
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 66
KELEVAN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
World Health Orgnization
Geneva, 1986
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
ISBN 92 4 154266 7
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1992
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR KELEVAN
1. SUMMARY AND CONCLUSIONS
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Man-made sources
3.2. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution
4.2. Biotransformation
4.3. Abiotic degradation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Soil
5.1.3. Food and animal feed
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution, storage, metabolic transformation,
and excretion
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic organisms
7.2. Terrestrial organisms
7.3. Microorganisms
7.4. Appraisal
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposures
8.2. Short-term exposures
8.2.1. Oral
8.2.2. Dermal
8.2.3. Inhalation
8.3. Long-term exposure
8.4. Reproduction studies
8.5. Mutagenicity
8.6. Carcinogenicity
8.7. Other studies
9. EFFECTS ON MAN
10. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON
THE ENVIRONMENT
10.1. Evaluation of the health risks for man
10.2. Evaluation of environmental effects
10.3. Conclusions and recommendations
REFERENCES
TASK GROUP MEETING ON ENVIRONMENTAL HEALTH CRITERIA FOR THE
ORGANOCHLORINE PESTICIDES
Members
Dr Z. Adamis, National Institute of Occupational Health,
Budapest, Hungarya
Dr L. Albert, Environmental Pollution Programme, National
Institute of Biological Resource Research, Xalapa, Mexico
(Vice-Chairman) b
Dr Sakdiprayoon Deema, Ministry of Agriculture and
Cooperatives, Bangkok, Thailandb
Dr R. Goulding, Chairman of the Scientific Sub-committee,
UK Pesticides Safety Precautions Scheme, Ministry of
Agriculture, Fisheries and Food, London, United Kingdom
(Chairman) a
Dr Y. Hayashi, Pathology Division, National Institute of
Hygienic Sciences, Tokyo, Japanb
Dr S.K. Kashyap, National Institute of Occupational Health
(Indian Council of Medical Research), Meghaninager,
Ahmedabad, Indiaa
Dr R. Kimbrough, Center for Environmental Health, Centers for
Disease Control, Atlanta, Georgia, USA (Rapporteur) b
Mr Y.T. Mosuro, Federal Ministry of Health, Food and Drug
Administration and Laboratory Services, Oshodi, Nigeriab
Dr Y. Osman, Occupational Health Department, Ministry of
Health, Khartoum, Sudanb
Dr L. Rosival, Centre of Hygiene, Research Institute of Preventive
Medicine, Bratislava, Czechoslovakia (Chairman) b
Dr F.W. van der Kreek, Ministry of Welfare, Health, and
Culture, Leidschendam, Netherlandsb
Dr D.C. Villeneuve, Environmental Contaminants Section,
Environmental Health Centre, Tunney's Pasture, Ottawa,
Ontario, Canada (Rapporteur) a
Dr D. Wassermann, Department of Occupational Health, The
Hebrew University, Haddassah Medical School, Jerusalem,
Israel (Vice-Chairman) a
Dr Xue Shou Zheng, School of Public Health, Shanghai Medical
University, Shanghai, Chinab
---------------------------------------------------------------------------
a Present at first Task Group meeting.
b Present at second Task Group meeting.
Representatives of Other Organizations
Dr A. Berlin, Health and Safety Directorate, Commission of the
European Communities, Luxembourgb
Mrs M. Th. van der Venne, Health and Safety Directorate,
Commission of the European Communities, Luxembourga
Observers
Dr C.J. Calo, European Chemical Industry Ecology and
Toxicology Centre (ECETOC), Brussels, Belgiuma
Dr D.M. Whitacre, International Group of National Associations
of Agrochemical Manufacturers (GIFAP), Brussels, Belgiuma
Dr A.A. van Kolfschoten, International Group of National Associations
of Agrochemical Manufacturers (GIFAP), Brussels, Belgiumb
Secretariat
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood
Experimental Station, Huntingdon, United Kingdomb
Dr M. Gilbert, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerlanda,b
Ms B. Goelzer, Office of Occupational Health, World Health
Organization, Geneva, Switzerlanda
Dr Y. Hasegawa, Division of Environmental Health, Environmental
Hazards and Food Protection, World Health Organization, Geneva,
Switzerlanda
Dr K.W. Jager, International Programme on Chemical Safety,
World Health Organization, Geneva, Switzerland (Secretary)a,b
Mr B. Labarthe, International Register for Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerlanda
Dr I.M. Lindquist, International Labour Organisation, Geneva,
Swizterlanda
Dr A. Pelfrene, Insecticides Development and Safe Use Unit,
World Health Organization, Geneva, Switzerlandb
Dr M. Vandekar, Pesticides Development and Safe Use Unit,
World Health Organization, Geneva, Switzerlanda
---------------------------------------------------------------------------
a Present at first Task Group meeting.
b Present at second Task Group meeting.
Secretariat (contd.)
Dr T. Vermeire, National Institute for Public Health and
Environmental Hygiene, Bilthoven, Netherlands (Temporary
Adviser) b
Mr J.D. Wilbourn, International Agency for Research on Cancer,
Lyons, Francea
---------------------------------------------------------------------------
a Present at first Task Group meeting.
b Present at second Task Group meeting.
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly
delaying their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda, which will appear in subsequent volumes.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone no. 988400 -
985850).
ENVIRONMENTAL HEALTH CRITERIA FOR KELEVAN
A WHO Task Group on Environmental Health Criteria for
Organochlorine Pesticides other than DDT met in Geneva on
28 November - 2 December, 1983. Dr K.W. Jager opened the meeting
on behalf of the Director-General.
The Task Group reviewed and revised the draft criteria
document. The Task Group concluded that the data on kelevan
were too sparse to make an evaluation of the health risks for
man or the effects on the environment. It recommended that
the draft should be recirculated to the IPCS and IRPTC focal
points with a request for further information.
A second WHO Task Group was held in Geneva on 9 - 13
December, 1985 to review and revise an amended draft and to
make an evaluation of the risks of kelevan for human health
and the environment.
The first drafts of the kelevan document were prepared by
DR D.C. VILLENEUVE of Canada and DR S. DOBSON of the United
Kingdom.
The present draft was prepared by the IPCS Secretariat,
updating the preliminary hazard assessment with new information
received in more than 50 replies from Focal Points.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
* * *
Partial financial support for the publication of this criteria
document was kindly provided by the United States Department of
Health and Human Services, through a contract from the National
Institute of Environmental Health Sciences, Research Triangle Park,
North Carolina, USA - a WHO Collaborating Centre for Environmental
Health Effects. The United Kingdom Department of Health and Social
Security generously supported the costs of printing.
1. SUMMARY AND CONCLUSIONS
Technical kelevan is a brownish solid substance with a
molecular formula of C17H12Cl10O4.
It is a chlordecone derivative and can be oxidized to
chlordecone before determination by gas chromatography with
electron capture detection.
Kelevan has been used in a number of countries as an
insecticide, mainly for the control of the potato beetle and the
banana root borer.
It is degraded quite rapidly by biotransformation and abiotic
degradation to other caged structure products. The half-life of
kelevan in soil has been reported to be 5 - 12 weeks. However, its
major metabolite, chlordecone, persists in the soil for several
years.
There is very little leaching of kelevan and its caged-
structure metabolites from the upper 10 cm of soil into lower
layers and into drainage water. Carrots, grown after an early
potato crop that had been treated with 300 g kelevan/ha, contained
up to 0.02 mg kelevan/kg and 0.04 mg chlordecone/kg; no residues
(< 0.01 mg/kg) were found in the potatoes.
There are no data on levels of exposure to kelevan for the
general population or in the work-place.
A few data are available on the environmental toxicity of
kelevan. The toxic threshold level for rainbow trout is of the
order of 0.1 mg/litre, and the oral LD50 for honey bees is > 1
mg/bee. Domestic hens dosed with 20 mg kelevan/bird per day for 8
weeks did not show any adverse effects. A soil level of 2500 mg
kelevan/kg did not affect the microflora over a 30-month period.
However, the available data are too few to make an informed
assessment of kelevan's likely impact on the environment,
especially on a long-term basis.
Kelevan is absorbed by experimental animals following
ingestion, inhalation, and via the skin. It accumulates in the
liver, brain, and in adipose tissue. It is metabolized to a
certain extent to chlordecone, both compounds being mainly excreted
with the bile into the faeces.
It is moderately toxic according to the scale of Hodge &
Sterner (1956) in single exposures (oral LD50 values for the rat
range from 240 to 550 mg/kg body weight). Symptoms of poisoning
include apathy, tremors, CNS hypersensitivity, and tonic-clonic
convulsions. The no-observed-adverse-effect level in a 90-day oral
study on rats was 5 mg/kg body weight. At higher levels (300 mg/kg
diet in females and 1000 mg/kg diet in both sexes), liver hypertrophy
occurred. In a 10-month oral study on rats, 0.28 mg/kg body weight
per day was considered to be a threshold dose. Oral exposure to 14
mg/kg body weight per day for 4 months caused necrosis of the liver
and kidneys in rats.
No abnormalities were found in reproduction studies on mice
when low doses (5 mg/kg diet) were given from 30 days prior to
mating to 90 days after mating. Teratogenic effects have not been
adequately evaluated.
Kelevan was not mutagenic in systems using microorganisms.
No carcinogenicity studies are available for kelevan, but there
is sufficient evidence of carcinogenicity for chlordecone, a major
metabolite, from studies on rats and mice.
No adverse health effects on human beings have been reported
from exposure to kelevan.
In view of the sparsity of available data, it is quite
impossible at this stage to arrive at an informed evaluation of
keleven with regard to its danger for workers, the possible
consumer hazards from food residues, or its impact on the
environment.
Therefore, since kelevan is converted to chlordecone in the
mammalian body and in the environment, and the toxicity data
available are similar to those on chlordecone, the evaluation of
chlordecone should largely apply to kelevan, which, in practice,
means that, unless kelevan is indispensable, it should not be used.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
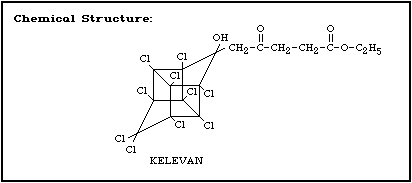 Molecular formula: C17H12Cl10O4
CAS chemical name: 1,3,4-metheno-1 H-cyclobuta; cd pentalene-
2-pentanoic acid, 1,1a,3,3a,4,5,5a,5b,
6-decachloro-octa-hydro-2-hydroxy-gamma-
oxo-ethyl ester
Trade names: Despirol, Elevat, GC-9160, General
Chemicals 9160
CAS registry number: 4234-79-1
Technical grade kelevan contains 94 - 98% pure kelevan, 0.1 -
2% chlordecone, and 0.5 - 4.0% inorganic salts (Maier-Bode, 1976).
2.2. Physical and Chemical Properties
The technical material is a brownish substance.
Some physical and chemical properties of kelevan are given
in Table 1.
2.3. Analytical Methods
Kelevan can be extracted from plant or animal tissues, or soils
using methylene chloride, isopropanol, or acetone. It can be
oxidized by refluxing with chromium trioxide in glacial acetic acid
to yield chlordecone. The chlordecone is then determined by gas-
liquid chromatography (GLC) techniques (Westlake et al., 1970). An
analytical method using liquid chromatography/mass spectrometry has
been described by Cairns et al. (1982).
Table 1. Some physical and chemical properties of kelevan
---------------------------------------------------------------------------
Physical state solid, powder
Colour white
Relative molecular mass 634.79
Melting point 91 °C
Vapour pressure (20 °C) < 0.0014 Pa (= < 10-2 mm Hg)
Solubility in water (20 °C) 5.5 mg/litre
(readily soluble in most
organic solvents)
Decomposition > 170 °C
---------------------------------------------------------------------------
From: Maier-Bode (1976).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Man-Made Sources
Kelevan is a condensation product of chlordecone and ethyl
levulinate (Gilbert et al., 1966).
The synthesis and insecticidal action of kelevan were reported
by Gilbert et al. (1966). Its synthesis has also been described by
Heys et al. (1979). The only information available in relation to
the production of kelevan was reported by Cannon et al. (1978), who
stated that approximately 99% of the production of chlordecone was
exported to the Federal Republic of Germany, where it served as a
raw material in the manufacture of another pesticide compound
kelevan.
3.2. Uses
Reference has been made to the use of kelevan in central and
southeastern Europe (Maier-Bode, 1976) and in South America (Cannon
et al., 1978).
Kelevan has mainly been used in the control of the potato
beetle (Leptinotarsa decemlineata) on potatoes, the banana root
borer on bananas, and Tanymecus palliatus on beets and corn.
Both dust and wettable-powder formulations have been used (Maier-
Bode, 1976).
Responses received from 49 countries throughout the world
indicated that kelevan had never been registered for use or used in
33 of them. In Spain, registration expired in 1975. In the Federal
Republic of Germany, the use of kelevan has been forbidden since
1982. In Hungary and the USSR, kelevan is still registered, but is
no longer used (personal communications to the IPCS and IRPTC,
1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution
Kelevan (determined as chlordecone) was shown in laboratory
studies to have a half-life in soil of 6 - 12 weeks under dark
conditions, and 5 - 10 weeks in diffused daylight (unpublished data
summarized by Maier-Bode, 1976). Analysis of soil samples in
various regions of Europe, where kelevan has been used to control
the potato beetle, confirmed this relatively rapid degradation
(unpublished data summarized by Maier-Bode, 1976). Soil treatment
was carried out using 14C-kelevan at 1.5 kg/ha and, though initial
residues in the soil were approximately 2 mg/kg, potatoes grown in
the soil contained residues of < 0.001 mg/kg, after peeling
(Klein, 1972).
Kelevan residues resulting from use in the field were predicted
on the basis of the volatilization, mineralization, and conversion
rates obtained from laboratory tests. Field residues of the parent
compound kelevan actually found in the field test were far lower
than calculated (Scheunert et al., 1983).
14C-Kelevan was applied to a "potato field model ecosystem".
The system was left to grow and ripen for 77 days. Approximately
95% of the applied radioactivity was recovered: 50.9% in soil,
42.4% in, and on, the potato plant, 1.6% in the air, and less than
0.001% in drainage water. Of approximately 50.9% contained in
soil, 38% was between 0 and 5 cm deep, 12.9% between 5 and 10 cm,
0.01% between 10 and 15 cm, and less than 0.001% between 15 and 20
cm deep. Of the total of recovered radioactivity, 24.4% was
unchanged kelevan, 40.5% kelvanic acid, 7.4% chlordecone, and 22.6%
different non-identified kelevan metabolites. Neither intact
kelevan nor its metabolites could be identified in potato fields
containing less than 0.03% of the initial radioactivity, or in
drainage water (Figge & Rehm, 1977; Figge, 1978).
When 5.4 mg of 14C-kelevan was sprayed on potato leaves, 6.9%
of the radioactivity was recovered from the plant, 26.3% from the
soil, 0.9% in drainage water, and 65.9% was lost to the air over 11
weeks. Much of the kelevan had been converted to kelevanic acid
including 68% of material recovered from soil and 65% of material
from the plant (Sandrock et al., 1974).
4.2. Biotransformation
Benigni et al. (1979) showed that kelevan was converted into
chlordecone in Nicotinea alata cell cultures and also in field
tests on potatoes and beets, and that the amount converted was
proportional to the length of treatment (see also Carere & Morpurgo,
1981).
In a laboratory study on 2 soil types, between 61 and 64% of
applied kelevan was degraded by microorganisms and physical and
chemical processes to kelevanic acid, in 4.5 months. In a second
study, under both laboratory and field conditions, one-third of
applied kelevan was degraded by microorganisms to chlordecone and
other unidentified products, over 30 months (Figge et al., 1983).
4.3. Abiotic degradation
Parlar et al. (1972) and Begum et al. (1973) studied the
decomposition of kelevan in the solid state or dissolved in
acetone, methanol, or n-hexane under the influence of ultra-
violet radiation (UVR). Several dechlorination products were
isolated, but the main degradation product was chlordecone. In
the gaseous phase, both mirex and chlordecone were formed.
Several photolysis products of kelevan have been described by
Wilson & Zehr (1978). These mainly concern modifications in the
side chain.
Kelevan slowly hydrolyses in water forming products such as
kelevanic acid and the carboxylic ester without the side chain,
which are more readily soluble in water (Sandrock et al., 1974).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
No data are available concerning the concentrations of kelevan
in air, water, or food.
5.1. Environmental Levels
5.1.1. Water
In laboratory experiments designed to ascertain runoff
characteristics of kelevan from soil, no traces of kelevan were
found in the runoff water (unpublished data summarized by Maier-
Bode, 1976).
5.1.2. Soil
Sandrock et al. (1974) studied the metabolism of 14C-kelevan in
potatoes and soil, 11 weeks and again one year after application on
leaves. Kelevanic acid (the principal metabolite), unmetabolized
kelevan, chlordecone, and chlordecone acetic acid were identified
in the soil, 11 weeks after application. Over 90% of the quantity
applied was metabolized during the first crop growth period; the
metabolites were products in which the side chain was shortened or
eliminated, without apparent changes in the carbon skeleton.
Chlordecone acetic acid was identified as the principal metabolite
in the soil after 1 year.
A dust or a suspension of 150 g kelevan aia/ha was applied
to 3 different soils in Slovakia. Three months after application,
approximately 30% of the kelevan was recovered as chlordecone.
Kelevan residues in potatoes grown in all three soil types ranged
from 0.001 - 0.004 mg/kg, whereas chlordecone was present in
traces only (Madaric & Sackmauerova, 1974).
5.1.3. Food and animal feed
When 14C-kelevan was applied to potato plants, neither the
compound nor any of its caged structure products were detectable in
the potato tubers (unpublished data summarized by Maier-Bode,
1976). Furthermore, detectable residues (> 0.01 mg/kg) were not
found in other crops growing in the same field including winter
wheat, winter rye, summer barley, and silo corn, even though the
field had been treated with 150 g kelevan/ha, the year before.
Carrots that had been planted in a field treated earlier in the
season with 150 and 300 g kelevan/ha, showed residues of 0.02 and
0.04 mg kelevan/kg. Rape, planted in a field treated with 250 and
150 g kelevan/ha, showed residues in seed of 0.07 mg/kg at harvest
(unpublished data summarized by Maier-Bode, 1976). Field studies
using 14C-kelevan showed that total residues, both in soil and
potatoes, mainly comprised hydrophilic metabolites including more
---------------------------------------------------------------------------
a ai = active ingredient.
than 80% kelevanic acid. Chordecone was also identified, but it
was uncertain whether it was an impurity or a metabolite (Klein,
1972).
Kelevan applied at 0.3 kg/ha increased the potato yield. At 0.6
kg/ha, its residues could be detected in the soil and the potato
roots during the vegetative period (Krasnykh, 1980).
Alfalfa, contaminated by spray-drift from the aerial spraying
of potatoes with kelevan, contained 4.8 mg kelevan/kg, directly
after spraying, 1.1 mg/kg, 3 days later, and 0.1 mg/kg, after 5 - 7
days. Fourteen days after spraying, kelevan could no longer be
detected (Jonas, 1983).
Residues of up to 0.02 and 0.04 mg/kg, respectively, of kelevan
and chlordecone were found in carrots planted after a crop of early
potatoes treated with the recommended application rate of 300 g
kelevan/ha. No residues were found in the potatoes (< 0.01
mg/kg). Residues were also found in the leaves and roots of
sugarbeets and in the seed and straw of summer and winter rape (up
to 0.06 mg/kg) (Maier-Bode, 1976).
Two groups of two, 200-kg steers were fed 0.05 or 0.1 mg
kelevan/kg feed for 6 weeks. No kelevan was detected in muscle,
kidneys, heart, or body fat, 2 and 4 weeks, respectively, after
this feeding period. The liver, however, contained 0.02 - 0.1 mg
kelevan/kg. Biopsies during the feeding period showed concentrations
of up to 1.6 mg kelevan/kg in body fat (Jonas, 1983).
6. KINETICS AND METABOLISM
6.1. Absorption
Kelevan can enter the body orally and by inhalation. It is
also absorbed through the skin, as shown by acute and short-term
dermal toxicity studies on rabbits (section 8.1, 8.2) (Maier-Bode,
1976).
6.2. Distribution, Storage, Metabolic Transformation, and
Excretion
Several unpublished studies have been summarized by Maier-Bode
(1976). Male rats were administered 14C-kelevan intra-gastrically
at 4.75 mg/kg body weight. As little as 3 h after administration,
14C activity was found in all tissues examined, but primarily in
the liver. The resulting pattern of accumulation was similar to
that of chlordecone in that it was greater in the heart, brain,
liver, etc. than in adipose tissue. The levels of kelevan in mg/kg
were as follows: serum 1.4, liver 20.9, heart 2.7, kidney 3.5,
brain 1.1, fat 0.84, muscle 0.87. After 14 days, levels in all
tissues were below 0.1 mg/kg, except for the liver, which contained
1.8 mg/kg. The liver still contained 0.3 mg/kg, 110 days later.
The data also indicated that kelevan is excreted through the liver
with the bile into the faeces and is not excreted to any great
extent in the urine. It appears primarily as unchanged kelevan and
also as chlordecone (Maier-Bode, 1976).
These studies indicate that chlordecone is a metabolite of
kelevan in the rat and suggest that kelevanic acid is an
intermediate.
In another study, a single oral dose of 4.75 mg 14C-kelevan/kg
body weight, in carboxymethylcellulose, was given to 60 male rats
by gavage (Maier-Bode, 1976). A considerable portion of the 14C was
excreted through the liver with the bile into the intestine. Eight
and 16 weeks later 14C could still be detected in organs and tissues.
In a study on rats administered a single oral dose of 1.52 mg
14C-kelevan/kg body weight, it was possible to identify14C-
chlordecone (as a transformation product partly as chlordecone-
arginine) by thin-layer chromatography in the faeces and urine of
the rats (Maier-Bode 1976).
Daily doses of a kelevan suspension in water were given to 15
male and 15 female rats by gavage. The total dose in the course of
8 weeks was 10 mg kelevan/kg body weight (Maier-Bode, 1976). Three
male and 3 female animals were killed and the tissues analysed, 1,
2, 4, 8, and 10 weeks after the first application. The liver,
brain, and body fat were analysed for kelevan and chlordecone. The
concentrations of kelevan and chlordecone, separately and combined,
were approximately constant. The conclusion of the author was that
there was no accumulation of kelevan or its metabolite chlordecone.
The faeces collected from the surviving rats during the entire 10-
week test period contained an average of 2.25 mg kelevan/kg and
0.84 mg chlordecone/kg. Neither kelevan nor chlordecone was found
(< 0.02 mg/litre) in the urine of the animals.
On the basis of the studies, the Task Group concluded that
chlordecone is a metabolite of kelevan, but that the extent of this
transformation is not known. It has to be taken into account that
chlordecone is also an impurity of kelevan.
The amounts of kelevan and chlordecone in the liver, brain, and
body fat are almost equal. Because of the short duration of the
study, nothing can be said of the half-life of kelevan and
chlordecone, but on the basis of the 2-week depletion period, it
appears that both half-lives are long.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Studies on the toxicity of kelevan (LC50) for juvenile fish
(rainbow trout) are reported in Table 2; no studies on other
aquatic organisms are available. Two separate studies indicate a
toxic threshold for rainbow trout of 0.1 mg/litre. Symptoms of
sublethal poisoning include disturbed swimming coordination (Maier-
Bode 1976).
7.2. Terrestrial Organisms
Under laboratory conditions, the toxicity for bees of kelevan,
at concentrations used in agriculture, was low (Tomaszewska, 1981).
The LD50 of Despirol (wettable powder 50% kelevan) for honey bees
was > 1 mg/bee (the maximum level tested) after testing orally, by
inhalation, by prolonged contact, or by spraying. The toxicity of
kelevan was lower for beneficial insects than for target species
(Maier-Bode, 1976).
Soil microarthropods (Collembola and Acarina) showed no change
in absolute or relative, species to species, population numbers
within 75 days of a single spraying of a potato crop at a rate of
300 g/ha (Hrlec & Ostrec, 1981).
Five pheasants and 3 domestic doves dosed with kelevan as
Despirol at 10 g/kg diet for 10 days did not show any effects
during the dosing period or during the 10-day period following
dosing. The amount of insecticide ingested averaged 101 mg/kg per
day for pheasants and 250 mg/kg per day for doves (Maier-Bode,
1976). When large numbers of laying domestic hens were dosed at up
to 20 mg/bird per day, for 8 weeks, no effects were observed on
laying activity; histological examination of tissues at the end of
the study did not reveal any differences between treated and
control birds (Maier-Bode, 1976).
7.3. Microorganisms
A laboratory study on microorganisms in two types of soil
treated with between 500 and 2500 mg 14C-kelevan/kg showed that,
whilst the organisms degraded kelevan, the insecticide caused no
change in either total or relative numbers of microorganisms. The
organisms were neither selected nor decimated by kelevan or its
degradation products over a 30-month period (Figge et al., 1983).
Table 2. Toxicity of kelevan for fisha
----------------------------------------------------------------------------------------------------
Species Life stage Length Water hardness Temperature Exposure time LC50
(cm) (dH01)b (°C) (h) (mg/litre)
----------------------------------------------------------------------------------------------------
Rainbow trout juvenile 7 - 9c 11 10 24 > 2
(Salmo gairdnerii)
Rainbow trout juvenile 7 - 9c 11 10 72 1.0
Rainbow trout juvenile 6 - 10 1 14 96 1.5
Rainbow trout juvenile 6 - 10 9 14 96 2.2
----------------------------------------------------------------------------------------------------
a From: Maier-Bode (1976).
b 1 degree of hardness (dH0) corresponds to 10.0 mg Ca0/litre water.
c For the studies on 7 - 9 cm trout, pH 6; pH not stated for other tests.
7.4. Appraisal
The data on kelevan are few. There is no information on its
immediate metabolite, kelevanic acid. Thus, it is difficult to
come to firm conclusions about the environmental significance of
kelevan. However, there are considerable data on the metabolite,
chlordecone. This is known to be stable and persistent. It
bioaccumulates and is more toxic for aquatic organisms than
kelevan. Chlordecone has severe sublethal effects on birds (WHO,
1984). The interpretation of data for kelevan, therefore, needs to
take into account the significance of chlordecone.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Data on the acute toxicity of kelevan are given in Table 3. A
similar pattern of response to acutely toxic doses of kelevan was
seen in the three species and included apathy, tremor, hyper-
sensitivity, and tonic-clonic convulsions.
Table 3. Acute toxicity of kelevan
-------------------------------------------------------------------
Animal Sex Route Vehicle LD50 Reference
(mg/kg
body weight)
-------------------------------------------------------------------
Rat M & F oral corn oil, 240 - 290 Maier-Bode (1976)
soybean oil
Rat oral 255 - 325 Kenaga & Allison
(1969)
Dog M & F oral corn oil, 400 - 550 Maier-Bode (1976)
soybean oil
Dog oral 400 - 500 Kenaga & Allison
(1969)
Rabbit M dermal corn oil 251 Maier-Bode (1976)
Rabbit dermal 188 - 314 Kenaga & Allison
(1969)
-------------------------------------------------------------------
8.2. Short-Term Exposures
8.2.1. Oral
Male rats were administered kelevan by oral intubation at 29
mg/kg body weight for 20 consecutive days (Medical College,
Virginia, 1968). No effects were observed on behaviour, organ
weights, or in the histopathological examination of liver and
kidneys. In a 90-day study (Medical College, Virginia, 1968), male
and female rats were fed kelevan incorporated in the diet at levels
of 0, 10, 30, 100, 300, or 1000 mg/kg. No animals died during the
course of the study, but animals fed 1000 mg/kg showed a reduced
weight gain. No treatment-related abnormalities were seen in food
consumption, haematology, urinalysis, or histopathology. Dose-
related liver hypertrophy was observed in females at 300 mg/kg diet
and in both sexes at 1000 mg/kg diet. Thus, 100 mg kelevan/kg
diet, equivalent to 5 mg/kg body weight per day, was a no-observed-
adverse-effect level in this study.
Albino rats were exposed to daily doses of 14 mg kelevan/kg
body weight for 4 months, or, 2.8 or 0.28 mg/kg body weight for 10
months. Hyperaemia of internal organs and necrosis of the liver
and kidneys were described, as well as lymphoid infiltration of
interstitial tissue in the lung. At 0.28 mg/kg, these changes were
reversible, and the author regarded this dose as a threshold dose
(Boreiko, 1980).
8.2.2. Dermal
Male and female rabbits were administered 25 or 50 mg
Despirol/kg body weight (12.5 or 25 mg kelevan/kg body weight), 5
days per week, for 9 weeks. The material was administered as an
aqueous paste to the shaved skin of the animals. There was no
difference between the treatment groups and controls. Only a
slight erythema was observed at the highest dose of kelevan
(Medical College, Virginia, 1968).
Kelevan, applied to the skin of rats, rabbits, and guinea-pigs,
in either one dose of 2000 mg/kg or 20 doses of 100 mg/kg caused
dystrophic changes in the liver and kidneys (Sasinovich et al.,
1977).
8.2.3. Inhalation
No adequately reported studies available.
8.3. Long-Term Exposure
No studies available.
8.4. Reproduction Studies
Reproduction was investigated in 100 male and 100 female BALB/C
mice fed kelevan in the diet at 5 mg/kg, from 30 days prior to
mating to 90 days after mating, over several litters (Ware & Good,
1967). No effects of treatment were observed on mortality, number
of females producing litters, pregnancy period, number of litters,
litter size, and sex ratio.
Thirty pregnant CD-1 mice were given the minimal toxic dose of
kelevan of 125 mg/kg body weight in 0.5 ml corn oil, by gavage,
from day 8 to day 12 of pregnancy. Four animals died. There were
no significant maternal weight changes nor effects on litter size
or pup weights (Chernoff & Kavlock, 1983).
It is known that chlordecone affects reproduction (WHO, 1984).
The dose of 5 mg kelevan/kg per day may have been too low to elicit
an effect on reproduction. The dose of 125 mg/kg apparently caused
sufficient toxicity in the dams to kill four of the animals. The
validity of both these studies, which were the only studies
available for evaluation, is limited.
8.5. Mutagenicity
Benigni et al. (1979) tested the mutagenic activity of kelevan
and its metabolite chlordecone on Aspergillus nidulans. As pure
compounds, both were negative (Carere & Morpurgo, 1981).
Kelevan and its degradation products did not show any mutagenic
activity in the Ames test with Salmonella typhimurium (Jaszczuk &
Syrowatka, 1980).
8.6. Carcinogenicity
No carcinogenicity studies are available for kelevan. However,
there is sufficient evidence of the carcinogenicity of its
metabolite chlordecone in animals (IARC, 1979; WHO, 1984)
8.7. Other Studies
A single intragastric dose of 140 mg kelevan/kg body weight
(approximately half the LD50), given to rats, decreased the rate
of bile secretion on the first day. From day 3 onwards, it
increased again, but it had not reached the level of the controls
on day 5 (Glukhova et al., 1978, 1979). Kelevan sharply increased
the levels of alanine aminotransferase and aspartate aminotransferase
in blood serum and caused structural changes in the liver at this
dose level.
9. EFFECTS ON MAN
No adverse health effects on human beings from exposure to
kelevan have been reported.
10. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of the Health Risks for Man
Kelevan, which is developed from chlordecone, is metabolized
in the mammalian body and in the environment back to chlordecone.
The acute toxicity of kelevan is similar to that of chlordecone,
which may well be its active metabolite.
Data on kelevan are sparse; several reports have not been
published and are not available for scrutiny and others lack
sufficient detail or are inadequate. No information exists on
actual human exposure.
The acute toxicity of kelevan in test animals is moderate (oral
LD50s ranging from 240 to 550 mg/kg body weight, according to the
scale of Hodge & Sterner (1956)), and similar to that of chlordecone.
However, the no-observed-adverse-effect level of 5 mg/kg body weight
per day observed in a 90-day oral study on the rat and a threshold
level of 0.28 mg/kg body weight per day observed in a 10-month oral
rat study, are very similar to those obtained with chlordecone (WHO,
1984). The pathological findings of liver hypertrophy and necrosis
of liver and kidneys are also similar.
Kelevan is not mutagenic in systems using microorganisms.
No carcinogenicity studies are available. However, there
is sufficient evidence of the carcinogenicity of its metabolite
chlordecone for mice and rats (IARC, 1979; WHO, 1984).
No adverse effects on human health due to exposure to
kelevan have been reported.
10.2. Evaluation of Environmental Effects
There have not been any reports of adverse effects on the
environment due to exposure to kelevan. Available information
suggests that the probability of deleterious effects on terrestrial
organisms from kelevan is low. Its metabolite, chlordecone, is
toxic for birds and microorganisms, though there is no indication
of this for the parent compound. Aquatic data for kelevan are
limited to one species and one life stage; it is moderately to
highly toxic for juvenile rainbow trout. It is possible, but
improbable, that local concentrations of kelevan after recommended
agricultural use could exceed the toxic threshold for trout fry.
The compound gives concern with aquatic organisms because its
degradation product is both more persistent and more toxic for fish
than the parent compound.
10.3. Conclusions and Recommendations
In view of the sparsity of available data, it is impossible to
arrive at an informed evaluation of kelevan with regard to its
danger for workers, the possible consumer hazards from food
residues, or its impact on the environment. Thus, as kelevan is
converted to chlordecone in the mammalian body and in the
environment, and as the available toxicity data are similar to
those on chlordecone, the evaluation for chlordecone (WHO, 1984)
should also largely apply to kelevan, unless further data to the
contrary become available. In practice, this means that, unless
kelevan is indispensable, it should not be used.
REFERENCES
ANONYMOUS (1978a) Kepone/mirex/hexachlorocyclopentadiene -
an environmental assessment, Washington DC, US Department of
Commerce, US National Technical Information Service (PB
280-289) (NCR).
BEGUM, S., GOEB, S., PARLAR, H., & KORTE, F. (1973) Reaction
behaviour of kelevan in solution, as a solid, and in gaseous
phase under UV irradiation. Chemosphere, 2: 235-238.
BENIGNI, R., BIGNAMI, M., CAMONI, I., CARERE, A., CONTI, G.,
IACHETTA, R., MORPURGO, G., & ORTALI, V.A. (1979) A new in
vitro method for testing plant metabolism in mutagenicity
studies. J. Toxicol. environ. Health, 5: 809-819.
BOREIKO, N.R. (1980) [Effects of the pesticide Despirol on
the morphology of internal organs of laboratory animals.] Gig.
i Sanit., 45: 68-69 (in Russian).
CAIRNS, T., SIEGMUND, E.G., & DOOSE, G.M. (1982) Liquid
chromatography/mass spectrometry of Kepone hydrate, kelevan,
and mirex. Anal. Chem., 54: 953-957.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W.,
HAYES, C., STRAUB, W.E., LANDRIGAN, P.J., & LIDDLE, J.A.
(1978) Epidemic kepone poisoning in chemical workers. Am. J.
Epidemiol., 107: 529-537.
CARERE, A. & MORPURGO, G. (1981) Comparison of the mutagenic
activity of pesticides in vitro in various short-term assays.
Prog. in mutat. Res., 2: 87-104.
CHERNOFF, N. & KAVLOCK, R.J. (1983) In: Waters, M.D.,
Sandhu, S.S., Lewton, J., Claxton, L., Chernoff, N., & Wesnow,
S., ed. A teratology test system which utilizes postnatal
growth and viability in the mouse. III. Short-term bioassays
in the analysis of complex mixtures, New York, Plenum Press.
FIGGE, K. (1978) [Environmental ecological studies using
radioactive compounds.] Org. Verunreinig., 208-230 (in German).
FIGGE, K. & REHM, H. (1977) [On the behaviour of the
insecticide kelevan and its metabolites in the potato field
ecosystem.] Z. Pflanzenkr. Pflanzenschutz, 84(7/8): 385-409
(in German).
FIGGE, K., RHEM, H., & SCHOENWALDER, H. (1983) [Degradation
and biocidal effects of chemical plant-protection agents and
pesticides in the soil by the example of the insecticide
Kelevan.] Z. Pflanzenernaehr Bodenkd, 146: 316-340 (in German).
GILBERT, E., LOMBARDO, E.P., RUMANOWSKI, E.J., & WALKER, G.L.
(1966) Preparation and insecticidal evaluation of alcoholic
analogs of kepone. J. agric. food Chem., 14: 111-118.
GLUKHOVA, L.G., VASILENKO, T.E., & APON, N.I. (1978)
[Comparative characteristics of the effects of organochlorine
and organophosphorus toxic chemicals on the bile-forming
function of the liver.] Vrach. Delo., 11: 130-131 (in Russian).
GLUKHOVA, L.G., VASILENKO, T.E., APON, N.I., & BOREIKO, N.P.
(1979) [Functional state of the liver under the acute effects
of phosalone and Despirol.] Vrach. Delo., 10: 108-109 (in
Russian).
HEYS, J.R., DUNCAN, W.P., PERRY, W.C., EBERT, D.A.,
RADOLOVICH, G., & HAILE, C.L. (1979) Synthesis of carbon-14
and carbon-13 labelled chlorinated polycyclic pesticides. J.
labelled Comp. Radiopharm., 16: 295-306.
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., Handbook of toxicology,
Philadelphia, Pennsylvania, W.B. Saunders Company, Vol. 1.
HRLEC, G. & OSTREC, L. (1981) [Residue influence of
pesticides and their activity on soil microarthropods.]
Agrohemija, 7-8: 291-296 (in Serbian).
IARC (1979) Some halogenated hydrocarbons, Lyons,
International Agency for Research on Cancer (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Vol. 20).
JASZCZUK, E. & SYROWATKA, T. (1980) [Mutagenic action of
certain pesticides on Salmonella typhimurium.] Rocz. Panstw.
Zekl. Hig., 31: 305-311 (in Polish).
JONAS, K. (1983) [Analysis and the toxicological behaviour
of residues of the insecticide kelevan.] Mh. Vet. Med., 38:
223-225 (in German).
KENAGA, E.E. & ALLISON, W.E. (1969) Commercial and
experimental organic insecticides: 1969 revision, Vol. 15(2),
pp. 85-148 (Bulletin of the Entomological Society of America
(96).
KLEIN, W. (1972) Cyclodiene and other residues in growing
crops: status report. In: Isotope tracer studies of chemical
residues in food and the agricultural environment. Proceedings
and Report of Research Coordination Meetings, Ispra, Italy,
Vienna, Austria, IAEA, 156 pp.
KRASNYKH, A.A. (1980) [Despirol residues in soil and
potato.] Khim. Sel'sk Khoz., 18: 53-54 (in Russian).
MADARIC, A. & SACKMAUEROVA, M. (1974) Residues of kelevan
(Despirol) in the soil and in potatoes. In: Proceedings of the
3rd International Congress on Pesticide Chemicals, Helsinki
3-9 July, 1974.
MAIER-BODE, H. (1976) The insecticide kelevan. Res. Rev.,
63: 45-76.
MEDICAL COLLEGE, VIRGINIA (1965-68) Reports to the Allied
Chemical Corporation.
PARLAR, H., KLEIN, W., & KORTE, F. (1972) Photochlorination
reactions of kelevan. Chemosphere, 1: 129-132.
SANDROCK, K., BIENICK, D., KLEIN, W., & KORTE, F. (1974)
Isolation and structural resolution of 14C-kelevan metabolites
and balance in potatoes and soil. Chemosphere, 3(5): 199-204.
SASINOVICH, L.M., OVSYANNIKOVA, L.M., & BADAEVA, L.N. (1977)
Characteristics of the skin-absorptive effect of new
pesticides belonging to different chemical groups. Gig. Tr.
Prof. Zabol., 7: 34-36.
SCHEUNERT, I., VOCKEL, D., SCHMITZER, J., VISWANATHAN, R.,
KLEIN, W., & KORTE, F. (1983) Fate of chemicals in plant-
soil systems: comparison of laboratory test data with results
of open-air long-term experiments. Ecol. environ. Saf., 7:
390-399.
TOMASZEWSKA, B., BOHOSIEWICZ, M., & JOPEK, Z. (1981)
Toxicity of Despirol for bees under laboratory conditions.
Zesz. Nauk. Akad. Roln. Wrpclawin. Weter.: 117-120.
WARE, G.W. & GOOD, E.E. (1967) Effects of insecticides on
reproduction in the laboratory mouse. III. Tranid and GC-9160.
J. environ. Entomol., 60: 530-532.
WESTLAKE, A., WESTLAKE, W.E., & GUNTHER, F.A. (1970)
Determination of residues of GC-9160 in cabbage, lettuce, and
citrus fruit. J. agric. food Chem., 18: 159-165.
WHO (1984) Environmental Health Criteria 43: Chlordecone,
Geneva, World Health Organization, 57 pp.
WILSON, N.K. & ZEHR, R.D. (1978) Structures of some kepone
photoproducts and related chlorinated pentacyclodecanes by
carbon-13 and proton nuclear magnetic resonance. J. organ.
Chem., 44: 1278-1282.
Molecular formula: C17H12Cl10O4
CAS chemical name: 1,3,4-metheno-1 H-cyclobuta; cd pentalene-
2-pentanoic acid, 1,1a,3,3a,4,5,5a,5b,
6-decachloro-octa-hydro-2-hydroxy-gamma-
oxo-ethyl ester
Trade names: Despirol, Elevat, GC-9160, General
Chemicals 9160
CAS registry number: 4234-79-1
Technical grade kelevan contains 94 - 98% pure kelevan, 0.1 -
2% chlordecone, and 0.5 - 4.0% inorganic salts (Maier-Bode, 1976).
2.2. Physical and Chemical Properties
The technical material is a brownish substance.
Some physical and chemical properties of kelevan are given
in Table 1.
2.3. Analytical Methods
Kelevan can be extracted from plant or animal tissues, or soils
using methylene chloride, isopropanol, or acetone. It can be
oxidized by refluxing with chromium trioxide in glacial acetic acid
to yield chlordecone. The chlordecone is then determined by gas-
liquid chromatography (GLC) techniques (Westlake et al., 1970). An
analytical method using liquid chromatography/mass spectrometry has
been described by Cairns et al. (1982).
Table 1. Some physical and chemical properties of kelevan
---------------------------------------------------------------------------
Physical state solid, powder
Colour white
Relative molecular mass 634.79
Melting point 91 °C
Vapour pressure (20 °C) < 0.0014 Pa (= < 10-2 mm Hg)
Solubility in water (20 °C) 5.5 mg/litre
(readily soluble in most
organic solvents)
Decomposition > 170 °C
---------------------------------------------------------------------------
From: Maier-Bode (1976).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Man-Made Sources
Kelevan is a condensation product of chlordecone and ethyl
levulinate (Gilbert et al., 1966).
The synthesis and insecticidal action of kelevan were reported
by Gilbert et al. (1966). Its synthesis has also been described by
Heys et al. (1979). The only information available in relation to
the production of kelevan was reported by Cannon et al. (1978), who
stated that approximately 99% of the production of chlordecone was
exported to the Federal Republic of Germany, where it served as a
raw material in the manufacture of another pesticide compound
kelevan.
3.2. Uses
Reference has been made to the use of kelevan in central and
southeastern Europe (Maier-Bode, 1976) and in South America (Cannon
et al., 1978).
Kelevan has mainly been used in the control of the potato
beetle (Leptinotarsa decemlineata) on potatoes, the banana root
borer on bananas, and Tanymecus palliatus on beets and corn.
Both dust and wettable-powder formulations have been used (Maier-
Bode, 1976).
Responses received from 49 countries throughout the world
indicated that kelevan had never been registered for use or used in
33 of them. In Spain, registration expired in 1975. In the Federal
Republic of Germany, the use of kelevan has been forbidden since
1982. In Hungary and the USSR, kelevan is still registered, but is
no longer used (personal communications to the IPCS and IRPTC,
1985).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and Distribution
Kelevan (determined as chlordecone) was shown in laboratory
studies to have a half-life in soil of 6 - 12 weeks under dark
conditions, and 5 - 10 weeks in diffused daylight (unpublished data
summarized by Maier-Bode, 1976). Analysis of soil samples in
various regions of Europe, where kelevan has been used to control
the potato beetle, confirmed this relatively rapid degradation
(unpublished data summarized by Maier-Bode, 1976). Soil treatment
was carried out using 14C-kelevan at 1.5 kg/ha and, though initial
residues in the soil were approximately 2 mg/kg, potatoes grown in
the soil contained residues of < 0.001 mg/kg, after peeling
(Klein, 1972).
Kelevan residues resulting from use in the field were predicted
on the basis of the volatilization, mineralization, and conversion
rates obtained from laboratory tests. Field residues of the parent
compound kelevan actually found in the field test were far lower
than calculated (Scheunert et al., 1983).
14C-Kelevan was applied to a "potato field model ecosystem".
The system was left to grow and ripen for 77 days. Approximately
95% of the applied radioactivity was recovered: 50.9% in soil,
42.4% in, and on, the potato plant, 1.6% in the air, and less than
0.001% in drainage water. Of approximately 50.9% contained in
soil, 38% was between 0 and 5 cm deep, 12.9% between 5 and 10 cm,
0.01% between 10 and 15 cm, and less than 0.001% between 15 and 20
cm deep. Of the total of recovered radioactivity, 24.4% was
unchanged kelevan, 40.5% kelvanic acid, 7.4% chlordecone, and 22.6%
different non-identified kelevan metabolites. Neither intact
kelevan nor its metabolites could be identified in potato fields
containing less than 0.03% of the initial radioactivity, or in
drainage water (Figge & Rehm, 1977; Figge, 1978).
When 5.4 mg of 14C-kelevan was sprayed on potato leaves, 6.9%
of the radioactivity was recovered from the plant, 26.3% from the
soil, 0.9% in drainage water, and 65.9% was lost to the air over 11
weeks. Much of the kelevan had been converted to kelevanic acid
including 68% of material recovered from soil and 65% of material
from the plant (Sandrock et al., 1974).
4.2. Biotransformation
Benigni et al. (1979) showed that kelevan was converted into
chlordecone in Nicotinea alata cell cultures and also in field
tests on potatoes and beets, and that the amount converted was
proportional to the length of treatment (see also Carere & Morpurgo,
1981).
In a laboratory study on 2 soil types, between 61 and 64% of
applied kelevan was degraded by microorganisms and physical and
chemical processes to kelevanic acid, in 4.5 months. In a second
study, under both laboratory and field conditions, one-third of
applied kelevan was degraded by microorganisms to chlordecone and
other unidentified products, over 30 months (Figge et al., 1983).
4.3. Abiotic degradation
Parlar et al. (1972) and Begum et al. (1973) studied the
decomposition of kelevan in the solid state or dissolved in
acetone, methanol, or n-hexane under the influence of ultra-
violet radiation (UVR). Several dechlorination products were
isolated, but the main degradation product was chlordecone. In
the gaseous phase, both mirex and chlordecone were formed.
Several photolysis products of kelevan have been described by
Wilson & Zehr (1978). These mainly concern modifications in the
side chain.
Kelevan slowly hydrolyses in water forming products such as
kelevanic acid and the carboxylic ester without the side chain,
which are more readily soluble in water (Sandrock et al., 1974).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
No data are available concerning the concentrations of kelevan
in air, water, or food.
5.1. Environmental Levels
5.1.1. Water
In laboratory experiments designed to ascertain runoff
characteristics of kelevan from soil, no traces of kelevan were
found in the runoff water (unpublished data summarized by Maier-
Bode, 1976).
5.1.2. Soil
Sandrock et al. (1974) studied the metabolism of 14C-kelevan in
potatoes and soil, 11 weeks and again one year after application on
leaves. Kelevanic acid (the principal metabolite), unmetabolized
kelevan, chlordecone, and chlordecone acetic acid were identified
in the soil, 11 weeks after application. Over 90% of the quantity
applied was metabolized during the first crop growth period; the
metabolites were products in which the side chain was shortened or
eliminated, without apparent changes in the carbon skeleton.
Chlordecone acetic acid was identified as the principal metabolite
in the soil after 1 year.
A dust or a suspension of 150 g kelevan aia/ha was applied
to 3 different soils in Slovakia. Three months after application,
approximately 30% of the kelevan was recovered as chlordecone.
Kelevan residues in potatoes grown in all three soil types ranged
from 0.001 - 0.004 mg/kg, whereas chlordecone was present in
traces only (Madaric & Sackmauerova, 1974).
5.1.3. Food and animal feed
When 14C-kelevan was applied to potato plants, neither the
compound nor any of its caged structure products were detectable in
the potato tubers (unpublished data summarized by Maier-Bode,
1976). Furthermore, detectable residues (> 0.01 mg/kg) were not
found in other crops growing in the same field including winter
wheat, winter rye, summer barley, and silo corn, even though the
field had been treated with 150 g kelevan/ha, the year before.
Carrots that had been planted in a field treated earlier in the
season with 150 and 300 g kelevan/ha, showed residues of 0.02 and
0.04 mg kelevan/kg. Rape, planted in a field treated with 250 and
150 g kelevan/ha, showed residues in seed of 0.07 mg/kg at harvest
(unpublished data summarized by Maier-Bode, 1976). Field studies
using 14C-kelevan showed that total residues, both in soil and
potatoes, mainly comprised hydrophilic metabolites including more
---------------------------------------------------------------------------
a ai = active ingredient.
than 80% kelevanic acid. Chordecone was also identified, but it
was uncertain whether it was an impurity or a metabolite (Klein,
1972).
Kelevan applied at 0.3 kg/ha increased the potato yield. At 0.6
kg/ha, its residues could be detected in the soil and the potato
roots during the vegetative period (Krasnykh, 1980).
Alfalfa, contaminated by spray-drift from the aerial spraying
of potatoes with kelevan, contained 4.8 mg kelevan/kg, directly
after spraying, 1.1 mg/kg, 3 days later, and 0.1 mg/kg, after 5 - 7
days. Fourteen days after spraying, kelevan could no longer be
detected (Jonas, 1983).
Residues of up to 0.02 and 0.04 mg/kg, respectively, of kelevan
and chlordecone were found in carrots planted after a crop of early
potatoes treated with the recommended application rate of 300 g
kelevan/ha. No residues were found in the potatoes (< 0.01
mg/kg). Residues were also found in the leaves and roots of
sugarbeets and in the seed and straw of summer and winter rape (up
to 0.06 mg/kg) (Maier-Bode, 1976).
Two groups of two, 200-kg steers were fed 0.05 or 0.1 mg
kelevan/kg feed for 6 weeks. No kelevan was detected in muscle,
kidneys, heart, or body fat, 2 and 4 weeks, respectively, after
this feeding period. The liver, however, contained 0.02 - 0.1 mg
kelevan/kg. Biopsies during the feeding period showed concentrations
of up to 1.6 mg kelevan/kg in body fat (Jonas, 1983).
6. KINETICS AND METABOLISM
6.1. Absorption
Kelevan can enter the body orally and by inhalation. It is
also absorbed through the skin, as shown by acute and short-term
dermal toxicity studies on rabbits (section 8.1, 8.2) (Maier-Bode,
1976).
6.2. Distribution, Storage, Metabolic Transformation, and
Excretion
Several unpublished studies have been summarized by Maier-Bode
(1976). Male rats were administered 14C-kelevan intra-gastrically
at 4.75 mg/kg body weight. As little as 3 h after administration,
14C activity was found in all tissues examined, but primarily in
the liver. The resulting pattern of accumulation was similar to
that of chlordecone in that it was greater in the heart, brain,
liver, etc. than in adipose tissue. The levels of kelevan in mg/kg
were as follows: serum 1.4, liver 20.9, heart 2.7, kidney 3.5,
brain 1.1, fat 0.84, muscle 0.87. After 14 days, levels in all
tissues were below 0.1 mg/kg, except for the liver, which contained
1.8 mg/kg. The liver still contained 0.3 mg/kg, 110 days later.
The data also indicated that kelevan is excreted through the liver
with the bile into the faeces and is not excreted to any great
extent in the urine. It appears primarily as unchanged kelevan and
also as chlordecone (Maier-Bode, 1976).
These studies indicate that chlordecone is a metabolite of
kelevan in the rat and suggest that kelevanic acid is an
intermediate.
In another study, a single oral dose of 4.75 mg 14C-kelevan/kg
body weight, in carboxymethylcellulose, was given to 60 male rats
by gavage (Maier-Bode, 1976). A considerable portion of the 14C was
excreted through the liver with the bile into the intestine. Eight
and 16 weeks later 14C could still be detected in organs and tissues.
In a study on rats administered a single oral dose of 1.52 mg
14C-kelevan/kg body weight, it was possible to identify14C-
chlordecone (as a transformation product partly as chlordecone-
arginine) by thin-layer chromatography in the faeces and urine of
the rats (Maier-Bode 1976).
Daily doses of a kelevan suspension in water were given to 15
male and 15 female rats by gavage. The total dose in the course of
8 weeks was 10 mg kelevan/kg body weight (Maier-Bode, 1976). Three
male and 3 female animals were killed and the tissues analysed, 1,
2, 4, 8, and 10 weeks after the first application. The liver,
brain, and body fat were analysed for kelevan and chlordecone. The
concentrations of kelevan and chlordecone, separately and combined,
were approximately constant. The conclusion of the author was that
there was no accumulation of kelevan or its metabolite chlordecone.
The faeces collected from the surviving rats during the entire 10-
week test period contained an average of 2.25 mg kelevan/kg and
0.84 mg chlordecone/kg. Neither kelevan nor chlordecone was found
(< 0.02 mg/litre) in the urine of the animals.
On the basis of the studies, the Task Group concluded that
chlordecone is a metabolite of kelevan, but that the extent of this
transformation is not known. It has to be taken into account that
chlordecone is also an impurity of kelevan.
The amounts of kelevan and chlordecone in the liver, brain, and
body fat are almost equal. Because of the short duration of the
study, nothing can be said of the half-life of kelevan and
chlordecone, but on the basis of the 2-week depletion period, it
appears that both half-lives are long.
7. EFFECTS ON ORGANISMS IN THE ENVIRONMENT
7.1. Aquatic Organisms
Studies on the toxicity of kelevan (LC50) for juvenile fish
(rainbow trout) are reported in Table 2; no studies on other
aquatic organisms are available. Two separate studies indicate a
toxic threshold for rainbow trout of 0.1 mg/litre. Symptoms of
sublethal poisoning include disturbed swimming coordination (Maier-
Bode 1976).
7.2. Terrestrial Organisms
Under laboratory conditions, the toxicity for bees of kelevan,
at concentrations used in agriculture, was low (Tomaszewska, 1981).
The LD50 of Despirol (wettable powder 50% kelevan) for honey bees
was > 1 mg/bee (the maximum level tested) after testing orally, by
inhalation, by prolonged contact, or by spraying. The toxicity of
kelevan was lower for beneficial insects than for target species
(Maier-Bode, 1976).
Soil microarthropods (Collembola and Acarina) showed no change
in absolute or relative, species to species, population numbers
within 75 days of a single spraying of a potato crop at a rate of
300 g/ha (Hrlec & Ostrec, 1981).
Five pheasants and 3 domestic doves dosed with kelevan as
Despirol at 10 g/kg diet for 10 days did not show any effects
during the dosing period or during the 10-day period following
dosing. The amount of insecticide ingested averaged 101 mg/kg per
day for pheasants and 250 mg/kg per day for doves (Maier-Bode,
1976). When large numbers of laying domestic hens were dosed at up
to 20 mg/bird per day, for 8 weeks, no effects were observed on
laying activity; histological examination of tissues at the end of
the study did not reveal any differences between treated and
control birds (Maier-Bode, 1976).
7.3. Microorganisms
A laboratory study on microorganisms in two types of soil
treated with between 500 and 2500 mg 14C-kelevan/kg showed that,
whilst the organisms degraded kelevan, the insecticide caused no
change in either total or relative numbers of microorganisms. The
organisms were neither selected nor decimated by kelevan or its
degradation products over a 30-month period (Figge et al., 1983).
Table 2. Toxicity of kelevan for fisha
----------------------------------------------------------------------------------------------------
Species Life stage Length Water hardness Temperature Exposure time LC50
(cm) (dH01)b (°C) (h) (mg/litre)
----------------------------------------------------------------------------------------------------
Rainbow trout juvenile 7 - 9c 11 10 24 > 2
(Salmo gairdnerii)
Rainbow trout juvenile 7 - 9c 11 10 72 1.0
Rainbow trout juvenile 6 - 10 1 14 96 1.5
Rainbow trout juvenile 6 - 10 9 14 96 2.2
----------------------------------------------------------------------------------------------------
a From: Maier-Bode (1976).
b 1 degree of hardness (dH0) corresponds to 10.0 mg Ca0/litre water.
c For the studies on 7 - 9 cm trout, pH 6; pH not stated for other tests.
7.4. Appraisal
The data on kelevan are few. There is no information on its
immediate metabolite, kelevanic acid. Thus, it is difficult to
come to firm conclusions about the environmental significance of
kelevan. However, there are considerable data on the metabolite,
chlordecone. This is known to be stable and persistent. It
bioaccumulates and is more toxic for aquatic organisms than
kelevan. Chlordecone has severe sublethal effects on birds (WHO,
1984). The interpretation of data for kelevan, therefore, needs to
take into account the significance of chlordecone.
8. EFFECTS ON EXPERIMENTAL ANIMALS AND IN VITRO TEST SYSTEMS
8.1. Single Exposures
Data on the acute toxicity of kelevan are given in Table 3. A
similar pattern of response to acutely toxic doses of kelevan was
seen in the three species and included apathy, tremor, hyper-
sensitivity, and tonic-clonic convulsions.
Table 3. Acute toxicity of kelevan
-------------------------------------------------------------------
Animal Sex Route Vehicle LD50 Reference
(mg/kg
body weight)
-------------------------------------------------------------------
Rat M & F oral corn oil, 240 - 290 Maier-Bode (1976)
soybean oil
Rat oral 255 - 325 Kenaga & Allison
(1969)
Dog M & F oral corn oil, 400 - 550 Maier-Bode (1976)
soybean oil
Dog oral 400 - 500 Kenaga & Allison
(1969)
Rabbit M dermal corn oil 251 Maier-Bode (1976)
Rabbit dermal 188 - 314 Kenaga & Allison
(1969)
-------------------------------------------------------------------
8.2. Short-Term Exposures
8.2.1. Oral
Male rats were administered kelevan by oral intubation at 29
mg/kg body weight for 20 consecutive days (Medical College,
Virginia, 1968). No effects were observed on behaviour, organ
weights, or in the histopathological examination of liver and
kidneys. In a 90-day study (Medical College, Virginia, 1968), male
and female rats were fed kelevan incorporated in the diet at levels
of 0, 10, 30, 100, 300, or 1000 mg/kg. No animals died during the
course of the study, but animals fed 1000 mg/kg showed a reduced
weight gain. No treatment-related abnormalities were seen in food
consumption, haematology, urinalysis, or histopathology. Dose-
related liver hypertrophy was observed in females at 300 mg/kg diet
and in both sexes at 1000 mg/kg diet. Thus, 100 mg kelevan/kg
diet, equivalent to 5 mg/kg body weight per day, was a no-observed-
adverse-effect level in this study.
Albino rats were exposed to daily doses of 14 mg kelevan/kg
body weight for 4 months, or, 2.8 or 0.28 mg/kg body weight for 10
months. Hyperaemia of internal organs and necrosis of the liver
and kidneys were described, as well as lymphoid infiltration of
interstitial tissue in the lung. At 0.28 mg/kg, these changes were
reversible, and the author regarded this dose as a threshold dose
(Boreiko, 1980).
8.2.2. Dermal
Male and female rabbits were administered 25 or 50 mg
Despirol/kg body weight (12.5 or 25 mg kelevan/kg body weight), 5
days per week, for 9 weeks. The material was administered as an
aqueous paste to the shaved skin of the animals. There was no
difference between the treatment groups and controls. Only a
slight erythema was observed at the highest dose of kelevan
(Medical College, Virginia, 1968).
Kelevan, applied to the skin of rats, rabbits, and guinea-pigs,
in either one dose of 2000 mg/kg or 20 doses of 100 mg/kg caused
dystrophic changes in the liver and kidneys (Sasinovich et al.,
1977).
8.2.3. Inhalation
No adequately reported studies available.
8.3. Long-Term Exposure
No studies available.
8.4. Reproduction Studies
Reproduction was investigated in 100 male and 100 female BALB/C
mice fed kelevan in the diet at 5 mg/kg, from 30 days prior to
mating to 90 days after mating, over several litters (Ware & Good,
1967). No effects of treatment were observed on mortality, number
of females producing litters, pregnancy period, number of litters,
litter size, and sex ratio.
Thirty pregnant CD-1 mice were given the minimal toxic dose of
kelevan of 125 mg/kg body weight in 0.5 ml corn oil, by gavage,
from day 8 to day 12 of pregnancy. Four animals died. There were
no significant maternal weight changes nor effects on litter size
or pup weights (Chernoff & Kavlock, 1983).
It is known that chlordecone affects reproduction (WHO, 1984).
The dose of 5 mg kelevan/kg per day may have been too low to elicit
an effect on reproduction. The dose of 125 mg/kg apparently caused
sufficient toxicity in the dams to kill four of the animals. The
validity of both these studies, which were the only studies
available for evaluation, is limited.
8.5. Mutagenicity
Benigni et al. (1979) tested the mutagenic activity of kelevan
and its metabolite chlordecone on Aspergillus nidulans. As pure
compounds, both were negative (Carere & Morpurgo, 1981).
Kelevan and its degradation products did not show any mutagenic
activity in the Ames test with Salmonella typhimurium (Jaszczuk &
Syrowatka, 1980).
8.6. Carcinogenicity
No carcinogenicity studies are available for kelevan. However,
there is sufficient evidence of the carcinogenicity of its
metabolite chlordecone in animals (IARC, 1979; WHO, 1984)
8.7. Other Studies
A single intragastric dose of 140 mg kelevan/kg body weight
(approximately half the LD50), given to rats, decreased the rate
of bile secretion on the first day. From day 3 onwards, it
increased again, but it had not reached the level of the controls
on day 5 (Glukhova et al., 1978, 1979). Kelevan sharply increased
the levels of alanine aminotransferase and aspartate aminotransferase
in blood serum and caused structural changes in the liver at this
dose level.
9. EFFECTS ON MAN
No adverse health effects on human beings from exposure to
kelevan have been reported.
10. EVALUATION OF THE HEALTH RISKS FOR MAN AND EFFECTS ON THE
ENVIRONMENT
10.1. Evaluation of the Health Risks for Man
Kelevan, which is developed from chlordecone, is metabolized
in the mammalian body and in the environment back to chlordecone.
The acute toxicity of kelevan is similar to that of chlordecone,
which may well be its active metabolite.
Data on kelevan are sparse; several reports have not been
published and are not available for scrutiny and others lack
sufficient detail or are inadequate. No information exists on
actual human exposure.
The acute toxicity of kelevan in test animals is moderate (oral
LD50s ranging from 240 to 550 mg/kg body weight, according to the
scale of Hodge & Sterner (1956)), and similar to that of chlordecone.
However, the no-observed-adverse-effect level of 5 mg/kg body weight
per day observed in a 90-day oral study on the rat and a threshold
level of 0.28 mg/kg body weight per day observed in a 10-month oral
rat study, are very similar to those obtained with chlordecone (WHO,
1984). The pathological findings of liver hypertrophy and necrosis
of liver and kidneys are also similar.
Kelevan is not mutagenic in systems using microorganisms.
No carcinogenicity studies are available. However, there
is sufficient evidence of the carcinogenicity of its metabolite
chlordecone for mice and rats (IARC, 1979; WHO, 1984).
No adverse effects on human health due to exposure to
kelevan have been reported.
10.2. Evaluation of Environmental Effects
There have not been any reports of adverse effects on the
environment due to exposure to kelevan. Available information
suggests that the probability of deleterious effects on terrestrial
organisms from kelevan is low. Its metabolite, chlordecone, is
toxic for birds and microorganisms, though there is no indication
of this for the parent compound. Aquatic data for kelevan are
limited to one species and one life stage; it is moderately to
highly toxic for juvenile rainbow trout. It is possible, but
improbable, that local concentrations of kelevan after recommended
agricultural use could exceed the toxic threshold for trout fry.
The compound gives concern with aquatic organisms because its
degradation product is both more persistent and more toxic for fish
than the parent compound.
10.3. Conclusions and Recommendations
In view of the sparsity of available data, it is impossible to
arrive at an informed evaluation of kelevan with regard to its
danger for workers, the possible consumer hazards from food
residues, or its impact on the environment. Thus, as kelevan is
converted to chlordecone in the mammalian body and in the
environment, and as the available toxicity data are similar to
those on chlordecone, the evaluation for chlordecone (WHO, 1984)
should also largely apply to kelevan, unless further data to the
contrary become available. In practice, this means that, unless
kelevan is indispensable, it should not be used.
REFERENCES
ANONYMOUS (1978a) Kepone/mirex/hexachlorocyclopentadiene -
an environmental assessment, Washington DC, US Department of
Commerce, US National Technical Information Service (PB
280-289) (NCR).
BEGUM, S., GOEB, S., PARLAR, H., & KORTE, F. (1973) Reaction
behaviour of kelevan in solution, as a solid, and in gaseous
phase under UV irradiation. Chemosphere, 2: 235-238.
BENIGNI, R., BIGNAMI, M., CAMONI, I., CARERE, A., CONTI, G.,
IACHETTA, R., MORPURGO, G., & ORTALI, V.A. (1979) A new in
vitro method for testing plant metabolism in mutagenicity
studies. J. Toxicol. environ. Health, 5: 809-819.
BOREIKO, N.R. (1980) [Effects of the pesticide Despirol on
the morphology of internal organs of laboratory animals.] Gig.
i Sanit., 45: 68-69 (in Russian).
CAIRNS, T., SIEGMUND, E.G., & DOOSE, G.M. (1982) Liquid
chromatography/mass spectrometry of Kepone hydrate, kelevan,
and mirex. Anal. Chem., 54: 953-957.
CANNON, S.B., VEAZEY, J.M., Jr, JACKSON, R.S., BURSE, V.W.,
HAYES, C., STRAUB, W.E., LANDRIGAN, P.J., & LIDDLE, J.A.
(1978) Epidemic kepone poisoning in chemical workers. Am. J.
Epidemiol., 107: 529-537.
CARERE, A. & MORPURGO, G. (1981) Comparison of the mutagenic
activity of pesticides in vitro in various short-term assays.
Prog. in mutat. Res., 2: 87-104.
CHERNOFF, N. & KAVLOCK, R.J. (1983) In: Waters, M.D.,
Sandhu, S.S., Lewton, J., Claxton, L., Chernoff, N., & Wesnow,
S., ed. A teratology test system which utilizes postnatal
growth and viability in the mouse. III. Short-term bioassays
in the analysis of complex mixtures, New York, Plenum Press.
FIGGE, K. (1978) [Environmental ecological studies using
radioactive compounds.] Org. Verunreinig., 208-230 (in German).
FIGGE, K. & REHM, H. (1977) [On the behaviour of the
insecticide kelevan and its metabolites in the potato field
ecosystem.] Z. Pflanzenkr. Pflanzenschutz, 84(7/8): 385-409
(in German).
FIGGE, K., RHEM, H., & SCHOENWALDER, H. (1983) [Degradation
and biocidal effects of chemical plant-protection agents and
pesticides in the soil by the example of the insecticide
Kelevan.] Z. Pflanzenernaehr Bodenkd, 146: 316-340 (in German).
GILBERT, E., LOMBARDO, E.P., RUMANOWSKI, E.J., & WALKER, G.L.
(1966) Preparation and insecticidal evaluation of alcoholic
analogs of kepone. J. agric. food Chem., 14: 111-118.
GLUKHOVA, L.G., VASILENKO, T.E., & APON, N.I. (1978)
[Comparative characteristics of the effects of organochlorine
and organophosphorus toxic chemicals on the bile-forming
function of the liver.] Vrach. Delo., 11: 130-131 (in Russian).
GLUKHOVA, L.G., VASILENKO, T.E., APON, N.I., & BOREIKO, N.P.
(1979) [Functional state of the liver under the acute effects
of phosalone and Despirol.] Vrach. Delo., 10: 108-109 (in
Russian).
HEYS, J.R., DUNCAN, W.P., PERRY, W.C., EBERT, D.A.,
RADOLOVICH, G., & HAILE, C.L. (1979) Synthesis of carbon-14
and carbon-13 labelled chlorinated polycyclic pesticides. J.
labelled Comp. Radiopharm., 16: 295-306.
HODGE, H.C. & STERNER, J.H. (1956) Combined tabulation of
toxicity classes. In: Spector, W.S., Handbook of toxicology,
Philadelphia, Pennsylvania, W.B. Saunders Company, Vol. 1.
HRLEC, G. & OSTREC, L. (1981) [Residue influence of
pesticides and their activity on soil microarthropods.]
Agrohemija, 7-8: 291-296 (in Serbian).
IARC (1979) Some halogenated hydrocarbons, Lyons,
International Agency for Research on Cancer (Monographs on the
Evaluation of the Carcinogenic Risk of Chemicals to Humans,
Vol. 20).
JASZCZUK, E. & SYROWATKA, T. (1980) [Mutagenic action of
certain pesticides on Salmonella typhimurium.] Rocz. Panstw.
Zekl. Hig., 31: 305-311 (in Polish).
JONAS, K. (1983) [Analysis and the toxicological behaviour
of residues of the insecticide kelevan.] Mh. Vet. Med., 38:
223-225 (in German).
KENAGA, E.E. & ALLISON, W.E. (1969) Commercial and
experimental organic insecticides: 1969 revision, Vol. 15(2),
pp. 85-148 (Bulletin of the Entomological Society of America
(96).
KLEIN, W. (1972) Cyclodiene and other residues in growing
crops: status report. In: Isotope tracer studies of chemical
residues in food and the agricultural environment. Proceedings
and Report of Research Coordination Meetings, Ispra, Italy,
Vienna, Austria, IAEA, 156 pp.
KRASNYKH, A.A. (1980) [Despirol residues in soil and
potato.] Khim. Sel'sk Khoz., 18: 53-54 (in Russian).
MADARIC, A. & SACKMAUEROVA, M. (1974) Residues of kelevan
(Despirol) in the soil and in potatoes. In: Proceedings of the
3rd International Congress on Pesticide Chemicals, Helsinki
3-9 July, 1974.
MAIER-BODE, H. (1976) The insecticide kelevan. Res. Rev.,
63: 45-76.
MEDICAL COLLEGE, VIRGINIA (1965-68) Reports to the Allied
Chemical Corporation.
PARLAR, H., KLEIN, W., & KORTE, F. (1972) Photochlorination
reactions of kelevan. Chemosphere, 1: 129-132.
SANDROCK, K., BIENICK, D., KLEIN, W., & KORTE, F. (1974)
Isolation and structural resolution of 14C-kelevan metabolites
and balance in potatoes and soil. Chemosphere, 3(5): 199-204.
SASINOVICH, L.M., OVSYANNIKOVA, L.M., & BADAEVA, L.N. (1977)
Characteristics of the skin-absorptive effect of new
pesticides belonging to different chemical groups. Gig. Tr.
Prof. Zabol., 7: 34-36.
SCHEUNERT, I., VOCKEL, D., SCHMITZER, J., VISWANATHAN, R.,
KLEIN, W., & KORTE, F. (1983) Fate of chemicals in plant-
soil systems: comparison of laboratory test data with results
of open-air long-term experiments. Ecol. environ. Saf., 7:
390-399.
TOMASZEWSKA, B., BOHOSIEWICZ, M., & JOPEK, Z. (1981)
Toxicity of Despirol for bees under laboratory conditions.
Zesz. Nauk. Akad. Roln. Wrpclawin. Weter.: 117-120.
WARE, G.W. & GOOD, E.E. (1967) Effects of insecticides on
reproduction in the laboratory mouse. III. Tranid and GC-9160.
J. environ. Entomol., 60: 530-532.
WESTLAKE, A., WESTLAKE, W.E., & GUNTHER, F.A. (1970)
Determination of residues of GC-9160 in cabbage, lettuce, and
citrus fruit. J. agric. food Chem., 18: 159-165.
WHO (1984) Environmental Health Criteria 43: Chlordecone,
Geneva, World Health Organization, 57 pp.
WILSON, N.K. & ZEHR, R.D. (1978) Structures of some kepone
photoproducts and related chlorinated pentacyclodecanes by
carbon-13 and proton nuclear magnetic resonance. J. organ.
Chem., 44: 1278-1282.
 Molecular formula: C17H12Cl10O4
CAS chemical name: 1,3,4-metheno-1 H-cyclobuta; cd pentalene-
2-pentanoic acid, 1,1a,3,3a,4,5,5a,5b,
6-decachloro-octa-hydro-2-hydroxy-gamma-
oxo-ethyl ester
Trade names: Despirol, Elevat, GC-9160, General
Chemicals 9160
CAS registry number:
Molecular formula: C17H12Cl10O4
CAS chemical name: 1,3,4-metheno-1 H-cyclobuta; cd pentalene-
2-pentanoic acid, 1,1a,3,3a,4,5,5a,5b,
6-decachloro-octa-hydro-2-hydroxy-gamma-
oxo-ethyl ester
Trade names: Despirol, Elevat, GC-9160, General
Chemicals 9160
CAS registry number: 