
UNITED NATIONS ENVIRONMENT PROGRAMME
INTERNATIONAL LABOUR ORGANISATION
WORLD HEALTH ORGANIZATION
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 184
Diflubenzuron
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
First draft prepared by Dr M. Tasheva, Sofia, Bulgaria
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 1996
The International Programme on Chemical Safety (IPCS) is a joint
venture of the United Nations Environment Programme, the International
Labour Organisation, and the World Health Organization. The main
objective of the IPCS is to carry out and disseminate evaluations of
the effects of chemicals on human health and the quality of the
environment. Supporting activities include the development of
epidemiological, experimental laboratory, and risk-assessment methods
that could produce internationally comparable results, and the
development of manpower in the field of toxicology. Other activities
carried out by the IPCS include the development of know-how for coping
with chemical accidents, coordination of laboratory testing and
epidemiological studies, and promotion of research on the mechanisms
of the biological action of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization and the Organisation for
Economic Co-operation and Development (Participating Organizations),
following recommendations made by the 1992 UN Conference on
Environment and Development to strengthen cooperation and increase
coordination in the field of chemical safety. The purpose of the IOMC
is to promote coordination of the policies and activities pursued by
the Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing in Publication Data
Diflubenzuron.
(Environmental health criteria ; 184)
1. Diflubenzuron - adverse effects 2. Diflubenzuron - toxicity
3. Insecticides - adverse effects 4. Insecticides - toxicity
5. Environmental exposure I. Series
ISBN 92 4 157184 1 (NLM Classification: WA 240)
ISSN 0250-863X
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1996
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved. The designations
employed and the presentation of the material in this publication do
not imply the expression of any opinion whatsoever on the part of the
Secretariat of the World Health Organization concerning the legal
status of any country, territory, city or area or of its authorities,
or concerning the delimitation of its frontiers or boundaries. The
mention of specific companies or of certain manufacturers' products
does not imply that they are endorsed or recommended by the World
Health Organization in preference to others of a similar nature that
are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
Preamble
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1. Summary
1.1.1. Identity, physical and chemical properties, and
analytical methods
1.1.2. Sources of human and environmental exposure
1.1.3. Environmental transport, distribution and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism in laboratory animals
1.1.6. Effects on laboratory mammals and in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Evaluation
1.2.1. Evaluation of human health risks
1.2.2. Evaluation of effects on the environment
1.2.3. Toxicological criteria for setting guidance values
1.3. Conclusions and recommendations
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factor
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Production levels and processes
3.2.2. Formulations
3.2.3. Uses
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION AND FATE
4.1. Appraisal
4.2. Transport and distribution between media
4.2.1. Soil mobility
4.2.2. Dissipation
4.2.3. Evaporation
4.2.4. Crop residue data
4.3. Transformation
4.3.1. Abiotic degradation
4.3.1.1 Photolysis
4.3.1.2 Hydrolysis
4.3.2. Biodegradation
4.3.2.1 Water
4.3.2.2 Soil
4.4. Bioaccumulation and biomagnification
4.5. Interaction with other physical, chemical or
biological factors
4.6. Ultimate fate following use
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air
5.1.2. Water
5.1.3. Food and feed
5.1.4. Forest plants and litter
5.1.5. Aquatic organisms
5.2. General population exposure
5.3. Occupational exposure during manufacture, formulation or use
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1. Absorption
6.2. Distribution
6.3. Metabolic transformation
6.3.1. Metabolites - distribution, excretion, retention
and turnover
6.4. Elimination and excretion
6.5. Retention and turnover
6.5.1. Biological half-life
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.3. Long-term exposure
7.4. Skin and eye irritation; sensitization
7.5. Reproductive toxicity, embryotoxicity and teratogenicity
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.8. Other special studies
7.8.1. Special studies on met- and sulfhaemoglobin
formation
7.9. Toxicity of metabolites
7.9.1. Carcinogenicity studies with 4-chloroaniline
8. EFFECTS ON HUMANS
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Laboratory experiments
9.1.1. Microorganisms
9.1.1.1 Water
9.1.1.2 Soil
9.1.2. Aquatic organisms
9.1.2.1 Microorganisms
9.1.2.2 Plants
9.1.2.3 Invertebrates
9.1.2.4 Vertebrates
9.1.3. Terrestrial organisms
9.1.3.1 Plants
9.1.3.2 Invertebrates
9.1.3.3 Vertebrates
9.2. Field observations
9.2.1. Microorganisms
9.2.1.1 Water
9.2.1.2 Soil
9.2.2. Aquatic organisms
9.2.2.1 Plant
9.2.2.2 Invertebrates
9.2.2.3 Vertebrates
9.2.3. Terrestrial organisms
9.2.3.1 Invertebrates
9.2.3.2 Vertebrates
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the criteria
monographs as accurately as possible without unduly delaying their
publication. In the interest of all users of the Environmental Health
Criteria monographs, readers are requested to communicate any errors
that may have occurred to the Director of the International Programme
on Chemical Safety, World Health Organization, Geneva, Switzerland, in
order that they may be included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from the
International Register of Potentially Toxic Chemicals, Case postale
356, 1219 Châtelaine, Geneva, Switzerland (Telephone No. 9799111).
* * *
This publication was made possible by grant number 5 U01
ES02617-15 from the National Institute of Environmental Health
Sciences, National Institutes of Health, USA, and by financial support
from the European Commission.
The proprietary information contained in this document cannot
replace documentation for registration purposes, because the latter
has to be closely linked to the source, the manufacturing route, and
the purity/impurities of the substance to be registered. The data
should be used in accordance with paragraphs 82-84 and recommendations
paragraph 90 of the Second FAO Government Consultation (1982).
Environmental Health Criteria
PREAMBLE
Objectives
In 1973 the WHO Environmental Health Criteria Programme was
initiated with the following objectives:
(i) to assess information on the relationship between exposure to
environmental pollutants and human health, and to provide
guidelines for setting exposure limits;
(ii) to identify new or potential pollutants;
(iii) to identify gaps in knowledge concerning the health effects of
pollutants;
(iv) to promote the harmonization of toxicological and
epidemiological methods in order to have internationally
comparable results.
The first Environmental Health Criteria (EHC) monograph, on
mercury, was published in 1976 and since that time an ever-increasing
number of assessments of chemicals and of physical effects have been
produced. In addition, many EHC monographs have been devoted to
evaluating toxicological methodology, e.g., for genetic, neurotoxic,
teratogenic and nephrotoxic effects. Other publications have been
concerned with epidemiological guidelines, evaluation of short-term
tests for carcinogens, biomarkers, effects on the elderly and so
forth.
Since its inauguration the EHC Programme has widened its scope,
and the importance of environmental effects, in addition to health
effects, has been increasingly emphasized in the total evaluation of
chemicals.
The original impetus for the Programme came from World Health
Assembly resolutions and the recommendations of the 1972 UN Conference
on the Human Environment. Subsequently the work became an integral
part of the International Programme on Chemical Safety (IPCS), a
cooperative programme of UNEP, ILO and WHO. In this manner, with the
strong support of the new partners, the importance of occupational
health and environmental effects was fully recognized. The EHC
monographs have become widely established, used and recognized
throughout the world.
The recommendations of the 1992 UN Conference on Environment and
Development and the subsequent establishment of the Intergovernmental
Forum on Chemical Safety with the priorities for action in the six
programme areas of Chapter 19, Agenda 21, all lend further weight to
the need for EHC assessments of the risks of chemicals.
Scope
The criteria monographs are intended to provide critical reviews
on the effect on human health and the environment of chemicals and of
combinations of chemicals and physical and biological agents. As
such, they include and review studies that are of direct relevance for
the evaluation. However, they do not describe every study carried
out. Worldwide data are used and are quoted from original studies,
not from abstracts or reviews. Both published and unpublished reports
are considered and it is incumbent on the authors to assess all the
articles cited in the references. Preference is always given to
published data. Unpublished data are only used when relevant
published data are absent or when they are pivotal to the risk
assessment. A detailed policy statement is available that describes
the procedures used for unpublished proprietary data so that this
information can be used in the evaluation without compromising its
confidential nature (WHO (1990) Revised Guidelines for the Preparation
of Environmental Health Criteria Monographs. PCS/90.69, Geneva, World
Health Organization).
In the evaluation of human health risks, sound human data,
whenever available, are preferred to animal data. Animal and
in vitro studies provide support and are used mainly to supply
evidence missing from human studies. It is mandatory that research on
human subjects is conducted in full accord with ethical principles,
including the provisions of the Helsinki Declaration.
The EHC monographs are intended to assist national and
international authorities in making risk assessments and subsequent
risk management decisions. They represent a thorough evaluation of
risks and are not, in any sense, recommendations for regulation or
standard setting. These latter are the exclusive purview of national
and regional governments.
Content
The layout of EHC monographs for chemicals is outlined below.
* Summary - a review of the salient facts and the risk evaluation
of the chemical
* Identity - physical and chemical properties, analytical methods
* Sources of exposure
* Environmental transport, distribution and transformation
* Environmental levels and human exposure
* Kinetics and metabolism in laboratory animals and humans
* Effects on laboratory mammals and in vitro test systems
* Effects on humans
* Effects on other organisms in the laboratory and field
* Evaluation of human health risks and effects on the environment
* Conclusions and recommendations for protection of human health
and the environment
* Further research
* Previous evaluations by international bodies, e.g., IARC, JECFA,
JMPR
Selection of chemicals
Since the inception of the EHC Programme, the IPCS has organized
meetings of scientists to establish lists of priority chemicals for
subsequent evaluation. Such meetings have been held in: Ispra, Italy,
1980; Oxford, United Kingdom, 1984; Berlin, Germany, 1987; and North
Carolina, USA, 1995. The selection of chemicals has been based on the
following criteria: the existence of scientific evidence that the
substance presents a hazard to human health and/or the environment;
the possible use, persistence, accumulation or degradation of the
substance shows that there may be significant human or environmental
exposure; the size and nature of populations at risk (both human and
other species) and risks for environment; international concern, i.e.
the substance is of major interest to several countries; adequate data
on the hazards are available.
If an EHC monograph is proposed for a chemical not on the
priority list, the IPCS Secretariat consults with the Cooperating
Organizations and all the Participating Institutions before embarking
on the preparation of the monograph.
Procedures
The order of procedures that result in the publication of an EHC
monograph is shown in the flow chart. A designated staff member of
IPCS, responsible for the scientific quality of the document, serves
as Responsible Officer (RO). The IPCS Editor is responsible for
layout and language. The first draft, prepared by consultants or,
more usually, staff from an IPCS Participating Institution, is based
initially on data provided from the International Register of
Potentially Toxic Chemicals, and reference data bases such as Medline
and Toxline.
The draft document, when received by the RO, may require an
initial review by a small panel of experts to determine its scientific
quality and objectivity. Once the RO finds the document acceptable as
a first draft, it is distributed, in its unedited form, to well over
150 EHC contact points throughout the world who are asked to comment
on its completeness and accuracy and, where necessary, provide
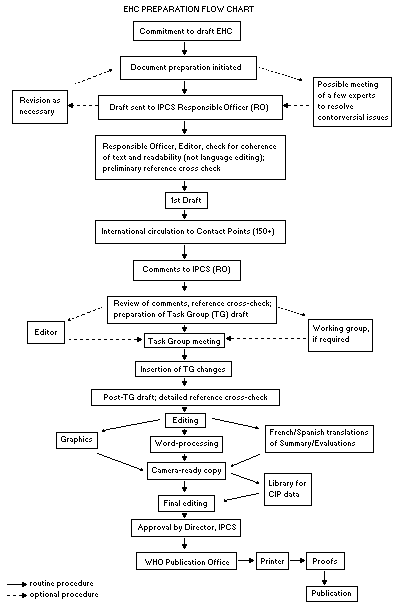 additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme,
Châtelaine, Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
The Core Assessment Group (CAG) of the Joint Meeting on Pesticide
Residues met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel of the WHO welcomed the participants on behalf of WHO,
and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to diflubenzuron.
The first draft of the monograph was prepared by Dr M. Tasheva,
Sofia, Bulgaria. The second draft, incorporating comments received
following circulation of the first draft to the IPCS contact points
for Environmental Health Criteria monographs, was prepared by the IPCS
Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that Solvay-Duphar, BV, made available to the IPCS its
proprietary toxicological information on diflubenzuron is gratefully
acknowledged. This allowed the CAG to make its evaluation on a more
complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ADI acceptable daily intake
a.i. active ingredient
AP alkaline phosphatase
bw body weight
4-CPU 4-chlorophenylurea
DFB diflubenzuron
2,6-DFBA 2,6-difluorobenzoic acid
ECD electron capture detection
G granular formulation
GC gas chromatography
GLC gas-liquid chromatography
Hb haemoglobin
HPLC high performance liquid chromatography
MATC maximum acceptable toxicant concentration
MCH mean cell haemoglobin
MCHC mean cell haemoglobin concentration
MCV mean cell volume
NOAEC no-observed-adverse-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus detector
PCA para-chloroaniline (4-chloroaniline)
PCV packed cell volume
SAP serum alkaline phosphatase
SGOT serum glutamic-oxaloacetic transaminase (aspartate
aminotransferase)
SGPT serum glutamic-pyruvic transaminase (alanine
aminotransferase)
TLC thin-layer chromatography
WP wettable powder
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Diflubenzuron is a member of the benzoylphenylurea group of
insecticides. Its insecticidal action is due to interaction with
chitin synthesis and/or deposition. It forms odourless white crystals
with a melting point of 230-232°C. It is sparingly soluble in water
(0.2 mg/litre at 20°C) and is virtually non-volatile. It is
relatively stable in acidic and neutral media but it hydrolyses in
alkaline conditions.
Diflubenzuron is produced by the reaction of 2,6-difluoro-
benzamide with 4-chlorophenylisocyanate.
Diflubenzuron residues may be measured in water, biological
samples and soils by HPLC with UV detection or by GC with ECD for
analysis of the intact molecule or following derivatization of the
liberated 4-chloroaniline with trifluoroacetic anhydride.
1.1.2 Sources of human and environmental exposure
Diflubenzuron is a synthetic compound used in agriculture,
forestry and public health programmes to control insect pests and
vectors. Different formulations of diflubenzuron are available for
these uses. There is no relevant information on human exposure to
diflubenzuron.
1.1.3 Environmental transport, distribution and transformation
Diflubenzuron is usually applied directly to plants and water.
Uptake of diflubenzuron through plant leaves does not occur.
The adsorption of diflubenzuron to soil is rapid. It is
immobilized in the top 10 cm layer of soil to which it is applied. It
is unlikely to leach. Diflubenzuron is degraded in soils of various
types and origin under aerobic or anaerobic conditions with a half-
life of a few days. The rate of degradation depends greatly on the
diflubenzuron particle size. The main metabolic pathway (over 90%) is
hydrolysis leading to 2,6-difluorobenzoic acid and 4-chlorophenylurea;
these are degraded with half-lives of about 4 and 6 weeks,
respectively. Free 4-chloroaniline has not been detected in soils.
Diflubenzuron degrades rapidly in neutral or alkaline waters.
Studies of application of diflubenzuron to water show rapid partition
to sediment; the parent compound and 4-chlorophenylurea may persist on
sediment for more than 30 days.
Diflubenzuron does not bioaccumulate in fish.
1.1.4 Environmental levels and human exposure
Exposure of the general population to diflubenzuron via water or
food as a result of its use in agriculture, against forest insects or
in mosquito control is negligible.
1.1.5 Kinetics and metabolism in laboratory animals
In experimental animals, diflubenzuron is absorbed from the
digestive tract and to a lesser extent through the skin. There is a
saturable absorption mechanism in the rat gastrointestinal tract.
Consequently a large proportion of orally administered diflubenzuron
is found in the faeces. Diflubenzuron has widespread distribution in
the tissues, but it does not accumulate.
The metabolic fate of diflubenzuron has been studied in various
species. The major route of metabolism in mammals is via
hydroxylation. Hydrolysis of diflubenzuron may occur at any of the
three carbon-nitrogen bonds. In pigs and chickens the major route of
hydrolysis is at the ureido bridge. In rats and cows the major
metabolic pathway is hydroxylation. The major metabolites in sheep,
swine and chickens are 2,6-difluorobenzoic acid and 4-chloro-
phenylurea; minor metabolites are 2,6-difluorobenzamide and
4-chloroaniline. In rats and cattle 80% of the metabolites are
2,6-difluoro-3-hydroxydiflubenzuron, 4-chloro-2-hydroxy-diflubenzuron
and 4-chloro-3-hydroxydiflubenzuron. The metabolic studies indicate
that little or no 4-chloroaniline is formed in rats or cattle.
The major route of elimination is via the faeces, ranging from 70
to 85% in cats, pigs and cattle. In sheep elimination is roughly
equally distributed between the urine and faeces. Urinary excretion
in rats and mice decreases proportionally with increasing dosage
level. Less than 1% of an oral dose is recovered in exhaled air.
Only trace residues are found in milk.
No human studies on the kinetics and metabolism of diflubenzuron,
including the extent of biotransformation to 4-chloroaniline, are
available.
1.1.6 Effects on laboratory mammals and in vitro test systems
Diflubenzuron has low acute toxicity by any route of exposure.
It has been classified by WHO as a "product unlikely to present an
acute hazard in normal use", based on an acute oral LD50 of more than
4640 mg/kg body weight in rats. The acute dermal LD50 in rats is
greater than 10 000 mg/kg body weight while the acute inhalational
LC50 for rats is greater than 2.49 mg/litre. No signs of
intoxication have been observed during the 14-day period following
single administration of diflubenzuron by various routes to a variety
of animal species.
Diflubenzuron is not a skin irritant (in rabbits) and not a skin
sensitizer (in guinea-pigs). It is marginally irritating to the eyes
of rabbits.
Diflubenzuron causes methaemoglobinaemia and sulfhaemo-
globinaemia. Dose-related methaemoglobinaemia has been demonstrated
after oral, dermal or inhalatory exposure to diflubenzuron in various
species. This effect is the most sensitive toxicological end-point in
experimental animals. The NOEL based on methaemoglobin formation is
2 mg/kg body weight per day in rats and dogs and 2.4 mg/kg body weight
per day in mice. In long-term toxicity studies with mice and rats,
treatment-related changes were principally associated with oxidation
of haemoglobin or with hepatocyte changes.
In carcinogenicity studies in mice and rats at dietary levels up
to 10 000 mg/kg in the diet, there were no treatment-related effects
on tumour incidence. Specifically, there were no mesenchymal
neoplasms of the spleen or liver as observed in carcinogenicity
studies with 4-chloroaniline.
In several reproductive toxicity studies on rats, mice, rabbits
and three avian species, no effects were seen on reproduction and
there was no embryotoxicity. Teratogenicity studies in rats and
rabbits demonstrated no teratogenic effects.
Diflubenzuron and its main metabolites have been examined in a
variety of in vitro and in vivo mutagenicity tests. Neither
diflubenzuron nor its major metabolites have a mutagenic effect.
The minor metabolite, 4-chloroaniline, was shown to be positive
in several in vitro mutagenicity assays using various end-points.
It is carcinogenic in rats and mice. The neoplastic lesions related
to administration of 4-chloroaniline were benign and malignant
mesenchymal tumours in the spleens of male rats and haemangiomas and
haemangiosarcomas, primarily in the spleen and liver of male mice.
1.1.7 Effects on humans
The diflubenzuron metabolite, 4-chloroaniline, has been reported
to cause methaemoglobinaemia in exposed workers and in neonates
inadvertently exposed. Some individuals who are deficient in
NADH-methaemoglobin reductase may be particularly sensitive to
4-chloroaniline and hence to diflubenzuron exposure.
1.1.8 Effects on other organisms in the laboratory and field
All chitin-synthesizing organisms show susceptibility to
diflubenzuron.
Bacteria were not affected by diflubenzuron at concentrations of
500 mg/kg soil; some stimulation of nitrogen fixation was seen.
Diflubenzuron acetone solutions were degraded; the acetone was used
as carbon source. Algal biomass increased at a diflubenzuron
concentration of 1 µg/litre. There were no adverse effects at
concentration above the limit of diflubenzuron solubility. Fungi were
temporarily affected at 0.1 µg/litre in laboratory streams.
Aquatic invertebrates show variable responses to diflubenzuron.
Molluscs are insensitive, the LC50 being greater than 200 mg/litre.
LC50 values for other invertebrates ranged from 1 to > 1000
µg/litre, reflecting the effects of the compound on juvenile,
moulting stages. A MATC for Daphnia has been estimated at > 40 and
< 93 ng/litre; as expected, larval mayflies and other aquatic insect
juveniles are highly susceptible. Overspray of water bodies would be
expected to kill some aquatic invertebrates.
In ecosystems and field experiments where diflubenzuron was
applied directly to the water, the effects on most taxa were less
severe than predictions from acute laboratory tests. No effects on
aquatic organisms have been found after aerial applications to
forests.
The LC50 values for fish are > 150 mg/litre. In field
experiments, fish kills have never been recorded.
The oral and contact LD50 for honey-bees is greater than
30 µg/bee. Honey-bee colonies were not affected after aerial
application of 350 g diflubenzuron/ha.
A 5-day dietary study on the mallard duck and bobwhite quail with
levels of up to 4640 mg/kg feed revealed no observable signs of
toxicity. Small songbirds in the forest ecosystem were not affected
after aerial application of diflubenzuron at 350 g/ha.
Small mammal species showed no reductions in numbers after
application of diflubenzuron at 67 g/ha to a forest.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The primary manifestation of diflubenzuron toxicity is
methaemoglobin induction. This toxicity occurs in a range of test
animal species. It is attributable to the metabolite, 4-chloroaniline,
which is known to induce methaemoglobin formation in several animal
species and in humans.
Diflubenzuron does not cause other toxicities on chronic dietary
administration. It is not mutagenic or carcinogenic in mice or rats.
However, its metabolite, 4-chloroaniline, is mutagenic in vitro and
is carcinogenic in mice and male rats. Although 4-chloroaniline is a
minor urinary metabolite of diflubenzuron in rats, the extent to which
it is formed in vivo in various animal species remains unknown.
Similarly, the comparative degree of absorption of its parent compound
in various species is unknown.
The sensitivity of human haemoglobin to methaemoglobin formation
by 4-chloroaniline in vivo is not known. However, since induction
of methaemoglobinaemia is consistently the most sensitive measure of
diflubenzuron toxicity in the various animal species tested, it may be
used as the basis to estimate the levels causing no toxicological
effect.
1.2.2 Evaluation of effects on the environment
Diflubenzuron adsorbs readily to soil with little subsequent
desorption. Its mobility in soil is very low, practically all
residues remaining within 15 cm of the top, even in sandy loam soils;
diflubenzuron does not leach. It is only partly removed from foliage
by heavy rainfall. Nevertheless, some diflubenzuron may be present in
surface water shortly after application, due to flooding of treatment
areas or agricultural run-off.
Dissipation of diflubenzuron from water is rapid. Adsorption to
sediment occurs within 4 days; both parent compound and 4-chloro-
phenylurea metabolite may persist on sediment for at least 30 days.
Uptake of diflubenzuron by plants through the leaves after aerial
application does not occur. Some uptake of soil residues does occur
in plants and this may be translocated. At the highest application
rate (1 kg a.i./ha), following 1 month ageing of residues, up to
1 mg/kg residue may be found in various crops.
Photolysis of diflubenzuron is slow with a calculated half-life
of 40 days. Under environmental conditions abiotic degradation in
water and soil represents a minimum route of break-down. Aerobic
degradation in water is a microbial process with a half-life of a few
days under both laboratory and field conditions. In the field,
degradation of diflubenzuron applied at practical rates is influenced
by pH, temperature, formulation, organic matter content and depth of
the water.
Degradation in soil through microbial hydrolysis is a rapid
process, with a half-life of a few days, depending on diflubenzuron
particle size. The major break-down products are 2,6-difluorobenzoic
acid and 4-chlorophenylurea; a minor metabolite is parachloroaniline.
All these are irreversibly bound to soil and/or further metabolized.
The half-life of diflubenzuron residues on citrus fruits is
significantly decreased by high temperature and humidity.
Anaerobic degradation in water and sediment is slower than
aerobic.
Fish bioconcentrate diflubenzuron and some bioaccumulation takes
place during extended exposure up to a plateau, depending on the water
concentration, owing to fast degradation of diflubenzuron and
excretion of metabolites; the depuration half-life is less than one
day. The 4-chloroaniline metabolite has not been detected in fish.
Fish are not sensitive to diflubenzuron, the LC50 values being
> 150 mg/litre. Metabolites of diflubenzuron are also of low
toxicity to fish. Chronic exposure has shown no effects on fish at
recommended application rates; the compound does not persist in water
and no chronic exposure is expected.
Diflubenzuron is not phytotoxic to duckweed at the diflubenzuron
solubility limit concentration.
Honey-bees were not affected by topical applications of
> 30 µg/bee or dietary concentrations of up to 1000 mg/kg diet.
Brood in hives was reduced when bees were fed syrup at 59 mg
diflubenzuron/kg. Brood was also reduced following exposure of
flying colonies.
Earthworms were not affected at a concentration of 780 mg/kg
soil, which is at least three orders of magnitude above reported soil
residues.
Diflubenzuron has low acute toxicity to birds, the oral and
dietary LD(LC)50 values being greater than 3000 mg/kg diet.
Following recommended application rates diflubenzuron is not expected
to pose a hazard to birds.
Extensive field studies have shown minimal or reversible effects
on most aquatic invertebrates; daphnids were most seriously affected,
with short-term reductions in populations of up to 75% following a
single application of diflubenzuron. Fish were not affected by water
overspraying. Neither bird nor mammal populations were adversely
affected following forest spraying with diflubenzuron.
A summary of risk quotients for birds, fish and aquatic
invertebrates is given in Table 1.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on diflubenzuron of relevance for
setting guidance values are shown in Table 2.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based on
application rates of 2.5 kg a.i./ha of diflubenzuron to soybeans (worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 3762 73.7-535.7 51.0-7.0
Acute fish (stream) 150 0.0007 214 300
Acute fish (pond) 150 0.01 15 000
Acute aquatic
invertebrate (stream) 0.005 0.0007 7.1
Acute aquatic
invertebrate (pond) 0.005 0.01 0.5
a Estimated environmental concentration in the terrestrial environment (for bird
exposure) is based on the stated application rate and the assumption of
deposition on short grass using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a
single application and estimated runoff into water; no direct overspray is
included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below
1.0 indicate likely exposure to toxic concentrations by organisms in the
different risk categories.
Table 2. Toxicological criteria for estimating guidance values for diflubenzuron
Exposure scenario Relevant route/effect/ Result/remarks
(technical species
diflubenzuron)
Short-term dermal, irritation, rabbit non-irritant
(1-7 days)
ocular, irritation, rabbit marginal, high dose
dermal, sensitization, non-sensitizing
guinea-pig
inhalational, toxicity, rat LC50 > 2.49 mg/litre
(single exposure)
Mid-term
(1-26 weeks)
3 weeks; 5 days dermal, irritation, rabbit NOEL = 70 mg/kg body
per week weight per day
3 weeks; 5 days inhalational, methaemoglobin NOAEL = < 0.12 mg/litre
per week formation, rat
Long-term dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, rat per day
dietary, methaemoglobin NOEL = 2.4 mg/kg body weight
formation, mouse per day
dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, dog per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of diflubenzuron,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 2 mg/kg body weight per day derived in long-term
toxicity studies on mice, rats and dogs and applying a 100-fold
uncertainty factor, that 0.02 mg/kg body weight per day will probably
not cause adverse effects in humans whatever the route of exposure.
Biomonitoring of 4-chloroaniline during occupational exposures
needs to be carried out.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Molecular structure
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme,
Châtelaine, Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
The Core Assessment Group (CAG) of the Joint Meeting on Pesticide
Residues met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel of the WHO welcomed the participants on behalf of WHO,
and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to diflubenzuron.
The first draft of the monograph was prepared by Dr M. Tasheva,
Sofia, Bulgaria. The second draft, incorporating comments received
following circulation of the first draft to the IPCS contact points
for Environmental Health Criteria monographs, was prepared by the IPCS
Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that Solvay-Duphar, BV, made available to the IPCS its
proprietary toxicological information on diflubenzuron is gratefully
acknowledged. This allowed the CAG to make its evaluation on a more
complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ADI acceptable daily intake
a.i. active ingredient
AP alkaline phosphatase
bw body weight
4-CPU 4-chlorophenylurea
DFB diflubenzuron
2,6-DFBA 2,6-difluorobenzoic acid
ECD electron capture detection
G granular formulation
GC gas chromatography
GLC gas-liquid chromatography
Hb haemoglobin
HPLC high performance liquid chromatography
MATC maximum acceptable toxicant concentration
MCH mean cell haemoglobin
MCHC mean cell haemoglobin concentration
MCV mean cell volume
NOAEC no-observed-adverse-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus detector
PCA para-chloroaniline (4-chloroaniline)
PCV packed cell volume
SAP serum alkaline phosphatase
SGOT serum glutamic-oxaloacetic transaminase (aspartate
aminotransferase)
SGPT serum glutamic-pyruvic transaminase (alanine
aminotransferase)
TLC thin-layer chromatography
WP wettable powder
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Diflubenzuron is a member of the benzoylphenylurea group of
insecticides. Its insecticidal action is due to interaction with
chitin synthesis and/or deposition. It forms odourless white crystals
with a melting point of 230-232°C. It is sparingly soluble in water
(0.2 mg/litre at 20°C) and is virtually non-volatile. It is
relatively stable in acidic and neutral media but it hydrolyses in
alkaline conditions.
Diflubenzuron is produced by the reaction of 2,6-difluoro-
benzamide with 4-chlorophenylisocyanate.
Diflubenzuron residues may be measured in water, biological
samples and soils by HPLC with UV detection or by GC with ECD for
analysis of the intact molecule or following derivatization of the
liberated 4-chloroaniline with trifluoroacetic anhydride.
1.1.2 Sources of human and environmental exposure
Diflubenzuron is a synthetic compound used in agriculture,
forestry and public health programmes to control insect pests and
vectors. Different formulations of diflubenzuron are available for
these uses. There is no relevant information on human exposure to
diflubenzuron.
1.1.3 Environmental transport, distribution and transformation
Diflubenzuron is usually applied directly to plants and water.
Uptake of diflubenzuron through plant leaves does not occur.
The adsorption of diflubenzuron to soil is rapid. It is
immobilized in the top 10 cm layer of soil to which it is applied. It
is unlikely to leach. Diflubenzuron is degraded in soils of various
types and origin under aerobic or anaerobic conditions with a half-
life of a few days. The rate of degradation depends greatly on the
diflubenzuron particle size. The main metabolic pathway (over 90%) is
hydrolysis leading to 2,6-difluorobenzoic acid and 4-chlorophenylurea;
these are degraded with half-lives of about 4 and 6 weeks,
respectively. Free 4-chloroaniline has not been detected in soils.
Diflubenzuron degrades rapidly in neutral or alkaline waters.
Studies of application of diflubenzuron to water show rapid partition
to sediment; the parent compound and 4-chlorophenylurea may persist on
sediment for more than 30 days.
Diflubenzuron does not bioaccumulate in fish.
1.1.4 Environmental levels and human exposure
Exposure of the general population to diflubenzuron via water or
food as a result of its use in agriculture, against forest insects or
in mosquito control is negligible.
1.1.5 Kinetics and metabolism in laboratory animals
In experimental animals, diflubenzuron is absorbed from the
digestive tract and to a lesser extent through the skin. There is a
saturable absorption mechanism in the rat gastrointestinal tract.
Consequently a large proportion of orally administered diflubenzuron
is found in the faeces. Diflubenzuron has widespread distribution in
the tissues, but it does not accumulate.
The metabolic fate of diflubenzuron has been studied in various
species. The major route of metabolism in mammals is via
hydroxylation. Hydrolysis of diflubenzuron may occur at any of the
three carbon-nitrogen bonds. In pigs and chickens the major route of
hydrolysis is at the ureido bridge. In rats and cows the major
metabolic pathway is hydroxylation. The major metabolites in sheep,
swine and chickens are 2,6-difluorobenzoic acid and 4-chloro-
phenylurea; minor metabolites are 2,6-difluorobenzamide and
4-chloroaniline. In rats and cattle 80% of the metabolites are
2,6-difluoro-3-hydroxydiflubenzuron, 4-chloro-2-hydroxy-diflubenzuron
and 4-chloro-3-hydroxydiflubenzuron. The metabolic studies indicate
that little or no 4-chloroaniline is formed in rats or cattle.
The major route of elimination is via the faeces, ranging from 70
to 85% in cats, pigs and cattle. In sheep elimination is roughly
equally distributed between the urine and faeces. Urinary excretion
in rats and mice decreases proportionally with increasing dosage
level. Less than 1% of an oral dose is recovered in exhaled air.
Only trace residues are found in milk.
No human studies on the kinetics and metabolism of diflubenzuron,
including the extent of biotransformation to 4-chloroaniline, are
available.
1.1.6 Effects on laboratory mammals and in vitro test systems
Diflubenzuron has low acute toxicity by any route of exposure.
It has been classified by WHO as a "product unlikely to present an
acute hazard in normal use", based on an acute oral LD50 of more than
4640 mg/kg body weight in rats. The acute dermal LD50 in rats is
greater than 10 000 mg/kg body weight while the acute inhalational
LC50 for rats is greater than 2.49 mg/litre. No signs of
intoxication have been observed during the 14-day period following
single administration of diflubenzuron by various routes to a variety
of animal species.
Diflubenzuron is not a skin irritant (in rabbits) and not a skin
sensitizer (in guinea-pigs). It is marginally irritating to the eyes
of rabbits.
Diflubenzuron causes methaemoglobinaemia and sulfhaemo-
globinaemia. Dose-related methaemoglobinaemia has been demonstrated
after oral, dermal or inhalatory exposure to diflubenzuron in various
species. This effect is the most sensitive toxicological end-point in
experimental animals. The NOEL based on methaemoglobin formation is
2 mg/kg body weight per day in rats and dogs and 2.4 mg/kg body weight
per day in mice. In long-term toxicity studies with mice and rats,
treatment-related changes were principally associated with oxidation
of haemoglobin or with hepatocyte changes.
In carcinogenicity studies in mice and rats at dietary levels up
to 10 000 mg/kg in the diet, there were no treatment-related effects
on tumour incidence. Specifically, there were no mesenchymal
neoplasms of the spleen or liver as observed in carcinogenicity
studies with 4-chloroaniline.
In several reproductive toxicity studies on rats, mice, rabbits
and three avian species, no effects were seen on reproduction and
there was no embryotoxicity. Teratogenicity studies in rats and
rabbits demonstrated no teratogenic effects.
Diflubenzuron and its main metabolites have been examined in a
variety of in vitro and in vivo mutagenicity tests. Neither
diflubenzuron nor its major metabolites have a mutagenic effect.
The minor metabolite, 4-chloroaniline, was shown to be positive
in several in vitro mutagenicity assays using various end-points.
It is carcinogenic in rats and mice. The neoplastic lesions related
to administration of 4-chloroaniline were benign and malignant
mesenchymal tumours in the spleens of male rats and haemangiomas and
haemangiosarcomas, primarily in the spleen and liver of male mice.
1.1.7 Effects on humans
The diflubenzuron metabolite, 4-chloroaniline, has been reported
to cause methaemoglobinaemia in exposed workers and in neonates
inadvertently exposed. Some individuals who are deficient in
NADH-methaemoglobin reductase may be particularly sensitive to
4-chloroaniline and hence to diflubenzuron exposure.
1.1.8 Effects on other organisms in the laboratory and field
All chitin-synthesizing organisms show susceptibility to
diflubenzuron.
Bacteria were not affected by diflubenzuron at concentrations of
500 mg/kg soil; some stimulation of nitrogen fixation was seen.
Diflubenzuron acetone solutions were degraded; the acetone was used
as carbon source. Algal biomass increased at a diflubenzuron
concentration of 1 µg/litre. There were no adverse effects at
concentration above the limit of diflubenzuron solubility. Fungi were
temporarily affected at 0.1 µg/litre in laboratory streams.
Aquatic invertebrates show variable responses to diflubenzuron.
Molluscs are insensitive, the LC50 being greater than 200 mg/litre.
LC50 values for other invertebrates ranged from 1 to > 1000
µg/litre, reflecting the effects of the compound on juvenile,
moulting stages. A MATC for Daphnia has been estimated at > 40 and
< 93 ng/litre; as expected, larval mayflies and other aquatic insect
juveniles are highly susceptible. Overspray of water bodies would be
expected to kill some aquatic invertebrates.
In ecosystems and field experiments where diflubenzuron was
applied directly to the water, the effects on most taxa were less
severe than predictions from acute laboratory tests. No effects on
aquatic organisms have been found after aerial applications to
forests.
The LC50 values for fish are > 150 mg/litre. In field
experiments, fish kills have never been recorded.
The oral and contact LD50 for honey-bees is greater than
30 µg/bee. Honey-bee colonies were not affected after aerial
application of 350 g diflubenzuron/ha.
A 5-day dietary study on the mallard duck and bobwhite quail with
levels of up to 4640 mg/kg feed revealed no observable signs of
toxicity. Small songbirds in the forest ecosystem were not affected
after aerial application of diflubenzuron at 350 g/ha.
Small mammal species showed no reductions in numbers after
application of diflubenzuron at 67 g/ha to a forest.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The primary manifestation of diflubenzuron toxicity is
methaemoglobin induction. This toxicity occurs in a range of test
animal species. It is attributable to the metabolite, 4-chloroaniline,
which is known to induce methaemoglobin formation in several animal
species and in humans.
Diflubenzuron does not cause other toxicities on chronic dietary
administration. It is not mutagenic or carcinogenic in mice or rats.
However, its metabolite, 4-chloroaniline, is mutagenic in vitro and
is carcinogenic in mice and male rats. Although 4-chloroaniline is a
minor urinary metabolite of diflubenzuron in rats, the extent to which
it is formed in vivo in various animal species remains unknown.
Similarly, the comparative degree of absorption of its parent compound
in various species is unknown.
The sensitivity of human haemoglobin to methaemoglobin formation
by 4-chloroaniline in vivo is not known. However, since induction
of methaemoglobinaemia is consistently the most sensitive measure of
diflubenzuron toxicity in the various animal species tested, it may be
used as the basis to estimate the levels causing no toxicological
effect.
1.2.2 Evaluation of effects on the environment
Diflubenzuron adsorbs readily to soil with little subsequent
desorption. Its mobility in soil is very low, practically all
residues remaining within 15 cm of the top, even in sandy loam soils;
diflubenzuron does not leach. It is only partly removed from foliage
by heavy rainfall. Nevertheless, some diflubenzuron may be present in
surface water shortly after application, due to flooding of treatment
areas or agricultural run-off.
Dissipation of diflubenzuron from water is rapid. Adsorption to
sediment occurs within 4 days; both parent compound and 4-chloro-
phenylurea metabolite may persist on sediment for at least 30 days.
Uptake of diflubenzuron by plants through the leaves after aerial
application does not occur. Some uptake of soil residues does occur
in plants and this may be translocated. At the highest application
rate (1 kg a.i./ha), following 1 month ageing of residues, up to
1 mg/kg residue may be found in various crops.
Photolysis of diflubenzuron is slow with a calculated half-life
of 40 days. Under environmental conditions abiotic degradation in
water and soil represents a minimum route of break-down. Aerobic
degradation in water is a microbial process with a half-life of a few
days under both laboratory and field conditions. In the field,
degradation of diflubenzuron applied at practical rates is influenced
by pH, temperature, formulation, organic matter content and depth of
the water.
Degradation in soil through microbial hydrolysis is a rapid
process, with a half-life of a few days, depending on diflubenzuron
particle size. The major break-down products are 2,6-difluorobenzoic
acid and 4-chlorophenylurea; a minor metabolite is parachloroaniline.
All these are irreversibly bound to soil and/or further metabolized.
The half-life of diflubenzuron residues on citrus fruits is
significantly decreased by high temperature and humidity.
Anaerobic degradation in water and sediment is slower than
aerobic.
Fish bioconcentrate diflubenzuron and some bioaccumulation takes
place during extended exposure up to a plateau, depending on the water
concentration, owing to fast degradation of diflubenzuron and
excretion of metabolites; the depuration half-life is less than one
day. The 4-chloroaniline metabolite has not been detected in fish.
Fish are not sensitive to diflubenzuron, the LC50 values being
> 150 mg/litre. Metabolites of diflubenzuron are also of low
toxicity to fish. Chronic exposure has shown no effects on fish at
recommended application rates; the compound does not persist in water
and no chronic exposure is expected.
Diflubenzuron is not phytotoxic to duckweed at the diflubenzuron
solubility limit concentration.
Honey-bees were not affected by topical applications of
> 30 µg/bee or dietary concentrations of up to 1000 mg/kg diet.
Brood in hives was reduced when bees were fed syrup at 59 mg
diflubenzuron/kg. Brood was also reduced following exposure of
flying colonies.
Earthworms were not affected at a concentration of 780 mg/kg
soil, which is at least three orders of magnitude above reported soil
residues.
Diflubenzuron has low acute toxicity to birds, the oral and
dietary LD(LC)50 values being greater than 3000 mg/kg diet.
Following recommended application rates diflubenzuron is not expected
to pose a hazard to birds.
Extensive field studies have shown minimal or reversible effects
on most aquatic invertebrates; daphnids were most seriously affected,
with short-term reductions in populations of up to 75% following a
single application of diflubenzuron. Fish were not affected by water
overspraying. Neither bird nor mammal populations were adversely
affected following forest spraying with diflubenzuron.
A summary of risk quotients for birds, fish and aquatic
invertebrates is given in Table 1.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on diflubenzuron of relevance for
setting guidance values are shown in Table 2.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based on
application rates of 2.5 kg a.i./ha of diflubenzuron to soybeans (worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 3762 73.7-535.7 51.0-7.0
Acute fish (stream) 150 0.0007 214 300
Acute fish (pond) 150 0.01 15 000
Acute aquatic
invertebrate (stream) 0.005 0.0007 7.1
Acute aquatic
invertebrate (pond) 0.005 0.01 0.5
a Estimated environmental concentration in the terrestrial environment (for bird
exposure) is based on the stated application rate and the assumption of
deposition on short grass using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a
single application and estimated runoff into water; no direct overspray is
included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below
1.0 indicate likely exposure to toxic concentrations by organisms in the
different risk categories.
Table 2. Toxicological criteria for estimating guidance values for diflubenzuron
Exposure scenario Relevant route/effect/ Result/remarks
(technical species
diflubenzuron)
Short-term dermal, irritation, rabbit non-irritant
(1-7 days)
ocular, irritation, rabbit marginal, high dose
dermal, sensitization, non-sensitizing
guinea-pig
inhalational, toxicity, rat LC50 > 2.49 mg/litre
(single exposure)
Mid-term
(1-26 weeks)
3 weeks; 5 days dermal, irritation, rabbit NOEL = 70 mg/kg body
per week weight per day
3 weeks; 5 days inhalational, methaemoglobin NOAEL = < 0.12 mg/litre
per week formation, rat
Long-term dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, rat per day
dietary, methaemoglobin NOEL = 2.4 mg/kg body weight
formation, mouse per day
dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, dog per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of diflubenzuron,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 2 mg/kg body weight per day derived in long-term
toxicity studies on mice, rats and dogs and applying a 100-fold
uncertainty factor, that 0.02 mg/kg body weight per day will probably
not cause adverse effects in humans whatever the route of exposure.
Biomonitoring of 4-chloroaniline during occupational exposures
needs to be carried out.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Molecular structure
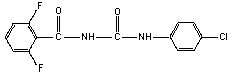 Empirical formula C14H9ClF2N2O2
Common name Diflubenzuron
Common trade names Dimilin; Micromite; Vigilante
Common abbreviation DFB
IUPAC name 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)-
urea
CAS chemical name N-[[(4-chlorophenyl) amino] carbonyl]-
2,6-difluorobenzamide
CAS registry number 35367-38-5
RTECS registry number YS6200000
Technical diflubenzuron contains > 95% pure compound.
2.2 Physical and chemical properties
Diflubenzuron is an odourless white crystalline solid. It is
almost insoluble in water and poorly soluble in apolar organic
solvents. In polar to very polar solvents, the solubility is moderate
to good, e.g., in acetone it is 6.5 g/litre at 20°C. Diflubenzuron is
highly soluble in N-methylpyrolidone (200 g/litre), dimethyl-
sulfoxide and dimethylformamide (both 120 g/litre).
Some physical and chemical properties of diflubenzuron are given
in Table 3.
Table 3. Physical and chemical properties of diflubenzuron
Relative molecular mass 310.7
Melting point technical > 95% 210-230°C
> 99% pure 230-232°C
Vapour pressure at 25°C 0.00012 mPa
Volatility
solid material < 4%
from water pH 5.6 < 2% (virtually non-volatile)
Specific gravity 1.56
n-Octanol/water partition coefficient
(log Kow) 5000
Solubility in water (at 25°C and pH 5.6) 8 × 10-5 g/litre
Stability in water (0.0001 g/litre 4% decomposition after 3 weeks at pH 5
in the dark) 8% decomposition after 3 weeks at pH 7
26% decomposition after 3 weeks at pH 91
2.3 Conversion factor
1 ppm = 12.7 mg/m3 at 25°C
1 mg/m3 = 0.079 ppm at 25°C
2.4 Analytical methods
Analytical methods for determining diflubenzuron in crops, soil,
water and biological samples are summarized in Table 4.
A review of the analytical methods has been presented by Rabenort
et al. (1978). Two general types of assay procedures for
diflubenzuron are available: high performance liquid chromatography
(HPLC) and gas chromatography (GC).
Table 4. Methods for the determination of diflubenzuron residues
Sample type Extraction/clean-up Analytical Limit of Comments Reference
method detection
Crops, soil, water dichloromethane; clean-up HPLC 0.03 mg/kg Rabenort et al. (1978)
on a Florisil column
Milk ethyl acetate HPLC 0.1 mg/kg Corley et al. (1974)
Crops acetone (n-hexane) HPLC 0.01 mg/kg Nakayama (1977a)
Apples acetonitrile HPLC 0.008 mg/kg Goto (1977a)
Tea acetone/dichloromethane HPLC 0.1 mg/kg Nakayama (1977b)
Tea acetone or water HPLC 0.2 mg/kg Goto (1977b)
Crops, soil, sediment; acetonitrile HPLC 0.05 mg/kg the procedures involve Celite Di Prima et al. (1978)
aquatic and forest liquid-liquid partition, and
foliage; fish and Florisil-aluminasilica gel
shellfish; animal column chromatography;
tissues 20 g sample
Crops acetone-hexane GLC-ECD 0.20 mg/kg Lawrence & Sundaram
(1+4) (1976); Di Prima (1976)
Soybean acetonitrile for process GC-ECD 0.05 mg/kg after hydrolysis and Lawrence & Sundaram
fractions, hulls and meal; derivatization (1976); Di Prima (1976)
hexane-acetonitrile for
oil
Water dichloromethane TLC 0.1 mg/kg Singh & Kaira (1989)
Table 4 (Con't)
Sample type Extraction/clean-up Analytical Limit of Comments Reference
method detection
Water & soil hexane/ethyl acetate; GC/ECD 0.05 ng 100 ml sample of water Smith et al. (1983)
evaporate to dryness; or 10 g sample of soil
dissolve residue in
benzene; derivatize with
trifluoroacetic anhydride
(with trimethylamine as
catalyst); LC on Florisil/
hexane: ethylether
(9:1 v/v)
Water ethyl acetate, KCl; GC/ECD 20 µg/litre % DEGS-LAC 728 on Cooke & Ober (1980)
derivatize with Chromosorb W-AW at 165°C
trifluoroacetic anhydride;
LC on Florisil
Exposure pads methylene chloride or HPLC/UV 3 ng 103.2 cm2 pads Bogus et al. (1985)
other solvents; clean-up (254 nm)
on SEPPAC C18; elute with
methanol
The HPLC method is recommended by CIPAC as a method of choice
(van Rossum et al., 1984). An alternative method for analysis of
residues in crops, soil, mud and water using Celite column
chromatography has been described by Di Prima et al. (1978). A gas
chromatographic method used on the acetylated derivative of
diflubenzuron was described by Worobey & Webster (1977) but has not
been applied to crop samples. The formation of 4-chloroaniline from
diflubenzuron under acidic conditions provides the basis for the GC
method.
Most of the recommended extraction procedures use acetonitrile or
acetone followed by n-hexane or dichloromethane.
Wie & Hammock (1982, 1984) developed three enzyme-linked
immunosorbent assays (ELISA) for diflubenzuron. All three assays were
based on antibodies raised against an N-carboxypropyl hapten of
diflubenzuron, while a diflubenzuron phenylacetic acid derivative
coupled to a carrier other than the immunizing antigen was used as the
coating antigen. None of these assays demonstrated significant cross-
reactivity with benzamide, urea, phenylurea or aniline components of
diflubenzuron. Each of the three assays was shown to be as sensitive
as the recommended HPLC methodology for the analysis of diflubenzuron
in water. Using ELISA, DFB was detected in milk at a level of
1-2 µg/litre without any sample extraction procedure.
Wimmer et al. (1991) developed a gas chromatography/mass
spectrometry (GC/MS) method using deuterated diflubenzuron as internal
standard and claimed high sensitivity.
The Joint FAO/WHO Codex Alimentarius Commission has given
recommendations for the methods of analysis to be used in determining
diflubenzuron residues (FAO/WHO, 1989).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Diflubenzuron does not occur naturally in the environment.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Diflubenzuron was first commercialized by Philips-Duphar BV, The
Netherlands (now Solvay Duphar BV). Solvay Duphar BV produces
diflubenzuron under the trade name Dimilin, but production figures are
not available.
Diflubenzuron is synthesized by the reaction of 2,6-difluoro-
benzamide with p-chlorophenyl isocyanate.
3.2.2 Formulations
Technical diflubenzuron is made into diflubenzuron 90%
concentrate by air-milling with a grinding aid and sufficient kaolin
to attain 90% active material. This is the product from which all
other formulations are made; these are listed below.
Dry products
* Dimilin 25W: a 25% wettable powder (more or less the standard
product)
* Dimilin 5W: a local Italian formulation containing 5% active
ingredient
* Various granular formulations used locally in specific
situations; these products are expected to be removed from the
market within 2 or 3 years
Water-based products
* Dimilin SC-48: a suspension concentrate containing 48% active
ingredient
* Dimilin SC-15: a suspension concentrate containing 15% active
ingredient for the French market
* Dimilin 4L, a suspension concentrate (0.4 kg/litre) containing
48% active ingredient for the USA market
Oil-based products
* Dimilin ODC-45: an oil-based dispersible concentrate containing
45% active ingredient to be diluted with mineral or vegetable oil
for spraying operations; this formulation may not be mixed with
water
* Dimilin OF-6: a dispersion in oil ready for direct spraying,
containing 6% active ingredient; this product must not be diluted
or mixed with water
* Dimilin 2F: an oil-based suspension concentrate containing 24%
active ingredient; it must not be diluted with water for spraying
and is a local formulation development for the USA market
The all-round formulations are Dimilin 25W, Dimilin 5W, Dimilin
SC-48, Dimilin SC-15 and Dimilin 4L. Dimilin ODC-45 was developed
specially for aerial spraying operations on non-food crops and
forestry. Dimilin OF-6 was developed for broadcast aerial spraying
operations to control locusts and grasshoppers. Dimilin 2F was
developed for those purposes where oil must be added to improve spray
deposit tenacity on crops such as cotton.
3.2.3 Uses
Diflubenzuron was the first benzoylphenylurea to be discovered.
Its insecticidal properties were first described by van Daalen et al.
(1972).
Diflubenzuron is effective as a stomach and contact insecticide,
acting by inhibiting chitin synthesis and so interfering with the
formation of the cuticle. Thus, all stages of insects that form new
cuticles should be susceptible to diflubenzuron exposure. It has no
systemic activity and does not penetrate plant tissue. Consequently,
plant sucking insects are generally unaffected, and this forms the
basis of its selectivity.
The recommended application rates for diflubenzuron are given in
Table 5.
Diflubenzuron is effective at a concentration of 15-300 mg
a.i./litre of water against leaf-feeding larvae and leaf miners in
forestry (Lymantria dispar, Thaumethopoea pityocampa), top fruit
( Cydia pomonella, Psylla spp), citrus (Phyllocoptruta oleivora),
field crops including cotton and soybeans (Anthonomus grandis,
Anticarsia gemmatalis), and horticultural crops (Pieris
brassicae). It is also effective against the larvae of Sciaridae
and Phoridae in mushrooms (1 g/m2 casing at case mixing or
as a drench in 2.5 litre of water to the finished casing), against
mosquito larvae (20-45 g/ha water surface) and against fly
larvae (Stomoxys calcitrans, Musca domestica) as a surface
application in animal housings (0.5-1.0 g/m2 surface) (Worthing &
Walker, 1987).
Table 5. Recommended application rates for diflubenzuron on
different cropsa
Pest Crop Rate/concentration
Apple rust mite apples/pears 0.01-0.015% a.i.
Codling moth apples/pears 0.01-0.015% a.i.
Leaf miners apples/pears 0.01-0.015% a.i.
Leaf rollers 0.01-0.02% a.i.
Pear suckers 0.01 (+0.3% crop oil)% a.i.
0.02-0.03% (without oil) a.i.
Winter moth 0.02% a.i.
Plum fruit moth plum 0.02% a.i.
Olive moth plum 0.01-0.02% a.i.
Citrus rust mite citrus fruit 0.0075-0.0125% a.i.
Citrus weevil citrus fruit 0.015-0.03% a.i.
Cotton ball weevil cotton 70 g/ha a.i.
Army worms cotton 150-300 g/ha a.i.
Army worms maize and Sorghum 70-150 g/ha a.i.
Cotton leaf worms 75-150 g/ha a.i.
Beet army worms peanuts 150-300 g/ha a.i.
Rice water weevil rice 75-150 g/ha a.i.
Fall army worms rice 70-100 g/ha a.i.
Mosquitoes up to 100 g/ha a.i.
Rice leaf rollers rice 75-250 g/ha a.i.
Various pests peanuts up to 75 g/ha a.i.
Various pests oil palm 50-150 g/ha a.i.
Various pests soybean 20-150 g/ha
a Solvay Duphar (1994)
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION AND FATE
4.1 Appraisal
Diflubenzuron is hydrolysed and photolysed slowly (see section
2.2). Residues in the aquatic environment may decrease rapidly, due
to adsorption by organic and inorganic matter. This process greatly
reduces the availability of diflubenzuron to aquatic organisms.
4.2 Transport and distribution between media
Diflubenzuron is generally applied either directly on plants or
on water for mosquito control.
4.2.1 Soil mobility
Diflubenzuron and its two formulations, Dimilin WP-25 and Dimilin
SC-48, were applied separately at 17.23, 51.69 and 155.07 µg a.i.
(corresponding to 70, 210 and 630 g a.i./ha) to the top layers of
columns (30 × 5.6 cm internal diameter) packed with either sandy or
clay loam forest soils. Water (1.25 litre) equivalent to 50.8 cm of
precipitation (representing an average annual rainfall) was allowed to
leach through each column. After leaching, the columns were divided
into five segments from bottom to top as follows: two 10-cm
increments, one 5-cm increment and two 2.5 cm increments.
Diflubenzuron residues in soils were extracted and analysed by HPLC.
Diflubenzuron mobility was low and did not increase with dosage. At a
deposit rate equivalent to 70 g a.i./ha, nearly all the residues were
found within the top 2.5 cm of the column. Even at 630 g a.i./ha,
only about 9% of the technical diflubenzuron, 7% of Dimilin SC-48 and
4% of Dimilin WP-25 moved below the 2.5 cm level in sandy loam. The
mobility of diflubenzuron in clay loam was lower than in sandy loam.
No residues were found below the 10 cm level or in the leachates in
either soil type at any dosage levels. The mobility of diflubenzuron
was also influenced by the additives present in the formulation, the
mobilities being in the following order: technical diflubenzuron
> Dimilin SC-48 > Dimilin WP-25 (Sundaram & Nott, 1989).
Helling (1985) investigated the movement of 14C-labelled
diflubenzuron in five soils and classified it as immobile in all of
them. After six treatments of cotton fields with 14C-labelled
diflubenzuron, most radioactivity was detected in the top 10 cm layer
of soil (Bull & Ivie, 1978). Diflubenzuron was found to adsorb very
rapidly to eight soil types (greater than 87% of the initial amount),
and there was only limited desorption (Booth et al., 1987).
Fourteen days after a single foliar application of 14C-labelled
diflubenzuron to field-grown cotton, only just over 10% of the dose
was absorbed into the plants. After 21 days and following a heavy
rainfall, approximately 23% of the applied diflubenzuron remained on
the treated leaf surfaces (Bull & Ivie, 1978).
No leaching occurred when 14C-labelled diflubenzuron was applied
to soil at the rate of 134.52 g/ha in an area with a normal rainfall
of 32 cm (Danhaus et al., 1976).
4.2.2 Dissipation
Diflubenzuron might enter an estuary either as a result of
flooding of treated supra-tidal mosquito breeding lagoons during
spring tides or from agricultural run-off after significant rainfall
(Cunningham & Myers, 1986).
Following aerial application at 67.26 g/ha to a watershed,
diflubenzuron was found to reach the stream channel. It was also
washed from the foliage as a result of several subsequent rainfalls
(Jones & Konchenderfer, 1988). However, these discharges were very
short-lived.
No residues were found in sediments from a lake treated with
diflubenzuron, suggesting rapid dissipation before or upon reaching
the bottom sediment (Apperson et al., 1978).
Pritchard & Bourquin (1981) demonstrated some affinity of
diflubenzuron for sediments, i.e. a partition coefficient of 380 in
simulated estuarine conditions. According to Cunningham & Myers
(1986), sediment appeared to be a major site for diflubenzuron
adsorption in a supra-tidal salt marsh. Carringer et al. (1975) found
that the organic content of soil was the most important factor in
determining adsorption and dissipation of diflubenzuron, and that
adsorption was inversely related to the water solubility of
diflubenzuron.
4.2.3 Evaporation
When diflubenzuron was applied as Dimilin WP 80 at a
concentration of 75 g/ha a.i. to bare soil (less than 1.5% organic
matter) and red kidney bean leaves, no significant evaporation was
measured under the following simulated climatological conditions: wind
speed 1-2 m/s; temperature 20-21°C; relative humidity 25-45% (van der
Laan-Straathof & Thus, 1994).
4.2.4 Crop residue data
When soybean and maize (corn) seedlings and potato tubers were
planted into soil treated with 3H- or 14C-labelled diflubenzuron,
only small amounts of radioactivity were taken up (Nimmo & de Wilde,
1976a). When 3H- or 14C-labelled diflubenzuron was applied to soil
in which the seedlings of wheat and rice were already present, the
14C residues in rice and wheat leaves were between 0.1 and 0.5 µg/kg.
The residues consisted mainly of 4-chlorophenylurea and polar
conjugates. The 14C residues in the wheat seeds were 0.02-0.04 mg/kg
and 3H residues were lower (Nimmo & de Wilde, 1976b).
The fate of diflubenzuron was studied following application to
soybeans both in greenhouse and field conditions. It was found that
75 to 100% of the total residues in soybean plants consisted of
unaltered diflubenzuron. There was no significant absorption or
translocation of residues. Less than 0.05 mg/kg of the total residues
was found in harvested soybean seed (Gustafson & Wargo, 1976).
The diflubenzuron spray residue on aerial parts of plants is
essentially stable. Leaf permeation does not occur and the compound
is not translocated to other parts of the plant. It has been
demonstrated that there is virtually no absorption, translocation or
metabolism of foliar-applied diflubenzuron on greenhouse cotton plants
(Nimmo & de Wilde, 1974; Nimmo, 1976a,b; Mansager et al., 1979).
Plant metabolism studies in corn, soybean, cabbage and apples
have demonstrated that no degradation products are found in plant
tissues. The only residue component present was the parent compound
diflubenzuron. Similar results were reported for cotton. Studies on
citrus fruits, apples and soybeans have confirmed that the only
residue component is the parent compound diflubenzuron. It can be
concluded that plants do not metabolize diflubenzuron (Nimmo & de
Wilde, 1974; Nimmo et al., 1978; Bull & Ivie, 1978; Nigg, 1989;
Joustra et al., 1989; Serra & Joustra, 1990; van Kampen & Joustra,
1991; Thus & van der Laan, 1993).
4.3 Transformation
4.3.1 Abiotic degradation
Under environmental conditions abiotic degradation of
diflubenzuron represents a very minor route of breakdown, owing to
the stability of the substance.
4.3.1.1 Photolysis
On the basis of results from a 15-day photolysis experiment, a
photolytic half-life of 40 days was calculated for diflubenzuron by
regression analysis (Boelhouwers et al., 1988a,b). After one week of
storage at 50°C or after one day at 100°C, there was no significant
decomposition (< 2%). The solid is stable to sunlight.
4.3.1.2 Hydrolysis
Abiotic hydrolysis of diflubenzuron in solution does not occur at
normal pH values. At pH 9 the hydrolytic half-life is 32.5 days,
4-chlorophenyl urea (4-CPU) and 2,6-difluorobenzoic acid (2,6-DFBA)
being the degradation products (Boelhouwers et al., 1988a).
High temperature (121°C) increases the degradation of
diflubenzuron in aqueous media at levels greatly above its solubility
in water and result in its rapid degradation to as many as seven
identified products: 4-CPU, 2,6-DFBA, 2,6-difluorobenzamide,
4-chloroaniline, N,N'-bis (4-chlorophenyl) urea, 1-(4-chlorophenyl)-
5-fluoro-2,4 (1H,3H)-quinazolinedione and 2-[(4-chlorophenyl) amino]-
6-fluorobenzoic acid. 4-Chloroaniline, N,N'-bis (4-chlorophenyl)
urea and 2[(4-chlorophenyl) amino]-6-fluorobenzoic acid were not
detected at lower temperatures (0.1 mg [14C]-diflubenzuron/litre
water or buffer at 36°C). 4-Chloroaniline was a major degradation
product of diflubenzuron in heat-treated samples, but it was not seen
at lower temperatures (Ivie et al., 1980).
The heat-induced degradation of diflubenzuron increased with
increasing pH (Schaefer & Dupras, 1976). Nigg et al. (1986) found
that high temperature and humidity significantly decreased the half-
life of diflubenzuron residues on citrus fruit.
4.3.2 Biodegradation
4.3.2.1 Water
a) Laboratory studies
Degradation in water can also occur through microbial action,
since in sterile water no breakdown or hydrolysis occurs (Boelhouwers
et al., 1988a). In freshly sampled ditch water, Nimmo & De Wilde
(1975a) demonstrated 50% degradation in 1-4 weeks. The breakdown
products were the same as the primary soil metabolites (4-CPU and
2,6-DFBA). Ivie et al. (1980) reported the same metabolites. Anton
et al. (1993) calculated the half-life of diflubenzuron in aerated and
unaerated tap water to be less than half a day and less than one day,
respectively.
When diflubenzuron (1.3 mg/litre) was added to an anaerobic silt
loam/water system, disappearance from the water phase showed a half-
life of 18 days and from the total system a half-life of 34 days.
The metabolites were 4-CPU and 2,6-DFBA, and almost no bound residue
was formed (Thus et al., 1991). After 90 days less than 2% of added
diflubenzuron remained in the system (Thus & van Dyk, 1991).
In another study, van der Laan-Straathof & Thus (1993) calculated
the half-life of diflubenzuron in water to be 2.5 days. Of the two
degradation products, 4-CPU underwent no further degradation but
2,6-DFBA was mineralized.
b) Outdoor models
Schaefer et al. (1980) reported that, in pasture water with a pH
of 8.2 and afternoon temperatures as high as 38-40°C, there was a
decline from an initial nominal concentration of 30 µg/litre to a
one-hour measured concentration of 20.3 µg/litre and subsequently to
21.6, 13.6, 4.4, and 2.4 µg/litre on days 1, 2, 3, and 4 respectively.
Schaefer & Dupras (1976) applied two formulations of
diflubenzuron (a wettable powder and a flowable formulation) to
artificial ponds of 1 m2 surface area containing 318 litres of pond
water. An initial concentration of 80 µg/litre decreased to 50%
within about 2 days. The diflubenzuron residue level after one week
was 2-3 µg/litre.
The half-life of diflubenzuron (1 µg/litre) in the aqueous
fraction of sludge experiments was 4-15 h (Booth et al., 1987), and
the half-life in sea water was reported to be less than 4 days
(Schimmel et al., 1983). Cunningham & Myers (1986) estimated a half-
life of less than 1 day for residues of diflubenzuron in water
following three applications of 0.4% granules and three applications
of 25% WP at a rate of 45 g a.i./ha to a supra-tidal salt marsh.
Madder & Lockhart (1980) studied model ponds (20 m2) to which
Dimilin WP-25 was applied at 56 g/ha (equivalent to 11.2 µg/litre).
For an unexplained reason, the measured concentration reached a
maximum value of about 17.5 µg/litre, 4 days after treatment. It
decreased by around 50% during the next 5 days. A residue of
2 µg/litre remained 2 weeks after application. On the basis of a
bioassay, a diflubenzuron half-life of about 3 days was calculated.
Collwell & Schaefer (1980) applied diflubenzuron to five
experimental ponds (each 100 m2) at a mean concentration of
13 µg/litre. The residue levels in water declined to an average of
7.2 µg/litre after 24 h.
In a study by Sarkar (1982), a 3 × 1 × 0.3 m open tank containing
water was sprayed with a dispersion of Dimilin WP-25. Three
subsequent applications were made, giving diflubenzuron concentrations
of 25, 35 and 50 µg/litre, respectively. These concentrations
decreased to 50% in about 3-4 days.
Pritchard & Bourquin (1981) studied the environmental fate of
diflubenzuron under simulated estuarine conditions in a laboratory
continuous-flow estuarine system and a static test system. The
hydrolytic half-life of diflubenzuron was 17 days in the static test
system, whereas the biological half-life was 5 days. 4-Chloroaniline
was not detected in either of the systems.
Thus & van der Laan-Straathof (1994) studied the fate of
diflubenzuron in two model ditch systems. Diflubenzuron was added at
a concentration of 0.94 mg/kg to two sediments (sandy loam and silt
loam), both of which were covered with aerated surface water. It
disappeared rapidly from the water phase through degradation and
adsorption to the sediment, the half-lives being 1.9 and 1.1 days,
respectively. Dissipation of diflubenzuron from the complete sandy
loam and silt loam systems occurred with half-lives of 25 and 10 days,
respectively. The metabolites (> 1% of the added diflubenzuron)
consisted of CO2, 4-CPU and 2,6-DFBA.
c) Field studies
Apperson et al. (1978) described the treatment of three farm
ponds with diflubenzuron levels of 2.5, 5 and 10 µg/litre, and a lake
with 5 µg/litre. Shortly after the application, a rapid decline in
diflubenzuron residues occurred, resulting in half-life values of only
a few days. In the lake no residues were found in the sediment
samples, suggesting that diflubenzuron was rapidly dissipated before,
or upon reaching, the bottom sediment.
Hester (1982) applied diflubenzuron at 0.045 kg a.i./ha to
specially constructed estuarine ponds. The water residue levels
decreased rapidly from 7.5 to 2 µg/litre in 2-3 days (study II) and
from 3.3 to 0.6 µg/litre in 7 days (study I).
d) Discussion and appraisal
The rate of decrease in diflubenzuron concentration after
application of the formulated product to natural waters depends on the
combined action of many environmental factors. Factors affecting the
degradation rate of diflubenzuron include the acidity (pH), the
relative local abundance of soil and organic debris, and the water
depth.
Half-life values vary from less than 4 days to 4 weeks in
laboratory experiments.
The use of artificial ponds or basins, preferably outdoors,
yields more relevant data and fairly consistent results. Dissipation
half-life values vary from 1-5 days after diflubenzuron has been
applied at recommended rates.
The dissipation half-life of diflubenzuron in the aquatic
environment is between one day and one week in most cases, depending
on the properties of the applied formulation and on the
characteristics of the application site. The presence of organic
sediments (hydrosoil, plant debris) and a relatively high local
temperature are factors that particularly accelerate the disappearance
of diflubenzuron.
4.3.2.2 Soil
a) Mobility in soil
Diflubenzuron is immobile in soil, as demonstrated by Helling
(1985) in column leaching experiments and Booth et al. (1987) in
adsorption-desorption studies with eight soil types.
The work of Carringer et al. (1975) suggests that soil organic
matter is an important parameter in soil adsorption. Due to its
immobility in soil, diflubenzuron is not likely to contaminate
groundwater by vertical movement in soil or to contaminate open water
by lateral movement in groundwater.
This has been confirmed in studies carried out in field soils
with growth of citrus fruits (Verhey, 1991a; Kramer, 1991), apple
(Kramer, 1990, Verhey, 1991b), soybean (Kramer, 1992b) and cotton
(Kramer, 1992a). After three applications of diflubenzuron (Dimilin
25W) at normal rates, most residue was found in the top 15 cm of soil
and no residue was encountered below 30 cm.
b) Degradation in soil
The rates of disappearance of technical diflubenzuron applied at
10 mg/kg on quartz sand to natural sandy loam and muck soils were
significantly greater than for the corresponding sterilized soils
(e.g., 2-12% and 80-87% diflubenzuron, respectively, remaining at
12 weeks), demonstrating that soil microorganisms play a major role in
their degradation (Chapman et al., 1985).
Diflubenzuron is very rapidly hydrolysed in soil. The half-life
time is 2 days to one week. The primary metabolites are 2,6-DFBA and
4-CPU. The process is microbial, since in sterilized soil no breakdown
occurs. The rate of breakdown is strongly dependent on the particle
size of diflubenzuron (see Fig. 1) (Nimmo et al., 1984, 1986).
The half-life in water in alkaline pastures is 1 day and in
neutral lake water it is from 10 to 15 days (Nimmo & de Wilde, 1975a).
Metabolic routes other than 4-CPU and 2,6-DFBA are virtually
irrelevant. Both primary metabolites are further metabolized,
2,6-DFBA with a half-life of about 4 weeks and 4-CPU with a
dissipation time of 1 to 3 months. Radiolabelling of both primary
metabolites and of a carbon atom in the ureido bridge shows carbon
dioxide development from mineralization. However, both the benzoic
acid ring carbon and the ureido bridge carbon are mineralized
much faster than the aniline moiety carbon, suggesting that
para-chloroaniline (PCA) is a major secondary metabolite that is
virtually irreversibly bound to soil (Bollag et al., 1978; Mansager et
al., 1979; Nimmo et al., 1984, 1986, 1990).
Empirical formula C14H9ClF2N2O2
Common name Diflubenzuron
Common trade names Dimilin; Micromite; Vigilante
Common abbreviation DFB
IUPAC name 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)-
urea
CAS chemical name N-[[(4-chlorophenyl) amino] carbonyl]-
2,6-difluorobenzamide
CAS registry number 35367-38-5
RTECS registry number YS6200000
Technical diflubenzuron contains > 95% pure compound.
2.2 Physical and chemical properties
Diflubenzuron is an odourless white crystalline solid. It is
almost insoluble in water and poorly soluble in apolar organic
solvents. In polar to very polar solvents, the solubility is moderate
to good, e.g., in acetone it is 6.5 g/litre at 20°C. Diflubenzuron is
highly soluble in N-methylpyrolidone (200 g/litre), dimethyl-
sulfoxide and dimethylformamide (both 120 g/litre).
Some physical and chemical properties of diflubenzuron are given
in Table 3.
Table 3. Physical and chemical properties of diflubenzuron
Relative molecular mass 310.7
Melting point technical > 95% 210-230°C
> 99% pure 230-232°C
Vapour pressure at 25°C 0.00012 mPa
Volatility
solid material < 4%
from water pH 5.6 < 2% (virtually non-volatile)
Specific gravity 1.56
n-Octanol/water partition coefficient
(log Kow) 5000
Solubility in water (at 25°C and pH 5.6) 8 × 10-5 g/litre
Stability in water (0.0001 g/litre 4% decomposition after 3 weeks at pH 5
in the dark) 8% decomposition after 3 weeks at pH 7
26% decomposition after 3 weeks at pH 91
2.3 Conversion factor
1 ppm = 12.7 mg/m3 at 25°C
1 mg/m3 = 0.079 ppm at 25°C
2.4 Analytical methods
Analytical methods for determining diflubenzuron in crops, soil,
water and biological samples are summarized in Table 4.
A review of the analytical methods has been presented by Rabenort
et al. (1978). Two general types of assay procedures for
diflubenzuron are available: high performance liquid chromatography
(HPLC) and gas chromatography (GC).
Table 4. Methods for the determination of diflubenzuron residues
Sample type Extraction/clean-up Analytical Limit of Comments Reference
method detection
Crops, soil, water dichloromethane; clean-up HPLC 0.03 mg/kg Rabenort et al. (1978)
on a Florisil column
Milk ethyl acetate HPLC 0.1 mg/kg Corley et al. (1974)
Crops acetone (n-hexane) HPLC 0.01 mg/kg Nakayama (1977a)
Apples acetonitrile HPLC 0.008 mg/kg Goto (1977a)
Tea acetone/dichloromethane HPLC 0.1 mg/kg Nakayama (1977b)
Tea acetone or water HPLC 0.2 mg/kg Goto (1977b)
Crops, soil, sediment; acetonitrile HPLC 0.05 mg/kg the procedures involve Celite Di Prima et al. (1978)
aquatic and forest liquid-liquid partition, and
foliage; fish and Florisil-aluminasilica gel
shellfish; animal column chromatography;
tissues 20 g sample
Crops acetone-hexane GLC-ECD 0.20 mg/kg Lawrence & Sundaram
(1+4) (1976); Di Prima (1976)
Soybean acetonitrile for process GC-ECD 0.05 mg/kg after hydrolysis and Lawrence & Sundaram
fractions, hulls and meal; derivatization (1976); Di Prima (1976)
hexane-acetonitrile for
oil
Water dichloromethane TLC 0.1 mg/kg Singh & Kaira (1989)
Table 4 (Con't)
Sample type Extraction/clean-up Analytical Limit of Comments Reference
method detection
Water & soil hexane/ethyl acetate; GC/ECD 0.05 ng 100 ml sample of water Smith et al. (1983)
evaporate to dryness; or 10 g sample of soil
dissolve residue in
benzene; derivatize with
trifluoroacetic anhydride
(with trimethylamine as
catalyst); LC on Florisil/
hexane: ethylether
(9:1 v/v)
Water ethyl acetate, KCl; GC/ECD 20 µg/litre % DEGS-LAC 728 on Cooke & Ober (1980)
derivatize with Chromosorb W-AW at 165°C
trifluoroacetic anhydride;
LC on Florisil
Exposure pads methylene chloride or HPLC/UV 3 ng 103.2 cm2 pads Bogus et al. (1985)
other solvents; clean-up (254 nm)
on SEPPAC C18; elute with
methanol
The HPLC method is recommended by CIPAC as a method of choice
(van Rossum et al., 1984). An alternative method for analysis of
residues in crops, soil, mud and water using Celite column
chromatography has been described by Di Prima et al. (1978). A gas
chromatographic method used on the acetylated derivative of
diflubenzuron was described by Worobey & Webster (1977) but has not
been applied to crop samples. The formation of 4-chloroaniline from
diflubenzuron under acidic conditions provides the basis for the GC
method.
Most of the recommended extraction procedures use acetonitrile or
acetone followed by n-hexane or dichloromethane.
Wie & Hammock (1982, 1984) developed three enzyme-linked
immunosorbent assays (ELISA) for diflubenzuron. All three assays were
based on antibodies raised against an N-carboxypropyl hapten of
diflubenzuron, while a diflubenzuron phenylacetic acid derivative
coupled to a carrier other than the immunizing antigen was used as the
coating antigen. None of these assays demonstrated significant cross-
reactivity with benzamide, urea, phenylurea or aniline components of
diflubenzuron. Each of the three assays was shown to be as sensitive
as the recommended HPLC methodology for the analysis of diflubenzuron
in water. Using ELISA, DFB was detected in milk at a level of
1-2 µg/litre without any sample extraction procedure.
Wimmer et al. (1991) developed a gas chromatography/mass
spectrometry (GC/MS) method using deuterated diflubenzuron as internal
standard and claimed high sensitivity.
The Joint FAO/WHO Codex Alimentarius Commission has given
recommendations for the methods of analysis to be used in determining
diflubenzuron residues (FAO/WHO, 1989).
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Diflubenzuron does not occur naturally in the environment.
3.2 Anthropogenic sources
3.2.1 Production levels and processes
Diflubenzuron was first commercialized by Philips-Duphar BV, The
Netherlands (now Solvay Duphar BV). Solvay Duphar BV produces
diflubenzuron under the trade name Dimilin, but production figures are
not available.
Diflubenzuron is synthesized by the reaction of 2,6-difluoro-
benzamide with p-chlorophenyl isocyanate.
3.2.2 Formulations
Technical diflubenzuron is made into diflubenzuron 90%
concentrate by air-milling with a grinding aid and sufficient kaolin
to attain 90% active material. This is the product from which all
other formulations are made; these are listed below.
Dry products
* Dimilin 25W: a 25% wettable powder (more or less the standard
product)
* Dimilin 5W: a local Italian formulation containing 5% active
ingredient
* Various granular formulations used locally in specific
situations; these products are expected to be removed from the
market within 2 or 3 years
Water-based products
* Dimilin SC-48: a suspension concentrate containing 48% active
ingredient
* Dimilin SC-15: a suspension concentrate containing 15% active
ingredient for the French market
* Dimilin 4L, a suspension concentrate (0.4 kg/litre) containing
48% active ingredient for the USA market
Oil-based products
* Dimilin ODC-45: an oil-based dispersible concentrate containing
45% active ingredient to be diluted with mineral or vegetable oil
for spraying operations; this formulation may not be mixed with
water
* Dimilin OF-6: a dispersion in oil ready for direct spraying,
containing 6% active ingredient; this product must not be diluted
or mixed with water
* Dimilin 2F: an oil-based suspension concentrate containing 24%
active ingredient; it must not be diluted with water for spraying
and is a local formulation development for the USA market
The all-round formulations are Dimilin 25W, Dimilin 5W, Dimilin
SC-48, Dimilin SC-15 and Dimilin 4L. Dimilin ODC-45 was developed
specially for aerial spraying operations on non-food crops and
forestry. Dimilin OF-6 was developed for broadcast aerial spraying
operations to control locusts and grasshoppers. Dimilin 2F was
developed for those purposes where oil must be added to improve spray
deposit tenacity on crops such as cotton.
3.2.3 Uses
Diflubenzuron was the first benzoylphenylurea to be discovered.
Its insecticidal properties were first described by van Daalen et al.
(1972).
Diflubenzuron is effective as a stomach and contact insecticide,
acting by inhibiting chitin synthesis and so interfering with the
formation of the cuticle. Thus, all stages of insects that form new
cuticles should be susceptible to diflubenzuron exposure. It has no
systemic activity and does not penetrate plant tissue. Consequently,
plant sucking insects are generally unaffected, and this forms the
basis of its selectivity.
The recommended application rates for diflubenzuron are given in
Table 5.
Diflubenzuron is effective at a concentration of 15-300 mg
a.i./litre of water against leaf-feeding larvae and leaf miners in
forestry (Lymantria dispar, Thaumethopoea pityocampa), top fruit
( Cydia pomonella, Psylla spp), citrus (Phyllocoptruta oleivora),
field crops including cotton and soybeans (Anthonomus grandis,
Anticarsia gemmatalis), and horticultural crops (Pieris
brassicae). It is also effective against the larvae of Sciaridae
and Phoridae in mushrooms (1 g/m2 casing at case mixing or
as a drench in 2.5 litre of water to the finished casing), against
mosquito larvae (20-45 g/ha water surface) and against fly
larvae (Stomoxys calcitrans, Musca domestica) as a surface
application in animal housings (0.5-1.0 g/m2 surface) (Worthing &
Walker, 1987).
Table 5. Recommended application rates for diflubenzuron on
different cropsa
Pest Crop Rate/concentration
Apple rust mite apples/pears 0.01-0.015% a.i.
Codling moth apples/pears 0.01-0.015% a.i.
Leaf miners apples/pears 0.01-0.015% a.i.
Leaf rollers 0.01-0.02% a.i.
Pear suckers 0.01 (+0.3% crop oil)% a.i.
0.02-0.03% (without oil) a.i.
Winter moth 0.02% a.i.
Plum fruit moth plum 0.02% a.i.
Olive moth plum 0.01-0.02% a.i.
Citrus rust mite citrus fruit 0.0075-0.0125% a.i.
Citrus weevil citrus fruit 0.015-0.03% a.i.
Cotton ball weevil cotton 70 g/ha a.i.
Army worms cotton 150-300 g/ha a.i.
Army worms maize and Sorghum 70-150 g/ha a.i.
Cotton leaf worms 75-150 g/ha a.i.
Beet army worms peanuts 150-300 g/ha a.i.
Rice water weevil rice 75-150 g/ha a.i.
Fall army worms rice 70-100 g/ha a.i.
Mosquitoes up to 100 g/ha a.i.
Rice leaf rollers rice 75-250 g/ha a.i.
Various pests peanuts up to 75 g/ha a.i.
Various pests oil palm 50-150 g/ha a.i.
Various pests soybean 20-150 g/ha
a Solvay Duphar (1994)
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, TRANSFORMATION AND FATE
4.1 Appraisal
Diflubenzuron is hydrolysed and photolysed slowly (see section
2.2). Residues in the aquatic environment may decrease rapidly, due
to adsorption by organic and inorganic matter. This process greatly
reduces the availability of diflubenzuron to aquatic organisms.
4.2 Transport and distribution between media
Diflubenzuron is generally applied either directly on plants or
on water for mosquito control.
4.2.1 Soil mobility
Diflubenzuron and its two formulations, Dimilin WP-25 and Dimilin
SC-48, were applied separately at 17.23, 51.69 and 155.07 µg a.i.
(corresponding to 70, 210 and 630 g a.i./ha) to the top layers of
columns (30 × 5.6 cm internal diameter) packed with either sandy or
clay loam forest soils. Water (1.25 litre) equivalent to 50.8 cm of
precipitation (representing an average annual rainfall) was allowed to
leach through each column. After leaching, the columns were divided
into five segments from bottom to top as follows: two 10-cm
increments, one 5-cm increment and two 2.5 cm increments.
Diflubenzuron residues in soils were extracted and analysed by HPLC.
Diflubenzuron mobility was low and did not increase with dosage. At a
deposit rate equivalent to 70 g a.i./ha, nearly all the residues were
found within the top 2.5 cm of the column. Even at 630 g a.i./ha,
only about 9% of the technical diflubenzuron, 7% of Dimilin SC-48 and
4% of Dimilin WP-25 moved below the 2.5 cm level in sandy loam. The
mobility of diflubenzuron in clay loam was lower than in sandy loam.
No residues were found below the 10 cm level or in the leachates in
either soil type at any dosage levels. The mobility of diflubenzuron
was also influenced by the additives present in the formulation, the
mobilities being in the following order: technical diflubenzuron
> Dimilin SC-48 > Dimilin WP-25 (Sundaram & Nott, 1989).
Helling (1985) investigated the movement of 14C-labelled
diflubenzuron in five soils and classified it as immobile in all of
them. After six treatments of cotton fields with 14C-labelled
diflubenzuron, most radioactivity was detected in the top 10 cm layer
of soil (Bull & Ivie, 1978). Diflubenzuron was found to adsorb very
rapidly to eight soil types (greater than 87% of the initial amount),
and there was only limited desorption (Booth et al., 1987).
Fourteen days after a single foliar application of 14C-labelled
diflubenzuron to field-grown cotton, only just over 10% of the dose
was absorbed into the plants. After 21 days and following a heavy
rainfall, approximately 23% of the applied diflubenzuron remained on
the treated leaf surfaces (Bull & Ivie, 1978).
No leaching occurred when 14C-labelled diflubenzuron was applied
to soil at the rate of 134.52 g/ha in an area with a normal rainfall
of 32 cm (Danhaus et al., 1976).
4.2.2 Dissipation
Diflubenzuron might enter an estuary either as a result of
flooding of treated supra-tidal mosquito breeding lagoons during
spring tides or from agricultural run-off after significant rainfall
(Cunningham & Myers, 1986).
Following aerial application at 67.26 g/ha to a watershed,
diflubenzuron was found to reach the stream channel. It was also
washed from the foliage as a result of several subsequent rainfalls
(Jones & Konchenderfer, 1988). However, these discharges were very
short-lived.
No residues were found in sediments from a lake treated with
diflubenzuron, suggesting rapid dissipation before or upon reaching
the bottom sediment (Apperson et al., 1978).
Pritchard & Bourquin (1981) demonstrated some affinity of
diflubenzuron for sediments, i.e. a partition coefficient of 380 in
simulated estuarine conditions. According to Cunningham & Myers
(1986), sediment appeared to be a major site for diflubenzuron
adsorption in a supra-tidal salt marsh. Carringer et al. (1975) found
that the organic content of soil was the most important factor in
determining adsorption and dissipation of diflubenzuron, and that
adsorption was inversely related to the water solubility of
diflubenzuron.
4.2.3 Evaporation
When diflubenzuron was applied as Dimilin WP 80 at a
concentration of 75 g/ha a.i. to bare soil (less than 1.5% organic
matter) and red kidney bean leaves, no significant evaporation was
measured under the following simulated climatological conditions: wind
speed 1-2 m/s; temperature 20-21°C; relative humidity 25-45% (van der
Laan-Straathof & Thus, 1994).
4.2.4 Crop residue data
When soybean and maize (corn) seedlings and potato tubers were
planted into soil treated with 3H- or 14C-labelled diflubenzuron,
only small amounts of radioactivity were taken up (Nimmo & de Wilde,
1976a). When 3H- or 14C-labelled diflubenzuron was applied to soil
in which the seedlings of wheat and rice were already present, the
14C residues in rice and wheat leaves were between 0.1 and 0.5 µg/kg.
The residues consisted mainly of 4-chlorophenylurea and polar
conjugates. The 14C residues in the wheat seeds were 0.02-0.04 mg/kg
and 3H residues were lower (Nimmo & de Wilde, 1976b).
The fate of diflubenzuron was studied following application to
soybeans both in greenhouse and field conditions. It was found that
75 to 100% of the total residues in soybean plants consisted of
unaltered diflubenzuron. There was no significant absorption or
translocation of residues. Less than 0.05 mg/kg of the total residues
was found in harvested soybean seed (Gustafson & Wargo, 1976).
The diflubenzuron spray residue on aerial parts of plants is
essentially stable. Leaf permeation does not occur and the compound
is not translocated to other parts of the plant. It has been
demonstrated that there is virtually no absorption, translocation or
metabolism of foliar-applied diflubenzuron on greenhouse cotton plants
(Nimmo & de Wilde, 1974; Nimmo, 1976a,b; Mansager et al., 1979).
Plant metabolism studies in corn, soybean, cabbage and apples
have demonstrated that no degradation products are found in plant
tissues. The only residue component present was the parent compound
diflubenzuron. Similar results were reported for cotton. Studies on
citrus fruits, apples and soybeans have confirmed that the only
residue component is the parent compound diflubenzuron. It can be
concluded that plants do not metabolize diflubenzuron (Nimmo & de
Wilde, 1974; Nimmo et al., 1978; Bull & Ivie, 1978; Nigg, 1989;
Joustra et al., 1989; Serra & Joustra, 1990; van Kampen & Joustra,
1991; Thus & van der Laan, 1993).
4.3 Transformation
4.3.1 Abiotic degradation
Under environmental conditions abiotic degradation of
diflubenzuron represents a very minor route of breakdown, owing to
the stability of the substance.
4.3.1.1 Photolysis
On the basis of results from a 15-day photolysis experiment, a
photolytic half-life of 40 days was calculated for diflubenzuron by
regression analysis (Boelhouwers et al., 1988a,b). After one week of
storage at 50°C or after one day at 100°C, there was no significant
decomposition (< 2%). The solid is stable to sunlight.
4.3.1.2 Hydrolysis
Abiotic hydrolysis of diflubenzuron in solution does not occur at
normal pH values. At pH 9 the hydrolytic half-life is 32.5 days,
4-chlorophenyl urea (4-CPU) and 2,6-difluorobenzoic acid (2,6-DFBA)
being the degradation products (Boelhouwers et al., 1988a).
High temperature (121°C) increases the degradation of
diflubenzuron in aqueous media at levels greatly above its solubility
in water and result in its rapid degradation to as many as seven
identified products: 4-CPU, 2,6-DFBA, 2,6-difluorobenzamide,
4-chloroaniline, N,N'-bis (4-chlorophenyl) urea, 1-(4-chlorophenyl)-
5-fluoro-2,4 (1H,3H)-quinazolinedione and 2-[(4-chlorophenyl) amino]-
6-fluorobenzoic acid. 4-Chloroaniline, N,N'-bis (4-chlorophenyl)
urea and 2[(4-chlorophenyl) amino]-6-fluorobenzoic acid were not
detected at lower temperatures (0.1 mg [14C]-diflubenzuron/litre
water or buffer at 36°C). 4-Chloroaniline was a major degradation
product of diflubenzuron in heat-treated samples, but it was not seen
at lower temperatures (Ivie et al., 1980).
The heat-induced degradation of diflubenzuron increased with
increasing pH (Schaefer & Dupras, 1976). Nigg et al. (1986) found
that high temperature and humidity significantly decreased the half-
life of diflubenzuron residues on citrus fruit.
4.3.2 Biodegradation
4.3.2.1 Water
a) Laboratory studies
Degradation in water can also occur through microbial action,
since in sterile water no breakdown or hydrolysis occurs (Boelhouwers
et al., 1988a). In freshly sampled ditch water, Nimmo & De Wilde
(1975a) demonstrated 50% degradation in 1-4 weeks. The breakdown
products were the same as the primary soil metabolites (4-CPU and
2,6-DFBA). Ivie et al. (1980) reported the same metabolites. Anton
et al. (1993) calculated the half-life of diflubenzuron in aerated and
unaerated tap water to be less than half a day and less than one day,
respectively.
When diflubenzuron (1.3 mg/litre) was added to an anaerobic silt
loam/water system, disappearance from the water phase showed a half-
life of 18 days and from the total system a half-life of 34 days.
The metabolites were 4-CPU and 2,6-DFBA, and almost no bound residue
was formed (Thus et al., 1991). After 90 days less than 2% of added
diflubenzuron remained in the system (Thus & van Dyk, 1991).
In another study, van der Laan-Straathof & Thus (1993) calculated
the half-life of diflubenzuron in water to be 2.5 days. Of the two
degradation products, 4-CPU underwent no further degradation but
2,6-DFBA was mineralized.
b) Outdoor models
Schaefer et al. (1980) reported that, in pasture water with a pH
of 8.2 and afternoon temperatures as high as 38-40°C, there was a
decline from an initial nominal concentration of 30 µg/litre to a
one-hour measured concentration of 20.3 µg/litre and subsequently to
21.6, 13.6, 4.4, and 2.4 µg/litre on days 1, 2, 3, and 4 respectively.
Schaefer & Dupras (1976) applied two formulations of
diflubenzuron (a wettable powder and a flowable formulation) to
artificial ponds of 1 m2 surface area containing 318 litres of pond
water. An initial concentration of 80 µg/litre decreased to 50%
within about 2 days. The diflubenzuron residue level after one week
was 2-3 µg/litre.
The half-life of diflubenzuron (1 µg/litre) in the aqueous
fraction of sludge experiments was 4-15 h (Booth et al., 1987), and
the half-life in sea water was reported to be less than 4 days
(Schimmel et al., 1983). Cunningham & Myers (1986) estimated a half-
life of less than 1 day for residues of diflubenzuron in water
following three applications of 0.4% granules and three applications
of 25% WP at a rate of 45 g a.i./ha to a supra-tidal salt marsh.
Madder & Lockhart (1980) studied model ponds (20 m2) to which
Dimilin WP-25 was applied at 56 g/ha (equivalent to 11.2 µg/litre).
For an unexplained reason, the measured concentration reached a
maximum value of about 17.5 µg/litre, 4 days after treatment. It
decreased by around 50% during the next 5 days. A residue of
2 µg/litre remained 2 weeks after application. On the basis of a
bioassay, a diflubenzuron half-life of about 3 days was calculated.
Collwell & Schaefer (1980) applied diflubenzuron to five
experimental ponds (each 100 m2) at a mean concentration of
13 µg/litre. The residue levels in water declined to an average of
7.2 µg/litre after 24 h.
In a study by Sarkar (1982), a 3 × 1 × 0.3 m open tank containing
water was sprayed with a dispersion of Dimilin WP-25. Three
subsequent applications were made, giving diflubenzuron concentrations
of 25, 35 and 50 µg/litre, respectively. These concentrations
decreased to 50% in about 3-4 days.
Pritchard & Bourquin (1981) studied the environmental fate of
diflubenzuron under simulated estuarine conditions in a laboratory
continuous-flow estuarine system and a static test system. The
hydrolytic half-life of diflubenzuron was 17 days in the static test
system, whereas the biological half-life was 5 days. 4-Chloroaniline
was not detected in either of the systems.
Thus & van der Laan-Straathof (1994) studied the fate of
diflubenzuron in two model ditch systems. Diflubenzuron was added at
a concentration of 0.94 mg/kg to two sediments (sandy loam and silt
loam), both of which were covered with aerated surface water. It
disappeared rapidly from the water phase through degradation and
adsorption to the sediment, the half-lives being 1.9 and 1.1 days,
respectively. Dissipation of diflubenzuron from the complete sandy
loam and silt loam systems occurred with half-lives of 25 and 10 days,
respectively. The metabolites (> 1% of the added diflubenzuron)
consisted of CO2, 4-CPU and 2,6-DFBA.
c) Field studies
Apperson et al. (1978) described the treatment of three farm
ponds with diflubenzuron levels of 2.5, 5 and 10 µg/litre, and a lake
with 5 µg/litre. Shortly after the application, a rapid decline in
diflubenzuron residues occurred, resulting in half-life values of only
a few days. In the lake no residues were found in the sediment
samples, suggesting that diflubenzuron was rapidly dissipated before,
or upon reaching, the bottom sediment.
Hester (1982) applied diflubenzuron at 0.045 kg a.i./ha to
specially constructed estuarine ponds. The water residue levels
decreased rapidly from 7.5 to 2 µg/litre in 2-3 days (study II) and
from 3.3 to 0.6 µg/litre in 7 days (study I).
d) Discussion and appraisal
The rate of decrease in diflubenzuron concentration after
application of the formulated product to natural waters depends on the
combined action of many environmental factors. Factors affecting the
degradation rate of diflubenzuron include the acidity (pH), the
relative local abundance of soil and organic debris, and the water
depth.
Half-life values vary from less than 4 days to 4 weeks in
laboratory experiments.
The use of artificial ponds or basins, preferably outdoors,
yields more relevant data and fairly consistent results. Dissipation
half-life values vary from 1-5 days after diflubenzuron has been
applied at recommended rates.
The dissipation half-life of diflubenzuron in the aquatic
environment is between one day and one week in most cases, depending
on the properties of the applied formulation and on the
characteristics of the application site. The presence of organic
sediments (hydrosoil, plant debris) and a relatively high local
temperature are factors that particularly accelerate the disappearance
of diflubenzuron.
4.3.2.2 Soil
a) Mobility in soil
Diflubenzuron is immobile in soil, as demonstrated by Helling
(1985) in column leaching experiments and Booth et al. (1987) in
adsorption-desorption studies with eight soil types.
The work of Carringer et al. (1975) suggests that soil organic
matter is an important parameter in soil adsorption. Due to its
immobility in soil, diflubenzuron is not likely to contaminate
groundwater by vertical movement in soil or to contaminate open water
by lateral movement in groundwater.
This has been confirmed in studies carried out in field soils
with growth of citrus fruits (Verhey, 1991a; Kramer, 1991), apple
(Kramer, 1990, Verhey, 1991b), soybean (Kramer, 1992b) and cotton
(Kramer, 1992a). After three applications of diflubenzuron (Dimilin
25W) at normal rates, most residue was found in the top 15 cm of soil
and no residue was encountered below 30 cm.
b) Degradation in soil
The rates of disappearance of technical diflubenzuron applied at
10 mg/kg on quartz sand to natural sandy loam and muck soils were
significantly greater than for the corresponding sterilized soils
(e.g., 2-12% and 80-87% diflubenzuron, respectively, remaining at
12 weeks), demonstrating that soil microorganisms play a major role in
their degradation (Chapman et al., 1985).
Diflubenzuron is very rapidly hydrolysed in soil. The half-life
time is 2 days to one week. The primary metabolites are 2,6-DFBA and
4-CPU. The process is microbial, since in sterilized soil no breakdown
occurs. The rate of breakdown is strongly dependent on the particle
size of diflubenzuron (see Fig. 1) (Nimmo et al., 1984, 1986).
The half-life in water in alkaline pastures is 1 day and in
neutral lake water it is from 10 to 15 days (Nimmo & de Wilde, 1975a).
Metabolic routes other than 4-CPU and 2,6-DFBA are virtually
irrelevant. Both primary metabolites are further metabolized,
2,6-DFBA with a half-life of about 4 weeks and 4-CPU with a
dissipation time of 1 to 3 months. Radiolabelling of both primary
metabolites and of a carbon atom in the ureido bridge shows carbon
dioxide development from mineralization. However, both the benzoic
acid ring carbon and the ureido bridge carbon are mineralized
much faster than the aniline moiety carbon, suggesting that
para-chloroaniline (PCA) is a major secondary metabolite that is
virtually irreversibly bound to soil (Bollag et al., 1978; Mansager et
al., 1979; Nimmo et al., 1984, 1986, 1990).
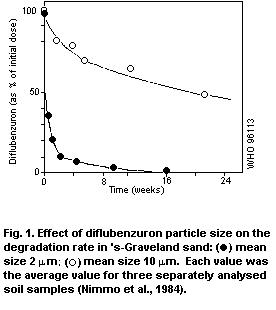 Even as a bound residue PCA is metabolized. Apparently, the
breakdown of 4-CPU in soil is a complex process in which PCA is a
transient metabolite or intermediate. The breakdown process leads to
products beyond the aniline structure. If PCA is applied to soil,
6 weeks incubation at 25°C yields 60% breakdown products of a
different nature (Bollag et al., 1978). The aniline itself is firmly
bound to soil and immobilized (Hsu & Bartha, 1974; Moreale & van
Bladel, 1976; Bollag et al., 1978; Simmons et al., 1989).
Fig. 2 shows metabolic pathways in soil.
The main metabolic pathway (over 90%) is hydrolysis, leading to
2,6-DFBA and 4-CPU. The second site of cleavage occurs at CN bonds 2
and 3. Both reactions lead to the formation of 2,6-difluorobenzamide
(DFBAM), which readily hydrolyses to 2,6-DFBA (Verloop & Ferrell,
1977; Nimmo et al., 1984).
The major metabolite in an activated sludge system is 4-CPU.
This is the major metabolite reported in most soil metabolism
experiments (Booth et al., 1987). 4-CPU was found to be converted
into bound residues with a half-life of 5-10 weeks. In the bound
residues, 4-CPU and PCA were present in roughly equal amounts after
2 months (Verloop & Ferrell, 1977). Free PCA was not found in soil
(Nimmo et al., 1986). The soil type and characteristics appear to
have no influence on the rate of degradation (Nimmo et al., 1984).
Metcalf et al. (1975) found no significant degradation of
diflubenzuron in a silty clay loam after incubation at 26.7°C for
periods of 1, 2 and 4 weeks. However, the authors did not take into
account the particle size of the soil, and used techniques that have a
negative influence on breakdown.
The rate of degradation of 14C- or 3H-diflubenzuron applied to
a mushroom growth medium (dose 2 g/m2) was between 30-50% in one
month. The main degradation products, 4-CPU and 2,6-DFBA, were
absorbed from the growth medium by the mushrooms, resulting in
residue levels of 0.1-0.6 mg/kg and 1-3 mg/kg, respectively (Nimmo &
de Wilde, 1977a). Free PCA or its further possible degradation
products were not present in the extractable residues (Nimmo & de
Wilde, 1975a; Verloop & Ferrell, 1977). Organic matter in soil
significantly contributed to the adsorption of chloroaniline compounds
and their immobilization (Hsu & Bartha, 1974; Moreale & van Bladel,
1976; Bollag et al., 1978).
Nimmo & de Wilde (1975a) found a degradation half-life of
0.5-1 week at a diflubenzuron concentration of 1 mg/kg (corresponding
to an application dose of approximately 300 g/ha). 2,6-DFBA was
degraded with a half-life of approximately 4 weeks, and 4-CPU with a
half-life of 2-3 months.
Even as a bound residue PCA is metabolized. Apparently, the
breakdown of 4-CPU in soil is a complex process in which PCA is a
transient metabolite or intermediate. The breakdown process leads to
products beyond the aniline structure. If PCA is applied to soil,
6 weeks incubation at 25°C yields 60% breakdown products of a
different nature (Bollag et al., 1978). The aniline itself is firmly
bound to soil and immobilized (Hsu & Bartha, 1974; Moreale & van
Bladel, 1976; Bollag et al., 1978; Simmons et al., 1989).
Fig. 2 shows metabolic pathways in soil.
The main metabolic pathway (over 90%) is hydrolysis, leading to
2,6-DFBA and 4-CPU. The second site of cleavage occurs at CN bonds 2
and 3. Both reactions lead to the formation of 2,6-difluorobenzamide
(DFBAM), which readily hydrolyses to 2,6-DFBA (Verloop & Ferrell,
1977; Nimmo et al., 1984).
The major metabolite in an activated sludge system is 4-CPU.
This is the major metabolite reported in most soil metabolism
experiments (Booth et al., 1987). 4-CPU was found to be converted
into bound residues with a half-life of 5-10 weeks. In the bound
residues, 4-CPU and PCA were present in roughly equal amounts after
2 months (Verloop & Ferrell, 1977). Free PCA was not found in soil
(Nimmo et al., 1986). The soil type and characteristics appear to
have no influence on the rate of degradation (Nimmo et al., 1984).
Metcalf et al. (1975) found no significant degradation of
diflubenzuron in a silty clay loam after incubation at 26.7°C for
periods of 1, 2 and 4 weeks. However, the authors did not take into
account the particle size of the soil, and used techniques that have a
negative influence on breakdown.
The rate of degradation of 14C- or 3H-diflubenzuron applied to
a mushroom growth medium (dose 2 g/m2) was between 30-50% in one
month. The main degradation products, 4-CPU and 2,6-DFBA, were
absorbed from the growth medium by the mushrooms, resulting in
residue levels of 0.1-0.6 mg/kg and 1-3 mg/kg, respectively (Nimmo &
de Wilde, 1977a). Free PCA or its further possible degradation
products were not present in the extractable residues (Nimmo & de
Wilde, 1975a; Verloop & Ferrell, 1977). Organic matter in soil
significantly contributed to the adsorption of chloroaniline compounds
and their immobilization (Hsu & Bartha, 1974; Moreale & van Bladel,
1976; Bollag et al., 1978).
Nimmo & de Wilde (1975a) found a degradation half-life of
0.5-1 week at a diflubenzuron concentration of 1 mg/kg (corresponding
to an application dose of approximately 300 g/ha). 2,6-DFBA was
degraded with a half-life of approximately 4 weeks, and 4-CPU with a
half-life of 2-3 months.
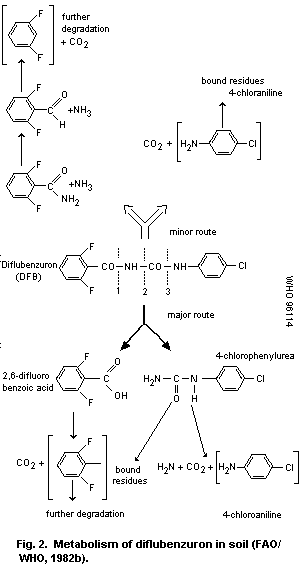 Walstra & Joustra (1990) applied 0.69 mg diflubenzuron/kg to
sandy loam. When incubated in the dark at 24°C, they obtained a half-
life for diflubenzuron of 50 h.
Diflubenzuron was found to be rapidly degraded by four soil fungi
( Fusarium sp., Cephalosporium sp., Penicillium sp. and Rhodotorula
sp.), the half-lives being 7, 13, 14 and 18 days, respectively
(Seuferer et al., 1979).
Several degradation studies on diflubenzuron (Dimilin 25 W) in
field soils have been conducted (Kramer, 1990, 1991, 1992a,b; Verhey,
1991a,b). Most of the degradation half-lives were between one and two
weeks, except in the case of the two Verhey studies, which yielded
half-lives of more than two months. In all studies, the metabolites
were 4-CPU and 2,6-DFBA.
No degradation of diflubenzuron by the soil microorganism
Pseudomonas putida was observed (Booth & Ferrell, 1977).
4.4 Bioaccumulation and biomagnification
Metcalf et al. (1975) studied the fate of 14C-diflubenzuron in a
laboratory model ecosystem. Diflubenzuron was clearly persistent in
some organisms, such as algae (Oedogonium cardiacum), snails
( Physa sp.), caterpillars (Estigmene acrea) and mosquito larvae
(Culex pipiens quinquefasciatus). The fish Gambusia affinis was
able to degrade diflubenzuron more efficiently. Diflubenzuron did not
biomagnify in the fish through food chain transfer. The biomagnifi-
cation was about 40-fold greater in mosquito larvae than in Gambusia
affinis.
When the bluegill sunfish (Lepomis macrochirus) was exposed to
10 µg diflubenzuron/litre for 24 h the tissues contained an average of
264 µg/kg. After 24 to 48 h of exposure, fish degraded and eliminated
the diflubenzuron. The excretory products were neither the parent
compound nor 4-CPU. The amount of diflubenzuron remaining in fish
tissues at various times was dependant on the reduction of residue
concentration in water. However, the potential for degradation and
elimination was very great (Schaefer et al., 1979).
A dynamic 42-day study was conducted by Burgess (1989) in order
to evaluate the bioconcentration of 14C-diflubenzuron by bluegill
sunfish (Lepomis macrochirus). A flow-through proportional diluter
system was used for a 28-day exposure period. Radioanalysis of
fillet, whole fish and visceral portions was performed throughout the
exposure period. Daily bioconcentration factors ranged from 34 to 200,
78 to 360, and 100 to 550 for fillet, whole fish and viscera,
respectively. Uptake tissue concentrations of 14C-diflubenzuron
ranged from 0.25 to 1.7 mg/kg for fillet, 0.58 to 3.3 mg/kg for whole
fish, and 0.75 to 4.7 mg/kg for viscera. To measure the elimination
of 14C-diflubenzuron, the test fish were placed in clean water for 14
days. Radioanalysis throughout the depuration period indicated 99%
depuration for each of fillet, whole fish and viscera. The fillet
concentration of 14C-diflubenzuron decreased from 1.6 mg/kg on day 28
of exposure to 0.012 mg/kg by day 14 of the depuration period. Whole
fish levels decreased from 3.3 mg/kg on day 28 of exposure to
0.038 mg/kg by the end of the study; whereas, viscera concentrations
dropped from 4.4 mg/kg on day 28 of exposure to 0.056 mg/kg by day 14
of depuration. BIOFAC modelling estimated the uptake rate constant
(K1) to be 370 (± 57) mg/kg fish per mg/litre water per day, the
depuration rate constant (K2) 1.2 (± 0.18) day-1, the time for 50%
depuration 0.60 (± 0.09) days, the bioconcentration factor (BCF) 320
(± 70), and the time to reach 90% or steady state 2.0 (± 0.31) days.
The BIOFAC-calculated BCF value was the same as the observed mean
whole fish BCF of 320 for days 3, 7, 14, 21 and 28. Fig. 3 shows the
accumulation, plateauing and depuration in this study.
During the study, no mortality or abnormal behaviour was observed
in the test fish. This appeared to indicate that the test fish were
in good health and would provide acceptable data for defining the
uptake/depuration potential of 14C-diflubenzuron. Analysis of fish
revealed parent compound (80%), 2,6-difluorobenzamide (10-13%) and
three other minor metabolites (one of which probably was 4-CPU). PCA
was demonstrated to be absent (sensitivity limit 0.01 mg/kg).
White crappies (Pomoxis annularis) contained residues from
355.1 to 62.2 µg/kg at 4 and 21 days, respectively, following
treatment of a lake with 5 µg diflubenzuron/litre (Apperson et al.,
1978).
Channel catfish (Ictalurus punctatus) did not bioaccumulate
diflubenzuron residues (less than 0.05 mg/kg) from treated soil in a
simulated lake ecosystem (Booth & Ferrell, 1977).
Assuming a biomagnification of 50-160, and that fish are capable
of rapidly depleting residues from the body, the likelihood of fish
accumulating significant residues of diflubenzuron is low (Apperson et
al., 1978; Schaefer et al., 1980).
4.5 Interaction with other physical, chemical or biological factors
Schaefer & Dupras (1976) reported that application of the
technical grade compound in an ethanol carrier or as a flowable liquid
formulation resulted in higher concentrations in the upper water
levels of mosquito ponds for a period of 3 days following spray
treatment than in the case of spray treatment with wettable powder
formulation (the actual formulation used for mosquito control
spraying).
Walstra & Joustra (1990) applied 0.69 mg diflubenzuron/kg to
sandy loam. When incubated in the dark at 24°C, they obtained a half-
life for diflubenzuron of 50 h.
Diflubenzuron was found to be rapidly degraded by four soil fungi
( Fusarium sp., Cephalosporium sp., Penicillium sp. and Rhodotorula
sp.), the half-lives being 7, 13, 14 and 18 days, respectively
(Seuferer et al., 1979).
Several degradation studies on diflubenzuron (Dimilin 25 W) in
field soils have been conducted (Kramer, 1990, 1991, 1992a,b; Verhey,
1991a,b). Most of the degradation half-lives were between one and two
weeks, except in the case of the two Verhey studies, which yielded
half-lives of more than two months. In all studies, the metabolites
were 4-CPU and 2,6-DFBA.
No degradation of diflubenzuron by the soil microorganism
Pseudomonas putida was observed (Booth & Ferrell, 1977).
4.4 Bioaccumulation and biomagnification
Metcalf et al. (1975) studied the fate of 14C-diflubenzuron in a
laboratory model ecosystem. Diflubenzuron was clearly persistent in
some organisms, such as algae (Oedogonium cardiacum), snails
( Physa sp.), caterpillars (Estigmene acrea) and mosquito larvae
(Culex pipiens quinquefasciatus). The fish Gambusia affinis was
able to degrade diflubenzuron more efficiently. Diflubenzuron did not
biomagnify in the fish through food chain transfer. The biomagnifi-
cation was about 40-fold greater in mosquito larvae than in Gambusia
affinis.
When the bluegill sunfish (Lepomis macrochirus) was exposed to
10 µg diflubenzuron/litre for 24 h the tissues contained an average of
264 µg/kg. After 24 to 48 h of exposure, fish degraded and eliminated
the diflubenzuron. The excretory products were neither the parent
compound nor 4-CPU. The amount of diflubenzuron remaining in fish
tissues at various times was dependant on the reduction of residue
concentration in water. However, the potential for degradation and
elimination was very great (Schaefer et al., 1979).
A dynamic 42-day study was conducted by Burgess (1989) in order
to evaluate the bioconcentration of 14C-diflubenzuron by bluegill
sunfish (Lepomis macrochirus). A flow-through proportional diluter
system was used for a 28-day exposure period. Radioanalysis of
fillet, whole fish and visceral portions was performed throughout the
exposure period. Daily bioconcentration factors ranged from 34 to 200,
78 to 360, and 100 to 550 for fillet, whole fish and viscera,
respectively. Uptake tissue concentrations of 14C-diflubenzuron
ranged from 0.25 to 1.7 mg/kg for fillet, 0.58 to 3.3 mg/kg for whole
fish, and 0.75 to 4.7 mg/kg for viscera. To measure the elimination
of 14C-diflubenzuron, the test fish were placed in clean water for 14
days. Radioanalysis throughout the depuration period indicated 99%
depuration for each of fillet, whole fish and viscera. The fillet
concentration of 14C-diflubenzuron decreased from 1.6 mg/kg on day 28
of exposure to 0.012 mg/kg by day 14 of the depuration period. Whole
fish levels decreased from 3.3 mg/kg on day 28 of exposure to
0.038 mg/kg by the end of the study; whereas, viscera concentrations
dropped from 4.4 mg/kg on day 28 of exposure to 0.056 mg/kg by day 14
of depuration. BIOFAC modelling estimated the uptake rate constant
(K1) to be 370 (± 57) mg/kg fish per mg/litre water per day, the
depuration rate constant (K2) 1.2 (± 0.18) day-1, the time for 50%
depuration 0.60 (± 0.09) days, the bioconcentration factor (BCF) 320
(± 70), and the time to reach 90% or steady state 2.0 (± 0.31) days.
The BIOFAC-calculated BCF value was the same as the observed mean
whole fish BCF of 320 for days 3, 7, 14, 21 and 28. Fig. 3 shows the
accumulation, plateauing and depuration in this study.
During the study, no mortality or abnormal behaviour was observed
in the test fish. This appeared to indicate that the test fish were
in good health and would provide acceptable data for defining the
uptake/depuration potential of 14C-diflubenzuron. Analysis of fish
revealed parent compound (80%), 2,6-difluorobenzamide (10-13%) and
three other minor metabolites (one of which probably was 4-CPU). PCA
was demonstrated to be absent (sensitivity limit 0.01 mg/kg).
White crappies (Pomoxis annularis) contained residues from
355.1 to 62.2 µg/kg at 4 and 21 days, respectively, following
treatment of a lake with 5 µg diflubenzuron/litre (Apperson et al.,
1978).
Channel catfish (Ictalurus punctatus) did not bioaccumulate
diflubenzuron residues (less than 0.05 mg/kg) from treated soil in a
simulated lake ecosystem (Booth & Ferrell, 1977).
Assuming a biomagnification of 50-160, and that fish are capable
of rapidly depleting residues from the body, the likelihood of fish
accumulating significant residues of diflubenzuron is low (Apperson et
al., 1978; Schaefer et al., 1980).
4.5 Interaction with other physical, chemical or biological factors
Schaefer & Dupras (1976) reported that application of the
technical grade compound in an ethanol carrier or as a flowable liquid
formulation resulted in higher concentrations in the upper water
levels of mosquito ponds for a period of 3 days following spray
treatment than in the case of spray treatment with wettable powder
formulation (the actual formulation used for mosquito control
spraying).
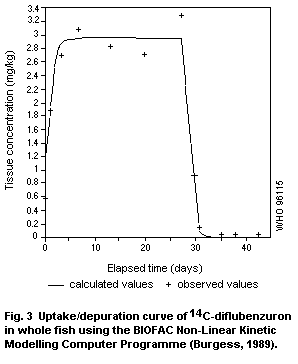 Seuferer et al. (1979) reported that the soil microorganisms
Rhodotorula sp., Penicillium sp. and Cephalosporium sp. cannot
utilize diflubenzuron as a sole carbon and energy source. However,
accelerated breakdown of diflubenzuron occurred in the presence of
these organisms.
4.6 Ultimate fate following use
It appears that after direct spraying diflubenzuron is persistent
on foliage, it remains almost completely at the site of application on
the surface, and it does not penetrate the plants.
Diflubenzuron is readily degraded in soils of various types and
origin under aerobic or anaerobic conditions with a half-life in the
range of 0.5 to 1 week. It is metabolized by microorganisms
principally to 4-CPU and 2,6-DFBA. The latter is unstable with a
half-life of 3-5 days (Nimmo et al., 1984) to 4 weeks (Verloop &
Ferrell, 1977). The half-life of 4-CPU is about 6 weeks (Nimmo et
al., 1984). Free PCA has not been detected in soil.
In spite of rapid degradation in soil, small amounts of residue
(up to 1 mg/kg, depending on ageing time and growth stage of plants)
may be taken up by crops in treated soil (Thus et al., 1994).
Field applications of diflubenzuron produce soil residues which
might possibly lead to residues in rotational crops by re-uptake from
soil.
Studies with direct applications to field water show a moderate
persistence of diflubenzuron in water. Half-life values average one
week or less. This rapid rate of loss may be more dependent on
adsorption to organic matter than on microbial degradation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
No information is available on air concentrations of
diflubenzuron.
5.1.2 Water
A total of 1160 ha of insect-infested forest in Finland was
sprayed with diflubenzuron (25% WP) from a fixed-winged aircraft at an
application rate of 75 g a.i. in 50 litres water per ha. The residues
in "run-off" water (gathered in specially dug pits adjacent to the
sprayed area) decreased from 5 µg/litre one day after spraying to
0.1 µg/litre after 2 months. The concentration in water in open pits
was 0.1 µg/litre 1 and 7 days after application and 0.2 µg/litre
1 month after application. After 2 months no residues were detected.
All water samples taken from outside the treated area contained less
than 0.1 µg/litre (the limit of sensitivity) (Mutanen et al., 1988).
Diflubenzuron was found in the water of the Fraser River, Canada,
up to 71 days following application with diflubenzuron (1% granular
formulation) at a rate of 4.5 kg/ha (45 g a.i./ha). The peak value
was 1.8 µg/litre 8 days after treatment (Wan & Wilson, 1977).
After aerial application of diflubenzuron (25% WP formulation) to
two forest ponds in Canada, the maximum residue levels in water,
sediment, aquatic plants and fish were 13.82 µg/litre (at 1 h),
0.24 mg/kg (at 1 day), 0.36 mg/kg (at 1 day) and 0.11 mg/kg
(at 1 day), respectively. The rate of dissipation was rapid, non-
detectable levels being reached in 20 days for water, 5 days in
aquatic plants and 3 days in fish (Kingsbury et al., 1987).
A pond in Salt Lake County, Utah, USA, was treated with three
applications of diflubenzuron at a rate of 280.25 g a.i./ha.
Diflubenzuron was found at less than 0.05 mg/litre 4 days following
treatment (Booth et at., 1987).
Residues in three farm ponds in California treated with
diflubenzuron (2.5, 5 and 10 µg/litre) averaged 1.9, 4.6 and
9.8 µg/litre, respectively, 1-4 h after the applications. They
declined steadily averaging 0.5, 0.3 and 0.2 µg/litre, respectively,
2 weeks later. Residues in a small lake treated at 5 µg/litre
averaged 3.3 µg/litre following treatment and 0.4 µg/litre after
35 days. No residues were found in sediment samples taken post-
treatment (Apperson et al., 1978).
One hour after a single application of 45 g diflubenzuron/ha to
brackish water pools the residues in water and in sediment were
3.6 µg/litre and 80 µg/kg, respectively. The concentration in
sediment increased to 520 µg/kg after 1 day and reached its maximum of
780 µg/kg 4 days following application (Hester et al., 1986). After
6 applications of diflubenzuron at a rate of 145.73 g/ha to Utah Lake,
USA, the residues in sediments were less than 0.05 mg/kg (Booth et
al., 1987).
Other field studies with similar results have been reported by
Anon (1980), Smith & Edmunds (1985), Van Den Berg (1986), Huber &
Collins (1987), Jones & Kochenderfer (1988), Huber & Manchester
(1988), Downey (1990) and Sundaram et al. (1991). It is clear that a
variety of application scenarios will result in measurable residues of
diflubenzuron in water (Table 6).
The overall conclusion is that diflubenzuron residues in stagnant
water dissipate rapidly within days. In flowing water, e.g., in
wooded areas, diflubenzuron residues may peak shortly after rainfall
but such peak concentrations are very transient in nature.
5.1.3 Food and feed
Data on residues in food resulting from treatment with
diflubenzuron have been summarized by FAO/WHO (1982a,b, 1985a,b,
1986a,b).
Residue data obtained from various countries showed residues in
apples below 1.0 mg diflubenzuron/kg at 2 weeks after the last
application at recommended rates. Residues in whole citrus fruit were
below 0.5 mg/kg 1 week after the last application at the recommended
rate. Residues in soybean seed and cottonseed were generally below
the limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron-treated soil, high
levels of the metabolite 2,6-DFBA are taken up from the soil.
Diflubenzuron was found at a level of 0.1 mg/kg, while the 2,6-DFBA
level was around 1 mg/kg (see chapter 4).
Residues in wild mushrooms after aerial application to forests in
Finland were on average 0.07 mg/kg 1 week after spraying with 75 g
diflubenzuron in 50 litre water per ha. In bilberries the residues
decreased on average from 0.2 mg/kg 1 day after spraying to 0.09 mg/kg
after 1 month (Mutanen et al., 1988).
Diflubenzuron applied as a wettable powder spray to growing
alfalfa at 20-100 g/ha showed initial residue levels of 1.8-8.5 mg/kg.
Residues of 0.3-1.5 mg/kg remained 22 days after applications (Lauren
et al., 1984).
Table 6. Summary and comparison of experimental parameters among key studies designed to measure environmental concentrations of
diflubenzuron in water
Medium Formulation a.i.% Method of Application Maximum Time for Minimum Time for References
treated application rate a.i. concentration maximum concentrationa minimum
concentration concentration
Farm ponds 25 WP 25 hand sprayer 2.5-10 µg/litre 1.9-9.8 µg/litre 1-4 h 0.5-0.2 µg/litre 14 days Apperson
(0.06-0.2 ha) from boat et al.
(1978)
Small lake 25 WP 25 hand sprayer 5 µg/litre 3.3 µg/litre 4 h 0.4 µg/litre 35 days Apperson
(18.6 ha) from boat et al.
(1978)
Pond W-25 25 hand-operated 0.28 kg/ha 56 µg/litre 96 h < 0.01 µg/litre 40 days Booth
spray et al.
applicator (1987)
Brackish 25 WP 25 clothes 0.045 kg/ha 7.5 µg/litre 48-72 h < 0.3 µg/litre 25-30 days Hester
pools sprinkler (1986)
Forest ponds 25 WP 25 aircraft 0.07 kg/ha 13.82 µg/litre 1 h < DL 20 days Kingsbury
(25 ha) (four et al.
atomizers) (1987)
Field plot 25 WP 25 fixed-wing 0.075 kg in 50 5.0 µg/litre 24 h < DL 60 days Mutanen
(1160 ha) aircraft litre water/ha et al.
(1988)
Fixed plots granular 1.0 aircraft 0.023 kg/ha, 1.8 µg/litre 192 h < DL 60 days Wan &
(3-40 ha) 0.46 kg/ha Wilson
(1977)
a DL = determination limit
After two soil applications of 67.26 g/ha, the residues of
diflubenzuron in the rotational crops (wheat, cabbage and onions) were
less than 0.01 mg/kg (Danhaus & Sieck, 1976).
Mian & Mulla (1983) studied the persistence of diflubenzuron in
stored wheat after applications of 1, 5 and 10 mg/kg. The residue
levels were 0.59, 2.75 and 5.00 mg/kg, respectively, 23 months after
treatment.
5.1.4 Forest plants and litter
The level of diflubenzuron residues in pine needles was on
average 3.0 mg/kg 1 day after application to the forest in Finland at
a rate of 75 g diflubenzuron in 50 litres water per ha. The level had
decreased to 0.2-0.3 mg/kg or was not detectable 2 months later
(Mutanen et al., 1988).
Booth et al. (1987) found diflubenzuron residues of less than
0.05 mg/kg in the forest litter 1, 4, 10 and 21 days after treatment
with 0.28 kg a.i./ha.
Sundaram (1986) studied the residues in a forest in Canada after
simulated aerial spraying of diflubenzuron in acetone and in fuel oil:
Arotex 3470 mixture, each at 90 g a.i. in 18 litre/ha. The residue
levels 1 h after application varied, respectively, from 23.8 to
30.6 µg/g in foliage and from 3.08 to 4.60 µg/g in litter. Forty-five
days after spraying the residue levels in foliage were 0.80 and
3.9 µg/g, respectively, for the above-mentioned formulations.
Spray deposit patterns and persistence of diflubenzuron in white
pine ( Pinus strobus L.) and sugar maple ( Acer saccharum Marsh.)
canopies, forest litter and soil were studied after aerial application
of a 250 g/kg wettable powder formulation (Dimilin WP-25) at
70 g a.i./ha, using three volume rates (2.5, 5 and 10 litres/ha), over
three blocks in a mixed forest near Kaladar, Ontario, Canada, during
1986 (Sundaram, 1991). In the spray block that received 10 litres/ha,
diflubenzuron persisted in foliage as long as 120 days after
treatment, but it lasted for only about a week in forest litter and
soil samples. At 2.5 and 5 litres/ha, diflubenzuron failed to persist
in foliage as long, and residues in litter and soil, which were barely
above the quantification limit, persisted only for a few days.
5.1.5 Aquatic organisms
Residues in fish are given in section 4.4.
5.2 General population exposure
Exposure of the general population to diflubenzuron via food and
drinking-water may occur.
Twelve volunteers with whole body dosimeters were exposed for 4 h
to Dimilin 25 W after simulated indoor treatment of carpets at
0.16 g/m2. Average deposition was 5.3 ± 2.3 µg diflubenzuron/cm2
carpet. Total dermal exposure ranged from 0.053 to 0.25 mg/kg body
weight per day to (average 0.15 ± 0.066 mg/kg body weight per day).
Assuming a dermal absorption of 0.2%, the total exposure via the
dermal route was calculated to be 0.0003 mg/kg body weight per day.
Air concentrations ranged from 10.2 to 32.4 µg/m3 during the first
4 h and were < 1 µg/m3 at 12-16 h. The total respiratory exposure
was calculated to be 0.0011 mg/kg body weight per day. The total
exposure, via the dermal and respiratory route, was calculated to be
0.0014 mg/kg body weight (Honeycutt, 1993).
5.3 Occupational exposure during manufacture, formulation or use
In a US Department of Agriculture report, human exposure via a
variety of exposure scenarios was estimated using standardized methods
and assumptions. The exposure scenarios included mixing and loading by
workers, via aircraft or truck spillage, and general public exposure
via the diet or resulting from occupational aerial spraying. Dermal
absorption of diflubenzuron was assumed to be 10%. Estimated
realistic doses for humans were < 0.003 mg/kg body weight per day
except where aircraft or truck spillages occurred, in which case
exposures were significantly higher. Estimated worst-case doses for
humans were < 0.01 mg/kg body weight per day, except where aircraft
or truck spillages occurred (USDA, 1985).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Diflubenzuron is absorbed from the digestive tract but only
poorly absorbed through the skin. Willems et al. (1980) found that in
rats the relative intestinal absorption diminished greatly with
increasing dose. Following a dose of 4 mg/kg body weight 42.5% was
absorbed, but only 3.7% of a 900 mg/kg body weight dose was absorbed.
Dermal absorption of 14C-diflubenzuron was only 0.2% when it was
applied to the shaved skin of rabbits as an aqueous micro-suspension
of 150 mg/kg (De Lange, 1979).
When applied dermally to cattle 14C-diflubenzuron was not
absorbed or degraded through the skin to any detectable degree (Ivie,
1978).
6.2 Distribution
Body tissues show little tendency to retain diflubenzuron.
Analysis of tissues for radiocarbon residues, 4 days (for sheep) or
7 days (for cows) after a single oral dose of 10 mg/kg body weight,
indicated that only the liver contained appreciable levels of
radioactivity, ranging from 2 to 4 mg/kg diflubenzuron equivalents
(Ivie, 1977).
More than one third of an oral diflubenzuron dose appeared in the
bile of a cannulated sheep (Ivie, 1977).
The highest 14C-diflubenzuron residue present in pig tissues
after a single oral dose of 5 mg/kg body weight was 0.43 mg/kg in the
gall bladder. All other tissue residue levels were found to be less
than 0.30 mg/kg (Opdycke et al., 1982a).
Twenty-two dairy cows were fed 14C-diflubenzuron (labelled in
both phenyl moieties) in a diet at dose levels of 0.05, 0.5, 5, 25 and
250 mg/kg feed for 28 days. Residues in blood, fat and muscle were
below the detection limit (0.0067-0.04 mg/kg) at all dose levels. They
were only detected following a dose of 250 mg/kg in the liver and
kidney where residues were 6.040 and 1.038 mg/kg, respectively.
Residues in milk were found at dose levels of 5 and 250 mg/kg, where
the highest levels of diflubenzuron were 0.009 and 0.20 mg/kg,
respectively (Smith & Merricks, 1976a).
In a study by Miller et al. (1976a), two dairy cows were fed
diflubenzuron at 0.25 or 1 mg/kg body weight per day for 4 months. A
third cow received an increased dosage of 8 to 16 mg/kg body weight
per day, the highest value being maintained for three months. In the
fat, liver and milk of the third cow, residues were 0.2, 0.13 and
0.02 mg/kg, respectively.
When dairy bull calves (four treated and four controls) received
diflubenzuron at 1.0 to 2.8 mg/kg body weight, residues were detected
only in the tissue samples of one bull (0.02 mg/kg in liver and
kidney, 0.04 mg/kg in the subcutaneous fat, and 0.08 mg/kg in the
renal and omental fat (Miller et al., 1979).
The maximum total residue in eggs 3 days after a single dose of
5 mg/kg 14C-diflubenzuron to hens was 0.248 mg/kg (Opdycke, 1976).
When laying hens were administered 14C-diflubenzuron at dose
levels 0.05, 0.5, and 5.0 mg/kg feed for 28 days, dose-related
residues ranging from 0.007 mg/kg at the lowest to 1.2 mg/kg at the
highest dose level were found in kidney, liver and fat. After 7 days
of withdrawal, residues in all tissues and eggs were below the
detection limit (0.0006-0.032 mg/kg) for all dose levels (Smith &
Merricks, 1976b).
When diflubenzuron was fed to white leghorn and black sex-linked
cross hens at a level of 10 mg/kg feed for 15 weeks, detectable
residues were found in eggs, liver and visceral fat. Residues were
significantly higher in eggs from white leghorn hens than in eggs from
black sex-linked cross hens, the average levels being 0.55 and
0.38 mg/kg, respectively (Miller et al., 1976b).
6.3 Metabolic transformation
The metabolic fate of diflubenzuron has been studied in various
species. Metabolic pathways of diflubenzuron are shown in Fig. 4
In rats and cows the major metabolic pathway involves
hydroxylation of the phenyl moieties of the compound. About 80% of the
metabolites in rat urine were identified as 2,6-difluoro-3-
hydroxydiflubenzuron and 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the benzoyl
ureido bridge. The major part was excreted as 2,6-DFBA and
constituted more than half of the urinary metabolites. 4-CPU was not
detected in bile or urine in a significant quantity (De Lange et al.,
1975; Willems et al., 1980).
The major metabolite in cow urine was 2,6-difluoro-3-hydroxy-
diflubenzuron (45%). Relatively small quantities of 4-chloro-2-
hydroxy- (1.6%) and 4-chloro-3-hydroxydiflubenzuron (3.7%) and the
scission products 4-CPU (0.6%), 2,6-DFBA (6.0%) and 2,6-difluoro-
hippuric acid (6.9%) were present (Ivie, 1978).
Seuferer et al. (1979) reported that the soil microorganisms
Rhodotorula sp., Penicillium sp. and Cephalosporium sp. cannot
utilize diflubenzuron as a sole carbon and energy source. However,
accelerated breakdown of diflubenzuron occurred in the presence of
these organisms.
4.6 Ultimate fate following use
It appears that after direct spraying diflubenzuron is persistent
on foliage, it remains almost completely at the site of application on
the surface, and it does not penetrate the plants.
Diflubenzuron is readily degraded in soils of various types and
origin under aerobic or anaerobic conditions with a half-life in the
range of 0.5 to 1 week. It is metabolized by microorganisms
principally to 4-CPU and 2,6-DFBA. The latter is unstable with a
half-life of 3-5 days (Nimmo et al., 1984) to 4 weeks (Verloop &
Ferrell, 1977). The half-life of 4-CPU is about 6 weeks (Nimmo et
al., 1984). Free PCA has not been detected in soil.
In spite of rapid degradation in soil, small amounts of residue
(up to 1 mg/kg, depending on ageing time and growth stage of plants)
may be taken up by crops in treated soil (Thus et al., 1994).
Field applications of diflubenzuron produce soil residues which
might possibly lead to residues in rotational crops by re-uptake from
soil.
Studies with direct applications to field water show a moderate
persistence of diflubenzuron in water. Half-life values average one
week or less. This rapid rate of loss may be more dependent on
adsorption to organic matter than on microbial degradation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
No information is available on air concentrations of
diflubenzuron.
5.1.2 Water
A total of 1160 ha of insect-infested forest in Finland was
sprayed with diflubenzuron (25% WP) from a fixed-winged aircraft at an
application rate of 75 g a.i. in 50 litres water per ha. The residues
in "run-off" water (gathered in specially dug pits adjacent to the
sprayed area) decreased from 5 µg/litre one day after spraying to
0.1 µg/litre after 2 months. The concentration in water in open pits
was 0.1 µg/litre 1 and 7 days after application and 0.2 µg/litre
1 month after application. After 2 months no residues were detected.
All water samples taken from outside the treated area contained less
than 0.1 µg/litre (the limit of sensitivity) (Mutanen et al., 1988).
Diflubenzuron was found in the water of the Fraser River, Canada,
up to 71 days following application with diflubenzuron (1% granular
formulation) at a rate of 4.5 kg/ha (45 g a.i./ha). The peak value
was 1.8 µg/litre 8 days after treatment (Wan & Wilson, 1977).
After aerial application of diflubenzuron (25% WP formulation) to
two forest ponds in Canada, the maximum residue levels in water,
sediment, aquatic plants and fish were 13.82 µg/litre (at 1 h),
0.24 mg/kg (at 1 day), 0.36 mg/kg (at 1 day) and 0.11 mg/kg
(at 1 day), respectively. The rate of dissipation was rapid, non-
detectable levels being reached in 20 days for water, 5 days in
aquatic plants and 3 days in fish (Kingsbury et al., 1987).
A pond in Salt Lake County, Utah, USA, was treated with three
applications of diflubenzuron at a rate of 280.25 g a.i./ha.
Diflubenzuron was found at less than 0.05 mg/litre 4 days following
treatment (Booth et at., 1987).
Residues in three farm ponds in California treated with
diflubenzuron (2.5, 5 and 10 µg/litre) averaged 1.9, 4.6 and
9.8 µg/litre, respectively, 1-4 h after the applications. They
declined steadily averaging 0.5, 0.3 and 0.2 µg/litre, respectively,
2 weeks later. Residues in a small lake treated at 5 µg/litre
averaged 3.3 µg/litre following treatment and 0.4 µg/litre after
35 days. No residues were found in sediment samples taken post-
treatment (Apperson et al., 1978).
One hour after a single application of 45 g diflubenzuron/ha to
brackish water pools the residues in water and in sediment were
3.6 µg/litre and 80 µg/kg, respectively. The concentration in
sediment increased to 520 µg/kg after 1 day and reached its maximum of
780 µg/kg 4 days following application (Hester et al., 1986). After
6 applications of diflubenzuron at a rate of 145.73 g/ha to Utah Lake,
USA, the residues in sediments were less than 0.05 mg/kg (Booth et
al., 1987).
Other field studies with similar results have been reported by
Anon (1980), Smith & Edmunds (1985), Van Den Berg (1986), Huber &
Collins (1987), Jones & Kochenderfer (1988), Huber & Manchester
(1988), Downey (1990) and Sundaram et al. (1991). It is clear that a
variety of application scenarios will result in measurable residues of
diflubenzuron in water (Table 6).
The overall conclusion is that diflubenzuron residues in stagnant
water dissipate rapidly within days. In flowing water, e.g., in
wooded areas, diflubenzuron residues may peak shortly after rainfall
but such peak concentrations are very transient in nature.
5.1.3 Food and feed
Data on residues in food resulting from treatment with
diflubenzuron have been summarized by FAO/WHO (1982a,b, 1985a,b,
1986a,b).
Residue data obtained from various countries showed residues in
apples below 1.0 mg diflubenzuron/kg at 2 weeks after the last
application at recommended rates. Residues in whole citrus fruit were
below 0.5 mg/kg 1 week after the last application at the recommended
rate. Residues in soybean seed and cottonseed were generally below
the limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron-treated soil, high
levels of the metabolite 2,6-DFBA are taken up from the soil.
Diflubenzuron was found at a level of 0.1 mg/kg, while the 2,6-DFBA
level was around 1 mg/kg (see chapter 4).
Residues in wild mushrooms after aerial application to forests in
Finland were on average 0.07 mg/kg 1 week after spraying with 75 g
diflubenzuron in 50 litre water per ha. In bilberries the residues
decreased on average from 0.2 mg/kg 1 day after spraying to 0.09 mg/kg
after 1 month (Mutanen et al., 1988).
Diflubenzuron applied as a wettable powder spray to growing
alfalfa at 20-100 g/ha showed initial residue levels of 1.8-8.5 mg/kg.
Residues of 0.3-1.5 mg/kg remained 22 days after applications (Lauren
et al., 1984).
Table 6. Summary and comparison of experimental parameters among key studies designed to measure environmental concentrations of
diflubenzuron in water
Medium Formulation a.i.% Method of Application Maximum Time for Minimum Time for References
treated application rate a.i. concentration maximum concentrationa minimum
concentration concentration
Farm ponds 25 WP 25 hand sprayer 2.5-10 µg/litre 1.9-9.8 µg/litre 1-4 h 0.5-0.2 µg/litre 14 days Apperson
(0.06-0.2 ha) from boat et al.
(1978)
Small lake 25 WP 25 hand sprayer 5 µg/litre 3.3 µg/litre 4 h 0.4 µg/litre 35 days Apperson
(18.6 ha) from boat et al.
(1978)
Pond W-25 25 hand-operated 0.28 kg/ha 56 µg/litre 96 h < 0.01 µg/litre 40 days Booth
spray et al.
applicator (1987)
Brackish 25 WP 25 clothes 0.045 kg/ha 7.5 µg/litre 48-72 h < 0.3 µg/litre 25-30 days Hester
pools sprinkler (1986)
Forest ponds 25 WP 25 aircraft 0.07 kg/ha 13.82 µg/litre 1 h < DL 20 days Kingsbury
(25 ha) (four et al.
atomizers) (1987)
Field plot 25 WP 25 fixed-wing 0.075 kg in 50 5.0 µg/litre 24 h < DL 60 days Mutanen
(1160 ha) aircraft litre water/ha et al.
(1988)
Fixed plots granular 1.0 aircraft 0.023 kg/ha, 1.8 µg/litre 192 h < DL 60 days Wan &
(3-40 ha) 0.46 kg/ha Wilson
(1977)
a DL = determination limit
After two soil applications of 67.26 g/ha, the residues of
diflubenzuron in the rotational crops (wheat, cabbage and onions) were
less than 0.01 mg/kg (Danhaus & Sieck, 1976).
Mian & Mulla (1983) studied the persistence of diflubenzuron in
stored wheat after applications of 1, 5 and 10 mg/kg. The residue
levels were 0.59, 2.75 and 5.00 mg/kg, respectively, 23 months after
treatment.
5.1.4 Forest plants and litter
The level of diflubenzuron residues in pine needles was on
average 3.0 mg/kg 1 day after application to the forest in Finland at
a rate of 75 g diflubenzuron in 50 litres water per ha. The level had
decreased to 0.2-0.3 mg/kg or was not detectable 2 months later
(Mutanen et al., 1988).
Booth et al. (1987) found diflubenzuron residues of less than
0.05 mg/kg in the forest litter 1, 4, 10 and 21 days after treatment
with 0.28 kg a.i./ha.
Sundaram (1986) studied the residues in a forest in Canada after
simulated aerial spraying of diflubenzuron in acetone and in fuel oil:
Arotex 3470 mixture, each at 90 g a.i. in 18 litre/ha. The residue
levels 1 h after application varied, respectively, from 23.8 to
30.6 µg/g in foliage and from 3.08 to 4.60 µg/g in litter. Forty-five
days after spraying the residue levels in foliage were 0.80 and
3.9 µg/g, respectively, for the above-mentioned formulations.
Spray deposit patterns and persistence of diflubenzuron in white
pine ( Pinus strobus L.) and sugar maple ( Acer saccharum Marsh.)
canopies, forest litter and soil were studied after aerial application
of a 250 g/kg wettable powder formulation (Dimilin WP-25) at
70 g a.i./ha, using three volume rates (2.5, 5 and 10 litres/ha), over
three blocks in a mixed forest near Kaladar, Ontario, Canada, during
1986 (Sundaram, 1991). In the spray block that received 10 litres/ha,
diflubenzuron persisted in foliage as long as 120 days after
treatment, but it lasted for only about a week in forest litter and
soil samples. At 2.5 and 5 litres/ha, diflubenzuron failed to persist
in foliage as long, and residues in litter and soil, which were barely
above the quantification limit, persisted only for a few days.
5.1.5 Aquatic organisms
Residues in fish are given in section 4.4.
5.2 General population exposure
Exposure of the general population to diflubenzuron via food and
drinking-water may occur.
Twelve volunteers with whole body dosimeters were exposed for 4 h
to Dimilin 25 W after simulated indoor treatment of carpets at
0.16 g/m2. Average deposition was 5.3 ± 2.3 µg diflubenzuron/cm2
carpet. Total dermal exposure ranged from 0.053 to 0.25 mg/kg body
weight per day to (average 0.15 ± 0.066 mg/kg body weight per day).
Assuming a dermal absorption of 0.2%, the total exposure via the
dermal route was calculated to be 0.0003 mg/kg body weight per day.
Air concentrations ranged from 10.2 to 32.4 µg/m3 during the first
4 h and were < 1 µg/m3 at 12-16 h. The total respiratory exposure
was calculated to be 0.0011 mg/kg body weight per day. The total
exposure, via the dermal and respiratory route, was calculated to be
0.0014 mg/kg body weight (Honeycutt, 1993).
5.3 Occupational exposure during manufacture, formulation or use
In a US Department of Agriculture report, human exposure via a
variety of exposure scenarios was estimated using standardized methods
and assumptions. The exposure scenarios included mixing and loading by
workers, via aircraft or truck spillage, and general public exposure
via the diet or resulting from occupational aerial spraying. Dermal
absorption of diflubenzuron was assumed to be 10%. Estimated
realistic doses for humans were < 0.003 mg/kg body weight per day
except where aircraft or truck spillages occurred, in which case
exposures were significantly higher. Estimated worst-case doses for
humans were < 0.01 mg/kg body weight per day, except where aircraft
or truck spillages occurred (USDA, 1985).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Diflubenzuron is absorbed from the digestive tract but only
poorly absorbed through the skin. Willems et al. (1980) found that in
rats the relative intestinal absorption diminished greatly with
increasing dose. Following a dose of 4 mg/kg body weight 42.5% was
absorbed, but only 3.7% of a 900 mg/kg body weight dose was absorbed.
Dermal absorption of 14C-diflubenzuron was only 0.2% when it was
applied to the shaved skin of rabbits as an aqueous micro-suspension
of 150 mg/kg (De Lange, 1979).
When applied dermally to cattle 14C-diflubenzuron was not
absorbed or degraded through the skin to any detectable degree (Ivie,
1978).
6.2 Distribution
Body tissues show little tendency to retain diflubenzuron.
Analysis of tissues for radiocarbon residues, 4 days (for sheep) or
7 days (for cows) after a single oral dose of 10 mg/kg body weight,
indicated that only the liver contained appreciable levels of
radioactivity, ranging from 2 to 4 mg/kg diflubenzuron equivalents
(Ivie, 1977).
More than one third of an oral diflubenzuron dose appeared in the
bile of a cannulated sheep (Ivie, 1977).
The highest 14C-diflubenzuron residue present in pig tissues
after a single oral dose of 5 mg/kg body weight was 0.43 mg/kg in the
gall bladder. All other tissue residue levels were found to be less
than 0.30 mg/kg (Opdycke et al., 1982a).
Twenty-two dairy cows were fed 14C-diflubenzuron (labelled in
both phenyl moieties) in a diet at dose levels of 0.05, 0.5, 5, 25 and
250 mg/kg feed for 28 days. Residues in blood, fat and muscle were
below the detection limit (0.0067-0.04 mg/kg) at all dose levels. They
were only detected following a dose of 250 mg/kg in the liver and
kidney where residues were 6.040 and 1.038 mg/kg, respectively.
Residues in milk were found at dose levels of 5 and 250 mg/kg, where
the highest levels of diflubenzuron were 0.009 and 0.20 mg/kg,
respectively (Smith & Merricks, 1976a).
In a study by Miller et al. (1976a), two dairy cows were fed
diflubenzuron at 0.25 or 1 mg/kg body weight per day for 4 months. A
third cow received an increased dosage of 8 to 16 mg/kg body weight
per day, the highest value being maintained for three months. In the
fat, liver and milk of the third cow, residues were 0.2, 0.13 and
0.02 mg/kg, respectively.
When dairy bull calves (four treated and four controls) received
diflubenzuron at 1.0 to 2.8 mg/kg body weight, residues were detected
only in the tissue samples of one bull (0.02 mg/kg in liver and
kidney, 0.04 mg/kg in the subcutaneous fat, and 0.08 mg/kg in the
renal and omental fat (Miller et al., 1979).
The maximum total residue in eggs 3 days after a single dose of
5 mg/kg 14C-diflubenzuron to hens was 0.248 mg/kg (Opdycke, 1976).
When laying hens were administered 14C-diflubenzuron at dose
levels 0.05, 0.5, and 5.0 mg/kg feed for 28 days, dose-related
residues ranging from 0.007 mg/kg at the lowest to 1.2 mg/kg at the
highest dose level were found in kidney, liver and fat. After 7 days
of withdrawal, residues in all tissues and eggs were below the
detection limit (0.0006-0.032 mg/kg) for all dose levels (Smith &
Merricks, 1976b).
When diflubenzuron was fed to white leghorn and black sex-linked
cross hens at a level of 10 mg/kg feed for 15 weeks, detectable
residues were found in eggs, liver and visceral fat. Residues were
significantly higher in eggs from white leghorn hens than in eggs from
black sex-linked cross hens, the average levels being 0.55 and
0.38 mg/kg, respectively (Miller et al., 1976b).
6.3 Metabolic transformation
The metabolic fate of diflubenzuron has been studied in various
species. Metabolic pathways of diflubenzuron are shown in Fig. 4
In rats and cows the major metabolic pathway involves
hydroxylation of the phenyl moieties of the compound. About 80% of the
metabolites in rat urine were identified as 2,6-difluoro-3-
hydroxydiflubenzuron and 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the benzoyl
ureido bridge. The major part was excreted as 2,6-DFBA and
constituted more than half of the urinary metabolites. 4-CPU was not
detected in bile or urine in a significant quantity (De Lange et al.,
1975; Willems et al., 1980).
The major metabolite in cow urine was 2,6-difluoro-3-hydroxy-
diflubenzuron (45%). Relatively small quantities of 4-chloro-2-
hydroxy- (1.6%) and 4-chloro-3-hydroxydiflubenzuron (3.7%) and the
scission products 4-CPU (0.6%), 2,6-DFBA (6.0%) and 2,6-difluoro-
hippuric acid (6.9%) were present (Ivie, 1978).
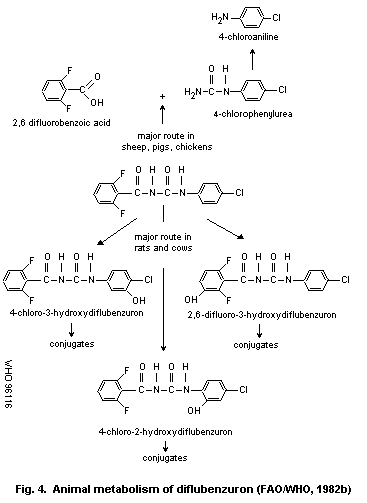 The major metabolites (approximately 50%) in sheep urine were
2,6-DFBA and 2,6-difluorohippuric acid (Ivie, 1978).
14C-Diflubenzuron uniformly radiolabelled in both rings was
administered to a pig as an oral dose of 5 mg/kg body weight. Of the
administered dose, 82% was eliminated in faeces as parent compound and
5% was recovered in urine. Identification of the metabolic products
in urine revealed 2,6-DFBA (0.28% of the dose), 4-CPU (0.82%), PCA
(1.03%) and 2,6-difluorobenzamide (0.83%). Cleavage of the urea
moiety between the benzoyl carbon and urea nitrogen was shown to be
the primary degradation pathway in pigs (Opdycke et al., 1982a).
In chickens only small quantities of the metabolites 2,6-DFBA,
4-CPU and PCA were found in excreta and tissues (Opdycke, 1976).
Neither induction nor inhibition of mixed-function oxidase activity
altered diflubenzuron metabolism in chickens (Opdycke et al., 1982b).
After 4 days daily doses of 7.8 g diflubenzuron/kg body weight,
De Bree et al. (1977) found PCA at a level of 30 ng/ml in rat plasma
and 323 ng/g in erythrocytes. PCA, estimated by the concentration in
the urine, represented at most 0.01% of the dose actually absorbed.
6.3.1 Metabolites - distribution, excretion, retention and turnover
When 14C-PCA was administered orally as single doses of 0.3,
3.0 or 30.0 mg/kg to male Fischer-344 rats, approximately 75% of the
administered radioactivity was excreted in the urine within 24 h,
while approximately 10% appeared in the faeces. Excretion was
virtually complete (92-97%) 7 days after dosing. The highest tissue
levels of radioactivity following a single intravenous dose of
3.0 mg/kg were found in the liver, fat, muscle and skin. Tissue
levels peaked within 5-60 min after dosing. By 3 days, concentrations
in all tissues except the blood had declined to < 0.3% of the dose
(Sipes & Carter, 1988). At this time, the only tissue containing more
than 1% of the dose was the cellular compartment of blood, which
contained 1-2% of the dose. The decline of PCA concentration in all
tissues, except for urine, faeces and intestinal contents, was
biexponential. The t alpha 1/2 for fat, muscle and skin was about
1.5 h, while the tß1/2 was approx. 43-59 h. The t alpha 1/2 for
liver was 3.5 h. Levels of unchanged PCA in all tissues peaked after
5 min following intravenous administration. The highest amount of
unchanged PCA was attained in muscle (15% of radioactivity in the
tissue) followed by skin (6%), fat (3%) and liver (2%). The decline of
PCA in all tissues, except for the liver, followed biexponential kinetics
with an estimated t alpha 1/2 of 8 min and a tß1/2 of 3 to 5 h.
PCA is rapidly metabolized to p-chloroacetanilide (PCAA) as the
initial step in the metabolism and excretion of PCA. The decline of PCAA
was monoexponential, the appearance half-life being approx. 6 min in the
testes and 15 min in the brain. The elimination half-life in the
brain, kidney, testes, muscle, skin and fat was around 1.0 to 2.0 h.
The elimination of PCA does not depend on either the dose or route of
administration. Approximately 4% of the urinary radioactivity in the
0-24 h urine sample was unchanged PCA; less than 1% was found in the
faeces. PCAA was not detected in either urine or faeces over a 3-day
period (Sipes & Carter, 1988).
After a single intravenous dose of 14C-PCA (3 mg/kg), maximal
tissue levels were reached within 15 min in most tissues. At this
time, most of the radioactivity was located in muscle (34%), fat
(14%), skin (12%), liver (8%) and blood (7%). Elimination half-lives
from tissues ranged between 1.5 and 4 h. By 8 h, approximately 90% of
the administered dose had been eliminated into urine and faeces. By
3 days, concentrations in all tissues, except blood, had declined to
< 0.3% of the dose (US NTP, 1989).
6.4 Elimination and excretion
After oral administration to rats of 5 mg diflubenzuron labelled
with 3H in the benzoyl and with 14C in the aniline moiety, 95% of
the 3H and 70-75% of the 14C radioactivity were retrieved in urine
and faeces. 2,6-DFBA was shown to constitute more than half of the
urinary metabolites (De Lange et al., 1977). Up to 1% of an oral dose
of 5 mg 14C-diflubenzuron labelled at the benzoyl moiety was
recovered in the expired air of rats (De Lange et al., 1974; Willems
et al., 1980).
When 14C-diflubenzuron, labelled in the aniline moiety, was
administered by gavage (4, 16, 48, 128, 900 and 1000 mg/kg body
weight) to rats, the urinary excretion was complete after 48-72 h.
Urinary excretion after single oral administration of diflubenzuron
relatively decreased with increasing dose level, being 27.6% of the
dose at 4 mg/kg and 1% at 1000 mg/kg (De Lange et al., 1977).
When 14C-diflubenzuron was administered at single oral doses of
12.5, 63.5, 202.5 and 925 mg/kg body weight to Swiss mice, the
excretion was almost completed within 48 h. The cumulative percentage
of the dose excreted in the urine decreased from 15% at the dose level
of 12.5 mg/kg to approximately 2% at 925 mg/kg (De Lange & Post,
1978).
Hawkins et al. (1980) studied the excretion of radioactivity in
urine and faeces after oral administration of 3H/14C-diflubenzuron
(7 mg/kg) to male cats. The radioactive dose was given on day 10 of a
15-day dosing regime of non-radioactive diflubenzuron (days 1-9 and
days 11-15). The excretion of radioactivity in urine accounted for
9.5 and 9.6% of the 14C and 3H doses, respectively, during 6 days
after dosing. The elimination of radioactivity in faeces accounted
for 77.3 and 71.6% of the 14C and 3H doses, respectively, during 6
days after dosing.
After an oral administration of 14C-diflubenzuron (5 mg/kg) to
female pigs, 82% of the dose was eliminated via faeces and 5% via
urine in 11 days (Opdycke, 1976).
About 85% of a single oral dose of 14C-diflubenzuron (10 mg/kg
body weight) administered to a cow was recovered in the faeces during
the first 4 days after treatment. About 15% was recovered in urine
and only about 0.2% was secreted in the milk (Ivie, 1977, 1978).
Sheep excreted 41% of the dose (10 mg/kg) in the urine and 43% in
the faeces during the 4 days after treatment. Bile-cannulated sheep
eliminated 24% of the dose in the urine, 32% in the faeces and 36% in
the bile. Sheep treated with 500 mg 14C-diflubenzuron/kg as a single
oral dose eliminated a much smaller proportion of the 14C in urine
and bile. This was probably due to reduced absorption from the
gastrointestinal tract when the sheep were given an exaggerated dose
(Ivie, 1977).
An oral dose of 5 mg 14C-diflubenzuron/kg administered to white
leghorn hens and Rhode Island red-barred Plymouth Rock buff cross hens
was rapidly excreted unaltered within the first 8 h. Up to 91 and
82%, respectively, were excreted within 13 days (Opdycke, 1976).
6.5 Retention and turnover
6.5.1 Biological half-life
From the studies of Willems et al. (1980) and Ivie (1978), the
half-life of diflubenzuron appears to be 12 h in rat and sheep and
18-20 h in the cow.
Diflubenzuron has been shown to pass intact through the
intestinal tract and remained active in the manure (Nimmo & de Wilde,
1977b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of diflubenzuron and its formulations to
different species is summarized in Table 7. No signs of intoxication
were observed during the 14-day period following a single
administration of diflubenzuron.
Van Eldik (1974) reported an intraperitoneal LD50 of
> 2150 mg/kg in rats and mice.
7.2 Short-term exposure
Rats (5 of each sex per group) were fed on a diet containing
diflubenzuron at concentrations of 0, 800, 4000, 20 000 and
100 000 mg/kg feed for 4 weeks. Behaviour, body weight, food and
water consumption were not affected by the treatment. There was a
dose-related increase in the met- and sulfhaemoglobin content of the
blood in all treated groups except for the methaemoglobin value for
females in the 800-mg/kg dose group. Lower erythrocyte, packed cell
volume (PCV) and haemoglobin values were observed in both sexes of the
100 000-mg/kg dose group. There was a dose-related increase in spleen
and liver weights. Only the dose level of 800 mg/kg did not affect
the liver weight (Palmer et al., 1977).
Five male Swiss-albino rats were given 96.7 mg diflubenzuron/kg
body weight per day, dissolved in corn oil, in their diet for 48 days.
The total dose was 4640 mg/kg body weight. Five controls were given
corn oil only in their diet. At the end of the study the treated
groups showed a significantly lower mean haemoglobin concentration
than the controls, and decreases in MCH and MCHC values (Berberian &
Enan, 1989).
Diflubenzuron was administered to Sprague-Dawley rats of the CD
strain (20 of each sex per group) at dietary levels of 10 000 and
100 000 mg/kg feed for 9 weeks, followed by a 4-week withdrawal
period. Lower values for red blood cell parameters were recorded at
both dose levels. An increase in reticulocyte count and a pallor of
the extremities and eyes were observed. The formation of
methaemoglobin occurred in both males and females, with approximately
5-8% of the available haemoglobin being transformed to methaemoglobin.
After a withdrawal period of 4 weeks methaemoglobin comprised less
than 2% of the available haemoglobin in tested animals compared with
approximately 0.6% in controls. Higher values for SGPT were recorded
as well as heavier liver, spleen and adrenal weights. Minor
enlargement of centrilobular hepatocytes in the highest dose group was
observed, but this finding disappeared after the withdrawal period
(Hunter et al., 1979).
Table 7. Acute toxicity of diflubenzuron and its formulations
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Diflubenzuron mouse: > 4640 rat: > 10 000 rabbit: > 30 mg/ rabbit: 40 mg rabbit: non- guinea-pig: non-
technical mg/kg body weight mg/kg body weight litre nominal instilled in eye; irritant (Taylor, sensitizing
(Koopman, 1977c) marginal irritant 1973a) (Prinsen, 1992)
(Davies &
rat: > 4640 mg/kg rabbit: > 4 ml/kg > 3.75 mg/litre Ligget, 1973)
body weight body weight of 50% actual (Berczy et
(van Eldik, 1973a; in gum tragacanth al., 1975a)
Koopman, 1977a) (Davies &
Halliday, 1974)
Diflubenzuron mouse: > 5000 rat: > 2000 mg/kg rat: > 13.8 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: 25%
90% concentrate mg/kg body weight body weight litre nominal instilled in eye; non-irritant w/w in paraffin
(Koopman & Pot, (Koopman, 1984a) very slight (OECD Guideline (Grade 1); weak
1986) > 2.49 mg/litre irritant (Koopman, 404) (Koopman, sensitizer (OECD
actual (Greenough 1984c) 1984d) Guideline 406)
rat: > 5000 mg/kg & McDonald, (Kynoch & Smith,
body weight 1986) 1986)
(Koopman, 1984b)
Dimilin WP 25% rat: > 40 000 rat: > 20 000 rat: > 150 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: non-
mg/kg body weight mg/kg body weight litre nominal instilled in eye; non-irritant to sensitizing
(Janssen & Pot, slight to only minimally (Kynoch & Elliott,
mice: > 40 000 1987e) > 3.5 mg/litre moderate, irritant 1978,a,b)
mg/kg body weight actual (Arts, transientsient eye (Taylor, 1973b;
(van Eldik, 1973b; 1991) irritation (Snoeij Chandran, 1981;
Koopman, 1977b) & Busé-Pot, 1991) Snoeij &
Busé-Pot, 1990)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin SC-48 rat: > 5000 mg/kg rat: > 2000 mg/kg rabbit: 0.1 ml rabbit: non- guinea-pig: weak
body weight body weight - instilled in eye; irritant (Janssen sensitizer
(Janssen & Pot, (Janssen & Pot, slight irritant & Pot, 1987b) (Kynoch &
1987c) 1987d) (Janssen & Pot, Parcell, 1987)
1987a)
Dimilin SC-15 albino rats: 0.1 rabbit: minimally
- - - ml instilled in irritant -
eye; non-irritant (Prinsen, 1989a)
(Prinsen, 1989b)
Dimilin 4F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 1.9 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight actual (Jackson instilled in eye; minimally irritant sensitizing
(Spanjers, 1988a) (Spanjers, 1988b) et al., 1990) non-irritant (Prinsen, 1988a) (Prinsen, 1989c)
(Prinsen, 1988b)
Dimilin 2F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 4.4 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig:
body weight body weight actual very instilled in eye; moderate irritant moderate (Grade
(Koopman, 1985d) (Koopman, 1985c) slightly irritant moderate irritant OECD Guideline III) (Kynoch &
(Zwart, 1985) OECD Guideline 404 (Koopman, Parcell, 1987)
405 (Koopman, 1985a)
1985b)
Dimilin ODC 45 rat: > 37 300 mg/kg rat: > 37 300 mg/kg rabbit: 0.1 ml rabbit: 0.5 ml
body weight body weight instilled in eye; moderate irritant
(Koopman & (Koopman, 1980b) marginally (Koopman,
Jongeling, 1979) irritant (Koopman, 1980c)
1980a)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin OF 6 rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 95.7 mg/ rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight litre nominal: instilled in eye; topical; mild sensitizing
(Besten et al., (Besten et al., > 2.17 mg/litre non-irritant irritant (Prinsen, 1993)
1993a) 1993b) actual (Janssen & (Janssen & van (Janssen & van
van Doorn, 1993a) Doorn, 1993c) Doorn, 1993b)
Wistar rats (10 of each sex per group) were fed diflubenzuron in
the diet for 13 weeks at concentrations of 0, 3.125, 12.5, 50 or
200 mg/kg feed. Behaviour, growth and food intake were unaffected by
the treatment. At the highest dose level the PCV value, the
haemoglobin concentration and the number of erythrocytes were
decreased. There was an increase in the SGPT and SGOT activities in
the males of the highest dose group at the end of the experiment. A
slight increase in the number of normally occurring scattered small
foci of necrotic parenchymal cells was observed, accompanied by
mononuclear inflammatory cell infiltration and proliferation of
reticuloendothelial system cells in the liver of both males end
females of the 50 and 200 mg/kg groups (Kemp et al., 1973a,b).
Absence of toxic effects in chronic/oncogenicity studies at low
dose levels necessitated re-evaluation of liver histopathology in the
90-day feeding study; "piece meal" liver cell necrosis was reported at
the two highest dose levels, i.e. 50 and 200 mg/kg feed. The original
slides of the study were re-evaluated by four histopathologists in
four different laboratories. They independently and unanimously
agreed that the lesions in the livers of treated rats were found to
the same extent in the livers of untreated rats. This showed that the
high dose levels in the study did not demonstrate a treatment-related
necrotic effect in the liver (Offringa, 1977).
Technical grade diflubenzuron was administered in the diet to
male and female 21- to 28-day-old Sprague-Dawley rats (40 of each sex
per group) at dose levels of 0, 160, 400, 2000, 10 000 and
50 000 mg/kg feed for 13 weeks. No apparent treatment-related effects
were noted on mortality, clinical observations, body weight gain, food
consumption, clinical chemistry or urinalysis. A treatment-related
significant increase in methaemoglobin concentration was noted in all
treated groups. Sulfhaemoglobin values showed increases at dose levels
of 2000 mg/kg or more. A significant treatment-related decrease in
haemoglobin, PCV and erythrocyte count was observed in males and
females at all dose levels by the end of the study. An increase was
noted in the reticulocyte count at all dose levels except 160 mg/kg,
and the number of the Heinz bodies was higher in the 10 000 and
50 000 mg/kg groups. After 7 weeks, spleen weights were increased in
the females at all dose levels, but after 13 weeks no effect was found
at 160 mg/kg. With the exception of the lowest dose level, all
treated groups showed a higher liver weight. The administration of
diflubenzuron resulted in a dose-related increase in the incidence of
chronic hepatitis and haemosiderosis of the liver. It was also
associated at all dose levels with haemosiderosis and congestion of
the spleen and mild erythroid hyperplasia of the bone marrow. The
severity of the lesions tended to increase with the dose. Liver
lesions were more severe in males than in females and were more severe
at 13 weeks than at 7 weeks. A no-observed-effect level (NOEL) was
not established (Burdock et al., 1980b; Goodman, 1980b).
Diflubenzuron was given to CFLP mice for 6 weeks at levels of 16
and 50 mg/kg feed. There were no clinical signs and no effects on
food consumption, body weight, blood chemistry or macroscopic
pathology. In three of the eight animals given 50 mg/kg, foci of
liver cell necrosis, with or without inflammatory cell filtration,
were noted. Other organs were not examined microscopically (Hunter et
al., 1974).
Diflubenzuron was administered to Swiss Webster mice in a 30-day
oral intubation study. Groups of five mice each received either no
treatment, vehicle control (Polyethylene glycol 400) or diflubenzuron
suspensions at dose levels of 125, 500 or 2000 mg/kg body weight.
Hepatic glutathione- S-transferase activity and morphological
characteristics were studied. Diflubenzuron was shown to elicit
hepatocellular changes at all dose levels. The activities of three
glutathione- S-transferases ( S-aryl, S-aralkyl and S-epoxide)
were irregularly altered in a non-dose-related manner. Light
microscopy revealed radial arrays of hepatocellular vacuolization
between the portal and central vein areas. There was evidence of an
increase in the amount of endoplasmic reticulum (Young et al., 1986).
Male and female mice of the B6C3F1 strain (40 of each sex per
group) received diflubenzuron (97.2% a.i.) in the diet at dose levels
of 0, 16, 50, 400, 2000, 10 000 or 50 000 mg/kg feed for 13 weeks. An
additional group of 100 of each sex served as a control. No
compound-related effects were apparent with respect to clinical signs,
survival, growth rates, total food consumption or gross pathology.
Significant treatment-related increases in met- and sulfhaemoglobin
concentrations were noted in all treated groups, except in the group
fed 16 mg/kg. At the higher dose levels, there was a decrease in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects on the weights of liver and spleen were
noted. In the females, adrenal weight was decreased (but not in a
dose-related fashion) at all dose levels after 7 weeks and increased
after 13 weeks in higher dose levels. Higher adrenal weight was
observed in treated males than in the controls. Histopathological
examination revealed treatment-related centrilobular hypertrophy of
hepatocytes, with or without cell necrosis, haemosiderosis of the
liver and spleen, extramedullary haematopoiesis and mild chronic
hepatitis in treated animals of both sexes, some of which effects were
observed at the lowest dose level (16 mg/kg). The liver lesions were
more severe in males than in females, being most severe in the high-
dose males. The NOEL for methaemoglobin formation was 16 mg/kg feed
(Burdock et al., 1980a; Goodman, 1980a).
HC/CFLP mice (40 of each sex per group) were fed diflubenzuron
(97.2% purity) for 14 weeks at levels of 0, 80, 400, 2000, 10 000 and
50 000 mg/kg feed. On the second day of treatment, the majority of
mice treated with 10 000 or 50 000 mg/kg showed dark eyes and/or
prominent caudal blood vessels. On day 5, blue/grey discoloration of
the extremities was noted for the majority of mice treated with
50 000 mg/kg. Mice in the lowest dose group exhibited no clinical
signs. Mortality, food consumption, water consumption and body weight
changes were not significantly affected by the treatment. Lower PCV
and red blood cell counts were found at all dose levels except
80 mg/kg. The total white blood cell count, lymphocyte count,
haemoglobin concentration, incidence of Heinz bodies and red blood
cell count were increased in all treated groups. At week 7, there was
an increase in the number of reticulocytes in treated mice,
particularly in males treated at 10 000 or 50 000 mg/kg. At week 14,
the reticulocyte counts were similar to those of the controls. A
treatment-related increase in both met- and sulfhaemoglobin was
recorded in all treated groups at weeks 7 and 14 of the investigation.
Plasma glutamic-pyruvic transaminase values were increased at all dose
levels, with the exception of 80 mg/kg feed. Lower blood cholesterol
levels were noted in the 2000, 10 000 and 50 000 mg/kg groups.
Macroscopic examination showed dark discoloration and/or enlargement
of the spleen and pale subcapsular areas of the liver in all dose
groups after both 7 and 14 weeks. Histopathological examination of
the spleen revealed increased haemosiderosis at all dose levels except
80 mg/kg. In the liver, areas of focal necrosis and/or fibrosis in
the parenchyma, with or without associated inflammatory cells,
fibroblasts or pigment-laden macrophages, were observed. At higher
dose levels necrotic and fatty hepatocytes and brown pigment-laden
Kupffer cells were found. A NOEL was not established (Colley et al.,
1981a,b).
Diflubenzuron was fed to groups of three male and three female
beagle dogs for 13 weeks at concentrations of 0, 10, 20, 40 and
160 mg/kg diet. No effect of the treatment on behaviour, body weight
or food and water consumption was observed. Elevated SAP and SGPT
values were recorded for some dogs receiving 40 or 160 mg
diflubenzuron/kg feed. After 6 weeks, methaemoglobin and another
abnormal pigment, probably sulfhaemoglobin, were demonstrated in dogs
given 160 mg/kg. After 12 weeks of administration, some recovery was
observed. Organ weights and gross and microscopic evaluation did not
reveal any treatment-related effects. The NOEL for methaemoglobin
formation was 40 mg/kg feed (Chesterman et al., 1974).
Diflubenzuron was administered daily in gelatin capsules to male
and female beagle dogs (6 of each sex per group) at dose levels of 2,
10, 50 or 250 mg/kg body weight per day for 52 weeks. There were no
treatment-related effects on mortality, food consumption or body
weight gain. Dose-related marginal increases in methaemoglobin and
sulfhaemoglobin were recorded from 10 mg/kg upwards. At 50 and
250 mg/kg, haemoglobin concentration and MCHC were decreased whereas
reticulocyte and platelet counts were increased. Heinz bodies were
also detected in several animals receiving 50 and 250 mg/kg. Dose-
related increases in liver and spleen weights were found in the 50 and
250 mg/kg males. Histopathological evaluation of the liver revealed
an increase in the incidence of pigmented macrophages and Kupffer cell
siderosis at 50 and 250 mg/kg in both males and females. The NOEL
based on the increase in met- and sulfhaemoglobin was 2 mg/kg body
weight (Greenough et al., 1985).
In a study by Berczy et al. (1975c), rats were exposed daily for
a one-hour period to technical diflubenzuron dust at nominal
concentrations of 0, 0.5, 5.0 and 50 mg/litre air, respectively (the
actual concentrations were 0, 0.12, 0.87 and 1.85 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days per week. The methaemoglobin levels in male rats at the two
lower concentrations and female rats of all test groups were
significantly higher than those of controls.
Rabbits were exposed daily for one-hour periods to technical
diflubenzuron dust at concentrations of 0.5, 5.0 and 25 mg/litre air
(the measured concentrations were 0.15, 0.75 and 1.99 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days each week. There were no signs of irritation in animals
exposed to 0.5 mg/litre, but mild transient respiratory irritation was
seen at the two higher concentrations. Haematological examination,
biochemistry tests and macroscopic inspection revealed no treatment-
related abnormalities (Berczy et al., 1975b).
Diflubenzuron, at levels of 4.64, 10 and 21.5% weight/volume was
applied daily to the intact and abraded skin of rabbits at a dosage
level of 1.5 ml/kg body weight, 5 days a week, for 3 consecutive weeks
(equivalent to 69.6, 150 and 322.5 mg diflubenzuron/kg body weight per
day). The sulfhaemoglobin level was increased in one rabbit out of 20
in the 10% group, and in 5 rabbits out of 20 at the high treatment
level. The 10% treatment level was considered to be the NOEL based on
sulfhaemoglobin formation (Davies et al., 1975).
7.3 Long-term exposure
Diflubenzuron was administered to Sprague-Dawley rats (60 of each
sex per group) in their diet at levels 0, 10, 20, 40 and 160 mg/kg
feed for 104 weeks. There were no treatment-related effects on body
weight gain, food intake, renal function or on macroscopic and
microscopic pathology. In rats treated with 160 mg/kg, significantly
higher methaemoglobin levels were recorded. The tumour profile of
treated rats was similar to that of the controls. The NOEL based on
methaemoglobin was 40 mg/kg, equivalent to a mean intake of 1.43 and
1.73 mg/kg per day for males and females, respectively (Hunter et
al.,1976).
When diflubenzuron was administered in the diet to male and
female Sprague-Dawley rats (50 of each sex per group) at dosage levels
of 0, 156, 625, 2500 and 10 000 mg/kg feed for 104 weeks, there was no
evidence of an effect on mortality or treatment-related clinical
signs. Significantly increased absolute methaemoglobin and
sulfhaemoglobin values were observed in all male treatment groups.
However, increases in relative methaemoglobin values (% of total
haemoglobin) were only noted in the 156, 2500 and 10 000 mg/kg groups,
while sulfhaemoglobin increases were noted in the 156, 625 and
10 000 mg/kg females. There was a significant increase in absolute
and relative spleen weights in the two highest dose groups of both
sexes, together with haemosiderosis in spleen and liver. There was no
evidence of carcinogenicity after 2 years of feeding diflubenzuron
(Burdock et al., 1984).
In a study by Hunter et al. (1975), diflubenzuron was
administered to CFLP mice for 80 weeks at dietary levels of 0, 4, 8,
16 and 50 mg/kg feed. There were no overt signs of reaction to
treatment. Behaviour, mortality, food and water consumption and body
weight were unaffected by the treatment. The macroscopic changes
observed were those commonly seen in mice of this strain and age. No
histopathological changes were seen that were considered to be related
to the administration of diflubenzuron. The incidence of liver cell
tumours was higher in this study than it was in other studies
performed in these laboratories using this strain of mouse. The
increase was seen in both treated and control groups of either sex and
showed no evidence of a treatment-related effect. There was no
evidence of a treatment-related effect on tumour incidence in the CFLP
mouse.
Male and female HC/CFLP mice (88 of each sex per group) were fed
diflubenzuron at dietary levels of 0, 16, 80, 400, 2000 and
10 000 mg/kg feed for 91 weeks. There was no indication of a
treatment-related effect on survival, food consumption or body weight
gain. Treatment-related elevations of MCH and MCHC values were
recorded from week 26 onwards in mice given 10 000 mg/kg. The
incidence of Heinz bodies increased in a dose-related manner among
mice given 400, 2000 or 10 000 mg/kg from week 52 onwards. Dose-
related increases in methaemoglobin levels were found from week 26
onwards and in sulfhaemoglobin levels from week 52 onwards in the mice
fed 80 mg/kg or more. Elevated alkaline phosphatase (AP) activities
were seen at weeks 24, 76 and 89 among male mice receiving 2000 and
10 000 mg/kg and at week 84 among mice of the 400 mg/kg dose group.
An increased incidence of splenic and/or hepatic enlargement was seen
among mice treated with 10 000 mg/kg. Cyanosis of the skin was noted
among mice at 400, 2000 and 10 000 mg/kg. Increased liver and spleen
weights were found among mice given 2000 and 10 000 mg/kg. An
increased incidence of hepatocyte enlargement and increased
extramedullary haematopoiesis in the liver and spleen were seen in
mice at high dose levels. In the 400, 2000 and 10 000 mg/kg groups,
there was an increased incidence of siderocytosis in the spleen and of
pigmented Kupffer cells in the liver. There was no treatment-related
effect on tumour incidence (Colley et al., 1984).
7.4 Skin and eye irritation; sensitization
Relevant data are given in Table 7.
Administration of technical diflubenzuron to the intact and
abraded skin of albino rabbits did not produce irritation of the skin
after exposure times of 24 and 72 h (Taylor, 1973a,b).
Diflubenzuron was found to be a moderate irritant to the rabbit
skin after application of 0.5 ml of 45% oil dispersible concentrate
for 24 h (Koopman, 1980a; Prinsen, 1990).
Diflubenzuron (both technical and 45% oil dispersible
concentrate) was considered to be marginally irritant to the rabbit
eye (Davies & Ligget, 1973; Koopman, 1980b).
Diflubenzuron (48% water-based paste) was not found to be a
dermal sensitizer in guinea-pigs (Kynoch & Parcell, 1987).
Technical diflubenzuron was studied for skin sensitization in a
maximization test on guinea-pigs and was found to be non-sensitizing
(Prinsen, 1992). However, some formulations are mild sensitizers
(Table 7).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Diflubenzuron was fed to pathogen-free rats of the CFY strain
(20 of each sex per group) at dietary levels of 0, 1000 and
100 000 mg/kg for one generation and one litter. The animals were
maintained on their respective diets for 9 weeks prior to mating.
There were no clear effects on mating performance, pregnancy rate,
duration of gestation, litter size, offspring mortality, litter weight
or the type and distribution of abnormalities. Dose-related effects
of diflubenzuron were demonstrated at 17 weeks in adults and consisted
of reduced values for PCV, haemoglobin, total red cells count and
MCHC, increased values for methaemoglobin, MCV, spleen weight and
siderocyte incidence in the spleen, and the occurrence of iron-
pigment-containing Kupffer cells in the liver. A dose-related effect
on the liver was also shown by increased weight and SGPT activity and
centrilobular hepatocyte enlargement. Reduced blood glucose
concentrations were recorded in both treated groups. At the highest
dosed level, the offspring showed increased liver and spleen weights
for both sexes (Palmer et al., 1978).
In a three-generation reproductive study with rats fed
diflubenzuron at concentrations of 10, 20, 40 and 160 mg/kg feed, no
adverse treatment-related effects on mating performance, pregnancy
rate, duration of gestation or litter parameters (total loss, size,
mean pup weight, mortality, abnormalities) were found (Palmer & Hill,
1975a).
After gavage administration of diflubenzuron at doses of 1, 2 and
4 mg/kg body weight per day to pregnant rats during days 6-15 of
gestation, no effects were observed on embryonic or fetal development
(Palmer & Hill, 1975b).
Treatment of pregnant New Zealand white rabbits at oral doses of
1, 2 and 4 mg/kg body weight per day during days 6-18 of gestation did
not affect embryonic or fetal development as assessed by the incidence
of major malformations, minor anomalies and skeletal variants (Palmer
& Hill, 1975c).
In a study by Booth (1977), pregnant Swiss mice were fed a diet
containing 50 mg/kg of diflubenzuron (partly 14C) for a period of
17 days. Some of these mice were killed at day 17 after conception,
while the others were allowed to give birth. The results of this
study showed that mucopolysaccharide synthesis in embryonic mouse-limb
cartilage was normal. Diflubenzuron did not pass through embryonic
membranes nor was it passed from mother to suckling young mice.
Analysis of 226 embryos showed no gross teratogenic effects.
Two groups of 24 timed-mated female rats of the Crl:CD (SD) BR
strain were dosed once daily by the oral route between days 6 and 15
of pregnancy. One group was dosed with diflubenzuron at 1000 mg/kg
per day, while the other group was given the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 20 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths or treatment-related changes in clinical condition. Treatment
did not affect maternal growth or food consumption. There were no
maternal abnormalities at necropsy that were considered to be related
to treatment. The mean numbers of corpora lutea, implantations and
live fetuses were similar in all groups. Both pre- and post-
implantation losses were unaffected by treatment. Fetal weights and
sex ratio were unaffected by diflubenzuron treatment. The incidences
of major external/visceral and skeletal abnormalities, and minor
external/visceral abnormalities were not affected by diflubenzuron
treatment. The incidence of fetuses with minor skeletal abnormalities
was slightly higher in the treated group, but was within the usual
background range. There were intergroup differences in the
proportions of fetuses with specific minor abnormalities and variants
of skeletal ossification. For some bones, the differences achieved
statistical significance. However, on balance, treated group fetuses
were considered to be similarly ossified to the control fetuses. Oral
administration of diflubenzuron at a dose level of 1000 mg/kg per day
did not elicit maternal toxicity or any evidence of embryotoxicity
(Kavanagh, 1988a).
Two groups of 16 timed-mated female New Zealand White rabbits
were dosed once daily by the oral route from days 7 to 19 of
pregnancy, inclusive. One group was dosed with diflubenzuron at
1000 mg/kg per day and the other group with the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 28 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths, changes in clinical condition or abnormalities at maternal
necropsy considered to be related to diflubenzuron treatment. Two
animals were killed prematurely during the study, one from the control
group and one from the treated group. The changes in clinical
condition and abnormalities observed at necropsy in these animals were
considered to be unrelated to treatment. Treatment did not affect
maternal growth or food consumption. The mean numbers of corpora
lutea, implantations and live fetuses were similar in all groups. Both
pre- and post-implantation losses were unaffected by treatment. Fetal
weights and sex ratio were unaffected by diflubenzuron treatment. The
incidence of both major and minor external/visceral and skeletal
abnormalities, as well as the numbers of skeletal variants, was
unaffected by diflubenzuron treatment. Oral administration of
diflubenzuron at a dose level of 1000 mg/kg per day did not elicit
maternal toxicity or any evidence of embryotoxicity (Kavanagh, 1988b).
In a study by Kubena (1982), diflubenzuron was fed at levels of
0, 2.5, 25 and 250 mg/kg feed to male and female layer-breed chickens
from 1 day of age through a laying cycle. Characteristics measured
were egg production, egg weight, eggshell weight, fertility,
hatchability and effects on the progeny. Feeding diflubenzuron at
levels up to 250 mg/kg feed did not affect these characteristics.
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller & Booth, 1976).
7.6 Mutagenicity and related end-points
Diflubenzuron was examined by in vitro and in vivo
mutagenicity tests. The results are summarized in Table 8.
Mutagenicity tests have also been carried out with the major
metabolites of diflubenzuron (Table 9).
Table 8. Mutagenicity tests with diflubenzuron
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Microorganisms
Reverse Salmonella typhimurium
mutation TA98, TA100, 10-1000 µg/plate +/- negative Bryant (1976)
TA98, TA100, TA1537, TA1978 1000 µg/spot +/- negative Bryant (1976)
Reverse S. typhimurium 0.1-500 µg/plate +/- negative Brusick & Weir
mutation TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Reverse S. typhimurium 10, 100 or 1000 µg/plate; +/- negative McGregor et al.
mutation TA98, TA100, 19, 186, 1860 µg DFB/plate (1979)
TA1535, TA1537 (as Dimilin W-25)
Reverse S. typhimurium up to 5000 µg/plate +/-b negative Moriya et al.
mutation TA98, TA100, TA1535, (1983)
TA1537, TA1538
Escherichia coli. WP2 hcr
Reverse S. typhimurium up to 1000 µg/plate +/- negative Koorn (1990)
mutation TA98, TA100, TA1535,
TA1537, TA1538
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammalian cells in vitro
Forward L5178Y mouse lymphoma 1.17-300 µg/ml +/- negative McGregor et al.
mutation cells (1979)
Chromosomal Chinese hamster ovary up to 200 µg/ml +/- negative Taalman & Hoorn
aberrations cells (1986)
Unscheduled human diploid WI-38 cells 50-1000 µg/ml +/- negative Brusick & Weir
DNA synthesis (blocked in the G1 phase) (1977c)
DNA repair rat hepatocytes up to 333 µg/ml negative Enninga (1990)
(unscheduled
DNA synthesis)
Cell transformation BALB-3T3 cells, 0.02-0.312 µg/ml - negative Brusick & Weir
in vitro (1977b)
Cell transformation pregnant hamster, ip 10, 200 and 500 mg/kg negative Quarles et al.
(transplacental injection on 10th day of body weight (1980)
transformation) gestation, fetal cell culture
3 days after injection
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammals
Micronucleus mouse bone marrow 15, 150, 1500 mg/kg body negative McGregor et al.
weight 30 and 6 h before (1979)
necropsy
Dominant male mice mated to 1000 and 2000 mg/kg body negative Arnold et al.
lethal 3 females weekly for 6 weeks weight intraperitoneal (1974)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b Source of S9 not indicated
Table 9. Mutagenicity tests with diflubenzuron metabolites
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
A. 4-chlorophenylurea
Microorganisms
Reverse mutation Salmonella typhimurium
and differential TA98, TA100, TA1535, 1000 µg/spot +/- negativeb Dorough (1977)
killing TA1537, TA1538, TA1978
Reverse mutation TA98, TA100 10, 100, 500, negative
1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 6.25-50 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Cell BALB-3T3 cells, 0.019-0.312 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977a)
0.312 mg/ml
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
B. 2,6-Difluorobenzoic acid
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negative Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Reverse mutation S. typhimurium 10, 100, 500,
TA98, TA100 1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977b)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 75-500 µg/ml +/- positive Matheson & Brusick
DNA synthesis (blocked in the G1 phase) with (1978a)
activation
Cell BALB-3T3 cells, 0.156-2.5 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977b)
2.5 mg/ml
C. 4-Chloroaniline (PCA)
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negativeb Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Reverse mutation TA98, TA100 10, 100, 500, positive in
1000 µg/plate TA98 at 500
and 1000 µg
with
activation
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977c)
TA1537, TA1538
Reverse mutation S. typhimurium
TA98, TA100, TA1530, 0-1500 µg/plate +/- negative Gilbert et al. (1980)
TA1535, TA1537, TA1538
Reverse S. typhimurium C3076, 1000 µg/plate +/- negative Thompson et al.
mutation D3052, G46, TA98, TA100, (1983)
TA1535, TA1537, TA1538,
Escherichia coli WP2,
WP2 uvrA
Reverse S. typhimurium 3333 µg/plate +/- positivec Dunkel et al. (1985)
mutation TA98, TA100, TA1535,
TA1537, TA1538,
Escherichia coli
Reverse S. typhimurium 1666 µg/plate +/- positived Mortelmans et al.
mutation TA97, TA98, TA100, TA1535 negative (1986)
DNA damage Escherichia coli polA+/polA- 250 µg/plate - positive Rosenkranz & Poirier
(1979)
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Mutation Aspergillus nidulans 200 µg/ml - positive Prasad (1970)
Mammalian cells
Forward mutation L5178Y mouse lymphoma +/- positived Caspary et al. (1988)
tk+/- cells
Unscheduled human WI-38 cells 250-1000 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Unscheduled rat primary hepatocytes 5-50 µg/ml - positive Williams et al.
DNA synthesis (1982)
Unscheduled rat primary hepatocytes 50 nmol/ml - negative Thompson et al.
DNA synthesis (1983)
Sister chromatid Chinese hamster ovary 1600 µg/ml +/- positived US NTP (1989)
exchange cells
Chromosomal Chinese hamster ovary cells 1000 µg/ml +/- positived US NTP (1989)
aberrations and negative
Cell BALB-3T3 cells, 0.039-0.625 mg/ml not stated negative Matheson & Brusick
transformation in vitro (1978c)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b No increase in revertants, strains TA1538/TA1978 positive for differential killing
c Tested in three independent laboratories
d Tested in two independent laboratories
According to the findings presented, neither diflubenzuron nor
its major metabolites may be considered to have mutagenic effect.
Several positive effects were, however, obtained with PCA.
7.7 Carcinogenicity
Long-term carcinogenicity studies were described in section 7.3.
In both mouse and rat oncogenicity studies, diflubenzuron at dose
levels up to 10 000 mg/kg feed caused no change in tumour profile or
onset of tumours. In the rat oncogenicity study, the incidence of
sarcoma in the spleen and phaeochromocytomas was not increased. In
the mouse oncogenicity study, the incidence of hepatocellular
neoplasms or haemangiosarcomas in spleen and liver was not increased.
Therefore, diflubenzuron, in combination with its metabolites as
generated in the animal metabolic system, is not oncogenic.
Significantly, there were no non-neoplastic or neoplastic lesions of
the vasculature, including that of liver and spleen, in B6C3F1 male
mice treated with diflubenzuron. Similarly, there were no fibrotic or
carcinomatous lesions in the spleen of treated male F-344/N rats.
7.8 Other special studies
Diflubenzuron has been studied in mice for its growth-inhibiting
activity in serially transplanted B16 malignant melanoma and CA1025
skin carcinoma. A single 800 mg/kg intraperitoneal injection of
diflubenzuron induced rapid (24 h) decreases in tumour volume in 78%
and 66% of the tumours, respectively, while in control mice 85% of the
melanomas and 91% of the skin carcinomas increased in volume over the
same time period (Jenkins et al., 1984). These observations were
later confirmed, and it was suggested that the activity was due to
derivatives of a hydroxylated metabolite (Jenkins et al., 1986).
Studies of nucleoside uptake by Harding Passey melanoma cells
in vitro indicated a rapid (< 5 min) inhibition of the uptake of
uridine, adenosine and cytidine, but not of thymidine, by
diflubenzuron; this could not be reversed by washing. De novo
nucleic acid synthesis was not impaired and in vitro cell growth was
unaffected (Mayer et al., 1984).
El-Sebae et al. (1988) tested the effect of diflubenzuron on
protein and RNA biosynthesis by rabbit liver and muscle tissues kept
in an incubation medium. The synthesis of protein and RNA was
significantly stimulated in the liver and inhibited in the muscle by
graded doses. The maximum effect on both tissues was reached at 5 µg
diflubenzuron/ml for protein synthesis and at 0.2 µg/ml for RNA
synthesis, the effect on protein synthesis being more pronounced than
that on RNA synthesis in both tissues.
7.8.1 Special studies on met- and sulfhaemoglobin formation
The ability of diflubenzuron to induce methaemoglobin and
sulfhaemoglobin formation has been recognized since the initial
toxicity studies on the compound. Methaemoglobinaemia has been
demonstrated after oral, dermal and inhalatory exposure to
diflubenzuron in various species (see section 7.2 and 7.3). It is the
most sensitive parameter in this case.
Fifteen male Wistar rats received diflubenzuron (technical) by
gastric intubation at a dose level of 5000 mg/kg body weight per day
for 8 days. No effect was observed on Heinz body formation, whereas
met- and sulfhaemoglobin levels were significantly increased when
compared with the control group. The increase in the methaemoglobin
level was about 6% (Keet, 1977a).
When diflubenzuron was administered to male Wistar rats for 28
days at oral doses of 100 and 500 mg/kg body weight, it induced
elevation of methaemoglobin concentration and reticulocytes count in
both of the treated groups. However, there was no dose-response
relationship at the dose levels investigated (Tasheva & Hristeva,
1991, 1993).
Technical diflubenzuron was administered by gastric intubation to
male Swiss mice daily for a period of 14 days at dose levels of 0, 8,
40, 200, 1000 and 5000 mg/kg body weight. Body weight measurement and
macroscopic evaluation did not reveal any effect of the treatment. At
dose levels of 1000 and 5000 mg/kg the percentages of methaemoglobin
and erythrocytes containing Heinz bodies were increased. The
sulfhaemoglobin level was statistically significantly increased at
200, 1000 and 5000 mg/kg in comparison to the control group. The NOEL
was considered to be 40 mg/kg body weight based on sulfhaemoglobin
(Keet, 1977b).
When female mice were fed 0, 50, 200, 400, 1000 and 2000 mg
diflubenzuron/kg feed for 30 days, sulfhaemoglobin was demonstrated in
the blood from 200 mg/kg onwards (being 13% of total haemoglobin at
2000 mg/kg). Mice fed 1000 and 2000 mg/kg showed signs of cyanosis
after 3 weeks. Recovery was completed after a 3-week withdrawal
period (Bentley et al., 1979).
When 15 male New Zealand White rabbits were fed technical or
analytically pure diflubenzuron (640 mg/kg feed) for 21 or 18 days,
respectively, the methaemoglobin and sulfhaemoglobin levels were
significantly increased (Keet, 1977c).
Male and female cats (24 of each sex per group) received
diflubenzuron orally for 21 days at dose levels of 0, 30, 70, 100, 300
and 1000 mg/kg body weight and were observed for the subsequent 14-day
period. A dose-related elevation of methaemoglobin level was observed
with ceiling values at 300 and 1000 mg/kg. For male cats the NOEL was
30 mg/kg, but no NOEL was achieved for females. Increased
sulfhaemoglobin and Heinz bodies were observed in all treated groups.
NOEL values for sulfhaemoglobin were not achieved. The haemoglobin
concentration, reticulocyte number and organ weights were not affected
by the treatment (Schwartz & Borzelleca, 1981).
After dermal application of technical diflubenzuron at a dose
level of 1.5 ml/kg body weight for 18 days to rabbits, the
methaemoglobin level was increased (Keet, 1977c).
The available data demonstrate that dose-response relationship
for production of methaemoglobin exists. This is considered to be the
most sensitive end-point after repeated exposure in experimental
animals.
Table 10 summarizes the effects on methaemoglobinaemia as
determined in various studies.
7.9 Toxicity of metabolites
In rat metabolic studies it was shown that about 20% of absorbed
diflubenzuron is metabolized to 2,6-DFBA and its counterpart 4-CPU.
Only a small fraction of the 4-CPU is metabolized to PCA (see
section 6).
The acute oral toxicity of the major metabolites of diflubenzuron
is summarized in Table 11.
Loss of activity, catatony, paralysis and severe bradypnoea were
observed in rats treated with the metabolite 4-CPU. The minimum
symptomatic dose level was 100 mg/kg body weight. At autopsy, the
animals showed congested blood vessels and haemorrhage in the
gastrointestinal tract (Koelman-Klaus, 1978a).
Rats dosed with 2,6-DFBA showed slight increase in startle
response, activity, abdominal and limb tone, slight decrease in
grooming activity, slightly abnormal gait and body posture, mild
restlessness, irritation and aggressivity, pilo-erection and increased
alertness. The minimum symptomatic dose level was 464 mg/kg body
weight (Koelman-Klaus, 1978b).
Mutagenicity tests have been carried out with 2,6-DFBA, 4-CPU and
PCA (see section 7.6 and Table 9).
Table 10. Summary of the effects on methaemoglobinaemia in various species
Species Route Duration No-observed-effect level Reference
Rat diet 4 weeks male: not achieved; female: 800 mg/kg feed Palmer et al. (1977)
(equivalent to 45 mg/kg per day)
Rat diet 13 weeks not established - lowest dose tested 160 mg/kg feed Burdock et al. (1980b)
Rat diet 104 weeks 40 mg/kg feed (equivalent to 2 mg/kg Hunter et al. (1976)
per day body weight)
Mouse oral 2 weeks 200 mg/kg body weight Keet (1977b)
Mouse diet 13 weeks male/female: 16 mg/kg feed (equivalent to Burdock et al. (1980a)
2.4 mg/kg body weight per day)
Mouse diet 14 weeks not established - lowest dose tested 80 mg/kg feed Colley et al. (1981a,b)
Mouse diet 91 weeks male/female: 16 mg/kg feed (equivalent to Colley et al. (1984)
2.4 mg/kg body weight per day)
Cat oral 3 weeks male: 30 mg/kg body weight; female: not achieved Schwartz & Borzelleca (1981)
Dog diet 13 weeks male/female: 40 mg/kg feed Chesterman et al. (1974)
Dog oral (capsules) 52 weeks male/female: 2 mg/kg body weight Greenough et al. (1985)
Table 11. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50 Reference
(mg/kg)
4-Chlorophenylurea rat male oral 1080 Koelman-Klaus
female oral 1210 (1978a)
2,6-Difluorobenzoic rat male, oral 4640 Koelman-Klaus
acid female (1978b)
7.9.1 Carcinogenicity studies with 4-chloroaniline
The diflubenzuron metabolite, 4-chloroaniline (PCA), has been
assayed for carcinogenicity by the US NCI (1979) and by the US NTP
(1989) using Fischer-344 rats and B6C3F1 mice on both occasions. In
the earlier of these studies, technical-grade PCA was administered at
dietary concentrations of 250 and 500 mg/kg to rats and 2500 and
5000 mg/kg to mice. Groups of 50 male and 50 female animals of each
species were randomized to the treatment groups at approximately six
weeks of age. The control groups consisted of 20 animals of each sex
and species. All animals which survived were treated for 78 weeks and
observed, untreated, for a further 24 weeks (rats) or 13 weeks (mice).
Survival in all groups was good and it was judged that there were
adequate numbers at risk for late-developing tumours. In rats, the
most significant findings were treatment-related proliferative splenic
capsular and parenchymal lesions in males and females and, in male
rats of the high-dose group, the occurrence of several types of
unusual splenic neoplasms (i.e. fibroma, fibrosarcoma, sarcoma NOS,
haemangiosarcoma and osteosarcoma) which appeared to arise from areas
of capsular or parenchymal fibrosis. These neoplasms were combined
for analysis because it was considered that the fibromas were a benign
form of sarcoma and that the neoplasms had a common cellular origin.
The combined incidences were 0/20 control, 0/49 low-dose and 10/49
high-dose rats. This result indicated a carcinogenic effect of
treatment in male rats. There was no similar effect in the spleen of
females. In mice of each sex, there was increased incidence of
haemangiomas and haemangiosarcomas in various organs. The combined
incidences were 2/20 in control males, 10/50 in low-dose males and
14/50 in high-dose males, and 0/18 in control females, 3/49 in low-
dose females and 8/42 in high-dose females. In addition, there was,
in female mice only, a non-significant increase in hepatocellular
carcinomas and adenomas combined (0/18 control, 1/49 low-dose and 6/41
high-dose). The smaller number of control group animals in these
experiments significantly reduced the statistical power. It was
recognized that PCA is unstable in feed and so the animals in the
early experiments may have received lower doses than intended.
Consequently, PCA was administered by gavage with an aqueous vehicle
containing hydrochloric acid in the later experiments (US NTP, 1989).
Groups of 49 or 50 rats and mice of each sex (7 to 8 weeks old)
were administered PCA at dose levels of 2, 6 or 18 mg/kg (rats) or 3,
10, or 30 mg/kg (mice) on 5 days per week for 103 weeks. Vehicle
control groups of 50 males and 50 females received deionized water by
gavage. Survival was adequate for analysis, although variable. It was
lower, for example, in the vehicle control groups of rats, which was
attributable to the higher incidence of mononuclear cell leukaemia in
these groups. Significant, non-neoplastic findings in rats included
treatment-related increases in the incidence of splenic fibrosis
(males: 3/49 control, 11/50 low-dose, 12/50 mid-dose, 41/50 high-dose;
females: 1/50 control, 2/50 low-dose, 3/50 mid-dose, 42/50 high-dose),
lipocytic infiltration of the spleen (males: 0/49, 0/50, 0/50, 24/50;
females: 0/50, 0/50, 0/50, 11/50) and adrenal medullary hyperplasia
in female rats (4/50, 4/50, 7/50, 24/50). In addition, the
methaemoglobin level was consistently increased in the mid- and high-
dose groups of male rats at 6, 12, 18 and 24 months. There was no
NOEL for this parameter in male rats. Bone-marrow hyperplasia was
also observed. The combined incidences of uncommon splenic sarcomas
(fibrosarcomas, osteosarcomas or haemangiosarcomas) were increased in
male rats but not in females (males: 0/49, 1/50, 3/50, 38/50; females:
0/50, 0/50, 1/50, 1/50). There was also a small increase in
phaeochromocytomas or malignant phaeochromocytomas in male rats
(13/49, 14/48, 15/48, 26/49). The incidences of mononuclear cell
leukaemia were reduced in male and female rats of the treatment groups
(males: 21/49, 3/50, 2/50, 3/50; females: 10/50, 2/50, 1/50, 1/50).
This reduction may be related to splenic toxicity, since splenectomy
of Fischer rats at one to two months of age markedly reduces the
incidence of mononuclear cell leukaemia later in life (Boorman et al.,
1990). However, similar reductions in the incidence of this neoplasm
are not seen with several other aniline-like chemicals that also cause
splenic toxicity. In male mice, but not in females, the incidence of
haemangiosarcomas of the liver and spleen was slightly greater in the
high-dose group than in the controls (males: 4/50, 4/49, 1/50, 10/50)
and the incidences of hepatocellular carcinomas or adenomas (combined)
were also increased in treated male mice (males: 11/50, 21/49, 20/50,
21/50). The incidences of malignant lymphomas were slightly reduced
in treated male and female mice (males: 10/50, 3/49, 9/50, 3/50;
females: 19/50, 12/50, 5/50, 10/50).
There are various points of similarity between the two studies
described above: (a) in male and female rats there was splenic
toxicity; (b) in male rats, there was a treatment-related increase in
uncommon splenic sarcomas; and (c) in male mice, there was a
treatment-related increase in haemangiosarcomas and haemangiomas
combined. The reductions in the incidence of mononuclear cell
leukaemia in rats and malignant lymphomas in mice seen in the latter
study were not observable in the earlier study because their incidence
in the control groups of that study was already low. It is concluded
that PCA is carcinogenic in both mice and rats.
8. EFFECTS ON HUMANS
No data concerning the effects of diflubenzuron on human health
are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Diflubenzuron was tested for its effects on morphogenesis in
Streptomyces spp. Exposure to 400 mg diflubenzuron/litre resulted in
reduced dominance of spore hairs and reduced width of the outer wall,
and prevented formation of the inner spore wall in S. babergiensis.
In S. coelicolor, 1600 mg diflubenzuron/litre altered the structure
of the fibrillar pattern of spore envelopes. Exposure to diflubenzuron
resulted in small increases in exported protein and in an approximately
20% increase in chitinase in both Streptomyces spp (Smucker &
Simon, 1986).
9.1.1.1 Water
Aquatic bacterial biomass and density were not affected by
diflubenzuron (1.0 µg/litre), although after three months of
continuous exposure some decline in species diversity was found.
Owing to detrimental effects on aquatic arthropods, e.g., filter
feeders that may use bacteria as a food source, such effects may very
well be secondary in nature and not a direct effect of diflubenzuron
(Hansen & Garton, 1982b).
In a test on the alga Selenastrum capricornutum, a nominal
concentration of 0.20 mg diflubenzuron/litre did not reduce the
biomass and can be considered a no-observed-effect concentration
(NOEC) (Berends & Thus, 1992b).
9.1.1.2 Soil
Soil microorganisms were able to use diflubenzuron as carbon
source when added as an acetone solution (Seuferer et al., 1979; see
section 4.3.2.2).
The effect of diflubenzuron (100, 200, 300, 400 and 500 mg/kg
soil) was studied in non-sterile soil incubated under aerobic
conditions and in sterilized soil inoculated with Azotobacter
vinelandii. The presence of diflubenzuron had a stimulatory effect
on nitrogen fixation in both non-sterile and sterile soil (Martinez-
Toledo et al., 1988a). At similar concentrations, diflubenzuron did
not affect the growth of Azotobacter vinelandii in culture media,
either with or without a nitrogen source (Martinez-Toledo et al.,
1988b).
9.1.2 Aquatic organisms
9.1.2.1 Microorganisms
Cyanobacteria (the blue-green alga Plectonema boryanum) grew
rapidly in the presence of diflubenzuron (initial concentration
0.1 mg/litre) with no visible signs of inhibited growth (Booth &
Ferrell, 1977). Concentrations of 1, 10, 50 and 100 mg/litre did not
affect the growth of six species of fungi: Rizopus arrhizus,
Aspergillus niger, Fusarium oxysporum, Mycorrhizae-Rhizopogan
vinicolor, Pythum debaranum and Trichoderma viride (Booth et al.,
1987). However, these authors autoclaved the medium containing
diflubenzuron, which led to extensive breakdown of the pesticide
(Willems et al., 1977).
Five-day lethality tests on the algae Selenastrum capricornutum
and Anabaena flos-aquae resulted in NOAEC values above the exposure
concentrations of 300 and 330 µg/litre, respectively (Thompson &
Swigert, 1993b,c). Similarly, tests on the diatoms Navicula
pelliculosa and Skeletonema costatum led to NOAEC values of 380
and 270 µg/litre, respectively (Thompson & Swigert, 1993d,e).
Algae were affected at 1.0 µg/litre by technical diflubenzuron in
dimethylformamide added to laboratory stream channels: the alga
biomass increased and chlorophyll and phaeocitin levels were elevated.
Fungi were only affected temporarily by 0.1 µg/litre under the same
circumstances. Changes in species diversity had disappeared after
2 months of continuous dosing (Hansen & Garton, 1982 b).
9.1.2.2 Plants
In a 14-day toxicity test on diflubenzuron in duckweed
(Lemna gibba), the NOAEC was higher than the tested concentration
(190 µg/litre) (Thompson & Swigert, 1993a).
9.1.2.3 Invertebrates
The acute toxicity of diflubenzuron to a number of non-target
aquatic invertebrates is presented in Table 12. Comprehensive eviews
on the subject have been published recently, e.g., by Fischer & Hall
(1992) and by Cunningham (1986) on the effects of diflubenzuron on
estuarine crustaceans. The acute LC50 for insects ranges from
1 µg/litre (Diptera) to 250 µg/litre (Coleoptera), and mayflies
have an LC90 of 1 to 10 µg/litre. Other aquatic arthropods such as
water fleas, scuds and sow bugs have LC50 values of 5 to 15 µg/litre.
Table 12. Acute toxicity of diflubenzuron for non-target aquatic invertebrates
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Daphnia magna 1st instar stat 22 40 7.2 48-h EC50 15 Julin & Sanders (1978)
(Water flea)
Daphnia magna 24 h stat 20 50 48-h LC50 4.55 Hansen & Garton (1982a)
(Water flea) stat 20 100 48-h LC50 6.89 Hansen & Garton (1982a)
stat 20 200 48-h LC50 4.42 Hansen & Garton (1982a)
Daphnia magna 0-24 h 20 294 8.1 48-h EC50 7.1 Kuijpers (1988)
(Water flea) 20 294 8.1 24-h EC50 68 Kuijpers (1988)
Gammarus pulex mature stat 12 40 7.2 96-h LC50 30 Julin & Sanders (1978)
(Scud)
Hyallela azteca 2-4 mm flow 20 25 96-h LC50 1.84 Hansen & Garton (1982a)
(Amphipod)
Chironomus plumosus 4th instar stat 22 40 7.2 48-h EC50 560 Julin & Sanders (1978)
(Midge)
Cricotopus sp. 4th instar flow 20 25 EC50 (moulting 1.72 Hansen & Garton (1982a)
(Midge) success)
Tanytarsus dissimilis 2nd instar flow 20 25 EC50 (moulting 1.02 Hansen & Garton (1982a)
(Midge) success)
Acartia tonsa adult constant 20 10b 5-day LC50 > 1000 Tester & Costlow
(Copepod) daily (1981)
replenishment
Table 12 (Con't)
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Mysidopsis bahia adult intermittent 24-25 24-27b 96-h LC50 2.06 Nimmo et al. (1980)
(Mysid shrimp) flow
adult continuous 24-26 23-29b 21-day LC50 1.24 Nimmo et al. (1980)
flow
Palaemonetes pugio larvae stat 22 20b 96-h LC50 1.44 Wilson & Costlow
(Grass Shrimp) (1987)
post larval renewal 22 20 96-h LC50 1.62 Wilson & Costlow (1987)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Salinity (expressed as parts per thousand)
Concentrations of diflubenzuron causing significant mortality of
several freshwater invertebrates are presented in Table 13.
Table 13. Concentrations of diflubenzuron causing significant
mortality of freshwater invertebratesa
Organisms Concentration
(µg/litre)
Water flea (Daphnia magna) 2.0
Amphipod (Hyalella azteca) 2.0
Snail (Juga plicifera) > 36
Snail (Physa spp.) > 36
Caddis fly (Clisforonia magnifica) 0.1
Midge (Tanytarsus dissimilis) 4.9
Midge (Cricotopus spp.) 1.6
a From: Nebeker et al. (1983)
The 96-h LC50 for the snails Juga plicifera and Physa sp.
was > 45 µg/litre (Hansen & Garton, 1982a).
The 48-h EC50 values of diflubenzuron metabolites for midge
larvae were > 100 mg/litre for 4-CPU and 2,6-DFBA and 43 mg/litre for
PCA (Julin & Sanders, 1978).
Diflubenzuron suspended in water at a concentration of
200 mg/litre was not directly toxic to the freshwater clam Anodonta
cygnea during 3 months of treatment. Diflubenzuron produced
disturbances in the calcification process in the lamellar layer of the
shell. Positive PAS reaction of the secretory cells on the outer
mantle epithelium has been observed (Machado et al., 1990).
Daphnia magna was continuously exposed to 14C-diflubenzuron at
concentrations of 5.6, 14, 23, 40 and 93 ng/litre. After 21 days of
exposure daphnid survival at the highest concentration (93 ng/litre)
was 50%. In the remaining concentrations it ranged from 93 to 98%,
which was comparable to the survival (99%) of the control organisms.
Reproduction and body length were affected only at the highest
concentration. The maximum acceptable toxicant concentration (MATC)
of 14C-diflubenzuron for Daphnia magna was > 40 and < 93 ng/litre
(Surprenant, 1988).
Technical diflubenzuron in dimethylformamide at 0.1, 1, 10 and
50 µg/litre was added continuously to complex laboratory stream
channels supplied periodically with field-collected microorganisms for
5 months. LC50 values ranging from 1.0 to 1.8 µg/litre were
determined for four insect and crustacean species ( Tanytarsus
dissimilis, Cricotopus sp. and Hyalella azteca). A chronic effect
level was obtained only for Daphnia magna (0.06 µg/litre). Mayflies
and stoneflies were the most sensitive. They were severely affected
at 1 µg/litre within one month (the sampling interval) and numbers
were two to three orders of magnitude lower, almost leading to their
elimination. The survival of chironomids was reduced by 10 µg/litre
(Hansen & Garton, 1982a,b) (see also section 9.1.1.1).
Benthic communities in outdoor experimental streams were exposed
to 1 or 10 mg/litre of diflubenzuron for 30 min. The effect was
assessed daily by examining drifting pupal exuviae over a period of
one month following the treatment. No drift of macrobenthos was
induced at the time of application. However, diflubenzuron affected
the emergence of all species examined. High larval mortality for a
species of chironomid was observed directly in the stream treated with
diflubenzuron, where numbers of mayfly nymphs and caddisfly larvae
were also decreased (Yasuno & Satake, 1990).
Technical diflubenzuron in acetone was applied at 5 µg/litre to
two aquaria in a simulated field test outdoors. Daphnid numbers were
markedly reduced three days after treatment, but recovered slowly.
Copepods numbers were moderately reduced, exceeding the control
numbers after 18 days. The seed shrimp population showed no harmful
effect (Miura & Takahashi, 1974a,b).
In a study by Collwell & Schaefer (1980), diflubenzuron was
applied to experimental ponds (mean concentration of 13.2 µg/litre) in
California. An hour after treatment, cladoceran ( Ceriodaphnia sp.,
Diaphanosoma sp., Chydorus sp., Bosmina sp. and Daphnia sp.)
numbers were strongly reduced and did not return to pretreatment
levels until more than 5 weeks after treatment. Copepod ( Diaptomus
sp. and Cyclops sp.) numbers were also reduced but to a lesser
extent and for a shorter period than for the cladocerans. The
rotifers increased in abundance in both the control and treated ponds
during the first 8 days following treatment.
Using laboratory tests to study mortality, Miura & Takahashi
(1974a) found that crustaceans, especially the tadpole shrimp
(T. longicaudatus), clam shrimp ( Eulimnadia spp.) and water fleas
( Daphnia and Moina spp.), were highly susceptible to diflubenzuron
at levels below 0.01 mg/litre. Copepods, Cyclops and Diaptomus
spp. showed some tolerance, whereas seed shrimp ( Cypricerus and
Cypridopsis spp.) tolerated as much as 0.5 mg/litre. Among aquatic
insects tested, mayfly nymphs ( Callibaetis spp.) were most
susceptible. Aquatic midge larvae (G. holoprasinus) also
showed susceptibility. However, dytiscid ( T. bassillaris and
Laccorhilus spp.) and hydrophilid beetles ( H. triangularis and
T. lateralis (mosquito predators)) demonstrated a strong tolerance.
Mosquito fish (Gambusia affinis) showed no effect at a relatively
high dose of
1 mg/litre.
When larvae of the crab Rhithropanopeus harrisii were exposed
to sublethal concentrations of diflubenzuron (0.05, 0.1, 0.3 and
0.5 µg/litre), swimming speed increased in stage I, II and III zoeae,
0.3 µg/litre being the lowest effective concentration. Phototaxis was
altered in stage IV only at concentrations as low as 0.1 µg/litre
(Forward & Costlow, 1978).
Christiansen et al. (1978) showed that nearly 100% of
Rhithropanopeus harrisii larvae at each of the four zoeal stages
died when moulting to the succeeding stage after only 3 days of
exposure to 10 µg diflubenzuron/litre. This concentration was also
lethal for larvae of Sesarma reticulatum (Say).
Christiansen & Costlow (1980) exposed larvae of the estuarine
crab Rhithropanopeus harrisii in laboratory conditions to 10 µg
diflubenzuron/litre as an indicator of persistence of
diflubenzuron in brackish water. Disturbance of endocuticle
deposition seemed to occur as soon as newly hatched larvae (less than
12 h old) were exposed to diflubenzuron. Diflubenzuron not only
affected endocuticle deposition in the larvae, but also exocuticle
deposition. The only part of the crab larval exoskeleton that did not
seem to be affected by diflubenzuron was the epicuticle (Cristiansen
et al., 1978; Cristiansen & Costlow, 1982).
Diflubenzuron, at concentrations of 0.02 and 0.2 mg/litre,
enhanced mortality during moulting of the crab Carcinus
mediterraneus (Czerniavsky) (Cardinal et al., 1979).
Cirripede crustaceans, (barnacles, Balanus eburneus) exposed to
concentrations of 1 to 1000 µg/litre over a 28-day period showed a
dose-dependent mortality. Heavy mortality occurred during the second
week of exposure. Lethal and sublethal effects were observed at
concentrations as low as 50 µg/litre (Gulka et al., 1980). Disruption
of the exoskeleton of B. eburneus caused by diflubenzuron was
similar to that observed in insects. Development of barnacles exposed
to diflubenzuron for 10 days or more at 750 and 1000 µg/litre was
delayed in the premoult phase of cuticle secretion (Gulka et al.,
1982).
Wilson & Costlow (1986) found that diflubenzuron concentrations
of 2.5 and 5 µg/litre were lethal to larvae of the grass shrimp
(Palaemonetes pugio), causing 100% mortality on days 14 and 6,
respectively. Wilson et al. (1985) studied the effects of
diflubenzuron upon phototaxis of larvae of P. pugio. The depression
in positive phototaxis and elevation in negative phototaxis were most
pronounced at 0.5 µg/litre, and the lowest test concentration to
affect phototaxis was 0.3 µg/litre. The alterations in photo
responses varied with the embryonic stage at which exposure to
diflubenzuron commenced. This study was carried out at optimal
salinity and temperature, but in the estuary these conditions
fluctuate daily, which may amplify the observed effects.
Nimmo et al. (1980) observed that chronic exposure of the mysid
shrimp Mysidopsis bahia to 0.075 µg diflubenzuron/litre reduced the
reproductive success of both parents and progeny even after they were
transferred to uncontaminated sea water.
Diflubenzuron reduced the reproductive life span of adult brine
shrimps (Artemia salina) at levels of 2-10 µg/litre and caused death
of immature shrimps within 3 days at concentrations above 10 µg/litre
(Cunningham, 1976).
Fiddler crabs (Uca pugilator) were exposed to diflubenzuron
(Dimilin WP-25%) at 0.5, 5 and 50 µg/litre for 1, 2, 3 and 4 weeks
after multiple autonomy of one chela and five walking legs. Exposure
to diflubenzuron retarded the rate of limb regeneration in a dose-
dependent fashion. The effects of diflubenzuron were seen even in
crabs exposed for only one week. Significant retardation was evident
by day 21 at 5.0 µg/litre but was not statistically significant at
0.5 µg/litre. At 5 and 50 µg/litre moult-associated mortality was
seen. The number of setae on regenerated limbs was less than the
number on the intact limbs. The effects were reduced in experiments
in which sediment was present (Weis et al., 1987).
The burrowing activity of Uca pugilator in sand under
laboratory conditions was not altered when the sand was contaminated
with 1 mg diflubenzuron/litre, indicating a lack of avoidance of
diflubenzuron-contaminated sand. However, exposure for 1 week to 0.5,
5.0 or 50 µg/litre led to a decrease in the amount of burrowing
activity. The behavioral response was not changed after exposure for
1, 2 or 3 weeks and was not concentration-related (Weis & Perlmutter,
1987).
Survival, moulting and behaviour of juvenile fiddler crabs were
significantly affected by exposure to diflubenzuron (0.2, 2, 20 and
200 µg/litre) for 24 h weekly during 10 weeks. All crabs in the 200
and 20 µg/litre groups died after 8 and 23 weeks, respectively. The
no-observed-effect concentrations (NOEC) for moulting (time to the
first moult), survival (time until death), and behaviour (ability to
escape from the test container) were 20, 2 and 0.2 µg/litre,
respectively (Cunningham & Myers, 1987).
Larvae of the stone crab (Menippe mercenaria) were exposed to
0.5, 1.0, 3.0, and 6.0 µg diflubenzuron/litre in combination with
different temperature and salinity. All of these concentrations were
lethal to the larvae. Evidence for synergistic effects of
diflubenzuron and temperature or salinity was observed. Tolerance of
the megalopa of the blue crab Callinectes sapidus to diflubenzuron
at concentrations of 0.5, 1.0, 3.0 and 6.0 µg/litre was slightly
higher than for Menippe mercenaria but was also dependent upon
temperature and salinity. At 20°C the percentage survival at a
concentration of 1 µg/litre was similar to that observed for the
controls (Costlow, 1979).
Tester & Costlow (1981) reported that the marine copepod
Acartia tonsa exposed to 1 and 10 µg diflubenzuron/litre for 36 h
failed to produce viable nauplii even after they had been placed in
clean sea water. No viable nauplii were produced by these females for
at least 30 h after treatment ended.
Adult crustaceans were more resistant to exposure than their
larvae at high concentrations (100-200 µg/litre) of technical grade
diflubenzuron. Adults also exhibited significant mortality associated
with moulting (Cunningham, 1976; Cardinal et al., 1979; Gulka et al.,
1980).
When larvae of horseshoe crabs (Limulus polyphemus) were
exposed to 5 and 50 µg diflubenzuron/litre, the crabs in the
50 µg/litre group exhibited severe mortality immediately after
ecdysis. The larval stages were shown to be quite resistant to
diflubenzuron, compared with other crustacean larvae (Weis & Ma,
1987).
9.1.2.4 Vertebrates
Data on the acute toxicity of diflubenzuron and its metabolites
for fish are presented in Tables 14 and 15, respectively.
A 96-h acute toxicity test on juvenile sheepshead minnow
(Cyprinodon variegatus) in a flow-through system resulted in no
mortality at the nominal exposure concentration of 130 µg
diflubenzuron/litre (Graves & Swigert, 1993). A similar test under
semi-static conditions on zebra fish (Brachydanio rerio) and rainbow
trout (Oncorhynchus mykiss) at a nominal diflubenzuron concentration
of 200 µg/litre also showed no mortality, nor changes in the appearance
Table 14. Acute toxity of diflubenzuron to fish
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Coho salmon 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus kisutch) Pridmore (1978)
Rainbow trout 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus mykiss) Pridmore (1978)
Rainbow trout 1.2 g stat 12 40 7.2 96-h LC50 240 Julin & Sanders
(Oncorhynchus mykiss) (1978)
Rainbow trout not flow-through 96-h LC50 140 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Rainbow troutb not flow-through 96-h LC50 195 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Fathead minnow 0.87 g stat 22 40 7.2 96-h LC50 430 Julin & Sanders
(Pimephales promelas) (1978)
Channel catfish 2.2 g stat 22 40 7.2 96-h LC50 370 Julin & Sanders
(Ictalurus punctatus) (1978)
Bluegill 0.5 g stat 22 40 7.2 96-h LC50 660 Julin & Sanders
(Lepomis macrochirus) (1978)
Bluegill not flow-through 96-h LC50 135 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Bluegillb not flow-through 96-h LC50 230 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Table 14. (Con't)
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Mummichog adult 2.7 g stat 24 22 ppt 8.0 96-h LC50 32 990 Lee & Scott
(Fundulus heteroclitus) renewal salinity (1989)
Mummichogb not flow-through 96-h LC50 255 Marshall & Hieb
(Fundulus heteroclitus) reported (1973)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Formulated product 25 WP
Table 15. Acute toxicity of metabolites of diflubenzuron to fish (from: Julin & Sanders, 1978)
Concentrations (mg/litre)
Organism Water Effect
temperature measured
(°C) 4-Chlorophenyl 2,6-Difluorobenzoic 4-Chloroaniline
urea acid
Rainbow trout 12 96-h LC50 72 (57-90) > 100 14 (11-16)
(Oncorhynchus mykiss)
Channel catfish 22 96-h LC50 > 100 > 100 23 (18-29)
(Ictalurus punctatus)
Fathead minnow 22 96-h LC50 > 100 69 (55-87) 12 (7-18)
(Pimephales promelas)
Bluegill 22 96-h LC50 > 100 > 100 2.4 (1.8-3.2)
(Lepomis macrochirus)
or behaviour of the fish (Berends & van der Laan-Straathof, 1994a,b).
All these test concentrations were above the diflubenzuron solubility
level of 80 µg/litre.
Nebeker et al. (1983) found no significant reduction in
survival of fathead minnow (Pimephales promelas) or guppy
(Poecilia reticulata) as a result of exposure to diflubenzuron at
concentrations below 36 µg/litre during acute (96-h) and chronic
tests.
No acute response resulted from exposure of fish to a
diflubenzuron concentration of 45 µg/litre, and no chronic effects
were observed at this concentration, the highest one tested (Hansen &
Garton, 1982a,b).
Diflubenzuron was not toxic to either rainbow trout or coho
salmon exposed at concentrations up to 150 mg/litre for a 96-h period.
A 15-min exposure to 1 g/litre did not result in any fish mortality
(McKague & Pridmore, 1978).
Madder & Lockhart (1978) found a dose-related decrease of
glutamic-oxaloacetic transaminase activity in rainbow trout
(Oncorhynchus mykiss) exposed to diflubenzuron at concentrations of
0.625, 1.25, 2.5, 5 and 10 mg/litre.
At a concentration of 0.01 mg/litre, diflubenzuron had a
repellent effect on precocious male Atlantic salmon parr (Granett et
al., 1978).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Photosynthesis, respiration and leaf ultrastructure of soybeans
were unaffected by diflubenzuron at doses up to a level of
0.269 kg a.i./ha (Hatzios & Penner, 1978).
Diflubenzuron is used as an insecticide in forestry, agriculture
and horticulture. No phytotoxicity was reported in the field studies
cited in section 9.2.
9.1.3.2 Invertebrates
The oral and contact LD50 values of diflubenzuron for honey-bees
are greater than 30 µg/bee (Stevenson, 1978).
Diflubenzuron did not show any toxicity to bees at concentrations
up to 1000 mg/kg in the diet (Yu et al., 1984).
Barker & Taber (1977) found that diflubenzuron reduced brood
production when fed for 10 days to honey-bees at 59 mg/kg in sugar
syrup, but not at 5.9 or 0.59 mg/kg.
Barker & Waller (1978) confirmed that 60 mg/kg in sugar syrup or
100 mg/litre in water caused colonies to produce less brood.
Stoner & Wilson (1982) found that diflubenzuron fed to flying
colonies at 1 or 10 mg/kg for a year significantly reduced the amount
of sealed brood.
Nation et al. (1986) reported that diflubenzuron did not cause
reduction in pollen consumption or brood production when fed for 10
weeks to caged colonies of honey-bees at 10 mg/kg, but it caused more
than 50% reduction in the amount of syrup stored.
Gordon & Cornect (1986) showed that, at concentrations that are
effective in suppressing egg hatching and larva development of the
cabbage maggot Delia radicum, diflubenzuron did not adversely
affect eggs, first-instar larvae or adults of the rove beetle
Aleochara bilineata, an important predator and parasitoid of the
cabbage maggot.
When the acute toxicity of diflubenzuron to the earthworm
Eisenia fetida was tested in artificial soil to which diflubenzuron
had been added, 780 mg/kg dry soil was the no-observed-effect
concentration (NOEC) for the 14-day test period (Berends et al.,
1992). For the WP-25 formulation, the NOEC was 1.0 g/kg (Berends &
Thus, 1992a).
9.1.3.3 Vertebrates
a) Birds
The acute oral LD50 of technical diflubenzuron for red-winged
blackbirds (Agelaius phoeniceus) is 3762 mg/kg body weight. In an
8-day dietary LD50 study on mallard duck and bobwhite quail using
technical diflubenzuron, levels up to 4640 mg/kg in the feed gave no
observable signs of toxicity (Maas et al., 1980).
When diflubenzuron was fed to mature White leghorn hens at
dietary levels of 10, 50, 100, and 500 mg/kg for 8 weeks, there were
no adverse effects on feed consumption, body weight, egg production,
egg weight, eggshell thickness, fertility, hatchability or progeny
performance. The highest dose used was 50 times higher than the
efficacy level for fly control (Cecil et al., 1981).
In chronic toxicity tests, groups consisting of 10 male and 10
female one-day-old chicks of barred Plymouth rocks and white leghorn
hens, Nicholas white turkeys, mallard ducks and ring-necked pheasants
were given diets containing diflubenzuron levels of 0.25, 1.25, 25 and
250 mg/kg for 91 days after hatching. No differences between control
and treatment groups were observed in mortality, food consumption,
body weight, comb and wattle development, weight of inner organs,
serum hormone levels or general behaviour (Maas et al., 1980).
There was no effect of diflubenzuron on the content of hyaluronic
acid in the skin of Hubbard broiler chickens when they were fed at
levels of 2.5 and 250 mg/kg in the diet for 98 days after hatching
(Deul & Jong, 1977). In an experiment with the same dose levels and
duration, diflubenzuron had no effect on hyaluronic acid synthesis or
comb deposition in either growing broilers or layers (Crookshank et
al., 1978).
White leghorn and black sex-linked cross hens were fed
diflubenzuron at a level of 10 mg/kg in the ration for 15 weeks.
Diflubenzuron had no effect on body weight gain, egg production,
fertility or hatchability (Miller et al., 1976b).
In a feeding study with 2.5, 25 and 250 mg/kg, diflubenzuron did
not affect bobwhite quail reproduction (Booth et al., 1987).
Diflubenzuron did not show significant teratogenic activity on chick
embryos over time when injected at 10 mg/egg (Booth et al., 1987).
A one-generation bobwhite quail (Colinus virginianus)
reproduction study was conducted in which a diflubenzuron-containing
diet was administered ad libitum to young adults (24 weeks old at
test initiation) approaching their first breeding season. Dietary
concentrations of 250, 500 or 1000 mg/kg did not result in treatment-
related mortality, overt signs of toxicity or effects upon adult body
weight or feed consumption during the 21-week exposure period. There
were no apparent treatment-related effects upon reproductive
parameters at 250 or 500 mg/kg. There may have been a slight reduction
in the number of eggs laid, although this was not statistically
significant or dose-related at 1000 mg/kg. On the basis of a possible
effect on egg production at 1000 mg/kg, the NOEC for diflubenzuron in
this study was above 500 mg/kg (Beavers et al., 1990a).
In a one-generation reproduction study on the mallard duck
(Anas platyrhynchos), diets containing diflubenzuron were
administered ad libitum to young adults (27 weeks old at test
initiation) approaching their first breeding season. Dietary
diflubenzuron concentrations of 250, 500 and 1000 mg/kg did not
result in treatment-related mortality, overt signs of toxicity or
effects upon adult body weight or feed consumption during the 20-week
exposure period. There were no apparent treatment-related effects
upon reproductive performance at any of the concentration tested. At
1000 mg/kg there was a slight, but statistically significant,
reduction in mean eggshell thickness. On the basis of the effect upon
eggshell thickness at 1000 mg/kg, the NOEC for diflubenzuron in this
study was 500 mg/kg (Beavers et al., 1990b).
b) Mammals
In studies by Ross et al. (1977a,b), diflubenzuron was
administered in the feed to sheep (3 of each sex per group) as a model
for ruminant wildlife at concentrations of 500, 2500 and 10 000 mg/kg
feed for 13 weeks. No treatment-related effects were observed on food
consumption, body weight gain, haematological parameters or
urinalysis. Increase in met- and sulfhaemoglobin levels were observed
at 13 weeks and there was a reduction in the weight of the thyroid.
No histopathological abnormalities were observed. Both the plasma and
the erythrocyte cholinesterase activities were unaffected by the
treatment after 6 weeks.
9.2 Field observations
9.2.1 Microorganisms
9.2.1.1 Water
The laboratory data available (see section 9.1.1.1) make it
unlikely that detrimental effects will occur.
Rotifers were unaffected by diflubenzuron (28 and 56 g a.i./ha)
in both experimental and naturally treated ponds (Ali & Lord, 1980).
9.2.1.2 Soil
One aerial application of diflubenzuron at 67.26 g/ha had no
adverse effects upon populations of bacteria, actynomycetes or fungi
in leaf litter and forest soil (Wang, 1975; Kurczewski et al., 1975).
9.2.2 Aquatic organisms
9.2.2.1 Plant
The laboratory data available (see section 9.1.2.2) make it
unlikely that detrimental effects will occur.
9.2.2.2 Invertebrates
Recent field studies have demonstrated that effects on aquatic
fauna are limited and transient, and that recovery is evident after
3 months (Huber & Collins, 1987; Kingsbury et al., 1987; Huber &
Manchester, 1988; Ali et al., 1988; Ali & Kok-Yokomi, 1989; Sundaram
et al., 1991).
Diflubenzuron (25% WP) applied at 33.63 and 134.52 g a.i./ha,
4 times at 2-week intervals, had no adverse effect on freshwater clams
10 days after final treatment (Jackson, 1976).
When diflubenzuron (25% WP) was applied at 1.121-280.25 g a.i./ha
to flooded rice fields in Louisiana, USA, significant reduction of
Tropisternus spp. and Libellulidae was found 80 days after
treatment. Significantly more chironomid and baetid immatures
occurred due to reduction in the number of predators (Steelman et al.,
1975).
Mulla et al. (1975) found that at an application rate of 28 and
56 g a.i./ha diflubenzuron reduced slightly, and only for a short
time, the number of mayfly ( Beatis sp.) in the treated ponds. The
numbers, however, appeared to be within natural fluctuation limits
equal to the check ponds or the pretreatment levels during all other
sampling periods. At these application rates, diflubenzuron caused a
short-term reduction in copepod populations, but they started to
increase on the 11th or 15th day of post-treatment sampling. There
was little or no effect on the ostracod population.
In a forestry spraying programme at 0.0672 kg a.i./ha, aquatic
arthropods were studied in a water shed (White Deer Creek). Sampling
at three test stations and one control station in the watershed
revealed a rich fauna, dominated numerically by Ephemeroptera,
Chironomida, Trichoptera and Plecoptera in descending order of
abundance. Significant differences in larval abundance in the surber
samples were found to occur from one sampling day to the next, or
between pre-spray and post-spray periods, for most of the organisms
examined. In all cases, control and test stations showed similar
patterns of variation, so treatment effects were discussed as a
probable cause. The number of organisms tended to be higher at the
upstream stations. Chironomida and Trichoptera pupal abundance in the
surber samples was examined for decreases after spraying but numbers
were too low to permit statistical analysis. Pupal abundance showed
some direct relationship to larval abundances. Nymphal drift rates
were examined for possible increases after spraying, but most of the
significant changes were decreases during the post-spray period.
These decreases also occurred at the control station. The drift rates
of nymphal and pupal exuviae also did not indicate a treatment effect.
Individual species abundances in the surber samples were examined for
possible selective effects of diflubenzuron. Comparisons were made
between pre-spray and post-spray periods at both test and control
stations, but no evidence of a treatment-related decrease in the
abundance of any of these organisms was found. The net effect was
usually an increase in the density of these organisms during the post-
spray period. No organisms showing abnormal ecdysis or pupation were
found in any of the samples. It was concluded that spraying with
diflubenzuron had no adverse effect on the macrobenthic community in
White Deer Creek (White, 1975).
In a study by Booth (1975), diflubenzuron (25% WP) was applied at
44.84 g a.i./ha to small ponds in Utah. Biosamples taken 30 and 80
days later showed that, although larva and immature aquatic insect
populations were decreased at 30 days, the total number of adults was
not significantly different from the control number at 80 days.
Booth & Ferrell (1977) examined the effect of diflubenzuron on
over 20 different species (corixidae and collembolans) after multiple
pond applications at 45 g a.i./ha. Only immature corixidae (water
boatmen) and collembolans (springtails) were significantly affected.
Segmented worms (Oligochaeta) and midges (Chironomidae)
were unaffected by six applications of diflubenzuron at a rate of
145.73 g a.i./ha to Utah Lake (Booth et al., 1987).
Diflubenzuron (28 and 56 g a.i./ha) reduced numbers of
Chaoborus sp. and Baetis sp. in both experimental and natural
treated ponds. Chaoborus sp. recovered within 1-3 weeks (Ali &
Lord, 1980).
The application of granular diflubenzuron at 0.11 kg a.i./ha
(about 3.7 µg/litre) to residential-recreational lakes caused a 62-75%
reduction of Daphnia pulex and Daphnia galeata during 7 days
following treatment. The populations recovered in the second week
after treatment. A 30% reduction in Diaptomus spp. was noted 2
days after the treatment. Hyalella azteca was affected markedly,
with a maximum reduction of 97% after 3 weeks of treatment. No
detectable effects on Cyprinotus sp., Cyclops sp. or Bosmina
longirostris were observed (Ali & Mulla, 1978a). Ali & Mulla
(1978b) also studied the impact of diflubenzuron on invertebrates in a
residential-recreational lake after two treatments with 25% WP
formulation at 156 g a.i./ha (about 12 µg/litre). Diflubenzuron
concentrations in water were not measured but were based on nominal
initial concentrations. The results from this study are summarized in
Table 16.
Diflubenzuron (25% WP) at rates of 0.02, 0.025, 0.03, 0.035,
0.04, 0.045 and 0.05 lb a.i./acre was applied to 19 irrigated pastures
and spring ponds (in some cases up to 4 times) at 2- to 3-week
intervals in California. Cladoceran and nymphal mayfly populations
were temporarily and slightly reduced, but recovered within a short
period of time. Even repeated treatment of the same pastures did not
eliminate the populations. The impact on copepod populations was
inconclusive. Adult beetles demonstrated high tolerance to
Table 16. Effect of diflubenzuron on invertebrates in Vallage Grove Lake, Californiaa
Species First application (April) Second application (August)
Effect Recovery Effect Recovery
Daphnia leavis elimination within no recovery 6 months
Birge 1 week after treatment
Ceriodaphnia sp. elimination within no recovery 6 months
1 week after treatment
Bosmina longirostris elimination within recovery after elimination after reappearance in small numbers
(O.F. Muller) 1 week 11 weeks 1 week 8-9 weeks after treatment
Cyclops spp. elimination within recovery within elimination within recovery after 4 weeks
1 week 6-7 weeks 1-2 weeks
Diaptomus sp. elimination within recovery after absent prior to reappearance in small numbers
1 week 4 months treatment 1-2 months later
Hyalella azteca elimination within no recovery 6 months
(Saussure) 4 weeks after treatment
Caenis sp. elimination within recovery within elimination within recovery in 4-5 weeks
3 weeks 6-7 weeks 2-3 weeks
Physa sp. no adverse effects no adverse effects
Cypridopsis sp. no adverse effects no adverse effects
a From: Ali & Mulla (1978b)
diflubenzuron, but few dead larvae ( Laccophilus spp., Hydrophilus
triangularis and Tropisternus lateralis) were observed. Spiders
( Pardosa spp. and Lycosa spp.) showed no apparent effects. There
were no deleterious effects on planarian, rotifer, seed shrimp and
fresh water flagellate populations (Miura, 1974).
Diflubenzuron (25 WP formulation) was applied 3 times at 2.5-week
intervals to man-made pools in Manitoba, Canada at 56 g/a.i. ha (this
produced initial concentrations of 0.02 mg/litre). The treatment
produced no detectable effect on chironomid larvae in terms of numbers
or composition, although dead larvae were noted after treatment.
Daphnid populations were significantly reduced on most sampling dates.
Repetitive treatments prevented the recovery of cladocerans. Despite
these reductions, daphnids were not annihilated. At a higher rate
(0.22 kg a.i./ha, about 7.4 µg/litre), both species of Daphnia were
eliminated for 3 months after treatment. Populations of Diaptomus
spp. declined to zero 7 days after treatment, but recovered during the
second week following treatment. Diflubenzuron caused reductions in
the number of H. azteca (32-100%) during the 2´ months following
treatment. Seed shrimp populations were stressed for only 2 weeks,
and there were no observable effects on oligochaetes at a treatment
level of 0.22 kg a.i./ha (about 7.4 µg/litre). Copepods only
occasionally showed significant reduction, and recovered within 10
days after treatment. Of the ten other non-target invertebrates (i.e.
Chaoboridae, Tipulidae, Ephemeroptera, Corixidae, Nonectidae,
Gerridae, Dystiscidae, Hydrophilidae, Hydrarina and Gastropoda),
only mayfly nymphs (Ephemeroptera) were significantly reduced
(Madder, 1977).
When diflubenzuron at a rate of 34.2 g/ha was applied aerially to
a forested area in the USA, there were no significant effects on the
population structure of the benthic macro-invertebrate community.
Some decreases in the number of mayflies Heptagenia sp. and
Rhithrogenia sp. were noted in treatment streams, but the total taxa
richness values remained high. An increase in abundance of the
caddisfly Lepidostoma sp. was found. Allonarcys sp. was not
affected by the treatment (Huber & Collins, 1987; Huber & Manchester,
1988).
After aerial application of diflubenzuron to two forest ponds in
Canada the greatest effect was on crustacean zooplankton, especially
cladocerans, with limited effect on pond benthos. Recovery of
population of even the most severely affected organism such as
Daphnids was well established by 3 months after treatment. The
maximum residue found in water was 13.82 µg/litre 1 h after
application (Kingsbury et al., 1987).
Diflubenzuron WP-25 was applied at a rate of 70 g active
ingredient in 10, 5 or 2.5 litre/ha to three spray blocks in a mixed
boreal forest near Kaladar, Ontario, Canada. Water, sediment and
aquatic plants were collected from two ponds and a stream at intervals
up to 30 days after treatment for analysis of diflubenzuron residues.
The duration of detectable residues was different for each substrate,
but in all cases it was less than 2 weeks. Zooplankton and benthic
invertebrate populations were monitored for up to 110 days after
spraying in two ponds in the high-volume rate block and in control
ponds. Significant mortality occurred in two groups of caged
macroinvertebrates (amphipoda and immature corixidae) 1 to 6 days
after the ponds were treated with diflubenzuron. Three taxa of
littoral insects ( Caenis, Celithemis and Coenagrion) were
significantly reduced in abundance in the treated ponds 21 to 34 days
after treatment but recovered to pre-treatment levels by the end of
the season. Of the six remaining groups studied, only one (immature
corixidae) may have been slightly affected by treatment. Zooplankton
(cladocera and copepoda) population numbers were reduced 3 days after
treatment and remained suppressed for 2-3 months (Sundaram et al.,
1991).
Blumberg (1986) found no effect on population numbers in a
forested ecosystem after aerial application of diflubenzuron (25 WP)
at rate 140 g a.i./ha.
When diflubenzuron (33.63 and 134.52 g a.i./ha) was applied 4
times at 2-week intervals to small ponds in Virginia, numbers of
daphnids were markedly reduced at both treatment levels. There was no
appreciable effect on two other invertebrate species ( Chironomidae
or Chaoborus) (Birdsong, 1977).
In a study by Rodrigues (1982), diflubenzuron (25% WP) was
applied at a rate of 1 mg/litre per 30 min to three forest streams in
New York. The reduction of chironomid larvae 15 days after treatment
ranged from 35 to 91% at different sites in the three streams. Of the
two stonefly families present in the three streams, Nemouridae were
affected more than Leuctridae, with decreases of 75-94% and 70%,
respectively. Ephemeroptera were reduced, but Trichoptera and
Coleoptera were unaffected.
Following aerial application at 67.26 g a.i./ha to a watershed,
diflubenzuron reached the stream channel (also as a result of wash-off
from foliage following several subsequent rainfalls), but the
invertebrate populations were not affected (Jones & Konchenderfer,
1988).
After two applications of 44.8, 112 and 224 g a.i./ha, grass
shrimp showed mortality of 86.6, 100, and 100%, respectively. At the
two lowest application rates, fiddler crabs ( Uca spp.) showed
mortalities of 53.3 and 66.6%, respectively (McAlonan, 1976).
Diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied 4
times at 2-week intervals to man-made ponds in North Carolina,
Arkansas and Texas, USA. In North Carolina no apparent effect on
phytoplankton was found. There was a marked decrease in the numbers
of crustaceans ( Cladocera and Copepoda), as well as of certain
species from benthic communities such as Hexagenia and Chaoborus
in Arkansas. A relative decrease in copepod numbers was observed, but
the authors attributed this partly to natural mortality during winter.
In Texas there was a marked decrease in the numbers of all crustaceans
and a corresponding increase in rotifer populations, particularly
Asplanchina (Aquatic Environmental sciences, 1976a,b,c).
When diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied
4 times at 2-week intervals to man-made ponds in Alabama, USA, daphnid
populations were reduced to 50% of the pre-treatment level at a rate
of 134.52 g/ha but increased at 33.63 g/ha to a level of 15% of that
in a control pond. Gastropod numbers decreased in the benthic samples
but increased in the periphyton samples from the suspended plate
samplers in the treated ponds (Jackson, 1976).
Diflubenzuron at 33.63 and 134.52 g/ha was applied 4 times at
2-week intervals to a tidally influenced salt marsh in Virginia, which
was sampled biologically 14 times over a 70-day period. Three species
of arthropods, Cyathura polita, Ceratopogonidae sp., and Psychodidae
sp., showed significant reductions in numbers. Oligochaetes, Hanynkia
speciosa and the molluscs were not significantly affected (Matta,
1976).
Six applications of diflubenzuron (28 g a.i./ha) over an 18-month
period caused statistically significant differences in the population
density of aquatic organisms in a Louisiana coastal marsh. None of the
organisms affected were completely eliminated from the ecosystem.
Populations of five taxa (nymphs of Trichocorixa louisianae Jaczewski
and Buenoa spp., Coenagrionidae naiad spp., Berosus infuscatus Le Conte
adults, and Hyalella azteca Saussure) were significantly reduced.
Population of 15 taxa, i.e. Physa sp., Ceanis sp. and Callibaetis sp.
naiads, Noteridae larvae, Hydrovatus cuspidatus, Kunze adults,
Hydrovatus sp. larvae, Dytiscidae larvae, Mesovelia mulsanti Jaczewski
adults, Trichocorixa louisiana adults, larvae of Chironomidae, Ephudridae,
Dolichopodiae and Tabanidae, and the fish Gambusia affinis (Baird and
Griard) and Jordanella floridae (Goode and Bean), showed significant
increases after exposure to diflubenzuron. The 27 remaining aquatic
organisms (members of the Hemiptera, Coleoptera, Mysidacea, Decapoda,
Diptera and Odonata) showed no statistically significant differences
when compared with untreated populations (Farlow et al., 1978).
After treatment with diflubenzuron (as 1% granular formulation)
at rate of 4.5 g a.i./ha to a marsh habitat on the Fraser River,
Canada, the population of cladocerans appeared to be depressed for
about 5 days but they recovered 2 weeks after treatment. There was no
effect on copepods or ostracods. Significant reduction in the numbers
of water beetles and zooplankton occurred (Wan & Wilson, 1977).
In a field study by Hester et al. (1986), WP-25 formulation was
evaluated in natural salt water pools to determine its toxicity to
juvenile stages of three estuarine crustaceans exposed to a single
application of 45 g diflubenzuron/ha. One hour after application,
water residues of diflubenzuron averaged 3.6 µg/litre and mortality
was 46.5, 60.7 and 40.6% for Callinectes sp., Palaemonetes pugio
and Uca pugilator, respectively, over the 10-day observation period.
When these species were introduced into the pools 7 days after
treatment, mortality was not significantly greater in the treated
group than in the controls over the 21-day observation period. The
maximum concentration after organisms were introduced was
0.69 µg/litre, and mortalities of 22.2, 1.5 and 4.2% for Callinectes
sp., Palaemonetes pugio and Uca pugilator, respectively, were
reported.
In a study by Hester (1982), diflubenzuron (25% WP) at the rate
of 0.045 kg a.i./ha was applied to tidal ponds. Residue analysis
showed 2 to 5 µg/litre after 1 h. The mortality of three
estuarine decapods: blue crabs ( Callinectes sp.), grass shrimp
(Palaemonetes pugio) and fiddler crabs (Uca pugilator) was 44,
61 and 41%, respectively. When these decapods were introduced to the
ponds 7 days after application, the residues in water were
0.4-0.7 µg/litre and mortality was 22, 1.5 and 4%, respectively.
9.2.2.3 Vertebrates
During a forestry spraying programme at 0.067 kg diflu-
benzuron/ha, a survey on fish was performed in the watershed.
Observations made on caged brown trout in the stream from day -7 to
day +6 revealed no immediate mortality or erratic behaviour
attributable to treatment. Barrier seines placed at the upstream and
downstream boundaries of the spray area did not collect any dead or
dying fish during the 1-week period they were in the stream. Visual
observations made by walking along the stream during this same period
also revealed no dead or dying fish. Post-spray population
estimations revealed increases in both test and control areas.
However, statistical analysis indicated no significant differences
pre- and post-spray between stations. The treatment had no observable
effect on the development of fish larvae. Immediate mortality was not
observed in caged fish or stream fish collected by barrier seines.
Delayed mortality attributable to spraying was not shown by population
estimations taken 6 weeks following spray, nor were any dead or dying
fish seen during this period. There appeared to be no adverse effect
of diflubenzuron sprayed at 0.067 kg a.i./ha on the fish species
observed in this study (White, 1975).
No effect on fish populations ( Micropterus salmoides, Lepomis
macrochirus and Gambusia affinis) was observed after four
applications of diflubenzuron (33.69 and 134.52 g a.i./ha) at 2-week
intervals to small ponds in Virginia (Birdsong, 1977).
Killifish (Fundulus heteroclitus) showed no effect after three
applications of diflubenzuron at rates from 11.12 to 224.2 g a.i./ha
to replicated semi-natural pools (McAlonan, 1976). Diflubenzuron
revealed no toxic effect on bullheads or sunfish after aerial
application of 35 g/ha in Canada (Buckner et al., 1975).
Growth of bluegill sunfish ( Lepomis macrochirus Rafinesque) was
not affected by diflubenzuron applications at rates of 2.5-10 µg/litre
to ponds and lakes in California (Apperson et al., 1978). Ten days
after the fourth treatment to man-made ponds with diflubenzuron at
33.63 and 134.52 g a.i./ha no apparent adverse effect on largemouth
bass was observed (Jackson, 1976).
No death of fish ( Pomoxis nigromaculatus and Ictalurus
nebulosis) occurred after application of diflubenzuron (mean
concentration of 13.2 µg/litre one hour after treatment) to
experimental ponds in California. For 1 month following treatment,
stomach content analyses showed alteration in the diet of the fish
(Collwell & Schaefer, 1980).
9.2.3 Terrestrial organisms
9.2.3.1 Invertebrates
Honey-bee colonies remained normal after an aerial application of
350 g diflubenzuron/ha in Canada (Buckner et al., 1975).
A comparative study of the effect of diflubenzuron on carabid
fauna in oak woods in Westphalia in 1987-1988 was reported. The total
number of captured beetles in treated areas was lower in 1989.
Evaluation of the 12 beetle species most frequently caught indicated
lower numbers than in control areas. The decrease coincided with the
diflubenzuron treatment in the middle of May. No higher active
density of species reproducing in late summer or autumn was observed
in the untreated areas (Klenner, 1990).
In a forestry spraying programme (0.0672 kg diflubenzuron per
ha), a survey of microarthropods collected from leaf litter and soil
in control and sprayed areas of the Bald Eagle State Forest,
Pennsylvania, USA, showed little or no effect of diflubenzuron on the
spray plot fauna. Increases and decreases in both the control and
spray population sizes during the 6-week study period could seemingly
be explained on the basis of seasonal population cycles, increased
soil moisture condition and differential extraction efficiencies
(White, 1975).
9.2.3.2 Vertebrates
Small song birds in a forest ecosystem were unaffected after an
aerial application of 350 g diflubenzuron/ha in Canada (Buckner et
al., 1975).
In a forestry spraying programme (0.0672 kg diflubenzuron/ha)
surveys were made on birds and small mammals. Effects on birds were
assessed using single male mapping surveys. The survey methodology
was designed to minimize bias due to differences between observers,
plots, times of day and abundance of birds. Vegetation and
defoliation types were identified and mapped and defoliation was
sampled at 136 sites. Four hundred and sixteen surveys were conducted
on treated plots, defoliated plots, and undisturbed plots. No
differences were observed in the number of individuals singing, the
amount of time each individual spent singing or the song repetition
rate during song bouts. The results show that diflubenzuron did not
lead to the death or emigration of singing birds and probably had no
direct adverse physiological effects on birds since their vocal
behaviour changed little, if at all. The singing male surveys did not
detect a presumed food shortage among canopy feeders caused by the
defoliation. Small mammals studied in this project were censused as
thoroughly as possible. The short pre-spray sampling period for the
treated plot was a handicap in evaluating the pre-spray population
levels; however, post-spray sampling compensated for this to some
degree. Peromyscus, Clethrionomys and Sorex consume arthropods to
varying degrees and may be exposed to diflubenzuron present as
residues. Diflubenzuron had no demonstrable effects on any of these
small mammals sampled in this study. The application of diflubenzuron
at the rate of 0.067 kg/ha provided good foliage protection within the
spray sites and apparently made the dying gypsy moth larvae
unpalatable. Other contact and stomach pesticides generally do not
have this effect on caterpillars and are consequently consumed by
insectivorous mammals. The reduced exposure by non-ingestion is no
doubt a beneficial aspect. None of the species of small mammals
showed reductions in numbers from pre-spray to post-spray periods that
could be attributed to the compound. Stomach analysis did not
indicate any change in the feeding habits of any of the small mammals
from either plot in the study. Changes in reproduction could not be
detected. However, of the Peromyscus and Clethrionomys animals
that were dissected and examined, 7 out of 12 females were in a
healthy condition or had gravid reproductive organs. Others were
either virgins or placental scars could not easily be seen, as in
Sorex. The body weight of all the small mammals in the study was
normal for each species. Juveniles were easily distinguished by
weight and gonad development. A regression comparing gonad weights
and body weights of Clethrionomys indicated a strong correlation.
Since the body weight of small mammals increases with age, this was to
be expected (White, 1975).
Diflubenzuron sprayed at 0.14 and 0.28 kg/ha over forests in
north-eastern Oregon, USA, did not have any detectable adverse effect
on bird population numbers, nesting or bird behaviour (Richmond et
al., 1979).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diflubenzuron was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1981, 1984, and 1985 (FAO/WHO, 1982a,b;
1985a,b; 1986a,b). In 1985, JMPR established an Acceptable Daily
Intake (ADI) for human beings of 0-0.02 mg/kg body weight per day,
based on the fact that the following levels produced no toxicological
effects:
rat: 2 mg/kg body weight (40 mg/kg diet)
mouse: 2.4 mg/kg body weight (16 mg/kg diet)
dog: 2 mg/kg body weight per day
Diflubenzuron has been classified as "a product unlikely to
present an acute hazard in normal use", on the basis of an acute oral
LD50 for the rat that is greater than 4640 mg/kg body weight (WHO,
1994).
REFERENCES
Ali A & Kok-Yokomi ML (1989) Field studies on the impact of a new
benzoylphenylurea insect growth regulator (UC-84572) on selected
aquatic nontarget invertebrates. Bull Environ Contam Toxicol,
42: 134-141.
Ali A & Lord J (1980) Impact of experimental insect growth regulators
on some nontarget aquatic invertebrates. Mosq News, 40(4): 564-571.
Ali A & Mulla MS (1978a) Effects of chironomid larvicides and
diflubenzuron on nontarget invertebrates in residential-recreational
lake. Environ Entomol, 7: 21-27.
Ali A & Mulla MS (1978b) Impact of the insect growth regulator
diflubenzuron on invertebrates in a residential-recreational lake.
Arch Environ Contam Toxicol, 7: 483-491.
Ali A, Nigg HN, Stamper JH, Kok-Yokomi ML, & Weaver M (1988)
Diflubenzuron application to citrus and its impact on invertebrates in
an adjacent pond. Bull Environ Contam Toxicol, 41: 781-790.
Anon (1980) Final report: Environmental monitoring of the 1979 Gypsy
moth control program in five eastern States. Hyattsville, Maryland, US
Department of Agriculture, Animal an Plant Health Inspection Service,
25 pp (Proprietary report No. 56683/18/1983, submitted to WHO by
Duphar BV, Crop Protection Division, 'sGraveland, The Netherlands).
Anton FA, Cuadra LM, Gutierrez P, Laborda E, & Laborda P (1993)
Degradational behavior of the pesticides glyphosate and diflubenzuron
in water. Bull Environ Contam Toxicol, 51: 882-888.
Apperson CS, Schaefer CH, Colwell AE, Werner GH, Anderson NL, Dupras
EF, & Longanecke DR (1978) Effects of diflubenzuron on Chaoborus
astictopus and nontarget organisms and persistence of diflubenzuron
in lentic habitats. J Econ Entomol, 71(3): 521-527.
Aquatic Environmental Sciences (1976a) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
North Carolina. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-13, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976b) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Arkansas. Tarrytown, New York, Union Carbide Corporation, 9 pp
(Unpublished proprietary report NTP-8, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976c) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Texas. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-7, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Arnold D, Kennedy SL, & Keplinger ML (1974) Mutagenic study with TH
6040 in albino mice. Northbrook, Illinois, Industrial Bio-Test
Laboratories Inc. (IBT No. 622-05068) (Unpublished proprietary report,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Arts JHE (1991) Acute (4-hour) inhalation toxicity of dimilin WP 25%
in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report TNO No. 56645/64/1991, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Barker RY & Taber S (1977) Effects of diflubenzuron fed to caged honey
bees. Environ Entomol, 6: 167-168.
Barker RY & Waller GD (1978) Effects of diflubenzuron wettable powder
on caged honey bee colonies. Environ Entomol, 7: 534-535.
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990a) A one-generation
reproduction study with the bobwhite ( Colinus virginianus. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/08/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990b) A one-generation
reproduction study with the mallard ( Anas platyrhynchos. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/07/1990, submitted to WHO by Solvay Duphar, BV, 'sGraveland,
The Netherlands).
Bentley JP, Weber GH, & Gould D (1979) The effect of diflubenzuron
feeding on glucosaminoglycan and sulfhemoglobin biosynthesis in mice.
Pestic Biochem Physiol, 10: 162-167.
Berberian IG & Enan EE (1989) Hematological studies on White male rats
exposed to some antimoulding compounds. Bull Environ Contam Toxicol,
43: 60-65.
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975a) Acute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/198/74988, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975b) Subacute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/199/7551, submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975c) Subacute inhalation
toxicity to the rat of DU 112307 insecticide powder. (Evaluation of
methaemoglobinaemia). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/197/ 741013, submitted to WHO
by Solvay Duphar BV, Weesp, The Netherlands).
Berends AG & Thus JLG (1992a) The acute toxicity of dimilin WP-25 to
the earthworm Eisenia fetida 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 16 pp (Proprietary
report No. 56635/26/1992).
Berends AG & Thus JLG (1992b) The toxicity of diflubenzuron to the
alga Selenastrum capricornutum 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 23 pp (Proprietary
report No. 56635/49/1992).
Berends AG & van den Laan-Straathof JMTh (1994a) The acute toxicity of
diflubenzuron to Zebra fish ( Brachydanio rerio. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/57/93).
Berends AG & van den Laan-Straathof JMTh (1994b) The acute toxicity of
diflubenzuron to rainbow trout ( Oncorhynchus mykiss. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/02/94).
Berends AG, Thus JLG, & Jansen WAJ (1992) The acute toxicity of
diflubenzuron to the earthworm Eisenia fetida 'sGraveland, The
Netherlands, Solvay Duphar BV, Environmental Research Department,
16 pp (Proprietary report No. 56635/22/1992).
Besten C Den, Buse TE, & Koopman TSM (1993a) Acute oral toxicity study
with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay Duphar
BV (Proprietary report No. 56345/51/1993).
Besten C Den, Buse TE, & Koopman TSM (1993b) Acute dermal toxicity
study with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56345/52/1993).
Birdsong RS (1977) Field test of Dimilin on non-target organisms in
Virginia. Nonfolk, Virginia, Environmental Consultants, Inc. (NTP-11)
(Unpublished proprietary report, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Blumerg AY (1986) Survey of aquatic soil and soil surface invertebrate
fauna in a North Carolina forest (pre and post application of Dimilin
WP-25) Weesp, The Netherlands, Duphar BV (Proprietary report
No. 56635/24/1986).
Boelhouwers EJ, Joustra KD, Nijssen OA, & Stegman KH (1988a)
Hydrolysis of 14C-labelled diflubenzuron in buffer solutions at pH 5,
pH 7, and pH 9. 'sGraveland, The Netherlands, Solvay Duphar
(Proprietary report No 56630/137/1988).
Boelhouwers EJ, Joustra KD, & Stegman KH (1988b) Photodegradation of
14C-labelled diflubenzuron in water. 'sGraveland, The Netherlands,
Solvay Duphar BV (Proprietary report No. 56630/35/1988).
Bogus ER, Gallagher PA, Cameron EA, & Mumma RO (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed phase solid support. J Agric Food Chem, 33(6): 1018-1021.
Bollag JM, Blattmann P, & Laanio T (1978) Adsorption and
transformation of four substituted anilines in soil. J Agric Food
Chem, 26(6): 1302-1306.
Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, & MacKenzie WF
(1990) Pathology of the Fischer rat - Reference and atlas. New York,
London, Academic Press.
Booth GM (1975) The impact of Dimilin W-25 on non-target invertebrates
in ponds located in Salt Lake county, Utah. Provo, Utah, Brigham Young
University (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Booth GM (1977) Biochemical effects of diflubenzuron on mouse embryos.
Provo, Utah, Brigham Young University (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Booth GM & Ferrell D (1977) Degradation of Dimilin by aquatic
foodwebs. In: Khan MAQ ed. Pesticides in aquatic environments. New
York, Plenum Publishing Corporation, pp 221-243.
Booth GM, Alder DC, Lee ML, Carter W, Whitmore RC, & Seegmiller RE
(1987) Environmental fate and properties of 1-(4-chlorophenyl)-
3-(2,6-difluorobenzoyl) urea (diflubenzuron, Dimilin). In: Wright JE &
Retnakaran ed. Chitin and benzoylphenyl ureas. Dordrecht, The
Netherlands, Junk Publishers, pp 141-204.
Brusick DJ & Weir RJ (1977a) Mutagenic evaluation of diflubenzuron
technical batch FL 4/605201. Final report (Project No. 2683).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/34/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Brusick DJ & Weir RJ (1977b) Evaluation of diflubenzuron: In vitro
malignant transformation in BALB/3T3 cells. Final Report (Project
No 2688). Kensington, Maryland, Litton Bionetics Inc. (Unpublished
proprietary report No. 56645/35/1977, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Brusick DJ & Weir RJ (1977c) Evaluation of diflubenzuron: Unscheduled
DNA synthesis in WI-38 cells. Final Report (Project No 2688).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/36/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Bryant H (1976) Activity of TH-6040 in the Ames Salmonella
typhimurium mutagenesis assay. Report to H.W. Dorough, University of
Kentucky, Lexington, USA (Unpublished proprietary report submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Buckner CH, McLeod BB, & Kingsbury PD (1975) The effect of an
experimental application of Dimilin upon selected forest fauna.
Ottawa, Ontario, Chemical Control Research Institute
(Report No. CC-X-97).
Bull DL & Ivie GW (1978) Fate of diflubenzuron in cotton, soil, and
rotational crops. J Agric Food Chem, 26(3): 515-520.
Burdock GA, Serota DG, Alsaker RD, & Purvis DS (1980a) Ninety-day
subchronic toxicity study in mice - Diflubenzuron technical. Final
report (Project No. 533-120). Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Burdock GA, Serota DG, Purvis D, & Alsaker RD (1980b) Subchronic
dietary toxicity study in rats - Diflubenzuron. Final report: Volume 1
(Project No. 533-119). Vienna, Virginia, Hazleton Laboratories America
Inc. (Unpublished proprietary report submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Burdock GA, Wolfe GW, Hepner KE, Alsaker RD, Koka M, & Phipps RB
(1984) Oncogenicity study in rats on diflubenzuron. Final report.
Vienna, Virginia, Hazleton Laboratories America Inc. (Unpublished
proprietary report No. 56645/08/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Burgess D (1989) Uptake, depuration and bioconcentration of
14C-diflubenzuron by Bluegill sunfish ( Lepomis macrochirus.
Columbia, Missouri, Analytical Bio-Chemistry Laboratories, Inc.
(Proprietary report No. 56635/16/1989, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Cardinal H, Vernet G, & Sinegre G (1979) Quelques effets d'un
inhibiteur de croissance: le diflubenzuron sur un crabe Carcinus
mediterraneus(Czerniavsky). C C R Soc Biol, 173: 1105-1108.
Carringer RD, Weber JB, & Monaco TJ (1975) Adsorption-desorption of
selected pesticides by organic matter and montmorillonite. J Agric
Food Chem, 23: 568-572.
Caspary WJ, Daston DS, Myhr BC, Mitchell AD, Rudd CJ, & Lee PS (1988)
Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay:
interlaboratory reproducibility and assessment. Environ Mol Mutagen,
12(Suppl 13): 195-229.
Cecil HC, Miller RW, & Corley C (1981) Feeding three insect growth
regulators to white Leghorn hens: Residues in eggs and tissues and
effects on production and reproduction. Poult Sci, 60: 2017-2027.
Chandran VR (1981) Primary skin irritation study in rabbit with
Dimilin 25 WP. Padappai, Tamil Nadu, India, Coromandel Indag Research
Centre (Proprietary report No. 31, submitted to WHO by Solvay Duphar
BV, 'sGraveland, The Netherlands).
Chapman RA, Tu CM, Harris CR, & Harris C (1985) Persistence of
diflubenzuron and Bay Sir 8514 in natural and sterile sandy loam and
organic soils. J Environ Sci Health, B20(5): 489-497.
Chesterman H, Heywood R, Barker MH, Street AE, & Cherry CP (1974)
DU 112307 toxicity in repeated dietary administration to beagle dogs
(Repeated administration for 13 weeks). Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR169/74157, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Christiansen ME & Costlow JD (1980) Persistence of the insect growth
regulator Dimilin in brackish water: A laboratory evaluation using
larvae of an estuarine crab as an indicator. Helgoländer Meeresunters,
33: 327-332.
Christiansen ME & Costlow JD (1982) Ultrastructural study of the
exoskeleton of the estuarine crab Rhithropanopeus harrsii Effect of
the insect growth regulator Dimilin (diflubenzuron) on the formation
of the larval cuticle. Mar Biol, 66: 217-226.
Christiansen ME, Costlow JD, & Monroe RJ (1978) Effects of the insect
growth regulator Dimilin (TH6040) on larval development of two
estuarine crabs. Mar Biol, 50: 29-36.
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981a) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume I. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/294/80185, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981b) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume II. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/294/80185, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Colley J, Heywood R, Street AE, & Gopinath C (1984) The effect of
diflubenzuron given by oral administration with the feed on toxicity
and tumour development in male and female HC/CFLP mice. Final report.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. 56645/32/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Collwell AE & Schaefer CH (1980) Diets of Ictalurus nebulosus and
Pomoxis nigromaculatus altered by diflubenzuron. Can J Fish Aquat
Sci 37(4): 632-639.
Cooke M & Ober AG (1980) OV-17-QF-1 Capillary column for
organochlorine pesticide analysis. J Chromatogr, 195: 265-269.
Corley C, Miller RW, & Hill K (1974) Determination of
N-(4-chlorophenyl)-N-(2,6-difluorobenzoyl)-urea in milk by
high-speed liquid chromatography. J AOAC, 57(6): 1269-1271.
Costlow YD (1979) Effect of Dimilin on development of larvae of the
stone crab Menippe mercenaria and the blue crab Callinectes
sapidus In: Vernberg WB, Calabrese A, Thurburg FP, & Vernberg FJ ed.
Marine pollution: Functional responses. New York, London, Academic
Press, pp 355-363.
Crookshank HR, Sowa BA, Kubena L, Holman GM, Smalley HE, & Morison R
(1978) Effect of diflubenzuron (Dimilin) on the hyaluronic acid
concentration in the chicken combs. Poult Sci, 57: 804-806.
Cunningham PA (I976) Effects of Dimilin (TH-6040) on reproduction in
the brine shrimp, Artemia salina Environ Entomol, 5: 701-706.
Cunningham PA (1986) A review of toxicity testing and degradation
studies used to predict the effects of diflubenzuron (Dimilin) in
estuarine crustaceans. Environ Pollut, A40: 63-86.
Cunningham PA & Myers LE (I986) Dynamics of diflubenzuron (Dimilin)
concentrations in water and sediment of a supratidal saltmarsh site
following repetitive aerial applications for mosquito control. Environ
Pollut, A41(1): 63-88.
Cunningham PA & Myers LE (I987) Effects of diflubenzuron (Dimilin) on
survival, molting, and behaviour of juvenile fiddler crabs, Uca
pugilator Arch Environ Contam Toxicol, 16: 745-752.
Danhaus RG & Sieck RF (1976) Rotation crop uptake of 14C-residues
following soil application of Dimilin. Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Danhaus RG, Wargo JP, & Sieck RF (1976) 14C-Dimilin field soil
leaching study. Colorado Springs, Colorado, Analytical Development
Corporation (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Davies RE & Halliday JC (1974) Acute percutaneous toxicity to rabbits
of DU 112307 technical. Huntingdon, England Huntingdon Research Centre
(Unpublished proprietary report No. 2171/D175/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE & Ligget MP (1973) Irritant effects of DU 112307 technical
on rabbit eye mucosa. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 2170/176D/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE, Elliot PH, Street AE, Heywood R, & Prentice DE (1975)
Effect of repeated applications of DU 112307 to the skin of rabbits
for three weeks. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR200/74851, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
De Bree H, De Lange N, Overmars H, & Post LC (1977) Diflubenzuron:
analysis of metabolites connected with methaemoglobinaemia. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/08/1977).
De Lange N (1979) Diflubenzuron: dermal absorption in the rabbit.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56654/09/1979).
De Lange N & Post LC (1978) Diflubenzuron: intestinal absorption in
the mouse in relation to dosage level. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56654/04/1978).
De Lange N, Overmans H, & Post LC (1974) PH 60-40: excretion of
radioactivity and metabolite patterns in rats following oral
administration. Weesp, The Netherlands, Philips-Duphar BV (Unpublished
proprietary report No. 56654/20/1974).
De Lange N, Overmans H, Willems AGM, & Post LS (1975) Diflubenzuron
(PH 60-40): balance studies in the rat, and identification of urinary
metabolites. Weesp, The Netherlands, Philips Duphar BV (Unpublished
proprietary report No. 56654/22/1975).
De Lange N, Fronik GM, & Post LC (1977) Diflubenzuron: intestinal
absorption in the rat in relation to dosage level. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/10/1977).
Deul DH & Jong BJ de (1977) The possible influence of DU 112307 on the
in vivo synthesis of hyaluronic acid in chicken skin. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56635/01/1977).
Di Prima SJ (1976) Determination of diflubenzuron residues in soybean
process fractions (meal, oil, hulls) by gas chromatography. Thompson-
Hayward Chemical Company (Unpublished proprietary report No. AM-13,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Di Prima SJ, Cannizaro RD, Roger JC, & Ferrell CD (1978) Analysis of
diflubenzuron residues in environmental samples by high-pressure
liquid chromatography. J Agric Food Chem, 26(4): 968-971.
Dorough HW (1977) Screening of selected Thompson-Hayward Chemicals for
activity in the Ames Salmonella mutagenicity test. Lexington,
University of Kentucky, Department of Entomology (Unpublished report
No. MTM-91).
Downer DM (1990) Analysis of diflubenzuron in streamwater samples from
little sluice mountain gypsy moth sprayblock. Harrisonburg, Virginia,
US Forest Service, 30 pp (Unpublished proprietary report submitted to
WHO by Duphar BV, Weesp, The Netherlands).
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1985) Reproducibility of microbial
mutagenicity assays: II. Testing of carcinogens and noncarcinogens in
Salmonella typhimurium and Escherichia coli Environ Mol Mutagen,
7(Suppl 5): 1-248.
El-Sebae AH, Salem MH, Al-Assar MRS, & Enan EE (1988) In vitro
effect of profenofos, fenvalerate and dimilin on protein and RNA
biosynthesis by rabbit liver and muscle tissues. J Environ Sci Health,
B23(5): 439-451.
Enninga IC (1990) Evaluation of DNA repair inducing ability of
diflubenzuron in a primary culture of rat hepatocytes (with
independent repeat). Report RCC NOTOX BV, 'sHertogenbos, The
Netherlands No. 56645/114/1990. Proprietary report, submitted to WHO
by Solvay Duphar, BV, 'sGraveland, The Netherlands.
FAO/WHO (1982a) Pesticides residues in food - 1981. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticides Residues in
Food and Environment and WHO Expert Group on Pesticides Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 37).
FAO/WHO (1982b) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985a) Pesticide residues in food - 1984. Report of the Joint
Meeting on Pesticide Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) Pesticide residues in food - 1984. Evaluations: The
Monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO, Plant Production and Protection Paper 67).
FAO/WHO (1986a) Pesticide residues in food - 1985. Report of the Joint
Meeting of the Residues in Food and the Environment and a WHO Expert
Group on Pesticides Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations:
Part II - Toxicology. Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of pesticide
residues, 4th ed. Rome, Codex Committee on Pesticide Residues, Food
and Agriculture Organization of the United Nations.
Farlow JE, Breaud TP, Steelman CD, & Schilling PE (1978) Effect of
the insect growth regulator diflubenzuron on non-target aquatic
populations in a Louisiana intermediate marsh. Environ Entomol,
7: 199-204.
Fischer SA & Hall LW Jr (1992) Environmental concentrations and
aquatic toxicity data on diflubenzuron (Dimilin). Crit Rev Toxicol,
22(1): 45-79.
Forward RB & Costlow JD (1978) Sublethal effects of insect growth
regulators upon crab larval behaviour. Water Air Soil Pollut,
9: 227-238.
Gilbert P, Saint-Ruf G, Poncelet F, & Mercier M (1980) Genetic effects
of chlorinated anilines and azobenzenes on Salmonella typhimurium
Arch Environ Contam Toxicol, 9: 533-541.
Goodman DG (1980a) Histopathologic evaluation of mice administered
diflubenzuron in the diet. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Goodman DG (1980b) Histopathologic evaluation of rats administered
diflubenzuron in the diet. Washington, DC, Clement Associates
(Unpublished proprietary report submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Gordon R & Cornect M (1986) Toxicity of the insect growth regulator
diflubenzuron to the rove beetle Aleochara bilineata a parasitoid
and predator of the cabbage maggot Delia radicum Entomol Exp Appl,
42: 179-185.
Goto M (1977a) Crop residue analysis of Dimilin. Japan, Institute
of Pesticide Residues (Unpublished proprietary report
No. K/Resid./004/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Goto M (1977b) Crop residue analysis of Dimilin. Japan, Institute of
Pesticide Residues (Unpublished proprietary report No.
K/Resid./007/1977, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Granett J, Morang S, & Hatch R (1978) Reduced movement of precocious
male Atlantic salmon parr into sublethal Dimilin-G1 and carrier
concentrations. Bull Environ Contam Toxicol, 19(4): 462-464.
Graves WC & Swigert JP (1993) Diflubenzuron: A 96-h flow through acute
toxicity test with the sheepshead minnow ( Cyprinodon variegatus.
Final report. Wildlife International Ltd. USA (Unpublished proprietary
report No. 56835/13/93, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Greenough RJ, Goburdhun R, Hundson R, & MacNaughtan F (1985)
Diflubenzuron. 52 week oral toxicity study in dogs: Volumes 1 and 2
(Project No. 630146,2728). Musselburgh, Scotland, Inveresk Research
International (Unpublished proprietary report No. 56645/32/1985,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Greenough RJ & McDonald P (1986) Diflubenzuron VC 90 acute inhalation
toxicity study in rats (limit test). Musselburgh, Scotland, Inveresk
Research International (Proprietary report No. 56645/41/1986,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Gulka G, Doscher CM, & Watabe N (1980) Toxicity and molt-accelerating
effects of diflubenzuron on the barnacle, Balanus eburneus Bull
Environ Contam Toxicol, 25: 477-481.
Gulka G, Gulka CM, & Watabe N (1982) Histopathological effects of
diflubenzuron on the cirripede crustacean, Balanus eburneus Arch
Environ Contam Toxicol, 11: 11-16.
Gustafson DE & Wargo JP (1976) Fate of Dimilin following application
to soybeans (ADC Project No 222). Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hansen SR & Garton RR (1982a) Ability of standard toxicity test to
predict the effects of the insecticide diflubenzuron on laboratory
stream communities. Can J Fish Aquat Sci, 39: 1273-1288.
Hansen SR & Garton RR (1982b) The effects of diflubenzuron on a
complex laboratory stream community. Arch Environ Contam Toxicol,
11: 1-10.
Hatzios KK & Penner D (1978) The effect of diflubenzuron on soybean
photosynthesis, respiration and leaf ultrastructure. Pestic Biochem
Physiol, 9: 65-69.
Hawkins DR, Jackson AJS, & Roberts NL (1980) Excretion of radio-
activity after oral administration of 3H/14C-diflubenzuron to
cats (PDR/302/80443). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 56654/14/1980, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands.
Helling CS (1985) Soil mobility of three Thompson-Hayward pesticides.
Interim report. Beltsville, Maryland, US Department of Agriculture,
Agricultural Research Centre (Unpublished proprietary report No. E-34,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hester PG (1982) Efficacy of diflubenzuron on three estuarine decapods
( Callinectes sp., Palaemonetes pugio and Uca sp.) (Unpublished
document submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hester PG, Olson MA, & Floore TG (1986) Effects of diflubenzuron on
three estuarine decapods, Callinectes sp., Palaemonetes pugio and
Uca pugilator J Fla Anti-Mosq Assoc, 57: 8-14.
Honeycutt R (1993) Indoor occupant exposure study with Dimilin 25 W.
Colorado Springs, Colorado, Analytical Research and Development
Corporation (Report No. 56635/56/1992, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hsu TS & Bartha R (1974) Interaction of pesticide-derived chloroaniline
residues with soil organic matter. Soil Sci, 1974: 444-452.
Huber CM & Collins DL (1987) Final report on the 1987 spray project to
eradicate Gypsy moth from the Tusquitee ranger district on the
Nantahala national forest. Asheville, North Carolina, Field Office,
Forest Pest Management (Unpublished report No. 88-1-4, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Huber CM & Manchester EH (1988) Final report on the eradication of
the Gypsy moth from the Tusquitee ranger district on the Nantahala
national forest. Forest Pest Management (Unpublished report
No. 89-1-7, submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hunter B, Batham P, Street AE, & Cherry CP (1974) DU 112307
preliminary assessment of the toxicity to male mice in dietary
administration for 6 weeks. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/174/74199, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hunter B, Batham P, Offer JM, & Prentice DE (1975) Tumorigenicity
study of DU 112307 to mice. Dietary administration for 80 weeks. Final
report. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/170/75685, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Colley J, Street AE, Heywood R, Prentice DE, & Offer J
(1976) Effects of DU 112307 in dietary administration to rats for 104
weeks. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/171/75945, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Jordan J, Heywod R, Street AE, Prentice DE, Wight DG, &
Gibson WA (1979) DU 112307 toxicity to rats in dietary administration
for nine weeks followed by a four week withdrawal period (final
report). Huntingdon, England, Huntingdon Research Centre (Unpublished
report No. PDR/248/77883).
Ivie GW (1977) Metabolism of insect growth regulators in animals.
In: Ivie GW & Dorough HW ed. Fate of pesticides in large animals. New
York, London, Academic Press, pp 111-125.
Ivie GW (1978) Fate of diflubenzuron in cattle and sheep. J Agric Food
Chem, 26(1): 81-89.
Ivie GW, Bull DL, & Veech JA (1980) Fate of diflubenzuron in water.
J Agric Food Chem, 28(2): 330-337.
Jackson GA (1976) Dimilin (TH 6040): Biological impact on pond
organisms. Marion, Alabama, Southeastern Fish Cultural Laboratory
(Unpublished proprietary report NTP-42, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Jackson GC, Hardy CJ, Simms F, Mullins PA, & Gopinath Ch (1990)
Dimilin 4F acute inhalation toxicity study in rats 4-hour exposure.
Huntingdon, England, Huntingdon Research Centre (Proprietary report
No. 56645/68/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Jagannath DR & Brusick DJ (1977a) Mutagenicity evaluation of
4-chlorophenyl urea, final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/42/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977b) Mutagenicity evaluation of
2,6-difluorobenzoic acid. Final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/40/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977c) Mutagenicity evaluation of
4-chloroanilin. Final report. Kensington, Maryland, Litton Bionetics
Inc. (Unpublished proprietary report No. 56645/41/1977, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Janssen PJM & Pot TE (1987a) Primary irritation study of dimilin SC-48
to the eye of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/46/1987).
Janssen PJM & Pot TE (1987b) Primary irritation study of dimilin SC-48
to the skin of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/45/1987).
Janssen PJM & Pot TE (1987c) Acute oral toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/44/1987).
Janssen PJM & Pot TE (1987d) Acute dermal toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/43/1987).
Janssen PJM & Pot TE (1987e) Acute dermal toxicity study with dimilin
WP-25 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/58/1987).
Janssen PJM & van Doorn WM (1993a) Acute inhalation toxicity study
with dimilin OF-6 in male and female rats. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/01/1993).
Janssen PJM & van Doorn WM (1993b) Primary irritation study of dimilin
OF-6 to the skin of female rabbits. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/30/1993).
Janssen PJM & van Doorn WM (1993c) Primary irritation study of dimilin
OF-6 to the eye of female rabbits. Weesp, The Netherlands, Solvay
Duphar BV, Department of Toxicology (Proprietary report
No. 56345/31/1993).
Jenkins VK, Mayer RT, & Perry RR (1984) Effects of diflubenzuron on
growth of malignant melanoma and skin carcinoma tumors in mice. Invest
New Drugs, 2: 19-27.
Jenkins VK, Perry RR, Ahmer AE, & Ives K (1986) Role of metabolism in
effects of diflubenzuron on growth of B16 melanomas in mice. Invest
New Drugs, 4: 325-335.
Jones AS & Konchenderfer JN (1988) Persistence of diflubenzuron
(Dimilin) in a small eastern watershed and its impact on invertebrates
in a headwater stream. Research Triangle Park, North Carolina,
Southeastern Forest Experiment Station (Unpublished proprietary report
No. 56637/26/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Joustra KD, van Kampen WG, & Walstra P (1989) Metabolism of [14C]
diflubenzuron in citrus fruit (Analytical report). Weesp, The
Netherlands, Duphar BV, Analytical Development Department (Proprietary
report No. 56630/86/1989).
Julin AM & Sanders HO (1978) Toxicity of IGR, diflubenzuron, to
freshwater intervertebrates and fishes. Mosq News, 38: 256-259.
Kavanagh P (1988a) Diflubenzuron oral (gavage) rat teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/11/87)
(Proprietary report No. 56645/68/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kavanagh P (1988b) Diflubenzuron oral (gavage) rabbit teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/12/87)
(Proprietary report No. 56645/79/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Keet CMJF (1977a) The methaemoglobin, sulphaemoglobin and Heinz body
forming properties of DU 112307 after oral administration to male rats
during 8 days. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/15/1977).
Keet CMJF (1977b) The effect of DU 112307 (tech) in male mice after
daily oral administration (14-days) on body weight, methaemoglobin,
sulphaemoglobin and Heinz body formation. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/33/1977).
Keet CMJF (1977c) The methaemoglobin and sulphaemoglobin forming
properties of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/02/1977).
Kemp A, van der Heijden CA, & van Eldik A (1973a) Dietary
administration of DU 112307 to male and female rats for three months.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56645/13A/1973).
Kemp A, van der Heijden CA, & van Eldik A (1973b) Appendix III to
report No. 56645/13A/1973 individual data: dietary administration of
DU 112307 to male and female rats for 3 months. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56645/13B/1973).
Kingsbury P, Sundaram KMS, Holmers KMS, Nott R, & Kreutzweiser D
(1987) Aquatic fate and impact studies with Dimilin. Ottawa, Ontario,
Forest Pest Management Institute (Unpublished report No. 78, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Klenner MF (1990) [A comparative study of the carabid fauna in the
Dimilin-treated and untreated oak stands in Westphalia.] Mitt BBA
(Berlin-Dahlem), 266: 279 (in German).
Koelman-Klaus HJS (1978a) Acute oral toxicity study of
4-chlorophenylurea in male and female rats. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/09/1978).
Koelman-Klaus HJS (1978b) Acute oral toxicity study of
2,6-difluorobenzoic acid in male and female rats. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56645/25/1978).
Koopman TSM (1977a) Acute oral toxicity study with DU 112307 technical
in mice. 'sGraveland, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/04/1977).
Koopman TSM (1977b) Acute oral toxicity study with DU 112307 WP 25%
in mice and rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/03/1977).
Koopman TSM (1977c) Acute dermal toxicity study with DU 112307
technical in rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/07/1977).
Koopman TSM (1980a) Primary irritation of Dimilin ODC-45 to the rabbit
eye. 'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/02/1980).
Koopman TSM (1980b) Acute dermal toxicity study of dimilin ODC-45 in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/04/1980).
Koopman TSM (1980c) Primary irritation of Dimilin ODC-45 to the rabbit
skin. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/03/1980).
Koopman TSM (1984a) Acute dermal toxicity study with diflubenzuron
VC-90 in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/31/1984).
Koopman TSM (1984b) Acute oral toxicity study with diflubenzuron VC-90
in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/30/1984).
Koopman TSM (1984c) Primary irritation study of diflubenzuron VC-90 to
the rabbit eye. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/29/1984).
Koopman TSM (1984d) Primary irritation study of diflubenzuron VC-90 to
the rabbit skin. 'sGraveland, The Netherlands, Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/44/1984).
Koopman TSM (1985a) Primary irritation study of dimilin 2F to the
skin of the male rabbit. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report
No. 56645/93/1985).
Koopman TSM (1985b) Primary irritation study of dimilin 2F to
the eye of the male rabbit. 'sGraveland, The Netherlands,
Duphar BV, Department of Toxicology (Unpublished proprietary report
No. 56645/91/1985).
Koopman TSM (1985c) Acute dermal toxicity study with dimilin 2F in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/95/1985).
Koopman TSM (1985d) Acute oral toxicity study with dimilin 2F in rats.
'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/94/1985).
Koopman TSM & Jongeling AJ (1979) Acute oral toxicity study with
Dimilin ODC 45% in rats. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report No.
56645/38/1979).
Koopman TSM & Pot TE (1986) Acute oral toxicity study with
diflubenzuron VC-90% in mice. 'sGraveland, The Netherlands, Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/39/1986).
Koorn JC (1990) Study to examine the possible mutagenic activity of
diflubenzuron in the Ames Salmonella microsome assay. 'sGraveland,
The Netherlands, Duphar BV, Department of Toxicology (Proprietary
report No. 56645/74/1990).
Kramer HT (1990) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in an apple orchard in Phelps (New York). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-271,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1991) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in a citrus orchard in Oviedo (Florida). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-270,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992a) Field dissipation study with diflubenzuron
insecticide applied for control of insects on cotton (a bare soil
application in Arkansas). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-110, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992b) Field dissipation study with diflubenzuron
insecticide applied for control of insects on soybeans (a bare soil
application in Louisiana). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-116, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kubena LF (1982) The influence of diflubenzuron on several
reproductive characteristics in male and female layer-breed chickens.
Poult Sci, 61: 268-271.
Kuijpers LAM (1988) The acute toxicity of diflubenzuron to Daphnia
magna Weesp, The Netherlands, Duphar BV (Unpublished proprietary
report No. 56635/26/1988).
Kurczewski F, Wang C, Grimble D, Smith R, & Brezner J (1975)
Environmental impact of dimilin. A final report: Effects of dimilin
upon microorganisms in leaf litter and forest soil. Syracuse, New
York, State University of New York, pp 28-43.
Kynoch SR & Elliot PH (1978a) Screening test for delayed contact
hypersensitivity with Dimilin WP 25%. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 0911/D258/78, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Elliot PH (1978b) Screening test for delayed contact
hypersensitivity with dummy formulation for dimilin WP 25% in the
albino guinea-pig. Huntingdon, England, Huntingdon Research Centre
(Proprietary report No. 9112/D258/78, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin 2F. Huntingdon, England, Huntingdon Research
Centre (Proprietary report No. 56645/13/1987, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin SC-48. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 56645/72/1987, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Smith PA (1986) Delayed contact hypersensitivity in the
guinea-pig with diflubenzuron VC-90. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 86558D/PDR 432SS, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Lauren DR, Agnew MP, & Henzell RF (1984) Field life of the insect
growth regulator diflubenzuron on lucerne. N Z J Agric Res,
27: 425-429.
Lawrence JF & Sundaram KMS (1976) Gas-liquid chromatographic analysis
of N-(4-chlorophenyl-N-2,6-difluorobenzoyl) urea insecticide after
chemical derivatization. J AOAC, 59(4): 938-941.
Lee BM & Scott GI (1989) Acute toxicity of temephos, fenoxycarb,
diflubenzuron and methoprene and Bacillus thuringiensis var.
Israelensis to the mummichug ( Fundulus heteroclitus. Bull Environ
Contam Toxicol, 43: 827-832.
Maas W, Van Hes R, Grosscurt AC, & Deul DH (1980) [Benzoylphenylurea
insecticides.] In: Wegler R ed. [Chemistry of plant protection
chemicals and pesticides.] Berlin, Heidelberg, Springer Verlag, vol 6,
pp 432-470 (in German).
McAlonan WG (1976) Effects of two insect growth regulators on some
selected salt-marsh non-target organisms. University of Delaware, USA
(Thesis) (NTP-53).
Machado J, Coimbra J, Castilho F, & Sa C (1990) Effects of
diflubenzuron on shell formation of the freshwater clam, Anodonta
cygnea Arch Environ Contam Toxicol, 19: 35-39.
McGregor JT, Gould DH, Mitchell AD, & Sterling GP (1979) Mutagenicity
tests of diflubenzuron in the micronucleus test in mice, the L5178Y
mouse lymphoma forward mutation assay, and the Ames Salmonella
reverse mutation test. Mutat Res, 66: 45-53.
McKague AB & Pridmore RB (1978) Toxicity of Altosid and Dimilin to
juvenile rainbow trout and coho salmon. Bull Environ Contam Toxicol,
20: 167-169.
Madder DJ (1977) The disappearance from, efficacy in and effect on
non-target organisms of diflubenzuron, methoprene and chlorpyrifos in
a lentic ecosystem. Canada, University of Manitoba, Faculty of
Graduate Studies, 139 pp (Thesis) (NTP-71).
Madder DJ & Lockhart WL (1978) A preliminary study of the effects of
diflubenzuron and methoprene on rainbow trout ( Salmo gairdneri. Bull
Environ Contam Toxicol, 20: 66-70.
Madder DJ & Lockhart WL (1980) Studies on the dissipation of
diflubenzuron and methoprene from shallow prairie pools. Can Entomol,
112: 173-177.
Mansager ER, Still GC, & Frear DS (1979) Fate of 14C diflubenzuron on
cotton and in soil. Pestic Biochem Physiol, 12: 172-182.
Marshall BF & Hieb BF (1973) 96-h LC50 Salmo gairdneri (n.m.
Oncorhynchus mykiss, Lepomis machrochirus and Fundulus heteroclitus
Marine Research Institute (Unpublished study submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Martinez-Toledo MV, Rubia T, De La Moreno J, & Gonzalez-Lopez J
(1988a) Effect of diflubenzuron on azotobacter nitrogen fixation in
soil. Chemosphere, 17(4): 829-834.
Martinez-Toledo MV, Gonzalez-Lopez J, Rubia T, De La Moreno J, &
Ramos-Cormenzana A (1988b) Diflubenzuron and the acetylene-reduction
activity of Azotobacter vinelandii Soil Biol Biochem,
20(2): 255-256.
Matheson DW & Brusick DJ (1977a) Evaluation of 4-chlorophenylurea
in vitromalignant transformation in BALB/3T3 cells. Kensington,
Maryland, Litton Bionetics Inc. (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brucisk DJ (1977b) Evaluation of 2,6-diflurobenzoic acid
in vitromalignant transformation in BALB/3T3 cells. Final report.
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/10/1978, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Matheson DW & Brusick DJ (1978a) Mutagenicity evaluation of
2,6-difluorobenzoic acid in unscheduled DNA synthesis in the human WI-
38 cells assay. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/16/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978b) Mutagenicity evaluation of
4-chlorophenylurea in unscheduled DNA synthesis in the human WI-38
cells assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/24/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978c) Mutagenicity evaluation of
4-chloroanilin in the in vitro transformation of BALB/3T3 cells
assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/23/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matta JF (1976) The effect of dimilin on non-target organisms in a
marsh community. Norfolk, Virginia, Environmental Consultants (Report
prepared for Thompson-Hayward Chemical Company) (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Mayer RT, Netter KJ, Leising HB, & Schachtschabel DD (1994) Inhibition
of the uptake of nucleosides in cultured Harding-Passey melanoma cells
by diflubenzuron. Toxicology, 30: 1-6.
Metcalf RL, Lu PY, & Browlus S (1975) Degradation and environmental
fate of 1-(2,6-difluorobenzoyl)-3-(4-chlorophenyl) urea. J Agric Food
Chem, 23(3): 359-364.
Mian LS & Mulla MS (1983) Persistence of three IGRs in stored wheat.
J Econ Entomol, 76(3): 622-625.
Miller RW, Corley C, Oehler DD, & Pickens LG (1976a) Feeding TH 6040
to cattle: residues in tissues and milk and breakdown in manure.
J Agric Food Chem, 24(3): 687-688.
Miller RW, Corley C, & Shufeld SR (1976b) Effects of feeding TH 6040
to two breeds of chickens. J Econ Entomol, 69(6): 741-743.
Miller RW, Cecil HC, Carey AM, Corley C, & Kiddy CA (1979) Effects of
feeding diflubenzuron to young male Holstein cattle. Bull Environ
Contam Toxicol, 23: 482-486.
Miura T (1974) Biological activity of TH 6040 against organisms
associated with mosquito breeding habitats in irrigated pastures.
Fresno, California, University of California, Mosquito Control
Research Laboratory (Unpublished report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Miura T & Takahashi RM (1974a) Insect developmental inhibitors effects
of candidate mosquito control agents on nontarget aquatic organisms.
Environ Entomol, 3(4): 631-636.
Miura T & Takahashi RM (1974b) Toxicity of TH 6040 to freshwater
crustacea and the use of a tolerance index as a method of expressing
side effects on nontargets. Proceedings of the Forty-Second Annual
Conference of the California Mosquito and Vector Control Association,
pp 177-180.
Moreale A & Van Bladel R (1976) Influence of soil properties on
adsorption of pesticide-derived aniline and p-chloroaniline. J Soil
Sci, 27: 48-57.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Mulla MS, Majori G, & Darwazeh HA (1975) Effects of the insect growth
regulator Dimilin (TH-6040) on mosquitoes and some nontarget
organisms. Mosq News, 35: 211-216.
Mutanen RM, Siltanen HT, Kuukka VP, Annila EA, & Varama MMO (1988)
Residues of diflubenzuron and two of its metabolites in a forest
ecosystem after control of the pine looper moth, Bupalus
piniarius. L Pestic Sci, 23: 131-140.
Nakayama K (1977a) Crop residue analysis of Dimilin (apple).
Japan, Institute of Pesticides (Unpublished proprietary report
No. K/Resid/003/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Nakayama K (1977b) Crop residue analysis of Dimilin. Japan, Institute
of Pesticides (Unpublished proprietary report No. K/Resid/006/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Nation JL, Robinson FA, Ju SJ, & Bolten AB (1986) Influence upon honey
bees of chronic exposure to very low levels of selected insecticides
in their diet. J Agric Res, 25(3): 170-177.
Nebeker AV, McKinney P, & Cairns MA (1983) Acute and chronic effects
of diflubenzuron (Dimilin) on freshwater fish and invertebrates.
Environ Toxicol Chem, 2: 329-336.
Nigg HN (1989) Metabolism of 14C-diflubenzuron in citrus fruits.
Field operations report. Lake Alfred, Florida, Citrus Research and
Education Center (Proprietary report No. 56635/13/1989, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Nigg HN, Canizzaro RD, & Stamper JH (1986) Diflubenzuron surface
residues in Florida citrus. Bull Environ Contam Toxicol,
36(6): 833-838.
Nimmo WB & de Wilde PC (1974) Fate of diflubenzuron on leaves of corn,
soybean, cabbage and apples. 'sGraveland, The Netherlands, Philips-
Duphar BV, Crop Protection Division (Unpublished proprietary report
No. 56635/16A/1974).
Nimmo WB & de Wilde PC (1975a) Degradation of diflubenzuron in soil
and natural water. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/37/1975).
Nimmo WB & de Wilde PC (1975b) Degradation of diflubenzuron in sterile
water at pH 5, 7, 9 and 12. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/32/1975).
Nimmo WB & de Wilde PC (1976a) Uptake of diflubenzuron treated soil by
soybean, maize and potato. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/04/I976).
Nimmo WB & de Wilde PC (1976b) Uptake of diflubenzuron and its
metabolites from the soil by rice and wheat. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/08/1976).
Nimmo WB & de Wilde PC (1977a) Degradation of diflubenzuron in
mushroom growth medium and uptake of its metabolites in the mushrooms.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56635/21A/1977).
Nimmo WB & de Wilde PC (1977b) Degradation of diflubenzuron in calf
and chicken manure. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/27/1977).
Nimmo WB, Willems AGM, & de Wilde PC (1978) Fate of diflubenzuron
applied to leaves and fruits of apple trees. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/33/1978).
Nimmo WB, Hamaker TL, More JC, & Wood RA (1980) Acute and chronic
effects of Dimilin on survival and reproduction of Misidopsis bahia
In: Eaton JG, Parrish PR, & Hendricks AC ed. Aquatic toxicology.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 366-376 (Special Technical Publication 707).
Nimmo WB, Wilde PC, & Verloop A (1984) The degradation of
diflubenzuron and its chief metabolites in soils. Part I: Hydrolytic
cleavage of diflubenzuron. Pestic Sci, 15: 574-585.
Nimmo WB, Willems AGM, Joustra KD, & Verloop A (1986) The degradation
of diflubenzuron and its chief metabolites in soils. Part II: Fate of
4-chlorophenylurea. Pestic Sci, 17: 403-411.
Nimmo WB, Joustra KD, & Willems AGM (1990) The degradation of
diflubenzuron and its chief metabolites in soils. Part III: Fate of
2,6-difluorobenzoic acid. Pestic Sci, 29: 39-45.
Offringa OR (1977) Addendum report to the chronic studies with
DU 112307: (A) Dietary administration to rats for 104 weeks - (B)
Dietary administration to mice for 80 weeks. Weesp, The Netherlands,
Philips-Duphar BV, Department of Toxicology (Report No. 56645/
16/1977).
Opdycke JC (1976) Metabolism and fate of diflubenzuron in chickens and
swine. University of Maryland, Faculty of the Graduate School
(Thesis).
Opdycke JC, Miller RW, & Menzer RE (1982a) Metabolism and fate of
diflubenzuron in swine. J Agric Food Chem, 30(6): 1223-1227.
Opdycke JC, Miller RW, & Menzer RE (1982b) In vivo and liver
microsomal metabolism of diflubenzuron by two breeds of chickens.
J Agric Food Chem, 30(6):1227-1233.
Palmer AK & Hill PA (1975a) Effect of DU 112307 on reproductive
function of multiple generations in the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/173/75954, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK & Hill PA (1975b) Effect of DU 112307 on pregnancy of the
rat. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/192/74978, submitted to WHO by Solvay-
Duphar, B.V., Weesp, The Netherlands).
Palmer AK & Hill PA (1975c) Effect of DU 112307 on pregnancy of the
New Zealand white rabbit. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/193/74937, submitted to
WHO by Solvay-Duphar BV, Weesp, The Netherlands).
Palmer AK, Allen PA, Street EA, & Prentice DG (1977) Preliminary
assessment of the effect of DU 112307 on the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/243/77208, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK, Allen PA, Heywood R, Street EA, Gibson WA, Offer JM, &
Jolly DW (1978) Effect of dietary administration of DU 112307 on
reproductive function of one generation in the rat. Huntingdon,
England, Huntingdon Research Centre (Report No. PDR 244/78653). 1978.
Unpublished proprietary report, submitted to WHO by Solvay-Duphar,
B.V., Weesp, The Netherlands.
Prasad I (1970) Mutagenic effects of the herbicide 3',4'-dichloro-
propionalide and its degradation products. Can J Microbiol,
16: 369-372.
Prinsen MK (1988a) Primary dermal irritation/corrosion study with
dimilin 4F in albino rabbits. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
93/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1988b) Primary eye irritation study with dimilin 4F in
albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/92/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Prinsen MK (1989a) Acute dermal irritation/corrosion study with
dimilin SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
150/1989, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Prinsen MK (1989b) Acute eye irritation/corrosion study with dimilin
SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/151/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1989c) Sensitization study with dimilin 4F in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/23/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1990) Acute dermal irritation/corrosion study with dimilin
ODC-45 in albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology
and Nutrition Institute (Unpublished proprietary report
No. 56645/99/1990, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1992) Sensitization study with diflubenzuron technical in
guinea-pigs. The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report No. 56645/26/1992, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1993) Sensitization study with Dimilin OF-6 in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56345/03/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Pritchard PH & Bourquin AW (1981) Fate of Dimilin in laboratory
estuarine ecosystems. Washington, DC, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished report).
Quarles JM, Norman JO, & Kubena LF (1980) Absence of transformation by
diflubenzuron in host mediated transplacental carcinogenassay. Bull
Environ Contam Toxicol, 25: 252-256.
Rabenort B, de Wilde PC, de Boer FG, Korver PK, Di Prima SJ, &
Cannizzaro RD (1978) Diflubenzuron. In: Analytical methods for
pesticides and plant growth regulators. New York, London, Academic
Press, vol 10, pp 57-72.
Richmond ML, Henny CJ, Floyd RL, Mannan RW, Finch DM, & de Weese LR
(1979) Effects of Sevin-4-oil, Dimilin and orthene on forest birds in
Northeastern Oregon. Berkeley, California, Pacific Southwest Forest
and Range Experiment Station (Research Paper PSW-148).
Rodrigues CS (1982) Effects of insecticides including insect growth
regulators on black fly (Diptera: Simuliidae) larvae and associated
nontarget stream invertebrates. Ontario, Canada, University of Guelph
(Thesis).
Rosenkranz HS & Poirier LA (1979) Evaluation of the mutagenicity and
DNA-modifying activity of carcinogens and noncarcinogens in microbial
systems. J Natl Cancer Inst, 62: 873-892.
Ross DB, Prentice DE, Newman AJ, Street AE, Hepworth PL, & Roberts NL
(1977a) DU 112307 13 weeks oral toxicity study in the sheep.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR229/77226, submitted to WHO by Solvay-Duphar
BV, Weesp, The Netherlands).
Ross DB, Street AE, & Roberts NL (1977b) Blood red cell and plasma
cholinesterase value following six weeks inclusion of DU 112307 in the
diet of sheep. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR229A/77225, submitted to WHO by
Solvay-Duphar BV, Weesp, The Netherlands).
Sarkar DK (1982) Persistence of dimilin 25 WP in water. Padappai,
Tamil Nadu, India, Coromandel Indag Research Centre (Proprietary
report No. 124, submitted to WHO by Solvay-Duphar BV, 'sGraveland, The
Netherlands).
Schaefer CH & Dupras EF (1976) Factors affecting the stability of
Dimilin in water and the persistence of Dimilin in field waters.
J Agric Food Chem, 24(4): 733-739.
Schaefer CH, Dupras EF, Stewart RJ, Davidson LW, & Colwell AE (1979)
The accumulation and elimination of diflubenzuron by fish. Bull
Environ Contam Toxicol, 21: 249-254.
Schaefer CH, Colwell AE, & Dupras EF (1980) The occurrence of
p-chloroaniline and p-chlorophenylurea from the degradation of
diflubenzuron in water and fish. In: Proceedings and papers of the
Forty-Eighth Annual Conference of the California Mosquito and Vector
Control Association, 20-23 January 1980, pp 84-89.
Schimmel SC, Garnas RL, Patrick JM, & Moore JC (1983) Acute toxicity,
bioconcentration and persistence of AC 222,705, Benthiocarb etc. in
the estuarine environment. J Agric Food Chem, 31(1): 104-113.
Schwartz SL & Borzelleca JF (1981) Effects of diflubenzuron on
methemoglobin, sulfhemoglobin and Heinz body production in cats.
Richmond, Virginia, Toxicology and Pharmacology, Inc. (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Seegmiller RE & Booth GM (1976) Teratogenic and biochemical effects of
TH-6040 on chicken embryos. Provo, Utah, Brigham Young University
(Unpublished report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Serra GE & Joustra KD (1990) Diflubenzuron: Nature of residues on
apples. Greenhouse operations report. Weesp, The Netherlands, Duphar
BV (Proprietary report No. 56630/43/1990).
Seuferer SL, Braymer HD, & Dunn JJ (1979) Metabolism of diflubenzuron
by soil microorganisms and mutagenicity of the metabolites. Pestic
Biochem Physiol, 10: 174-180.
Simmons KE, Minard RD, & Bollag JM (1989) Oxidative co-oligomerization
of guaiacol and 4-chloroaniline. Environ Sci Technol, 23(1): 115-121.
Singh PP & Kaira RL (1989) Determination of diflubenzuron residues by
thin-layer chromatography. Chromatographia, 27(1/2): 53-54.
Sipes JG & Carter DE (1988) Pharmacokinetics of xenobiotics:
p-chloroaniline. Tucson, Arizona, University of Tucson (Unpublished
report No. MRJDN 410882-01, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith JT & Edmunds CP (1985) Environmental monitoring of the 1984
gypsy moth control program in Tennessee, USA. Weesp, The Netherlands,
Duphar BV, 25 pp (Proprietary report No. 56637/27/1985).
Smith KS & Merricks DL (1976a) TH 6040 tissue residue and metabolism
study in dairy cows. Reading, Pennsylvania, Cannon Laboratories, Inc.
(Unpublished proprietary report No. 5E-7372, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Smith KS & Merricks DL (1976b) Tissue residue and metabolism study in
poultry. Reading, Pennsylvania, Cannon Laboratories, Inc. (Unpublished
proprietary report No. 5E-7372, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith S, Willis GH, & McDowell LL (1983) Electron-capture gas
chromatographic determination of diflubenzuron and permethrin in soil
and water. J Agric Food Chem, 31: 610-612.
Smucker RA & Simon SL (1986) Some effects of diflubenzuron on growth
and sporogenesis in Streptomyces spp. Appl Environ Microbiol,
51(1): 25-31.
Snoeij NJ & Buse-Pot TE (1990) Primary irritation study of dimilin WP
25% to the skin of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/09/1991).
Snoeij NJ & Buse-Pot TE (1991) Primary irritation study of dimilin WP
25% to the eye of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/13/1991).
Solvay Duphar (1994) Recommendations for field use (diflubenzuron) -
Guidelines. Weesp, The Netherlands, Solvay Duphar BV.
Spanjers MTh (1988a) Determination of the acute oral toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/85/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Spanjers MTh (1988b) Determination of the acute dermal toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/84/1988, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Steelman CD, Farlow JE, Breaud TP, & Schilling PE (1975) Effects of
growth regulators on Psorophora columbiae (Dyar and Knab) and non-
target aquatic insect species in rice fields. Mosq News, 35(1): 67-76.
Stevenson JH (1978) The acute toxicity of formulated pesticides to
worker honey bees. ( Apis mellifera L.. Plant Pathol, 27: 38-40.
Stoner A & Wilson WT (1982) Diflubenzuron (Dimilin): effect of long-
term feeding of low dose in sugar-cake or sucrose syrup on honey bees
in standard-size field colonies. Am Bee J, 122: 579-582.
Sundaram KMS (1986) Persistence and degradation of diflubenzuron in
conifer foliage, forest litter and soil, following simulated aerial
application. Sault Ste Marie, Ontario, Canada, Forest Pest Management
Institute (Information report FPM-X-74).
Sundaram KMS (1991) Spray deposit patterns and persistence of
diflubenzuron in some terrestrial components of a forest ecosystem
after application at three volume rates under field and laboratory
conditions. Pestic Sci, 32: 275-293.
Sundaram KMS & Nott R (1989) Mobility of diflubenzuron in two types of
forest soils. J Environ Sci Health, B24(1): 65-86.
Sundaram KMS, Holmes SB, Kreutzweiser DP, Sundaram A, & Kingsbury PD
(1991) Environmental persistence and impact of diflubenzuron in a
forest aquatic environment following aerial application. Arch Environ
Contam Toxicol, 20: 313-324.
Surprenant DC (1988) The chronic toxicity of 14C-diflubenzuron to
Daphnia magna under flow-through conditions. Wareham, Massachusetts,
Springborn Life Sciences, Inc. (Proprietary report No. 56635/22/1988,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Taalman RDFM & Hoorn AJW (1986) Mutagenicity evaluation of
diflubenzuron technical (Assay No. E-9481). Veenendaal, The
Netherlands, Hazleton Biotechnologies Corporation (Proprietary report
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Tasheva M & Hristeva V (1991) Biochemical and hematological changes in
rats following 28 days oral administration of diflubenzuron and
triflumuron. In: Abstracts from the 1991 Eurotox Congress, Maastricht,
The Netherlands, 1-4 September 1991, p 68.
Tasheva M & Hristeva V (1993) Comparative study on the effects of five
benzoylphenylurea insecticides on haematological parameters in rats.
J Appl Toxicol, 13(1): 67-68.
Taylor RE (1973a) Primary skin irritation study TH-6040 technical
(albino rabbit). Lincoln, Nebraska, Harris Laboratories (Proprietary
report submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Taylor RE (1973b) Primary skin irritation study TH-6040 W25 wettable
powder (albino rabbit). Lincoln, Nebraska, Harris Laboratories
(Proprietary report submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Tester PA & Costlow JD (1981) Effect of insect growth regulator
Dimilin (TH 6040) on fecundity and egg viability of the marine copepod
Acartia tonsa Mar Ecol Prog Ser, 5: 297-302.
Thompson SG & Swigert JP (1993a) Diflubenzuron: A 14-day toxicity test
with Duckweed ( Lemna gibba G3). Euston, Maryland, Wildlife
International Ltd (Proprietary report No. 56835/22/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993b) Diflubenzuron: A 5-day toxicity test
with freshwater alga ( Selenastrum capricornutum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/23/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993c) Diflubenzuron: A 5-day toxicity test
with the freshwater alga ( Anabaena flos-aquae. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/21/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993d) Diflubenzuron: A 5-day toxicity test
with the freshwater diatom ( Navicula pelliculosa. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/24/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993e) Diflubenzuron: A 5-day toxicity test
with the marine diatom ( Skeletonema costatum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/25/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson CZ, Hill LE, Epp JK, & Probst GS (1983) The induction of
bacterial mutation and hepatocyte unscheduled DNA synthesis by
monosubstituted anilines. Environ Mutagen, 5: 803-811.
Thus JLG & van der Laan-Straathof JMTh (1994) Fate of diflubenzuron in
model ditch systems. 'sGraveland, The Netherlands, Solvay Duphar
BV, Environmental Research Department (Proprietary report
No. 56835/55/93).
Thus LG & van der Laan JMT (1993) Nature of residues of diflubenzuron
in soybeans. 'sGraveland, The Netherlands, Solvay Duphar BV,
Environmental Research Department (Proprietary report
No. 56635/57/1992).
Thus JLG & van Dyk NRM (1991) Anaerobic aquatic metabolism of
diflubenzuron -Addendum report. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56635/54/91).
Thus JLG, van Dyk NRM, Rompa-van der Veldt CAH, & Walstra P (1991)
Anaerobic aquatic metabolism of diflubenzuron - Addendum report.
'sGraveland, The Netherlands, Solvay Duphar BV (Proprietary report
No. 56635/34/91).
USDA (1985) Gypsy moth suppression and eradication projects: Final
addendum to the final environmental impact statement as supplemented -
1985. Bromall, Pennsylvania, US Department of Agriculture, Forest
Service (Submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
US NCI (1979) Bioassay of p-chloroaniline for possible
carcinogenicity. Bethesda, Maryland, US National Cancer Institute
(Technical Report Series No. 189, NIH Publication No. 79-1745).
US NTP (1989) Toxicology and carcinogenesis studies of para-
chloroaniline hydrochloride in F344/N rats and B6C3F1 mice (gavage
studies). Research Triangle Park, North Carolina, US National
Toxicology Program (Technical Report Series No. 351).
Van Daalen JJ, Meltzer J, Mulder R, & Wellinga K (1972) A selective
insecticide with a novel mode of action. Naturwissenschaften,
59: 312-313.
Van den Berg G (1986) Dissipation of diflubenzuron residues after
application of dimilin WP-25 in a forestry area in North Carolina and
some ecological effects. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division, 11 pp (Proprietary report No. 56637/47/1986).
Van der Laan-Straathof JMTh & Thus JLG (1993) Determination of the
biodegradability of 14C-diflubenzuron in an adapted modified Sturm
test. Weesp, The Netherlands, Solvay-Duphar (Proprietary research
report No. 56835/41/93).
Van der Laan-Straathof JMTh & Thus JLG (1994) Evaporation of
diflubenzuron from bare soil and red kidney bean leaves after
application of dimilin WSE-80. Weesp, The Netherlands, Solvay-Duphar
(Proprietary research report No. 56835/17/94).
Van Eldik A (1973a) Acute toxicity studies with DU 112307 in mice and
rats. Weesp, The Netherlands, Philips-Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/14/1973).
Van Eldik A (1973b) Acute toxicity studies with DU 112307 25% WP in
mice and rats. Weesp, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Proprietary report No. 56645/15A/1973).
Van Eldik A (1974) Acute toxicity studies with DU 112307 in mice after
intraperitoneal administration. Weesp, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/01/1974).
Van Kampen WG & Joustra KD (1991) Diflubenzuron: residues on apples.
Weesp, The Netherlands, Duphar BV, Analytical Development Department
(Proprietary analytical report No. 56630/46/1990).
Van Rossum A, De Reijke A, & Zeeman J (1984) Diflubenzuron (update).
In: Analytical methods for pesticides and plants growth regulators.
New York, London, Academic Press, vol 13, pp 165-169.
Verhey ME (1991a) Analysis of field dissipation soil samples from
Madera, California for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 019, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verhey ME (1991b) Analysis of field dissipation soil samples from
Woodburn, Oregon, for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 020, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verloop A & Ferrell CD (1977) Benzoylphenyl ureas - a new group of
larvicides interfering with chitin deposition. In: Plimmer JR ed.
Pesticide chemistry in the 20th century. Washington, DC, American
Chemical Society, pp 237-270 (ACS Symposium Series No. 37).
Walstra P & Joustra KD (1990) Aerobic soil metabolism of diflubenzuron
in sandy loam. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division (Proprietary report No. 56635/65/1990).
Wan MTK & Wilson DM (1977) Impact of insect growth regulators on
selected non-target organisms co-existing with mosquito larvae.
Washington, DC, Environmental Protection Service, Pollution Abatement
Branch (Unpublished report No. EPS-5-PR-77-1/NTP81).
Wang CJK (1975) Effects of Dimilin upon microorganisms in leaf litter
and forest soil. In: Evaluation of Dimilin against the Gypsy moth
effects on non-target organisms 1975. Report expanded Gypsy moth
research and applications program, Hamden USA (Unpublished report
No. NTP-18, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Weis JS & Ma A (1987) Effects of the pesticide diflubenzuron on larval
horseshoe crabs, Limulus polyphemus Bull Environ Contam Toxicol,
39: 224-228.
Weis JS & Perlmutter J (1987) Burrowing behaviour by the fiddler crab
Uca pugilator: inhibition by insecticide diflubenzuron. Mar Ecol
Prog Ser, 38: 109-113.
Weis JS, Cohen R, & Kwiatkowski JK (1987) Effects of diflubenzuron on
limb regeneration and molting in the fiddler crab, Uca pugilator
Aquat Toxicol, 10(5-6): 279-290.
White WB (1975) Evaluation of Dimilin against the Gypsy moth and
effects on non-target organisms, 1975 (Compiled by the expanded Gypsy
moth research and applications program). Upper Darby, Pennsylvania, US
Department of Agriculture, Forest Service (Unpublished report).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, p 64 (WHO/PCS/94.2).
Wie SJ & Hammock BD (1982) Development of enzyme-linked immunosorbent
assays for residue analysis of diflubenzuron and Sir 8541. J Agric
Food Chem, 30: 949-957.
Wie SJ & Hammock BD (1984) Comparison of coating and immunizing
antigen structure on the sensitivity and specificity of
immunoassays for benzoylphenylurea insecticides. J Agric Food Chem,
32(6): 1294-1301.
Willems AGM, Joustra KD, & Thus JLG (1977) Stability of diflubenzuron
in aqueous solution during heat sterilization. 'sGraveland, The
Netherlands, Philips-Duphar BV, Crop Protection Division (Proprietary
report No. 56635/35/1977).
Willems AGM, Overmars H, Scherpenisse P, de Lange N, & Post LC (1980)
Diflubenzuron: intestinal absorption and metabolism in the rat.
Xenobiotica, 10(2): 103-112.
Williams GM, Laspia MF, & Dunkel VC (1982) Reliability of the
hepatocyte primary culture/DNA repair test in testing of coded
carcinogens and noncarcinogens. Mutat Res, 97: 359-370.
Wilson JEH & Costlow JD (1986) Comparative toxicity of two Dimilin
formulations to the grass shrimp Palaemonetes pugio Bull Environ
Contam Toxicol, 36: 858-865.
Wilson JEH & Costlow JD (1987) Acute toxicity of diflubenzuron (DFB)
to various life stages of the grass shrimp, Palaemonetes pugio Water
Air Soil Pollut, 33: 411-417.
Wilson JEH, Forward RB, & Costlow JD (1985) Effects of embryonic
exposure to sublethal concentrations of Dimilin on the photobehavior
of grass shrimp larvae. In: Marine pollution and physiology: Recent
advances. Colombia, South Carolina, University of South Carolina
Press, pp 377-396.
Wimmer MJ, Smith RR, & Jones JP (1991) Analysis of diflubenzuron by
gas chromatography/mass spectrometry using deuterated diflubenzuron as
internal standard. J Agric Food Chem, 39: 280-286.
Worobey BL & Webster GRB (1977) Gas-liquid chromatographic
determination of diflubenzuron (Dimilin) in water as its
trifluoroacetyl derivative. J Assoc Off Anal Chem, 60(1): 213-217.
Worthing CR & Walker SB (1987) The pesticide manual: A world
compendium. Croydon, British Crop Protection Council, pp 4720-4721.
Yasuno M & Satake K (1990) Effects of diflubenzuron and methoprene on
the emergence of insects and their density in an outdoor experimental
stream. Chemosphere, 21(10-11): 1321-1335.
Young MF, Trombetta LD, & Carson S (1986) Effects of diflubenzuron on
the mouse liver. J Appl Toxicol, 6(5): 343-348.
Yu SJ, Robinson FA, & Nation JL (1984) Detoxication capacity in the
honey bee, Apis mellifera L. Pestic Biochem Physiol, 22: 360-368.
Zwart A (1985) Acute inhalation toxicity study of dimilin 2F,
diflubenzuron in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. V85.347/250925, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le diflubenzuron appartient au groupe des insecticides dérivés de
la benzoylphénylurée. Son activité insecticide est due à une
interaction avec la synthèse et le dépôt de la chitine. Il forme des
cristaux blancs inodores dont le point de fusion est de 230-232°C.
Il est légèrement soluble dans l'eau (0,2 mg/litre à 20°C)
et pratiquement non volatil. Il est relativement stable en milieu
acide ou neutre mais s'hydrolyse en milieu alcalin.
Le diflubenzuron s'obtient par réaction du 2,6-difluoro-
benzamide sur l'isocyanate de 4-chlorophényle.
Le dosage des résidus de diflubenzuron présents dans l'eau, les
échantillons biologiques,et le sol peut s'effectuer par
chromatographie liquide à haute performance avec détection par UV ou
encore par chromatographie en phase gazeuse avec détection par capture
d'électrons, soit directement sur la molécule initiale, soit sur un
dérivé (libération de 4-chloroaniline et action de l'anhydride
trifluoracétique).
2. Sources d'exposition humaine et environnementale
Le diflubenzuron est un produit de synthèse utilisé en
agriculture, en foresterie et dans les programmes de santé publique
pour détruire les ravageurs et les vecteurs de maladies. Différentes
formulations existent à cet usage. On ne possède pas de
renseignements au sujet de cas d'exposition humaine au diflubenzuron
qui auraient pu se produire.
3. Transport, distribution et transformation dans l'environnement
En général, le diflubenzuron est appliqué directement sur les
végétaux et les eaux à traiter. Le feuillage ne constitue pas une
porte d'entrée dans la plante.
Le diflubenzuron est rapidement adsorbé sur les particules du
sol. Il reste fixé dans les 10 premiers cm du sol sur lequel il est
épandu. Il est peu probable qu'il subisse un lessivage. Dans divers
types de sol, il subit une décomposition aérobie ou anaérobie avec une
demi-vie de quelques jours. La vitesse de décomposition dépend
largement de la taille des particules de diflubenzuron. La principale
voie métabolique (plus de 90%) esst l'hydrolyse qui conduit à la
formation d'acide 2,6-difluorobenzoïque et de 4-chlorophénylurée; ces
deux composés sont dégradés à leur tour, respectivement en 4 et 6
semaines. On n'a pas décelé la présence de 4-chloroaniline libre
dans le sol.
Le diflubenzuron se décompose rapidement dans les eaux neutres ou
alcalines. On constate qu'une fois épandu sur l'eau, il se répartit
rapidement entre celle-ci et les sédiments.Le composé initial et la
4-chlorophénylurée peuvent persister plus de 30 jours dans les
sédiments.
Le diflubenzuron ne s'accumule pas chez les poissons.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation en agriculture, en foresterie ou pour la
démoustication n'entraîne qu'une exposition négligeable de la
population générale par l'intermédiaire de la nourriture ou de l'eau.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez l'animal de laboratoire, le diflubenzuron est absorbé au
niveau des voies digestives et, à un moindre degré, au niveau cutané.
Chez le rat, il existe un mécanisme d'absorption saturable. Dans ces
conditions, une forte proportion du diflubenzuron administré par voie
orale se retrouve dans les matières fécales. Le diflubenzuron se
répartit largement dans les tissus, mais il ne s'y accumule pas.
Le métabolisme du diflubenzuron a été étudié chez diverses
espèces animales. Chez les mammifères, la principale voie métabolique
comporte une hydroxylation. L'hydrolyse peut se produire au niveau de
l'une quelconque des trois liaisons carbone-azote. Chez le porc et le
poulet, l'hydrolyse s'effectue principalement au niveau du pont
uréido. Chez le rat et la vache, l'hydroxylation constitue la
principale voie métabolique. Chez le mouton, le porc et le poulet,
les principaux métabolites sont le 2,6-difluorobenzamide et la
4-chlorophénylurée; on trouve aussi, en moindre proportion, du
2,6-difluorobenzamide et de la 4-chloroaniline. Chez le rat et les
bovins, 80% des métabolites sont constitués de 2,6-difluoro-3-
hydroxydiflubenzuron, de 4-chloro-2-hydroxydifluorobenzuron et de
4-chloro-3-hydroxydiflubenzuron. Les études de métabolisme indiquent
qu'il ne se forme pratiquement pas de 4-chloroaniline chez le rat et
les bovins.
Chez les chats, les porcs et les bovins, l'élimination
s'effectue principalement par la voie fécale, à hauteur de 70 à 85%.
Chez les ovins, la voie urinaire et la voie fécale ont à peu près la
même importance de ce point de vue. Chez le rat et la souris,
l'excrétion urinaire décroît proportionnellement à l'augmentation de
la dose. Moins de 1% de la dose administrée par la voie orale se
retrouve dans l'air expiré. Le diflubenzuron n'est présent qu'à
l'état de résidus dans le lait.
Il n'existe pas d'étude sur la cinétique et le métabolisme du
diflubenzuron chez l'homme et notamment, sur son degré de
biotransformation en 4-chloroaniline.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Quel que soit le mode d'exposition, le diflubenzuron présente une
faible toxicité aiguë. En se basant sur le fait que sa DL50 aiguë
par voie orale est supérieure à 4640 mg/kg de poids corporel chez le
rat, l'OMS estime qu'il s'agit d'un produit qui ne présente
vraisemblablement pas de risque d'intoxication aiguë en utilisation
normale. Chez ce même animal, la DL50 aiguë par voie percutanée est
supérieure à 10 000 mg/kg de poids corporel et la CL50 dépasse 2,49
mg/litre par la voie respiratoire. Au cours d'une période de deux
semaines pendant laquelle diverses espèces animales avaient reçu du
diflubenzuron en une seule prise et selon divers modes
d'administration, on n'a constaté aucun signe d'intoxication.
Le diflubenzuron n'est pas irritant pour la peau (chez le lapin)
et ne provoque pas non plus de sensibilisation cutanée (chez le
cobaye). Il est légèrement irritant pour la muqueuse oculaire chez le
lapin.
Le diflubenzuron provoque une méthémoglobinémie et une
sulfhémoglobinémie. Une méthémoglobinémie liée à la dose a été mise
en évidence après exposition d'animaux de diverses espèces au
diflubenzuron par la voie orale, percutanée ou respiratoire. Cet
effet constitue le point d'aboutissement toxicologique le plus
sensible chez les animaux de laboratoire. En prenant comme critère la
méthémoglobinémie, la dose sans effet observable est de 2 mg/kg de
poids corporel par jour chez les rats et les chiens et de
2,4 mg/kg de poids corporel par jour chez les souris. Les études de
toxicité à long terme effectuées sur des souris et des rats
ont montré que les modifications imputables au traitement
correspondaient principalement à l'oxydation de l'hémoglobine et à une
altération des hépatocytes.
Des études de cancérogénicité effectuées sur des rats et des
souris à des doses allant jusqu'à 10 000 mg/kg de nourriture, n'ont
pas révélé de modification de l'incidence tumorale qui soit imputable
au traitement. En particulier, on n'a pas observé de néoplasmes au
niveau du mésenchyme splénique ou hépatique lors d'études de
cancérogénicité utilisant de la 4-chloroaniline.
Plusieurs études toxicologiques portant sur la fonction de
reproduction ont été menées sur des rats, des souris, des lapins et
trois espèces d'oiseaux; elles n'ont pas mis en évidence d'effets
pathogènes et le produit ne s'est pas révélé embryotoxique. Les
études de tératogénicité effectuées sur des rats et des lapins se sont
également révélées négatives.
Le diflubenzuron et ses principaux métabolites ont également été
soumis à diverses épreuves de mutagénicité in vivo e in vitro
Ni le diflubenzuron ni ses principaux métabolites n'ont donné de
résultat positif dans ces épreuves.
Le métabolite secondaire, c'est-à-dire la 4-chloroaniline,
a donné des résultats positifs dans plusieurs épreuves de mutagénicité
in vitro portant sur divers points d'aboutissement toxicologiques.
Il est cancérogène pour le rat et la souris. Les tumeurs imputables à
l'administration de 4-chloroaniline se sont révélées bénignes; quant
aux tumeurs malignes observées, il s'agissait de tumeurs du mésenchyme
splénique chez les rats mâles ainsi que d'hémangiomes et
d'hémangiosarcomes spléniques ou hépatiques chez les souris mâles.
7. Effets sur l'homme
On a signalé des cas de méthémoglobinémie chez des travailleurs
exposés de par leur profession et des nouveau-nés exposés par
inadvertance à de la 4-chloroaniline, un métabolite secondaire du
diflubenzuron. Les sujets qui présentent un déficit en NADH-
méthémoglobine-réductase peuvent être particulièrement sensibles à la
4-chloroaniline et par conséquent à une exposition au diflubenzuron.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Tous les organismes qui synthétisent la chitine sont sensibles au
diflubenzuron.
A la concentration de 500 mg/kg de terre, les bactéries n'ont pas
souffert d'une exposition au diflubenzuron. Il y a eu une certaine
stimulation de la fixation d'azote. Les bactéries décomposent les
solutions de diflubenzuron dans l'acétone, solvant qu'elles utilisent
comme source de carbone. Une concentration de diflubenzuron de 1
µg/litre a provoqué un accroissement de la biomasse algaire. Aucun
effet nocif n'a été observé à des concentration supérieures à la
limite de solubilité du diflubenzuron. Des champignons placés dans un
courant créé en laboratoire ont été temporairement affectés à la
concentration de 0,1 µg/litre.
Les invertébrés aquatiques présentent des réactions variées au
diflubenzuron. Les mollusques n'y sont pas sensibles, avec une CL50
supérieure à 200 mg/litre. Chez les autres invertébrés, la CL50 peut
aller de 1 à > 1000 µg/litre, ce qui peut refléter la sensibilité de
ces organismes au moment de la mue. On estime que pour la daphnie, la
concentration tolérable maximale en produit toxique est supérieure à
40 ng/litre et inférieure à 93 ng/litre. Comme prévu, les larves
d'éphémères et autres formes pré-imaginales d'insectes divers, sont
très sesnsibles au diflubenzuron. Le traitement des eaux de surface
par le diflubenzuron est donc probablement susceptible de causer une
certaine mortalité parmi les insectes aquatiques.
Lors de traitements expérimentaux effectués sur le terrain et
dans divers écosystèmes, on a constaté que la plupart des organismes
avaient moins souffert que ne le laissaient prévoir les études
toxicologiques en laboratoire. Aucun effet n'a été constaté sur les
organismes aquatiques après traitement de forêts par voie aérienne.
Pour les poissons, la CL50 est supérieure à 150 mg/litre. Les
essais effectués sur le terrain n'ont enregistré aucune mortalité chez
les poissons.
La DL50 par voie orale et par contact est supérieure à 30 µg par
insecte chez l'abeille mellifique. Après épandage de diflubenzuron
par voie aérienne à raison de 350 g/ha, on n'a pas constaté de dommage
parmi les colonies d'abeilles des alentours.
Une étude alimentaire de 5 jours sur des colverts et des
gallinacés du genre colin avec des doses allant jusqu'à 4640 mg/kg de
nourriture, n'a pas révélé de signes de toxicité. Après épandage de
diflubenzuron par voie aérienne sur des forêts à raison de 350 g/ha,
on n'a pas constaté de dommages parmi les oiseaux chanteurs de
l'écosystème forestier.
Après épandage de diflubenzuron à raison de 67 g/ha sur une
forêt, on n'a pas observé de réduction dans l'effectif des populations
de petits mammifères.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El diflubenzurón pertenece al grupo de los insecticidas
derivados de la benzoilfenilurea. Su acción insecticida se debe
a la interacción con la síntesis y/o deposición de quitina. Forma
cristales blancos inodoros con un punto de fusión de 230-232°C. Es
bastante soluble en agua (0,2 mg/litro a 20°C) y prácticamente
involátil. Es relativamente estable en medios ácidos y neutros, pero
se hidroliza en condiciones alcalinas.
El diflubenzurón se produce haciendo reaccionar 2,6-difluoro-
benzamida con 4-clorofenilisocianato.
Los residuos de diflubenzurón pueden medirse en el agua, en
muestras biológicas y en el suelo mediante cromatografía líquida de
alta resolución con detección de radiación ultravioleta o mediante
cromatografía de gases con detector de captura de electrones para el
análisis de la molécula intacta o tras la derivatización de la
4-cloroanilina liberada con anhídrido trifluoracético.
2. Fuentes de exposición humana y ambiental
El diflubenzurón es un compuesto sintético utilizado en la
agricultura, en la silvicultura y en programas de salud pública para
combatir plagas de insectos y vectores. Para esos usos existen
diferentes formulaciones de diflubenzurón. No se dispone de
información pertinente sobre la exposición humana a este producto.
3. Transporte, distribución y transformación en el medio ambiente
El diflubenzurón suele aplicarse directamente a las plantas y al
agua. No se produce absorción a través de las hojas.
La adsorción del diflubenzurón en el suelo es rápida. El
producto se inmoviliza en la capa superior de 10 cm del suelo al que
se aplica, y las probabilidades de lixiviación son escasas. El
diflubenzurón se degrada en suelos de diversos tipos y orígenes en
condiciones aerobias o anaerobias, con una semivida de pocos días. La
velocidad de degradación depende en gran medida del tamaño de las
partículas de diflubenzurón. La principal ruta metabólica (más del
90%) es la hidrólisis, que produce 2,6-ácido difluorobenzoico y
4-clorofenilurea; estos productos se degradan con semividas del orden
de cuatro y seis semanas, respectivamente. No se ha detectado
4-cloroanilina libre en los suelos.
El diflubenzurón se degrada rápidamente en aguas neutras o
alcalinas. Estudios de aplicación al agua revelan que el
diflubenzurón se concentra rápidamente en el sedimento; el compuesto
de origen y la 4-clorofenilurea pueden persistir en el sedimento por
más de 30 días.
El diflubenzurón no es objeto de bioacumulación en los peces.
4. Niveles ambientales y exposición humana
La exposición de la población general al diflubenzurón por medio
del agua o los alimentos de resultas de su utilización en la
agricultura, contra insectos forestales o en la lucha contra los
mosquitos, es insignificante.
5. Cinética y metabolismo en animales de laboratorio
En animales de experimentación, el diflubenzurón se absorbe en el
tubo digestivo y, en menor medida, a través de la piel. En el tubo
digestivo de la rata existe un mecanismo de absorción saturable, por
lo que una gran proporción del diflubenzurón administrado oralmente
aparece en las heces. El diflubenzurón tiene una amplia distribución
en los tejidos, pero no se acumula.
El destino metabólico del diflubenzurón ha sido estudiado en
diversas especies. La principal vía metabólica en los mamíferos es la
hidroxilación. La hidrólisis del diflubenzurón puede producirse en
cualquiera de los tres enlaces carbono-nitrógeno. En los cerdos y los
pollos, la principal ruta de hidrólisis es el puente ureido. En las
ratas y las vacas, la principal vía metabólica es la hidroxilación.
En las ovejas, los cerdos y los pollos, los metabolitos más
importantes son el 2,6-ácido difluorobenzoico y la 4-clorofenilurea;
los metabolitos secundarios son la 2,6-difluorobenzamida y la
4-cloroanilina. En las ratas y el ganado vacuno, el 80% de los
metabolitos está constituido por 2,6-difluoro-3-hidroxidiflubenzurón,
4-cloro-2-hidroxi-diflubenzurón y 4-cloro-3-hidroxidiflubenzurón. Los
estudios metabólicos indican que en las ratas o el ganado vacuno se
forman cantidades mínimas o nulas de 4-cloroanilina.
La principal ruta de eliminación es a través de las heces, con
porcentajes de entre el 70 y el 85% en los gatos, los cerdos y el
ganado vacuno. En el ganado ovino, la eliminación se distribuye
aproximadamente por igual entre la orina y las heces. En las ratas y
ratones, la excreción urinaria disminuye proporcionalmente al aumento
de la dosis. Menos del 1% de una dosis oral se recupera en el aire
exhalado. En la leche sólo se han hallado residuos ínfimos.
No se dispone de ningún estudio humano de la cinética y el
metabolismo del diflubenzurón, incluido el alcance de la
biotransformación en 4-cloroanilina.
6. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
El diflubenzurón tiene una toxicidad aguda baja por cualquier vía
de exposición. La OMS lo ha clasificado como producto con pocas
probabilidades de presentar un riesgo agudo en el uso normal, sobre la
base de una DL50 aguda por vía oral de más de 4640 mg/kg de peso
corporal en las ratas. La DL50 aguda por vía cutánea en las ratas es
superior a 10 000 mg/kg de peso corporal, mientras que la CL50 aguda
por inhalación en las ratas excede de 2,49 mg/litro. No se han
observado signos de intoxicación en los 14 días siguientes a una
administración única de diflubenzurón por diversas rutas a una
variedad de especies animales.
El diflubenzurón no provoca irritación cutánea (en el conejo) ni
sensibilización de la piel (en el cobayo). Produce una ligera
irritación a los ojos en el conejo.
El diflubenzurón causa metahemoglobinemia y sulfohemoglobinemia.
Tras la exposición oral, cutánea o por inhalación de diversas especies
al diflubenzurón se ha demostrado la presencia de metahemoglobinemia
dosisdependiente. Este efecto es la variable de evaluación
toxicológica más sensible en los animales de experimentación. El NOEL
basado en la formación de metahemoglobina es de 2 mg/kg de peso
corporal por día en las ratas y los perros, y de 2,4 mg/kg de peso
corporal por día en los ratones. En estudios de toxicidad a largo
plazo realizados con ratones y ratas, los cambios relacionados con el
tratamiento se han asociado principalmente a la oxidación de la
hemoglobina o a alteraciones de los hepatocitos.
En estudios de la carcinogenicidad en ratones y ratas con niveles
de hasta 10 000 mg/kg en la alimentación, no se observaron efectos
relacionados con el tratamiento en la incidencia de tumores.
Específicamente, no se registraron neoplasias mesenquimatosas del bazo
o el hígado como las observadas en los estudios de carcinogenicidad
con 4-cloroanilina.
En varios estudios de la toxicidad reproductiva en ratas,
ratones, conejos y tres especies aviarias no se observó ningún efecto
en la reproducción, ni tampoco embriotoxicidad. Los estudios de
teratogenicidad en ratas y conejos no revelaron ningún efecto
teratogénico.
El diflubenzurón y sus principales metabolitos han sido sometidos
a una serie de ensayos de mutagenicidad in vitro e in vivo Ni el
diflubenzurón ni sus principales metabolitos tienen efecto mutagénico.
El metabolito secundario 4-cloroanilina ha dado un resultado
positivo en varios ensayos de mutagenicidad in vitro con diversas
variables de valoración. Es carcinógeno en las ratas y los ratones.
Las lesiones neoplásicas relacionadas con la administración de
4-cloroanilina fueron tumores mesenquimatosos benignos y malignos en
el bazo de ratas macho, y hemangiomas y hemangiosarcomas,
principalmente en el bazo y el hígado de ratones macho.
7. Efectos en el ser humano
Se ha notificado que el metabolito 4-cloroanilina del
diflubenzurón ha causado metahemoglobinemia en trabajadores sometidos
a exposición y en neonatos expuestos por inadvertencia. Algunas
personas con carencia de NADH metahemoglobina reductasa pueden ser
particularmente sensibles a la 4-cloroanilina y, por lo tanto, a la
exposición al diflubenzurón.
8. Efectos sobre otros organismos en el laboratorio y en el terreno
Todos los organismos que sintetizan quitina presentan sensibilidad
al diflubenzurón.
Las bacterias no resultaron afectadas por el diflubenzurón a
concentraciones de 500 mg/kg de suelo; se observó cierta estimulación
de la fijación de nitrógeno. Las soluciones de diflubenzurón-acetona
se degradaron; la acetona se utilizó como fuente de carbono. La
biomasa de algas aumentó a una concentración de diflubenzurón de
1 µg/litro. No se observaron efectos adversos a concentraciones
superiores al límite de solubilidad del diflubenzurón. Los hongos
resultaron temporalmente afectados a 0,1 µg/litro en condiciones de
laboratorio.
Los invertebrados acuáticos presentan respuestas variables al
diflubenzurón. Los moluscos son insensibles, con una CL50 superior a
200 mg/litro. Los valores de la CL50 de otros invertebrados van
desde 1 hasta más de 1000 µg/litro, en función de los efectos del
compuesto en las fases juvenil y de muda. Para Daphnia se ha
estimado una MATC de > 40 y < 93 ng/litro; como era de prever, las
larvas de mosca de mayo y otros insectos acuáticos juveniles son
sumamente sensibles. El rociamiento de masas de agua matará
probablemente algunos invertebrados acuáticos.
En los ecosistemas y experimentos de campo en que se aplicó
diflubenzurón directamente al agua, los efectos en la mayoría de los
grupos taxonómicos fueron menos graves de lo previsto a partir de las
pruebas de laboratorio sobre efectos agudos. No se han observado
efectos en los organismos acuáticos después de aplicaciones aéreas a
los bosques.
Los valores de la CL50 para los peces son de > 150 mg/litro.
En los experimentos prácticos no se ha registrado nunca la muerte de
peces.
La DL50 oral y por contacto en la abeja de miel es superior a
30 µg/individuo. Las colonias de abejas no resultaron afectadas tras
la aplicación aérea de 350 g de diflubenzurón/hectárea.
Un estudio de alimentación de cinco días de duración en patos
silvestres y codornices con niveles de hasta 4640 mg/kg de pienso no
reveló ningún signo observable de toxicidad. Las pequeñas aves
canoras del ecosistema forestal no resultaron afectadas por la
aplicación aérea de diflubenzurón a razón de 350 g/hectárea.
Las especies mamíferas pequeñas no sufrieron mermas de las
poblaciones tras la aplicación de diflubenzurón en un bosque a razón
de 67 g/hectárea.
The major metabolites (approximately 50%) in sheep urine were
2,6-DFBA and 2,6-difluorohippuric acid (Ivie, 1978).
14C-Diflubenzuron uniformly radiolabelled in both rings was
administered to a pig as an oral dose of 5 mg/kg body weight. Of the
administered dose, 82% was eliminated in faeces as parent compound and
5% was recovered in urine. Identification of the metabolic products
in urine revealed 2,6-DFBA (0.28% of the dose), 4-CPU (0.82%), PCA
(1.03%) and 2,6-difluorobenzamide (0.83%). Cleavage of the urea
moiety between the benzoyl carbon and urea nitrogen was shown to be
the primary degradation pathway in pigs (Opdycke et al., 1982a).
In chickens only small quantities of the metabolites 2,6-DFBA,
4-CPU and PCA were found in excreta and tissues (Opdycke, 1976).
Neither induction nor inhibition of mixed-function oxidase activity
altered diflubenzuron metabolism in chickens (Opdycke et al., 1982b).
After 4 days daily doses of 7.8 g diflubenzuron/kg body weight,
De Bree et al. (1977) found PCA at a level of 30 ng/ml in rat plasma
and 323 ng/g in erythrocytes. PCA, estimated by the concentration in
the urine, represented at most 0.01% of the dose actually absorbed.
6.3.1 Metabolites - distribution, excretion, retention and turnover
When 14C-PCA was administered orally as single doses of 0.3,
3.0 or 30.0 mg/kg to male Fischer-344 rats, approximately 75% of the
administered radioactivity was excreted in the urine within 24 h,
while approximately 10% appeared in the faeces. Excretion was
virtually complete (92-97%) 7 days after dosing. The highest tissue
levels of radioactivity following a single intravenous dose of
3.0 mg/kg were found in the liver, fat, muscle and skin. Tissue
levels peaked within 5-60 min after dosing. By 3 days, concentrations
in all tissues except the blood had declined to < 0.3% of the dose
(Sipes & Carter, 1988). At this time, the only tissue containing more
than 1% of the dose was the cellular compartment of blood, which
contained 1-2% of the dose. The decline of PCA concentration in all
tissues, except for urine, faeces and intestinal contents, was
biexponential. The t alpha 1/2 for fat, muscle and skin was about
1.5 h, while the tß1/2 was approx. 43-59 h. The t alpha 1/2 for
liver was 3.5 h. Levels of unchanged PCA in all tissues peaked after
5 min following intravenous administration. The highest amount of
unchanged PCA was attained in muscle (15% of radioactivity in the
tissue) followed by skin (6%), fat (3%) and liver (2%). The decline of
PCA in all tissues, except for the liver, followed biexponential kinetics
with an estimated t alpha 1/2 of 8 min and a tß1/2 of 3 to 5 h.
PCA is rapidly metabolized to p-chloroacetanilide (PCAA) as the
initial step in the metabolism and excretion of PCA. The decline of PCAA
was monoexponential, the appearance half-life being approx. 6 min in the
testes and 15 min in the brain. The elimination half-life in the
brain, kidney, testes, muscle, skin and fat was around 1.0 to 2.0 h.
The elimination of PCA does not depend on either the dose or route of
administration. Approximately 4% of the urinary radioactivity in the
0-24 h urine sample was unchanged PCA; less than 1% was found in the
faeces. PCAA was not detected in either urine or faeces over a 3-day
period (Sipes & Carter, 1988).
After a single intravenous dose of 14C-PCA (3 mg/kg), maximal
tissue levels were reached within 15 min in most tissues. At this
time, most of the radioactivity was located in muscle (34%), fat
(14%), skin (12%), liver (8%) and blood (7%). Elimination half-lives
from tissues ranged between 1.5 and 4 h. By 8 h, approximately 90% of
the administered dose had been eliminated into urine and faeces. By
3 days, concentrations in all tissues, except blood, had declined to
< 0.3% of the dose (US NTP, 1989).
6.4 Elimination and excretion
After oral administration to rats of 5 mg diflubenzuron labelled
with 3H in the benzoyl and with 14C in the aniline moiety, 95% of
the 3H and 70-75% of the 14C radioactivity were retrieved in urine
and faeces. 2,6-DFBA was shown to constitute more than half of the
urinary metabolites (De Lange et al., 1977). Up to 1% of an oral dose
of 5 mg 14C-diflubenzuron labelled at the benzoyl moiety was
recovered in the expired air of rats (De Lange et al., 1974; Willems
et al., 1980).
When 14C-diflubenzuron, labelled in the aniline moiety, was
administered by gavage (4, 16, 48, 128, 900 and 1000 mg/kg body
weight) to rats, the urinary excretion was complete after 48-72 h.
Urinary excretion after single oral administration of diflubenzuron
relatively decreased with increasing dose level, being 27.6% of the
dose at 4 mg/kg and 1% at 1000 mg/kg (De Lange et al., 1977).
When 14C-diflubenzuron was administered at single oral doses of
12.5, 63.5, 202.5 and 925 mg/kg body weight to Swiss mice, the
excretion was almost completed within 48 h. The cumulative percentage
of the dose excreted in the urine decreased from 15% at the dose level
of 12.5 mg/kg to approximately 2% at 925 mg/kg (De Lange & Post,
1978).
Hawkins et al. (1980) studied the excretion of radioactivity in
urine and faeces after oral administration of 3H/14C-diflubenzuron
(7 mg/kg) to male cats. The radioactive dose was given on day 10 of a
15-day dosing regime of non-radioactive diflubenzuron (days 1-9 and
days 11-15). The excretion of radioactivity in urine accounted for
9.5 and 9.6% of the 14C and 3H doses, respectively, during 6 days
after dosing. The elimination of radioactivity in faeces accounted
for 77.3 and 71.6% of the 14C and 3H doses, respectively, during 6
days after dosing.
After an oral administration of 14C-diflubenzuron (5 mg/kg) to
female pigs, 82% of the dose was eliminated via faeces and 5% via
urine in 11 days (Opdycke, 1976).
About 85% of a single oral dose of 14C-diflubenzuron (10 mg/kg
body weight) administered to a cow was recovered in the faeces during
the first 4 days after treatment. About 15% was recovered in urine
and only about 0.2% was secreted in the milk (Ivie, 1977, 1978).
Sheep excreted 41% of the dose (10 mg/kg) in the urine and 43% in
the faeces during the 4 days after treatment. Bile-cannulated sheep
eliminated 24% of the dose in the urine, 32% in the faeces and 36% in
the bile. Sheep treated with 500 mg 14C-diflubenzuron/kg as a single
oral dose eliminated a much smaller proportion of the 14C in urine
and bile. This was probably due to reduced absorption from the
gastrointestinal tract when the sheep were given an exaggerated dose
(Ivie, 1977).
An oral dose of 5 mg 14C-diflubenzuron/kg administered to white
leghorn hens and Rhode Island red-barred Plymouth Rock buff cross hens
was rapidly excreted unaltered within the first 8 h. Up to 91 and
82%, respectively, were excreted within 13 days (Opdycke, 1976).
6.5 Retention and turnover
6.5.1 Biological half-life
From the studies of Willems et al. (1980) and Ivie (1978), the
half-life of diflubenzuron appears to be 12 h in rat and sheep and
18-20 h in the cow.
Diflubenzuron has been shown to pass intact through the
intestinal tract and remained active in the manure (Nimmo & de Wilde,
1977b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of diflubenzuron and its formulations to
different species is summarized in Table 7. No signs of intoxication
were observed during the 14-day period following a single
administration of diflubenzuron.
Van Eldik (1974) reported an intraperitoneal LD50 of
> 2150 mg/kg in rats and mice.
7.2 Short-term exposure
Rats (5 of each sex per group) were fed on a diet containing
diflubenzuron at concentrations of 0, 800, 4000, 20 000 and
100 000 mg/kg feed for 4 weeks. Behaviour, body weight, food and
water consumption were not affected by the treatment. There was a
dose-related increase in the met- and sulfhaemoglobin content of the
blood in all treated groups except for the methaemoglobin value for
females in the 800-mg/kg dose group. Lower erythrocyte, packed cell
volume (PCV) and haemoglobin values were observed in both sexes of the
100 000-mg/kg dose group. There was a dose-related increase in spleen
and liver weights. Only the dose level of 800 mg/kg did not affect
the liver weight (Palmer et al., 1977).
Five male Swiss-albino rats were given 96.7 mg diflubenzuron/kg
body weight per day, dissolved in corn oil, in their diet for 48 days.
The total dose was 4640 mg/kg body weight. Five controls were given
corn oil only in their diet. At the end of the study the treated
groups showed a significantly lower mean haemoglobin concentration
than the controls, and decreases in MCH and MCHC values (Berberian &
Enan, 1989).
Diflubenzuron was administered to Sprague-Dawley rats of the CD
strain (20 of each sex per group) at dietary levels of 10 000 and
100 000 mg/kg feed for 9 weeks, followed by a 4-week withdrawal
period. Lower values for red blood cell parameters were recorded at
both dose levels. An increase in reticulocyte count and a pallor of
the extremities and eyes were observed. The formation of
methaemoglobin occurred in both males and females, with approximately
5-8% of the available haemoglobin being transformed to methaemoglobin.
After a withdrawal period of 4 weeks methaemoglobin comprised less
than 2% of the available haemoglobin in tested animals compared with
approximately 0.6% in controls. Higher values for SGPT were recorded
as well as heavier liver, spleen and adrenal weights. Minor
enlargement of centrilobular hepatocytes in the highest dose group was
observed, but this finding disappeared after the withdrawal period
(Hunter et al., 1979).
Table 7. Acute toxicity of diflubenzuron and its formulations
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Diflubenzuron mouse: > 4640 rat: > 10 000 rabbit: > 30 mg/ rabbit: 40 mg rabbit: non- guinea-pig: non-
technical mg/kg body weight mg/kg body weight litre nominal instilled in eye; irritant (Taylor, sensitizing
(Koopman, 1977c) marginal irritant 1973a) (Prinsen, 1992)
(Davies &
rat: > 4640 mg/kg rabbit: > 4 ml/kg > 3.75 mg/litre Ligget, 1973)
body weight body weight of 50% actual (Berczy et
(van Eldik, 1973a; in gum tragacanth al., 1975a)
Koopman, 1977a) (Davies &
Halliday, 1974)
Diflubenzuron mouse: > 5000 rat: > 2000 mg/kg rat: > 13.8 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: 25%
90% concentrate mg/kg body weight body weight litre nominal instilled in eye; non-irritant w/w in paraffin
(Koopman & Pot, (Koopman, 1984a) very slight (OECD Guideline (Grade 1); weak
1986) > 2.49 mg/litre irritant (Koopman, 404) (Koopman, sensitizer (OECD
actual (Greenough 1984c) 1984d) Guideline 406)
rat: > 5000 mg/kg & McDonald, (Kynoch & Smith,
body weight 1986) 1986)
(Koopman, 1984b)
Dimilin WP 25% rat: > 40 000 rat: > 20 000 rat: > 150 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: non-
mg/kg body weight mg/kg body weight litre nominal instilled in eye; non-irritant to sensitizing
(Janssen & Pot, slight to only minimally (Kynoch & Elliott,
mice: > 40 000 1987e) > 3.5 mg/litre moderate, irritant 1978,a,b)
mg/kg body weight actual (Arts, transientsient eye (Taylor, 1973b;
(van Eldik, 1973b; 1991) irritation (Snoeij Chandran, 1981;
Koopman, 1977b) & Busé-Pot, 1991) Snoeij &
Busé-Pot, 1990)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin SC-48 rat: > 5000 mg/kg rat: > 2000 mg/kg rabbit: 0.1 ml rabbit: non- guinea-pig: weak
body weight body weight - instilled in eye; irritant (Janssen sensitizer
(Janssen & Pot, (Janssen & Pot, slight irritant & Pot, 1987b) (Kynoch &
1987c) 1987d) (Janssen & Pot, Parcell, 1987)
1987a)
Dimilin SC-15 albino rats: 0.1 rabbit: minimally
- - - ml instilled in irritant -
eye; non-irritant (Prinsen, 1989a)
(Prinsen, 1989b)
Dimilin 4F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 1.9 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight actual (Jackson instilled in eye; minimally irritant sensitizing
(Spanjers, 1988a) (Spanjers, 1988b) et al., 1990) non-irritant (Prinsen, 1988a) (Prinsen, 1989c)
(Prinsen, 1988b)
Dimilin 2F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 4.4 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig:
body weight body weight actual very instilled in eye; moderate irritant moderate (Grade
(Koopman, 1985d) (Koopman, 1985c) slightly irritant moderate irritant OECD Guideline III) (Kynoch &
(Zwart, 1985) OECD Guideline 404 (Koopman, Parcell, 1987)
405 (Koopman, 1985a)
1985b)
Dimilin ODC 45 rat: > 37 300 mg/kg rat: > 37 300 mg/kg rabbit: 0.1 ml rabbit: 0.5 ml
body weight body weight instilled in eye; moderate irritant
(Koopman & (Koopman, 1980b) marginally (Koopman,
Jongeling, 1979) irritant (Koopman, 1980c)
1980a)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin OF 6 rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 95.7 mg/ rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight litre nominal: instilled in eye; topical; mild sensitizing
(Besten et al., (Besten et al., > 2.17 mg/litre non-irritant irritant (Prinsen, 1993)
1993a) 1993b) actual (Janssen & (Janssen & van (Janssen & van
van Doorn, 1993a) Doorn, 1993c) Doorn, 1993b)
Wistar rats (10 of each sex per group) were fed diflubenzuron in
the diet for 13 weeks at concentrations of 0, 3.125, 12.5, 50 or
200 mg/kg feed. Behaviour, growth and food intake were unaffected by
the treatment. At the highest dose level the PCV value, the
haemoglobin concentration and the number of erythrocytes were
decreased. There was an increase in the SGPT and SGOT activities in
the males of the highest dose group at the end of the experiment. A
slight increase in the number of normally occurring scattered small
foci of necrotic parenchymal cells was observed, accompanied by
mononuclear inflammatory cell infiltration and proliferation of
reticuloendothelial system cells in the liver of both males end
females of the 50 and 200 mg/kg groups (Kemp et al., 1973a,b).
Absence of toxic effects in chronic/oncogenicity studies at low
dose levels necessitated re-evaluation of liver histopathology in the
90-day feeding study; "piece meal" liver cell necrosis was reported at
the two highest dose levels, i.e. 50 and 200 mg/kg feed. The original
slides of the study were re-evaluated by four histopathologists in
four different laboratories. They independently and unanimously
agreed that the lesions in the livers of treated rats were found to
the same extent in the livers of untreated rats. This showed that the
high dose levels in the study did not demonstrate a treatment-related
necrotic effect in the liver (Offringa, 1977).
Technical grade diflubenzuron was administered in the diet to
male and female 21- to 28-day-old Sprague-Dawley rats (40 of each sex
per group) at dose levels of 0, 160, 400, 2000, 10 000 and
50 000 mg/kg feed for 13 weeks. No apparent treatment-related effects
were noted on mortality, clinical observations, body weight gain, food
consumption, clinical chemistry or urinalysis. A treatment-related
significant increase in methaemoglobin concentration was noted in all
treated groups. Sulfhaemoglobin values showed increases at dose levels
of 2000 mg/kg or more. A significant treatment-related decrease in
haemoglobin, PCV and erythrocyte count was observed in males and
females at all dose levels by the end of the study. An increase was
noted in the reticulocyte count at all dose levels except 160 mg/kg,
and the number of the Heinz bodies was higher in the 10 000 and
50 000 mg/kg groups. After 7 weeks, spleen weights were increased in
the females at all dose levels, but after 13 weeks no effect was found
at 160 mg/kg. With the exception of the lowest dose level, all
treated groups showed a higher liver weight. The administration of
diflubenzuron resulted in a dose-related increase in the incidence of
chronic hepatitis and haemosiderosis of the liver. It was also
associated at all dose levels with haemosiderosis and congestion of
the spleen and mild erythroid hyperplasia of the bone marrow. The
severity of the lesions tended to increase with the dose. Liver
lesions were more severe in males than in females and were more severe
at 13 weeks than at 7 weeks. A no-observed-effect level (NOEL) was
not established (Burdock et al., 1980b; Goodman, 1980b).
Diflubenzuron was given to CFLP mice for 6 weeks at levels of 16
and 50 mg/kg feed. There were no clinical signs and no effects on
food consumption, body weight, blood chemistry or macroscopic
pathology. In three of the eight animals given 50 mg/kg, foci of
liver cell necrosis, with or without inflammatory cell filtration,
were noted. Other organs were not examined microscopically (Hunter et
al., 1974).
Diflubenzuron was administered to Swiss Webster mice in a 30-day
oral intubation study. Groups of five mice each received either no
treatment, vehicle control (Polyethylene glycol 400) or diflubenzuron
suspensions at dose levels of 125, 500 or 2000 mg/kg body weight.
Hepatic glutathione- S-transferase activity and morphological
characteristics were studied. Diflubenzuron was shown to elicit
hepatocellular changes at all dose levels. The activities of three
glutathione- S-transferases ( S-aryl, S-aralkyl and S-epoxide)
were irregularly altered in a non-dose-related manner. Light
microscopy revealed radial arrays of hepatocellular vacuolization
between the portal and central vein areas. There was evidence of an
increase in the amount of endoplasmic reticulum (Young et al., 1986).
Male and female mice of the B6C3F1 strain (40 of each sex per
group) received diflubenzuron (97.2% a.i.) in the diet at dose levels
of 0, 16, 50, 400, 2000, 10 000 or 50 000 mg/kg feed for 13 weeks. An
additional group of 100 of each sex served as a control. No
compound-related effects were apparent with respect to clinical signs,
survival, growth rates, total food consumption or gross pathology.
Significant treatment-related increases in met- and sulfhaemoglobin
concentrations were noted in all treated groups, except in the group
fed 16 mg/kg. At the higher dose levels, there was a decrease in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects on the weights of liver and spleen were
noted. In the females, adrenal weight was decreased (but not in a
dose-related fashion) at all dose levels after 7 weeks and increased
after 13 weeks in higher dose levels. Higher adrenal weight was
observed in treated males than in the controls. Histopathological
examination revealed treatment-related centrilobular hypertrophy of
hepatocytes, with or without cell necrosis, haemosiderosis of the
liver and spleen, extramedullary haematopoiesis and mild chronic
hepatitis in treated animals of both sexes, some of which effects were
observed at the lowest dose level (16 mg/kg). The liver lesions were
more severe in males than in females, being most severe in the high-
dose males. The NOEL for methaemoglobin formation was 16 mg/kg feed
(Burdock et al., 1980a; Goodman, 1980a).
HC/CFLP mice (40 of each sex per group) were fed diflubenzuron
(97.2% purity) for 14 weeks at levels of 0, 80, 400, 2000, 10 000 and
50 000 mg/kg feed. On the second day of treatment, the majority of
mice treated with 10 000 or 50 000 mg/kg showed dark eyes and/or
prominent caudal blood vessels. On day 5, blue/grey discoloration of
the extremities was noted for the majority of mice treated with
50 000 mg/kg. Mice in the lowest dose group exhibited no clinical
signs. Mortality, food consumption, water consumption and body weight
changes were not significantly affected by the treatment. Lower PCV
and red blood cell counts were found at all dose levels except
80 mg/kg. The total white blood cell count, lymphocyte count,
haemoglobin concentration, incidence of Heinz bodies and red blood
cell count were increased in all treated groups. At week 7, there was
an increase in the number of reticulocytes in treated mice,
particularly in males treated at 10 000 or 50 000 mg/kg. At week 14,
the reticulocyte counts were similar to those of the controls. A
treatment-related increase in both met- and sulfhaemoglobin was
recorded in all treated groups at weeks 7 and 14 of the investigation.
Plasma glutamic-pyruvic transaminase values were increased at all dose
levels, with the exception of 80 mg/kg feed. Lower blood cholesterol
levels were noted in the 2000, 10 000 and 50 000 mg/kg groups.
Macroscopic examination showed dark discoloration and/or enlargement
of the spleen and pale subcapsular areas of the liver in all dose
groups after both 7 and 14 weeks. Histopathological examination of
the spleen revealed increased haemosiderosis at all dose levels except
80 mg/kg. In the liver, areas of focal necrosis and/or fibrosis in
the parenchyma, with or without associated inflammatory cells,
fibroblasts or pigment-laden macrophages, were observed. At higher
dose levels necrotic and fatty hepatocytes and brown pigment-laden
Kupffer cells were found. A NOEL was not established (Colley et al.,
1981a,b).
Diflubenzuron was fed to groups of three male and three female
beagle dogs for 13 weeks at concentrations of 0, 10, 20, 40 and
160 mg/kg diet. No effect of the treatment on behaviour, body weight
or food and water consumption was observed. Elevated SAP and SGPT
values were recorded for some dogs receiving 40 or 160 mg
diflubenzuron/kg feed. After 6 weeks, methaemoglobin and another
abnormal pigment, probably sulfhaemoglobin, were demonstrated in dogs
given 160 mg/kg. After 12 weeks of administration, some recovery was
observed. Organ weights and gross and microscopic evaluation did not
reveal any treatment-related effects. The NOEL for methaemoglobin
formation was 40 mg/kg feed (Chesterman et al., 1974).
Diflubenzuron was administered daily in gelatin capsules to male
and female beagle dogs (6 of each sex per group) at dose levels of 2,
10, 50 or 250 mg/kg body weight per day for 52 weeks. There were no
treatment-related effects on mortality, food consumption or body
weight gain. Dose-related marginal increases in methaemoglobin and
sulfhaemoglobin were recorded from 10 mg/kg upwards. At 50 and
250 mg/kg, haemoglobin concentration and MCHC were decreased whereas
reticulocyte and platelet counts were increased. Heinz bodies were
also detected in several animals receiving 50 and 250 mg/kg. Dose-
related increases in liver and spleen weights were found in the 50 and
250 mg/kg males. Histopathological evaluation of the liver revealed
an increase in the incidence of pigmented macrophages and Kupffer cell
siderosis at 50 and 250 mg/kg in both males and females. The NOEL
based on the increase in met- and sulfhaemoglobin was 2 mg/kg body
weight (Greenough et al., 1985).
In a study by Berczy et al. (1975c), rats were exposed daily for
a one-hour period to technical diflubenzuron dust at nominal
concentrations of 0, 0.5, 5.0 and 50 mg/litre air, respectively (the
actual concentrations were 0, 0.12, 0.87 and 1.85 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days per week. The methaemoglobin levels in male rats at the two
lower concentrations and female rats of all test groups were
significantly higher than those of controls.
Rabbits were exposed daily for one-hour periods to technical
diflubenzuron dust at concentrations of 0.5, 5.0 and 25 mg/litre air
(the measured concentrations were 0.15, 0.75 and 1.99 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days each week. There were no signs of irritation in animals
exposed to 0.5 mg/litre, but mild transient respiratory irritation was
seen at the two higher concentrations. Haematological examination,
biochemistry tests and macroscopic inspection revealed no treatment-
related abnormalities (Berczy et al., 1975b).
Diflubenzuron, at levels of 4.64, 10 and 21.5% weight/volume was
applied daily to the intact and abraded skin of rabbits at a dosage
level of 1.5 ml/kg body weight, 5 days a week, for 3 consecutive weeks
(equivalent to 69.6, 150 and 322.5 mg diflubenzuron/kg body weight per
day). The sulfhaemoglobin level was increased in one rabbit out of 20
in the 10% group, and in 5 rabbits out of 20 at the high treatment
level. The 10% treatment level was considered to be the NOEL based on
sulfhaemoglobin formation (Davies et al., 1975).
7.3 Long-term exposure
Diflubenzuron was administered to Sprague-Dawley rats (60 of each
sex per group) in their diet at levels 0, 10, 20, 40 and 160 mg/kg
feed for 104 weeks. There were no treatment-related effects on body
weight gain, food intake, renal function or on macroscopic and
microscopic pathology. In rats treated with 160 mg/kg, significantly
higher methaemoglobin levels were recorded. The tumour profile of
treated rats was similar to that of the controls. The NOEL based on
methaemoglobin was 40 mg/kg, equivalent to a mean intake of 1.43 and
1.73 mg/kg per day for males and females, respectively (Hunter et
al.,1976).
When diflubenzuron was administered in the diet to male and
female Sprague-Dawley rats (50 of each sex per group) at dosage levels
of 0, 156, 625, 2500 and 10 000 mg/kg feed for 104 weeks, there was no
evidence of an effect on mortality or treatment-related clinical
signs. Significantly increased absolute methaemoglobin and
sulfhaemoglobin values were observed in all male treatment groups.
However, increases in relative methaemoglobin values (% of total
haemoglobin) were only noted in the 156, 2500 and 10 000 mg/kg groups,
while sulfhaemoglobin increases were noted in the 156, 625 and
10 000 mg/kg females. There was a significant increase in absolute
and relative spleen weights in the two highest dose groups of both
sexes, together with haemosiderosis in spleen and liver. There was no
evidence of carcinogenicity after 2 years of feeding diflubenzuron
(Burdock et al., 1984).
In a study by Hunter et al. (1975), diflubenzuron was
administered to CFLP mice for 80 weeks at dietary levels of 0, 4, 8,
16 and 50 mg/kg feed. There were no overt signs of reaction to
treatment. Behaviour, mortality, food and water consumption and body
weight were unaffected by the treatment. The macroscopic changes
observed were those commonly seen in mice of this strain and age. No
histopathological changes were seen that were considered to be related
to the administration of diflubenzuron. The incidence of liver cell
tumours was higher in this study than it was in other studies
performed in these laboratories using this strain of mouse. The
increase was seen in both treated and control groups of either sex and
showed no evidence of a treatment-related effect. There was no
evidence of a treatment-related effect on tumour incidence in the CFLP
mouse.
Male and female HC/CFLP mice (88 of each sex per group) were fed
diflubenzuron at dietary levels of 0, 16, 80, 400, 2000 and
10 000 mg/kg feed for 91 weeks. There was no indication of a
treatment-related effect on survival, food consumption or body weight
gain. Treatment-related elevations of MCH and MCHC values were
recorded from week 26 onwards in mice given 10 000 mg/kg. The
incidence of Heinz bodies increased in a dose-related manner among
mice given 400, 2000 or 10 000 mg/kg from week 52 onwards. Dose-
related increases in methaemoglobin levels were found from week 26
onwards and in sulfhaemoglobin levels from week 52 onwards in the mice
fed 80 mg/kg or more. Elevated alkaline phosphatase (AP) activities
were seen at weeks 24, 76 and 89 among male mice receiving 2000 and
10 000 mg/kg and at week 84 among mice of the 400 mg/kg dose group.
An increased incidence of splenic and/or hepatic enlargement was seen
among mice treated with 10 000 mg/kg. Cyanosis of the skin was noted
among mice at 400, 2000 and 10 000 mg/kg. Increased liver and spleen
weights were found among mice given 2000 and 10 000 mg/kg. An
increased incidence of hepatocyte enlargement and increased
extramedullary haematopoiesis in the liver and spleen were seen in
mice at high dose levels. In the 400, 2000 and 10 000 mg/kg groups,
there was an increased incidence of siderocytosis in the spleen and of
pigmented Kupffer cells in the liver. There was no treatment-related
effect on tumour incidence (Colley et al., 1984).
7.4 Skin and eye irritation; sensitization
Relevant data are given in Table 7.
Administration of technical diflubenzuron to the intact and
abraded skin of albino rabbits did not produce irritation of the skin
after exposure times of 24 and 72 h (Taylor, 1973a,b).
Diflubenzuron was found to be a moderate irritant to the rabbit
skin after application of 0.5 ml of 45% oil dispersible concentrate
for 24 h (Koopman, 1980a; Prinsen, 1990).
Diflubenzuron (both technical and 45% oil dispersible
concentrate) was considered to be marginally irritant to the rabbit
eye (Davies & Ligget, 1973; Koopman, 1980b).
Diflubenzuron (48% water-based paste) was not found to be a
dermal sensitizer in guinea-pigs (Kynoch & Parcell, 1987).
Technical diflubenzuron was studied for skin sensitization in a
maximization test on guinea-pigs and was found to be non-sensitizing
(Prinsen, 1992). However, some formulations are mild sensitizers
(Table 7).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Diflubenzuron was fed to pathogen-free rats of the CFY strain
(20 of each sex per group) at dietary levels of 0, 1000 and
100 000 mg/kg for one generation and one litter. The animals were
maintained on their respective diets for 9 weeks prior to mating.
There were no clear effects on mating performance, pregnancy rate,
duration of gestation, litter size, offspring mortality, litter weight
or the type and distribution of abnormalities. Dose-related effects
of diflubenzuron were demonstrated at 17 weeks in adults and consisted
of reduced values for PCV, haemoglobin, total red cells count and
MCHC, increased values for methaemoglobin, MCV, spleen weight and
siderocyte incidence in the spleen, and the occurrence of iron-
pigment-containing Kupffer cells in the liver. A dose-related effect
on the liver was also shown by increased weight and SGPT activity and
centrilobular hepatocyte enlargement. Reduced blood glucose
concentrations were recorded in both treated groups. At the highest
dosed level, the offspring showed increased liver and spleen weights
for both sexes (Palmer et al., 1978).
In a three-generation reproductive study with rats fed
diflubenzuron at concentrations of 10, 20, 40 and 160 mg/kg feed, no
adverse treatment-related effects on mating performance, pregnancy
rate, duration of gestation or litter parameters (total loss, size,
mean pup weight, mortality, abnormalities) were found (Palmer & Hill,
1975a).
After gavage administration of diflubenzuron at doses of 1, 2 and
4 mg/kg body weight per day to pregnant rats during days 6-15 of
gestation, no effects were observed on embryonic or fetal development
(Palmer & Hill, 1975b).
Treatment of pregnant New Zealand white rabbits at oral doses of
1, 2 and 4 mg/kg body weight per day during days 6-18 of gestation did
not affect embryonic or fetal development as assessed by the incidence
of major malformations, minor anomalies and skeletal variants (Palmer
& Hill, 1975c).
In a study by Booth (1977), pregnant Swiss mice were fed a diet
containing 50 mg/kg of diflubenzuron (partly 14C) for a period of
17 days. Some of these mice were killed at day 17 after conception,
while the others were allowed to give birth. The results of this
study showed that mucopolysaccharide synthesis in embryonic mouse-limb
cartilage was normal. Diflubenzuron did not pass through embryonic
membranes nor was it passed from mother to suckling young mice.
Analysis of 226 embryos showed no gross teratogenic effects.
Two groups of 24 timed-mated female rats of the Crl:CD (SD) BR
strain were dosed once daily by the oral route between days 6 and 15
of pregnancy. One group was dosed with diflubenzuron at 1000 mg/kg
per day, while the other group was given the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 20 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths or treatment-related changes in clinical condition. Treatment
did not affect maternal growth or food consumption. There were no
maternal abnormalities at necropsy that were considered to be related
to treatment. The mean numbers of corpora lutea, implantations and
live fetuses were similar in all groups. Both pre- and post-
implantation losses were unaffected by treatment. Fetal weights and
sex ratio were unaffected by diflubenzuron treatment. The incidences
of major external/visceral and skeletal abnormalities, and minor
external/visceral abnormalities were not affected by diflubenzuron
treatment. The incidence of fetuses with minor skeletal abnormalities
was slightly higher in the treated group, but was within the usual
background range. There were intergroup differences in the
proportions of fetuses with specific minor abnormalities and variants
of skeletal ossification. For some bones, the differences achieved
statistical significance. However, on balance, treated group fetuses
were considered to be similarly ossified to the control fetuses. Oral
administration of diflubenzuron at a dose level of 1000 mg/kg per day
did not elicit maternal toxicity or any evidence of embryotoxicity
(Kavanagh, 1988a).
Two groups of 16 timed-mated female New Zealand White rabbits
were dosed once daily by the oral route from days 7 to 19 of
pregnancy, inclusive. One group was dosed with diflubenzuron at
1000 mg/kg per day and the other group with the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 28 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths, changes in clinical condition or abnormalities at maternal
necropsy considered to be related to diflubenzuron treatment. Two
animals were killed prematurely during the study, one from the control
group and one from the treated group. The changes in clinical
condition and abnormalities observed at necropsy in these animals were
considered to be unrelated to treatment. Treatment did not affect
maternal growth or food consumption. The mean numbers of corpora
lutea, implantations and live fetuses were similar in all groups. Both
pre- and post-implantation losses were unaffected by treatment. Fetal
weights and sex ratio were unaffected by diflubenzuron treatment. The
incidence of both major and minor external/visceral and skeletal
abnormalities, as well as the numbers of skeletal variants, was
unaffected by diflubenzuron treatment. Oral administration of
diflubenzuron at a dose level of 1000 mg/kg per day did not elicit
maternal toxicity or any evidence of embryotoxicity (Kavanagh, 1988b).
In a study by Kubena (1982), diflubenzuron was fed at levels of
0, 2.5, 25 and 250 mg/kg feed to male and female layer-breed chickens
from 1 day of age through a laying cycle. Characteristics measured
were egg production, egg weight, eggshell weight, fertility,
hatchability and effects on the progeny. Feeding diflubenzuron at
levels up to 250 mg/kg feed did not affect these characteristics.
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller & Booth, 1976).
7.6 Mutagenicity and related end-points
Diflubenzuron was examined by in vitro and in vivo
mutagenicity tests. The results are summarized in Table 8.
Mutagenicity tests have also been carried out with the major
metabolites of diflubenzuron (Table 9).
Table 8. Mutagenicity tests with diflubenzuron
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Microorganisms
Reverse Salmonella typhimurium
mutation TA98, TA100, 10-1000 µg/plate +/- negative Bryant (1976)
TA98, TA100, TA1537, TA1978 1000 µg/spot +/- negative Bryant (1976)
Reverse S. typhimurium 0.1-500 µg/plate +/- negative Brusick & Weir
mutation TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Reverse S. typhimurium 10, 100 or 1000 µg/plate; +/- negative McGregor et al.
mutation TA98, TA100, 19, 186, 1860 µg DFB/plate (1979)
TA1535, TA1537 (as Dimilin W-25)
Reverse S. typhimurium up to 5000 µg/plate +/-b negative Moriya et al.
mutation TA98, TA100, TA1535, (1983)
TA1537, TA1538
Escherichia coli. WP2 hcr
Reverse S. typhimurium up to 1000 µg/plate +/- negative Koorn (1990)
mutation TA98, TA100, TA1535,
TA1537, TA1538
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammalian cells in vitro
Forward L5178Y mouse lymphoma 1.17-300 µg/ml +/- negative McGregor et al.
mutation cells (1979)
Chromosomal Chinese hamster ovary up to 200 µg/ml +/- negative Taalman & Hoorn
aberrations cells (1986)
Unscheduled human diploid WI-38 cells 50-1000 µg/ml +/- negative Brusick & Weir
DNA synthesis (blocked in the G1 phase) (1977c)
DNA repair rat hepatocytes up to 333 µg/ml negative Enninga (1990)
(unscheduled
DNA synthesis)
Cell transformation BALB-3T3 cells, 0.02-0.312 µg/ml - negative Brusick & Weir
in vitro (1977b)
Cell transformation pregnant hamster, ip 10, 200 and 500 mg/kg negative Quarles et al.
(transplacental injection on 10th day of body weight (1980)
transformation) gestation, fetal cell culture
3 days after injection
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammals
Micronucleus mouse bone marrow 15, 150, 1500 mg/kg body negative McGregor et al.
weight 30 and 6 h before (1979)
necropsy
Dominant male mice mated to 1000 and 2000 mg/kg body negative Arnold et al.
lethal 3 females weekly for 6 weeks weight intraperitoneal (1974)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b Source of S9 not indicated
Table 9. Mutagenicity tests with diflubenzuron metabolites
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
A. 4-chlorophenylurea
Microorganisms
Reverse mutation Salmonella typhimurium
and differential TA98, TA100, TA1535, 1000 µg/spot +/- negativeb Dorough (1977)
killing TA1537, TA1538, TA1978
Reverse mutation TA98, TA100 10, 100, 500, negative
1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 6.25-50 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Cell BALB-3T3 cells, 0.019-0.312 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977a)
0.312 mg/ml
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
B. 2,6-Difluorobenzoic acid
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negative Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Reverse mutation S. typhimurium 10, 100, 500,
TA98, TA100 1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977b)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 75-500 µg/ml +/- positive Matheson & Brusick
DNA synthesis (blocked in the G1 phase) with (1978a)
activation
Cell BALB-3T3 cells, 0.156-2.5 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977b)
2.5 mg/ml
C. 4-Chloroaniline (PCA)
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negativeb Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Reverse mutation TA98, TA100 10, 100, 500, positive in
1000 µg/plate TA98 at 500
and 1000 µg
with
activation
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977c)
TA1537, TA1538
Reverse mutation S. typhimurium
TA98, TA100, TA1530, 0-1500 µg/plate +/- negative Gilbert et al. (1980)
TA1535, TA1537, TA1538
Reverse S. typhimurium C3076, 1000 µg/plate +/- negative Thompson et al.
mutation D3052, G46, TA98, TA100, (1983)
TA1535, TA1537, TA1538,
Escherichia coli WP2,
WP2 uvrA
Reverse S. typhimurium 3333 µg/plate +/- positivec Dunkel et al. (1985)
mutation TA98, TA100, TA1535,
TA1537, TA1538,
Escherichia coli
Reverse S. typhimurium 1666 µg/plate +/- positived Mortelmans et al.
mutation TA97, TA98, TA100, TA1535 negative (1986)
DNA damage Escherichia coli polA+/polA- 250 µg/plate - positive Rosenkranz & Poirier
(1979)
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Mutation Aspergillus nidulans 200 µg/ml - positive Prasad (1970)
Mammalian cells
Forward mutation L5178Y mouse lymphoma +/- positived Caspary et al. (1988)
tk+/- cells
Unscheduled human WI-38 cells 250-1000 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Unscheduled rat primary hepatocytes 5-50 µg/ml - positive Williams et al.
DNA synthesis (1982)
Unscheduled rat primary hepatocytes 50 nmol/ml - negative Thompson et al.
DNA synthesis (1983)
Sister chromatid Chinese hamster ovary 1600 µg/ml +/- positived US NTP (1989)
exchange cells
Chromosomal Chinese hamster ovary cells 1000 µg/ml +/- positived US NTP (1989)
aberrations and negative
Cell BALB-3T3 cells, 0.039-0.625 mg/ml not stated negative Matheson & Brusick
transformation in vitro (1978c)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b No increase in revertants, strains TA1538/TA1978 positive for differential killing
c Tested in three independent laboratories
d Tested in two independent laboratories
According to the findings presented, neither diflubenzuron nor
its major metabolites may be considered to have mutagenic effect.
Several positive effects were, however, obtained with PCA.
7.7 Carcinogenicity
Long-term carcinogenicity studies were described in section 7.3.
In both mouse and rat oncogenicity studies, diflubenzuron at dose
levels up to 10 000 mg/kg feed caused no change in tumour profile or
onset of tumours. In the rat oncogenicity study, the incidence of
sarcoma in the spleen and phaeochromocytomas was not increased. In
the mouse oncogenicity study, the incidence of hepatocellular
neoplasms or haemangiosarcomas in spleen and liver was not increased.
Therefore, diflubenzuron, in combination with its metabolites as
generated in the animal metabolic system, is not oncogenic.
Significantly, there were no non-neoplastic or neoplastic lesions of
the vasculature, including that of liver and spleen, in B6C3F1 male
mice treated with diflubenzuron. Similarly, there were no fibrotic or
carcinomatous lesions in the spleen of treated male F-344/N rats.
7.8 Other special studies
Diflubenzuron has been studied in mice for its growth-inhibiting
activity in serially transplanted B16 malignant melanoma and CA1025
skin carcinoma. A single 800 mg/kg intraperitoneal injection of
diflubenzuron induced rapid (24 h) decreases in tumour volume in 78%
and 66% of the tumours, respectively, while in control mice 85% of the
melanomas and 91% of the skin carcinomas increased in volume over the
same time period (Jenkins et al., 1984). These observations were
later confirmed, and it was suggested that the activity was due to
derivatives of a hydroxylated metabolite (Jenkins et al., 1986).
Studies of nucleoside uptake by Harding Passey melanoma cells
in vitro indicated a rapid (< 5 min) inhibition of the uptake of
uridine, adenosine and cytidine, but not of thymidine, by
diflubenzuron; this could not be reversed by washing. De novo
nucleic acid synthesis was not impaired and in vitro cell growth was
unaffected (Mayer et al., 1984).
El-Sebae et al. (1988) tested the effect of diflubenzuron on
protein and RNA biosynthesis by rabbit liver and muscle tissues kept
in an incubation medium. The synthesis of protein and RNA was
significantly stimulated in the liver and inhibited in the muscle by
graded doses. The maximum effect on both tissues was reached at 5 µg
diflubenzuron/ml for protein synthesis and at 0.2 µg/ml for RNA
synthesis, the effect on protein synthesis being more pronounced than
that on RNA synthesis in both tissues.
7.8.1 Special studies on met- and sulfhaemoglobin formation
The ability of diflubenzuron to induce methaemoglobin and
sulfhaemoglobin formation has been recognized since the initial
toxicity studies on the compound. Methaemoglobinaemia has been
demonstrated after oral, dermal and inhalatory exposure to
diflubenzuron in various species (see section 7.2 and 7.3). It is the
most sensitive parameter in this case.
Fifteen male Wistar rats received diflubenzuron (technical) by
gastric intubation at a dose level of 5000 mg/kg body weight per day
for 8 days. No effect was observed on Heinz body formation, whereas
met- and sulfhaemoglobin levels were significantly increased when
compared with the control group. The increase in the methaemoglobin
level was about 6% (Keet, 1977a).
When diflubenzuron was administered to male Wistar rats for 28
days at oral doses of 100 and 500 mg/kg body weight, it induced
elevation of methaemoglobin concentration and reticulocytes count in
both of the treated groups. However, there was no dose-response
relationship at the dose levels investigated (Tasheva & Hristeva,
1991, 1993).
Technical diflubenzuron was administered by gastric intubation to
male Swiss mice daily for a period of 14 days at dose levels of 0, 8,
40, 200, 1000 and 5000 mg/kg body weight. Body weight measurement and
macroscopic evaluation did not reveal any effect of the treatment. At
dose levels of 1000 and 5000 mg/kg the percentages of methaemoglobin
and erythrocytes containing Heinz bodies were increased. The
sulfhaemoglobin level was statistically significantly increased at
200, 1000 and 5000 mg/kg in comparison to the control group. The NOEL
was considered to be 40 mg/kg body weight based on sulfhaemoglobin
(Keet, 1977b).
When female mice were fed 0, 50, 200, 400, 1000 and 2000 mg
diflubenzuron/kg feed for 30 days, sulfhaemoglobin was demonstrated in
the blood from 200 mg/kg onwards (being 13% of total haemoglobin at
2000 mg/kg). Mice fed 1000 and 2000 mg/kg showed signs of cyanosis
after 3 weeks. Recovery was completed after a 3-week withdrawal
period (Bentley et al., 1979).
When 15 male New Zealand White rabbits were fed technical or
analytically pure diflubenzuron (640 mg/kg feed) for 21 or 18 days,
respectively, the methaemoglobin and sulfhaemoglobin levels were
significantly increased (Keet, 1977c).
Male and female cats (24 of each sex per group) received
diflubenzuron orally for 21 days at dose levels of 0, 30, 70, 100, 300
and 1000 mg/kg body weight and were observed for the subsequent 14-day
period. A dose-related elevation of methaemoglobin level was observed
with ceiling values at 300 and 1000 mg/kg. For male cats the NOEL was
30 mg/kg, but no NOEL was achieved for females. Increased
sulfhaemoglobin and Heinz bodies were observed in all treated groups.
NOEL values for sulfhaemoglobin were not achieved. The haemoglobin
concentration, reticulocyte number and organ weights were not affected
by the treatment (Schwartz & Borzelleca, 1981).
After dermal application of technical diflubenzuron at a dose
level of 1.5 ml/kg body weight for 18 days to rabbits, the
methaemoglobin level was increased (Keet, 1977c).
The available data demonstrate that dose-response relationship
for production of methaemoglobin exists. This is considered to be the
most sensitive end-point after repeated exposure in experimental
animals.
Table 10 summarizes the effects on methaemoglobinaemia as
determined in various studies.
7.9 Toxicity of metabolites
In rat metabolic studies it was shown that about 20% of absorbed
diflubenzuron is metabolized to 2,6-DFBA and its counterpart 4-CPU.
Only a small fraction of the 4-CPU is metabolized to PCA (see
section 6).
The acute oral toxicity of the major metabolites of diflubenzuron
is summarized in Table 11.
Loss of activity, catatony, paralysis and severe bradypnoea were
observed in rats treated with the metabolite 4-CPU. The minimum
symptomatic dose level was 100 mg/kg body weight. At autopsy, the
animals showed congested blood vessels and haemorrhage in the
gastrointestinal tract (Koelman-Klaus, 1978a).
Rats dosed with 2,6-DFBA showed slight increase in startle
response, activity, abdominal and limb tone, slight decrease in
grooming activity, slightly abnormal gait and body posture, mild
restlessness, irritation and aggressivity, pilo-erection and increased
alertness. The minimum symptomatic dose level was 464 mg/kg body
weight (Koelman-Klaus, 1978b).
Mutagenicity tests have been carried out with 2,6-DFBA, 4-CPU and
PCA (see section 7.6 and Table 9).
Table 10. Summary of the effects on methaemoglobinaemia in various species
Species Route Duration No-observed-effect level Reference
Rat diet 4 weeks male: not achieved; female: 800 mg/kg feed Palmer et al. (1977)
(equivalent to 45 mg/kg per day)
Rat diet 13 weeks not established - lowest dose tested 160 mg/kg feed Burdock et al. (1980b)
Rat diet 104 weeks 40 mg/kg feed (equivalent to 2 mg/kg Hunter et al. (1976)
per day body weight)
Mouse oral 2 weeks 200 mg/kg body weight Keet (1977b)
Mouse diet 13 weeks male/female: 16 mg/kg feed (equivalent to Burdock et al. (1980a)
2.4 mg/kg body weight per day)
Mouse diet 14 weeks not established - lowest dose tested 80 mg/kg feed Colley et al. (1981a,b)
Mouse diet 91 weeks male/female: 16 mg/kg feed (equivalent to Colley et al. (1984)
2.4 mg/kg body weight per day)
Cat oral 3 weeks male: 30 mg/kg body weight; female: not achieved Schwartz & Borzelleca (1981)
Dog diet 13 weeks male/female: 40 mg/kg feed Chesterman et al. (1974)
Dog oral (capsules) 52 weeks male/female: 2 mg/kg body weight Greenough et al. (1985)
Table 11. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50 Reference
(mg/kg)
4-Chlorophenylurea rat male oral 1080 Koelman-Klaus
female oral 1210 (1978a)
2,6-Difluorobenzoic rat male, oral 4640 Koelman-Klaus
acid female (1978b)
7.9.1 Carcinogenicity studies with 4-chloroaniline
The diflubenzuron metabolite, 4-chloroaniline (PCA), has been
assayed for carcinogenicity by the US NCI (1979) and by the US NTP
(1989) using Fischer-344 rats and B6C3F1 mice on both occasions. In
the earlier of these studies, technical-grade PCA was administered at
dietary concentrations of 250 and 500 mg/kg to rats and 2500 and
5000 mg/kg to mice. Groups of 50 male and 50 female animals of each
species were randomized to the treatment groups at approximately six
weeks of age. The control groups consisted of 20 animals of each sex
and species. All animals which survived were treated for 78 weeks and
observed, untreated, for a further 24 weeks (rats) or 13 weeks (mice).
Survival in all groups was good and it was judged that there were
adequate numbers at risk for late-developing tumours. In rats, the
most significant findings were treatment-related proliferative splenic
capsular and parenchymal lesions in males and females and, in male
rats of the high-dose group, the occurrence of several types of
unusual splenic neoplasms (i.e. fibroma, fibrosarcoma, sarcoma NOS,
haemangiosarcoma and osteosarcoma) which appeared to arise from areas
of capsular or parenchymal fibrosis. These neoplasms were combined
for analysis because it was considered that the fibromas were a benign
form of sarcoma and that the neoplasms had a common cellular origin.
The combined incidences were 0/20 control, 0/49 low-dose and 10/49
high-dose rats. This result indicated a carcinogenic effect of
treatment in male rats. There was no similar effect in the spleen of
females. In mice of each sex, there was increased incidence of
haemangiomas and haemangiosarcomas in various organs. The combined
incidences were 2/20 in control males, 10/50 in low-dose males and
14/50 in high-dose males, and 0/18 in control females, 3/49 in low-
dose females and 8/42 in high-dose females. In addition, there was,
in female mice only, a non-significant increase in hepatocellular
carcinomas and adenomas combined (0/18 control, 1/49 low-dose and 6/41
high-dose). The smaller number of control group animals in these
experiments significantly reduced the statistical power. It was
recognized that PCA is unstable in feed and so the animals in the
early experiments may have received lower doses than intended.
Consequently, PCA was administered by gavage with an aqueous vehicle
containing hydrochloric acid in the later experiments (US NTP, 1989).
Groups of 49 or 50 rats and mice of each sex (7 to 8 weeks old)
were administered PCA at dose levels of 2, 6 or 18 mg/kg (rats) or 3,
10, or 30 mg/kg (mice) on 5 days per week for 103 weeks. Vehicle
control groups of 50 males and 50 females received deionized water by
gavage. Survival was adequate for analysis, although variable. It was
lower, for example, in the vehicle control groups of rats, which was
attributable to the higher incidence of mononuclear cell leukaemia in
these groups. Significant, non-neoplastic findings in rats included
treatment-related increases in the incidence of splenic fibrosis
(males: 3/49 control, 11/50 low-dose, 12/50 mid-dose, 41/50 high-dose;
females: 1/50 control, 2/50 low-dose, 3/50 mid-dose, 42/50 high-dose),
lipocytic infiltration of the spleen (males: 0/49, 0/50, 0/50, 24/50;
females: 0/50, 0/50, 0/50, 11/50) and adrenal medullary hyperplasia
in female rats (4/50, 4/50, 7/50, 24/50). In addition, the
methaemoglobin level was consistently increased in the mid- and high-
dose groups of male rats at 6, 12, 18 and 24 months. There was no
NOEL for this parameter in male rats. Bone-marrow hyperplasia was
also observed. The combined incidences of uncommon splenic sarcomas
(fibrosarcomas, osteosarcomas or haemangiosarcomas) were increased in
male rats but not in females (males: 0/49, 1/50, 3/50, 38/50; females:
0/50, 0/50, 1/50, 1/50). There was also a small increase in
phaeochromocytomas or malignant phaeochromocytomas in male rats
(13/49, 14/48, 15/48, 26/49). The incidences of mononuclear cell
leukaemia were reduced in male and female rats of the treatment groups
(males: 21/49, 3/50, 2/50, 3/50; females: 10/50, 2/50, 1/50, 1/50).
This reduction may be related to splenic toxicity, since splenectomy
of Fischer rats at one to two months of age markedly reduces the
incidence of mononuclear cell leukaemia later in life (Boorman et al.,
1990). However, similar reductions in the incidence of this neoplasm
are not seen with several other aniline-like chemicals that also cause
splenic toxicity. In male mice, but not in females, the incidence of
haemangiosarcomas of the liver and spleen was slightly greater in the
high-dose group than in the controls (males: 4/50, 4/49, 1/50, 10/50)
and the incidences of hepatocellular carcinomas or adenomas (combined)
were also increased in treated male mice (males: 11/50, 21/49, 20/50,
21/50). The incidences of malignant lymphomas were slightly reduced
in treated male and female mice (males: 10/50, 3/49, 9/50, 3/50;
females: 19/50, 12/50, 5/50, 10/50).
There are various points of similarity between the two studies
described above: (a) in male and female rats there was splenic
toxicity; (b) in male rats, there was a treatment-related increase in
uncommon splenic sarcomas; and (c) in male mice, there was a
treatment-related increase in haemangiosarcomas and haemangiomas
combined. The reductions in the incidence of mononuclear cell
leukaemia in rats and malignant lymphomas in mice seen in the latter
study were not observable in the earlier study because their incidence
in the control groups of that study was already low. It is concluded
that PCA is carcinogenic in both mice and rats.
8. EFFECTS ON HUMANS
No data concerning the effects of diflubenzuron on human health
are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Diflubenzuron was tested for its effects on morphogenesis in
Streptomyces spp. Exposure to 400 mg diflubenzuron/litre resulted in
reduced dominance of spore hairs and reduced width of the outer wall,
and prevented formation of the inner spore wall in S. babergiensis.
In S. coelicolor, 1600 mg diflubenzuron/litre altered the structure
of the fibrillar pattern of spore envelopes. Exposure to diflubenzuron
resulted in small increases in exported protein and in an approximately
20% increase in chitinase in both Streptomyces spp (Smucker &
Simon, 1986).
9.1.1.1 Water
Aquatic bacterial biomass and density were not affected by
diflubenzuron (1.0 µg/litre), although after three months of
continuous exposure some decline in species diversity was found.
Owing to detrimental effects on aquatic arthropods, e.g., filter
feeders that may use bacteria as a food source, such effects may very
well be secondary in nature and not a direct effect of diflubenzuron
(Hansen & Garton, 1982b).
In a test on the alga Selenastrum capricornutum, a nominal
concentration of 0.20 mg diflubenzuron/litre did not reduce the
biomass and can be considered a no-observed-effect concentration
(NOEC) (Berends & Thus, 1992b).
9.1.1.2 Soil
Soil microorganisms were able to use diflubenzuron as carbon
source when added as an acetone solution (Seuferer et al., 1979; see
section 4.3.2.2).
The effect of diflubenzuron (100, 200, 300, 400 and 500 mg/kg
soil) was studied in non-sterile soil incubated under aerobic
conditions and in sterilized soil inoculated with Azotobacter
vinelandii. The presence of diflubenzuron had a stimulatory effect
on nitrogen fixation in both non-sterile and sterile soil (Martinez-
Toledo et al., 1988a). At similar concentrations, diflubenzuron did
not affect the growth of Azotobacter vinelandii in culture media,
either with or without a nitrogen source (Martinez-Toledo et al.,
1988b).
9.1.2 Aquatic organisms
9.1.2.1 Microorganisms
Cyanobacteria (the blue-green alga Plectonema boryanum) grew
rapidly in the presence of diflubenzuron (initial concentration
0.1 mg/litre) with no visible signs of inhibited growth (Booth &
Ferrell, 1977). Concentrations of 1, 10, 50 and 100 mg/litre did not
affect the growth of six species of fungi: Rizopus arrhizus,
Aspergillus niger, Fusarium oxysporum, Mycorrhizae-Rhizopogan
vinicolor, Pythum debaranum and Trichoderma viride (Booth et al.,
1987). However, these authors autoclaved the medium containing
diflubenzuron, which led to extensive breakdown of the pesticide
(Willems et al., 1977).
Five-day lethality tests on the algae Selenastrum capricornutum
and Anabaena flos-aquae resulted in NOAEC values above the exposure
concentrations of 300 and 330 µg/litre, respectively (Thompson &
Swigert, 1993b,c). Similarly, tests on the diatoms Navicula
pelliculosa and Skeletonema costatum led to NOAEC values of 380
and 270 µg/litre, respectively (Thompson & Swigert, 1993d,e).
Algae were affected at 1.0 µg/litre by technical diflubenzuron in
dimethylformamide added to laboratory stream channels: the alga
biomass increased and chlorophyll and phaeocitin levels were elevated.
Fungi were only affected temporarily by 0.1 µg/litre under the same
circumstances. Changes in species diversity had disappeared after
2 months of continuous dosing (Hansen & Garton, 1982 b).
9.1.2.2 Plants
In a 14-day toxicity test on diflubenzuron in duckweed
(Lemna gibba), the NOAEC was higher than the tested concentration
(190 µg/litre) (Thompson & Swigert, 1993a).
9.1.2.3 Invertebrates
The acute toxicity of diflubenzuron to a number of non-target
aquatic invertebrates is presented in Table 12. Comprehensive eviews
on the subject have been published recently, e.g., by Fischer & Hall
(1992) and by Cunningham (1986) on the effects of diflubenzuron on
estuarine crustaceans. The acute LC50 for insects ranges from
1 µg/litre (Diptera) to 250 µg/litre (Coleoptera), and mayflies
have an LC90 of 1 to 10 µg/litre. Other aquatic arthropods such as
water fleas, scuds and sow bugs have LC50 values of 5 to 15 µg/litre.
Table 12. Acute toxicity of diflubenzuron for non-target aquatic invertebrates
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Daphnia magna 1st instar stat 22 40 7.2 48-h EC50 15 Julin & Sanders (1978)
(Water flea)
Daphnia magna 24 h stat 20 50 48-h LC50 4.55 Hansen & Garton (1982a)
(Water flea) stat 20 100 48-h LC50 6.89 Hansen & Garton (1982a)
stat 20 200 48-h LC50 4.42 Hansen & Garton (1982a)
Daphnia magna 0-24 h 20 294 8.1 48-h EC50 7.1 Kuijpers (1988)
(Water flea) 20 294 8.1 24-h EC50 68 Kuijpers (1988)
Gammarus pulex mature stat 12 40 7.2 96-h LC50 30 Julin & Sanders (1978)
(Scud)
Hyallela azteca 2-4 mm flow 20 25 96-h LC50 1.84 Hansen & Garton (1982a)
(Amphipod)
Chironomus plumosus 4th instar stat 22 40 7.2 48-h EC50 560 Julin & Sanders (1978)
(Midge)
Cricotopus sp. 4th instar flow 20 25 EC50 (moulting 1.72 Hansen & Garton (1982a)
(Midge) success)
Tanytarsus dissimilis 2nd instar flow 20 25 EC50 (moulting 1.02 Hansen & Garton (1982a)
(Midge) success)
Acartia tonsa adult constant 20 10b 5-day LC50 > 1000 Tester & Costlow
(Copepod) daily (1981)
replenishment
Table 12 (Con't)
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Mysidopsis bahia adult intermittent 24-25 24-27b 96-h LC50 2.06 Nimmo et al. (1980)
(Mysid shrimp) flow
adult continuous 24-26 23-29b 21-day LC50 1.24 Nimmo et al. (1980)
flow
Palaemonetes pugio larvae stat 22 20b 96-h LC50 1.44 Wilson & Costlow
(Grass Shrimp) (1987)
post larval renewal 22 20 96-h LC50 1.62 Wilson & Costlow (1987)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Salinity (expressed as parts per thousand)
Concentrations of diflubenzuron causing significant mortality of
several freshwater invertebrates are presented in Table 13.
Table 13. Concentrations of diflubenzuron causing significant
mortality of freshwater invertebratesa
Organisms Concentration
(µg/litre)
Water flea (Daphnia magna) 2.0
Amphipod (Hyalella azteca) 2.0
Snail (Juga plicifera) > 36
Snail (Physa spp.) > 36
Caddis fly (Clisforonia magnifica) 0.1
Midge (Tanytarsus dissimilis) 4.9
Midge (Cricotopus spp.) 1.6
a From: Nebeker et al. (1983)
The 96-h LC50 for the snails Juga plicifera and Physa sp.
was > 45 µg/litre (Hansen & Garton, 1982a).
The 48-h EC50 values of diflubenzuron metabolites for midge
larvae were > 100 mg/litre for 4-CPU and 2,6-DFBA and 43 mg/litre for
PCA (Julin & Sanders, 1978).
Diflubenzuron suspended in water at a concentration of
200 mg/litre was not directly toxic to the freshwater clam Anodonta
cygnea during 3 months of treatment. Diflubenzuron produced
disturbances in the calcification process in the lamellar layer of the
shell. Positive PAS reaction of the secretory cells on the outer
mantle epithelium has been observed (Machado et al., 1990).
Daphnia magna was continuously exposed to 14C-diflubenzuron at
concentrations of 5.6, 14, 23, 40 and 93 ng/litre. After 21 days of
exposure daphnid survival at the highest concentration (93 ng/litre)
was 50%. In the remaining concentrations it ranged from 93 to 98%,
which was comparable to the survival (99%) of the control organisms.
Reproduction and body length were affected only at the highest
concentration. The maximum acceptable toxicant concentration (MATC)
of 14C-diflubenzuron for Daphnia magna was > 40 and < 93 ng/litre
(Surprenant, 1988).
Technical diflubenzuron in dimethylformamide at 0.1, 1, 10 and
50 µg/litre was added continuously to complex laboratory stream
channels supplied periodically with field-collected microorganisms for
5 months. LC50 values ranging from 1.0 to 1.8 µg/litre were
determined for four insect and crustacean species ( Tanytarsus
dissimilis, Cricotopus sp. and Hyalella azteca). A chronic effect
level was obtained only for Daphnia magna (0.06 µg/litre). Mayflies
and stoneflies were the most sensitive. They were severely affected
at 1 µg/litre within one month (the sampling interval) and numbers
were two to three orders of magnitude lower, almost leading to their
elimination. The survival of chironomids was reduced by 10 µg/litre
(Hansen & Garton, 1982a,b) (see also section 9.1.1.1).
Benthic communities in outdoor experimental streams were exposed
to 1 or 10 mg/litre of diflubenzuron for 30 min. The effect was
assessed daily by examining drifting pupal exuviae over a period of
one month following the treatment. No drift of macrobenthos was
induced at the time of application. However, diflubenzuron affected
the emergence of all species examined. High larval mortality for a
species of chironomid was observed directly in the stream treated with
diflubenzuron, where numbers of mayfly nymphs and caddisfly larvae
were also decreased (Yasuno & Satake, 1990).
Technical diflubenzuron in acetone was applied at 5 µg/litre to
two aquaria in a simulated field test outdoors. Daphnid numbers were
markedly reduced three days after treatment, but recovered slowly.
Copepods numbers were moderately reduced, exceeding the control
numbers after 18 days. The seed shrimp population showed no harmful
effect (Miura & Takahashi, 1974a,b).
In a study by Collwell & Schaefer (1980), diflubenzuron was
applied to experimental ponds (mean concentration of 13.2 µg/litre) in
California. An hour after treatment, cladoceran ( Ceriodaphnia sp.,
Diaphanosoma sp., Chydorus sp., Bosmina sp. and Daphnia sp.)
numbers were strongly reduced and did not return to pretreatment
levels until more than 5 weeks after treatment. Copepod ( Diaptomus
sp. and Cyclops sp.) numbers were also reduced but to a lesser
extent and for a shorter period than for the cladocerans. The
rotifers increased in abundance in both the control and treated ponds
during the first 8 days following treatment.
Using laboratory tests to study mortality, Miura & Takahashi
(1974a) found that crustaceans, especially the tadpole shrimp
(T. longicaudatus), clam shrimp ( Eulimnadia spp.) and water fleas
( Daphnia and Moina spp.), were highly susceptible to diflubenzuron
at levels below 0.01 mg/litre. Copepods, Cyclops and Diaptomus
spp. showed some tolerance, whereas seed shrimp ( Cypricerus and
Cypridopsis spp.) tolerated as much as 0.5 mg/litre. Among aquatic
insects tested, mayfly nymphs ( Callibaetis spp.) were most
susceptible. Aquatic midge larvae (G. holoprasinus) also
showed susceptibility. However, dytiscid ( T. bassillaris and
Laccorhilus spp.) and hydrophilid beetles ( H. triangularis and
T. lateralis (mosquito predators)) demonstrated a strong tolerance.
Mosquito fish (Gambusia affinis) showed no effect at a relatively
high dose of
1 mg/litre.
When larvae of the crab Rhithropanopeus harrisii were exposed
to sublethal concentrations of diflubenzuron (0.05, 0.1, 0.3 and
0.5 µg/litre), swimming speed increased in stage I, II and III zoeae,
0.3 µg/litre being the lowest effective concentration. Phototaxis was
altered in stage IV only at concentrations as low as 0.1 µg/litre
(Forward & Costlow, 1978).
Christiansen et al. (1978) showed that nearly 100% of
Rhithropanopeus harrisii larvae at each of the four zoeal stages
died when moulting to the succeeding stage after only 3 days of
exposure to 10 µg diflubenzuron/litre. This concentration was also
lethal for larvae of Sesarma reticulatum (Say).
Christiansen & Costlow (1980) exposed larvae of the estuarine
crab Rhithropanopeus harrisii in laboratory conditions to 10 µg
diflubenzuron/litre as an indicator of persistence of
diflubenzuron in brackish water. Disturbance of endocuticle
deposition seemed to occur as soon as newly hatched larvae (less than
12 h old) were exposed to diflubenzuron. Diflubenzuron not only
affected endocuticle deposition in the larvae, but also exocuticle
deposition. The only part of the crab larval exoskeleton that did not
seem to be affected by diflubenzuron was the epicuticle (Cristiansen
et al., 1978; Cristiansen & Costlow, 1982).
Diflubenzuron, at concentrations of 0.02 and 0.2 mg/litre,
enhanced mortality during moulting of the crab Carcinus
mediterraneus (Czerniavsky) (Cardinal et al., 1979).
Cirripede crustaceans, (barnacles, Balanus eburneus) exposed to
concentrations of 1 to 1000 µg/litre over a 28-day period showed a
dose-dependent mortality. Heavy mortality occurred during the second
week of exposure. Lethal and sublethal effects were observed at
concentrations as low as 50 µg/litre (Gulka et al., 1980). Disruption
of the exoskeleton of B. eburneus caused by diflubenzuron was
similar to that observed in insects. Development of barnacles exposed
to diflubenzuron for 10 days or more at 750 and 1000 µg/litre was
delayed in the premoult phase of cuticle secretion (Gulka et al.,
1982).
Wilson & Costlow (1986) found that diflubenzuron concentrations
of 2.5 and 5 µg/litre were lethal to larvae of the grass shrimp
(Palaemonetes pugio), causing 100% mortality on days 14 and 6,
respectively. Wilson et al. (1985) studied the effects of
diflubenzuron upon phototaxis of larvae of P. pugio. The depression
in positive phototaxis and elevation in negative phototaxis were most
pronounced at 0.5 µg/litre, and the lowest test concentration to
affect phototaxis was 0.3 µg/litre. The alterations in photo
responses varied with the embryonic stage at which exposure to
diflubenzuron commenced. This study was carried out at optimal
salinity and temperature, but in the estuary these conditions
fluctuate daily, which may amplify the observed effects.
Nimmo et al. (1980) observed that chronic exposure of the mysid
shrimp Mysidopsis bahia to 0.075 µg diflubenzuron/litre reduced the
reproductive success of both parents and progeny even after they were
transferred to uncontaminated sea water.
Diflubenzuron reduced the reproductive life span of adult brine
shrimps (Artemia salina) at levels of 2-10 µg/litre and caused death
of immature shrimps within 3 days at concentrations above 10 µg/litre
(Cunningham, 1976).
Fiddler crabs (Uca pugilator) were exposed to diflubenzuron
(Dimilin WP-25%) at 0.5, 5 and 50 µg/litre for 1, 2, 3 and 4 weeks
after multiple autonomy of one chela and five walking legs. Exposure
to diflubenzuron retarded the rate of limb regeneration in a dose-
dependent fashion. The effects of diflubenzuron were seen even in
crabs exposed for only one week. Significant retardation was evident
by day 21 at 5.0 µg/litre but was not statistically significant at
0.5 µg/litre. At 5 and 50 µg/litre moult-associated mortality was
seen. The number of setae on regenerated limbs was less than the
number on the intact limbs. The effects were reduced in experiments
in which sediment was present (Weis et al., 1987).
The burrowing activity of Uca pugilator in sand under
laboratory conditions was not altered when the sand was contaminated
with 1 mg diflubenzuron/litre, indicating a lack of avoidance of
diflubenzuron-contaminated sand. However, exposure for 1 week to 0.5,
5.0 or 50 µg/litre led to a decrease in the amount of burrowing
activity. The behavioral response was not changed after exposure for
1, 2 or 3 weeks and was not concentration-related (Weis & Perlmutter,
1987).
Survival, moulting and behaviour of juvenile fiddler crabs were
significantly affected by exposure to diflubenzuron (0.2, 2, 20 and
200 µg/litre) for 24 h weekly during 10 weeks. All crabs in the 200
and 20 µg/litre groups died after 8 and 23 weeks, respectively. The
no-observed-effect concentrations (NOEC) for moulting (time to the
first moult), survival (time until death), and behaviour (ability to
escape from the test container) were 20, 2 and 0.2 µg/litre,
respectively (Cunningham & Myers, 1987).
Larvae of the stone crab (Menippe mercenaria) were exposed to
0.5, 1.0, 3.0, and 6.0 µg diflubenzuron/litre in combination with
different temperature and salinity. All of these concentrations were
lethal to the larvae. Evidence for synergistic effects of
diflubenzuron and temperature or salinity was observed. Tolerance of
the megalopa of the blue crab Callinectes sapidus to diflubenzuron
at concentrations of 0.5, 1.0, 3.0 and 6.0 µg/litre was slightly
higher than for Menippe mercenaria but was also dependent upon
temperature and salinity. At 20°C the percentage survival at a
concentration of 1 µg/litre was similar to that observed for the
controls (Costlow, 1979).
Tester & Costlow (1981) reported that the marine copepod
Acartia tonsa exposed to 1 and 10 µg diflubenzuron/litre for 36 h
failed to produce viable nauplii even after they had been placed in
clean sea water. No viable nauplii were produced by these females for
at least 30 h after treatment ended.
Adult crustaceans were more resistant to exposure than their
larvae at high concentrations (100-200 µg/litre) of technical grade
diflubenzuron. Adults also exhibited significant mortality associated
with moulting (Cunningham, 1976; Cardinal et al., 1979; Gulka et al.,
1980).
When larvae of horseshoe crabs (Limulus polyphemus) were
exposed to 5 and 50 µg diflubenzuron/litre, the crabs in the
50 µg/litre group exhibited severe mortality immediately after
ecdysis. The larval stages were shown to be quite resistant to
diflubenzuron, compared with other crustacean larvae (Weis & Ma,
1987).
9.1.2.4 Vertebrates
Data on the acute toxicity of diflubenzuron and its metabolites
for fish are presented in Tables 14 and 15, respectively.
A 96-h acute toxicity test on juvenile sheepshead minnow
(Cyprinodon variegatus) in a flow-through system resulted in no
mortality at the nominal exposure concentration of 130 µg
diflubenzuron/litre (Graves & Swigert, 1993). A similar test under
semi-static conditions on zebra fish (Brachydanio rerio) and rainbow
trout (Oncorhynchus mykiss) at a nominal diflubenzuron concentration
of 200 µg/litre also showed no mortality, nor changes in the appearance
Table 14. Acute toxity of diflubenzuron to fish
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Coho salmon 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus kisutch) Pridmore (1978)
Rainbow trout 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus mykiss) Pridmore (1978)
Rainbow trout 1.2 g stat 12 40 7.2 96-h LC50 240 Julin & Sanders
(Oncorhynchus mykiss) (1978)
Rainbow trout not flow-through 96-h LC50 140 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Rainbow troutb not flow-through 96-h LC50 195 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Fathead minnow 0.87 g stat 22 40 7.2 96-h LC50 430 Julin & Sanders
(Pimephales promelas) (1978)
Channel catfish 2.2 g stat 22 40 7.2 96-h LC50 370 Julin & Sanders
(Ictalurus punctatus) (1978)
Bluegill 0.5 g stat 22 40 7.2 96-h LC50 660 Julin & Sanders
(Lepomis macrochirus) (1978)
Bluegill not flow-through 96-h LC50 135 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Bluegillb not flow-through 96-h LC50 230 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Table 14. (Con't)
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Mummichog adult 2.7 g stat 24 22 ppt 8.0 96-h LC50 32 990 Lee & Scott
(Fundulus heteroclitus) renewal salinity (1989)
Mummichogb not flow-through 96-h LC50 255 Marshall & Hieb
(Fundulus heteroclitus) reported (1973)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Formulated product 25 WP
Table 15. Acute toxicity of metabolites of diflubenzuron to fish (from: Julin & Sanders, 1978)
Concentrations (mg/litre)
Organism Water Effect
temperature measured
(°C) 4-Chlorophenyl 2,6-Difluorobenzoic 4-Chloroaniline
urea acid
Rainbow trout 12 96-h LC50 72 (57-90) > 100 14 (11-16)
(Oncorhynchus mykiss)
Channel catfish 22 96-h LC50 > 100 > 100 23 (18-29)
(Ictalurus punctatus)
Fathead minnow 22 96-h LC50 > 100 69 (55-87) 12 (7-18)
(Pimephales promelas)
Bluegill 22 96-h LC50 > 100 > 100 2.4 (1.8-3.2)
(Lepomis macrochirus)
or behaviour of the fish (Berends & van der Laan-Straathof, 1994a,b).
All these test concentrations were above the diflubenzuron solubility
level of 80 µg/litre.
Nebeker et al. (1983) found no significant reduction in
survival of fathead minnow (Pimephales promelas) or guppy
(Poecilia reticulata) as a result of exposure to diflubenzuron at
concentrations below 36 µg/litre during acute (96-h) and chronic
tests.
No acute response resulted from exposure of fish to a
diflubenzuron concentration of 45 µg/litre, and no chronic effects
were observed at this concentration, the highest one tested (Hansen &
Garton, 1982a,b).
Diflubenzuron was not toxic to either rainbow trout or coho
salmon exposed at concentrations up to 150 mg/litre for a 96-h period.
A 15-min exposure to 1 g/litre did not result in any fish mortality
(McKague & Pridmore, 1978).
Madder & Lockhart (1978) found a dose-related decrease of
glutamic-oxaloacetic transaminase activity in rainbow trout
(Oncorhynchus mykiss) exposed to diflubenzuron at concentrations of
0.625, 1.25, 2.5, 5 and 10 mg/litre.
At a concentration of 0.01 mg/litre, diflubenzuron had a
repellent effect on precocious male Atlantic salmon parr (Granett et
al., 1978).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Photosynthesis, respiration and leaf ultrastructure of soybeans
were unaffected by diflubenzuron at doses up to a level of
0.269 kg a.i./ha (Hatzios & Penner, 1978).
Diflubenzuron is used as an insecticide in forestry, agriculture
and horticulture. No phytotoxicity was reported in the field studies
cited in section 9.2.
9.1.3.2 Invertebrates
The oral and contact LD50 values of diflubenzuron for honey-bees
are greater than 30 µg/bee (Stevenson, 1978).
Diflubenzuron did not show any toxicity to bees at concentrations
up to 1000 mg/kg in the diet (Yu et al., 1984).
Barker & Taber (1977) found that diflubenzuron reduced brood
production when fed for 10 days to honey-bees at 59 mg/kg in sugar
syrup, but not at 5.9 or 0.59 mg/kg.
Barker & Waller (1978) confirmed that 60 mg/kg in sugar syrup or
100 mg/litre in water caused colonies to produce less brood.
Stoner & Wilson (1982) found that diflubenzuron fed to flying
colonies at 1 or 10 mg/kg for a year significantly reduced the amount
of sealed brood.
Nation et al. (1986) reported that diflubenzuron did not cause
reduction in pollen consumption or brood production when fed for 10
weeks to caged colonies of honey-bees at 10 mg/kg, but it caused more
than 50% reduction in the amount of syrup stored.
Gordon & Cornect (1986) showed that, at concentrations that are
effective in suppressing egg hatching and larva development of the
cabbage maggot Delia radicum, diflubenzuron did not adversely
affect eggs, first-instar larvae or adults of the rove beetle
Aleochara bilineata, an important predator and parasitoid of the
cabbage maggot.
When the acute toxicity of diflubenzuron to the earthworm
Eisenia fetida was tested in artificial soil to which diflubenzuron
had been added, 780 mg/kg dry soil was the no-observed-effect
concentration (NOEC) for the 14-day test period (Berends et al.,
1992). For the WP-25 formulation, the NOEC was 1.0 g/kg (Berends &
Thus, 1992a).
9.1.3.3 Vertebrates
a) Birds
The acute oral LD50 of technical diflubenzuron for red-winged
blackbirds (Agelaius phoeniceus) is 3762 mg/kg body weight. In an
8-day dietary LD50 study on mallard duck and bobwhite quail using
technical diflubenzuron, levels up to 4640 mg/kg in the feed gave no
observable signs of toxicity (Maas et al., 1980).
When diflubenzuron was fed to mature White leghorn hens at
dietary levels of 10, 50, 100, and 500 mg/kg for 8 weeks, there were
no adverse effects on feed consumption, body weight, egg production,
egg weight, eggshell thickness, fertility, hatchability or progeny
performance. The highest dose used was 50 times higher than the
efficacy level for fly control (Cecil et al., 1981).
In chronic toxicity tests, groups consisting of 10 male and 10
female one-day-old chicks of barred Plymouth rocks and white leghorn
hens, Nicholas white turkeys, mallard ducks and ring-necked pheasants
were given diets containing diflubenzuron levels of 0.25, 1.25, 25 and
250 mg/kg for 91 days after hatching. No differences between control
and treatment groups were observed in mortality, food consumption,
body weight, comb and wattle development, weight of inner organs,
serum hormone levels or general behaviour (Maas et al., 1980).
There was no effect of diflubenzuron on the content of hyaluronic
acid in the skin of Hubbard broiler chickens when they were fed at
levels of 2.5 and 250 mg/kg in the diet for 98 days after hatching
(Deul & Jong, 1977). In an experiment with the same dose levels and
duration, diflubenzuron had no effect on hyaluronic acid synthesis or
comb deposition in either growing broilers or layers (Crookshank et
al., 1978).
White leghorn and black sex-linked cross hens were fed
diflubenzuron at a level of 10 mg/kg in the ration for 15 weeks.
Diflubenzuron had no effect on body weight gain, egg production,
fertility or hatchability (Miller et al., 1976b).
In a feeding study with 2.5, 25 and 250 mg/kg, diflubenzuron did
not affect bobwhite quail reproduction (Booth et al., 1987).
Diflubenzuron did not show significant teratogenic activity on chick
embryos over time when injected at 10 mg/egg (Booth et al., 1987).
A one-generation bobwhite quail (Colinus virginianus)
reproduction study was conducted in which a diflubenzuron-containing
diet was administered ad libitum to young adults (24 weeks old at
test initiation) approaching their first breeding season. Dietary
concentrations of 250, 500 or 1000 mg/kg did not result in treatment-
related mortality, overt signs of toxicity or effects upon adult body
weight or feed consumption during the 21-week exposure period. There
were no apparent treatment-related effects upon reproductive
parameters at 250 or 500 mg/kg. There may have been a slight reduction
in the number of eggs laid, although this was not statistically
significant or dose-related at 1000 mg/kg. On the basis of a possible
effect on egg production at 1000 mg/kg, the NOEC for diflubenzuron in
this study was above 500 mg/kg (Beavers et al., 1990a).
In a one-generation reproduction study on the mallard duck
(Anas platyrhynchos), diets containing diflubenzuron were
administered ad libitum to young adults (27 weeks old at test
initiation) approaching their first breeding season. Dietary
diflubenzuron concentrations of 250, 500 and 1000 mg/kg did not
result in treatment-related mortality, overt signs of toxicity or
effects upon adult body weight or feed consumption during the 20-week
exposure period. There were no apparent treatment-related effects
upon reproductive performance at any of the concentration tested. At
1000 mg/kg there was a slight, but statistically significant,
reduction in mean eggshell thickness. On the basis of the effect upon
eggshell thickness at 1000 mg/kg, the NOEC for diflubenzuron in this
study was 500 mg/kg (Beavers et al., 1990b).
b) Mammals
In studies by Ross et al. (1977a,b), diflubenzuron was
administered in the feed to sheep (3 of each sex per group) as a model
for ruminant wildlife at concentrations of 500, 2500 and 10 000 mg/kg
feed for 13 weeks. No treatment-related effects were observed on food
consumption, body weight gain, haematological parameters or
urinalysis. Increase in met- and sulfhaemoglobin levels were observed
at 13 weeks and there was a reduction in the weight of the thyroid.
No histopathological abnormalities were observed. Both the plasma and
the erythrocyte cholinesterase activities were unaffected by the
treatment after 6 weeks.
9.2 Field observations
9.2.1 Microorganisms
9.2.1.1 Water
The laboratory data available (see section 9.1.1.1) make it
unlikely that detrimental effects will occur.
Rotifers were unaffected by diflubenzuron (28 and 56 g a.i./ha)
in both experimental and naturally treated ponds (Ali & Lord, 1980).
9.2.1.2 Soil
One aerial application of diflubenzuron at 67.26 g/ha had no
adverse effects upon populations of bacteria, actynomycetes or fungi
in leaf litter and forest soil (Wang, 1975; Kurczewski et al., 1975).
9.2.2 Aquatic organisms
9.2.2.1 Plant
The laboratory data available (see section 9.1.2.2) make it
unlikely that detrimental effects will occur.
9.2.2.2 Invertebrates
Recent field studies have demonstrated that effects on aquatic
fauna are limited and transient, and that recovery is evident after
3 months (Huber & Collins, 1987; Kingsbury et al., 1987; Huber &
Manchester, 1988; Ali et al., 1988; Ali & Kok-Yokomi, 1989; Sundaram
et al., 1991).
Diflubenzuron (25% WP) applied at 33.63 and 134.52 g a.i./ha,
4 times at 2-week intervals, had no adverse effect on freshwater clams
10 days after final treatment (Jackson, 1976).
When diflubenzuron (25% WP) was applied at 1.121-280.25 g a.i./ha
to flooded rice fields in Louisiana, USA, significant reduction of
Tropisternus spp. and Libellulidae was found 80 days after
treatment. Significantly more chironomid and baetid immatures
occurred due to reduction in the number of predators (Steelman et al.,
1975).
Mulla et al. (1975) found that at an application rate of 28 and
56 g a.i./ha diflubenzuron reduced slightly, and only for a short
time, the number of mayfly ( Beatis sp.) in the treated ponds. The
numbers, however, appeared to be within natural fluctuation limits
equal to the check ponds or the pretreatment levels during all other
sampling periods. At these application rates, diflubenzuron caused a
short-term reduction in copepod populations, but they started to
increase on the 11th or 15th day of post-treatment sampling. There
was little or no effect on the ostracod population.
In a forestry spraying programme at 0.0672 kg a.i./ha, aquatic
arthropods were studied in a water shed (White Deer Creek). Sampling
at three test stations and one control station in the watershed
revealed a rich fauna, dominated numerically by Ephemeroptera,
Chironomida, Trichoptera and Plecoptera in descending order of
abundance. Significant differences in larval abundance in the surber
samples were found to occur from one sampling day to the next, or
between pre-spray and post-spray periods, for most of the organisms
examined. In all cases, control and test stations showed similar
patterns of variation, so treatment effects were discussed as a
probable cause. The number of organisms tended to be higher at the
upstream stations. Chironomida and Trichoptera pupal abundance in the
surber samples was examined for decreases after spraying but numbers
were too low to permit statistical analysis. Pupal abundance showed
some direct relationship to larval abundances. Nymphal drift rates
were examined for possible increases after spraying, but most of the
significant changes were decreases during the post-spray period.
These decreases also occurred at the control station. The drift rates
of nymphal and pupal exuviae also did not indicate a treatment effect.
Individual species abundances in the surber samples were examined for
possible selective effects of diflubenzuron. Comparisons were made
between pre-spray and post-spray periods at both test and control
stations, but no evidence of a treatment-related decrease in the
abundance of any of these organisms was found. The net effect was
usually an increase in the density of these organisms during the post-
spray period. No organisms showing abnormal ecdysis or pupation were
found in any of the samples. It was concluded that spraying with
diflubenzuron had no adverse effect on the macrobenthic community in
White Deer Creek (White, 1975).
In a study by Booth (1975), diflubenzuron (25% WP) was applied at
44.84 g a.i./ha to small ponds in Utah. Biosamples taken 30 and 80
days later showed that, although larva and immature aquatic insect
populations were decreased at 30 days, the total number of adults was
not significantly different from the control number at 80 days.
Booth & Ferrell (1977) examined the effect of diflubenzuron on
over 20 different species (corixidae and collembolans) after multiple
pond applications at 45 g a.i./ha. Only immature corixidae (water
boatmen) and collembolans (springtails) were significantly affected.
Segmented worms (Oligochaeta) and midges (Chironomidae)
were unaffected by six applications of diflubenzuron at a rate of
145.73 g a.i./ha to Utah Lake (Booth et al., 1987).
Diflubenzuron (28 and 56 g a.i./ha) reduced numbers of
Chaoborus sp. and Baetis sp. in both experimental and natural
treated ponds. Chaoborus sp. recovered within 1-3 weeks (Ali &
Lord, 1980).
The application of granular diflubenzuron at 0.11 kg a.i./ha
(about 3.7 µg/litre) to residential-recreational lakes caused a 62-75%
reduction of Daphnia pulex and Daphnia galeata during 7 days
following treatment. The populations recovered in the second week
after treatment. A 30% reduction in Diaptomus spp. was noted 2
days after the treatment. Hyalella azteca was affected markedly,
with a maximum reduction of 97% after 3 weeks of treatment. No
detectable effects on Cyprinotus sp., Cyclops sp. or Bosmina
longirostris were observed (Ali & Mulla, 1978a). Ali & Mulla
(1978b) also studied the impact of diflubenzuron on invertebrates in a
residential-recreational lake after two treatments with 25% WP
formulation at 156 g a.i./ha (about 12 µg/litre). Diflubenzuron
concentrations in water were not measured but were based on nominal
initial concentrations. The results from this study are summarized in
Table 16.
Diflubenzuron (25% WP) at rates of 0.02, 0.025, 0.03, 0.035,
0.04, 0.045 and 0.05 lb a.i./acre was applied to 19 irrigated pastures
and spring ponds (in some cases up to 4 times) at 2- to 3-week
intervals in California. Cladoceran and nymphal mayfly populations
were temporarily and slightly reduced, but recovered within a short
period of time. Even repeated treatment of the same pastures did not
eliminate the populations. The impact on copepod populations was
inconclusive. Adult beetles demonstrated high tolerance to
Table 16. Effect of diflubenzuron on invertebrates in Vallage Grove Lake, Californiaa
Species First application (April) Second application (August)
Effect Recovery Effect Recovery
Daphnia leavis elimination within no recovery 6 months
Birge 1 week after treatment
Ceriodaphnia sp. elimination within no recovery 6 months
1 week after treatment
Bosmina longirostris elimination within recovery after elimination after reappearance in small numbers
(O.F. Muller) 1 week 11 weeks 1 week 8-9 weeks after treatment
Cyclops spp. elimination within recovery within elimination within recovery after 4 weeks
1 week 6-7 weeks 1-2 weeks
Diaptomus sp. elimination within recovery after absent prior to reappearance in small numbers
1 week 4 months treatment 1-2 months later
Hyalella azteca elimination within no recovery 6 months
(Saussure) 4 weeks after treatment
Caenis sp. elimination within recovery within elimination within recovery in 4-5 weeks
3 weeks 6-7 weeks 2-3 weeks
Physa sp. no adverse effects no adverse effects
Cypridopsis sp. no adverse effects no adverse effects
a From: Ali & Mulla (1978b)
diflubenzuron, but few dead larvae ( Laccophilus spp., Hydrophilus
triangularis and Tropisternus lateralis) were observed. Spiders
( Pardosa spp. and Lycosa spp.) showed no apparent effects. There
were no deleterious effects on planarian, rotifer, seed shrimp and
fresh water flagellate populations (Miura, 1974).
Diflubenzuron (25 WP formulation) was applied 3 times at 2.5-week
intervals to man-made pools in Manitoba, Canada at 56 g/a.i. ha (this
produced initial concentrations of 0.02 mg/litre). The treatment
produced no detectable effect on chironomid larvae in terms of numbers
or composition, although dead larvae were noted after treatment.
Daphnid populations were significantly reduced on most sampling dates.
Repetitive treatments prevented the recovery of cladocerans. Despite
these reductions, daphnids were not annihilated. At a higher rate
(0.22 kg a.i./ha, about 7.4 µg/litre), both species of Daphnia were
eliminated for 3 months after treatment. Populations of Diaptomus
spp. declined to zero 7 days after treatment, but recovered during the
second week following treatment. Diflubenzuron caused reductions in
the number of H. azteca (32-100%) during the 2´ months following
treatment. Seed shrimp populations were stressed for only 2 weeks,
and there were no observable effects on oligochaetes at a treatment
level of 0.22 kg a.i./ha (about 7.4 µg/litre). Copepods only
occasionally showed significant reduction, and recovered within 10
days after treatment. Of the ten other non-target invertebrates (i.e.
Chaoboridae, Tipulidae, Ephemeroptera, Corixidae, Nonectidae,
Gerridae, Dystiscidae, Hydrophilidae, Hydrarina and Gastropoda),
only mayfly nymphs (Ephemeroptera) were significantly reduced
(Madder, 1977).
When diflubenzuron at a rate of 34.2 g/ha was applied aerially to
a forested area in the USA, there were no significant effects on the
population structure of the benthic macro-invertebrate community.
Some decreases in the number of mayflies Heptagenia sp. and
Rhithrogenia sp. were noted in treatment streams, but the total taxa
richness values remained high. An increase in abundance of the
caddisfly Lepidostoma sp. was found. Allonarcys sp. was not
affected by the treatment (Huber & Collins, 1987; Huber & Manchester,
1988).
After aerial application of diflubenzuron to two forest ponds in
Canada the greatest effect was on crustacean zooplankton, especially
cladocerans, with limited effect on pond benthos. Recovery of
population of even the most severely affected organism such as
Daphnids was well established by 3 months after treatment. The
maximum residue found in water was 13.82 µg/litre 1 h after
application (Kingsbury et al., 1987).
Diflubenzuron WP-25 was applied at a rate of 70 g active
ingredient in 10, 5 or 2.5 litre/ha to three spray blocks in a mixed
boreal forest near Kaladar, Ontario, Canada. Water, sediment and
aquatic plants were collected from two ponds and a stream at intervals
up to 30 days after treatment for analysis of diflubenzuron residues.
The duration of detectable residues was different for each substrate,
but in all cases it was less than 2 weeks. Zooplankton and benthic
invertebrate populations were monitored for up to 110 days after
spraying in two ponds in the high-volume rate block and in control
ponds. Significant mortality occurred in two groups of caged
macroinvertebrates (amphipoda and immature corixidae) 1 to 6 days
after the ponds were treated with diflubenzuron. Three taxa of
littoral insects ( Caenis, Celithemis and Coenagrion) were
significantly reduced in abundance in the treated ponds 21 to 34 days
after treatment but recovered to pre-treatment levels by the end of
the season. Of the six remaining groups studied, only one (immature
corixidae) may have been slightly affected by treatment. Zooplankton
(cladocera and copepoda) population numbers were reduced 3 days after
treatment and remained suppressed for 2-3 months (Sundaram et al.,
1991).
Blumberg (1986) found no effect on population numbers in a
forested ecosystem after aerial application of diflubenzuron (25 WP)
at rate 140 g a.i./ha.
When diflubenzuron (33.63 and 134.52 g a.i./ha) was applied 4
times at 2-week intervals to small ponds in Virginia, numbers of
daphnids were markedly reduced at both treatment levels. There was no
appreciable effect on two other invertebrate species ( Chironomidae
or Chaoborus) (Birdsong, 1977).
In a study by Rodrigues (1982), diflubenzuron (25% WP) was
applied at a rate of 1 mg/litre per 30 min to three forest streams in
New York. The reduction of chironomid larvae 15 days after treatment
ranged from 35 to 91% at different sites in the three streams. Of the
two stonefly families present in the three streams, Nemouridae were
affected more than Leuctridae, with decreases of 75-94% and 70%,
respectively. Ephemeroptera were reduced, but Trichoptera and
Coleoptera were unaffected.
Following aerial application at 67.26 g a.i./ha to a watershed,
diflubenzuron reached the stream channel (also as a result of wash-off
from foliage following several subsequent rainfalls), but the
invertebrate populations were not affected (Jones & Konchenderfer,
1988).
After two applications of 44.8, 112 and 224 g a.i./ha, grass
shrimp showed mortality of 86.6, 100, and 100%, respectively. At the
two lowest application rates, fiddler crabs ( Uca spp.) showed
mortalities of 53.3 and 66.6%, respectively (McAlonan, 1976).
Diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied 4
times at 2-week intervals to man-made ponds in North Carolina,
Arkansas and Texas, USA. In North Carolina no apparent effect on
phytoplankton was found. There was a marked decrease in the numbers
of crustaceans ( Cladocera and Copepoda), as well as of certain
species from benthic communities such as Hexagenia and Chaoborus
in Arkansas. A relative decrease in copepod numbers was observed, but
the authors attributed this partly to natural mortality during winter.
In Texas there was a marked decrease in the numbers of all crustaceans
and a corresponding increase in rotifer populations, particularly
Asplanchina (Aquatic Environmental sciences, 1976a,b,c).
When diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied
4 times at 2-week intervals to man-made ponds in Alabama, USA, daphnid
populations were reduced to 50% of the pre-treatment level at a rate
of 134.52 g/ha but increased at 33.63 g/ha to a level of 15% of that
in a control pond. Gastropod numbers decreased in the benthic samples
but increased in the periphyton samples from the suspended plate
samplers in the treated ponds (Jackson, 1976).
Diflubenzuron at 33.63 and 134.52 g/ha was applied 4 times at
2-week intervals to a tidally influenced salt marsh in Virginia, which
was sampled biologically 14 times over a 70-day period. Three species
of arthropods, Cyathura polita, Ceratopogonidae sp., and Psychodidae
sp., showed significant reductions in numbers. Oligochaetes, Hanynkia
speciosa and the molluscs were not significantly affected (Matta,
1976).
Six applications of diflubenzuron (28 g a.i./ha) over an 18-month
period caused statistically significant differences in the population
density of aquatic organisms in a Louisiana coastal marsh. None of the
organisms affected were completely eliminated from the ecosystem.
Populations of five taxa (nymphs of Trichocorixa louisianae Jaczewski
and Buenoa spp., Coenagrionidae naiad spp., Berosus infuscatus Le Conte
adults, and Hyalella azteca Saussure) were significantly reduced.
Population of 15 taxa, i.e. Physa sp., Ceanis sp. and Callibaetis sp.
naiads, Noteridae larvae, Hydrovatus cuspidatus, Kunze adults,
Hydrovatus sp. larvae, Dytiscidae larvae, Mesovelia mulsanti Jaczewski
adults, Trichocorixa louisiana adults, larvae of Chironomidae, Ephudridae,
Dolichopodiae and Tabanidae, and the fish Gambusia affinis (Baird and
Griard) and Jordanella floridae (Goode and Bean), showed significant
increases after exposure to diflubenzuron. The 27 remaining aquatic
organisms (members of the Hemiptera, Coleoptera, Mysidacea, Decapoda,
Diptera and Odonata) showed no statistically significant differences
when compared with untreated populations (Farlow et al., 1978).
After treatment with diflubenzuron (as 1% granular formulation)
at rate of 4.5 g a.i./ha to a marsh habitat on the Fraser River,
Canada, the population of cladocerans appeared to be depressed for
about 5 days but they recovered 2 weeks after treatment. There was no
effect on copepods or ostracods. Significant reduction in the numbers
of water beetles and zooplankton occurred (Wan & Wilson, 1977).
In a field study by Hester et al. (1986), WP-25 formulation was
evaluated in natural salt water pools to determine its toxicity to
juvenile stages of three estuarine crustaceans exposed to a single
application of 45 g diflubenzuron/ha. One hour after application,
water residues of diflubenzuron averaged 3.6 µg/litre and mortality
was 46.5, 60.7 and 40.6% for Callinectes sp., Palaemonetes pugio
and Uca pugilator, respectively, over the 10-day observation period.
When these species were introduced into the pools 7 days after
treatment, mortality was not significantly greater in the treated
group than in the controls over the 21-day observation period. The
maximum concentration after organisms were introduced was
0.69 µg/litre, and mortalities of 22.2, 1.5 and 4.2% for Callinectes
sp., Palaemonetes pugio and Uca pugilator, respectively, were
reported.
In a study by Hester (1982), diflubenzuron (25% WP) at the rate
of 0.045 kg a.i./ha was applied to tidal ponds. Residue analysis
showed 2 to 5 µg/litre after 1 h. The mortality of three
estuarine decapods: blue crabs ( Callinectes sp.), grass shrimp
(Palaemonetes pugio) and fiddler crabs (Uca pugilator) was 44,
61 and 41%, respectively. When these decapods were introduced to the
ponds 7 days after application, the residues in water were
0.4-0.7 µg/litre and mortality was 22, 1.5 and 4%, respectively.
9.2.2.3 Vertebrates
During a forestry spraying programme at 0.067 kg diflu-
benzuron/ha, a survey on fish was performed in the watershed.
Observations made on caged brown trout in the stream from day -7 to
day +6 revealed no immediate mortality or erratic behaviour
attributable to treatment. Barrier seines placed at the upstream and
downstream boundaries of the spray area did not collect any dead or
dying fish during the 1-week period they were in the stream. Visual
observations made by walking along the stream during this same period
also revealed no dead or dying fish. Post-spray population
estimations revealed increases in both test and control areas.
However, statistical analysis indicated no significant differences
pre- and post-spray between stations. The treatment had no observable
effect on the development of fish larvae. Immediate mortality was not
observed in caged fish or stream fish collected by barrier seines.
Delayed mortality attributable to spraying was not shown by population
estimations taken 6 weeks following spray, nor were any dead or dying
fish seen during this period. There appeared to be no adverse effect
of diflubenzuron sprayed at 0.067 kg a.i./ha on the fish species
observed in this study (White, 1975).
No effect on fish populations ( Micropterus salmoides, Lepomis
macrochirus and Gambusia affinis) was observed after four
applications of diflubenzuron (33.69 and 134.52 g a.i./ha) at 2-week
intervals to small ponds in Virginia (Birdsong, 1977).
Killifish (Fundulus heteroclitus) showed no effect after three
applications of diflubenzuron at rates from 11.12 to 224.2 g a.i./ha
to replicated semi-natural pools (McAlonan, 1976). Diflubenzuron
revealed no toxic effect on bullheads or sunfish after aerial
application of 35 g/ha in Canada (Buckner et al., 1975).
Growth of bluegill sunfish ( Lepomis macrochirus Rafinesque) was
not affected by diflubenzuron applications at rates of 2.5-10 µg/litre
to ponds and lakes in California (Apperson et al., 1978). Ten days
after the fourth treatment to man-made ponds with diflubenzuron at
33.63 and 134.52 g a.i./ha no apparent adverse effect on largemouth
bass was observed (Jackson, 1976).
No death of fish ( Pomoxis nigromaculatus and Ictalurus
nebulosis) occurred after application of diflubenzuron (mean
concentration of 13.2 µg/litre one hour after treatment) to
experimental ponds in California. For 1 month following treatment,
stomach content analyses showed alteration in the diet of the fish
(Collwell & Schaefer, 1980).
9.2.3 Terrestrial organisms
9.2.3.1 Invertebrates
Honey-bee colonies remained normal after an aerial application of
350 g diflubenzuron/ha in Canada (Buckner et al., 1975).
A comparative study of the effect of diflubenzuron on carabid
fauna in oak woods in Westphalia in 1987-1988 was reported. The total
number of captured beetles in treated areas was lower in 1989.
Evaluation of the 12 beetle species most frequently caught indicated
lower numbers than in control areas. The decrease coincided with the
diflubenzuron treatment in the middle of May. No higher active
density of species reproducing in late summer or autumn was observed
in the untreated areas (Klenner, 1990).
In a forestry spraying programme (0.0672 kg diflubenzuron per
ha), a survey of microarthropods collected from leaf litter and soil
in control and sprayed areas of the Bald Eagle State Forest,
Pennsylvania, USA, showed little or no effect of diflubenzuron on the
spray plot fauna. Increases and decreases in both the control and
spray population sizes during the 6-week study period could seemingly
be explained on the basis of seasonal population cycles, increased
soil moisture condition and differential extraction efficiencies
(White, 1975).
9.2.3.2 Vertebrates
Small song birds in a forest ecosystem were unaffected after an
aerial application of 350 g diflubenzuron/ha in Canada (Buckner et
al., 1975).
In a forestry spraying programme (0.0672 kg diflubenzuron/ha)
surveys were made on birds and small mammals. Effects on birds were
assessed using single male mapping surveys. The survey methodology
was designed to minimize bias due to differences between observers,
plots, times of day and abundance of birds. Vegetation and
defoliation types were identified and mapped and defoliation was
sampled at 136 sites. Four hundred and sixteen surveys were conducted
on treated plots, defoliated plots, and undisturbed plots. No
differences were observed in the number of individuals singing, the
amount of time each individual spent singing or the song repetition
rate during song bouts. The results show that diflubenzuron did not
lead to the death or emigration of singing birds and probably had no
direct adverse physiological effects on birds since their vocal
behaviour changed little, if at all. The singing male surveys did not
detect a presumed food shortage among canopy feeders caused by the
defoliation. Small mammals studied in this project were censused as
thoroughly as possible. The short pre-spray sampling period for the
treated plot was a handicap in evaluating the pre-spray population
levels; however, post-spray sampling compensated for this to some
degree. Peromyscus, Clethrionomys and Sorex consume arthropods to
varying degrees and may be exposed to diflubenzuron present as
residues. Diflubenzuron had no demonstrable effects on any of these
small mammals sampled in this study. The application of diflubenzuron
at the rate of 0.067 kg/ha provided good foliage protection within the
spray sites and apparently made the dying gypsy moth larvae
unpalatable. Other contact and stomach pesticides generally do not
have this effect on caterpillars and are consequently consumed by
insectivorous mammals. The reduced exposure by non-ingestion is no
doubt a beneficial aspect. None of the species of small mammals
showed reductions in numbers from pre-spray to post-spray periods that
could be attributed to the compound. Stomach analysis did not
indicate any change in the feeding habits of any of the small mammals
from either plot in the study. Changes in reproduction could not be
detected. However, of the Peromyscus and Clethrionomys animals
that were dissected and examined, 7 out of 12 females were in a
healthy condition or had gravid reproductive organs. Others were
either virgins or placental scars could not easily be seen, as in
Sorex. The body weight of all the small mammals in the study was
normal for each species. Juveniles were easily distinguished by
weight and gonad development. A regression comparing gonad weights
and body weights of Clethrionomys indicated a strong correlation.
Since the body weight of small mammals increases with age, this was to
be expected (White, 1975).
Diflubenzuron sprayed at 0.14 and 0.28 kg/ha over forests in
north-eastern Oregon, USA, did not have any detectable adverse effect
on bird population numbers, nesting or bird behaviour (Richmond et
al., 1979).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diflubenzuron was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1981, 1984, and 1985 (FAO/WHO, 1982a,b;
1985a,b; 1986a,b). In 1985, JMPR established an Acceptable Daily
Intake (ADI) for human beings of 0-0.02 mg/kg body weight per day,
based on the fact that the following levels produced no toxicological
effects:
rat: 2 mg/kg body weight (40 mg/kg diet)
mouse: 2.4 mg/kg body weight (16 mg/kg diet)
dog: 2 mg/kg body weight per day
Diflubenzuron has been classified as "a product unlikely to
present an acute hazard in normal use", on the basis of an acute oral
LD50 for the rat that is greater than 4640 mg/kg body weight (WHO,
1994).
REFERENCES
Ali A & Kok-Yokomi ML (1989) Field studies on the impact of a new
benzoylphenylurea insect growth regulator (UC-84572) on selected
aquatic nontarget invertebrates. Bull Environ Contam Toxicol,
42: 134-141.
Ali A & Lord J (1980) Impact of experimental insect growth regulators
on some nontarget aquatic invertebrates. Mosq News, 40(4): 564-571.
Ali A & Mulla MS (1978a) Effects of chironomid larvicides and
diflubenzuron on nontarget invertebrates in residential-recreational
lake. Environ Entomol, 7: 21-27.
Ali A & Mulla MS (1978b) Impact of the insect growth regulator
diflubenzuron on invertebrates in a residential-recreational lake.
Arch Environ Contam Toxicol, 7: 483-491.
Ali A, Nigg HN, Stamper JH, Kok-Yokomi ML, & Weaver M (1988)
Diflubenzuron application to citrus and its impact on invertebrates in
an adjacent pond. Bull Environ Contam Toxicol, 41: 781-790.
Anon (1980) Final report: Environmental monitoring of the 1979 Gypsy
moth control program in five eastern States. Hyattsville, Maryland, US
Department of Agriculture, Animal an Plant Health Inspection Service,
25 pp (Proprietary report No. 56683/18/1983, submitted to WHO by
Duphar BV, Crop Protection Division, 'sGraveland, The Netherlands).
Anton FA, Cuadra LM, Gutierrez P, Laborda E, & Laborda P (1993)
Degradational behavior of the pesticides glyphosate and diflubenzuron
in water. Bull Environ Contam Toxicol, 51: 882-888.
Apperson CS, Schaefer CH, Colwell AE, Werner GH, Anderson NL, Dupras
EF, & Longanecke DR (1978) Effects of diflubenzuron on Chaoborus
astictopus and nontarget organisms and persistence of diflubenzuron
in lentic habitats. J Econ Entomol, 71(3): 521-527.
Aquatic Environmental Sciences (1976a) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
North Carolina. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-13, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976b) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Arkansas. Tarrytown, New York, Union Carbide Corporation, 9 pp
(Unpublished proprietary report NTP-8, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976c) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Texas. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-7, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Arnold D, Kennedy SL, & Keplinger ML (1974) Mutagenic study with TH
6040 in albino mice. Northbrook, Illinois, Industrial Bio-Test
Laboratories Inc. (IBT No. 622-05068) (Unpublished proprietary report,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Arts JHE (1991) Acute (4-hour) inhalation toxicity of dimilin WP 25%
in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report TNO No. 56645/64/1991, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Barker RY & Taber S (1977) Effects of diflubenzuron fed to caged honey
bees. Environ Entomol, 6: 167-168.
Barker RY & Waller GD (1978) Effects of diflubenzuron wettable powder
on caged honey bee colonies. Environ Entomol, 7: 534-535.
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990a) A one-generation
reproduction study with the bobwhite ( Colinus virginianus. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/08/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990b) A one-generation
reproduction study with the mallard ( Anas platyrhynchos. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/07/1990, submitted to WHO by Solvay Duphar, BV, 'sGraveland,
The Netherlands).
Bentley JP, Weber GH, & Gould D (1979) The effect of diflubenzuron
feeding on glucosaminoglycan and sulfhemoglobin biosynthesis in mice.
Pestic Biochem Physiol, 10: 162-167.
Berberian IG & Enan EE (1989) Hematological studies on White male rats
exposed to some antimoulding compounds. Bull Environ Contam Toxicol,
43: 60-65.
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975a) Acute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/198/74988, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975b) Subacute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/199/7551, submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975c) Subacute inhalation
toxicity to the rat of DU 112307 insecticide powder. (Evaluation of
methaemoglobinaemia). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/197/ 741013, submitted to WHO
by Solvay Duphar BV, Weesp, The Netherlands).
Berends AG & Thus JLG (1992a) The acute toxicity of dimilin WP-25 to
the earthworm Eisenia fetida 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 16 pp (Proprietary
report No. 56635/26/1992).
Berends AG & Thus JLG (1992b) The toxicity of diflubenzuron to the
alga Selenastrum capricornutum 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 23 pp (Proprietary
report No. 56635/49/1992).
Berends AG & van den Laan-Straathof JMTh (1994a) The acute toxicity of
diflubenzuron to Zebra fish ( Brachydanio rerio. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/57/93).
Berends AG & van den Laan-Straathof JMTh (1994b) The acute toxicity of
diflubenzuron to rainbow trout ( Oncorhynchus mykiss. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/02/94).
Berends AG, Thus JLG, & Jansen WAJ (1992) The acute toxicity of
diflubenzuron to the earthworm Eisenia fetida 'sGraveland, The
Netherlands, Solvay Duphar BV, Environmental Research Department,
16 pp (Proprietary report No. 56635/22/1992).
Besten C Den, Buse TE, & Koopman TSM (1993a) Acute oral toxicity study
with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay Duphar
BV (Proprietary report No. 56345/51/1993).
Besten C Den, Buse TE, & Koopman TSM (1993b) Acute dermal toxicity
study with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56345/52/1993).
Birdsong RS (1977) Field test of Dimilin on non-target organisms in
Virginia. Nonfolk, Virginia, Environmental Consultants, Inc. (NTP-11)
(Unpublished proprietary report, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Blumerg AY (1986) Survey of aquatic soil and soil surface invertebrate
fauna in a North Carolina forest (pre and post application of Dimilin
WP-25) Weesp, The Netherlands, Duphar BV (Proprietary report
No. 56635/24/1986).
Boelhouwers EJ, Joustra KD, Nijssen OA, & Stegman KH (1988a)
Hydrolysis of 14C-labelled diflubenzuron in buffer solutions at pH 5,
pH 7, and pH 9. 'sGraveland, The Netherlands, Solvay Duphar
(Proprietary report No 56630/137/1988).
Boelhouwers EJ, Joustra KD, & Stegman KH (1988b) Photodegradation of
14C-labelled diflubenzuron in water. 'sGraveland, The Netherlands,
Solvay Duphar BV (Proprietary report No. 56630/35/1988).
Bogus ER, Gallagher PA, Cameron EA, & Mumma RO (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed phase solid support. J Agric Food Chem, 33(6): 1018-1021.
Bollag JM, Blattmann P, & Laanio T (1978) Adsorption and
transformation of four substituted anilines in soil. J Agric Food
Chem, 26(6): 1302-1306.
Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, & MacKenzie WF
(1990) Pathology of the Fischer rat - Reference and atlas. New York,
London, Academic Press.
Booth GM (1975) The impact of Dimilin W-25 on non-target invertebrates
in ponds located in Salt Lake county, Utah. Provo, Utah, Brigham Young
University (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Booth GM (1977) Biochemical effects of diflubenzuron on mouse embryos.
Provo, Utah, Brigham Young University (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Booth GM & Ferrell D (1977) Degradation of Dimilin by aquatic
foodwebs. In: Khan MAQ ed. Pesticides in aquatic environments. New
York, Plenum Publishing Corporation, pp 221-243.
Booth GM, Alder DC, Lee ML, Carter W, Whitmore RC, & Seegmiller RE
(1987) Environmental fate and properties of 1-(4-chlorophenyl)-
3-(2,6-difluorobenzoyl) urea (diflubenzuron, Dimilin). In: Wright JE &
Retnakaran ed. Chitin and benzoylphenyl ureas. Dordrecht, The
Netherlands, Junk Publishers, pp 141-204.
Brusick DJ & Weir RJ (1977a) Mutagenic evaluation of diflubenzuron
technical batch FL 4/605201. Final report (Project No. 2683).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/34/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Brusick DJ & Weir RJ (1977b) Evaluation of diflubenzuron: In vitro
malignant transformation in BALB/3T3 cells. Final Report (Project
No 2688). Kensington, Maryland, Litton Bionetics Inc. (Unpublished
proprietary report No. 56645/35/1977, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Brusick DJ & Weir RJ (1977c) Evaluation of diflubenzuron: Unscheduled
DNA synthesis in WI-38 cells. Final Report (Project No 2688).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/36/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Bryant H (1976) Activity of TH-6040 in the Ames Salmonella
typhimurium mutagenesis assay. Report to H.W. Dorough, University of
Kentucky, Lexington, USA (Unpublished proprietary report submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Buckner CH, McLeod BB, & Kingsbury PD (1975) The effect of an
experimental application of Dimilin upon selected forest fauna.
Ottawa, Ontario, Chemical Control Research Institute
(Report No. CC-X-97).
Bull DL & Ivie GW (1978) Fate of diflubenzuron in cotton, soil, and
rotational crops. J Agric Food Chem, 26(3): 515-520.
Burdock GA, Serota DG, Alsaker RD, & Purvis DS (1980a) Ninety-day
subchronic toxicity study in mice - Diflubenzuron technical. Final
report (Project No. 533-120). Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Burdock GA, Serota DG, Purvis D, & Alsaker RD (1980b) Subchronic
dietary toxicity study in rats - Diflubenzuron. Final report: Volume 1
(Project No. 533-119). Vienna, Virginia, Hazleton Laboratories America
Inc. (Unpublished proprietary report submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Burdock GA, Wolfe GW, Hepner KE, Alsaker RD, Koka M, & Phipps RB
(1984) Oncogenicity study in rats on diflubenzuron. Final report.
Vienna, Virginia, Hazleton Laboratories America Inc. (Unpublished
proprietary report No. 56645/08/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Burgess D (1989) Uptake, depuration and bioconcentration of
14C-diflubenzuron by Bluegill sunfish ( Lepomis macrochirus.
Columbia, Missouri, Analytical Bio-Chemistry Laboratories, Inc.
(Proprietary report No. 56635/16/1989, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Cardinal H, Vernet G, & Sinegre G (1979) Quelques effets d'un
inhibiteur de croissance: le diflubenzuron sur un crabe Carcinus
mediterraneus(Czerniavsky). C C R Soc Biol, 173: 1105-1108.
Carringer RD, Weber JB, & Monaco TJ (1975) Adsorption-desorption of
selected pesticides by organic matter and montmorillonite. J Agric
Food Chem, 23: 568-572.
Caspary WJ, Daston DS, Myhr BC, Mitchell AD, Rudd CJ, & Lee PS (1988)
Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay:
interlaboratory reproducibility and assessment. Environ Mol Mutagen,
12(Suppl 13): 195-229.
Cecil HC, Miller RW, & Corley C (1981) Feeding three insect growth
regulators to white Leghorn hens: Residues in eggs and tissues and
effects on production and reproduction. Poult Sci, 60: 2017-2027.
Chandran VR (1981) Primary skin irritation study in rabbit with
Dimilin 25 WP. Padappai, Tamil Nadu, India, Coromandel Indag Research
Centre (Proprietary report No. 31, submitted to WHO by Solvay Duphar
BV, 'sGraveland, The Netherlands).
Chapman RA, Tu CM, Harris CR, & Harris C (1985) Persistence of
diflubenzuron and Bay Sir 8514 in natural and sterile sandy loam and
organic soils. J Environ Sci Health, B20(5): 489-497.
Chesterman H, Heywood R, Barker MH, Street AE, & Cherry CP (1974)
DU 112307 toxicity in repeated dietary administration to beagle dogs
(Repeated administration for 13 weeks). Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR169/74157, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Christiansen ME & Costlow JD (1980) Persistence of the insect growth
regulator Dimilin in brackish water: A laboratory evaluation using
larvae of an estuarine crab as an indicator. Helgoländer Meeresunters,
33: 327-332.
Christiansen ME & Costlow JD (1982) Ultrastructural study of the
exoskeleton of the estuarine crab Rhithropanopeus harrsii Effect of
the insect growth regulator Dimilin (diflubenzuron) on the formation
of the larval cuticle. Mar Biol, 66: 217-226.
Christiansen ME, Costlow JD, & Monroe RJ (1978) Effects of the insect
growth regulator Dimilin (TH6040) on larval development of two
estuarine crabs. Mar Biol, 50: 29-36.
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981a) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume I. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/294/80185, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981b) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume II. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/294/80185, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Colley J, Heywood R, Street AE, & Gopinath C (1984) The effect of
diflubenzuron given by oral administration with the feed on toxicity
and tumour development in male and female HC/CFLP mice. Final report.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. 56645/32/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Collwell AE & Schaefer CH (1980) Diets of Ictalurus nebulosus and
Pomoxis nigromaculatus altered by diflubenzuron. Can J Fish Aquat
Sci 37(4): 632-639.
Cooke M & Ober AG (1980) OV-17-QF-1 Capillary column for
organochlorine pesticide analysis. J Chromatogr, 195: 265-269.
Corley C, Miller RW, & Hill K (1974) Determination of
N-(4-chlorophenyl)-N-(2,6-difluorobenzoyl)-urea in milk by
high-speed liquid chromatography. J AOAC, 57(6): 1269-1271.
Costlow YD (1979) Effect of Dimilin on development of larvae of the
stone crab Menippe mercenaria and the blue crab Callinectes
sapidus In: Vernberg WB, Calabrese A, Thurburg FP, & Vernberg FJ ed.
Marine pollution: Functional responses. New York, London, Academic
Press, pp 355-363.
Crookshank HR, Sowa BA, Kubena L, Holman GM, Smalley HE, & Morison R
(1978) Effect of diflubenzuron (Dimilin) on the hyaluronic acid
concentration in the chicken combs. Poult Sci, 57: 804-806.
Cunningham PA (I976) Effects of Dimilin (TH-6040) on reproduction in
the brine shrimp, Artemia salina Environ Entomol, 5: 701-706.
Cunningham PA (1986) A review of toxicity testing and degradation
studies used to predict the effects of diflubenzuron (Dimilin) in
estuarine crustaceans. Environ Pollut, A40: 63-86.
Cunningham PA & Myers LE (I986) Dynamics of diflubenzuron (Dimilin)
concentrations in water and sediment of a supratidal saltmarsh site
following repetitive aerial applications for mosquito control. Environ
Pollut, A41(1): 63-88.
Cunningham PA & Myers LE (I987) Effects of diflubenzuron (Dimilin) on
survival, molting, and behaviour of juvenile fiddler crabs, Uca
pugilator Arch Environ Contam Toxicol, 16: 745-752.
Danhaus RG & Sieck RF (1976) Rotation crop uptake of 14C-residues
following soil application of Dimilin. Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Danhaus RG, Wargo JP, & Sieck RF (1976) 14C-Dimilin field soil
leaching study. Colorado Springs, Colorado, Analytical Development
Corporation (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Davies RE & Halliday JC (1974) Acute percutaneous toxicity to rabbits
of DU 112307 technical. Huntingdon, England Huntingdon Research Centre
(Unpublished proprietary report No. 2171/D175/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE & Ligget MP (1973) Irritant effects of DU 112307 technical
on rabbit eye mucosa. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 2170/176D/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE, Elliot PH, Street AE, Heywood R, & Prentice DE (1975)
Effect of repeated applications of DU 112307 to the skin of rabbits
for three weeks. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR200/74851, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
De Bree H, De Lange N, Overmars H, & Post LC (1977) Diflubenzuron:
analysis of metabolites connected with methaemoglobinaemia. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/08/1977).
De Lange N (1979) Diflubenzuron: dermal absorption in the rabbit.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56654/09/1979).
De Lange N & Post LC (1978) Diflubenzuron: intestinal absorption in
the mouse in relation to dosage level. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56654/04/1978).
De Lange N, Overmans H, & Post LC (1974) PH 60-40: excretion of
radioactivity and metabolite patterns in rats following oral
administration. Weesp, The Netherlands, Philips-Duphar BV (Unpublished
proprietary report No. 56654/20/1974).
De Lange N, Overmans H, Willems AGM, & Post LS (1975) Diflubenzuron
(PH 60-40): balance studies in the rat, and identification of urinary
metabolites. Weesp, The Netherlands, Philips Duphar BV (Unpublished
proprietary report No. 56654/22/1975).
De Lange N, Fronik GM, & Post LC (1977) Diflubenzuron: intestinal
absorption in the rat in relation to dosage level. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/10/1977).
Deul DH & Jong BJ de (1977) The possible influence of DU 112307 on the
in vivo synthesis of hyaluronic acid in chicken skin. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56635/01/1977).
Di Prima SJ (1976) Determination of diflubenzuron residues in soybean
process fractions (meal, oil, hulls) by gas chromatography. Thompson-
Hayward Chemical Company (Unpublished proprietary report No. AM-13,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Di Prima SJ, Cannizaro RD, Roger JC, & Ferrell CD (1978) Analysis of
diflubenzuron residues in environmental samples by high-pressure
liquid chromatography. J Agric Food Chem, 26(4): 968-971.
Dorough HW (1977) Screening of selected Thompson-Hayward Chemicals for
activity in the Ames Salmonella mutagenicity test. Lexington,
University of Kentucky, Department of Entomology (Unpublished report
No. MTM-91).
Downer DM (1990) Analysis of diflubenzuron in streamwater samples from
little sluice mountain gypsy moth sprayblock. Harrisonburg, Virginia,
US Forest Service, 30 pp (Unpublished proprietary report submitted to
WHO by Duphar BV, Weesp, The Netherlands).
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1985) Reproducibility of microbial
mutagenicity assays: II. Testing of carcinogens and noncarcinogens in
Salmonella typhimurium and Escherichia coli Environ Mol Mutagen,
7(Suppl 5): 1-248.
El-Sebae AH, Salem MH, Al-Assar MRS, & Enan EE (1988) In vitro
effect of profenofos, fenvalerate and dimilin on protein and RNA
biosynthesis by rabbit liver and muscle tissues. J Environ Sci Health,
B23(5): 439-451.
Enninga IC (1990) Evaluation of DNA repair inducing ability of
diflubenzuron in a primary culture of rat hepatocytes (with
independent repeat). Report RCC NOTOX BV, 'sHertogenbos, The
Netherlands No. 56645/114/1990. Proprietary report, submitted to WHO
by Solvay Duphar, BV, 'sGraveland, The Netherlands.
FAO/WHO (1982a) Pesticides residues in food - 1981. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticides Residues in
Food and Environment and WHO Expert Group on Pesticides Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 37).
FAO/WHO (1982b) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985a) Pesticide residues in food - 1984. Report of the Joint
Meeting on Pesticide Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) Pesticide residues in food - 1984. Evaluations: The
Monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO, Plant Production and Protection Paper 67).
FAO/WHO (1986a) Pesticide residues in food - 1985. Report of the Joint
Meeting of the Residues in Food and the Environment and a WHO Expert
Group on Pesticides Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations:
Part II - Toxicology. Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of pesticide
residues, 4th ed. Rome, Codex Committee on Pesticide Residues, Food
and Agriculture Organization of the United Nations.
Farlow JE, Breaud TP, Steelman CD, & Schilling PE (1978) Effect of
the insect growth regulator diflubenzuron on non-target aquatic
populations in a Louisiana intermediate marsh. Environ Entomol,
7: 199-204.
Fischer SA & Hall LW Jr (1992) Environmental concentrations and
aquatic toxicity data on diflubenzuron (Dimilin). Crit Rev Toxicol,
22(1): 45-79.
Forward RB & Costlow JD (1978) Sublethal effects of insect growth
regulators upon crab larval behaviour. Water Air Soil Pollut,
9: 227-238.
Gilbert P, Saint-Ruf G, Poncelet F, & Mercier M (1980) Genetic effects
of chlorinated anilines and azobenzenes on Salmonella typhimurium
Arch Environ Contam Toxicol, 9: 533-541.
Goodman DG (1980a) Histopathologic evaluation of mice administered
diflubenzuron in the diet. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Goodman DG (1980b) Histopathologic evaluation of rats administered
diflubenzuron in the diet. Washington, DC, Clement Associates
(Unpublished proprietary report submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Gordon R & Cornect M (1986) Toxicity of the insect growth regulator
diflubenzuron to the rove beetle Aleochara bilineata a parasitoid
and predator of the cabbage maggot Delia radicum Entomol Exp Appl,
42: 179-185.
Goto M (1977a) Crop residue analysis of Dimilin. Japan, Institute
of Pesticide Residues (Unpublished proprietary report
No. K/Resid./004/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Goto M (1977b) Crop residue analysis of Dimilin. Japan, Institute of
Pesticide Residues (Unpublished proprietary report No.
K/Resid./007/1977, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Granett J, Morang S, & Hatch R (1978) Reduced movement of precocious
male Atlantic salmon parr into sublethal Dimilin-G1 and carrier
concentrations. Bull Environ Contam Toxicol, 19(4): 462-464.
Graves WC & Swigert JP (1993) Diflubenzuron: A 96-h flow through acute
toxicity test with the sheepshead minnow ( Cyprinodon variegatus.
Final report. Wildlife International Ltd. USA (Unpublished proprietary
report No. 56835/13/93, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Greenough RJ, Goburdhun R, Hundson R, & MacNaughtan F (1985)
Diflubenzuron. 52 week oral toxicity study in dogs: Volumes 1 and 2
(Project No. 630146,2728). Musselburgh, Scotland, Inveresk Research
International (Unpublished proprietary report No. 56645/32/1985,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Greenough RJ & McDonald P (1986) Diflubenzuron VC 90 acute inhalation
toxicity study in rats (limit test). Musselburgh, Scotland, Inveresk
Research International (Proprietary report No. 56645/41/1986,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Gulka G, Doscher CM, & Watabe N (1980) Toxicity and molt-accelerating
effects of diflubenzuron on the barnacle, Balanus eburneus Bull
Environ Contam Toxicol, 25: 477-481.
Gulka G, Gulka CM, & Watabe N (1982) Histopathological effects of
diflubenzuron on the cirripede crustacean, Balanus eburneus Arch
Environ Contam Toxicol, 11: 11-16.
Gustafson DE & Wargo JP (1976) Fate of Dimilin following application
to soybeans (ADC Project No 222). Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hansen SR & Garton RR (1982a) Ability of standard toxicity test to
predict the effects of the insecticide diflubenzuron on laboratory
stream communities. Can J Fish Aquat Sci, 39: 1273-1288.
Hansen SR & Garton RR (1982b) The effects of diflubenzuron on a
complex laboratory stream community. Arch Environ Contam Toxicol,
11: 1-10.
Hatzios KK & Penner D (1978) The effect of diflubenzuron on soybean
photosynthesis, respiration and leaf ultrastructure. Pestic Biochem
Physiol, 9: 65-69.
Hawkins DR, Jackson AJS, & Roberts NL (1980) Excretion of radio-
activity after oral administration of 3H/14C-diflubenzuron to
cats (PDR/302/80443). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 56654/14/1980, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands.
Helling CS (1985) Soil mobility of three Thompson-Hayward pesticides.
Interim report. Beltsville, Maryland, US Department of Agriculture,
Agricultural Research Centre (Unpublished proprietary report No. E-34,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hester PG (1982) Efficacy of diflubenzuron on three estuarine decapods
( Callinectes sp., Palaemonetes pugio and Uca sp.) (Unpublished
document submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hester PG, Olson MA, & Floore TG (1986) Effects of diflubenzuron on
three estuarine decapods, Callinectes sp., Palaemonetes pugio and
Uca pugilator J Fla Anti-Mosq Assoc, 57: 8-14.
Honeycutt R (1993) Indoor occupant exposure study with Dimilin 25 W.
Colorado Springs, Colorado, Analytical Research and Development
Corporation (Report No. 56635/56/1992, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hsu TS & Bartha R (1974) Interaction of pesticide-derived chloroaniline
residues with soil organic matter. Soil Sci, 1974: 444-452.
Huber CM & Collins DL (1987) Final report on the 1987 spray project to
eradicate Gypsy moth from the Tusquitee ranger district on the
Nantahala national forest. Asheville, North Carolina, Field Office,
Forest Pest Management (Unpublished report No. 88-1-4, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Huber CM & Manchester EH (1988) Final report on the eradication of
the Gypsy moth from the Tusquitee ranger district on the Nantahala
national forest. Forest Pest Management (Unpublished report
No. 89-1-7, submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hunter B, Batham P, Street AE, & Cherry CP (1974) DU 112307
preliminary assessment of the toxicity to male mice in dietary
administration for 6 weeks. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/174/74199, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hunter B, Batham P, Offer JM, & Prentice DE (1975) Tumorigenicity
study of DU 112307 to mice. Dietary administration for 80 weeks. Final
report. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/170/75685, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Colley J, Street AE, Heywood R, Prentice DE, & Offer J
(1976) Effects of DU 112307 in dietary administration to rats for 104
weeks. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/171/75945, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Jordan J, Heywod R, Street AE, Prentice DE, Wight DG, &
Gibson WA (1979) DU 112307 toxicity to rats in dietary administration
for nine weeks followed by a four week withdrawal period (final
report). Huntingdon, England, Huntingdon Research Centre (Unpublished
report No. PDR/248/77883).
Ivie GW (1977) Metabolism of insect growth regulators in animals.
In: Ivie GW & Dorough HW ed. Fate of pesticides in large animals. New
York, London, Academic Press, pp 111-125.
Ivie GW (1978) Fate of diflubenzuron in cattle and sheep. J Agric Food
Chem, 26(1): 81-89.
Ivie GW, Bull DL, & Veech JA (1980) Fate of diflubenzuron in water.
J Agric Food Chem, 28(2): 330-337.
Jackson GA (1976) Dimilin (TH 6040): Biological impact on pond
organisms. Marion, Alabama, Southeastern Fish Cultural Laboratory
(Unpublished proprietary report NTP-42, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Jackson GC, Hardy CJ, Simms F, Mullins PA, & Gopinath Ch (1990)
Dimilin 4F acute inhalation toxicity study in rats 4-hour exposure.
Huntingdon, England, Huntingdon Research Centre (Proprietary report
No. 56645/68/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Jagannath DR & Brusick DJ (1977a) Mutagenicity evaluation of
4-chlorophenyl urea, final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/42/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977b) Mutagenicity evaluation of
2,6-difluorobenzoic acid. Final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/40/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977c) Mutagenicity evaluation of
4-chloroanilin. Final report. Kensington, Maryland, Litton Bionetics
Inc. (Unpublished proprietary report No. 56645/41/1977, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Janssen PJM & Pot TE (1987a) Primary irritation study of dimilin SC-48
to the eye of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/46/1987).
Janssen PJM & Pot TE (1987b) Primary irritation study of dimilin SC-48
to the skin of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/45/1987).
Janssen PJM & Pot TE (1987c) Acute oral toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/44/1987).
Janssen PJM & Pot TE (1987d) Acute dermal toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/43/1987).
Janssen PJM & Pot TE (1987e) Acute dermal toxicity study with dimilin
WP-25 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/58/1987).
Janssen PJM & van Doorn WM (1993a) Acute inhalation toxicity study
with dimilin OF-6 in male and female rats. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/01/1993).
Janssen PJM & van Doorn WM (1993b) Primary irritation study of dimilin
OF-6 to the skin of female rabbits. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/30/1993).
Janssen PJM & van Doorn WM (1993c) Primary irritation study of dimilin
OF-6 to the eye of female rabbits. Weesp, The Netherlands, Solvay
Duphar BV, Department of Toxicology (Proprietary report
No. 56345/31/1993).
Jenkins VK, Mayer RT, & Perry RR (1984) Effects of diflubenzuron on
growth of malignant melanoma and skin carcinoma tumors in mice. Invest
New Drugs, 2: 19-27.
Jenkins VK, Perry RR, Ahmer AE, & Ives K (1986) Role of metabolism in
effects of diflubenzuron on growth of B16 melanomas in mice. Invest
New Drugs, 4: 325-335.
Jones AS & Konchenderfer JN (1988) Persistence of diflubenzuron
(Dimilin) in a small eastern watershed and its impact on invertebrates
in a headwater stream. Research Triangle Park, North Carolina,
Southeastern Forest Experiment Station (Unpublished proprietary report
No. 56637/26/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Joustra KD, van Kampen WG, & Walstra P (1989) Metabolism of [14C]
diflubenzuron in citrus fruit (Analytical report). Weesp, The
Netherlands, Duphar BV, Analytical Development Department (Proprietary
report No. 56630/86/1989).
Julin AM & Sanders HO (1978) Toxicity of IGR, diflubenzuron, to
freshwater intervertebrates and fishes. Mosq News, 38: 256-259.
Kavanagh P (1988a) Diflubenzuron oral (gavage) rat teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/11/87)
(Proprietary report No. 56645/68/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kavanagh P (1988b) Diflubenzuron oral (gavage) rabbit teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/12/87)
(Proprietary report No. 56645/79/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Keet CMJF (1977a) The methaemoglobin, sulphaemoglobin and Heinz body
forming properties of DU 112307 after oral administration to male rats
during 8 days. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/15/1977).
Keet CMJF (1977b) The effect of DU 112307 (tech) in male mice after
daily oral administration (14-days) on body weight, methaemoglobin,
sulphaemoglobin and Heinz body formation. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/33/1977).
Keet CMJF (1977c) The methaemoglobin and sulphaemoglobin forming
properties of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/02/1977).
Kemp A, van der Heijden CA, & van Eldik A (1973a) Dietary
administration of DU 112307 to male and female rats for three months.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56645/13A/1973).
Kemp A, van der Heijden CA, & van Eldik A (1973b) Appendix III to
report No. 56645/13A/1973 individual data: dietary administration of
DU 112307 to male and female rats for 3 months. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56645/13B/1973).
Kingsbury P, Sundaram KMS, Holmers KMS, Nott R, & Kreutzweiser D
(1987) Aquatic fate and impact studies with Dimilin. Ottawa, Ontario,
Forest Pest Management Institute (Unpublished report No. 78, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Klenner MF (1990) [A comparative study of the carabid fauna in the
Dimilin-treated and untreated oak stands in Westphalia.] Mitt BBA
(Berlin-Dahlem), 266: 279 (in German).
Koelman-Klaus HJS (1978a) Acute oral toxicity study of
4-chlorophenylurea in male and female rats. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/09/1978).
Koelman-Klaus HJS (1978b) Acute oral toxicity study of
2,6-difluorobenzoic acid in male and female rats. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56645/25/1978).
Koopman TSM (1977a) Acute oral toxicity study with DU 112307 technical
in mice. 'sGraveland, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/04/1977).
Koopman TSM (1977b) Acute oral toxicity study with DU 112307 WP 25%
in mice and rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/03/1977).
Koopman TSM (1977c) Acute dermal toxicity study with DU 112307
technical in rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/07/1977).
Koopman TSM (1980a) Primary irritation of Dimilin ODC-45 to the rabbit
eye. 'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/02/1980).
Koopman TSM (1980b) Acute dermal toxicity study of dimilin ODC-45 in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/04/1980).
Koopman TSM (1980c) Primary irritation of Dimilin ODC-45 to the rabbit
skin. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/03/1980).
Koopman TSM (1984a) Acute dermal toxicity study with diflubenzuron
VC-90 in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/31/1984).
Koopman TSM (1984b) Acute oral toxicity study with diflubenzuron VC-90
in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/30/1984).
Koopman TSM (1984c) Primary irritation study of diflubenzuron VC-90 to
the rabbit eye. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/29/1984).
Koopman TSM (1984d) Primary irritation study of diflubenzuron VC-90 to
the rabbit skin. 'sGraveland, The Netherlands, Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/44/1984).
Koopman TSM (1985a) Primary irritation study of dimilin 2F to the
skin of the male rabbit. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report
No. 56645/93/1985).
Koopman TSM (1985b) Primary irritation study of dimilin 2F to
the eye of the male rabbit. 'sGraveland, The Netherlands,
Duphar BV, Department of Toxicology (Unpublished proprietary report
No. 56645/91/1985).
Koopman TSM (1985c) Acute dermal toxicity study with dimilin 2F in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/95/1985).
Koopman TSM (1985d) Acute oral toxicity study with dimilin 2F in rats.
'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/94/1985).
Koopman TSM & Jongeling AJ (1979) Acute oral toxicity study with
Dimilin ODC 45% in rats. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report No.
56645/38/1979).
Koopman TSM & Pot TE (1986) Acute oral toxicity study with
diflubenzuron VC-90% in mice. 'sGraveland, The Netherlands, Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/39/1986).
Koorn JC (1990) Study to examine the possible mutagenic activity of
diflubenzuron in the Ames Salmonella microsome assay. 'sGraveland,
The Netherlands, Duphar BV, Department of Toxicology (Proprietary
report No. 56645/74/1990).
Kramer HT (1990) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in an apple orchard in Phelps (New York). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-271,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1991) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in a citrus orchard in Oviedo (Florida). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-270,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992a) Field dissipation study with diflubenzuron
insecticide applied for control of insects on cotton (a bare soil
application in Arkansas). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-110, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992b) Field dissipation study with diflubenzuron
insecticide applied for control of insects on soybeans (a bare soil
application in Louisiana). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-116, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kubena LF (1982) The influence of diflubenzuron on several
reproductive characteristics in male and female layer-breed chickens.
Poult Sci, 61: 268-271.
Kuijpers LAM (1988) The acute toxicity of diflubenzuron to Daphnia
magna Weesp, The Netherlands, Duphar BV (Unpublished proprietary
report No. 56635/26/1988).
Kurczewski F, Wang C, Grimble D, Smith R, & Brezner J (1975)
Environmental impact of dimilin. A final report: Effects of dimilin
upon microorganisms in leaf litter and forest soil. Syracuse, New
York, State University of New York, pp 28-43.
Kynoch SR & Elliot PH (1978a) Screening test for delayed contact
hypersensitivity with Dimilin WP 25%. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 0911/D258/78, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Elliot PH (1978b) Screening test for delayed contact
hypersensitivity with dummy formulation for dimilin WP 25% in the
albino guinea-pig. Huntingdon, England, Huntingdon Research Centre
(Proprietary report No. 9112/D258/78, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin 2F. Huntingdon, England, Huntingdon Research
Centre (Proprietary report No. 56645/13/1987, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin SC-48. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 56645/72/1987, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Smith PA (1986) Delayed contact hypersensitivity in the
guinea-pig with diflubenzuron VC-90. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 86558D/PDR 432SS, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Lauren DR, Agnew MP, & Henzell RF (1984) Field life of the insect
growth regulator diflubenzuron on lucerne. N Z J Agric Res,
27: 425-429.
Lawrence JF & Sundaram KMS (1976) Gas-liquid chromatographic analysis
of N-(4-chlorophenyl-N-2,6-difluorobenzoyl) urea insecticide after
chemical derivatization. J AOAC, 59(4): 938-941.
Lee BM & Scott GI (1989) Acute toxicity of temephos, fenoxycarb,
diflubenzuron and methoprene and Bacillus thuringiensis var.
Israelensis to the mummichug ( Fundulus heteroclitus. Bull Environ
Contam Toxicol, 43: 827-832.
Maas W, Van Hes R, Grosscurt AC, & Deul DH (1980) [Benzoylphenylurea
insecticides.] In: Wegler R ed. [Chemistry of plant protection
chemicals and pesticides.] Berlin, Heidelberg, Springer Verlag, vol 6,
pp 432-470 (in German).
McAlonan WG (1976) Effects of two insect growth regulators on some
selected salt-marsh non-target organisms. University of Delaware, USA
(Thesis) (NTP-53).
Machado J, Coimbra J, Castilho F, & Sa C (1990) Effects of
diflubenzuron on shell formation of the freshwater clam, Anodonta
cygnea Arch Environ Contam Toxicol, 19: 35-39.
McGregor JT, Gould DH, Mitchell AD, & Sterling GP (1979) Mutagenicity
tests of diflubenzuron in the micronucleus test in mice, the L5178Y
mouse lymphoma forward mutation assay, and the Ames Salmonella
reverse mutation test. Mutat Res, 66: 45-53.
McKague AB & Pridmore RB (1978) Toxicity of Altosid and Dimilin to
juvenile rainbow trout and coho salmon. Bull Environ Contam Toxicol,
20: 167-169.
Madder DJ (1977) The disappearance from, efficacy in and effect on
non-target organisms of diflubenzuron, methoprene and chlorpyrifos in
a lentic ecosystem. Canada, University of Manitoba, Faculty of
Graduate Studies, 139 pp (Thesis) (NTP-71).
Madder DJ & Lockhart WL (1978) A preliminary study of the effects of
diflubenzuron and methoprene on rainbow trout ( Salmo gairdneri. Bull
Environ Contam Toxicol, 20: 66-70.
Madder DJ & Lockhart WL (1980) Studies on the dissipation of
diflubenzuron and methoprene from shallow prairie pools. Can Entomol,
112: 173-177.
Mansager ER, Still GC, & Frear DS (1979) Fate of 14C diflubenzuron on
cotton and in soil. Pestic Biochem Physiol, 12: 172-182.
Marshall BF & Hieb BF (1973) 96-h LC50 Salmo gairdneri (n.m.
Oncorhynchus mykiss, Lepomis machrochirus and Fundulus heteroclitus
Marine Research Institute (Unpublished study submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Martinez-Toledo MV, Rubia T, De La Moreno J, & Gonzalez-Lopez J
(1988a) Effect of diflubenzuron on azotobacter nitrogen fixation in
soil. Chemosphere, 17(4): 829-834.
Martinez-Toledo MV, Gonzalez-Lopez J, Rubia T, De La Moreno J, &
Ramos-Cormenzana A (1988b) Diflubenzuron and the acetylene-reduction
activity of Azotobacter vinelandii Soil Biol Biochem,
20(2): 255-256.
Matheson DW & Brusick DJ (1977a) Evaluation of 4-chlorophenylurea
in vitromalignant transformation in BALB/3T3 cells. Kensington,
Maryland, Litton Bionetics Inc. (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brucisk DJ (1977b) Evaluation of 2,6-diflurobenzoic acid
in vitromalignant transformation in BALB/3T3 cells. Final report.
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/10/1978, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Matheson DW & Brusick DJ (1978a) Mutagenicity evaluation of
2,6-difluorobenzoic acid in unscheduled DNA synthesis in the human WI-
38 cells assay. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/16/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978b) Mutagenicity evaluation of
4-chlorophenylurea in unscheduled DNA synthesis in the human WI-38
cells assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/24/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978c) Mutagenicity evaluation of
4-chloroanilin in the in vitro transformation of BALB/3T3 cells
assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/23/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matta JF (1976) The effect of dimilin on non-target organisms in a
marsh community. Norfolk, Virginia, Environmental Consultants (Report
prepared for Thompson-Hayward Chemical Company) (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Mayer RT, Netter KJ, Leising HB, & Schachtschabel DD (1994) Inhibition
of the uptake of nucleosides in cultured Harding-Passey melanoma cells
by diflubenzuron. Toxicology, 30: 1-6.
Metcalf RL, Lu PY, & Browlus S (1975) Degradation and environmental
fate of 1-(2,6-difluorobenzoyl)-3-(4-chlorophenyl) urea. J Agric Food
Chem, 23(3): 359-364.
Mian LS & Mulla MS (1983) Persistence of three IGRs in stored wheat.
J Econ Entomol, 76(3): 622-625.
Miller RW, Corley C, Oehler DD, & Pickens LG (1976a) Feeding TH 6040
to cattle: residues in tissues and milk and breakdown in manure.
J Agric Food Chem, 24(3): 687-688.
Miller RW, Corley C, & Shufeld SR (1976b) Effects of feeding TH 6040
to two breeds of chickens. J Econ Entomol, 69(6): 741-743.
Miller RW, Cecil HC, Carey AM, Corley C, & Kiddy CA (1979) Effects of
feeding diflubenzuron to young male Holstein cattle. Bull Environ
Contam Toxicol, 23: 482-486.
Miura T (1974) Biological activity of TH 6040 against organisms
associated with mosquito breeding habitats in irrigated pastures.
Fresno, California, University of California, Mosquito Control
Research Laboratory (Unpublished report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Miura T & Takahashi RM (1974a) Insect developmental inhibitors effects
of candidate mosquito control agents on nontarget aquatic organisms.
Environ Entomol, 3(4): 631-636.
Miura T & Takahashi RM (1974b) Toxicity of TH 6040 to freshwater
crustacea and the use of a tolerance index as a method of expressing
side effects on nontargets. Proceedings of the Forty-Second Annual
Conference of the California Mosquito and Vector Control Association,
pp 177-180.
Moreale A & Van Bladel R (1976) Influence of soil properties on
adsorption of pesticide-derived aniline and p-chloroaniline. J Soil
Sci, 27: 48-57.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Mulla MS, Majori G, & Darwazeh HA (1975) Effects of the insect growth
regulator Dimilin (TH-6040) on mosquitoes and some nontarget
organisms. Mosq News, 35: 211-216.
Mutanen RM, Siltanen HT, Kuukka VP, Annila EA, & Varama MMO (1988)
Residues of diflubenzuron and two of its metabolites in a forest
ecosystem after control of the pine looper moth, Bupalus
piniarius. L Pestic Sci, 23: 131-140.
Nakayama K (1977a) Crop residue analysis of Dimilin (apple).
Japan, Institute of Pesticides (Unpublished proprietary report
No. K/Resid/003/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Nakayama K (1977b) Crop residue analysis of Dimilin. Japan, Institute
of Pesticides (Unpublished proprietary report No. K/Resid/006/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Nation JL, Robinson FA, Ju SJ, & Bolten AB (1986) Influence upon honey
bees of chronic exposure to very low levels of selected insecticides
in their diet. J Agric Res, 25(3): 170-177.
Nebeker AV, McKinney P, & Cairns MA (1983) Acute and chronic effects
of diflubenzuron (Dimilin) on freshwater fish and invertebrates.
Environ Toxicol Chem, 2: 329-336.
Nigg HN (1989) Metabolism of 14C-diflubenzuron in citrus fruits.
Field operations report. Lake Alfred, Florida, Citrus Research and
Education Center (Proprietary report No. 56635/13/1989, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Nigg HN, Canizzaro RD, & Stamper JH (1986) Diflubenzuron surface
residues in Florida citrus. Bull Environ Contam Toxicol,
36(6): 833-838.
Nimmo WB & de Wilde PC (1974) Fate of diflubenzuron on leaves of corn,
soybean, cabbage and apples. 'sGraveland, The Netherlands, Philips-
Duphar BV, Crop Protection Division (Unpublished proprietary report
No. 56635/16A/1974).
Nimmo WB & de Wilde PC (1975a) Degradation of diflubenzuron in soil
and natural water. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/37/1975).
Nimmo WB & de Wilde PC (1975b) Degradation of diflubenzuron in sterile
water at pH 5, 7, 9 and 12. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/32/1975).
Nimmo WB & de Wilde PC (1976a) Uptake of diflubenzuron treated soil by
soybean, maize and potato. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/04/I976).
Nimmo WB & de Wilde PC (1976b) Uptake of diflubenzuron and its
metabolites from the soil by rice and wheat. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/08/1976).
Nimmo WB & de Wilde PC (1977a) Degradation of diflubenzuron in
mushroom growth medium and uptake of its metabolites in the mushrooms.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56635/21A/1977).
Nimmo WB & de Wilde PC (1977b) Degradation of diflubenzuron in calf
and chicken manure. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/27/1977).
Nimmo WB, Willems AGM, & de Wilde PC (1978) Fate of diflubenzuron
applied to leaves and fruits of apple trees. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/33/1978).
Nimmo WB, Hamaker TL, More JC, & Wood RA (1980) Acute and chronic
effects of Dimilin on survival and reproduction of Misidopsis bahia
In: Eaton JG, Parrish PR, & Hendricks AC ed. Aquatic toxicology.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 366-376 (Special Technical Publication 707).
Nimmo WB, Wilde PC, & Verloop A (1984) The degradation of
diflubenzuron and its chief metabolites in soils. Part I: Hydrolytic
cleavage of diflubenzuron. Pestic Sci, 15: 574-585.
Nimmo WB, Willems AGM, Joustra KD, & Verloop A (1986) The degradation
of diflubenzuron and its chief metabolites in soils. Part II: Fate of
4-chlorophenylurea. Pestic Sci, 17: 403-411.
Nimmo WB, Joustra KD, & Willems AGM (1990) The degradation of
diflubenzuron and its chief metabolites in soils. Part III: Fate of
2,6-difluorobenzoic acid. Pestic Sci, 29: 39-45.
Offringa OR (1977) Addendum report to the chronic studies with
DU 112307: (A) Dietary administration to rats for 104 weeks - (B)
Dietary administration to mice for 80 weeks. Weesp, The Netherlands,
Philips-Duphar BV, Department of Toxicology (Report No. 56645/
16/1977).
Opdycke JC (1976) Metabolism and fate of diflubenzuron in chickens and
swine. University of Maryland, Faculty of the Graduate School
(Thesis).
Opdycke JC, Miller RW, & Menzer RE (1982a) Metabolism and fate of
diflubenzuron in swine. J Agric Food Chem, 30(6): 1223-1227.
Opdycke JC, Miller RW, & Menzer RE (1982b) In vivo and liver
microsomal metabolism of diflubenzuron by two breeds of chickens.
J Agric Food Chem, 30(6):1227-1233.
Palmer AK & Hill PA (1975a) Effect of DU 112307 on reproductive
function of multiple generations in the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/173/75954, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK & Hill PA (1975b) Effect of DU 112307 on pregnancy of the
rat. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/192/74978, submitted to WHO by Solvay-
Duphar, B.V., Weesp, The Netherlands).
Palmer AK & Hill PA (1975c) Effect of DU 112307 on pregnancy of the
New Zealand white rabbit. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/193/74937, submitted to
WHO by Solvay-Duphar BV, Weesp, The Netherlands).
Palmer AK, Allen PA, Street EA, & Prentice DG (1977) Preliminary
assessment of the effect of DU 112307 on the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/243/77208, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK, Allen PA, Heywood R, Street EA, Gibson WA, Offer JM, &
Jolly DW (1978) Effect of dietary administration of DU 112307 on
reproductive function of one generation in the rat. Huntingdon,
England, Huntingdon Research Centre (Report No. PDR 244/78653). 1978.
Unpublished proprietary report, submitted to WHO by Solvay-Duphar,
B.V., Weesp, The Netherlands.
Prasad I (1970) Mutagenic effects of the herbicide 3',4'-dichloro-
propionalide and its degradation products. Can J Microbiol,
16: 369-372.
Prinsen MK (1988a) Primary dermal irritation/corrosion study with
dimilin 4F in albino rabbits. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
93/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1988b) Primary eye irritation study with dimilin 4F in
albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/92/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Prinsen MK (1989a) Acute dermal irritation/corrosion study with
dimilin SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
150/1989, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Prinsen MK (1989b) Acute eye irritation/corrosion study with dimilin
SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/151/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1989c) Sensitization study with dimilin 4F in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/23/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1990) Acute dermal irritation/corrosion study with dimilin
ODC-45 in albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology
and Nutrition Institute (Unpublished proprietary report
No. 56645/99/1990, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1992) Sensitization study with diflubenzuron technical in
guinea-pigs. The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report No. 56645/26/1992, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1993) Sensitization study with Dimilin OF-6 in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56345/03/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Pritchard PH & Bourquin AW (1981) Fate of Dimilin in laboratory
estuarine ecosystems. Washington, DC, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished report).
Quarles JM, Norman JO, & Kubena LF (1980) Absence of transformation by
diflubenzuron in host mediated transplacental carcinogenassay. Bull
Environ Contam Toxicol, 25: 252-256.
Rabenort B, de Wilde PC, de Boer FG, Korver PK, Di Prima SJ, &
Cannizzaro RD (1978) Diflubenzuron. In: Analytical methods for
pesticides and plant growth regulators. New York, London, Academic
Press, vol 10, pp 57-72.
Richmond ML, Henny CJ, Floyd RL, Mannan RW, Finch DM, & de Weese LR
(1979) Effects of Sevin-4-oil, Dimilin and orthene on forest birds in
Northeastern Oregon. Berkeley, California, Pacific Southwest Forest
and Range Experiment Station (Research Paper PSW-148).
Rodrigues CS (1982) Effects of insecticides including insect growth
regulators on black fly (Diptera: Simuliidae) larvae and associated
nontarget stream invertebrates. Ontario, Canada, University of Guelph
(Thesis).
Rosenkranz HS & Poirier LA (1979) Evaluation of the mutagenicity and
DNA-modifying activity of carcinogens and noncarcinogens in microbial
systems. J Natl Cancer Inst, 62: 873-892.
Ross DB, Prentice DE, Newman AJ, Street AE, Hepworth PL, & Roberts NL
(1977a) DU 112307 13 weeks oral toxicity study in the sheep.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR229/77226, submitted to WHO by Solvay-Duphar
BV, Weesp, The Netherlands).
Ross DB, Street AE, & Roberts NL (1977b) Blood red cell and plasma
cholinesterase value following six weeks inclusion of DU 112307 in the
diet of sheep. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR229A/77225, submitted to WHO by
Solvay-Duphar BV, Weesp, The Netherlands).
Sarkar DK (1982) Persistence of dimilin 25 WP in water. Padappai,
Tamil Nadu, India, Coromandel Indag Research Centre (Proprietary
report No. 124, submitted to WHO by Solvay-Duphar BV, 'sGraveland, The
Netherlands).
Schaefer CH & Dupras EF (1976) Factors affecting the stability of
Dimilin in water and the persistence of Dimilin in field waters.
J Agric Food Chem, 24(4): 733-739.
Schaefer CH, Dupras EF, Stewart RJ, Davidson LW, & Colwell AE (1979)
The accumulation and elimination of diflubenzuron by fish. Bull
Environ Contam Toxicol, 21: 249-254.
Schaefer CH, Colwell AE, & Dupras EF (1980) The occurrence of
p-chloroaniline and p-chlorophenylurea from the degradation of
diflubenzuron in water and fish. In: Proceedings and papers of the
Forty-Eighth Annual Conference of the California Mosquito and Vector
Control Association, 20-23 January 1980, pp 84-89.
Schimmel SC, Garnas RL, Patrick JM, & Moore JC (1983) Acute toxicity,
bioconcentration and persistence of AC 222,705, Benthiocarb etc. in
the estuarine environment. J Agric Food Chem, 31(1): 104-113.
Schwartz SL & Borzelleca JF (1981) Effects of diflubenzuron on
methemoglobin, sulfhemoglobin and Heinz body production in cats.
Richmond, Virginia, Toxicology and Pharmacology, Inc. (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Seegmiller RE & Booth GM (1976) Teratogenic and biochemical effects of
TH-6040 on chicken embryos. Provo, Utah, Brigham Young University
(Unpublished report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Serra GE & Joustra KD (1990) Diflubenzuron: Nature of residues on
apples. Greenhouse operations report. Weesp, The Netherlands, Duphar
BV (Proprietary report No. 56630/43/1990).
Seuferer SL, Braymer HD, & Dunn JJ (1979) Metabolism of diflubenzuron
by soil microorganisms and mutagenicity of the metabolites. Pestic
Biochem Physiol, 10: 174-180.
Simmons KE, Minard RD, & Bollag JM (1989) Oxidative co-oligomerization
of guaiacol and 4-chloroaniline. Environ Sci Technol, 23(1): 115-121.
Singh PP & Kaira RL (1989) Determination of diflubenzuron residues by
thin-layer chromatography. Chromatographia, 27(1/2): 53-54.
Sipes JG & Carter DE (1988) Pharmacokinetics of xenobiotics:
p-chloroaniline. Tucson, Arizona, University of Tucson (Unpublished
report No. MRJDN 410882-01, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith JT & Edmunds CP (1985) Environmental monitoring of the 1984
gypsy moth control program in Tennessee, USA. Weesp, The Netherlands,
Duphar BV, 25 pp (Proprietary report No. 56637/27/1985).
Smith KS & Merricks DL (1976a) TH 6040 tissue residue and metabolism
study in dairy cows. Reading, Pennsylvania, Cannon Laboratories, Inc.
(Unpublished proprietary report No. 5E-7372, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Smith KS & Merricks DL (1976b) Tissue residue and metabolism study in
poultry. Reading, Pennsylvania, Cannon Laboratories, Inc. (Unpublished
proprietary report No. 5E-7372, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith S, Willis GH, & McDowell LL (1983) Electron-capture gas
chromatographic determination of diflubenzuron and permethrin in soil
and water. J Agric Food Chem, 31: 610-612.
Smucker RA & Simon SL (1986) Some effects of diflubenzuron on growth
and sporogenesis in Streptomyces spp. Appl Environ Microbiol,
51(1): 25-31.
Snoeij NJ & Buse-Pot TE (1990) Primary irritation study of dimilin WP
25% to the skin of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/09/1991).
Snoeij NJ & Buse-Pot TE (1991) Primary irritation study of dimilin WP
25% to the eye of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/13/1991).
Solvay Duphar (1994) Recommendations for field use (diflubenzuron) -
Guidelines. Weesp, The Netherlands, Solvay Duphar BV.
Spanjers MTh (1988a) Determination of the acute oral toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/85/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Spanjers MTh (1988b) Determination of the acute dermal toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/84/1988, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Steelman CD, Farlow JE, Breaud TP, & Schilling PE (1975) Effects of
growth regulators on Psorophora columbiae (Dyar and Knab) and non-
target aquatic insect species in rice fields. Mosq News, 35(1): 67-76.
Stevenson JH (1978) The acute toxicity of formulated pesticides to
worker honey bees. ( Apis mellifera L.. Plant Pathol, 27: 38-40.
Stoner A & Wilson WT (1982) Diflubenzuron (Dimilin): effect of long-
term feeding of low dose in sugar-cake or sucrose syrup on honey bees
in standard-size field colonies. Am Bee J, 122: 579-582.
Sundaram KMS (1986) Persistence and degradation of diflubenzuron in
conifer foliage, forest litter and soil, following simulated aerial
application. Sault Ste Marie, Ontario, Canada, Forest Pest Management
Institute (Information report FPM-X-74).
Sundaram KMS (1991) Spray deposit patterns and persistence of
diflubenzuron in some terrestrial components of a forest ecosystem
after application at three volume rates under field and laboratory
conditions. Pestic Sci, 32: 275-293.
Sundaram KMS & Nott R (1989) Mobility of diflubenzuron in two types of
forest soils. J Environ Sci Health, B24(1): 65-86.
Sundaram KMS, Holmes SB, Kreutzweiser DP, Sundaram A, & Kingsbury PD
(1991) Environmental persistence and impact of diflubenzuron in a
forest aquatic environment following aerial application. Arch Environ
Contam Toxicol, 20: 313-324.
Surprenant DC (1988) The chronic toxicity of 14C-diflubenzuron to
Daphnia magna under flow-through conditions. Wareham, Massachusetts,
Springborn Life Sciences, Inc. (Proprietary report No. 56635/22/1988,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Taalman RDFM & Hoorn AJW (1986) Mutagenicity evaluation of
diflubenzuron technical (Assay No. E-9481). Veenendaal, The
Netherlands, Hazleton Biotechnologies Corporation (Proprietary report
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Tasheva M & Hristeva V (1991) Biochemical and hematological changes in
rats following 28 days oral administration of diflubenzuron and
triflumuron. In: Abstracts from the 1991 Eurotox Congress, Maastricht,
The Netherlands, 1-4 September 1991, p 68.
Tasheva M & Hristeva V (1993) Comparative study on the effects of five
benzoylphenylurea insecticides on haematological parameters in rats.
J Appl Toxicol, 13(1): 67-68.
Taylor RE (1973a) Primary skin irritation study TH-6040 technical
(albino rabbit). Lincoln, Nebraska, Harris Laboratories (Proprietary
report submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Taylor RE (1973b) Primary skin irritation study TH-6040 W25 wettable
powder (albino rabbit). Lincoln, Nebraska, Harris Laboratories
(Proprietary report submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Tester PA & Costlow JD (1981) Effect of insect growth regulator
Dimilin (TH 6040) on fecundity and egg viability of the marine copepod
Acartia tonsa Mar Ecol Prog Ser, 5: 297-302.
Thompson SG & Swigert JP (1993a) Diflubenzuron: A 14-day toxicity test
with Duckweed ( Lemna gibba G3). Euston, Maryland, Wildlife
International Ltd (Proprietary report No. 56835/22/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993b) Diflubenzuron: A 5-day toxicity test
with freshwater alga ( Selenastrum capricornutum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/23/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993c) Diflubenzuron: A 5-day toxicity test
with the freshwater alga ( Anabaena flos-aquae. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/21/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993d) Diflubenzuron: A 5-day toxicity test
with the freshwater diatom ( Navicula pelliculosa. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/24/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993e) Diflubenzuron: A 5-day toxicity test
with the marine diatom ( Skeletonema costatum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/25/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson CZ, Hill LE, Epp JK, & Probst GS (1983) The induction of
bacterial mutation and hepatocyte unscheduled DNA synthesis by
monosubstituted anilines. Environ Mutagen, 5: 803-811.
Thus JLG & van der Laan-Straathof JMTh (1994) Fate of diflubenzuron in
model ditch systems. 'sGraveland, The Netherlands, Solvay Duphar
BV, Environmental Research Department (Proprietary report
No. 56835/55/93).
Thus LG & van der Laan JMT (1993) Nature of residues of diflubenzuron
in soybeans. 'sGraveland, The Netherlands, Solvay Duphar BV,
Environmental Research Department (Proprietary report
No. 56635/57/1992).
Thus JLG & van Dyk NRM (1991) Anaerobic aquatic metabolism of
diflubenzuron -Addendum report. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56635/54/91).
Thus JLG, van Dyk NRM, Rompa-van der Veldt CAH, & Walstra P (1991)
Anaerobic aquatic metabolism of diflubenzuron - Addendum report.
'sGraveland, The Netherlands, Solvay Duphar BV (Proprietary report
No. 56635/34/91).
USDA (1985) Gypsy moth suppression and eradication projects: Final
addendum to the final environmental impact statement as supplemented -
1985. Bromall, Pennsylvania, US Department of Agriculture, Forest
Service (Submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
US NCI (1979) Bioassay of p-chloroaniline for possible
carcinogenicity. Bethesda, Maryland, US National Cancer Institute
(Technical Report Series No. 189, NIH Publication No. 79-1745).
US NTP (1989) Toxicology and carcinogenesis studies of para-
chloroaniline hydrochloride in F344/N rats and B6C3F1 mice (gavage
studies). Research Triangle Park, North Carolina, US National
Toxicology Program (Technical Report Series No. 351).
Van Daalen JJ, Meltzer J, Mulder R, & Wellinga K (1972) A selective
insecticide with a novel mode of action. Naturwissenschaften,
59: 312-313.
Van den Berg G (1986) Dissipation of diflubenzuron residues after
application of dimilin WP-25 in a forestry area in North Carolina and
some ecological effects. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division, 11 pp (Proprietary report No. 56637/47/1986).
Van der Laan-Straathof JMTh & Thus JLG (1993) Determination of the
biodegradability of 14C-diflubenzuron in an adapted modified Sturm
test. Weesp, The Netherlands, Solvay-Duphar (Proprietary research
report No. 56835/41/93).
Van der Laan-Straathof JMTh & Thus JLG (1994) Evaporation of
diflubenzuron from bare soil and red kidney bean leaves after
application of dimilin WSE-80. Weesp, The Netherlands, Solvay-Duphar
(Proprietary research report No. 56835/17/94).
Van Eldik A (1973a) Acute toxicity studies with DU 112307 in mice and
rats. Weesp, The Netherlands, Philips-Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/14/1973).
Van Eldik A (1973b) Acute toxicity studies with DU 112307 25% WP in
mice and rats. Weesp, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Proprietary report No. 56645/15A/1973).
Van Eldik A (1974) Acute toxicity studies with DU 112307 in mice after
intraperitoneal administration. Weesp, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/01/1974).
Van Kampen WG & Joustra KD (1991) Diflubenzuron: residues on apples.
Weesp, The Netherlands, Duphar BV, Analytical Development Department
(Proprietary analytical report No. 56630/46/1990).
Van Rossum A, De Reijke A, & Zeeman J (1984) Diflubenzuron (update).
In: Analytical methods for pesticides and plants growth regulators.
New York, London, Academic Press, vol 13, pp 165-169.
Verhey ME (1991a) Analysis of field dissipation soil samples from
Madera, California for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 019, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verhey ME (1991b) Analysis of field dissipation soil samples from
Woodburn, Oregon, for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 020, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verloop A & Ferrell CD (1977) Benzoylphenyl ureas - a new group of
larvicides interfering with chitin deposition. In: Plimmer JR ed.
Pesticide chemistry in the 20th century. Washington, DC, American
Chemical Society, pp 237-270 (ACS Symposium Series No. 37).
Walstra P & Joustra KD (1990) Aerobic soil metabolism of diflubenzuron
in sandy loam. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division (Proprietary report No. 56635/65/1990).
Wan MTK & Wilson DM (1977) Impact of insect growth regulators on
selected non-target organisms co-existing with mosquito larvae.
Washington, DC, Environmental Protection Service, Pollution Abatement
Branch (Unpublished report No. EPS-5-PR-77-1/NTP81).
Wang CJK (1975) Effects of Dimilin upon microorganisms in leaf litter
and forest soil. In: Evaluation of Dimilin against the Gypsy moth
effects on non-target organisms 1975. Report expanded Gypsy moth
research and applications program, Hamden USA (Unpublished report
No. NTP-18, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Weis JS & Ma A (1987) Effects of the pesticide diflubenzuron on larval
horseshoe crabs, Limulus polyphemus Bull Environ Contam Toxicol,
39: 224-228.
Weis JS & Perlmutter J (1987) Burrowing behaviour by the fiddler crab
Uca pugilator: inhibition by insecticide diflubenzuron. Mar Ecol
Prog Ser, 38: 109-113.
Weis JS, Cohen R, & Kwiatkowski JK (1987) Effects of diflubenzuron on
limb regeneration and molting in the fiddler crab, Uca pugilator
Aquat Toxicol, 10(5-6): 279-290.
White WB (1975) Evaluation of Dimilin against the Gypsy moth and
effects on non-target organisms, 1975 (Compiled by the expanded Gypsy
moth research and applications program). Upper Darby, Pennsylvania, US
Department of Agriculture, Forest Service (Unpublished report).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, p 64 (WHO/PCS/94.2).
Wie SJ & Hammock BD (1982) Development of enzyme-linked immunosorbent
assays for residue analysis of diflubenzuron and Sir 8541. J Agric
Food Chem, 30: 949-957.
Wie SJ & Hammock BD (1984) Comparison of coating and immunizing
antigen structure on the sensitivity and specificity of
immunoassays for benzoylphenylurea insecticides. J Agric Food Chem,
32(6): 1294-1301.
Willems AGM, Joustra KD, & Thus JLG (1977) Stability of diflubenzuron
in aqueous solution during heat sterilization. 'sGraveland, The
Netherlands, Philips-Duphar BV, Crop Protection Division (Proprietary
report No. 56635/35/1977).
Willems AGM, Overmars H, Scherpenisse P, de Lange N, & Post LC (1980)
Diflubenzuron: intestinal absorption and metabolism in the rat.
Xenobiotica, 10(2): 103-112.
Williams GM, Laspia MF, & Dunkel VC (1982) Reliability of the
hepatocyte primary culture/DNA repair test in testing of coded
carcinogens and noncarcinogens. Mutat Res, 97: 359-370.
Wilson JEH & Costlow JD (1986) Comparative toxicity of two Dimilin
formulations to the grass shrimp Palaemonetes pugio Bull Environ
Contam Toxicol, 36: 858-865.
Wilson JEH & Costlow JD (1987) Acute toxicity of diflubenzuron (DFB)
to various life stages of the grass shrimp, Palaemonetes pugio Water
Air Soil Pollut, 33: 411-417.
Wilson JEH, Forward RB, & Costlow JD (1985) Effects of embryonic
exposure to sublethal concentrations of Dimilin on the photobehavior
of grass shrimp larvae. In: Marine pollution and physiology: Recent
advances. Colombia, South Carolina, University of South Carolina
Press, pp 377-396.
Wimmer MJ, Smith RR, & Jones JP (1991) Analysis of diflubenzuron by
gas chromatography/mass spectrometry using deuterated diflubenzuron as
internal standard. J Agric Food Chem, 39: 280-286.
Worobey BL & Webster GRB (1977) Gas-liquid chromatographic
determination of diflubenzuron (Dimilin) in water as its
trifluoroacetyl derivative. J Assoc Off Anal Chem, 60(1): 213-217.
Worthing CR & Walker SB (1987) The pesticide manual: A world
compendium. Croydon, British Crop Protection Council, pp 4720-4721.
Yasuno M & Satake K (1990) Effects of diflubenzuron and methoprene on
the emergence of insects and their density in an outdoor experimental
stream. Chemosphere, 21(10-11): 1321-1335.
Young MF, Trombetta LD, & Carson S (1986) Effects of diflubenzuron on
the mouse liver. J Appl Toxicol, 6(5): 343-348.
Yu SJ, Robinson FA, & Nation JL (1984) Detoxication capacity in the
honey bee, Apis mellifera L. Pestic Biochem Physiol, 22: 360-368.
Zwart A (1985) Acute inhalation toxicity study of dimilin 2F,
diflubenzuron in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. V85.347/250925, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le diflubenzuron appartient au groupe des insecticides dérivés de
la benzoylphénylurée. Son activité insecticide est due à une
interaction avec la synthèse et le dépôt de la chitine. Il forme des
cristaux blancs inodores dont le point de fusion est de 230-232°C.
Il est légèrement soluble dans l'eau (0,2 mg/litre à 20°C)
et pratiquement non volatil. Il est relativement stable en milieu
acide ou neutre mais s'hydrolyse en milieu alcalin.
Le diflubenzuron s'obtient par réaction du 2,6-difluoro-
benzamide sur l'isocyanate de 4-chlorophényle.
Le dosage des résidus de diflubenzuron présents dans l'eau, les
échantillons biologiques,et le sol peut s'effectuer par
chromatographie liquide à haute performance avec détection par UV ou
encore par chromatographie en phase gazeuse avec détection par capture
d'électrons, soit directement sur la molécule initiale, soit sur un
dérivé (libération de 4-chloroaniline et action de l'anhydride
trifluoracétique).
2. Sources d'exposition humaine et environnementale
Le diflubenzuron est un produit de synthèse utilisé en
agriculture, en foresterie et dans les programmes de santé publique
pour détruire les ravageurs et les vecteurs de maladies. Différentes
formulations existent à cet usage. On ne possède pas de
renseignements au sujet de cas d'exposition humaine au diflubenzuron
qui auraient pu se produire.
3. Transport, distribution et transformation dans l'environnement
En général, le diflubenzuron est appliqué directement sur les
végétaux et les eaux à traiter. Le feuillage ne constitue pas une
porte d'entrée dans la plante.
Le diflubenzuron est rapidement adsorbé sur les particules du
sol. Il reste fixé dans les 10 premiers cm du sol sur lequel il est
épandu. Il est peu probable qu'il subisse un lessivage. Dans divers
types de sol, il subit une décomposition aérobie ou anaérobie avec une
demi-vie de quelques jours. La vitesse de décomposition dépend
largement de la taille des particules de diflubenzuron. La principale
voie métabolique (plus de 90%) esst l'hydrolyse qui conduit à la
formation d'acide 2,6-difluorobenzoïque et de 4-chlorophénylurée; ces
deux composés sont dégradés à leur tour, respectivement en 4 et 6
semaines. On n'a pas décelé la présence de 4-chloroaniline libre
dans le sol.
Le diflubenzuron se décompose rapidement dans les eaux neutres ou
alcalines. On constate qu'une fois épandu sur l'eau, il se répartit
rapidement entre celle-ci et les sédiments.Le composé initial et la
4-chlorophénylurée peuvent persister plus de 30 jours dans les
sédiments.
Le diflubenzuron ne s'accumule pas chez les poissons.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation en agriculture, en foresterie ou pour la
démoustication n'entraîne qu'une exposition négligeable de la
population générale par l'intermédiaire de la nourriture ou de l'eau.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez l'animal de laboratoire, le diflubenzuron est absorbé au
niveau des voies digestives et, à un moindre degré, au niveau cutané.
Chez le rat, il existe un mécanisme d'absorption saturable. Dans ces
conditions, une forte proportion du diflubenzuron administré par voie
orale se retrouve dans les matières fécales. Le diflubenzuron se
répartit largement dans les tissus, mais il ne s'y accumule pas.
Le métabolisme du diflubenzuron a été étudié chez diverses
espèces animales. Chez les mammifères, la principale voie métabolique
comporte une hydroxylation. L'hydrolyse peut se produire au niveau de
l'une quelconque des trois liaisons carbone-azote. Chez le porc et le
poulet, l'hydrolyse s'effectue principalement au niveau du pont
uréido. Chez le rat et la vache, l'hydroxylation constitue la
principale voie métabolique. Chez le mouton, le porc et le poulet,
les principaux métabolites sont le 2,6-difluorobenzamide et la
4-chlorophénylurée; on trouve aussi, en moindre proportion, du
2,6-difluorobenzamide et de la 4-chloroaniline. Chez le rat et les
bovins, 80% des métabolites sont constitués de 2,6-difluoro-3-
hydroxydiflubenzuron, de 4-chloro-2-hydroxydifluorobenzuron et de
4-chloro-3-hydroxydiflubenzuron. Les études de métabolisme indiquent
qu'il ne se forme pratiquement pas de 4-chloroaniline chez le rat et
les bovins.
Chez les chats, les porcs et les bovins, l'élimination
s'effectue principalement par la voie fécale, à hauteur de 70 à 85%.
Chez les ovins, la voie urinaire et la voie fécale ont à peu près la
même importance de ce point de vue. Chez le rat et la souris,
l'excrétion urinaire décroît proportionnellement à l'augmentation de
la dose. Moins de 1% de la dose administrée par la voie orale se
retrouve dans l'air expiré. Le diflubenzuron n'est présent qu'à
l'état de résidus dans le lait.
Il n'existe pas d'étude sur la cinétique et le métabolisme du
diflubenzuron chez l'homme et notamment, sur son degré de
biotransformation en 4-chloroaniline.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Quel que soit le mode d'exposition, le diflubenzuron présente une
faible toxicité aiguë. En se basant sur le fait que sa DL50 aiguë
par voie orale est supérieure à 4640 mg/kg de poids corporel chez le
rat, l'OMS estime qu'il s'agit d'un produit qui ne présente
vraisemblablement pas de risque d'intoxication aiguë en utilisation
normale. Chez ce même animal, la DL50 aiguë par voie percutanée est
supérieure à 10 000 mg/kg de poids corporel et la CL50 dépasse 2,49
mg/litre par la voie respiratoire. Au cours d'une période de deux
semaines pendant laquelle diverses espèces animales avaient reçu du
diflubenzuron en une seule prise et selon divers modes
d'administration, on n'a constaté aucun signe d'intoxication.
Le diflubenzuron n'est pas irritant pour la peau (chez le lapin)
et ne provoque pas non plus de sensibilisation cutanée (chez le
cobaye). Il est légèrement irritant pour la muqueuse oculaire chez le
lapin.
Le diflubenzuron provoque une méthémoglobinémie et une
sulfhémoglobinémie. Une méthémoglobinémie liée à la dose a été mise
en évidence après exposition d'animaux de diverses espèces au
diflubenzuron par la voie orale, percutanée ou respiratoire. Cet
effet constitue le point d'aboutissement toxicologique le plus
sensible chez les animaux de laboratoire. En prenant comme critère la
méthémoglobinémie, la dose sans effet observable est de 2 mg/kg de
poids corporel par jour chez les rats et les chiens et de
2,4 mg/kg de poids corporel par jour chez les souris. Les études de
toxicité à long terme effectuées sur des souris et des rats
ont montré que les modifications imputables au traitement
correspondaient principalement à l'oxydation de l'hémoglobine et à une
altération des hépatocytes.
Des études de cancérogénicité effectuées sur des rats et des
souris à des doses allant jusqu'à 10 000 mg/kg de nourriture, n'ont
pas révélé de modification de l'incidence tumorale qui soit imputable
au traitement. En particulier, on n'a pas observé de néoplasmes au
niveau du mésenchyme splénique ou hépatique lors d'études de
cancérogénicité utilisant de la 4-chloroaniline.
Plusieurs études toxicologiques portant sur la fonction de
reproduction ont été menées sur des rats, des souris, des lapins et
trois espèces d'oiseaux; elles n'ont pas mis en évidence d'effets
pathogènes et le produit ne s'est pas révélé embryotoxique. Les
études de tératogénicité effectuées sur des rats et des lapins se sont
également révélées négatives.
Le diflubenzuron et ses principaux métabolites ont également été
soumis à diverses épreuves de mutagénicité in vivo e in vitro
Ni le diflubenzuron ni ses principaux métabolites n'ont donné de
résultat positif dans ces épreuves.
Le métabolite secondaire, c'est-à-dire la 4-chloroaniline,
a donné des résultats positifs dans plusieurs épreuves de mutagénicité
in vitro portant sur divers points d'aboutissement toxicologiques.
Il est cancérogène pour le rat et la souris. Les tumeurs imputables à
l'administration de 4-chloroaniline se sont révélées bénignes; quant
aux tumeurs malignes observées, il s'agissait de tumeurs du mésenchyme
splénique chez les rats mâles ainsi que d'hémangiomes et
d'hémangiosarcomes spléniques ou hépatiques chez les souris mâles.
7. Effets sur l'homme
On a signalé des cas de méthémoglobinémie chez des travailleurs
exposés de par leur profession et des nouveau-nés exposés par
inadvertance à de la 4-chloroaniline, un métabolite secondaire du
diflubenzuron. Les sujets qui présentent un déficit en NADH-
méthémoglobine-réductase peuvent être particulièrement sensibles à la
4-chloroaniline et par conséquent à une exposition au diflubenzuron.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Tous les organismes qui synthétisent la chitine sont sensibles au
diflubenzuron.
A la concentration de 500 mg/kg de terre, les bactéries n'ont pas
souffert d'une exposition au diflubenzuron. Il y a eu une certaine
stimulation de la fixation d'azote. Les bactéries décomposent les
solutions de diflubenzuron dans l'acétone, solvant qu'elles utilisent
comme source de carbone. Une concentration de diflubenzuron de 1
µg/litre a provoqué un accroissement de la biomasse algaire. Aucun
effet nocif n'a été observé à des concentration supérieures à la
limite de solubilité du diflubenzuron. Des champignons placés dans un
courant créé en laboratoire ont été temporairement affectés à la
concentration de 0,1 µg/litre.
Les invertébrés aquatiques présentent des réactions variées au
diflubenzuron. Les mollusques n'y sont pas sensibles, avec une CL50
supérieure à 200 mg/litre. Chez les autres invertébrés, la CL50 peut
aller de 1 à > 1000 µg/litre, ce qui peut refléter la sensibilité de
ces organismes au moment de la mue. On estime que pour la daphnie, la
concentration tolérable maximale en produit toxique est supérieure à
40 ng/litre et inférieure à 93 ng/litre. Comme prévu, les larves
d'éphémères et autres formes pré-imaginales d'insectes divers, sont
très sesnsibles au diflubenzuron. Le traitement des eaux de surface
par le diflubenzuron est donc probablement susceptible de causer une
certaine mortalité parmi les insectes aquatiques.
Lors de traitements expérimentaux effectués sur le terrain et
dans divers écosystèmes, on a constaté que la plupart des organismes
avaient moins souffert que ne le laissaient prévoir les études
toxicologiques en laboratoire. Aucun effet n'a été constaté sur les
organismes aquatiques après traitement de forêts par voie aérienne.
Pour les poissons, la CL50 est supérieure à 150 mg/litre. Les
essais effectués sur le terrain n'ont enregistré aucune mortalité chez
les poissons.
La DL50 par voie orale et par contact est supérieure à 30 µg par
insecte chez l'abeille mellifique. Après épandage de diflubenzuron
par voie aérienne à raison de 350 g/ha, on n'a pas constaté de dommage
parmi les colonies d'abeilles des alentours.
Une étude alimentaire de 5 jours sur des colverts et des
gallinacés du genre colin avec des doses allant jusqu'à 4640 mg/kg de
nourriture, n'a pas révélé de signes de toxicité. Après épandage de
diflubenzuron par voie aérienne sur des forêts à raison de 350 g/ha,
on n'a pas constaté de dommages parmi les oiseaux chanteurs de
l'écosystème forestier.
Après épandage de diflubenzuron à raison de 67 g/ha sur une
forêt, on n'a pas observé de réduction dans l'effectif des populations
de petits mammifères.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El diflubenzurón pertenece al grupo de los insecticidas
derivados de la benzoilfenilurea. Su acción insecticida se debe
a la interacción con la síntesis y/o deposición de quitina. Forma
cristales blancos inodoros con un punto de fusión de 230-232°C. Es
bastante soluble en agua (0,2 mg/litro a 20°C) y prácticamente
involátil. Es relativamente estable en medios ácidos y neutros, pero
se hidroliza en condiciones alcalinas.
El diflubenzurón se produce haciendo reaccionar 2,6-difluoro-
benzamida con 4-clorofenilisocianato.
Los residuos de diflubenzurón pueden medirse en el agua, en
muestras biológicas y en el suelo mediante cromatografía líquida de
alta resolución con detección de radiación ultravioleta o mediante
cromatografía de gases con detector de captura de electrones para el
análisis de la molécula intacta o tras la derivatización de la
4-cloroanilina liberada con anhídrido trifluoracético.
2. Fuentes de exposición humana y ambiental
El diflubenzurón es un compuesto sintético utilizado en la
agricultura, en la silvicultura y en programas de salud pública para
combatir plagas de insectos y vectores. Para esos usos existen
diferentes formulaciones de diflubenzurón. No se dispone de
información pertinente sobre la exposición humana a este producto.
3. Transporte, distribución y transformación en el medio ambiente
El diflubenzurón suele aplicarse directamente a las plantas y al
agua. No se produce absorción a través de las hojas.
La adsorción del diflubenzurón en el suelo es rápida. El
producto se inmoviliza en la capa superior de 10 cm del suelo al que
se aplica, y las probabilidades de lixiviación son escasas. El
diflubenzurón se degrada en suelos de diversos tipos y orígenes en
condiciones aerobias o anaerobias, con una semivida de pocos días. La
velocidad de degradación depende en gran medida del tamaño de las
partículas de diflubenzurón. La principal ruta metabólica (más del
90%) es la hidrólisis, que produce 2,6-ácido difluorobenzoico y
4-clorofenilurea; estos productos se degradan con semividas del orden
de cuatro y seis semanas, respectivamente. No se ha detectado
4-cloroanilina libre en los suelos.
El diflubenzurón se degrada rápidamente en aguas neutras o
alcalinas. Estudios de aplicación al agua revelan que el
diflubenzurón se concentra rápidamente en el sedimento; el compuesto
de origen y la 4-clorofenilurea pueden persistir en el sedimento por
más de 30 días.
El diflubenzurón no es objeto de bioacumulación en los peces.
4. Niveles ambientales y exposición humana
La exposición de la población general al diflubenzurón por medio
del agua o los alimentos de resultas de su utilización en la
agricultura, contra insectos forestales o en la lucha contra los
mosquitos, es insignificante.
5. Cinética y metabolismo en animales de laboratorio
En animales de experimentación, el diflubenzurón se absorbe en el
tubo digestivo y, en menor medida, a través de la piel. En el tubo
digestivo de la rata existe un mecanismo de absorción saturable, por
lo que una gran proporción del diflubenzurón administrado oralmente
aparece en las heces. El diflubenzurón tiene una amplia distribución
en los tejidos, pero no se acumula.
El destino metabólico del diflubenzurón ha sido estudiado en
diversas especies. La principal vía metabólica en los mamíferos es la
hidroxilación. La hidrólisis del diflubenzurón puede producirse en
cualquiera de los tres enlaces carbono-nitrógeno. En los cerdos y los
pollos, la principal ruta de hidrólisis es el puente ureido. En las
ratas y las vacas, la principal vía metabólica es la hidroxilación.
En las ovejas, los cerdos y los pollos, los metabolitos más
importantes son el 2,6-ácido difluorobenzoico y la 4-clorofenilurea;
los metabolitos secundarios son la 2,6-difluorobenzamida y la
4-cloroanilina. En las ratas y el ganado vacuno, el 80% de los
metabolitos está constituido por 2,6-difluoro-3-hidroxidiflubenzurón,
4-cloro-2-hidroxi-diflubenzurón y 4-cloro-3-hidroxidiflubenzurón. Los
estudios metabólicos indican que en las ratas o el ganado vacuno se
forman cantidades mínimas o nulas de 4-cloroanilina.
La principal ruta de eliminación es a través de las heces, con
porcentajes de entre el 70 y el 85% en los gatos, los cerdos y el
ganado vacuno. En el ganado ovino, la eliminación se distribuye
aproximadamente por igual entre la orina y las heces. En las ratas y
ratones, la excreción urinaria disminuye proporcionalmente al aumento
de la dosis. Menos del 1% de una dosis oral se recupera en el aire
exhalado. En la leche sólo se han hallado residuos ínfimos.
No se dispone de ningún estudio humano de la cinética y el
metabolismo del diflubenzurón, incluido el alcance de la
biotransformación en 4-cloroanilina.
6. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
El diflubenzurón tiene una toxicidad aguda baja por cualquier vía
de exposición. La OMS lo ha clasificado como producto con pocas
probabilidades de presentar un riesgo agudo en el uso normal, sobre la
base de una DL50 aguda por vía oral de más de 4640 mg/kg de peso
corporal en las ratas. La DL50 aguda por vía cutánea en las ratas es
superior a 10 000 mg/kg de peso corporal, mientras que la CL50 aguda
por inhalación en las ratas excede de 2,49 mg/litro. No se han
observado signos de intoxicación en los 14 días siguientes a una
administración única de diflubenzurón por diversas rutas a una
variedad de especies animales.
El diflubenzurón no provoca irritación cutánea (en el conejo) ni
sensibilización de la piel (en el cobayo). Produce una ligera
irritación a los ojos en el conejo.
El diflubenzurón causa metahemoglobinemia y sulfohemoglobinemia.
Tras la exposición oral, cutánea o por inhalación de diversas especies
al diflubenzurón se ha demostrado la presencia de metahemoglobinemia
dosisdependiente. Este efecto es la variable de evaluación
toxicológica más sensible en los animales de experimentación. El NOEL
basado en la formación de metahemoglobina es de 2 mg/kg de peso
corporal por día en las ratas y los perros, y de 2,4 mg/kg de peso
corporal por día en los ratones. En estudios de toxicidad a largo
plazo realizados con ratones y ratas, los cambios relacionados con el
tratamiento se han asociado principalmente a la oxidación de la
hemoglobina o a alteraciones de los hepatocitos.
En estudios de la carcinogenicidad en ratones y ratas con niveles
de hasta 10 000 mg/kg en la alimentación, no se observaron efectos
relacionados con el tratamiento en la incidencia de tumores.
Específicamente, no se registraron neoplasias mesenquimatosas del bazo
o el hígado como las observadas en los estudios de carcinogenicidad
con 4-cloroanilina.
En varios estudios de la toxicidad reproductiva en ratas,
ratones, conejos y tres especies aviarias no se observó ningún efecto
en la reproducción, ni tampoco embriotoxicidad. Los estudios de
teratogenicidad en ratas y conejos no revelaron ningún efecto
teratogénico.
El diflubenzurón y sus principales metabolitos han sido sometidos
a una serie de ensayos de mutagenicidad in vitro e in vivo Ni el
diflubenzurón ni sus principales metabolitos tienen efecto mutagénico.
El metabolito secundario 4-cloroanilina ha dado un resultado
positivo en varios ensayos de mutagenicidad in vitro con diversas
variables de valoración. Es carcinógeno en las ratas y los ratones.
Las lesiones neoplásicas relacionadas con la administración de
4-cloroanilina fueron tumores mesenquimatosos benignos y malignos en
el bazo de ratas macho, y hemangiomas y hemangiosarcomas,
principalmente en el bazo y el hígado de ratones macho.
7. Efectos en el ser humano
Se ha notificado que el metabolito 4-cloroanilina del
diflubenzurón ha causado metahemoglobinemia en trabajadores sometidos
a exposición y en neonatos expuestos por inadvertencia. Algunas
personas con carencia de NADH metahemoglobina reductasa pueden ser
particularmente sensibles a la 4-cloroanilina y, por lo tanto, a la
exposición al diflubenzurón.
8. Efectos sobre otros organismos en el laboratorio y en el terreno
Todos los organismos que sintetizan quitina presentan sensibilidad
al diflubenzurón.
Las bacterias no resultaron afectadas por el diflubenzurón a
concentraciones de 500 mg/kg de suelo; se observó cierta estimulación
de la fijación de nitrógeno. Las soluciones de diflubenzurón-acetona
se degradaron; la acetona se utilizó como fuente de carbono. La
biomasa de algas aumentó a una concentración de diflubenzurón de
1 µg/litro. No se observaron efectos adversos a concentraciones
superiores al límite de solubilidad del diflubenzurón. Los hongos
resultaron temporalmente afectados a 0,1 µg/litro en condiciones de
laboratorio.
Los invertebrados acuáticos presentan respuestas variables al
diflubenzurón. Los moluscos son insensibles, con una CL50 superior a
200 mg/litro. Los valores de la CL50 de otros invertebrados van
desde 1 hasta más de 1000 µg/litro, en función de los efectos del
compuesto en las fases juvenil y de muda. Para Daphnia se ha
estimado una MATC de > 40 y < 93 ng/litro; como era de prever, las
larvas de mosca de mayo y otros insectos acuáticos juveniles son
sumamente sensibles. El rociamiento de masas de agua matará
probablemente algunos invertebrados acuáticos.
En los ecosistemas y experimentos de campo en que se aplicó
diflubenzurón directamente al agua, los efectos en la mayoría de los
grupos taxonómicos fueron menos graves de lo previsto a partir de las
pruebas de laboratorio sobre efectos agudos. No se han observado
efectos en los organismos acuáticos después de aplicaciones aéreas a
los bosques.
Los valores de la CL50 para los peces son de > 150 mg/litro.
En los experimentos prácticos no se ha registrado nunca la muerte de
peces.
La DL50 oral y por contacto en la abeja de miel es superior a
30 µg/individuo. Las colonias de abejas no resultaron afectadas tras
la aplicación aérea de 350 g de diflubenzurón/hectárea.
Un estudio de alimentación de cinco días de duración en patos
silvestres y codornices con niveles de hasta 4640 mg/kg de pienso no
reveló ningún signo observable de toxicidad. Las pequeñas aves
canoras del ecosistema forestal no resultaron afectadas por la
aplicación aérea de diflubenzurón a razón de 350 g/hectárea.
Las especies mamíferas pequeñas no sufrieron mermas de las
poblaciones tras la aplicación de diflubenzurón en un bosque a razón
de 67 g/hectárea.
 additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme,
Châtelaine, Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
The Core Assessment Group (CAG) of the Joint Meeting on Pesticide
Residues met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel of the WHO welcomed the participants on behalf of WHO,
and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to diflubenzuron.
The first draft of the monograph was prepared by Dr M. Tasheva,
Sofia, Bulgaria. The second draft, incorporating comments received
following circulation of the first draft to the IPCS contact points
for Environmental Health Criteria monographs, was prepared by the IPCS
Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that Solvay-Duphar, BV, made available to the IPCS its
proprietary toxicological information on diflubenzuron is gratefully
acknowledged. This allowed the CAG to make its evaluation on a more
complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ADI acceptable daily intake
a.i. active ingredient
AP alkaline phosphatase
bw body weight
4-CPU 4-chlorophenylurea
DFB diflubenzuron
2,6-DFBA 2,6-difluorobenzoic acid
ECD electron capture detection
G granular formulation
GC gas chromatography
GLC gas-liquid chromatography
Hb haemoglobin
HPLC high performance liquid chromatography
MATC maximum acceptable toxicant concentration
MCH mean cell haemoglobin
MCHC mean cell haemoglobin concentration
MCV mean cell volume
NOAEC no-observed-adverse-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus detector
PCA para-chloroaniline (4-chloroaniline)
PCV packed cell volume
SAP serum alkaline phosphatase
SGOT serum glutamic-oxaloacetic transaminase (aspartate
aminotransferase)
SGPT serum glutamic-pyruvic transaminase (alanine
aminotransferase)
TLC thin-layer chromatography
WP wettable powder
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Diflubenzuron is a member of the benzoylphenylurea group of
insecticides. Its insecticidal action is due to interaction with
chitin synthesis and/or deposition. It forms odourless white crystals
with a melting point of 230-232°C. It is sparingly soluble in water
(0.2 mg/litre at 20°C) and is virtually non-volatile. It is
relatively stable in acidic and neutral media but it hydrolyses in
alkaline conditions.
Diflubenzuron is produced by the reaction of 2,6-difluoro-
benzamide with 4-chlorophenylisocyanate.
Diflubenzuron residues may be measured in water, biological
samples and soils by HPLC with UV detection or by GC with ECD for
analysis of the intact molecule or following derivatization of the
liberated 4-chloroaniline with trifluoroacetic anhydride.
1.1.2 Sources of human and environmental exposure
Diflubenzuron is a synthetic compound used in agriculture,
forestry and public health programmes to control insect pests and
vectors. Different formulations of diflubenzuron are available for
these uses. There is no relevant information on human exposure to
diflubenzuron.
1.1.3 Environmental transport, distribution and transformation
Diflubenzuron is usually applied directly to plants and water.
Uptake of diflubenzuron through plant leaves does not occur.
The adsorption of diflubenzuron to soil is rapid. It is
immobilized in the top 10 cm layer of soil to which it is applied. It
is unlikely to leach. Diflubenzuron is degraded in soils of various
types and origin under aerobic or anaerobic conditions with a half-
life of a few days. The rate of degradation depends greatly on the
diflubenzuron particle size. The main metabolic pathway (over 90%) is
hydrolysis leading to 2,6-difluorobenzoic acid and 4-chlorophenylurea;
these are degraded with half-lives of about 4 and 6 weeks,
respectively. Free 4-chloroaniline has not been detected in soils.
Diflubenzuron degrades rapidly in neutral or alkaline waters.
Studies of application of diflubenzuron to water show rapid partition
to sediment; the parent compound and 4-chlorophenylurea may persist on
sediment for more than 30 days.
Diflubenzuron does not bioaccumulate in fish.
1.1.4 Environmental levels and human exposure
Exposure of the general population to diflubenzuron via water or
food as a result of its use in agriculture, against forest insects or
in mosquito control is negligible.
1.1.5 Kinetics and metabolism in laboratory animals
In experimental animals, diflubenzuron is absorbed from the
digestive tract and to a lesser extent through the skin. There is a
saturable absorption mechanism in the rat gastrointestinal tract.
Consequently a large proportion of orally administered diflubenzuron
is found in the faeces. Diflubenzuron has widespread distribution in
the tissues, but it does not accumulate.
The metabolic fate of diflubenzuron has been studied in various
species. The major route of metabolism in mammals is via
hydroxylation. Hydrolysis of diflubenzuron may occur at any of the
three carbon-nitrogen bonds. In pigs and chickens the major route of
hydrolysis is at the ureido bridge. In rats and cows the major
metabolic pathway is hydroxylation. The major metabolites in sheep,
swine and chickens are 2,6-difluorobenzoic acid and 4-chloro-
phenylurea; minor metabolites are 2,6-difluorobenzamide and
4-chloroaniline. In rats and cattle 80% of the metabolites are
2,6-difluoro-3-hydroxydiflubenzuron, 4-chloro-2-hydroxy-diflubenzuron
and 4-chloro-3-hydroxydiflubenzuron. The metabolic studies indicate
that little or no 4-chloroaniline is formed in rats or cattle.
The major route of elimination is via the faeces, ranging from 70
to 85% in cats, pigs and cattle. In sheep elimination is roughly
equally distributed between the urine and faeces. Urinary excretion
in rats and mice decreases proportionally with increasing dosage
level. Less than 1% of an oral dose is recovered in exhaled air.
Only trace residues are found in milk.
No human studies on the kinetics and metabolism of diflubenzuron,
including the extent of biotransformation to 4-chloroaniline, are
available.
1.1.6 Effects on laboratory mammals and in vitro test systems
Diflubenzuron has low acute toxicity by any route of exposure.
It has been classified by WHO as a "product unlikely to present an
acute hazard in normal use", based on an acute oral LD50 of more than
4640 mg/kg body weight in rats. The acute dermal LD50 in rats is
greater than 10 000 mg/kg body weight while the acute inhalational
LC50 for rats is greater than 2.49 mg/litre. No signs of
intoxication have been observed during the 14-day period following
single administration of diflubenzuron by various routes to a variety
of animal species.
Diflubenzuron is not a skin irritant (in rabbits) and not a skin
sensitizer (in guinea-pigs). It is marginally irritating to the eyes
of rabbits.
Diflubenzuron causes methaemoglobinaemia and sulfhaemo-
globinaemia. Dose-related methaemoglobinaemia has been demonstrated
after oral, dermal or inhalatory exposure to diflubenzuron in various
species. This effect is the most sensitive toxicological end-point in
experimental animals. The NOEL based on methaemoglobin formation is
2 mg/kg body weight per day in rats and dogs and 2.4 mg/kg body weight
per day in mice. In long-term toxicity studies with mice and rats,
treatment-related changes were principally associated with oxidation
of haemoglobin or with hepatocyte changes.
In carcinogenicity studies in mice and rats at dietary levels up
to 10 000 mg/kg in the diet, there were no treatment-related effects
on tumour incidence. Specifically, there were no mesenchymal
neoplasms of the spleen or liver as observed in carcinogenicity
studies with 4-chloroaniline.
In several reproductive toxicity studies on rats, mice, rabbits
and three avian species, no effects were seen on reproduction and
there was no embryotoxicity. Teratogenicity studies in rats and
rabbits demonstrated no teratogenic effects.
Diflubenzuron and its main metabolites have been examined in a
variety of in vitro and in vivo mutagenicity tests. Neither
diflubenzuron nor its major metabolites have a mutagenic effect.
The minor metabolite, 4-chloroaniline, was shown to be positive
in several in vitro mutagenicity assays using various end-points.
It is carcinogenic in rats and mice. The neoplastic lesions related
to administration of 4-chloroaniline were benign and malignant
mesenchymal tumours in the spleens of male rats and haemangiomas and
haemangiosarcomas, primarily in the spleen and liver of male mice.
1.1.7 Effects on humans
The diflubenzuron metabolite, 4-chloroaniline, has been reported
to cause methaemoglobinaemia in exposed workers and in neonates
inadvertently exposed. Some individuals who are deficient in
NADH-methaemoglobin reductase may be particularly sensitive to
4-chloroaniline and hence to diflubenzuron exposure.
1.1.8 Effects on other organisms in the laboratory and field
All chitin-synthesizing organisms show susceptibility to
diflubenzuron.
Bacteria were not affected by diflubenzuron at concentrations of
500 mg/kg soil; some stimulation of nitrogen fixation was seen.
Diflubenzuron acetone solutions were degraded; the acetone was used
as carbon source. Algal biomass increased at a diflubenzuron
concentration of 1 µg/litre. There were no adverse effects at
concentration above the limit of diflubenzuron solubility. Fungi were
temporarily affected at 0.1 µg/litre in laboratory streams.
Aquatic invertebrates show variable responses to diflubenzuron.
Molluscs are insensitive, the LC50 being greater than 200 mg/litre.
LC50 values for other invertebrates ranged from 1 to > 1000
µg/litre, reflecting the effects of the compound on juvenile,
moulting stages. A MATC for Daphnia has been estimated at > 40 and
< 93 ng/litre; as expected, larval mayflies and other aquatic insect
juveniles are highly susceptible. Overspray of water bodies would be
expected to kill some aquatic invertebrates.
In ecosystems and field experiments where diflubenzuron was
applied directly to the water, the effects on most taxa were less
severe than predictions from acute laboratory tests. No effects on
aquatic organisms have been found after aerial applications to
forests.
The LC50 values for fish are > 150 mg/litre. In field
experiments, fish kills have never been recorded.
The oral and contact LD50 for honey-bees is greater than
30 µg/bee. Honey-bee colonies were not affected after aerial
application of 350 g diflubenzuron/ha.
A 5-day dietary study on the mallard duck and bobwhite quail with
levels of up to 4640 mg/kg feed revealed no observable signs of
toxicity. Small songbirds in the forest ecosystem were not affected
after aerial application of diflubenzuron at 350 g/ha.
Small mammal species showed no reductions in numbers after
application of diflubenzuron at 67 g/ha to a forest.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The primary manifestation of diflubenzuron toxicity is
methaemoglobin induction. This toxicity occurs in a range of test
animal species. It is attributable to the metabolite, 4-chloroaniline,
which is known to induce methaemoglobin formation in several animal
species and in humans.
Diflubenzuron does not cause other toxicities on chronic dietary
administration. It is not mutagenic or carcinogenic in mice or rats.
However, its metabolite, 4-chloroaniline, is mutagenic in vitro and
is carcinogenic in mice and male rats. Although 4-chloroaniline is a
minor urinary metabolite of diflubenzuron in rats, the extent to which
it is formed in vivo in various animal species remains unknown.
Similarly, the comparative degree of absorption of its parent compound
in various species is unknown.
The sensitivity of human haemoglobin to methaemoglobin formation
by 4-chloroaniline in vivo is not known. However, since induction
of methaemoglobinaemia is consistently the most sensitive measure of
diflubenzuron toxicity in the various animal species tested, it may be
used as the basis to estimate the levels causing no toxicological
effect.
1.2.2 Evaluation of effects on the environment
Diflubenzuron adsorbs readily to soil with little subsequent
desorption. Its mobility in soil is very low, practically all
residues remaining within 15 cm of the top, even in sandy loam soils;
diflubenzuron does not leach. It is only partly removed from foliage
by heavy rainfall. Nevertheless, some diflubenzuron may be present in
surface water shortly after application, due to flooding of treatment
areas or agricultural run-off.
Dissipation of diflubenzuron from water is rapid. Adsorption to
sediment occurs within 4 days; both parent compound and 4-chloro-
phenylurea metabolite may persist on sediment for at least 30 days.
Uptake of diflubenzuron by plants through the leaves after aerial
application does not occur. Some uptake of soil residues does occur
in plants and this may be translocated. At the highest application
rate (1 kg a.i./ha), following 1 month ageing of residues, up to
1 mg/kg residue may be found in various crops.
Photolysis of diflubenzuron is slow with a calculated half-life
of 40 days. Under environmental conditions abiotic degradation in
water and soil represents a minimum route of break-down. Aerobic
degradation in water is a microbial process with a half-life of a few
days under both laboratory and field conditions. In the field,
degradation of diflubenzuron applied at practical rates is influenced
by pH, temperature, formulation, organic matter content and depth of
the water.
Degradation in soil through microbial hydrolysis is a rapid
process, with a half-life of a few days, depending on diflubenzuron
particle size. The major break-down products are 2,6-difluorobenzoic
acid and 4-chlorophenylurea; a minor metabolite is parachloroaniline.
All these are irreversibly bound to soil and/or further metabolized.
The half-life of diflubenzuron residues on citrus fruits is
significantly decreased by high temperature and humidity.
Anaerobic degradation in water and sediment is slower than
aerobic.
Fish bioconcentrate diflubenzuron and some bioaccumulation takes
place during extended exposure up to a plateau, depending on the water
concentration, owing to fast degradation of diflubenzuron and
excretion of metabolites; the depuration half-life is less than one
day. The 4-chloroaniline metabolite has not been detected in fish.
Fish are not sensitive to diflubenzuron, the LC50 values being
> 150 mg/litre. Metabolites of diflubenzuron are also of low
toxicity to fish. Chronic exposure has shown no effects on fish at
recommended application rates; the compound does not persist in water
and no chronic exposure is expected.
Diflubenzuron is not phytotoxic to duckweed at the diflubenzuron
solubility limit concentration.
Honey-bees were not affected by topical applications of
> 30 µg/bee or dietary concentrations of up to 1000 mg/kg diet.
Brood in hives was reduced when bees were fed syrup at 59 mg
diflubenzuron/kg. Brood was also reduced following exposure of
flying colonies.
Earthworms were not affected at a concentration of 780 mg/kg
soil, which is at least three orders of magnitude above reported soil
residues.
Diflubenzuron has low acute toxicity to birds, the oral and
dietary LD(LC)50 values being greater than 3000 mg/kg diet.
Following recommended application rates diflubenzuron is not expected
to pose a hazard to birds.
Extensive field studies have shown minimal or reversible effects
on most aquatic invertebrates; daphnids were most seriously affected,
with short-term reductions in populations of up to 75% following a
single application of diflubenzuron. Fish were not affected by water
overspraying. Neither bird nor mammal populations were adversely
affected following forest spraying with diflubenzuron.
A summary of risk quotients for birds, fish and aquatic
invertebrates is given in Table 1.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on diflubenzuron of relevance for
setting guidance values are shown in Table 2.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based on
application rates of 2.5 kg a.i./ha of diflubenzuron to soybeans (worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 3762 73.7-535.7 51.0-7.0
Acute fish (stream) 150 0.0007 214 300
Acute fish (pond) 150 0.01 15 000
Acute aquatic
invertebrate (stream) 0.005 0.0007 7.1
Acute aquatic
invertebrate (pond) 0.005 0.01 0.5
a Estimated environmental concentration in the terrestrial environment (for bird
exposure) is based on the stated application rate and the assumption of
deposition on short grass using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a
single application and estimated runoff into water; no direct overspray is
included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below
1.0 indicate likely exposure to toxic concentrations by organisms in the
different risk categories.
Table 2. Toxicological criteria for estimating guidance values for diflubenzuron
Exposure scenario Relevant route/effect/ Result/remarks
(technical species
diflubenzuron)
Short-term dermal, irritation, rabbit non-irritant
(1-7 days)
ocular, irritation, rabbit marginal, high dose
dermal, sensitization, non-sensitizing
guinea-pig
inhalational, toxicity, rat LC50 > 2.49 mg/litre
(single exposure)
Mid-term
(1-26 weeks)
3 weeks; 5 days dermal, irritation, rabbit NOEL = 70 mg/kg body
per week weight per day
3 weeks; 5 days inhalational, methaemoglobin NOAEL = < 0.12 mg/litre
per week formation, rat
Long-term dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, rat per day
dietary, methaemoglobin NOEL = 2.4 mg/kg body weight
formation, mouse per day
dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, dog per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of diflubenzuron,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 2 mg/kg body weight per day derived in long-term
toxicity studies on mice, rats and dogs and applying a 100-fold
uncertainty factor, that 0.02 mg/kg body weight per day will probably
not cause adverse effects in humans whatever the route of exposure.
Biomonitoring of 4-chloroaniline during occupational exposures
needs to be carried out.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Molecular structure
additional material. The contact points, usually designated by
governments, may be Participating Institutions, IPCS Focal Points, or
individual scientists known for their particular expertise. Generally
some four months are allowed before the comments are considered by the
RO and author(s). A second draft incorporating comments received and
approved by the Director, IPCS, is then distributed to Task Group
members, who carry out the peer review, at least six weeks before
their meeting.
The Task Group members serve as individual scientists, not as
representatives of any organization, government or industry. Their
function is to evaluate the accuracy, significance and relevance of
the information in the document and to assess the health and
environmental risks from exposure to the chemical. A summary and
recommendations for further research and improved safety aspects are
also required. The composition of the Task Group is dictated by the
range of expertise required for the subject of the meeting and by the
need for a balanced geographical distribution.
The three cooperating organizations of the IPCS recognize
the important role played by nongovernmental organizations.
Representatives from relevant national and international associations
may be invited to join the Task Group as observers. While observers
may provide a valuable contribution to the process, they can only
speak at the invitation of the Chairperson.
Observers do not participate in the final evaluation of the chemical;
this is the sole responsibility of the Task Group members. When the
Task Group considers it to be appropriate, it may meet in camera.
All individuals who as authors, consultants or advisers
participate in the preparation of the EHC monograph must, in addition
to serving in their personal capacity as scientists, inform the RO if
at any time a conflict of interest, whether actual or potential, could
be perceived in their work. They are required to sign a conflict of
interest statement. Such a procedure ensures the transparency and
probity of the process.
When the Task Group has completed its review and the RO is
satisfied as to the scientific correctness and completeness of the
document, it then goes for language editing, reference checking, and
preparation of camera-ready copy. After approval by the Director,
IPCS, the monograph is submitted to the WHO Office of Publications for
printing. At this time a copy of the final draft is sent to the
Chairperson and Rapporteur of the Task Group to check for any errors.
It is accepted that the following criteria should initiate the
updating of an EHC monograph: new data are available that would
substantially change the evaluation; there is public concern for
health or environmental effects of the agent because of greater
exposure; an appreciable time period has elapsed since the last
evaluation.
All Participating Institutions are informed, through the EHC
progress report, of the authors and institutions proposed for the
drafting of the documents. A comprehensive file of all comments
received on drafts of each EHC monograph is maintained and is
available on request. The Chairpersons of Task Groups are briefed
before each meeting on their role and responsibility in ensuring that
these rules are followed.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
Members
Dr T. Bailey, US Environmental Protection Agency, Washington DC, USA
Dr A.L. Black, Department of Human Services and Health, Canberra,
Australia
Mr D.J. Clegg, Carp, Ontario, Canada
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, Cambridgeshire, United Kingdom
(Vice-Chairman)
Dr P.E.T. Douben, Her Majesty's Inspectorate of Pollution, London,
United Kingdom (EHC Joint Rapporteur)
Dr P. Fenner-Crisp, US Environmental Protection Agency, Washington DC,
USA
Dr R. Hailey, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, USA
Ms K. Hughes, Environmental Health Directorate, Health Canada,
Ottawa, Ontario, Canada (EHC Joint Rapporteur)
Dr D. Kanungo, Central Insecticides Laboratory, Government of India,
Ministry of Agriculture & Cooperation, Directorate of Plant
Protection, Quarantine & Storage, Faridabad, Haryana, India
Dr L. Landner, MFG, European Environmental Research Group Ltd,
Stockholm, Sweden
Dr M.H. Litchfield, Melrose Consultancy, Denmans Lane, Fontwell,
Arundel, West Sussex, United Kingdom (CAG Joint Rapporteur)
Professor M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy (Chairman)
Professor D.R. Mattison, University of Pittsburgh, Graduate School of
Public Health, Pittsburgh, Pennsylvania, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr P. Sinhaseni, Chulalongkorn University, Bangkok, Thailand
Dr S.A. Soliman, King Saud University, Bureidah, Saudi Arabia
Dr M. Tasheva, National Centre of Hygiene, Medical Ecology and
Nutrition, Sofia, Bulgaria (CAG Joint Rapporteur)
Mr J.R. Taylor, Pesticides Safety Directorate, Ministry of
Agriculture, Fisheries and Food, York, United Kingdom
Dr H.M. Temmink, Wageningen Agricultural University, Wageningen, The
Netherlands
Dr M.I. Willems, TNO Nutrition and Food Research Institute, Zeist,
The Netherlands
Representatives of GIFAPa (Groupement International des
Associations Nationales de Fabricants de Produits Agrochimiques)
Dr M. Bliss, Jr., ISK Biosciences Corporation, Mentor, Ohio, USA
Dr A.C. Dykstra, Registration Department BPID, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr H. Frazier, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr R. Gardiner, GIFAP, Brussels, Belgium
Dr B. Julin, Regulatory Affairs, Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr S.M. Kennedy (Environmental Science), Du Pont de Nemours (Belgium),
Agricultural Products Department, Mercure Centre, Brussels,
Belgium
Dr J. Killeen, ISK Biosciences Corporation, Mentor, Ohio, USA
Dr Th. S.M. Koopman, Toxicology Department, Solvay-Duphar BV, CP
Weesp, The Netherlands
Dr R.L. Mull, Du Pont Agricultural Products, Wilmington, Delaware, USA
Dr J.L.G. Thus, Environmental Research Department, Solvay-Duphar BV,
CP Weesp, The Netherlands
Secretariat
Ms A. Sundén Byléhn, International Register of Potentially
Toxic Chemicals, United Nations Environment Programme,
Châtelaine, Switzerland
Dr P. Chamberlain, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
a Participated as required for exchange of information.
Dr J. Herrman, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr K. Jager, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Dr P. Jenkins, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr W. Kreisel, World Health Organization, Geneva, Switzerland
Dr M. Mercier, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr M.I. Mikheev, Occupational Health, World Health Organization,
Geneva, Switzerland
Dr G. Moy, Food Safety, World Health Organization, Geneva, Switzerland
Mr I. Obadia, International Labour Organisation, Geneva, Switzerland
Dr R. Pletina, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland
Dr E. Smith, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland (EHC Secretary)
Mr J. Wilbourn, International Agency for Research on Cancer, Lyon,
France
ENVIRONMENTAL HEALTH CRITERIA FOR DIFLUBENZURON
The Core Assessment Group (CAG) of the Joint Meeting on Pesticide
Residues met in Geneva from 25 October to 3 November 1994.
Dr W. Kreisel of the WHO welcomed the participants on behalf of WHO,
and Dr M. Mercier, Director, IPCS, on behalf of the IPCS and its
cooperating organizations (UNEP/ILO/WHO). The Group reviewed and
revised the draft monograph and made an evaluation of the risks for
human health and the environment from exposure to diflubenzuron.
The first draft of the monograph was prepared by Dr M. Tasheva,
Sofia, Bulgaria. The second draft, incorporating comments received
following circulation of the first draft to the IPCS contact points
for Environmental Health Criteria monographs, was prepared by the IPCS
Secretariat.
Dr K.W. Jager and Dr P.G. Jenkins, both members of the IPCS
Central Unit, were responsible for the overall scientific content and
technical editing, respectively.
The fact that Solvay-Duphar, BV, made available to the IPCS its
proprietary toxicological information on diflubenzuron is gratefully
acknowledged. This allowed the CAG to make its evaluation on a more
complete database.
The efforts of all who helped in the preparation and finalization
of the monograph are gratefully acknowledged.
ABBREVIATIONS
ADI acceptable daily intake
a.i. active ingredient
AP alkaline phosphatase
bw body weight
4-CPU 4-chlorophenylurea
DFB diflubenzuron
2,6-DFBA 2,6-difluorobenzoic acid
ECD electron capture detection
G granular formulation
GC gas chromatography
GLC gas-liquid chromatography
Hb haemoglobin
HPLC high performance liquid chromatography
MATC maximum acceptable toxicant concentration
MCH mean cell haemoglobin
MCHC mean cell haemoglobin concentration
MCV mean cell volume
NOAEC no-observed-adverse-effect concentration
NOEL no-observed-effect level
NPD nitrogen-phosphorus detector
PCA para-chloroaniline (4-chloroaniline)
PCV packed cell volume
SAP serum alkaline phosphatase
SGOT serum glutamic-oxaloacetic transaminase (aspartate
aminotransferase)
SGPT serum glutamic-pyruvic transaminase (alanine
aminotransferase)
TLC thin-layer chromatography
WP wettable powder
1. SUMMARY AND EVALUATION; CONCLUSIONS AND RECOMMENDATIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Diflubenzuron is a member of the benzoylphenylurea group of
insecticides. Its insecticidal action is due to interaction with
chitin synthesis and/or deposition. It forms odourless white crystals
with a melting point of 230-232°C. It is sparingly soluble in water
(0.2 mg/litre at 20°C) and is virtually non-volatile. It is
relatively stable in acidic and neutral media but it hydrolyses in
alkaline conditions.
Diflubenzuron is produced by the reaction of 2,6-difluoro-
benzamide with 4-chlorophenylisocyanate.
Diflubenzuron residues may be measured in water, biological
samples and soils by HPLC with UV detection or by GC with ECD for
analysis of the intact molecule or following derivatization of the
liberated 4-chloroaniline with trifluoroacetic anhydride.
1.1.2 Sources of human and environmental exposure
Diflubenzuron is a synthetic compound used in agriculture,
forestry and public health programmes to control insect pests and
vectors. Different formulations of diflubenzuron are available for
these uses. There is no relevant information on human exposure to
diflubenzuron.
1.1.3 Environmental transport, distribution and transformation
Diflubenzuron is usually applied directly to plants and water.
Uptake of diflubenzuron through plant leaves does not occur.
The adsorption of diflubenzuron to soil is rapid. It is
immobilized in the top 10 cm layer of soil to which it is applied. It
is unlikely to leach. Diflubenzuron is degraded in soils of various
types and origin under aerobic or anaerobic conditions with a half-
life of a few days. The rate of degradation depends greatly on the
diflubenzuron particle size. The main metabolic pathway (over 90%) is
hydrolysis leading to 2,6-difluorobenzoic acid and 4-chlorophenylurea;
these are degraded with half-lives of about 4 and 6 weeks,
respectively. Free 4-chloroaniline has not been detected in soils.
Diflubenzuron degrades rapidly in neutral or alkaline waters.
Studies of application of diflubenzuron to water show rapid partition
to sediment; the parent compound and 4-chlorophenylurea may persist on
sediment for more than 30 days.
Diflubenzuron does not bioaccumulate in fish.
1.1.4 Environmental levels and human exposure
Exposure of the general population to diflubenzuron via water or
food as a result of its use in agriculture, against forest insects or
in mosquito control is negligible.
1.1.5 Kinetics and metabolism in laboratory animals
In experimental animals, diflubenzuron is absorbed from the
digestive tract and to a lesser extent through the skin. There is a
saturable absorption mechanism in the rat gastrointestinal tract.
Consequently a large proportion of orally administered diflubenzuron
is found in the faeces. Diflubenzuron has widespread distribution in
the tissues, but it does not accumulate.
The metabolic fate of diflubenzuron has been studied in various
species. The major route of metabolism in mammals is via
hydroxylation. Hydrolysis of diflubenzuron may occur at any of the
three carbon-nitrogen bonds. In pigs and chickens the major route of
hydrolysis is at the ureido bridge. In rats and cows the major
metabolic pathway is hydroxylation. The major metabolites in sheep,
swine and chickens are 2,6-difluorobenzoic acid and 4-chloro-
phenylurea; minor metabolites are 2,6-difluorobenzamide and
4-chloroaniline. In rats and cattle 80% of the metabolites are
2,6-difluoro-3-hydroxydiflubenzuron, 4-chloro-2-hydroxy-diflubenzuron
and 4-chloro-3-hydroxydiflubenzuron. The metabolic studies indicate
that little or no 4-chloroaniline is formed in rats or cattle.
The major route of elimination is via the faeces, ranging from 70
to 85% in cats, pigs and cattle. In sheep elimination is roughly
equally distributed between the urine and faeces. Urinary excretion
in rats and mice decreases proportionally with increasing dosage
level. Less than 1% of an oral dose is recovered in exhaled air.
Only trace residues are found in milk.
No human studies on the kinetics and metabolism of diflubenzuron,
including the extent of biotransformation to 4-chloroaniline, are
available.
1.1.6 Effects on laboratory mammals and in vitro test systems
Diflubenzuron has low acute toxicity by any route of exposure.
It has been classified by WHO as a "product unlikely to present an
acute hazard in normal use", based on an acute oral LD50 of more than
4640 mg/kg body weight in rats. The acute dermal LD50 in rats is
greater than 10 000 mg/kg body weight while the acute inhalational
LC50 for rats is greater than 2.49 mg/litre. No signs of
intoxication have been observed during the 14-day period following
single administration of diflubenzuron by various routes to a variety
of animal species.
Diflubenzuron is not a skin irritant (in rabbits) and not a skin
sensitizer (in guinea-pigs). It is marginally irritating to the eyes
of rabbits.
Diflubenzuron causes methaemoglobinaemia and sulfhaemo-
globinaemia. Dose-related methaemoglobinaemia has been demonstrated
after oral, dermal or inhalatory exposure to diflubenzuron in various
species. This effect is the most sensitive toxicological end-point in
experimental animals. The NOEL based on methaemoglobin formation is
2 mg/kg body weight per day in rats and dogs and 2.4 mg/kg body weight
per day in mice. In long-term toxicity studies with mice and rats,
treatment-related changes were principally associated with oxidation
of haemoglobin or with hepatocyte changes.
In carcinogenicity studies in mice and rats at dietary levels up
to 10 000 mg/kg in the diet, there were no treatment-related effects
on tumour incidence. Specifically, there were no mesenchymal
neoplasms of the spleen or liver as observed in carcinogenicity
studies with 4-chloroaniline.
In several reproductive toxicity studies on rats, mice, rabbits
and three avian species, no effects were seen on reproduction and
there was no embryotoxicity. Teratogenicity studies in rats and
rabbits demonstrated no teratogenic effects.
Diflubenzuron and its main metabolites have been examined in a
variety of in vitro and in vivo mutagenicity tests. Neither
diflubenzuron nor its major metabolites have a mutagenic effect.
The minor metabolite, 4-chloroaniline, was shown to be positive
in several in vitro mutagenicity assays using various end-points.
It is carcinogenic in rats and mice. The neoplastic lesions related
to administration of 4-chloroaniline were benign and malignant
mesenchymal tumours in the spleens of male rats and haemangiomas and
haemangiosarcomas, primarily in the spleen and liver of male mice.
1.1.7 Effects on humans
The diflubenzuron metabolite, 4-chloroaniline, has been reported
to cause methaemoglobinaemia in exposed workers and in neonates
inadvertently exposed. Some individuals who are deficient in
NADH-methaemoglobin reductase may be particularly sensitive to
4-chloroaniline and hence to diflubenzuron exposure.
1.1.8 Effects on other organisms in the laboratory and field
All chitin-synthesizing organisms show susceptibility to
diflubenzuron.
Bacteria were not affected by diflubenzuron at concentrations of
500 mg/kg soil; some stimulation of nitrogen fixation was seen.
Diflubenzuron acetone solutions were degraded; the acetone was used
as carbon source. Algal biomass increased at a diflubenzuron
concentration of 1 µg/litre. There were no adverse effects at
concentration above the limit of diflubenzuron solubility. Fungi were
temporarily affected at 0.1 µg/litre in laboratory streams.
Aquatic invertebrates show variable responses to diflubenzuron.
Molluscs are insensitive, the LC50 being greater than 200 mg/litre.
LC50 values for other invertebrates ranged from 1 to > 1000
µg/litre, reflecting the effects of the compound on juvenile,
moulting stages. A MATC for Daphnia has been estimated at > 40 and
< 93 ng/litre; as expected, larval mayflies and other aquatic insect
juveniles are highly susceptible. Overspray of water bodies would be
expected to kill some aquatic invertebrates.
In ecosystems and field experiments where diflubenzuron was
applied directly to the water, the effects on most taxa were less
severe than predictions from acute laboratory tests. No effects on
aquatic organisms have been found after aerial applications to
forests.
The LC50 values for fish are > 150 mg/litre. In field
experiments, fish kills have never been recorded.
The oral and contact LD50 for honey-bees is greater than
30 µg/bee. Honey-bee colonies were not affected after aerial
application of 350 g diflubenzuron/ha.
A 5-day dietary study on the mallard duck and bobwhite quail with
levels of up to 4640 mg/kg feed revealed no observable signs of
toxicity. Small songbirds in the forest ecosystem were not affected
after aerial application of diflubenzuron at 350 g/ha.
Small mammal species showed no reductions in numbers after
application of diflubenzuron at 67 g/ha to a forest.
1.2 Evaluation
1.2.1 Evaluation of human health risks
The primary manifestation of diflubenzuron toxicity is
methaemoglobin induction. This toxicity occurs in a range of test
animal species. It is attributable to the metabolite, 4-chloroaniline,
which is known to induce methaemoglobin formation in several animal
species and in humans.
Diflubenzuron does not cause other toxicities on chronic dietary
administration. It is not mutagenic or carcinogenic in mice or rats.
However, its metabolite, 4-chloroaniline, is mutagenic in vitro and
is carcinogenic in mice and male rats. Although 4-chloroaniline is a
minor urinary metabolite of diflubenzuron in rats, the extent to which
it is formed in vivo in various animal species remains unknown.
Similarly, the comparative degree of absorption of its parent compound
in various species is unknown.
The sensitivity of human haemoglobin to methaemoglobin formation
by 4-chloroaniline in vivo is not known. However, since induction
of methaemoglobinaemia is consistently the most sensitive measure of
diflubenzuron toxicity in the various animal species tested, it may be
used as the basis to estimate the levels causing no toxicological
effect.
1.2.2 Evaluation of effects on the environment
Diflubenzuron adsorbs readily to soil with little subsequent
desorption. Its mobility in soil is very low, practically all
residues remaining within 15 cm of the top, even in sandy loam soils;
diflubenzuron does not leach. It is only partly removed from foliage
by heavy rainfall. Nevertheless, some diflubenzuron may be present in
surface water shortly after application, due to flooding of treatment
areas or agricultural run-off.
Dissipation of diflubenzuron from water is rapid. Adsorption to
sediment occurs within 4 days; both parent compound and 4-chloro-
phenylurea metabolite may persist on sediment for at least 30 days.
Uptake of diflubenzuron by plants through the leaves after aerial
application does not occur. Some uptake of soil residues does occur
in plants and this may be translocated. At the highest application
rate (1 kg a.i./ha), following 1 month ageing of residues, up to
1 mg/kg residue may be found in various crops.
Photolysis of diflubenzuron is slow with a calculated half-life
of 40 days. Under environmental conditions abiotic degradation in
water and soil represents a minimum route of break-down. Aerobic
degradation in water is a microbial process with a half-life of a few
days under both laboratory and field conditions. In the field,
degradation of diflubenzuron applied at practical rates is influenced
by pH, temperature, formulation, organic matter content and depth of
the water.
Degradation in soil through microbial hydrolysis is a rapid
process, with a half-life of a few days, depending on diflubenzuron
particle size. The major break-down products are 2,6-difluorobenzoic
acid and 4-chlorophenylurea; a minor metabolite is parachloroaniline.
All these are irreversibly bound to soil and/or further metabolized.
The half-life of diflubenzuron residues on citrus fruits is
significantly decreased by high temperature and humidity.
Anaerobic degradation in water and sediment is slower than
aerobic.
Fish bioconcentrate diflubenzuron and some bioaccumulation takes
place during extended exposure up to a plateau, depending on the water
concentration, owing to fast degradation of diflubenzuron and
excretion of metabolites; the depuration half-life is less than one
day. The 4-chloroaniline metabolite has not been detected in fish.
Fish are not sensitive to diflubenzuron, the LC50 values being
> 150 mg/litre. Metabolites of diflubenzuron are also of low
toxicity to fish. Chronic exposure has shown no effects on fish at
recommended application rates; the compound does not persist in water
and no chronic exposure is expected.
Diflubenzuron is not phytotoxic to duckweed at the diflubenzuron
solubility limit concentration.
Honey-bees were not affected by topical applications of
> 30 µg/bee or dietary concentrations of up to 1000 mg/kg diet.
Brood in hives was reduced when bees were fed syrup at 59 mg
diflubenzuron/kg. Brood was also reduced following exposure of
flying colonies.
Earthworms were not affected at a concentration of 780 mg/kg
soil, which is at least three orders of magnitude above reported soil
residues.
Diflubenzuron has low acute toxicity to birds, the oral and
dietary LD(LC)50 values being greater than 3000 mg/kg diet.
Following recommended application rates diflubenzuron is not expected
to pose a hazard to birds.
Extensive field studies have shown minimal or reversible effects
on most aquatic invertebrates; daphnids were most seriously affected,
with short-term reductions in populations of up to 75% following a
single application of diflubenzuron. Fish were not affected by water
overspraying. Neither bird nor mammal populations were adversely
affected following forest spraying with diflubenzuron.
A summary of risk quotients for birds, fish and aquatic
invertebrates is given in Table 1.
1.2.3 Toxicological criteria for setting guidance values
The toxicological studies on diflubenzuron of relevance for
setting guidance values are shown in Table 2.
Table 1. Toxicity/exposure ratios for birds, fish and aquatic invertebrates based on
application rates of 2.5 kg a.i./ha of diflubenzuron to soybeans (worst case)
Risk category LC50 (mg/litre Estimated exposure Toxicity/exposure
or mg/kg diet) (mg/litre or ratio (TER)c
mg/kg diet)a,b
Acute bird 3762 73.7-535.7 51.0-7.0
Acute fish (stream) 150 0.0007 214 300
Acute fish (pond) 150 0.01 15 000
Acute aquatic
invertebrate (stream) 0.005 0.0007 7.1
Acute aquatic
invertebrate (pond) 0.005 0.01 0.5
a Estimated environmental concentration in the terrestrial environment (for bird
exposure) is based on the stated application rate and the assumption of
deposition on short grass using the US EPA nomogram.
b Aquatic exposure concentrations were taken from the STREAM model based on a
single application and estimated runoff into water; no direct overspray is
included.
c TER is the toxicity (as LC50) divided by the exposure; values at or below
1.0 indicate likely exposure to toxic concentrations by organisms in the
different risk categories.
Table 2. Toxicological criteria for estimating guidance values for diflubenzuron
Exposure scenario Relevant route/effect/ Result/remarks
(technical species
diflubenzuron)
Short-term dermal, irritation, rabbit non-irritant
(1-7 days)
ocular, irritation, rabbit marginal, high dose
dermal, sensitization, non-sensitizing
guinea-pig
inhalational, toxicity, rat LC50 > 2.49 mg/litre
(single exposure)
Mid-term
(1-26 weeks)
3 weeks; 5 days dermal, irritation, rabbit NOEL = 70 mg/kg body
per week weight per day
3 weeks; 5 days inhalational, methaemoglobin NOAEL = < 0.12 mg/litre
per week formation, rat
Long-term dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, rat per day
dietary, methaemoglobin NOEL = 2.4 mg/kg body weight
formation, mouse per day
dietary, methaemoglobin NOEL = 2 mg/kg body weight
formation, dog per day
1.3 Conclusions and recommendations
Considering the toxicological characteristics of diflubenzuron,
both qualitatively and quantitatively, it was concluded, on the basis
of the NOEL of 2 mg/kg body weight per day derived in long-term
toxicity studies on mice, rats and dogs and applying a 100-fold
uncertainty factor, that 0.02 mg/kg body weight per day will probably
not cause adverse effects in humans whatever the route of exposure.
Biomonitoring of 4-chloroaniline during occupational exposures
needs to be carried out.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, ANALYTICAL METHODS
2.1 Identity
Molecular structure
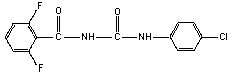 Empirical formula C14H9ClF2N2O2
Common name Diflubenzuron
Common trade names Dimilin; Micromite; Vigilante
Common abbreviation DFB
IUPAC name 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)-
urea
CAS chemical name N-[[(4-chlorophenyl) amino] carbonyl]-
2,6-difluorobenzamide
CAS registry number
Empirical formula C14H9ClF2N2O2
Common name Diflubenzuron
Common trade names Dimilin; Micromite; Vigilante
Common abbreviation DFB
IUPAC name 1-(4-chlorophenyl)-3-(2,6-difluorobenzoyl)-
urea
CAS chemical name N-[[(4-chlorophenyl) amino] carbonyl]-
2,6-difluorobenzamide
CAS registry number  Even as a bound residue PCA is metabolized. Apparently, the
breakdown of 4-CPU in soil is a complex process in which PCA is a
transient metabolite or intermediate. The breakdown process leads to
products beyond the aniline structure. If PCA is applied to soil,
6 weeks incubation at 25°C yields 60% breakdown products of a
different nature (Bollag et al., 1978). The aniline itself is firmly
bound to soil and immobilized (Hsu & Bartha, 1974; Moreale & van
Bladel, 1976; Bollag et al., 1978; Simmons et al., 1989).
Fig. 2 shows metabolic pathways in soil.
The main metabolic pathway (over 90%) is hydrolysis, leading to
2,6-DFBA and 4-CPU. The second site of cleavage occurs at CN bonds 2
and 3. Both reactions lead to the formation of 2,6-difluorobenzamide
(DFBAM), which readily hydrolyses to 2,6-DFBA (Verloop & Ferrell,
1977; Nimmo et al., 1984).
The major metabolite in an activated sludge system is 4-CPU.
This is the major metabolite reported in most soil metabolism
experiments (Booth et al., 1987). 4-CPU was found to be converted
into bound residues with a half-life of 5-10 weeks. In the bound
residues, 4-CPU and PCA were present in roughly equal amounts after
2 months (Verloop & Ferrell, 1977). Free PCA was not found in soil
(Nimmo et al., 1986). The soil type and characteristics appear to
have no influence on the rate of degradation (Nimmo et al., 1984).
Metcalf et al. (1975) found no significant degradation of
diflubenzuron in a silty clay loam after incubation at 26.7°C for
periods of 1, 2 and 4 weeks. However, the authors did not take into
account the particle size of the soil, and used techniques that have a
negative influence on breakdown.
The rate of degradation of 14C- or 3H-diflubenzuron applied to
a mushroom growth medium (dose 2 g/m2) was between 30-50% in one
month. The main degradation products, 4-CPU and 2,6-DFBA, were
absorbed from the growth medium by the mushrooms, resulting in
residue levels of 0.1-0.6 mg/kg and 1-3 mg/kg, respectively (Nimmo &
de Wilde, 1977a). Free PCA or its further possible degradation
products were not present in the extractable residues (Nimmo & de
Wilde, 1975a; Verloop & Ferrell, 1977). Organic matter in soil
significantly contributed to the adsorption of chloroaniline compounds
and their immobilization (Hsu & Bartha, 1974; Moreale & van Bladel,
1976; Bollag et al., 1978).
Nimmo & de Wilde (1975a) found a degradation half-life of
0.5-1 week at a diflubenzuron concentration of 1 mg/kg (corresponding
to an application dose of approximately 300 g/ha). 2,6-DFBA was
degraded with a half-life of approximately 4 weeks, and 4-CPU with a
half-life of 2-3 months.
Even as a bound residue PCA is metabolized. Apparently, the
breakdown of 4-CPU in soil is a complex process in which PCA is a
transient metabolite or intermediate. The breakdown process leads to
products beyond the aniline structure. If PCA is applied to soil,
6 weeks incubation at 25°C yields 60% breakdown products of a
different nature (Bollag et al., 1978). The aniline itself is firmly
bound to soil and immobilized (Hsu & Bartha, 1974; Moreale & van
Bladel, 1976; Bollag et al., 1978; Simmons et al., 1989).
Fig. 2 shows metabolic pathways in soil.
The main metabolic pathway (over 90%) is hydrolysis, leading to
2,6-DFBA and 4-CPU. The second site of cleavage occurs at CN bonds 2
and 3. Both reactions lead to the formation of 2,6-difluorobenzamide
(DFBAM), which readily hydrolyses to 2,6-DFBA (Verloop & Ferrell,
1977; Nimmo et al., 1984).
The major metabolite in an activated sludge system is 4-CPU.
This is the major metabolite reported in most soil metabolism
experiments (Booth et al., 1987). 4-CPU was found to be converted
into bound residues with a half-life of 5-10 weeks. In the bound
residues, 4-CPU and PCA were present in roughly equal amounts after
2 months (Verloop & Ferrell, 1977). Free PCA was not found in soil
(Nimmo et al., 1986). The soil type and characteristics appear to
have no influence on the rate of degradation (Nimmo et al., 1984).
Metcalf et al. (1975) found no significant degradation of
diflubenzuron in a silty clay loam after incubation at 26.7°C for
periods of 1, 2 and 4 weeks. However, the authors did not take into
account the particle size of the soil, and used techniques that have a
negative influence on breakdown.
The rate of degradation of 14C- or 3H-diflubenzuron applied to
a mushroom growth medium (dose 2 g/m2) was between 30-50% in one
month. The main degradation products, 4-CPU and 2,6-DFBA, were
absorbed from the growth medium by the mushrooms, resulting in
residue levels of 0.1-0.6 mg/kg and 1-3 mg/kg, respectively (Nimmo &
de Wilde, 1977a). Free PCA or its further possible degradation
products were not present in the extractable residues (Nimmo & de
Wilde, 1975a; Verloop & Ferrell, 1977). Organic matter in soil
significantly contributed to the adsorption of chloroaniline compounds
and their immobilization (Hsu & Bartha, 1974; Moreale & van Bladel,
1976; Bollag et al., 1978).
Nimmo & de Wilde (1975a) found a degradation half-life of
0.5-1 week at a diflubenzuron concentration of 1 mg/kg (corresponding
to an application dose of approximately 300 g/ha). 2,6-DFBA was
degraded with a half-life of approximately 4 weeks, and 4-CPU with a
half-life of 2-3 months.
 Walstra & Joustra (1990) applied 0.69 mg diflubenzuron/kg to
sandy loam. When incubated in the dark at 24°C, they obtained a half-
life for diflubenzuron of 50 h.
Diflubenzuron was found to be rapidly degraded by four soil fungi
( Fusarium sp., Cephalosporium sp., Penicillium sp. and Rhodotorula
sp.), the half-lives being 7, 13, 14 and 18 days, respectively
(Seuferer et al., 1979).
Several degradation studies on diflubenzuron (Dimilin 25 W) in
field soils have been conducted (Kramer, 1990, 1991, 1992a,b; Verhey,
1991a,b). Most of the degradation half-lives were between one and two
weeks, except in the case of the two Verhey studies, which yielded
half-lives of more than two months. In all studies, the metabolites
were 4-CPU and 2,6-DFBA.
No degradation of diflubenzuron by the soil microorganism
Pseudomonas putida was observed (Booth & Ferrell, 1977).
4.4 Bioaccumulation and biomagnification
Metcalf et al. (1975) studied the fate of 14C-diflubenzuron in a
laboratory model ecosystem. Diflubenzuron was clearly persistent in
some organisms, such as algae (Oedogonium cardiacum), snails
( Physa sp.), caterpillars (Estigmene acrea) and mosquito larvae
(Culex pipiens quinquefasciatus). The fish Gambusia affinis was
able to degrade diflubenzuron more efficiently. Diflubenzuron did not
biomagnify in the fish through food chain transfer. The biomagnifi-
cation was about 40-fold greater in mosquito larvae than in Gambusia
affinis.
When the bluegill sunfish (Lepomis macrochirus) was exposed to
10 µg diflubenzuron/litre for 24 h the tissues contained an average of
264 µg/kg. After 24 to 48 h of exposure, fish degraded and eliminated
the diflubenzuron. The excretory products were neither the parent
compound nor 4-CPU. The amount of diflubenzuron remaining in fish
tissues at various times was dependant on the reduction of residue
concentration in water. However, the potential for degradation and
elimination was very great (Schaefer et al., 1979).
A dynamic 42-day study was conducted by Burgess (1989) in order
to evaluate the bioconcentration of 14C-diflubenzuron by bluegill
sunfish (Lepomis macrochirus). A flow-through proportional diluter
system was used for a 28-day exposure period. Radioanalysis of
fillet, whole fish and visceral portions was performed throughout the
exposure period. Daily bioconcentration factors ranged from 34 to 200,
78 to 360, and 100 to 550 for fillet, whole fish and viscera,
respectively. Uptake tissue concentrations of 14C-diflubenzuron
ranged from 0.25 to 1.7 mg/kg for fillet, 0.58 to 3.3 mg/kg for whole
fish, and 0.75 to 4.7 mg/kg for viscera. To measure the elimination
of 14C-diflubenzuron, the test fish were placed in clean water for 14
days. Radioanalysis throughout the depuration period indicated 99%
depuration for each of fillet, whole fish and viscera. The fillet
concentration of 14C-diflubenzuron decreased from 1.6 mg/kg on day 28
of exposure to 0.012 mg/kg by day 14 of the depuration period. Whole
fish levels decreased from 3.3 mg/kg on day 28 of exposure to
0.038 mg/kg by the end of the study; whereas, viscera concentrations
dropped from 4.4 mg/kg on day 28 of exposure to 0.056 mg/kg by day 14
of depuration. BIOFAC modelling estimated the uptake rate constant
(K1) to be 370 (± 57) mg/kg fish per mg/litre water per day, the
depuration rate constant (K2) 1.2 (± 0.18) day-1, the time for 50%
depuration 0.60 (± 0.09) days, the bioconcentration factor (BCF) 320
(± 70), and the time to reach 90% or steady state 2.0 (± 0.31) days.
The BIOFAC-calculated BCF value was the same as the observed mean
whole fish BCF of 320 for days 3, 7, 14, 21 and 28. Fig. 3 shows the
accumulation, plateauing and depuration in this study.
During the study, no mortality or abnormal behaviour was observed
in the test fish. This appeared to indicate that the test fish were
in good health and would provide acceptable data for defining the
uptake/depuration potential of 14C-diflubenzuron. Analysis of fish
revealed parent compound (80%), 2,6-difluorobenzamide (10-13%) and
three other minor metabolites (one of which probably was 4-CPU). PCA
was demonstrated to be absent (sensitivity limit 0.01 mg/kg).
White crappies (Pomoxis annularis) contained residues from
355.1 to 62.2 µg/kg at 4 and 21 days, respectively, following
treatment of a lake with 5 µg diflubenzuron/litre (Apperson et al.,
1978).
Channel catfish (Ictalurus punctatus) did not bioaccumulate
diflubenzuron residues (less than 0.05 mg/kg) from treated soil in a
simulated lake ecosystem (Booth & Ferrell, 1977).
Assuming a biomagnification of 50-160, and that fish are capable
of rapidly depleting residues from the body, the likelihood of fish
accumulating significant residues of diflubenzuron is low (Apperson et
al., 1978; Schaefer et al., 1980).
4.5 Interaction with other physical, chemical or biological factors
Schaefer & Dupras (1976) reported that application of the
technical grade compound in an ethanol carrier or as a flowable liquid
formulation resulted in higher concentrations in the upper water
levels of mosquito ponds for a period of 3 days following spray
treatment than in the case of spray treatment with wettable powder
formulation (the actual formulation used for mosquito control
spraying).
Walstra & Joustra (1990) applied 0.69 mg diflubenzuron/kg to
sandy loam. When incubated in the dark at 24°C, they obtained a half-
life for diflubenzuron of 50 h.
Diflubenzuron was found to be rapidly degraded by four soil fungi
( Fusarium sp., Cephalosporium sp., Penicillium sp. and Rhodotorula
sp.), the half-lives being 7, 13, 14 and 18 days, respectively
(Seuferer et al., 1979).
Several degradation studies on diflubenzuron (Dimilin 25 W) in
field soils have been conducted (Kramer, 1990, 1991, 1992a,b; Verhey,
1991a,b). Most of the degradation half-lives were between one and two
weeks, except in the case of the two Verhey studies, which yielded
half-lives of more than two months. In all studies, the metabolites
were 4-CPU and 2,6-DFBA.
No degradation of diflubenzuron by the soil microorganism
Pseudomonas putida was observed (Booth & Ferrell, 1977).
4.4 Bioaccumulation and biomagnification
Metcalf et al. (1975) studied the fate of 14C-diflubenzuron in a
laboratory model ecosystem. Diflubenzuron was clearly persistent in
some organisms, such as algae (Oedogonium cardiacum), snails
( Physa sp.), caterpillars (Estigmene acrea) and mosquito larvae
(Culex pipiens quinquefasciatus). The fish Gambusia affinis was
able to degrade diflubenzuron more efficiently. Diflubenzuron did not
biomagnify in the fish through food chain transfer. The biomagnifi-
cation was about 40-fold greater in mosquito larvae than in Gambusia
affinis.
When the bluegill sunfish (Lepomis macrochirus) was exposed to
10 µg diflubenzuron/litre for 24 h the tissues contained an average of
264 µg/kg. After 24 to 48 h of exposure, fish degraded and eliminated
the diflubenzuron. The excretory products were neither the parent
compound nor 4-CPU. The amount of diflubenzuron remaining in fish
tissues at various times was dependant on the reduction of residue
concentration in water. However, the potential for degradation and
elimination was very great (Schaefer et al., 1979).
A dynamic 42-day study was conducted by Burgess (1989) in order
to evaluate the bioconcentration of 14C-diflubenzuron by bluegill
sunfish (Lepomis macrochirus). A flow-through proportional diluter
system was used for a 28-day exposure period. Radioanalysis of
fillet, whole fish and visceral portions was performed throughout the
exposure period. Daily bioconcentration factors ranged from 34 to 200,
78 to 360, and 100 to 550 for fillet, whole fish and viscera,
respectively. Uptake tissue concentrations of 14C-diflubenzuron
ranged from 0.25 to 1.7 mg/kg for fillet, 0.58 to 3.3 mg/kg for whole
fish, and 0.75 to 4.7 mg/kg for viscera. To measure the elimination
of 14C-diflubenzuron, the test fish were placed in clean water for 14
days. Radioanalysis throughout the depuration period indicated 99%
depuration for each of fillet, whole fish and viscera. The fillet
concentration of 14C-diflubenzuron decreased from 1.6 mg/kg on day 28
of exposure to 0.012 mg/kg by day 14 of the depuration period. Whole
fish levels decreased from 3.3 mg/kg on day 28 of exposure to
0.038 mg/kg by the end of the study; whereas, viscera concentrations
dropped from 4.4 mg/kg on day 28 of exposure to 0.056 mg/kg by day 14
of depuration. BIOFAC modelling estimated the uptake rate constant
(K1) to be 370 (± 57) mg/kg fish per mg/litre water per day, the
depuration rate constant (K2) 1.2 (± 0.18) day-1, the time for 50%
depuration 0.60 (± 0.09) days, the bioconcentration factor (BCF) 320
(± 70), and the time to reach 90% or steady state 2.0 (± 0.31) days.
The BIOFAC-calculated BCF value was the same as the observed mean
whole fish BCF of 320 for days 3, 7, 14, 21 and 28. Fig. 3 shows the
accumulation, plateauing and depuration in this study.
During the study, no mortality or abnormal behaviour was observed
in the test fish. This appeared to indicate that the test fish were
in good health and would provide acceptable data for defining the
uptake/depuration potential of 14C-diflubenzuron. Analysis of fish
revealed parent compound (80%), 2,6-difluorobenzamide (10-13%) and
three other minor metabolites (one of which probably was 4-CPU). PCA
was demonstrated to be absent (sensitivity limit 0.01 mg/kg).
White crappies (Pomoxis annularis) contained residues from
355.1 to 62.2 µg/kg at 4 and 21 days, respectively, following
treatment of a lake with 5 µg diflubenzuron/litre (Apperson et al.,
1978).
Channel catfish (Ictalurus punctatus) did not bioaccumulate
diflubenzuron residues (less than 0.05 mg/kg) from treated soil in a
simulated lake ecosystem (Booth & Ferrell, 1977).
Assuming a biomagnification of 50-160, and that fish are capable
of rapidly depleting residues from the body, the likelihood of fish
accumulating significant residues of diflubenzuron is low (Apperson et
al., 1978; Schaefer et al., 1980).
4.5 Interaction with other physical, chemical or biological factors
Schaefer & Dupras (1976) reported that application of the
technical grade compound in an ethanol carrier or as a flowable liquid
formulation resulted in higher concentrations in the upper water
levels of mosquito ponds for a period of 3 days following spray
treatment than in the case of spray treatment with wettable powder
formulation (the actual formulation used for mosquito control
spraying).
 Seuferer et al. (1979) reported that the soil microorganisms
Rhodotorula sp., Penicillium sp. and Cephalosporium sp. cannot
utilize diflubenzuron as a sole carbon and energy source. However,
accelerated breakdown of diflubenzuron occurred in the presence of
these organisms.
4.6 Ultimate fate following use
It appears that after direct spraying diflubenzuron is persistent
on foliage, it remains almost completely at the site of application on
the surface, and it does not penetrate the plants.
Diflubenzuron is readily degraded in soils of various types and
origin under aerobic or anaerobic conditions with a half-life in the
range of 0.5 to 1 week. It is metabolized by microorganisms
principally to 4-CPU and 2,6-DFBA. The latter is unstable with a
half-life of 3-5 days (Nimmo et al., 1984) to 4 weeks (Verloop &
Ferrell, 1977). The half-life of 4-CPU is about 6 weeks (Nimmo et
al., 1984). Free PCA has not been detected in soil.
In spite of rapid degradation in soil, small amounts of residue
(up to 1 mg/kg, depending on ageing time and growth stage of plants)
may be taken up by crops in treated soil (Thus et al., 1994).
Field applications of diflubenzuron produce soil residues which
might possibly lead to residues in rotational crops by re-uptake from
soil.
Studies with direct applications to field water show a moderate
persistence of diflubenzuron in water. Half-life values average one
week or less. This rapid rate of loss may be more dependent on
adsorption to organic matter than on microbial degradation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
No information is available on air concentrations of
diflubenzuron.
5.1.2 Water
A total of 1160 ha of insect-infested forest in Finland was
sprayed with diflubenzuron (25% WP) from a fixed-winged aircraft at an
application rate of 75 g a.i. in 50 litres water per ha. The residues
in "run-off" water (gathered in specially dug pits adjacent to the
sprayed area) decreased from 5 µg/litre one day after spraying to
0.1 µg/litre after 2 months. The concentration in water in open pits
was 0.1 µg/litre 1 and 7 days after application and 0.2 µg/litre
1 month after application. After 2 months no residues were detected.
All water samples taken from outside the treated area contained less
than 0.1 µg/litre (the limit of sensitivity) (Mutanen et al., 1988).
Diflubenzuron was found in the water of the Fraser River, Canada,
up to 71 days following application with diflubenzuron (1% granular
formulation) at a rate of 4.5 kg/ha (45 g a.i./ha). The peak value
was 1.8 µg/litre 8 days after treatment (Wan & Wilson, 1977).
After aerial application of diflubenzuron (25% WP formulation) to
two forest ponds in Canada, the maximum residue levels in water,
sediment, aquatic plants and fish were 13.82 µg/litre (at 1 h),
0.24 mg/kg (at 1 day), 0.36 mg/kg (at 1 day) and 0.11 mg/kg
(at 1 day), respectively. The rate of dissipation was rapid, non-
detectable levels being reached in 20 days for water, 5 days in
aquatic plants and 3 days in fish (Kingsbury et al., 1987).
A pond in Salt Lake County, Utah, USA, was treated with three
applications of diflubenzuron at a rate of 280.25 g a.i./ha.
Diflubenzuron was found at less than 0.05 mg/litre 4 days following
treatment (Booth et at., 1987).
Residues in three farm ponds in California treated with
diflubenzuron (2.5, 5 and 10 µg/litre) averaged 1.9, 4.6 and
9.8 µg/litre, respectively, 1-4 h after the applications. They
declined steadily averaging 0.5, 0.3 and 0.2 µg/litre, respectively,
2 weeks later. Residues in a small lake treated at 5 µg/litre
averaged 3.3 µg/litre following treatment and 0.4 µg/litre after
35 days. No residues were found in sediment samples taken post-
treatment (Apperson et al., 1978).
One hour after a single application of 45 g diflubenzuron/ha to
brackish water pools the residues in water and in sediment were
3.6 µg/litre and 80 µg/kg, respectively. The concentration in
sediment increased to 520 µg/kg after 1 day and reached its maximum of
780 µg/kg 4 days following application (Hester et al., 1986). After
6 applications of diflubenzuron at a rate of 145.73 g/ha to Utah Lake,
USA, the residues in sediments were less than 0.05 mg/kg (Booth et
al., 1987).
Other field studies with similar results have been reported by
Anon (1980), Smith & Edmunds (1985), Van Den Berg (1986), Huber &
Collins (1987), Jones & Kochenderfer (1988), Huber & Manchester
(1988), Downey (1990) and Sundaram et al. (1991). It is clear that a
variety of application scenarios will result in measurable residues of
diflubenzuron in water (Table 6).
The overall conclusion is that diflubenzuron residues in stagnant
water dissipate rapidly within days. In flowing water, e.g., in
wooded areas, diflubenzuron residues may peak shortly after rainfall
but such peak concentrations are very transient in nature.
5.1.3 Food and feed
Data on residues in food resulting from treatment with
diflubenzuron have been summarized by FAO/WHO (1982a,b, 1985a,b,
1986a,b).
Residue data obtained from various countries showed residues in
apples below 1.0 mg diflubenzuron/kg at 2 weeks after the last
application at recommended rates. Residues in whole citrus fruit were
below 0.5 mg/kg 1 week after the last application at the recommended
rate. Residues in soybean seed and cottonseed were generally below
the limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron-treated soil, high
levels of the metabolite 2,6-DFBA are taken up from the soil.
Diflubenzuron was found at a level of 0.1 mg/kg, while the 2,6-DFBA
level was around 1 mg/kg (see chapter 4).
Residues in wild mushrooms after aerial application to forests in
Finland were on average 0.07 mg/kg 1 week after spraying with 75 g
diflubenzuron in 50 litre water per ha. In bilberries the residues
decreased on average from 0.2 mg/kg 1 day after spraying to 0.09 mg/kg
after 1 month (Mutanen et al., 1988).
Diflubenzuron applied as a wettable powder spray to growing
alfalfa at 20-100 g/ha showed initial residue levels of 1.8-8.5 mg/kg.
Residues of 0.3-1.5 mg/kg remained 22 days after applications (Lauren
et al., 1984).
Table 6. Summary and comparison of experimental parameters among key studies designed to measure environmental concentrations of
diflubenzuron in water
Medium Formulation a.i.% Method of Application Maximum Time for Minimum Time for References
treated application rate a.i. concentration maximum concentrationa minimum
concentration concentration
Farm ponds 25 WP 25 hand sprayer 2.5-10 µg/litre 1.9-9.8 µg/litre 1-4 h 0.5-0.2 µg/litre 14 days Apperson
(0.06-0.2 ha) from boat et al.
(1978)
Small lake 25 WP 25 hand sprayer 5 µg/litre 3.3 µg/litre 4 h 0.4 µg/litre 35 days Apperson
(18.6 ha) from boat et al.
(1978)
Pond W-25 25 hand-operated 0.28 kg/ha 56 µg/litre 96 h < 0.01 µg/litre 40 days Booth
spray et al.
applicator (1987)
Brackish 25 WP 25 clothes 0.045 kg/ha 7.5 µg/litre 48-72 h < 0.3 µg/litre 25-30 days Hester
pools sprinkler (1986)
Forest ponds 25 WP 25 aircraft 0.07 kg/ha 13.82 µg/litre 1 h < DL 20 days Kingsbury
(25 ha) (four et al.
atomizers) (1987)
Field plot 25 WP 25 fixed-wing 0.075 kg in 50 5.0 µg/litre 24 h < DL 60 days Mutanen
(1160 ha) aircraft litre water/ha et al.
(1988)
Fixed plots granular 1.0 aircraft 0.023 kg/ha, 1.8 µg/litre 192 h < DL 60 days Wan &
(3-40 ha) 0.46 kg/ha Wilson
(1977)
a DL = determination limit
After two soil applications of 67.26 g/ha, the residues of
diflubenzuron in the rotational crops (wheat, cabbage and onions) were
less than 0.01 mg/kg (Danhaus & Sieck, 1976).
Mian & Mulla (1983) studied the persistence of diflubenzuron in
stored wheat after applications of 1, 5 and 10 mg/kg. The residue
levels were 0.59, 2.75 and 5.00 mg/kg, respectively, 23 months after
treatment.
5.1.4 Forest plants and litter
The level of diflubenzuron residues in pine needles was on
average 3.0 mg/kg 1 day after application to the forest in Finland at
a rate of 75 g diflubenzuron in 50 litres water per ha. The level had
decreased to 0.2-0.3 mg/kg or was not detectable 2 months later
(Mutanen et al., 1988).
Booth et al. (1987) found diflubenzuron residues of less than
0.05 mg/kg in the forest litter 1, 4, 10 and 21 days after treatment
with 0.28 kg a.i./ha.
Sundaram (1986) studied the residues in a forest in Canada after
simulated aerial spraying of diflubenzuron in acetone and in fuel oil:
Arotex 3470 mixture, each at 90 g a.i. in 18 litre/ha. The residue
levels 1 h after application varied, respectively, from 23.8 to
30.6 µg/g in foliage and from 3.08 to 4.60 µg/g in litter. Forty-five
days after spraying the residue levels in foliage were 0.80 and
3.9 µg/g, respectively, for the above-mentioned formulations.
Spray deposit patterns and persistence of diflubenzuron in white
pine ( Pinus strobus L.) and sugar maple ( Acer saccharum Marsh.)
canopies, forest litter and soil were studied after aerial application
of a 250 g/kg wettable powder formulation (Dimilin WP-25) at
70 g a.i./ha, using three volume rates (2.5, 5 and 10 litres/ha), over
three blocks in a mixed forest near Kaladar, Ontario, Canada, during
1986 (Sundaram, 1991). In the spray block that received 10 litres/ha,
diflubenzuron persisted in foliage as long as 120 days after
treatment, but it lasted for only about a week in forest litter and
soil samples. At 2.5 and 5 litres/ha, diflubenzuron failed to persist
in foliage as long, and residues in litter and soil, which were barely
above the quantification limit, persisted only for a few days.
5.1.5 Aquatic organisms
Residues in fish are given in section 4.4.
5.2 General population exposure
Exposure of the general population to diflubenzuron via food and
drinking-water may occur.
Twelve volunteers with whole body dosimeters were exposed for 4 h
to Dimilin 25 W after simulated indoor treatment of carpets at
0.16 g/m2. Average deposition was 5.3 ± 2.3 µg diflubenzuron/cm2
carpet. Total dermal exposure ranged from 0.053 to 0.25 mg/kg body
weight per day to (average 0.15 ± 0.066 mg/kg body weight per day).
Assuming a dermal absorption of 0.2%, the total exposure via the
dermal route was calculated to be 0.0003 mg/kg body weight per day.
Air concentrations ranged from 10.2 to 32.4 µg/m3 during the first
4 h and were < 1 µg/m3 at 12-16 h. The total respiratory exposure
was calculated to be 0.0011 mg/kg body weight per day. The total
exposure, via the dermal and respiratory route, was calculated to be
0.0014 mg/kg body weight (Honeycutt, 1993).
5.3 Occupational exposure during manufacture, formulation or use
In a US Department of Agriculture report, human exposure via a
variety of exposure scenarios was estimated using standardized methods
and assumptions. The exposure scenarios included mixing and loading by
workers, via aircraft or truck spillage, and general public exposure
via the diet or resulting from occupational aerial spraying. Dermal
absorption of diflubenzuron was assumed to be 10%. Estimated
realistic doses for humans were < 0.003 mg/kg body weight per day
except where aircraft or truck spillages occurred, in which case
exposures were significantly higher. Estimated worst-case doses for
humans were < 0.01 mg/kg body weight per day, except where aircraft
or truck spillages occurred (USDA, 1985).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Diflubenzuron is absorbed from the digestive tract but only
poorly absorbed through the skin. Willems et al. (1980) found that in
rats the relative intestinal absorption diminished greatly with
increasing dose. Following a dose of 4 mg/kg body weight 42.5% was
absorbed, but only 3.7% of a 900 mg/kg body weight dose was absorbed.
Dermal absorption of 14C-diflubenzuron was only 0.2% when it was
applied to the shaved skin of rabbits as an aqueous micro-suspension
of 150 mg/kg (De Lange, 1979).
When applied dermally to cattle 14C-diflubenzuron was not
absorbed or degraded through the skin to any detectable degree (Ivie,
1978).
6.2 Distribution
Body tissues show little tendency to retain diflubenzuron.
Analysis of tissues for radiocarbon residues, 4 days (for sheep) or
7 days (for cows) after a single oral dose of 10 mg/kg body weight,
indicated that only the liver contained appreciable levels of
radioactivity, ranging from 2 to 4 mg/kg diflubenzuron equivalents
(Ivie, 1977).
More than one third of an oral diflubenzuron dose appeared in the
bile of a cannulated sheep (Ivie, 1977).
The highest 14C-diflubenzuron residue present in pig tissues
after a single oral dose of 5 mg/kg body weight was 0.43 mg/kg in the
gall bladder. All other tissue residue levels were found to be less
than 0.30 mg/kg (Opdycke et al., 1982a).
Twenty-two dairy cows were fed 14C-diflubenzuron (labelled in
both phenyl moieties) in a diet at dose levels of 0.05, 0.5, 5, 25 and
250 mg/kg feed for 28 days. Residues in blood, fat and muscle were
below the detection limit (0.0067-0.04 mg/kg) at all dose levels. They
were only detected following a dose of 250 mg/kg in the liver and
kidney where residues were 6.040 and 1.038 mg/kg, respectively.
Residues in milk were found at dose levels of 5 and 250 mg/kg, where
the highest levels of diflubenzuron were 0.009 and 0.20 mg/kg,
respectively (Smith & Merricks, 1976a).
In a study by Miller et al. (1976a), two dairy cows were fed
diflubenzuron at 0.25 or 1 mg/kg body weight per day for 4 months. A
third cow received an increased dosage of 8 to 16 mg/kg body weight
per day, the highest value being maintained for three months. In the
fat, liver and milk of the third cow, residues were 0.2, 0.13 and
0.02 mg/kg, respectively.
When dairy bull calves (four treated and four controls) received
diflubenzuron at 1.0 to 2.8 mg/kg body weight, residues were detected
only in the tissue samples of one bull (0.02 mg/kg in liver and
kidney, 0.04 mg/kg in the subcutaneous fat, and 0.08 mg/kg in the
renal and omental fat (Miller et al., 1979).
The maximum total residue in eggs 3 days after a single dose of
5 mg/kg 14C-diflubenzuron to hens was 0.248 mg/kg (Opdycke, 1976).
When laying hens were administered 14C-diflubenzuron at dose
levels 0.05, 0.5, and 5.0 mg/kg feed for 28 days, dose-related
residues ranging from 0.007 mg/kg at the lowest to 1.2 mg/kg at the
highest dose level were found in kidney, liver and fat. After 7 days
of withdrawal, residues in all tissues and eggs were below the
detection limit (0.0006-0.032 mg/kg) for all dose levels (Smith &
Merricks, 1976b).
When diflubenzuron was fed to white leghorn and black sex-linked
cross hens at a level of 10 mg/kg feed for 15 weeks, detectable
residues were found in eggs, liver and visceral fat. Residues were
significantly higher in eggs from white leghorn hens than in eggs from
black sex-linked cross hens, the average levels being 0.55 and
0.38 mg/kg, respectively (Miller et al., 1976b).
6.3 Metabolic transformation
The metabolic fate of diflubenzuron has been studied in various
species. Metabolic pathways of diflubenzuron are shown in Fig. 4
In rats and cows the major metabolic pathway involves
hydroxylation of the phenyl moieties of the compound. About 80% of the
metabolites in rat urine were identified as 2,6-difluoro-3-
hydroxydiflubenzuron and 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the benzoyl
ureido bridge. The major part was excreted as 2,6-DFBA and
constituted more than half of the urinary metabolites. 4-CPU was not
detected in bile or urine in a significant quantity (De Lange et al.,
1975; Willems et al., 1980).
The major metabolite in cow urine was 2,6-difluoro-3-hydroxy-
diflubenzuron (45%). Relatively small quantities of 4-chloro-2-
hydroxy- (1.6%) and 4-chloro-3-hydroxydiflubenzuron (3.7%) and the
scission products 4-CPU (0.6%), 2,6-DFBA (6.0%) and 2,6-difluoro-
hippuric acid (6.9%) were present (Ivie, 1978).
Seuferer et al. (1979) reported that the soil microorganisms
Rhodotorula sp., Penicillium sp. and Cephalosporium sp. cannot
utilize diflubenzuron as a sole carbon and energy source. However,
accelerated breakdown of diflubenzuron occurred in the presence of
these organisms.
4.6 Ultimate fate following use
It appears that after direct spraying diflubenzuron is persistent
on foliage, it remains almost completely at the site of application on
the surface, and it does not penetrate the plants.
Diflubenzuron is readily degraded in soils of various types and
origin under aerobic or anaerobic conditions with a half-life in the
range of 0.5 to 1 week. It is metabolized by microorganisms
principally to 4-CPU and 2,6-DFBA. The latter is unstable with a
half-life of 3-5 days (Nimmo et al., 1984) to 4 weeks (Verloop &
Ferrell, 1977). The half-life of 4-CPU is about 6 weeks (Nimmo et
al., 1984). Free PCA has not been detected in soil.
In spite of rapid degradation in soil, small amounts of residue
(up to 1 mg/kg, depending on ageing time and growth stage of plants)
may be taken up by crops in treated soil (Thus et al., 1994).
Field applications of diflubenzuron produce soil residues which
might possibly lead to residues in rotational crops by re-uptake from
soil.
Studies with direct applications to field water show a moderate
persistence of diflubenzuron in water. Half-life values average one
week or less. This rapid rate of loss may be more dependent on
adsorption to organic matter than on microbial degradation.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air
No information is available on air concentrations of
diflubenzuron.
5.1.2 Water
A total of 1160 ha of insect-infested forest in Finland was
sprayed with diflubenzuron (25% WP) from a fixed-winged aircraft at an
application rate of 75 g a.i. in 50 litres water per ha. The residues
in "run-off" water (gathered in specially dug pits adjacent to the
sprayed area) decreased from 5 µg/litre one day after spraying to
0.1 µg/litre after 2 months. The concentration in water in open pits
was 0.1 µg/litre 1 and 7 days after application and 0.2 µg/litre
1 month after application. After 2 months no residues were detected.
All water samples taken from outside the treated area contained less
than 0.1 µg/litre (the limit of sensitivity) (Mutanen et al., 1988).
Diflubenzuron was found in the water of the Fraser River, Canada,
up to 71 days following application with diflubenzuron (1% granular
formulation) at a rate of 4.5 kg/ha (45 g a.i./ha). The peak value
was 1.8 µg/litre 8 days after treatment (Wan & Wilson, 1977).
After aerial application of diflubenzuron (25% WP formulation) to
two forest ponds in Canada, the maximum residue levels in water,
sediment, aquatic plants and fish were 13.82 µg/litre (at 1 h),
0.24 mg/kg (at 1 day), 0.36 mg/kg (at 1 day) and 0.11 mg/kg
(at 1 day), respectively. The rate of dissipation was rapid, non-
detectable levels being reached in 20 days for water, 5 days in
aquatic plants and 3 days in fish (Kingsbury et al., 1987).
A pond in Salt Lake County, Utah, USA, was treated with three
applications of diflubenzuron at a rate of 280.25 g a.i./ha.
Diflubenzuron was found at less than 0.05 mg/litre 4 days following
treatment (Booth et at., 1987).
Residues in three farm ponds in California treated with
diflubenzuron (2.5, 5 and 10 µg/litre) averaged 1.9, 4.6 and
9.8 µg/litre, respectively, 1-4 h after the applications. They
declined steadily averaging 0.5, 0.3 and 0.2 µg/litre, respectively,
2 weeks later. Residues in a small lake treated at 5 µg/litre
averaged 3.3 µg/litre following treatment and 0.4 µg/litre after
35 days. No residues were found in sediment samples taken post-
treatment (Apperson et al., 1978).
One hour after a single application of 45 g diflubenzuron/ha to
brackish water pools the residues in water and in sediment were
3.6 µg/litre and 80 µg/kg, respectively. The concentration in
sediment increased to 520 µg/kg after 1 day and reached its maximum of
780 µg/kg 4 days following application (Hester et al., 1986). After
6 applications of diflubenzuron at a rate of 145.73 g/ha to Utah Lake,
USA, the residues in sediments were less than 0.05 mg/kg (Booth et
al., 1987).
Other field studies with similar results have been reported by
Anon (1980), Smith & Edmunds (1985), Van Den Berg (1986), Huber &
Collins (1987), Jones & Kochenderfer (1988), Huber & Manchester
(1988), Downey (1990) and Sundaram et al. (1991). It is clear that a
variety of application scenarios will result in measurable residues of
diflubenzuron in water (Table 6).
The overall conclusion is that diflubenzuron residues in stagnant
water dissipate rapidly within days. In flowing water, e.g., in
wooded areas, diflubenzuron residues may peak shortly after rainfall
but such peak concentrations are very transient in nature.
5.1.3 Food and feed
Data on residues in food resulting from treatment with
diflubenzuron have been summarized by FAO/WHO (1982a,b, 1985a,b,
1986a,b).
Residue data obtained from various countries showed residues in
apples below 1.0 mg diflubenzuron/kg at 2 weeks after the last
application at recommended rates. Residues in whole citrus fruit were
below 0.5 mg/kg 1 week after the last application at the recommended
rate. Residues in soybean seed and cottonseed were generally below
the limit of determination (0.05 mg/kg).
Mushrooms have a residue pattern different from other plant
material. In mushrooms growing on diflubenzuron-treated soil, high
levels of the metabolite 2,6-DFBA are taken up from the soil.
Diflubenzuron was found at a level of 0.1 mg/kg, while the 2,6-DFBA
level was around 1 mg/kg (see chapter 4).
Residues in wild mushrooms after aerial application to forests in
Finland were on average 0.07 mg/kg 1 week after spraying with 75 g
diflubenzuron in 50 litre water per ha. In bilberries the residues
decreased on average from 0.2 mg/kg 1 day after spraying to 0.09 mg/kg
after 1 month (Mutanen et al., 1988).
Diflubenzuron applied as a wettable powder spray to growing
alfalfa at 20-100 g/ha showed initial residue levels of 1.8-8.5 mg/kg.
Residues of 0.3-1.5 mg/kg remained 22 days after applications (Lauren
et al., 1984).
Table 6. Summary and comparison of experimental parameters among key studies designed to measure environmental concentrations of
diflubenzuron in water
Medium Formulation a.i.% Method of Application Maximum Time for Minimum Time for References
treated application rate a.i. concentration maximum concentrationa minimum
concentration concentration
Farm ponds 25 WP 25 hand sprayer 2.5-10 µg/litre 1.9-9.8 µg/litre 1-4 h 0.5-0.2 µg/litre 14 days Apperson
(0.06-0.2 ha) from boat et al.
(1978)
Small lake 25 WP 25 hand sprayer 5 µg/litre 3.3 µg/litre 4 h 0.4 µg/litre 35 days Apperson
(18.6 ha) from boat et al.
(1978)
Pond W-25 25 hand-operated 0.28 kg/ha 56 µg/litre 96 h < 0.01 µg/litre 40 days Booth
spray et al.
applicator (1987)
Brackish 25 WP 25 clothes 0.045 kg/ha 7.5 µg/litre 48-72 h < 0.3 µg/litre 25-30 days Hester
pools sprinkler (1986)
Forest ponds 25 WP 25 aircraft 0.07 kg/ha 13.82 µg/litre 1 h < DL 20 days Kingsbury
(25 ha) (four et al.
atomizers) (1987)
Field plot 25 WP 25 fixed-wing 0.075 kg in 50 5.0 µg/litre 24 h < DL 60 days Mutanen
(1160 ha) aircraft litre water/ha et al.
(1988)
Fixed plots granular 1.0 aircraft 0.023 kg/ha, 1.8 µg/litre 192 h < DL 60 days Wan &
(3-40 ha) 0.46 kg/ha Wilson
(1977)
a DL = determination limit
After two soil applications of 67.26 g/ha, the residues of
diflubenzuron in the rotational crops (wheat, cabbage and onions) were
less than 0.01 mg/kg (Danhaus & Sieck, 1976).
Mian & Mulla (1983) studied the persistence of diflubenzuron in
stored wheat after applications of 1, 5 and 10 mg/kg. The residue
levels were 0.59, 2.75 and 5.00 mg/kg, respectively, 23 months after
treatment.
5.1.4 Forest plants and litter
The level of diflubenzuron residues in pine needles was on
average 3.0 mg/kg 1 day after application to the forest in Finland at
a rate of 75 g diflubenzuron in 50 litres water per ha. The level had
decreased to 0.2-0.3 mg/kg or was not detectable 2 months later
(Mutanen et al., 1988).
Booth et al. (1987) found diflubenzuron residues of less than
0.05 mg/kg in the forest litter 1, 4, 10 and 21 days after treatment
with 0.28 kg a.i./ha.
Sundaram (1986) studied the residues in a forest in Canada after
simulated aerial spraying of diflubenzuron in acetone and in fuel oil:
Arotex 3470 mixture, each at 90 g a.i. in 18 litre/ha. The residue
levels 1 h after application varied, respectively, from 23.8 to
30.6 µg/g in foliage and from 3.08 to 4.60 µg/g in litter. Forty-five
days after spraying the residue levels in foliage were 0.80 and
3.9 µg/g, respectively, for the above-mentioned formulations.
Spray deposit patterns and persistence of diflubenzuron in white
pine ( Pinus strobus L.) and sugar maple ( Acer saccharum Marsh.)
canopies, forest litter and soil were studied after aerial application
of a 250 g/kg wettable powder formulation (Dimilin WP-25) at
70 g a.i./ha, using three volume rates (2.5, 5 and 10 litres/ha), over
three blocks in a mixed forest near Kaladar, Ontario, Canada, during
1986 (Sundaram, 1991). In the spray block that received 10 litres/ha,
diflubenzuron persisted in foliage as long as 120 days after
treatment, but it lasted for only about a week in forest litter and
soil samples. At 2.5 and 5 litres/ha, diflubenzuron failed to persist
in foliage as long, and residues in litter and soil, which were barely
above the quantification limit, persisted only for a few days.
5.1.5 Aquatic organisms
Residues in fish are given in section 4.4.
5.2 General population exposure
Exposure of the general population to diflubenzuron via food and
drinking-water may occur.
Twelve volunteers with whole body dosimeters were exposed for 4 h
to Dimilin 25 W after simulated indoor treatment of carpets at
0.16 g/m2. Average deposition was 5.3 ± 2.3 µg diflubenzuron/cm2
carpet. Total dermal exposure ranged from 0.053 to 0.25 mg/kg body
weight per day to (average 0.15 ± 0.066 mg/kg body weight per day).
Assuming a dermal absorption of 0.2%, the total exposure via the
dermal route was calculated to be 0.0003 mg/kg body weight per day.
Air concentrations ranged from 10.2 to 32.4 µg/m3 during the first
4 h and were < 1 µg/m3 at 12-16 h. The total respiratory exposure
was calculated to be 0.0011 mg/kg body weight per day. The total
exposure, via the dermal and respiratory route, was calculated to be
0.0014 mg/kg body weight (Honeycutt, 1993).
5.3 Occupational exposure during manufacture, formulation or use
In a US Department of Agriculture report, human exposure via a
variety of exposure scenarios was estimated using standardized methods
and assumptions. The exposure scenarios included mixing and loading by
workers, via aircraft or truck spillage, and general public exposure
via the diet or resulting from occupational aerial spraying. Dermal
absorption of diflubenzuron was assumed to be 10%. Estimated
realistic doses for humans were < 0.003 mg/kg body weight per day
except where aircraft or truck spillages occurred, in which case
exposures were significantly higher. Estimated worst-case doses for
humans were < 0.01 mg/kg body weight per day, except where aircraft
or truck spillages occurred (USDA, 1985).
6. KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
6.1 Absorption
Diflubenzuron is absorbed from the digestive tract but only
poorly absorbed through the skin. Willems et al. (1980) found that in
rats the relative intestinal absorption diminished greatly with
increasing dose. Following a dose of 4 mg/kg body weight 42.5% was
absorbed, but only 3.7% of a 900 mg/kg body weight dose was absorbed.
Dermal absorption of 14C-diflubenzuron was only 0.2% when it was
applied to the shaved skin of rabbits as an aqueous micro-suspension
of 150 mg/kg (De Lange, 1979).
When applied dermally to cattle 14C-diflubenzuron was not
absorbed or degraded through the skin to any detectable degree (Ivie,
1978).
6.2 Distribution
Body tissues show little tendency to retain diflubenzuron.
Analysis of tissues for radiocarbon residues, 4 days (for sheep) or
7 days (for cows) after a single oral dose of 10 mg/kg body weight,
indicated that only the liver contained appreciable levels of
radioactivity, ranging from 2 to 4 mg/kg diflubenzuron equivalents
(Ivie, 1977).
More than one third of an oral diflubenzuron dose appeared in the
bile of a cannulated sheep (Ivie, 1977).
The highest 14C-diflubenzuron residue present in pig tissues
after a single oral dose of 5 mg/kg body weight was 0.43 mg/kg in the
gall bladder. All other tissue residue levels were found to be less
than 0.30 mg/kg (Opdycke et al., 1982a).
Twenty-two dairy cows were fed 14C-diflubenzuron (labelled in
both phenyl moieties) in a diet at dose levels of 0.05, 0.5, 5, 25 and
250 mg/kg feed for 28 days. Residues in blood, fat and muscle were
below the detection limit (0.0067-0.04 mg/kg) at all dose levels. They
were only detected following a dose of 250 mg/kg in the liver and
kidney where residues were 6.040 and 1.038 mg/kg, respectively.
Residues in milk were found at dose levels of 5 and 250 mg/kg, where
the highest levels of diflubenzuron were 0.009 and 0.20 mg/kg,
respectively (Smith & Merricks, 1976a).
In a study by Miller et al. (1976a), two dairy cows were fed
diflubenzuron at 0.25 or 1 mg/kg body weight per day for 4 months. A
third cow received an increased dosage of 8 to 16 mg/kg body weight
per day, the highest value being maintained for three months. In the
fat, liver and milk of the third cow, residues were 0.2, 0.13 and
0.02 mg/kg, respectively.
When dairy bull calves (four treated and four controls) received
diflubenzuron at 1.0 to 2.8 mg/kg body weight, residues were detected
only in the tissue samples of one bull (0.02 mg/kg in liver and
kidney, 0.04 mg/kg in the subcutaneous fat, and 0.08 mg/kg in the
renal and omental fat (Miller et al., 1979).
The maximum total residue in eggs 3 days after a single dose of
5 mg/kg 14C-diflubenzuron to hens was 0.248 mg/kg (Opdycke, 1976).
When laying hens were administered 14C-diflubenzuron at dose
levels 0.05, 0.5, and 5.0 mg/kg feed for 28 days, dose-related
residues ranging from 0.007 mg/kg at the lowest to 1.2 mg/kg at the
highest dose level were found in kidney, liver and fat. After 7 days
of withdrawal, residues in all tissues and eggs were below the
detection limit (0.0006-0.032 mg/kg) for all dose levels (Smith &
Merricks, 1976b).
When diflubenzuron was fed to white leghorn and black sex-linked
cross hens at a level of 10 mg/kg feed for 15 weeks, detectable
residues were found in eggs, liver and visceral fat. Residues were
significantly higher in eggs from white leghorn hens than in eggs from
black sex-linked cross hens, the average levels being 0.55 and
0.38 mg/kg, respectively (Miller et al., 1976b).
6.3 Metabolic transformation
The metabolic fate of diflubenzuron has been studied in various
species. Metabolic pathways of diflubenzuron are shown in Fig. 4
In rats and cows the major metabolic pathway involves
hydroxylation of the phenyl moieties of the compound. About 80% of the
metabolites in rat urine were identified as 2,6-difluoro-3-
hydroxydiflubenzuron and 4-chloro-2-hydroxy- and 4-chloro-3-
hydroxydiflubenzuron. About 20% underwent scission of the benzoyl
ureido bridge. The major part was excreted as 2,6-DFBA and
constituted more than half of the urinary metabolites. 4-CPU was not
detected in bile or urine in a significant quantity (De Lange et al.,
1975; Willems et al., 1980).
The major metabolite in cow urine was 2,6-difluoro-3-hydroxy-
diflubenzuron (45%). Relatively small quantities of 4-chloro-2-
hydroxy- (1.6%) and 4-chloro-3-hydroxydiflubenzuron (3.7%) and the
scission products 4-CPU (0.6%), 2,6-DFBA (6.0%) and 2,6-difluoro-
hippuric acid (6.9%) were present (Ivie, 1978).
 The major metabolites (approximately 50%) in sheep urine were
2,6-DFBA and 2,6-difluorohippuric acid (Ivie, 1978).
14C-Diflubenzuron uniformly radiolabelled in both rings was
administered to a pig as an oral dose of 5 mg/kg body weight. Of the
administered dose, 82% was eliminated in faeces as parent compound and
5% was recovered in urine. Identification of the metabolic products
in urine revealed 2,6-DFBA (0.28% of the dose), 4-CPU (0.82%), PCA
(1.03%) and 2,6-difluorobenzamide (0.83%). Cleavage of the urea
moiety between the benzoyl carbon and urea nitrogen was shown to be
the primary degradation pathway in pigs (Opdycke et al., 1982a).
In chickens only small quantities of the metabolites 2,6-DFBA,
4-CPU and PCA were found in excreta and tissues (Opdycke, 1976).
Neither induction nor inhibition of mixed-function oxidase activity
altered diflubenzuron metabolism in chickens (Opdycke et al., 1982b).
After 4 days daily doses of 7.8 g diflubenzuron/kg body weight,
De Bree et al. (1977) found PCA at a level of 30 ng/ml in rat plasma
and 323 ng/g in erythrocytes. PCA, estimated by the concentration in
the urine, represented at most 0.01% of the dose actually absorbed.
6.3.1 Metabolites - distribution, excretion, retention and turnover
When 14C-PCA was administered orally as single doses of 0.3,
3.0 or 30.0 mg/kg to male Fischer-344 rats, approximately 75% of the
administered radioactivity was excreted in the urine within 24 h,
while approximately 10% appeared in the faeces. Excretion was
virtually complete (92-97%) 7 days after dosing. The highest tissue
levels of radioactivity following a single intravenous dose of
3.0 mg/kg were found in the liver, fat, muscle and skin. Tissue
levels peaked within 5-60 min after dosing. By 3 days, concentrations
in all tissues except the blood had declined to < 0.3% of the dose
(Sipes & Carter, 1988). At this time, the only tissue containing more
than 1% of the dose was the cellular compartment of blood, which
contained 1-2% of the dose. The decline of PCA concentration in all
tissues, except for urine, faeces and intestinal contents, was
biexponential. The t alpha 1/2 for fat, muscle and skin was about
1.5 h, while the tß1/2 was approx. 43-59 h. The t alpha 1/2 for
liver was 3.5 h. Levels of unchanged PCA in all tissues peaked after
5 min following intravenous administration. The highest amount of
unchanged PCA was attained in muscle (15% of radioactivity in the
tissue) followed by skin (6%), fat (3%) and liver (2%). The decline of
PCA in all tissues, except for the liver, followed biexponential kinetics
with an estimated t alpha 1/2 of 8 min and a tß1/2 of 3 to 5 h.
PCA is rapidly metabolized to p-chloroacetanilide (PCAA) as the
initial step in the metabolism and excretion of PCA. The decline of PCAA
was monoexponential, the appearance half-life being approx. 6 min in the
testes and 15 min in the brain. The elimination half-life in the
brain, kidney, testes, muscle, skin and fat was around 1.0 to 2.0 h.
The elimination of PCA does not depend on either the dose or route of
administration. Approximately 4% of the urinary radioactivity in the
0-24 h urine sample was unchanged PCA; less than 1% was found in the
faeces. PCAA was not detected in either urine or faeces over a 3-day
period (Sipes & Carter, 1988).
After a single intravenous dose of 14C-PCA (3 mg/kg), maximal
tissue levels were reached within 15 min in most tissues. At this
time, most of the radioactivity was located in muscle (34%), fat
(14%), skin (12%), liver (8%) and blood (7%). Elimination half-lives
from tissues ranged between 1.5 and 4 h. By 8 h, approximately 90% of
the administered dose had been eliminated into urine and faeces. By
3 days, concentrations in all tissues, except blood, had declined to
< 0.3% of the dose (US NTP, 1989).
6.4 Elimination and excretion
After oral administration to rats of 5 mg diflubenzuron labelled
with 3H in the benzoyl and with 14C in the aniline moiety, 95% of
the 3H and 70-75% of the 14C radioactivity were retrieved in urine
and faeces. 2,6-DFBA was shown to constitute more than half of the
urinary metabolites (De Lange et al., 1977). Up to 1% of an oral dose
of 5 mg 14C-diflubenzuron labelled at the benzoyl moiety was
recovered in the expired air of rats (De Lange et al., 1974; Willems
et al., 1980).
When 14C-diflubenzuron, labelled in the aniline moiety, was
administered by gavage (4, 16, 48, 128, 900 and 1000 mg/kg body
weight) to rats, the urinary excretion was complete after 48-72 h.
Urinary excretion after single oral administration of diflubenzuron
relatively decreased with increasing dose level, being 27.6% of the
dose at 4 mg/kg and 1% at 1000 mg/kg (De Lange et al., 1977).
When 14C-diflubenzuron was administered at single oral doses of
12.5, 63.5, 202.5 and 925 mg/kg body weight to Swiss mice, the
excretion was almost completed within 48 h. The cumulative percentage
of the dose excreted in the urine decreased from 15% at the dose level
of 12.5 mg/kg to approximately 2% at 925 mg/kg (De Lange & Post,
1978).
Hawkins et al. (1980) studied the excretion of radioactivity in
urine and faeces after oral administration of 3H/14C-diflubenzuron
(7 mg/kg) to male cats. The radioactive dose was given on day 10 of a
15-day dosing regime of non-radioactive diflubenzuron (days 1-9 and
days 11-15). The excretion of radioactivity in urine accounted for
9.5 and 9.6% of the 14C and 3H doses, respectively, during 6 days
after dosing. The elimination of radioactivity in faeces accounted
for 77.3 and 71.6% of the 14C and 3H doses, respectively, during 6
days after dosing.
After an oral administration of 14C-diflubenzuron (5 mg/kg) to
female pigs, 82% of the dose was eliminated via faeces and 5% via
urine in 11 days (Opdycke, 1976).
About 85% of a single oral dose of 14C-diflubenzuron (10 mg/kg
body weight) administered to a cow was recovered in the faeces during
the first 4 days after treatment. About 15% was recovered in urine
and only about 0.2% was secreted in the milk (Ivie, 1977, 1978).
Sheep excreted 41% of the dose (10 mg/kg) in the urine and 43% in
the faeces during the 4 days after treatment. Bile-cannulated sheep
eliminated 24% of the dose in the urine, 32% in the faeces and 36% in
the bile. Sheep treated with 500 mg 14C-diflubenzuron/kg as a single
oral dose eliminated a much smaller proportion of the 14C in urine
and bile. This was probably due to reduced absorption from the
gastrointestinal tract when the sheep were given an exaggerated dose
(Ivie, 1977).
An oral dose of 5 mg 14C-diflubenzuron/kg administered to white
leghorn hens and Rhode Island red-barred Plymouth Rock buff cross hens
was rapidly excreted unaltered within the first 8 h. Up to 91 and
82%, respectively, were excreted within 13 days (Opdycke, 1976).
6.5 Retention and turnover
6.5.1 Biological half-life
From the studies of Willems et al. (1980) and Ivie (1978), the
half-life of diflubenzuron appears to be 12 h in rat and sheep and
18-20 h in the cow.
Diflubenzuron has been shown to pass intact through the
intestinal tract and remained active in the manure (Nimmo & de Wilde,
1977b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of diflubenzuron and its formulations to
different species is summarized in Table 7. No signs of intoxication
were observed during the 14-day period following a single
administration of diflubenzuron.
Van Eldik (1974) reported an intraperitoneal LD50 of
> 2150 mg/kg in rats and mice.
7.2 Short-term exposure
Rats (5 of each sex per group) were fed on a diet containing
diflubenzuron at concentrations of 0, 800, 4000, 20 000 and
100 000 mg/kg feed for 4 weeks. Behaviour, body weight, food and
water consumption were not affected by the treatment. There was a
dose-related increase in the met- and sulfhaemoglobin content of the
blood in all treated groups except for the methaemoglobin value for
females in the 800-mg/kg dose group. Lower erythrocyte, packed cell
volume (PCV) and haemoglobin values were observed in both sexes of the
100 000-mg/kg dose group. There was a dose-related increase in spleen
and liver weights. Only the dose level of 800 mg/kg did not affect
the liver weight (Palmer et al., 1977).
Five male Swiss-albino rats were given 96.7 mg diflubenzuron/kg
body weight per day, dissolved in corn oil, in their diet for 48 days.
The total dose was 4640 mg/kg body weight. Five controls were given
corn oil only in their diet. At the end of the study the treated
groups showed a significantly lower mean haemoglobin concentration
than the controls, and decreases in MCH and MCHC values (Berberian &
Enan, 1989).
Diflubenzuron was administered to Sprague-Dawley rats of the CD
strain (20 of each sex per group) at dietary levels of 10 000 and
100 000 mg/kg feed for 9 weeks, followed by a 4-week withdrawal
period. Lower values for red blood cell parameters were recorded at
both dose levels. An increase in reticulocyte count and a pallor of
the extremities and eyes were observed. The formation of
methaemoglobin occurred in both males and females, with approximately
5-8% of the available haemoglobin being transformed to methaemoglobin.
After a withdrawal period of 4 weeks methaemoglobin comprised less
than 2% of the available haemoglobin in tested animals compared with
approximately 0.6% in controls. Higher values for SGPT were recorded
as well as heavier liver, spleen and adrenal weights. Minor
enlargement of centrilobular hepatocytes in the highest dose group was
observed, but this finding disappeared after the withdrawal period
(Hunter et al., 1979).
Table 7. Acute toxicity of diflubenzuron and its formulations
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Diflubenzuron mouse: > 4640 rat: > 10 000 rabbit: > 30 mg/ rabbit: 40 mg rabbit: non- guinea-pig: non-
technical mg/kg body weight mg/kg body weight litre nominal instilled in eye; irritant (Taylor, sensitizing
(Koopman, 1977c) marginal irritant 1973a) (Prinsen, 1992)
(Davies &
rat: > 4640 mg/kg rabbit: > 4 ml/kg > 3.75 mg/litre Ligget, 1973)
body weight body weight of 50% actual (Berczy et
(van Eldik, 1973a; in gum tragacanth al., 1975a)
Koopman, 1977a) (Davies &
Halliday, 1974)
Diflubenzuron mouse: > 5000 rat: > 2000 mg/kg rat: > 13.8 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: 25%
90% concentrate mg/kg body weight body weight litre nominal instilled in eye; non-irritant w/w in paraffin
(Koopman & Pot, (Koopman, 1984a) very slight (OECD Guideline (Grade 1); weak
1986) > 2.49 mg/litre irritant (Koopman, 404) (Koopman, sensitizer (OECD
actual (Greenough 1984c) 1984d) Guideline 406)
rat: > 5000 mg/kg & McDonald, (Kynoch & Smith,
body weight 1986) 1986)
(Koopman, 1984b)
Dimilin WP 25% rat: > 40 000 rat: > 20 000 rat: > 150 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: non-
mg/kg body weight mg/kg body weight litre nominal instilled in eye; non-irritant to sensitizing
(Janssen & Pot, slight to only minimally (Kynoch & Elliott,
mice: > 40 000 1987e) > 3.5 mg/litre moderate, irritant 1978,a,b)
mg/kg body weight actual (Arts, transientsient eye (Taylor, 1973b;
(van Eldik, 1973b; 1991) irritation (Snoeij Chandran, 1981;
Koopman, 1977b) & Busé-Pot, 1991) Snoeij &
Busé-Pot, 1990)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin SC-48 rat: > 5000 mg/kg rat: > 2000 mg/kg rabbit: 0.1 ml rabbit: non- guinea-pig: weak
body weight body weight - instilled in eye; irritant (Janssen sensitizer
(Janssen & Pot, (Janssen & Pot, slight irritant & Pot, 1987b) (Kynoch &
1987c) 1987d) (Janssen & Pot, Parcell, 1987)
1987a)
Dimilin SC-15 albino rats: 0.1 rabbit: minimally
- - - ml instilled in irritant -
eye; non-irritant (Prinsen, 1989a)
(Prinsen, 1989b)
Dimilin 4F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 1.9 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight actual (Jackson instilled in eye; minimally irritant sensitizing
(Spanjers, 1988a) (Spanjers, 1988b) et al., 1990) non-irritant (Prinsen, 1988a) (Prinsen, 1989c)
(Prinsen, 1988b)
Dimilin 2F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 4.4 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig:
body weight body weight actual very instilled in eye; moderate irritant moderate (Grade
(Koopman, 1985d) (Koopman, 1985c) slightly irritant moderate irritant OECD Guideline III) (Kynoch &
(Zwart, 1985) OECD Guideline 404 (Koopman, Parcell, 1987)
405 (Koopman, 1985a)
1985b)
Dimilin ODC 45 rat: > 37 300 mg/kg rat: > 37 300 mg/kg rabbit: 0.1 ml rabbit: 0.5 ml
body weight body weight instilled in eye; moderate irritant
(Koopman & (Koopman, 1980b) marginally (Koopman,
Jongeling, 1979) irritant (Koopman, 1980c)
1980a)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin OF 6 rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 95.7 mg/ rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight litre nominal: instilled in eye; topical; mild sensitizing
(Besten et al., (Besten et al., > 2.17 mg/litre non-irritant irritant (Prinsen, 1993)
1993a) 1993b) actual (Janssen & (Janssen & van (Janssen & van
van Doorn, 1993a) Doorn, 1993c) Doorn, 1993b)
Wistar rats (10 of each sex per group) were fed diflubenzuron in
the diet for 13 weeks at concentrations of 0, 3.125, 12.5, 50 or
200 mg/kg feed. Behaviour, growth and food intake were unaffected by
the treatment. At the highest dose level the PCV value, the
haemoglobin concentration and the number of erythrocytes were
decreased. There was an increase in the SGPT and SGOT activities in
the males of the highest dose group at the end of the experiment. A
slight increase in the number of normally occurring scattered small
foci of necrotic parenchymal cells was observed, accompanied by
mononuclear inflammatory cell infiltration and proliferation of
reticuloendothelial system cells in the liver of both males end
females of the 50 and 200 mg/kg groups (Kemp et al., 1973a,b).
Absence of toxic effects in chronic/oncogenicity studies at low
dose levels necessitated re-evaluation of liver histopathology in the
90-day feeding study; "piece meal" liver cell necrosis was reported at
the two highest dose levels, i.e. 50 and 200 mg/kg feed. The original
slides of the study were re-evaluated by four histopathologists in
four different laboratories. They independently and unanimously
agreed that the lesions in the livers of treated rats were found to
the same extent in the livers of untreated rats. This showed that the
high dose levels in the study did not demonstrate a treatment-related
necrotic effect in the liver (Offringa, 1977).
Technical grade diflubenzuron was administered in the diet to
male and female 21- to 28-day-old Sprague-Dawley rats (40 of each sex
per group) at dose levels of 0, 160, 400, 2000, 10 000 and
50 000 mg/kg feed for 13 weeks. No apparent treatment-related effects
were noted on mortality, clinical observations, body weight gain, food
consumption, clinical chemistry or urinalysis. A treatment-related
significant increase in methaemoglobin concentration was noted in all
treated groups. Sulfhaemoglobin values showed increases at dose levels
of 2000 mg/kg or more. A significant treatment-related decrease in
haemoglobin, PCV and erythrocyte count was observed in males and
females at all dose levels by the end of the study. An increase was
noted in the reticulocyte count at all dose levels except 160 mg/kg,
and the number of the Heinz bodies was higher in the 10 000 and
50 000 mg/kg groups. After 7 weeks, spleen weights were increased in
the females at all dose levels, but after 13 weeks no effect was found
at 160 mg/kg. With the exception of the lowest dose level, all
treated groups showed a higher liver weight. The administration of
diflubenzuron resulted in a dose-related increase in the incidence of
chronic hepatitis and haemosiderosis of the liver. It was also
associated at all dose levels with haemosiderosis and congestion of
the spleen and mild erythroid hyperplasia of the bone marrow. The
severity of the lesions tended to increase with the dose. Liver
lesions were more severe in males than in females and were more severe
at 13 weeks than at 7 weeks. A no-observed-effect level (NOEL) was
not established (Burdock et al., 1980b; Goodman, 1980b).
Diflubenzuron was given to CFLP mice for 6 weeks at levels of 16
and 50 mg/kg feed. There were no clinical signs and no effects on
food consumption, body weight, blood chemistry or macroscopic
pathology. In three of the eight animals given 50 mg/kg, foci of
liver cell necrosis, with or without inflammatory cell filtration,
were noted. Other organs were not examined microscopically (Hunter et
al., 1974).
Diflubenzuron was administered to Swiss Webster mice in a 30-day
oral intubation study. Groups of five mice each received either no
treatment, vehicle control (Polyethylene glycol 400) or diflubenzuron
suspensions at dose levels of 125, 500 or 2000 mg/kg body weight.
Hepatic glutathione- S-transferase activity and morphological
characteristics were studied. Diflubenzuron was shown to elicit
hepatocellular changes at all dose levels. The activities of three
glutathione- S-transferases ( S-aryl, S-aralkyl and S-epoxide)
were irregularly altered in a non-dose-related manner. Light
microscopy revealed radial arrays of hepatocellular vacuolization
between the portal and central vein areas. There was evidence of an
increase in the amount of endoplasmic reticulum (Young et al., 1986).
Male and female mice of the B6C3F1 strain (40 of each sex per
group) received diflubenzuron (97.2% a.i.) in the diet at dose levels
of 0, 16, 50, 400, 2000, 10 000 or 50 000 mg/kg feed for 13 weeks. An
additional group of 100 of each sex served as a control. No
compound-related effects were apparent with respect to clinical signs,
survival, growth rates, total food consumption or gross pathology.
Significant treatment-related increases in met- and sulfhaemoglobin
concentrations were noted in all treated groups, except in the group
fed 16 mg/kg. At the higher dose levels, there was a decrease in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects on the weights of liver and spleen were
noted. In the females, adrenal weight was decreased (but not in a
dose-related fashion) at all dose levels after 7 weeks and increased
after 13 weeks in higher dose levels. Higher adrenal weight was
observed in treated males than in the controls. Histopathological
examination revealed treatment-related centrilobular hypertrophy of
hepatocytes, with or without cell necrosis, haemosiderosis of the
liver and spleen, extramedullary haematopoiesis and mild chronic
hepatitis in treated animals of both sexes, some of which effects were
observed at the lowest dose level (16 mg/kg). The liver lesions were
more severe in males than in females, being most severe in the high-
dose males. The NOEL for methaemoglobin formation was 16 mg/kg feed
(Burdock et al., 1980a; Goodman, 1980a).
HC/CFLP mice (40 of each sex per group) were fed diflubenzuron
(97.2% purity) for 14 weeks at levels of 0, 80, 400, 2000, 10 000 and
50 000 mg/kg feed. On the second day of treatment, the majority of
mice treated with 10 000 or 50 000 mg/kg showed dark eyes and/or
prominent caudal blood vessels. On day 5, blue/grey discoloration of
the extremities was noted for the majority of mice treated with
50 000 mg/kg. Mice in the lowest dose group exhibited no clinical
signs. Mortality, food consumption, water consumption and body weight
changes were not significantly affected by the treatment. Lower PCV
and red blood cell counts were found at all dose levels except
80 mg/kg. The total white blood cell count, lymphocyte count,
haemoglobin concentration, incidence of Heinz bodies and red blood
cell count were increased in all treated groups. At week 7, there was
an increase in the number of reticulocytes in treated mice,
particularly in males treated at 10 000 or 50 000 mg/kg. At week 14,
the reticulocyte counts were similar to those of the controls. A
treatment-related increase in both met- and sulfhaemoglobin was
recorded in all treated groups at weeks 7 and 14 of the investigation.
Plasma glutamic-pyruvic transaminase values were increased at all dose
levels, with the exception of 80 mg/kg feed. Lower blood cholesterol
levels were noted in the 2000, 10 000 and 50 000 mg/kg groups.
Macroscopic examination showed dark discoloration and/or enlargement
of the spleen and pale subcapsular areas of the liver in all dose
groups after both 7 and 14 weeks. Histopathological examination of
the spleen revealed increased haemosiderosis at all dose levels except
80 mg/kg. In the liver, areas of focal necrosis and/or fibrosis in
the parenchyma, with or without associated inflammatory cells,
fibroblasts or pigment-laden macrophages, were observed. At higher
dose levels necrotic and fatty hepatocytes and brown pigment-laden
Kupffer cells were found. A NOEL was not established (Colley et al.,
1981a,b).
Diflubenzuron was fed to groups of three male and three female
beagle dogs for 13 weeks at concentrations of 0, 10, 20, 40 and
160 mg/kg diet. No effect of the treatment on behaviour, body weight
or food and water consumption was observed. Elevated SAP and SGPT
values were recorded for some dogs receiving 40 or 160 mg
diflubenzuron/kg feed. After 6 weeks, methaemoglobin and another
abnormal pigment, probably sulfhaemoglobin, were demonstrated in dogs
given 160 mg/kg. After 12 weeks of administration, some recovery was
observed. Organ weights and gross and microscopic evaluation did not
reveal any treatment-related effects. The NOEL for methaemoglobin
formation was 40 mg/kg feed (Chesterman et al., 1974).
Diflubenzuron was administered daily in gelatin capsules to male
and female beagle dogs (6 of each sex per group) at dose levels of 2,
10, 50 or 250 mg/kg body weight per day for 52 weeks. There were no
treatment-related effects on mortality, food consumption or body
weight gain. Dose-related marginal increases in methaemoglobin and
sulfhaemoglobin were recorded from 10 mg/kg upwards. At 50 and
250 mg/kg, haemoglobin concentration and MCHC were decreased whereas
reticulocyte and platelet counts were increased. Heinz bodies were
also detected in several animals receiving 50 and 250 mg/kg. Dose-
related increases in liver and spleen weights were found in the 50 and
250 mg/kg males. Histopathological evaluation of the liver revealed
an increase in the incidence of pigmented macrophages and Kupffer cell
siderosis at 50 and 250 mg/kg in both males and females. The NOEL
based on the increase in met- and sulfhaemoglobin was 2 mg/kg body
weight (Greenough et al., 1985).
In a study by Berczy et al. (1975c), rats were exposed daily for
a one-hour period to technical diflubenzuron dust at nominal
concentrations of 0, 0.5, 5.0 and 50 mg/litre air, respectively (the
actual concentrations were 0, 0.12, 0.87 and 1.85 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days per week. The methaemoglobin levels in male rats at the two
lower concentrations and female rats of all test groups were
significantly higher than those of controls.
Rabbits were exposed daily for one-hour periods to technical
diflubenzuron dust at concentrations of 0.5, 5.0 and 25 mg/litre air
(the measured concentrations were 0.15, 0.75 and 1.99 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days each week. There were no signs of irritation in animals
exposed to 0.5 mg/litre, but mild transient respiratory irritation was
seen at the two higher concentrations. Haematological examination,
biochemistry tests and macroscopic inspection revealed no treatment-
related abnormalities (Berczy et al., 1975b).
Diflubenzuron, at levels of 4.64, 10 and 21.5% weight/volume was
applied daily to the intact and abraded skin of rabbits at a dosage
level of 1.5 ml/kg body weight, 5 days a week, for 3 consecutive weeks
(equivalent to 69.6, 150 and 322.5 mg diflubenzuron/kg body weight per
day). The sulfhaemoglobin level was increased in one rabbit out of 20
in the 10% group, and in 5 rabbits out of 20 at the high treatment
level. The 10% treatment level was considered to be the NOEL based on
sulfhaemoglobin formation (Davies et al., 1975).
7.3 Long-term exposure
Diflubenzuron was administered to Sprague-Dawley rats (60 of each
sex per group) in their diet at levels 0, 10, 20, 40 and 160 mg/kg
feed for 104 weeks. There were no treatment-related effects on body
weight gain, food intake, renal function or on macroscopic and
microscopic pathology. In rats treated with 160 mg/kg, significantly
higher methaemoglobin levels were recorded. The tumour profile of
treated rats was similar to that of the controls. The NOEL based on
methaemoglobin was 40 mg/kg, equivalent to a mean intake of 1.43 and
1.73 mg/kg per day for males and females, respectively (Hunter et
al.,1976).
When diflubenzuron was administered in the diet to male and
female Sprague-Dawley rats (50 of each sex per group) at dosage levels
of 0, 156, 625, 2500 and 10 000 mg/kg feed for 104 weeks, there was no
evidence of an effect on mortality or treatment-related clinical
signs. Significantly increased absolute methaemoglobin and
sulfhaemoglobin values were observed in all male treatment groups.
However, increases in relative methaemoglobin values (% of total
haemoglobin) were only noted in the 156, 2500 and 10 000 mg/kg groups,
while sulfhaemoglobin increases were noted in the 156, 625 and
10 000 mg/kg females. There was a significant increase in absolute
and relative spleen weights in the two highest dose groups of both
sexes, together with haemosiderosis in spleen and liver. There was no
evidence of carcinogenicity after 2 years of feeding diflubenzuron
(Burdock et al., 1984).
In a study by Hunter et al. (1975), diflubenzuron was
administered to CFLP mice for 80 weeks at dietary levels of 0, 4, 8,
16 and 50 mg/kg feed. There were no overt signs of reaction to
treatment. Behaviour, mortality, food and water consumption and body
weight were unaffected by the treatment. The macroscopic changes
observed were those commonly seen in mice of this strain and age. No
histopathological changes were seen that were considered to be related
to the administration of diflubenzuron. The incidence of liver cell
tumours was higher in this study than it was in other studies
performed in these laboratories using this strain of mouse. The
increase was seen in both treated and control groups of either sex and
showed no evidence of a treatment-related effect. There was no
evidence of a treatment-related effect on tumour incidence in the CFLP
mouse.
Male and female HC/CFLP mice (88 of each sex per group) were fed
diflubenzuron at dietary levels of 0, 16, 80, 400, 2000 and
10 000 mg/kg feed for 91 weeks. There was no indication of a
treatment-related effect on survival, food consumption or body weight
gain. Treatment-related elevations of MCH and MCHC values were
recorded from week 26 onwards in mice given 10 000 mg/kg. The
incidence of Heinz bodies increased in a dose-related manner among
mice given 400, 2000 or 10 000 mg/kg from week 52 onwards. Dose-
related increases in methaemoglobin levels were found from week 26
onwards and in sulfhaemoglobin levels from week 52 onwards in the mice
fed 80 mg/kg or more. Elevated alkaline phosphatase (AP) activities
were seen at weeks 24, 76 and 89 among male mice receiving 2000 and
10 000 mg/kg and at week 84 among mice of the 400 mg/kg dose group.
An increased incidence of splenic and/or hepatic enlargement was seen
among mice treated with 10 000 mg/kg. Cyanosis of the skin was noted
among mice at 400, 2000 and 10 000 mg/kg. Increased liver and spleen
weights were found among mice given 2000 and 10 000 mg/kg. An
increased incidence of hepatocyte enlargement and increased
extramedullary haematopoiesis in the liver and spleen were seen in
mice at high dose levels. In the 400, 2000 and 10 000 mg/kg groups,
there was an increased incidence of siderocytosis in the spleen and of
pigmented Kupffer cells in the liver. There was no treatment-related
effect on tumour incidence (Colley et al., 1984).
7.4 Skin and eye irritation; sensitization
Relevant data are given in Table 7.
Administration of technical diflubenzuron to the intact and
abraded skin of albino rabbits did not produce irritation of the skin
after exposure times of 24 and 72 h (Taylor, 1973a,b).
Diflubenzuron was found to be a moderate irritant to the rabbit
skin after application of 0.5 ml of 45% oil dispersible concentrate
for 24 h (Koopman, 1980a; Prinsen, 1990).
Diflubenzuron (both technical and 45% oil dispersible
concentrate) was considered to be marginally irritant to the rabbit
eye (Davies & Ligget, 1973; Koopman, 1980b).
Diflubenzuron (48% water-based paste) was not found to be a
dermal sensitizer in guinea-pigs (Kynoch & Parcell, 1987).
Technical diflubenzuron was studied for skin sensitization in a
maximization test on guinea-pigs and was found to be non-sensitizing
(Prinsen, 1992). However, some formulations are mild sensitizers
(Table 7).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Diflubenzuron was fed to pathogen-free rats of the CFY strain
(20 of each sex per group) at dietary levels of 0, 1000 and
100 000 mg/kg for one generation and one litter. The animals were
maintained on their respective diets for 9 weeks prior to mating.
There were no clear effects on mating performance, pregnancy rate,
duration of gestation, litter size, offspring mortality, litter weight
or the type and distribution of abnormalities. Dose-related effects
of diflubenzuron were demonstrated at 17 weeks in adults and consisted
of reduced values for PCV, haemoglobin, total red cells count and
MCHC, increased values for methaemoglobin, MCV, spleen weight and
siderocyte incidence in the spleen, and the occurrence of iron-
pigment-containing Kupffer cells in the liver. A dose-related effect
on the liver was also shown by increased weight and SGPT activity and
centrilobular hepatocyte enlargement. Reduced blood glucose
concentrations were recorded in both treated groups. At the highest
dosed level, the offspring showed increased liver and spleen weights
for both sexes (Palmer et al., 1978).
In a three-generation reproductive study with rats fed
diflubenzuron at concentrations of 10, 20, 40 and 160 mg/kg feed, no
adverse treatment-related effects on mating performance, pregnancy
rate, duration of gestation or litter parameters (total loss, size,
mean pup weight, mortality, abnormalities) were found (Palmer & Hill,
1975a).
After gavage administration of diflubenzuron at doses of 1, 2 and
4 mg/kg body weight per day to pregnant rats during days 6-15 of
gestation, no effects were observed on embryonic or fetal development
(Palmer & Hill, 1975b).
Treatment of pregnant New Zealand white rabbits at oral doses of
1, 2 and 4 mg/kg body weight per day during days 6-18 of gestation did
not affect embryonic or fetal development as assessed by the incidence
of major malformations, minor anomalies and skeletal variants (Palmer
& Hill, 1975c).
In a study by Booth (1977), pregnant Swiss mice were fed a diet
containing 50 mg/kg of diflubenzuron (partly 14C) for a period of
17 days. Some of these mice were killed at day 17 after conception,
while the others were allowed to give birth. The results of this
study showed that mucopolysaccharide synthesis in embryonic mouse-limb
cartilage was normal. Diflubenzuron did not pass through embryonic
membranes nor was it passed from mother to suckling young mice.
Analysis of 226 embryos showed no gross teratogenic effects.
Two groups of 24 timed-mated female rats of the Crl:CD (SD) BR
strain were dosed once daily by the oral route between days 6 and 15
of pregnancy. One group was dosed with diflubenzuron at 1000 mg/kg
per day, while the other group was given the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 20 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths or treatment-related changes in clinical condition. Treatment
did not affect maternal growth or food consumption. There were no
maternal abnormalities at necropsy that were considered to be related
to treatment. The mean numbers of corpora lutea, implantations and
live fetuses were similar in all groups. Both pre- and post-
implantation losses were unaffected by treatment. Fetal weights and
sex ratio were unaffected by diflubenzuron treatment. The incidences
of major external/visceral and skeletal abnormalities, and minor
external/visceral abnormalities were not affected by diflubenzuron
treatment. The incidence of fetuses with minor skeletal abnormalities
was slightly higher in the treated group, but was within the usual
background range. There were intergroup differences in the
proportions of fetuses with specific minor abnormalities and variants
of skeletal ossification. For some bones, the differences achieved
statistical significance. However, on balance, treated group fetuses
were considered to be similarly ossified to the control fetuses. Oral
administration of diflubenzuron at a dose level of 1000 mg/kg per day
did not elicit maternal toxicity or any evidence of embryotoxicity
(Kavanagh, 1988a).
Two groups of 16 timed-mated female New Zealand White rabbits
were dosed once daily by the oral route from days 7 to 19 of
pregnancy, inclusive. One group was dosed with diflubenzuron at
1000 mg/kg per day and the other group with the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 28 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths, changes in clinical condition or abnormalities at maternal
necropsy considered to be related to diflubenzuron treatment. Two
animals were killed prematurely during the study, one from the control
group and one from the treated group. The changes in clinical
condition and abnormalities observed at necropsy in these animals were
considered to be unrelated to treatment. Treatment did not affect
maternal growth or food consumption. The mean numbers of corpora
lutea, implantations and live fetuses were similar in all groups. Both
pre- and post-implantation losses were unaffected by treatment. Fetal
weights and sex ratio were unaffected by diflubenzuron treatment. The
incidence of both major and minor external/visceral and skeletal
abnormalities, as well as the numbers of skeletal variants, was
unaffected by diflubenzuron treatment. Oral administration of
diflubenzuron at a dose level of 1000 mg/kg per day did not elicit
maternal toxicity or any evidence of embryotoxicity (Kavanagh, 1988b).
In a study by Kubena (1982), diflubenzuron was fed at levels of
0, 2.5, 25 and 250 mg/kg feed to male and female layer-breed chickens
from 1 day of age through a laying cycle. Characteristics measured
were egg production, egg weight, eggshell weight, fertility,
hatchability and effects on the progeny. Feeding diflubenzuron at
levels up to 250 mg/kg feed did not affect these characteristics.
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller & Booth, 1976).
7.6 Mutagenicity and related end-points
Diflubenzuron was examined by in vitro and in vivo
mutagenicity tests. The results are summarized in Table 8.
Mutagenicity tests have also been carried out with the major
metabolites of diflubenzuron (Table 9).
Table 8. Mutagenicity tests with diflubenzuron
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Microorganisms
Reverse Salmonella typhimurium
mutation TA98, TA100, 10-1000 µg/plate +/- negative Bryant (1976)
TA98, TA100, TA1537, TA1978 1000 µg/spot +/- negative Bryant (1976)
Reverse S. typhimurium 0.1-500 µg/plate +/- negative Brusick & Weir
mutation TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Reverse S. typhimurium 10, 100 or 1000 µg/plate; +/- negative McGregor et al.
mutation TA98, TA100, 19, 186, 1860 µg DFB/plate (1979)
TA1535, TA1537 (as Dimilin W-25)
Reverse S. typhimurium up to 5000 µg/plate +/-b negative Moriya et al.
mutation TA98, TA100, TA1535, (1983)
TA1537, TA1538
Escherichia coli. WP2 hcr
Reverse S. typhimurium up to 1000 µg/plate +/- negative Koorn (1990)
mutation TA98, TA100, TA1535,
TA1537, TA1538
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammalian cells in vitro
Forward L5178Y mouse lymphoma 1.17-300 µg/ml +/- negative McGregor et al.
mutation cells (1979)
Chromosomal Chinese hamster ovary up to 200 µg/ml +/- negative Taalman & Hoorn
aberrations cells (1986)
Unscheduled human diploid WI-38 cells 50-1000 µg/ml +/- negative Brusick & Weir
DNA synthesis (blocked in the G1 phase) (1977c)
DNA repair rat hepatocytes up to 333 µg/ml negative Enninga (1990)
(unscheduled
DNA synthesis)
Cell transformation BALB-3T3 cells, 0.02-0.312 µg/ml - negative Brusick & Weir
in vitro (1977b)
Cell transformation pregnant hamster, ip 10, 200 and 500 mg/kg negative Quarles et al.
(transplacental injection on 10th day of body weight (1980)
transformation) gestation, fetal cell culture
3 days after injection
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammals
Micronucleus mouse bone marrow 15, 150, 1500 mg/kg body negative McGregor et al.
weight 30 and 6 h before (1979)
necropsy
Dominant male mice mated to 1000 and 2000 mg/kg body negative Arnold et al.
lethal 3 females weekly for 6 weeks weight intraperitoneal (1974)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b Source of S9 not indicated
Table 9. Mutagenicity tests with diflubenzuron metabolites
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
A. 4-chlorophenylurea
Microorganisms
Reverse mutation Salmonella typhimurium
and differential TA98, TA100, TA1535, 1000 µg/spot +/- negativeb Dorough (1977)
killing TA1537, TA1538, TA1978
Reverse mutation TA98, TA100 10, 100, 500, negative
1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 6.25-50 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Cell BALB-3T3 cells, 0.019-0.312 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977a)
0.312 mg/ml
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
B. 2,6-Difluorobenzoic acid
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negative Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Reverse mutation S. typhimurium 10, 100, 500,
TA98, TA100 1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977b)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 75-500 µg/ml +/- positive Matheson & Brusick
DNA synthesis (blocked in the G1 phase) with (1978a)
activation
Cell BALB-3T3 cells, 0.156-2.5 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977b)
2.5 mg/ml
C. 4-Chloroaniline (PCA)
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negativeb Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Reverse mutation TA98, TA100 10, 100, 500, positive in
1000 µg/plate TA98 at 500
and 1000 µg
with
activation
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977c)
TA1537, TA1538
Reverse mutation S. typhimurium
TA98, TA100, TA1530, 0-1500 µg/plate +/- negative Gilbert et al. (1980)
TA1535, TA1537, TA1538
Reverse S. typhimurium C3076, 1000 µg/plate +/- negative Thompson et al.
mutation D3052, G46, TA98, TA100, (1983)
TA1535, TA1537, TA1538,
Escherichia coli WP2,
WP2 uvrA
Reverse S. typhimurium 3333 µg/plate +/- positivec Dunkel et al. (1985)
mutation TA98, TA100, TA1535,
TA1537, TA1538,
Escherichia coli
Reverse S. typhimurium 1666 µg/plate +/- positived Mortelmans et al.
mutation TA97, TA98, TA100, TA1535 negative (1986)
DNA damage Escherichia coli polA+/polA- 250 µg/plate - positive Rosenkranz & Poirier
(1979)
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Mutation Aspergillus nidulans 200 µg/ml - positive Prasad (1970)
Mammalian cells
Forward mutation L5178Y mouse lymphoma +/- positived Caspary et al. (1988)
tk+/- cells
Unscheduled human WI-38 cells 250-1000 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Unscheduled rat primary hepatocytes 5-50 µg/ml - positive Williams et al.
DNA synthesis (1982)
Unscheduled rat primary hepatocytes 50 nmol/ml - negative Thompson et al.
DNA synthesis (1983)
Sister chromatid Chinese hamster ovary 1600 µg/ml +/- positived US NTP (1989)
exchange cells
Chromosomal Chinese hamster ovary cells 1000 µg/ml +/- positived US NTP (1989)
aberrations and negative
Cell BALB-3T3 cells, 0.039-0.625 mg/ml not stated negative Matheson & Brusick
transformation in vitro (1978c)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b No increase in revertants, strains TA1538/TA1978 positive for differential killing
c Tested in three independent laboratories
d Tested in two independent laboratories
According to the findings presented, neither diflubenzuron nor
its major metabolites may be considered to have mutagenic effect.
Several positive effects were, however, obtained with PCA.
7.7 Carcinogenicity
Long-term carcinogenicity studies were described in section 7.3.
In both mouse and rat oncogenicity studies, diflubenzuron at dose
levels up to 10 000 mg/kg feed caused no change in tumour profile or
onset of tumours. In the rat oncogenicity study, the incidence of
sarcoma in the spleen and phaeochromocytomas was not increased. In
the mouse oncogenicity study, the incidence of hepatocellular
neoplasms or haemangiosarcomas in spleen and liver was not increased.
Therefore, diflubenzuron, in combination with its metabolites as
generated in the animal metabolic system, is not oncogenic.
Significantly, there were no non-neoplastic or neoplastic lesions of
the vasculature, including that of liver and spleen, in B6C3F1 male
mice treated with diflubenzuron. Similarly, there were no fibrotic or
carcinomatous lesions in the spleen of treated male F-344/N rats.
7.8 Other special studies
Diflubenzuron has been studied in mice for its growth-inhibiting
activity in serially transplanted B16 malignant melanoma and CA1025
skin carcinoma. A single 800 mg/kg intraperitoneal injection of
diflubenzuron induced rapid (24 h) decreases in tumour volume in 78%
and 66% of the tumours, respectively, while in control mice 85% of the
melanomas and 91% of the skin carcinomas increased in volume over the
same time period (Jenkins et al., 1984). These observations were
later confirmed, and it was suggested that the activity was due to
derivatives of a hydroxylated metabolite (Jenkins et al., 1986).
Studies of nucleoside uptake by Harding Passey melanoma cells
in vitro indicated a rapid (< 5 min) inhibition of the uptake of
uridine, adenosine and cytidine, but not of thymidine, by
diflubenzuron; this could not be reversed by washing. De novo
nucleic acid synthesis was not impaired and in vitro cell growth was
unaffected (Mayer et al., 1984).
El-Sebae et al. (1988) tested the effect of diflubenzuron on
protein and RNA biosynthesis by rabbit liver and muscle tissues kept
in an incubation medium. The synthesis of protein and RNA was
significantly stimulated in the liver and inhibited in the muscle by
graded doses. The maximum effect on both tissues was reached at 5 µg
diflubenzuron/ml for protein synthesis and at 0.2 µg/ml for RNA
synthesis, the effect on protein synthesis being more pronounced than
that on RNA synthesis in both tissues.
7.8.1 Special studies on met- and sulfhaemoglobin formation
The ability of diflubenzuron to induce methaemoglobin and
sulfhaemoglobin formation has been recognized since the initial
toxicity studies on the compound. Methaemoglobinaemia has been
demonstrated after oral, dermal and inhalatory exposure to
diflubenzuron in various species (see section 7.2 and 7.3). It is the
most sensitive parameter in this case.
Fifteen male Wistar rats received diflubenzuron (technical) by
gastric intubation at a dose level of 5000 mg/kg body weight per day
for 8 days. No effect was observed on Heinz body formation, whereas
met- and sulfhaemoglobin levels were significantly increased when
compared with the control group. The increase in the methaemoglobin
level was about 6% (Keet, 1977a).
When diflubenzuron was administered to male Wistar rats for 28
days at oral doses of 100 and 500 mg/kg body weight, it induced
elevation of methaemoglobin concentration and reticulocytes count in
both of the treated groups. However, there was no dose-response
relationship at the dose levels investigated (Tasheva & Hristeva,
1991, 1993).
Technical diflubenzuron was administered by gastric intubation to
male Swiss mice daily for a period of 14 days at dose levels of 0, 8,
40, 200, 1000 and 5000 mg/kg body weight. Body weight measurement and
macroscopic evaluation did not reveal any effect of the treatment. At
dose levels of 1000 and 5000 mg/kg the percentages of methaemoglobin
and erythrocytes containing Heinz bodies were increased. The
sulfhaemoglobin level was statistically significantly increased at
200, 1000 and 5000 mg/kg in comparison to the control group. The NOEL
was considered to be 40 mg/kg body weight based on sulfhaemoglobin
(Keet, 1977b).
When female mice were fed 0, 50, 200, 400, 1000 and 2000 mg
diflubenzuron/kg feed for 30 days, sulfhaemoglobin was demonstrated in
the blood from 200 mg/kg onwards (being 13% of total haemoglobin at
2000 mg/kg). Mice fed 1000 and 2000 mg/kg showed signs of cyanosis
after 3 weeks. Recovery was completed after a 3-week withdrawal
period (Bentley et al., 1979).
When 15 male New Zealand White rabbits were fed technical or
analytically pure diflubenzuron (640 mg/kg feed) for 21 or 18 days,
respectively, the methaemoglobin and sulfhaemoglobin levels were
significantly increased (Keet, 1977c).
Male and female cats (24 of each sex per group) received
diflubenzuron orally for 21 days at dose levels of 0, 30, 70, 100, 300
and 1000 mg/kg body weight and were observed for the subsequent 14-day
period. A dose-related elevation of methaemoglobin level was observed
with ceiling values at 300 and 1000 mg/kg. For male cats the NOEL was
30 mg/kg, but no NOEL was achieved for females. Increased
sulfhaemoglobin and Heinz bodies were observed in all treated groups.
NOEL values for sulfhaemoglobin were not achieved. The haemoglobin
concentration, reticulocyte number and organ weights were not affected
by the treatment (Schwartz & Borzelleca, 1981).
After dermal application of technical diflubenzuron at a dose
level of 1.5 ml/kg body weight for 18 days to rabbits, the
methaemoglobin level was increased (Keet, 1977c).
The available data demonstrate that dose-response relationship
for production of methaemoglobin exists. This is considered to be the
most sensitive end-point after repeated exposure in experimental
animals.
Table 10 summarizes the effects on methaemoglobinaemia as
determined in various studies.
7.9 Toxicity of metabolites
In rat metabolic studies it was shown that about 20% of absorbed
diflubenzuron is metabolized to 2,6-DFBA and its counterpart 4-CPU.
Only a small fraction of the 4-CPU is metabolized to PCA (see
section 6).
The acute oral toxicity of the major metabolites of diflubenzuron
is summarized in Table 11.
Loss of activity, catatony, paralysis and severe bradypnoea were
observed in rats treated with the metabolite 4-CPU. The minimum
symptomatic dose level was 100 mg/kg body weight. At autopsy, the
animals showed congested blood vessels and haemorrhage in the
gastrointestinal tract (Koelman-Klaus, 1978a).
Rats dosed with 2,6-DFBA showed slight increase in startle
response, activity, abdominal and limb tone, slight decrease in
grooming activity, slightly abnormal gait and body posture, mild
restlessness, irritation and aggressivity, pilo-erection and increased
alertness. The minimum symptomatic dose level was 464 mg/kg body
weight (Koelman-Klaus, 1978b).
Mutagenicity tests have been carried out with 2,6-DFBA, 4-CPU and
PCA (see section 7.6 and Table 9).
Table 10. Summary of the effects on methaemoglobinaemia in various species
Species Route Duration No-observed-effect level Reference
Rat diet 4 weeks male: not achieved; female: 800 mg/kg feed Palmer et al. (1977)
(equivalent to 45 mg/kg per day)
Rat diet 13 weeks not established - lowest dose tested 160 mg/kg feed Burdock et al. (1980b)
Rat diet 104 weeks 40 mg/kg feed (equivalent to 2 mg/kg Hunter et al. (1976)
per day body weight)
Mouse oral 2 weeks 200 mg/kg body weight Keet (1977b)
Mouse diet 13 weeks male/female: 16 mg/kg feed (equivalent to Burdock et al. (1980a)
2.4 mg/kg body weight per day)
Mouse diet 14 weeks not established - lowest dose tested 80 mg/kg feed Colley et al. (1981a,b)
Mouse diet 91 weeks male/female: 16 mg/kg feed (equivalent to Colley et al. (1984)
2.4 mg/kg body weight per day)
Cat oral 3 weeks male: 30 mg/kg body weight; female: not achieved Schwartz & Borzelleca (1981)
Dog diet 13 weeks male/female: 40 mg/kg feed Chesterman et al. (1974)
Dog oral (capsules) 52 weeks male/female: 2 mg/kg body weight Greenough et al. (1985)
Table 11. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50 Reference
(mg/kg)
4-Chlorophenylurea rat male oral 1080 Koelman-Klaus
female oral 1210 (1978a)
2,6-Difluorobenzoic rat male, oral 4640 Koelman-Klaus
acid female (1978b)
7.9.1 Carcinogenicity studies with 4-chloroaniline
The diflubenzuron metabolite, 4-chloroaniline (PCA), has been
assayed for carcinogenicity by the US NCI (1979) and by the US NTP
(1989) using Fischer-344 rats and B6C3F1 mice on both occasions. In
the earlier of these studies, technical-grade PCA was administered at
dietary concentrations of 250 and 500 mg/kg to rats and 2500 and
5000 mg/kg to mice. Groups of 50 male and 50 female animals of each
species were randomized to the treatment groups at approximately six
weeks of age. The control groups consisted of 20 animals of each sex
and species. All animals which survived were treated for 78 weeks and
observed, untreated, for a further 24 weeks (rats) or 13 weeks (mice).
Survival in all groups was good and it was judged that there were
adequate numbers at risk for late-developing tumours. In rats, the
most significant findings were treatment-related proliferative splenic
capsular and parenchymal lesions in males and females and, in male
rats of the high-dose group, the occurrence of several types of
unusual splenic neoplasms (i.e. fibroma, fibrosarcoma, sarcoma NOS,
haemangiosarcoma and osteosarcoma) which appeared to arise from areas
of capsular or parenchymal fibrosis. These neoplasms were combined
for analysis because it was considered that the fibromas were a benign
form of sarcoma and that the neoplasms had a common cellular origin.
The combined incidences were 0/20 control, 0/49 low-dose and 10/49
high-dose rats. This result indicated a carcinogenic effect of
treatment in male rats. There was no similar effect in the spleen of
females. In mice of each sex, there was increased incidence of
haemangiomas and haemangiosarcomas in various organs. The combined
incidences were 2/20 in control males, 10/50 in low-dose males and
14/50 in high-dose males, and 0/18 in control females, 3/49 in low-
dose females and 8/42 in high-dose females. In addition, there was,
in female mice only, a non-significant increase in hepatocellular
carcinomas and adenomas combined (0/18 control, 1/49 low-dose and 6/41
high-dose). The smaller number of control group animals in these
experiments significantly reduced the statistical power. It was
recognized that PCA is unstable in feed and so the animals in the
early experiments may have received lower doses than intended.
Consequently, PCA was administered by gavage with an aqueous vehicle
containing hydrochloric acid in the later experiments (US NTP, 1989).
Groups of 49 or 50 rats and mice of each sex (7 to 8 weeks old)
were administered PCA at dose levels of 2, 6 or 18 mg/kg (rats) or 3,
10, or 30 mg/kg (mice) on 5 days per week for 103 weeks. Vehicle
control groups of 50 males and 50 females received deionized water by
gavage. Survival was adequate for analysis, although variable. It was
lower, for example, in the vehicle control groups of rats, which was
attributable to the higher incidence of mononuclear cell leukaemia in
these groups. Significant, non-neoplastic findings in rats included
treatment-related increases in the incidence of splenic fibrosis
(males: 3/49 control, 11/50 low-dose, 12/50 mid-dose, 41/50 high-dose;
females: 1/50 control, 2/50 low-dose, 3/50 mid-dose, 42/50 high-dose),
lipocytic infiltration of the spleen (males: 0/49, 0/50, 0/50, 24/50;
females: 0/50, 0/50, 0/50, 11/50) and adrenal medullary hyperplasia
in female rats (4/50, 4/50, 7/50, 24/50). In addition, the
methaemoglobin level was consistently increased in the mid- and high-
dose groups of male rats at 6, 12, 18 and 24 months. There was no
NOEL for this parameter in male rats. Bone-marrow hyperplasia was
also observed. The combined incidences of uncommon splenic sarcomas
(fibrosarcomas, osteosarcomas or haemangiosarcomas) were increased in
male rats but not in females (males: 0/49, 1/50, 3/50, 38/50; females:
0/50, 0/50, 1/50, 1/50). There was also a small increase in
phaeochromocytomas or malignant phaeochromocytomas in male rats
(13/49, 14/48, 15/48, 26/49). The incidences of mononuclear cell
leukaemia were reduced in male and female rats of the treatment groups
(males: 21/49, 3/50, 2/50, 3/50; females: 10/50, 2/50, 1/50, 1/50).
This reduction may be related to splenic toxicity, since splenectomy
of Fischer rats at one to two months of age markedly reduces the
incidence of mononuclear cell leukaemia later in life (Boorman et al.,
1990). However, similar reductions in the incidence of this neoplasm
are not seen with several other aniline-like chemicals that also cause
splenic toxicity. In male mice, but not in females, the incidence of
haemangiosarcomas of the liver and spleen was slightly greater in the
high-dose group than in the controls (males: 4/50, 4/49, 1/50, 10/50)
and the incidences of hepatocellular carcinomas or adenomas (combined)
were also increased in treated male mice (males: 11/50, 21/49, 20/50,
21/50). The incidences of malignant lymphomas were slightly reduced
in treated male and female mice (males: 10/50, 3/49, 9/50, 3/50;
females: 19/50, 12/50, 5/50, 10/50).
There are various points of similarity between the two studies
described above: (a) in male and female rats there was splenic
toxicity; (b) in male rats, there was a treatment-related increase in
uncommon splenic sarcomas; and (c) in male mice, there was a
treatment-related increase in haemangiosarcomas and haemangiomas
combined. The reductions in the incidence of mononuclear cell
leukaemia in rats and malignant lymphomas in mice seen in the latter
study were not observable in the earlier study because their incidence
in the control groups of that study was already low. It is concluded
that PCA is carcinogenic in both mice and rats.
8. EFFECTS ON HUMANS
No data concerning the effects of diflubenzuron on human health
are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Diflubenzuron was tested for its effects on morphogenesis in
Streptomyces spp. Exposure to 400 mg diflubenzuron/litre resulted in
reduced dominance of spore hairs and reduced width of the outer wall,
and prevented formation of the inner spore wall in S. babergiensis.
In S. coelicolor, 1600 mg diflubenzuron/litre altered the structure
of the fibrillar pattern of spore envelopes. Exposure to diflubenzuron
resulted in small increases in exported protein and in an approximately
20% increase in chitinase in both Streptomyces spp (Smucker &
Simon, 1986).
9.1.1.1 Water
Aquatic bacterial biomass and density were not affected by
diflubenzuron (1.0 µg/litre), although after three months of
continuous exposure some decline in species diversity was found.
Owing to detrimental effects on aquatic arthropods, e.g., filter
feeders that may use bacteria as a food source, such effects may very
well be secondary in nature and not a direct effect of diflubenzuron
(Hansen & Garton, 1982b).
In a test on the alga Selenastrum capricornutum, a nominal
concentration of 0.20 mg diflubenzuron/litre did not reduce the
biomass and can be considered a no-observed-effect concentration
(NOEC) (Berends & Thus, 1992b).
9.1.1.2 Soil
Soil microorganisms were able to use diflubenzuron as carbon
source when added as an acetone solution (Seuferer et al., 1979; see
section 4.3.2.2).
The effect of diflubenzuron (100, 200, 300, 400 and 500 mg/kg
soil) was studied in non-sterile soil incubated under aerobic
conditions and in sterilized soil inoculated with Azotobacter
vinelandii. The presence of diflubenzuron had a stimulatory effect
on nitrogen fixation in both non-sterile and sterile soil (Martinez-
Toledo et al., 1988a). At similar concentrations, diflubenzuron did
not affect the growth of Azotobacter vinelandii in culture media,
either with or without a nitrogen source (Martinez-Toledo et al.,
1988b).
9.1.2 Aquatic organisms
9.1.2.1 Microorganisms
Cyanobacteria (the blue-green alga Plectonema boryanum) grew
rapidly in the presence of diflubenzuron (initial concentration
0.1 mg/litre) with no visible signs of inhibited growth (Booth &
Ferrell, 1977). Concentrations of 1, 10, 50 and 100 mg/litre did not
affect the growth of six species of fungi: Rizopus arrhizus,
Aspergillus niger, Fusarium oxysporum, Mycorrhizae-Rhizopogan
vinicolor, Pythum debaranum and Trichoderma viride (Booth et al.,
1987). However, these authors autoclaved the medium containing
diflubenzuron, which led to extensive breakdown of the pesticide
(Willems et al., 1977).
Five-day lethality tests on the algae Selenastrum capricornutum
and Anabaena flos-aquae resulted in NOAEC values above the exposure
concentrations of 300 and 330 µg/litre, respectively (Thompson &
Swigert, 1993b,c). Similarly, tests on the diatoms Navicula
pelliculosa and Skeletonema costatum led to NOAEC values of 380
and 270 µg/litre, respectively (Thompson & Swigert, 1993d,e).
Algae were affected at 1.0 µg/litre by technical diflubenzuron in
dimethylformamide added to laboratory stream channels: the alga
biomass increased and chlorophyll and phaeocitin levels were elevated.
Fungi were only affected temporarily by 0.1 µg/litre under the same
circumstances. Changes in species diversity had disappeared after
2 months of continuous dosing (Hansen & Garton, 1982 b).
9.1.2.2 Plants
In a 14-day toxicity test on diflubenzuron in duckweed
(Lemna gibba), the NOAEC was higher than the tested concentration
(190 µg/litre) (Thompson & Swigert, 1993a).
9.1.2.3 Invertebrates
The acute toxicity of diflubenzuron to a number of non-target
aquatic invertebrates is presented in Table 12. Comprehensive eviews
on the subject have been published recently, e.g., by Fischer & Hall
(1992) and by Cunningham (1986) on the effects of diflubenzuron on
estuarine crustaceans. The acute LC50 for insects ranges from
1 µg/litre (Diptera) to 250 µg/litre (Coleoptera), and mayflies
have an LC90 of 1 to 10 µg/litre. Other aquatic arthropods such as
water fleas, scuds and sow bugs have LC50 values of 5 to 15 µg/litre.
Table 12. Acute toxicity of diflubenzuron for non-target aquatic invertebrates
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Daphnia magna 1st instar stat 22 40 7.2 48-h EC50 15 Julin & Sanders (1978)
(Water flea)
Daphnia magna 24 h stat 20 50 48-h LC50 4.55 Hansen & Garton (1982a)
(Water flea) stat 20 100 48-h LC50 6.89 Hansen & Garton (1982a)
stat 20 200 48-h LC50 4.42 Hansen & Garton (1982a)
Daphnia magna 0-24 h 20 294 8.1 48-h EC50 7.1 Kuijpers (1988)
(Water flea) 20 294 8.1 24-h EC50 68 Kuijpers (1988)
Gammarus pulex mature stat 12 40 7.2 96-h LC50 30 Julin & Sanders (1978)
(Scud)
Hyallela azteca 2-4 mm flow 20 25 96-h LC50 1.84 Hansen & Garton (1982a)
(Amphipod)
Chironomus plumosus 4th instar stat 22 40 7.2 48-h EC50 560 Julin & Sanders (1978)
(Midge)
Cricotopus sp. 4th instar flow 20 25 EC50 (moulting 1.72 Hansen & Garton (1982a)
(Midge) success)
Tanytarsus dissimilis 2nd instar flow 20 25 EC50 (moulting 1.02 Hansen & Garton (1982a)
(Midge) success)
Acartia tonsa adult constant 20 10b 5-day LC50 > 1000 Tester & Costlow
(Copepod) daily (1981)
replenishment
Table 12 (Con't)
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Mysidopsis bahia adult intermittent 24-25 24-27b 96-h LC50 2.06 Nimmo et al. (1980)
(Mysid shrimp) flow
adult continuous 24-26 23-29b 21-day LC50 1.24 Nimmo et al. (1980)
flow
Palaemonetes pugio larvae stat 22 20b 96-h LC50 1.44 Wilson & Costlow
(Grass Shrimp) (1987)
post larval renewal 22 20 96-h LC50 1.62 Wilson & Costlow (1987)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Salinity (expressed as parts per thousand)
Concentrations of diflubenzuron causing significant mortality of
several freshwater invertebrates are presented in Table 13.
Table 13. Concentrations of diflubenzuron causing significant
mortality of freshwater invertebratesa
Organisms Concentration
(µg/litre)
Water flea (Daphnia magna) 2.0
Amphipod (Hyalella azteca) 2.0
Snail (Juga plicifera) > 36
Snail (Physa spp.) > 36
Caddis fly (Clisforonia magnifica) 0.1
Midge (Tanytarsus dissimilis) 4.9
Midge (Cricotopus spp.) 1.6
a From: Nebeker et al. (1983)
The 96-h LC50 for the snails Juga plicifera and Physa sp.
was > 45 µg/litre (Hansen & Garton, 1982a).
The 48-h EC50 values of diflubenzuron metabolites for midge
larvae were > 100 mg/litre for 4-CPU and 2,6-DFBA and 43 mg/litre for
PCA (Julin & Sanders, 1978).
Diflubenzuron suspended in water at a concentration of
200 mg/litre was not directly toxic to the freshwater clam Anodonta
cygnea during 3 months of treatment. Diflubenzuron produced
disturbances in the calcification process in the lamellar layer of the
shell. Positive PAS reaction of the secretory cells on the outer
mantle epithelium has been observed (Machado et al., 1990).
Daphnia magna was continuously exposed to 14C-diflubenzuron at
concentrations of 5.6, 14, 23, 40 and 93 ng/litre. After 21 days of
exposure daphnid survival at the highest concentration (93 ng/litre)
was 50%. In the remaining concentrations it ranged from 93 to 98%,
which was comparable to the survival (99%) of the control organisms.
Reproduction and body length were affected only at the highest
concentration. The maximum acceptable toxicant concentration (MATC)
of 14C-diflubenzuron for Daphnia magna was > 40 and < 93 ng/litre
(Surprenant, 1988).
Technical diflubenzuron in dimethylformamide at 0.1, 1, 10 and
50 µg/litre was added continuously to complex laboratory stream
channels supplied periodically with field-collected microorganisms for
5 months. LC50 values ranging from 1.0 to 1.8 µg/litre were
determined for four insect and crustacean species ( Tanytarsus
dissimilis, Cricotopus sp. and Hyalella azteca). A chronic effect
level was obtained only for Daphnia magna (0.06 µg/litre). Mayflies
and stoneflies were the most sensitive. They were severely affected
at 1 µg/litre within one month (the sampling interval) and numbers
were two to three orders of magnitude lower, almost leading to their
elimination. The survival of chironomids was reduced by 10 µg/litre
(Hansen & Garton, 1982a,b) (see also section 9.1.1.1).
Benthic communities in outdoor experimental streams were exposed
to 1 or 10 mg/litre of diflubenzuron for 30 min. The effect was
assessed daily by examining drifting pupal exuviae over a period of
one month following the treatment. No drift of macrobenthos was
induced at the time of application. However, diflubenzuron affected
the emergence of all species examined. High larval mortality for a
species of chironomid was observed directly in the stream treated with
diflubenzuron, where numbers of mayfly nymphs and caddisfly larvae
were also decreased (Yasuno & Satake, 1990).
Technical diflubenzuron in acetone was applied at 5 µg/litre to
two aquaria in a simulated field test outdoors. Daphnid numbers were
markedly reduced three days after treatment, but recovered slowly.
Copepods numbers were moderately reduced, exceeding the control
numbers after 18 days. The seed shrimp population showed no harmful
effect (Miura & Takahashi, 1974a,b).
In a study by Collwell & Schaefer (1980), diflubenzuron was
applied to experimental ponds (mean concentration of 13.2 µg/litre) in
California. An hour after treatment, cladoceran ( Ceriodaphnia sp.,
Diaphanosoma sp., Chydorus sp., Bosmina sp. and Daphnia sp.)
numbers were strongly reduced and did not return to pretreatment
levels until more than 5 weeks after treatment. Copepod ( Diaptomus
sp. and Cyclops sp.) numbers were also reduced but to a lesser
extent and for a shorter period than for the cladocerans. The
rotifers increased in abundance in both the control and treated ponds
during the first 8 days following treatment.
Using laboratory tests to study mortality, Miura & Takahashi
(1974a) found that crustaceans, especially the tadpole shrimp
(T. longicaudatus), clam shrimp ( Eulimnadia spp.) and water fleas
( Daphnia and Moina spp.), were highly susceptible to diflubenzuron
at levels below 0.01 mg/litre. Copepods, Cyclops and Diaptomus
spp. showed some tolerance, whereas seed shrimp ( Cypricerus and
Cypridopsis spp.) tolerated as much as 0.5 mg/litre. Among aquatic
insects tested, mayfly nymphs ( Callibaetis spp.) were most
susceptible. Aquatic midge larvae (G. holoprasinus) also
showed susceptibility. However, dytiscid ( T. bassillaris and
Laccorhilus spp.) and hydrophilid beetles ( H. triangularis and
T. lateralis (mosquito predators)) demonstrated a strong tolerance.
Mosquito fish (Gambusia affinis) showed no effect at a relatively
high dose of
1 mg/litre.
When larvae of the crab Rhithropanopeus harrisii were exposed
to sublethal concentrations of diflubenzuron (0.05, 0.1, 0.3 and
0.5 µg/litre), swimming speed increased in stage I, II and III zoeae,
0.3 µg/litre being the lowest effective concentration. Phototaxis was
altered in stage IV only at concentrations as low as 0.1 µg/litre
(Forward & Costlow, 1978).
Christiansen et al. (1978) showed that nearly 100% of
Rhithropanopeus harrisii larvae at each of the four zoeal stages
died when moulting to the succeeding stage after only 3 days of
exposure to 10 µg diflubenzuron/litre. This concentration was also
lethal for larvae of Sesarma reticulatum (Say).
Christiansen & Costlow (1980) exposed larvae of the estuarine
crab Rhithropanopeus harrisii in laboratory conditions to 10 µg
diflubenzuron/litre as an indicator of persistence of
diflubenzuron in brackish water. Disturbance of endocuticle
deposition seemed to occur as soon as newly hatched larvae (less than
12 h old) were exposed to diflubenzuron. Diflubenzuron not only
affected endocuticle deposition in the larvae, but also exocuticle
deposition. The only part of the crab larval exoskeleton that did not
seem to be affected by diflubenzuron was the epicuticle (Cristiansen
et al., 1978; Cristiansen & Costlow, 1982).
Diflubenzuron, at concentrations of 0.02 and 0.2 mg/litre,
enhanced mortality during moulting of the crab Carcinus
mediterraneus (Czerniavsky) (Cardinal et al., 1979).
Cirripede crustaceans, (barnacles, Balanus eburneus) exposed to
concentrations of 1 to 1000 µg/litre over a 28-day period showed a
dose-dependent mortality. Heavy mortality occurred during the second
week of exposure. Lethal and sublethal effects were observed at
concentrations as low as 50 µg/litre (Gulka et al., 1980). Disruption
of the exoskeleton of B. eburneus caused by diflubenzuron was
similar to that observed in insects. Development of barnacles exposed
to diflubenzuron for 10 days or more at 750 and 1000 µg/litre was
delayed in the premoult phase of cuticle secretion (Gulka et al.,
1982).
Wilson & Costlow (1986) found that diflubenzuron concentrations
of 2.5 and 5 µg/litre were lethal to larvae of the grass shrimp
(Palaemonetes pugio), causing 100% mortality on days 14 and 6,
respectively. Wilson et al. (1985) studied the effects of
diflubenzuron upon phototaxis of larvae of P. pugio. The depression
in positive phototaxis and elevation in negative phototaxis were most
pronounced at 0.5 µg/litre, and the lowest test concentration to
affect phototaxis was 0.3 µg/litre. The alterations in photo
responses varied with the embryonic stage at which exposure to
diflubenzuron commenced. This study was carried out at optimal
salinity and temperature, but in the estuary these conditions
fluctuate daily, which may amplify the observed effects.
Nimmo et al. (1980) observed that chronic exposure of the mysid
shrimp Mysidopsis bahia to 0.075 µg diflubenzuron/litre reduced the
reproductive success of both parents and progeny even after they were
transferred to uncontaminated sea water.
Diflubenzuron reduced the reproductive life span of adult brine
shrimps (Artemia salina) at levels of 2-10 µg/litre and caused death
of immature shrimps within 3 days at concentrations above 10 µg/litre
(Cunningham, 1976).
Fiddler crabs (Uca pugilator) were exposed to diflubenzuron
(Dimilin WP-25%) at 0.5, 5 and 50 µg/litre for 1, 2, 3 and 4 weeks
after multiple autonomy of one chela and five walking legs. Exposure
to diflubenzuron retarded the rate of limb regeneration in a dose-
dependent fashion. The effects of diflubenzuron were seen even in
crabs exposed for only one week. Significant retardation was evident
by day 21 at 5.0 µg/litre but was not statistically significant at
0.5 µg/litre. At 5 and 50 µg/litre moult-associated mortality was
seen. The number of setae on regenerated limbs was less than the
number on the intact limbs. The effects were reduced in experiments
in which sediment was present (Weis et al., 1987).
The burrowing activity of Uca pugilator in sand under
laboratory conditions was not altered when the sand was contaminated
with 1 mg diflubenzuron/litre, indicating a lack of avoidance of
diflubenzuron-contaminated sand. However, exposure for 1 week to 0.5,
5.0 or 50 µg/litre led to a decrease in the amount of burrowing
activity. The behavioral response was not changed after exposure for
1, 2 or 3 weeks and was not concentration-related (Weis & Perlmutter,
1987).
Survival, moulting and behaviour of juvenile fiddler crabs were
significantly affected by exposure to diflubenzuron (0.2, 2, 20 and
200 µg/litre) for 24 h weekly during 10 weeks. All crabs in the 200
and 20 µg/litre groups died after 8 and 23 weeks, respectively. The
no-observed-effect concentrations (NOEC) for moulting (time to the
first moult), survival (time until death), and behaviour (ability to
escape from the test container) were 20, 2 and 0.2 µg/litre,
respectively (Cunningham & Myers, 1987).
Larvae of the stone crab (Menippe mercenaria) were exposed to
0.5, 1.0, 3.0, and 6.0 µg diflubenzuron/litre in combination with
different temperature and salinity. All of these concentrations were
lethal to the larvae. Evidence for synergistic effects of
diflubenzuron and temperature or salinity was observed. Tolerance of
the megalopa of the blue crab Callinectes sapidus to diflubenzuron
at concentrations of 0.5, 1.0, 3.0 and 6.0 µg/litre was slightly
higher than for Menippe mercenaria but was also dependent upon
temperature and salinity. At 20°C the percentage survival at a
concentration of 1 µg/litre was similar to that observed for the
controls (Costlow, 1979).
Tester & Costlow (1981) reported that the marine copepod
Acartia tonsa exposed to 1 and 10 µg diflubenzuron/litre for 36 h
failed to produce viable nauplii even after they had been placed in
clean sea water. No viable nauplii were produced by these females for
at least 30 h after treatment ended.
Adult crustaceans were more resistant to exposure than their
larvae at high concentrations (100-200 µg/litre) of technical grade
diflubenzuron. Adults also exhibited significant mortality associated
with moulting (Cunningham, 1976; Cardinal et al., 1979; Gulka et al.,
1980).
When larvae of horseshoe crabs (Limulus polyphemus) were
exposed to 5 and 50 µg diflubenzuron/litre, the crabs in the
50 µg/litre group exhibited severe mortality immediately after
ecdysis. The larval stages were shown to be quite resistant to
diflubenzuron, compared with other crustacean larvae (Weis & Ma,
1987).
9.1.2.4 Vertebrates
Data on the acute toxicity of diflubenzuron and its metabolites
for fish are presented in Tables 14 and 15, respectively.
A 96-h acute toxicity test on juvenile sheepshead minnow
(Cyprinodon variegatus) in a flow-through system resulted in no
mortality at the nominal exposure concentration of 130 µg
diflubenzuron/litre (Graves & Swigert, 1993). A similar test under
semi-static conditions on zebra fish (Brachydanio rerio) and rainbow
trout (Oncorhynchus mykiss) at a nominal diflubenzuron concentration
of 200 µg/litre also showed no mortality, nor changes in the appearance
Table 14. Acute toxity of diflubenzuron to fish
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Coho salmon 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus kisutch) Pridmore (1978)
Rainbow trout 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus mykiss) Pridmore (1978)
Rainbow trout 1.2 g stat 12 40 7.2 96-h LC50 240 Julin & Sanders
(Oncorhynchus mykiss) (1978)
Rainbow trout not flow-through 96-h LC50 140 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Rainbow troutb not flow-through 96-h LC50 195 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Fathead minnow 0.87 g stat 22 40 7.2 96-h LC50 430 Julin & Sanders
(Pimephales promelas) (1978)
Channel catfish 2.2 g stat 22 40 7.2 96-h LC50 370 Julin & Sanders
(Ictalurus punctatus) (1978)
Bluegill 0.5 g stat 22 40 7.2 96-h LC50 660 Julin & Sanders
(Lepomis macrochirus) (1978)
Bluegill not flow-through 96-h LC50 135 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Bluegillb not flow-through 96-h LC50 230 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Table 14. (Con't)
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Mummichog adult 2.7 g stat 24 22 ppt 8.0 96-h LC50 32 990 Lee & Scott
(Fundulus heteroclitus) renewal salinity (1989)
Mummichogb not flow-through 96-h LC50 255 Marshall & Hieb
(Fundulus heteroclitus) reported (1973)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Formulated product 25 WP
Table 15. Acute toxicity of metabolites of diflubenzuron to fish (from: Julin & Sanders, 1978)
Concentrations (mg/litre)
Organism Water Effect
temperature measured
(°C) 4-Chlorophenyl 2,6-Difluorobenzoic 4-Chloroaniline
urea acid
Rainbow trout 12 96-h LC50 72 (57-90) > 100 14 (11-16)
(Oncorhynchus mykiss)
Channel catfish 22 96-h LC50 > 100 > 100 23 (18-29)
(Ictalurus punctatus)
Fathead minnow 22 96-h LC50 > 100 69 (55-87) 12 (7-18)
(Pimephales promelas)
Bluegill 22 96-h LC50 > 100 > 100 2.4 (1.8-3.2)
(Lepomis macrochirus)
or behaviour of the fish (Berends & van der Laan-Straathof, 1994a,b).
All these test concentrations were above the diflubenzuron solubility
level of 80 µg/litre.
Nebeker et al. (1983) found no significant reduction in
survival of fathead minnow (Pimephales promelas) or guppy
(Poecilia reticulata) as a result of exposure to diflubenzuron at
concentrations below 36 µg/litre during acute (96-h) and chronic
tests.
No acute response resulted from exposure of fish to a
diflubenzuron concentration of 45 µg/litre, and no chronic effects
were observed at this concentration, the highest one tested (Hansen &
Garton, 1982a,b).
Diflubenzuron was not toxic to either rainbow trout or coho
salmon exposed at concentrations up to 150 mg/litre for a 96-h period.
A 15-min exposure to 1 g/litre did not result in any fish mortality
(McKague & Pridmore, 1978).
Madder & Lockhart (1978) found a dose-related decrease of
glutamic-oxaloacetic transaminase activity in rainbow trout
(Oncorhynchus mykiss) exposed to diflubenzuron at concentrations of
0.625, 1.25, 2.5, 5 and 10 mg/litre.
At a concentration of 0.01 mg/litre, diflubenzuron had a
repellent effect on precocious male Atlantic salmon parr (Granett et
al., 1978).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Photosynthesis, respiration and leaf ultrastructure of soybeans
were unaffected by diflubenzuron at doses up to a level of
0.269 kg a.i./ha (Hatzios & Penner, 1978).
Diflubenzuron is used as an insecticide in forestry, agriculture
and horticulture. No phytotoxicity was reported in the field studies
cited in section 9.2.
9.1.3.2 Invertebrates
The oral and contact LD50 values of diflubenzuron for honey-bees
are greater than 30 µg/bee (Stevenson, 1978).
Diflubenzuron did not show any toxicity to bees at concentrations
up to 1000 mg/kg in the diet (Yu et al., 1984).
Barker & Taber (1977) found that diflubenzuron reduced brood
production when fed for 10 days to honey-bees at 59 mg/kg in sugar
syrup, but not at 5.9 or 0.59 mg/kg.
Barker & Waller (1978) confirmed that 60 mg/kg in sugar syrup or
100 mg/litre in water caused colonies to produce less brood.
Stoner & Wilson (1982) found that diflubenzuron fed to flying
colonies at 1 or 10 mg/kg for a year significantly reduced the amount
of sealed brood.
Nation et al. (1986) reported that diflubenzuron did not cause
reduction in pollen consumption or brood production when fed for 10
weeks to caged colonies of honey-bees at 10 mg/kg, but it caused more
than 50% reduction in the amount of syrup stored.
Gordon & Cornect (1986) showed that, at concentrations that are
effective in suppressing egg hatching and larva development of the
cabbage maggot Delia radicum, diflubenzuron did not adversely
affect eggs, first-instar larvae or adults of the rove beetle
Aleochara bilineata, an important predator and parasitoid of the
cabbage maggot.
When the acute toxicity of diflubenzuron to the earthworm
Eisenia fetida was tested in artificial soil to which diflubenzuron
had been added, 780 mg/kg dry soil was the no-observed-effect
concentration (NOEC) for the 14-day test period (Berends et al.,
1992). For the WP-25 formulation, the NOEC was 1.0 g/kg (Berends &
Thus, 1992a).
9.1.3.3 Vertebrates
a) Birds
The acute oral LD50 of technical diflubenzuron for red-winged
blackbirds (Agelaius phoeniceus) is 3762 mg/kg body weight. In an
8-day dietary LD50 study on mallard duck and bobwhite quail using
technical diflubenzuron, levels up to 4640 mg/kg in the feed gave no
observable signs of toxicity (Maas et al., 1980).
When diflubenzuron was fed to mature White leghorn hens at
dietary levels of 10, 50, 100, and 500 mg/kg for 8 weeks, there were
no adverse effects on feed consumption, body weight, egg production,
egg weight, eggshell thickness, fertility, hatchability or progeny
performance. The highest dose used was 50 times higher than the
efficacy level for fly control (Cecil et al., 1981).
In chronic toxicity tests, groups consisting of 10 male and 10
female one-day-old chicks of barred Plymouth rocks and white leghorn
hens, Nicholas white turkeys, mallard ducks and ring-necked pheasants
were given diets containing diflubenzuron levels of 0.25, 1.25, 25 and
250 mg/kg for 91 days after hatching. No differences between control
and treatment groups were observed in mortality, food consumption,
body weight, comb and wattle development, weight of inner organs,
serum hormone levels or general behaviour (Maas et al., 1980).
There was no effect of diflubenzuron on the content of hyaluronic
acid in the skin of Hubbard broiler chickens when they were fed at
levels of 2.5 and 250 mg/kg in the diet for 98 days after hatching
(Deul & Jong, 1977). In an experiment with the same dose levels and
duration, diflubenzuron had no effect on hyaluronic acid synthesis or
comb deposition in either growing broilers or layers (Crookshank et
al., 1978).
White leghorn and black sex-linked cross hens were fed
diflubenzuron at a level of 10 mg/kg in the ration for 15 weeks.
Diflubenzuron had no effect on body weight gain, egg production,
fertility or hatchability (Miller et al., 1976b).
In a feeding study with 2.5, 25 and 250 mg/kg, diflubenzuron did
not affect bobwhite quail reproduction (Booth et al., 1987).
Diflubenzuron did not show significant teratogenic activity on chick
embryos over time when injected at 10 mg/egg (Booth et al., 1987).
A one-generation bobwhite quail (Colinus virginianus)
reproduction study was conducted in which a diflubenzuron-containing
diet was administered ad libitum to young adults (24 weeks old at
test initiation) approaching their first breeding season. Dietary
concentrations of 250, 500 or 1000 mg/kg did not result in treatment-
related mortality, overt signs of toxicity or effects upon adult body
weight or feed consumption during the 21-week exposure period. There
were no apparent treatment-related effects upon reproductive
parameters at 250 or 500 mg/kg. There may have been a slight reduction
in the number of eggs laid, although this was not statistically
significant or dose-related at 1000 mg/kg. On the basis of a possible
effect on egg production at 1000 mg/kg, the NOEC for diflubenzuron in
this study was above 500 mg/kg (Beavers et al., 1990a).
In a one-generation reproduction study on the mallard duck
(Anas platyrhynchos), diets containing diflubenzuron were
administered ad libitum to young adults (27 weeks old at test
initiation) approaching their first breeding season. Dietary
diflubenzuron concentrations of 250, 500 and 1000 mg/kg did not
result in treatment-related mortality, overt signs of toxicity or
effects upon adult body weight or feed consumption during the 20-week
exposure period. There were no apparent treatment-related effects
upon reproductive performance at any of the concentration tested. At
1000 mg/kg there was a slight, but statistically significant,
reduction in mean eggshell thickness. On the basis of the effect upon
eggshell thickness at 1000 mg/kg, the NOEC for diflubenzuron in this
study was 500 mg/kg (Beavers et al., 1990b).
b) Mammals
In studies by Ross et al. (1977a,b), diflubenzuron was
administered in the feed to sheep (3 of each sex per group) as a model
for ruminant wildlife at concentrations of 500, 2500 and 10 000 mg/kg
feed for 13 weeks. No treatment-related effects were observed on food
consumption, body weight gain, haematological parameters or
urinalysis. Increase in met- and sulfhaemoglobin levels were observed
at 13 weeks and there was a reduction in the weight of the thyroid.
No histopathological abnormalities were observed. Both the plasma and
the erythrocyte cholinesterase activities were unaffected by the
treatment after 6 weeks.
9.2 Field observations
9.2.1 Microorganisms
9.2.1.1 Water
The laboratory data available (see section 9.1.1.1) make it
unlikely that detrimental effects will occur.
Rotifers were unaffected by diflubenzuron (28 and 56 g a.i./ha)
in both experimental and naturally treated ponds (Ali & Lord, 1980).
9.2.1.2 Soil
One aerial application of diflubenzuron at 67.26 g/ha had no
adverse effects upon populations of bacteria, actynomycetes or fungi
in leaf litter and forest soil (Wang, 1975; Kurczewski et al., 1975).
9.2.2 Aquatic organisms
9.2.2.1 Plant
The laboratory data available (see section 9.1.2.2) make it
unlikely that detrimental effects will occur.
9.2.2.2 Invertebrates
Recent field studies have demonstrated that effects on aquatic
fauna are limited and transient, and that recovery is evident after
3 months (Huber & Collins, 1987; Kingsbury et al., 1987; Huber &
Manchester, 1988; Ali et al., 1988; Ali & Kok-Yokomi, 1989; Sundaram
et al., 1991).
Diflubenzuron (25% WP) applied at 33.63 and 134.52 g a.i./ha,
4 times at 2-week intervals, had no adverse effect on freshwater clams
10 days after final treatment (Jackson, 1976).
When diflubenzuron (25% WP) was applied at 1.121-280.25 g a.i./ha
to flooded rice fields in Louisiana, USA, significant reduction of
Tropisternus spp. and Libellulidae was found 80 days after
treatment. Significantly more chironomid and baetid immatures
occurred due to reduction in the number of predators (Steelman et al.,
1975).
Mulla et al. (1975) found that at an application rate of 28 and
56 g a.i./ha diflubenzuron reduced slightly, and only for a short
time, the number of mayfly ( Beatis sp.) in the treated ponds. The
numbers, however, appeared to be within natural fluctuation limits
equal to the check ponds or the pretreatment levels during all other
sampling periods. At these application rates, diflubenzuron caused a
short-term reduction in copepod populations, but they started to
increase on the 11th or 15th day of post-treatment sampling. There
was little or no effect on the ostracod population.
In a forestry spraying programme at 0.0672 kg a.i./ha, aquatic
arthropods were studied in a water shed (White Deer Creek). Sampling
at three test stations and one control station in the watershed
revealed a rich fauna, dominated numerically by Ephemeroptera,
Chironomida, Trichoptera and Plecoptera in descending order of
abundance. Significant differences in larval abundance in the surber
samples were found to occur from one sampling day to the next, or
between pre-spray and post-spray periods, for most of the organisms
examined. In all cases, control and test stations showed similar
patterns of variation, so treatment effects were discussed as a
probable cause. The number of organisms tended to be higher at the
upstream stations. Chironomida and Trichoptera pupal abundance in the
surber samples was examined for decreases after spraying but numbers
were too low to permit statistical analysis. Pupal abundance showed
some direct relationship to larval abundances. Nymphal drift rates
were examined for possible increases after spraying, but most of the
significant changes were decreases during the post-spray period.
These decreases also occurred at the control station. The drift rates
of nymphal and pupal exuviae also did not indicate a treatment effect.
Individual species abundances in the surber samples were examined for
possible selective effects of diflubenzuron. Comparisons were made
between pre-spray and post-spray periods at both test and control
stations, but no evidence of a treatment-related decrease in the
abundance of any of these organisms was found. The net effect was
usually an increase in the density of these organisms during the post-
spray period. No organisms showing abnormal ecdysis or pupation were
found in any of the samples. It was concluded that spraying with
diflubenzuron had no adverse effect on the macrobenthic community in
White Deer Creek (White, 1975).
In a study by Booth (1975), diflubenzuron (25% WP) was applied at
44.84 g a.i./ha to small ponds in Utah. Biosamples taken 30 and 80
days later showed that, although larva and immature aquatic insect
populations were decreased at 30 days, the total number of adults was
not significantly different from the control number at 80 days.
Booth & Ferrell (1977) examined the effect of diflubenzuron on
over 20 different species (corixidae and collembolans) after multiple
pond applications at 45 g a.i./ha. Only immature corixidae (water
boatmen) and collembolans (springtails) were significantly affected.
Segmented worms (Oligochaeta) and midges (Chironomidae)
were unaffected by six applications of diflubenzuron at a rate of
145.73 g a.i./ha to Utah Lake (Booth et al., 1987).
Diflubenzuron (28 and 56 g a.i./ha) reduced numbers of
Chaoborus sp. and Baetis sp. in both experimental and natural
treated ponds. Chaoborus sp. recovered within 1-3 weeks (Ali &
Lord, 1980).
The application of granular diflubenzuron at 0.11 kg a.i./ha
(about 3.7 µg/litre) to residential-recreational lakes caused a 62-75%
reduction of Daphnia pulex and Daphnia galeata during 7 days
following treatment. The populations recovered in the second week
after treatment. A 30% reduction in Diaptomus spp. was noted 2
days after the treatment. Hyalella azteca was affected markedly,
with a maximum reduction of 97% after 3 weeks of treatment. No
detectable effects on Cyprinotus sp., Cyclops sp. or Bosmina
longirostris were observed (Ali & Mulla, 1978a). Ali & Mulla
(1978b) also studied the impact of diflubenzuron on invertebrates in a
residential-recreational lake after two treatments with 25% WP
formulation at 156 g a.i./ha (about 12 µg/litre). Diflubenzuron
concentrations in water were not measured but were based on nominal
initial concentrations. The results from this study are summarized in
Table 16.
Diflubenzuron (25% WP) at rates of 0.02, 0.025, 0.03, 0.035,
0.04, 0.045 and 0.05 lb a.i./acre was applied to 19 irrigated pastures
and spring ponds (in some cases up to 4 times) at 2- to 3-week
intervals in California. Cladoceran and nymphal mayfly populations
were temporarily and slightly reduced, but recovered within a short
period of time. Even repeated treatment of the same pastures did not
eliminate the populations. The impact on copepod populations was
inconclusive. Adult beetles demonstrated high tolerance to
Table 16. Effect of diflubenzuron on invertebrates in Vallage Grove Lake, Californiaa
Species First application (April) Second application (August)
Effect Recovery Effect Recovery
Daphnia leavis elimination within no recovery 6 months
Birge 1 week after treatment
Ceriodaphnia sp. elimination within no recovery 6 months
1 week after treatment
Bosmina longirostris elimination within recovery after elimination after reappearance in small numbers
(O.F. Muller) 1 week 11 weeks 1 week 8-9 weeks after treatment
Cyclops spp. elimination within recovery within elimination within recovery after 4 weeks
1 week 6-7 weeks 1-2 weeks
Diaptomus sp. elimination within recovery after absent prior to reappearance in small numbers
1 week 4 months treatment 1-2 months later
Hyalella azteca elimination within no recovery 6 months
(Saussure) 4 weeks after treatment
Caenis sp. elimination within recovery within elimination within recovery in 4-5 weeks
3 weeks 6-7 weeks 2-3 weeks
Physa sp. no adverse effects no adverse effects
Cypridopsis sp. no adverse effects no adverse effects
a From: Ali & Mulla (1978b)
diflubenzuron, but few dead larvae ( Laccophilus spp., Hydrophilus
triangularis and Tropisternus lateralis) were observed. Spiders
( Pardosa spp. and Lycosa spp.) showed no apparent effects. There
were no deleterious effects on planarian, rotifer, seed shrimp and
fresh water flagellate populations (Miura, 1974).
Diflubenzuron (25 WP formulation) was applied 3 times at 2.5-week
intervals to man-made pools in Manitoba, Canada at 56 g/a.i. ha (this
produced initial concentrations of 0.02 mg/litre). The treatment
produced no detectable effect on chironomid larvae in terms of numbers
or composition, although dead larvae were noted after treatment.
Daphnid populations were significantly reduced on most sampling dates.
Repetitive treatments prevented the recovery of cladocerans. Despite
these reductions, daphnids were not annihilated. At a higher rate
(0.22 kg a.i./ha, about 7.4 µg/litre), both species of Daphnia were
eliminated for 3 months after treatment. Populations of Diaptomus
spp. declined to zero 7 days after treatment, but recovered during the
second week following treatment. Diflubenzuron caused reductions in
the number of H. azteca (32-100%) during the 2´ months following
treatment. Seed shrimp populations were stressed for only 2 weeks,
and there were no observable effects on oligochaetes at a treatment
level of 0.22 kg a.i./ha (about 7.4 µg/litre). Copepods only
occasionally showed significant reduction, and recovered within 10
days after treatment. Of the ten other non-target invertebrates (i.e.
Chaoboridae, Tipulidae, Ephemeroptera, Corixidae, Nonectidae,
Gerridae, Dystiscidae, Hydrophilidae, Hydrarina and Gastropoda),
only mayfly nymphs (Ephemeroptera) were significantly reduced
(Madder, 1977).
When diflubenzuron at a rate of 34.2 g/ha was applied aerially to
a forested area in the USA, there were no significant effects on the
population structure of the benthic macro-invertebrate community.
Some decreases in the number of mayflies Heptagenia sp. and
Rhithrogenia sp. were noted in treatment streams, but the total taxa
richness values remained high. An increase in abundance of the
caddisfly Lepidostoma sp. was found. Allonarcys sp. was not
affected by the treatment (Huber & Collins, 1987; Huber & Manchester,
1988).
After aerial application of diflubenzuron to two forest ponds in
Canada the greatest effect was on crustacean zooplankton, especially
cladocerans, with limited effect on pond benthos. Recovery of
population of even the most severely affected organism such as
Daphnids was well established by 3 months after treatment. The
maximum residue found in water was 13.82 µg/litre 1 h after
application (Kingsbury et al., 1987).
Diflubenzuron WP-25 was applied at a rate of 70 g active
ingredient in 10, 5 or 2.5 litre/ha to three spray blocks in a mixed
boreal forest near Kaladar, Ontario, Canada. Water, sediment and
aquatic plants were collected from two ponds and a stream at intervals
up to 30 days after treatment for analysis of diflubenzuron residues.
The duration of detectable residues was different for each substrate,
but in all cases it was less than 2 weeks. Zooplankton and benthic
invertebrate populations were monitored for up to 110 days after
spraying in two ponds in the high-volume rate block and in control
ponds. Significant mortality occurred in two groups of caged
macroinvertebrates (amphipoda and immature corixidae) 1 to 6 days
after the ponds were treated with diflubenzuron. Three taxa of
littoral insects ( Caenis, Celithemis and Coenagrion) were
significantly reduced in abundance in the treated ponds 21 to 34 days
after treatment but recovered to pre-treatment levels by the end of
the season. Of the six remaining groups studied, only one (immature
corixidae) may have been slightly affected by treatment. Zooplankton
(cladocera and copepoda) population numbers were reduced 3 days after
treatment and remained suppressed for 2-3 months (Sundaram et al.,
1991).
Blumberg (1986) found no effect on population numbers in a
forested ecosystem after aerial application of diflubenzuron (25 WP)
at rate 140 g a.i./ha.
When diflubenzuron (33.63 and 134.52 g a.i./ha) was applied 4
times at 2-week intervals to small ponds in Virginia, numbers of
daphnids were markedly reduced at both treatment levels. There was no
appreciable effect on two other invertebrate species ( Chironomidae
or Chaoborus) (Birdsong, 1977).
In a study by Rodrigues (1982), diflubenzuron (25% WP) was
applied at a rate of 1 mg/litre per 30 min to three forest streams in
New York. The reduction of chironomid larvae 15 days after treatment
ranged from 35 to 91% at different sites in the three streams. Of the
two stonefly families present in the three streams, Nemouridae were
affected more than Leuctridae, with decreases of 75-94% and 70%,
respectively. Ephemeroptera were reduced, but Trichoptera and
Coleoptera were unaffected.
Following aerial application at 67.26 g a.i./ha to a watershed,
diflubenzuron reached the stream channel (also as a result of wash-off
from foliage following several subsequent rainfalls), but the
invertebrate populations were not affected (Jones & Konchenderfer,
1988).
After two applications of 44.8, 112 and 224 g a.i./ha, grass
shrimp showed mortality of 86.6, 100, and 100%, respectively. At the
two lowest application rates, fiddler crabs ( Uca spp.) showed
mortalities of 53.3 and 66.6%, respectively (McAlonan, 1976).
Diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied 4
times at 2-week intervals to man-made ponds in North Carolina,
Arkansas and Texas, USA. In North Carolina no apparent effect on
phytoplankton was found. There was a marked decrease in the numbers
of crustaceans ( Cladocera and Copepoda), as well as of certain
species from benthic communities such as Hexagenia and Chaoborus
in Arkansas. A relative decrease in copepod numbers was observed, but
the authors attributed this partly to natural mortality during winter.
In Texas there was a marked decrease in the numbers of all crustaceans
and a corresponding increase in rotifer populations, particularly
Asplanchina (Aquatic Environmental sciences, 1976a,b,c).
When diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied
4 times at 2-week intervals to man-made ponds in Alabama, USA, daphnid
populations were reduced to 50% of the pre-treatment level at a rate
of 134.52 g/ha but increased at 33.63 g/ha to a level of 15% of that
in a control pond. Gastropod numbers decreased in the benthic samples
but increased in the periphyton samples from the suspended plate
samplers in the treated ponds (Jackson, 1976).
Diflubenzuron at 33.63 and 134.52 g/ha was applied 4 times at
2-week intervals to a tidally influenced salt marsh in Virginia, which
was sampled biologically 14 times over a 70-day period. Three species
of arthropods, Cyathura polita, Ceratopogonidae sp., and Psychodidae
sp., showed significant reductions in numbers. Oligochaetes, Hanynkia
speciosa and the molluscs were not significantly affected (Matta,
1976).
Six applications of diflubenzuron (28 g a.i./ha) over an 18-month
period caused statistically significant differences in the population
density of aquatic organisms in a Louisiana coastal marsh. None of the
organisms affected were completely eliminated from the ecosystem.
Populations of five taxa (nymphs of Trichocorixa louisianae Jaczewski
and Buenoa spp., Coenagrionidae naiad spp., Berosus infuscatus Le Conte
adults, and Hyalella azteca Saussure) were significantly reduced.
Population of 15 taxa, i.e. Physa sp., Ceanis sp. and Callibaetis sp.
naiads, Noteridae larvae, Hydrovatus cuspidatus, Kunze adults,
Hydrovatus sp. larvae, Dytiscidae larvae, Mesovelia mulsanti Jaczewski
adults, Trichocorixa louisiana adults, larvae of Chironomidae, Ephudridae,
Dolichopodiae and Tabanidae, and the fish Gambusia affinis (Baird and
Griard) and Jordanella floridae (Goode and Bean), showed significant
increases after exposure to diflubenzuron. The 27 remaining aquatic
organisms (members of the Hemiptera, Coleoptera, Mysidacea, Decapoda,
Diptera and Odonata) showed no statistically significant differences
when compared with untreated populations (Farlow et al., 1978).
After treatment with diflubenzuron (as 1% granular formulation)
at rate of 4.5 g a.i./ha to a marsh habitat on the Fraser River,
Canada, the population of cladocerans appeared to be depressed for
about 5 days but they recovered 2 weeks after treatment. There was no
effect on copepods or ostracods. Significant reduction in the numbers
of water beetles and zooplankton occurred (Wan & Wilson, 1977).
In a field study by Hester et al. (1986), WP-25 formulation was
evaluated in natural salt water pools to determine its toxicity to
juvenile stages of three estuarine crustaceans exposed to a single
application of 45 g diflubenzuron/ha. One hour after application,
water residues of diflubenzuron averaged 3.6 µg/litre and mortality
was 46.5, 60.7 and 40.6% for Callinectes sp., Palaemonetes pugio
and Uca pugilator, respectively, over the 10-day observation period.
When these species were introduced into the pools 7 days after
treatment, mortality was not significantly greater in the treated
group than in the controls over the 21-day observation period. The
maximum concentration after organisms were introduced was
0.69 µg/litre, and mortalities of 22.2, 1.5 and 4.2% for Callinectes
sp., Palaemonetes pugio and Uca pugilator, respectively, were
reported.
In a study by Hester (1982), diflubenzuron (25% WP) at the rate
of 0.045 kg a.i./ha was applied to tidal ponds. Residue analysis
showed 2 to 5 µg/litre after 1 h. The mortality of three
estuarine decapods: blue crabs ( Callinectes sp.), grass shrimp
(Palaemonetes pugio) and fiddler crabs (Uca pugilator) was 44,
61 and 41%, respectively. When these decapods were introduced to the
ponds 7 days after application, the residues in water were
0.4-0.7 µg/litre and mortality was 22, 1.5 and 4%, respectively.
9.2.2.3 Vertebrates
During a forestry spraying programme at 0.067 kg diflu-
benzuron/ha, a survey on fish was performed in the watershed.
Observations made on caged brown trout in the stream from day -7 to
day +6 revealed no immediate mortality or erratic behaviour
attributable to treatment. Barrier seines placed at the upstream and
downstream boundaries of the spray area did not collect any dead or
dying fish during the 1-week period they were in the stream. Visual
observations made by walking along the stream during this same period
also revealed no dead or dying fish. Post-spray population
estimations revealed increases in both test and control areas.
However, statistical analysis indicated no significant differences
pre- and post-spray between stations. The treatment had no observable
effect on the development of fish larvae. Immediate mortality was not
observed in caged fish or stream fish collected by barrier seines.
Delayed mortality attributable to spraying was not shown by population
estimations taken 6 weeks following spray, nor were any dead or dying
fish seen during this period. There appeared to be no adverse effect
of diflubenzuron sprayed at 0.067 kg a.i./ha on the fish species
observed in this study (White, 1975).
No effect on fish populations ( Micropterus salmoides, Lepomis
macrochirus and Gambusia affinis) was observed after four
applications of diflubenzuron (33.69 and 134.52 g a.i./ha) at 2-week
intervals to small ponds in Virginia (Birdsong, 1977).
Killifish (Fundulus heteroclitus) showed no effect after three
applications of diflubenzuron at rates from 11.12 to 224.2 g a.i./ha
to replicated semi-natural pools (McAlonan, 1976). Diflubenzuron
revealed no toxic effect on bullheads or sunfish after aerial
application of 35 g/ha in Canada (Buckner et al., 1975).
Growth of bluegill sunfish ( Lepomis macrochirus Rafinesque) was
not affected by diflubenzuron applications at rates of 2.5-10 µg/litre
to ponds and lakes in California (Apperson et al., 1978). Ten days
after the fourth treatment to man-made ponds with diflubenzuron at
33.63 and 134.52 g a.i./ha no apparent adverse effect on largemouth
bass was observed (Jackson, 1976).
No death of fish ( Pomoxis nigromaculatus and Ictalurus
nebulosis) occurred after application of diflubenzuron (mean
concentration of 13.2 µg/litre one hour after treatment) to
experimental ponds in California. For 1 month following treatment,
stomach content analyses showed alteration in the diet of the fish
(Collwell & Schaefer, 1980).
9.2.3 Terrestrial organisms
9.2.3.1 Invertebrates
Honey-bee colonies remained normal after an aerial application of
350 g diflubenzuron/ha in Canada (Buckner et al., 1975).
A comparative study of the effect of diflubenzuron on carabid
fauna in oak woods in Westphalia in 1987-1988 was reported. The total
number of captured beetles in treated areas was lower in 1989.
Evaluation of the 12 beetle species most frequently caught indicated
lower numbers than in control areas. The decrease coincided with the
diflubenzuron treatment in the middle of May. No higher active
density of species reproducing in late summer or autumn was observed
in the untreated areas (Klenner, 1990).
In a forestry spraying programme (0.0672 kg diflubenzuron per
ha), a survey of microarthropods collected from leaf litter and soil
in control and sprayed areas of the Bald Eagle State Forest,
Pennsylvania, USA, showed little or no effect of diflubenzuron on the
spray plot fauna. Increases and decreases in both the control and
spray population sizes during the 6-week study period could seemingly
be explained on the basis of seasonal population cycles, increased
soil moisture condition and differential extraction efficiencies
(White, 1975).
9.2.3.2 Vertebrates
Small song birds in a forest ecosystem were unaffected after an
aerial application of 350 g diflubenzuron/ha in Canada (Buckner et
al., 1975).
In a forestry spraying programme (0.0672 kg diflubenzuron/ha)
surveys were made on birds and small mammals. Effects on birds were
assessed using single male mapping surveys. The survey methodology
was designed to minimize bias due to differences between observers,
plots, times of day and abundance of birds. Vegetation and
defoliation types were identified and mapped and defoliation was
sampled at 136 sites. Four hundred and sixteen surveys were conducted
on treated plots, defoliated plots, and undisturbed plots. No
differences were observed in the number of individuals singing, the
amount of time each individual spent singing or the song repetition
rate during song bouts. The results show that diflubenzuron did not
lead to the death or emigration of singing birds and probably had no
direct adverse physiological effects on birds since their vocal
behaviour changed little, if at all. The singing male surveys did not
detect a presumed food shortage among canopy feeders caused by the
defoliation. Small mammals studied in this project were censused as
thoroughly as possible. The short pre-spray sampling period for the
treated plot was a handicap in evaluating the pre-spray population
levels; however, post-spray sampling compensated for this to some
degree. Peromyscus, Clethrionomys and Sorex consume arthropods to
varying degrees and may be exposed to diflubenzuron present as
residues. Diflubenzuron had no demonstrable effects on any of these
small mammals sampled in this study. The application of diflubenzuron
at the rate of 0.067 kg/ha provided good foliage protection within the
spray sites and apparently made the dying gypsy moth larvae
unpalatable. Other contact and stomach pesticides generally do not
have this effect on caterpillars and are consequently consumed by
insectivorous mammals. The reduced exposure by non-ingestion is no
doubt a beneficial aspect. None of the species of small mammals
showed reductions in numbers from pre-spray to post-spray periods that
could be attributed to the compound. Stomach analysis did not
indicate any change in the feeding habits of any of the small mammals
from either plot in the study. Changes in reproduction could not be
detected. However, of the Peromyscus and Clethrionomys animals
that were dissected and examined, 7 out of 12 females were in a
healthy condition or had gravid reproductive organs. Others were
either virgins or placental scars could not easily be seen, as in
Sorex. The body weight of all the small mammals in the study was
normal for each species. Juveniles were easily distinguished by
weight and gonad development. A regression comparing gonad weights
and body weights of Clethrionomys indicated a strong correlation.
Since the body weight of small mammals increases with age, this was to
be expected (White, 1975).
Diflubenzuron sprayed at 0.14 and 0.28 kg/ha over forests in
north-eastern Oregon, USA, did not have any detectable adverse effect
on bird population numbers, nesting or bird behaviour (Richmond et
al., 1979).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diflubenzuron was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1981, 1984, and 1985 (FAO/WHO, 1982a,b;
1985a,b; 1986a,b). In 1985, JMPR established an Acceptable Daily
Intake (ADI) for human beings of 0-0.02 mg/kg body weight per day,
based on the fact that the following levels produced no toxicological
effects:
rat: 2 mg/kg body weight (40 mg/kg diet)
mouse: 2.4 mg/kg body weight (16 mg/kg diet)
dog: 2 mg/kg body weight per day
Diflubenzuron has been classified as "a product unlikely to
present an acute hazard in normal use", on the basis of an acute oral
LD50 for the rat that is greater than 4640 mg/kg body weight (WHO,
1994).
REFERENCES
Ali A & Kok-Yokomi ML (1989) Field studies on the impact of a new
benzoylphenylurea insect growth regulator (UC-84572) on selected
aquatic nontarget invertebrates. Bull Environ Contam Toxicol,
42: 134-141.
Ali A & Lord J (1980) Impact of experimental insect growth regulators
on some nontarget aquatic invertebrates. Mosq News, 40(4): 564-571.
Ali A & Mulla MS (1978a) Effects of chironomid larvicides and
diflubenzuron on nontarget invertebrates in residential-recreational
lake. Environ Entomol, 7: 21-27.
Ali A & Mulla MS (1978b) Impact of the insect growth regulator
diflubenzuron on invertebrates in a residential-recreational lake.
Arch Environ Contam Toxicol, 7: 483-491.
Ali A, Nigg HN, Stamper JH, Kok-Yokomi ML, & Weaver M (1988)
Diflubenzuron application to citrus and its impact on invertebrates in
an adjacent pond. Bull Environ Contam Toxicol, 41: 781-790.
Anon (1980) Final report: Environmental monitoring of the 1979 Gypsy
moth control program in five eastern States. Hyattsville, Maryland, US
Department of Agriculture, Animal an Plant Health Inspection Service,
25 pp (Proprietary report No. 56683/18/1983, submitted to WHO by
Duphar BV, Crop Protection Division, 'sGraveland, The Netherlands).
Anton FA, Cuadra LM, Gutierrez P, Laborda E, & Laborda P (1993)
Degradational behavior of the pesticides glyphosate and diflubenzuron
in water. Bull Environ Contam Toxicol, 51: 882-888.
Apperson CS, Schaefer CH, Colwell AE, Werner GH, Anderson NL, Dupras
EF, & Longanecke DR (1978) Effects of diflubenzuron on Chaoborus
astictopus and nontarget organisms and persistence of diflubenzuron
in lentic habitats. J Econ Entomol, 71(3): 521-527.
Aquatic Environmental Sciences (1976a) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
North Carolina. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-13, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976b) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Arkansas. Tarrytown, New York, Union Carbide Corporation, 9 pp
(Unpublished proprietary report NTP-8, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976c) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Texas. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-7, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Arnold D, Kennedy SL, & Keplinger ML (1974) Mutagenic study with TH
6040 in albino mice. Northbrook, Illinois, Industrial Bio-Test
Laboratories Inc. (IBT No. 622-05068) (Unpublished proprietary report,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Arts JHE (1991) Acute (4-hour) inhalation toxicity of dimilin WP 25%
in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report TNO No. 56645/64/1991, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Barker RY & Taber S (1977) Effects of diflubenzuron fed to caged honey
bees. Environ Entomol, 6: 167-168.
Barker RY & Waller GD (1978) Effects of diflubenzuron wettable powder
on caged honey bee colonies. Environ Entomol, 7: 534-535.
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990a) A one-generation
reproduction study with the bobwhite ( Colinus virginianus. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/08/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990b) A one-generation
reproduction study with the mallard ( Anas platyrhynchos. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/07/1990, submitted to WHO by Solvay Duphar, BV, 'sGraveland,
The Netherlands).
Bentley JP, Weber GH, & Gould D (1979) The effect of diflubenzuron
feeding on glucosaminoglycan and sulfhemoglobin biosynthesis in mice.
Pestic Biochem Physiol, 10: 162-167.
Berberian IG & Enan EE (1989) Hematological studies on White male rats
exposed to some antimoulding compounds. Bull Environ Contam Toxicol,
43: 60-65.
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975a) Acute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/198/74988, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975b) Subacute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/199/7551, submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975c) Subacute inhalation
toxicity to the rat of DU 112307 insecticide powder. (Evaluation of
methaemoglobinaemia). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/197/ 741013, submitted to WHO
by Solvay Duphar BV, Weesp, The Netherlands).
Berends AG & Thus JLG (1992a) The acute toxicity of dimilin WP-25 to
the earthworm Eisenia fetida 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 16 pp (Proprietary
report No. 56635/26/1992).
Berends AG & Thus JLG (1992b) The toxicity of diflubenzuron to the
alga Selenastrum capricornutum 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 23 pp (Proprietary
report No. 56635/49/1992).
Berends AG & van den Laan-Straathof JMTh (1994a) The acute toxicity of
diflubenzuron to Zebra fish ( Brachydanio rerio. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/57/93).
Berends AG & van den Laan-Straathof JMTh (1994b) The acute toxicity of
diflubenzuron to rainbow trout ( Oncorhynchus mykiss. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/02/94).
Berends AG, Thus JLG, & Jansen WAJ (1992) The acute toxicity of
diflubenzuron to the earthworm Eisenia fetida 'sGraveland, The
Netherlands, Solvay Duphar BV, Environmental Research Department,
16 pp (Proprietary report No. 56635/22/1992).
Besten C Den, Buse TE, & Koopman TSM (1993a) Acute oral toxicity study
with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay Duphar
BV (Proprietary report No. 56345/51/1993).
Besten C Den, Buse TE, & Koopman TSM (1993b) Acute dermal toxicity
study with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56345/52/1993).
Birdsong RS (1977) Field test of Dimilin on non-target organisms in
Virginia. Nonfolk, Virginia, Environmental Consultants, Inc. (NTP-11)
(Unpublished proprietary report, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Blumerg AY (1986) Survey of aquatic soil and soil surface invertebrate
fauna in a North Carolina forest (pre and post application of Dimilin
WP-25) Weesp, The Netherlands, Duphar BV (Proprietary report
No. 56635/24/1986).
Boelhouwers EJ, Joustra KD, Nijssen OA, & Stegman KH (1988a)
Hydrolysis of 14C-labelled diflubenzuron in buffer solutions at pH 5,
pH 7, and pH 9. 'sGraveland, The Netherlands, Solvay Duphar
(Proprietary report No 56630/137/1988).
Boelhouwers EJ, Joustra KD, & Stegman KH (1988b) Photodegradation of
14C-labelled diflubenzuron in water. 'sGraveland, The Netherlands,
Solvay Duphar BV (Proprietary report No. 56630/35/1988).
Bogus ER, Gallagher PA, Cameron EA, & Mumma RO (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed phase solid support. J Agric Food Chem, 33(6): 1018-1021.
Bollag JM, Blattmann P, & Laanio T (1978) Adsorption and
transformation of four substituted anilines in soil. J Agric Food
Chem, 26(6): 1302-1306.
Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, & MacKenzie WF
(1990) Pathology of the Fischer rat - Reference and atlas. New York,
London, Academic Press.
Booth GM (1975) The impact of Dimilin W-25 on non-target invertebrates
in ponds located in Salt Lake county, Utah. Provo, Utah, Brigham Young
University (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Booth GM (1977) Biochemical effects of diflubenzuron on mouse embryos.
Provo, Utah, Brigham Young University (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Booth GM & Ferrell D (1977) Degradation of Dimilin by aquatic
foodwebs. In: Khan MAQ ed. Pesticides in aquatic environments. New
York, Plenum Publishing Corporation, pp 221-243.
Booth GM, Alder DC, Lee ML, Carter W, Whitmore RC, & Seegmiller RE
(1987) Environmental fate and properties of 1-(4-chlorophenyl)-
3-(2,6-difluorobenzoyl) urea (diflubenzuron, Dimilin). In: Wright JE &
Retnakaran ed. Chitin and benzoylphenyl ureas. Dordrecht, The
Netherlands, Junk Publishers, pp 141-204.
Brusick DJ & Weir RJ (1977a) Mutagenic evaluation of diflubenzuron
technical batch FL 4/605201. Final report (Project No. 2683).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/34/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Brusick DJ & Weir RJ (1977b) Evaluation of diflubenzuron: In vitro
malignant transformation in BALB/3T3 cells. Final Report (Project
No 2688). Kensington, Maryland, Litton Bionetics Inc. (Unpublished
proprietary report No. 56645/35/1977, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Brusick DJ & Weir RJ (1977c) Evaluation of diflubenzuron: Unscheduled
DNA synthesis in WI-38 cells. Final Report (Project No 2688).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/36/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Bryant H (1976) Activity of TH-6040 in the Ames Salmonella
typhimurium mutagenesis assay. Report to H.W. Dorough, University of
Kentucky, Lexington, USA (Unpublished proprietary report submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Buckner CH, McLeod BB, & Kingsbury PD (1975) The effect of an
experimental application of Dimilin upon selected forest fauna.
Ottawa, Ontario, Chemical Control Research Institute
(Report No. CC-X-97).
Bull DL & Ivie GW (1978) Fate of diflubenzuron in cotton, soil, and
rotational crops. J Agric Food Chem, 26(3): 515-520.
Burdock GA, Serota DG, Alsaker RD, & Purvis DS (1980a) Ninety-day
subchronic toxicity study in mice - Diflubenzuron technical. Final
report (Project No. 533-120). Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Burdock GA, Serota DG, Purvis D, & Alsaker RD (1980b) Subchronic
dietary toxicity study in rats - Diflubenzuron. Final report: Volume 1
(Project No. 533-119). Vienna, Virginia, Hazleton Laboratories America
Inc. (Unpublished proprietary report submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Burdock GA, Wolfe GW, Hepner KE, Alsaker RD, Koka M, & Phipps RB
(1984) Oncogenicity study in rats on diflubenzuron. Final report.
Vienna, Virginia, Hazleton Laboratories America Inc. (Unpublished
proprietary report No. 56645/08/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Burgess D (1989) Uptake, depuration and bioconcentration of
14C-diflubenzuron by Bluegill sunfish ( Lepomis macrochirus.
Columbia, Missouri, Analytical Bio-Chemistry Laboratories, Inc.
(Proprietary report No. 56635/16/1989, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Cardinal H, Vernet G, & Sinegre G (1979) Quelques effets d'un
inhibiteur de croissance: le diflubenzuron sur un crabe Carcinus
mediterraneus(Czerniavsky). C C R Soc Biol, 173: 1105-1108.
Carringer RD, Weber JB, & Monaco TJ (1975) Adsorption-desorption of
selected pesticides by organic matter and montmorillonite. J Agric
Food Chem, 23: 568-572.
Caspary WJ, Daston DS, Myhr BC, Mitchell AD, Rudd CJ, & Lee PS (1988)
Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay:
interlaboratory reproducibility and assessment. Environ Mol Mutagen,
12(Suppl 13): 195-229.
Cecil HC, Miller RW, & Corley C (1981) Feeding three insect growth
regulators to white Leghorn hens: Residues in eggs and tissues and
effects on production and reproduction. Poult Sci, 60: 2017-2027.
Chandran VR (1981) Primary skin irritation study in rabbit with
Dimilin 25 WP. Padappai, Tamil Nadu, India, Coromandel Indag Research
Centre (Proprietary report No. 31, submitted to WHO by Solvay Duphar
BV, 'sGraveland, The Netherlands).
Chapman RA, Tu CM, Harris CR, & Harris C (1985) Persistence of
diflubenzuron and Bay Sir 8514 in natural and sterile sandy loam and
organic soils. J Environ Sci Health, B20(5): 489-497.
Chesterman H, Heywood R, Barker MH, Street AE, & Cherry CP (1974)
DU 112307 toxicity in repeated dietary administration to beagle dogs
(Repeated administration for 13 weeks). Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR169/74157, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Christiansen ME & Costlow JD (1980) Persistence of the insect growth
regulator Dimilin in brackish water: A laboratory evaluation using
larvae of an estuarine crab as an indicator. Helgoländer Meeresunters,
33: 327-332.
Christiansen ME & Costlow JD (1982) Ultrastructural study of the
exoskeleton of the estuarine crab Rhithropanopeus harrsii Effect of
the insect growth regulator Dimilin (diflubenzuron) on the formation
of the larval cuticle. Mar Biol, 66: 217-226.
Christiansen ME, Costlow JD, & Monroe RJ (1978) Effects of the insect
growth regulator Dimilin (TH6040) on larval development of two
estuarine crabs. Mar Biol, 50: 29-36.
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981a) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume I. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/294/80185, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981b) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume II. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/294/80185, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Colley J, Heywood R, Street AE, & Gopinath C (1984) The effect of
diflubenzuron given by oral administration with the feed on toxicity
and tumour development in male and female HC/CFLP mice. Final report.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. 56645/32/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Collwell AE & Schaefer CH (1980) Diets of Ictalurus nebulosus and
Pomoxis nigromaculatus altered by diflubenzuron. Can J Fish Aquat
Sci 37(4): 632-639.
Cooke M & Ober AG (1980) OV-17-QF-1 Capillary column for
organochlorine pesticide analysis. J Chromatogr, 195: 265-269.
Corley C, Miller RW, & Hill K (1974) Determination of
N-(4-chlorophenyl)-N-(2,6-difluorobenzoyl)-urea in milk by
high-speed liquid chromatography. J AOAC, 57(6): 1269-1271.
Costlow YD (1979) Effect of Dimilin on development of larvae of the
stone crab Menippe mercenaria and the blue crab Callinectes
sapidus In: Vernberg WB, Calabrese A, Thurburg FP, & Vernberg FJ ed.
Marine pollution: Functional responses. New York, London, Academic
Press, pp 355-363.
Crookshank HR, Sowa BA, Kubena L, Holman GM, Smalley HE, & Morison R
(1978) Effect of diflubenzuron (Dimilin) on the hyaluronic acid
concentration in the chicken combs. Poult Sci, 57: 804-806.
Cunningham PA (I976) Effects of Dimilin (TH-6040) on reproduction in
the brine shrimp, Artemia salina Environ Entomol, 5: 701-706.
Cunningham PA (1986) A review of toxicity testing and degradation
studies used to predict the effects of diflubenzuron (Dimilin) in
estuarine crustaceans. Environ Pollut, A40: 63-86.
Cunningham PA & Myers LE (I986) Dynamics of diflubenzuron (Dimilin)
concentrations in water and sediment of a supratidal saltmarsh site
following repetitive aerial applications for mosquito control. Environ
Pollut, A41(1): 63-88.
Cunningham PA & Myers LE (I987) Effects of diflubenzuron (Dimilin) on
survival, molting, and behaviour of juvenile fiddler crabs, Uca
pugilator Arch Environ Contam Toxicol, 16: 745-752.
Danhaus RG & Sieck RF (1976) Rotation crop uptake of 14C-residues
following soil application of Dimilin. Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Danhaus RG, Wargo JP, & Sieck RF (1976) 14C-Dimilin field soil
leaching study. Colorado Springs, Colorado, Analytical Development
Corporation (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Davies RE & Halliday JC (1974) Acute percutaneous toxicity to rabbits
of DU 112307 technical. Huntingdon, England Huntingdon Research Centre
(Unpublished proprietary report No. 2171/D175/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE & Ligget MP (1973) Irritant effects of DU 112307 technical
on rabbit eye mucosa. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 2170/176D/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE, Elliot PH, Street AE, Heywood R, & Prentice DE (1975)
Effect of repeated applications of DU 112307 to the skin of rabbits
for three weeks. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR200/74851, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
De Bree H, De Lange N, Overmars H, & Post LC (1977) Diflubenzuron:
analysis of metabolites connected with methaemoglobinaemia. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/08/1977).
De Lange N (1979) Diflubenzuron: dermal absorption in the rabbit.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56654/09/1979).
De Lange N & Post LC (1978) Diflubenzuron: intestinal absorption in
the mouse in relation to dosage level. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56654/04/1978).
De Lange N, Overmans H, & Post LC (1974) PH 60-40: excretion of
radioactivity and metabolite patterns in rats following oral
administration. Weesp, The Netherlands, Philips-Duphar BV (Unpublished
proprietary report No. 56654/20/1974).
De Lange N, Overmans H, Willems AGM, & Post LS (1975) Diflubenzuron
(PH 60-40): balance studies in the rat, and identification of urinary
metabolites. Weesp, The Netherlands, Philips Duphar BV (Unpublished
proprietary report No. 56654/22/1975).
De Lange N, Fronik GM, & Post LC (1977) Diflubenzuron: intestinal
absorption in the rat in relation to dosage level. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/10/1977).
Deul DH & Jong BJ de (1977) The possible influence of DU 112307 on the
in vivo synthesis of hyaluronic acid in chicken skin. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56635/01/1977).
Di Prima SJ (1976) Determination of diflubenzuron residues in soybean
process fractions (meal, oil, hulls) by gas chromatography. Thompson-
Hayward Chemical Company (Unpublished proprietary report No. AM-13,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Di Prima SJ, Cannizaro RD, Roger JC, & Ferrell CD (1978) Analysis of
diflubenzuron residues in environmental samples by high-pressure
liquid chromatography. J Agric Food Chem, 26(4): 968-971.
Dorough HW (1977) Screening of selected Thompson-Hayward Chemicals for
activity in the Ames Salmonella mutagenicity test. Lexington,
University of Kentucky, Department of Entomology (Unpublished report
No. MTM-91).
Downer DM (1990) Analysis of diflubenzuron in streamwater samples from
little sluice mountain gypsy moth sprayblock. Harrisonburg, Virginia,
US Forest Service, 30 pp (Unpublished proprietary report submitted to
WHO by Duphar BV, Weesp, The Netherlands).
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1985) Reproducibility of microbial
mutagenicity assays: II. Testing of carcinogens and noncarcinogens in
Salmonella typhimurium and Escherichia coli Environ Mol Mutagen,
7(Suppl 5): 1-248.
El-Sebae AH, Salem MH, Al-Assar MRS, & Enan EE (1988) In vitro
effect of profenofos, fenvalerate and dimilin on protein and RNA
biosynthesis by rabbit liver and muscle tissues. J Environ Sci Health,
B23(5): 439-451.
Enninga IC (1990) Evaluation of DNA repair inducing ability of
diflubenzuron in a primary culture of rat hepatocytes (with
independent repeat). Report RCC NOTOX BV, 'sHertogenbos, The
Netherlands No. 56645/114/1990. Proprietary report, submitted to WHO
by Solvay Duphar, BV, 'sGraveland, The Netherlands.
FAO/WHO (1982a) Pesticides residues in food - 1981. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticides Residues in
Food and Environment and WHO Expert Group on Pesticides Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 37).
FAO/WHO (1982b) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985a) Pesticide residues in food - 1984. Report of the Joint
Meeting on Pesticide Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) Pesticide residues in food - 1984. Evaluations: The
Monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO, Plant Production and Protection Paper 67).
FAO/WHO (1986a) Pesticide residues in food - 1985. Report of the Joint
Meeting of the Residues in Food and the Environment and a WHO Expert
Group on Pesticides Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations:
Part II - Toxicology. Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of pesticide
residues, 4th ed. Rome, Codex Committee on Pesticide Residues, Food
and Agriculture Organization of the United Nations.
Farlow JE, Breaud TP, Steelman CD, & Schilling PE (1978) Effect of
the insect growth regulator diflubenzuron on non-target aquatic
populations in a Louisiana intermediate marsh. Environ Entomol,
7: 199-204.
Fischer SA & Hall LW Jr (1992) Environmental concentrations and
aquatic toxicity data on diflubenzuron (Dimilin). Crit Rev Toxicol,
22(1): 45-79.
Forward RB & Costlow JD (1978) Sublethal effects of insect growth
regulators upon crab larval behaviour. Water Air Soil Pollut,
9: 227-238.
Gilbert P, Saint-Ruf G, Poncelet F, & Mercier M (1980) Genetic effects
of chlorinated anilines and azobenzenes on Salmonella typhimurium
Arch Environ Contam Toxicol, 9: 533-541.
Goodman DG (1980a) Histopathologic evaluation of mice administered
diflubenzuron in the diet. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Goodman DG (1980b) Histopathologic evaluation of rats administered
diflubenzuron in the diet. Washington, DC, Clement Associates
(Unpublished proprietary report submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Gordon R & Cornect M (1986) Toxicity of the insect growth regulator
diflubenzuron to the rove beetle Aleochara bilineata a parasitoid
and predator of the cabbage maggot Delia radicum Entomol Exp Appl,
42: 179-185.
Goto M (1977a) Crop residue analysis of Dimilin. Japan, Institute
of Pesticide Residues (Unpublished proprietary report
No. K/Resid./004/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Goto M (1977b) Crop residue analysis of Dimilin. Japan, Institute of
Pesticide Residues (Unpublished proprietary report No.
K/Resid./007/1977, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Granett J, Morang S, & Hatch R (1978) Reduced movement of precocious
male Atlantic salmon parr into sublethal Dimilin-G1 and carrier
concentrations. Bull Environ Contam Toxicol, 19(4): 462-464.
Graves WC & Swigert JP (1993) Diflubenzuron: A 96-h flow through acute
toxicity test with the sheepshead minnow ( Cyprinodon variegatus.
Final report. Wildlife International Ltd. USA (Unpublished proprietary
report No. 56835/13/93, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Greenough RJ, Goburdhun R, Hundson R, & MacNaughtan F (1985)
Diflubenzuron. 52 week oral toxicity study in dogs: Volumes 1 and 2
(Project No. 630146,2728). Musselburgh, Scotland, Inveresk Research
International (Unpublished proprietary report No. 56645/32/1985,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Greenough RJ & McDonald P (1986) Diflubenzuron VC 90 acute inhalation
toxicity study in rats (limit test). Musselburgh, Scotland, Inveresk
Research International (Proprietary report No. 56645/41/1986,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Gulka G, Doscher CM, & Watabe N (1980) Toxicity and molt-accelerating
effects of diflubenzuron on the barnacle, Balanus eburneus Bull
Environ Contam Toxicol, 25: 477-481.
Gulka G, Gulka CM, & Watabe N (1982) Histopathological effects of
diflubenzuron on the cirripede crustacean, Balanus eburneus Arch
Environ Contam Toxicol, 11: 11-16.
Gustafson DE & Wargo JP (1976) Fate of Dimilin following application
to soybeans (ADC Project No 222). Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hansen SR & Garton RR (1982a) Ability of standard toxicity test to
predict the effects of the insecticide diflubenzuron on laboratory
stream communities. Can J Fish Aquat Sci, 39: 1273-1288.
Hansen SR & Garton RR (1982b) The effects of diflubenzuron on a
complex laboratory stream community. Arch Environ Contam Toxicol,
11: 1-10.
Hatzios KK & Penner D (1978) The effect of diflubenzuron on soybean
photosynthesis, respiration and leaf ultrastructure. Pestic Biochem
Physiol, 9: 65-69.
Hawkins DR, Jackson AJS, & Roberts NL (1980) Excretion of radio-
activity after oral administration of 3H/14C-diflubenzuron to
cats (PDR/302/80443). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 56654/14/1980, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands.
Helling CS (1985) Soil mobility of three Thompson-Hayward pesticides.
Interim report. Beltsville, Maryland, US Department of Agriculture,
Agricultural Research Centre (Unpublished proprietary report No. E-34,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hester PG (1982) Efficacy of diflubenzuron on three estuarine decapods
( Callinectes sp., Palaemonetes pugio and Uca sp.) (Unpublished
document submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hester PG, Olson MA, & Floore TG (1986) Effects of diflubenzuron on
three estuarine decapods, Callinectes sp., Palaemonetes pugio and
Uca pugilator J Fla Anti-Mosq Assoc, 57: 8-14.
Honeycutt R (1993) Indoor occupant exposure study with Dimilin 25 W.
Colorado Springs, Colorado, Analytical Research and Development
Corporation (Report No. 56635/56/1992, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hsu TS & Bartha R (1974) Interaction of pesticide-derived chloroaniline
residues with soil organic matter. Soil Sci, 1974: 444-452.
Huber CM & Collins DL (1987) Final report on the 1987 spray project to
eradicate Gypsy moth from the Tusquitee ranger district on the
Nantahala national forest. Asheville, North Carolina, Field Office,
Forest Pest Management (Unpublished report No. 88-1-4, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Huber CM & Manchester EH (1988) Final report on the eradication of
the Gypsy moth from the Tusquitee ranger district on the Nantahala
national forest. Forest Pest Management (Unpublished report
No. 89-1-7, submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hunter B, Batham P, Street AE, & Cherry CP (1974) DU 112307
preliminary assessment of the toxicity to male mice in dietary
administration for 6 weeks. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/174/74199, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hunter B, Batham P, Offer JM, & Prentice DE (1975) Tumorigenicity
study of DU 112307 to mice. Dietary administration for 80 weeks. Final
report. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/170/75685, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Colley J, Street AE, Heywood R, Prentice DE, & Offer J
(1976) Effects of DU 112307 in dietary administration to rats for 104
weeks. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/171/75945, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Jordan J, Heywod R, Street AE, Prentice DE, Wight DG, &
Gibson WA (1979) DU 112307 toxicity to rats in dietary administration
for nine weeks followed by a four week withdrawal period (final
report). Huntingdon, England, Huntingdon Research Centre (Unpublished
report No. PDR/248/77883).
Ivie GW (1977) Metabolism of insect growth regulators in animals.
In: Ivie GW & Dorough HW ed. Fate of pesticides in large animals. New
York, London, Academic Press, pp 111-125.
Ivie GW (1978) Fate of diflubenzuron in cattle and sheep. J Agric Food
Chem, 26(1): 81-89.
Ivie GW, Bull DL, & Veech JA (1980) Fate of diflubenzuron in water.
J Agric Food Chem, 28(2): 330-337.
Jackson GA (1976) Dimilin (TH 6040): Biological impact on pond
organisms. Marion, Alabama, Southeastern Fish Cultural Laboratory
(Unpublished proprietary report NTP-42, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Jackson GC, Hardy CJ, Simms F, Mullins PA, & Gopinath Ch (1990)
Dimilin 4F acute inhalation toxicity study in rats 4-hour exposure.
Huntingdon, England, Huntingdon Research Centre (Proprietary report
No. 56645/68/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Jagannath DR & Brusick DJ (1977a) Mutagenicity evaluation of
4-chlorophenyl urea, final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/42/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977b) Mutagenicity evaluation of
2,6-difluorobenzoic acid. Final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/40/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977c) Mutagenicity evaluation of
4-chloroanilin. Final report. Kensington, Maryland, Litton Bionetics
Inc. (Unpublished proprietary report No. 56645/41/1977, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Janssen PJM & Pot TE (1987a) Primary irritation study of dimilin SC-48
to the eye of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/46/1987).
Janssen PJM & Pot TE (1987b) Primary irritation study of dimilin SC-48
to the skin of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/45/1987).
Janssen PJM & Pot TE (1987c) Acute oral toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/44/1987).
Janssen PJM & Pot TE (1987d) Acute dermal toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/43/1987).
Janssen PJM & Pot TE (1987e) Acute dermal toxicity study with dimilin
WP-25 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/58/1987).
Janssen PJM & van Doorn WM (1993a) Acute inhalation toxicity study
with dimilin OF-6 in male and female rats. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/01/1993).
Janssen PJM & van Doorn WM (1993b) Primary irritation study of dimilin
OF-6 to the skin of female rabbits. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/30/1993).
Janssen PJM & van Doorn WM (1993c) Primary irritation study of dimilin
OF-6 to the eye of female rabbits. Weesp, The Netherlands, Solvay
Duphar BV, Department of Toxicology (Proprietary report
No. 56345/31/1993).
Jenkins VK, Mayer RT, & Perry RR (1984) Effects of diflubenzuron on
growth of malignant melanoma and skin carcinoma tumors in mice. Invest
New Drugs, 2: 19-27.
Jenkins VK, Perry RR, Ahmer AE, & Ives K (1986) Role of metabolism in
effects of diflubenzuron on growth of B16 melanomas in mice. Invest
New Drugs, 4: 325-335.
Jones AS & Konchenderfer JN (1988) Persistence of diflubenzuron
(Dimilin) in a small eastern watershed and its impact on invertebrates
in a headwater stream. Research Triangle Park, North Carolina,
Southeastern Forest Experiment Station (Unpublished proprietary report
No. 56637/26/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Joustra KD, van Kampen WG, & Walstra P (1989) Metabolism of [14C]
diflubenzuron in citrus fruit (Analytical report). Weesp, The
Netherlands, Duphar BV, Analytical Development Department (Proprietary
report No. 56630/86/1989).
Julin AM & Sanders HO (1978) Toxicity of IGR, diflubenzuron, to
freshwater intervertebrates and fishes. Mosq News, 38: 256-259.
Kavanagh P (1988a) Diflubenzuron oral (gavage) rat teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/11/87)
(Proprietary report No. 56645/68/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kavanagh P (1988b) Diflubenzuron oral (gavage) rabbit teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/12/87)
(Proprietary report No. 56645/79/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Keet CMJF (1977a) The methaemoglobin, sulphaemoglobin and Heinz body
forming properties of DU 112307 after oral administration to male rats
during 8 days. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/15/1977).
Keet CMJF (1977b) The effect of DU 112307 (tech) in male mice after
daily oral administration (14-days) on body weight, methaemoglobin,
sulphaemoglobin and Heinz body formation. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/33/1977).
Keet CMJF (1977c) The methaemoglobin and sulphaemoglobin forming
properties of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/02/1977).
Kemp A, van der Heijden CA, & van Eldik A (1973a) Dietary
administration of DU 112307 to male and female rats for three months.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56645/13A/1973).
Kemp A, van der Heijden CA, & van Eldik A (1973b) Appendix III to
report No. 56645/13A/1973 individual data: dietary administration of
DU 112307 to male and female rats for 3 months. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56645/13B/1973).
Kingsbury P, Sundaram KMS, Holmers KMS, Nott R, & Kreutzweiser D
(1987) Aquatic fate and impact studies with Dimilin. Ottawa, Ontario,
Forest Pest Management Institute (Unpublished report No. 78, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Klenner MF (1990) [A comparative study of the carabid fauna in the
Dimilin-treated and untreated oak stands in Westphalia.] Mitt BBA
(Berlin-Dahlem), 266: 279 (in German).
Koelman-Klaus HJS (1978a) Acute oral toxicity study of
4-chlorophenylurea in male and female rats. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/09/1978).
Koelman-Klaus HJS (1978b) Acute oral toxicity study of
2,6-difluorobenzoic acid in male and female rats. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56645/25/1978).
Koopman TSM (1977a) Acute oral toxicity study with DU 112307 technical
in mice. 'sGraveland, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/04/1977).
Koopman TSM (1977b) Acute oral toxicity study with DU 112307 WP 25%
in mice and rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/03/1977).
Koopman TSM (1977c) Acute dermal toxicity study with DU 112307
technical in rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/07/1977).
Koopman TSM (1980a) Primary irritation of Dimilin ODC-45 to the rabbit
eye. 'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/02/1980).
Koopman TSM (1980b) Acute dermal toxicity study of dimilin ODC-45 in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/04/1980).
Koopman TSM (1980c) Primary irritation of Dimilin ODC-45 to the rabbit
skin. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/03/1980).
Koopman TSM (1984a) Acute dermal toxicity study with diflubenzuron
VC-90 in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/31/1984).
Koopman TSM (1984b) Acute oral toxicity study with diflubenzuron VC-90
in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/30/1984).
Koopman TSM (1984c) Primary irritation study of diflubenzuron VC-90 to
the rabbit eye. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/29/1984).
Koopman TSM (1984d) Primary irritation study of diflubenzuron VC-90 to
the rabbit skin. 'sGraveland, The Netherlands, Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/44/1984).
Koopman TSM (1985a) Primary irritation study of dimilin 2F to the
skin of the male rabbit. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report
No. 56645/93/1985).
Koopman TSM (1985b) Primary irritation study of dimilin 2F to
the eye of the male rabbit. 'sGraveland, The Netherlands,
Duphar BV, Department of Toxicology (Unpublished proprietary report
No. 56645/91/1985).
Koopman TSM (1985c) Acute dermal toxicity study with dimilin 2F in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/95/1985).
Koopman TSM (1985d) Acute oral toxicity study with dimilin 2F in rats.
'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/94/1985).
Koopman TSM & Jongeling AJ (1979) Acute oral toxicity study with
Dimilin ODC 45% in rats. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report No.
56645/38/1979).
Koopman TSM & Pot TE (1986) Acute oral toxicity study with
diflubenzuron VC-90% in mice. 'sGraveland, The Netherlands, Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/39/1986).
Koorn JC (1990) Study to examine the possible mutagenic activity of
diflubenzuron in the Ames Salmonella microsome assay. 'sGraveland,
The Netherlands, Duphar BV, Department of Toxicology (Proprietary
report No. 56645/74/1990).
Kramer HT (1990) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in an apple orchard in Phelps (New York). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-271,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1991) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in a citrus orchard in Oviedo (Florida). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-270,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992a) Field dissipation study with diflubenzuron
insecticide applied for control of insects on cotton (a bare soil
application in Arkansas). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-110, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992b) Field dissipation study with diflubenzuron
insecticide applied for control of insects on soybeans (a bare soil
application in Louisiana). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-116, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kubena LF (1982) The influence of diflubenzuron on several
reproductive characteristics in male and female layer-breed chickens.
Poult Sci, 61: 268-271.
Kuijpers LAM (1988) The acute toxicity of diflubenzuron to Daphnia
magna Weesp, The Netherlands, Duphar BV (Unpublished proprietary
report No. 56635/26/1988).
Kurczewski F, Wang C, Grimble D, Smith R, & Brezner J (1975)
Environmental impact of dimilin. A final report: Effects of dimilin
upon microorganisms in leaf litter and forest soil. Syracuse, New
York, State University of New York, pp 28-43.
Kynoch SR & Elliot PH (1978a) Screening test for delayed contact
hypersensitivity with Dimilin WP 25%. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 0911/D258/78, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Elliot PH (1978b) Screening test for delayed contact
hypersensitivity with dummy formulation for dimilin WP 25% in the
albino guinea-pig. Huntingdon, England, Huntingdon Research Centre
(Proprietary report No. 9112/D258/78, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin 2F. Huntingdon, England, Huntingdon Research
Centre (Proprietary report No. 56645/13/1987, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin SC-48. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 56645/72/1987, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Smith PA (1986) Delayed contact hypersensitivity in the
guinea-pig with diflubenzuron VC-90. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 86558D/PDR 432SS, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Lauren DR, Agnew MP, & Henzell RF (1984) Field life of the insect
growth regulator diflubenzuron on lucerne. N Z J Agric Res,
27: 425-429.
Lawrence JF & Sundaram KMS (1976) Gas-liquid chromatographic analysis
of N-(4-chlorophenyl-N-2,6-difluorobenzoyl) urea insecticide after
chemical derivatization. J AOAC, 59(4): 938-941.
Lee BM & Scott GI (1989) Acute toxicity of temephos, fenoxycarb,
diflubenzuron and methoprene and Bacillus thuringiensis var.
Israelensis to the mummichug ( Fundulus heteroclitus. Bull Environ
Contam Toxicol, 43: 827-832.
Maas W, Van Hes R, Grosscurt AC, & Deul DH (1980) [Benzoylphenylurea
insecticides.] In: Wegler R ed. [Chemistry of plant protection
chemicals and pesticides.] Berlin, Heidelberg, Springer Verlag, vol 6,
pp 432-470 (in German).
McAlonan WG (1976) Effects of two insect growth regulators on some
selected salt-marsh non-target organisms. University of Delaware, USA
(Thesis) (NTP-53).
Machado J, Coimbra J, Castilho F, & Sa C (1990) Effects of
diflubenzuron on shell formation of the freshwater clam, Anodonta
cygnea Arch Environ Contam Toxicol, 19: 35-39.
McGregor JT, Gould DH, Mitchell AD, & Sterling GP (1979) Mutagenicity
tests of diflubenzuron in the micronucleus test in mice, the L5178Y
mouse lymphoma forward mutation assay, and the Ames Salmonella
reverse mutation test. Mutat Res, 66: 45-53.
McKague AB & Pridmore RB (1978) Toxicity of Altosid and Dimilin to
juvenile rainbow trout and coho salmon. Bull Environ Contam Toxicol,
20: 167-169.
Madder DJ (1977) The disappearance from, efficacy in and effect on
non-target organisms of diflubenzuron, methoprene and chlorpyrifos in
a lentic ecosystem. Canada, University of Manitoba, Faculty of
Graduate Studies, 139 pp (Thesis) (NTP-71).
Madder DJ & Lockhart WL (1978) A preliminary study of the effects of
diflubenzuron and methoprene on rainbow trout ( Salmo gairdneri. Bull
Environ Contam Toxicol, 20: 66-70.
Madder DJ & Lockhart WL (1980) Studies on the dissipation of
diflubenzuron and methoprene from shallow prairie pools. Can Entomol,
112: 173-177.
Mansager ER, Still GC, & Frear DS (1979) Fate of 14C diflubenzuron on
cotton and in soil. Pestic Biochem Physiol, 12: 172-182.
Marshall BF & Hieb BF (1973) 96-h LC50 Salmo gairdneri (n.m.
Oncorhynchus mykiss, Lepomis machrochirus and Fundulus heteroclitus
Marine Research Institute (Unpublished study submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Martinez-Toledo MV, Rubia T, De La Moreno J, & Gonzalez-Lopez J
(1988a) Effect of diflubenzuron on azotobacter nitrogen fixation in
soil. Chemosphere, 17(4): 829-834.
Martinez-Toledo MV, Gonzalez-Lopez J, Rubia T, De La Moreno J, &
Ramos-Cormenzana A (1988b) Diflubenzuron and the acetylene-reduction
activity of Azotobacter vinelandii Soil Biol Biochem,
20(2): 255-256.
Matheson DW & Brusick DJ (1977a) Evaluation of 4-chlorophenylurea
in vitromalignant transformation in BALB/3T3 cells. Kensington,
Maryland, Litton Bionetics Inc. (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brucisk DJ (1977b) Evaluation of 2,6-diflurobenzoic acid
in vitromalignant transformation in BALB/3T3 cells. Final report.
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/10/1978, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Matheson DW & Brusick DJ (1978a) Mutagenicity evaluation of
2,6-difluorobenzoic acid in unscheduled DNA synthesis in the human WI-
38 cells assay. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/16/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978b) Mutagenicity evaluation of
4-chlorophenylurea in unscheduled DNA synthesis in the human WI-38
cells assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/24/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978c) Mutagenicity evaluation of
4-chloroanilin in the in vitro transformation of BALB/3T3 cells
assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/23/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matta JF (1976) The effect of dimilin on non-target organisms in a
marsh community. Norfolk, Virginia, Environmental Consultants (Report
prepared for Thompson-Hayward Chemical Company) (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Mayer RT, Netter KJ, Leising HB, & Schachtschabel DD (1994) Inhibition
of the uptake of nucleosides in cultured Harding-Passey melanoma cells
by diflubenzuron. Toxicology, 30: 1-6.
Metcalf RL, Lu PY, & Browlus S (1975) Degradation and environmental
fate of 1-(2,6-difluorobenzoyl)-3-(4-chlorophenyl) urea. J Agric Food
Chem, 23(3): 359-364.
Mian LS & Mulla MS (1983) Persistence of three IGRs in stored wheat.
J Econ Entomol, 76(3): 622-625.
Miller RW, Corley C, Oehler DD, & Pickens LG (1976a) Feeding TH 6040
to cattle: residues in tissues and milk and breakdown in manure.
J Agric Food Chem, 24(3): 687-688.
Miller RW, Corley C, & Shufeld SR (1976b) Effects of feeding TH 6040
to two breeds of chickens. J Econ Entomol, 69(6): 741-743.
Miller RW, Cecil HC, Carey AM, Corley C, & Kiddy CA (1979) Effects of
feeding diflubenzuron to young male Holstein cattle. Bull Environ
Contam Toxicol, 23: 482-486.
Miura T (1974) Biological activity of TH 6040 against organisms
associated with mosquito breeding habitats in irrigated pastures.
Fresno, California, University of California, Mosquito Control
Research Laboratory (Unpublished report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Miura T & Takahashi RM (1974a) Insect developmental inhibitors effects
of candidate mosquito control agents on nontarget aquatic organisms.
Environ Entomol, 3(4): 631-636.
Miura T & Takahashi RM (1974b) Toxicity of TH 6040 to freshwater
crustacea and the use of a tolerance index as a method of expressing
side effects on nontargets. Proceedings of the Forty-Second Annual
Conference of the California Mosquito and Vector Control Association,
pp 177-180.
Moreale A & Van Bladel R (1976) Influence of soil properties on
adsorption of pesticide-derived aniline and p-chloroaniline. J Soil
Sci, 27: 48-57.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Mulla MS, Majori G, & Darwazeh HA (1975) Effects of the insect growth
regulator Dimilin (TH-6040) on mosquitoes and some nontarget
organisms. Mosq News, 35: 211-216.
Mutanen RM, Siltanen HT, Kuukka VP, Annila EA, & Varama MMO (1988)
Residues of diflubenzuron and two of its metabolites in a forest
ecosystem after control of the pine looper moth, Bupalus
piniarius. L Pestic Sci, 23: 131-140.
Nakayama K (1977a) Crop residue analysis of Dimilin (apple).
Japan, Institute of Pesticides (Unpublished proprietary report
No. K/Resid/003/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Nakayama K (1977b) Crop residue analysis of Dimilin. Japan, Institute
of Pesticides (Unpublished proprietary report No. K/Resid/006/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Nation JL, Robinson FA, Ju SJ, & Bolten AB (1986) Influence upon honey
bees of chronic exposure to very low levels of selected insecticides
in their diet. J Agric Res, 25(3): 170-177.
Nebeker AV, McKinney P, & Cairns MA (1983) Acute and chronic effects
of diflubenzuron (Dimilin) on freshwater fish and invertebrates.
Environ Toxicol Chem, 2: 329-336.
Nigg HN (1989) Metabolism of 14C-diflubenzuron in citrus fruits.
Field operations report. Lake Alfred, Florida, Citrus Research and
Education Center (Proprietary report No. 56635/13/1989, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Nigg HN, Canizzaro RD, & Stamper JH (1986) Diflubenzuron surface
residues in Florida citrus. Bull Environ Contam Toxicol,
36(6): 833-838.
Nimmo WB & de Wilde PC (1974) Fate of diflubenzuron on leaves of corn,
soybean, cabbage and apples. 'sGraveland, The Netherlands, Philips-
Duphar BV, Crop Protection Division (Unpublished proprietary report
No. 56635/16A/1974).
Nimmo WB & de Wilde PC (1975a) Degradation of diflubenzuron in soil
and natural water. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/37/1975).
Nimmo WB & de Wilde PC (1975b) Degradation of diflubenzuron in sterile
water at pH 5, 7, 9 and 12. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/32/1975).
Nimmo WB & de Wilde PC (1976a) Uptake of diflubenzuron treated soil by
soybean, maize and potato. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/04/I976).
Nimmo WB & de Wilde PC (1976b) Uptake of diflubenzuron and its
metabolites from the soil by rice and wheat. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/08/1976).
Nimmo WB & de Wilde PC (1977a) Degradation of diflubenzuron in
mushroom growth medium and uptake of its metabolites in the mushrooms.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56635/21A/1977).
Nimmo WB & de Wilde PC (1977b) Degradation of diflubenzuron in calf
and chicken manure. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/27/1977).
Nimmo WB, Willems AGM, & de Wilde PC (1978) Fate of diflubenzuron
applied to leaves and fruits of apple trees. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/33/1978).
Nimmo WB, Hamaker TL, More JC, & Wood RA (1980) Acute and chronic
effects of Dimilin on survival and reproduction of Misidopsis bahia
In: Eaton JG, Parrish PR, & Hendricks AC ed. Aquatic toxicology.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 366-376 (Special Technical Publication 707).
Nimmo WB, Wilde PC, & Verloop A (1984) The degradation of
diflubenzuron and its chief metabolites in soils. Part I: Hydrolytic
cleavage of diflubenzuron. Pestic Sci, 15: 574-585.
Nimmo WB, Willems AGM, Joustra KD, & Verloop A (1986) The degradation
of diflubenzuron and its chief metabolites in soils. Part II: Fate of
4-chlorophenylurea. Pestic Sci, 17: 403-411.
Nimmo WB, Joustra KD, & Willems AGM (1990) The degradation of
diflubenzuron and its chief metabolites in soils. Part III: Fate of
2,6-difluorobenzoic acid. Pestic Sci, 29: 39-45.
Offringa OR (1977) Addendum report to the chronic studies with
DU 112307: (A) Dietary administration to rats for 104 weeks - (B)
Dietary administration to mice for 80 weeks. Weesp, The Netherlands,
Philips-Duphar BV, Department of Toxicology (Report No. 56645/
16/1977).
Opdycke JC (1976) Metabolism and fate of diflubenzuron in chickens and
swine. University of Maryland, Faculty of the Graduate School
(Thesis).
Opdycke JC, Miller RW, & Menzer RE (1982a) Metabolism and fate of
diflubenzuron in swine. J Agric Food Chem, 30(6): 1223-1227.
Opdycke JC, Miller RW, & Menzer RE (1982b) In vivo and liver
microsomal metabolism of diflubenzuron by two breeds of chickens.
J Agric Food Chem, 30(6):1227-1233.
Palmer AK & Hill PA (1975a) Effect of DU 112307 on reproductive
function of multiple generations in the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/173/75954, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK & Hill PA (1975b) Effect of DU 112307 on pregnancy of the
rat. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/192/74978, submitted to WHO by Solvay-
Duphar, B.V., Weesp, The Netherlands).
Palmer AK & Hill PA (1975c) Effect of DU 112307 on pregnancy of the
New Zealand white rabbit. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/193/74937, submitted to
WHO by Solvay-Duphar BV, Weesp, The Netherlands).
Palmer AK, Allen PA, Street EA, & Prentice DG (1977) Preliminary
assessment of the effect of DU 112307 on the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/243/77208, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK, Allen PA, Heywood R, Street EA, Gibson WA, Offer JM, &
Jolly DW (1978) Effect of dietary administration of DU 112307 on
reproductive function of one generation in the rat. Huntingdon,
England, Huntingdon Research Centre (Report No. PDR 244/78653). 1978.
Unpublished proprietary report, submitted to WHO by Solvay-Duphar,
B.V., Weesp, The Netherlands.
Prasad I (1970) Mutagenic effects of the herbicide 3',4'-dichloro-
propionalide and its degradation products. Can J Microbiol,
16: 369-372.
Prinsen MK (1988a) Primary dermal irritation/corrosion study with
dimilin 4F in albino rabbits. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
93/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1988b) Primary eye irritation study with dimilin 4F in
albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/92/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Prinsen MK (1989a) Acute dermal irritation/corrosion study with
dimilin SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
150/1989, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Prinsen MK (1989b) Acute eye irritation/corrosion study with dimilin
SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/151/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1989c) Sensitization study with dimilin 4F in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/23/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1990) Acute dermal irritation/corrosion study with dimilin
ODC-45 in albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology
and Nutrition Institute (Unpublished proprietary report
No. 56645/99/1990, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1992) Sensitization study with diflubenzuron technical in
guinea-pigs. The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report No. 56645/26/1992, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1993) Sensitization study with Dimilin OF-6 in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56345/03/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Pritchard PH & Bourquin AW (1981) Fate of Dimilin in laboratory
estuarine ecosystems. Washington, DC, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished report).
Quarles JM, Norman JO, & Kubena LF (1980) Absence of transformation by
diflubenzuron in host mediated transplacental carcinogenassay. Bull
Environ Contam Toxicol, 25: 252-256.
Rabenort B, de Wilde PC, de Boer FG, Korver PK, Di Prima SJ, &
Cannizzaro RD (1978) Diflubenzuron. In: Analytical methods for
pesticides and plant growth regulators. New York, London, Academic
Press, vol 10, pp 57-72.
Richmond ML, Henny CJ, Floyd RL, Mannan RW, Finch DM, & de Weese LR
(1979) Effects of Sevin-4-oil, Dimilin and orthene on forest birds in
Northeastern Oregon. Berkeley, California, Pacific Southwest Forest
and Range Experiment Station (Research Paper PSW-148).
Rodrigues CS (1982) Effects of insecticides including insect growth
regulators on black fly (Diptera: Simuliidae) larvae and associated
nontarget stream invertebrates. Ontario, Canada, University of Guelph
(Thesis).
Rosenkranz HS & Poirier LA (1979) Evaluation of the mutagenicity and
DNA-modifying activity of carcinogens and noncarcinogens in microbial
systems. J Natl Cancer Inst, 62: 873-892.
Ross DB, Prentice DE, Newman AJ, Street AE, Hepworth PL, & Roberts NL
(1977a) DU 112307 13 weeks oral toxicity study in the sheep.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR229/77226, submitted to WHO by Solvay-Duphar
BV, Weesp, The Netherlands).
Ross DB, Street AE, & Roberts NL (1977b) Blood red cell and plasma
cholinesterase value following six weeks inclusion of DU 112307 in the
diet of sheep. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR229A/77225, submitted to WHO by
Solvay-Duphar BV, Weesp, The Netherlands).
Sarkar DK (1982) Persistence of dimilin 25 WP in water. Padappai,
Tamil Nadu, India, Coromandel Indag Research Centre (Proprietary
report No. 124, submitted to WHO by Solvay-Duphar BV, 'sGraveland, The
Netherlands).
Schaefer CH & Dupras EF (1976) Factors affecting the stability of
Dimilin in water and the persistence of Dimilin in field waters.
J Agric Food Chem, 24(4): 733-739.
Schaefer CH, Dupras EF, Stewart RJ, Davidson LW, & Colwell AE (1979)
The accumulation and elimination of diflubenzuron by fish. Bull
Environ Contam Toxicol, 21: 249-254.
Schaefer CH, Colwell AE, & Dupras EF (1980) The occurrence of
p-chloroaniline and p-chlorophenylurea from the degradation of
diflubenzuron in water and fish. In: Proceedings and papers of the
Forty-Eighth Annual Conference of the California Mosquito and Vector
Control Association, 20-23 January 1980, pp 84-89.
Schimmel SC, Garnas RL, Patrick JM, & Moore JC (1983) Acute toxicity,
bioconcentration and persistence of AC 222,705, Benthiocarb etc. in
the estuarine environment. J Agric Food Chem, 31(1): 104-113.
Schwartz SL & Borzelleca JF (1981) Effects of diflubenzuron on
methemoglobin, sulfhemoglobin and Heinz body production in cats.
Richmond, Virginia, Toxicology and Pharmacology, Inc. (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Seegmiller RE & Booth GM (1976) Teratogenic and biochemical effects of
TH-6040 on chicken embryos. Provo, Utah, Brigham Young University
(Unpublished report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Serra GE & Joustra KD (1990) Diflubenzuron: Nature of residues on
apples. Greenhouse operations report. Weesp, The Netherlands, Duphar
BV (Proprietary report No. 56630/43/1990).
Seuferer SL, Braymer HD, & Dunn JJ (1979) Metabolism of diflubenzuron
by soil microorganisms and mutagenicity of the metabolites. Pestic
Biochem Physiol, 10: 174-180.
Simmons KE, Minard RD, & Bollag JM (1989) Oxidative co-oligomerization
of guaiacol and 4-chloroaniline. Environ Sci Technol, 23(1): 115-121.
Singh PP & Kaira RL (1989) Determination of diflubenzuron residues by
thin-layer chromatography. Chromatographia, 27(1/2): 53-54.
Sipes JG & Carter DE (1988) Pharmacokinetics of xenobiotics:
p-chloroaniline. Tucson, Arizona, University of Tucson (Unpublished
report No. MRJDN 410882-01, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith JT & Edmunds CP (1985) Environmental monitoring of the 1984
gypsy moth control program in Tennessee, USA. Weesp, The Netherlands,
Duphar BV, 25 pp (Proprietary report No. 56637/27/1985).
Smith KS & Merricks DL (1976a) TH 6040 tissue residue and metabolism
study in dairy cows. Reading, Pennsylvania, Cannon Laboratories, Inc.
(Unpublished proprietary report No. 5E-7372, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Smith KS & Merricks DL (1976b) Tissue residue and metabolism study in
poultry. Reading, Pennsylvania, Cannon Laboratories, Inc. (Unpublished
proprietary report No. 5E-7372, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith S, Willis GH, & McDowell LL (1983) Electron-capture gas
chromatographic determination of diflubenzuron and permethrin in soil
and water. J Agric Food Chem, 31: 610-612.
Smucker RA & Simon SL (1986) Some effects of diflubenzuron on growth
and sporogenesis in Streptomyces spp. Appl Environ Microbiol,
51(1): 25-31.
Snoeij NJ & Buse-Pot TE (1990) Primary irritation study of dimilin WP
25% to the skin of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/09/1991).
Snoeij NJ & Buse-Pot TE (1991) Primary irritation study of dimilin WP
25% to the eye of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/13/1991).
Solvay Duphar (1994) Recommendations for field use (diflubenzuron) -
Guidelines. Weesp, The Netherlands, Solvay Duphar BV.
Spanjers MTh (1988a) Determination of the acute oral toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/85/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Spanjers MTh (1988b) Determination of the acute dermal toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/84/1988, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Steelman CD, Farlow JE, Breaud TP, & Schilling PE (1975) Effects of
growth regulators on Psorophora columbiae (Dyar and Knab) and non-
target aquatic insect species in rice fields. Mosq News, 35(1): 67-76.
Stevenson JH (1978) The acute toxicity of formulated pesticides to
worker honey bees. ( Apis mellifera L.. Plant Pathol, 27: 38-40.
Stoner A & Wilson WT (1982) Diflubenzuron (Dimilin): effect of long-
term feeding of low dose in sugar-cake or sucrose syrup on honey bees
in standard-size field colonies. Am Bee J, 122: 579-582.
Sundaram KMS (1986) Persistence and degradation of diflubenzuron in
conifer foliage, forest litter and soil, following simulated aerial
application. Sault Ste Marie, Ontario, Canada, Forest Pest Management
Institute (Information report FPM-X-74).
Sundaram KMS (1991) Spray deposit patterns and persistence of
diflubenzuron in some terrestrial components of a forest ecosystem
after application at three volume rates under field and laboratory
conditions. Pestic Sci, 32: 275-293.
Sundaram KMS & Nott R (1989) Mobility of diflubenzuron in two types of
forest soils. J Environ Sci Health, B24(1): 65-86.
Sundaram KMS, Holmes SB, Kreutzweiser DP, Sundaram A, & Kingsbury PD
(1991) Environmental persistence and impact of diflubenzuron in a
forest aquatic environment following aerial application. Arch Environ
Contam Toxicol, 20: 313-324.
Surprenant DC (1988) The chronic toxicity of 14C-diflubenzuron to
Daphnia magna under flow-through conditions. Wareham, Massachusetts,
Springborn Life Sciences, Inc. (Proprietary report No. 56635/22/1988,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Taalman RDFM & Hoorn AJW (1986) Mutagenicity evaluation of
diflubenzuron technical (Assay No. E-9481). Veenendaal, The
Netherlands, Hazleton Biotechnologies Corporation (Proprietary report
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Tasheva M & Hristeva V (1991) Biochemical and hematological changes in
rats following 28 days oral administration of diflubenzuron and
triflumuron. In: Abstracts from the 1991 Eurotox Congress, Maastricht,
The Netherlands, 1-4 September 1991, p 68.
Tasheva M & Hristeva V (1993) Comparative study on the effects of five
benzoylphenylurea insecticides on haematological parameters in rats.
J Appl Toxicol, 13(1): 67-68.
Taylor RE (1973a) Primary skin irritation study TH-6040 technical
(albino rabbit). Lincoln, Nebraska, Harris Laboratories (Proprietary
report submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Taylor RE (1973b) Primary skin irritation study TH-6040 W25 wettable
powder (albino rabbit). Lincoln, Nebraska, Harris Laboratories
(Proprietary report submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Tester PA & Costlow JD (1981) Effect of insect growth regulator
Dimilin (TH 6040) on fecundity and egg viability of the marine copepod
Acartia tonsa Mar Ecol Prog Ser, 5: 297-302.
Thompson SG & Swigert JP (1993a) Diflubenzuron: A 14-day toxicity test
with Duckweed ( Lemna gibba G3). Euston, Maryland, Wildlife
International Ltd (Proprietary report No. 56835/22/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993b) Diflubenzuron: A 5-day toxicity test
with freshwater alga ( Selenastrum capricornutum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/23/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993c) Diflubenzuron: A 5-day toxicity test
with the freshwater alga ( Anabaena flos-aquae. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/21/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993d) Diflubenzuron: A 5-day toxicity test
with the freshwater diatom ( Navicula pelliculosa. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/24/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993e) Diflubenzuron: A 5-day toxicity test
with the marine diatom ( Skeletonema costatum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/25/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson CZ, Hill LE, Epp JK, & Probst GS (1983) The induction of
bacterial mutation and hepatocyte unscheduled DNA synthesis by
monosubstituted anilines. Environ Mutagen, 5: 803-811.
Thus JLG & van der Laan-Straathof JMTh (1994) Fate of diflubenzuron in
model ditch systems. 'sGraveland, The Netherlands, Solvay Duphar
BV, Environmental Research Department (Proprietary report
No. 56835/55/93).
Thus LG & van der Laan JMT (1993) Nature of residues of diflubenzuron
in soybeans. 'sGraveland, The Netherlands, Solvay Duphar BV,
Environmental Research Department (Proprietary report
No. 56635/57/1992).
Thus JLG & van Dyk NRM (1991) Anaerobic aquatic metabolism of
diflubenzuron -Addendum report. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56635/54/91).
Thus JLG, van Dyk NRM, Rompa-van der Veldt CAH, & Walstra P (1991)
Anaerobic aquatic metabolism of diflubenzuron - Addendum report.
'sGraveland, The Netherlands, Solvay Duphar BV (Proprietary report
No. 56635/34/91).
USDA (1985) Gypsy moth suppression and eradication projects: Final
addendum to the final environmental impact statement as supplemented -
1985. Bromall, Pennsylvania, US Department of Agriculture, Forest
Service (Submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
US NCI (1979) Bioassay of p-chloroaniline for possible
carcinogenicity. Bethesda, Maryland, US National Cancer Institute
(Technical Report Series No. 189, NIH Publication No. 79-1745).
US NTP (1989) Toxicology and carcinogenesis studies of para-
chloroaniline hydrochloride in F344/N rats and B6C3F1 mice (gavage
studies). Research Triangle Park, North Carolina, US National
Toxicology Program (Technical Report Series No. 351).
Van Daalen JJ, Meltzer J, Mulder R, & Wellinga K (1972) A selective
insecticide with a novel mode of action. Naturwissenschaften,
59: 312-313.
Van den Berg G (1986) Dissipation of diflubenzuron residues after
application of dimilin WP-25 in a forestry area in North Carolina and
some ecological effects. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division, 11 pp (Proprietary report No. 56637/47/1986).
Van der Laan-Straathof JMTh & Thus JLG (1993) Determination of the
biodegradability of 14C-diflubenzuron in an adapted modified Sturm
test. Weesp, The Netherlands, Solvay-Duphar (Proprietary research
report No. 56835/41/93).
Van der Laan-Straathof JMTh & Thus JLG (1994) Evaporation of
diflubenzuron from bare soil and red kidney bean leaves after
application of dimilin WSE-80. Weesp, The Netherlands, Solvay-Duphar
(Proprietary research report No. 56835/17/94).
Van Eldik A (1973a) Acute toxicity studies with DU 112307 in mice and
rats. Weesp, The Netherlands, Philips-Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/14/1973).
Van Eldik A (1973b) Acute toxicity studies with DU 112307 25% WP in
mice and rats. Weesp, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Proprietary report No. 56645/15A/1973).
Van Eldik A (1974) Acute toxicity studies with DU 112307 in mice after
intraperitoneal administration. Weesp, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/01/1974).
Van Kampen WG & Joustra KD (1991) Diflubenzuron: residues on apples.
Weesp, The Netherlands, Duphar BV, Analytical Development Department
(Proprietary analytical report No. 56630/46/1990).
Van Rossum A, De Reijke A, & Zeeman J (1984) Diflubenzuron (update).
In: Analytical methods for pesticides and plants growth regulators.
New York, London, Academic Press, vol 13, pp 165-169.
Verhey ME (1991a) Analysis of field dissipation soil samples from
Madera, California for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 019, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verhey ME (1991b) Analysis of field dissipation soil samples from
Woodburn, Oregon, for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 020, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verloop A & Ferrell CD (1977) Benzoylphenyl ureas - a new group of
larvicides interfering with chitin deposition. In: Plimmer JR ed.
Pesticide chemistry in the 20th century. Washington, DC, American
Chemical Society, pp 237-270 (ACS Symposium Series No. 37).
Walstra P & Joustra KD (1990) Aerobic soil metabolism of diflubenzuron
in sandy loam. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division (Proprietary report No. 56635/65/1990).
Wan MTK & Wilson DM (1977) Impact of insect growth regulators on
selected non-target organisms co-existing with mosquito larvae.
Washington, DC, Environmental Protection Service, Pollution Abatement
Branch (Unpublished report No. EPS-5-PR-77-1/NTP81).
Wang CJK (1975) Effects of Dimilin upon microorganisms in leaf litter
and forest soil. In: Evaluation of Dimilin against the Gypsy moth
effects on non-target organisms 1975. Report expanded Gypsy moth
research and applications program, Hamden USA (Unpublished report
No. NTP-18, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Weis JS & Ma A (1987) Effects of the pesticide diflubenzuron on larval
horseshoe crabs, Limulus polyphemus Bull Environ Contam Toxicol,
39: 224-228.
Weis JS & Perlmutter J (1987) Burrowing behaviour by the fiddler crab
Uca pugilator: inhibition by insecticide diflubenzuron. Mar Ecol
Prog Ser, 38: 109-113.
Weis JS, Cohen R, & Kwiatkowski JK (1987) Effects of diflubenzuron on
limb regeneration and molting in the fiddler crab, Uca pugilator
Aquat Toxicol, 10(5-6): 279-290.
White WB (1975) Evaluation of Dimilin against the Gypsy moth and
effects on non-target organisms, 1975 (Compiled by the expanded Gypsy
moth research and applications program). Upper Darby, Pennsylvania, US
Department of Agriculture, Forest Service (Unpublished report).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, p 64 (WHO/PCS/94.2).
Wie SJ & Hammock BD (1982) Development of enzyme-linked immunosorbent
assays for residue analysis of diflubenzuron and Sir 8541. J Agric
Food Chem, 30: 949-957.
Wie SJ & Hammock BD (1984) Comparison of coating and immunizing
antigen structure on the sensitivity and specificity of
immunoassays for benzoylphenylurea insecticides. J Agric Food Chem,
32(6): 1294-1301.
Willems AGM, Joustra KD, & Thus JLG (1977) Stability of diflubenzuron
in aqueous solution during heat sterilization. 'sGraveland, The
Netherlands, Philips-Duphar BV, Crop Protection Division (Proprietary
report No. 56635/35/1977).
Willems AGM, Overmars H, Scherpenisse P, de Lange N, & Post LC (1980)
Diflubenzuron: intestinal absorption and metabolism in the rat.
Xenobiotica, 10(2): 103-112.
Williams GM, Laspia MF, & Dunkel VC (1982) Reliability of the
hepatocyte primary culture/DNA repair test in testing of coded
carcinogens and noncarcinogens. Mutat Res, 97: 359-370.
Wilson JEH & Costlow JD (1986) Comparative toxicity of two Dimilin
formulations to the grass shrimp Palaemonetes pugio Bull Environ
Contam Toxicol, 36: 858-865.
Wilson JEH & Costlow JD (1987) Acute toxicity of diflubenzuron (DFB)
to various life stages of the grass shrimp, Palaemonetes pugio Water
Air Soil Pollut, 33: 411-417.
Wilson JEH, Forward RB, & Costlow JD (1985) Effects of embryonic
exposure to sublethal concentrations of Dimilin on the photobehavior
of grass shrimp larvae. In: Marine pollution and physiology: Recent
advances. Colombia, South Carolina, University of South Carolina
Press, pp 377-396.
Wimmer MJ, Smith RR, & Jones JP (1991) Analysis of diflubenzuron by
gas chromatography/mass spectrometry using deuterated diflubenzuron as
internal standard. J Agric Food Chem, 39: 280-286.
Worobey BL & Webster GRB (1977) Gas-liquid chromatographic
determination of diflubenzuron (Dimilin) in water as its
trifluoroacetyl derivative. J Assoc Off Anal Chem, 60(1): 213-217.
Worthing CR & Walker SB (1987) The pesticide manual: A world
compendium. Croydon, British Crop Protection Council, pp 4720-4721.
Yasuno M & Satake K (1990) Effects of diflubenzuron and methoprene on
the emergence of insects and their density in an outdoor experimental
stream. Chemosphere, 21(10-11): 1321-1335.
Young MF, Trombetta LD, & Carson S (1986) Effects of diflubenzuron on
the mouse liver. J Appl Toxicol, 6(5): 343-348.
Yu SJ, Robinson FA, & Nation JL (1984) Detoxication capacity in the
honey bee, Apis mellifera L. Pestic Biochem Physiol, 22: 360-368.
Zwart A (1985) Acute inhalation toxicity study of dimilin 2F,
diflubenzuron in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. V85.347/250925, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le diflubenzuron appartient au groupe des insecticides dérivés de
la benzoylphénylurée. Son activité insecticide est due à une
interaction avec la synthèse et le dépôt de la chitine. Il forme des
cristaux blancs inodores dont le point de fusion est de 230-232°C.
Il est légèrement soluble dans l'eau (0,2 mg/litre à 20°C)
et pratiquement non volatil. Il est relativement stable en milieu
acide ou neutre mais s'hydrolyse en milieu alcalin.
Le diflubenzuron s'obtient par réaction du 2,6-difluoro-
benzamide sur l'isocyanate de 4-chlorophényle.
Le dosage des résidus de diflubenzuron présents dans l'eau, les
échantillons biologiques,et le sol peut s'effectuer par
chromatographie liquide à haute performance avec détection par UV ou
encore par chromatographie en phase gazeuse avec détection par capture
d'électrons, soit directement sur la molécule initiale, soit sur un
dérivé (libération de 4-chloroaniline et action de l'anhydride
trifluoracétique).
2. Sources d'exposition humaine et environnementale
Le diflubenzuron est un produit de synthèse utilisé en
agriculture, en foresterie et dans les programmes de santé publique
pour détruire les ravageurs et les vecteurs de maladies. Différentes
formulations existent à cet usage. On ne possède pas de
renseignements au sujet de cas d'exposition humaine au diflubenzuron
qui auraient pu se produire.
3. Transport, distribution et transformation dans l'environnement
En général, le diflubenzuron est appliqué directement sur les
végétaux et les eaux à traiter. Le feuillage ne constitue pas une
porte d'entrée dans la plante.
Le diflubenzuron est rapidement adsorbé sur les particules du
sol. Il reste fixé dans les 10 premiers cm du sol sur lequel il est
épandu. Il est peu probable qu'il subisse un lessivage. Dans divers
types de sol, il subit une décomposition aérobie ou anaérobie avec une
demi-vie de quelques jours. La vitesse de décomposition dépend
largement de la taille des particules de diflubenzuron. La principale
voie métabolique (plus de 90%) esst l'hydrolyse qui conduit à la
formation d'acide 2,6-difluorobenzoïque et de 4-chlorophénylurée; ces
deux composés sont dégradés à leur tour, respectivement en 4 et 6
semaines. On n'a pas décelé la présence de 4-chloroaniline libre
dans le sol.
Le diflubenzuron se décompose rapidement dans les eaux neutres ou
alcalines. On constate qu'une fois épandu sur l'eau, il se répartit
rapidement entre celle-ci et les sédiments.Le composé initial et la
4-chlorophénylurée peuvent persister plus de 30 jours dans les
sédiments.
Le diflubenzuron ne s'accumule pas chez les poissons.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation en agriculture, en foresterie ou pour la
démoustication n'entraîne qu'une exposition négligeable de la
population générale par l'intermédiaire de la nourriture ou de l'eau.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez l'animal de laboratoire, le diflubenzuron est absorbé au
niveau des voies digestives et, à un moindre degré, au niveau cutané.
Chez le rat, il existe un mécanisme d'absorption saturable. Dans ces
conditions, une forte proportion du diflubenzuron administré par voie
orale se retrouve dans les matières fécales. Le diflubenzuron se
répartit largement dans les tissus, mais il ne s'y accumule pas.
Le métabolisme du diflubenzuron a été étudié chez diverses
espèces animales. Chez les mammifères, la principale voie métabolique
comporte une hydroxylation. L'hydrolyse peut se produire au niveau de
l'une quelconque des trois liaisons carbone-azote. Chez le porc et le
poulet, l'hydrolyse s'effectue principalement au niveau du pont
uréido. Chez le rat et la vache, l'hydroxylation constitue la
principale voie métabolique. Chez le mouton, le porc et le poulet,
les principaux métabolites sont le 2,6-difluorobenzamide et la
4-chlorophénylurée; on trouve aussi, en moindre proportion, du
2,6-difluorobenzamide et de la 4-chloroaniline. Chez le rat et les
bovins, 80% des métabolites sont constitués de 2,6-difluoro-3-
hydroxydiflubenzuron, de 4-chloro-2-hydroxydifluorobenzuron et de
4-chloro-3-hydroxydiflubenzuron. Les études de métabolisme indiquent
qu'il ne se forme pratiquement pas de 4-chloroaniline chez le rat et
les bovins.
Chez les chats, les porcs et les bovins, l'élimination
s'effectue principalement par la voie fécale, à hauteur de 70 à 85%.
Chez les ovins, la voie urinaire et la voie fécale ont à peu près la
même importance de ce point de vue. Chez le rat et la souris,
l'excrétion urinaire décroît proportionnellement à l'augmentation de
la dose. Moins de 1% de la dose administrée par la voie orale se
retrouve dans l'air expiré. Le diflubenzuron n'est présent qu'à
l'état de résidus dans le lait.
Il n'existe pas d'étude sur la cinétique et le métabolisme du
diflubenzuron chez l'homme et notamment, sur son degré de
biotransformation en 4-chloroaniline.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Quel que soit le mode d'exposition, le diflubenzuron présente une
faible toxicité aiguë. En se basant sur le fait que sa DL50 aiguë
par voie orale est supérieure à 4640 mg/kg de poids corporel chez le
rat, l'OMS estime qu'il s'agit d'un produit qui ne présente
vraisemblablement pas de risque d'intoxication aiguë en utilisation
normale. Chez ce même animal, la DL50 aiguë par voie percutanée est
supérieure à 10 000 mg/kg de poids corporel et la CL50 dépasse 2,49
mg/litre par la voie respiratoire. Au cours d'une période de deux
semaines pendant laquelle diverses espèces animales avaient reçu du
diflubenzuron en une seule prise et selon divers modes
d'administration, on n'a constaté aucun signe d'intoxication.
Le diflubenzuron n'est pas irritant pour la peau (chez le lapin)
et ne provoque pas non plus de sensibilisation cutanée (chez le
cobaye). Il est légèrement irritant pour la muqueuse oculaire chez le
lapin.
Le diflubenzuron provoque une méthémoglobinémie et une
sulfhémoglobinémie. Une méthémoglobinémie liée à la dose a été mise
en évidence après exposition d'animaux de diverses espèces au
diflubenzuron par la voie orale, percutanée ou respiratoire. Cet
effet constitue le point d'aboutissement toxicologique le plus
sensible chez les animaux de laboratoire. En prenant comme critère la
méthémoglobinémie, la dose sans effet observable est de 2 mg/kg de
poids corporel par jour chez les rats et les chiens et de
2,4 mg/kg de poids corporel par jour chez les souris. Les études de
toxicité à long terme effectuées sur des souris et des rats
ont montré que les modifications imputables au traitement
correspondaient principalement à l'oxydation de l'hémoglobine et à une
altération des hépatocytes.
Des études de cancérogénicité effectuées sur des rats et des
souris à des doses allant jusqu'à 10 000 mg/kg de nourriture, n'ont
pas révélé de modification de l'incidence tumorale qui soit imputable
au traitement. En particulier, on n'a pas observé de néoplasmes au
niveau du mésenchyme splénique ou hépatique lors d'études de
cancérogénicité utilisant de la 4-chloroaniline.
Plusieurs études toxicologiques portant sur la fonction de
reproduction ont été menées sur des rats, des souris, des lapins et
trois espèces d'oiseaux; elles n'ont pas mis en évidence d'effets
pathogènes et le produit ne s'est pas révélé embryotoxique. Les
études de tératogénicité effectuées sur des rats et des lapins se sont
également révélées négatives.
Le diflubenzuron et ses principaux métabolites ont également été
soumis à diverses épreuves de mutagénicité in vivo e in vitro
Ni le diflubenzuron ni ses principaux métabolites n'ont donné de
résultat positif dans ces épreuves.
Le métabolite secondaire, c'est-à-dire la 4-chloroaniline,
a donné des résultats positifs dans plusieurs épreuves de mutagénicité
in vitro portant sur divers points d'aboutissement toxicologiques.
Il est cancérogène pour le rat et la souris. Les tumeurs imputables à
l'administration de 4-chloroaniline se sont révélées bénignes; quant
aux tumeurs malignes observées, il s'agissait de tumeurs du mésenchyme
splénique chez les rats mâles ainsi que d'hémangiomes et
d'hémangiosarcomes spléniques ou hépatiques chez les souris mâles.
7. Effets sur l'homme
On a signalé des cas de méthémoglobinémie chez des travailleurs
exposés de par leur profession et des nouveau-nés exposés par
inadvertance à de la 4-chloroaniline, un métabolite secondaire du
diflubenzuron. Les sujets qui présentent un déficit en NADH-
méthémoglobine-réductase peuvent être particulièrement sensibles à la
4-chloroaniline et par conséquent à une exposition au diflubenzuron.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Tous les organismes qui synthétisent la chitine sont sensibles au
diflubenzuron.
A la concentration de 500 mg/kg de terre, les bactéries n'ont pas
souffert d'une exposition au diflubenzuron. Il y a eu une certaine
stimulation de la fixation d'azote. Les bactéries décomposent les
solutions de diflubenzuron dans l'acétone, solvant qu'elles utilisent
comme source de carbone. Une concentration de diflubenzuron de 1
µg/litre a provoqué un accroissement de la biomasse algaire. Aucun
effet nocif n'a été observé à des concentration supérieures à la
limite de solubilité du diflubenzuron. Des champignons placés dans un
courant créé en laboratoire ont été temporairement affectés à la
concentration de 0,1 µg/litre.
Les invertébrés aquatiques présentent des réactions variées au
diflubenzuron. Les mollusques n'y sont pas sensibles, avec une CL50
supérieure à 200 mg/litre. Chez les autres invertébrés, la CL50 peut
aller de 1 à > 1000 µg/litre, ce qui peut refléter la sensibilité de
ces organismes au moment de la mue. On estime que pour la daphnie, la
concentration tolérable maximale en produit toxique est supérieure à
40 ng/litre et inférieure à 93 ng/litre. Comme prévu, les larves
d'éphémères et autres formes pré-imaginales d'insectes divers, sont
très sesnsibles au diflubenzuron. Le traitement des eaux de surface
par le diflubenzuron est donc probablement susceptible de causer une
certaine mortalité parmi les insectes aquatiques.
Lors de traitements expérimentaux effectués sur le terrain et
dans divers écosystèmes, on a constaté que la plupart des organismes
avaient moins souffert que ne le laissaient prévoir les études
toxicologiques en laboratoire. Aucun effet n'a été constaté sur les
organismes aquatiques après traitement de forêts par voie aérienne.
Pour les poissons, la CL50 est supérieure à 150 mg/litre. Les
essais effectués sur le terrain n'ont enregistré aucune mortalité chez
les poissons.
La DL50 par voie orale et par contact est supérieure à 30 µg par
insecte chez l'abeille mellifique. Après épandage de diflubenzuron
par voie aérienne à raison de 350 g/ha, on n'a pas constaté de dommage
parmi les colonies d'abeilles des alentours.
Une étude alimentaire de 5 jours sur des colverts et des
gallinacés du genre colin avec des doses allant jusqu'à 4640 mg/kg de
nourriture, n'a pas révélé de signes de toxicité. Après épandage de
diflubenzuron par voie aérienne sur des forêts à raison de 350 g/ha,
on n'a pas constaté de dommages parmi les oiseaux chanteurs de
l'écosystème forestier.
Après épandage de diflubenzuron à raison de 67 g/ha sur une
forêt, on n'a pas observé de réduction dans l'effectif des populations
de petits mammifères.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El diflubenzurón pertenece al grupo de los insecticidas
derivados de la benzoilfenilurea. Su acción insecticida se debe
a la interacción con la síntesis y/o deposición de quitina. Forma
cristales blancos inodoros con un punto de fusión de 230-232°C. Es
bastante soluble en agua (0,2 mg/litro a 20°C) y prácticamente
involátil. Es relativamente estable en medios ácidos y neutros, pero
se hidroliza en condiciones alcalinas.
El diflubenzurón se produce haciendo reaccionar 2,6-difluoro-
benzamida con 4-clorofenilisocianato.
Los residuos de diflubenzurón pueden medirse en el agua, en
muestras biológicas y en el suelo mediante cromatografía líquida de
alta resolución con detección de radiación ultravioleta o mediante
cromatografía de gases con detector de captura de electrones para el
análisis de la molécula intacta o tras la derivatización de la
4-cloroanilina liberada con anhídrido trifluoracético.
2. Fuentes de exposición humana y ambiental
El diflubenzurón es un compuesto sintético utilizado en la
agricultura, en la silvicultura y en programas de salud pública para
combatir plagas de insectos y vectores. Para esos usos existen
diferentes formulaciones de diflubenzurón. No se dispone de
información pertinente sobre la exposición humana a este producto.
3. Transporte, distribución y transformación en el medio ambiente
El diflubenzurón suele aplicarse directamente a las plantas y al
agua. No se produce absorción a través de las hojas.
La adsorción del diflubenzurón en el suelo es rápida. El
producto se inmoviliza en la capa superior de 10 cm del suelo al que
se aplica, y las probabilidades de lixiviación son escasas. El
diflubenzurón se degrada en suelos de diversos tipos y orígenes en
condiciones aerobias o anaerobias, con una semivida de pocos días. La
velocidad de degradación depende en gran medida del tamaño de las
partículas de diflubenzurón. La principal ruta metabólica (más del
90%) es la hidrólisis, que produce 2,6-ácido difluorobenzoico y
4-clorofenilurea; estos productos se degradan con semividas del orden
de cuatro y seis semanas, respectivamente. No se ha detectado
4-cloroanilina libre en los suelos.
El diflubenzurón se degrada rápidamente en aguas neutras o
alcalinas. Estudios de aplicación al agua revelan que el
diflubenzurón se concentra rápidamente en el sedimento; el compuesto
de origen y la 4-clorofenilurea pueden persistir en el sedimento por
más de 30 días.
El diflubenzurón no es objeto de bioacumulación en los peces.
4. Niveles ambientales y exposición humana
La exposición de la población general al diflubenzurón por medio
del agua o los alimentos de resultas de su utilización en la
agricultura, contra insectos forestales o en la lucha contra los
mosquitos, es insignificante.
5. Cinética y metabolismo en animales de laboratorio
En animales de experimentación, el diflubenzurón se absorbe en el
tubo digestivo y, en menor medida, a través de la piel. En el tubo
digestivo de la rata existe un mecanismo de absorción saturable, por
lo que una gran proporción del diflubenzurón administrado oralmente
aparece en las heces. El diflubenzurón tiene una amplia distribución
en los tejidos, pero no se acumula.
El destino metabólico del diflubenzurón ha sido estudiado en
diversas especies. La principal vía metabólica en los mamíferos es la
hidroxilación. La hidrólisis del diflubenzurón puede producirse en
cualquiera de los tres enlaces carbono-nitrógeno. En los cerdos y los
pollos, la principal ruta de hidrólisis es el puente ureido. En las
ratas y las vacas, la principal vía metabólica es la hidroxilación.
En las ovejas, los cerdos y los pollos, los metabolitos más
importantes son el 2,6-ácido difluorobenzoico y la 4-clorofenilurea;
los metabolitos secundarios son la 2,6-difluorobenzamida y la
4-cloroanilina. En las ratas y el ganado vacuno, el 80% de los
metabolitos está constituido por 2,6-difluoro-3-hidroxidiflubenzurón,
4-cloro-2-hidroxi-diflubenzurón y 4-cloro-3-hidroxidiflubenzurón. Los
estudios metabólicos indican que en las ratas o el ganado vacuno se
forman cantidades mínimas o nulas de 4-cloroanilina.
La principal ruta de eliminación es a través de las heces, con
porcentajes de entre el 70 y el 85% en los gatos, los cerdos y el
ganado vacuno. En el ganado ovino, la eliminación se distribuye
aproximadamente por igual entre la orina y las heces. En las ratas y
ratones, la excreción urinaria disminuye proporcionalmente al aumento
de la dosis. Menos del 1% de una dosis oral se recupera en el aire
exhalado. En la leche sólo se han hallado residuos ínfimos.
No se dispone de ningún estudio humano de la cinética y el
metabolismo del diflubenzurón, incluido el alcance de la
biotransformación en 4-cloroanilina.
6. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
El diflubenzurón tiene una toxicidad aguda baja por cualquier vía
de exposición. La OMS lo ha clasificado como producto con pocas
probabilidades de presentar un riesgo agudo en el uso normal, sobre la
base de una DL50 aguda por vía oral de más de 4640 mg/kg de peso
corporal en las ratas. La DL50 aguda por vía cutánea en las ratas es
superior a 10 000 mg/kg de peso corporal, mientras que la CL50 aguda
por inhalación en las ratas excede de 2,49 mg/litro. No se han
observado signos de intoxicación en los 14 días siguientes a una
administración única de diflubenzurón por diversas rutas a una
variedad de especies animales.
El diflubenzurón no provoca irritación cutánea (en el conejo) ni
sensibilización de la piel (en el cobayo). Produce una ligera
irritación a los ojos en el conejo.
El diflubenzurón causa metahemoglobinemia y sulfohemoglobinemia.
Tras la exposición oral, cutánea o por inhalación de diversas especies
al diflubenzurón se ha demostrado la presencia de metahemoglobinemia
dosisdependiente. Este efecto es la variable de evaluación
toxicológica más sensible en los animales de experimentación. El NOEL
basado en la formación de metahemoglobina es de 2 mg/kg de peso
corporal por día en las ratas y los perros, y de 2,4 mg/kg de peso
corporal por día en los ratones. En estudios de toxicidad a largo
plazo realizados con ratones y ratas, los cambios relacionados con el
tratamiento se han asociado principalmente a la oxidación de la
hemoglobina o a alteraciones de los hepatocitos.
En estudios de la carcinogenicidad en ratones y ratas con niveles
de hasta 10 000 mg/kg en la alimentación, no se observaron efectos
relacionados con el tratamiento en la incidencia de tumores.
Específicamente, no se registraron neoplasias mesenquimatosas del bazo
o el hígado como las observadas en los estudios de carcinogenicidad
con 4-cloroanilina.
En varios estudios de la toxicidad reproductiva en ratas,
ratones, conejos y tres especies aviarias no se observó ningún efecto
en la reproducción, ni tampoco embriotoxicidad. Los estudios de
teratogenicidad en ratas y conejos no revelaron ningún efecto
teratogénico.
El diflubenzurón y sus principales metabolitos han sido sometidos
a una serie de ensayos de mutagenicidad in vitro e in vivo Ni el
diflubenzurón ni sus principales metabolitos tienen efecto mutagénico.
El metabolito secundario 4-cloroanilina ha dado un resultado
positivo en varios ensayos de mutagenicidad in vitro con diversas
variables de valoración. Es carcinógeno en las ratas y los ratones.
Las lesiones neoplásicas relacionadas con la administración de
4-cloroanilina fueron tumores mesenquimatosos benignos y malignos en
el bazo de ratas macho, y hemangiomas y hemangiosarcomas,
principalmente en el bazo y el hígado de ratones macho.
7. Efectos en el ser humano
Se ha notificado que el metabolito 4-cloroanilina del
diflubenzurón ha causado metahemoglobinemia en trabajadores sometidos
a exposición y en neonatos expuestos por inadvertencia. Algunas
personas con carencia de NADH metahemoglobina reductasa pueden ser
particularmente sensibles a la 4-cloroanilina y, por lo tanto, a la
exposición al diflubenzurón.
8. Efectos sobre otros organismos en el laboratorio y en el terreno
Todos los organismos que sintetizan quitina presentan sensibilidad
al diflubenzurón.
Las bacterias no resultaron afectadas por el diflubenzurón a
concentraciones de 500 mg/kg de suelo; se observó cierta estimulación
de la fijación de nitrógeno. Las soluciones de diflubenzurón-acetona
se degradaron; la acetona se utilizó como fuente de carbono. La
biomasa de algas aumentó a una concentración de diflubenzurón de
1 µg/litro. No se observaron efectos adversos a concentraciones
superiores al límite de solubilidad del diflubenzurón. Los hongos
resultaron temporalmente afectados a 0,1 µg/litro en condiciones de
laboratorio.
Los invertebrados acuáticos presentan respuestas variables al
diflubenzurón. Los moluscos son insensibles, con una CL50 superior a
200 mg/litro. Los valores de la CL50 de otros invertebrados van
desde 1 hasta más de 1000 µg/litro, en función de los efectos del
compuesto en las fases juvenil y de muda. Para Daphnia se ha
estimado una MATC de > 40 y < 93 ng/litro; como era de prever, las
larvas de mosca de mayo y otros insectos acuáticos juveniles son
sumamente sensibles. El rociamiento de masas de agua matará
probablemente algunos invertebrados acuáticos.
En los ecosistemas y experimentos de campo en que se aplicó
diflubenzurón directamente al agua, los efectos en la mayoría de los
grupos taxonómicos fueron menos graves de lo previsto a partir de las
pruebas de laboratorio sobre efectos agudos. No se han observado
efectos en los organismos acuáticos después de aplicaciones aéreas a
los bosques.
Los valores de la CL50 para los peces son de > 150 mg/litro.
En los experimentos prácticos no se ha registrado nunca la muerte de
peces.
La DL50 oral y por contacto en la abeja de miel es superior a
30 µg/individuo. Las colonias de abejas no resultaron afectadas tras
la aplicación aérea de 350 g de diflubenzurón/hectárea.
Un estudio de alimentación de cinco días de duración en patos
silvestres y codornices con niveles de hasta 4640 mg/kg de pienso no
reveló ningún signo observable de toxicidad. Las pequeñas aves
canoras del ecosistema forestal no resultaron afectadas por la
aplicación aérea de diflubenzurón a razón de 350 g/hectárea.
Las especies mamíferas pequeñas no sufrieron mermas de las
poblaciones tras la aplicación de diflubenzurón en un bosque a razón
de 67 g/hectárea.
The major metabolites (approximately 50%) in sheep urine were
2,6-DFBA and 2,6-difluorohippuric acid (Ivie, 1978).
14C-Diflubenzuron uniformly radiolabelled in both rings was
administered to a pig as an oral dose of 5 mg/kg body weight. Of the
administered dose, 82% was eliminated in faeces as parent compound and
5% was recovered in urine. Identification of the metabolic products
in urine revealed 2,6-DFBA (0.28% of the dose), 4-CPU (0.82%), PCA
(1.03%) and 2,6-difluorobenzamide (0.83%). Cleavage of the urea
moiety between the benzoyl carbon and urea nitrogen was shown to be
the primary degradation pathway in pigs (Opdycke et al., 1982a).
In chickens only small quantities of the metabolites 2,6-DFBA,
4-CPU and PCA were found in excreta and tissues (Opdycke, 1976).
Neither induction nor inhibition of mixed-function oxidase activity
altered diflubenzuron metabolism in chickens (Opdycke et al., 1982b).
After 4 days daily doses of 7.8 g diflubenzuron/kg body weight,
De Bree et al. (1977) found PCA at a level of 30 ng/ml in rat plasma
and 323 ng/g in erythrocytes. PCA, estimated by the concentration in
the urine, represented at most 0.01% of the dose actually absorbed.
6.3.1 Metabolites - distribution, excretion, retention and turnover
When 14C-PCA was administered orally as single doses of 0.3,
3.0 or 30.0 mg/kg to male Fischer-344 rats, approximately 75% of the
administered radioactivity was excreted in the urine within 24 h,
while approximately 10% appeared in the faeces. Excretion was
virtually complete (92-97%) 7 days after dosing. The highest tissue
levels of radioactivity following a single intravenous dose of
3.0 mg/kg were found in the liver, fat, muscle and skin. Tissue
levels peaked within 5-60 min after dosing. By 3 days, concentrations
in all tissues except the blood had declined to < 0.3% of the dose
(Sipes & Carter, 1988). At this time, the only tissue containing more
than 1% of the dose was the cellular compartment of blood, which
contained 1-2% of the dose. The decline of PCA concentration in all
tissues, except for urine, faeces and intestinal contents, was
biexponential. The t alpha 1/2 for fat, muscle and skin was about
1.5 h, while the tß1/2 was approx. 43-59 h. The t alpha 1/2 for
liver was 3.5 h. Levels of unchanged PCA in all tissues peaked after
5 min following intravenous administration. The highest amount of
unchanged PCA was attained in muscle (15% of radioactivity in the
tissue) followed by skin (6%), fat (3%) and liver (2%). The decline of
PCA in all tissues, except for the liver, followed biexponential kinetics
with an estimated t alpha 1/2 of 8 min and a tß1/2 of 3 to 5 h.
PCA is rapidly metabolized to p-chloroacetanilide (PCAA) as the
initial step in the metabolism and excretion of PCA. The decline of PCAA
was monoexponential, the appearance half-life being approx. 6 min in the
testes and 15 min in the brain. The elimination half-life in the
brain, kidney, testes, muscle, skin and fat was around 1.0 to 2.0 h.
The elimination of PCA does not depend on either the dose or route of
administration. Approximately 4% of the urinary radioactivity in the
0-24 h urine sample was unchanged PCA; less than 1% was found in the
faeces. PCAA was not detected in either urine or faeces over a 3-day
period (Sipes & Carter, 1988).
After a single intravenous dose of 14C-PCA (3 mg/kg), maximal
tissue levels were reached within 15 min in most tissues. At this
time, most of the radioactivity was located in muscle (34%), fat
(14%), skin (12%), liver (8%) and blood (7%). Elimination half-lives
from tissues ranged between 1.5 and 4 h. By 8 h, approximately 90% of
the administered dose had been eliminated into urine and faeces. By
3 days, concentrations in all tissues, except blood, had declined to
< 0.3% of the dose (US NTP, 1989).
6.4 Elimination and excretion
After oral administration to rats of 5 mg diflubenzuron labelled
with 3H in the benzoyl and with 14C in the aniline moiety, 95% of
the 3H and 70-75% of the 14C radioactivity were retrieved in urine
and faeces. 2,6-DFBA was shown to constitute more than half of the
urinary metabolites (De Lange et al., 1977). Up to 1% of an oral dose
of 5 mg 14C-diflubenzuron labelled at the benzoyl moiety was
recovered in the expired air of rats (De Lange et al., 1974; Willems
et al., 1980).
When 14C-diflubenzuron, labelled in the aniline moiety, was
administered by gavage (4, 16, 48, 128, 900 and 1000 mg/kg body
weight) to rats, the urinary excretion was complete after 48-72 h.
Urinary excretion after single oral administration of diflubenzuron
relatively decreased with increasing dose level, being 27.6% of the
dose at 4 mg/kg and 1% at 1000 mg/kg (De Lange et al., 1977).
When 14C-diflubenzuron was administered at single oral doses of
12.5, 63.5, 202.5 and 925 mg/kg body weight to Swiss mice, the
excretion was almost completed within 48 h. The cumulative percentage
of the dose excreted in the urine decreased from 15% at the dose level
of 12.5 mg/kg to approximately 2% at 925 mg/kg (De Lange & Post,
1978).
Hawkins et al. (1980) studied the excretion of radioactivity in
urine and faeces after oral administration of 3H/14C-diflubenzuron
(7 mg/kg) to male cats. The radioactive dose was given on day 10 of a
15-day dosing regime of non-radioactive diflubenzuron (days 1-9 and
days 11-15). The excretion of radioactivity in urine accounted for
9.5 and 9.6% of the 14C and 3H doses, respectively, during 6 days
after dosing. The elimination of radioactivity in faeces accounted
for 77.3 and 71.6% of the 14C and 3H doses, respectively, during 6
days after dosing.
After an oral administration of 14C-diflubenzuron (5 mg/kg) to
female pigs, 82% of the dose was eliminated via faeces and 5% via
urine in 11 days (Opdycke, 1976).
About 85% of a single oral dose of 14C-diflubenzuron (10 mg/kg
body weight) administered to a cow was recovered in the faeces during
the first 4 days after treatment. About 15% was recovered in urine
and only about 0.2% was secreted in the milk (Ivie, 1977, 1978).
Sheep excreted 41% of the dose (10 mg/kg) in the urine and 43% in
the faeces during the 4 days after treatment. Bile-cannulated sheep
eliminated 24% of the dose in the urine, 32% in the faeces and 36% in
the bile. Sheep treated with 500 mg 14C-diflubenzuron/kg as a single
oral dose eliminated a much smaller proportion of the 14C in urine
and bile. This was probably due to reduced absorption from the
gastrointestinal tract when the sheep were given an exaggerated dose
(Ivie, 1977).
An oral dose of 5 mg 14C-diflubenzuron/kg administered to white
leghorn hens and Rhode Island red-barred Plymouth Rock buff cross hens
was rapidly excreted unaltered within the first 8 h. Up to 91 and
82%, respectively, were excreted within 13 days (Opdycke, 1976).
6.5 Retention and turnover
6.5.1 Biological half-life
From the studies of Willems et al. (1980) and Ivie (1978), the
half-life of diflubenzuron appears to be 12 h in rat and sheep and
18-20 h in the cow.
Diflubenzuron has been shown to pass intact through the
intestinal tract and remained active in the manure (Nimmo & de Wilde,
1977b).
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of diflubenzuron and its formulations to
different species is summarized in Table 7. No signs of intoxication
were observed during the 14-day period following a single
administration of diflubenzuron.
Van Eldik (1974) reported an intraperitoneal LD50 of
> 2150 mg/kg in rats and mice.
7.2 Short-term exposure
Rats (5 of each sex per group) were fed on a diet containing
diflubenzuron at concentrations of 0, 800, 4000, 20 000 and
100 000 mg/kg feed for 4 weeks. Behaviour, body weight, food and
water consumption were not affected by the treatment. There was a
dose-related increase in the met- and sulfhaemoglobin content of the
blood in all treated groups except for the methaemoglobin value for
females in the 800-mg/kg dose group. Lower erythrocyte, packed cell
volume (PCV) and haemoglobin values were observed in both sexes of the
100 000-mg/kg dose group. There was a dose-related increase in spleen
and liver weights. Only the dose level of 800 mg/kg did not affect
the liver weight (Palmer et al., 1977).
Five male Swiss-albino rats were given 96.7 mg diflubenzuron/kg
body weight per day, dissolved in corn oil, in their diet for 48 days.
The total dose was 4640 mg/kg body weight. Five controls were given
corn oil only in their diet. At the end of the study the treated
groups showed a significantly lower mean haemoglobin concentration
than the controls, and decreases in MCH and MCHC values (Berberian &
Enan, 1989).
Diflubenzuron was administered to Sprague-Dawley rats of the CD
strain (20 of each sex per group) at dietary levels of 10 000 and
100 000 mg/kg feed for 9 weeks, followed by a 4-week withdrawal
period. Lower values for red blood cell parameters were recorded at
both dose levels. An increase in reticulocyte count and a pallor of
the extremities and eyes were observed. The formation of
methaemoglobin occurred in both males and females, with approximately
5-8% of the available haemoglobin being transformed to methaemoglobin.
After a withdrawal period of 4 weeks methaemoglobin comprised less
than 2% of the available haemoglobin in tested animals compared with
approximately 0.6% in controls. Higher values for SGPT were recorded
as well as heavier liver, spleen and adrenal weights. Minor
enlargement of centrilobular hepatocytes in the highest dose group was
observed, but this finding disappeared after the withdrawal period
(Hunter et al., 1979).
Table 7. Acute toxicity of diflubenzuron and its formulations
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Diflubenzuron mouse: > 4640 rat: > 10 000 rabbit: > 30 mg/ rabbit: 40 mg rabbit: non- guinea-pig: non-
technical mg/kg body weight mg/kg body weight litre nominal instilled in eye; irritant (Taylor, sensitizing
(Koopman, 1977c) marginal irritant 1973a) (Prinsen, 1992)
(Davies &
rat: > 4640 mg/kg rabbit: > 4 ml/kg > 3.75 mg/litre Ligget, 1973)
body weight body weight of 50% actual (Berczy et
(van Eldik, 1973a; in gum tragacanth al., 1975a)
Koopman, 1977a) (Davies &
Halliday, 1974)
Diflubenzuron mouse: > 5000 rat: > 2000 mg/kg rat: > 13.8 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: 25%
90% concentrate mg/kg body weight body weight litre nominal instilled in eye; non-irritant w/w in paraffin
(Koopman & Pot, (Koopman, 1984a) very slight (OECD Guideline (Grade 1); weak
1986) > 2.49 mg/litre irritant (Koopman, 404) (Koopman, sensitizer (OECD
actual (Greenough 1984c) 1984d) Guideline 406)
rat: > 5000 mg/kg & McDonald, (Kynoch & Smith,
body weight 1986) 1986)
(Koopman, 1984b)
Dimilin WP 25% rat: > 40 000 rat: > 20 000 rat: > 150 mg/ rabbit: 100 mg rabbit: 500 mg guinea-pig: non-
mg/kg body weight mg/kg body weight litre nominal instilled in eye; non-irritant to sensitizing
(Janssen & Pot, slight to only minimally (Kynoch & Elliott,
mice: > 40 000 1987e) > 3.5 mg/litre moderate, irritant 1978,a,b)
mg/kg body weight actual (Arts, transientsient eye (Taylor, 1973b;
(van Eldik, 1973b; 1991) irritation (Snoeij Chandran, 1981;
Koopman, 1977b) & Busé-Pot, 1991) Snoeij &
Busé-Pot, 1990)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin SC-48 rat: > 5000 mg/kg rat: > 2000 mg/kg rabbit: 0.1 ml rabbit: non- guinea-pig: weak
body weight body weight - instilled in eye; irritant (Janssen sensitizer
(Janssen & Pot, (Janssen & Pot, slight irritant & Pot, 1987b) (Kynoch &
1987c) 1987d) (Janssen & Pot, Parcell, 1987)
1987a)
Dimilin SC-15 albino rats: 0.1 rabbit: minimally
- - - ml instilled in irritant -
eye; non-irritant (Prinsen, 1989a)
(Prinsen, 1989b)
Dimilin 4F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 1.9 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight actual (Jackson instilled in eye; minimally irritant sensitizing
(Spanjers, 1988a) (Spanjers, 1988b) et al., 1990) non-irritant (Prinsen, 1988a) (Prinsen, 1989c)
(Prinsen, 1988b)
Dimilin 2F rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 4.4 mg/litre rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig:
body weight body weight actual very instilled in eye; moderate irritant moderate (Grade
(Koopman, 1985d) (Koopman, 1985c) slightly irritant moderate irritant OECD Guideline III) (Kynoch &
(Zwart, 1985) OECD Guideline 404 (Koopman, Parcell, 1987)
405 (Koopman, 1985a)
1985b)
Dimilin ODC 45 rat: > 37 300 mg/kg rat: > 37 300 mg/kg rabbit: 0.1 ml rabbit: 0.5 ml
body weight body weight instilled in eye; moderate irritant
(Koopman & (Koopman, 1980b) marginally (Koopman,
Jongeling, 1979) irritant (Koopman, 1980c)
1980a)
Table 7. (Con't)
Acute oral LD50 Acute dermal LD50 Acute inhalation Primary eye Primary skin Dermal
LC50 irritation irritation sensitization
Dimilin OF 6 rat: > 5000 mg/kg rat: > 2000 mg/kg rat: > 95.7 mg/ rabbit: 0.1 ml rabbit: 0.5 ml guinea-pig: non-
body weight body weight litre nominal: instilled in eye; topical; mild sensitizing
(Besten et al., (Besten et al., > 2.17 mg/litre non-irritant irritant (Prinsen, 1993)
1993a) 1993b) actual (Janssen & (Janssen & van (Janssen & van
van Doorn, 1993a) Doorn, 1993c) Doorn, 1993b)
Wistar rats (10 of each sex per group) were fed diflubenzuron in
the diet for 13 weeks at concentrations of 0, 3.125, 12.5, 50 or
200 mg/kg feed. Behaviour, growth and food intake were unaffected by
the treatment. At the highest dose level the PCV value, the
haemoglobin concentration and the number of erythrocytes were
decreased. There was an increase in the SGPT and SGOT activities in
the males of the highest dose group at the end of the experiment. A
slight increase in the number of normally occurring scattered small
foci of necrotic parenchymal cells was observed, accompanied by
mononuclear inflammatory cell infiltration and proliferation of
reticuloendothelial system cells in the liver of both males end
females of the 50 and 200 mg/kg groups (Kemp et al., 1973a,b).
Absence of toxic effects in chronic/oncogenicity studies at low
dose levels necessitated re-evaluation of liver histopathology in the
90-day feeding study; "piece meal" liver cell necrosis was reported at
the two highest dose levels, i.e. 50 and 200 mg/kg feed. The original
slides of the study were re-evaluated by four histopathologists in
four different laboratories. They independently and unanimously
agreed that the lesions in the livers of treated rats were found to
the same extent in the livers of untreated rats. This showed that the
high dose levels in the study did not demonstrate a treatment-related
necrotic effect in the liver (Offringa, 1977).
Technical grade diflubenzuron was administered in the diet to
male and female 21- to 28-day-old Sprague-Dawley rats (40 of each sex
per group) at dose levels of 0, 160, 400, 2000, 10 000 and
50 000 mg/kg feed for 13 weeks. No apparent treatment-related effects
were noted on mortality, clinical observations, body weight gain, food
consumption, clinical chemistry or urinalysis. A treatment-related
significant increase in methaemoglobin concentration was noted in all
treated groups. Sulfhaemoglobin values showed increases at dose levels
of 2000 mg/kg or more. A significant treatment-related decrease in
haemoglobin, PCV and erythrocyte count was observed in males and
females at all dose levels by the end of the study. An increase was
noted in the reticulocyte count at all dose levels except 160 mg/kg,
and the number of the Heinz bodies was higher in the 10 000 and
50 000 mg/kg groups. After 7 weeks, spleen weights were increased in
the females at all dose levels, but after 13 weeks no effect was found
at 160 mg/kg. With the exception of the lowest dose level, all
treated groups showed a higher liver weight. The administration of
diflubenzuron resulted in a dose-related increase in the incidence of
chronic hepatitis and haemosiderosis of the liver. It was also
associated at all dose levels with haemosiderosis and congestion of
the spleen and mild erythroid hyperplasia of the bone marrow. The
severity of the lesions tended to increase with the dose. Liver
lesions were more severe in males than in females and were more severe
at 13 weeks than at 7 weeks. A no-observed-effect level (NOEL) was
not established (Burdock et al., 1980b; Goodman, 1980b).
Diflubenzuron was given to CFLP mice for 6 weeks at levels of 16
and 50 mg/kg feed. There were no clinical signs and no effects on
food consumption, body weight, blood chemistry or macroscopic
pathology. In three of the eight animals given 50 mg/kg, foci of
liver cell necrosis, with or without inflammatory cell filtration,
were noted. Other organs were not examined microscopically (Hunter et
al., 1974).
Diflubenzuron was administered to Swiss Webster mice in a 30-day
oral intubation study. Groups of five mice each received either no
treatment, vehicle control (Polyethylene glycol 400) or diflubenzuron
suspensions at dose levels of 125, 500 or 2000 mg/kg body weight.
Hepatic glutathione- S-transferase activity and morphological
characteristics were studied. Diflubenzuron was shown to elicit
hepatocellular changes at all dose levels. The activities of three
glutathione- S-transferases ( S-aryl, S-aralkyl and S-epoxide)
were irregularly altered in a non-dose-related manner. Light
microscopy revealed radial arrays of hepatocellular vacuolization
between the portal and central vein areas. There was evidence of an
increase in the amount of endoplasmic reticulum (Young et al., 1986).
Male and female mice of the B6C3F1 strain (40 of each sex per
group) received diflubenzuron (97.2% a.i.) in the diet at dose levels
of 0, 16, 50, 400, 2000, 10 000 or 50 000 mg/kg feed for 13 weeks. An
additional group of 100 of each sex served as a control. No
compound-related effects were apparent with respect to clinical signs,
survival, growth rates, total food consumption or gross pathology.
Significant treatment-related increases in met- and sulfhaemoglobin
concentrations were noted in all treated groups, except in the group
fed 16 mg/kg. At the higher dose levels, there was a decrease in
haematocrit and erythrocyte counts and an increase in reticulocyte,
platelet and Heinz body counts. Significantly higher alkaline
phosphatase activity was noted in the 10 000 and 50 000 mg/kg groups.
Compound-related effects on the weights of liver and spleen were
noted. In the females, adrenal weight was decreased (but not in a
dose-related fashion) at all dose levels after 7 weeks and increased
after 13 weeks in higher dose levels. Higher adrenal weight was
observed in treated males than in the controls. Histopathological
examination revealed treatment-related centrilobular hypertrophy of
hepatocytes, with or without cell necrosis, haemosiderosis of the
liver and spleen, extramedullary haematopoiesis and mild chronic
hepatitis in treated animals of both sexes, some of which effects were
observed at the lowest dose level (16 mg/kg). The liver lesions were
more severe in males than in females, being most severe in the high-
dose males. The NOEL for methaemoglobin formation was 16 mg/kg feed
(Burdock et al., 1980a; Goodman, 1980a).
HC/CFLP mice (40 of each sex per group) were fed diflubenzuron
(97.2% purity) for 14 weeks at levels of 0, 80, 400, 2000, 10 000 and
50 000 mg/kg feed. On the second day of treatment, the majority of
mice treated with 10 000 or 50 000 mg/kg showed dark eyes and/or
prominent caudal blood vessels. On day 5, blue/grey discoloration of
the extremities was noted for the majority of mice treated with
50 000 mg/kg. Mice in the lowest dose group exhibited no clinical
signs. Mortality, food consumption, water consumption and body weight
changes were not significantly affected by the treatment. Lower PCV
and red blood cell counts were found at all dose levels except
80 mg/kg. The total white blood cell count, lymphocyte count,
haemoglobin concentration, incidence of Heinz bodies and red blood
cell count were increased in all treated groups. At week 7, there was
an increase in the number of reticulocytes in treated mice,
particularly in males treated at 10 000 or 50 000 mg/kg. At week 14,
the reticulocyte counts were similar to those of the controls. A
treatment-related increase in both met- and sulfhaemoglobin was
recorded in all treated groups at weeks 7 and 14 of the investigation.
Plasma glutamic-pyruvic transaminase values were increased at all dose
levels, with the exception of 80 mg/kg feed. Lower blood cholesterol
levels were noted in the 2000, 10 000 and 50 000 mg/kg groups.
Macroscopic examination showed dark discoloration and/or enlargement
of the spleen and pale subcapsular areas of the liver in all dose
groups after both 7 and 14 weeks. Histopathological examination of
the spleen revealed increased haemosiderosis at all dose levels except
80 mg/kg. In the liver, areas of focal necrosis and/or fibrosis in
the parenchyma, with or without associated inflammatory cells,
fibroblasts or pigment-laden macrophages, were observed. At higher
dose levels necrotic and fatty hepatocytes and brown pigment-laden
Kupffer cells were found. A NOEL was not established (Colley et al.,
1981a,b).
Diflubenzuron was fed to groups of three male and three female
beagle dogs for 13 weeks at concentrations of 0, 10, 20, 40 and
160 mg/kg diet. No effect of the treatment on behaviour, body weight
or food and water consumption was observed. Elevated SAP and SGPT
values were recorded for some dogs receiving 40 or 160 mg
diflubenzuron/kg feed. After 6 weeks, methaemoglobin and another
abnormal pigment, probably sulfhaemoglobin, were demonstrated in dogs
given 160 mg/kg. After 12 weeks of administration, some recovery was
observed. Organ weights and gross and microscopic evaluation did not
reveal any treatment-related effects. The NOEL for methaemoglobin
formation was 40 mg/kg feed (Chesterman et al., 1974).
Diflubenzuron was administered daily in gelatin capsules to male
and female beagle dogs (6 of each sex per group) at dose levels of 2,
10, 50 or 250 mg/kg body weight per day for 52 weeks. There were no
treatment-related effects on mortality, food consumption or body
weight gain. Dose-related marginal increases in methaemoglobin and
sulfhaemoglobin were recorded from 10 mg/kg upwards. At 50 and
250 mg/kg, haemoglobin concentration and MCHC were decreased whereas
reticulocyte and platelet counts were increased. Heinz bodies were
also detected in several animals receiving 50 and 250 mg/kg. Dose-
related increases in liver and spleen weights were found in the 50 and
250 mg/kg males. Histopathological evaluation of the liver revealed
an increase in the incidence of pigmented macrophages and Kupffer cell
siderosis at 50 and 250 mg/kg in both males and females. The NOEL
based on the increase in met- and sulfhaemoglobin was 2 mg/kg body
weight (Greenough et al., 1985).
In a study by Berczy et al. (1975c), rats were exposed daily for
a one-hour period to technical diflubenzuron dust at nominal
concentrations of 0, 0.5, 5.0 and 50 mg/litre air, respectively (the
actual concentrations were 0, 0.12, 0.87 and 1.85 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days per week. The methaemoglobin levels in male rats at the two
lower concentrations and female rats of all test groups were
significantly higher than those of controls.
Rabbits were exposed daily for one-hour periods to technical
diflubenzuron dust at concentrations of 0.5, 5.0 and 25 mg/litre air
(the measured concentrations were 0.15, 0.75 and 1.99 mg/litre,
respectively). Exposures were repeated over a period of 3 weeks,
5 days each week. There were no signs of irritation in animals
exposed to 0.5 mg/litre, but mild transient respiratory irritation was
seen at the two higher concentrations. Haematological examination,
biochemistry tests and macroscopic inspection revealed no treatment-
related abnormalities (Berczy et al., 1975b).
Diflubenzuron, at levels of 4.64, 10 and 21.5% weight/volume was
applied daily to the intact and abraded skin of rabbits at a dosage
level of 1.5 ml/kg body weight, 5 days a week, for 3 consecutive weeks
(equivalent to 69.6, 150 and 322.5 mg diflubenzuron/kg body weight per
day). The sulfhaemoglobin level was increased in one rabbit out of 20
in the 10% group, and in 5 rabbits out of 20 at the high treatment
level. The 10% treatment level was considered to be the NOEL based on
sulfhaemoglobin formation (Davies et al., 1975).
7.3 Long-term exposure
Diflubenzuron was administered to Sprague-Dawley rats (60 of each
sex per group) in their diet at levels 0, 10, 20, 40 and 160 mg/kg
feed for 104 weeks. There were no treatment-related effects on body
weight gain, food intake, renal function or on macroscopic and
microscopic pathology. In rats treated with 160 mg/kg, significantly
higher methaemoglobin levels were recorded. The tumour profile of
treated rats was similar to that of the controls. The NOEL based on
methaemoglobin was 40 mg/kg, equivalent to a mean intake of 1.43 and
1.73 mg/kg per day for males and females, respectively (Hunter et
al.,1976).
When diflubenzuron was administered in the diet to male and
female Sprague-Dawley rats (50 of each sex per group) at dosage levels
of 0, 156, 625, 2500 and 10 000 mg/kg feed for 104 weeks, there was no
evidence of an effect on mortality or treatment-related clinical
signs. Significantly increased absolute methaemoglobin and
sulfhaemoglobin values were observed in all male treatment groups.
However, increases in relative methaemoglobin values (% of total
haemoglobin) were only noted in the 156, 2500 and 10 000 mg/kg groups,
while sulfhaemoglobin increases were noted in the 156, 625 and
10 000 mg/kg females. There was a significant increase in absolute
and relative spleen weights in the two highest dose groups of both
sexes, together with haemosiderosis in spleen and liver. There was no
evidence of carcinogenicity after 2 years of feeding diflubenzuron
(Burdock et al., 1984).
In a study by Hunter et al. (1975), diflubenzuron was
administered to CFLP mice for 80 weeks at dietary levels of 0, 4, 8,
16 and 50 mg/kg feed. There were no overt signs of reaction to
treatment. Behaviour, mortality, food and water consumption and body
weight were unaffected by the treatment. The macroscopic changes
observed were those commonly seen in mice of this strain and age. No
histopathological changes were seen that were considered to be related
to the administration of diflubenzuron. The incidence of liver cell
tumours was higher in this study than it was in other studies
performed in these laboratories using this strain of mouse. The
increase was seen in both treated and control groups of either sex and
showed no evidence of a treatment-related effect. There was no
evidence of a treatment-related effect on tumour incidence in the CFLP
mouse.
Male and female HC/CFLP mice (88 of each sex per group) were fed
diflubenzuron at dietary levels of 0, 16, 80, 400, 2000 and
10 000 mg/kg feed for 91 weeks. There was no indication of a
treatment-related effect on survival, food consumption or body weight
gain. Treatment-related elevations of MCH and MCHC values were
recorded from week 26 onwards in mice given 10 000 mg/kg. The
incidence of Heinz bodies increased in a dose-related manner among
mice given 400, 2000 or 10 000 mg/kg from week 52 onwards. Dose-
related increases in methaemoglobin levels were found from week 26
onwards and in sulfhaemoglobin levels from week 52 onwards in the mice
fed 80 mg/kg or more. Elevated alkaline phosphatase (AP) activities
were seen at weeks 24, 76 and 89 among male mice receiving 2000 and
10 000 mg/kg and at week 84 among mice of the 400 mg/kg dose group.
An increased incidence of splenic and/or hepatic enlargement was seen
among mice treated with 10 000 mg/kg. Cyanosis of the skin was noted
among mice at 400, 2000 and 10 000 mg/kg. Increased liver and spleen
weights were found among mice given 2000 and 10 000 mg/kg. An
increased incidence of hepatocyte enlargement and increased
extramedullary haematopoiesis in the liver and spleen were seen in
mice at high dose levels. In the 400, 2000 and 10 000 mg/kg groups,
there was an increased incidence of siderocytosis in the spleen and of
pigmented Kupffer cells in the liver. There was no treatment-related
effect on tumour incidence (Colley et al., 1984).
7.4 Skin and eye irritation; sensitization
Relevant data are given in Table 7.
Administration of technical diflubenzuron to the intact and
abraded skin of albino rabbits did not produce irritation of the skin
after exposure times of 24 and 72 h (Taylor, 1973a,b).
Diflubenzuron was found to be a moderate irritant to the rabbit
skin after application of 0.5 ml of 45% oil dispersible concentrate
for 24 h (Koopman, 1980a; Prinsen, 1990).
Diflubenzuron (both technical and 45% oil dispersible
concentrate) was considered to be marginally irritant to the rabbit
eye (Davies & Ligget, 1973; Koopman, 1980b).
Diflubenzuron (48% water-based paste) was not found to be a
dermal sensitizer in guinea-pigs (Kynoch & Parcell, 1987).
Technical diflubenzuron was studied for skin sensitization in a
maximization test on guinea-pigs and was found to be non-sensitizing
(Prinsen, 1992). However, some formulations are mild sensitizers
(Table 7).
7.5 Reproductive toxicity, embryotoxicity and teratogenicity
Diflubenzuron was fed to pathogen-free rats of the CFY strain
(20 of each sex per group) at dietary levels of 0, 1000 and
100 000 mg/kg for one generation and one litter. The animals were
maintained on their respective diets for 9 weeks prior to mating.
There were no clear effects on mating performance, pregnancy rate,
duration of gestation, litter size, offspring mortality, litter weight
or the type and distribution of abnormalities. Dose-related effects
of diflubenzuron were demonstrated at 17 weeks in adults and consisted
of reduced values for PCV, haemoglobin, total red cells count and
MCHC, increased values for methaemoglobin, MCV, spleen weight and
siderocyte incidence in the spleen, and the occurrence of iron-
pigment-containing Kupffer cells in the liver. A dose-related effect
on the liver was also shown by increased weight and SGPT activity and
centrilobular hepatocyte enlargement. Reduced blood glucose
concentrations were recorded in both treated groups. At the highest
dosed level, the offspring showed increased liver and spleen weights
for both sexes (Palmer et al., 1978).
In a three-generation reproductive study with rats fed
diflubenzuron at concentrations of 10, 20, 40 and 160 mg/kg feed, no
adverse treatment-related effects on mating performance, pregnancy
rate, duration of gestation or litter parameters (total loss, size,
mean pup weight, mortality, abnormalities) were found (Palmer & Hill,
1975a).
After gavage administration of diflubenzuron at doses of 1, 2 and
4 mg/kg body weight per day to pregnant rats during days 6-15 of
gestation, no effects were observed on embryonic or fetal development
(Palmer & Hill, 1975b).
Treatment of pregnant New Zealand white rabbits at oral doses of
1, 2 and 4 mg/kg body weight per day during days 6-18 of gestation did
not affect embryonic or fetal development as assessed by the incidence
of major malformations, minor anomalies and skeletal variants (Palmer
& Hill, 1975c).
In a study by Booth (1977), pregnant Swiss mice were fed a diet
containing 50 mg/kg of diflubenzuron (partly 14C) for a period of
17 days. Some of these mice were killed at day 17 after conception,
while the others were allowed to give birth. The results of this
study showed that mucopolysaccharide synthesis in embryonic mouse-limb
cartilage was normal. Diflubenzuron did not pass through embryonic
membranes nor was it passed from mother to suckling young mice.
Analysis of 226 embryos showed no gross teratogenic effects.
Two groups of 24 timed-mated female rats of the Crl:CD (SD) BR
strain were dosed once daily by the oral route between days 6 and 15
of pregnancy. One group was dosed with diflubenzuron at 1000 mg/kg
per day, while the other group was given the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 20 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths or treatment-related changes in clinical condition. Treatment
did not affect maternal growth or food consumption. There were no
maternal abnormalities at necropsy that were considered to be related
to treatment. The mean numbers of corpora lutea, implantations and
live fetuses were similar in all groups. Both pre- and post-
implantation losses were unaffected by treatment. Fetal weights and
sex ratio were unaffected by diflubenzuron treatment. The incidences
of major external/visceral and skeletal abnormalities, and minor
external/visceral abnormalities were not affected by diflubenzuron
treatment. The incidence of fetuses with minor skeletal abnormalities
was slightly higher in the treated group, but was within the usual
background range. There were intergroup differences in the
proportions of fetuses with specific minor abnormalities and variants
of skeletal ossification. For some bones, the differences achieved
statistical significance. However, on balance, treated group fetuses
were considered to be similarly ossified to the control fetuses. Oral
administration of diflubenzuron at a dose level of 1000 mg/kg per day
did not elicit maternal toxicity or any evidence of embryotoxicity
(Kavanagh, 1988a).
Two groups of 16 timed-mated female New Zealand White rabbits
were dosed once daily by the oral route from days 7 to 19 of
pregnancy, inclusive. One group was dosed with diflubenzuron at
1000 mg/kg per day and the other group with the vehicle (1.0% gum
tragacanth) only. Clinical signs, body weights and food consumption
were recorded. The females were killed on day 28 of pregnancy and a
necropsy was performed. The fetuses were subjected to detailed
external, visceral and skeletal examinations. There were no maternal
deaths, changes in clinical condition or abnormalities at maternal
necropsy considered to be related to diflubenzuron treatment. Two
animals were killed prematurely during the study, one from the control
group and one from the treated group. The changes in clinical
condition and abnormalities observed at necropsy in these animals were
considered to be unrelated to treatment. Treatment did not affect
maternal growth or food consumption. The mean numbers of corpora
lutea, implantations and live fetuses were similar in all groups. Both
pre- and post-implantation losses were unaffected by treatment. Fetal
weights and sex ratio were unaffected by diflubenzuron treatment. The
incidence of both major and minor external/visceral and skeletal
abnormalities, as well as the numbers of skeletal variants, was
unaffected by diflubenzuron treatment. Oral administration of
diflubenzuron at a dose level of 1000 mg/kg per day did not elicit
maternal toxicity or any evidence of embryotoxicity (Kavanagh, 1988b).
In a study by Kubena (1982), diflubenzuron was fed at levels of
0, 2.5, 25 and 250 mg/kg feed to male and female layer-breed chickens
from 1 day of age through a laying cycle. Characteristics measured
were egg production, egg weight, eggshell weight, fertility,
hatchability and effects on the progeny. Feeding diflubenzuron at
levels up to 250 mg/kg feed did not affect these characteristics.
Groups of chicken eggs were injected near the embryonic coelom
with a suspension of 10 mg diflubenzuron in 0.1 ml of peanut oil.
Diflubenzuron did not cause significant malformations in the embryos
(Seegmiller & Booth, 1976).
7.6 Mutagenicity and related end-points
Diflubenzuron was examined by in vitro and in vivo
mutagenicity tests. The results are summarized in Table 8.
Mutagenicity tests have also been carried out with the major
metabolites of diflubenzuron (Table 9).
Table 8. Mutagenicity tests with diflubenzuron
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Microorganisms
Reverse Salmonella typhimurium
mutation TA98, TA100, 10-1000 µg/plate +/- negative Bryant (1976)
TA98, TA100, TA1537, TA1978 1000 µg/spot +/- negative Bryant (1976)
Reverse S. typhimurium 0.1-500 µg/plate +/- negative Brusick & Weir
mutation TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Reverse S. typhimurium 10, 100 or 1000 µg/plate; +/- negative McGregor et al.
mutation TA98, TA100, 19, 186, 1860 µg DFB/plate (1979)
TA1535, TA1537 (as Dimilin W-25)
Reverse S. typhimurium up to 5000 µg/plate +/-b negative Moriya et al.
mutation TA98, TA100, TA1535, (1983)
TA1537, TA1538
Escherichia coli. WP2 hcr
Reverse S. typhimurium up to 1000 µg/plate +/- negative Koorn (1990)
mutation TA98, TA100, TA1535,
TA1537, TA1538
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammalian cells in vitro
Forward L5178Y mouse lymphoma 1.17-300 µg/ml +/- negative McGregor et al.
mutation cells (1979)
Chromosomal Chinese hamster ovary up to 200 µg/ml +/- negative Taalman & Hoorn
aberrations cells (1986)
Unscheduled human diploid WI-38 cells 50-1000 µg/ml +/- negative Brusick & Weir
DNA synthesis (blocked in the G1 phase) (1977c)
DNA repair rat hepatocytes up to 333 µg/ml negative Enninga (1990)
(unscheduled
DNA synthesis)
Cell transformation BALB-3T3 cells, 0.02-0.312 µg/ml - negative Brusick & Weir
in vitro (1977b)
Cell transformation pregnant hamster, ip 10, 200 and 500 mg/kg negative Quarles et al.
(transplacental injection on 10th day of body weight (1980)
transformation) gestation, fetal cell culture
3 days after injection
Table 8. (Con't)
End-point Organisms, Dose level, Metabolic activationa Results References
cells, strains concentration (presence/absence = +/-)
Mammals
Micronucleus mouse bone marrow 15, 150, 1500 mg/kg body negative McGregor et al.
weight 30 and 6 h before (1979)
necropsy
Dominant male mice mated to 1000 and 2000 mg/kg body negative Arnold et al.
lethal 3 females weekly for 6 weeks weight intraperitoneal (1974)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b Source of S9 not indicated
Table 9. Mutagenicity tests with diflubenzuron metabolites
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
A. 4-chlorophenylurea
Microorganisms
Reverse mutation Salmonella typhimurium
and differential TA98, TA100, TA1535, 1000 µg/spot +/- negativeb Dorough (1977)
killing TA1537, TA1538, TA1978
Reverse mutation TA98, TA100 10, 100, 500, negative
1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977a)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 6.25-50 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Cell BALB-3T3 cells, 0.019-0.312 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977a)
0.312 mg/ml
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
B. 2,6-Difluorobenzoic acid
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negative Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Reverse mutation S. typhimurium 10, 100, 500,
TA98, TA100 1000 µg/plate
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977b)
TA1537, TA1538
Mammalian cells
Unscheduled human WI-38 cells 75-500 µg/ml +/- positive Matheson & Brusick
DNA synthesis (blocked in the G1 phase) with (1978a)
activation
Cell BALB-3T3 cells, 0.156-2.5 mg/ml not stated weak Matheson & Brusick
transformation in vitro positive at (1977b)
2.5 mg/ml
C. 4-Chloroaniline (PCA)
Microorganisms
Reverse mutation S. typhimurium 1000 µg/spot +/- negativeb Dorough (1977)
and differential TA98, TA100, TA1535,
killing TA1537, TA1538, TA1978
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Reverse mutation TA98, TA100 10, 100, 500, positive in
1000 µg/plate TA98 at 500
and 1000 µg
with
activation
Reverse mutation S. typhimurium 0.1-500 µg/plate +/- negative Jagannath & Brusick
TA98, TA100, TA1535, (1977c)
TA1537, TA1538
Reverse mutation S. typhimurium
TA98, TA100, TA1530, 0-1500 µg/plate +/- negative Gilbert et al. (1980)
TA1535, TA1537, TA1538
Reverse S. typhimurium C3076, 1000 µg/plate +/- negative Thompson et al.
mutation D3052, G46, TA98, TA100, (1983)
TA1535, TA1537, TA1538,
Escherichia coli WP2,
WP2 uvrA
Reverse S. typhimurium 3333 µg/plate +/- positivec Dunkel et al. (1985)
mutation TA98, TA100, TA1535,
TA1537, TA1538,
Escherichia coli
Reverse S. typhimurium 1666 µg/plate +/- positived Mortelmans et al.
mutation TA97, TA98, TA100, TA1535 negative (1986)
DNA damage Escherichia coli polA+/polA- 250 µg/plate - positive Rosenkranz & Poirier
(1979)
Table 9. (Con't)
End-point Test system Dose level Metabolic activationa Results References
(presence/absence = +/-)
Mutation Aspergillus nidulans 200 µg/ml - positive Prasad (1970)
Mammalian cells
Forward mutation L5178Y mouse lymphoma +/- positived Caspary et al. (1988)
tk+/- cells
Unscheduled human WI-38 cells 250-1000 µg/ml +/- negative Matheson & Brusick
DNA synthesis (blocked in the G1 phase) (1978b)
Unscheduled rat primary hepatocytes 5-50 µg/ml - positive Williams et al.
DNA synthesis (1982)
Unscheduled rat primary hepatocytes 50 nmol/ml - negative Thompson et al.
DNA synthesis (1983)
Sister chromatid Chinese hamster ovary 1600 µg/ml +/- positived US NTP (1989)
exchange cells
Chromosomal Chinese hamster ovary cells 1000 µg/ml +/- positived US NTP (1989)
aberrations and negative
Cell BALB-3T3 cells, 0.039-0.625 mg/ml not stated negative Matheson & Brusick
transformation in vitro (1978c)
a Unless indicated otherwise, S9 was obtained from livers of rats treated with Arochlor-1254
b No increase in revertants, strains TA1538/TA1978 positive for differential killing
c Tested in three independent laboratories
d Tested in two independent laboratories
According to the findings presented, neither diflubenzuron nor
its major metabolites may be considered to have mutagenic effect.
Several positive effects were, however, obtained with PCA.
7.7 Carcinogenicity
Long-term carcinogenicity studies were described in section 7.3.
In both mouse and rat oncogenicity studies, diflubenzuron at dose
levels up to 10 000 mg/kg feed caused no change in tumour profile or
onset of tumours. In the rat oncogenicity study, the incidence of
sarcoma in the spleen and phaeochromocytomas was not increased. In
the mouse oncogenicity study, the incidence of hepatocellular
neoplasms or haemangiosarcomas in spleen and liver was not increased.
Therefore, diflubenzuron, in combination with its metabolites as
generated in the animal metabolic system, is not oncogenic.
Significantly, there were no non-neoplastic or neoplastic lesions of
the vasculature, including that of liver and spleen, in B6C3F1 male
mice treated with diflubenzuron. Similarly, there were no fibrotic or
carcinomatous lesions in the spleen of treated male F-344/N rats.
7.8 Other special studies
Diflubenzuron has been studied in mice for its growth-inhibiting
activity in serially transplanted B16 malignant melanoma and CA1025
skin carcinoma. A single 800 mg/kg intraperitoneal injection of
diflubenzuron induced rapid (24 h) decreases in tumour volume in 78%
and 66% of the tumours, respectively, while in control mice 85% of the
melanomas and 91% of the skin carcinomas increased in volume over the
same time period (Jenkins et al., 1984). These observations were
later confirmed, and it was suggested that the activity was due to
derivatives of a hydroxylated metabolite (Jenkins et al., 1986).
Studies of nucleoside uptake by Harding Passey melanoma cells
in vitro indicated a rapid (< 5 min) inhibition of the uptake of
uridine, adenosine and cytidine, but not of thymidine, by
diflubenzuron; this could not be reversed by washing. De novo
nucleic acid synthesis was not impaired and in vitro cell growth was
unaffected (Mayer et al., 1984).
El-Sebae et al. (1988) tested the effect of diflubenzuron on
protein and RNA biosynthesis by rabbit liver and muscle tissues kept
in an incubation medium. The synthesis of protein and RNA was
significantly stimulated in the liver and inhibited in the muscle by
graded doses. The maximum effect on both tissues was reached at 5 µg
diflubenzuron/ml for protein synthesis and at 0.2 µg/ml for RNA
synthesis, the effect on protein synthesis being more pronounced than
that on RNA synthesis in both tissues.
7.8.1 Special studies on met- and sulfhaemoglobin formation
The ability of diflubenzuron to induce methaemoglobin and
sulfhaemoglobin formation has been recognized since the initial
toxicity studies on the compound. Methaemoglobinaemia has been
demonstrated after oral, dermal and inhalatory exposure to
diflubenzuron in various species (see section 7.2 and 7.3). It is the
most sensitive parameter in this case.
Fifteen male Wistar rats received diflubenzuron (technical) by
gastric intubation at a dose level of 5000 mg/kg body weight per day
for 8 days. No effect was observed on Heinz body formation, whereas
met- and sulfhaemoglobin levels were significantly increased when
compared with the control group. The increase in the methaemoglobin
level was about 6% (Keet, 1977a).
When diflubenzuron was administered to male Wistar rats for 28
days at oral doses of 100 and 500 mg/kg body weight, it induced
elevation of methaemoglobin concentration and reticulocytes count in
both of the treated groups. However, there was no dose-response
relationship at the dose levels investigated (Tasheva & Hristeva,
1991, 1993).
Technical diflubenzuron was administered by gastric intubation to
male Swiss mice daily for a period of 14 days at dose levels of 0, 8,
40, 200, 1000 and 5000 mg/kg body weight. Body weight measurement and
macroscopic evaluation did not reveal any effect of the treatment. At
dose levels of 1000 and 5000 mg/kg the percentages of methaemoglobin
and erythrocytes containing Heinz bodies were increased. The
sulfhaemoglobin level was statistically significantly increased at
200, 1000 and 5000 mg/kg in comparison to the control group. The NOEL
was considered to be 40 mg/kg body weight based on sulfhaemoglobin
(Keet, 1977b).
When female mice were fed 0, 50, 200, 400, 1000 and 2000 mg
diflubenzuron/kg feed for 30 days, sulfhaemoglobin was demonstrated in
the blood from 200 mg/kg onwards (being 13% of total haemoglobin at
2000 mg/kg). Mice fed 1000 and 2000 mg/kg showed signs of cyanosis
after 3 weeks. Recovery was completed after a 3-week withdrawal
period (Bentley et al., 1979).
When 15 male New Zealand White rabbits were fed technical or
analytically pure diflubenzuron (640 mg/kg feed) for 21 or 18 days,
respectively, the methaemoglobin and sulfhaemoglobin levels were
significantly increased (Keet, 1977c).
Male and female cats (24 of each sex per group) received
diflubenzuron orally for 21 days at dose levels of 0, 30, 70, 100, 300
and 1000 mg/kg body weight and were observed for the subsequent 14-day
period. A dose-related elevation of methaemoglobin level was observed
with ceiling values at 300 and 1000 mg/kg. For male cats the NOEL was
30 mg/kg, but no NOEL was achieved for females. Increased
sulfhaemoglobin and Heinz bodies were observed in all treated groups.
NOEL values for sulfhaemoglobin were not achieved. The haemoglobin
concentration, reticulocyte number and organ weights were not affected
by the treatment (Schwartz & Borzelleca, 1981).
After dermal application of technical diflubenzuron at a dose
level of 1.5 ml/kg body weight for 18 days to rabbits, the
methaemoglobin level was increased (Keet, 1977c).
The available data demonstrate that dose-response relationship
for production of methaemoglobin exists. This is considered to be the
most sensitive end-point after repeated exposure in experimental
animals.
Table 10 summarizes the effects on methaemoglobinaemia as
determined in various studies.
7.9 Toxicity of metabolites
In rat metabolic studies it was shown that about 20% of absorbed
diflubenzuron is metabolized to 2,6-DFBA and its counterpart 4-CPU.
Only a small fraction of the 4-CPU is metabolized to PCA (see
section 6).
The acute oral toxicity of the major metabolites of diflubenzuron
is summarized in Table 11.
Loss of activity, catatony, paralysis and severe bradypnoea were
observed in rats treated with the metabolite 4-CPU. The minimum
symptomatic dose level was 100 mg/kg body weight. At autopsy, the
animals showed congested blood vessels and haemorrhage in the
gastrointestinal tract (Koelman-Klaus, 1978a).
Rats dosed with 2,6-DFBA showed slight increase in startle
response, activity, abdominal and limb tone, slight decrease in
grooming activity, slightly abnormal gait and body posture, mild
restlessness, irritation and aggressivity, pilo-erection and increased
alertness. The minimum symptomatic dose level was 464 mg/kg body
weight (Koelman-Klaus, 1978b).
Mutagenicity tests have been carried out with 2,6-DFBA, 4-CPU and
PCA (see section 7.6 and Table 9).
Table 10. Summary of the effects on methaemoglobinaemia in various species
Species Route Duration No-observed-effect level Reference
Rat diet 4 weeks male: not achieved; female: 800 mg/kg feed Palmer et al. (1977)
(equivalent to 45 mg/kg per day)
Rat diet 13 weeks not established - lowest dose tested 160 mg/kg feed Burdock et al. (1980b)
Rat diet 104 weeks 40 mg/kg feed (equivalent to 2 mg/kg Hunter et al. (1976)
per day body weight)
Mouse oral 2 weeks 200 mg/kg body weight Keet (1977b)
Mouse diet 13 weeks male/female: 16 mg/kg feed (equivalent to Burdock et al. (1980a)
2.4 mg/kg body weight per day)
Mouse diet 14 weeks not established - lowest dose tested 80 mg/kg feed Colley et al. (1981a,b)
Mouse diet 91 weeks male/female: 16 mg/kg feed (equivalent to Colley et al. (1984)
2.4 mg/kg body weight per day)
Cat oral 3 weeks male: 30 mg/kg body weight; female: not achieved Schwartz & Borzelleca (1981)
Dog diet 13 weeks male/female: 40 mg/kg feed Chesterman et al. (1974)
Dog oral (capsules) 52 weeks male/female: 2 mg/kg body weight Greenough et al. (1985)
Table 11. Acute toxicity of diflubenzuron metabolites
Metabolite Species Sex Route LD50 Reference
(mg/kg)
4-Chlorophenylurea rat male oral 1080 Koelman-Klaus
female oral 1210 (1978a)
2,6-Difluorobenzoic rat male, oral 4640 Koelman-Klaus
acid female (1978b)
7.9.1 Carcinogenicity studies with 4-chloroaniline
The diflubenzuron metabolite, 4-chloroaniline (PCA), has been
assayed for carcinogenicity by the US NCI (1979) and by the US NTP
(1989) using Fischer-344 rats and B6C3F1 mice on both occasions. In
the earlier of these studies, technical-grade PCA was administered at
dietary concentrations of 250 and 500 mg/kg to rats and 2500 and
5000 mg/kg to mice. Groups of 50 male and 50 female animals of each
species were randomized to the treatment groups at approximately six
weeks of age. The control groups consisted of 20 animals of each sex
and species. All animals which survived were treated for 78 weeks and
observed, untreated, for a further 24 weeks (rats) or 13 weeks (mice).
Survival in all groups was good and it was judged that there were
adequate numbers at risk for late-developing tumours. In rats, the
most significant findings were treatment-related proliferative splenic
capsular and parenchymal lesions in males and females and, in male
rats of the high-dose group, the occurrence of several types of
unusual splenic neoplasms (i.e. fibroma, fibrosarcoma, sarcoma NOS,
haemangiosarcoma and osteosarcoma) which appeared to arise from areas
of capsular or parenchymal fibrosis. These neoplasms were combined
for analysis because it was considered that the fibromas were a benign
form of sarcoma and that the neoplasms had a common cellular origin.
The combined incidences were 0/20 control, 0/49 low-dose and 10/49
high-dose rats. This result indicated a carcinogenic effect of
treatment in male rats. There was no similar effect in the spleen of
females. In mice of each sex, there was increased incidence of
haemangiomas and haemangiosarcomas in various organs. The combined
incidences were 2/20 in control males, 10/50 in low-dose males and
14/50 in high-dose males, and 0/18 in control females, 3/49 in low-
dose females and 8/42 in high-dose females. In addition, there was,
in female mice only, a non-significant increase in hepatocellular
carcinomas and adenomas combined (0/18 control, 1/49 low-dose and 6/41
high-dose). The smaller number of control group animals in these
experiments significantly reduced the statistical power. It was
recognized that PCA is unstable in feed and so the animals in the
early experiments may have received lower doses than intended.
Consequently, PCA was administered by gavage with an aqueous vehicle
containing hydrochloric acid in the later experiments (US NTP, 1989).
Groups of 49 or 50 rats and mice of each sex (7 to 8 weeks old)
were administered PCA at dose levels of 2, 6 or 18 mg/kg (rats) or 3,
10, or 30 mg/kg (mice) on 5 days per week for 103 weeks. Vehicle
control groups of 50 males and 50 females received deionized water by
gavage. Survival was adequate for analysis, although variable. It was
lower, for example, in the vehicle control groups of rats, which was
attributable to the higher incidence of mononuclear cell leukaemia in
these groups. Significant, non-neoplastic findings in rats included
treatment-related increases in the incidence of splenic fibrosis
(males: 3/49 control, 11/50 low-dose, 12/50 mid-dose, 41/50 high-dose;
females: 1/50 control, 2/50 low-dose, 3/50 mid-dose, 42/50 high-dose),
lipocytic infiltration of the spleen (males: 0/49, 0/50, 0/50, 24/50;
females: 0/50, 0/50, 0/50, 11/50) and adrenal medullary hyperplasia
in female rats (4/50, 4/50, 7/50, 24/50). In addition, the
methaemoglobin level was consistently increased in the mid- and high-
dose groups of male rats at 6, 12, 18 and 24 months. There was no
NOEL for this parameter in male rats. Bone-marrow hyperplasia was
also observed. The combined incidences of uncommon splenic sarcomas
(fibrosarcomas, osteosarcomas or haemangiosarcomas) were increased in
male rats but not in females (males: 0/49, 1/50, 3/50, 38/50; females:
0/50, 0/50, 1/50, 1/50). There was also a small increase in
phaeochromocytomas or malignant phaeochromocytomas in male rats
(13/49, 14/48, 15/48, 26/49). The incidences of mononuclear cell
leukaemia were reduced in male and female rats of the treatment groups
(males: 21/49, 3/50, 2/50, 3/50; females: 10/50, 2/50, 1/50, 1/50).
This reduction may be related to splenic toxicity, since splenectomy
of Fischer rats at one to two months of age markedly reduces the
incidence of mononuclear cell leukaemia later in life (Boorman et al.,
1990). However, similar reductions in the incidence of this neoplasm
are not seen with several other aniline-like chemicals that also cause
splenic toxicity. In male mice, but not in females, the incidence of
haemangiosarcomas of the liver and spleen was slightly greater in the
high-dose group than in the controls (males: 4/50, 4/49, 1/50, 10/50)
and the incidences of hepatocellular carcinomas or adenomas (combined)
were also increased in treated male mice (males: 11/50, 21/49, 20/50,
21/50). The incidences of malignant lymphomas were slightly reduced
in treated male and female mice (males: 10/50, 3/49, 9/50, 3/50;
females: 19/50, 12/50, 5/50, 10/50).
There are various points of similarity between the two studies
described above: (a) in male and female rats there was splenic
toxicity; (b) in male rats, there was a treatment-related increase in
uncommon splenic sarcomas; and (c) in male mice, there was a
treatment-related increase in haemangiosarcomas and haemangiomas
combined. The reductions in the incidence of mononuclear cell
leukaemia in rats and malignant lymphomas in mice seen in the latter
study were not observable in the earlier study because their incidence
in the control groups of that study was already low. It is concluded
that PCA is carcinogenic in both mice and rats.
8. EFFECTS ON HUMANS
No data concerning the effects of diflubenzuron on human health
are available.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Laboratory experiments
9.1.1 Microorganisms
Diflubenzuron was tested for its effects on morphogenesis in
Streptomyces spp. Exposure to 400 mg diflubenzuron/litre resulted in
reduced dominance of spore hairs and reduced width of the outer wall,
and prevented formation of the inner spore wall in S. babergiensis.
In S. coelicolor, 1600 mg diflubenzuron/litre altered the structure
of the fibrillar pattern of spore envelopes. Exposure to diflubenzuron
resulted in small increases in exported protein and in an approximately
20% increase in chitinase in both Streptomyces spp (Smucker &
Simon, 1986).
9.1.1.1 Water
Aquatic bacterial biomass and density were not affected by
diflubenzuron (1.0 µg/litre), although after three months of
continuous exposure some decline in species diversity was found.
Owing to detrimental effects on aquatic arthropods, e.g., filter
feeders that may use bacteria as a food source, such effects may very
well be secondary in nature and not a direct effect of diflubenzuron
(Hansen & Garton, 1982b).
In a test on the alga Selenastrum capricornutum, a nominal
concentration of 0.20 mg diflubenzuron/litre did not reduce the
biomass and can be considered a no-observed-effect concentration
(NOEC) (Berends & Thus, 1992b).
9.1.1.2 Soil
Soil microorganisms were able to use diflubenzuron as carbon
source when added as an acetone solution (Seuferer et al., 1979; see
section 4.3.2.2).
The effect of diflubenzuron (100, 200, 300, 400 and 500 mg/kg
soil) was studied in non-sterile soil incubated under aerobic
conditions and in sterilized soil inoculated with Azotobacter
vinelandii. The presence of diflubenzuron had a stimulatory effect
on nitrogen fixation in both non-sterile and sterile soil (Martinez-
Toledo et al., 1988a). At similar concentrations, diflubenzuron did
not affect the growth of Azotobacter vinelandii in culture media,
either with or without a nitrogen source (Martinez-Toledo et al.,
1988b).
9.1.2 Aquatic organisms
9.1.2.1 Microorganisms
Cyanobacteria (the blue-green alga Plectonema boryanum) grew
rapidly in the presence of diflubenzuron (initial concentration
0.1 mg/litre) with no visible signs of inhibited growth (Booth &
Ferrell, 1977). Concentrations of 1, 10, 50 and 100 mg/litre did not
affect the growth of six species of fungi: Rizopus arrhizus,
Aspergillus niger, Fusarium oxysporum, Mycorrhizae-Rhizopogan
vinicolor, Pythum debaranum and Trichoderma viride (Booth et al.,
1987). However, these authors autoclaved the medium containing
diflubenzuron, which led to extensive breakdown of the pesticide
(Willems et al., 1977).
Five-day lethality tests on the algae Selenastrum capricornutum
and Anabaena flos-aquae resulted in NOAEC values above the exposure
concentrations of 300 and 330 µg/litre, respectively (Thompson &
Swigert, 1993b,c). Similarly, tests on the diatoms Navicula
pelliculosa and Skeletonema costatum led to NOAEC values of 380
and 270 µg/litre, respectively (Thompson & Swigert, 1993d,e).
Algae were affected at 1.0 µg/litre by technical diflubenzuron in
dimethylformamide added to laboratory stream channels: the alga
biomass increased and chlorophyll and phaeocitin levels were elevated.
Fungi were only affected temporarily by 0.1 µg/litre under the same
circumstances. Changes in species diversity had disappeared after
2 months of continuous dosing (Hansen & Garton, 1982 b).
9.1.2.2 Plants
In a 14-day toxicity test on diflubenzuron in duckweed
(Lemna gibba), the NOAEC was higher than the tested concentration
(190 µg/litre) (Thompson & Swigert, 1993a).
9.1.2.3 Invertebrates
The acute toxicity of diflubenzuron to a number of non-target
aquatic invertebrates is presented in Table 12. Comprehensive eviews
on the subject have been published recently, e.g., by Fischer & Hall
(1992) and by Cunningham (1986) on the effects of diflubenzuron on
estuarine crustaceans. The acute LC50 for insects ranges from
1 µg/litre (Diptera) to 250 µg/litre (Coleoptera), and mayflies
have an LC90 of 1 to 10 µg/litre. Other aquatic arthropods such as
water fleas, scuds and sow bugs have LC50 values of 5 to 15 µg/litre.
Table 12. Acute toxicity of diflubenzuron for non-target aquatic invertebrates
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Daphnia magna 1st instar stat 22 40 7.2 48-h EC50 15 Julin & Sanders (1978)
(Water flea)
Daphnia magna 24 h stat 20 50 48-h LC50 4.55 Hansen & Garton (1982a)
(Water flea) stat 20 100 48-h LC50 6.89 Hansen & Garton (1982a)
stat 20 200 48-h LC50 4.42 Hansen & Garton (1982a)
Daphnia magna 0-24 h 20 294 8.1 48-h EC50 7.1 Kuijpers (1988)
(Water flea) 20 294 8.1 24-h EC50 68 Kuijpers (1988)
Gammarus pulex mature stat 12 40 7.2 96-h LC50 30 Julin & Sanders (1978)
(Scud)
Hyallela azteca 2-4 mm flow 20 25 96-h LC50 1.84 Hansen & Garton (1982a)
(Amphipod)
Chironomus plumosus 4th instar stat 22 40 7.2 48-h EC50 560 Julin & Sanders (1978)
(Midge)
Cricotopus sp. 4th instar flow 20 25 EC50 (moulting 1.72 Hansen & Garton (1982a)
(Midge) success)
Tanytarsus dissimilis 2nd instar flow 20 25 EC50 (moulting 1.02 Hansen & Garton (1982a)
(Midge) success)
Acartia tonsa adult constant 20 10b 5-day LC50 > 1000 Tester & Costlow
(Copepod) daily (1981)
replenishment
Table 12 (Con't)
Species Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (µg/litre)
Mysidopsis bahia adult intermittent 24-25 24-27b 96-h LC50 2.06 Nimmo et al. (1980)
(Mysid shrimp) flow
adult continuous 24-26 23-29b 21-day LC50 1.24 Nimmo et al. (1980)
flow
Palaemonetes pugio larvae stat 22 20b 96-h LC50 1.44 Wilson & Costlow
(Grass Shrimp) (1987)
post larval renewal 22 20 96-h LC50 1.62 Wilson & Costlow (1987)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Salinity (expressed as parts per thousand)
Concentrations of diflubenzuron causing significant mortality of
several freshwater invertebrates are presented in Table 13.
Table 13. Concentrations of diflubenzuron causing significant
mortality of freshwater invertebratesa
Organisms Concentration
(µg/litre)
Water flea (Daphnia magna) 2.0
Amphipod (Hyalella azteca) 2.0
Snail (Juga plicifera) > 36
Snail (Physa spp.) > 36
Caddis fly (Clisforonia magnifica) 0.1
Midge (Tanytarsus dissimilis) 4.9
Midge (Cricotopus spp.) 1.6
a From: Nebeker et al. (1983)
The 96-h LC50 for the snails Juga plicifera and Physa sp.
was > 45 µg/litre (Hansen & Garton, 1982a).
The 48-h EC50 values of diflubenzuron metabolites for midge
larvae were > 100 mg/litre for 4-CPU and 2,6-DFBA and 43 mg/litre for
PCA (Julin & Sanders, 1978).
Diflubenzuron suspended in water at a concentration of
200 mg/litre was not directly toxic to the freshwater clam Anodonta
cygnea during 3 months of treatment. Diflubenzuron produced
disturbances in the calcification process in the lamellar layer of the
shell. Positive PAS reaction of the secretory cells on the outer
mantle epithelium has been observed (Machado et al., 1990).
Daphnia magna was continuously exposed to 14C-diflubenzuron at
concentrations of 5.6, 14, 23, 40 and 93 ng/litre. After 21 days of
exposure daphnid survival at the highest concentration (93 ng/litre)
was 50%. In the remaining concentrations it ranged from 93 to 98%,
which was comparable to the survival (99%) of the control organisms.
Reproduction and body length were affected only at the highest
concentration. The maximum acceptable toxicant concentration (MATC)
of 14C-diflubenzuron for Daphnia magna was > 40 and < 93 ng/litre
(Surprenant, 1988).
Technical diflubenzuron in dimethylformamide at 0.1, 1, 10 and
50 µg/litre was added continuously to complex laboratory stream
channels supplied periodically with field-collected microorganisms for
5 months. LC50 values ranging from 1.0 to 1.8 µg/litre were
determined for four insect and crustacean species ( Tanytarsus
dissimilis, Cricotopus sp. and Hyalella azteca). A chronic effect
level was obtained only for Daphnia magna (0.06 µg/litre). Mayflies
and stoneflies were the most sensitive. They were severely affected
at 1 µg/litre within one month (the sampling interval) and numbers
were two to three orders of magnitude lower, almost leading to their
elimination. The survival of chironomids was reduced by 10 µg/litre
(Hansen & Garton, 1982a,b) (see also section 9.1.1.1).
Benthic communities in outdoor experimental streams were exposed
to 1 or 10 mg/litre of diflubenzuron for 30 min. The effect was
assessed daily by examining drifting pupal exuviae over a period of
one month following the treatment. No drift of macrobenthos was
induced at the time of application. However, diflubenzuron affected
the emergence of all species examined. High larval mortality for a
species of chironomid was observed directly in the stream treated with
diflubenzuron, where numbers of mayfly nymphs and caddisfly larvae
were also decreased (Yasuno & Satake, 1990).
Technical diflubenzuron in acetone was applied at 5 µg/litre to
two aquaria in a simulated field test outdoors. Daphnid numbers were
markedly reduced three days after treatment, but recovered slowly.
Copepods numbers were moderately reduced, exceeding the control
numbers after 18 days. The seed shrimp population showed no harmful
effect (Miura & Takahashi, 1974a,b).
In a study by Collwell & Schaefer (1980), diflubenzuron was
applied to experimental ponds (mean concentration of 13.2 µg/litre) in
California. An hour after treatment, cladoceran ( Ceriodaphnia sp.,
Diaphanosoma sp., Chydorus sp., Bosmina sp. and Daphnia sp.)
numbers were strongly reduced and did not return to pretreatment
levels until more than 5 weeks after treatment. Copepod ( Diaptomus
sp. and Cyclops sp.) numbers were also reduced but to a lesser
extent and for a shorter period than for the cladocerans. The
rotifers increased in abundance in both the control and treated ponds
during the first 8 days following treatment.
Using laboratory tests to study mortality, Miura & Takahashi
(1974a) found that crustaceans, especially the tadpole shrimp
(T. longicaudatus), clam shrimp ( Eulimnadia spp.) and water fleas
( Daphnia and Moina spp.), were highly susceptible to diflubenzuron
at levels below 0.01 mg/litre. Copepods, Cyclops and Diaptomus
spp. showed some tolerance, whereas seed shrimp ( Cypricerus and
Cypridopsis spp.) tolerated as much as 0.5 mg/litre. Among aquatic
insects tested, mayfly nymphs ( Callibaetis spp.) were most
susceptible. Aquatic midge larvae (G. holoprasinus) also
showed susceptibility. However, dytiscid ( T. bassillaris and
Laccorhilus spp.) and hydrophilid beetles ( H. triangularis and
T. lateralis (mosquito predators)) demonstrated a strong tolerance.
Mosquito fish (Gambusia affinis) showed no effect at a relatively
high dose of
1 mg/litre.
When larvae of the crab Rhithropanopeus harrisii were exposed
to sublethal concentrations of diflubenzuron (0.05, 0.1, 0.3 and
0.5 µg/litre), swimming speed increased in stage I, II and III zoeae,
0.3 µg/litre being the lowest effective concentration. Phototaxis was
altered in stage IV only at concentrations as low as 0.1 µg/litre
(Forward & Costlow, 1978).
Christiansen et al. (1978) showed that nearly 100% of
Rhithropanopeus harrisii larvae at each of the four zoeal stages
died when moulting to the succeeding stage after only 3 days of
exposure to 10 µg diflubenzuron/litre. This concentration was also
lethal for larvae of Sesarma reticulatum (Say).
Christiansen & Costlow (1980) exposed larvae of the estuarine
crab Rhithropanopeus harrisii in laboratory conditions to 10 µg
diflubenzuron/litre as an indicator of persistence of
diflubenzuron in brackish water. Disturbance of endocuticle
deposition seemed to occur as soon as newly hatched larvae (less than
12 h old) were exposed to diflubenzuron. Diflubenzuron not only
affected endocuticle deposition in the larvae, but also exocuticle
deposition. The only part of the crab larval exoskeleton that did not
seem to be affected by diflubenzuron was the epicuticle (Cristiansen
et al., 1978; Cristiansen & Costlow, 1982).
Diflubenzuron, at concentrations of 0.02 and 0.2 mg/litre,
enhanced mortality during moulting of the crab Carcinus
mediterraneus (Czerniavsky) (Cardinal et al., 1979).
Cirripede crustaceans, (barnacles, Balanus eburneus) exposed to
concentrations of 1 to 1000 µg/litre over a 28-day period showed a
dose-dependent mortality. Heavy mortality occurred during the second
week of exposure. Lethal and sublethal effects were observed at
concentrations as low as 50 µg/litre (Gulka et al., 1980). Disruption
of the exoskeleton of B. eburneus caused by diflubenzuron was
similar to that observed in insects. Development of barnacles exposed
to diflubenzuron for 10 days or more at 750 and 1000 µg/litre was
delayed in the premoult phase of cuticle secretion (Gulka et al.,
1982).
Wilson & Costlow (1986) found that diflubenzuron concentrations
of 2.5 and 5 µg/litre were lethal to larvae of the grass shrimp
(Palaemonetes pugio), causing 100% mortality on days 14 and 6,
respectively. Wilson et al. (1985) studied the effects of
diflubenzuron upon phototaxis of larvae of P. pugio. The depression
in positive phototaxis and elevation in negative phototaxis were most
pronounced at 0.5 µg/litre, and the lowest test concentration to
affect phototaxis was 0.3 µg/litre. The alterations in photo
responses varied with the embryonic stage at which exposure to
diflubenzuron commenced. This study was carried out at optimal
salinity and temperature, but in the estuary these conditions
fluctuate daily, which may amplify the observed effects.
Nimmo et al. (1980) observed that chronic exposure of the mysid
shrimp Mysidopsis bahia to 0.075 µg diflubenzuron/litre reduced the
reproductive success of both parents and progeny even after they were
transferred to uncontaminated sea water.
Diflubenzuron reduced the reproductive life span of adult brine
shrimps (Artemia salina) at levels of 2-10 µg/litre and caused death
of immature shrimps within 3 days at concentrations above 10 µg/litre
(Cunningham, 1976).
Fiddler crabs (Uca pugilator) were exposed to diflubenzuron
(Dimilin WP-25%) at 0.5, 5 and 50 µg/litre for 1, 2, 3 and 4 weeks
after multiple autonomy of one chela and five walking legs. Exposure
to diflubenzuron retarded the rate of limb regeneration in a dose-
dependent fashion. The effects of diflubenzuron were seen even in
crabs exposed for only one week. Significant retardation was evident
by day 21 at 5.0 µg/litre but was not statistically significant at
0.5 µg/litre. At 5 and 50 µg/litre moult-associated mortality was
seen. The number of setae on regenerated limbs was less than the
number on the intact limbs. The effects were reduced in experiments
in which sediment was present (Weis et al., 1987).
The burrowing activity of Uca pugilator in sand under
laboratory conditions was not altered when the sand was contaminated
with 1 mg diflubenzuron/litre, indicating a lack of avoidance of
diflubenzuron-contaminated sand. However, exposure for 1 week to 0.5,
5.0 or 50 µg/litre led to a decrease in the amount of burrowing
activity. The behavioral response was not changed after exposure for
1, 2 or 3 weeks and was not concentration-related (Weis & Perlmutter,
1987).
Survival, moulting and behaviour of juvenile fiddler crabs were
significantly affected by exposure to diflubenzuron (0.2, 2, 20 and
200 µg/litre) for 24 h weekly during 10 weeks. All crabs in the 200
and 20 µg/litre groups died after 8 and 23 weeks, respectively. The
no-observed-effect concentrations (NOEC) for moulting (time to the
first moult), survival (time until death), and behaviour (ability to
escape from the test container) were 20, 2 and 0.2 µg/litre,
respectively (Cunningham & Myers, 1987).
Larvae of the stone crab (Menippe mercenaria) were exposed to
0.5, 1.0, 3.0, and 6.0 µg diflubenzuron/litre in combination with
different temperature and salinity. All of these concentrations were
lethal to the larvae. Evidence for synergistic effects of
diflubenzuron and temperature or salinity was observed. Tolerance of
the megalopa of the blue crab Callinectes sapidus to diflubenzuron
at concentrations of 0.5, 1.0, 3.0 and 6.0 µg/litre was slightly
higher than for Menippe mercenaria but was also dependent upon
temperature and salinity. At 20°C the percentage survival at a
concentration of 1 µg/litre was similar to that observed for the
controls (Costlow, 1979).
Tester & Costlow (1981) reported that the marine copepod
Acartia tonsa exposed to 1 and 10 µg diflubenzuron/litre for 36 h
failed to produce viable nauplii even after they had been placed in
clean sea water. No viable nauplii were produced by these females for
at least 30 h after treatment ended.
Adult crustaceans were more resistant to exposure than their
larvae at high concentrations (100-200 µg/litre) of technical grade
diflubenzuron. Adults also exhibited significant mortality associated
with moulting (Cunningham, 1976; Cardinal et al., 1979; Gulka et al.,
1980).
When larvae of horseshoe crabs (Limulus polyphemus) were
exposed to 5 and 50 µg diflubenzuron/litre, the crabs in the
50 µg/litre group exhibited severe mortality immediately after
ecdysis. The larval stages were shown to be quite resistant to
diflubenzuron, compared with other crustacean larvae (Weis & Ma,
1987).
9.1.2.4 Vertebrates
Data on the acute toxicity of diflubenzuron and its metabolites
for fish are presented in Tables 14 and 15, respectively.
A 96-h acute toxicity test on juvenile sheepshead minnow
(Cyprinodon variegatus) in a flow-through system resulted in no
mortality at the nominal exposure concentration of 130 µg
diflubenzuron/litre (Graves & Swigert, 1993). A similar test under
semi-static conditions on zebra fish (Brachydanio rerio) and rainbow
trout (Oncorhynchus mykiss) at a nominal diflubenzuron concentration
of 200 µg/litre also showed no mortality, nor changes in the appearance
Table 14. Acute toxity of diflubenzuron to fish
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Coho salmon 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus kisutch) Pridmore (1978)
Rainbow trout 1 g stat 11 4.55 6.5 96-h LC50 > 150 McKague &
(Oncorhynchus mykiss) Pridmore (1978)
Rainbow trout 1.2 g stat 12 40 7.2 96-h LC50 240 Julin & Sanders
(Oncorhynchus mykiss) (1978)
Rainbow trout not flow-through 96-h LC50 140 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Rainbow troutb not flow-through 96-h LC50 195 Marshall & Hieb
(Oncorhynchus mykiss) reported (1973)
Fathead minnow 0.87 g stat 22 40 7.2 96-h LC50 430 Julin & Sanders
(Pimephales promelas) (1978)
Channel catfish 2.2 g stat 22 40 7.2 96-h LC50 370 Julin & Sanders
(Ictalurus punctatus) (1978)
Bluegill 0.5 g stat 22 40 7.2 96-h LC50 660 Julin & Sanders
(Lepomis macrochirus) (1978)
Bluegill not flow-through 96-h LC50 135 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Bluegillb not flow-through 96-h LC50 230 Marshall & Hieb
(Lepomis macrochirus) reported (1973)
Table 14. (Con't)
Organism Size/age Stat/flowa Temperature Hardness pH Parameter Concentration Reference
(°C) (mg/litre) (mg/litre)
Mummichog adult 2.7 g stat 24 22 ppt 8.0 96-h LC50 32 990 Lee & Scott
(Fundulus heteroclitus) renewal salinity (1989)
Mummichogb not flow-through 96-h LC50 255 Marshall & Hieb
(Fundulus heteroclitus) reported (1973)
a Stat = static conditions (water unchanged for duration of test); flow = flow-through conditions (diflubenzuron concentration in water
continuously maintained)
b Formulated product 25 WP
Table 15. Acute toxicity of metabolites of diflubenzuron to fish (from: Julin & Sanders, 1978)
Concentrations (mg/litre)
Organism Water Effect
temperature measured
(°C) 4-Chlorophenyl 2,6-Difluorobenzoic 4-Chloroaniline
urea acid
Rainbow trout 12 96-h LC50 72 (57-90) > 100 14 (11-16)
(Oncorhynchus mykiss)
Channel catfish 22 96-h LC50 > 100 > 100 23 (18-29)
(Ictalurus punctatus)
Fathead minnow 22 96-h LC50 > 100 69 (55-87) 12 (7-18)
(Pimephales promelas)
Bluegill 22 96-h LC50 > 100 > 100 2.4 (1.8-3.2)
(Lepomis macrochirus)
or behaviour of the fish (Berends & van der Laan-Straathof, 1994a,b).
All these test concentrations were above the diflubenzuron solubility
level of 80 µg/litre.
Nebeker et al. (1983) found no significant reduction in
survival of fathead minnow (Pimephales promelas) or guppy
(Poecilia reticulata) as a result of exposure to diflubenzuron at
concentrations below 36 µg/litre during acute (96-h) and chronic
tests.
No acute response resulted from exposure of fish to a
diflubenzuron concentration of 45 µg/litre, and no chronic effects
were observed at this concentration, the highest one tested (Hansen &
Garton, 1982a,b).
Diflubenzuron was not toxic to either rainbow trout or coho
salmon exposed at concentrations up to 150 mg/litre for a 96-h period.
A 15-min exposure to 1 g/litre did not result in any fish mortality
(McKague & Pridmore, 1978).
Madder & Lockhart (1978) found a dose-related decrease of
glutamic-oxaloacetic transaminase activity in rainbow trout
(Oncorhynchus mykiss) exposed to diflubenzuron at concentrations of
0.625, 1.25, 2.5, 5 and 10 mg/litre.
At a concentration of 0.01 mg/litre, diflubenzuron had a
repellent effect on precocious male Atlantic salmon parr (Granett et
al., 1978).
9.1.3 Terrestrial organisms
9.1.3.1 Plants
Photosynthesis, respiration and leaf ultrastructure of soybeans
were unaffected by diflubenzuron at doses up to a level of
0.269 kg a.i./ha (Hatzios & Penner, 1978).
Diflubenzuron is used as an insecticide in forestry, agriculture
and horticulture. No phytotoxicity was reported in the field studies
cited in section 9.2.
9.1.3.2 Invertebrates
The oral and contact LD50 values of diflubenzuron for honey-bees
are greater than 30 µg/bee (Stevenson, 1978).
Diflubenzuron did not show any toxicity to bees at concentrations
up to 1000 mg/kg in the diet (Yu et al., 1984).
Barker & Taber (1977) found that diflubenzuron reduced brood
production when fed for 10 days to honey-bees at 59 mg/kg in sugar
syrup, but not at 5.9 or 0.59 mg/kg.
Barker & Waller (1978) confirmed that 60 mg/kg in sugar syrup or
100 mg/litre in water caused colonies to produce less brood.
Stoner & Wilson (1982) found that diflubenzuron fed to flying
colonies at 1 or 10 mg/kg for a year significantly reduced the amount
of sealed brood.
Nation et al. (1986) reported that diflubenzuron did not cause
reduction in pollen consumption or brood production when fed for 10
weeks to caged colonies of honey-bees at 10 mg/kg, but it caused more
than 50% reduction in the amount of syrup stored.
Gordon & Cornect (1986) showed that, at concentrations that are
effective in suppressing egg hatching and larva development of the
cabbage maggot Delia radicum, diflubenzuron did not adversely
affect eggs, first-instar larvae or adults of the rove beetle
Aleochara bilineata, an important predator and parasitoid of the
cabbage maggot.
When the acute toxicity of diflubenzuron to the earthworm
Eisenia fetida was tested in artificial soil to which diflubenzuron
had been added, 780 mg/kg dry soil was the no-observed-effect
concentration (NOEC) for the 14-day test period (Berends et al.,
1992). For the WP-25 formulation, the NOEC was 1.0 g/kg (Berends &
Thus, 1992a).
9.1.3.3 Vertebrates
a) Birds
The acute oral LD50 of technical diflubenzuron for red-winged
blackbirds (Agelaius phoeniceus) is 3762 mg/kg body weight. In an
8-day dietary LD50 study on mallard duck and bobwhite quail using
technical diflubenzuron, levels up to 4640 mg/kg in the feed gave no
observable signs of toxicity (Maas et al., 1980).
When diflubenzuron was fed to mature White leghorn hens at
dietary levels of 10, 50, 100, and 500 mg/kg for 8 weeks, there were
no adverse effects on feed consumption, body weight, egg production,
egg weight, eggshell thickness, fertility, hatchability or progeny
performance. The highest dose used was 50 times higher than the
efficacy level for fly control (Cecil et al., 1981).
In chronic toxicity tests, groups consisting of 10 male and 10
female one-day-old chicks of barred Plymouth rocks and white leghorn
hens, Nicholas white turkeys, mallard ducks and ring-necked pheasants
were given diets containing diflubenzuron levels of 0.25, 1.25, 25 and
250 mg/kg for 91 days after hatching. No differences between control
and treatment groups were observed in mortality, food consumption,
body weight, comb and wattle development, weight of inner organs,
serum hormone levels or general behaviour (Maas et al., 1980).
There was no effect of diflubenzuron on the content of hyaluronic
acid in the skin of Hubbard broiler chickens when they were fed at
levels of 2.5 and 250 mg/kg in the diet for 98 days after hatching
(Deul & Jong, 1977). In an experiment with the same dose levels and
duration, diflubenzuron had no effect on hyaluronic acid synthesis or
comb deposition in either growing broilers or layers (Crookshank et
al., 1978).
White leghorn and black sex-linked cross hens were fed
diflubenzuron at a level of 10 mg/kg in the ration for 15 weeks.
Diflubenzuron had no effect on body weight gain, egg production,
fertility or hatchability (Miller et al., 1976b).
In a feeding study with 2.5, 25 and 250 mg/kg, diflubenzuron did
not affect bobwhite quail reproduction (Booth et al., 1987).
Diflubenzuron did not show significant teratogenic activity on chick
embryos over time when injected at 10 mg/egg (Booth et al., 1987).
A one-generation bobwhite quail (Colinus virginianus)
reproduction study was conducted in which a diflubenzuron-containing
diet was administered ad libitum to young adults (24 weeks old at
test initiation) approaching their first breeding season. Dietary
concentrations of 250, 500 or 1000 mg/kg did not result in treatment-
related mortality, overt signs of toxicity or effects upon adult body
weight or feed consumption during the 21-week exposure period. There
were no apparent treatment-related effects upon reproductive
parameters at 250 or 500 mg/kg. There may have been a slight reduction
in the number of eggs laid, although this was not statistically
significant or dose-related at 1000 mg/kg. On the basis of a possible
effect on egg production at 1000 mg/kg, the NOEC for diflubenzuron in
this study was above 500 mg/kg (Beavers et al., 1990a).
In a one-generation reproduction study on the mallard duck
(Anas platyrhynchos), diets containing diflubenzuron were
administered ad libitum to young adults (27 weeks old at test
initiation) approaching their first breeding season. Dietary
diflubenzuron concentrations of 250, 500 and 1000 mg/kg did not
result in treatment-related mortality, overt signs of toxicity or
effects upon adult body weight or feed consumption during the 20-week
exposure period. There were no apparent treatment-related effects
upon reproductive performance at any of the concentration tested. At
1000 mg/kg there was a slight, but statistically significant,
reduction in mean eggshell thickness. On the basis of the effect upon
eggshell thickness at 1000 mg/kg, the NOEC for diflubenzuron in this
study was 500 mg/kg (Beavers et al., 1990b).
b) Mammals
In studies by Ross et al. (1977a,b), diflubenzuron was
administered in the feed to sheep (3 of each sex per group) as a model
for ruminant wildlife at concentrations of 500, 2500 and 10 000 mg/kg
feed for 13 weeks. No treatment-related effects were observed on food
consumption, body weight gain, haematological parameters or
urinalysis. Increase in met- and sulfhaemoglobin levels were observed
at 13 weeks and there was a reduction in the weight of the thyroid.
No histopathological abnormalities were observed. Both the plasma and
the erythrocyte cholinesterase activities were unaffected by the
treatment after 6 weeks.
9.2 Field observations
9.2.1 Microorganisms
9.2.1.1 Water
The laboratory data available (see section 9.1.1.1) make it
unlikely that detrimental effects will occur.
Rotifers were unaffected by diflubenzuron (28 and 56 g a.i./ha)
in both experimental and naturally treated ponds (Ali & Lord, 1980).
9.2.1.2 Soil
One aerial application of diflubenzuron at 67.26 g/ha had no
adverse effects upon populations of bacteria, actynomycetes or fungi
in leaf litter and forest soil (Wang, 1975; Kurczewski et al., 1975).
9.2.2 Aquatic organisms
9.2.2.1 Plant
The laboratory data available (see section 9.1.2.2) make it
unlikely that detrimental effects will occur.
9.2.2.2 Invertebrates
Recent field studies have demonstrated that effects on aquatic
fauna are limited and transient, and that recovery is evident after
3 months (Huber & Collins, 1987; Kingsbury et al., 1987; Huber &
Manchester, 1988; Ali et al., 1988; Ali & Kok-Yokomi, 1989; Sundaram
et al., 1991).
Diflubenzuron (25% WP) applied at 33.63 and 134.52 g a.i./ha,
4 times at 2-week intervals, had no adverse effect on freshwater clams
10 days after final treatment (Jackson, 1976).
When diflubenzuron (25% WP) was applied at 1.121-280.25 g a.i./ha
to flooded rice fields in Louisiana, USA, significant reduction of
Tropisternus spp. and Libellulidae was found 80 days after
treatment. Significantly more chironomid and baetid immatures
occurred due to reduction in the number of predators (Steelman et al.,
1975).
Mulla et al. (1975) found that at an application rate of 28 and
56 g a.i./ha diflubenzuron reduced slightly, and only for a short
time, the number of mayfly ( Beatis sp.) in the treated ponds. The
numbers, however, appeared to be within natural fluctuation limits
equal to the check ponds or the pretreatment levels during all other
sampling periods. At these application rates, diflubenzuron caused a
short-term reduction in copepod populations, but they started to
increase on the 11th or 15th day of post-treatment sampling. There
was little or no effect on the ostracod population.
In a forestry spraying programme at 0.0672 kg a.i./ha, aquatic
arthropods were studied in a water shed (White Deer Creek). Sampling
at three test stations and one control station in the watershed
revealed a rich fauna, dominated numerically by Ephemeroptera,
Chironomida, Trichoptera and Plecoptera in descending order of
abundance. Significant differences in larval abundance in the surber
samples were found to occur from one sampling day to the next, or
between pre-spray and post-spray periods, for most of the organisms
examined. In all cases, control and test stations showed similar
patterns of variation, so treatment effects were discussed as a
probable cause. The number of organisms tended to be higher at the
upstream stations. Chironomida and Trichoptera pupal abundance in the
surber samples was examined for decreases after spraying but numbers
were too low to permit statistical analysis. Pupal abundance showed
some direct relationship to larval abundances. Nymphal drift rates
were examined for possible increases after spraying, but most of the
significant changes were decreases during the post-spray period.
These decreases also occurred at the control station. The drift rates
of nymphal and pupal exuviae also did not indicate a treatment effect.
Individual species abundances in the surber samples were examined for
possible selective effects of diflubenzuron. Comparisons were made
between pre-spray and post-spray periods at both test and control
stations, but no evidence of a treatment-related decrease in the
abundance of any of these organisms was found. The net effect was
usually an increase in the density of these organisms during the post-
spray period. No organisms showing abnormal ecdysis or pupation were
found in any of the samples. It was concluded that spraying with
diflubenzuron had no adverse effect on the macrobenthic community in
White Deer Creek (White, 1975).
In a study by Booth (1975), diflubenzuron (25% WP) was applied at
44.84 g a.i./ha to small ponds in Utah. Biosamples taken 30 and 80
days later showed that, although larva and immature aquatic insect
populations were decreased at 30 days, the total number of adults was
not significantly different from the control number at 80 days.
Booth & Ferrell (1977) examined the effect of diflubenzuron on
over 20 different species (corixidae and collembolans) after multiple
pond applications at 45 g a.i./ha. Only immature corixidae (water
boatmen) and collembolans (springtails) were significantly affected.
Segmented worms (Oligochaeta) and midges (Chironomidae)
were unaffected by six applications of diflubenzuron at a rate of
145.73 g a.i./ha to Utah Lake (Booth et al., 1987).
Diflubenzuron (28 and 56 g a.i./ha) reduced numbers of
Chaoborus sp. and Baetis sp. in both experimental and natural
treated ponds. Chaoborus sp. recovered within 1-3 weeks (Ali &
Lord, 1980).
The application of granular diflubenzuron at 0.11 kg a.i./ha
(about 3.7 µg/litre) to residential-recreational lakes caused a 62-75%
reduction of Daphnia pulex and Daphnia galeata during 7 days
following treatment. The populations recovered in the second week
after treatment. A 30% reduction in Diaptomus spp. was noted 2
days after the treatment. Hyalella azteca was affected markedly,
with a maximum reduction of 97% after 3 weeks of treatment. No
detectable effects on Cyprinotus sp., Cyclops sp. or Bosmina
longirostris were observed (Ali & Mulla, 1978a). Ali & Mulla
(1978b) also studied the impact of diflubenzuron on invertebrates in a
residential-recreational lake after two treatments with 25% WP
formulation at 156 g a.i./ha (about 12 µg/litre). Diflubenzuron
concentrations in water were not measured but were based on nominal
initial concentrations. The results from this study are summarized in
Table 16.
Diflubenzuron (25% WP) at rates of 0.02, 0.025, 0.03, 0.035,
0.04, 0.045 and 0.05 lb a.i./acre was applied to 19 irrigated pastures
and spring ponds (in some cases up to 4 times) at 2- to 3-week
intervals in California. Cladoceran and nymphal mayfly populations
were temporarily and slightly reduced, but recovered within a short
period of time. Even repeated treatment of the same pastures did not
eliminate the populations. The impact on copepod populations was
inconclusive. Adult beetles demonstrated high tolerance to
Table 16. Effect of diflubenzuron on invertebrates in Vallage Grove Lake, Californiaa
Species First application (April) Second application (August)
Effect Recovery Effect Recovery
Daphnia leavis elimination within no recovery 6 months
Birge 1 week after treatment
Ceriodaphnia sp. elimination within no recovery 6 months
1 week after treatment
Bosmina longirostris elimination within recovery after elimination after reappearance in small numbers
(O.F. Muller) 1 week 11 weeks 1 week 8-9 weeks after treatment
Cyclops spp. elimination within recovery within elimination within recovery after 4 weeks
1 week 6-7 weeks 1-2 weeks
Diaptomus sp. elimination within recovery after absent prior to reappearance in small numbers
1 week 4 months treatment 1-2 months later
Hyalella azteca elimination within no recovery 6 months
(Saussure) 4 weeks after treatment
Caenis sp. elimination within recovery within elimination within recovery in 4-5 weeks
3 weeks 6-7 weeks 2-3 weeks
Physa sp. no adverse effects no adverse effects
Cypridopsis sp. no adverse effects no adverse effects
a From: Ali & Mulla (1978b)
diflubenzuron, but few dead larvae ( Laccophilus spp., Hydrophilus
triangularis and Tropisternus lateralis) were observed. Spiders
( Pardosa spp. and Lycosa spp.) showed no apparent effects. There
were no deleterious effects on planarian, rotifer, seed shrimp and
fresh water flagellate populations (Miura, 1974).
Diflubenzuron (25 WP formulation) was applied 3 times at 2.5-week
intervals to man-made pools in Manitoba, Canada at 56 g/a.i. ha (this
produced initial concentrations of 0.02 mg/litre). The treatment
produced no detectable effect on chironomid larvae in terms of numbers
or composition, although dead larvae were noted after treatment.
Daphnid populations were significantly reduced on most sampling dates.
Repetitive treatments prevented the recovery of cladocerans. Despite
these reductions, daphnids were not annihilated. At a higher rate
(0.22 kg a.i./ha, about 7.4 µg/litre), both species of Daphnia were
eliminated for 3 months after treatment. Populations of Diaptomus
spp. declined to zero 7 days after treatment, but recovered during the
second week following treatment. Diflubenzuron caused reductions in
the number of H. azteca (32-100%) during the 2´ months following
treatment. Seed shrimp populations were stressed for only 2 weeks,
and there were no observable effects on oligochaetes at a treatment
level of 0.22 kg a.i./ha (about 7.4 µg/litre). Copepods only
occasionally showed significant reduction, and recovered within 10
days after treatment. Of the ten other non-target invertebrates (i.e.
Chaoboridae, Tipulidae, Ephemeroptera, Corixidae, Nonectidae,
Gerridae, Dystiscidae, Hydrophilidae, Hydrarina and Gastropoda),
only mayfly nymphs (Ephemeroptera) were significantly reduced
(Madder, 1977).
When diflubenzuron at a rate of 34.2 g/ha was applied aerially to
a forested area in the USA, there were no significant effects on the
population structure of the benthic macro-invertebrate community.
Some decreases in the number of mayflies Heptagenia sp. and
Rhithrogenia sp. were noted in treatment streams, but the total taxa
richness values remained high. An increase in abundance of the
caddisfly Lepidostoma sp. was found. Allonarcys sp. was not
affected by the treatment (Huber & Collins, 1987; Huber & Manchester,
1988).
After aerial application of diflubenzuron to two forest ponds in
Canada the greatest effect was on crustacean zooplankton, especially
cladocerans, with limited effect on pond benthos. Recovery of
population of even the most severely affected organism such as
Daphnids was well established by 3 months after treatment. The
maximum residue found in water was 13.82 µg/litre 1 h after
application (Kingsbury et al., 1987).
Diflubenzuron WP-25 was applied at a rate of 70 g active
ingredient in 10, 5 or 2.5 litre/ha to three spray blocks in a mixed
boreal forest near Kaladar, Ontario, Canada. Water, sediment and
aquatic plants were collected from two ponds and a stream at intervals
up to 30 days after treatment for analysis of diflubenzuron residues.
The duration of detectable residues was different for each substrate,
but in all cases it was less than 2 weeks. Zooplankton and benthic
invertebrate populations were monitored for up to 110 days after
spraying in two ponds in the high-volume rate block and in control
ponds. Significant mortality occurred in two groups of caged
macroinvertebrates (amphipoda and immature corixidae) 1 to 6 days
after the ponds were treated with diflubenzuron. Three taxa of
littoral insects ( Caenis, Celithemis and Coenagrion) were
significantly reduced in abundance in the treated ponds 21 to 34 days
after treatment but recovered to pre-treatment levels by the end of
the season. Of the six remaining groups studied, only one (immature
corixidae) may have been slightly affected by treatment. Zooplankton
(cladocera and copepoda) population numbers were reduced 3 days after
treatment and remained suppressed for 2-3 months (Sundaram et al.,
1991).
Blumberg (1986) found no effect on population numbers in a
forested ecosystem after aerial application of diflubenzuron (25 WP)
at rate 140 g a.i./ha.
When diflubenzuron (33.63 and 134.52 g a.i./ha) was applied 4
times at 2-week intervals to small ponds in Virginia, numbers of
daphnids were markedly reduced at both treatment levels. There was no
appreciable effect on two other invertebrate species ( Chironomidae
or Chaoborus) (Birdsong, 1977).
In a study by Rodrigues (1982), diflubenzuron (25% WP) was
applied at a rate of 1 mg/litre per 30 min to three forest streams in
New York. The reduction of chironomid larvae 15 days after treatment
ranged from 35 to 91% at different sites in the three streams. Of the
two stonefly families present in the three streams, Nemouridae were
affected more than Leuctridae, with decreases of 75-94% and 70%,
respectively. Ephemeroptera were reduced, but Trichoptera and
Coleoptera were unaffected.
Following aerial application at 67.26 g a.i./ha to a watershed,
diflubenzuron reached the stream channel (also as a result of wash-off
from foliage following several subsequent rainfalls), but the
invertebrate populations were not affected (Jones & Konchenderfer,
1988).
After two applications of 44.8, 112 and 224 g a.i./ha, grass
shrimp showed mortality of 86.6, 100, and 100%, respectively. At the
two lowest application rates, fiddler crabs ( Uca spp.) showed
mortalities of 53.3 and 66.6%, respectively (McAlonan, 1976).
Diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied 4
times at 2-week intervals to man-made ponds in North Carolina,
Arkansas and Texas, USA. In North Carolina no apparent effect on
phytoplankton was found. There was a marked decrease in the numbers
of crustaceans ( Cladocera and Copepoda), as well as of certain
species from benthic communities such as Hexagenia and Chaoborus
in Arkansas. A relative decrease in copepod numbers was observed, but
the authors attributed this partly to natural mortality during winter.
In Texas there was a marked decrease in the numbers of all crustaceans
and a corresponding increase in rotifer populations, particularly
Asplanchina (Aquatic Environmental sciences, 1976a,b,c).
When diflubenzuron (25% WP) at 33.63 and 134.52 g/ha was applied
4 times at 2-week intervals to man-made ponds in Alabama, USA, daphnid
populations were reduced to 50% of the pre-treatment level at a rate
of 134.52 g/ha but increased at 33.63 g/ha to a level of 15% of that
in a control pond. Gastropod numbers decreased in the benthic samples
but increased in the periphyton samples from the suspended plate
samplers in the treated ponds (Jackson, 1976).
Diflubenzuron at 33.63 and 134.52 g/ha was applied 4 times at
2-week intervals to a tidally influenced salt marsh in Virginia, which
was sampled biologically 14 times over a 70-day period. Three species
of arthropods, Cyathura polita, Ceratopogonidae sp., and Psychodidae
sp., showed significant reductions in numbers. Oligochaetes, Hanynkia
speciosa and the molluscs were not significantly affected (Matta,
1976).
Six applications of diflubenzuron (28 g a.i./ha) over an 18-month
period caused statistically significant differences in the population
density of aquatic organisms in a Louisiana coastal marsh. None of the
organisms affected were completely eliminated from the ecosystem.
Populations of five taxa (nymphs of Trichocorixa louisianae Jaczewski
and Buenoa spp., Coenagrionidae naiad spp., Berosus infuscatus Le Conte
adults, and Hyalella azteca Saussure) were significantly reduced.
Population of 15 taxa, i.e. Physa sp., Ceanis sp. and Callibaetis sp.
naiads, Noteridae larvae, Hydrovatus cuspidatus, Kunze adults,
Hydrovatus sp. larvae, Dytiscidae larvae, Mesovelia mulsanti Jaczewski
adults, Trichocorixa louisiana adults, larvae of Chironomidae, Ephudridae,
Dolichopodiae and Tabanidae, and the fish Gambusia affinis (Baird and
Griard) and Jordanella floridae (Goode and Bean), showed significant
increases after exposure to diflubenzuron. The 27 remaining aquatic
organisms (members of the Hemiptera, Coleoptera, Mysidacea, Decapoda,
Diptera and Odonata) showed no statistically significant differences
when compared with untreated populations (Farlow et al., 1978).
After treatment with diflubenzuron (as 1% granular formulation)
at rate of 4.5 g a.i./ha to a marsh habitat on the Fraser River,
Canada, the population of cladocerans appeared to be depressed for
about 5 days but they recovered 2 weeks after treatment. There was no
effect on copepods or ostracods. Significant reduction in the numbers
of water beetles and zooplankton occurred (Wan & Wilson, 1977).
In a field study by Hester et al. (1986), WP-25 formulation was
evaluated in natural salt water pools to determine its toxicity to
juvenile stages of three estuarine crustaceans exposed to a single
application of 45 g diflubenzuron/ha. One hour after application,
water residues of diflubenzuron averaged 3.6 µg/litre and mortality
was 46.5, 60.7 and 40.6% for Callinectes sp., Palaemonetes pugio
and Uca pugilator, respectively, over the 10-day observation period.
When these species were introduced into the pools 7 days after
treatment, mortality was not significantly greater in the treated
group than in the controls over the 21-day observation period. The
maximum concentration after organisms were introduced was
0.69 µg/litre, and mortalities of 22.2, 1.5 and 4.2% for Callinectes
sp., Palaemonetes pugio and Uca pugilator, respectively, were
reported.
In a study by Hester (1982), diflubenzuron (25% WP) at the rate
of 0.045 kg a.i./ha was applied to tidal ponds. Residue analysis
showed 2 to 5 µg/litre after 1 h. The mortality of three
estuarine decapods: blue crabs ( Callinectes sp.), grass shrimp
(Palaemonetes pugio) and fiddler crabs (Uca pugilator) was 44,
61 and 41%, respectively. When these decapods were introduced to the
ponds 7 days after application, the residues in water were
0.4-0.7 µg/litre and mortality was 22, 1.5 and 4%, respectively.
9.2.2.3 Vertebrates
During a forestry spraying programme at 0.067 kg diflu-
benzuron/ha, a survey on fish was performed in the watershed.
Observations made on caged brown trout in the stream from day -7 to
day +6 revealed no immediate mortality or erratic behaviour
attributable to treatment. Barrier seines placed at the upstream and
downstream boundaries of the spray area did not collect any dead or
dying fish during the 1-week period they were in the stream. Visual
observations made by walking along the stream during this same period
also revealed no dead or dying fish. Post-spray population
estimations revealed increases in both test and control areas.
However, statistical analysis indicated no significant differences
pre- and post-spray between stations. The treatment had no observable
effect on the development of fish larvae. Immediate mortality was not
observed in caged fish or stream fish collected by barrier seines.
Delayed mortality attributable to spraying was not shown by population
estimations taken 6 weeks following spray, nor were any dead or dying
fish seen during this period. There appeared to be no adverse effect
of diflubenzuron sprayed at 0.067 kg a.i./ha on the fish species
observed in this study (White, 1975).
No effect on fish populations ( Micropterus salmoides, Lepomis
macrochirus and Gambusia affinis) was observed after four
applications of diflubenzuron (33.69 and 134.52 g a.i./ha) at 2-week
intervals to small ponds in Virginia (Birdsong, 1977).
Killifish (Fundulus heteroclitus) showed no effect after three
applications of diflubenzuron at rates from 11.12 to 224.2 g a.i./ha
to replicated semi-natural pools (McAlonan, 1976). Diflubenzuron
revealed no toxic effect on bullheads or sunfish after aerial
application of 35 g/ha in Canada (Buckner et al., 1975).
Growth of bluegill sunfish ( Lepomis macrochirus Rafinesque) was
not affected by diflubenzuron applications at rates of 2.5-10 µg/litre
to ponds and lakes in California (Apperson et al., 1978). Ten days
after the fourth treatment to man-made ponds with diflubenzuron at
33.63 and 134.52 g a.i./ha no apparent adverse effect on largemouth
bass was observed (Jackson, 1976).
No death of fish ( Pomoxis nigromaculatus and Ictalurus
nebulosis) occurred after application of diflubenzuron (mean
concentration of 13.2 µg/litre one hour after treatment) to
experimental ponds in California. For 1 month following treatment,
stomach content analyses showed alteration in the diet of the fish
(Collwell & Schaefer, 1980).
9.2.3 Terrestrial organisms
9.2.3.1 Invertebrates
Honey-bee colonies remained normal after an aerial application of
350 g diflubenzuron/ha in Canada (Buckner et al., 1975).
A comparative study of the effect of diflubenzuron on carabid
fauna in oak woods in Westphalia in 1987-1988 was reported. The total
number of captured beetles in treated areas was lower in 1989.
Evaluation of the 12 beetle species most frequently caught indicated
lower numbers than in control areas. The decrease coincided with the
diflubenzuron treatment in the middle of May. No higher active
density of species reproducing in late summer or autumn was observed
in the untreated areas (Klenner, 1990).
In a forestry spraying programme (0.0672 kg diflubenzuron per
ha), a survey of microarthropods collected from leaf litter and soil
in control and sprayed areas of the Bald Eagle State Forest,
Pennsylvania, USA, showed little or no effect of diflubenzuron on the
spray plot fauna. Increases and decreases in both the control and
spray population sizes during the 6-week study period could seemingly
be explained on the basis of seasonal population cycles, increased
soil moisture condition and differential extraction efficiencies
(White, 1975).
9.2.3.2 Vertebrates
Small song birds in a forest ecosystem were unaffected after an
aerial application of 350 g diflubenzuron/ha in Canada (Buckner et
al., 1975).
In a forestry spraying programme (0.0672 kg diflubenzuron/ha)
surveys were made on birds and small mammals. Effects on birds were
assessed using single male mapping surveys. The survey methodology
was designed to minimize bias due to differences between observers,
plots, times of day and abundance of birds. Vegetation and
defoliation types were identified and mapped and defoliation was
sampled at 136 sites. Four hundred and sixteen surveys were conducted
on treated plots, defoliated plots, and undisturbed plots. No
differences were observed in the number of individuals singing, the
amount of time each individual spent singing or the song repetition
rate during song bouts. The results show that diflubenzuron did not
lead to the death or emigration of singing birds and probably had no
direct adverse physiological effects on birds since their vocal
behaviour changed little, if at all. The singing male surveys did not
detect a presumed food shortage among canopy feeders caused by the
defoliation. Small mammals studied in this project were censused as
thoroughly as possible. The short pre-spray sampling period for the
treated plot was a handicap in evaluating the pre-spray population
levels; however, post-spray sampling compensated for this to some
degree. Peromyscus, Clethrionomys and Sorex consume arthropods to
varying degrees and may be exposed to diflubenzuron present as
residues. Diflubenzuron had no demonstrable effects on any of these
small mammals sampled in this study. The application of diflubenzuron
at the rate of 0.067 kg/ha provided good foliage protection within the
spray sites and apparently made the dying gypsy moth larvae
unpalatable. Other contact and stomach pesticides generally do not
have this effect on caterpillars and are consequently consumed by
insectivorous mammals. The reduced exposure by non-ingestion is no
doubt a beneficial aspect. None of the species of small mammals
showed reductions in numbers from pre-spray to post-spray periods that
could be attributed to the compound. Stomach analysis did not
indicate any change in the feeding habits of any of the small mammals
from either plot in the study. Changes in reproduction could not be
detected. However, of the Peromyscus and Clethrionomys animals
that were dissected and examined, 7 out of 12 females were in a
healthy condition or had gravid reproductive organs. Others were
either virgins or placental scars could not easily be seen, as in
Sorex. The body weight of all the small mammals in the study was
normal for each species. Juveniles were easily distinguished by
weight and gonad development. A regression comparing gonad weights
and body weights of Clethrionomys indicated a strong correlation.
Since the body weight of small mammals increases with age, this was to
be expected (White, 1975).
Diflubenzuron sprayed at 0.14 and 0.28 kg/ha over forests in
north-eastern Oregon, USA, did not have any detectable adverse effect
on bird population numbers, nesting or bird behaviour (Richmond et
al., 1979).
10. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Diflubenzuron was evaluated by the Joint FAO/WHO Meeting on
Pesticide Residues (JMPR) in 1981, 1984, and 1985 (FAO/WHO, 1982a,b;
1985a,b; 1986a,b). In 1985, JMPR established an Acceptable Daily
Intake (ADI) for human beings of 0-0.02 mg/kg body weight per day,
based on the fact that the following levels produced no toxicological
effects:
rat: 2 mg/kg body weight (40 mg/kg diet)
mouse: 2.4 mg/kg body weight (16 mg/kg diet)
dog: 2 mg/kg body weight per day
Diflubenzuron has been classified as "a product unlikely to
present an acute hazard in normal use", on the basis of an acute oral
LD50 for the rat that is greater than 4640 mg/kg body weight (WHO,
1994).
REFERENCES
Ali A & Kok-Yokomi ML (1989) Field studies on the impact of a new
benzoylphenylurea insect growth regulator (UC-84572) on selected
aquatic nontarget invertebrates. Bull Environ Contam Toxicol,
42: 134-141.
Ali A & Lord J (1980) Impact of experimental insect growth regulators
on some nontarget aquatic invertebrates. Mosq News, 40(4): 564-571.
Ali A & Mulla MS (1978a) Effects of chironomid larvicides and
diflubenzuron on nontarget invertebrates in residential-recreational
lake. Environ Entomol, 7: 21-27.
Ali A & Mulla MS (1978b) Impact of the insect growth regulator
diflubenzuron on invertebrates in a residential-recreational lake.
Arch Environ Contam Toxicol, 7: 483-491.
Ali A, Nigg HN, Stamper JH, Kok-Yokomi ML, & Weaver M (1988)
Diflubenzuron application to citrus and its impact on invertebrates in
an adjacent pond. Bull Environ Contam Toxicol, 41: 781-790.
Anon (1980) Final report: Environmental monitoring of the 1979 Gypsy
moth control program in five eastern States. Hyattsville, Maryland, US
Department of Agriculture, Animal an Plant Health Inspection Service,
25 pp (Proprietary report No. 56683/18/1983, submitted to WHO by
Duphar BV, Crop Protection Division, 'sGraveland, The Netherlands).
Anton FA, Cuadra LM, Gutierrez P, Laborda E, & Laborda P (1993)
Degradational behavior of the pesticides glyphosate and diflubenzuron
in water. Bull Environ Contam Toxicol, 51: 882-888.
Apperson CS, Schaefer CH, Colwell AE, Werner GH, Anderson NL, Dupras
EF, & Longanecke DR (1978) Effects of diflubenzuron on Chaoborus
astictopus and nontarget organisms and persistence of diflubenzuron
in lentic habitats. J Econ Entomol, 71(3): 521-527.
Aquatic Environmental Sciences (1976a) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
North Carolina. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-13, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976b) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Arkansas. Tarrytown, New York, Union Carbide Corporation, 9 pp
(Unpublished proprietary report NTP-8, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Aquatic Environmental Sciences (1976c) The effect of the mosquito
larvicide Dimilin on the freshwater environment of three test ponds in
Texas. Tarrytown, New York, Union Carbide Corporation, 12 pp
(Unpublished proprietary report NTP-7, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Arnold D, Kennedy SL, & Keplinger ML (1974) Mutagenic study with TH
6040 in albino mice. Northbrook, Illinois, Industrial Bio-Test
Laboratories Inc. (IBT No. 622-05068) (Unpublished proprietary report,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Arts JHE (1991) Acute (4-hour) inhalation toxicity of dimilin WP 25%
in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report TNO No. 56645/64/1991, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Barker RY & Taber S (1977) Effects of diflubenzuron fed to caged honey
bees. Environ Entomol, 6: 167-168.
Barker RY & Waller GD (1978) Effects of diflubenzuron wettable powder
on caged honey bee colonies. Environ Entomol, 7: 534-535.
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990a) A one-generation
reproduction study with the bobwhite ( Colinus virginianus. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/08/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Beavers JB, Corbitt A, Hawrot R, & Jaber MJ (1990b) A one-generation
reproduction study with the mallard ( Anas platyrhynchos. Easton,
Maryland, Wildlife International Ltd. (Proprietary report
No. 56645/07/1990, submitted to WHO by Solvay Duphar, BV, 'sGraveland,
The Netherlands).
Bentley JP, Weber GH, & Gould D (1979) The effect of diflubenzuron
feeding on glucosaminoglycan and sulfhemoglobin biosynthesis in mice.
Pestic Biochem Physiol, 10: 162-167.
Berberian IG & Enan EE (1989) Hematological studies on White male rats
exposed to some antimoulding compounds. Bull Environ Contam Toxicol,
43: 60-65.
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975a) Acute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/198/74988, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975b) Subacute inhalation
toxicity to the rabbit of DU 112307 technical grade powder.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/199/7551, submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Berczy ZS, Cobb LM, Street AE, & Cherry CP (1975c) Subacute inhalation
toxicity to the rat of DU 112307 insecticide powder. (Evaluation of
methaemoglobinaemia). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/197/ 741013, submitted to WHO
by Solvay Duphar BV, Weesp, The Netherlands).
Berends AG & Thus JLG (1992a) The acute toxicity of dimilin WP-25 to
the earthworm Eisenia fetida 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 16 pp (Proprietary
report No. 56635/26/1992).
Berends AG & Thus JLG (1992b) The toxicity of diflubenzuron to the
alga Selenastrum capricornutum 'sGraveland, The Netherlands, Solvay
Duphar BV, Environmental Research Department, 23 pp (Proprietary
report No. 56635/49/1992).
Berends AG & van den Laan-Straathof JMTh (1994a) The acute toxicity of
diflubenzuron to Zebra fish ( Brachydanio rerio. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/57/93).
Berends AG & van den Laan-Straathof JMTh (1994b) The acute toxicity of
diflubenzuron to rainbow trout ( Oncorhynchus mykiss. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56835/02/94).
Berends AG, Thus JLG, & Jansen WAJ (1992) The acute toxicity of
diflubenzuron to the earthworm Eisenia fetida 'sGraveland, The
Netherlands, Solvay Duphar BV, Environmental Research Department,
16 pp (Proprietary report No. 56635/22/1992).
Besten C Den, Buse TE, & Koopman TSM (1993a) Acute oral toxicity study
with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay Duphar
BV (Proprietary report No. 56345/51/1993).
Besten C Den, Buse TE, & Koopman TSM (1993b) Acute dermal toxicity
study with Dimilin OF-6 in rats. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56345/52/1993).
Birdsong RS (1977) Field test of Dimilin on non-target organisms in
Virginia. Nonfolk, Virginia, Environmental Consultants, Inc. (NTP-11)
(Unpublished proprietary report, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Blumerg AY (1986) Survey of aquatic soil and soil surface invertebrate
fauna in a North Carolina forest (pre and post application of Dimilin
WP-25) Weesp, The Netherlands, Duphar BV (Proprietary report
No. 56635/24/1986).
Boelhouwers EJ, Joustra KD, Nijssen OA, & Stegman KH (1988a)
Hydrolysis of 14C-labelled diflubenzuron in buffer solutions at pH 5,
pH 7, and pH 9. 'sGraveland, The Netherlands, Solvay Duphar
(Proprietary report No 56630/137/1988).
Boelhouwers EJ, Joustra KD, & Stegman KH (1988b) Photodegradation of
14C-labelled diflubenzuron in water. 'sGraveland, The Netherlands,
Solvay Duphar BV (Proprietary report No. 56630/35/1988).
Bogus ER, Gallagher PA, Cameron EA, & Mumma RO (1985) Analysis of
pesticide exposure pads using selective absorption and elution of
reversed phase solid support. J Agric Food Chem, 33(6): 1018-1021.
Bollag JM, Blattmann P, & Laanio T (1978) Adsorption and
transformation of four substituted anilines in soil. J Agric Food
Chem, 26(6): 1302-1306.
Boorman GA, Eustis SL, Elwell MR, Montgomery CA Jr, & MacKenzie WF
(1990) Pathology of the Fischer rat - Reference and atlas. New York,
London, Academic Press.
Booth GM (1975) The impact of Dimilin W-25 on non-target invertebrates
in ponds located in Salt Lake county, Utah. Provo, Utah, Brigham Young
University (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Booth GM (1977) Biochemical effects of diflubenzuron on mouse embryos.
Provo, Utah, Brigham Young University (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Booth GM & Ferrell D (1977) Degradation of Dimilin by aquatic
foodwebs. In: Khan MAQ ed. Pesticides in aquatic environments. New
York, Plenum Publishing Corporation, pp 221-243.
Booth GM, Alder DC, Lee ML, Carter W, Whitmore RC, & Seegmiller RE
(1987) Environmental fate and properties of 1-(4-chlorophenyl)-
3-(2,6-difluorobenzoyl) urea (diflubenzuron, Dimilin). In: Wright JE &
Retnakaran ed. Chitin and benzoylphenyl ureas. Dordrecht, The
Netherlands, Junk Publishers, pp 141-204.
Brusick DJ & Weir RJ (1977a) Mutagenic evaluation of diflubenzuron
technical batch FL 4/605201. Final report (Project No. 2683).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/34/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Brusick DJ & Weir RJ (1977b) Evaluation of diflubenzuron: In vitro
malignant transformation in BALB/3T3 cells. Final Report (Project
No 2688). Kensington, Maryland, Litton Bionetics Inc. (Unpublished
proprietary report No. 56645/35/1977, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Brusick DJ & Weir RJ (1977c) Evaluation of diflubenzuron: Unscheduled
DNA synthesis in WI-38 cells. Final Report (Project No 2688).
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/36/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Bryant H (1976) Activity of TH-6040 in the Ames Salmonella
typhimurium mutagenesis assay. Report to H.W. Dorough, University of
Kentucky, Lexington, USA (Unpublished proprietary report submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Buckner CH, McLeod BB, & Kingsbury PD (1975) The effect of an
experimental application of Dimilin upon selected forest fauna.
Ottawa, Ontario, Chemical Control Research Institute
(Report No. CC-X-97).
Bull DL & Ivie GW (1978) Fate of diflubenzuron in cotton, soil, and
rotational crops. J Agric Food Chem, 26(3): 515-520.
Burdock GA, Serota DG, Alsaker RD, & Purvis DS (1980a) Ninety-day
subchronic toxicity study in mice - Diflubenzuron technical. Final
report (Project No. 533-120). Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Burdock GA, Serota DG, Purvis D, & Alsaker RD (1980b) Subchronic
dietary toxicity study in rats - Diflubenzuron. Final report: Volume 1
(Project No. 533-119). Vienna, Virginia, Hazleton Laboratories America
Inc. (Unpublished proprietary report submitted to WHO by Solvay Duphar
BV, Weesp, The Netherlands).
Burdock GA, Wolfe GW, Hepner KE, Alsaker RD, Koka M, & Phipps RB
(1984) Oncogenicity study in rats on diflubenzuron. Final report.
Vienna, Virginia, Hazleton Laboratories America Inc. (Unpublished
proprietary report No. 56645/08/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Burgess D (1989) Uptake, depuration and bioconcentration of
14C-diflubenzuron by Bluegill sunfish ( Lepomis macrochirus.
Columbia, Missouri, Analytical Bio-Chemistry Laboratories, Inc.
(Proprietary report No. 56635/16/1989, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Cardinal H, Vernet G, & Sinegre G (1979) Quelques effets d'un
inhibiteur de croissance: le diflubenzuron sur un crabe Carcinus
mediterraneus(Czerniavsky). C C R Soc Biol, 173: 1105-1108.
Carringer RD, Weber JB, & Monaco TJ (1975) Adsorption-desorption of
selected pesticides by organic matter and montmorillonite. J Agric
Food Chem, 23: 568-572.
Caspary WJ, Daston DS, Myhr BC, Mitchell AD, Rudd CJ, & Lee PS (1988)
Evaluation of the L5178Y mouse lymphoma cell mutagenesis assay:
interlaboratory reproducibility and assessment. Environ Mol Mutagen,
12(Suppl 13): 195-229.
Cecil HC, Miller RW, & Corley C (1981) Feeding three insect growth
regulators to white Leghorn hens: Residues in eggs and tissues and
effects on production and reproduction. Poult Sci, 60: 2017-2027.
Chandran VR (1981) Primary skin irritation study in rabbit with
Dimilin 25 WP. Padappai, Tamil Nadu, India, Coromandel Indag Research
Centre (Proprietary report No. 31, submitted to WHO by Solvay Duphar
BV, 'sGraveland, The Netherlands).
Chapman RA, Tu CM, Harris CR, & Harris C (1985) Persistence of
diflubenzuron and Bay Sir 8514 in natural and sterile sandy loam and
organic soils. J Environ Sci Health, B20(5): 489-497.
Chesterman H, Heywood R, Barker MH, Street AE, & Cherry CP (1974)
DU 112307 toxicity in repeated dietary administration to beagle dogs
(Repeated administration for 13 weeks). Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR169/74157, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Christiansen ME & Costlow JD (1980) Persistence of the insect growth
regulator Dimilin in brackish water: A laboratory evaluation using
larvae of an estuarine crab as an indicator. Helgoländer Meeresunters,
33: 327-332.
Christiansen ME & Costlow JD (1982) Ultrastructural study of the
exoskeleton of the estuarine crab Rhithropanopeus harrsii Effect of
the insect growth regulator Dimilin (diflubenzuron) on the formation
of the larval cuticle. Mar Biol, 66: 217-226.
Christiansen ME, Costlow JD, & Monroe RJ (1978) Effects of the insect
growth regulator Dimilin (TH6040) on larval development of two
estuarine crabs. Mar Biol, 50: 29-36.
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981a) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume I. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/294/80185, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Colley JC, Batham P, Heywood R, Street AE, Gopinath C, Cherry CP,
Gibson WA, & Prentice DE (1981b) The effects of dietary administration
of diflubenzuron to male and female HC/CFLP mice for 14 weeks:
Volume II. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR/294/80185, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Colley J, Heywood R, Street AE, & Gopinath C (1984) The effect of
diflubenzuron given by oral administration with the feed on toxicity
and tumour development in male and female HC/CFLP mice. Final report.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. 56645/32/1984, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Collwell AE & Schaefer CH (1980) Diets of Ictalurus nebulosus and
Pomoxis nigromaculatus altered by diflubenzuron. Can J Fish Aquat
Sci 37(4): 632-639.
Cooke M & Ober AG (1980) OV-17-QF-1 Capillary column for
organochlorine pesticide analysis. J Chromatogr, 195: 265-269.
Corley C, Miller RW, & Hill K (1974) Determination of
N-(4-chlorophenyl)-N-(2,6-difluorobenzoyl)-urea in milk by
high-speed liquid chromatography. J AOAC, 57(6): 1269-1271.
Costlow YD (1979) Effect of Dimilin on development of larvae of the
stone crab Menippe mercenaria and the blue crab Callinectes
sapidus In: Vernberg WB, Calabrese A, Thurburg FP, & Vernberg FJ ed.
Marine pollution: Functional responses. New York, London, Academic
Press, pp 355-363.
Crookshank HR, Sowa BA, Kubena L, Holman GM, Smalley HE, & Morison R
(1978) Effect of diflubenzuron (Dimilin) on the hyaluronic acid
concentration in the chicken combs. Poult Sci, 57: 804-806.
Cunningham PA (I976) Effects of Dimilin (TH-6040) on reproduction in
the brine shrimp, Artemia salina Environ Entomol, 5: 701-706.
Cunningham PA (1986) A review of toxicity testing and degradation
studies used to predict the effects of diflubenzuron (Dimilin) in
estuarine crustaceans. Environ Pollut, A40: 63-86.
Cunningham PA & Myers LE (I986) Dynamics of diflubenzuron (Dimilin)
concentrations in water and sediment of a supratidal saltmarsh site
following repetitive aerial applications for mosquito control. Environ
Pollut, A41(1): 63-88.
Cunningham PA & Myers LE (I987) Effects of diflubenzuron (Dimilin) on
survival, molting, and behaviour of juvenile fiddler crabs, Uca
pugilator Arch Environ Contam Toxicol, 16: 745-752.
Danhaus RG & Sieck RF (1976) Rotation crop uptake of 14C-residues
following soil application of Dimilin. Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Danhaus RG, Wargo JP, & Sieck RF (1976) 14C-Dimilin field soil
leaching study. Colorado Springs, Colorado, Analytical Development
Corporation (Unpublished proprietary report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Davies RE & Halliday JC (1974) Acute percutaneous toxicity to rabbits
of DU 112307 technical. Huntingdon, England Huntingdon Research Centre
(Unpublished proprietary report No. 2171/D175/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE & Ligget MP (1973) Irritant effects of DU 112307 technical
on rabbit eye mucosa. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 2170/176D/73, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Davies RE, Elliot PH, Street AE, Heywood R, & Prentice DE (1975)
Effect of repeated applications of DU 112307 to the skin of rabbits
for three weeks. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR200/74851, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
De Bree H, De Lange N, Overmars H, & Post LC (1977) Diflubenzuron:
analysis of metabolites connected with methaemoglobinaemia. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/08/1977).
De Lange N (1979) Diflubenzuron: dermal absorption in the rabbit.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56654/09/1979).
De Lange N & Post LC (1978) Diflubenzuron: intestinal absorption in
the mouse in relation to dosage level. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56654/04/1978).
De Lange N, Overmans H, & Post LC (1974) PH 60-40: excretion of
radioactivity and metabolite patterns in rats following oral
administration. Weesp, The Netherlands, Philips-Duphar BV (Unpublished
proprietary report No. 56654/20/1974).
De Lange N, Overmans H, Willems AGM, & Post LS (1975) Diflubenzuron
(PH 60-40): balance studies in the rat, and identification of urinary
metabolites. Weesp, The Netherlands, Philips Duphar BV (Unpublished
proprietary report No. 56654/22/1975).
De Lange N, Fronik GM, & Post LC (1977) Diflubenzuron: intestinal
absorption in the rat in relation to dosage level. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56654/10/1977).
Deul DH & Jong BJ de (1977) The possible influence of DU 112307 on the
in vivo synthesis of hyaluronic acid in chicken skin. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56635/01/1977).
Di Prima SJ (1976) Determination of diflubenzuron residues in soybean
process fractions (meal, oil, hulls) by gas chromatography. Thompson-
Hayward Chemical Company (Unpublished proprietary report No. AM-13,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Di Prima SJ, Cannizaro RD, Roger JC, & Ferrell CD (1978) Analysis of
diflubenzuron residues in environmental samples by high-pressure
liquid chromatography. J Agric Food Chem, 26(4): 968-971.
Dorough HW (1977) Screening of selected Thompson-Hayward Chemicals for
activity in the Ames Salmonella mutagenicity test. Lexington,
University of Kentucky, Department of Entomology (Unpublished report
No. MTM-91).
Downer DM (1990) Analysis of diflubenzuron in streamwater samples from
little sluice mountain gypsy moth sprayblock. Harrisonburg, Virginia,
US Forest Service, 30 pp (Unpublished proprietary report submitted to
WHO by Duphar BV, Weesp, The Netherlands).
Dunkel VC, Zeiger E, Brusick D, McCoy E, McGregor D, Mortelmans K,
Rosenkranz HS, & Simmon VF (1985) Reproducibility of microbial
mutagenicity assays: II. Testing of carcinogens and noncarcinogens in
Salmonella typhimurium and Escherichia coli Environ Mol Mutagen,
7(Suppl 5): 1-248.
El-Sebae AH, Salem MH, Al-Assar MRS, & Enan EE (1988) In vitro
effect of profenofos, fenvalerate and dimilin on protein and RNA
biosynthesis by rabbit liver and muscle tissues. J Environ Sci Health,
B23(5): 439-451.
Enninga IC (1990) Evaluation of DNA repair inducing ability of
diflubenzuron in a primary culture of rat hepatocytes (with
independent repeat). Report RCC NOTOX BV, 'sHertogenbos, The
Netherlands No. 56645/114/1990. Proprietary report, submitted to WHO
by Solvay Duphar, BV, 'sGraveland, The Netherlands.
FAO/WHO (1982a) Pesticides residues in food - 1981. Report of the
Joint Meeting of the FAO Panel of Experts on Pesticides Residues in
Food and Environment and WHO Expert Group on Pesticides Residues.
Rome, Food and Agriculture Organization of the United Nations (FAO
Plant Production and Protection Paper 37).
FAO/WHO (1982b) Pesticide residues in food - 1981. Evaluations: The
monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO Plant Production and Protection Paper 42).
FAO/WHO (1985a) Pesticide residues in food - 1984. Report of the Joint
Meeting on Pesticide Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 62).
FAO/WHO (1985b) Pesticide residues in food - 1984. Evaluations: The
Monographs. Rome, Food and Agriculture Organization of the United
Nations (FAO, Plant Production and Protection Paper 67).
FAO/WHO (1986a) Pesticide residues in food - 1985. Report of the Joint
Meeting of the Residues in Food and the Environment and a WHO Expert
Group on Pesticides Residues. Rome, Food and Agriculture Organization
of the United Nations (FAO Plant Production and Protection Paper 68).
FAO/WHO (1986b) Pesticide residues in food - 1985. Evaluations:
Part II - Toxicology. Rome, Food and Agriculture Organization of the
United Nations (FAO Plant Production and Protection Paper 72/2).
FAO/WHO (1989) Guide to Codex recommendations concerning pesticide
residues. Part 8: Recommendations for methods of analysis of pesticide
residues, 4th ed. Rome, Codex Committee on Pesticide Residues, Food
and Agriculture Organization of the United Nations.
Farlow JE, Breaud TP, Steelman CD, & Schilling PE (1978) Effect of
the insect growth regulator diflubenzuron on non-target aquatic
populations in a Louisiana intermediate marsh. Environ Entomol,
7: 199-204.
Fischer SA & Hall LW Jr (1992) Environmental concentrations and
aquatic toxicity data on diflubenzuron (Dimilin). Crit Rev Toxicol,
22(1): 45-79.
Forward RB & Costlow JD (1978) Sublethal effects of insect growth
regulators upon crab larval behaviour. Water Air Soil Pollut,
9: 227-238.
Gilbert P, Saint-Ruf G, Poncelet F, & Mercier M (1980) Genetic effects
of chlorinated anilines and azobenzenes on Salmonella typhimurium
Arch Environ Contam Toxicol, 9: 533-541.
Goodman DG (1980a) Histopathologic evaluation of mice administered
diflubenzuron in the diet. Vienna, Virginia, Hazleton Laboratories
America Inc. (Unpublished proprietary report submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Goodman DG (1980b) Histopathologic evaluation of rats administered
diflubenzuron in the diet. Washington, DC, Clement Associates
(Unpublished proprietary report submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Gordon R & Cornect M (1986) Toxicity of the insect growth regulator
diflubenzuron to the rove beetle Aleochara bilineata a parasitoid
and predator of the cabbage maggot Delia radicum Entomol Exp Appl,
42: 179-185.
Goto M (1977a) Crop residue analysis of Dimilin. Japan, Institute
of Pesticide Residues (Unpublished proprietary report
No. K/Resid./004/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Goto M (1977b) Crop residue analysis of Dimilin. Japan, Institute of
Pesticide Residues (Unpublished proprietary report No.
K/Resid./007/1977, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Granett J, Morang S, & Hatch R (1978) Reduced movement of precocious
male Atlantic salmon parr into sublethal Dimilin-G1 and carrier
concentrations. Bull Environ Contam Toxicol, 19(4): 462-464.
Graves WC & Swigert JP (1993) Diflubenzuron: A 96-h flow through acute
toxicity test with the sheepshead minnow ( Cyprinodon variegatus.
Final report. Wildlife International Ltd. USA (Unpublished proprietary
report No. 56835/13/93, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Greenough RJ, Goburdhun R, Hundson R, & MacNaughtan F (1985)
Diflubenzuron. 52 week oral toxicity study in dogs: Volumes 1 and 2
(Project No. 630146,2728). Musselburgh, Scotland, Inveresk Research
International (Unpublished proprietary report No. 56645/32/1985,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Greenough RJ & McDonald P (1986) Diflubenzuron VC 90 acute inhalation
toxicity study in rats (limit test). Musselburgh, Scotland, Inveresk
Research International (Proprietary report No. 56645/41/1986,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Gulka G, Doscher CM, & Watabe N (1980) Toxicity and molt-accelerating
effects of diflubenzuron on the barnacle, Balanus eburneus Bull
Environ Contam Toxicol, 25: 477-481.
Gulka G, Gulka CM, & Watabe N (1982) Histopathological effects of
diflubenzuron on the cirripede crustacean, Balanus eburneus Arch
Environ Contam Toxicol, 11: 11-16.
Gustafson DE & Wargo JP (1976) Fate of Dimilin following application
to soybeans (ADC Project No 222). Colorado Springs, Colorado,
Analytical Development Corporation (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hansen SR & Garton RR (1982a) Ability of standard toxicity test to
predict the effects of the insecticide diflubenzuron on laboratory
stream communities. Can J Fish Aquat Sci, 39: 1273-1288.
Hansen SR & Garton RR (1982b) The effects of diflubenzuron on a
complex laboratory stream community. Arch Environ Contam Toxicol,
11: 1-10.
Hatzios KK & Penner D (1978) The effect of diflubenzuron on soybean
photosynthesis, respiration and leaf ultrastructure. Pestic Biochem
Physiol, 9: 65-69.
Hawkins DR, Jackson AJS, & Roberts NL (1980) Excretion of radio-
activity after oral administration of 3H/14C-diflubenzuron to
cats (PDR/302/80443). Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. 56654/14/1980, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands.
Helling CS (1985) Soil mobility of three Thompson-Hayward pesticides.
Interim report. Beltsville, Maryland, US Department of Agriculture,
Agricultural Research Centre (Unpublished proprietary report No. E-34,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hester PG (1982) Efficacy of diflubenzuron on three estuarine decapods
( Callinectes sp., Palaemonetes pugio and Uca sp.) (Unpublished
document submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hester PG, Olson MA, & Floore TG (1986) Effects of diflubenzuron on
three estuarine decapods, Callinectes sp., Palaemonetes pugio and
Uca pugilator J Fla Anti-Mosq Assoc, 57: 8-14.
Honeycutt R (1993) Indoor occupant exposure study with Dimilin 25 W.
Colorado Springs, Colorado, Analytical Research and Development
Corporation (Report No. 56635/56/1992, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hsu TS & Bartha R (1974) Interaction of pesticide-derived chloroaniline
residues with soil organic matter. Soil Sci, 1974: 444-452.
Huber CM & Collins DL (1987) Final report on the 1987 spray project to
eradicate Gypsy moth from the Tusquitee ranger district on the
Nantahala national forest. Asheville, North Carolina, Field Office,
Forest Pest Management (Unpublished report No. 88-1-4, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Huber CM & Manchester EH (1988) Final report on the eradication of
the Gypsy moth from the Tusquitee ranger district on the Nantahala
national forest. Forest Pest Management (Unpublished report
No. 89-1-7, submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Hunter B, Batham P, Street AE, & Cherry CP (1974) DU 112307
preliminary assessment of the toxicity to male mice in dietary
administration for 6 weeks. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/174/74199, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Hunter B, Batham P, Offer JM, & Prentice DE (1975) Tumorigenicity
study of DU 112307 to mice. Dietary administration for 80 weeks. Final
report. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/170/75685, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Colley J, Street AE, Heywood R, Prentice DE, & Offer J
(1976) Effects of DU 112307 in dietary administration to rats for 104
weeks. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/171/75945, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Hunter B, Jordan J, Heywod R, Street AE, Prentice DE, Wight DG, &
Gibson WA (1979) DU 112307 toxicity to rats in dietary administration
for nine weeks followed by a four week withdrawal period (final
report). Huntingdon, England, Huntingdon Research Centre (Unpublished
report No. PDR/248/77883).
Ivie GW (1977) Metabolism of insect growth regulators in animals.
In: Ivie GW & Dorough HW ed. Fate of pesticides in large animals. New
York, London, Academic Press, pp 111-125.
Ivie GW (1978) Fate of diflubenzuron in cattle and sheep. J Agric Food
Chem, 26(1): 81-89.
Ivie GW, Bull DL, & Veech JA (1980) Fate of diflubenzuron in water.
J Agric Food Chem, 28(2): 330-337.
Jackson GA (1976) Dimilin (TH 6040): Biological impact on pond
organisms. Marion, Alabama, Southeastern Fish Cultural Laboratory
(Unpublished proprietary report NTP-42, submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Jackson GC, Hardy CJ, Simms F, Mullins PA, & Gopinath Ch (1990)
Dimilin 4F acute inhalation toxicity study in rats 4-hour exposure.
Huntingdon, England, Huntingdon Research Centre (Proprietary report
No. 56645/68/1990, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Jagannath DR & Brusick DJ (1977a) Mutagenicity evaluation of
4-chlorophenyl urea, final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/42/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977b) Mutagenicity evaluation of
2,6-difluorobenzoic acid. Final report. Kensington, Maryland, Litton
Bionetics Inc. (Unpublished proprietary report No. 56645/40/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Jagannath DR & Brusick DJ (1977c) Mutagenicity evaluation of
4-chloroanilin. Final report. Kensington, Maryland, Litton Bionetics
Inc. (Unpublished proprietary report No. 56645/41/1977, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Janssen PJM & Pot TE (1987a) Primary irritation study of dimilin SC-48
to the eye of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/46/1987).
Janssen PJM & Pot TE (1987b) Primary irritation study of dimilin SC-48
to the skin of the male rabbit. Weesp, The Netherlands, Solvay Duphar
BV, Department of Toxicology (Proprietary report No. 56645/45/1987).
Janssen PJM & Pot TE (1987c) Acute oral toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/44/1987).
Janssen PJM & Pot TE (1987d) Acute dermal toxicity study with dimilin
SC-48 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/43/1987).
Janssen PJM & Pot TE (1987e) Acute dermal toxicity study with dimilin
WP-25 in rats. Weesp, The Netherlands, Solvay Duphar BV, Department of
Toxicology (Proprietary report No. 56645/58/1987).
Janssen PJM & van Doorn WM (1993a) Acute inhalation toxicity study
with dimilin OF-6 in male and female rats. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/01/1993).
Janssen PJM & van Doorn WM (1993b) Primary irritation study of dimilin
OF-6 to the skin of female rabbits. Weesp, The Netherlands,
Solvay Duphar BV, Department of Toxicology (Proprietary report
No. 56345/30/1993).
Janssen PJM & van Doorn WM (1993c) Primary irritation study of dimilin
OF-6 to the eye of female rabbits. Weesp, The Netherlands, Solvay
Duphar BV, Department of Toxicology (Proprietary report
No. 56345/31/1993).
Jenkins VK, Mayer RT, & Perry RR (1984) Effects of diflubenzuron on
growth of malignant melanoma and skin carcinoma tumors in mice. Invest
New Drugs, 2: 19-27.
Jenkins VK, Perry RR, Ahmer AE, & Ives K (1986) Role of metabolism in
effects of diflubenzuron on growth of B16 melanomas in mice. Invest
New Drugs, 4: 325-335.
Jones AS & Konchenderfer JN (1988) Persistence of diflubenzuron
(Dimilin) in a small eastern watershed and its impact on invertebrates
in a headwater stream. Research Triangle Park, North Carolina,
Southeastern Forest Experiment Station (Unpublished proprietary report
No. 56637/26/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Joustra KD, van Kampen WG, & Walstra P (1989) Metabolism of [14C]
diflubenzuron in citrus fruit (Analytical report). Weesp, The
Netherlands, Duphar BV, Analytical Development Department (Proprietary
report No. 56630/86/1989).
Julin AM & Sanders HO (1978) Toxicity of IGR, diflubenzuron, to
freshwater intervertebrates and fishes. Mosq News, 38: 256-259.
Kavanagh P (1988a) Diflubenzuron oral (gavage) rat teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/11/87)
(Proprietary report No. 56645/68/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kavanagh P (1988b) Diflubenzuron oral (gavage) rabbit teratology limit
study. Ledbury, England, Toxicol Laboratories Ltd. (PHD/12/87)
(Proprietary report No. 56645/79/1987, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Keet CMJF (1977a) The methaemoglobin, sulphaemoglobin and Heinz body
forming properties of DU 112307 after oral administration to male rats
during 8 days. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/15/1977).
Keet CMJF (1977b) The effect of DU 112307 (tech) in male mice after
daily oral administration (14-days) on body weight, methaemoglobin,
sulphaemoglobin and Heinz body formation. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/33/1977).
Keet CMJF (1977c) The methaemoglobin and sulphaemoglobin forming
properties of DU 112307 in male rabbits after prolonged dietary and
dermal administration. Weesp, The Netherlands, Duphar BV (Unpublished
proprietary report No. 56645/02/1977).
Kemp A, van der Heijden CA, & van Eldik A (1973a) Dietary
administration of DU 112307 to male and female rats for three months.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56645/13A/1973).
Kemp A, van der Heijden CA, & van Eldik A (1973b) Appendix III to
report No. 56645/13A/1973 individual data: dietary administration of
DU 112307 to male and female rats for 3 months. Weesp, The
Netherlands, Philips-Duphar BV (Unpublished proprietary report
No. 56645/13B/1973).
Kingsbury P, Sundaram KMS, Holmers KMS, Nott R, & Kreutzweiser D
(1987) Aquatic fate and impact studies with Dimilin. Ottawa, Ontario,
Forest Pest Management Institute (Unpublished report No. 78, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Klenner MF (1990) [A comparative study of the carabid fauna in the
Dimilin-treated and untreated oak stands in Westphalia.] Mitt BBA
(Berlin-Dahlem), 266: 279 (in German).
Koelman-Klaus HJS (1978a) Acute oral toxicity study of
4-chlorophenylurea in male and female rats. Weesp, The Netherlands,
Duphar BV (Unpublished proprietary report No. 56645/09/1978).
Koelman-Klaus HJS (1978b) Acute oral toxicity study of
2,6-difluorobenzoic acid in male and female rats. Weesp, The
Netherlands, Duphar BV (Unpublished proprietary report
No. 56645/25/1978).
Koopman TSM (1977a) Acute oral toxicity study with DU 112307 technical
in mice. 'sGraveland, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/04/1977).
Koopman TSM (1977b) Acute oral toxicity study with DU 112307 WP 25%
in mice and rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/03/1977).
Koopman TSM (1977c) Acute dermal toxicity study with DU 112307
technical in rats. 'sGraveland, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/07/1977).
Koopman TSM (1980a) Primary irritation of Dimilin ODC-45 to the rabbit
eye. 'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/02/1980).
Koopman TSM (1980b) Acute dermal toxicity study of dimilin ODC-45 in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/04/1980).
Koopman TSM (1980c) Primary irritation of Dimilin ODC-45 to the rabbit
skin. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/03/1980).
Koopman TSM (1984a) Acute dermal toxicity study with diflubenzuron
VC-90 in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/31/1984).
Koopman TSM (1984b) Acute oral toxicity study with diflubenzuron VC-90
in rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/30/1984).
Koopman TSM (1984c) Primary irritation study of diflubenzuron VC-90 to
the rabbit eye. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/29/1984).
Koopman TSM (1984d) Primary irritation study of diflubenzuron VC-90 to
the rabbit skin. 'sGraveland, The Netherlands, Duphar BV, Department
of Toxicology (Unpublished proprietary report No. 56645/44/1984).
Koopman TSM (1985a) Primary irritation study of dimilin 2F to the
skin of the male rabbit. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report
No. 56645/93/1985).
Koopman TSM (1985b) Primary irritation study of dimilin 2F to
the eye of the male rabbit. 'sGraveland, The Netherlands,
Duphar BV, Department of Toxicology (Unpublished proprietary report
No. 56645/91/1985).
Koopman TSM (1985c) Acute dermal toxicity study with dimilin 2F in
rats. 'sGraveland, The Netherlands, Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/95/1985).
Koopman TSM (1985d) Acute oral toxicity study with dimilin 2F in rats.
'sGraveland, The Netherlands, Duphar BV, Department of Toxicology
(Unpublished proprietary report No. 56645/94/1985).
Koopman TSM & Jongeling AJ (1979) Acute oral toxicity study with
Dimilin ODC 45% in rats. 'sGraveland, The Netherlands, Duphar BV,
Department of Toxicology (Unpublished proprietary report No.
56645/38/1979).
Koopman TSM & Pot TE (1986) Acute oral toxicity study with
diflubenzuron VC-90% in mice. 'sGraveland, The Netherlands, Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/39/1986).
Koorn JC (1990) Study to examine the possible mutagenic activity of
diflubenzuron in the Ames Salmonella microsome assay. 'sGraveland,
The Netherlands, Duphar BV, Department of Toxicology (Proprietary
report No. 56645/74/1990).
Kramer HT (1990) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in an apple orchard in Phelps (New York). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-271,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1991) Determination of diflubenzuron and the metabolites
4-chlorophenyl urea and 2,6-difluorobenzoic acid in soil from a
dissipation trial in a citrus orchard in Oviedo (Florida). Madison,
Wisconsin, Hazleton Laboratories (Proprietary report No. HLA-6012-270,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992a) Field dissipation study with diflubenzuron
insecticide applied for control of insects on cotton (a bare soil
application in Arkansas). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-110, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kramer HT (1992b) Field dissipation study with diflubenzuron
insecticide applied for control of insects on soybeans (a bare soil
application in Louisiana). Madison, Wisconsin, Hazleton Laboratories
(Proprietary report No. HLA-6248-116, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kubena LF (1982) The influence of diflubenzuron on several
reproductive characteristics in male and female layer-breed chickens.
Poult Sci, 61: 268-271.
Kuijpers LAM (1988) The acute toxicity of diflubenzuron to Daphnia
magna Weesp, The Netherlands, Duphar BV (Unpublished proprietary
report No. 56635/26/1988).
Kurczewski F, Wang C, Grimble D, Smith R, & Brezner J (1975)
Environmental impact of dimilin. A final report: Effects of dimilin
upon microorganisms in leaf litter and forest soil. Syracuse, New
York, State University of New York, pp 28-43.
Kynoch SR & Elliot PH (1978a) Screening test for delayed contact
hypersensitivity with Dimilin WP 25%. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 0911/D258/78, submitted to WHO
by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Elliot PH (1978b) Screening test for delayed contact
hypersensitivity with dummy formulation for dimilin WP 25% in the
albino guinea-pig. Huntingdon, England, Huntingdon Research Centre
(Proprietary report No. 9112/D258/78, submitted to WHO by Solvay
Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin 2F. Huntingdon, England, Huntingdon Research
Centre (Proprietary report No. 56645/13/1987, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Parcell BI (1987) Delayed contact hypersensitivity in the
guinea-pig with Dimilin SC-48. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 56645/72/1987, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Kynoch SR & Smith PA (1986) Delayed contact hypersensitivity in the
guinea-pig with diflubenzuron VC-90. Huntingdon, England, Huntingdon
Research Centre (Proprietary report No. 86558D/PDR 432SS, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Lauren DR, Agnew MP, & Henzell RF (1984) Field life of the insect
growth regulator diflubenzuron on lucerne. N Z J Agric Res,
27: 425-429.
Lawrence JF & Sundaram KMS (1976) Gas-liquid chromatographic analysis
of N-(4-chlorophenyl-N-2,6-difluorobenzoyl) urea insecticide after
chemical derivatization. J AOAC, 59(4): 938-941.
Lee BM & Scott GI (1989) Acute toxicity of temephos, fenoxycarb,
diflubenzuron and methoprene and Bacillus thuringiensis var.
Israelensis to the mummichug ( Fundulus heteroclitus. Bull Environ
Contam Toxicol, 43: 827-832.
Maas W, Van Hes R, Grosscurt AC, & Deul DH (1980) [Benzoylphenylurea
insecticides.] In: Wegler R ed. [Chemistry of plant protection
chemicals and pesticides.] Berlin, Heidelberg, Springer Verlag, vol 6,
pp 432-470 (in German).
McAlonan WG (1976) Effects of two insect growth regulators on some
selected salt-marsh non-target organisms. University of Delaware, USA
(Thesis) (NTP-53).
Machado J, Coimbra J, Castilho F, & Sa C (1990) Effects of
diflubenzuron on shell formation of the freshwater clam, Anodonta
cygnea Arch Environ Contam Toxicol, 19: 35-39.
McGregor JT, Gould DH, Mitchell AD, & Sterling GP (1979) Mutagenicity
tests of diflubenzuron in the micronucleus test in mice, the L5178Y
mouse lymphoma forward mutation assay, and the Ames Salmonella
reverse mutation test. Mutat Res, 66: 45-53.
McKague AB & Pridmore RB (1978) Toxicity of Altosid and Dimilin to
juvenile rainbow trout and coho salmon. Bull Environ Contam Toxicol,
20: 167-169.
Madder DJ (1977) The disappearance from, efficacy in and effect on
non-target organisms of diflubenzuron, methoprene and chlorpyrifos in
a lentic ecosystem. Canada, University of Manitoba, Faculty of
Graduate Studies, 139 pp (Thesis) (NTP-71).
Madder DJ & Lockhart WL (1978) A preliminary study of the effects of
diflubenzuron and methoprene on rainbow trout ( Salmo gairdneri. Bull
Environ Contam Toxicol, 20: 66-70.
Madder DJ & Lockhart WL (1980) Studies on the dissipation of
diflubenzuron and methoprene from shallow prairie pools. Can Entomol,
112: 173-177.
Mansager ER, Still GC, & Frear DS (1979) Fate of 14C diflubenzuron on
cotton and in soil. Pestic Biochem Physiol, 12: 172-182.
Marshall BF & Hieb BF (1973) 96-h LC50 Salmo gairdneri (n.m.
Oncorhynchus mykiss, Lepomis machrochirus and Fundulus heteroclitus
Marine Research Institute (Unpublished study submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Martinez-Toledo MV, Rubia T, De La Moreno J, & Gonzalez-Lopez J
(1988a) Effect of diflubenzuron on azotobacter nitrogen fixation in
soil. Chemosphere, 17(4): 829-834.
Martinez-Toledo MV, Gonzalez-Lopez J, Rubia T, De La Moreno J, &
Ramos-Cormenzana A (1988b) Diflubenzuron and the acetylene-reduction
activity of Azotobacter vinelandii Soil Biol Biochem,
20(2): 255-256.
Matheson DW & Brusick DJ (1977a) Evaluation of 4-chlorophenylurea
in vitromalignant transformation in BALB/3T3 cells. Kensington,
Maryland, Litton Bionetics Inc. (Unpublished proprietary report
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brucisk DJ (1977b) Evaluation of 2,6-diflurobenzoic acid
in vitromalignant transformation in BALB/3T3 cells. Final report.
Kensington, Maryland, Litton Bionetics Inc. (Unpublished proprietary
report No. 56645/10/1978, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Matheson DW & Brusick DJ (1978a) Mutagenicity evaluation of
2,6-difluorobenzoic acid in unscheduled DNA synthesis in the human WI-
38 cells assay. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/16/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978b) Mutagenicity evaluation of
4-chlorophenylurea in unscheduled DNA synthesis in the human WI-38
cells assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/24/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matheson DW & Brusick DJ (1978c) Mutagenicity evaluation of
4-chloroanilin in the in vitro transformation of BALB/3T3 cells
assay. Final report. Kensington, Maryland, Litton Bionetics Inc.
(Unpublished proprietary report No. 56645/23/1978, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Matta JF (1976) The effect of dimilin on non-target organisms in a
marsh community. Norfolk, Virginia, Environmental Consultants (Report
prepared for Thompson-Hayward Chemical Company) (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Mayer RT, Netter KJ, Leising HB, & Schachtschabel DD (1994) Inhibition
of the uptake of nucleosides in cultured Harding-Passey melanoma cells
by diflubenzuron. Toxicology, 30: 1-6.
Metcalf RL, Lu PY, & Browlus S (1975) Degradation and environmental
fate of 1-(2,6-difluorobenzoyl)-3-(4-chlorophenyl) urea. J Agric Food
Chem, 23(3): 359-364.
Mian LS & Mulla MS (1983) Persistence of three IGRs in stored wheat.
J Econ Entomol, 76(3): 622-625.
Miller RW, Corley C, Oehler DD, & Pickens LG (1976a) Feeding TH 6040
to cattle: residues in tissues and milk and breakdown in manure.
J Agric Food Chem, 24(3): 687-688.
Miller RW, Corley C, & Shufeld SR (1976b) Effects of feeding TH 6040
to two breeds of chickens. J Econ Entomol, 69(6): 741-743.
Miller RW, Cecil HC, Carey AM, Corley C, & Kiddy CA (1979) Effects of
feeding diflubenzuron to young male Holstein cattle. Bull Environ
Contam Toxicol, 23: 482-486.
Miura T (1974) Biological activity of TH 6040 against organisms
associated with mosquito breeding habitats in irrigated pastures.
Fresno, California, University of California, Mosquito Control
Research Laboratory (Unpublished report submitted to WHO by Solvay
Duphar BV, Weesp, The Netherlands).
Miura T & Takahashi RM (1974a) Insect developmental inhibitors effects
of candidate mosquito control agents on nontarget aquatic organisms.
Environ Entomol, 3(4): 631-636.
Miura T & Takahashi RM (1974b) Toxicity of TH 6040 to freshwater
crustacea and the use of a tolerance index as a method of expressing
side effects on nontargets. Proceedings of the Forty-Second Annual
Conference of the California Mosquito and Vector Control Association,
pp 177-180.
Moreale A & Van Bladel R (1976) Influence of soil properties on
adsorption of pesticide-derived aniline and p-chloroaniline. J Soil
Sci, 27: 48-57.
Moriya M, Ohta T, Watanabe K, Miyazawa T, Kato K, & Shirasu Y (1983)
Further mutagenicity studies on pesticides in bacterial reversion
assay systems. Mutat Res, 116: 185-216.
Mortelmans K, Haworth S, Lawlor T, Speck W, Tainer B, & Zeiger E
(1986) Salmonella mutagenicity tests: II. Results from the testing
of 270 chemicals. Environ Mutagen, 8(Suppl 7): 1-119.
Mulla MS, Majori G, & Darwazeh HA (1975) Effects of the insect growth
regulator Dimilin (TH-6040) on mosquitoes and some nontarget
organisms. Mosq News, 35: 211-216.
Mutanen RM, Siltanen HT, Kuukka VP, Annila EA, & Varama MMO (1988)
Residues of diflubenzuron and two of its metabolites in a forest
ecosystem after control of the pine looper moth, Bupalus
piniarius. L Pestic Sci, 23: 131-140.
Nakayama K (1977a) Crop residue analysis of Dimilin (apple).
Japan, Institute of Pesticides (Unpublished proprietary report
No. K/Resid/003/1977, submitted to WHO by Solvay Duphar BV, Weesp,
The Netherlands).
Nakayama K (1977b) Crop residue analysis of Dimilin. Japan, Institute
of Pesticides (Unpublished proprietary report No. K/Resid/006/1977,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Nation JL, Robinson FA, Ju SJ, & Bolten AB (1986) Influence upon honey
bees of chronic exposure to very low levels of selected insecticides
in their diet. J Agric Res, 25(3): 170-177.
Nebeker AV, McKinney P, & Cairns MA (1983) Acute and chronic effects
of diflubenzuron (Dimilin) on freshwater fish and invertebrates.
Environ Toxicol Chem, 2: 329-336.
Nigg HN (1989) Metabolism of 14C-diflubenzuron in citrus fruits.
Field operations report. Lake Alfred, Florida, Citrus Research and
Education Center (Proprietary report No. 56635/13/1989, submitted to
WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Nigg HN, Canizzaro RD, & Stamper JH (1986) Diflubenzuron surface
residues in Florida citrus. Bull Environ Contam Toxicol,
36(6): 833-838.
Nimmo WB & de Wilde PC (1974) Fate of diflubenzuron on leaves of corn,
soybean, cabbage and apples. 'sGraveland, The Netherlands, Philips-
Duphar BV, Crop Protection Division (Unpublished proprietary report
No. 56635/16A/1974).
Nimmo WB & de Wilde PC (1975a) Degradation of diflubenzuron in soil
and natural water. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/37/1975).
Nimmo WB & de Wilde PC (1975b) Degradation of diflubenzuron in sterile
water at pH 5, 7, 9 and 12. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/32/1975).
Nimmo WB & de Wilde PC (1976a) Uptake of diflubenzuron treated soil by
soybean, maize and potato. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/04/I976).
Nimmo WB & de Wilde PC (1976b) Uptake of diflubenzuron and its
metabolites from the soil by rice and wheat. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/08/1976).
Nimmo WB & de Wilde PC (1977a) Degradation of diflubenzuron in
mushroom growth medium and uptake of its metabolites in the mushrooms.
Weesp, The Netherlands, Philips-Duphar BV (Unpublished proprietary
report No. 56635/21A/1977).
Nimmo WB & de Wilde PC (1977b) Degradation of diflubenzuron in calf
and chicken manure. Weesp, The Netherlands, Philips-Duphar BV
(Unpublished proprietary report No. 56635/27/1977).
Nimmo WB, Willems AGM, & de Wilde PC (1978) Fate of diflubenzuron
applied to leaves and fruits of apple trees. Weesp, The Netherlands,
Philips-Duphar BV (Unpublished proprietary report No. 56635/33/1978).
Nimmo WB, Hamaker TL, More JC, & Wood RA (1980) Acute and chronic
effects of Dimilin on survival and reproduction of Misidopsis bahia
In: Eaton JG, Parrish PR, & Hendricks AC ed. Aquatic toxicology.
Philadelphia, Pennsylvania, American Society for Testing and
Materials, pp 366-376 (Special Technical Publication 707).
Nimmo WB, Wilde PC, & Verloop A (1984) The degradation of
diflubenzuron and its chief metabolites in soils. Part I: Hydrolytic
cleavage of diflubenzuron. Pestic Sci, 15: 574-585.
Nimmo WB, Willems AGM, Joustra KD, & Verloop A (1986) The degradation
of diflubenzuron and its chief metabolites in soils. Part II: Fate of
4-chlorophenylurea. Pestic Sci, 17: 403-411.
Nimmo WB, Joustra KD, & Willems AGM (1990) The degradation of
diflubenzuron and its chief metabolites in soils. Part III: Fate of
2,6-difluorobenzoic acid. Pestic Sci, 29: 39-45.
Offringa OR (1977) Addendum report to the chronic studies with
DU 112307: (A) Dietary administration to rats for 104 weeks - (B)
Dietary administration to mice for 80 weeks. Weesp, The Netherlands,
Philips-Duphar BV, Department of Toxicology (Report No. 56645/
16/1977).
Opdycke JC (1976) Metabolism and fate of diflubenzuron in chickens and
swine. University of Maryland, Faculty of the Graduate School
(Thesis).
Opdycke JC, Miller RW, & Menzer RE (1982a) Metabolism and fate of
diflubenzuron in swine. J Agric Food Chem, 30(6): 1223-1227.
Opdycke JC, Miller RW, & Menzer RE (1982b) In vivo and liver
microsomal metabolism of diflubenzuron by two breeds of chickens.
J Agric Food Chem, 30(6):1227-1233.
Palmer AK & Hill PA (1975a) Effect of DU 112307 on reproductive
function of multiple generations in the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/173/75954, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK & Hill PA (1975b) Effect of DU 112307 on pregnancy of the
rat. Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR/192/74978, submitted to WHO by Solvay-
Duphar, B.V., Weesp, The Netherlands).
Palmer AK & Hill PA (1975c) Effect of DU 112307 on pregnancy of the
New Zealand white rabbit. Huntingdon, England, Huntingdon Research
Centre (Unpublished proprietary report No. PDR/193/74937, submitted to
WHO by Solvay-Duphar BV, Weesp, The Netherlands).
Palmer AK, Allen PA, Street EA, & Prentice DG (1977) Preliminary
assessment of the effect of DU 112307 on the rat. Huntingdon, England,
Huntingdon Research Centre (Unpublished proprietary report
No. PDR/243/77208, submitted to WHO by Solvay-Duphar BV, Weesp, The
Netherlands).
Palmer AK, Allen PA, Heywood R, Street EA, Gibson WA, Offer JM, &
Jolly DW (1978) Effect of dietary administration of DU 112307 on
reproductive function of one generation in the rat. Huntingdon,
England, Huntingdon Research Centre (Report No. PDR 244/78653). 1978.
Unpublished proprietary report, submitted to WHO by Solvay-Duphar,
B.V., Weesp, The Netherlands.
Prasad I (1970) Mutagenic effects of the herbicide 3',4'-dichloro-
propionalide and its degradation products. Can J Microbiol,
16: 369-372.
Prinsen MK (1988a) Primary dermal irritation/corrosion study with
dimilin 4F in albino rabbits. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
93/1988, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1988b) Primary eye irritation study with dimilin 4F in
albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/92/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Prinsen MK (1989a) Acute dermal irritation/corrosion study with
dimilin SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO
Toxicology and Nutrition Institute (Proprietary report No. 56645/
150/1989, submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Prinsen MK (1989b) Acute eye irritation/corrosion study with dimilin
SC 15% in albino rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/151/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1989c) Sensitization study with dimilin 4F in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/23/1989, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1990) Acute dermal irritation/corrosion study with dimilin
ODC-45 in albino rabbits. Zeist, The Netherlands, TNO-CIVO Toxicology
and Nutrition Institute (Unpublished proprietary report
No. 56645/99/1990, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Prinsen MK (1992) Sensitization study with diflubenzuron technical in
guinea-pigs. The Netherlands, TNO-CIVO Toxicology and Nutrition
Institute (Proprietary report No. 56645/26/1992, submitted to WHO by
Solvay Duphar BV, 'sGraveland, The Netherlands).
Prinsen MK (1993) Sensitization study with Dimilin OF-6 in guinea-pigs
(maximization test). Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56345/03/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Pritchard PH & Bourquin AW (1981) Fate of Dimilin in laboratory
estuarine ecosystems. Washington, DC, US Environmental Protection
Agency, Environmental Research Laboratory (Unpublished report).
Quarles JM, Norman JO, & Kubena LF (1980) Absence of transformation by
diflubenzuron in host mediated transplacental carcinogenassay. Bull
Environ Contam Toxicol, 25: 252-256.
Rabenort B, de Wilde PC, de Boer FG, Korver PK, Di Prima SJ, &
Cannizzaro RD (1978) Diflubenzuron. In: Analytical methods for
pesticides and plant growth regulators. New York, London, Academic
Press, vol 10, pp 57-72.
Richmond ML, Henny CJ, Floyd RL, Mannan RW, Finch DM, & de Weese LR
(1979) Effects of Sevin-4-oil, Dimilin and orthene on forest birds in
Northeastern Oregon. Berkeley, California, Pacific Southwest Forest
and Range Experiment Station (Research Paper PSW-148).
Rodrigues CS (1982) Effects of insecticides including insect growth
regulators on black fly (Diptera: Simuliidae) larvae and associated
nontarget stream invertebrates. Ontario, Canada, University of Guelph
(Thesis).
Rosenkranz HS & Poirier LA (1979) Evaluation of the mutagenicity and
DNA-modifying activity of carcinogens and noncarcinogens in microbial
systems. J Natl Cancer Inst, 62: 873-892.
Ross DB, Prentice DE, Newman AJ, Street AE, Hepworth PL, & Roberts NL
(1977a) DU 112307 13 weeks oral toxicity study in the sheep.
Huntingdon, England, Huntingdon Research Centre (Unpublished
proprietary report No. PDR229/77226, submitted to WHO by Solvay-Duphar
BV, Weesp, The Netherlands).
Ross DB, Street AE, & Roberts NL (1977b) Blood red cell and plasma
cholinesterase value following six weeks inclusion of DU 112307 in the
diet of sheep. Huntingdon, England, Huntingdon Research Centre
(Unpublished proprietary report No. PDR229A/77225, submitted to WHO by
Solvay-Duphar BV, Weesp, The Netherlands).
Sarkar DK (1982) Persistence of dimilin 25 WP in water. Padappai,
Tamil Nadu, India, Coromandel Indag Research Centre (Proprietary
report No. 124, submitted to WHO by Solvay-Duphar BV, 'sGraveland, The
Netherlands).
Schaefer CH & Dupras EF (1976) Factors affecting the stability of
Dimilin in water and the persistence of Dimilin in field waters.
J Agric Food Chem, 24(4): 733-739.
Schaefer CH, Dupras EF, Stewart RJ, Davidson LW, & Colwell AE (1979)
The accumulation and elimination of diflubenzuron by fish. Bull
Environ Contam Toxicol, 21: 249-254.
Schaefer CH, Colwell AE, & Dupras EF (1980) The occurrence of
p-chloroaniline and p-chlorophenylurea from the degradation of
diflubenzuron in water and fish. In: Proceedings and papers of the
Forty-Eighth Annual Conference of the California Mosquito and Vector
Control Association, 20-23 January 1980, pp 84-89.
Schimmel SC, Garnas RL, Patrick JM, & Moore JC (1983) Acute toxicity,
bioconcentration and persistence of AC 222,705, Benthiocarb etc. in
the estuarine environment. J Agric Food Chem, 31(1): 104-113.
Schwartz SL & Borzelleca JF (1981) Effects of diflubenzuron on
methemoglobin, sulfhemoglobin and Heinz body production in cats.
Richmond, Virginia, Toxicology and Pharmacology, Inc. (Unpublished
proprietary report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Seegmiller RE & Booth GM (1976) Teratogenic and biochemical effects of
TH-6040 on chicken embryos. Provo, Utah, Brigham Young University
(Unpublished report submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Serra GE & Joustra KD (1990) Diflubenzuron: Nature of residues on
apples. Greenhouse operations report. Weesp, The Netherlands, Duphar
BV (Proprietary report No. 56630/43/1990).
Seuferer SL, Braymer HD, & Dunn JJ (1979) Metabolism of diflubenzuron
by soil microorganisms and mutagenicity of the metabolites. Pestic
Biochem Physiol, 10: 174-180.
Simmons KE, Minard RD, & Bollag JM (1989) Oxidative co-oligomerization
of guaiacol and 4-chloroaniline. Environ Sci Technol, 23(1): 115-121.
Singh PP & Kaira RL (1989) Determination of diflubenzuron residues by
thin-layer chromatography. Chromatographia, 27(1/2): 53-54.
Sipes JG & Carter DE (1988) Pharmacokinetics of xenobiotics:
p-chloroaniline. Tucson, Arizona, University of Tucson (Unpublished
report No. MRJDN 410882-01, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith JT & Edmunds CP (1985) Environmental monitoring of the 1984
gypsy moth control program in Tennessee, USA. Weesp, The Netherlands,
Duphar BV, 25 pp (Proprietary report No. 56637/27/1985).
Smith KS & Merricks DL (1976a) TH 6040 tissue residue and metabolism
study in dairy cows. Reading, Pennsylvania, Cannon Laboratories, Inc.
(Unpublished proprietary report No. 5E-7372, submitted to WHO by
Solvay Duphar BV, Weesp, The Netherlands).
Smith KS & Merricks DL (1976b) Tissue residue and metabolism study in
poultry. Reading, Pennsylvania, Cannon Laboratories, Inc. (Unpublished
proprietary report No. 5E-7372, submitted to WHO by Solvay Duphar BV,
Weesp, The Netherlands).
Smith S, Willis GH, & McDowell LL (1983) Electron-capture gas
chromatographic determination of diflubenzuron and permethrin in soil
and water. J Agric Food Chem, 31: 610-612.
Smucker RA & Simon SL (1986) Some effects of diflubenzuron on growth
and sporogenesis in Streptomyces spp. Appl Environ Microbiol,
51(1): 25-31.
Snoeij NJ & Buse-Pot TE (1990) Primary irritation study of dimilin WP
25% to the skin of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/09/1991).
Snoeij NJ & Buse-Pot TE (1991) Primary irritation study of dimilin WP
25% to the eye of male rabbits. Weesp, The Netherlands, Duphar BV,
Department of Toxicology (Proprietary report No. 56645/13/1991).
Solvay Duphar (1994) Recommendations for field use (diflubenzuron) -
Guidelines. Weesp, The Netherlands, Solvay Duphar BV.
Spanjers MTh (1988a) Determination of the acute oral toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/85/1988, submitted
to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Spanjers MTh (1988b) Determination of the acute dermal toxicity of
"Dimilin 4F" in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. 56645/84/1988, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Steelman CD, Farlow JE, Breaud TP, & Schilling PE (1975) Effects of
growth regulators on Psorophora columbiae (Dyar and Knab) and non-
target aquatic insect species in rice fields. Mosq News, 35(1): 67-76.
Stevenson JH (1978) The acute toxicity of formulated pesticides to
worker honey bees. ( Apis mellifera L.. Plant Pathol, 27: 38-40.
Stoner A & Wilson WT (1982) Diflubenzuron (Dimilin): effect of long-
term feeding of low dose in sugar-cake or sucrose syrup on honey bees
in standard-size field colonies. Am Bee J, 122: 579-582.
Sundaram KMS (1986) Persistence and degradation of diflubenzuron in
conifer foliage, forest litter and soil, following simulated aerial
application. Sault Ste Marie, Ontario, Canada, Forest Pest Management
Institute (Information report FPM-X-74).
Sundaram KMS (1991) Spray deposit patterns and persistence of
diflubenzuron in some terrestrial components of a forest ecosystem
after application at three volume rates under field and laboratory
conditions. Pestic Sci, 32: 275-293.
Sundaram KMS & Nott R (1989) Mobility of diflubenzuron in two types of
forest soils. J Environ Sci Health, B24(1): 65-86.
Sundaram KMS, Holmes SB, Kreutzweiser DP, Sundaram A, & Kingsbury PD
(1991) Environmental persistence and impact of diflubenzuron in a
forest aquatic environment following aerial application. Arch Environ
Contam Toxicol, 20: 313-324.
Surprenant DC (1988) The chronic toxicity of 14C-diflubenzuron to
Daphnia magna under flow-through conditions. Wareham, Massachusetts,
Springborn Life Sciences, Inc. (Proprietary report No. 56635/22/1988,
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Taalman RDFM & Hoorn AJW (1986) Mutagenicity evaluation of
diflubenzuron technical (Assay No. E-9481). Veenendaal, The
Netherlands, Hazleton Biotechnologies Corporation (Proprietary report
submitted to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
Tasheva M & Hristeva V (1991) Biochemical and hematological changes in
rats following 28 days oral administration of diflubenzuron and
triflumuron. In: Abstracts from the 1991 Eurotox Congress, Maastricht,
The Netherlands, 1-4 September 1991, p 68.
Tasheva M & Hristeva V (1993) Comparative study on the effects of five
benzoylphenylurea insecticides on haematological parameters in rats.
J Appl Toxicol, 13(1): 67-68.
Taylor RE (1973a) Primary skin irritation study TH-6040 technical
(albino rabbit). Lincoln, Nebraska, Harris Laboratories (Proprietary
report submitted to WHO by Solvay Duphar BV, 'sGraveland, The
Netherlands).
Taylor RE (1973b) Primary skin irritation study TH-6040 W25 wettable
powder (albino rabbit). Lincoln, Nebraska, Harris Laboratories
(Proprietary report submitted to WHO by Solvay Duphar BV, 'sGraveland,
The Netherlands).
Tester PA & Costlow JD (1981) Effect of insect growth regulator
Dimilin (TH 6040) on fecundity and egg viability of the marine copepod
Acartia tonsa Mar Ecol Prog Ser, 5: 297-302.
Thompson SG & Swigert JP (1993a) Diflubenzuron: A 14-day toxicity test
with Duckweed ( Lemna gibba G3). Euston, Maryland, Wildlife
International Ltd (Proprietary report No. 56835/22/93, submitted to
WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993b) Diflubenzuron: A 5-day toxicity test
with freshwater alga ( Selenastrum capricornutum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/23/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993c) Diflubenzuron: A 5-day toxicity test
with the freshwater alga ( Anabaena flos-aquae. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/21/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993d) Diflubenzuron: A 5-day toxicity test
with the freshwater diatom ( Navicula pelliculosa. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/24/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson SG & Swigert JP (1993e) Diflubenzuron: A 5-day toxicity test
with the marine diatom ( Skeletonema costatum. Euston, Maryland,
Wildlife International Ltd (Proprietary report No. 56835/25/93,
submitted to WHO by Solvay Duphar BV, Weesp, The Netherlands).
Thompson CZ, Hill LE, Epp JK, & Probst GS (1983) The induction of
bacterial mutation and hepatocyte unscheduled DNA synthesis by
monosubstituted anilines. Environ Mutagen, 5: 803-811.
Thus JLG & van der Laan-Straathof JMTh (1994) Fate of diflubenzuron in
model ditch systems. 'sGraveland, The Netherlands, Solvay Duphar
BV, Environmental Research Department (Proprietary report
No. 56835/55/93).
Thus LG & van der Laan JMT (1993) Nature of residues of diflubenzuron
in soybeans. 'sGraveland, The Netherlands, Solvay Duphar BV,
Environmental Research Department (Proprietary report
No. 56635/57/1992).
Thus JLG & van Dyk NRM (1991) Anaerobic aquatic metabolism of
diflubenzuron -Addendum report. 'sGraveland, The Netherlands, Solvay
Duphar BV (Proprietary report No. 56635/54/91).
Thus JLG, van Dyk NRM, Rompa-van der Veldt CAH, & Walstra P (1991)
Anaerobic aquatic metabolism of diflubenzuron - Addendum report.
'sGraveland, The Netherlands, Solvay Duphar BV (Proprietary report
No. 56635/34/91).
USDA (1985) Gypsy moth suppression and eradication projects: Final
addendum to the final environmental impact statement as supplemented -
1985. Bromall, Pennsylvania, US Department of Agriculture, Forest
Service (Submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
US NCI (1979) Bioassay of p-chloroaniline for possible
carcinogenicity. Bethesda, Maryland, US National Cancer Institute
(Technical Report Series No. 189, NIH Publication No. 79-1745).
US NTP (1989) Toxicology and carcinogenesis studies of para-
chloroaniline hydrochloride in F344/N rats and B6C3F1 mice (gavage
studies). Research Triangle Park, North Carolina, US National
Toxicology Program (Technical Report Series No. 351).
Van Daalen JJ, Meltzer J, Mulder R, & Wellinga K (1972) A selective
insecticide with a novel mode of action. Naturwissenschaften,
59: 312-313.
Van den Berg G (1986) Dissipation of diflubenzuron residues after
application of dimilin WP-25 in a forestry area in North Carolina and
some ecological effects. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division, 11 pp (Proprietary report No. 56637/47/1986).
Van der Laan-Straathof JMTh & Thus JLG (1993) Determination of the
biodegradability of 14C-diflubenzuron in an adapted modified Sturm
test. Weesp, The Netherlands, Solvay-Duphar (Proprietary research
report No. 56835/41/93).
Van der Laan-Straathof JMTh & Thus JLG (1994) Evaporation of
diflubenzuron from bare soil and red kidney bean leaves after
application of dimilin WSE-80. Weesp, The Netherlands, Solvay-Duphar
(Proprietary research report No. 56835/17/94).
Van Eldik A (1973a) Acute toxicity studies with DU 112307 in mice and
rats. Weesp, The Netherlands, Philips-Duphar BV, Department of
Toxicology (Unpublished proprietary report No. 56645/14/1973).
Van Eldik A (1973b) Acute toxicity studies with DU 112307 25% WP in
mice and rats. Weesp, The Netherlands, Philips-Duphar BV, Department
of Toxicology (Proprietary report No. 56645/15A/1973).
Van Eldik A (1974) Acute toxicity studies with DU 112307 in mice after
intraperitoneal administration. Weesp, The Netherlands, Philips-Duphar
BV, Department of Toxicology (Unpublished proprietary report
No. 56645/01/1974).
Van Kampen WG & Joustra KD (1991) Diflubenzuron: residues on apples.
Weesp, The Netherlands, Duphar BV, Analytical Development Department
(Proprietary analytical report No. 56630/46/1990).
Van Rossum A, De Reijke A, & Zeeman J (1984) Diflubenzuron (update).
In: Analytical methods for pesticides and plants growth regulators.
New York, London, Academic Press, vol 13, pp 165-169.
Verhey ME (1991a) Analysis of field dissipation soil samples from
Madera, California for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 019, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verhey ME (1991b) Analysis of field dissipation soil samples from
Woodburn, Oregon, for residues of diflubenzuron, 4-chlorophenylurea
and 2,6-difluorobenzoic acid. Colorado Springs, Colorado,
Analytical Research and Development Corporation (Proprietary report
No. 1085/C 303 60 020, submitted to WHO by Solvay-Duphar BV,
'sGraveland, The Netherlands).
Verloop A & Ferrell CD (1977) Benzoylphenyl ureas - a new group of
larvicides interfering with chitin deposition. In: Plimmer JR ed.
Pesticide chemistry in the 20th century. Washington, DC, American
Chemical Society, pp 237-270 (ACS Symposium Series No. 37).
Walstra P & Joustra KD (1990) Aerobic soil metabolism of diflubenzuron
in sandy loam. 'sGraveland, The Netherlands, Duphar BV, Crop
Protection Division (Proprietary report No. 56635/65/1990).
Wan MTK & Wilson DM (1977) Impact of insect growth regulators on
selected non-target organisms co-existing with mosquito larvae.
Washington, DC, Environmental Protection Service, Pollution Abatement
Branch (Unpublished report No. EPS-5-PR-77-1/NTP81).
Wang CJK (1975) Effects of Dimilin upon microorganisms in leaf litter
and forest soil. In: Evaluation of Dimilin against the Gypsy moth
effects on non-target organisms 1975. Report expanded Gypsy moth
research and applications program, Hamden USA (Unpublished report
No. NTP-18, submitted to WHO by Solvay Duphar BV, Weesp, The
Netherlands).
Weis JS & Ma A (1987) Effects of the pesticide diflubenzuron on larval
horseshoe crabs, Limulus polyphemus Bull Environ Contam Toxicol,
39: 224-228.
Weis JS & Perlmutter J (1987) Burrowing behaviour by the fiddler crab
Uca pugilator: inhibition by insecticide diflubenzuron. Mar Ecol
Prog Ser, 38: 109-113.
Weis JS, Cohen R, & Kwiatkowski JK (1987) Effects of diflubenzuron on
limb regeneration and molting in the fiddler crab, Uca pugilator
Aquat Toxicol, 10(5-6): 279-290.
White WB (1975) Evaluation of Dimilin against the Gypsy moth and
effects on non-target organisms, 1975 (Compiled by the expanded Gypsy
moth research and applications program). Upper Darby, Pennsylvania, US
Department of Agriculture, Forest Service (Unpublished report).
WHO (1994) The WHO recommended classification of pesticides by hazard
and guidelines to classification 1994-1995. Geneva, World Health
Organization, p 64 (WHO/PCS/94.2).
Wie SJ & Hammock BD (1982) Development of enzyme-linked immunosorbent
assays for residue analysis of diflubenzuron and Sir 8541. J Agric
Food Chem, 30: 949-957.
Wie SJ & Hammock BD (1984) Comparison of coating and immunizing
antigen structure on the sensitivity and specificity of
immunoassays for benzoylphenylurea insecticides. J Agric Food Chem,
32(6): 1294-1301.
Willems AGM, Joustra KD, & Thus JLG (1977) Stability of diflubenzuron
in aqueous solution during heat sterilization. 'sGraveland, The
Netherlands, Philips-Duphar BV, Crop Protection Division (Proprietary
report No. 56635/35/1977).
Willems AGM, Overmars H, Scherpenisse P, de Lange N, & Post LC (1980)
Diflubenzuron: intestinal absorption and metabolism in the rat.
Xenobiotica, 10(2): 103-112.
Williams GM, Laspia MF, & Dunkel VC (1982) Reliability of the
hepatocyte primary culture/DNA repair test in testing of coded
carcinogens and noncarcinogens. Mutat Res, 97: 359-370.
Wilson JEH & Costlow JD (1986) Comparative toxicity of two Dimilin
formulations to the grass shrimp Palaemonetes pugio Bull Environ
Contam Toxicol, 36: 858-865.
Wilson JEH & Costlow JD (1987) Acute toxicity of diflubenzuron (DFB)
to various life stages of the grass shrimp, Palaemonetes pugio Water
Air Soil Pollut, 33: 411-417.
Wilson JEH, Forward RB, & Costlow JD (1985) Effects of embryonic
exposure to sublethal concentrations of Dimilin on the photobehavior
of grass shrimp larvae. In: Marine pollution and physiology: Recent
advances. Colombia, South Carolina, University of South Carolina
Press, pp 377-396.
Wimmer MJ, Smith RR, & Jones JP (1991) Analysis of diflubenzuron by
gas chromatography/mass spectrometry using deuterated diflubenzuron as
internal standard. J Agric Food Chem, 39: 280-286.
Worobey BL & Webster GRB (1977) Gas-liquid chromatographic
determination of diflubenzuron (Dimilin) in water as its
trifluoroacetyl derivative. J Assoc Off Anal Chem, 60(1): 213-217.
Worthing CR & Walker SB (1987) The pesticide manual: A world
compendium. Croydon, British Crop Protection Council, pp 4720-4721.
Yasuno M & Satake K (1990) Effects of diflubenzuron and methoprene on
the emergence of insects and their density in an outdoor experimental
stream. Chemosphere, 21(10-11): 1321-1335.
Young MF, Trombetta LD, & Carson S (1986) Effects of diflubenzuron on
the mouse liver. J Appl Toxicol, 6(5): 343-348.
Yu SJ, Robinson FA, & Nation JL (1984) Detoxication capacity in the
honey bee, Apis mellifera L. Pestic Biochem Physiol, 22: 360-368.
Zwart A (1985) Acute inhalation toxicity study of dimilin 2F,
diflubenzuron in rats. Zeist, The Netherlands, TNO-CIVO Toxicology and
Nutrition Institute (Proprietary report No. V85.347/250925, submitted
to WHO by Solvay Duphar BV, 'sGraveland, The Netherlands).
RESUME
1. Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le diflubenzuron appartient au groupe des insecticides dérivés de
la benzoylphénylurée. Son activité insecticide est due à une
interaction avec la synthèse et le dépôt de la chitine. Il forme des
cristaux blancs inodores dont le point de fusion est de 230-232°C.
Il est légèrement soluble dans l'eau (0,2 mg/litre à 20°C)
et pratiquement non volatil. Il est relativement stable en milieu
acide ou neutre mais s'hydrolyse en milieu alcalin.
Le diflubenzuron s'obtient par réaction du 2,6-difluoro-
benzamide sur l'isocyanate de 4-chlorophényle.
Le dosage des résidus de diflubenzuron présents dans l'eau, les
échantillons biologiques,et le sol peut s'effectuer par
chromatographie liquide à haute performance avec détection par UV ou
encore par chromatographie en phase gazeuse avec détection par capture
d'électrons, soit directement sur la molécule initiale, soit sur un
dérivé (libération de 4-chloroaniline et action de l'anhydride
trifluoracétique).
2. Sources d'exposition humaine et environnementale
Le diflubenzuron est un produit de synthèse utilisé en
agriculture, en foresterie et dans les programmes de santé publique
pour détruire les ravageurs et les vecteurs de maladies. Différentes
formulations existent à cet usage. On ne possède pas de
renseignements au sujet de cas d'exposition humaine au diflubenzuron
qui auraient pu se produire.
3. Transport, distribution et transformation dans l'environnement
En général, le diflubenzuron est appliqué directement sur les
végétaux et les eaux à traiter. Le feuillage ne constitue pas une
porte d'entrée dans la plante.
Le diflubenzuron est rapidement adsorbé sur les particules du
sol. Il reste fixé dans les 10 premiers cm du sol sur lequel il est
épandu. Il est peu probable qu'il subisse un lessivage. Dans divers
types de sol, il subit une décomposition aérobie ou anaérobie avec une
demi-vie de quelques jours. La vitesse de décomposition dépend
largement de la taille des particules de diflubenzuron. La principale
voie métabolique (plus de 90%) esst l'hydrolyse qui conduit à la
formation d'acide 2,6-difluorobenzoïque et de 4-chlorophénylurée; ces
deux composés sont dégradés à leur tour, respectivement en 4 et 6
semaines. On n'a pas décelé la présence de 4-chloroaniline libre
dans le sol.
Le diflubenzuron se décompose rapidement dans les eaux neutres ou
alcalines. On constate qu'une fois épandu sur l'eau, il se répartit
rapidement entre celle-ci et les sédiments.Le composé initial et la
4-chlorophénylurée peuvent persister plus de 30 jours dans les
sédiments.
Le diflubenzuron ne s'accumule pas chez les poissons.
4. Concentrations dans l'environnement et exposition humaine
L'utilisation en agriculture, en foresterie ou pour la
démoustication n'entraîne qu'une exposition négligeable de la
population générale par l'intermédiaire de la nourriture ou de l'eau.
5. Cinétique et métabolisme chez les animaux de laboratoire
Chez l'animal de laboratoire, le diflubenzuron est absorbé au
niveau des voies digestives et, à un moindre degré, au niveau cutané.
Chez le rat, il existe un mécanisme d'absorption saturable. Dans ces
conditions, une forte proportion du diflubenzuron administré par voie
orale se retrouve dans les matières fécales. Le diflubenzuron se
répartit largement dans les tissus, mais il ne s'y accumule pas.
Le métabolisme du diflubenzuron a été étudié chez diverses
espèces animales. Chez les mammifères, la principale voie métabolique
comporte une hydroxylation. L'hydrolyse peut se produire au niveau de
l'une quelconque des trois liaisons carbone-azote. Chez le porc et le
poulet, l'hydrolyse s'effectue principalement au niveau du pont
uréido. Chez le rat et la vache, l'hydroxylation constitue la
principale voie métabolique. Chez le mouton, le porc et le poulet,
les principaux métabolites sont le 2,6-difluorobenzamide et la
4-chlorophénylurée; on trouve aussi, en moindre proportion, du
2,6-difluorobenzamide et de la 4-chloroaniline. Chez le rat et les
bovins, 80% des métabolites sont constitués de 2,6-difluoro-3-
hydroxydiflubenzuron, de 4-chloro-2-hydroxydifluorobenzuron et de
4-chloro-3-hydroxydiflubenzuron. Les études de métabolisme indiquent
qu'il ne se forme pratiquement pas de 4-chloroaniline chez le rat et
les bovins.
Chez les chats, les porcs et les bovins, l'élimination
s'effectue principalement par la voie fécale, à hauteur de 70 à 85%.
Chez les ovins, la voie urinaire et la voie fécale ont à peu près la
même importance de ce point de vue. Chez le rat et la souris,
l'excrétion urinaire décroît proportionnellement à l'augmentation de
la dose. Moins de 1% de la dose administrée par la voie orale se
retrouve dans l'air expiré. Le diflubenzuron n'est présent qu'à
l'état de résidus dans le lait.
Il n'existe pas d'étude sur la cinétique et le métabolisme du
diflubenzuron chez l'homme et notamment, sur son degré de
biotransformation en 4-chloroaniline.
6. Effets sur les mammifères de laboratoire et les systèmes
d'épreuve in vitro
Quel que soit le mode d'exposition, le diflubenzuron présente une
faible toxicité aiguë. En se basant sur le fait que sa DL50 aiguë
par voie orale est supérieure à 4640 mg/kg de poids corporel chez le
rat, l'OMS estime qu'il s'agit d'un produit qui ne présente
vraisemblablement pas de risque d'intoxication aiguë en utilisation
normale. Chez ce même animal, la DL50 aiguë par voie percutanée est
supérieure à 10 000 mg/kg de poids corporel et la CL50 dépasse 2,49
mg/litre par la voie respiratoire. Au cours d'une période de deux
semaines pendant laquelle diverses espèces animales avaient reçu du
diflubenzuron en une seule prise et selon divers modes
d'administration, on n'a constaté aucun signe d'intoxication.
Le diflubenzuron n'est pas irritant pour la peau (chez le lapin)
et ne provoque pas non plus de sensibilisation cutanée (chez le
cobaye). Il est légèrement irritant pour la muqueuse oculaire chez le
lapin.
Le diflubenzuron provoque une méthémoglobinémie et une
sulfhémoglobinémie. Une méthémoglobinémie liée à la dose a été mise
en évidence après exposition d'animaux de diverses espèces au
diflubenzuron par la voie orale, percutanée ou respiratoire. Cet
effet constitue le point d'aboutissement toxicologique le plus
sensible chez les animaux de laboratoire. En prenant comme critère la
méthémoglobinémie, la dose sans effet observable est de 2 mg/kg de
poids corporel par jour chez les rats et les chiens et de
2,4 mg/kg de poids corporel par jour chez les souris. Les études de
toxicité à long terme effectuées sur des souris et des rats
ont montré que les modifications imputables au traitement
correspondaient principalement à l'oxydation de l'hémoglobine et à une
altération des hépatocytes.
Des études de cancérogénicité effectuées sur des rats et des
souris à des doses allant jusqu'à 10 000 mg/kg de nourriture, n'ont
pas révélé de modification de l'incidence tumorale qui soit imputable
au traitement. En particulier, on n'a pas observé de néoplasmes au
niveau du mésenchyme splénique ou hépatique lors d'études de
cancérogénicité utilisant de la 4-chloroaniline.
Plusieurs études toxicologiques portant sur la fonction de
reproduction ont été menées sur des rats, des souris, des lapins et
trois espèces d'oiseaux; elles n'ont pas mis en évidence d'effets
pathogènes et le produit ne s'est pas révélé embryotoxique. Les
études de tératogénicité effectuées sur des rats et des lapins se sont
également révélées négatives.
Le diflubenzuron et ses principaux métabolites ont également été
soumis à diverses épreuves de mutagénicité in vivo e in vitro
Ni le diflubenzuron ni ses principaux métabolites n'ont donné de
résultat positif dans ces épreuves.
Le métabolite secondaire, c'est-à-dire la 4-chloroaniline,
a donné des résultats positifs dans plusieurs épreuves de mutagénicité
in vitro portant sur divers points d'aboutissement toxicologiques.
Il est cancérogène pour le rat et la souris. Les tumeurs imputables à
l'administration de 4-chloroaniline se sont révélées bénignes; quant
aux tumeurs malignes observées, il s'agissait de tumeurs du mésenchyme
splénique chez les rats mâles ainsi que d'hémangiomes et
d'hémangiosarcomes spléniques ou hépatiques chez les souris mâles.
7. Effets sur l'homme
On a signalé des cas de méthémoglobinémie chez des travailleurs
exposés de par leur profession et des nouveau-nés exposés par
inadvertance à de la 4-chloroaniline, un métabolite secondaire du
diflubenzuron. Les sujets qui présentent un déficit en NADH-
méthémoglobine-réductase peuvent être particulièrement sensibles à la
4-chloroaniline et par conséquent à une exposition au diflubenzuron.
8. Effets sur les autres êtres vivants au laboratoire et dans leur
milieu naturel
Tous les organismes qui synthétisent la chitine sont sensibles au
diflubenzuron.
A la concentration de 500 mg/kg de terre, les bactéries n'ont pas
souffert d'une exposition au diflubenzuron. Il y a eu une certaine
stimulation de la fixation d'azote. Les bactéries décomposent les
solutions de diflubenzuron dans l'acétone, solvant qu'elles utilisent
comme source de carbone. Une concentration de diflubenzuron de 1
µg/litre a provoqué un accroissement de la biomasse algaire. Aucun
effet nocif n'a été observé à des concentration supérieures à la
limite de solubilité du diflubenzuron. Des champignons placés dans un
courant créé en laboratoire ont été temporairement affectés à la
concentration de 0,1 µg/litre.
Les invertébrés aquatiques présentent des réactions variées au
diflubenzuron. Les mollusques n'y sont pas sensibles, avec une CL50
supérieure à 200 mg/litre. Chez les autres invertébrés, la CL50 peut
aller de 1 à > 1000 µg/litre, ce qui peut refléter la sensibilité de
ces organismes au moment de la mue. On estime que pour la daphnie, la
concentration tolérable maximale en produit toxique est supérieure à
40 ng/litre et inférieure à 93 ng/litre. Comme prévu, les larves
d'éphémères et autres formes pré-imaginales d'insectes divers, sont
très sesnsibles au diflubenzuron. Le traitement des eaux de surface
par le diflubenzuron est donc probablement susceptible de causer une
certaine mortalité parmi les insectes aquatiques.
Lors de traitements expérimentaux effectués sur le terrain et
dans divers écosystèmes, on a constaté que la plupart des organismes
avaient moins souffert que ne le laissaient prévoir les études
toxicologiques en laboratoire. Aucun effet n'a été constaté sur les
organismes aquatiques après traitement de forêts par voie aérienne.
Pour les poissons, la CL50 est supérieure à 150 mg/litre. Les
essais effectués sur le terrain n'ont enregistré aucune mortalité chez
les poissons.
La DL50 par voie orale et par contact est supérieure à 30 µg par
insecte chez l'abeille mellifique. Après épandage de diflubenzuron
par voie aérienne à raison de 350 g/ha, on n'a pas constaté de dommage
parmi les colonies d'abeilles des alentours.
Une étude alimentaire de 5 jours sur des colverts et des
gallinacés du genre colin avec des doses allant jusqu'à 4640 mg/kg de
nourriture, n'a pas révélé de signes de toxicité. Après épandage de
diflubenzuron par voie aérienne sur des forêts à raison de 350 g/ha,
on n'a pas constaté de dommages parmi les oiseaux chanteurs de
l'écosystème forestier.
Après épandage de diflubenzuron à raison de 67 g/ha sur une
forêt, on n'a pas observé de réduction dans l'effectif des populations
de petits mammifères.
RESUMEN
1. Identidad, propiedades físicas y químicas y métodos analíticos
El diflubenzurón pertenece al grupo de los insecticidas
derivados de la benzoilfenilurea. Su acción insecticida se debe
a la interacción con la síntesis y/o deposición de quitina. Forma
cristales blancos inodoros con un punto de fusión de 230-232°C. Es
bastante soluble en agua (0,2 mg/litro a 20°C) y prácticamente
involátil. Es relativamente estable en medios ácidos y neutros, pero
se hidroliza en condiciones alcalinas.
El diflubenzurón se produce haciendo reaccionar 2,6-difluoro-
benzamida con 4-clorofenilisocianato.
Los residuos de diflubenzurón pueden medirse en el agua, en
muestras biológicas y en el suelo mediante cromatografía líquida de
alta resolución con detección de radiación ultravioleta o mediante
cromatografía de gases con detector de captura de electrones para el
análisis de la molécula intacta o tras la derivatización de la
4-cloroanilina liberada con anhídrido trifluoracético.
2. Fuentes de exposición humana y ambiental
El diflubenzurón es un compuesto sintético utilizado en la
agricultura, en la silvicultura y en programas de salud pública para
combatir plagas de insectos y vectores. Para esos usos existen
diferentes formulaciones de diflubenzurón. No se dispone de
información pertinente sobre la exposición humana a este producto.
3. Transporte, distribución y transformación en el medio ambiente
El diflubenzurón suele aplicarse directamente a las plantas y al
agua. No se produce absorción a través de las hojas.
La adsorción del diflubenzurón en el suelo es rápida. El
producto se inmoviliza en la capa superior de 10 cm del suelo al que
se aplica, y las probabilidades de lixiviación son escasas. El
diflubenzurón se degrada en suelos de diversos tipos y orígenes en
condiciones aerobias o anaerobias, con una semivida de pocos días. La
velocidad de degradación depende en gran medida del tamaño de las
partículas de diflubenzurón. La principal ruta metabólica (más del
90%) es la hidrólisis, que produce 2,6-ácido difluorobenzoico y
4-clorofenilurea; estos productos se degradan con semividas del orden
de cuatro y seis semanas, respectivamente. No se ha detectado
4-cloroanilina libre en los suelos.
El diflubenzurón se degrada rápidamente en aguas neutras o
alcalinas. Estudios de aplicación al agua revelan que el
diflubenzurón se concentra rápidamente en el sedimento; el compuesto
de origen y la 4-clorofenilurea pueden persistir en el sedimento por
más de 30 días.
El diflubenzurón no es objeto de bioacumulación en los peces.
4. Niveles ambientales y exposición humana
La exposición de la población general al diflubenzurón por medio
del agua o los alimentos de resultas de su utilización en la
agricultura, contra insectos forestales o en la lucha contra los
mosquitos, es insignificante.
5. Cinética y metabolismo en animales de laboratorio
En animales de experimentación, el diflubenzurón se absorbe en el
tubo digestivo y, en menor medida, a través de la piel. En el tubo
digestivo de la rata existe un mecanismo de absorción saturable, por
lo que una gran proporción del diflubenzurón administrado oralmente
aparece en las heces. El diflubenzurón tiene una amplia distribución
en los tejidos, pero no se acumula.
El destino metabólico del diflubenzurón ha sido estudiado en
diversas especies. La principal vía metabólica en los mamíferos es la
hidroxilación. La hidrólisis del diflubenzurón puede producirse en
cualquiera de los tres enlaces carbono-nitrógeno. En los cerdos y los
pollos, la principal ruta de hidrólisis es el puente ureido. En las
ratas y las vacas, la principal vía metabólica es la hidroxilación.
En las ovejas, los cerdos y los pollos, los metabolitos más
importantes son el 2,6-ácido difluorobenzoico y la 4-clorofenilurea;
los metabolitos secundarios son la 2,6-difluorobenzamida y la
4-cloroanilina. En las ratas y el ganado vacuno, el 80% de los
metabolitos está constituido por 2,6-difluoro-3-hidroxidiflubenzurón,
4-cloro-2-hidroxi-diflubenzurón y 4-cloro-3-hidroxidiflubenzurón. Los
estudios metabólicos indican que en las ratas o el ganado vacuno se
forman cantidades mínimas o nulas de 4-cloroanilina.
La principal ruta de eliminación es a través de las heces, con
porcentajes de entre el 70 y el 85% en los gatos, los cerdos y el
ganado vacuno. En el ganado ovino, la eliminación se distribuye
aproximadamente por igual entre la orina y las heces. En las ratas y
ratones, la excreción urinaria disminuye proporcionalmente al aumento
de la dosis. Menos del 1% de una dosis oral se recupera en el aire
exhalado. En la leche sólo se han hallado residuos ínfimos.
No se dispone de ningún estudio humano de la cinética y el
metabolismo del diflubenzurón, incluido el alcance de la
biotransformación en 4-cloroanilina.
6. Efectos en mamíferos de laboratorio y en sistemas de pruebas
in vitro
El diflubenzurón tiene una toxicidad aguda baja por cualquier vía
de exposición. La OMS lo ha clasificado como producto con pocas
probabilidades de presentar un riesgo agudo en el uso normal, sobre la
base de una DL50 aguda por vía oral de más de 4640 mg/kg de peso
corporal en las ratas. La DL50 aguda por vía cutánea en las ratas es
superior a 10 000 mg/kg de peso corporal, mientras que la CL50 aguda
por inhalación en las ratas excede de 2,49 mg/litro. No se han
observado signos de intoxicación en los 14 días siguientes a una
administración única de diflubenzurón por diversas rutas a una
variedad de especies animales.
El diflubenzurón no provoca irritación cutánea (en el conejo) ni
sensibilización de la piel (en el cobayo). Produce una ligera
irritación a los ojos en el conejo.
El diflubenzurón causa metahemoglobinemia y sulfohemoglobinemia.
Tras la exposición oral, cutánea o por inhalación de diversas especies
al diflubenzurón se ha demostrado la presencia de metahemoglobinemia
dosisdependiente. Este efecto es la variable de evaluación
toxicológica más sensible en los animales de experimentación. El NOEL
basado en la formación de metahemoglobina es de 2 mg/kg de peso
corporal por día en las ratas y los perros, y de 2,4 mg/kg de peso
corporal por día en los ratones. En estudios de toxicidad a largo
plazo realizados con ratones y ratas, los cambios relacionados con el
tratamiento se han asociado principalmente a la oxidación de la
hemoglobina o a alteraciones de los hepatocitos.
En estudios de la carcinogenicidad en ratones y ratas con niveles
de hasta 10 000 mg/kg en la alimentación, no se observaron efectos
relacionados con el tratamiento en la incidencia de tumores.
Específicamente, no se registraron neoplasias mesenquimatosas del bazo
o el hígado como las observadas en los estudios de carcinogenicidad
con 4-cloroanilina.
En varios estudios de la toxicidad reproductiva en ratas,
ratones, conejos y tres especies aviarias no se observó ningún efecto
en la reproducción, ni tampoco embriotoxicidad. Los estudios de
teratogenicidad en ratas y conejos no revelaron ningún efecto
teratogénico.
El diflubenzurón y sus principales metabolitos han sido sometidos
a una serie de ensayos de mutagenicidad in vitro e in vivo Ni el
diflubenzurón ni sus principales metabolitos tienen efecto mutagénico.
El metabolito secundario 4-cloroanilina ha dado un resultado
positivo en varios ensayos de mutagenicidad in vitro con diversas
variables de valoración. Es carcinógeno en las ratas y los ratones.
Las lesiones neoplásicas relacionadas con la administración de
4-cloroanilina fueron tumores mesenquimatosos benignos y malignos en
el bazo de ratas macho, y hemangiomas y hemangiosarcomas,
principalmente en el bazo y el hígado de ratones macho.
7. Efectos en el ser humano
Se ha notificado que el metabolito 4-cloroanilina del
diflubenzurón ha causado metahemoglobinemia en trabajadores sometidos
a exposición y en neonatos expuestos por inadvertencia. Algunas
personas con carencia de NADH metahemoglobina reductasa pueden ser
particularmente sensibles a la 4-cloroanilina y, por lo tanto, a la
exposición al diflubenzurón.
8. Efectos sobre otros organismos en el laboratorio y en el terreno
Todos los organismos que sintetizan quitina presentan sensibilidad
al diflubenzurón.
Las bacterias no resultaron afectadas por el diflubenzurón a
concentraciones de 500 mg/kg de suelo; se observó cierta estimulación
de la fijación de nitrógeno. Las soluciones de diflubenzurón-acetona
se degradaron; la acetona se utilizó como fuente de carbono. La
biomasa de algas aumentó a una concentración de diflubenzurón de
1 µg/litro. No se observaron efectos adversos a concentraciones
superiores al límite de solubilidad del diflubenzurón. Los hongos
resultaron temporalmente afectados a 0,1 µg/litro en condiciones de
laboratorio.
Los invertebrados acuáticos presentan respuestas variables al
diflubenzurón. Los moluscos son insensibles, con una CL50 superior a
200 mg/litro. Los valores de la CL50 de otros invertebrados van
desde 1 hasta más de 1000 µg/litro, en función de los efectos del
compuesto en las fases juvenil y de muda. Para Daphnia se ha
estimado una MATC de > 40 y < 93 ng/litro; como era de prever, las
larvas de mosca de mayo y otros insectos acuáticos juveniles son
sumamente sensibles. El rociamiento de masas de agua matará
probablemente algunos invertebrados acuáticos.
En los ecosistemas y experimentos de campo en que se aplicó
diflubenzurón directamente al agua, los efectos en la mayoría de los
grupos taxonómicos fueron menos graves de lo previsto a partir de las
pruebas de laboratorio sobre efectos agudos. No se han observado
efectos en los organismos acuáticos después de aplicaciones aéreas a
los bosques.
Los valores de la CL50 para los peces son de > 150 mg/litro.
En los experimentos prácticos no se ha registrado nunca la muerte de
peces.
La DL50 oral y por contacto en la abeja de miel es superior a
30 µg/individuo. Las colonias de abejas no resultaron afectadas tras
la aplicación aérea de 350 g de diflubenzurón/hectárea.
Un estudio de alimentación de cinco días de duración en patos
silvestres y codornices con niveles de hasta 4640 mg/kg de pienso no
reveló ningún signo observable de toxicidad. Las pequeñas aves
canoras del ecosistema forestal no resultaron afectadas por la
aplicación aérea de diflubenzurón a razón de 350 g/hectárea.
Las especies mamíferas pequeñas no sufrieron mermas de las
poblaciones tras la aplicación de diflubenzurón en un bosque a razón
de 67 g/hectárea.
