
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 148
BENOMYL
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr L.W. Hershberger and
Dr G.T. Arce, E.I. Du Pont de Nemours and
Company, Wilmington, Delaware, USA
World Health Orgnization
Geneva, 1993
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Benomyl.
(Environmental health criteria ; 148)
1.Benomyl - adverse effects 2.Benomyl - toxicity
3.Environmental exposure I.Series
ISBN 92 4 157148 9 (LC Classification: SB 951.3)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1993
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR BENOMYL
1. SUMMARY AND CONCLUSIONS
1.1. Summary
1.1.1. Identity, physical and chemical properties, and
analytical methods
1.1.2. Sources of human and environmental exposure
1.1.3. Environmental transport, distribution and
transformation
1.1.4. Environmental levels and human exposure
1.1.5. Kinetics and metabolism
1.1.6. Effects on laboratory mammals; in vitro test
systems
1.1.7. Effects on humans
1.1.8. Effects on other organisms in the laboratory and
field
1.2. Conclusions
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1. Chemical identity
2.1.1. Primary constituent
2.1.2. Technical product
2.2. Physical and chemical properties
2.3. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural occurrence
3.2. Anthropogenic sources
3.2.1. Uses
3.2.2. Worldwide sales
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1. Transport and distribution between media
4.1.1. Air
4.1.2. Water
4.1.3. Soil
4.1.4. Leaching
4.1.5. Crop uptake
4.2. Transformation
4.2.1. Biodegradation
4.2.1.1 Water
4.2.1.2 Soil
4.2.1.3 Crops
4.2.2. Abiotic degradation
4.2.3. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Air, water and soil
5.1.2. Food and feed
5.1.3. Terrestrial and aquatic organisms
5.2. General population exposure
5.2.1. USA
5.2.2. Sweden
5.2.3. Maximum residue limits
5.3. Occupational exposure during manufacture, formulation or
use
5.3.1. Use
6. KINETICS AND METABOLISM
6.1. Absorption
6.2. Distribution and accumulation
6.3. Metabolic transformation
6.4. Elimination and excretion
6.5. Reaction with body components
7. EFFECTS ON LABORATORY MAMMALS; IN VITRO TEST SYSTEMS
7.1. Single exposure
7.2. Short-term exposure
7.2.1. Gavage
7.2.2. Feeding
7.2.2.1 Rat
7.2.2.2 Dog
7.2.3. Dermal
7.2.4. Inhalation
7.3. Skin and eye irritation; sensitization
7.3.1. Dermal
7.3.2. Eye
7.3.3. Sensitization
7.4. Long-term exposure
7.4.1. Rat
7.4.2. Mouse
7.5. Reproduction, embryotoxicity and teratogenicity
7.5.1. Reproduction
7.5.1.1 Rat feeding studies
7.5.1.2 Rat gavage studies
7.5.1.3 Dog inhalation studies
7.5.2. Teratogenicity and embryotoxicity
7.5.2.1 Mouse gavage studies
7.5.2.2 Rat gavage studies
7.5.2.3 Rat feeding studies
7.5.2.4 Rabbit feeding studies
7.6. Mutagenicity and related end-points
7.7. Carcinogenicity
7.7.1. Rat
7.7.2. Mouse
7.8. Special studies
7.8.1. Neurotoxicity
7.8.2. Effects in tissue culture
7.9. Factors modifying toxicity; toxicity of metabolites
7.10. Mechanisms of toxicity - mode of action
8. EFFECTS ON HUMANS
8.1. General population exposure
8.2. Occupational exposure
8.2.1. Acute toxicity
8.2.2. Effects of short- and long-term exposure
9. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
9.1. Microorganisms
9.2. Aquatic organisms
9.3. Terrestrial organisms
9.4. Population and ecosystem effects
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
10.1. Evaluation of human health risks
10.2. Evaluation of effects on the environment
10.3. Conclusions
11. FURTHER RESEARCH
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME ET CONCLUSIONS
RESUMEN Y CONCLUSIONES
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR BENOMYL AND
CARBENDAZIM
Members
Dr G. Burin, Office of Pesticide Programmes, US Environmental
Protection Agency, Washington, D.C., USA
Dr R. Cooper, Reproductive Toxicology Branch, US Environmental
Protection Agency, Research Triangle Park, North Carolina, USA
Dr I. Desi, Department of Public Health, Albert Szent-Györgyi
University Medical School, Szeged, Hungary
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntingdon, United Kingdom
Dr A. Helweg, Department for Pesticide Analysis and Ecotoxicology,
Danish Research Service for Plant and Soil Science, Flakkebjerg,
Slagelse, Denmark
Dr M. Lotti, Institute of Occupational Medicine, University of
Padua, Padua, Italy ( Chairman)
Dr K. Maita, Toxicology Division, Institute of Environmental
Toxicology, Kodaira-Shi, Tokyo, Japan
Dr F. Matsumura, Department of Environmental Toxicology, Institute
of Toxicology and Environmental Health, University of California,
Davis, California, USA
Dr T.K. Pandita, Microbiology and Cell Biology Laboratory, Indian
Institute of Science, Bangalore, Indiaa
Dr C. Sonich-Mullin, Environmental Criteria and Assessment Office,
US Environmental Protection Agency, Cincinnati, Ohio, USA
Dr P.P. Yao, Institute of Occupational Medicine, Chinese Academy of
Preventive Medicine, Beijing, China
a Invited but unable to attend the meeting
Secretariat
Dr B.H. Chen, International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland ( Secretary)
Dr L.W. Hershberger, Dupont Agricultural Products, Walker's Mill,
Barley Mill Plaza, Wilmington, Delaware, USA ( Rapporteur)
Mr P. Howe, Institute of Terrestrial Ecology, Monks Wood, Abbots
Ripton, Huntington, United Kingdom
NOTE TO READERS OF THE CRITERIA MONOGRAPHS
Every effort has been made to present information in the
criteria monographs as accurately as possible without unduly
delaying their publication. In the interest of all users of the
Environmental Health Criteria monographs, readers are kindly
requested to communicate any errors that may have occurred to the
Director of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR BENOMYL
A WHO Task Group on Environmental Health Criteria for Benomyl
and Carbendazim, sponsored by the US Environmental Protection
Agency, met in Cincinnati, USA, from 14 to 19 September 1992. On
behalf of the host agency, Dr T. Harvey opened the meeting and
welcomed the participants. Dr B.H. Chen of the International
Programme on Chemical Safety (IPCS) welcomed the participants on
behalf of the Director, IPCS, and the three IPCS cooperating
organizations (UNEP/ILO/WHO). The Task Group reviewed and revised
the draft criteria monograph and made an evaluation of the risks for
human health and the environment from exposure to benomyl.
The first draft of this monograph was prepared by Dr L.W.
Hershberger and Dr G.T. Arce of E.I. Du Pont de Nemours and Company,
Wilmington, Delaware, USA. The second draft was prepared by Dr L.W.
Hershberger incorporating comments received following the
circulation of the first draft to the IPCS Contact Points for
Environmental Health Criteria monographs. Dr M. Lotti (Institute of
Occupational Medicine, University of Padua, Italy) made a
considerable contribution to the preparation of the final text. Dr
B.H. Chen and Dr P.G. Jenkins, both members of the IPCS Central
Unit, were responsible for the overall scientific content and
technical editing, respectively.
The efforts of all who helped in the preparation and
finalization of the monograph are gratefully acknowledged.
Financial support for the meeting was provided by the US
Environmental Protection Agency, Cincinnati, USA.
ABBREVIATIONS
ADI acceptable daily intake
a.i. active ingredient
BIC butyl isocyanate
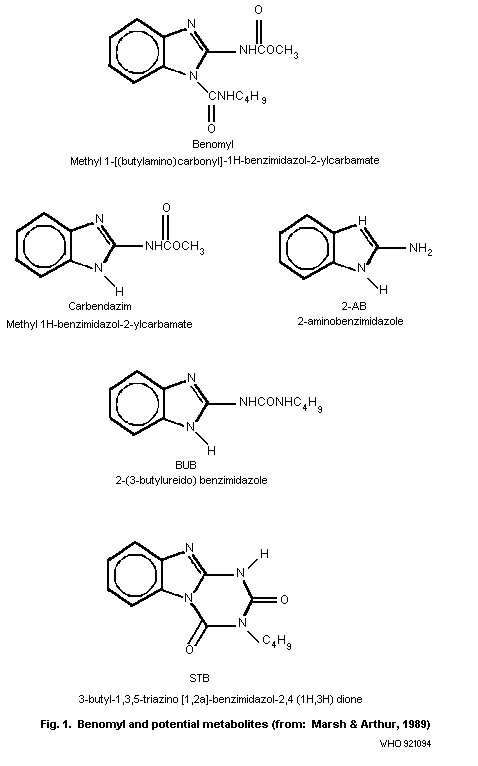
BUB 2-(3-butylureido)benzimidazole
EEC European Economic Community
HPLC high performance liquid chromatography
Koc Distribution coefficient between pesticide adsorbed to soil
organic carbon and pesticide in solution
Kom Distribution coefficient between pesticide adsorbed to soil
organic matter and pesticide in solution
MRL maximum residue limits
NOEL no-observed-effect level
OECD Organisation for Economic Co-operation and Development
STB 3-butyl-1,3,5-triazino[1,2a]-benzimidazol-2,4(1H,3H)dione
2-AB 2-aminobenzimidazole
5-HBC methyl (5-hydroxy-1H-benzimidazol-2-yl)-carbamate
1. SUMMARY AND CONCLUSIONS
1.1 Summary
1.1.1 Identity, physical and chemical properties, and analytical
methods
Benomyl, a tan crystalline solid, is a systemic fungicide
belonging to the benzimidazole family. It decomposes just above its
melting point of 140 °C and has a vapour pressure of < 5 x 10-6
Pa (< 3.7 x 10-8 mmHg) at 25 °C. Benomyl is virtually insoluble
in water at pH 5 and 25 °C, the solubility being 3.6 mg/litre. It is
stable under normal storage conditions but decomposes to carbendazim
in water.
Residual and environmental analyses are performed by extraction
with an organic solvent, purification of the extract by a
liquid-liquid partitioning procedure, and conversion of the residue
to carbendazim. Measurement of residues may be determined by high
performance liquid chromatography or immunoassay.
1.1.2 Sources of human and environmental exposure
In 1988, the estimated worldwide use of benomyl was
approximately 1700 tonnes. It is a widely used fungicide registered
for use on over 70 crops in 50 countries. Benomyl is formulated as a
wettable powder.
1.1.3 Environmental transport, distribution and transformation
Benomyl is rapidly converted to carbendazim in the environment
with half-lives of 2 and 19 h in water and in soil, respectively.
Data from studies on both benomyl and carbendazim are therefore
relevant for the evaluation of environmental effects.
Carbendazim decomposes in the environment with half-lives of 6
to 12 months on bare soil, 3 to 6 months on turf, and 2 and 25
months in water under aerobic and anaerobic conditions,
respectively.
Carbendazim is mainly decomposed by microorganisms.
2-Aminobenzimidazole (2-AB) is a major degradation product and is
further decomposed by microbial activity.
When phenyl-14C-labelled benomyl was decomposed, only 9% of
the 14C was evolved as CO2 during 1 year of incubation. The
remaining 14C was recovered mainly as carbendazim and bound
residues. The fate of a possible degradation product
(1,2-diaminobenzene) may further clarify the degradation pathway of
benzimidazole fungicides in the environment.
Field and column studies have shown that carbendazim remains in
the soil surface layer. There is no available determination of
carbendazim adsorption in soil, but it is expected to be as strongly
adsorbed to soil as benomyl, with Koc values ranging from 1000 to
3600. The log Kow values for benomyl and carbendazim are 1.36 and
1.49, respectively.
No risk of leaching was apparent when this was evaluated in a
screening model based on adsorption and persistence data. This
statement is supported by analyses of well-water in the USA where
benomyl was not found in any of 495 wells and carbendazim not in any
of 212 wells (limit of detection not available). Surface run-off of
benomyl and carbendazim is expected to consist only of fungicide
adsorbed to soil particles, and these compounds are expected to be
strongly adsorbed to sediments in the aqueous environment.
Benomyl in solutions, plants and soil degrades to carbendazim
(methyl-1H-benzimidazol-2-carbamate) and to 2-AB, STB
(3-butyl-1,3,5-triazino[1,2a]-benzimidazol-2,4(1H,3H)dione) and BBU
(1-(2-benzimidazolyl)-3- n-butylurea). There is little or no
photolysis of benomyl.
In animal systems, benomyl is metabolized to carbendazim and
other polar metabolites, which are rapidly excreted. Neither benomyl
nor carbendazim has been observed to accumulate in any biological
system.
1.1.4 Environmental levels and human exposure
No environmental monitoring data for benomyl appear to be
available. However, the following can be summarized from
environmental fate studies.
Since benomyl and carbendazim remain stable for several weeks
on plant material, they may become accessible to organisms feeding
on leaf litter. Soil and sediments may contain residues of
carbendazim for up to 3 years. However, the strong adsorption of
carbendazim to soil and sediment particles reduces the exposure of
terrestrial and aquatic organisms.
The main source of exposure for the general human population is
residues of benomyl and carbendazim in food crops. Dietary exposure
analysis in the USA (combined benomyl and carbendazim) and the
Netherlands (carbendazim) yielded an expected mean intake of about
one-tenth of the recommended Acceptable Daily Intake (ADI) for
benomyl of 0.02 mg/kg body weight and for carbendazim of 0.01 mg/kg
body weight.
Occupational exposures during the manufacturing process are
below Threshold Limit Values. Agricultural workers engaged in
pesticide mixing and loading or re-entering benomyl-treated fields
are expected to be exposed dermally to a few mg of benomyl per hour.
This type of exposure could be reduced by the use of protective
devices. Furthermore, since dermal absorption is expected to be low,
the probability of benomyl having systemic toxic effects on human
populations through this route is very low.
1.1.5 Kinetics and metabolism
Benomyl is readily absorbed in animal experiments after oral
and inhalation exposure, but much less so following dermal exposure.
Absorbed benomyl is rapidly metabolized and excreted in the urine
and faeces. In rats fed 14C-labelled benomyl, its metabolites
carbendazim and methyl(5-hydroxy-1H-benzimidazol-2-yl)-carbamate
(5-HBC) were found in the blood and in small amounts in the testes,
kidneys and livers. The tissue distribution showed no
bioconcentration. In urine the primary metabolite was 5-HBC, some
carbendazim also being present. By 72 h after administration, 98% of
the given amount had been excreted. In cows dosed by capsule for 5
days with radiolabelled benomyl at a dose equivalent to 50 mg/kg
diet, there was a benomyl equivalent level of 4 mg/kg in the liver,
0.25 mg/kg in the kidney and no significant levels in other tissues
or fat. During feeding, 65% of the radiolabel was excreted in the
urine, 21% in the faeces and 0.4% in the milk. The major metabolite
in the milk was 5-HBC. Similar metabolism and elimination patterns
were found in other animals.
Benomyl does not inhibit acetyl cholinesterase in vitro. It
has been shown to induce liver epoxyhydrolase, gamma-glutamyl
transpeptidase and glutathione- S-transferase in in vivo studies
on mice and rats.
1.1.6 Effects on laboratory mammals; in vitro test systems
1.1.6.1 Single exposure
Benomyl has low acute toxicity with an oral LD50 in the rat
of > 10 000 mg/kg and an inhalation 4-h LC50 of > 4 mg/litre.
Carbendazim, like its parent compound benomyl, has an LD50 in rats
of > 10 000 mg/kg. Dogs, exposed via inhalation for 4 h at 1.65
mg/litre and examined 28 days after exposure, showed decreased liver
weight. A single dose of benomyl to rats by gavage showed
reproductive effects at 70 days after exposure (see section
1.1.6.5).
1.1.6.2 Short-term exposure
Short-term gavage, dietary or dermal administration of benomyl
for up to 90 days slightly increased liver weights in the rat (125
mg/kg per day, dietary) and produced effects on male reproductive
organs (decreased testis and epididymal weights, decreased sperm
production) in the rat (45 mg/kg per day, gavage; no-observed-effect
level (NOEL) = 15 mg/kg), rabbit (1000 mg/kg per day, oral; 500
mg/kg body weight per day, dermal) and beagle dog (62.5 mg/kg; NOEL
= 18.4 mg/kg per day, dietary). Liver and testicular effects were
not observed in rats exposed via inhalation to benomyl
concentrations of up to 200 mg/m3 for 90 days.
1.1.6.3 Skin and eye irritation and sensitization
Application to the skin of the rabbit and guinea-pig produced
either mild or no irritation and moderate skin sensitization.
Application to the eyes of rats produced temporary mild conjunctival
irritation.
1.1.6.4 Long-term exposure
A long-term feeding study in rats did not demonstrate any
compound-related effects at dose levels up to and including 2500
mg/kg diet (125 mg/kg body weight per day). This study was not
considered adequate to evaluate reproductive effects. In the CD-1
mouse, liver weights were increased at dose levels of 1500 mg/kg
diet or more. Male mice had decreased absolute testes weights and
thymic atrophy at a level of 5000 mg/kg diet.
1.1.6.5 Reproduction, embryotoxicity, and teratogenicity
Benomyl causes a decrease in testis and epididymis weight, a
reduction in caudal sperm reserves, a decrease in sperm production,
and a lowering of male fertility rates. At higher doses, there is
hypospermatogenesis with generalized disruption of all stages of
spermatogenesis. Benomyl does not effect copulatory behaviour,
seminal vesicles, sperm mobility or related reproductive hormones.
The lowest benomyl concentration shown to induce a statistically
significant spermatogenic effect in male rats was 45 mg/kg per day.
The NOEL for these effects was 15 mg/kg per day.
A single dose of benomyl (100 mg/kg or more) administered to
rats by gavage showed effects, at 70 days aftr exposure, which
included decreased testis weight and seminiferous tubular atrophy.
When administered via gavage from days 7 to 16 of gestation to
ChD-CD rats and Wistar rats, benomyl was found to be teratogenic at
62.5 mg/kg for both strains, but not at 30 mg/kg for ChR-CD rats and
not at 31.2 mg/kg for Wistar rats. When Sprague-Dawley rats were
administered by gavage on days 7 to 21 of gestation, benomyl was
found to be teratogenic at 31.2 mg/kg. The effects were
microphthalmia, hydrocephaly, and encephaloceles. Postnatal
development of rats was adversely affected at dose levels greater
than 15.6 mg/kg.
In mice, gavage dosing at a concentration of 50 mg/kg or more
induced supernumery ribs and other skeletal and visceral anomalies.
A NOEL was not established in the mouse because no doses lower than
50 mg/kg were tested. Except for a marginal increase in supernumery
ribs in rabbits, no teratogenic effects were observed at dose levels
as high as 500 mg/kg diet.
1.1.6.6 Mutagenicity and related end-points
Studies in somatic and germ cells show that benomyl does not
cause gene mutations or structural chromosomal damage (aberrations)
and it does not interact directly with DNA (causing DNA damage and
repair). This has been demonstrated in both mammalian and
non-mammalian systems.
Benomyl does, however, cause numerical chromosome aberrations
(aneuploidy and/or polyploidy) in experimental systems in vitro
and in vivo.
1.1.6.7 Carcinogenicity
Benomyl or carbendazim caused liver tumours in two strains of
mice (CD-1 and Swiss (SPF)) that have a high spontaneous rate of
liver tumours. In contrast, carbendazim was not carcinogenic in
NMRKf mice, which have a low spontaneous rate of such tumours.
The first carcinogenicity study using CD-1 mice showed a
statistically significant dose-related increase of hepatocellular
neoplasia in females, and a statistically significant response was
also observed in the mid-dose (1500 mg/kg) males but not in the
high-dose males because of the high mortality rate. A second
carcinogenicity study of carbendazim in a genetically related mouse
strain, SPF mice (Swiss random strain), at doses of 0, 150, 300 and
1000 mg/kg (increased to 5000 mg/kg during the study) showed an
increase in the incidence of combined hepatocellular adenomas and
carcinomas. A third study carried out in NMRKf mice at doses of 0,
50, 150, 300 and 1000 mg/kg (increased to 5000 mg/kg during the
study) showed no carcinogenic effects.
Carcinogenicity studies with both benomyl and carbendazim were
negative in rats.
1.1.6.8 Mechanism of toxicity - mode of action
The biological effects of benomyl and carbendazim are thought
to be the result of their interaction with cell microtubules. These
structures are involved in vital functions such as cell division,
which is inhibited by benomyl and carbendazim. Benomyl and
carbendazim toxicity in mammals is linked with microtubular
dysfunction.
Benomyl and carbendazim, like other benzimidazole compounds,
display selective toxicity for species. This selectivity is, at
least in part, explained by the different binding of benomyl and
carbendazim to tubulins of target and non-target species.
1.1.7 Effects on humans
Benomyl causes contact dermatitis and dermal sensitization. No
other effects have been reported.
1.1.8 Effects on other organisms in the laboratory and field
Benomyl has little effect on soil microbial activity at
recommended application rates. Some adverse effects have been
reported for groups of fungi.
The 72-h EC50, based on total growth, for the green alga
Selenastrum capricornutum was calculated to be 2.0 mg/litre; the
no-observed-effect concentration (NOEC) was 0.5 mg/litre. The
toxicity of benomyl to aquatic invertebrates and fish varies widely,
96-h LC50 values ranging from 0.006 mg/litre for the channel
catfish (yolk-sac fry) to > 100 mg/litre for the crayfish.
Benomyl is toxic to earthworms in laboratory experiments at
realistic exposure concentrations and as a result of recommended
usage in the field. It is of low toxicity to birds and its
degradation product carbendazim is "relatively non-toxic" to
honey-bees.
1.2 Conclusions
Benomyl causes dermal sensitization in humans. Both benomyl and
carbendazim represent a very low risk for acute poisoning in humans.
Given the current exposures and the low rate of dermal absorption of
these two compounds, it is unlikely that they would cause systemic
toxicity effects either in the general population or in
occupationally exposed subjects. These conclusions are drawn from
animal data and the limited human data available, and are supported
by the understanding of the mode of action of carbendazim and
benomyl in both target and non-target species.
Further elucidation of the mechanism of toxicity of benomyl and
carbendazim in mammals will perhaps permit a better definition of
no-observed-effect levels. Binding studies on tubulins of target
cells (testis and embryonic tissues) will facilitate inter-species
comparisons.
Carbendazim is strongly adsorbed to soil organic matter and
remains in the soil for up to 3 years. Carbendazim persists on leaf
surfaces and, therefore, in leaf litter. Earthworms have been shown
to be adversely affected (population and reproductive effects) at
recommended application rates. There is no information on other soil
or litter arthropods that would be similarly exposed.
The high toxicity to aquatic organisms in laboratory tests is
unlikely to be seen in the field because of the low bioavailability
of sediment-bound residues of carbendazim. However, no information
is available on sediment-living species, which would receive the
highest exposure.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND ANALYTICAL
METHODS
2.1 Chemical identity
2.1.1 Primary constituent
Chemical structure:
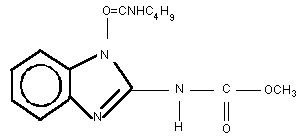 Molecular formula: C14H18N4O3
Common name: Benomyl
CAS chemical name: Carbamic acid, [1-(butylamino)carbonyl]-1H-
benzimidazol-2-yl]-, methyl ester
IUPAC chemical name: Methyl 1-[(butylamino)carbonyl]-1H-
benzimidazol-2-ylcarbamate
CAS registry number: 17804-35-2
Relative molecular mass: 290.3
Synonym: Methyl 1-(butylcarbamoyl)-2-benzimida-
zolecarbamate
2.1.2 Technical product
Major trade names: Benlate, Tersan, Fungicide 1991, Fundazol
Purity: > 95% (FAO specifications)
2.2 Physical and chemical properties
Table 1. Some physical and chemical properties of Benomyl
Physical state Crystalline solid
Colour Tan
Odour Negligible
Melting point/boiling point/ Decomposes just after
flash point melting at 140 °C
Explosion limits LEL = 0.05 g/litre in air
Vapour pressure < 5.0 x 10-6 Pa (< 3.7 x
10-8 mmHg) at 25 °Ca
Density 0.38 g/cm3
Log n-octanol/water partition
coefficient 1.36
Solubility in water 3.6 mg/litre (at pH 5 and 25
°C)
Solubility in organic solvents Chloroform 9.4
(g/100 g solvent at 25 °C) Dimethylformamide 5.3
Acetone 1.8
Xylene 1.0
Ethanol 0.4
Heptane 40
Henry's constant < 4.2 x 10-9 atm-m3/mol
at pH 5 and 25 °C
Soil/water partition coefficient 1090 mg/g (Kom); 1860 mg/g
(Koc)b
a Barefoot (1988)
b Koc = Distribution coefficient between pesticide adsorbed
to soil organic carbon and pesticide in solution.
Kom = Distribution coefficient between pesticide adsorbed to
soil organic matter and pesticide in solution.
2.3 Analytical methods
Most methods for determining benomyl and its by-product
residues in plant and animal tissue and in soil involve isolation of
the residue by extraction with an organic solvent, purification of
the extract by a liquid-liquid partitioning procedure, and
conversion of the residue to carbendazim. Residues may be measured
by procedures using high-speed cation-exchange liquid
chromatography, reversed phase HPLC, and immunoassay. One method for
analysis of water samples can distinguish between benomyl and
carbendazim. Recoveries of benomyl, carbendazim and 2-AB
(2-aminobenzimidazole) from various types of soils average 92, 88
and 71%, respectively. The lower limit of sensitivity of the method
is 0.05 ppm for each of these components. The recoveries and
sensitivities for plant tissues are similar. Table 2 outlines
various analytical methods for soil, water, plant and animal tissue.
Table 2. Analytical Methods for Benomyl
Analytical method Medium Detection limit Comments Reference
Strong cation exchange/HPLC soil 0.05 mg/kg acidic methanol extraction converts Kirkland et al. (1973)
residual benomyl to carbendazim
Strong cation exchange/HPLC plant 0.05 mg/kg acidic methanol extraction converts Kirkland et al. (1973)
residual benomyl to carbendazim
Strong cation exchange/HPLC animal 0.01 mg/kg (milk) acidic aqueous hydrolysis followed Kirkland (1973)
0.05 mg/kg by organic extraction converts
(tissue) benomyl to carbendazim and frees
metabolites from conjugates
Reversed phase HPLC water 9.0 x 10-6 g/litre on-line HPLC with preconcentration; Marvin et al. (1991)
benomyl and carbendazim
determined separately
Reversed phase blueberries 0.03 mg/kg acidic methanol extraction converts Bushway et al. (1991)
HPLC/fluorescence detection residual benomyl to carbendazim
Radioimmunoassay plant 0.05-1.0 mg/kg ethyl acetate extraction converts Newsome & Shields
(dependent on crop) residual benomyl to carbendazim (1981)
Enzyme-linked immunosorbent plant 0.50 mg/kg ethyl acetate extraction converts Newsome & Collins
assay (ELISA) residual benomyl to carbendazim (1987)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Benomyl does not occur naturally.
3.2 Anthropogenic sources
3.2.1 Uses
Benomyl is one of the most widely used members of a family of
fungicides known as benzimidazoles. It is registered in more than 50
countries for use on more than 70 crops, including cereals, cotton,
grapes, bananas and other fruits, ornamentals, plantation crops,
sugar beet, soybeans, tobacco, turf, vegetables, mushrooms and many
other crops, and is used under most climatic conditions. Registered
benomyl usage specifies rates from 0.1 to 2.0 kg a.i./ha and
applications from once per year to spray intervals ranging from 7 to
14 days (FAO/WHO, 1985a; 1988a). Benomyl is effective at low usage
rates against more than 190 different fungal diseases such as leaf
spots, blotches and blights; fruit spots and rots; sooty moulds;
scabs; bulb, corn and tuber decays; blossom blights; powdery
mildews; certain rusts; and common soilborne crown and root rots.
A key limitation to the use of benomyl and other benzimidazoles
is the development of fungal resistance. Resistance management can
be achieved by using benomyl in combination with a non-benzimidazole
companion fungicide as a tank mix or it may be used alternately with
a non-benzimidazole fungicide (Delp, 1980; Staub & Sozzi, 1984).
Benomyl is formulated as a wettable powder and dry flowable or
dispersible granules. In some countries the latter formulation is no
longer available.
3.2.2 Worldwide sales
In 1991, the estimated worldwide sales of benomyl was US$ 290
million. This was about 50% of the worldwide market for
benzimidazole products. Carbendazim (20%) and thiophanatemethyl
(20%) account for most of the rest of the benzimidazole market
(County NatWest WoodMac).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Benomyl has a vapour pressure of < 5.0 x 10-6 Pa (< 3.7 x
10-8 mmHg) and a solubility in water of 3.6 mg/litre at pH 5 and
25 °C. As a result, it has a Henry's constant of < 4.2 x 10-9
atm-m3/mol. Benomyl is essentially non-volatile from water
surfaces.
4.1.2 Water
The half-life of benomyl in surface water and sediment under
aerobic conditions has been shown to be approximately 2 h. Its
metabolite carbendazim had a half-life of 61 days under non-sterile
conditions. After 30 days, 22% of the applied radioactivity was
bound to sediments and < 1% of the applied radioactivity was
evolved as carbon dioxide (Arthur et al., 1989a).
4.1.3 Soil
Radiolabelled benomyl was found to be strongly adsorbed (Ka =
6.1 and 13 µg/g) to two different sandy loam soils and very strongly
adsorbed (Ka = 50 and 90 µg/g) to two different silt loam soils.
Adsorption was not significantly affected by the benomyl
concentration over the range 0.2-2.3 ppm. Adsorbed radioactivity was
not readily desorbed from any of the test soils. The Ka, corrected
for the organic matter content of the soils, was 2-4 times higher on
the silt loam than on the sandy loam soil. This difference suggests
that variables other than percentage organic matter (i.e. cation
exchange capacity, particle size or compound degradation) influence
adsorption. The ease of desorption appears to be inversely related
to the organic content of the soils (Priester, 1985). The structure
of benomyl and its soil degradation products, i.e. carbendazim
(methyl 1H-benzimidazol-2-ylcarbamate), 2-AB (2-aminobenzimidazole),
STB (3-butyl-1,3,5-triazino[1,2a]-benzimidazol-2,4(1H,3H)dione), and
BBU (1-(2-benzimidazolyl)-3-n-butylurea), which is also known as
2-(3-butylureido)-benzimidazole (BUB), are given in Fig. 1. The
major proportion of each of the metabolites was found in the
uppermost (0-12.7 cm) soil layer. The extent of mobility correlated
with the type and characteristics of the soil to which benomyl was
applied. The 14C label was less mobile in soils of lower sand
content and higher silt or clay content. It was also found to be
less mobile on soils of higher organic content and lower pH (Chang,
1985). In a soil column leaching experiment in rice paddy soil,
benomyl did not leach significantly. Approximately 94% was found in
the top 5 cm, 9% in the next 5-10 cm, and less than 1% was detected
in any lower segments (Ryan, 1989). These data indicate that
benomyl, carbendazim, BUB and STB are highly immobile.
Molecular formula: C14H18N4O3
Common name: Benomyl
CAS chemical name: Carbamic acid, [1-(butylamino)carbonyl]-1H-
benzimidazol-2-yl]-, methyl ester
IUPAC chemical name: Methyl 1-[(butylamino)carbonyl]-1H-
benzimidazol-2-ylcarbamate
CAS registry number: 17804-35-2
Relative molecular mass: 290.3
Synonym: Methyl 1-(butylcarbamoyl)-2-benzimida-
zolecarbamate
2.1.2 Technical product
Major trade names: Benlate, Tersan, Fungicide 1991, Fundazol
Purity: > 95% (FAO specifications)
2.2 Physical and chemical properties
Table 1. Some physical and chemical properties of Benomyl
Physical state Crystalline solid
Colour Tan
Odour Negligible
Melting point/boiling point/ Decomposes just after
flash point melting at 140 °C
Explosion limits LEL = 0.05 g/litre in air
Vapour pressure < 5.0 x 10-6 Pa (< 3.7 x
10-8 mmHg) at 25 °Ca
Density 0.38 g/cm3
Log n-octanol/water partition
coefficient 1.36
Solubility in water 3.6 mg/litre (at pH 5 and 25
°C)
Solubility in organic solvents Chloroform 9.4
(g/100 g solvent at 25 °C) Dimethylformamide 5.3
Acetone 1.8
Xylene 1.0
Ethanol 0.4
Heptane 40
Henry's constant < 4.2 x 10-9 atm-m3/mol
at pH 5 and 25 °C
Soil/water partition coefficient 1090 mg/g (Kom); 1860 mg/g
(Koc)b
a Barefoot (1988)
b Koc = Distribution coefficient between pesticide adsorbed
to soil organic carbon and pesticide in solution.
Kom = Distribution coefficient between pesticide adsorbed to
soil organic matter and pesticide in solution.
2.3 Analytical methods
Most methods for determining benomyl and its by-product
residues in plant and animal tissue and in soil involve isolation of
the residue by extraction with an organic solvent, purification of
the extract by a liquid-liquid partitioning procedure, and
conversion of the residue to carbendazim. Residues may be measured
by procedures using high-speed cation-exchange liquid
chromatography, reversed phase HPLC, and immunoassay. One method for
analysis of water samples can distinguish between benomyl and
carbendazim. Recoveries of benomyl, carbendazim and 2-AB
(2-aminobenzimidazole) from various types of soils average 92, 88
and 71%, respectively. The lower limit of sensitivity of the method
is 0.05 ppm for each of these components. The recoveries and
sensitivities for plant tissues are similar. Table 2 outlines
various analytical methods for soil, water, plant and animal tissue.
Table 2. Analytical Methods for Benomyl
Analytical method Medium Detection limit Comments Reference
Strong cation exchange/HPLC soil 0.05 mg/kg acidic methanol extraction converts Kirkland et al. (1973)
residual benomyl to carbendazim
Strong cation exchange/HPLC plant 0.05 mg/kg acidic methanol extraction converts Kirkland et al. (1973)
residual benomyl to carbendazim
Strong cation exchange/HPLC animal 0.01 mg/kg (milk) acidic aqueous hydrolysis followed Kirkland (1973)
0.05 mg/kg by organic extraction converts
(tissue) benomyl to carbendazim and frees
metabolites from conjugates
Reversed phase HPLC water 9.0 x 10-6 g/litre on-line HPLC with preconcentration; Marvin et al. (1991)
benomyl and carbendazim
determined separately
Reversed phase blueberries 0.03 mg/kg acidic methanol extraction converts Bushway et al. (1991)
HPLC/fluorescence detection residual benomyl to carbendazim
Radioimmunoassay plant 0.05-1.0 mg/kg ethyl acetate extraction converts Newsome & Shields
(dependent on crop) residual benomyl to carbendazim (1981)
Enzyme-linked immunosorbent plant 0.50 mg/kg ethyl acetate extraction converts Newsome & Collins
assay (ELISA) residual benomyl to carbendazim (1987)
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural occurrence
Benomyl does not occur naturally.
3.2 Anthropogenic sources
3.2.1 Uses
Benomyl is one of the most widely used members of a family of
fungicides known as benzimidazoles. It is registered in more than 50
countries for use on more than 70 crops, including cereals, cotton,
grapes, bananas and other fruits, ornamentals, plantation crops,
sugar beet, soybeans, tobacco, turf, vegetables, mushrooms and many
other crops, and is used under most climatic conditions. Registered
benomyl usage specifies rates from 0.1 to 2.0 kg a.i./ha and
applications from once per year to spray intervals ranging from 7 to
14 days (FAO/WHO, 1985a; 1988a). Benomyl is effective at low usage
rates against more than 190 different fungal diseases such as leaf
spots, blotches and blights; fruit spots and rots; sooty moulds;
scabs; bulb, corn and tuber decays; blossom blights; powdery
mildews; certain rusts; and common soilborne crown and root rots.
A key limitation to the use of benomyl and other benzimidazoles
is the development of fungal resistance. Resistance management can
be achieved by using benomyl in combination with a non-benzimidazole
companion fungicide as a tank mix or it may be used alternately with
a non-benzimidazole fungicide (Delp, 1980; Staub & Sozzi, 1984).
Benomyl is formulated as a wettable powder and dry flowable or
dispersible granules. In some countries the latter formulation is no
longer available.
3.2.2 Worldwide sales
In 1991, the estimated worldwide sales of benomyl was US$ 290
million. This was about 50% of the worldwide market for
benzimidazole products. Carbendazim (20%) and thiophanatemethyl
(20%) account for most of the rest of the benzimidazole market
(County NatWest WoodMac).
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION AND TRANSFORMATION
4.1 Transport and distribution between media
4.1.1 Air
Benomyl has a vapour pressure of < 5.0 x 10-6 Pa (< 3.7 x
10-8 mmHg) and a solubility in water of 3.6 mg/litre at pH 5 and
25 °C. As a result, it has a Henry's constant of < 4.2 x 10-9
atm-m3/mol. Benomyl is essentially non-volatile from water
surfaces.
4.1.2 Water
The half-life of benomyl in surface water and sediment under
aerobic conditions has been shown to be approximately 2 h. Its
metabolite carbendazim had a half-life of 61 days under non-sterile
conditions. After 30 days, 22% of the applied radioactivity was
bound to sediments and < 1% of the applied radioactivity was
evolved as carbon dioxide (Arthur et al., 1989a).
4.1.3 Soil
Radiolabelled benomyl was found to be strongly adsorbed (Ka =
6.1 and 13 µg/g) to two different sandy loam soils and very strongly
adsorbed (Ka = 50 and 90 µg/g) to two different silt loam soils.
Adsorption was not significantly affected by the benomyl
concentration over the range 0.2-2.3 ppm. Adsorbed radioactivity was
not readily desorbed from any of the test soils. The Ka, corrected
for the organic matter content of the soils, was 2-4 times higher on
the silt loam than on the sandy loam soil. This difference suggests
that variables other than percentage organic matter (i.e. cation
exchange capacity, particle size or compound degradation) influence
adsorption. The ease of desorption appears to be inversely related
to the organic content of the soils (Priester, 1985). The structure
of benomyl and its soil degradation products, i.e. carbendazim
(methyl 1H-benzimidazol-2-ylcarbamate), 2-AB (2-aminobenzimidazole),
STB (3-butyl-1,3,5-triazino[1,2a]-benzimidazol-2,4(1H,3H)dione), and
BBU (1-(2-benzimidazolyl)-3-n-butylurea), which is also known as
2-(3-butylureido)-benzimidazole (BUB), are given in Fig. 1. The
major proportion of each of the metabolites was found in the
uppermost (0-12.7 cm) soil layer. The extent of mobility correlated
with the type and characteristics of the soil to which benomyl was
applied. The 14C label was less mobile in soils of lower sand
content and higher silt or clay content. It was also found to be
less mobile on soils of higher organic content and lower pH (Chang,
1985). In a soil column leaching experiment in rice paddy soil,
benomyl did not leach significantly. Approximately 94% was found in
the top 5 cm, 9% in the next 5-10 cm, and less than 1% was detected
in any lower segments (Ryan, 1989). These data indicate that
benomyl, carbendazim, BUB and STB are highly immobile.
 Similar mobility results have been observed in the field.
Benomyl and its degradates were studied on bare soil and turf in
four areas of the USA. Carbendazim and 2-AB were the major and minor
degradates, respectively. After 1 and 2 years of outdoor exposure,
the half-life of total benzimidazole-containing residues was about 3
to 6 months on turf and about 6 to 12 months on bare soil (Baude et
al., 1974). Under these conditions, benomyl, carbendazim and 2-AB
showed little or no downward movement.
4.1.4 Leaching
To evaluate the risk of pollution of ground and drainage water,
screening models based on adsorption and persistence can be used,
together with existing analyses of groundwater samples. Gustafson
(1989) proposed the use of the equation GUS = log T´ (4 - log
Koc); GUS values < 1.8 = "improbable leachers", GUS values of
1.8-2.8 = "transition" and GUS values > 2.8 = "probable leachers".
For benomyl, Kom values of 550, 620, 2100 and 1100 (mean 1093)
were found in four different soils (Priester, 1985). A Kom of 1093
is equal to a Koc of 1857 since Koc = Kom x 1.7. The half-life
of 320 days given by Marsh & Arthur (1989) seems in good agreement
with field half-lives of 6 to 12 months (Baude et al., 1974).
When the calculation of the GUS value is based on a Koc of
1857 and a T0.5 of 320 days, a value of 1.83 is obtained.
According to this value, benomyl/carbendazim lies between the
"improbable leachers" and "transition", and, therefore, would not be
expected to occur in ground water. The adsorption of benomyl and of
carbendazim is expected to be of the same order of magnitude since
the Kow values are almost identical (log Kow = 1.49 and 1.36 for
carbendazim and benomyl, respectively). In ground water studies in
the USA (Parsons & Witt, 1988), benomyl was not found in any of 495
wells tested and carbendazim not in any of 212 wells (detection
limit not reported).
In an EEC survey (Fielding, 1992), the presence of carbendazim
in groundwater in the Netherlands and in Italy was investigated.
Carbendazim was found in one of two samples from the Netherlands
(0.1 µg/litre), and the level was above 0.1 µg/litre in 23 of 70
samples in Italy. Detection of the non-polar DDT and lindane in many
wells in the Italian study may indicate macropore transport or
artifacts such as direct pollution of wells.
4.1.5 Crop uptake
Various greenhouse and outdoor tests, in which benomyl was
applied to several crops (apples, bananas, cucumbers, grapes and
oranges), indicate that benomyl and carbendazim remain on plant
surfaces as major components of the total residue (Baude et al.,
1973). Benomyl is primarily converted to carbendazim once inside
plant tissues.
Although benomyl is systemic when applied directly to plant
foliage, crop uptake of soil residues is extremely low, even when
the crop is planted in the same growing season as the benomyl
treatment. In a greenhouse crop-rotation study, [2-14C]-
carbendazim, the more persistent benomyl metabolite, was applied to
a loamy sand soil. Aging periods of 30, 120 or 145 days were used
and the crops studied were beets, barley and cabbage. Radioactivity
did not accumulate in these crops grown to maturity in a loamy sand
soil treated 30 days earlier with 1 kg a.i./ha or 120 to 145 days
earlier with 3 kg a.i./ha. Accumulation factors, calculated as the
ratio of radioactivity in the crop to that in the corresponding
soil, were very low in beet foliage (0.04) and beet roots (0.03),
low in cabbage and barley grain (0.2), and ranged from 0.9 to 1.2 in
barley straw (Rhodes, 1987).
4.2 Transformation
Numerous field studies to determine the fate and behaviour of
benomyl in soil have shown the instability of benomyl under various
conditions. In solutions, plants, and soil, it degrades to
carbendazim. The conversion of benomyl under alkaline conditions to
STB and BBU has also been reported (section 4.1). The environmental
fate of benomyl has been thoroughly reviewed by Zbozinek (1984).
4.2.1 Biodegradation
4.2.1.1 Water
Anaerobic aquatic degradation studies in pond water and
sediment showed a half-life of 2 h for benomyl and 743 days for its
degradation product carbendazim. Some (1-8%) transformation to STB
occurred. After one year 36% of the applied radioactivity was bound
to the sediment (Arthur et al., 1989b).
4.2.1.2 Soil
In a study by Marsh & Arthur (1989), non-sterile and sterile
samples of Keyport silt loam soil were treated with [phenyl(U)-
14C]benomyl at a concentration of approximately 7.0 mg/kg. This is
equivalent to the expected soil residues in the surface 10 cm of
topsoil when benomyl is applied at 8 kg a.i./ha. Distilled water was
added to each sample until it reached 75% of its moisture-holding
capacity at 0.33 bar. The soils were incubated in the dark at
approximately 25 °C. The non-sterile soil flasks were sampled after
0.1, 0.2, 1, 3, 7, 14, 30, 60, 120, 270 and 365 days. Samples of
sterilized soil were taken after 14, 30, 120, 270 and 365 days.
The half-life of benomyl in non-sterile silt loam was 19 h, but
this was not determined in the sterilized soil. Benomyl was rapidly
converted to carbendazim. The carbendazim had a half-life of 320
days under non-sterile aerobic conditions (Marsh & Arthur, 1989).
This is in close agreement with reported half-lives of 6-12 months
for benzimidazoles applied to bare soil (Baude et al., 1974).
After 365 days of incubation, 9% of the 14C was evolved as
14CO2, 34% could still be recovered as carbendazim, and 36% was
not extractable. The total recovery of 14C was 88%.
In the sterilized soil, the half-life of carbendazim was
approximately 1000 days (Marsh & Arthur, 1989).
When the degradation of 2-14C-carbendazim (20 mg/kg) was
determined, 33% of the 14C label added was evolved as 14CO2
during 270 days. Identical or even faster 14C evolution was
observed from 2-14C-labelled 2-AB (Helweg, 1977). The relatively
low 14C evolution from phenyl-14C-labelled benomyl/carbendazim
may be caused by the formation of strongly adsorbed degradation
products or compounds that are readily incorporated into soil
organic matter. Thus, most of the remaining radioactivity was
accounted for in the organic fraction of the soil.
To elucidate the reason for the low 14C evolution from
phenyl-14C-labelled fungicide, the fate of a possible degradation
product, 1,2-diaminobenzene, needs to be determined.
4.2.1.3 Crops
Metabolism studies in various crops (soybeans, rice, sugar beet
and peaches) using [phenyl(U)-14C]benomyl have shown that the only
species of significance in plant tissues are benomyl, carbendazim
and 2-AB. Soybeans were treated twice with 1 kg a.i./ha and
harvested 35 days later. Rice was treated twice with 2 kg a.i./ha
and harvested at 21 days, sugar beet was treated with 0.5 kg a.i./ha
five times and harvested at 21 days, and peaches were treated twice
at 1 kg a.i./ha and harvested 20 min after spraying. Soybeans, rice
and sugar beet were treated at twice the recommended application
rate. The concentration of radiolabelled compounds in mature
soybeans was 0.7 mg/kg and consisted of 0.42 mg 2-AB/kg, 0.05 mg
benomyl/kg and 0.14 mg carbendazim per kg (Bolton et al., 1986a).
Levels in the rice grain were 2.7 and 7.3 mg/kg for benomyl and
carbendazim, respectively (Bolton et al., 1986b). Sugar beet tops
retained 99% of the total recovered radioactivity, 6.8 mg/kg being
present as carbendazim and 0.4 mg/kg as benomyl. The roots retained
only 0.01 mg carbendazim per kg (Tolle, 1988). After the first
application to peaches, benomyl was present at 0.65 mg/kg and
carbendazim at 0.72 mg/kg. The second application resulted in 0.33
mg benomyl/kg and 0.92 mg carbendazim/kg. No other radioactive
metabolites were found in peaches (Stevenson, 1985).
Chiba & Veres (1981) applied benomyl to apple trees as Benlate
50% WP at a rate of 1.7 kg/ha. Three successive applications were
made in 1977 and a single spray was applied in 1979. Between 3 and 7
days after application there was a marked reduction of about 50% in
benomyl residues from an initial level of about 110 mg/kg. This fall
in benomyl was accompanied by a doubling in the level of carbendazim
residues over the same period due to benomyl degradation to
carbendazim. Within 46 days of the single application in 1979,
benomyl residues fell to 0.63 mg/kg foliage and carbendazim was
present at 1.2 mg/kg. Following the three sprayings in 1977 (at 0,
13 and 27 days after the initial application), residue levels were
2.6 and 17.1 mg/kg foliage for benomyl and carbendazim 83 days after
the first spraying. Both experiments showed an exponential fall in
benomyl residues but the rate of decline was much slower in the case
of the more persistent metabolite.
4.2.2 Abiotic degradation
In a study by Wheeler (1985), the hydrolysis of benomyl was
studied in sterilized aqueous solutions maintained at 25 °C in the
dark for 30 days at pH 5, 7 and 9. In pH 5 buffer, the major product
was carbendazim, whereas at pH 7 and 9 carbendazim and STB were the
major products. STB represented approximately 25% of the total
radioactivity at pH 7 and approximately 80% at pH 9. The half-lives
of benomyl in the pH 5, 7 and 9 solutions were approximately 3.5,
1.5 and less than 1 h, respectively. There was no further
degradation of carbendazim at pH 5 and 7 over 30 days. At pH 9,
however, carbendazim was slowly hydrolysed to 2-AB with a half-life
of 54 days (Priester, 1984).
Aqueous photolysis studies conducted in natural sunlight have
shown that benomyl is mainly degraded by hydrolysis rather than
photolysis (Powley, 1985).
4.2.3 Bioaccumulation
Although only low concentrations of benomyl or its metabolites
would be expected in natural waters, studies have evaluated the
metabolism and bioaccumulation in fish. Bluegill sunfish ( Lepomis
macrochirus) were exposed to radiolabelled carbendazim
concentrations of 0.018 or 0.17 mg/litre for 4 weeks in a dynamic
study designed to measure the bioaccumulation of 14C residues in
edible tissue, viscera, remaining carcass and whole fish. A two-week
depuration phase followed the exposure phase. Results were similar
at the two exposure concentrations, the peak whole fish
bioconcentration factors (BCFs) being 27 and 23 at the low and high
exposure levels, respectively. The radioactivity was concentrated
more in the viscera than in other tissues, the peak viscera BCFs
being 460 and 380 for the low and high exposure levels,
respectively. Very little bioconcentration occurred in the muscle
tissue (BCF = < 4) or the remaining carcass (BCF = < 7). During
the 14-day depuration phase, > 94% of the peak level of
radioactivity was lost from the whole fish, viscera and muscle. The
rate of loss from the carcass tissue was lower (77% and 82% loss for
the low and high exposure levels, respectively) (Hutton et al.,
1984).
When rainbow trout ( Oncorhynchus mykiss), channel catfish
( Ictalurus punctatus) and bluegill sunfish ( Lepomis macrochirus)
were injected intraperitoneally with carbendazim, branchial and
biliary excretion were the major pathways for the elimination
(Palawski & Knowles, 1986). In a separate experiment, the three fish
species were exposed to 45 µg carbendazim/litre for 96 h, except in
the case of catfish, which were exposed for 48 h. This was followed
by a 96-h depuration phase. Rainbow trout had the highest uptake
rate constant (1.78 per h) and bioconcentration factor (159) of the
three species. Much less carbendazim was accumulated by channel
catfish than by the other two species, but this residue level (0.44
µg/g) appeared to be lethal after 48 h of exposure. The elimination
rate constant and the biological half-life of carbendazim were
similar for rainbow trout and bluegill sunfish. However, the
elimination rate constant was greater and the biological half-life
shorter in channel catfish (13 h) than in the other two species.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air, water and soil
The environmental levels in air, water and soil are discussed
in detail in section 4.
5.1.2 Food and feed
Levels of benomyl in food and feed are indicated in section
5.2.
5.1.3 Terrestrial and aquatic organisms
Benomyl levels in terrestrial and aquatic organisms are
discussed in detail in sections 4 and 6.
5.2 General population exposure
The principal exposure of the general population to benomyl is
through dietary exposure. It was recommended by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1988b) that all
maximum residue limits (MRLs) for benomyl, thiophanate-methyl and
carbendazim be listed as carbendazim (see Table 5).
5.2.1 USA
A system called the Dietary Risk Evaluation System (DRES),
which was developed by the US Environmental Protection Agency, was
used to quantify the intake of residues occurring in various
commodities. The system assumes a diet consistent with the 1977-1978
USDA Nationwide Food Consumption Survey. This survey was a
stratified probability survey in which 3-day dietary records of
approximately 30 000 individuals were collected. The dietary intake
of residues resulting from registered food crop uses of benomyl was
then estimated using mean residue levels found in controlled field
trials and adjusting for the effects of food processing, e.g.,
washing and cooking, on residues of benomyl and its metabolites.
Based on this analysis, the total dietary exposure was
determined for the general population and for a number of population
subgroups. The exposure of the average person to residues resulting
from benomyl use was estimated to be 0.218 µg/kg body weight per
day. The highest exposure was found in the population subgroup
entitled "non-hispanic other than black or white", the estimated
exposure being 1.479 µg/kg body weight per day. The lowest exposure
was found in the > 20-year-old males where the estimated exposure
was 0.144 µg/kg body weight per day (Eickhoff et al., 1989). These
estimates are below the benomyl ADI allocated by JMPR (0-0.02 mg/kg
body weight per day) (FAO/WHO, 1985a,b).
5.2.2 Sweden
Residue monitoring data for benzimidazole fungicides, i.e.
benomyl, carbendazim and thiophanate-methyl, on food crops from
Sweden is shown in Table 3 (FAO/WHO, 1988b). No further analysis to
determine dietary intake was performed.
5.2.3 Maximum residue limits
National MRLs for certain commodities are listed in Table 4
(FAO/WHO, 1988a).
A complete list of MRLs for carbendazim, including new
proposals and an indication of the source of the data (application
of benomyl, carbendazim, or thiophanate-methyl) on which the MRL is
based, is given in Table 5 (FAO/WHO, 1988b).
5.3 Occupational exposure during manufacture, formulation or use
The levels of inhalation exposure to benomyl and carbendazim
experienced by workers in a major manufacturing facility (DuPont)
were reviewed from 1986 to 1989. The average levels of benomyl and
carbendazim were less than 0.2 mg/m3 and 0.3 mg/m3,
respectively. Table 6 lists established inhalation exposure limits
for benomyl and carbendazim.
5.3.1 Use
Potential dermal and respiratory exposure to benomyl wettable
powder formulation under actual use situations has been determined
for: a) tank loading and mixing for aerial application; b) re-entry
into treated fields; and c) home use (garden, ornamental and
greenhouse). For crop treatments, approximately 17 kg benomyl
(formulation) was handled per cycle. Maximum exposure occurred in
the loading and mixing operation for aerial application, where
dermal exposure was 26 mg benomyl per mixing cycle, primarily to
hands and forearms (90%) and respiratory exposure averaged 0.08 mg
benomyl. Re-entry data revealed dermal and respiratory exposures of
5.9 mg/h and < 0.002 mg/h, respectively. Home-use situations
(application of 7 to 8 litres of benomyl in hand-held compressed air
sprayers) produced exposures of 1 mg and 0.003 mg per application
cycle for dermal and respiratory routes, respectively (Everhart &
Holt, 1982). Similar average dermal exposure levels (5.39 mg/h) for
strawberry harvesters were reported by Zweig et al (1983).
Table 3. Benomyl/carbendazim/thiophanate-methyl residues in food in Swedena
Samples Swedish/imported No. of samples Samples with residues Residue level Median value
>0.20 mg/kg (mg/kg) (mg/kg)
1986
Pineapples imported 3 1 0.69
Grapes imported 20 3 0.17-0.35 0.26
Strawberries imported 7 1 0.29
Mangoes imported 17 4 0.20-1.82 0.70
Papayas imported 5 2 0.25-0.45
Pears Swedish 17 3 0.32-0.62 0.43
imported 45 7 0.20-0.45 0.34
Apples Swedish 78 17 0.20-0.72 0.40
imported 91 30 0.21-0.74 0.39
1987
Grapes imported 28 3 0.52-0.87 0.60
Strawberries imported 7 0.23
Mangoes imported 14 5 0.29-1.30 0.66
Papayas imported 4 2 0.86-1.14
Pears Swedish 14 1 0.52
imported 62 13 0.21-0.45 0.29
Apples Swedish 61 25 0.20-1.17 0.45
imported 94 12 0.21-0.82 0.36
a From: FAO/WHO (1988b)
Table 4. National Maximum Residue Limits (mg/kg) for certain commoditiesa
banana cereal cherries citrus bean cucumber peach pome fruit strawberries grapes
Australia 1 0.05 5 10 3 3 5 5 6 2
Austria 0.2 0.5 7 1 0.5 2 1.5 3
Belgium 2 0.5 2 2 0.5 2 5 5 2
Brazil 1 0.5 10 10 2 0.5 10 5 5 10
Bulgaria 0.5 10 5 5 10
Canada 5 10 1 0.5 10 5 5 5
Denmark 2 0.1 2 5 2 2 2 2 5 5
France 1 1.5 6
Finland 0.2 1 2 0.5 0.5 1 1 1
Germany 0.2 0.5 2 7 1 0.5 2 2 3
Hungary 2 1
Israel 10 10 10 5 10
Italy 0.5 0.5 1 1
Mexico 10 2 1 15 7 5 10
Netherlands 3 0.1 3 4 3 3 3 3 3 3
New Zealand 5 1 5 5 2 2 5 5 5 5
Spain (guidelines) 1 0.5 5 7 2 2 5 5 1 5
Switzerland 1 0.2 3 7 0.2 0.1 3 3 3 3
United Kingdom 1 0.5 10 0.5 10 5 5 10
(proposed)
USA 1 0.2 15 10 2 1 15 7 5 10
USSR 1 0.5 10 10 2 0.5 10 5 5 5
Yugoslavia 0.1 7 0.5 0.1 2 0.5 2
a From: FAO/WHO (1988a)
Table 5. Proposed Maximum Residue Limits for carbendazim from any
sourcea
Commodity MRL (mg/kg) Applicationb
Apricot 10c B,C
Asparagus 0.1d B,T
Avocado 0.5 B
Banana 1c B,C,T
Barley straw and fodder, dry 2 B
Bean fodder 50 C
Beans, dry 2 B
Berries and other small fruit 5 B,C,T
Brussel sprouts 0.5 B
Broad bean 2 T
Carrot 5c C,T
Cattle meat 0.1d B
Celery 2 B,C
Cereal grains 0.5 B,C,T
Cherries 10c B,C,T
Citrus fruits 10c B,C,T
Coffee beans 0.1d C
Common beane 2 C
Cucumber 0.5 B,C,T
Eggs (poultry) 0.1d B,T
Egg plant 0.5 C
Gherkin 2 C,T
Hops, dry 50 C
Lettuce, head 5 B,C,T
Mango 2 B
Melons, except watermelons 2c B,C
Milk 0.1d B
Mushrooms 1 B,C,T
Nectarine 2 B
Onion, bulb 2 C,T
Peach 10c B,C,T
Peanut 0.1d B,C
Peanut fodder 5 B,C
Peppers 5 C
Pineapple 20c B
Plums (including prunes) 2c B,C,T
Pome fruit 5c B,C,T
Potato 3c,f B,C
Poultry meat 0.1d B,T
Rape seed 0.05d C
Rice straw and fodder, dry 15 B,C,T
Sheep meat 0.1d B
Soya bean, dry 0.2 C
Soya bean fodder 0.1d C
Squash, summer 0.5 B
Table 5 (contd).
Commodity MRL (mg/kg) Applicationb
Sugar beat 0.1d B,C,T
Sugar beat leaves on tops 10 B,C,T
Swedeg 0.1d C
Sweet potato 1 B
Taro 0.1d B
Tomato 5 B,C,T
Tree nuts 0.1d B
Wheat straw and fodder, dry 5 B
Winter squash 0.5 B
a From: FAO/WHO (1988b)
b B = benomyl; C = carbendazim; T = thiophanate-methyl
c MRL based on post-harvest use
d At or about the limit of detection
e JMPR recommended 2 mg/kg for dry, dwarf, lima and snap beans. These
are all covered by "VP 0526, Common bean" and "VP 0071, Beans,
dry" in the new classification
f washed before analysis
g Described as rutabagas in 1983 recommendation
Table 6. Established inhalation exposure limitsa
Country and agency Compound TWAb STELc
(mg/m3) (mg/m3)
Australia benomyl 10 -
Belgium benomyl 10 -
Denmark benomyl 5 -
Finland benomyl 10 30
France benomyl 10 -
Switzerland benomyl 10 -
United Kingdom benomyl 10 15
USA: ACGIHd benomyl 10 -
USA: NIOSHe/OSHAf benomyl 10 -
(inhalable dust)
USA: NIOSH/OSHA benomyl 5 -
(respirable dust)
USSR carbendazim - 0.1
a From: ILO (1991)
b Time-weighted average
c Short-term exposure limit
d American Conference of Governmental Industrial Hygienists
e National Institute of Occupational Safety and Health
f Occupational Safety and Health Administration
Air concentrations of benomyl ranged from 0.0074 to 0.053
mg/m3 (average 0.027 mg/m3) during its application in
greenhouses. Spraying tall plants (over 1.5 m) caused three times
higher concentrations in air than spraying low plants. No detectable
amounts of benomyl or its metabolites (carbendazim, 4-HBC and 5-HBC)
were found in the urine of applicators during the 48 h following the
application. However, information describing protective clothing,
ventilation, and other hygienic factors was not reported (Liesivuori
& Jääskeläinen, 1984).
6. KINETICS AND METABOLISM
Benomyl is extensively metabolized by animals, as described in
detail in section 6.3. Metabolite names and structures are given in
Table 6 and Figures 2 and 3.
6.1 Absorption
Absorption in ChR-CD male rats was monitored after dermal
application of 0.1, 1, 10, and 100 mg benomyl (as 2-14C-Benlate 50
WP) at 0.5, 1, 2, 4 and 10 h intervals. Four rats were used for each
treatment and time interval. Benomyl was slowly absorbed across an
area of skin (16% of the animal), appearing in the blood and urine
within 30 min after treatment and reaching a maximum between 2 and 4
h after dosing (Belasco, 1979b). The concentration of benomyl and
its metabolites in the blood peaked at 0.05 mg/litre (2 h sample) in
the low-dose group (0.1 mg) and at 0.10 mg/litre (4 h sample) in the
high-dose group (100 mg). This represented a 20-fold increase in
blood concentration after a 1000-fold dose increase. Thus,
absorption into the bloodstream was non-linear with respect to dose.
An in vitro study on the penetration of formulated benomyl
(Benlate 50 WP) through human skin showed that benomyl penetrates
human skin poorly when it is applied as a recommended spray strength
solution. Much less penetration was detected when dry concentrated
benomyl was applied (Ward & Scott, 1992).
In a rat gavage study, the absorption of carbendazim given in
the form of a corn oil suspension was estimated to be approximately
80% (Monson, 1990).
6.2 Distribution and accumulation
Blood levels of benomyl and its metabolites in male rats were
measured 6 and 18 h after exposure in male rats. The rats were
exposed to time-weighted averages of 0.32 and 3.3 mg/litre of air
for 0.5, 1, 2 and 6 h. The methodology did not distinguish between
benomyl and carbendazim. At both exposure levels, the blood
concentrations of benomyl/carbendazim were greater than that of
5-HBC 6 h after exposure; the levels were 0.39-2.3 mg/litre and
0.25-1.2 mg/litre, respectively. At 18 h after exposure, only 5-HBC
was detected in the blood (1.1 mg/litre) and this only at the
highest dose. Urinary metabolites consisted primarily of 5-HBC, and
limited amounts of benomyl/carbendazim were also detected (Turney,
1979).
Table 7. Chemical names of benomyl and its metabolites in animalsa
Common or abbreviated Chemical name
name
Benomyl Carbamic acid, [1-(butylamino)carbonyl]-
1H-benzimidazol-2-yl]-, methyl ester
Carbendazim (MBC) methyl (1-H-benzimidazol-2-yl)carbamate
5-HBC methyl (5-hydroxy-1H-benzimidazol-2-yl)-
carbamate
4-HBC methyl (4-hydroxy-1H-benzimidazol-2-yl)-
carbamate
5-HBC-Sb 2-[(methoxycarbonyl)amino]-1H-benzimidazol-5-
yl hydrogen sulfate
5-HBC-Gc [2-[(methoxycarbonyl)amino]-1H-benzimidazol-
5-yl] ß-D-glucopyranosiduronic acid
MBC-4,5-epoxide
MBC-5,6-epoxide
MBC-4,5-dihydrodiol (4,5-dihydro-4,5-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-5,6-dihydrodiol (5,6-dihydro-5,6-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-4,5-diol
MBC-5,6-diol
5-OH-6-GS-MBCd S-[5,6-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5-OH-4-GS-MBC S-[4,5-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5,6-HOBC-N-oxide methyl (6-hydroxy-5-oxo-5H-benzimidazol-2-
yl)-carbamate-N-oxide
Table 7 (contd).
Common or abbreviated Chemical name
name
5,6-HOBC-N-oxide-G [2-[(methoxycarbonyl)amino]-6-oxo-6H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid-N-oxide
5,6-DHBC methyl (5,6-dihydroxy-1H-benzimidazol-2-yl)
carbamate
5,6-DHBC-G [6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid
5,6-DHBC-S 6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl 5-(hydrogen sulfate)
2-AB 2-aminobenzimidazole
2-AB dihydrodiol 2-amino-4,5-dihydro-4,5-dihydroxy-1H-
benzimidazol
5-HAB 5-hydroxy-2-aminobenzimidazole
a From: Krechniak & Klosowska (1986); Monson (1986a,b); Monson (1990)
b S = conjugate with sulfuric acid
c G = conjugate with glucuronic acid
d GS = conjugate with glutathione
In a study by Han (1979), ten male ChR-CD rats were given 1 and
10 µg benomyl intravenously (as 14C-Benlate 50% WP). Radioactivity
was found in the urine as 5-HBC at 6, 12 and 24 h after dosing, and
there was little radioactivity in the blood or faeces at these
sampling times. No radioactivity (< 0.1%) was found in any tissue
after 24 h except in blood, which contained trace quantities of
14C residues.
In a further study, three groups of five rats of each sex were
gavaged with [phenyl(U)-14C] carbendazim. One group received a
single dose of 14C-carbendazim (50 mg/kg). The second group
received a single dose of 14C-carbendazim (50 mg/kg) following 14
days of pre-conditioning with non-radiolabelled carbendazim (50
mg/kg per day). The third group received a single dose of
14C-carbendazim (1000 mg/kg). For all groups, > 98% of the
recovered radioactivity was excreted in the urine or faeces by the
time of sacrifice (72 h after 14C dosing). The 14C remaining in
tissues was < 1% of the applied dose (Monson, 1990).
In a study by Belasco et al. (1969), 14C-benomyl was
administered to male ChR-CD rats and the blood and testes were
analysed. The fungicide was given by gavage as: (a) a single dose of
1000 mg/kg to five rats, which were sacrificed either 1, 2, 4, 7 or
24 h later; (b) 10 repeated doses of 200 mg/kg per day to two rats,
which were sacrificed either 1 or 24 h after the last dose. In
addition, blood and testes from rats fed 2500 mg/kg diet for one
year were analysed. In rats given 1000 mg/kg, results show that: (a)
the total 14C radioactivity (calculated as benomyl) ranged from 3
to 13 ppm in the blood and from 2 to 4 ppm in the testes; (b) 5-HBC
appeared in the blood and testes as early as 1 h after dosing; and
(c) the concentration of benomyl and/or carbendazim decreased with
time and there was a corresponding increase in the concentration of
5-HBC in the blood and testes. Analyses of blood and testes from
rats given 10 repeated oral doses of 200 mg/kg per day showed that
one hour after the last dose no benomyl/carbendazim (< 0.1 ppm) was
detected and only low levels of 5-HBC were found (1.5 ppm blood and
0.3 ppm in testes). No benomyl/carbendazim or 5-HBC (< 0.1 ppm) was
found 24 h after the last dose. With rats fed 2500 mg benomyl/kg
diet for one year, no benomyl/carbendazim (< 0.1 ppm) was detected
in blood or testes. Only a minimal amount of 5-HBC was found in
blood (0.2 ppm) and none was found in the testes (< 0.1 ppm)
(Belasco et al., 1969).
In a series of metabolic studies, benomyl and/or Benlate (50%
benomyl formulation) were administered either by gavage or in the
diet to pregnant ChR-CD rats to determine the concentrations of
benomyl, carbendazim and two carbendazim metabolites (4-and 5-HBC)
in maternal blood and embryonic tissue (Culik, 1981a,b). Dosing took
place on days 7 to 16 of gestation at levels of 125 mg/kg body
weight per day via gavage or 5000-10 000 mg/kg diet (approximately
400-800 mg/kg body weight). Blood samples from the dams and tissue
samples from their embryos were examined on the first, sixth and
tenth days of dietary administration and on days 12 and 16 of gavage
administration. Embryos and maternal blood were analysed 1, 2, 4, 8
and 24 h after gavage.
The levels of benomyl/carbendazim in maternal blood and
embryonic tissues, 24 h following each dose, markedly decreased with
the number of treatments. The level of benomyl (one hour after
treatment) ranged from 0.98 to 8.4 mg/kg with a mean value of 5.0
mg/kg on the first day of treatment. After 10 treatments, the levels
of benomyl/carbendazim ranged from < 0.12 to 0.39 mg/kg (one hour
after last treatment). In the embryo there was 0.13 mg/kg
benomyl/carbendazim after the tenth treatment compared with a mean
of 1.9 mg/kg after the first treatment. The half-life of benomyl in
maternal blood was approximately 45 min and was less in the embryos.
The level of 5-HBC (0.84-2.9 mg/kg) 2 h following the last gavage
increased with the number of exposures, the half-life in the blood
being 2-3 h in the dam and 4-8 h in the embryo. 4-HBC was not
detected.
In the dietary studies, the levels of benomyl, carbendazim and
4-HBC were too low to be measured in the embryonic tissue. 4-HBC
could not be detected in the dams. Irrespective of the dose level
(5000 and 10 000 mg/kg diet active ingredient) of benomyl or
Benlate, the level of benomyl/carbendazim in maternal blood was very
low. In three separate groups of animals, the mean highest blood
concentrations of benomyl/carbendazim were 0.35, 0.61 and 0.23 mg/kg
in each group of dams. The mean highest value of 5-HBC (5000 mg
benomyl/kg diet) was 0.44 mg/kg in the blood and 0.33 mg/kg in the
embryos. Animals fed benomyl or Benlate at a level of 10 000 mg/kg
diet had 5-HBC levels an order of magnitude higher (Culik, 1981a,b).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approxi mately
17 h after the tenth dose, the cow was sacrificed and organ, tissue
and blood samples were subsequently collected. 14C residue levels
in the milk averaged 0.2 mg/kg (calculated as benomyl), 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids. Small amounts of
radioactivity were detected in the liver (4.12 mg/kg) and kidney
(0.25 mg/kg), most of which was bound. No significant levels of
radioactivity (0.06 mg/kg) were detected in other tissues or fat
(Monson, 1985).
Lactating and non-lactating goats were given daily capsule
doses of [2-14C]-benomyl, equivalent to 36 and 88 mg/kg,
respectively, in the total daily diet, for five days. Milk residues
accounted for approximately 2% of the total dose. Approximately 25%
of the milk radioactivity was incorporated into the natural milk
components casein and whey protein. There were no detectable
residues in muscle tissue and fat (< 0.01 mg/kg). However,
radioactivity detected in liver and kidney amounted to 3.8 and 0.09
mg/kg (calculated as benomyl equivalents), respectively (Han, 1980).
In a study by Johnson (1988), the total 14C residue and
metabolic fate of carbendazim in the liver was examined in
non-lactating female goats. Twelve goats were administered a
feed-rate-equivalent dose of [phenyl(U)-14C]-carbendazim (> 50
mg/kg), once a day, for up to 30 days. Within 2 weeks of dose
initiation, a plateau of 14C residues in the liver was achieved at
a level of 9.48 mg/kg (group mean of the total radiolabelled liver
residues for goats sacrificed 2, 3, and 4 weeks after initiation of
dosing). The total 14C residue levels in the liver decreased to
5.17, 3.55 and 1.67 mg/kg (calculated as carbendazim equivalents) 1,
2 and 3 weeks, respectively, after dosing ceased. The elimination
half-life for total 14C residues from the liver, based on this
depuration data, was calculated to be approximately 9 days. The
half-life for removal of carbendazim from the general circulation,
based on 14C-carbendazim equivalent whole blood levels, was
approximately 10 h. The level of bound, non-extractable 14C
residues in the liver of goats sacrificed after 28 days was 1.0
mg/kg.
The results of this study suggest that levels of carbendazim-
derived residues do not accumulate beyond 2 weeks when goats are
exposed to a constant feed level of 50 mg carbendazim/kg.
Furthermore, discontinuation of exposure results in a clearing of
residues from the liver (Johnson, 1988).
The metabolism of benomyl was studied in laying hens by Monson
(1986a). Two hens were individually dosed daily for three
consecutive days with 3.5 mg [2-14C]-benomyl at a rate equivalent
to 29 mg/kg in the daily feed, and two hens were individually dosed
with 3.29 mg [phenyl(U)-14C]-benomyl at rates equivalent to 27
mg/kg in the daily feed. Faeces and eggs from the previous 24 h were
collected just before each dosing. Twenty-two hours after the third
dose, the hens were killed and samples of muscle (breast and thigh),
liver, kidney and fat were analysed. The concentration of
radioactivity (calculated as benomyl equivalents) in the tissues and
day-3 eggs of the [2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-
dosed hens, respectively, was as follows: liver (0.54 and 0.41
mg/kg), kidney (0.28 and 0.16 mg/kg), thigh and breast muscle (both
0.01 mg/kg), fat (0.05 and 0.02 mg/kg) and eggs (0.08 and 0.05
mg/kg).
The distribution of benomyl in this study was comparable to
that in a 20-hen [2-14C]-carbendazim metabolism study. The
concentrations of radioactivity, calculated as mg carbendazim/kg, in
the high-dose laying hens (dose equivalent 120 mg/kg carbendazim in
the diet) were liver (2.63), kidney (1.74), thigh muscle (0.06),
breast muscle (0.05), fat (0.03), day-6 eggs (0.63) (Monson, 1986b).
This study is discussed in detail in the Environmental Health
Criteria monograph on Carbendazim (WHO, 1993).
When bluegill sunfish were exposed to benomyl, carbendazim and
2-AB at nominal concentrations of 0.05 mg/litre (measured
concentrations of 0.01 to 0.04 mg/litre) and 5.0 mg/litre (measured
concentrations of 2 to 5 mg/litre), no residues were found in the
tissues of fish exposed to low levels of these three compounds.
Detectable residues were found in the tissues of fish exposed to the
high levels, but there was no build-up or bioconcentration with time
(DuPont, 1972).
6.3 Metabolic transformation
Benomyl is extensively metabolized by rats to carbendazim,
which is then further metabolized. Studies with rats administered
benomyl intravenously (Han, 1979), dermally (Belasco, 1979b) or by
inhalation (FAO/WHO, 1985a) showed that 5-HBC is the main urinary
metabolite, some carbendazim also being present.
In a rat gavage study (Monson, 1990; see section 6.2),
carbendazim was extensively metabolized. Three dosing regimens (five
rats of each sex per group) were used: a single oral dose of 50
mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with non-radiolabelled carbendazim at 50
mg/kg for 14 days (pre-conditioned low dose); and a single oral dose
of 1000 mg/kg (high dose). The 48-h urine from the low-dose and the
high-dose rats and the 14-day urine from the pre-conditioned
low-dose group were collected. The total recovery from urine was
61.5 and 61.7% of given doses for the low-dose and pre-conditioned
low-dose male groups, 53.2 and 59.3% for the low-dose and
pre-conditioned low-dose female groups, and 39 and 41% for both male
and female high-dose groups, respectively. 5-HBC-S (21-43% of given
dose) was identified as the main metabolite, except in the case of
the pre-conditioned low-dose and high-dose female rat groups
(5.5-10%), while in all female rat groups 5,6-HOBC-N-oxide-G
(10-19%) was predominant. Both 5,6-DHBC-S and 5,6-DHBC-G were
identified as minor metabolites.
In the same study, the faeces were collected at the same
periods as the urine. The total recovery from faeces was about 24%
for the low-dose and pre-conditioned low-dose male groups, 33-38%
for the low-dose and pre-conditioned low-dose female groups, and
higher (> 60%) for both male and female high-dose groups. Unchanged
carbendazim was about 10-15% of the given dose in the faeces of
high-dose rats (Monson, 1990). The proposed metabolic pathway for
benomyl in rats is given in Fig. 2.
When a lactating Holstein cow was dosed by capsule twice daily
(515 mg per dose), equivalent to 50 mg/kg diet, for 5 consecutive
days with [2-14C]-benomyl, the major metabolites of whole milk
were 5-HBC (0.06 mg/litre), 4-HBC (0.03 mg/litre) and MBC-4,5-
dihydrodiol (< 0.07 mg/litre). The proportions of radioactive
residues in the urine were 46% 5-HBC, 3% 4-HBC, and 50% polar
aqueous-soluble metabolites, which included MBC-4,5-dihydrodiol,
2-AB-dihydrodiol and 5-OH-4-GS-MBC (Monson, 1985).
Similar mobility results have been observed in the field.
Benomyl and its degradates were studied on bare soil and turf in
four areas of the USA. Carbendazim and 2-AB were the major and minor
degradates, respectively. After 1 and 2 years of outdoor exposure,
the half-life of total benzimidazole-containing residues was about 3
to 6 months on turf and about 6 to 12 months on bare soil (Baude et
al., 1974). Under these conditions, benomyl, carbendazim and 2-AB
showed little or no downward movement.
4.1.4 Leaching
To evaluate the risk of pollution of ground and drainage water,
screening models based on adsorption and persistence can be used,
together with existing analyses of groundwater samples. Gustafson
(1989) proposed the use of the equation GUS = log T´ (4 - log
Koc); GUS values < 1.8 = "improbable leachers", GUS values of
1.8-2.8 = "transition" and GUS values > 2.8 = "probable leachers".
For benomyl, Kom values of 550, 620, 2100 and 1100 (mean 1093)
were found in four different soils (Priester, 1985). A Kom of 1093
is equal to a Koc of 1857 since Koc = Kom x 1.7. The half-life
of 320 days given by Marsh & Arthur (1989) seems in good agreement
with field half-lives of 6 to 12 months (Baude et al., 1974).
When the calculation of the GUS value is based on a Koc of
1857 and a T0.5 of 320 days, a value of 1.83 is obtained.
According to this value, benomyl/carbendazim lies between the
"improbable leachers" and "transition", and, therefore, would not be
expected to occur in ground water. The adsorption of benomyl and of
carbendazim is expected to be of the same order of magnitude since
the Kow values are almost identical (log Kow = 1.49 and 1.36 for
carbendazim and benomyl, respectively). In ground water studies in
the USA (Parsons & Witt, 1988), benomyl was not found in any of 495
wells tested and carbendazim not in any of 212 wells (detection
limit not reported).
In an EEC survey (Fielding, 1992), the presence of carbendazim
in groundwater in the Netherlands and in Italy was investigated.
Carbendazim was found in one of two samples from the Netherlands
(0.1 µg/litre), and the level was above 0.1 µg/litre in 23 of 70
samples in Italy. Detection of the non-polar DDT and lindane in many
wells in the Italian study may indicate macropore transport or
artifacts such as direct pollution of wells.
4.1.5 Crop uptake
Various greenhouse and outdoor tests, in which benomyl was
applied to several crops (apples, bananas, cucumbers, grapes and
oranges), indicate that benomyl and carbendazim remain on plant
surfaces as major components of the total residue (Baude et al.,
1973). Benomyl is primarily converted to carbendazim once inside
plant tissues.
Although benomyl is systemic when applied directly to plant
foliage, crop uptake of soil residues is extremely low, even when
the crop is planted in the same growing season as the benomyl
treatment. In a greenhouse crop-rotation study, [2-14C]-
carbendazim, the more persistent benomyl metabolite, was applied to
a loamy sand soil. Aging periods of 30, 120 or 145 days were used
and the crops studied were beets, barley and cabbage. Radioactivity
did not accumulate in these crops grown to maturity in a loamy sand
soil treated 30 days earlier with 1 kg a.i./ha or 120 to 145 days
earlier with 3 kg a.i./ha. Accumulation factors, calculated as the
ratio of radioactivity in the crop to that in the corresponding
soil, were very low in beet foliage (0.04) and beet roots (0.03),
low in cabbage and barley grain (0.2), and ranged from 0.9 to 1.2 in
barley straw (Rhodes, 1987).
4.2 Transformation
Numerous field studies to determine the fate and behaviour of
benomyl in soil have shown the instability of benomyl under various
conditions. In solutions, plants, and soil, it degrades to
carbendazim. The conversion of benomyl under alkaline conditions to
STB and BBU has also been reported (section 4.1). The environmental
fate of benomyl has been thoroughly reviewed by Zbozinek (1984).
4.2.1 Biodegradation
4.2.1.1 Water
Anaerobic aquatic degradation studies in pond water and
sediment showed a half-life of 2 h for benomyl and 743 days for its
degradation product carbendazim. Some (1-8%) transformation to STB
occurred. After one year 36% of the applied radioactivity was bound
to the sediment (Arthur et al., 1989b).
4.2.1.2 Soil
In a study by Marsh & Arthur (1989), non-sterile and sterile
samples of Keyport silt loam soil were treated with [phenyl(U)-
14C]benomyl at a concentration of approximately 7.0 mg/kg. This is
equivalent to the expected soil residues in the surface 10 cm of
topsoil when benomyl is applied at 8 kg a.i./ha. Distilled water was
added to each sample until it reached 75% of its moisture-holding
capacity at 0.33 bar. The soils were incubated in the dark at
approximately 25 °C. The non-sterile soil flasks were sampled after
0.1, 0.2, 1, 3, 7, 14, 30, 60, 120, 270 and 365 days. Samples of
sterilized soil were taken after 14, 30, 120, 270 and 365 days.
The half-life of benomyl in non-sterile silt loam was 19 h, but
this was not determined in the sterilized soil. Benomyl was rapidly
converted to carbendazim. The carbendazim had a half-life of 320
days under non-sterile aerobic conditions (Marsh & Arthur, 1989).
This is in close agreement with reported half-lives of 6-12 months
for benzimidazoles applied to bare soil (Baude et al., 1974).
After 365 days of incubation, 9% of the 14C was evolved as
14CO2, 34% could still be recovered as carbendazim, and 36% was
not extractable. The total recovery of 14C was 88%.
In the sterilized soil, the half-life of carbendazim was
approximately 1000 days (Marsh & Arthur, 1989).
When the degradation of 2-14C-carbendazim (20 mg/kg) was
determined, 33% of the 14C label added was evolved as 14CO2
during 270 days. Identical or even faster 14C evolution was
observed from 2-14C-labelled 2-AB (Helweg, 1977). The relatively
low 14C evolution from phenyl-14C-labelled benomyl/carbendazim
may be caused by the formation of strongly adsorbed degradation
products or compounds that are readily incorporated into soil
organic matter. Thus, most of the remaining radioactivity was
accounted for in the organic fraction of the soil.
To elucidate the reason for the low 14C evolution from
phenyl-14C-labelled fungicide, the fate of a possible degradation
product, 1,2-diaminobenzene, needs to be determined.
4.2.1.3 Crops
Metabolism studies in various crops (soybeans, rice, sugar beet
and peaches) using [phenyl(U)-14C]benomyl have shown that the only
species of significance in plant tissues are benomyl, carbendazim
and 2-AB. Soybeans were treated twice with 1 kg a.i./ha and
harvested 35 days later. Rice was treated twice with 2 kg a.i./ha
and harvested at 21 days, sugar beet was treated with 0.5 kg a.i./ha
five times and harvested at 21 days, and peaches were treated twice
at 1 kg a.i./ha and harvested 20 min after spraying. Soybeans, rice
and sugar beet were treated at twice the recommended application
rate. The concentration of radiolabelled compounds in mature
soybeans was 0.7 mg/kg and consisted of 0.42 mg 2-AB/kg, 0.05 mg
benomyl/kg and 0.14 mg carbendazim per kg (Bolton et al., 1986a).
Levels in the rice grain were 2.7 and 7.3 mg/kg for benomyl and
carbendazim, respectively (Bolton et al., 1986b). Sugar beet tops
retained 99% of the total recovered radioactivity, 6.8 mg/kg being
present as carbendazim and 0.4 mg/kg as benomyl. The roots retained
only 0.01 mg carbendazim per kg (Tolle, 1988). After the first
application to peaches, benomyl was present at 0.65 mg/kg and
carbendazim at 0.72 mg/kg. The second application resulted in 0.33
mg benomyl/kg and 0.92 mg carbendazim/kg. No other radioactive
metabolites were found in peaches (Stevenson, 1985).
Chiba & Veres (1981) applied benomyl to apple trees as Benlate
50% WP at a rate of 1.7 kg/ha. Three successive applications were
made in 1977 and a single spray was applied in 1979. Between 3 and 7
days after application there was a marked reduction of about 50% in
benomyl residues from an initial level of about 110 mg/kg. This fall
in benomyl was accompanied by a doubling in the level of carbendazim
residues over the same period due to benomyl degradation to
carbendazim. Within 46 days of the single application in 1979,
benomyl residues fell to 0.63 mg/kg foliage and carbendazim was
present at 1.2 mg/kg. Following the three sprayings in 1977 (at 0,
13 and 27 days after the initial application), residue levels were
2.6 and 17.1 mg/kg foliage for benomyl and carbendazim 83 days after
the first spraying. Both experiments showed an exponential fall in
benomyl residues but the rate of decline was much slower in the case
of the more persistent metabolite.
4.2.2 Abiotic degradation
In a study by Wheeler (1985), the hydrolysis of benomyl was
studied in sterilized aqueous solutions maintained at 25 °C in the
dark for 30 days at pH 5, 7 and 9. In pH 5 buffer, the major product
was carbendazim, whereas at pH 7 and 9 carbendazim and STB were the
major products. STB represented approximately 25% of the total
radioactivity at pH 7 and approximately 80% at pH 9. The half-lives
of benomyl in the pH 5, 7 and 9 solutions were approximately 3.5,
1.5 and less than 1 h, respectively. There was no further
degradation of carbendazim at pH 5 and 7 over 30 days. At pH 9,
however, carbendazim was slowly hydrolysed to 2-AB with a half-life
of 54 days (Priester, 1984).
Aqueous photolysis studies conducted in natural sunlight have
shown that benomyl is mainly degraded by hydrolysis rather than
photolysis (Powley, 1985).
4.2.3 Bioaccumulation
Although only low concentrations of benomyl or its metabolites
would be expected in natural waters, studies have evaluated the
metabolism and bioaccumulation in fish. Bluegill sunfish ( Lepomis
macrochirus) were exposed to radiolabelled carbendazim
concentrations of 0.018 or 0.17 mg/litre for 4 weeks in a dynamic
study designed to measure the bioaccumulation of 14C residues in
edible tissue, viscera, remaining carcass and whole fish. A two-week
depuration phase followed the exposure phase. Results were similar
at the two exposure concentrations, the peak whole fish
bioconcentration factors (BCFs) being 27 and 23 at the low and high
exposure levels, respectively. The radioactivity was concentrated
more in the viscera than in other tissues, the peak viscera BCFs
being 460 and 380 for the low and high exposure levels,
respectively. Very little bioconcentration occurred in the muscle
tissue (BCF = < 4) or the remaining carcass (BCF = < 7). During
the 14-day depuration phase, > 94% of the peak level of
radioactivity was lost from the whole fish, viscera and muscle. The
rate of loss from the carcass tissue was lower (77% and 82% loss for
the low and high exposure levels, respectively) (Hutton et al.,
1984).
When rainbow trout ( Oncorhynchus mykiss), channel catfish
( Ictalurus punctatus) and bluegill sunfish ( Lepomis macrochirus)
were injected intraperitoneally with carbendazim, branchial and
biliary excretion were the major pathways for the elimination
(Palawski & Knowles, 1986). In a separate experiment, the three fish
species were exposed to 45 µg carbendazim/litre for 96 h, except in
the case of catfish, which were exposed for 48 h. This was followed
by a 96-h depuration phase. Rainbow trout had the highest uptake
rate constant (1.78 per h) and bioconcentration factor (159) of the
three species. Much less carbendazim was accumulated by channel
catfish than by the other two species, but this residue level (0.44
µg/g) appeared to be lethal after 48 h of exposure. The elimination
rate constant and the biological half-life of carbendazim were
similar for rainbow trout and bluegill sunfish. However, the
elimination rate constant was greater and the biological half-life
shorter in channel catfish (13 h) than in the other two species.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air, water and soil
The environmental levels in air, water and soil are discussed
in detail in section 4.
5.1.2 Food and feed
Levels of benomyl in food and feed are indicated in section
5.2.
5.1.3 Terrestrial and aquatic organisms
Benomyl levels in terrestrial and aquatic organisms are
discussed in detail in sections 4 and 6.
5.2 General population exposure
The principal exposure of the general population to benomyl is
through dietary exposure. It was recommended by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1988b) that all
maximum residue limits (MRLs) for benomyl, thiophanate-methyl and
carbendazim be listed as carbendazim (see Table 5).
5.2.1 USA
A system called the Dietary Risk Evaluation System (DRES),
which was developed by the US Environmental Protection Agency, was
used to quantify the intake of residues occurring in various
commodities. The system assumes a diet consistent with the 1977-1978
USDA Nationwide Food Consumption Survey. This survey was a
stratified probability survey in which 3-day dietary records of
approximately 30 000 individuals were collected. The dietary intake
of residues resulting from registered food crop uses of benomyl was
then estimated using mean residue levels found in controlled field
trials and adjusting for the effects of food processing, e.g.,
washing and cooking, on residues of benomyl and its metabolites.
Based on this analysis, the total dietary exposure was
determined for the general population and for a number of population
subgroups. The exposure of the average person to residues resulting
from benomyl use was estimated to be 0.218 µg/kg body weight per
day. The highest exposure was found in the population subgroup
entitled "non-hispanic other than black or white", the estimated
exposure being 1.479 µg/kg body weight per day. The lowest exposure
was found in the > 20-year-old males where the estimated exposure
was 0.144 µg/kg body weight per day (Eickhoff et al., 1989). These
estimates are below the benomyl ADI allocated by JMPR (0-0.02 mg/kg
body weight per day) (FAO/WHO, 1985a,b).
5.2.2 Sweden
Residue monitoring data for benzimidazole fungicides, i.e.
benomyl, carbendazim and thiophanate-methyl, on food crops from
Sweden is shown in Table 3 (FAO/WHO, 1988b). No further analysis to
determine dietary intake was performed.
5.2.3 Maximum residue limits
National MRLs for certain commodities are listed in Table 4
(FAO/WHO, 1988a).
A complete list of MRLs for carbendazim, including new
proposals and an indication of the source of the data (application
of benomyl, carbendazim, or thiophanate-methyl) on which the MRL is
based, is given in Table 5 (FAO/WHO, 1988b).
5.3 Occupational exposure during manufacture, formulation or use
The levels of inhalation exposure to benomyl and carbendazim
experienced by workers in a major manufacturing facility (DuPont)
were reviewed from 1986 to 1989. The average levels of benomyl and
carbendazim were less than 0.2 mg/m3 and 0.3 mg/m3,
respectively. Table 6 lists established inhalation exposure limits
for benomyl and carbendazim.
5.3.1 Use
Potential dermal and respiratory exposure to benomyl wettable
powder formulation under actual use situations has been determined
for: a) tank loading and mixing for aerial application; b) re-entry
into treated fields; and c) home use (garden, ornamental and
greenhouse). For crop treatments, approximately 17 kg benomyl
(formulation) was handled per cycle. Maximum exposure occurred in
the loading and mixing operation for aerial application, where
dermal exposure was 26 mg benomyl per mixing cycle, primarily to
hands and forearms (90%) and respiratory exposure averaged 0.08 mg
benomyl. Re-entry data revealed dermal and respiratory exposures of
5.9 mg/h and < 0.002 mg/h, respectively. Home-use situations
(application of 7 to 8 litres of benomyl in hand-held compressed air
sprayers) produced exposures of 1 mg and 0.003 mg per application
cycle for dermal and respiratory routes, respectively (Everhart &
Holt, 1982). Similar average dermal exposure levels (5.39 mg/h) for
strawberry harvesters were reported by Zweig et al (1983).
Table 3. Benomyl/carbendazim/thiophanate-methyl residues in food in Swedena
Samples Swedish/imported No. of samples Samples with residues Residue level Median value
>0.20 mg/kg (mg/kg) (mg/kg)
1986
Pineapples imported 3 1 0.69
Grapes imported 20 3 0.17-0.35 0.26
Strawberries imported 7 1 0.29
Mangoes imported 17 4 0.20-1.82 0.70
Papayas imported 5 2 0.25-0.45
Pears Swedish 17 3 0.32-0.62 0.43
imported 45 7 0.20-0.45 0.34
Apples Swedish 78 17 0.20-0.72 0.40
imported 91 30 0.21-0.74 0.39
1987
Grapes imported 28 3 0.52-0.87 0.60
Strawberries imported 7 0.23
Mangoes imported 14 5 0.29-1.30 0.66
Papayas imported 4 2 0.86-1.14
Pears Swedish 14 1 0.52
imported 62 13 0.21-0.45 0.29
Apples Swedish 61 25 0.20-1.17 0.45
imported 94 12 0.21-0.82 0.36
a From: FAO/WHO (1988b)
Table 4. National Maximum Residue Limits (mg/kg) for certain commoditiesa
banana cereal cherries citrus bean cucumber peach pome fruit strawberries grapes
Australia 1 0.05 5 10 3 3 5 5 6 2
Austria 0.2 0.5 7 1 0.5 2 1.5 3
Belgium 2 0.5 2 2 0.5 2 5 5 2
Brazil 1 0.5 10 10 2 0.5 10 5 5 10
Bulgaria 0.5 10 5 5 10
Canada 5 10 1 0.5 10 5 5 5
Denmark 2 0.1 2 5 2 2 2 2 5 5
France 1 1.5 6
Finland 0.2 1 2 0.5 0.5 1 1 1
Germany 0.2 0.5 2 7 1 0.5 2 2 3
Hungary 2 1
Israel 10 10 10 5 10
Italy 0.5 0.5 1 1
Mexico 10 2 1 15 7 5 10
Netherlands 3 0.1 3 4 3 3 3 3 3 3
New Zealand 5 1 5 5 2 2 5 5 5 5
Spain (guidelines) 1 0.5 5 7 2 2 5 5 1 5
Switzerland 1 0.2 3 7 0.2 0.1 3 3 3 3
United Kingdom 1 0.5 10 0.5 10 5 5 10
(proposed)
USA 1 0.2 15 10 2 1 15 7 5 10
USSR 1 0.5 10 10 2 0.5 10 5 5 5
Yugoslavia 0.1 7 0.5 0.1 2 0.5 2
a From: FAO/WHO (1988a)
Table 5. Proposed Maximum Residue Limits for carbendazim from any
sourcea
Commodity MRL (mg/kg) Applicationb
Apricot 10c B,C
Asparagus 0.1d B,T
Avocado 0.5 B
Banana 1c B,C,T
Barley straw and fodder, dry 2 B
Bean fodder 50 C
Beans, dry 2 B
Berries and other small fruit 5 B,C,T
Brussel sprouts 0.5 B
Broad bean 2 T
Carrot 5c C,T
Cattle meat 0.1d B
Celery 2 B,C
Cereal grains 0.5 B,C,T
Cherries 10c B,C,T
Citrus fruits 10c B,C,T
Coffee beans 0.1d C
Common beane 2 C
Cucumber 0.5 B,C,T
Eggs (poultry) 0.1d B,T
Egg plant 0.5 C
Gherkin 2 C,T
Hops, dry 50 C
Lettuce, head 5 B,C,T
Mango 2 B
Melons, except watermelons 2c B,C
Milk 0.1d B
Mushrooms 1 B,C,T
Nectarine 2 B
Onion, bulb 2 C,T
Peach 10c B,C,T
Peanut 0.1d B,C
Peanut fodder 5 B,C
Peppers 5 C
Pineapple 20c B
Plums (including prunes) 2c B,C,T
Pome fruit 5c B,C,T
Potato 3c,f B,C
Poultry meat 0.1d B,T
Rape seed 0.05d C
Rice straw and fodder, dry 15 B,C,T
Sheep meat 0.1d B
Soya bean, dry 0.2 C
Soya bean fodder 0.1d C
Squash, summer 0.5 B
Table 5 (contd).
Commodity MRL (mg/kg) Applicationb
Sugar beat 0.1d B,C,T
Sugar beat leaves on tops 10 B,C,T
Swedeg 0.1d C
Sweet potato 1 B
Taro 0.1d B
Tomato 5 B,C,T
Tree nuts 0.1d B
Wheat straw and fodder, dry 5 B
Winter squash 0.5 B
a From: FAO/WHO (1988b)
b B = benomyl; C = carbendazim; T = thiophanate-methyl
c MRL based on post-harvest use
d At or about the limit of detection
e JMPR recommended 2 mg/kg for dry, dwarf, lima and snap beans. These
are all covered by "VP 0526, Common bean" and "VP 0071, Beans,
dry" in the new classification
f washed before analysis
g Described as rutabagas in 1983 recommendation
Table 6. Established inhalation exposure limitsa
Country and agency Compound TWAb STELc
(mg/m3) (mg/m3)
Australia benomyl 10 -
Belgium benomyl 10 -
Denmark benomyl 5 -
Finland benomyl 10 30
France benomyl 10 -
Switzerland benomyl 10 -
United Kingdom benomyl 10 15
USA: ACGIHd benomyl 10 -
USA: NIOSHe/OSHAf benomyl 10 -
(inhalable dust)
USA: NIOSH/OSHA benomyl 5 -
(respirable dust)
USSR carbendazim - 0.1
a From: ILO (1991)
b Time-weighted average
c Short-term exposure limit
d American Conference of Governmental Industrial Hygienists
e National Institute of Occupational Safety and Health
f Occupational Safety and Health Administration
Air concentrations of benomyl ranged from 0.0074 to 0.053
mg/m3 (average 0.027 mg/m3) during its application in
greenhouses. Spraying tall plants (over 1.5 m) caused three times
higher concentrations in air than spraying low plants. No detectable
amounts of benomyl or its metabolites (carbendazim, 4-HBC and 5-HBC)
were found in the urine of applicators during the 48 h following the
application. However, information describing protective clothing,
ventilation, and other hygienic factors was not reported (Liesivuori
& Jääskeläinen, 1984).
6. KINETICS AND METABOLISM
Benomyl is extensively metabolized by animals, as described in
detail in section 6.3. Metabolite names and structures are given in
Table 6 and Figures 2 and 3.
6.1 Absorption
Absorption in ChR-CD male rats was monitored after dermal
application of 0.1, 1, 10, and 100 mg benomyl (as 2-14C-Benlate 50
WP) at 0.5, 1, 2, 4 and 10 h intervals. Four rats were used for each
treatment and time interval. Benomyl was slowly absorbed across an
area of skin (16% of the animal), appearing in the blood and urine
within 30 min after treatment and reaching a maximum between 2 and 4
h after dosing (Belasco, 1979b). The concentration of benomyl and
its metabolites in the blood peaked at 0.05 mg/litre (2 h sample) in
the low-dose group (0.1 mg) and at 0.10 mg/litre (4 h sample) in the
high-dose group (100 mg). This represented a 20-fold increase in
blood concentration after a 1000-fold dose increase. Thus,
absorption into the bloodstream was non-linear with respect to dose.
An in vitro study on the penetration of formulated benomyl
(Benlate 50 WP) through human skin showed that benomyl penetrates
human skin poorly when it is applied as a recommended spray strength
solution. Much less penetration was detected when dry concentrated
benomyl was applied (Ward & Scott, 1992).
In a rat gavage study, the absorption of carbendazim given in
the form of a corn oil suspension was estimated to be approximately
80% (Monson, 1990).
6.2 Distribution and accumulation
Blood levels of benomyl and its metabolites in male rats were
measured 6 and 18 h after exposure in male rats. The rats were
exposed to time-weighted averages of 0.32 and 3.3 mg/litre of air
for 0.5, 1, 2 and 6 h. The methodology did not distinguish between
benomyl and carbendazim. At both exposure levels, the blood
concentrations of benomyl/carbendazim were greater than that of
5-HBC 6 h after exposure; the levels were 0.39-2.3 mg/litre and
0.25-1.2 mg/litre, respectively. At 18 h after exposure, only 5-HBC
was detected in the blood (1.1 mg/litre) and this only at the
highest dose. Urinary metabolites consisted primarily of 5-HBC, and
limited amounts of benomyl/carbendazim were also detected (Turney,
1979).
Table 7. Chemical names of benomyl and its metabolites in animalsa
Common or abbreviated Chemical name
name
Benomyl Carbamic acid, [1-(butylamino)carbonyl]-
1H-benzimidazol-2-yl]-, methyl ester
Carbendazim (MBC) methyl (1-H-benzimidazol-2-yl)carbamate
5-HBC methyl (5-hydroxy-1H-benzimidazol-2-yl)-
carbamate
4-HBC methyl (4-hydroxy-1H-benzimidazol-2-yl)-
carbamate
5-HBC-Sb 2-[(methoxycarbonyl)amino]-1H-benzimidazol-5-
yl hydrogen sulfate
5-HBC-Gc [2-[(methoxycarbonyl)amino]-1H-benzimidazol-
5-yl] ß-D-glucopyranosiduronic acid
MBC-4,5-epoxide
MBC-5,6-epoxide
MBC-4,5-dihydrodiol (4,5-dihydro-4,5-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-5,6-dihydrodiol (5,6-dihydro-5,6-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-4,5-diol
MBC-5,6-diol
5-OH-6-GS-MBCd S-[5,6-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5-OH-4-GS-MBC S-[4,5-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5,6-HOBC-N-oxide methyl (6-hydroxy-5-oxo-5H-benzimidazol-2-
yl)-carbamate-N-oxide
Table 7 (contd).
Common or abbreviated Chemical name
name
5,6-HOBC-N-oxide-G [2-[(methoxycarbonyl)amino]-6-oxo-6H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid-N-oxide
5,6-DHBC methyl (5,6-dihydroxy-1H-benzimidazol-2-yl)
carbamate
5,6-DHBC-G [6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid
5,6-DHBC-S 6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl 5-(hydrogen sulfate)
2-AB 2-aminobenzimidazole
2-AB dihydrodiol 2-amino-4,5-dihydro-4,5-dihydroxy-1H-
benzimidazol
5-HAB 5-hydroxy-2-aminobenzimidazole
a From: Krechniak & Klosowska (1986); Monson (1986a,b); Monson (1990)
b S = conjugate with sulfuric acid
c G = conjugate with glucuronic acid
d GS = conjugate with glutathione
In a study by Han (1979), ten male ChR-CD rats were given 1 and
10 µg benomyl intravenously (as 14C-Benlate 50% WP). Radioactivity
was found in the urine as 5-HBC at 6, 12 and 24 h after dosing, and
there was little radioactivity in the blood or faeces at these
sampling times. No radioactivity (< 0.1%) was found in any tissue
after 24 h except in blood, which contained trace quantities of
14C residues.
In a further study, three groups of five rats of each sex were
gavaged with [phenyl(U)-14C] carbendazim. One group received a
single dose of 14C-carbendazim (50 mg/kg). The second group
received a single dose of 14C-carbendazim (50 mg/kg) following 14
days of pre-conditioning with non-radiolabelled carbendazim (50
mg/kg per day). The third group received a single dose of
14C-carbendazim (1000 mg/kg). For all groups, > 98% of the
recovered radioactivity was excreted in the urine or faeces by the
time of sacrifice (72 h after 14C dosing). The 14C remaining in
tissues was < 1% of the applied dose (Monson, 1990).
In a study by Belasco et al. (1969), 14C-benomyl was
administered to male ChR-CD rats and the blood and testes were
analysed. The fungicide was given by gavage as: (a) a single dose of
1000 mg/kg to five rats, which were sacrificed either 1, 2, 4, 7 or
24 h later; (b) 10 repeated doses of 200 mg/kg per day to two rats,
which were sacrificed either 1 or 24 h after the last dose. In
addition, blood and testes from rats fed 2500 mg/kg diet for one
year were analysed. In rats given 1000 mg/kg, results show that: (a)
the total 14C radioactivity (calculated as benomyl) ranged from 3
to 13 ppm in the blood and from 2 to 4 ppm in the testes; (b) 5-HBC
appeared in the blood and testes as early as 1 h after dosing; and
(c) the concentration of benomyl and/or carbendazim decreased with
time and there was a corresponding increase in the concentration of
5-HBC in the blood and testes. Analyses of blood and testes from
rats given 10 repeated oral doses of 200 mg/kg per day showed that
one hour after the last dose no benomyl/carbendazim (< 0.1 ppm) was
detected and only low levels of 5-HBC were found (1.5 ppm blood and
0.3 ppm in testes). No benomyl/carbendazim or 5-HBC (< 0.1 ppm) was
found 24 h after the last dose. With rats fed 2500 mg benomyl/kg
diet for one year, no benomyl/carbendazim (< 0.1 ppm) was detected
in blood or testes. Only a minimal amount of 5-HBC was found in
blood (0.2 ppm) and none was found in the testes (< 0.1 ppm)
(Belasco et al., 1969).
In a series of metabolic studies, benomyl and/or Benlate (50%
benomyl formulation) were administered either by gavage or in the
diet to pregnant ChR-CD rats to determine the concentrations of
benomyl, carbendazim and two carbendazim metabolites (4-and 5-HBC)
in maternal blood and embryonic tissue (Culik, 1981a,b). Dosing took
place on days 7 to 16 of gestation at levels of 125 mg/kg body
weight per day via gavage or 5000-10 000 mg/kg diet (approximately
400-800 mg/kg body weight). Blood samples from the dams and tissue
samples from their embryos were examined on the first, sixth and
tenth days of dietary administration and on days 12 and 16 of gavage
administration. Embryos and maternal blood were analysed 1, 2, 4, 8
and 24 h after gavage.
The levels of benomyl/carbendazim in maternal blood and
embryonic tissues, 24 h following each dose, markedly decreased with
the number of treatments. The level of benomyl (one hour after
treatment) ranged from 0.98 to 8.4 mg/kg with a mean value of 5.0
mg/kg on the first day of treatment. After 10 treatments, the levels
of benomyl/carbendazim ranged from < 0.12 to 0.39 mg/kg (one hour
after last treatment). In the embryo there was 0.13 mg/kg
benomyl/carbendazim after the tenth treatment compared with a mean
of 1.9 mg/kg after the first treatment. The half-life of benomyl in
maternal blood was approximately 45 min and was less in the embryos.
The level of 5-HBC (0.84-2.9 mg/kg) 2 h following the last gavage
increased with the number of exposures, the half-life in the blood
being 2-3 h in the dam and 4-8 h in the embryo. 4-HBC was not
detected.
In the dietary studies, the levels of benomyl, carbendazim and
4-HBC were too low to be measured in the embryonic tissue. 4-HBC
could not be detected in the dams. Irrespective of the dose level
(5000 and 10 000 mg/kg diet active ingredient) of benomyl or
Benlate, the level of benomyl/carbendazim in maternal blood was very
low. In three separate groups of animals, the mean highest blood
concentrations of benomyl/carbendazim were 0.35, 0.61 and 0.23 mg/kg
in each group of dams. The mean highest value of 5-HBC (5000 mg
benomyl/kg diet) was 0.44 mg/kg in the blood and 0.33 mg/kg in the
embryos. Animals fed benomyl or Benlate at a level of 10 000 mg/kg
diet had 5-HBC levels an order of magnitude higher (Culik, 1981a,b).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approxi mately
17 h after the tenth dose, the cow was sacrificed and organ, tissue
and blood samples were subsequently collected. 14C residue levels
in the milk averaged 0.2 mg/kg (calculated as benomyl), 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids. Small amounts of
radioactivity were detected in the liver (4.12 mg/kg) and kidney
(0.25 mg/kg), most of which was bound. No significant levels of
radioactivity (0.06 mg/kg) were detected in other tissues or fat
(Monson, 1985).
Lactating and non-lactating goats were given daily capsule
doses of [2-14C]-benomyl, equivalent to 36 and 88 mg/kg,
respectively, in the total daily diet, for five days. Milk residues
accounted for approximately 2% of the total dose. Approximately 25%
of the milk radioactivity was incorporated into the natural milk
components casein and whey protein. There were no detectable
residues in muscle tissue and fat (< 0.01 mg/kg). However,
radioactivity detected in liver and kidney amounted to 3.8 and 0.09
mg/kg (calculated as benomyl equivalents), respectively (Han, 1980).
In a study by Johnson (1988), the total 14C residue and
metabolic fate of carbendazim in the liver was examined in
non-lactating female goats. Twelve goats were administered a
feed-rate-equivalent dose of [phenyl(U)-14C]-carbendazim (> 50
mg/kg), once a day, for up to 30 days. Within 2 weeks of dose
initiation, a plateau of 14C residues in the liver was achieved at
a level of 9.48 mg/kg (group mean of the total radiolabelled liver
residues for goats sacrificed 2, 3, and 4 weeks after initiation of
dosing). The total 14C residue levels in the liver decreased to
5.17, 3.55 and 1.67 mg/kg (calculated as carbendazim equivalents) 1,
2 and 3 weeks, respectively, after dosing ceased. The elimination
half-life for total 14C residues from the liver, based on this
depuration data, was calculated to be approximately 9 days. The
half-life for removal of carbendazim from the general circulation,
based on 14C-carbendazim equivalent whole blood levels, was
approximately 10 h. The level of bound, non-extractable 14C
residues in the liver of goats sacrificed after 28 days was 1.0
mg/kg.
The results of this study suggest that levels of carbendazim-
derived residues do not accumulate beyond 2 weeks when goats are
exposed to a constant feed level of 50 mg carbendazim/kg.
Furthermore, discontinuation of exposure results in a clearing of
residues from the liver (Johnson, 1988).
The metabolism of benomyl was studied in laying hens by Monson
(1986a). Two hens were individually dosed daily for three
consecutive days with 3.5 mg [2-14C]-benomyl at a rate equivalent
to 29 mg/kg in the daily feed, and two hens were individually dosed
with 3.29 mg [phenyl(U)-14C]-benomyl at rates equivalent to 27
mg/kg in the daily feed. Faeces and eggs from the previous 24 h were
collected just before each dosing. Twenty-two hours after the third
dose, the hens were killed and samples of muscle (breast and thigh),
liver, kidney and fat were analysed. The concentration of
radioactivity (calculated as benomyl equivalents) in the tissues and
day-3 eggs of the [2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-
dosed hens, respectively, was as follows: liver (0.54 and 0.41
mg/kg), kidney (0.28 and 0.16 mg/kg), thigh and breast muscle (both
0.01 mg/kg), fat (0.05 and 0.02 mg/kg) and eggs (0.08 and 0.05
mg/kg).
The distribution of benomyl in this study was comparable to
that in a 20-hen [2-14C]-carbendazim metabolism study. The
concentrations of radioactivity, calculated as mg carbendazim/kg, in
the high-dose laying hens (dose equivalent 120 mg/kg carbendazim in
the diet) were liver (2.63), kidney (1.74), thigh muscle (0.06),
breast muscle (0.05), fat (0.03), day-6 eggs (0.63) (Monson, 1986b).
This study is discussed in detail in the Environmental Health
Criteria monograph on Carbendazim (WHO, 1993).
When bluegill sunfish were exposed to benomyl, carbendazim and
2-AB at nominal concentrations of 0.05 mg/litre (measured
concentrations of 0.01 to 0.04 mg/litre) and 5.0 mg/litre (measured
concentrations of 2 to 5 mg/litre), no residues were found in the
tissues of fish exposed to low levels of these three compounds.
Detectable residues were found in the tissues of fish exposed to the
high levels, but there was no build-up or bioconcentration with time
(DuPont, 1972).
6.3 Metabolic transformation
Benomyl is extensively metabolized by rats to carbendazim,
which is then further metabolized. Studies with rats administered
benomyl intravenously (Han, 1979), dermally (Belasco, 1979b) or by
inhalation (FAO/WHO, 1985a) showed that 5-HBC is the main urinary
metabolite, some carbendazim also being present.
In a rat gavage study (Monson, 1990; see section 6.2),
carbendazim was extensively metabolized. Three dosing regimens (five
rats of each sex per group) were used: a single oral dose of 50
mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with non-radiolabelled carbendazim at 50
mg/kg for 14 days (pre-conditioned low dose); and a single oral dose
of 1000 mg/kg (high dose). The 48-h urine from the low-dose and the
high-dose rats and the 14-day urine from the pre-conditioned
low-dose group were collected. The total recovery from urine was
61.5 and 61.7% of given doses for the low-dose and pre-conditioned
low-dose male groups, 53.2 and 59.3% for the low-dose and
pre-conditioned low-dose female groups, and 39 and 41% for both male
and female high-dose groups, respectively. 5-HBC-S (21-43% of given
dose) was identified as the main metabolite, except in the case of
the pre-conditioned low-dose and high-dose female rat groups
(5.5-10%), while in all female rat groups 5,6-HOBC-N-oxide-G
(10-19%) was predominant. Both 5,6-DHBC-S and 5,6-DHBC-G were
identified as minor metabolites.
In the same study, the faeces were collected at the same
periods as the urine. The total recovery from faeces was about 24%
for the low-dose and pre-conditioned low-dose male groups, 33-38%
for the low-dose and pre-conditioned low-dose female groups, and
higher (> 60%) for both male and female high-dose groups. Unchanged
carbendazim was about 10-15% of the given dose in the faeces of
high-dose rats (Monson, 1990). The proposed metabolic pathway for
benomyl in rats is given in Fig. 2.
When a lactating Holstein cow was dosed by capsule twice daily
(515 mg per dose), equivalent to 50 mg/kg diet, for 5 consecutive
days with [2-14C]-benomyl, the major metabolites of whole milk
were 5-HBC (0.06 mg/litre), 4-HBC (0.03 mg/litre) and MBC-4,5-
dihydrodiol (< 0.07 mg/litre). The proportions of radioactive
residues in the urine were 46% 5-HBC, 3% 4-HBC, and 50% polar
aqueous-soluble metabolites, which included MBC-4,5-dihydrodiol,
2-AB-dihydrodiol and 5-OH-4-GS-MBC (Monson, 1985).
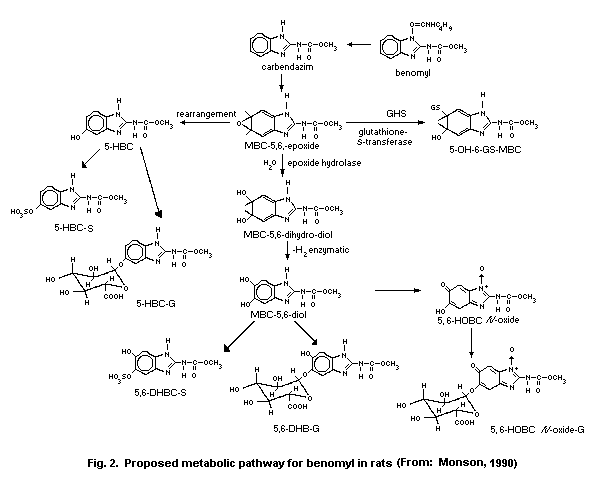 Lactating and non-lactating goats were given five consecutive
daily doses of 2-14C-benomyl by capsule at rates equivalent to 36
and 88 mg/kg, respectively, in the total daily diet. The main
metabolite in milk was 5-HBC, and there were minor amounts of 4-HBC
and 5-HAB. The principal metabolites in urine and faeces were 5-HBC
and 4-HBC. The main identified metabolite in the kidney and liver
was 5-HBC (about 6% of the residue). Much of the liver residue was
incorporated into glycogen, protein, fatty acids and cholesterol,
and accounted for approximately 35% of the liver residues. Further
characterization of the bound liver tissue residues following
enzymatic and trifluoroacetic anhydride hydrolysis identified
5-hydroxy-benzimidazole moieties as the principal (at least 77%)
14C residue in goat liver. No free benomyl, carbendazim or 5-HBC
was detected in the liver (Han, 1980; Hardesty, 1982).
In a further study, the total 14C residue and metabolic fate
of carbendazim in the liver were examined in non-lactating female
goats. Twelve goats were administered a dose equivalent to 50 mg/kg
feed once a day for up to 30 days. Extraction of composite liver
homogenate from goats sacrificed 4 weeks after initiation of dosing
("plateau level") indicated that the major ethyl acetate extractable
and identifiable radiolabelled residues in the liver were 5-HBC (2
to 3 mg/kg) and carbendazim (approximately 0.2 mg/kg) (Johnson,
1988).
The metabolism of [2-14C]-benomyl and [phenyl(U)-14C]-
benomyl has been studied in laying hens (see section 6.2 for a
detailed description of the study). Benomyl was extensively
metabolized to carbendazim, 5-HBC, MBC-4,5-dihydrodiol and a
metabolite tentatively identified as 5-OH-4-GS-MBC. The metabolic
profile observed in hens indicates that the benzimidazole ring is
not broken during metabolism (Monson, 1986a). The proposed metabolic
pathway for benomyl in the laying hen is given in Fig. 3.
Monson (1991) analysed the release and characterization of
bound benomyl and carbendazim metabolites in diary cow, goat, hen
and rat liver after treatment with 14C-benomyl or
14C-carbendazim via Raney nickel desulfurization and acid
dehydration. Using this technique, he was able to show that bound
14C residue was released from the liver of cows (76% bound before
desulfurization and 36% bound after desulfurization) and hens (58%
bound before desulfurization and 19% bound after desulfurization).
The major part of the reduced residue was identified as 5-HBC,
5,6-HOBC or carbendazim, suggesting that the bound liver residue
consisted of conjugates of benzimidazole-related products and not
natural products resulting from breakdown and incorporation.
In fish, benomyl and carbendazim are metabolized to 5-HBC
(Dupont, 1972).
Lactating and non-lactating goats were given five consecutive
daily doses of 2-14C-benomyl by capsule at rates equivalent to 36
and 88 mg/kg, respectively, in the total daily diet. The main
metabolite in milk was 5-HBC, and there were minor amounts of 4-HBC
and 5-HAB. The principal metabolites in urine and faeces were 5-HBC
and 4-HBC. The main identified metabolite in the kidney and liver
was 5-HBC (about 6% of the residue). Much of the liver residue was
incorporated into glycogen, protein, fatty acids and cholesterol,
and accounted for approximately 35% of the liver residues. Further
characterization of the bound liver tissue residues following
enzymatic and trifluoroacetic anhydride hydrolysis identified
5-hydroxy-benzimidazole moieties as the principal (at least 77%)
14C residue in goat liver. No free benomyl, carbendazim or 5-HBC
was detected in the liver (Han, 1980; Hardesty, 1982).
In a further study, the total 14C residue and metabolic fate
of carbendazim in the liver were examined in non-lactating female
goats. Twelve goats were administered a dose equivalent to 50 mg/kg
feed once a day for up to 30 days. Extraction of composite liver
homogenate from goats sacrificed 4 weeks after initiation of dosing
("plateau level") indicated that the major ethyl acetate extractable
and identifiable radiolabelled residues in the liver were 5-HBC (2
to 3 mg/kg) and carbendazim (approximately 0.2 mg/kg) (Johnson,
1988).
The metabolism of [2-14C]-benomyl and [phenyl(U)-14C]-
benomyl has been studied in laying hens (see section 6.2 for a
detailed description of the study). Benomyl was extensively
metabolized to carbendazim, 5-HBC, MBC-4,5-dihydrodiol and a
metabolite tentatively identified as 5-OH-4-GS-MBC. The metabolic
profile observed in hens indicates that the benzimidazole ring is
not broken during metabolism (Monson, 1986a). The proposed metabolic
pathway for benomyl in the laying hen is given in Fig. 3.
Monson (1991) analysed the release and characterization of
bound benomyl and carbendazim metabolites in diary cow, goat, hen
and rat liver after treatment with 14C-benomyl or
14C-carbendazim via Raney nickel desulfurization and acid
dehydration. Using this technique, he was able to show that bound
14C residue was released from the liver of cows (76% bound before
desulfurization and 36% bound after desulfurization) and hens (58%
bound before desulfurization and 19% bound after desulfurization).
The major part of the reduced residue was identified as 5-HBC,
5,6-HOBC or carbendazim, suggesting that the bound liver residue
consisted of conjugates of benzimidazole-related products and not
natural products resulting from breakdown and incorporation.
In fish, benomyl and carbendazim are metabolized to 5-HBC
(Dupont, 1972).
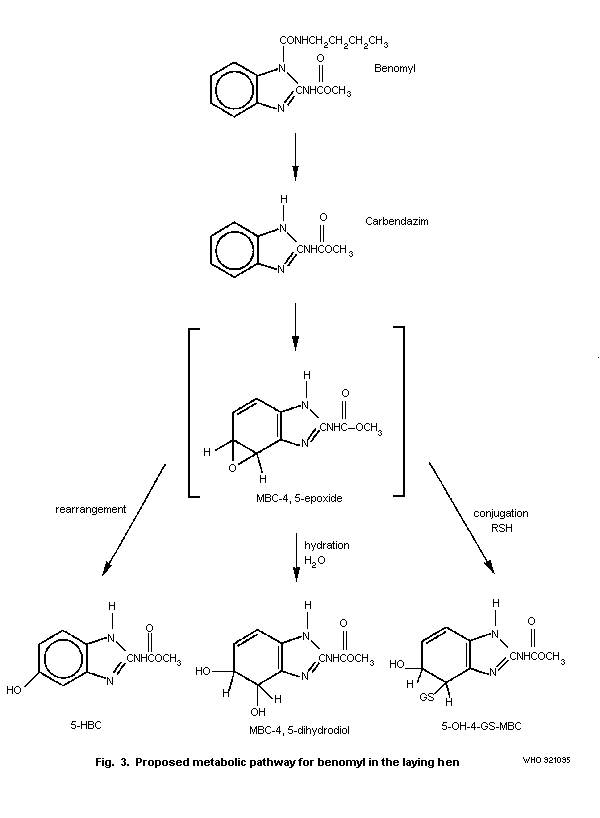 6.4 Elimination and excretion
Absorbed benomyl and carbendazim are rapidly excreted in the
urine and faeces.
In a study where rats were administered 1 or 10 µg formulated
14C-benomyl (50% wettable powder) in a single intravenous dose by
tail injection, more than 80% of the dose was excreted in the urine
and faeces within 6 h after injection and the total urine and faeces
recovery was > 95% in 24 h (Han, 1979).
[Phenyl(U)-14C]-carbendazim was administered by gavage to
Sprague-Dawley rats using three dosing regimens: a single oral dose
of 50 mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with unlabelled carbendazim of 50 mg/kg for
14 days (pre-conditioned low dose); and a single oral dose of 1000
mg/kg (high dose). Each dosing group consisted of five animals of
each sex. A preliminary study conducted with two rats of each sex,
each rat having received a single oral dose of 50 mg/kg,
demonstrated that 95% of the radioactivity excreted in the urine and
faeces was recovered within 72 h after dosing and that < 1% of the
dose was expired as volatile metabolites. In the full study, > 98%
of the recovered radioactivity was excreted by the time of sacrifice
(i.e. 72 h after dosing) for each dosing group. Urinary excretion
accounted for 62% to 66% of the dose in males and 54% to 62% of the
dose in low-dose and pre-conditioned low-dose female groups. In the
high-dose group, this pathway accounted for 41% of the dose in all
animals. Elimination of radiolabel in faeces accounted for virtually
all of the remaining radiolabel. There were no apparent differences
between male and female rats with respect to the extent of
absorption and extent and rate of elimination of 14C-carbendazim
equivalents within a given treatment group (Monson, 1990).
In a study by Han (1978), two male ChR-CD-1 mice were fed a
diet of non-radiolabelled benomyl (2500 mg/kg) for 21 days and were
then gavaged with 2.5 mg [2-14C]-benomyl in corn oil. An identical
experiment was performed with one male ChR-CD hamster. More than 90%
of the radioactivity was eliminated in the urine and faeces within
72 h (Han, 1978).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approximately
17 h after the tenth dose, the cow was sacrificed and organs,
tissues and blood were collected for analysis. At sacrifice, 65% of
the radiolabel had been excreted in the urine, 21% in the faeces and
0.4% in the milk. Carbon-14 residue levels in the milk averaged 0.2
mg/litre (calculated as benomyl equivalents) with 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids (Monson, 1985).
Lactating and non-lactating goats were given 5 consecutive
daily doses of [2-14C]-benomyl by capsule at rates equivalent to
36 and 88 mg/kg, respectively, in the total daily diet. Most of the
radioactivity (96%) had been eliminated in the urine and faeces by
the time of sacrifice (Han, 1980).
The excretion of benomyl was studied in laying hens dosed daily
for three consecutive days with 3.5 mg [2-14C]-benomyl or 3.29 mg
[phenyl(U)-14C]-benomyl. At sacrifice (22 h after the last dose),
an average of 107% and 95% of the dose had been excreted for the
[2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-dosed birds,
respectively (Monson, 1986a).
In a similar study on 14C-carbendazim, groups of laying hens
were fed at a rate equivalent to 5 and 120 mg/kg diet. At sacrifice,
24 h after the sixth daily dose, an average of 95% of the dose had
been excreted by the low-dose birds and 92% by the high-dose birds
(Monson, 1986b).
6.5 Reaction with body components
An in vitro study using acetyl cholinesterase from bovine
erythrocytes showed that benomyl did not inhibit this enzyme. The
acetyl cholinesterase inhibition constant (KI) for benomyl was
greater than 1 x 10-3 mol/litre (Belasco, 1970). Another in vitro
study by Krupka (1974) verified that benomyl did not inhibit either
acetyl cholinesterase or butyryl cholinesterase.
In a study by Guengerich (1981), the effects of benomyl and
carbendazim on hepatic enzymes were studied in male and female
Crl-CD rats and CD-1 mice. The treatment groups included animals fed
for 28 days with diets that contained benomyl or carbendazim at
concentrations of 0, 10, 30, 100, 300, 1000 or 3000 mg/kg. In these
studies, microsomal epoxide hydrolase and cytosolic glutathione- S-
transferase were monitored in subcellular fractions isolated from
the livers of animals in each treatment group. Liver weights were
also recorded. Elevated mean absolute liver weights were observed at
1000 and 3000 mg carbendazim/kg in both male and female rats and at
300 mg carbendazim/kg in female rats. However, the only
significantly elevated liver weight was found in females after a
dose of 3000 mg benomyl/kg. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced
epoxide hydrolase in both sexes of rats and mice at 1000 and 3000
mg/kg. Induction of glutathione- S-transferase was observed at 3000
mg/kg in the case of both benomyl and carbendazim. In general, the
level of induction seemed to be slightly greater in females than
males. There did not appear to be any substantial difference in
enzyme induction between rats and mice.
In a study by Shukla et al. (1989), levels of gamma-glutamyl
transpeptidase (GGT) were evaluated after benomyl exposure. Female
albino rats (eight per group) and female Swiss albino mice (eight
per group) were given 1000 and 4000 mg benomyl/kg feed for 15 days,
and blood and liver GGT levels were analysed. Benomyl exposure
increased the activity of both blood and liver GGT in both rats and
mice, and the degree of induction was dose related (Shukla et al.,
1989).
7. EFFECTS ON LABORATORY MAMMALS; IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of benomyl in several animal species is
summarized in Table 8. Benomyl has an oral LD50 in the rat of >
10 000 mg/kg and an inhalation 4-h LC50 > 4 mg/litre. Several
other minor metabolites were evaluated and the approximate lethal
doses were 3400 mg/kg for 2-AB, 7500 mg/kg for 5-HBC, 17 000 mg/kg
for BUB and 17 000 mg/kg for STB.
In a study by Littlefield & Busey (1969), three groups of male
dogs (around 10 dogs/group) were exposed to benomyl at air
concentrations of 0, 0.65 and 1.65 mg/litre. One half of the dogs in
each group were killed on day 14 and the remainder on day 28. The
liver weight of the high-dose dogs was significantly decreased on
day 28. For further discussion of single dose toxic effects, see
section 7.5.1.
7.2 Short-term exposure
7.2.1 Gavage
In a 14-day rat study, benomyl (200 and 3400 mg/kg in peanut
oil) was given by gavage five times a week for two weeks to six male
ChR-CD rats per group. Four out of six rats died after 5, 7, 8 and 9
doses, respectively, of 3400 mg/kg. No clinical signs of toxicity
were observed in the group treated with 200 mg/kg per day.
Degeneration of germinal epithelium, multinucleated giant cells and
reduction or absence of sperm were observed in the testes after
multiple doses of 3400 mg/kg per day. Less than 10% of the
testicular tubules were affected in only two out of six animals
dosed with 200 mg/kg. At the high dose level, there was erosion and
thickening of the squamous mucosa of the stomach with submucosal
inflammation and a decrease in the large globular-shaped vacuoles
located centrolobularly in the liver (Sherman & Krauss, 1966).
7.2.2 Feeding
7.2.2.1 Rat
In a 90-day study by Sherman et al. (1967), groups of rats
(4-week-old ChR-CD rats, 16 rats of each sex per group) were fed
Benlate 70 WP (72% benomyl) in the diet at levels of 0, 100, 500 and
2500 mg benomyl/kg. The animals were observed daily for behavioural
changes and body weights, and food consumption was recorded at
weekly intervals. Haematological examinations were conducted on six
male and six female rats in each group at 30, 60 and 90 days.
Routine urine and plasma alkaline phosphatase and glutamic pyruvic
transaminase activity analyses were performed on the same animals.
After 96-103 days of continuous feeding, 10 male and 10 female rats
in each group were killed, and selected organs were weighed and
examined microscopically. The remaining six male and female animals
in each group after the terminal sacrifice were used in a
one-generation reproductive study. No effect was observed with
respect to reproduction or lactation in the delivery or rearing of
the F1A litters. There were no compound-related effects on weight
gain, food consumption, food efficiency, clinical signs, or on
haematology, biochemistry or urinalysis determinations. The
liver-to-body weight ratio in females was slightly increased at 2500
mg/kg, compared with control rats. Gross and microscopic
examinations of tissues and organs showed no significant effects
attributable to the presence of benomyl in the diet at levels up to
and including 2500 mg/kg.
7.2.2.2 Dog
Groups of beagle dogs (four males and four females per group;
7-9 months old) were administered benomyl 50% wettable powder in the
diet at dosage levels of 0, 100, 500 and 2500 mg/kg diet (based on
active ingredient) for three months (this corresponded to treatment
levels of 0, 3.8, 18.4 and 84 mg/kg body weight). Food consumption
and body weight data were recorded weekly, and clinical laboratory
examinations (including haematology, biochemistry and urinalysis)
were performed pre-test and after 1, 2 and 3 months of feeding. At
the conclusion of the study, selected organs were weighed and
subjected to gross and microscopic examinations. No mortality or
adverse clinical effects were observed over the course of the study,
and growth and food consumption were not effected by the treatment.
Urine parameters showed no differences from the control, and there
were no dose-related effects on the haematological values. Alkaline
phosphatase and glutamic pyruvic transaminase activities were
increased in high-dose males and females. There were statistically
significant decreases in the albumin/globulin ratio in either males
or females fed 2500 mg/kg diet. Organ-to-body weight ratio changes
were observed in the high-dose males and females for the thymus
(decreased) and thyroid (increased). One of the four females fed
2500 mg/kg diet had an enlarged spleen at the end of the exposure
period, as well as a decreased erythrocyte count, haemoglobin
concentration and haematocrit value. Histopathological examination
revealed myeloid hyperplasia of the spleen and bone marrow and
erythroid hyperplasia of bone marrow. This did not appear to be
compound related since group mean values were not significantly
different. Three out of four males fed 2500 mg/kg diet had reduced
relative prostate weights when compared with controls. Microscopic
examinations of tissues and organs did not indicate changes in dogs
fed benomyl for 90 days. The no-observed-effect level (NOEL) was 500
mg/kg diet (Sherman, 1968).
Table 8. Acute toxicity of benomyl and its metabolites for laboratory mammals
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl ratb M/F 10/dose oral peanut oil LD50 > 10 000 Sherman (1969a)
rabbitc M 1/dose oral 50% wettable powder ALD > 3400 Fritz (1969)
dogd M 1 oral evaporated milk and ALD > 1000 Sherman (1969b)
water (1:1)
Benlate OD (50% rat M 10/dose oral corn oil LD50 > 12 000 Hostetler (1977)
benomyl)
Fungicide 1991 rabbit M/F 4/dose dermal (4 h) 50% wettable powder LD50 > 10 000 Busey (1968a)
(50% benomyl)
rat M 6/dose inhalation (4 h) 50% wettable powder LC50 > 4.01 mg/litre Busey (1968b)
(analytical)
dog M 10/dose inhalation 50% wettable powder LC50 > 1.65 mg/litre Littlefield & Busey
(analytical) (1969)
Benlate fungicide rat M/F 10/dose oral aqueous suspension LD50 > 10 000 Sherman (1969a)
(52-53% benomyl)
rat M 5/dose inhalation 50% wettable powder LC50 > 0.82 mg/litre Hornberger (1969)
Benlate PNW (50% rabbit M/F 10/dose dermal 50% wettable powder LD50 > 2000 Gargus & Zoetis
benomyl) (1983c)
Benlate 50 DF (50% rat M/F 5/dose oral aqueous suspension LD50 > 5000 Sarver (1987)
benomyl)
rabbit M/F 5/dose dermal 50% dry flowable LD50 > 2000 Brock (1987)
Table 8 (contd).
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl metabolites
2-Benzimidazole carbamic rat M/F 10/dose oral corn oil LD50 > 10 000 Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole- rat M 1/dose oral corn oil ALD > 7500 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole rat M 1/dose oral peanut oil ALD > 3400 Fritz & Sherman
(1969)
Benzimidazole 2- rat M 1/dose oral corn oil ALD > 17 000 Dashiell (1972)
(3-butylureido)
S-Triazine, 3-butyl- rat M 1/dose oral corn oil ALD > 17 000 Barbo & Carroll
benzimidazole (1,2a), (1972)
-2,4(1H,3H)-dione
a Based on active ingredient; ALD = approximate lethal dose
b ChR-CD or Crl:CD rats
c New Zealand white rabbits
d Beagle dogs
7.2.3 Dermal
In a study on groups of five male and five female New Zealand
albino rabbits, weighing 2 to 2.4 kg, 15 dermal applications of a
50% benomyl formulation (equivalent to 1000 mg/kg) were made on both
abraded and intact abdominal skin sites. The animals were exposed
for 6 h/day, 5 days/week for 3 weeks. After each daily application,
the abdomen was washed with tap water. Observations were made daily
for mortality and toxic effects and weekly for body weight changes.
Gross necropsy and microscopic examinations were performed. Slight
erythema, oedema and atonia were observed at both abraded and intact
skin sites. Slight to moderate desquamation occurred throughout the
exposure period. No apparent compound-related body weight or organ
weight changes were reported. Microscopic examination of the males
demonstrated that benomyl produced degeneration of the spermatogenic
elements of the seminiferous tubules of the testes, the changes
including vacuolated and multi-nucleated spermatocytes (Busey,
1968d).
In a separate repeated-dose dermal study, groups of five male
and five female New Zealand albino rabbits, weighing 3 kg, were
exposed to doses of benomyl equivalent to 0, 50, 250, 500, 1000 and
5000 mg/kg applied to non-occluded abraded dorsal skin sites 6 h a
day, five days a week, for three weeks. Test material was removed by
washing the skin site and drying with a towel. There were decreased
body weight gains for both males and females at the two highest dose
levels. Mild to moderate skin irritation was reported for all groups
but was most notable at the highest dose level. Diarrhoea, oliguria
and haematuria were observed in males and females at 1000 and 5000
mg/kg. Decreased average testicular weights and testes-to-body
weight ratios were observed at 1000 mg/kg only. There were no
histopathological changes reported (Hood, 1969).
7.2.4 Inhalation
In an inhalation study, groups of 20 male and 20 female CD rats
were exposed, nose-only, 6 h a day for 90 days, to 0, 10, 50 and 200
mg benomyl/m3. At 45 and 90 days, blood and urine samples were
collected from 10 rats of each sex per group for clinical analysis
and then killed for pathological examination. After approximately 45
days of exposure, test-compound-related degeneration of the
olfactory epithelium was observed in all males and in eight of the
ten females exposed to 200 mg/m3. Two male rats exposed to 50
mg/m3 had similar but less severe olfactory degeneration. After
approximately 90 days of exposure, all of the animals showed
olfactory degeneration at 200 mg/m3, along with three males
exposed to 50 mg/m3. No other compound-related pathological
effects were observed. Male rats exposed to 200 mg/m3 had
depressed mean body weights compared to controls and this correlated
with a reduction in food consumption (Warheit et al., 1989).
7.3 Skin and eye irritation; sensitization
7.3.1 Dermal
A 50% wettable powder applied to the clipped intact and abraded
abdomen of albino rabbits produced moderate to marked erythema,
slight oedema and slight desquamation. Exposure was for 24 h to
occluded skin sites at doses > 0.5 g/animal. Albino guinea-pigs
similarly exposed to 10, 25 and 40% dilutions of technical grade
benomyl in dimethyl phthalate presented only mild irritation of both
intact and abraded skin sites (Majut, 1966; Busey, 1968a; Colburn,
1969; Frank, 1969).
When "Benlate" 50 DF (50% benomyl, 0.5 g Benlate 50 DF) was
evaluated for primary dermal irritation potential in six male New
Zealand white rabbits, no dermal irritation was observed at 4 or 24
h after application. By 48 h, slight to mild erythema was observed
in two rabbits and was still evident at 72 h. The primary irritation
scores ranged from 0-1 (not an irritant) (Vick & Brock, 1987).
In a study by Desi (1979), benomyl (98% purity) was applied to
a shaved area of the back of four albino rabbits at 5 mg/cm2.
Draize scores (Draize et al., 1944) were assessed 4 and 72 h after
application. Lesions produced by this method were classified as
"mild irritation".
7.3.2 Eye
The eye irritation properties of benomyl were examined in
albino rabbits in several tests using technical grade benomyl, 50%
wettable powder and a suspension in mineral oil. Mild conjunctival
irritation and minor transitory corneal opacity were reported after
48 to 96 h in all tests (Reinke, 1966; Frank, 1972). Similar results
were obtained with Benlate PNW (a 50% wettable powder) (Gargus &
Zoetis, 1983a,b).
Another eye irritation experiment was performed with 5 mg pure
benomyl using four albino rabbits (Desi, 1979). Results assessed
according to Draize (Draize et al., 1944) indicated that benomyl is
a mild eye irritant.
7.3.3 Sensitization
Albino guinea-pigs exposed to benomyl, either technical
material or a 50% sucrose formulation, produced mild to moderate
skin erythema during the challenge phase following both intradermal
injections or repeat applications to abraded skin (Majut, 1966;
Colburn, 1969; Frank, 1969).
In another sensitization study, Benlate PNW (50% benomyl
prepared as a 0.1% solution in saline) was injected weekly (four
injections) into ten albino guinea-pigs (Hartley strain). Ten
control animals were injected with saline. Fourteen days after the
final injection, 8% or 80% Benlate PNW saline solutions were applied
to the backs of the induced animals and saline was applied to the
backs of the control animals. No significant increase in score
occurred in any of the control animals at either challenge
concentration. Benlate PNW produced an unequivocal and significant
(two-step) increase at two of ten sites challenged with an 8%
suspension, and at seven of ten sites challenged with an 80%
suspension (Gargus & Zoetis, 1984).
Technical benomyl produced sensitization in all ten animals
tested in a guinea-pig maximization test (Matsushita et al., 1977).
7.4 Long-term exposure
7.4.1 Rat
Groups of weanling rats (36 male and 36 female Charles River
albino rats/group) were fed benomyl (50-70% a.i.) in the diet for
104 weeks at levels of 0, 100, 500 and 2500 mg/kg. Growth, as
observed by body weight changes and food consumption data, was
recorded weekly for the first year and twice a month thereafter.
Daily observations were made of clinical effects and mortality. At
periodic intervals during the study, haematological, urinalysis and
selected clinical chemistry examinations were performed. After one
year each group was reduced to 30 males and 30 females by interim
sacrifice for gross and microscopic evaluations. At the conclusion
of the study, all surviving animals were sacrificed and gross
examinations of tissues and organs were made. Initially, microscopic
examinations of tissues and organs from the control and 2500 mg/kg
groups were conducted, as were liver, kidney and testes examinations
of animals in the 100 and 500 mg/kg dose groups. In follow-up
pathological evaluations, all of the tissues and organs of the
control and low-, intermediate- and high-dose groups were examined
microscopically. There was no mortality attributable to benomyl in
the diet. Survival decreased to approximately 50% during the second
year, but was comparable among all groups. Body weight, food
consumption and food efficiency were unaffected by treatment. The
average daily dose for the 2500 mg/kg group was 330 mg/kg body
weight per day initially, 91-106 mg/kg body weight per day at one
year and 70-85 mg/kg body weight per day at two years. There were no
compound-related clinical manifestations of toxicity.
Haematological, urine and liver function tests were unaffected by
treatment. There were no differences in organ weight or organ-to-
body-weight ratios between control and treated groups (Sherman,
1969c; Lee, 1977).
7.4.2 Mouse
In a study by Weichman et al. (1982), male and female CD-1 mice
(80 males and 80 females per group) were administered benomyl (99%
a.i.) in the diet at levels of 0, 500, 1500 and 5000 mg/kg (the
highest levels was reduced from 7500 mg/kg after 37 weeks) for two
years. The mice were 6-7 weeks old at the start of the study. Median
survival time was unaffected by treatment. Male and female mice fed
1500 or 5000 mg/kg exhibited dose-related body weight decreases.
Food consumption was variable throughout the study, although
high-dose females appeared to consume less food. The average daily
intake of benomyl for males was 1079 mg/kg body weight per day
initially, 878 mg/kg body weight per day for 1 year and 679 mg/kg
body weight per day for 2 years; for females it was 1442 mg/kg body
weight per day initially, 1192 mg/kg body weight per day for 1 year,
and 959 mg/kg body weight per day for 2 years. There were no
apparent differences between treatment and control groups with
respect to palpable mass, number of mice affected or latency period
of discovery. Haematology examinations revealed no abnormalities
except for slightly decreased erythrocyte counts in the case of
males at 1500 mg/kg and females at 5000 mg/kg. Haemoglobin and
haematocrit values were also slightly depressed in 1500-mg/kg males.
Significant compound-related changes were seen in the absolute
and relative liver weights for males at 1500 and 5000 mg/kg for
females at 5000 mg/kg. Male mice also presented decreased absolute
testes weights at the highest dose level. Non-neoplastic organ
changes in males (5000 mg/kg) were confined to the liver
(degeneration, pigment, cytomegaly), thymus (atrophy), testes,
epididymis (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini) and prostate. In female mice,
splenic haemosiderosis was significantly increased at 5000 mg/kg, as
was submucosal lymphocytic infiltration of the trachea at 1500 mg/kg
(Weichman et al., 1982).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A number of reproduction studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in the Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.5.1.1 Rat feeding studies
A 2-generation reproduction study was conducted using Crl:CD
rats (Mebus, 1990). Throughout the study, animals were fed diets
containing 0, 100, 500, 3000 or 10 000 mg benomyl/kg. P1 parental
rats received the test diets for 71 days (mean daily intake 0,
approx. 6, approx. 30, approx. 190 or approx. 350 mg/kg per day,
respectively) before being bred to animals from the same dietary
concentration group for production of the F1 parental rats. F1
rats were mated after being maintained on their respective diets
(mean daily intake 0, approx. 8, approx. 20, approx. 250, or approx.
1000 mg/kg per day, respectively) for at least 105 days after
weaning for production of the F2A litter. F1 dams were mated
again, to different non-sibling males, at least 1 week after weaning
the F2A litter, to produce the F2B litters.
The following indices of reproductive function were calculated
for the P1 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental
generation rats were sacrificed for gross and histopathological
(gross lesions and target organs only) examination. Complete
histopathological examination was conducted on control and high-dose
animals. Twenty F2A and 20 F2B weanlings were also given a gross
pathological examination.
There were no compound-related effects on parental mortality at
any benomyl concentration. Mean body weights, body weight gains and
overall food consumption of P1 and F1 male and female rats were
significantly lower in the animals receiving 10 000 mg/kg than they
were in controls.
At 10 000 mg/kg, there was a significant compound-related
decrease in the number of F2A and F2B offspring alive prior to
culling (on day 4). In addition, male and female offspring of rats
fed 10 000 mg/kg weighed consistently less at birth than did the
offspring of control rats. With the exception of the 14-day male
pups in the F2B generation, F2A and F2B offspring in the 3000
mg/kg group also had compound-related significantly depressed body
weights on days 14 and 21 of lactation.
Testicular sperm counts in P1 and F1 rats were decreased in
the 3000 and 10 000 mg/kg groups. This was accompanied by decreased
testicular weight and histopathological changes in the testes at 10
000 mg/kg. Microscopic observations included atrophy and
degeneration of the seminiferous tubules in the testes of rats in
the 3000 and 10 000 mg/kg groups and oligospermia in the
epididymides of the high-dose P1 generation and the 3000 and 10
000 mg/kg F1 generation. However, there were no compound-related
differences in mating indices, fertility indices or gestation length
which could be attributed to benomyl feeding (Mebus, 1990).
In a feeding study by Barnes et al. (1983), adult male Wistar
rats (27 animals/group) were fed 0, 1, 6.3 or 203 mg/kg for 70 days
(13 animals/group) and allowed to recover for 70 days (14
animals/group). Ejaculated sperm counts were reportedly depressed in
the 203-mg/kg group during the feeding phase. There was also a
decrease in relative testicular weights and a slightly lowered male
fertility index in all treated groups. Benomyl did not alter
copulatory behaviour and did not induce dominant lethal mutation.
Plasma testosterone and gonadotropin levels remained unchanged
throughout the study. Identical studies conducted during the
recovery phase showed all of the treatment-related effects to be
completely reversible.
7.5.1.2 Rat gavage studies
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal Sprague-Dawley male rats
(33 days old), which were gavaged daily for 10 days at doses of 0 or
200 mg/kg per day. Eight animals per group were killed at 3, 17, 31,
45 and 59 days after the last treatment. Selected tissues, including
liver, kidneys, testes, seminal vesicles and epididymides, were
removed, weighed and examined histo logically. Samples of seminal
fluid from the vas deferens were also examined. Observation
intervals were pre-selected to coincide with stages of
spermatogenesis. Data were presented on tissue weights, total
epididymal sperm counts, vas deferens sperm concentrations and
testicular histology. There were no effects related to treatment
(Carter, 1982).
In a similar study, adult male Sprague-Dawley rats (65 days
old) received 10 daily treatments of 0, 200 or 400 mg benomyl/kg per
day by gavage, and, 14 days after the last treatment, body weight,
tissue weights, total epididymal sperm counts and sperm
concentration in the vas deferens were measured and histological
investigations of the testes were carried out. Production of
testosterone by the Leydig cells was stimulated by subcutaneous
injections of human chorionic gonadotrophin (HCG) 2 h prior to
sacrifice. There were no compound-related effects on body weight or
on liver, kidney, adrenal, testis or seminal vesicle weights.
Caudate epididymal weights were, however, depressed by treatment
with benomyl. There were also treatment-related reductions in
epididymal sperm count (caput and caudate) as well as in the vas
deferens sperm concentration. The study was designed to evaluate
alterations in spermatozoa undergoing spermatogenesis in the
seminiferous tubules of the testes during exposure to benomyl.
Animals exposed to 400 mg/kg per day presented histological evidence
of hypospermatocytogenesis with generalized disruption of all stages
of spermatogenesis, when compared with controls (Carter & Laskey,
1982).
Linder et al. (1988) evaluated the effect of administering 1,
5, 15 or 45 mg benomyl/kg per day by gavage daily to 102-day old
Wistar male rats (12/group). The males were bred to untreated
females after 62 days of dosing and killed after 76-79 days.
Reproductive behaviour, seminal vesicle weight, prostate weight,
sperm motility, and serum gonadotropin hormones and serum gonadal
hormone levels were no different from those in controls at any dose.
At necropsy, males exposed to 45 mg/kg had decreased testis and
epididymal weights, reduced cauda sperm reserves, decreased sperm
production, increased numbers of decapitated spermatozoa and
increased numbers of seminiferous tubules containing multinucleated
giant cells.
In a study by Hess et al. (1991), adult male Sprague-Dawley
rats (100 days of age, 20 rats/dose) were given a single dose of
benomyl in corn oil (0, 25, 50, 100, 200, 400 or 800 mg/kg body
weight). Eight animals/group were sacrificed at 2 days and 12
animals/group at 70 days (except for the 800 mg/kg group) after
treatment. The testis and excurrent ducts were examined each time to
determine benomyl effects on spermatogenesis and on the epididymis.
The primary effects seen at day 2 were testicular swelling and
occlusions of the efferent ductules. Premature release of germ cells
(sloughing) was the most sensitive short-term response to benomyl.
Sloughing was detected in all treatment groups but was statistically
significant (p < 0.05) at doses of 100 mg/kg to 800 mg/kg.
Occlusions of the efferent ductules of the testis were dose
dependent and correlated with the increase in testis weight on day
2. The greatest increase in testes weight was observed in the 400
mg/kg group. Long-term effects (70 days) were seen in the 100, 200
and 400 mg/kg groups, e.g., decreased testis weight (400 mg/kg),
dose-dependent increases in seminiferous tubular atrophy, and
increases in the number of reproductive tracts containing occluded
efferent ductules. No long-term effects were seen in the 0, 25 or 50
mg/kg groups.
7.5.1.3 Dog inhalation studies
Three groups (10 dogs per group) of sexually mature male dogs
were exposed to benomyl at air concentrations (aerosol/cloud) of 0,
0.65 and 1.65 mg/litre for a 4-h period. One half of the dogs in
each group were killed on day 14 and the remainder on day 28
following the exposure. Histopathological examination revealed a
reduction in spermatogonic activity on day 14, but not on day 28, in
the high-dose group (Littlefield & Busey, 1969).
7.5.2 Teratogenicity and embryotoxicity
7.5.2.1 Mouse gavage studies
Groups of pregnant CD-1 mice (20-25 mice/group) were
administered benomyl via gavage at dose levels of 0, 50, 100 and 200
mg/kg per day on days 7 to 17 of gestation. Animals were killed on
day 18, pups were delivered by Caesarean section, the number of
live, dead and resorbed fetuses was determined, and fetuses were
examined for gross abnormalities. Half of the fetuses were examined
for visceral abnormalities and the other half for skeletal
abnormalities. No maternal toxicity was observed. Fetal development
was adversely affected by treatment at all dose levels. The high
dose caused an increased supraoccipital score, decreased numbers of
caudal and sternal ossifications and increased incidences of
enlarged lateral ventricles and enlarged renal pelvis. The latter,
while not significant at the lower doses, did demonstrate
dose-related increases at all other doses. The occurrence of
supernumerary ribs and subnormal vertebral centrums was
significantly increased in a dose-related manner at all dose levels.
There was an increase in the number of abnormal litters and fetuses,
which was significantly different from the controls, at levels of
100 and 200 mg/kg per day. Fetal weights were also decreased at
these dose levels. Major abnormalities included exencephaly,
hydrocephaly, cleft palate, hydronephrosis, polydactyly,
oligodactyly, umbilical hernia, fused ribs, fused vertebrae and
short/kinky tail (Kavlock et al., 1982).
7.5.2.2 Rat gavage studies
In a study by Staples (1980), benomyl (99.2% a.i.) was adminis
tered by gavage to groups of pregnant rats (ChR-CD) at dose levels
of 0, 3, 10, 30, 62.5 and 125 mg/kg per day from days 7 to 16 of
gestation. There were 60 dams in the control group and 27 in each of
the other test groups; they were observed daily for signs of
toxicity and changes in behaviour. No clinical signs of toxicity or
mortality were observed among dams in any dose group. Body weight
gain was comparable to controls, as were the incidences of
pregnancy, corpora lutea and implantation sites, and the sex ratio.
However, fetal body weight was significantly decreased at the two
highest dose levels. There was also an increased incidence of
embryo/fetal mortality at 125 mg/kg per day.
The malformations observed included microphthalmia,
anophthalmia and hydrocephaly (distended lateral ventricles). These
appeared to be compound related at the higher dose levels.
Microphthalmia was seen in two fetuses from different litters at 10
mg/kg per day and in one fetus from the control group.
Microphthalmia/anopthalmia was observed in only 4-6 out of 4935
fetuses examined in the historical data base at this laboratory.
Histological examination of eyes revealed pathological changes
consisting of irregular lenses, retro-bulbar glandular adnexa,
distorted or compressed retinal layers and thickened nerve fibres in
the 10, 62.5 and 125 mg/kg per day treatment groups. Major skeletal
malformations observed in the 125 mg/kg dose group included fused
ribs, fused sternebrae and fused thoracic arches. Additional
skeletal variations were also increased at 62.5 and 125 mg/kg per
day; these included misaligned and unossified sternebrae and
bipartite vertebral centra (Staples, 1980).
In order to determine a no-effect level for microphthalmia and
hydrocephaly, groups of 50 pregnant rats of the same strain and from
the same supplier were administered benomyl (99.1% a.i.) via gavage
at dosage levels of 0, 3, 6.25, 10, 20, 30 and 62.5 mg/kg per day
from days 7 to 16 of gestation (Staples, 1982). Each group contained
50 animals except the high-dose group, which contained 20 female
rats. Reproductive status was determined on a per-litter basis
following gross pathological evaluation. The number of implantation
sites, resorptions, dead, live and stunted fetuses, and the mean
weight of live fetuses per litter were determined. Only fetal heads
were fixed and examined microscopically. Microphthalmia was
determined on the basis of the smallest eye in the control group (<
1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two animals with malformations,
both in the 62.5 mg/kg per day group. One fetus exhibited internal
hydrocephaly and another, in a separate litter, unilateral
microphthalmia. There was no teratogenic response at doses up to 30
mg/kg.
The teratogenic potential of benomyl was examined in groups of
Wistar rats (12 to 30 pregnant rats/group) orally gavaged at dose
levels of 0, 15.6, 31.2, 62.5 and 125 mg/kg per day on days 7 to 16
of gestation. Major anomalies were observed primarily at levels of
62.5 mg/kg per day or more and included encephaloceles,
hydrocephaly, microphthalmia, fused vertebrae and fused ribs. A dose
level of 31.2 mg/kg per day appeared to be without adverse effects
on the developing rat fetus in this evaluation. A significant
reduction in maternal body weight was observed at the highest dose
level (Kavlock et al., 1982).
Kavlock et al. (1982) also evaluated the effect of low levels
of benomyl as the pups aged. Benomyl was administered via gavage to
groups of Wistar rats at dose levels of 0, 15.6 and 31.2 mg/kg per
day from day 7 of gestation to day 15 of lactation (day 22 of
gestation was considered day 0 of lactation). The litters were
weighed on days 8, 15, 22, 29 and 100 after parturition, and
locomotor activity was evaluated periodically throughout the study.
At 100 days of age, several organs were weighed, including the
adrenals, liver, kidney, ovaries, testes and the ventral prostate
plus seminal vesicles. There were no compound-related effects either
on litter size at birth or weaning, or on body weights of fetuses.
Growth, survival and locomotor activity values were comparable with
those of the controls throughout the study. Organ weights were
comparable with those of controls, except that testes and ventral
prostate/seminal vesicle weights were significantly reduced at 31.2
mg/kg per day (but not at 15.6 mg/kg) (Kavlock et al., 1982).
In other studies (Zeman et al., 1986; Hoogenboom et al., 1991),
benomyl produced ocular and craniocerebral malformations in
Sprague-Dawley rats when administered by gavage at doses of 31.2 and
62.4 mg/kg per day on day 7-21 of gestation. Ocular anomalies
(retinal dysplasia, cataracts, microphthalmia and anophthalmia)
occurred in 43.3% of fetuses when the dams were administered 62.4
mg/kg per day. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose level
of benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
(consisting primarily of hydrocephaly) occurred in fetuses from dams
administered 31.2 mg/kg per day in combination with the semipurified
diet (Zeman et al., 1986).
7.5.2.3 Rat feeding studies
In a study by Sherman et al. (1972), groups of rats (26-28
pregnant ChR-CD rats/group) were administered a benomyl formulation
(53.5% a.i.) in the diet at dosages of 0, 100, 500, 2500 and 5000
mg/kg from day 6 to day 15 of gestation. Average doses were
equivalent to 0, 8.6, 43.5, 209.5 and 372.9 mg/kg body weight per
day. On day 20 of gestation, all pregnant animals were sacrificed
and fetuses were delivered by Caesarean section. There was no
mortality attributable to benomyl, no clinical signs of toxicity and
no adverse effects on the body weight of dams. Dams in the
highest-dose group had a reduced food intake during the period
benomyl was administered in the diet, but the intake returned to a
level similar to the controls for the remainder of the study. Except
for three litters at the highest dose, where there were incidences
of hydronephrosis and retarded ossification (interparietal and
occipital bones), there were no effects on fetal development related
to benomyl administration.
In a similar study, groups of pregnant Wistar rats (27-28
rats/group) were fed benomyl at dose levels of 0, 1690, 3380 and
6760 mg/kg diet (time-weighted doses of 0, 169, 298 and 505 mg/kg
body weight per day, respectively) from days 7 to 16 of gestation.
No dose-related anomalies or major malformations were associated
with exposure to benomyl at any of the dose levels used. Reduced
fetal weight was observed at the two highest dose levels (Kavlock et
al., 1982).
7.5.2.4 Rabbit feeding studies
Groups of rabbits (15 artificially inseminated New Zealand
albino rabbits/group) were administered a benomyl formulation (50%
a.i.) in the diet at dose levels of 0, 100 and 500 mg/kg diet from
day 8 to day 16 of gestation. Mortality, clinical observations and
food consumption were determined daily and body weights were
measured weekly. There were 12 pregnant does in the control group,
13 in the low-dose group and 9 in the high-dose group. Of these, 6,
7 and 5, respectively, were sacrificed on day 29 or 30 and fetuses
were delivered by Caesarean section; the remaining does gave birth
normally. Maternal toxicity was not observed at any dose level.
Except for a marginal increase in rudimentary ribs at 500 mg/kg,
developmental toxicity was not observed. The numbers of litters and
of fetuses examined were less than adequate to assess the fetotoxic
or teratogenic potential of benomyl to pregnant rabbits (Busey,
1968c; FAO/WHO, 1985a).
7.6 Mutagenicity and related end-points
Numerous studies have been conducted to assess the mutagenic
potential of benomyl, its metabolite carbendazim, and several
benomyl formulations. Many of the results are conflicting and many
of the study reports do not provide sufficient detail to evaluate
the reasons for the conflicting data. This summary will cover only
those studies where sufficient experimental detail and data were
reported (Table 9).
Studies on somatic and germ cells have shown that benomyl does
not cause gene mutations or structural chromosomal damage
(aberrations) and that it does not interact directly with DNA. This
has been demonstrated in both mammalian and non-mammalian systems.
However, in the mammalian in vitro studies for gene mutations and
structural chromosome aberrations, some positive results were
obtained with benomyl. These positive results appear to have been a
consequence of the inherent sensitivity of some in vitro mammalian
test systems to cytotoxic agents. Results in mammalian in vivo
studies for gene mutations and structural chromosome aberrations
were negative.
Benomyl does cause numerical chromosomal aberrations
(aneuploidy and/or polyploidy) in experimental systems both in
vitro and in vivo (Table 9).
7.7 Carcinogenicity
A number of carcinogenicity studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.7.1 Rat
In a two-year study on benomyl, groups of Charles River albino
weanling rats (36 male and 36 female rats) were fed benomyl (50-70%
a.i.) in the diet at levels of 0, 100, 500 and 2500 mg/kg diet. At
the end of the study all surviving animals were sacrificed and gross
and microscopic examinations of tissues and organs were carried out.
The most frequently observed tumours involved the pituitary, but
these were equally distributed among control and treated groups.
Mammary, adrenal and other tumours were also observed; these were
scattered among all groups. There were no adverse effects or
significant histopathological changes at any dose levels in this
study that could be attributable to benomyl (Sherman, 1969c; Lee,
1977).
Table 9. Studies on mutagenicity of benomyl
End points/Tests Species, strains Concentrationb Activation Result Reference
1. DNA damage and repair
Mitotic gene conversion Saccharomyces cerevisiae, NR with and negative Siebert et al. (1970);
D4 & D7 without de Bertoldi et al. (1980)
Mitotic gene conversion Aspergillus nidulans, 0.35-2.8 mM without negative de Bertoldi & Griselli
D7 (1980)
Mitotic crossing-over test A. nidulans, P NR without negative Bignami et al. (1977)
A. nidulans, D7 NR without negative de Bertoldi & Griselli
(1980)
Non-disjunction A. nidulans, D7 0.35-2.8 mM without positive de Bertoldi & Griselli
(1980)
Sister chromatid exchanges human lymphocyte 0.05-2.0 µg/ml NR slight increase in Georgieva et al. (1990)
(SCE) cultures SCE, no dose-response
relationship
Unscheduled DNA synthesis B6C3F1 male mice & 0.5-500 µg/ml NR negative Tong (1981)
Fisher-344 male rat
hepatocytes
2. Gene mutation
a) Bacterial & fungal Salmonella typhimurium 0.125-1.0 µg/ml NR positive(TA1535) Kappas et al. (1976)
gene mutation TA1535, TA1538 0.125-1.0 µg/ml NR negative(TA1538)
Escherichia coli, 0.125-1.0 µg/ml NR positive Kappas et al. (1976)
WP2 uvra
E. coli, WP2 uvra 2.5-10.0 µg/ml NR positive Kappas et al. (1976)
E. coli, WP2 0.125-1.0 µg/ml NR negative Kappas et al. (1976)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Point mutation A. nidulans 0.25-0.4 µg/ml NR positive Kappas & Bridges (1981)
Spot test S. typhimurium, his, 50-5000 µg per negative Fiscor et al. (1978)
G 46 & TA1535, TA1530 spot
TA1950
S. typhimurium, TA100 50-2000 µg per with negative Fiscor et al. (1978)
plate
Host-mediated assay S. typhimurium, his, 3 consecutive negative Fiscor et al. (1978)
G 46 subcutaneous
injections of
500 mg/kg in mice
TA1950 an oral dose of 4000 negative Fiscor et al. (1978)
mg/kg in rats or mice
Spot test S. typhimurium, TA1535, 20 µg/spot with and negative Carere et al. (1978)
TA1536, TA1537, TA1538 without
Forward spot mutation Streptomyces coelicolor 500 µg/spot without negative Carere et al. (1978)
assay
Ames plate incorporation S. typhimurium, TA1537 500 µg/plate without marginal Russell (1978a)
test positive
S. typhimurium, TA1535, 10-500 µg/ with negative Donovan & Krahn (1981);
TA1537, TA98, TA100 plate Rickard (1983a,b)
10-200 µg/plate without negative
TA1535, TA1537, TA98, 10-500 µg/plate with and negative Russell (1978b)
TA100 without
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
TA1513, TA1537, up to 1000 µg/ with and negative Shirasu et al. (1978)
TA1538, TA98, TA100 plate without
E. coli, WP2 hcr up to 500 µg/ with and negative Shirasu et al. (1978)
plate without
recA spot test B. subtilis, M45 & H-17 2000 µg/plate NR negative Shirasu et al. (1978)
Host-mediated assay S. typhimurium, his, 2000 mg/kg negative Shirasu et al. (1978)
(ICR mice) G46
b) In vitro mammalian
gene mutaion
HGPRTa gene Chinese hamster ovary 17 & 172 µM with negative Fitzpatrick (1980)
cells 3 & 120 µM without negative
Mouse lymphoma Mutant colonies in 0.5-20 µM without negative Amacher et al. (1979)
L5178Y TK+/- gene RPMl1640/horse serum
mutation assays (MLY) 2.5-25 µM with negative
MLY > 2.5 µM negative McCooey et al. (1983a)
MLY carbendazim, without negative McCooey et al. (1983b)
25-200 µM
carbendazim, with negative McCooey et al. (1983b)
12.5-200 µM
MLY N-butylisocyanate without negative McCooey et al. (1983b)
2.5-25 µM
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) Insect germ cell
gene mutation
Sex-linked recessive adult male fed benomyl suspension negative Lamb & Lilly (1980)
lethals D. melanogaster (1.5 mg/ml)
3. Chromosomal effects
a) Yeast and fungi A. nidulans 0.25 µg/ml without increased frequency of Hastie (1970)
(diploid) 0.5 µg/ml segregants
A. nidulans 0.25 µg/ml without no increased frequency Hastie (1970)
(haploid) 0.5 µg/ml of segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(diploid) segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(haploid) segregants
A. nidulans 0.35 to 2.8 mM without increased De Bertoldi & Griselli
(diploid) nondisjunction (1980)
Saccharomyces cerevisiae, 30 µg/ml without induced chromosome Albertini (1991)
D61.M malsegregation
b) In vitro mammalian human lymphocytes 1.0-100.0 µg/ml with negative Pilinskaya (1983)
assays
human lymphocytes 1.0-100.0 µg/ml without weak positive at Pilinskaya (1983)
10.0 µg/ml only
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
human/mouse mono- < 15 µg/ml without induced aneuploidy Athwal & Sandhu (1985);
chromosomal hybrid and polyploidy Sandhu et al. (1988)
cells (R3-5)
human/mouse mono- 15 µg/ml without slight increase in Athwal & Sandhu (1985);
chromosomal hybrid structural Sandhu et al. (1988)
cells (R3-5) chromosomes; 1.5 µg/ml
threshold (polyploidy)
V79/AP4 Chinese NR without dose-related increase Rainaldi et al. (1989)
hamster cells in numerical
chromosomal
aberrations
Chinese hamster NR without dose-related increase Eastmond & Tucker
ovary cells in numerical (1989)
chromosomal
aberrations
human lymphocytes NR without dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations; 0.1 µg/ml
threshold (aneuploidy)
Chinese hamster- NR without dose-related increase Zelesco et al. (1990)
human hybrid cells in numerical
chromosomal
(EUBI) aberrations; 2.0 µg/ml
threshold (aneuploidy)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) In vivo mammalian rats up to 500 mg/kg no chromosomal Ruziscka et al. (1976)
assays for 8 days by gavage aberrations in bone
marrow, increase in
chromosomal
aberrations in
embryonic cells at
200 and 500 mg/kg doses
Bone marrow micro- ICR mice 2 gavage doses of increase in Seiler (1976)
nucleus test 9, 500 or 1000 micronucleated
mg/kg 24 h apart polychromatic
erythrocytes at
1000 mg/kg only
Bone marrow micro- BDF1 mice single gavage dose increase in Sasaki (1990)
nucleus test of 0, 1250, 2500 or micronucleated
5000 mg/kg polychromatic
erythrocytes at
2500 and 5000 mg/kg
Bone marrow chromosomal B6D2F2/Cr-1Br mice single gavage dose no increase in Stahl (1990)
aberrations of 0, 625, 1250, 2500, structural chromosomal
or 5000 mg/kg aberrations
d) In vivo germ cell
chromosomal aberration
Dominant lethal test ChR-CD rat 0, 500, 2500 or negative Culik & Gibson (1974)
5000 mg/kg feeding
for 7 days
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Dominant lethal test Wistar rats 0, 1, 6.3 or 203 negative Barnes et al. (1983)
mg/kg feeding for
70 days
Dominant lethal test Wistar rats 70 daily gavage doses negative Georgieva et al. (1990)
of 0, 10 or 50 mg/kg
a HGPRT = hypoxanthine-guanine phosphoribosyl transferase
b NR = not reported
7.7.2 Mouse
Male and female CD-1 mice (80 males and 80 females per group)
were fed benomyl (99% a.i.) at dose levels of 0, 500, 1500 and 5000
mg/kg diet (the highest level was reduced from 7500 mg/kg after 37
weeks) for two years. The incidence of hepatocellular adenomas and
carcinomas (see Table 10) in female mice was increased in a
dose-dependent manner. In male mice, numbers of hepatocellular
adenomas and carcinomas were significantly increased at 500 and 1500
mg/kg but not at 5000 mg/kg dose. The increased number of lung
alveogenic carcinomas in male mice was still within the range of
historical controls (Weichman et al., 1982; Frame & van Pelt, 1990;
Hardisty, 1990).
7.8 Special studies
7.8.1 Neurotoxicity
Studies performed using White Leghorn hens (10/group) gave no
indication of neurotoxic potential with single oral doses of benomyl
at levels up to 5000 mg/kg (Goldenthal, 1978; Jessup & Dean, 1979;
Jessup, 1979).
Studies performed using adult male CFY rats (10 rats/group)
gave no indication of altered EEG potentials or behaviour (learning
ability assessed with a 4-choice T-maze) after treatment with 250 or
500 mg benomyl/kg per day for 3 months when compared with controls
(Desi, 1983).
7.8.2 Effects in tissue culture
In a study by Desi et al. (1977), primary monkey kidney cells
were incubated with benomyl (Fundazol 50 WP) at levels of 1, 10, 50,
100, 250 and 400 mg/kg. Growth inhibition and syncytium-like
appearance of the cell monolayer was observed at 250 mg/kg, and at
400 mg/kg all the cells were killed.
7.9 Factors modifying toxicity; toxicity of metabolites
Benomyl degrades to butyl isocyanate (BIC) and carbendazim
quite rapidly in water and in many organic solvents used in
toxicological testing. The biological activity of benomyl is
essentially that of carbendazim both in a toxicological sense and in
its use as a fungicide. A detailed discussion of carbendazim
toxicology is given in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
Table 10. Incidence and latency of hepatic tumours in benomyl-treated CD-1 micea
Male mice Female miceb
Benomyl concentration (mg/kg diet): 0 500 1500 5000 0 500 1500 5000
Hepatocellular adenoma
Number of animals with tumours 9 8 10 10 2 2 5 7
Latent time to first tumour (days) 530 556 541 627 744 641 650 644
Average latent period (days) 687 707 695 726 744 688 717 722
Hepatocellular carcinoma
Number of animals with tumours 14 26c 41d 17 2 7 7 14e
Latent time to first tumour (days) 545 470 590 508 744 640 736 426
Average latent period (days) 693 705 721 711 744 708 736 695
a From: Wiechman (1982)
b P < 0.05 Dose/response increase of adenomas and carcinomas
c P < 0.05
d P < 0.001
e P < 0.01
BIC is toxic by inhalation. In 4-month subchronic inhalation
studies, the no-observed-effect levels (NOEL) for BIC in rats and
mice were determined to be 0.32 and 1.5 ppm, respectively (Gurova et
al., 1976). An industrial hygiene survey found that workers
experienced severe eye irritation and lacrimation at BIC exposure
levels of 5-10 ppb (Kelly, 1989).
7.10 Mechanisms of toxicity - Mode of action
Biochemical studies on the mechanism of action of benzimidazole
compounds have shown that their biological effects are caused by
interactions with cell microtubules (Davidse & Flach, 1977). These
cellular structures are present in all eukariotic cells and are
involved in several vital functions, such as intracellular
transports and cell division. Benzimidazol compounds have been used
as anticancer drugs and as antihelminthic drugs in animals and
humans because they act as spindle poisons by interfering with the
formation and/or functioning of microtubules. However, eukaryotes
are known to be unequally sensitive to each benzimidazol compound,
which explains the use of these compounds in helminthiases.
Selective toxicity of benomyl and carbendazim for fungi has been
explained by comparing their binding to fungal and mammalian
tubulin. The different sensitivity of several fungi has also been
explained by the different affinity of benomyl and carbendazim for
fungal tubulin.
Benomyl has been found to bind to fungal tubulin but not to
porcine brain tubulin, indicating that mammalian tubulin has no, or
at least low, affinity for benomyl (Davidse & Flach, 1977). This is
in agreement with the observation that benomyl at concentrations
that are lethal for sensitive fungi does not interact with in vitro
microtubule assembly in these brain extracts. In vitro ID50
values for several mycelial extracts of various fungal species
sensitive to benomyl were all below 5 µmol/litre (Davidse & Flach,
1977). In vitro rat brain tubulin polymerization was inhibited to
about 20% at benomyl or carbendazim concentrations of 25 µmol/litre
(De Brabander et al., 1976b). For comparison, a standard antitubulin
drug in humans such as vincristine inhibited 50% tubulin assembly at
0.1 µmol/litre in the same experiment. The assembly of sheep and
calf brain microtubule was also found to be unaffected by
carbendazim concentrations higher than 100 µmol/litre (Ireland et
al., 1979).
Mitotic arrest by benzimidazole and six analogues at metaphase
was evaluated in human lymphocyte cultures. Structure-activity
relationships indicate that antimitotic activity is related to C6
substitution of the benzimidazole moiety (Holden et al., 1980). In
this study, however, benomyl and carbendazim were not tested. The
question of whether all C6 unsubstituted benzimidazoles, such as
benomyl and carbendazim, have no effect on mitosis of human
lymphocytes in cell cultures is therefore unresolved.
A link between the effects of benomyl and carbendazim on brain
tubulin and their teratogenic effects has been postulated (Ellis et
al., 1987, 1988).
8. EFFECTS ON HUMANS
8.1 General population exposure
No references to benomyl poisoning in the general population
have been documented in the scientific literature. Recent data used
to estimate dietary exposure based on food consumption patterns
within the USA indicate exposures well below the NOELs in animal
toxicity tests.
8.2 Occupational exposure
8.2.1 Acute toxicity
Benomyl has a very low acute toxicity. No inadvertent poisoning
of agricultural or factory workers has been documented (Goulding,
1983).
8.2.2 Effects of short- and long-term exposure
Benomyl causes contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et al., 1985). In
controlled patch tests of agricultural, ex-agricultural and
non-agricultural workers (total of 200 subjects), only one
agricultural worker showed any contact dermatitis to 0.1% benomyl
(Lisi et al., 1986).
A survey of cross-sensitization between benomyl and other
pesticides was conducted in Japan on a group of 126 farmers who
applied benomyl to their crops. Thirty-nine of the farmers gave
positive test results with benomyl, the highest incidence being
among female farmers. There were cross-reactions between benomyl and
other pesticides, such as diazinon, saturn, daconil and Z-bordeaux
(Matsushita & Aoyama, 1981).
Selected blood profiles from 50 factory workers involved in the
manufacture of benomyl were compared to those of a control group of
48 workers who were not exposed to carbendazim. White blood cell
count, red blood cell count and haemoglobin and haematocrit values
were comparable among the two groups. There were no quantitative
estimates of exposure given for the factory workers (Everhart, 1979;
FAO/WHO, 1985a).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers exposed to benomyl between 1970 and 1977. The
workers ranged from 19 to 64 years of age (79% were between 20 and
39, and 78% of the spouses were similarly aged between 20 and 39
years). Exposure duration ranged from less than one month to 95
months, and more than 51% of the workers were potentially exposed
from 1 to 5 months. The birth rates of exposed workers' spouses were
compared with those of four comparison populations from the same
county, state, region and country (USA). There was no reduction in
fertility as shown by the birth rates for the study population,
which were generally higher than those of the comparison
populations. Spermatogenesis among workers was not examined (Gooch,
1978; FAO/WHO, 1985a).
In studies on agricultural spraymen using benomyl (Fundazol 50
WP), 14 spraymen working in greenhouses were followed, some of them
for two years. General medical check-up and routine blood and urine
tests were performed. Electrocardiograms were recorded and blood
cholinesterase activity was monitored. Lymphocytes from peripheral
blood were examined for chromosomal aberrations both before and
during the study. There was no difference in structural chromosomal
aberration between the spraymen and controls. After benomyl
exposure, numerical chromosomal aberrations were higher in the
spraymen than in controls. However, the spraymen had a higher level
of numerical chromosome aberration than the controls even before
benomyl exposure (Desi et al., 1990; Nehez et al., 1992).
9. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Soil respiration has been found to be little influenced by
benomyl at concentrations below 10 mg/kg, which is the maximum soil
concentration expected after use at recommended application rates
(Hofer et al., 1971; van Fassen, 1974; Peeples, 1974; Weeks &
Hedrick, 1975).
A study on the influence of benomyl on soil nitrogen
mineralization showed that the release of ammonia was not decreased
by benomyl, whereas the influence of the fungicide on nitrification
varied from a stimulation (van Fassen, 1974), through no effect
(Mazur & Hughes, 1975), to a decreased nitrification (Hofer et al.,
1971; Wainwright & Pugh, 1974). The differences may be related to
the soil composition since Hofer et al. (1971) found a greater
effect in sandy than in organic soil.
Benomyl, in combination with eleven other pesticides that were
used in an orchard spray programme, had only a minimal and
short-term effect on respiration, ammonification and nitrification
at concentrations expected after recommended use of benomyl over a
spraying season. Ten times the recommended application rates had a
pronounced effect on both respiration and nitrification, which
lasted for more than 4 weeks (Helweg, 1985).
The influence of benomyl and carbendazim on soil microbial
activity was studied in Sweden following repeated annual
applications during autumn to winter cereals for a period of 3 to 5
years. The effects of the fungicides on straw decomposition, balance
of straw fungal flora and nitrogen mineralization in the soil were
investigated in field and laboratory experiments. The decomposition
of straw in the field was not affected in clay soils by annual
applications of up to 2 kg/ha. In sandy soils, rates of up to 0.5
kg/ha had no effect, but in one case at 2 kg/ha the initial stages
of straw decomposition were slightly inhibited. All doses tested in
both clay and sandy soils caused changes in the composition of the
straw fungal flora (Torstensson & Wessen, 1984).
Benomyl had no effect on soil bacterial populations in
laboratory studies, but fungi and actinomycetes populations were
reduced (Siegel, 1975). Under greenhouse conditions (Kaastra-Howeler
& Gams, 1973) or field conditions (Peeples, 1974) at application
rates of up to 89.6 kg a.i./ha, little effect on microbial
populations was observed following benomyl treatment.
9.2 Aquatic organisms
The effect of benomyl was monitored using the green alga
Selenastrum capricornutum in an OECD guideline test (201). The
EC50 (based on total growth) at 72 h was 2.0 mg/litre and at 120 h
was 3.1 mg/litre. The no-observed-effect concentration (NOEC) was
0.5 mg/litre. To study whether benomyl was algistatic or algicidal,
organisms were recultured at the end of the initial 120 h
incubation. Regrowth occurred in the control but not in the test
cultures (8.0 mg/litre) after a period of 7 days. Benomyl was,
therefore, considered to be algicidal (Douglas & Handley, 1988). In
another study using Chlorella pyrenoidosa, the 48-h EC50 for
growth inhibition was calculated as 1.4 mg/litre (Canton, 1976).
The acute toxicity of benomyl to a variety of aquatic organisms
is summarized in Table 11. For 96-h tests, LC50 values ranged from
0.006 mg/litre for channel catfish ( Ictalurus punctatus; yolk-sac
fry) to > 100 mg/litre for crayfish ( Procambarus sp.) (Mayer &
Ellersieck, 1986).
9.3 Terrestrial organisms
Several field studies have investigated the toxicity of benomyl
to earthworms (Table 12). In one study, the 48-h LC50 for E.
foetida was 9.1 µg/cm3 soil (Roberts & Dorough, 1984).
Van Gestel et al. (1992) exposed red earthworms ( Eisenia
andrei) to benomyl added to artificial soil and used final
concentrations of 0, 0.1, 0.32, 1.0, 3.2 and 10 mg/kg dry soil. The
worms had been acclimatized for 1 week in the artificial soil, which
contained 4 g/kg cow dung as food for the worms. At the highest
concentration of 10 mg/kg, high mortality occurred; LC50 values of
6.0 and 5.7 mg/kg dry soil were calculated after 3 and 6 weeks of
incubation, respectively. Growth was significantly reduced at 3.2
mg/kg soil. EC50 values for the effect of benomyl on cocoon
production did not differ significantly for the two test periods,
and the EC50 was 1.6 (1.2-2.3) mg/kg for the entire 6-week test
period.
Zoran et al. (1986) and Drewes et al. (1987) have shown that
the conduction velocity for medial and lateral giant nerve fibres is
affected by exposure of earthworms to sublethal concentrations of
benomyl. Zoran et al. (1986) have also shown that segmental
replication is affected in amputated earthworms exposed to sublethal
concentrations of benomyl.
The benomyl metabolite carbendazim (99.3% purity) was evaluated
for acute contact toxicity after thoracic application to honey-bees
(Apis mellifera). Each treatment level consisted of four replicates
of ten bees each. Forty bees served as positive control (using
carbaryl) and forty as negative control. No deaths occurred after
application of carbendazim at 50 µg/bee, the highest rate tested.
Carbendazim is, therefore, classified as "relatively non-toxic" to
the honey-bee (Meade, 1984).
Table 11. Toxicity of benomyl to aquatic organisms
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Freshwater
Water flea adult stat 25 3 14 Yoshida & Nishiuchi (1972)
(Daphnia magna) < 24 h stat 20 87 8.5 48 0.11c Hutton (1989)
< 24 h stat 20 48 0.64 Canton (1976)
< 24 h stat 17 40 7.4 48 2.8 Mayer & Ellersieck (1986)
Scud
(Gammarus pseudolimnaeus) adult stat 17 40 7.4 96 0.75 Mayer & Ellersieck (1986)
Crayfish
(Orconectes nais) instar stat 22 40 7.4 96 >10 Mayer & Ellersieck (1986)
Crayfish
(Procambarus sp.) immature stat 22 40 7.4 96 >100 Mayer & Ellersieck (1986)
Midge
(Chironomus plumosus) instar stat 22 40 7.4 48 7.0 Mayer & Ellersieck (1986)
Rainbow trout 3 months stat 15 48 0.48 Canton (1976)
(Oncorhynchus mykiss) 0.8 g stat 7 44 7.4 96 0.17 Mayer & Ellersieck (1986)
0.8 g stat 12 44 7.4 96 0.20 Mayer & Ellersieck (1986)
0.8 g stat 17 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1.2 g stat 12 44 6.5 96 0.16 Mayer & Ellersieck (1986)
1.2 g stat 12 44 7.5 96 0.19 Mayer & Ellersieck (1986)
1.2 g stat 12 44 8.5 96 0.88 Mayer & Ellersieck (1986)
0.6 g stat 12 44 7.4 96 0.23 Mayer & Ellersieck (1986)
0.6 g stat 12 320 7.4 96 0.60 Mayer & Ellersieck (1986)
fingerling stat 12 44 7.4 96 0.12 Mayer & Ellersieck (1986)
swimup fry stat 12 44 7.4 96 0.16 Mayer & Ellersieck (1986)
yolk-sac fry stat 12 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1 g stat 12 44 7.4 96 0.31c Mayer & Ellersieck (1986)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Fathead minnow 0.9 g stat 22 44 7.4 96 2.2 Mayer & Ellersieck (1986)
(Pimephales promelas) 0.5 g stat 22 45 7.1 96 1.3 Mayer & Ellersieck (1986)
0.5 g stat 22 44 7.4 96 1.9c Mayer & Ellersieck (1986)
Channel catfish 1.2 g stat 22 44 7.4 96 0.029 Mayer & Ellersieck (1986)
(Ictalurus punctatus) 0.05 g stat 22 44 7.4 96 0.013 Mayer & Ellersieck (1986)
0.15 g stat 22 44 7.4 96 0.024 Mayer & Ellersieck (1986)
swimup fry stat 22 44 7.4 96 0.012 Mayer & Ellersieck (1986)
yolk-sac fry stat 22 44 7.4 96 0.006 Mayer & Ellersieck (1986)
1.2 g stat 22 44 7.4 96 0.028c Mayer & Ellersieck (1986)
Bluegill
(Lepomis macrochirus) 0.9 g stat 12 44 7.4 96 0.75 Mayer & Ellersieck (1986)
0.9 g stat 17 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.9 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 6.5 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.5 96 1.2 Mayer & Ellersieck (1986)
0.6 g stat 22 44 8.5 96 6.4 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 320 7.4 96 2.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.2c Mayer & Ellersieck (1986)
Carp
(Cyprinus carpio) 5 cm stat 25 48 7.5 Yoshida & Nishiuchi (1972)
Killifish
(Fundulus sp.) 2.5 cm stat 25 48 11 Yoshida & Nishiuchi (1972)
Loach 10 cm stat 25 48 14 Yoshida & Nishiuchi (1972)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Guppy
(Poecilia reticulata) 3 weeks stat 24 48 3.4 Canton (1976)
Tadpole
(Bufo sp.) < 1 month stat 25 48 4.3 Yoshida & Nishiuchi (1972)
Estuarine and Marine
Eastern oyster
(Crassostrea virginica) 25-50 mm flow 17-19 96 1.37d Boeri (1988a)
Grass shrimp
(Palaemonetes pugio) 18 mm stat 18 96 45.8c Bionomics Inc. (1972)
Mysid shrimp
(Mysidopsis bahia) stat 23 96 0.175 Boeri (1988c)
Dungeness crab
(Cancer magister) larvae 96 7.6 Armstrong et al. (1976)
Sheepshead minnow
(Cyprinodon variegatus) 0.14 g stat 22 96 3.88 Boeri (1988b)
a stat = static conditons (water unchanged for duration of test); flow = flow-through conditions (benomyl concentration in water
continuously maintained)
b hardness given as mg CaCO3/litre
c 50% wettable powder
d EC50 based on rate of shell deposition
Table 12. Summary of earthworm toxicity data on benomyl in field studiesa
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass 0.125 0.9 63 8% reduction in number Ammon (1985)
1.25 3.6 43% reduction in number
Grass 7.8 22.2 21 95% reduction in number Tomlin & Gore (1974)
91% reduction in biomass
Grass 0.56 1.6 49 79% reduction in cast Keogh & Whitehead (1975)
production
Grass 0.56 1.6 13 50% reduction in number Tomlin et al. (1980,
(0.18)b 28 40% reduction in number 1981)
180 70% reduction in number
328 67% reduction in number
1.12 (0.90)b 28 35% reduction in number
(0.20)b 180 46% reduction in number
328 50% reduction in number
2.24 6.4 13 75% reduction in number
28 50% reduction in number
(0.30)b 180 50% reduction in number
328 64% reduction in number
Grass/loam 2.0 5.7 30 70% reduction in number Edwards & Brown (1982)
180 22% reduction in number
365 1% reduction in number
Grass/sandy loam 5.0 14.3 30 89% reduction in number Edwards & Brown (1982)
180 59% reduction in number
365 32% reduction in number
Table 12 (contd).
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass/loamy sand 10.0 28.6 30 80% reduction in number
180 89% reduction in number
365 89% reduction in number
Grass/sandy loam 5.0 14.3 30 91% reduction in number; Edwards & Brown (1982)
99% reduction in L. terrestris;
29% reduction in L. festivus
180 90% reduction in L. terrestris;
200% increase in L. festivus
365 99% reduction in L. terrestris;
1457% increase in L. festivus
a From: Van Gestel (1992)
b Results of analysis of the top 15-cm layer carried out by the author, recalculated as the concentration in the top 2.5 cm layer
The acute toxicity for several birds is listed in Table 13.
Benomyl is of low toxicity to birds.
Table 13. Acute toxicity of benomyl to birds
Species LD50a 5-day LC50b Reference
(mg/kg) (mg/kg)
Bobwhite quail > 10 000 Busey (1968e)
Mallard duck > 10 000 Busey (1968e)
Japanese quail > 5000 c Hill & Camardese
(1986)
Starling > 100 Schafer (1972)
Redwinged blackbird 100 Schafer (1972)
a LD50 = single oral dose expressed as mg/kg body weight
b LC50 = 5-day dietary exposure followed by 3 days on a "clean" diet
expressed as mg/kg diet
c 50% active ingredient; no overt signs of toxicity were observed
9.4 Population and ecosystem effects
Under certain conditions benomyl may have an effect on
populations of earthworms. In apple orchards where foliage has been
treated repeatedly at a rate of 0.28 kg/ha and has fallen to the
ground, earthworms may be eliminated after two years of benomyl use
(up to 13 sprayings). The earthworms Lumbricus terrestris and
Allolobophora chlorotica were most affected. Populations of other
species recovered within two years of the termination of spraying.
Orchard yields were unaffected, as were earthworm populations
adjacent to the orchards, because of the immobility of benomyl in
the soil (Stringer & Wright, 1973; Stringer & Lyons, 1974).
Van Gestel (1992) has summarized reports of the toxicity of
benomyl on earthworms from field studies with different soil types,
application rates and crops (Table 12). Estimated soil
concentrations in Table 12, for the various uses of the fungicide,
are based on application rates; they assume no mobility of the
compound beyond the top 2.5 cm of soil and homogeneous distribution
of benomyl in this layer. For orchard application, it was further
assumed that 50% of the applied active ingredient reached the soil.
Reported effects include reduced numbers and reduced activity of
worms. Application rates are within the recommended rates for
benomyl as a fungicide on these crops.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Benomyl and carbendazim are two different fungicides in their
own right. However, carbendazim is also the main metabolite of
benomyl in mammals and the degradation product of benomyl in the
environment. Butyl isocyanate is the chemical moiety removed from
benomyl when carbendazim is formed. Given the similar toxicities
caused by benomyl and carbendazim and the different toxicological
profile of butyl isocyanate, the two fungicides are evaluated
together in this monograph.
10.1 Evaluation of human health risks
The two primary routes of exposure to humans are through diet
and through manufacturing or use of the product.
There is limited information on actual dietary exposure to
benomyl and carbendazim. Dietary exposure has been estimated in the
Netherlands and the USA. In the USA, exposure based on dietary
habits, measured residue levels, and the percentage of crop treated
has been calculated for various subgroups of population. These
calculations indicate that the estimated benomyl exposure is
0.144-1.479 µg/kg per day (section 5.2.1). In the Netherlands, the
mean dietary intake was estimated to be 0.83 µg/kg per day (0.05
mg/day per person). These levels of exposure are below the
recommended ADI of 0.01 (carbendazim) and 0.02 (benomyl) mg/kg body
weight.
The average air levels of benomyl and carbendazim have been
determined in a manufacturing facility (section 5.3) and found to be
less than 0.2 and 0.3 mg/m3, respectively. Both values are below
the Threshold Limit Value of 5-10 mg benomyl/m3 established by a
number of governmental agencies.
In one study, potential respiratory and dermal exposure to
benomyl wettable powder formulation was determined under several
agricultural use situations (section 5.3). The highest rates of
exposure occurred in situations of mixing and loading in preparation
for aerial application; dermal and respiratory exposures were,
respectively, 26 and 0.08 mg/person per cycle. Home users and
agricultural workers re-entering treated fields were estimated to be
exposed to about 1 mg/person per cycle and 5.9 mg/person per hour,
principally through the dermal route.
Because of the low mammalian toxicity, acute benomyl or
carbendazim poisonings are unlikely to occur under conditions of
normal use.
Several studies of agricultural workers (section 8.2) have
shown some cases of contact dermatitis after exposure to benomyl.
These effects can be significantly reduced or eliminated by wearing
long-sleeved shirts, long trousers and gloves.
There is little information on health effects in humans as a
consequence of exposure to either benomyl or carbendazim. Two
studies have been conducted on factory workers involved in the
manufacture of benomyl (section 8.2). In one study, haematological
profiles from 50 factory workers involved in the manufacture of
benomyl were comparable to those from a control group of 48 workers.
A second study found no decrease in the birth rate of the wives of
298 factory workers exposed to benomyl.
Extensive studies of various species of laboratory animals show
reproductive, developmental, mutagenic and carcinogenic effects
associated with both benomyl and carbendazim. The effects observed
on rat fetuses were microphthalmia, hydrocephaly and encephaloceles.
The no-observed-effect levels (NOEL) for developmental toxicity are
equal to or greater than 10 mg/kg body weight per day, depending
upon the species and route of administration. Similarly, the NOEL of
benomyl for reproductive effects in the male rat appears to be 15
mg/kg body weight per day after gavage dosing. In feeding studies
with both benomyl and carbendazim, the NOEL appears to be 500 mg/kg
diet (equivalent to 25 mg/kg body weight per day). However, one
benomyl feeding study reported a NOEL of less than 1 mg/kg diet
(0.05 mg/kg body weight per day) for male reproductive effects. The
reason for the discrepancy between the NOEL in this latter study and
other investigations is unknown.
The only consistent genotoxic effect noted in animal studies is
the induction of numerical chromosomal aberrations. These effects
are consistent with the interaction of benomyl and carbendazim with
microtubule formation.
Rat carcinogenicity studies did not show any carcinogenic
effect for either compound. Benomyl and carbendazim induce
hepatocellular tumours in CD-1 and SPF Swiss mice but not in NMRKf
mice. This finding in mice is not considered to be a result of a
direct genotoxic action. Rather, it appears to be associated with
liver toxicity in strains of mice that are highly susceptible to
tumour formation at this site.
Benomyl and carbendazim are spindle poisons. Effects on target
cells are consequences of binding to microtubules, giving toxicities
similar to those of other spindle poisons such as colchicine and
vincristine. Benzimidazol compounds in general and benomyl and
carbendazim in particular have selective effects on the microtubules
of different eukaryotes. Reasons for this selectivity include the
binding capability to different tubulins and pharmacokinetic
differences across species. In vitro concentrations of benomyl
used to kill sensitive fungi were found to be ineffec tive in
disturbing mammalian microtubular functions. These studies on the
mechanism of action of benomyl and carbendazim indicate a selective
effect of these compounds for target species.
In summary, the LD50, as determined in a number of test
species, for benomyl ranges from > 2000 to > 12 000 mg/kg and for
carbendazim from > 2000 to > 15 000 mg/kg. There are no known
reports of human poisoning for either compound. This, coupled with
the low estimated environmental levels of both compounds, would
suggest that the possibility of acute poisoning by benomyl or
carbendazim is very remote. Similarly, the data available on test
species make it unlikely that either benomyl or carbendazim is
carcinogenic for humans. The NOELs for both reproductive and
teratogenic effects of benomyl and carbendazim (i.e. 10-15 mg/kg) do
raise a possibility that an accidental ingestion of either fungicide
could adversely alter reproductive outcome in humans, but the
likelihood that such poisoning would occur is remote. The
selectivity of these two benzamidazol compounds for the tubulin of
the target species (fungi) and their relative ineffectiveness to
disturb mammalian microtubule function further reduce the
possibility of their having toxic effects in humans.
10.2 Evaluation of effects on the environment
Benomyl is rapidly converted to carbendazim in various
environmental compartments, the half-lives being 2 and 19 h in water
and soil, respectively. Therefore, data from studies on both benomyl
and carbendazim are relevant for the evaluation of environmental
effects.
Carbendazim persists on leaf surfaces and in leaf litter. In
soil the half-life is between 3 and 12 months, and the compound may
be detected for up to 3 years. However, in many cases, major
residues will be lost within a single season. Residues of
carbendazim and its metabolites are strongly bound or incorporated
into soil organic matter. The strong adsorption (Koc =
approximately 2000) of carbendazim to soil and sediment particles
reduces its bioavailability to terrestrial and aquatic organisms.
Similarly, the mobility of carbendazim in soil is limited, and it is
not expected to leach to ground water.
Benomyl and carbendazim are highly toxic to some aquatic
organisms in laboratory tests, the most sensitive species being the
channel catfish with a 96-h LC50 for yolk-sac fry of 0.006 mg
benomyl/litre. However, this toxicity is unlikely to be manifest in
the environment for most aquatic organisms because of the low
bioavailability in surface waters. The exposure of sediment-living
organisms could be greater, but no test results are available for
these organisms.
Benomyl and carbendazim affect groups of fungi in soil but do
not seem to modify the overall microbial activity of the soil when
used at normal field rates.
In both the laboratory and field, benomyl and carbendazim
applied at recommended rates cause deaths and sublethal reproductive
effects on earthworms of many different species. Surface-feeding
species eating leaf litter are most at risk. Populations may take
more than 2 years to recover. There are no studies available on
other litter and soil invertebrates.
Benomyl and carbendazim have low toxicity for birds and
carbendazim is classified as "relatively non-toxic" to honey-bees.
10.3 Conclusions
Benomyl causes dermal sensitization in humans. Both benomyl and
carbendazim represent a very low risk for acute poisoning in humans.
Given the current exposures and the low rate of dermal absorption of
benomyl and carbendazim, it is unlikely that they would cause
systemic toxicity effects either in the general population or in
occupationally exposed subjects. These conclusions are drawn from
animal data and from the limited human data available, but these
extrapolations are supported by the understanding of the mode of
action of carbendazim and benomyl in both target and non-target
species.
Further elucidation of the mechanism of toxicity of carbendazim
and benomyl in mammals will perhaps permit a better determination of
no-observed-effect levels. Binding studies on tubulins of target
cells (testis and embryonic tissues) will facilitate comparisons
across species.
Carbendazim is strongly adsorbed to soil organic matter and
remains in the soil for up to 3 years. It persists on leaf surfaces
and, therefore, in leaf litter. Earthworms have been shown to be
adversely affected (population and reproductive effects) at
recommended application rates. There is no information on other soil
or litter arthropods that would be similarly exposed.
The high toxicity to aquatic organisms in laboratory tests is
unlikely to be seen in the field because of the low bioavailability
of sediment-bound residues of carbendazim. However, no information
is available on sediment-living species which would receive the
highest exposure.
11. FURTHER RESEARCH
1. Comparative binding studies of carbendazim to tubulins of
target tissues from various species should be undertaken.
2. Further clarification of the fate of 1,2-diaminobenzene and
bound residues in the environment is needed.
3. The effects of benomyl and carbendazim on sediment-dwelling
organisms needs to be investigated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Benomyl was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1973, 1975, 1978, 1983 and 1988. The 1978 meeting
agreed that the MRLs for benomyl, carbendazim and thiophanate-methyl
should be combined and expressed as carbendazim. Benomyl residues
were last evaluated by the 1988 meeting (FAO/WHO, 1988a,b) and the
MRLs were updated at that time. These MRLs (expressed as
carbendazim) are listed in Table 4. The 1983 meeting (FAO/WHO,
1985a) evaluated benomyl toxicology and set the following benomyl
NOEL levels and ADI:
Rat: 2500 mg/kg in the diet, equivalent to 125 mg/kg body
weight
Dog: 100 mg/kg (carbendazim) in the diet, equivalent to 2.5
mg/kg body weight
Rat: teratology - 30 mg/kg body weight per day
The estimated ADI for benomyl was established at 0-0.02 mg/kg
body weight.
Benomyl has not been evaluated by the International Agency for
Research on Cancer (IARC).
REFERENCES
Albertini S (1991) Reevaluation of the 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae aneuploidy
test systems by the Gene-Tox Program using strain D61.m of
Saccharomyces cerevisiae. Mutat Res, 260: 165-180.
Amacher DE, Paillet S, & Ray VA (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Application
to genetic toxicological testing. Mutat Res, 64: 391-406.
Ammon HU (1985) Worm toxicity tests using Tubifex tubifex. In:
Comportement et effets secondaires des pesticides dans le sol. Les
Colloques de l'INRA 31, Versailles, 4-8 juin 1984. Paris, Institut
national de la Recherche agronomique, pp 303-317.
Armstrong DA, Buchanan DU, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp. in laboratory-reared larvae of the Dungeness crab,
Cancer magister, and possible chemical treatments. J Invertebr
Pathol, 28: 329-336.
Arthur MF, Schweitzer KL, Fadel LC, Marsh BH, & Marsh SS (1989a)
Aerobic aquatic metabolism of [phenyl(U)-14C]benomyl in
Greenville, Mississippi, water and sediment (Battelle Study No.
N-0966-7301). Columbus, Ohio, Battelle, Environmental Sciences
Department (Unpublished report No. AMR-1452-89, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Arthur MF, Marsh BH, Fadel LC, & Zwick TC (1989b) Anaerobic aquatic
metabolism of [phenyl(U)-14C]benomyl in West Jefferson, Ohio, pond
water and sediment (Battelle Study No. NO799-8800). Columbus, Ohio,
Battelle, Environmental Sciences Department (Unpublished report No.
AMR 770-87, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Athwal RS & Sandhu SS (1985) Use of human X mouse hybrid cell line
to detect aneuploidy induced by environmental chemicals. Mutat Res,
149: 73-81.
Barbo EC & Carroll KS (1972) Oral ALD test (S-triazine,
3-butylbenzimidazolo[1,2-a],-2,4[1H,3H]-dione. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 227-72).
Barefoot AC (1988) Vapor pressure of benomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Research & Development
Division, Experimental Station (Unpublished report No. AMR 1078-88).
Barnes TB, Verlangieri AJ, & Wilson MC (1983) Reproductive toxicity
of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate (benomyl) in
male Wistar rats. Toxicology, 28: 103-115.
Baude JF, Gardiner JA, & Han JCY (1973) Characterization of residues
on plants following foliar spray applications of benomyl. J Agric
Food Chem, 21: 1084-1090.
Baude FJ, Pease HL, & Holt RF (1974) Fate of benomyl on field soil
and turf. J Agric Food Chem, 22: 413.
Belasco IJ (1979a) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX-4).
Belasco IJ (1979b) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied with supplement.
Wilmington, Delaware, Du Pont de Nemours and Co., Inc. (Unpublished
report No. B/ME-47 and supplement No. HLR 117-79).
Belasco IJ, Kirkland JJ, Pease HL, & Sherman H (1969) Studies with
2-14C-labelled methyl-1-(butylcarbamoyl)-2-benzimidazolecarbamate
(benomyl) in rats. Wilmington, Delaware, Du Pont de Nemours and Co.,
Inc., Biochemical Department, Research Division, Experimental
Station (Unpublished report No. B/ME-36).
Bignami M, Aulicino F, Velcich A, Carere A, & Morpurgo G (1977)
Mutagenic and recombinogenic action of pesticides in Aspergillus
nidulans. Mutat Res, 46: 395-402.
Bionomics, Inc. (1972) Acute toxicity of Benlate to grass shrimp
( Palaemonetes vulgaris). Wareham, Massachusetts, Bionomics, Inc.
(Unpublished report No. HOL 275-72, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Boeri RL (1988a) Flow through acute toxicity of benomyl technical to
the eastern oyster, Crassostrea virginica. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 13-89, prepared
for E.I. Du Pont de Nemours and Co., Inc.).
Boeri RL (1988b) Static acute toxicity of benomyl technical to the
sheepshead minnow, Cyprinodon variegatus. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 9-89, prepared for
Du Pont de Nemours and Co., Inc.).
Boeri RL (1988c) Static acute toxicity of benomyl technical to the
mysid, Mysidopsis bahia. Marblehead, Massachusetts, Enseco
(Unpublished report No. HLO 828-88, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Bolton EE, Anderson JJ, & Koeppe MK (1986a) Metabolism of
[phenyl(U)-14C] benomyl in field-grown soybeans. Wilmington,
Delaware, Du Pont de Nemours and Co., Inc., Agricultural Products
Department, Experimental Station (Unpublished report No.
AMR-531-86).
Bolton EE, Anderson JJ, & Koeppe MK (1986b) Metabolism of
[phenyl(U)-14C] benomyl in paddy rice. Wilmington, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Agricultural Products Department,
Experimental Station (Unpublished report No. AMR-507-86).
Brock WJ (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 505-87).
Busey WM (l968a) Acute dermal LD50 test and dermal irritation test
on rabbits using a wettable powder formulation (50% benomyl) with
histological addendum. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Falls Church, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. MRO 1126-1,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968c) Teratology study in rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 1079, prepared for
E.I Du Pont de Nemours and Co., Inc.). Busey WM (l968d) Repeated
dermal application test on rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 298-68, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (1968e) Acute dietary administration - mallard ducklings
and bobwhite quail: Fungicide 1991. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Bushway RJ, Hurst HL, Kugabalasooriar J, & Perkins LB (1991)
Determination of carbendazim in blueberries by reversed-phase high
performance liquid chromatography. J Chromatogr, 555: 321-324.
Canton JH (1976) The toxicology of benomyl, thiophanate-methyl, and
BCM to four freshwater organisms. Bull Environ Contam Toxicol, 16:
214-218.
Carere A, Ortali VA, Cardamone G, Torracca AM, & Raschetti R (1978)
Microbiological mutagenicity studies of pesticides in vitro. Mutat
Res, 57: 277-826.
Carter SD (l982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Raleigh, North Carolina, North Carolina
State University (Thesis).
Carter SD & Laskey JW (l982) Effect of benomyl on reproduction in
the male rat. Toxicol Lett, 11: 87-94.
Chang WM (1985) Soil column leaching studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Agricultural Chemicals Department, Experimental Station
(Unpublished report No. AMR 426-85).
Chiba M & Veres DF (1981) Fate of benomyl and its degradation
compound methyl 2-benzimidazole carbamate on apple foliage. J Agric
Food Chem, 29: 588-590.
Clermont Y (1972) Kinetics of spermatogenesis in mammals:
"seminiferous epithelium cycle and spermatogonial renewal". Physiol
Rev, 52: 198-236.
Colburn CW (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 84-69).
County Natwest Woodmac (1992) The fungicide market. London, County
Natwest Woodmac, Agrochemical Service, pp 49-51.
Culik R (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the concepti
of rats exposed to benomyl and Benlate by diet. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 916-80).
Culik R (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. HLR 970-80).
Culik R & Gibson JR (1974) "Benlate" dominant lethal study in male
rats with supplemental statistical report (22-77). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 72-74).
Cummings AM, Harris ST, & Rehnberg GL (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat. Fundam
Appl Toxicol, 15: 528-535.
Dashiell OL (1972) Acute oral test (benzimidazole,
2-(3-butylureido)). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 456-72).
Davidse LC & Flach W (1977) Differential binding of methyl
benzimidazol-2-yl carbamate to fungal tubulin as a mechanism of
resistance to this antimitotic agent in mutant strains of
Aspergillus nidulans. J Cell Biol, 72: 174-193.
De Bertoldi M & Griselli M (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat Res, 74:
303-324.
De Bertoldi M, Griselli M, Giovannetti M, & Barale R (1980)
Mutagenicity of pesticides evaluated by means of gene-conversion in
Saccharomyces cerevisiae and in Aspergillus nidulans. Environ
Mutagen, 2: 359-370.
De Brabander M, Van de Veire R, Aerts F, Geuens S, & Hoebeke J
(1976a) A new culture model facilitating rapid quantitative testing
of mitotic spindle inhibition in mammalian cells. J Natl Cancer
Inst, 56: 357-363.
De Brabander M, Van de Veire R, Aerts F, Brogers M, & Janssen PAJ
(1976b) The effect of methyl [5(2-thienylcarbonyl)-1H-benzimidazol-
2-yl carbamate (17934; NSC 238159), a new synthetic antitumoral drug
interfering with microtubules, on mammalian cells cultured in
vitro. Cancer Res, 36: 905-916.
Delp CJ (1980) Coping with resistance to plant disease control
agents. Plant Dis, 64: 652-657.
Dési I (1979) Hygienic-toxicological evaluations of pesticides.
Budapest, National Institute of Hygiene (D.Sc. Thesis).
Dési I (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav Toxicol Teratol, 5: 503-515.
Dési I, Dura G, Szlobodnyik J, & Csuka I (1977) Testing of pesticide
toxicity in tissue culture. J Toxicol Environ Health, 2: 1053-1066.
Dési I, Nehéz M, Palotas M, Tempfli A, Hogye A, & Vetro G (1990)
Experience of health status surveillance of pesticide workers in
Hungary. Med Lav, 81(6): 517-523.
Donovan SD & Krahn DF (1981) Mutagenic evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 343-81).
Douglass MT & Handley JW (1988) The algistatic activity of benomyl
technical. Huntingdon, United Kingdom, Huntingdon Research Centre
Ltd (Unpublished report No. DPT 171(n)/88371, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Draize JH, Woodard G, & Gawery HO (1944) New methods for the study
of irritation and toxicity of substances applied topically to the
skin and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Drewes CD, Zoran MJ, & Callahan CA (1987) Sublethal neurotoxic
effects of the fungicide benomyl on earthworms (Eisenia fetida).
Pestic Sci, 19: 197-208.
Du Pont (1972) Residue studies - fish: benomyl, MBC, and 2-AB.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report).
Du Pont (1987) Determination of octanol water partition coefficient
for benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/PC-55-CA).
Eastmond DA & Tucker JD (1989) Kinetochlore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat Res, 224: 517-525.
Edwards PJ & Brown SM (1982) Use of grassland plots to study the
effect of pesticides on earthworms. Pedobiologia, 24: 145-150
Eickhoff JC, Petersen BJ, & Chaisson CF (1989) Anticipated residues
of benomyl in food crops and potential dietary exposure and risk
assessment. Washington, DC, Technical Assessment System, Inc.
(Unpublished report No. TAS 000-005, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Ellis WG, Semple JL, Hoogenboom ER, Kavlock RJ, & Zeman FJ (1987)
Benomyl-induced craniocerebral anomalies in fetuses of adequately
nourished and protein -deprived rats. Teratog Carcinog Mutagen, 7:
357-375.
Ellis WG, De Roos F, Kavlock RJ, & Zeman FJ (1988) Relationship of
periventricular overgrowth to hydrocephalus in brains of fetal rats
exposed to benomyl. Teratog Carcinog Mutagen, 8: 377-391.
Evans EL & Mitchell AD (1980) An evaluation of the effect of benomyl
on sister chromatic exchange frequencies in cultured Chinese hamster
ovary cells. Menlo Park, California, SRI International (Unpublished
report prepared for E.I. Du Pont de Nemours and Co., Inc.).
Everhart LP (1979) Benlate dust exposure survey. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Biochemical
Department (Unpublished report No. B/TOX 6).
Everhart LP & Holt RF (1982) Potential benlate fungicide exposure
during mixer/loader operations, crop harvest and home use. J Agric
Food Chem, 30: 222-227.
FAO/WHO (1985a) Benomyl. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 7-46 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1985b) Carbendazim. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 89-121 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1988a) Benomyl. In: Pesticide residues in food -
Evaluations 1988. Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 5-15 (FAO Plant Production
and Protection Paper 93/1).
FAO/WHO (1988b) Carbendazim. In: Pesticide Residues in Food -
Evaluations 1988: Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 41-54 (FAO Plant Production
and Protection Paper 93/1).
Fielding M, Barcelo D, Helweg A, Galassi S, Torstensson L, van
Zoonen P, Wolter R, & Angeletti G (1992) Pesticides in ground and
drinking water. Brussels, Commission of the European Communities,
Directorate-General for Science, Research and Development,
Environmental and Waste Recycling, 135 pp (Water Pollution Research
Report 27).
Fiscor G, Bordas S, & Stewart SJ (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl-N'-nitro-N-nitrosoguanine in Salmonella typhimurium in
vitro and in rodent host-mediated assays. Mutat Res, 51: 151-164.
Fitzpatrick K (1980) Chinese hamster ovary cell assay for
mutagenicity. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 438-80).
Frame SR & Van Pelt CS (1990) Oncogenicity studies with benomyl and
MBC in mice: Supplemental peer review. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 20-82 and supplement No. HLR 70-82).
Frank KM (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 85-69).
Frank KM (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 233-72).
Fritz SB (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 109-69).
Fritz SB & Sherman H (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 51-69).
Gargus JL & Zoetis T (1983a) Primary irritation study in rabbits
(Benlate PNW). Vienna, Virginia, Hazelton Laboratories, Inc.
(Unpublished report No. HLO 510-83, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983b) Eye irritation test in rabbits (EPA
pesticide registration - Benlate PNW). Vienna, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 511-83, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983c) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines - Benlate PNW).
Vienna, Virginia, Hazelton Laboratories, Inc. (Unpublished report
No. HLO 512-83, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Vienna, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. HLO 67-84,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Georgieva V, Vachkova R, Tzoneva M, & Kappas A (1990) Genotoxic
activity of benomyl in different test systems. Environ Mol Mutagen,
16: 32-36.
Goldenthal EI (1978) Neurotoxicity study in hens [using technical
benomyl (< 95% benomyl). Mattawan, Michigan, International Research
and Development Corporation (Unpublished report and addendum No. HLO
28-79, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gooch JJ (1978) Fertility of workers potentially exposed to benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX 7).
Goodman NC (1975) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 847-74).
Goulding R (1983) Poisoning on the farm. J Soc Occup Med, 33: 60-65.
Guengerich FP (1981) Enzyme induction with Du Pont compounds H11,
202-02 and H10, 962-02. Nashville, Tennessee, Vanderbilt University,
School of Medicine (Unpublished report No. HLO 850-81, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gurova AI, Alekseeva NP, Gorlova OE, & Chernyshova RA (1976) [Data
for substantiation of the maximum permissible level of
m-(trifluoromethyl) phenyl isocyanate and butyl isocyanate in the
air of work areas.] Gig Tr Prof Zabol, 3: 53-55 (in Russian).
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 80: 339-357.
Han JCY (1978) Metabolism of 14C-labeled benomyl in the mouse and
hamster. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-65).
Han JCY (1979) 2-14C-Benomyl (50% WP) rat study - intravenous
injection. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-41).
Han JCY (1980) Metabolism of 2-14C-benomyl in the lactating nanny
goat. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report No. B/ME-39).
Hardesty PT (1982) Attempts to characterize liver residues from
14C-benomyl dosed goat. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Biochemicals Department (Unpublished report
No. AMR 71-82).
Hardisty JF (1990) Oncogenicity studies with benomyl and MBC in
mice. Peer-review of liver neoplasms. Research Triangle Park, North
Carolina, Experimental Pathology Laboratories, Inc. (Unpublished
report No. 129-012, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Hastie AC (1970) "Benlate"-induced instability of Aspergillus
diploids. Nature (Lond), 226: 771.
Helweg A (1977) Degradation and absorption of carbendazim and
2-aminobenzimidazole in soil. Pestic Sci, 8: 71-78.
Helweg A (1985) Side effects caused by pesticide combinations. In:
Jensen V, Kjoller A, & Sorensen LH ed. Proceedings of the FEMS
Symposium on Microbial Communities in Soil. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 385-395.
Hess RA, Moore BJ, Forrer J, Linder RE, & Abuel-Atta AA (1991) The
fungicide benomyl (methyl) 1-(butylcarbomyl)-2-benzimidazole
carbamate causes testicular dysfunction by inducing the sloughing of
germ cells and occlusion of efferent ductules. Fundam Appl Toxicol,
17: 733-745.
Hill EF & Camardese MB (1986) Lethal toxicities of environmental
contaminants and pesticides to Coturnix. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
Hofer I, Beck T, & Wallnöfer P (1971) [The influence of the
fungicide benomyl on soil microflora.] Z Pflanzenkr Pflanzenschutz,
78: 399-405 (in German).
Holden HE, Crider PA, & Wahrenburg MG (1980) Mitotic arrest by
benzimidazole analogs in human lymphocyte cultures. Environ Mutat,
2: 67-73.
Hood DB (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 211-69).
Hoogenboom ER, Ransdell JF, Ellis WG, Kavlock RJ, & Zeman FJ (1991)
Effects on the fetal rat eye of maternal benomyl exposure and
protein malnutrition. Curr Eye Res, 10: 601-612.
Hornberger CS (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
95-69).
Hostetler KH (1977) Oral LD50 test (Benlate OD). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 527-77).
Hutton DG (1989) Static acute 48-hour EC50 of Benlate fungicide 50
DF to Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 541-88).
Hutton DG, Kasprzak DJ, & Priester TM (1984) Laboratory studies of
[2-14C] carbendazim bioconcentration in Bluegill sunfish. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 428-84).
ILO (1991) Occupational exposure limits for airborne toxic
substances, 3rd ed. Geneva, International Labour Office
(Occupational Safety and Health Series No. 37).
Ireland CM, Gull K, Gutteridge WE, & Pogson CI (1979) The
interaction of benzimidazole carbamates with mammalian microtubule
protein. Biochem Pharmacol, 28: 2680-2682.
Jessup CD (1979) Acute delayed neurotoxicity study in chickens using
technical benomyl (> 95% benomyl). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report No. HLO 674-79, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Jessup DC & Dean W (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl). Mattawan,
Michigan, International Research and Development Corporation
(Unpublished report No. HLO 29-79, prepared for E.I. Du Pont de
Nemours and Co., Inc).
Johnson JD (1988) Determination of the plateau level of bound
[phenyl(U)-14C] carbendazim residues in goat liver. Columbus,
Ohio, Batelle, Columbus Division (Unpublished report No. AMR 779-87,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Kaastra-Howeler LH & Gams W (1973) Preliminary study on the effect
of benomyl on the fungal flora in a greenhouse soil. Neth J Plant
Pathol, 79: 516-518.
Kappas A & Bridges BA (1981) Induction of point mutations by benomyl
in DNA-repair-deficient Aspergillus nidulas. Mutat Res, 91:
115-118.
Kappas A, Georgopoulos SG, & Hastie AC (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat Res, 26: 17-27.
Kappas A, Green MHL, Bridges BA, Rogers AM, & Muriel WJ (1976)
Benomyl - a novel type of base analogue mutagen? Mutat Res, 40:
379-382.
Kavlock RJ, Chernoff N, Gray LE, Gray JA, & Whitehouse D (1982)
Teratogenic effects of benomyl in the Wistar rat and CD-1 mouse,
with emphasis on the route of administration. Toxicol Appl
Pharmacol, 62: 44-54.
Kelly DP (1989) Butyl isocyanate industrial hygiene survey. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. BENO/TOX 18).
Keogh RG & Whitehead PH (1975) Observations on some effects of
pasture spraying with benomyl and carbendazim on earthworm activity
and litter removal from pasture. NZ J Exp Agric, 3: 103-104.
Kirkland JJ (1973) Method for high-speed liquid chromatographic
analysis of benomyl and/or metabolite residue in cow milk, urine,
feces and tissues. J Agric Food Chem, 21: 171-177.
Kirkland JJ, Holt RF, & Pease HL (1973) Determination of benomyl
residues in soils and plant tissues by high speed cation exchange
liquid chromatography. J Agric Food Chem, 21: 368-371.
Krechniak J & Klosowska B (1986) The fate of 14C-carbendazim in
rat. Xenobiotica, 16(9): 809-815.
Krupka RM (1974) On the anti-cholinesterase activity of benomyl.
Pestic Sci, 5: 211-216.
Kuehne G, Heise H, Plottke B, & Puskeiler T (1985) Dermatitis after
benlate contact. Z Gesamte Hyg Grenzgeb, 31: 710-711.
Lamb MJ & Lilly LJ (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17:
83-95.
Lee KP (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 66-77).
Liesivuori J & Jääskeläinen S (1984) Exposure of greenhouse workers
to pesticides. Tampore, Finland, National Board of Labor Protection,
pp VIII-IX (Research Report No. 46).
Linder RE, Rehnberg GL, Strader LF, & Diggs JP (1988) Evaluation of
reproductive parameters in adult male Wistar Rats after subchronic
exposure. J Toxicol Environ Health, 25: 285-298.
Lisi P, Caraffini S, & Assalve D (1986) A test series for pesticide
dermatitis. Contact Dermatitis, 15: 266-269.
Littlefield NA & Busey WM (1969) Four-hour acute inhalation exposure
test in dogs using a wettable powder formulation (50% benomyl).
Falls Church, Virginia, Hazelton Laboratories, Inc. (Unpublished
report No. HLR 192-69, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983a) L5178Y mouse
lymphoma cell assay for mutagenicity (benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 86-83).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983b) L5178Y mouse
lymphoma cell assay for mutagenicity. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc. (Unpublished report No. HLR 253-83).
Majut JC (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 174-06).
Marsh BH & Arthur MF (1989) Aerobic metabolism of
[phenyl(U)-14C]benomyl in Keyport Silt Loam. Columbus, Ohio,
Battelle Memorial Institute (Unpublished report No. AMR 1112-88,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Marvin CH, Birndle ID, Hall CD, & Chiba M (1991) Rapid on-line
precolumn high performance liquid chromatographic method for the
determination of benomyl, carbendazim and aldicarb species in
drinking water. J Chromatogr, 555: 147-154.
Matsushita T & Aoyama K (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind Health,
19: 77-83.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp 45-56 (Resource Publication No. 160).
Mazur AR & Hughes TD (1975) Nitrogen transformation in soil as
affected by the fungicides benomyl, dyrene and maneb. Agron J, 67:
755-758.
Meade AB (1984) Acute contact LD50 toxicity study in honey bees
( Apis mellifera) with INE 965-212 (MBC). Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc. (Unpublished report No. AMB
84-5).
Mebus CA (1990) Reproductive and fertility effects with DPX-1991-529
(benomyl). Multigeneration reproduction study in rats. Newark,
Delaware, Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 765-90).
Monson KD (1985) Metabolism of [2-14C]benomyl in the lactating
dairy cow. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc. (Unpublished report No. AMR 247-84).
Monson KD (1986a) Metabolism of [2-14C]benomyl and [phenyl(U)-
14C]benomyl in laying hens. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc. (Unpublished report No. AMR 391-85).
Monson KD (1986b) Metabolism of [2-14C]carbendazim laying hens.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 264-84).
Monson KD (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 1141-88).
Monson KD (1991) Release and characterization of bound benomyl and
carbendazim metabolites in animal tissues via Raney nickel
desulfurization and acid dehydration. J Agric Food Chem, 39:
1808-1811.
Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, & Linder RE (in
press) Acute and long-term effects of the fungicide carbendazim
(methyl 2-benzimidazole carbamate, MBC) on the male reproductive
system in the rat. J Androl.
Nehéz M, Palotas M, Zimanyi M, Boros P, Mohos G, Vetro G, & Desi I
(1992) [Repeated cytogenetic examinations of workers using
pesticides in Csongrad County.] Egeszsegtudomany, 36: 40-47 (in
Hungarian).
Newsome WH & Collins PG (1987) Enzyme-linked immunosorbent assay of
benomyl and thiobendazole in some foods. J Assoc Off Anal Chem, 70:
1025-1027.
Newsome WH & Shields JB (1981) A radioimmunoassay for benomyl and
methyl 2-benzimidazolecarbamate on food. J Agric Food Chem, 29:
220-222.
Palawski DU & Knowles CO (1986) Toxicological studies of benomyl and
carbendazim in rainbow trout, channel catfish and bluegills. Environ
Toxicol Chem, 5: 1039-1046.
Parsons DW & Witt JM (1988) Pesticides in groundwater in the United
States of America: A report of a 1988 survey of State lead agencies.
Corvallis, Oregon, Oregon State University Extension Service.
Peeples JL (1974) Microbial activity in benomyl-treated soil.
Phytopathology, 64: 857-860.
Pilinskaya MA (1983) Investigation of the cytogenetic action of the
pesticides captan and benomyl in a culture of human peripheral blood
lymphocytes in the absence and presence of a system of metabolic
activation. Cytol Genet, 17: 29-33.
Powley CR (1985) Aqueous photolysis of [phenyl-14C(U)]benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 420-85).
Priester TM (1984) Hydrolysis of carbendazim [2-14C]. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 265-84).
Priester TM (1985) Batch equilibrium (adsorption/deabsorption) and
soil thin-layer chromatography studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 425-85).
Rainaldi G, Flori L, Colella CM, Mariani T, Piras A, Simi S, &
Simili M (1989) Analysis by BrUdR-labelling technique of induced
aneuploidy in mammalian cells in culture. Mutat Res, 177: 255-260.
Reinke RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
81-66).
Rhodes BC (1987) Greenhouse crop-rotation study with
[2-14C]carbendazim. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Inc. (Unpublished report No. AMR 495-86).
Rickard LB (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 97-83).
Rickard LB (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 98-83).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to
the earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Russell JF (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 18-78).
Russell JF (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 31-78).
Ruzicska P, Peter S, Laczi J, & Czeizel E (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP. Egeszegtudomany, 20:
74-83.
Ryan DL (1989) Soil column leaching of [phenyl(U)-14C]benomyl in a
rice paddy soil. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 1512-89).
Sandhu SS, Gudi RD, & Athwal RS (1988) A monochromosomal hybrid cell
assay for evaluating the genotoxicity of environmental chemicals.
Cell Biol Toxicol, 4: 495-506.
Sarver JW (1987) Acute oral toxicity study with IN-T1991 in male and
female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. HLR 334-87).
Sasaki YFX (1990) Benomyl: Micronucleus test in mice. Tokyo,
Institute of Environmental Toxicology, Kodaira Laboratories
(Unpublished report No. IET 89-0046, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Seiler JP (1976) The mutagenicity of benzimidazole and benzimidazole
derivatives. VI. Cytogenetic effects of benzimidazole derivatives in
the bone marrow of the mouse and the Chinese hamster. Mutat Res, 40:
339-348.
Sherman H (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 269-68).
Sherman H (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 17-69).
Sherman H (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
168-69).
Sherman H (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 232-69).
Sherman H (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 48-70).
Sherman H (1972) Long-term feeding studies in rats and dogs with
2-benzimadazolecarbamic acid, methyl ester (INE-965) (50% and 70%
MBC wettable powder formulations): Parts I and II. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 195-72).
Sherman H & Krauss WC (1966) Acute oral test [benomyl]. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 100-66).
Sherman H, Barnes JR, & Krauss WC (1967) Ninety-day feeding study
with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell, Laboratory (Unpublished report No. HLR 11-67).
Sherman H, Culik R, & Jackson RA (1975) Reproduction, teratogenic
and mutagenic studies with benomyl. Toxicol Appl Pharmacol, 32:
305-315.
Shirasu Y, Moriya M, & Kato K (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Tokyo, Institute of
Environmental Toxicology, Kodaira Laboratories (Unpublished report
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Shukla Y, Antony M, & Mehrota NK (1989) Studies on gamma-glutamyl
transpeptidase in rodents exposed to benomyl. Bull Environ Contam
Toxicol, 42: 301-306.
Siebert D, Zimmermann FK, & Lemperle E (1970) Genetic effects of
fungicides. Mutat Res, 10: 533-543.
Siegel MR (1975) Benomyl-soil microbial interactions.
Phytopathology, 65: 219-220.
Snee DA (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test described in HLR 43-69).
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. 134-69).
Stahl RG Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 401-90).
Staples RE (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl):
Parts I, II and III. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 649-80).
Staples RE (1982) Teratogenicity study in the rat using technical
benomyl (>95% benomyl) administered by gavage and supplement with
individual animal data. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
587-82).
Staub T & Sozzi D (1984) Fungicide resistance: A continuing
challenge. Plant Dis, 68: 1026-1031.
Stevenson IE (1985) Metabolism of [phenyl(U)-14C]benomyl in
peaches. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 443-85).
Stringer A & Lyons C (1974) The effect of benomyl and
thiophanate-methyl on earthworm populations in apple orchards.
Pestic Sci, 5: 189-196.
Stringer A & Wright MA (1973) The effect of benomyl and some related
compounds on Lumbricus terrestris and other earthworms. Pestic
Sci, 4: 165-170.
Tolle DA (1988) Metabolism of [phenyl(U)-14C] benomyl in sugar
beets. West Jefferson, Ohio, Battelle Columbus DW (Unpublished
report No. AMR 620-86, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a
fungicide on the numbers and biomass of earthworms in pasture. Bull
Environ Contam Toxicol, 12: 487-492.
Tomlin AD, Tolman JH, & Thorn GD (1980/1981) Suppression of
earthworm ( Lumbricus terrestris) populations around an airport by
soil application of the fungicide benomyl. Prot Ecol, 2: 319-323.
Tong C (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Valhalla, New York, Naylor Dana Institute (Unpublished report No.
HLO 741-81, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Torstensson L & Wessen B (1984) Interactions between the fungicide
benomyl and soil microorganisms. Soil Biol Biochem, 16: 445-452.
Turney RT (1979) Rat inhalation study - Benlate. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 116-79).
Van Faassen HG (1974) Effect of the fungicide benomyl on some
metabolic processes and on numbers of bacteria and actinomycetes in
the soil. Soil Biol Biochem, 6: 131-133. Van Gestel CAM (1992)
Validation of earthworm toxicity tests by comparison with field
studies: A review of benomyl, carbendazim, carbofuran, and carbaryl.
Ecotoxicol Environ Saf, 23: 221-236.
Van Gestel CAM, Dirven-van Breeman EM, Baerselman R, Emans HJB,
Janssen JAM, Postuma R, & van Vliet PJM (1992) Comparison of
sublethal and lethal criteria for nine different chemicals in
standardized toxicity tests using the earthworm Eisenia andrei.
Ecotoxicol Environ Saf, 23: 206-220.
Van Joost TH, Naafs B, & van Ketel WG (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9: 153-154.
Vick DA & Brock WJ (1987) Primary dermal irritation study with
benlate 50 DF fungicide in rabbits. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 300-87).
Wainwright M & Pugh GJF (1974) The effect of fungicides on certain
chemical and microbial properties of soils. Soil Biol Biochem, 6:
263-267.
Ward RS & Scott RC (1992) Benomyl: in vitro absorption of a 500 g
kg-1 WP formulation through human epidermis. Fernhurst, Naslemere,
Surrey, Imperial Chemical Industries (ICI) (Unpublished report No.
CTL/P/3659).
Warheit DB, Kelly DP, Carakostas MC, & Singer AW (1989) A 90-day
inhalation toxicity study with benomyl in rats. Fundam Appl Toxicol,
12: 333-345.
Weeks RE & Hedrick HG (1975) Influence of a systemic fungicide on
oxygen uptake by soil microorganisms. Soil Sci, 119: 280-284.
Wheeler J (1985) Hydrolysis of [phenyl-14C(U)]benomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 419-85).
WHO (1993) Environmental Health Criteria 149: Carbendazim. Geneva,
World Health Organization.
Wiechman BE (1982) Long term feeding study with methyl
l-(butylcarbamoyl)-2-benzimidazolecarbamate in mice (INT-1991; >95%
benomyl): Parts I, II and III. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 20-82).
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Bull Agric Chem Insp Stn, 12: 122-128.
Zbozinek JV (1984) Environmental transformations of DPA, SOPP,
benomyl and TBZ. Residue Rev, 92: 113-155.
Zelesco PA, Barbieri I, & Graves JAM (1990) Use of a cell hybrid
test system to demonstrate that benomyl induces aneuploidy and
polyploidy. Mutat Res, 242: 329-335.
Zeman FJ, Hoogenboom ER, Kavlock RJ, & Semple JL (1986) Effects on
the fetus of maternal benomyl exposure in the protein-deprived rat.
J Toxicol Environ Health, 17: 405-417.
Zoran MJ, Heppner TJ, & Drewes CD (1986) Teratogenic effects of the
fungicide benomyl on posterior segmental regeneration in the
earthworm, Eisenia fetida. Pestic Sci, 17: 641-652.
Zweig G, Gao R, & Popendorf W (1983) Simultaneous dermal exposure to
captan and benomyl by strawberry harvesters. J Agric Food Chem, 31:
1109-1113.
RESUME ET CONCLUSIONS
1. Résumé
1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le bénomyl, un solide cristallin de couleur ambrée, est un
fongicide endothérapique qui appartient à la famille du
benzimidazole. Il se décompose juste au-dessus de son point de
fusion de 140 °C et sa tension de vapeur est < 5 x 10-6 Pa
(< 3,7 x 10-8 mmHg) à 25 °C. Le bénomyl est pratiquement
insoluble dans l'eau à pH 5 et à 25 °C, sa solubilité étant de 3,6
mg/litre. Il est stable dans les conditions normales de stockage
mais se décompose en carbendazime dans l'eau.
L'analyse des résidus de même que celle des prélèvements
effectués dans l'environnement comporte une extraction au moyen d'un
solvant organique, une purification de l'extrait par partage
liquide-liquide et la transformation du résidu obtenu en
carbendazime. Le dosage de ces résidus peut s'effectuer par
chromatographie en phase liquide à haute performance ou par titrage
immunologique.
1.2 Sources d'exposition humaine et environnementale
En 1988, on estimait à environ 1700 tonnes la quantité de
bénomyl utilisée dans le monde. Il s'agit d'un fongicide très
largement utilisé, homologué dans 50 pays pour le traitement de plus
de 70 cultures. Le bénomyl est présenté sous forme de poudre
mouillable.
1.3 Transport, distribution et transformation dans l'environnement
Le bénomyl se transforme rapidement en carbendazime dans
l'environnement avec une demi-vie respective de 2 et 19 heures dans
l'eau et le sol. On peut donc utiliser aussi bien les résultats des
études sur le bénomyl que sur le carbendazime pour l'évaluation des
effets sur l'environnement.
Dans l'environnement, le carbendazime se décompose avec une
demi-vie de 6 à 12 mois sur le sol nu, de 3 à 6 mois sur le gazon et
de 2 à 25 mois dans l'eau en aérobiose et en anaérobiose,
respectivement.
Le carbendazime est principalement décomposé par les
microorganismes. Le 2-aminobenzimidazole (2-AB) en est l'un des
principaux produits de dégradation et il est à son tour décomposé
par les microorganismes.
Lors de la décomposition de bénomyl marqué au 14C sur le
noyau phényle, on a constaté que 9% seulement du carbone-14 étaient
éliminés sous forme de CO2 en une année d'incubation. Le
carbone-14 restant était principalement récupéré sous forme de
carbendazime et de résidus liés. L'étude de la destinée d'un
éventuel produit de dégradation (1,2-diaminobenzène) pourrait
peut-être permettre de mieux définir la voie de dégradation des
fongicides benzymidazoliques dans l'environnement.
Des études effectuées sur le terrain ou sur colonnes ont montré
que le carbendazime restait dans les couches superficielles du sol.
On n'a pas mesuré l'adsorption du carbendazime dans le sol, mais on
pense qu'elle doit être aussi forte que dans le cas du bénomyl, avec
des valeurs de Koc allant de 1000 à 3600. Les valeurs de log Kow
sont respectivement de 1,36 et de 1,49 pour le bénomyl et le
carbendazime.
Un modèle de criblage basé sur les données d'adsorption et de
persistance n'a pas révélé de risque de lessivage. Cette observation
est corroborée par des analyses d'eau de puits effectuées aux
Etats-Unis, analyses qui n'ont pas permis de déceler ce composé dans
l'un quelconque des 495 puits étudiés ni le carbendazime dans 212
autres (la limite de détection n'a pas été précisée). On estime que
le bénomyl et le carbendazime entraînés par ruissellement
correspondent uniquement à la fraction adsorbée aux particules de
sol; d'ailleurs ces composés sont sans doute fortement adsorbés aux
sédiments présents dans l'environnement aquatique.
En solution, dans les végétaux et le sol, le bénomyl subit une
décomposition en carbendazime (méthyl-1H-benzimidazole-2-carbamate)
en 2-AB, STB (3-butyl-1,3,5-triazino[1,2a]-benzimidazole-
2,4(1H,3H)dione) ainsi qu'en BBU (1-(2-benzimidazolyl)-3-n-
butylurée). La photolyse du bénomyl est pratiquement inexistante.
Chez l'animal, le bénomyl est métabolisé en carbendazime ainsi
qu'en d'autres métabolites polaires qui sont rapidement excrétés. On
n'a pas observé d'accumulation de bénomyl ni de carbendazime dans
aucun système biologique.
1.4 Concentrations dans l'environnement et exposition humaine
Il ne semble pas qu'il existe de données résultant d'une
surveillance du bénomyl dans l'environnement. Toutefois on peut
récapituler ainsi les données tirées d'études portant sur la
destinée de ce produit dans l'environnement.
Comme le bénomyl et le carbendazime restent stables pendant des
semaines sur les végétaux, ils peuvent être ingérés par des
organismes qui se nourrissent de feuilles mortes. Des résidus de
carbendazime peuvent subsister jusqu'à 3 ans dans le sol et les
sédiments. Toutefois, la forte adsorption du carbendazime aux
particules de sol et de sédiments réduit l'exposition des organismes
terrestres et aquatiques.
Ce sont les résidus de bénomyl et de carbendazime présents sur
les cultures vivrières qui constituent la principale source
d'exposition de la population humaine dans son ensemble. L'analyse
de l'exposition par voie alimentaire qui a été effectuée aux
Etats-Unis (bénomyl et carbendazime associés) et aux Pays-Bas
(carbendazime seul) a montré que la quantité moyenne ingérée était
vraisemblablement de l'ordre d'un dixième de la dose journalière
acceptable (DJA) qui est, pour le bénomyl de 0,02 mg/kg de poids
corporel, et pour le carbendazime de 0,01 mg/kg de poids corporel.
L'exposition professionnelle au cours de la production est
inférieure à la valeur-seuil. Les ouvriers agricoles qui préparent
les mélanges, effectuent le remplissage, ou retournent dans les
champs traités par du bénomyl, courent un risque d'exposition
cutanée de quelques mg de bénomyl à l'heure. Le port de dispositifs
de protection permettrait de réduire encore cette exposition. En
outre, étant donné que l'absorption percutanée est vraisemblablement
faible, il est très peu probable que le bénomyl puisse avoir des
effets toxiques généraux sur les populations humaines en étant
absorbé par cette voie.
1.5 Cinétique et métabolisme
Après exposition par voie orale ou respiratoire, le bénomyl est
rapidement absorbé par l'organisme animal mais cette absorption est
bien moindre après exposition par voie cutanée. Une fois absorbé, le
bénomyl est rapidement métabolisé, puis excrété dans les urines et
les matières fécales. Après avoir administré du bénomyl marqué au
carbone-14 à des rats, on a retrouvé dans le sang, et en petites
quantités dans les testicules, les reins et le foie, deux de ses
métabolites, le carbendazime et le carbamate de méthyl-5-hydroxy-1H-
benzimidazole-2-yle (5-HBC). La distribution tissulaire des composés
n'était pas révélatrice d'une bioconcentration. Dans l'urine, le
principal métabolite était le 5-HBC à côté d'un peu de carbendazime.
Dans les 72 heures suivant l'administration, 98% de la quantité
administrée avaient été excrétés. Chez des vaches recevant pendant 5
jours des capsules contenant du bénomyl radiomarqué à raison de 50
mg/kg de nourriture, les concentrations équivalentes de bénomyl
retrouvées dans les divers organes étaient de 4 mg/kg dans le foie,
et de 0,25 mg/kg dans les reins; dans les autres tissus et les
graisses, les valeurs n'étaient pas significatives. Pendant la
période d'administration, 65% du composé radiomarqué ont été
excrétés dans les urines, 21% dans les matières fécales et 0,4% dans
le lait. Le principal métabolite présent dans le lait était le
5-HBC. Chez d'autres animaux, on a constaté un métabolisme et des
modalités d'élimination similaires.
Le bénomyl n'inhibe pas l'acétylcholinestérase in vitro. On a
montré qu'il induisait l'époxyhydrolase hépatique, la gamma-
glutamyle transpeptidase ainsi que la glutation-S-transférase chez
la souris et le rat in vivo.
1.6 Effets sur les mammifères de laboratoire et sur les systèmes
d'épreuve in vitro
1.6.1 Exposition unique
Le bénomyl présente une faible toxicité aiguë, la DL50 par
voie orale chez le rat étant > 10 000 mg/kg, et la CL50 à 4 h par
voie respiratoire étant > 4 mg/litre. Des chiens exposés par voie
respiratoire pendant 4 heures à la dose de 1,65 mg/litre et examinés
28 jours après l'exposition, présentaient une diminution du poids du
foie. Une dose unique administrée à des rats par gavage a entraîné
des effets sur la reproduction 70 jours après l'exposition (voir
Section 1.6.5).
1.6.2 Exposition de brève durée
Administré par gavage pendant de brèves périodes allant jusqu'à
90 jours, en mélange à la nourriture ou en application cutanée, le
bénomyl a légèrement augmenté le poids du foie chez le rat (à la
dose quotidienne de 125 mg/kg, en mélange à la nourriture) et a
produit des effets sur les organes reproducteurs mâles chez le rat:
diminution du poids des testicules et de l'épididyme, réduction de
la spermatogenèse (dose quotidienne: 45 mg/kg, par gavage, dose sans
effet observable = 15 mg/kg), chez le lapin (dose quotidienne: 1000
mg/kg, par voie orale; 500 mg/kg, en appli cation cutanée) et chez
le chien beagle (62,5 mg/kg, dose sans effet observable = 18,4 mg/kg
par jour, en mélange à la nourriture). Chez les rats exposés par la
voie respiratoire à du bénomyl à des concentrations allant jusqu'à
200 mg/m3 pendant 90 jours, on n'a pas observé d'effets sur le
foie ni les testicules.
1.6.3 Irritation et sensibilisation au niveau de la peau et
des yeux
L'application de bénomyl sur l'épiderme de lapins ou de cobayes
n'a produit que peu ou pas d'irritation et une sensibilisation
modérée de la peau. Chez le rat, l'instillation oculaire a produit
une irritation légère et temporaire de la conjonctive.
1.6.4 Exposition de longue durée
A l'issue d'une étude prolongée d'alimentation chez des rats,
on n'a pas pu mettre en évidence d'effets imputables à ce composé à
des doses allant jusqu'à 2500 mg/kg de nourriture (soit 125 mg/kg de
poids corporel par jour). Cette étude n'a pas été jugée suffi sante
pour permettre une évaluation des effets sur la reproduction. Chez
la souris CD-1, le poids du foie était en augmentation à partir de
la dose de 1500 mg/kg de nourriture. A la dose de 5000 mg/kg de
nourriture, on notait chez les souris mâles une réduction du poids
absolu des testicules et une atrophie du thymus.
1.6.5 Reproduction, embryotoxicité et tératogénicité
Le bénomyl détermine une diminution du poids des testicules et
de l'épidydime, avec réduction des réserves de spermatozoïdes au
niveau de la queue de l'épidydime, une diminution de la production
des spermatozoïdes et une réduction de la fécondité des mâles. A
plus fortes doses, il y a diminution de la spermatogénèse qui est
perturbée à tous ses stades. Le bénomyl n'affecte pas le
comportement copulatoire, les vésicules séminales, la mobilité des
spermatozoïdes ni les hormones sexuelles correspondantes. La
concentration de bénomyl la plus faible qui produise un effet
statistiquement significatif sur la spermatogénèse du rat mâle a été
chiffrée à 45 mg/kg par jour. La dose sans effet observable pour ces
effets est de 15 mg/kg par jour.
Une seule dose de bénomyl (100 mg/kg ou davantage) administrée
par gavage à des rats a produit, 70 jours après l'exposition, des
effets consistant notamment en une réduction du poids des testicules
et une atrophie des tubes séminifères.
Administré par gavage à des rats ChD-CD et Wistar du 7e au
16e jour de la gestation, le bénomyl s'est révélé tératogène pour
les 2 souches à 62,5 mg/kg, mais pas à 30 mg/kg pour les ChR-CD, ni
à 31,2 mg/kg pour les Wistar. Administré par gavage à des rats
Sprague-Dawley du 7e au 21e jour de gestation, le bénomyl s'est
révélé tératogène à 31,2 mg/kg. Les effets observés étaient une
microphthalmie, une hydrocéphalie et une encéphalocèle. Le
développement post-natal des rats était également perturbé aux doses
supérieures à 15,6 mg/kg.
Chez la souris, l'administration par gavage à des
concentrations supérieures ou égales à 50 mg/kg a provoqué
l'apparition de côtes surnuméraires et autres anomalies du squelette
et des viscères. On n'a pas établi, chez la souris, la valeur de la
dose sans effet observable car les doses administrées étaient toutes
supérieures à 50 mg/kg. A part une augmentation marginale de la
fréquence des côtes surnuméraires chez le lapin, on n'a pas observé,
chez cet animal, d'effets tératogènes à des doses atteignant 500
mg/kg de nourriture.
1.6.6 Mutagénicité et autres points d'aboutissement des effets
toxiques
L'étude des cellules somatiques et germinales montre que le
bénomyl n'entraîne pas de mutations géniques ni d'anomalies
structurales chromosomiques et qu'il n'y a pas non plus
d'interaction directe avec l'ADN (qui provoquerait des lésions de
l'ADN et leur réparation). Ces faits ont été mis en évidence à la
fois sur des cellules mammaliennes et non mammaliennes.
Cependant le bénomyl provoque des aberrations dans le nombre
des chromosomes (aneuploïdie et/ou polyploïdie) dans les systèmes
d'épreuve (tant in vitro qu' in vivo).
1.6.7 Cancérogénicité
Chez les souris CD-1 et Swiss axéniques, qui présentent un taux
important de tumeurs spontanées du foie, on a observé ce type de
tumeurs après administration de bénomyl ou de carbendazime. En
revanche, le carbendazime ne s'est pas révélé cancérogène chez les
souris NMRKf, qui n'ont qu'un faible taux de tumeurs hépatiques
spontanées.
La première étude de cancérogénicité portant sur des souris
CD-1 a fait ressortir l'existence d'une augmentation liée à la dose
et statistiquement significative des néoplasmes hépatocellulaires
chez les femelles et l'on a également observé chez les mâles, aux
doses moyennes (1500 mg/kg), une réaction statistiquement
significative qui ne s'observait plus aux doses élevées en raison
d'un fort taux de mortalité. Une deuxième étude de cancérogénicité
portant sur le carbendazime a été effectuée chez une souche
génétiquement apparentée de souris Swiss axéniques et exogames à des
doses de 0, 150, 300 et 1000 mg/kg (la dernière dose étant portée à
5000 mg/kg au cours de l'étude); elle a révélé un accroissement dans
l'incidence de l'ensemble des adénomes et carcinomes
hépatocellulaires. Une troisième étude effectuée cette fois sur des
souris NMRKf à des doses 0, 50, 150, 300 et 1000 mg/kg (portées
ensuite à 5000 mg/kg) n'a pas fait ressortir d'effets cancérogènes.
Les études de cancérogénicité portant sur le bénomyl et le
carbendazime ont donné des résultats négatifs chez le rat.
1.6.8 Mécanisme de la toxicité - mode d'action
On pense que les effets biologiques du bénomyl et du
carbendazime résultent de leur interaction avec les microtubules
cellulaires. Ces structures interviennent dans des fonctions aussi
importantes que la division cellulaire, qui est inhibée par ces deux
substances. La toxicité du bénomyl et du carbendazime pour les
mammifères est donc liée à une perturbation des fonctions du système
microtubulaire.
Comme les autres dérivés du benzimidazole, le bénomyl et le
carbendazime sont plus ou moins toxiques selon les espèces. Cette
sélectivité toxicologique s'explique au moins en partie par le fait
que le bénomyl et le carbendazime ne se lient pas de la même manière
aux tubulines des espèces visées et des espèces non visées.
1.7 Effets sur l'homme
Le bénomyl provoque des dermatites de contact ainsi qu'une
sensibilisation cutanée. On n'a pas fait état d'autres effets.
1.8 Effets sur les autres êtres vivant au laboratoire et dans
leur milieu naturel
Le bénomyl n'a guère d'effet sur l'activité microbienne du sol
aux doses d'emploi recommandées. On a cependant signalé l'existence
d'effets nocifs vis-à-vis de certains groupes de champignons.
On a calculé que la CE50 à 72 heures, fondée la croissance
totale, pour les algues bleu-vert du genre Selenastrum
capricornutum, était égale à 2,0 mg/litre; la concentration sans
effet observable était de 0,5 mg/litre. La toxicité du bénomyl pour
les invertébrés aquatiques et les poissons varie dans de larges
proportions, les valeurs de la CL50 à 96 heures allant de 0,006
mg/litre pour des poissons-chats du genre Ictalurus (alevins
porteurs de leur sac vitellin) à plus de 100 mg/litre pour les
écrevisses.
Le bénomyl s'est révélé toxique pour les lombrics lors
d'expériences de laboratoire qui reproduisaient les conditions
réelles d'exposition résultant de l'utilisation recommandée sur le
terrain. Il est peu toxique pour les oiseaux et son produit de
dégradation, le carbendazime, est "relativement non toxique" pour
les abeilles.
2. Conclusions
Le bénomyl provoque une sensibilisation cutanée chez l'homme.
Le bénomyl et le carbendazime ne font courir à l'homme qu'un très
faible risque d'intoxication aiguë. Etant donné les conditions
actuelles d'exposition et le faible taux d'absorption percutanée de
ces deux composés, il est improbable qu'ils entraînent des effets
toxiques généraux dans la population ou chez les personnes exposées
de par leur profession. Ces conclusions sont tirées de données
relatives à l'animal et, dans une moindre mesure, à l'homme; elles
reposent sur la connaissance du mode d'action du carbendazime et du
bénomyl, tant chez les espèces visées que chez les espèces non
visées.
Grâce à une meilleure connaissance du mécanisme de la toxicité
du bénomyl et du carbendazime pour les mammifères, on pourra
peut-être mieux définir les doses sans effet observable. Des études
portant sur la liaison de ces composés aux tubulines des cellules
cibles (tissus testiculaires et embryonnaires) faciliteront sans
doute les comparaisons interspécifiques.
Le carbendazime est fortement adsorbé aux matières organiques
du sol et il y persiste pendant des périodes pouvant atteindre 3
ans. Il persiste également à la surface des feuilles et se retrouve
par conséquent dans les feuilles mortes. On a montré que les
lombrics pouvaient souffrir (dans leur population et dans leur
reproduction) de ces composés aux doses d'emploi recommandées. On ne
possède aucun renseignement sur les autres arthropodes qui vivent
dans le sol ou les débris organiques et qui pourraient être exposés
de la même manière.
Il est improbable que la forte toxicité vis-à-vis des
organismes aquatiques révélée par les épreuves de laboratoire
s'observe également dans le milieu naturel, du fait de la faible
biodisponi-bilité des résidus de carbendazime liés aux sédiments.
Toutefois on ne possède aucune donnée sur les espèces vivant sur les
sédiments et qui seraient donc les plus exposées.
RESUMEN Y CONCLUSIONES
1. Resumen
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
El benomilo es un sólido cristalino de color tostado y acción
fungicida sistémica que pertenece a la familia del bencimidazol. Se
descompone a una temperatura apenas superior a 140 °C,
correspondiente a su punto de fusión, y su presión de vapor a 25 °C
es < 5 x 10-6 Pa (< 3,7 x 10-8 mmHg). Prácticamente es
insoluble en agua a pH 5 y 25 °C, siendo su solubilidad 3,6
mg/litro. Es un compuesto estable en condiciones de almacenamiento
normales, pero en agua se descompone y forma carbendazima.
Los análisis de las muestras procedentes de residuos y del
medio ambiente se realizan mediante extracción con un disolvente
orgánico, purificación del extracto obtenido utilizando un
procedimiento de reparto líquido-líquido y conversión del residuo en
carbendazima. La valoración de los residuos se puede realizar
mediante cromatografía líquida de alto rendimiento o inmunoensayo.
1.2 Fuentes de exposición humana y ambiental
En 1988, el uso mundial estimado de benomilo fue unas 1700
toneladas. Es un fungicida muy utilizado, que se encuentra
registrado en 50 países en los que se permite su uso en más de 70
cultivos. El benomilo está formulado como polvo humectable.
1.3 Transporte, distribución y transformación en el medio ambiente
En el medio ambiente, el benomilo se transforma rápidamente en
carbendazima con una semivida de 2 y 19 h en el agua y el suelo
respectivamente. Por consiguiente, para la evaluación de los efectos
sobre el medio ambiente son importantes los datos obtenidos de los
estudios realizados con ambos compuestos.
La carbendazima se descompone en el medio ambiente con una
semivida de 6 a 12 meses en el suelo desnudo, de 3 a 6 meses en el
césped y de 2 y 25 meses en el agua en condiciones aerobias y
anaerobias respectivamente.
La carbendazima se descompone principalmente por acción de los
microorganismos. Un producto importante de su degradación es el
2-aminobencimidazol (2-AB), que luego se descompone de nuevo por la
actividad microbiana.
En la descomposición del benomilo marcado con un grupo fenilo
con 14C, sólo el 9% del 14C formó CO2 durante un año de
incubación. El resto del 14C se recuperó sobre todo como
carbendazima y en productos unidos a residuos. El destino de un
posible producto de degradación (1,2-diaminobenceno) puede aclarar
ulteriormente la vía de degradación de los fungicidas
bencimidazólicos en el medio ambiente.
En estudios de campo y de columna se ha puesto de manifiesto
que la carbendazima queda retenida en la capa superficial del suelo.
Aunque no se dispone de datos sobre su adsorción en el suelo, se
considera que ésta puede ser tan intensa como la del benomilo, con
valores de Koc que oscilan entre 1000 y 3600. Los valores del log
Kow para el benomilo y la carbendazima son respectivamente 1,36 y
1,49.
En la evaluación realizada en un modelo de selección, basado en
datos de adsorción y persistencia, se puso de manifiesto que no
había riesgo de lixiviación. En los Estados Unidos se han efectuado
análisis de agua de pozos que confirman esto, puesto que no se
encontraron trazas de benomilo en ninguno de los 495 pozos
muestreados ni se detectó carbendazima en ninguno de los 212 (no se
dispone del límite de detección). Es de suponer que la escorrentía
superficial de ambos compuestos se deba solamente al fungicida
adsorbido en las partículas del suelo y que en el medio acuoso estén
fuertemente adsorbidos en los sedimentos.
En solución, en las plantas y en el suelo, el benomilo se
degrada a carbendazima (1H-bencimidazol-2-carbamato de metilo) y a
2-AB, STB (3-butil-1,3,5-triazino[1,2a]-bencimidazol-2,4(1H,3H)
diona) y BBU (1-(2-bencimidazolil)-3-n-butilurea). La fotolisis del
benomilo es escasa o nula.
En los animales, el benomilo se descompone formando
carbendazima y otros metabolitos polares, que se excretan
rápidamente. No se ha observado que el benomilo o la carbendazima se
acumulen en ningún sistema biológico.
1.4 Niveles medioambientales y exposición humana
No parece que se disponga de datos de vigilancia ambiental para
el benomilo. Sin embargo, los estudios realizados sobre su destino
en el medio ambiente pueden resumirse como sigue.
Puesto que el benomilo y la carbendazima se mantienen estables
en las plantas durante varias semanas, pueden pasar a los organismos
que se alimentan de las hojas caídas. El suelo y los sedimentos
pueden conservar residuos de carbendazima hasta tres años. Sin
embargo, la fuerte adsorción de este compuesto en las partículas del
suelo y en los sedimentos reduce la exposición de los organismos
terrestres y acuáticos.
La principal fuente de exposición para la población humana
general se debe a los residuos de benomilo y carbendazima en los
cultivos alimentarios. En análisis de la exposición a través de los
sedimentos realizados en los Estados Unidos (benomilo y carbendazima
combinados) y en los Países Bajos (con carbendazima) se obtuvo una
ingesta media prevista de alrededor del 10 por ciento de la ingesta
diaria admisible (IDA) recomendada, de 0,02 mg/kg de peso corporal
para el benomilo y de 0,01 mg/kg de peso corporal para la
carbendazima.
La exposición profesional durante el proceso de fabricación es
inferior al valor umbral límite. Se considera que los trabajadores
agrícolas que se ocupan de mezclar y cargar los plaguicidas o que
entran en campos tratados con benomilo sufren exposición cutánea a
unos mg de benomilo por hora. Este forma de exposición se podría
reducir con algún tipo de protección. Por otra parte, puesto que la
absorción cutánea prevista es baja, la probabilidad de que el
benomilo tenga efectos tóxicos sistémicos sobre la población humana
a través de esta vía es muy escasa.
1.5 Cinética y metabolismo
En experimentos con animales se ha puesto de manifiesto que
éstos absorben fácilmente el benomilo tras la exposición oral y
respiratoria, pero mucho menos después de una exposición cutánea. El
benomilo absorbido se metaboliza rápidamente y se excreta en la
orina y las heces. En ratas alimentadas con benomilo marcado con
14C, se encontraron en la sangre, y en pequeña cantidad en los
testículos, los riñones y el hígado, los metabolitos carbendazima y
(5-hidroxi-1H-bencimidazol-2-il)-carbamato de metilo (5-HBC). La
distribución en los tejidos demostraba la ausencia de
bioconcentración. El metabolito principal en la orina era el 5-HBC,
acompañado de una pequeña cantidad de carbendazima. A las 72 h de la
administración ya se había excretado el 98% de la cantidad
suministrada. En vacas tratadas durante 5 días con cápsulas con
benomilo marcado, en dosis equivalentes a una alimentación de 50
mg/kg, se detectó en el hígado una concentración de esta sustancia
equivalente a 4 mg/kg, en los riñones 0,25 mg/kg y en el tejido
adiposo o en otros niveles no significativos. Cuando se administró
con los alimentos, el 65% del compuesto marcado se excretó en la
orina, el 21% en las heces y el 0,4% en la leche. El principal
metabolito en la leche fue el 5-HBC. El metabolismo y los sistemas
de eliminación fueron semejante en otros animales.
El benomilo no inhibe la acetilcolinesterasa in vitro. Se ha
demostrado en estudios in vivo en ratas y ratones que induce la
epoxihidrolasa hepática, la gamma-glutamil transpeptidasa y la
glutatión-S-transferasa.
1.6 Efectos en los mamíferos de laboratorio y en sistemas
de prueba in vitro
1.6.1 Exposición única
El benomilo tiene una toxicidad aguda baja, con una DL50 por
vía oral en ratas de > 10 000 mg/kg y una CL50 por inhalación
durante 4 h de > 4 mg/litro. La carbendazima, al igual que la
sustancia de la que se deriva, tiene una DL50 en ratas de > 10
000 mg/kg. Los perros, expuestos por inhalación a 1,65 mg/litro
durante 4 h y examinados 28 días después, mostraron una dismi nución
del peso del hígado. La administración por sonda de una sola dosis
de benomilo a ratas mostró efectos en la reproducción a los 70 días
de la exposición (véase el apartado 1.6.5).
1.6.2 Exposición breve
La administración de benomilo mediante sonda, en los alimentos
o por exposición cutánea durante un período máximo de 90 días en las
ratas aumentó ligeramente el peso del hígado (125 mg/kg al día, con
los alimentos) y tuvo efectos sobre los órganos reproductores
masculinos (disminución del peso de los testículos y los epidídimos,
y reducción de la espermatogénesis) en las ratas (45 mg/kg al día,
administrado por sonda; nivel sin efecto observado (NOEL) =
15mg/kg), los conejos (1000 mg/kg al día, por vía oral; 500 mg/kg de
peso corporal al día, por vía cutánea) y los perros (62,5 mg/kg;
NOEL = 18,4 mg/kg al día, con los alimentos). No se observaron
efectos hepáticos ni testiculares en la ratas expuestas por
inhalación a concentraciones de benomilo de hasta 200 mg/m3
durante 90 días.
1.6.3 Irritación y sensibilización cutánea y ocular
La aplicación cutánea a conejos y cobayos ocasionó una
irritación leve o nula y una sensibilización moderada de la piel. Su
aplicación ocular en ratas produjo de manera transitoria una
irritación conjuntival ligera.
1.6.4 Exposición prolongada
En un estudio de alimentación de larga duración en ratas, con
dosis de hasta 2500 mg/kg de alimentos (125 mg/kg de peso corporal
al día) no se puso de manifiesto ningún efecto relacionado con el
compuesto. Este estudio no se consideró adecuado para evaluar los
efectos sobre la reproducción. En el ratón CD-1 se observó que, con
dosis de 1500 mg/kg de alimento o superiores, se producía un aumento
de peso del hígado. En los ratones macho, las dosis de hasta 5000
mg/kg de alimento provocaron una disminución en términos absolutos
del peso de los testículos y la atrofia del timo.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
El benomilo produce una disminución del peso de los testículos
y el epidídimo, de la producción de esperma y de la tasa de
fecundidad de los machos. A dosis más elevadas, provoca
hipoespermatogénesis, con interrupción general de todas las fases de
la espermatogénesis. No afecta, en cambio, al comportamiento
copulatorio, las vesículas seminales, la movilidad del esperma o las
hormonas de la reproducción asociadas. La concentración más baja del
benomilo capaz de inducir un efecto espermatogénico estadísticamente
significativo en ratas macho fue de 45 mg/kg por día. El NOEL para
estos efectos fue de 15 mg/kg por día.
La administración por sonda de una sola dosis de benomilo (100
mg/kg o más) mostró efectos en ratas a los 70 días de la exposición,
que incluyeron descenso del peso de los testículos y atrofia de los
túbulos seminíferos. Administrado por sonda a ratas ChD-CD y Wistar
durante los días 7 a 16 de la gestación, el benomilo resultó
teratogénico a 62,5 mg/kg en ambas estirpes, pero no a 30 mg/kg en
ratas ChD-CD y no a 31,2 mg en ratas Wistar. Administrado por sonda
a ratas Sprague-Dawley en los días 7 a 21 de la gestación, el
benomilo resultó teratogénico en dosis de 31,2 mg/kg. Los efectos
que produjo fueron microftal mia, hidrocefalia y encafaloceles. Las
dosis superiores a 15,6 mg/kg tuvieron un efecto negativo sobre el
desarrollo posnatal.
La administración por sonda de 50 mg/kg o concentraciones
superiores indujo en ratones la aparición de costillas
supernumerarias y de otras anomalías esqueléticas y viscerales. No
se ha determinado el NOEL en los ratones porque no se ensayaron
dosis inferiores a 50 mg/kg. A excepción de un aumento marginal de
las costillas supernumerarias en conejos, no se observaron efectos
teratogénicos incluso a dosis de hasta 500 mg/kg de alimento.
1.6.6 Mutagenicidad y otros efectos finales afines
En unos estudios realizados en células somáticas y germinales
se ha observado que no provoca mutaciones genéticas ni daños en la
estructura de los cromosomas (aberraciones) y tampoco tiene un
efecto directo sobre el ADN (causante de daños y la reparación del
ADN). Esto se ha demostrado tanto en mamíferos como en otros
animales.
El benomilo, sin embargo, produce aberraciones cromosómicas
numéricas (aneuploidía o poliploidía) en sistemas experimentales in
vitro e in vivo.
1.6.7 Carcinogenicidad
En el primer estudio de carcinogenicidad con ratones CD-1 se
puso de manifiesto un aumento estadísticamente significativo de
neoplasia hepatocelular relacionado con la dosis en las hembras y
también en los machos tratados con una dosis de nivel medio (1500
mg/kg) se observó una respuesta estadísticamente significativa, pero
no en los que recibieron dosis elevadas, a causa del elevado índice
de mortalidad. En un segundo estudio sobre la carcinogenicidad de la
carbendazima en una raza de ratones genéticamente relacionada con la
anterior, los ratones SPF (raza aleatoria suiza), con dosis de 0,
150, 300 y 1000 mg/kg (que se aumentó a 5000 mg/kg durante el
estudio) se puso de manifiesto un aumento en el número de casos de
adenomas y carcinomas hepatocelulares combinados. En un tercer
estudio realizado en ratones NMRKf con dosis de 0, 50, 150, 300 y
1000 mg/kg (que se aumentó a 5000 mg/kg durante el estudio) no se
produjeron efectos carcinogénicos. El benomilo y la carbendazima
causaron tumores hepáticos en dos estirpes de ratones (CD-1 y suizos
(SPF)) que presentaban una tasa alta de tumores hepáticos de
formación espontánea. Por el contrario, la carbendazima no resultó
carcinogénica en ratones NMRKf, que presentan una tasa baja de esos
tumores espontáneos.
Los estudios de carcinogenidad del benomilo y la carbendazima
en ratas fueron negativos.
1.6.8 Mecanismo de toxicidad, modo de acción
Se considera que los efectos biológicos de estos compuestos son
el resultado de su interacción con los microtúbulos celulares. Estas
estructuras participan en funciones esenciales, como la división
celular, que inhiben el benomilo y la carbendazima. La toxicidad de
estos productos en los mamíferos está vinculada a una disfunción
microtubular.
El benomilo y la carbendazima, al igual que otros compuestos
del bencimidazol, tienen una toxicidad selectiva para distintas
especies. Esta se explica, por lo menos en parte, porque el benomilo
y la carbendazima se unen de manera distinta a los microtúbulos de
las especies específicas en las que actúan y en las que no.
1.7 Efectos en el ser humano
El benomilo causa dermatitis por contacto y sensibilización
cutánea. No se ha informado de otros efectos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Con las dosis de aplicación recomendadas, el benomilo tiene
pocos efectos sobre la actividad microbiana del suelo. Se han
notificado algunos efectos adversos sobre ciertos grupos de hongos.
La CE50 a las 72 h, basada en el crecimiento total, para el
alga verde Selenastrum capricornutum fue de 2,0 mg/litro; la
concen tración sin efecto observado (NOEC) fue de 0,5 mg/litro. La
toxicidad del benomilo para los invertebrados acuáticos y los peces
varía ampliamente, con una CL50 a las 96 h que oscila entre 0,006
mg/litro para Ictalurus punctatus (alevines con saco vitelino) y
> 100 mg/litro para los cangrejos de río.
No observaron efectos tóxicos en experimentos de laboratorio en
las lombrices de tierra expuestas a concentraciones normales de
benomilo y como resultado del uso de la dosis de aplicación
recomendada en el campo. Tiene una toxicidad baja para las aves, y
la carbendazima, producto de su degradación, es "relativamente no
tóxica" para las abejas de miel.
2. Conclusiones
El benomilo causa sensibilización cutánea en el ser humano.
Tanto el benomilo como la carbendazima representan un riesgo muy
pequeño de intoxicación aguda. Dados los niveles de exposición
actuales y el bajo índice de absorción cutánea de estos dos
compuestos, no es probable que pudieran tener efectos de toxicidad
sistémica en la población general o en personas expuestas
profesionalmente. Estas son las conclusiones que se pueden sacar de
los datos obtenidos en animales y de los limitados datos sobre el
ser humano de que se dispone, pero estas extrapolaciones están
respaldadas por el conocimiento del modo de acción de la
carbendazima y el benomilo en especies en las que actúan y en las
que no.
Una mayor clarificación del mecanismo de toxicidad de ambos
compuestos en los mamíferos permitirá quizás definir mejor los
niveles sin efectos observados. El estudio de su unión a los
microtúbulos de las células destinatarias (tejidos testicular y
embrionario) facilitará la comparación entre distintas especies.
La carbendazima se adsorbe fuertemente en la materia orgánica
del suelo que la conserva durante un período de hasta 3 años.
Persiste en la superficie de las hojas y, por consiguiente, en las
hojas caídas. Se ha demostrado que las dosis recomendadas de
aplicación afectan negativamente a las lombrices de tierra (con
efectos sobre la población y la reproducción). No se dispone de
datos acerca de sus efectos sobre otros artrópodos del suelo o de la
maleza, que estarían igualmente expuestos.
No es probable que se pueda observar en el medio ambiente la
elevada toxicidad demostrada en las pruebas de laboratorio para los
organismos acuáticos debido a la baja biodisponibilidad de los
residuos de carbendazima unidos a los sedimentos. Sin embargo, no se
dispone de información acerca de sus efectos en las especies que
viven en los sedimentos, que sufrirían la exposición más intensa.
6.4 Elimination and excretion
Absorbed benomyl and carbendazim are rapidly excreted in the
urine and faeces.
In a study where rats were administered 1 or 10 µg formulated
14C-benomyl (50% wettable powder) in a single intravenous dose by
tail injection, more than 80% of the dose was excreted in the urine
and faeces within 6 h after injection and the total urine and faeces
recovery was > 95% in 24 h (Han, 1979).
[Phenyl(U)-14C]-carbendazim was administered by gavage to
Sprague-Dawley rats using three dosing regimens: a single oral dose
of 50 mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with unlabelled carbendazim of 50 mg/kg for
14 days (pre-conditioned low dose); and a single oral dose of 1000
mg/kg (high dose). Each dosing group consisted of five animals of
each sex. A preliminary study conducted with two rats of each sex,
each rat having received a single oral dose of 50 mg/kg,
demonstrated that 95% of the radioactivity excreted in the urine and
faeces was recovered within 72 h after dosing and that < 1% of the
dose was expired as volatile metabolites. In the full study, > 98%
of the recovered radioactivity was excreted by the time of sacrifice
(i.e. 72 h after dosing) for each dosing group. Urinary excretion
accounted for 62% to 66% of the dose in males and 54% to 62% of the
dose in low-dose and pre-conditioned low-dose female groups. In the
high-dose group, this pathway accounted for 41% of the dose in all
animals. Elimination of radiolabel in faeces accounted for virtually
all of the remaining radiolabel. There were no apparent differences
between male and female rats with respect to the extent of
absorption and extent and rate of elimination of 14C-carbendazim
equivalents within a given treatment group (Monson, 1990).
In a study by Han (1978), two male ChR-CD-1 mice were fed a
diet of non-radiolabelled benomyl (2500 mg/kg) for 21 days and were
then gavaged with 2.5 mg [2-14C]-benomyl in corn oil. An identical
experiment was performed with one male ChR-CD hamster. More than 90%
of the radioactivity was eliminated in the urine and faeces within
72 h (Han, 1978).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approximately
17 h after the tenth dose, the cow was sacrificed and organs,
tissues and blood were collected for analysis. At sacrifice, 65% of
the radiolabel had been excreted in the urine, 21% in the faeces and
0.4% in the milk. Carbon-14 residue levels in the milk averaged 0.2
mg/litre (calculated as benomyl equivalents) with 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids (Monson, 1985).
Lactating and non-lactating goats were given 5 consecutive
daily doses of [2-14C]-benomyl by capsule at rates equivalent to
36 and 88 mg/kg, respectively, in the total daily diet. Most of the
radioactivity (96%) had been eliminated in the urine and faeces by
the time of sacrifice (Han, 1980).
The excretion of benomyl was studied in laying hens dosed daily
for three consecutive days with 3.5 mg [2-14C]-benomyl or 3.29 mg
[phenyl(U)-14C]-benomyl. At sacrifice (22 h after the last dose),
an average of 107% and 95% of the dose had been excreted for the
[2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-dosed birds,
respectively (Monson, 1986a).
In a similar study on 14C-carbendazim, groups of laying hens
were fed at a rate equivalent to 5 and 120 mg/kg diet. At sacrifice,
24 h after the sixth daily dose, an average of 95% of the dose had
been excreted by the low-dose birds and 92% by the high-dose birds
(Monson, 1986b).
6.5 Reaction with body components
An in vitro study using acetyl cholinesterase from bovine
erythrocytes showed that benomyl did not inhibit this enzyme. The
acetyl cholinesterase inhibition constant (KI) for benomyl was
greater than 1 x 10-3 mol/litre (Belasco, 1970). Another in vitro
study by Krupka (1974) verified that benomyl did not inhibit either
acetyl cholinesterase or butyryl cholinesterase.
In a study by Guengerich (1981), the effects of benomyl and
carbendazim on hepatic enzymes were studied in male and female
Crl-CD rats and CD-1 mice. The treatment groups included animals fed
for 28 days with diets that contained benomyl or carbendazim at
concentrations of 0, 10, 30, 100, 300, 1000 or 3000 mg/kg. In these
studies, microsomal epoxide hydrolase and cytosolic glutathione- S-
transferase were monitored in subcellular fractions isolated from
the livers of animals in each treatment group. Liver weights were
also recorded. Elevated mean absolute liver weights were observed at
1000 and 3000 mg carbendazim/kg in both male and female rats and at
300 mg carbendazim/kg in female rats. However, the only
significantly elevated liver weight was found in females after a
dose of 3000 mg benomyl/kg. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced
epoxide hydrolase in both sexes of rats and mice at 1000 and 3000
mg/kg. Induction of glutathione- S-transferase was observed at 3000
mg/kg in the case of both benomyl and carbendazim. In general, the
level of induction seemed to be slightly greater in females than
males. There did not appear to be any substantial difference in
enzyme induction between rats and mice.
In a study by Shukla et al. (1989), levels of gamma-glutamyl
transpeptidase (GGT) were evaluated after benomyl exposure. Female
albino rats (eight per group) and female Swiss albino mice (eight
per group) were given 1000 and 4000 mg benomyl/kg feed for 15 days,
and blood and liver GGT levels were analysed. Benomyl exposure
increased the activity of both blood and liver GGT in both rats and
mice, and the degree of induction was dose related (Shukla et al.,
1989).
7. EFFECTS ON LABORATORY MAMMALS; IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of benomyl in several animal species is
summarized in Table 8. Benomyl has an oral LD50 in the rat of >
10 000 mg/kg and an inhalation 4-h LC50 > 4 mg/litre. Several
other minor metabolites were evaluated and the approximate lethal
doses were 3400 mg/kg for 2-AB, 7500 mg/kg for 5-HBC, 17 000 mg/kg
for BUB and 17 000 mg/kg for STB.
In a study by Littlefield & Busey (1969), three groups of male
dogs (around 10 dogs/group) were exposed to benomyl at air
concentrations of 0, 0.65 and 1.65 mg/litre. One half of the dogs in
each group were killed on day 14 and the remainder on day 28. The
liver weight of the high-dose dogs was significantly decreased on
day 28. For further discussion of single dose toxic effects, see
section 7.5.1.
7.2 Short-term exposure
7.2.1 Gavage
In a 14-day rat study, benomyl (200 and 3400 mg/kg in peanut
oil) was given by gavage five times a week for two weeks to six male
ChR-CD rats per group. Four out of six rats died after 5, 7, 8 and 9
doses, respectively, of 3400 mg/kg. No clinical signs of toxicity
were observed in the group treated with 200 mg/kg per day.
Degeneration of germinal epithelium, multinucleated giant cells and
reduction or absence of sperm were observed in the testes after
multiple doses of 3400 mg/kg per day. Less than 10% of the
testicular tubules were affected in only two out of six animals
dosed with 200 mg/kg. At the high dose level, there was erosion and
thickening of the squamous mucosa of the stomach with submucosal
inflammation and a decrease in the large globular-shaped vacuoles
located centrolobularly in the liver (Sherman & Krauss, 1966).
7.2.2 Feeding
7.2.2.1 Rat
In a 90-day study by Sherman et al. (1967), groups of rats
(4-week-old ChR-CD rats, 16 rats of each sex per group) were fed
Benlate 70 WP (72% benomyl) in the diet at levels of 0, 100, 500 and
2500 mg benomyl/kg. The animals were observed daily for behavioural
changes and body weights, and food consumption was recorded at
weekly intervals. Haematological examinations were conducted on six
male and six female rats in each group at 30, 60 and 90 days.
Routine urine and plasma alkaline phosphatase and glutamic pyruvic
transaminase activity analyses were performed on the same animals.
After 96-103 days of continuous feeding, 10 male and 10 female rats
in each group were killed, and selected organs were weighed and
examined microscopically. The remaining six male and female animals
in each group after the terminal sacrifice were used in a
one-generation reproductive study. No effect was observed with
respect to reproduction or lactation in the delivery or rearing of
the F1A litters. There were no compound-related effects on weight
gain, food consumption, food efficiency, clinical signs, or on
haematology, biochemistry or urinalysis determinations. The
liver-to-body weight ratio in females was slightly increased at 2500
mg/kg, compared with control rats. Gross and microscopic
examinations of tissues and organs showed no significant effects
attributable to the presence of benomyl in the diet at levels up to
and including 2500 mg/kg.
7.2.2.2 Dog
Groups of beagle dogs (four males and four females per group;
7-9 months old) were administered benomyl 50% wettable powder in the
diet at dosage levels of 0, 100, 500 and 2500 mg/kg diet (based on
active ingredient) for three months (this corresponded to treatment
levels of 0, 3.8, 18.4 and 84 mg/kg body weight). Food consumption
and body weight data were recorded weekly, and clinical laboratory
examinations (including haematology, biochemistry and urinalysis)
were performed pre-test and after 1, 2 and 3 months of feeding. At
the conclusion of the study, selected organs were weighed and
subjected to gross and microscopic examinations. No mortality or
adverse clinical effects were observed over the course of the study,
and growth and food consumption were not effected by the treatment.
Urine parameters showed no differences from the control, and there
were no dose-related effects on the haematological values. Alkaline
phosphatase and glutamic pyruvic transaminase activities were
increased in high-dose males and females. There were statistically
significant decreases in the albumin/globulin ratio in either males
or females fed 2500 mg/kg diet. Organ-to-body weight ratio changes
were observed in the high-dose males and females for the thymus
(decreased) and thyroid (increased). One of the four females fed
2500 mg/kg diet had an enlarged spleen at the end of the exposure
period, as well as a decreased erythrocyte count, haemoglobin
concentration and haematocrit value. Histopathological examination
revealed myeloid hyperplasia of the spleen and bone marrow and
erythroid hyperplasia of bone marrow. This did not appear to be
compound related since group mean values were not significantly
different. Three out of four males fed 2500 mg/kg diet had reduced
relative prostate weights when compared with controls. Microscopic
examinations of tissues and organs did not indicate changes in dogs
fed benomyl for 90 days. The no-observed-effect level (NOEL) was 500
mg/kg diet (Sherman, 1968).
Table 8. Acute toxicity of benomyl and its metabolites for laboratory mammals
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl ratb M/F 10/dose oral peanut oil LD50 > 10 000 Sherman (1969a)
rabbitc M 1/dose oral 50% wettable powder ALD > 3400 Fritz (1969)
dogd M 1 oral evaporated milk and ALD > 1000 Sherman (1969b)
water (1:1)
Benlate OD (50% rat M 10/dose oral corn oil LD50 > 12 000 Hostetler (1977)
benomyl)
Fungicide 1991 rabbit M/F 4/dose dermal (4 h) 50% wettable powder LD50 > 10 000 Busey (1968a)
(50% benomyl)
rat M 6/dose inhalation (4 h) 50% wettable powder LC50 > 4.01 mg/litre Busey (1968b)
(analytical)
dog M 10/dose inhalation 50% wettable powder LC50 > 1.65 mg/litre Littlefield & Busey
(analytical) (1969)
Benlate fungicide rat M/F 10/dose oral aqueous suspension LD50 > 10 000 Sherman (1969a)
(52-53% benomyl)
rat M 5/dose inhalation 50% wettable powder LC50 > 0.82 mg/litre Hornberger (1969)
Benlate PNW (50% rabbit M/F 10/dose dermal 50% wettable powder LD50 > 2000 Gargus & Zoetis
benomyl) (1983c)
Benlate 50 DF (50% rat M/F 5/dose oral aqueous suspension LD50 > 5000 Sarver (1987)
benomyl)
rabbit M/F 5/dose dermal 50% dry flowable LD50 > 2000 Brock (1987)
Table 8 (contd).
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl metabolites
2-Benzimidazole carbamic rat M/F 10/dose oral corn oil LD50 > 10 000 Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole- rat M 1/dose oral corn oil ALD > 7500 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole rat M 1/dose oral peanut oil ALD > 3400 Fritz & Sherman
(1969)
Benzimidazole 2- rat M 1/dose oral corn oil ALD > 17 000 Dashiell (1972)
(3-butylureido)
S-Triazine, 3-butyl- rat M 1/dose oral corn oil ALD > 17 000 Barbo & Carroll
benzimidazole (1,2a), (1972)
-2,4(1H,3H)-dione
a Based on active ingredient; ALD = approximate lethal dose
b ChR-CD or Crl:CD rats
c New Zealand white rabbits
d Beagle dogs
7.2.3 Dermal
In a study on groups of five male and five female New Zealand
albino rabbits, weighing 2 to 2.4 kg, 15 dermal applications of a
50% benomyl formulation (equivalent to 1000 mg/kg) were made on both
abraded and intact abdominal skin sites. The animals were exposed
for 6 h/day, 5 days/week for 3 weeks. After each daily application,
the abdomen was washed with tap water. Observations were made daily
for mortality and toxic effects and weekly for body weight changes.
Gross necropsy and microscopic examinations were performed. Slight
erythema, oedema and atonia were observed at both abraded and intact
skin sites. Slight to moderate desquamation occurred throughout the
exposure period. No apparent compound-related body weight or organ
weight changes were reported. Microscopic examination of the males
demonstrated that benomyl produced degeneration of the spermatogenic
elements of the seminiferous tubules of the testes, the changes
including vacuolated and multi-nucleated spermatocytes (Busey,
1968d).
In a separate repeated-dose dermal study, groups of five male
and five female New Zealand albino rabbits, weighing 3 kg, were
exposed to doses of benomyl equivalent to 0, 50, 250, 500, 1000 and
5000 mg/kg applied to non-occluded abraded dorsal skin sites 6 h a
day, five days a week, for three weeks. Test material was removed by
washing the skin site and drying with a towel. There were decreased
body weight gains for both males and females at the two highest dose
levels. Mild to moderate skin irritation was reported for all groups
but was most notable at the highest dose level. Diarrhoea, oliguria
and haematuria were observed in males and females at 1000 and 5000
mg/kg. Decreased average testicular weights and testes-to-body
weight ratios were observed at 1000 mg/kg only. There were no
histopathological changes reported (Hood, 1969).
7.2.4 Inhalation
In an inhalation study, groups of 20 male and 20 female CD rats
were exposed, nose-only, 6 h a day for 90 days, to 0, 10, 50 and 200
mg benomyl/m3. At 45 and 90 days, blood and urine samples were
collected from 10 rats of each sex per group for clinical analysis
and then killed for pathological examination. After approximately 45
days of exposure, test-compound-related degeneration of the
olfactory epithelium was observed in all males and in eight of the
ten females exposed to 200 mg/m3. Two male rats exposed to 50
mg/m3 had similar but less severe olfactory degeneration. After
approximately 90 days of exposure, all of the animals showed
olfactory degeneration at 200 mg/m3, along with three males
exposed to 50 mg/m3. No other compound-related pathological
effects were observed. Male rats exposed to 200 mg/m3 had
depressed mean body weights compared to controls and this correlated
with a reduction in food consumption (Warheit et al., 1989).
7.3 Skin and eye irritation; sensitization
7.3.1 Dermal
A 50% wettable powder applied to the clipped intact and abraded
abdomen of albino rabbits produced moderate to marked erythema,
slight oedema and slight desquamation. Exposure was for 24 h to
occluded skin sites at doses > 0.5 g/animal. Albino guinea-pigs
similarly exposed to 10, 25 and 40% dilutions of technical grade
benomyl in dimethyl phthalate presented only mild irritation of both
intact and abraded skin sites (Majut, 1966; Busey, 1968a; Colburn,
1969; Frank, 1969).
When "Benlate" 50 DF (50% benomyl, 0.5 g Benlate 50 DF) was
evaluated for primary dermal irritation potential in six male New
Zealand white rabbits, no dermal irritation was observed at 4 or 24
h after application. By 48 h, slight to mild erythema was observed
in two rabbits and was still evident at 72 h. The primary irritation
scores ranged from 0-1 (not an irritant) (Vick & Brock, 1987).
In a study by Desi (1979), benomyl (98% purity) was applied to
a shaved area of the back of four albino rabbits at 5 mg/cm2.
Draize scores (Draize et al., 1944) were assessed 4 and 72 h after
application. Lesions produced by this method were classified as
"mild irritation".
7.3.2 Eye
The eye irritation properties of benomyl were examined in
albino rabbits in several tests using technical grade benomyl, 50%
wettable powder and a suspension in mineral oil. Mild conjunctival
irritation and minor transitory corneal opacity were reported after
48 to 96 h in all tests (Reinke, 1966; Frank, 1972). Similar results
were obtained with Benlate PNW (a 50% wettable powder) (Gargus &
Zoetis, 1983a,b).
Another eye irritation experiment was performed with 5 mg pure
benomyl using four albino rabbits (Desi, 1979). Results assessed
according to Draize (Draize et al., 1944) indicated that benomyl is
a mild eye irritant.
7.3.3 Sensitization
Albino guinea-pigs exposed to benomyl, either technical
material or a 50% sucrose formulation, produced mild to moderate
skin erythema during the challenge phase following both intradermal
injections or repeat applications to abraded skin (Majut, 1966;
Colburn, 1969; Frank, 1969).
In another sensitization study, Benlate PNW (50% benomyl
prepared as a 0.1% solution in saline) was injected weekly (four
injections) into ten albino guinea-pigs (Hartley strain). Ten
control animals were injected with saline. Fourteen days after the
final injection, 8% or 80% Benlate PNW saline solutions were applied
to the backs of the induced animals and saline was applied to the
backs of the control animals. No significant increase in score
occurred in any of the control animals at either challenge
concentration. Benlate PNW produced an unequivocal and significant
(two-step) increase at two of ten sites challenged with an 8%
suspension, and at seven of ten sites challenged with an 80%
suspension (Gargus & Zoetis, 1984).
Technical benomyl produced sensitization in all ten animals
tested in a guinea-pig maximization test (Matsushita et al., 1977).
7.4 Long-term exposure
7.4.1 Rat
Groups of weanling rats (36 male and 36 female Charles River
albino rats/group) were fed benomyl (50-70% a.i.) in the diet for
104 weeks at levels of 0, 100, 500 and 2500 mg/kg. Growth, as
observed by body weight changes and food consumption data, was
recorded weekly for the first year and twice a month thereafter.
Daily observations were made of clinical effects and mortality. At
periodic intervals during the study, haematological, urinalysis and
selected clinical chemistry examinations were performed. After one
year each group was reduced to 30 males and 30 females by interim
sacrifice for gross and microscopic evaluations. At the conclusion
of the study, all surviving animals were sacrificed and gross
examinations of tissues and organs were made. Initially, microscopic
examinations of tissues and organs from the control and 2500 mg/kg
groups were conducted, as were liver, kidney and testes examinations
of animals in the 100 and 500 mg/kg dose groups. In follow-up
pathological evaluations, all of the tissues and organs of the
control and low-, intermediate- and high-dose groups were examined
microscopically. There was no mortality attributable to benomyl in
the diet. Survival decreased to approximately 50% during the second
year, but was comparable among all groups. Body weight, food
consumption and food efficiency were unaffected by treatment. The
average daily dose for the 2500 mg/kg group was 330 mg/kg body
weight per day initially, 91-106 mg/kg body weight per day at one
year and 70-85 mg/kg body weight per day at two years. There were no
compound-related clinical manifestations of toxicity.
Haematological, urine and liver function tests were unaffected by
treatment. There were no differences in organ weight or organ-to-
body-weight ratios between control and treated groups (Sherman,
1969c; Lee, 1977).
7.4.2 Mouse
In a study by Weichman et al. (1982), male and female CD-1 mice
(80 males and 80 females per group) were administered benomyl (99%
a.i.) in the diet at levels of 0, 500, 1500 and 5000 mg/kg (the
highest levels was reduced from 7500 mg/kg after 37 weeks) for two
years. The mice were 6-7 weeks old at the start of the study. Median
survival time was unaffected by treatment. Male and female mice fed
1500 or 5000 mg/kg exhibited dose-related body weight decreases.
Food consumption was variable throughout the study, although
high-dose females appeared to consume less food. The average daily
intake of benomyl for males was 1079 mg/kg body weight per day
initially, 878 mg/kg body weight per day for 1 year and 679 mg/kg
body weight per day for 2 years; for females it was 1442 mg/kg body
weight per day initially, 1192 mg/kg body weight per day for 1 year,
and 959 mg/kg body weight per day for 2 years. There were no
apparent differences between treatment and control groups with
respect to palpable mass, number of mice affected or latency period
of discovery. Haematology examinations revealed no abnormalities
except for slightly decreased erythrocyte counts in the case of
males at 1500 mg/kg and females at 5000 mg/kg. Haemoglobin and
haematocrit values were also slightly depressed in 1500-mg/kg males.
Significant compound-related changes were seen in the absolute
and relative liver weights for males at 1500 and 5000 mg/kg for
females at 5000 mg/kg. Male mice also presented decreased absolute
testes weights at the highest dose level. Non-neoplastic organ
changes in males (5000 mg/kg) were confined to the liver
(degeneration, pigment, cytomegaly), thymus (atrophy), testes,
epididymis (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini) and prostate. In female mice,
splenic haemosiderosis was significantly increased at 5000 mg/kg, as
was submucosal lymphocytic infiltration of the trachea at 1500 mg/kg
(Weichman et al., 1982).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A number of reproduction studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in the Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.5.1.1 Rat feeding studies
A 2-generation reproduction study was conducted using Crl:CD
rats (Mebus, 1990). Throughout the study, animals were fed diets
containing 0, 100, 500, 3000 or 10 000 mg benomyl/kg. P1 parental
rats received the test diets for 71 days (mean daily intake 0,
approx. 6, approx. 30, approx. 190 or approx. 350 mg/kg per day,
respectively) before being bred to animals from the same dietary
concentration group for production of the F1 parental rats. F1
rats were mated after being maintained on their respective diets
(mean daily intake 0, approx. 8, approx. 20, approx. 250, or approx.
1000 mg/kg per day, respectively) for at least 105 days after
weaning for production of the F2A litter. F1 dams were mated
again, to different non-sibling males, at least 1 week after weaning
the F2A litter, to produce the F2B litters.
The following indices of reproductive function were calculated
for the P1 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental
generation rats were sacrificed for gross and histopathological
(gross lesions and target organs only) examination. Complete
histopathological examination was conducted on control and high-dose
animals. Twenty F2A and 20 F2B weanlings were also given a gross
pathological examination.
There were no compound-related effects on parental mortality at
any benomyl concentration. Mean body weights, body weight gains and
overall food consumption of P1 and F1 male and female rats were
significantly lower in the animals receiving 10 000 mg/kg than they
were in controls.
At 10 000 mg/kg, there was a significant compound-related
decrease in the number of F2A and F2B offspring alive prior to
culling (on day 4). In addition, male and female offspring of rats
fed 10 000 mg/kg weighed consistently less at birth than did the
offspring of control rats. With the exception of the 14-day male
pups in the F2B generation, F2A and F2B offspring in the 3000
mg/kg group also had compound-related significantly depressed body
weights on days 14 and 21 of lactation.
Testicular sperm counts in P1 and F1 rats were decreased in
the 3000 and 10 000 mg/kg groups. This was accompanied by decreased
testicular weight and histopathological changes in the testes at 10
000 mg/kg. Microscopic observations included atrophy and
degeneration of the seminiferous tubules in the testes of rats in
the 3000 and 10 000 mg/kg groups and oligospermia in the
epididymides of the high-dose P1 generation and the 3000 and 10
000 mg/kg F1 generation. However, there were no compound-related
differences in mating indices, fertility indices or gestation length
which could be attributed to benomyl feeding (Mebus, 1990).
In a feeding study by Barnes et al. (1983), adult male Wistar
rats (27 animals/group) were fed 0, 1, 6.3 or 203 mg/kg for 70 days
(13 animals/group) and allowed to recover for 70 days (14
animals/group). Ejaculated sperm counts were reportedly depressed in
the 203-mg/kg group during the feeding phase. There was also a
decrease in relative testicular weights and a slightly lowered male
fertility index in all treated groups. Benomyl did not alter
copulatory behaviour and did not induce dominant lethal mutation.
Plasma testosterone and gonadotropin levels remained unchanged
throughout the study. Identical studies conducted during the
recovery phase showed all of the treatment-related effects to be
completely reversible.
7.5.1.2 Rat gavage studies
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal Sprague-Dawley male rats
(33 days old), which were gavaged daily for 10 days at doses of 0 or
200 mg/kg per day. Eight animals per group were killed at 3, 17, 31,
45 and 59 days after the last treatment. Selected tissues, including
liver, kidneys, testes, seminal vesicles and epididymides, were
removed, weighed and examined histo logically. Samples of seminal
fluid from the vas deferens were also examined. Observation
intervals were pre-selected to coincide with stages of
spermatogenesis. Data were presented on tissue weights, total
epididymal sperm counts, vas deferens sperm concentrations and
testicular histology. There were no effects related to treatment
(Carter, 1982).
In a similar study, adult male Sprague-Dawley rats (65 days
old) received 10 daily treatments of 0, 200 or 400 mg benomyl/kg per
day by gavage, and, 14 days after the last treatment, body weight,
tissue weights, total epididymal sperm counts and sperm
concentration in the vas deferens were measured and histological
investigations of the testes were carried out. Production of
testosterone by the Leydig cells was stimulated by subcutaneous
injections of human chorionic gonadotrophin (HCG) 2 h prior to
sacrifice. There were no compound-related effects on body weight or
on liver, kidney, adrenal, testis or seminal vesicle weights.
Caudate epididymal weights were, however, depressed by treatment
with benomyl. There were also treatment-related reductions in
epididymal sperm count (caput and caudate) as well as in the vas
deferens sperm concentration. The study was designed to evaluate
alterations in spermatozoa undergoing spermatogenesis in the
seminiferous tubules of the testes during exposure to benomyl.
Animals exposed to 400 mg/kg per day presented histological evidence
of hypospermatocytogenesis with generalized disruption of all stages
of spermatogenesis, when compared with controls (Carter & Laskey,
1982).
Linder et al. (1988) evaluated the effect of administering 1,
5, 15 or 45 mg benomyl/kg per day by gavage daily to 102-day old
Wistar male rats (12/group). The males were bred to untreated
females after 62 days of dosing and killed after 76-79 days.
Reproductive behaviour, seminal vesicle weight, prostate weight,
sperm motility, and serum gonadotropin hormones and serum gonadal
hormone levels were no different from those in controls at any dose.
At necropsy, males exposed to 45 mg/kg had decreased testis and
epididymal weights, reduced cauda sperm reserves, decreased sperm
production, increased numbers of decapitated spermatozoa and
increased numbers of seminiferous tubules containing multinucleated
giant cells.
In a study by Hess et al. (1991), adult male Sprague-Dawley
rats (100 days of age, 20 rats/dose) were given a single dose of
benomyl in corn oil (0, 25, 50, 100, 200, 400 or 800 mg/kg body
weight). Eight animals/group were sacrificed at 2 days and 12
animals/group at 70 days (except for the 800 mg/kg group) after
treatment. The testis and excurrent ducts were examined each time to
determine benomyl effects on spermatogenesis and on the epididymis.
The primary effects seen at day 2 were testicular swelling and
occlusions of the efferent ductules. Premature release of germ cells
(sloughing) was the most sensitive short-term response to benomyl.
Sloughing was detected in all treatment groups but was statistically
significant (p < 0.05) at doses of 100 mg/kg to 800 mg/kg.
Occlusions of the efferent ductules of the testis were dose
dependent and correlated with the increase in testis weight on day
2. The greatest increase in testes weight was observed in the 400
mg/kg group. Long-term effects (70 days) were seen in the 100, 200
and 400 mg/kg groups, e.g., decreased testis weight (400 mg/kg),
dose-dependent increases in seminiferous tubular atrophy, and
increases in the number of reproductive tracts containing occluded
efferent ductules. No long-term effects were seen in the 0, 25 or 50
mg/kg groups.
7.5.1.3 Dog inhalation studies
Three groups (10 dogs per group) of sexually mature male dogs
were exposed to benomyl at air concentrations (aerosol/cloud) of 0,
0.65 and 1.65 mg/litre for a 4-h period. One half of the dogs in
each group were killed on day 14 and the remainder on day 28
following the exposure. Histopathological examination revealed a
reduction in spermatogonic activity on day 14, but not on day 28, in
the high-dose group (Littlefield & Busey, 1969).
7.5.2 Teratogenicity and embryotoxicity
7.5.2.1 Mouse gavage studies
Groups of pregnant CD-1 mice (20-25 mice/group) were
administered benomyl via gavage at dose levels of 0, 50, 100 and 200
mg/kg per day on days 7 to 17 of gestation. Animals were killed on
day 18, pups were delivered by Caesarean section, the number of
live, dead and resorbed fetuses was determined, and fetuses were
examined for gross abnormalities. Half of the fetuses were examined
for visceral abnormalities and the other half for skeletal
abnormalities. No maternal toxicity was observed. Fetal development
was adversely affected by treatment at all dose levels. The high
dose caused an increased supraoccipital score, decreased numbers of
caudal and sternal ossifications and increased incidences of
enlarged lateral ventricles and enlarged renal pelvis. The latter,
while not significant at the lower doses, did demonstrate
dose-related increases at all other doses. The occurrence of
supernumerary ribs and subnormal vertebral centrums was
significantly increased in a dose-related manner at all dose levels.
There was an increase in the number of abnormal litters and fetuses,
which was significantly different from the controls, at levels of
100 and 200 mg/kg per day. Fetal weights were also decreased at
these dose levels. Major abnormalities included exencephaly,
hydrocephaly, cleft palate, hydronephrosis, polydactyly,
oligodactyly, umbilical hernia, fused ribs, fused vertebrae and
short/kinky tail (Kavlock et al., 1982).
7.5.2.2 Rat gavage studies
In a study by Staples (1980), benomyl (99.2% a.i.) was adminis
tered by gavage to groups of pregnant rats (ChR-CD) at dose levels
of 0, 3, 10, 30, 62.5 and 125 mg/kg per day from days 7 to 16 of
gestation. There were 60 dams in the control group and 27 in each of
the other test groups; they were observed daily for signs of
toxicity and changes in behaviour. No clinical signs of toxicity or
mortality were observed among dams in any dose group. Body weight
gain was comparable to controls, as were the incidences of
pregnancy, corpora lutea and implantation sites, and the sex ratio.
However, fetal body weight was significantly decreased at the two
highest dose levels. There was also an increased incidence of
embryo/fetal mortality at 125 mg/kg per day.
The malformations observed included microphthalmia,
anophthalmia and hydrocephaly (distended lateral ventricles). These
appeared to be compound related at the higher dose levels.
Microphthalmia was seen in two fetuses from different litters at 10
mg/kg per day and in one fetus from the control group.
Microphthalmia/anopthalmia was observed in only 4-6 out of 4935
fetuses examined in the historical data base at this laboratory.
Histological examination of eyes revealed pathological changes
consisting of irregular lenses, retro-bulbar glandular adnexa,
distorted or compressed retinal layers and thickened nerve fibres in
the 10, 62.5 and 125 mg/kg per day treatment groups. Major skeletal
malformations observed in the 125 mg/kg dose group included fused
ribs, fused sternebrae and fused thoracic arches. Additional
skeletal variations were also increased at 62.5 and 125 mg/kg per
day; these included misaligned and unossified sternebrae and
bipartite vertebral centra (Staples, 1980).
In order to determine a no-effect level for microphthalmia and
hydrocephaly, groups of 50 pregnant rats of the same strain and from
the same supplier were administered benomyl (99.1% a.i.) via gavage
at dosage levels of 0, 3, 6.25, 10, 20, 30 and 62.5 mg/kg per day
from days 7 to 16 of gestation (Staples, 1982). Each group contained
50 animals except the high-dose group, which contained 20 female
rats. Reproductive status was determined on a per-litter basis
following gross pathological evaluation. The number of implantation
sites, resorptions, dead, live and stunted fetuses, and the mean
weight of live fetuses per litter were determined. Only fetal heads
were fixed and examined microscopically. Microphthalmia was
determined on the basis of the smallest eye in the control group (<
1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two animals with malformations,
both in the 62.5 mg/kg per day group. One fetus exhibited internal
hydrocephaly and another, in a separate litter, unilateral
microphthalmia. There was no teratogenic response at doses up to 30
mg/kg.
The teratogenic potential of benomyl was examined in groups of
Wistar rats (12 to 30 pregnant rats/group) orally gavaged at dose
levels of 0, 15.6, 31.2, 62.5 and 125 mg/kg per day on days 7 to 16
of gestation. Major anomalies were observed primarily at levels of
62.5 mg/kg per day or more and included encephaloceles,
hydrocephaly, microphthalmia, fused vertebrae and fused ribs. A dose
level of 31.2 mg/kg per day appeared to be without adverse effects
on the developing rat fetus in this evaluation. A significant
reduction in maternal body weight was observed at the highest dose
level (Kavlock et al., 1982).
Kavlock et al. (1982) also evaluated the effect of low levels
of benomyl as the pups aged. Benomyl was administered via gavage to
groups of Wistar rats at dose levels of 0, 15.6 and 31.2 mg/kg per
day from day 7 of gestation to day 15 of lactation (day 22 of
gestation was considered day 0 of lactation). The litters were
weighed on days 8, 15, 22, 29 and 100 after parturition, and
locomotor activity was evaluated periodically throughout the study.
At 100 days of age, several organs were weighed, including the
adrenals, liver, kidney, ovaries, testes and the ventral prostate
plus seminal vesicles. There were no compound-related effects either
on litter size at birth or weaning, or on body weights of fetuses.
Growth, survival and locomotor activity values were comparable with
those of the controls throughout the study. Organ weights were
comparable with those of controls, except that testes and ventral
prostate/seminal vesicle weights were significantly reduced at 31.2
mg/kg per day (but not at 15.6 mg/kg) (Kavlock et al., 1982).
In other studies (Zeman et al., 1986; Hoogenboom et al., 1991),
benomyl produced ocular and craniocerebral malformations in
Sprague-Dawley rats when administered by gavage at doses of 31.2 and
62.4 mg/kg per day on day 7-21 of gestation. Ocular anomalies
(retinal dysplasia, cataracts, microphthalmia and anophthalmia)
occurred in 43.3% of fetuses when the dams were administered 62.4
mg/kg per day. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose level
of benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
(consisting primarily of hydrocephaly) occurred in fetuses from dams
administered 31.2 mg/kg per day in combination with the semipurified
diet (Zeman et al., 1986).
7.5.2.3 Rat feeding studies
In a study by Sherman et al. (1972), groups of rats (26-28
pregnant ChR-CD rats/group) were administered a benomyl formulation
(53.5% a.i.) in the diet at dosages of 0, 100, 500, 2500 and 5000
mg/kg from day 6 to day 15 of gestation. Average doses were
equivalent to 0, 8.6, 43.5, 209.5 and 372.9 mg/kg body weight per
day. On day 20 of gestation, all pregnant animals were sacrificed
and fetuses were delivered by Caesarean section. There was no
mortality attributable to benomyl, no clinical signs of toxicity and
no adverse effects on the body weight of dams. Dams in the
highest-dose group had a reduced food intake during the period
benomyl was administered in the diet, but the intake returned to a
level similar to the controls for the remainder of the study. Except
for three litters at the highest dose, where there were incidences
of hydronephrosis and retarded ossification (interparietal and
occipital bones), there were no effects on fetal development related
to benomyl administration.
In a similar study, groups of pregnant Wistar rats (27-28
rats/group) were fed benomyl at dose levels of 0, 1690, 3380 and
6760 mg/kg diet (time-weighted doses of 0, 169, 298 and 505 mg/kg
body weight per day, respectively) from days 7 to 16 of gestation.
No dose-related anomalies or major malformations were associated
with exposure to benomyl at any of the dose levels used. Reduced
fetal weight was observed at the two highest dose levels (Kavlock et
al., 1982).
7.5.2.4 Rabbit feeding studies
Groups of rabbits (15 artificially inseminated New Zealand
albino rabbits/group) were administered a benomyl formulation (50%
a.i.) in the diet at dose levels of 0, 100 and 500 mg/kg diet from
day 8 to day 16 of gestation. Mortality, clinical observations and
food consumption were determined daily and body weights were
measured weekly. There were 12 pregnant does in the control group,
13 in the low-dose group and 9 in the high-dose group. Of these, 6,
7 and 5, respectively, were sacrificed on day 29 or 30 and fetuses
were delivered by Caesarean section; the remaining does gave birth
normally. Maternal toxicity was not observed at any dose level.
Except for a marginal increase in rudimentary ribs at 500 mg/kg,
developmental toxicity was not observed. The numbers of litters and
of fetuses examined were less than adequate to assess the fetotoxic
or teratogenic potential of benomyl to pregnant rabbits (Busey,
1968c; FAO/WHO, 1985a).
7.6 Mutagenicity and related end-points
Numerous studies have been conducted to assess the mutagenic
potential of benomyl, its metabolite carbendazim, and several
benomyl formulations. Many of the results are conflicting and many
of the study reports do not provide sufficient detail to evaluate
the reasons for the conflicting data. This summary will cover only
those studies where sufficient experimental detail and data were
reported (Table 9).
Studies on somatic and germ cells have shown that benomyl does
not cause gene mutations or structural chromosomal damage
(aberrations) and that it does not interact directly with DNA. This
has been demonstrated in both mammalian and non-mammalian systems.
However, in the mammalian in vitro studies for gene mutations and
structural chromosome aberrations, some positive results were
obtained with benomyl. These positive results appear to have been a
consequence of the inherent sensitivity of some in vitro mammalian
test systems to cytotoxic agents. Results in mammalian in vivo
studies for gene mutations and structural chromosome aberrations
were negative.
Benomyl does cause numerical chromosomal aberrations
(aneuploidy and/or polyploidy) in experimental systems both in
vitro and in vivo (Table 9).
7.7 Carcinogenicity
A number of carcinogenicity studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.7.1 Rat
In a two-year study on benomyl, groups of Charles River albino
weanling rats (36 male and 36 female rats) were fed benomyl (50-70%
a.i.) in the diet at levels of 0, 100, 500 and 2500 mg/kg diet. At
the end of the study all surviving animals were sacrificed and gross
and microscopic examinations of tissues and organs were carried out.
The most frequently observed tumours involved the pituitary, but
these were equally distributed among control and treated groups.
Mammary, adrenal and other tumours were also observed; these were
scattered among all groups. There were no adverse effects or
significant histopathological changes at any dose levels in this
study that could be attributable to benomyl (Sherman, 1969c; Lee,
1977).
Table 9. Studies on mutagenicity of benomyl
End points/Tests Species, strains Concentrationb Activation Result Reference
1. DNA damage and repair
Mitotic gene conversion Saccharomyces cerevisiae, NR with and negative Siebert et al. (1970);
D4 & D7 without de Bertoldi et al. (1980)
Mitotic gene conversion Aspergillus nidulans, 0.35-2.8 mM without negative de Bertoldi & Griselli
D7 (1980)
Mitotic crossing-over test A. nidulans, P NR without negative Bignami et al. (1977)
A. nidulans, D7 NR without negative de Bertoldi & Griselli
(1980)
Non-disjunction A. nidulans, D7 0.35-2.8 mM without positive de Bertoldi & Griselli
(1980)
Sister chromatid exchanges human lymphocyte 0.05-2.0 µg/ml NR slight increase in Georgieva et al. (1990)
(SCE) cultures SCE, no dose-response
relationship
Unscheduled DNA synthesis B6C3F1 male mice & 0.5-500 µg/ml NR negative Tong (1981)
Fisher-344 male rat
hepatocytes
2. Gene mutation
a) Bacterial & fungal Salmonella typhimurium 0.125-1.0 µg/ml NR positive(TA1535) Kappas et al. (1976)
gene mutation TA1535, TA1538 0.125-1.0 µg/ml NR negative(TA1538)
Escherichia coli, 0.125-1.0 µg/ml NR positive Kappas et al. (1976)
WP2 uvra
E. coli, WP2 uvra 2.5-10.0 µg/ml NR positive Kappas et al. (1976)
E. coli, WP2 0.125-1.0 µg/ml NR negative Kappas et al. (1976)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Point mutation A. nidulans 0.25-0.4 µg/ml NR positive Kappas & Bridges (1981)
Spot test S. typhimurium, his, 50-5000 µg per negative Fiscor et al. (1978)
G 46 & TA1535, TA1530 spot
TA1950
S. typhimurium, TA100 50-2000 µg per with negative Fiscor et al. (1978)
plate
Host-mediated assay S. typhimurium, his, 3 consecutive negative Fiscor et al. (1978)
G 46 subcutaneous
injections of
500 mg/kg in mice
TA1950 an oral dose of 4000 negative Fiscor et al. (1978)
mg/kg in rats or mice
Spot test S. typhimurium, TA1535, 20 µg/spot with and negative Carere et al. (1978)
TA1536, TA1537, TA1538 without
Forward spot mutation Streptomyces coelicolor 500 µg/spot without negative Carere et al. (1978)
assay
Ames plate incorporation S. typhimurium, TA1537 500 µg/plate without marginal Russell (1978a)
test positive
S. typhimurium, TA1535, 10-500 µg/ with negative Donovan & Krahn (1981);
TA1537, TA98, TA100 plate Rickard (1983a,b)
10-200 µg/plate without negative
TA1535, TA1537, TA98, 10-500 µg/plate with and negative Russell (1978b)
TA100 without
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
TA1513, TA1537, up to 1000 µg/ with and negative Shirasu et al. (1978)
TA1538, TA98, TA100 plate without
E. coli, WP2 hcr up to 500 µg/ with and negative Shirasu et al. (1978)
plate without
recA spot test B. subtilis, M45 & H-17 2000 µg/plate NR negative Shirasu et al. (1978)
Host-mediated assay S. typhimurium, his, 2000 mg/kg negative Shirasu et al. (1978)
(ICR mice) G46
b) In vitro mammalian
gene mutaion
HGPRTa gene Chinese hamster ovary 17 & 172 µM with negative Fitzpatrick (1980)
cells 3 & 120 µM without negative
Mouse lymphoma Mutant colonies in 0.5-20 µM without negative Amacher et al. (1979)
L5178Y TK+/- gene RPMl1640/horse serum
mutation assays (MLY) 2.5-25 µM with negative
MLY > 2.5 µM negative McCooey et al. (1983a)
MLY carbendazim, without negative McCooey et al. (1983b)
25-200 µM
carbendazim, with negative McCooey et al. (1983b)
12.5-200 µM
MLY N-butylisocyanate without negative McCooey et al. (1983b)
2.5-25 µM
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) Insect germ cell
gene mutation
Sex-linked recessive adult male fed benomyl suspension negative Lamb & Lilly (1980)
lethals D. melanogaster (1.5 mg/ml)
3. Chromosomal effects
a) Yeast and fungi A. nidulans 0.25 µg/ml without increased frequency of Hastie (1970)
(diploid) 0.5 µg/ml segregants
A. nidulans 0.25 µg/ml without no increased frequency Hastie (1970)
(haploid) 0.5 µg/ml of segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(diploid) segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(haploid) segregants
A. nidulans 0.35 to 2.8 mM without increased De Bertoldi & Griselli
(diploid) nondisjunction (1980)
Saccharomyces cerevisiae, 30 µg/ml without induced chromosome Albertini (1991)
D61.M malsegregation
b) In vitro mammalian human lymphocytes 1.0-100.0 µg/ml with negative Pilinskaya (1983)
assays
human lymphocytes 1.0-100.0 µg/ml without weak positive at Pilinskaya (1983)
10.0 µg/ml only
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
human/mouse mono- < 15 µg/ml without induced aneuploidy Athwal & Sandhu (1985);
chromosomal hybrid and polyploidy Sandhu et al. (1988)
cells (R3-5)
human/mouse mono- 15 µg/ml without slight increase in Athwal & Sandhu (1985);
chromosomal hybrid structural Sandhu et al. (1988)
cells (R3-5) chromosomes; 1.5 µg/ml
threshold (polyploidy)
V79/AP4 Chinese NR without dose-related increase Rainaldi et al. (1989)
hamster cells in numerical
chromosomal
aberrations
Chinese hamster NR without dose-related increase Eastmond & Tucker
ovary cells in numerical (1989)
chromosomal
aberrations
human lymphocytes NR without dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations; 0.1 µg/ml
threshold (aneuploidy)
Chinese hamster- NR without dose-related increase Zelesco et al. (1990)
human hybrid cells in numerical
chromosomal
(EUBI) aberrations; 2.0 µg/ml
threshold (aneuploidy)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) In vivo mammalian rats up to 500 mg/kg no chromosomal Ruziscka et al. (1976)
assays for 8 days by gavage aberrations in bone
marrow, increase in
chromosomal
aberrations in
embryonic cells at
200 and 500 mg/kg doses
Bone marrow micro- ICR mice 2 gavage doses of increase in Seiler (1976)
nucleus test 9, 500 or 1000 micronucleated
mg/kg 24 h apart polychromatic
erythrocytes at
1000 mg/kg only
Bone marrow micro- BDF1 mice single gavage dose increase in Sasaki (1990)
nucleus test of 0, 1250, 2500 or micronucleated
5000 mg/kg polychromatic
erythrocytes at
2500 and 5000 mg/kg
Bone marrow chromosomal B6D2F2/Cr-1Br mice single gavage dose no increase in Stahl (1990)
aberrations of 0, 625, 1250, 2500, structural chromosomal
or 5000 mg/kg aberrations
d) In vivo germ cell
chromosomal aberration
Dominant lethal test ChR-CD rat 0, 500, 2500 or negative Culik & Gibson (1974)
5000 mg/kg feeding
for 7 days
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Dominant lethal test Wistar rats 0, 1, 6.3 or 203 negative Barnes et al. (1983)
mg/kg feeding for
70 days
Dominant lethal test Wistar rats 70 daily gavage doses negative Georgieva et al. (1990)
of 0, 10 or 50 mg/kg
a HGPRT = hypoxanthine-guanine phosphoribosyl transferase
b NR = not reported
7.7.2 Mouse
Male and female CD-1 mice (80 males and 80 females per group)
were fed benomyl (99% a.i.) at dose levels of 0, 500, 1500 and 5000
mg/kg diet (the highest level was reduced from 7500 mg/kg after 37
weeks) for two years. The incidence of hepatocellular adenomas and
carcinomas (see Table 10) in female mice was increased in a
dose-dependent manner. In male mice, numbers of hepatocellular
adenomas and carcinomas were significantly increased at 500 and 1500
mg/kg but not at 5000 mg/kg dose. The increased number of lung
alveogenic carcinomas in male mice was still within the range of
historical controls (Weichman et al., 1982; Frame & van Pelt, 1990;
Hardisty, 1990).
7.8 Special studies
7.8.1 Neurotoxicity
Studies performed using White Leghorn hens (10/group) gave no
indication of neurotoxic potential with single oral doses of benomyl
at levels up to 5000 mg/kg (Goldenthal, 1978; Jessup & Dean, 1979;
Jessup, 1979).
Studies performed using adult male CFY rats (10 rats/group)
gave no indication of altered EEG potentials or behaviour (learning
ability assessed with a 4-choice T-maze) after treatment with 250 or
500 mg benomyl/kg per day for 3 months when compared with controls
(Desi, 1983).
7.8.2 Effects in tissue culture
In a study by Desi et al. (1977), primary monkey kidney cells
were incubated with benomyl (Fundazol 50 WP) at levels of 1, 10, 50,
100, 250 and 400 mg/kg. Growth inhibition and syncytium-like
appearance of the cell monolayer was observed at 250 mg/kg, and at
400 mg/kg all the cells were killed.
7.9 Factors modifying toxicity; toxicity of metabolites
Benomyl degrades to butyl isocyanate (BIC) and carbendazim
quite rapidly in water and in many organic solvents used in
toxicological testing. The biological activity of benomyl is
essentially that of carbendazim both in a toxicological sense and in
its use as a fungicide. A detailed discussion of carbendazim
toxicology is given in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
Table 10. Incidence and latency of hepatic tumours in benomyl-treated CD-1 micea
Male mice Female miceb
Benomyl concentration (mg/kg diet): 0 500 1500 5000 0 500 1500 5000
Hepatocellular adenoma
Number of animals with tumours 9 8 10 10 2 2 5 7
Latent time to first tumour (days) 530 556 541 627 744 641 650 644
Average latent period (days) 687 707 695 726 744 688 717 722
Hepatocellular carcinoma
Number of animals with tumours 14 26c 41d 17 2 7 7 14e
Latent time to first tumour (days) 545 470 590 508 744 640 736 426
Average latent period (days) 693 705 721 711 744 708 736 695
a From: Wiechman (1982)
b P < 0.05 Dose/response increase of adenomas and carcinomas
c P < 0.05
d P < 0.001
e P < 0.01
BIC is toxic by inhalation. In 4-month subchronic inhalation
studies, the no-observed-effect levels (NOEL) for BIC in rats and
mice were determined to be 0.32 and 1.5 ppm, respectively (Gurova et
al., 1976). An industrial hygiene survey found that workers
experienced severe eye irritation and lacrimation at BIC exposure
levels of 5-10 ppb (Kelly, 1989).
7.10 Mechanisms of toxicity - Mode of action
Biochemical studies on the mechanism of action of benzimidazole
compounds have shown that their biological effects are caused by
interactions with cell microtubules (Davidse & Flach, 1977). These
cellular structures are present in all eukariotic cells and are
involved in several vital functions, such as intracellular
transports and cell division. Benzimidazol compounds have been used
as anticancer drugs and as antihelminthic drugs in animals and
humans because they act as spindle poisons by interfering with the
formation and/or functioning of microtubules. However, eukaryotes
are known to be unequally sensitive to each benzimidazol compound,
which explains the use of these compounds in helminthiases.
Selective toxicity of benomyl and carbendazim for fungi has been
explained by comparing their binding to fungal and mammalian
tubulin. The different sensitivity of several fungi has also been
explained by the different affinity of benomyl and carbendazim for
fungal tubulin.
Benomyl has been found to bind to fungal tubulin but not to
porcine brain tubulin, indicating that mammalian tubulin has no, or
at least low, affinity for benomyl (Davidse & Flach, 1977). This is
in agreement with the observation that benomyl at concentrations
that are lethal for sensitive fungi does not interact with in vitro
microtubule assembly in these brain extracts. In vitro ID50
values for several mycelial extracts of various fungal species
sensitive to benomyl were all below 5 µmol/litre (Davidse & Flach,
1977). In vitro rat brain tubulin polymerization was inhibited to
about 20% at benomyl or carbendazim concentrations of 25 µmol/litre
(De Brabander et al., 1976b). For comparison, a standard antitubulin
drug in humans such as vincristine inhibited 50% tubulin assembly at
0.1 µmol/litre in the same experiment. The assembly of sheep and
calf brain microtubule was also found to be unaffected by
carbendazim concentrations higher than 100 µmol/litre (Ireland et
al., 1979).
Mitotic arrest by benzimidazole and six analogues at metaphase
was evaluated in human lymphocyte cultures. Structure-activity
relationships indicate that antimitotic activity is related to C6
substitution of the benzimidazole moiety (Holden et al., 1980). In
this study, however, benomyl and carbendazim were not tested. The
question of whether all C6 unsubstituted benzimidazoles, such as
benomyl and carbendazim, have no effect on mitosis of human
lymphocytes in cell cultures is therefore unresolved.
A link between the effects of benomyl and carbendazim on brain
tubulin and their teratogenic effects has been postulated (Ellis et
al., 1987, 1988).
8. EFFECTS ON HUMANS
8.1 General population exposure
No references to benomyl poisoning in the general population
have been documented in the scientific literature. Recent data used
to estimate dietary exposure based on food consumption patterns
within the USA indicate exposures well below the NOELs in animal
toxicity tests.
8.2 Occupational exposure
8.2.1 Acute toxicity
Benomyl has a very low acute toxicity. No inadvertent poisoning
of agricultural or factory workers has been documented (Goulding,
1983).
8.2.2 Effects of short- and long-term exposure
Benomyl causes contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et al., 1985). In
controlled patch tests of agricultural, ex-agricultural and
non-agricultural workers (total of 200 subjects), only one
agricultural worker showed any contact dermatitis to 0.1% benomyl
(Lisi et al., 1986).
A survey of cross-sensitization between benomyl and other
pesticides was conducted in Japan on a group of 126 farmers who
applied benomyl to their crops. Thirty-nine of the farmers gave
positive test results with benomyl, the highest incidence being
among female farmers. There were cross-reactions between benomyl and
other pesticides, such as diazinon, saturn, daconil and Z-bordeaux
(Matsushita & Aoyama, 1981).
Selected blood profiles from 50 factory workers involved in the
manufacture of benomyl were compared to those of a control group of
48 workers who were not exposed to carbendazim. White blood cell
count, red blood cell count and haemoglobin and haematocrit values
were comparable among the two groups. There were no quantitative
estimates of exposure given for the factory workers (Everhart, 1979;
FAO/WHO, 1985a).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers exposed to benomyl between 1970 and 1977. The
workers ranged from 19 to 64 years of age (79% were between 20 and
39, and 78% of the spouses were similarly aged between 20 and 39
years). Exposure duration ranged from less than one month to 95
months, and more than 51% of the workers were potentially exposed
from 1 to 5 months. The birth rates of exposed workers' spouses were
compared with those of four comparison populations from the same
county, state, region and country (USA). There was no reduction in
fertility as shown by the birth rates for the study population,
which were generally higher than those of the comparison
populations. Spermatogenesis among workers was not examined (Gooch,
1978; FAO/WHO, 1985a).
In studies on agricultural spraymen using benomyl (Fundazol 50
WP), 14 spraymen working in greenhouses were followed, some of them
for two years. General medical check-up and routine blood and urine
tests were performed. Electrocardiograms were recorded and blood
cholinesterase activity was monitored. Lymphocytes from peripheral
blood were examined for chromosomal aberrations both before and
during the study. There was no difference in structural chromosomal
aberration between the spraymen and controls. After benomyl
exposure, numerical chromosomal aberrations were higher in the
spraymen than in controls. However, the spraymen had a higher level
of numerical chromosome aberration than the controls even before
benomyl exposure (Desi et al., 1990; Nehez et al., 1992).
9. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Soil respiration has been found to be little influenced by
benomyl at concentrations below 10 mg/kg, which is the maximum soil
concentration expected after use at recommended application rates
(Hofer et al., 1971; van Fassen, 1974; Peeples, 1974; Weeks &
Hedrick, 1975).
A study on the influence of benomyl on soil nitrogen
mineralization showed that the release of ammonia was not decreased
by benomyl, whereas the influence of the fungicide on nitrification
varied from a stimulation (van Fassen, 1974), through no effect
(Mazur & Hughes, 1975), to a decreased nitrification (Hofer et al.,
1971; Wainwright & Pugh, 1974). The differences may be related to
the soil composition since Hofer et al. (1971) found a greater
effect in sandy than in organic soil.
Benomyl, in combination with eleven other pesticides that were
used in an orchard spray programme, had only a minimal and
short-term effect on respiration, ammonification and nitrification
at concentrations expected after recommended use of benomyl over a
spraying season. Ten times the recommended application rates had a
pronounced effect on both respiration and nitrification, which
lasted for more than 4 weeks (Helweg, 1985).
The influence of benomyl and carbendazim on soil microbial
activity was studied in Sweden following repeated annual
applications during autumn to winter cereals for a period of 3 to 5
years. The effects of the fungicides on straw decomposition, balance
of straw fungal flora and nitrogen mineralization in the soil were
investigated in field and laboratory experiments. The decomposition
of straw in the field was not affected in clay soils by annual
applications of up to 2 kg/ha. In sandy soils, rates of up to 0.5
kg/ha had no effect, but in one case at 2 kg/ha the initial stages
of straw decomposition were slightly inhibited. All doses tested in
both clay and sandy soils caused changes in the composition of the
straw fungal flora (Torstensson & Wessen, 1984).
Benomyl had no effect on soil bacterial populations in
laboratory studies, but fungi and actinomycetes populations were
reduced (Siegel, 1975). Under greenhouse conditions (Kaastra-Howeler
& Gams, 1973) or field conditions (Peeples, 1974) at application
rates of up to 89.6 kg a.i./ha, little effect on microbial
populations was observed following benomyl treatment.
9.2 Aquatic organisms
The effect of benomyl was monitored using the green alga
Selenastrum capricornutum in an OECD guideline test (201). The
EC50 (based on total growth) at 72 h was 2.0 mg/litre and at 120 h
was 3.1 mg/litre. The no-observed-effect concentration (NOEC) was
0.5 mg/litre. To study whether benomyl was algistatic or algicidal,
organisms were recultured at the end of the initial 120 h
incubation. Regrowth occurred in the control but not in the test
cultures (8.0 mg/litre) after a period of 7 days. Benomyl was,
therefore, considered to be algicidal (Douglas & Handley, 1988). In
another study using Chlorella pyrenoidosa, the 48-h EC50 for
growth inhibition was calculated as 1.4 mg/litre (Canton, 1976).
The acute toxicity of benomyl to a variety of aquatic organisms
is summarized in Table 11. For 96-h tests, LC50 values ranged from
0.006 mg/litre for channel catfish ( Ictalurus punctatus; yolk-sac
fry) to > 100 mg/litre for crayfish ( Procambarus sp.) (Mayer &
Ellersieck, 1986).
9.3 Terrestrial organisms
Several field studies have investigated the toxicity of benomyl
to earthworms (Table 12). In one study, the 48-h LC50 for E.
foetida was 9.1 µg/cm3 soil (Roberts & Dorough, 1984).
Van Gestel et al. (1992) exposed red earthworms ( Eisenia
andrei) to benomyl added to artificial soil and used final
concentrations of 0, 0.1, 0.32, 1.0, 3.2 and 10 mg/kg dry soil. The
worms had been acclimatized for 1 week in the artificial soil, which
contained 4 g/kg cow dung as food for the worms. At the highest
concentration of 10 mg/kg, high mortality occurred; LC50 values of
6.0 and 5.7 mg/kg dry soil were calculated after 3 and 6 weeks of
incubation, respectively. Growth was significantly reduced at 3.2
mg/kg soil. EC50 values for the effect of benomyl on cocoon
production did not differ significantly for the two test periods,
and the EC50 was 1.6 (1.2-2.3) mg/kg for the entire 6-week test
period.
Zoran et al. (1986) and Drewes et al. (1987) have shown that
the conduction velocity for medial and lateral giant nerve fibres is
affected by exposure of earthworms to sublethal concentrations of
benomyl. Zoran et al. (1986) have also shown that segmental
replication is affected in amputated earthworms exposed to sublethal
concentrations of benomyl.
The benomyl metabolite carbendazim (99.3% purity) was evaluated
for acute contact toxicity after thoracic application to honey-bees
(Apis mellifera). Each treatment level consisted of four replicates
of ten bees each. Forty bees served as positive control (using
carbaryl) and forty as negative control. No deaths occurred after
application of carbendazim at 50 µg/bee, the highest rate tested.
Carbendazim is, therefore, classified as "relatively non-toxic" to
the honey-bee (Meade, 1984).
Table 11. Toxicity of benomyl to aquatic organisms
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Freshwater
Water flea adult stat 25 3 14 Yoshida & Nishiuchi (1972)
(Daphnia magna) < 24 h stat 20 87 8.5 48 0.11c Hutton (1989)
< 24 h stat 20 48 0.64 Canton (1976)
< 24 h stat 17 40 7.4 48 2.8 Mayer & Ellersieck (1986)
Scud
(Gammarus pseudolimnaeus) adult stat 17 40 7.4 96 0.75 Mayer & Ellersieck (1986)
Crayfish
(Orconectes nais) instar stat 22 40 7.4 96 >10 Mayer & Ellersieck (1986)
Crayfish
(Procambarus sp.) immature stat 22 40 7.4 96 >100 Mayer & Ellersieck (1986)
Midge
(Chironomus plumosus) instar stat 22 40 7.4 48 7.0 Mayer & Ellersieck (1986)
Rainbow trout 3 months stat 15 48 0.48 Canton (1976)
(Oncorhynchus mykiss) 0.8 g stat 7 44 7.4 96 0.17 Mayer & Ellersieck (1986)
0.8 g stat 12 44 7.4 96 0.20 Mayer & Ellersieck (1986)
0.8 g stat 17 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1.2 g stat 12 44 6.5 96 0.16 Mayer & Ellersieck (1986)
1.2 g stat 12 44 7.5 96 0.19 Mayer & Ellersieck (1986)
1.2 g stat 12 44 8.5 96 0.88 Mayer & Ellersieck (1986)
0.6 g stat 12 44 7.4 96 0.23 Mayer & Ellersieck (1986)
0.6 g stat 12 320 7.4 96 0.60 Mayer & Ellersieck (1986)
fingerling stat 12 44 7.4 96 0.12 Mayer & Ellersieck (1986)
swimup fry stat 12 44 7.4 96 0.16 Mayer & Ellersieck (1986)
yolk-sac fry stat 12 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1 g stat 12 44 7.4 96 0.31c Mayer & Ellersieck (1986)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Fathead minnow 0.9 g stat 22 44 7.4 96 2.2 Mayer & Ellersieck (1986)
(Pimephales promelas) 0.5 g stat 22 45 7.1 96 1.3 Mayer & Ellersieck (1986)
0.5 g stat 22 44 7.4 96 1.9c Mayer & Ellersieck (1986)
Channel catfish 1.2 g stat 22 44 7.4 96 0.029 Mayer & Ellersieck (1986)
(Ictalurus punctatus) 0.05 g stat 22 44 7.4 96 0.013 Mayer & Ellersieck (1986)
0.15 g stat 22 44 7.4 96 0.024 Mayer & Ellersieck (1986)
swimup fry stat 22 44 7.4 96 0.012 Mayer & Ellersieck (1986)
yolk-sac fry stat 22 44 7.4 96 0.006 Mayer & Ellersieck (1986)
1.2 g stat 22 44 7.4 96 0.028c Mayer & Ellersieck (1986)
Bluegill
(Lepomis macrochirus) 0.9 g stat 12 44 7.4 96 0.75 Mayer & Ellersieck (1986)
0.9 g stat 17 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.9 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 6.5 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.5 96 1.2 Mayer & Ellersieck (1986)
0.6 g stat 22 44 8.5 96 6.4 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 320 7.4 96 2.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.2c Mayer & Ellersieck (1986)
Carp
(Cyprinus carpio) 5 cm stat 25 48 7.5 Yoshida & Nishiuchi (1972)
Killifish
(Fundulus sp.) 2.5 cm stat 25 48 11 Yoshida & Nishiuchi (1972)
Loach 10 cm stat 25 48 14 Yoshida & Nishiuchi (1972)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Guppy
(Poecilia reticulata) 3 weeks stat 24 48 3.4 Canton (1976)
Tadpole
(Bufo sp.) < 1 month stat 25 48 4.3 Yoshida & Nishiuchi (1972)
Estuarine and Marine
Eastern oyster
(Crassostrea virginica) 25-50 mm flow 17-19 96 1.37d Boeri (1988a)
Grass shrimp
(Palaemonetes pugio) 18 mm stat 18 96 45.8c Bionomics Inc. (1972)
Mysid shrimp
(Mysidopsis bahia) stat 23 96 0.175 Boeri (1988c)
Dungeness crab
(Cancer magister) larvae 96 7.6 Armstrong et al. (1976)
Sheepshead minnow
(Cyprinodon variegatus) 0.14 g stat 22 96 3.88 Boeri (1988b)
a stat = static conditons (water unchanged for duration of test); flow = flow-through conditions (benomyl concentration in water
continuously maintained)
b hardness given as mg CaCO3/litre
c 50% wettable powder
d EC50 based on rate of shell deposition
Table 12. Summary of earthworm toxicity data on benomyl in field studiesa
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass 0.125 0.9 63 8% reduction in number Ammon (1985)
1.25 3.6 43% reduction in number
Grass 7.8 22.2 21 95% reduction in number Tomlin & Gore (1974)
91% reduction in biomass
Grass 0.56 1.6 49 79% reduction in cast Keogh & Whitehead (1975)
production
Grass 0.56 1.6 13 50% reduction in number Tomlin et al. (1980,
(0.18)b 28 40% reduction in number 1981)
180 70% reduction in number
328 67% reduction in number
1.12 (0.90)b 28 35% reduction in number
(0.20)b 180 46% reduction in number
328 50% reduction in number
2.24 6.4 13 75% reduction in number
28 50% reduction in number
(0.30)b 180 50% reduction in number
328 64% reduction in number
Grass/loam 2.0 5.7 30 70% reduction in number Edwards & Brown (1982)
180 22% reduction in number
365 1% reduction in number
Grass/sandy loam 5.0 14.3 30 89% reduction in number Edwards & Brown (1982)
180 59% reduction in number
365 32% reduction in number
Table 12 (contd).
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass/loamy sand 10.0 28.6 30 80% reduction in number
180 89% reduction in number
365 89% reduction in number
Grass/sandy loam 5.0 14.3 30 91% reduction in number; Edwards & Brown (1982)
99% reduction in L. terrestris;
29% reduction in L. festivus
180 90% reduction in L. terrestris;
200% increase in L. festivus
365 99% reduction in L. terrestris;
1457% increase in L. festivus
a From: Van Gestel (1992)
b Results of analysis of the top 15-cm layer carried out by the author, recalculated as the concentration in the top 2.5 cm layer
The acute toxicity for several birds is listed in Table 13.
Benomyl is of low toxicity to birds.
Table 13. Acute toxicity of benomyl to birds
Species LD50a 5-day LC50b Reference
(mg/kg) (mg/kg)
Bobwhite quail > 10 000 Busey (1968e)
Mallard duck > 10 000 Busey (1968e)
Japanese quail > 5000 c Hill & Camardese
(1986)
Starling > 100 Schafer (1972)
Redwinged blackbird 100 Schafer (1972)
a LD50 = single oral dose expressed as mg/kg body weight
b LC50 = 5-day dietary exposure followed by 3 days on a "clean" diet
expressed as mg/kg diet
c 50% active ingredient; no overt signs of toxicity were observed
9.4 Population and ecosystem effects
Under certain conditions benomyl may have an effect on
populations of earthworms. In apple orchards where foliage has been
treated repeatedly at a rate of 0.28 kg/ha and has fallen to the
ground, earthworms may be eliminated after two years of benomyl use
(up to 13 sprayings). The earthworms Lumbricus terrestris and
Allolobophora chlorotica were most affected. Populations of other
species recovered within two years of the termination of spraying.
Orchard yields were unaffected, as were earthworm populations
adjacent to the orchards, because of the immobility of benomyl in
the soil (Stringer & Wright, 1973; Stringer & Lyons, 1974).
Van Gestel (1992) has summarized reports of the toxicity of
benomyl on earthworms from field studies with different soil types,
application rates and crops (Table 12). Estimated soil
concentrations in Table 12, for the various uses of the fungicide,
are based on application rates; they assume no mobility of the
compound beyond the top 2.5 cm of soil and homogeneous distribution
of benomyl in this layer. For orchard application, it was further
assumed that 50% of the applied active ingredient reached the soil.
Reported effects include reduced numbers and reduced activity of
worms. Application rates are within the recommended rates for
benomyl as a fungicide on these crops.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Benomyl and carbendazim are two different fungicides in their
own right. However, carbendazim is also the main metabolite of
benomyl in mammals and the degradation product of benomyl in the
environment. Butyl isocyanate is the chemical moiety removed from
benomyl when carbendazim is formed. Given the similar toxicities
caused by benomyl and carbendazim and the different toxicological
profile of butyl isocyanate, the two fungicides are evaluated
together in this monograph.
10.1 Evaluation of human health risks
The two primary routes of exposure to humans are through diet
and through manufacturing or use of the product.
There is limited information on actual dietary exposure to
benomyl and carbendazim. Dietary exposure has been estimated in the
Netherlands and the USA. In the USA, exposure based on dietary
habits, measured residue levels, and the percentage of crop treated
has been calculated for various subgroups of population. These
calculations indicate that the estimated benomyl exposure is
0.144-1.479 µg/kg per day (section 5.2.1). In the Netherlands, the
mean dietary intake was estimated to be 0.83 µg/kg per day (0.05
mg/day per person). These levels of exposure are below the
recommended ADI of 0.01 (carbendazim) and 0.02 (benomyl) mg/kg body
weight.
The average air levels of benomyl and carbendazim have been
determined in a manufacturing facility (section 5.3) and found to be
less than 0.2 and 0.3 mg/m3, respectively. Both values are below
the Threshold Limit Value of 5-10 mg benomyl/m3 established by a
number of governmental agencies.
In one study, potential respiratory and dermal exposure to
benomyl wettable powder formulation was determined under several
agricultural use situations (section 5.3). The highest rates of
exposure occurred in situations of mixing and loading in preparation
for aerial application; dermal and respiratory exposures were,
respectively, 26 and 0.08 mg/person per cycle. Home users and
agricultural workers re-entering treated fields were estimated to be
exposed to about 1 mg/person per cycle and 5.9 mg/person per hour,
principally through the dermal route.
Because of the low mammalian toxicity, acute benomyl or
carbendazim poisonings are unlikely to occur under conditions of
normal use.
Several studies of agricultural workers (section 8.2) have
shown some cases of contact dermatitis after exposure to benomyl.
These effects can be significantly reduced or eliminated by wearing
long-sleeved shirts, long trousers and gloves.
There is little information on health effects in humans as a
consequence of exposure to either benomyl or carbendazim. Two
studies have been conducted on factory workers involved in the
manufacture of benomyl (section 8.2). In one study, haematological
profiles from 50 factory workers involved in the manufacture of
benomyl were comparable to those from a control group of 48 workers.
A second study found no decrease in the birth rate of the wives of
298 factory workers exposed to benomyl.
Extensive studies of various species of laboratory animals show
reproductive, developmental, mutagenic and carcinogenic effects
associated with both benomyl and carbendazim. The effects observed
on rat fetuses were microphthalmia, hydrocephaly and encephaloceles.
The no-observed-effect levels (NOEL) for developmental toxicity are
equal to or greater than 10 mg/kg body weight per day, depending
upon the species and route of administration. Similarly, the NOEL of
benomyl for reproductive effects in the male rat appears to be 15
mg/kg body weight per day after gavage dosing. In feeding studies
with both benomyl and carbendazim, the NOEL appears to be 500 mg/kg
diet (equivalent to 25 mg/kg body weight per day). However, one
benomyl feeding study reported a NOEL of less than 1 mg/kg diet
(0.05 mg/kg body weight per day) for male reproductive effects. The
reason for the discrepancy between the NOEL in this latter study and
other investigations is unknown.
The only consistent genotoxic effect noted in animal studies is
the induction of numerical chromosomal aberrations. These effects
are consistent with the interaction of benomyl and carbendazim with
microtubule formation.
Rat carcinogenicity studies did not show any carcinogenic
effect for either compound. Benomyl and carbendazim induce
hepatocellular tumours in CD-1 and SPF Swiss mice but not in NMRKf
mice. This finding in mice is not considered to be a result of a
direct genotoxic action. Rather, it appears to be associated with
liver toxicity in strains of mice that are highly susceptible to
tumour formation at this site.
Benomyl and carbendazim are spindle poisons. Effects on target
cells are consequences of binding to microtubules, giving toxicities
similar to those of other spindle poisons such as colchicine and
vincristine. Benzimidazol compounds in general and benomyl and
carbendazim in particular have selective effects on the microtubules
of different eukaryotes. Reasons for this selectivity include the
binding capability to different tubulins and pharmacokinetic
differences across species. In vitro concentrations of benomyl
used to kill sensitive fungi were found to be ineffec tive in
disturbing mammalian microtubular functions. These studies on the
mechanism of action of benomyl and carbendazim indicate a selective
effect of these compounds for target species.
In summary, the LD50, as determined in a number of test
species, for benomyl ranges from > 2000 to > 12 000 mg/kg and for
carbendazim from > 2000 to > 15 000 mg/kg. There are no known
reports of human poisoning for either compound. This, coupled with
the low estimated environmental levels of both compounds, would
suggest that the possibility of acute poisoning by benomyl or
carbendazim is very remote. Similarly, the data available on test
species make it unlikely that either benomyl or carbendazim is
carcinogenic for humans. The NOELs for both reproductive and
teratogenic effects of benomyl and carbendazim (i.e. 10-15 mg/kg) do
raise a possibility that an accidental ingestion of either fungicide
could adversely alter reproductive outcome in humans, but the
likelihood that such poisoning would occur is remote. The
selectivity of these two benzamidazol compounds for the tubulin of
the target species (fungi) and their relative ineffectiveness to
disturb mammalian microtubule function further reduce the
possibility of their having toxic effects in humans.
10.2 Evaluation of effects on the environment
Benomyl is rapidly converted to carbendazim in various
environmental compartments, the half-lives being 2 and 19 h in water
and soil, respectively. Therefore, data from studies on both benomyl
and carbendazim are relevant for the evaluation of environmental
effects.
Carbendazim persists on leaf surfaces and in leaf litter. In
soil the half-life is between 3 and 12 months, and the compound may
be detected for up to 3 years. However, in many cases, major
residues will be lost within a single season. Residues of
carbendazim and its metabolites are strongly bound or incorporated
into soil organic matter. The strong adsorption (Koc =
approximately 2000) of carbendazim to soil and sediment particles
reduces its bioavailability to terrestrial and aquatic organisms.
Similarly, the mobility of carbendazim in soil is limited, and it is
not expected to leach to ground water.
Benomyl and carbendazim are highly toxic to some aquatic
organisms in laboratory tests, the most sensitive species being the
channel catfish with a 96-h LC50 for yolk-sac fry of 0.006 mg
benomyl/litre. However, this toxicity is unlikely to be manifest in
the environment for most aquatic organisms because of the low
bioavailability in surface waters. The exposure of sediment-living
organisms could be greater, but no test results are available for
these organisms.
Benomyl and carbendazim affect groups of fungi in soil but do
not seem to modify the overall microbial activity of the soil when
used at normal field rates.
In both the laboratory and field, benomyl and carbendazim
applied at recommended rates cause deaths and sublethal reproductive
effects on earthworms of many different species. Surface-feeding
species eating leaf litter are most at risk. Populations may take
more than 2 years to recover. There are no studies available on
other litter and soil invertebrates.
Benomyl and carbendazim have low toxicity for birds and
carbendazim is classified as "relatively non-toxic" to honey-bees.
10.3 Conclusions
Benomyl causes dermal sensitization in humans. Both benomyl and
carbendazim represent a very low risk for acute poisoning in humans.
Given the current exposures and the low rate of dermal absorption of
benomyl and carbendazim, it is unlikely that they would cause
systemic toxicity effects either in the general population or in
occupationally exposed subjects. These conclusions are drawn from
animal data and from the limited human data available, but these
extrapolations are supported by the understanding of the mode of
action of carbendazim and benomyl in both target and non-target
species.
Further elucidation of the mechanism of toxicity of carbendazim
and benomyl in mammals will perhaps permit a better determination of
no-observed-effect levels. Binding studies on tubulins of target
cells (testis and embryonic tissues) will facilitate comparisons
across species.
Carbendazim is strongly adsorbed to soil organic matter and
remains in the soil for up to 3 years. It persists on leaf surfaces
and, therefore, in leaf litter. Earthworms have been shown to be
adversely affected (population and reproductive effects) at
recommended application rates. There is no information on other soil
or litter arthropods that would be similarly exposed.
The high toxicity to aquatic organisms in laboratory tests is
unlikely to be seen in the field because of the low bioavailability
of sediment-bound residues of carbendazim. However, no information
is available on sediment-living species which would receive the
highest exposure.
11. FURTHER RESEARCH
1. Comparative binding studies of carbendazim to tubulins of
target tissues from various species should be undertaken.
2. Further clarification of the fate of 1,2-diaminobenzene and
bound residues in the environment is needed.
3. The effects of benomyl and carbendazim on sediment-dwelling
organisms needs to be investigated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Benomyl was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1973, 1975, 1978, 1983 and 1988. The 1978 meeting
agreed that the MRLs for benomyl, carbendazim and thiophanate-methyl
should be combined and expressed as carbendazim. Benomyl residues
were last evaluated by the 1988 meeting (FAO/WHO, 1988a,b) and the
MRLs were updated at that time. These MRLs (expressed as
carbendazim) are listed in Table 4. The 1983 meeting (FAO/WHO,
1985a) evaluated benomyl toxicology and set the following benomyl
NOEL levels and ADI:
Rat: 2500 mg/kg in the diet, equivalent to 125 mg/kg body
weight
Dog: 100 mg/kg (carbendazim) in the diet, equivalent to 2.5
mg/kg body weight
Rat: teratology - 30 mg/kg body weight per day
The estimated ADI for benomyl was established at 0-0.02 mg/kg
body weight.
Benomyl has not been evaluated by the International Agency for
Research on Cancer (IARC).
REFERENCES
Albertini S (1991) Reevaluation of the 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae aneuploidy
test systems by the Gene-Tox Program using strain D61.m of
Saccharomyces cerevisiae. Mutat Res, 260: 165-180.
Amacher DE, Paillet S, & Ray VA (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Application
to genetic toxicological testing. Mutat Res, 64: 391-406.
Ammon HU (1985) Worm toxicity tests using Tubifex tubifex. In:
Comportement et effets secondaires des pesticides dans le sol. Les
Colloques de l'INRA 31, Versailles, 4-8 juin 1984. Paris, Institut
national de la Recherche agronomique, pp 303-317.
Armstrong DA, Buchanan DU, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp. in laboratory-reared larvae of the Dungeness crab,
Cancer magister, and possible chemical treatments. J Invertebr
Pathol, 28: 329-336.
Arthur MF, Schweitzer KL, Fadel LC, Marsh BH, & Marsh SS (1989a)
Aerobic aquatic metabolism of [phenyl(U)-14C]benomyl in
Greenville, Mississippi, water and sediment (Battelle Study No.
N-0966-7301). Columbus, Ohio, Battelle, Environmental Sciences
Department (Unpublished report No. AMR-1452-89, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Arthur MF, Marsh BH, Fadel LC, & Zwick TC (1989b) Anaerobic aquatic
metabolism of [phenyl(U)-14C]benomyl in West Jefferson, Ohio, pond
water and sediment (Battelle Study No. NO799-8800). Columbus, Ohio,
Battelle, Environmental Sciences Department (Unpublished report No.
AMR 770-87, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Athwal RS & Sandhu SS (1985) Use of human X mouse hybrid cell line
to detect aneuploidy induced by environmental chemicals. Mutat Res,
149: 73-81.
Barbo EC & Carroll KS (1972) Oral ALD test (S-triazine,
3-butylbenzimidazolo[1,2-a],-2,4[1H,3H]-dione. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 227-72).
Barefoot AC (1988) Vapor pressure of benomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Research & Development
Division, Experimental Station (Unpublished report No. AMR 1078-88).
Barnes TB, Verlangieri AJ, & Wilson MC (1983) Reproductive toxicity
of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate (benomyl) in
male Wistar rats. Toxicology, 28: 103-115.
Baude JF, Gardiner JA, & Han JCY (1973) Characterization of residues
on plants following foliar spray applications of benomyl. J Agric
Food Chem, 21: 1084-1090.
Baude FJ, Pease HL, & Holt RF (1974) Fate of benomyl on field soil
and turf. J Agric Food Chem, 22: 413.
Belasco IJ (1979a) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX-4).
Belasco IJ (1979b) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied with supplement.
Wilmington, Delaware, Du Pont de Nemours and Co., Inc. (Unpublished
report No. B/ME-47 and supplement No. HLR 117-79).
Belasco IJ, Kirkland JJ, Pease HL, & Sherman H (1969) Studies with
2-14C-labelled methyl-1-(butylcarbamoyl)-2-benzimidazolecarbamate
(benomyl) in rats. Wilmington, Delaware, Du Pont de Nemours and Co.,
Inc., Biochemical Department, Research Division, Experimental
Station (Unpublished report No. B/ME-36).
Bignami M, Aulicino F, Velcich A, Carere A, & Morpurgo G (1977)
Mutagenic and recombinogenic action of pesticides in Aspergillus
nidulans. Mutat Res, 46: 395-402.
Bionomics, Inc. (1972) Acute toxicity of Benlate to grass shrimp
( Palaemonetes vulgaris). Wareham, Massachusetts, Bionomics, Inc.
(Unpublished report No. HOL 275-72, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Boeri RL (1988a) Flow through acute toxicity of benomyl technical to
the eastern oyster, Crassostrea virginica. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 13-89, prepared
for E.I. Du Pont de Nemours and Co., Inc.).
Boeri RL (1988b) Static acute toxicity of benomyl technical to the
sheepshead minnow, Cyprinodon variegatus. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 9-89, prepared for
Du Pont de Nemours and Co., Inc.).
Boeri RL (1988c) Static acute toxicity of benomyl technical to the
mysid, Mysidopsis bahia. Marblehead, Massachusetts, Enseco
(Unpublished report No. HLO 828-88, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Bolton EE, Anderson JJ, & Koeppe MK (1986a) Metabolism of
[phenyl(U)-14C] benomyl in field-grown soybeans. Wilmington,
Delaware, Du Pont de Nemours and Co., Inc., Agricultural Products
Department, Experimental Station (Unpublished report No.
AMR-531-86).
Bolton EE, Anderson JJ, & Koeppe MK (1986b) Metabolism of
[phenyl(U)-14C] benomyl in paddy rice. Wilmington, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Agricultural Products Department,
Experimental Station (Unpublished report No. AMR-507-86).
Brock WJ (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 505-87).
Busey WM (l968a) Acute dermal LD50 test and dermal irritation test
on rabbits using a wettable powder formulation (50% benomyl) with
histological addendum. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Falls Church, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. MRO 1126-1,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968c) Teratology study in rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 1079, prepared for
E.I Du Pont de Nemours and Co., Inc.). Busey WM (l968d) Repeated
dermal application test on rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 298-68, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (1968e) Acute dietary administration - mallard ducklings
and bobwhite quail: Fungicide 1991. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Bushway RJ, Hurst HL, Kugabalasooriar J, & Perkins LB (1991)
Determination of carbendazim in blueberries by reversed-phase high
performance liquid chromatography. J Chromatogr, 555: 321-324.
Canton JH (1976) The toxicology of benomyl, thiophanate-methyl, and
BCM to four freshwater organisms. Bull Environ Contam Toxicol, 16:
214-218.
Carere A, Ortali VA, Cardamone G, Torracca AM, & Raschetti R (1978)
Microbiological mutagenicity studies of pesticides in vitro. Mutat
Res, 57: 277-826.
Carter SD (l982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Raleigh, North Carolina, North Carolina
State University (Thesis).
Carter SD & Laskey JW (l982) Effect of benomyl on reproduction in
the male rat. Toxicol Lett, 11: 87-94.
Chang WM (1985) Soil column leaching studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Agricultural Chemicals Department, Experimental Station
(Unpublished report No. AMR 426-85).
Chiba M & Veres DF (1981) Fate of benomyl and its degradation
compound methyl 2-benzimidazole carbamate on apple foliage. J Agric
Food Chem, 29: 588-590.
Clermont Y (1972) Kinetics of spermatogenesis in mammals:
"seminiferous epithelium cycle and spermatogonial renewal". Physiol
Rev, 52: 198-236.
Colburn CW (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 84-69).
County Natwest Woodmac (1992) The fungicide market. London, County
Natwest Woodmac, Agrochemical Service, pp 49-51.
Culik R (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the concepti
of rats exposed to benomyl and Benlate by diet. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 916-80).
Culik R (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. HLR 970-80).
Culik R & Gibson JR (1974) "Benlate" dominant lethal study in male
rats with supplemental statistical report (22-77). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 72-74).
Cummings AM, Harris ST, & Rehnberg GL (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat. Fundam
Appl Toxicol, 15: 528-535.
Dashiell OL (1972) Acute oral test (benzimidazole,
2-(3-butylureido)). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 456-72).
Davidse LC & Flach W (1977) Differential binding of methyl
benzimidazol-2-yl carbamate to fungal tubulin as a mechanism of
resistance to this antimitotic agent in mutant strains of
Aspergillus nidulans. J Cell Biol, 72: 174-193.
De Bertoldi M & Griselli M (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat Res, 74:
303-324.
De Bertoldi M, Griselli M, Giovannetti M, & Barale R (1980)
Mutagenicity of pesticides evaluated by means of gene-conversion in
Saccharomyces cerevisiae and in Aspergillus nidulans. Environ
Mutagen, 2: 359-370.
De Brabander M, Van de Veire R, Aerts F, Geuens S, & Hoebeke J
(1976a) A new culture model facilitating rapid quantitative testing
of mitotic spindle inhibition in mammalian cells. J Natl Cancer
Inst, 56: 357-363.
De Brabander M, Van de Veire R, Aerts F, Brogers M, & Janssen PAJ
(1976b) The effect of methyl [5(2-thienylcarbonyl)-1H-benzimidazol-
2-yl carbamate (17934; NSC 238159), a new synthetic antitumoral drug
interfering with microtubules, on mammalian cells cultured in
vitro. Cancer Res, 36: 905-916.
Delp CJ (1980) Coping with resistance to plant disease control
agents. Plant Dis, 64: 652-657.
Dési I (1979) Hygienic-toxicological evaluations of pesticides.
Budapest, National Institute of Hygiene (D.Sc. Thesis).
Dési I (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav Toxicol Teratol, 5: 503-515.
Dési I, Dura G, Szlobodnyik J, & Csuka I (1977) Testing of pesticide
toxicity in tissue culture. J Toxicol Environ Health, 2: 1053-1066.
Dési I, Nehéz M, Palotas M, Tempfli A, Hogye A, & Vetro G (1990)
Experience of health status surveillance of pesticide workers in
Hungary. Med Lav, 81(6): 517-523.
Donovan SD & Krahn DF (1981) Mutagenic evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 343-81).
Douglass MT & Handley JW (1988) The algistatic activity of benomyl
technical. Huntingdon, United Kingdom, Huntingdon Research Centre
Ltd (Unpublished report No. DPT 171(n)/88371, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Draize JH, Woodard G, & Gawery HO (1944) New methods for the study
of irritation and toxicity of substances applied topically to the
skin and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Drewes CD, Zoran MJ, & Callahan CA (1987) Sublethal neurotoxic
effects of the fungicide benomyl on earthworms (Eisenia fetida).
Pestic Sci, 19: 197-208.
Du Pont (1972) Residue studies - fish: benomyl, MBC, and 2-AB.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report).
Du Pont (1987) Determination of octanol water partition coefficient
for benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/PC-55-CA).
Eastmond DA & Tucker JD (1989) Kinetochlore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat Res, 224: 517-525.
Edwards PJ & Brown SM (1982) Use of grassland plots to study the
effect of pesticides on earthworms. Pedobiologia, 24: 145-150
Eickhoff JC, Petersen BJ, & Chaisson CF (1989) Anticipated residues
of benomyl in food crops and potential dietary exposure and risk
assessment. Washington, DC, Technical Assessment System, Inc.
(Unpublished report No. TAS 000-005, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Ellis WG, Semple JL, Hoogenboom ER, Kavlock RJ, & Zeman FJ (1987)
Benomyl-induced craniocerebral anomalies in fetuses of adequately
nourished and protein -deprived rats. Teratog Carcinog Mutagen, 7:
357-375.
Ellis WG, De Roos F, Kavlock RJ, & Zeman FJ (1988) Relationship of
periventricular overgrowth to hydrocephalus in brains of fetal rats
exposed to benomyl. Teratog Carcinog Mutagen, 8: 377-391.
Evans EL & Mitchell AD (1980) An evaluation of the effect of benomyl
on sister chromatic exchange frequencies in cultured Chinese hamster
ovary cells. Menlo Park, California, SRI International (Unpublished
report prepared for E.I. Du Pont de Nemours and Co., Inc.).
Everhart LP (1979) Benlate dust exposure survey. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Biochemical
Department (Unpublished report No. B/TOX 6).
Everhart LP & Holt RF (1982) Potential benlate fungicide exposure
during mixer/loader operations, crop harvest and home use. J Agric
Food Chem, 30: 222-227.
FAO/WHO (1985a) Benomyl. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 7-46 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1985b) Carbendazim. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 89-121 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1988a) Benomyl. In: Pesticide residues in food -
Evaluations 1988. Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 5-15 (FAO Plant Production
and Protection Paper 93/1).
FAO/WHO (1988b) Carbendazim. In: Pesticide Residues in Food -
Evaluations 1988: Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 41-54 (FAO Plant Production
and Protection Paper 93/1).
Fielding M, Barcelo D, Helweg A, Galassi S, Torstensson L, van
Zoonen P, Wolter R, & Angeletti G (1992) Pesticides in ground and
drinking water. Brussels, Commission of the European Communities,
Directorate-General for Science, Research and Development,
Environmental and Waste Recycling, 135 pp (Water Pollution Research
Report 27).
Fiscor G, Bordas S, & Stewart SJ (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl-N'-nitro-N-nitrosoguanine in Salmonella typhimurium in
vitro and in rodent host-mediated assays. Mutat Res, 51: 151-164.
Fitzpatrick K (1980) Chinese hamster ovary cell assay for
mutagenicity. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 438-80).
Frame SR & Van Pelt CS (1990) Oncogenicity studies with benomyl and
MBC in mice: Supplemental peer review. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 20-82 and supplement No. HLR 70-82).
Frank KM (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 85-69).
Frank KM (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 233-72).
Fritz SB (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 109-69).
Fritz SB & Sherman H (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 51-69).
Gargus JL & Zoetis T (1983a) Primary irritation study in rabbits
(Benlate PNW). Vienna, Virginia, Hazelton Laboratories, Inc.
(Unpublished report No. HLO 510-83, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983b) Eye irritation test in rabbits (EPA
pesticide registration - Benlate PNW). Vienna, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 511-83, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983c) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines - Benlate PNW).
Vienna, Virginia, Hazelton Laboratories, Inc. (Unpublished report
No. HLO 512-83, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Vienna, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. HLO 67-84,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Georgieva V, Vachkova R, Tzoneva M, & Kappas A (1990) Genotoxic
activity of benomyl in different test systems. Environ Mol Mutagen,
16: 32-36.
Goldenthal EI (1978) Neurotoxicity study in hens [using technical
benomyl (< 95% benomyl). Mattawan, Michigan, International Research
and Development Corporation (Unpublished report and addendum No. HLO
28-79, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gooch JJ (1978) Fertility of workers potentially exposed to benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX 7).
Goodman NC (1975) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 847-74).
Goulding R (1983) Poisoning on the farm. J Soc Occup Med, 33: 60-65.
Guengerich FP (1981) Enzyme induction with Du Pont compounds H11,
202-02 and H10, 962-02. Nashville, Tennessee, Vanderbilt University,
School of Medicine (Unpublished report No. HLO 850-81, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gurova AI, Alekseeva NP, Gorlova OE, & Chernyshova RA (1976) [Data
for substantiation of the maximum permissible level of
m-(trifluoromethyl) phenyl isocyanate and butyl isocyanate in the
air of work areas.] Gig Tr Prof Zabol, 3: 53-55 (in Russian).
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 80: 339-357.
Han JCY (1978) Metabolism of 14C-labeled benomyl in the mouse and
hamster. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-65).
Han JCY (1979) 2-14C-Benomyl (50% WP) rat study - intravenous
injection. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-41).
Han JCY (1980) Metabolism of 2-14C-benomyl in the lactating nanny
goat. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report No. B/ME-39).
Hardesty PT (1982) Attempts to characterize liver residues from
14C-benomyl dosed goat. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Biochemicals Department (Unpublished report
No. AMR 71-82).
Hardisty JF (1990) Oncogenicity studies with benomyl and MBC in
mice. Peer-review of liver neoplasms. Research Triangle Park, North
Carolina, Experimental Pathology Laboratories, Inc. (Unpublished
report No. 129-012, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Hastie AC (1970) "Benlate"-induced instability of Aspergillus
diploids. Nature (Lond), 226: 771.
Helweg A (1977) Degradation and absorption of carbendazim and
2-aminobenzimidazole in soil. Pestic Sci, 8: 71-78.
Helweg A (1985) Side effects caused by pesticide combinations. In:
Jensen V, Kjoller A, & Sorensen LH ed. Proceedings of the FEMS
Symposium on Microbial Communities in Soil. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 385-395.
Hess RA, Moore BJ, Forrer J, Linder RE, & Abuel-Atta AA (1991) The
fungicide benomyl (methyl) 1-(butylcarbomyl)-2-benzimidazole
carbamate causes testicular dysfunction by inducing the sloughing of
germ cells and occlusion of efferent ductules. Fundam Appl Toxicol,
17: 733-745.
Hill EF & Camardese MB (1986) Lethal toxicities of environmental
contaminants and pesticides to Coturnix. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
Hofer I, Beck T, & Wallnöfer P (1971) [The influence of the
fungicide benomyl on soil microflora.] Z Pflanzenkr Pflanzenschutz,
78: 399-405 (in German).
Holden HE, Crider PA, & Wahrenburg MG (1980) Mitotic arrest by
benzimidazole analogs in human lymphocyte cultures. Environ Mutat,
2: 67-73.
Hood DB (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 211-69).
Hoogenboom ER, Ransdell JF, Ellis WG, Kavlock RJ, & Zeman FJ (1991)
Effects on the fetal rat eye of maternal benomyl exposure and
protein malnutrition. Curr Eye Res, 10: 601-612.
Hornberger CS (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
95-69).
Hostetler KH (1977) Oral LD50 test (Benlate OD). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 527-77).
Hutton DG (1989) Static acute 48-hour EC50 of Benlate fungicide 50
DF to Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 541-88).
Hutton DG, Kasprzak DJ, & Priester TM (1984) Laboratory studies of
[2-14C] carbendazim bioconcentration in Bluegill sunfish. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 428-84).
ILO (1991) Occupational exposure limits for airborne toxic
substances, 3rd ed. Geneva, International Labour Office
(Occupational Safety and Health Series No. 37).
Ireland CM, Gull K, Gutteridge WE, & Pogson CI (1979) The
interaction of benzimidazole carbamates with mammalian microtubule
protein. Biochem Pharmacol, 28: 2680-2682.
Jessup CD (1979) Acute delayed neurotoxicity study in chickens using
technical benomyl (> 95% benomyl). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report No. HLO 674-79, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Jessup DC & Dean W (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl). Mattawan,
Michigan, International Research and Development Corporation
(Unpublished report No. HLO 29-79, prepared for E.I. Du Pont de
Nemours and Co., Inc).
Johnson JD (1988) Determination of the plateau level of bound
[phenyl(U)-14C] carbendazim residues in goat liver. Columbus,
Ohio, Batelle, Columbus Division (Unpublished report No. AMR 779-87,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Kaastra-Howeler LH & Gams W (1973) Preliminary study on the effect
of benomyl on the fungal flora in a greenhouse soil. Neth J Plant
Pathol, 79: 516-518.
Kappas A & Bridges BA (1981) Induction of point mutations by benomyl
in DNA-repair-deficient Aspergillus nidulas. Mutat Res, 91:
115-118.
Kappas A, Georgopoulos SG, & Hastie AC (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat Res, 26: 17-27.
Kappas A, Green MHL, Bridges BA, Rogers AM, & Muriel WJ (1976)
Benomyl - a novel type of base analogue mutagen? Mutat Res, 40:
379-382.
Kavlock RJ, Chernoff N, Gray LE, Gray JA, & Whitehouse D (1982)
Teratogenic effects of benomyl in the Wistar rat and CD-1 mouse,
with emphasis on the route of administration. Toxicol Appl
Pharmacol, 62: 44-54.
Kelly DP (1989) Butyl isocyanate industrial hygiene survey. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. BENO/TOX 18).
Keogh RG & Whitehead PH (1975) Observations on some effects of
pasture spraying with benomyl and carbendazim on earthworm activity
and litter removal from pasture. NZ J Exp Agric, 3: 103-104.
Kirkland JJ (1973) Method for high-speed liquid chromatographic
analysis of benomyl and/or metabolite residue in cow milk, urine,
feces and tissues. J Agric Food Chem, 21: 171-177.
Kirkland JJ, Holt RF, & Pease HL (1973) Determination of benomyl
residues in soils and plant tissues by high speed cation exchange
liquid chromatography. J Agric Food Chem, 21: 368-371.
Krechniak J & Klosowska B (1986) The fate of 14C-carbendazim in
rat. Xenobiotica, 16(9): 809-815.
Krupka RM (1974) On the anti-cholinesterase activity of benomyl.
Pestic Sci, 5: 211-216.
Kuehne G, Heise H, Plottke B, & Puskeiler T (1985) Dermatitis after
benlate contact. Z Gesamte Hyg Grenzgeb, 31: 710-711.
Lamb MJ & Lilly LJ (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17:
83-95.
Lee KP (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 66-77).
Liesivuori J & Jääskeläinen S (1984) Exposure of greenhouse workers
to pesticides. Tampore, Finland, National Board of Labor Protection,
pp VIII-IX (Research Report No. 46).
Linder RE, Rehnberg GL, Strader LF, & Diggs JP (1988) Evaluation of
reproductive parameters in adult male Wistar Rats after subchronic
exposure. J Toxicol Environ Health, 25: 285-298.
Lisi P, Caraffini S, & Assalve D (1986) A test series for pesticide
dermatitis. Contact Dermatitis, 15: 266-269.
Littlefield NA & Busey WM (1969) Four-hour acute inhalation exposure
test in dogs using a wettable powder formulation (50% benomyl).
Falls Church, Virginia, Hazelton Laboratories, Inc. (Unpublished
report No. HLR 192-69, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983a) L5178Y mouse
lymphoma cell assay for mutagenicity (benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 86-83).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983b) L5178Y mouse
lymphoma cell assay for mutagenicity. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc. (Unpublished report No. HLR 253-83).
Majut JC (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 174-06).
Marsh BH & Arthur MF (1989) Aerobic metabolism of
[phenyl(U)-14C]benomyl in Keyport Silt Loam. Columbus, Ohio,
Battelle Memorial Institute (Unpublished report No. AMR 1112-88,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Marvin CH, Birndle ID, Hall CD, & Chiba M (1991) Rapid on-line
precolumn high performance liquid chromatographic method for the
determination of benomyl, carbendazim and aldicarb species in
drinking water. J Chromatogr, 555: 147-154.
Matsushita T & Aoyama K (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind Health,
19: 77-83.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp 45-56 (Resource Publication No. 160).
Mazur AR & Hughes TD (1975) Nitrogen transformation in soil as
affected by the fungicides benomyl, dyrene and maneb. Agron J, 67:
755-758.
Meade AB (1984) Acute contact LD50 toxicity study in honey bees
( Apis mellifera) with INE 965-212 (MBC). Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc. (Unpublished report No. AMB
84-5).
Mebus CA (1990) Reproductive and fertility effects with DPX-1991-529
(benomyl). Multigeneration reproduction study in rats. Newark,
Delaware, Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 765-90).
Monson KD (1985) Metabolism of [2-14C]benomyl in the lactating
dairy cow. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc. (Unpublished report No. AMR 247-84).
Monson KD (1986a) Metabolism of [2-14C]benomyl and [phenyl(U)-
14C]benomyl in laying hens. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc. (Unpublished report No. AMR 391-85).
Monson KD (1986b) Metabolism of [2-14C]carbendazim laying hens.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 264-84).
Monson KD (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 1141-88).
Monson KD (1991) Release and characterization of bound benomyl and
carbendazim metabolites in animal tissues via Raney nickel
desulfurization and acid dehydration. J Agric Food Chem, 39:
1808-1811.
Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, & Linder RE (in
press) Acute and long-term effects of the fungicide carbendazim
(methyl 2-benzimidazole carbamate, MBC) on the male reproductive
system in the rat. J Androl.
Nehéz M, Palotas M, Zimanyi M, Boros P, Mohos G, Vetro G, & Desi I
(1992) [Repeated cytogenetic examinations of workers using
pesticides in Csongrad County.] Egeszsegtudomany, 36: 40-47 (in
Hungarian).
Newsome WH & Collins PG (1987) Enzyme-linked immunosorbent assay of
benomyl and thiobendazole in some foods. J Assoc Off Anal Chem, 70:
1025-1027.
Newsome WH & Shields JB (1981) A radioimmunoassay for benomyl and
methyl 2-benzimidazolecarbamate on food. J Agric Food Chem, 29:
220-222.
Palawski DU & Knowles CO (1986) Toxicological studies of benomyl and
carbendazim in rainbow trout, channel catfish and bluegills. Environ
Toxicol Chem, 5: 1039-1046.
Parsons DW & Witt JM (1988) Pesticides in groundwater in the United
States of America: A report of a 1988 survey of State lead agencies.
Corvallis, Oregon, Oregon State University Extension Service.
Peeples JL (1974) Microbial activity in benomyl-treated soil.
Phytopathology, 64: 857-860.
Pilinskaya MA (1983) Investigation of the cytogenetic action of the
pesticides captan and benomyl in a culture of human peripheral blood
lymphocytes in the absence and presence of a system of metabolic
activation. Cytol Genet, 17: 29-33.
Powley CR (1985) Aqueous photolysis of [phenyl-14C(U)]benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 420-85).
Priester TM (1984) Hydrolysis of carbendazim [2-14C]. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 265-84).
Priester TM (1985) Batch equilibrium (adsorption/deabsorption) and
soil thin-layer chromatography studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 425-85).
Rainaldi G, Flori L, Colella CM, Mariani T, Piras A, Simi S, &
Simili M (1989) Analysis by BrUdR-labelling technique of induced
aneuploidy in mammalian cells in culture. Mutat Res, 177: 255-260.
Reinke RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
81-66).
Rhodes BC (1987) Greenhouse crop-rotation study with
[2-14C]carbendazim. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Inc. (Unpublished report No. AMR 495-86).
Rickard LB (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 97-83).
Rickard LB (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 98-83).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to
the earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Russell JF (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 18-78).
Russell JF (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 31-78).
Ruzicska P, Peter S, Laczi J, & Czeizel E (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP. Egeszegtudomany, 20:
74-83.
Ryan DL (1989) Soil column leaching of [phenyl(U)-14C]benomyl in a
rice paddy soil. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 1512-89).
Sandhu SS, Gudi RD, & Athwal RS (1988) A monochromosomal hybrid cell
assay for evaluating the genotoxicity of environmental chemicals.
Cell Biol Toxicol, 4: 495-506.
Sarver JW (1987) Acute oral toxicity study with IN-T1991 in male and
female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. HLR 334-87).
Sasaki YFX (1990) Benomyl: Micronucleus test in mice. Tokyo,
Institute of Environmental Toxicology, Kodaira Laboratories
(Unpublished report No. IET 89-0046, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Seiler JP (1976) The mutagenicity of benzimidazole and benzimidazole
derivatives. VI. Cytogenetic effects of benzimidazole derivatives in
the bone marrow of the mouse and the Chinese hamster. Mutat Res, 40:
339-348.
Sherman H (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 269-68).
Sherman H (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 17-69).
Sherman H (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
168-69).
Sherman H (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 232-69).
Sherman H (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 48-70).
Sherman H (1972) Long-term feeding studies in rats and dogs with
2-benzimadazolecarbamic acid, methyl ester (INE-965) (50% and 70%
MBC wettable powder formulations): Parts I and II. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 195-72).
Sherman H & Krauss WC (1966) Acute oral test [benomyl]. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 100-66).
Sherman H, Barnes JR, & Krauss WC (1967) Ninety-day feeding study
with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell, Laboratory (Unpublished report No. HLR 11-67).
Sherman H, Culik R, & Jackson RA (1975) Reproduction, teratogenic
and mutagenic studies with benomyl. Toxicol Appl Pharmacol, 32:
305-315.
Shirasu Y, Moriya M, & Kato K (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Tokyo, Institute of
Environmental Toxicology, Kodaira Laboratories (Unpublished report
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Shukla Y, Antony M, & Mehrota NK (1989) Studies on gamma-glutamyl
transpeptidase in rodents exposed to benomyl. Bull Environ Contam
Toxicol, 42: 301-306.
Siebert D, Zimmermann FK, & Lemperle E (1970) Genetic effects of
fungicides. Mutat Res, 10: 533-543.
Siegel MR (1975) Benomyl-soil microbial interactions.
Phytopathology, 65: 219-220.
Snee DA (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test described in HLR 43-69).
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. 134-69).
Stahl RG Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 401-90).
Staples RE (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl):
Parts I, II and III. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 649-80).
Staples RE (1982) Teratogenicity study in the rat using technical
benomyl (>95% benomyl) administered by gavage and supplement with
individual animal data. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
587-82).
Staub T & Sozzi D (1984) Fungicide resistance: A continuing
challenge. Plant Dis, 68: 1026-1031.
Stevenson IE (1985) Metabolism of [phenyl(U)-14C]benomyl in
peaches. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 443-85).
Stringer A & Lyons C (1974) The effect of benomyl and
thiophanate-methyl on earthworm populations in apple orchards.
Pestic Sci, 5: 189-196.
Stringer A & Wright MA (1973) The effect of benomyl and some related
compounds on Lumbricus terrestris and other earthworms. Pestic
Sci, 4: 165-170.
Tolle DA (1988) Metabolism of [phenyl(U)-14C] benomyl in sugar
beets. West Jefferson, Ohio, Battelle Columbus DW (Unpublished
report No. AMR 620-86, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a
fungicide on the numbers and biomass of earthworms in pasture. Bull
Environ Contam Toxicol, 12: 487-492.
Tomlin AD, Tolman JH, & Thorn GD (1980/1981) Suppression of
earthworm ( Lumbricus terrestris) populations around an airport by
soil application of the fungicide benomyl. Prot Ecol, 2: 319-323.
Tong C (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Valhalla, New York, Naylor Dana Institute (Unpublished report No.
HLO 741-81, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Torstensson L & Wessen B (1984) Interactions between the fungicide
benomyl and soil microorganisms. Soil Biol Biochem, 16: 445-452.
Turney RT (1979) Rat inhalation study - Benlate. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 116-79).
Van Faassen HG (1974) Effect of the fungicide benomyl on some
metabolic processes and on numbers of bacteria and actinomycetes in
the soil. Soil Biol Biochem, 6: 131-133. Van Gestel CAM (1992)
Validation of earthworm toxicity tests by comparison with field
studies: A review of benomyl, carbendazim, carbofuran, and carbaryl.
Ecotoxicol Environ Saf, 23: 221-236.
Van Gestel CAM, Dirven-van Breeman EM, Baerselman R, Emans HJB,
Janssen JAM, Postuma R, & van Vliet PJM (1992) Comparison of
sublethal and lethal criteria for nine different chemicals in
standardized toxicity tests using the earthworm Eisenia andrei.
Ecotoxicol Environ Saf, 23: 206-220.
Van Joost TH, Naafs B, & van Ketel WG (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9: 153-154.
Vick DA & Brock WJ (1987) Primary dermal irritation study with
benlate 50 DF fungicide in rabbits. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 300-87).
Wainwright M & Pugh GJF (1974) The effect of fungicides on certain
chemical and microbial properties of soils. Soil Biol Biochem, 6:
263-267.
Ward RS & Scott RC (1992) Benomyl: in vitro absorption of a 500 g
kg-1 WP formulation through human epidermis. Fernhurst, Naslemere,
Surrey, Imperial Chemical Industries (ICI) (Unpublished report No.
CTL/P/3659).
Warheit DB, Kelly DP, Carakostas MC, & Singer AW (1989) A 90-day
inhalation toxicity study with benomyl in rats. Fundam Appl Toxicol,
12: 333-345.
Weeks RE & Hedrick HG (1975) Influence of a systemic fungicide on
oxygen uptake by soil microorganisms. Soil Sci, 119: 280-284.
Wheeler J (1985) Hydrolysis of [phenyl-14C(U)]benomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 419-85).
WHO (1993) Environmental Health Criteria 149: Carbendazim. Geneva,
World Health Organization.
Wiechman BE (1982) Long term feeding study with methyl
l-(butylcarbamoyl)-2-benzimidazolecarbamate in mice (INT-1991; >95%
benomyl): Parts I, II and III. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 20-82).
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Bull Agric Chem Insp Stn, 12: 122-128.
Zbozinek JV (1984) Environmental transformations of DPA, SOPP,
benomyl and TBZ. Residue Rev, 92: 113-155.
Zelesco PA, Barbieri I, & Graves JAM (1990) Use of a cell hybrid
test system to demonstrate that benomyl induces aneuploidy and
polyploidy. Mutat Res, 242: 329-335.
Zeman FJ, Hoogenboom ER, Kavlock RJ, & Semple JL (1986) Effects on
the fetus of maternal benomyl exposure in the protein-deprived rat.
J Toxicol Environ Health, 17: 405-417.
Zoran MJ, Heppner TJ, & Drewes CD (1986) Teratogenic effects of the
fungicide benomyl on posterior segmental regeneration in the
earthworm, Eisenia fetida. Pestic Sci, 17: 641-652.
Zweig G, Gao R, & Popendorf W (1983) Simultaneous dermal exposure to
captan and benomyl by strawberry harvesters. J Agric Food Chem, 31:
1109-1113.
RESUME ET CONCLUSIONS
1. Résumé
1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le bénomyl, un solide cristallin de couleur ambrée, est un
fongicide endothérapique qui appartient à la famille du
benzimidazole. Il se décompose juste au-dessus de son point de
fusion de 140 °C et sa tension de vapeur est < 5 x 10-6 Pa
(< 3,7 x 10-8 mmHg) à 25 °C. Le bénomyl est pratiquement
insoluble dans l'eau à pH 5 et à 25 °C, sa solubilité étant de 3,6
mg/litre. Il est stable dans les conditions normales de stockage
mais se décompose en carbendazime dans l'eau.
L'analyse des résidus de même que celle des prélèvements
effectués dans l'environnement comporte une extraction au moyen d'un
solvant organique, une purification de l'extrait par partage
liquide-liquide et la transformation du résidu obtenu en
carbendazime. Le dosage de ces résidus peut s'effectuer par
chromatographie en phase liquide à haute performance ou par titrage
immunologique.
1.2 Sources d'exposition humaine et environnementale
En 1988, on estimait à environ 1700 tonnes la quantité de
bénomyl utilisée dans le monde. Il s'agit d'un fongicide très
largement utilisé, homologué dans 50 pays pour le traitement de plus
de 70 cultures. Le bénomyl est présenté sous forme de poudre
mouillable.
1.3 Transport, distribution et transformation dans l'environnement
Le bénomyl se transforme rapidement en carbendazime dans
l'environnement avec une demi-vie respective de 2 et 19 heures dans
l'eau et le sol. On peut donc utiliser aussi bien les résultats des
études sur le bénomyl que sur le carbendazime pour l'évaluation des
effets sur l'environnement.
Dans l'environnement, le carbendazime se décompose avec une
demi-vie de 6 à 12 mois sur le sol nu, de 3 à 6 mois sur le gazon et
de 2 à 25 mois dans l'eau en aérobiose et en anaérobiose,
respectivement.
Le carbendazime est principalement décomposé par les
microorganismes. Le 2-aminobenzimidazole (2-AB) en est l'un des
principaux produits de dégradation et il est à son tour décomposé
par les microorganismes.
Lors de la décomposition de bénomyl marqué au 14C sur le
noyau phényle, on a constaté que 9% seulement du carbone-14 étaient
éliminés sous forme de CO2 en une année d'incubation. Le
carbone-14 restant était principalement récupéré sous forme de
carbendazime et de résidus liés. L'étude de la destinée d'un
éventuel produit de dégradation (1,2-diaminobenzène) pourrait
peut-être permettre de mieux définir la voie de dégradation des
fongicides benzymidazoliques dans l'environnement.
Des études effectuées sur le terrain ou sur colonnes ont montré
que le carbendazime restait dans les couches superficielles du sol.
On n'a pas mesuré l'adsorption du carbendazime dans le sol, mais on
pense qu'elle doit être aussi forte que dans le cas du bénomyl, avec
des valeurs de Koc allant de 1000 à 3600. Les valeurs de log Kow
sont respectivement de 1,36 et de 1,49 pour le bénomyl et le
carbendazime.
Un modèle de criblage basé sur les données d'adsorption et de
persistance n'a pas révélé de risque de lessivage. Cette observation
est corroborée par des analyses d'eau de puits effectuées aux
Etats-Unis, analyses qui n'ont pas permis de déceler ce composé dans
l'un quelconque des 495 puits étudiés ni le carbendazime dans 212
autres (la limite de détection n'a pas été précisée). On estime que
le bénomyl et le carbendazime entraînés par ruissellement
correspondent uniquement à la fraction adsorbée aux particules de
sol; d'ailleurs ces composés sont sans doute fortement adsorbés aux
sédiments présents dans l'environnement aquatique.
En solution, dans les végétaux et le sol, le bénomyl subit une
décomposition en carbendazime (méthyl-1H-benzimidazole-2-carbamate)
en 2-AB, STB (3-butyl-1,3,5-triazino[1,2a]-benzimidazole-
2,4(1H,3H)dione) ainsi qu'en BBU (1-(2-benzimidazolyl)-3-n-
butylurée). La photolyse du bénomyl est pratiquement inexistante.
Chez l'animal, le bénomyl est métabolisé en carbendazime ainsi
qu'en d'autres métabolites polaires qui sont rapidement excrétés. On
n'a pas observé d'accumulation de bénomyl ni de carbendazime dans
aucun système biologique.
1.4 Concentrations dans l'environnement et exposition humaine
Il ne semble pas qu'il existe de données résultant d'une
surveillance du bénomyl dans l'environnement. Toutefois on peut
récapituler ainsi les données tirées d'études portant sur la
destinée de ce produit dans l'environnement.
Comme le bénomyl et le carbendazime restent stables pendant des
semaines sur les végétaux, ils peuvent être ingérés par des
organismes qui se nourrissent de feuilles mortes. Des résidus de
carbendazime peuvent subsister jusqu'à 3 ans dans le sol et les
sédiments. Toutefois, la forte adsorption du carbendazime aux
particules de sol et de sédiments réduit l'exposition des organismes
terrestres et aquatiques.
Ce sont les résidus de bénomyl et de carbendazime présents sur
les cultures vivrières qui constituent la principale source
d'exposition de la population humaine dans son ensemble. L'analyse
de l'exposition par voie alimentaire qui a été effectuée aux
Etats-Unis (bénomyl et carbendazime associés) et aux Pays-Bas
(carbendazime seul) a montré que la quantité moyenne ingérée était
vraisemblablement de l'ordre d'un dixième de la dose journalière
acceptable (DJA) qui est, pour le bénomyl de 0,02 mg/kg de poids
corporel, et pour le carbendazime de 0,01 mg/kg de poids corporel.
L'exposition professionnelle au cours de la production est
inférieure à la valeur-seuil. Les ouvriers agricoles qui préparent
les mélanges, effectuent le remplissage, ou retournent dans les
champs traités par du bénomyl, courent un risque d'exposition
cutanée de quelques mg de bénomyl à l'heure. Le port de dispositifs
de protection permettrait de réduire encore cette exposition. En
outre, étant donné que l'absorption percutanée est vraisemblablement
faible, il est très peu probable que le bénomyl puisse avoir des
effets toxiques généraux sur les populations humaines en étant
absorbé par cette voie.
1.5 Cinétique et métabolisme
Après exposition par voie orale ou respiratoire, le bénomyl est
rapidement absorbé par l'organisme animal mais cette absorption est
bien moindre après exposition par voie cutanée. Une fois absorbé, le
bénomyl est rapidement métabolisé, puis excrété dans les urines et
les matières fécales. Après avoir administré du bénomyl marqué au
carbone-14 à des rats, on a retrouvé dans le sang, et en petites
quantités dans les testicules, les reins et le foie, deux de ses
métabolites, le carbendazime et le carbamate de méthyl-5-hydroxy-1H-
benzimidazole-2-yle (5-HBC). La distribution tissulaire des composés
n'était pas révélatrice d'une bioconcentration. Dans l'urine, le
principal métabolite était le 5-HBC à côté d'un peu de carbendazime.
Dans les 72 heures suivant l'administration, 98% de la quantité
administrée avaient été excrétés. Chez des vaches recevant pendant 5
jours des capsules contenant du bénomyl radiomarqué à raison de 50
mg/kg de nourriture, les concentrations équivalentes de bénomyl
retrouvées dans les divers organes étaient de 4 mg/kg dans le foie,
et de 0,25 mg/kg dans les reins; dans les autres tissus et les
graisses, les valeurs n'étaient pas significatives. Pendant la
période d'administration, 65% du composé radiomarqué ont été
excrétés dans les urines, 21% dans les matières fécales et 0,4% dans
le lait. Le principal métabolite présent dans le lait était le
5-HBC. Chez d'autres animaux, on a constaté un métabolisme et des
modalités d'élimination similaires.
Le bénomyl n'inhibe pas l'acétylcholinestérase in vitro. On a
montré qu'il induisait l'époxyhydrolase hépatique, la gamma-
glutamyle transpeptidase ainsi que la glutation-S-transférase chez
la souris et le rat in vivo.
1.6 Effets sur les mammifères de laboratoire et sur les systèmes
d'épreuve in vitro
1.6.1 Exposition unique
Le bénomyl présente une faible toxicité aiguë, la DL50 par
voie orale chez le rat étant > 10 000 mg/kg, et la CL50 à 4 h par
voie respiratoire étant > 4 mg/litre. Des chiens exposés par voie
respiratoire pendant 4 heures à la dose de 1,65 mg/litre et examinés
28 jours après l'exposition, présentaient une diminution du poids du
foie. Une dose unique administrée à des rats par gavage a entraîné
des effets sur la reproduction 70 jours après l'exposition (voir
Section 1.6.5).
1.6.2 Exposition de brève durée
Administré par gavage pendant de brèves périodes allant jusqu'à
90 jours, en mélange à la nourriture ou en application cutanée, le
bénomyl a légèrement augmenté le poids du foie chez le rat (à la
dose quotidienne de 125 mg/kg, en mélange à la nourriture) et a
produit des effets sur les organes reproducteurs mâles chez le rat:
diminution du poids des testicules et de l'épididyme, réduction de
la spermatogenèse (dose quotidienne: 45 mg/kg, par gavage, dose sans
effet observable = 15 mg/kg), chez le lapin (dose quotidienne: 1000
mg/kg, par voie orale; 500 mg/kg, en appli cation cutanée) et chez
le chien beagle (62,5 mg/kg, dose sans effet observable = 18,4 mg/kg
par jour, en mélange à la nourriture). Chez les rats exposés par la
voie respiratoire à du bénomyl à des concentrations allant jusqu'à
200 mg/m3 pendant 90 jours, on n'a pas observé d'effets sur le
foie ni les testicules.
1.6.3 Irritation et sensibilisation au niveau de la peau et
des yeux
L'application de bénomyl sur l'épiderme de lapins ou de cobayes
n'a produit que peu ou pas d'irritation et une sensibilisation
modérée de la peau. Chez le rat, l'instillation oculaire a produit
une irritation légère et temporaire de la conjonctive.
1.6.4 Exposition de longue durée
A l'issue d'une étude prolongée d'alimentation chez des rats,
on n'a pas pu mettre en évidence d'effets imputables à ce composé à
des doses allant jusqu'à 2500 mg/kg de nourriture (soit 125 mg/kg de
poids corporel par jour). Cette étude n'a pas été jugée suffi sante
pour permettre une évaluation des effets sur la reproduction. Chez
la souris CD-1, le poids du foie était en augmentation à partir de
la dose de 1500 mg/kg de nourriture. A la dose de 5000 mg/kg de
nourriture, on notait chez les souris mâles une réduction du poids
absolu des testicules et une atrophie du thymus.
1.6.5 Reproduction, embryotoxicité et tératogénicité
Le bénomyl détermine une diminution du poids des testicules et
de l'épidydime, avec réduction des réserves de spermatozoïdes au
niveau de la queue de l'épidydime, une diminution de la production
des spermatozoïdes et une réduction de la fécondité des mâles. A
plus fortes doses, il y a diminution de la spermatogénèse qui est
perturbée à tous ses stades. Le bénomyl n'affecte pas le
comportement copulatoire, les vésicules séminales, la mobilité des
spermatozoïdes ni les hormones sexuelles correspondantes. La
concentration de bénomyl la plus faible qui produise un effet
statistiquement significatif sur la spermatogénèse du rat mâle a été
chiffrée à 45 mg/kg par jour. La dose sans effet observable pour ces
effets est de 15 mg/kg par jour.
Une seule dose de bénomyl (100 mg/kg ou davantage) administrée
par gavage à des rats a produit, 70 jours après l'exposition, des
effets consistant notamment en une réduction du poids des testicules
et une atrophie des tubes séminifères.
Administré par gavage à des rats ChD-CD et Wistar du 7e au
16e jour de la gestation, le bénomyl s'est révélé tératogène pour
les 2 souches à 62,5 mg/kg, mais pas à 30 mg/kg pour les ChR-CD, ni
à 31,2 mg/kg pour les Wistar. Administré par gavage à des rats
Sprague-Dawley du 7e au 21e jour de gestation, le bénomyl s'est
révélé tératogène à 31,2 mg/kg. Les effets observés étaient une
microphthalmie, une hydrocéphalie et une encéphalocèle. Le
développement post-natal des rats était également perturbé aux doses
supérieures à 15,6 mg/kg.
Chez la souris, l'administration par gavage à des
concentrations supérieures ou égales à 50 mg/kg a provoqué
l'apparition de côtes surnuméraires et autres anomalies du squelette
et des viscères. On n'a pas établi, chez la souris, la valeur de la
dose sans effet observable car les doses administrées étaient toutes
supérieures à 50 mg/kg. A part une augmentation marginale de la
fréquence des côtes surnuméraires chez le lapin, on n'a pas observé,
chez cet animal, d'effets tératogènes à des doses atteignant 500
mg/kg de nourriture.
1.6.6 Mutagénicité et autres points d'aboutissement des effets
toxiques
L'étude des cellules somatiques et germinales montre que le
bénomyl n'entraîne pas de mutations géniques ni d'anomalies
structurales chromosomiques et qu'il n'y a pas non plus
d'interaction directe avec l'ADN (qui provoquerait des lésions de
l'ADN et leur réparation). Ces faits ont été mis en évidence à la
fois sur des cellules mammaliennes et non mammaliennes.
Cependant le bénomyl provoque des aberrations dans le nombre
des chromosomes (aneuploïdie et/ou polyploïdie) dans les systèmes
d'épreuve (tant in vitro qu' in vivo).
1.6.7 Cancérogénicité
Chez les souris CD-1 et Swiss axéniques, qui présentent un taux
important de tumeurs spontanées du foie, on a observé ce type de
tumeurs après administration de bénomyl ou de carbendazime. En
revanche, le carbendazime ne s'est pas révélé cancérogène chez les
souris NMRKf, qui n'ont qu'un faible taux de tumeurs hépatiques
spontanées.
La première étude de cancérogénicité portant sur des souris
CD-1 a fait ressortir l'existence d'une augmentation liée à la dose
et statistiquement significative des néoplasmes hépatocellulaires
chez les femelles et l'on a également observé chez les mâles, aux
doses moyennes (1500 mg/kg), une réaction statistiquement
significative qui ne s'observait plus aux doses élevées en raison
d'un fort taux de mortalité. Une deuxième étude de cancérogénicité
portant sur le carbendazime a été effectuée chez une souche
génétiquement apparentée de souris Swiss axéniques et exogames à des
doses de 0, 150, 300 et 1000 mg/kg (la dernière dose étant portée à
5000 mg/kg au cours de l'étude); elle a révélé un accroissement dans
l'incidence de l'ensemble des adénomes et carcinomes
hépatocellulaires. Une troisième étude effectuée cette fois sur des
souris NMRKf à des doses 0, 50, 150, 300 et 1000 mg/kg (portées
ensuite à 5000 mg/kg) n'a pas fait ressortir d'effets cancérogènes.
Les études de cancérogénicité portant sur le bénomyl et le
carbendazime ont donné des résultats négatifs chez le rat.
1.6.8 Mécanisme de la toxicité - mode d'action
On pense que les effets biologiques du bénomyl et du
carbendazime résultent de leur interaction avec les microtubules
cellulaires. Ces structures interviennent dans des fonctions aussi
importantes que la division cellulaire, qui est inhibée par ces deux
substances. La toxicité du bénomyl et du carbendazime pour les
mammifères est donc liée à une perturbation des fonctions du système
microtubulaire.
Comme les autres dérivés du benzimidazole, le bénomyl et le
carbendazime sont plus ou moins toxiques selon les espèces. Cette
sélectivité toxicologique s'explique au moins en partie par le fait
que le bénomyl et le carbendazime ne se lient pas de la même manière
aux tubulines des espèces visées et des espèces non visées.
1.7 Effets sur l'homme
Le bénomyl provoque des dermatites de contact ainsi qu'une
sensibilisation cutanée. On n'a pas fait état d'autres effets.
1.8 Effets sur les autres êtres vivant au laboratoire et dans
leur milieu naturel
Le bénomyl n'a guère d'effet sur l'activité microbienne du sol
aux doses d'emploi recommandées. On a cependant signalé l'existence
d'effets nocifs vis-à-vis de certains groupes de champignons.
On a calculé que la CE50 à 72 heures, fondée la croissance
totale, pour les algues bleu-vert du genre Selenastrum
capricornutum, était égale à 2,0 mg/litre; la concentration sans
effet observable était de 0,5 mg/litre. La toxicité du bénomyl pour
les invertébrés aquatiques et les poissons varie dans de larges
proportions, les valeurs de la CL50 à 96 heures allant de 0,006
mg/litre pour des poissons-chats du genre Ictalurus (alevins
porteurs de leur sac vitellin) à plus de 100 mg/litre pour les
écrevisses.
Le bénomyl s'est révélé toxique pour les lombrics lors
d'expériences de laboratoire qui reproduisaient les conditions
réelles d'exposition résultant de l'utilisation recommandée sur le
terrain. Il est peu toxique pour les oiseaux et son produit de
dégradation, le carbendazime, est "relativement non toxique" pour
les abeilles.
2. Conclusions
Le bénomyl provoque une sensibilisation cutanée chez l'homme.
Le bénomyl et le carbendazime ne font courir à l'homme qu'un très
faible risque d'intoxication aiguë. Etant donné les conditions
actuelles d'exposition et le faible taux d'absorption percutanée de
ces deux composés, il est improbable qu'ils entraînent des effets
toxiques généraux dans la population ou chez les personnes exposées
de par leur profession. Ces conclusions sont tirées de données
relatives à l'animal et, dans une moindre mesure, à l'homme; elles
reposent sur la connaissance du mode d'action du carbendazime et du
bénomyl, tant chez les espèces visées que chez les espèces non
visées.
Grâce à une meilleure connaissance du mécanisme de la toxicité
du bénomyl et du carbendazime pour les mammifères, on pourra
peut-être mieux définir les doses sans effet observable. Des études
portant sur la liaison de ces composés aux tubulines des cellules
cibles (tissus testiculaires et embryonnaires) faciliteront sans
doute les comparaisons interspécifiques.
Le carbendazime est fortement adsorbé aux matières organiques
du sol et il y persiste pendant des périodes pouvant atteindre 3
ans. Il persiste également à la surface des feuilles et se retrouve
par conséquent dans les feuilles mortes. On a montré que les
lombrics pouvaient souffrir (dans leur population et dans leur
reproduction) de ces composés aux doses d'emploi recommandées. On ne
possède aucun renseignement sur les autres arthropodes qui vivent
dans le sol ou les débris organiques et qui pourraient être exposés
de la même manière.
Il est improbable que la forte toxicité vis-à-vis des
organismes aquatiques révélée par les épreuves de laboratoire
s'observe également dans le milieu naturel, du fait de la faible
biodisponi-bilité des résidus de carbendazime liés aux sédiments.
Toutefois on ne possède aucune donnée sur les espèces vivant sur les
sédiments et qui seraient donc les plus exposées.
RESUMEN Y CONCLUSIONES
1. Resumen
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
El benomilo es un sólido cristalino de color tostado y acción
fungicida sistémica que pertenece a la familia del bencimidazol. Se
descompone a una temperatura apenas superior a 140 °C,
correspondiente a su punto de fusión, y su presión de vapor a 25 °C
es < 5 x 10-6 Pa (< 3,7 x 10-8 mmHg). Prácticamente es
insoluble en agua a pH 5 y 25 °C, siendo su solubilidad 3,6
mg/litro. Es un compuesto estable en condiciones de almacenamiento
normales, pero en agua se descompone y forma carbendazima.
Los análisis de las muestras procedentes de residuos y del
medio ambiente se realizan mediante extracción con un disolvente
orgánico, purificación del extracto obtenido utilizando un
procedimiento de reparto líquido-líquido y conversión del residuo en
carbendazima. La valoración de los residuos se puede realizar
mediante cromatografía líquida de alto rendimiento o inmunoensayo.
1.2 Fuentes de exposición humana y ambiental
En 1988, el uso mundial estimado de benomilo fue unas 1700
toneladas. Es un fungicida muy utilizado, que se encuentra
registrado en 50 países en los que se permite su uso en más de 70
cultivos. El benomilo está formulado como polvo humectable.
1.3 Transporte, distribución y transformación en el medio ambiente
En el medio ambiente, el benomilo se transforma rápidamente en
carbendazima con una semivida de 2 y 19 h en el agua y el suelo
respectivamente. Por consiguiente, para la evaluación de los efectos
sobre el medio ambiente son importantes los datos obtenidos de los
estudios realizados con ambos compuestos.
La carbendazima se descompone en el medio ambiente con una
semivida de 6 a 12 meses en el suelo desnudo, de 3 a 6 meses en el
césped y de 2 y 25 meses en el agua en condiciones aerobias y
anaerobias respectivamente.
La carbendazima se descompone principalmente por acción de los
microorganismos. Un producto importante de su degradación es el
2-aminobencimidazol (2-AB), que luego se descompone de nuevo por la
actividad microbiana.
En la descomposición del benomilo marcado con un grupo fenilo
con 14C, sólo el 9% del 14C formó CO2 durante un año de
incubación. El resto del 14C se recuperó sobre todo como
carbendazima y en productos unidos a residuos. El destino de un
posible producto de degradación (1,2-diaminobenceno) puede aclarar
ulteriormente la vía de degradación de los fungicidas
bencimidazólicos en el medio ambiente.
En estudios de campo y de columna se ha puesto de manifiesto
que la carbendazima queda retenida en la capa superficial del suelo.
Aunque no se dispone de datos sobre su adsorción en el suelo, se
considera que ésta puede ser tan intensa como la del benomilo, con
valores de Koc que oscilan entre 1000 y 3600. Los valores del log
Kow para el benomilo y la carbendazima son respectivamente 1,36 y
1,49.
En la evaluación realizada en un modelo de selección, basado en
datos de adsorción y persistencia, se puso de manifiesto que no
había riesgo de lixiviación. En los Estados Unidos se han efectuado
análisis de agua de pozos que confirman esto, puesto que no se
encontraron trazas de benomilo en ninguno de los 495 pozos
muestreados ni se detectó carbendazima en ninguno de los 212 (no se
dispone del límite de detección). Es de suponer que la escorrentía
superficial de ambos compuestos se deba solamente al fungicida
adsorbido en las partículas del suelo y que en el medio acuoso estén
fuertemente adsorbidos en los sedimentos.
En solución, en las plantas y en el suelo, el benomilo se
degrada a carbendazima (1H-bencimidazol-2-carbamato de metilo) y a
2-AB, STB (3-butil-1,3,5-triazino[1,2a]-bencimidazol-2,4(1H,3H)
diona) y BBU (1-(2-bencimidazolil)-3-n-butilurea). La fotolisis del
benomilo es escasa o nula.
En los animales, el benomilo se descompone formando
carbendazima y otros metabolitos polares, que se excretan
rápidamente. No se ha observado que el benomilo o la carbendazima se
acumulen en ningún sistema biológico.
1.4 Niveles medioambientales y exposición humana
No parece que se disponga de datos de vigilancia ambiental para
el benomilo. Sin embargo, los estudios realizados sobre su destino
en el medio ambiente pueden resumirse como sigue.
Puesto que el benomilo y la carbendazima se mantienen estables
en las plantas durante varias semanas, pueden pasar a los organismos
que se alimentan de las hojas caídas. El suelo y los sedimentos
pueden conservar residuos de carbendazima hasta tres años. Sin
embargo, la fuerte adsorción de este compuesto en las partículas del
suelo y en los sedimentos reduce la exposición de los organismos
terrestres y acuáticos.
La principal fuente de exposición para la población humana
general se debe a los residuos de benomilo y carbendazima en los
cultivos alimentarios. En análisis de la exposición a través de los
sedimentos realizados en los Estados Unidos (benomilo y carbendazima
combinados) y en los Países Bajos (con carbendazima) se obtuvo una
ingesta media prevista de alrededor del 10 por ciento de la ingesta
diaria admisible (IDA) recomendada, de 0,02 mg/kg de peso corporal
para el benomilo y de 0,01 mg/kg de peso corporal para la
carbendazima.
La exposición profesional durante el proceso de fabricación es
inferior al valor umbral límite. Se considera que los trabajadores
agrícolas que se ocupan de mezclar y cargar los plaguicidas o que
entran en campos tratados con benomilo sufren exposición cutánea a
unos mg de benomilo por hora. Este forma de exposición se podría
reducir con algún tipo de protección. Por otra parte, puesto que la
absorción cutánea prevista es baja, la probabilidad de que el
benomilo tenga efectos tóxicos sistémicos sobre la población humana
a través de esta vía es muy escasa.
1.5 Cinética y metabolismo
En experimentos con animales se ha puesto de manifiesto que
éstos absorben fácilmente el benomilo tras la exposición oral y
respiratoria, pero mucho menos después de una exposición cutánea. El
benomilo absorbido se metaboliza rápidamente y se excreta en la
orina y las heces. En ratas alimentadas con benomilo marcado con
14C, se encontraron en la sangre, y en pequeña cantidad en los
testículos, los riñones y el hígado, los metabolitos carbendazima y
(5-hidroxi-1H-bencimidazol-2-il)-carbamato de metilo (5-HBC). La
distribución en los tejidos demostraba la ausencia de
bioconcentración. El metabolito principal en la orina era el 5-HBC,
acompañado de una pequeña cantidad de carbendazima. A las 72 h de la
administración ya se había excretado el 98% de la cantidad
suministrada. En vacas tratadas durante 5 días con cápsulas con
benomilo marcado, en dosis equivalentes a una alimentación de 50
mg/kg, se detectó en el hígado una concentración de esta sustancia
equivalente a 4 mg/kg, en los riñones 0,25 mg/kg y en el tejido
adiposo o en otros niveles no significativos. Cuando se administró
con los alimentos, el 65% del compuesto marcado se excretó en la
orina, el 21% en las heces y el 0,4% en la leche. El principal
metabolito en la leche fue el 5-HBC. El metabolismo y los sistemas
de eliminación fueron semejante en otros animales.
El benomilo no inhibe la acetilcolinesterasa in vitro. Se ha
demostrado en estudios in vivo en ratas y ratones que induce la
epoxihidrolasa hepática, la gamma-glutamil transpeptidasa y la
glutatión-S-transferasa.
1.6 Efectos en los mamíferos de laboratorio y en sistemas
de prueba in vitro
1.6.1 Exposición única
El benomilo tiene una toxicidad aguda baja, con una DL50 por
vía oral en ratas de > 10 000 mg/kg y una CL50 por inhalación
durante 4 h de > 4 mg/litro. La carbendazima, al igual que la
sustancia de la que se deriva, tiene una DL50 en ratas de > 10
000 mg/kg. Los perros, expuestos por inhalación a 1,65 mg/litro
durante 4 h y examinados 28 días después, mostraron una dismi nución
del peso del hígado. La administración por sonda de una sola dosis
de benomilo a ratas mostró efectos en la reproducción a los 70 días
de la exposición (véase el apartado 1.6.5).
1.6.2 Exposición breve
La administración de benomilo mediante sonda, en los alimentos
o por exposición cutánea durante un período máximo de 90 días en las
ratas aumentó ligeramente el peso del hígado (125 mg/kg al día, con
los alimentos) y tuvo efectos sobre los órganos reproductores
masculinos (disminución del peso de los testículos y los epidídimos,
y reducción de la espermatogénesis) en las ratas (45 mg/kg al día,
administrado por sonda; nivel sin efecto observado (NOEL) =
15mg/kg), los conejos (1000 mg/kg al día, por vía oral; 500 mg/kg de
peso corporal al día, por vía cutánea) y los perros (62,5 mg/kg;
NOEL = 18,4 mg/kg al día, con los alimentos). No se observaron
efectos hepáticos ni testiculares en la ratas expuestas por
inhalación a concentraciones de benomilo de hasta 200 mg/m3
durante 90 días.
1.6.3 Irritación y sensibilización cutánea y ocular
La aplicación cutánea a conejos y cobayos ocasionó una
irritación leve o nula y una sensibilización moderada de la piel. Su
aplicación ocular en ratas produjo de manera transitoria una
irritación conjuntival ligera.
1.6.4 Exposición prolongada
En un estudio de alimentación de larga duración en ratas, con
dosis de hasta 2500 mg/kg de alimentos (125 mg/kg de peso corporal
al día) no se puso de manifiesto ningún efecto relacionado con el
compuesto. Este estudio no se consideró adecuado para evaluar los
efectos sobre la reproducción. En el ratón CD-1 se observó que, con
dosis de 1500 mg/kg de alimento o superiores, se producía un aumento
de peso del hígado. En los ratones macho, las dosis de hasta 5000
mg/kg de alimento provocaron una disminución en términos absolutos
del peso de los testículos y la atrofia del timo.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
El benomilo produce una disminución del peso de los testículos
y el epidídimo, de la producción de esperma y de la tasa de
fecundidad de los machos. A dosis más elevadas, provoca
hipoespermatogénesis, con interrupción general de todas las fases de
la espermatogénesis. No afecta, en cambio, al comportamiento
copulatorio, las vesículas seminales, la movilidad del esperma o las
hormonas de la reproducción asociadas. La concentración más baja del
benomilo capaz de inducir un efecto espermatogénico estadísticamente
significativo en ratas macho fue de 45 mg/kg por día. El NOEL para
estos efectos fue de 15 mg/kg por día.
La administración por sonda de una sola dosis de benomilo (100
mg/kg o más) mostró efectos en ratas a los 70 días de la exposición,
que incluyeron descenso del peso de los testículos y atrofia de los
túbulos seminíferos. Administrado por sonda a ratas ChD-CD y Wistar
durante los días 7 a 16 de la gestación, el benomilo resultó
teratogénico a 62,5 mg/kg en ambas estirpes, pero no a 30 mg/kg en
ratas ChD-CD y no a 31,2 mg en ratas Wistar. Administrado por sonda
a ratas Sprague-Dawley en los días 7 a 21 de la gestación, el
benomilo resultó teratogénico en dosis de 31,2 mg/kg. Los efectos
que produjo fueron microftal mia, hidrocefalia y encafaloceles. Las
dosis superiores a 15,6 mg/kg tuvieron un efecto negativo sobre el
desarrollo posnatal.
La administración por sonda de 50 mg/kg o concentraciones
superiores indujo en ratones la aparición de costillas
supernumerarias y de otras anomalías esqueléticas y viscerales. No
se ha determinado el NOEL en los ratones porque no se ensayaron
dosis inferiores a 50 mg/kg. A excepción de un aumento marginal de
las costillas supernumerarias en conejos, no se observaron efectos
teratogénicos incluso a dosis de hasta 500 mg/kg de alimento.
1.6.6 Mutagenicidad y otros efectos finales afines
En unos estudios realizados en células somáticas y germinales
se ha observado que no provoca mutaciones genéticas ni daños en la
estructura de los cromosomas (aberraciones) y tampoco tiene un
efecto directo sobre el ADN (causante de daños y la reparación del
ADN). Esto se ha demostrado tanto en mamíferos como en otros
animales.
El benomilo, sin embargo, produce aberraciones cromosómicas
numéricas (aneuploidía o poliploidía) en sistemas experimentales in
vitro e in vivo.
1.6.7 Carcinogenicidad
En el primer estudio de carcinogenicidad con ratones CD-1 se
puso de manifiesto un aumento estadísticamente significativo de
neoplasia hepatocelular relacionado con la dosis en las hembras y
también en los machos tratados con una dosis de nivel medio (1500
mg/kg) se observó una respuesta estadísticamente significativa, pero
no en los que recibieron dosis elevadas, a causa del elevado índice
de mortalidad. En un segundo estudio sobre la carcinogenicidad de la
carbendazima en una raza de ratones genéticamente relacionada con la
anterior, los ratones SPF (raza aleatoria suiza), con dosis de 0,
150, 300 y 1000 mg/kg (que se aumentó a 5000 mg/kg durante el
estudio) se puso de manifiesto un aumento en el número de casos de
adenomas y carcinomas hepatocelulares combinados. En un tercer
estudio realizado en ratones NMRKf con dosis de 0, 50, 150, 300 y
1000 mg/kg (que se aumentó a 5000 mg/kg durante el estudio) no se
produjeron efectos carcinogénicos. El benomilo y la carbendazima
causaron tumores hepáticos en dos estirpes de ratones (CD-1 y suizos
(SPF)) que presentaban una tasa alta de tumores hepáticos de
formación espontánea. Por el contrario, la carbendazima no resultó
carcinogénica en ratones NMRKf, que presentan una tasa baja de esos
tumores espontáneos.
Los estudios de carcinogenidad del benomilo y la carbendazima
en ratas fueron negativos.
1.6.8 Mecanismo de toxicidad, modo de acción
Se considera que los efectos biológicos de estos compuestos son
el resultado de su interacción con los microtúbulos celulares. Estas
estructuras participan en funciones esenciales, como la división
celular, que inhiben el benomilo y la carbendazima. La toxicidad de
estos productos en los mamíferos está vinculada a una disfunción
microtubular.
El benomilo y la carbendazima, al igual que otros compuestos
del bencimidazol, tienen una toxicidad selectiva para distintas
especies. Esta se explica, por lo menos en parte, porque el benomilo
y la carbendazima se unen de manera distinta a los microtúbulos de
las especies específicas en las que actúan y en las que no.
1.7 Efectos en el ser humano
El benomilo causa dermatitis por contacto y sensibilización
cutánea. No se ha informado de otros efectos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Con las dosis de aplicación recomendadas, el benomilo tiene
pocos efectos sobre la actividad microbiana del suelo. Se han
notificado algunos efectos adversos sobre ciertos grupos de hongos.
La CE50 a las 72 h, basada en el crecimiento total, para el
alga verde Selenastrum capricornutum fue de 2,0 mg/litro; la
concen tración sin efecto observado (NOEC) fue de 0,5 mg/litro. La
toxicidad del benomilo para los invertebrados acuáticos y los peces
varía ampliamente, con una CL50 a las 96 h que oscila entre 0,006
mg/litro para Ictalurus punctatus (alevines con saco vitelino) y
> 100 mg/litro para los cangrejos de río.
No observaron efectos tóxicos en experimentos de laboratorio en
las lombrices de tierra expuestas a concentraciones normales de
benomilo y como resultado del uso de la dosis de aplicación
recomendada en el campo. Tiene una toxicidad baja para las aves, y
la carbendazima, producto de su degradación, es "relativamente no
tóxica" para las abejas de miel.
2. Conclusiones
El benomilo causa sensibilización cutánea en el ser humano.
Tanto el benomilo como la carbendazima representan un riesgo muy
pequeño de intoxicación aguda. Dados los niveles de exposición
actuales y el bajo índice de absorción cutánea de estos dos
compuestos, no es probable que pudieran tener efectos de toxicidad
sistémica en la población general o en personas expuestas
profesionalmente. Estas son las conclusiones que se pueden sacar de
los datos obtenidos en animales y de los limitados datos sobre el
ser humano de que se dispone, pero estas extrapolaciones están
respaldadas por el conocimiento del modo de acción de la
carbendazima y el benomilo en especies en las que actúan y en las
que no.
Una mayor clarificación del mecanismo de toxicidad de ambos
compuestos en los mamíferos permitirá quizás definir mejor los
niveles sin efectos observados. El estudio de su unión a los
microtúbulos de las células destinatarias (tejidos testicular y
embrionario) facilitará la comparación entre distintas especies.
La carbendazima se adsorbe fuertemente en la materia orgánica
del suelo que la conserva durante un período de hasta 3 años.
Persiste en la superficie de las hojas y, por consiguiente, en las
hojas caídas. Se ha demostrado que las dosis recomendadas de
aplicación afectan negativamente a las lombrices de tierra (con
efectos sobre la población y la reproducción). No se dispone de
datos acerca de sus efectos sobre otros artrópodos del suelo o de la
maleza, que estarían igualmente expuestos.
No es probable que se pueda observar en el medio ambiente la
elevada toxicidad demostrada en las pruebas de laboratorio para los
organismos acuáticos debido a la baja biodisponibilidad de los
residuos de carbendazima unidos a los sedimentos. Sin embargo, no se
dispone de información acerca de sus efectos en las especies que
viven en los sedimentos, que sufrirían la exposición más intensa.
See Also:
Toxicological Abbreviations
Benomyl (HSG 81, 1993)
Benomyl (ICSC)
Benomyl (WHO Pesticide Residues Series 3)
Benomyl (WHO Pesticide Residues Series 5)
Benomyl (Pesticide residues in food: 1983 evaluations)
Benomyl (JMPR Evaluation 1995 Part II Toxicological and environmental)
 Molecular formula: C14H18N4O3
Common name: Benomyl
CAS chemical name: Carbamic acid, [1-(butylamino)carbonyl]-1H-
benzimidazol-2-yl]-, methyl ester
IUPAC chemical name: Methyl 1-[(butylamino)carbonyl]-1H-
benzimidazol-2-ylcarbamate
CAS registry number:
Molecular formula: C14H18N4O3
Common name: Benomyl
CAS chemical name: Carbamic acid, [1-(butylamino)carbonyl]-1H-
benzimidazol-2-yl]-, methyl ester
IUPAC chemical name: Methyl 1-[(butylamino)carbonyl]-1H-
benzimidazol-2-ylcarbamate
CAS registry number:  Similar mobility results have been observed in the field.
Benomyl and its degradates were studied on bare soil and turf in
four areas of the USA. Carbendazim and 2-AB were the major and minor
degradates, respectively. After 1 and 2 years of outdoor exposure,
the half-life of total benzimidazole-containing residues was about 3
to 6 months on turf and about 6 to 12 months on bare soil (Baude et
al., 1974). Under these conditions, benomyl, carbendazim and 2-AB
showed little or no downward movement.
4.1.4 Leaching
To evaluate the risk of pollution of ground and drainage water,
screening models based on adsorption and persistence can be used,
together with existing analyses of groundwater samples. Gustafson
(1989) proposed the use of the equation GUS = log T´ (4 - log
Koc); GUS values < 1.8 = "improbable leachers", GUS values of
1.8-2.8 = "transition" and GUS values > 2.8 = "probable leachers".
For benomyl, Kom values of 550, 620, 2100 and 1100 (mean 1093)
were found in four different soils (Priester, 1985). A Kom of 1093
is equal to a Koc of 1857 since Koc = Kom x 1.7. The half-life
of 320 days given by Marsh & Arthur (1989) seems in good agreement
with field half-lives of 6 to 12 months (Baude et al., 1974).
When the calculation of the GUS value is based on a Koc of
1857 and a T0.5 of 320 days, a value of 1.83 is obtained.
According to this value, benomyl/carbendazim lies between the
"improbable leachers" and "transition", and, therefore, would not be
expected to occur in ground water. The adsorption of benomyl and of
carbendazim is expected to be of the same order of magnitude since
the Kow values are almost identical (log Kow = 1.49 and 1.36 for
carbendazim and benomyl, respectively). In ground water studies in
the USA (Parsons & Witt, 1988), benomyl was not found in any of 495
wells tested and carbendazim not in any of 212 wells (detection
limit not reported).
In an EEC survey (Fielding, 1992), the presence of carbendazim
in groundwater in the Netherlands and in Italy was investigated.
Carbendazim was found in one of two samples from the Netherlands
(0.1 µg/litre), and the level was above 0.1 µg/litre in 23 of 70
samples in Italy. Detection of the non-polar DDT and lindane in many
wells in the Italian study may indicate macropore transport or
artifacts such as direct pollution of wells.
4.1.5 Crop uptake
Various greenhouse and outdoor tests, in which benomyl was
applied to several crops (apples, bananas, cucumbers, grapes and
oranges), indicate that benomyl and carbendazim remain on plant
surfaces as major components of the total residue (Baude et al.,
1973). Benomyl is primarily converted to carbendazim once inside
plant tissues.
Although benomyl is systemic when applied directly to plant
foliage, crop uptake of soil residues is extremely low, even when
the crop is planted in the same growing season as the benomyl
treatment. In a greenhouse crop-rotation study, [2-14C]-
carbendazim, the more persistent benomyl metabolite, was applied to
a loamy sand soil. Aging periods of 30, 120 or 145 days were used
and the crops studied were beets, barley and cabbage. Radioactivity
did not accumulate in these crops grown to maturity in a loamy sand
soil treated 30 days earlier with 1 kg a.i./ha or 120 to 145 days
earlier with 3 kg a.i./ha. Accumulation factors, calculated as the
ratio of radioactivity in the crop to that in the corresponding
soil, were very low in beet foliage (0.04) and beet roots (0.03),
low in cabbage and barley grain (0.2), and ranged from 0.9 to 1.2 in
barley straw (Rhodes, 1987).
4.2 Transformation
Numerous field studies to determine the fate and behaviour of
benomyl in soil have shown the instability of benomyl under various
conditions. In solutions, plants, and soil, it degrades to
carbendazim. The conversion of benomyl under alkaline conditions to
STB and BBU has also been reported (section 4.1). The environmental
fate of benomyl has been thoroughly reviewed by Zbozinek (1984).
4.2.1 Biodegradation
4.2.1.1 Water
Anaerobic aquatic degradation studies in pond water and
sediment showed a half-life of 2 h for benomyl and 743 days for its
degradation product carbendazim. Some (1-8%) transformation to STB
occurred. After one year 36% of the applied radioactivity was bound
to the sediment (Arthur et al., 1989b).
4.2.1.2 Soil
In a study by Marsh & Arthur (1989), non-sterile and sterile
samples of Keyport silt loam soil were treated with [phenyl(U)-
14C]benomyl at a concentration of approximately 7.0 mg/kg. This is
equivalent to the expected soil residues in the surface 10 cm of
topsoil when benomyl is applied at 8 kg a.i./ha. Distilled water was
added to each sample until it reached 75% of its moisture-holding
capacity at 0.33 bar. The soils were incubated in the dark at
approximately 25 °C. The non-sterile soil flasks were sampled after
0.1, 0.2, 1, 3, 7, 14, 30, 60, 120, 270 and 365 days. Samples of
sterilized soil were taken after 14, 30, 120, 270 and 365 days.
The half-life of benomyl in non-sterile silt loam was 19 h, but
this was not determined in the sterilized soil. Benomyl was rapidly
converted to carbendazim. The carbendazim had a half-life of 320
days under non-sterile aerobic conditions (Marsh & Arthur, 1989).
This is in close agreement with reported half-lives of 6-12 months
for benzimidazoles applied to bare soil (Baude et al., 1974).
After 365 days of incubation, 9% of the 14C was evolved as
14CO2, 34% could still be recovered as carbendazim, and 36% was
not extractable. The total recovery of 14C was 88%.
In the sterilized soil, the half-life of carbendazim was
approximately 1000 days (Marsh & Arthur, 1989).
When the degradation of 2-14C-carbendazim (20 mg/kg) was
determined, 33% of the 14C label added was evolved as 14CO2
during 270 days. Identical or even faster 14C evolution was
observed from 2-14C-labelled 2-AB (Helweg, 1977). The relatively
low 14C evolution from phenyl-14C-labelled benomyl/carbendazim
may be caused by the formation of strongly adsorbed degradation
products or compounds that are readily incorporated into soil
organic matter. Thus, most of the remaining radioactivity was
accounted for in the organic fraction of the soil.
To elucidate the reason for the low 14C evolution from
phenyl-14C-labelled fungicide, the fate of a possible degradation
product, 1,2-diaminobenzene, needs to be determined.
4.2.1.3 Crops
Metabolism studies in various crops (soybeans, rice, sugar beet
and peaches) using [phenyl(U)-14C]benomyl have shown that the only
species of significance in plant tissues are benomyl, carbendazim
and 2-AB. Soybeans were treated twice with 1 kg a.i./ha and
harvested 35 days later. Rice was treated twice with 2 kg a.i./ha
and harvested at 21 days, sugar beet was treated with 0.5 kg a.i./ha
five times and harvested at 21 days, and peaches were treated twice
at 1 kg a.i./ha and harvested 20 min after spraying. Soybeans, rice
and sugar beet were treated at twice the recommended application
rate. The concentration of radiolabelled compounds in mature
soybeans was 0.7 mg/kg and consisted of 0.42 mg 2-AB/kg, 0.05 mg
benomyl/kg and 0.14 mg carbendazim per kg (Bolton et al., 1986a).
Levels in the rice grain were 2.7 and 7.3 mg/kg for benomyl and
carbendazim, respectively (Bolton et al., 1986b). Sugar beet tops
retained 99% of the total recovered radioactivity, 6.8 mg/kg being
present as carbendazim and 0.4 mg/kg as benomyl. The roots retained
only 0.01 mg carbendazim per kg (Tolle, 1988). After the first
application to peaches, benomyl was present at 0.65 mg/kg and
carbendazim at 0.72 mg/kg. The second application resulted in 0.33
mg benomyl/kg and 0.92 mg carbendazim/kg. No other radioactive
metabolites were found in peaches (Stevenson, 1985).
Chiba & Veres (1981) applied benomyl to apple trees as Benlate
50% WP at a rate of 1.7 kg/ha. Three successive applications were
made in 1977 and a single spray was applied in 1979. Between 3 and 7
days after application there was a marked reduction of about 50% in
benomyl residues from an initial level of about 110 mg/kg. This fall
in benomyl was accompanied by a doubling in the level of carbendazim
residues over the same period due to benomyl degradation to
carbendazim. Within 46 days of the single application in 1979,
benomyl residues fell to 0.63 mg/kg foliage and carbendazim was
present at 1.2 mg/kg. Following the three sprayings in 1977 (at 0,
13 and 27 days after the initial application), residue levels were
2.6 and 17.1 mg/kg foliage for benomyl and carbendazim 83 days after
the first spraying. Both experiments showed an exponential fall in
benomyl residues but the rate of decline was much slower in the case
of the more persistent metabolite.
4.2.2 Abiotic degradation
In a study by Wheeler (1985), the hydrolysis of benomyl was
studied in sterilized aqueous solutions maintained at 25 °C in the
dark for 30 days at pH 5, 7 and 9. In pH 5 buffer, the major product
was carbendazim, whereas at pH 7 and 9 carbendazim and STB were the
major products. STB represented approximately 25% of the total
radioactivity at pH 7 and approximately 80% at pH 9. The half-lives
of benomyl in the pH 5, 7 and 9 solutions were approximately 3.5,
1.5 and less than 1 h, respectively. There was no further
degradation of carbendazim at pH 5 and 7 over 30 days. At pH 9,
however, carbendazim was slowly hydrolysed to 2-AB with a half-life
of 54 days (Priester, 1984).
Aqueous photolysis studies conducted in natural sunlight have
shown that benomyl is mainly degraded by hydrolysis rather than
photolysis (Powley, 1985).
4.2.3 Bioaccumulation
Although only low concentrations of benomyl or its metabolites
would be expected in natural waters, studies have evaluated the
metabolism and bioaccumulation in fish. Bluegill sunfish ( Lepomis
macrochirus) were exposed to radiolabelled carbendazim
concentrations of 0.018 or 0.17 mg/litre for 4 weeks in a dynamic
study designed to measure the bioaccumulation of 14C residues in
edible tissue, viscera, remaining carcass and whole fish. A two-week
depuration phase followed the exposure phase. Results were similar
at the two exposure concentrations, the peak whole fish
bioconcentration factors (BCFs) being 27 and 23 at the low and high
exposure levels, respectively. The radioactivity was concentrated
more in the viscera than in other tissues, the peak viscera BCFs
being 460 and 380 for the low and high exposure levels,
respectively. Very little bioconcentration occurred in the muscle
tissue (BCF = < 4) or the remaining carcass (BCF = < 7). During
the 14-day depuration phase, > 94% of the peak level of
radioactivity was lost from the whole fish, viscera and muscle. The
rate of loss from the carcass tissue was lower (77% and 82% loss for
the low and high exposure levels, respectively) (Hutton et al.,
1984).
When rainbow trout ( Oncorhynchus mykiss), channel catfish
( Ictalurus punctatus) and bluegill sunfish ( Lepomis macrochirus)
were injected intraperitoneally with carbendazim, branchial and
biliary excretion were the major pathways for the elimination
(Palawski & Knowles, 1986). In a separate experiment, the three fish
species were exposed to 45 µg carbendazim/litre for 96 h, except in
the case of catfish, which were exposed for 48 h. This was followed
by a 96-h depuration phase. Rainbow trout had the highest uptake
rate constant (1.78 per h) and bioconcentration factor (159) of the
three species. Much less carbendazim was accumulated by channel
catfish than by the other two species, but this residue level (0.44
µg/g) appeared to be lethal after 48 h of exposure. The elimination
rate constant and the biological half-life of carbendazim were
similar for rainbow trout and bluegill sunfish. However, the
elimination rate constant was greater and the biological half-life
shorter in channel catfish (13 h) than in the other two species.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air, water and soil
The environmental levels in air, water and soil are discussed
in detail in section 4.
5.1.2 Food and feed
Levels of benomyl in food and feed are indicated in section
5.2.
5.1.3 Terrestrial and aquatic organisms
Benomyl levels in terrestrial and aquatic organisms are
discussed in detail in sections 4 and 6.
5.2 General population exposure
The principal exposure of the general population to benomyl is
through dietary exposure. It was recommended by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1988b) that all
maximum residue limits (MRLs) for benomyl, thiophanate-methyl and
carbendazim be listed as carbendazim (see Table 5).
5.2.1 USA
A system called the Dietary Risk Evaluation System (DRES),
which was developed by the US Environmental Protection Agency, was
used to quantify the intake of residues occurring in various
commodities. The system assumes a diet consistent with the 1977-1978
USDA Nationwide Food Consumption Survey. This survey was a
stratified probability survey in which 3-day dietary records of
approximately 30 000 individuals were collected. The dietary intake
of residues resulting from registered food crop uses of benomyl was
then estimated using mean residue levels found in controlled field
trials and adjusting for the effects of food processing, e.g.,
washing and cooking, on residues of benomyl and its metabolites.
Based on this analysis, the total dietary exposure was
determined for the general population and for a number of population
subgroups. The exposure of the average person to residues resulting
from benomyl use was estimated to be 0.218 µg/kg body weight per
day. The highest exposure was found in the population subgroup
entitled "non-hispanic other than black or white", the estimated
exposure being 1.479 µg/kg body weight per day. The lowest exposure
was found in the > 20-year-old males where the estimated exposure
was 0.144 µg/kg body weight per day (Eickhoff et al., 1989). These
estimates are below the benomyl ADI allocated by JMPR (0-0.02 mg/kg
body weight per day) (FAO/WHO, 1985a,b).
5.2.2 Sweden
Residue monitoring data for benzimidazole fungicides, i.e.
benomyl, carbendazim and thiophanate-methyl, on food crops from
Sweden is shown in Table 3 (FAO/WHO, 1988b). No further analysis to
determine dietary intake was performed.
5.2.3 Maximum residue limits
National MRLs for certain commodities are listed in Table 4
(FAO/WHO, 1988a).
A complete list of MRLs for carbendazim, including new
proposals and an indication of the source of the data (application
of benomyl, carbendazim, or thiophanate-methyl) on which the MRL is
based, is given in Table 5 (FAO/WHO, 1988b).
5.3 Occupational exposure during manufacture, formulation or use
The levels of inhalation exposure to benomyl and carbendazim
experienced by workers in a major manufacturing facility (DuPont)
were reviewed from 1986 to 1989. The average levels of benomyl and
carbendazim were less than 0.2 mg/m3 and 0.3 mg/m3,
respectively. Table 6 lists established inhalation exposure limits
for benomyl and carbendazim.
5.3.1 Use
Potential dermal and respiratory exposure to benomyl wettable
powder formulation under actual use situations has been determined
for: a) tank loading and mixing for aerial application; b) re-entry
into treated fields; and c) home use (garden, ornamental and
greenhouse). For crop treatments, approximately 17 kg benomyl
(formulation) was handled per cycle. Maximum exposure occurred in
the loading and mixing operation for aerial application, where
dermal exposure was 26 mg benomyl per mixing cycle, primarily to
hands and forearms (90%) and respiratory exposure averaged 0.08 mg
benomyl. Re-entry data revealed dermal and respiratory exposures of
5.9 mg/h and < 0.002 mg/h, respectively. Home-use situations
(application of 7 to 8 litres of benomyl in hand-held compressed air
sprayers) produced exposures of 1 mg and 0.003 mg per application
cycle for dermal and respiratory routes, respectively (Everhart &
Holt, 1982). Similar average dermal exposure levels (5.39 mg/h) for
strawberry harvesters were reported by Zweig et al (1983).
Table 3. Benomyl/carbendazim/thiophanate-methyl residues in food in Swedena
Samples Swedish/imported No. of samples Samples with residues Residue level Median value
>0.20 mg/kg (mg/kg) (mg/kg)
1986
Pineapples imported 3 1 0.69
Grapes imported 20 3 0.17-0.35 0.26
Strawberries imported 7 1 0.29
Mangoes imported 17 4 0.20-1.82 0.70
Papayas imported 5 2 0.25-0.45
Pears Swedish 17 3 0.32-0.62 0.43
imported 45 7 0.20-0.45 0.34
Apples Swedish 78 17 0.20-0.72 0.40
imported 91 30 0.21-0.74 0.39
1987
Grapes imported 28 3 0.52-0.87 0.60
Strawberries imported 7 0.23
Mangoes imported 14 5 0.29-1.30 0.66
Papayas imported 4 2 0.86-1.14
Pears Swedish 14 1 0.52
imported 62 13 0.21-0.45 0.29
Apples Swedish 61 25 0.20-1.17 0.45
imported 94 12 0.21-0.82 0.36
a From: FAO/WHO (1988b)
Table 4. National Maximum Residue Limits (mg/kg) for certain commoditiesa
banana cereal cherries citrus bean cucumber peach pome fruit strawberries grapes
Australia 1 0.05 5 10 3 3 5 5 6 2
Austria 0.2 0.5 7 1 0.5 2 1.5 3
Belgium 2 0.5 2 2 0.5 2 5 5 2
Brazil 1 0.5 10 10 2 0.5 10 5 5 10
Bulgaria 0.5 10 5 5 10
Canada 5 10 1 0.5 10 5 5 5
Denmark 2 0.1 2 5 2 2 2 2 5 5
France 1 1.5 6
Finland 0.2 1 2 0.5 0.5 1 1 1
Germany 0.2 0.5 2 7 1 0.5 2 2 3
Hungary 2 1
Israel 10 10 10 5 10
Italy 0.5 0.5 1 1
Mexico 10 2 1 15 7 5 10
Netherlands 3 0.1 3 4 3 3 3 3 3 3
New Zealand 5 1 5 5 2 2 5 5 5 5
Spain (guidelines) 1 0.5 5 7 2 2 5 5 1 5
Switzerland 1 0.2 3 7 0.2 0.1 3 3 3 3
United Kingdom 1 0.5 10 0.5 10 5 5 10
(proposed)
USA 1 0.2 15 10 2 1 15 7 5 10
USSR 1 0.5 10 10 2 0.5 10 5 5 5
Yugoslavia 0.1 7 0.5 0.1 2 0.5 2
a From: FAO/WHO (1988a)
Table 5. Proposed Maximum Residue Limits for carbendazim from any
sourcea
Commodity MRL (mg/kg) Applicationb
Apricot 10c B,C
Asparagus 0.1d B,T
Avocado 0.5 B
Banana 1c B,C,T
Barley straw and fodder, dry 2 B
Bean fodder 50 C
Beans, dry 2 B
Berries and other small fruit 5 B,C,T
Brussel sprouts 0.5 B
Broad bean 2 T
Carrot 5c C,T
Cattle meat 0.1d B
Celery 2 B,C
Cereal grains 0.5 B,C,T
Cherries 10c B,C,T
Citrus fruits 10c B,C,T
Coffee beans 0.1d C
Common beane 2 C
Cucumber 0.5 B,C,T
Eggs (poultry) 0.1d B,T
Egg plant 0.5 C
Gherkin 2 C,T
Hops, dry 50 C
Lettuce, head 5 B,C,T
Mango 2 B
Melons, except watermelons 2c B,C
Milk 0.1d B
Mushrooms 1 B,C,T
Nectarine 2 B
Onion, bulb 2 C,T
Peach 10c B,C,T
Peanut 0.1d B,C
Peanut fodder 5 B,C
Peppers 5 C
Pineapple 20c B
Plums (including prunes) 2c B,C,T
Pome fruit 5c B,C,T
Potato 3c,f B,C
Poultry meat 0.1d B,T
Rape seed 0.05d C
Rice straw and fodder, dry 15 B,C,T
Sheep meat 0.1d B
Soya bean, dry 0.2 C
Soya bean fodder 0.1d C
Squash, summer 0.5 B
Table 5 (contd).
Commodity MRL (mg/kg) Applicationb
Sugar beat 0.1d B,C,T
Sugar beat leaves on tops 10 B,C,T
Swedeg 0.1d C
Sweet potato 1 B
Taro 0.1d B
Tomato 5 B,C,T
Tree nuts 0.1d B
Wheat straw and fodder, dry 5 B
Winter squash 0.5 B
a From: FAO/WHO (1988b)
b B = benomyl; C = carbendazim; T = thiophanate-methyl
c MRL based on post-harvest use
d At or about the limit of detection
e JMPR recommended 2 mg/kg for dry, dwarf, lima and snap beans. These
are all covered by "VP 0526, Common bean" and "VP 0071, Beans,
dry" in the new classification
f washed before analysis
g Described as rutabagas in 1983 recommendation
Table 6. Established inhalation exposure limitsa
Country and agency Compound TWAb STELc
(mg/m3) (mg/m3)
Australia benomyl 10 -
Belgium benomyl 10 -
Denmark benomyl 5 -
Finland benomyl 10 30
France benomyl 10 -
Switzerland benomyl 10 -
United Kingdom benomyl 10 15
USA: ACGIHd benomyl 10 -
USA: NIOSHe/OSHAf benomyl 10 -
(inhalable dust)
USA: NIOSH/OSHA benomyl 5 -
(respirable dust)
USSR carbendazim - 0.1
a From: ILO (1991)
b Time-weighted average
c Short-term exposure limit
d American Conference of Governmental Industrial Hygienists
e National Institute of Occupational Safety and Health
f Occupational Safety and Health Administration
Air concentrations of benomyl ranged from 0.0074 to 0.053
mg/m3 (average 0.027 mg/m3) during its application in
greenhouses. Spraying tall plants (over 1.5 m) caused three times
higher concentrations in air than spraying low plants. No detectable
amounts of benomyl or its metabolites (carbendazim, 4-HBC and 5-HBC)
were found in the urine of applicators during the 48 h following the
application. However, information describing protective clothing,
ventilation, and other hygienic factors was not reported (Liesivuori
& Jääskeläinen, 1984).
6. KINETICS AND METABOLISM
Benomyl is extensively metabolized by animals, as described in
detail in section 6.3. Metabolite names and structures are given in
Table 6 and Figures 2 and 3.
6.1 Absorption
Absorption in ChR-CD male rats was monitored after dermal
application of 0.1, 1, 10, and 100 mg benomyl (as 2-14C-Benlate 50
WP) at 0.5, 1, 2, 4 and 10 h intervals. Four rats were used for each
treatment and time interval. Benomyl was slowly absorbed across an
area of skin (16% of the animal), appearing in the blood and urine
within 30 min after treatment and reaching a maximum between 2 and 4
h after dosing (Belasco, 1979b). The concentration of benomyl and
its metabolites in the blood peaked at 0.05 mg/litre (2 h sample) in
the low-dose group (0.1 mg) and at 0.10 mg/litre (4 h sample) in the
high-dose group (100 mg). This represented a 20-fold increase in
blood concentration after a 1000-fold dose increase. Thus,
absorption into the bloodstream was non-linear with respect to dose.
An in vitro study on the penetration of formulated benomyl
(Benlate 50 WP) through human skin showed that benomyl penetrates
human skin poorly when it is applied as a recommended spray strength
solution. Much less penetration was detected when dry concentrated
benomyl was applied (Ward & Scott, 1992).
In a rat gavage study, the absorption of carbendazim given in
the form of a corn oil suspension was estimated to be approximately
80% (Monson, 1990).
6.2 Distribution and accumulation
Blood levels of benomyl and its metabolites in male rats were
measured 6 and 18 h after exposure in male rats. The rats were
exposed to time-weighted averages of 0.32 and 3.3 mg/litre of air
for 0.5, 1, 2 and 6 h. The methodology did not distinguish between
benomyl and carbendazim. At both exposure levels, the blood
concentrations of benomyl/carbendazim were greater than that of
5-HBC 6 h after exposure; the levels were 0.39-2.3 mg/litre and
0.25-1.2 mg/litre, respectively. At 18 h after exposure, only 5-HBC
was detected in the blood (1.1 mg/litre) and this only at the
highest dose. Urinary metabolites consisted primarily of 5-HBC, and
limited amounts of benomyl/carbendazim were also detected (Turney,
1979).
Table 7. Chemical names of benomyl and its metabolites in animalsa
Common or abbreviated Chemical name
name
Benomyl Carbamic acid, [1-(butylamino)carbonyl]-
1H-benzimidazol-2-yl]-, methyl ester
Carbendazim (MBC) methyl (1-H-benzimidazol-2-yl)carbamate
5-HBC methyl (5-hydroxy-1H-benzimidazol-2-yl)-
carbamate
4-HBC methyl (4-hydroxy-1H-benzimidazol-2-yl)-
carbamate
5-HBC-Sb 2-[(methoxycarbonyl)amino]-1H-benzimidazol-5-
yl hydrogen sulfate
5-HBC-Gc [2-[(methoxycarbonyl)amino]-1H-benzimidazol-
5-yl] ß-D-glucopyranosiduronic acid
MBC-4,5-epoxide
MBC-5,6-epoxide
MBC-4,5-dihydrodiol (4,5-dihydro-4,5-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-5,6-dihydrodiol (5,6-dihydro-5,6-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-4,5-diol
MBC-5,6-diol
5-OH-6-GS-MBCd S-[5,6-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5-OH-4-GS-MBC S-[4,5-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5,6-HOBC-N-oxide methyl (6-hydroxy-5-oxo-5H-benzimidazol-2-
yl)-carbamate-N-oxide
Table 7 (contd).
Common or abbreviated Chemical name
name
5,6-HOBC-N-oxide-G [2-[(methoxycarbonyl)amino]-6-oxo-6H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid-N-oxide
5,6-DHBC methyl (5,6-dihydroxy-1H-benzimidazol-2-yl)
carbamate
5,6-DHBC-G [6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid
5,6-DHBC-S 6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl 5-(hydrogen sulfate)
2-AB 2-aminobenzimidazole
2-AB dihydrodiol 2-amino-4,5-dihydro-4,5-dihydroxy-1H-
benzimidazol
5-HAB 5-hydroxy-2-aminobenzimidazole
a From: Krechniak & Klosowska (1986); Monson (1986a,b); Monson (1990)
b S = conjugate with sulfuric acid
c G = conjugate with glucuronic acid
d GS = conjugate with glutathione
In a study by Han (1979), ten male ChR-CD rats were given 1 and
10 µg benomyl intravenously (as 14C-Benlate 50% WP). Radioactivity
was found in the urine as 5-HBC at 6, 12 and 24 h after dosing, and
there was little radioactivity in the blood or faeces at these
sampling times. No radioactivity (< 0.1%) was found in any tissue
after 24 h except in blood, which contained trace quantities of
14C residues.
In a further study, three groups of five rats of each sex were
gavaged with [phenyl(U)-14C] carbendazim. One group received a
single dose of 14C-carbendazim (50 mg/kg). The second group
received a single dose of 14C-carbendazim (50 mg/kg) following 14
days of pre-conditioning with non-radiolabelled carbendazim (50
mg/kg per day). The third group received a single dose of
14C-carbendazim (1000 mg/kg). For all groups, > 98% of the
recovered radioactivity was excreted in the urine or faeces by the
time of sacrifice (72 h after 14C dosing). The 14C remaining in
tissues was < 1% of the applied dose (Monson, 1990).
In a study by Belasco et al. (1969), 14C-benomyl was
administered to male ChR-CD rats and the blood and testes were
analysed. The fungicide was given by gavage as: (a) a single dose of
1000 mg/kg to five rats, which were sacrificed either 1, 2, 4, 7 or
24 h later; (b) 10 repeated doses of 200 mg/kg per day to two rats,
which were sacrificed either 1 or 24 h after the last dose. In
addition, blood and testes from rats fed 2500 mg/kg diet for one
year were analysed. In rats given 1000 mg/kg, results show that: (a)
the total 14C radioactivity (calculated as benomyl) ranged from 3
to 13 ppm in the blood and from 2 to 4 ppm in the testes; (b) 5-HBC
appeared in the blood and testes as early as 1 h after dosing; and
(c) the concentration of benomyl and/or carbendazim decreased with
time and there was a corresponding increase in the concentration of
5-HBC in the blood and testes. Analyses of blood and testes from
rats given 10 repeated oral doses of 200 mg/kg per day showed that
one hour after the last dose no benomyl/carbendazim (< 0.1 ppm) was
detected and only low levels of 5-HBC were found (1.5 ppm blood and
0.3 ppm in testes). No benomyl/carbendazim or 5-HBC (< 0.1 ppm) was
found 24 h after the last dose. With rats fed 2500 mg benomyl/kg
diet for one year, no benomyl/carbendazim (< 0.1 ppm) was detected
in blood or testes. Only a minimal amount of 5-HBC was found in
blood (0.2 ppm) and none was found in the testes (< 0.1 ppm)
(Belasco et al., 1969).
In a series of metabolic studies, benomyl and/or Benlate (50%
benomyl formulation) were administered either by gavage or in the
diet to pregnant ChR-CD rats to determine the concentrations of
benomyl, carbendazim and two carbendazim metabolites (4-and 5-HBC)
in maternal blood and embryonic tissue (Culik, 1981a,b). Dosing took
place on days 7 to 16 of gestation at levels of 125 mg/kg body
weight per day via gavage or 5000-10 000 mg/kg diet (approximately
400-800 mg/kg body weight). Blood samples from the dams and tissue
samples from their embryos were examined on the first, sixth and
tenth days of dietary administration and on days 12 and 16 of gavage
administration. Embryos and maternal blood were analysed 1, 2, 4, 8
and 24 h after gavage.
The levels of benomyl/carbendazim in maternal blood and
embryonic tissues, 24 h following each dose, markedly decreased with
the number of treatments. The level of benomyl (one hour after
treatment) ranged from 0.98 to 8.4 mg/kg with a mean value of 5.0
mg/kg on the first day of treatment. After 10 treatments, the levels
of benomyl/carbendazim ranged from < 0.12 to 0.39 mg/kg (one hour
after last treatment). In the embryo there was 0.13 mg/kg
benomyl/carbendazim after the tenth treatment compared with a mean
of 1.9 mg/kg after the first treatment. The half-life of benomyl in
maternal blood was approximately 45 min and was less in the embryos.
The level of 5-HBC (0.84-2.9 mg/kg) 2 h following the last gavage
increased with the number of exposures, the half-life in the blood
being 2-3 h in the dam and 4-8 h in the embryo. 4-HBC was not
detected.
In the dietary studies, the levels of benomyl, carbendazim and
4-HBC were too low to be measured in the embryonic tissue. 4-HBC
could not be detected in the dams. Irrespective of the dose level
(5000 and 10 000 mg/kg diet active ingredient) of benomyl or
Benlate, the level of benomyl/carbendazim in maternal blood was very
low. In three separate groups of animals, the mean highest blood
concentrations of benomyl/carbendazim were 0.35, 0.61 and 0.23 mg/kg
in each group of dams. The mean highest value of 5-HBC (5000 mg
benomyl/kg diet) was 0.44 mg/kg in the blood and 0.33 mg/kg in the
embryos. Animals fed benomyl or Benlate at a level of 10 000 mg/kg
diet had 5-HBC levels an order of magnitude higher (Culik, 1981a,b).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approxi mately
17 h after the tenth dose, the cow was sacrificed and organ, tissue
and blood samples were subsequently collected. 14C residue levels
in the milk averaged 0.2 mg/kg (calculated as benomyl), 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids. Small amounts of
radioactivity were detected in the liver (4.12 mg/kg) and kidney
(0.25 mg/kg), most of which was bound. No significant levels of
radioactivity (0.06 mg/kg) were detected in other tissues or fat
(Monson, 1985).
Lactating and non-lactating goats were given daily capsule
doses of [2-14C]-benomyl, equivalent to 36 and 88 mg/kg,
respectively, in the total daily diet, for five days. Milk residues
accounted for approximately 2% of the total dose. Approximately 25%
of the milk radioactivity was incorporated into the natural milk
components casein and whey protein. There were no detectable
residues in muscle tissue and fat (< 0.01 mg/kg). However,
radioactivity detected in liver and kidney amounted to 3.8 and 0.09
mg/kg (calculated as benomyl equivalents), respectively (Han, 1980).
In a study by Johnson (1988), the total 14C residue and
metabolic fate of carbendazim in the liver was examined in
non-lactating female goats. Twelve goats were administered a
feed-rate-equivalent dose of [phenyl(U)-14C]-carbendazim (> 50
mg/kg), once a day, for up to 30 days. Within 2 weeks of dose
initiation, a plateau of 14C residues in the liver was achieved at
a level of 9.48 mg/kg (group mean of the total radiolabelled liver
residues for goats sacrificed 2, 3, and 4 weeks after initiation of
dosing). The total 14C residue levels in the liver decreased to
5.17, 3.55 and 1.67 mg/kg (calculated as carbendazim equivalents) 1,
2 and 3 weeks, respectively, after dosing ceased. The elimination
half-life for total 14C residues from the liver, based on this
depuration data, was calculated to be approximately 9 days. The
half-life for removal of carbendazim from the general circulation,
based on 14C-carbendazim equivalent whole blood levels, was
approximately 10 h. The level of bound, non-extractable 14C
residues in the liver of goats sacrificed after 28 days was 1.0
mg/kg.
The results of this study suggest that levels of carbendazim-
derived residues do not accumulate beyond 2 weeks when goats are
exposed to a constant feed level of 50 mg carbendazim/kg.
Furthermore, discontinuation of exposure results in a clearing of
residues from the liver (Johnson, 1988).
The metabolism of benomyl was studied in laying hens by Monson
(1986a). Two hens were individually dosed daily for three
consecutive days with 3.5 mg [2-14C]-benomyl at a rate equivalent
to 29 mg/kg in the daily feed, and two hens were individually dosed
with 3.29 mg [phenyl(U)-14C]-benomyl at rates equivalent to 27
mg/kg in the daily feed. Faeces and eggs from the previous 24 h were
collected just before each dosing. Twenty-two hours after the third
dose, the hens were killed and samples of muscle (breast and thigh),
liver, kidney and fat were analysed. The concentration of
radioactivity (calculated as benomyl equivalents) in the tissues and
day-3 eggs of the [2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-
dosed hens, respectively, was as follows: liver (0.54 and 0.41
mg/kg), kidney (0.28 and 0.16 mg/kg), thigh and breast muscle (both
0.01 mg/kg), fat (0.05 and 0.02 mg/kg) and eggs (0.08 and 0.05
mg/kg).
The distribution of benomyl in this study was comparable to
that in a 20-hen [2-14C]-carbendazim metabolism study. The
concentrations of radioactivity, calculated as mg carbendazim/kg, in
the high-dose laying hens (dose equivalent 120 mg/kg carbendazim in
the diet) were liver (2.63), kidney (1.74), thigh muscle (0.06),
breast muscle (0.05), fat (0.03), day-6 eggs (0.63) (Monson, 1986b).
This study is discussed in detail in the Environmental Health
Criteria monograph on Carbendazim (WHO, 1993).
When bluegill sunfish were exposed to benomyl, carbendazim and
2-AB at nominal concentrations of 0.05 mg/litre (measured
concentrations of 0.01 to 0.04 mg/litre) and 5.0 mg/litre (measured
concentrations of 2 to 5 mg/litre), no residues were found in the
tissues of fish exposed to low levels of these three compounds.
Detectable residues were found in the tissues of fish exposed to the
high levels, but there was no build-up or bioconcentration with time
(DuPont, 1972).
6.3 Metabolic transformation
Benomyl is extensively metabolized by rats to carbendazim,
which is then further metabolized. Studies with rats administered
benomyl intravenously (Han, 1979), dermally (Belasco, 1979b) or by
inhalation (FAO/WHO, 1985a) showed that 5-HBC is the main urinary
metabolite, some carbendazim also being present.
In a rat gavage study (Monson, 1990; see section 6.2),
carbendazim was extensively metabolized. Three dosing regimens (five
rats of each sex per group) were used: a single oral dose of 50
mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with non-radiolabelled carbendazim at 50
mg/kg for 14 days (pre-conditioned low dose); and a single oral dose
of 1000 mg/kg (high dose). The 48-h urine from the low-dose and the
high-dose rats and the 14-day urine from the pre-conditioned
low-dose group were collected. The total recovery from urine was
61.5 and 61.7% of given doses for the low-dose and pre-conditioned
low-dose male groups, 53.2 and 59.3% for the low-dose and
pre-conditioned low-dose female groups, and 39 and 41% for both male
and female high-dose groups, respectively. 5-HBC-S (21-43% of given
dose) was identified as the main metabolite, except in the case of
the pre-conditioned low-dose and high-dose female rat groups
(5.5-10%), while in all female rat groups 5,6-HOBC-N-oxide-G
(10-19%) was predominant. Both 5,6-DHBC-S and 5,6-DHBC-G were
identified as minor metabolites.
In the same study, the faeces were collected at the same
periods as the urine. The total recovery from faeces was about 24%
for the low-dose and pre-conditioned low-dose male groups, 33-38%
for the low-dose and pre-conditioned low-dose female groups, and
higher (> 60%) for both male and female high-dose groups. Unchanged
carbendazim was about 10-15% of the given dose in the faeces of
high-dose rats (Monson, 1990). The proposed metabolic pathway for
benomyl in rats is given in Fig. 2.
When a lactating Holstein cow was dosed by capsule twice daily
(515 mg per dose), equivalent to 50 mg/kg diet, for 5 consecutive
days with [2-14C]-benomyl, the major metabolites of whole milk
were 5-HBC (0.06 mg/litre), 4-HBC (0.03 mg/litre) and MBC-4,5-
dihydrodiol (< 0.07 mg/litre). The proportions of radioactive
residues in the urine were 46% 5-HBC, 3% 4-HBC, and 50% polar
aqueous-soluble metabolites, which included MBC-4,5-dihydrodiol,
2-AB-dihydrodiol and 5-OH-4-GS-MBC (Monson, 1985).
Similar mobility results have been observed in the field.
Benomyl and its degradates were studied on bare soil and turf in
four areas of the USA. Carbendazim and 2-AB were the major and minor
degradates, respectively. After 1 and 2 years of outdoor exposure,
the half-life of total benzimidazole-containing residues was about 3
to 6 months on turf and about 6 to 12 months on bare soil (Baude et
al., 1974). Under these conditions, benomyl, carbendazim and 2-AB
showed little or no downward movement.
4.1.4 Leaching
To evaluate the risk of pollution of ground and drainage water,
screening models based on adsorption and persistence can be used,
together with existing analyses of groundwater samples. Gustafson
(1989) proposed the use of the equation GUS = log T´ (4 - log
Koc); GUS values < 1.8 = "improbable leachers", GUS values of
1.8-2.8 = "transition" and GUS values > 2.8 = "probable leachers".
For benomyl, Kom values of 550, 620, 2100 and 1100 (mean 1093)
were found in four different soils (Priester, 1985). A Kom of 1093
is equal to a Koc of 1857 since Koc = Kom x 1.7. The half-life
of 320 days given by Marsh & Arthur (1989) seems in good agreement
with field half-lives of 6 to 12 months (Baude et al., 1974).
When the calculation of the GUS value is based on a Koc of
1857 and a T0.5 of 320 days, a value of 1.83 is obtained.
According to this value, benomyl/carbendazim lies between the
"improbable leachers" and "transition", and, therefore, would not be
expected to occur in ground water. The adsorption of benomyl and of
carbendazim is expected to be of the same order of magnitude since
the Kow values are almost identical (log Kow = 1.49 and 1.36 for
carbendazim and benomyl, respectively). In ground water studies in
the USA (Parsons & Witt, 1988), benomyl was not found in any of 495
wells tested and carbendazim not in any of 212 wells (detection
limit not reported).
In an EEC survey (Fielding, 1992), the presence of carbendazim
in groundwater in the Netherlands and in Italy was investigated.
Carbendazim was found in one of two samples from the Netherlands
(0.1 µg/litre), and the level was above 0.1 µg/litre in 23 of 70
samples in Italy. Detection of the non-polar DDT and lindane in many
wells in the Italian study may indicate macropore transport or
artifacts such as direct pollution of wells.
4.1.5 Crop uptake
Various greenhouse and outdoor tests, in which benomyl was
applied to several crops (apples, bananas, cucumbers, grapes and
oranges), indicate that benomyl and carbendazim remain on plant
surfaces as major components of the total residue (Baude et al.,
1973). Benomyl is primarily converted to carbendazim once inside
plant tissues.
Although benomyl is systemic when applied directly to plant
foliage, crop uptake of soil residues is extremely low, even when
the crop is planted in the same growing season as the benomyl
treatment. In a greenhouse crop-rotation study, [2-14C]-
carbendazim, the more persistent benomyl metabolite, was applied to
a loamy sand soil. Aging periods of 30, 120 or 145 days were used
and the crops studied were beets, barley and cabbage. Radioactivity
did not accumulate in these crops grown to maturity in a loamy sand
soil treated 30 days earlier with 1 kg a.i./ha or 120 to 145 days
earlier with 3 kg a.i./ha. Accumulation factors, calculated as the
ratio of radioactivity in the crop to that in the corresponding
soil, were very low in beet foliage (0.04) and beet roots (0.03),
low in cabbage and barley grain (0.2), and ranged from 0.9 to 1.2 in
barley straw (Rhodes, 1987).
4.2 Transformation
Numerous field studies to determine the fate and behaviour of
benomyl in soil have shown the instability of benomyl under various
conditions. In solutions, plants, and soil, it degrades to
carbendazim. The conversion of benomyl under alkaline conditions to
STB and BBU has also been reported (section 4.1). The environmental
fate of benomyl has been thoroughly reviewed by Zbozinek (1984).
4.2.1 Biodegradation
4.2.1.1 Water
Anaerobic aquatic degradation studies in pond water and
sediment showed a half-life of 2 h for benomyl and 743 days for its
degradation product carbendazim. Some (1-8%) transformation to STB
occurred. After one year 36% of the applied radioactivity was bound
to the sediment (Arthur et al., 1989b).
4.2.1.2 Soil
In a study by Marsh & Arthur (1989), non-sterile and sterile
samples of Keyport silt loam soil were treated with [phenyl(U)-
14C]benomyl at a concentration of approximately 7.0 mg/kg. This is
equivalent to the expected soil residues in the surface 10 cm of
topsoil when benomyl is applied at 8 kg a.i./ha. Distilled water was
added to each sample until it reached 75% of its moisture-holding
capacity at 0.33 bar. The soils were incubated in the dark at
approximately 25 °C. The non-sterile soil flasks were sampled after
0.1, 0.2, 1, 3, 7, 14, 30, 60, 120, 270 and 365 days. Samples of
sterilized soil were taken after 14, 30, 120, 270 and 365 days.
The half-life of benomyl in non-sterile silt loam was 19 h, but
this was not determined in the sterilized soil. Benomyl was rapidly
converted to carbendazim. The carbendazim had a half-life of 320
days under non-sterile aerobic conditions (Marsh & Arthur, 1989).
This is in close agreement with reported half-lives of 6-12 months
for benzimidazoles applied to bare soil (Baude et al., 1974).
After 365 days of incubation, 9% of the 14C was evolved as
14CO2, 34% could still be recovered as carbendazim, and 36% was
not extractable. The total recovery of 14C was 88%.
In the sterilized soil, the half-life of carbendazim was
approximately 1000 days (Marsh & Arthur, 1989).
When the degradation of 2-14C-carbendazim (20 mg/kg) was
determined, 33% of the 14C label added was evolved as 14CO2
during 270 days. Identical or even faster 14C evolution was
observed from 2-14C-labelled 2-AB (Helweg, 1977). The relatively
low 14C evolution from phenyl-14C-labelled benomyl/carbendazim
may be caused by the formation of strongly adsorbed degradation
products or compounds that are readily incorporated into soil
organic matter. Thus, most of the remaining radioactivity was
accounted for in the organic fraction of the soil.
To elucidate the reason for the low 14C evolution from
phenyl-14C-labelled fungicide, the fate of a possible degradation
product, 1,2-diaminobenzene, needs to be determined.
4.2.1.3 Crops
Metabolism studies in various crops (soybeans, rice, sugar beet
and peaches) using [phenyl(U)-14C]benomyl have shown that the only
species of significance in plant tissues are benomyl, carbendazim
and 2-AB. Soybeans were treated twice with 1 kg a.i./ha and
harvested 35 days later. Rice was treated twice with 2 kg a.i./ha
and harvested at 21 days, sugar beet was treated with 0.5 kg a.i./ha
five times and harvested at 21 days, and peaches were treated twice
at 1 kg a.i./ha and harvested 20 min after spraying. Soybeans, rice
and sugar beet were treated at twice the recommended application
rate. The concentration of radiolabelled compounds in mature
soybeans was 0.7 mg/kg and consisted of 0.42 mg 2-AB/kg, 0.05 mg
benomyl/kg and 0.14 mg carbendazim per kg (Bolton et al., 1986a).
Levels in the rice grain were 2.7 and 7.3 mg/kg for benomyl and
carbendazim, respectively (Bolton et al., 1986b). Sugar beet tops
retained 99% of the total recovered radioactivity, 6.8 mg/kg being
present as carbendazim and 0.4 mg/kg as benomyl. The roots retained
only 0.01 mg carbendazim per kg (Tolle, 1988). After the first
application to peaches, benomyl was present at 0.65 mg/kg and
carbendazim at 0.72 mg/kg. The second application resulted in 0.33
mg benomyl/kg and 0.92 mg carbendazim/kg. No other radioactive
metabolites were found in peaches (Stevenson, 1985).
Chiba & Veres (1981) applied benomyl to apple trees as Benlate
50% WP at a rate of 1.7 kg/ha. Three successive applications were
made in 1977 and a single spray was applied in 1979. Between 3 and 7
days after application there was a marked reduction of about 50% in
benomyl residues from an initial level of about 110 mg/kg. This fall
in benomyl was accompanied by a doubling in the level of carbendazim
residues over the same period due to benomyl degradation to
carbendazim. Within 46 days of the single application in 1979,
benomyl residues fell to 0.63 mg/kg foliage and carbendazim was
present at 1.2 mg/kg. Following the three sprayings in 1977 (at 0,
13 and 27 days after the initial application), residue levels were
2.6 and 17.1 mg/kg foliage for benomyl and carbendazim 83 days after
the first spraying. Both experiments showed an exponential fall in
benomyl residues but the rate of decline was much slower in the case
of the more persistent metabolite.
4.2.2 Abiotic degradation
In a study by Wheeler (1985), the hydrolysis of benomyl was
studied in sterilized aqueous solutions maintained at 25 °C in the
dark for 30 days at pH 5, 7 and 9. In pH 5 buffer, the major product
was carbendazim, whereas at pH 7 and 9 carbendazim and STB were the
major products. STB represented approximately 25% of the total
radioactivity at pH 7 and approximately 80% at pH 9. The half-lives
of benomyl in the pH 5, 7 and 9 solutions were approximately 3.5,
1.5 and less than 1 h, respectively. There was no further
degradation of carbendazim at pH 5 and 7 over 30 days. At pH 9,
however, carbendazim was slowly hydrolysed to 2-AB with a half-life
of 54 days (Priester, 1984).
Aqueous photolysis studies conducted in natural sunlight have
shown that benomyl is mainly degraded by hydrolysis rather than
photolysis (Powley, 1985).
4.2.3 Bioaccumulation
Although only low concentrations of benomyl or its metabolites
would be expected in natural waters, studies have evaluated the
metabolism and bioaccumulation in fish. Bluegill sunfish ( Lepomis
macrochirus) were exposed to radiolabelled carbendazim
concentrations of 0.018 or 0.17 mg/litre for 4 weeks in a dynamic
study designed to measure the bioaccumulation of 14C residues in
edible tissue, viscera, remaining carcass and whole fish. A two-week
depuration phase followed the exposure phase. Results were similar
at the two exposure concentrations, the peak whole fish
bioconcentration factors (BCFs) being 27 and 23 at the low and high
exposure levels, respectively. The radioactivity was concentrated
more in the viscera than in other tissues, the peak viscera BCFs
being 460 and 380 for the low and high exposure levels,
respectively. Very little bioconcentration occurred in the muscle
tissue (BCF = < 4) or the remaining carcass (BCF = < 7). During
the 14-day depuration phase, > 94% of the peak level of
radioactivity was lost from the whole fish, viscera and muscle. The
rate of loss from the carcass tissue was lower (77% and 82% loss for
the low and high exposure levels, respectively) (Hutton et al.,
1984).
When rainbow trout ( Oncorhynchus mykiss), channel catfish
( Ictalurus punctatus) and bluegill sunfish ( Lepomis macrochirus)
were injected intraperitoneally with carbendazim, branchial and
biliary excretion were the major pathways for the elimination
(Palawski & Knowles, 1986). In a separate experiment, the three fish
species were exposed to 45 µg carbendazim/litre for 96 h, except in
the case of catfish, which were exposed for 48 h. This was followed
by a 96-h depuration phase. Rainbow trout had the highest uptake
rate constant (1.78 per h) and bioconcentration factor (159) of the
three species. Much less carbendazim was accumulated by channel
catfish than by the other two species, but this residue level (0.44
µg/g) appeared to be lethal after 48 h of exposure. The elimination
rate constant and the biological half-life of carbendazim were
similar for rainbow trout and bluegill sunfish. However, the
elimination rate constant was greater and the biological half-life
shorter in channel catfish (13 h) than in the other two species.
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Air, water and soil
The environmental levels in air, water and soil are discussed
in detail in section 4.
5.1.2 Food and feed
Levels of benomyl in food and feed are indicated in section
5.2.
5.1.3 Terrestrial and aquatic organisms
Benomyl levels in terrestrial and aquatic organisms are
discussed in detail in sections 4 and 6.
5.2 General population exposure
The principal exposure of the general population to benomyl is
through dietary exposure. It was recommended by the Joint FAO/WHO
Meeting on Pesticide Residues (JMPR) (FAO/WHO, 1988b) that all
maximum residue limits (MRLs) for benomyl, thiophanate-methyl and
carbendazim be listed as carbendazim (see Table 5).
5.2.1 USA
A system called the Dietary Risk Evaluation System (DRES),
which was developed by the US Environmental Protection Agency, was
used to quantify the intake of residues occurring in various
commodities. The system assumes a diet consistent with the 1977-1978
USDA Nationwide Food Consumption Survey. This survey was a
stratified probability survey in which 3-day dietary records of
approximately 30 000 individuals were collected. The dietary intake
of residues resulting from registered food crop uses of benomyl was
then estimated using mean residue levels found in controlled field
trials and adjusting for the effects of food processing, e.g.,
washing and cooking, on residues of benomyl and its metabolites.
Based on this analysis, the total dietary exposure was
determined for the general population and for a number of population
subgroups. The exposure of the average person to residues resulting
from benomyl use was estimated to be 0.218 µg/kg body weight per
day. The highest exposure was found in the population subgroup
entitled "non-hispanic other than black or white", the estimated
exposure being 1.479 µg/kg body weight per day. The lowest exposure
was found in the > 20-year-old males where the estimated exposure
was 0.144 µg/kg body weight per day (Eickhoff et al., 1989). These
estimates are below the benomyl ADI allocated by JMPR (0-0.02 mg/kg
body weight per day) (FAO/WHO, 1985a,b).
5.2.2 Sweden
Residue monitoring data for benzimidazole fungicides, i.e.
benomyl, carbendazim and thiophanate-methyl, on food crops from
Sweden is shown in Table 3 (FAO/WHO, 1988b). No further analysis to
determine dietary intake was performed.
5.2.3 Maximum residue limits
National MRLs for certain commodities are listed in Table 4
(FAO/WHO, 1988a).
A complete list of MRLs for carbendazim, including new
proposals and an indication of the source of the data (application
of benomyl, carbendazim, or thiophanate-methyl) on which the MRL is
based, is given in Table 5 (FAO/WHO, 1988b).
5.3 Occupational exposure during manufacture, formulation or use
The levels of inhalation exposure to benomyl and carbendazim
experienced by workers in a major manufacturing facility (DuPont)
were reviewed from 1986 to 1989. The average levels of benomyl and
carbendazim were less than 0.2 mg/m3 and 0.3 mg/m3,
respectively. Table 6 lists established inhalation exposure limits
for benomyl and carbendazim.
5.3.1 Use
Potential dermal and respiratory exposure to benomyl wettable
powder formulation under actual use situations has been determined
for: a) tank loading and mixing for aerial application; b) re-entry
into treated fields; and c) home use (garden, ornamental and
greenhouse). For crop treatments, approximately 17 kg benomyl
(formulation) was handled per cycle. Maximum exposure occurred in
the loading and mixing operation for aerial application, where
dermal exposure was 26 mg benomyl per mixing cycle, primarily to
hands and forearms (90%) and respiratory exposure averaged 0.08 mg
benomyl. Re-entry data revealed dermal and respiratory exposures of
5.9 mg/h and < 0.002 mg/h, respectively. Home-use situations
(application of 7 to 8 litres of benomyl in hand-held compressed air
sprayers) produced exposures of 1 mg and 0.003 mg per application
cycle for dermal and respiratory routes, respectively (Everhart &
Holt, 1982). Similar average dermal exposure levels (5.39 mg/h) for
strawberry harvesters were reported by Zweig et al (1983).
Table 3. Benomyl/carbendazim/thiophanate-methyl residues in food in Swedena
Samples Swedish/imported No. of samples Samples with residues Residue level Median value
>0.20 mg/kg (mg/kg) (mg/kg)
1986
Pineapples imported 3 1 0.69
Grapes imported 20 3 0.17-0.35 0.26
Strawberries imported 7 1 0.29
Mangoes imported 17 4 0.20-1.82 0.70
Papayas imported 5 2 0.25-0.45
Pears Swedish 17 3 0.32-0.62 0.43
imported 45 7 0.20-0.45 0.34
Apples Swedish 78 17 0.20-0.72 0.40
imported 91 30 0.21-0.74 0.39
1987
Grapes imported 28 3 0.52-0.87 0.60
Strawberries imported 7 0.23
Mangoes imported 14 5 0.29-1.30 0.66
Papayas imported 4 2 0.86-1.14
Pears Swedish 14 1 0.52
imported 62 13 0.21-0.45 0.29
Apples Swedish 61 25 0.20-1.17 0.45
imported 94 12 0.21-0.82 0.36
a From: FAO/WHO (1988b)
Table 4. National Maximum Residue Limits (mg/kg) for certain commoditiesa
banana cereal cherries citrus bean cucumber peach pome fruit strawberries grapes
Australia 1 0.05 5 10 3 3 5 5 6 2
Austria 0.2 0.5 7 1 0.5 2 1.5 3
Belgium 2 0.5 2 2 0.5 2 5 5 2
Brazil 1 0.5 10 10 2 0.5 10 5 5 10
Bulgaria 0.5 10 5 5 10
Canada 5 10 1 0.5 10 5 5 5
Denmark 2 0.1 2 5 2 2 2 2 5 5
France 1 1.5 6
Finland 0.2 1 2 0.5 0.5 1 1 1
Germany 0.2 0.5 2 7 1 0.5 2 2 3
Hungary 2 1
Israel 10 10 10 5 10
Italy 0.5 0.5 1 1
Mexico 10 2 1 15 7 5 10
Netherlands 3 0.1 3 4 3 3 3 3 3 3
New Zealand 5 1 5 5 2 2 5 5 5 5
Spain (guidelines) 1 0.5 5 7 2 2 5 5 1 5
Switzerland 1 0.2 3 7 0.2 0.1 3 3 3 3
United Kingdom 1 0.5 10 0.5 10 5 5 10
(proposed)
USA 1 0.2 15 10 2 1 15 7 5 10
USSR 1 0.5 10 10 2 0.5 10 5 5 5
Yugoslavia 0.1 7 0.5 0.1 2 0.5 2
a From: FAO/WHO (1988a)
Table 5. Proposed Maximum Residue Limits for carbendazim from any
sourcea
Commodity MRL (mg/kg) Applicationb
Apricot 10c B,C
Asparagus 0.1d B,T
Avocado 0.5 B
Banana 1c B,C,T
Barley straw and fodder, dry 2 B
Bean fodder 50 C
Beans, dry 2 B
Berries and other small fruit 5 B,C,T
Brussel sprouts 0.5 B
Broad bean 2 T
Carrot 5c C,T
Cattle meat 0.1d B
Celery 2 B,C
Cereal grains 0.5 B,C,T
Cherries 10c B,C,T
Citrus fruits 10c B,C,T
Coffee beans 0.1d C
Common beane 2 C
Cucumber 0.5 B,C,T
Eggs (poultry) 0.1d B,T
Egg plant 0.5 C
Gherkin 2 C,T
Hops, dry 50 C
Lettuce, head 5 B,C,T
Mango 2 B
Melons, except watermelons 2c B,C
Milk 0.1d B
Mushrooms 1 B,C,T
Nectarine 2 B
Onion, bulb 2 C,T
Peach 10c B,C,T
Peanut 0.1d B,C
Peanut fodder 5 B,C
Peppers 5 C
Pineapple 20c B
Plums (including prunes) 2c B,C,T
Pome fruit 5c B,C,T
Potato 3c,f B,C
Poultry meat 0.1d B,T
Rape seed 0.05d C
Rice straw and fodder, dry 15 B,C,T
Sheep meat 0.1d B
Soya bean, dry 0.2 C
Soya bean fodder 0.1d C
Squash, summer 0.5 B
Table 5 (contd).
Commodity MRL (mg/kg) Applicationb
Sugar beat 0.1d B,C,T
Sugar beat leaves on tops 10 B,C,T
Swedeg 0.1d C
Sweet potato 1 B
Taro 0.1d B
Tomato 5 B,C,T
Tree nuts 0.1d B
Wheat straw and fodder, dry 5 B
Winter squash 0.5 B
a From: FAO/WHO (1988b)
b B = benomyl; C = carbendazim; T = thiophanate-methyl
c MRL based on post-harvest use
d At or about the limit of detection
e JMPR recommended 2 mg/kg for dry, dwarf, lima and snap beans. These
are all covered by "VP 0526, Common bean" and "VP 0071, Beans,
dry" in the new classification
f washed before analysis
g Described as rutabagas in 1983 recommendation
Table 6. Established inhalation exposure limitsa
Country and agency Compound TWAb STELc
(mg/m3) (mg/m3)
Australia benomyl 10 -
Belgium benomyl 10 -
Denmark benomyl 5 -
Finland benomyl 10 30
France benomyl 10 -
Switzerland benomyl 10 -
United Kingdom benomyl 10 15
USA: ACGIHd benomyl 10 -
USA: NIOSHe/OSHAf benomyl 10 -
(inhalable dust)
USA: NIOSH/OSHA benomyl 5 -
(respirable dust)
USSR carbendazim - 0.1
a From: ILO (1991)
b Time-weighted average
c Short-term exposure limit
d American Conference of Governmental Industrial Hygienists
e National Institute of Occupational Safety and Health
f Occupational Safety and Health Administration
Air concentrations of benomyl ranged from 0.0074 to 0.053
mg/m3 (average 0.027 mg/m3) during its application in
greenhouses. Spraying tall plants (over 1.5 m) caused three times
higher concentrations in air than spraying low plants. No detectable
amounts of benomyl or its metabolites (carbendazim, 4-HBC and 5-HBC)
were found in the urine of applicators during the 48 h following the
application. However, information describing protective clothing,
ventilation, and other hygienic factors was not reported (Liesivuori
& Jääskeläinen, 1984).
6. KINETICS AND METABOLISM
Benomyl is extensively metabolized by animals, as described in
detail in section 6.3. Metabolite names and structures are given in
Table 6 and Figures 2 and 3.
6.1 Absorption
Absorption in ChR-CD male rats was monitored after dermal
application of 0.1, 1, 10, and 100 mg benomyl (as 2-14C-Benlate 50
WP) at 0.5, 1, 2, 4 and 10 h intervals. Four rats were used for each
treatment and time interval. Benomyl was slowly absorbed across an
area of skin (16% of the animal), appearing in the blood and urine
within 30 min after treatment and reaching a maximum between 2 and 4
h after dosing (Belasco, 1979b). The concentration of benomyl and
its metabolites in the blood peaked at 0.05 mg/litre (2 h sample) in
the low-dose group (0.1 mg) and at 0.10 mg/litre (4 h sample) in the
high-dose group (100 mg). This represented a 20-fold increase in
blood concentration after a 1000-fold dose increase. Thus,
absorption into the bloodstream was non-linear with respect to dose.
An in vitro study on the penetration of formulated benomyl
(Benlate 50 WP) through human skin showed that benomyl penetrates
human skin poorly when it is applied as a recommended spray strength
solution. Much less penetration was detected when dry concentrated
benomyl was applied (Ward & Scott, 1992).
In a rat gavage study, the absorption of carbendazim given in
the form of a corn oil suspension was estimated to be approximately
80% (Monson, 1990).
6.2 Distribution and accumulation
Blood levels of benomyl and its metabolites in male rats were
measured 6 and 18 h after exposure in male rats. The rats were
exposed to time-weighted averages of 0.32 and 3.3 mg/litre of air
for 0.5, 1, 2 and 6 h. The methodology did not distinguish between
benomyl and carbendazim. At both exposure levels, the blood
concentrations of benomyl/carbendazim were greater than that of
5-HBC 6 h after exposure; the levels were 0.39-2.3 mg/litre and
0.25-1.2 mg/litre, respectively. At 18 h after exposure, only 5-HBC
was detected in the blood (1.1 mg/litre) and this only at the
highest dose. Urinary metabolites consisted primarily of 5-HBC, and
limited amounts of benomyl/carbendazim were also detected (Turney,
1979).
Table 7. Chemical names of benomyl and its metabolites in animalsa
Common or abbreviated Chemical name
name
Benomyl Carbamic acid, [1-(butylamino)carbonyl]-
1H-benzimidazol-2-yl]-, methyl ester
Carbendazim (MBC) methyl (1-H-benzimidazol-2-yl)carbamate
5-HBC methyl (5-hydroxy-1H-benzimidazol-2-yl)-
carbamate
4-HBC methyl (4-hydroxy-1H-benzimidazol-2-yl)-
carbamate
5-HBC-Sb 2-[(methoxycarbonyl)amino]-1H-benzimidazol-5-
yl hydrogen sulfate
5-HBC-Gc [2-[(methoxycarbonyl)amino]-1H-benzimidazol-
5-yl] ß-D-glucopyranosiduronic acid
MBC-4,5-epoxide
MBC-5,6-epoxide
MBC-4,5-dihydrodiol (4,5-dihydro-4,5-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-5,6-dihydrodiol (5,6-dihydro-5,6-dihydroxy-1H-benzimidazol-
2-yl) carbamate
MBC-4,5-diol
MBC-5,6-diol
5-OH-6-GS-MBCd S-[5,6-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5-OH-4-GS-MBC S-[4,5-dihydro-5-hydroxy-2-(methoxycarbonyl
amino)-1H-benzimidazol-4-yl]glutathione
5,6-HOBC-N-oxide methyl (6-hydroxy-5-oxo-5H-benzimidazol-2-
yl)-carbamate-N-oxide
Table 7 (contd).
Common or abbreviated Chemical name
name
5,6-HOBC-N-oxide-G [2-[(methoxycarbonyl)amino]-6-oxo-6H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid-N-oxide
5,6-DHBC methyl (5,6-dihydroxy-1H-benzimidazol-2-yl)
carbamate
5,6-DHBC-G [6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl] ß-D-glucopyranosiduronic
acid
5,6-DHBC-S 6-hydroxy-2-[(methoxycarbonyl)amino]-1H-
benzimidazol-5-yl 5-(hydrogen sulfate)
2-AB 2-aminobenzimidazole
2-AB dihydrodiol 2-amino-4,5-dihydro-4,5-dihydroxy-1H-
benzimidazol
5-HAB 5-hydroxy-2-aminobenzimidazole
a From: Krechniak & Klosowska (1986); Monson (1986a,b); Monson (1990)
b S = conjugate with sulfuric acid
c G = conjugate with glucuronic acid
d GS = conjugate with glutathione
In a study by Han (1979), ten male ChR-CD rats were given 1 and
10 µg benomyl intravenously (as 14C-Benlate 50% WP). Radioactivity
was found in the urine as 5-HBC at 6, 12 and 24 h after dosing, and
there was little radioactivity in the blood or faeces at these
sampling times. No radioactivity (< 0.1%) was found in any tissue
after 24 h except in blood, which contained trace quantities of
14C residues.
In a further study, three groups of five rats of each sex were
gavaged with [phenyl(U)-14C] carbendazim. One group received a
single dose of 14C-carbendazim (50 mg/kg). The second group
received a single dose of 14C-carbendazim (50 mg/kg) following 14
days of pre-conditioning with non-radiolabelled carbendazim (50
mg/kg per day). The third group received a single dose of
14C-carbendazim (1000 mg/kg). For all groups, > 98% of the
recovered radioactivity was excreted in the urine or faeces by the
time of sacrifice (72 h after 14C dosing). The 14C remaining in
tissues was < 1% of the applied dose (Monson, 1990).
In a study by Belasco et al. (1969), 14C-benomyl was
administered to male ChR-CD rats and the blood and testes were
analysed. The fungicide was given by gavage as: (a) a single dose of
1000 mg/kg to five rats, which were sacrificed either 1, 2, 4, 7 or
24 h later; (b) 10 repeated doses of 200 mg/kg per day to two rats,
which were sacrificed either 1 or 24 h after the last dose. In
addition, blood and testes from rats fed 2500 mg/kg diet for one
year were analysed. In rats given 1000 mg/kg, results show that: (a)
the total 14C radioactivity (calculated as benomyl) ranged from 3
to 13 ppm in the blood and from 2 to 4 ppm in the testes; (b) 5-HBC
appeared in the blood and testes as early as 1 h after dosing; and
(c) the concentration of benomyl and/or carbendazim decreased with
time and there was a corresponding increase in the concentration of
5-HBC in the blood and testes. Analyses of blood and testes from
rats given 10 repeated oral doses of 200 mg/kg per day showed that
one hour after the last dose no benomyl/carbendazim (< 0.1 ppm) was
detected and only low levels of 5-HBC were found (1.5 ppm blood and
0.3 ppm in testes). No benomyl/carbendazim or 5-HBC (< 0.1 ppm) was
found 24 h after the last dose. With rats fed 2500 mg benomyl/kg
diet for one year, no benomyl/carbendazim (< 0.1 ppm) was detected
in blood or testes. Only a minimal amount of 5-HBC was found in
blood (0.2 ppm) and none was found in the testes (< 0.1 ppm)
(Belasco et al., 1969).
In a series of metabolic studies, benomyl and/or Benlate (50%
benomyl formulation) were administered either by gavage or in the
diet to pregnant ChR-CD rats to determine the concentrations of
benomyl, carbendazim and two carbendazim metabolites (4-and 5-HBC)
in maternal blood and embryonic tissue (Culik, 1981a,b). Dosing took
place on days 7 to 16 of gestation at levels of 125 mg/kg body
weight per day via gavage or 5000-10 000 mg/kg diet (approximately
400-800 mg/kg body weight). Blood samples from the dams and tissue
samples from their embryos were examined on the first, sixth and
tenth days of dietary administration and on days 12 and 16 of gavage
administration. Embryos and maternal blood were analysed 1, 2, 4, 8
and 24 h after gavage.
The levels of benomyl/carbendazim in maternal blood and
embryonic tissues, 24 h following each dose, markedly decreased with
the number of treatments. The level of benomyl (one hour after
treatment) ranged from 0.98 to 8.4 mg/kg with a mean value of 5.0
mg/kg on the first day of treatment. After 10 treatments, the levels
of benomyl/carbendazim ranged from < 0.12 to 0.39 mg/kg (one hour
after last treatment). In the embryo there was 0.13 mg/kg
benomyl/carbendazim after the tenth treatment compared with a mean
of 1.9 mg/kg after the first treatment. The half-life of benomyl in
maternal blood was approximately 45 min and was less in the embryos.
The level of 5-HBC (0.84-2.9 mg/kg) 2 h following the last gavage
increased with the number of exposures, the half-life in the blood
being 2-3 h in the dam and 4-8 h in the embryo. 4-HBC was not
detected.
In the dietary studies, the levels of benomyl, carbendazim and
4-HBC were too low to be measured in the embryonic tissue. 4-HBC
could not be detected in the dams. Irrespective of the dose level
(5000 and 10 000 mg/kg diet active ingredient) of benomyl or
Benlate, the level of benomyl/carbendazim in maternal blood was very
low. In three separate groups of animals, the mean highest blood
concentrations of benomyl/carbendazim were 0.35, 0.61 and 0.23 mg/kg
in each group of dams. The mean highest value of 5-HBC (5000 mg
benomyl/kg diet) was 0.44 mg/kg in the blood and 0.33 mg/kg in the
embryos. Animals fed benomyl or Benlate at a level of 10 000 mg/kg
diet had 5-HBC levels an order of magnitude higher (Culik, 1981a,b).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approxi mately
17 h after the tenth dose, the cow was sacrificed and organ, tissue
and blood samples were subsequently collected. 14C residue levels
in the milk averaged 0.2 mg/kg (calculated as benomyl), 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids. Small amounts of
radioactivity were detected in the liver (4.12 mg/kg) and kidney
(0.25 mg/kg), most of which was bound. No significant levels of
radioactivity (0.06 mg/kg) were detected in other tissues or fat
(Monson, 1985).
Lactating and non-lactating goats were given daily capsule
doses of [2-14C]-benomyl, equivalent to 36 and 88 mg/kg,
respectively, in the total daily diet, for five days. Milk residues
accounted for approximately 2% of the total dose. Approximately 25%
of the milk radioactivity was incorporated into the natural milk
components casein and whey protein. There were no detectable
residues in muscle tissue and fat (< 0.01 mg/kg). However,
radioactivity detected in liver and kidney amounted to 3.8 and 0.09
mg/kg (calculated as benomyl equivalents), respectively (Han, 1980).
In a study by Johnson (1988), the total 14C residue and
metabolic fate of carbendazim in the liver was examined in
non-lactating female goats. Twelve goats were administered a
feed-rate-equivalent dose of [phenyl(U)-14C]-carbendazim (> 50
mg/kg), once a day, for up to 30 days. Within 2 weeks of dose
initiation, a plateau of 14C residues in the liver was achieved at
a level of 9.48 mg/kg (group mean of the total radiolabelled liver
residues for goats sacrificed 2, 3, and 4 weeks after initiation of
dosing). The total 14C residue levels in the liver decreased to
5.17, 3.55 and 1.67 mg/kg (calculated as carbendazim equivalents) 1,
2 and 3 weeks, respectively, after dosing ceased. The elimination
half-life for total 14C residues from the liver, based on this
depuration data, was calculated to be approximately 9 days. The
half-life for removal of carbendazim from the general circulation,
based on 14C-carbendazim equivalent whole blood levels, was
approximately 10 h. The level of bound, non-extractable 14C
residues in the liver of goats sacrificed after 28 days was 1.0
mg/kg.
The results of this study suggest that levels of carbendazim-
derived residues do not accumulate beyond 2 weeks when goats are
exposed to a constant feed level of 50 mg carbendazim/kg.
Furthermore, discontinuation of exposure results in a clearing of
residues from the liver (Johnson, 1988).
The metabolism of benomyl was studied in laying hens by Monson
(1986a). Two hens were individually dosed daily for three
consecutive days with 3.5 mg [2-14C]-benomyl at a rate equivalent
to 29 mg/kg in the daily feed, and two hens were individually dosed
with 3.29 mg [phenyl(U)-14C]-benomyl at rates equivalent to 27
mg/kg in the daily feed. Faeces and eggs from the previous 24 h were
collected just before each dosing. Twenty-two hours after the third
dose, the hens were killed and samples of muscle (breast and thigh),
liver, kidney and fat were analysed. The concentration of
radioactivity (calculated as benomyl equivalents) in the tissues and
day-3 eggs of the [2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-
dosed hens, respectively, was as follows: liver (0.54 and 0.41
mg/kg), kidney (0.28 and 0.16 mg/kg), thigh and breast muscle (both
0.01 mg/kg), fat (0.05 and 0.02 mg/kg) and eggs (0.08 and 0.05
mg/kg).
The distribution of benomyl in this study was comparable to
that in a 20-hen [2-14C]-carbendazim metabolism study. The
concentrations of radioactivity, calculated as mg carbendazim/kg, in
the high-dose laying hens (dose equivalent 120 mg/kg carbendazim in
the diet) were liver (2.63), kidney (1.74), thigh muscle (0.06),
breast muscle (0.05), fat (0.03), day-6 eggs (0.63) (Monson, 1986b).
This study is discussed in detail in the Environmental Health
Criteria monograph on Carbendazim (WHO, 1993).
When bluegill sunfish were exposed to benomyl, carbendazim and
2-AB at nominal concentrations of 0.05 mg/litre (measured
concentrations of 0.01 to 0.04 mg/litre) and 5.0 mg/litre (measured
concentrations of 2 to 5 mg/litre), no residues were found in the
tissues of fish exposed to low levels of these three compounds.
Detectable residues were found in the tissues of fish exposed to the
high levels, but there was no build-up or bioconcentration with time
(DuPont, 1972).
6.3 Metabolic transformation
Benomyl is extensively metabolized by rats to carbendazim,
which is then further metabolized. Studies with rats administered
benomyl intravenously (Han, 1979), dermally (Belasco, 1979b) or by
inhalation (FAO/WHO, 1985a) showed that 5-HBC is the main urinary
metabolite, some carbendazim also being present.
In a rat gavage study (Monson, 1990; see section 6.2),
carbendazim was extensively metabolized. Three dosing regimens (five
rats of each sex per group) were used: a single oral dose of 50
mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with non-radiolabelled carbendazim at 50
mg/kg for 14 days (pre-conditioned low dose); and a single oral dose
of 1000 mg/kg (high dose). The 48-h urine from the low-dose and the
high-dose rats and the 14-day urine from the pre-conditioned
low-dose group were collected. The total recovery from urine was
61.5 and 61.7% of given doses for the low-dose and pre-conditioned
low-dose male groups, 53.2 and 59.3% for the low-dose and
pre-conditioned low-dose female groups, and 39 and 41% for both male
and female high-dose groups, respectively. 5-HBC-S (21-43% of given
dose) was identified as the main metabolite, except in the case of
the pre-conditioned low-dose and high-dose female rat groups
(5.5-10%), while in all female rat groups 5,6-HOBC-N-oxide-G
(10-19%) was predominant. Both 5,6-DHBC-S and 5,6-DHBC-G were
identified as minor metabolites.
In the same study, the faeces were collected at the same
periods as the urine. The total recovery from faeces was about 24%
for the low-dose and pre-conditioned low-dose male groups, 33-38%
for the low-dose and pre-conditioned low-dose female groups, and
higher (> 60%) for both male and female high-dose groups. Unchanged
carbendazim was about 10-15% of the given dose in the faeces of
high-dose rats (Monson, 1990). The proposed metabolic pathway for
benomyl in rats is given in Fig. 2.
When a lactating Holstein cow was dosed by capsule twice daily
(515 mg per dose), equivalent to 50 mg/kg diet, for 5 consecutive
days with [2-14C]-benomyl, the major metabolites of whole milk
were 5-HBC (0.06 mg/litre), 4-HBC (0.03 mg/litre) and MBC-4,5-
dihydrodiol (< 0.07 mg/litre). The proportions of radioactive
residues in the urine were 46% 5-HBC, 3% 4-HBC, and 50% polar
aqueous-soluble metabolites, which included MBC-4,5-dihydrodiol,
2-AB-dihydrodiol and 5-OH-4-GS-MBC (Monson, 1985).
 Lactating and non-lactating goats were given five consecutive
daily doses of 2-14C-benomyl by capsule at rates equivalent to 36
and 88 mg/kg, respectively, in the total daily diet. The main
metabolite in milk was 5-HBC, and there were minor amounts of 4-HBC
and 5-HAB. The principal metabolites in urine and faeces were 5-HBC
and 4-HBC. The main identified metabolite in the kidney and liver
was 5-HBC (about 6% of the residue). Much of the liver residue was
incorporated into glycogen, protein, fatty acids and cholesterol,
and accounted for approximately 35% of the liver residues. Further
characterization of the bound liver tissue residues following
enzymatic and trifluoroacetic anhydride hydrolysis identified
5-hydroxy-benzimidazole moieties as the principal (at least 77%)
14C residue in goat liver. No free benomyl, carbendazim or 5-HBC
was detected in the liver (Han, 1980; Hardesty, 1982).
In a further study, the total 14C residue and metabolic fate
of carbendazim in the liver were examined in non-lactating female
goats. Twelve goats were administered a dose equivalent to 50 mg/kg
feed once a day for up to 30 days. Extraction of composite liver
homogenate from goats sacrificed 4 weeks after initiation of dosing
("plateau level") indicated that the major ethyl acetate extractable
and identifiable radiolabelled residues in the liver were 5-HBC (2
to 3 mg/kg) and carbendazim (approximately 0.2 mg/kg) (Johnson,
1988).
The metabolism of [2-14C]-benomyl and [phenyl(U)-14C]-
benomyl has been studied in laying hens (see section 6.2 for a
detailed description of the study). Benomyl was extensively
metabolized to carbendazim, 5-HBC, MBC-4,5-dihydrodiol and a
metabolite tentatively identified as 5-OH-4-GS-MBC. The metabolic
profile observed in hens indicates that the benzimidazole ring is
not broken during metabolism (Monson, 1986a). The proposed metabolic
pathway for benomyl in the laying hen is given in Fig. 3.
Monson (1991) analysed the release and characterization of
bound benomyl and carbendazim metabolites in diary cow, goat, hen
and rat liver after treatment with 14C-benomyl or
14C-carbendazim via Raney nickel desulfurization and acid
dehydration. Using this technique, he was able to show that bound
14C residue was released from the liver of cows (76% bound before
desulfurization and 36% bound after desulfurization) and hens (58%
bound before desulfurization and 19% bound after desulfurization).
The major part of the reduced residue was identified as 5-HBC,
5,6-HOBC or carbendazim, suggesting that the bound liver residue
consisted of conjugates of benzimidazole-related products and not
natural products resulting from breakdown and incorporation.
In fish, benomyl and carbendazim are metabolized to 5-HBC
(Dupont, 1972).
Lactating and non-lactating goats were given five consecutive
daily doses of 2-14C-benomyl by capsule at rates equivalent to 36
and 88 mg/kg, respectively, in the total daily diet. The main
metabolite in milk was 5-HBC, and there were minor amounts of 4-HBC
and 5-HAB. The principal metabolites in urine and faeces were 5-HBC
and 4-HBC. The main identified metabolite in the kidney and liver
was 5-HBC (about 6% of the residue). Much of the liver residue was
incorporated into glycogen, protein, fatty acids and cholesterol,
and accounted for approximately 35% of the liver residues. Further
characterization of the bound liver tissue residues following
enzymatic and trifluoroacetic anhydride hydrolysis identified
5-hydroxy-benzimidazole moieties as the principal (at least 77%)
14C residue in goat liver. No free benomyl, carbendazim or 5-HBC
was detected in the liver (Han, 1980; Hardesty, 1982).
In a further study, the total 14C residue and metabolic fate
of carbendazim in the liver were examined in non-lactating female
goats. Twelve goats were administered a dose equivalent to 50 mg/kg
feed once a day for up to 30 days. Extraction of composite liver
homogenate from goats sacrificed 4 weeks after initiation of dosing
("plateau level") indicated that the major ethyl acetate extractable
and identifiable radiolabelled residues in the liver were 5-HBC (2
to 3 mg/kg) and carbendazim (approximately 0.2 mg/kg) (Johnson,
1988).
The metabolism of [2-14C]-benomyl and [phenyl(U)-14C]-
benomyl has been studied in laying hens (see section 6.2 for a
detailed description of the study). Benomyl was extensively
metabolized to carbendazim, 5-HBC, MBC-4,5-dihydrodiol and a
metabolite tentatively identified as 5-OH-4-GS-MBC. The metabolic
profile observed in hens indicates that the benzimidazole ring is
not broken during metabolism (Monson, 1986a). The proposed metabolic
pathway for benomyl in the laying hen is given in Fig. 3.
Monson (1991) analysed the release and characterization of
bound benomyl and carbendazim metabolites in diary cow, goat, hen
and rat liver after treatment with 14C-benomyl or
14C-carbendazim via Raney nickel desulfurization and acid
dehydration. Using this technique, he was able to show that bound
14C residue was released from the liver of cows (76% bound before
desulfurization and 36% bound after desulfurization) and hens (58%
bound before desulfurization and 19% bound after desulfurization).
The major part of the reduced residue was identified as 5-HBC,
5,6-HOBC or carbendazim, suggesting that the bound liver residue
consisted of conjugates of benzimidazole-related products and not
natural products resulting from breakdown and incorporation.
In fish, benomyl and carbendazim are metabolized to 5-HBC
(Dupont, 1972).
 6.4 Elimination and excretion
Absorbed benomyl and carbendazim are rapidly excreted in the
urine and faeces.
In a study where rats were administered 1 or 10 µg formulated
14C-benomyl (50% wettable powder) in a single intravenous dose by
tail injection, more than 80% of the dose was excreted in the urine
and faeces within 6 h after injection and the total urine and faeces
recovery was > 95% in 24 h (Han, 1979).
[Phenyl(U)-14C]-carbendazim was administered by gavage to
Sprague-Dawley rats using three dosing regimens: a single oral dose
of 50 mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with unlabelled carbendazim of 50 mg/kg for
14 days (pre-conditioned low dose); and a single oral dose of 1000
mg/kg (high dose). Each dosing group consisted of five animals of
each sex. A preliminary study conducted with two rats of each sex,
each rat having received a single oral dose of 50 mg/kg,
demonstrated that 95% of the radioactivity excreted in the urine and
faeces was recovered within 72 h after dosing and that < 1% of the
dose was expired as volatile metabolites. In the full study, > 98%
of the recovered radioactivity was excreted by the time of sacrifice
(i.e. 72 h after dosing) for each dosing group. Urinary excretion
accounted for 62% to 66% of the dose in males and 54% to 62% of the
dose in low-dose and pre-conditioned low-dose female groups. In the
high-dose group, this pathway accounted for 41% of the dose in all
animals. Elimination of radiolabel in faeces accounted for virtually
all of the remaining radiolabel. There were no apparent differences
between male and female rats with respect to the extent of
absorption and extent and rate of elimination of 14C-carbendazim
equivalents within a given treatment group (Monson, 1990).
In a study by Han (1978), two male ChR-CD-1 mice were fed a
diet of non-radiolabelled benomyl (2500 mg/kg) for 21 days and were
then gavaged with 2.5 mg [2-14C]-benomyl in corn oil. An identical
experiment was performed with one male ChR-CD hamster. More than 90%
of the radioactivity was eliminated in the urine and faeces within
72 h (Han, 1978).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approximately
17 h after the tenth dose, the cow was sacrificed and organs,
tissues and blood were collected for analysis. At sacrifice, 65% of
the radiolabel had been excreted in the urine, 21% in the faeces and
0.4% in the milk. Carbon-14 residue levels in the milk averaged 0.2
mg/litre (calculated as benomyl equivalents) with 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids (Monson, 1985).
Lactating and non-lactating goats were given 5 consecutive
daily doses of [2-14C]-benomyl by capsule at rates equivalent to
36 and 88 mg/kg, respectively, in the total daily diet. Most of the
radioactivity (96%) had been eliminated in the urine and faeces by
the time of sacrifice (Han, 1980).
The excretion of benomyl was studied in laying hens dosed daily
for three consecutive days with 3.5 mg [2-14C]-benomyl or 3.29 mg
[phenyl(U)-14C]-benomyl. At sacrifice (22 h after the last dose),
an average of 107% and 95% of the dose had been excreted for the
[2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-dosed birds,
respectively (Monson, 1986a).
In a similar study on 14C-carbendazim, groups of laying hens
were fed at a rate equivalent to 5 and 120 mg/kg diet. At sacrifice,
24 h after the sixth daily dose, an average of 95% of the dose had
been excreted by the low-dose birds and 92% by the high-dose birds
(Monson, 1986b).
6.5 Reaction with body components
An in vitro study using acetyl cholinesterase from bovine
erythrocytes showed that benomyl did not inhibit this enzyme. The
acetyl cholinesterase inhibition constant (KI) for benomyl was
greater than 1 x 10-3 mol/litre (Belasco, 1970). Another in vitro
study by Krupka (1974) verified that benomyl did not inhibit either
acetyl cholinesterase or butyryl cholinesterase.
In a study by Guengerich (1981), the effects of benomyl and
carbendazim on hepatic enzymes were studied in male and female
Crl-CD rats and CD-1 mice. The treatment groups included animals fed
for 28 days with diets that contained benomyl or carbendazim at
concentrations of 0, 10, 30, 100, 300, 1000 or 3000 mg/kg. In these
studies, microsomal epoxide hydrolase and cytosolic glutathione- S-
transferase were monitored in subcellular fractions isolated from
the livers of animals in each treatment group. Liver weights were
also recorded. Elevated mean absolute liver weights were observed at
1000 and 3000 mg carbendazim/kg in both male and female rats and at
300 mg carbendazim/kg in female rats. However, the only
significantly elevated liver weight was found in females after a
dose of 3000 mg benomyl/kg. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced
epoxide hydrolase in both sexes of rats and mice at 1000 and 3000
mg/kg. Induction of glutathione- S-transferase was observed at 3000
mg/kg in the case of both benomyl and carbendazim. In general, the
level of induction seemed to be slightly greater in females than
males. There did not appear to be any substantial difference in
enzyme induction between rats and mice.
In a study by Shukla et al. (1989), levels of gamma-glutamyl
transpeptidase (GGT) were evaluated after benomyl exposure. Female
albino rats (eight per group) and female Swiss albino mice (eight
per group) were given 1000 and 4000 mg benomyl/kg feed for 15 days,
and blood and liver GGT levels were analysed. Benomyl exposure
increased the activity of both blood and liver GGT in both rats and
mice, and the degree of induction was dose related (Shukla et al.,
1989).
7. EFFECTS ON LABORATORY MAMMALS; IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of benomyl in several animal species is
summarized in Table 8. Benomyl has an oral LD50 in the rat of >
10 000 mg/kg and an inhalation 4-h LC50 > 4 mg/litre. Several
other minor metabolites were evaluated and the approximate lethal
doses were 3400 mg/kg for 2-AB, 7500 mg/kg for 5-HBC, 17 000 mg/kg
for BUB and 17 000 mg/kg for STB.
In a study by Littlefield & Busey (1969), three groups of male
dogs (around 10 dogs/group) were exposed to benomyl at air
concentrations of 0, 0.65 and 1.65 mg/litre. One half of the dogs in
each group were killed on day 14 and the remainder on day 28. The
liver weight of the high-dose dogs was significantly decreased on
day 28. For further discussion of single dose toxic effects, see
section 7.5.1.
7.2 Short-term exposure
7.2.1 Gavage
In a 14-day rat study, benomyl (200 and 3400 mg/kg in peanut
oil) was given by gavage five times a week for two weeks to six male
ChR-CD rats per group. Four out of six rats died after 5, 7, 8 and 9
doses, respectively, of 3400 mg/kg. No clinical signs of toxicity
were observed in the group treated with 200 mg/kg per day.
Degeneration of germinal epithelium, multinucleated giant cells and
reduction or absence of sperm were observed in the testes after
multiple doses of 3400 mg/kg per day. Less than 10% of the
testicular tubules were affected in only two out of six animals
dosed with 200 mg/kg. At the high dose level, there was erosion and
thickening of the squamous mucosa of the stomach with submucosal
inflammation and a decrease in the large globular-shaped vacuoles
located centrolobularly in the liver (Sherman & Krauss, 1966).
7.2.2 Feeding
7.2.2.1 Rat
In a 90-day study by Sherman et al. (1967), groups of rats
(4-week-old ChR-CD rats, 16 rats of each sex per group) were fed
Benlate 70 WP (72% benomyl) in the diet at levels of 0, 100, 500 and
2500 mg benomyl/kg. The animals were observed daily for behavioural
changes and body weights, and food consumption was recorded at
weekly intervals. Haematological examinations were conducted on six
male and six female rats in each group at 30, 60 and 90 days.
Routine urine and plasma alkaline phosphatase and glutamic pyruvic
transaminase activity analyses were performed on the same animals.
After 96-103 days of continuous feeding, 10 male and 10 female rats
in each group were killed, and selected organs were weighed and
examined microscopically. The remaining six male and female animals
in each group after the terminal sacrifice were used in a
one-generation reproductive study. No effect was observed with
respect to reproduction or lactation in the delivery or rearing of
the F1A litters. There were no compound-related effects on weight
gain, food consumption, food efficiency, clinical signs, or on
haematology, biochemistry or urinalysis determinations. The
liver-to-body weight ratio in females was slightly increased at 2500
mg/kg, compared with control rats. Gross and microscopic
examinations of tissues and organs showed no significant effects
attributable to the presence of benomyl in the diet at levels up to
and including 2500 mg/kg.
7.2.2.2 Dog
Groups of beagle dogs (four males and four females per group;
7-9 months old) were administered benomyl 50% wettable powder in the
diet at dosage levels of 0, 100, 500 and 2500 mg/kg diet (based on
active ingredient) for three months (this corresponded to treatment
levels of 0, 3.8, 18.4 and 84 mg/kg body weight). Food consumption
and body weight data were recorded weekly, and clinical laboratory
examinations (including haematology, biochemistry and urinalysis)
were performed pre-test and after 1, 2 and 3 months of feeding. At
the conclusion of the study, selected organs were weighed and
subjected to gross and microscopic examinations. No mortality or
adverse clinical effects were observed over the course of the study,
and growth and food consumption were not effected by the treatment.
Urine parameters showed no differences from the control, and there
were no dose-related effects on the haematological values. Alkaline
phosphatase and glutamic pyruvic transaminase activities were
increased in high-dose males and females. There were statistically
significant decreases in the albumin/globulin ratio in either males
or females fed 2500 mg/kg diet. Organ-to-body weight ratio changes
were observed in the high-dose males and females for the thymus
(decreased) and thyroid (increased). One of the four females fed
2500 mg/kg diet had an enlarged spleen at the end of the exposure
period, as well as a decreased erythrocyte count, haemoglobin
concentration and haematocrit value. Histopathological examination
revealed myeloid hyperplasia of the spleen and bone marrow and
erythroid hyperplasia of bone marrow. This did not appear to be
compound related since group mean values were not significantly
different. Three out of four males fed 2500 mg/kg diet had reduced
relative prostate weights when compared with controls. Microscopic
examinations of tissues and organs did not indicate changes in dogs
fed benomyl for 90 days. The no-observed-effect level (NOEL) was 500
mg/kg diet (Sherman, 1968).
Table 8. Acute toxicity of benomyl and its metabolites for laboratory mammals
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl ratb M/F 10/dose oral peanut oil LD50 > 10 000 Sherman (1969a)
rabbitc M 1/dose oral 50% wettable powder ALD > 3400 Fritz (1969)
dogd M 1 oral evaporated milk and ALD > 1000 Sherman (1969b)
water (1:1)
Benlate OD (50% rat M 10/dose oral corn oil LD50 > 12 000 Hostetler (1977)
benomyl)
Fungicide 1991 rabbit M/F 4/dose dermal (4 h) 50% wettable powder LD50 > 10 000 Busey (1968a)
(50% benomyl)
rat M 6/dose inhalation (4 h) 50% wettable powder LC50 > 4.01 mg/litre Busey (1968b)
(analytical)
dog M 10/dose inhalation 50% wettable powder LC50 > 1.65 mg/litre Littlefield & Busey
(analytical) (1969)
Benlate fungicide rat M/F 10/dose oral aqueous suspension LD50 > 10 000 Sherman (1969a)
(52-53% benomyl)
rat M 5/dose inhalation 50% wettable powder LC50 > 0.82 mg/litre Hornberger (1969)
Benlate PNW (50% rabbit M/F 10/dose dermal 50% wettable powder LD50 > 2000 Gargus & Zoetis
benomyl) (1983c)
Benlate 50 DF (50% rat M/F 5/dose oral aqueous suspension LD50 > 5000 Sarver (1987)
benomyl)
rabbit M/F 5/dose dermal 50% dry flowable LD50 > 2000 Brock (1987)
Table 8 (contd).
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl metabolites
2-Benzimidazole carbamic rat M/F 10/dose oral corn oil LD50 > 10 000 Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole- rat M 1/dose oral corn oil ALD > 7500 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole rat M 1/dose oral peanut oil ALD > 3400 Fritz & Sherman
(1969)
Benzimidazole 2- rat M 1/dose oral corn oil ALD > 17 000 Dashiell (1972)
(3-butylureido)
S-Triazine, 3-butyl- rat M 1/dose oral corn oil ALD > 17 000 Barbo & Carroll
benzimidazole (1,2a), (1972)
-2,4(1H,3H)-dione
a Based on active ingredient; ALD = approximate lethal dose
b ChR-CD or Crl:CD rats
c New Zealand white rabbits
d Beagle dogs
7.2.3 Dermal
In a study on groups of five male and five female New Zealand
albino rabbits, weighing 2 to 2.4 kg, 15 dermal applications of a
50% benomyl formulation (equivalent to 1000 mg/kg) were made on both
abraded and intact abdominal skin sites. The animals were exposed
for 6 h/day, 5 days/week for 3 weeks. After each daily application,
the abdomen was washed with tap water. Observations were made daily
for mortality and toxic effects and weekly for body weight changes.
Gross necropsy and microscopic examinations were performed. Slight
erythema, oedema and atonia were observed at both abraded and intact
skin sites. Slight to moderate desquamation occurred throughout the
exposure period. No apparent compound-related body weight or organ
weight changes were reported. Microscopic examination of the males
demonstrated that benomyl produced degeneration of the spermatogenic
elements of the seminiferous tubules of the testes, the changes
including vacuolated and multi-nucleated spermatocytes (Busey,
1968d).
In a separate repeated-dose dermal study, groups of five male
and five female New Zealand albino rabbits, weighing 3 kg, were
exposed to doses of benomyl equivalent to 0, 50, 250, 500, 1000 and
5000 mg/kg applied to non-occluded abraded dorsal skin sites 6 h a
day, five days a week, for three weeks. Test material was removed by
washing the skin site and drying with a towel. There were decreased
body weight gains for both males and females at the two highest dose
levels. Mild to moderate skin irritation was reported for all groups
but was most notable at the highest dose level. Diarrhoea, oliguria
and haematuria were observed in males and females at 1000 and 5000
mg/kg. Decreased average testicular weights and testes-to-body
weight ratios were observed at 1000 mg/kg only. There were no
histopathological changes reported (Hood, 1969).
7.2.4 Inhalation
In an inhalation study, groups of 20 male and 20 female CD rats
were exposed, nose-only, 6 h a day for 90 days, to 0, 10, 50 and 200
mg benomyl/m3. At 45 and 90 days, blood and urine samples were
collected from 10 rats of each sex per group for clinical analysis
and then killed for pathological examination. After approximately 45
days of exposure, test-compound-related degeneration of the
olfactory epithelium was observed in all males and in eight of the
ten females exposed to 200 mg/m3. Two male rats exposed to 50
mg/m3 had similar but less severe olfactory degeneration. After
approximately 90 days of exposure, all of the animals showed
olfactory degeneration at 200 mg/m3, along with three males
exposed to 50 mg/m3. No other compound-related pathological
effects were observed. Male rats exposed to 200 mg/m3 had
depressed mean body weights compared to controls and this correlated
with a reduction in food consumption (Warheit et al., 1989).
7.3 Skin and eye irritation; sensitization
7.3.1 Dermal
A 50% wettable powder applied to the clipped intact and abraded
abdomen of albino rabbits produced moderate to marked erythema,
slight oedema and slight desquamation. Exposure was for 24 h to
occluded skin sites at doses > 0.5 g/animal. Albino guinea-pigs
similarly exposed to 10, 25 and 40% dilutions of technical grade
benomyl in dimethyl phthalate presented only mild irritation of both
intact and abraded skin sites (Majut, 1966; Busey, 1968a; Colburn,
1969; Frank, 1969).
When "Benlate" 50 DF (50% benomyl, 0.5 g Benlate 50 DF) was
evaluated for primary dermal irritation potential in six male New
Zealand white rabbits, no dermal irritation was observed at 4 or 24
h after application. By 48 h, slight to mild erythema was observed
in two rabbits and was still evident at 72 h. The primary irritation
scores ranged from 0-1 (not an irritant) (Vick & Brock, 1987).
In a study by Desi (1979), benomyl (98% purity) was applied to
a shaved area of the back of four albino rabbits at 5 mg/cm2.
Draize scores (Draize et al., 1944) were assessed 4 and 72 h after
application. Lesions produced by this method were classified as
"mild irritation".
7.3.2 Eye
The eye irritation properties of benomyl were examined in
albino rabbits in several tests using technical grade benomyl, 50%
wettable powder and a suspension in mineral oil. Mild conjunctival
irritation and minor transitory corneal opacity were reported after
48 to 96 h in all tests (Reinke, 1966; Frank, 1972). Similar results
were obtained with Benlate PNW (a 50% wettable powder) (Gargus &
Zoetis, 1983a,b).
Another eye irritation experiment was performed with 5 mg pure
benomyl using four albino rabbits (Desi, 1979). Results assessed
according to Draize (Draize et al., 1944) indicated that benomyl is
a mild eye irritant.
7.3.3 Sensitization
Albino guinea-pigs exposed to benomyl, either technical
material or a 50% sucrose formulation, produced mild to moderate
skin erythema during the challenge phase following both intradermal
injections or repeat applications to abraded skin (Majut, 1966;
Colburn, 1969; Frank, 1969).
In another sensitization study, Benlate PNW (50% benomyl
prepared as a 0.1% solution in saline) was injected weekly (four
injections) into ten albino guinea-pigs (Hartley strain). Ten
control animals were injected with saline. Fourteen days after the
final injection, 8% or 80% Benlate PNW saline solutions were applied
to the backs of the induced animals and saline was applied to the
backs of the control animals. No significant increase in score
occurred in any of the control animals at either challenge
concentration. Benlate PNW produced an unequivocal and significant
(two-step) increase at two of ten sites challenged with an 8%
suspension, and at seven of ten sites challenged with an 80%
suspension (Gargus & Zoetis, 1984).
Technical benomyl produced sensitization in all ten animals
tested in a guinea-pig maximization test (Matsushita et al., 1977).
7.4 Long-term exposure
7.4.1 Rat
Groups of weanling rats (36 male and 36 female Charles River
albino rats/group) were fed benomyl (50-70% a.i.) in the diet for
104 weeks at levels of 0, 100, 500 and 2500 mg/kg. Growth, as
observed by body weight changes and food consumption data, was
recorded weekly for the first year and twice a month thereafter.
Daily observations were made of clinical effects and mortality. At
periodic intervals during the study, haematological, urinalysis and
selected clinical chemistry examinations were performed. After one
year each group was reduced to 30 males and 30 females by interim
sacrifice for gross and microscopic evaluations. At the conclusion
of the study, all surviving animals were sacrificed and gross
examinations of tissues and organs were made. Initially, microscopic
examinations of tissues and organs from the control and 2500 mg/kg
groups were conducted, as were liver, kidney and testes examinations
of animals in the 100 and 500 mg/kg dose groups. In follow-up
pathological evaluations, all of the tissues and organs of the
control and low-, intermediate- and high-dose groups were examined
microscopically. There was no mortality attributable to benomyl in
the diet. Survival decreased to approximately 50% during the second
year, but was comparable among all groups. Body weight, food
consumption and food efficiency were unaffected by treatment. The
average daily dose for the 2500 mg/kg group was 330 mg/kg body
weight per day initially, 91-106 mg/kg body weight per day at one
year and 70-85 mg/kg body weight per day at two years. There were no
compound-related clinical manifestations of toxicity.
Haematological, urine and liver function tests were unaffected by
treatment. There were no differences in organ weight or organ-to-
body-weight ratios between control and treated groups (Sherman,
1969c; Lee, 1977).
7.4.2 Mouse
In a study by Weichman et al. (1982), male and female CD-1 mice
(80 males and 80 females per group) were administered benomyl (99%
a.i.) in the diet at levels of 0, 500, 1500 and 5000 mg/kg (the
highest levels was reduced from 7500 mg/kg after 37 weeks) for two
years. The mice were 6-7 weeks old at the start of the study. Median
survival time was unaffected by treatment. Male and female mice fed
1500 or 5000 mg/kg exhibited dose-related body weight decreases.
Food consumption was variable throughout the study, although
high-dose females appeared to consume less food. The average daily
intake of benomyl for males was 1079 mg/kg body weight per day
initially, 878 mg/kg body weight per day for 1 year and 679 mg/kg
body weight per day for 2 years; for females it was 1442 mg/kg body
weight per day initially, 1192 mg/kg body weight per day for 1 year,
and 959 mg/kg body weight per day for 2 years. There were no
apparent differences between treatment and control groups with
respect to palpable mass, number of mice affected or latency period
of discovery. Haematology examinations revealed no abnormalities
except for slightly decreased erythrocyte counts in the case of
males at 1500 mg/kg and females at 5000 mg/kg. Haemoglobin and
haematocrit values were also slightly depressed in 1500-mg/kg males.
Significant compound-related changes were seen in the absolute
and relative liver weights for males at 1500 and 5000 mg/kg for
females at 5000 mg/kg. Male mice also presented decreased absolute
testes weights at the highest dose level. Non-neoplastic organ
changes in males (5000 mg/kg) were confined to the liver
(degeneration, pigment, cytomegaly), thymus (atrophy), testes,
epididymis (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini) and prostate. In female mice,
splenic haemosiderosis was significantly increased at 5000 mg/kg, as
was submucosal lymphocytic infiltration of the trachea at 1500 mg/kg
(Weichman et al., 1982).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A number of reproduction studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in the Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.5.1.1 Rat feeding studies
A 2-generation reproduction study was conducted using Crl:CD
rats (Mebus, 1990). Throughout the study, animals were fed diets
containing 0, 100, 500, 3000 or 10 000 mg benomyl/kg. P1 parental
rats received the test diets for 71 days (mean daily intake 0,
approx. 6, approx. 30, approx. 190 or approx. 350 mg/kg per day,
respectively) before being bred to animals from the same dietary
concentration group for production of the F1 parental rats. F1
rats were mated after being maintained on their respective diets
(mean daily intake 0, approx. 8, approx. 20, approx. 250, or approx.
1000 mg/kg per day, respectively) for at least 105 days after
weaning for production of the F2A litter. F1 dams were mated
again, to different non-sibling males, at least 1 week after weaning
the F2A litter, to produce the F2B litters.
The following indices of reproductive function were calculated
for the P1 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental
generation rats were sacrificed for gross and histopathological
(gross lesions and target organs only) examination. Complete
histopathological examination was conducted on control and high-dose
animals. Twenty F2A and 20 F2B weanlings were also given a gross
pathological examination.
There were no compound-related effects on parental mortality at
any benomyl concentration. Mean body weights, body weight gains and
overall food consumption of P1 and F1 male and female rats were
significantly lower in the animals receiving 10 000 mg/kg than they
were in controls.
At 10 000 mg/kg, there was a significant compound-related
decrease in the number of F2A and F2B offspring alive prior to
culling (on day 4). In addition, male and female offspring of rats
fed 10 000 mg/kg weighed consistently less at birth than did the
offspring of control rats. With the exception of the 14-day male
pups in the F2B generation, F2A and F2B offspring in the 3000
mg/kg group also had compound-related significantly depressed body
weights on days 14 and 21 of lactation.
Testicular sperm counts in P1 and F1 rats were decreased in
the 3000 and 10 000 mg/kg groups. This was accompanied by decreased
testicular weight and histopathological changes in the testes at 10
000 mg/kg. Microscopic observations included atrophy and
degeneration of the seminiferous tubules in the testes of rats in
the 3000 and 10 000 mg/kg groups and oligospermia in the
epididymides of the high-dose P1 generation and the 3000 and 10
000 mg/kg F1 generation. However, there were no compound-related
differences in mating indices, fertility indices or gestation length
which could be attributed to benomyl feeding (Mebus, 1990).
In a feeding study by Barnes et al. (1983), adult male Wistar
rats (27 animals/group) were fed 0, 1, 6.3 or 203 mg/kg for 70 days
(13 animals/group) and allowed to recover for 70 days (14
animals/group). Ejaculated sperm counts were reportedly depressed in
the 203-mg/kg group during the feeding phase. There was also a
decrease in relative testicular weights and a slightly lowered male
fertility index in all treated groups. Benomyl did not alter
copulatory behaviour and did not induce dominant lethal mutation.
Plasma testosterone and gonadotropin levels remained unchanged
throughout the study. Identical studies conducted during the
recovery phase showed all of the treatment-related effects to be
completely reversible.
7.5.1.2 Rat gavage studies
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal Sprague-Dawley male rats
(33 days old), which were gavaged daily for 10 days at doses of 0 or
200 mg/kg per day. Eight animals per group were killed at 3, 17, 31,
45 and 59 days after the last treatment. Selected tissues, including
liver, kidneys, testes, seminal vesicles and epididymides, were
removed, weighed and examined histo logically. Samples of seminal
fluid from the vas deferens were also examined. Observation
intervals were pre-selected to coincide with stages of
spermatogenesis. Data were presented on tissue weights, total
epididymal sperm counts, vas deferens sperm concentrations and
testicular histology. There were no effects related to treatment
(Carter, 1982).
In a similar study, adult male Sprague-Dawley rats (65 days
old) received 10 daily treatments of 0, 200 or 400 mg benomyl/kg per
day by gavage, and, 14 days after the last treatment, body weight,
tissue weights, total epididymal sperm counts and sperm
concentration in the vas deferens were measured and histological
investigations of the testes were carried out. Production of
testosterone by the Leydig cells was stimulated by subcutaneous
injections of human chorionic gonadotrophin (HCG) 2 h prior to
sacrifice. There were no compound-related effects on body weight or
on liver, kidney, adrenal, testis or seminal vesicle weights.
Caudate epididymal weights were, however, depressed by treatment
with benomyl. There were also treatment-related reductions in
epididymal sperm count (caput and caudate) as well as in the vas
deferens sperm concentration. The study was designed to evaluate
alterations in spermatozoa undergoing spermatogenesis in the
seminiferous tubules of the testes during exposure to benomyl.
Animals exposed to 400 mg/kg per day presented histological evidence
of hypospermatocytogenesis with generalized disruption of all stages
of spermatogenesis, when compared with controls (Carter & Laskey,
1982).
Linder et al. (1988) evaluated the effect of administering 1,
5, 15 or 45 mg benomyl/kg per day by gavage daily to 102-day old
Wistar male rats (12/group). The males were bred to untreated
females after 62 days of dosing and killed after 76-79 days.
Reproductive behaviour, seminal vesicle weight, prostate weight,
sperm motility, and serum gonadotropin hormones and serum gonadal
hormone levels were no different from those in controls at any dose.
At necropsy, males exposed to 45 mg/kg had decreased testis and
epididymal weights, reduced cauda sperm reserves, decreased sperm
production, increased numbers of decapitated spermatozoa and
increased numbers of seminiferous tubules containing multinucleated
giant cells.
In a study by Hess et al. (1991), adult male Sprague-Dawley
rats (100 days of age, 20 rats/dose) were given a single dose of
benomyl in corn oil (0, 25, 50, 100, 200, 400 or 800 mg/kg body
weight). Eight animals/group were sacrificed at 2 days and 12
animals/group at 70 days (except for the 800 mg/kg group) after
treatment. The testis and excurrent ducts were examined each time to
determine benomyl effects on spermatogenesis and on the epididymis.
The primary effects seen at day 2 were testicular swelling and
occlusions of the efferent ductules. Premature release of germ cells
(sloughing) was the most sensitive short-term response to benomyl.
Sloughing was detected in all treatment groups but was statistically
significant (p < 0.05) at doses of 100 mg/kg to 800 mg/kg.
Occlusions of the efferent ductules of the testis were dose
dependent and correlated with the increase in testis weight on day
2. The greatest increase in testes weight was observed in the 400
mg/kg group. Long-term effects (70 days) were seen in the 100, 200
and 400 mg/kg groups, e.g., decreased testis weight (400 mg/kg),
dose-dependent increases in seminiferous tubular atrophy, and
increases in the number of reproductive tracts containing occluded
efferent ductules. No long-term effects were seen in the 0, 25 or 50
mg/kg groups.
7.5.1.3 Dog inhalation studies
Three groups (10 dogs per group) of sexually mature male dogs
were exposed to benomyl at air concentrations (aerosol/cloud) of 0,
0.65 and 1.65 mg/litre for a 4-h period. One half of the dogs in
each group were killed on day 14 and the remainder on day 28
following the exposure. Histopathological examination revealed a
reduction in spermatogonic activity on day 14, but not on day 28, in
the high-dose group (Littlefield & Busey, 1969).
7.5.2 Teratogenicity and embryotoxicity
7.5.2.1 Mouse gavage studies
Groups of pregnant CD-1 mice (20-25 mice/group) were
administered benomyl via gavage at dose levels of 0, 50, 100 and 200
mg/kg per day on days 7 to 17 of gestation. Animals were killed on
day 18, pups were delivered by Caesarean section, the number of
live, dead and resorbed fetuses was determined, and fetuses were
examined for gross abnormalities. Half of the fetuses were examined
for visceral abnormalities and the other half for skeletal
abnormalities. No maternal toxicity was observed. Fetal development
was adversely affected by treatment at all dose levels. The high
dose caused an increased supraoccipital score, decreased numbers of
caudal and sternal ossifications and increased incidences of
enlarged lateral ventricles and enlarged renal pelvis. The latter,
while not significant at the lower doses, did demonstrate
dose-related increases at all other doses. The occurrence of
supernumerary ribs and subnormal vertebral centrums was
significantly increased in a dose-related manner at all dose levels.
There was an increase in the number of abnormal litters and fetuses,
which was significantly different from the controls, at levels of
100 and 200 mg/kg per day. Fetal weights were also decreased at
these dose levels. Major abnormalities included exencephaly,
hydrocephaly, cleft palate, hydronephrosis, polydactyly,
oligodactyly, umbilical hernia, fused ribs, fused vertebrae and
short/kinky tail (Kavlock et al., 1982).
7.5.2.2 Rat gavage studies
In a study by Staples (1980), benomyl (99.2% a.i.) was adminis
tered by gavage to groups of pregnant rats (ChR-CD) at dose levels
of 0, 3, 10, 30, 62.5 and 125 mg/kg per day from days 7 to 16 of
gestation. There were 60 dams in the control group and 27 in each of
the other test groups; they were observed daily for signs of
toxicity and changes in behaviour. No clinical signs of toxicity or
mortality were observed among dams in any dose group. Body weight
gain was comparable to controls, as were the incidences of
pregnancy, corpora lutea and implantation sites, and the sex ratio.
However, fetal body weight was significantly decreased at the two
highest dose levels. There was also an increased incidence of
embryo/fetal mortality at 125 mg/kg per day.
The malformations observed included microphthalmia,
anophthalmia and hydrocephaly (distended lateral ventricles). These
appeared to be compound related at the higher dose levels.
Microphthalmia was seen in two fetuses from different litters at 10
mg/kg per day and in one fetus from the control group.
Microphthalmia/anopthalmia was observed in only 4-6 out of 4935
fetuses examined in the historical data base at this laboratory.
Histological examination of eyes revealed pathological changes
consisting of irregular lenses, retro-bulbar glandular adnexa,
distorted or compressed retinal layers and thickened nerve fibres in
the 10, 62.5 and 125 mg/kg per day treatment groups. Major skeletal
malformations observed in the 125 mg/kg dose group included fused
ribs, fused sternebrae and fused thoracic arches. Additional
skeletal variations were also increased at 62.5 and 125 mg/kg per
day; these included misaligned and unossified sternebrae and
bipartite vertebral centra (Staples, 1980).
In order to determine a no-effect level for microphthalmia and
hydrocephaly, groups of 50 pregnant rats of the same strain and from
the same supplier were administered benomyl (99.1% a.i.) via gavage
at dosage levels of 0, 3, 6.25, 10, 20, 30 and 62.5 mg/kg per day
from days 7 to 16 of gestation (Staples, 1982). Each group contained
50 animals except the high-dose group, which contained 20 female
rats. Reproductive status was determined on a per-litter basis
following gross pathological evaluation. The number of implantation
sites, resorptions, dead, live and stunted fetuses, and the mean
weight of live fetuses per litter were determined. Only fetal heads
were fixed and examined microscopically. Microphthalmia was
determined on the basis of the smallest eye in the control group (<
1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two animals with malformations,
both in the 62.5 mg/kg per day group. One fetus exhibited internal
hydrocephaly and another, in a separate litter, unilateral
microphthalmia. There was no teratogenic response at doses up to 30
mg/kg.
The teratogenic potential of benomyl was examined in groups of
Wistar rats (12 to 30 pregnant rats/group) orally gavaged at dose
levels of 0, 15.6, 31.2, 62.5 and 125 mg/kg per day on days 7 to 16
of gestation. Major anomalies were observed primarily at levels of
62.5 mg/kg per day or more and included encephaloceles,
hydrocephaly, microphthalmia, fused vertebrae and fused ribs. A dose
level of 31.2 mg/kg per day appeared to be without adverse effects
on the developing rat fetus in this evaluation. A significant
reduction in maternal body weight was observed at the highest dose
level (Kavlock et al., 1982).
Kavlock et al. (1982) also evaluated the effect of low levels
of benomyl as the pups aged. Benomyl was administered via gavage to
groups of Wistar rats at dose levels of 0, 15.6 and 31.2 mg/kg per
day from day 7 of gestation to day 15 of lactation (day 22 of
gestation was considered day 0 of lactation). The litters were
weighed on days 8, 15, 22, 29 and 100 after parturition, and
locomotor activity was evaluated periodically throughout the study.
At 100 days of age, several organs were weighed, including the
adrenals, liver, kidney, ovaries, testes and the ventral prostate
plus seminal vesicles. There were no compound-related effects either
on litter size at birth or weaning, or on body weights of fetuses.
Growth, survival and locomotor activity values were comparable with
those of the controls throughout the study. Organ weights were
comparable with those of controls, except that testes and ventral
prostate/seminal vesicle weights were significantly reduced at 31.2
mg/kg per day (but not at 15.6 mg/kg) (Kavlock et al., 1982).
In other studies (Zeman et al., 1986; Hoogenboom et al., 1991),
benomyl produced ocular and craniocerebral malformations in
Sprague-Dawley rats when administered by gavage at doses of 31.2 and
62.4 mg/kg per day on day 7-21 of gestation. Ocular anomalies
(retinal dysplasia, cataracts, microphthalmia and anophthalmia)
occurred in 43.3% of fetuses when the dams were administered 62.4
mg/kg per day. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose level
of benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
(consisting primarily of hydrocephaly) occurred in fetuses from dams
administered 31.2 mg/kg per day in combination with the semipurified
diet (Zeman et al., 1986).
7.5.2.3 Rat feeding studies
In a study by Sherman et al. (1972), groups of rats (26-28
pregnant ChR-CD rats/group) were administered a benomyl formulation
(53.5% a.i.) in the diet at dosages of 0, 100, 500, 2500 and 5000
mg/kg from day 6 to day 15 of gestation. Average doses were
equivalent to 0, 8.6, 43.5, 209.5 and 372.9 mg/kg body weight per
day. On day 20 of gestation, all pregnant animals were sacrificed
and fetuses were delivered by Caesarean section. There was no
mortality attributable to benomyl, no clinical signs of toxicity and
no adverse effects on the body weight of dams. Dams in the
highest-dose group had a reduced food intake during the period
benomyl was administered in the diet, but the intake returned to a
level similar to the controls for the remainder of the study. Except
for three litters at the highest dose, where there were incidences
of hydronephrosis and retarded ossification (interparietal and
occipital bones), there were no effects on fetal development related
to benomyl administration.
In a similar study, groups of pregnant Wistar rats (27-28
rats/group) were fed benomyl at dose levels of 0, 1690, 3380 and
6760 mg/kg diet (time-weighted doses of 0, 169, 298 and 505 mg/kg
body weight per day, respectively) from days 7 to 16 of gestation.
No dose-related anomalies or major malformations were associated
with exposure to benomyl at any of the dose levels used. Reduced
fetal weight was observed at the two highest dose levels (Kavlock et
al., 1982).
7.5.2.4 Rabbit feeding studies
Groups of rabbits (15 artificially inseminated New Zealand
albino rabbits/group) were administered a benomyl formulation (50%
a.i.) in the diet at dose levels of 0, 100 and 500 mg/kg diet from
day 8 to day 16 of gestation. Mortality, clinical observations and
food consumption were determined daily and body weights were
measured weekly. There were 12 pregnant does in the control group,
13 in the low-dose group and 9 in the high-dose group. Of these, 6,
7 and 5, respectively, were sacrificed on day 29 or 30 and fetuses
were delivered by Caesarean section; the remaining does gave birth
normally. Maternal toxicity was not observed at any dose level.
Except for a marginal increase in rudimentary ribs at 500 mg/kg,
developmental toxicity was not observed. The numbers of litters and
of fetuses examined were less than adequate to assess the fetotoxic
or teratogenic potential of benomyl to pregnant rabbits (Busey,
1968c; FAO/WHO, 1985a).
7.6 Mutagenicity and related end-points
Numerous studies have been conducted to assess the mutagenic
potential of benomyl, its metabolite carbendazim, and several
benomyl formulations. Many of the results are conflicting and many
of the study reports do not provide sufficient detail to evaluate
the reasons for the conflicting data. This summary will cover only
those studies where sufficient experimental detail and data were
reported (Table 9).
Studies on somatic and germ cells have shown that benomyl does
not cause gene mutations or structural chromosomal damage
(aberrations) and that it does not interact directly with DNA. This
has been demonstrated in both mammalian and non-mammalian systems.
However, in the mammalian in vitro studies for gene mutations and
structural chromosome aberrations, some positive results were
obtained with benomyl. These positive results appear to have been a
consequence of the inherent sensitivity of some in vitro mammalian
test systems to cytotoxic agents. Results in mammalian in vivo
studies for gene mutations and structural chromosome aberrations
were negative.
Benomyl does cause numerical chromosomal aberrations
(aneuploidy and/or polyploidy) in experimental systems both in
vitro and in vivo (Table 9).
7.7 Carcinogenicity
A number of carcinogenicity studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.7.1 Rat
In a two-year study on benomyl, groups of Charles River albino
weanling rats (36 male and 36 female rats) were fed benomyl (50-70%
a.i.) in the diet at levels of 0, 100, 500 and 2500 mg/kg diet. At
the end of the study all surviving animals were sacrificed and gross
and microscopic examinations of tissues and organs were carried out.
The most frequently observed tumours involved the pituitary, but
these were equally distributed among control and treated groups.
Mammary, adrenal and other tumours were also observed; these were
scattered among all groups. There were no adverse effects or
significant histopathological changes at any dose levels in this
study that could be attributable to benomyl (Sherman, 1969c; Lee,
1977).
Table 9. Studies on mutagenicity of benomyl
End points/Tests Species, strains Concentrationb Activation Result Reference
1. DNA damage and repair
Mitotic gene conversion Saccharomyces cerevisiae, NR with and negative Siebert et al. (1970);
D4 & D7 without de Bertoldi et al. (1980)
Mitotic gene conversion Aspergillus nidulans, 0.35-2.8 mM without negative de Bertoldi & Griselli
D7 (1980)
Mitotic crossing-over test A. nidulans, P NR without negative Bignami et al. (1977)
A. nidulans, D7 NR without negative de Bertoldi & Griselli
(1980)
Non-disjunction A. nidulans, D7 0.35-2.8 mM without positive de Bertoldi & Griselli
(1980)
Sister chromatid exchanges human lymphocyte 0.05-2.0 µg/ml NR slight increase in Georgieva et al. (1990)
(SCE) cultures SCE, no dose-response
relationship
Unscheduled DNA synthesis B6C3F1 male mice & 0.5-500 µg/ml NR negative Tong (1981)
Fisher-344 male rat
hepatocytes
2. Gene mutation
a) Bacterial & fungal Salmonella typhimurium 0.125-1.0 µg/ml NR positive(TA1535) Kappas et al. (1976)
gene mutation TA1535, TA1538 0.125-1.0 µg/ml NR negative(TA1538)
Escherichia coli, 0.125-1.0 µg/ml NR positive Kappas et al. (1976)
WP2 uvra
E. coli, WP2 uvra 2.5-10.0 µg/ml NR positive Kappas et al. (1976)
E. coli, WP2 0.125-1.0 µg/ml NR negative Kappas et al. (1976)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Point mutation A. nidulans 0.25-0.4 µg/ml NR positive Kappas & Bridges (1981)
Spot test S. typhimurium, his, 50-5000 µg per negative Fiscor et al. (1978)
G 46 & TA1535, TA1530 spot
TA1950
S. typhimurium, TA100 50-2000 µg per with negative Fiscor et al. (1978)
plate
Host-mediated assay S. typhimurium, his, 3 consecutive negative Fiscor et al. (1978)
G 46 subcutaneous
injections of
500 mg/kg in mice
TA1950 an oral dose of 4000 negative Fiscor et al. (1978)
mg/kg in rats or mice
Spot test S. typhimurium, TA1535, 20 µg/spot with and negative Carere et al. (1978)
TA1536, TA1537, TA1538 without
Forward spot mutation Streptomyces coelicolor 500 µg/spot without negative Carere et al. (1978)
assay
Ames plate incorporation S. typhimurium, TA1537 500 µg/plate without marginal Russell (1978a)
test positive
S. typhimurium, TA1535, 10-500 µg/ with negative Donovan & Krahn (1981);
TA1537, TA98, TA100 plate Rickard (1983a,b)
10-200 µg/plate without negative
TA1535, TA1537, TA98, 10-500 µg/plate with and negative Russell (1978b)
TA100 without
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
TA1513, TA1537, up to 1000 µg/ with and negative Shirasu et al. (1978)
TA1538, TA98, TA100 plate without
E. coli, WP2 hcr up to 500 µg/ with and negative Shirasu et al. (1978)
plate without
recA spot test B. subtilis, M45 & H-17 2000 µg/plate NR negative Shirasu et al. (1978)
Host-mediated assay S. typhimurium, his, 2000 mg/kg negative Shirasu et al. (1978)
(ICR mice) G46
b) In vitro mammalian
gene mutaion
HGPRTa gene Chinese hamster ovary 17 & 172 µM with negative Fitzpatrick (1980)
cells 3 & 120 µM without negative
Mouse lymphoma Mutant colonies in 0.5-20 µM without negative Amacher et al. (1979)
L5178Y TK+/- gene RPMl1640/horse serum
mutation assays (MLY) 2.5-25 µM with negative
MLY > 2.5 µM negative McCooey et al. (1983a)
MLY carbendazim, without negative McCooey et al. (1983b)
25-200 µM
carbendazim, with negative McCooey et al. (1983b)
12.5-200 µM
MLY N-butylisocyanate without negative McCooey et al. (1983b)
2.5-25 µM
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) Insect germ cell
gene mutation
Sex-linked recessive adult male fed benomyl suspension negative Lamb & Lilly (1980)
lethals D. melanogaster (1.5 mg/ml)
3. Chromosomal effects
a) Yeast and fungi A. nidulans 0.25 µg/ml without increased frequency of Hastie (1970)
(diploid) 0.5 µg/ml segregants
A. nidulans 0.25 µg/ml without no increased frequency Hastie (1970)
(haploid) 0.5 µg/ml of segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(diploid) segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(haploid) segregants
A. nidulans 0.35 to 2.8 mM without increased De Bertoldi & Griselli
(diploid) nondisjunction (1980)
Saccharomyces cerevisiae, 30 µg/ml without induced chromosome Albertini (1991)
D61.M malsegregation
b) In vitro mammalian human lymphocytes 1.0-100.0 µg/ml with negative Pilinskaya (1983)
assays
human lymphocytes 1.0-100.0 µg/ml without weak positive at Pilinskaya (1983)
10.0 µg/ml only
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
human/mouse mono- < 15 µg/ml without induced aneuploidy Athwal & Sandhu (1985);
chromosomal hybrid and polyploidy Sandhu et al. (1988)
cells (R3-5)
human/mouse mono- 15 µg/ml without slight increase in Athwal & Sandhu (1985);
chromosomal hybrid structural Sandhu et al. (1988)
cells (R3-5) chromosomes; 1.5 µg/ml
threshold (polyploidy)
V79/AP4 Chinese NR without dose-related increase Rainaldi et al. (1989)
hamster cells in numerical
chromosomal
aberrations
Chinese hamster NR without dose-related increase Eastmond & Tucker
ovary cells in numerical (1989)
chromosomal
aberrations
human lymphocytes NR without dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations; 0.1 µg/ml
threshold (aneuploidy)
Chinese hamster- NR without dose-related increase Zelesco et al. (1990)
human hybrid cells in numerical
chromosomal
(EUBI) aberrations; 2.0 µg/ml
threshold (aneuploidy)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) In vivo mammalian rats up to 500 mg/kg no chromosomal Ruziscka et al. (1976)
assays for 8 days by gavage aberrations in bone
marrow, increase in
chromosomal
aberrations in
embryonic cells at
200 and 500 mg/kg doses
Bone marrow micro- ICR mice 2 gavage doses of increase in Seiler (1976)
nucleus test 9, 500 or 1000 micronucleated
mg/kg 24 h apart polychromatic
erythrocytes at
1000 mg/kg only
Bone marrow micro- BDF1 mice single gavage dose increase in Sasaki (1990)
nucleus test of 0, 1250, 2500 or micronucleated
5000 mg/kg polychromatic
erythrocytes at
2500 and 5000 mg/kg
Bone marrow chromosomal B6D2F2/Cr-1Br mice single gavage dose no increase in Stahl (1990)
aberrations of 0, 625, 1250, 2500, structural chromosomal
or 5000 mg/kg aberrations
d) In vivo germ cell
chromosomal aberration
Dominant lethal test ChR-CD rat 0, 500, 2500 or negative Culik & Gibson (1974)
5000 mg/kg feeding
for 7 days
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Dominant lethal test Wistar rats 0, 1, 6.3 or 203 negative Barnes et al. (1983)
mg/kg feeding for
70 days
Dominant lethal test Wistar rats 70 daily gavage doses negative Georgieva et al. (1990)
of 0, 10 or 50 mg/kg
a HGPRT = hypoxanthine-guanine phosphoribosyl transferase
b NR = not reported
7.7.2 Mouse
Male and female CD-1 mice (80 males and 80 females per group)
were fed benomyl (99% a.i.) at dose levels of 0, 500, 1500 and 5000
mg/kg diet (the highest level was reduced from 7500 mg/kg after 37
weeks) for two years. The incidence of hepatocellular adenomas and
carcinomas (see Table 10) in female mice was increased in a
dose-dependent manner. In male mice, numbers of hepatocellular
adenomas and carcinomas were significantly increased at 500 and 1500
mg/kg but not at 5000 mg/kg dose. The increased number of lung
alveogenic carcinomas in male mice was still within the range of
historical controls (Weichman et al., 1982; Frame & van Pelt, 1990;
Hardisty, 1990).
7.8 Special studies
7.8.1 Neurotoxicity
Studies performed using White Leghorn hens (10/group) gave no
indication of neurotoxic potential with single oral doses of benomyl
at levels up to 5000 mg/kg (Goldenthal, 1978; Jessup & Dean, 1979;
Jessup, 1979).
Studies performed using adult male CFY rats (10 rats/group)
gave no indication of altered EEG potentials or behaviour (learning
ability assessed with a 4-choice T-maze) after treatment with 250 or
500 mg benomyl/kg per day for 3 months when compared with controls
(Desi, 1983).
7.8.2 Effects in tissue culture
In a study by Desi et al. (1977), primary monkey kidney cells
were incubated with benomyl (Fundazol 50 WP) at levels of 1, 10, 50,
100, 250 and 400 mg/kg. Growth inhibition and syncytium-like
appearance of the cell monolayer was observed at 250 mg/kg, and at
400 mg/kg all the cells were killed.
7.9 Factors modifying toxicity; toxicity of metabolites
Benomyl degrades to butyl isocyanate (BIC) and carbendazim
quite rapidly in water and in many organic solvents used in
toxicological testing. The biological activity of benomyl is
essentially that of carbendazim both in a toxicological sense and in
its use as a fungicide. A detailed discussion of carbendazim
toxicology is given in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
Table 10. Incidence and latency of hepatic tumours in benomyl-treated CD-1 micea
Male mice Female miceb
Benomyl concentration (mg/kg diet): 0 500 1500 5000 0 500 1500 5000
Hepatocellular adenoma
Number of animals with tumours 9 8 10 10 2 2 5 7
Latent time to first tumour (days) 530 556 541 627 744 641 650 644
Average latent period (days) 687 707 695 726 744 688 717 722
Hepatocellular carcinoma
Number of animals with tumours 14 26c 41d 17 2 7 7 14e
Latent time to first tumour (days) 545 470 590 508 744 640 736 426
Average latent period (days) 693 705 721 711 744 708 736 695
a From: Wiechman (1982)
b P < 0.05 Dose/response increase of adenomas and carcinomas
c P < 0.05
d P < 0.001
e P < 0.01
BIC is toxic by inhalation. In 4-month subchronic inhalation
studies, the no-observed-effect levels (NOEL) for BIC in rats and
mice were determined to be 0.32 and 1.5 ppm, respectively (Gurova et
al., 1976). An industrial hygiene survey found that workers
experienced severe eye irritation and lacrimation at BIC exposure
levels of 5-10 ppb (Kelly, 1989).
7.10 Mechanisms of toxicity - Mode of action
Biochemical studies on the mechanism of action of benzimidazole
compounds have shown that their biological effects are caused by
interactions with cell microtubules (Davidse & Flach, 1977). These
cellular structures are present in all eukariotic cells and are
involved in several vital functions, such as intracellular
transports and cell division. Benzimidazol compounds have been used
as anticancer drugs and as antihelminthic drugs in animals and
humans because they act as spindle poisons by interfering with the
formation and/or functioning of microtubules. However, eukaryotes
are known to be unequally sensitive to each benzimidazol compound,
which explains the use of these compounds in helminthiases.
Selective toxicity of benomyl and carbendazim for fungi has been
explained by comparing their binding to fungal and mammalian
tubulin. The different sensitivity of several fungi has also been
explained by the different affinity of benomyl and carbendazim for
fungal tubulin.
Benomyl has been found to bind to fungal tubulin but not to
porcine brain tubulin, indicating that mammalian tubulin has no, or
at least low, affinity for benomyl (Davidse & Flach, 1977). This is
in agreement with the observation that benomyl at concentrations
that are lethal for sensitive fungi does not interact with in vitro
microtubule assembly in these brain extracts. In vitro ID50
values for several mycelial extracts of various fungal species
sensitive to benomyl were all below 5 µmol/litre (Davidse & Flach,
1977). In vitro rat brain tubulin polymerization was inhibited to
about 20% at benomyl or carbendazim concentrations of 25 µmol/litre
(De Brabander et al., 1976b). For comparison, a standard antitubulin
drug in humans such as vincristine inhibited 50% tubulin assembly at
0.1 µmol/litre in the same experiment. The assembly of sheep and
calf brain microtubule was also found to be unaffected by
carbendazim concentrations higher than 100 µmol/litre (Ireland et
al., 1979).
Mitotic arrest by benzimidazole and six analogues at metaphase
was evaluated in human lymphocyte cultures. Structure-activity
relationships indicate that antimitotic activity is related to C6
substitution of the benzimidazole moiety (Holden et al., 1980). In
this study, however, benomyl and carbendazim were not tested. The
question of whether all C6 unsubstituted benzimidazoles, such as
benomyl and carbendazim, have no effect on mitosis of human
lymphocytes in cell cultures is therefore unresolved.
A link between the effects of benomyl and carbendazim on brain
tubulin and their teratogenic effects has been postulated (Ellis et
al., 1987, 1988).
8. EFFECTS ON HUMANS
8.1 General population exposure
No references to benomyl poisoning in the general population
have been documented in the scientific literature. Recent data used
to estimate dietary exposure based on food consumption patterns
within the USA indicate exposures well below the NOELs in animal
toxicity tests.
8.2 Occupational exposure
8.2.1 Acute toxicity
Benomyl has a very low acute toxicity. No inadvertent poisoning
of agricultural or factory workers has been documented (Goulding,
1983).
8.2.2 Effects of short- and long-term exposure
Benomyl causes contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et al., 1985). In
controlled patch tests of agricultural, ex-agricultural and
non-agricultural workers (total of 200 subjects), only one
agricultural worker showed any contact dermatitis to 0.1% benomyl
(Lisi et al., 1986).
A survey of cross-sensitization between benomyl and other
pesticides was conducted in Japan on a group of 126 farmers who
applied benomyl to their crops. Thirty-nine of the farmers gave
positive test results with benomyl, the highest incidence being
among female farmers. There were cross-reactions between benomyl and
other pesticides, such as diazinon, saturn, daconil and Z-bordeaux
(Matsushita & Aoyama, 1981).
Selected blood profiles from 50 factory workers involved in the
manufacture of benomyl were compared to those of a control group of
48 workers who were not exposed to carbendazim. White blood cell
count, red blood cell count and haemoglobin and haematocrit values
were comparable among the two groups. There were no quantitative
estimates of exposure given for the factory workers (Everhart, 1979;
FAO/WHO, 1985a).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers exposed to benomyl between 1970 and 1977. The
workers ranged from 19 to 64 years of age (79% were between 20 and
39, and 78% of the spouses were similarly aged between 20 and 39
years). Exposure duration ranged from less than one month to 95
months, and more than 51% of the workers were potentially exposed
from 1 to 5 months. The birth rates of exposed workers' spouses were
compared with those of four comparison populations from the same
county, state, region and country (USA). There was no reduction in
fertility as shown by the birth rates for the study population,
which were generally higher than those of the comparison
populations. Spermatogenesis among workers was not examined (Gooch,
1978; FAO/WHO, 1985a).
In studies on agricultural spraymen using benomyl (Fundazol 50
WP), 14 spraymen working in greenhouses were followed, some of them
for two years. General medical check-up and routine blood and urine
tests were performed. Electrocardiograms were recorded and blood
cholinesterase activity was monitored. Lymphocytes from peripheral
blood were examined for chromosomal aberrations both before and
during the study. There was no difference in structural chromosomal
aberration between the spraymen and controls. After benomyl
exposure, numerical chromosomal aberrations were higher in the
spraymen than in controls. However, the spraymen had a higher level
of numerical chromosome aberration than the controls even before
benomyl exposure (Desi et al., 1990; Nehez et al., 1992).
9. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Soil respiration has been found to be little influenced by
benomyl at concentrations below 10 mg/kg, which is the maximum soil
concentration expected after use at recommended application rates
(Hofer et al., 1971; van Fassen, 1974; Peeples, 1974; Weeks &
Hedrick, 1975).
A study on the influence of benomyl on soil nitrogen
mineralization showed that the release of ammonia was not decreased
by benomyl, whereas the influence of the fungicide on nitrification
varied from a stimulation (van Fassen, 1974), through no effect
(Mazur & Hughes, 1975), to a decreased nitrification (Hofer et al.,
1971; Wainwright & Pugh, 1974). The differences may be related to
the soil composition since Hofer et al. (1971) found a greater
effect in sandy than in organic soil.
Benomyl, in combination with eleven other pesticides that were
used in an orchard spray programme, had only a minimal and
short-term effect on respiration, ammonification and nitrification
at concentrations expected after recommended use of benomyl over a
spraying season. Ten times the recommended application rates had a
pronounced effect on both respiration and nitrification, which
lasted for more than 4 weeks (Helweg, 1985).
The influence of benomyl and carbendazim on soil microbial
activity was studied in Sweden following repeated annual
applications during autumn to winter cereals for a period of 3 to 5
years. The effects of the fungicides on straw decomposition, balance
of straw fungal flora and nitrogen mineralization in the soil were
investigated in field and laboratory experiments. The decomposition
of straw in the field was not affected in clay soils by annual
applications of up to 2 kg/ha. In sandy soils, rates of up to 0.5
kg/ha had no effect, but in one case at 2 kg/ha the initial stages
of straw decomposition were slightly inhibited. All doses tested in
both clay and sandy soils caused changes in the composition of the
straw fungal flora (Torstensson & Wessen, 1984).
Benomyl had no effect on soil bacterial populations in
laboratory studies, but fungi and actinomycetes populations were
reduced (Siegel, 1975). Under greenhouse conditions (Kaastra-Howeler
& Gams, 1973) or field conditions (Peeples, 1974) at application
rates of up to 89.6 kg a.i./ha, little effect on microbial
populations was observed following benomyl treatment.
9.2 Aquatic organisms
The effect of benomyl was monitored using the green alga
Selenastrum capricornutum in an OECD guideline test (201). The
EC50 (based on total growth) at 72 h was 2.0 mg/litre and at 120 h
was 3.1 mg/litre. The no-observed-effect concentration (NOEC) was
0.5 mg/litre. To study whether benomyl was algistatic or algicidal,
organisms were recultured at the end of the initial 120 h
incubation. Regrowth occurred in the control but not in the test
cultures (8.0 mg/litre) after a period of 7 days. Benomyl was,
therefore, considered to be algicidal (Douglas & Handley, 1988). In
another study using Chlorella pyrenoidosa, the 48-h EC50 for
growth inhibition was calculated as 1.4 mg/litre (Canton, 1976).
The acute toxicity of benomyl to a variety of aquatic organisms
is summarized in Table 11. For 96-h tests, LC50 values ranged from
0.006 mg/litre for channel catfish ( Ictalurus punctatus; yolk-sac
fry) to > 100 mg/litre for crayfish ( Procambarus sp.) (Mayer &
Ellersieck, 1986).
9.3 Terrestrial organisms
Several field studies have investigated the toxicity of benomyl
to earthworms (Table 12). In one study, the 48-h LC50 for E.
foetida was 9.1 µg/cm3 soil (Roberts & Dorough, 1984).
Van Gestel et al. (1992) exposed red earthworms ( Eisenia
andrei) to benomyl added to artificial soil and used final
concentrations of 0, 0.1, 0.32, 1.0, 3.2 and 10 mg/kg dry soil. The
worms had been acclimatized for 1 week in the artificial soil, which
contained 4 g/kg cow dung as food for the worms. At the highest
concentration of 10 mg/kg, high mortality occurred; LC50 values of
6.0 and 5.7 mg/kg dry soil were calculated after 3 and 6 weeks of
incubation, respectively. Growth was significantly reduced at 3.2
mg/kg soil. EC50 values for the effect of benomyl on cocoon
production did not differ significantly for the two test periods,
and the EC50 was 1.6 (1.2-2.3) mg/kg for the entire 6-week test
period.
Zoran et al. (1986) and Drewes et al. (1987) have shown that
the conduction velocity for medial and lateral giant nerve fibres is
affected by exposure of earthworms to sublethal concentrations of
benomyl. Zoran et al. (1986) have also shown that segmental
replication is affected in amputated earthworms exposed to sublethal
concentrations of benomyl.
The benomyl metabolite carbendazim (99.3% purity) was evaluated
for acute contact toxicity after thoracic application to honey-bees
(Apis mellifera). Each treatment level consisted of four replicates
of ten bees each. Forty bees served as positive control (using
carbaryl) and forty as negative control. No deaths occurred after
application of carbendazim at 50 µg/bee, the highest rate tested.
Carbendazim is, therefore, classified as "relatively non-toxic" to
the honey-bee (Meade, 1984).
Table 11. Toxicity of benomyl to aquatic organisms
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Freshwater
Water flea adult stat 25 3 14 Yoshida & Nishiuchi (1972)
(Daphnia magna) < 24 h stat 20 87 8.5 48 0.11c Hutton (1989)
< 24 h stat 20 48 0.64 Canton (1976)
< 24 h stat 17 40 7.4 48 2.8 Mayer & Ellersieck (1986)
Scud
(Gammarus pseudolimnaeus) adult stat 17 40 7.4 96 0.75 Mayer & Ellersieck (1986)
Crayfish
(Orconectes nais) instar stat 22 40 7.4 96 >10 Mayer & Ellersieck (1986)
Crayfish
(Procambarus sp.) immature stat 22 40 7.4 96 >100 Mayer & Ellersieck (1986)
Midge
(Chironomus plumosus) instar stat 22 40 7.4 48 7.0 Mayer & Ellersieck (1986)
Rainbow trout 3 months stat 15 48 0.48 Canton (1976)
(Oncorhynchus mykiss) 0.8 g stat 7 44 7.4 96 0.17 Mayer & Ellersieck (1986)
0.8 g stat 12 44 7.4 96 0.20 Mayer & Ellersieck (1986)
0.8 g stat 17 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1.2 g stat 12 44 6.5 96 0.16 Mayer & Ellersieck (1986)
1.2 g stat 12 44 7.5 96 0.19 Mayer & Ellersieck (1986)
1.2 g stat 12 44 8.5 96 0.88 Mayer & Ellersieck (1986)
0.6 g stat 12 44 7.4 96 0.23 Mayer & Ellersieck (1986)
0.6 g stat 12 320 7.4 96 0.60 Mayer & Ellersieck (1986)
fingerling stat 12 44 7.4 96 0.12 Mayer & Ellersieck (1986)
swimup fry stat 12 44 7.4 96 0.16 Mayer & Ellersieck (1986)
yolk-sac fry stat 12 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1 g stat 12 44 7.4 96 0.31c Mayer & Ellersieck (1986)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Fathead minnow 0.9 g stat 22 44 7.4 96 2.2 Mayer & Ellersieck (1986)
(Pimephales promelas) 0.5 g stat 22 45 7.1 96 1.3 Mayer & Ellersieck (1986)
0.5 g stat 22 44 7.4 96 1.9c Mayer & Ellersieck (1986)
Channel catfish 1.2 g stat 22 44 7.4 96 0.029 Mayer & Ellersieck (1986)
(Ictalurus punctatus) 0.05 g stat 22 44 7.4 96 0.013 Mayer & Ellersieck (1986)
0.15 g stat 22 44 7.4 96 0.024 Mayer & Ellersieck (1986)
swimup fry stat 22 44 7.4 96 0.012 Mayer & Ellersieck (1986)
yolk-sac fry stat 22 44 7.4 96 0.006 Mayer & Ellersieck (1986)
1.2 g stat 22 44 7.4 96 0.028c Mayer & Ellersieck (1986)
Bluegill
(Lepomis macrochirus) 0.9 g stat 12 44 7.4 96 0.75 Mayer & Ellersieck (1986)
0.9 g stat 17 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.9 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 6.5 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.5 96 1.2 Mayer & Ellersieck (1986)
0.6 g stat 22 44 8.5 96 6.4 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 320 7.4 96 2.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.2c Mayer & Ellersieck (1986)
Carp
(Cyprinus carpio) 5 cm stat 25 48 7.5 Yoshida & Nishiuchi (1972)
Killifish
(Fundulus sp.) 2.5 cm stat 25 48 11 Yoshida & Nishiuchi (1972)
Loach 10 cm stat 25 48 14 Yoshida & Nishiuchi (1972)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Guppy
(Poecilia reticulata) 3 weeks stat 24 48 3.4 Canton (1976)
Tadpole
(Bufo sp.) < 1 month stat 25 48 4.3 Yoshida & Nishiuchi (1972)
Estuarine and Marine
Eastern oyster
(Crassostrea virginica) 25-50 mm flow 17-19 96 1.37d Boeri (1988a)
Grass shrimp
(Palaemonetes pugio) 18 mm stat 18 96 45.8c Bionomics Inc. (1972)
Mysid shrimp
(Mysidopsis bahia) stat 23 96 0.175 Boeri (1988c)
Dungeness crab
(Cancer magister) larvae 96 7.6 Armstrong et al. (1976)
Sheepshead minnow
(Cyprinodon variegatus) 0.14 g stat 22 96 3.88 Boeri (1988b)
a stat = static conditons (water unchanged for duration of test); flow = flow-through conditions (benomyl concentration in water
continuously maintained)
b hardness given as mg CaCO3/litre
c 50% wettable powder
d EC50 based on rate of shell deposition
Table 12. Summary of earthworm toxicity data on benomyl in field studiesa
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass 0.125 0.9 63 8% reduction in number Ammon (1985)
1.25 3.6 43% reduction in number
Grass 7.8 22.2 21 95% reduction in number Tomlin & Gore (1974)
91% reduction in biomass
Grass 0.56 1.6 49 79% reduction in cast Keogh & Whitehead (1975)
production
Grass 0.56 1.6 13 50% reduction in number Tomlin et al. (1980,
(0.18)b 28 40% reduction in number 1981)
180 70% reduction in number
328 67% reduction in number
1.12 (0.90)b 28 35% reduction in number
(0.20)b 180 46% reduction in number
328 50% reduction in number
2.24 6.4 13 75% reduction in number
28 50% reduction in number
(0.30)b 180 50% reduction in number
328 64% reduction in number
Grass/loam 2.0 5.7 30 70% reduction in number Edwards & Brown (1982)
180 22% reduction in number
365 1% reduction in number
Grass/sandy loam 5.0 14.3 30 89% reduction in number Edwards & Brown (1982)
180 59% reduction in number
365 32% reduction in number
Table 12 (contd).
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass/loamy sand 10.0 28.6 30 80% reduction in number
180 89% reduction in number
365 89% reduction in number
Grass/sandy loam 5.0 14.3 30 91% reduction in number; Edwards & Brown (1982)
99% reduction in L. terrestris;
29% reduction in L. festivus
180 90% reduction in L. terrestris;
200% increase in L. festivus
365 99% reduction in L. terrestris;
1457% increase in L. festivus
a From: Van Gestel (1992)
b Results of analysis of the top 15-cm layer carried out by the author, recalculated as the concentration in the top 2.5 cm layer
The acute toxicity for several birds is listed in Table 13.
Benomyl is of low toxicity to birds.
Table 13. Acute toxicity of benomyl to birds
Species LD50a 5-day LC50b Reference
(mg/kg) (mg/kg)
Bobwhite quail > 10 000 Busey (1968e)
Mallard duck > 10 000 Busey (1968e)
Japanese quail > 5000 c Hill & Camardese
(1986)
Starling > 100 Schafer (1972)
Redwinged blackbird 100 Schafer (1972)
a LD50 = single oral dose expressed as mg/kg body weight
b LC50 = 5-day dietary exposure followed by 3 days on a "clean" diet
expressed as mg/kg diet
c 50% active ingredient; no overt signs of toxicity were observed
9.4 Population and ecosystem effects
Under certain conditions benomyl may have an effect on
populations of earthworms. In apple orchards where foliage has been
treated repeatedly at a rate of 0.28 kg/ha and has fallen to the
ground, earthworms may be eliminated after two years of benomyl use
(up to 13 sprayings). The earthworms Lumbricus terrestris and
Allolobophora chlorotica were most affected. Populations of other
species recovered within two years of the termination of spraying.
Orchard yields were unaffected, as were earthworm populations
adjacent to the orchards, because of the immobility of benomyl in
the soil (Stringer & Wright, 1973; Stringer & Lyons, 1974).
Van Gestel (1992) has summarized reports of the toxicity of
benomyl on earthworms from field studies with different soil types,
application rates and crops (Table 12). Estimated soil
concentrations in Table 12, for the various uses of the fungicide,
are based on application rates; they assume no mobility of the
compound beyond the top 2.5 cm of soil and homogeneous distribution
of benomyl in this layer. For orchard application, it was further
assumed that 50% of the applied active ingredient reached the soil.
Reported effects include reduced numbers and reduced activity of
worms. Application rates are within the recommended rates for
benomyl as a fungicide on these crops.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Benomyl and carbendazim are two different fungicides in their
own right. However, carbendazim is also the main metabolite of
benomyl in mammals and the degradation product of benomyl in the
environment. Butyl isocyanate is the chemical moiety removed from
benomyl when carbendazim is formed. Given the similar toxicities
caused by benomyl and carbendazim and the different toxicological
profile of butyl isocyanate, the two fungicides are evaluated
together in this monograph.
10.1 Evaluation of human health risks
The two primary routes of exposure to humans are through diet
and through manufacturing or use of the product.
There is limited information on actual dietary exposure to
benomyl and carbendazim. Dietary exposure has been estimated in the
Netherlands and the USA. In the USA, exposure based on dietary
habits, measured residue levels, and the percentage of crop treated
has been calculated for various subgroups of population. These
calculations indicate that the estimated benomyl exposure is
0.144-1.479 µg/kg per day (section 5.2.1). In the Netherlands, the
mean dietary intake was estimated to be 0.83 µg/kg per day (0.05
mg/day per person). These levels of exposure are below the
recommended ADI of 0.01 (carbendazim) and 0.02 (benomyl) mg/kg body
weight.
The average air levels of benomyl and carbendazim have been
determined in a manufacturing facility (section 5.3) and found to be
less than 0.2 and 0.3 mg/m3, respectively. Both values are below
the Threshold Limit Value of 5-10 mg benomyl/m3 established by a
number of governmental agencies.
In one study, potential respiratory and dermal exposure to
benomyl wettable powder formulation was determined under several
agricultural use situations (section 5.3). The highest rates of
exposure occurred in situations of mixing and loading in preparation
for aerial application; dermal and respiratory exposures were,
respectively, 26 and 0.08 mg/person per cycle. Home users and
agricultural workers re-entering treated fields were estimated to be
exposed to about 1 mg/person per cycle and 5.9 mg/person per hour,
principally through the dermal route.
Because of the low mammalian toxicity, acute benomyl or
carbendazim poisonings are unlikely to occur under conditions of
normal use.
Several studies of agricultural workers (section 8.2) have
shown some cases of contact dermatitis after exposure to benomyl.
These effects can be significantly reduced or eliminated by wearing
long-sleeved shirts, long trousers and gloves.
There is little information on health effects in humans as a
consequence of exposure to either benomyl or carbendazim. Two
studies have been conducted on factory workers involved in the
manufacture of benomyl (section 8.2). In one study, haematological
profiles from 50 factory workers involved in the manufacture of
benomyl were comparable to those from a control group of 48 workers.
A second study found no decrease in the birth rate of the wives of
298 factory workers exposed to benomyl.
Extensive studies of various species of laboratory animals show
reproductive, developmental, mutagenic and carcinogenic effects
associated with both benomyl and carbendazim. The effects observed
on rat fetuses were microphthalmia, hydrocephaly and encephaloceles.
The no-observed-effect levels (NOEL) for developmental toxicity are
equal to or greater than 10 mg/kg body weight per day, depending
upon the species and route of administration. Similarly, the NOEL of
benomyl for reproductive effects in the male rat appears to be 15
mg/kg body weight per day after gavage dosing. In feeding studies
with both benomyl and carbendazim, the NOEL appears to be 500 mg/kg
diet (equivalent to 25 mg/kg body weight per day). However, one
benomyl feeding study reported a NOEL of less than 1 mg/kg diet
(0.05 mg/kg body weight per day) for male reproductive effects. The
reason for the discrepancy between the NOEL in this latter study and
other investigations is unknown.
The only consistent genotoxic effect noted in animal studies is
the induction of numerical chromosomal aberrations. These effects
are consistent with the interaction of benomyl and carbendazim with
microtubule formation.
Rat carcinogenicity studies did not show any carcinogenic
effect for either compound. Benomyl and carbendazim induce
hepatocellular tumours in CD-1 and SPF Swiss mice but not in NMRKf
mice. This finding in mice is not considered to be a result of a
direct genotoxic action. Rather, it appears to be associated with
liver toxicity in strains of mice that are highly susceptible to
tumour formation at this site.
Benomyl and carbendazim are spindle poisons. Effects on target
cells are consequences of binding to microtubules, giving toxicities
similar to those of other spindle poisons such as colchicine and
vincristine. Benzimidazol compounds in general and benomyl and
carbendazim in particular have selective effects on the microtubules
of different eukaryotes. Reasons for this selectivity include the
binding capability to different tubulins and pharmacokinetic
differences across species. In vitro concentrations of benomyl
used to kill sensitive fungi were found to be ineffec tive in
disturbing mammalian microtubular functions. These studies on the
mechanism of action of benomyl and carbendazim indicate a selective
effect of these compounds for target species.
In summary, the LD50, as determined in a number of test
species, for benomyl ranges from > 2000 to > 12 000 mg/kg and for
carbendazim from > 2000 to > 15 000 mg/kg. There are no known
reports of human poisoning for either compound. This, coupled with
the low estimated environmental levels of both compounds, would
suggest that the possibility of acute poisoning by benomyl or
carbendazim is very remote. Similarly, the data available on test
species make it unlikely that either benomyl or carbendazim is
carcinogenic for humans. The NOELs for both reproductive and
teratogenic effects of benomyl and carbendazim (i.e. 10-15 mg/kg) do
raise a possibility that an accidental ingestion of either fungicide
could adversely alter reproductive outcome in humans, but the
likelihood that such poisoning would occur is remote. The
selectivity of these two benzamidazol compounds for the tubulin of
the target species (fungi) and their relative ineffectiveness to
disturb mammalian microtubule function further reduce the
possibility of their having toxic effects in humans.
10.2 Evaluation of effects on the environment
Benomyl is rapidly converted to carbendazim in various
environmental compartments, the half-lives being 2 and 19 h in water
and soil, respectively. Therefore, data from studies on both benomyl
and carbendazim are relevant for the evaluation of environmental
effects.
Carbendazim persists on leaf surfaces and in leaf litter. In
soil the half-life is between 3 and 12 months, and the compound may
be detected for up to 3 years. However, in many cases, major
residues will be lost within a single season. Residues of
carbendazim and its metabolites are strongly bound or incorporated
into soil organic matter. The strong adsorption (Koc =
approximately 2000) of carbendazim to soil and sediment particles
reduces its bioavailability to terrestrial and aquatic organisms.
Similarly, the mobility of carbendazim in soil is limited, and it is
not expected to leach to ground water.
Benomyl and carbendazim are highly toxic to some aquatic
organisms in laboratory tests, the most sensitive species being the
channel catfish with a 96-h LC50 for yolk-sac fry of 0.006 mg
benomyl/litre. However, this toxicity is unlikely to be manifest in
the environment for most aquatic organisms because of the low
bioavailability in surface waters. The exposure of sediment-living
organisms could be greater, but no test results are available for
these organisms.
Benomyl and carbendazim affect groups of fungi in soil but do
not seem to modify the overall microbial activity of the soil when
used at normal field rates.
In both the laboratory and field, benomyl and carbendazim
applied at recommended rates cause deaths and sublethal reproductive
effects on earthworms of many different species. Surface-feeding
species eating leaf litter are most at risk. Populations may take
more than 2 years to recover. There are no studies available on
other litter and soil invertebrates.
Benomyl and carbendazim have low toxicity for birds and
carbendazim is classified as "relatively non-toxic" to honey-bees.
10.3 Conclusions
Benomyl causes dermal sensitization in humans. Both benomyl and
carbendazim represent a very low risk for acute poisoning in humans.
Given the current exposures and the low rate of dermal absorption of
benomyl and carbendazim, it is unlikely that they would cause
systemic toxicity effects either in the general population or in
occupationally exposed subjects. These conclusions are drawn from
animal data and from the limited human data available, but these
extrapolations are supported by the understanding of the mode of
action of carbendazim and benomyl in both target and non-target
species.
Further elucidation of the mechanism of toxicity of carbendazim
and benomyl in mammals will perhaps permit a better determination of
no-observed-effect levels. Binding studies on tubulins of target
cells (testis and embryonic tissues) will facilitate comparisons
across species.
Carbendazim is strongly adsorbed to soil organic matter and
remains in the soil for up to 3 years. It persists on leaf surfaces
and, therefore, in leaf litter. Earthworms have been shown to be
adversely affected (population and reproductive effects) at
recommended application rates. There is no information on other soil
or litter arthropods that would be similarly exposed.
The high toxicity to aquatic organisms in laboratory tests is
unlikely to be seen in the field because of the low bioavailability
of sediment-bound residues of carbendazim. However, no information
is available on sediment-living species which would receive the
highest exposure.
11. FURTHER RESEARCH
1. Comparative binding studies of carbendazim to tubulins of
target tissues from various species should be undertaken.
2. Further clarification of the fate of 1,2-diaminobenzene and
bound residues in the environment is needed.
3. The effects of benomyl and carbendazim on sediment-dwelling
organisms needs to be investigated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Benomyl was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1973, 1975, 1978, 1983 and 1988. The 1978 meeting
agreed that the MRLs for benomyl, carbendazim and thiophanate-methyl
should be combined and expressed as carbendazim. Benomyl residues
were last evaluated by the 1988 meeting (FAO/WHO, 1988a,b) and the
MRLs were updated at that time. These MRLs (expressed as
carbendazim) are listed in Table 4. The 1983 meeting (FAO/WHO,
1985a) evaluated benomyl toxicology and set the following benomyl
NOEL levels and ADI:
Rat: 2500 mg/kg in the diet, equivalent to 125 mg/kg body
weight
Dog: 100 mg/kg (carbendazim) in the diet, equivalent to 2.5
mg/kg body weight
Rat: teratology - 30 mg/kg body weight per day
The estimated ADI for benomyl was established at 0-0.02 mg/kg
body weight.
Benomyl has not been evaluated by the International Agency for
Research on Cancer (IARC).
REFERENCES
Albertini S (1991) Reevaluation of the 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae aneuploidy
test systems by the Gene-Tox Program using strain D61.m of
Saccharomyces cerevisiae. Mutat Res, 260: 165-180.
Amacher DE, Paillet S, & Ray VA (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Application
to genetic toxicological testing. Mutat Res, 64: 391-406.
Ammon HU (1985) Worm toxicity tests using Tubifex tubifex. In:
Comportement et effets secondaires des pesticides dans le sol. Les
Colloques de l'INRA 31, Versailles, 4-8 juin 1984. Paris, Institut
national de la Recherche agronomique, pp 303-317.
Armstrong DA, Buchanan DU, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp. in laboratory-reared larvae of the Dungeness crab,
Cancer magister, and possible chemical treatments. J Invertebr
Pathol, 28: 329-336.
Arthur MF, Schweitzer KL, Fadel LC, Marsh BH, & Marsh SS (1989a)
Aerobic aquatic metabolism of [phenyl(U)-14C]benomyl in
Greenville, Mississippi, water and sediment (Battelle Study No.
N-0966-7301). Columbus, Ohio, Battelle, Environmental Sciences
Department (Unpublished report No. AMR-1452-89, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Arthur MF, Marsh BH, Fadel LC, & Zwick TC (1989b) Anaerobic aquatic
metabolism of [phenyl(U)-14C]benomyl in West Jefferson, Ohio, pond
water and sediment (Battelle Study No. NO799-8800). Columbus, Ohio,
Battelle, Environmental Sciences Department (Unpublished report No.
AMR 770-87, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Athwal RS & Sandhu SS (1985) Use of human X mouse hybrid cell line
to detect aneuploidy induced by environmental chemicals. Mutat Res,
149: 73-81.
Barbo EC & Carroll KS (1972) Oral ALD test (S-triazine,
3-butylbenzimidazolo[1,2-a],-2,4[1H,3H]-dione. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 227-72).
Barefoot AC (1988) Vapor pressure of benomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Research & Development
Division, Experimental Station (Unpublished report No. AMR 1078-88).
Barnes TB, Verlangieri AJ, & Wilson MC (1983) Reproductive toxicity
of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate (benomyl) in
male Wistar rats. Toxicology, 28: 103-115.
Baude JF, Gardiner JA, & Han JCY (1973) Characterization of residues
on plants following foliar spray applications of benomyl. J Agric
Food Chem, 21: 1084-1090.
Baude FJ, Pease HL, & Holt RF (1974) Fate of benomyl on field soil
and turf. J Agric Food Chem, 22: 413.
Belasco IJ (1979a) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX-4).
Belasco IJ (1979b) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied with supplement.
Wilmington, Delaware, Du Pont de Nemours and Co., Inc. (Unpublished
report No. B/ME-47 and supplement No. HLR 117-79).
Belasco IJ, Kirkland JJ, Pease HL, & Sherman H (1969) Studies with
2-14C-labelled methyl-1-(butylcarbamoyl)-2-benzimidazolecarbamate
(benomyl) in rats. Wilmington, Delaware, Du Pont de Nemours and Co.,
Inc., Biochemical Department, Research Division, Experimental
Station (Unpublished report No. B/ME-36).
Bignami M, Aulicino F, Velcich A, Carere A, & Morpurgo G (1977)
Mutagenic and recombinogenic action of pesticides in Aspergillus
nidulans. Mutat Res, 46: 395-402.
Bionomics, Inc. (1972) Acute toxicity of Benlate to grass shrimp
( Palaemonetes vulgaris). Wareham, Massachusetts, Bionomics, Inc.
(Unpublished report No. HOL 275-72, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Boeri RL (1988a) Flow through acute toxicity of benomyl technical to
the eastern oyster, Crassostrea virginica. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 13-89, prepared
for E.I. Du Pont de Nemours and Co., Inc.).
Boeri RL (1988b) Static acute toxicity of benomyl technical to the
sheepshead minnow, Cyprinodon variegatus. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 9-89, prepared for
Du Pont de Nemours and Co., Inc.).
Boeri RL (1988c) Static acute toxicity of benomyl technical to the
mysid, Mysidopsis bahia. Marblehead, Massachusetts, Enseco
(Unpublished report No. HLO 828-88, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Bolton EE, Anderson JJ, & Koeppe MK (1986a) Metabolism of
[phenyl(U)-14C] benomyl in field-grown soybeans. Wilmington,
Delaware, Du Pont de Nemours and Co., Inc., Agricultural Products
Department, Experimental Station (Unpublished report No.
AMR-531-86).
Bolton EE, Anderson JJ, & Koeppe MK (1986b) Metabolism of
[phenyl(U)-14C] benomyl in paddy rice. Wilmington, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Agricultural Products Department,
Experimental Station (Unpublished report No. AMR-507-86).
Brock WJ (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 505-87).
Busey WM (l968a) Acute dermal LD50 test and dermal irritation test
on rabbits using a wettable powder formulation (50% benomyl) with
histological addendum. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Falls Church, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. MRO 1126-1,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968c) Teratology study in rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 1079, prepared for
E.I Du Pont de Nemours and Co., Inc.). Busey WM (l968d) Repeated
dermal application test on rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 298-68, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (1968e) Acute dietary administration - mallard ducklings
and bobwhite quail: Fungicide 1991. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Bushway RJ, Hurst HL, Kugabalasooriar J, & Perkins LB (1991)
Determination of carbendazim in blueberries by reversed-phase high
performance liquid chromatography. J Chromatogr, 555: 321-324.
Canton JH (1976) The toxicology of benomyl, thiophanate-methyl, and
BCM to four freshwater organisms. Bull Environ Contam Toxicol, 16:
214-218.
Carere A, Ortali VA, Cardamone G, Torracca AM, & Raschetti R (1978)
Microbiological mutagenicity studies of pesticides in vitro. Mutat
Res, 57: 277-826.
Carter SD (l982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Raleigh, North Carolina, North Carolina
State University (Thesis).
Carter SD & Laskey JW (l982) Effect of benomyl on reproduction in
the male rat. Toxicol Lett, 11: 87-94.
Chang WM (1985) Soil column leaching studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Agricultural Chemicals Department, Experimental Station
(Unpublished report No. AMR 426-85).
Chiba M & Veres DF (1981) Fate of benomyl and its degradation
compound methyl 2-benzimidazole carbamate on apple foliage. J Agric
Food Chem, 29: 588-590.
Clermont Y (1972) Kinetics of spermatogenesis in mammals:
"seminiferous epithelium cycle and spermatogonial renewal". Physiol
Rev, 52: 198-236.
Colburn CW (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 84-69).
County Natwest Woodmac (1992) The fungicide market. London, County
Natwest Woodmac, Agrochemical Service, pp 49-51.
Culik R (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the concepti
of rats exposed to benomyl and Benlate by diet. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 916-80).
Culik R (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. HLR 970-80).
Culik R & Gibson JR (1974) "Benlate" dominant lethal study in male
rats with supplemental statistical report (22-77). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 72-74).
Cummings AM, Harris ST, & Rehnberg GL (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat. Fundam
Appl Toxicol, 15: 528-535.
Dashiell OL (1972) Acute oral test (benzimidazole,
2-(3-butylureido)). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 456-72).
Davidse LC & Flach W (1977) Differential binding of methyl
benzimidazol-2-yl carbamate to fungal tubulin as a mechanism of
resistance to this antimitotic agent in mutant strains of
Aspergillus nidulans. J Cell Biol, 72: 174-193.
De Bertoldi M & Griselli M (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat Res, 74:
303-324.
De Bertoldi M, Griselli M, Giovannetti M, & Barale R (1980)
Mutagenicity of pesticides evaluated by means of gene-conversion in
Saccharomyces cerevisiae and in Aspergillus nidulans. Environ
Mutagen, 2: 359-370.
De Brabander M, Van de Veire R, Aerts F, Geuens S, & Hoebeke J
(1976a) A new culture model facilitating rapid quantitative testing
of mitotic spindle inhibition in mammalian cells. J Natl Cancer
Inst, 56: 357-363.
De Brabander M, Van de Veire R, Aerts F, Brogers M, & Janssen PAJ
(1976b) The effect of methyl [5(2-thienylcarbonyl)-1H-benzimidazol-
2-yl carbamate (17934; NSC 238159), a new synthetic antitumoral drug
interfering with microtubules, on mammalian cells cultured in
vitro. Cancer Res, 36: 905-916.
Delp CJ (1980) Coping with resistance to plant disease control
agents. Plant Dis, 64: 652-657.
Dési I (1979) Hygienic-toxicological evaluations of pesticides.
Budapest, National Institute of Hygiene (D.Sc. Thesis).
Dési I (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav Toxicol Teratol, 5: 503-515.
Dési I, Dura G, Szlobodnyik J, & Csuka I (1977) Testing of pesticide
toxicity in tissue culture. J Toxicol Environ Health, 2: 1053-1066.
Dési I, Nehéz M, Palotas M, Tempfli A, Hogye A, & Vetro G (1990)
Experience of health status surveillance of pesticide workers in
Hungary. Med Lav, 81(6): 517-523.
Donovan SD & Krahn DF (1981) Mutagenic evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 343-81).
Douglass MT & Handley JW (1988) The algistatic activity of benomyl
technical. Huntingdon, United Kingdom, Huntingdon Research Centre
Ltd (Unpublished report No. DPT 171(n)/88371, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Draize JH, Woodard G, & Gawery HO (1944) New methods for the study
of irritation and toxicity of substances applied topically to the
skin and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Drewes CD, Zoran MJ, & Callahan CA (1987) Sublethal neurotoxic
effects of the fungicide benomyl on earthworms (Eisenia fetida).
Pestic Sci, 19: 197-208.
Du Pont (1972) Residue studies - fish: benomyl, MBC, and 2-AB.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report).
Du Pont (1987) Determination of octanol water partition coefficient
for benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/PC-55-CA).
Eastmond DA & Tucker JD (1989) Kinetochlore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat Res, 224: 517-525.
Edwards PJ & Brown SM (1982) Use of grassland plots to study the
effect of pesticides on earthworms. Pedobiologia, 24: 145-150
Eickhoff JC, Petersen BJ, & Chaisson CF (1989) Anticipated residues
of benomyl in food crops and potential dietary exposure and risk
assessment. Washington, DC, Technical Assessment System, Inc.
(Unpublished report No. TAS 000-005, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Ellis WG, Semple JL, Hoogenboom ER, Kavlock RJ, & Zeman FJ (1987)
Benomyl-induced craniocerebral anomalies in fetuses of adequately
nourished and protein -deprived rats. Teratog Carcinog Mutagen, 7:
357-375.
Ellis WG, De Roos F, Kavlock RJ, & Zeman FJ (1988) Relationship of
periventricular overgrowth to hydrocephalus in brains of fetal rats
exposed to benomyl. Teratog Carcinog Mutagen, 8: 377-391.
Evans EL & Mitchell AD (1980) An evaluation of the effect of benomyl
on sister chromatic exchange frequencies in cultured Chinese hamster
ovary cells. Menlo Park, California, SRI International (Unpublished
report prepared for E.I. Du Pont de Nemours and Co., Inc.).
Everhart LP (1979) Benlate dust exposure survey. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Biochemical
Department (Unpublished report No. B/TOX 6).
Everhart LP & Holt RF (1982) Potential benlate fungicide exposure
during mixer/loader operations, crop harvest and home use. J Agric
Food Chem, 30: 222-227.
FAO/WHO (1985a) Benomyl. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 7-46 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1985b) Carbendazim. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 89-121 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1988a) Benomyl. In: Pesticide residues in food -
Evaluations 1988. Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 5-15 (FAO Plant Production
and Protection Paper 93/1).
FAO/WHO (1988b) Carbendazim. In: Pesticide Residues in Food -
Evaluations 1988: Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 41-54 (FAO Plant Production
and Protection Paper 93/1).
Fielding M, Barcelo D, Helweg A, Galassi S, Torstensson L, van
Zoonen P, Wolter R, & Angeletti G (1992) Pesticides in ground and
drinking water. Brussels, Commission of the European Communities,
Directorate-General for Science, Research and Development,
Environmental and Waste Recycling, 135 pp (Water Pollution Research
Report 27).
Fiscor G, Bordas S, & Stewart SJ (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl-N'-nitro-N-nitrosoguanine in Salmonella typhimurium in
vitro and in rodent host-mediated assays. Mutat Res, 51: 151-164.
Fitzpatrick K (1980) Chinese hamster ovary cell assay for
mutagenicity. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 438-80).
Frame SR & Van Pelt CS (1990) Oncogenicity studies with benomyl and
MBC in mice: Supplemental peer review. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 20-82 and supplement No. HLR 70-82).
Frank KM (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 85-69).
Frank KM (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 233-72).
Fritz SB (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 109-69).
Fritz SB & Sherman H (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 51-69).
Gargus JL & Zoetis T (1983a) Primary irritation study in rabbits
(Benlate PNW). Vienna, Virginia, Hazelton Laboratories, Inc.
(Unpublished report No. HLO 510-83, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983b) Eye irritation test in rabbits (EPA
pesticide registration - Benlate PNW). Vienna, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 511-83, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983c) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines - Benlate PNW).
Vienna, Virginia, Hazelton Laboratories, Inc. (Unpublished report
No. HLO 512-83, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Vienna, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. HLO 67-84,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Georgieva V, Vachkova R, Tzoneva M, & Kappas A (1990) Genotoxic
activity of benomyl in different test systems. Environ Mol Mutagen,
16: 32-36.
Goldenthal EI (1978) Neurotoxicity study in hens [using technical
benomyl (< 95% benomyl). Mattawan, Michigan, International Research
and Development Corporation (Unpublished report and addendum No. HLO
28-79, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gooch JJ (1978) Fertility of workers potentially exposed to benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX 7).
Goodman NC (1975) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 847-74).
Goulding R (1983) Poisoning on the farm. J Soc Occup Med, 33: 60-65.
Guengerich FP (1981) Enzyme induction with Du Pont compounds H11,
202-02 and H10, 962-02. Nashville, Tennessee, Vanderbilt University,
School of Medicine (Unpublished report No. HLO 850-81, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gurova AI, Alekseeva NP, Gorlova OE, & Chernyshova RA (1976) [Data
for substantiation of the maximum permissible level of
m-(trifluoromethyl) phenyl isocyanate and butyl isocyanate in the
air of work areas.] Gig Tr Prof Zabol, 3: 53-55 (in Russian).
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 80: 339-357.
Han JCY (1978) Metabolism of 14C-labeled benomyl in the mouse and
hamster. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-65).
Han JCY (1979) 2-14C-Benomyl (50% WP) rat study - intravenous
injection. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-41).
Han JCY (1980) Metabolism of 2-14C-benomyl in the lactating nanny
goat. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report No. B/ME-39).
Hardesty PT (1982) Attempts to characterize liver residues from
14C-benomyl dosed goat. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Biochemicals Department (Unpublished report
No. AMR 71-82).
Hardisty JF (1990) Oncogenicity studies with benomyl and MBC in
mice. Peer-review of liver neoplasms. Research Triangle Park, North
Carolina, Experimental Pathology Laboratories, Inc. (Unpublished
report No. 129-012, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Hastie AC (1970) "Benlate"-induced instability of Aspergillus
diploids. Nature (Lond), 226: 771.
Helweg A (1977) Degradation and absorption of carbendazim and
2-aminobenzimidazole in soil. Pestic Sci, 8: 71-78.
Helweg A (1985) Side effects caused by pesticide combinations. In:
Jensen V, Kjoller A, & Sorensen LH ed. Proceedings of the FEMS
Symposium on Microbial Communities in Soil. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 385-395.
Hess RA, Moore BJ, Forrer J, Linder RE, & Abuel-Atta AA (1991) The
fungicide benomyl (methyl) 1-(butylcarbomyl)-2-benzimidazole
carbamate causes testicular dysfunction by inducing the sloughing of
germ cells and occlusion of efferent ductules. Fundam Appl Toxicol,
17: 733-745.
Hill EF & Camardese MB (1986) Lethal toxicities of environmental
contaminants and pesticides to Coturnix. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
Hofer I, Beck T, & Wallnöfer P (1971) [The influence of the
fungicide benomyl on soil microflora.] Z Pflanzenkr Pflanzenschutz,
78: 399-405 (in German).
Holden HE, Crider PA, & Wahrenburg MG (1980) Mitotic arrest by
benzimidazole analogs in human lymphocyte cultures. Environ Mutat,
2: 67-73.
Hood DB (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 211-69).
Hoogenboom ER, Ransdell JF, Ellis WG, Kavlock RJ, & Zeman FJ (1991)
Effects on the fetal rat eye of maternal benomyl exposure and
protein malnutrition. Curr Eye Res, 10: 601-612.
Hornberger CS (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
95-69).
Hostetler KH (1977) Oral LD50 test (Benlate OD). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 527-77).
Hutton DG (1989) Static acute 48-hour EC50 of Benlate fungicide 50
DF to Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 541-88).
Hutton DG, Kasprzak DJ, & Priester TM (1984) Laboratory studies of
[2-14C] carbendazim bioconcentration in Bluegill sunfish. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 428-84).
ILO (1991) Occupational exposure limits for airborne toxic
substances, 3rd ed. Geneva, International Labour Office
(Occupational Safety and Health Series No. 37).
Ireland CM, Gull K, Gutteridge WE, & Pogson CI (1979) The
interaction of benzimidazole carbamates with mammalian microtubule
protein. Biochem Pharmacol, 28: 2680-2682.
Jessup CD (1979) Acute delayed neurotoxicity study in chickens using
technical benomyl (> 95% benomyl). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report No. HLO 674-79, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Jessup DC & Dean W (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl). Mattawan,
Michigan, International Research and Development Corporation
(Unpublished report No. HLO 29-79, prepared for E.I. Du Pont de
Nemours and Co., Inc).
Johnson JD (1988) Determination of the plateau level of bound
[phenyl(U)-14C] carbendazim residues in goat liver. Columbus,
Ohio, Batelle, Columbus Division (Unpublished report No. AMR 779-87,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Kaastra-Howeler LH & Gams W (1973) Preliminary study on the effect
of benomyl on the fungal flora in a greenhouse soil. Neth J Plant
Pathol, 79: 516-518.
Kappas A & Bridges BA (1981) Induction of point mutations by benomyl
in DNA-repair-deficient Aspergillus nidulas. Mutat Res, 91:
115-118.
Kappas A, Georgopoulos SG, & Hastie AC (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat Res, 26: 17-27.
Kappas A, Green MHL, Bridges BA, Rogers AM, & Muriel WJ (1976)
Benomyl - a novel type of base analogue mutagen? Mutat Res, 40:
379-382.
Kavlock RJ, Chernoff N, Gray LE, Gray JA, & Whitehouse D (1982)
Teratogenic effects of benomyl in the Wistar rat and CD-1 mouse,
with emphasis on the route of administration. Toxicol Appl
Pharmacol, 62: 44-54.
Kelly DP (1989) Butyl isocyanate industrial hygiene survey. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. BENO/TOX 18).
Keogh RG & Whitehead PH (1975) Observations on some effects of
pasture spraying with benomyl and carbendazim on earthworm activity
and litter removal from pasture. NZ J Exp Agric, 3: 103-104.
Kirkland JJ (1973) Method for high-speed liquid chromatographic
analysis of benomyl and/or metabolite residue in cow milk, urine,
feces and tissues. J Agric Food Chem, 21: 171-177.
Kirkland JJ, Holt RF, & Pease HL (1973) Determination of benomyl
residues in soils and plant tissues by high speed cation exchange
liquid chromatography. J Agric Food Chem, 21: 368-371.
Krechniak J & Klosowska B (1986) The fate of 14C-carbendazim in
rat. Xenobiotica, 16(9): 809-815.
Krupka RM (1974) On the anti-cholinesterase activity of benomyl.
Pestic Sci, 5: 211-216.
Kuehne G, Heise H, Plottke B, & Puskeiler T (1985) Dermatitis after
benlate contact. Z Gesamte Hyg Grenzgeb, 31: 710-711.
Lamb MJ & Lilly LJ (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17:
83-95.
Lee KP (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 66-77).
Liesivuori J & Jääskeläinen S (1984) Exposure of greenhouse workers
to pesticides. Tampore, Finland, National Board of Labor Protection,
pp VIII-IX (Research Report No. 46).
Linder RE, Rehnberg GL, Strader LF, & Diggs JP (1988) Evaluation of
reproductive parameters in adult male Wistar Rats after subchronic
exposure. J Toxicol Environ Health, 25: 285-298.
Lisi P, Caraffini S, & Assalve D (1986) A test series for pesticide
dermatitis. Contact Dermatitis, 15: 266-269.
Littlefield NA & Busey WM (1969) Four-hour acute inhalation exposure
test in dogs using a wettable powder formulation (50% benomyl).
Falls Church, Virginia, Hazelton Laboratories, Inc. (Unpublished
report No. HLR 192-69, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983a) L5178Y mouse
lymphoma cell assay for mutagenicity (benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 86-83).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983b) L5178Y mouse
lymphoma cell assay for mutagenicity. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc. (Unpublished report No. HLR 253-83).
Majut JC (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 174-06).
Marsh BH & Arthur MF (1989) Aerobic metabolism of
[phenyl(U)-14C]benomyl in Keyport Silt Loam. Columbus, Ohio,
Battelle Memorial Institute (Unpublished report No. AMR 1112-88,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Marvin CH, Birndle ID, Hall CD, & Chiba M (1991) Rapid on-line
precolumn high performance liquid chromatographic method for the
determination of benomyl, carbendazim and aldicarb species in
drinking water. J Chromatogr, 555: 147-154.
Matsushita T & Aoyama K (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind Health,
19: 77-83.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp 45-56 (Resource Publication No. 160).
Mazur AR & Hughes TD (1975) Nitrogen transformation in soil as
affected by the fungicides benomyl, dyrene and maneb. Agron J, 67:
755-758.
Meade AB (1984) Acute contact LD50 toxicity study in honey bees
( Apis mellifera) with INE 965-212 (MBC). Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc. (Unpublished report No. AMB
84-5).
Mebus CA (1990) Reproductive and fertility effects with DPX-1991-529
(benomyl). Multigeneration reproduction study in rats. Newark,
Delaware, Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 765-90).
Monson KD (1985) Metabolism of [2-14C]benomyl in the lactating
dairy cow. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc. (Unpublished report No. AMR 247-84).
Monson KD (1986a) Metabolism of [2-14C]benomyl and [phenyl(U)-
14C]benomyl in laying hens. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc. (Unpublished report No. AMR 391-85).
Monson KD (1986b) Metabolism of [2-14C]carbendazim laying hens.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 264-84).
Monson KD (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 1141-88).
Monson KD (1991) Release and characterization of bound benomyl and
carbendazim metabolites in animal tissues via Raney nickel
desulfurization and acid dehydration. J Agric Food Chem, 39:
1808-1811.
Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, & Linder RE (in
press) Acute and long-term effects of the fungicide carbendazim
(methyl 2-benzimidazole carbamate, MBC) on the male reproductive
system in the rat. J Androl.
Nehéz M, Palotas M, Zimanyi M, Boros P, Mohos G, Vetro G, & Desi I
(1992) [Repeated cytogenetic examinations of workers using
pesticides in Csongrad County.] Egeszsegtudomany, 36: 40-47 (in
Hungarian).
Newsome WH & Collins PG (1987) Enzyme-linked immunosorbent assay of
benomyl and thiobendazole in some foods. J Assoc Off Anal Chem, 70:
1025-1027.
Newsome WH & Shields JB (1981) A radioimmunoassay for benomyl and
methyl 2-benzimidazolecarbamate on food. J Agric Food Chem, 29:
220-222.
Palawski DU & Knowles CO (1986) Toxicological studies of benomyl and
carbendazim in rainbow trout, channel catfish and bluegills. Environ
Toxicol Chem, 5: 1039-1046.
Parsons DW & Witt JM (1988) Pesticides in groundwater in the United
States of America: A report of a 1988 survey of State lead agencies.
Corvallis, Oregon, Oregon State University Extension Service.
Peeples JL (1974) Microbial activity in benomyl-treated soil.
Phytopathology, 64: 857-860.
Pilinskaya MA (1983) Investigation of the cytogenetic action of the
pesticides captan and benomyl in a culture of human peripheral blood
lymphocytes in the absence and presence of a system of metabolic
activation. Cytol Genet, 17: 29-33.
Powley CR (1985) Aqueous photolysis of [phenyl-14C(U)]benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 420-85).
Priester TM (1984) Hydrolysis of carbendazim [2-14C]. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 265-84).
Priester TM (1985) Batch equilibrium (adsorption/deabsorption) and
soil thin-layer chromatography studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 425-85).
Rainaldi G, Flori L, Colella CM, Mariani T, Piras A, Simi S, &
Simili M (1989) Analysis by BrUdR-labelling technique of induced
aneuploidy in mammalian cells in culture. Mutat Res, 177: 255-260.
Reinke RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
81-66).
Rhodes BC (1987) Greenhouse crop-rotation study with
[2-14C]carbendazim. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Inc. (Unpublished report No. AMR 495-86).
Rickard LB (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 97-83).
Rickard LB (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 98-83).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to
the earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Russell JF (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 18-78).
Russell JF (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 31-78).
Ruzicska P, Peter S, Laczi J, & Czeizel E (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP. Egeszegtudomany, 20:
74-83.
Ryan DL (1989) Soil column leaching of [phenyl(U)-14C]benomyl in a
rice paddy soil. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 1512-89).
Sandhu SS, Gudi RD, & Athwal RS (1988) A monochromosomal hybrid cell
assay for evaluating the genotoxicity of environmental chemicals.
Cell Biol Toxicol, 4: 495-506.
Sarver JW (1987) Acute oral toxicity study with IN-T1991 in male and
female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. HLR 334-87).
Sasaki YFX (1990) Benomyl: Micronucleus test in mice. Tokyo,
Institute of Environmental Toxicology, Kodaira Laboratories
(Unpublished report No. IET 89-0046, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Seiler JP (1976) The mutagenicity of benzimidazole and benzimidazole
derivatives. VI. Cytogenetic effects of benzimidazole derivatives in
the bone marrow of the mouse and the Chinese hamster. Mutat Res, 40:
339-348.
Sherman H (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 269-68).
Sherman H (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 17-69).
Sherman H (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
168-69).
Sherman H (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 232-69).
Sherman H (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 48-70).
Sherman H (1972) Long-term feeding studies in rats and dogs with
2-benzimadazolecarbamic acid, methyl ester (INE-965) (50% and 70%
MBC wettable powder formulations): Parts I and II. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 195-72).
Sherman H & Krauss WC (1966) Acute oral test [benomyl]. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 100-66).
Sherman H, Barnes JR, & Krauss WC (1967) Ninety-day feeding study
with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell, Laboratory (Unpublished report No. HLR 11-67).
Sherman H, Culik R, & Jackson RA (1975) Reproduction, teratogenic
and mutagenic studies with benomyl. Toxicol Appl Pharmacol, 32:
305-315.
Shirasu Y, Moriya M, & Kato K (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Tokyo, Institute of
Environmental Toxicology, Kodaira Laboratories (Unpublished report
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Shukla Y, Antony M, & Mehrota NK (1989) Studies on gamma-glutamyl
transpeptidase in rodents exposed to benomyl. Bull Environ Contam
Toxicol, 42: 301-306.
Siebert D, Zimmermann FK, & Lemperle E (1970) Genetic effects of
fungicides. Mutat Res, 10: 533-543.
Siegel MR (1975) Benomyl-soil microbial interactions.
Phytopathology, 65: 219-220.
Snee DA (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test described in HLR 43-69).
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. 134-69).
Stahl RG Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 401-90).
Staples RE (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl):
Parts I, II and III. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 649-80).
Staples RE (1982) Teratogenicity study in the rat using technical
benomyl (>95% benomyl) administered by gavage and supplement with
individual animal data. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
587-82).
Staub T & Sozzi D (1984) Fungicide resistance: A continuing
challenge. Plant Dis, 68: 1026-1031.
Stevenson IE (1985) Metabolism of [phenyl(U)-14C]benomyl in
peaches. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 443-85).
Stringer A & Lyons C (1974) The effect of benomyl and
thiophanate-methyl on earthworm populations in apple orchards.
Pestic Sci, 5: 189-196.
Stringer A & Wright MA (1973) The effect of benomyl and some related
compounds on Lumbricus terrestris and other earthworms. Pestic
Sci, 4: 165-170.
Tolle DA (1988) Metabolism of [phenyl(U)-14C] benomyl in sugar
beets. West Jefferson, Ohio, Battelle Columbus DW (Unpublished
report No. AMR 620-86, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a
fungicide on the numbers and biomass of earthworms in pasture. Bull
Environ Contam Toxicol, 12: 487-492.
Tomlin AD, Tolman JH, & Thorn GD (1980/1981) Suppression of
earthworm ( Lumbricus terrestris) populations around an airport by
soil application of the fungicide benomyl. Prot Ecol, 2: 319-323.
Tong C (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Valhalla, New York, Naylor Dana Institute (Unpublished report No.
HLO 741-81, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Torstensson L & Wessen B (1984) Interactions between the fungicide
benomyl and soil microorganisms. Soil Biol Biochem, 16: 445-452.
Turney RT (1979) Rat inhalation study - Benlate. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 116-79).
Van Faassen HG (1974) Effect of the fungicide benomyl on some
metabolic processes and on numbers of bacteria and actinomycetes in
the soil. Soil Biol Biochem, 6: 131-133. Van Gestel CAM (1992)
Validation of earthworm toxicity tests by comparison with field
studies: A review of benomyl, carbendazim, carbofuran, and carbaryl.
Ecotoxicol Environ Saf, 23: 221-236.
Van Gestel CAM, Dirven-van Breeman EM, Baerselman R, Emans HJB,
Janssen JAM, Postuma R, & van Vliet PJM (1992) Comparison of
sublethal and lethal criteria for nine different chemicals in
standardized toxicity tests using the earthworm Eisenia andrei.
Ecotoxicol Environ Saf, 23: 206-220.
Van Joost TH, Naafs B, & van Ketel WG (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9: 153-154.
Vick DA & Brock WJ (1987) Primary dermal irritation study with
benlate 50 DF fungicide in rabbits. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 300-87).
Wainwright M & Pugh GJF (1974) The effect of fungicides on certain
chemical and microbial properties of soils. Soil Biol Biochem, 6:
263-267.
Ward RS & Scott RC (1992) Benomyl: in vitro absorption of a 500 g
kg-1 WP formulation through human epidermis. Fernhurst, Naslemere,
Surrey, Imperial Chemical Industries (ICI) (Unpublished report No.
CTL/P/3659).
Warheit DB, Kelly DP, Carakostas MC, & Singer AW (1989) A 90-day
inhalation toxicity study with benomyl in rats. Fundam Appl Toxicol,
12: 333-345.
Weeks RE & Hedrick HG (1975) Influence of a systemic fungicide on
oxygen uptake by soil microorganisms. Soil Sci, 119: 280-284.
Wheeler J (1985) Hydrolysis of [phenyl-14C(U)]benomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 419-85).
WHO (1993) Environmental Health Criteria 149: Carbendazim. Geneva,
World Health Organization.
Wiechman BE (1982) Long term feeding study with methyl
l-(butylcarbamoyl)-2-benzimidazolecarbamate in mice (INT-1991; >95%
benomyl): Parts I, II and III. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 20-82).
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Bull Agric Chem Insp Stn, 12: 122-128.
Zbozinek JV (1984) Environmental transformations of DPA, SOPP,
benomyl and TBZ. Residue Rev, 92: 113-155.
Zelesco PA, Barbieri I, & Graves JAM (1990) Use of a cell hybrid
test system to demonstrate that benomyl induces aneuploidy and
polyploidy. Mutat Res, 242: 329-335.
Zeman FJ, Hoogenboom ER, Kavlock RJ, & Semple JL (1986) Effects on
the fetus of maternal benomyl exposure in the protein-deprived rat.
J Toxicol Environ Health, 17: 405-417.
Zoran MJ, Heppner TJ, & Drewes CD (1986) Teratogenic effects of the
fungicide benomyl on posterior segmental regeneration in the
earthworm, Eisenia fetida. Pestic Sci, 17: 641-652.
Zweig G, Gao R, & Popendorf W (1983) Simultaneous dermal exposure to
captan and benomyl by strawberry harvesters. J Agric Food Chem, 31:
1109-1113.
RESUME ET CONCLUSIONS
1. Résumé
1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le bénomyl, un solide cristallin de couleur ambrée, est un
fongicide endothérapique qui appartient à la famille du
benzimidazole. Il se décompose juste au-dessus de son point de
fusion de 140 °C et sa tension de vapeur est < 5 x 10-6 Pa
(< 3,7 x 10-8 mmHg) à 25 °C. Le bénomyl est pratiquement
insoluble dans l'eau à pH 5 et à 25 °C, sa solubilité étant de 3,6
mg/litre. Il est stable dans les conditions normales de stockage
mais se décompose en carbendazime dans l'eau.
L'analyse des résidus de même que celle des prélèvements
effectués dans l'environnement comporte une extraction au moyen d'un
solvant organique, une purification de l'extrait par partage
liquide-liquide et la transformation du résidu obtenu en
carbendazime. Le dosage de ces résidus peut s'effectuer par
chromatographie en phase liquide à haute performance ou par titrage
immunologique.
1.2 Sources d'exposition humaine et environnementale
En 1988, on estimait à environ 1700 tonnes la quantité de
bénomyl utilisée dans le monde. Il s'agit d'un fongicide très
largement utilisé, homologué dans 50 pays pour le traitement de plus
de 70 cultures. Le bénomyl est présenté sous forme de poudre
mouillable.
1.3 Transport, distribution et transformation dans l'environnement
Le bénomyl se transforme rapidement en carbendazime dans
l'environnement avec une demi-vie respective de 2 et 19 heures dans
l'eau et le sol. On peut donc utiliser aussi bien les résultats des
études sur le bénomyl que sur le carbendazime pour l'évaluation des
effets sur l'environnement.
Dans l'environnement, le carbendazime se décompose avec une
demi-vie de 6 à 12 mois sur le sol nu, de 3 à 6 mois sur le gazon et
de 2 à 25 mois dans l'eau en aérobiose et en anaérobiose,
respectivement.
Le carbendazime est principalement décomposé par les
microorganismes. Le 2-aminobenzimidazole (2-AB) en est l'un des
principaux produits de dégradation et il est à son tour décomposé
par les microorganismes.
Lors de la décomposition de bénomyl marqué au 14C sur le
noyau phényle, on a constaté que 9% seulement du carbone-14 étaient
éliminés sous forme de CO2 en une année d'incubation. Le
carbone-14 restant était principalement récupéré sous forme de
carbendazime et de résidus liés. L'étude de la destinée d'un
éventuel produit de dégradation (1,2-diaminobenzène) pourrait
peut-être permettre de mieux définir la voie de dégradation des
fongicides benzymidazoliques dans l'environnement.
Des études effectuées sur le terrain ou sur colonnes ont montré
que le carbendazime restait dans les couches superficielles du sol.
On n'a pas mesuré l'adsorption du carbendazime dans le sol, mais on
pense qu'elle doit être aussi forte que dans le cas du bénomyl, avec
des valeurs de Koc allant de 1000 à 3600. Les valeurs de log Kow
sont respectivement de 1,36 et de 1,49 pour le bénomyl et le
carbendazime.
Un modèle de criblage basé sur les données d'adsorption et de
persistance n'a pas révélé de risque de lessivage. Cette observation
est corroborée par des analyses d'eau de puits effectuées aux
Etats-Unis, analyses qui n'ont pas permis de déceler ce composé dans
l'un quelconque des 495 puits étudiés ni le carbendazime dans 212
autres (la limite de détection n'a pas été précisée). On estime que
le bénomyl et le carbendazime entraînés par ruissellement
correspondent uniquement à la fraction adsorbée aux particules de
sol; d'ailleurs ces composés sont sans doute fortement adsorbés aux
sédiments présents dans l'environnement aquatique.
En solution, dans les végétaux et le sol, le bénomyl subit une
décomposition en carbendazime (méthyl-1H-benzimidazole-2-carbamate)
en 2-AB, STB (3-butyl-1,3,5-triazino[1,2a]-benzimidazole-
2,4(1H,3H)dione) ainsi qu'en BBU (1-(2-benzimidazolyl)-3-n-
butylurée). La photolyse du bénomyl est pratiquement inexistante.
Chez l'animal, le bénomyl est métabolisé en carbendazime ainsi
qu'en d'autres métabolites polaires qui sont rapidement excrétés. On
n'a pas observé d'accumulation de bénomyl ni de carbendazime dans
aucun système biologique.
1.4 Concentrations dans l'environnement et exposition humaine
Il ne semble pas qu'il existe de données résultant d'une
surveillance du bénomyl dans l'environnement. Toutefois on peut
récapituler ainsi les données tirées d'études portant sur la
destinée de ce produit dans l'environnement.
Comme le bénomyl et le carbendazime restent stables pendant des
semaines sur les végétaux, ils peuvent être ingérés par des
organismes qui se nourrissent de feuilles mortes. Des résidus de
carbendazime peuvent subsister jusqu'à 3 ans dans le sol et les
sédiments. Toutefois, la forte adsorption du carbendazime aux
particules de sol et de sédiments réduit l'exposition des organismes
terrestres et aquatiques.
Ce sont les résidus de bénomyl et de carbendazime présents sur
les cultures vivrières qui constituent la principale source
d'exposition de la population humaine dans son ensemble. L'analyse
de l'exposition par voie alimentaire qui a été effectuée aux
Etats-Unis (bénomyl et carbendazime associés) et aux Pays-Bas
(carbendazime seul) a montré que la quantité moyenne ingérée était
vraisemblablement de l'ordre d'un dixième de la dose journalière
acceptable (DJA) qui est, pour le bénomyl de 0,02 mg/kg de poids
corporel, et pour le carbendazime de 0,01 mg/kg de poids corporel.
L'exposition professionnelle au cours de la production est
inférieure à la valeur-seuil. Les ouvriers agricoles qui préparent
les mélanges, effectuent le remplissage, ou retournent dans les
champs traités par du bénomyl, courent un risque d'exposition
cutanée de quelques mg de bénomyl à l'heure. Le port de dispositifs
de protection permettrait de réduire encore cette exposition. En
outre, étant donné que l'absorption percutanée est vraisemblablement
faible, il est très peu probable que le bénomyl puisse avoir des
effets toxiques généraux sur les populations humaines en étant
absorbé par cette voie.
1.5 Cinétique et métabolisme
Après exposition par voie orale ou respiratoire, le bénomyl est
rapidement absorbé par l'organisme animal mais cette absorption est
bien moindre après exposition par voie cutanée. Une fois absorbé, le
bénomyl est rapidement métabolisé, puis excrété dans les urines et
les matières fécales. Après avoir administré du bénomyl marqué au
carbone-14 à des rats, on a retrouvé dans le sang, et en petites
quantités dans les testicules, les reins et le foie, deux de ses
métabolites, le carbendazime et le carbamate de méthyl-5-hydroxy-1H-
benzimidazole-2-yle (5-HBC). La distribution tissulaire des composés
n'était pas révélatrice d'une bioconcentration. Dans l'urine, le
principal métabolite était le 5-HBC à côté d'un peu de carbendazime.
Dans les 72 heures suivant l'administration, 98% de la quantité
administrée avaient été excrétés. Chez des vaches recevant pendant 5
jours des capsules contenant du bénomyl radiomarqué à raison de 50
mg/kg de nourriture, les concentrations équivalentes de bénomyl
retrouvées dans les divers organes étaient de 4 mg/kg dans le foie,
et de 0,25 mg/kg dans les reins; dans les autres tissus et les
graisses, les valeurs n'étaient pas significatives. Pendant la
période d'administration, 65% du composé radiomarqué ont été
excrétés dans les urines, 21% dans les matières fécales et 0,4% dans
le lait. Le principal métabolite présent dans le lait était le
5-HBC. Chez d'autres animaux, on a constaté un métabolisme et des
modalités d'élimination similaires.
Le bénomyl n'inhibe pas l'acétylcholinestérase in vitro. On a
montré qu'il induisait l'époxyhydrolase hépatique, la gamma-
glutamyle transpeptidase ainsi que la glutation-S-transférase chez
la souris et le rat in vivo.
1.6 Effets sur les mammifères de laboratoire et sur les systèmes
d'épreuve in vitro
1.6.1 Exposition unique
Le bénomyl présente une faible toxicité aiguë, la DL50 par
voie orale chez le rat étant > 10 000 mg/kg, et la CL50 à 4 h par
voie respiratoire étant > 4 mg/litre. Des chiens exposés par voie
respiratoire pendant 4 heures à la dose de 1,65 mg/litre et examinés
28 jours après l'exposition, présentaient une diminution du poids du
foie. Une dose unique administrée à des rats par gavage a entraîné
des effets sur la reproduction 70 jours après l'exposition (voir
Section 1.6.5).
1.6.2 Exposition de brève durée
Administré par gavage pendant de brèves périodes allant jusqu'à
90 jours, en mélange à la nourriture ou en application cutanée, le
bénomyl a légèrement augmenté le poids du foie chez le rat (à la
dose quotidienne de 125 mg/kg, en mélange à la nourriture) et a
produit des effets sur les organes reproducteurs mâles chez le rat:
diminution du poids des testicules et de l'épididyme, réduction de
la spermatogenèse (dose quotidienne: 45 mg/kg, par gavage, dose sans
effet observable = 15 mg/kg), chez le lapin (dose quotidienne: 1000
mg/kg, par voie orale; 500 mg/kg, en appli cation cutanée) et chez
le chien beagle (62,5 mg/kg, dose sans effet observable = 18,4 mg/kg
par jour, en mélange à la nourriture). Chez les rats exposés par la
voie respiratoire à du bénomyl à des concentrations allant jusqu'à
200 mg/m3 pendant 90 jours, on n'a pas observé d'effets sur le
foie ni les testicules.
1.6.3 Irritation et sensibilisation au niveau de la peau et
des yeux
L'application de bénomyl sur l'épiderme de lapins ou de cobayes
n'a produit que peu ou pas d'irritation et une sensibilisation
modérée de la peau. Chez le rat, l'instillation oculaire a produit
une irritation légère et temporaire de la conjonctive.
1.6.4 Exposition de longue durée
A l'issue d'une étude prolongée d'alimentation chez des rats,
on n'a pas pu mettre en évidence d'effets imputables à ce composé à
des doses allant jusqu'à 2500 mg/kg de nourriture (soit 125 mg/kg de
poids corporel par jour). Cette étude n'a pas été jugée suffi sante
pour permettre une évaluation des effets sur la reproduction. Chez
la souris CD-1, le poids du foie était en augmentation à partir de
la dose de 1500 mg/kg de nourriture. A la dose de 5000 mg/kg de
nourriture, on notait chez les souris mâles une réduction du poids
absolu des testicules et une atrophie du thymus.
1.6.5 Reproduction, embryotoxicité et tératogénicité
Le bénomyl détermine une diminution du poids des testicules et
de l'épidydime, avec réduction des réserves de spermatozoïdes au
niveau de la queue de l'épidydime, une diminution de la production
des spermatozoïdes et une réduction de la fécondité des mâles. A
plus fortes doses, il y a diminution de la spermatogénèse qui est
perturbée à tous ses stades. Le bénomyl n'affecte pas le
comportement copulatoire, les vésicules séminales, la mobilité des
spermatozoïdes ni les hormones sexuelles correspondantes. La
concentration de bénomyl la plus faible qui produise un effet
statistiquement significatif sur la spermatogénèse du rat mâle a été
chiffrée à 45 mg/kg par jour. La dose sans effet observable pour ces
effets est de 15 mg/kg par jour.
Une seule dose de bénomyl (100 mg/kg ou davantage) administrée
par gavage à des rats a produit, 70 jours après l'exposition, des
effets consistant notamment en une réduction du poids des testicules
et une atrophie des tubes séminifères.
Administré par gavage à des rats ChD-CD et Wistar du 7e au
16e jour de la gestation, le bénomyl s'est révélé tératogène pour
les 2 souches à 62,5 mg/kg, mais pas à 30 mg/kg pour les ChR-CD, ni
à 31,2 mg/kg pour les Wistar. Administré par gavage à des rats
Sprague-Dawley du 7e au 21e jour de gestation, le bénomyl s'est
révélé tératogène à 31,2 mg/kg. Les effets observés étaient une
microphthalmie, une hydrocéphalie et une encéphalocèle. Le
développement post-natal des rats était également perturbé aux doses
supérieures à 15,6 mg/kg.
Chez la souris, l'administration par gavage à des
concentrations supérieures ou égales à 50 mg/kg a provoqué
l'apparition de côtes surnuméraires et autres anomalies du squelette
et des viscères. On n'a pas établi, chez la souris, la valeur de la
dose sans effet observable car les doses administrées étaient toutes
supérieures à 50 mg/kg. A part une augmentation marginale de la
fréquence des côtes surnuméraires chez le lapin, on n'a pas observé,
chez cet animal, d'effets tératogènes à des doses atteignant 500
mg/kg de nourriture.
1.6.6 Mutagénicité et autres points d'aboutissement des effets
toxiques
L'étude des cellules somatiques et germinales montre que le
bénomyl n'entraîne pas de mutations géniques ni d'anomalies
structurales chromosomiques et qu'il n'y a pas non plus
d'interaction directe avec l'ADN (qui provoquerait des lésions de
l'ADN et leur réparation). Ces faits ont été mis en évidence à la
fois sur des cellules mammaliennes et non mammaliennes.
Cependant le bénomyl provoque des aberrations dans le nombre
des chromosomes (aneuploïdie et/ou polyploïdie) dans les systèmes
d'épreuve (tant in vitro qu' in vivo).
1.6.7 Cancérogénicité
Chez les souris CD-1 et Swiss axéniques, qui présentent un taux
important de tumeurs spontanées du foie, on a observé ce type de
tumeurs après administration de bénomyl ou de carbendazime. En
revanche, le carbendazime ne s'est pas révélé cancérogène chez les
souris NMRKf, qui n'ont qu'un faible taux de tumeurs hépatiques
spontanées.
La première étude de cancérogénicité portant sur des souris
CD-1 a fait ressortir l'existence d'une augmentation liée à la dose
et statistiquement significative des néoplasmes hépatocellulaires
chez les femelles et l'on a également observé chez les mâles, aux
doses moyennes (1500 mg/kg), une réaction statistiquement
significative qui ne s'observait plus aux doses élevées en raison
d'un fort taux de mortalité. Une deuxième étude de cancérogénicité
portant sur le carbendazime a été effectuée chez une souche
génétiquement apparentée de souris Swiss axéniques et exogames à des
doses de 0, 150, 300 et 1000 mg/kg (la dernière dose étant portée à
5000 mg/kg au cours de l'étude); elle a révélé un accroissement dans
l'incidence de l'ensemble des adénomes et carcinomes
hépatocellulaires. Une troisième étude effectuée cette fois sur des
souris NMRKf à des doses 0, 50, 150, 300 et 1000 mg/kg (portées
ensuite à 5000 mg/kg) n'a pas fait ressortir d'effets cancérogènes.
Les études de cancérogénicité portant sur le bénomyl et le
carbendazime ont donné des résultats négatifs chez le rat.
1.6.8 Mécanisme de la toxicité - mode d'action
On pense que les effets biologiques du bénomyl et du
carbendazime résultent de leur interaction avec les microtubules
cellulaires. Ces structures interviennent dans des fonctions aussi
importantes que la division cellulaire, qui est inhibée par ces deux
substances. La toxicité du bénomyl et du carbendazime pour les
mammifères est donc liée à une perturbation des fonctions du système
microtubulaire.
Comme les autres dérivés du benzimidazole, le bénomyl et le
carbendazime sont plus ou moins toxiques selon les espèces. Cette
sélectivité toxicologique s'explique au moins en partie par le fait
que le bénomyl et le carbendazime ne se lient pas de la même manière
aux tubulines des espèces visées et des espèces non visées.
1.7 Effets sur l'homme
Le bénomyl provoque des dermatites de contact ainsi qu'une
sensibilisation cutanée. On n'a pas fait état d'autres effets.
1.8 Effets sur les autres êtres vivant au laboratoire et dans
leur milieu naturel
Le bénomyl n'a guère d'effet sur l'activité microbienne du sol
aux doses d'emploi recommandées. On a cependant signalé l'existence
d'effets nocifs vis-à-vis de certains groupes de champignons.
On a calculé que la CE50 à 72 heures, fondée la croissance
totale, pour les algues bleu-vert du genre Selenastrum
capricornutum, était égale à 2,0 mg/litre; la concentration sans
effet observable était de 0,5 mg/litre. La toxicité du bénomyl pour
les invertébrés aquatiques et les poissons varie dans de larges
proportions, les valeurs de la CL50 à 96 heures allant de 0,006
mg/litre pour des poissons-chats du genre Ictalurus (alevins
porteurs de leur sac vitellin) à plus de 100 mg/litre pour les
écrevisses.
Le bénomyl s'est révélé toxique pour les lombrics lors
d'expériences de laboratoire qui reproduisaient les conditions
réelles d'exposition résultant de l'utilisation recommandée sur le
terrain. Il est peu toxique pour les oiseaux et son produit de
dégradation, le carbendazime, est "relativement non toxique" pour
les abeilles.
2. Conclusions
Le bénomyl provoque une sensibilisation cutanée chez l'homme.
Le bénomyl et le carbendazime ne font courir à l'homme qu'un très
faible risque d'intoxication aiguë. Etant donné les conditions
actuelles d'exposition et le faible taux d'absorption percutanée de
ces deux composés, il est improbable qu'ils entraînent des effets
toxiques généraux dans la population ou chez les personnes exposées
de par leur profession. Ces conclusions sont tirées de données
relatives à l'animal et, dans une moindre mesure, à l'homme; elles
reposent sur la connaissance du mode d'action du carbendazime et du
bénomyl, tant chez les espèces visées que chez les espèces non
visées.
Grâce à une meilleure connaissance du mécanisme de la toxicité
du bénomyl et du carbendazime pour les mammifères, on pourra
peut-être mieux définir les doses sans effet observable. Des études
portant sur la liaison de ces composés aux tubulines des cellules
cibles (tissus testiculaires et embryonnaires) faciliteront sans
doute les comparaisons interspécifiques.
Le carbendazime est fortement adsorbé aux matières organiques
du sol et il y persiste pendant des périodes pouvant atteindre 3
ans. Il persiste également à la surface des feuilles et se retrouve
par conséquent dans les feuilles mortes. On a montré que les
lombrics pouvaient souffrir (dans leur population et dans leur
reproduction) de ces composés aux doses d'emploi recommandées. On ne
possède aucun renseignement sur les autres arthropodes qui vivent
dans le sol ou les débris organiques et qui pourraient être exposés
de la même manière.
Il est improbable que la forte toxicité vis-à-vis des
organismes aquatiques révélée par les épreuves de laboratoire
s'observe également dans le milieu naturel, du fait de la faible
biodisponi-bilité des résidus de carbendazime liés aux sédiments.
Toutefois on ne possède aucune donnée sur les espèces vivant sur les
sédiments et qui seraient donc les plus exposées.
RESUMEN Y CONCLUSIONES
1. Resumen
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
El benomilo es un sólido cristalino de color tostado y acción
fungicida sistémica que pertenece a la familia del bencimidazol. Se
descompone a una temperatura apenas superior a 140 °C,
correspondiente a su punto de fusión, y su presión de vapor a 25 °C
es < 5 x 10-6 Pa (< 3,7 x 10-8 mmHg). Prácticamente es
insoluble en agua a pH 5 y 25 °C, siendo su solubilidad 3,6
mg/litro. Es un compuesto estable en condiciones de almacenamiento
normales, pero en agua se descompone y forma carbendazima.
Los análisis de las muestras procedentes de residuos y del
medio ambiente se realizan mediante extracción con un disolvente
orgánico, purificación del extracto obtenido utilizando un
procedimiento de reparto líquido-líquido y conversión del residuo en
carbendazima. La valoración de los residuos se puede realizar
mediante cromatografía líquida de alto rendimiento o inmunoensayo.
1.2 Fuentes de exposición humana y ambiental
En 1988, el uso mundial estimado de benomilo fue unas 1700
toneladas. Es un fungicida muy utilizado, que se encuentra
registrado en 50 países en los que se permite su uso en más de 70
cultivos. El benomilo está formulado como polvo humectable.
1.3 Transporte, distribución y transformación en el medio ambiente
En el medio ambiente, el benomilo se transforma rápidamente en
carbendazima con una semivida de 2 y 19 h en el agua y el suelo
respectivamente. Por consiguiente, para la evaluación de los efectos
sobre el medio ambiente son importantes los datos obtenidos de los
estudios realizados con ambos compuestos.
La carbendazima se descompone en el medio ambiente con una
semivida de 6 a 12 meses en el suelo desnudo, de 3 a 6 meses en el
césped y de 2 y 25 meses en el agua en condiciones aerobias y
anaerobias respectivamente.
La carbendazima se descompone principalmente por acción de los
microorganismos. Un producto importante de su degradación es el
2-aminobencimidazol (2-AB), que luego se descompone de nuevo por la
actividad microbiana.
En la descomposición del benomilo marcado con un grupo fenilo
con 14C, sólo el 9% del 14C formó CO2 durante un año de
incubación. El resto del 14C se recuperó sobre todo como
carbendazima y en productos unidos a residuos. El destino de un
posible producto de degradación (1,2-diaminobenceno) puede aclarar
ulteriormente la vía de degradación de los fungicidas
bencimidazólicos en el medio ambiente.
En estudios de campo y de columna se ha puesto de manifiesto
que la carbendazima queda retenida en la capa superficial del suelo.
Aunque no se dispone de datos sobre su adsorción en el suelo, se
considera que ésta puede ser tan intensa como la del benomilo, con
valores de Koc que oscilan entre 1000 y 3600. Los valores del log
Kow para el benomilo y la carbendazima son respectivamente 1,36 y
1,49.
En la evaluación realizada en un modelo de selección, basado en
datos de adsorción y persistencia, se puso de manifiesto que no
había riesgo de lixiviación. En los Estados Unidos se han efectuado
análisis de agua de pozos que confirman esto, puesto que no se
encontraron trazas de benomilo en ninguno de los 495 pozos
muestreados ni se detectó carbendazima en ninguno de los 212 (no se
dispone del límite de detección). Es de suponer que la escorrentía
superficial de ambos compuestos se deba solamente al fungicida
adsorbido en las partículas del suelo y que en el medio acuoso estén
fuertemente adsorbidos en los sedimentos.
En solución, en las plantas y en el suelo, el benomilo se
degrada a carbendazima (1H-bencimidazol-2-carbamato de metilo) y a
2-AB, STB (3-butil-1,3,5-triazino[1,2a]-bencimidazol-2,4(1H,3H)
diona) y BBU (1-(2-bencimidazolil)-3-n-butilurea). La fotolisis del
benomilo es escasa o nula.
En los animales, el benomilo se descompone formando
carbendazima y otros metabolitos polares, que se excretan
rápidamente. No se ha observado que el benomilo o la carbendazima se
acumulen en ningún sistema biológico.
1.4 Niveles medioambientales y exposición humana
No parece que se disponga de datos de vigilancia ambiental para
el benomilo. Sin embargo, los estudios realizados sobre su destino
en el medio ambiente pueden resumirse como sigue.
Puesto que el benomilo y la carbendazima se mantienen estables
en las plantas durante varias semanas, pueden pasar a los organismos
que se alimentan de las hojas caídas. El suelo y los sedimentos
pueden conservar residuos de carbendazima hasta tres años. Sin
embargo, la fuerte adsorción de este compuesto en las partículas del
suelo y en los sedimentos reduce la exposición de los organismos
terrestres y acuáticos.
La principal fuente de exposición para la población humana
general se debe a los residuos de benomilo y carbendazima en los
cultivos alimentarios. En análisis de la exposición a través de los
sedimentos realizados en los Estados Unidos (benomilo y carbendazima
combinados) y en los Países Bajos (con carbendazima) se obtuvo una
ingesta media prevista de alrededor del 10 por ciento de la ingesta
diaria admisible (IDA) recomendada, de 0,02 mg/kg de peso corporal
para el benomilo y de 0,01 mg/kg de peso corporal para la
carbendazima.
La exposición profesional durante el proceso de fabricación es
inferior al valor umbral límite. Se considera que los trabajadores
agrícolas que se ocupan de mezclar y cargar los plaguicidas o que
entran en campos tratados con benomilo sufren exposición cutánea a
unos mg de benomilo por hora. Este forma de exposición se podría
reducir con algún tipo de protección. Por otra parte, puesto que la
absorción cutánea prevista es baja, la probabilidad de que el
benomilo tenga efectos tóxicos sistémicos sobre la población humana
a través de esta vía es muy escasa.
1.5 Cinética y metabolismo
En experimentos con animales se ha puesto de manifiesto que
éstos absorben fácilmente el benomilo tras la exposición oral y
respiratoria, pero mucho menos después de una exposición cutánea. El
benomilo absorbido se metaboliza rápidamente y se excreta en la
orina y las heces. En ratas alimentadas con benomilo marcado con
14C, se encontraron en la sangre, y en pequeña cantidad en los
testículos, los riñones y el hígado, los metabolitos carbendazima y
(5-hidroxi-1H-bencimidazol-2-il)-carbamato de metilo (5-HBC). La
distribución en los tejidos demostraba la ausencia de
bioconcentración. El metabolito principal en la orina era el 5-HBC,
acompañado de una pequeña cantidad de carbendazima. A las 72 h de la
administración ya se había excretado el 98% de la cantidad
suministrada. En vacas tratadas durante 5 días con cápsulas con
benomilo marcado, en dosis equivalentes a una alimentación de 50
mg/kg, se detectó en el hígado una concentración de esta sustancia
equivalente a 4 mg/kg, en los riñones 0,25 mg/kg y en el tejido
adiposo o en otros niveles no significativos. Cuando se administró
con los alimentos, el 65% del compuesto marcado se excretó en la
orina, el 21% en las heces y el 0,4% en la leche. El principal
metabolito en la leche fue el 5-HBC. El metabolismo y los sistemas
de eliminación fueron semejante en otros animales.
El benomilo no inhibe la acetilcolinesterasa in vitro. Se ha
demostrado en estudios in vivo en ratas y ratones que induce la
epoxihidrolasa hepática, la gamma-glutamil transpeptidasa y la
glutatión-S-transferasa.
1.6 Efectos en los mamíferos de laboratorio y en sistemas
de prueba in vitro
1.6.1 Exposición única
El benomilo tiene una toxicidad aguda baja, con una DL50 por
vía oral en ratas de > 10 000 mg/kg y una CL50 por inhalación
durante 4 h de > 4 mg/litro. La carbendazima, al igual que la
sustancia de la que se deriva, tiene una DL50 en ratas de > 10
000 mg/kg. Los perros, expuestos por inhalación a 1,65 mg/litro
durante 4 h y examinados 28 días después, mostraron una dismi nución
del peso del hígado. La administración por sonda de una sola dosis
de benomilo a ratas mostró efectos en la reproducción a los 70 días
de la exposición (véase el apartado 1.6.5).
1.6.2 Exposición breve
La administración de benomilo mediante sonda, en los alimentos
o por exposición cutánea durante un período máximo de 90 días en las
ratas aumentó ligeramente el peso del hígado (125 mg/kg al día, con
los alimentos) y tuvo efectos sobre los órganos reproductores
masculinos (disminución del peso de los testículos y los epidídimos,
y reducción de la espermatogénesis) en las ratas (45 mg/kg al día,
administrado por sonda; nivel sin efecto observado (NOEL) =
15mg/kg), los conejos (1000 mg/kg al día, por vía oral; 500 mg/kg de
peso corporal al día, por vía cutánea) y los perros (62,5 mg/kg;
NOEL = 18,4 mg/kg al día, con los alimentos). No se observaron
efectos hepáticos ni testiculares en la ratas expuestas por
inhalación a concentraciones de benomilo de hasta 200 mg/m3
durante 90 días.
1.6.3 Irritación y sensibilización cutánea y ocular
La aplicación cutánea a conejos y cobayos ocasionó una
irritación leve o nula y una sensibilización moderada de la piel. Su
aplicación ocular en ratas produjo de manera transitoria una
irritación conjuntival ligera.
1.6.4 Exposición prolongada
En un estudio de alimentación de larga duración en ratas, con
dosis de hasta 2500 mg/kg de alimentos (125 mg/kg de peso corporal
al día) no se puso de manifiesto ningún efecto relacionado con el
compuesto. Este estudio no se consideró adecuado para evaluar los
efectos sobre la reproducción. En el ratón CD-1 se observó que, con
dosis de 1500 mg/kg de alimento o superiores, se producía un aumento
de peso del hígado. En los ratones macho, las dosis de hasta 5000
mg/kg de alimento provocaron una disminución en términos absolutos
del peso de los testículos y la atrofia del timo.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
El benomilo produce una disminución del peso de los testículos
y el epidídimo, de la producción de esperma y de la tasa de
fecundidad de los machos. A dosis más elevadas, provoca
hipoespermatogénesis, con interrupción general de todas las fases de
la espermatogénesis. No afecta, en cambio, al comportamiento
copulatorio, las vesículas seminales, la movilidad del esperma o las
hormonas de la reproducción asociadas. La concentración más baja del
benomilo capaz de inducir un efecto espermatogénico estadísticamente
significativo en ratas macho fue de 45 mg/kg por día. El NOEL para
estos efectos fue de 15 mg/kg por día.
La administración por sonda de una sola dosis de benomilo (100
mg/kg o más) mostró efectos en ratas a los 70 días de la exposición,
que incluyeron descenso del peso de los testículos y atrofia de los
túbulos seminíferos. Administrado por sonda a ratas ChD-CD y Wistar
durante los días 7 a 16 de la gestación, el benomilo resultó
teratogénico a 62,5 mg/kg en ambas estirpes, pero no a 30 mg/kg en
ratas ChD-CD y no a 31,2 mg en ratas Wistar. Administrado por sonda
a ratas Sprague-Dawley en los días 7 a 21 de la gestación, el
benomilo resultó teratogénico en dosis de 31,2 mg/kg. Los efectos
que produjo fueron microftal mia, hidrocefalia y encafaloceles. Las
dosis superiores a 15,6 mg/kg tuvieron un efecto negativo sobre el
desarrollo posnatal.
La administración por sonda de 50 mg/kg o concentraciones
superiores indujo en ratones la aparición de costillas
supernumerarias y de otras anomalías esqueléticas y viscerales. No
se ha determinado el NOEL en los ratones porque no se ensayaron
dosis inferiores a 50 mg/kg. A excepción de un aumento marginal de
las costillas supernumerarias en conejos, no se observaron efectos
teratogénicos incluso a dosis de hasta 500 mg/kg de alimento.
1.6.6 Mutagenicidad y otros efectos finales afines
En unos estudios realizados en células somáticas y germinales
se ha observado que no provoca mutaciones genéticas ni daños en la
estructura de los cromosomas (aberraciones) y tampoco tiene un
efecto directo sobre el ADN (causante de daños y la reparación del
ADN). Esto se ha demostrado tanto en mamíferos como en otros
animales.
El benomilo, sin embargo, produce aberraciones cromosómicas
numéricas (aneuploidía o poliploidía) en sistemas experimentales in
vitro e in vivo.
1.6.7 Carcinogenicidad
En el primer estudio de carcinogenicidad con ratones CD-1 se
puso de manifiesto un aumento estadísticamente significativo de
neoplasia hepatocelular relacionado con la dosis en las hembras y
también en los machos tratados con una dosis de nivel medio (1500
mg/kg) se observó una respuesta estadísticamente significativa, pero
no en los que recibieron dosis elevadas, a causa del elevado índice
de mortalidad. En un segundo estudio sobre la carcinogenicidad de la
carbendazima en una raza de ratones genéticamente relacionada con la
anterior, los ratones SPF (raza aleatoria suiza), con dosis de 0,
150, 300 y 1000 mg/kg (que se aumentó a 5000 mg/kg durante el
estudio) se puso de manifiesto un aumento en el número de casos de
adenomas y carcinomas hepatocelulares combinados. En un tercer
estudio realizado en ratones NMRKf con dosis de 0, 50, 150, 300 y
1000 mg/kg (que se aumentó a 5000 mg/kg durante el estudio) no se
produjeron efectos carcinogénicos. El benomilo y la carbendazima
causaron tumores hepáticos en dos estirpes de ratones (CD-1 y suizos
(SPF)) que presentaban una tasa alta de tumores hepáticos de
formación espontánea. Por el contrario, la carbendazima no resultó
carcinogénica en ratones NMRKf, que presentan una tasa baja de esos
tumores espontáneos.
Los estudios de carcinogenidad del benomilo y la carbendazima
en ratas fueron negativos.
1.6.8 Mecanismo de toxicidad, modo de acción
Se considera que los efectos biológicos de estos compuestos son
el resultado de su interacción con los microtúbulos celulares. Estas
estructuras participan en funciones esenciales, como la división
celular, que inhiben el benomilo y la carbendazima. La toxicidad de
estos productos en los mamíferos está vinculada a una disfunción
microtubular.
El benomilo y la carbendazima, al igual que otros compuestos
del bencimidazol, tienen una toxicidad selectiva para distintas
especies. Esta se explica, por lo menos en parte, porque el benomilo
y la carbendazima se unen de manera distinta a los microtúbulos de
las especies específicas en las que actúan y en las que no.
1.7 Efectos en el ser humano
El benomilo causa dermatitis por contacto y sensibilización
cutánea. No se ha informado de otros efectos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Con las dosis de aplicación recomendadas, el benomilo tiene
pocos efectos sobre la actividad microbiana del suelo. Se han
notificado algunos efectos adversos sobre ciertos grupos de hongos.
La CE50 a las 72 h, basada en el crecimiento total, para el
alga verde Selenastrum capricornutum fue de 2,0 mg/litro; la
concen tración sin efecto observado (NOEC) fue de 0,5 mg/litro. La
toxicidad del benomilo para los invertebrados acuáticos y los peces
varía ampliamente, con una CL50 a las 96 h que oscila entre 0,006
mg/litro para Ictalurus punctatus (alevines con saco vitelino) y
> 100 mg/litro para los cangrejos de río.
No observaron efectos tóxicos en experimentos de laboratorio en
las lombrices de tierra expuestas a concentraciones normales de
benomilo y como resultado del uso de la dosis de aplicación
recomendada en el campo. Tiene una toxicidad baja para las aves, y
la carbendazima, producto de su degradación, es "relativamente no
tóxica" para las abejas de miel.
2. Conclusiones
El benomilo causa sensibilización cutánea en el ser humano.
Tanto el benomilo como la carbendazima representan un riesgo muy
pequeño de intoxicación aguda. Dados los niveles de exposición
actuales y el bajo índice de absorción cutánea de estos dos
compuestos, no es probable que pudieran tener efectos de toxicidad
sistémica en la población general o en personas expuestas
profesionalmente. Estas son las conclusiones que se pueden sacar de
los datos obtenidos en animales y de los limitados datos sobre el
ser humano de que se dispone, pero estas extrapolaciones están
respaldadas por el conocimiento del modo de acción de la
carbendazima y el benomilo en especies en las que actúan y en las
que no.
Una mayor clarificación del mecanismo de toxicidad de ambos
compuestos en los mamíferos permitirá quizás definir mejor los
niveles sin efectos observados. El estudio de su unión a los
microtúbulos de las células destinatarias (tejidos testicular y
embrionario) facilitará la comparación entre distintas especies.
La carbendazima se adsorbe fuertemente en la materia orgánica
del suelo que la conserva durante un período de hasta 3 años.
Persiste en la superficie de las hojas y, por consiguiente, en las
hojas caídas. Se ha demostrado que las dosis recomendadas de
aplicación afectan negativamente a las lombrices de tierra (con
efectos sobre la población y la reproducción). No se dispone de
datos acerca de sus efectos sobre otros artrópodos del suelo o de la
maleza, que estarían igualmente expuestos.
No es probable que se pueda observar en el medio ambiente la
elevada toxicidad demostrada en las pruebas de laboratorio para los
organismos acuáticos debido a la baja biodisponibilidad de los
residuos de carbendazima unidos a los sedimentos. Sin embargo, no se
dispone de información acerca de sus efectos en las especies que
viven en los sedimentos, que sufrirían la exposición más intensa.
6.4 Elimination and excretion
Absorbed benomyl and carbendazim are rapidly excreted in the
urine and faeces.
In a study where rats were administered 1 or 10 µg formulated
14C-benomyl (50% wettable powder) in a single intravenous dose by
tail injection, more than 80% of the dose was excreted in the urine
and faeces within 6 h after injection and the total urine and faeces
recovery was > 95% in 24 h (Han, 1979).
[Phenyl(U)-14C]-carbendazim was administered by gavage to
Sprague-Dawley rats using three dosing regimens: a single oral dose
of 50 mg/kg (low dose); a single oral dose of 50 mg/kg following
pre-conditioning gavage with unlabelled carbendazim of 50 mg/kg for
14 days (pre-conditioned low dose); and a single oral dose of 1000
mg/kg (high dose). Each dosing group consisted of five animals of
each sex. A preliminary study conducted with two rats of each sex,
each rat having received a single oral dose of 50 mg/kg,
demonstrated that 95% of the radioactivity excreted in the urine and
faeces was recovered within 72 h after dosing and that < 1% of the
dose was expired as volatile metabolites. In the full study, > 98%
of the recovered radioactivity was excreted by the time of sacrifice
(i.e. 72 h after dosing) for each dosing group. Urinary excretion
accounted for 62% to 66% of the dose in males and 54% to 62% of the
dose in low-dose and pre-conditioned low-dose female groups. In the
high-dose group, this pathway accounted for 41% of the dose in all
animals. Elimination of radiolabel in faeces accounted for virtually
all of the remaining radiolabel. There were no apparent differences
between male and female rats with respect to the extent of
absorption and extent and rate of elimination of 14C-carbendazim
equivalents within a given treatment group (Monson, 1990).
In a study by Han (1978), two male ChR-CD-1 mice were fed a
diet of non-radiolabelled benomyl (2500 mg/kg) for 21 days and were
then gavaged with 2.5 mg [2-14C]-benomyl in corn oil. An identical
experiment was performed with one male ChR-CD hamster. More than 90%
of the radioactivity was eliminated in the urine and faeces within
72 h (Han, 1978).
A lactating Holstein cow was dosed by capsule twice daily (515
mg [2-14C]-benomyl each dose), equivalent to 50 mg/kg in the
average total daily feed, for 5 consecutive days, and samples of
urine, faeces and milk were collected at each dosing. Approximately
17 h after the tenth dose, the cow was sacrificed and organs,
tissues and blood were collected for analysis. At sacrifice, 65% of
the radiolabel had been excreted in the urine, 21% in the faeces and
0.4% in the milk. Carbon-14 residue levels in the milk averaged 0.2
mg/litre (calculated as benomyl equivalents) with 49% of the
radioactive metabolites being extractable in ethyl acetate, 36%
soluble in water, and 8% isolated as solids (Monson, 1985).
Lactating and non-lactating goats were given 5 consecutive
daily doses of [2-14C]-benomyl by capsule at rates equivalent to
36 and 88 mg/kg, respectively, in the total daily diet. Most of the
radioactivity (96%) had been eliminated in the urine and faeces by
the time of sacrifice (Han, 1980).
The excretion of benomyl was studied in laying hens dosed daily
for three consecutive days with 3.5 mg [2-14C]-benomyl or 3.29 mg
[phenyl(U)-14C]-benomyl. At sacrifice (22 h after the last dose),
an average of 107% and 95% of the dose had been excreted for the
[2-14C]-benomyl- and [phenyl(U)-14C]-benomyl-dosed birds,
respectively (Monson, 1986a).
In a similar study on 14C-carbendazim, groups of laying hens
were fed at a rate equivalent to 5 and 120 mg/kg diet. At sacrifice,
24 h after the sixth daily dose, an average of 95% of the dose had
been excreted by the low-dose birds and 92% by the high-dose birds
(Monson, 1986b).
6.5 Reaction with body components
An in vitro study using acetyl cholinesterase from bovine
erythrocytes showed that benomyl did not inhibit this enzyme. The
acetyl cholinesterase inhibition constant (KI) for benomyl was
greater than 1 x 10-3 mol/litre (Belasco, 1970). Another in vitro
study by Krupka (1974) verified that benomyl did not inhibit either
acetyl cholinesterase or butyryl cholinesterase.
In a study by Guengerich (1981), the effects of benomyl and
carbendazim on hepatic enzymes were studied in male and female
Crl-CD rats and CD-1 mice. The treatment groups included animals fed
for 28 days with diets that contained benomyl or carbendazim at
concentrations of 0, 10, 30, 100, 300, 1000 or 3000 mg/kg. In these
studies, microsomal epoxide hydrolase and cytosolic glutathione- S-
transferase were monitored in subcellular fractions isolated from
the livers of animals in each treatment group. Liver weights were
also recorded. Elevated mean absolute liver weights were observed at
1000 and 3000 mg carbendazim/kg in both male and female rats and at
300 mg carbendazim/kg in female rats. However, the only
significantly elevated liver weight was found in females after a
dose of 3000 mg benomyl/kg. No apparent liver toxicity or effect on
body weight was observed. Both benomyl and carbendazim induced
epoxide hydrolase in both sexes of rats and mice at 1000 and 3000
mg/kg. Induction of glutathione- S-transferase was observed at 3000
mg/kg in the case of both benomyl and carbendazim. In general, the
level of induction seemed to be slightly greater in females than
males. There did not appear to be any substantial difference in
enzyme induction between rats and mice.
In a study by Shukla et al. (1989), levels of gamma-glutamyl
transpeptidase (GGT) were evaluated after benomyl exposure. Female
albino rats (eight per group) and female Swiss albino mice (eight
per group) were given 1000 and 4000 mg benomyl/kg feed for 15 days,
and blood and liver GGT levels were analysed. Benomyl exposure
increased the activity of both blood and liver GGT in both rats and
mice, and the degree of induction was dose related (Shukla et al.,
1989).
7. EFFECTS ON LABORATORY MAMMALS; IN VITRO TEST SYSTEMS
7.1 Single exposure
The acute toxicity of benomyl in several animal species is
summarized in Table 8. Benomyl has an oral LD50 in the rat of >
10 000 mg/kg and an inhalation 4-h LC50 > 4 mg/litre. Several
other minor metabolites were evaluated and the approximate lethal
doses were 3400 mg/kg for 2-AB, 7500 mg/kg for 5-HBC, 17 000 mg/kg
for BUB and 17 000 mg/kg for STB.
In a study by Littlefield & Busey (1969), three groups of male
dogs (around 10 dogs/group) were exposed to benomyl at air
concentrations of 0, 0.65 and 1.65 mg/litre. One half of the dogs in
each group were killed on day 14 and the remainder on day 28. The
liver weight of the high-dose dogs was significantly decreased on
day 28. For further discussion of single dose toxic effects, see
section 7.5.1.
7.2 Short-term exposure
7.2.1 Gavage
In a 14-day rat study, benomyl (200 and 3400 mg/kg in peanut
oil) was given by gavage five times a week for two weeks to six male
ChR-CD rats per group. Four out of six rats died after 5, 7, 8 and 9
doses, respectively, of 3400 mg/kg. No clinical signs of toxicity
were observed in the group treated with 200 mg/kg per day.
Degeneration of germinal epithelium, multinucleated giant cells and
reduction or absence of sperm were observed in the testes after
multiple doses of 3400 mg/kg per day. Less than 10% of the
testicular tubules were affected in only two out of six animals
dosed with 200 mg/kg. At the high dose level, there was erosion and
thickening of the squamous mucosa of the stomach with submucosal
inflammation and a decrease in the large globular-shaped vacuoles
located centrolobularly in the liver (Sherman & Krauss, 1966).
7.2.2 Feeding
7.2.2.1 Rat
In a 90-day study by Sherman et al. (1967), groups of rats
(4-week-old ChR-CD rats, 16 rats of each sex per group) were fed
Benlate 70 WP (72% benomyl) in the diet at levels of 0, 100, 500 and
2500 mg benomyl/kg. The animals were observed daily for behavioural
changes and body weights, and food consumption was recorded at
weekly intervals. Haematological examinations were conducted on six
male and six female rats in each group at 30, 60 and 90 days.
Routine urine and plasma alkaline phosphatase and glutamic pyruvic
transaminase activity analyses were performed on the same animals.
After 96-103 days of continuous feeding, 10 male and 10 female rats
in each group were killed, and selected organs were weighed and
examined microscopically. The remaining six male and female animals
in each group after the terminal sacrifice were used in a
one-generation reproductive study. No effect was observed with
respect to reproduction or lactation in the delivery or rearing of
the F1A litters. There were no compound-related effects on weight
gain, food consumption, food efficiency, clinical signs, or on
haematology, biochemistry or urinalysis determinations. The
liver-to-body weight ratio in females was slightly increased at 2500
mg/kg, compared with control rats. Gross and microscopic
examinations of tissues and organs showed no significant effects
attributable to the presence of benomyl in the diet at levels up to
and including 2500 mg/kg.
7.2.2.2 Dog
Groups of beagle dogs (four males and four females per group;
7-9 months old) were administered benomyl 50% wettable powder in the
diet at dosage levels of 0, 100, 500 and 2500 mg/kg diet (based on
active ingredient) for three months (this corresponded to treatment
levels of 0, 3.8, 18.4 and 84 mg/kg body weight). Food consumption
and body weight data were recorded weekly, and clinical laboratory
examinations (including haematology, biochemistry and urinalysis)
were performed pre-test and after 1, 2 and 3 months of feeding. At
the conclusion of the study, selected organs were weighed and
subjected to gross and microscopic examinations. No mortality or
adverse clinical effects were observed over the course of the study,
and growth and food consumption were not effected by the treatment.
Urine parameters showed no differences from the control, and there
were no dose-related effects on the haematological values. Alkaline
phosphatase and glutamic pyruvic transaminase activities were
increased in high-dose males and females. There were statistically
significant decreases in the albumin/globulin ratio in either males
or females fed 2500 mg/kg diet. Organ-to-body weight ratio changes
were observed in the high-dose males and females for the thymus
(decreased) and thyroid (increased). One of the four females fed
2500 mg/kg diet had an enlarged spleen at the end of the exposure
period, as well as a decreased erythrocyte count, haemoglobin
concentration and haematocrit value. Histopathological examination
revealed myeloid hyperplasia of the spleen and bone marrow and
erythroid hyperplasia of bone marrow. This did not appear to be
compound related since group mean values were not significantly
different. Three out of four males fed 2500 mg/kg diet had reduced
relative prostate weights when compared with controls. Microscopic
examinations of tissues and organs did not indicate changes in dogs
fed benomyl for 90 days. The no-observed-effect level (NOEL) was 500
mg/kg diet (Sherman, 1968).
Table 8. Acute toxicity of benomyl and its metabolites for laboratory mammals
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl ratb M/F 10/dose oral peanut oil LD50 > 10 000 Sherman (1969a)
rabbitc M 1/dose oral 50% wettable powder ALD > 3400 Fritz (1969)
dogd M 1 oral evaporated milk and ALD > 1000 Sherman (1969b)
water (1:1)
Benlate OD (50% rat M 10/dose oral corn oil LD50 > 12 000 Hostetler (1977)
benomyl)
Fungicide 1991 rabbit M/F 4/dose dermal (4 h) 50% wettable powder LD50 > 10 000 Busey (1968a)
(50% benomyl)
rat M 6/dose inhalation (4 h) 50% wettable powder LC50 > 4.01 mg/litre Busey (1968b)
(analytical)
dog M 10/dose inhalation 50% wettable powder LC50 > 1.65 mg/litre Littlefield & Busey
(analytical) (1969)
Benlate fungicide rat M/F 10/dose oral aqueous suspension LD50 > 10 000 Sherman (1969a)
(52-53% benomyl)
rat M 5/dose inhalation 50% wettable powder LC50 > 0.82 mg/litre Hornberger (1969)
Benlate PNW (50% rabbit M/F 10/dose dermal 50% wettable powder LD50 > 2000 Gargus & Zoetis
benomyl) (1983c)
Benlate 50 DF (50% rat M/F 5/dose oral aqueous suspension LD50 > 5000 Sarver (1987)
benomyl)
rabbit M/F 5/dose dermal 50% dry flowable LD50 > 2000 Brock (1987)
Table 8 (contd).
Chemical Species Sex Number of Route Vehicle Concentrationa Reference
animals (mg/kg body weight)
Benomyl metabolites
2-Benzimidazole carbamic rat M/F 10/dose oral corn oil LD50 > 10 000 Goodman (1975)
acid, methyl ester
5-Hydroxy-2-benzimidazole- rat M 1/dose oral corn oil ALD > 7500 Snee (1969)
carbamic acid, methyl ester
2-Aminobenzimidazole rat M 1/dose oral peanut oil ALD > 3400 Fritz & Sherman
(1969)
Benzimidazole 2- rat M 1/dose oral corn oil ALD > 17 000 Dashiell (1972)
(3-butylureido)
S-Triazine, 3-butyl- rat M 1/dose oral corn oil ALD > 17 000 Barbo & Carroll
benzimidazole (1,2a), (1972)
-2,4(1H,3H)-dione
a Based on active ingredient; ALD = approximate lethal dose
b ChR-CD or Crl:CD rats
c New Zealand white rabbits
d Beagle dogs
7.2.3 Dermal
In a study on groups of five male and five female New Zealand
albino rabbits, weighing 2 to 2.4 kg, 15 dermal applications of a
50% benomyl formulation (equivalent to 1000 mg/kg) were made on both
abraded and intact abdominal skin sites. The animals were exposed
for 6 h/day, 5 days/week for 3 weeks. After each daily application,
the abdomen was washed with tap water. Observations were made daily
for mortality and toxic effects and weekly for body weight changes.
Gross necropsy and microscopic examinations were performed. Slight
erythema, oedema and atonia were observed at both abraded and intact
skin sites. Slight to moderate desquamation occurred throughout the
exposure period. No apparent compound-related body weight or organ
weight changes were reported. Microscopic examination of the males
demonstrated that benomyl produced degeneration of the spermatogenic
elements of the seminiferous tubules of the testes, the changes
including vacuolated and multi-nucleated spermatocytes (Busey,
1968d).
In a separate repeated-dose dermal study, groups of five male
and five female New Zealand albino rabbits, weighing 3 kg, were
exposed to doses of benomyl equivalent to 0, 50, 250, 500, 1000 and
5000 mg/kg applied to non-occluded abraded dorsal skin sites 6 h a
day, five days a week, for three weeks. Test material was removed by
washing the skin site and drying with a towel. There were decreased
body weight gains for both males and females at the two highest dose
levels. Mild to moderate skin irritation was reported for all groups
but was most notable at the highest dose level. Diarrhoea, oliguria
and haematuria were observed in males and females at 1000 and 5000
mg/kg. Decreased average testicular weights and testes-to-body
weight ratios were observed at 1000 mg/kg only. There were no
histopathological changes reported (Hood, 1969).
7.2.4 Inhalation
In an inhalation study, groups of 20 male and 20 female CD rats
were exposed, nose-only, 6 h a day for 90 days, to 0, 10, 50 and 200
mg benomyl/m3. At 45 and 90 days, blood and urine samples were
collected from 10 rats of each sex per group for clinical analysis
and then killed for pathological examination. After approximately 45
days of exposure, test-compound-related degeneration of the
olfactory epithelium was observed in all males and in eight of the
ten females exposed to 200 mg/m3. Two male rats exposed to 50
mg/m3 had similar but less severe olfactory degeneration. After
approximately 90 days of exposure, all of the animals showed
olfactory degeneration at 200 mg/m3, along with three males
exposed to 50 mg/m3. No other compound-related pathological
effects were observed. Male rats exposed to 200 mg/m3 had
depressed mean body weights compared to controls and this correlated
with a reduction in food consumption (Warheit et al., 1989).
7.3 Skin and eye irritation; sensitization
7.3.1 Dermal
A 50% wettable powder applied to the clipped intact and abraded
abdomen of albino rabbits produced moderate to marked erythema,
slight oedema and slight desquamation. Exposure was for 24 h to
occluded skin sites at doses > 0.5 g/animal. Albino guinea-pigs
similarly exposed to 10, 25 and 40% dilutions of technical grade
benomyl in dimethyl phthalate presented only mild irritation of both
intact and abraded skin sites (Majut, 1966; Busey, 1968a; Colburn,
1969; Frank, 1969).
When "Benlate" 50 DF (50% benomyl, 0.5 g Benlate 50 DF) was
evaluated for primary dermal irritation potential in six male New
Zealand white rabbits, no dermal irritation was observed at 4 or 24
h after application. By 48 h, slight to mild erythema was observed
in two rabbits and was still evident at 72 h. The primary irritation
scores ranged from 0-1 (not an irritant) (Vick & Brock, 1987).
In a study by Desi (1979), benomyl (98% purity) was applied to
a shaved area of the back of four albino rabbits at 5 mg/cm2.
Draize scores (Draize et al., 1944) were assessed 4 and 72 h after
application. Lesions produced by this method were classified as
"mild irritation".
7.3.2 Eye
The eye irritation properties of benomyl were examined in
albino rabbits in several tests using technical grade benomyl, 50%
wettable powder and a suspension in mineral oil. Mild conjunctival
irritation and minor transitory corneal opacity were reported after
48 to 96 h in all tests (Reinke, 1966; Frank, 1972). Similar results
were obtained with Benlate PNW (a 50% wettable powder) (Gargus &
Zoetis, 1983a,b).
Another eye irritation experiment was performed with 5 mg pure
benomyl using four albino rabbits (Desi, 1979). Results assessed
according to Draize (Draize et al., 1944) indicated that benomyl is
a mild eye irritant.
7.3.3 Sensitization
Albino guinea-pigs exposed to benomyl, either technical
material or a 50% sucrose formulation, produced mild to moderate
skin erythema during the challenge phase following both intradermal
injections or repeat applications to abraded skin (Majut, 1966;
Colburn, 1969; Frank, 1969).
In another sensitization study, Benlate PNW (50% benomyl
prepared as a 0.1% solution in saline) was injected weekly (four
injections) into ten albino guinea-pigs (Hartley strain). Ten
control animals were injected with saline. Fourteen days after the
final injection, 8% or 80% Benlate PNW saline solutions were applied
to the backs of the induced animals and saline was applied to the
backs of the control animals. No significant increase in score
occurred in any of the control animals at either challenge
concentration. Benlate PNW produced an unequivocal and significant
(two-step) increase at two of ten sites challenged with an 8%
suspension, and at seven of ten sites challenged with an 80%
suspension (Gargus & Zoetis, 1984).
Technical benomyl produced sensitization in all ten animals
tested in a guinea-pig maximization test (Matsushita et al., 1977).
7.4 Long-term exposure
7.4.1 Rat
Groups of weanling rats (36 male and 36 female Charles River
albino rats/group) were fed benomyl (50-70% a.i.) in the diet for
104 weeks at levels of 0, 100, 500 and 2500 mg/kg. Growth, as
observed by body weight changes and food consumption data, was
recorded weekly for the first year and twice a month thereafter.
Daily observations were made of clinical effects and mortality. At
periodic intervals during the study, haematological, urinalysis and
selected clinical chemistry examinations were performed. After one
year each group was reduced to 30 males and 30 females by interim
sacrifice for gross and microscopic evaluations. At the conclusion
of the study, all surviving animals were sacrificed and gross
examinations of tissues and organs were made. Initially, microscopic
examinations of tissues and organs from the control and 2500 mg/kg
groups were conducted, as were liver, kidney and testes examinations
of animals in the 100 and 500 mg/kg dose groups. In follow-up
pathological evaluations, all of the tissues and organs of the
control and low-, intermediate- and high-dose groups were examined
microscopically. There was no mortality attributable to benomyl in
the diet. Survival decreased to approximately 50% during the second
year, but was comparable among all groups. Body weight, food
consumption and food efficiency were unaffected by treatment. The
average daily dose for the 2500 mg/kg group was 330 mg/kg body
weight per day initially, 91-106 mg/kg body weight per day at one
year and 70-85 mg/kg body weight per day at two years. There were no
compound-related clinical manifestations of toxicity.
Haematological, urine and liver function tests were unaffected by
treatment. There were no differences in organ weight or organ-to-
body-weight ratios between control and treated groups (Sherman,
1969c; Lee, 1977).
7.4.2 Mouse
In a study by Weichman et al. (1982), male and female CD-1 mice
(80 males and 80 females per group) were administered benomyl (99%
a.i.) in the diet at levels of 0, 500, 1500 and 5000 mg/kg (the
highest levels was reduced from 7500 mg/kg after 37 weeks) for two
years. The mice were 6-7 weeks old at the start of the study. Median
survival time was unaffected by treatment. Male and female mice fed
1500 or 5000 mg/kg exhibited dose-related body weight decreases.
Food consumption was variable throughout the study, although
high-dose females appeared to consume less food. The average daily
intake of benomyl for males was 1079 mg/kg body weight per day
initially, 878 mg/kg body weight per day for 1 year and 679 mg/kg
body weight per day for 2 years; for females it was 1442 mg/kg body
weight per day initially, 1192 mg/kg body weight per day for 1 year,
and 959 mg/kg body weight per day for 2 years. There were no
apparent differences between treatment and control groups with
respect to palpable mass, number of mice affected or latency period
of discovery. Haematology examinations revealed no abnormalities
except for slightly decreased erythrocyte counts in the case of
males at 1500 mg/kg and females at 5000 mg/kg. Haemoglobin and
haematocrit values were also slightly depressed in 1500-mg/kg males.
Significant compound-related changes were seen in the absolute
and relative liver weights for males at 1500 and 5000 mg/kg for
females at 5000 mg/kg. Male mice also presented decreased absolute
testes weights at the highest dose level. Non-neoplastic organ
changes in males (5000 mg/kg) were confined to the liver
(degeneration, pigment, cytomegaly), thymus (atrophy), testes,
epididymis (degeneration of seminiferous tubules, atrophy,
aspermatogenesis, distended acini) and prostate. In female mice,
splenic haemosiderosis was significantly increased at 5000 mg/kg, as
was submucosal lymphocytic infiltration of the trachea at 1500 mg/kg
(Weichman et al., 1982).
7.5 Reproduction, embryotoxicity and teratogenicity
7.5.1 Reproduction
A number of reproduction studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in the Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.5.1.1 Rat feeding studies
A 2-generation reproduction study was conducted using Crl:CD
rats (Mebus, 1990). Throughout the study, animals were fed diets
containing 0, 100, 500, 3000 or 10 000 mg benomyl/kg. P1 parental
rats received the test diets for 71 days (mean daily intake 0,
approx. 6, approx. 30, approx. 190 or approx. 350 mg/kg per day,
respectively) before being bred to animals from the same dietary
concentration group for production of the F1 parental rats. F1
rats were mated after being maintained on their respective diets
(mean daily intake 0, approx. 8, approx. 20, approx. 250, or approx.
1000 mg/kg per day, respectively) for at least 105 days after
weaning for production of the F2A litter. F1 dams were mated
again, to different non-sibling males, at least 1 week after weaning
the F2A litter, to produce the F2B litters.
The following indices of reproductive function were calculated
for the P1 and F1 adults: mating, fertility, gestation,
viability, lactation, percent of pups born alive and percent litter
survival. In addition, mean body weights, body weight gains, food
consumption and food efficiency were measured, and clinical
observations were recorded. After litter production, all parental
generation rats were sacrificed for gross and histopathological
(gross lesions and target organs only) examination. Complete
histopathological examination was conducted on control and high-dose
animals. Twenty F2A and 20 F2B weanlings were also given a gross
pathological examination.
There were no compound-related effects on parental mortality at
any benomyl concentration. Mean body weights, body weight gains and
overall food consumption of P1 and F1 male and female rats were
significantly lower in the animals receiving 10 000 mg/kg than they
were in controls.
At 10 000 mg/kg, there was a significant compound-related
decrease in the number of F2A and F2B offspring alive prior to
culling (on day 4). In addition, male and female offspring of rats
fed 10 000 mg/kg weighed consistently less at birth than did the
offspring of control rats. With the exception of the 14-day male
pups in the F2B generation, F2A and F2B offspring in the 3000
mg/kg group also had compound-related significantly depressed body
weights on days 14 and 21 of lactation.
Testicular sperm counts in P1 and F1 rats were decreased in
the 3000 and 10 000 mg/kg groups. This was accompanied by decreased
testicular weight and histopathological changes in the testes at 10
000 mg/kg. Microscopic observations included atrophy and
degeneration of the seminiferous tubules in the testes of rats in
the 3000 and 10 000 mg/kg groups and oligospermia in the
epididymides of the high-dose P1 generation and the 3000 and 10
000 mg/kg F1 generation. However, there were no compound-related
differences in mating indices, fertility indices or gestation length
which could be attributed to benomyl feeding (Mebus, 1990).
In a feeding study by Barnes et al. (1983), adult male Wistar
rats (27 animals/group) were fed 0, 1, 6.3 or 203 mg/kg for 70 days
(13 animals/group) and allowed to recover for 70 days (14
animals/group). Ejaculated sperm counts were reportedly depressed in
the 203-mg/kg group during the feeding phase. There was also a
decrease in relative testicular weights and a slightly lowered male
fertility index in all treated groups. Benomyl did not alter
copulatory behaviour and did not induce dominant lethal mutation.
Plasma testosterone and gonadotropin levels remained unchanged
throughout the study. Identical studies conducted during the
recovery phase showed all of the treatment-related effects to be
completely reversible.
7.5.1.2 Rat gavage studies
The effects of exposure to benomyl on male reproductive
development was evaluated in prepubertal Sprague-Dawley male rats
(33 days old), which were gavaged daily for 10 days at doses of 0 or
200 mg/kg per day. Eight animals per group were killed at 3, 17, 31,
45 and 59 days after the last treatment. Selected tissues, including
liver, kidneys, testes, seminal vesicles and epididymides, were
removed, weighed and examined histo logically. Samples of seminal
fluid from the vas deferens were also examined. Observation
intervals were pre-selected to coincide with stages of
spermatogenesis. Data were presented on tissue weights, total
epididymal sperm counts, vas deferens sperm concentrations and
testicular histology. There were no effects related to treatment
(Carter, 1982).
In a similar study, adult male Sprague-Dawley rats (65 days
old) received 10 daily treatments of 0, 200 or 400 mg benomyl/kg per
day by gavage, and, 14 days after the last treatment, body weight,
tissue weights, total epididymal sperm counts and sperm
concentration in the vas deferens were measured and histological
investigations of the testes were carried out. Production of
testosterone by the Leydig cells was stimulated by subcutaneous
injections of human chorionic gonadotrophin (HCG) 2 h prior to
sacrifice. There were no compound-related effects on body weight or
on liver, kidney, adrenal, testis or seminal vesicle weights.
Caudate epididymal weights were, however, depressed by treatment
with benomyl. There were also treatment-related reductions in
epididymal sperm count (caput and caudate) as well as in the vas
deferens sperm concentration. The study was designed to evaluate
alterations in spermatozoa undergoing spermatogenesis in the
seminiferous tubules of the testes during exposure to benomyl.
Animals exposed to 400 mg/kg per day presented histological evidence
of hypospermatocytogenesis with generalized disruption of all stages
of spermatogenesis, when compared with controls (Carter & Laskey,
1982).
Linder et al. (1988) evaluated the effect of administering 1,
5, 15 or 45 mg benomyl/kg per day by gavage daily to 102-day old
Wistar male rats (12/group). The males were bred to untreated
females after 62 days of dosing and killed after 76-79 days.
Reproductive behaviour, seminal vesicle weight, prostate weight,
sperm motility, and serum gonadotropin hormones and serum gonadal
hormone levels were no different from those in controls at any dose.
At necropsy, males exposed to 45 mg/kg had decreased testis and
epididymal weights, reduced cauda sperm reserves, decreased sperm
production, increased numbers of decapitated spermatozoa and
increased numbers of seminiferous tubules containing multinucleated
giant cells.
In a study by Hess et al. (1991), adult male Sprague-Dawley
rats (100 days of age, 20 rats/dose) were given a single dose of
benomyl in corn oil (0, 25, 50, 100, 200, 400 or 800 mg/kg body
weight). Eight animals/group were sacrificed at 2 days and 12
animals/group at 70 days (except for the 800 mg/kg group) after
treatment. The testis and excurrent ducts were examined each time to
determine benomyl effects on spermatogenesis and on the epididymis.
The primary effects seen at day 2 were testicular swelling and
occlusions of the efferent ductules. Premature release of germ cells
(sloughing) was the most sensitive short-term response to benomyl.
Sloughing was detected in all treatment groups but was statistically
significant (p < 0.05) at doses of 100 mg/kg to 800 mg/kg.
Occlusions of the efferent ductules of the testis were dose
dependent and correlated with the increase in testis weight on day
2. The greatest increase in testes weight was observed in the 400
mg/kg group. Long-term effects (70 days) were seen in the 100, 200
and 400 mg/kg groups, e.g., decreased testis weight (400 mg/kg),
dose-dependent increases in seminiferous tubular atrophy, and
increases in the number of reproductive tracts containing occluded
efferent ductules. No long-term effects were seen in the 0, 25 or 50
mg/kg groups.
7.5.1.3 Dog inhalation studies
Three groups (10 dogs per group) of sexually mature male dogs
were exposed to benomyl at air concentrations (aerosol/cloud) of 0,
0.65 and 1.65 mg/litre for a 4-h period. One half of the dogs in
each group were killed on day 14 and the remainder on day 28
following the exposure. Histopathological examination revealed a
reduction in spermatogonic activity on day 14, but not on day 28, in
the high-dose group (Littlefield & Busey, 1969).
7.5.2 Teratogenicity and embryotoxicity
7.5.2.1 Mouse gavage studies
Groups of pregnant CD-1 mice (20-25 mice/group) were
administered benomyl via gavage at dose levels of 0, 50, 100 and 200
mg/kg per day on days 7 to 17 of gestation. Animals were killed on
day 18, pups were delivered by Caesarean section, the number of
live, dead and resorbed fetuses was determined, and fetuses were
examined for gross abnormalities. Half of the fetuses were examined
for visceral abnormalities and the other half for skeletal
abnormalities. No maternal toxicity was observed. Fetal development
was adversely affected by treatment at all dose levels. The high
dose caused an increased supraoccipital score, decreased numbers of
caudal and sternal ossifications and increased incidences of
enlarged lateral ventricles and enlarged renal pelvis. The latter,
while not significant at the lower doses, did demonstrate
dose-related increases at all other doses. The occurrence of
supernumerary ribs and subnormal vertebral centrums was
significantly increased in a dose-related manner at all dose levels.
There was an increase in the number of abnormal litters and fetuses,
which was significantly different from the controls, at levels of
100 and 200 mg/kg per day. Fetal weights were also decreased at
these dose levels. Major abnormalities included exencephaly,
hydrocephaly, cleft palate, hydronephrosis, polydactyly,
oligodactyly, umbilical hernia, fused ribs, fused vertebrae and
short/kinky tail (Kavlock et al., 1982).
7.5.2.2 Rat gavage studies
In a study by Staples (1980), benomyl (99.2% a.i.) was adminis
tered by gavage to groups of pregnant rats (ChR-CD) at dose levels
of 0, 3, 10, 30, 62.5 and 125 mg/kg per day from days 7 to 16 of
gestation. There were 60 dams in the control group and 27 in each of
the other test groups; they were observed daily for signs of
toxicity and changes in behaviour. No clinical signs of toxicity or
mortality were observed among dams in any dose group. Body weight
gain was comparable to controls, as were the incidences of
pregnancy, corpora lutea and implantation sites, and the sex ratio.
However, fetal body weight was significantly decreased at the two
highest dose levels. There was also an increased incidence of
embryo/fetal mortality at 125 mg/kg per day.
The malformations observed included microphthalmia,
anophthalmia and hydrocephaly (distended lateral ventricles). These
appeared to be compound related at the higher dose levels.
Microphthalmia was seen in two fetuses from different litters at 10
mg/kg per day and in one fetus from the control group.
Microphthalmia/anopthalmia was observed in only 4-6 out of 4935
fetuses examined in the historical data base at this laboratory.
Histological examination of eyes revealed pathological changes
consisting of irregular lenses, retro-bulbar glandular adnexa,
distorted or compressed retinal layers and thickened nerve fibres in
the 10, 62.5 and 125 mg/kg per day treatment groups. Major skeletal
malformations observed in the 125 mg/kg dose group included fused
ribs, fused sternebrae and fused thoracic arches. Additional
skeletal variations were also increased at 62.5 and 125 mg/kg per
day; these included misaligned and unossified sternebrae and
bipartite vertebral centra (Staples, 1980).
In order to determine a no-effect level for microphthalmia and
hydrocephaly, groups of 50 pregnant rats of the same strain and from
the same supplier were administered benomyl (99.1% a.i.) via gavage
at dosage levels of 0, 3, 6.25, 10, 20, 30 and 62.5 mg/kg per day
from days 7 to 16 of gestation (Staples, 1982). Each group contained
50 animals except the high-dose group, which contained 20 female
rats. Reproductive status was determined on a per-litter basis
following gross pathological evaluation. The number of implantation
sites, resorptions, dead, live and stunted fetuses, and the mean
weight of live fetuses per litter were determined. Only fetal heads
were fixed and examined microscopically. Microphthalmia was
determined on the basis of the smallest eye in the control group (<
1.8 mm). Mean fetal body weight was significantly lower in the
high-dose group. There were only two animals with malformations,
both in the 62.5 mg/kg per day group. One fetus exhibited internal
hydrocephaly and another, in a separate litter, unilateral
microphthalmia. There was no teratogenic response at doses up to 30
mg/kg.
The teratogenic potential of benomyl was examined in groups of
Wistar rats (12 to 30 pregnant rats/group) orally gavaged at dose
levels of 0, 15.6, 31.2, 62.5 and 125 mg/kg per day on days 7 to 16
of gestation. Major anomalies were observed primarily at levels of
62.5 mg/kg per day or more and included encephaloceles,
hydrocephaly, microphthalmia, fused vertebrae and fused ribs. A dose
level of 31.2 mg/kg per day appeared to be without adverse effects
on the developing rat fetus in this evaluation. A significant
reduction in maternal body weight was observed at the highest dose
level (Kavlock et al., 1982).
Kavlock et al. (1982) also evaluated the effect of low levels
of benomyl as the pups aged. Benomyl was administered via gavage to
groups of Wistar rats at dose levels of 0, 15.6 and 31.2 mg/kg per
day from day 7 of gestation to day 15 of lactation (day 22 of
gestation was considered day 0 of lactation). The litters were
weighed on days 8, 15, 22, 29 and 100 after parturition, and
locomotor activity was evaluated periodically throughout the study.
At 100 days of age, several organs were weighed, including the
adrenals, liver, kidney, ovaries, testes and the ventral prostate
plus seminal vesicles. There were no compound-related effects either
on litter size at birth or weaning, or on body weights of fetuses.
Growth, survival and locomotor activity values were comparable with
those of the controls throughout the study. Organ weights were
comparable with those of controls, except that testes and ventral
prostate/seminal vesicle weights were significantly reduced at 31.2
mg/kg per day (but not at 15.6 mg/kg) (Kavlock et al., 1982).
In other studies (Zeman et al., 1986; Hoogenboom et al., 1991),
benomyl produced ocular and craniocerebral malformations in
Sprague-Dawley rats when administered by gavage at doses of 31.2 and
62.4 mg/kg per day on day 7-21 of gestation. Ocular anomalies
(retinal dysplasia, cataracts, microphthalmia and anophthalmia)
occurred in 43.3% of fetuses when the dams were administered 62.4
mg/kg per day. The occurrence increased to 62.5% when the dams were
given a semipurified protein-deficient diet and the same dose level
of benomyl (Hoogenboom et al., 1991). Craniocerebral malformations
(consisting primarily of hydrocephaly) occurred in fetuses from dams
administered 31.2 mg/kg per day in combination with the semipurified
diet (Zeman et al., 1986).
7.5.2.3 Rat feeding studies
In a study by Sherman et al. (1972), groups of rats (26-28
pregnant ChR-CD rats/group) were administered a benomyl formulation
(53.5% a.i.) in the diet at dosages of 0, 100, 500, 2500 and 5000
mg/kg from day 6 to day 15 of gestation. Average doses were
equivalent to 0, 8.6, 43.5, 209.5 and 372.9 mg/kg body weight per
day. On day 20 of gestation, all pregnant animals were sacrificed
and fetuses were delivered by Caesarean section. There was no
mortality attributable to benomyl, no clinical signs of toxicity and
no adverse effects on the body weight of dams. Dams in the
highest-dose group had a reduced food intake during the period
benomyl was administered in the diet, but the intake returned to a
level similar to the controls for the remainder of the study. Except
for three litters at the highest dose, where there were incidences
of hydronephrosis and retarded ossification (interparietal and
occipital bones), there were no effects on fetal development related
to benomyl administration.
In a similar study, groups of pregnant Wistar rats (27-28
rats/group) were fed benomyl at dose levels of 0, 1690, 3380 and
6760 mg/kg diet (time-weighted doses of 0, 169, 298 and 505 mg/kg
body weight per day, respectively) from days 7 to 16 of gestation.
No dose-related anomalies or major malformations were associated
with exposure to benomyl at any of the dose levels used. Reduced
fetal weight was observed at the two highest dose levels (Kavlock et
al., 1982).
7.5.2.4 Rabbit feeding studies
Groups of rabbits (15 artificially inseminated New Zealand
albino rabbits/group) were administered a benomyl formulation (50%
a.i.) in the diet at dose levels of 0, 100 and 500 mg/kg diet from
day 8 to day 16 of gestation. Mortality, clinical observations and
food consumption were determined daily and body weights were
measured weekly. There were 12 pregnant does in the control group,
13 in the low-dose group and 9 in the high-dose group. Of these, 6,
7 and 5, respectively, were sacrificed on day 29 or 30 and fetuses
were delivered by Caesarean section; the remaining does gave birth
normally. Maternal toxicity was not observed at any dose level.
Except for a marginal increase in rudimentary ribs at 500 mg/kg,
developmental toxicity was not observed. The numbers of litters and
of fetuses examined were less than adequate to assess the fetotoxic
or teratogenic potential of benomyl to pregnant rabbits (Busey,
1968c; FAO/WHO, 1985a).
7.6 Mutagenicity and related end-points
Numerous studies have been conducted to assess the mutagenic
potential of benomyl, its metabolite carbendazim, and several
benomyl formulations. Many of the results are conflicting and many
of the study reports do not provide sufficient detail to evaluate
the reasons for the conflicting data. This summary will cover only
those studies where sufficient experimental detail and data were
reported (Table 9).
Studies on somatic and germ cells have shown that benomyl does
not cause gene mutations or structural chromosomal damage
(aberrations) and that it does not interact directly with DNA. This
has been demonstrated in both mammalian and non-mammalian systems.
However, in the mammalian in vitro studies for gene mutations and
structural chromosome aberrations, some positive results were
obtained with benomyl. These positive results appear to have been a
consequence of the inherent sensitivity of some in vitro mammalian
test systems to cytotoxic agents. Results in mammalian in vivo
studies for gene mutations and structural chromosome aberrations
were negative.
Benomyl does cause numerical chromosomal aberrations
(aneuploidy and/or polyploidy) in experimental systems both in
vitro and in vivo (Table 9).
7.7 Carcinogenicity
A number of carcinogenicity studies have been conducted on
carbendazim, the main metabolite of benomyl. A description of these
studies can be found in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
7.7.1 Rat
In a two-year study on benomyl, groups of Charles River albino
weanling rats (36 male and 36 female rats) were fed benomyl (50-70%
a.i.) in the diet at levels of 0, 100, 500 and 2500 mg/kg diet. At
the end of the study all surviving animals were sacrificed and gross
and microscopic examinations of tissues and organs were carried out.
The most frequently observed tumours involved the pituitary, but
these were equally distributed among control and treated groups.
Mammary, adrenal and other tumours were also observed; these were
scattered among all groups. There were no adverse effects or
significant histopathological changes at any dose levels in this
study that could be attributable to benomyl (Sherman, 1969c; Lee,
1977).
Table 9. Studies on mutagenicity of benomyl
End points/Tests Species, strains Concentrationb Activation Result Reference
1. DNA damage and repair
Mitotic gene conversion Saccharomyces cerevisiae, NR with and negative Siebert et al. (1970);
D4 & D7 without de Bertoldi et al. (1980)
Mitotic gene conversion Aspergillus nidulans, 0.35-2.8 mM without negative de Bertoldi & Griselli
D7 (1980)
Mitotic crossing-over test A. nidulans, P NR without negative Bignami et al. (1977)
A. nidulans, D7 NR without negative de Bertoldi & Griselli
(1980)
Non-disjunction A. nidulans, D7 0.35-2.8 mM without positive de Bertoldi & Griselli
(1980)
Sister chromatid exchanges human lymphocyte 0.05-2.0 µg/ml NR slight increase in Georgieva et al. (1990)
(SCE) cultures SCE, no dose-response
relationship
Unscheduled DNA synthesis B6C3F1 male mice & 0.5-500 µg/ml NR negative Tong (1981)
Fisher-344 male rat
hepatocytes
2. Gene mutation
a) Bacterial & fungal Salmonella typhimurium 0.125-1.0 µg/ml NR positive(TA1535) Kappas et al. (1976)
gene mutation TA1535, TA1538 0.125-1.0 µg/ml NR negative(TA1538)
Escherichia coli, 0.125-1.0 µg/ml NR positive Kappas et al. (1976)
WP2 uvra
E. coli, WP2 uvra 2.5-10.0 µg/ml NR positive Kappas et al. (1976)
E. coli, WP2 0.125-1.0 µg/ml NR negative Kappas et al. (1976)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Point mutation A. nidulans 0.25-0.4 µg/ml NR positive Kappas & Bridges (1981)
Spot test S. typhimurium, his, 50-5000 µg per negative Fiscor et al. (1978)
G 46 & TA1535, TA1530 spot
TA1950
S. typhimurium, TA100 50-2000 µg per with negative Fiscor et al. (1978)
plate
Host-mediated assay S. typhimurium, his, 3 consecutive negative Fiscor et al. (1978)
G 46 subcutaneous
injections of
500 mg/kg in mice
TA1950 an oral dose of 4000 negative Fiscor et al. (1978)
mg/kg in rats or mice
Spot test S. typhimurium, TA1535, 20 µg/spot with and negative Carere et al. (1978)
TA1536, TA1537, TA1538 without
Forward spot mutation Streptomyces coelicolor 500 µg/spot without negative Carere et al. (1978)
assay
Ames plate incorporation S. typhimurium, TA1537 500 µg/plate without marginal Russell (1978a)
test positive
S. typhimurium, TA1535, 10-500 µg/ with negative Donovan & Krahn (1981);
TA1537, TA98, TA100 plate Rickard (1983a,b)
10-200 µg/plate without negative
TA1535, TA1537, TA98, 10-500 µg/plate with and negative Russell (1978b)
TA100 without
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
TA1513, TA1537, up to 1000 µg/ with and negative Shirasu et al. (1978)
TA1538, TA98, TA100 plate without
E. coli, WP2 hcr up to 500 µg/ with and negative Shirasu et al. (1978)
plate without
recA spot test B. subtilis, M45 & H-17 2000 µg/plate NR negative Shirasu et al. (1978)
Host-mediated assay S. typhimurium, his, 2000 mg/kg negative Shirasu et al. (1978)
(ICR mice) G46
b) In vitro mammalian
gene mutaion
HGPRTa gene Chinese hamster ovary 17 & 172 µM with negative Fitzpatrick (1980)
cells 3 & 120 µM without negative
Mouse lymphoma Mutant colonies in 0.5-20 µM without negative Amacher et al. (1979)
L5178Y TK+/- gene RPMl1640/horse serum
mutation assays (MLY) 2.5-25 µM with negative
MLY > 2.5 µM negative McCooey et al. (1983a)
MLY carbendazim, without negative McCooey et al. (1983b)
25-200 µM
carbendazim, with negative McCooey et al. (1983b)
12.5-200 µM
MLY N-butylisocyanate without negative McCooey et al. (1983b)
2.5-25 µM
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) Insect germ cell
gene mutation
Sex-linked recessive adult male fed benomyl suspension negative Lamb & Lilly (1980)
lethals D. melanogaster (1.5 mg/ml)
3. Chromosomal effects
a) Yeast and fungi A. nidulans 0.25 µg/ml without increased frequency of Hastie (1970)
(diploid) 0.5 µg/ml segregants
A. nidulans 0.25 µg/ml without no increased frequency Hastie (1970)
(haploid) 0.5 µg/ml of segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(diploid) segregants
A. nidulans 0.75 to 1.75 µM without increased frequency of Kappas (1974)
(haploid) segregants
A. nidulans 0.35 to 2.8 mM without increased De Bertoldi & Griselli
(diploid) nondisjunction (1980)
Saccharomyces cerevisiae, 30 µg/ml without induced chromosome Albertini (1991)
D61.M malsegregation
b) In vitro mammalian human lymphocytes 1.0-100.0 µg/ml with negative Pilinskaya (1983)
assays
human lymphocytes 1.0-100.0 µg/ml without weak positive at Pilinskaya (1983)
10.0 µg/ml only
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
human/mouse mono- < 15 µg/ml without induced aneuploidy Athwal & Sandhu (1985);
chromosomal hybrid and polyploidy Sandhu et al. (1988)
cells (R3-5)
human/mouse mono- 15 µg/ml without slight increase in Athwal & Sandhu (1985);
chromosomal hybrid structural Sandhu et al. (1988)
cells (R3-5) chromosomes; 1.5 µg/ml
threshold (polyploidy)
V79/AP4 Chinese NR without dose-related increase Rainaldi et al. (1989)
hamster cells in numerical
chromosomal
aberrations
Chinese hamster NR without dose-related increase Eastmond & Tucker
ovary cells in numerical (1989)
chromosomal
aberrations
human lymphocytes NR without dose-related increase Georgieva et al. (1990)
in numerical
chromosomal
aberrations; 0.1 µg/ml
threshold (aneuploidy)
Chinese hamster- NR without dose-related increase Zelesco et al. (1990)
human hybrid cells in numerical
chromosomal
(EUBI) aberrations; 2.0 µg/ml
threshold (aneuploidy)
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
c) In vivo mammalian rats up to 500 mg/kg no chromosomal Ruziscka et al. (1976)
assays for 8 days by gavage aberrations in bone
marrow, increase in
chromosomal
aberrations in
embryonic cells at
200 and 500 mg/kg doses
Bone marrow micro- ICR mice 2 gavage doses of increase in Seiler (1976)
nucleus test 9, 500 or 1000 micronucleated
mg/kg 24 h apart polychromatic
erythrocytes at
1000 mg/kg only
Bone marrow micro- BDF1 mice single gavage dose increase in Sasaki (1990)
nucleus test of 0, 1250, 2500 or micronucleated
5000 mg/kg polychromatic
erythrocytes at
2500 and 5000 mg/kg
Bone marrow chromosomal B6D2F2/Cr-1Br mice single gavage dose no increase in Stahl (1990)
aberrations of 0, 625, 1250, 2500, structural chromosomal
or 5000 mg/kg aberrations
d) In vivo germ cell
chromosomal aberration
Dominant lethal test ChR-CD rat 0, 500, 2500 or negative Culik & Gibson (1974)
5000 mg/kg feeding
for 7 days
Table 9 (contd).
End points/Tests Species, strains Concentrationb Activation Result Reference
Dominant lethal test Wistar rats 0, 1, 6.3 or 203 negative Barnes et al. (1983)
mg/kg feeding for
70 days
Dominant lethal test Wistar rats 70 daily gavage doses negative Georgieva et al. (1990)
of 0, 10 or 50 mg/kg
a HGPRT = hypoxanthine-guanine phosphoribosyl transferase
b NR = not reported
7.7.2 Mouse
Male and female CD-1 mice (80 males and 80 females per group)
were fed benomyl (99% a.i.) at dose levels of 0, 500, 1500 and 5000
mg/kg diet (the highest level was reduced from 7500 mg/kg after 37
weeks) for two years. The incidence of hepatocellular adenomas and
carcinomas (see Table 10) in female mice was increased in a
dose-dependent manner. In male mice, numbers of hepatocellular
adenomas and carcinomas were significantly increased at 500 and 1500
mg/kg but not at 5000 mg/kg dose. The increased number of lung
alveogenic carcinomas in male mice was still within the range of
historical controls (Weichman et al., 1982; Frame & van Pelt, 1990;
Hardisty, 1990).
7.8 Special studies
7.8.1 Neurotoxicity
Studies performed using White Leghorn hens (10/group) gave no
indication of neurotoxic potential with single oral doses of benomyl
at levels up to 5000 mg/kg (Goldenthal, 1978; Jessup & Dean, 1979;
Jessup, 1979).
Studies performed using adult male CFY rats (10 rats/group)
gave no indication of altered EEG potentials or behaviour (learning
ability assessed with a 4-choice T-maze) after treatment with 250 or
500 mg benomyl/kg per day for 3 months when compared with controls
(Desi, 1983).
7.8.2 Effects in tissue culture
In a study by Desi et al. (1977), primary monkey kidney cells
were incubated with benomyl (Fundazol 50 WP) at levels of 1, 10, 50,
100, 250 and 400 mg/kg. Growth inhibition and syncytium-like
appearance of the cell monolayer was observed at 250 mg/kg, and at
400 mg/kg all the cells were killed.
7.9 Factors modifying toxicity; toxicity of metabolites
Benomyl degrades to butyl isocyanate (BIC) and carbendazim
quite rapidly in water and in many organic solvents used in
toxicological testing. The biological activity of benomyl is
essentially that of carbendazim both in a toxicological sense and in
its use as a fungicide. A detailed discussion of carbendazim
toxicology is given in Environmental Health Criteria 149:
Carbendazim (WHO, 1993).
Table 10. Incidence and latency of hepatic tumours in benomyl-treated CD-1 micea
Male mice Female miceb
Benomyl concentration (mg/kg diet): 0 500 1500 5000 0 500 1500 5000
Hepatocellular adenoma
Number of animals with tumours 9 8 10 10 2 2 5 7
Latent time to first tumour (days) 530 556 541 627 744 641 650 644
Average latent period (days) 687 707 695 726 744 688 717 722
Hepatocellular carcinoma
Number of animals with tumours 14 26c 41d 17 2 7 7 14e
Latent time to first tumour (days) 545 470 590 508 744 640 736 426
Average latent period (days) 693 705 721 711 744 708 736 695
a From: Wiechman (1982)
b P < 0.05 Dose/response increase of adenomas and carcinomas
c P < 0.05
d P < 0.001
e P < 0.01
BIC is toxic by inhalation. In 4-month subchronic inhalation
studies, the no-observed-effect levels (NOEL) for BIC in rats and
mice were determined to be 0.32 and 1.5 ppm, respectively (Gurova et
al., 1976). An industrial hygiene survey found that workers
experienced severe eye irritation and lacrimation at BIC exposure
levels of 5-10 ppb (Kelly, 1989).
7.10 Mechanisms of toxicity - Mode of action
Biochemical studies on the mechanism of action of benzimidazole
compounds have shown that their biological effects are caused by
interactions with cell microtubules (Davidse & Flach, 1977). These
cellular structures are present in all eukariotic cells and are
involved in several vital functions, such as intracellular
transports and cell division. Benzimidazol compounds have been used
as anticancer drugs and as antihelminthic drugs in animals and
humans because they act as spindle poisons by interfering with the
formation and/or functioning of microtubules. However, eukaryotes
are known to be unequally sensitive to each benzimidazol compound,
which explains the use of these compounds in helminthiases.
Selective toxicity of benomyl and carbendazim for fungi has been
explained by comparing their binding to fungal and mammalian
tubulin. The different sensitivity of several fungi has also been
explained by the different affinity of benomyl and carbendazim for
fungal tubulin.
Benomyl has been found to bind to fungal tubulin but not to
porcine brain tubulin, indicating that mammalian tubulin has no, or
at least low, affinity for benomyl (Davidse & Flach, 1977). This is
in agreement with the observation that benomyl at concentrations
that are lethal for sensitive fungi does not interact with in vitro
microtubule assembly in these brain extracts. In vitro ID50
values for several mycelial extracts of various fungal species
sensitive to benomyl were all below 5 µmol/litre (Davidse & Flach,
1977). In vitro rat brain tubulin polymerization was inhibited to
about 20% at benomyl or carbendazim concentrations of 25 µmol/litre
(De Brabander et al., 1976b). For comparison, a standard antitubulin
drug in humans such as vincristine inhibited 50% tubulin assembly at
0.1 µmol/litre in the same experiment. The assembly of sheep and
calf brain microtubule was also found to be unaffected by
carbendazim concentrations higher than 100 µmol/litre (Ireland et
al., 1979).
Mitotic arrest by benzimidazole and six analogues at metaphase
was evaluated in human lymphocyte cultures. Structure-activity
relationships indicate that antimitotic activity is related to C6
substitution of the benzimidazole moiety (Holden et al., 1980). In
this study, however, benomyl and carbendazim were not tested. The
question of whether all C6 unsubstituted benzimidazoles, such as
benomyl and carbendazim, have no effect on mitosis of human
lymphocytes in cell cultures is therefore unresolved.
A link between the effects of benomyl and carbendazim on brain
tubulin and their teratogenic effects has been postulated (Ellis et
al., 1987, 1988).
8. EFFECTS ON HUMANS
8.1 General population exposure
No references to benomyl poisoning in the general population
have been documented in the scientific literature. Recent data used
to estimate dietary exposure based on food consumption patterns
within the USA indicate exposures well below the NOELs in animal
toxicity tests.
8.2 Occupational exposure
8.2.1 Acute toxicity
Benomyl has a very low acute toxicity. No inadvertent poisoning
of agricultural or factory workers has been documented (Goulding,
1983).
8.2.2 Effects of short- and long-term exposure
Benomyl causes contact dermatitis and dermal sensitization in
some farm workers (van Joost et al., 1983; Kuehne et al., 1985). In
controlled patch tests of agricultural, ex-agricultural and
non-agricultural workers (total of 200 subjects), only one
agricultural worker showed any contact dermatitis to 0.1% benomyl
(Lisi et al., 1986).
A survey of cross-sensitization between benomyl and other
pesticides was conducted in Japan on a group of 126 farmers who
applied benomyl to their crops. Thirty-nine of the farmers gave
positive test results with benomyl, the highest incidence being
among female farmers. There were cross-reactions between benomyl and
other pesticides, such as diazinon, saturn, daconil and Z-bordeaux
(Matsushita & Aoyama, 1981).
Selected blood profiles from 50 factory workers involved in the
manufacture of benomyl were compared to those of a control group of
48 workers who were not exposed to carbendazim. White blood cell
count, red blood cell count and haemoglobin and haematocrit values
were comparable among the two groups. There were no quantitative
estimates of exposure given for the factory workers (Everhart, 1979;
FAO/WHO, 1985a).
A study was performed to determine whether exposure to benomyl
and carbendazim had an adverse effect on the fertility of 298 male
manufacturing workers exposed to benomyl between 1970 and 1977. The
workers ranged from 19 to 64 years of age (79% were between 20 and
39, and 78% of the spouses were similarly aged between 20 and 39
years). Exposure duration ranged from less than one month to 95
months, and more than 51% of the workers were potentially exposed
from 1 to 5 months. The birth rates of exposed workers' spouses were
compared with those of four comparison populations from the same
county, state, region and country (USA). There was no reduction in
fertility as shown by the birth rates for the study population,
which were generally higher than those of the comparison
populations. Spermatogenesis among workers was not examined (Gooch,
1978; FAO/WHO, 1985a).
In studies on agricultural spraymen using benomyl (Fundazol 50
WP), 14 spraymen working in greenhouses were followed, some of them
for two years. General medical check-up and routine blood and urine
tests were performed. Electrocardiograms were recorded and blood
cholinesterase activity was monitored. Lymphocytes from peripheral
blood were examined for chromosomal aberrations both before and
during the study. There was no difference in structural chromosomal
aberration between the spraymen and controls. After benomyl
exposure, numerical chromosomal aberrations were higher in the
spraymen than in controls. However, the spraymen had a higher level
of numerical chromosome aberration than the controls even before
benomyl exposure (Desi et al., 1990; Nehez et al., 1992).
9. EFFECTS ON ORGANISMS IN THE LABORATORY AND FIELD
9.1 Microorganisms
Soil respiration has been found to be little influenced by
benomyl at concentrations below 10 mg/kg, which is the maximum soil
concentration expected after use at recommended application rates
(Hofer et al., 1971; van Fassen, 1974; Peeples, 1974; Weeks &
Hedrick, 1975).
A study on the influence of benomyl on soil nitrogen
mineralization showed that the release of ammonia was not decreased
by benomyl, whereas the influence of the fungicide on nitrification
varied from a stimulation (van Fassen, 1974), through no effect
(Mazur & Hughes, 1975), to a decreased nitrification (Hofer et al.,
1971; Wainwright & Pugh, 1974). The differences may be related to
the soil composition since Hofer et al. (1971) found a greater
effect in sandy than in organic soil.
Benomyl, in combination with eleven other pesticides that were
used in an orchard spray programme, had only a minimal and
short-term effect on respiration, ammonification and nitrification
at concentrations expected after recommended use of benomyl over a
spraying season. Ten times the recommended application rates had a
pronounced effect on both respiration and nitrification, which
lasted for more than 4 weeks (Helweg, 1985).
The influence of benomyl and carbendazim on soil microbial
activity was studied in Sweden following repeated annual
applications during autumn to winter cereals for a period of 3 to 5
years. The effects of the fungicides on straw decomposition, balance
of straw fungal flora and nitrogen mineralization in the soil were
investigated in field and laboratory experiments. The decomposition
of straw in the field was not affected in clay soils by annual
applications of up to 2 kg/ha. In sandy soils, rates of up to 0.5
kg/ha had no effect, but in one case at 2 kg/ha the initial stages
of straw decomposition were slightly inhibited. All doses tested in
both clay and sandy soils caused changes in the composition of the
straw fungal flora (Torstensson & Wessen, 1984).
Benomyl had no effect on soil bacterial populations in
laboratory studies, but fungi and actinomycetes populations were
reduced (Siegel, 1975). Under greenhouse conditions (Kaastra-Howeler
& Gams, 1973) or field conditions (Peeples, 1974) at application
rates of up to 89.6 kg a.i./ha, little effect on microbial
populations was observed following benomyl treatment.
9.2 Aquatic organisms
The effect of benomyl was monitored using the green alga
Selenastrum capricornutum in an OECD guideline test (201). The
EC50 (based on total growth) at 72 h was 2.0 mg/litre and at 120 h
was 3.1 mg/litre. The no-observed-effect concentration (NOEC) was
0.5 mg/litre. To study whether benomyl was algistatic or algicidal,
organisms were recultured at the end of the initial 120 h
incubation. Regrowth occurred in the control but not in the test
cultures (8.0 mg/litre) after a period of 7 days. Benomyl was,
therefore, considered to be algicidal (Douglas & Handley, 1988). In
another study using Chlorella pyrenoidosa, the 48-h EC50 for
growth inhibition was calculated as 1.4 mg/litre (Canton, 1976).
The acute toxicity of benomyl to a variety of aquatic organisms
is summarized in Table 11. For 96-h tests, LC50 values ranged from
0.006 mg/litre for channel catfish ( Ictalurus punctatus; yolk-sac
fry) to > 100 mg/litre for crayfish ( Procambarus sp.) (Mayer &
Ellersieck, 1986).
9.3 Terrestrial organisms
Several field studies have investigated the toxicity of benomyl
to earthworms (Table 12). In one study, the 48-h LC50 for E.
foetida was 9.1 µg/cm3 soil (Roberts & Dorough, 1984).
Van Gestel et al. (1992) exposed red earthworms ( Eisenia
andrei) to benomyl added to artificial soil and used final
concentrations of 0, 0.1, 0.32, 1.0, 3.2 and 10 mg/kg dry soil. The
worms had been acclimatized for 1 week in the artificial soil, which
contained 4 g/kg cow dung as food for the worms. At the highest
concentration of 10 mg/kg, high mortality occurred; LC50 values of
6.0 and 5.7 mg/kg dry soil were calculated after 3 and 6 weeks of
incubation, respectively. Growth was significantly reduced at 3.2
mg/kg soil. EC50 values for the effect of benomyl on cocoon
production did not differ significantly for the two test periods,
and the EC50 was 1.6 (1.2-2.3) mg/kg for the entire 6-week test
period.
Zoran et al. (1986) and Drewes et al. (1987) have shown that
the conduction velocity for medial and lateral giant nerve fibres is
affected by exposure of earthworms to sublethal concentrations of
benomyl. Zoran et al. (1986) have also shown that segmental
replication is affected in amputated earthworms exposed to sublethal
concentrations of benomyl.
The benomyl metabolite carbendazim (99.3% purity) was evaluated
for acute contact toxicity after thoracic application to honey-bees
(Apis mellifera). Each treatment level consisted of four replicates
of ten bees each. Forty bees served as positive control (using
carbaryl) and forty as negative control. No deaths occurred after
application of carbendazim at 50 µg/bee, the highest rate tested.
Carbendazim is, therefore, classified as "relatively non-toxic" to
the honey-bee (Meade, 1984).
Table 11. Toxicity of benomyl to aquatic organisms
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Freshwater
Water flea adult stat 25 3 14 Yoshida & Nishiuchi (1972)
(Daphnia magna) < 24 h stat 20 87 8.5 48 0.11c Hutton (1989)
< 24 h stat 20 48 0.64 Canton (1976)
< 24 h stat 17 40 7.4 48 2.8 Mayer & Ellersieck (1986)
Scud
(Gammarus pseudolimnaeus) adult stat 17 40 7.4 96 0.75 Mayer & Ellersieck (1986)
Crayfish
(Orconectes nais) instar stat 22 40 7.4 96 >10 Mayer & Ellersieck (1986)
Crayfish
(Procambarus sp.) immature stat 22 40 7.4 96 >100 Mayer & Ellersieck (1986)
Midge
(Chironomus plumosus) instar stat 22 40 7.4 48 7.0 Mayer & Ellersieck (1986)
Rainbow trout 3 months stat 15 48 0.48 Canton (1976)
(Oncorhynchus mykiss) 0.8 g stat 7 44 7.4 96 0.17 Mayer & Ellersieck (1986)
0.8 g stat 12 44 7.4 96 0.20 Mayer & Ellersieck (1986)
0.8 g stat 17 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1.2 g stat 12 44 6.5 96 0.16 Mayer & Ellersieck (1986)
1.2 g stat 12 44 7.5 96 0.19 Mayer & Ellersieck (1986)
1.2 g stat 12 44 8.5 96 0.88 Mayer & Ellersieck (1986)
0.6 g stat 12 44 7.4 96 0.23 Mayer & Ellersieck (1986)
0.6 g stat 12 320 7.4 96 0.60 Mayer & Ellersieck (1986)
fingerling stat 12 44 7.4 96 0.12 Mayer & Ellersieck (1986)
swimup fry stat 12 44 7.4 96 0.16 Mayer & Ellersieck (1986)
yolk-sac fry stat 12 44 7.4 96 0.28 Mayer & Ellersieck (1986)
1 g stat 12 44 7.4 96 0.31c Mayer & Ellersieck (1986)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Fathead minnow 0.9 g stat 22 44 7.4 96 2.2 Mayer & Ellersieck (1986)
(Pimephales promelas) 0.5 g stat 22 45 7.1 96 1.3 Mayer & Ellersieck (1986)
0.5 g stat 22 44 7.4 96 1.9c Mayer & Ellersieck (1986)
Channel catfish 1.2 g stat 22 44 7.4 96 0.029 Mayer & Ellersieck (1986)
(Ictalurus punctatus) 0.05 g stat 22 44 7.4 96 0.013 Mayer & Ellersieck (1986)
0.15 g stat 22 44 7.4 96 0.024 Mayer & Ellersieck (1986)
swimup fry stat 22 44 7.4 96 0.012 Mayer & Ellersieck (1986)
yolk-sac fry stat 22 44 7.4 96 0.006 Mayer & Ellersieck (1986)
1.2 g stat 22 44 7.4 96 0.028c Mayer & Ellersieck (1986)
Bluegill
(Lepomis macrochirus) 0.9 g stat 12 44 7.4 96 0.75 Mayer & Ellersieck (1986)
0.9 g stat 17 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.9 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 6.5 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.5 96 1.2 Mayer & Ellersieck (1986)
0.6 g stat 22 44 8.5 96 6.4 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.3 Mayer & Ellersieck (1986)
0.6 g stat 22 320 7.4 96 2.3 Mayer & Ellersieck (1986)
0.6 g stat 22 44 7.4 96 1.2c Mayer & Ellersieck (1986)
Carp
(Cyprinus carpio) 5 cm stat 25 48 7.5 Yoshida & Nishiuchi (1972)
Killifish
(Fundulus sp.) 2.5 cm stat 25 48 11 Yoshida & Nishiuchi (1972)
Loach 10 cm stat 25 48 14 Yoshida & Nishiuchi (1972)
Table 11 (contd).
Organism Size/ Stat/ Temperature Hardnessb pH Duration LC50 Reference
age flow (°C) (mg/litre) (h) (mg/litre)
Guppy
(Poecilia reticulata) 3 weeks stat 24 48 3.4 Canton (1976)
Tadpole
(Bufo sp.) < 1 month stat 25 48 4.3 Yoshida & Nishiuchi (1972)
Estuarine and Marine
Eastern oyster
(Crassostrea virginica) 25-50 mm flow 17-19 96 1.37d Boeri (1988a)
Grass shrimp
(Palaemonetes pugio) 18 mm stat 18 96 45.8c Bionomics Inc. (1972)
Mysid shrimp
(Mysidopsis bahia) stat 23 96 0.175 Boeri (1988c)
Dungeness crab
(Cancer magister) larvae 96 7.6 Armstrong et al. (1976)
Sheepshead minnow
(Cyprinodon variegatus) 0.14 g stat 22 96 3.88 Boeri (1988b)
a stat = static conditons (water unchanged for duration of test); flow = flow-through conditions (benomyl concentration in water
continuously maintained)
b hardness given as mg CaCO3/litre
c 50% wettable powder
d EC50 based on rate of shell deposition
Table 12. Summary of earthworm toxicity data on benomyl in field studiesa
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass 0.125 0.9 63 8% reduction in number Ammon (1985)
1.25 3.6 43% reduction in number
Grass 7.8 22.2 21 95% reduction in number Tomlin & Gore (1974)
91% reduction in biomass
Grass 0.56 1.6 49 79% reduction in cast Keogh & Whitehead (1975)
production
Grass 0.56 1.6 13 50% reduction in number Tomlin et al. (1980,
(0.18)b 28 40% reduction in number 1981)
180 70% reduction in number
328 67% reduction in number
1.12 (0.90)b 28 35% reduction in number
(0.20)b 180 46% reduction in number
328 50% reduction in number
2.24 6.4 13 75% reduction in number
28 50% reduction in number
(0.30)b 180 50% reduction in number
328 64% reduction in number
Grass/loam 2.0 5.7 30 70% reduction in number Edwards & Brown (1982)
180 22% reduction in number
365 1% reduction in number
Grass/sandy loam 5.0 14.3 30 89% reduction in number Edwards & Brown (1982)
180 59% reduction in number
365 32% reduction in number
Table 12 (contd).
Crop/soil Dosage Estimated soil Time Effect Reference
type (kg/ha) concentration (mg/kg) (days)
Grass/loamy sand 10.0 28.6 30 80% reduction in number
180 89% reduction in number
365 89% reduction in number
Grass/sandy loam 5.0 14.3 30 91% reduction in number; Edwards & Brown (1982)
99% reduction in L. terrestris;
29% reduction in L. festivus
180 90% reduction in L. terrestris;
200% increase in L. festivus
365 99% reduction in L. terrestris;
1457% increase in L. festivus
a From: Van Gestel (1992)
b Results of analysis of the top 15-cm layer carried out by the author, recalculated as the concentration in the top 2.5 cm layer
The acute toxicity for several birds is listed in Table 13.
Benomyl is of low toxicity to birds.
Table 13. Acute toxicity of benomyl to birds
Species LD50a 5-day LC50b Reference
(mg/kg) (mg/kg)
Bobwhite quail > 10 000 Busey (1968e)
Mallard duck > 10 000 Busey (1968e)
Japanese quail > 5000 c Hill & Camardese
(1986)
Starling > 100 Schafer (1972)
Redwinged blackbird 100 Schafer (1972)
a LD50 = single oral dose expressed as mg/kg body weight
b LC50 = 5-day dietary exposure followed by 3 days on a "clean" diet
expressed as mg/kg diet
c 50% active ingredient; no overt signs of toxicity were observed
9.4 Population and ecosystem effects
Under certain conditions benomyl may have an effect on
populations of earthworms. In apple orchards where foliage has been
treated repeatedly at a rate of 0.28 kg/ha and has fallen to the
ground, earthworms may be eliminated after two years of benomyl use
(up to 13 sprayings). The earthworms Lumbricus terrestris and
Allolobophora chlorotica were most affected. Populations of other
species recovered within two years of the termination of spraying.
Orchard yields were unaffected, as were earthworm populations
adjacent to the orchards, because of the immobility of benomyl in
the soil (Stringer & Wright, 1973; Stringer & Lyons, 1974).
Van Gestel (1992) has summarized reports of the toxicity of
benomyl on earthworms from field studies with different soil types,
application rates and crops (Table 12). Estimated soil
concentrations in Table 12, for the various uses of the fungicide,
are based on application rates; they assume no mobility of the
compound beyond the top 2.5 cm of soil and homogeneous distribution
of benomyl in this layer. For orchard application, it was further
assumed that 50% of the applied active ingredient reached the soil.
Reported effects include reduced numbers and reduced activity of
worms. Application rates are within the recommended rates for
benomyl as a fungicide on these crops.
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE ENVIRONMENT
Benomyl and carbendazim are two different fungicides in their
own right. However, carbendazim is also the main metabolite of
benomyl in mammals and the degradation product of benomyl in the
environment. Butyl isocyanate is the chemical moiety removed from
benomyl when carbendazim is formed. Given the similar toxicities
caused by benomyl and carbendazim and the different toxicological
profile of butyl isocyanate, the two fungicides are evaluated
together in this monograph.
10.1 Evaluation of human health risks
The two primary routes of exposure to humans are through diet
and through manufacturing or use of the product.
There is limited information on actual dietary exposure to
benomyl and carbendazim. Dietary exposure has been estimated in the
Netherlands and the USA. In the USA, exposure based on dietary
habits, measured residue levels, and the percentage of crop treated
has been calculated for various subgroups of population. These
calculations indicate that the estimated benomyl exposure is
0.144-1.479 µg/kg per day (section 5.2.1). In the Netherlands, the
mean dietary intake was estimated to be 0.83 µg/kg per day (0.05
mg/day per person). These levels of exposure are below the
recommended ADI of 0.01 (carbendazim) and 0.02 (benomyl) mg/kg body
weight.
The average air levels of benomyl and carbendazim have been
determined in a manufacturing facility (section 5.3) and found to be
less than 0.2 and 0.3 mg/m3, respectively. Both values are below
the Threshold Limit Value of 5-10 mg benomyl/m3 established by a
number of governmental agencies.
In one study, potential respiratory and dermal exposure to
benomyl wettable powder formulation was determined under several
agricultural use situations (section 5.3). The highest rates of
exposure occurred in situations of mixing and loading in preparation
for aerial application; dermal and respiratory exposures were,
respectively, 26 and 0.08 mg/person per cycle. Home users and
agricultural workers re-entering treated fields were estimated to be
exposed to about 1 mg/person per cycle and 5.9 mg/person per hour,
principally through the dermal route.
Because of the low mammalian toxicity, acute benomyl or
carbendazim poisonings are unlikely to occur under conditions of
normal use.
Several studies of agricultural workers (section 8.2) have
shown some cases of contact dermatitis after exposure to benomyl.
These effects can be significantly reduced or eliminated by wearing
long-sleeved shirts, long trousers and gloves.
There is little information on health effects in humans as a
consequence of exposure to either benomyl or carbendazim. Two
studies have been conducted on factory workers involved in the
manufacture of benomyl (section 8.2). In one study, haematological
profiles from 50 factory workers involved in the manufacture of
benomyl were comparable to those from a control group of 48 workers.
A second study found no decrease in the birth rate of the wives of
298 factory workers exposed to benomyl.
Extensive studies of various species of laboratory animals show
reproductive, developmental, mutagenic and carcinogenic effects
associated with both benomyl and carbendazim. The effects observed
on rat fetuses were microphthalmia, hydrocephaly and encephaloceles.
The no-observed-effect levels (NOEL) for developmental toxicity are
equal to or greater than 10 mg/kg body weight per day, depending
upon the species and route of administration. Similarly, the NOEL of
benomyl for reproductive effects in the male rat appears to be 15
mg/kg body weight per day after gavage dosing. In feeding studies
with both benomyl and carbendazim, the NOEL appears to be 500 mg/kg
diet (equivalent to 25 mg/kg body weight per day). However, one
benomyl feeding study reported a NOEL of less than 1 mg/kg diet
(0.05 mg/kg body weight per day) for male reproductive effects. The
reason for the discrepancy between the NOEL in this latter study and
other investigations is unknown.
The only consistent genotoxic effect noted in animal studies is
the induction of numerical chromosomal aberrations. These effects
are consistent with the interaction of benomyl and carbendazim with
microtubule formation.
Rat carcinogenicity studies did not show any carcinogenic
effect for either compound. Benomyl and carbendazim induce
hepatocellular tumours in CD-1 and SPF Swiss mice but not in NMRKf
mice. This finding in mice is not considered to be a result of a
direct genotoxic action. Rather, it appears to be associated with
liver toxicity in strains of mice that are highly susceptible to
tumour formation at this site.
Benomyl and carbendazim are spindle poisons. Effects on target
cells are consequences of binding to microtubules, giving toxicities
similar to those of other spindle poisons such as colchicine and
vincristine. Benzimidazol compounds in general and benomyl and
carbendazim in particular have selective effects on the microtubules
of different eukaryotes. Reasons for this selectivity include the
binding capability to different tubulins and pharmacokinetic
differences across species. In vitro concentrations of benomyl
used to kill sensitive fungi were found to be ineffec tive in
disturbing mammalian microtubular functions. These studies on the
mechanism of action of benomyl and carbendazim indicate a selective
effect of these compounds for target species.
In summary, the LD50, as determined in a number of test
species, for benomyl ranges from > 2000 to > 12 000 mg/kg and for
carbendazim from > 2000 to > 15 000 mg/kg. There are no known
reports of human poisoning for either compound. This, coupled with
the low estimated environmental levels of both compounds, would
suggest that the possibility of acute poisoning by benomyl or
carbendazim is very remote. Similarly, the data available on test
species make it unlikely that either benomyl or carbendazim is
carcinogenic for humans. The NOELs for both reproductive and
teratogenic effects of benomyl and carbendazim (i.e. 10-15 mg/kg) do
raise a possibility that an accidental ingestion of either fungicide
could adversely alter reproductive outcome in humans, but the
likelihood that such poisoning would occur is remote. The
selectivity of these two benzamidazol compounds for the tubulin of
the target species (fungi) and their relative ineffectiveness to
disturb mammalian microtubule function further reduce the
possibility of their having toxic effects in humans.
10.2 Evaluation of effects on the environment
Benomyl is rapidly converted to carbendazim in various
environmental compartments, the half-lives being 2 and 19 h in water
and soil, respectively. Therefore, data from studies on both benomyl
and carbendazim are relevant for the evaluation of environmental
effects.
Carbendazim persists on leaf surfaces and in leaf litter. In
soil the half-life is between 3 and 12 months, and the compound may
be detected for up to 3 years. However, in many cases, major
residues will be lost within a single season. Residues of
carbendazim and its metabolites are strongly bound or incorporated
into soil organic matter. The strong adsorption (Koc =
approximately 2000) of carbendazim to soil and sediment particles
reduces its bioavailability to terrestrial and aquatic organisms.
Similarly, the mobility of carbendazim in soil is limited, and it is
not expected to leach to ground water.
Benomyl and carbendazim are highly toxic to some aquatic
organisms in laboratory tests, the most sensitive species being the
channel catfish with a 96-h LC50 for yolk-sac fry of 0.006 mg
benomyl/litre. However, this toxicity is unlikely to be manifest in
the environment for most aquatic organisms because of the low
bioavailability in surface waters. The exposure of sediment-living
organisms could be greater, but no test results are available for
these organisms.
Benomyl and carbendazim affect groups of fungi in soil but do
not seem to modify the overall microbial activity of the soil when
used at normal field rates.
In both the laboratory and field, benomyl and carbendazim
applied at recommended rates cause deaths and sublethal reproductive
effects on earthworms of many different species. Surface-feeding
species eating leaf litter are most at risk. Populations may take
more than 2 years to recover. There are no studies available on
other litter and soil invertebrates.
Benomyl and carbendazim have low toxicity for birds and
carbendazim is classified as "relatively non-toxic" to honey-bees.
10.3 Conclusions
Benomyl causes dermal sensitization in humans. Both benomyl and
carbendazim represent a very low risk for acute poisoning in humans.
Given the current exposures and the low rate of dermal absorption of
benomyl and carbendazim, it is unlikely that they would cause
systemic toxicity effects either in the general population or in
occupationally exposed subjects. These conclusions are drawn from
animal data and from the limited human data available, but these
extrapolations are supported by the understanding of the mode of
action of carbendazim and benomyl in both target and non-target
species.
Further elucidation of the mechanism of toxicity of carbendazim
and benomyl in mammals will perhaps permit a better determination of
no-observed-effect levels. Binding studies on tubulins of target
cells (testis and embryonic tissues) will facilitate comparisons
across species.
Carbendazim is strongly adsorbed to soil organic matter and
remains in the soil for up to 3 years. It persists on leaf surfaces
and, therefore, in leaf litter. Earthworms have been shown to be
adversely affected (population and reproductive effects) at
recommended application rates. There is no information on other soil
or litter arthropods that would be similarly exposed.
The high toxicity to aquatic organisms in laboratory tests is
unlikely to be seen in the field because of the low bioavailability
of sediment-bound residues of carbendazim. However, no information
is available on sediment-living species which would receive the
highest exposure.
11. FURTHER RESEARCH
1. Comparative binding studies of carbendazim to tubulins of
target tissues from various species should be undertaken.
2. Further clarification of the fate of 1,2-diaminobenzene and
bound residues in the environment is needed.
3. The effects of benomyl and carbendazim on sediment-dwelling
organisms needs to be investigated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Benomyl was evaluated by the Joint FAO/WHO Meeting on Pesticide
Residues (JMPR) in 1973, 1975, 1978, 1983 and 1988. The 1978 meeting
agreed that the MRLs for benomyl, carbendazim and thiophanate-methyl
should be combined and expressed as carbendazim. Benomyl residues
were last evaluated by the 1988 meeting (FAO/WHO, 1988a,b) and the
MRLs were updated at that time. These MRLs (expressed as
carbendazim) are listed in Table 4. The 1983 meeting (FAO/WHO,
1985a) evaluated benomyl toxicology and set the following benomyl
NOEL levels and ADI:
Rat: 2500 mg/kg in the diet, equivalent to 125 mg/kg body
weight
Dog: 100 mg/kg (carbendazim) in the diet, equivalent to 2.5
mg/kg body weight
Rat: teratology - 30 mg/kg body weight per day
The estimated ADI for benomyl was established at 0-0.02 mg/kg
body weight.
Benomyl has not been evaluated by the International Agency for
Research on Cancer (IARC).
REFERENCES
Albertini S (1991) Reevaluation of the 9 compounds reported
conclusive positive in yeast Saccharomyces cerevisiae aneuploidy
test systems by the Gene-Tox Program using strain D61.m of
Saccharomyces cerevisiae. Mutat Res, 260: 165-180.
Amacher DE, Paillet S, & Ray VA (1979) Point mutations at the
thymidine kinase locus in L5178Y mouse lymphoma cells: Application
to genetic toxicological testing. Mutat Res, 64: 391-406.
Ammon HU (1985) Worm toxicity tests using Tubifex tubifex. In:
Comportement et effets secondaires des pesticides dans le sol. Les
Colloques de l'INRA 31, Versailles, 4-8 juin 1984. Paris, Institut
national de la Recherche agronomique, pp 303-317.
Armstrong DA, Buchanan DU, & Caldwell RS (1976) A mycosis caused by
Lagenidium sp. in laboratory-reared larvae of the Dungeness crab,
Cancer magister, and possible chemical treatments. J Invertebr
Pathol, 28: 329-336.
Arthur MF, Schweitzer KL, Fadel LC, Marsh BH, & Marsh SS (1989a)
Aerobic aquatic metabolism of [phenyl(U)-14C]benomyl in
Greenville, Mississippi, water and sediment (Battelle Study No.
N-0966-7301). Columbus, Ohio, Battelle, Environmental Sciences
Department (Unpublished report No. AMR-1452-89, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Arthur MF, Marsh BH, Fadel LC, & Zwick TC (1989b) Anaerobic aquatic
metabolism of [phenyl(U)-14C]benomyl in West Jefferson, Ohio, pond
water and sediment (Battelle Study No. NO799-8800). Columbus, Ohio,
Battelle, Environmental Sciences Department (Unpublished report No.
AMR 770-87, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Athwal RS & Sandhu SS (1985) Use of human X mouse hybrid cell line
to detect aneuploidy induced by environmental chemicals. Mutat Res,
149: 73-81.
Barbo EC & Carroll KS (1972) Oral ALD test (S-triazine,
3-butylbenzimidazolo[1,2-a],-2,4[1H,3H]-dione. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 227-72).
Barefoot AC (1988) Vapor pressure of benomyl. Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Research & Development
Division, Experimental Station (Unpublished report No. AMR 1078-88).
Barnes TB, Verlangieri AJ, & Wilson MC (1983) Reproductive toxicity
of methyl-1-(butylcarbamoyl)-2-benzimidazole carbamate (benomyl) in
male Wistar rats. Toxicology, 28: 103-115.
Baude JF, Gardiner JA, & Han JCY (1973) Characterization of residues
on plants following foliar spray applications of benomyl. J Agric
Food Chem, 21: 1084-1090.
Baude FJ, Pease HL, & Holt RF (1974) Fate of benomyl on field soil
and turf. J Agric Food Chem, 22: 413.
Belasco IJ (1979a) Study showing the absence of acetylcholinesterase
inhibition with a wettable powder formulation (50% Benomyl).
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX-4).
Belasco IJ (1979b) 2-14C-Benomyl (50 WP) adsorption through rat
skin. Part II: Effect of time and dose applied with supplement.
Wilmington, Delaware, Du Pont de Nemours and Co., Inc. (Unpublished
report No. B/ME-47 and supplement No. HLR 117-79).
Belasco IJ, Kirkland JJ, Pease HL, & Sherman H (1969) Studies with
2-14C-labelled methyl-1-(butylcarbamoyl)-2-benzimidazolecarbamate
(benomyl) in rats. Wilmington, Delaware, Du Pont de Nemours and Co.,
Inc., Biochemical Department, Research Division, Experimental
Station (Unpublished report No. B/ME-36).
Bignami M, Aulicino F, Velcich A, Carere A, & Morpurgo G (1977)
Mutagenic and recombinogenic action of pesticides in Aspergillus
nidulans. Mutat Res, 46: 395-402.
Bionomics, Inc. (1972) Acute toxicity of Benlate to grass shrimp
( Palaemonetes vulgaris). Wareham, Massachusetts, Bionomics, Inc.
(Unpublished report No. HOL 275-72, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Boeri RL (1988a) Flow through acute toxicity of benomyl technical to
the eastern oyster, Crassostrea virginica. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 13-89, prepared
for E.I. Du Pont de Nemours and Co., Inc.).
Boeri RL (1988b) Static acute toxicity of benomyl technical to the
sheepshead minnow, Cyprinodon variegatus. Marblehead,
Massachusetts, Enseco (Unpublished report No. HLO 9-89, prepared for
Du Pont de Nemours and Co., Inc.).
Boeri RL (1988c) Static acute toxicity of benomyl technical to the
mysid, Mysidopsis bahia. Marblehead, Massachusetts, Enseco
(Unpublished report No. HLO 828-88, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Bolton EE, Anderson JJ, & Koeppe MK (1986a) Metabolism of
[phenyl(U)-14C] benomyl in field-grown soybeans. Wilmington,
Delaware, Du Pont de Nemours and Co., Inc., Agricultural Products
Department, Experimental Station (Unpublished report No.
AMR-531-86).
Bolton EE, Anderson JJ, & Koeppe MK (1986b) Metabolism of
[phenyl(U)-14C] benomyl in paddy rice. Wilmington, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Agricultural Products Department,
Experimental Station (Unpublished report No. AMR-507-86).
Brock WJ (1987) Acute dermal toxicity study with Benlate 50 DF
fungicide in rabbits. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 505-87).
Busey WM (l968a) Acute dermal LD50 test and dermal irritation test
on rabbits using a wettable powder formulation (50% benomyl) with
histological addendum. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968b) Acute inhalation exposure test in rats using a
wettable powder formulation (50% benomyl). Falls Church, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. MRO 1126-1,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (l968c) Teratology study in rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 1079, prepared for
E.I Du Pont de Nemours and Co., Inc.). Busey WM (l968d) Repeated
dermal application test on rabbits using a wettable powder
formulation (50% benomyl). Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 298-68, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Busey WM (1968e) Acute dietary administration - mallard ducklings
and bobwhite quail: Fungicide 1991. Falls Church, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. MRO 581-239, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Bushway RJ, Hurst HL, Kugabalasooriar J, & Perkins LB (1991)
Determination of carbendazim in blueberries by reversed-phase high
performance liquid chromatography. J Chromatogr, 555: 321-324.
Canton JH (1976) The toxicology of benomyl, thiophanate-methyl, and
BCM to four freshwater organisms. Bull Environ Contam Toxicol, 16:
214-218.
Carere A, Ortali VA, Cardamone G, Torracca AM, & Raschetti R (1978)
Microbiological mutagenicity studies of pesticides in vitro. Mutat
Res, 57: 277-826.
Carter SD (l982) Effect of benomyl on the reproductive development
in the prepubertal male rat. Raleigh, North Carolina, North Carolina
State University (Thesis).
Carter SD & Laskey JW (l982) Effect of benomyl on reproduction in
the male rat. Toxicol Lett, 11: 87-94.
Chang WM (1985) Soil column leaching studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Agricultural Chemicals Department, Experimental Station
(Unpublished report No. AMR 426-85).
Chiba M & Veres DF (1981) Fate of benomyl and its degradation
compound methyl 2-benzimidazole carbamate on apple foliage. J Agric
Food Chem, 29: 588-590.
Clermont Y (1972) Kinetics of spermatogenesis in mammals:
"seminiferous epithelium cycle and spermatogonial renewal". Physiol
Rev, 52: 198-236.
Colburn CW (1969) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (> 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 84-69).
County Natwest Woodmac (1992) The fungicide market. London, County
Natwest Woodmac, Agrochemical Service, pp 49-51.
Culik R (1981a) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) concentrations in maternal blood and in the concepti
of rats exposed to benomyl and Benlate by diet. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 916-80).
Culik R (1981b) Determination of benomyl/methyl-2-benzimidazole
carbamate (MBC) 4-OH MBC and 5-OH MBC concentrations in maternal
blood and in the concepti of rats exposed to benomyl by gavage.
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. HLR 970-80).
Culik R & Gibson JR (1974) "Benlate" dominant lethal study in male
rats with supplemental statistical report (22-77). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 72-74).
Cummings AM, Harris ST, & Rehnberg GL (1990) Effects of methyl
benzimidazole carbamate during early pregnancy in the rat. Fundam
Appl Toxicol, 15: 528-535.
Dashiell OL (1972) Acute oral test (benzimidazole,
2-(3-butylureido)). Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 456-72).
Davidse LC & Flach W (1977) Differential binding of methyl
benzimidazol-2-yl carbamate to fungal tubulin as a mechanism of
resistance to this antimitotic agent in mutant strains of
Aspergillus nidulans. J Cell Biol, 72: 174-193.
De Bertoldi M & Griselli M (1980) Different test systems in
Aspergillus nidulans for the evaluation of mitotic gene
conversion, crossing-over and nondisjunction. Mutat Res, 74:
303-324.
De Bertoldi M, Griselli M, Giovannetti M, & Barale R (1980)
Mutagenicity of pesticides evaluated by means of gene-conversion in
Saccharomyces cerevisiae and in Aspergillus nidulans. Environ
Mutagen, 2: 359-370.
De Brabander M, Van de Veire R, Aerts F, Geuens S, & Hoebeke J
(1976a) A new culture model facilitating rapid quantitative testing
of mitotic spindle inhibition in mammalian cells. J Natl Cancer
Inst, 56: 357-363.
De Brabander M, Van de Veire R, Aerts F, Brogers M, & Janssen PAJ
(1976b) The effect of methyl [5(2-thienylcarbonyl)-1H-benzimidazol-
2-yl carbamate (17934; NSC 238159), a new synthetic antitumoral drug
interfering with microtubules, on mammalian cells cultured in
vitro. Cancer Res, 36: 905-916.
Delp CJ (1980) Coping with resistance to plant disease control
agents. Plant Dis, 64: 652-657.
Dési I (1979) Hygienic-toxicological evaluations of pesticides.
Budapest, National Institute of Hygiene (D.Sc. Thesis).
Dési I (1983) Neurotoxicological investigation of pesticides in
animal experiments. Neurobehav Toxicol Teratol, 5: 503-515.
Dési I, Dura G, Szlobodnyik J, & Csuka I (1977) Testing of pesticide
toxicity in tissue culture. J Toxicol Environ Health, 2: 1053-1066.
Dési I, Nehéz M, Palotas M, Tempfli A, Hogye A, & Vetro G (1990)
Experience of health status surveillance of pesticide workers in
Hungary. Med Lav, 81(6): 517-523.
Donovan SD & Krahn DF (1981) Mutagenic evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 343-81).
Douglass MT & Handley JW (1988) The algistatic activity of benomyl
technical. Huntingdon, United Kingdom, Huntingdon Research Centre
Ltd (Unpublished report No. DPT 171(n)/88371, prepared for E.I. Du
Pont de Nemours and Co., Inc.).
Draize JH, Woodard G, & Gawery HO (1944) New methods for the study
of irritation and toxicity of substances applied topically to the
skin and mucous membranes. J Pharmacol Exp Ther, 82: 377-390.
Drewes CD, Zoran MJ, & Callahan CA (1987) Sublethal neurotoxic
effects of the fungicide benomyl on earthworms (Eisenia fetida).
Pestic Sci, 19: 197-208.
Du Pont (1972) Residue studies - fish: benomyl, MBC, and 2-AB.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report).
Du Pont (1987) Determination of octanol water partition coefficient
for benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/PC-55-CA).
Eastmond DA & Tucker JD (1989) Kinetochlore localization in
micronucleated cytokinesis-blocked Chinese hamster ovary cells: A
new and rapid assay for identifying aneuploidy-inducing agents.
Mutat Res, 224: 517-525.
Edwards PJ & Brown SM (1982) Use of grassland plots to study the
effect of pesticides on earthworms. Pedobiologia, 24: 145-150
Eickhoff JC, Petersen BJ, & Chaisson CF (1989) Anticipated residues
of benomyl in food crops and potential dietary exposure and risk
assessment. Washington, DC, Technical Assessment System, Inc.
(Unpublished report No. TAS 000-005, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Ellis WG, Semple JL, Hoogenboom ER, Kavlock RJ, & Zeman FJ (1987)
Benomyl-induced craniocerebral anomalies in fetuses of adequately
nourished and protein -deprived rats. Teratog Carcinog Mutagen, 7:
357-375.
Ellis WG, De Roos F, Kavlock RJ, & Zeman FJ (1988) Relationship of
periventricular overgrowth to hydrocephalus in brains of fetal rats
exposed to benomyl. Teratog Carcinog Mutagen, 8: 377-391.
Evans EL & Mitchell AD (1980) An evaluation of the effect of benomyl
on sister chromatic exchange frequencies in cultured Chinese hamster
ovary cells. Menlo Park, California, SRI International (Unpublished
report prepared for E.I. Du Pont de Nemours and Co., Inc.).
Everhart LP (1979) Benlate dust exposure survey. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Biochemical
Department (Unpublished report No. B/TOX 6).
Everhart LP & Holt RF (1982) Potential benlate fungicide exposure
during mixer/loader operations, crop harvest and home use. J Agric
Food Chem, 30: 222-227.
FAO/WHO (1985a) Benomyl. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 7-46 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1985b) Carbendazim. In: Pesticide residues in food -
Evaluations 1983. Rome, Food and Agriculture Organization of the
United Nations, pp 89-121 (FAO Plant Production and Protection Paper
61).
FAO/WHO (1988a) Benomyl. In: Pesticide residues in food -
Evaluations 1988. Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 5-15 (FAO Plant Production
and Protection Paper 93/1).
FAO/WHO (1988b) Carbendazim. In: Pesticide Residues in Food -
Evaluations 1988: Part I: Residues. Rome, Food and Agriculture
Organization of the United Nations, pp 41-54 (FAO Plant Production
and Protection Paper 93/1).
Fielding M, Barcelo D, Helweg A, Galassi S, Torstensson L, van
Zoonen P, Wolter R, & Angeletti G (1992) Pesticides in ground and
drinking water. Brussels, Commission of the European Communities,
Directorate-General for Science, Research and Development,
Environmental and Waste Recycling, 135 pp (Water Pollution Research
Report 27).
Fiscor G, Bordas S, & Stewart SJ (1978) Mutagenicity testing of
benomyl, methyl-2-benzimidazole carbamate, streptozotocin and
N-methyl-N'-nitro-N-nitrosoguanine in Salmonella typhimurium in
vitro and in rodent host-mediated assays. Mutat Res, 51: 151-164.
Fitzpatrick K (1980) Chinese hamster ovary cell assay for
mutagenicity. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 438-80).
Frame SR & Van Pelt CS (1990) Oncogenicity studies with benomyl and
MBC in mice: Supplemental peer review. Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 20-82 and supplement No. HLR 70-82).
Frank KM (1969) Skin irritation and sensitization tests on guinea
pigs using a wettable powder formulation (50% benomyl). Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 85-69).
Frank KM (1972) Eye irritation test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 233-72).
Fritz SB (1969) Acute oral ALD test in rabbits using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 109-69).
Fritz SB & Sherman H (1969) Acute oral ALD test in rats using
technical 2-AB (> 95% 2-AB). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 51-69).
Gargus JL & Zoetis T (1983a) Primary irritation study in rabbits
(Benlate PNW). Vienna, Virginia, Hazelton Laboratories, Inc.
(Unpublished report No. HLO 510-83, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983b) Eye irritation test in rabbits (EPA
pesticide registration - Benlate PNW). Vienna, Virginia, Hazelton
Laboratories, Inc. (Unpublished report No. HLO 511-83, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1983c) Acute skin absorption LD50 test on
rabbits (EPA pesticide registration guidelines - Benlate PNW).
Vienna, Virginia, Hazelton Laboratories, Inc. (Unpublished report
No. HLO 512-83, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gargus JL & Zoetis T (1984) Primary skin irritation and
sensitization test on guinea pigs (Benlate PNW). Vienna, Virginia,
Hazelton Laboratories, Inc. (Unpublished report No. HLO 67-84,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Georgieva V, Vachkova R, Tzoneva M, & Kappas A (1990) Genotoxic
activity of benomyl in different test systems. Environ Mol Mutagen,
16: 32-36.
Goldenthal EI (1978) Neurotoxicity study in hens [using technical
benomyl (< 95% benomyl). Mattawan, Michigan, International Research
and Development Corporation (Unpublished report and addendum No. HLO
28-79, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Gooch JJ (1978) Fertility of workers potentially exposed to benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. B/TOX 7).
Goodman NC (1975) Intraperitoneal LD50 test in rats. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 847-74).
Goulding R (1983) Poisoning on the farm. J Soc Occup Med, 33: 60-65.
Guengerich FP (1981) Enzyme induction with Du Pont compounds H11,
202-02 and H10, 962-02. Nashville, Tennessee, Vanderbilt University,
School of Medicine (Unpublished report No. HLO 850-81, prepared for
E.I. Du Pont de Nemours and Co., Inc.).
Gurova AI, Alekseeva NP, Gorlova OE, & Chernyshova RA (1976) [Data
for substantiation of the maximum permissible level of
m-(trifluoromethyl) phenyl isocyanate and butyl isocyanate in the
air of work areas.] Gig Tr Prof Zabol, 3: 53-55 (in Russian).
Gustafson DI (1989) Groundwater ubiquity score: A simple method for
assessing pesticide leachability. Environ Toxicol Chem, 80: 339-357.
Han JCY (1978) Metabolism of 14C-labeled benomyl in the mouse and
hamster. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-65).
Han JCY (1979) 2-14C-Benomyl (50% WP) rat study - intravenous
injection. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Biochemicals Department (Unpublished report No. B/ME-41).
Han JCY (1980) Metabolism of 2-14C-benomyl in the lactating nanny
goat. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Biochemicals Department (Unpublished report No. B/ME-39).
Hardesty PT (1982) Attempts to characterize liver residues from
14C-benomyl dosed goat. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Biochemicals Department (Unpublished report
No. AMR 71-82).
Hardisty JF (1990) Oncogenicity studies with benomyl and MBC in
mice. Peer-review of liver neoplasms. Research Triangle Park, North
Carolina, Experimental Pathology Laboratories, Inc. (Unpublished
report No. 129-012, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Hastie AC (1970) "Benlate"-induced instability of Aspergillus
diploids. Nature (Lond), 226: 771.
Helweg A (1977) Degradation and absorption of carbendazim and
2-aminobenzimidazole in soil. Pestic Sci, 8: 71-78.
Helweg A (1985) Side effects caused by pesticide combinations. In:
Jensen V, Kjoller A, & Sorensen LH ed. Proceedings of the FEMS
Symposium on Microbial Communities in Soil. Amsterdam, Oxford, New
York, Elsevier Science Publishers, pp 385-395.
Hess RA, Moore BJ, Forrer J, Linder RE, & Abuel-Atta AA (1991) The
fungicide benomyl (methyl) 1-(butylcarbomyl)-2-benzimidazole
carbamate causes testicular dysfunction by inducing the sloughing of
germ cells and occlusion of efferent ductules. Fundam Appl Toxicol,
17: 733-745.
Hill EF & Camardese MB (1986) Lethal toxicities of environmental
contaminants and pesticides to Coturnix. Washington, DC, US
Department of the Interior, Fish and Wildlife Service (Fish and
Wildlife Technical Report No. 2).
Hofer I, Beck T, & Wallnöfer P (1971) [The influence of the
fungicide benomyl on soil microflora.] Z Pflanzenkr Pflanzenschutz,
78: 399-405 (in German).
Holden HE, Crider PA, & Wahrenburg MG (1980) Mitotic arrest by
benzimidazole analogs in human lymphocyte cultures. Environ Mutat,
2: 67-73.
Hood DB (1969) Fifteen exposure dermal tests on rabbits using a
wettable powder formulation (50% benomyl). Newark, Delaware, E.I. Du
Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 211-69).
Hoogenboom ER, Ransdell JF, Ellis WG, Kavlock RJ, & Zeman FJ (1991)
Effects on the fetal rat eye of maternal benomyl exposure and
protein malnutrition. Curr Eye Res, 10: 601-612.
Hornberger CS (1969) Acute dust inhalation test in rats using a
wettable powder formulation (50% benomyl) with report on
spermatogenesis effects. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
95-69).
Hostetler KH (1977) Oral LD50 test (Benlate OD). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 527-77).
Hutton DG (1989) Static acute 48-hour EC50 of Benlate fungicide 50
DF to Daphnia magna. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 541-88).
Hutton DG, Kasprzak DJ, & Priester TM (1984) Laboratory studies of
[2-14C] carbendazim bioconcentration in Bluegill sunfish. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 428-84).
ILO (1991) Occupational exposure limits for airborne toxic
substances, 3rd ed. Geneva, International Labour Office
(Occupational Safety and Health Series No. 37).
Ireland CM, Gull K, Gutteridge WE, & Pogson CI (1979) The
interaction of benzimidazole carbamates with mammalian microtubule
protein. Biochem Pharmacol, 28: 2680-2682.
Jessup CD (1979) Acute delayed neurotoxicity study in chickens using
technical benomyl (> 95% benomyl). Mattawan, Michigan,
International Research and Development Corporation (Unpublished
report No. HLO 674-79, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Jessup DC & Dean W (1979) Acute delayed neurotoxicity study in
chickens using technical benomyl (less than 95% benomyl). Mattawan,
Michigan, International Research and Development Corporation
(Unpublished report No. HLO 29-79, prepared for E.I. Du Pont de
Nemours and Co., Inc).
Johnson JD (1988) Determination of the plateau level of bound
[phenyl(U)-14C] carbendazim residues in goat liver. Columbus,
Ohio, Batelle, Columbus Division (Unpublished report No. AMR 779-87,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Kaastra-Howeler LH & Gams W (1973) Preliminary study on the effect
of benomyl on the fungal flora in a greenhouse soil. Neth J Plant
Pathol, 79: 516-518.
Kappas A & Bridges BA (1981) Induction of point mutations by benomyl
in DNA-repair-deficient Aspergillus nidulas. Mutat Res, 91:
115-118.
Kappas A, Georgopoulos SG, & Hastie AC (1974) On the genetic
activity of benzimidazole and thiophanate fungicides on diploid
Aspergillus nidulans. Mutat Res, 26: 17-27.
Kappas A, Green MHL, Bridges BA, Rogers AM, & Muriel WJ (1976)
Benomyl - a novel type of base analogue mutagen? Mutat Res, 40:
379-382.
Kavlock RJ, Chernoff N, Gray LE, Gray JA, & Whitehouse D (1982)
Teratogenic effects of benomyl in the Wistar rat and CD-1 mouse,
with emphasis on the route of administration. Toxicol Appl
Pharmacol, 62: 44-54.
Kelly DP (1989) Butyl isocyanate industrial hygiene survey. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. BENO/TOX 18).
Keogh RG & Whitehead PH (1975) Observations on some effects of
pasture spraying with benomyl and carbendazim on earthworm activity
and litter removal from pasture. NZ J Exp Agric, 3: 103-104.
Kirkland JJ (1973) Method for high-speed liquid chromatographic
analysis of benomyl and/or metabolite residue in cow milk, urine,
feces and tissues. J Agric Food Chem, 21: 171-177.
Kirkland JJ, Holt RF, & Pease HL (1973) Determination of benomyl
residues in soils and plant tissues by high speed cation exchange
liquid chromatography. J Agric Food Chem, 21: 368-371.
Krechniak J & Klosowska B (1986) The fate of 14C-carbendazim in
rat. Xenobiotica, 16(9): 809-815.
Krupka RM (1974) On the anti-cholinesterase activity of benomyl.
Pestic Sci, 5: 211-216.
Kuehne G, Heise H, Plottke B, & Puskeiler T (1985) Dermatitis after
benlate contact. Z Gesamte Hyg Grenzgeb, 31: 710-711.
Lamb MJ & Lilly LJ (1980) An investigation of some genetic
toxicological effects of the fungicide benomyl. Toxicology, 17:
83-95.
Lee KP (1977) The two-year feeding study in rats with benomyl with
supplemental pathology report. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 66-77).
Liesivuori J & Jääskeläinen S (1984) Exposure of greenhouse workers
to pesticides. Tampore, Finland, National Board of Labor Protection,
pp VIII-IX (Research Report No. 46).
Linder RE, Rehnberg GL, Strader LF, & Diggs JP (1988) Evaluation of
reproductive parameters in adult male Wistar Rats after subchronic
exposure. J Toxicol Environ Health, 25: 285-298.
Lisi P, Caraffini S, & Assalve D (1986) A test series for pesticide
dermatitis. Contact Dermatitis, 15: 266-269.
Littlefield NA & Busey WM (1969) Four-hour acute inhalation exposure
test in dogs using a wettable powder formulation (50% benomyl).
Falls Church, Virginia, Hazelton Laboratories, Inc. (Unpublished
report No. HLR 192-69, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983a) L5178Y mouse
lymphoma cell assay for mutagenicity (benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 86-83).
McCooey KT, Arce GT, Sarrif AM, & Krahn DF (1983b) L5178Y mouse
lymphoma cell assay for mutagenicity. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc. (Unpublished report No. HLR 253-83).
Majut JC (1966) Skin irritation and sensitization tests on guinea
pigs using technical benomyl (< 95% benomyl). Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 174-06).
Marsh BH & Arthur MF (1989) Aerobic metabolism of
[phenyl(U)-14C]benomyl in Keyport Silt Loam. Columbus, Ohio,
Battelle Memorial Institute (Unpublished report No. AMR 1112-88,
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Marvin CH, Birndle ID, Hall CD, & Chiba M (1991) Rapid on-line
precolumn high performance liquid chromatographic method for the
determination of benomyl, carbendazim and aldicarb species in
drinking water. J Chromatogr, 555: 147-154.
Matsushita T & Aoyama K (1981) Cross reactions between some
pesticides and the fungicide benomyl in contact allergy. Ind Health,
19: 77-83.
Mayer FL & Ellersieck MR (1986) Manual of acute toxicity:
Interpretation and data base for 410 chemicals and 66 species of
freshwater animals. Washington, DC, US Department of the Interior,
Fish and Wildlife Service, pp 45-56 (Resource Publication No. 160).
Mazur AR & Hughes TD (1975) Nitrogen transformation in soil as
affected by the fungicides benomyl, dyrene and maneb. Agron J, 67:
755-758.
Meade AB (1984) Acute contact LD50 toxicity study in honey bees
( Apis mellifera) with INE 965-212 (MBC). Wilmington, Delaware,
E.I. Du Pont de Nemours and Co., Inc. (Unpublished report No. AMB
84-5).
Mebus CA (1990) Reproductive and fertility effects with DPX-1991-529
(benomyl). Multigeneration reproduction study in rats. Newark,
Delaware, Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 765-90).
Monson KD (1985) Metabolism of [2-14C]benomyl in the lactating
dairy cow. Wilmington, Delaware, E.I. Du Pont de Nemours and Co.,
Inc. (Unpublished report No. AMR 247-84).
Monson KD (1986a) Metabolism of [2-14C]benomyl and [phenyl(U)-
14C]benomyl in laying hens. Wilmington, Delaware, E.I. Du Pont de
Nemours and Co., Inc. (Unpublished report No. AMR 391-85).
Monson KD (1986b) Metabolism of [2-14C]carbendazim laying hens.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 264-84).
Monson KD (1990) Metabolism of [phenyl(U)-14C]carbendazim in rats.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 1141-88).
Monson KD (1991) Release and characterization of bound benomyl and
carbendazim metabolites in animal tissues via Raney nickel
desulfurization and acid dehydration. J Agric Food Chem, 39:
1808-1811.
Nakai M, Hess RA, Moore BJ, Guttroff RF, Strader LF, & Linder RE (in
press) Acute and long-term effects of the fungicide carbendazim
(methyl 2-benzimidazole carbamate, MBC) on the male reproductive
system in the rat. J Androl.
Nehéz M, Palotas M, Zimanyi M, Boros P, Mohos G, Vetro G, & Desi I
(1992) [Repeated cytogenetic examinations of workers using
pesticides in Csongrad County.] Egeszsegtudomany, 36: 40-47 (in
Hungarian).
Newsome WH & Collins PG (1987) Enzyme-linked immunosorbent assay of
benomyl and thiobendazole in some foods. J Assoc Off Anal Chem, 70:
1025-1027.
Newsome WH & Shields JB (1981) A radioimmunoassay for benomyl and
methyl 2-benzimidazolecarbamate on food. J Agric Food Chem, 29:
220-222.
Palawski DU & Knowles CO (1986) Toxicological studies of benomyl and
carbendazim in rainbow trout, channel catfish and bluegills. Environ
Toxicol Chem, 5: 1039-1046.
Parsons DW & Witt JM (1988) Pesticides in groundwater in the United
States of America: A report of a 1988 survey of State lead agencies.
Corvallis, Oregon, Oregon State University Extension Service.
Peeples JL (1974) Microbial activity in benomyl-treated soil.
Phytopathology, 64: 857-860.
Pilinskaya MA (1983) Investigation of the cytogenetic action of the
pesticides captan and benomyl in a culture of human peripheral blood
lymphocytes in the absence and presence of a system of metabolic
activation. Cytol Genet, 17: 29-33.
Powley CR (1985) Aqueous photolysis of [phenyl-14C(U)]benomyl.
Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 420-85).
Priester TM (1984) Hydrolysis of carbendazim [2-14C]. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 265-84).
Priester TM (1985) Batch equilibrium (adsorption/deabsorption) and
soil thin-layer chromatography studies with [phenyl-
14C(U)]benomyl. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 425-85).
Rainaldi G, Flori L, Colella CM, Mariani T, Piras A, Simi S, &
Simili M (1989) Analysis by BrUdR-labelling technique of induced
aneuploidy in mammalian cells in culture. Mutat Res, 177: 255-260.
Reinke RE (1966) Eye irritation test in rabbits using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
81-66).
Rhodes BC (1987) Greenhouse crop-rotation study with
[2-14C]carbendazim. Wilmington, Delaware, E.I. Du Pont de Nemours
and Co., Inc. (Unpublished report No. AMR 495-86).
Rickard LB (1983a) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 97-83).
Rickard LB (1983b) Mutagenicity evaluation in Salmonella
typhimurium. Newark, Delaware, E.I. Du Pont de Nemours and Co.,
Inc., Haskell Laboratory (Unpublished report No. HLR 98-83).
Roberts BL & Dorough HW (1984) Relative toxicities of chemicals to
the earthworm Eisenia foetida. Environ Toxicol Chem, 3: 67-78.
Russell JF (1978a) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 18-78).
Russell JF (1978b) Mutagenic activity of 2-benzimidazolecarbamic
acid, 1-(butylcarbamoyl)-methyl ester in the Salmonella/microsome
assay. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 31-78).
Ruzicska P, Peter S, Laczi J, & Czeizel E (1976) Study of the
chromosome mutagenicity of Fundazol 50 WP. Egeszegtudomany, 20:
74-83.
Ryan DL (1989) Soil column leaching of [phenyl(U)-14C]benomyl in a
rice paddy soil. Wilmington, Delaware, E.I. Du Pont de Nemours and
Co., Inc. (Unpublished report No. AMR 1512-89).
Sandhu SS, Gudi RD, & Athwal RS (1988) A monochromosomal hybrid cell
assay for evaluating the genotoxicity of environmental chemicals.
Cell Biol Toxicol, 4: 495-506.
Sarver JW (1987) Acute oral toxicity study with IN-T1991 in male and
female rats. Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. HLR 334-87).
Sasaki YFX (1990) Benomyl: Micronucleus test in mice. Tokyo,
Institute of Environmental Toxicology, Kodaira Laboratories
(Unpublished report No. IET 89-0046, prepared for E.I. Du Pont de
Nemours and Co., Inc.).
Schafer EW (1972) The acute oral toxicity of 369 pesticidal
pharmaceuticals and other chemicals to wild birds. Toxicol Appl
Pharmacol, 21: 315-330.
Seiler JP (1976) The mutagenicity of benzimidazole and benzimidazole
derivatives. VI. Cytogenetic effects of benzimidazole derivatives in
the bone marrow of the mouse and the Chinese hamster. Mutat Res, 40:
339-348.
Sherman H (1968) Three-month feeding study in dogs using a wettable
powder formulation (50% benomyl). Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 269-68).
Sherman H (1969a) Acute oral LD50 test in rats using technical
benomyl (> 95% benomyl) and a wettable powder formulation (50%
benomyl). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 17-69).
Sherman H (1969b) Acute oral ALD test in a dog using technical
benomyl (> 95% benomyl). Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
168-69).
Sherman H (1969c) Long term feeding study in rats with
1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 232-69).
Sherman H (1970) Long-term feeding study in dogs with
1-butycarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell Laboratory (Unpublished report No. HLR 48-70).
Sherman H (1972) Long-term feeding studies in rats and dogs with
2-benzimadazolecarbamic acid, methyl ester (INE-965) (50% and 70%
MBC wettable powder formulations): Parts I and II. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 195-72).
Sherman H & Krauss WC (1966) Acute oral test [benomyl]. Newark,
Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 100-66).
Sherman H, Barnes JR, & Krauss WC (1967) Ninety-day feeding study
with 1-butylcarbamoyl-2-benzimidazolecarbamic acid, methyl ester
(INT-1991). Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc.,
Haskell, Laboratory (Unpublished report No. HLR 11-67).
Sherman H, Culik R, & Jackson RA (1975) Reproduction, teratogenic
and mutagenic studies with benomyl. Toxicol Appl Pharmacol, 32:
305-315.
Shirasu Y, Moriya M, & Kato K (1978) Mutagenicity testing on
fungicide 1991 in microbial systems. Tokyo, Institute of
Environmental Toxicology, Kodaira Laboratories (Unpublished report
prepared for E.I. Du Pont de Nemours and Co., Inc.).
Shukla Y, Antony M, & Mehrota NK (1989) Studies on gamma-glutamyl
transpeptidase in rodents exposed to benomyl. Bull Environ Contam
Toxicol, 42: 301-306.
Siebert D, Zimmermann FK, & Lemperle E (1970) Genetic effects of
fungicides. Mutat Res, 10: 533-543.
Siegel MR (1975) Benomyl-soil microbial interactions.
Phytopathology, 65: 219-220.
Snee DA (1969) Acute oral ALD test in rats and ten-dose subacute
oral test in rats using technical 5-HBC (> 95% 5-HBC) (with
pathology on the acute oral ALD test described in HLR 43-69).
Newark, Delaware, E.I. Du Pont de Nemours and Co., Inc., Haskell
Laboratory (Unpublished report No. 134-69).
Stahl RG Jr (1990) In vivo evaluation of INT-1991-259 for
chromosome aberrations in mouse bone marrow. Newark, Delaware, E.I.
Du Pont de Nemours and Co., Inc., Haskell Laboratory (Unpublished
report No. HLR 401-90).
Staples RE (1980) Teratogenicity study in the rat after
administration by gavage of technical benomyl (> 95% benomyl):
Parts I, II and III. Newark, Delaware, E.I. Du Pont de Nemours and
Co., Inc., Haskell Laboratory (Unpublished report No. HLR 649-80).
Staples RE (1982) Teratogenicity study in the rat using technical
benomyl (>95% benomyl) administered by gavage and supplement with
individual animal data. Newark, Delaware, E.I. Du Pont de Nemours
and Co., Inc., Haskell Laboratory (Unpublished report No. HLR
587-82).
Staub T & Sozzi D (1984) Fungicide resistance: A continuing
challenge. Plant Dis, 68: 1026-1031.
Stevenson IE (1985) Metabolism of [phenyl(U)-14C]benomyl in
peaches. Wilmington, Delaware, E.I. Du Pont de Nemours and Co., Inc.
(Unpublished report No. AMR 443-85).
Stringer A & Lyons C (1974) The effect of benomyl and
thiophanate-methyl on earthworm populations in apple orchards.
Pestic Sci, 5: 189-196.
Stringer A & Wright MA (1973) The effect of benomyl and some related
compounds on Lumbricus terrestris and other earthworms. Pestic
Sci, 4: 165-170.
Tolle DA (1988) Metabolism of [phenyl(U)-14C] benomyl in sugar
beets. West Jefferson, Ohio, Battelle Columbus DW (Unpublished
report No. AMR 620-86, prepared for E.I. Du Pont de Nemours and Co.,
Inc.).
Tomlin AD & Gore FL (1974) Effects of six insecticides and a
fungicide on the numbers and biomass of earthworms in pasture. Bull
Environ Contam Toxicol, 12: 487-492.
Tomlin AD, Tolman JH, & Thorn GD (1980/1981) Suppression of
earthworm ( Lumbricus terrestris) populations around an airport by
soil application of the fungicide benomyl. Prot Ecol, 2: 319-323.
Tong C (1981) Hepatocyte primary culture/DNA repair assay on
compound 10, 962-02 (benomyl) using mouse hepatocytes in culture.
Valhalla, New York, Naylor Dana Institute (Unpublished report No.
HLO 741-81, prepared for E.I. Du Pont de Nemours and Co., Inc.).
Torstensson L & Wessen B (1984) Interactions between the fungicide
benomyl and soil microorganisms. Soil Biol Biochem, 16: 445-452.
Turney RT (1979) Rat inhalation study - Benlate. Newark, Delaware,
E.I. Du Pont de Nemours and Co., Inc., Haskell Laboratory
(Unpublished report No. HLR 116-79).
Van Faassen HG (1974) Effect of the fungicide benomyl on some
metabolic processes and on numbers of bacteria and actinomycetes in
the soil. Soil Biol Biochem, 6: 131-133. Van Gestel CAM (1992)
Validation of earthworm toxicity tests by comparison with field
studies: A review of benomyl, carbendazim, carbofuran, and carbaryl.
Ecotoxicol Environ Saf, 23: 221-236.
Van Gestel CAM, Dirven-van Breeman EM, Baerselman R, Emans HJB,
Janssen JAM, Postuma R, & van Vliet PJM (1992) Comparison of
sublethal and lethal criteria for nine different chemicals in
standardized toxicity tests using the earthworm Eisenia andrei.
Ecotoxicol Environ Saf, 23: 206-220.
Van Joost TH, Naafs B, & van Ketel WG (1983) Sensitization to
benomyl and related pesticides. Contact Dermatitis, 9: 153-154.
Vick DA & Brock WJ (1987) Primary dermal irritation study with
benlate 50 DF fungicide in rabbits. Newark, Delaware, E.I. Du Pont
de Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 300-87).
Wainwright M & Pugh GJF (1974) The effect of fungicides on certain
chemical and microbial properties of soils. Soil Biol Biochem, 6:
263-267.
Ward RS & Scott RC (1992) Benomyl: in vitro absorption of a 500 g
kg-1 WP formulation through human epidermis. Fernhurst, Naslemere,
Surrey, Imperial Chemical Industries (ICI) (Unpublished report No.
CTL/P/3659).
Warheit DB, Kelly DP, Carakostas MC, & Singer AW (1989) A 90-day
inhalation toxicity study with benomyl in rats. Fundam Appl Toxicol,
12: 333-345.
Weeks RE & Hedrick HG (1975) Influence of a systemic fungicide on
oxygen uptake by soil microorganisms. Soil Sci, 119: 280-284.
Wheeler J (1985) Hydrolysis of [phenyl-14C(U)]benomyl. Wilmington,
Delaware, E.I. Du Pont de Nemours and Co., Inc. (Unpublished report
No. AMR 419-85).
WHO (1993) Environmental Health Criteria 149: Carbendazim. Geneva,
World Health Organization.
Wiechman BE (1982) Long term feeding study with methyl
l-(butylcarbamoyl)-2-benzimidazolecarbamate in mice (INT-1991; >95%
benomyl): Parts I, II and III. Newark, Delaware, E.I. Du Pont de
Nemours and Co., Inc., Haskell Laboratory (Unpublished report No.
HLR 20-82).
Yoshida K & Nishiuchi Y (1972) Toxicity of pesticides to some water
organisms. Bull Agric Chem Insp Stn, 12: 122-128.
Zbozinek JV (1984) Environmental transformations of DPA, SOPP,
benomyl and TBZ. Residue Rev, 92: 113-155.
Zelesco PA, Barbieri I, & Graves JAM (1990) Use of a cell hybrid
test system to demonstrate that benomyl induces aneuploidy and
polyploidy. Mutat Res, 242: 329-335.
Zeman FJ, Hoogenboom ER, Kavlock RJ, & Semple JL (1986) Effects on
the fetus of maternal benomyl exposure in the protein-deprived rat.
J Toxicol Environ Health, 17: 405-417.
Zoran MJ, Heppner TJ, & Drewes CD (1986) Teratogenic effects of the
fungicide benomyl on posterior segmental regeneration in the
earthworm, Eisenia fetida. Pestic Sci, 17: 641-652.
Zweig G, Gao R, & Popendorf W (1983) Simultaneous dermal exposure to
captan and benomyl by strawberry harvesters. J Agric Food Chem, 31:
1109-1113.
RESUME ET CONCLUSIONS
1. Résumé
1.1 Identité, propriétés physiques et chimiques et méthodes
d'analyse
Le bénomyl, un solide cristallin de couleur ambrée, est un
fongicide endothérapique qui appartient à la famille du
benzimidazole. Il se décompose juste au-dessus de son point de
fusion de 140 °C et sa tension de vapeur est < 5 x 10-6 Pa
(< 3,7 x 10-8 mmHg) à 25 °C. Le bénomyl est pratiquement
insoluble dans l'eau à pH 5 et à 25 °C, sa solubilité étant de 3,6
mg/litre. Il est stable dans les conditions normales de stockage
mais se décompose en carbendazime dans l'eau.
L'analyse des résidus de même que celle des prélèvements
effectués dans l'environnement comporte une extraction au moyen d'un
solvant organique, une purification de l'extrait par partage
liquide-liquide et la transformation du résidu obtenu en
carbendazime. Le dosage de ces résidus peut s'effectuer par
chromatographie en phase liquide à haute performance ou par titrage
immunologique.
1.2 Sources d'exposition humaine et environnementale
En 1988, on estimait à environ 1700 tonnes la quantité de
bénomyl utilisée dans le monde. Il s'agit d'un fongicide très
largement utilisé, homologué dans 50 pays pour le traitement de plus
de 70 cultures. Le bénomyl est présenté sous forme de poudre
mouillable.
1.3 Transport, distribution et transformation dans l'environnement
Le bénomyl se transforme rapidement en carbendazime dans
l'environnement avec une demi-vie respective de 2 et 19 heures dans
l'eau et le sol. On peut donc utiliser aussi bien les résultats des
études sur le bénomyl que sur le carbendazime pour l'évaluation des
effets sur l'environnement.
Dans l'environnement, le carbendazime se décompose avec une
demi-vie de 6 à 12 mois sur le sol nu, de 3 à 6 mois sur le gazon et
de 2 à 25 mois dans l'eau en aérobiose et en anaérobiose,
respectivement.
Le carbendazime est principalement décomposé par les
microorganismes. Le 2-aminobenzimidazole (2-AB) en est l'un des
principaux produits de dégradation et il est à son tour décomposé
par les microorganismes.
Lors de la décomposition de bénomyl marqué au 14C sur le
noyau phényle, on a constaté que 9% seulement du carbone-14 étaient
éliminés sous forme de CO2 en une année d'incubation. Le
carbone-14 restant était principalement récupéré sous forme de
carbendazime et de résidus liés. L'étude de la destinée d'un
éventuel produit de dégradation (1,2-diaminobenzène) pourrait
peut-être permettre de mieux définir la voie de dégradation des
fongicides benzymidazoliques dans l'environnement.
Des études effectuées sur le terrain ou sur colonnes ont montré
que le carbendazime restait dans les couches superficielles du sol.
On n'a pas mesuré l'adsorption du carbendazime dans le sol, mais on
pense qu'elle doit être aussi forte que dans le cas du bénomyl, avec
des valeurs de Koc allant de 1000 à 3600. Les valeurs de log Kow
sont respectivement de 1,36 et de 1,49 pour le bénomyl et le
carbendazime.
Un modèle de criblage basé sur les données d'adsorption et de
persistance n'a pas révélé de risque de lessivage. Cette observation
est corroborée par des analyses d'eau de puits effectuées aux
Etats-Unis, analyses qui n'ont pas permis de déceler ce composé dans
l'un quelconque des 495 puits étudiés ni le carbendazime dans 212
autres (la limite de détection n'a pas été précisée). On estime que
le bénomyl et le carbendazime entraînés par ruissellement
correspondent uniquement à la fraction adsorbée aux particules de
sol; d'ailleurs ces composés sont sans doute fortement adsorbés aux
sédiments présents dans l'environnement aquatique.
En solution, dans les végétaux et le sol, le bénomyl subit une
décomposition en carbendazime (méthyl-1H-benzimidazole-2-carbamate)
en 2-AB, STB (3-butyl-1,3,5-triazino[1,2a]-benzimidazole-
2,4(1H,3H)dione) ainsi qu'en BBU (1-(2-benzimidazolyl)-3-n-
butylurée). La photolyse du bénomyl est pratiquement inexistante.
Chez l'animal, le bénomyl est métabolisé en carbendazime ainsi
qu'en d'autres métabolites polaires qui sont rapidement excrétés. On
n'a pas observé d'accumulation de bénomyl ni de carbendazime dans
aucun système biologique.
1.4 Concentrations dans l'environnement et exposition humaine
Il ne semble pas qu'il existe de données résultant d'une
surveillance du bénomyl dans l'environnement. Toutefois on peut
récapituler ainsi les données tirées d'études portant sur la
destinée de ce produit dans l'environnement.
Comme le bénomyl et le carbendazime restent stables pendant des
semaines sur les végétaux, ils peuvent être ingérés par des
organismes qui se nourrissent de feuilles mortes. Des résidus de
carbendazime peuvent subsister jusqu'à 3 ans dans le sol et les
sédiments. Toutefois, la forte adsorption du carbendazime aux
particules de sol et de sédiments réduit l'exposition des organismes
terrestres et aquatiques.
Ce sont les résidus de bénomyl et de carbendazime présents sur
les cultures vivrières qui constituent la principale source
d'exposition de la population humaine dans son ensemble. L'analyse
de l'exposition par voie alimentaire qui a été effectuée aux
Etats-Unis (bénomyl et carbendazime associés) et aux Pays-Bas
(carbendazime seul) a montré que la quantité moyenne ingérée était
vraisemblablement de l'ordre d'un dixième de la dose journalière
acceptable (DJA) qui est, pour le bénomyl de 0,02 mg/kg de poids
corporel, et pour le carbendazime de 0,01 mg/kg de poids corporel.
L'exposition professionnelle au cours de la production est
inférieure à la valeur-seuil. Les ouvriers agricoles qui préparent
les mélanges, effectuent le remplissage, ou retournent dans les
champs traités par du bénomyl, courent un risque d'exposition
cutanée de quelques mg de bénomyl à l'heure. Le port de dispositifs
de protection permettrait de réduire encore cette exposition. En
outre, étant donné que l'absorption percutanée est vraisemblablement
faible, il est très peu probable que le bénomyl puisse avoir des
effets toxiques généraux sur les populations humaines en étant
absorbé par cette voie.
1.5 Cinétique et métabolisme
Après exposition par voie orale ou respiratoire, le bénomyl est
rapidement absorbé par l'organisme animal mais cette absorption est
bien moindre après exposition par voie cutanée. Une fois absorbé, le
bénomyl est rapidement métabolisé, puis excrété dans les urines et
les matières fécales. Après avoir administré du bénomyl marqué au
carbone-14 à des rats, on a retrouvé dans le sang, et en petites
quantités dans les testicules, les reins et le foie, deux de ses
métabolites, le carbendazime et le carbamate de méthyl-5-hydroxy-1H-
benzimidazole-2-yle (5-HBC). La distribution tissulaire des composés
n'était pas révélatrice d'une bioconcentration. Dans l'urine, le
principal métabolite était le 5-HBC à côté d'un peu de carbendazime.
Dans les 72 heures suivant l'administration, 98% de la quantité
administrée avaient été excrétés. Chez des vaches recevant pendant 5
jours des capsules contenant du bénomyl radiomarqué à raison de 50
mg/kg de nourriture, les concentrations équivalentes de bénomyl
retrouvées dans les divers organes étaient de 4 mg/kg dans le foie,
et de 0,25 mg/kg dans les reins; dans les autres tissus et les
graisses, les valeurs n'étaient pas significatives. Pendant la
période d'administration, 65% du composé radiomarqué ont été
excrétés dans les urines, 21% dans les matières fécales et 0,4% dans
le lait. Le principal métabolite présent dans le lait était le
5-HBC. Chez d'autres animaux, on a constaté un métabolisme et des
modalités d'élimination similaires.
Le bénomyl n'inhibe pas l'acétylcholinestérase in vitro. On a
montré qu'il induisait l'époxyhydrolase hépatique, la gamma-
glutamyle transpeptidase ainsi que la glutation-S-transférase chez
la souris et le rat in vivo.
1.6 Effets sur les mammifères de laboratoire et sur les systèmes
d'épreuve in vitro
1.6.1 Exposition unique
Le bénomyl présente une faible toxicité aiguë, la DL50 par
voie orale chez le rat étant > 10 000 mg/kg, et la CL50 à 4 h par
voie respiratoire étant > 4 mg/litre. Des chiens exposés par voie
respiratoire pendant 4 heures à la dose de 1,65 mg/litre et examinés
28 jours après l'exposition, présentaient une diminution du poids du
foie. Une dose unique administrée à des rats par gavage a entraîné
des effets sur la reproduction 70 jours après l'exposition (voir
Section 1.6.5).
1.6.2 Exposition de brève durée
Administré par gavage pendant de brèves périodes allant jusqu'à
90 jours, en mélange à la nourriture ou en application cutanée, le
bénomyl a légèrement augmenté le poids du foie chez le rat (à la
dose quotidienne de 125 mg/kg, en mélange à la nourriture) et a
produit des effets sur les organes reproducteurs mâles chez le rat:
diminution du poids des testicules et de l'épididyme, réduction de
la spermatogenèse (dose quotidienne: 45 mg/kg, par gavage, dose sans
effet observable = 15 mg/kg), chez le lapin (dose quotidienne: 1000
mg/kg, par voie orale; 500 mg/kg, en appli cation cutanée) et chez
le chien beagle (62,5 mg/kg, dose sans effet observable = 18,4 mg/kg
par jour, en mélange à la nourriture). Chez les rats exposés par la
voie respiratoire à du bénomyl à des concentrations allant jusqu'à
200 mg/m3 pendant 90 jours, on n'a pas observé d'effets sur le
foie ni les testicules.
1.6.3 Irritation et sensibilisation au niveau de la peau et
des yeux
L'application de bénomyl sur l'épiderme de lapins ou de cobayes
n'a produit que peu ou pas d'irritation et une sensibilisation
modérée de la peau. Chez le rat, l'instillation oculaire a produit
une irritation légère et temporaire de la conjonctive.
1.6.4 Exposition de longue durée
A l'issue d'une étude prolongée d'alimentation chez des rats,
on n'a pas pu mettre en évidence d'effets imputables à ce composé à
des doses allant jusqu'à 2500 mg/kg de nourriture (soit 125 mg/kg de
poids corporel par jour). Cette étude n'a pas été jugée suffi sante
pour permettre une évaluation des effets sur la reproduction. Chez
la souris CD-1, le poids du foie était en augmentation à partir de
la dose de 1500 mg/kg de nourriture. A la dose de 5000 mg/kg de
nourriture, on notait chez les souris mâles une réduction du poids
absolu des testicules et une atrophie du thymus.
1.6.5 Reproduction, embryotoxicité et tératogénicité
Le bénomyl détermine une diminution du poids des testicules et
de l'épidydime, avec réduction des réserves de spermatozoïdes au
niveau de la queue de l'épidydime, une diminution de la production
des spermatozoïdes et une réduction de la fécondité des mâles. A
plus fortes doses, il y a diminution de la spermatogénèse qui est
perturbée à tous ses stades. Le bénomyl n'affecte pas le
comportement copulatoire, les vésicules séminales, la mobilité des
spermatozoïdes ni les hormones sexuelles correspondantes. La
concentration de bénomyl la plus faible qui produise un effet
statistiquement significatif sur la spermatogénèse du rat mâle a été
chiffrée à 45 mg/kg par jour. La dose sans effet observable pour ces
effets est de 15 mg/kg par jour.
Une seule dose de bénomyl (100 mg/kg ou davantage) administrée
par gavage à des rats a produit, 70 jours après l'exposition, des
effets consistant notamment en une réduction du poids des testicules
et une atrophie des tubes séminifères.
Administré par gavage à des rats ChD-CD et Wistar du 7e au
16e jour de la gestation, le bénomyl s'est révélé tératogène pour
les 2 souches à 62,5 mg/kg, mais pas à 30 mg/kg pour les ChR-CD, ni
à 31,2 mg/kg pour les Wistar. Administré par gavage à des rats
Sprague-Dawley du 7e au 21e jour de gestation, le bénomyl s'est
révélé tératogène à 31,2 mg/kg. Les effets observés étaient une
microphthalmie, une hydrocéphalie et une encéphalocèle. Le
développement post-natal des rats était également perturbé aux doses
supérieures à 15,6 mg/kg.
Chez la souris, l'administration par gavage à des
concentrations supérieures ou égales à 50 mg/kg a provoqué
l'apparition de côtes surnuméraires et autres anomalies du squelette
et des viscères. On n'a pas établi, chez la souris, la valeur de la
dose sans effet observable car les doses administrées étaient toutes
supérieures à 50 mg/kg. A part une augmentation marginale de la
fréquence des côtes surnuméraires chez le lapin, on n'a pas observé,
chez cet animal, d'effets tératogènes à des doses atteignant 500
mg/kg de nourriture.
1.6.6 Mutagénicité et autres points d'aboutissement des effets
toxiques
L'étude des cellules somatiques et germinales montre que le
bénomyl n'entraîne pas de mutations géniques ni d'anomalies
structurales chromosomiques et qu'il n'y a pas non plus
d'interaction directe avec l'ADN (qui provoquerait des lésions de
l'ADN et leur réparation). Ces faits ont été mis en évidence à la
fois sur des cellules mammaliennes et non mammaliennes.
Cependant le bénomyl provoque des aberrations dans le nombre
des chromosomes (aneuploïdie et/ou polyploïdie) dans les systèmes
d'épreuve (tant in vitro qu' in vivo).
1.6.7 Cancérogénicité
Chez les souris CD-1 et Swiss axéniques, qui présentent un taux
important de tumeurs spontanées du foie, on a observé ce type de
tumeurs après administration de bénomyl ou de carbendazime. En
revanche, le carbendazime ne s'est pas révélé cancérogène chez les
souris NMRKf, qui n'ont qu'un faible taux de tumeurs hépatiques
spontanées.
La première étude de cancérogénicité portant sur des souris
CD-1 a fait ressortir l'existence d'une augmentation liée à la dose
et statistiquement significative des néoplasmes hépatocellulaires
chez les femelles et l'on a également observé chez les mâles, aux
doses moyennes (1500 mg/kg), une réaction statistiquement
significative qui ne s'observait plus aux doses élevées en raison
d'un fort taux de mortalité. Une deuxième étude de cancérogénicité
portant sur le carbendazime a été effectuée chez une souche
génétiquement apparentée de souris Swiss axéniques et exogames à des
doses de 0, 150, 300 et 1000 mg/kg (la dernière dose étant portée à
5000 mg/kg au cours de l'étude); elle a révélé un accroissement dans
l'incidence de l'ensemble des adénomes et carcinomes
hépatocellulaires. Une troisième étude effectuée cette fois sur des
souris NMRKf à des doses 0, 50, 150, 300 et 1000 mg/kg (portées
ensuite à 5000 mg/kg) n'a pas fait ressortir d'effets cancérogènes.
Les études de cancérogénicité portant sur le bénomyl et le
carbendazime ont donné des résultats négatifs chez le rat.
1.6.8 Mécanisme de la toxicité - mode d'action
On pense que les effets biologiques du bénomyl et du
carbendazime résultent de leur interaction avec les microtubules
cellulaires. Ces structures interviennent dans des fonctions aussi
importantes que la division cellulaire, qui est inhibée par ces deux
substances. La toxicité du bénomyl et du carbendazime pour les
mammifères est donc liée à une perturbation des fonctions du système
microtubulaire.
Comme les autres dérivés du benzimidazole, le bénomyl et le
carbendazime sont plus ou moins toxiques selon les espèces. Cette
sélectivité toxicologique s'explique au moins en partie par le fait
que le bénomyl et le carbendazime ne se lient pas de la même manière
aux tubulines des espèces visées et des espèces non visées.
1.7 Effets sur l'homme
Le bénomyl provoque des dermatites de contact ainsi qu'une
sensibilisation cutanée. On n'a pas fait état d'autres effets.
1.8 Effets sur les autres êtres vivant au laboratoire et dans
leur milieu naturel
Le bénomyl n'a guère d'effet sur l'activité microbienne du sol
aux doses d'emploi recommandées. On a cependant signalé l'existence
d'effets nocifs vis-à-vis de certains groupes de champignons.
On a calculé que la CE50 à 72 heures, fondée la croissance
totale, pour les algues bleu-vert du genre Selenastrum
capricornutum, était égale à 2,0 mg/litre; la concentration sans
effet observable était de 0,5 mg/litre. La toxicité du bénomyl pour
les invertébrés aquatiques et les poissons varie dans de larges
proportions, les valeurs de la CL50 à 96 heures allant de 0,006
mg/litre pour des poissons-chats du genre Ictalurus (alevins
porteurs de leur sac vitellin) à plus de 100 mg/litre pour les
écrevisses.
Le bénomyl s'est révélé toxique pour les lombrics lors
d'expériences de laboratoire qui reproduisaient les conditions
réelles d'exposition résultant de l'utilisation recommandée sur le
terrain. Il est peu toxique pour les oiseaux et son produit de
dégradation, le carbendazime, est "relativement non toxique" pour
les abeilles.
2. Conclusions
Le bénomyl provoque une sensibilisation cutanée chez l'homme.
Le bénomyl et le carbendazime ne font courir à l'homme qu'un très
faible risque d'intoxication aiguë. Etant donné les conditions
actuelles d'exposition et le faible taux d'absorption percutanée de
ces deux composés, il est improbable qu'ils entraînent des effets
toxiques généraux dans la population ou chez les personnes exposées
de par leur profession. Ces conclusions sont tirées de données
relatives à l'animal et, dans une moindre mesure, à l'homme; elles
reposent sur la connaissance du mode d'action du carbendazime et du
bénomyl, tant chez les espèces visées que chez les espèces non
visées.
Grâce à une meilleure connaissance du mécanisme de la toxicité
du bénomyl et du carbendazime pour les mammifères, on pourra
peut-être mieux définir les doses sans effet observable. Des études
portant sur la liaison de ces composés aux tubulines des cellules
cibles (tissus testiculaires et embryonnaires) faciliteront sans
doute les comparaisons interspécifiques.
Le carbendazime est fortement adsorbé aux matières organiques
du sol et il y persiste pendant des périodes pouvant atteindre 3
ans. Il persiste également à la surface des feuilles et se retrouve
par conséquent dans les feuilles mortes. On a montré que les
lombrics pouvaient souffrir (dans leur population et dans leur
reproduction) de ces composés aux doses d'emploi recommandées. On ne
possède aucun renseignement sur les autres arthropodes qui vivent
dans le sol ou les débris organiques et qui pourraient être exposés
de la même manière.
Il est improbable que la forte toxicité vis-à-vis des
organismes aquatiques révélée par les épreuves de laboratoire
s'observe également dans le milieu naturel, du fait de la faible
biodisponi-bilité des résidus de carbendazime liés aux sédiments.
Toutefois on ne possède aucune donnée sur les espèces vivant sur les
sédiments et qui seraient donc les plus exposées.
RESUMEN Y CONCLUSIONES
1. Resumen
1.1 Identidad, propiedades físicas y químicas y métodos analíticos
El benomilo es un sólido cristalino de color tostado y acción
fungicida sistémica que pertenece a la familia del bencimidazol. Se
descompone a una temperatura apenas superior a 140 °C,
correspondiente a su punto de fusión, y su presión de vapor a 25 °C
es < 5 x 10-6 Pa (< 3,7 x 10-8 mmHg). Prácticamente es
insoluble en agua a pH 5 y 25 °C, siendo su solubilidad 3,6
mg/litro. Es un compuesto estable en condiciones de almacenamiento
normales, pero en agua se descompone y forma carbendazima.
Los análisis de las muestras procedentes de residuos y del
medio ambiente se realizan mediante extracción con un disolvente
orgánico, purificación del extracto obtenido utilizando un
procedimiento de reparto líquido-líquido y conversión del residuo en
carbendazima. La valoración de los residuos se puede realizar
mediante cromatografía líquida de alto rendimiento o inmunoensayo.
1.2 Fuentes de exposición humana y ambiental
En 1988, el uso mundial estimado de benomilo fue unas 1700
toneladas. Es un fungicida muy utilizado, que se encuentra
registrado en 50 países en los que se permite su uso en más de 70
cultivos. El benomilo está formulado como polvo humectable.
1.3 Transporte, distribución y transformación en el medio ambiente
En el medio ambiente, el benomilo se transforma rápidamente en
carbendazima con una semivida de 2 y 19 h en el agua y el suelo
respectivamente. Por consiguiente, para la evaluación de los efectos
sobre el medio ambiente son importantes los datos obtenidos de los
estudios realizados con ambos compuestos.
La carbendazima se descompone en el medio ambiente con una
semivida de 6 a 12 meses en el suelo desnudo, de 3 a 6 meses en el
césped y de 2 y 25 meses en el agua en condiciones aerobias y
anaerobias respectivamente.
La carbendazima se descompone principalmente por acción de los
microorganismos. Un producto importante de su degradación es el
2-aminobencimidazol (2-AB), que luego se descompone de nuevo por la
actividad microbiana.
En la descomposición del benomilo marcado con un grupo fenilo
con 14C, sólo el 9% del 14C formó CO2 durante un año de
incubación. El resto del 14C se recuperó sobre todo como
carbendazima y en productos unidos a residuos. El destino de un
posible producto de degradación (1,2-diaminobenceno) puede aclarar
ulteriormente la vía de degradación de los fungicidas
bencimidazólicos en el medio ambiente.
En estudios de campo y de columna se ha puesto de manifiesto
que la carbendazima queda retenida en la capa superficial del suelo.
Aunque no se dispone de datos sobre su adsorción en el suelo, se
considera que ésta puede ser tan intensa como la del benomilo, con
valores de Koc que oscilan entre 1000 y 3600. Los valores del log
Kow para el benomilo y la carbendazima son respectivamente 1,36 y
1,49.
En la evaluación realizada en un modelo de selección, basado en
datos de adsorción y persistencia, se puso de manifiesto que no
había riesgo de lixiviación. En los Estados Unidos se han efectuado
análisis de agua de pozos que confirman esto, puesto que no se
encontraron trazas de benomilo en ninguno de los 495 pozos
muestreados ni se detectó carbendazima en ninguno de los 212 (no se
dispone del límite de detección). Es de suponer que la escorrentía
superficial de ambos compuestos se deba solamente al fungicida
adsorbido en las partículas del suelo y que en el medio acuoso estén
fuertemente adsorbidos en los sedimentos.
En solución, en las plantas y en el suelo, el benomilo se
degrada a carbendazima (1H-bencimidazol-2-carbamato de metilo) y a
2-AB, STB (3-butil-1,3,5-triazino[1,2a]-bencimidazol-2,4(1H,3H)
diona) y BBU (1-(2-bencimidazolil)-3-n-butilurea). La fotolisis del
benomilo es escasa o nula.
En los animales, el benomilo se descompone formando
carbendazima y otros metabolitos polares, que se excretan
rápidamente. No se ha observado que el benomilo o la carbendazima se
acumulen en ningún sistema biológico.
1.4 Niveles medioambientales y exposición humana
No parece que se disponga de datos de vigilancia ambiental para
el benomilo. Sin embargo, los estudios realizados sobre su destino
en el medio ambiente pueden resumirse como sigue.
Puesto que el benomilo y la carbendazima se mantienen estables
en las plantas durante varias semanas, pueden pasar a los organismos
que se alimentan de las hojas caídas. El suelo y los sedimentos
pueden conservar residuos de carbendazima hasta tres años. Sin
embargo, la fuerte adsorción de este compuesto en las partículas del
suelo y en los sedimentos reduce la exposición de los organismos
terrestres y acuáticos.
La principal fuente de exposición para la población humana
general se debe a los residuos de benomilo y carbendazima en los
cultivos alimentarios. En análisis de la exposición a través de los
sedimentos realizados en los Estados Unidos (benomilo y carbendazima
combinados) y en los Países Bajos (con carbendazima) se obtuvo una
ingesta media prevista de alrededor del 10 por ciento de la ingesta
diaria admisible (IDA) recomendada, de 0,02 mg/kg de peso corporal
para el benomilo y de 0,01 mg/kg de peso corporal para la
carbendazima.
La exposición profesional durante el proceso de fabricación es
inferior al valor umbral límite. Se considera que los trabajadores
agrícolas que se ocupan de mezclar y cargar los plaguicidas o que
entran en campos tratados con benomilo sufren exposición cutánea a
unos mg de benomilo por hora. Este forma de exposición se podría
reducir con algún tipo de protección. Por otra parte, puesto que la
absorción cutánea prevista es baja, la probabilidad de que el
benomilo tenga efectos tóxicos sistémicos sobre la población humana
a través de esta vía es muy escasa.
1.5 Cinética y metabolismo
En experimentos con animales se ha puesto de manifiesto que
éstos absorben fácilmente el benomilo tras la exposición oral y
respiratoria, pero mucho menos después de una exposición cutánea. El
benomilo absorbido se metaboliza rápidamente y se excreta en la
orina y las heces. En ratas alimentadas con benomilo marcado con
14C, se encontraron en la sangre, y en pequeña cantidad en los
testículos, los riñones y el hígado, los metabolitos carbendazima y
(5-hidroxi-1H-bencimidazol-2-il)-carbamato de metilo (5-HBC). La
distribución en los tejidos demostraba la ausencia de
bioconcentración. El metabolito principal en la orina era el 5-HBC,
acompañado de una pequeña cantidad de carbendazima. A las 72 h de la
administración ya se había excretado el 98% de la cantidad
suministrada. En vacas tratadas durante 5 días con cápsulas con
benomilo marcado, en dosis equivalentes a una alimentación de 50
mg/kg, se detectó en el hígado una concentración de esta sustancia
equivalente a 4 mg/kg, en los riñones 0,25 mg/kg y en el tejido
adiposo o en otros niveles no significativos. Cuando se administró
con los alimentos, el 65% del compuesto marcado se excretó en la
orina, el 21% en las heces y el 0,4% en la leche. El principal
metabolito en la leche fue el 5-HBC. El metabolismo y los sistemas
de eliminación fueron semejante en otros animales.
El benomilo no inhibe la acetilcolinesterasa in vitro. Se ha
demostrado en estudios in vivo en ratas y ratones que induce la
epoxihidrolasa hepática, la gamma-glutamil transpeptidasa y la
glutatión-S-transferasa.
1.6 Efectos en los mamíferos de laboratorio y en sistemas
de prueba in vitro
1.6.1 Exposición única
El benomilo tiene una toxicidad aguda baja, con una DL50 por
vía oral en ratas de > 10 000 mg/kg y una CL50 por inhalación
durante 4 h de > 4 mg/litro. La carbendazima, al igual que la
sustancia de la que se deriva, tiene una DL50 en ratas de > 10
000 mg/kg. Los perros, expuestos por inhalación a 1,65 mg/litro
durante 4 h y examinados 28 días después, mostraron una dismi nución
del peso del hígado. La administración por sonda de una sola dosis
de benomilo a ratas mostró efectos en la reproducción a los 70 días
de la exposición (véase el apartado 1.6.5).
1.6.2 Exposición breve
La administración de benomilo mediante sonda, en los alimentos
o por exposición cutánea durante un período máximo de 90 días en las
ratas aumentó ligeramente el peso del hígado (125 mg/kg al día, con
los alimentos) y tuvo efectos sobre los órganos reproductores
masculinos (disminución del peso de los testículos y los epidídimos,
y reducción de la espermatogénesis) en las ratas (45 mg/kg al día,
administrado por sonda; nivel sin efecto observado (NOEL) =
15mg/kg), los conejos (1000 mg/kg al día, por vía oral; 500 mg/kg de
peso corporal al día, por vía cutánea) y los perros (62,5 mg/kg;
NOEL = 18,4 mg/kg al día, con los alimentos). No se observaron
efectos hepáticos ni testiculares en la ratas expuestas por
inhalación a concentraciones de benomilo de hasta 200 mg/m3
durante 90 días.
1.6.3 Irritación y sensibilización cutánea y ocular
La aplicación cutánea a conejos y cobayos ocasionó una
irritación leve o nula y una sensibilización moderada de la piel. Su
aplicación ocular en ratas produjo de manera transitoria una
irritación conjuntival ligera.
1.6.4 Exposición prolongada
En un estudio de alimentación de larga duración en ratas, con
dosis de hasta 2500 mg/kg de alimentos (125 mg/kg de peso corporal
al día) no se puso de manifiesto ningún efecto relacionado con el
compuesto. Este estudio no se consideró adecuado para evaluar los
efectos sobre la reproducción. En el ratón CD-1 se observó que, con
dosis de 1500 mg/kg de alimento o superiores, se producía un aumento
de peso del hígado. En los ratones macho, las dosis de hasta 5000
mg/kg de alimento provocaron una disminución en términos absolutos
del peso de los testículos y la atrofia del timo.
1.6.5 Reproducción, embriotoxicidad y teratogenicidad
El benomilo produce una disminución del peso de los testículos
y el epidídimo, de la producción de esperma y de la tasa de
fecundidad de los machos. A dosis más elevadas, provoca
hipoespermatogénesis, con interrupción general de todas las fases de
la espermatogénesis. No afecta, en cambio, al comportamiento
copulatorio, las vesículas seminales, la movilidad del esperma o las
hormonas de la reproducción asociadas. La concentración más baja del
benomilo capaz de inducir un efecto espermatogénico estadísticamente
significativo en ratas macho fue de 45 mg/kg por día. El NOEL para
estos efectos fue de 15 mg/kg por día.
La administración por sonda de una sola dosis de benomilo (100
mg/kg o más) mostró efectos en ratas a los 70 días de la exposición,
que incluyeron descenso del peso de los testículos y atrofia de los
túbulos seminíferos. Administrado por sonda a ratas ChD-CD y Wistar
durante los días 7 a 16 de la gestación, el benomilo resultó
teratogénico a 62,5 mg/kg en ambas estirpes, pero no a 30 mg/kg en
ratas ChD-CD y no a 31,2 mg en ratas Wistar. Administrado por sonda
a ratas Sprague-Dawley en los días 7 a 21 de la gestación, el
benomilo resultó teratogénico en dosis de 31,2 mg/kg. Los efectos
que produjo fueron microftal mia, hidrocefalia y encafaloceles. Las
dosis superiores a 15,6 mg/kg tuvieron un efecto negativo sobre el
desarrollo posnatal.
La administración por sonda de 50 mg/kg o concentraciones
superiores indujo en ratones la aparición de costillas
supernumerarias y de otras anomalías esqueléticas y viscerales. No
se ha determinado el NOEL en los ratones porque no se ensayaron
dosis inferiores a 50 mg/kg. A excepción de un aumento marginal de
las costillas supernumerarias en conejos, no se observaron efectos
teratogénicos incluso a dosis de hasta 500 mg/kg de alimento.
1.6.6 Mutagenicidad y otros efectos finales afines
En unos estudios realizados en células somáticas y germinales
se ha observado que no provoca mutaciones genéticas ni daños en la
estructura de los cromosomas (aberraciones) y tampoco tiene un
efecto directo sobre el ADN (causante de daños y la reparación del
ADN). Esto se ha demostrado tanto en mamíferos como en otros
animales.
El benomilo, sin embargo, produce aberraciones cromosómicas
numéricas (aneuploidía o poliploidía) en sistemas experimentales in
vitro e in vivo.
1.6.7 Carcinogenicidad
En el primer estudio de carcinogenicidad con ratones CD-1 se
puso de manifiesto un aumento estadísticamente significativo de
neoplasia hepatocelular relacionado con la dosis en las hembras y
también en los machos tratados con una dosis de nivel medio (1500
mg/kg) se observó una respuesta estadísticamente significativa, pero
no en los que recibieron dosis elevadas, a causa del elevado índice
de mortalidad. En un segundo estudio sobre la carcinogenicidad de la
carbendazima en una raza de ratones genéticamente relacionada con la
anterior, los ratones SPF (raza aleatoria suiza), con dosis de 0,
150, 300 y 1000 mg/kg (que se aumentó a 5000 mg/kg durante el
estudio) se puso de manifiesto un aumento en el número de casos de
adenomas y carcinomas hepatocelulares combinados. En un tercer
estudio realizado en ratones NMRKf con dosis de 0, 50, 150, 300 y
1000 mg/kg (que se aumentó a 5000 mg/kg durante el estudio) no se
produjeron efectos carcinogénicos. El benomilo y la carbendazima
causaron tumores hepáticos en dos estirpes de ratones (CD-1 y suizos
(SPF)) que presentaban una tasa alta de tumores hepáticos de
formación espontánea. Por el contrario, la carbendazima no resultó
carcinogénica en ratones NMRKf, que presentan una tasa baja de esos
tumores espontáneos.
Los estudios de carcinogenidad del benomilo y la carbendazima
en ratas fueron negativos.
1.6.8 Mecanismo de toxicidad, modo de acción
Se considera que los efectos biológicos de estos compuestos son
el resultado de su interacción con los microtúbulos celulares. Estas
estructuras participan en funciones esenciales, como la división
celular, que inhiben el benomilo y la carbendazima. La toxicidad de
estos productos en los mamíferos está vinculada a una disfunción
microtubular.
El benomilo y la carbendazima, al igual que otros compuestos
del bencimidazol, tienen una toxicidad selectiva para distintas
especies. Esta se explica, por lo menos en parte, porque el benomilo
y la carbendazima se unen de manera distinta a los microtúbulos de
las especies específicas en las que actúan y en las que no.
1.7 Efectos en el ser humano
El benomilo causa dermatitis por contacto y sensibilización
cutánea. No se ha informado de otros efectos.
1.8 Efectos en otros organismos en el laboratorio y en el medio
ambiente
Con las dosis de aplicación recomendadas, el benomilo tiene
pocos efectos sobre la actividad microbiana del suelo. Se han
notificado algunos efectos adversos sobre ciertos grupos de hongos.
La CE50 a las 72 h, basada en el crecimiento total, para el
alga verde Selenastrum capricornutum fue de 2,0 mg/litro; la
concen tración sin efecto observado (NOEC) fue de 0,5 mg/litro. La
toxicidad del benomilo para los invertebrados acuáticos y los peces
varía ampliamente, con una CL50 a las 96 h que oscila entre 0,006
mg/litro para Ictalurus punctatus (alevines con saco vitelino) y
> 100 mg/litro para los cangrejos de río.
No observaron efectos tóxicos en experimentos de laboratorio en
las lombrices de tierra expuestas a concentraciones normales de
benomilo y como resultado del uso de la dosis de aplicación
recomendada en el campo. Tiene una toxicidad baja para las aves, y
la carbendazima, producto de su degradación, es "relativamente no
tóxica" para las abejas de miel.
2. Conclusiones
El benomilo causa sensibilización cutánea en el ser humano.
Tanto el benomilo como la carbendazima representan un riesgo muy
pequeño de intoxicación aguda. Dados los niveles de exposición
actuales y el bajo índice de absorción cutánea de estos dos
compuestos, no es probable que pudieran tener efectos de toxicidad
sistémica en la población general o en personas expuestas
profesionalmente. Estas son las conclusiones que se pueden sacar de
los datos obtenidos en animales y de los limitados datos sobre el
ser humano de que se dispone, pero estas extrapolaciones están
respaldadas por el conocimiento del modo de acción de la
carbendazima y el benomilo en especies en las que actúan y en las
que no.
Una mayor clarificación del mecanismo de toxicidad de ambos
compuestos en los mamíferos permitirá quizás definir mejor los
niveles sin efectos observados. El estudio de su unión a los
microtúbulos de las células destinatarias (tejidos testicular y
embrionario) facilitará la comparación entre distintas especies.
La carbendazima se adsorbe fuertemente en la materia orgánica
del suelo que la conserva durante un período de hasta 3 años.
Persiste en la superficie de las hojas y, por consiguiente, en las
hojas caídas. Se ha demostrado que las dosis recomendadas de
aplicación afectan negativamente a las lombrices de tierra (con
efectos sobre la población y la reproducción). No se dispone de
datos acerca de sus efectos sobre otros artrópodos del suelo o de la
maleza, que estarían igualmente expuestos.
No es probable que se pueda observar en el medio ambiente la
elevada toxicidad demostrada en las pruebas de laboratorio para los
organismos acuáticos debido a la baja biodisponibilidad de los
residuos de carbendazima unidos a los sedimentos. Sin embargo, no se
dispone de información acerca de sus efectos en las especies que
viven en los sedimentos, que sufrirían la exposición más intensa.
