52: 161-169.
GRIFFITH, J. & DUNCAN, R.C. (1985) Serum organochlorine residues in
Florida citrus workers compared to the national health and
nutrition examination survey sample. Bull. environ. Contam.
Toxicol., 35: 411-417.
GRIFFITHS, D.C., RAW, F., & LOFTY, J.R. (1967) The effects on soil
fauna of insecticides tested against wireworms ( Agriotes ssp.) in
wheat. Ann. appl. Biol., 60: 479-490.
GROSCH, D.S. & VALCOVIC, I.R. (1967) Chlorinated hydrocarbon
insecticides are not mutagenic in Bracon hebetor tests. J. econ.
Entomol., 60(4): 1177-1179.
GUERZONI, M.E., DEL CUPOLO, L., & PONTI, I. (1976) Mutagenic
activity of pesticides. Riv. Sci. Tec. Aliment. Nutr. Um., 6:
161-165.
GUTENMANN, W.H., GREENWOOD, R.A., GYRISCO G.G., & LITTLE, R.J.
(1972) Studies of aldrin and chlordane in silt loam soils and their
possible translocation in field corn in New York. J. econ.
Entomol., 65(3): 842-844.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of the Netherlands. Sci. total
Environ., 43: 193-219.
GUPTA, H.C.L. & KAVADIA, V.S. (1979) Dissipation of aldrin residues
in clay loam soil under the cover of root crops. Indian J. plant
Prot., 7(1): 43-49.
GUPTA, H.C.L., KUSHWAHA, K.S., KAVADIA, V.S., & SRIVASTAVA, B.P.
(1979) Aldrin residues in soils and its translocation in maize and
pearl millet. Indian J. Entomol., 41(1): 47-57.
GUPTA, P.C. (1975) Neurotoxicity of chronic chlorinated hydrocarbon
insecticide poisoning: a clinical and electroencephalographic study
in man. Indian J. med. Res., 63(4): 601-606.
HAAN, C.T. (1971) Movement of pesticides by runoff and erosion.
Trans. Am. Soc. Agric. Eng., 14: 445-447, 449.
HAEGELE, M.A. & TUCKER, R.K. (1974) Effects of 15 common
environmental pollutants on eggshell thickness in mallards and
Cournix. Bull. environ. Contam. Toxicol., 11(1): 98-102.
HAGLEY, E.A.C. (1965) Effect of insecticides on the growth of
vegetable seedlings. J. econ. Entomol., 58(4): 777-778.
HAMILTON, H.E., MORGAN, D.P., & SIMMONS, A. (1978) A pesticide
(dieldrin)-induced immunohemolytic anemia. Environ. Res., 17:
155-164.
HARGESHEIMER, E.E. (1984) Rapid determination of organochlorine
pesticides and polychlorinated biphenyls using selected ion
monitoring mass spectrometry. J. Assoc. Off. Anal. Chem., 67(6):
1067-1075.
HARR, J.R., CLAEYS, R.R., BONE, J.F., & MCCORCLE, T.W. (1970)
Dieldrin toxicosis: rat reproduction. Am. J. vet. Res., 31: 181-189.
HARRIS, C.I. (1969) Movement of pesticides in soil. J. agric. food
Chem., 17(1): 80-82.
HARRIS, C.R. (1964) Influence of soil type and soil moisture on the
toxicity of insecticides in soils to insects. Nature (Lond.), 202:
724.
HARRIS, C.R. (1972) Behaviour of dieldrin in soil. Laboratory
studies influencing biological activity. J. econ. Entomol., 65: 8-13.
HARRIS, C.R. & SANS, W.W. (1967) Absorption of organochlorine
insecticide residues from agricultural soils by root crops. J.
agric. food Chem., 15: 86-93.
HARRIS, C.R., SANS, W.W., & MILES, J.R.W. (1966) Exploratory
studies on occurrence of organochlorine insecticide residues in
agricultural soils in Southwestern Ontario. J. agric. food Chem.,
14(4): 398-403.
HARRISON, R.B., HOLMES, D.C., ROBURN, J., & TATTON, J.O'G. (1967)
The fate of some organochlorine pesticides on leaves. J. Sci. Food
Agric., 18: 10.
HARVEY, G.R., STEINHAVER, W.G., & TEAL, J.M. (1973) Polychlorobiphenyls
in North Atlantic Ocean water. Science, 180: 643-644.
HARVEY, G.R., STEINHAVER, W.G., & MIKLAS, H.P. (1974) Decline of
PCB concentrations in North Atlantic surface water. Nature (Lond.),
252: 387-388.
HASELTINE, S.D., HEINZ, G.H., REICHEL, W.L., & MOORE, J.F. (1981)
Organochlorine and metal residues in eggs of waterfowl nesting on
islands in Lake Michigan off Door County, Wisconsin, 1977-78.
Pestic. monit. J., 15(2): 90-97.
HASHEMY-TONKABONY, S.E. & SOLEIMANI-AMIRI, M.J. (1978) Chlorinated
pesticide residues in the body fat of people in Iran. Environ.
Res., 16: 419-422.
HATHWAY, D.E., MOSS, J.A., ROSE, J.A., & WILLIAMS, D.J.M. (1967)
Transport of dieldrin from mother to blastocyst and from mother to
foetus in pregnant rabbits. Eur. J. Pharmacol., 1: 167-175.
HAVERA, S.P. & DUZAN, R.E. (1986) Organochlorine and PCB residues
in tissues of raptors from Illinois, 1966-81. Bull. environ.
Contam. Toxicol., 36: 23-32.
HAYES, W.J. (1957) Dieldrin poisoning in man, Washington, DC, US
Department of Health, Eduction and Welfare, Public Health Service,
Vol. 72, pp. 1087-1091 (Public Health Report No. 12).
HAYES, W.J. (1959) The toxicity of dieldrin to man. Report on a
survey. Bull. World Health Organ., 20: 891-912.
HAYES, W.J., Jr (1963) Clinical handbook on economic poisons.
Emergency information for treating poisoning, Washington, DC, US
Government Printing Office (Public Health Service Publication No.
476).
HAYES, W.J. (1974) Distribution of dieldrin following a single oral
dose. Toxicol. appl. Pharmacol., 28: 485-492.
HAYES, W.J. (1982) Chlorinated hydrocarbon insecticides. In:
Pesticides studied in man, Baltimore, Maryland, Williams and
Wilkins, pp. 172-283.
HAYES, W.J. & CURLEY, A. (1968) Storage and excretion of dieldrin
and related compounds. Arch. environ. Health, 16(2): 155-162.
HAYES, W.J., Jr, DALE, W.E., & BURSE, V.W. (1965) Chlorinated
hydrocarbon pesticides in the fat of people in New Orleans. Life
Sci., 4: 1611-1615.
HEATH, D.F. & VANDEKAR, M. (1964) Toxicity and metabolism of
dieldrin in rats. Br. J. ind. Med., 21: 269-279.
HEESCHEN, W. (1972) Analyses for residues in milk and milk
products. In: Coulston, F. & Korte, F., ed. Environmental quality
and safety, Stuttgart, G. Thieme Verlag, Vol. 1, p. 229.
HEINZ, G.H. & JOHNSON, R.W. (1981) Diagnostic brain residues of
dieldrin: some new insights. In: Lamb, D.W. & Kenaga, E.E., ed.
Avian and mammalian wildlife toxicology. Second Conference,
Philadelphia, Pennsylvania, American Society of Testing Materials,
pp. 72-92 (STP757).
HELENE, C.G., LORD, K.A., & RUEGG, E.F. (1981) The persistence,
leaching, and volatilization of 14C aldrin in two Brazilian soils
Cienc. Cult., 33: 101-105.
HENDERSON, C., PICKERING, Q.H., & TARZWELL, C.M. (1959) Relative
toxicity of ten chlorinated hydrocarbon insecticides to four
species of fish. Trans. Am. Fish. Soc., 88: 23-32.
HENDERSON, C., JOHNSON, W.L., & INGLIS, A. (1969) Organochlorine
insecticide residues in fish. Pestic. monit. J., 3(3): 145-171.
HENDERSON, C., INGLIS, A., & JOHNSON, W.L. (1971) Organochlorine
insecticide residues in fish - fall 1969. Pestic. monit. J., 5(1):
1-11.
HENDERSON, G.L. & CROSBY, D.G. (1968) The photodecomposition of
dieldrin residues in water. Bull. environ. Contam. Toxicol., 3:
131-134.
HERRERA MARTEACHE, A., POLO VILLAR, L.M., JODRAL VILLAREJO,
M., POLO VILLAR, G., MALLOL, J., & POZO LORA, R. (1978)
[Organochlorine pesticide residues in human fat in Spain.] Rev.
Sanid. Hig. publica., 52: 1125-1144 (in Spanish).
HERZEL, F. (1971) [The behaviour of some persistent insecticides in
the soil.] Bundesgesundheitsblatt, 14(3): 23-28 (in German).
HERZEL, F. (1972) Organochlorine insecticides in surface waters in
Germany: 1970 and 1971. Pestic. monit. J., 6(3): 179-187.
HEYNDRICKX, A. & MAES, R. (1969) The excretion of chlorinated
hydrocarbon insecticides in human mother milk. J. Pharm. Belg., 24:
459-463.
HICKEY, J.J., ed. (1969) Peregrine falcon populations: their
biology and decline, Madison, Wisconsin, University of Wisconsin
Press.
HICKEY, J.J. & ANDERSON, D.W. (1968) Chlorinated hydrocarbons and
eggshell changes in raptorial and fish-eating birds. Science, 162:
271-273.
HILL, D.W. & MCCARTY, P.L. (1967) Anaerobic degradation of selected
chlorinated hydrocarbon pesticides. J. Water Pollut. Control Fed.,
39: 1259-1277.
HILL, E.F., HEATH, R.G., SPANN, J.W., & WILLIAMS, J.D. (1975)
Lethal dietary toxicities of environmental pollutants to birds,
Washington, DC, US Department of the Interior, Fish and Wildlife
Service (Special Scientific Report: Wildlife No. 191).
HILL, E.F., SPANN, J.W., & WILLIAMS, J.D. (1977) Responsiveness of
6 to 14 generations of birds to dietary dieldrin toxicity. Toxicol.
appl. Pharmacol., 42: 425-431.
HINDIN, E., MAY, D.S., & DUNSTAN, G.S. (1964) Collection and
analysis of synthetic organic pesticides from surface and ground
water. Residue Rev., 7: 130-156.
HODGE, H.C., BOYCE, A.M., DEICHMANN, W.B., & KRAYBILL, H.F. (1967)
Toxicology and no-effect levels of aldrin and dieldrin. Toxicol.
appl. Pharmacol., 10: 613-675.
HOERSCHELMANN, H., POLZHOFER, K., FIGGE, K., & BALLSCHMITER,
K. (1979) [Organochlorine pesticides and polychlorinated biphenyls
in birds eggs from the Falkland Islands and from northern Germany.]
Environ. Pollut., 13: 247-269 (in German).
HOFFMAN, W.S., ADLER, H., FISHBEIN, W.I., & BAUER, F.C. (1967)
Relation of pesticide concentrations in fat to pathological changes
in tissues. Arch. environ. Health, 15: 758-765.
HOGAN, R.L. & ROELOFS, E.W. (1971) Concentrations of dieldrin in
the blood and brain of the green sunfish Lepomis cyanellus at
death. J. Fish Res. Board Can., 28(4): 610-612.
HOLDEN, A.V. (1975) The accumulation of oceanic contaminants in
marine mammals, Rapp. P.-V. Réun. Cons. int. Explor. Mer, 169:
353-361.
HOLDRINET, M.V.H., BRAUN, H.E., FRANK, R., STOPPS, G.E., SMOUT,
M.S., & MCWADE, J.W. (1977) Organochlorine residues in human
adipose tissue and milk from Ontario residents, 1969-1974. Can. J.
public Health, 68: 74-80.
HOLT, R.L., CRUSE, S., & GREEN, E.S. (1986) Pesticide and
polychlorinated biphenyl residues in human adipose tissue from
North East Louisiana. Bull. environ. Contam. Toxicol., 36: 651-655.
HOOFTMAN, R.N. & VINK, G.J. (1980) The determination of toxic
effects of pollutants with the marine polychaete worm Ophryotrocha
diadema. Ecotoxicol. environ. Saf., 4: 252-262.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1962) Electro-
encephalograms in insecticide toxicity. Arch. environ. Health, 4:
86-94.
HOOGENDAM, I., VERSTEEG, J.P.J., & DE VLIEGER, M. (1965) Nine
years' toxicity control in insecticide plants. Arch. environ.
Health, 10: 441-448.
HORNABROOK, R.W., DYMENT, P.G., GOMES, E.D., & WISEMAN, J.S. (1972)
DDT residues in human milk from New Guinea natives. Med. J. Aust.,
1: 1297-1300.
HUDSON, R.H., TUCKER, R.K., & HAEGELE, M.A. (1984) Handbook of
toxicity of pesticides to wildlife, 2nd ed., US Department of the
Interior, Fish and Wildlife Service, pp. 9-10 (Publication No.
153).
HUNT, P.F., STEVENSON, D.E., THORPE, E., & WALKER, A.I.T. (1975)
Mouse data. Letter to the editor. Food Cosmet. Toxicol., 13:
597-599.
HUNTER, C.G. & ROBINSON, J. (1967) Pharmacodynamics of dieldrin
(HEOD). I. Ingestion by human subjects for 18 months. Arch.
environ. Health, 15(5): 614-626.
HUNTER, C.G. & ROBINSON, J. (1968) Aldrin, dieldrin, and man. Food
Cosmet. Toxicol., 6: 253-260.
HUNTER, C.G., ROBINSON, J., & RICHARDSON, A. (1963) Chlorinated
insecticide content of human body fat in southern England. Br. med.
J., 1: 221-224.
HUNTER, C.G., ROBINSON, J., & JAGER, K.W. (1967) Aldrin and
dieldrin: the safety of present exposures of the general
populations of the United Kingdom and the United States. Food
Cosmet. Toxicol., 5: 781-787.
HUNTER, C.G., ROBINSON, J., & ROBERTS, M. (1969) Pharmaco-dynamics
of dieldrin (HEOD). II. Ingestion by human subjects for 18 to 24
months, and post-exposure for eight months. Arch. environ. Health,
18(1): 12-21.
HUTSON, D.H. (1976) Comparative metabolism of dieldrin in the rat
(CFE) and in two strains of mouse (CF1 and LACG). Food Cosmet.
Toxicol., 14: 577-591.
IARC (1974) Some organochlorine pesticides, Lyons, International
Agency for Research on Cancer, 241 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans, Vol.
5).
IARC (1982) Chemicals, industrial processes, and industries
associated with cancer in humans, Lyons, International Agency for
Research on Cancer, Vol. 1-29, 292 pp (IARC Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Man, Suppl. 4).
IARC (1987) Overall evaluations of carcinogenicity. An update of
IARC Monographs, Lyons, International Agency for Research on
Cancer, pp. 1-42 (Monographs on the Evaluation of Carcinogenic
Risks to Humans, Suppl. 7).
IATROPOULOS, M.J., MILLING, A., MUELLER, W.F., NOHYNEK, G., ROZMAN,
K., COULSTON, F., & KORTE, F. (1975) Absorption, transport, and
organotropism of dichlorobiphenyl (DCB), dieldrin, and
hexachlorobenzene (HCB) in rats. Environ. Res., 10: 384-389.
IBRAHIM, T.M. (1964) A toxicological study of the action of the
insecticide dieldrin and related substances on the contraction of
striated muscle in the rat, Utrecht, Rijks Universiteit (Thesis).
ICHINOSE, R. & KURIHARA, N. (1985) Uptake of dieldrin, lindane, and
DDT by isolated hepatocytes. Pestic. Biochem. Physiol., 23: 116-122.
IGUE, K., FARMER, W.J., SPENCER, W.F., & MARTIN, J.P. (1972)
Volatility of organochlorine insecticides from soil. II. Effect of
relative humidity and soil water content on dieldrin volatility.
Soil Sci. Soc. Am. Proc., 36: 447-450.
ITO, N., TSUDA, H., HASEGAWA, R., & IMAIDA, K. (1983) Comparison of
the promoting effects of various agents in induction of
preneoplastic lesions in rat liver. Environ. Health Perspect., 50:
131-138.
IVEY, M.C., CLABORN, H.V., MANN, H.D., RADELEFF, R.D., & WOODARD,
G.T. (1961) Aldrin and dieldrin content of body tissues of
livestock receiving aldrin in their diet. J. agric. food Chem.,
9(5): 374-376.
IVIE, G.W. & CASIDA, J.E. (1970) Enhancement of photo-alteration of
cyclodiene insecticide chemical residues by rotenone. Science, 167:
1620-1622.
JAGER, K.W. (1970) Aldrin, dieldrin, endrin, and telodrin: an
epidemiological and toxicological study of long-term occupational
exposure, Amsterdam, London, New York, Elsevier Publishing Company,
234 pp.
JANSEN, J.D. (1979) The predictive value of tests for carcinogenic
or mutagenic activity. In: Deichmann, W.B., ed. Toxicology and
occupational medicine, Amsterdam, Elsevier/North Holland, pp.
71-80.
JEFFERIES, D.J. (1969) Causes of badger mortality in eastern
counties of England. J. Zool. (Lond.), 157: 429-436.
JEFFERIES, D.J. (1972) Organochlorine insecticide residues in
British bats and their significance. J. Zool. (Lond.), 166:
245-263.
JEFFERIES, D.J. (1975) Different activity patterns of male and
female badgers (Meles meles) as shown by road mortality. J. Zool.
(Lond.), 177: 504-506.
JEFFERIES, D.J. & DAVIS, B.N.K. (1968) Dynamics of dieldrin in
soil, earthworms, and song thrushes. J. wildl. Manage., 32(3):
441-456.
JEFFERIES, D.J. & PENDLEBURY, J.B. (1968) Population fluctuations
of stoats, weasels and hedgehogs in recent years. J. Zool. (Lond.),
156: 513-517.
JEFFERIES, D.J., STAINSBY, B., & FRENCH, M.C. (1973) The ecology of
small mammals in arable fields drilled with winter wheat and the
increase in their dieldrin and mercury residues. J. Zool. (Lond.),
171: 513-539.
JEFFERIES, D.J., FRENCH, M.C., & STEBBING, R.E. (1974) Pollution
and mammals: otters, Huntingdon, Natural Environment Research
Council, Institute of Terrestrial Ecology, Monks Wood Experimental
Station, pp. 13-15 (Report for 1972-73).
JENSEN, L.D. & GAUFIN, A.R. (1966) Acute and long-term effects of
organic insecticides on two species of stonefly naiads. J. Water
Pollut. Control Fed., 38(8): 1273-1286.
JOHNSON, L.G. & MORRIS, R.L. (1971) Chlorinated hydrocarbon
pesticides in Iowa rivers. Pestic. monit. J., 4(4): 216-219.
JOHNSON, R.D. & MANSKE, D.D. (1977) Pesticide and other chemical
residues in total diet samples. XI. Pestic. monit. J., 11(3): 116-131.
JOHNSON, W.W. & FINLEY, M.T. (1980) Handbook of acute toxicity of
chemicals to fish and aquatic invertebrates, Washington, DC, US
Department of the Interior, Fish and Wildlife Service, pp. 1-3, 8,
10, 29-30 (Resource Publication No. 137).
JOHNSTON, B.T., SAUNDERS C.R., SANDERS, H.O., & CAMPBELL, R.S.
(1971) Biological magnification and degradation of DDT and aldrin
by fresh-water invertebrates. J. Fish Res. Board Can., 28: 705-709.
JOHNSTON, D.W. (1975) Organochlorine pesticide residues in small
migratory birds, 1964-1973. Pestic. monit. J., 9(2): 79-88.
JOLLY, D.W. (1954) Studies on the acute toxicity of dieldrin to
sheep. Vet. Rec., 66: 444-447.
JONAS, R.B. & PFAENDER, F.K. (1976) Chlorinated hydrocarbon
pesticides in Western North Atlantic Ocean. Environ. Sci. Technol.,
10(8): 770-773.
JONES, D.M., BENNETT, D., & ELGAR, K.E. (1978) Deaths of owls
traced to insecticide-treated timber. Nature (Lond.), 272: 52.
JOY, R.M. (1976) The alteration by dieldrin of cortical
excitability conditioned by sensory stimuli. Toxicol. appl.
Pharmacol., 38(2): 357-368.
JOY, R.M. (1977) Contrasting actions of dieldrin and aldrin-
transdiol, its metabolite, on cat CNS functions. Toxicol. appl.
Pharmacol., 42: 137-148.
JOY, R.M. (1982) Mode of action of lindane, dieldrin, and related
insecticides in the central nervous system. Neurobehav. Toxicol.
Teratol., 4: 813-823.
JUNK, G.A., SPALDING, R.F., & RICHARD, J.J. (1980) Aerial,
vertical, and temporal differences in groundwater chemistry. II.
Organic constituents. J. environ. Qual., 9: 479-483.
JURY, W.A., SPENCER, W.F., & FARMER, W.J. (1983) Use of models for
assessing relative volatility, mobility, and persistence of
pesticides and other trace organics in soil systems. In: Saxena,
J., ed. Hazard assessment of chemicals, current development, Vol.
2, New York, London, Academic Press, pp 1-43.
KADIS, V.W., BREITKREITZ, W.E., & JONASSON, O.J. (1970) Insecticide
levels in human tissues of Alberta residents. Can. J. public
Health, 61(5): 413-416.
KAISER, T.E., REICHEL, W.L., LOCKE, L.N., CROMARTIE, E., KRYNITSKY,
A.J., LAMONT, T.G., MULHERN, B.M., PROUTY, R.M., STAFFORD, C.J., &
SWINEFORD, D.M. (1980) Organochlorine pesticide, PCB, and PBB
residues and necropsy data for bald eagles from 29 states - 1975-
77. Pestic. monit. J., 13(4): 145-149.
KANITZ, S. & CASTELLO, G. (1966) [Presence of residues of some
disinfectants in human fatty tissue and in certain foods.] G. Ig.
Med. prev., 7: 1-19 (in Italian).
KANJA, L., SKARE, J.K., NAFSTAD, I., MAITAI, C.K., & LOKKEN, P.
(1986) Organochlorine pesticides in human milk from different areas
of Kenya, 1983-1985. J. Toxicol. environ. Health, 19: 449-464.
KATZ, M. (1961) Acute toxicity of some organic insecticides to
three species of salmonids and to the three-spine stickleback.
Trans. Am. Fish. Soc., 90(2): 264-268.
KAWATSKI, J.A. & SCHMULBACH, J.C. (1972) Uptake and elimination of
14C-aldrin and 14C-dieldrin by the ostracod Chlamydotheca arcuata
(Sars). Int. J. environ. anal. Chem., 1: 283-291.
KAZANTZIS, G., MCLAUGHLIN, A.I.G., & PRIOR, P.F. (1964) Poisoning
in industrial workers by the insecticide aldrin. Br. J. ind. Med.,
21: 46-51.
KEANE, W.T. & ZAVON, M.R. (1969a) Validity of a critical blood
level for prevention of dieldrin intoxication. Arch. environ.
Health, 19: 36-44.
KEANE, W.T. & ZAVON, M.R. (1969b) The total body burden of
dieldrin. Bull. environ. Contam. Toxicol., 4(1): 1-16.
KELLER, J. & ALFARO, J.F. (1966) Effect of water application rate
on leaching. Soil Sci., 102(2): 107-114.
KEPLINGER, M.L., DEICHMANN, W.B., & SALA, F. (1970) Effects of
combinations of pesticides on reproduction in mice. In: Pesticides
symposia, Miami Beach, Florida, Halos and Associates Inc., pp.
125-138.
KHAIRY, M. (1960) Effects of chronic dieldrin ingestion on the
muscular efficiency of rats. Br. J. ind. Med., 17: 146-148.
KHAN, M.A.Q., COELLO, W., KHAN, A.A., & PINTO, H. (1972a) Some
characteristics of the microsomal mixed-function oxidase in the
fresh-water crayfish Cambarus. Life Sci., 11(2): 405-415.
KHAN, M.A.Q., KAMAL, A., WOLIN, R.J., & RUNNELS, J. (1972b) In vivo
and in vitro epoxidation of aldrin by aquatic food chain organisms.
Bull. environ. Contam. Toxicol., 8(4): 219-228.
KING, P.H. & MCCARTY, P.L. (1968) A chromatographic model for
predicting pesticide migration in soils. Soil Sci., 106(4): 248-261.
KINOSHITA, F.K. & KEMPF, C.K. (1970) Quantitative measurement of
hepatic microsomal enzyme induction after dietary intake of
chlorinated hydrocarbon insecticides. Toxicol. appl. Pharmacol.,
17(1): 288.
KITSELMAN, C.H. (1953) Long-term studies on dogs fed aldrin and
dieldrin in sublethal dosages, with reference to the
histopathological findings and reproduction. J. Am. Vet. Med.
Assoc., 123: 28-30.
KLAASSEN, H.E. & KADOUM, A.M. (1973) Pesticide residues in natural
fish populations of the Smoky Hill River of Western Kansas - 1967-
1969, Pestic. monit. J., 7(1): 53-61.
KLAUNIG, J.E., GOLDBLATT, P.J., HINTON, D.E., LIPSKY, M.M., &
TRUMP, B. (1984) Carcinogen-induced unscheduled DNA synthesis in
mouse hepatocytes. Toxicol. Pathol., 12(2): 119-125.
KLEIN, A.K., LINK, J.D., & IVES, N.F. (1968) Isolation and
purification of metabolites found in the urine of male rats fed
aldrin and dieldrin. J. Assoc. Off. Agric. Chem., 51: 895-898.
KLEIN, A.K., DAILEY, R.E., WALTON, M.S., BECK, V., & LINK, J.D.
(1970) Metabolites isolated from urine of rats fed 14C-
photodieldrin. J. agric. food Chem., 18(4): 705-708.
KLEIN, M.L. & LINCER, J.L. (1974) Behavioural effects of dieldrin
upon the fiddler crab Uca pugilator. In: Vernberg, F.J. &
Vernberg, W.B., ed. Pollution and physiology of marine organisms,
New York, London, Academic Press, pp. 181-196.
KLEIN, W., KOHLI, J., WEISGERBER, I., & KORTE, F. (1973) Fate of
aldrin-14C in potatoes and soil under outdoor conditions. J. agric.
food Chem., 21(2): 152-156.
KLEMMER, H.W., RASHAD, M.N., & MI, M.P. (1973) Age, sex, and race
effects on the distribution of organochlorine pesticide residues in
serum. In: Deichmann, W.B., ed. Pesticides and the environment,
New York, International Medical Book Corporation, pp. 53-61.
KLEVAY, L.M. (1970) Dieldrin excretion by the isolated perfused rat
liver: a sexual difference. Toxicol. appl. Pharmacol., 17: 813-815.
KOEMAN, J.H. (1971) [The occurrence and the toxicological
implications of some chlorinated hydrocarbons in the Dutch coastal
area in the period 1965-70], Utrecht, Rijks Universiteit (Thesis)
(in Dutch).
KOEMAN, J.H. & PENNINGS, J.H. (1970) An orientational survey on the
side effects and environmental distribution of insecticides used in
tsetse control in Africa. Bull. environ. Contam. Toxicol., 5(2):
164-170.
KOEMAN, J.H., OSKAMP, A.A.G., VEEN, J., BROUWER, E., ROOTH, J.,
ZWART, P., VAN DE BROCK, E., & VAN GENDEREN, H. (1967) Insecticides
as a factor in the mortality of the sandwich tern (Sterna
sandvicensis). A preliminary communication. Meded. Fac. Landouwwet.
Rijksuniv. Gent, 32: 841-854.
KOEMAN, J.H., VINK, J.A.J., & DE GOEIJ, J.J.M. (1969) Causes of
mortality in birds of prey and owls in the Netherlands in the
winter of 1968-69. Ardea, 57: 67-76.
KOEMAN, J.H., PEETERS, W.H.M., & PENNINGS, J.H. (1971) OECD
collaborative study 1969/71: pesticide residues in the environment,
Utrecht, The Netherlands, Institute of Veterinary Pharmacology and
Toxicology (Unpublished report).
KOEMAN, J.H., VAN BEUSEKOM, C.F., & DE GOEIJ, J.J.M. (1972)
Eggshell and population changes in the sparrow hawk (Accipiter
nisus). TNO Nieuws, 27: 542-550.
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1972) [Contributions to
ecological chemistry ILI(1). Transformation and residue behaviour
of dieldrin 14C in onions after seed treatment.] Chem. Mikrobiol.
Technol. Lebensm., 1: 149-150 (in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973a) [Contributions to
ecological chemistry LVIII. Transport of aldrin-14C and
transformation products in the soil.] Chemosphere, 3: 125-130
(in German).
KOHLI, J., WEISGERBER, I., & KLEIN, W. (1973) [Contributions to
ecological chemistry LIX. Residue behaviour and transformation of
14C-dieldrin in crops, soil and seepage water after application to
the soil.] Chemosphere, 4: 153-156 (in German).
KORSCHGEN, L.J. (1971) Disappearance and persistence of aldrin
after five annual applications. J. wildl. Manage., 35(3): 494-500.
KORTE, F. (1965) Metabolism of chlorinated insecticides. In:
Radioisotopes in the detection of pesticide residues. Proceedings
of the International Atomic Energy Panel, Vienna, International
Atomic Energy Agency, p. 38.
KORTE, F. & ARENT, H. (1965) Metabolism of insecticides. IX(1).
Isolation and identification of dieldrin metabolites from urine of
rabbits after oral administration of dieldrin-14C. Life Sci., 4:
2017-2026.
KRAUL, I. & KARLOG, O. (1976) Persistent organochlorinated
compounds in human organs collected in Denmark, 1972-73. Acta
pharmacol. toxicol., 38: 38-48.
KRAYBILL, H.F. (1977) Global distribution of carcinogenic
pollutants in water. In: Proceedings of the Conference on Aquatic
Pollutants and Biological Effects with Emphasis on Neoplasia, 27-29
September, New York, New York Academy of Sciences.
KRIEGER, R.I. & WILKINSON, C.F. (1969) Microsomal mixed-function
oxidases in insects. I. Localization and properties of an enzyme
system affecting aldrin epoxidation in larvae of the southern
armyworm (Prodenia eridania). Biochem. Pharmacol., 18: 1403-1415.
KURATA, M., HIROSE, K., & UMEDA, M. (1982) Inhibition of metabolic
cooperation in Chinese hamster cells by organochlorine pesticides.
Gann, 73: 217-221.
KURIHARA, N., HORI, N., & ICHINOSE, R. (1984) Cytochrome P-450
content and aldrin epoxidation to dieldrin in isolated rat
hepatocytes. Pestic. Biochem. Physiol., 21: 63-73.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, N.S. (1978a) Persistence
and dissipation of aldrin and dieldrin in soil: a review.
Pesticides, 12(6): 14-18.
KUSHWAHA, K.S., GUPTA, H.C.L., & KAVADIA, V.S. (1978) Effect of
temperature on the degradation of aldrin residues in sandy loam
soil. Ann. arid Zone, 17(2): 200-206.
KUTZ, F.W., YOBS, A.R., & YANG, H.S.C. (1976) National pesticide
monitoring programs. In: Lee, R.E., ed. Air pollution from
pesticides and agricultural processes, Cleveland, Ohio, CRC Press,
pp. 95-136.
KUTZ, F.W., STRASSMAN, S., & YOBS, A.R. (1979) Survey of pesticide
residues and their metabolites in the general population of the
United States. In: Berlin, A., Wolff, A.H., & Hasegawa, Y., ed. Use
of biological specimens to assess human exposure to environmental
pollutants, The Hague, Martinus Nijhoff, pp. 267-274.
LANE, C.E. & LIVINGSTON, R.J. (1970) Some acute and chronic effects
of dieldrin on the sailfin molly Poecilia latipinna. Trans. Am.
Fish. Soc., 99(3): 489-495.
LANE, C.E., SEBA, D.B., & HEARN, W.L. (1970) Possible metabolites
of dieldrin in the sailfin molly (Poecilia latipinna). Proc. Soc.
Exp. Biol. Med., 133: 1375-1377.
LAUBSCHER, J.A., DUTT, G.R., & ROAN, C.C. (1971) Chlorinated
insecticide residues in wildlife and soil as a function of distance
from application. Pestic. monit. J., 5(3): 251-258.
LAWRENCE, L.J. & CASIDA, J.E. (1984) Interactions of lindane,
toxaphene, and cyclodiene with brain-specific t-butyl-
bicyclophosphorothionate receptor. Life Sci., 35: 171-178.
LAY, J.P., WEISGERBER, I., & KLEIN, W. (1975) Conversion of the
aldrin/dieldrin metabolite dihydrochlordene dicarboxylic acid-14C
in rats. Pestic. Biochem. Physiol., 5: 226-232.
LEHMAN, A.J. (1951) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Q. Bull. Assoc. Food Drug Off., 15: 122-133.
LEHMAN, A.J. (1952) Chemicals in foods: a report to the Association
of Food and Drug Officials on current developments. Part II.
Pesticides. Section II. Dermal toxicity. Q. Bull. Assoc. Food Drug
Off. (USA), 16: 3-9.
LEHNER, P.N. & EGBERT, A. (1969) Dieldrin and eggshell thickness in
ducks. Nature (Lond.), 224: 1218-1219.
LENARDON, A.M., DE HEVIA, M.I.M., FUSE, J.A., DE NOCHETTO, C.B., &
DEPETRIS, P.J. (1984) Organochlorine and organophosphorus
pesticides in the Parana river (Argentina). Sci. total Environ., 34:
289-297.
LENON, H., CURRY, L., MILLER, A., & PATULSKI, D. (1972) Insecticide
residues in water and sediment from cisterns on the US and British
Virgin Islands. Pestic. monit. J., 6: 188-193.
LEWIS, R.G. (1976) Sampling and analysis of airborne pesticides.
In: Lee, R.E., Jr, ed. Air pollution from pesticides and
agricultural processes, Cleveland, Ohio, CRC Press, pp. 51-94.
LEWIS, R.G. & LEE, R.E., Jr (1976) Air pollution from pesticides:
sources, occurrence, and dispersion. In: Lee, R.E., Jr, ed. Air
pollution from pesticides and agricultural processes, Cleveland,
Ohio, CRC Press, pp. 5-50.
LICHTENBERG, J.J., EICHELBERGER, J.W., DRESSMAN, R.C., &
LONGBOTTOM, J.E. (1970) Pesticides in surface waters of the United
States: a 5-year summary, 1964-68. Pestic. monit. J., 4(2): 71-86.
LICHTENSTEIN, E.P. (1959) Absorption of some chlorinated
hydrocarbon insecticides from soils into various crops. J. agric.
food Chem., 7: 430-433.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1959) Breakdown of lindane and
aldrin in soils. J. econ. Entomol., 52(1): 118-124.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1960) Epoxidation of aldrin and
heptachlor in soils as influenced by autoclaving moisture and soil
types. J. econ. Entomol., 53(2): 192-197.
LICHTENSTEIN, E.P. & SCHULZ, K.R. (1970) Volatilization of
insecticides from various substrates. J. agric. food Chem., 18(5):
814-818.
LICHTENSTEIN, E.P., MUELLER, C.H., MYRDAL, G.R., & SCHULZ, K.R.
(1962) Vertical distribution and persistence of insecticidal
residues in soils as influenced by mode of application and cover
crop. J. econ. Entomol., 55(2): 215-219.
LICHTENSTEIN, E.P., SCHULZ, K.R., FUHREMANN, T.W., & LIANG, T.T.
(1970) Degradation of aldrin and heptachlor in field soils during a
ten-year period. Translocation into crops. J. agric. food Chem.,
18(1): 100-106.
LICHTENSTEIN, E.P., FUHREMANN, T.W., & SCHULZ, K.R. (1971)
Persistence and vertical distribution of DDT, lindane, and aldrin
residues, 10 and 15 years after a single soil application. J.
agric. food Chem., 19(4): 718-721.
LINDER, R.L. & DAHLGREN, R.B. (1970) Occurrence of organochlorine
insecticides in pheasants of South Dakota. Pestic. monit. J., 3(4):
227-232.
LINDER, R.L., DAHLGREN, R.B., & GREICHUS, Y.A. (1970) Residues in
the brain of adult pheasants given dieldrin. J. wildl. Manage., 34:
954-956.
LINDSTROM, F.T, BOERSMA, L., & STOCKARD, D. (1971) A theory on the
mass transport of previously distributed chemicals in a water
saturated sorbing porous medium: isothermal cases. Soil Sci., 112(5):
291-300.
LISS, P.S. & SLATER, P.G. (1974) Flux of gases across the air-sea
interface. Nature (Lond.), 247: 181-184.
LLOYD, C., BOGAN, J.A., BOURNE, W.R.P., DAWSON, P., & PARSLOW,
J.L.F. (1974) Seabird mortality in the North Irish Sea and Firth of
Clyde early in 1974. Mar. Pollut. Bull., 5: 136-140.
LOCKIE, J.D. & RATCLIFFE, D.A. (1964) Insecticides and Scottish
golden eagles. Br. Birds, 57(3): 89-102.
LOCKIE, J.D., RATCLIFFE, D.A., & BALHARRY, R. (1969) Breeding
success and organochlorine residues in golden eagles in west
Scotland. J. appl. Ecol., 6: 381-389.
LONG, K.R., BEAT, V.B., GOMBART, A.K., SHEETS, R.F., HAMILTON,
H.E., FALABALLA, F., BONDERMAN, D.P., & CHOI, U.Y. (1969) The
epidemiology of pesticides in a rural area. Am. Ind. Hyg. Assoc.
J., 30: 298-304.
LOOSE, L.D. (1982) Macrophage induction of T-suppressor cells in
pesticide-exposed and protozoan-infected mice, Envir. Health
Perspect., 43: 89-97.
LOOSE, L.D., SILKWORTH, J.B., CHARBONNEAU, T., & BLUMENSTOCK,
F. (1981) Environmental chemical-induced macrophage dysfunction.
Environ. Health Perspect., 39: 79-91.
LOTZ, F., KEMPNEY, J., & DE KEMPNEY, R.S.G. (1983) [Light-induced
breakdown of absorbed chemicals: a simple device for comparative
tests.] Chemosphere, 12: 873-878 (in German).
LOWDEN, G.F., SAUNDERS, C.L., & EDWARDS, R.W. (1969) Organochlorine
insecticides in water. Part II. Water Treat. Exam., 18: 275-287.
LU, F.C., JESSUP, D.C., & LAVALLEE, A. (1965) Toxicity of
pesticides in young versus adult rats. Food Cosmet. Toxicol., 3:
591-596.
LUCKENS, M.M. & DAVIS, W.H. (1965) Toxicity of dieldrin and endrin
to bats. Nature (Lond.), 207(4999): 879-880.
LUDKE, J.R., GIBSON, J.R., & LUSK, C.I. (1972) Mixed-function
oxidase activity in fresh-water fish: aldrin epoxidation and
parathion activation. Toxicol. appl. Pharmacol., 21: 89-97.
LUDWIG, G. & KORTE, F. (1965) Metabolism of insecticides. X.
Detection of dieldrin metabolites by GLC analysis. Life Sci., 4:
2027-2031.
LUDWIG, G., WEIS, J., & KORTE, F. (1964) Excretion and distribution
of aldrin-14C and its metabolites after oral administration for a
long period of time. Life Sci., 3: 123-130.
MCCANN, J., CHOI, E., YAMASAKI, E., & AMES, B.N. (1975) Detection
of carcinogens as mutagens in the Salmonella/microsome test: assay
of 300 chemicals. Proc. Natl Acad. Sci. (USA), 72(12): 5135-5139.
MACCUAIG, R.D. (1975) Occurrence and movements of pesticide
residues in Ethiopia. Environ. Qual. Saf., 3(suppl.): 850-851.
MACCUAIG, R.D. (1976) The occurrence of insecticides in the blood
of staff of a locust control organization. Bull. environ. Contam.
Toxicol., 15: 162-170.
MACDONALD, R. (1982) Toxicology of sprays: the 4-h acute inhalation
toxicity of aqueous sprays prepared from aldrin 48% (w/v) EC,
Sittingbourne, Shell Research (SBGR.82.036) (Unpublished
proprietary report).
MACEK, K.J. (1975) Acute toxicity of pesticide mixtures to
bluegills. Bull. environ. Contam. Toxicol., 14(6): 648-652.
MACEK, K.J., HUTCHINSON, C., & COPE, O.B. (1969) The effects of
temperature on the susceptibility of bluegills and rainbow trout to
selected pesticides. Bull. environ. Contam. Toxicol., 4(3): 174-183.
MCEWEN, L.C. & BROWN, R.L. (1966) Acute toxicity of dieldrin and
malathion to wild sharp-tailed grouse. J. wildl. Manage., 30(3):
604-611.
MACKAY, D. & LEINONEN, P.J. (1975) Rate of evaporation of low-
solubility contaminants from water bodies to atmosphere. Environ.
Sci. Technol., 9(13): 1178-1180.
MACKAY, D. & WOLKOFF, A.W. (1973) Rate of evaporation of low-
solubility contaminants to atmosphere. Environ. Sci. Technol., 7(7):
611-614.
MACKAY, D., SHIU, W.Y., & SUTHERLAND, R.P. (1979) Determination of
air-water Henry's Law constants for hydrophobic pollutants.
Environ. Sci. Technol., 13(3): 333-337.
MCKINNEY, J.D., MATTHEWS, H.B., & WILSON, N.K. (1973) Determination
of optical purity and prochirality of chlorinated polycyclodiene
pesticide metabolites. Tetrahedron Lett., 21: 1895-1898.
MCLEESE, D.W. & METCALFE, C.D. (1980) Toxicities of eight
organochlorine compounds in sediment and seawater to Crangon
septemspinosa. Bull. environ. Contam. Toxicol., 25: 921-928.
MCLEESE, D.W., BURRIDGE, L.E., & VAN DINTER, J. (1982) Toxicities
of five organochlorine compounds in water and sediment to Nereis
virens. Bull. environ. Contam. Toxicol., 28: 216-220.
MAJUMDAR, S.K., KOPELMAN, H.A., & SCHNITMAN, M.J. (1976) Dieldrin-
induced chromosome damage in mouse bone-marrow and WI-38 human lung
cells. J. Hered., 67(5): 303-307.
MAJUMDAR, S.K., MAHARAM, L.G., & VIGLIANTI, G.A. (1977)
Mutagenicity of dieldrin in the Salmonella/microsome test. J.
Hered., 68: 184-185.
MANIGOLD, D.B. & SCHULZE, J.A. (1969) Pesticides in selected
western streams: a progress report. Pestic. monit. J., 3(2): 124-135.
MARCHANT, J.H. (1980) Recent trends in sparrowhawk numbers in
Britain. Bird Stud., 27: 152-154.
MARLOW, R.G. & WALLACE, B.G. (1983) Assessment of exposure
following the use of aldrin as a termiticide in homes. Part II. Air
concentrations and kitchen wipes after one year, Sittingbourne,
Shell Research (SBGR.83.285).
MARLOW, R.G., WALLACE, B.G., & MOORE, J.P. (1982) Assessment of
exposure following the use of aldrin as a termiticide in homes,
Sittingbourne, Shell Research (SBGR.82.370).
MARSHALL, T.C., DOROUGH, H.W., & SWIM, H.E. (1976) Screening of
pesticides for mutagenic potential using Salmonella typhimurium
mutants. J. agric. food Chem., 24(3): 560-563.
MASON, J.W. & ROWE, D.R. (1976) The accumulation and loss of
dieldrin and endrin in the eastern oyster. Arch. environ. Contam.
Toxicol., 4: 349-360.
MASTROMATTEO, E. (1971) Pesticides and man's health: the picture in
Ontario. In: Proceedings of the Working Conference on
Epidemiological Toxicology of Pesticides, Amsterdam, 8-10 September
1971 (Unpublished paper).
MATSUMURA, F. & BOUSH, G.M. (1967) Dieldrin degradation by soil
microorganisms. Science, 156: 959-961.
MATTHEWS, H.B. & MATSUMURA, F. (1969) Metabolic fate of dieldrin in
the rat. J. agric. food Chem., 17: 845-852.
MATTHEWS, H.B., MCKINNEY, J.D., & LUCIER, G.W. (1971) Dieldrin
metabolism, excretion, and storage in male and female rats. J.
agric. food Chem., 19(6): 1244-1248.
MATTRAW, H.C., Jr (1975) Occurrence of chlorinated hydrocarbon
insecticides, southern Florida, 1968-72. Pestic. monit. J., 9(2):
106-114.
MAYER, R., LETEY, J., & FARMER, W.J. (1974) Models for predicting
volatilization of soil-incorporated pesticides. Soil Sci. Soc. Am.
Proc., 38: 563-568.
MEHENDALE, H.M. & EL-BASSIOUNI, E.A. (1975) Uptake and disposition
of aldrin and dieldrin by isolated perfused rabbit lung. Drug
Metab. Disp., 3: 543.
MEHENDALE, H.M., SKRENTNY, R.F., & DOROUGH, H.W. (1972) Oxidative
metabolism of aldrin by subcellular root fractions of several plant
species. J. agric. food Chem., 20(2): 398-402.
MEHRLE, P.M. & BLOOMFIELD, R.A. (1974) Ammonia detoxifying
mechanisms of rainbow trout altered by dietary dieldrin. Toxicol.
appl. Pharmacol., 27: 355-365.
MEIERHENRY, E.F., RUEBNER, B.H., GERSHWIN, M.E., HSIEH, L.S., &
FRENCH, S.W. (1983) Dieldrin-induced mallory bodies in hepatic
tumours of mice of different strains. Hepatology, 3(1): 90-95.
MEITH-AVCIN, N., WARLEN, S.M., & BARBER, R.T. (1973) Organochlorine
insecticide residues in a bathyl-demersal fish from 2500 metres.
Environ. Lett., 5(4): 215-221.
MELNIKOV, N.N. (1971) Chemistry of pesticides. Residue Rev., 36:
60-61.
MENDENHALL, V.M., KLAAS, E.E., & MCLANE, M.A.R. (1983) Breeding
success of barn owls (Tyto alba) fed low levels of DDE and
dieldrin. Arch. environ. Contam. Toxicol., 12: 235-240.
MENZEL, D.W., ANDERSON, J., & RANDTKE, A. (1970) Marine
phytoplankton vary in their response to chlorinated hydrocarbons.
Science, 167: 1724-1726.
MES, J., CAMPBELL, D.S., ROBINSON, R.N., & DAVIES, D.J.A. (1977)
Polychlorinated biphenyl and organochlorine pesticide residues in
adipose tissue of Canadians. Bull. environ. Contam. Toxicol., 17(2):
196-203.
MES, J., DAVIES, D.J., & TURTON, D. (1982) Polychlorinated biphenyl
and other chlorinated hydrocarbon residues in adipose tissue of
Canadians. Bull. environ. Contam. Toxicol., 8: 97-104.
MES, J., DOYLE, J.A., ADAMS, B.R., DAVIES, D.J., & TURTON, D.
(1984) Polychlorinated biphenyls and organochlorine pesticides in
milk and blood of Canadian women during lactation. Bull. environ.
Contam. Toxicol., 13: 217-233.
MES, J., DAVIES, D.J., & TURTON, D. (1985) Environmental
contaminants in human fat: a comparison between accidental and non-
accidental causes of death. Ecotoxicol. environ. Saf., 10: 70-74.
METCALFE, R.L., KAPOOR, I.P., LU, P-Y., SCHUTH, C.K., & SHERMAN, P.
(1973) Model ecosystem studies of the environment and fate of six
organochlorine pesticides. Environ. Health Perspect., 4: 35-44.
MICK, D.L., LONG, K.R., & BONDERMAN, D.P. (1972) Aldrin and
dieldrin in the blood of pesticide formulators. Am. Ind. Hyg.
Assoc. J., 33(2): 94-99.
MILES, J.R.W. & HARRIS, C.R. (1971) Insecticide residues in a
stream and a controlled drainage system in agricultural areas of
southwestern Ontario, 1970. Pestic. monit. J., 5(3): 289-294.
MILES, J.R.W. & HARRIS, C.R. (1973) Organochlorine insecticide
residues in streams draining agricultural, urban-agricultural, and
resort areas of Ontario, Canada - 1971. Pestic. monit. J., 6(4):
363-368.
MILLER, G.J. & FOX, J.A. (1973) Chlorinated hydrocarbon pesticide
residues in Queensland human milks. Med. J. Aust., 2: 261-264.
MILLER, G.C. & ZEPP, R.G. (1983) Extrapolating photolysis rates
from the laboratory to the environment. Residue Rev., 85: 89-110.
MINISTRY OF WELFARE, HEALTH AND CULTURE (1983) Surveillance
programme: man and nutrition, The Hague, Netherlands, Ministry of
Welfare, Health and Culture, State Supervisory Public Health
Service.
MOERSDORF, K., LUDWIG, G., VOGEL, J., & KORTE, F. (1963) [Excretion
of aldrin-14C and dieldrin-14C and their metabolites through the
bile.] Med. Exp., 8: 90 (in German).
MOFFET, G.B. & YARBROUGH, J.D. (1972) The effects of DDT,
toxaphene, and dieldrin on succinic dehydrogenase activity in
insecticide-resistant and susceptible Gambusia affinis. J. agric.
food Chem., 20(3): 558-560.
MORGAN, D.P. & ROAN, C.C. (1969) Renal function in persons
occupationally exposed to pesticides. Arch. environ. Health, 19:
633-636.
MORGAN, D.P. & ROAN, C.C. (1970) Chlorinated hydrocarbon pesticide
residue in human tissues. Arch. environ. Health, 20: 452-457.
MORGAN, D.P. & ROAN, C.C. (1973) Adrenocortical function in persons
occupationally exposed to pesticides. J. occup. Med., 15(1): 26-28.
MORGAN, D.P. & ROAN, C.C. (1974) Liver function in workers having
high tissue stores of chlorinated hydrocarbon pesticides. Arch.
environ. Health, 29: 14-17.
MORITA, H. & UMEDA, M. (1984) Detection of mutagenicity of various
compounds by FM 3A cell system. Paper presented at the 12th Annual
Meeting of the Mutagenicity Society, Japan. Mutat. Res., 130(5): 371
(No. 33).
MORIYA, M., OHTA, T., WATANABE, K., MIYAZAWA, T., KATO, K., &
SHIRASU, Y. (1983) Further mutagenicity studies on pesticides in
bacterial reversion assay systems. Mutat. Res., 116: 185-216.
MOSSING, M.L., REDETZKE, K.A., & APPLEGATE, H.G. (1985)
Organochlorine pesticides in blood of persons from El Paso, Texas.
J. environ. Health, 47(6): 312-313.
MOZA, P., WEISGERBER, I., & KLEIN, W. (1972) [Leaching a water-
soluble aldrin-14C breakdown product out of soil.] Chemosphere, 5:
191-195 (in German).
MUELLER, W. & LEACH, R.M., Jr (1974) Effects of chemicals on
eggshell formation. Annu. Rev. Pharmacol., 14: 289-303.
MUELLER, W., NOHYNEK, G., WOODS, G., KORTE, F., & COULSTON, F.
(1975a) Comparative metabolism of dieldrin-14C in mouse, rat,
rabbit, rhesus monkey, and chimpanzee. Chemosphere, 4(2): 89-92.
MUELLER, W., WOODS, G., KORTE, F., & COULSTON, F. (1975b)
Metabolism and organ distribution of dieldrin-14C in rhesus monkeys
after single oral and intravenous administration. Chemosphere, 2:
93-98.
MUELLER, P., NAGEL, P., & FLACKE, W. (1981) Ecological side effects
of dieldrin application against tsetse flies in Adamaoua,
Cameroon. Oecologia, 50(2): 187-194.
MUIR, C.M.C. (1970) The acute oral and percutaneous toxicities to
rats of formulations of aldrin, dieldrin, or endrin,
Sittingbourne, Shell Research (TLGR.0020.70) (Unpublished
proprietary report).
MUIRHEAD, E.E., GROVES, M., GUY, R., HALDEN, E.R., & BASS, R.K.
(1959) Acquired hemolytic anemia, exposure to insecticides and
positive Coombs test dependent on insecticide preparations. Vox
sang., 4: 277-292.
MULHERN, B.M., REICHEL, W.L., LOCKE, L.N., LAMONT, T.G., BELISLE,
A., CROMARTIE, E., BAGLEY, G.E., & PROUTY, R.M. (1970)
Organochlorine residues and autopsy data from bald eagles 1966-68.
Pestic. monit. J., 4(3): 141-144.
MULLER, H.D. & LOCKMAN, D.C. (1972) Fecundity and progeny growth
following subacute insecticide ingestion by the mallard. Poult.
Sci., 51: 239-241.
MULLINS, D.E., JOHNSEN, R.E., & STARR, R.I. (1971) Persistence of
organochlorine insecticide residues in agricultural soils of
Colorado. Pestic. monit. J., 5(3): 268-275.
MURPHY, D.A. & KORSCHGEN, L.J. (1970) Reproduction, growth, and
tissue residues of deer fed dieldrin. J. wildl. Manage., 34(4):
887-903.
MURPHY, R. & HARVEY, C. (1985) Residues and metabolites of selected
persistent halogenated hydrocarbons in blood specimens from a
general population survey. Environ. Health Perspect., 60: 115-120.
MURTON, R.K. & VIZOSO, M. (1963) Dressed cereal seed as a hazard to
woodpigeons. Ann. appl. Biol., 52: 503-517.
NASH, R.G. (1983) Comparative volatilization and dissipation rates
of several pesticides from soil. J. agric. food Chem., 31(2): 210-217.
NASH, R.G., BEALL, M.L., Jr, & WOOLSON, E.A. (1970) Plant uptake of
chlorinated insecticides from soils. Agron. J., 62: 369-372.
NATIONAL FOOD ADMINISTRATION (1982) Summary and assessment of data
received from the FAO/WHO Collaborating Centres for Food
Contamination Monitoring, Uppsala, Global Environmental Monitoring
System (GEMS), Joint FAO/WHO Food and Animal Feed Contamination
Monitoring Programme, pp. 26-29.
NCI (1977) Bioassay of photodieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-817).
NCI (1978a) Bioassay of aldrin and dieldrin for possible
carcinogenicity, Bethesda, Maryland, National Cancer Institute
(DHEW Publication No. (NIH) 78-821).
NCI (1978b) Bioassay of dieldrin for possible carcinogenicity,
Bethesda, Maryland, National Cancer Institute (DHEW Publication No.
(NIH) 78-822).
NEILL, D.D., MULLER, H.D., & SHUTZE, J.V. (1971) Pesticide effects
on the fecundity of the gray partridge. Bull. environ. Contam.
Toxicol., 6(6): 546-551.
NELSON, E. (1953) Aldrin poisoning. Rocky Mt. med. J., 50: 483-486.
NEWTON, I. (1973a) Studies of sparrowhawks. Br. Birds, 66: 271-278.
NEWTON, I. (1973b) Success of sparrowhawks in an area of pesticide
usage. Bird Stud., 20: 1-8.
NEWTON, I. (1974) Changes attributed to pesticides in the nesting
success of the sparrowhawks in Britain. J. appl. Ecol., 11: 95-102.
NEWTON, I. (1976) Breeding of sparrowhawks (Accipiter nisus) in
different environments. J. anim. Ecol., 45: 831-849.
NEWTON, I. (1979) Population ecology of raptors, Berkhamsted,
Poyser T. and A.D., Ltd.
NEWTON, I. & BOGAN, J. (1974) Organochlorine residues, eggshell
thinning, and hatching success in British sparrowhawks. Nature
(Lond.), 249: 582-583.
NEWTON, I. & BOGAN, J. (1978) The role of different organochlorine
compounds in the breeding of British sparrowhawks. J. appl. Ecol.,
15: 105-116.
NEWTON, I. & HAAS, M.B. (1984) The return of the sparrowhawk. Br.
Birds, 77: 47-70.
NEWTON, I., MARQUISS, M., & MOSS, D. (1979) Habitat, female age,
organochlorine compounds, and breeding of European sparrowhawks. J.
appl. Ecol., 16: 777-793.
NISHIMURA, N., NISHIMURA, H., & OSHIMA, H. (1982) Survey on
mutagenicity of pesticides by the Salmonella microsome test. J.
Aichi Med. Univ. Assoc., 10(4): 305-312.
NOHYNEK, G.J., MUELLER, W.F., COULSTON, F., & KORTE, F. (1979)
Metabolism, excretion, and tissue distribution of 14C-photodieldrin
in non-human primates following oral administration and intravenous
injection. Ecotoxicol. environ. Saf., 3: 1-9.
O'CONNOR, R.J. (1982) Habitat occupancy and regulation of clutch
size in the European kestrel Falco tinnunculus. Bird Stud., 29:
17-26.
ODA, J. & MUELLER, W. (1972) Identification of a mammalian
breakdown product of dieldrin. Environ. Qual. Saf., 1: 248-249.
OHLENDORF, H.M., BARTONEK, J.C., DIVOKY, G.J., KLAAS, E.E., &
KRYNITSKY, A.J. (1982) Organochlorine residues in eggs of Alaskan
seabirds, Washington, DC, US Department of the Interior, Fish and
Wildlife Service (Special Scientific Report: Wildlife No. 245).
OLOFFS, P.C., HARDWICK, D.F., SZETO, S.Y., & MOERMAN, D.G. (1974)
DDT, dieldrin, and heptachlorepoxide in humans with liver
cirrhosis. Clin. Biochem., 7: 297-306.
ONSAGER, J.A., RUSK, H.W., & BUTLER, L.I. (1970) Residues of
aldrin, dieldrin, chlordane, and DDT in soil and sugar beets. J.
econ. Entomol., 63(4): 1143-1146.
ORTEGA, P., HAYES, W.J., & DURHAM, W.F. (1957) Pathologic changes
in the liver of rats after feeding low levels of various
insecticides. Am. Med. Assoc. Arch. Pathol., 64(6): 614-622.
OTTOLENGHI, A.D., HASEMAN, J.K., & SUGGS, F. (1974) Teratogenic
effects of aldrin, dieldrin, and endrin in hamsters and mice.
Teratology, 9: 11-16.
PACCAGNELLA, B., GHEZZO, F., PRATI, L., FEDRAZZONI, U., & BELLONI,
G. (1971) Epidemiological study of long-term effects of pesticides
on human health. Bull. World Health Organ., 45: 181-199.
PAINTER, R.B. (1981) DNA synthesis inhibition in mammalian cells as
a test for mutagenic carcinogens. In: Stich, H.F. & San, R.H.C.,
ed. Short-term tests for chemical carcinogens, New York, Springer-
Verlag.
PARK, P.O. & MCKONE, C.E. (1966) The persistence and distribution
of soil-applied insecticides in an irrigated soil in northern
Tanzania. Trop. Agric. Trin., 43(2): 133-142.
PARSLOW, J.L.F. (1973) Breeding birds of Britain and Ireland: a
historical survey, Berkhamsted, Poyser T. and A.D., Ltd.
PARSLOW, J.L.F. & JEFFERIES, D.J. (1973) Relationship between
organochlorine residues in livers and whole bodies of guillemots.
Environ. Pollut., 5: 87-101.
PARSONS, A.M. & MOORE, D.J. (1966) Some reactions of dieldrin and
the proton magnetic resonance spectra of the products. J. Chem.
Soc., C: 2026-2031.
PATEL, T.B. & RAO, V.N. (1958) Dieldrin poisoning in man. A report
of 20 cases in Bombay State. Br. med. J., 1: 919-921.
PATON, R., LUKE, B.G., & ROBERTS, G. (1984) Studies on the sorption
of organochlorine insecticides by flour stored on or near treated
laminated timber or plywood as used in freight containers. Pestic.
Sci., 15: 624-629.
PEARCE, P.A., PEAKALL, D.B., & REYNOLDS, L.M. (1979) Shell thinning
and residues of organochlorines and mercury in seabird eggs,
eastern Canada, 1970-76. Pestic. monit. J., 13(2): 61-68.
PEARSON, J.E., TINSLEY, K., & HERNANDEZ, T. (1973) Distribution of
dieldrin in the turtle. Bull. environ. Contam. Toxicol., 10(6):
360-364.
PETROCELLI, S.R., HANKS, A.R., & ANDERSON, J.W. (1973) Uptake and
accumulation of an organochlorine insecticide (dieldrin) by an
estuarine mollusc Rangia cuneata. Bull. environ. Contam. Toxicol.,
10(5): 315-320.
PETROCELLI, S.R., ANDERSON, J.W., & HANKS, A.R. (1975)
Biomagnification of dieldrin residues by food-chain transfer from
clams to blue crabs under controlled conditions. Bull. environ.
Contam. Toxicol., 13(1): 108-116.
PIONKE, H.B. & CHESTERS, G. (1973) Pesticide-sediment-water
interactions. J. environ. Qual., 2(1): 29-45.
POLISHUK, Z.W., WASSERMANN, M., WASSERMANN, D., GRONER, Y.,
LAZAROVICI, S., & TOMATIS, L. (1970) Effects of pregnancy on
storage of organochlorine insecticides. Arch. environ. Health, 20:
215-217.
POLISHUK, Z.W., RON, M., WASSERMANN, M., CUCOS, S., WASSERMANN, D.,
& LEMESCH, C. (1977) Organochlorine compounds in human blood plasma
and milk. Pestic. monit. J., 10(4): 121-129.
PORTER, R.D. & WIEMEYER, S.N. (1969) Dieldrin and DDT: effects on
sparrowhawk eggshells and reproduction. Science, 165: 199-200.
POTTER, J.C., DIXON, L.D., BARBER, G.F., & MARXMILLER, R.L. (1972)
Residues of 14 C-dieldrin and its metabolites in milk from cows fed
dieldrin-14 C, Modesto, California, Shell Development Company
(TIR-24-109-72) (Unpublished proprietary report).
POWELL, A.J.B., STEVENS, T., & MCCULLY, K.A. (1970) Effects of
commercial processing on residues of aldrin and dieldrin in
tomatoes and residues in subsequent crops grown on the treated
plots. J. agric. food Chem., 18(2): 224-227.
POWERS, C.D, ROWLAND, R.G., & WURSTER, C.F. (1977) Dieldrin-induced
destruction of marine algal cells with concomitant decrease in size
of survivors and their progeny. Environ. Pollut., 12: 17-25.
PRESTT, I. (1965) An enquiry into the recent breeding status of
some of the smaller birds of prey and crows in Britain. Bird Stud.,
12(3): 196-221.
PRESTT, I. & BELL, A.A. (1966) An objective method of recording
breeding distribution of common birds of prey in Britain. Bird
Stud., 13(4): 277-283.
PRINCI, F. (1954) Toxicity of the chlorinated hydrocarbon
insecticides. In: Proceedings of the XI International Congress di
Medicina del Lavoro, Pipola, Napoli, Tipografia Saverio, pp.
253-272.
PRINCI, F. & SPURBECK, G.H. (1951) A study of workers exposed to
the insecticides chlordane, aldrin, dieldrin. Arch. ind. Hyg.
occup. Med., 3: 64-72.
PROBST, A.H. & EVERLY, R.T. (1957) Effect of soil insecticides on
emergence, growth, yield, and chemical composition of soybeans.
Agron. J., 1957: 385-387.
PROBST, G.S., MCMAHON, R.E., HILL, L.E., THOMPSON, C.Z., EPP, J.K.,
& NEAL, S.B. (1981) Chemically-induced unscheduled DNA synthesis in
primary rat hepatocyte cultures: a comparison with bacterial
mutagenicity using 218 compounds. Environ. Mutagenesis, 3: 11-32.
PROSPERO, J.M. & SEBA, D.B. (1972) Some additional measurements of
pesticides in the lower atmosphere of the northern equatorial
Atlantic Ocean. Atmos. Environ., 6(5): 363-364.
PROUTY, R.M., REICHEL, W.L., LOCKE, L.N., BELISLE, A.A., CROMARTIE,
E., KAISER, T.E., LAMONT, T.G., MULHERN, B.M., & SWINEFORD, D.M.
(1977) Residues of organochlorine pesticides and polychlorinated
biphenyls and autopsy data for bald eagles, 1973-74. Pestic. monit.
J., 11(3): 134-137.
PURCHASE, I.F.H., LONGSTAFF, E., ASHBY, J., STYLES, J.A., ANDERSON,
D., LEFEVRE, P.A., & WESTWOOD, F.R. (1978) An evaluation of 6
short-term tests for detecting organic chemical carcinogens. Br. J.
Cancer, 37: 873-903.
RADELEFF, R.D., WOODARD, G.T., NICKERSON, W.J., & BUSHLAND, R.C.
(1955) The acute toxicity of chlorinated hydrocarbon and organic
phosphorus insecticides to livestock, Kerville, Texas, US
Department of Agriculture, Agricultural Research Service,
(Technical Bulletin 1122).
RADELEFF, R.D., NICKERSON, W.J., & WELLS, R.W. (1960) Acute toxic
effects upon livestock and meat and milk residues of dieldrin. J.
econ. Entomol., 53(3): 425-429.
RADOMSKI, J.L. & FISEROVA-BERGEROVA, V. (1965) The determination of
pesticides in tissues with the electron-capture detector without
prior clean-up. Ind. Med. Surg., 32: 934-939.
RADOMSKI, J.L., DEICHMANN, W.B., & CLIZER, E.E. (1968) Pesticide
concentrations in the liver, brain, and adipose tissue of terminal
hospital patients. Food Cosmet. Toxicol., 6: 209-220.
RADOMSKI, J.L., ASTOLFI, E., DEICHMANN, W.B., & REY, A.A. (1971)
Blood levels of organochlorine pesticides in Argentina:
occupationally and non-occupationally exposed adults, children, and
newborn infants. Toxicol. appl. Pharmacol., 20: 186-193.
RATCLIFFE, D.A. (1963) The status of the peregrine in Great
Britain. Bird Stud., 10: 56-90.
RATCLIFFE, D.A. (1965) The peregrine situation in Great Britain,
1963-64. Bird Stud., 12(2): 66-82.
RATCLIFFE, D.A. (1967a) Decrease in eggshell weight in certain
birds of prey. Nature (Lond.), 215: 208-210.
RATCLIFFE, D.A. (1967b) The peregrine situation in Great Britain,
1965-66. Bird Stud., 14(4): 238-245.
RATCLIFFE, D.A. (1970) Changes attributable to pesticides in egg
breakage frequency and eggshell thickness in some British birds. J.
appl. Ecol., 7: 67-115.
RATCLIFFE, D.A. (1972) The peregrine population of Great Britain in
1971. Bird Stud., 19: 117-156.
RATCLIFFE, D.A. (1980) The peregrine falcon, Calton, T. and A.D.
Poyser.
RATCLIFFE, D.A. (1984) The peregrine breeding population of the
United Kingdom in 1981. Bird Stud., 31(1): 1-18.
REICHEL, W.L., CROMARTIE, E., LAMONT, T.G., MULHERN, B.M., &
PROUTY, R.M. (1969) Pesticide residues in eagles. Pestic. monit.
J., 3(3): 142-144.
REIDINGER, R.F. & CRABTREE, D.G. (1974) Organochlorine residues in
golden eagles, United States, March 1964-July 1971. Pestic. monit.
J., 8(1): 37-43.
REINERT, R.E. (1972) Accumulation of dieldrin in an alga
(Scenedesmus obliquus), Daphnia magna, and the guppy (Poecilia
reticulata). J. Fish. Res. Board Can., 29(10): 1413-1418.
REINKE, J., UTHE, J.F., & JAMIESON, D. (1972) Organochlorine
pesticide residues in commercially caught fish in Canada, 1970.
Pestic. monit. J., 6(1): 43-49.
REIMOLD, R.J. (1975) Chlorinated hydrocarbon pesticides and mercury
in coastal biota - Puerto Rico and the US Virgin Islands 1972-1974.
Pestic. monit. J., 9(1): 39-43.
REYNOLDS, C.M. (1974) The census of heronries, 1969-73. Bird Stud.,
21: 129-134.
RIBBENS, P.H. (1985) Mortality study of industrial workers exposed
to aldrin, dieldrin, and endrin. Ind. Arch. occup. environ. Health,
56: 75-79.
RICE, C.P. & SIKKA, H.C. (1973) Fate of dieldrin in selected
species of marine algae. Bull. environ. Contam. Toxicol., 9(2):
116-123.
RICHARD, J.J., JUNK, G.A., AVERY, M.J., NEHRING, N.L., FRITZ, J.S.,
& SVEC, H.J. (1975) Analysis of various Iowa waters for selected
pesticides: atrazine, DDE, and dieldrin - 1974. Pestic. monit. J.,
9(3): 117-123.
RICHARDSON, A. (1971) The isolation and identification of a
metabolite of HEOD (dieldrin) from human faeces, Sittingbourne,
Shell Research (TLGR.0021.71).
RICHARDSON, A. & ROBINSON, J. (1971) The identification of a major
metabolite of HEOD (dieldrin) in human faeces. Xenobiotica, 1(3):
213-219.
RICHARDSON, A., ROBINSON, J., BUSH, B., & DAVIES, J.M. (1967a)
Determination of dieldrin (HEOD) in blood. Arch. environ. Health,
14(5): 703-708.
RICHARDSON, L.A., LANE, J.R., GARDNER, W.S., PEELER, J.T., &
CAMPBELL, J.E. (1967b) Relationship of dietary intake to
concentration of dieldrin and endrin in dogs. Bull. environ.
Contam. Toxicol., 2(4): 207-219.
RICHARDSON, A., BALDWIN, M.K., & ROBINSON, J. (1968) Metabolites of
dieldrin (HEOD) in the urine and faeces of rats. Chem. Ind., 1968:
588-589.
RICKARD, D.G. & DULLEY, M.E.R. (1983) The levels of some heavy
metals and chlorinated hydrocarbons in fish from the tidal Thames.
Environ. Pollut. Ser. B., 5: 101-119.
RISEBROUGH, R.W., HUGGETT, R.J., GRIFFIN, J.J., & GOLDBERG, E.D.
(1968) Pesticides: transatlantic movements in the northeast trades.
Science, 159(3820): 1233-1236.
RITCEY, W.R., SAVARY, G., & MCCULLY, K.A. (1973) Organochlorine
insecticide residues in human adipose tissue of Canadians. Can. J.
public Health, 64: 380-386.
ROBINSON, J. (1969) Organochlorine insecticides and bird
populations in Britain. In: Miller, M.W. & Berg, G.G., ed. Chemical
fallout, Springfield, Illinois, C.C. Thomas, pp. 113-169.
ROBINSON, J. & CRABTREE, A.N. (1969) The effect of dieldrin on
homing pigeons ( Columba livia var.). Meded. Fac. Landbouwwet.
Rijksuniv. Gent, 34(3): 413-427.
ROBINSON, J. & HUNTER, C.G. (1966) Organochlorine insecticides:
concentrations in human blood and adipose tissue. Arch. environ.
Health, 13: 558-563.
ROBINSON, J. & ROBERTS, M. (1969) Estimation of the exposure of the
general population to dieldrin (HEOD). Food Cosmet. Toxicol., 7:
501-514.
ROBINSON, J., RICHARDSON, A., & ELGAR, K.E. (1966a) Chemical
identity in ultramicroanalysis. In: Proceedings of the American
Chemical Society Meeting, New York, 11-16 September, 1966,
Washington, DC, American Chemical Society.
ROBINSON, J., RICHARDSON, A., BUSH, B., & ELGAR, K.E. (1966b) A
photoisomerization product of dieldrin. Bull. environ. Contam.
Toxicol., 1(4): 127-132.
ROBINSON, J., RICHARDSON, A., & DAVIES, J.M. (1967a) Comparison of
analytical methods for determination of dieldrin (HEOD) in blood.
Arch. environ. Health, 15(1): 67-69.
ROBINSON, J., BROWN, V.K.H., RICHARDSON, A., & ROBERTS, M. (1967b)
Residues of dieldrin (HEOD) in the tissues of experimentally
poisoned birds. Life Sci., 6: 1207-1220.
ROBINSON, J., ROBERTS, M., BALDWIN, M., & WALKER, A.I.T. (1969) The
pharmacokinetics of HEOD (dieldrin) in the rat. Food Cosmet.
Toxicol., 7: 317-332.
ROBURN, J. (1963) Effect of sunlight and ultraviolet radiation on
chlorinated pesticide residues. J. chem. Ind., 1: 1555-1556.
ROCCHI, P., PEROCCO, P., ALBERGHINI, W., FINI, A., & PRODI, G.
(1980) Effect of pesticides on scheduled and unscheduled DNA
synthesis of rat thymocytes and human lymphocytes. Arch. Toxicol.,
45: 101-108.
ROHWER, D. (1983a) [Pesticides in breast milk (problems with
residues of polychlorinated hydrocarbons).] Wiss. Inf., 9(3):
197-201 (in German).
ROHWER, D. (1983b) [Pesticides in breast milk.] Geburtshilfe
Frauenheilkd., 43: 160-163 (in German).
ROSE, G.P. (1982) Toxicity of insecticides: the acute oral and
percutaneous toxicity, skin and eye irritancy, and skin sensitizing
potential of a 480 g/litre emulsifiable concentrate of aldrin (EF
5159), Sittingbourne, Shell Research (SBGR.81.319) (Unpublished
proprietary report).
ROSE, G.P. (1984a) Toxicology of insecticides: the acute
percutaneous toxicity of the 480 g/litre aldrin emulsifiable
concentrate formulation CF 06323, Sittingbourne, Shell Research
(SBGR.84.047) (Unpublished proprietary report).
ROSE, G.P. (1984b) Toxicology of insecticides: the acute
percutaneous toxicity of the 200 g/litre dieldrin emulsifiable
concentrate formulation CF 06271, Sittingbourne, Shell Research
(SBGR.84.045) (Unpublished proprietary report).
ROSE, G.P. (1984c) Toxicology of insecticides (organochlorines):
the acute percutaneous toxicity of the 680 g/l dieldrin suspension
concentrate SF 06349, Sittingbourne, Shell Research (SBGR.84.210)
(Unpublished proprietary report).
ROSEN, J.D. & CAREY, W.F. (1968) Preparation of the photoisomers of
aldrin and dieldrin. J. agric. food Chem., 16: 536-537.
ROSEN, J.D., SUTHERLAND, D.J., & LIPTON, G.R. (1966) The photochemical
isomerization of dieldrin and endrin and effects on toxicity. Bull.
environ. Contam. Toxicol., 1(4): 133-140.
ROSEWELL, K.T., MUIR, D.C.G., & BAKER, B.E. (1979) Organochlorine
residues in Harp Seal (Phagophilus groenlandicus) tissues, Gulf of
St. Lawrence, 1971, 1973. Pestic. monit. J., 12: 189-192.
ROSS, R.D. & CROSBY, D.G. (1974) Photosensitizers in agricultural
water samples, Washington, DC, American Chemical Society (ACS
Meeting, Abstract No. 167, Section PEST 67).
ROSS, R.D. & CROSBY, D.G. (1975) The photooxidation of aldrin in
water. Chemosphere, 4: 227.
ROSS, R.D. & CROSBY, D.G. (1985) Photooxidant activity in natural
waters. Environ. Toxicol. Chem., 4: 773-778.
ROSS, W.R., VAN LEEUWEN, J., & GRABOW, W.O.K. (1976) Studies on the
disinfection and chemical oxidation with ozone and chlorine in
water reclamation. In: Proceedings of the Second Symposium on Ozone
Technology, Montreal, International Ozone Institute, pp. 479-513.
ROWE, D.B., CANTER, L.W., SNIJDER, P.J., & MASON, J.W. (1971)
Dieldrin and endrin concentrations in a Louisiana estuary. Pestic.
monit. J., 4(4): 177-183.
RUEBNER, B.H., GERSHWIN, M.E., MEIERHENRY, E.F., HSIEH, L.S., &
DUNN, P.L. (1984a) Irreversibility of liver tumours in C3H mice. J.
Natl Cancer Inst., 73(2): 493-498.
RUEBNER, B.H., GERSHWIN, M.E., FRENCH, S.W., MEIERHENRY, E., DUNN,
P., & HSIEH, L.S. (1984b) Mouse hepatic neoplasia: Differences
among strains and carcinogens. In: Popp J.A., ed. Current
perspectives in mouse liver neoplasia, Washington, DC, Hemisphere.
SAHA, J.G. & SUMNER, A.K. (1971) Organochlorine insecticide
residues in soil from vegetable farms in Saskatchewan. Pestic.
monit. J., 5(1): 28-31.
SAHA, J.G., KARAPALLY, J.C., & JANSEN, W.K. (1971) Influence of the
type of mineral soil on the uptake of dieldrin by wheat seedlings.
J. agric. food Chem., 19: 842-845.
SAND, P.F., WIERSMA, G.B., & LANDRY, J.L. (1972) Pesticide residues
in sweet potatoes and soil - 1969. Pestic. monit. J., 5(4): 342-344.
SANDERS, H.O. (1970) Pesticide toxicities to tadpoles of the
western chorus frog Pseudacris triseriata and Fowler's Toad Bufo
woodhousii fowleri. Copeia, 2: 246-251.
SANDERS, H.O. & COPE, O.B. (1968) The relative toxicities of
several pesticides to naiads of three species of stoneflies.
Limnol. Oceanogr., 13: 112-117.
SANDHU, S.-S., WARREN, W.J., & NELSON, P. (1978) Pesticidal residue
in rural potable water. Am. Water Works Assoc. J., 70(1): 41-45.
SANDIFER, S.H., CUPP, C.M., WILKINS, R.T., LOADHOLT, B., & SCHUMAN,
S.H. (1981) A case-control study of persons with elevated blood
levels of dieldrin. Arch. environ. Contam. Toxicol., 10(1): 35-45.
SANGER, A.M.H. (1959) Aldrin, dieldrin, and endrin toxicity to
bees. Span, 2(2): 59-63.
SASCHENBRECKER, P.W. (1976) Levels of terminal pesticide residues
in Canadian meat, Can. Vet. J., 17(6): 158-163.
SAVAGE, E.P., KEEFE, T.J., TESSARI, J.D., WHEELER, H.W., APPLEHAUS,
F.M., GOES, E.A., & FORD, S.A. (1981) National study of chlorinated
hydrocarbon insecticide residues in human milk, USA. I. Geographic
distribution of dieldrin, heptachlor, heptachlor epoxide,
chlordane, oxychlordane, and mirex. Am. J. Epidemiol., 113: 413-422.
SCHAFER, M.L. (1968) Pesticides in blood. Residue Rev., 24: 19-39.
SCHAUBERGER, C.W. & WILDMAN, R.B. (1977) Accumulation of aldrin and
dieldrin by blue-green algae and related effects on photosynthetic
pigments. Bull. environ. Contam. Toxicol., 17(5): 534-541.
SCHULZE, J.A., MANIGOLD, D.B., & ANDREWS, F.L. (1973) Pesticides in
selected western streams - 1968-71. Pestic. monit. J., 7(1): 73-84.
SEAL, W.L., DAWSEY, L.H., & CAVIN, G.E. (1967) Monitoring for
chlorinated hydrocarbon pesticides in soil and root crops in the
eastern states in 1965. Pestic. monit. J., 1(3): 22-25.
SELBY, L.A., NEWELL, K.W., HAUSER, G.A., & JUNKER, G. (1969a)
Comparison of chlorinated hydrocarbon pesticides in maternal blood
and placental tissues. Environ. Res., 2(4): 247-255.
SELBY, L.A., NEWELL, K.W., WAGGENSPACK, C., HAUSER, G.A., & JUNKER,
G. (1969b) Estimating pesticide exposure in man as related to
measurable intake: environmental versus chemical index. Am. J.
Epidemiol., 89(3): 241-253.
SETHUNATHAN, N. (1973) Microbial degradation of insecticides in
flooded soil and in anaerobic cultures. Residue Rev., 47: 143-165.
SHAH, P.V. & GUTHRIE, F.E. (1976) Dermal absorption, distribution,
and the fate of six pesticides in the rabbit. In: Watson, D.L. &
Brown, A.W.A., ed. Pesticide management and insecticide resistance,
New York, London, Academic Press, pp. 547-554.
SHAKOORI, A.R., RASUL, Y.G., & ALI, S.S. (1986) The effect of long-
term administration of dieldrin on biochemical components in blood
serum of albino rats. Toxicol. Lett., 9(4): 35.
SHANNON, L.R. (1977a) Equilibrium between uptake and elimination of
dieldrin by channel catfish Ictalurus punctatus. Bull. environ.
Contam. Toxicol., 17(3): 278-284.
SHANNON, L.R. (1977b) Accumulation and elimination of dieldrin in
muscle tissue of channel catfish. Bull. environ. Contam. Toxicol.,
17(6): 637-644.
SHAROM, M.S., MILES, J.R.W., HARRIS, C.R., & MCEWEN, F.L. (1980)
Behaviour of 12 insecticides in soil and aqueous suspensions of
soil and sediment, Water Res., 14: 1095-1100.
SHEETS, T.J., JACKSON, M.D., & PHELPS, L.D. (1970) Water monitoring
system for pesticides in North Carolina, US Clearinghouse, 109 pp
(P.B. Report No. 189291).
SHELL (1976) Chemicals for plant protection, veterinary uses, and
public health, London, Shell International Chemical Company, Ltd,
pp. 3-4, 9-10.
SHELL (1984) Shell guide to pesticide safety, London, Shell
International Chemical Company, Ltd, Agrochemical Division, pp.
27-29.
SHELLENBERGER, T.E. (1978) A multi-generation toxicity evaluation
of p,p'-DDT and dieldrin with Japanese quail. Effects on growth and
reproduction. Drug chem. Toxicol., 1(2): 137-146.
SHELLENBERGER, T.E. & NEWELL, G.W. (1965) Toxicological evaluations
of agricultural chemicals with Japanese quail (Coturnix coturnix
japonica). Lab. anim. Care, 15(2): 119-130.
SHERMAN, M. & ROSENBERG, M.M. (1953) Acute toxicity of four
chlorinated dimethanonaphthalene insecticides to chicks. J. econ.
Entomol., 46(6): 1067-1070.
SHIRASU, Y. (1975) Significance of mutagenicity testing on
pesticides. Environ. Qual. Saf., 4: 226-231.
SIERRA, M. & SANTIAGO, D. (1987) Organochlorine pesticide levels in
barn owls collected in Leon, Spain. Bull. environ. Contam.
Toxicol., 38: 261-265.
SIERRA, M., TERAN, M.T., GALLEGO, A., DIEZ, M.J., & SANTIAGO, D.
(1987) Organochlorine contamination in three species of diurnal
raptors in Leon, Spain. Bull. environ. Contam. Toxicol., 38: 254-260.
SILBERGELD, E.K. (1973) Dieldrin. Effects of chronic sublethal
exposure on adaptation to thermal stress in fresh-water fish.
Environ. Sci. Technol., 7(9): 846-849.
SIMMON, V.F., KAUHANEN, K., & TARDIFF, R.C. (1977) Mutagenic
activity of chemicals identified in drinking-water. In: Scott, D.,
Bridges, B.A., & Sobels, F.H., ed. Progress in genetic toxicology,
Amsterdam, Elsevier Science Publishers, pp. 249-258.
SINA, J.F., BEAN, C.L., DYSART, G.R., TAYLOR, V.I., & BRADLEY, M.O.
(1983) Evaluation of the alkaline elution/rat hepatocyte assay as a
predictor of carcinogenic/mutagenic potential. Mutat. Res., 113:
357-391.
SINGH, K.K., JHA, G.J., SINGH, P.N., & CHAUHAN, H.V.S. (1985)
Pathophysiology of acute aldrin intoxication in goats. Toxicol.
Abstr., 8(7): 27 (No. 4086-x8).
SIYALI, D.S. (1972) Hexachlorobenzene and other organochlorine
pesticides in human blood. Med. J. Aust., 2: 1063-1066.
SIYALI, D.S. (1973) Polychlorinated biphenyls, hexachlorobenzene,
and other organochlorine pesticides in human milk. Med. J. Aust.,
2: 815-818.
SIYALI, D.S. & SIMSON, R.E. (1973) Chlorinated hydrocarbon
pesticides in human blood and fat. Med. J. Aust., 1: 212-213.
SLATER, R.M. & SPEDDING, D.J. (1981) Transport of dieldrin between
air and water. Arch. environ. Contam. Toxicol., 10: 25-33.
SMITH, J.H., BOMBERGER, D.C., Jr, & HAYNES, D.L. (1981)
Volatilization rates of intermediate and low volatility chemicals
from water. Chemosphere, 10(3): 281-289.
SMITH, R.M. & COLE, C.F. (1973) Effects of egg concentrations of
DDT and dieldrin on development in winter flounder
(Pseudopleuronectes americanus). J. Fish. Res. Board Can., 30(12):
1894-1898.
SMITH, S.W.C. (1978) Pesticide residues in the total diet. Food
Technol. Aust., 1978: 349-352.
SPALDING, R.F., JUNK, C.A., & RICHARD, J.R. (1980) Pesticides in
groundwater beneath irrigated farmland in Nebraska, August 1978.
Pestic. monit. J., 14: 70-73.
SPARR, B.I., APPLEBY, W.G., DEVRIES, D.M., OSMUN, J.V., MCBRIDE,
J.M., FOSTER, G.L., & GOULD R.F., ed. (1966) Organic pesticides in
the environment, Washington, DC, American Chemical Society, pp.
146-162 (Advances in Chemistry Series No. 60).
SPENCER, W.F. & CLIATH, M.M. (1973) Pesticide volatilization as
related to water loss from soil. J. environ. Qual., 2(2): 284-289.
SPENCER, W.F. & CLIATH, M.M. (1975) Environmental dynamics of
pesticides. In: Haque, R. & Freed, V.H. ed., New York, London,
Plenum Press, pp. 61-78.
SPENCER, W.F., CLIATH, M.M., & FARMER, W.J. (1969) Vapor density of
soil-applied dieldrin as related to soil-water content,
temperature, and dieldrin concentration. Soil Sci. Soc. Am. Proc.,
33: 509-511.
SPENCER, W.F., FARMER, W.J., & CLIATH, M.M. (1973) Pesticide
volatilization. Residue Rev., 49: 1-47.
SPIOTTA, E.J. (1951) Aldrin poisoning in man. Report of a case.
Arch. ind. Hyg. occup. Med., 4: 560-566.
STACEY, C.I. & TATUM, T. (1985) House treatment with organochlorine
pesticides and their levels in human milk - Perth, Western
Australia. Bull. environ. Contam. Toxicol., 35: 202-208.
STACEY, C.I. & THOMAS, B.W. (1975) Organochlorine pesticide
residues in human milk, Western Australia, 1970-71. Pestic. monit.
J., 9(2): 64-66.
STACEY, C.I., PERRIMAN, W.S., & WHITNEY, S. (1985) Organochlorine
pesticides residue levels in human milk: Western Australia,
1979-80. Arch. environ. Health, 40: 102-108.
STANLEY, C.W., BARNEY, J.E., HELTON, M.R., & YOBS, A.R. (1971)
Measurement of atmospheric levels of pesticides. Environ. Sci.
Technol., 5(5): 430-435.
STANLEY, P.I. & BUNYAN, P.J. (1979) Hazards to wintering geese and
other wildlife from the use of dieldrin, chlorfenvinphos, and
carbophenothion as wheat seed treatments. Proc. R. Soc. Lond. Ser
B, 205: 31-45.
STEVENS, L.J., COLLIER, C.W., & WOODHAM, D.W. (1970) Monitoring
pesticides in soils from areas of regular, limited, and no
pesticide use. Pestic. monit. J., 4(3): 145-164.
STEVENSON, D.E. & WALKER, A.I.T. (1969) Hepatic lesions produced in
mice by dieldrin and other hepatic enzyme-inducing compounds. J.
Eur. Toxicol., 2: 83-84.
STEVENSON, D.E., THORPE, E., HUNT, P.F., & WALKER, A.I.T. (1976)
The toxic effects of dieldrin in rats: a re-evaluation of data
obtained in a two-year feeding study. Toxicol. appl. Pharmacol., 36:
247-254.
STEWART, D.J. & STEIN, R.A. (1974) Short-term fate of dietary
dieldrin in the digestive tract of juvenile lake trout (Salvelinus
namaycush). Bull. environ. Contam. Toxicol., 11(6): 563-566.
STEWART, D.K.R. & FOX, C.J.S. (1971) Persistence of organochlorine
insecticides and their metabolites in Nova Scotian soils. J. econ.
Entomol., 64(2): 367-371.
STEWART, D.K.R. & GAUL, S.O. (1977) Dihydrochlordene dicarboxylic
acid residues in soil treated with high rates of aldrin. Bull.
environ. Contam. Toxicol., 17(6): 712-713.
STICKEL, W.H. (1975) Some effects of pollutants in terrestrial
ecosystems. Environ. Sci. Res., 7: 25-74.
STICKEL, W.H., STICKEL, L.F., & SPANN, J.W. (1969) Tissue residues
of dieldrin in relation to mortality in birds and mammals. In:
Miller, M.W. & Berg, G.G., ed. Chemical fallout, Springfield,
Illinois, C.C. Thomas, pp. 174-200.
SUNDARAM, K.S., DAMODARAN, V.N., & VENKITASUBRAMANIAM, T.A.
(1978) Absorption of dieldrin through monkey and dog skin. Indian
J. exp. Biol., 16: 101-103.
SUZUKI, M., YAMATO, Y., & WATANABE, T. (1974) Photodieldrin
residues in field soils. Bull. environ. Contam. Toxicol., 12:
275-279.
SWENBERG, J.A., PETZOLD, G.L., & HARBACH, P.R. (1976) In vitro DNA
damage-alkaline elution assay of predicting carcinogenic potential.
Biochem. Biophys. Res. Commun., 72(2): 732-738.
SZARO, R.C., COON, N.C., & KOLBE, E. (1979) Pesticide and PCB of
common eider, herring gull, and great black-backed gull eggs. Bull.
environ. Contam. Toxicol., 22: 394-399.
TABOR, E.C. (1966) Contamination of urban air through the use of
insecticides. Trans. N.Y. Acad. Sci., 28: 569-578.
TAKEI, G.H., KAUAHIKAUA, S.M., & LEONG, G.H. (1983) Analyses of
human milk samples collected in Hawaii for residues of
organochlorine pesticides and polychlorobiphenyls. Bull. environ.
Contam. Toxicol., 30: 606-613.
TANNOCK, J., HOWELLS, W.W., & PHELPS, R.J. (1983) Chlorinated
hydrocarbon pesticide residues in eggs of some birds in Zimbabwe.
Environ. Pollut. Ser. B, 5: 147-155.
TARRANT, K.R. & TATTON, J.O'G. (1968) Organochlorine pesticides in
rainwater in the British Isles. Nature (Lond.), 219: 725-727.
TATTON, J.O'G. & RUZICKA, J.H.A. (1967) Organochlorine pesticides
in Antarctica. Nature (Lond.), 215: 346-348.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., & FREEMAN, H.P. (1972)
Measurement of volatilization of dieldrin and heptachlor from a
soil in the field. In: Abstracts of the 163rd National Meeting of
the American Chemical Society, Boston, Massachusetts, Washington,
DC, American Chemical Society.
TAYLOR, A.W., GLOTFELTY, D.E., GLASS, B.L., FREEMAN, H.P., &
EDWARDS, W.M. (1976) Volatilization of dieldrin and heptachlor from
a maize field. J. agric. food Chem., 24(3): 625-631.
TEJEDOR, M.C., MURADO, M.A., & BALUJA, G. (1974) [Contamination of
the environment by organochlorine pesticides.] An. Quim., 70:
1177-1183 (in Spanish).
TENNEKES, H.A., WRIGHT, A.S., DIX, K.M., & KOEMAN, J.H. (1981)
Effects of dieldrin, diet and bedding on enzyme function and tumour
incidence in livers of male CF-1 mice. Cancer Res., 41: 3615-3620.
TENNEKES, H.A., RAVENZWAAY, B., & KUNZ, H.W. (1985) Quantitative
aspects of enhanced liver tumour formation in CF-1 mice by
dieldrin. Carcinogenesis, 6(10): 1457-1462.
TERRIERE, L.C. & YU, S.J. (1976) Microsomal oxidases in the flesh
fly ( Sarcophaga bullata Parker) and the black blow fly ( Phormia
regina Meigen). Pestic. Biochem. Physiol., 6: 223-228.
THOMPSON, A.R., EDWARDS, C.A., EDWARDS, M.J., & BEYNON, K.I. (1970)
Movement of dieldrin through soils. II. In sloping troughs and soil
columns. Pestic. Sci., 1(5): 174-178.
THOMPSON, J.F. (1976) Manual of analytical quality control for
pesticides and related compounds in human and environmental
samples, Research Triangle Park, North Carolina, US Environmental
Protection Agency, Office of Research and Development, Health
Effects Research Laboratory (EPA-600/1-76-017).
THORPE, E. (1973) The toxicology of dieldrin (HEOD):
transplantation of liver tumours in mice, Sittingbourne, Shell
Research (TLGR.0041.73) (Unpublished proprietary report).
THORPE, E. & HUNT, P.F. (1975) Toxicology of dieldrin (HEOD): study
of the pathological changes in 3 strains of mice following
prolonged ingestion of dieldrin, Sittingbourne, Shell Research
(TLGR.0012.75) (Unpublished proprietary report).
THORPE, E. & WALKER, A.I.T. (1973) The toxicology of dieldrin
(HEOD). II. Comparative long-term oral toxicity studies in mice
with dieldrin, DDT, phenobarbitone, beta-BHC, and gamma-BHC. Food
Cosmet. Toxicol., 11: 433-442.
TREON, J.F. & CLEVELAND, F.P. (1955) Toxicity of certain
chlorinated hydrocarbon insecticides for laboratory animals, with
special reference to aldrin and dieldrin. J. agric. food Chem.,
3(5): 402-408.
TREON, J.F., DUTRA, F.R., SHAFFER, F.E., CLEVELAND, F.P., WAGNER,
W., & GAHEGAN, T. (1951) The toxicity of aldrin, dieldrin, and DDT
when fed to rats over the period of six months, Cincinnati, Ohio,
Kettering Laboratory.
TREON, J.F., HARTMAN, L., GAHEGAN, T., & NEDDERMANN, G. (1953) The
immediate and cumulative toxicity of aldrin, dieldrin, and DDT when
maintained in contact with the skin of rabbits, Cincinnati, Ohio,
Kettering Laboratory.
TROSKO, J.E., JONE, C., & CHANG, C.C. (in press) Inhibition of gap-
functional mediated intercellular communication in vitro by aldrin,
dieldrin and toxaphene: a possible cellular mechanism for their
tumour-promoting and neurotoxic effects. Mol. Toxicol.
TU, C.M. (1981) Effects of some pesticides on enzyme activities in
an organic soil. Bull. environ. Contam. Toxicol., 27: 109-114.
TU, C.M. & MILES, J.R.W. (1976) Interactions between insecticides
and soil microbes. Residue Rev., 64: 17-65.
TUCKER, R.K. & CRABTREE, D.G. (1970) Handbook of toxicity of
pesticides to wildlife, Washington, DC, US Department of the
Interior, Bureau of Sport Fishing and Wildlife, pp. 16-17, 46-47
(Resource Publication No. 84).
TUCKER, R.K. & HAEGELE, M.A. (1971) Comparative acute oral toxicity
of pesticides to six species of birds. Toxicol. appl. Pharmacol.,
20(1): 57-65.
TUINSTRA, L.G.M.TH. (1971) Organochlorine insecticide residues in
human milk in the Leiden region. Ned. Melk. Zuiveltijdschr., 25:
24-32.
TURNER, B.C., GLOTFELTY, D.E., & TAYLOR, A. (1977) Photodieldrin
formation and volatilization from grass. J. agric. food Chem., 25:
548-550.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., THEARLE, R.J.P., EGAN, H.,
EVANS, W.H., & SOUTAR, N.M. (1963) The effects on birds of certain
chlorinated insecticides used as seed dressings. J. Sci. Food
Agric., 14(8): 567-577.
TURTLE, E.E., TAYLOR, A., WRIGHT, E.N., MURTON, R.K., BRADY, J.,
THEARLE, R.J.P., JONES, F.J.S., REA, R.E., KIRBY, D.R., SMITH,
B.M., & RUTTER, M.E. (1965) Pesticides and wildlife. Report of the
Infestation Control Laboratory for 1962-64, London, Her Majesty's
Stationary Office, pp. 23-47.
UK-HMSO (1986) Food surveillance paper, London, Her Majesty's
Stationary Office, (Document No. 16).
UK-MAFF (1982-85) Report of the Working Party on Pesticide
Residues, London, Ministry of Agriculture, Fisheries and Food
(Food Surveillance Paper No. 16).
US EPA (1983) Environmental news, Washington, DC, US Environmental
Protection Agency, Office of Public Affairs.
UYETA, M., TAUE, S., CHIKAZAWA, K., & NISHIMOTO, T. (1971)
Pesticides translocated in food - organochlorine pesticides in the
total diet. J. Food Hyg. Soc. Jpn. 12: 445.
UZOUKWU, M. & SLEIGHT, S.D. (1972) Effects of dieldrin in pregnant
sows. J. Am. Vet. Med. Assoc., 160(12): 1641-1643.
VAN DEN BERCKEN, J. (1972) An electrophysiological investigation
into the action of DDT, dieldrin, and allethrin in the clawed toad
Xenopus laevis, Utrecht, Rijks Universiteit (Thesis).
VAN DEN BERCKEN, J. & NARAHASHI, T. (1974) Effects of aldrin-
transdiol, a metabolite of the insecticide dieldrin, on nerve
membrane. Eur. J. Pharmacol., 27: 255-258.
VAN DEN BROEK, W.L.F. (1979) Seasonal levels of chlorinated
hydrocarbons and heavy metals in fish and brown shrimps from the
Medway estuary, Kent. Environ. Pollut., 19: 21-38.
VAN DIJCK, P. & VAN DE VOORDE, H. (1976) Mutagenicity versus
carcinogenicity of organochlorine insecticides. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 41(2): 1491-1498.
VAN DUURSEN, P. (1985) Open letter. Shell Post, 1985(671).
VAN GELDER, G.A. & CUNNINGHAM, W.L. (1975) The effects of low-level
dieldrin exposure on the EEG and learning ability of the squirrel
monkey. Toxicol. appl. Pharmacol., 33(1): 142 (Abstract No. 50).
VAN GENDEREN, H. (1965) The toxicology of the chlorinated
hydrocarbon insecticides. A progress report with particular
reference to the qualitative aspects of the action in warm-blooded
animals. Opzoekingsstn Staat Gent, 30(3): 1321-1335.
VAN GENDEREN, H. (1979) [Dieldrin: a troublesome insecticide.] K.
Ned. Akad. Wet. (Amsterdam) 88(3): 24-32 (in Dutch).
VAN GENUCHTEN, M.TH., DAVIDSON, J.M., & WIERENGA, P.J. (1974) An
evaluation of kinetic and equilibrium equations for the prediction
of pesticide movement through porous media. Soil Sci. Soc. Am.
Proc., 38: 29-35.
VAN HAVER, W., VANDEZANDE, A., & GORDTS, L. (1978) [Organochlorine
pesticides in human fatty tissue.] Arch. Belg. Méd. soc. Hyg. Méd.
Trav. Méd. lég., 36: 147-155 (in Dutch).
VAN LEEUWEN, C.J. (1986) Ecotoxicological aspects of
dithiocarbamates, Utrecht, University of Utrecht (Thesis).
VAN RAALTE, H.G.S. (1965) Aspects of pesticide toxicity. In:
Proceedings of the Conference on Occupational Health, Caracas,
Venezuela (Unpublished paper).
VAN RAALTE, H.G.S. (1977) Human experience with dieldrin in
perspective. Ecotoxicol. environ. Saf., 1: 203-210.
VERMA, S.R. & TONK, I.P. (1984) Biomonitoring of the contamination
of water by a sublethal concentration of pesticides: a system
analysis approach. Acta hydrochim. hydrobiol., 12(4): 399-409.
VERSTEEG, J.P.J. & JAGER, K.W. (1973) Long-term occupational
exposure to the insecticides aldrin, dieldrin, endrin, and
telodrin. Br. J. ind. Med., 30: 201-202.
VIRGO, B.B. & BELLWARD, G.D. (1975) Effects of dietary dieldrin on
reproduction in the Swiss-Vancouver (SWV) mouse. Environ. Physiol.
Biochem., 5: 440-450.
VIRGO, B.B. & BELLWARD, G.D. (1977) Effects of dietary dieldrin on
offspring viability, maternal behaviour, and milk production in the
mouse. Res. Commun. chem. Pathol. Pharmacol., 17(3): 399-409.
VOERMAN, S. & BESEMER, A.F.H. (1975) Persistence of dieldrin,
lindane, and DDT in a light sandy soil and their uptake by grass.
Bull. environ. Contam. Toxicol., 13(4): 501-505.
VOUTSINOU-TALIADOURI, F. & SATSMADJIS, J. (1982) Influence of
metropolitan waste on the concentration of chlorinated hydrocarbons
and metals in striped mullet. Mar. Pollut. Bull., 13(8): 266-269.
VREMAN, K. & POORTVLIET, L.J. (1982) Pesticide levels in milk and
milk products in relation to their milk fat content. Neth. Milk
Dairy J., 36: 145-148.
VREMAN, K., POORTVLIET, L.J., & VAN DEN HOEK, J. (1980) Transfer of
organochlorine pesticides from feed into the milk and body fat of
cows. Long-term experiment with intake at low levels. Neth. Milk
Dairy J., 34: 87-105.
WADE, M.H., MOYER, J.W., & HINE, C.H. (1979) Mutagenic action of a
series of epoxides. Mutat. Res., 66: 367-371.
WADE, M.H., TROSKO, J.E., & SCHINDLER, M. (1986) A fluorescence
photobleaching assay of gap junction-mediated communication between
human cells. Science, 232: 525-528.
WALKER, A.I.T., NEILL, C.H., STEVENSON, D.E., & ROBINSON, J.
(1969a) The toxicity of dieldrin (HEOD) to Japanese quail (Coturnix
coturnix japonica). Toxicol. appl. Pharmacol., 15: 69-73.
WALKER, A.I.T., STEVENSON, D.E., ROBINSON, J., THORPE, E., &
ROBERTS, M. (1969b) The toxicology and pharmacodynamics of dieldrin
(HEOD): two-year oral exposure of rats and dogs. Toxicol. appl.
Pharmacol., 15: 345-373.
WALKER, A.I.T., THORPE, E., ROBINSON, J., & BALDWIN, M.K. (1971)
Toxicity studies on the photoisomerisation product of dieldrin.
Meded. Fac. Landbouwwet. Rijksuniv. Gent, 36(1): 398-409.
WALKER, A.I.T., THORPE, E., & STEVENSON, D.E. (1972) The toxicology
of dieldrin (HEOD). I. Long-term oral toxicity studies in mice.
Food Cosmet. Toxicol., 11: 415-432.
WALTON, M.S., BECK-BASTONE, V., & BARON, R.L. (1971) Subchronic
toxicity of photodieldrin, a photodecomposition product of
dieldrin. Toxicol. appl. Pharmacol., 20(1): 82-88.
WARNICK, S.L. (1972) Organochlorine pesticide levels in human serum
and adipose tissue, Utah, fiscal years 1967-71. Pestic. monit. J.,
6(1): 9-13.
WARNICK, S.L. & CARTER, J.E. (1972) Some findings in a study of
workers occupationally exposed to pesticides. Arch. environ.
Health, 25: 265-270.
WASSERMANN, M., CURNOW, D.H., FORTE, P.N., & GRONER, Y. (1968)
Storage of organochlorine pesticides in the body fat of people in
western Australia. Int. J. ind. Med. Surg., 37(4): 295-300.
WASSERMANN, M., FRANCONE, M.P., WASSERMANN, D., MARIANI, F., &
GRONER, Y. (1969) [Organochlorine pesticide content in the fatty
tissue of the general population in the Republic of Argentina.]
Sem. med., 134: 459-462 (in Spanish).
WASSERMANN, M., WASSERMANN, D, LAZAROVICI, S., COETZEE, A.M., &
TOMATIS, L. (1970) Present state of the storage of the
organochlorine insecticides in the general population of South
Africa. South Afr. med. J., 44: 646-648.
WASSERMANN, M., NOGUEIRA, D.P., TOMATIS, L., ATHIE, E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972a) Storage of organochlorine
insecticides in people of Sao Paulo, Brazil. Ind. Med. Surg., 41(3):
22-25.
WASSERMANN, M., ROGOFF, M.G., TOMATIS, L., DAY, N.E., WASSERMANN,
D., DJAVAHERIAN, M., & GUTTEL, C. (1972b) Storage of organochlorine
insecticides in the adipose tissue of people in Kenya. Ann. Soc.
Belg. Méd. Trop., 52(6): 509-514.
WASSERMANN, M., SOFOLUWE, G.O., TOMATIS, L., DAY, N.E., WASSERMANN,
D., & LAZAROVICI, S. (1972c) Storage of organochlorine insecticides
in people in Nigeria. Environ. Physiol. Biochem., 2: 59-67.
WASSERMANN, M., TRISHNANANDA, M., TOMATIS, L., DAY, N.E.,
WASSERMANN, D., RUNGPITARANGSI, V., CHIAMSAKOL, V.,
DJAVAHERIAN, M., & CUCOS, S. (1972d) Storage of organochlorine
insecticides in the adipose tissue of people from Thailand.
Southeast Asian J. trop. Med. public Health, 3(2): 280-285.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., &
DJAVAHERIAN, M. (1974a) Storage of organochlorine insecticides in
adipose tissue of Ugandans. Bull. environ. Contam. Toxicol., 12(4):
501-508.
WASSERMANN, M., TOMATIS, L., WASSERMANN, D., DAY, N.E., GRONER, Y.,
LAZAROVICI, S., & ROSENFELD, D. (1974b) Epidemiology of
organochlorine insecticides in adipose tissue of Israelis. Pestic.
monit. J., 8(1): 1-7.
WATSON, M., BENSON, W.W., & GABICA, J. (1970) Serum organochlorine
pesticide levels in people of southern Idaho. Pestic. monit. J.,
4(2): 47-50.
WEDBERG, J.L., MOORE, S., III, AMORE, F.J., & MCAVOY, H. (1978)
Residues in food and feed. Organochlorine insecticide residues in
bovine milk and manufactured milk products in Illinois 1971-76.
Pestic. monit. J., 11: 161-164.
WEGMAN, R.C.C. & GREVE, P.A. (1974) Levels of organochlorine
pesticides and inorganic bromide in human milk. Meded. Fac.
Landbouwwet. Rijksuniv. Gent, 39: 1301-1310.
WEGMAN, R.C.C. & GREVE, P.A. (1978) Organochlorines, cholinesterase
inhibitors, and aromatic amines in Dutch water samples, September
1969-December 1975. Pestic. monit. J., 12(3): 149-162.
WEISGERBER, I., KOHLI, J., KAUL, R., KLEIN, W., & KORTE, F. (1974)
Fate of aldrin-14C in maize, wheat, and soils under outdoor
conditions. J. agric. food Chem., 22(4): 609-612.
WEISGERBER, I., BIENIEK, D., KOHLI, J., & KLEIN, W. (1975)
Isolation and identification of three unreported photodieldrin-14C
metabolites in soil. J. agric. food Chem., 23: 873-877.
WELLS, M.R. & YARBROUGH, J.D. (1973) In vivo and in vitro retention
of 14C-aldrin and 14C-dieldrin in cellular fractions from brain and
liver tissues of insecticide-resistant and susceptible Gambusia.
Toxicol. appl. Pharmacol., 24: 190-196.
WELLS, M.R., PHILLIPS, J.B., & MURPHY, G.G. (1974) ATPase activity
in tissues of the map turtle Graptemys geographica following in
vitro treatment with aldrin and dieldrin. Bull. environ. Contam.
Toxicol., 11(6): 572-576.
WESSELS, C.L. (1978) Residues in soybean plants of aldrin and
dieldrin following soil application and of endosulphan and DDT
following foliar application. Rhodesian. J. agric. Res., 16:
205-210.
WHEATLEY, G.A. & HARDMAN, J.A. (1965) Indications of the presence
of organochlorine insecticides in rainwater in central England.
Nature (Lond.), 207: 486-487.
WHEATLEY, G.A. & HARDMAN, J.A. (1968) Organochlorine insecticide
residues in earthworms from arable soils. J. Sci. Food Agric., 19:
219-225.
WHEATLEY, G.A., HARDMAN, J.A., & STRICKLAND, A.H. (1962) Residues
of chlorinated hydrocarbon insecticides in some farm soils in
England. Plant Pathol., 11: 81-90.
WHITE, D.H. (1976) Nationwide residues of organochlorine in
starling, 1974. Pestic. monit. J., 10(1): 10-17.
WHITE, D.H., KING, K.A., & PROUTY, R.M. (1980) Significance of
organochlorine and heavy metal residues in wintering shorebirds at
Corpus Christi, Texas, 1976-77. Pestic. monit. J., 14(2): 58-63.
WHO (1958) Note by Secretariat on aldrin poisoning in Kenya,
Geneva, World Health Organization, p. 3 (Information Circular on
the Toxicity of Pesticides to Man No. 1).
WHO (1977) Outbreak of food poisoning of chemical origin, Geneva,
World Health Organization, p. 217 (Weekly Epidemiological Record No.
52).
WHO (1984) Guidelines for drinking-water quality, Geneva, World
Health Organization, Vol. 1, p. 69.
WHO (1988) The WHO recommended classification of pesticides by
hazard. Guidelines to classification 1988-89, Geneva, World Health
Organization (Unpublished document VBC/88.953).
WHO/FAO (1975-85) Data sheets on pesticides, Geneva, World Health
Organization (Unpublished documents).
WIEMEYER, S.N., MULHERN, B.M., LIGAS, F.J., HENSEL, R.J., MATHISEN,
J.E., ROBARDS, F.C., & POSTUPALSKY, S. (1972) Residues of
organochlorine pesticides, polychlorinated biphenyls, and mercury
in bald eagle eggs and changes in shell thickness, 1969 and 1970.
Pestic. monit. J., 6(1): 50-55.
WIERSMA, G.B., MITCHELL, W.G., & STANFORD, C.L. (1972) Pesticide
residues in onions and soil - 1969. Pestic. monit. J., 5(4):
345-347.
WIESE, I.H. & BASSON, N.C.J. (1966) The degradation of some
persistent chlorinated hydrocarbon insecticides applied to
different soil types. South Afr. J. agric. Sci., 9: 945-969.
WIESE, I.H., BASSON, N.C.J., VAN DER VIJVER, J.H., & VAN DER MERWE,
J.H. (1969) Toxicology and dynamics of dieldrin in the crowned
guinea-fowl Numida meleagris L. Phytophylactica, 1: 161-176.
WIESE, I.H., BASSON, N.C.J., BASSON, P.A., NAUDE, T.W., & MAARTENS,
B.P. (1973) The toxicology and pathology of dieldrin and photo-
dieldrin poisoning in two antelope species. Onderstepoort J. vet.
Res., 40(1): 31-40.
WILLIAMS, D.T., BENOIT, F.M., MCNEIL, E.E., & OTSON, R. (1978)
Organochlorine pesticide levels in Ottawa drinking water, 1976.
Pestic. monit. J., 12(3): 163-166.
WILLIAMS, D.T., LEBEL, G.L., & JUNKINS, E. (1984) A comparison of
organochlorine residues in human adipose tissue autopsy samples
from two Ontario municipalities. J. Toxicol. environ. Health, 13:
19-29.
WILLIAMS, G.M. (1982) Organochlorine pesticides and inhibition of
intercellular communication as the mechanism for their liver tumor
production. In: Miyamoto, J. & Kearney, P.C., ed. Pesticide
chemistry: human welfare and the environment. Oxford, New York,
Pergamon Press, Vol. 3, pp. 475-478.
WILLIAMS, R. & HOLDEN, A.V. (1973) Organochlorine residues from
plankton. Mar. Pollut. Bull., 4(7): 109-111.
WILLIAMS, S., MILLS, P.A., & MCDOWELL, R.E. (1964) Residues in milk
of cows fed rations containing low concentrations of five
chlorinated hydrocarbon pesticides. J. Assoc. Off. Agric. Chem.,
47(6): 1124-1128.
WILLIS, G.H., PARR, J.F., SMITH, S., & CARROLL, B.R. (1972)
Volatilization of dieldrin from fallow soil as affected by
different soil water regimes. J. environ. Qual., 1(2): 193-196.
WINTHROP, G.J. & FELICE, J.F. (1957) [A clinical toxicological
study of spraymen of a chlorinated hydrocarbon insecticide.] Bol.
Sanit. Panama, 43: 512-517 (in Spanish with English summary).
WIT, S.L. (1971) [Persistent insecticides in Dutch body fat.] Chem.
Weekbl., 67(5): 11-14 (in Dutch).
WOLFE, H.R., DURHAM, W.F., & ARMSTRONG, J.F. (1963) Health hazards
of the pesticides endrin and dieldrin. Arch. environ. Health, 6:
458-464.
WORTHING, C.R. & WALKER, S.B. (1983) The pesticide manual: a world
compendium, 7th ed., Croydon, British Crop Protection Council.
WRIGHT, A.S., POTTER, D., WOODER, M.F., DONNINGER, C., & GREENLAND,
R.D. (1972) The effects of dieldrin on the subcellular structure
and function of mammalian liver cells. Food Cosmet. Toxicol., 10:
311-332.
WRIGHT, A.S., AKINTONWA, D.A.A., & WOODER, M.F. (1977) Studies on
the interactions of dieldrin with mammalian liver cells at the
subcellular level. Ecotoxicol. environ. Saf., 1: 7-16, 427.
WRIGHT, A.S., DONNINGER, C., GREENLAND, R.D., STEMMER, K.L., &
ZAVON, M.R. (1978) The effects of prolonged ingestion of dieldrin
on the livers of male rhesus monkeys. Ecotoxicol. environ. Saf., 1:
477-502.
WUENSCHER, K. & ACKER, L. (1969) [The occurrence of chlorinated
insecticides in human fatty tissues.] Med. Ernähr., 10(4): 75-80
(in German).
WUTHRICH, C., MULLER, F., BLASER, O., & MAREK, B. (1985)
[Subjection of the population to pesticides and other foreign
substances through food.] Mitt. Geb. Lebensm. Hyg., 76: 260-276
(in German).
WYLLIE, J., GABICA, J., & BENSON, W.W. (1972) Comparative
organochlorine pesticide residues in serum and biopsied lipoid
tissue: a survey of 200 persons in southern Idaho - 1970. Pestic.
monit. J., 6(2): 84-88.
YAKUSHIJI, T., WATANABE, I., KUWABARA, K., YOSHIDA, S., KOYAMA, K.,
& KUNITA, N. (1979) Levels of polychlorinated biphenyls (PCBs) and
organochlorine pesticides in human milk and blood collected in
Osaka prefecture from 1972 to 1977. Int. Arch. occup. environ.
Health, 43: 1-15.
YAP, H.H., DESAIAH, D., CUTKOMP, L.K., & KOCH, R.B. (1975) In vitro
inhibition of fish brain ATPase activity by cyclodiene insecticides
and related compounds. Bull. environ. Contam. Toxicol., 14(2):
163-167.
YARON, B., SWOBODA, A.R., & THOMAS, G.W. (1967) Aldrin adsorption
by soils and clays. J. agric. food Chem., 15(4): 671-675.
YOSHIMURA, M., YAMADA, T., SUGIYAMA, S., NODA, H., & MITSUKUNI, Y.
(1979) Organochlorinated pesticides in human organs and tissues.
Jpn. J. leg. Med., 33(2): 91-102.
YU, S.J., KIIGEMAJI, M., & TERRIERE, L.C. (1971) Oxidative
metabolism of aldrin and isodrin by bean root fractions. J. agric.
food Chem., 19: 5-9.
ZABIK, M.E., HOOJJAT, P., & WEAVER, C.M. (1979) Polychlorinated
biphenyls, dieldrin, and DDT in lake trout cooked by broiling,
roasting, or microwave. Bull. environ. Contam. Toxicol., 21:
136-143.
ZAVON, M.R. & HAMMAN, R.E. (1961) Human experience with dieldrin in
malaria control programs. Am. J. public Health, 51(7): 1026-1032.
ZAVON, M.R. & STEMMER, K.L. (1975) The effect of dieldrin ingestion
on rhesus monkeys. A six-year study, Cincinnati, Ohio, Kettering
Laboratory.
ZAVON, M.R., HINE, C.H., & PARKER, K.D. (1965) Chlorinated
hydrocarbon insecticides in human body fat in the United States. J.
Am. Med. Assoc., 193(10): 837-839.
ZELLE, B. & LOHMAN, P.H.M. (1977) Repair of DNA in cultured human
cells treated with dieldrin and 4-nitroquinoline-N-oxide,
Rijswijk, The Netherlands, Medical Biological Laboratory TNO.
ZHONG-XIANG, L., KAVANAGH, T., TROSKO, J.E., & CHANG, C.C. (1986)
Inhibition of gap junctional intercellular communication in human
teratocarcinoma cells by organochlorine pesticides. Toxicol. appl.
Pharmacol., 83: 10-19.
ZIMMERLI, B. & MAREK, B. (1973) [Subjection of the Swiss population
to pesticides.] Mitt. Geb. Lebensm. Hyg., 64(4): 459-479
(in German).
APPENDIX I. NOMENCLATURE
Two major systems are currently used for the nomenclature of
these compounds: "polyhydroaromatic" names used by Chemical
Abstracts (American Chemical Society) and IUPAC and the von
Baeyer/IUPAC system for polycylic aliphatic compounds. That the
latter system should be used for the cyclodiene insecticides was
proposed by Benson (1969) and Bedford (1974). The "polyaromatic"
system has, unfortunately, been subject to historical variation,
and there are differences between the IUPAC, British and American
conventions for defining the 3-dimensional stereochemistry in this
system. As a consequence of the differences in the numbering of the
carbon atoms in the two major systems, and the modification of the
Chemical Abstracts "polyaromatic" name for dieldrin since 1971,
considerable confusion can occur regarding the nomenclature of
metabolites.
The various alternative names for aldrin, dieldrin, and photo-
dieldrin are summarized in Table 48. A useful discussion of
nomenclature is given by Brooks (1974).
For convenience, in view of the much more extensive usage in
the literature of the former Chemical Abstracts names for aldrin
and dieldrin, the names of their metabolites in this review are
based (if appropriate) on the former Chemical Abstracts names of
the parent compounds given in Table 48. The names of the
metabolites are given in Table 49, together with some alternative
names based on either the current Chemical Abstracts name for
dieldrin or the von Baeyer/IUPAC system.
The possible misunderstandings that may occur, particularly
for those not familiar with the various conventions of chemical
nomenclature, are illustrated by the different names that may be
given to the major faecal metabolite of dieldrin. This one
compound may be designated:
(a) 9-hydroxy dieldrin (former CA system);
(b) 8-hydroxy dieldrin (current CA system); or
(c) 12-hydroxy dieldrin (von Baeyer/IUPAC system).
Table 48. Alternative chemical names for aldrin, dieldrin, and photodieldrin
---------------------------------------------------------------------------------------------------------------
Compounda Polyhydroaromatic name Polycyclic aliphatic name
Chemical Abstracts IUPAC (von Baeyer/IUPAC)
---------------------------------------------------------------------------------------------------------------
Aldrin Formerly: Formerly: 1,8,9,10,11,11-hexachloro-2,3-7,6-
(HHDN) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- endo-2,1-7,8-exo-tetracyclo[6.2.13,6
(I) 1,4,4a,5,8,8a-hexahydro- 1,4,4a,5,8,8a-hexahydro- 02,7] dodeca-4,9-diene
endo-1,4-exo-5,8- exo-1,4-endo-5,8-
dimethanonaphthalene dimethanonaphthalene
Currently: Currently:
1,2,3,4,10,10-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1 alpha,4 alpha,4a-beta, hexachloro-1,4,4a,5,8,8a-
5 alpha,8a,8a beta-hexahydro- hexahydro-1,4:5,8-
1,4:5,8-dimethanonaphthalene dimethanonaphthalene
Dieldrin Formerly: Formerly: 1,8,9,10.11,11-hexachloro-4,5-exo-
(HEOD) 1,2,3,4,10,10-hexachloro- 1,2,3,4,10,10-hexachloro- epoxy-2,3-7,6-endo-2,1-7,8-exo-
(II) 6,7-epoxy-1,4,4a,5,6,7,8,8a- 6,7-epoxy-1,4,4a,5,6,7,8,8a- tetracyclo[6.2.1.13,6.02,7] dodec-
octahydro-endo-1,4-exo- octahydro-exo-1,4-endo-5,8- 9-ene
5,8-dimethanohaphthalene dimethanonaphthalene
Currently: Currently:
3,4,5,6,9,9-hexachloro- (IR,4S,5S,8R)-1,2,3,4,10,10-
1a alpha,2 beta,2a alpha, hexachloro-1,4,4a,5,6,7,8,8a-
3 beta,6 beta,6a alpha,7 beta, octahydro-6,7 epoxy-1,4:5,8-
7a alpha-octahydro-2,7:3,6- dimethanonaphthalene
dimethanonaphth[2,3-b]oxirene
Photo- 1,1,2,3,3a,7a-hexachloro- 3,exo-4,5,6,6,7-hexachloro-11,12-
dieldrin 6,7-epoxy-2,4,7-metheno- exo-epoxy-pentacyclo[6.4.0.02,10.
(III) decahydro-3H-cyclopenta[a]- 03,7.05,9]-dodecane
pentalene
---------------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
Table 49. Chemical nomenclature of metabolites of aldrin and dieldrin
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
9-Hydroxy dieldrin 9-hydroxy-1,2,3,4,10,10-hexa- 9-(syn-epoxy)hydroxy-1,2,3,4,10,10-hexa-
(VI) (9-Hydroxy HEOD) chloro-6,7-epoxy-1,4,4a,5,6,7, chloro-6,7,-epoxy-1,4,4a,5,6,7,8,8a-octa-
8,8a-octahydro-1,4-endo-5,8-exo- hydro-1,4-endo,exo-5,8-dimethanonaphthalene
dimethanonaphthalene
8-hydroxy-3,4,5,6,9,9-hexachloro-1a alpha,
2 beta, 2a alpha, 3 beta, 6 beta, 6a alpha,
7 beta, 7a alpha-octahydro-2,7:3,6-
dimethanonapth[2,3-b]oxirene
1,8,9,10,11,11-hexachloro-4,5-exo-epoxy-12-
(synepoxy)hydroxy-2,3-7,6-endo-2,1-7,8-exo-
tetracyclo[6.2.1.13,602,7]dodec-9-ene
Aldrin trans-diol (IV) trans-6,7-dihydroxy-1,2,3,4,10,10- 1,8,9,10,11,11-hexachloro-4,5-trans-
hexachloro-1,4,4a,6,7,5,8,8a-hexa- dihydroxy-2,3-7,6-endo-2,1-7,8-exo-
hydro-1,4-endo-5,8-exo-dimethano- tetracyclo[6.2.1.13,6.02,7]dodec-9-ene
naphthalene
Aldrin dicarboxylic 4,5,6,7,8,8-hexachloro-4,7-methano- 1,7,8,9,10,10-hexachloro-2,3-6,5-endo-
acid (V) 3a,4,7,7a-tetrahydro-indane-1,3- tricyclo[5.2.1.02,6]dec-8-ene-3,5-exo-
dicarboxylic acid dicarboxylic acid
Bridged pentachloro- 3,5,6,6,7-pentachloro-11,12-exo-
ketone (VII) (PCK, epoxy-pentacyclo[6.4.0.02,10.03,7
Klein's metabolite) .05,9]dodecan-4-one
Dechloro-aldrin 4,5,6,7,8-pentachloro-4,7-methano-
dicarboxylic 3a,4,7,7a-tetrahydro-indane-1,3-
acid (VIII) dicarboxylic acid
Dieldrin ketone (IX) 1,2,3,4,10,10-hexachloro-1,4,4a,5, 1,8,9,10,11,11-hexachloro-2,3-7,6-endo-
6,7,8,8a-octahydro-6-keto-endo- 2,1-7,8-exo-tetracyclo[6.2.1.13,6.02,7]-
1,4-exo-5,8-dimethanonaphthalene dodec-9-en-4-one
Photodieldrin 3-exo-4,5,6,6,7-hexachloro-
ketone (X) pentacyclo[6.4.0.02,10.03,7.05,9]
dodecan-11-one
---------------------------------------------------------------------------------------------------------
Table 49. (contd.)
---------------------------------------------------------------------------------------------------------
Trivial name(s)a Chemical name used in this review Alternative chemical names
---------------------------------------------------------------------------------------------------------
Photodieldrin trans- 3,exo-4,5,6.6,7-hexachloro-11,12
diol (XI) (caged dihydroxy-pentacyclo[6.4.0.02,10.
aldrin trans-diol) 03,7.05,9]dodecane
Photoaldrin dicarbo- 1,7,8,exo-9,10,10-hexachlorotetra-
xylic acid (XII) cyclo[5.2.1.02,6.04,8]decane-3,5-
(caged aldrin acid) exo,exo-dicarboxylic acid
Photoaldrin (XIII) 3,exo-4,5,6,6,7-hexachloropenta-
cyclo[6.4.0.02,10.03,7.05,9]dodec-
11-ene
---------------------------------------------------------------------------------------------------------
a Roman numerals in parentheses refer to the structures in Fig. 2 of the main document.
RESUME
1. Généralités
L'aldrine et la dieldrine qui sont l'une et l'autre des
pesticides organochlorés fabriqués industriellement depuis 1950,
ont été utilisés dans le monde entier jusqu'au début des années 70
comme insecticides en agriculture, contre de nombreux ravageurs
présents dans le sol, et pour le traitement des semences. Ces
insecticides étaient actifs contre les termites, les sauterelles,
les xylophages, les coléoptères et les ravageurs des textiles. La
dieldrine a également été employée en santé publique, pour la lutte
contre la mouche tsé-tsé et d'autres vecteurs de maladies
tropicales invalidantes. L'aldrine comme la dieldrine agissent par
contact et par ingestion.
Depuis le début des années 70, ces deux composés sont
interdits ou font l'objet de limitations rigoureuses dans un
certain nombre de pays, spécialement en agriculture. Néanmoins,
ils continuent d'être employés pour la destruction des termites
dans d'autres pays. La production annuelle mondiale, estimée à
13 000 tonnes en 1972, est tombée à moins de 2500 tonnes en 1984.
L'aldrine et la dieldrine de qualité technique ont une pureté
respective de 90% et plus de 95%. Les principales impuretés sont,
pour l'aldrine, l'octachlorocyclopentène, l'hexachlorobutadiène et
des produits de polymérisation et, pour la dieldrine, des
polychloroépoxy-octahydrodiméthanonaphtalènes.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans la plupart des alcanes, des
hydrocarbures aromatiques et des hydrocarbures halogénés, ainsi que
dans les esters, les cétones et les alcools.
La tension de vapeur de l'aldrine est de 6,5 x 10-5 mmHg à
25°C et celle de la dieldrine de 3,2 x 10-6 mmHg à 25°C.
Les méthodes utilisables pour le dosage de l'aldrine et de la
dieldrine dans les aliments, les aliments pour animaux et le milieu
sont décrites à la section 2.
2. Transport, distribution et transformation dans l'environnement
L'aldrine est principalement utilisée comme insecticide épandu
au niveau du sol. Les sols ainsi traités constituent donc dans
l'environnement une source importante d'aldrine et de son produit
de réaction, la dieldrine.
L'aldrine n'a qu'une faible capacité de migration à partir des
zones traitées, par volatilisation ou lessivage. Elle s'adsorbe
préférentiellement, et rapidement, sur les sols riches en matières
organiques, mais faiblement sur les sols argileux. Il est rare que
l'aldrine et la dielrine pénètrent au-delà des 20 premiers
centimètres de la couche de sol traité. L'aldrine adhère si
solidement aux particules du sol que seules des traces peuvent être
retirées par l'eau. C'est pourquoi il n'y a généralement pas
contamination des eaux souterraines.
L'aldrine s'élimine du sol selon une cinétique qui évoque une
réaction du premier ordre. Immédiatement après épandage, on
observe une courte période d'élimination rapide par volatilisation,
suivie d'une seconde période, plus longue, de décroissance
exponentielle, principalement du fait de la transformation en
dieldrine, plus lente à se dissiper. Néanmoins, il peut y avoir
une certaine migration du fait de l'érosion du sol, sous l'action
du vent, des eaux de ruissellement de du déplacement des sédiments.
Les observations faites sur les résidus d'aldrine dans la nature
montrent que, apparemment, ce composé est essentiellement retenu
dans le sol et que pour 97%, le résidu essentiel n'est pas le
composé d'origine mais l'époxyde correspondant, la dieldrine.
La photodieldrine est un produit de photodégradation de la
dieldrine, peu répandu dans l'environnement.
Après épandage sur le sol, l'aldrine disparaît lentement dans
les régions tempérées puisque, dans le cas type, il faut un an pour
qu'elle s'élimine aux trois quarts. La vitesse d'élimination
diminue ensuite à mesure que l'aldrine se transforme en dieldrine.
Il semblerait que la vitesse d'élimination soit plus élevée en
anaérobiose, comme c'est le cas dans les rizières, qu'en aérobiose.
Dans les régions tropicales, la dieldrine s'élimine du sol très
rapidement, jusqu'à hauteur de 90% au cours du premier mois, alors
que, dans le sol des régions tempérées, la dieldrine a une demi-vie
d'environ 5 ans. La volatilisation semble être le principal
mécanisme d'élimination à partir du sol, bien que la teneur
atmosphérique de la dieldrine et de l'aldrine soit généralement
faible. Une partie de la dieldrine est éliminée de l'atmosphère
par les précipitations, mais la concentration de ce produit est
très faible dans les eaux souterraines par suite de son adsorption
énergique sur les particules telluriques. On trouve de la
dieldrine en petites quantités dans les eaux de surface qui sont
contaminées par les eaux de ruissellement provenant de terres
agricoles.
3. Concentrations environnementales et exposition humaine
On trouve de l'aldrine et de la dieldrine dans l'atmosphère en
phase vapeur, adsorbées sur des poussières ou dans les eaux de
pluie, à des teneurs variables selon la situation. Ces produits
s'observent principalement dans les régions agricoles où leur
concentration atmosphérique moyenne est de l'ordre de 1 - 2 ng/m3
avec des maximums d'environ 40 ng/m3. Dans l'eau de pluie, on
relève des concentrations de l'ordre de 10 - 20 ng/litre ou parfois
plus.
Dans les habitations traitées avec ces produits contre les
termites, on observe une concentration dans l'air allant de 0,04 à
7 µg/m3, selon le moment de l'échantillonnage (c'est-à-dire le
nombre de jours après l'épandage) et le type d'habitation. Au bout
de 8 semaines, la concentration a très nettement diminué. Lorsqu'on
traite le bois en profondeur dans ces maisons, la concentration de
la dieldrine dans l'air va de 0,01 à 0,5 µg/m3. On a constaté que
l'aldrine et la dieldrine migraient dans les produits alimentaires
à partir de panneaux lamellés et de contreplaqués traités, ainsi
que par contact direct ou sorption à partir de l'atmosphère.
On a signalé la présence de dieldrine en milieu aquatique.
Mais les concentrations étaient très faibles, le plus souvent
inférieures à 5 ng/litre. Les concentrations plus élevées ont
généralement étéattribuées au rejet d'effluents industriels ou à
l'érosion du sol à la suite de l'utilisation de ces produits en
agriculture. Dans les cours d'eau, les sédiments peuvent présenter
des teneurs beaucoup plus élevées (allant jusqu'à 1 mg/kg).
On trouve rarement de l'aldrine dans les aliments, alors que
la dieldrine est plus courante, spécialement dans les produits
laitiers, les produits carnés, le poisson, les huiles et les
graisses, les pommes de terre et certains autres légumes
(principalement des légumes-racines). Des limites maximales de
résidus (LMR) de l'ordre de 0,02 - 0,2 mg/kg de produit ont été
recommandées lors des réunions conjointes successives FAO/OMS sur
les résidus de pesticides. Les études récentes réalisées dans
différents pays montrent que la concentration effective de la
dieldrine dans les denrées alimentaires est généralement plus
faible. Le recul est net au Royaume-Uni. En 1966 - 67, la
concentration moyenne des résidus de dieldrine observée lors d'une
étude sur la ration totale était de 0,004 mg/kg d'aliments tandis
que, pendant la période 1975 - 77, elle n'était plus que de 0,0015
mg/kg pour tomber à 0,0005 mg/kg en 1981. Cette évolution en
baisse est confirmée dans d'autres pays, par exemple aux Etats-Unis
d'Amérique. Cela tient peut-être à l'interdiction ou à la
limitation d'emploi de ces composés.
Dans un grand nombre de travaux publiés, on a recherché la
présence de dieldrine dans le tissu adipeux, les organes, le sang
et d'autres tissus, chez des sujets de la population générale. Au
cours des 25 dernières années, des enquêtes ont été réalisées dans
de nombreux pays, partout dans le monde. La plupart des
concentrations moyennes observées dans le tissu adipeux se situent
entre 0,1 et 0,4 mg/kg. Aux Etats-Unis d'Amérique, aux Pays-Bas et
au Royaume-Uni, on note une diminution de la concentration dans les
tissus adipeux depuis le milieu de la décennie 70. La
concentration sanguine varie de 1 à 2 µg/litre. Dans le foie, elle
est inférieure à 0,4 mg/kg tandis que, dans les autres tissus, à
savoir les reins, l'encéphale et les gonades, elle est inférieure à
0,1 mg/kg.
A la suite d'une exposition par voie transplacentaire, on
trouve de la dieldrine dans le sang, les tissus adipeux et d'autres
tissus du foetus et du nouveau-né. Les concentrations sont 2 à 10
fois plus faibles que chez la mère. Il n'existe aucune différence
entre le nourrisson et l'adulte pour ce qui est des concentrations
relatives de la dieldrine dans le cerveau, le foie et les tissus
adipeux. La dieldrine est également excrétée dans le lait
maternel. Depuis une quinzaine d'années, on recherche dans divers
pays la présence de pesticides organochlorés dans le lait de femme.
Dans la plupart des pays, la concentration plafonne à 6 µg/litre,
sauf cas exceptionnels.
4. Cinétique et métabolisme
Chez les animaux comme chez l'homme, l'aldrine et la dieldrine
passent rapidement des voies digestives dans le courant sanguin.
L'absorption a également lieu au niveau de la peau ou des poumons
après inhalation de la vapeur. Une étude sur des volontaires a
montré que la quantité résorbée par la peau intacte représente
7 - 8% de la dose appliquée. Selon des études d'inhalation
conduites sur des volontaires, le taux d'absorption et de rétention
de l'aldrine dans l'organisme peut atteindre 50% de la vapeur
inhalée. Après absorption, le composé se répartit rapidement dans
tous les organes et tissus, et il existe un échange permanent entre
le sang et les autres tissus. Entre-temps,l'aldrine se transforme
en dieldrine, principalement au niveau du foie mais aussi, dans une
moindre proportion, dans d'autres tissus comme les poumons. Cette
conversion est très rapide.
Après administration par voie orale d'une dose de 10 mg
d'aldrine par kg de poids corporel à des rats de 1 jour, on a
retrouvé ce composé dans le foie des animaux d'expérience 2 heures
après l'administration. Au cours des quelques heures suivantes, la
dieldrine s'est concentrée dans une beaucoup plus large mesure dans
les tissus lipidiques.
Comme l'ont montré de nombreuses études effectuées avec de
l'aldrine ou de la dieldrine marquées au 14C, une partie du produit
ingéré passe telle quelle dans l'intestin d'où elle est éliminée de
l'organisme, une partie est excrétée telle quelle à partir du foie
dans la bile, une autre fraction est stockée dans les divers
organes et tissus, en particulier le tissu adipeux, et une dernière
fraction est métabolisée dans le foie en produits à caractère
hydrophile et polaire plus prononcé. Chez l'homme et la plupart des
animaux, les métabolites sont principalement éliminés dans les
excreta, par l'intermédiaire de la bile. On a établi par ailleurs
que la biodégradation de l'aldrine et de la dieldrine aboutit aux
mêmes métabolites.
La plupart des connaissances actuelles sur le catabolisme de
la dieldrine chez les mammifères proviennent d'études chez la
souris, le rat, le lapin, le mouton, le chien, les petits singes,
le chimpanzé et l'homme. Dans l'ensemble, on n'observe que des
différences quantitatives entre les diverses espèces et les
mécanismes sont apparemment semblable chez le rat et les primates.
Le principal métabolite, sauf chez le rat, est le dérivé
hydroxylé en 9. On le trouve dans les déjections ainsi que dans
l'urine, sous forme libre ou conjuguée. On a découvert et
identifié chez les animaux d'expérience trois autres métabolites,
présents en petites quantités. Il s'agit d'un dérivé 6,7-
dihydroxylé en position trans, d'un acide dicarboxylique dérivé du
composé dihydroxylé et d'une pentachlorocétone pontée.
Seul le composé hydroxylé en 9 a été mis en évidence chez
l'homme, dans les matières fécales, tandis que ni ce composé ni les
autres métabolites n'apparaissaient dans le sang ou les autres
tissus. On a observé la présence de dieldrine dans les selles
d'ouvriers professionnellement exposés, tandis que, dans la
population générale, les quantités étaient inférieures au seuil de
détection. L'examen des urines de cinq ouvriers a montré que
l'excrétion de la dieldrine et de ses quatre métabolites par voie
urinaire était minime par rapport à l'élimination du métabolite
hydroxylé en 9 par voie fécale.
La transformation de l'aldrine en dieldrine dans le foie, sous
l'action de mono-oxygénases à fonction mixte (aldrine-époxydase) et
la distribution, puis le dépôt ultérieur, de la dieldrine
(principalement dans les tissus à contenu lipidique, tels que le
tissu adipeux, le foie, les reins, le coeur et le cerveau) sont
beaucoup plus rapides que le catabolisme et l'élimination finale de
la dieldrine intacte et de ses métabolites. Dans ces conditions,
pour un apport quotidien moyen déterminé d'aldrine ou de dieldrine,
il y a accumulation lente de dieldrine dans l'organisme. Mais
cette accumulation n'est pas indéfinie. Quand l'administration se
poursuit, on finit par aboutir à un état d'équilibre dynamique, la
quantité excrétée compensantexactement l'apport. La quantité
stockée maximale dépend de l'apport quotidien, tout comme on l'a
montré chez le rat, le chien et l'homme.
Quand l'apport d'aldrine/dieldrine est réduit ou interrompu,
la charge de l'organisme diminue. Chez l'homme, la demi-vie
biologique est de l'ordre de 9 à 12 mois. Chez le rat, le chien et
l'homme, on a démontré l'existence de relations significatives
entre la concentration de la dieldrine dans le sang et sa
concentration dans d'autres tissus.
De nombreuses études sur la concentration de la dieldrine dans
divers tissus, dont le sang et les tissus adipeux, aussi bien dans
la population générale que dans des catégories particulières, ont
été réalisées dans plusieurs pays et ont montré que, à l'équilibre,
les concentrations respectives dans les tissus adipeux, le foie, le
cerveau et le sang sont sensiblement proportionnelles à 150, 15, 3
et 1.
La dieldrine est transportée par le placenta jusqu'au foetus.
Il y a accumulation dans les mêmes organes et tissus que chez
l'adulte, mais en quantités moindres. Il existe apparemment un
équilibre entre les concentrations chez la mère et chez le foetus.
Chez le rat et le chien, la photodieldrine est également
métabolisée sous forme de pentachlorocétone pontée. On a retrouvé
les deux composés dans les tissus adipeux, le foie et les reins des
animaux à qui l'on avait administré de la photodieldrine à forte
dose. Chez l'homme, aucun résidu de ces composés n'a été mis en
évidence dans le tissu adipeux, les reins ni le lait maternel.
L'accumulation de photodieldrine dans les tissus adipeux des
animaux d'expérience était beaucoup moins importante que celle de
la dieldrine.
5. Effets sur les êtres vivants dans leur milieu natural
5.1 Accumulation
La plupart des résidus présents chez les êtres vivants sont
des résidus de dieldrine, car l'aldrine se transforme facilement
chez eux en dieldrine.
Les champignons, les streptomycètes et les bactéries
concentrent la dieldrine du milieu ambiant dans une proportion qui
peut aller en 4 h de 0,3 à plus de 100. Les protozoaires absorbent
la dieldrine davantage que les algues. Celles-ci absorbent très
rapidement la dieldrine présente dans le milieu de culture, les
concentrations maximales étant souvent atteintes en quelques
heures.
De nombreuses espèces d'invertébrés aquatiques concentrent
fortement la dieldrine à partir d'une eau à très faible teneur.
L'équilibre est atteint en quelques jours. Lorsqu'on les remet en
eau pure, la dieldrine s'élimine rapidement, avec une demi-vie de
60 - 120 h.
Pour les poissons entiers, le facteur de bioconcentration
dépasse 10 000. Chez une espèce de poissons, la demi-vie
d'élimination de la dieldrine accumulée s'est établie à 16 jours.
La bioconcentration de dieldrine chez les organismes
aquatiques se fait principalement à partir de l'eau et non par
ingestion d'aliments.
Les lombrics absorbent la dieldrine présente dans le sol et
laconcentrent jusqu'à un facteur maximal d'environ 170. Pour la
plupart des types de sol, il n'existe guère de corrélation entre la
concentra-tion atteinte chez le lombric et la concentration dans le
sol.
De nombreux travaux ont été consacrés à la présence de la
dieldrine dans les tissus ou dans les oeufs d'espèces non visées.
Les concentrations observées sont extrêmement variables, allant de
0,001 mg/kg à 100 mg/kg de tissu, mais elles restent le plus
souvent inférieures à 1 mg/kg de tissu.
Chez les oiseaux, il y a accumulation rapide de dieldrine
aussi bien dans les tissus que dans les oeufs. De même, on a
montré que diverses espèces de mammifères accumulent la dieldrine,
en particulier dans les graisses.
5.2 Toxicité pour les micro-organismes
La dieldrine a des effets très variables sur les algues uni-
cellulaires, avec une action sensible sur certaines espèces dès la
concentration de 10 µg/litre tandis que d'autres espèces ne sont
pas touchées même à la concentration de 1000 µg/litre. L'aldrine
et la dieldrine n'ont que peu d'effets sur les bactéries
terricoles, même à des concentrations très supérieures aux valeurs
habituelles. Dans la plupart des études, aucun effet n'a été
constaté après exposition à une concentration de 2000 mg/kg de
terre. Des effets ont été signalés sur la photosynthèse chez
différentes espèces d'algues, avec une action plus marquée de
l'aldrine que de la dieldrine à concentrations égales. Mais ces
effets minimes sur la biochimie des algues n'étaient que
transitoires.
5.3 Toxicité pour les organismes aquatiques
L'aldrine et la dieldrine sont extrêmement toxiques pour les
crustacés aquatiques, avec des valeurs de la DL50 à 96 h
inférieures à 50 µg/litre. Cependant, les quelques résultats plus
élevés signalés (jusqu'à 4300 µg/litre) illustrent les différences
de sensibilité selon les espèces. Les daphnies sont moins
sensibles à la dieldrine qu'à l'aldrine, avec des DL50 à 48 h de
23 - 32 µg/litre dans le premier cas et de 190 - 330 µg/litre dans
le second. Les mollusques sont nettement plus résistants, les CL50
à 48 h pouvant atteindre plus de 10 000 µg/litre. Des études de
plusieurs semaines ont confirmé la résistance relative des daphnies
et des mollusques. Les invertébrés aquatiques les plus sensibles
sont les stades larvaires des insectes, avec des CL50 à 96 h de
0,5 - 39 µg/litre pour la dieldrine et de 1,3 - 180 µg/litre pour
l'aldrine.
Lors d'épreuves de toxicité aiguë, l'aldrine comme la
dieldrine se sont montrées très toxiques vis-à-vis des poissons.
Chez diverses espèces de poisson, on a relevé des valeurs de la
CL50 à 96 h allant de 2,2 à 53 µg/litre pour l'aldrine et de 1,1 à
41 µg/litre pour la dieldrine. De nombreuses études ont montré que
la toxicité augmente avec la température. Dans une étude prolongée
sur Poecilia latipinna, on a obtenu un taux de mortalité de 100% en
présence d'une concentration de dieldrine égale ou supérieure à 3
µg/litre. L'addition de dieldrine à la nourriture de truites arc-
en-ciel jusqu'à des concentrations de 430 µg/kg de poids corporel
par jour, n'a exercé aucune influence sur la mortalité mais a
entraîné des modifications enzymatiques. Des altérations
morphologiques ont été observées aumicroscope électronique dans les
mitochondries hépatiques. Le mécanisme de détoxification de
l'ammoniaque chez les poissons est sensible à la dieldrine, la dose
sans effet nocif apparent étant inférieure à 14 µg/kg de poids
corporel par jour. La sensibilité à la dieldrine s'est révélée
variable selon le stade de développement des poissons. Les oeufs
étaient résistants et les formes juvéniles moins sensibles que les
adultes.
La toxicité aiguë de l'aldrine comme de la dieldrine est
élevée pour les larves d'amphibiens, avec des CL50 à 85 h de
l'ordre de 100 µg/litre.
5.4 Toxicité pour les organismes terrestres
La dieldrine est peu toxique pour les végétaux supérieurs
puisque les cultures ne sont affectées que par des doses
supérieures à 22 kg/ha. La phytotoxicité de l'aldrine est plus
importante, notamment pour les tomates et les concombres, mais
uniquement à des doses plusieurs fois supérieures aux valeurs
recommandées. Le chou est la plante cultivée la plus sensible à
l'aldrine.
Vis-à-vis des abeilles, la DL50 par voie orale varie, selon
les observations publiées, de 0,24 à 0,45 µg/abeille pour l'aldrine
et de 0,15 à 0,32 µg/abeille pour la dieldrine. Les quantités
toxiques par contact vont de 0,15 à 0,80 µg/abeille pour l'aldrine
et de 0,15 à 0,41 µg/abeille pour la dieldrine. D'après deux
études, la dieldrine est relativement plus toxique vis-à-vis des
insectes prédateurs qui se nourrissent de ravageurs.
Selon des études effectuées en laboratoire, le lombric
supporte des doses d'aldrine de 13 mg/kg en sol artificiel, le taux
de mortalité étant inférieur à 1%. La CL50 à six semaines était de
60 mg d'aldrine par kg de sol.
Pour 13 espèces d'oiseaux, la toxicité aiguë de l'aldrine et
de la dieldrine variait de plus du simple au décuple, avec des
valeurs de 6,6 - 520 mg/kg de poids corporel pour l'aldrine et de
6,9 - 381 mg/kg de poids corporel pour la dieldrine. Chez quatre
espèces d'oiseaux, la toxicité subaiguë par voie orale
correspondait à des doses comprises entre 34 et 155 mg/kg pour
l'aldrine et 37 et 169 mg/kg pour la dieldrine. Des épreuves
répétées au cours d'une certaine période n'ont révélé aucun signe
de résistance acquise chez ces espèces. D'après des études sur la
reproduction de plusieurs espèces de volaille, une concentration de
la dieldrine dépassant 10 mg/kg dans les aliments provoque une
certaine mortalité chez les adultes. Aucun effet ne s'est fait
sentir sur la production des oeufs, la fécondité, le taux
d'éclosion ni la survie des poussins en présence de dieldrine dans
les aliments à des concentrations qui ne sont pas toxiques pour la
mère. La dieldrine n'a aucune influence directe sur l'épaisseur de
la coquille des oeufs. Cependant, la diminution de la consommation
de nourriture, qui constitue un symptôme de l'intoxication par la
dieldrine, peut entraîner une diminution de l'épaisseur de la
coquille.
Chez le mammifères non élevés au laboratoire, la réponse à la
dieldrine varie selon les espèces. Chez quatre espèces de
campagnols, on a observé des valeurs de la DL50 aiguë, allant de
100 à 210 mg/kg de poids corporel, ce qui montre que ces animaux
sont moins sensibles àla dieldrine que les animaux de laboratoire.
Des musaraignes ont survécu à la consommation d'une nourriture
contenant 50 mg de dieldrine par kg mais sont mortes quand la
concentration est passée à 200 mg/kg. Des damalisques (une espèce
d'antilope) ont survécu 90 jours à une nourriture contenant de la
dieldrine à raison de 5 ou 15 mg/kg mais sont toutes mortes dans
les 24 jours pour une concentration égale ou supérieure à 25 mg/kg.
Tous les damalisques d'une région où l'on avait pulvérisé de la
dieldrine à raison de 0,16 kg/ha sont mortes et le calcul a montré
que l'apport alimentaire était de 1,82 mg/kg par jour. Trente pour
cent des springboks ont survécu aux épandages, sans manifester
d'effets tardifs. Les signes toxicologiques de l'intoxication par
la dieldrine étaient sensiblement les mêmes que chez les mammifères
de laboratoire.
5.5 Effets sur les populations et les écosystèmes
Certaines études donnent à penser que des populations de
mammifères ont été intoxiquées par de la dieldrine. Il est
probable que de petits mammifères sont morts après avoir mangé des
semences enrobées de dieldrine mais les populations se sont
reconstituées par immigration. Des chauves-souris ont été tuées
par la dieldrine contenue dans les agents de protection du bois.
Des résidus de dieldrine ont été signalés chez de nombreuses
espèces d'oiseaux. Partout dans le monde, c'est chez les oiseaux
de proie que les résidus sont les plus abondants car les animaux se
situent en fin de chaîne alimentaire. La teneur en dieldrine des
tissus et des oeufs d'oiseau suit l'évolution de l'emploi de
l'aldrine et de la dieldrine, et elle a diminué à la suite des
restrictions imposées à leur usage. Il n'est pas facile de repérer
les effets de la dieldrine car les résidus de cet insecticide
s'accompagnent de résidus d'autres organochlorés. La dieldrine est
plus toxique que le DDT pour les oiseaux et il est probable qu'elle
a provoqué chez les adultes une plus forte mortalité que le DDT.
Il est encore plus difficile de démontrer l'existence d'effets de
la dieldrine sur la reproduction à l'état naturel. En outre, il se
peut que les effets interviennent longtemps après l'exposition.
6. Effets sur les animaux d'expérience et les systèmes d'épreuve
in vitro
L'aldrine et la dieldrine sont extrêmement toxiques : pour ces
deux composés, la DL50 varie, chez la souris et chez le rat, de 40
à 70 mg/kg de poids corporel. Par voie percutanée, la dose toxique
se situe entre 40 et 150 mg/kg de poids corporel selon l'espèce en
cause et le solvant utilisé. On a constaté que l'aldrine et la
dieldrine de qualité technique déterminent chez le lapin une
irritation cutanée légère à intense, mais la cause en est le
solvant. Dans l'épreuve de maximalisation de Magnusson & Kligman
chez le cobaye, l'aldrine a provoqué un effet de sensibilisation.
Pourtant, au cours de 20 années de fabrication et de préparation
des formules, aucun cas de sensibilisation cutanée n'a été observé
dans un groupe comptant plus de 1000 travailleurs.
L'aldrine, comme la dieldrine, a une faible tension de vapeur
de sorte que, en principe, il n'y a aucun effet aigu par
inhalation. Les effets observés lors des études de toxicité aiguë
après exposition par toutes les voies possibles concernent le
système nerveux central etconsistent en hyperexcitabilité,
tremblements et convulsions.
Des études d'exposition par voie orale, de courte ou longue
durée, ont été réalisées avec l'aldrine et la dieldrine, chez la
souris, le rat, le chien, le hamster et les petits singes. Chez le
rat et la souris, le foie est le principal organecible : on observe
une augmentation de son poids par rapport au poids du corps et une
hypertrophie des hépatocytes centrilobulaires, la réversibilité
étant possible à un stade précoce. Au microscope, ces altérations
se traduisent par une augmentation de l'oxyphilie cytoplasmique et
une migration périphérique des granules basophiles. Ces
altérations ne se rencontrent pas au niveau du foie chez le hamster
et le singe. Chez le chien, l'atteinte hépatique est peu prononcée
(dégénérescence graisseuse et légère atrophie des hépatocytes);
elle s'accompagne d'une atteinte rénale consistant dans une
vacuolisation de l'épithélium des tubules distaux et une
dégénérescence tubulaire. Chez le rat, la dose sans effet nocif
observable se situe dans l'ensemble, d'après les résultats dont on
dispose sur le court et le long terme, aux alentours de 0,5 mg/kg
de nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
En augmentant les quantités incorporées à la nourriture, jusqu'à
obtenir l'équivalent de 0,05 mg/kg de poids corporel ou davantage,
on observe une hépatomégalie et des altérations histologiques
d'importance proportionnée à la dose. Chez le chien une dose de
0,04 - 0,2 mg/kg de poids corporel s'est révélée sans effet.
Plusieurs études de cancérogénicité à long terme ont été
effectuées sur différentes souches de souris, avec de l'aldrine ou
de la dieldrine. Chaque fois, on a observé des tumeurs
hépatocellulaires bénignes ou malignes. Apparemment, les femelles
étaient plus sensibles que les mâles. Aucun autre type de tumeur
ne s'est manifesté dans ces études.
Des études à long terme sur d'autres espèces (rat, hamster)
n'ont révélé aucune augmentation de l'incidence tumorale.
L'administration de photodieldrine incorporée à la nourriture,
jusqu'à une concentration de 7,5 mg/kg d'aliments, ne s'est pas
révélé tumorigène.
En outre, on a publié un certain nombre d'études spéciales qui
n'ont, jusqu'ici, pas permis d'élucider le mécanisme de la
production des tumeurs hépatiques chez la souris.
Dans la plupart des études de reproduction (sur 1 à 6
générations), réalisées avec l'aldrine ou la dieldrine sur des
souris et des rats, le principal effet constaté a été
l'augmentation du taux de mortalité dans la descendance, avant
sevrage. La capacité génésique n'a été atteinte qu'à des doses
toxiques pour la mère. Les études sur le chien étaient trop
limitées pour permettre les conclusions catégoriques, si ce n'est
qu'on a noté une augmentation systématique de la mortalité des
chiots à la mamelle.
D'après les résultats de ces études sur la reproduction, on
peut conclure que, de ce point de vue, les doses sans effet nocif
décelable sont de 2 mg de dieldrine par kg de nourriture chez le
rat et de 3 mg de dieldrine par kg de nourriture chez la souris,
soit l'équivalent quotidien de 0,1 et 0,4 mg/kg de poids corporel
respectivement.
Aucun signe de tératogénicité n'a été observé chez la souris,
le rat ou le lapin, après administration par voie orale de doses
d'aldrineet de dieldrine atteignant 6 mg/kg de poids corporel.
L'administration d'une dose unique d'aldrine et de dieldrine,
représentant environ la moitié de la DL50, a provoqué des effets
toxiques intenses chez le foetus de souris et de hamster, ainsi
qu'une incidence accrue d'anomalies tératogènes. La signification
de ces observations est douteuse en présence d'effets toxiques
probables chez les femelles gravides.
Les études de mutagénicité in vivo ou in vitro ont été
nombreuses, mais elles ont presque toujours donné des résultats
négatifs.
La toxicité aiguë de la photodieldrine par voie orale est plus
élevée que celle de la dieldrine chez la souris, le rat et le
cobaye. Lors d'études de toxicité aiguë ou à long terme, on a
observé des symptômes d'intoxication et des effets sur les organes
cibles analogues à ceux de la dieldrine, tant sur le plan
quantitatif que sur le plan qualitatif. La photodieldrine ne s'est
pas montrée tumorigène chez la souris ni chez le rat.
Comme la plupart des autres substances chimiques, l'aldrine et
la dieldrine exercent leurs effets toxiques selon plusieurs
mécanismes. Les organes cibles sont le système nerveux central et
le foie. Chez l'homme et les autres vertébrés, l'intoxication
secondaire à une exposition aiguë ou chronique, se caractérise par
des mouvements musculaires involontaires et des convulsions
épileptiformes. En cas de survie, la récupération est totale après
une courte durée marquée par des symptômes résiduels. Au niveau du
foie, on observe une activité accrue des enzymes microsomales de
biotransformation, en particulier du système enzymatique cytochrome
P-450/monooxygénase. Cette induction des enzymes mircosomales est
réversible et, au-delà d'un certain niveau, elle semble liée aux
altérations cytoplasmiques au niveau du foie et à l'hépatomégalie
chez les rongeurs.
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut penser que, en pratique, ces produits ne contribuent guère à
l'incidence des cancers humains.
7. Effets chez l'homme
L'aldrine et la dieldrine sont très toxiques pour l'homme. Il
y a eu de graves cas d'intoxication accidentelle ou professionnelle
mais il est rare qu'ils aient fait des victimes. La plus faible
dose ayant provoqué une issue fatale a été estimée à 10 mg/kg de
poids corporel. Les personnes ayant survécu à une intoxication
aiguë ou subaiguë se sont complètement rétablies. Aucun effet
irréversible ni atteinte anatomopathologique résiduelle n'a été
signalée.
Les effets nocifs de l'aldrine et de la dieldrine sont
fonction de la concentration de la dieldrine dans le sang. Le
dosage de la dieldrine dans le sang permet de diagnostiquer avec
précision une exposition à l'aldrine/dieldrine. Chez les
travailleurs, le taux sanguin au-dessous duquel on n'observe aucun
effet nocif (dose limite sans effet nocif décelable) est de 105
µg/litre de sang. Cela correspond à un apport quotidien de
dieldrine de 0,02 mg/kg de poids corporel.
L'exposition environnementale (principalement par
l'intermédiairedes aliments mais aussi, dans une faible mesure, par
voie respiratoire) entraîne l'apparition de dieldrine à une très
faible concentration dans les organes, le sang et le lait maternel.
Autant qu'on puisse en juger d'après des études épidémiologiques et
cliniques poussées, il n'existe aucun raison de penser que les taux
couramment observés dans l'organisme constituent une menace pour la
santé de la population en général. Lors d'une étude poursuivie
pendant plus de 20 ans auprès de 1000 travailleurs de l'industrie,
employés dans une fabrique d'insecticides à base d'aldrine/dieldrine,
aucune augmentation de l'incidence des cancers n'a été observée chez
les sujets fortement exposés à ces deux produits. Phénomène encore
plus significatif, aucun signe avant-coureur, sous forme d'une
altération de la fonction hépatique, n'a été observé.
Une étude épidémiologique sur la mortalité a été réalisée dans
une unité de production aux Etats-Unis, sur une cohorte de 870
travailleurs exposés à l'aldrine, à la dieldrine et à l'endrine.
Malgré près de 25 000 années-homme d'observation, il n'a pas été
possible de repérer un risque particulier de cancer attributable au
travail dans cette usine.
EVALUATION DES DANGERS POUR LA SANTE DE L'HOMME ET DES EFFETS SUR
L'ENVIRONNEMENT
1. Evaluation des dangers pour la santé de l'homme
L'aldrine et la dieldrine sont des pesticides organochlorés
qui ont été utilisés partout dans le monde entre 1950 et le début
des années 70 comme insecticides en agriculture et pour le
traitement des semences, pour la destruction des ravageurs
terricoles et d'autres types d'insectes (par exemple les termites,
les sauterelles et les ravageurs des textiles) ainsi que pour la
lutte contre les glossines et autres vecteurs de maladies. Chez
les insectes, ces composés exercent leur effet toxique par contact
et par voie digestive. A partir des années 70, on en a limité ou
interdit l'emploi dans plusieurs pays, spécialement en agriculture.
Pourtant, ils continuent d'être utilisés dans d'autres pays pour la
destruction des termites.
Les deux composés sont pratiquement insolubles dans l'eau et
modérément à très solubles dans de nombreux solvants organiques.
Leur tension de vapeur est faible.
On trouve souvent de la dieldrine dans des produits laitiers
ou carnés, le poisson, les huiles et les graisses et certains
légumes, notamment des légumes-racines. La limite maximale de
résidus recommandée par les instances compétentes de la FAO/OMS
lors des réunions conjointes sur les résidus de pesticides varie de
0,02 à 0,2 mg/kg de produit. Des mesures récentes ont montré que
les teneurs effectives sont plus faibles, comme l'ont d'ailleurs
confirmé les études sur la ration globale. Comme l'utilisation de
ces deux composés fait maintenant l'objet de restrictions, on
observe une diminution lente mais régulière de la teneur en résidus
des différentes denrées alimentaires.
Les quantités ingérées par l'homme avec sa ration quotidienne
se traduisent, malgré la faible concentration de ces produits dans
les aliments, par la présence de dieldrine dans le tissu adipeux et
dans certains autres tissus et organes. Des enquêtes à l'échelle
mondiale montrent que les teneurs moyennes varient de 0,1 à 0,4
mg/kg de tissu adipeux. Depuis le début des années 70, cette
concentration diminue lentement.
Comme le foetus est exposé par voie transplacentaire, ses
tissus adipeux contiennent également de la dieldrine, mais à une
concentration qui n'est que de 10 à 50% de la concentration chez la
mère. Il semble exister un équilibre entre les concentrations
foetales et les concentrations maternelles. La dieldrine est
également excrétée dans le lait. Une exposition est possible par
inhalation dans les habitations où l'on utilise ce produit pour la
destruction des termites. Après traitement, on observe des
concentrations atmosphériques allant de 0,01 à 7 µg/m3, selon le
mode d'épandage, la concentration utilisée, les modalités de
l'aération et le moment où les échantillons sont prélevés. En
pareilles circonstances, les aliments peuvent aussi être
contaminés, par contact direct, ou par sorption à partir de l'air
ambiant.
Le métabolisme s'effectue principalement dans le foie où
l'aldrine se transforme rapidement en dieldrine. Le catabolisme de
la dieldrineest plus lent que celui de ses métabolites hydrophiles
qui sont excrétés dans la bile et dans les urines. La structure de
ces métabolites a été établie. Chez toutes les espèces étudiées,
notamment l'homme, on a montré que les quantités
d'aldrine/dieldrine accumulées se stabilisent à un niveau qui est
fonction de l'apport puisqu'il existe une relation linéaire entre
les quantités accumulées et le logarithme de l'apport. Quand
l'exposition prend fin, la concentration de la dieldrine dans les
tissus de l'organisme diminue selon une loi exponentielle. La
toxicité aiguë de l'aldrine et de la dieldrine est importante chez
les mammifères par voie orale, tandis que la toxicité par voie
cutanée est modérée. Aucune sensibilisation cutanée n'a été
observée. Les effets constatés à la suite d'une exposition
expérimentale aiguë ou de courte ou longue durée intéressent le
système nerveux central. Le foie est également un organe cible.
Chez les souris et les rats, on observe à ce niveau des altérations
désignées sous le nom de "foie de rongeur sous insecticide
organochloré".
Apparemment, l'aldrine et la dieldrine ne sont pas tératogènes
à des doses inférieures à celles qui sont toxiques chez la femelle
gravide et chez le foetus. On n'a pas fait état de toxicité pour
la fonction de reproduction chez le mâle ou la femelle.
De nombreuses études de mutagénicité in vitro et in vivo ont
montré que ni l'aldrine ni la dieldrine ne sont mutagènes.
Lors d'études à long terme, ces deux produits ont déterminé
chez la souris des tumeurs hépatiques, bénignes ou malignes. En
revanche, aucune augmentation de l'incidence des tumeurs hépatiques
ou autres n'a été observée chez le rat ni le hamster.
Selon le CIRC (1987), il n'existe pas de preuves suffisantes
d'un pouvoir cancérogène chez l'homme et les preuves de
cancérogénicité chez l'animal d'expérience sont limitées.
L'aldrine comme la dieldrine ont été classées dans le groupe 3, à
savoir celui des produits chimiques dont il est impossible de
préciser le pouvoir cancérogène chez l'homme.
Compte tenu des résultats obtenus lors des études de toxicité
de courte ou de longue durée, la dose globale sans effet nocif
décelable se situe chez le rat à 0,5 mg de dieldrine par kg de
nourriture, soit l'équivalent de 0,025 mg/kg de poids corporel.
Chez le chien, la dose sans effet nocif décelable est de 0,04 mg/kg
de poids corporel. Lors des réunions conjointes FAO/OMS de 1966 et
1977 sur les résidus de pesticides, on a fixé la dose journalière
admissible (DJA) à 0,1 µg/kg de poids corporel, en tenant compte de
la non-cancérogénicité de ces deux substances pour l'homme.
L'aldrine et la dieldrine sont très toxiques pour l'homme. On
connaît des cas d'intoxication accidentelle ou professionnelle,
mais qui ont rarement fait des victimes. Les survivants à une
intoxication aiguë ou subaiguë se sont entièrement rétablis. Les
effets nocifs sont fonction de la concentration sanguine de la
dieldrine, dont le dosage permet de diagnostiquer avec précision
une exposition à l'aldrine/dieldrine. Pour taux sanguins inférieur
à 105 µg/litre, aucun effet indésirable n'est à craindre. Cette
concentration constitue la dose limite sans effet nocif décelable
et correspond à un apport quotidien de 0,02 mg de dieldrine par kg
de poids corporel.
L'exposition liée à l'environnement, principalement par la voie
alimentaire, entraîne la présence de faibles concentrations de
dieldrine dans l'organisme. D'après des études épidémiologiques et
cliniques poussées, ces teneurs ne constituent pas une menace pour
la santé humaine.
Aucun signe avant-coureur d'une altération de la fonction
hépatique n'a été observé lors d'une enquête de 20 ans portant sur
plus de 1000 ouvriers de l'industrie exposés à l'aldrine et à la
dieldrine. Dans cette étude ainsi que dans une autre effectuée aux
Etats-Unis d'Amérique, aucun risque particulier de cancer n'a été
repéré chez les personnes profesionnellement exposées à l'aldrine
et à la dieldrine (parfois à de fortes concentrations).
Dans l'ensemble, d'après les observations faites sur l'aldrine
et la dieldrine, notamment dans le cadre des études sur l'homme, on
peut estimer que, en pratique, ces produits chimiques ne
contribuent que très peu, sinon pas du tout, à l'incidence des
cancers humains.
La photodieldrine, produit qui résulte de la dégradation de la
dieldrine sous l'action de la lumière, est analogue à la dieldrine
pour ce qui est de sa toxicité sur une courte durée. Elle n'est ni
tératogène ni cancérogène chez la souris et le rat. L'accumulation
de photodieldrine dans les tissus adipeux d'animaux d'expérience
s'est révélée inférieure à celle de la dieldrine.
2. Evaluation des effets sur l'environnement
La principale source de dieldrine (jusqu'à 97%) dans
l'environnement est l'aldrine, un insecticide épandu au niveau du
sol. L'aldrine et son produit de réaction, la dieldrine sont
rapidement absorbé par les sols, spécialement ceux qui sont riches
en matières organiques. De ce fait, la pénétration est limitée et
il n'y a généralement aucune contamination des eaux souterraines.
Les deux composés sont entraînés principalement du fait de
l'érosion (sous l'action du vent) et du transport des sédiments
(eaux superficielles de ruissellement), mais non par lessivage.
L'emploi d'aldrine et de dieldrine en agriculture donne lieu à
la présence de résidus (principalement de dieldrine) dans le sol où
ils peuvent persister plusieurs années; la demi-vie de la dieldrine
est estimée à 4 - 7 ans. La persistance de ces composés est
moindre dans les régions tropicales que dans les régions tempérées.
L'aldrine et la dieldrine passent, par volatilisation, des
récoltes et du sol traités à l'atmosphère; elles peuvent aussi y
pénétrer directement lors de l'épandage. La dieldrine retourne au
sol ou dans les étendues d'eau par les précipitations ou par dépôt
de particules sèches. Les composés se rencontrent donc soit en
phase vapeur (à des concentrations très faibles, en général de
l'ordre de 1 - 2 ng/m3), soit adsorbés sur des particules de
poussière, soit encore dans les eaux de pluie (à des concentrations
de l'ordre de 10 - 20 ng/litre).
Plusieurs auteurs ont signalé la présence de dieldrine en
milieu aquatique. Dans les eaux de surface, les concentrations
sont le plus souvent très faibles, inférieures à 5 ng/litre. Mais
des valeurs plus élevées s'observent dans les régions soumises à
l'érosion ou danscelles où l'on utilise ce produit en agriculture.
Dans ces régions, les sédiments des cours d'eau peuvent renfermer
jusqu'à 1 mg de dieldrine par kilogramme. La forte capacité qu'ont
les organismes aquatiques à concentrer la dieldrine à partir de
teneurs très faibles peut aboutir à l'accumulation de doses
toxiques. La concentration de ce produit tout au long de la chaîne
alimentaire aquatique est moins importante qu'une absorption
directe à partir de l'eau.
Comme la dieldrine est très répandue dans l'environnement et
qu'elle y persiste, on observe des concentrations très variées chez
les organismes non visés. Alors qu'auparavant les valeurs
observées allaient de 0,001 mg à 100 mg/kg de tissu, elles sont
aujourd'hui le plus souvent inférieures à 1 mg/kg de tissu.
Dans les écosystèmes terrestres, l'aldrine et la dieldrine
s'accumulent chez divers organismes, principalement sous forme de
dieldrine. Cette dernière est probablement responsable de la mort
de mammifères dans la nature et de la raréfaction de certaines
espèces, comme la loutre. Certains petits mammifères périssent
sans doute après avoir mangé des céréales traitées mais il est
probable que leurs populations se reconstituent par immigration à
partir des zones voisines. Les oiseaux de proie qui mangent de
petits mammifères et de petits oiseaux contaminés par la dieldrine
absorbent et concentrent cet insecticide dans leurs tissus et leurs
oeufs. Des oiseaux granivores ont été tués par la consommation de
céréales traitées. Il est probable que la raréfaction des oiseaux
de proie s'explique par la présence dans leurs tissus de résidus de
dieldrine (entre autres organochlorés). Les effets de la dieldrine
se manifestent avec un certain retard car les résidus s'accumulent
dans les graisses pendant l'hiver d'où ils ne se libèrent qu'au
printemps. Le fait de n'utiliser la dieldrine qu'à certaines
époques de l'année n'a pas réduit la mortalité des oiseaux.
La large utilisation d'aldrine et de dieldrine, parallèlement
à celle d'autres organochlorés, a exercé des effets très nocifs sur
l'environnement mais grâce à des restrictions draconiennes,
particulièrement en ce qui concerne les semences traitées, les
populations d'oiseaux commencent à se reconstituer.
3. Conclusions
a) L'aldrine et la dieldrine ont donné lieu à des études
poussées et variées sur le plan toxicologique, clinique et
épidémiologique. La charge de l'organisme résulte principalement
de l'ingestion de résidus présents dans la nourriture (les
quantités ingérées semblant diminuer de façon générale et tomber
en-dessous des DJA fixées) et, dans une moindre mesure, de
l'inhalation de ces produits. D'après l'étude des données, on a
tout lieu de penser que la charge de l'organisme résultant du
niveau actuel d'exposition ne menace en aucun cas la santé de la
population dans son ensemble.
b) La dieldrine se rencontre presque partout dans le lait
maternel. Mais, sa concentration dans le sang et dans le tissu
adipeux des nourrissons n'augmente pas avec l'âge au cours des six
premiers mois de leur vie et le taux sanguin n'est pas plus élevé
que chez un enfant nourri au biberon. Dans ces conditions,
l'allaitement au sein reste la méthode de choix pour nourir les
nourissons malgré la présence de résidus de dieldrine.
c) Lors du traitement de locaux, notamment pour la destruction
des termites, l'exposition des occupants ne semble pas présenter
des risques pour leur santé, pour autant que le traitement
s'effectue correctement.
d) Malgré leur toxicité élevée, l'aldrine et la dieldrine
peuvent être manipulées sans danger dans la mesure où l'on observe
toujours les précautions recommandées en vue de réduire au minimum
l'exposition des opérateurs. Dans le cas contraire, il y a risque
d'intoxication.
e) Pendant la période où l'on a massivement utilisé l'aldrine
et la dieldrine, c'est-à-dire de 1950 au début des années 70, il
est certain que cette pratique a eu des effets dommageables sur
diverses espèces. Ces effets sont imputables en partie à la
dieldrine à côté d'autres organochlorés. Depuis qu'on a limité de
façon draconienne l'utilisation de ces produits, les espèces
touchées se sont reconstituées.
RECOMMENDATIONS
1. Il faut effectuer des études de tératogénicité
complémentaires sur le hamster, bien conçues, avec des doses
réalistes de dieldrine.
2. Dans l'étude du mécanisme de la cancérogenèse, on
s'efforcera d'expliquer pourquoi les réactions hépatiques sont si
différentes chez la souris et chez les autres espèces.
3. On continuera d'utiliser la dieldrine pour l'étude des
mécanismes neurotoxiques, à la fois sur le plan expérimental et sur
le plan clinique.
4. Pour des raisons écologiques, toute reprise d'une
utilisation massive d'aldrine et de dieldrine est exclue, et on
n'utilisera ces produits que s'il n'existe pas de produit moins
nocif d'efficacité équivalente.
5. Afin de préserver la santé et le bien-être des travailleurs
et de la population en général, il convient de ne confier la
manipulation et l'épandage de l'aldrine et de la dieldrine qu'à des
opérateurs compétents et dûment formés, qui devront appliquer les
mesures de sécurité qui s'imposent.
6. En raison du risque d'intoxication accidentelle par
l'aldrine, spécialement chez les enfants, il faut en interdire
l'utilisation sous forme de granulés contre les fourmis.
