
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 127
ACROLEIN
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organisation, or the World Health Organization.
Published under the joint sponsorship of
the United Nations Environment Programme,
the International Labour Organisation,
and the World Health Organization
First draft prepared by Dr T. Vermeire,
National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
World Health Orgnization
Geneva, 1992
The International Programme on Chemical Safety (IPCS) is a
joint venture of the United Nations Environment Programme, the
International Labour Organisation, and the World Health
Organization. The main objective of the IPCS is to carry out and
disseminate evaluations of the effects of chemicals on human health
and the quality of the environment. Supporting activities include
the development of epidemiological, experimental laboratory, and
risk-assessment methods that could produce internationally
comparable results, and the development of manpower in the field of
toxicology. Other activities carried out by the IPCS include the
development of know-how for coping with chemical accidents,
coordination of laboratory testing and epidemiological studies, and
promotion of research on the mechanisms of the biological action of
chemicals.
WHO Library Cataloguing in Publication Data
Acrolein.
(Environmental health criteria ; 127)
1.Acrolein - adverse effects 2.Acrolein - toxicity
3.Environmental exposure 4.Environmental pollutants
I.Series
ISBN 92 4 157127 6 (LC Classification: QD 305.A6)
ISSN 0250-863X
The World Health Organization welcomes requests for permission
to reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made
to the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 1991
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material
in this publication do not imply the expression of any opinion
whatsoever on the part of the Secretariat of the World Health
Organization concerning the legal status of any country, territory,
city or area or of its authorities, or concerning the delimitation
of its frontiers or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar
nature that are not mentioned. Errors and omissions excepted, the
names of proprietary products are distinguished by initial capital
letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ACROLEIN
1. SUMMARY
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
2.1. Identity
2.2. Physical and chemical properties
2.3. Conversion factors
2.4. Analytical methods
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1. Natural sources
3.2. Anthropogenic sources
3.2.1. Production
3.2.1.1 Production levels and processes
3.2.1.2 Emissions
3.2.2. Uses
3.2.3. Waste disposal
3.2.4. Other sources
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1. Transport and distribution between media
4.2. Abiotic degradation
4.2.1. Photolysis
4.2.2. Photooxidation
4.2.3. Hydration
4.3. Biotransformation
4.3.1. Biodegration
4.3.2. Bioaccumulation
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1. Environmental levels
5.1.1. Water
5.1.2. Air
5.2. General population exposure
5.2.1. Air
5.2.2. Food
5.3. Occupational exposure
6. KINETICS AND METABOLISM
6.1. Absorption and distribution
6.2. Reaction with body components
6.2.1. Tracer-binding studies
6.2.2. Adduct formation
6.2.2.1 Interactions with sulfhydryl groups
6.2.2.2 In vitro interactions with nucleic
acids
6.3. Metabolism and excretion
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1. Single exposure
7.1.1. Mortality
7.1.2. Effects on the respiratory tract
7.1.3. Effects on skin and eyes
7.1.4. Systemic effects
7.1.5. Cytotoxicity in vitro
7.2. Short-term exposure
7.2.1. Continuous inhalation exposure
7.2.2. Repeated inhalation exposure
7.2.3. Repeated intraperitoneal exposure
7.3. Biochemical effects and mechanisms of toxicity
7.3.1. Protein and non-protein sulfhydryl depletion
7.3.2. Inhibition of macromolecular synthesis
7.3.3. Effects on microsomal oxidation
7.3.4. Other biochemical effects
7.4. Immunotoxicity and host resistance
7.5. Reproductive toxicity, embryotoxicity, and teratogenicity
7.6. Mutagenicity and related end-points
7.6.1. DNA damage
7.6.2. Mutation and chromosomal effects
7.6.3. Cell transformation
7.7. Carcinogenicity
7.7.1. Inhalation exposure
7.7.2. Oral exposure
7.7.3. Skin exposure
7.8. Interacting agents
8. EFFECTS ON HUMANS
8.1. Single exposure
8.1.1. Poisoning incidents
8.1.2. Controlled experiments
8.1.2.1 Vapour exposure
8.1.2.2 Dermal exposure
8.2. Long-term exposure
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1. Aquatic organisms
9.2. Terrestrial organisms
9.2.1. Birds
9.2.2. Plants
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON
THE ENVIRONMENT
10.1. Evaluation of human health risks
10.1.1. Exposure
10.1.2. Health effects
10.2. Evaluation of effects on the environment
11. FURTHER RESEARCH
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
RESUME
RESUMEN
WHO TASK GROUP ON ENVIRONMENTAL HEALTH
CRITERIA FOR ACROLEIN
Members
Dr G. Damgard-Nielsen, National Institution of Occupational Health,
Copenhagen, Denmark
Dr I. Dewhurst, Division of Toxicology and Environmental Health,
Department of Health, London, United Kingdom
Dr R. Drew, Toxicology Information Services, Safety Occupational
Health and Environmental Protection, ICI Australia, Melbourne,
Victoria, Australia
Dr B. Gilbert, Technology Development Company (CODETEC), Cidade
Universitaria, Campinas, Brazil ( Rapporteur)
Dr K. Hemminki, Institute of Occupational Health, Helsinki ( Chairman)
Dr R. Maronpot, Chemical Pathology Branch, Division of Toxicology,
Research and Testing, National Institute of Environmental Health
Sciences, Research Triangle Park, North Carolina, USA
Dr M. Noweir, Industrial Engineering Department, College of
Engineering, King Abdul Aziz University, Jeddah, Saudi Arabia
Dr M. Wallén, National Chemicals Inspectorate, Solna, Sweden
Secretariat
Ms B. Labarthe, International Register of Potentially Toxic
Chemicals, United Nations Environment Programme, Geneva,
Switzerland
Dr T. Ng, Office of Occupational Health, World Health Organization,
Switzerland
Dr G. Nordberg, International Agency for Research on Cancer, Lyon,
France
Professor F. Valic, IPCS Consultant, World Health Organization,
Geneva, Switzerland ( Responsible Officer and Secretary)a
Dr T. Vermeire, National Institute of Public Health and
Environmental Protection, Bilthoven, The Netherlands
a Vice-rector, University of Zagreb, Zagreb, Yugoslavia
NOTE TO READERS OF THE CRITERIA DOCUMENTS
Every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication. In the interest of all users of the
environmental health criteria documents, readers are kindly
requested to communicate any errors that may have occurred to the
Manager of the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland, in order that they may be
included in corrigenda.
* * *
A detailed data profile and a legal file can be obtained from
the International Register of Potentially Toxic Chemicals, Palais
des Nations, 1211 Geneva 10, Switzerland (Telephone No. 7988400 or
7985850).
ENVIRONMENTAL HEALTH CRITERIA FOR ACROLEIN
A WHO Task Group on Environmental Health Criteria for Acrolein
met in Geneva from 7 to 11 May 1990. Dr M. Mercier, Manager, IPCS,
opened the meeting and welcomed the participants on behalf of the
heads of the three IPCS cooperating organizations (UNEP/ILO/WHO).
The Task Group reviewed and revised the draft monograph and made an
evaluation of the risks for human health and the environment from
exposure to acrolein.
The first draft of this monograph was prepared by Dr T.
Vermeire, National Institute of Public Health and Environmental
Protection, Bilthoven, Netherlands. Professor F. Valic was
responsible for the overall scientific content, and Dr P.G. Jenkins,
IPCS, for the technical editing.
The efforts of all who helped in the preparation and
finalization of the document are gratefully acknowledged.
ABBREVIATIONS
BOD biochemical oxygen demand
COD chemical oxygen demand
EEC European Economic Community
HPLC high-performance liquid chromatography
LOAEL lowest-observed-adverse-effect level
NAD nicotinamide adenine dinucleotide
NADPH reduced nicotinamide adenine dinucleotide phosphate
NIOSH National Institute for Occupational Safety and Health
(USA)
NOAEL no-observed-adverse-effect level
1. SUMMARY
Acrolein is a volatile highly flammable liquid with a pungent,
choking, disagreeable odour. It is a very reactive compound.
The world production of isolated acrolein was estimated to be
59 000 tonnes in 1975. A still larger amount of acrolein is
produced and consumed as an intermediate in the synthesis of acrylic
acid and its esters.
Analytical methods are available for the determination of
acrolein in various media. The minimum detection limits that have
been reported are 0.1 µg/m3 air (gas chromatography/mass
spectrometry), 0.1 µg/litre water (high-pressure liquid
chromatography), 2.8 µg/litre biological media (fluorimetry),
590 µg/kg fish (gas chromatography/mass spectrometry), and
1.4 µg/m3 exhaust gas (high-pressure liquid chromatography).
Acrolein has been detected in some plant and animal sources
including foods and beverages. The substance is primarily used as
intermediate in chemical synthesis but also as an aquatic biocide.
Emissions of acrolein may occur at sites of production or use.
Important acrolein emissions into the air arise from incomplete
combustion or pyrolysis of organic materials such as fuels,
synthetic polymers, food, and tobacco. Acrolein may make up 3-10% of
total vehicle exhaust aldehydes. Smoking one cigarette yields
3-228 µg acrolein. Acrolein is a product of photochemical oxidation
of specific organic air pollutants.
Exposure of the general population will predominantly occur via
air. Oral exposure may occur via alcoholic beverages or heated
foodstuffs.
Average acrolein levels of up to approximately 15 µg/m3 and
maximum levels of up to 32 µg/m3 have been measured in urban air.
Near industries and close to exhaust pipes, levels that are ten to
one hundred times higher may occur. Extremely high air levels in
the mg/m3 range can be found as a result of fires. In indoor air,
smoking one cigarette per m3 of room-space in 10-13 min was found
to lead to acrolein vapour concentrations of 450-840 µg/m3.
Workplace levels of over 1000 µg/m3 were reported in situations
involving the heating of organic materials, e.g., welding or heating
of organic materials.
Acrolein is degraded in the atmosphere by reaction with
hydroxyl radicals. Atmospheric residence times are about one day.
In surface water, acrolein dissipates in a few days. Acrolein has a
low soil adsorption potential. Both aerobic and anaerobic
degradation have been reported, although the toxicity of the
compound to microorganisms may prevent biodegradation. Based on the
physical and chemical properties, bioaccumulation of acrolein would
not be expected to occur.
Acrolein is very toxic to aquatic organisms. Acute EC50 and
LC50 values for bacteria, algae, crustacea, and fish are between
0.02 and 2.5 mg/litre, bacteria being the most sensitive species.
The 60-day no-observed-adverse-effect level (NOAEL) for fish has
been determined to be 0.0114 mg/litre. Effective control of aquatic
plants by acrolein has been achieved at dosages of between 4 and
26 mg/litre.h. Adverse effects on crops grown on soil irrigated by
acrolein-treated water have been observed at concentrations of
15 mg/litre or more.
In animals and humans the reactivity of acrolein effectively
confines the substance to the site of exposure, and pathological
findings are also limited to these sites. A retention of 80-85%
acrolein was found in the respiratory tract of dogs exposed to
400-600 mg/m3. Acrolein reacts directly with protein and
non-protein sulfhydryl groups and with primary and secondary amines.
It may also be metabolized to mercapturic acids, acrylic acid,
glycidaldehyde or glyceraldehyde. Evidence for the last three
metabolites has only been obtained in vitro.
Acrolein is a cytotoxic agent. In vitro cytotoxicity has
been observed at levels as low as 0.1 mg/litre. The substance is
highly toxic to experimental animals and humans following a single
exposure via different routes. The vapour is irritating to the eyes
and respiratory tract. Liquid acrolein is a corrosive substance.
The NOAEL for irritant dermatitis from ethanolic acrolein was found
to be 0.1%. Experiments with human volunteers, exposed to acrolein
vapour, show a lowest-observed-adverse-effect level (LOAEL) of
0.13 mg/m3, at which level eyes may become irritated within 5 min.
In addition, respiratory tract effects are evident from 0.7 mg/m3.
At higher single exposure levels, degeneration of the respiratory
epithelium, inflammatory sequelae, and perturbation of respiratory
function develop.
The toxicological effects from continuous inhalation exposure
at concentrations from 0.5 to 4.1 mg/m3 have been studied in rats,
dogs, guinea-pigs, and monkeys. Both respiratory tract function and
histopathological effects were seen when animals were exposed to
acrolein at levels of 0.5 mg/m3 or more for 90 days.
The toxicological effects from repeated inhalation exposure to
acrolein vapour at concentrations ranging from 0.39 mg/m3 to 11.2
mg/m3 have been studied in a variety of laboratory animals.
Exposure durations ranged from 5 days to as long as 52 weeks. In
general, body weight gain reduction, decrement of pulmonary
function, and pathological changes in nose, upper airways, and lungs
have been documented in most species exposed to concentrations of
1.6 mg/m3 or more for 8 h/day. Pathological changes include
inflammation, metaplasia, and hyperplasia of the respiratory tract.
Significant mortality has been observed following repeated exposures
to acrolein vapour at concentrations above 9.07 mg/m3. In
experimental animals acrolein has been shown to deplete tissue
glutathione and in in vitro studies, to inhibit enzymes by
reacting with sulfhydryl groups at active sites. There is limited
evidence that acrolein can depress pulmonary host defences in mice
and rats.
Acrolein can induce teratogenic and embryotoxic effects if
administered directly into the amnion. However, the fact that no
effect was found in rabbits injected intravenously with 3 mg/kg
suggests that human exposure to acrolein is unlikely to affect the
developing embryo.
Acrolein has been shown to interact with nucleic acids
in vitro and to inhibit their synthesis both in vitro and
in vivo. Without activation it induced gene mutations in bacteria
and fungi and caused sister chromatid exchanges in mammalian cells.
In all cases these effects occurred within a very narrow dose range
governed by the reactivity, volatility, and cytotoxicity of
acrolein. A dominant lethal test in mice was negative. The
available data show that acrolein is a weak mutagen to some
bacteria, fungi, and cultured mammalian cells.
In hamsters that were exposed for 52 weeks to acrolein vapour
at a level of 9.2 mg/m3 for 7 h/day and 5 days/week and were
observed for another 29 weeks, no tumours were found. When hamsters
were exposed to acrolein vapour similarly for 52 weeks, and, in
addition, to intratracheal doses of benzo[a]pyrene weekly or to
subcutaneous doses of diethylnitrosamine once every three weeks, no
clear co-carcinogenic action of acrolein was observed. Oral exposure
of rats to acrolein in drinking-water at doses of between 5 and
50 mg/kg body weight per day (5 days/week for 104-124 weeks) did not
induce tumours. In view of the limited nature of all these tests,
the data for determining the carcinogenicity of acrolein to
experimental animals are considered inadequate. In consequence, an
evaluation of the carcinogenicity of acrolein to humans is also
considered impossible.
The threshold levels of acrolein causing irritation and health
effects are 0.07 mg/m3 for odour perception, 0.13 mg/m3 for eye
irritation, 0.3 mg/m3 for nasal irritation and eye blinking, and
0.7 mg/m3 for decreased respiratory rate. As the level of
acrolein rarely exceeds 0.03 mg/m3 in urban air, it is not likely
to reach annoyance or harmful levels in normal circumstances.
In view of the high toxicity of acrolein to aquatic organisms,
the substance presents a risk to aquatic life at or near sites of
industrial discharges, spills, and biocidal use.
2. IDENTITY, PHYSICAL AND CHEMICAL PROPERTIES, AND
ANALYTICAL METHODS
2.1 Identity
Chemical formula: C3H4O
Chemical structure:
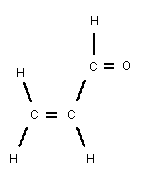 Relative molecular 56.06
mass:
Common name: acrolein
Common synonyms: acraldehyde, acrylaldehyde (IUPAC name),
acrylic aldehyde, propenal, prop-2-enal,
prop-2-en-1-al
Common trade Acquinite, Aqualin, Aqualine, Biocide,
names: Magnicide-H, NSC 8819, Slimicide
CAS chemical name: 2-propenal
CAS registry 107-02-8
number:
RTECS registry AS 1050000
number:
Specifications: commercial acrolein contains 95.5% or
more of the compound and, as main
impurities, water (up to 3.0% by weight)
and other carbonyl compounds (up to 1.5%
by weight), mainly propanal and acetone.
Hydroquinone is added as an inhibitor of
polymerization (0.1-0.25% by weight)
(Hess et al., 1978).
2.2 Physical and chemical properties
Acrolein is a volatile, highly flammable, lacrimatory liquid at
ordinary temperature and pressure. Its odour is described as burnt
sweet, pungent, choking, and disagreeable (Hess et al., 1978;
Hawley, 1981). The compound is highly soluble in water and in
organic solvents such as ethanol and diethylether. The extreme
reactivity of acrolein can be attributed to the conjugation of a
carbonyl group with a vinyl group within its structure. Reactions
shown by acrolein include Diels-Alder condensations, dimerization
and polymerization, additions to the carbon-carbon double bond,
carbonyl additions, oxidation, and reduction. In the absence of an
inhibitor, acrolein is subject to highly exothermic polymerization
catalysed by light and air at room temperature to an insoluble,
cross-linked solid. Highly exothermic polymerization also occurs in
the presence of traces of acids or strong bases even when an
inhibitor is present. Inhibited acrolein undergoes dimerization
above 150 °C. Some physical and chemical data on acrolein are
presented in Table 1.
Table 1. Some physical and chemical data on acrolein
Physical state mobile liquid
Colour colourless (pure) or
yellowish (commercial)
Odour perception threshold 0.07 mg/m3 a
Odour recognition threshold 0.48 mg/m3 b
Melting point -87 °C
Boiling point (at 101.3 kPa) 52.7 °C
Water solubility (at 20 °C) 206 g/litre
Log n-octanol-water partition 0.9c
coefficient
Relative density (at 20 °C) 0.8427
Relative vapour density 1.94
Vapour pressure (at 20 °C) 29.3 kPa (220 mmHg)
Flash point (open cup) -18 °C
Flash point (closed cup) -26 °C
Flammability limits 2.8-31.0% by volume
a Sinkuvene (1970) (see Table 12)
b Leonardos et al. (1969) (see Table 12)
c Experimentally derived by Veith et al. (1980)
2.3 Conversion factors
At 25 °C and 101.3 kPa (760 mmHg), 1 ppm of acrolein =
2.29 mg/m3 air and 1 mg of acrolein per m3 air = 0.44 ppm.
2.4 Analytical methods
A summary of relevant methods of sampling and analysis is
presented in Table 2.
Tejada (1986) presented data showing that the air analysis HPLC
method employing a 2,4-dinitrophenylhydrazine-coated SP cartridge
(Kuwata et al., 1983) is equivalent to that using impingers with
2,4-dinitrophenylhydrazine in acetonitrile (Lipari & Swarin, 1982).
The latter method was also evaluated in several laboratories and was
found adequate for the evaluation of the working environment (Perez
et al., 1984). Nevertheless, the separation of
2,4-dinitrophenylhydrazine derivatives of acrolein and acetone by
HPLC can present difficulties (Olson & Swarin, 1985). A highly
sensitive electrochemical detection method was found by Jacobs &
Kissinger (1982) to be suitable and was later improved by Facchini
et al. (1986).
A personal sampling device for firemen, which employs molecular
sieves, was described by Treitman et al. (1980). Other sampling
methods using solid sorbents coated with 2,4-dinitrophenylhydrazine,
as applied by Kuwata et al. (1983) for location monitoring, were
found suitable for personal sampling procedures (Andersson et al.,
1981; Rietz, 1985).
The NIOSH procedure for industrial air monitoring involves
absorption onto N-hydroxymethylpiperazine-coated XAD-2 resin and gas
chromatographic analysis of the toluene eluate (US-NIOSH, 1984).
This method has been validated by a Shell Development Company
analytical laboratory and was not revised by NIOSH in 1989.
Table 2. Sampling, preparation, and analysis of acrolein
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air absorption in ethanolic UV spectrometry 20 µg/m3 0.02 m3 suitable for location Manita &
solution of monitoring; designed Goldberg
thiosemicar-bazide for analysis of ambient (1970)
and hydrochloric acid air; interference from
other alpha, ß-unsaturated
aldehydes
air absorption in ethanolic colorimetry 20 µg/m3 0.05 m3 suitable for location Cohen &
solution of monitoring; designed Altshuller
4-hexylresor-cinol, for analysis of ambient (1961), Katz
mercuric chloride, and and industrial air and (1977), Harke
trichloroacetic acid exhaust gas; slight et al. (1972)
interference from dienes
and alpha, ß-unsaturated
aldehydes; also suitable
for analysis of smoke
air absorption in aqueous colorimetry 20 µg/m3 0.06 m3 suitable for location Pfaffli (1982),
sodium bisulfite; monitoring; designed Katz (1977),
addition of ethanolic for analysis of ambient Ayer & Yeager
solution of and industrial air and (1982)
4-hexylresorcinol, cigarette smoke
mercuric chloride, and
trichloroacetic acid;
heating
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air collection on molecular fluorimetry 2 µg/m3 0.06 m3 suitable for location Suzuki & Imai
sieve 3A and 13X; monitoring; designed (1982)
desorption by heat; for analysis of ambient
collection in water; air; interference from
reaction with aqueous croton-aldehyde and
o-aminobiphenyl-sulfuric methylvinyl ketone
acid; heating
air adsorption on Poropak N; gas chromatography < 600 0.003-0.008 suitable for personal Campbell & Moore
desorption by heat with flame ionization µg/m3 m3 monitoring (1979)
detection
air adsorption on Tenax GC gas chromatography 0.1 0.006-0.019 suitable for location Krost et al.
desorption by heat; with mass µg/m3 m3 and personal (1982)
cryofocussing spectrometric (breakthrough designed for
detection volume) analysis of ambient air
air cryogradient sampling on gas chromatography 0.1 µg/m3 0.003 m3 suitable for location Jonsson & Berg
siloxane-coated with flame monitoring; designed (1983)
chromosorb W AW; ionization and mass for analysis of ambient
desorption by heat spectrometric air
detection
air absorption into ethanol; gas chromatography 1 µg/m3 0.003-0.04 suitable for location Nishikawa et al.
reaction with aqueous with electron m3 monitoring; designed (1986)
methoxyamine capture detection for analysis of ambient
hydrochlo-ride-sodium air
acetate; bromination;
adsorption on SP-cartridge;
elution by diethyl ether
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air absorption into aqueous gas chromatography 435 0.01 m3 designed for analysis Saito et al.
2,4-DNPH hydrochloride; with flame µg/m3 of exhaust gas (1983)
extraction by chloroform; ionization detection
and anthracene as
internal standard
air collection in cold trap; gas chromatography designed for analysis Rathkamp et al.
warming trap of tobacco smoke (1973)
air direct introduction gas chromatography 0.1 2 cm3 designed for analysis Richter &
g/m3 of tobacco smoke Erfuhrth (1979)
air adsorption on HPLC with UV 0.5 0.1 m3 suitable for location Kuwata et al.
2,4-DNPH-phosphoric acid detection µg/m3 monitoring; designed (1983)
coated SP-cartridge; for analysis of
elution by acetonitrile industrial and ambient
air
air absorption into solution HPLC with UV 11 0.02 m3 suitable for location Lipari & Swarin
of 2,4-DNPH-perchloric detection µg/m3 monitoring; designed (1982)
acid in acetonitrile; for analysis of exhaust
gas
air absorption into solution HPLC with 1.4 0.02 m3 suitable for location Swarin & Lipari
of 2-diphenylacetyl-1,3- fluorescence µg/m3 monitoring; designed (1983)
indandione-1-hydrazone detection for analysis of exhaust
and hydrochloric acid in gas
acetonitrile
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air absorption into aqueous HPLC with 10 µg/ 1 cigarette designed for analysis Manning et al.
2,4-DNPH-hydrochloric UV detection cigarette of cigarette smoke (1983)
acid and chloroform gas phase
air absorption into gas chromatography 229 0.05 m3 suitable for US-NIOSH (1984)
2-(hydroxymethyl) with µg/m3 personal monitoring
piperidine on XAD-2; nitrogen-specific
elution by toluene detector
water addition of colorimetry 400 0.0025 slight interference Cohen &
4-hexyl-resorcinol-mercuric µg/litre litre from dienes and alpha, Altshuller (1961)
chloride solution and ß-unsaturated aldehydes
trichloroacetic acid to
sample in ethanol
water reaction with methoxylamine gas chromatography 0.4 designed for analysis Nishikawa et al.
hydrochloride-sodium with electron µg/litre of rain water (1987a)
acetate; bromination; capture detection
adsorption on SP
cartridge; elution by
diethyl ether
water reaction with 2,4-DNPH; HPLC with 29 designed for analysis Facchini et al.
with addition of electro-chemical µg/litre of fog and rain water (1986)
iso-octane detection
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
water low pressure distillation; HPLC with UV < 0.1 1000 ml designed for analysis Greenhoff &
cryofocussing into aqueous detection µg/litre of beer Wheeler (1981)
2,4-DNPH-hydro-chloric acid;
extraction by chloroform;
TLC and magnesia-silica-gel
column chromatography
biological reaction with aqueous fluorimetry 2.8 2 ml designed for analysis Alarcon (1968)
media m-aminophenol-hydroxyl- µg/litre of biological media
amine-hydrochloride-
hydrochloric acid;
heating
tissue homogenization; reaction HPLC with UV Boor & Ansari
with aqueous detection (1986)
2,4-DNPH-sulfuric acid;
extraction by chloroform
food ultrasonic homogenization gas chromatography 590 1000 mg designed for analysis Easley et al.
in cooled water; purging with mass µg/kg of volatile organic (1981)
by helium; trapping on spectrometric compounds in fish
Tenax GC-silica-gel-charcoal; detection
desorption by heat
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural sources
Acrolein is reported to occur naturally, e.g., in the essential
oil extracted from the wood of oak trees (IARC, 1979), in tomatoes
(Hayase et al., 1984), and in certain other foods (section
5.2.2.).
3.2 Anthropogenic sources
3.2.1 Production
3.2.1.1 Production levels and processes
In 1975, the worldwide production of acrolein was estimated to
be 59 000 tonnes, although at this time production figures probably
only related to isolated acrolein (Hess et al., 1978). It is
mainly produced in the USA, Japan, France, and Germany. In
addition, acrolein is produced as an unisolated intermediate in the
synthesis of acrylic acid and its esters. In 1983, 216 000 to
242 000 tonnes of acrolein was reported to be used in the USA for
this purpose, amounting to 91-93% of the total production in that
country (Beauchamp et al.,1985). Formerly acrolein was produced by
vapour phase condensation of acetaldehyde and formaldehyde (Hess
et al., 1978). Although this process is now virtually obsolete,
some production via this pathway has continued in the USSR (IRPTC,
1984). Worldwide, most acrolein is now produced by the direct
catalytic oxidation of propene. Catalysts containing bismuth,
molybdenum, and other metal oxides enable a conversion of propene of
over 90% and have a high selectivity for acrolein. By-products are
acrylic acid, acetic acid, acetaldehyde, and carbon oxides (Hess
et al., 1978; Ohara et al., 1987). Another catalyst used for
this process, cuprous oxide, has a lower performance (Hess et al.,
1978; IRPTC, 1984).
3.2.1.2 Emissions
Closed-systems are used in production facilities, and releases
of acrolein to the environment are expected to be low, especially
when the compound is directly converted to acrylic acid and its
esters. The compound is emitted via exhaust fumes, process waters
and waste, and following leakage of equipment. Production losses in
the USA in 1978 were estimated to be 35 tonnes or approximately 0.1%
of the amount of isolated acrolein produced (Beauchamp et al.,
1985).
The air emission factor of acrolein in the synthesis of
acrylonitrile in the Netherlands has been reported to be 0.1-0.3 kg
per tonne of acrylonitrile (DGEP, 1988). Acrolein has also been
identified in the process streams of plants manufacturing acrylic
acid (Serth et al., 1978). The application of acrolein as a
biocide brings the chemical directly into the aquatic environment.
3.2.2 Uses
The principal use of acrolein is as an intermediate in the
synthesis of numerous chemicals, in particular acrylic acid and its
lower alkyl esters and DL-methionine, an essential amino acid used
as a feed supplement for poultry and cattle. In the USA, in 1983, 91
to 93% of the total quantity of acrolein produced was converted to
acrylic acid and its esters, and 5% to methionine (Beauchamp
et al., 1985). Other derivatives of acrolein are:
2-hydroxyadipaldehyde, 1,2,6-hexanetriol, lysine, glutaraldehyde,
tetrahydro-benzaldehyde, pentanediols, 1,4-butanediol,
tetrahydrofuran, pyridine, 3-picoline, allyl alcohol, glycerol,
quinoline, homopolymers, and copolymers (Hess et al., 1978).
Among the direct uses of acrolein, its application as a biocide
is the most significant one. Acrolein at a concentration of
6-10 mg/litre in water is used as an algicide, molluscicide, and
herbicide in recirculating process water systems, irrigation
channels, cooling water towers, and water treatment ponds (Hess
et al., 1978). About 66 tonnes of acrolein is reported to be used
annually in Australia to control submersed plants in about 4000 km
of irrigation channels (Bowmer & Sainty, 1977; Bowmer & Smith,
1984). Acrolein protects feed lines for subsurface injection of
waste water, liquid hydrocarbon fuels and oil wells against the
growth of microorganisms, and at 0.4-0.6 mg/litre it controls slime
formation in the paper industry. The substance can also be used as
a tissue fixative, warning agent in methyl chloride refrigerants,
leather tanning agent, and for the immobilization of enzymes via
polymerization, etherification of food starch, and the production of
perfumes and colloidal metals (Hess et al., 1978; IARC, 1985).
3.2.3 Waste disposal
Acrolein wastes mainly arise during production and processing
of the compound and its derivatives.
Aqueous wastes with low concentrations of acrolein are usually
neutralized with sodium hydroxide and fed to a sewage treatment
plant for biological secondary treatment. Concentrated wastes are
reprocessed whenever possible or burnt in special waste incinerators
(IRPTC, 1985).
3.2.4 Other sources
Incomplete combustion and thermal degradation (pyrolysis) of
organic substances such as fuels, tobacco, fats, synthetic and
natural polymers, and foodstuffs frequently result in the emission
of aldehydes. Reported levels are presented in section 5.1.2.
Emission rates for several of such sources are presented in Table 3.
The major sources of aldehydes in ambient air formed by
incomplete combustion and/or thermal degradation are residential
wood burning, burning of coal, oil or natural gas in power plants,
burning of fuels in automobiles, and burning of refuse and
vegetation (Lipari et al., 1984). Formaldehyde is the major
aldehyde emitted, but acrolein may make up 3 to 10 % of total
automobile exhaust aldehydes and 1 to 13% of total wood-smoke
aldehydes (Fracchia et al., 1967; Oberdorfer, 1971; Lipari
et al., 1984). Modern catalytic converters in automobiles almost
completely remove these aldehydes from exhaust gases. Acrolein may
constitute up to 7% of the aldehydes in cigarette smoke (Rickert
et al., 1980).
Aldehydes are also formed by photochemical oxidation of
hydrocarbons in the atmosphere. Leach et al. (1964) concluded
that formaldehyde and acrolein would constitute 50% and 5%,
respectively, of the total aldehyde present in irradiated diluted
car exhaust. Acrolein was considered to be mainly a product of
oxidation of 1,3-butadiene (Schuck & Renzetti, 1960; Leach et al.,
1964), but propene (Graedel et al., 1976; Takeuchi & Ibusuki,
1986), 1,3-pentadiene, 2-methyl-1,3-pentadiene (Altshuller &
Bufalini, 1965), and crotonaldehyde (IRPTC, 1984) have also been
implicated. The photooxidation of 1,3-butadiene in an irradiated
smog chamber, also containing nitrogen monoxide and air, gave rise
to the formation of acrolein (55% yield based on 1,3-buta-diene
initial concentrations). The rate of formation of acrolein was the
same as that of 1,3-butadiene consumption. (Maldotti et al.,
1980). Cancer chemotherapy patients receiving cyclo-phosphamide are
exposed to acrolein, which results from the metabolism of this drug.
Table 3. Emission rates of aldehydes
Source Total Formaldehyde Acrolein Unit Reference
aldehydes
Residential wood burning 0.6-2.3 0.089-0.708 0.021-0.132 g/kg Lipari et al. (1984)
Power plants - coal 0.002 g/kg Natusch (1978)
- oil 0.1 g/kg
- natural gas 0.2 g/kg
Automobiles - petrol 0.01-0.08 g/km Lipari et al. (1984)
0.4-2.3 0.2-1.6 0.01-0.16 g/litre Guicherit & Schulting
(1985)
8.4-63 4-38 1-2 mg/min Lies et al. (1986)
- diesel 0.021 g/km Lipari et al. (1984)
1-2 0.5-1.4 0.03-0.20 g/litre Guicherit & Schulting
(1985)
0.0080 0.0002 g/litre Smythe & Karasek (1973)
44 18 3 mg/min Lies et al. (1986)
Vegetation burning 0.003 g/kg Lipari et al. (1984)
Cigarette smoking 82-1203 3-228 µg/cigarette see section 5.2.1
Pyrolysis of flue-cured tobacco 42-82 µg/g Baker et al. (1984)
Heating in air (at up to 400 °C) of
- polyethylene up to 75 up to 20 g/kg Morikawa (1976)
- polypropylene up to 54 up to 8 g/kg
- cellulose up to 27 up to 3 g/kg
- glucose up to 18 up to 1 g/kg
- wood up to 15 up to 1 g/kg
Smouldering cellulosic materials 0.66-10.02 0.46-1.74 g/kg
Hot wire cutting (50 cm long at 215 °C)
of PVC wrapping film 27-151 ng/cut Boettner & Ball (1980)
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
Acrolein is released into the atmosphere during the production
of the compound itself and its derivatives, in industrial and
non-industrial processes involving incomplete combustion and/or
thermal degradation of organic substances, and, indirectly, by
photochemical oxidation of hydrocarbons in the atmosphere. Emissions
to water and soil occur during production of the compound itself and
its derivatives, and through biocidal use, spills, and waste
disposal (chapter 3).
Intercompartmental transport of acrolein should be limited in
view of its high reactivity, as is discussed in sections 4.2. and
4.3. Considering the high vapour pressure of acrolein, some
transfer across the water-air and soil-air boundaries can be
expected. In a laboratory experiment Bowmer et al. (1974)
explained a difference of 10% between the amount of total aldehydes
(acrolein and non-volatile degradation products, see section 4.2) in
an open tank and that in closed bottles by volatilization. It was
noted that volatilization may be greatly increased by turbulence.
Adsorption to soil, often involving probable reaction with soil
components, may impair the transfer of a compound to air or ground
water. The tendency of untreated acrolein to adsorb to soil
particles can be expressed in terms of Koc, the ratio of the
amount of chemical adsorbed (per unit weight of organic carbon) to
the concentration of the chemical in solution at equilibrium. Based
on the available empirical relationships derived for estimating
Koc, a low soil adsorption potential is expected (Lyman et al.,
1982). Experimentally, acrolein showed a limited (30% of a 0.1%
solution) adsorbability to activated carbon (Giusti et al., 1974).
4.2 Abiotic degradation
Once in the atmosphere, acrolein may photodissociate or react
with hydroxyl radicals and ozone. In water, photolysis or hydration
may occur. These processes will be discussed in the following
sections.
4.2.1 Photolysis
Acrolein shows a moderate absorption of light within the solar
spectrum at 315 nm (with a molar extinction coefficient of 26
litre/mol per cm) and therefore would be expected to be
photoreactive (Lyman et al., 1982). However, irradiation of an
acrolein-air mixture by artificial sunlight did not result in any
detectable photolysis (Maldotti et al., 1980). Irradiation of
acrolein vapour in high vacuum apparatus at 313 nm and 30-200 °C
resulted in the formation of trace amounts of ethene and carbon
oxides (Osborne et al., 1962; Coomber & Pitts, 1969).
4.2.2 Photooxidation
Experimentally determined rate constants for the pseudo first
order reaction between acrolein and hydroxyl radicals in the
atmosphere are presented in Table 4. Also shown are the atmospheric
residence times, which can be derived from the rate constants
assuming a 12-h daytime average hydroxyl radical concentration of
2 x 10-15 mol/litre (Lyman et al., 1982). The estimated
atmospheric residence time of acrolein of approximately 20 h will
decrease with increasing hydroxyl radical concentrations in more
polluted atmospheres and increase with the decline in temperature,
and consequently the rate of reaction, at higher altitudes. Other
variations will be caused by seasonal, altitudinal, diurnal, and
geographical fluctuations in the hydroxyl radical concentration.
Other potentially significant gas-phase reactions in the
atmosphere may occur between acrolein and ozone or nitrate radicals.
Experimentally determined rate constants and atmospheric residence
times for these reactions are shown in Table 4. The atmospheric
residence times were estimated assuming a 24-h average ozone
concentration of 1.6 x 10-9 mol/litre (Lyman et al., 1982) and a
12-h night-time average nitrate radical concentration of 4.0 x
10-12 mol/litre (Atkinson et al., 1987). It can be concluded
that the tropospheric removal processes for acrolein are dominated
by the reaction with hydroxyl radicals. Carbon monoxide,
formaldehyde, glycoaldehyde, ketene, and peroxypropenyl nitrate have
been identified as products of the reaction between acrolein and
hydroxyl radicals (Edney et al., 1982), and glyoxal was also
suggested to be one of the reaction products (Edney et al., 1982,
1986b).
As discussed in section 3.2.4, acrolein is also formed by the
photochemical degradation of hydrocarbons in general and
1,3-butadiene in particular. When mixtures of acrolein or
1,3-butadiene with nitrogen monoxide and air were irradiated in a
smog chamber, the time required for the half-conversion of
1,3-butadiene to acrolein was always shorter than that required for
the half conversion of acrolein. It was concluded that in a real
atmospheric environment, with continuous emissions of 1,3-butadiene,
acrolein will be continuously formed (Bignozzi et al., 1980).
Table 4. Rate constants and calculated atmospheric residence times for gas-phase reactions of acrolein.
Reactant Temperature Technique used Rate constant Atmospheric Reference
(°C) (litre/mol per sec) residence time
(h)
OH radical 25 relative rate 16 x 109 17 Maldotti et al. (1980)
25 relative rate 11.4 x 109 24 Kerr & Sheppard (1981)
23 absolute rate 20.6 x 109 13 Edney et al. (1982)
26 relative rate 11.4 x 109 24 Atkinson et al. (1983)
23 relative rate 12.3 x 109 23 Edney et al. (1986a)
O3 23 absolute rate 16.9 x 104 1029 Atkinson et al. (1981)
NO3 25 relative rate 35.5 x 104 391 Atkinson et al. (1987)
4.2.3 Hydration
Acrolein does not contain hydrolysable groups but it does react
with water in a reversible hydration reaction to 3-hydroxypropanal.
The equilibrium constant is pH independent and increases appreciably
with increasing initial acrolein concentration, presumably because
of the reversible dimerization of 3-hydroxypropanal (Hall & Stern,
1950). In more dilute solutions the equilibrium constant was found
to approach 12 at 20 °C (Pressman & Lucas, 1942; Hall & Stern,
1950), indicating that approximately 92% of acrolein is in the
hydrated form at equilibrium. This agrees well with the equilibrium
concentrations found in buffered solutions of acrolein at 21 °C
(Bowmer & Higgins, 1976).
The hydration of acrolein is a first order reaction with
respect to acrolein. The rate constants are independent of the
initial acrolein concentrations but increase with increasing acid
concentrations (Pressman & Lucas, 1942; Hall & Stern, 1950) and also
when the pH is raised from 5 to 9 (Bowmer & Higgins, 1976). In
dilute buffered solutions of acrolein in distilled water the rate
constant is 0.015 h-1 at 21 °C and pH 7, corresponding to a
half-life of 46 h. However, although in laboratory experiments an
equilibrium is reached with 8% of the original acrolein and 85% of
total aldehydes still present, these do not persist in river waters
so that other methods of dissipation must exist (Bowmer et al.,
1974; Bowmer & Higgins, 1976; see also section 4.3.1).
The dissipation of acrolein in field experiments in irrigation
channels also followed first order kinetics and was faster than
could be predicted assuming hydration alone. First order rate
constants, based on the data thought to be most reliable varied
between 0.104 and 0.208 h-1 at pH values of 7.1 to 7.5 and
temperatures of 16 to 24 °C. From these rate constants, half-lives
of between 3 and 7 h can be calculated (O'Loughlin & Bowmer, 1975;
Bowmer & Higgins, 1976; Bowmer & Sainty, 1977). The latter data
agree better than the laboratory data with the results of bioassays
with bacteria and fish, which show that aged acrolein solutions
become biocidally inactive after approximately 120 to 180 h at a pH
of 7 (Kissel et al., 1978). Apparently processes other than
hydration also contribute to acrolein dissipation, e.g., catalysis
other than acid-base catalysis, adsorption, and volatilization
(Bowmer & Higgins, 1976).
4.3 Biotransformation
4.3.1 Biodegradation
No biological degradation of acrolein was observed in two BOD5
tests with unacclimated microorganisms (Stack, 1957; Bridie et al.,
1979a) or in an anaerobic digestion test with unacclimated
acetate-enriched cultures (Chou et al., 1978). In two of these
cases this was explained by the toxicity of the test compound to
microorganisms (Stack, 1957; Chou et al., 1978). The BOD5 of
acrolein in river water containing microorganisms acclimated to
acrolein over 100 days was found to be 30% of the theoretical oxygen
demand (Stack, 1957). Applying methane fermentation in a mixed
reactor with a 20-day retention time, seeded by an acetate-enriched
culture, a 42% reduction in COD was achieved after 70-90 days of
acclimation to a final daily feed concentration of 10 g/litre (Chou
et al., 1978). In a static-culture flask-screening procedure,
acrolein (at a concentration of 5 or 10 mg/litre medium) was
completely degraded aerobically within 7 days, as shown by gas
chromatography and by determination of dissolved organic carbon and
total organic carbon (Tabak et al., 1981).
As discussed in section 4.2.3, acrolein in water is in
equilibrium with its hydration product. Bowmer & Higgins (1976)
observed rapid dissipation of this product in irrigation water after
a lag period of 100 h at acrolein levels below 2-3 mg/litre and
suggested that this could be due to biodegradation.
4.3.2 Bioaccumulation
On the basis of the high water solubility and chemical
reactivity of acrolein and its low experimentally determined log
n-octanol-water partition coefficient of 0.9 (Veith et al.,
1980), no bioaccumulation would be expected. Following the exposure
of Bluegill sunfish to 14C-labelled acrolein (13 µg/litre water)
for 28 days, the half-time for removal of radiolabel taken up by the
fish was more than 7 days (Barrows et al, 1980). Although the
accumulation of acrolein derived radioactively in this study was
described by the authors as bioaccumulation, it does not represent
bioaccumulation of acrolein per se but rather incorporation of the
radioactive carbon into tissues following the reaction of acrolein
with protein sulfhydryl groups or metabolism of absorbed acrolein
and incorporation of label into intermediary metabolites (see
chapter 6) (Barrows et al., 1980).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
Concentrations of acrolein measured in various types of water
at different locations are summarized in Table 5.
5.1.2 Air
Concentrations of acrolein measured in air at different
locations are summarized in Table 6. Sources of acrolein (see
chapter 3) are reflected in the levels found.
5.2 General population exposure
5.2.1 Air
The general population can be exposed to acrolein in indoor and
outdoor air (Table 6). Levels of up to 32 µg/m3 have been
measured in outdoor urban air in Japan, Sweden, and the USA. In
addition, both smokers and non-smokers are exposed to acrolein as
the product of pyrolysis of tobacco. An extensive data base shows a
delivery of 3-228 µg of acrolein per cigarette to the smoker via the
gas-phase of mainstream smoke, the amount depending on the type of
cigarette and smoking conditions (Artho & Koch, 1969; Testa &
Joigny, 1972; Rathkamp et al., 1973; Rylander, 1973; Guerin
et al., 1974; Hoffmann et al., 1975; Richter & Erfuhrth, 1979;
Magin, 1980; Rickert et al., 1980; Manning et al., 1983; Baker
et al., 1984). The delivery of total aldehydes was found to be
82-1255 µg per cigarette (Rickert et al., 1980), consisting mainly
of acetaldehyde (Harke et al., 1972; Rathkamp et al., 1973). In
the mainstream smoke of marijuana cigarettes, 92 µg of acrolein per
cigarette was found (Hoffmann et al., 1975). Non-smokers are
mainly exposed to the side-stream smoke of tobacco products.
Smoking 1 cigarette per m3 of room-space in 10-13 min was found to
lead to acrolein levels in the gas-phase of side-stream smoke of
0.84 mg/m3 (Jermini et al., 1976), 0.59 mg/m3 (derived from
Harke et al., 1972), and 0.45 mg/m3 (derived from Hugod et al.,
1978). In one of these experiments it was observed that the
presence of people in the room reduced the acrolein levels, probably
by respiratory uptake and condensation onto hair, skin, and
clothing, (Hugod et al., 1978). Evidence has also been presented
that acrolein is associated with smoke particles. The fraction of
acrolein thus associated can be deduced to be 20-75% of the total
(Hugod et al., 1978; Ayer & Yeager, 1982).
The 30-min average acrolein levels measured in air grab-samples
from four restaurants were between 11 and 23 µg/m3, the maximum
being 41 µg/m3 (Fischer et al., 1978).
Table 5. Environmental levels of acrolein in water
Type of water Location Detection limit Levels observeda Reference
(µg/litre) (µg/litre)
Surface water USA, irrigation canal, not reported Bartley & Gangstad (1974)
point of application 100
16 km downstream 50
32 km downstream 35
64 km downstream 30
Ground water USA, water in community 0.1-3.0 nd Krill & Sonzogni (1986)
and private wells
Fog water Italy, Po valley 29 nd-120 Facchini et al. (1986)
Rain water Italy, Po valley 29 nd Facchini et al. (1986)
Rain water USA, 4 urban locations not reported nd Grosjean & Wright (1983)
USA, 1 urban location 50b
Rain water Japan, source unknown 0.04 nd (2 samples) Nishikawa et al. (1987a)
1.5-3.1 (3 samples)
a nd = not detected
b includes acetone
Table 6. Environmental levels of acrolein in air
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Not defined The Netherlands 0.001 Guicherit & Schulting (1985)
Urban Los Angeles, USA 7 nd-0.025 Renzetti & Bryan (1961)
Urban Los Angeles, USA 0.002-0.032 Altshuller & McPherson (1963)
(average, 0.016)
Urban, busy road Sweden 0.1 0.012 Jonsson & Berg (1983)
Urban Japan 0.5 nd Kuwata et al. (1983)
Urban Japan 1 0.002-0.004 Nishikawa et al. (1986)
Urban, highway USSR nd-0.022 Sinkuvene (1970)
Residential, USSR
100 m from highway nd-0.013
Industrial, USSR 2.5 (max. of Plotnikova (1957)
50 m from petrochemical plant 25/25 samples)
2000 m from petrochemical plant 0.64 (max. of
21/27 samples)
1000 m from oil-seed mill USSR 0.1-0.2 Chraiber et al. (1964)
150 m from oil-seed mill USSR 0.32 Zorin (1966)
Near coal coking plant Czechoslovakia 0.004-0.009 Masek (1972)
(average, 0.007)
Table 6 (contd).
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Near pitch coking plant Czechoslovakia 0.101-0.37
(average, 0.223)
Enamelled wire plants (two), USSR Vorob'eva et al. (1982)
300 m from plants 0.28-0.36
1000 m from plants 0.14-0.46
"control area" 0.001-0.23
Coffee roasting outlet USA 200 0.59 Levaggi & Feldstein (1970)
Incinerator 0.5 0.5-0.6 Kuwata et al. (1983)
Fire-fighters' personal monitors Boston, USA 1150 > 6.9 (10% of samples) Treitman et al. (1980)
in over 200 structural fires (1-litre sample) > 0.69 (50% of samples)
Enclosed space of 8 m3 containing Japan > 69 (44% of samples) Morikawa & Yanai (1986)
burning household combustibles 1370 (max)
(15% synthetics)
Enclosed space, pyrolysis of 2-5 g USA Potts et al. (1978)
of polyethylene foam in 147 litres;
chamber at 380 °C 128-355
chamber at 340 °C < 4.6
chamber at 380 °C, red oak 18.32-412.2
chamber at 245 °C, wax candles 98.47-249.61
chamber combustion of 2-5 g of 4.58-52.67
polyethylene foam
Cooking area, heating of sunflower USSR 1.1 (max) Turuk-Pchelina (1960)
oil at 160-170 °C
Table 6 (contd).
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Beside exhaust of cars, 0.46-27.71 Cohen & Altshuller (1961),
unidentified fuel Seizinger & Dimitriades (1972),
Nishikawa et al. (1986, 1987b)
Beside exhaust of engines, 0.130-50.6 Sinkuvene (1970),
gasoline Saito et al. (1983)
diesel 0.58-7.2 Sinkuvene (1970),
Klochkovskii et al. (1981),
Saito et al. (1983)
Beside exhaust of cars, up to 6.1 Hoshika & Takata (1976)
gasoline Lipari & Swarin (1982)
diesel 0.5-2.1 Smythe & Karasek (1973),
Lipari & Swarin (1982),
Swarin & Lipari (1983)
ethanol 11 nd Lipari & Swarin (1982)
Near jet engine nd-0.12 Miyamoto (1986)
a max = maximum; nd = not detected
5.2.2 Food
In newly prepared beer, acrolein was found at a level of
2 µg/litre in one study (Greenhoff & Wheeler, 1981) but was not
detected in another (Bohmann, 1985). Aging can raise the level to
5 µg/litre (Greenhoff & Wheeler, 1981). Higher concentrations were
reported in another study (Diaz Marot et al, 1983). However, in
this case the eight compounds identified after a single
chromatographic procedure, except for acetaldehyde, did not include
the principal components identified after three successive
chromatographic procedures by the earlier authors (Greenhoff &
Wheeler, 1981) so that superimposition of acrolein and other
compounds may have occurred.
The identification of acrolein in wines (Sponholz, 1982)
followed adjustment of the pH to 8 and distillation procedures that
might have generated acrolein from a precursor. Similar
restrictions may apply to determinations in brandies (Rosenthaler &
Vegezzi, 1955; Postel & Adam, 1983). Heated and aged bone grease
contained an average level of 4.2 mg/kg (Maslowska & Bazylak, 1985).
Acrolein was further detected as a volatile in "peppery" rums and
whiskies (Mills et al., 1954; Lencrerot et al., 1984), apple
eau-de-vie (Subden et al., 1986), in white bread (Mulders & Dhont,
1972), cooked potatoes (Tajima et al., 1967), ripe tomatoes
(Hayase et al., 1984), vegetable oils (Snyder et al., 1985), raw
chicken breast muscle (Grey & Shrimpton, 1966), turkey meat
(Hrdlicka & Kuca, 1964), sour salted pork (Cantoni et al., 1969),
heated beef fat (Umano & Shibamoto, 1987), cooked horse mackerel
(Shimomura et al., 1971), and as a product of the thermal
degradation of amino acids (Alarcon, 1976).
5.3 Occupational exposure
Concentrations of acrolein measured at different places of work
are summarized in Table 7.
Table 7. Occupational exposure levels
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Production plant for acrolein USSR 0.1-8.2 Kantemirova (1975, 1977)
and methyl mercaptopropionic
aldehyde
Plant manufacturing disposable USA 20 nd-0.07 Schutte (1977)
microscope drapes, polyethylene
sheets cut by a hot wire
Workshop where metals, coated USSR 0.11-0.57 (venting) Protsenko et al. (1973)
with anti-corrosion primers 0.73-1.04
are welded (no venting)
Workshop where metals are gas-cut 0.31-1.04
Workshop where metals (no primer) nd
are welded
Coal-coking plants Czechoslovakia 0.002-0.55 Masek (1972)
Pitch-coking plants 0.11-0.493
Rubber vulcanization plant USSR 0.44-1.5 Volkova & Bagdinov (1969)
Expresser and forepress shops USSR 2-10b Chraiber et al. (1964)
in oil seed mills
Plant producing thermoplastics Finland 20 nd Pfaffli (1982)
Engine workshops, welding Denmark 15 0.031-0.605c Rietz (1985)
a nd = not detected c 3 out of 13 samples
b It should be noted that these levels exceed normal tolerance.
6. KINETICS AND METABOLISM
6.1 Absorption and distribution
The reactivity of acrolein towards free thiol groups (section
6.3) effectively reduces the bioavailability of the substance.
Controlled experiments on systemic absorption and kinetics have not
been conducted, but there are indications that acrolein is not
highly absorbed into the system since toxicological findings are
restricted to the site of exposure (see chapters 8 & 9). The fact
that McNulty et al. (1984) saw no reduction in liver glutathione
following inhalation exposure also suggests that inhaled acrolein
does not reach the liver to any great extent (section 7.3.1).
Experiments with mongrel dogs showed a high retention of
inhaled acrolein vapour in the respiratory tract. The inhaled
vapour concentrations were measured to be between 400 and
600 mg/m3. Retention was calculated by subtracting the amount
recovered in exhaled air from the amount inhaled. The total tract
retention at different ventilation rates was 80 to 85%. Upper tract
retention, measured after severing the trachea just above the
bifurcation, was 72 to 85% and was also independent of the
ventilation rate. Lower-tract retention, measured after tracheal
cannulation, was 64 to 71% and slightly decreased as ventilation
rate increased (Egle, 1972). Evidence for systemic absorption of
acrolein from the gastrointestinal tract was reported by Draminski
et al. (1983), who identified a low level of acrolein-derived
conjugates in the urine of rats after the ingestion of a single dose
of 10 mg/kg body weight. This dose killed 50% of the animals in
this study.
6.2 Reaction with body components
6.2.1 Tracer-binding studies
The in vitro binding of 14C-labelled acrolein to protein
has been investigated using rat liver microsomes. Acrolein was
found to bind to microsomal protein in the absence of NADPH or in
the presence of both NADPH and a mixed-function oxidase inhibitor.
Incubation following the addition of free sulfhydryl-containing
compounds reduced binding by 70-90%, while the addition of lysine
reduced binding by 12%. Using gel electrophoresis-fluorography it
was shown that acrolein, incubated with a reconstituted cytochrome
P-450 system, migrated mostly with cytochrome P-450. It was
concluded that acrolein is capable of alkylating free sulfhydryl
groups in cytochrome P-450 (Marinello et al., 1984).
When rats received tritium-labelled acrolein intraperitoneally
24 h after partial hepatectomy, the percentages of total liver
radioactivity recovered in the acid-soluble fraction, lipids,
proteins, RNA, and DNA were approximately 94, 3.5, 1.2, 0.6, and
0.4%, respectively, during the first 5 h after exposure.
Distribution of label was stable for at least 24 h. Acrolein was
bound to DNA at a rate of 1 molecule per 40 000 nucleotides.
A similar DNA-binding rate was observed for the green alga
Dunaliella bioculata at a 10 times higher acrolein concentration
(Munsch et al., 1974a). In in vitro studies, labelled acrolein
was found to bind to native calf thymus DNA and other DNA polymerase
templates at rates of 0.5-1 molecule per 1000 nucleotides (Munsch
et al., 1974b). In a follow-up experiment with Dunaliella
bioculata, quantitative autoradiography and electron microscopy
showed that the preferential area of cellular fixation for acrolein
was the nucleus. This fixation was stable for at least 2 days,
while that in the plastid and cytoplasm decreased initially (Marano
& Demèstere, 1976). As no adducts were identified in these studies,
these data were considered unsuitable for evaluation.
6.2.2 Adduct formation
The findings of the tracer-binding studies (section 6.2.1) are
not surprising considering the reactivity of acrolein, which makes
the molecule a likely candidate for interactions with protein and
non-protein sulfhydryl groups and with primary and secondary amine
groups such as occur in proteins and nucleic acids. These reactions
are most likely to be initiated by nucleophilic Michael addition to
the double bond (Beauchamp et al., 1985; Shapiro et al., 1986).
Beauchamp et al. (1985) discussed extensively the interactions
with protein sulfhydryl groups and primary and secondary amine
groups.
6.2.2.1 Interactions with sulfhydryl groups
The non-enzymatic reaction between equimolar amounts of
acrolein and glutathione, cysteine or acetylcysteine in a buffered
aqueous solution proceeds rapidly to near-completion, forming stable
adducts (Esterbauer et al., 1975; Alarcon, 1976).
Acrolein-acetylcysteine and acrolein-cysteine adducts yield on
reduction S-(3-hydroxypropyl)mercapturic acid and
S-(3-hydroxypropyl)-cysteine, respectively (Alarcon, 1976). The
reaction between glutathione and acrolein may be catalysed by
glutathione S-transferase, as was shown for acrolein-diethylacetal
and crotonaldehyde (Boyland & Chasseaud, 1967). Biochemical and
toxicological investigations provide more evidence for the
interaction, either enzymatic or non-enzymatic, between acrolein and
free sulfhydryl groups. In summary, it has been observed that:
* acrolein exposure of whole organisms or tissue fractions
results in glutathione depletion (section 7.3.1);
* co-exposure of organisms to acrolein and free
sulfhydryl-containing compounds protects against the
biological effects of acrolein (sections 7.3.3, 7.3.4, and
7.5);
* acrolein can inhibit enzymes containing free sulfhydryl
groups on their active site (section 7.3);
* glutathione conjugates appear in the urine of
acrolein-dosed rats (section 6.3).
6.2.2.2 In vitro interactions with nucleic acids
Non-catalytic reactions occur between acrolein and cytidine
monophosphate (Descroix, 1972), deoxyguanosine (Hemminki et al.,
1980), and deoxyadenosine (Lutz et al., 1982). Chung et al.
(1984) have identified the nucleotides resulting from the reaction
between acrolein and deoxyguanosine or calf thymus DNA (at 37 °C and
pH 7) in phosphate buffer. The adducts identified were the 6- and
8-hydroxy derivatives of cyclic 1,N2-propano-deoxyguanosine. These
adducts were shown to be formed in a dose-dependent fashion in
Salmonella typhimurium TA100 and TA104 following exposure to
acrolein and identification of the DNA adducts by an immunoassay
(Foiles et al., 1989; see also section 7.6.2). Shapiro et al.
(1986) reported that acrolein reacts with cytosine and adenosine
derivatives (at 25 °C and pH 4.2), yielding cyclic 3,N4 adducts of
cytosine derivatives and 1,N6 adducts of adenosine derivatives.
The reaction between guanosine and acrolein yields the cyclic 1,N2
adduct (at 55 °C and pH 4).
The demonstration that acrolein can cause or enhance the
formation of complexes between DNA strands (DNA-DNA crosslinking)
and between DNA and cellular proteins (DNA-protein crosslinking) is
indirect evidence that acrolein interacts with nucleic acids. This
subject is discussed further in section 7.6.1. However, no studies
have demonstrated unequivocally the interaction of acrolein with DNA
following in vivo administration to animals.
6.3 Metabolism and excretion
Acrolein is expected to be eliminated from the body via
glutathione conjugation (section 6.2.2.1). Draminski et al.
(1983) administered acrolein in corn oil orally to Wistar rats at a
dose of 10 mg/kg body weight. The urinary metabolites identified by
gas chromatography with mass spectrometric detection were
S-carboxylethyl-mercapturic acid and its methyl ester, the latter
possibly being the result of methylation of the urine samples prior
to gas chromatography. In expired air a volatile compound was
detected by gas chromatography, which was not identified; it was
reported that its retention time did not correspond to that of
methyl acrylate, acrolein or allyl alcohol. The reduced form of
S-carboxylethyl-mercapturic acid, i.e. S-hydroxypropyl-mercapturic
acid, was identified by paper and gas chromatography as the sole
metabolite in the urine of CFE rats injected subcutaneously with a
1% solution of acrolein in arachis oil at a dose of approximately
20 mg/kg body weight (Kaye, 1973). This metabolite was collected
within 24 h and accounted for 10.5% of the total dose (uncorrected
for a recovery of 58%). These data indicate that conjugation with
glutathione may dominate the metabolism of acrolein.
Data obtained in vitro show that acrolein can also be a
substrate of liver aldehyde dehydrogenase (EC 1.2.1.5) and lung or
liver microsomal epoxidase (EC 1.14.14.1) (Patel et al., 1980).
Acrolein, at concentrations of approximately 200 mg/litre medium,
was oxidized to acrylic acid by rat liver S9 supernatant, cytosol,
and microsomes, but not by lung fractions, in the presence of NAD+
or NADP+. The reaction proceeded faster with NAD+ as cofactor
than with NADP+ and was completely inhibited by disulfiram (Patel
et al., 1980). Rikans (1987) studied the kinetics of this
reaction: mitochondrial and cytosolic rat liver fractions each
contained two aldehyde dehydrogenase activities with Km values of
22-39 mg/litre and 0.8-1.4 mg/litre. Microsomes contained a high
Km activity. Incubation of rat liver or lung microsomes in the
presence of acrolein and NADPH yielded glycidaldehyde and its
hydration product glyceraldehyde, showing involvement of microsomal
cytochrome P-450-dependent epoxidase (Patel et al., 1980).
Postulated pathways of acrolein metabolism are summarized in
Figure 1.
In a human study, the intravenous injection of 1g
cyclophosphamide resulted in the excretion of 1.5% acrolein
mercapturic acid adduct in the urine (Alarcon, 1976).
As for the fate of the primary metabolites of acrolein, it has
been proposed that acrylic acid is methylated and subsequently
conjugated to yield S-carboxyl-ethylmercapturic acid, which is a
known metabolite of methyl acrylate (Draminski et al., 1983).
However, methyl acrylate has never been reported as a metabolite of
either acrolein or acrylic acid. It seems more likely that acrylic
acid is incorporated into normal cellular metabolism via the
propionate degradative pathway (Kutzman et al., 1982; Debethizy
et al., 1987). Glycidaldehyde has been shown to be a substrate
for lung and liver cytosolic glutathione S-transferase (EC
2.5.1.18) and can also be hydrated to glyceraldehyde (Patel et al.,
1980). Glyceraldehyde can be metabolized via the glycolytic
pathways.
Relative molecular 56.06
mass:
Common name: acrolein
Common synonyms: acraldehyde, acrylaldehyde (IUPAC name),
acrylic aldehyde, propenal, prop-2-enal,
prop-2-en-1-al
Common trade Acquinite, Aqualin, Aqualine, Biocide,
names: Magnicide-H, NSC 8819, Slimicide
CAS chemical name: 2-propenal
CAS registry 107-02-8
number:
RTECS registry AS 1050000
number:
Specifications: commercial acrolein contains 95.5% or
more of the compound and, as main
impurities, water (up to 3.0% by weight)
and other carbonyl compounds (up to 1.5%
by weight), mainly propanal and acetone.
Hydroquinone is added as an inhibitor of
polymerization (0.1-0.25% by weight)
(Hess et al., 1978).
2.2 Physical and chemical properties
Acrolein is a volatile, highly flammable, lacrimatory liquid at
ordinary temperature and pressure. Its odour is described as burnt
sweet, pungent, choking, and disagreeable (Hess et al., 1978;
Hawley, 1981). The compound is highly soluble in water and in
organic solvents such as ethanol and diethylether. The extreme
reactivity of acrolein can be attributed to the conjugation of a
carbonyl group with a vinyl group within its structure. Reactions
shown by acrolein include Diels-Alder condensations, dimerization
and polymerization, additions to the carbon-carbon double bond,
carbonyl additions, oxidation, and reduction. In the absence of an
inhibitor, acrolein is subject to highly exothermic polymerization
catalysed by light and air at room temperature to an insoluble,
cross-linked solid. Highly exothermic polymerization also occurs in
the presence of traces of acids or strong bases even when an
inhibitor is present. Inhibited acrolein undergoes dimerization
above 150 °C. Some physical and chemical data on acrolein are
presented in Table 1.
Table 1. Some physical and chemical data on acrolein
Physical state mobile liquid
Colour colourless (pure) or
yellowish (commercial)
Odour perception threshold 0.07 mg/m3 a
Odour recognition threshold 0.48 mg/m3 b
Melting point -87 °C
Boiling point (at 101.3 kPa) 52.7 °C
Water solubility (at 20 °C) 206 g/litre
Log n-octanol-water partition 0.9c
coefficient
Relative density (at 20 °C) 0.8427
Relative vapour density 1.94
Vapour pressure (at 20 °C) 29.3 kPa (220 mmHg)
Flash point (open cup) -18 °C
Flash point (closed cup) -26 °C
Flammability limits 2.8-31.0% by volume
a Sinkuvene (1970) (see Table 12)
b Leonardos et al. (1969) (see Table 12)
c Experimentally derived by Veith et al. (1980)
2.3 Conversion factors
At 25 °C and 101.3 kPa (760 mmHg), 1 ppm of acrolein =
2.29 mg/m3 air and 1 mg of acrolein per m3 air = 0.44 ppm.
2.4 Analytical methods
A summary of relevant methods of sampling and analysis is
presented in Table 2.
Tejada (1986) presented data showing that the air analysis HPLC
method employing a 2,4-dinitrophenylhydrazine-coated SP cartridge
(Kuwata et al., 1983) is equivalent to that using impingers with
2,4-dinitrophenylhydrazine in acetonitrile (Lipari & Swarin, 1982).
The latter method was also evaluated in several laboratories and was
found adequate for the evaluation of the working environment (Perez
et al., 1984). Nevertheless, the separation of
2,4-dinitrophenylhydrazine derivatives of acrolein and acetone by
HPLC can present difficulties (Olson & Swarin, 1985). A highly
sensitive electrochemical detection method was found by Jacobs &
Kissinger (1982) to be suitable and was later improved by Facchini
et al. (1986).
A personal sampling device for firemen, which employs molecular
sieves, was described by Treitman et al. (1980). Other sampling
methods using solid sorbents coated with 2,4-dinitrophenylhydrazine,
as applied by Kuwata et al. (1983) for location monitoring, were
found suitable for personal sampling procedures (Andersson et al.,
1981; Rietz, 1985).
The NIOSH procedure for industrial air monitoring involves
absorption onto N-hydroxymethylpiperazine-coated XAD-2 resin and gas
chromatographic analysis of the toluene eluate (US-NIOSH, 1984).
This method has been validated by a Shell Development Company
analytical laboratory and was not revised by NIOSH in 1989.
Table 2. Sampling, preparation, and analysis of acrolein
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air absorption in ethanolic UV spectrometry 20 µg/m3 0.02 m3 suitable for location Manita &
solution of monitoring; designed Goldberg
thiosemicar-bazide for analysis of ambient (1970)
and hydrochloric acid air; interference from
other alpha, ß-unsaturated
aldehydes
air absorption in ethanolic colorimetry 20 µg/m3 0.05 m3 suitable for location Cohen &
solution of monitoring; designed Altshuller
4-hexylresor-cinol, for analysis of ambient (1961), Katz
mercuric chloride, and and industrial air and (1977), Harke
trichloroacetic acid exhaust gas; slight et al. (1972)
interference from dienes
and alpha, ß-unsaturated
aldehydes; also suitable
for analysis of smoke
air absorption in aqueous colorimetry 20 µg/m3 0.06 m3 suitable for location Pfaffli (1982),
sodium bisulfite; monitoring; designed Katz (1977),
addition of ethanolic for analysis of ambient Ayer & Yeager
solution of and industrial air and (1982)
4-hexylresorcinol, cigarette smoke
mercuric chloride, and
trichloroacetic acid;
heating
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air collection on molecular fluorimetry 2 µg/m3 0.06 m3 suitable for location Suzuki & Imai
sieve 3A and 13X; monitoring; designed (1982)
desorption by heat; for analysis of ambient
collection in water; air; interference from
reaction with aqueous croton-aldehyde and
o-aminobiphenyl-sulfuric methylvinyl ketone
acid; heating
air adsorption on Poropak N; gas chromatography < 600 0.003-0.008 suitable for personal Campbell & Moore
desorption by heat with flame ionization µg/m3 m3 monitoring (1979)
detection
air adsorption on Tenax GC gas chromatography 0.1 0.006-0.019 suitable for location Krost et al.
desorption by heat; with mass µg/m3 m3 and personal (1982)
cryofocussing spectrometric (breakthrough designed for
detection volume) analysis of ambient air
air cryogradient sampling on gas chromatography 0.1 µg/m3 0.003 m3 suitable for location Jonsson & Berg
siloxane-coated with flame monitoring; designed (1983)
chromosorb W AW; ionization and mass for analysis of ambient
desorption by heat spectrometric air
detection
air absorption into ethanol; gas chromatography 1 µg/m3 0.003-0.04 suitable for location Nishikawa et al.
reaction with aqueous with electron m3 monitoring; designed (1986)
methoxyamine capture detection for analysis of ambient
hydrochlo-ride-sodium air
acetate; bromination;
adsorption on SP-cartridge;
elution by diethyl ether
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air absorption into aqueous gas chromatography 435 0.01 m3 designed for analysis Saito et al.
2,4-DNPH hydrochloride; with flame µg/m3 of exhaust gas (1983)
extraction by chloroform; ionization detection
and anthracene as
internal standard
air collection in cold trap; gas chromatography designed for analysis Rathkamp et al.
warming trap of tobacco smoke (1973)
air direct introduction gas chromatography 0.1 2 cm3 designed for analysis Richter &
g/m3 of tobacco smoke Erfuhrth (1979)
air adsorption on HPLC with UV 0.5 0.1 m3 suitable for location Kuwata et al.
2,4-DNPH-phosphoric acid detection µg/m3 monitoring; designed (1983)
coated SP-cartridge; for analysis of
elution by acetonitrile industrial and ambient
air
air absorption into solution HPLC with UV 11 0.02 m3 suitable for location Lipari & Swarin
of 2,4-DNPH-perchloric detection µg/m3 monitoring; designed (1982)
acid in acetonitrile; for analysis of exhaust
gas
air absorption into solution HPLC with 1.4 0.02 m3 suitable for location Swarin & Lipari
of 2-diphenylacetyl-1,3- fluorescence µg/m3 monitoring; designed (1983)
indandione-1-hydrazone detection for analysis of exhaust
and hydrochloric acid in gas
acetonitrile
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
air absorption into aqueous HPLC with 10 µg/ 1 cigarette designed for analysis Manning et al.
2,4-DNPH-hydrochloric UV detection cigarette of cigarette smoke (1983)
acid and chloroform gas phase
air absorption into gas chromatography 229 0.05 m3 suitable for US-NIOSH (1984)
2-(hydroxymethyl) with µg/m3 personal monitoring
piperidine on XAD-2; nitrogen-specific
elution by toluene detector
water addition of colorimetry 400 0.0025 slight interference Cohen &
4-hexyl-resorcinol-mercuric µg/litre litre from dienes and alpha, Altshuller (1961)
chloride solution and ß-unsaturated aldehydes
trichloroacetic acid to
sample in ethanol
water reaction with methoxylamine gas chromatography 0.4 designed for analysis Nishikawa et al.
hydrochloride-sodium with electron µg/litre of rain water (1987a)
acetate; bromination; capture detection
adsorption on SP
cartridge; elution by
diethyl ether
water reaction with 2,4-DNPH; HPLC with 29 designed for analysis Facchini et al.
with addition of electro-chemical µg/litre of fog and rain water (1986)
iso-octane detection
Table 2 (contd).
Medium Sampling method Analytical method Detection Sample Comments Reference
limit size
water low pressure distillation; HPLC with UV < 0.1 1000 ml designed for analysis Greenhoff &
cryofocussing into aqueous detection µg/litre of beer Wheeler (1981)
2,4-DNPH-hydro-chloric acid;
extraction by chloroform;
TLC and magnesia-silica-gel
column chromatography
biological reaction with aqueous fluorimetry 2.8 2 ml designed for analysis Alarcon (1968)
media m-aminophenol-hydroxyl- µg/litre of biological media
amine-hydrochloride-
hydrochloric acid;
heating
tissue homogenization; reaction HPLC with UV Boor & Ansari
with aqueous detection (1986)
2,4-DNPH-sulfuric acid;
extraction by chloroform
food ultrasonic homogenization gas chromatography 590 1000 mg designed for analysis Easley et al.
in cooled water; purging with mass µg/kg of volatile organic (1981)
by helium; trapping on spectrometric compounds in fish
Tenax GC-silica-gel-charcoal; detection
desorption by heat
3. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
3.1 Natural sources
Acrolein is reported to occur naturally, e.g., in the essential
oil extracted from the wood of oak trees (IARC, 1979), in tomatoes
(Hayase et al., 1984), and in certain other foods (section
5.2.2.).
3.2 Anthropogenic sources
3.2.1 Production
3.2.1.1 Production levels and processes
In 1975, the worldwide production of acrolein was estimated to
be 59 000 tonnes, although at this time production figures probably
only related to isolated acrolein (Hess et al., 1978). It is
mainly produced in the USA, Japan, France, and Germany. In
addition, acrolein is produced as an unisolated intermediate in the
synthesis of acrylic acid and its esters. In 1983, 216 000 to
242 000 tonnes of acrolein was reported to be used in the USA for
this purpose, amounting to 91-93% of the total production in that
country (Beauchamp et al.,1985). Formerly acrolein was produced by
vapour phase condensation of acetaldehyde and formaldehyde (Hess
et al., 1978). Although this process is now virtually obsolete,
some production via this pathway has continued in the USSR (IRPTC,
1984). Worldwide, most acrolein is now produced by the direct
catalytic oxidation of propene. Catalysts containing bismuth,
molybdenum, and other metal oxides enable a conversion of propene of
over 90% and have a high selectivity for acrolein. By-products are
acrylic acid, acetic acid, acetaldehyde, and carbon oxides (Hess
et al., 1978; Ohara et al., 1987). Another catalyst used for
this process, cuprous oxide, has a lower performance (Hess et al.,
1978; IRPTC, 1984).
3.2.1.2 Emissions
Closed-systems are used in production facilities, and releases
of acrolein to the environment are expected to be low, especially
when the compound is directly converted to acrylic acid and its
esters. The compound is emitted via exhaust fumes, process waters
and waste, and following leakage of equipment. Production losses in
the USA in 1978 were estimated to be 35 tonnes or approximately 0.1%
of the amount of isolated acrolein produced (Beauchamp et al.,
1985).
The air emission factor of acrolein in the synthesis of
acrylonitrile in the Netherlands has been reported to be 0.1-0.3 kg
per tonne of acrylonitrile (DGEP, 1988). Acrolein has also been
identified in the process streams of plants manufacturing acrylic
acid (Serth et al., 1978). The application of acrolein as a
biocide brings the chemical directly into the aquatic environment.
3.2.2 Uses
The principal use of acrolein is as an intermediate in the
synthesis of numerous chemicals, in particular acrylic acid and its
lower alkyl esters and DL-methionine, an essential amino acid used
as a feed supplement for poultry and cattle. In the USA, in 1983, 91
to 93% of the total quantity of acrolein produced was converted to
acrylic acid and its esters, and 5% to methionine (Beauchamp
et al., 1985). Other derivatives of acrolein are:
2-hydroxyadipaldehyde, 1,2,6-hexanetriol, lysine, glutaraldehyde,
tetrahydro-benzaldehyde, pentanediols, 1,4-butanediol,
tetrahydrofuran, pyridine, 3-picoline, allyl alcohol, glycerol,
quinoline, homopolymers, and copolymers (Hess et al., 1978).
Among the direct uses of acrolein, its application as a biocide
is the most significant one. Acrolein at a concentration of
6-10 mg/litre in water is used as an algicide, molluscicide, and
herbicide in recirculating process water systems, irrigation
channels, cooling water towers, and water treatment ponds (Hess
et al., 1978). About 66 tonnes of acrolein is reported to be used
annually in Australia to control submersed plants in about 4000 km
of irrigation channels (Bowmer & Sainty, 1977; Bowmer & Smith,
1984). Acrolein protects feed lines for subsurface injection of
waste water, liquid hydrocarbon fuels and oil wells against the
growth of microorganisms, and at 0.4-0.6 mg/litre it controls slime
formation in the paper industry. The substance can also be used as
a tissue fixative, warning agent in methyl chloride refrigerants,
leather tanning agent, and for the immobilization of enzymes via
polymerization, etherification of food starch, and the production of
perfumes and colloidal metals (Hess et al., 1978; IARC, 1985).
3.2.3 Waste disposal
Acrolein wastes mainly arise during production and processing
of the compound and its derivatives.
Aqueous wastes with low concentrations of acrolein are usually
neutralized with sodium hydroxide and fed to a sewage treatment
plant for biological secondary treatment. Concentrated wastes are
reprocessed whenever possible or burnt in special waste incinerators
(IRPTC, 1985).
3.2.4 Other sources
Incomplete combustion and thermal degradation (pyrolysis) of
organic substances such as fuels, tobacco, fats, synthetic and
natural polymers, and foodstuffs frequently result in the emission
of aldehydes. Reported levels are presented in section 5.1.2.
Emission rates for several of such sources are presented in Table 3.
The major sources of aldehydes in ambient air formed by
incomplete combustion and/or thermal degradation are residential
wood burning, burning of coal, oil or natural gas in power plants,
burning of fuels in automobiles, and burning of refuse and
vegetation (Lipari et al., 1984). Formaldehyde is the major
aldehyde emitted, but acrolein may make up 3 to 10 % of total
automobile exhaust aldehydes and 1 to 13% of total wood-smoke
aldehydes (Fracchia et al., 1967; Oberdorfer, 1971; Lipari
et al., 1984). Modern catalytic converters in automobiles almost
completely remove these aldehydes from exhaust gases. Acrolein may
constitute up to 7% of the aldehydes in cigarette smoke (Rickert
et al., 1980).
Aldehydes are also formed by photochemical oxidation of
hydrocarbons in the atmosphere. Leach et al. (1964) concluded
that formaldehyde and acrolein would constitute 50% and 5%,
respectively, of the total aldehyde present in irradiated diluted
car exhaust. Acrolein was considered to be mainly a product of
oxidation of 1,3-butadiene (Schuck & Renzetti, 1960; Leach et al.,
1964), but propene (Graedel et al., 1976; Takeuchi & Ibusuki,
1986), 1,3-pentadiene, 2-methyl-1,3-pentadiene (Altshuller &
Bufalini, 1965), and crotonaldehyde (IRPTC, 1984) have also been
implicated. The photooxidation of 1,3-butadiene in an irradiated
smog chamber, also containing nitrogen monoxide and air, gave rise
to the formation of acrolein (55% yield based on 1,3-buta-diene
initial concentrations). The rate of formation of acrolein was the
same as that of 1,3-butadiene consumption. (Maldotti et al.,
1980). Cancer chemotherapy patients receiving cyclo-phosphamide are
exposed to acrolein, which results from the metabolism of this drug.
Table 3. Emission rates of aldehydes
Source Total Formaldehyde Acrolein Unit Reference
aldehydes
Residential wood burning 0.6-2.3 0.089-0.708 0.021-0.132 g/kg Lipari et al. (1984)
Power plants - coal 0.002 g/kg Natusch (1978)
- oil 0.1 g/kg
- natural gas 0.2 g/kg
Automobiles - petrol 0.01-0.08 g/km Lipari et al. (1984)
0.4-2.3 0.2-1.6 0.01-0.16 g/litre Guicherit & Schulting
(1985)
8.4-63 4-38 1-2 mg/min Lies et al. (1986)
- diesel 0.021 g/km Lipari et al. (1984)
1-2 0.5-1.4 0.03-0.20 g/litre Guicherit & Schulting
(1985)
0.0080 0.0002 g/litre Smythe & Karasek (1973)
44 18 3 mg/min Lies et al. (1986)
Vegetation burning 0.003 g/kg Lipari et al. (1984)
Cigarette smoking 82-1203 3-228 µg/cigarette see section 5.2.1
Pyrolysis of flue-cured tobacco 42-82 µg/g Baker et al. (1984)
Heating in air (at up to 400 °C) of
- polyethylene up to 75 up to 20 g/kg Morikawa (1976)
- polypropylene up to 54 up to 8 g/kg
- cellulose up to 27 up to 3 g/kg
- glucose up to 18 up to 1 g/kg
- wood up to 15 up to 1 g/kg
Smouldering cellulosic materials 0.66-10.02 0.46-1.74 g/kg
Hot wire cutting (50 cm long at 215 °C)
of PVC wrapping film 27-151 ng/cut Boettner & Ball (1980)
4. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
4.1 Transport and distribution between media
Acrolein is released into the atmosphere during the production
of the compound itself and its derivatives, in industrial and
non-industrial processes involving incomplete combustion and/or
thermal degradation of organic substances, and, indirectly, by
photochemical oxidation of hydrocarbons in the atmosphere. Emissions
to water and soil occur during production of the compound itself and
its derivatives, and through biocidal use, spills, and waste
disposal (chapter 3).
Intercompartmental transport of acrolein should be limited in
view of its high reactivity, as is discussed in sections 4.2. and
4.3. Considering the high vapour pressure of acrolein, some
transfer across the water-air and soil-air boundaries can be
expected. In a laboratory experiment Bowmer et al. (1974)
explained a difference of 10% between the amount of total aldehydes
(acrolein and non-volatile degradation products, see section 4.2) in
an open tank and that in closed bottles by volatilization. It was
noted that volatilization may be greatly increased by turbulence.
Adsorption to soil, often involving probable reaction with soil
components, may impair the transfer of a compound to air or ground
water. The tendency of untreated acrolein to adsorb to soil
particles can be expressed in terms of Koc, the ratio of the
amount of chemical adsorbed (per unit weight of organic carbon) to
the concentration of the chemical in solution at equilibrium. Based
on the available empirical relationships derived for estimating
Koc, a low soil adsorption potential is expected (Lyman et al.,
1982). Experimentally, acrolein showed a limited (30% of a 0.1%
solution) adsorbability to activated carbon (Giusti et al., 1974).
4.2 Abiotic degradation
Once in the atmosphere, acrolein may photodissociate or react
with hydroxyl radicals and ozone. In water, photolysis or hydration
may occur. These processes will be discussed in the following
sections.
4.2.1 Photolysis
Acrolein shows a moderate absorption of light within the solar
spectrum at 315 nm (with a molar extinction coefficient of 26
litre/mol per cm) and therefore would be expected to be
photoreactive (Lyman et al., 1982). However, irradiation of an
acrolein-air mixture by artificial sunlight did not result in any
detectable photolysis (Maldotti et al., 1980). Irradiation of
acrolein vapour in high vacuum apparatus at 313 nm and 30-200 °C
resulted in the formation of trace amounts of ethene and carbon
oxides (Osborne et al., 1962; Coomber & Pitts, 1969).
4.2.2 Photooxidation
Experimentally determined rate constants for the pseudo first
order reaction between acrolein and hydroxyl radicals in the
atmosphere are presented in Table 4. Also shown are the atmospheric
residence times, which can be derived from the rate constants
assuming a 12-h daytime average hydroxyl radical concentration of
2 x 10-15 mol/litre (Lyman et al., 1982). The estimated
atmospheric residence time of acrolein of approximately 20 h will
decrease with increasing hydroxyl radical concentrations in more
polluted atmospheres and increase with the decline in temperature,
and consequently the rate of reaction, at higher altitudes. Other
variations will be caused by seasonal, altitudinal, diurnal, and
geographical fluctuations in the hydroxyl radical concentration.
Other potentially significant gas-phase reactions in the
atmosphere may occur between acrolein and ozone or nitrate radicals.
Experimentally determined rate constants and atmospheric residence
times for these reactions are shown in Table 4. The atmospheric
residence times were estimated assuming a 24-h average ozone
concentration of 1.6 x 10-9 mol/litre (Lyman et al., 1982) and a
12-h night-time average nitrate radical concentration of 4.0 x
10-12 mol/litre (Atkinson et al., 1987). It can be concluded
that the tropospheric removal processes for acrolein are dominated
by the reaction with hydroxyl radicals. Carbon monoxide,
formaldehyde, glycoaldehyde, ketene, and peroxypropenyl nitrate have
been identified as products of the reaction between acrolein and
hydroxyl radicals (Edney et al., 1982), and glyoxal was also
suggested to be one of the reaction products (Edney et al., 1982,
1986b).
As discussed in section 3.2.4, acrolein is also formed by the
photochemical degradation of hydrocarbons in general and
1,3-butadiene in particular. When mixtures of acrolein or
1,3-butadiene with nitrogen monoxide and air were irradiated in a
smog chamber, the time required for the half-conversion of
1,3-butadiene to acrolein was always shorter than that required for
the half conversion of acrolein. It was concluded that in a real
atmospheric environment, with continuous emissions of 1,3-butadiene,
acrolein will be continuously formed (Bignozzi et al., 1980).
Table 4. Rate constants and calculated atmospheric residence times for gas-phase reactions of acrolein.
Reactant Temperature Technique used Rate constant Atmospheric Reference
(°C) (litre/mol per sec) residence time
(h)
OH radical 25 relative rate 16 x 109 17 Maldotti et al. (1980)
25 relative rate 11.4 x 109 24 Kerr & Sheppard (1981)
23 absolute rate 20.6 x 109 13 Edney et al. (1982)
26 relative rate 11.4 x 109 24 Atkinson et al. (1983)
23 relative rate 12.3 x 109 23 Edney et al. (1986a)
O3 23 absolute rate 16.9 x 104 1029 Atkinson et al. (1981)
NO3 25 relative rate 35.5 x 104 391 Atkinson et al. (1987)
4.2.3 Hydration
Acrolein does not contain hydrolysable groups but it does react
with water in a reversible hydration reaction to 3-hydroxypropanal.
The equilibrium constant is pH independent and increases appreciably
with increasing initial acrolein concentration, presumably because
of the reversible dimerization of 3-hydroxypropanal (Hall & Stern,
1950). In more dilute solutions the equilibrium constant was found
to approach 12 at 20 °C (Pressman & Lucas, 1942; Hall & Stern,
1950), indicating that approximately 92% of acrolein is in the
hydrated form at equilibrium. This agrees well with the equilibrium
concentrations found in buffered solutions of acrolein at 21 °C
(Bowmer & Higgins, 1976).
The hydration of acrolein is a first order reaction with
respect to acrolein. The rate constants are independent of the
initial acrolein concentrations but increase with increasing acid
concentrations (Pressman & Lucas, 1942; Hall & Stern, 1950) and also
when the pH is raised from 5 to 9 (Bowmer & Higgins, 1976). In
dilute buffered solutions of acrolein in distilled water the rate
constant is 0.015 h-1 at 21 °C and pH 7, corresponding to a
half-life of 46 h. However, although in laboratory experiments an
equilibrium is reached with 8% of the original acrolein and 85% of
total aldehydes still present, these do not persist in river waters
so that other methods of dissipation must exist (Bowmer et al.,
1974; Bowmer & Higgins, 1976; see also section 4.3.1).
The dissipation of acrolein in field experiments in irrigation
channels also followed first order kinetics and was faster than
could be predicted assuming hydration alone. First order rate
constants, based on the data thought to be most reliable varied
between 0.104 and 0.208 h-1 at pH values of 7.1 to 7.5 and
temperatures of 16 to 24 °C. From these rate constants, half-lives
of between 3 and 7 h can be calculated (O'Loughlin & Bowmer, 1975;
Bowmer & Higgins, 1976; Bowmer & Sainty, 1977). The latter data
agree better than the laboratory data with the results of bioassays
with bacteria and fish, which show that aged acrolein solutions
become biocidally inactive after approximately 120 to 180 h at a pH
of 7 (Kissel et al., 1978). Apparently processes other than
hydration also contribute to acrolein dissipation, e.g., catalysis
other than acid-base catalysis, adsorption, and volatilization
(Bowmer & Higgins, 1976).
4.3 Biotransformation
4.3.1 Biodegradation
No biological degradation of acrolein was observed in two BOD5
tests with unacclimated microorganisms (Stack, 1957; Bridie et al.,
1979a) or in an anaerobic digestion test with unacclimated
acetate-enriched cultures (Chou et al., 1978). In two of these
cases this was explained by the toxicity of the test compound to
microorganisms (Stack, 1957; Chou et al., 1978). The BOD5 of
acrolein in river water containing microorganisms acclimated to
acrolein over 100 days was found to be 30% of the theoretical oxygen
demand (Stack, 1957). Applying methane fermentation in a mixed
reactor with a 20-day retention time, seeded by an acetate-enriched
culture, a 42% reduction in COD was achieved after 70-90 days of
acclimation to a final daily feed concentration of 10 g/litre (Chou
et al., 1978). In a static-culture flask-screening procedure,
acrolein (at a concentration of 5 or 10 mg/litre medium) was
completely degraded aerobically within 7 days, as shown by gas
chromatography and by determination of dissolved organic carbon and
total organic carbon (Tabak et al., 1981).
As discussed in section 4.2.3, acrolein in water is in
equilibrium with its hydration product. Bowmer & Higgins (1976)
observed rapid dissipation of this product in irrigation water after
a lag period of 100 h at acrolein levels below 2-3 mg/litre and
suggested that this could be due to biodegradation.
4.3.2 Bioaccumulation
On the basis of the high water solubility and chemical
reactivity of acrolein and its low experimentally determined log
n-octanol-water partition coefficient of 0.9 (Veith et al.,
1980), no bioaccumulation would be expected. Following the exposure
of Bluegill sunfish to 14C-labelled acrolein (13 µg/litre water)
for 28 days, the half-time for removal of radiolabel taken up by the
fish was more than 7 days (Barrows et al, 1980). Although the
accumulation of acrolein derived radioactively in this study was
described by the authors as bioaccumulation, it does not represent
bioaccumulation of acrolein per se but rather incorporation of the
radioactive carbon into tissues following the reaction of acrolein
with protein sulfhydryl groups or metabolism of absorbed acrolein
and incorporation of label into intermediary metabolites (see
chapter 6) (Barrows et al., 1980).
5. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
5.1 Environmental levels
5.1.1 Water
Concentrations of acrolein measured in various types of water
at different locations are summarized in Table 5.
5.1.2 Air
Concentrations of acrolein measured in air at different
locations are summarized in Table 6. Sources of acrolein (see
chapter 3) are reflected in the levels found.
5.2 General population exposure
5.2.1 Air
The general population can be exposed to acrolein in indoor and
outdoor air (Table 6). Levels of up to 32 µg/m3 have been
measured in outdoor urban air in Japan, Sweden, and the USA. In
addition, both smokers and non-smokers are exposed to acrolein as
the product of pyrolysis of tobacco. An extensive data base shows a
delivery of 3-228 µg of acrolein per cigarette to the smoker via the
gas-phase of mainstream smoke, the amount depending on the type of
cigarette and smoking conditions (Artho & Koch, 1969; Testa &
Joigny, 1972; Rathkamp et al., 1973; Rylander, 1973; Guerin
et al., 1974; Hoffmann et al., 1975; Richter & Erfuhrth, 1979;
Magin, 1980; Rickert et al., 1980; Manning et al., 1983; Baker
et al., 1984). The delivery of total aldehydes was found to be
82-1255 µg per cigarette (Rickert et al., 1980), consisting mainly
of acetaldehyde (Harke et al., 1972; Rathkamp et al., 1973). In
the mainstream smoke of marijuana cigarettes, 92 µg of acrolein per
cigarette was found (Hoffmann et al., 1975). Non-smokers are
mainly exposed to the side-stream smoke of tobacco products.
Smoking 1 cigarette per m3 of room-space in 10-13 min was found to
lead to acrolein levels in the gas-phase of side-stream smoke of
0.84 mg/m3 (Jermini et al., 1976), 0.59 mg/m3 (derived from
Harke et al., 1972), and 0.45 mg/m3 (derived from Hugod et al.,
1978). In one of these experiments it was observed that the
presence of people in the room reduced the acrolein levels, probably
by respiratory uptake and condensation onto hair, skin, and
clothing, (Hugod et al., 1978). Evidence has also been presented
that acrolein is associated with smoke particles. The fraction of
acrolein thus associated can be deduced to be 20-75% of the total
(Hugod et al., 1978; Ayer & Yeager, 1982).
The 30-min average acrolein levels measured in air grab-samples
from four restaurants were between 11 and 23 µg/m3, the maximum
being 41 µg/m3 (Fischer et al., 1978).
Table 5. Environmental levels of acrolein in water
Type of water Location Detection limit Levels observeda Reference
(µg/litre) (µg/litre)
Surface water USA, irrigation canal, not reported Bartley & Gangstad (1974)
point of application 100
16 km downstream 50
32 km downstream 35
64 km downstream 30
Ground water USA, water in community 0.1-3.0 nd Krill & Sonzogni (1986)
and private wells
Fog water Italy, Po valley 29 nd-120 Facchini et al. (1986)
Rain water Italy, Po valley 29 nd Facchini et al. (1986)
Rain water USA, 4 urban locations not reported nd Grosjean & Wright (1983)
USA, 1 urban location 50b
Rain water Japan, source unknown 0.04 nd (2 samples) Nishikawa et al. (1987a)
1.5-3.1 (3 samples)
a nd = not detected
b includes acetone
Table 6. Environmental levels of acrolein in air
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Not defined The Netherlands 0.001 Guicherit & Schulting (1985)
Urban Los Angeles, USA 7 nd-0.025 Renzetti & Bryan (1961)
Urban Los Angeles, USA 0.002-0.032 Altshuller & McPherson (1963)
(average, 0.016)
Urban, busy road Sweden 0.1 0.012 Jonsson & Berg (1983)
Urban Japan 0.5 nd Kuwata et al. (1983)
Urban Japan 1 0.002-0.004 Nishikawa et al. (1986)
Urban, highway USSR nd-0.022 Sinkuvene (1970)
Residential, USSR
100 m from highway nd-0.013
Industrial, USSR 2.5 (max. of Plotnikova (1957)
50 m from petrochemical plant 25/25 samples)
2000 m from petrochemical plant 0.64 (max. of
21/27 samples)
1000 m from oil-seed mill USSR 0.1-0.2 Chraiber et al. (1964)
150 m from oil-seed mill USSR 0.32 Zorin (1966)
Near coal coking plant Czechoslovakia 0.004-0.009 Masek (1972)
(average, 0.007)
Table 6 (contd).
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Near pitch coking plant Czechoslovakia 0.101-0.37
(average, 0.223)
Enamelled wire plants (two), USSR Vorob'eva et al. (1982)
300 m from plants 0.28-0.36
1000 m from plants 0.14-0.46
"control area" 0.001-0.23
Coffee roasting outlet USA 200 0.59 Levaggi & Feldstein (1970)
Incinerator 0.5 0.5-0.6 Kuwata et al. (1983)
Fire-fighters' personal monitors Boston, USA 1150 > 6.9 (10% of samples) Treitman et al. (1980)
in over 200 structural fires (1-litre sample) > 0.69 (50% of samples)
Enclosed space of 8 m3 containing Japan > 69 (44% of samples) Morikawa & Yanai (1986)
burning household combustibles 1370 (max)
(15% synthetics)
Enclosed space, pyrolysis of 2-5 g USA Potts et al. (1978)
of polyethylene foam in 147 litres;
chamber at 380 °C 128-355
chamber at 340 °C < 4.6
chamber at 380 °C, red oak 18.32-412.2
chamber at 245 °C, wax candles 98.47-249.61
chamber combustion of 2-5 g of 4.58-52.67
polyethylene foam
Cooking area, heating of sunflower USSR 1.1 (max) Turuk-Pchelina (1960)
oil at 160-170 °C
Table 6 (contd).
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Beside exhaust of cars, 0.46-27.71 Cohen & Altshuller (1961),
unidentified fuel Seizinger & Dimitriades (1972),
Nishikawa et al. (1986, 1987b)
Beside exhaust of engines, 0.130-50.6 Sinkuvene (1970),
gasoline Saito et al. (1983)
diesel 0.58-7.2 Sinkuvene (1970),
Klochkovskii et al. (1981),
Saito et al. (1983)
Beside exhaust of cars, up to 6.1 Hoshika & Takata (1976)
gasoline Lipari & Swarin (1982)
diesel 0.5-2.1 Smythe & Karasek (1973),
Lipari & Swarin (1982),
Swarin & Lipari (1983)
ethanol 11 nd Lipari & Swarin (1982)
Near jet engine nd-0.12 Miyamoto (1986)
a max = maximum; nd = not detected
5.2.2 Food
In newly prepared beer, acrolein was found at a level of
2 µg/litre in one study (Greenhoff & Wheeler, 1981) but was not
detected in another (Bohmann, 1985). Aging can raise the level to
5 µg/litre (Greenhoff & Wheeler, 1981). Higher concentrations were
reported in another study (Diaz Marot et al, 1983). However, in
this case the eight compounds identified after a single
chromatographic procedure, except for acetaldehyde, did not include
the principal components identified after three successive
chromatographic procedures by the earlier authors (Greenhoff &
Wheeler, 1981) so that superimposition of acrolein and other
compounds may have occurred.
The identification of acrolein in wines (Sponholz, 1982)
followed adjustment of the pH to 8 and distillation procedures that
might have generated acrolein from a precursor. Similar
restrictions may apply to determinations in brandies (Rosenthaler &
Vegezzi, 1955; Postel & Adam, 1983). Heated and aged bone grease
contained an average level of 4.2 mg/kg (Maslowska & Bazylak, 1985).
Acrolein was further detected as a volatile in "peppery" rums and
whiskies (Mills et al., 1954; Lencrerot et al., 1984), apple
eau-de-vie (Subden et al., 1986), in white bread (Mulders & Dhont,
1972), cooked potatoes (Tajima et al., 1967), ripe tomatoes
(Hayase et al., 1984), vegetable oils (Snyder et al., 1985), raw
chicken breast muscle (Grey & Shrimpton, 1966), turkey meat
(Hrdlicka & Kuca, 1964), sour salted pork (Cantoni et al., 1969),
heated beef fat (Umano & Shibamoto, 1987), cooked horse mackerel
(Shimomura et al., 1971), and as a product of the thermal
degradation of amino acids (Alarcon, 1976).
5.3 Occupational exposure
Concentrations of acrolein measured at different places of work
are summarized in Table 7.
Table 7. Occupational exposure levels
Type of site Country Detection limit Levels observeda Reference
(µg/m3) (mg/m3)
Production plant for acrolein USSR 0.1-8.2 Kantemirova (1975, 1977)
and methyl mercaptopropionic
aldehyde
Plant manufacturing disposable USA 20 nd-0.07 Schutte (1977)
microscope drapes, polyethylene
sheets cut by a hot wire
Workshop where metals, coated USSR 0.11-0.57 (venting) Protsenko et al. (1973)
with anti-corrosion primers 0.73-1.04
are welded (no venting)
Workshop where metals are gas-cut 0.31-1.04
Workshop where metals (no primer) nd
are welded
Coal-coking plants Czechoslovakia 0.002-0.55 Masek (1972)
Pitch-coking plants 0.11-0.493
Rubber vulcanization plant USSR 0.44-1.5 Volkova & Bagdinov (1969)
Expresser and forepress shops USSR 2-10b Chraiber et al. (1964)
in oil seed mills
Plant producing thermoplastics Finland 20 nd Pfaffli (1982)
Engine workshops, welding Denmark 15 0.031-0.605c Rietz (1985)
a nd = not detected c 3 out of 13 samples
b It should be noted that these levels exceed normal tolerance.
6. KINETICS AND METABOLISM
6.1 Absorption and distribution
The reactivity of acrolein towards free thiol groups (section
6.3) effectively reduces the bioavailability of the substance.
Controlled experiments on systemic absorption and kinetics have not
been conducted, but there are indications that acrolein is not
highly absorbed into the system since toxicological findings are
restricted to the site of exposure (see chapters 8 & 9). The fact
that McNulty et al. (1984) saw no reduction in liver glutathione
following inhalation exposure also suggests that inhaled acrolein
does not reach the liver to any great extent (section 7.3.1).
Experiments with mongrel dogs showed a high retention of
inhaled acrolein vapour in the respiratory tract. The inhaled
vapour concentrations were measured to be between 400 and
600 mg/m3. Retention was calculated by subtracting the amount
recovered in exhaled air from the amount inhaled. The total tract
retention at different ventilation rates was 80 to 85%. Upper tract
retention, measured after severing the trachea just above the
bifurcation, was 72 to 85% and was also independent of the
ventilation rate. Lower-tract retention, measured after tracheal
cannulation, was 64 to 71% and slightly decreased as ventilation
rate increased (Egle, 1972). Evidence for systemic absorption of
acrolein from the gastrointestinal tract was reported by Draminski
et al. (1983), who identified a low level of acrolein-derived
conjugates in the urine of rats after the ingestion of a single dose
of 10 mg/kg body weight. This dose killed 50% of the animals in
this study.
6.2 Reaction with body components
6.2.1 Tracer-binding studies
The in vitro binding of 14C-labelled acrolein to protein
has been investigated using rat liver microsomes. Acrolein was
found to bind to microsomal protein in the absence of NADPH or in
the presence of both NADPH and a mixed-function oxidase inhibitor.
Incubation following the addition of free sulfhydryl-containing
compounds reduced binding by 70-90%, while the addition of lysine
reduced binding by 12%. Using gel electrophoresis-fluorography it
was shown that acrolein, incubated with a reconstituted cytochrome
P-450 system, migrated mostly with cytochrome P-450. It was
concluded that acrolein is capable of alkylating free sulfhydryl
groups in cytochrome P-450 (Marinello et al., 1984).
When rats received tritium-labelled acrolein intraperitoneally
24 h after partial hepatectomy, the percentages of total liver
radioactivity recovered in the acid-soluble fraction, lipids,
proteins, RNA, and DNA were approximately 94, 3.5, 1.2, 0.6, and
0.4%, respectively, during the first 5 h after exposure.
Distribution of label was stable for at least 24 h. Acrolein was
bound to DNA at a rate of 1 molecule per 40 000 nucleotides.
A similar DNA-binding rate was observed for the green alga
Dunaliella bioculata at a 10 times higher acrolein concentration
(Munsch et al., 1974a). In in vitro studies, labelled acrolein
was found to bind to native calf thymus DNA and other DNA polymerase
templates at rates of 0.5-1 molecule per 1000 nucleotides (Munsch
et al., 1974b). In a follow-up experiment with Dunaliella
bioculata, quantitative autoradiography and electron microscopy
showed that the preferential area of cellular fixation for acrolein
was the nucleus. This fixation was stable for at least 2 days,
while that in the plastid and cytoplasm decreased initially (Marano
& Demèstere, 1976). As no adducts were identified in these studies,
these data were considered unsuitable for evaluation.
6.2.2 Adduct formation
The findings of the tracer-binding studies (section 6.2.1) are
not surprising considering the reactivity of acrolein, which makes
the molecule a likely candidate for interactions with protein and
non-protein sulfhydryl groups and with primary and secondary amine
groups such as occur in proteins and nucleic acids. These reactions
are most likely to be initiated by nucleophilic Michael addition to
the double bond (Beauchamp et al., 1985; Shapiro et al., 1986).
Beauchamp et al. (1985) discussed extensively the interactions
with protein sulfhydryl groups and primary and secondary amine
groups.
6.2.2.1 Interactions with sulfhydryl groups
The non-enzymatic reaction between equimolar amounts of
acrolein and glutathione, cysteine or acetylcysteine in a buffered
aqueous solution proceeds rapidly to near-completion, forming stable
adducts (Esterbauer et al., 1975; Alarcon, 1976).
Acrolein-acetylcysteine and acrolein-cysteine adducts yield on
reduction S-(3-hydroxypropyl)mercapturic acid and
S-(3-hydroxypropyl)-cysteine, respectively (Alarcon, 1976). The
reaction between glutathione and acrolein may be catalysed by
glutathione S-transferase, as was shown for acrolein-diethylacetal
and crotonaldehyde (Boyland & Chasseaud, 1967). Biochemical and
toxicological investigations provide more evidence for the
interaction, either enzymatic or non-enzymatic, between acrolein and
free sulfhydryl groups. In summary, it has been observed that:
* acrolein exposure of whole organisms or tissue fractions
results in glutathione depletion (section 7.3.1);
* co-exposure of organisms to acrolein and free
sulfhydryl-containing compounds protects against the
biological effects of acrolein (sections 7.3.3, 7.3.4, and
7.5);
* acrolein can inhibit enzymes containing free sulfhydryl
groups on their active site (section 7.3);
* glutathione conjugates appear in the urine of
acrolein-dosed rats (section 6.3).
6.2.2.2 In vitro interactions with nucleic acids
Non-catalytic reactions occur between acrolein and cytidine
monophosphate (Descroix, 1972), deoxyguanosine (Hemminki et al.,
1980), and deoxyadenosine (Lutz et al., 1982). Chung et al.
(1984) have identified the nucleotides resulting from the reaction
between acrolein and deoxyguanosine or calf thymus DNA (at 37 °C and
pH 7) in phosphate buffer. The adducts identified were the 6- and
8-hydroxy derivatives of cyclic 1,N2-propano-deoxyguanosine. These
adducts were shown to be formed in a dose-dependent fashion in
Salmonella typhimurium TA100 and TA104 following exposure to
acrolein and identification of the DNA adducts by an immunoassay
(Foiles et al., 1989; see also section 7.6.2). Shapiro et al.
(1986) reported that acrolein reacts with cytosine and adenosine
derivatives (at 25 °C and pH 4.2), yielding cyclic 3,N4 adducts of
cytosine derivatives and 1,N6 adducts of adenosine derivatives.
The reaction between guanosine and acrolein yields the cyclic 1,N2
adduct (at 55 °C and pH 4).
The demonstration that acrolein can cause or enhance the
formation of complexes between DNA strands (DNA-DNA crosslinking)
and between DNA and cellular proteins (DNA-protein crosslinking) is
indirect evidence that acrolein interacts with nucleic acids. This
subject is discussed further in section 7.6.1. However, no studies
have demonstrated unequivocally the interaction of acrolein with DNA
following in vivo administration to animals.
6.3 Metabolism and excretion
Acrolein is expected to be eliminated from the body via
glutathione conjugation (section 6.2.2.1). Draminski et al.
(1983) administered acrolein in corn oil orally to Wistar rats at a
dose of 10 mg/kg body weight. The urinary metabolites identified by
gas chromatography with mass spectrometric detection were
S-carboxylethyl-mercapturic acid and its methyl ester, the latter
possibly being the result of methylation of the urine samples prior
to gas chromatography. In expired air a volatile compound was
detected by gas chromatography, which was not identified; it was
reported that its retention time did not correspond to that of
methyl acrylate, acrolein or allyl alcohol. The reduced form of
S-carboxylethyl-mercapturic acid, i.e. S-hydroxypropyl-mercapturic
acid, was identified by paper and gas chromatography as the sole
metabolite in the urine of CFE rats injected subcutaneously with a
1% solution of acrolein in arachis oil at a dose of approximately
20 mg/kg body weight (Kaye, 1973). This metabolite was collected
within 24 h and accounted for 10.5% of the total dose (uncorrected
for a recovery of 58%). These data indicate that conjugation with
glutathione may dominate the metabolism of acrolein.
Data obtained in vitro show that acrolein can also be a
substrate of liver aldehyde dehydrogenase (EC 1.2.1.5) and lung or
liver microsomal epoxidase (EC 1.14.14.1) (Patel et al., 1980).
Acrolein, at concentrations of approximately 200 mg/litre medium,
was oxidized to acrylic acid by rat liver S9 supernatant, cytosol,
and microsomes, but not by lung fractions, in the presence of NAD+
or NADP+. The reaction proceeded faster with NAD+ as cofactor
than with NADP+ and was completely inhibited by disulfiram (Patel
et al., 1980). Rikans (1987) studied the kinetics of this
reaction: mitochondrial and cytosolic rat liver fractions each
contained two aldehyde dehydrogenase activities with Km values of
22-39 mg/litre and 0.8-1.4 mg/litre. Microsomes contained a high
Km activity. Incubation of rat liver or lung microsomes in the
presence of acrolein and NADPH yielded glycidaldehyde and its
hydration product glyceraldehyde, showing involvement of microsomal
cytochrome P-450-dependent epoxidase (Patel et al., 1980).
Postulated pathways of acrolein metabolism are summarized in
Figure 1.
In a human study, the intravenous injection of 1g
cyclophosphamide resulted in the excretion of 1.5% acrolein
mercapturic acid adduct in the urine (Alarcon, 1976).
As for the fate of the primary metabolites of acrolein, it has
been proposed that acrylic acid is methylated and subsequently
conjugated to yield S-carboxyl-ethylmercapturic acid, which is a
known metabolite of methyl acrylate (Draminski et al., 1983).
However, methyl acrylate has never been reported as a metabolite of
either acrolein or acrylic acid. It seems more likely that acrylic
acid is incorporated into normal cellular metabolism via the
propionate degradative pathway (Kutzman et al., 1982; Debethizy
et al., 1987). Glycidaldehyde has been shown to be a substrate
for lung and liver cytosolic glutathione S-transferase (EC
2.5.1.18) and can also be hydrated to glyceraldehyde (Patel et al.,
1980). Glyceraldehyde can be metabolized via the glycolytic
pathways.
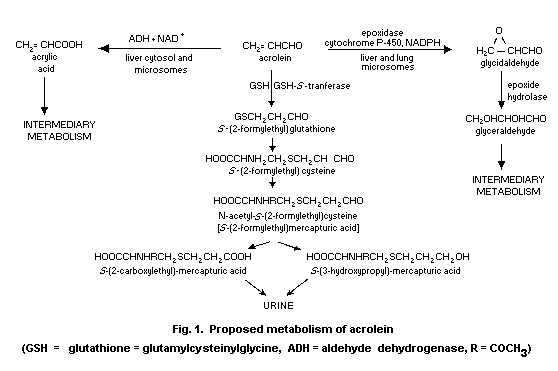 7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Mortality
The available acute mortality data are summarized in Table 8.
Most tests for the determination of the acute toxicity of acrolein
do not comply with present standards. Nevertheless, retesting is
not justified for ethical reasons and in view of the overt high
toxicity of acrolein following inhalation or oral exposure (Hodge &
Sterner, 1943).
In addition to the data in Table 8, an oral LD95 of
11.2 mg/kg body weight for Charles River rats, observed for 24 h,
has been reported (Sprince et al., 1979). Draminski et al.
(1983) reported the deaths of 5/10 rats given 10 mg/kg body weight
in corn oil by gavage.
7.1.2 Effects on the respiratory tract
In vapour exposure tests, the effects observed in experimental
animals have almost exclusively been local effects on the
respiratory tract and eyes.
In the LC50 studies, effects on the respiratory tract were
clinically observed as nasal irritation and respiratory distress in
rats (Skog, 1950; Potts et al., 1978; Crane et al., 1986),
hamsters (Kruysse, 1971), mice, guinea-pigs, and rabbits (Salem &
Cullumbine, 1960) at exposure levels of between 25 mg/m3 for 4 h
and 95 150 mg/m3 for 3 min. Rats exposed for 10 min to
concentrations of 750 or 1000 mg/m3 suffered asphyxiation
(Catilina et al., 1966).
Histopathological investigations in experiments with
vapour-exposed rats (Skog, 1950; Catilina et al., 1966; Potts
et al., 1978; Ballantyne et al., 1989), hamsters (Kilburn &
McKenzie, 1978), guinea-pigs (Dahlgren et al., 1972; Jousserandot
et al, 1981), and rabbits (Beeley et al., 1986) revealed varying
degrees of degeneration of the respiratory epithelium consisting of
deciliation (see also in vitro work on cytotoxicity discussed in
7.1.5), exfoliation, necrosis, mucus secretion, and vacuolization.
Also observed were acute inflammatory changes consisting of
infiltration of white blood cells into the mucosa, hyperaemia,
haemorrhages, and intercellular oedema. Proliferative changes of
the respiratory epithelium, in the form of early stratification and
hyperplasia, were observed in hamsters 96 h after exposure to
13.7 mg/m3 for 4 h (Kilburn & McKenzie, 1978).
Table 8. Acute mortality caused by acrolein
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Rat (Wistar) male inhalation (10 min) 8 750 Catilina et al. (1966)b
Rat (Wistar) not reported oral 14 46 (39-56) Smyth et al. (1951)g
Rat (unspecified not inhalation (30 min) 21 300 Skog (1950)b,c
strain) reported
Rat (Sprague-Dawley) male inhalation (30 min) 14 95-217 Potts et al. (1978)d
Rat (Sprague-Dawley) male and inhalation (1 h) 14 65 (60-68) Ballantyne et al. (1989)
female inhalation (4 h) 14 20.8 (17.5-24.8)
Rat (Sherman) male and inhalation (4 h) 14 18 Carpenter et al. (1949)b,e
female
Hamster (Syrian golden) male and inhalation (4 h) 14 58 (54-62) Kruysse (1971)
female
Table 8 (contd)
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Mouse (unspecified male inhalation (6 h) 1 151 Philippin et al. (1970)f
strain)
Mouse (NMRI) not reported intraperitoneal 6 7 Warholm et al. (1984)g
a Where available, 95% confidence limits are given in parentheses.
b Determination of acrolein levels was not reported.
c No mortality at 100 mg/m3, 100% mortality at 700 mg/m3.
d Approximate value: no mortality at 33 mg/m3, 1/7 and 7/7 died at 95 and 217 mg/m3, respectively.
e Approximate value: 2-4/6 died.
f No mortality at 71 mg/m3, 100% mortality at 273 mg/m3.
g The vehicle was water.
Functional changes in the respiratory system following acrolein
vapour exposure have been investigated in guinea-pigs and mice. A
rapidly reversible increase in respiratory rate was observed in
intact guinea-pigs during exposure to 39 mg/m3 for 60 min (Davis
et al., 1967) and to 0.8 mg/m3 or more for 2 h (Murphy et al.,
1963) followed by a decrease in respiratory rate and an increase in
tidal volume. No changes in pulmonary compliance were reported.
Davis et al. (1967) did not observe these effects in
tracheotomized animals and concluded that they were caused by reflex
stimulation of upper airway receptors and not by
bronchoconstriction. Murphy et al. (1963), observing that
anticholinergic bronchodilators, aminophylline and isoproterenol,
but not antihistaminics, reduced the acrolein-induced increase in
respiratory resistance, concluded that acrolein caused
bronchoconstriction mediated through reflex cholinergic stimulation.
In another experiment, an increase in respiratory resistance was
also observed in anaesthetized, tracheotomized guinea-pigs with
transected medulla during exposure to 43 mg/m3 for up to 5 min
(Guillerm et al., 1967b). The effect was not reversed by atropine.
It was concluded by the authors that acrolein did not cause
bronchoconstriction via reflex stimulation, but probably via
histamine release. When anaesthetized mice were exposed to 300 or
600 mg/m3 for 5 min via a tracheal cannula, respiratory
resistance, respiratory rate, and tidal volume decreased and
pulmonary compliance increased at an unspecified time after exposure
(Watanabe & Aviado, 1974).
The concentration that produces a 50% decrease in respiratory
rate (RD50) as a result of reflex stimulation of trigeminal nerve
endings in the nasal mucosa (sensory irritation) has been used as an
index of upper respiratory tract irritation. This effect reduces
the penetration of noxious chemicals into the lower respiratory
tract. The rate of respiration was measured in a body
plethysmograph, only the animals' heads being exposed to the
acrolein vapour. Depending on the strain, RD50 values for mice
ranged from 2.4 to 6.6 mg/m3 (Kane & Alarie, 1977; Nielsen
et al., 1984; Steinhagen & Barrow, 1984). In rats a RD50 of
13.7 mg/m3 was found (Babiuk et al., 1985).
7.1.3 Effects on skin and eyes
Animal skin irritation tests have not been performed and skin
irritation has not been mentioned as an effect in the acute
inhalation tests reported.
One special in vivo eye irritation test involved
vapour-exposed and control rabbits. At analysed concentrations of
acrolein (method not specified) between 4.3 and 5.9 mg/m3,
maintained over 4 h, slight chemosis was observed but no iritis
(Mettier et al., 1960). Eye irritation was observed clinically in
rodents in several acute inhalation tests, but was not graded (Skog,
1950; Salem & Cullumbine, 1960; Kruysse, 1971; Potts et al.,
1978).
7.1.4 Systemic effects
With respect to systemic effects, most studies have been
performed at concentrations far above the lethal dose. When rats
were exposed to concentrations of acrolein between 1214 and
95 150 mg/m3 during various periods of time, incapacitation,
indicated by the inability to walk in a rotating cage, and
convulsions were observed after 2.8 min at the highest concentration
and after 27 to 34 min at the lowest concentration. These effects
were followed by death after several minutes. Cyanosis of the
extremities and agitation were observed at levels of 22 900 mg/m3
or more (Crane et al., 1986).
The effects of acrolein on the cardiovascular system were
investigated by Egle & Hudgins (1974). Rats anaesthetized by sodium
pentobarbital and exposed only via the mouth and nose to
concentrations between 10 and 5000 mg/m3 for 1 min showed an
increase in blood pressure at all exposure levels. The heart rate
was increased at concentrations from 50 mg/m3 to 500 mg/m3 but
decreased at 2500 and 5000 mg/m3. Intravenous experiments
suggested that increased blood pressor responses resulted from the
release of catecholamines from sympathetic nerve endings and from
the adrenal medulla and that the decreased heart rate effect was
mediated by the vagus nerve (Egle & Hudgins, 1974).
In an acute oral test with rats exposed at 11.2 mg/kg body
weight, decreased reflexes, body sag, poor body tone, lethargy,
stupor, and tremors were observed, as well as respiratory distress
(Sprince et al., 1979).
Because acrolein was shown to induce acute cytotoxicity of the
rat urinary bladder mucosa when instilled directly into the bladder
lumen (Chaviano et al., 1985), this end-point was investigated
in vivo. Two days after a single oral or intraperitoneal dose of
25 mg/kg body weight to ten rats per group, focal simple hyperplasia
of the urinary bladder was detected in the three surviving rats
dosed intraperitoneally. None of the orally exposed rats showed
this effect, but all exhibited severe erosive haemorrhagic
gastritis. Both orally and intraperitoneally exposed rats showed
eosinophilic degeneration of hepatocytes. No abnormalities were
observed in sections of lungs, kidneys, and spleen. Acrolein was
also administered intraperitoneally at single doses of 0.5, 1, 2, 4,
or 6 mg/kg body weight. Proliferation of the bladder mucosa was
evaluated autoradiographically by measuring [3H-methyl]thymidine
incorporation in exposed versus control rats 5 days after the
intraperitoneal injection of acrolein and was found to be increased
nearly two-fold at the highest dose. Body weight gain was decreased
at the two highest doses. Histopathological evaluation of the liver
and urinary bladder did not reveal abnormalities (Sakata et al.,
1989).
7.1.5 Cytotoxicity in vitro
As shown in Table 9, mammalian cell viability is affected by
acrolein in vitro at nominal concentrations of 0.1 mg/litre or
more (not corrected for interaction with culture medium components
or volatilization). The concentration at which formaldehyde
exhibited a similar degree of cytotoxicity was about 6 to 100 times
higher (Holmberg & Malmfors, 1974; Pilotti et al., 1975; Koerker
et al., 1976; Krokan et al., 1985).
Acrolein is a known inhibitor of respiratory tract ciliary
movement in vitro. After a 20-min exposure to an acrolein
concentration of 34-46 mg/m3, the ciliary beating frequency of
excised sheep trachea decreased by 30% (Guillerm et al., 1967a).
Exposure to 13 mg/m3 for 1 h is the greatest exposure that does
not stop ciliary activity in excised rabbit trachea (Dalhamn &
Rosengren, 1971). The no-observed-effect-level for longer exposure
periods would be expected to be lower than 13 mg/m3. Other
in vitro investigations into the inhibition of ciliary movement by
acrolein were reviewed by Izard & Libermann (1978).
7.2 Short-term exposure
7.2.1 Continuous inhalation exposure
In two subchronic inhalation studies with rats, changes in
weight gain, longevity, behaviour, and several physiological
parameters were reported (Gusev et al., 1966; Sinkuvene, 1970).
Unfortunately, the reports did not give sufficient details on the
exposure conditions and protocols and the studies are thus of
limited value in evaluating the toxicological properties of
acrolein.
Table 9. In vitro cytotoxicity of acrolein
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Rat cardiac fibroblasts/myocytes 4 increased lactate Toraason et al. (1989)
dehydrogenase release > 2.8
Rat cardiac myocytes 2 abolished myocine beat > 2.8
dehydrogenase
4 decreased ATP levels > 0.56
Mouse Ehrlich Landschutz 5 92% survivala 1 Holmberg & Malmfors (1974)
Diploid ascites tumour cells 5 53% survivala 5
Mouse B P8 ascites sarcoma cells 48 20% growth rate inhibition 0.6 Pilotti et al. (1975)
48 94% growth rate inhibition 5.6
Mouse C1300 neuroblastoma cells 24 50% survivala 1.7 Koerker et al. (1976)
Mouse L 1210 leukaemia cells 1 70-80% survivala 1.1 Wrabetz et al. (1980)
1 < 15% survivala 2.8
Chinese hamster ovary cells 5 100% mitotis inhibition 0.6 Au et al. (1980)
Adult human bronchial 1 92% colony-forming efficiency 0.06 Krokan et al. (1985)
fibro-blasts 1 45% colony-forming efficiency 0.2
Table 9 (contd).
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Adult human lymphocytes 48 decreased replicative index 0.6 Wilmer et al. (1986)
48 100% mitosis inhibition 2.2
Human K562 chromic myeloid 1 marked reduction in > 0.3 Crook et al. (1986a,b)
leukaemia cells colony-forming ability
Human bronchial epithelial cells 1 20% colony-forming efficiency 0.06 Grafström et al. (1988)
1 50% colony-forming efficiency 0.06-0.17
1 50% survivala 0.34
3 clonal growth rate inhibition > 0.17
3 increase in cross-linkage
envelope formation > 0.06
3 decreased plasminogen
activator activity > 0.56
Human fibroblasts 5 63% cell count reduction < 0.017 Curren et al. (1988)
DNA-repair deficient human
fibroblasts 5 63% cell count reduction 0.045
a measured as dye exclusion
Groups of 7 or 8 Sprague-Dawley rats of both sexes, 7 or 8
Princeton or Hartley-derived guinea-pigs of both sexes, 2 male
pure-bred Beagle dogs, and 9 male squirrel-monkeys were exposed to a
vapourized acrolein-ethanol-water mixture for 90 days (Lyon et al.,
1970). The measured acrolein concentrations were 0, 0.5 (two groups
for each species), 2.3, and 4.1 mg/m3 and the ethanol
concentrations were below 18.7 mg/m3. Pathological investigations
did not include weighing of tissues and organs or examination of the
tracheas at the lowest exposure level. There was no
treatment-related mortality. One monkey died at 0.5 mg/m3 and one
at 2.3 mg/m3 due to accidental infections. Body weight gain
reduction was only found in rats at 2.3 and 4.1 mg/m3.
Clinically, ocular discharge and salivation were observed in dogs at
2.3 and 4.1 mg/m3 and in monkeys at 4.1 mg/m3. Monkeys kept
their eyes closed at 2.3 mg/m3. No adverse effects on
haematological or biochemical parameters were observed in any of the
animals. At necropsy, occasional pulmonary haemorrhage and focal
necrosis in the liver were found in three rats at 2.3 mg/m3.
Pulmonary inflammation and occasional focal liver necrosis were also
observed in guinea-pigs at this concentration. Sections of lung
from two of the four dogs exposed at 0.5 mg/m3 revealed focal
vacuolization, hyperaemia, and increased secretion of bronchiolar
epithelial cells, slight bronchoconstriction, and moderate
emphysema. At 2.3 mg/m3, focal inflammatory reactions involved
lung, kidney, and liver. Bronchiolitis and early broncho-pneumonia
were seen in one dog. At 4.1 mg/m3, both dogs had confluent
bronchopneumonia. All nine monkeys at 4.1 mg/m3 showed squamous
metaplasia and six of them showed basal cell hyperplasia in the
trachea. None of the species revealed other treatment-related
changes (Lyon et al., 1970).
Bouley et al. (1975) exposed a total of 173 male SPF-OFA rats
to a measured acrolein vapour concentration of 1.26 mg/m3 for a
period of 15 to 180 days and used control groups of equal size. No
mortality occurred. Sneezing was observed from day 7 to day 21 in
the treated animals, and body weight gain and food consumption were
reduced. There was an increase in relative lung weight in rats
killed on day 77 but not in rats killed on days 15 or 32. The
relative liver weight was decreased at day 15 but not thereafter,
and the number of alveolar macrophages was decreased at days 10 and
26 but not at days 60 or 180. When groups of 16 rats were infected
by one LD50 dose of airborne Salmonella enteriditis on day 18 or
day 63, mortality increased from 53% in controls to 94% in the
exposed rats infected on day 18. No changes were observed in
biochemical parameters, including the amount of liver DNA per mg of
protein in a group of partially hepatectomized rats, or in the
response of spleen lymphocytes to phytohaemagglutinin in rats
exposed for 39 to 57 days. Other end-points were not investigated.
7.2.2 Repeated inhalation exposure
Lyon et al. (1970) exposed groups of rats, guinea-pigs, dogs,
and monkeys to acrolein vapour at concentrations of 0, 1.6. and
8.5 mg/m3 for 8 h per day and 5 days per week over 6 weeks. With
the exception of the exposure levels, period, and frequency, the
protocol was the same as that for the continuous inhalation exposure
described in section 7.2.1. Two deaths occurred among the nine
monkeys at 8.5 mg/m3. There was body weight gain reduction in
rats and body weight loss (not statistically significant) in monkeys
at 8.5 mg/m3. Clinically, eye irritation and salivation were
observed in dogs and monkeys and difficult breathing in dogs at
8.5 mg/m3. No adverse effects on haematological or biochemical
parameters were observed in any of the animals. At necropsy,
sections of lung from all animals exposed to 1.6 mg/m3 showed
chronic inflammatory changes. Additionally, some showed emphysema.
At 8.5 mg/m3, squamous metaplasia and basal cell hyperplasia were
observed in the trachea of both dogs and monkeys. In addition,
bronchopneumonia was noted in dogs and necrotizing bronchitis and
bronchiolitis in monkeys. Focal calcification of the tubular
epithelium was noted in the kidneys of rats and monkeys at
8.5 mg/m3.
Groups of male Sprague-Dawley rats were also exposed to
acrolein vapour at measured concentrations of 0, 0.39, 2.45, and
6.82 mg/m3 for 6 h per day and 5 days per week over 3 weeks (Leach
et al., 1987). Subgroups were used for immunological
investigations (section 7.4) and for histopathological examination
of nasal turbinates and lungs. Body weight gain was depressed from
week 1 up to the end of the exposure period at 6.82 mg/m3.
Absolute, but not relative, spleen weight was reduced at this
exposure level. There were no histological effects on the lungs,
but the respiratory epithelium of the nasal turbinates showed
exfoliation, erosion, and necrosis, as well as dysplasia and
squamous metaplasia at 6.82 mg/m3. In addition, the mucous
membrane covering the septum and lining the floor of the cavity
showed hyperplasia and dysplasia (Leach et al., 1987).
Another experiment involved Dahl rats of two lines, one
susceptible (DS) and one resistant (DR) to salt-induced hypertension
(Kutzman et al., 1984). Groups of 10 female rats of each line were
exposed to measured acrolein concentrations of 0, 0.89, 3.21, and
9.07 mg/m3 for 6 h per day and 5 days per week over 61-63 days.
One week after the exposure, survivors were killed for pathological
and compositional analysis of the lung following behavioural and
clinical chemistry testing. At 9.07 mg/m3, all DS rats died
within 11 days and 4 DR rats died within the exposure period.
Reduced body weights were measured in the surviving DR rats during
the first 3 weeks, followed by an almost normal body weight gain.
Biochemical changes were found in DR rats at 9.07 mg/m3 and
included increases in lung hydroxyproline and elastin, serum
phosphorus, and in the activities of serum alkaline phosphatase,
alanine aminotransferase (EC 2.6.1.2), and aspartate
aminotransferase (EC 2.6.1.1). No effects were observed on
exploratory behaviour, locomotor activity, blood pressure, lung
protein, blood urea nitrogen, or on serum creatinine, uric acid, or
calcium. At necropsy of survivors, DR rats exposed to 9.07 mg/m3
had increased relative weights of several organs, especially the
lungs. It was noted by the authors that the exposed rats gained a
considerable amount of weight during the week following exposure.
In both rat lines, concentration-related increases were observed in
lymphoid aggregates in pulmonary parenchyma, in collections of
intra-alveolar macrophages with foamy cytoplasm, and in
hyperplastic/metaplastic terminal bronchiolar epithelial changes.
Multifocal interstitial pneumonitis and squamous metaplasia of the
tracheal epithelium were also found in DR rats exposed to
9.07 mg/m3. In contrast, dead and moribund rats, especially those
of the DS strain, mainly exhibited severe bronchial and bronchiolar
epithelial necrosis with exfoliation, oedema, haemorrhage, and
varying degrees of bronchopneumonia. Adverse effects were absent in
nasal turbinates and in non-pulmonary tissues (Kutzman et al.,
1984).
In follow-up studies, groups of 32 to 57 male Fischer-344 rats
were exposed in exactly the same way as described for the Dahl rats.
Surviving rats were tested for pulmonary function one week after
exposure and then killed and examined for compositional analysis,
morphometry, and (in nine rats per group) pathological changes in
the lung (Kutzman et al., 1985; Costa et al., 1986). At
9.07 mg/m3, 56% mortality occurred. After an initial body weight
loss over the first 10 days, weight gain became comparable to that
of controls. There was an increase in the relative weight of
several organs, especially the lungs. Lungs also showed an increase
in water content and in the levels of elastin and hydroxyproline,
but not in the levels of protein and DNA. The hydroxyproline level
was also elevated at 3.21 mg/m3. Histologically, surviving rats
treated with 3.21 or 9.07 mg/m3 demonstrated an exposure-related
increase in effects on the respiratory tract consisting of
bronchiolar epithelial necrosis with exfoliation, bronchiolar
mucopurulent plugs, an increase in bronchiolar and alveolar
macrophages, and focal pneumonitis. At 3.21 mg/m3, there was
type II cell hyperplasia and at 9.07 mg/m3 tracheal,
peribronchial, and alveolar oedema and acute rhinitis. The severity
of lung lesions was highly variable and three of the nine rats
examined at 9.07 mg/m3 did not exhibit histological damage.
Moribund rats mainly showed severe acute bronchopneumonia and focal
alveolar and tracheal oedema with exfoliation in the bronchi and
bronchioles (Kutzman et al., 1985). In another report from the
same research group, the results of pulmonary function testing and
morphometry disclosed air-flow dysfunction at 9.07 mg/m3, which
was correlated with the presence of focal peribronchial lesions and
the lung elastin concentrations. In contrast, the rats exposed at
0.89 mg/m3 exhibited enhanced flow-volume dynamics, whereas no
effects on lung function were present in the 3.21-mg/m3 group
(Costa et al., 1986).
Groups of six Wistar rats and ten Syrian golden hamsters of
both sexes were exposed to acrolein vapour at measured
concentrations of 0, 0.9, 3.2, and 11.2 mg/m3 for 6 h per day and
5 days per week over 13 weeks (Feron et al., 1978). Within the
first month of exposure to 11.2 mg/m3, half the number of rats of
each sex died. One hamster died at this exposure level because of
renal failure. A treatment-related decrease in body weight gain and
food intake was observed in rats exposed to 3.2 mg/m3 or more.
Hamsters showed decreased body weight gain at 11.2 mg/m3, but food
intake was not examined. At this exposure level, all animals kept
their eyes closed, rats showed bristling hair, and hamsters showed
salivation and nasal discharge. Haematological investigation and
urinalysis in week 12 showed no changes in rats. In hamsters,
urinalysis revealed no changes, but females showed increases in the
number of erythrocytes, packed cell volume, haemoglobin content, and
number of lymphocytes and a decrease in the number of neutrophilic
leucocytes. Changes in relative organ weights, which were
considered by the authors to be related to the treatment, were found
in the lung, heart, and kidneys of both species and in the adrenals
of rats exposed to 11.2 mg/m3. Histological changes were confined
to the respiratory tract. In the nose, rats exhibited an
exposure-related increase in squamous metaplasia and neutrophilic
infiltration of the mucosa at levels of 0.9 mg/m3 or more (at
0.9 mg/m3 each effect was observed in one male) and occasional
necrotizing rhinitis at 11.2 mg/m3. Hamsters also showed these
effects at 11.2 mg/m3 but only minimal inflammatory changes at
3.2 mg/m3. In the larynx and trachea of rats exposed to
11.2 mg/m3, squamous metaplasia was also observed and was
accompanied by hyperplasia in bronchi and bronchioli. At this
exposure level, the larynx of hamsters was slightly thickened and
focal hyperplasia and metaplasia were found in the trachea.
Inflammatory changes were present in the bronchi, bronchioli, and
alveoli of rats and included haemorrhage, oedema, accumulations of
alveolar macrophages, an increase in mucus-producing cells in the
bronchioli, and bronchopneumonia. The authors noted considerable
variation between individual rats in the degree of the lesions
(Feron et al., 1978).
Feron & Kruysse (1977) exposed groups of 36 Syrian golden
hamsters of both sexes to acrolein vapour at measured levels of 0
and 9.2 mg/m3 for 7 h per day and 5 days per week over 52 weeks.
Except for 6 males and 6 females, the hamsters were observed for a
further 29 weeks after the exposure period. Overall mortality was
38% in exposed hamsters and 33% in controls. Body weight was
slightly and reversibly decreased at the end of the exposure period.
The other effects observed at the end of the exposure period were
essentially similar (but less severe) to those described above for
hamsters exposed to 11.2 mg/m3 for 13 weeks, but hyperplasia was
not observed. Histological changes were restricted to the anterior
half of the nasomaxillary turbinates and were still found in 20% of
the animals at week 81. At that time they mainly consisted of a
thickened submucosa and exudation into the lumen. Epithelial
metaplasia, but not hyperplasia, was noted. No tumours were found.
Histopathological examination of the respiratory tract of male
Swiss-Webster mice was the object of a study involving groups of 16
to 24 male mice exposed to measured concentrations of 3.9 mg/m3
for 6 h per day during 5 days (Buckley et al., 1984). The lesions
observed were restricted to the nose and were most severe in the
anterior respiratory epithelium and on the free margins of the
nasomaxillary turbinates and the adjacent nasal septum. They
consisted of severe deciliation, moderate exfoliation, erosion,
ulceration and necrosis, severe squamous metaplasia, moderate
neutrophilic infiltration, and a slight serofibrinous exudate.
Lesions in the olfactory epithelium were largely confined to the
dorsal meatus and consisted of moderate ulceration and necrosis, and
slight squamous metaplasia. The nasal squamous epithelium was not
affected (Buckley et al., 1984).
One special investigation concerned the effects of acrolein
vapour on the respiratory functions of male Swiss mice exposed to
100 mg/m3 for two daily periods of 30 min each for 5 weeks. Body
weights were not affected. There was a decrease in pulmonary
compliance, but no effects were found on pulmonary resistance,
respiratory volume, or functional residual capacity. The lungs
showed an increase in phospholipid content (Watanabe & Aviado,1974).
In summary, the toxicological effects on a variety of
laboratory animals from repeated inhalation exposure to acrolein
vapour at concentrations ranging from 0.39 mg/m3 to 11.2 mg/m3
have been studied. Exposure durations ranged from 5 days to as long
as 52 weeks. In general, body weight gain reduction, decrement of
pulmonary function, and pathological changes in nose, upper airways,
and lungs have been documented in most species exposed to acrolein
concentrations of 1.6 mg/m3 or more. Pathological changes include
inflammation, metaplasia, and hyperplasia of the respiratory tract.
Significant mortality has been observed following repeated exposures
to acrolein vapour at concentrations above 9.07 mg/m3.
7.2.3 Repeated intraperitoneal exposure
Groups of ten intact or adrenalectomized NMRI mice were
injected intraperitoneally with saline or acrolein in water at daily
doses of 4 to 16 mg/kg body weight for 1 to 6 days. One week after
the last injection the mice were killed for autopsy.
Clinical signs of toxicity were hunched posture, inactivity,
and ruffled fur. Total body weight and relative thymus and spleen
weights showed a dose-related reduction, while the adrenals showed
an increase in relative weight. Histologically, thymic necrosis and
splenic atrophy were the only changes observed. These changes were
absent in controls and in adrenalectomized mice. The levels of
reduced glutathione and the activity of glutathione S-transferase
in liver cytosol were unchanged, but the rate of glutathione
synthesis was increased. Repeated exposure to acrolein caused a
progressively less pronounced effect on mortality (Warholm et al.,
1984).
7.3 Biochemical effects and mechanisms of toxicity
7.3.1 Protein and non-protein sulfhydryl depletion
A dose-related non-protein sulfhydryl (reduced glutathione)
depletion was observed in the nasal respiratory mucosa of male
Fischer rats after nose-only exposure for 3 h to acrolein vapour at
concentrations of 0.23-11.4 mg/m3 (McNulty et al., 1984; Lam
et al., 1985). Depletion of glutathione in the liver was not
observed at these exposure levels (McNulty et al., 1984). The
glutathione depletion in the nasal mucosa appeared irreversible at
11.4 mg/m3 (McNulty et al., 1984). In female C3Hf/HeHa mice,
intraperitoneally exposed once to doses between 20 and 80 mg/kg body
weight and killed 2 h later, a dose-related decrease in liver
glutathione levels was observed. These doses are however extremely
high considering the fact that a dose of 4.5 mg/kg body weight was
lethal within 1.7 h (Gurtoo et al., 1981a).
A dose-related in vitro glutathione depletion has been
observed in human bronchial fibroblasts (Krokan et al., 1985),
human bronchial epithelial cells (Grafström et al., 1988), human
chronic myeloid leukemia cells (Crook et al., 1986b), and human
and rat phagocytic cells (Witz et al., 1987) from the lowest
acrolein concentration tested (56 µg/litre). The effect has also
been reported to occur in isolated rat hepatocytes (Zitting &
Heinonen, 1980; Dawson et al., 1984; Dore & Montaldo, 1984; Ku &
Billings, 1986) and in rat liver or lung microsomal suspensions
(Patel et al., 1984), the lowest-observed-effect level being
1400 µg/litre (Dawson et al., 1984). Ku & Billings (1986)
observed that both mitochondrial and cytosolic glutathione levels
were decreased.
As a result of acrolein exposure, there was a decrease in the
level of both membrane surface and soluble protein sulfhydryl groups
in in vitro human and rat phagocytic cells (Witz et al., 1987)
and a decrease in the level of soluble protein sulfhydryl compounds
in human bronchial epithelial cells (Grafström et al., 1988).
Acrolein has also been shown to cause a decrease in membrane surface
protein sulfhydryl groups in rat hepatocytes (Ku & Billings, 1986)
and to reduce the protein sulfhydryl content of liver and lung
microsomal preparations (Patel et al., 1984).
7.3.2 Inhibition of macromolecular synthesis
When partially hepatectomized Wistar rats were exposed
intraperitoneally to a single acrolein dose of 0.5, 1.6, 2.0, or
2.7 mg/kg body weight, a dose-related inhibition of the synthesis of
DNA and RNA was measured in liver and lung cells (Munsch &
Frayssinet, 1971).
Inhibition of DNA, RNA, and/or protein synthesis has been
observed in Escherichia coli (Kimes & Morris, 1971), the slime
mold Physarum polycephalum (Leuchtenberger et al., 1968), the
alga Dunaliella bioculata (Marano & Puiseux-Dao, 1982), and in
in vitro mammalian cells such as mouse kidney cells (Leuchtenberger
et al., 1968) and polyoma transformed Chinese hamster cells
(Alarcon, 1972). Acrolein was shown to inhibit RNA polymerase in
isolated rat liver nuclei (Moule & Frayssinet, 1971) and isolated
rat liver DNA polymerase (Munsch et al., 1973). The activity of
the latter enzyme is associated with at least one functional
sulfhydryl group, and preincubation of the enzyme with
2-mercaptoethanol protected against the inhibitory action of
acrolein. Since acrolein did not inhibit isolated Escherichia coli
polymerase I, devoid of sulfhydryl groups in its active centre,
Munsch et al. (1973) suggested that the inhibitory action of
acrolein is caused by a reaction with sulfhydryl groups.
7.3.3 Effects on microsomal oxidation
In in vitro studies, acrolein has been shown to convert rat
liver cytochrome P-450 to cytochrome P-420 and to inhibit rat liver
NADPH-cytochrome-c reductase (EC 1.6.2.4) in a time- and
concentration-related fashion (Marinello et al., 1978; Ivanetich
et al., 1978; Berrigan et al., 1980; Gurtoo et al., 1981b;
Marinello et al., 1981; Patel et al., 1984; Cooper et al.,
1987). A concomitant decrease occurred in the activity of several
monooxygenases: benzphetamine N-demethylase, aniline hydroxylase,
ethylmorphine N-demethylase (Patel et al., 1984), and
7-ethoxyresorufin O-deethylase (Cooper et al., 1987). The
lowest-observed-effect levels reported were 2 mg/litre for
inactivation of cytochrome P-450 (Gurtoo et al., 1981b) and
25 mg/litre for inhibition of NADPH-cytochrome- c reductase
(Marinello et al.,1981). It was also shown that the addition of
sulfhydryl-containing agents, such as cysteine, acetylcysteine,
glutathione, dithiothreitol, and semicarbazide, reduced the above
effects, suggesting that acrolein produces them by reacting with
sulfhydryl groups at the active sites.
7.3.4 Other biochemical effects
In vivo studies with Holtzman rats have shown that rat liver
alkaline phosphatase (EC 3.1.3.1) and tyrosine aminotransferase
(EC 2.6.1.5) activities are increased markedly after inhalation of
acrolein for 4 h at a concentration of 14.7 mg/m3 or after a
single intraperitoneal injection of acrolein in water at doses of
1.5-6 mg/kg body weight (Murphy et al., 1964; Murphy, 1965). The
increase in alkaline phosphatase activity following intraperitoneal
injection was shown to be dose related (Murphy, 1965). The effects
were reduced by prior adrenalectomy or hypophysectomy or by
pretreatment with protein synthesis inhibitors such as actinomycin
D, puromycin, and ethionine, suggesting that the irritant action of
acrolein stimulates the pituitary-adrenal system to release
glucocorticoids, which act to increase the synthesis of adaptive
liver enzymes (Murphy, 1965; Murphy & Porter, 1966). Increased
plasma and adrenal levels of corticosterone were measured in
Holtzman rats one hour after a single intraperitoneal injection
(3 mg/kg body weight) of acrolein in water (Szot & Murphy, 1971).
The hypersecretion of glucocorticoids could also explain the
observed increase in liver glycogen level following intraperitoneal
exposure to acrolein at a dose of 1.5 mg/kg body weight (Murphy &
Porter, 1966).
At a concentration of 5.6 mg/litre, acrolein produced an 80%
inhibition of the noradrenaline-induced oxygen consumption of
isolated hamster brown fat cells (Pettersson et al., 1980). In
addition, Zollner (1973) observed an acrolein-induced inhibition of
the respiration of intact rat liver mitochondria and found evidence
for an inhibition at three different sites: glutamate transport,
inorganic phosphorus transport, and the enzyme succinic
dehydrogenase (EC 1.3.5.1).
Several sulfhydryl-sensitive enzymes have been shown to be
inhibited by acrolein in vitro, e.g., rabbit muscle L-lactate
dehydrogenase (EC 1.1.1.27), yeast glucose-6-phosphate
dehydro-genase (EC 1.1.1.49), and yeast alcohol dehydrogenase
(EC 1.1.1.1) (Benedict & Stedman, 1969), porcine lung
15-hydroxyprosta-glandin dehydrogenase (EC 1.1.1.141) (Liu & Tai,
1985), rat liver or urothelium S-adenosyl-L-methionine-
DNA(cytosine-5)- methyltransferase (EC 2.1.1.37) (Cox et al.,
1988), and O6-methylguanine-DNA methyltransferase (EC 2.1.1.63)
in cultured human bronchial fibroblasts (Krokan et al, 1985). In
two of these studies glutathione was shown to afford protection
against inhibition of the enzyme (Liu & Tai, 1985; Cox et al.,
1988).
It has been suggested that the formation of a Schiff base
between acrolein and sensitive amine groups is responsible for the
observed inhibition in vitro of Salmonella typhimurium
deoxyribose-5-phosphate aldolase (EC 4.1.2.4) at a concentration of
approximately 14 mg/litre (Wilton, 1976) and human plasma
alpha1-proteinase inhibitor (Gan & Ansari, 1987).
Acrolein was shown to cause a concentration-dependent increase
in lipid peroxidation in isolated rat hepatocytes at levels that
also decreased glutathione concentrations (Zitting & Heinonen,
1980). Preincubation of washed rat liver microsomes with acrolein
abolished the protective effect of glutathione against
iron/ascorbate-induced lipid peroxidation (Haenen et al, 1988). The
authors claimed that the protective effect of glutathione was
mediated by vitamin E scavenging membrane lipid radicals. It was
suggested that acrolein was inhibiting a glutathione-dependent
reductase enzyme responsible for reducing vitamin E radicals back to
vitamin E.
7.4 Immunotoxicity and host resistance
Acrolein has been found to depress pulmonary host defenses in a
number of tests.
In female Swiss mice, exposed to measured concentrations of
1.1, 6.9, and 14.2 mg/m3 for 8 h, a concentration-related increase
in the survival of Staphylococcus aureus was seen at levels of
6.9 mg/m3 or more (Astry & Jakab, 1983). A concentration- and
time-related increase in the survival of S. aureus and Proteus
mirabilis was found in male Swiss CD-1 mice exposed to measured
concentrations of 2.3 to 4.6 mg/m3 for 24 h. When the mice were
also infected with Sendai virus, intrapulmonary bacterial death was
further suppressed (Jakab, 1977). An increased survival of
Klebsiella pneumoniae, but no increased mortality from pneumonia
following challenges with Streptococcus zooepidemicus, was
observed in female CD-1 mice after exposure to acrolein at a
measured concentration of 0.23 mg/m3 for 3 h per day over 5 days
(Aranyi et al., 1986). In female CR/CD-1 mice, exposure to a
measured acrolein concentration of 4.6 mg/m3, for one period of
6 h or for 7 consecutive daily periods of 8 h, resulted in an
increased mortality from Streptococcus pyogenes and Salmonella
typhimurium, respectively, but not from influenza A virus (Campbell
et al., 1981).
Sherwood et al. (1986) exposed groups of 33 male
Sprague-Dawley rats to acrolein vapour at analysed concentrations of
0.39, 2.45 or 6.82 mg/m3 for 3 weeks (6 h per day and 5 days per
week). The relative pulmonary bactericidal activity to
K. pneumoniae was not affected nor was the number of alveolar
cells. However, the number of peritoneal macrophages was decreased
at concentrations of 2.45 mg/m3 or more, and alveolar and
peritoneal macrophages had altered phagocytic and enzymic patterns
at > 0.39 mg/m3.
When SPF-OFA rats were exposed continuously to a measured
acrolein concentration of 1.26 mg/m3 for 18 days, they exhibited
an increased mortality from an infection by Salmonella enteritidis.
However, no such effect was observed following 63 days of exposure
(Bouley et al., 1975).
Acrolein has been shown to inhibit in vitro protein synthesis
(Leffingwell & Low, 1979), and phagocytosis and ATPase (EC
3.6.1.37-38) activity (Low et al., 1977) in rabbit pulmonary
alveolar macrophages. Inhibition of a graft-versus-host reaction in
rats was found after Wistar rat spleen cells were incubated
in vitro with acrolein and injected into all four feet of hybrid
F1 rats. In addition, a decreased mitogen response of human
peripheral lymphocytes was recorded (Whitehouse et al., 1974).
Acrolein was also found to inhibit in vitro chemotaxis of human
polymorphonuclear leucocytes (Bridges et al., 1977).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Two in vivo exposure studies have been reported. In one, 3
male and 21 female SPF-OFA rats were exposed continuously to
acrolein vapour at a measured concentration of 1.26 mg/m3 for 25
days and allowed to mate on day 4. It should be noted that the
exposure period did not cover the complete spermatogenic period of
60 days. The number of pregnant animals and the number and mean
weight of the fetuses were unaffected in comparison to the control
rats (Bouley et al., 1985). In the second study, groups of 12 to
16 New Zealand rabbits were injected (into the ear vein) with a
solution of acrolein in saline (3, 4.5 or 6 mg/kg body weight) on
the 9th day of gestation. At 4.5 and 6 mg/kg body weight, maternal
toxicity was indicated by the death of 3 and 6 dams, respectively,
and embryotoxicity by a dose-related increase in resorptions which
was significant at 6 mg/kg body weight. It was also reported that
the number of malformed and retarded fetuses increased in a
dose-related manner, although the increases were statistically
non-significant. No effects on maternal toxicity, embryotoxicity or
fetuses were noted at 3 mg/kg body weight (Claussen et al., 1980).
A clear effect on the development of the embryo in vivo was
observed only when acrolein was administered close to the target
site by intra-amniotic injection. Using groups of 12 to 19 pregnant
New Zealand rabbits, 0, 10, 20, or 40 µl of a 0.84% solution of
acrolein in saline was injected into the amnion of all embryos in
one of the uterine horns on the 9th day of gestation. The embryos in
the other uterine horn received saline only and served as controls.
The dams were killed on day 28 of gestation. There was a
dose-related increase in the rate of resorptions and malformations,
significant at doses of 20 µl or more per embryo. Malformations
included deformed and asymmetric vertebrae, spina bifida, deformed
and fused ribs, and lack or fusion of sternum segments. No effect
was observed on the number of implantations and fetuses or on fetal
growth (Claussen et al., 1980). A similar study was carried out
on pregnant Sprague-Dawley rats injected with acrolein doses of 0,
0.1, 1.0, 2.5, 5.0, 10.0 or 100 µg per fetus in 10 ml of saline on
the 13th day of pregnancy. The dams were killed on day 20 of
gestation. A dose-related increase in the percentage of dead and
resorbed fetuses per litter was observed at all dose levels. The
total number of litters at each dose level varied from 4 to 18. The
percentage of malformed fetuses per litter also was increased in a
dose-related manner at doses of up to 5 µg per fetus. The increase
was significant only up to this dose level, probably because at
higher doses there were few surviving fetuses. Treatment-related
effects included oedema, micrognathia, hindlimb and forelimb
defects, and hydrocephaly (Slott & Hales, 1985). These results
confirmed the findings of an earlier, identical test using dose
levels of 0.1, 10, and 100 µg per fetus (Hales, 1982).
Acrolein was also shown to be embryotoxic and teratogenic in
the rat whole embryo culture system. As with embryos exposed in
vivo, the concentration range for teratogenicity was very narrow
(Slott & Hales, 1986). Schmid et al. (1981) and Mirkes et al.
(1984) observed embryotoxicity but no teratogenicity in the same
test system, this being probably the result of the different test
conditions used (Slott & Hales, 1986). Depletion of glutathione by
buthionine sulfoximine enhanced the embryotoxicity and
teratogenicity of acrolein in the in vitro studies of Slott &
Hales (1987a), whereas exogenous glutathione afforded protection
against these effects (Slott & Hales, 1987b).
In a mouse limb bud culture system, acrolein induced impairment
of limb bud differentiation, indicative of a teratogenic action
(Stahlmann et al., 1985). When acrolein was injected into chicken
eggs, embryotoxic and teratogenic effects were observed (Kankaanpaa
et al., 1979; Korhonen et al., 1983; Chhibber & Gilani, 1986).
In summary, acrolein can induce teratogenic and embryotoxic
effects if administered directly to the embryos or fetuses.
However, the fact that no effect was found in rabbits injected
intravenously with 3 mg/kg suggests that neither skin contact nor
inhalation of acrolein is likely to affect the developing embryo.
7.6 Mutagenicity and related end-points
7.6.1 DNA damage
In vitro studies have revealed interactions between acrolein
and DNA and RNA (Munsch et al., 1974b; section 6.2.1). Acrolein
has also been found to react with purine and pyrimidine bases or
intact DNA in vitro, and several adducts have been identified
(Descroix, 1972; Hemminki et al., 1980; Lutz et al., 1982; Chung
et al., 1984; Shapiro et al., 1986; section 6.2.2.2). Cyclic
deoxyguanosine DNA adducts were formed in a dose-dependent fashion
in acrolein-exposed Salmonella typhimurium TA100 and TA104. This
adduct formation correlated with the induction of reverse mutations
in these strains (section 7.6.2; Foiles et al., 1989).
No data on the formation of DNA adducts following exposure of
animals to acrolein are available.
Incubation of Fischer-344 rat nasal mucosal homogenate with
acrolein resulted in a concentration-dependent increase in
DNA-protein cross-linking, which was not observed following
inhalation exposure of rats to acrolein at a concentration of
4.6 mg/m3 for 6 h (Lam et al., 1985). According to the authors
this could be explained by the preferential reaction of acrolein
with sulfhydryl groups. DNA-protein cross-linking and single strand
breaks were observed in vitro in human bronchial fibroblasts at
cytotoxic concentrations of 1.7 mg/litre or more (Grafström et al.,
1986, 1988), and there was indirect evidence for some formation of
DNA interstrand cross-linking (Grafström et al., 1988). No
DNA-protein or DNA interstrand cross-linking was induced by acrolein
in mouse L1210 leukemia cells at cytotoxic levels that produced
single strand breaks and/or alkali-labile sites in these cells
(Erickson et al., 1980) or in human chronic myeloid leukemia cells
(Crook et al., 1986a). In non-mammalian assays, Fleer & Brendel
(1982) did not find DNA interstrand cross-linking or single strand
breaks in MB1072-2B yeast cells and Kubinski et al. (1981)
observed DNA-cell binding in Escherichia coli in the presence of a
rat liver S9 fraction. These studies demonstrate that effects on
DNA occur only at cytotoxic concentrations of acrolein.
Results of DNA repair tests are not available. Acrolein has
been demonstrated to inhibit O6-methylguanine-DNA
methyltransferase (EC 2.1.1.63.; section 7.3.4) and, therefore, can
be expected to reduce the capacity for repair of O6-guanine
alkylations in DNA (Krokan et al., 1985).
7.6.2 Mutation and chromosomal effects
The results of tests for the induction of gene mutations and
chromosome damage by acrolein are summarized in Table 10.
In point mutation assays with Salmonella typhimurium, the
positive or equivocal responses obtained were all observed within a
narrow dose range of up to 10-56 µg per plate, higher doses being
toxic. Clearly positive, dose-related increases in revertant
colonies per plate at 2-5 times the background rate were observed in
the absence of metabolic activation only in TA100 (Lutz et al.,
1982; Foiles et al., 1989; Hoffman et al., 1989), TA104 (Marnett
et al., 1985; Foiles et al., 1989; Hoffman et al., 1989), and
TA98 (Lijinsky & Andrews, 1980). Khudoley et al. (1986) reported
positive results in strains TA98 and TA100 without specifying dose
levels or revertant rates. Some evidence for indirect mutagenicity
was found in strains TA1535 (Hales, 1982) and TA100 (Haworth
et al., 1983), the slight increase in TA100 revertants being dose
related. However, negative results, both with and without metabolic
activation, have also been obtained in these strains. Some of these
negative results were clearly related to the incubation conditions,
which were probably highly toxic, e.g., those obtained in spot tests
(Andersen et al., 1972; Florin et al., 1980). In Salmonella
typhimurium TA100 and TA104, strains that show a clear mutagenic
response to acrolein, DNA-acrolein adducts have also been identified
(section 7.6.1).
Acrolein did not induce sex-linked recessive lethality in
Drosophila melanogaster adults (Zimmering et al., 1985), but
induced a 12-fold increase in sex-linked recessive lethality in
hatching eggs and larva at an exposure level that was not reported
but caused over 75% larval death (Rapoport, 1948). In the latter
test, treatment of adults was reported to be less effective.
Three cytogenetic tests have been carried out with acrolein,
two in Chinese hamster ovary cells (Au et al., 1980; Galloway
et al., 1987) and one in human lymphocytes (Wilmer et al.,
1986). Acrolein was shown to induce sister chromatid exchanges in
the absence of a metabolic activating system in all three studies.
The lowest effective concentration was 56 µg/litre (Galloway
et al., 1987). No increase in chromosome aberrations was reported
in one study (Galloway et al., 1987), while chromosome breakage
was reported in another study at cytotoxic concentrations (Au
et al., 1980).
Three properties of acrolein make it difficult to test for
mutagenicity: its high cytotoxicity, which prevents the expression
of any mutagenic activity, and its high reactivity and volatility,
which prevent it reaching the target sites. However, acrolein can
be considered to be a weak mutagen in some bacterial and fungal test
systems in the absence of metabolic activating systems and to
induce sister chromatid exchange in cultured mammalian cells.
7.6.3 Cell transformation
Acrolein (0.4 µg/ml) has been found not to exhibit transforming
potential in C3H/10T1/2 cells but to initiate the process of
transformation. The latter was measured by exposing cultures to
acrolein for 24 h and, subsequently, to a phorbol ester for 6 weeks
(Abernethy et al., 1983).
Table 10. Tests for gene mutation and chromosomal damage by acrolein
Test description Organism Species/strain/cell type Resulta Reference
Gene mutations
Reverse mutations bacteria Salmonella typhimurium TA1535 ±(+S9) Hales (1982)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Lijinsky & Andrews (1980)
Salmonella typhimurium TA100 +(-S9) Lutz et al. (1982); Khudoley et al., 1986;
Foiles et al. (1989); Hoffman et al. (1988)
±(+S9) Haworth et al. (1983)
- Florin et al. (1980); Loquet et al. (1981);
Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium TA104 +(-S9) Marnett et al. (1985); Foiles et al. (1989);
Hoffman et al. (1989)
Salmonella typhimurium TA102 - Marnett et al. (1985)
Salmonella typhimurium TA98 +(-S9) Lijinsky & Andrews (1980); Khudoley et al. (1986)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Basu & Marnett (1984)
Salmonella typhimurium TA1537 - Florin et al. (1980); Haworth et al. (1983);
Lijinsky & Andrews (1980)
Salmonella typhimurium TA1538 - Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium, 8 strains - Andersen et al. (1972)
Escherichia coli 343/113 - Ellenberger & Mohn (1976, 1977)b
Escherichia coli WP2 uvrA ±(-S9) Hemminki et al. (1980)
yeast Saccharomyces cerevisiae S211, S138 - Izard (1973)
Forward mutations yeast Saccharomyces cerevisiae N123 +(-S9) Izard (1973)c
Table 10 (contd).
Test description Organism Species/strain/cell type Resulta Reference
Forward mutations man normal fibroblasts -(-S9) Curren et al. (1988)
DNA-repair-deficient fibroblasts +(-S9) Curren et al. (1988)
hamster V79 cells +(-S9) Smith et al. (1990)
Sex-linked lethal insect Drosophila melanogaster, hatching + Rapoport (1948)d
mutations eggs and young larva
Drosophila melanogaster, adults - Zimmering et al. (1985)e
Chromosomal damage
Aberrations hamster ovary cells in vitro ±(+S9) Au et al. (1980)
±(-S9) Galloway et al. (1987)
Sister chromatid hamster ovary cells in vitro +(-S9) Au et al. (1980)
exchanges +(-S9) Galloway et al. (1987)
man lymphocytes in vitro +(-S9) Wilmer et al. (1986)
Dominant lethal mouse germ cells - Epstein & Shafner (1968)
mutations
(ip exposure)
a + = >2 x background rate or statistically significant (P < 0.05); ± = equivocal; - = negative.
b Details for this test were not reported.
c Plate test for petite mutations (production of a respiratory-deficient mutant).
d Doses were not reported. Treatment of adults was found to be less effective.
e Exposure via feeding solution or via injection.
7.7 Carcinogenicity
7.7.1 Inhalation exposure
Inhalation experiments of appropriate duration specifically
designed to assess the carcinogenicity of acrolein vapour have not
been conducted.
An 81-week study (52 weeks of acrolein exposure at 9.2 mg/m3
followed by 29 weeks without exposure) on groups of 36 Syrian golden
hamsters of both sexes is described in section 7.2.2. The effects
of treatment included a persistent and statistically significant
reduction in body weight in females, an increased relative brain
weight in males and females at 52 weeks, and an increased relative
lung weight in females at 52 weeks. Apart from one small tracheal
papilloma in an acrolein-exposed female, no respiratory tract
tumours were observed in control or treated hamsters (Feron &
Kruysse, 1977). In order to elucidate a possible co-carcinogenic
action of acrolein, Feron & Kruysse (1977) also exposed groups of 30
Syrian golden hamsters of both sexes to measured acrolein vapour
concentrations of 0 or 9.2 mg/m3, 7 h per day and 5 days per week
for 52 weeks, and, for the same period, either weekly to an
intratracheal dose of benzo [a]pyrene or once every 3 weeks to a
subcutaneous dose of diethylnitrosamine. Total dose levels were 18.2
or 36.4 mg benzo [a]pyrene and 2.1 µl diethylnitrosamine. Survivors
were killed at week 81, and all hamsters were subjected to
postmortem examination. The mortality rate in the groups treated
with benzo [a]pyrene was slightly higher than in other groups. The
incidence of benzo [a]pyrene-induced respiratory tract tumours was
slightly (but statistically insignificantly) higher in females also
exposed to acrolein vapour. In these females, at the higher dose
level of benzo [a]pyrene, respiratory tract tumours occurred
earlier and the number of malignant tumours was slightly increased.
Taken together, these observations might suggest an enhancing effect
of acrolein on benzo [a]pyrene carcinogenesis in the respiratory
tract, but the effect cannot be considered proven.
In a study by Le Bouffant et al. (1980), rats, 20 animals per
group, were exposed to 18.3 mg/m3, 1 h/day and 5 days/week, for 10
or 18 months. No tumours or metaplasias were found.
7.7.2 Oral exposure
In a study by Lijinsky & Reuber (1987), groups of 20
Fischer-344 rats of both sexes were exposed to weekly prepared
acrolein of unspecified purity in drinking-water. Each cage of four
rats received 80 ml of acrolein solutions at concentrations of 100
or 250 mg/litre for 124 weeks (males only) or 625 mg/litre for 104
weeks (both sexes) for 5 days per week (this was estimated by the
Task Group to be equivalent to approximately 5, 12.5, and 50 mg/kg
body weight per day, respectively). Total doses were 1200, 3100,
and 6500 mg per rat, respectively. Controls were left untreated.
Survivors were killed at week 123-132 and all rats were subjected to
postmortem examination. The mean survival time was about 120 weeks
for experimental and control groups. There was a marginal, but not
statistically significant, increase in the incidence of adrenal
cortical adenomas (5/20) in female rats at 625 mg/litre, compared to
concurrent controls (1/20), and a decrease in the incidence of
pituitary neoplasms in both sexes at 625 mg/litre. In addition, 2
of 20 females given 625 mg/litre had hyperplastic nodules of the
adrenal cortex. The authors cited historic control values for
adrenal cortical adenomas or carcinomas in female Fischer-344 rats
from other laboratories as 1.3% at 26 months of age and 4.8% in a
lifespan study. Because of limited numbers of animals used and
concerns regarding the purity and stability of acrolein in the dosed
drinking-water, the authors of this study did not consider it to be
a definitive carcinogenicity bioassay. In addition, the Task Group
considered the historical control values quoted by the authors to be
of limited use in evaluating the importance of the tumour incidence
found in this study.
Acrolein appeared to be too toxic to Syrian golden hamsters
following oral exposure by gavage in corn oil to conduct an
effective carcinogenicity study (Lijinsky & Reuber, 1987).
7.7.3 Skin exposure
In a study by Salaman & Roe (1956), a group of 15 S strain mice
of unspecified sex and age received weekly doses of 0.5% acrolein in
acetone for 10 weeks. The total dose was 12.6 mg per rat, although
the purity of the acrolein was not reported. The control group
comprised 20 mice. From day 25 after the first acrolein treatment,
the mice received once per week a skin application of 0.17% croton
oil (0.085% in weeks 2 and 3) for 18 weeks. Croton oil and acrolein
were applied alternately at 3 or 4 day intervals. At the end of
treatment, the mortality rate and the incidence of skin papillomata
were similar to those of the controls treated only with croton oil.
However, this study must be considered inadequate because of the
limited number of animals used and the short duration of the
experiment.
7.8 Interacting agents
Free sulfhydryl-containing compounds have been found to give
protection against the adverse effects of acrolein in vitro, e.g.,
the inhibition of enzymes involved in macromolecular synthesis
(Munsch et al., 1973), liver microsomal cytochrome P-450s
(Marinello et al., 1978; Berrigan et al., 1980; Gurtoo et al.,
1981b, Patel et al., 1984; Cooper et al., 1987), and several
other sulfhydryl-sensitive enzymes (Liu & Tai, 1985; Cox et al.,
1988), the adverse effects on rabbit alveolar macrophages (Low et
al., 1977; Leffingwell & Low, 1979), and the impairment of mouse
limb bud differentiation (Stahlmann et al., 1985). Free
sulfhydryl-containing agents protected against the acute lethal
effects of acrolein in Charles River rats (Sprince et al., 1979)
and in DBA/2J mice (Gurtoo et al., 1981a).
When Swiss-Webster mice were exposed to acrolein-formaldehyde
mixtures, the percentage decrease in respiratory rate was found to
be less than the sum of the percentage decreases due to each
compound alone (Kane & Alarie,1978). In acrolein-exposed Fischer-344
rats, pretreatment with formaldehyde resulted in a lower percentage
decrease in respiratory rate compared to non-pretreated rats (Babiuk
et al., 1985). It was suggested in both investigations that
acrolein and formaldehyde competed for the same receptor
(competitive agonism). In a comparable experiment, the maximum
percentage decrease in the respiratory rate of Swiss-Webster mice
exposed to a mixture of acrolein and sulfur dioxide was lower than
that of acrolein alone. This antagonistic effect was thought to be
caused by a chemical reaction in the air phase between the two
compounds, which reduced the effective concentrations (Kane &
Alarie, 1979).
In Fischer-344 rats exposed to formaldehyde vapour
(7.4 mg/m3) once for 6 h, co-exposure to acrolein vapour
(4.6 mg/m3) resulted in a higher increase in DNA-protein
cross-linking than was observed with formaldehyde alone. Acrolein
alone did not increase DNA-protein cross-linking in this experiment
(Lam et al., 1985).
In a study by Hales et al. (1988), anaesthetized,
artificially ventilated mongrel dogs were exposed to acrolein or
hydrochloric acid with added synthetic smoke composed of carbon
particles for 10 min. The dogs were exposed to smoke with or
without analytically determined acrolein concentrations of <
458 mg/m3, 458-687 mg/m3 or > 687 mg/m3. Smoke with
acrolein, but not smoke with hydrochloric acid, produced
non-cardiogenic, peribronchiolar pulmonary oedema in a
concentration- and time-related fashion. Both acrolein and
hydrochloric acid produced airway damage consisting of mucosal
degeneration and desquamation and inflammatory cell infiltration.
Acrolein at levels above 458 mg/m3 also caused fibrin deposition
in the alveolar spaces that juxtaposed injured bronchioles.
8. EFFECTS ON HUMANS
8.1 Single exposure
8.1.1 Poisoning incidents
One man was exposed dermally and by inhalation when acrolein
was sprayed into his face following an accident in a chemical plant.
Immediately, his face and eyelids were burnt. Within 20 h he was
hospitalized with fever, cough, frothy sputum, cyanosis, and acute
respiratory failure. Two months after the accident, the opening of
the right bronchus was obstructed and the upper trachea showed
slight oedema with haemorrhagic spots. At 18 months he had developed
chronic bronchitis and emphysema, which might have been a sequel of
the accidental exposure (Champeix et al., 1966).
One case of attempted oral suicidal intoxication has been
reported. The man swallowed approximately 1.5 g of acrolein in a
glass of orange juice. Blood was found in his stomach and the number
of red and white blood cells was increased. Gastroscopic
examination showed shrinkage of the stomach and a massive chronic
gastritis with erosions and ulceration. Further examination of the
stomach revealed regenerating mucous membranes, few mucous glands,
granulation and scarring of the serosa, shrinkage and stenosis of
the pylorus, lymphadenitis, and haemosiderin deposition in lymph
nodes. The man was successfully treated by gastrectomy (Schielke,
1987).
Two cases of suspected exposure to acrolein have been reported.
The death of two young boys who inhaled smoke from an overheated
frier for approximately 2 h was thought to be related to acrolein
exposure, although other chemicals might also have been involved.
One of the boys was found dead, while the other suffered from acute
respiratory failure. Following oxygen therapy, the second boy died
due to asphyxia. At autopsy a massive cellular desquamation of the
bronchial lining was observed. The tracheal and bronchial lumina
were filled with debris and the lungs showed multiple infarcts
(Gosselin et al., 1979).
Four female factory workers operating a machine for cutting and
sealing polyethylene bags and a fifth sitting next to the machine
complained of a burning sensation in the eyes, a feeling of dryness
and irritation in the nose and throat, and itching and irritation of
the skin of the face, neck and forearms. These complaints were
related to the smoke developed. The presence of formaldehyde and
"acrolein and/or other aldehydes" in the smoke was suspected and
confirmed. During heavy smoke exposure, itching eruptions developed
on exposed skin. Drowsiness and headache was also experienced. All
symptoms were reversible (Hovding, 1969).
8.1.2 Controlled experiments
8.1.2.1 Vapour exposure
Several studies with volunteers have been conducted with the
object of establing thresholds for odour perception and recognition
and for effects on the eyes, nose, respiratory tract, and nervous
system. The results of these studies are summarized in Table 11. The
exposure period was up to 60 min. In most cases the concentration
of acrolein was determined colorimetrically, although a few reports
did not include a description of the analytical method (Plotnikova,
1957; Sinkuvene, 1970; Harada, 1977). Sinkuvene (1970) reported the
threshold for changes in the electrical activity of the brain
cortex, as measured by electro-encephalography, to be 0.05 mg/m3.
However, this result cannot be evaluated since experimental data
were not provided. The odour perception threshold for sensitive
people was 0.07 mg/m3.
In studies by Weber-Tschopp et al. (1977), groups of human
volunteer students of both sexes were exposed either for 60 min to
acrolein at a concentration of 0.69 mg/m3 or to gradually
increasing acrolein concentrations from 0 up to 1.37 mg/m3 over
35 min followed by a 5-min exposure to 1.37 mg/m3. In further
experiments with side-stream cigarette smoke instead of pure
acrolein vapour, it was noted that the effects of pure acrolein
vapour were small compared to those produced by side-stream smoke
with the same acrolein vapour concentration. It was concluded that
acrolein was only to a minor extent responsible for the effects
observed (Weber-Tschopp et al., 1976). It must be noted, however,
that a significant part of the acrolein in side-stream cigarette
smoke may be associated with particulate matter (Ayer & Yeager,
1982) and would not have been measured. This may have resulted in an
underestimation of the acrolein concentration in the smoke. Many of
the studies considered in this section are old and the analytical
techniques are often poorly described; the absolute figures reported
may, therefore, be suspect.
8.1.2.2 Dermal exposure
In an investigation into irritant dermatitis possibly caused by
contaminants present in diallylglycol carbonate monomer, patch tests
were conducted with acrolein in ethanol at concentrations of 0.01,
0.1, 1, and 10% on groups of 8, 10, 48, and 20 volunteers,
respectively. At 1%, six positive reactions were recorded, four
cases of serious oedema with bullae and two of erythema. At 10%,
all subjects showed positive reactions with bullae, necrosis,
inflammatory cell infiltrate, and papillary oedema (Lacroix et al.,
1976).
Table 11. Thresholds for acute effects of acrolein on humans
Concentration Exposure Effect Reference
(mg/m3) period (min)
0.05 changes in electrical activity of brain cortex Sinkuvene (1970)
0.07 odour perception by most sensitive individuals Sinkuvene (1970)
0.13 5 no or medium subjective eye irritation Darley et al. (1960)a
0.21 5 increased incidence of subjective eye irritation Weber-Tschopp et al. (1977)b
0.34 10 increased incidence of subjective nasal irritation Weber-Tschopp et al. (1977)b
0.34 30 time-related increase in eye-blink frequency van Eick (1977)a
0.39 10 increased incidence of subjective annoyance Weber-Tschopp et al. (1977)b
0.48 odour recognition Leonardos et al. (1969)
0.59 15 increase in eye-blink frequency Weber-Tschopp et al. (1977)b
0.6 10 increase in sensitivity to light Plotnikova (1957)
0.69 40 decrease in respiratory rate; increased incidence of Weber-Tschopp et al. (1977)c
subjective general irritation of eyes, nose, and neck
0.69 10 increase in eye-blink frequency Weber-Tschopp et al. (1977)c
1 3 slight subjective conjunctival irritation Plotnikova (1957)
1 3 stinging sensation in nose Plotnikova (1957)
1.1 5 increased incidence of subjective eye irritation Stephens et al. (1961)a
1.1 5 increase in tear volume, pH,and lysozyme activity Harada (1977)
1.37 35 decrease in respiratory rate Weber-Tschopp et al. (1977)b
1.5 3 pneumographic changes in rhythm and amplitude of Plotnikova (1957)
respiratory movements
Table 11 (contd).
Concentration Exposure Effect Reference
(mg/m3) period (min)
1.7 3 reflex action on optical chronaxy Plotnikova (1957)
1.88 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 20 seconds
2.80 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 5 seconds
3 5 medium to severe subjective eye irritation Darley et al. (1960)a
4 2-3 acute subjective conjunctival and nasal irritation; Plotnikova (1957)
painful sensation in nasopharyngeal region
a exposure of eyes only
b exposure to gradually increasing concentrations up to 1.37 mg/m3
c exposure to a fixed concentration
8.2 Long-term exposure
No data are available on the long-term exposure of humans to
acrolein.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
A summary of acute aquatic toxicity data is presented in
Table 12. In most of these studies, the amount of acrolein added was
reported but the concentrations present were not measured. In these
cases, the actual concentrations may have been lower than the
nominal ones in view of the volatility of the substance and its
hydration rate (see section 4.2).
One of the studies in Table 12 (Lorz et al., 1979) is a
comparatively detailed examination of the acute toxicity of acrolein
to Coho salmon. Within 144 h of exposure to 0.075 mg/litre or more,
all fish died. In surviving fish the activity of gill
Na+,K+-ATPase (EC 3.6.1.37) and the tolerance to subsequent
sea-water exposure were not affected at concentrations up to
0.05 mg/litre. A histological examination of the gills, kidneys, and
liver at 0, 0.05, and 0.1 mg/litre revealed concentration-dependent
adverse effects.
A 3-generation 64-day test with the crustacean Daphnia magna
was conducted in a flow-through open system with well water at
20 °C, a pH between 7.0 and 7.3, a dissolved oxygen concentration of
7.5 mg/litre, and a water hardness of 35 mg CaCO3/litre. The
highest concentration that did not result in mortality was
0.0169 mg/litre (acrolein concentrations were measured in this
study). Survival was reduced at levels of 0.0336 mg/litre or more,
but the number of young per female was not affected even at the
highest concentration tested, 0.0427 mg/litre (Macek et al.,
1976).
Macek et al. (1976) also reported on a 60-day test with
fathead minnow (Pimephales promelas) in a flow-through open system
with well water at 25 °C, a pH between 6.6 and 6.8, a dissolved
oxygen concentration of 8.2 mg/litre, and a water hardness of 32 mg
CaCO3/litre. The highest concentration without adverse effects
was 0.0114 mg/litre (acrolein concentrations were measured in this
study). At 0.0417 mg/litre, there was increased mortality among
offspring. No adverse effects were found on survival and mortality
of adults, number of spawnings and number of eggs per female, number
of eggs per spawn, length of offspring, or hatchability.
It is clear from Table 12 why acrolein is also used as an
algicide, slimicide, and molluscicide.
Table 12. Acute aquatic toxicity of acrolein
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Fresh water
alga Enteromorpha 25 stat, closed 24 h 50% inhibition 1.8c Fritz-
intestinalis of photosynthesis Sheridan
(1982)
alga Cladophora 25 stat, closed 24 h 50% inhibition 1.00c
glomerata of photosynthesis
alga Anabaena 25 stat, closed 24 h 50% inhibition 0.69c
of photosynthesis
bacterium Proteus 37 7.0 stat, closed 2 h 50% growth 0.02 Brown &
vulgaris reduction Fowler
(1967)
bacterium Pseudomonas 25 7.0 stat, closed 16 h TT 0.21 Bringmann
putida & Kuhn
(1977)
protozoan Entosiphon 25 6.9 stat, closed 72 h TT 0.85 Bringmann
sulcatum (1978)
protozoan Chilomonas 20 6.9 stat, closed 48 h TT 1.7 Bringmann
paramecium et al.
(1980)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
protozoan Uronema 25 6.8 stat, closed 20 h TT 0.44 Bringmann
parduczi & Kuhn
(1980)
mollusc snail 21-25 flow, open 48 h 99-100% 20-25 Unrau et
(Bulinus mortality al. (1965)d
truncatus)
mollusc snail stat, open 3 h 100% mortality 10 Ferguson
(Biomphalaria 24 h 10% mortality 1.25 et al.
glabrata), eggs (1961)
mollusc snail (Biomphalaria stat, open 24 h 98% mortality 10 Ferguson
glabrata), adults 24 h 35% mortality 2.5 et al.
(1961)
crustacean water flea 20 7.0- 7.5 35 stat, open 48 h LC50 0.057 Macek et
(Daphnia magna) 7.3 al. (1976)
crustacean water flea 22 7.0- 154 stat, closed 48 h EC50f 0.093 Randall &
(Daphnia magna) 8.2 Knopp (1980)
crustacean water flea 22 7.4- 6-9 173 stat, closed 48 h LC50 0.083 LeBlanc
(Daphnia magna) 9.4 (1980)
fish harlequin fish 20 7.2 20 flow, open 48 h LC50 0.06 Alabaster
(Rasbora (1969)
heteromorpha)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
fish fathead minnow 25 6.6- 8.2 32 flow, open 144 h LC50 0.084 Macek et
(Pimephales 6.8 al.
promelas) (1976)e
fish golden orfe 20 7-8 > 5 200-300 stat 48 h LC50 0.25 & Juhnke &
(Leuciscus idus 2.5 Ludemann
melanotus) (1978)
fish goldfish 20 6-8 > 4 108 stat, open 24 h LC50 < 0.08 Bridie
(Carassius et al.
auratus) (1979)c e
fish Bluegill 21-23 6.5- 10- 32-48 stat, closed 96 h LC50 0.09 Buccafusco
sunfish 7.9 0.3 et al.
(Leopomis (1981)
macrochirus)
fish Coho salmon 10 7.4- > 10 100 stat, open 96 h LC50 0.068 Lorz et al.
(Oncorhynchus 7.6 (1979)g
kisutch)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Marine
mollusc common mussel 15 stat, closed 6 h 40% mortality 0.6 Rijstenbil
(Mytilus edulis) 6 h 70% mortality 1.0 & van Galen
8 h 70% detached 0.57 (1981)e h
mussels
a static or flow-through test, open or closed system
b TT = toxic threshold for inhibition of cell multiplication
c exposure to Magnacide-H (92% acrolein, 8% inert ingredients)
d field study, resurgence of snails was delayed by 8 to 12 months
e analysis for acrolein was reported
f the effect was complete immobilization
g static-renewal test
h static-renewal test (1.6% salinity)
9.2 Terrestrial organisms
9.2.1 Birds
The LD50 for the adult starling (Sturnus vulgaris) was
reported to be > 100 mg/kg body weight. The birds were observed for
7 days after dosing, but only two birds per dose were tested
(Schafer, 1972).
9.2.2 Plants
Acrolein is used as biocide, particularly to control aquatic
plants such as Elodea canadensis, Vallisneria spiralis
(ribbonweed), and Potamogeton tricarinatus (floating pondweed).
In Australia, a maximum concentration of about 15 mg/litre over a
period not exceeding a few hours has been imposed. In the USA,
acrolein is injected into larger channels over longer periods at low
concentrations (approximately 0.1 mg/litre over 48 h) (Bowmer &
Sainty, 1977). It has been shown that the dosage of acrolein
required for control, as defined by the product of time and
concentration required for 80% reduction in biomass, is independent
of the separate values of concentration and time, provided that the
concentration exceeds 0.1 mg/litre and the dosage exceeds 2 mg/litre
per h. In tank experiments, the minimum dosages required for 80%
control of ribbonweed and floating pondweed were about 4 and
26 mg/litre per h, respectively (Bowmer & Sainty, 1977). The
effective dosage (> 80% kill) for Elodea canadensis was 8 to
10 mg/litre per h (Van Overbeek et al., 1959; Bowmer & Smith,
1984). Sublethal concentrations of acrolein stimulated the growth
of Elodea (Bowmer & Smith, 1984).
Elongation of pollen tubes of lily seeds (Lilium longiflorum)
was inhibited completely after a 5-h exposure to acrolein vapour at
a measured concentration of 0.91 mg/m3, a temperature of 28 °C,
and a relative humidity of 60%. A 10% inhibition was found after
1 h (Masaru er al., 1976).
The nature and extent of adverse effects on various crops grown
in soil irrigated by acrolein-treated water have been investigated
in two studies. Acrolein concentrations varied between 15 and
50 mg/litre of supply water. Most furrow-irrigated crops, including
beans, clover, corn, and millet, did not show any damage.
Significant damage to foliage was observed in cotton at acrolein
concentrations of 25 mg/litre or more, but there was no evidence of
chronic or residual phytotoxicity. Slight damage to the foliage of
cucumbers and tomatoes was observed at 40 mg/litre. Vegetable
seedlings in contact with treated water were damaged even at the
lowest concentrations used (Unrau et al., 1965; Ferguson et al.,
1965).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Exposure of the general population to acrolein occurs mainly
via air. Exposure via water would only be significant in cases of
ingestion of, or skin contact with, acrolein deliberately applied as
a biocide to irrigation water. Oral exposure to acrolein may also
occur via alcoholic beverages or heated foodstuffs (chapters 3 and
5).
In urban areas, average levels of up to 15 µg/m3 and maximum
levels of up to 32 µg/m3 have been measured away from industrial
sources. Near industries and close to the exhaust pipes of vehicles,
engines, and combustion appliances, levels ten to one hundred times
higher may occur. Extremely high levels of acrolein in the mg/m3
range can be found as a result of fires (section 5.2.1).
Major indoor sources of acrolein are combustion appliances and
tobacco smoking (section 3.2.4). Levels of acrolein in smoke from
indoor open fires for cooking or heating purposes have not been
reported. Smoking one cigarette per m3 of room-space in 10-13 min
has been shown to lead to acrolein vapour concentrations of
450-840 µg/m3 (section 5.2.1). Recent occupational exposure levels
of acrolein in the air at sites of its production or processing are
not available. Workplace levels of over 1000 µg/m3 have been
reported in situations involving the heating of organic materials
(section 5.3).
In summary, the main source of exposure of the general
population to acrolein is via tobacco smoke. General environmental
pollution by vehicle exhaust and the smoke of burning organic
materials is the next most important source.
10.1.2 Health effects
Owing to the reactivity of acrolein, retention at the site of
entry into the body, usually the respiratory tract, is high
(section 6.1). Primary pathological findings are limited
principally to these sites (sections 7 and 8). Any acrolein absorbed
is liable to react directly with protein and non-protein sulfhydryl
groups or with primary and secondary amines such as those found in
proteins and nucleic acids (sections 6.2 and 7.3). Acrolein may also
be metabolized to mercapturic acids, acrylic acid, glycidaldehyde or
glyceraldehyde (section 6.3). Evidence for the last three
metabolites has only been obtained in vitro.
Acrolein is a cytotoxic agent (section 7.1.5) highly toxic to
experimental animals and man following acute exposure via different
routes (sections 7.1.1 and 8.1.1). The vapour is very irritating to
the eyes and the respiratory tract. Liquid acrolein is a corrosive
substance. The no-observed-adverse-effect level for irritant
dermatitis from ethanolic acrolein was found to be 0.1% (section
8.1.2.2). The odour perception threshold for the most sensitive
individuals is reported to be 0.07 mg/m3 (8.1.2.1). Experiments
with human volunteers show a lowest-observed-adverse-effect level of
0.13 mg/m3, at which level eyes may become irritated after 5 min.
In addition to irritation of the eyes, changes in respiratory tract
function are evident at or above 0.7 mg/m3 (40-min exposure)
(section 8.1.2.1). At higher concentrations, degeneration of the
respiratory epithelium and irritation of all exposed mucosa develop.
Oedematous changes in the tracheal and bronchial mucosa and
bronchial obstruction can be expected after very high exposure to
acrolein vapour (section 8.1).
There are no human toxicological data from long-term exposure
to acrolein. The toxicity from exposure to acrolein vapour has been
relatively well investigated in several animal studies for exposure
periods of up to 52 weeks (section 7.2). Both respiratory tract
function and histopathological effects have been observed at
0.5-0.8 mg/m3 (continuous exposure). Toxicological effects in the
respiratory tract have been documented in most animal species
exposed repeatedly to acrolein concentrations of 1.6-3.2 mg/m3 or
more, and mortality has occurred following exposure to
concentrations above 9 mg/m3. There is limited evidence that
acrolein can depress pulmonary host defenses in mice and rats.
Acrolein can induce teratogenic and embryotoxic effects if
administered directly into the amnion. However, the fact that no
effect was found in rabbits injected intravenously with 3 mg/kg
suggests that human exposure to acrolein is unlikely to affect the
developing embryo (section 7.5).
Acrolein has been shown to interact with DNA and RNA in vitro
and to inhibit their synthesis both in vivo and in vitro. In
vitro, it induces gene mutations in bacteria and fungi and sister
chromatid exchanges in mammalian cells (section 7.6). There is
inadequate evidence to allow the mutagenic potential in humans to be
assessed reliably.
One long-term drinking-water study with rats (130 weeks) and
two inhalation tests, one with hamsters (81 weeks) and the other
with rats (40 or 70 weeks), failed to demonstrate carcinogenic or
clear co-carcinogenic effects of acrolein (section 7.7). Due to the
shortcomings of the tests used, acrolein cannot be considered to
have been adequately tested for carcinogenicity and no conclusions
concerning its carcinogenicity are possible.
The threshold levels of acrolein that cause irritation and
health effects are 0.07 mg/m3 for odour perception, 0.13 mg/m3
for eye irritation, 0.3 mg/m3 for nasal irritation and eye
blinking, and 0.7 mg/m3 for decreased respiratory rate. Since
the level of acrolein rarely exceeds 0.030-0.040 µg/m3 in polluted
urban air or smoke-filled restaurants, acrolein alone is unlikely to
reach annoyance or harmful levels in normal circumstances. Provided
that acrolein concentrations are maintained below 0.05 mg/m3, most
of the population will be spared from any known annoyance or health
effects. However, in polluted urban areas and smoke-filled rooms,
acrolein is present in combination with other irritating aldehydes,
and control of acrolein alone is not sufficient to prevent annoyance
or harmful effects.
10.2 Evaluation of effects on the environment
Acrolein is released into the environment during production of
the compound itself and its derivatives, in processes involving
incomplete combustion and/or pyrolysis of organic substances, by
photochemical oxidation of specific air pollutants, and through
biocidal use, spills, and waste disposal (chapter 3).
Degradation in the atmosphere begins mainly by reaction with
hydroxyl radicals. The calculated atmospheric residence time is
approximately one day (section 4.2). Photolysis does not occur to a
significant degree (section 4.2.1). In natural water, acrolein
dissipates fairly rapidly as a result of catalysed hydration,
reactions with organic material, and volatilization (sections 4.2
and 4.3). Acrolein has a low soil adsorption potential
(section 4.1). Aerobic and anaerobic biodegradation of the compound
has been reported, although its toxicity to microorganisms may
prevent biodegradation (section 4.3.1). Based on its physical and
chemical properties, bioaccumulation would not be expected to occur
(section 4.3.2). It can be concluded that acrolein is unlikely to
persist in any environmental compartment.
Acrolein is very toxic to aquatic organisms. Acute EC50 or
LC50 values for various species range between 0.02 and
2.5 mg/litre. The 60-day NOAEL for fish (fathead minnow) is
0.0114 mg/litre (section 9.1).
In view of the high toxicity of acrolein to aquatic organisms,
the substance presents a risk to aquatic life at or near sites of
industrial discharges, spills, and biocidal use.
11. FURTHER RESEARCH
a) Human exposure characteristics should be further evaluated.
This applies to exposure due to environmental and
occupational air, as well as to intake from food and beverages.
b) These evaluations should include determinations of other
chemicals that occur with acrolein and that interact or have
biological effects similar to those due to acrolein exposure.
c) The most important target organ for airborne acrolein exposure
is the respiratory system. Therefore, further studies
including epidemiological studies should focus on this system
and particularly on the occupational environment. Possible
decreases in host resistance to respiratory infections should
be investigated.
d) The uptake of acrolein in the different parts of the
respiratory system should be examined further. The metabolism
and excretion of acrolein, as well as of its metabolites from
the respiratory system, should be given high priority as there
is an almost total lack of information about these processes.
e) The efficacy of sulfhydryl compounds, such as N-acetylcysteine
or 2-mercaptoethylsulfonic acid sodium salt (MESNA) as
antidotes for acrolein poisoning should be evaluated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Evidence for the potential carcinogenicity of acrolein has been
evaluated by the International Agency for Research on Cancer (IARC,
1979, 1985, 1987). The evidence for carcinogenicity was considered
to be inadequate both in animals and in humans. Thus no evaluation
could be made of the carcinogenicity of acrolein to humans.
Regulatory standards established by national bodies in various
countries and the EEC are summarized in the data profile of the
International Register of Potentially Toxic Chemicals (IRPTC, 1990)
and are tabulated in the Health and Safety Guide for Acrolein (WHO,
1991).
REFERENCES
ABERNETHY, D.J., FRAZELLE, J.H., & BOREIKO, C.J. (1983) Relative
cytotoxicity and transforming potential of respiratory irritants in
the C3H/10T1/2 cell transformation system. Environ. Mutagen., 5:
419 (abstract).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents, and miscellaneous
substances. Int. Pest Control, 11: 29-35.
ALARCON, R.A. (1968) Fluorometric determination of acrolein and
related compounds with m-aminophenol. Anal. Chem., 40: 1704-1708.
ALARCON, R.A. (1972) Acrolein, a component of a universal
cell-growth regulatory system: a theory. J theor. Biol., 37:
159-167.
ALARCON, R.A. (1976) Formation of acrolein from various amino-acids
and polyamines under degradation at 100 °C. Environ. Res., 12:
317-326.
ALTSHULLER, A.P. & BUFALINI, J.J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ALTSHULLER, A.P. & MCPHERSON, S.P. (1963) Spectrophotometric
analysis of aldehydes in the Los Angeles atmosphere. J. Air Pollut.
Control Assoc., 13: 109-111.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20: 649-656.
ANDERSSON, K., HALLGREN, C., LEVIN, J.-O., & NILSSON, C.-A. (1981)
Solid chemosorbent for sampling sub-ppm levels of acrolein and
glutaraldehyde in air. Chemosphere, 10: 275-280.
ARANYI, C., O'SHEA, W.J., GRAHAM, J.A., & MILLER, F.J. (1986) The
effects of inhalation of organic chemical air contaminants on murine
lung host defenses. Fundam. appl. Toxicol., 6: 713-720.
ARTHO, A. & KOCH, R. (1969) [The concentration of acrolein and
hydrogen cyanide in cigarette smoke]. Mitt. Lebensm. Hyg. (Bern),
60: 379-388 (in German).
ASTRY, C. & JAKAB, G.J. (1983) The effects of acrolein exposure on
pulmonary antibacterial defenses. Toxicol. appl. Pharmacol., 67:
49-54.
ATKINSON, R., ASCHMANN, S.M., WINER, A.M., & PITTS, J.N. (1981) Rate
constants for the gas-phase reactions of O3 with a series of
carbonyls at 296 K. Int. J. chem. Kinet., 13: 1133-1150.
ATKINSON, R., ASCHMANN, S.M., & PITTS, J.N. (1983) Kinetics of the
gas-phase reactions of OH radicals with a series of
alpha,ß-unsaturated carbonyls at 299 + 2 K. Int. J. chem. Kinet.,
15: 75-81.
ATKINSON, R., ASCHMANN, S.M., & GOODMAN, M.A. (1987) Kinetics of the
gas-phase reactions of NO3 radicals with a series of alkynes,
haloalkenes, and alpha,ß-unsaturated aldehydes. Int. J. chem.
Kinet., 19: 299-307.
AU, W., SOKOVA, O.I., KOPNIN, B., & ARRIGHI, F.E. (1980) Cytogenetic
toxicity of cyclophosphamide and its metabolites in vitro.
Cytogenet. cell Genet., 26: 108-116.
AYER, H.E. & YEAGER, D.W. (1982) Irritants in cigarette smoke
plumes. Am. J. public Health, 72: 1283-1285.
BABIUK, C., STEINHAGEN, W.H., & BARROW, C.S. (1985) Sensory
irritation response to inhaled aldehydes after formaldehyde
pretreatment. Toxicol. appl. Pharmacol., 79: 143-149.
BAKER, R.R., DYMOND, H.F., & SHILLABEER, P.K. (1984) Determination
of alpha,ß-unsaturated compounds formed by a burning cigarette.
Anal. Proc. (Lond.), 21: 135-137.
BALLANTYNE, B., DODD, D.E., PRITTS, I.M., NACHREIMER, D.J., &
FOWLER, E.H. (1989) Acute vapour inhalation toxicity of acrolein
and its influence as a trace contaminent in
2-methoxy-3,4-dihydro-2H-pyran. Hum. Toxicol., 8: 229-235.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J. (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Proceedings of the
1978 Symposium on dynamics, exposure and hazard assessment of toxic
chemicals, Ann Arbor, Michigan, Ann Arbor Science, pp. 379-392.
BARTLEY, T.R. & GANGSTAD, E.O. (1974) Environmental aspects of
aquatic plant control. J. Irrig. Drain. Div., 100: 231-244.
BASU, A.K. & MARNETT, L.J. (1984) Molecular requirements for the
mutagenicity of malondialdehyde and related acroleins. Cancer Res.,
44: 2848-2854.
BEAUCHAMP, R.O., ANDJELKOVICH, D.A., KLIGERMAN, A.D., MORGAN, K.T.,
& HECK, H. d'A. (1985) A critical review of the literature on
acrolein toxicity. CRC crit. Rev. Toxicol., 14: 309-380.
BEELEY, J.M., CROW, J., JONES, J.G., MINTY, B., LYNCH, R.D., &
PRYCE, D.P. (1986) Mortality and lung histopathology after
inhalation lung injury. The effect of corticosteroids. Am. Rev.
respir. Dis., 133: 191-196.
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9: 119-127.
BENEDICT, R.C. & STEDMAN, R.L. (1969) Composition studies on
tobacco. XXXVII. Inhibition of lactic, alcohol and
glucose-6-phosphate dehydrogenases by cigarette smoke and components
thereof. Tob. Sci., 13: 166-168.
BERRIGAN, M.J., GURTOO, H.L., SHARMA, S.D., STRUCK, R.F., &
MARINELLO, A.J. (1980) Protection by N-acetylcysteine of
cyclophosphamide metabolism - related in vivo depression of mixed
function oxygenase activity and in vitro denaturation of
cytochrome P-450. Biochem. biophys. Res. Commun., 93: 797-803.
BIGNOZZI, C.A., CHIORBOLI, C., MALDOTTI, A., & CARASSITI, V. (1980).
Atmospheric reactivity of 1,3-butadiene - nitrogen monoxide and
acrolein - nitrogen monoxide systems. Ann. Chim. (Rome), 70:
453-461.
BOETTNER, E.A. & BALL, G.L. (1980). Thermal degradation products
from PVC film in food-wrapping operations. Am. Ind. Hyg. Assoc. J.,
41: 513-522. BOHMANN, J.-J. (1985) [Aging behaviour of beer.]
Monatsschr. Brauwiss., 4: 175-180 (in German).
BOOR, P.J. & ANSARI, G.A.S. (1986) High-performance liquid
chromatographic method for quantitation of acrolein in biological
samples. J. Chromatogr., 375: 159-164.
BOULEY, G., DUBREUIL, A., GODIN, J., & BOUDENE, C. (1975) Effets,
chez le rat, d'une faible dose d'acroléine inhalée en continu. Eur.
J. Toxicol., 8: 291-297.
BOWMER, K.H. & HIGGINS, M.L. (1976) Some aspects of the persistence
and fate of acrolein herbicide in water. Arch.environ. Contam.
Toxicol., 5: 87-96.
BOWMER, K.H. & SAINTY, G.R. (1977) Management of aquatic plants with
acrolein. J. aquat. Plant Manage., 15: 40-46.
BOWMER, K.H. & SMITH, G.H. (1984) Herbicides for injection into
flowing water: acrolein and endothal-amine. Weed Res., 24: 201-211.
BOWMER, K.H., LANG, A.R.G., HIGGINS, M.L., PILLAY, A.R., & TCHAN,
Y.T. (1974) Loss of acrolein from water by volatilization and
degradation. Weed Res., 14: 325-328.
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed conjugations
of glutathione with unsaturated compounds. Biochem. J., 104:
95-102.
BRIDGES, R.B., KRAAL, J.H., HUANG, L.J.T., & CHANCELLOR, M.B. (1977)
Effects of cigarette smoke components on in vitro chemotaxis of
human polymorphonuclear leukocytes. Infect. Immun., 16: 240-248.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRINGMANN, G. (1978) [Determination of the harmful biological action
of water-endangering substances on protozoa; I. Bacteria fed
flagellates.] Z. Wasser-Abwasser Forsch., 11: 210-215 (in German).
BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the harmful
action of water-endangering substances on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z. Wasser-Abwasser Forsch., 10:
87-98 (in German).
BRINGMANN, G. & KUHN, R. (1980) [Determination of the harmful
biological action of water-endangering substances on protozoa; II.
Bacteria fed ciliates.] Z. Wasser-Abwasser Forsch., 13: 26-31 (in
German).
BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa; III Saprozoic flagellates.] Z. Wasser-Abwasser Forsch.,
13: 170-173 (in German).
BROWN, P.W. & FOWLER, C.A. (1967) The toxicity of tobacco smoke
solutions to Proteus vulgaris. Beitr. Tabakforsch., 4: 78-83.
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity
of priority pollutants to Bluegill (Lepomis macrochirus). Bull.
environ. Contam. Toxicol., 26: 446-452.
BUCKLEY, L.A., JIANG, X.Z., JAMES, R.A., MORGAN, K.T., & BARROW,
C.S. (1984) Respiratory tract lesions induced by sensory irritants
at the RD50 concentration. Toxicol. appl. Pharmacol., 74:
417-429.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative determination
of acrylonitrile, acrolein, acetonitrile and acetone in workplace
air. Am. Ind. Hyg. Assoc. J., 40: 904-909.
CAMPBELL, K.I., GEORGE, E.L., & WASHINGTON, I.S. (1981) Enhanced
susceptibility to infection in mice after exposure to dilute exhaust
from light duty diesel engines. Environ. Int., 5: 377-382.
CANTONI, C., BIANCHI, M.A., RENON, P., & CALCINARDI, C. (1969)
[Bacterial and chemical alterations during souring in salted pork.]
Atti. Soc. Ital. Sci. Vet., 23: 752-756 (in Italian).
CARPENTER, C.P., SMYTH, H.F., POZZANI, U.C. (1949) The assay of
acute vapor toxicity, and the grading and interpretation of results
on 96 compounds. J. ind. Hyg. Toxicol., 31: 343-346.
CATILINA, P., THIEBLOT, L., & CHAMPEIX, J. (1966) Lesions
respiratoires expérimentales par inhalation d'acroléine chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc. (Paris), 27: 857-867.
CHAMPEIX, J., COURTIAL, L., PERCHE, E., & CATILINA, P. (1966)
Broncho-pneumopathie aiguë par vapeurs d'acroléine. Arch. Mal.
prof. Méd. Trav. Sécur. Soc., 27: 794-796.
CHAVIAMO, A.H., GILL, W.B., RUGGIERO, U.J., & VERMEULEN, C.W. (1985)
Experimental cytoran cystitis and prevention by acetylcysteine. J.
Urol., 134: 598-600.
CHHIBBER, G. & GILANI, S.H. (1986) Acrolein and embryogenesis: an
experimental study. Environ. Res., 39: 44-49.
CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation and
degradation of petrochemical wastewater components by methane
fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
CHRAIBER, L.B., SOSNOVSKY, S.I., TATARKIN, Y.N., & VINNIKOVA, L.I.
(1964) [Air pollution with acrolein vapors in expeller and forepress
shops of butter and fat mills of Uzbekistan.] Gig. Tr. prof.
Zabol., 1(11): 49-50 (in Russian).
CHUNG, F.-L., YOUNG, R., & HECHT, S.S. (1984) Formation of cyclic
1,N2-propanodeoxyguanosine adducts in DNA upon reaction with
acrolein or crotonaldehyde. Cancer Res., 44: 990-995.
CLAUSSEN, U., HELLMANN, W., & PACHE, G. (1980) The embryotoxicity of
the cyclophosphamide metabolite acrolein in rabbits, tested in vivo
by i.v. injection and by the yolk-sac method. Drug Res., 30:
2080-2083.
COHEN, I.R. & ALTSHULLER, A.P. (1961) A new spectrophotometric
method for the determination of acrolein in combustion gases and in
the atmosphere. Anal. Chem., 33: 726-733.
COOMBER, J.W. & PITTS, J.N. (1969) Molecular structure and
photochemical reactivity. XII. The vapor-phase photochemistry of
acrolein at 3130 A. J. Am. Chem. Soc., 91: 547-550.
COOPER, K.O., WITMER, C.M., & WITZ, G. (1987) Inhibition of
microsomal cytochrome c reductase activity by a series of
alpha,ß-unsaturated aldehydes. Biochem. Pharmacol., 36: 627-631.
COSTA, D.L., KUTZMAN, R.S., LEHMANN, J.R., & DREW, R.T. (1986)
Altered lung function and structure in the rat after subchronic
exposure to acrolein. Am. Rev. respir. Dis., 133: 286-291.
COX, R., GOORHA, S., & IRVING, C.C. (1988) Inhibition of DNA
methylase activity by acrolein. Carcinogenesis, 9: 463-465.
CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & ABBOTT, J.K. (1986)
Inhalation toxicology: VII. Times to incapacitiation and death for
rats exposed continuously to atmospheric acrolein vapor, Washington,
D.C., Federal Aviation Administration, Office of Aviation Medicine
(DOT/FAA/AM-86/5)
CROOK, T.R., SOUHAMI, R.L., & MCLEAN, A.E.M. (1986a) Cytotoxicity,
DNA cross-linking, and single strand breaks induced by activated
cyclophosphamide and acrolein in human leukemia cells. Cancer Res.,
46: 5029-5034.
CROOK, T.R., SOUHAMI, R.L., WHYMAN, G.D., & MCLEAN, A.E.M. (1986b)
Glutathione depletion as a determinant of sensitivity of human
leukemia cells to cyclophosphamide. Cancer Res., 46: 5035-5038.
CURREN, R.D., YANG, L.L., CONKLIN, P.M., GRAFSTROM, R.C., & HARRIS,
C.C. (1988) Mutagenesis of xeroderma pigmentosum fibroblasts by
acrolein. Mutat. Res., 209: 17-22.
DAHLGREN, S.E., DALEN, H., & DALHAMM, T. (1972) Ultrastructural
observations on chemically induced inflammation in guinea pig
trachea. Virchows Arch. Abt. B Zellpathol., 11: 211-223.
DALHAMN, T. & ROSENGREN, A. (1971) Effect of different aldehydes on
tracheal mucosa. Arch. Otolarygnol., 83: 496-500.
DARLEY, E.F., MIDDLETON, J.T., & GARBER, M.J. (1960) Plant damage
and eye irritation from ozone-hydrocarbon reactions. J. agric. food
Chem., 8: 483-485.
DAVIS, T.R.A., BATTISTA, S.P., & KENSLER, C.J. (1967) Mechanism of
respiratory effects during exposure of guinea pigs to irritants.
Arch. environ. Health, 15: 412-419.
DAWSON, J.R., NORBECK, K., ANUNDI, I., & MOLDEUS, P. (1984) The
effectiveness of N-acetylcysteine in isolated hepatocytes, against
the toxicity of paracetamol, acrolein, and paraquat. Arch.
Toxicol., 55: 11-15.
DEBETHIZY, J.D., UDINSKY, J.R., SCRIBNER, H.E., & FREDERICK, C.B.
(1987) The disposition and metabolism of acrylic acid and ethyl
acrylate in male Sprague-Dawley rats. Fundam. appl. Pharmacol., 8:
549-561.
DESCROIX, H. (1972) Etude complémentaire des interactions entre
acroléine et cytidine-monophosphate. C. R. Acad. Sci. Paris, D274:
2362-2365.
DGEP (1988) Unpublished Review of literature data on acrolein,
Leidschendam, The Netherlands, Ministry of Housing, Physical
Planning and Environment, Directorate-General of Environmental
Protection.
DIAZ MAROT, A., GASSIOT MATAS, M., & FERRER MARTIN, M. (1983)
[Stripping and high performance liquid chromatography in the
analysis of carbonyl compounds in beers at ppb level.] Afinidad,
40: 21-24 (in Spanish).
DORE, M. & MONTALDO, C. (1984) [Study on the conjungation in vitro
of allyl alcohol and its metabolites with reduced glutathione.]
Boll. Soc. Ital. Biol. Sper., 60: 1497-1501 (in Italian).
DRAMINSKI, W., EDER, E., & HENSCHLER, D. (1983) A new pathway of
acrolein metabolism in rats. Arch. Toxicol., 52: 243-247.
EASLEY, D.M., KLEOPFER, R.D., & CARASEA, A.M. (1981) Gas
chromatographic-mass spectrometric determination of volatile organic
compounds in fish. J. Assoc. Off. Anal. Chem., 64: 653-656.
EDNEY, E.O., MITCHELL, S., & BUFALINI, J.J. (1982) Atmospheric
chemistry of several toxic compounds, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Atmospheric Chemistry
and Physics Division, Environmental Sciences Research Laboratory,
Office of Research and Development (EPA-600/3-82-092, PB 83-146340).
EDNEY, E.O., KLEINDIENST, T.E., & CORSE, E.W. (1986a) Room
temperature rate constants for the reaction of OH with selected
chlorinated and oxygenated hydrocarbons. Int. J. chem. Kinet., 18:
1355-1371.
EDNEY, E.O., SHEPSON, P.B., KLEINDIENST, T.E. & CORSE, E.W. (1986b)
The photooxidation of allyl chloride. Int. J. chem. Kinet., 18:
597-608.
EGLE, J.L. (1972) Retention of inhaled formaldehyde,
propionaldehyde, and acrolein in the dog. Arch. environ. Health,
25: 119-124.
EGLE, J.L. & HUDGINS, P.M. (1974) Dose-dependent sympathomimetic and
cardioinhibitory effects of acrolein and formaldehyde in the
anesthetized rat. Toxicol. appl. Pharmacol., 28: 358-366.
ELLENBERGER, J. & MOHN, G.R. (1976) Comparative mutagenicity testing
of cyclphosphamide and some of its metabolites. Mutat. Res., 37:
120 (abstract).
ELLENBERGER, J. & MOHN, G.R. (1977) Mutagenic activity of major
mammalian metabolites of cyclophosphamide toward several genes of
Escherichia coli. J. Toxicol. environ. Health, 3: 637-650.
EPSTEIN, S.S. & SHAFNER H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ERICKSON, L.C., RAMONAS, L.M., ZAHARKO, D.S., & KOHN, K.W. (1980)
Cytotoxicity and DNA cross-linking activity of
4-sulfidocyclophosphamides in mouse leukemia cells in vitro.
Cancer Res., 40: 4216-4220.
ESTERBAUER, H., ZOLLNER, H., & SCHOLZ, N. (1975) Reaction of
glutathione with conjugated carbonyls. Z. Naturforsch., 30:
466-473.
FACCHINI, M.C., CHIAVARI, G., & FUZZI, S. (1986) An improved HPLC
method for carbonyl compound speciation in the atmospheric liquid
phase. Chemosphere, 15: 667-674.
FERGUSON, F.F., RICHARDS, C.S., & PALMER, J.R. (1961) Control of
Australorbis glabratus by acrolein in Puerto Rico. Public Health
Rep., 76: 461-468.
FERGUSON, F.F., DAWOOD, I.K., & BLONDEAU, R. (1965) Preliminary
field trials of acrolein in the Sudan. Bull. World Health Organ.,
32: 243-248.
FERON, V.J. & KRUYSSE, A. (1977) Effects of exposure to acrolein
vapor in hamsters simultaneously treated with benzo [a]pyrene or
diethylnitrosamine. J. Toxicol. environ. Health, 3: 379-394.
FERON, V.J., KRUYSSE, A., TIL, H.P., & IMMEL, H.R. (1978) Repeated
exposure to acrolein vapour: subacute studies in hamsters, rats and
rabbits. Toxicology, 9: 47-57.
FISCHER, T., WEBER, A., & GRANDJEAN, E. (1978) [Air pollution due to
tobacco smoke in restaurants.] Int. Arch. occup. environ. Health,
41: 267-280 (in German).
FLEER, R. & BRENDEL, M. (1982) Toxicity, interstrand cross-links and
DNA fragmentation induced by `activated' cyclophosphamide in yeast:
comparative studies on 4-hydroperoxy-cyclophosphamide, its
monofunctional analogue, acrolein, phosphoramide mustard, and
non-nitrogen mustard. Chem.-biol. Interact., 39: 1-15.
FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity using the
Ames' test. Toxicology, 18; 219-232.
FOILES, P.G., AKERKAR, S.A., & CHUNG, F.-L. (1989) Application of an
immunoassay for cyclic acrolein deoxyguanosine adducts to assess
their formation in DNA of Salmonella typhimurium under conditions
of mutation induction by acrolein. Carcinogenesis, 10: 87-90.
FRACCHIA, M.F., SCHUETTE, F.J., & MUELLER, P.K. (1967) A method for
sampling and determination of organic carbonyl compounds in
automobile exhaust. Environ. Sci. Technol., 1: 915-922.
FRITZ-SHERIDAN, R.P. (1982) Impact of the herbicide Magnacide-H
(2-propenal) on algae. Bull. environ. Contam. Toxicol., 28:
245-249.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO,
J., MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. mol.
Mutagen., 10(supplement): 1-175.
GAN, J.C. & ANSARI, G.A.S. (1987) Plausible mechanism of
inactivation of plasma alpha1-proteinase inhibitor by acrolein.
Res. Commun. chem. Pathol. Pharmacol., 55: 419-422.
GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated carbon
adsorption of petrochemicals. J. Water Pollut. Control Fed., 46:
947-965.
GOSSELIN, B., WATTEL, F., CHOPIN, C., DEGAND, P., FRUCHART, J.C.,
VAN DER LOO, D., & CRASQUIN, O. (1979) Intoxication aiguë par
l'acroléine, une observation. Nouv. Presse méd., 8: 2469-2472.
GRAEDEL, T.E., FARROW, L.A., & WEBER, T.A. (1976) Kinetic studies of
the photochemistry of the urban atmosphere. Atmos. Environ., 10:
1095-1116.
GRAFSTROM, R.C., CURREN, R.D., YANG, L.L., & HARRIS, C.C. (1986)
Aldehyde-induced inhibition of DNA repair and potentiation of
N-nitrosocompound-induced mutagenesis in cultured human cells.
Prog. clin. biol. Res., 209A: 255-264.
GRAFSTROM, R.C., DYPBUKT, J.M., WILLEY, J.C., SUNDQVIST, K., EDMAN,
C., ATZORI, L., & HARRIS, C.C. (1988) Pathobiological effects of
acrolein in cultured human bronchial epithelial cells. Cancer Res.,
48: 1717-1721.
GREENHOFF, K. & WHEELER, R.E. (1981) Analysis of beer carbonyls at
the part per billion level by combined liquid chromatography and
high pressure liquid chromatography. J. Inst. Brew., 86: 35-41.
GREY, T.C. & SHRIMPTON, D.H. (1966) Volatile components of raw
chicken breast muscle. Br. poult. Sci., 8: 23-33.
GROSJEAN, D. & WRIGHT ,B. (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos. Environ., 17: 2093-2096.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
chromatogr. Sci., 12: 385-391.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of The Netherlands. Sci. total
Environ., 43: 193-219.
GUILLERM, R., BADRE, R., & HEE, J. (1967a) Détermination du seuil
d'action des polluants irritants de l'atmosphère - Comparaison de
deux méthodes. Ann. occup. Hyg., 10: 127-133.
GUILLERM, R., SAINDELLE, A., FALTOT, P., & HEE, J. (1967b) Action de
la fumée de cigarette et de quelques-uns de ses constituants sur les
resistances ventilatoires chez le cobaye. Arch. int. Pharmacodyn.
Ther., 167: 101-114.
GURTOO, H.L., HIPKENS, J.H., & SHARMA, S.D. (1981a) Role of
glutathione in the metabolism-dependent toxicity and chemotherapy of
cyclophosphamide. Cancer Res., 41: 3584-3591.
GURTOO, H.L., MARINELLO, A.J., STRUCK, R.F., PAUL, B., & DAHMS, R.P.
(1981b) Studies on the mechanism of denaturation of cytochrome P-450
by cyclophosphamide and its metabolites. J. biol. Chem., 256:
11691-11701.
GUSEV, M.I., SVECHNIKOVA, A.I., DRONOV, I.S., GREBENSKOVA, M.D., &
GOLOVINA, A.I. (1966) [Substantiation of the daily average maximum
permissible concentration of acrolein in the atmosphere.] Gig. i
Sanit., 31: 9-13 (in Russian).
HAENEN, G.R.M.M., VERMEULEN, N.P.E., TAI TIN TSOI, J.N.L., RAGETLI,
H.M.N., TIMMERMAN, H., & BAST, A. (1988) Activation of the
microsomal glutathione- S-transferase dependent protection against
lipid peroxidation by acrolein. Biochem. Pharmacol., 37: 1933-1938.
HALES, B.F. (1982) Comparison of the mutagenicity and teratogenicity
of cyclophosphamide and its active metabolites,
4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein.
Cancer Res., 42: 3016-3021.
HALES, C.A., BARKIN, P.W., JUNG, W., TRAUTMAN, E., LAMBORGHINI, D.,
HERRIG, N., & BURKE, J. (1988) Synthetic smoke with acrolein but not
HCL produces pulmonary edema. J. appl. Physiol., 64: 1121-1133.
HALL, R.H., & STERN, E.S. (1950) Acid-catalysed hydration of
acraldehyde. Kinetics of the reaction and isolation of
ß-hydroxypropaldehyde. J. Chem. Soc., 1950: 490-498.
HARADA, M. (1977) [Biochemical analysis of tear fluid in
photochemical smog.] Nippon Ganka Gakkai Zasshi, 81: 275-286 (in
Japanese).
HARKE, H.-P., BAARS, A., FRAHM, B., PETERS, H., & SCHULTZ, C. (1972)
[On the problem of passive smoking. Relation between the
concentration of smoke constituents in the air of several large
rooms and the number of smoked cigarettes and time.] Int. Arch.
Arbeitsmed., 29: 323-339 (in German).
HAWLEY, G.G. (1981) The condensed chemical dictionary, 10th ed.,
New York, Van Nostrand Reinhold Co.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HAYASE, F., CHUNG, T.-Y., & KATO, H. (1984) Changes of volatile
components of tomato fruits during ripening. Food Chem., 14:
113-124.
HEMMINKI, K., FALCK, K., & VAINIO, H. (1980) Comparison of
alkylation rates and mutagenicity of directly acting industrial and
laboratory chemicals. Arch. Toxicol., 46: 277-285.
HESS, L.G., KURTZ, A.N., & STANTON, D.B. (1978) Acrolein and
derivatives. In: Kirk, R.E. & Othmer, D.F. ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol 1:
pp. 277-297.
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity classes.
Ind. Hyg. Q., 10: 93-96.
HOFFMAN, C., BASTIAN, H., WIEDENMANN, M., DEININGER, C., & EDER, E.
(1989) Detection of acrolein congener-DNA adducts isolated from
cellular systems. Arch. Toxicol., Suppl. 13: 219-223.
HOFFMANN, D., BRUNNEMANN, K.D., GORI, G.B., & WYNDER, E.L. (1975) On
the carcinogenicity of marijuana smoke. Recent Adv. Phytochem., 9:
63-81.
HOLMBERG, B. & MALMFORS, T. (1974) The cytotoxicity of some organic
solvents. Environ. Res., 7: 183-192.
HOSHIKA, Y. & TAKATA, Y. (1976) Gas chromatographic separation of
carbonyl compounds as their 2,4-dinitrophenylhydrazones using glass
capillary columns. J. Chromatogr., 120: 379-389.
HOVDING, G. (1969) Occupational dermatitis from pyrolysis products
of polythene. Acta dermatovenereol, 49: 147-149.
HRDLICKA, J. & KUCA, J. (1965) The changes of carbonyl compounds in
the heat-processing of meat. Poult. Sci., 44: 7-31.
HUGOD, C., HAWKINS, L.H., & ASTRUP, P. (1978) Exposure of passive
smokers to tobacco smoke constituents. Int. Arch. occup. environ.
Health, 42: 21-29.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein, Lyons, International Agency for Research on Cancer, pp.
479-495 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Man, Vol. 19).
IARC (1985) Allyl compounds, aldehydes, epoxides and peroxides,
Lyons, International Agency for Research on Cancer, pp. 133-161
(IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1985) Treatment and disposal methods for waste chemicals,
Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
IRPTC (1990) Data profile on acrolein, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ, I.D., &
BRADSHAW, J.J. (1978) Organic compounds - Their interaction with and
degradation of hepatic microsomal drug-metabolizing enzymes in
vitro. Drug Metab. Disp., 6: 218-225.
IZARD, C. (1973) Effets de l'acroléine sur la division cellulaire,
le cycle et la synthèse de l'ADN, chez Vicia faba. C. R. Acad.
Sci. Paris Ser. D, 276: 1745-1747.
IZARD, C. & LIBERMANN, C. (1978) Acrolein. Mutat. Res., 47:
115-138.
IZMEROV, N.F., ed. (1984) Acrolein. Moscow, Centre of International
Projects, GKNT, 15 pp (IRPTC Scientific Reviews of Soviet Literature
on Toxicity and Hazards of Chemicals 50).
JACOBS, W.A. & KISSINGER, P.T. (1982) Determination of carbonyl 2,4-
dinitrophenylhydrazones by liquid chromatography/electrochemistry.
J. liq. Chromatogr., 5: 669-676.
JAKAB, G.J. (1977) Adverse effect of a cigarette smoke component,
acrolein, on pulmonary defenses and on viral-bacterial interactions
in the lung. Am. Rev. respir. Dis., 115: 33-38.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of several gas-phase components of side-stream smoke
of cigarettes in indoor air as contribution towards the problem of
passive smoking.] Int. Arch. occup. environ. Health, 36: 169-181
(in German).
JONSSON, A. & BERG, S. (1983) Determination of low-molecular-weight
oxygenated hydrocarbons in ambient air by cryogradient sampling and
two-dimensional gas chromatography. J. Chromatogr., 279: 307-322.
JOUSSERANDOT, P., GREMAIN, J., & DU BOISTESSELIN, R. (1981)
L'utilisation de méthodes histologiques pour la démonstration de
l'activite anti-inflammatoire de la fusafungine. Sem. Hop. Paris,
57: 143-150.
JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of 200
chemical compounds for acute toxicity towards fish by means of the
goldfish test.] Z. Wasser-Abwasser Forsch., 11: 161-164 (in
German).
KANE, L.E. & ALARIE, Y. (1977) Sensory irritation to formaldehyde
and acrolein during single and repeated exposures in mice. Am. Ind.
Hyg. Assoc. J., 38: 509-521.
KANE, L.E. & ALARIE, Y. (1978) Evaluation of sensory irritation from
acrolein-formaldehyde mixtures. Am Ind. Hyg. Assoc. J., 39:
270-274.
KANE, L.E. & ALARIE, Y. (1979) Interactions of sulfur dioxide and
acrolein as sensory irritants. Toxicol. appl. Pharmacol., 48:
305-315.
KANKAANPAA, J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acrylamide in
developing chick embryos. Toxicol. Lett., 4: 93-96.
KANTEMIROVA, A.E. (1975) [The disease incidence rate, including
temporal disability of workers engaged in the production of acrolein
and methylmercaptopropione (MMP) aldehyde.] Trans. Volgograd med.
Inst., 26(4): 79-85 (in Russian).
KANTEMIROVA, A.E. (1977) [The disease incidence rate, involving
temporal disability of workers engaged in the production of acrolein
and MMP aldehyde.] Trans. Volgograd med. Inst., 27(5): 32-33 (in
Russian).
KATZ, M. (1977) Methods of air sampling and analysis, 2nd ed.,
Washington, DC, American Public Health Association.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134: 1093-1101.
KERR, J.A. & SHEPPARD (1981) Kinetics of the reactions of hydroxyl
radicals with aldehydes studied under atmospheric conditions.
Environ. Sci. Technol., 15: 960-963.
KHUDOLEY, V.V., MIZGIREV, I.V., & PLISS, G.B. (1986) [Evaluation of
mutagenic activity of carcinogens and other chemical agents with
Salmonella typhimurium assays.] Vopr. Onkol., 32: 73-80 (in
Russian).
KILBURN, K.H. & MCKENZIE, W.N. (1978) Leukocyte recruitment to
airways by aldehyde-carbon combinations that mimic cigarette smoke.
Lab. Invest., 38: 134-142.
KIMES, B.W. & MORRIS, D.R. (1971) Inhibition of nucleic acid and
protein synthesis in Escherichia coli by oxidized polyamines and
acrolein. Biochim. Biophys. Acta, 228: 235-244.
KISSEL, C.L., BRADY, J.L., GUERRA, A.M., PAU, J.K., ROCKIE, B.A., &
CASERIO, F.F. (1978) Analysis of acrolein in aged aqueous media.
Comparison of various analytical methods with bioassays. J. agric.
food Chem., 26: 1338-1343.
KLOCHKOVSKII, S.P., LUKASHENKO, R.D., PODVYSOTSKII, K.S., &
KAGRAMANYAN, N.P. (1981) [Acrolein and formaldehyde content in the
air of quarries.] Bezoppasnost. Tr. Prom-St. 1981(12):38 (in
Russian).
KOERKER, R.L., BERLIN, A.J., & SCHNEIDER, F.H. (1976) The
cytotoxicity of short-chain alcohols and aldehydes in cultured
neuroblastoma cells. Toxicol. appl. Pharmacol., 37: 281-288.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1983) Embryotoxic effects
of acrolein, methylacrylates, guanidines and resorcinol on three day
chicken embryos. Acta pharmacol. toxicol., 52: 95-99.
KRILL, R.M. & SONZOGNI, W.C. (1986) Chemical monitoring of
Wisconsin's groundwater. J. Am. Water Works Assoc., 78: 70-75
KROKAN, H., GRAFSTROM, R.C., SUNDQVIST, K., ESTERBAUER, H., &
HARRIS, C.C. (1985) Cytotoxicity, thiol depletion and inhibition of
O6-methylguanine-DNA methyltransferase by various aldehydes in
cultured human bronchial fibroblasts. Carcinogenesis, 6: 1755-1759.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A. (1982)
Collection and analysis of hazardous organic emissions. Anal.
Chem., 54: 810-817.
KRUYSSE, A. (1971) Acute inhalation toxicity of acrolein in
hamsters, Zeist, The Netherlands, Central Institute for Nutrition
and Food Research, TNO (Report No. R3516).
KU, R.H. & BILLINGS, R.E. (1986) The role of mitochondrial
glutathione and cellular protein sulfhydryls in formaldehyde
toxicity in glutathione-depleted rat hepatocytes. Arch. Biochem.
Biophys., 247: 183-189.
KUBINSKI, H., GUTZKE, G.E., & KUBINSKI, Z.O. (1981) DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
KUTZMAN, R.S., MEYER, G.-J., & WOLF, A.P. (1982) The biodistribution
and metabolic fate of [11C]-acrylic acid in the rat after acute
inhalation exposure or stomach intubation. J. Toxicol. environ.
Health, 10: 969-979.
KUTZMAN, R.S., WEHNER, R.W., & HABER, S.B. (1984) Selected responses
of hypertension sensitive and resistant rats to inhaled acrolein.
Toxicology, 31: 53-65.
KUTZMAN, R.S., POPENOE, E.A., SCHMAELER, M., & DREW, R.T. (1985)
Changes in rat lung structure and composition as a result of
subchronic exposure to acrolein. Toxicology, 34: 139-151.
KUWATA, K., UEBORI, M., YAMASAKI, H., KUGE, Y., & KISO, Y. (1983)
Determination of aliphatic aldehydes in air by liquid
chromatography. Anal. Chem., 55: 2013-2016.
LACROIX, M., BURCKEL, H., FOUSSEREAU, J., GROSSHANS, E., CAVELIER,
C., LIMASSET, J.C., DUCOS, P., GRADINSKI, D., & DUPRAT, P. (1976)
Irritant dermatitis from diallylglycol carbonate monomer in the
optical industry. Contact dermatitis, 2: 183-195.
LAM, C.-W., CASANOVA, M., & HECK, H.D'A. (1985) Depletion of nasal
mucosal glutathione by acrolein and enhancement of
formaldehyde-induced DNA-protein cross-linking by simultaneous
exposure to acrolein. Arch. Toxicol., 58: 67-71.
LEACH, P.W., LENG, L.J., BELLAR, T.A., SIGSBY, J.E., & ALTSHULLER,
A.P. (1964) Effects of HC/NOx ratios on irradiated auto exhaust,
part II. J. Air Pollut. Control Assoc., 14: 176-183.
LEACH, C.L., HATOUM, N.S., RATAJCZAK, H.V., & GERHART, J.M. (1987)
The pathologic and immunologic effects of inhaled acrolein in rats.
Toxicol. Lett., 39: 189-198.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24:
684-691.
LE BOUFFANT, L., MARTIN, J.C., DANIEL, H., HENIN, J.P., & NORMAND,
C. (1980) Action of intensive cigarette smoke inhalations on the rat
lung. Role of particulate and gaseous cofactors. J. Natl Cancer
Inst., 64: 273-281.
LEFFINGWELL, C.M. & LOW, R.B. (1979) Cigarette smoke components and
alveolar macrophage protein synthesis. Arch. environ. Health, 34:
97-102.
LENCREROT, P., PARFAIT, A., & JOURET, C. (1984) Rôle des
corynebactéries dans la production d'acroléine (2-propenal) dans les
rhums. Ind. aliment. agric., 101: 763-765.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determinations of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEUCHTENBERGER, C., SCHUMACHER, M., & HADIMANN, T. (1968) Further
cytological and cytochemical studies on the biological significance
of the gas phase of fresh cigarette smoke. Z. Präventivmed., 13:
130-141.
LEVAGGI, D.A. & FELDSTEIN, M. (1970) The determination of
formaldehyde, acrolein, and low molecular weight aldehydes in
industrial emissions on a single collection sample. J. Air Pollut.
Control Assoc., 20: 312-313.
LIES, K.-H., POSTULKA, A., GRING, H., & HARTUNG, A. (1986) Aldehyde
emissions from passenger cars. Staub-Reinhalt. Luft, 46: 136-139.
LIJINSKY, W. & ANDREWS, A.W. (1980) Mutagenicity of vinyl compounds
in Salmonella typhimurium. Teratogen. Carcinogen. Mutagen., 1:
259-267.
LIJINSKI, W. & REUBER, M.D. (1987) Chronic carcinogenesis studies of
acrolein and related compounds. Toxicol. ind. Health, 3: 337-345.
LIPARI, F. & SWARIN, S.J. (1982) Determination of formaldehyde and
other aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. J. Chromatogr., 247: 297-306.
LIPARI, F., DASCH, J.M., & SCRUGGS, W.F. (1984) Aldehyde emissions
from wood-burning fireplaces. Environ. Sci. Technol., 18: 326-330.
LIU, Y. & TAI, H.-H. (1985) Inactivation of pulmonary NAD-dependent
15-hydroxyprostaglandin dehydrogenase by acrolein. Biochem.
Pharmacol., 34: 4275-4278.
LOQUET, C., TOUSSAINT, G., & LETALAER, J.Y. (1981) Studies on
mutagenic constituents of apple brandy and various alcoholic
beverages collected in Western France, a high incidence area for
oesophageal cancer. Mutat. Res., 88: 155-164.
LORZ, H.W., GLENN, S., WILLIAMS, R.H., KUNKEL, C.M., NORRIS, L.A., &
LOPER, B.R. (1979) Effects of selected herbicides on Smolting
Salmon, Corvallis, Oregon, USA Environmental Protection Agency,
Environment Research Laboratory Corvallis, Office of Research and
Development (Report EPA-600/3-79-071).
LOW, E.S., LOW, R.B., & GREEN, G.M. (1977) Correlated effects of
cigarette smoke components on alveolar macrophage adenosine
triphosphatase activity and phagocytosis. Am. Rev. respir. Dis.,
115: 963-970.
LUTZ, D., EDER, E., NEUDECKER, T., & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha,ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93: 305-315.
LYMAN, W.J., REEHL, W.F., & ROSENBLATT, D.H. (1982) Handbook of
chemical property estimation methods, New York, McGraw-Hill Book
Company.
LYON, J.P., JENKINS, L.J., JONES, R.A., COON, R.A., & SIEGEL, J.
(1970) Repeated and continuous exposure of laboratory animals to
acrolein. Toxicol. appl. Pharmacol., 17: 726-732.
MACEK, K.J., LINDBERG, M.A., SAUTER, S., BUXTON, K.S., & COSTA, P.A.
(1976) Toxicity of four pesticides to water fleas and fathead
minnows, Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory, Office of Research and
Development, (Ecological Research Series EPA-600/3-76-099).
MCNULTY, M.J., HECK, H.D'A., & CASANOVA-SCHMITZ, M. (1984) Depletion
of glutathione in rat respiratory mucosa by inhaled acrolein. Fed.
Proc., 43: 575 (abstract).
MAGIN, D.F. (1980) Gas chromatography of simple monocarbonyls in
cigarette whole smoke as the benzyloxime derivatives. J.
Chromatogr., 202: 255-261.
MALDOTTI, A., CHIORBOLI, C., BIGNOZZI, C.A., BARTOCCI, C., &
CARASSITI, V. (1980) Photooxidation of 1,3-butadiene containing
systems: rate constant determination for the reaction of acrolein
with OH radicals. Int. J. chem. Kinet., 12: 905-913.
MANITA, M.D. & GOLDBERG, E.K. (1970) [Spectrophotometric analysis of
airborne acrolein using the semithiocarbazide reagent.] Gig. i
Sanit., 35(5): 63-65 (in Russian).
MANNING, D.L., MASKARINEC, M.P., JENKINS, R.A., & MARSHALL, A.H.
(1983) High performance liquid chromatographic determination of
selected gas phase carbonyl in tobacco smoke. J. Assoc. Off. Anal.
Chem., 66: 8-12.
MARANO, F. & DEMESTERE, M. (1976) Ultrastructural autoradiographic
study of the intracellular fixation of 3H-acrolein. Experientia
(Basel), 32: 501-503.
MARANO, F. & PUISEUX-DAO, S. (1982) Acrolein and cell cycle.
Toxicol. Lett., 14: 143-149.
MARINELLO, A.J., GURTOO, H.L., STRUCK, R.F., & PAUL, B. (1978)
Denaturation of cytochrome P-450 by cyclophosphamide metabolites.
Biochem. Biophys. Res. Commun., 83: 1347-1353.
MARINELLO, A.J., BERRIGAN, M.J., STRUCK, R.F., GUENGERICH, F.P., &
GURTOO, H.L. (1981) Inhibition of NADPH-cytochrome P450 reductase by
cyclophosphamide and its metabolites. Biochem. biophys. Res.
Commun., 99: 399-406.
MARINELLO, A.J., BANSAL, S.K., PAUL, B., KOSER, P.L., LOVE, J.,
STRUCK, R.F., & GURTOO, H.L. (1984) Metabolism and binding of
cyclophosphamide and its metabolite acrolein to rat hepatic
microsomal cytochrome P-450. Cancer Res., 44: 4615-4621.
MARNETT, L.J., HURD, H.K., HOLLSTEIN, M.C., LEVIN, D.E., ESTERBAUER,
H., & AMES, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA 104. Mutat. Res., 148:
25-34.
MASARU, N., SYOZO, F., & SABURO, K. (1976) Effects of exposure to
various injurious gases on germination of lily pollen. Environ.
Pollut., 11: 181-187.
MASEK, V. (1972) [Aldehydes in the air at working places in coal and
pitch coking plants.] Staub-Reinhalt. Luft, 32: 335-336 (in
German).
MASLOWSKA, J. & BAZYLAK, G. (1985) Thermofractographic determination
of monocarbonyl compounds in animal waste fats used as feed fat.
Anim. Feed Sci. Technol., 13: 227-236.
METTIER, S.R., BOYER, H.K., HINE, C.H., & MCEWEN, W.K. (1960) A
study of the effects of air pollutants on the eye. Am. Med. Assoc.
Arch. ind. Health, 21: 13-18.
MILLS, D.E., BAUGH, W.D., & CONNER, H.A. (1954) Studies on the
formation of acrolein in distillery mashes. Appl. Microbiol., 2:
9-13.
MIRKES, P.E., GREENAWAY, J.C., ROGERS, J.G., & BRUNDRETT, R.B.
(1984) Role of acrolein in cyclophosphamide teratogenicity in rat
embryos in vitro. Toxicol. appl. Pharmacol., 72: 281-291.
MIYAMOTO, Y. (1986) Eye and respiratory irritants in jet engine
exhaust. Aviat. Space environ. Med., 57: 1104-1108.
MORIKAWA, T. (1976) Acrolein, formaldehyde, and volatile fatty acids
from smoldering combustion. J. Combust. Toxicol., 3: 135-150.
MORIKAWA, T. & YANAI, E. (1986) Toxic gases evolution from
air-controlled fires in a semi-full scale room. J. Fire Sci., 4:
299-314.
MOULE, Y. & FRAYSSINET, C. (1971) Effects of acrolein on
transcription in vitro. Fed. Eur. Biochem. Soc. Lett., 16:
216-218.
MULDERS, E.J. & DHONT, J.H. (1972) The odour of white bread. III.
Identification of volatile carbony compounds and fatty acids. Z.
Lebensm. Unters. Forsch., 150: 228-232.
MUNSCH, N. & FRAYSSINET, C. (1971) Action de l'acroléine sur les
syntheses d'acides nucléiques in vivo. Biochimie, 53: 243-248.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1973) Effects of
acrolein on DNA synthesis in vitro. Fed. Eur. Biochem. Soc.
Lett., 30: 286-289.
MUNSCH, N., MARANO, F., & FRAYSSINET, C. (1974a) Incorporation
d'acroleine 3H dans le foie du rat et chez Dunaliella bioculata.
Biochimie, 56: 1433-1436.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1974b) In vitro
binding of 3H-acrolein to regenerating rat liver DNA polymerase.
Experientia, 30: 1234-1236.
MURPHY, S.D. (1965) Mechanism of the effect of acrolein on rat liver
enzymes. Toxicol. appl. Pharmacol., 7: 833-843.
MURPHY, S.D. & PORTER, S. (1966) Effects of toxic chemicals on some
adaptive liver enzymes, liver glycogen, and blood glucose in fasted
rats. Biochem. Pharmacol., 15: 1665-1676.
MURPHY, S.D., KLINGSHIRN, D.A., & ULRICH, C.E. (1963) Respiratory
response of guinea pigs during acrolein inhalation and its
modification by drugs. J. Pharmacol. exp. Ther., 141: 79-83.
MURPHY, S.D., DAVIS, H.V., & ZARATZIAN, V.L. (1964) Biochemical
effects in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
NATUSCH, D.F.S. (1978) Potentially carcinogenic species emitted to
the atmosphere by fossil-fueled power plants. Environ. Health
Perspect., 22: 79-90.
NIELSEN, G.D., BAKBO, J.C., & HOLST, E. (1984) Sensory irritation
and pulmonary irritation by airborne allyl acetate, allyl alcohol,
and allyl ether compared to acrolein. Acta pharmacol. toxicol., 54:
292-298.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1986) Determination of
micro amounts of acrolein in air by gas chromatography. J.
Chromatogr., 370: 327-332.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987a) Gas chromatographic
determination of acrolein in rain water using bromination of
O-methyloxime. Analyst, 112: 45-48.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987b) Determination of
acrolein and crotonaldehyde in automobile exhaust gas by gas
chromatography with electron-capture detection. Analyst, 112:
859-862.
OBERDORFER, P.E. (1971) The determination of aldehydes in automobile
exhaust gas. In: Vehicle emissions, Part III, Society of Automotive
Engineers Prog. Tech., Vol. 14, pp. 32-42.
OHARA, T., SATO, T., SHIMUZU, N., PRESCHER, G., SCHWIND, H., &
WEIBURG, O. (1987) Acrolein and methacrolein. In: Encyclopedia of
chemical technology, Deerfield Beech, Florida, Ullmann Verlag
Chemie.
O'LOUGHLIN, E.M. & BOWMER, K.H. (1975) Dilution and decay of aquatic
herbicides in flowing channels. J. Hydrol., 26: 217-235.
OLSON, K.L. & SWARIN, S.J. (1985) Determination of aldehydes and
ketones by derivatization and liquid chromatography-mass
spectrometry. J. Chromatogr., 333: 337-347.
OSBORNE, A.D., PITTS, J.N., & DARLEY, E.F. (1962) On the stability
of acrolein towards photooxidation in the near ultra-violet. Int.
J. Air Water Pollut., 6: 1-3.
PATEL, J.M., WOOD, J.C., & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparations. Drug Metab. Disposal, 8: 305-308.
PATEL, J.M., ORTIZ, E., KOLMSTETTER, C., & LEIBMAN, K.C. (1984)
Selective inactivation of rat lung and liver microsomal
NADPH-cytochrome c reductase by acrolein. Drug Metab. Disposal, 12:
460-463.
PEREZ, J.M., LIPARI, F., & SEIZINGER, D.E. (1984) Cooperative
development of analytical methods for diesel emissions and
particulates - solvent extractables, aldehydes and sulfate methods.
In: Diesel exhaust emissions, Society of Automotive Engineers, pp.
95-114 (Special Publication No. 578).
PETTERSSON, B., CURVALL, M., & ENZELL, C.R. (1980) Effects of
tobacco smoke compounds on the noradrenaline induced oxidative
metabolism in isolated brown fat cells. Toxicology, 18: 1-15.
PFAFFLI, P. (1982) III. Industrial hygiene measurements. Scand. J
Work Environ. Health, 8(suppl. 2): 27-43.
PHILIPPIN, C., GILGEN, A., & GRANDJEAN, E. (1970) Etude
toxicologique et physiologique de l'acroléine chez la souris. Int.
Arch. Arbeitsmed., 26: 281-305.
PILOTTI, A., ANCKER, K., ARRHENIUS, E., & ENZELL, C. (1975) Effects
of tobacco and tobacco smoke constituents on cell multiplication in
vitro. Toxicology, 5: 49-62.
PLOTNIKOVA, M.M. (1957) [Data on hygienic evaluation of acrolein as
a pollution of the atmosphere.] Gig. i Sanit., 22(6): 10-15 (in
Russian).
POSTEL, W. & ADAM, L. (1983) [Gas chromatographic characterization
of raspberry brandy.] Dtsch. Lebensm. Rundschau, 79: 117-122 (in
German).
POTTS, W.J., LEDERER, T.S., & QUAST, J.F. (1978) A study of the
inhalation toxicity of smoke produced upon pyrolysis and combustion
of polyethylene foams. Part I. Laboratory studies. J. Combust.
Toxicol., 5: 408-433.
PRESSMAN, D. & LUCAS, H.J. (1942) Hydration of unsaturated
compounds. XI. Acrolein and acrylic acid. J. Am. Chem. Soc., 64:
1953-1957.
PROTSENKO, G.A., TRUBILKO, V.I., & SAVCHENKOV, V.A. (1973) Working
conditions when metals to which primer has been applied are welded
evaluated from the health and hygiene aspect. Avt. Svarka, 2:
65-68.
RANDALL, T.L. & KNOPP, P.V. (1980) Detoxification of specific
organic substances by wet oxidation. J. Water Pollut. Control Fed.,
52: 2117-2130.
RAPOPORT, I.A. (1948) [Mutations under the influence of unsaturated
aldehydes.] Dokl. Akad. Nauk. SSSR, 16: 713-715 (in Russian).
RATHKAMP, G., TSO, T.C., & HOFFMANN, D. (1973) Chemical studies on
tobacco smoke. XX: Smoke analysis of cigarettes made from bright
tobaccos differing in variety and stalk positions. Beitr.
Tabakforsch., 7: 179-189.
RENZETTI, N.A. & BRYAN, R.J. (1961) Atmospheric sampling for
aldehydes and eye irritation in Los Angeles smog. J. Air Pollut.
Control Assoc., 11: 421-424, 427.
RICHTER, M. & ERFURTH, I. (1979) [On the gas chromatographic
determination of acrolein in its original state in the main stream
smoke of cigarettes.] Ber. Inst. Tabaksforsch. 26: 36-45 (in
German).
RICKERT, W.S., ROBINSON, J.C., & YOUNG, J.C. (1980) Estmating the
hazards of "less hazardous" cigarettes. I. Tar, nicotine, carbon
monoxide, acrolein, hydrogen cyanide, and total aldehyde deliveries
of Canadian cigarettes. J. Toxicol. environ. Health, 6: 351-365.
RIETZ, B. (1985) Determination of three aldehydes in the air of
working environments. Anal. Lett., 18(A19): 2369-2379.
RIJSTENBIL, J.W. & VAN GALEN, G.C. (1981) Chemical control of mussel
settlement in a cooling water system using acrolein. Environ,
Pollut., A25: 187-195.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver aldehyde
dehydrogenases. Relation to allyl alcohol hepatoxicity. Metab.
Disposal, 15: 356-362.
ROSENTHALER, L. & VEGEZZI, G. (1955) [Acrolein in spirits.] Z.
Lebensm. Unters. Forsch., 102: 117-123 (in German).
RYLANDER, R. (1973) Toxicity of cigarette smoke components: free
lung cell response in acute exposures. Am. Rev. respir. Dis., 108:
1279-1282.
SAITO, T., TAKASHINA, T., YANAGISAWA, S., & SHIRAI, T. (1983)
[Determination of trace low molecular weight aliphatic compounds in
auto exhaust by gas chromatography with a glass capillary column.]
Bunseki Kagaku, 32: 33-38 (in Japanese).
SAKATA, T., SMITH, R.A., GARLAND, E.M., & COHEN, S.M. (1989) Rat
urinary bladder epithelial lesions induced by acrolein. J. environ.
Pathol. Toxicol., 9: 159-170.
SALAMAN, M.H. & ROE, F.J.C. (1956) Further tests for tumour-
initiating activity: N,N-di-(2-chloroethyl)-p-aminophenylbutyric
acid (CB1348) as an initiator of skin tumour formation in the mouse.
Br. J. Cancer, 10: 363-378.
SALEM, H. & CULLUMBINE, H. (1960) Inhalation toxicities of some
aldehydes. Toxicol. appl. Pharmacol., 2: 183-187.
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHIELKE, D.-J. (1987) [Gastrectomy following a rare caustic
lesion.] Chirurg, 58: 50-52 (in German).
SCHMID, B.P., GOULDING, E., KITCHIN, K., & SANYAL, M.K. (1981)
Assessment of the teratogenic potential of acrolein and
cyclophosphamide in a rat embryo culture system. Toxicology, 22:
235-243.
SCHUCK, E.A. & RENZETTI, N.A. (1960) Eye irritants formed during
photo-oxidation of hydrocarbons in the presence of oxides of
nitrogen. J. Air Pollut. Control Assoc., 10: 389-392.
SCHUTTE, N.P. (1977) Hazard evaluation and technical assistance
report No. TA 77-11: Xomed Company, Cincinnati, Ohio, Cincinnati,
Ohio, USA, National Institute for Occupational Safety and Health
(NIOSH-TR-TA-77-11, PB 278834).
SEIZINGER, D.E. & DIMITRIADES, B. (1972) Oxygenates in exhaust from
simple hydrocarbon fuels. J. Air Pollut. Control Assoc., 22: 47-51.
SERTH, R.W., TIERNEY, D.R., & HUGHES, T.W. (1978) Source
assessment: acrylic acid manufacture, Cincinnati, Ohio, US
Environmental Protection Agency, Industrial Environmental Research
Laboratory (Report EPA-600/2-78-004w).
SHAPIRO, R., SODUM, R.S., EVERETT, D.W., & KUNDU, S.K. (1986)
Reactions of nucleosides with glyoxal and acrolein. In: The role of
cyclic nucleic acid adducts in carcinogenesis and mutagenesis,
Proceedings of a meeting organized by the IARC and co-sponsored by
the US National Cancer Institute, and the Lawrence Berkely
Laboratory at the University of California, Lyon, 17-19 September,
Lyon, International Agency for Research on Cancer, pp. 165-173 (IARC
Scientific Publications No. 70).
SHERWOOD, R.L., LEACH, C.L., HATOUM, N.S., & ARANYI, C. (1986)
Effects of acrolein on macrophage functions in rats. Toxicol.
Lett., 32: 41-49.
SHIMOMURA, M., YOSHIMATSU, F., & MATSUMOTO, F. (1971) [Fish odour of
cooked horse mackerel.] Kaseigaku Zasshi, 2: 106-112 (in Japanese).
SIM, V.M. & PATTLE, R.E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1908-1913.
SINKUVENE, D. (1970) [Hygienic assessment of acrolein as an
atmospheric pollutant.] Gig. i Sanit., 35(3): 6-10 (in Russian).
SKOG, E. (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde,
propionaldehyde and butyraldehyde, as well as of acrolein and
crotonaldehyde. Acta pharmacol., 6: 299-318.
SLOTT, V.L. & HALES, B.F. (1985) Teratogenicity and embryolethality
of acrolein and structurally related compounds in rats. Teratology,
32: 65-72.
SLOTT, V.L. & HALES, B.F. (1986) The embryolethality and
teratogenicity of acrolein in cultured rat embryos. Teratology, 34:
155-163.
SLOTT, V.L. & HALES, B.F. (1987a) Enhancement of the embryotoxicity
of acrolein, but not phosphoramide mustard, by glutathione depletion
in rat embryos in vitro. Biochem. Pharmacol., 36: 2019-2025.
SLOTT, V.L. & HALES, B.F. (1987b) Protection of rat embryos in
culture against the embryotoxicity of acrolein using exogenous
glutathione., Biochem. Pharmacol., 36: 2087-2194.
SMITH, R.A., COHEN, S.M., & LAWSON, T.A. (1990) Acrolein
mutagenicity in the V79 assay. Carcinogenesis, 11: 497-498.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SMYTHE, R.J. & KARASEK, F.W. (1973) The analysis of diesel engine
exhaust for low-molecular-weight carbonyl compounds. J.
Chromatogr., 86: 228-231.
SNYDER, J.M., FRANKEL, E.N., & SELKE, E. (1985) Capillary gas
chromatographic analyses of headspace volatiles from vegetable oils.
J. Am. Oil Chem. Soc., 6: 1675-1679.
SPONHOLZ, W.-R. (1982) [Analysis and occurrence of aldehydes in
wines.] Z. Lebensm. Unters. Forsch., 174: 458-462 (in German).
SPRINCE, H., PARKER, C.M., & SMITH, G.G. (1979) Comparison of
protection by L-ascorbic acid, L-cysteine, and adrenergic-blocking
agents against acetaldehyde, acrolein, and formaldehyde toxicity:
implications in smoking. Agents Actions, 9: 407-414.
STACK, V.T. (1957) Toxicity of alpha,ß-unsaturated carbonyl
compounds to microorganisms. Ind. eng. Chem., 49: 913-917.
STAHLMANN, R., BLUTH, U., & NEUBERT, D. (1985) Effects of the
cyclophosphamide metabolite acrolein in mammalian limb bud cultures.
Arch. Toxicol., 57: 163-167.
STEINHAGEN, W.H. & BARROW, C.S. (1984) Sensory irritation
structure-activity study of inhaled aldehydes in B6C3F1 and
Swiss-Webster mice. Toxicol. appl. Pharmacol., 72: 495-503.
STEPHENS, E.R., DARLEY, E.F., TAYLOR, O.C., & SCOTT, W.E. (1961)
Photochemical reaction products in air pollution. Int. J. Air Water
Pollut., 4: 79-100.
SUBDEN, R.E., KRIZUS, A., & AKHTAR, M. (1986) Mutagen content of
Canadian apple eau-de-vie. Can. Inst. Food Sci. Technol. J., 19:
134-136.
SUZUKI, Y. & IMAI, S. (1982) Determination of traces of gaseous
acrolein by collection on molecular sieves and fluorimetry with
o-aminobiphenyl. Anal. Chim. Acta, 136: 155-162.
SWARIN, S.J. & LIPARI, F. (1983) Determination of formaldehyde and
other aldehydes by high performance liquid chromatography with
fluorescence detection. J. liq. Chromatogr., 6: 425-444.
SZOT, R.J. & MURPHY, S.D. (1971) Relationships between cyclic
variations in adrenocortical secretory activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant compounds.
J. Water Pollut. Control Fed., 53: 1503-1517.
TAJIMA, M., KIDA, K., & FUJIMAKI, M. (1967) Effect of gamma
irradiation on volatile compounds from cooked potato. Agric. biol.
Chem., 31: 935-938.
TAKEUCHI, K. & IBUSUKI, T. (1986) Heterogeneous photochemical
reactions of a propylene-nitrogen dioxide-metal oxide-dry air
system. Atmos. Environ., 20: 1155-1160.
TEJADA, S.B. (1986) Evaluation of silica gel cartridges coated in
situ with acidified 2,4-dinitrophenylhydrazine for sampling
aldehydes and ketones in air. Int. J. environ. anal. Chem., 26:
167-185.
TESTA, A. & JOIGNY, C. (1972) Dosage par chromatographie en phase
gazeuse de l'acroléine et d'autres composés alpha,ß-insaturés de la
phase gazeuse de la fumée de cigarette. Ann. Serv. Exploit. ind.
Tab. Allumettes - Div. Etud. Equip., 10: 67-81.
TORAASON, M., LUKEN, M.E., BREITENSTEIN, M., KRUEGER, J.A., &
BIAGINI, R.E. (1989) Comparative toxicity of allylamine and acrolein
in cultured myocytes and fibroblasts from neonatal rat heart.
Toxicology, 56: 107-117.
TREITMAN, R.D., BURGESS, W.A., & GOLD, A. (1980) Air contaminants
encountered by firefighters. Am. Ind. Hyg. Assoc. J., 41: 796-802.
TURUK-PCHELINA, Z.F. (1960) [Acrolein releases into the air while
cooking.] Gig. i Sanit., 25(5): 96-97 (in Russian).
UMANO, K. & SHIBAMOTO, T. (1987) Analysis of headspace volatiles
from overheated beef fat. J. agric. food Chem., 35: 14-18.
UNRAU, G.O., FAROOQ, M., DAWOOD, I.K., MIGUEL, L.C., & DAZO, B.C.
(1965) Field trials in Egypt with acrolein herbicide-molluscicide.
World Health Organ. Bull., 32: 249-260.
US-NIOSH (1984) NIOSH manual of analytical methods, 3rd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (DHHS (NIOSH) Publication No. 84-100. Method 2501).
VAN EICK, A.J. (1977) [The effect of acrolein in air on the eye
blinking frequency of man and guinea pig], Rijswijk, The
Netherlands, Technical and Physical Research, Medical Biological
Laboratory, TNO-MBL, (Report No. A76/K/098) (in Dutch).
VAN OVERBEEK, J., HUGHES, W.J., & BLONDEAU, R. (1959) Acrolein for
the control of water weeds and disease-carrying water snails.
Science, 129: 335-336.
VEITH, G.D., MACEK, K.J., PETROCELLI, S.R., & CARROL, J. (1980) An
evaluation of using partition coefficients and water solubility to
estimate bioconcentration factors for organic chemicals in fish.
Aquat. Toxicol.: 116-129.
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene problems
in vulcanization processes of rubber production]. Gig. i Sanit.,
34: 33-40 (in Russian).
VOROB'EVA, A.I., VOLKOTRUB, L.P., FEOKTISTOVA, N.F., BOBIN, V.I.,
SHESTAKOVA, N.A., & USHAKOVA, N.S. (1982) [Characteristics of
atmospheric pollution from enameled wire manufacturing plants.]
Gig. i Sanit., 1982(6): 66-67 (in Russian).
WARHOLM, M., HOLMBERG, B., & MALMFORS, T. (1984) The cytotoxicity of
some organic solvents. Environ. Res., 7: 183-192.
WATANABE, T. & AVIADO, D.M. (1974) Functional and biochemical
effects on the lung following inhalation of cigarette smoke and
constituents. II. Skatole, acrolein, and acetaldehyde. Toxicol.
appl. Pharmacol. 30: 201-209.
WEBER-TSCHOPP, A., FISCHER, T., & GRANDJEAN, E. (1976) [Objective
and subjective physiological effects of passive smoking.] Int. Arch.
occup. environ. Health, 37: 277-288 (in German).
WEBER-TSCHOPP, A., FISCHER, T., GIERER, R., & GRANDJEAN, E. (1977)
[Experimental irritation by acrolein in human beings.] Z.
Arbeitswiss., 32: 166-171 (in German).
WHITEHOUSE, M.W., BECK, F.W.J., DROGE, M.M., & STRUCK, R.F. (1974)
Lymphocyte deactivation by (potential immunosuppressant) alkylating
metabolites of cyclophosphamide. Agents Actions, 4: 117-123.
WHO (1991) Health and Safety Guide 67: Acrolein, Geneva, World
Health Organization.
WILMER, J.L., EREXSON, G.L., & KLIGERMAN, A.D. (1986) Attenuation of
cytogenetic damage by 2-mercaptoethanesulphonate in cultured human
lymphocytes exposed to cyclophosphamide and its reactive
metabolites. Cancer Res., 46: 203-210.
WILTON, D.C. (1976) Acrolein, an irreversible active-site-directed
inhibitor of deoxyribose 5-phosphate aldolase? Biochem. J., 153:
495-497.
WITZ, G., LAWRIE, N.J., AMORUSO, M.A., & GOLDSTEIN, B.D. (1987)
Inhibition by reactive aldehydes of superoxide anion radical
production from stimulated polymorphonuclear leukocytes and
pulmonary alveolar macrophages. Biochem. Pharmacol., 36: 721-726.
WRABETZ, E., PETER, G., & HOHORST, H.J. (1980) Does acrolein
contribute to the cytotoxicity of cyclophosphamide? J. Cancer Res.,
98: 119-126.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20
coded compounds tested for the National Toxicology Program.
Environ. Mutagen., 7: 87-100.
ZITTING, A. & HEINONEN, T. (1980) Decrease of reduced glutathione in
isolated rat hepatocytes caused by acrolein, acrylonitril, and the
thermal degradation products of styrene copolymers. Toxicology, 17:
333-341.
ZOLLNER, H. (1973) Inhibition of some mitochondrial functions by
acrolein and methylvinylketone. Biochem. Pharmacol., 22: 1171-1178.
ZORIN, V.M. (1966) [Acrolein-induced air pollution.] Zdravookhr.
Belorussii, 1966(7): 43-44 (in Russian).
RESUME
L'acroléine est un liquide volatil extrêmement inflammable dont
l'odeur, âcre et suffocante, est très désagréable. C'est un composé
très réactif.
En 1975, on estime que la production mondiale d'acroléine en
tant que telle était de 59 000 tonnes. On en produit et consomme
encore davantage comme intermédiaire pour la synthèse de l'acide
acrylique et de ses esters.
On dispose d'un certain nombre de méthodes d'analyse pour la
recherche et le dosage de l'acroléine dans divers milieux. On a fait
état de limites inférieures de détection de l'ordre de 0,1 µg/m3
dans l'air (chromatographie en phase gazeuse/spectrométrie de
masse), de 0,1 µg/l dans l'eau (chromatographie liquide à haute
pression), de 2,8 µg/litre dans les milieux biologiques
(fluorimétrie), de 590 µg/kg dans le poisson (chromatographie en
phase gazeuse/spectrométrie de masse) et de 1,4 µg/m3 dans les gaz
d'échappement (chromatographie liquide à haute pression).
On a trouvé de l'acroléine dans certains produits d'origine
végétale et animale et notamment dans des denrées alimentaires et
des boissons. L'acroléine est utilisée principalement comme
intermédiaire en synthèse organique, mais également comme produit
biocide en milieu aquatique.
Des émissions d'acroléine peuvent se produire sur les lieux de
production ou d'utilisation. Elles peuvent être importantes dans
l'air à la suite de la combustion ou de la pyrolyse incomplète de
produits organiques tels que combustibles, polymères de synthèse,
dans certains aliments et le tabac. L'acroléine peut représenter
jusqu'à 3-10 % des aldéhydes totaux présents dans les gaz
d'échappement des véhicules à moteur. La consommation d'une
cigarette fournit de 3 à 228 µg d'acroléine. L'acroléine est un
produit d'oxydation photochimique de certains polluants organiques
de l'atmosphère.
La population générale est essentiellement exposée par
l'intermédiaire de l'air. Une exposition peut également se produire
par voie orale par suite de la consommation de boissons alcoolisées
ou de denrées alimentaires chauffées.
On a mesuré dans l'air des villes des concentrations moyennes
d'acroléine atteignant environ 15 µg/m3 avec des maxima allant
jusqu'à 32 µg/m3. A proximité d'installations industrielles et de
pots d'échappement, des concentrations dix à cent fois plus élevées
sont possibles. Les incendies peuvent donner naissance à des teneurs
très élevées d'acroléine, de l'ordre du mg/m3 d'air. A
l'intérieur des habitations, on a observé que la consommation d'une
cigarette par m3 d'air dans un local en l'espace de 10 à
13 minutes produisait des concentrations en vapeurs d'acroléine de
l'ordre de 450 à 840 µg/m3. Sur les lieux de travail, on a signalé
des teneurs dépassant 100 µg/m3 dans des cas où l'on élevait la
température de certains produits organiques, par exemple lors du
chauffage ou du soudage de ces substances.
Dans l'atmosphère, l'acroléine est dégradée par réaction avec
les radicaux hydroxyles. Sa durée de séjour dans l'atmosphère est de
l'ordre d'une journée. Dans les eaux de surface, l'acroléine se
dissipe en quelques jours. Elle est faiblement adsorbée aux
particules du sol. On a fait état de dégradation aérobie et
anaérobie, encore que la toxicité du composé pour les
micro-organismes puisse faire obstacle à sa biodégradation. Compte
tenu des propriétés physiques et chimiques de l'acroléine, il ne
semble pas que cette substance ait une tendance à la
bioaccumulation.
L'acroléine est extrêmement toxique pour les organismes
aquatiques. Pour les bactéries, les algues, les crustacés et les
poissons, sa toxicité aiguë, estimée d'après les valeurs de la CE50
et de la CL50, se situe entre 0,02 et 2,5 mg/litre, les bactéries
étant l'espèce la plus sensible. Pour le poisson, on a fixé à
0,0114 mg/litre la dose sans effet nocif observable à 60 jours.
L'acroléine détruit efficacement les végétaux aquatiques à des doses
comprises entre 4 et 26 mg/litre.h. A partir de 15 mg/litre, on
observe des effets nocifs sur les cultures irriguées au moyen d'eau
traitée à l'acroléine.
Chez l'homme et l'animal, l'acroléine reste confinée sur son
site d'exposition en raison de sa réactivité et les observations
pathologiques sont également limitées à ce site. Chez des chiens
exposés à des doses de 400 à 600 mg/m3, on a observé un taux de
rétention de 80 à 85 % au niveau des voies respiratoires.
L'acroléine réagit directement sur les groupements sulfhydryles
protéiques et non protéiques ainsi que sur les amines primaires et
secondaires. Elle peut également être métabolisée en acide
mercapturique, en acide acrylique, en glycidaldéhyde ou en
glycéraldéhyde. Les trois derniers métabolites n'ont été observés
qu' in vitro.
L'acroléine est un agent cytotoxique. Sa cytotoxicité s'observe
in vitro dès 0,1 mg/litre. Elle est extrêmement toxique pour les
animaux de laboratoire et l'homme, à la suite d'une seule exposition
quelle qu'en soit la voie. Sa vapeur est irritante pour l'oeil et
les muqueuses respiratoires. Le liquide est corrosif et on a
constaté qu'en solution éthanolique le seuil d'apparition d'une
dermatite d'irritation était de 0,1%. L'expérimentation sur des
volontaires humains exposés à des vapeurs d'acroléine a permis de
fixer à 0,13 mg/m3 la dose la plus faible produisant des effets
nocifs observables; à cette dose, une irritation des yeux se produit
en l'espace de cinq minutes. En outre, les effets au niveau des
voies respiratoires deviennent évidents à partir de 0,7 mg/m3. Une
seule exposition à des doses plus élevées entraîne une
dégénérescence de l'épithélium respiratoire, des séquelles
inflammatoires et une perturbation de la fonction respiratoire.
On a étudié sur des rats, des chiens, des cobayes et des singes
les effets toxicologiques de l'inhalation continue d'acroléine à des
concentrations de 0,5 à 4,1 mg/m3. Des effets histopathologiques
et des effets sur la fonction respiratoire ont été observés chez les
animaux exposés à des teneurs supérieures ou égales à 0,5 mg/m3
pendant 90 jours.
On a étudié sur divers animaux de laboratoire les effets
toxicologiques d'expositions répétées par la voie respiratoire à des
vapeurs d'acroléine, à des concentrations allant de 0,39 mg/m3 à
11,2 mg/m3. La durée de l'exposition allait de cinq jours à
52 semaines. En général, on a fait état chez la plupart des espèces
exposées huit heures par jour à des concentrations de 1,6 mg/m3 ou
davantage, d'une réduction du gain de poids, d'une diminution de la
fonction respiratoire et de modifications pathologiques au niveau du
nez, des voies respiratoires supérieures et des poumons. Parmi les
modifications anatomopathologiques figuraient une inflammation, une
métaplasie et une hyperplasie des voies respiratoires. On a observé
une mortalité importante après expositions répétées à des vapeurs
d'acroléine à des concentrations dépassant 9,07 mg/m3. Chez
l'animal d'expérience, on a montré que l'acroléine provoquait une
déplétion du glutathion tissulaire in vivo et une inhibition des
enzymes in vitro par réaction sur les groupements sulfhydryles au
niveau des sites actifs. Il existe quelques données selon lesquelles
l'acroléine est susceptible d'amoindrir les défenses pulmonaires de
l'hôte chez la souris et le rat.
L'acroléine peut produire des effets tératogènes et
embryotoxiques lorsqu'on l'introduit directement dans l'amnios.
Toutefois, l'absence d'effets chez des lapins à qui elle avait été
injectée par voie intraveineuse à la dose de 3 mg/kg incite à penser
que l'exposition de l'homme à l'acroléine ne devrait pas avoir
d'effet nocif sur le développement de l'embryon.
On a montré que l'acroléine interagissait avec les acides
nucléiques in vitro et en inhibait la synthèse tant in vitro
qu' in vivo. Sans avoir besoin d'être activée, elle produit des
mutations géniques chez les bactéries et les champignons et induit
des échanges entre chromatides soeurs dans les cellules
mammaliennes. Dans tous les cas, ces effets se sont produits dans un
intervalle de dose extrêmement limité qui était fonction de la
réactivité, de la volatilité et de la cytotoxicité de l'acroléine.
Une épreuve de mutation létale dominante chez la souris a donné des
résultats négatifs. Les données disponibles montrent que l'acroléine
est faiblement mutagène pour certains champignons et bactéries et
certaines cultures de cellules mammaliennes.
Des hamsters ont été exposés pendant 52 semaines à des vapeurs
d'acroléine à la dose de 9,2 mg/m3, 7 heures par jour et 5 jours
par semaine, puis ont été placés en observation pendant les 29
semaines suivantes; aucune tumeur n'a été observée. En exposant ces
hamsters dans les mêmes conditions à des vapeurs d'acroléine et
pendant la même durée avec, en outre, des doses intra-trachéennes
hebdomadaires de benzo[a]pyrène ou des doses sous-cutanées une fois
toutes les trois semaines de diéthylnitrosamine, on n'a pas non plus
observé d'effets co-cancérogènes bien nets attribuables à
l'acroléine. Des rats exposés par voie orale à de l'acroléine dans
leur eau de boisson à des doses comprises entre 5 et 50 mg/kg par kg
de poids corporel (quotidiennement, cinq jours par semaine pendant
100 à 124 semaines) n'ont pas présenté de tumeur. En raison du
caractère limité de toutes ces épreuves, on estime que les données
qui permettraient d'évaluer la cancérogénicité de l'acroléine chez
l'animal d'expérience sont encore insuffisantes. De ce fait, il est
impossible pour l'instant d'évaluer la cancérogénicité de
l'acroléine pour l'homme.
Les différents seuils de concentration auxquels apparaissent
les différents effets de l'acroléine sont les suivants : perception
d'une odeur, 0,007 mg/m3, irritation oculaire, 0,3 mg/m3,
irritation du nez et clignement des yeux, 0,03 mg/m3, réduction de
la fréquence respiratoire, 0,7 mg/m3. Comme la concentration de
l'acroléine dépasse rarement 0,03 mg/m3 dans l'air des villes,
elle n'est pas susceptible de constituer une nuisance dans les
circonstances normales.
Du fait de sa forte toxicité pour les organismes aquatiques,
l'acroléine présente un danger pour la faune et la flore aquatique à
proximité ou sur les sites de décharge de déchets industriels, en
cas de déversements et là où l'on utilise ce produit comme biocide.
1. RESUMEN
La acroleína es un líquido volátil, sumamente inflamable, con
un olor pungente, asfixiante y desagradable. Se trata de un
compuesto muy reactivo.
La producción mundial de acroleína aislada se calculó en 59 000
toneladas en 1975. Se produce y consume una cantidad aún mayor de
acroleína como intermediaria en la síntesis de ácido acrílico y sus
ésteres.
Se dispone de métodos analíticos para determinar la acroleína
presente en diversos medios. Los límites mínimos de detección que
se han comunicado son 0,1 µg/m3 de aire (cromatografía
gaseosa/spectrometría de masas), 0,1 µg/litro de agua (cromatografía
líquida a alta presión), 2,8 µg/litro de medio biológico
(fluorimetría), 590 µg/kg en peces (cromatografía
gaseosa/espectrometría de masas), y 1,4 µg/m3 de gases de escape
(cromatografía líquida a alta presión).
La acroleína se ha detectado en algunos vegetales y animales,
inclusive en alimentos y bebidas. La sustancia se usa
principalmente como intermediaria en la síntesis química aunque
también como biocida acuático.
Pueden producirse emisiones de acroleína en sus lugares de
producción o de uso. Las emisiones importantes a la atmósfera se
deben a la combustión incompleta o la pirólisis de materiales
orgánicos como ser combustibles, polímeros sintéticos, alimentos y
tabaco. La acroleína puede representar el 3-10% de los aldehídos
totales presentes en los escapes de automóviles. El humo de un
cigarrillo libera 3-228 µg de acroleína. La acroleína es uno de los
productos de la oxidación fotoquímica de ciertos contaminantes
orgánicos de la atmósfera.
La exposición de la población general se produce principalmente
por el aire. La exposición por vía oral puede producirse por el
consumo de bebidas alcohólicas o alimentos calentados.
En la atmósfera urbana se han medido niveles promedio de
acroleína de hasta unos 15 µg/m3 y niveles máximos de hasta
32 µg/m3. En las cercanías de las industrias y junto a los caños
de escape pueden registrarse niveles entre 10 y 100 veces
superiores. Como resultado de incendios pueden hallarse niveles
sumamente elevados en el aire, del orden de mg/m3. En el aire
cerrado de interiores, el consumo de un cigarrillo por m3 de
volumen de la habitación produjo en 10-13 minutos concentraciones de
vapor de acroleína de 450-840 µg/m3. En el medio ambiente laboral
se han detectado niveles de más de 1000 µg/m3 en situaciones que
entrañaban aumento de temperatura de materiales orgánicos, por
ejemplo durante la soldadura o el calentamiento.
La acroleína se degrada en la atmósfera por reacción con
radicales hidroxilo. El tiempo de persistencia en la atmósfera es
de aproximadamente un día. En aguas de superficie, la acroleína se
disipa en pocos días. Tiene un bajo potencial de adsorción en el
suelo. Se ha observado su degradación en condiciones aerobias y
anaerobias, si bien la toxicidad del compuesto para los
microorganismos puede impedir la biodegradación. En vista de sus
propiedades físicas y químicas, es improbable que se produzca
bioacumulación de acroleína.
La acroleína es sumamente tóxica para los organismos acuáticos.
Los valores de la CE50 y la CL50 correspondientes a bacterias,
algas, crustáceos y peces se encuentran entre 0,02 y 2,5 mg/litro,
siendo las bacterias los organismos más sensibles. En peces se ha
determinado que el nivel sin observación de efectos adversos (NOAEL)
a 60 días es de 0,0114 mg/litro. Se ha conseguido combatir
eficazmente los vegetales acuáticos con dosis de acroleína
comprendidas entre 4 y 26 mg/litro.h. Se han observado efectos
adversos en cultivos que crecen en suelos irrigados con agua tratada
con acroleína en concentraciones de 15 mg/litro o más.
En el animale y en el ser humano la reactividad de la acroleína
limita efectivamente la sustancia al lugar de exposición; los
hallazgos patológicos se limitan asimismo a esos lugares. En el
tracto respiratorio de perros expuestos a 400-600 mg/m3 se
encontró una retención del 80-85% de acroleína. La acroleína
reacciona directamente con los grupos sulfhidrilo contenidos en
radicales proteicos o no proteicos y con aminas primarias y
secundarias. También puede ser metabolizado a ácidos mercaptúricos,
ácido acrílico, glicidaldehído o gliceraldehído. Estos tres últimos
metabolitos sólo se han encontrado in vitro.
La acroleína es un agente citotóxico. Se ha observado
citotoxicidad in vitro con niveles de solamente 0,1 mg/litro. La
sustancia es sumamente tóxica para los animales de experimentación y
el ser humano tras una exposición única por diferentes vías. El
vapor es irritante para los ojos y el tracto respiratorio. En
estado líquido es corrosiva. Con respecto a la dermatitis
irritante, se encontró que el NOAEL de la acroleína etanólica era de
0,1%. Los experimentos con voluntarios humanos expuestos a vapores
de acroleína mostraron un nivel mínimo de observación de efectos
(LOAEL) de 0,13 mg/m3, dosis con la que los ojos pueden irritarse
al cabo de cinco minutos. Además, se observan efectos en el tracto
respiratorio a partir de 0,7 mg/m3. Con exposiciones aisladas a
niveles más altos, aparecen: degeneración del epitelio respiratorio,
secuelas inflamatorias y trastorno de la función respiratoria.
Los efectos toxicológicos de la exposición por inhalación
continua de concentraciones comprendidas entre 0,5 y 4,1 mg/m3 se
han estudiado en la rata, el perro, el cobayo y el mono. Se
observaron efectos sobre la función respiratoria y trastornos
histopatológicos cuando se expuso a los animales a niveles de
acroleína de 0,5 mg/m3 o más, durante 90 días.
Los efectos toxicológicos de la inhalación repetida de vapores
de acroleína en concentraciones comprendidas entre 0,39 mg/m3 y
11,2 mg/m3 se han estudiado en diversos animales de laboratorio.
Las duraciones de la exposición variaron entre 5 días y hasta 52
semanas. En general, se han documentado: reducción de la
adquisición de peso corporal, disminución de la función pulmonar y
cambios patológicos en la nariz, las vías aéreas superiores y los
pulmones en la mayoría de las especies expuestas a concentraciones
de 1,6 mg/m3 o más, durante 8 h/día. Entre los cambios
patológicos se observaron inflamación, metaplasia e hiperplasia del
tracto respiratorio. Se ha observado un nivel significativo de
mortalidad tras la exposición repetida a concentraciones de vapor de
acroleína superiores a 9,07 mg/m3. En animales de
experimentación, se ha demostrado que la acroleína agota el
glutatión tisular y que in vitro inhibe enzimas reaccionando con
los grupos sulfhidrilo de los sitios activos. Hay limitada
evidencia de que la acroleína pueda deprimir las defensas pulmonares
en el ratón y la rata.
La acroleína puede inducir efectos teratogénicos y
embriotóxicos si se administra directamente en el amnios. No
obstante, el hecho de que no se observaran efectos en ratones a los
que se inyectó 3 mg/kg por vía intravenosa sugiere que la exposición
humana a la acroleína tiene pocas probabilidades de afectar al
embrión en desarrollo.
Se ha demostrado que la acroleína interacciona con los ácidos
nucleicos in vitro y que inhibe su síntesis tanto in vitro como
in vivo. Sin activación, indujo mutaciones génicas en bacterias y
hongos y provocó intercambios de cromátidas hermanas en células de
mamíferos. En todos los casos esos efectos se produjeron en un
margen muy reducido de concentraciones, limitado por la reactividad,
la volatilidad y la citotoxicidad de la acroleína. Un ensayo de
letalidad dominante en ratones dio resultado negativo. Los datos
disponibles muestran que la acroleína es un mutágeno débil para
ciertas bacterias, hongos y cultivo celular de mamífero.
No se encontraron tumores en hámsters expuestos durante 52
semanas a vapores de acroleína con una concentración de 9,2 mg/m3
durante 7 h/día, 5 días a la semana, y observados durante 29 semanas
más. Cuando se expusieron hámsters a vapores de acroleína en las
mismas condiciones durante 52 semanas y, además, a dosis
intratraqueales de benzo[a]pireno semanalmente o a dosis subcutáneas
de dietilnitrosamina una vez cada tres semanas, no se observó una
acción cocarcinogénica clara de la acroleína. La exposición de
ratas por vía oral a la acroleína en el agua de bebida, en dosis
comprendidas entre 5 y 50 mg/kg de peso corporal al día
(5 días/semana durante 104-124 semanas) no indujo tumores. Dado el
carácter limitado de todos esos ensayos, se considera que no se
dispone de datos suficientes para determinar la carcinogenicidad de
la acroleína en los animales de experimentación. En consecuencia,
se considera asimismo imposible evaluar la carcinogenicidad de la
acroleína para el ser humano.
Los umbrales de acroleína que causan irritación y efectos en la
salud son 0,07 mg/m3 en el caso de la percepción del olor,
0,13 mg/m3 en la irritación ocular, 0,3 mg/m3 en la irritación
nasal y el parpadeo, y 0,7 mg/m3 en la disminución del ritmo
respiratorio. Puesto que el nivel de acroleína raras veces supera
los 0,03 mg/m3 en el aire urbano, es poco probable que alcance
niveles molestos o nocivos en circunstancias normales.
En vista de la elevada toxicidad de la acroleína para los
organismos acuáticos, la sustancia representa un riesgo para la vida
acuática en las proximidades de las zonas donde se producen vertidos
y escapes industriales, y en los lugares donde se usa como biocida.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Mortality
The available acute mortality data are summarized in Table 8.
Most tests for the determination of the acute toxicity of acrolein
do not comply with present standards. Nevertheless, retesting is
not justified for ethical reasons and in view of the overt high
toxicity of acrolein following inhalation or oral exposure (Hodge &
Sterner, 1943).
In addition to the data in Table 8, an oral LD95 of
11.2 mg/kg body weight for Charles River rats, observed for 24 h,
has been reported (Sprince et al., 1979). Draminski et al.
(1983) reported the deaths of 5/10 rats given 10 mg/kg body weight
in corn oil by gavage.
7.1.2 Effects on the respiratory tract
In vapour exposure tests, the effects observed in experimental
animals have almost exclusively been local effects on the
respiratory tract and eyes.
In the LC50 studies, effects on the respiratory tract were
clinically observed as nasal irritation and respiratory distress in
rats (Skog, 1950; Potts et al., 1978; Crane et al., 1986),
hamsters (Kruysse, 1971), mice, guinea-pigs, and rabbits (Salem &
Cullumbine, 1960) at exposure levels of between 25 mg/m3 for 4 h
and 95 150 mg/m3 for 3 min. Rats exposed for 10 min to
concentrations of 750 or 1000 mg/m3 suffered asphyxiation
(Catilina et al., 1966).
Histopathological investigations in experiments with
vapour-exposed rats (Skog, 1950; Catilina et al., 1966; Potts
et al., 1978; Ballantyne et al., 1989), hamsters (Kilburn &
McKenzie, 1978), guinea-pigs (Dahlgren et al., 1972; Jousserandot
et al, 1981), and rabbits (Beeley et al., 1986) revealed varying
degrees of degeneration of the respiratory epithelium consisting of
deciliation (see also in vitro work on cytotoxicity discussed in
7.1.5), exfoliation, necrosis, mucus secretion, and vacuolization.
Also observed were acute inflammatory changes consisting of
infiltration of white blood cells into the mucosa, hyperaemia,
haemorrhages, and intercellular oedema. Proliferative changes of
the respiratory epithelium, in the form of early stratification and
hyperplasia, were observed in hamsters 96 h after exposure to
13.7 mg/m3 for 4 h (Kilburn & McKenzie, 1978).
Table 8. Acute mortality caused by acrolein
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Rat (Wistar) male inhalation (10 min) 8 750 Catilina et al. (1966)b
Rat (Wistar) not reported oral 14 46 (39-56) Smyth et al. (1951)g
Rat (unspecified not inhalation (30 min) 21 300 Skog (1950)b,c
strain) reported
Rat (Sprague-Dawley) male inhalation (30 min) 14 95-217 Potts et al. (1978)d
Rat (Sprague-Dawley) male and inhalation (1 h) 14 65 (60-68) Ballantyne et al. (1989)
female inhalation (4 h) 14 20.8 (17.5-24.8)
Rat (Sherman) male and inhalation (4 h) 14 18 Carpenter et al. (1949)b,e
female
Hamster (Syrian golden) male and inhalation (4 h) 14 58 (54-62) Kruysse (1971)
female
Table 8 (contd)
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Mouse (unspecified male inhalation (6 h) 1 151 Philippin et al. (1970)f
strain)
Mouse (NMRI) not reported intraperitoneal 6 7 Warholm et al. (1984)g
a Where available, 95% confidence limits are given in parentheses.
b Determination of acrolein levels was not reported.
c No mortality at 100 mg/m3, 100% mortality at 700 mg/m3.
d Approximate value: no mortality at 33 mg/m3, 1/7 and 7/7 died at 95 and 217 mg/m3, respectively.
e Approximate value: 2-4/6 died.
f No mortality at 71 mg/m3, 100% mortality at 273 mg/m3.
g The vehicle was water.
Functional changes in the respiratory system following acrolein
vapour exposure have been investigated in guinea-pigs and mice. A
rapidly reversible increase in respiratory rate was observed in
intact guinea-pigs during exposure to 39 mg/m3 for 60 min (Davis
et al., 1967) and to 0.8 mg/m3 or more for 2 h (Murphy et al.,
1963) followed by a decrease in respiratory rate and an increase in
tidal volume. No changes in pulmonary compliance were reported.
Davis et al. (1967) did not observe these effects in
tracheotomized animals and concluded that they were caused by reflex
stimulation of upper airway receptors and not by
bronchoconstriction. Murphy et al. (1963), observing that
anticholinergic bronchodilators, aminophylline and isoproterenol,
but not antihistaminics, reduced the acrolein-induced increase in
respiratory resistance, concluded that acrolein caused
bronchoconstriction mediated through reflex cholinergic stimulation.
In another experiment, an increase in respiratory resistance was
also observed in anaesthetized, tracheotomized guinea-pigs with
transected medulla during exposure to 43 mg/m3 for up to 5 min
(Guillerm et al., 1967b). The effect was not reversed by atropine.
It was concluded by the authors that acrolein did not cause
bronchoconstriction via reflex stimulation, but probably via
histamine release. When anaesthetized mice were exposed to 300 or
600 mg/m3 for 5 min via a tracheal cannula, respiratory
resistance, respiratory rate, and tidal volume decreased and
pulmonary compliance increased at an unspecified time after exposure
(Watanabe & Aviado, 1974).
The concentration that produces a 50% decrease in respiratory
rate (RD50) as a result of reflex stimulation of trigeminal nerve
endings in the nasal mucosa (sensory irritation) has been used as an
index of upper respiratory tract irritation. This effect reduces
the penetration of noxious chemicals into the lower respiratory
tract. The rate of respiration was measured in a body
plethysmograph, only the animals' heads being exposed to the
acrolein vapour. Depending on the strain, RD50 values for mice
ranged from 2.4 to 6.6 mg/m3 (Kane & Alarie, 1977; Nielsen
et al., 1984; Steinhagen & Barrow, 1984). In rats a RD50 of
13.7 mg/m3 was found (Babiuk et al., 1985).
7.1.3 Effects on skin and eyes
Animal skin irritation tests have not been performed and skin
irritation has not been mentioned as an effect in the acute
inhalation tests reported.
One special in vivo eye irritation test involved
vapour-exposed and control rabbits. At analysed concentrations of
acrolein (method not specified) between 4.3 and 5.9 mg/m3,
maintained over 4 h, slight chemosis was observed but no iritis
(Mettier et al., 1960). Eye irritation was observed clinically in
rodents in several acute inhalation tests, but was not graded (Skog,
1950; Salem & Cullumbine, 1960; Kruysse, 1971; Potts et al.,
1978).
7.1.4 Systemic effects
With respect to systemic effects, most studies have been
performed at concentrations far above the lethal dose. When rats
were exposed to concentrations of acrolein between 1214 and
95 150 mg/m3 during various periods of time, incapacitation,
indicated by the inability to walk in a rotating cage, and
convulsions were observed after 2.8 min at the highest concentration
and after 27 to 34 min at the lowest concentration. These effects
were followed by death after several minutes. Cyanosis of the
extremities and agitation were observed at levels of 22 900 mg/m3
or more (Crane et al., 1986).
The effects of acrolein on the cardiovascular system were
investigated by Egle & Hudgins (1974). Rats anaesthetized by sodium
pentobarbital and exposed only via the mouth and nose to
concentrations between 10 and 5000 mg/m3 for 1 min showed an
increase in blood pressure at all exposure levels. The heart rate
was increased at concentrations from 50 mg/m3 to 500 mg/m3 but
decreased at 2500 and 5000 mg/m3. Intravenous experiments
suggested that increased blood pressor responses resulted from the
release of catecholamines from sympathetic nerve endings and from
the adrenal medulla and that the decreased heart rate effect was
mediated by the vagus nerve (Egle & Hudgins, 1974).
In an acute oral test with rats exposed at 11.2 mg/kg body
weight, decreased reflexes, body sag, poor body tone, lethargy,
stupor, and tremors were observed, as well as respiratory distress
(Sprince et al., 1979).
Because acrolein was shown to induce acute cytotoxicity of the
rat urinary bladder mucosa when instilled directly into the bladder
lumen (Chaviano et al., 1985), this end-point was investigated
in vivo. Two days after a single oral or intraperitoneal dose of
25 mg/kg body weight to ten rats per group, focal simple hyperplasia
of the urinary bladder was detected in the three surviving rats
dosed intraperitoneally. None of the orally exposed rats showed
this effect, but all exhibited severe erosive haemorrhagic
gastritis. Both orally and intraperitoneally exposed rats showed
eosinophilic degeneration of hepatocytes. No abnormalities were
observed in sections of lungs, kidneys, and spleen. Acrolein was
also administered intraperitoneally at single doses of 0.5, 1, 2, 4,
or 6 mg/kg body weight. Proliferation of the bladder mucosa was
evaluated autoradiographically by measuring [3H-methyl]thymidine
incorporation in exposed versus control rats 5 days after the
intraperitoneal injection of acrolein and was found to be increased
nearly two-fold at the highest dose. Body weight gain was decreased
at the two highest doses. Histopathological evaluation of the liver
and urinary bladder did not reveal abnormalities (Sakata et al.,
1989).
7.1.5 Cytotoxicity in vitro
As shown in Table 9, mammalian cell viability is affected by
acrolein in vitro at nominal concentrations of 0.1 mg/litre or
more (not corrected for interaction with culture medium components
or volatilization). The concentration at which formaldehyde
exhibited a similar degree of cytotoxicity was about 6 to 100 times
higher (Holmberg & Malmfors, 1974; Pilotti et al., 1975; Koerker
et al., 1976; Krokan et al., 1985).
Acrolein is a known inhibitor of respiratory tract ciliary
movement in vitro. After a 20-min exposure to an acrolein
concentration of 34-46 mg/m3, the ciliary beating frequency of
excised sheep trachea decreased by 30% (Guillerm et al., 1967a).
Exposure to 13 mg/m3 for 1 h is the greatest exposure that does
not stop ciliary activity in excised rabbit trachea (Dalhamn &
Rosengren, 1971). The no-observed-effect-level for longer exposure
periods would be expected to be lower than 13 mg/m3. Other
in vitro investigations into the inhibition of ciliary movement by
acrolein were reviewed by Izard & Libermann (1978).
7.2 Short-term exposure
7.2.1 Continuous inhalation exposure
In two subchronic inhalation studies with rats, changes in
weight gain, longevity, behaviour, and several physiological
parameters were reported (Gusev et al., 1966; Sinkuvene, 1970).
Unfortunately, the reports did not give sufficient details on the
exposure conditions and protocols and the studies are thus of
limited value in evaluating the toxicological properties of
acrolein.
Table 9. In vitro cytotoxicity of acrolein
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Rat cardiac fibroblasts/myocytes 4 increased lactate Toraason et al. (1989)
dehydrogenase release > 2.8
Rat cardiac myocytes 2 abolished myocine beat > 2.8
dehydrogenase
4 decreased ATP levels > 0.56
Mouse Ehrlich Landschutz 5 92% survivala 1 Holmberg & Malmfors (1974)
Diploid ascites tumour cells 5 53% survivala 5
Mouse B P8 ascites sarcoma cells 48 20% growth rate inhibition 0.6 Pilotti et al. (1975)
48 94% growth rate inhibition 5.6
Mouse C1300 neuroblastoma cells 24 50% survivala 1.7 Koerker et al. (1976)
Mouse L 1210 leukaemia cells 1 70-80% survivala 1.1 Wrabetz et al. (1980)
1 < 15% survivala 2.8
Chinese hamster ovary cells 5 100% mitotis inhibition 0.6 Au et al. (1980)
Adult human bronchial 1 92% colony-forming efficiency 0.06 Krokan et al. (1985)
fibro-blasts 1 45% colony-forming efficiency 0.2
Table 9 (contd).
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Adult human lymphocytes 48 decreased replicative index 0.6 Wilmer et al. (1986)
48 100% mitosis inhibition 2.2
Human K562 chromic myeloid 1 marked reduction in > 0.3 Crook et al. (1986a,b)
leukaemia cells colony-forming ability
Human bronchial epithelial cells 1 20% colony-forming efficiency 0.06 Grafström et al. (1988)
1 50% colony-forming efficiency 0.06-0.17
1 50% survivala 0.34
3 clonal growth rate inhibition > 0.17
3 increase in cross-linkage
envelope formation > 0.06
3 decreased plasminogen
activator activity > 0.56
Human fibroblasts 5 63% cell count reduction < 0.017 Curren et al. (1988)
DNA-repair deficient human
fibroblasts 5 63% cell count reduction 0.045
a measured as dye exclusion
Groups of 7 or 8 Sprague-Dawley rats of both sexes, 7 or 8
Princeton or Hartley-derived guinea-pigs of both sexes, 2 male
pure-bred Beagle dogs, and 9 male squirrel-monkeys were exposed to a
vapourized acrolein-ethanol-water mixture for 90 days (Lyon et al.,
1970). The measured acrolein concentrations were 0, 0.5 (two groups
for each species), 2.3, and 4.1 mg/m3 and the ethanol
concentrations were below 18.7 mg/m3. Pathological investigations
did not include weighing of tissues and organs or examination of the
tracheas at the lowest exposure level. There was no
treatment-related mortality. One monkey died at 0.5 mg/m3 and one
at 2.3 mg/m3 due to accidental infections. Body weight gain
reduction was only found in rats at 2.3 and 4.1 mg/m3.
Clinically, ocular discharge and salivation were observed in dogs at
2.3 and 4.1 mg/m3 and in monkeys at 4.1 mg/m3. Monkeys kept
their eyes closed at 2.3 mg/m3. No adverse effects on
haematological or biochemical parameters were observed in any of the
animals. At necropsy, occasional pulmonary haemorrhage and focal
necrosis in the liver were found in three rats at 2.3 mg/m3.
Pulmonary inflammation and occasional focal liver necrosis were also
observed in guinea-pigs at this concentration. Sections of lung
from two of the four dogs exposed at 0.5 mg/m3 revealed focal
vacuolization, hyperaemia, and increased secretion of bronchiolar
epithelial cells, slight bronchoconstriction, and moderate
emphysema. At 2.3 mg/m3, focal inflammatory reactions involved
lung, kidney, and liver. Bronchiolitis and early broncho-pneumonia
were seen in one dog. At 4.1 mg/m3, both dogs had confluent
bronchopneumonia. All nine monkeys at 4.1 mg/m3 showed squamous
metaplasia and six of them showed basal cell hyperplasia in the
trachea. None of the species revealed other treatment-related
changes (Lyon et al., 1970).
Bouley et al. (1975) exposed a total of 173 male SPF-OFA rats
to a measured acrolein vapour concentration of 1.26 mg/m3 for a
period of 15 to 180 days and used control groups of equal size. No
mortality occurred. Sneezing was observed from day 7 to day 21 in
the treated animals, and body weight gain and food consumption were
reduced. There was an increase in relative lung weight in rats
killed on day 77 but not in rats killed on days 15 or 32. The
relative liver weight was decreased at day 15 but not thereafter,
and the number of alveolar macrophages was decreased at days 10 and
26 but not at days 60 or 180. When groups of 16 rats were infected
by one LD50 dose of airborne Salmonella enteriditis on day 18 or
day 63, mortality increased from 53% in controls to 94% in the
exposed rats infected on day 18. No changes were observed in
biochemical parameters, including the amount of liver DNA per mg of
protein in a group of partially hepatectomized rats, or in the
response of spleen lymphocytes to phytohaemagglutinin in rats
exposed for 39 to 57 days. Other end-points were not investigated.
7.2.2 Repeated inhalation exposure
Lyon et al. (1970) exposed groups of rats, guinea-pigs, dogs,
and monkeys to acrolein vapour at concentrations of 0, 1.6. and
8.5 mg/m3 for 8 h per day and 5 days per week over 6 weeks. With
the exception of the exposure levels, period, and frequency, the
protocol was the same as that for the continuous inhalation exposure
described in section 7.2.1. Two deaths occurred among the nine
monkeys at 8.5 mg/m3. There was body weight gain reduction in
rats and body weight loss (not statistically significant) in monkeys
at 8.5 mg/m3. Clinically, eye irritation and salivation were
observed in dogs and monkeys and difficult breathing in dogs at
8.5 mg/m3. No adverse effects on haematological or biochemical
parameters were observed in any of the animals. At necropsy,
sections of lung from all animals exposed to 1.6 mg/m3 showed
chronic inflammatory changes. Additionally, some showed emphysema.
At 8.5 mg/m3, squamous metaplasia and basal cell hyperplasia were
observed in the trachea of both dogs and monkeys. In addition,
bronchopneumonia was noted in dogs and necrotizing bronchitis and
bronchiolitis in monkeys. Focal calcification of the tubular
epithelium was noted in the kidneys of rats and monkeys at
8.5 mg/m3.
Groups of male Sprague-Dawley rats were also exposed to
acrolein vapour at measured concentrations of 0, 0.39, 2.45, and
6.82 mg/m3 for 6 h per day and 5 days per week over 3 weeks (Leach
et al., 1987). Subgroups were used for immunological
investigations (section 7.4) and for histopathological examination
of nasal turbinates and lungs. Body weight gain was depressed from
week 1 up to the end of the exposure period at 6.82 mg/m3.
Absolute, but not relative, spleen weight was reduced at this
exposure level. There were no histological effects on the lungs,
but the respiratory epithelium of the nasal turbinates showed
exfoliation, erosion, and necrosis, as well as dysplasia and
squamous metaplasia at 6.82 mg/m3. In addition, the mucous
membrane covering the septum and lining the floor of the cavity
showed hyperplasia and dysplasia (Leach et al., 1987).
Another experiment involved Dahl rats of two lines, one
susceptible (DS) and one resistant (DR) to salt-induced hypertension
(Kutzman et al., 1984). Groups of 10 female rats of each line were
exposed to measured acrolein concentrations of 0, 0.89, 3.21, and
9.07 mg/m3 for 6 h per day and 5 days per week over 61-63 days.
One week after the exposure, survivors were killed for pathological
and compositional analysis of the lung following behavioural and
clinical chemistry testing. At 9.07 mg/m3, all DS rats died
within 11 days and 4 DR rats died within the exposure period.
Reduced body weights were measured in the surviving DR rats during
the first 3 weeks, followed by an almost normal body weight gain.
Biochemical changes were found in DR rats at 9.07 mg/m3 and
included increases in lung hydroxyproline and elastin, serum
phosphorus, and in the activities of serum alkaline phosphatase,
alanine aminotransferase (EC 2.6.1.2), and aspartate
aminotransferase (EC 2.6.1.1). No effects were observed on
exploratory behaviour, locomotor activity, blood pressure, lung
protein, blood urea nitrogen, or on serum creatinine, uric acid, or
calcium. At necropsy of survivors, DR rats exposed to 9.07 mg/m3
had increased relative weights of several organs, especially the
lungs. It was noted by the authors that the exposed rats gained a
considerable amount of weight during the week following exposure.
In both rat lines, concentration-related increases were observed in
lymphoid aggregates in pulmonary parenchyma, in collections of
intra-alveolar macrophages with foamy cytoplasm, and in
hyperplastic/metaplastic terminal bronchiolar epithelial changes.
Multifocal interstitial pneumonitis and squamous metaplasia of the
tracheal epithelium were also found in DR rats exposed to
9.07 mg/m3. In contrast, dead and moribund rats, especially those
of the DS strain, mainly exhibited severe bronchial and bronchiolar
epithelial necrosis with exfoliation, oedema, haemorrhage, and
varying degrees of bronchopneumonia. Adverse effects were absent in
nasal turbinates and in non-pulmonary tissues (Kutzman et al.,
1984).
In follow-up studies, groups of 32 to 57 male Fischer-344 rats
were exposed in exactly the same way as described for the Dahl rats.
Surviving rats were tested for pulmonary function one week after
exposure and then killed and examined for compositional analysis,
morphometry, and (in nine rats per group) pathological changes in
the lung (Kutzman et al., 1985; Costa et al., 1986). At
9.07 mg/m3, 56% mortality occurred. After an initial body weight
loss over the first 10 days, weight gain became comparable to that
of controls. There was an increase in the relative weight of
several organs, especially the lungs. Lungs also showed an increase
in water content and in the levels of elastin and hydroxyproline,
but not in the levels of protein and DNA. The hydroxyproline level
was also elevated at 3.21 mg/m3. Histologically, surviving rats
treated with 3.21 or 9.07 mg/m3 demonstrated an exposure-related
increase in effects on the respiratory tract consisting of
bronchiolar epithelial necrosis with exfoliation, bronchiolar
mucopurulent plugs, an increase in bronchiolar and alveolar
macrophages, and focal pneumonitis. At 3.21 mg/m3, there was
type II cell hyperplasia and at 9.07 mg/m3 tracheal,
peribronchial, and alveolar oedema and acute rhinitis. The severity
of lung lesions was highly variable and three of the nine rats
examined at 9.07 mg/m3 did not exhibit histological damage.
Moribund rats mainly showed severe acute bronchopneumonia and focal
alveolar and tracheal oedema with exfoliation in the bronchi and
bronchioles (Kutzman et al., 1985). In another report from the
same research group, the results of pulmonary function testing and
morphometry disclosed air-flow dysfunction at 9.07 mg/m3, which
was correlated with the presence of focal peribronchial lesions and
the lung elastin concentrations. In contrast, the rats exposed at
0.89 mg/m3 exhibited enhanced flow-volume dynamics, whereas no
effects on lung function were present in the 3.21-mg/m3 group
(Costa et al., 1986).
Groups of six Wistar rats and ten Syrian golden hamsters of
both sexes were exposed to acrolein vapour at measured
concentrations of 0, 0.9, 3.2, and 11.2 mg/m3 for 6 h per day and
5 days per week over 13 weeks (Feron et al., 1978). Within the
first month of exposure to 11.2 mg/m3, half the number of rats of
each sex died. One hamster died at this exposure level because of
renal failure. A treatment-related decrease in body weight gain and
food intake was observed in rats exposed to 3.2 mg/m3 or more.
Hamsters showed decreased body weight gain at 11.2 mg/m3, but food
intake was not examined. At this exposure level, all animals kept
their eyes closed, rats showed bristling hair, and hamsters showed
salivation and nasal discharge. Haematological investigation and
urinalysis in week 12 showed no changes in rats. In hamsters,
urinalysis revealed no changes, but females showed increases in the
number of erythrocytes, packed cell volume, haemoglobin content, and
number of lymphocytes and a decrease in the number of neutrophilic
leucocytes. Changes in relative organ weights, which were
considered by the authors to be related to the treatment, were found
in the lung, heart, and kidneys of both species and in the adrenals
of rats exposed to 11.2 mg/m3. Histological changes were confined
to the respiratory tract. In the nose, rats exhibited an
exposure-related increase in squamous metaplasia and neutrophilic
infiltration of the mucosa at levels of 0.9 mg/m3 or more (at
0.9 mg/m3 each effect was observed in one male) and occasional
necrotizing rhinitis at 11.2 mg/m3. Hamsters also showed these
effects at 11.2 mg/m3 but only minimal inflammatory changes at
3.2 mg/m3. In the larynx and trachea of rats exposed to
11.2 mg/m3, squamous metaplasia was also observed and was
accompanied by hyperplasia in bronchi and bronchioli. At this
exposure level, the larynx of hamsters was slightly thickened and
focal hyperplasia and metaplasia were found in the trachea.
Inflammatory changes were present in the bronchi, bronchioli, and
alveoli of rats and included haemorrhage, oedema, accumulations of
alveolar macrophages, an increase in mucus-producing cells in the
bronchioli, and bronchopneumonia. The authors noted considerable
variation between individual rats in the degree of the lesions
(Feron et al., 1978).
Feron & Kruysse (1977) exposed groups of 36 Syrian golden
hamsters of both sexes to acrolein vapour at measured levels of 0
and 9.2 mg/m3 for 7 h per day and 5 days per week over 52 weeks.
Except for 6 males and 6 females, the hamsters were observed for a
further 29 weeks after the exposure period. Overall mortality was
38% in exposed hamsters and 33% in controls. Body weight was
slightly and reversibly decreased at the end of the exposure period.
The other effects observed at the end of the exposure period were
essentially similar (but less severe) to those described above for
hamsters exposed to 11.2 mg/m3 for 13 weeks, but hyperplasia was
not observed. Histological changes were restricted to the anterior
half of the nasomaxillary turbinates and were still found in 20% of
the animals at week 81. At that time they mainly consisted of a
thickened submucosa and exudation into the lumen. Epithelial
metaplasia, but not hyperplasia, was noted. No tumours were found.
Histopathological examination of the respiratory tract of male
Swiss-Webster mice was the object of a study involving groups of 16
to 24 male mice exposed to measured concentrations of 3.9 mg/m3
for 6 h per day during 5 days (Buckley et al., 1984). The lesions
observed were restricted to the nose and were most severe in the
anterior respiratory epithelium and on the free margins of the
nasomaxillary turbinates and the adjacent nasal septum. They
consisted of severe deciliation, moderate exfoliation, erosion,
ulceration and necrosis, severe squamous metaplasia, moderate
neutrophilic infiltration, and a slight serofibrinous exudate.
Lesions in the olfactory epithelium were largely confined to the
dorsal meatus and consisted of moderate ulceration and necrosis, and
slight squamous metaplasia. The nasal squamous epithelium was not
affected (Buckley et al., 1984).
One special investigation concerned the effects of acrolein
vapour on the respiratory functions of male Swiss mice exposed to
100 mg/m3 for two daily periods of 30 min each for 5 weeks. Body
weights were not affected. There was a decrease in pulmonary
compliance, but no effects were found on pulmonary resistance,
respiratory volume, or functional residual capacity. The lungs
showed an increase in phospholipid content (Watanabe & Aviado,1974).
In summary, the toxicological effects on a variety of
laboratory animals from repeated inhalation exposure to acrolein
vapour at concentrations ranging from 0.39 mg/m3 to 11.2 mg/m3
have been studied. Exposure durations ranged from 5 days to as long
as 52 weeks. In general, body weight gain reduction, decrement of
pulmonary function, and pathological changes in nose, upper airways,
and lungs have been documented in most species exposed to acrolein
concentrations of 1.6 mg/m3 or more. Pathological changes include
inflammation, metaplasia, and hyperplasia of the respiratory tract.
Significant mortality has been observed following repeated exposures
to acrolein vapour at concentrations above 9.07 mg/m3.
7.2.3 Repeated intraperitoneal exposure
Groups of ten intact or adrenalectomized NMRI mice were
injected intraperitoneally with saline or acrolein in water at daily
doses of 4 to 16 mg/kg body weight for 1 to 6 days. One week after
the last injection the mice were killed for autopsy.
Clinical signs of toxicity were hunched posture, inactivity,
and ruffled fur. Total body weight and relative thymus and spleen
weights showed a dose-related reduction, while the adrenals showed
an increase in relative weight. Histologically, thymic necrosis and
splenic atrophy were the only changes observed. These changes were
absent in controls and in adrenalectomized mice. The levels of
reduced glutathione and the activity of glutathione S-transferase
in liver cytosol were unchanged, but the rate of glutathione
synthesis was increased. Repeated exposure to acrolein caused a
progressively less pronounced effect on mortality (Warholm et al.,
1984).
7.3 Biochemical effects and mechanisms of toxicity
7.3.1 Protein and non-protein sulfhydryl depletion
A dose-related non-protein sulfhydryl (reduced glutathione)
depletion was observed in the nasal respiratory mucosa of male
Fischer rats after nose-only exposure for 3 h to acrolein vapour at
concentrations of 0.23-11.4 mg/m3 (McNulty et al., 1984; Lam
et al., 1985). Depletion of glutathione in the liver was not
observed at these exposure levels (McNulty et al., 1984). The
glutathione depletion in the nasal mucosa appeared irreversible at
11.4 mg/m3 (McNulty et al., 1984). In female C3Hf/HeHa mice,
intraperitoneally exposed once to doses between 20 and 80 mg/kg body
weight and killed 2 h later, a dose-related decrease in liver
glutathione levels was observed. These doses are however extremely
high considering the fact that a dose of 4.5 mg/kg body weight was
lethal within 1.7 h (Gurtoo et al., 1981a).
A dose-related in vitro glutathione depletion has been
observed in human bronchial fibroblasts (Krokan et al., 1985),
human bronchial epithelial cells (Grafström et al., 1988), human
chronic myeloid leukemia cells (Crook et al., 1986b), and human
and rat phagocytic cells (Witz et al., 1987) from the lowest
acrolein concentration tested (56 µg/litre). The effect has also
been reported to occur in isolated rat hepatocytes (Zitting &
Heinonen, 1980; Dawson et al., 1984; Dore & Montaldo, 1984; Ku &
Billings, 1986) and in rat liver or lung microsomal suspensions
(Patel et al., 1984), the lowest-observed-effect level being
1400 µg/litre (Dawson et al., 1984). Ku & Billings (1986)
observed that both mitochondrial and cytosolic glutathione levels
were decreased.
As a result of acrolein exposure, there was a decrease in the
level of both membrane surface and soluble protein sulfhydryl groups
in in vitro human and rat phagocytic cells (Witz et al., 1987)
and a decrease in the level of soluble protein sulfhydryl compounds
in human bronchial epithelial cells (Grafström et al., 1988).
Acrolein has also been shown to cause a decrease in membrane surface
protein sulfhydryl groups in rat hepatocytes (Ku & Billings, 1986)
and to reduce the protein sulfhydryl content of liver and lung
microsomal preparations (Patel et al., 1984).
7.3.2 Inhibition of macromolecular synthesis
When partially hepatectomized Wistar rats were exposed
intraperitoneally to a single acrolein dose of 0.5, 1.6, 2.0, or
2.7 mg/kg body weight, a dose-related inhibition of the synthesis of
DNA and RNA was measured in liver and lung cells (Munsch &
Frayssinet, 1971).
Inhibition of DNA, RNA, and/or protein synthesis has been
observed in Escherichia coli (Kimes & Morris, 1971), the slime
mold Physarum polycephalum (Leuchtenberger et al., 1968), the
alga Dunaliella bioculata (Marano & Puiseux-Dao, 1982), and in
in vitro mammalian cells such as mouse kidney cells (Leuchtenberger
et al., 1968) and polyoma transformed Chinese hamster cells
(Alarcon, 1972). Acrolein was shown to inhibit RNA polymerase in
isolated rat liver nuclei (Moule & Frayssinet, 1971) and isolated
rat liver DNA polymerase (Munsch et al., 1973). The activity of
the latter enzyme is associated with at least one functional
sulfhydryl group, and preincubation of the enzyme with
2-mercaptoethanol protected against the inhibitory action of
acrolein. Since acrolein did not inhibit isolated Escherichia coli
polymerase I, devoid of sulfhydryl groups in its active centre,
Munsch et al. (1973) suggested that the inhibitory action of
acrolein is caused by a reaction with sulfhydryl groups.
7.3.3 Effects on microsomal oxidation
In in vitro studies, acrolein has been shown to convert rat
liver cytochrome P-450 to cytochrome P-420 and to inhibit rat liver
NADPH-cytochrome-c reductase (EC 1.6.2.4) in a time- and
concentration-related fashion (Marinello et al., 1978; Ivanetich
et al., 1978; Berrigan et al., 1980; Gurtoo et al., 1981b;
Marinello et al., 1981; Patel et al., 1984; Cooper et al.,
1987). A concomitant decrease occurred in the activity of several
monooxygenases: benzphetamine N-demethylase, aniline hydroxylase,
ethylmorphine N-demethylase (Patel et al., 1984), and
7-ethoxyresorufin O-deethylase (Cooper et al., 1987). The
lowest-observed-effect levels reported were 2 mg/litre for
inactivation of cytochrome P-450 (Gurtoo et al., 1981b) and
25 mg/litre for inhibition of NADPH-cytochrome- c reductase
(Marinello et al.,1981). It was also shown that the addition of
sulfhydryl-containing agents, such as cysteine, acetylcysteine,
glutathione, dithiothreitol, and semicarbazide, reduced the above
effects, suggesting that acrolein produces them by reacting with
sulfhydryl groups at the active sites.
7.3.4 Other biochemical effects
In vivo studies with Holtzman rats have shown that rat liver
alkaline phosphatase (EC 3.1.3.1) and tyrosine aminotransferase
(EC 2.6.1.5) activities are increased markedly after inhalation of
acrolein for 4 h at a concentration of 14.7 mg/m3 or after a
single intraperitoneal injection of acrolein in water at doses of
1.5-6 mg/kg body weight (Murphy et al., 1964; Murphy, 1965). The
increase in alkaline phosphatase activity following intraperitoneal
injection was shown to be dose related (Murphy, 1965). The effects
were reduced by prior adrenalectomy or hypophysectomy or by
pretreatment with protein synthesis inhibitors such as actinomycin
D, puromycin, and ethionine, suggesting that the irritant action of
acrolein stimulates the pituitary-adrenal system to release
glucocorticoids, which act to increase the synthesis of adaptive
liver enzymes (Murphy, 1965; Murphy & Porter, 1966). Increased
plasma and adrenal levels of corticosterone were measured in
Holtzman rats one hour after a single intraperitoneal injection
(3 mg/kg body weight) of acrolein in water (Szot & Murphy, 1971).
The hypersecretion of glucocorticoids could also explain the
observed increase in liver glycogen level following intraperitoneal
exposure to acrolein at a dose of 1.5 mg/kg body weight (Murphy &
Porter, 1966).
At a concentration of 5.6 mg/litre, acrolein produced an 80%
inhibition of the noradrenaline-induced oxygen consumption of
isolated hamster brown fat cells (Pettersson et al., 1980). In
addition, Zollner (1973) observed an acrolein-induced inhibition of
the respiration of intact rat liver mitochondria and found evidence
for an inhibition at three different sites: glutamate transport,
inorganic phosphorus transport, and the enzyme succinic
dehydrogenase (EC 1.3.5.1).
Several sulfhydryl-sensitive enzymes have been shown to be
inhibited by acrolein in vitro, e.g., rabbit muscle L-lactate
dehydrogenase (EC 1.1.1.27), yeast glucose-6-phosphate
dehydro-genase (EC 1.1.1.49), and yeast alcohol dehydrogenase
(EC 1.1.1.1) (Benedict & Stedman, 1969), porcine lung
15-hydroxyprosta-glandin dehydrogenase (EC 1.1.1.141) (Liu & Tai,
1985), rat liver or urothelium S-adenosyl-L-methionine-
DNA(cytosine-5)- methyltransferase (EC 2.1.1.37) (Cox et al.,
1988), and O6-methylguanine-DNA methyltransferase (EC 2.1.1.63)
in cultured human bronchial fibroblasts (Krokan et al, 1985). In
two of these studies glutathione was shown to afford protection
against inhibition of the enzyme (Liu & Tai, 1985; Cox et al.,
1988).
It has been suggested that the formation of a Schiff base
between acrolein and sensitive amine groups is responsible for the
observed inhibition in vitro of Salmonella typhimurium
deoxyribose-5-phosphate aldolase (EC 4.1.2.4) at a concentration of
approximately 14 mg/litre (Wilton, 1976) and human plasma
alpha1-proteinase inhibitor (Gan & Ansari, 1987).
Acrolein was shown to cause a concentration-dependent increase
in lipid peroxidation in isolated rat hepatocytes at levels that
also decreased glutathione concentrations (Zitting & Heinonen,
1980). Preincubation of washed rat liver microsomes with acrolein
abolished the protective effect of glutathione against
iron/ascorbate-induced lipid peroxidation (Haenen et al, 1988). The
authors claimed that the protective effect of glutathione was
mediated by vitamin E scavenging membrane lipid radicals. It was
suggested that acrolein was inhibiting a glutathione-dependent
reductase enzyme responsible for reducing vitamin E radicals back to
vitamin E.
7.4 Immunotoxicity and host resistance
Acrolein has been found to depress pulmonary host defenses in a
number of tests.
In female Swiss mice, exposed to measured concentrations of
1.1, 6.9, and 14.2 mg/m3 for 8 h, a concentration-related increase
in the survival of Staphylococcus aureus was seen at levels of
6.9 mg/m3 or more (Astry & Jakab, 1983). A concentration- and
time-related increase in the survival of S. aureus and Proteus
mirabilis was found in male Swiss CD-1 mice exposed to measured
concentrations of 2.3 to 4.6 mg/m3 for 24 h. When the mice were
also infected with Sendai virus, intrapulmonary bacterial death was
further suppressed (Jakab, 1977). An increased survival of
Klebsiella pneumoniae, but no increased mortality from pneumonia
following challenges with Streptococcus zooepidemicus, was
observed in female CD-1 mice after exposure to acrolein at a
measured concentration of 0.23 mg/m3 for 3 h per day over 5 days
(Aranyi et al., 1986). In female CR/CD-1 mice, exposure to a
measured acrolein concentration of 4.6 mg/m3, for one period of
6 h or for 7 consecutive daily periods of 8 h, resulted in an
increased mortality from Streptococcus pyogenes and Salmonella
typhimurium, respectively, but not from influenza A virus (Campbell
et al., 1981).
Sherwood et al. (1986) exposed groups of 33 male
Sprague-Dawley rats to acrolein vapour at analysed concentrations of
0.39, 2.45 or 6.82 mg/m3 for 3 weeks (6 h per day and 5 days per
week). The relative pulmonary bactericidal activity to
K. pneumoniae was not affected nor was the number of alveolar
cells. However, the number of peritoneal macrophages was decreased
at concentrations of 2.45 mg/m3 or more, and alveolar and
peritoneal macrophages had altered phagocytic and enzymic patterns
at > 0.39 mg/m3.
When SPF-OFA rats were exposed continuously to a measured
acrolein concentration of 1.26 mg/m3 for 18 days, they exhibited
an increased mortality from an infection by Salmonella enteritidis.
However, no such effect was observed following 63 days of exposure
(Bouley et al., 1975).
Acrolein has been shown to inhibit in vitro protein synthesis
(Leffingwell & Low, 1979), and phagocytosis and ATPase (EC
3.6.1.37-38) activity (Low et al., 1977) in rabbit pulmonary
alveolar macrophages. Inhibition of a graft-versus-host reaction in
rats was found after Wistar rat spleen cells were incubated
in vitro with acrolein and injected into all four feet of hybrid
F1 rats. In addition, a decreased mitogen response of human
peripheral lymphocytes was recorded (Whitehouse et al., 1974).
Acrolein was also found to inhibit in vitro chemotaxis of human
polymorphonuclear leucocytes (Bridges et al., 1977).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Two in vivo exposure studies have been reported. In one, 3
male and 21 female SPF-OFA rats were exposed continuously to
acrolein vapour at a measured concentration of 1.26 mg/m3 for 25
days and allowed to mate on day 4. It should be noted that the
exposure period did not cover the complete spermatogenic period of
60 days. The number of pregnant animals and the number and mean
weight of the fetuses were unaffected in comparison to the control
rats (Bouley et al., 1985). In the second study, groups of 12 to
16 New Zealand rabbits were injected (into the ear vein) with a
solution of acrolein in saline (3, 4.5 or 6 mg/kg body weight) on
the 9th day of gestation. At 4.5 and 6 mg/kg body weight, maternal
toxicity was indicated by the death of 3 and 6 dams, respectively,
and embryotoxicity by a dose-related increase in resorptions which
was significant at 6 mg/kg body weight. It was also reported that
the number of malformed and retarded fetuses increased in a
dose-related manner, although the increases were statistically
non-significant. No effects on maternal toxicity, embryotoxicity or
fetuses were noted at 3 mg/kg body weight (Claussen et al., 1980).
A clear effect on the development of the embryo in vivo was
observed only when acrolein was administered close to the target
site by intra-amniotic injection. Using groups of 12 to 19 pregnant
New Zealand rabbits, 0, 10, 20, or 40 µl of a 0.84% solution of
acrolein in saline was injected into the amnion of all embryos in
one of the uterine horns on the 9th day of gestation. The embryos in
the other uterine horn received saline only and served as controls.
The dams were killed on day 28 of gestation. There was a
dose-related increase in the rate of resorptions and malformations,
significant at doses of 20 µl or more per embryo. Malformations
included deformed and asymmetric vertebrae, spina bifida, deformed
and fused ribs, and lack or fusion of sternum segments. No effect
was observed on the number of implantations and fetuses or on fetal
growth (Claussen et al., 1980). A similar study was carried out
on pregnant Sprague-Dawley rats injected with acrolein doses of 0,
0.1, 1.0, 2.5, 5.0, 10.0 or 100 µg per fetus in 10 ml of saline on
the 13th day of pregnancy. The dams were killed on day 20 of
gestation. A dose-related increase in the percentage of dead and
resorbed fetuses per litter was observed at all dose levels. The
total number of litters at each dose level varied from 4 to 18. The
percentage of malformed fetuses per litter also was increased in a
dose-related manner at doses of up to 5 µg per fetus. The increase
was significant only up to this dose level, probably because at
higher doses there were few surviving fetuses. Treatment-related
effects included oedema, micrognathia, hindlimb and forelimb
defects, and hydrocephaly (Slott & Hales, 1985). These results
confirmed the findings of an earlier, identical test using dose
levels of 0.1, 10, and 100 µg per fetus (Hales, 1982).
Acrolein was also shown to be embryotoxic and teratogenic in
the rat whole embryo culture system. As with embryos exposed in
vivo, the concentration range for teratogenicity was very narrow
(Slott & Hales, 1986). Schmid et al. (1981) and Mirkes et al.
(1984) observed embryotoxicity but no teratogenicity in the same
test system, this being probably the result of the different test
conditions used (Slott & Hales, 1986). Depletion of glutathione by
buthionine sulfoximine enhanced the embryotoxicity and
teratogenicity of acrolein in the in vitro studies of Slott &
Hales (1987a), whereas exogenous glutathione afforded protection
against these effects (Slott & Hales, 1987b).
In a mouse limb bud culture system, acrolein induced impairment
of limb bud differentiation, indicative of a teratogenic action
(Stahlmann et al., 1985). When acrolein was injected into chicken
eggs, embryotoxic and teratogenic effects were observed (Kankaanpaa
et al., 1979; Korhonen et al., 1983; Chhibber & Gilani, 1986).
In summary, acrolein can induce teratogenic and embryotoxic
effects if administered directly to the embryos or fetuses.
However, the fact that no effect was found in rabbits injected
intravenously with 3 mg/kg suggests that neither skin contact nor
inhalation of acrolein is likely to affect the developing embryo.
7.6 Mutagenicity and related end-points
7.6.1 DNA damage
In vitro studies have revealed interactions between acrolein
and DNA and RNA (Munsch et al., 1974b; section 6.2.1). Acrolein
has also been found to react with purine and pyrimidine bases or
intact DNA in vitro, and several adducts have been identified
(Descroix, 1972; Hemminki et al., 1980; Lutz et al., 1982; Chung
et al., 1984; Shapiro et al., 1986; section 6.2.2.2). Cyclic
deoxyguanosine DNA adducts were formed in a dose-dependent fashion
in acrolein-exposed Salmonella typhimurium TA100 and TA104. This
adduct formation correlated with the induction of reverse mutations
in these strains (section 7.6.2; Foiles et al., 1989).
No data on the formation of DNA adducts following exposure of
animals to acrolein are available.
Incubation of Fischer-344 rat nasal mucosal homogenate with
acrolein resulted in a concentration-dependent increase in
DNA-protein cross-linking, which was not observed following
inhalation exposure of rats to acrolein at a concentration of
4.6 mg/m3 for 6 h (Lam et al., 1985). According to the authors
this could be explained by the preferential reaction of acrolein
with sulfhydryl groups. DNA-protein cross-linking and single strand
breaks were observed in vitro in human bronchial fibroblasts at
cytotoxic concentrations of 1.7 mg/litre or more (Grafström et al.,
1986, 1988), and there was indirect evidence for some formation of
DNA interstrand cross-linking (Grafström et al., 1988). No
DNA-protein or DNA interstrand cross-linking was induced by acrolein
in mouse L1210 leukemia cells at cytotoxic levels that produced
single strand breaks and/or alkali-labile sites in these cells
(Erickson et al., 1980) or in human chronic myeloid leukemia cells
(Crook et al., 1986a). In non-mammalian assays, Fleer & Brendel
(1982) did not find DNA interstrand cross-linking or single strand
breaks in MB1072-2B yeast cells and Kubinski et al. (1981)
observed DNA-cell binding in Escherichia coli in the presence of a
rat liver S9 fraction. These studies demonstrate that effects on
DNA occur only at cytotoxic concentrations of acrolein.
Results of DNA repair tests are not available. Acrolein has
been demonstrated to inhibit O6-methylguanine-DNA
methyltransferase (EC 2.1.1.63.; section 7.3.4) and, therefore, can
be expected to reduce the capacity for repair of O6-guanine
alkylations in DNA (Krokan et al., 1985).
7.6.2 Mutation and chromosomal effects
The results of tests for the induction of gene mutations and
chromosome damage by acrolein are summarized in Table 10.
In point mutation assays with Salmonella typhimurium, the
positive or equivocal responses obtained were all observed within a
narrow dose range of up to 10-56 µg per plate, higher doses being
toxic. Clearly positive, dose-related increases in revertant
colonies per plate at 2-5 times the background rate were observed in
the absence of metabolic activation only in TA100 (Lutz et al.,
1982; Foiles et al., 1989; Hoffman et al., 1989), TA104 (Marnett
et al., 1985; Foiles et al., 1989; Hoffman et al., 1989), and
TA98 (Lijinsky & Andrews, 1980). Khudoley et al. (1986) reported
positive results in strains TA98 and TA100 without specifying dose
levels or revertant rates. Some evidence for indirect mutagenicity
was found in strains TA1535 (Hales, 1982) and TA100 (Haworth
et al., 1983), the slight increase in TA100 revertants being dose
related. However, negative results, both with and without metabolic
activation, have also been obtained in these strains. Some of these
negative results were clearly related to the incubation conditions,
which were probably highly toxic, e.g., those obtained in spot tests
(Andersen et al., 1972; Florin et al., 1980). In Salmonella
typhimurium TA100 and TA104, strains that show a clear mutagenic
response to acrolein, DNA-acrolein adducts have also been identified
(section 7.6.1).
Acrolein did not induce sex-linked recessive lethality in
Drosophila melanogaster adults (Zimmering et al., 1985), but
induced a 12-fold increase in sex-linked recessive lethality in
hatching eggs and larva at an exposure level that was not reported
but caused over 75% larval death (Rapoport, 1948). In the latter
test, treatment of adults was reported to be less effective.
Three cytogenetic tests have been carried out with acrolein,
two in Chinese hamster ovary cells (Au et al., 1980; Galloway
et al., 1987) and one in human lymphocytes (Wilmer et al.,
1986). Acrolein was shown to induce sister chromatid exchanges in
the absence of a metabolic activating system in all three studies.
The lowest effective concentration was 56 µg/litre (Galloway
et al., 1987). No increase in chromosome aberrations was reported
in one study (Galloway et al., 1987), while chromosome breakage
was reported in another study at cytotoxic concentrations (Au
et al., 1980).
Three properties of acrolein make it difficult to test for
mutagenicity: its high cytotoxicity, which prevents the expression
of any mutagenic activity, and its high reactivity and volatility,
which prevent it reaching the target sites. However, acrolein can
be considered to be a weak mutagen in some bacterial and fungal test
systems in the absence of metabolic activating systems and to
induce sister chromatid exchange in cultured mammalian cells.
7.6.3 Cell transformation
Acrolein (0.4 µg/ml) has been found not to exhibit transforming
potential in C3H/10T1/2 cells but to initiate the process of
transformation. The latter was measured by exposing cultures to
acrolein for 24 h and, subsequently, to a phorbol ester for 6 weeks
(Abernethy et al., 1983).
Table 10. Tests for gene mutation and chromosomal damage by acrolein
Test description Organism Species/strain/cell type Resulta Reference
Gene mutations
Reverse mutations bacteria Salmonella typhimurium TA1535 ±(+S9) Hales (1982)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Lijinsky & Andrews (1980)
Salmonella typhimurium TA100 +(-S9) Lutz et al. (1982); Khudoley et al., 1986;
Foiles et al. (1989); Hoffman et al. (1988)
±(+S9) Haworth et al. (1983)
- Florin et al. (1980); Loquet et al. (1981);
Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium TA104 +(-S9) Marnett et al. (1985); Foiles et al. (1989);
Hoffman et al. (1989)
Salmonella typhimurium TA102 - Marnett et al. (1985)
Salmonella typhimurium TA98 +(-S9) Lijinsky & Andrews (1980); Khudoley et al. (1986)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Basu & Marnett (1984)
Salmonella typhimurium TA1537 - Florin et al. (1980); Haworth et al. (1983);
Lijinsky & Andrews (1980)
Salmonella typhimurium TA1538 - Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium, 8 strains - Andersen et al. (1972)
Escherichia coli 343/113 - Ellenberger & Mohn (1976, 1977)b
Escherichia coli WP2 uvrA ±(-S9) Hemminki et al. (1980)
yeast Saccharomyces cerevisiae S211, S138 - Izard (1973)
Forward mutations yeast Saccharomyces cerevisiae N123 +(-S9) Izard (1973)c
Table 10 (contd).
Test description Organism Species/strain/cell type Resulta Reference
Forward mutations man normal fibroblasts -(-S9) Curren et al. (1988)
DNA-repair-deficient fibroblasts +(-S9) Curren et al. (1988)
hamster V79 cells +(-S9) Smith et al. (1990)
Sex-linked lethal insect Drosophila melanogaster, hatching + Rapoport (1948)d
mutations eggs and young larva
Drosophila melanogaster, adults - Zimmering et al. (1985)e
Chromosomal damage
Aberrations hamster ovary cells in vitro ±(+S9) Au et al. (1980)
±(-S9) Galloway et al. (1987)
Sister chromatid hamster ovary cells in vitro +(-S9) Au et al. (1980)
exchanges +(-S9) Galloway et al. (1987)
man lymphocytes in vitro +(-S9) Wilmer et al. (1986)
Dominant lethal mouse germ cells - Epstein & Shafner (1968)
mutations
(ip exposure)
a + = >2 x background rate or statistically significant (P < 0.05); ± = equivocal; - = negative.
b Details for this test were not reported.
c Plate test for petite mutations (production of a respiratory-deficient mutant).
d Doses were not reported. Treatment of adults was found to be less effective.
e Exposure via feeding solution or via injection.
7.7 Carcinogenicity
7.7.1 Inhalation exposure
Inhalation experiments of appropriate duration specifically
designed to assess the carcinogenicity of acrolein vapour have not
been conducted.
An 81-week study (52 weeks of acrolein exposure at 9.2 mg/m3
followed by 29 weeks without exposure) on groups of 36 Syrian golden
hamsters of both sexes is described in section 7.2.2. The effects
of treatment included a persistent and statistically significant
reduction in body weight in females, an increased relative brain
weight in males and females at 52 weeks, and an increased relative
lung weight in females at 52 weeks. Apart from one small tracheal
papilloma in an acrolein-exposed female, no respiratory tract
tumours were observed in control or treated hamsters (Feron &
Kruysse, 1977). In order to elucidate a possible co-carcinogenic
action of acrolein, Feron & Kruysse (1977) also exposed groups of 30
Syrian golden hamsters of both sexes to measured acrolein vapour
concentrations of 0 or 9.2 mg/m3, 7 h per day and 5 days per week
for 52 weeks, and, for the same period, either weekly to an
intratracheal dose of benzo [a]pyrene or once every 3 weeks to a
subcutaneous dose of diethylnitrosamine. Total dose levels were 18.2
or 36.4 mg benzo [a]pyrene and 2.1 µl diethylnitrosamine. Survivors
were killed at week 81, and all hamsters were subjected to
postmortem examination. The mortality rate in the groups treated
with benzo [a]pyrene was slightly higher than in other groups. The
incidence of benzo [a]pyrene-induced respiratory tract tumours was
slightly (but statistically insignificantly) higher in females also
exposed to acrolein vapour. In these females, at the higher dose
level of benzo [a]pyrene, respiratory tract tumours occurred
earlier and the number of malignant tumours was slightly increased.
Taken together, these observations might suggest an enhancing effect
of acrolein on benzo [a]pyrene carcinogenesis in the respiratory
tract, but the effect cannot be considered proven.
In a study by Le Bouffant et al. (1980), rats, 20 animals per
group, were exposed to 18.3 mg/m3, 1 h/day and 5 days/week, for 10
or 18 months. No tumours or metaplasias were found.
7.7.2 Oral exposure
In a study by Lijinsky & Reuber (1987), groups of 20
Fischer-344 rats of both sexes were exposed to weekly prepared
acrolein of unspecified purity in drinking-water. Each cage of four
rats received 80 ml of acrolein solutions at concentrations of 100
or 250 mg/litre for 124 weeks (males only) or 625 mg/litre for 104
weeks (both sexes) for 5 days per week (this was estimated by the
Task Group to be equivalent to approximately 5, 12.5, and 50 mg/kg
body weight per day, respectively). Total doses were 1200, 3100,
and 6500 mg per rat, respectively. Controls were left untreated.
Survivors were killed at week 123-132 and all rats were subjected to
postmortem examination. The mean survival time was about 120 weeks
for experimental and control groups. There was a marginal, but not
statistically significant, increase in the incidence of adrenal
cortical adenomas (5/20) in female rats at 625 mg/litre, compared to
concurrent controls (1/20), and a decrease in the incidence of
pituitary neoplasms in both sexes at 625 mg/litre. In addition, 2
of 20 females given 625 mg/litre had hyperplastic nodules of the
adrenal cortex. The authors cited historic control values for
adrenal cortical adenomas or carcinomas in female Fischer-344 rats
from other laboratories as 1.3% at 26 months of age and 4.8% in a
lifespan study. Because of limited numbers of animals used and
concerns regarding the purity and stability of acrolein in the dosed
drinking-water, the authors of this study did not consider it to be
a definitive carcinogenicity bioassay. In addition, the Task Group
considered the historical control values quoted by the authors to be
of limited use in evaluating the importance of the tumour incidence
found in this study.
Acrolein appeared to be too toxic to Syrian golden hamsters
following oral exposure by gavage in corn oil to conduct an
effective carcinogenicity study (Lijinsky & Reuber, 1987).
7.7.3 Skin exposure
In a study by Salaman & Roe (1956), a group of 15 S strain mice
of unspecified sex and age received weekly doses of 0.5% acrolein in
acetone for 10 weeks. The total dose was 12.6 mg per rat, although
the purity of the acrolein was not reported. The control group
comprised 20 mice. From day 25 after the first acrolein treatment,
the mice received once per week a skin application of 0.17% croton
oil (0.085% in weeks 2 and 3) for 18 weeks. Croton oil and acrolein
were applied alternately at 3 or 4 day intervals. At the end of
treatment, the mortality rate and the incidence of skin papillomata
were similar to those of the controls treated only with croton oil.
However, this study must be considered inadequate because of the
limited number of animals used and the short duration of the
experiment.
7.8 Interacting agents
Free sulfhydryl-containing compounds have been found to give
protection against the adverse effects of acrolein in vitro, e.g.,
the inhibition of enzymes involved in macromolecular synthesis
(Munsch et al., 1973), liver microsomal cytochrome P-450s
(Marinello et al., 1978; Berrigan et al., 1980; Gurtoo et al.,
1981b, Patel et al., 1984; Cooper et al., 1987), and several
other sulfhydryl-sensitive enzymes (Liu & Tai, 1985; Cox et al.,
1988), the adverse effects on rabbit alveolar macrophages (Low et
al., 1977; Leffingwell & Low, 1979), and the impairment of mouse
limb bud differentiation (Stahlmann et al., 1985). Free
sulfhydryl-containing agents protected against the acute lethal
effects of acrolein in Charles River rats (Sprince et al., 1979)
and in DBA/2J mice (Gurtoo et al., 1981a).
When Swiss-Webster mice were exposed to acrolein-formaldehyde
mixtures, the percentage decrease in respiratory rate was found to
be less than the sum of the percentage decreases due to each
compound alone (Kane & Alarie,1978). In acrolein-exposed Fischer-344
rats, pretreatment with formaldehyde resulted in a lower percentage
decrease in respiratory rate compared to non-pretreated rats (Babiuk
et al., 1985). It was suggested in both investigations that
acrolein and formaldehyde competed for the same receptor
(competitive agonism). In a comparable experiment, the maximum
percentage decrease in the respiratory rate of Swiss-Webster mice
exposed to a mixture of acrolein and sulfur dioxide was lower than
that of acrolein alone. This antagonistic effect was thought to be
caused by a chemical reaction in the air phase between the two
compounds, which reduced the effective concentrations (Kane &
Alarie, 1979).
In Fischer-344 rats exposed to formaldehyde vapour
(7.4 mg/m3) once for 6 h, co-exposure to acrolein vapour
(4.6 mg/m3) resulted in a higher increase in DNA-protein
cross-linking than was observed with formaldehyde alone. Acrolein
alone did not increase DNA-protein cross-linking in this experiment
(Lam et al., 1985).
In a study by Hales et al. (1988), anaesthetized,
artificially ventilated mongrel dogs were exposed to acrolein or
hydrochloric acid with added synthetic smoke composed of carbon
particles for 10 min. The dogs were exposed to smoke with or
without analytically determined acrolein concentrations of <
458 mg/m3, 458-687 mg/m3 or > 687 mg/m3. Smoke with
acrolein, but not smoke with hydrochloric acid, produced
non-cardiogenic, peribronchiolar pulmonary oedema in a
concentration- and time-related fashion. Both acrolein and
hydrochloric acid produced airway damage consisting of mucosal
degeneration and desquamation and inflammatory cell infiltration.
Acrolein at levels above 458 mg/m3 also caused fibrin deposition
in the alveolar spaces that juxtaposed injured bronchioles.
8. EFFECTS ON HUMANS
8.1 Single exposure
8.1.1 Poisoning incidents
One man was exposed dermally and by inhalation when acrolein
was sprayed into his face following an accident in a chemical plant.
Immediately, his face and eyelids were burnt. Within 20 h he was
hospitalized with fever, cough, frothy sputum, cyanosis, and acute
respiratory failure. Two months after the accident, the opening of
the right bronchus was obstructed and the upper trachea showed
slight oedema with haemorrhagic spots. At 18 months he had developed
chronic bronchitis and emphysema, which might have been a sequel of
the accidental exposure (Champeix et al., 1966).
One case of attempted oral suicidal intoxication has been
reported. The man swallowed approximately 1.5 g of acrolein in a
glass of orange juice. Blood was found in his stomach and the number
of red and white blood cells was increased. Gastroscopic
examination showed shrinkage of the stomach and a massive chronic
gastritis with erosions and ulceration. Further examination of the
stomach revealed regenerating mucous membranes, few mucous glands,
granulation and scarring of the serosa, shrinkage and stenosis of
the pylorus, lymphadenitis, and haemosiderin deposition in lymph
nodes. The man was successfully treated by gastrectomy (Schielke,
1987).
Two cases of suspected exposure to acrolein have been reported.
The death of two young boys who inhaled smoke from an overheated
frier for approximately 2 h was thought to be related to acrolein
exposure, although other chemicals might also have been involved.
One of the boys was found dead, while the other suffered from acute
respiratory failure. Following oxygen therapy, the second boy died
due to asphyxia. At autopsy a massive cellular desquamation of the
bronchial lining was observed. The tracheal and bronchial lumina
were filled with debris and the lungs showed multiple infarcts
(Gosselin et al., 1979).
Four female factory workers operating a machine for cutting and
sealing polyethylene bags and a fifth sitting next to the machine
complained of a burning sensation in the eyes, a feeling of dryness
and irritation in the nose and throat, and itching and irritation of
the skin of the face, neck and forearms. These complaints were
related to the smoke developed. The presence of formaldehyde and
"acrolein and/or other aldehydes" in the smoke was suspected and
confirmed. During heavy smoke exposure, itching eruptions developed
on exposed skin. Drowsiness and headache was also experienced. All
symptoms were reversible (Hovding, 1969).
8.1.2 Controlled experiments
8.1.2.1 Vapour exposure
Several studies with volunteers have been conducted with the
object of establing thresholds for odour perception and recognition
and for effects on the eyes, nose, respiratory tract, and nervous
system. The results of these studies are summarized in Table 11. The
exposure period was up to 60 min. In most cases the concentration
of acrolein was determined colorimetrically, although a few reports
did not include a description of the analytical method (Plotnikova,
1957; Sinkuvene, 1970; Harada, 1977). Sinkuvene (1970) reported the
threshold for changes in the electrical activity of the brain
cortex, as measured by electro-encephalography, to be 0.05 mg/m3.
However, this result cannot be evaluated since experimental data
were not provided. The odour perception threshold for sensitive
people was 0.07 mg/m3.
In studies by Weber-Tschopp et al. (1977), groups of human
volunteer students of both sexes were exposed either for 60 min to
acrolein at a concentration of 0.69 mg/m3 or to gradually
increasing acrolein concentrations from 0 up to 1.37 mg/m3 over
35 min followed by a 5-min exposure to 1.37 mg/m3. In further
experiments with side-stream cigarette smoke instead of pure
acrolein vapour, it was noted that the effects of pure acrolein
vapour were small compared to those produced by side-stream smoke
with the same acrolein vapour concentration. It was concluded that
acrolein was only to a minor extent responsible for the effects
observed (Weber-Tschopp et al., 1976). It must be noted, however,
that a significant part of the acrolein in side-stream cigarette
smoke may be associated with particulate matter (Ayer & Yeager,
1982) and would not have been measured. This may have resulted in an
underestimation of the acrolein concentration in the smoke. Many of
the studies considered in this section are old and the analytical
techniques are often poorly described; the absolute figures reported
may, therefore, be suspect.
8.1.2.2 Dermal exposure
In an investigation into irritant dermatitis possibly caused by
contaminants present in diallylglycol carbonate monomer, patch tests
were conducted with acrolein in ethanol at concentrations of 0.01,
0.1, 1, and 10% on groups of 8, 10, 48, and 20 volunteers,
respectively. At 1%, six positive reactions were recorded, four
cases of serious oedema with bullae and two of erythema. At 10%,
all subjects showed positive reactions with bullae, necrosis,
inflammatory cell infiltrate, and papillary oedema (Lacroix et al.,
1976).
Table 11. Thresholds for acute effects of acrolein on humans
Concentration Exposure Effect Reference
(mg/m3) period (min)
0.05 changes in electrical activity of brain cortex Sinkuvene (1970)
0.07 odour perception by most sensitive individuals Sinkuvene (1970)
0.13 5 no or medium subjective eye irritation Darley et al. (1960)a
0.21 5 increased incidence of subjective eye irritation Weber-Tschopp et al. (1977)b
0.34 10 increased incidence of subjective nasal irritation Weber-Tschopp et al. (1977)b
0.34 30 time-related increase in eye-blink frequency van Eick (1977)a
0.39 10 increased incidence of subjective annoyance Weber-Tschopp et al. (1977)b
0.48 odour recognition Leonardos et al. (1969)
0.59 15 increase in eye-blink frequency Weber-Tschopp et al. (1977)b
0.6 10 increase in sensitivity to light Plotnikova (1957)
0.69 40 decrease in respiratory rate; increased incidence of Weber-Tschopp et al. (1977)c
subjective general irritation of eyes, nose, and neck
0.69 10 increase in eye-blink frequency Weber-Tschopp et al. (1977)c
1 3 slight subjective conjunctival irritation Plotnikova (1957)
1 3 stinging sensation in nose Plotnikova (1957)
1.1 5 increased incidence of subjective eye irritation Stephens et al. (1961)a
1.1 5 increase in tear volume, pH,and lysozyme activity Harada (1977)
1.37 35 decrease in respiratory rate Weber-Tschopp et al. (1977)b
1.5 3 pneumographic changes in rhythm and amplitude of Plotnikova (1957)
respiratory movements
Table 11 (contd).
Concentration Exposure Effect Reference
(mg/m3) period (min)
1.7 3 reflex action on optical chronaxy Plotnikova (1957)
1.88 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 20 seconds
2.80 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 5 seconds
3 5 medium to severe subjective eye irritation Darley et al. (1960)a
4 2-3 acute subjective conjunctival and nasal irritation; Plotnikova (1957)
painful sensation in nasopharyngeal region
a exposure of eyes only
b exposure to gradually increasing concentrations up to 1.37 mg/m3
c exposure to a fixed concentration
8.2 Long-term exposure
No data are available on the long-term exposure of humans to
acrolein.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
A summary of acute aquatic toxicity data is presented in
Table 12. In most of these studies, the amount of acrolein added was
reported but the concentrations present were not measured. In these
cases, the actual concentrations may have been lower than the
nominal ones in view of the volatility of the substance and its
hydration rate (see section 4.2).
One of the studies in Table 12 (Lorz et al., 1979) is a
comparatively detailed examination of the acute toxicity of acrolein
to Coho salmon. Within 144 h of exposure to 0.075 mg/litre or more,
all fish died. In surviving fish the activity of gill
Na+,K+-ATPase (EC 3.6.1.37) and the tolerance to subsequent
sea-water exposure were not affected at concentrations up to
0.05 mg/litre. A histological examination of the gills, kidneys, and
liver at 0, 0.05, and 0.1 mg/litre revealed concentration-dependent
adverse effects.
A 3-generation 64-day test with the crustacean Daphnia magna
was conducted in a flow-through open system with well water at
20 °C, a pH between 7.0 and 7.3, a dissolved oxygen concentration of
7.5 mg/litre, and a water hardness of 35 mg CaCO3/litre. The
highest concentration that did not result in mortality was
0.0169 mg/litre (acrolein concentrations were measured in this
study). Survival was reduced at levels of 0.0336 mg/litre or more,
but the number of young per female was not affected even at the
highest concentration tested, 0.0427 mg/litre (Macek et al.,
1976).
Macek et al. (1976) also reported on a 60-day test with
fathead minnow (Pimephales promelas) in a flow-through open system
with well water at 25 °C, a pH between 6.6 and 6.8, a dissolved
oxygen concentration of 8.2 mg/litre, and a water hardness of 32 mg
CaCO3/litre. The highest concentration without adverse effects
was 0.0114 mg/litre (acrolein concentrations were measured in this
study). At 0.0417 mg/litre, there was increased mortality among
offspring. No adverse effects were found on survival and mortality
of adults, number of spawnings and number of eggs per female, number
of eggs per spawn, length of offspring, or hatchability.
It is clear from Table 12 why acrolein is also used as an
algicide, slimicide, and molluscicide.
Table 12. Acute aquatic toxicity of acrolein
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Fresh water
alga Enteromorpha 25 stat, closed 24 h 50% inhibition 1.8c Fritz-
intestinalis of photosynthesis Sheridan
(1982)
alga Cladophora 25 stat, closed 24 h 50% inhibition 1.00c
glomerata of photosynthesis
alga Anabaena 25 stat, closed 24 h 50% inhibition 0.69c
of photosynthesis
bacterium Proteus 37 7.0 stat, closed 2 h 50% growth 0.02 Brown &
vulgaris reduction Fowler
(1967)
bacterium Pseudomonas 25 7.0 stat, closed 16 h TT 0.21 Bringmann
putida & Kuhn
(1977)
protozoan Entosiphon 25 6.9 stat, closed 72 h TT 0.85 Bringmann
sulcatum (1978)
protozoan Chilomonas 20 6.9 stat, closed 48 h TT 1.7 Bringmann
paramecium et al.
(1980)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
protozoan Uronema 25 6.8 stat, closed 20 h TT 0.44 Bringmann
parduczi & Kuhn
(1980)
mollusc snail 21-25 flow, open 48 h 99-100% 20-25 Unrau et
(Bulinus mortality al. (1965)d
truncatus)
mollusc snail stat, open 3 h 100% mortality 10 Ferguson
(Biomphalaria 24 h 10% mortality 1.25 et al.
glabrata), eggs (1961)
mollusc snail (Biomphalaria stat, open 24 h 98% mortality 10 Ferguson
glabrata), adults 24 h 35% mortality 2.5 et al.
(1961)
crustacean water flea 20 7.0- 7.5 35 stat, open 48 h LC50 0.057 Macek et
(Daphnia magna) 7.3 al. (1976)
crustacean water flea 22 7.0- 154 stat, closed 48 h EC50f 0.093 Randall &
(Daphnia magna) 8.2 Knopp (1980)
crustacean water flea 22 7.4- 6-9 173 stat, closed 48 h LC50 0.083 LeBlanc
(Daphnia magna) 9.4 (1980)
fish harlequin fish 20 7.2 20 flow, open 48 h LC50 0.06 Alabaster
(Rasbora (1969)
heteromorpha)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
fish fathead minnow 25 6.6- 8.2 32 flow, open 144 h LC50 0.084 Macek et
(Pimephales 6.8 al.
promelas) (1976)e
fish golden orfe 20 7-8 > 5 200-300 stat 48 h LC50 0.25 & Juhnke &
(Leuciscus idus 2.5 Ludemann
melanotus) (1978)
fish goldfish 20 6-8 > 4 108 stat, open 24 h LC50 < 0.08 Bridie
(Carassius et al.
auratus) (1979)c e
fish Bluegill 21-23 6.5- 10- 32-48 stat, closed 96 h LC50 0.09 Buccafusco
sunfish 7.9 0.3 et al.
(Leopomis (1981)
macrochirus)
fish Coho salmon 10 7.4- > 10 100 stat, open 96 h LC50 0.068 Lorz et al.
(Oncorhynchus 7.6 (1979)g
kisutch)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Marine
mollusc common mussel 15 stat, closed 6 h 40% mortality 0.6 Rijstenbil
(Mytilus edulis) 6 h 70% mortality 1.0 & van Galen
8 h 70% detached 0.57 (1981)e h
mussels
a static or flow-through test, open or closed system
b TT = toxic threshold for inhibition of cell multiplication
c exposure to Magnacide-H (92% acrolein, 8% inert ingredients)
d field study, resurgence of snails was delayed by 8 to 12 months
e analysis for acrolein was reported
f the effect was complete immobilization
g static-renewal test
h static-renewal test (1.6% salinity)
9.2 Terrestrial organisms
9.2.1 Birds
The LD50 for the adult starling (Sturnus vulgaris) was
reported to be > 100 mg/kg body weight. The birds were observed for
7 days after dosing, but only two birds per dose were tested
(Schafer, 1972).
9.2.2 Plants
Acrolein is used as biocide, particularly to control aquatic
plants such as Elodea canadensis, Vallisneria spiralis
(ribbonweed), and Potamogeton tricarinatus (floating pondweed).
In Australia, a maximum concentration of about 15 mg/litre over a
period not exceeding a few hours has been imposed. In the USA,
acrolein is injected into larger channels over longer periods at low
concentrations (approximately 0.1 mg/litre over 48 h) (Bowmer &
Sainty, 1977). It has been shown that the dosage of acrolein
required for control, as defined by the product of time and
concentration required for 80% reduction in biomass, is independent
of the separate values of concentration and time, provided that the
concentration exceeds 0.1 mg/litre and the dosage exceeds 2 mg/litre
per h. In tank experiments, the minimum dosages required for 80%
control of ribbonweed and floating pondweed were about 4 and
26 mg/litre per h, respectively (Bowmer & Sainty, 1977). The
effective dosage (> 80% kill) for Elodea canadensis was 8 to
10 mg/litre per h (Van Overbeek et al., 1959; Bowmer & Smith,
1984). Sublethal concentrations of acrolein stimulated the growth
of Elodea (Bowmer & Smith, 1984).
Elongation of pollen tubes of lily seeds (Lilium longiflorum)
was inhibited completely after a 5-h exposure to acrolein vapour at
a measured concentration of 0.91 mg/m3, a temperature of 28 °C,
and a relative humidity of 60%. A 10% inhibition was found after
1 h (Masaru er al., 1976).
The nature and extent of adverse effects on various crops grown
in soil irrigated by acrolein-treated water have been investigated
in two studies. Acrolein concentrations varied between 15 and
50 mg/litre of supply water. Most furrow-irrigated crops, including
beans, clover, corn, and millet, did not show any damage.
Significant damage to foliage was observed in cotton at acrolein
concentrations of 25 mg/litre or more, but there was no evidence of
chronic or residual phytotoxicity. Slight damage to the foliage of
cucumbers and tomatoes was observed at 40 mg/litre. Vegetable
seedlings in contact with treated water were damaged even at the
lowest concentrations used (Unrau et al., 1965; Ferguson et al.,
1965).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Exposure of the general population to acrolein occurs mainly
via air. Exposure via water would only be significant in cases of
ingestion of, or skin contact with, acrolein deliberately applied as
a biocide to irrigation water. Oral exposure to acrolein may also
occur via alcoholic beverages or heated foodstuffs (chapters 3 and
5).
In urban areas, average levels of up to 15 µg/m3 and maximum
levels of up to 32 µg/m3 have been measured away from industrial
sources. Near industries and close to the exhaust pipes of vehicles,
engines, and combustion appliances, levels ten to one hundred times
higher may occur. Extremely high levels of acrolein in the mg/m3
range can be found as a result of fires (section 5.2.1).
Major indoor sources of acrolein are combustion appliances and
tobacco smoking (section 3.2.4). Levels of acrolein in smoke from
indoor open fires for cooking or heating purposes have not been
reported. Smoking one cigarette per m3 of room-space in 10-13 min
has been shown to lead to acrolein vapour concentrations of
450-840 µg/m3 (section 5.2.1). Recent occupational exposure levels
of acrolein in the air at sites of its production or processing are
not available. Workplace levels of over 1000 µg/m3 have been
reported in situations involving the heating of organic materials
(section 5.3).
In summary, the main source of exposure of the general
population to acrolein is via tobacco smoke. General environmental
pollution by vehicle exhaust and the smoke of burning organic
materials is the next most important source.
10.1.2 Health effects
Owing to the reactivity of acrolein, retention at the site of
entry into the body, usually the respiratory tract, is high
(section 6.1). Primary pathological findings are limited
principally to these sites (sections 7 and 8). Any acrolein absorbed
is liable to react directly with protein and non-protein sulfhydryl
groups or with primary and secondary amines such as those found in
proteins and nucleic acids (sections 6.2 and 7.3). Acrolein may also
be metabolized to mercapturic acids, acrylic acid, glycidaldehyde or
glyceraldehyde (section 6.3). Evidence for the last three
metabolites has only been obtained in vitro.
Acrolein is a cytotoxic agent (section 7.1.5) highly toxic to
experimental animals and man following acute exposure via different
routes (sections 7.1.1 and 8.1.1). The vapour is very irritating to
the eyes and the respiratory tract. Liquid acrolein is a corrosive
substance. The no-observed-adverse-effect level for irritant
dermatitis from ethanolic acrolein was found to be 0.1% (section
8.1.2.2). The odour perception threshold for the most sensitive
individuals is reported to be 0.07 mg/m3 (8.1.2.1). Experiments
with human volunteers show a lowest-observed-adverse-effect level of
0.13 mg/m3, at which level eyes may become irritated after 5 min.
In addition to irritation of the eyes, changes in respiratory tract
function are evident at or above 0.7 mg/m3 (40-min exposure)
(section 8.1.2.1). At higher concentrations, degeneration of the
respiratory epithelium and irritation of all exposed mucosa develop.
Oedematous changes in the tracheal and bronchial mucosa and
bronchial obstruction can be expected after very high exposure to
acrolein vapour (section 8.1).
There are no human toxicological data from long-term exposure
to acrolein. The toxicity from exposure to acrolein vapour has been
relatively well investigated in several animal studies for exposure
periods of up to 52 weeks (section 7.2). Both respiratory tract
function and histopathological effects have been observed at
0.5-0.8 mg/m3 (continuous exposure). Toxicological effects in the
respiratory tract have been documented in most animal species
exposed repeatedly to acrolein concentrations of 1.6-3.2 mg/m3 or
more, and mortality has occurred following exposure to
concentrations above 9 mg/m3. There is limited evidence that
acrolein can depress pulmonary host defenses in mice and rats.
Acrolein can induce teratogenic and embryotoxic effects if
administered directly into the amnion. However, the fact that no
effect was found in rabbits injected intravenously with 3 mg/kg
suggests that human exposure to acrolein is unlikely to affect the
developing embryo (section 7.5).
Acrolein has been shown to interact with DNA and RNA in vitro
and to inhibit their synthesis both in vivo and in vitro. In
vitro, it induces gene mutations in bacteria and fungi and sister
chromatid exchanges in mammalian cells (section 7.6). There is
inadequate evidence to allow the mutagenic potential in humans to be
assessed reliably.
One long-term drinking-water study with rats (130 weeks) and
two inhalation tests, one with hamsters (81 weeks) and the other
with rats (40 or 70 weeks), failed to demonstrate carcinogenic or
clear co-carcinogenic effects of acrolein (section 7.7). Due to the
shortcomings of the tests used, acrolein cannot be considered to
have been adequately tested for carcinogenicity and no conclusions
concerning its carcinogenicity are possible.
The threshold levels of acrolein that cause irritation and
health effects are 0.07 mg/m3 for odour perception, 0.13 mg/m3
for eye irritation, 0.3 mg/m3 for nasal irritation and eye
blinking, and 0.7 mg/m3 for decreased respiratory rate. Since
the level of acrolein rarely exceeds 0.030-0.040 µg/m3 in polluted
urban air or smoke-filled restaurants, acrolein alone is unlikely to
reach annoyance or harmful levels in normal circumstances. Provided
that acrolein concentrations are maintained below 0.05 mg/m3, most
of the population will be spared from any known annoyance or health
effects. However, in polluted urban areas and smoke-filled rooms,
acrolein is present in combination with other irritating aldehydes,
and control of acrolein alone is not sufficient to prevent annoyance
or harmful effects.
10.2 Evaluation of effects on the environment
Acrolein is released into the environment during production of
the compound itself and its derivatives, in processes involving
incomplete combustion and/or pyrolysis of organic substances, by
photochemical oxidation of specific air pollutants, and through
biocidal use, spills, and waste disposal (chapter 3).
Degradation in the atmosphere begins mainly by reaction with
hydroxyl radicals. The calculated atmospheric residence time is
approximately one day (section 4.2). Photolysis does not occur to a
significant degree (section 4.2.1). In natural water, acrolein
dissipates fairly rapidly as a result of catalysed hydration,
reactions with organic material, and volatilization (sections 4.2
and 4.3). Acrolein has a low soil adsorption potential
(section 4.1). Aerobic and anaerobic biodegradation of the compound
has been reported, although its toxicity to microorganisms may
prevent biodegradation (section 4.3.1). Based on its physical and
chemical properties, bioaccumulation would not be expected to occur
(section 4.3.2). It can be concluded that acrolein is unlikely to
persist in any environmental compartment.
Acrolein is very toxic to aquatic organisms. Acute EC50 or
LC50 values for various species range between 0.02 and
2.5 mg/litre. The 60-day NOAEL for fish (fathead minnow) is
0.0114 mg/litre (section 9.1).
In view of the high toxicity of acrolein to aquatic organisms,
the substance presents a risk to aquatic life at or near sites of
industrial discharges, spills, and biocidal use.
11. FURTHER RESEARCH
a) Human exposure characteristics should be further evaluated.
This applies to exposure due to environmental and
occupational air, as well as to intake from food and beverages.
b) These evaluations should include determinations of other
chemicals that occur with acrolein and that interact or have
biological effects similar to those due to acrolein exposure.
c) The most important target organ for airborne acrolein exposure
is the respiratory system. Therefore, further studies
including epidemiological studies should focus on this system
and particularly on the occupational environment. Possible
decreases in host resistance to respiratory infections should
be investigated.
d) The uptake of acrolein in the different parts of the
respiratory system should be examined further. The metabolism
and excretion of acrolein, as well as of its metabolites from
the respiratory system, should be given high priority as there
is an almost total lack of information about these processes.
e) The efficacy of sulfhydryl compounds, such as N-acetylcysteine
or 2-mercaptoethylsulfonic acid sodium salt (MESNA) as
antidotes for acrolein poisoning should be evaluated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Evidence for the potential carcinogenicity of acrolein has been
evaluated by the International Agency for Research on Cancer (IARC,
1979, 1985, 1987). The evidence for carcinogenicity was considered
to be inadequate both in animals and in humans. Thus no evaluation
could be made of the carcinogenicity of acrolein to humans.
Regulatory standards established by national bodies in various
countries and the EEC are summarized in the data profile of the
International Register of Potentially Toxic Chemicals (IRPTC, 1990)
and are tabulated in the Health and Safety Guide for Acrolein (WHO,
1991).
REFERENCES
ABERNETHY, D.J., FRAZELLE, J.H., & BOREIKO, C.J. (1983) Relative
cytotoxicity and transforming potential of respiratory irritants in
the C3H/10T1/2 cell transformation system. Environ. Mutagen., 5:
419 (abstract).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents, and miscellaneous
substances. Int. Pest Control, 11: 29-35.
ALARCON, R.A. (1968) Fluorometric determination of acrolein and
related compounds with m-aminophenol. Anal. Chem., 40: 1704-1708.
ALARCON, R.A. (1972) Acrolein, a component of a universal
cell-growth regulatory system: a theory. J theor. Biol., 37:
159-167.
ALARCON, R.A. (1976) Formation of acrolein from various amino-acids
and polyamines under degradation at 100 °C. Environ. Res., 12:
317-326.
ALTSHULLER, A.P. & BUFALINI, J.J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ALTSHULLER, A.P. & MCPHERSON, S.P. (1963) Spectrophotometric
analysis of aldehydes in the Los Angeles atmosphere. J. Air Pollut.
Control Assoc., 13: 109-111.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20: 649-656.
ANDERSSON, K., HALLGREN, C., LEVIN, J.-O., & NILSSON, C.-A. (1981)
Solid chemosorbent for sampling sub-ppm levels of acrolein and
glutaraldehyde in air. Chemosphere, 10: 275-280.
ARANYI, C., O'SHEA, W.J., GRAHAM, J.A., & MILLER, F.J. (1986) The
effects of inhalation of organic chemical air contaminants on murine
lung host defenses. Fundam. appl. Toxicol., 6: 713-720.
ARTHO, A. & KOCH, R. (1969) [The concentration of acrolein and
hydrogen cyanide in cigarette smoke]. Mitt. Lebensm. Hyg. (Bern),
60: 379-388 (in German).
ASTRY, C. & JAKAB, G.J. (1983) The effects of acrolein exposure on
pulmonary antibacterial defenses. Toxicol. appl. Pharmacol., 67:
49-54.
ATKINSON, R., ASCHMANN, S.M., WINER, A.M., & PITTS, J.N. (1981) Rate
constants for the gas-phase reactions of O3 with a series of
carbonyls at 296 K. Int. J. chem. Kinet., 13: 1133-1150.
ATKINSON, R., ASCHMANN, S.M., & PITTS, J.N. (1983) Kinetics of the
gas-phase reactions of OH radicals with a series of
alpha,ß-unsaturated carbonyls at 299 + 2 K. Int. J. chem. Kinet.,
15: 75-81.
ATKINSON, R., ASCHMANN, S.M., & GOODMAN, M.A. (1987) Kinetics of the
gas-phase reactions of NO3 radicals with a series of alkynes,
haloalkenes, and alpha,ß-unsaturated aldehydes. Int. J. chem.
Kinet., 19: 299-307.
AU, W., SOKOVA, O.I., KOPNIN, B., & ARRIGHI, F.E. (1980) Cytogenetic
toxicity of cyclophosphamide and its metabolites in vitro.
Cytogenet. cell Genet., 26: 108-116.
AYER, H.E. & YEAGER, D.W. (1982) Irritants in cigarette smoke
plumes. Am. J. public Health, 72: 1283-1285.
BABIUK, C., STEINHAGEN, W.H., & BARROW, C.S. (1985) Sensory
irritation response to inhaled aldehydes after formaldehyde
pretreatment. Toxicol. appl. Pharmacol., 79: 143-149.
BAKER, R.R., DYMOND, H.F., & SHILLABEER, P.K. (1984) Determination
of alpha,ß-unsaturated compounds formed by a burning cigarette.
Anal. Proc. (Lond.), 21: 135-137.
BALLANTYNE, B., DODD, D.E., PRITTS, I.M., NACHREIMER, D.J., &
FOWLER, E.H. (1989) Acute vapour inhalation toxicity of acrolein
and its influence as a trace contaminent in
2-methoxy-3,4-dihydro-2H-pyran. Hum. Toxicol., 8: 229-235.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J. (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Proceedings of the
1978 Symposium on dynamics, exposure and hazard assessment of toxic
chemicals, Ann Arbor, Michigan, Ann Arbor Science, pp. 379-392.
BARTLEY, T.R. & GANGSTAD, E.O. (1974) Environmental aspects of
aquatic plant control. J. Irrig. Drain. Div., 100: 231-244.
BASU, A.K. & MARNETT, L.J. (1984) Molecular requirements for the
mutagenicity of malondialdehyde and related acroleins. Cancer Res.,
44: 2848-2854.
BEAUCHAMP, R.O., ANDJELKOVICH, D.A., KLIGERMAN, A.D., MORGAN, K.T.,
& HECK, H. d'A. (1985) A critical review of the literature on
acrolein toxicity. CRC crit. Rev. Toxicol., 14: 309-380.
BEELEY, J.M., CROW, J., JONES, J.G., MINTY, B., LYNCH, R.D., &
PRYCE, D.P. (1986) Mortality and lung histopathology after
inhalation lung injury. The effect of corticosteroids. Am. Rev.
respir. Dis., 133: 191-196.
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9: 119-127.
BENEDICT, R.C. & STEDMAN, R.L. (1969) Composition studies on
tobacco. XXXVII. Inhibition of lactic, alcohol and
glucose-6-phosphate dehydrogenases by cigarette smoke and components
thereof. Tob. Sci., 13: 166-168.
BERRIGAN, M.J., GURTOO, H.L., SHARMA, S.D., STRUCK, R.F., &
MARINELLO, A.J. (1980) Protection by N-acetylcysteine of
cyclophosphamide metabolism - related in vivo depression of mixed
function oxygenase activity and in vitro denaturation of
cytochrome P-450. Biochem. biophys. Res. Commun., 93: 797-803.
BIGNOZZI, C.A., CHIORBOLI, C., MALDOTTI, A., & CARASSITI, V. (1980).
Atmospheric reactivity of 1,3-butadiene - nitrogen monoxide and
acrolein - nitrogen monoxide systems. Ann. Chim. (Rome), 70:
453-461.
BOETTNER, E.A. & BALL, G.L. (1980). Thermal degradation products
from PVC film in food-wrapping operations. Am. Ind. Hyg. Assoc. J.,
41: 513-522. BOHMANN, J.-J. (1985) [Aging behaviour of beer.]
Monatsschr. Brauwiss., 4: 175-180 (in German).
BOOR, P.J. & ANSARI, G.A.S. (1986) High-performance liquid
chromatographic method for quantitation of acrolein in biological
samples. J. Chromatogr., 375: 159-164.
BOULEY, G., DUBREUIL, A., GODIN, J., & BOUDENE, C. (1975) Effets,
chez le rat, d'une faible dose d'acroléine inhalée en continu. Eur.
J. Toxicol., 8: 291-297.
BOWMER, K.H. & HIGGINS, M.L. (1976) Some aspects of the persistence
and fate of acrolein herbicide in water. Arch.environ. Contam.
Toxicol., 5: 87-96.
BOWMER, K.H. & SAINTY, G.R. (1977) Management of aquatic plants with
acrolein. J. aquat. Plant Manage., 15: 40-46.
BOWMER, K.H. & SMITH, G.H. (1984) Herbicides for injection into
flowing water: acrolein and endothal-amine. Weed Res., 24: 201-211.
BOWMER, K.H., LANG, A.R.G., HIGGINS, M.L., PILLAY, A.R., & TCHAN,
Y.T. (1974) Loss of acrolein from water by volatilization and
degradation. Weed Res., 14: 325-328.
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed conjugations
of glutathione with unsaturated compounds. Biochem. J., 104:
95-102.
BRIDGES, R.B., KRAAL, J.H., HUANG, L.J.T., & CHANCELLOR, M.B. (1977)
Effects of cigarette smoke components on in vitro chemotaxis of
human polymorphonuclear leukocytes. Infect. Immun., 16: 240-248.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRINGMANN, G. (1978) [Determination of the harmful biological action
of water-endangering substances on protozoa; I. Bacteria fed
flagellates.] Z. Wasser-Abwasser Forsch., 11: 210-215 (in German).
BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the harmful
action of water-endangering substances on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z. Wasser-Abwasser Forsch., 10:
87-98 (in German).
BRINGMANN, G. & KUHN, R. (1980) [Determination of the harmful
biological action of water-endangering substances on protozoa; II.
Bacteria fed ciliates.] Z. Wasser-Abwasser Forsch., 13: 26-31 (in
German).
BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa; III Saprozoic flagellates.] Z. Wasser-Abwasser Forsch.,
13: 170-173 (in German).
BROWN, P.W. & FOWLER, C.A. (1967) The toxicity of tobacco smoke
solutions to Proteus vulgaris. Beitr. Tabakforsch., 4: 78-83.
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity
of priority pollutants to Bluegill (Lepomis macrochirus). Bull.
environ. Contam. Toxicol., 26: 446-452.
BUCKLEY, L.A., JIANG, X.Z., JAMES, R.A., MORGAN, K.T., & BARROW,
C.S. (1984) Respiratory tract lesions induced by sensory irritants
at the RD50 concentration. Toxicol. appl. Pharmacol., 74:
417-429.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative determination
of acrylonitrile, acrolein, acetonitrile and acetone in workplace
air. Am. Ind. Hyg. Assoc. J., 40: 904-909.
CAMPBELL, K.I., GEORGE, E.L., & WASHINGTON, I.S. (1981) Enhanced
susceptibility to infection in mice after exposure to dilute exhaust
from light duty diesel engines. Environ. Int., 5: 377-382.
CANTONI, C., BIANCHI, M.A., RENON, P., & CALCINARDI, C. (1969)
[Bacterial and chemical alterations during souring in salted pork.]
Atti. Soc. Ital. Sci. Vet., 23: 752-756 (in Italian).
CARPENTER, C.P., SMYTH, H.F., POZZANI, U.C. (1949) The assay of
acute vapor toxicity, and the grading and interpretation of results
on 96 compounds. J. ind. Hyg. Toxicol., 31: 343-346.
CATILINA, P., THIEBLOT, L., & CHAMPEIX, J. (1966) Lesions
respiratoires expérimentales par inhalation d'acroléine chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc. (Paris), 27: 857-867.
CHAMPEIX, J., COURTIAL, L., PERCHE, E., & CATILINA, P. (1966)
Broncho-pneumopathie aiguë par vapeurs d'acroléine. Arch. Mal.
prof. Méd. Trav. Sécur. Soc., 27: 794-796.
CHAVIAMO, A.H., GILL, W.B., RUGGIERO, U.J., & VERMEULEN, C.W. (1985)
Experimental cytoran cystitis and prevention by acetylcysteine. J.
Urol., 134: 598-600.
CHHIBBER, G. & GILANI, S.H. (1986) Acrolein and embryogenesis: an
experimental study. Environ. Res., 39: 44-49.
CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation and
degradation of petrochemical wastewater components by methane
fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
CHRAIBER, L.B., SOSNOVSKY, S.I., TATARKIN, Y.N., & VINNIKOVA, L.I.
(1964) [Air pollution with acrolein vapors in expeller and forepress
shops of butter and fat mills of Uzbekistan.] Gig. Tr. prof.
Zabol., 1(11): 49-50 (in Russian).
CHUNG, F.-L., YOUNG, R., & HECHT, S.S. (1984) Formation of cyclic
1,N2-propanodeoxyguanosine adducts in DNA upon reaction with
acrolein or crotonaldehyde. Cancer Res., 44: 990-995.
CLAUSSEN, U., HELLMANN, W., & PACHE, G. (1980) The embryotoxicity of
the cyclophosphamide metabolite acrolein in rabbits, tested in vivo
by i.v. injection and by the yolk-sac method. Drug Res., 30:
2080-2083.
COHEN, I.R. & ALTSHULLER, A.P. (1961) A new spectrophotometric
method for the determination of acrolein in combustion gases and in
the atmosphere. Anal. Chem., 33: 726-733.
COOMBER, J.W. & PITTS, J.N. (1969) Molecular structure and
photochemical reactivity. XII. The vapor-phase photochemistry of
acrolein at 3130 A. J. Am. Chem. Soc., 91: 547-550.
COOPER, K.O., WITMER, C.M., & WITZ, G. (1987) Inhibition of
microsomal cytochrome c reductase activity by a series of
alpha,ß-unsaturated aldehydes. Biochem. Pharmacol., 36: 627-631.
COSTA, D.L., KUTZMAN, R.S., LEHMANN, J.R., & DREW, R.T. (1986)
Altered lung function and structure in the rat after subchronic
exposure to acrolein. Am. Rev. respir. Dis., 133: 286-291.
COX, R., GOORHA, S., & IRVING, C.C. (1988) Inhibition of DNA
methylase activity by acrolein. Carcinogenesis, 9: 463-465.
CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & ABBOTT, J.K. (1986)
Inhalation toxicology: VII. Times to incapacitiation and death for
rats exposed continuously to atmospheric acrolein vapor, Washington,
D.C., Federal Aviation Administration, Office of Aviation Medicine
(DOT/FAA/AM-86/5)
CROOK, T.R., SOUHAMI, R.L., & MCLEAN, A.E.M. (1986a) Cytotoxicity,
DNA cross-linking, and single strand breaks induced by activated
cyclophosphamide and acrolein in human leukemia cells. Cancer Res.,
46: 5029-5034.
CROOK, T.R., SOUHAMI, R.L., WHYMAN, G.D., & MCLEAN, A.E.M. (1986b)
Glutathione depletion as a determinant of sensitivity of human
leukemia cells to cyclophosphamide. Cancer Res., 46: 5035-5038.
CURREN, R.D., YANG, L.L., CONKLIN, P.M., GRAFSTROM, R.C., & HARRIS,
C.C. (1988) Mutagenesis of xeroderma pigmentosum fibroblasts by
acrolein. Mutat. Res., 209: 17-22.
DAHLGREN, S.E., DALEN, H., & DALHAMM, T. (1972) Ultrastructural
observations on chemically induced inflammation in guinea pig
trachea. Virchows Arch. Abt. B Zellpathol., 11: 211-223.
DALHAMN, T. & ROSENGREN, A. (1971) Effect of different aldehydes on
tracheal mucosa. Arch. Otolarygnol., 83: 496-500.
DARLEY, E.F., MIDDLETON, J.T., & GARBER, M.J. (1960) Plant damage
and eye irritation from ozone-hydrocarbon reactions. J. agric. food
Chem., 8: 483-485.
DAVIS, T.R.A., BATTISTA, S.P., & KENSLER, C.J. (1967) Mechanism of
respiratory effects during exposure of guinea pigs to irritants.
Arch. environ. Health, 15: 412-419.
DAWSON, J.R., NORBECK, K., ANUNDI, I., & MOLDEUS, P. (1984) The
effectiveness of N-acetylcysteine in isolated hepatocytes, against
the toxicity of paracetamol, acrolein, and paraquat. Arch.
Toxicol., 55: 11-15.
DEBETHIZY, J.D., UDINSKY, J.R., SCRIBNER, H.E., & FREDERICK, C.B.
(1987) The disposition and metabolism of acrylic acid and ethyl
acrylate in male Sprague-Dawley rats. Fundam. appl. Pharmacol., 8:
549-561.
DESCROIX, H. (1972) Etude complémentaire des interactions entre
acroléine et cytidine-monophosphate. C. R. Acad. Sci. Paris, D274:
2362-2365.
DGEP (1988) Unpublished Review of literature data on acrolein,
Leidschendam, The Netherlands, Ministry of Housing, Physical
Planning and Environment, Directorate-General of Environmental
Protection.
DIAZ MAROT, A., GASSIOT MATAS, M., & FERRER MARTIN, M. (1983)
[Stripping and high performance liquid chromatography in the
analysis of carbonyl compounds in beers at ppb level.] Afinidad,
40: 21-24 (in Spanish).
DORE, M. & MONTALDO, C. (1984) [Study on the conjungation in vitro
of allyl alcohol and its metabolites with reduced glutathione.]
Boll. Soc. Ital. Biol. Sper., 60: 1497-1501 (in Italian).
DRAMINSKI, W., EDER, E., & HENSCHLER, D. (1983) A new pathway of
acrolein metabolism in rats. Arch. Toxicol., 52: 243-247.
EASLEY, D.M., KLEOPFER, R.D., & CARASEA, A.M. (1981) Gas
chromatographic-mass spectrometric determination of volatile organic
compounds in fish. J. Assoc. Off. Anal. Chem., 64: 653-656.
EDNEY, E.O., MITCHELL, S., & BUFALINI, J.J. (1982) Atmospheric
chemistry of several toxic compounds, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Atmospheric Chemistry
and Physics Division, Environmental Sciences Research Laboratory,
Office of Research and Development (EPA-600/3-82-092, PB 83-146340).
EDNEY, E.O., KLEINDIENST, T.E., & CORSE, E.W. (1986a) Room
temperature rate constants for the reaction of OH with selected
chlorinated and oxygenated hydrocarbons. Int. J. chem. Kinet., 18:
1355-1371.
EDNEY, E.O., SHEPSON, P.B., KLEINDIENST, T.E. & CORSE, E.W. (1986b)
The photooxidation of allyl chloride. Int. J. chem. Kinet., 18:
597-608.
EGLE, J.L. (1972) Retention of inhaled formaldehyde,
propionaldehyde, and acrolein in the dog. Arch. environ. Health,
25: 119-124.
EGLE, J.L. & HUDGINS, P.M. (1974) Dose-dependent sympathomimetic and
cardioinhibitory effects of acrolein and formaldehyde in the
anesthetized rat. Toxicol. appl. Pharmacol., 28: 358-366.
ELLENBERGER, J. & MOHN, G.R. (1976) Comparative mutagenicity testing
of cyclphosphamide and some of its metabolites. Mutat. Res., 37:
120 (abstract).
ELLENBERGER, J. & MOHN, G.R. (1977) Mutagenic activity of major
mammalian metabolites of cyclophosphamide toward several genes of
Escherichia coli. J. Toxicol. environ. Health, 3: 637-650.
EPSTEIN, S.S. & SHAFNER H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ERICKSON, L.C., RAMONAS, L.M., ZAHARKO, D.S., & KOHN, K.W. (1980)
Cytotoxicity and DNA cross-linking activity of
4-sulfidocyclophosphamides in mouse leukemia cells in vitro.
Cancer Res., 40: 4216-4220.
ESTERBAUER, H., ZOLLNER, H., & SCHOLZ, N. (1975) Reaction of
glutathione with conjugated carbonyls. Z. Naturforsch., 30:
466-473.
FACCHINI, M.C., CHIAVARI, G., & FUZZI, S. (1986) An improved HPLC
method for carbonyl compound speciation in the atmospheric liquid
phase. Chemosphere, 15: 667-674.
FERGUSON, F.F., RICHARDS, C.S., & PALMER, J.R. (1961) Control of
Australorbis glabratus by acrolein in Puerto Rico. Public Health
Rep., 76: 461-468.
FERGUSON, F.F., DAWOOD, I.K., & BLONDEAU, R. (1965) Preliminary
field trials of acrolein in the Sudan. Bull. World Health Organ.,
32: 243-248.
FERON, V.J. & KRUYSSE, A. (1977) Effects of exposure to acrolein
vapor in hamsters simultaneously treated with benzo [a]pyrene or
diethylnitrosamine. J. Toxicol. environ. Health, 3: 379-394.
FERON, V.J., KRUYSSE, A., TIL, H.P., & IMMEL, H.R. (1978) Repeated
exposure to acrolein vapour: subacute studies in hamsters, rats and
rabbits. Toxicology, 9: 47-57.
FISCHER, T., WEBER, A., & GRANDJEAN, E. (1978) [Air pollution due to
tobacco smoke in restaurants.] Int. Arch. occup. environ. Health,
41: 267-280 (in German).
FLEER, R. & BRENDEL, M. (1982) Toxicity, interstrand cross-links and
DNA fragmentation induced by `activated' cyclophosphamide in yeast:
comparative studies on 4-hydroperoxy-cyclophosphamide, its
monofunctional analogue, acrolein, phosphoramide mustard, and
non-nitrogen mustard. Chem.-biol. Interact., 39: 1-15.
FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity using the
Ames' test. Toxicology, 18; 219-232.
FOILES, P.G., AKERKAR, S.A., & CHUNG, F.-L. (1989) Application of an
immunoassay for cyclic acrolein deoxyguanosine adducts to assess
their formation in DNA of Salmonella typhimurium under conditions
of mutation induction by acrolein. Carcinogenesis, 10: 87-90.
FRACCHIA, M.F., SCHUETTE, F.J., & MUELLER, P.K. (1967) A method for
sampling and determination of organic carbonyl compounds in
automobile exhaust. Environ. Sci. Technol., 1: 915-922.
FRITZ-SHERIDAN, R.P. (1982) Impact of the herbicide Magnacide-H
(2-propenal) on algae. Bull. environ. Contam. Toxicol., 28:
245-249.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO,
J., MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. mol.
Mutagen., 10(supplement): 1-175.
GAN, J.C. & ANSARI, G.A.S. (1987) Plausible mechanism of
inactivation of plasma alpha1-proteinase inhibitor by acrolein.
Res. Commun. chem. Pathol. Pharmacol., 55: 419-422.
GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated carbon
adsorption of petrochemicals. J. Water Pollut. Control Fed., 46:
947-965.
GOSSELIN, B., WATTEL, F., CHOPIN, C., DEGAND, P., FRUCHART, J.C.,
VAN DER LOO, D., & CRASQUIN, O. (1979) Intoxication aiguë par
l'acroléine, une observation. Nouv. Presse méd., 8: 2469-2472.
GRAEDEL, T.E., FARROW, L.A., & WEBER, T.A. (1976) Kinetic studies of
the photochemistry of the urban atmosphere. Atmos. Environ., 10:
1095-1116.
GRAFSTROM, R.C., CURREN, R.D., YANG, L.L., & HARRIS, C.C. (1986)
Aldehyde-induced inhibition of DNA repair and potentiation of
N-nitrosocompound-induced mutagenesis in cultured human cells.
Prog. clin. biol. Res., 209A: 255-264.
GRAFSTROM, R.C., DYPBUKT, J.M., WILLEY, J.C., SUNDQVIST, K., EDMAN,
C., ATZORI, L., & HARRIS, C.C. (1988) Pathobiological effects of
acrolein in cultured human bronchial epithelial cells. Cancer Res.,
48: 1717-1721.
GREENHOFF, K. & WHEELER, R.E. (1981) Analysis of beer carbonyls at
the part per billion level by combined liquid chromatography and
high pressure liquid chromatography. J. Inst. Brew., 86: 35-41.
GREY, T.C. & SHRIMPTON, D.H. (1966) Volatile components of raw
chicken breast muscle. Br. poult. Sci., 8: 23-33.
GROSJEAN, D. & WRIGHT ,B. (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos. Environ., 17: 2093-2096.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
chromatogr. Sci., 12: 385-391.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of The Netherlands. Sci. total
Environ., 43: 193-219.
GUILLERM, R., BADRE, R., & HEE, J. (1967a) Détermination du seuil
d'action des polluants irritants de l'atmosphère - Comparaison de
deux méthodes. Ann. occup. Hyg., 10: 127-133.
GUILLERM, R., SAINDELLE, A., FALTOT, P., & HEE, J. (1967b) Action de
la fumée de cigarette et de quelques-uns de ses constituants sur les
resistances ventilatoires chez le cobaye. Arch. int. Pharmacodyn.
Ther., 167: 101-114.
GURTOO, H.L., HIPKENS, J.H., & SHARMA, S.D. (1981a) Role of
glutathione in the metabolism-dependent toxicity and chemotherapy of
cyclophosphamide. Cancer Res., 41: 3584-3591.
GURTOO, H.L., MARINELLO, A.J., STRUCK, R.F., PAUL, B., & DAHMS, R.P.
(1981b) Studies on the mechanism of denaturation of cytochrome P-450
by cyclophosphamide and its metabolites. J. biol. Chem., 256:
11691-11701.
GUSEV, M.I., SVECHNIKOVA, A.I., DRONOV, I.S., GREBENSKOVA, M.D., &
GOLOVINA, A.I. (1966) [Substantiation of the daily average maximum
permissible concentration of acrolein in the atmosphere.] Gig. i
Sanit., 31: 9-13 (in Russian).
HAENEN, G.R.M.M., VERMEULEN, N.P.E., TAI TIN TSOI, J.N.L., RAGETLI,
H.M.N., TIMMERMAN, H., & BAST, A. (1988) Activation of the
microsomal glutathione- S-transferase dependent protection against
lipid peroxidation by acrolein. Biochem. Pharmacol., 37: 1933-1938.
HALES, B.F. (1982) Comparison of the mutagenicity and teratogenicity
of cyclophosphamide and its active metabolites,
4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein.
Cancer Res., 42: 3016-3021.
HALES, C.A., BARKIN, P.W., JUNG, W., TRAUTMAN, E., LAMBORGHINI, D.,
HERRIG, N., & BURKE, J. (1988) Synthetic smoke with acrolein but not
HCL produces pulmonary edema. J. appl. Physiol., 64: 1121-1133.
HALL, R.H., & STERN, E.S. (1950) Acid-catalysed hydration of
acraldehyde. Kinetics of the reaction and isolation of
ß-hydroxypropaldehyde. J. Chem. Soc., 1950: 490-498.
HARADA, M. (1977) [Biochemical analysis of tear fluid in
photochemical smog.] Nippon Ganka Gakkai Zasshi, 81: 275-286 (in
Japanese).
HARKE, H.-P., BAARS, A., FRAHM, B., PETERS, H., & SCHULTZ, C. (1972)
[On the problem of passive smoking. Relation between the
concentration of smoke constituents in the air of several large
rooms and the number of smoked cigarettes and time.] Int. Arch.
Arbeitsmed., 29: 323-339 (in German).
HAWLEY, G.G. (1981) The condensed chemical dictionary, 10th ed.,
New York, Van Nostrand Reinhold Co.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HAYASE, F., CHUNG, T.-Y., & KATO, H. (1984) Changes of volatile
components of tomato fruits during ripening. Food Chem., 14:
113-124.
HEMMINKI, K., FALCK, K., & VAINIO, H. (1980) Comparison of
alkylation rates and mutagenicity of directly acting industrial and
laboratory chemicals. Arch. Toxicol., 46: 277-285.
HESS, L.G., KURTZ, A.N., & STANTON, D.B. (1978) Acrolein and
derivatives. In: Kirk, R.E. & Othmer, D.F. ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol 1:
pp. 277-297.
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity classes.
Ind. Hyg. Q., 10: 93-96.
HOFFMAN, C., BASTIAN, H., WIEDENMANN, M., DEININGER, C., & EDER, E.
(1989) Detection of acrolein congener-DNA adducts isolated from
cellular systems. Arch. Toxicol., Suppl. 13: 219-223.
HOFFMANN, D., BRUNNEMANN, K.D., GORI, G.B., & WYNDER, E.L. (1975) On
the carcinogenicity of marijuana smoke. Recent Adv. Phytochem., 9:
63-81.
HOLMBERG, B. & MALMFORS, T. (1974) The cytotoxicity of some organic
solvents. Environ. Res., 7: 183-192.
HOSHIKA, Y. & TAKATA, Y. (1976) Gas chromatographic separation of
carbonyl compounds as their 2,4-dinitrophenylhydrazones using glass
capillary columns. J. Chromatogr., 120: 379-389.
HOVDING, G. (1969) Occupational dermatitis from pyrolysis products
of polythene. Acta dermatovenereol, 49: 147-149.
HRDLICKA, J. & KUCA, J. (1965) The changes of carbonyl compounds in
the heat-processing of meat. Poult. Sci., 44: 7-31.
HUGOD, C., HAWKINS, L.H., & ASTRUP, P. (1978) Exposure of passive
smokers to tobacco smoke constituents. Int. Arch. occup. environ.
Health, 42: 21-29.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein, Lyons, International Agency for Research on Cancer, pp.
479-495 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Man, Vol. 19).
IARC (1985) Allyl compounds, aldehydes, epoxides and peroxides,
Lyons, International Agency for Research on Cancer, pp. 133-161
(IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1985) Treatment and disposal methods for waste chemicals,
Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
IRPTC (1990) Data profile on acrolein, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ, I.D., &
BRADSHAW, J.J. (1978) Organic compounds - Their interaction with and
degradation of hepatic microsomal drug-metabolizing enzymes in
vitro. Drug Metab. Disp., 6: 218-225.
IZARD, C. (1973) Effets de l'acroléine sur la division cellulaire,
le cycle et la synthèse de l'ADN, chez Vicia faba. C. R. Acad.
Sci. Paris Ser. D, 276: 1745-1747.
IZARD, C. & LIBERMANN, C. (1978) Acrolein. Mutat. Res., 47:
115-138.
IZMEROV, N.F., ed. (1984) Acrolein. Moscow, Centre of International
Projects, GKNT, 15 pp (IRPTC Scientific Reviews of Soviet Literature
on Toxicity and Hazards of Chemicals 50).
JACOBS, W.A. & KISSINGER, P.T. (1982) Determination of carbonyl 2,4-
dinitrophenylhydrazones by liquid chromatography/electrochemistry.
J. liq. Chromatogr., 5: 669-676.
JAKAB, G.J. (1977) Adverse effect of a cigarette smoke component,
acrolein, on pulmonary defenses and on viral-bacterial interactions
in the lung. Am. Rev. respir. Dis., 115: 33-38.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of several gas-phase components of side-stream smoke
of cigarettes in indoor air as contribution towards the problem of
passive smoking.] Int. Arch. occup. environ. Health, 36: 169-181
(in German).
JONSSON, A. & BERG, S. (1983) Determination of low-molecular-weight
oxygenated hydrocarbons in ambient air by cryogradient sampling and
two-dimensional gas chromatography. J. Chromatogr., 279: 307-322.
JOUSSERANDOT, P., GREMAIN, J., & DU BOISTESSELIN, R. (1981)
L'utilisation de méthodes histologiques pour la démonstration de
l'activite anti-inflammatoire de la fusafungine. Sem. Hop. Paris,
57: 143-150.
JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of 200
chemical compounds for acute toxicity towards fish by means of the
goldfish test.] Z. Wasser-Abwasser Forsch., 11: 161-164 (in
German).
KANE, L.E. & ALARIE, Y. (1977) Sensory irritation to formaldehyde
and acrolein during single and repeated exposures in mice. Am. Ind.
Hyg. Assoc. J., 38: 509-521.
KANE, L.E. & ALARIE, Y. (1978) Evaluation of sensory irritation from
acrolein-formaldehyde mixtures. Am Ind. Hyg. Assoc. J., 39:
270-274.
KANE, L.E. & ALARIE, Y. (1979) Interactions of sulfur dioxide and
acrolein as sensory irritants. Toxicol. appl. Pharmacol., 48:
305-315.
KANKAANPAA, J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acrylamide in
developing chick embryos. Toxicol. Lett., 4: 93-96.
KANTEMIROVA, A.E. (1975) [The disease incidence rate, including
temporal disability of workers engaged in the production of acrolein
and methylmercaptopropione (MMP) aldehyde.] Trans. Volgograd med.
Inst., 26(4): 79-85 (in Russian).
KANTEMIROVA, A.E. (1977) [The disease incidence rate, involving
temporal disability of workers engaged in the production of acrolein
and MMP aldehyde.] Trans. Volgograd med. Inst., 27(5): 32-33 (in
Russian).
KATZ, M. (1977) Methods of air sampling and analysis, 2nd ed.,
Washington, DC, American Public Health Association.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134: 1093-1101.
KERR, J.A. & SHEPPARD (1981) Kinetics of the reactions of hydroxyl
radicals with aldehydes studied under atmospheric conditions.
Environ. Sci. Technol., 15: 960-963.
KHUDOLEY, V.V., MIZGIREV, I.V., & PLISS, G.B. (1986) [Evaluation of
mutagenic activity of carcinogens and other chemical agents with
Salmonella typhimurium assays.] Vopr. Onkol., 32: 73-80 (in
Russian).
KILBURN, K.H. & MCKENZIE, W.N. (1978) Leukocyte recruitment to
airways by aldehyde-carbon combinations that mimic cigarette smoke.
Lab. Invest., 38: 134-142.
KIMES, B.W. & MORRIS, D.R. (1971) Inhibition of nucleic acid and
protein synthesis in Escherichia coli by oxidized polyamines and
acrolein. Biochim. Biophys. Acta, 228: 235-244.
KISSEL, C.L., BRADY, J.L., GUERRA, A.M., PAU, J.K., ROCKIE, B.A., &
CASERIO, F.F. (1978) Analysis of acrolein in aged aqueous media.
Comparison of various analytical methods with bioassays. J. agric.
food Chem., 26: 1338-1343.
KLOCHKOVSKII, S.P., LUKASHENKO, R.D., PODVYSOTSKII, K.S., &
KAGRAMANYAN, N.P. (1981) [Acrolein and formaldehyde content in the
air of quarries.] Bezoppasnost. Tr. Prom-St. 1981(12):38 (in
Russian).
KOERKER, R.L., BERLIN, A.J., & SCHNEIDER, F.H. (1976) The
cytotoxicity of short-chain alcohols and aldehydes in cultured
neuroblastoma cells. Toxicol. appl. Pharmacol., 37: 281-288.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1983) Embryotoxic effects
of acrolein, methylacrylates, guanidines and resorcinol on three day
chicken embryos. Acta pharmacol. toxicol., 52: 95-99.
KRILL, R.M. & SONZOGNI, W.C. (1986) Chemical monitoring of
Wisconsin's groundwater. J. Am. Water Works Assoc., 78: 70-75
KROKAN, H., GRAFSTROM, R.C., SUNDQVIST, K., ESTERBAUER, H., &
HARRIS, C.C. (1985) Cytotoxicity, thiol depletion and inhibition of
O6-methylguanine-DNA methyltransferase by various aldehydes in
cultured human bronchial fibroblasts. Carcinogenesis, 6: 1755-1759.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A. (1982)
Collection and analysis of hazardous organic emissions. Anal.
Chem., 54: 810-817.
KRUYSSE, A. (1971) Acute inhalation toxicity of acrolein in
hamsters, Zeist, The Netherlands, Central Institute for Nutrition
and Food Research, TNO (Report No. R3516).
KU, R.H. & BILLINGS, R.E. (1986) The role of mitochondrial
glutathione and cellular protein sulfhydryls in formaldehyde
toxicity in glutathione-depleted rat hepatocytes. Arch. Biochem.
Biophys., 247: 183-189.
KUBINSKI, H., GUTZKE, G.E., & KUBINSKI, Z.O. (1981) DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
KUTZMAN, R.S., MEYER, G.-J., & WOLF, A.P. (1982) The biodistribution
and metabolic fate of [11C]-acrylic acid in the rat after acute
inhalation exposure or stomach intubation. J. Toxicol. environ.
Health, 10: 969-979.
KUTZMAN, R.S., WEHNER, R.W., & HABER, S.B. (1984) Selected responses
of hypertension sensitive and resistant rats to inhaled acrolein.
Toxicology, 31: 53-65.
KUTZMAN, R.S., POPENOE, E.A., SCHMAELER, M., & DREW, R.T. (1985)
Changes in rat lung structure and composition as a result of
subchronic exposure to acrolein. Toxicology, 34: 139-151.
KUWATA, K., UEBORI, M., YAMASAKI, H., KUGE, Y., & KISO, Y. (1983)
Determination of aliphatic aldehydes in air by liquid
chromatography. Anal. Chem., 55: 2013-2016.
LACROIX, M., BURCKEL, H., FOUSSEREAU, J., GROSSHANS, E., CAVELIER,
C., LIMASSET, J.C., DUCOS, P., GRADINSKI, D., & DUPRAT, P. (1976)
Irritant dermatitis from diallylglycol carbonate monomer in the
optical industry. Contact dermatitis, 2: 183-195.
LAM, C.-W., CASANOVA, M., & HECK, H.D'A. (1985) Depletion of nasal
mucosal glutathione by acrolein and enhancement of
formaldehyde-induced DNA-protein cross-linking by simultaneous
exposure to acrolein. Arch. Toxicol., 58: 67-71.
LEACH, P.W., LENG, L.J., BELLAR, T.A., SIGSBY, J.E., & ALTSHULLER,
A.P. (1964) Effects of HC/NOx ratios on irradiated auto exhaust,
part II. J. Air Pollut. Control Assoc., 14: 176-183.
LEACH, C.L., HATOUM, N.S., RATAJCZAK, H.V., & GERHART, J.M. (1987)
The pathologic and immunologic effects of inhaled acrolein in rats.
Toxicol. Lett., 39: 189-198.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24:
684-691.
LE BOUFFANT, L., MARTIN, J.C., DANIEL, H., HENIN, J.P., & NORMAND,
C. (1980) Action of intensive cigarette smoke inhalations on the rat
lung. Role of particulate and gaseous cofactors. J. Natl Cancer
Inst., 64: 273-281.
LEFFINGWELL, C.M. & LOW, R.B. (1979) Cigarette smoke components and
alveolar macrophage protein synthesis. Arch. environ. Health, 34:
97-102.
LENCREROT, P., PARFAIT, A., & JOURET, C. (1984) Rôle des
corynebactéries dans la production d'acroléine (2-propenal) dans les
rhums. Ind. aliment. agric., 101: 763-765.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determinations of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEUCHTENBERGER, C., SCHUMACHER, M., & HADIMANN, T. (1968) Further
cytological and cytochemical studies on the biological significance
of the gas phase of fresh cigarette smoke. Z. Präventivmed., 13:
130-141.
LEVAGGI, D.A. & FELDSTEIN, M. (1970) The determination of
formaldehyde, acrolein, and low molecular weight aldehydes in
industrial emissions on a single collection sample. J. Air Pollut.
Control Assoc., 20: 312-313.
LIES, K.-H., POSTULKA, A., GRING, H., & HARTUNG, A. (1986) Aldehyde
emissions from passenger cars. Staub-Reinhalt. Luft, 46: 136-139.
LIJINSKY, W. & ANDREWS, A.W. (1980) Mutagenicity of vinyl compounds
in Salmonella typhimurium. Teratogen. Carcinogen. Mutagen., 1:
259-267.
LIJINSKI, W. & REUBER, M.D. (1987) Chronic carcinogenesis studies of
acrolein and related compounds. Toxicol. ind. Health, 3: 337-345.
LIPARI, F. & SWARIN, S.J. (1982) Determination of formaldehyde and
other aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. J. Chromatogr., 247: 297-306.
LIPARI, F., DASCH, J.M., & SCRUGGS, W.F. (1984) Aldehyde emissions
from wood-burning fireplaces. Environ. Sci. Technol., 18: 326-330.
LIU, Y. & TAI, H.-H. (1985) Inactivation of pulmonary NAD-dependent
15-hydroxyprostaglandin dehydrogenase by acrolein. Biochem.
Pharmacol., 34: 4275-4278.
LOQUET, C., TOUSSAINT, G., & LETALAER, J.Y. (1981) Studies on
mutagenic constituents of apple brandy and various alcoholic
beverages collected in Western France, a high incidence area for
oesophageal cancer. Mutat. Res., 88: 155-164.
LORZ, H.W., GLENN, S., WILLIAMS, R.H., KUNKEL, C.M., NORRIS, L.A., &
LOPER, B.R. (1979) Effects of selected herbicides on Smolting
Salmon, Corvallis, Oregon, USA Environmental Protection Agency,
Environment Research Laboratory Corvallis, Office of Research and
Development (Report EPA-600/3-79-071).
LOW, E.S., LOW, R.B., & GREEN, G.M. (1977) Correlated effects of
cigarette smoke components on alveolar macrophage adenosine
triphosphatase activity and phagocytosis. Am. Rev. respir. Dis.,
115: 963-970.
LUTZ, D., EDER, E., NEUDECKER, T., & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha,ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93: 305-315.
LYMAN, W.J., REEHL, W.F., & ROSENBLATT, D.H. (1982) Handbook of
chemical property estimation methods, New York, McGraw-Hill Book
Company.
LYON, J.P., JENKINS, L.J., JONES, R.A., COON, R.A., & SIEGEL, J.
(1970) Repeated and continuous exposure of laboratory animals to
acrolein. Toxicol. appl. Pharmacol., 17: 726-732.
MACEK, K.J., LINDBERG, M.A., SAUTER, S., BUXTON, K.S., & COSTA, P.A.
(1976) Toxicity of four pesticides to water fleas and fathead
minnows, Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory, Office of Research and
Development, (Ecological Research Series EPA-600/3-76-099).
MCNULTY, M.J., HECK, H.D'A., & CASANOVA-SCHMITZ, M. (1984) Depletion
of glutathione in rat respiratory mucosa by inhaled acrolein. Fed.
Proc., 43: 575 (abstract).
MAGIN, D.F. (1980) Gas chromatography of simple monocarbonyls in
cigarette whole smoke as the benzyloxime derivatives. J.
Chromatogr., 202: 255-261.
MALDOTTI, A., CHIORBOLI, C., BIGNOZZI, C.A., BARTOCCI, C., &
CARASSITI, V. (1980) Photooxidation of 1,3-butadiene containing
systems: rate constant determination for the reaction of acrolein
with OH radicals. Int. J. chem. Kinet., 12: 905-913.
MANITA, M.D. & GOLDBERG, E.K. (1970) [Spectrophotometric analysis of
airborne acrolein using the semithiocarbazide reagent.] Gig. i
Sanit., 35(5): 63-65 (in Russian).
MANNING, D.L., MASKARINEC, M.P., JENKINS, R.A., & MARSHALL, A.H.
(1983) High performance liquid chromatographic determination of
selected gas phase carbonyl in tobacco smoke. J. Assoc. Off. Anal.
Chem., 66: 8-12.
MARANO, F. & DEMESTERE, M. (1976) Ultrastructural autoradiographic
study of the intracellular fixation of 3H-acrolein. Experientia
(Basel), 32: 501-503.
MARANO, F. & PUISEUX-DAO, S. (1982) Acrolein and cell cycle.
Toxicol. Lett., 14: 143-149.
MARINELLO, A.J., GURTOO, H.L., STRUCK, R.F., & PAUL, B. (1978)
Denaturation of cytochrome P-450 by cyclophosphamide metabolites.
Biochem. Biophys. Res. Commun., 83: 1347-1353.
MARINELLO, A.J., BERRIGAN, M.J., STRUCK, R.F., GUENGERICH, F.P., &
GURTOO, H.L. (1981) Inhibition of NADPH-cytochrome P450 reductase by
cyclophosphamide and its metabolites. Biochem. biophys. Res.
Commun., 99: 399-406.
MARINELLO, A.J., BANSAL, S.K., PAUL, B., KOSER, P.L., LOVE, J.,
STRUCK, R.F., & GURTOO, H.L. (1984) Metabolism and binding of
cyclophosphamide and its metabolite acrolein to rat hepatic
microsomal cytochrome P-450. Cancer Res., 44: 4615-4621.
MARNETT, L.J., HURD, H.K., HOLLSTEIN, M.C., LEVIN, D.E., ESTERBAUER,
H., & AMES, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA 104. Mutat. Res., 148:
25-34.
MASARU, N., SYOZO, F., & SABURO, K. (1976) Effects of exposure to
various injurious gases on germination of lily pollen. Environ.
Pollut., 11: 181-187.
MASEK, V. (1972) [Aldehydes in the air at working places in coal and
pitch coking plants.] Staub-Reinhalt. Luft, 32: 335-336 (in
German).
MASLOWSKA, J. & BAZYLAK, G. (1985) Thermofractographic determination
of monocarbonyl compounds in animal waste fats used as feed fat.
Anim. Feed Sci. Technol., 13: 227-236.
METTIER, S.R., BOYER, H.K., HINE, C.H., & MCEWEN, W.K. (1960) A
study of the effects of air pollutants on the eye. Am. Med. Assoc.
Arch. ind. Health, 21: 13-18.
MILLS, D.E., BAUGH, W.D., & CONNER, H.A. (1954) Studies on the
formation of acrolein in distillery mashes. Appl. Microbiol., 2:
9-13.
MIRKES, P.E., GREENAWAY, J.C., ROGERS, J.G., & BRUNDRETT, R.B.
(1984) Role of acrolein in cyclophosphamide teratogenicity in rat
embryos in vitro. Toxicol. appl. Pharmacol., 72: 281-291.
MIYAMOTO, Y. (1986) Eye and respiratory irritants in jet engine
exhaust. Aviat. Space environ. Med., 57: 1104-1108.
MORIKAWA, T. (1976) Acrolein, formaldehyde, and volatile fatty acids
from smoldering combustion. J. Combust. Toxicol., 3: 135-150.
MORIKAWA, T. & YANAI, E. (1986) Toxic gases evolution from
air-controlled fires in a semi-full scale room. J. Fire Sci., 4:
299-314.
MOULE, Y. & FRAYSSINET, C. (1971) Effects of acrolein on
transcription in vitro. Fed. Eur. Biochem. Soc. Lett., 16:
216-218.
MULDERS, E.J. & DHONT, J.H. (1972) The odour of white bread. III.
Identification of volatile carbony compounds and fatty acids. Z.
Lebensm. Unters. Forsch., 150: 228-232.
MUNSCH, N. & FRAYSSINET, C. (1971) Action de l'acroléine sur les
syntheses d'acides nucléiques in vivo. Biochimie, 53: 243-248.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1973) Effects of
acrolein on DNA synthesis in vitro. Fed. Eur. Biochem. Soc.
Lett., 30: 286-289.
MUNSCH, N., MARANO, F., & FRAYSSINET, C. (1974a) Incorporation
d'acroleine 3H dans le foie du rat et chez Dunaliella bioculata.
Biochimie, 56: 1433-1436.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1974b) In vitro
binding of 3H-acrolein to regenerating rat liver DNA polymerase.
Experientia, 30: 1234-1236.
MURPHY, S.D. (1965) Mechanism of the effect of acrolein on rat liver
enzymes. Toxicol. appl. Pharmacol., 7: 833-843.
MURPHY, S.D. & PORTER, S. (1966) Effects of toxic chemicals on some
adaptive liver enzymes, liver glycogen, and blood glucose in fasted
rats. Biochem. Pharmacol., 15: 1665-1676.
MURPHY, S.D., KLINGSHIRN, D.A., & ULRICH, C.E. (1963) Respiratory
response of guinea pigs during acrolein inhalation and its
modification by drugs. J. Pharmacol. exp. Ther., 141: 79-83.
MURPHY, S.D., DAVIS, H.V., & ZARATZIAN, V.L. (1964) Biochemical
effects in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
NATUSCH, D.F.S. (1978) Potentially carcinogenic species emitted to
the atmosphere by fossil-fueled power plants. Environ. Health
Perspect., 22: 79-90.
NIELSEN, G.D., BAKBO, J.C., & HOLST, E. (1984) Sensory irritation
and pulmonary irritation by airborne allyl acetate, allyl alcohol,
and allyl ether compared to acrolein. Acta pharmacol. toxicol., 54:
292-298.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1986) Determination of
micro amounts of acrolein in air by gas chromatography. J.
Chromatogr., 370: 327-332.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987a) Gas chromatographic
determination of acrolein in rain water using bromination of
O-methyloxime. Analyst, 112: 45-48.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987b) Determination of
acrolein and crotonaldehyde in automobile exhaust gas by gas
chromatography with electron-capture detection. Analyst, 112:
859-862.
OBERDORFER, P.E. (1971) The determination of aldehydes in automobile
exhaust gas. In: Vehicle emissions, Part III, Society of Automotive
Engineers Prog. Tech., Vol. 14, pp. 32-42.
OHARA, T., SATO, T., SHIMUZU, N., PRESCHER, G., SCHWIND, H., &
WEIBURG, O. (1987) Acrolein and methacrolein. In: Encyclopedia of
chemical technology, Deerfield Beech, Florida, Ullmann Verlag
Chemie.
O'LOUGHLIN, E.M. & BOWMER, K.H. (1975) Dilution and decay of aquatic
herbicides in flowing channels. J. Hydrol., 26: 217-235.
OLSON, K.L. & SWARIN, S.J. (1985) Determination of aldehydes and
ketones by derivatization and liquid chromatography-mass
spectrometry. J. Chromatogr., 333: 337-347.
OSBORNE, A.D., PITTS, J.N., & DARLEY, E.F. (1962) On the stability
of acrolein towards photooxidation in the near ultra-violet. Int.
J. Air Water Pollut., 6: 1-3.
PATEL, J.M., WOOD, J.C., & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparations. Drug Metab. Disposal, 8: 305-308.
PATEL, J.M., ORTIZ, E., KOLMSTETTER, C., & LEIBMAN, K.C. (1984)
Selective inactivation of rat lung and liver microsomal
NADPH-cytochrome c reductase by acrolein. Drug Metab. Disposal, 12:
460-463.
PEREZ, J.M., LIPARI, F., & SEIZINGER, D.E. (1984) Cooperative
development of analytical methods for diesel emissions and
particulates - solvent extractables, aldehydes and sulfate methods.
In: Diesel exhaust emissions, Society of Automotive Engineers, pp.
95-114 (Special Publication No. 578).
PETTERSSON, B., CURVALL, M., & ENZELL, C.R. (1980) Effects of
tobacco smoke compounds on the noradrenaline induced oxidative
metabolism in isolated brown fat cells. Toxicology, 18: 1-15.
PFAFFLI, P. (1982) III. Industrial hygiene measurements. Scand. J
Work Environ. Health, 8(suppl. 2): 27-43.
PHILIPPIN, C., GILGEN, A., & GRANDJEAN, E. (1970) Etude
toxicologique et physiologique de l'acroléine chez la souris. Int.
Arch. Arbeitsmed., 26: 281-305.
PILOTTI, A., ANCKER, K., ARRHENIUS, E., & ENZELL, C. (1975) Effects
of tobacco and tobacco smoke constituents on cell multiplication in
vitro. Toxicology, 5: 49-62.
PLOTNIKOVA, M.M. (1957) [Data on hygienic evaluation of acrolein as
a pollution of the atmosphere.] Gig. i Sanit., 22(6): 10-15 (in
Russian).
POSTEL, W. & ADAM, L. (1983) [Gas chromatographic characterization
of raspberry brandy.] Dtsch. Lebensm. Rundschau, 79: 117-122 (in
German).
POTTS, W.J., LEDERER, T.S., & QUAST, J.F. (1978) A study of the
inhalation toxicity of smoke produced upon pyrolysis and combustion
of polyethylene foams. Part I. Laboratory studies. J. Combust.
Toxicol., 5: 408-433.
PRESSMAN, D. & LUCAS, H.J. (1942) Hydration of unsaturated
compounds. XI. Acrolein and acrylic acid. J. Am. Chem. Soc., 64:
1953-1957.
PROTSENKO, G.A., TRUBILKO, V.I., & SAVCHENKOV, V.A. (1973) Working
conditions when metals to which primer has been applied are welded
evaluated from the health and hygiene aspect. Avt. Svarka, 2:
65-68.
RANDALL, T.L. & KNOPP, P.V. (1980) Detoxification of specific
organic substances by wet oxidation. J. Water Pollut. Control Fed.,
52: 2117-2130.
RAPOPORT, I.A. (1948) [Mutations under the influence of unsaturated
aldehydes.] Dokl. Akad. Nauk. SSSR, 16: 713-715 (in Russian).
RATHKAMP, G., TSO, T.C., & HOFFMANN, D. (1973) Chemical studies on
tobacco smoke. XX: Smoke analysis of cigarettes made from bright
tobaccos differing in variety and stalk positions. Beitr.
Tabakforsch., 7: 179-189.
RENZETTI, N.A. & BRYAN, R.J. (1961) Atmospheric sampling for
aldehydes and eye irritation in Los Angeles smog. J. Air Pollut.
Control Assoc., 11: 421-424, 427.
RICHTER, M. & ERFURTH, I. (1979) [On the gas chromatographic
determination of acrolein in its original state in the main stream
smoke of cigarettes.] Ber. Inst. Tabaksforsch. 26: 36-45 (in
German).
RICKERT, W.S., ROBINSON, J.C., & YOUNG, J.C. (1980) Estmating the
hazards of "less hazardous" cigarettes. I. Tar, nicotine, carbon
monoxide, acrolein, hydrogen cyanide, and total aldehyde deliveries
of Canadian cigarettes. J. Toxicol. environ. Health, 6: 351-365.
RIETZ, B. (1985) Determination of three aldehydes in the air of
working environments. Anal. Lett., 18(A19): 2369-2379.
RIJSTENBIL, J.W. & VAN GALEN, G.C. (1981) Chemical control of mussel
settlement in a cooling water system using acrolein. Environ,
Pollut., A25: 187-195.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver aldehyde
dehydrogenases. Relation to allyl alcohol hepatoxicity. Metab.
Disposal, 15: 356-362.
ROSENTHALER, L. & VEGEZZI, G. (1955) [Acrolein in spirits.] Z.
Lebensm. Unters. Forsch., 102: 117-123 (in German).
RYLANDER, R. (1973) Toxicity of cigarette smoke components: free
lung cell response in acute exposures. Am. Rev. respir. Dis., 108:
1279-1282.
SAITO, T., TAKASHINA, T., YANAGISAWA, S., & SHIRAI, T. (1983)
[Determination of trace low molecular weight aliphatic compounds in
auto exhaust by gas chromatography with a glass capillary column.]
Bunseki Kagaku, 32: 33-38 (in Japanese).
SAKATA, T., SMITH, R.A., GARLAND, E.M., & COHEN, S.M. (1989) Rat
urinary bladder epithelial lesions induced by acrolein. J. environ.
Pathol. Toxicol., 9: 159-170.
SALAMAN, M.H. & ROE, F.J.C. (1956) Further tests for tumour-
initiating activity: N,N-di-(2-chloroethyl)-p-aminophenylbutyric
acid (CB1348) as an initiator of skin tumour formation in the mouse.
Br. J. Cancer, 10: 363-378.
SALEM, H. & CULLUMBINE, H. (1960) Inhalation toxicities of some
aldehydes. Toxicol. appl. Pharmacol., 2: 183-187.
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHIELKE, D.-J. (1987) [Gastrectomy following a rare caustic
lesion.] Chirurg, 58: 50-52 (in German).
SCHMID, B.P., GOULDING, E., KITCHIN, K., & SANYAL, M.K. (1981)
Assessment of the teratogenic potential of acrolein and
cyclophosphamide in a rat embryo culture system. Toxicology, 22:
235-243.
SCHUCK, E.A. & RENZETTI, N.A. (1960) Eye irritants formed during
photo-oxidation of hydrocarbons in the presence of oxides of
nitrogen. J. Air Pollut. Control Assoc., 10: 389-392.
SCHUTTE, N.P. (1977) Hazard evaluation and technical assistance
report No. TA 77-11: Xomed Company, Cincinnati, Ohio, Cincinnati,
Ohio, USA, National Institute for Occupational Safety and Health
(NIOSH-TR-TA-77-11, PB 278834).
SEIZINGER, D.E. & DIMITRIADES, B. (1972) Oxygenates in exhaust from
simple hydrocarbon fuels. J. Air Pollut. Control Assoc., 22: 47-51.
SERTH, R.W., TIERNEY, D.R., & HUGHES, T.W. (1978) Source
assessment: acrylic acid manufacture, Cincinnati, Ohio, US
Environmental Protection Agency, Industrial Environmental Research
Laboratory (Report EPA-600/2-78-004w).
SHAPIRO, R., SODUM, R.S., EVERETT, D.W., & KUNDU, S.K. (1986)
Reactions of nucleosides with glyoxal and acrolein. In: The role of
cyclic nucleic acid adducts in carcinogenesis and mutagenesis,
Proceedings of a meeting organized by the IARC and co-sponsored by
the US National Cancer Institute, and the Lawrence Berkely
Laboratory at the University of California, Lyon, 17-19 September,
Lyon, International Agency for Research on Cancer, pp. 165-173 (IARC
Scientific Publications No. 70).
SHERWOOD, R.L., LEACH, C.L., HATOUM, N.S., & ARANYI, C. (1986)
Effects of acrolein on macrophage functions in rats. Toxicol.
Lett., 32: 41-49.
SHIMOMURA, M., YOSHIMATSU, F., & MATSUMOTO, F. (1971) [Fish odour of
cooked horse mackerel.] Kaseigaku Zasshi, 2: 106-112 (in Japanese).
SIM, V.M. & PATTLE, R.E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1908-1913.
SINKUVENE, D. (1970) [Hygienic assessment of acrolein as an
atmospheric pollutant.] Gig. i Sanit., 35(3): 6-10 (in Russian).
SKOG, E. (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde,
propionaldehyde and butyraldehyde, as well as of acrolein and
crotonaldehyde. Acta pharmacol., 6: 299-318.
SLOTT, V.L. & HALES, B.F. (1985) Teratogenicity and embryolethality
of acrolein and structurally related compounds in rats. Teratology,
32: 65-72.
SLOTT, V.L. & HALES, B.F. (1986) The embryolethality and
teratogenicity of acrolein in cultured rat embryos. Teratology, 34:
155-163.
SLOTT, V.L. & HALES, B.F. (1987a) Enhancement of the embryotoxicity
of acrolein, but not phosphoramide mustard, by glutathione depletion
in rat embryos in vitro. Biochem. Pharmacol., 36: 2019-2025.
SLOTT, V.L. & HALES, B.F. (1987b) Protection of rat embryos in
culture against the embryotoxicity of acrolein using exogenous
glutathione., Biochem. Pharmacol., 36: 2087-2194.
SMITH, R.A., COHEN, S.M., & LAWSON, T.A. (1990) Acrolein
mutagenicity in the V79 assay. Carcinogenesis, 11: 497-498.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SMYTHE, R.J. & KARASEK, F.W. (1973) The analysis of diesel engine
exhaust for low-molecular-weight carbonyl compounds. J.
Chromatogr., 86: 228-231.
SNYDER, J.M., FRANKEL, E.N., & SELKE, E. (1985) Capillary gas
chromatographic analyses of headspace volatiles from vegetable oils.
J. Am. Oil Chem. Soc., 6: 1675-1679.
SPONHOLZ, W.-R. (1982) [Analysis and occurrence of aldehydes in
wines.] Z. Lebensm. Unters. Forsch., 174: 458-462 (in German).
SPRINCE, H., PARKER, C.M., & SMITH, G.G. (1979) Comparison of
protection by L-ascorbic acid, L-cysteine, and adrenergic-blocking
agents against acetaldehyde, acrolein, and formaldehyde toxicity:
implications in smoking. Agents Actions, 9: 407-414.
STACK, V.T. (1957) Toxicity of alpha,ß-unsaturated carbonyl
compounds to microorganisms. Ind. eng. Chem., 49: 913-917.
STAHLMANN, R., BLUTH, U., & NEUBERT, D. (1985) Effects of the
cyclophosphamide metabolite acrolein in mammalian limb bud cultures.
Arch. Toxicol., 57: 163-167.
STEINHAGEN, W.H. & BARROW, C.S. (1984) Sensory irritation
structure-activity study of inhaled aldehydes in B6C3F1 and
Swiss-Webster mice. Toxicol. appl. Pharmacol., 72: 495-503.
STEPHENS, E.R., DARLEY, E.F., TAYLOR, O.C., & SCOTT, W.E. (1961)
Photochemical reaction products in air pollution. Int. J. Air Water
Pollut., 4: 79-100.
SUBDEN, R.E., KRIZUS, A., & AKHTAR, M. (1986) Mutagen content of
Canadian apple eau-de-vie. Can. Inst. Food Sci. Technol. J., 19:
134-136.
SUZUKI, Y. & IMAI, S. (1982) Determination of traces of gaseous
acrolein by collection on molecular sieves and fluorimetry with
o-aminobiphenyl. Anal. Chim. Acta, 136: 155-162.
SWARIN, S.J. & LIPARI, F. (1983) Determination of formaldehyde and
other aldehydes by high performance liquid chromatography with
fluorescence detection. J. liq. Chromatogr., 6: 425-444.
SZOT, R.J. & MURPHY, S.D. (1971) Relationships between cyclic
variations in adrenocortical secretory activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant compounds.
J. Water Pollut. Control Fed., 53: 1503-1517.
TAJIMA, M., KIDA, K., & FUJIMAKI, M. (1967) Effect of gamma
irradiation on volatile compounds from cooked potato. Agric. biol.
Chem., 31: 935-938.
TAKEUCHI, K. & IBUSUKI, T. (1986) Heterogeneous photochemical
reactions of a propylene-nitrogen dioxide-metal oxide-dry air
system. Atmos. Environ., 20: 1155-1160.
TEJADA, S.B. (1986) Evaluation of silica gel cartridges coated in
situ with acidified 2,4-dinitrophenylhydrazine for sampling
aldehydes and ketones in air. Int. J. environ. anal. Chem., 26:
167-185.
TESTA, A. & JOIGNY, C. (1972) Dosage par chromatographie en phase
gazeuse de l'acroléine et d'autres composés alpha,ß-insaturés de la
phase gazeuse de la fumée de cigarette. Ann. Serv. Exploit. ind.
Tab. Allumettes - Div. Etud. Equip., 10: 67-81.
TORAASON, M., LUKEN, M.E., BREITENSTEIN, M., KRUEGER, J.A., &
BIAGINI, R.E. (1989) Comparative toxicity of allylamine and acrolein
in cultured myocytes and fibroblasts from neonatal rat heart.
Toxicology, 56: 107-117.
TREITMAN, R.D., BURGESS, W.A., & GOLD, A. (1980) Air contaminants
encountered by firefighters. Am. Ind. Hyg. Assoc. J., 41: 796-802.
TURUK-PCHELINA, Z.F. (1960) [Acrolein releases into the air while
cooking.] Gig. i Sanit., 25(5): 96-97 (in Russian).
UMANO, K. & SHIBAMOTO, T. (1987) Analysis of headspace volatiles
from overheated beef fat. J. agric. food Chem., 35: 14-18.
UNRAU, G.O., FAROOQ, M., DAWOOD, I.K., MIGUEL, L.C., & DAZO, B.C.
(1965) Field trials in Egypt with acrolein herbicide-molluscicide.
World Health Organ. Bull., 32: 249-260.
US-NIOSH (1984) NIOSH manual of analytical methods, 3rd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (DHHS (NIOSH) Publication No. 84-100. Method 2501).
VAN EICK, A.J. (1977) [The effect of acrolein in air on the eye
blinking frequency of man and guinea pig], Rijswijk, The
Netherlands, Technical and Physical Research, Medical Biological
Laboratory, TNO-MBL, (Report No. A76/K/098) (in Dutch).
VAN OVERBEEK, J., HUGHES, W.J., & BLONDEAU, R. (1959) Acrolein for
the control of water weeds and disease-carrying water snails.
Science, 129: 335-336.
VEITH, G.D., MACEK, K.J., PETROCELLI, S.R., & CARROL, J. (1980) An
evaluation of using partition coefficients and water solubility to
estimate bioconcentration factors for organic chemicals in fish.
Aquat. Toxicol.: 116-129.
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene problems
in vulcanization processes of rubber production]. Gig. i Sanit.,
34: 33-40 (in Russian).
VOROB'EVA, A.I., VOLKOTRUB, L.P., FEOKTISTOVA, N.F., BOBIN, V.I.,
SHESTAKOVA, N.A., & USHAKOVA, N.S. (1982) [Characteristics of
atmospheric pollution from enameled wire manufacturing plants.]
Gig. i Sanit., 1982(6): 66-67 (in Russian).
WARHOLM, M., HOLMBERG, B., & MALMFORS, T. (1984) The cytotoxicity of
some organic solvents. Environ. Res., 7: 183-192.
WATANABE, T. & AVIADO, D.M. (1974) Functional and biochemical
effects on the lung following inhalation of cigarette smoke and
constituents. II. Skatole, acrolein, and acetaldehyde. Toxicol.
appl. Pharmacol. 30: 201-209.
WEBER-TSCHOPP, A., FISCHER, T., & GRANDJEAN, E. (1976) [Objective
and subjective physiological effects of passive smoking.] Int. Arch.
occup. environ. Health, 37: 277-288 (in German).
WEBER-TSCHOPP, A., FISCHER, T., GIERER, R., & GRANDJEAN, E. (1977)
[Experimental irritation by acrolein in human beings.] Z.
Arbeitswiss., 32: 166-171 (in German).
WHITEHOUSE, M.W., BECK, F.W.J., DROGE, M.M., & STRUCK, R.F. (1974)
Lymphocyte deactivation by (potential immunosuppressant) alkylating
metabolites of cyclophosphamide. Agents Actions, 4: 117-123.
WHO (1991) Health and Safety Guide 67: Acrolein, Geneva, World
Health Organization.
WILMER, J.L., EREXSON, G.L., & KLIGERMAN, A.D. (1986) Attenuation of
cytogenetic damage by 2-mercaptoethanesulphonate in cultured human
lymphocytes exposed to cyclophosphamide and its reactive
metabolites. Cancer Res., 46: 203-210.
WILTON, D.C. (1976) Acrolein, an irreversible active-site-directed
inhibitor of deoxyribose 5-phosphate aldolase? Biochem. J., 153:
495-497.
WITZ, G., LAWRIE, N.J., AMORUSO, M.A., & GOLDSTEIN, B.D. (1987)
Inhibition by reactive aldehydes of superoxide anion radical
production from stimulated polymorphonuclear leukocytes and
pulmonary alveolar macrophages. Biochem. Pharmacol., 36: 721-726.
WRABETZ, E., PETER, G., & HOHORST, H.J. (1980) Does acrolein
contribute to the cytotoxicity of cyclophosphamide? J. Cancer Res.,
98: 119-126.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20
coded compounds tested for the National Toxicology Program.
Environ. Mutagen., 7: 87-100.
ZITTING, A. & HEINONEN, T. (1980) Decrease of reduced glutathione in
isolated rat hepatocytes caused by acrolein, acrylonitril, and the
thermal degradation products of styrene copolymers. Toxicology, 17:
333-341.
ZOLLNER, H. (1973) Inhibition of some mitochondrial functions by
acrolein and methylvinylketone. Biochem. Pharmacol., 22: 1171-1178.
ZORIN, V.M. (1966) [Acrolein-induced air pollution.] Zdravookhr.
Belorussii, 1966(7): 43-44 (in Russian).
RESUME
L'acroléine est un liquide volatil extrêmement inflammable dont
l'odeur, âcre et suffocante, est très désagréable. C'est un composé
très réactif.
En 1975, on estime que la production mondiale d'acroléine en
tant que telle était de 59 000 tonnes. On en produit et consomme
encore davantage comme intermédiaire pour la synthèse de l'acide
acrylique et de ses esters.
On dispose d'un certain nombre de méthodes d'analyse pour la
recherche et le dosage de l'acroléine dans divers milieux. On a fait
état de limites inférieures de détection de l'ordre de 0,1 µg/m3
dans l'air (chromatographie en phase gazeuse/spectrométrie de
masse), de 0,1 µg/l dans l'eau (chromatographie liquide à haute
pression), de 2,8 µg/litre dans les milieux biologiques
(fluorimétrie), de 590 µg/kg dans le poisson (chromatographie en
phase gazeuse/spectrométrie de masse) et de 1,4 µg/m3 dans les gaz
d'échappement (chromatographie liquide à haute pression).
On a trouvé de l'acroléine dans certains produits d'origine
végétale et animale et notamment dans des denrées alimentaires et
des boissons. L'acroléine est utilisée principalement comme
intermédiaire en synthèse organique, mais également comme produit
biocide en milieu aquatique.
Des émissions d'acroléine peuvent se produire sur les lieux de
production ou d'utilisation. Elles peuvent être importantes dans
l'air à la suite de la combustion ou de la pyrolyse incomplète de
produits organiques tels que combustibles, polymères de synthèse,
dans certains aliments et le tabac. L'acroléine peut représenter
jusqu'à 3-10 % des aldéhydes totaux présents dans les gaz
d'échappement des véhicules à moteur. La consommation d'une
cigarette fournit de 3 à 228 µg d'acroléine. L'acroléine est un
produit d'oxydation photochimique de certains polluants organiques
de l'atmosphère.
La population générale est essentiellement exposée par
l'intermédiaire de l'air. Une exposition peut également se produire
par voie orale par suite de la consommation de boissons alcoolisées
ou de denrées alimentaires chauffées.
On a mesuré dans l'air des villes des concentrations moyennes
d'acroléine atteignant environ 15 µg/m3 avec des maxima allant
jusqu'à 32 µg/m3. A proximité d'installations industrielles et de
pots d'échappement, des concentrations dix à cent fois plus élevées
sont possibles. Les incendies peuvent donner naissance à des teneurs
très élevées d'acroléine, de l'ordre du mg/m3 d'air. A
l'intérieur des habitations, on a observé que la consommation d'une
cigarette par m3 d'air dans un local en l'espace de 10 à
13 minutes produisait des concentrations en vapeurs d'acroléine de
l'ordre de 450 à 840 µg/m3. Sur les lieux de travail, on a signalé
des teneurs dépassant 100 µg/m3 dans des cas où l'on élevait la
température de certains produits organiques, par exemple lors du
chauffage ou du soudage de ces substances.
Dans l'atmosphère, l'acroléine est dégradée par réaction avec
les radicaux hydroxyles. Sa durée de séjour dans l'atmosphère est de
l'ordre d'une journée. Dans les eaux de surface, l'acroléine se
dissipe en quelques jours. Elle est faiblement adsorbée aux
particules du sol. On a fait état de dégradation aérobie et
anaérobie, encore que la toxicité du composé pour les
micro-organismes puisse faire obstacle à sa biodégradation. Compte
tenu des propriétés physiques et chimiques de l'acroléine, il ne
semble pas que cette substance ait une tendance à la
bioaccumulation.
L'acroléine est extrêmement toxique pour les organismes
aquatiques. Pour les bactéries, les algues, les crustacés et les
poissons, sa toxicité aiguë, estimée d'après les valeurs de la CE50
et de la CL50, se situe entre 0,02 et 2,5 mg/litre, les bactéries
étant l'espèce la plus sensible. Pour le poisson, on a fixé à
0,0114 mg/litre la dose sans effet nocif observable à 60 jours.
L'acroléine détruit efficacement les végétaux aquatiques à des doses
comprises entre 4 et 26 mg/litre.h. A partir de 15 mg/litre, on
observe des effets nocifs sur les cultures irriguées au moyen d'eau
traitée à l'acroléine.
Chez l'homme et l'animal, l'acroléine reste confinée sur son
site d'exposition en raison de sa réactivité et les observations
pathologiques sont également limitées à ce site. Chez des chiens
exposés à des doses de 400 à 600 mg/m3, on a observé un taux de
rétention de 80 à 85 % au niveau des voies respiratoires.
L'acroléine réagit directement sur les groupements sulfhydryles
protéiques et non protéiques ainsi que sur les amines primaires et
secondaires. Elle peut également être métabolisée en acide
mercapturique, en acide acrylique, en glycidaldéhyde ou en
glycéraldéhyde. Les trois derniers métabolites n'ont été observés
qu' in vitro.
L'acroléine est un agent cytotoxique. Sa cytotoxicité s'observe
in vitro dès 0,1 mg/litre. Elle est extrêmement toxique pour les
animaux de laboratoire et l'homme, à la suite d'une seule exposition
quelle qu'en soit la voie. Sa vapeur est irritante pour l'oeil et
les muqueuses respiratoires. Le liquide est corrosif et on a
constaté qu'en solution éthanolique le seuil d'apparition d'une
dermatite d'irritation était de 0,1%. L'expérimentation sur des
volontaires humains exposés à des vapeurs d'acroléine a permis de
fixer à 0,13 mg/m3 la dose la plus faible produisant des effets
nocifs observables; à cette dose, une irritation des yeux se produit
en l'espace de cinq minutes. En outre, les effets au niveau des
voies respiratoires deviennent évidents à partir de 0,7 mg/m3. Une
seule exposition à des doses plus élevées entraîne une
dégénérescence de l'épithélium respiratoire, des séquelles
inflammatoires et une perturbation de la fonction respiratoire.
On a étudié sur des rats, des chiens, des cobayes et des singes
les effets toxicologiques de l'inhalation continue d'acroléine à des
concentrations de 0,5 à 4,1 mg/m3. Des effets histopathologiques
et des effets sur la fonction respiratoire ont été observés chez les
animaux exposés à des teneurs supérieures ou égales à 0,5 mg/m3
pendant 90 jours.
On a étudié sur divers animaux de laboratoire les effets
toxicologiques d'expositions répétées par la voie respiratoire à des
vapeurs d'acroléine, à des concentrations allant de 0,39 mg/m3 à
11,2 mg/m3. La durée de l'exposition allait de cinq jours à
52 semaines. En général, on a fait état chez la plupart des espèces
exposées huit heures par jour à des concentrations de 1,6 mg/m3 ou
davantage, d'une réduction du gain de poids, d'une diminution de la
fonction respiratoire et de modifications pathologiques au niveau du
nez, des voies respiratoires supérieures et des poumons. Parmi les
modifications anatomopathologiques figuraient une inflammation, une
métaplasie et une hyperplasie des voies respiratoires. On a observé
une mortalité importante après expositions répétées à des vapeurs
d'acroléine à des concentrations dépassant 9,07 mg/m3. Chez
l'animal d'expérience, on a montré que l'acroléine provoquait une
déplétion du glutathion tissulaire in vivo et une inhibition des
enzymes in vitro par réaction sur les groupements sulfhydryles au
niveau des sites actifs. Il existe quelques données selon lesquelles
l'acroléine est susceptible d'amoindrir les défenses pulmonaires de
l'hôte chez la souris et le rat.
L'acroléine peut produire des effets tératogènes et
embryotoxiques lorsqu'on l'introduit directement dans l'amnios.
Toutefois, l'absence d'effets chez des lapins à qui elle avait été
injectée par voie intraveineuse à la dose de 3 mg/kg incite à penser
que l'exposition de l'homme à l'acroléine ne devrait pas avoir
d'effet nocif sur le développement de l'embryon.
On a montré que l'acroléine interagissait avec les acides
nucléiques in vitro et en inhibait la synthèse tant in vitro
qu' in vivo. Sans avoir besoin d'être activée, elle produit des
mutations géniques chez les bactéries et les champignons et induit
des échanges entre chromatides soeurs dans les cellules
mammaliennes. Dans tous les cas, ces effets se sont produits dans un
intervalle de dose extrêmement limité qui était fonction de la
réactivité, de la volatilité et de la cytotoxicité de l'acroléine.
Une épreuve de mutation létale dominante chez la souris a donné des
résultats négatifs. Les données disponibles montrent que l'acroléine
est faiblement mutagène pour certains champignons et bactéries et
certaines cultures de cellules mammaliennes.
Des hamsters ont été exposés pendant 52 semaines à des vapeurs
d'acroléine à la dose de 9,2 mg/m3, 7 heures par jour et 5 jours
par semaine, puis ont été placés en observation pendant les 29
semaines suivantes; aucune tumeur n'a été observée. En exposant ces
hamsters dans les mêmes conditions à des vapeurs d'acroléine et
pendant la même durée avec, en outre, des doses intra-trachéennes
hebdomadaires de benzo[a]pyrène ou des doses sous-cutanées une fois
toutes les trois semaines de diéthylnitrosamine, on n'a pas non plus
observé d'effets co-cancérogènes bien nets attribuables à
l'acroléine. Des rats exposés par voie orale à de l'acroléine dans
leur eau de boisson à des doses comprises entre 5 et 50 mg/kg par kg
de poids corporel (quotidiennement, cinq jours par semaine pendant
100 à 124 semaines) n'ont pas présenté de tumeur. En raison du
caractère limité de toutes ces épreuves, on estime que les données
qui permettraient d'évaluer la cancérogénicité de l'acroléine chez
l'animal d'expérience sont encore insuffisantes. De ce fait, il est
impossible pour l'instant d'évaluer la cancérogénicité de
l'acroléine pour l'homme.
Les différents seuils de concentration auxquels apparaissent
les différents effets de l'acroléine sont les suivants : perception
d'une odeur, 0,007 mg/m3, irritation oculaire, 0,3 mg/m3,
irritation du nez et clignement des yeux, 0,03 mg/m3, réduction de
la fréquence respiratoire, 0,7 mg/m3. Comme la concentration de
l'acroléine dépasse rarement 0,03 mg/m3 dans l'air des villes,
elle n'est pas susceptible de constituer une nuisance dans les
circonstances normales.
Du fait de sa forte toxicité pour les organismes aquatiques,
l'acroléine présente un danger pour la faune et la flore aquatique à
proximité ou sur les sites de décharge de déchets industriels, en
cas de déversements et là où l'on utilise ce produit comme biocide.
1. RESUMEN
La acroleína es un líquido volátil, sumamente inflamable, con
un olor pungente, asfixiante y desagradable. Se trata de un
compuesto muy reactivo.
La producción mundial de acroleína aislada se calculó en 59 000
toneladas en 1975. Se produce y consume una cantidad aún mayor de
acroleína como intermediaria en la síntesis de ácido acrílico y sus
ésteres.
Se dispone de métodos analíticos para determinar la acroleína
presente en diversos medios. Los límites mínimos de detección que
se han comunicado son 0,1 µg/m3 de aire (cromatografía
gaseosa/spectrometría de masas), 0,1 µg/litro de agua (cromatografía
líquida a alta presión), 2,8 µg/litro de medio biológico
(fluorimetría), 590 µg/kg en peces (cromatografía
gaseosa/espectrometría de masas), y 1,4 µg/m3 de gases de escape
(cromatografía líquida a alta presión).
La acroleína se ha detectado en algunos vegetales y animales,
inclusive en alimentos y bebidas. La sustancia se usa
principalmente como intermediaria en la síntesis química aunque
también como biocida acuático.
Pueden producirse emisiones de acroleína en sus lugares de
producción o de uso. Las emisiones importantes a la atmósfera se
deben a la combustión incompleta o la pirólisis de materiales
orgánicos como ser combustibles, polímeros sintéticos, alimentos y
tabaco. La acroleína puede representar el 3-10% de los aldehídos
totales presentes en los escapes de automóviles. El humo de un
cigarrillo libera 3-228 µg de acroleína. La acroleína es uno de los
productos de la oxidación fotoquímica de ciertos contaminantes
orgánicos de la atmósfera.
La exposición de la población general se produce principalmente
por el aire. La exposición por vía oral puede producirse por el
consumo de bebidas alcohólicas o alimentos calentados.
En la atmósfera urbana se han medido niveles promedio de
acroleína de hasta unos 15 µg/m3 y niveles máximos de hasta
32 µg/m3. En las cercanías de las industrias y junto a los caños
de escape pueden registrarse niveles entre 10 y 100 veces
superiores. Como resultado de incendios pueden hallarse niveles
sumamente elevados en el aire, del orden de mg/m3. En el aire
cerrado de interiores, el consumo de un cigarrillo por m3 de
volumen de la habitación produjo en 10-13 minutos concentraciones de
vapor de acroleína de 450-840 µg/m3. En el medio ambiente laboral
se han detectado niveles de más de 1000 µg/m3 en situaciones que
entrañaban aumento de temperatura de materiales orgánicos, por
ejemplo durante la soldadura o el calentamiento.
La acroleína se degrada en la atmósfera por reacción con
radicales hidroxilo. El tiempo de persistencia en la atmósfera es
de aproximadamente un día. En aguas de superficie, la acroleína se
disipa en pocos días. Tiene un bajo potencial de adsorción en el
suelo. Se ha observado su degradación en condiciones aerobias y
anaerobias, si bien la toxicidad del compuesto para los
microorganismos puede impedir la biodegradación. En vista de sus
propiedades físicas y químicas, es improbable que se produzca
bioacumulación de acroleína.
La acroleína es sumamente tóxica para los organismos acuáticos.
Los valores de la CE50 y la CL50 correspondientes a bacterias,
algas, crustáceos y peces se encuentran entre 0,02 y 2,5 mg/litro,
siendo las bacterias los organismos más sensibles. En peces se ha
determinado que el nivel sin observación de efectos adversos (NOAEL)
a 60 días es de 0,0114 mg/litro. Se ha conseguido combatir
eficazmente los vegetales acuáticos con dosis de acroleína
comprendidas entre 4 y 26 mg/litro.h. Se han observado efectos
adversos en cultivos que crecen en suelos irrigados con agua tratada
con acroleína en concentraciones de 15 mg/litro o más.
En el animale y en el ser humano la reactividad de la acroleína
limita efectivamente la sustancia al lugar de exposición; los
hallazgos patológicos se limitan asimismo a esos lugares. En el
tracto respiratorio de perros expuestos a 400-600 mg/m3 se
encontró una retención del 80-85% de acroleína. La acroleína
reacciona directamente con los grupos sulfhidrilo contenidos en
radicales proteicos o no proteicos y con aminas primarias y
secundarias. También puede ser metabolizado a ácidos mercaptúricos,
ácido acrílico, glicidaldehído o gliceraldehído. Estos tres últimos
metabolitos sólo se han encontrado in vitro.
La acroleína es un agente citotóxico. Se ha observado
citotoxicidad in vitro con niveles de solamente 0,1 mg/litro. La
sustancia es sumamente tóxica para los animales de experimentación y
el ser humano tras una exposición única por diferentes vías. El
vapor es irritante para los ojos y el tracto respiratorio. En
estado líquido es corrosiva. Con respecto a la dermatitis
irritante, se encontró que el NOAEL de la acroleína etanólica era de
0,1%. Los experimentos con voluntarios humanos expuestos a vapores
de acroleína mostraron un nivel mínimo de observación de efectos
(LOAEL) de 0,13 mg/m3, dosis con la que los ojos pueden irritarse
al cabo de cinco minutos. Además, se observan efectos en el tracto
respiratorio a partir de 0,7 mg/m3. Con exposiciones aisladas a
niveles más altos, aparecen: degeneración del epitelio respiratorio,
secuelas inflamatorias y trastorno de la función respiratoria.
Los efectos toxicológicos de la exposición por inhalación
continua de concentraciones comprendidas entre 0,5 y 4,1 mg/m3 se
han estudiado en la rata, el perro, el cobayo y el mono. Se
observaron efectos sobre la función respiratoria y trastornos
histopatológicos cuando se expuso a los animales a niveles de
acroleína de 0,5 mg/m3 o más, durante 90 días.
Los efectos toxicológicos de la inhalación repetida de vapores
de acroleína en concentraciones comprendidas entre 0,39 mg/m3 y
11,2 mg/m3 se han estudiado en diversos animales de laboratorio.
Las duraciones de la exposición variaron entre 5 días y hasta 52
semanas. En general, se han documentado: reducción de la
adquisición de peso corporal, disminución de la función pulmonar y
cambios patológicos en la nariz, las vías aéreas superiores y los
pulmones en la mayoría de las especies expuestas a concentraciones
de 1,6 mg/m3 o más, durante 8 h/día. Entre los cambios
patológicos se observaron inflamación, metaplasia e hiperplasia del
tracto respiratorio. Se ha observado un nivel significativo de
mortalidad tras la exposición repetida a concentraciones de vapor de
acroleína superiores a 9,07 mg/m3. En animales de
experimentación, se ha demostrado que la acroleína agota el
glutatión tisular y que in vitro inhibe enzimas reaccionando con
los grupos sulfhidrilo de los sitios activos. Hay limitada
evidencia de que la acroleína pueda deprimir las defensas pulmonares
en el ratón y la rata.
La acroleína puede inducir efectos teratogénicos y
embriotóxicos si se administra directamente en el amnios. No
obstante, el hecho de que no se observaran efectos en ratones a los
que se inyectó 3 mg/kg por vía intravenosa sugiere que la exposición
humana a la acroleína tiene pocas probabilidades de afectar al
embrión en desarrollo.
Se ha demostrado que la acroleína interacciona con los ácidos
nucleicos in vitro y que inhibe su síntesis tanto in vitro como
in vivo. Sin activación, indujo mutaciones génicas en bacterias y
hongos y provocó intercambios de cromátidas hermanas en células de
mamíferos. En todos los casos esos efectos se produjeron en un
margen muy reducido de concentraciones, limitado por la reactividad,
la volatilidad y la citotoxicidad de la acroleína. Un ensayo de
letalidad dominante en ratones dio resultado negativo. Los datos
disponibles muestran que la acroleína es un mutágeno débil para
ciertas bacterias, hongos y cultivo celular de mamífero.
No se encontraron tumores en hámsters expuestos durante 52
semanas a vapores de acroleína con una concentración de 9,2 mg/m3
durante 7 h/día, 5 días a la semana, y observados durante 29 semanas
más. Cuando se expusieron hámsters a vapores de acroleína en las
mismas condiciones durante 52 semanas y, además, a dosis
intratraqueales de benzo[a]pireno semanalmente o a dosis subcutáneas
de dietilnitrosamina una vez cada tres semanas, no se observó una
acción cocarcinogénica clara de la acroleína. La exposición de
ratas por vía oral a la acroleína en el agua de bebida, en dosis
comprendidas entre 5 y 50 mg/kg de peso corporal al día
(5 días/semana durante 104-124 semanas) no indujo tumores. Dado el
carácter limitado de todos esos ensayos, se considera que no se
dispone de datos suficientes para determinar la carcinogenicidad de
la acroleína en los animales de experimentación. En consecuencia,
se considera asimismo imposible evaluar la carcinogenicidad de la
acroleína para el ser humano.
Los umbrales de acroleína que causan irritación y efectos en la
salud son 0,07 mg/m3 en el caso de la percepción del olor,
0,13 mg/m3 en la irritación ocular, 0,3 mg/m3 en la irritación
nasal y el parpadeo, y 0,7 mg/m3 en la disminución del ritmo
respiratorio. Puesto que el nivel de acroleína raras veces supera
los 0,03 mg/m3 en el aire urbano, es poco probable que alcance
niveles molestos o nocivos en circunstancias normales.
En vista de la elevada toxicidad de la acroleína para los
organismos acuáticos, la sustancia representa un riesgo para la vida
acuática en las proximidades de las zonas donde se producen vertidos
y escapes industriales, y en los lugares donde se usa como biocida.
 Relative molecular 56.06
mass:
Common name: acrolein
Common synonyms: acraldehyde, acrylaldehyde (IUPAC name),
acrylic aldehyde, propenal, prop-2-enal,
prop-2-en-1-al
Common trade Acquinite, Aqualin, Aqualine, Biocide,
names: Magnicide-H, NSC 8819, Slimicide
CAS chemical name: 2-propenal
CAS registry
Relative molecular 56.06
mass:
Common name: acrolein
Common synonyms: acraldehyde, acrylaldehyde (IUPAC name),
acrylic aldehyde, propenal, prop-2-enal,
prop-2-en-1-al
Common trade Acquinite, Aqualin, Aqualine, Biocide,
names: Magnicide-H, NSC 8819, Slimicide
CAS chemical name: 2-propenal
CAS registry  7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Mortality
The available acute mortality data are summarized in Table 8.
Most tests for the determination of the acute toxicity of acrolein
do not comply with present standards. Nevertheless, retesting is
not justified for ethical reasons and in view of the overt high
toxicity of acrolein following inhalation or oral exposure (Hodge &
Sterner, 1943).
In addition to the data in Table 8, an oral LD95 of
11.2 mg/kg body weight for Charles River rats, observed for 24 h,
has been reported (Sprince et al., 1979). Draminski et al.
(1983) reported the deaths of 5/10 rats given 10 mg/kg body weight
in corn oil by gavage.
7.1.2 Effects on the respiratory tract
In vapour exposure tests, the effects observed in experimental
animals have almost exclusively been local effects on the
respiratory tract and eyes.
In the LC50 studies, effects on the respiratory tract were
clinically observed as nasal irritation and respiratory distress in
rats (Skog, 1950; Potts et al., 1978; Crane et al., 1986),
hamsters (Kruysse, 1971), mice, guinea-pigs, and rabbits (Salem &
Cullumbine, 1960) at exposure levels of between 25 mg/m3 for 4 h
and 95 150 mg/m3 for 3 min. Rats exposed for 10 min to
concentrations of 750 or 1000 mg/m3 suffered asphyxiation
(Catilina et al., 1966).
Histopathological investigations in experiments with
vapour-exposed rats (Skog, 1950; Catilina et al., 1966; Potts
et al., 1978; Ballantyne et al., 1989), hamsters (Kilburn &
McKenzie, 1978), guinea-pigs (Dahlgren et al., 1972; Jousserandot
et al, 1981), and rabbits (Beeley et al., 1986) revealed varying
degrees of degeneration of the respiratory epithelium consisting of
deciliation (see also in vitro work on cytotoxicity discussed in
7.1.5), exfoliation, necrosis, mucus secretion, and vacuolization.
Also observed were acute inflammatory changes consisting of
infiltration of white blood cells into the mucosa, hyperaemia,
haemorrhages, and intercellular oedema. Proliferative changes of
the respiratory epithelium, in the form of early stratification and
hyperplasia, were observed in hamsters 96 h after exposure to
13.7 mg/m3 for 4 h (Kilburn & McKenzie, 1978).
Table 8. Acute mortality caused by acrolein
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Rat (Wistar) male inhalation (10 min) 8 750 Catilina et al. (1966)b
Rat (Wistar) not reported oral 14 46 (39-56) Smyth et al. (1951)g
Rat (unspecified not inhalation (30 min) 21 300 Skog (1950)b,c
strain) reported
Rat (Sprague-Dawley) male inhalation (30 min) 14 95-217 Potts et al. (1978)d
Rat (Sprague-Dawley) male and inhalation (1 h) 14 65 (60-68) Ballantyne et al. (1989)
female inhalation (4 h) 14 20.8 (17.5-24.8)
Rat (Sherman) male and inhalation (4 h) 14 18 Carpenter et al. (1949)b,e
female
Hamster (Syrian golden) male and inhalation (4 h) 14 58 (54-62) Kruysse (1971)
female
Table 8 (contd)
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Mouse (unspecified male inhalation (6 h) 1 151 Philippin et al. (1970)f
strain)
Mouse (NMRI) not reported intraperitoneal 6 7 Warholm et al. (1984)g
a Where available, 95% confidence limits are given in parentheses.
b Determination of acrolein levels was not reported.
c No mortality at 100 mg/m3, 100% mortality at 700 mg/m3.
d Approximate value: no mortality at 33 mg/m3, 1/7 and 7/7 died at 95 and 217 mg/m3, respectively.
e Approximate value: 2-4/6 died.
f No mortality at 71 mg/m3, 100% mortality at 273 mg/m3.
g The vehicle was water.
Functional changes in the respiratory system following acrolein
vapour exposure have been investigated in guinea-pigs and mice. A
rapidly reversible increase in respiratory rate was observed in
intact guinea-pigs during exposure to 39 mg/m3 for 60 min (Davis
et al., 1967) and to 0.8 mg/m3 or more for 2 h (Murphy et al.,
1963) followed by a decrease in respiratory rate and an increase in
tidal volume. No changes in pulmonary compliance were reported.
Davis et al. (1967) did not observe these effects in
tracheotomized animals and concluded that they were caused by reflex
stimulation of upper airway receptors and not by
bronchoconstriction. Murphy et al. (1963), observing that
anticholinergic bronchodilators, aminophylline and isoproterenol,
but not antihistaminics, reduced the acrolein-induced increase in
respiratory resistance, concluded that acrolein caused
bronchoconstriction mediated through reflex cholinergic stimulation.
In another experiment, an increase in respiratory resistance was
also observed in anaesthetized, tracheotomized guinea-pigs with
transected medulla during exposure to 43 mg/m3 for up to 5 min
(Guillerm et al., 1967b). The effect was not reversed by atropine.
It was concluded by the authors that acrolein did not cause
bronchoconstriction via reflex stimulation, but probably via
histamine release. When anaesthetized mice were exposed to 300 or
600 mg/m3 for 5 min via a tracheal cannula, respiratory
resistance, respiratory rate, and tidal volume decreased and
pulmonary compliance increased at an unspecified time after exposure
(Watanabe & Aviado, 1974).
The concentration that produces a 50% decrease in respiratory
rate (RD50) as a result of reflex stimulation of trigeminal nerve
endings in the nasal mucosa (sensory irritation) has been used as an
index of upper respiratory tract irritation. This effect reduces
the penetration of noxious chemicals into the lower respiratory
tract. The rate of respiration was measured in a body
plethysmograph, only the animals' heads being exposed to the
acrolein vapour. Depending on the strain, RD50 values for mice
ranged from 2.4 to 6.6 mg/m3 (Kane & Alarie, 1977; Nielsen
et al., 1984; Steinhagen & Barrow, 1984). In rats a RD50 of
13.7 mg/m3 was found (Babiuk et al., 1985).
7.1.3 Effects on skin and eyes
Animal skin irritation tests have not been performed and skin
irritation has not been mentioned as an effect in the acute
inhalation tests reported.
One special in vivo eye irritation test involved
vapour-exposed and control rabbits. At analysed concentrations of
acrolein (method not specified) between 4.3 and 5.9 mg/m3,
maintained over 4 h, slight chemosis was observed but no iritis
(Mettier et al., 1960). Eye irritation was observed clinically in
rodents in several acute inhalation tests, but was not graded (Skog,
1950; Salem & Cullumbine, 1960; Kruysse, 1971; Potts et al.,
1978).
7.1.4 Systemic effects
With respect to systemic effects, most studies have been
performed at concentrations far above the lethal dose. When rats
were exposed to concentrations of acrolein between 1214 and
95 150 mg/m3 during various periods of time, incapacitation,
indicated by the inability to walk in a rotating cage, and
convulsions were observed after 2.8 min at the highest concentration
and after 27 to 34 min at the lowest concentration. These effects
were followed by death after several minutes. Cyanosis of the
extremities and agitation were observed at levels of 22 900 mg/m3
or more (Crane et al., 1986).
The effects of acrolein on the cardiovascular system were
investigated by Egle & Hudgins (1974). Rats anaesthetized by sodium
pentobarbital and exposed only via the mouth and nose to
concentrations between 10 and 5000 mg/m3 for 1 min showed an
increase in blood pressure at all exposure levels. The heart rate
was increased at concentrations from 50 mg/m3 to 500 mg/m3 but
decreased at 2500 and 5000 mg/m3. Intravenous experiments
suggested that increased blood pressor responses resulted from the
release of catecholamines from sympathetic nerve endings and from
the adrenal medulla and that the decreased heart rate effect was
mediated by the vagus nerve (Egle & Hudgins, 1974).
In an acute oral test with rats exposed at 11.2 mg/kg body
weight, decreased reflexes, body sag, poor body tone, lethargy,
stupor, and tremors were observed, as well as respiratory distress
(Sprince et al., 1979).
Because acrolein was shown to induce acute cytotoxicity of the
rat urinary bladder mucosa when instilled directly into the bladder
lumen (Chaviano et al., 1985), this end-point was investigated
in vivo. Two days after a single oral or intraperitoneal dose of
25 mg/kg body weight to ten rats per group, focal simple hyperplasia
of the urinary bladder was detected in the three surviving rats
dosed intraperitoneally. None of the orally exposed rats showed
this effect, but all exhibited severe erosive haemorrhagic
gastritis. Both orally and intraperitoneally exposed rats showed
eosinophilic degeneration of hepatocytes. No abnormalities were
observed in sections of lungs, kidneys, and spleen. Acrolein was
also administered intraperitoneally at single doses of 0.5, 1, 2, 4,
or 6 mg/kg body weight. Proliferation of the bladder mucosa was
evaluated autoradiographically by measuring [3H-methyl]thymidine
incorporation in exposed versus control rats 5 days after the
intraperitoneal injection of acrolein and was found to be increased
nearly two-fold at the highest dose. Body weight gain was decreased
at the two highest doses. Histopathological evaluation of the liver
and urinary bladder did not reveal abnormalities (Sakata et al.,
1989).
7.1.5 Cytotoxicity in vitro
As shown in Table 9, mammalian cell viability is affected by
acrolein in vitro at nominal concentrations of 0.1 mg/litre or
more (not corrected for interaction with culture medium components
or volatilization). The concentration at which formaldehyde
exhibited a similar degree of cytotoxicity was about 6 to 100 times
higher (Holmberg & Malmfors, 1974; Pilotti et al., 1975; Koerker
et al., 1976; Krokan et al., 1985).
Acrolein is a known inhibitor of respiratory tract ciliary
movement in vitro. After a 20-min exposure to an acrolein
concentration of 34-46 mg/m3, the ciliary beating frequency of
excised sheep trachea decreased by 30% (Guillerm et al., 1967a).
Exposure to 13 mg/m3 for 1 h is the greatest exposure that does
not stop ciliary activity in excised rabbit trachea (Dalhamn &
Rosengren, 1971). The no-observed-effect-level for longer exposure
periods would be expected to be lower than 13 mg/m3. Other
in vitro investigations into the inhibition of ciliary movement by
acrolein were reviewed by Izard & Libermann (1978).
7.2 Short-term exposure
7.2.1 Continuous inhalation exposure
In two subchronic inhalation studies with rats, changes in
weight gain, longevity, behaviour, and several physiological
parameters were reported (Gusev et al., 1966; Sinkuvene, 1970).
Unfortunately, the reports did not give sufficient details on the
exposure conditions and protocols and the studies are thus of
limited value in evaluating the toxicological properties of
acrolein.
Table 9. In vitro cytotoxicity of acrolein
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Rat cardiac fibroblasts/myocytes 4 increased lactate Toraason et al. (1989)
dehydrogenase release > 2.8
Rat cardiac myocytes 2 abolished myocine beat > 2.8
dehydrogenase
4 decreased ATP levels > 0.56
Mouse Ehrlich Landschutz 5 92% survivala 1 Holmberg & Malmfors (1974)
Diploid ascites tumour cells 5 53% survivala 5
Mouse B P8 ascites sarcoma cells 48 20% growth rate inhibition 0.6 Pilotti et al. (1975)
48 94% growth rate inhibition 5.6
Mouse C1300 neuroblastoma cells 24 50% survivala 1.7 Koerker et al. (1976)
Mouse L 1210 leukaemia cells 1 70-80% survivala 1.1 Wrabetz et al. (1980)
1 < 15% survivala 2.8
Chinese hamster ovary cells 5 100% mitotis inhibition 0.6 Au et al. (1980)
Adult human bronchial 1 92% colony-forming efficiency 0.06 Krokan et al. (1985)
fibro-blasts 1 45% colony-forming efficiency 0.2
Table 9 (contd).
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Adult human lymphocytes 48 decreased replicative index 0.6 Wilmer et al. (1986)
48 100% mitosis inhibition 2.2
Human K562 chromic myeloid 1 marked reduction in > 0.3 Crook et al. (1986a,b)
leukaemia cells colony-forming ability
Human bronchial epithelial cells 1 20% colony-forming efficiency 0.06 Grafström et al. (1988)
1 50% colony-forming efficiency 0.06-0.17
1 50% survivala 0.34
3 clonal growth rate inhibition > 0.17
3 increase in cross-linkage
envelope formation > 0.06
3 decreased plasminogen
activator activity > 0.56
Human fibroblasts 5 63% cell count reduction < 0.017 Curren et al. (1988)
DNA-repair deficient human
fibroblasts 5 63% cell count reduction 0.045
a measured as dye exclusion
Groups of 7 or 8 Sprague-Dawley rats of both sexes, 7 or 8
Princeton or Hartley-derived guinea-pigs of both sexes, 2 male
pure-bred Beagle dogs, and 9 male squirrel-monkeys were exposed to a
vapourized acrolein-ethanol-water mixture for 90 days (Lyon et al.,
1970). The measured acrolein concentrations were 0, 0.5 (two groups
for each species), 2.3, and 4.1 mg/m3 and the ethanol
concentrations were below 18.7 mg/m3. Pathological investigations
did not include weighing of tissues and organs or examination of the
tracheas at the lowest exposure level. There was no
treatment-related mortality. One monkey died at 0.5 mg/m3 and one
at 2.3 mg/m3 due to accidental infections. Body weight gain
reduction was only found in rats at 2.3 and 4.1 mg/m3.
Clinically, ocular discharge and salivation were observed in dogs at
2.3 and 4.1 mg/m3 and in monkeys at 4.1 mg/m3. Monkeys kept
their eyes closed at 2.3 mg/m3. No adverse effects on
haematological or biochemical parameters were observed in any of the
animals. At necropsy, occasional pulmonary haemorrhage and focal
necrosis in the liver were found in three rats at 2.3 mg/m3.
Pulmonary inflammation and occasional focal liver necrosis were also
observed in guinea-pigs at this concentration. Sections of lung
from two of the four dogs exposed at 0.5 mg/m3 revealed focal
vacuolization, hyperaemia, and increased secretion of bronchiolar
epithelial cells, slight bronchoconstriction, and moderate
emphysema. At 2.3 mg/m3, focal inflammatory reactions involved
lung, kidney, and liver. Bronchiolitis and early broncho-pneumonia
were seen in one dog. At 4.1 mg/m3, both dogs had confluent
bronchopneumonia. All nine monkeys at 4.1 mg/m3 showed squamous
metaplasia and six of them showed basal cell hyperplasia in the
trachea. None of the species revealed other treatment-related
changes (Lyon et al., 1970).
Bouley et al. (1975) exposed a total of 173 male SPF-OFA rats
to a measured acrolein vapour concentration of 1.26 mg/m3 for a
period of 15 to 180 days and used control groups of equal size. No
mortality occurred. Sneezing was observed from day 7 to day 21 in
the treated animals, and body weight gain and food consumption were
reduced. There was an increase in relative lung weight in rats
killed on day 77 but not in rats killed on days 15 or 32. The
relative liver weight was decreased at day 15 but not thereafter,
and the number of alveolar macrophages was decreased at days 10 and
26 but not at days 60 or 180. When groups of 16 rats were infected
by one LD50 dose of airborne Salmonella enteriditis on day 18 or
day 63, mortality increased from 53% in controls to 94% in the
exposed rats infected on day 18. No changes were observed in
biochemical parameters, including the amount of liver DNA per mg of
protein in a group of partially hepatectomized rats, or in the
response of spleen lymphocytes to phytohaemagglutinin in rats
exposed for 39 to 57 days. Other end-points were not investigated.
7.2.2 Repeated inhalation exposure
Lyon et al. (1970) exposed groups of rats, guinea-pigs, dogs,
and monkeys to acrolein vapour at concentrations of 0, 1.6. and
8.5 mg/m3 for 8 h per day and 5 days per week over 6 weeks. With
the exception of the exposure levels, period, and frequency, the
protocol was the same as that for the continuous inhalation exposure
described in section 7.2.1. Two deaths occurred among the nine
monkeys at 8.5 mg/m3. There was body weight gain reduction in
rats and body weight loss (not statistically significant) in monkeys
at 8.5 mg/m3. Clinically, eye irritation and salivation were
observed in dogs and monkeys and difficult breathing in dogs at
8.5 mg/m3. No adverse effects on haematological or biochemical
parameters were observed in any of the animals. At necropsy,
sections of lung from all animals exposed to 1.6 mg/m3 showed
chronic inflammatory changes. Additionally, some showed emphysema.
At 8.5 mg/m3, squamous metaplasia and basal cell hyperplasia were
observed in the trachea of both dogs and monkeys. In addition,
bronchopneumonia was noted in dogs and necrotizing bronchitis and
bronchiolitis in monkeys. Focal calcification of the tubular
epithelium was noted in the kidneys of rats and monkeys at
8.5 mg/m3.
Groups of male Sprague-Dawley rats were also exposed to
acrolein vapour at measured concentrations of 0, 0.39, 2.45, and
6.82 mg/m3 for 6 h per day and 5 days per week over 3 weeks (Leach
et al., 1987). Subgroups were used for immunological
investigations (section 7.4) and for histopathological examination
of nasal turbinates and lungs. Body weight gain was depressed from
week 1 up to the end of the exposure period at 6.82 mg/m3.
Absolute, but not relative, spleen weight was reduced at this
exposure level. There were no histological effects on the lungs,
but the respiratory epithelium of the nasal turbinates showed
exfoliation, erosion, and necrosis, as well as dysplasia and
squamous metaplasia at 6.82 mg/m3. In addition, the mucous
membrane covering the septum and lining the floor of the cavity
showed hyperplasia and dysplasia (Leach et al., 1987).
Another experiment involved Dahl rats of two lines, one
susceptible (DS) and one resistant (DR) to salt-induced hypertension
(Kutzman et al., 1984). Groups of 10 female rats of each line were
exposed to measured acrolein concentrations of 0, 0.89, 3.21, and
9.07 mg/m3 for 6 h per day and 5 days per week over 61-63 days.
One week after the exposure, survivors were killed for pathological
and compositional analysis of the lung following behavioural and
clinical chemistry testing. At 9.07 mg/m3, all DS rats died
within 11 days and 4 DR rats died within the exposure period.
Reduced body weights were measured in the surviving DR rats during
the first 3 weeks, followed by an almost normal body weight gain.
Biochemical changes were found in DR rats at 9.07 mg/m3 and
included increases in lung hydroxyproline and elastin, serum
phosphorus, and in the activities of serum alkaline phosphatase,
alanine aminotransferase (EC 2.6.1.2), and aspartate
aminotransferase (EC 2.6.1.1). No effects were observed on
exploratory behaviour, locomotor activity, blood pressure, lung
protein, blood urea nitrogen, or on serum creatinine, uric acid, or
calcium. At necropsy of survivors, DR rats exposed to 9.07 mg/m3
had increased relative weights of several organs, especially the
lungs. It was noted by the authors that the exposed rats gained a
considerable amount of weight during the week following exposure.
In both rat lines, concentration-related increases were observed in
lymphoid aggregates in pulmonary parenchyma, in collections of
intra-alveolar macrophages with foamy cytoplasm, and in
hyperplastic/metaplastic terminal bronchiolar epithelial changes.
Multifocal interstitial pneumonitis and squamous metaplasia of the
tracheal epithelium were also found in DR rats exposed to
9.07 mg/m3. In contrast, dead and moribund rats, especially those
of the DS strain, mainly exhibited severe bronchial and bronchiolar
epithelial necrosis with exfoliation, oedema, haemorrhage, and
varying degrees of bronchopneumonia. Adverse effects were absent in
nasal turbinates and in non-pulmonary tissues (Kutzman et al.,
1984).
In follow-up studies, groups of 32 to 57 male Fischer-344 rats
were exposed in exactly the same way as described for the Dahl rats.
Surviving rats were tested for pulmonary function one week after
exposure and then killed and examined for compositional analysis,
morphometry, and (in nine rats per group) pathological changes in
the lung (Kutzman et al., 1985; Costa et al., 1986). At
9.07 mg/m3, 56% mortality occurred. After an initial body weight
loss over the first 10 days, weight gain became comparable to that
of controls. There was an increase in the relative weight of
several organs, especially the lungs. Lungs also showed an increase
in water content and in the levels of elastin and hydroxyproline,
but not in the levels of protein and DNA. The hydroxyproline level
was also elevated at 3.21 mg/m3. Histologically, surviving rats
treated with 3.21 or 9.07 mg/m3 demonstrated an exposure-related
increase in effects on the respiratory tract consisting of
bronchiolar epithelial necrosis with exfoliation, bronchiolar
mucopurulent plugs, an increase in bronchiolar and alveolar
macrophages, and focal pneumonitis. At 3.21 mg/m3, there was
type II cell hyperplasia and at 9.07 mg/m3 tracheal,
peribronchial, and alveolar oedema and acute rhinitis. The severity
of lung lesions was highly variable and three of the nine rats
examined at 9.07 mg/m3 did not exhibit histological damage.
Moribund rats mainly showed severe acute bronchopneumonia and focal
alveolar and tracheal oedema with exfoliation in the bronchi and
bronchioles (Kutzman et al., 1985). In another report from the
same research group, the results of pulmonary function testing and
morphometry disclosed air-flow dysfunction at 9.07 mg/m3, which
was correlated with the presence of focal peribronchial lesions and
the lung elastin concentrations. In contrast, the rats exposed at
0.89 mg/m3 exhibited enhanced flow-volume dynamics, whereas no
effects on lung function were present in the 3.21-mg/m3 group
(Costa et al., 1986).
Groups of six Wistar rats and ten Syrian golden hamsters of
both sexes were exposed to acrolein vapour at measured
concentrations of 0, 0.9, 3.2, and 11.2 mg/m3 for 6 h per day and
5 days per week over 13 weeks (Feron et al., 1978). Within the
first month of exposure to 11.2 mg/m3, half the number of rats of
each sex died. One hamster died at this exposure level because of
renal failure. A treatment-related decrease in body weight gain and
food intake was observed in rats exposed to 3.2 mg/m3 or more.
Hamsters showed decreased body weight gain at 11.2 mg/m3, but food
intake was not examined. At this exposure level, all animals kept
their eyes closed, rats showed bristling hair, and hamsters showed
salivation and nasal discharge. Haematological investigation and
urinalysis in week 12 showed no changes in rats. In hamsters,
urinalysis revealed no changes, but females showed increases in the
number of erythrocytes, packed cell volume, haemoglobin content, and
number of lymphocytes and a decrease in the number of neutrophilic
leucocytes. Changes in relative organ weights, which were
considered by the authors to be related to the treatment, were found
in the lung, heart, and kidneys of both species and in the adrenals
of rats exposed to 11.2 mg/m3. Histological changes were confined
to the respiratory tract. In the nose, rats exhibited an
exposure-related increase in squamous metaplasia and neutrophilic
infiltration of the mucosa at levels of 0.9 mg/m3 or more (at
0.9 mg/m3 each effect was observed in one male) and occasional
necrotizing rhinitis at 11.2 mg/m3. Hamsters also showed these
effects at 11.2 mg/m3 but only minimal inflammatory changes at
3.2 mg/m3. In the larynx and trachea of rats exposed to
11.2 mg/m3, squamous metaplasia was also observed and was
accompanied by hyperplasia in bronchi and bronchioli. At this
exposure level, the larynx of hamsters was slightly thickened and
focal hyperplasia and metaplasia were found in the trachea.
Inflammatory changes were present in the bronchi, bronchioli, and
alveoli of rats and included haemorrhage, oedema, accumulations of
alveolar macrophages, an increase in mucus-producing cells in the
bronchioli, and bronchopneumonia. The authors noted considerable
variation between individual rats in the degree of the lesions
(Feron et al., 1978).
Feron & Kruysse (1977) exposed groups of 36 Syrian golden
hamsters of both sexes to acrolein vapour at measured levels of 0
and 9.2 mg/m3 for 7 h per day and 5 days per week over 52 weeks.
Except for 6 males and 6 females, the hamsters were observed for a
further 29 weeks after the exposure period. Overall mortality was
38% in exposed hamsters and 33% in controls. Body weight was
slightly and reversibly decreased at the end of the exposure period.
The other effects observed at the end of the exposure period were
essentially similar (but less severe) to those described above for
hamsters exposed to 11.2 mg/m3 for 13 weeks, but hyperplasia was
not observed. Histological changes were restricted to the anterior
half of the nasomaxillary turbinates and were still found in 20% of
the animals at week 81. At that time they mainly consisted of a
thickened submucosa and exudation into the lumen. Epithelial
metaplasia, but not hyperplasia, was noted. No tumours were found.
Histopathological examination of the respiratory tract of male
Swiss-Webster mice was the object of a study involving groups of 16
to 24 male mice exposed to measured concentrations of 3.9 mg/m3
for 6 h per day during 5 days (Buckley et al., 1984). The lesions
observed were restricted to the nose and were most severe in the
anterior respiratory epithelium and on the free margins of the
nasomaxillary turbinates and the adjacent nasal septum. They
consisted of severe deciliation, moderate exfoliation, erosion,
ulceration and necrosis, severe squamous metaplasia, moderate
neutrophilic infiltration, and a slight serofibrinous exudate.
Lesions in the olfactory epithelium were largely confined to the
dorsal meatus and consisted of moderate ulceration and necrosis, and
slight squamous metaplasia. The nasal squamous epithelium was not
affected (Buckley et al., 1984).
One special investigation concerned the effects of acrolein
vapour on the respiratory functions of male Swiss mice exposed to
100 mg/m3 for two daily periods of 30 min each for 5 weeks. Body
weights were not affected. There was a decrease in pulmonary
compliance, but no effects were found on pulmonary resistance,
respiratory volume, or functional residual capacity. The lungs
showed an increase in phospholipid content (Watanabe & Aviado,1974).
In summary, the toxicological effects on a variety of
laboratory animals from repeated inhalation exposure to acrolein
vapour at concentrations ranging from 0.39 mg/m3 to 11.2 mg/m3
have been studied. Exposure durations ranged from 5 days to as long
as 52 weeks. In general, body weight gain reduction, decrement of
pulmonary function, and pathological changes in nose, upper airways,
and lungs have been documented in most species exposed to acrolein
concentrations of 1.6 mg/m3 or more. Pathological changes include
inflammation, metaplasia, and hyperplasia of the respiratory tract.
Significant mortality has been observed following repeated exposures
to acrolein vapour at concentrations above 9.07 mg/m3.
7.2.3 Repeated intraperitoneal exposure
Groups of ten intact or adrenalectomized NMRI mice were
injected intraperitoneally with saline or acrolein in water at daily
doses of 4 to 16 mg/kg body weight for 1 to 6 days. One week after
the last injection the mice were killed for autopsy.
Clinical signs of toxicity were hunched posture, inactivity,
and ruffled fur. Total body weight and relative thymus and spleen
weights showed a dose-related reduction, while the adrenals showed
an increase in relative weight. Histologically, thymic necrosis and
splenic atrophy were the only changes observed. These changes were
absent in controls and in adrenalectomized mice. The levels of
reduced glutathione and the activity of glutathione S-transferase
in liver cytosol were unchanged, but the rate of glutathione
synthesis was increased. Repeated exposure to acrolein caused a
progressively less pronounced effect on mortality (Warholm et al.,
1984).
7.3 Biochemical effects and mechanisms of toxicity
7.3.1 Protein and non-protein sulfhydryl depletion
A dose-related non-protein sulfhydryl (reduced glutathione)
depletion was observed in the nasal respiratory mucosa of male
Fischer rats after nose-only exposure for 3 h to acrolein vapour at
concentrations of 0.23-11.4 mg/m3 (McNulty et al., 1984; Lam
et al., 1985). Depletion of glutathione in the liver was not
observed at these exposure levels (McNulty et al., 1984). The
glutathione depletion in the nasal mucosa appeared irreversible at
11.4 mg/m3 (McNulty et al., 1984). In female C3Hf/HeHa mice,
intraperitoneally exposed once to doses between 20 and 80 mg/kg body
weight and killed 2 h later, a dose-related decrease in liver
glutathione levels was observed. These doses are however extremely
high considering the fact that a dose of 4.5 mg/kg body weight was
lethal within 1.7 h (Gurtoo et al., 1981a).
A dose-related in vitro glutathione depletion has been
observed in human bronchial fibroblasts (Krokan et al., 1985),
human bronchial epithelial cells (Grafström et al., 1988), human
chronic myeloid leukemia cells (Crook et al., 1986b), and human
and rat phagocytic cells (Witz et al., 1987) from the lowest
acrolein concentration tested (56 µg/litre). The effect has also
been reported to occur in isolated rat hepatocytes (Zitting &
Heinonen, 1980; Dawson et al., 1984; Dore & Montaldo, 1984; Ku &
Billings, 1986) and in rat liver or lung microsomal suspensions
(Patel et al., 1984), the lowest-observed-effect level being
1400 µg/litre (Dawson et al., 1984). Ku & Billings (1986)
observed that both mitochondrial and cytosolic glutathione levels
were decreased.
As a result of acrolein exposure, there was a decrease in the
level of both membrane surface and soluble protein sulfhydryl groups
in in vitro human and rat phagocytic cells (Witz et al., 1987)
and a decrease in the level of soluble protein sulfhydryl compounds
in human bronchial epithelial cells (Grafström et al., 1988).
Acrolein has also been shown to cause a decrease in membrane surface
protein sulfhydryl groups in rat hepatocytes (Ku & Billings, 1986)
and to reduce the protein sulfhydryl content of liver and lung
microsomal preparations (Patel et al., 1984).
7.3.2 Inhibition of macromolecular synthesis
When partially hepatectomized Wistar rats were exposed
intraperitoneally to a single acrolein dose of 0.5, 1.6, 2.0, or
2.7 mg/kg body weight, a dose-related inhibition of the synthesis of
DNA and RNA was measured in liver and lung cells (Munsch &
Frayssinet, 1971).
Inhibition of DNA, RNA, and/or protein synthesis has been
observed in Escherichia coli (Kimes & Morris, 1971), the slime
mold Physarum polycephalum (Leuchtenberger et al., 1968), the
alga Dunaliella bioculata (Marano & Puiseux-Dao, 1982), and in
in vitro mammalian cells such as mouse kidney cells (Leuchtenberger
et al., 1968) and polyoma transformed Chinese hamster cells
(Alarcon, 1972). Acrolein was shown to inhibit RNA polymerase in
isolated rat liver nuclei (Moule & Frayssinet, 1971) and isolated
rat liver DNA polymerase (Munsch et al., 1973). The activity of
the latter enzyme is associated with at least one functional
sulfhydryl group, and preincubation of the enzyme with
2-mercaptoethanol protected against the inhibitory action of
acrolein. Since acrolein did not inhibit isolated Escherichia coli
polymerase I, devoid of sulfhydryl groups in its active centre,
Munsch et al. (1973) suggested that the inhibitory action of
acrolein is caused by a reaction with sulfhydryl groups.
7.3.3 Effects on microsomal oxidation
In in vitro studies, acrolein has been shown to convert rat
liver cytochrome P-450 to cytochrome P-420 and to inhibit rat liver
NADPH-cytochrome-c reductase (EC 1.6.2.4) in a time- and
concentration-related fashion (Marinello et al., 1978; Ivanetich
et al., 1978; Berrigan et al., 1980; Gurtoo et al., 1981b;
Marinello et al., 1981; Patel et al., 1984; Cooper et al.,
1987). A concomitant decrease occurred in the activity of several
monooxygenases: benzphetamine N-demethylase, aniline hydroxylase,
ethylmorphine N-demethylase (Patel et al., 1984), and
7-ethoxyresorufin O-deethylase (Cooper et al., 1987). The
lowest-observed-effect levels reported were 2 mg/litre for
inactivation of cytochrome P-450 (Gurtoo et al., 1981b) and
25 mg/litre for inhibition of NADPH-cytochrome- c reductase
(Marinello et al.,1981). It was also shown that the addition of
sulfhydryl-containing agents, such as cysteine, acetylcysteine,
glutathione, dithiothreitol, and semicarbazide, reduced the above
effects, suggesting that acrolein produces them by reacting with
sulfhydryl groups at the active sites.
7.3.4 Other biochemical effects
In vivo studies with Holtzman rats have shown that rat liver
alkaline phosphatase (EC 3.1.3.1) and tyrosine aminotransferase
(EC 2.6.1.5) activities are increased markedly after inhalation of
acrolein for 4 h at a concentration of 14.7 mg/m3 or after a
single intraperitoneal injection of acrolein in water at doses of
1.5-6 mg/kg body weight (Murphy et al., 1964; Murphy, 1965). The
increase in alkaline phosphatase activity following intraperitoneal
injection was shown to be dose related (Murphy, 1965). The effects
were reduced by prior adrenalectomy or hypophysectomy or by
pretreatment with protein synthesis inhibitors such as actinomycin
D, puromycin, and ethionine, suggesting that the irritant action of
acrolein stimulates the pituitary-adrenal system to release
glucocorticoids, which act to increase the synthesis of adaptive
liver enzymes (Murphy, 1965; Murphy & Porter, 1966). Increased
plasma and adrenal levels of corticosterone were measured in
Holtzman rats one hour after a single intraperitoneal injection
(3 mg/kg body weight) of acrolein in water (Szot & Murphy, 1971).
The hypersecretion of glucocorticoids could also explain the
observed increase in liver glycogen level following intraperitoneal
exposure to acrolein at a dose of 1.5 mg/kg body weight (Murphy &
Porter, 1966).
At a concentration of 5.6 mg/litre, acrolein produced an 80%
inhibition of the noradrenaline-induced oxygen consumption of
isolated hamster brown fat cells (Pettersson et al., 1980). In
addition, Zollner (1973) observed an acrolein-induced inhibition of
the respiration of intact rat liver mitochondria and found evidence
for an inhibition at three different sites: glutamate transport,
inorganic phosphorus transport, and the enzyme succinic
dehydrogenase (EC 1.3.5.1).
Several sulfhydryl-sensitive enzymes have been shown to be
inhibited by acrolein in vitro, e.g., rabbit muscle L-lactate
dehydrogenase (EC 1.1.1.27), yeast glucose-6-phosphate
dehydro-genase (EC 1.1.1.49), and yeast alcohol dehydrogenase
(EC 1.1.1.1) (Benedict & Stedman, 1969), porcine lung
15-hydroxyprosta-glandin dehydrogenase (EC 1.1.1.141) (Liu & Tai,
1985), rat liver or urothelium S-adenosyl-L-methionine-
DNA(cytosine-5)- methyltransferase (EC 2.1.1.37) (Cox et al.,
1988), and O6-methylguanine-DNA methyltransferase (EC 2.1.1.63)
in cultured human bronchial fibroblasts (Krokan et al, 1985). In
two of these studies glutathione was shown to afford protection
against inhibition of the enzyme (Liu & Tai, 1985; Cox et al.,
1988).
It has been suggested that the formation of a Schiff base
between acrolein and sensitive amine groups is responsible for the
observed inhibition in vitro of Salmonella typhimurium
deoxyribose-5-phosphate aldolase (EC 4.1.2.4) at a concentration of
approximately 14 mg/litre (Wilton, 1976) and human plasma
alpha1-proteinase inhibitor (Gan & Ansari, 1987).
Acrolein was shown to cause a concentration-dependent increase
in lipid peroxidation in isolated rat hepatocytes at levels that
also decreased glutathione concentrations (Zitting & Heinonen,
1980). Preincubation of washed rat liver microsomes with acrolein
abolished the protective effect of glutathione against
iron/ascorbate-induced lipid peroxidation (Haenen et al, 1988). The
authors claimed that the protective effect of glutathione was
mediated by vitamin E scavenging membrane lipid radicals. It was
suggested that acrolein was inhibiting a glutathione-dependent
reductase enzyme responsible for reducing vitamin E radicals back to
vitamin E.
7.4 Immunotoxicity and host resistance
Acrolein has been found to depress pulmonary host defenses in a
number of tests.
In female Swiss mice, exposed to measured concentrations of
1.1, 6.9, and 14.2 mg/m3 for 8 h, a concentration-related increase
in the survival of Staphylococcus aureus was seen at levels of
6.9 mg/m3 or more (Astry & Jakab, 1983). A concentration- and
time-related increase in the survival of S. aureus and Proteus
mirabilis was found in male Swiss CD-1 mice exposed to measured
concentrations of 2.3 to 4.6 mg/m3 for 24 h. When the mice were
also infected with Sendai virus, intrapulmonary bacterial death was
further suppressed (Jakab, 1977). An increased survival of
Klebsiella pneumoniae, but no increased mortality from pneumonia
following challenges with Streptococcus zooepidemicus, was
observed in female CD-1 mice after exposure to acrolein at a
measured concentration of 0.23 mg/m3 for 3 h per day over 5 days
(Aranyi et al., 1986). In female CR/CD-1 mice, exposure to a
measured acrolein concentration of 4.6 mg/m3, for one period of
6 h or for 7 consecutive daily periods of 8 h, resulted in an
increased mortality from Streptococcus pyogenes and Salmonella
typhimurium, respectively, but not from influenza A virus (Campbell
et al., 1981).
Sherwood et al. (1986) exposed groups of 33 male
Sprague-Dawley rats to acrolein vapour at analysed concentrations of
0.39, 2.45 or 6.82 mg/m3 for 3 weeks (6 h per day and 5 days per
week). The relative pulmonary bactericidal activity to
K. pneumoniae was not affected nor was the number of alveolar
cells. However, the number of peritoneal macrophages was decreased
at concentrations of 2.45 mg/m3 or more, and alveolar and
peritoneal macrophages had altered phagocytic and enzymic patterns
at > 0.39 mg/m3.
When SPF-OFA rats were exposed continuously to a measured
acrolein concentration of 1.26 mg/m3 for 18 days, they exhibited
an increased mortality from an infection by Salmonella enteritidis.
However, no such effect was observed following 63 days of exposure
(Bouley et al., 1975).
Acrolein has been shown to inhibit in vitro protein synthesis
(Leffingwell & Low, 1979), and phagocytosis and ATPase (EC
3.6.1.37-38) activity (Low et al., 1977) in rabbit pulmonary
alveolar macrophages. Inhibition of a graft-versus-host reaction in
rats was found after Wistar rat spleen cells were incubated
in vitro with acrolein and injected into all four feet of hybrid
F1 rats. In addition, a decreased mitogen response of human
peripheral lymphocytes was recorded (Whitehouse et al., 1974).
Acrolein was also found to inhibit in vitro chemotaxis of human
polymorphonuclear leucocytes (Bridges et al., 1977).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Two in vivo exposure studies have been reported. In one, 3
male and 21 female SPF-OFA rats were exposed continuously to
acrolein vapour at a measured concentration of 1.26 mg/m3 for 25
days and allowed to mate on day 4. It should be noted that the
exposure period did not cover the complete spermatogenic period of
60 days. The number of pregnant animals and the number and mean
weight of the fetuses were unaffected in comparison to the control
rats (Bouley et al., 1985). In the second study, groups of 12 to
16 New Zealand rabbits were injected (into the ear vein) with a
solution of acrolein in saline (3, 4.5 or 6 mg/kg body weight) on
the 9th day of gestation. At 4.5 and 6 mg/kg body weight, maternal
toxicity was indicated by the death of 3 and 6 dams, respectively,
and embryotoxicity by a dose-related increase in resorptions which
was significant at 6 mg/kg body weight. It was also reported that
the number of malformed and retarded fetuses increased in a
dose-related manner, although the increases were statistically
non-significant. No effects on maternal toxicity, embryotoxicity or
fetuses were noted at 3 mg/kg body weight (Claussen et al., 1980).
A clear effect on the development of the embryo in vivo was
observed only when acrolein was administered close to the target
site by intra-amniotic injection. Using groups of 12 to 19 pregnant
New Zealand rabbits, 0, 10, 20, or 40 µl of a 0.84% solution of
acrolein in saline was injected into the amnion of all embryos in
one of the uterine horns on the 9th day of gestation. The embryos in
the other uterine horn received saline only and served as controls.
The dams were killed on day 28 of gestation. There was a
dose-related increase in the rate of resorptions and malformations,
significant at doses of 20 µl or more per embryo. Malformations
included deformed and asymmetric vertebrae, spina bifida, deformed
and fused ribs, and lack or fusion of sternum segments. No effect
was observed on the number of implantations and fetuses or on fetal
growth (Claussen et al., 1980). A similar study was carried out
on pregnant Sprague-Dawley rats injected with acrolein doses of 0,
0.1, 1.0, 2.5, 5.0, 10.0 or 100 µg per fetus in 10 ml of saline on
the 13th day of pregnancy. The dams were killed on day 20 of
gestation. A dose-related increase in the percentage of dead and
resorbed fetuses per litter was observed at all dose levels. The
total number of litters at each dose level varied from 4 to 18. The
percentage of malformed fetuses per litter also was increased in a
dose-related manner at doses of up to 5 µg per fetus. The increase
was significant only up to this dose level, probably because at
higher doses there were few surviving fetuses. Treatment-related
effects included oedema, micrognathia, hindlimb and forelimb
defects, and hydrocephaly (Slott & Hales, 1985). These results
confirmed the findings of an earlier, identical test using dose
levels of 0.1, 10, and 100 µg per fetus (Hales, 1982).
Acrolein was also shown to be embryotoxic and teratogenic in
the rat whole embryo culture system. As with embryos exposed in
vivo, the concentration range for teratogenicity was very narrow
(Slott & Hales, 1986). Schmid et al. (1981) and Mirkes et al.
(1984) observed embryotoxicity but no teratogenicity in the same
test system, this being probably the result of the different test
conditions used (Slott & Hales, 1986). Depletion of glutathione by
buthionine sulfoximine enhanced the embryotoxicity and
teratogenicity of acrolein in the in vitro studies of Slott &
Hales (1987a), whereas exogenous glutathione afforded protection
against these effects (Slott & Hales, 1987b).
In a mouse limb bud culture system, acrolein induced impairment
of limb bud differentiation, indicative of a teratogenic action
(Stahlmann et al., 1985). When acrolein was injected into chicken
eggs, embryotoxic and teratogenic effects were observed (Kankaanpaa
et al., 1979; Korhonen et al., 1983; Chhibber & Gilani, 1986).
In summary, acrolein can induce teratogenic and embryotoxic
effects if administered directly to the embryos or fetuses.
However, the fact that no effect was found in rabbits injected
intravenously with 3 mg/kg suggests that neither skin contact nor
inhalation of acrolein is likely to affect the developing embryo.
7.6 Mutagenicity and related end-points
7.6.1 DNA damage
In vitro studies have revealed interactions between acrolein
and DNA and RNA (Munsch et al., 1974b; section 6.2.1). Acrolein
has also been found to react with purine and pyrimidine bases or
intact DNA in vitro, and several adducts have been identified
(Descroix, 1972; Hemminki et al., 1980; Lutz et al., 1982; Chung
et al., 1984; Shapiro et al., 1986; section 6.2.2.2). Cyclic
deoxyguanosine DNA adducts were formed in a dose-dependent fashion
in acrolein-exposed Salmonella typhimurium TA100 and TA104. This
adduct formation correlated with the induction of reverse mutations
in these strains (section 7.6.2; Foiles et al., 1989).
No data on the formation of DNA adducts following exposure of
animals to acrolein are available.
Incubation of Fischer-344 rat nasal mucosal homogenate with
acrolein resulted in a concentration-dependent increase in
DNA-protein cross-linking, which was not observed following
inhalation exposure of rats to acrolein at a concentration of
4.6 mg/m3 for 6 h (Lam et al., 1985). According to the authors
this could be explained by the preferential reaction of acrolein
with sulfhydryl groups. DNA-protein cross-linking and single strand
breaks were observed in vitro in human bronchial fibroblasts at
cytotoxic concentrations of 1.7 mg/litre or more (Grafström et al.,
1986, 1988), and there was indirect evidence for some formation of
DNA interstrand cross-linking (Grafström et al., 1988). No
DNA-protein or DNA interstrand cross-linking was induced by acrolein
in mouse L1210 leukemia cells at cytotoxic levels that produced
single strand breaks and/or alkali-labile sites in these cells
(Erickson et al., 1980) or in human chronic myeloid leukemia cells
(Crook et al., 1986a). In non-mammalian assays, Fleer & Brendel
(1982) did not find DNA interstrand cross-linking or single strand
breaks in MB1072-2B yeast cells and Kubinski et al. (1981)
observed DNA-cell binding in Escherichia coli in the presence of a
rat liver S9 fraction. These studies demonstrate that effects on
DNA occur only at cytotoxic concentrations of acrolein.
Results of DNA repair tests are not available. Acrolein has
been demonstrated to inhibit O6-methylguanine-DNA
methyltransferase (EC 2.1.1.63.; section 7.3.4) and, therefore, can
be expected to reduce the capacity for repair of O6-guanine
alkylations in DNA (Krokan et al., 1985).
7.6.2 Mutation and chromosomal effects
The results of tests for the induction of gene mutations and
chromosome damage by acrolein are summarized in Table 10.
In point mutation assays with Salmonella typhimurium, the
positive or equivocal responses obtained were all observed within a
narrow dose range of up to 10-56 µg per plate, higher doses being
toxic. Clearly positive, dose-related increases in revertant
colonies per plate at 2-5 times the background rate were observed in
the absence of metabolic activation only in TA100 (Lutz et al.,
1982; Foiles et al., 1989; Hoffman et al., 1989), TA104 (Marnett
et al., 1985; Foiles et al., 1989; Hoffman et al., 1989), and
TA98 (Lijinsky & Andrews, 1980). Khudoley et al. (1986) reported
positive results in strains TA98 and TA100 without specifying dose
levels or revertant rates. Some evidence for indirect mutagenicity
was found in strains TA1535 (Hales, 1982) and TA100 (Haworth
et al., 1983), the slight increase in TA100 revertants being dose
related. However, negative results, both with and without metabolic
activation, have also been obtained in these strains. Some of these
negative results were clearly related to the incubation conditions,
which were probably highly toxic, e.g., those obtained in spot tests
(Andersen et al., 1972; Florin et al., 1980). In Salmonella
typhimurium TA100 and TA104, strains that show a clear mutagenic
response to acrolein, DNA-acrolein adducts have also been identified
(section 7.6.1).
Acrolein did not induce sex-linked recessive lethality in
Drosophila melanogaster adults (Zimmering et al., 1985), but
induced a 12-fold increase in sex-linked recessive lethality in
hatching eggs and larva at an exposure level that was not reported
but caused over 75% larval death (Rapoport, 1948). In the latter
test, treatment of adults was reported to be less effective.
Three cytogenetic tests have been carried out with acrolein,
two in Chinese hamster ovary cells (Au et al., 1980; Galloway
et al., 1987) and one in human lymphocytes (Wilmer et al.,
1986). Acrolein was shown to induce sister chromatid exchanges in
the absence of a metabolic activating system in all three studies.
The lowest effective concentration was 56 µg/litre (Galloway
et al., 1987). No increase in chromosome aberrations was reported
in one study (Galloway et al., 1987), while chromosome breakage
was reported in another study at cytotoxic concentrations (Au
et al., 1980).
Three properties of acrolein make it difficult to test for
mutagenicity: its high cytotoxicity, which prevents the expression
of any mutagenic activity, and its high reactivity and volatility,
which prevent it reaching the target sites. However, acrolein can
be considered to be a weak mutagen in some bacterial and fungal test
systems in the absence of metabolic activating systems and to
induce sister chromatid exchange in cultured mammalian cells.
7.6.3 Cell transformation
Acrolein (0.4 µg/ml) has been found not to exhibit transforming
potential in C3H/10T1/2 cells but to initiate the process of
transformation. The latter was measured by exposing cultures to
acrolein for 24 h and, subsequently, to a phorbol ester for 6 weeks
(Abernethy et al., 1983).
Table 10. Tests for gene mutation and chromosomal damage by acrolein
Test description Organism Species/strain/cell type Resulta Reference
Gene mutations
Reverse mutations bacteria Salmonella typhimurium TA1535 ±(+S9) Hales (1982)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Lijinsky & Andrews (1980)
Salmonella typhimurium TA100 +(-S9) Lutz et al. (1982); Khudoley et al., 1986;
Foiles et al. (1989); Hoffman et al. (1988)
±(+S9) Haworth et al. (1983)
- Florin et al. (1980); Loquet et al. (1981);
Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium TA104 +(-S9) Marnett et al. (1985); Foiles et al. (1989);
Hoffman et al. (1989)
Salmonella typhimurium TA102 - Marnett et al. (1985)
Salmonella typhimurium TA98 +(-S9) Lijinsky & Andrews (1980); Khudoley et al. (1986)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Basu & Marnett (1984)
Salmonella typhimurium TA1537 - Florin et al. (1980); Haworth et al. (1983);
Lijinsky & Andrews (1980)
Salmonella typhimurium TA1538 - Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium, 8 strains - Andersen et al. (1972)
Escherichia coli 343/113 - Ellenberger & Mohn (1976, 1977)b
Escherichia coli WP2 uvrA ±(-S9) Hemminki et al. (1980)
yeast Saccharomyces cerevisiae S211, S138 - Izard (1973)
Forward mutations yeast Saccharomyces cerevisiae N123 +(-S9) Izard (1973)c
Table 10 (contd).
Test description Organism Species/strain/cell type Resulta Reference
Forward mutations man normal fibroblasts -(-S9) Curren et al. (1988)
DNA-repair-deficient fibroblasts +(-S9) Curren et al. (1988)
hamster V79 cells +(-S9) Smith et al. (1990)
Sex-linked lethal insect Drosophila melanogaster, hatching + Rapoport (1948)d
mutations eggs and young larva
Drosophila melanogaster, adults - Zimmering et al. (1985)e
Chromosomal damage
Aberrations hamster ovary cells in vitro ±(+S9) Au et al. (1980)
±(-S9) Galloway et al. (1987)
Sister chromatid hamster ovary cells in vitro +(-S9) Au et al. (1980)
exchanges +(-S9) Galloway et al. (1987)
man lymphocytes in vitro +(-S9) Wilmer et al. (1986)
Dominant lethal mouse germ cells - Epstein & Shafner (1968)
mutations
(ip exposure)
a + = >2 x background rate or statistically significant (P < 0.05); ± = equivocal; - = negative.
b Details for this test were not reported.
c Plate test for petite mutations (production of a respiratory-deficient mutant).
d Doses were not reported. Treatment of adults was found to be less effective.
e Exposure via feeding solution or via injection.
7.7 Carcinogenicity
7.7.1 Inhalation exposure
Inhalation experiments of appropriate duration specifically
designed to assess the carcinogenicity of acrolein vapour have not
been conducted.
An 81-week study (52 weeks of acrolein exposure at 9.2 mg/m3
followed by 29 weeks without exposure) on groups of 36 Syrian golden
hamsters of both sexes is described in section 7.2.2. The effects
of treatment included a persistent and statistically significant
reduction in body weight in females, an increased relative brain
weight in males and females at 52 weeks, and an increased relative
lung weight in females at 52 weeks. Apart from one small tracheal
papilloma in an acrolein-exposed female, no respiratory tract
tumours were observed in control or treated hamsters (Feron &
Kruysse, 1977). In order to elucidate a possible co-carcinogenic
action of acrolein, Feron & Kruysse (1977) also exposed groups of 30
Syrian golden hamsters of both sexes to measured acrolein vapour
concentrations of 0 or 9.2 mg/m3, 7 h per day and 5 days per week
for 52 weeks, and, for the same period, either weekly to an
intratracheal dose of benzo [a]pyrene or once every 3 weeks to a
subcutaneous dose of diethylnitrosamine. Total dose levels were 18.2
or 36.4 mg benzo [a]pyrene and 2.1 µl diethylnitrosamine. Survivors
were killed at week 81, and all hamsters were subjected to
postmortem examination. The mortality rate in the groups treated
with benzo [a]pyrene was slightly higher than in other groups. The
incidence of benzo [a]pyrene-induced respiratory tract tumours was
slightly (but statistically insignificantly) higher in females also
exposed to acrolein vapour. In these females, at the higher dose
level of benzo [a]pyrene, respiratory tract tumours occurred
earlier and the number of malignant tumours was slightly increased.
Taken together, these observations might suggest an enhancing effect
of acrolein on benzo [a]pyrene carcinogenesis in the respiratory
tract, but the effect cannot be considered proven.
In a study by Le Bouffant et al. (1980), rats, 20 animals per
group, were exposed to 18.3 mg/m3, 1 h/day and 5 days/week, for 10
or 18 months. No tumours or metaplasias were found.
7.7.2 Oral exposure
In a study by Lijinsky & Reuber (1987), groups of 20
Fischer-344 rats of both sexes were exposed to weekly prepared
acrolein of unspecified purity in drinking-water. Each cage of four
rats received 80 ml of acrolein solutions at concentrations of 100
or 250 mg/litre for 124 weeks (males only) or 625 mg/litre for 104
weeks (both sexes) for 5 days per week (this was estimated by the
Task Group to be equivalent to approximately 5, 12.5, and 50 mg/kg
body weight per day, respectively). Total doses were 1200, 3100,
and 6500 mg per rat, respectively. Controls were left untreated.
Survivors were killed at week 123-132 and all rats were subjected to
postmortem examination. The mean survival time was about 120 weeks
for experimental and control groups. There was a marginal, but not
statistically significant, increase in the incidence of adrenal
cortical adenomas (5/20) in female rats at 625 mg/litre, compared to
concurrent controls (1/20), and a decrease in the incidence of
pituitary neoplasms in both sexes at 625 mg/litre. In addition, 2
of 20 females given 625 mg/litre had hyperplastic nodules of the
adrenal cortex. The authors cited historic control values for
adrenal cortical adenomas or carcinomas in female Fischer-344 rats
from other laboratories as 1.3% at 26 months of age and 4.8% in a
lifespan study. Because of limited numbers of animals used and
concerns regarding the purity and stability of acrolein in the dosed
drinking-water, the authors of this study did not consider it to be
a definitive carcinogenicity bioassay. In addition, the Task Group
considered the historical control values quoted by the authors to be
of limited use in evaluating the importance of the tumour incidence
found in this study.
Acrolein appeared to be too toxic to Syrian golden hamsters
following oral exposure by gavage in corn oil to conduct an
effective carcinogenicity study (Lijinsky & Reuber, 1987).
7.7.3 Skin exposure
In a study by Salaman & Roe (1956), a group of 15 S strain mice
of unspecified sex and age received weekly doses of 0.5% acrolein in
acetone for 10 weeks. The total dose was 12.6 mg per rat, although
the purity of the acrolein was not reported. The control group
comprised 20 mice. From day 25 after the first acrolein treatment,
the mice received once per week a skin application of 0.17% croton
oil (0.085% in weeks 2 and 3) for 18 weeks. Croton oil and acrolein
were applied alternately at 3 or 4 day intervals. At the end of
treatment, the mortality rate and the incidence of skin papillomata
were similar to those of the controls treated only with croton oil.
However, this study must be considered inadequate because of the
limited number of animals used and the short duration of the
experiment.
7.8 Interacting agents
Free sulfhydryl-containing compounds have been found to give
protection against the adverse effects of acrolein in vitro, e.g.,
the inhibition of enzymes involved in macromolecular synthesis
(Munsch et al., 1973), liver microsomal cytochrome P-450s
(Marinello et al., 1978; Berrigan et al., 1980; Gurtoo et al.,
1981b, Patel et al., 1984; Cooper et al., 1987), and several
other sulfhydryl-sensitive enzymes (Liu & Tai, 1985; Cox et al.,
1988), the adverse effects on rabbit alveolar macrophages (Low et
al., 1977; Leffingwell & Low, 1979), and the impairment of mouse
limb bud differentiation (Stahlmann et al., 1985). Free
sulfhydryl-containing agents protected against the acute lethal
effects of acrolein in Charles River rats (Sprince et al., 1979)
and in DBA/2J mice (Gurtoo et al., 1981a).
When Swiss-Webster mice were exposed to acrolein-formaldehyde
mixtures, the percentage decrease in respiratory rate was found to
be less than the sum of the percentage decreases due to each
compound alone (Kane & Alarie,1978). In acrolein-exposed Fischer-344
rats, pretreatment with formaldehyde resulted in a lower percentage
decrease in respiratory rate compared to non-pretreated rats (Babiuk
et al., 1985). It was suggested in both investigations that
acrolein and formaldehyde competed for the same receptor
(competitive agonism). In a comparable experiment, the maximum
percentage decrease in the respiratory rate of Swiss-Webster mice
exposed to a mixture of acrolein and sulfur dioxide was lower than
that of acrolein alone. This antagonistic effect was thought to be
caused by a chemical reaction in the air phase between the two
compounds, which reduced the effective concentrations (Kane &
Alarie, 1979).
In Fischer-344 rats exposed to formaldehyde vapour
(7.4 mg/m3) once for 6 h, co-exposure to acrolein vapour
(4.6 mg/m3) resulted in a higher increase in DNA-protein
cross-linking than was observed with formaldehyde alone. Acrolein
alone did not increase DNA-protein cross-linking in this experiment
(Lam et al., 1985).
In a study by Hales et al. (1988), anaesthetized,
artificially ventilated mongrel dogs were exposed to acrolein or
hydrochloric acid with added synthetic smoke composed of carbon
particles for 10 min. The dogs were exposed to smoke with or
without analytically determined acrolein concentrations of <
458 mg/m3, 458-687 mg/m3 or > 687 mg/m3. Smoke with
acrolein, but not smoke with hydrochloric acid, produced
non-cardiogenic, peribronchiolar pulmonary oedema in a
concentration- and time-related fashion. Both acrolein and
hydrochloric acid produced airway damage consisting of mucosal
degeneration and desquamation and inflammatory cell infiltration.
Acrolein at levels above 458 mg/m3 also caused fibrin deposition
in the alveolar spaces that juxtaposed injured bronchioles.
8. EFFECTS ON HUMANS
8.1 Single exposure
8.1.1 Poisoning incidents
One man was exposed dermally and by inhalation when acrolein
was sprayed into his face following an accident in a chemical plant.
Immediately, his face and eyelids were burnt. Within 20 h he was
hospitalized with fever, cough, frothy sputum, cyanosis, and acute
respiratory failure. Two months after the accident, the opening of
the right bronchus was obstructed and the upper trachea showed
slight oedema with haemorrhagic spots. At 18 months he had developed
chronic bronchitis and emphysema, which might have been a sequel of
the accidental exposure (Champeix et al., 1966).
One case of attempted oral suicidal intoxication has been
reported. The man swallowed approximately 1.5 g of acrolein in a
glass of orange juice. Blood was found in his stomach and the number
of red and white blood cells was increased. Gastroscopic
examination showed shrinkage of the stomach and a massive chronic
gastritis with erosions and ulceration. Further examination of the
stomach revealed regenerating mucous membranes, few mucous glands,
granulation and scarring of the serosa, shrinkage and stenosis of
the pylorus, lymphadenitis, and haemosiderin deposition in lymph
nodes. The man was successfully treated by gastrectomy (Schielke,
1987).
Two cases of suspected exposure to acrolein have been reported.
The death of two young boys who inhaled smoke from an overheated
frier for approximately 2 h was thought to be related to acrolein
exposure, although other chemicals might also have been involved.
One of the boys was found dead, while the other suffered from acute
respiratory failure. Following oxygen therapy, the second boy died
due to asphyxia. At autopsy a massive cellular desquamation of the
bronchial lining was observed. The tracheal and bronchial lumina
were filled with debris and the lungs showed multiple infarcts
(Gosselin et al., 1979).
Four female factory workers operating a machine for cutting and
sealing polyethylene bags and a fifth sitting next to the machine
complained of a burning sensation in the eyes, a feeling of dryness
and irritation in the nose and throat, and itching and irritation of
the skin of the face, neck and forearms. These complaints were
related to the smoke developed. The presence of formaldehyde and
"acrolein and/or other aldehydes" in the smoke was suspected and
confirmed. During heavy smoke exposure, itching eruptions developed
on exposed skin. Drowsiness and headache was also experienced. All
symptoms were reversible (Hovding, 1969).
8.1.2 Controlled experiments
8.1.2.1 Vapour exposure
Several studies with volunteers have been conducted with the
object of establing thresholds for odour perception and recognition
and for effects on the eyes, nose, respiratory tract, and nervous
system. The results of these studies are summarized in Table 11. The
exposure period was up to 60 min. In most cases the concentration
of acrolein was determined colorimetrically, although a few reports
did not include a description of the analytical method (Plotnikova,
1957; Sinkuvene, 1970; Harada, 1977). Sinkuvene (1970) reported the
threshold for changes in the electrical activity of the brain
cortex, as measured by electro-encephalography, to be 0.05 mg/m3.
However, this result cannot be evaluated since experimental data
were not provided. The odour perception threshold for sensitive
people was 0.07 mg/m3.
In studies by Weber-Tschopp et al. (1977), groups of human
volunteer students of both sexes were exposed either for 60 min to
acrolein at a concentration of 0.69 mg/m3 or to gradually
increasing acrolein concentrations from 0 up to 1.37 mg/m3 over
35 min followed by a 5-min exposure to 1.37 mg/m3. In further
experiments with side-stream cigarette smoke instead of pure
acrolein vapour, it was noted that the effects of pure acrolein
vapour were small compared to those produced by side-stream smoke
with the same acrolein vapour concentration. It was concluded that
acrolein was only to a minor extent responsible for the effects
observed (Weber-Tschopp et al., 1976). It must be noted, however,
that a significant part of the acrolein in side-stream cigarette
smoke may be associated with particulate matter (Ayer & Yeager,
1982) and would not have been measured. This may have resulted in an
underestimation of the acrolein concentration in the smoke. Many of
the studies considered in this section are old and the analytical
techniques are often poorly described; the absolute figures reported
may, therefore, be suspect.
8.1.2.2 Dermal exposure
In an investigation into irritant dermatitis possibly caused by
contaminants present in diallylglycol carbonate monomer, patch tests
were conducted with acrolein in ethanol at concentrations of 0.01,
0.1, 1, and 10% on groups of 8, 10, 48, and 20 volunteers,
respectively. At 1%, six positive reactions were recorded, four
cases of serious oedema with bullae and two of erythema. At 10%,
all subjects showed positive reactions with bullae, necrosis,
inflammatory cell infiltrate, and papillary oedema (Lacroix et al.,
1976).
Table 11. Thresholds for acute effects of acrolein on humans
Concentration Exposure Effect Reference
(mg/m3) period (min)
0.05 changes in electrical activity of brain cortex Sinkuvene (1970)
0.07 odour perception by most sensitive individuals Sinkuvene (1970)
0.13 5 no or medium subjective eye irritation Darley et al. (1960)a
0.21 5 increased incidence of subjective eye irritation Weber-Tschopp et al. (1977)b
0.34 10 increased incidence of subjective nasal irritation Weber-Tschopp et al. (1977)b
0.34 30 time-related increase in eye-blink frequency van Eick (1977)a
0.39 10 increased incidence of subjective annoyance Weber-Tschopp et al. (1977)b
0.48 odour recognition Leonardos et al. (1969)
0.59 15 increase in eye-blink frequency Weber-Tschopp et al. (1977)b
0.6 10 increase in sensitivity to light Plotnikova (1957)
0.69 40 decrease in respiratory rate; increased incidence of Weber-Tschopp et al. (1977)c
subjective general irritation of eyes, nose, and neck
0.69 10 increase in eye-blink frequency Weber-Tschopp et al. (1977)c
1 3 slight subjective conjunctival irritation Plotnikova (1957)
1 3 stinging sensation in nose Plotnikova (1957)
1.1 5 increased incidence of subjective eye irritation Stephens et al. (1961)a
1.1 5 increase in tear volume, pH,and lysozyme activity Harada (1977)
1.37 35 decrease in respiratory rate Weber-Tschopp et al. (1977)b
1.5 3 pneumographic changes in rhythm and amplitude of Plotnikova (1957)
respiratory movements
Table 11 (contd).
Concentration Exposure Effect Reference
(mg/m3) period (min)
1.7 3 reflex action on optical chronaxy Plotnikova (1957)
1.88 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 20 seconds
2.80 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 5 seconds
3 5 medium to severe subjective eye irritation Darley et al. (1960)a
4 2-3 acute subjective conjunctival and nasal irritation; Plotnikova (1957)
painful sensation in nasopharyngeal region
a exposure of eyes only
b exposure to gradually increasing concentrations up to 1.37 mg/m3
c exposure to a fixed concentration
8.2 Long-term exposure
No data are available on the long-term exposure of humans to
acrolein.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
A summary of acute aquatic toxicity data is presented in
Table 12. In most of these studies, the amount of acrolein added was
reported but the concentrations present were not measured. In these
cases, the actual concentrations may have been lower than the
nominal ones in view of the volatility of the substance and its
hydration rate (see section 4.2).
One of the studies in Table 12 (Lorz et al., 1979) is a
comparatively detailed examination of the acute toxicity of acrolein
to Coho salmon. Within 144 h of exposure to 0.075 mg/litre or more,
all fish died. In surviving fish the activity of gill
Na+,K+-ATPase (EC 3.6.1.37) and the tolerance to subsequent
sea-water exposure were not affected at concentrations up to
0.05 mg/litre. A histological examination of the gills, kidneys, and
liver at 0, 0.05, and 0.1 mg/litre revealed concentration-dependent
adverse effects.
A 3-generation 64-day test with the crustacean Daphnia magna
was conducted in a flow-through open system with well water at
20 °C, a pH between 7.0 and 7.3, a dissolved oxygen concentration of
7.5 mg/litre, and a water hardness of 35 mg CaCO3/litre. The
highest concentration that did not result in mortality was
0.0169 mg/litre (acrolein concentrations were measured in this
study). Survival was reduced at levels of 0.0336 mg/litre or more,
but the number of young per female was not affected even at the
highest concentration tested, 0.0427 mg/litre (Macek et al.,
1976).
Macek et al. (1976) also reported on a 60-day test with
fathead minnow (Pimephales promelas) in a flow-through open system
with well water at 25 °C, a pH between 6.6 and 6.8, a dissolved
oxygen concentration of 8.2 mg/litre, and a water hardness of 32 mg
CaCO3/litre. The highest concentration without adverse effects
was 0.0114 mg/litre (acrolein concentrations were measured in this
study). At 0.0417 mg/litre, there was increased mortality among
offspring. No adverse effects were found on survival and mortality
of adults, number of spawnings and number of eggs per female, number
of eggs per spawn, length of offspring, or hatchability.
It is clear from Table 12 why acrolein is also used as an
algicide, slimicide, and molluscicide.
Table 12. Acute aquatic toxicity of acrolein
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Fresh water
alga Enteromorpha 25 stat, closed 24 h 50% inhibition 1.8c Fritz-
intestinalis of photosynthesis Sheridan
(1982)
alga Cladophora 25 stat, closed 24 h 50% inhibition 1.00c
glomerata of photosynthesis
alga Anabaena 25 stat, closed 24 h 50% inhibition 0.69c
of photosynthesis
bacterium Proteus 37 7.0 stat, closed 2 h 50% growth 0.02 Brown &
vulgaris reduction Fowler
(1967)
bacterium Pseudomonas 25 7.0 stat, closed 16 h TT 0.21 Bringmann
putida & Kuhn
(1977)
protozoan Entosiphon 25 6.9 stat, closed 72 h TT 0.85 Bringmann
sulcatum (1978)
protozoan Chilomonas 20 6.9 stat, closed 48 h TT 1.7 Bringmann
paramecium et al.
(1980)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
protozoan Uronema 25 6.8 stat, closed 20 h TT 0.44 Bringmann
parduczi & Kuhn
(1980)
mollusc snail 21-25 flow, open 48 h 99-100% 20-25 Unrau et
(Bulinus mortality al. (1965)d
truncatus)
mollusc snail stat, open 3 h 100% mortality 10 Ferguson
(Biomphalaria 24 h 10% mortality 1.25 et al.
glabrata), eggs (1961)
mollusc snail (Biomphalaria stat, open 24 h 98% mortality 10 Ferguson
glabrata), adults 24 h 35% mortality 2.5 et al.
(1961)
crustacean water flea 20 7.0- 7.5 35 stat, open 48 h LC50 0.057 Macek et
(Daphnia magna) 7.3 al. (1976)
crustacean water flea 22 7.0- 154 stat, closed 48 h EC50f 0.093 Randall &
(Daphnia magna) 8.2 Knopp (1980)
crustacean water flea 22 7.4- 6-9 173 stat, closed 48 h LC50 0.083 LeBlanc
(Daphnia magna) 9.4 (1980)
fish harlequin fish 20 7.2 20 flow, open 48 h LC50 0.06 Alabaster
(Rasbora (1969)
heteromorpha)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
fish fathead minnow 25 6.6- 8.2 32 flow, open 144 h LC50 0.084 Macek et
(Pimephales 6.8 al.
promelas) (1976)e
fish golden orfe 20 7-8 > 5 200-300 stat 48 h LC50 0.25 & Juhnke &
(Leuciscus idus 2.5 Ludemann
melanotus) (1978)
fish goldfish 20 6-8 > 4 108 stat, open 24 h LC50 < 0.08 Bridie
(Carassius et al.
auratus) (1979)c e
fish Bluegill 21-23 6.5- 10- 32-48 stat, closed 96 h LC50 0.09 Buccafusco
sunfish 7.9 0.3 et al.
(Leopomis (1981)
macrochirus)
fish Coho salmon 10 7.4- > 10 100 stat, open 96 h LC50 0.068 Lorz et al.
(Oncorhynchus 7.6 (1979)g
kisutch)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Marine
mollusc common mussel 15 stat, closed 6 h 40% mortality 0.6 Rijstenbil
(Mytilus edulis) 6 h 70% mortality 1.0 & van Galen
8 h 70% detached 0.57 (1981)e h
mussels
a static or flow-through test, open or closed system
b TT = toxic threshold for inhibition of cell multiplication
c exposure to Magnacide-H (92% acrolein, 8% inert ingredients)
d field study, resurgence of snails was delayed by 8 to 12 months
e analysis for acrolein was reported
f the effect was complete immobilization
g static-renewal test
h static-renewal test (1.6% salinity)
9.2 Terrestrial organisms
9.2.1 Birds
The LD50 for the adult starling (Sturnus vulgaris) was
reported to be > 100 mg/kg body weight. The birds were observed for
7 days after dosing, but only two birds per dose were tested
(Schafer, 1972).
9.2.2 Plants
Acrolein is used as biocide, particularly to control aquatic
plants such as Elodea canadensis, Vallisneria spiralis
(ribbonweed), and Potamogeton tricarinatus (floating pondweed).
In Australia, a maximum concentration of about 15 mg/litre over a
period not exceeding a few hours has been imposed. In the USA,
acrolein is injected into larger channels over longer periods at low
concentrations (approximately 0.1 mg/litre over 48 h) (Bowmer &
Sainty, 1977). It has been shown that the dosage of acrolein
required for control, as defined by the product of time and
concentration required for 80% reduction in biomass, is independent
of the separate values of concentration and time, provided that the
concentration exceeds 0.1 mg/litre and the dosage exceeds 2 mg/litre
per h. In tank experiments, the minimum dosages required for 80%
control of ribbonweed and floating pondweed were about 4 and
26 mg/litre per h, respectively (Bowmer & Sainty, 1977). The
effective dosage (> 80% kill) for Elodea canadensis was 8 to
10 mg/litre per h (Van Overbeek et al., 1959; Bowmer & Smith,
1984). Sublethal concentrations of acrolein stimulated the growth
of Elodea (Bowmer & Smith, 1984).
Elongation of pollen tubes of lily seeds (Lilium longiflorum)
was inhibited completely after a 5-h exposure to acrolein vapour at
a measured concentration of 0.91 mg/m3, a temperature of 28 °C,
and a relative humidity of 60%. A 10% inhibition was found after
1 h (Masaru er al., 1976).
The nature and extent of adverse effects on various crops grown
in soil irrigated by acrolein-treated water have been investigated
in two studies. Acrolein concentrations varied between 15 and
50 mg/litre of supply water. Most furrow-irrigated crops, including
beans, clover, corn, and millet, did not show any damage.
Significant damage to foliage was observed in cotton at acrolein
concentrations of 25 mg/litre or more, but there was no evidence of
chronic or residual phytotoxicity. Slight damage to the foliage of
cucumbers and tomatoes was observed at 40 mg/litre. Vegetable
seedlings in contact with treated water were damaged even at the
lowest concentrations used (Unrau et al., 1965; Ferguson et al.,
1965).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Exposure of the general population to acrolein occurs mainly
via air. Exposure via water would only be significant in cases of
ingestion of, or skin contact with, acrolein deliberately applied as
a biocide to irrigation water. Oral exposure to acrolein may also
occur via alcoholic beverages or heated foodstuffs (chapters 3 and
5).
In urban areas, average levels of up to 15 µg/m3 and maximum
levels of up to 32 µg/m3 have been measured away from industrial
sources. Near industries and close to the exhaust pipes of vehicles,
engines, and combustion appliances, levels ten to one hundred times
higher may occur. Extremely high levels of acrolein in the mg/m3
range can be found as a result of fires (section 5.2.1).
Major indoor sources of acrolein are combustion appliances and
tobacco smoking (section 3.2.4). Levels of acrolein in smoke from
indoor open fires for cooking or heating purposes have not been
reported. Smoking one cigarette per m3 of room-space in 10-13 min
has been shown to lead to acrolein vapour concentrations of
450-840 µg/m3 (section 5.2.1). Recent occupational exposure levels
of acrolein in the air at sites of its production or processing are
not available. Workplace levels of over 1000 µg/m3 have been
reported in situations involving the heating of organic materials
(section 5.3).
In summary, the main source of exposure of the general
population to acrolein is via tobacco smoke. General environmental
pollution by vehicle exhaust and the smoke of burning organic
materials is the next most important source.
10.1.2 Health effects
Owing to the reactivity of acrolein, retention at the site of
entry into the body, usually the respiratory tract, is high
(section 6.1). Primary pathological findings are limited
principally to these sites (sections 7 and 8). Any acrolein absorbed
is liable to react directly with protein and non-protein sulfhydryl
groups or with primary and secondary amines such as those found in
proteins and nucleic acids (sections 6.2 and 7.3). Acrolein may also
be metabolized to mercapturic acids, acrylic acid, glycidaldehyde or
glyceraldehyde (section 6.3). Evidence for the last three
metabolites has only been obtained in vitro.
Acrolein is a cytotoxic agent (section 7.1.5) highly toxic to
experimental animals and man following acute exposure via different
routes (sections 7.1.1 and 8.1.1). The vapour is very irritating to
the eyes and the respiratory tract. Liquid acrolein is a corrosive
substance. The no-observed-adverse-effect level for irritant
dermatitis from ethanolic acrolein was found to be 0.1% (section
8.1.2.2). The odour perception threshold for the most sensitive
individuals is reported to be 0.07 mg/m3 (8.1.2.1). Experiments
with human volunteers show a lowest-observed-adverse-effect level of
0.13 mg/m3, at which level eyes may become irritated after 5 min.
In addition to irritation of the eyes, changes in respiratory tract
function are evident at or above 0.7 mg/m3 (40-min exposure)
(section 8.1.2.1). At higher concentrations, degeneration of the
respiratory epithelium and irritation of all exposed mucosa develop.
Oedematous changes in the tracheal and bronchial mucosa and
bronchial obstruction can be expected after very high exposure to
acrolein vapour (section 8.1).
There are no human toxicological data from long-term exposure
to acrolein. The toxicity from exposure to acrolein vapour has been
relatively well investigated in several animal studies for exposure
periods of up to 52 weeks (section 7.2). Both respiratory tract
function and histopathological effects have been observed at
0.5-0.8 mg/m3 (continuous exposure). Toxicological effects in the
respiratory tract have been documented in most animal species
exposed repeatedly to acrolein concentrations of 1.6-3.2 mg/m3 or
more, and mortality has occurred following exposure to
concentrations above 9 mg/m3. There is limited evidence that
acrolein can depress pulmonary host defenses in mice and rats.
Acrolein can induce teratogenic and embryotoxic effects if
administered directly into the amnion. However, the fact that no
effect was found in rabbits injected intravenously with 3 mg/kg
suggests that human exposure to acrolein is unlikely to affect the
developing embryo (section 7.5).
Acrolein has been shown to interact with DNA and RNA in vitro
and to inhibit their synthesis both in vivo and in vitro. In
vitro, it induces gene mutations in bacteria and fungi and sister
chromatid exchanges in mammalian cells (section 7.6). There is
inadequate evidence to allow the mutagenic potential in humans to be
assessed reliably.
One long-term drinking-water study with rats (130 weeks) and
two inhalation tests, one with hamsters (81 weeks) and the other
with rats (40 or 70 weeks), failed to demonstrate carcinogenic or
clear co-carcinogenic effects of acrolein (section 7.7). Due to the
shortcomings of the tests used, acrolein cannot be considered to
have been adequately tested for carcinogenicity and no conclusions
concerning its carcinogenicity are possible.
The threshold levels of acrolein that cause irritation and
health effects are 0.07 mg/m3 for odour perception, 0.13 mg/m3
for eye irritation, 0.3 mg/m3 for nasal irritation and eye
blinking, and 0.7 mg/m3 for decreased respiratory rate. Since
the level of acrolein rarely exceeds 0.030-0.040 µg/m3 in polluted
urban air or smoke-filled restaurants, acrolein alone is unlikely to
reach annoyance or harmful levels in normal circumstances. Provided
that acrolein concentrations are maintained below 0.05 mg/m3, most
of the population will be spared from any known annoyance or health
effects. However, in polluted urban areas and smoke-filled rooms,
acrolein is present in combination with other irritating aldehydes,
and control of acrolein alone is not sufficient to prevent annoyance
or harmful effects.
10.2 Evaluation of effects on the environment
Acrolein is released into the environment during production of
the compound itself and its derivatives, in processes involving
incomplete combustion and/or pyrolysis of organic substances, by
photochemical oxidation of specific air pollutants, and through
biocidal use, spills, and waste disposal (chapter 3).
Degradation in the atmosphere begins mainly by reaction with
hydroxyl radicals. The calculated atmospheric residence time is
approximately one day (section 4.2). Photolysis does not occur to a
significant degree (section 4.2.1). In natural water, acrolein
dissipates fairly rapidly as a result of catalysed hydration,
reactions with organic material, and volatilization (sections 4.2
and 4.3). Acrolein has a low soil adsorption potential
(section 4.1). Aerobic and anaerobic biodegradation of the compound
has been reported, although its toxicity to microorganisms may
prevent biodegradation (section 4.3.1). Based on its physical and
chemical properties, bioaccumulation would not be expected to occur
(section 4.3.2). It can be concluded that acrolein is unlikely to
persist in any environmental compartment.
Acrolein is very toxic to aquatic organisms. Acute EC50 or
LC50 values for various species range between 0.02 and
2.5 mg/litre. The 60-day NOAEL for fish (fathead minnow) is
0.0114 mg/litre (section 9.1).
In view of the high toxicity of acrolein to aquatic organisms,
the substance presents a risk to aquatic life at or near sites of
industrial discharges, spills, and biocidal use.
11. FURTHER RESEARCH
a) Human exposure characteristics should be further evaluated.
This applies to exposure due to environmental and
occupational air, as well as to intake from food and beverages.
b) These evaluations should include determinations of other
chemicals that occur with acrolein and that interact or have
biological effects similar to those due to acrolein exposure.
c) The most important target organ for airborne acrolein exposure
is the respiratory system. Therefore, further studies
including epidemiological studies should focus on this system
and particularly on the occupational environment. Possible
decreases in host resistance to respiratory infections should
be investigated.
d) The uptake of acrolein in the different parts of the
respiratory system should be examined further. The metabolism
and excretion of acrolein, as well as of its metabolites from
the respiratory system, should be given high priority as there
is an almost total lack of information about these processes.
e) The efficacy of sulfhydryl compounds, such as N-acetylcysteine
or 2-mercaptoethylsulfonic acid sodium salt (MESNA) as
antidotes for acrolein poisoning should be evaluated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Evidence for the potential carcinogenicity of acrolein has been
evaluated by the International Agency for Research on Cancer (IARC,
1979, 1985, 1987). The evidence for carcinogenicity was considered
to be inadequate both in animals and in humans. Thus no evaluation
could be made of the carcinogenicity of acrolein to humans.
Regulatory standards established by national bodies in various
countries and the EEC are summarized in the data profile of the
International Register of Potentially Toxic Chemicals (IRPTC, 1990)
and are tabulated in the Health and Safety Guide for Acrolein (WHO,
1991).
REFERENCES
ABERNETHY, D.J., FRAZELLE, J.H., & BOREIKO, C.J. (1983) Relative
cytotoxicity and transforming potential of respiratory irritants in
the C3H/10T1/2 cell transformation system. Environ. Mutagen., 5:
419 (abstract).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents, and miscellaneous
substances. Int. Pest Control, 11: 29-35.
ALARCON, R.A. (1968) Fluorometric determination of acrolein and
related compounds with m-aminophenol. Anal. Chem., 40: 1704-1708.
ALARCON, R.A. (1972) Acrolein, a component of a universal
cell-growth regulatory system: a theory. J theor. Biol., 37:
159-167.
ALARCON, R.A. (1976) Formation of acrolein from various amino-acids
and polyamines under degradation at 100 °C. Environ. Res., 12:
317-326.
ALTSHULLER, A.P. & BUFALINI, J.J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ALTSHULLER, A.P. & MCPHERSON, S.P. (1963) Spectrophotometric
analysis of aldehydes in the Los Angeles atmosphere. J. Air Pollut.
Control Assoc., 13: 109-111.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20: 649-656.
ANDERSSON, K., HALLGREN, C., LEVIN, J.-O., & NILSSON, C.-A. (1981)
Solid chemosorbent for sampling sub-ppm levels of acrolein and
glutaraldehyde in air. Chemosphere, 10: 275-280.
ARANYI, C., O'SHEA, W.J., GRAHAM, J.A., & MILLER, F.J. (1986) The
effects of inhalation of organic chemical air contaminants on murine
lung host defenses. Fundam. appl. Toxicol., 6: 713-720.
ARTHO, A. & KOCH, R. (1969) [The concentration of acrolein and
hydrogen cyanide in cigarette smoke]. Mitt. Lebensm. Hyg. (Bern),
60: 379-388 (in German).
ASTRY, C. & JAKAB, G.J. (1983) The effects of acrolein exposure on
pulmonary antibacterial defenses. Toxicol. appl. Pharmacol., 67:
49-54.
ATKINSON, R., ASCHMANN, S.M., WINER, A.M., & PITTS, J.N. (1981) Rate
constants for the gas-phase reactions of O3 with a series of
carbonyls at 296 K. Int. J. chem. Kinet., 13: 1133-1150.
ATKINSON, R., ASCHMANN, S.M., & PITTS, J.N. (1983) Kinetics of the
gas-phase reactions of OH radicals with a series of
alpha,ß-unsaturated carbonyls at 299 + 2 K. Int. J. chem. Kinet.,
15: 75-81.
ATKINSON, R., ASCHMANN, S.M., & GOODMAN, M.A. (1987) Kinetics of the
gas-phase reactions of NO3 radicals with a series of alkynes,
haloalkenes, and alpha,ß-unsaturated aldehydes. Int. J. chem.
Kinet., 19: 299-307.
AU, W., SOKOVA, O.I., KOPNIN, B., & ARRIGHI, F.E. (1980) Cytogenetic
toxicity of cyclophosphamide and its metabolites in vitro.
Cytogenet. cell Genet., 26: 108-116.
AYER, H.E. & YEAGER, D.W. (1982) Irritants in cigarette smoke
plumes. Am. J. public Health, 72: 1283-1285.
BABIUK, C., STEINHAGEN, W.H., & BARROW, C.S. (1985) Sensory
irritation response to inhaled aldehydes after formaldehyde
pretreatment. Toxicol. appl. Pharmacol., 79: 143-149.
BAKER, R.R., DYMOND, H.F., & SHILLABEER, P.K. (1984) Determination
of alpha,ß-unsaturated compounds formed by a burning cigarette.
Anal. Proc. (Lond.), 21: 135-137.
BALLANTYNE, B., DODD, D.E., PRITTS, I.M., NACHREIMER, D.J., &
FOWLER, E.H. (1989) Acute vapour inhalation toxicity of acrolein
and its influence as a trace contaminent in
2-methoxy-3,4-dihydro-2H-pyran. Hum. Toxicol., 8: 229-235.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J. (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Proceedings of the
1978 Symposium on dynamics, exposure and hazard assessment of toxic
chemicals, Ann Arbor, Michigan, Ann Arbor Science, pp. 379-392.
BARTLEY, T.R. & GANGSTAD, E.O. (1974) Environmental aspects of
aquatic plant control. J. Irrig. Drain. Div., 100: 231-244.
BASU, A.K. & MARNETT, L.J. (1984) Molecular requirements for the
mutagenicity of malondialdehyde and related acroleins. Cancer Res.,
44: 2848-2854.
BEAUCHAMP, R.O., ANDJELKOVICH, D.A., KLIGERMAN, A.D., MORGAN, K.T.,
& HECK, H. d'A. (1985) A critical review of the literature on
acrolein toxicity. CRC crit. Rev. Toxicol., 14: 309-380.
BEELEY, J.M., CROW, J., JONES, J.G., MINTY, B., LYNCH, R.D., &
PRYCE, D.P. (1986) Mortality and lung histopathology after
inhalation lung injury. The effect of corticosteroids. Am. Rev.
respir. Dis., 133: 191-196.
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9: 119-127.
BENEDICT, R.C. & STEDMAN, R.L. (1969) Composition studies on
tobacco. XXXVII. Inhibition of lactic, alcohol and
glucose-6-phosphate dehydrogenases by cigarette smoke and components
thereof. Tob. Sci., 13: 166-168.
BERRIGAN, M.J., GURTOO, H.L., SHARMA, S.D., STRUCK, R.F., &
MARINELLO, A.J. (1980) Protection by N-acetylcysteine of
cyclophosphamide metabolism - related in vivo depression of mixed
function oxygenase activity and in vitro denaturation of
cytochrome P-450. Biochem. biophys. Res. Commun., 93: 797-803.
BIGNOZZI, C.A., CHIORBOLI, C., MALDOTTI, A., & CARASSITI, V. (1980).
Atmospheric reactivity of 1,3-butadiene - nitrogen monoxide and
acrolein - nitrogen monoxide systems. Ann. Chim. (Rome), 70:
453-461.
BOETTNER, E.A. & BALL, G.L. (1980). Thermal degradation products
from PVC film in food-wrapping operations. Am. Ind. Hyg. Assoc. J.,
41: 513-522. BOHMANN, J.-J. (1985) [Aging behaviour of beer.]
Monatsschr. Brauwiss., 4: 175-180 (in German).
BOOR, P.J. & ANSARI, G.A.S. (1986) High-performance liquid
chromatographic method for quantitation of acrolein in biological
samples. J. Chromatogr., 375: 159-164.
BOULEY, G., DUBREUIL, A., GODIN, J., & BOUDENE, C. (1975) Effets,
chez le rat, d'une faible dose d'acroléine inhalée en continu. Eur.
J. Toxicol., 8: 291-297.
BOWMER, K.H. & HIGGINS, M.L. (1976) Some aspects of the persistence
and fate of acrolein herbicide in water. Arch.environ. Contam.
Toxicol., 5: 87-96.
BOWMER, K.H. & SAINTY, G.R. (1977) Management of aquatic plants with
acrolein. J. aquat. Plant Manage., 15: 40-46.
BOWMER, K.H. & SMITH, G.H. (1984) Herbicides for injection into
flowing water: acrolein and endothal-amine. Weed Res., 24: 201-211.
BOWMER, K.H., LANG, A.R.G., HIGGINS, M.L., PILLAY, A.R., & TCHAN,
Y.T. (1974) Loss of acrolein from water by volatilization and
degradation. Weed Res., 14: 325-328.
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed conjugations
of glutathione with unsaturated compounds. Biochem. J., 104:
95-102.
BRIDGES, R.B., KRAAL, J.H., HUANG, L.J.T., & CHANCELLOR, M.B. (1977)
Effects of cigarette smoke components on in vitro chemotaxis of
human polymorphonuclear leukocytes. Infect. Immun., 16: 240-248.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRINGMANN, G. (1978) [Determination of the harmful biological action
of water-endangering substances on protozoa; I. Bacteria fed
flagellates.] Z. Wasser-Abwasser Forsch., 11: 210-215 (in German).
BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the harmful
action of water-endangering substances on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z. Wasser-Abwasser Forsch., 10:
87-98 (in German).
BRINGMANN, G. & KUHN, R. (1980) [Determination of the harmful
biological action of water-endangering substances on protozoa; II.
Bacteria fed ciliates.] Z. Wasser-Abwasser Forsch., 13: 26-31 (in
German).
BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa; III Saprozoic flagellates.] Z. Wasser-Abwasser Forsch.,
13: 170-173 (in German).
BROWN, P.W. & FOWLER, C.A. (1967) The toxicity of tobacco smoke
solutions to Proteus vulgaris. Beitr. Tabakforsch., 4: 78-83.
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity
of priority pollutants to Bluegill (Lepomis macrochirus). Bull.
environ. Contam. Toxicol., 26: 446-452.
BUCKLEY, L.A., JIANG, X.Z., JAMES, R.A., MORGAN, K.T., & BARROW,
C.S. (1984) Respiratory tract lesions induced by sensory irritants
at the RD50 concentration. Toxicol. appl. Pharmacol., 74:
417-429.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative determination
of acrylonitrile, acrolein, acetonitrile and acetone in workplace
air. Am. Ind. Hyg. Assoc. J., 40: 904-909.
CAMPBELL, K.I., GEORGE, E.L., & WASHINGTON, I.S. (1981) Enhanced
susceptibility to infection in mice after exposure to dilute exhaust
from light duty diesel engines. Environ. Int., 5: 377-382.
CANTONI, C., BIANCHI, M.A., RENON, P., & CALCINARDI, C. (1969)
[Bacterial and chemical alterations during souring in salted pork.]
Atti. Soc. Ital. Sci. Vet., 23: 752-756 (in Italian).
CARPENTER, C.P., SMYTH, H.F., POZZANI, U.C. (1949) The assay of
acute vapor toxicity, and the grading and interpretation of results
on 96 compounds. J. ind. Hyg. Toxicol., 31: 343-346.
CATILINA, P., THIEBLOT, L., & CHAMPEIX, J. (1966) Lesions
respiratoires expérimentales par inhalation d'acroléine chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc. (Paris), 27: 857-867.
CHAMPEIX, J., COURTIAL, L., PERCHE, E., & CATILINA, P. (1966)
Broncho-pneumopathie aiguë par vapeurs d'acroléine. Arch. Mal.
prof. Méd. Trav. Sécur. Soc., 27: 794-796.
CHAVIAMO, A.H., GILL, W.B., RUGGIERO, U.J., & VERMEULEN, C.W. (1985)
Experimental cytoran cystitis and prevention by acetylcysteine. J.
Urol., 134: 598-600.
CHHIBBER, G. & GILANI, S.H. (1986) Acrolein and embryogenesis: an
experimental study. Environ. Res., 39: 44-49.
CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation and
degradation of petrochemical wastewater components by methane
fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
CHRAIBER, L.B., SOSNOVSKY, S.I., TATARKIN, Y.N., & VINNIKOVA, L.I.
(1964) [Air pollution with acrolein vapors in expeller and forepress
shops of butter and fat mills of Uzbekistan.] Gig. Tr. prof.
Zabol., 1(11): 49-50 (in Russian).
CHUNG, F.-L., YOUNG, R., & HECHT, S.S. (1984) Formation of cyclic
1,N2-propanodeoxyguanosine adducts in DNA upon reaction with
acrolein or crotonaldehyde. Cancer Res., 44: 990-995.
CLAUSSEN, U., HELLMANN, W., & PACHE, G. (1980) The embryotoxicity of
the cyclophosphamide metabolite acrolein in rabbits, tested in vivo
by i.v. injection and by the yolk-sac method. Drug Res., 30:
2080-2083.
COHEN, I.R. & ALTSHULLER, A.P. (1961) A new spectrophotometric
method for the determination of acrolein in combustion gases and in
the atmosphere. Anal. Chem., 33: 726-733.
COOMBER, J.W. & PITTS, J.N. (1969) Molecular structure and
photochemical reactivity. XII. The vapor-phase photochemistry of
acrolein at 3130 A. J. Am. Chem. Soc., 91: 547-550.
COOPER, K.O., WITMER, C.M., & WITZ, G. (1987) Inhibition of
microsomal cytochrome c reductase activity by a series of
alpha,ß-unsaturated aldehydes. Biochem. Pharmacol., 36: 627-631.
COSTA, D.L., KUTZMAN, R.S., LEHMANN, J.R., & DREW, R.T. (1986)
Altered lung function and structure in the rat after subchronic
exposure to acrolein. Am. Rev. respir. Dis., 133: 286-291.
COX, R., GOORHA, S., & IRVING, C.C. (1988) Inhibition of DNA
methylase activity by acrolein. Carcinogenesis, 9: 463-465.
CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & ABBOTT, J.K. (1986)
Inhalation toxicology: VII. Times to incapacitiation and death for
rats exposed continuously to atmospheric acrolein vapor, Washington,
D.C., Federal Aviation Administration, Office of Aviation Medicine
(DOT/FAA/AM-86/5)
CROOK, T.R., SOUHAMI, R.L., & MCLEAN, A.E.M. (1986a) Cytotoxicity,
DNA cross-linking, and single strand breaks induced by activated
cyclophosphamide and acrolein in human leukemia cells. Cancer Res.,
46: 5029-5034.
CROOK, T.R., SOUHAMI, R.L., WHYMAN, G.D., & MCLEAN, A.E.M. (1986b)
Glutathione depletion as a determinant of sensitivity of human
leukemia cells to cyclophosphamide. Cancer Res., 46: 5035-5038.
CURREN, R.D., YANG, L.L., CONKLIN, P.M., GRAFSTROM, R.C., & HARRIS,
C.C. (1988) Mutagenesis of xeroderma pigmentosum fibroblasts by
acrolein. Mutat. Res., 209: 17-22.
DAHLGREN, S.E., DALEN, H., & DALHAMM, T. (1972) Ultrastructural
observations on chemically induced inflammation in guinea pig
trachea. Virchows Arch. Abt. B Zellpathol., 11: 211-223.
DALHAMN, T. & ROSENGREN, A. (1971) Effect of different aldehydes on
tracheal mucosa. Arch. Otolarygnol., 83: 496-500.
DARLEY, E.F., MIDDLETON, J.T., & GARBER, M.J. (1960) Plant damage
and eye irritation from ozone-hydrocarbon reactions. J. agric. food
Chem., 8: 483-485.
DAVIS, T.R.A., BATTISTA, S.P., & KENSLER, C.J. (1967) Mechanism of
respiratory effects during exposure of guinea pigs to irritants.
Arch. environ. Health, 15: 412-419.
DAWSON, J.R., NORBECK, K., ANUNDI, I., & MOLDEUS, P. (1984) The
effectiveness of N-acetylcysteine in isolated hepatocytes, against
the toxicity of paracetamol, acrolein, and paraquat. Arch.
Toxicol., 55: 11-15.
DEBETHIZY, J.D., UDINSKY, J.R., SCRIBNER, H.E., & FREDERICK, C.B.
(1987) The disposition and metabolism of acrylic acid and ethyl
acrylate in male Sprague-Dawley rats. Fundam. appl. Pharmacol., 8:
549-561.
DESCROIX, H. (1972) Etude complémentaire des interactions entre
acroléine et cytidine-monophosphate. C. R. Acad. Sci. Paris, D274:
2362-2365.
DGEP (1988) Unpublished Review of literature data on acrolein,
Leidschendam, The Netherlands, Ministry of Housing, Physical
Planning and Environment, Directorate-General of Environmental
Protection.
DIAZ MAROT, A., GASSIOT MATAS, M., & FERRER MARTIN, M. (1983)
[Stripping and high performance liquid chromatography in the
analysis of carbonyl compounds in beers at ppb level.] Afinidad,
40: 21-24 (in Spanish).
DORE, M. & MONTALDO, C. (1984) [Study on the conjungation in vitro
of allyl alcohol and its metabolites with reduced glutathione.]
Boll. Soc. Ital. Biol. Sper., 60: 1497-1501 (in Italian).
DRAMINSKI, W., EDER, E., & HENSCHLER, D. (1983) A new pathway of
acrolein metabolism in rats. Arch. Toxicol., 52: 243-247.
EASLEY, D.M., KLEOPFER, R.D., & CARASEA, A.M. (1981) Gas
chromatographic-mass spectrometric determination of volatile organic
compounds in fish. J. Assoc. Off. Anal. Chem., 64: 653-656.
EDNEY, E.O., MITCHELL, S., & BUFALINI, J.J. (1982) Atmospheric
chemistry of several toxic compounds, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Atmospheric Chemistry
and Physics Division, Environmental Sciences Research Laboratory,
Office of Research and Development (EPA-600/3-82-092, PB 83-146340).
EDNEY, E.O., KLEINDIENST, T.E., & CORSE, E.W. (1986a) Room
temperature rate constants for the reaction of OH with selected
chlorinated and oxygenated hydrocarbons. Int. J. chem. Kinet., 18:
1355-1371.
EDNEY, E.O., SHEPSON, P.B., KLEINDIENST, T.E. & CORSE, E.W. (1986b)
The photooxidation of allyl chloride. Int. J. chem. Kinet., 18:
597-608.
EGLE, J.L. (1972) Retention of inhaled formaldehyde,
propionaldehyde, and acrolein in the dog. Arch. environ. Health,
25: 119-124.
EGLE, J.L. & HUDGINS, P.M. (1974) Dose-dependent sympathomimetic and
cardioinhibitory effects of acrolein and formaldehyde in the
anesthetized rat. Toxicol. appl. Pharmacol., 28: 358-366.
ELLENBERGER, J. & MOHN, G.R. (1976) Comparative mutagenicity testing
of cyclphosphamide and some of its metabolites. Mutat. Res., 37:
120 (abstract).
ELLENBERGER, J. & MOHN, G.R. (1977) Mutagenic activity of major
mammalian metabolites of cyclophosphamide toward several genes of
Escherichia coli. J. Toxicol. environ. Health, 3: 637-650.
EPSTEIN, S.S. & SHAFNER H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ERICKSON, L.C., RAMONAS, L.M., ZAHARKO, D.S., & KOHN, K.W. (1980)
Cytotoxicity and DNA cross-linking activity of
4-sulfidocyclophosphamides in mouse leukemia cells in vitro.
Cancer Res., 40: 4216-4220.
ESTERBAUER, H., ZOLLNER, H., & SCHOLZ, N. (1975) Reaction of
glutathione with conjugated carbonyls. Z. Naturforsch., 30:
466-473.
FACCHINI, M.C., CHIAVARI, G., & FUZZI, S. (1986) An improved HPLC
method for carbonyl compound speciation in the atmospheric liquid
phase. Chemosphere, 15: 667-674.
FERGUSON, F.F., RICHARDS, C.S., & PALMER, J.R. (1961) Control of
Australorbis glabratus by acrolein in Puerto Rico. Public Health
Rep., 76: 461-468.
FERGUSON, F.F., DAWOOD, I.K., & BLONDEAU, R. (1965) Preliminary
field trials of acrolein in the Sudan. Bull. World Health Organ.,
32: 243-248.
FERON, V.J. & KRUYSSE, A. (1977) Effects of exposure to acrolein
vapor in hamsters simultaneously treated with benzo [a]pyrene or
diethylnitrosamine. J. Toxicol. environ. Health, 3: 379-394.
FERON, V.J., KRUYSSE, A., TIL, H.P., & IMMEL, H.R. (1978) Repeated
exposure to acrolein vapour: subacute studies in hamsters, rats and
rabbits. Toxicology, 9: 47-57.
FISCHER, T., WEBER, A., & GRANDJEAN, E. (1978) [Air pollution due to
tobacco smoke in restaurants.] Int. Arch. occup. environ. Health,
41: 267-280 (in German).
FLEER, R. & BRENDEL, M. (1982) Toxicity, interstrand cross-links and
DNA fragmentation induced by `activated' cyclophosphamide in yeast:
comparative studies on 4-hydroperoxy-cyclophosphamide, its
monofunctional analogue, acrolein, phosphoramide mustard, and
non-nitrogen mustard. Chem.-biol. Interact., 39: 1-15.
FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity using the
Ames' test. Toxicology, 18; 219-232.
FOILES, P.G., AKERKAR, S.A., & CHUNG, F.-L. (1989) Application of an
immunoassay for cyclic acrolein deoxyguanosine adducts to assess
their formation in DNA of Salmonella typhimurium under conditions
of mutation induction by acrolein. Carcinogenesis, 10: 87-90.
FRACCHIA, M.F., SCHUETTE, F.J., & MUELLER, P.K. (1967) A method for
sampling and determination of organic carbonyl compounds in
automobile exhaust. Environ. Sci. Technol., 1: 915-922.
FRITZ-SHERIDAN, R.P. (1982) Impact of the herbicide Magnacide-H
(2-propenal) on algae. Bull. environ. Contam. Toxicol., 28:
245-249.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO,
J., MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. mol.
Mutagen., 10(supplement): 1-175.
GAN, J.C. & ANSARI, G.A.S. (1987) Plausible mechanism of
inactivation of plasma alpha1-proteinase inhibitor by acrolein.
Res. Commun. chem. Pathol. Pharmacol., 55: 419-422.
GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated carbon
adsorption of petrochemicals. J. Water Pollut. Control Fed., 46:
947-965.
GOSSELIN, B., WATTEL, F., CHOPIN, C., DEGAND, P., FRUCHART, J.C.,
VAN DER LOO, D., & CRASQUIN, O. (1979) Intoxication aiguë par
l'acroléine, une observation. Nouv. Presse méd., 8: 2469-2472.
GRAEDEL, T.E., FARROW, L.A., & WEBER, T.A. (1976) Kinetic studies of
the photochemistry of the urban atmosphere. Atmos. Environ., 10:
1095-1116.
GRAFSTROM, R.C., CURREN, R.D., YANG, L.L., & HARRIS, C.C. (1986)
Aldehyde-induced inhibition of DNA repair and potentiation of
N-nitrosocompound-induced mutagenesis in cultured human cells.
Prog. clin. biol. Res., 209A: 255-264.
GRAFSTROM, R.C., DYPBUKT, J.M., WILLEY, J.C., SUNDQVIST, K., EDMAN,
C., ATZORI, L., & HARRIS, C.C. (1988) Pathobiological effects of
acrolein in cultured human bronchial epithelial cells. Cancer Res.,
48: 1717-1721.
GREENHOFF, K. & WHEELER, R.E. (1981) Analysis of beer carbonyls at
the part per billion level by combined liquid chromatography and
high pressure liquid chromatography. J. Inst. Brew., 86: 35-41.
GREY, T.C. & SHRIMPTON, D.H. (1966) Volatile components of raw
chicken breast muscle. Br. poult. Sci., 8: 23-33.
GROSJEAN, D. & WRIGHT ,B. (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos. Environ., 17: 2093-2096.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
chromatogr. Sci., 12: 385-391.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of The Netherlands. Sci. total
Environ., 43: 193-219.
GUILLERM, R., BADRE, R., & HEE, J. (1967a) Détermination du seuil
d'action des polluants irritants de l'atmosphère - Comparaison de
deux méthodes. Ann. occup. Hyg., 10: 127-133.
GUILLERM, R., SAINDELLE, A., FALTOT, P., & HEE, J. (1967b) Action de
la fumée de cigarette et de quelques-uns de ses constituants sur les
resistances ventilatoires chez le cobaye. Arch. int. Pharmacodyn.
Ther., 167: 101-114.
GURTOO, H.L., HIPKENS, J.H., & SHARMA, S.D. (1981a) Role of
glutathione in the metabolism-dependent toxicity and chemotherapy of
cyclophosphamide. Cancer Res., 41: 3584-3591.
GURTOO, H.L., MARINELLO, A.J., STRUCK, R.F., PAUL, B., & DAHMS, R.P.
(1981b) Studies on the mechanism of denaturation of cytochrome P-450
by cyclophosphamide and its metabolites. J. biol. Chem., 256:
11691-11701.
GUSEV, M.I., SVECHNIKOVA, A.I., DRONOV, I.S., GREBENSKOVA, M.D., &
GOLOVINA, A.I. (1966) [Substantiation of the daily average maximum
permissible concentration of acrolein in the atmosphere.] Gig. i
Sanit., 31: 9-13 (in Russian).
HAENEN, G.R.M.M., VERMEULEN, N.P.E., TAI TIN TSOI, J.N.L., RAGETLI,
H.M.N., TIMMERMAN, H., & BAST, A. (1988) Activation of the
microsomal glutathione- S-transferase dependent protection against
lipid peroxidation by acrolein. Biochem. Pharmacol., 37: 1933-1938.
HALES, B.F. (1982) Comparison of the mutagenicity and teratogenicity
of cyclophosphamide and its active metabolites,
4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein.
Cancer Res., 42: 3016-3021.
HALES, C.A., BARKIN, P.W., JUNG, W., TRAUTMAN, E., LAMBORGHINI, D.,
HERRIG, N., & BURKE, J. (1988) Synthetic smoke with acrolein but not
HCL produces pulmonary edema. J. appl. Physiol., 64: 1121-1133.
HALL, R.H., & STERN, E.S. (1950) Acid-catalysed hydration of
acraldehyde. Kinetics of the reaction and isolation of
ß-hydroxypropaldehyde. J. Chem. Soc., 1950: 490-498.
HARADA, M. (1977) [Biochemical analysis of tear fluid in
photochemical smog.] Nippon Ganka Gakkai Zasshi, 81: 275-286 (in
Japanese).
HARKE, H.-P., BAARS, A., FRAHM, B., PETERS, H., & SCHULTZ, C. (1972)
[On the problem of passive smoking. Relation between the
concentration of smoke constituents in the air of several large
rooms and the number of smoked cigarettes and time.] Int. Arch.
Arbeitsmed., 29: 323-339 (in German).
HAWLEY, G.G. (1981) The condensed chemical dictionary, 10th ed.,
New York, Van Nostrand Reinhold Co.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HAYASE, F., CHUNG, T.-Y., & KATO, H. (1984) Changes of volatile
components of tomato fruits during ripening. Food Chem., 14:
113-124.
HEMMINKI, K., FALCK, K., & VAINIO, H. (1980) Comparison of
alkylation rates and mutagenicity of directly acting industrial and
laboratory chemicals. Arch. Toxicol., 46: 277-285.
HESS, L.G., KURTZ, A.N., & STANTON, D.B. (1978) Acrolein and
derivatives. In: Kirk, R.E. & Othmer, D.F. ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol 1:
pp. 277-297.
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity classes.
Ind. Hyg. Q., 10: 93-96.
HOFFMAN, C., BASTIAN, H., WIEDENMANN, M., DEININGER, C., & EDER, E.
(1989) Detection of acrolein congener-DNA adducts isolated from
cellular systems. Arch. Toxicol., Suppl. 13: 219-223.
HOFFMANN, D., BRUNNEMANN, K.D., GORI, G.B., & WYNDER, E.L. (1975) On
the carcinogenicity of marijuana smoke. Recent Adv. Phytochem., 9:
63-81.
HOLMBERG, B. & MALMFORS, T. (1974) The cytotoxicity of some organic
solvents. Environ. Res., 7: 183-192.
HOSHIKA, Y. & TAKATA, Y. (1976) Gas chromatographic separation of
carbonyl compounds as their 2,4-dinitrophenylhydrazones using glass
capillary columns. J. Chromatogr., 120: 379-389.
HOVDING, G. (1969) Occupational dermatitis from pyrolysis products
of polythene. Acta dermatovenereol, 49: 147-149.
HRDLICKA, J. & KUCA, J. (1965) The changes of carbonyl compounds in
the heat-processing of meat. Poult. Sci., 44: 7-31.
HUGOD, C., HAWKINS, L.H., & ASTRUP, P. (1978) Exposure of passive
smokers to tobacco smoke constituents. Int. Arch. occup. environ.
Health, 42: 21-29.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein, Lyons, International Agency for Research on Cancer, pp.
479-495 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Man, Vol. 19).
IARC (1985) Allyl compounds, aldehydes, epoxides and peroxides,
Lyons, International Agency for Research on Cancer, pp. 133-161
(IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1985) Treatment and disposal methods for waste chemicals,
Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
IRPTC (1990) Data profile on acrolein, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ, I.D., &
BRADSHAW, J.J. (1978) Organic compounds - Their interaction with and
degradation of hepatic microsomal drug-metabolizing enzymes in
vitro. Drug Metab. Disp., 6: 218-225.
IZARD, C. (1973) Effets de l'acroléine sur la division cellulaire,
le cycle et la synthèse de l'ADN, chez Vicia faba. C. R. Acad.
Sci. Paris Ser. D, 276: 1745-1747.
IZARD, C. & LIBERMANN, C. (1978) Acrolein. Mutat. Res., 47:
115-138.
IZMEROV, N.F., ed. (1984) Acrolein. Moscow, Centre of International
Projects, GKNT, 15 pp (IRPTC Scientific Reviews of Soviet Literature
on Toxicity and Hazards of Chemicals 50).
JACOBS, W.A. & KISSINGER, P.T. (1982) Determination of carbonyl 2,4-
dinitrophenylhydrazones by liquid chromatography/electrochemistry.
J. liq. Chromatogr., 5: 669-676.
JAKAB, G.J. (1977) Adverse effect of a cigarette smoke component,
acrolein, on pulmonary defenses and on viral-bacterial interactions
in the lung. Am. Rev. respir. Dis., 115: 33-38.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of several gas-phase components of side-stream smoke
of cigarettes in indoor air as contribution towards the problem of
passive smoking.] Int. Arch. occup. environ. Health, 36: 169-181
(in German).
JONSSON, A. & BERG, S. (1983) Determination of low-molecular-weight
oxygenated hydrocarbons in ambient air by cryogradient sampling and
two-dimensional gas chromatography. J. Chromatogr., 279: 307-322.
JOUSSERANDOT, P., GREMAIN, J., & DU BOISTESSELIN, R. (1981)
L'utilisation de méthodes histologiques pour la démonstration de
l'activite anti-inflammatoire de la fusafungine. Sem. Hop. Paris,
57: 143-150.
JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of 200
chemical compounds for acute toxicity towards fish by means of the
goldfish test.] Z. Wasser-Abwasser Forsch., 11: 161-164 (in
German).
KANE, L.E. & ALARIE, Y. (1977) Sensory irritation to formaldehyde
and acrolein during single and repeated exposures in mice. Am. Ind.
Hyg. Assoc. J., 38: 509-521.
KANE, L.E. & ALARIE, Y. (1978) Evaluation of sensory irritation from
acrolein-formaldehyde mixtures. Am Ind. Hyg. Assoc. J., 39:
270-274.
KANE, L.E. & ALARIE, Y. (1979) Interactions of sulfur dioxide and
acrolein as sensory irritants. Toxicol. appl. Pharmacol., 48:
305-315.
KANKAANPAA, J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acrylamide in
developing chick embryos. Toxicol. Lett., 4: 93-96.
KANTEMIROVA, A.E. (1975) [The disease incidence rate, including
temporal disability of workers engaged in the production of acrolein
and methylmercaptopropione (MMP) aldehyde.] Trans. Volgograd med.
Inst., 26(4): 79-85 (in Russian).
KANTEMIROVA, A.E. (1977) [The disease incidence rate, involving
temporal disability of workers engaged in the production of acrolein
and MMP aldehyde.] Trans. Volgograd med. Inst., 27(5): 32-33 (in
Russian).
KATZ, M. (1977) Methods of air sampling and analysis, 2nd ed.,
Washington, DC, American Public Health Association.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134: 1093-1101.
KERR, J.A. & SHEPPARD (1981) Kinetics of the reactions of hydroxyl
radicals with aldehydes studied under atmospheric conditions.
Environ. Sci. Technol., 15: 960-963.
KHUDOLEY, V.V., MIZGIREV, I.V., & PLISS, G.B. (1986) [Evaluation of
mutagenic activity of carcinogens and other chemical agents with
Salmonella typhimurium assays.] Vopr. Onkol., 32: 73-80 (in
Russian).
KILBURN, K.H. & MCKENZIE, W.N. (1978) Leukocyte recruitment to
airways by aldehyde-carbon combinations that mimic cigarette smoke.
Lab. Invest., 38: 134-142.
KIMES, B.W. & MORRIS, D.R. (1971) Inhibition of nucleic acid and
protein synthesis in Escherichia coli by oxidized polyamines and
acrolein. Biochim. Biophys. Acta, 228: 235-244.
KISSEL, C.L., BRADY, J.L., GUERRA, A.M., PAU, J.K., ROCKIE, B.A., &
CASERIO, F.F. (1978) Analysis of acrolein in aged aqueous media.
Comparison of various analytical methods with bioassays. J. agric.
food Chem., 26: 1338-1343.
KLOCHKOVSKII, S.P., LUKASHENKO, R.D., PODVYSOTSKII, K.S., &
KAGRAMANYAN, N.P. (1981) [Acrolein and formaldehyde content in the
air of quarries.] Bezoppasnost. Tr. Prom-St. 1981(12):38 (in
Russian).
KOERKER, R.L., BERLIN, A.J., & SCHNEIDER, F.H. (1976) The
cytotoxicity of short-chain alcohols and aldehydes in cultured
neuroblastoma cells. Toxicol. appl. Pharmacol., 37: 281-288.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1983) Embryotoxic effects
of acrolein, methylacrylates, guanidines and resorcinol on three day
chicken embryos. Acta pharmacol. toxicol., 52: 95-99.
KRILL, R.M. & SONZOGNI, W.C. (1986) Chemical monitoring of
Wisconsin's groundwater. J. Am. Water Works Assoc., 78: 70-75
KROKAN, H., GRAFSTROM, R.C., SUNDQVIST, K., ESTERBAUER, H., &
HARRIS, C.C. (1985) Cytotoxicity, thiol depletion and inhibition of
O6-methylguanine-DNA methyltransferase by various aldehydes in
cultured human bronchial fibroblasts. Carcinogenesis, 6: 1755-1759.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A. (1982)
Collection and analysis of hazardous organic emissions. Anal.
Chem., 54: 810-817.
KRUYSSE, A. (1971) Acute inhalation toxicity of acrolein in
hamsters, Zeist, The Netherlands, Central Institute for Nutrition
and Food Research, TNO (Report No. R3516).
KU, R.H. & BILLINGS, R.E. (1986) The role of mitochondrial
glutathione and cellular protein sulfhydryls in formaldehyde
toxicity in glutathione-depleted rat hepatocytes. Arch. Biochem.
Biophys., 247: 183-189.
KUBINSKI, H., GUTZKE, G.E., & KUBINSKI, Z.O. (1981) DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
KUTZMAN, R.S., MEYER, G.-J., & WOLF, A.P. (1982) The biodistribution
and metabolic fate of [11C]-acrylic acid in the rat after acute
inhalation exposure or stomach intubation. J. Toxicol. environ.
Health, 10: 969-979.
KUTZMAN, R.S., WEHNER, R.W., & HABER, S.B. (1984) Selected responses
of hypertension sensitive and resistant rats to inhaled acrolein.
Toxicology, 31: 53-65.
KUTZMAN, R.S., POPENOE, E.A., SCHMAELER, M., & DREW, R.T. (1985)
Changes in rat lung structure and composition as a result of
subchronic exposure to acrolein. Toxicology, 34: 139-151.
KUWATA, K., UEBORI, M., YAMASAKI, H., KUGE, Y., & KISO, Y. (1983)
Determination of aliphatic aldehydes in air by liquid
chromatography. Anal. Chem., 55: 2013-2016.
LACROIX, M., BURCKEL, H., FOUSSEREAU, J., GROSSHANS, E., CAVELIER,
C., LIMASSET, J.C., DUCOS, P., GRADINSKI, D., & DUPRAT, P. (1976)
Irritant dermatitis from diallylglycol carbonate monomer in the
optical industry. Contact dermatitis, 2: 183-195.
LAM, C.-W., CASANOVA, M., & HECK, H.D'A. (1985) Depletion of nasal
mucosal glutathione by acrolein and enhancement of
formaldehyde-induced DNA-protein cross-linking by simultaneous
exposure to acrolein. Arch. Toxicol., 58: 67-71.
LEACH, P.W., LENG, L.J., BELLAR, T.A., SIGSBY, J.E., & ALTSHULLER,
A.P. (1964) Effects of HC/NOx ratios on irradiated auto exhaust,
part II. J. Air Pollut. Control Assoc., 14: 176-183.
LEACH, C.L., HATOUM, N.S., RATAJCZAK, H.V., & GERHART, J.M. (1987)
The pathologic and immunologic effects of inhaled acrolein in rats.
Toxicol. Lett., 39: 189-198.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24:
684-691.
LE BOUFFANT, L., MARTIN, J.C., DANIEL, H., HENIN, J.P., & NORMAND,
C. (1980) Action of intensive cigarette smoke inhalations on the rat
lung. Role of particulate and gaseous cofactors. J. Natl Cancer
Inst., 64: 273-281.
LEFFINGWELL, C.M. & LOW, R.B. (1979) Cigarette smoke components and
alveolar macrophage protein synthesis. Arch. environ. Health, 34:
97-102.
LENCREROT, P., PARFAIT, A., & JOURET, C. (1984) Rôle des
corynebactéries dans la production d'acroléine (2-propenal) dans les
rhums. Ind. aliment. agric., 101: 763-765.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determinations of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEUCHTENBERGER, C., SCHUMACHER, M., & HADIMANN, T. (1968) Further
cytological and cytochemical studies on the biological significance
of the gas phase of fresh cigarette smoke. Z. Präventivmed., 13:
130-141.
LEVAGGI, D.A. & FELDSTEIN, M. (1970) The determination of
formaldehyde, acrolein, and low molecular weight aldehydes in
industrial emissions on a single collection sample. J. Air Pollut.
Control Assoc., 20: 312-313.
LIES, K.-H., POSTULKA, A., GRING, H., & HARTUNG, A. (1986) Aldehyde
emissions from passenger cars. Staub-Reinhalt. Luft, 46: 136-139.
LIJINSKY, W. & ANDREWS, A.W. (1980) Mutagenicity of vinyl compounds
in Salmonella typhimurium. Teratogen. Carcinogen. Mutagen., 1:
259-267.
LIJINSKI, W. & REUBER, M.D. (1987) Chronic carcinogenesis studies of
acrolein and related compounds. Toxicol. ind. Health, 3: 337-345.
LIPARI, F. & SWARIN, S.J. (1982) Determination of formaldehyde and
other aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. J. Chromatogr., 247: 297-306.
LIPARI, F., DASCH, J.M., & SCRUGGS, W.F. (1984) Aldehyde emissions
from wood-burning fireplaces. Environ. Sci. Technol., 18: 326-330.
LIU, Y. & TAI, H.-H. (1985) Inactivation of pulmonary NAD-dependent
15-hydroxyprostaglandin dehydrogenase by acrolein. Biochem.
Pharmacol., 34: 4275-4278.
LOQUET, C., TOUSSAINT, G., & LETALAER, J.Y. (1981) Studies on
mutagenic constituents of apple brandy and various alcoholic
beverages collected in Western France, a high incidence area for
oesophageal cancer. Mutat. Res., 88: 155-164.
LORZ, H.W., GLENN, S., WILLIAMS, R.H., KUNKEL, C.M., NORRIS, L.A., &
LOPER, B.R. (1979) Effects of selected herbicides on Smolting
Salmon, Corvallis, Oregon, USA Environmental Protection Agency,
Environment Research Laboratory Corvallis, Office of Research and
Development (Report EPA-600/3-79-071).
LOW, E.S., LOW, R.B., & GREEN, G.M. (1977) Correlated effects of
cigarette smoke components on alveolar macrophage adenosine
triphosphatase activity and phagocytosis. Am. Rev. respir. Dis.,
115: 963-970.
LUTZ, D., EDER, E., NEUDECKER, T., & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha,ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93: 305-315.
LYMAN, W.J., REEHL, W.F., & ROSENBLATT, D.H. (1982) Handbook of
chemical property estimation methods, New York, McGraw-Hill Book
Company.
LYON, J.P., JENKINS, L.J., JONES, R.A., COON, R.A., & SIEGEL, J.
(1970) Repeated and continuous exposure of laboratory animals to
acrolein. Toxicol. appl. Pharmacol., 17: 726-732.
MACEK, K.J., LINDBERG, M.A., SAUTER, S., BUXTON, K.S., & COSTA, P.A.
(1976) Toxicity of four pesticides to water fleas and fathead
minnows, Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory, Office of Research and
Development, (Ecological Research Series EPA-600/3-76-099).
MCNULTY, M.J., HECK, H.D'A., & CASANOVA-SCHMITZ, M. (1984) Depletion
of glutathione in rat respiratory mucosa by inhaled acrolein. Fed.
Proc., 43: 575 (abstract).
MAGIN, D.F. (1980) Gas chromatography of simple monocarbonyls in
cigarette whole smoke as the benzyloxime derivatives. J.
Chromatogr., 202: 255-261.
MALDOTTI, A., CHIORBOLI, C., BIGNOZZI, C.A., BARTOCCI, C., &
CARASSITI, V. (1980) Photooxidation of 1,3-butadiene containing
systems: rate constant determination for the reaction of acrolein
with OH radicals. Int. J. chem. Kinet., 12: 905-913.
MANITA, M.D. & GOLDBERG, E.K. (1970) [Spectrophotometric analysis of
airborne acrolein using the semithiocarbazide reagent.] Gig. i
Sanit., 35(5): 63-65 (in Russian).
MANNING, D.L., MASKARINEC, M.P., JENKINS, R.A., & MARSHALL, A.H.
(1983) High performance liquid chromatographic determination of
selected gas phase carbonyl in tobacco smoke. J. Assoc. Off. Anal.
Chem., 66: 8-12.
MARANO, F. & DEMESTERE, M. (1976) Ultrastructural autoradiographic
study of the intracellular fixation of 3H-acrolein. Experientia
(Basel), 32: 501-503.
MARANO, F. & PUISEUX-DAO, S. (1982) Acrolein and cell cycle.
Toxicol. Lett., 14: 143-149.
MARINELLO, A.J., GURTOO, H.L., STRUCK, R.F., & PAUL, B. (1978)
Denaturation of cytochrome P-450 by cyclophosphamide metabolites.
Biochem. Biophys. Res. Commun., 83: 1347-1353.
MARINELLO, A.J., BERRIGAN, M.J., STRUCK, R.F., GUENGERICH, F.P., &
GURTOO, H.L. (1981) Inhibition of NADPH-cytochrome P450 reductase by
cyclophosphamide and its metabolites. Biochem. biophys. Res.
Commun., 99: 399-406.
MARINELLO, A.J., BANSAL, S.K., PAUL, B., KOSER, P.L., LOVE, J.,
STRUCK, R.F., & GURTOO, H.L. (1984) Metabolism and binding of
cyclophosphamide and its metabolite acrolein to rat hepatic
microsomal cytochrome P-450. Cancer Res., 44: 4615-4621.
MARNETT, L.J., HURD, H.K., HOLLSTEIN, M.C., LEVIN, D.E., ESTERBAUER,
H., & AMES, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA 104. Mutat. Res., 148:
25-34.
MASARU, N., SYOZO, F., & SABURO, K. (1976) Effects of exposure to
various injurious gases on germination of lily pollen. Environ.
Pollut., 11: 181-187.
MASEK, V. (1972) [Aldehydes in the air at working places in coal and
pitch coking plants.] Staub-Reinhalt. Luft, 32: 335-336 (in
German).
MASLOWSKA, J. & BAZYLAK, G. (1985) Thermofractographic determination
of monocarbonyl compounds in animal waste fats used as feed fat.
Anim. Feed Sci. Technol., 13: 227-236.
METTIER, S.R., BOYER, H.K., HINE, C.H., & MCEWEN, W.K. (1960) A
study of the effects of air pollutants on the eye. Am. Med. Assoc.
Arch. ind. Health, 21: 13-18.
MILLS, D.E., BAUGH, W.D., & CONNER, H.A. (1954) Studies on the
formation of acrolein in distillery mashes. Appl. Microbiol., 2:
9-13.
MIRKES, P.E., GREENAWAY, J.C., ROGERS, J.G., & BRUNDRETT, R.B.
(1984) Role of acrolein in cyclophosphamide teratogenicity in rat
embryos in vitro. Toxicol. appl. Pharmacol., 72: 281-291.
MIYAMOTO, Y. (1986) Eye and respiratory irritants in jet engine
exhaust. Aviat. Space environ. Med., 57: 1104-1108.
MORIKAWA, T. (1976) Acrolein, formaldehyde, and volatile fatty acids
from smoldering combustion. J. Combust. Toxicol., 3: 135-150.
MORIKAWA, T. & YANAI, E. (1986) Toxic gases evolution from
air-controlled fires in a semi-full scale room. J. Fire Sci., 4:
299-314.
MOULE, Y. & FRAYSSINET, C. (1971) Effects of acrolein on
transcription in vitro. Fed. Eur. Biochem. Soc. Lett., 16:
216-218.
MULDERS, E.J. & DHONT, J.H. (1972) The odour of white bread. III.
Identification of volatile carbony compounds and fatty acids. Z.
Lebensm. Unters. Forsch., 150: 228-232.
MUNSCH, N. & FRAYSSINET, C. (1971) Action de l'acroléine sur les
syntheses d'acides nucléiques in vivo. Biochimie, 53: 243-248.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1973) Effects of
acrolein on DNA synthesis in vitro. Fed. Eur. Biochem. Soc.
Lett., 30: 286-289.
MUNSCH, N., MARANO, F., & FRAYSSINET, C. (1974a) Incorporation
d'acroleine 3H dans le foie du rat et chez Dunaliella bioculata.
Biochimie, 56: 1433-1436.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1974b) In vitro
binding of 3H-acrolein to regenerating rat liver DNA polymerase.
Experientia, 30: 1234-1236.
MURPHY, S.D. (1965) Mechanism of the effect of acrolein on rat liver
enzymes. Toxicol. appl. Pharmacol., 7: 833-843.
MURPHY, S.D. & PORTER, S. (1966) Effects of toxic chemicals on some
adaptive liver enzymes, liver glycogen, and blood glucose in fasted
rats. Biochem. Pharmacol., 15: 1665-1676.
MURPHY, S.D., KLINGSHIRN, D.A., & ULRICH, C.E. (1963) Respiratory
response of guinea pigs during acrolein inhalation and its
modification by drugs. J. Pharmacol. exp. Ther., 141: 79-83.
MURPHY, S.D., DAVIS, H.V., & ZARATZIAN, V.L. (1964) Biochemical
effects in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
NATUSCH, D.F.S. (1978) Potentially carcinogenic species emitted to
the atmosphere by fossil-fueled power plants. Environ. Health
Perspect., 22: 79-90.
NIELSEN, G.D., BAKBO, J.C., & HOLST, E. (1984) Sensory irritation
and pulmonary irritation by airborne allyl acetate, allyl alcohol,
and allyl ether compared to acrolein. Acta pharmacol. toxicol., 54:
292-298.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1986) Determination of
micro amounts of acrolein in air by gas chromatography. J.
Chromatogr., 370: 327-332.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987a) Gas chromatographic
determination of acrolein in rain water using bromination of
O-methyloxime. Analyst, 112: 45-48.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987b) Determination of
acrolein and crotonaldehyde in automobile exhaust gas by gas
chromatography with electron-capture detection. Analyst, 112:
859-862.
OBERDORFER, P.E. (1971) The determination of aldehydes in automobile
exhaust gas. In: Vehicle emissions, Part III, Society of Automotive
Engineers Prog. Tech., Vol. 14, pp. 32-42.
OHARA, T., SATO, T., SHIMUZU, N., PRESCHER, G., SCHWIND, H., &
WEIBURG, O. (1987) Acrolein and methacrolein. In: Encyclopedia of
chemical technology, Deerfield Beech, Florida, Ullmann Verlag
Chemie.
O'LOUGHLIN, E.M. & BOWMER, K.H. (1975) Dilution and decay of aquatic
herbicides in flowing channels. J. Hydrol., 26: 217-235.
OLSON, K.L. & SWARIN, S.J. (1985) Determination of aldehydes and
ketones by derivatization and liquid chromatography-mass
spectrometry. J. Chromatogr., 333: 337-347.
OSBORNE, A.D., PITTS, J.N., & DARLEY, E.F. (1962) On the stability
of acrolein towards photooxidation in the near ultra-violet. Int.
J. Air Water Pollut., 6: 1-3.
PATEL, J.M., WOOD, J.C., & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparations. Drug Metab. Disposal, 8: 305-308.
PATEL, J.M., ORTIZ, E., KOLMSTETTER, C., & LEIBMAN, K.C. (1984)
Selective inactivation of rat lung and liver microsomal
NADPH-cytochrome c reductase by acrolein. Drug Metab. Disposal, 12:
460-463.
PEREZ, J.M., LIPARI, F., & SEIZINGER, D.E. (1984) Cooperative
development of analytical methods for diesel emissions and
particulates - solvent extractables, aldehydes and sulfate methods.
In: Diesel exhaust emissions, Society of Automotive Engineers, pp.
95-114 (Special Publication No. 578).
PETTERSSON, B., CURVALL, M., & ENZELL, C.R. (1980) Effects of
tobacco smoke compounds on the noradrenaline induced oxidative
metabolism in isolated brown fat cells. Toxicology, 18: 1-15.
PFAFFLI, P. (1982) III. Industrial hygiene measurements. Scand. J
Work Environ. Health, 8(suppl. 2): 27-43.
PHILIPPIN, C., GILGEN, A., & GRANDJEAN, E. (1970) Etude
toxicologique et physiologique de l'acroléine chez la souris. Int.
Arch. Arbeitsmed., 26: 281-305.
PILOTTI, A., ANCKER, K., ARRHENIUS, E., & ENZELL, C. (1975) Effects
of tobacco and tobacco smoke constituents on cell multiplication in
vitro. Toxicology, 5: 49-62.
PLOTNIKOVA, M.M. (1957) [Data on hygienic evaluation of acrolein as
a pollution of the atmosphere.] Gig. i Sanit., 22(6): 10-15 (in
Russian).
POSTEL, W. & ADAM, L. (1983) [Gas chromatographic characterization
of raspberry brandy.] Dtsch. Lebensm. Rundschau, 79: 117-122 (in
German).
POTTS, W.J., LEDERER, T.S., & QUAST, J.F. (1978) A study of the
inhalation toxicity of smoke produced upon pyrolysis and combustion
of polyethylene foams. Part I. Laboratory studies. J. Combust.
Toxicol., 5: 408-433.
PRESSMAN, D. & LUCAS, H.J. (1942) Hydration of unsaturated
compounds. XI. Acrolein and acrylic acid. J. Am. Chem. Soc., 64:
1953-1957.
PROTSENKO, G.A., TRUBILKO, V.I., & SAVCHENKOV, V.A. (1973) Working
conditions when metals to which primer has been applied are welded
evaluated from the health and hygiene aspect. Avt. Svarka, 2:
65-68.
RANDALL, T.L. & KNOPP, P.V. (1980) Detoxification of specific
organic substances by wet oxidation. J. Water Pollut. Control Fed.,
52: 2117-2130.
RAPOPORT, I.A. (1948) [Mutations under the influence of unsaturated
aldehydes.] Dokl. Akad. Nauk. SSSR, 16: 713-715 (in Russian).
RATHKAMP, G., TSO, T.C., & HOFFMANN, D. (1973) Chemical studies on
tobacco smoke. XX: Smoke analysis of cigarettes made from bright
tobaccos differing in variety and stalk positions. Beitr.
Tabakforsch., 7: 179-189.
RENZETTI, N.A. & BRYAN, R.J. (1961) Atmospheric sampling for
aldehydes and eye irritation in Los Angeles smog. J. Air Pollut.
Control Assoc., 11: 421-424, 427.
RICHTER, M. & ERFURTH, I. (1979) [On the gas chromatographic
determination of acrolein in its original state in the main stream
smoke of cigarettes.] Ber. Inst. Tabaksforsch. 26: 36-45 (in
German).
RICKERT, W.S., ROBINSON, J.C., & YOUNG, J.C. (1980) Estmating the
hazards of "less hazardous" cigarettes. I. Tar, nicotine, carbon
monoxide, acrolein, hydrogen cyanide, and total aldehyde deliveries
of Canadian cigarettes. J. Toxicol. environ. Health, 6: 351-365.
RIETZ, B. (1985) Determination of three aldehydes in the air of
working environments. Anal. Lett., 18(A19): 2369-2379.
RIJSTENBIL, J.W. & VAN GALEN, G.C. (1981) Chemical control of mussel
settlement in a cooling water system using acrolein. Environ,
Pollut., A25: 187-195.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver aldehyde
dehydrogenases. Relation to allyl alcohol hepatoxicity. Metab.
Disposal, 15: 356-362.
ROSENTHALER, L. & VEGEZZI, G. (1955) [Acrolein in spirits.] Z.
Lebensm. Unters. Forsch., 102: 117-123 (in German).
RYLANDER, R. (1973) Toxicity of cigarette smoke components: free
lung cell response in acute exposures. Am. Rev. respir. Dis., 108:
1279-1282.
SAITO, T., TAKASHINA, T., YANAGISAWA, S., & SHIRAI, T. (1983)
[Determination of trace low molecular weight aliphatic compounds in
auto exhaust by gas chromatography with a glass capillary column.]
Bunseki Kagaku, 32: 33-38 (in Japanese).
SAKATA, T., SMITH, R.A., GARLAND, E.M., & COHEN, S.M. (1989) Rat
urinary bladder epithelial lesions induced by acrolein. J. environ.
Pathol. Toxicol., 9: 159-170.
SALAMAN, M.H. & ROE, F.J.C. (1956) Further tests for tumour-
initiating activity: N,N-di-(2-chloroethyl)-p-aminophenylbutyric
acid (CB1348) as an initiator of skin tumour formation in the mouse.
Br. J. Cancer, 10: 363-378.
SALEM, H. & CULLUMBINE, H. (1960) Inhalation toxicities of some
aldehydes. Toxicol. appl. Pharmacol., 2: 183-187.
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHIELKE, D.-J. (1987) [Gastrectomy following a rare caustic
lesion.] Chirurg, 58: 50-52 (in German).
SCHMID, B.P., GOULDING, E., KITCHIN, K., & SANYAL, M.K. (1981)
Assessment of the teratogenic potential of acrolein and
cyclophosphamide in a rat embryo culture system. Toxicology, 22:
235-243.
SCHUCK, E.A. & RENZETTI, N.A. (1960) Eye irritants formed during
photo-oxidation of hydrocarbons in the presence of oxides of
nitrogen. J. Air Pollut. Control Assoc., 10: 389-392.
SCHUTTE, N.P. (1977) Hazard evaluation and technical assistance
report No. TA 77-11: Xomed Company, Cincinnati, Ohio, Cincinnati,
Ohio, USA, National Institute for Occupational Safety and Health
(NIOSH-TR-TA-77-11, PB 278834).
SEIZINGER, D.E. & DIMITRIADES, B. (1972) Oxygenates in exhaust from
simple hydrocarbon fuels. J. Air Pollut. Control Assoc., 22: 47-51.
SERTH, R.W., TIERNEY, D.R., & HUGHES, T.W. (1978) Source
assessment: acrylic acid manufacture, Cincinnati, Ohio, US
Environmental Protection Agency, Industrial Environmental Research
Laboratory (Report EPA-600/2-78-004w).
SHAPIRO, R., SODUM, R.S., EVERETT, D.W., & KUNDU, S.K. (1986)
Reactions of nucleosides with glyoxal and acrolein. In: The role of
cyclic nucleic acid adducts in carcinogenesis and mutagenesis,
Proceedings of a meeting organized by the IARC and co-sponsored by
the US National Cancer Institute, and the Lawrence Berkely
Laboratory at the University of California, Lyon, 17-19 September,
Lyon, International Agency for Research on Cancer, pp. 165-173 (IARC
Scientific Publications No. 70).
SHERWOOD, R.L., LEACH, C.L., HATOUM, N.S., & ARANYI, C. (1986)
Effects of acrolein on macrophage functions in rats. Toxicol.
Lett., 32: 41-49.
SHIMOMURA, M., YOSHIMATSU, F., & MATSUMOTO, F. (1971) [Fish odour of
cooked horse mackerel.] Kaseigaku Zasshi, 2: 106-112 (in Japanese).
SIM, V.M. & PATTLE, R.E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1908-1913.
SINKUVENE, D. (1970) [Hygienic assessment of acrolein as an
atmospheric pollutant.] Gig. i Sanit., 35(3): 6-10 (in Russian).
SKOG, E. (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde,
propionaldehyde and butyraldehyde, as well as of acrolein and
crotonaldehyde. Acta pharmacol., 6: 299-318.
SLOTT, V.L. & HALES, B.F. (1985) Teratogenicity and embryolethality
of acrolein and structurally related compounds in rats. Teratology,
32: 65-72.
SLOTT, V.L. & HALES, B.F. (1986) The embryolethality and
teratogenicity of acrolein in cultured rat embryos. Teratology, 34:
155-163.
SLOTT, V.L. & HALES, B.F. (1987a) Enhancement of the embryotoxicity
of acrolein, but not phosphoramide mustard, by glutathione depletion
in rat embryos in vitro. Biochem. Pharmacol., 36: 2019-2025.
SLOTT, V.L. & HALES, B.F. (1987b) Protection of rat embryos in
culture against the embryotoxicity of acrolein using exogenous
glutathione., Biochem. Pharmacol., 36: 2087-2194.
SMITH, R.A., COHEN, S.M., & LAWSON, T.A. (1990) Acrolein
mutagenicity in the V79 assay. Carcinogenesis, 11: 497-498.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SMYTHE, R.J. & KARASEK, F.W. (1973) The analysis of diesel engine
exhaust for low-molecular-weight carbonyl compounds. J.
Chromatogr., 86: 228-231.
SNYDER, J.M., FRANKEL, E.N., & SELKE, E. (1985) Capillary gas
chromatographic analyses of headspace volatiles from vegetable oils.
J. Am. Oil Chem. Soc., 6: 1675-1679.
SPONHOLZ, W.-R. (1982) [Analysis and occurrence of aldehydes in
wines.] Z. Lebensm. Unters. Forsch., 174: 458-462 (in German).
SPRINCE, H., PARKER, C.M., & SMITH, G.G. (1979) Comparison of
protection by L-ascorbic acid, L-cysteine, and adrenergic-blocking
agents against acetaldehyde, acrolein, and formaldehyde toxicity:
implications in smoking. Agents Actions, 9: 407-414.
STACK, V.T. (1957) Toxicity of alpha,ß-unsaturated carbonyl
compounds to microorganisms. Ind. eng. Chem., 49: 913-917.
STAHLMANN, R., BLUTH, U., & NEUBERT, D. (1985) Effects of the
cyclophosphamide metabolite acrolein in mammalian limb bud cultures.
Arch. Toxicol., 57: 163-167.
STEINHAGEN, W.H. & BARROW, C.S. (1984) Sensory irritation
structure-activity study of inhaled aldehydes in B6C3F1 and
Swiss-Webster mice. Toxicol. appl. Pharmacol., 72: 495-503.
STEPHENS, E.R., DARLEY, E.F., TAYLOR, O.C., & SCOTT, W.E. (1961)
Photochemical reaction products in air pollution. Int. J. Air Water
Pollut., 4: 79-100.
SUBDEN, R.E., KRIZUS, A., & AKHTAR, M. (1986) Mutagen content of
Canadian apple eau-de-vie. Can. Inst. Food Sci. Technol. J., 19:
134-136.
SUZUKI, Y. & IMAI, S. (1982) Determination of traces of gaseous
acrolein by collection on molecular sieves and fluorimetry with
o-aminobiphenyl. Anal. Chim. Acta, 136: 155-162.
SWARIN, S.J. & LIPARI, F. (1983) Determination of formaldehyde and
other aldehydes by high performance liquid chromatography with
fluorescence detection. J. liq. Chromatogr., 6: 425-444.
SZOT, R.J. & MURPHY, S.D. (1971) Relationships between cyclic
variations in adrenocortical secretory activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant compounds.
J. Water Pollut. Control Fed., 53: 1503-1517.
TAJIMA, M., KIDA, K., & FUJIMAKI, M. (1967) Effect of gamma
irradiation on volatile compounds from cooked potato. Agric. biol.
Chem., 31: 935-938.
TAKEUCHI, K. & IBUSUKI, T. (1986) Heterogeneous photochemical
reactions of a propylene-nitrogen dioxide-metal oxide-dry air
system. Atmos. Environ., 20: 1155-1160.
TEJADA, S.B. (1986) Evaluation of silica gel cartridges coated in
situ with acidified 2,4-dinitrophenylhydrazine for sampling
aldehydes and ketones in air. Int. J. environ. anal. Chem., 26:
167-185.
TESTA, A. & JOIGNY, C. (1972) Dosage par chromatographie en phase
gazeuse de l'acroléine et d'autres composés alpha,ß-insaturés de la
phase gazeuse de la fumée de cigarette. Ann. Serv. Exploit. ind.
Tab. Allumettes - Div. Etud. Equip., 10: 67-81.
TORAASON, M., LUKEN, M.E., BREITENSTEIN, M., KRUEGER, J.A., &
BIAGINI, R.E. (1989) Comparative toxicity of allylamine and acrolein
in cultured myocytes and fibroblasts from neonatal rat heart.
Toxicology, 56: 107-117.
TREITMAN, R.D., BURGESS, W.A., & GOLD, A. (1980) Air contaminants
encountered by firefighters. Am. Ind. Hyg. Assoc. J., 41: 796-802.
TURUK-PCHELINA, Z.F. (1960) [Acrolein releases into the air while
cooking.] Gig. i Sanit., 25(5): 96-97 (in Russian).
UMANO, K. & SHIBAMOTO, T. (1987) Analysis of headspace volatiles
from overheated beef fat. J. agric. food Chem., 35: 14-18.
UNRAU, G.O., FAROOQ, M., DAWOOD, I.K., MIGUEL, L.C., & DAZO, B.C.
(1965) Field trials in Egypt with acrolein herbicide-molluscicide.
World Health Organ. Bull., 32: 249-260.
US-NIOSH (1984) NIOSH manual of analytical methods, 3rd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (DHHS (NIOSH) Publication No. 84-100. Method 2501).
VAN EICK, A.J. (1977) [The effect of acrolein in air on the eye
blinking frequency of man and guinea pig], Rijswijk, The
Netherlands, Technical and Physical Research, Medical Biological
Laboratory, TNO-MBL, (Report No. A76/K/098) (in Dutch).
VAN OVERBEEK, J., HUGHES, W.J., & BLONDEAU, R. (1959) Acrolein for
the control of water weeds and disease-carrying water snails.
Science, 129: 335-336.
VEITH, G.D., MACEK, K.J., PETROCELLI, S.R., & CARROL, J. (1980) An
evaluation of using partition coefficients and water solubility to
estimate bioconcentration factors for organic chemicals in fish.
Aquat. Toxicol.: 116-129.
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene problems
in vulcanization processes of rubber production]. Gig. i Sanit.,
34: 33-40 (in Russian).
VOROB'EVA, A.I., VOLKOTRUB, L.P., FEOKTISTOVA, N.F., BOBIN, V.I.,
SHESTAKOVA, N.A., & USHAKOVA, N.S. (1982) [Characteristics of
atmospheric pollution from enameled wire manufacturing plants.]
Gig. i Sanit., 1982(6): 66-67 (in Russian).
WARHOLM, M., HOLMBERG, B., & MALMFORS, T. (1984) The cytotoxicity of
some organic solvents. Environ. Res., 7: 183-192.
WATANABE, T. & AVIADO, D.M. (1974) Functional and biochemical
effects on the lung following inhalation of cigarette smoke and
constituents. II. Skatole, acrolein, and acetaldehyde. Toxicol.
appl. Pharmacol. 30: 201-209.
WEBER-TSCHOPP, A., FISCHER, T., & GRANDJEAN, E. (1976) [Objective
and subjective physiological effects of passive smoking.] Int. Arch.
occup. environ. Health, 37: 277-288 (in German).
WEBER-TSCHOPP, A., FISCHER, T., GIERER, R., & GRANDJEAN, E. (1977)
[Experimental irritation by acrolein in human beings.] Z.
Arbeitswiss., 32: 166-171 (in German).
WHITEHOUSE, M.W., BECK, F.W.J., DROGE, M.M., & STRUCK, R.F. (1974)
Lymphocyte deactivation by (potential immunosuppressant) alkylating
metabolites of cyclophosphamide. Agents Actions, 4: 117-123.
WHO (1991) Health and Safety Guide 67: Acrolein, Geneva, World
Health Organization.
WILMER, J.L., EREXSON, G.L., & KLIGERMAN, A.D. (1986) Attenuation of
cytogenetic damage by 2-mercaptoethanesulphonate in cultured human
lymphocytes exposed to cyclophosphamide and its reactive
metabolites. Cancer Res., 46: 203-210.
WILTON, D.C. (1976) Acrolein, an irreversible active-site-directed
inhibitor of deoxyribose 5-phosphate aldolase? Biochem. J., 153:
495-497.
WITZ, G., LAWRIE, N.J., AMORUSO, M.A., & GOLDSTEIN, B.D. (1987)
Inhibition by reactive aldehydes of superoxide anion radical
production from stimulated polymorphonuclear leukocytes and
pulmonary alveolar macrophages. Biochem. Pharmacol., 36: 721-726.
WRABETZ, E., PETER, G., & HOHORST, H.J. (1980) Does acrolein
contribute to the cytotoxicity of cyclophosphamide? J. Cancer Res.,
98: 119-126.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20
coded compounds tested for the National Toxicology Program.
Environ. Mutagen., 7: 87-100.
ZITTING, A. & HEINONEN, T. (1980) Decrease of reduced glutathione in
isolated rat hepatocytes caused by acrolein, acrylonitril, and the
thermal degradation products of styrene copolymers. Toxicology, 17:
333-341.
ZOLLNER, H. (1973) Inhibition of some mitochondrial functions by
acrolein and methylvinylketone. Biochem. Pharmacol., 22: 1171-1178.
ZORIN, V.M. (1966) [Acrolein-induced air pollution.] Zdravookhr.
Belorussii, 1966(7): 43-44 (in Russian).
RESUME
L'acroléine est un liquide volatil extrêmement inflammable dont
l'odeur, âcre et suffocante, est très désagréable. C'est un composé
très réactif.
En 1975, on estime que la production mondiale d'acroléine en
tant que telle était de 59 000 tonnes. On en produit et consomme
encore davantage comme intermédiaire pour la synthèse de l'acide
acrylique et de ses esters.
On dispose d'un certain nombre de méthodes d'analyse pour la
recherche et le dosage de l'acroléine dans divers milieux. On a fait
état de limites inférieures de détection de l'ordre de 0,1 µg/m3
dans l'air (chromatographie en phase gazeuse/spectrométrie de
masse), de 0,1 µg/l dans l'eau (chromatographie liquide à haute
pression), de 2,8 µg/litre dans les milieux biologiques
(fluorimétrie), de 590 µg/kg dans le poisson (chromatographie en
phase gazeuse/spectrométrie de masse) et de 1,4 µg/m3 dans les gaz
d'échappement (chromatographie liquide à haute pression).
On a trouvé de l'acroléine dans certains produits d'origine
végétale et animale et notamment dans des denrées alimentaires et
des boissons. L'acroléine est utilisée principalement comme
intermédiaire en synthèse organique, mais également comme produit
biocide en milieu aquatique.
Des émissions d'acroléine peuvent se produire sur les lieux de
production ou d'utilisation. Elles peuvent être importantes dans
l'air à la suite de la combustion ou de la pyrolyse incomplète de
produits organiques tels que combustibles, polymères de synthèse,
dans certains aliments et le tabac. L'acroléine peut représenter
jusqu'à 3-10 % des aldéhydes totaux présents dans les gaz
d'échappement des véhicules à moteur. La consommation d'une
cigarette fournit de 3 à 228 µg d'acroléine. L'acroléine est un
produit d'oxydation photochimique de certains polluants organiques
de l'atmosphère.
La population générale est essentiellement exposée par
l'intermédiaire de l'air. Une exposition peut également se produire
par voie orale par suite de la consommation de boissons alcoolisées
ou de denrées alimentaires chauffées.
On a mesuré dans l'air des villes des concentrations moyennes
d'acroléine atteignant environ 15 µg/m3 avec des maxima allant
jusqu'à 32 µg/m3. A proximité d'installations industrielles et de
pots d'échappement, des concentrations dix à cent fois plus élevées
sont possibles. Les incendies peuvent donner naissance à des teneurs
très élevées d'acroléine, de l'ordre du mg/m3 d'air. A
l'intérieur des habitations, on a observé que la consommation d'une
cigarette par m3 d'air dans un local en l'espace de 10 à
13 minutes produisait des concentrations en vapeurs d'acroléine de
l'ordre de 450 à 840 µg/m3. Sur les lieux de travail, on a signalé
des teneurs dépassant 100 µg/m3 dans des cas où l'on élevait la
température de certains produits organiques, par exemple lors du
chauffage ou du soudage de ces substances.
Dans l'atmosphère, l'acroléine est dégradée par réaction avec
les radicaux hydroxyles. Sa durée de séjour dans l'atmosphère est de
l'ordre d'une journée. Dans les eaux de surface, l'acroléine se
dissipe en quelques jours. Elle est faiblement adsorbée aux
particules du sol. On a fait état de dégradation aérobie et
anaérobie, encore que la toxicité du composé pour les
micro-organismes puisse faire obstacle à sa biodégradation. Compte
tenu des propriétés physiques et chimiques de l'acroléine, il ne
semble pas que cette substance ait une tendance à la
bioaccumulation.
L'acroléine est extrêmement toxique pour les organismes
aquatiques. Pour les bactéries, les algues, les crustacés et les
poissons, sa toxicité aiguë, estimée d'après les valeurs de la CE50
et de la CL50, se situe entre 0,02 et 2,5 mg/litre, les bactéries
étant l'espèce la plus sensible. Pour le poisson, on a fixé à
0,0114 mg/litre la dose sans effet nocif observable à 60 jours.
L'acroléine détruit efficacement les végétaux aquatiques à des doses
comprises entre 4 et 26 mg/litre.h. A partir de 15 mg/litre, on
observe des effets nocifs sur les cultures irriguées au moyen d'eau
traitée à l'acroléine.
Chez l'homme et l'animal, l'acroléine reste confinée sur son
site d'exposition en raison de sa réactivité et les observations
pathologiques sont également limitées à ce site. Chez des chiens
exposés à des doses de 400 à 600 mg/m3, on a observé un taux de
rétention de 80 à 85 % au niveau des voies respiratoires.
L'acroléine réagit directement sur les groupements sulfhydryles
protéiques et non protéiques ainsi que sur les amines primaires et
secondaires. Elle peut également être métabolisée en acide
mercapturique, en acide acrylique, en glycidaldéhyde ou en
glycéraldéhyde. Les trois derniers métabolites n'ont été observés
qu' in vitro.
L'acroléine est un agent cytotoxique. Sa cytotoxicité s'observe
in vitro dès 0,1 mg/litre. Elle est extrêmement toxique pour les
animaux de laboratoire et l'homme, à la suite d'une seule exposition
quelle qu'en soit la voie. Sa vapeur est irritante pour l'oeil et
les muqueuses respiratoires. Le liquide est corrosif et on a
constaté qu'en solution éthanolique le seuil d'apparition d'une
dermatite d'irritation était de 0,1%. L'expérimentation sur des
volontaires humains exposés à des vapeurs d'acroléine a permis de
fixer à 0,13 mg/m3 la dose la plus faible produisant des effets
nocifs observables; à cette dose, une irritation des yeux se produit
en l'espace de cinq minutes. En outre, les effets au niveau des
voies respiratoires deviennent évidents à partir de 0,7 mg/m3. Une
seule exposition à des doses plus élevées entraîne une
dégénérescence de l'épithélium respiratoire, des séquelles
inflammatoires et une perturbation de la fonction respiratoire.
On a étudié sur des rats, des chiens, des cobayes et des singes
les effets toxicologiques de l'inhalation continue d'acroléine à des
concentrations de 0,5 à 4,1 mg/m3. Des effets histopathologiques
et des effets sur la fonction respiratoire ont été observés chez les
animaux exposés à des teneurs supérieures ou égales à 0,5 mg/m3
pendant 90 jours.
On a étudié sur divers animaux de laboratoire les effets
toxicologiques d'expositions répétées par la voie respiratoire à des
vapeurs d'acroléine, à des concentrations allant de 0,39 mg/m3 à
11,2 mg/m3. La durée de l'exposition allait de cinq jours à
52 semaines. En général, on a fait état chez la plupart des espèces
exposées huit heures par jour à des concentrations de 1,6 mg/m3 ou
davantage, d'une réduction du gain de poids, d'une diminution de la
fonction respiratoire et de modifications pathologiques au niveau du
nez, des voies respiratoires supérieures et des poumons. Parmi les
modifications anatomopathologiques figuraient une inflammation, une
métaplasie et une hyperplasie des voies respiratoires. On a observé
une mortalité importante après expositions répétées à des vapeurs
d'acroléine à des concentrations dépassant 9,07 mg/m3. Chez
l'animal d'expérience, on a montré que l'acroléine provoquait une
déplétion du glutathion tissulaire in vivo et une inhibition des
enzymes in vitro par réaction sur les groupements sulfhydryles au
niveau des sites actifs. Il existe quelques données selon lesquelles
l'acroléine est susceptible d'amoindrir les défenses pulmonaires de
l'hôte chez la souris et le rat.
L'acroléine peut produire des effets tératogènes et
embryotoxiques lorsqu'on l'introduit directement dans l'amnios.
Toutefois, l'absence d'effets chez des lapins à qui elle avait été
injectée par voie intraveineuse à la dose de 3 mg/kg incite à penser
que l'exposition de l'homme à l'acroléine ne devrait pas avoir
d'effet nocif sur le développement de l'embryon.
On a montré que l'acroléine interagissait avec les acides
nucléiques in vitro et en inhibait la synthèse tant in vitro
qu' in vivo. Sans avoir besoin d'être activée, elle produit des
mutations géniques chez les bactéries et les champignons et induit
des échanges entre chromatides soeurs dans les cellules
mammaliennes. Dans tous les cas, ces effets se sont produits dans un
intervalle de dose extrêmement limité qui était fonction de la
réactivité, de la volatilité et de la cytotoxicité de l'acroléine.
Une épreuve de mutation létale dominante chez la souris a donné des
résultats négatifs. Les données disponibles montrent que l'acroléine
est faiblement mutagène pour certains champignons et bactéries et
certaines cultures de cellules mammaliennes.
Des hamsters ont été exposés pendant 52 semaines à des vapeurs
d'acroléine à la dose de 9,2 mg/m3, 7 heures par jour et 5 jours
par semaine, puis ont été placés en observation pendant les 29
semaines suivantes; aucune tumeur n'a été observée. En exposant ces
hamsters dans les mêmes conditions à des vapeurs d'acroléine et
pendant la même durée avec, en outre, des doses intra-trachéennes
hebdomadaires de benzo[a]pyrène ou des doses sous-cutanées une fois
toutes les trois semaines de diéthylnitrosamine, on n'a pas non plus
observé d'effets co-cancérogènes bien nets attribuables à
l'acroléine. Des rats exposés par voie orale à de l'acroléine dans
leur eau de boisson à des doses comprises entre 5 et 50 mg/kg par kg
de poids corporel (quotidiennement, cinq jours par semaine pendant
100 à 124 semaines) n'ont pas présenté de tumeur. En raison du
caractère limité de toutes ces épreuves, on estime que les données
qui permettraient d'évaluer la cancérogénicité de l'acroléine chez
l'animal d'expérience sont encore insuffisantes. De ce fait, il est
impossible pour l'instant d'évaluer la cancérogénicité de
l'acroléine pour l'homme.
Les différents seuils de concentration auxquels apparaissent
les différents effets de l'acroléine sont les suivants : perception
d'une odeur, 0,007 mg/m3, irritation oculaire, 0,3 mg/m3,
irritation du nez et clignement des yeux, 0,03 mg/m3, réduction de
la fréquence respiratoire, 0,7 mg/m3. Comme la concentration de
l'acroléine dépasse rarement 0,03 mg/m3 dans l'air des villes,
elle n'est pas susceptible de constituer une nuisance dans les
circonstances normales.
Du fait de sa forte toxicité pour les organismes aquatiques,
l'acroléine présente un danger pour la faune et la flore aquatique à
proximité ou sur les sites de décharge de déchets industriels, en
cas de déversements et là où l'on utilise ce produit comme biocide.
1. RESUMEN
La acroleína es un líquido volátil, sumamente inflamable, con
un olor pungente, asfixiante y desagradable. Se trata de un
compuesto muy reactivo.
La producción mundial de acroleína aislada se calculó en 59 000
toneladas en 1975. Se produce y consume una cantidad aún mayor de
acroleína como intermediaria en la síntesis de ácido acrílico y sus
ésteres.
Se dispone de métodos analíticos para determinar la acroleína
presente en diversos medios. Los límites mínimos de detección que
se han comunicado son 0,1 µg/m3 de aire (cromatografía
gaseosa/spectrometría de masas), 0,1 µg/litro de agua (cromatografía
líquida a alta presión), 2,8 µg/litro de medio biológico
(fluorimetría), 590 µg/kg en peces (cromatografía
gaseosa/espectrometría de masas), y 1,4 µg/m3 de gases de escape
(cromatografía líquida a alta presión).
La acroleína se ha detectado en algunos vegetales y animales,
inclusive en alimentos y bebidas. La sustancia se usa
principalmente como intermediaria en la síntesis química aunque
también como biocida acuático.
Pueden producirse emisiones de acroleína en sus lugares de
producción o de uso. Las emisiones importantes a la atmósfera se
deben a la combustión incompleta o la pirólisis de materiales
orgánicos como ser combustibles, polímeros sintéticos, alimentos y
tabaco. La acroleína puede representar el 3-10% de los aldehídos
totales presentes en los escapes de automóviles. El humo de un
cigarrillo libera 3-228 µg de acroleína. La acroleína es uno de los
productos de la oxidación fotoquímica de ciertos contaminantes
orgánicos de la atmósfera.
La exposición de la población general se produce principalmente
por el aire. La exposición por vía oral puede producirse por el
consumo de bebidas alcohólicas o alimentos calentados.
En la atmósfera urbana se han medido niveles promedio de
acroleína de hasta unos 15 µg/m3 y niveles máximos de hasta
32 µg/m3. En las cercanías de las industrias y junto a los caños
de escape pueden registrarse niveles entre 10 y 100 veces
superiores. Como resultado de incendios pueden hallarse niveles
sumamente elevados en el aire, del orden de mg/m3. En el aire
cerrado de interiores, el consumo de un cigarrillo por m3 de
volumen de la habitación produjo en 10-13 minutos concentraciones de
vapor de acroleína de 450-840 µg/m3. En el medio ambiente laboral
se han detectado niveles de más de 1000 µg/m3 en situaciones que
entrañaban aumento de temperatura de materiales orgánicos, por
ejemplo durante la soldadura o el calentamiento.
La acroleína se degrada en la atmósfera por reacción con
radicales hidroxilo. El tiempo de persistencia en la atmósfera es
de aproximadamente un día. En aguas de superficie, la acroleína se
disipa en pocos días. Tiene un bajo potencial de adsorción en el
suelo. Se ha observado su degradación en condiciones aerobias y
anaerobias, si bien la toxicidad del compuesto para los
microorganismos puede impedir la biodegradación. En vista de sus
propiedades físicas y químicas, es improbable que se produzca
bioacumulación de acroleína.
La acroleína es sumamente tóxica para los organismos acuáticos.
Los valores de la CE50 y la CL50 correspondientes a bacterias,
algas, crustáceos y peces se encuentran entre 0,02 y 2,5 mg/litro,
siendo las bacterias los organismos más sensibles. En peces se ha
determinado que el nivel sin observación de efectos adversos (NOAEL)
a 60 días es de 0,0114 mg/litro. Se ha conseguido combatir
eficazmente los vegetales acuáticos con dosis de acroleína
comprendidas entre 4 y 26 mg/litro.h. Se han observado efectos
adversos en cultivos que crecen en suelos irrigados con agua tratada
con acroleína en concentraciones de 15 mg/litro o más.
En el animale y en el ser humano la reactividad de la acroleína
limita efectivamente la sustancia al lugar de exposición; los
hallazgos patológicos se limitan asimismo a esos lugares. En el
tracto respiratorio de perros expuestos a 400-600 mg/m3 se
encontró una retención del 80-85% de acroleína. La acroleína
reacciona directamente con los grupos sulfhidrilo contenidos en
radicales proteicos o no proteicos y con aminas primarias y
secundarias. También puede ser metabolizado a ácidos mercaptúricos,
ácido acrílico, glicidaldehído o gliceraldehído. Estos tres últimos
metabolitos sólo se han encontrado in vitro.
La acroleína es un agente citotóxico. Se ha observado
citotoxicidad in vitro con niveles de solamente 0,1 mg/litro. La
sustancia es sumamente tóxica para los animales de experimentación y
el ser humano tras una exposición única por diferentes vías. El
vapor es irritante para los ojos y el tracto respiratorio. En
estado líquido es corrosiva. Con respecto a la dermatitis
irritante, se encontró que el NOAEL de la acroleína etanólica era de
0,1%. Los experimentos con voluntarios humanos expuestos a vapores
de acroleína mostraron un nivel mínimo de observación de efectos
(LOAEL) de 0,13 mg/m3, dosis con la que los ojos pueden irritarse
al cabo de cinco minutos. Además, se observan efectos en el tracto
respiratorio a partir de 0,7 mg/m3. Con exposiciones aisladas a
niveles más altos, aparecen: degeneración del epitelio respiratorio,
secuelas inflamatorias y trastorno de la función respiratoria.
Los efectos toxicológicos de la exposición por inhalación
continua de concentraciones comprendidas entre 0,5 y 4,1 mg/m3 se
han estudiado en la rata, el perro, el cobayo y el mono. Se
observaron efectos sobre la función respiratoria y trastornos
histopatológicos cuando se expuso a los animales a niveles de
acroleína de 0,5 mg/m3 o más, durante 90 días.
Los efectos toxicológicos de la inhalación repetida de vapores
de acroleína en concentraciones comprendidas entre 0,39 mg/m3 y
11,2 mg/m3 se han estudiado en diversos animales de laboratorio.
Las duraciones de la exposición variaron entre 5 días y hasta 52
semanas. En general, se han documentado: reducción de la
adquisición de peso corporal, disminución de la función pulmonar y
cambios patológicos en la nariz, las vías aéreas superiores y los
pulmones en la mayoría de las especies expuestas a concentraciones
de 1,6 mg/m3 o más, durante 8 h/día. Entre los cambios
patológicos se observaron inflamación, metaplasia e hiperplasia del
tracto respiratorio. Se ha observado un nivel significativo de
mortalidad tras la exposición repetida a concentraciones de vapor de
acroleína superiores a 9,07 mg/m3. En animales de
experimentación, se ha demostrado que la acroleína agota el
glutatión tisular y que in vitro inhibe enzimas reaccionando con
los grupos sulfhidrilo de los sitios activos. Hay limitada
evidencia de que la acroleína pueda deprimir las defensas pulmonares
en el ratón y la rata.
La acroleína puede inducir efectos teratogénicos y
embriotóxicos si se administra directamente en el amnios. No
obstante, el hecho de que no se observaran efectos en ratones a los
que se inyectó 3 mg/kg por vía intravenosa sugiere que la exposición
humana a la acroleína tiene pocas probabilidades de afectar al
embrión en desarrollo.
Se ha demostrado que la acroleína interacciona con los ácidos
nucleicos in vitro y que inhibe su síntesis tanto in vitro como
in vivo. Sin activación, indujo mutaciones génicas en bacterias y
hongos y provocó intercambios de cromátidas hermanas en células de
mamíferos. En todos los casos esos efectos se produjeron en un
margen muy reducido de concentraciones, limitado por la reactividad,
la volatilidad y la citotoxicidad de la acroleína. Un ensayo de
letalidad dominante en ratones dio resultado negativo. Los datos
disponibles muestran que la acroleína es un mutágeno débil para
ciertas bacterias, hongos y cultivo celular de mamífero.
No se encontraron tumores en hámsters expuestos durante 52
semanas a vapores de acroleína con una concentración de 9,2 mg/m3
durante 7 h/día, 5 días a la semana, y observados durante 29 semanas
más. Cuando se expusieron hámsters a vapores de acroleína en las
mismas condiciones durante 52 semanas y, además, a dosis
intratraqueales de benzo[a]pireno semanalmente o a dosis subcutáneas
de dietilnitrosamina una vez cada tres semanas, no se observó una
acción cocarcinogénica clara de la acroleína. La exposición de
ratas por vía oral a la acroleína en el agua de bebida, en dosis
comprendidas entre 5 y 50 mg/kg de peso corporal al día
(5 días/semana durante 104-124 semanas) no indujo tumores. Dado el
carácter limitado de todos esos ensayos, se considera que no se
dispone de datos suficientes para determinar la carcinogenicidad de
la acroleína en los animales de experimentación. En consecuencia,
se considera asimismo imposible evaluar la carcinogenicidad de la
acroleína para el ser humano.
Los umbrales de acroleína que causan irritación y efectos en la
salud son 0,07 mg/m3 en el caso de la percepción del olor,
0,13 mg/m3 en la irritación ocular, 0,3 mg/m3 en la irritación
nasal y el parpadeo, y 0,7 mg/m3 en la disminución del ritmo
respiratorio. Puesto que el nivel de acroleína raras veces supera
los 0,03 mg/m3 en el aire urbano, es poco probable que alcance
niveles molestos o nocivos en circunstancias normales.
En vista de la elevada toxicidad de la acroleína para los
organismos acuáticos, la sustancia representa un riesgo para la vida
acuática en las proximidades de las zonas donde se producen vertidos
y escapes industriales, y en los lugares donde se usa como biocida.
7. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
7.1 Single exposure
7.1.1 Mortality
The available acute mortality data are summarized in Table 8.
Most tests for the determination of the acute toxicity of acrolein
do not comply with present standards. Nevertheless, retesting is
not justified for ethical reasons and in view of the overt high
toxicity of acrolein following inhalation or oral exposure (Hodge &
Sterner, 1943).
In addition to the data in Table 8, an oral LD95 of
11.2 mg/kg body weight for Charles River rats, observed for 24 h,
has been reported (Sprince et al., 1979). Draminski et al.
(1983) reported the deaths of 5/10 rats given 10 mg/kg body weight
in corn oil by gavage.
7.1.2 Effects on the respiratory tract
In vapour exposure tests, the effects observed in experimental
animals have almost exclusively been local effects on the
respiratory tract and eyes.
In the LC50 studies, effects on the respiratory tract were
clinically observed as nasal irritation and respiratory distress in
rats (Skog, 1950; Potts et al., 1978; Crane et al., 1986),
hamsters (Kruysse, 1971), mice, guinea-pigs, and rabbits (Salem &
Cullumbine, 1960) at exposure levels of between 25 mg/m3 for 4 h
and 95 150 mg/m3 for 3 min. Rats exposed for 10 min to
concentrations of 750 or 1000 mg/m3 suffered asphyxiation
(Catilina et al., 1966).
Histopathological investigations in experiments with
vapour-exposed rats (Skog, 1950; Catilina et al., 1966; Potts
et al., 1978; Ballantyne et al., 1989), hamsters (Kilburn &
McKenzie, 1978), guinea-pigs (Dahlgren et al., 1972; Jousserandot
et al, 1981), and rabbits (Beeley et al., 1986) revealed varying
degrees of degeneration of the respiratory epithelium consisting of
deciliation (see also in vitro work on cytotoxicity discussed in
7.1.5), exfoliation, necrosis, mucus secretion, and vacuolization.
Also observed were acute inflammatory changes consisting of
infiltration of white blood cells into the mucosa, hyperaemia,
haemorrhages, and intercellular oedema. Proliferative changes of
the respiratory epithelium, in the form of early stratification and
hyperplasia, were observed in hamsters 96 h after exposure to
13.7 mg/m3 for 4 h (Kilburn & McKenzie, 1978).
Table 8. Acute mortality caused by acrolein
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Rat (Wistar) male inhalation (10 min) 8 750 Catilina et al. (1966)b
Rat (Wistar) not reported oral 14 46 (39-56) Smyth et al. (1951)g
Rat (unspecified not inhalation (30 min) 21 300 Skog (1950)b,c
strain) reported
Rat (Sprague-Dawley) male inhalation (30 min) 14 95-217 Potts et al. (1978)d
Rat (Sprague-Dawley) male and inhalation (1 h) 14 65 (60-68) Ballantyne et al. (1989)
female inhalation (4 h) 14 20.8 (17.5-24.8)
Rat (Sherman) male and inhalation (4 h) 14 18 Carpenter et al. (1949)b,e
female
Hamster (Syrian golden) male and inhalation (4 h) 14 58 (54-62) Kruysse (1971)
female
Table 8 (contd)
Species/strain Sex Route of exposure Observation LD (mg/kg bw) Reference
period (days) or LC50 (mg/m3)a
Mouse (unspecified male inhalation (6 h) 1 151 Philippin et al. (1970)f
strain)
Mouse (NMRI) not reported intraperitoneal 6 7 Warholm et al. (1984)g
a Where available, 95% confidence limits are given in parentheses.
b Determination of acrolein levels was not reported.
c No mortality at 100 mg/m3, 100% mortality at 700 mg/m3.
d Approximate value: no mortality at 33 mg/m3, 1/7 and 7/7 died at 95 and 217 mg/m3, respectively.
e Approximate value: 2-4/6 died.
f No mortality at 71 mg/m3, 100% mortality at 273 mg/m3.
g The vehicle was water.
Functional changes in the respiratory system following acrolein
vapour exposure have been investigated in guinea-pigs and mice. A
rapidly reversible increase in respiratory rate was observed in
intact guinea-pigs during exposure to 39 mg/m3 for 60 min (Davis
et al., 1967) and to 0.8 mg/m3 or more for 2 h (Murphy et al.,
1963) followed by a decrease in respiratory rate and an increase in
tidal volume. No changes in pulmonary compliance were reported.
Davis et al. (1967) did not observe these effects in
tracheotomized animals and concluded that they were caused by reflex
stimulation of upper airway receptors and not by
bronchoconstriction. Murphy et al. (1963), observing that
anticholinergic bronchodilators, aminophylline and isoproterenol,
but not antihistaminics, reduced the acrolein-induced increase in
respiratory resistance, concluded that acrolein caused
bronchoconstriction mediated through reflex cholinergic stimulation.
In another experiment, an increase in respiratory resistance was
also observed in anaesthetized, tracheotomized guinea-pigs with
transected medulla during exposure to 43 mg/m3 for up to 5 min
(Guillerm et al., 1967b). The effect was not reversed by atropine.
It was concluded by the authors that acrolein did not cause
bronchoconstriction via reflex stimulation, but probably via
histamine release. When anaesthetized mice were exposed to 300 or
600 mg/m3 for 5 min via a tracheal cannula, respiratory
resistance, respiratory rate, and tidal volume decreased and
pulmonary compliance increased at an unspecified time after exposure
(Watanabe & Aviado, 1974).
The concentration that produces a 50% decrease in respiratory
rate (RD50) as a result of reflex stimulation of trigeminal nerve
endings in the nasal mucosa (sensory irritation) has been used as an
index of upper respiratory tract irritation. This effect reduces
the penetration of noxious chemicals into the lower respiratory
tract. The rate of respiration was measured in a body
plethysmograph, only the animals' heads being exposed to the
acrolein vapour. Depending on the strain, RD50 values for mice
ranged from 2.4 to 6.6 mg/m3 (Kane & Alarie, 1977; Nielsen
et al., 1984; Steinhagen & Barrow, 1984). In rats a RD50 of
13.7 mg/m3 was found (Babiuk et al., 1985).
7.1.3 Effects on skin and eyes
Animal skin irritation tests have not been performed and skin
irritation has not been mentioned as an effect in the acute
inhalation tests reported.
One special in vivo eye irritation test involved
vapour-exposed and control rabbits. At analysed concentrations of
acrolein (method not specified) between 4.3 and 5.9 mg/m3,
maintained over 4 h, slight chemosis was observed but no iritis
(Mettier et al., 1960). Eye irritation was observed clinically in
rodents in several acute inhalation tests, but was not graded (Skog,
1950; Salem & Cullumbine, 1960; Kruysse, 1971; Potts et al.,
1978).
7.1.4 Systemic effects
With respect to systemic effects, most studies have been
performed at concentrations far above the lethal dose. When rats
were exposed to concentrations of acrolein between 1214 and
95 150 mg/m3 during various periods of time, incapacitation,
indicated by the inability to walk in a rotating cage, and
convulsions were observed after 2.8 min at the highest concentration
and after 27 to 34 min at the lowest concentration. These effects
were followed by death after several minutes. Cyanosis of the
extremities and agitation were observed at levels of 22 900 mg/m3
or more (Crane et al., 1986).
The effects of acrolein on the cardiovascular system were
investigated by Egle & Hudgins (1974). Rats anaesthetized by sodium
pentobarbital and exposed only via the mouth and nose to
concentrations between 10 and 5000 mg/m3 for 1 min showed an
increase in blood pressure at all exposure levels. The heart rate
was increased at concentrations from 50 mg/m3 to 500 mg/m3 but
decreased at 2500 and 5000 mg/m3. Intravenous experiments
suggested that increased blood pressor responses resulted from the
release of catecholamines from sympathetic nerve endings and from
the adrenal medulla and that the decreased heart rate effect was
mediated by the vagus nerve (Egle & Hudgins, 1974).
In an acute oral test with rats exposed at 11.2 mg/kg body
weight, decreased reflexes, body sag, poor body tone, lethargy,
stupor, and tremors were observed, as well as respiratory distress
(Sprince et al., 1979).
Because acrolein was shown to induce acute cytotoxicity of the
rat urinary bladder mucosa when instilled directly into the bladder
lumen (Chaviano et al., 1985), this end-point was investigated
in vivo. Two days after a single oral or intraperitoneal dose of
25 mg/kg body weight to ten rats per group, focal simple hyperplasia
of the urinary bladder was detected in the three surviving rats
dosed intraperitoneally. None of the orally exposed rats showed
this effect, but all exhibited severe erosive haemorrhagic
gastritis. Both orally and intraperitoneally exposed rats showed
eosinophilic degeneration of hepatocytes. No abnormalities were
observed in sections of lungs, kidneys, and spleen. Acrolein was
also administered intraperitoneally at single doses of 0.5, 1, 2, 4,
or 6 mg/kg body weight. Proliferation of the bladder mucosa was
evaluated autoradiographically by measuring [3H-methyl]thymidine
incorporation in exposed versus control rats 5 days after the
intraperitoneal injection of acrolein and was found to be increased
nearly two-fold at the highest dose. Body weight gain was decreased
at the two highest doses. Histopathological evaluation of the liver
and urinary bladder did not reveal abnormalities (Sakata et al.,
1989).
7.1.5 Cytotoxicity in vitro
As shown in Table 9, mammalian cell viability is affected by
acrolein in vitro at nominal concentrations of 0.1 mg/litre or
more (not corrected for interaction with culture medium components
or volatilization). The concentration at which formaldehyde
exhibited a similar degree of cytotoxicity was about 6 to 100 times
higher (Holmberg & Malmfors, 1974; Pilotti et al., 1975; Koerker
et al., 1976; Krokan et al., 1985).
Acrolein is a known inhibitor of respiratory tract ciliary
movement in vitro. After a 20-min exposure to an acrolein
concentration of 34-46 mg/m3, the ciliary beating frequency of
excised sheep trachea decreased by 30% (Guillerm et al., 1967a).
Exposure to 13 mg/m3 for 1 h is the greatest exposure that does
not stop ciliary activity in excised rabbit trachea (Dalhamn &
Rosengren, 1971). The no-observed-effect-level for longer exposure
periods would be expected to be lower than 13 mg/m3. Other
in vitro investigations into the inhibition of ciliary movement by
acrolein were reviewed by Izard & Libermann (1978).
7.2 Short-term exposure
7.2.1 Continuous inhalation exposure
In two subchronic inhalation studies with rats, changes in
weight gain, longevity, behaviour, and several physiological
parameters were reported (Gusev et al., 1966; Sinkuvene, 1970).
Unfortunately, the reports did not give sufficient details on the
exposure conditions and protocols and the studies are thus of
limited value in evaluating the toxicological properties of
acrolein.
Table 9. In vitro cytotoxicity of acrolein
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Rat cardiac fibroblasts/myocytes 4 increased lactate Toraason et al. (1989)
dehydrogenase release > 2.8
Rat cardiac myocytes 2 abolished myocine beat > 2.8
dehydrogenase
4 decreased ATP levels > 0.56
Mouse Ehrlich Landschutz 5 92% survivala 1 Holmberg & Malmfors (1974)
Diploid ascites tumour cells 5 53% survivala 5
Mouse B P8 ascites sarcoma cells 48 20% growth rate inhibition 0.6 Pilotti et al. (1975)
48 94% growth rate inhibition 5.6
Mouse C1300 neuroblastoma cells 24 50% survivala 1.7 Koerker et al. (1976)
Mouse L 1210 leukaemia cells 1 70-80% survivala 1.1 Wrabetz et al. (1980)
1 < 15% survivala 2.8
Chinese hamster ovary cells 5 100% mitotis inhibition 0.6 Au et al. (1980)
Adult human bronchial 1 92% colony-forming efficiency 0.06 Krokan et al. (1985)
fibro-blasts 1 45% colony-forming efficiency 0.2
Table 9 (contd).
Cell type Exposure Effect Concentration Reference
period (h) (mg/litre medium)
Adult human lymphocytes 48 decreased replicative index 0.6 Wilmer et al. (1986)
48 100% mitosis inhibition 2.2
Human K562 chromic myeloid 1 marked reduction in > 0.3 Crook et al. (1986a,b)
leukaemia cells colony-forming ability
Human bronchial epithelial cells 1 20% colony-forming efficiency 0.06 Grafström et al. (1988)
1 50% colony-forming efficiency 0.06-0.17
1 50% survivala 0.34
3 clonal growth rate inhibition > 0.17
3 increase in cross-linkage
envelope formation > 0.06
3 decreased plasminogen
activator activity > 0.56
Human fibroblasts 5 63% cell count reduction < 0.017 Curren et al. (1988)
DNA-repair deficient human
fibroblasts 5 63% cell count reduction 0.045
a measured as dye exclusion
Groups of 7 or 8 Sprague-Dawley rats of both sexes, 7 or 8
Princeton or Hartley-derived guinea-pigs of both sexes, 2 male
pure-bred Beagle dogs, and 9 male squirrel-monkeys were exposed to a
vapourized acrolein-ethanol-water mixture for 90 days (Lyon et al.,
1970). The measured acrolein concentrations were 0, 0.5 (two groups
for each species), 2.3, and 4.1 mg/m3 and the ethanol
concentrations were below 18.7 mg/m3. Pathological investigations
did not include weighing of tissues and organs or examination of the
tracheas at the lowest exposure level. There was no
treatment-related mortality. One monkey died at 0.5 mg/m3 and one
at 2.3 mg/m3 due to accidental infections. Body weight gain
reduction was only found in rats at 2.3 and 4.1 mg/m3.
Clinically, ocular discharge and salivation were observed in dogs at
2.3 and 4.1 mg/m3 and in monkeys at 4.1 mg/m3. Monkeys kept
their eyes closed at 2.3 mg/m3. No adverse effects on
haematological or biochemical parameters were observed in any of the
animals. At necropsy, occasional pulmonary haemorrhage and focal
necrosis in the liver were found in three rats at 2.3 mg/m3.
Pulmonary inflammation and occasional focal liver necrosis were also
observed in guinea-pigs at this concentration. Sections of lung
from two of the four dogs exposed at 0.5 mg/m3 revealed focal
vacuolization, hyperaemia, and increased secretion of bronchiolar
epithelial cells, slight bronchoconstriction, and moderate
emphysema. At 2.3 mg/m3, focal inflammatory reactions involved
lung, kidney, and liver. Bronchiolitis and early broncho-pneumonia
were seen in one dog. At 4.1 mg/m3, both dogs had confluent
bronchopneumonia. All nine monkeys at 4.1 mg/m3 showed squamous
metaplasia and six of them showed basal cell hyperplasia in the
trachea. None of the species revealed other treatment-related
changes (Lyon et al., 1970).
Bouley et al. (1975) exposed a total of 173 male SPF-OFA rats
to a measured acrolein vapour concentration of 1.26 mg/m3 for a
period of 15 to 180 days and used control groups of equal size. No
mortality occurred. Sneezing was observed from day 7 to day 21 in
the treated animals, and body weight gain and food consumption were
reduced. There was an increase in relative lung weight in rats
killed on day 77 but not in rats killed on days 15 or 32. The
relative liver weight was decreased at day 15 but not thereafter,
and the number of alveolar macrophages was decreased at days 10 and
26 but not at days 60 or 180. When groups of 16 rats were infected
by one LD50 dose of airborne Salmonella enteriditis on day 18 or
day 63, mortality increased from 53% in controls to 94% in the
exposed rats infected on day 18. No changes were observed in
biochemical parameters, including the amount of liver DNA per mg of
protein in a group of partially hepatectomized rats, or in the
response of spleen lymphocytes to phytohaemagglutinin in rats
exposed for 39 to 57 days. Other end-points were not investigated.
7.2.2 Repeated inhalation exposure
Lyon et al. (1970) exposed groups of rats, guinea-pigs, dogs,
and monkeys to acrolein vapour at concentrations of 0, 1.6. and
8.5 mg/m3 for 8 h per day and 5 days per week over 6 weeks. With
the exception of the exposure levels, period, and frequency, the
protocol was the same as that for the continuous inhalation exposure
described in section 7.2.1. Two deaths occurred among the nine
monkeys at 8.5 mg/m3. There was body weight gain reduction in
rats and body weight loss (not statistically significant) in monkeys
at 8.5 mg/m3. Clinically, eye irritation and salivation were
observed in dogs and monkeys and difficult breathing in dogs at
8.5 mg/m3. No adverse effects on haematological or biochemical
parameters were observed in any of the animals. At necropsy,
sections of lung from all animals exposed to 1.6 mg/m3 showed
chronic inflammatory changes. Additionally, some showed emphysema.
At 8.5 mg/m3, squamous metaplasia and basal cell hyperplasia were
observed in the trachea of both dogs and monkeys. In addition,
bronchopneumonia was noted in dogs and necrotizing bronchitis and
bronchiolitis in monkeys. Focal calcification of the tubular
epithelium was noted in the kidneys of rats and monkeys at
8.5 mg/m3.
Groups of male Sprague-Dawley rats were also exposed to
acrolein vapour at measured concentrations of 0, 0.39, 2.45, and
6.82 mg/m3 for 6 h per day and 5 days per week over 3 weeks (Leach
et al., 1987). Subgroups were used for immunological
investigations (section 7.4) and for histopathological examination
of nasal turbinates and lungs. Body weight gain was depressed from
week 1 up to the end of the exposure period at 6.82 mg/m3.
Absolute, but not relative, spleen weight was reduced at this
exposure level. There were no histological effects on the lungs,
but the respiratory epithelium of the nasal turbinates showed
exfoliation, erosion, and necrosis, as well as dysplasia and
squamous metaplasia at 6.82 mg/m3. In addition, the mucous
membrane covering the septum and lining the floor of the cavity
showed hyperplasia and dysplasia (Leach et al., 1987).
Another experiment involved Dahl rats of two lines, one
susceptible (DS) and one resistant (DR) to salt-induced hypertension
(Kutzman et al., 1984). Groups of 10 female rats of each line were
exposed to measured acrolein concentrations of 0, 0.89, 3.21, and
9.07 mg/m3 for 6 h per day and 5 days per week over 61-63 days.
One week after the exposure, survivors were killed for pathological
and compositional analysis of the lung following behavioural and
clinical chemistry testing. At 9.07 mg/m3, all DS rats died
within 11 days and 4 DR rats died within the exposure period.
Reduced body weights were measured in the surviving DR rats during
the first 3 weeks, followed by an almost normal body weight gain.
Biochemical changes were found in DR rats at 9.07 mg/m3 and
included increases in lung hydroxyproline and elastin, serum
phosphorus, and in the activities of serum alkaline phosphatase,
alanine aminotransferase (EC 2.6.1.2), and aspartate
aminotransferase (EC 2.6.1.1). No effects were observed on
exploratory behaviour, locomotor activity, blood pressure, lung
protein, blood urea nitrogen, or on serum creatinine, uric acid, or
calcium. At necropsy of survivors, DR rats exposed to 9.07 mg/m3
had increased relative weights of several organs, especially the
lungs. It was noted by the authors that the exposed rats gained a
considerable amount of weight during the week following exposure.
In both rat lines, concentration-related increases were observed in
lymphoid aggregates in pulmonary parenchyma, in collections of
intra-alveolar macrophages with foamy cytoplasm, and in
hyperplastic/metaplastic terminal bronchiolar epithelial changes.
Multifocal interstitial pneumonitis and squamous metaplasia of the
tracheal epithelium were also found in DR rats exposed to
9.07 mg/m3. In contrast, dead and moribund rats, especially those
of the DS strain, mainly exhibited severe bronchial and bronchiolar
epithelial necrosis with exfoliation, oedema, haemorrhage, and
varying degrees of bronchopneumonia. Adverse effects were absent in
nasal turbinates and in non-pulmonary tissues (Kutzman et al.,
1984).
In follow-up studies, groups of 32 to 57 male Fischer-344 rats
were exposed in exactly the same way as described for the Dahl rats.
Surviving rats were tested for pulmonary function one week after
exposure and then killed and examined for compositional analysis,
morphometry, and (in nine rats per group) pathological changes in
the lung (Kutzman et al., 1985; Costa et al., 1986). At
9.07 mg/m3, 56% mortality occurred. After an initial body weight
loss over the first 10 days, weight gain became comparable to that
of controls. There was an increase in the relative weight of
several organs, especially the lungs. Lungs also showed an increase
in water content and in the levels of elastin and hydroxyproline,
but not in the levels of protein and DNA. The hydroxyproline level
was also elevated at 3.21 mg/m3. Histologically, surviving rats
treated with 3.21 or 9.07 mg/m3 demonstrated an exposure-related
increase in effects on the respiratory tract consisting of
bronchiolar epithelial necrosis with exfoliation, bronchiolar
mucopurulent plugs, an increase in bronchiolar and alveolar
macrophages, and focal pneumonitis. At 3.21 mg/m3, there was
type II cell hyperplasia and at 9.07 mg/m3 tracheal,
peribronchial, and alveolar oedema and acute rhinitis. The severity
of lung lesions was highly variable and three of the nine rats
examined at 9.07 mg/m3 did not exhibit histological damage.
Moribund rats mainly showed severe acute bronchopneumonia and focal
alveolar and tracheal oedema with exfoliation in the bronchi and
bronchioles (Kutzman et al., 1985). In another report from the
same research group, the results of pulmonary function testing and
morphometry disclosed air-flow dysfunction at 9.07 mg/m3, which
was correlated with the presence of focal peribronchial lesions and
the lung elastin concentrations. In contrast, the rats exposed at
0.89 mg/m3 exhibited enhanced flow-volume dynamics, whereas no
effects on lung function were present in the 3.21-mg/m3 group
(Costa et al., 1986).
Groups of six Wistar rats and ten Syrian golden hamsters of
both sexes were exposed to acrolein vapour at measured
concentrations of 0, 0.9, 3.2, and 11.2 mg/m3 for 6 h per day and
5 days per week over 13 weeks (Feron et al., 1978). Within the
first month of exposure to 11.2 mg/m3, half the number of rats of
each sex died. One hamster died at this exposure level because of
renal failure. A treatment-related decrease in body weight gain and
food intake was observed in rats exposed to 3.2 mg/m3 or more.
Hamsters showed decreased body weight gain at 11.2 mg/m3, but food
intake was not examined. At this exposure level, all animals kept
their eyes closed, rats showed bristling hair, and hamsters showed
salivation and nasal discharge. Haematological investigation and
urinalysis in week 12 showed no changes in rats. In hamsters,
urinalysis revealed no changes, but females showed increases in the
number of erythrocytes, packed cell volume, haemoglobin content, and
number of lymphocytes and a decrease in the number of neutrophilic
leucocytes. Changes in relative organ weights, which were
considered by the authors to be related to the treatment, were found
in the lung, heart, and kidneys of both species and in the adrenals
of rats exposed to 11.2 mg/m3. Histological changes were confined
to the respiratory tract. In the nose, rats exhibited an
exposure-related increase in squamous metaplasia and neutrophilic
infiltration of the mucosa at levels of 0.9 mg/m3 or more (at
0.9 mg/m3 each effect was observed in one male) and occasional
necrotizing rhinitis at 11.2 mg/m3. Hamsters also showed these
effects at 11.2 mg/m3 but only minimal inflammatory changes at
3.2 mg/m3. In the larynx and trachea of rats exposed to
11.2 mg/m3, squamous metaplasia was also observed and was
accompanied by hyperplasia in bronchi and bronchioli. At this
exposure level, the larynx of hamsters was slightly thickened and
focal hyperplasia and metaplasia were found in the trachea.
Inflammatory changes were present in the bronchi, bronchioli, and
alveoli of rats and included haemorrhage, oedema, accumulations of
alveolar macrophages, an increase in mucus-producing cells in the
bronchioli, and bronchopneumonia. The authors noted considerable
variation between individual rats in the degree of the lesions
(Feron et al., 1978).
Feron & Kruysse (1977) exposed groups of 36 Syrian golden
hamsters of both sexes to acrolein vapour at measured levels of 0
and 9.2 mg/m3 for 7 h per day and 5 days per week over 52 weeks.
Except for 6 males and 6 females, the hamsters were observed for a
further 29 weeks after the exposure period. Overall mortality was
38% in exposed hamsters and 33% in controls. Body weight was
slightly and reversibly decreased at the end of the exposure period.
The other effects observed at the end of the exposure period were
essentially similar (but less severe) to those described above for
hamsters exposed to 11.2 mg/m3 for 13 weeks, but hyperplasia was
not observed. Histological changes were restricted to the anterior
half of the nasomaxillary turbinates and were still found in 20% of
the animals at week 81. At that time they mainly consisted of a
thickened submucosa and exudation into the lumen. Epithelial
metaplasia, but not hyperplasia, was noted. No tumours were found.
Histopathological examination of the respiratory tract of male
Swiss-Webster mice was the object of a study involving groups of 16
to 24 male mice exposed to measured concentrations of 3.9 mg/m3
for 6 h per day during 5 days (Buckley et al., 1984). The lesions
observed were restricted to the nose and were most severe in the
anterior respiratory epithelium and on the free margins of the
nasomaxillary turbinates and the adjacent nasal septum. They
consisted of severe deciliation, moderate exfoliation, erosion,
ulceration and necrosis, severe squamous metaplasia, moderate
neutrophilic infiltration, and a slight serofibrinous exudate.
Lesions in the olfactory epithelium were largely confined to the
dorsal meatus and consisted of moderate ulceration and necrosis, and
slight squamous metaplasia. The nasal squamous epithelium was not
affected (Buckley et al., 1984).
One special investigation concerned the effects of acrolein
vapour on the respiratory functions of male Swiss mice exposed to
100 mg/m3 for two daily periods of 30 min each for 5 weeks. Body
weights were not affected. There was a decrease in pulmonary
compliance, but no effects were found on pulmonary resistance,
respiratory volume, or functional residual capacity. The lungs
showed an increase in phospholipid content (Watanabe & Aviado,1974).
In summary, the toxicological effects on a variety of
laboratory animals from repeated inhalation exposure to acrolein
vapour at concentrations ranging from 0.39 mg/m3 to 11.2 mg/m3
have been studied. Exposure durations ranged from 5 days to as long
as 52 weeks. In general, body weight gain reduction, decrement of
pulmonary function, and pathological changes in nose, upper airways,
and lungs have been documented in most species exposed to acrolein
concentrations of 1.6 mg/m3 or more. Pathological changes include
inflammation, metaplasia, and hyperplasia of the respiratory tract.
Significant mortality has been observed following repeated exposures
to acrolein vapour at concentrations above 9.07 mg/m3.
7.2.3 Repeated intraperitoneal exposure
Groups of ten intact or adrenalectomized NMRI mice were
injected intraperitoneally with saline or acrolein in water at daily
doses of 4 to 16 mg/kg body weight for 1 to 6 days. One week after
the last injection the mice were killed for autopsy.
Clinical signs of toxicity were hunched posture, inactivity,
and ruffled fur. Total body weight and relative thymus and spleen
weights showed a dose-related reduction, while the adrenals showed
an increase in relative weight. Histologically, thymic necrosis and
splenic atrophy were the only changes observed. These changes were
absent in controls and in adrenalectomized mice. The levels of
reduced glutathione and the activity of glutathione S-transferase
in liver cytosol were unchanged, but the rate of glutathione
synthesis was increased. Repeated exposure to acrolein caused a
progressively less pronounced effect on mortality (Warholm et al.,
1984).
7.3 Biochemical effects and mechanisms of toxicity
7.3.1 Protein and non-protein sulfhydryl depletion
A dose-related non-protein sulfhydryl (reduced glutathione)
depletion was observed in the nasal respiratory mucosa of male
Fischer rats after nose-only exposure for 3 h to acrolein vapour at
concentrations of 0.23-11.4 mg/m3 (McNulty et al., 1984; Lam
et al., 1985). Depletion of glutathione in the liver was not
observed at these exposure levels (McNulty et al., 1984). The
glutathione depletion in the nasal mucosa appeared irreversible at
11.4 mg/m3 (McNulty et al., 1984). In female C3Hf/HeHa mice,
intraperitoneally exposed once to doses between 20 and 80 mg/kg body
weight and killed 2 h later, a dose-related decrease in liver
glutathione levels was observed. These doses are however extremely
high considering the fact that a dose of 4.5 mg/kg body weight was
lethal within 1.7 h (Gurtoo et al., 1981a).
A dose-related in vitro glutathione depletion has been
observed in human bronchial fibroblasts (Krokan et al., 1985),
human bronchial epithelial cells (Grafström et al., 1988), human
chronic myeloid leukemia cells (Crook et al., 1986b), and human
and rat phagocytic cells (Witz et al., 1987) from the lowest
acrolein concentration tested (56 µg/litre). The effect has also
been reported to occur in isolated rat hepatocytes (Zitting &
Heinonen, 1980; Dawson et al., 1984; Dore & Montaldo, 1984; Ku &
Billings, 1986) and in rat liver or lung microsomal suspensions
(Patel et al., 1984), the lowest-observed-effect level being
1400 µg/litre (Dawson et al., 1984). Ku & Billings (1986)
observed that both mitochondrial and cytosolic glutathione levels
were decreased.
As a result of acrolein exposure, there was a decrease in the
level of both membrane surface and soluble protein sulfhydryl groups
in in vitro human and rat phagocytic cells (Witz et al., 1987)
and a decrease in the level of soluble protein sulfhydryl compounds
in human bronchial epithelial cells (Grafström et al., 1988).
Acrolein has also been shown to cause a decrease in membrane surface
protein sulfhydryl groups in rat hepatocytes (Ku & Billings, 1986)
and to reduce the protein sulfhydryl content of liver and lung
microsomal preparations (Patel et al., 1984).
7.3.2 Inhibition of macromolecular synthesis
When partially hepatectomized Wistar rats were exposed
intraperitoneally to a single acrolein dose of 0.5, 1.6, 2.0, or
2.7 mg/kg body weight, a dose-related inhibition of the synthesis of
DNA and RNA was measured in liver and lung cells (Munsch &
Frayssinet, 1971).
Inhibition of DNA, RNA, and/or protein synthesis has been
observed in Escherichia coli (Kimes & Morris, 1971), the slime
mold Physarum polycephalum (Leuchtenberger et al., 1968), the
alga Dunaliella bioculata (Marano & Puiseux-Dao, 1982), and in
in vitro mammalian cells such as mouse kidney cells (Leuchtenberger
et al., 1968) and polyoma transformed Chinese hamster cells
(Alarcon, 1972). Acrolein was shown to inhibit RNA polymerase in
isolated rat liver nuclei (Moule & Frayssinet, 1971) and isolated
rat liver DNA polymerase (Munsch et al., 1973). The activity of
the latter enzyme is associated with at least one functional
sulfhydryl group, and preincubation of the enzyme with
2-mercaptoethanol protected against the inhibitory action of
acrolein. Since acrolein did not inhibit isolated Escherichia coli
polymerase I, devoid of sulfhydryl groups in its active centre,
Munsch et al. (1973) suggested that the inhibitory action of
acrolein is caused by a reaction with sulfhydryl groups.
7.3.3 Effects on microsomal oxidation
In in vitro studies, acrolein has been shown to convert rat
liver cytochrome P-450 to cytochrome P-420 and to inhibit rat liver
NADPH-cytochrome-c reductase (EC 1.6.2.4) in a time- and
concentration-related fashion (Marinello et al., 1978; Ivanetich
et al., 1978; Berrigan et al., 1980; Gurtoo et al., 1981b;
Marinello et al., 1981; Patel et al., 1984; Cooper et al.,
1987). A concomitant decrease occurred in the activity of several
monooxygenases: benzphetamine N-demethylase, aniline hydroxylase,
ethylmorphine N-demethylase (Patel et al., 1984), and
7-ethoxyresorufin O-deethylase (Cooper et al., 1987). The
lowest-observed-effect levels reported were 2 mg/litre for
inactivation of cytochrome P-450 (Gurtoo et al., 1981b) and
25 mg/litre for inhibition of NADPH-cytochrome- c reductase
(Marinello et al.,1981). It was also shown that the addition of
sulfhydryl-containing agents, such as cysteine, acetylcysteine,
glutathione, dithiothreitol, and semicarbazide, reduced the above
effects, suggesting that acrolein produces them by reacting with
sulfhydryl groups at the active sites.
7.3.4 Other biochemical effects
In vivo studies with Holtzman rats have shown that rat liver
alkaline phosphatase (EC 3.1.3.1) and tyrosine aminotransferase
(EC 2.6.1.5) activities are increased markedly after inhalation of
acrolein for 4 h at a concentration of 14.7 mg/m3 or after a
single intraperitoneal injection of acrolein in water at doses of
1.5-6 mg/kg body weight (Murphy et al., 1964; Murphy, 1965). The
increase in alkaline phosphatase activity following intraperitoneal
injection was shown to be dose related (Murphy, 1965). The effects
were reduced by prior adrenalectomy or hypophysectomy or by
pretreatment with protein synthesis inhibitors such as actinomycin
D, puromycin, and ethionine, suggesting that the irritant action of
acrolein stimulates the pituitary-adrenal system to release
glucocorticoids, which act to increase the synthesis of adaptive
liver enzymes (Murphy, 1965; Murphy & Porter, 1966). Increased
plasma and adrenal levels of corticosterone were measured in
Holtzman rats one hour after a single intraperitoneal injection
(3 mg/kg body weight) of acrolein in water (Szot & Murphy, 1971).
The hypersecretion of glucocorticoids could also explain the
observed increase in liver glycogen level following intraperitoneal
exposure to acrolein at a dose of 1.5 mg/kg body weight (Murphy &
Porter, 1966).
At a concentration of 5.6 mg/litre, acrolein produced an 80%
inhibition of the noradrenaline-induced oxygen consumption of
isolated hamster brown fat cells (Pettersson et al., 1980). In
addition, Zollner (1973) observed an acrolein-induced inhibition of
the respiration of intact rat liver mitochondria and found evidence
for an inhibition at three different sites: glutamate transport,
inorganic phosphorus transport, and the enzyme succinic
dehydrogenase (EC 1.3.5.1).
Several sulfhydryl-sensitive enzymes have been shown to be
inhibited by acrolein in vitro, e.g., rabbit muscle L-lactate
dehydrogenase (EC 1.1.1.27), yeast glucose-6-phosphate
dehydro-genase (EC 1.1.1.49), and yeast alcohol dehydrogenase
(EC 1.1.1.1) (Benedict & Stedman, 1969), porcine lung
15-hydroxyprosta-glandin dehydrogenase (EC 1.1.1.141) (Liu & Tai,
1985), rat liver or urothelium S-adenosyl-L-methionine-
DNA(cytosine-5)- methyltransferase (EC 2.1.1.37) (Cox et al.,
1988), and O6-methylguanine-DNA methyltransferase (EC 2.1.1.63)
in cultured human bronchial fibroblasts (Krokan et al, 1985). In
two of these studies glutathione was shown to afford protection
against inhibition of the enzyme (Liu & Tai, 1985; Cox et al.,
1988).
It has been suggested that the formation of a Schiff base
between acrolein and sensitive amine groups is responsible for the
observed inhibition in vitro of Salmonella typhimurium
deoxyribose-5-phosphate aldolase (EC 4.1.2.4) at a concentration of
approximately 14 mg/litre (Wilton, 1976) and human plasma
alpha1-proteinase inhibitor (Gan & Ansari, 1987).
Acrolein was shown to cause a concentration-dependent increase
in lipid peroxidation in isolated rat hepatocytes at levels that
also decreased glutathione concentrations (Zitting & Heinonen,
1980). Preincubation of washed rat liver microsomes with acrolein
abolished the protective effect of glutathione against
iron/ascorbate-induced lipid peroxidation (Haenen et al, 1988). The
authors claimed that the protective effect of glutathione was
mediated by vitamin E scavenging membrane lipid radicals. It was
suggested that acrolein was inhibiting a glutathione-dependent
reductase enzyme responsible for reducing vitamin E radicals back to
vitamin E.
7.4 Immunotoxicity and host resistance
Acrolein has been found to depress pulmonary host defenses in a
number of tests.
In female Swiss mice, exposed to measured concentrations of
1.1, 6.9, and 14.2 mg/m3 for 8 h, a concentration-related increase
in the survival of Staphylococcus aureus was seen at levels of
6.9 mg/m3 or more (Astry & Jakab, 1983). A concentration- and
time-related increase in the survival of S. aureus and Proteus
mirabilis was found in male Swiss CD-1 mice exposed to measured
concentrations of 2.3 to 4.6 mg/m3 for 24 h. When the mice were
also infected with Sendai virus, intrapulmonary bacterial death was
further suppressed (Jakab, 1977). An increased survival of
Klebsiella pneumoniae, but no increased mortality from pneumonia
following challenges with Streptococcus zooepidemicus, was
observed in female CD-1 mice after exposure to acrolein at a
measured concentration of 0.23 mg/m3 for 3 h per day over 5 days
(Aranyi et al., 1986). In female CR/CD-1 mice, exposure to a
measured acrolein concentration of 4.6 mg/m3, for one period of
6 h or for 7 consecutive daily periods of 8 h, resulted in an
increased mortality from Streptococcus pyogenes and Salmonella
typhimurium, respectively, but not from influenza A virus (Campbell
et al., 1981).
Sherwood et al. (1986) exposed groups of 33 male
Sprague-Dawley rats to acrolein vapour at analysed concentrations of
0.39, 2.45 or 6.82 mg/m3 for 3 weeks (6 h per day and 5 days per
week). The relative pulmonary bactericidal activity to
K. pneumoniae was not affected nor was the number of alveolar
cells. However, the number of peritoneal macrophages was decreased
at concentrations of 2.45 mg/m3 or more, and alveolar and
peritoneal macrophages had altered phagocytic and enzymic patterns
at > 0.39 mg/m3.
When SPF-OFA rats were exposed continuously to a measured
acrolein concentration of 1.26 mg/m3 for 18 days, they exhibited
an increased mortality from an infection by Salmonella enteritidis.
However, no such effect was observed following 63 days of exposure
(Bouley et al., 1975).
Acrolein has been shown to inhibit in vitro protein synthesis
(Leffingwell & Low, 1979), and phagocytosis and ATPase (EC
3.6.1.37-38) activity (Low et al., 1977) in rabbit pulmonary
alveolar macrophages. Inhibition of a graft-versus-host reaction in
rats was found after Wistar rat spleen cells were incubated
in vitro with acrolein and injected into all four feet of hybrid
F1 rats. In addition, a decreased mitogen response of human
peripheral lymphocytes was recorded (Whitehouse et al., 1974).
Acrolein was also found to inhibit in vitro chemotaxis of human
polymorphonuclear leucocytes (Bridges et al., 1977).
7.5 Reproductive toxicity, embryotoxicity, and teratogenicity
Two in vivo exposure studies have been reported. In one, 3
male and 21 female SPF-OFA rats were exposed continuously to
acrolein vapour at a measured concentration of 1.26 mg/m3 for 25
days and allowed to mate on day 4. It should be noted that the
exposure period did not cover the complete spermatogenic period of
60 days. The number of pregnant animals and the number and mean
weight of the fetuses were unaffected in comparison to the control
rats (Bouley et al., 1985). In the second study, groups of 12 to
16 New Zealand rabbits were injected (into the ear vein) with a
solution of acrolein in saline (3, 4.5 or 6 mg/kg body weight) on
the 9th day of gestation. At 4.5 and 6 mg/kg body weight, maternal
toxicity was indicated by the death of 3 and 6 dams, respectively,
and embryotoxicity by a dose-related increase in resorptions which
was significant at 6 mg/kg body weight. It was also reported that
the number of malformed and retarded fetuses increased in a
dose-related manner, although the increases were statistically
non-significant. No effects on maternal toxicity, embryotoxicity or
fetuses were noted at 3 mg/kg body weight (Claussen et al., 1980).
A clear effect on the development of the embryo in vivo was
observed only when acrolein was administered close to the target
site by intra-amniotic injection. Using groups of 12 to 19 pregnant
New Zealand rabbits, 0, 10, 20, or 40 µl of a 0.84% solution of
acrolein in saline was injected into the amnion of all embryos in
one of the uterine horns on the 9th day of gestation. The embryos in
the other uterine horn received saline only and served as controls.
The dams were killed on day 28 of gestation. There was a
dose-related increase in the rate of resorptions and malformations,
significant at doses of 20 µl or more per embryo. Malformations
included deformed and asymmetric vertebrae, spina bifida, deformed
and fused ribs, and lack or fusion of sternum segments. No effect
was observed on the number of implantations and fetuses or on fetal
growth (Claussen et al., 1980). A similar study was carried out
on pregnant Sprague-Dawley rats injected with acrolein doses of 0,
0.1, 1.0, 2.5, 5.0, 10.0 or 100 µg per fetus in 10 ml of saline on
the 13th day of pregnancy. The dams were killed on day 20 of
gestation. A dose-related increase in the percentage of dead and
resorbed fetuses per litter was observed at all dose levels. The
total number of litters at each dose level varied from 4 to 18. The
percentage of malformed fetuses per litter also was increased in a
dose-related manner at doses of up to 5 µg per fetus. The increase
was significant only up to this dose level, probably because at
higher doses there were few surviving fetuses. Treatment-related
effects included oedema, micrognathia, hindlimb and forelimb
defects, and hydrocephaly (Slott & Hales, 1985). These results
confirmed the findings of an earlier, identical test using dose
levels of 0.1, 10, and 100 µg per fetus (Hales, 1982).
Acrolein was also shown to be embryotoxic and teratogenic in
the rat whole embryo culture system. As with embryos exposed in
vivo, the concentration range for teratogenicity was very narrow
(Slott & Hales, 1986). Schmid et al. (1981) and Mirkes et al.
(1984) observed embryotoxicity but no teratogenicity in the same
test system, this being probably the result of the different test
conditions used (Slott & Hales, 1986). Depletion of glutathione by
buthionine sulfoximine enhanced the embryotoxicity and
teratogenicity of acrolein in the in vitro studies of Slott &
Hales (1987a), whereas exogenous glutathione afforded protection
against these effects (Slott & Hales, 1987b).
In a mouse limb bud culture system, acrolein induced impairment
of limb bud differentiation, indicative of a teratogenic action
(Stahlmann et al., 1985). When acrolein was injected into chicken
eggs, embryotoxic and teratogenic effects were observed (Kankaanpaa
et al., 1979; Korhonen et al., 1983; Chhibber & Gilani, 1986).
In summary, acrolein can induce teratogenic and embryotoxic
effects if administered directly to the embryos or fetuses.
However, the fact that no effect was found in rabbits injected
intravenously with 3 mg/kg suggests that neither skin contact nor
inhalation of acrolein is likely to affect the developing embryo.
7.6 Mutagenicity and related end-points
7.6.1 DNA damage
In vitro studies have revealed interactions between acrolein
and DNA and RNA (Munsch et al., 1974b; section 6.2.1). Acrolein
has also been found to react with purine and pyrimidine bases or
intact DNA in vitro, and several adducts have been identified
(Descroix, 1972; Hemminki et al., 1980; Lutz et al., 1982; Chung
et al., 1984; Shapiro et al., 1986; section 6.2.2.2). Cyclic
deoxyguanosine DNA adducts were formed in a dose-dependent fashion
in acrolein-exposed Salmonella typhimurium TA100 and TA104. This
adduct formation correlated with the induction of reverse mutations
in these strains (section 7.6.2; Foiles et al., 1989).
No data on the formation of DNA adducts following exposure of
animals to acrolein are available.
Incubation of Fischer-344 rat nasal mucosal homogenate with
acrolein resulted in a concentration-dependent increase in
DNA-protein cross-linking, which was not observed following
inhalation exposure of rats to acrolein at a concentration of
4.6 mg/m3 for 6 h (Lam et al., 1985). According to the authors
this could be explained by the preferential reaction of acrolein
with sulfhydryl groups. DNA-protein cross-linking and single strand
breaks were observed in vitro in human bronchial fibroblasts at
cytotoxic concentrations of 1.7 mg/litre or more (Grafström et al.,
1986, 1988), and there was indirect evidence for some formation of
DNA interstrand cross-linking (Grafström et al., 1988). No
DNA-protein or DNA interstrand cross-linking was induced by acrolein
in mouse L1210 leukemia cells at cytotoxic levels that produced
single strand breaks and/or alkali-labile sites in these cells
(Erickson et al., 1980) or in human chronic myeloid leukemia cells
(Crook et al., 1986a). In non-mammalian assays, Fleer & Brendel
(1982) did not find DNA interstrand cross-linking or single strand
breaks in MB1072-2B yeast cells and Kubinski et al. (1981)
observed DNA-cell binding in Escherichia coli in the presence of a
rat liver S9 fraction. These studies demonstrate that effects on
DNA occur only at cytotoxic concentrations of acrolein.
Results of DNA repair tests are not available. Acrolein has
been demonstrated to inhibit O6-methylguanine-DNA
methyltransferase (EC 2.1.1.63.; section 7.3.4) and, therefore, can
be expected to reduce the capacity for repair of O6-guanine
alkylations in DNA (Krokan et al., 1985).
7.6.2 Mutation and chromosomal effects
The results of tests for the induction of gene mutations and
chromosome damage by acrolein are summarized in Table 10.
In point mutation assays with Salmonella typhimurium, the
positive or equivocal responses obtained were all observed within a
narrow dose range of up to 10-56 µg per plate, higher doses being
toxic. Clearly positive, dose-related increases in revertant
colonies per plate at 2-5 times the background rate were observed in
the absence of metabolic activation only in TA100 (Lutz et al.,
1982; Foiles et al., 1989; Hoffman et al., 1989), TA104 (Marnett
et al., 1985; Foiles et al., 1989; Hoffman et al., 1989), and
TA98 (Lijinsky & Andrews, 1980). Khudoley et al. (1986) reported
positive results in strains TA98 and TA100 without specifying dose
levels or revertant rates. Some evidence for indirect mutagenicity
was found in strains TA1535 (Hales, 1982) and TA100 (Haworth
et al., 1983), the slight increase in TA100 revertants being dose
related. However, negative results, both with and without metabolic
activation, have also been obtained in these strains. Some of these
negative results were clearly related to the incubation conditions,
which were probably highly toxic, e.g., those obtained in spot tests
(Andersen et al., 1972; Florin et al., 1980). In Salmonella
typhimurium TA100 and TA104, strains that show a clear mutagenic
response to acrolein, DNA-acrolein adducts have also been identified
(section 7.6.1).
Acrolein did not induce sex-linked recessive lethality in
Drosophila melanogaster adults (Zimmering et al., 1985), but
induced a 12-fold increase in sex-linked recessive lethality in
hatching eggs and larva at an exposure level that was not reported
but caused over 75% larval death (Rapoport, 1948). In the latter
test, treatment of adults was reported to be less effective.
Three cytogenetic tests have been carried out with acrolein,
two in Chinese hamster ovary cells (Au et al., 1980; Galloway
et al., 1987) and one in human lymphocytes (Wilmer et al.,
1986). Acrolein was shown to induce sister chromatid exchanges in
the absence of a metabolic activating system in all three studies.
The lowest effective concentration was 56 µg/litre (Galloway
et al., 1987). No increase in chromosome aberrations was reported
in one study (Galloway et al., 1987), while chromosome breakage
was reported in another study at cytotoxic concentrations (Au
et al., 1980).
Three properties of acrolein make it difficult to test for
mutagenicity: its high cytotoxicity, which prevents the expression
of any mutagenic activity, and its high reactivity and volatility,
which prevent it reaching the target sites. However, acrolein can
be considered to be a weak mutagen in some bacterial and fungal test
systems in the absence of metabolic activating systems and to
induce sister chromatid exchange in cultured mammalian cells.
7.6.3 Cell transformation
Acrolein (0.4 µg/ml) has been found not to exhibit transforming
potential in C3H/10T1/2 cells but to initiate the process of
transformation. The latter was measured by exposing cultures to
acrolein for 24 h and, subsequently, to a phorbol ester for 6 weeks
(Abernethy et al., 1983).
Table 10. Tests for gene mutation and chromosomal damage by acrolein
Test description Organism Species/strain/cell type Resulta Reference
Gene mutations
Reverse mutations bacteria Salmonella typhimurium TA1535 ±(+S9) Hales (1982)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Lijinsky & Andrews (1980)
Salmonella typhimurium TA100 +(-S9) Lutz et al. (1982); Khudoley et al., 1986;
Foiles et al. (1989); Hoffman et al. (1988)
±(+S9) Haworth et al. (1983)
- Florin et al. (1980); Loquet et al. (1981);
Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium TA104 +(-S9) Marnett et al. (1985); Foiles et al. (1989);
Hoffman et al. (1989)
Salmonella typhimurium TA102 - Marnett et al. (1985)
Salmonella typhimurium TA98 +(-S9) Lijinsky & Andrews (1980); Khudoley et al. (1986)
- Florin et al. (1980); Loquet et al. (1981);
Haworth et al. (1983); Basu & Marnett (1984)
Salmonella typhimurium TA1537 - Florin et al. (1980); Haworth et al. (1983);
Lijinsky & Andrews (1980)
Salmonella typhimurium TA1538 - Basu & Marnett (1984); Lijinsky & Andrews (1980)
Salmonella typhimurium, 8 strains - Andersen et al. (1972)
Escherichia coli 343/113 - Ellenberger & Mohn (1976, 1977)b
Escherichia coli WP2 uvrA ±(-S9) Hemminki et al. (1980)
yeast Saccharomyces cerevisiae S211, S138 - Izard (1973)
Forward mutations yeast Saccharomyces cerevisiae N123 +(-S9) Izard (1973)c
Table 10 (contd).
Test description Organism Species/strain/cell type Resulta Reference
Forward mutations man normal fibroblasts -(-S9) Curren et al. (1988)
DNA-repair-deficient fibroblasts +(-S9) Curren et al. (1988)
hamster V79 cells +(-S9) Smith et al. (1990)
Sex-linked lethal insect Drosophila melanogaster, hatching + Rapoport (1948)d
mutations eggs and young larva
Drosophila melanogaster, adults - Zimmering et al. (1985)e
Chromosomal damage
Aberrations hamster ovary cells in vitro ±(+S9) Au et al. (1980)
±(-S9) Galloway et al. (1987)
Sister chromatid hamster ovary cells in vitro +(-S9) Au et al. (1980)
exchanges +(-S9) Galloway et al. (1987)
man lymphocytes in vitro +(-S9) Wilmer et al. (1986)
Dominant lethal mouse germ cells - Epstein & Shafner (1968)
mutations
(ip exposure)
a + = >2 x background rate or statistically significant (P < 0.05); ± = equivocal; - = negative.
b Details for this test were not reported.
c Plate test for petite mutations (production of a respiratory-deficient mutant).
d Doses were not reported. Treatment of adults was found to be less effective.
e Exposure via feeding solution or via injection.
7.7 Carcinogenicity
7.7.1 Inhalation exposure
Inhalation experiments of appropriate duration specifically
designed to assess the carcinogenicity of acrolein vapour have not
been conducted.
An 81-week study (52 weeks of acrolein exposure at 9.2 mg/m3
followed by 29 weeks without exposure) on groups of 36 Syrian golden
hamsters of both sexes is described in section 7.2.2. The effects
of treatment included a persistent and statistically significant
reduction in body weight in females, an increased relative brain
weight in males and females at 52 weeks, and an increased relative
lung weight in females at 52 weeks. Apart from one small tracheal
papilloma in an acrolein-exposed female, no respiratory tract
tumours were observed in control or treated hamsters (Feron &
Kruysse, 1977). In order to elucidate a possible co-carcinogenic
action of acrolein, Feron & Kruysse (1977) also exposed groups of 30
Syrian golden hamsters of both sexes to measured acrolein vapour
concentrations of 0 or 9.2 mg/m3, 7 h per day and 5 days per week
for 52 weeks, and, for the same period, either weekly to an
intratracheal dose of benzo [a]pyrene or once every 3 weeks to a
subcutaneous dose of diethylnitrosamine. Total dose levels were 18.2
or 36.4 mg benzo [a]pyrene and 2.1 µl diethylnitrosamine. Survivors
were killed at week 81, and all hamsters were subjected to
postmortem examination. The mortality rate in the groups treated
with benzo [a]pyrene was slightly higher than in other groups. The
incidence of benzo [a]pyrene-induced respiratory tract tumours was
slightly (but statistically insignificantly) higher in females also
exposed to acrolein vapour. In these females, at the higher dose
level of benzo [a]pyrene, respiratory tract tumours occurred
earlier and the number of malignant tumours was slightly increased.
Taken together, these observations might suggest an enhancing effect
of acrolein on benzo [a]pyrene carcinogenesis in the respiratory
tract, but the effect cannot be considered proven.
In a study by Le Bouffant et al. (1980), rats, 20 animals per
group, were exposed to 18.3 mg/m3, 1 h/day and 5 days/week, for 10
or 18 months. No tumours or metaplasias were found.
7.7.2 Oral exposure
In a study by Lijinsky & Reuber (1987), groups of 20
Fischer-344 rats of both sexes were exposed to weekly prepared
acrolein of unspecified purity in drinking-water. Each cage of four
rats received 80 ml of acrolein solutions at concentrations of 100
or 250 mg/litre for 124 weeks (males only) or 625 mg/litre for 104
weeks (both sexes) for 5 days per week (this was estimated by the
Task Group to be equivalent to approximately 5, 12.5, and 50 mg/kg
body weight per day, respectively). Total doses were 1200, 3100,
and 6500 mg per rat, respectively. Controls were left untreated.
Survivors were killed at week 123-132 and all rats were subjected to
postmortem examination. The mean survival time was about 120 weeks
for experimental and control groups. There was a marginal, but not
statistically significant, increase in the incidence of adrenal
cortical adenomas (5/20) in female rats at 625 mg/litre, compared to
concurrent controls (1/20), and a decrease in the incidence of
pituitary neoplasms in both sexes at 625 mg/litre. In addition, 2
of 20 females given 625 mg/litre had hyperplastic nodules of the
adrenal cortex. The authors cited historic control values for
adrenal cortical adenomas or carcinomas in female Fischer-344 rats
from other laboratories as 1.3% at 26 months of age and 4.8% in a
lifespan study. Because of limited numbers of animals used and
concerns regarding the purity and stability of acrolein in the dosed
drinking-water, the authors of this study did not consider it to be
a definitive carcinogenicity bioassay. In addition, the Task Group
considered the historical control values quoted by the authors to be
of limited use in evaluating the importance of the tumour incidence
found in this study.
Acrolein appeared to be too toxic to Syrian golden hamsters
following oral exposure by gavage in corn oil to conduct an
effective carcinogenicity study (Lijinsky & Reuber, 1987).
7.7.3 Skin exposure
In a study by Salaman & Roe (1956), a group of 15 S strain mice
of unspecified sex and age received weekly doses of 0.5% acrolein in
acetone for 10 weeks. The total dose was 12.6 mg per rat, although
the purity of the acrolein was not reported. The control group
comprised 20 mice. From day 25 after the first acrolein treatment,
the mice received once per week a skin application of 0.17% croton
oil (0.085% in weeks 2 and 3) for 18 weeks. Croton oil and acrolein
were applied alternately at 3 or 4 day intervals. At the end of
treatment, the mortality rate and the incidence of skin papillomata
were similar to those of the controls treated only with croton oil.
However, this study must be considered inadequate because of the
limited number of animals used and the short duration of the
experiment.
7.8 Interacting agents
Free sulfhydryl-containing compounds have been found to give
protection against the adverse effects of acrolein in vitro, e.g.,
the inhibition of enzymes involved in macromolecular synthesis
(Munsch et al., 1973), liver microsomal cytochrome P-450s
(Marinello et al., 1978; Berrigan et al., 1980; Gurtoo et al.,
1981b, Patel et al., 1984; Cooper et al., 1987), and several
other sulfhydryl-sensitive enzymes (Liu & Tai, 1985; Cox et al.,
1988), the adverse effects on rabbit alveolar macrophages (Low et
al., 1977; Leffingwell & Low, 1979), and the impairment of mouse
limb bud differentiation (Stahlmann et al., 1985). Free
sulfhydryl-containing agents protected against the acute lethal
effects of acrolein in Charles River rats (Sprince et al., 1979)
and in DBA/2J mice (Gurtoo et al., 1981a).
When Swiss-Webster mice were exposed to acrolein-formaldehyde
mixtures, the percentage decrease in respiratory rate was found to
be less than the sum of the percentage decreases due to each
compound alone (Kane & Alarie,1978). In acrolein-exposed Fischer-344
rats, pretreatment with formaldehyde resulted in a lower percentage
decrease in respiratory rate compared to non-pretreated rats (Babiuk
et al., 1985). It was suggested in both investigations that
acrolein and formaldehyde competed for the same receptor
(competitive agonism). In a comparable experiment, the maximum
percentage decrease in the respiratory rate of Swiss-Webster mice
exposed to a mixture of acrolein and sulfur dioxide was lower than
that of acrolein alone. This antagonistic effect was thought to be
caused by a chemical reaction in the air phase between the two
compounds, which reduced the effective concentrations (Kane &
Alarie, 1979).
In Fischer-344 rats exposed to formaldehyde vapour
(7.4 mg/m3) once for 6 h, co-exposure to acrolein vapour
(4.6 mg/m3) resulted in a higher increase in DNA-protein
cross-linking than was observed with formaldehyde alone. Acrolein
alone did not increase DNA-protein cross-linking in this experiment
(Lam et al., 1985).
In a study by Hales et al. (1988), anaesthetized,
artificially ventilated mongrel dogs were exposed to acrolein or
hydrochloric acid with added synthetic smoke composed of carbon
particles for 10 min. The dogs were exposed to smoke with or
without analytically determined acrolein concentrations of <
458 mg/m3, 458-687 mg/m3 or > 687 mg/m3. Smoke with
acrolein, but not smoke with hydrochloric acid, produced
non-cardiogenic, peribronchiolar pulmonary oedema in a
concentration- and time-related fashion. Both acrolein and
hydrochloric acid produced airway damage consisting of mucosal
degeneration and desquamation and inflammatory cell infiltration.
Acrolein at levels above 458 mg/m3 also caused fibrin deposition
in the alveolar spaces that juxtaposed injured bronchioles.
8. EFFECTS ON HUMANS
8.1 Single exposure
8.1.1 Poisoning incidents
One man was exposed dermally and by inhalation when acrolein
was sprayed into his face following an accident in a chemical plant.
Immediately, his face and eyelids were burnt. Within 20 h he was
hospitalized with fever, cough, frothy sputum, cyanosis, and acute
respiratory failure. Two months after the accident, the opening of
the right bronchus was obstructed and the upper trachea showed
slight oedema with haemorrhagic spots. At 18 months he had developed
chronic bronchitis and emphysema, which might have been a sequel of
the accidental exposure (Champeix et al., 1966).
One case of attempted oral suicidal intoxication has been
reported. The man swallowed approximately 1.5 g of acrolein in a
glass of orange juice. Blood was found in his stomach and the number
of red and white blood cells was increased. Gastroscopic
examination showed shrinkage of the stomach and a massive chronic
gastritis with erosions and ulceration. Further examination of the
stomach revealed regenerating mucous membranes, few mucous glands,
granulation and scarring of the serosa, shrinkage and stenosis of
the pylorus, lymphadenitis, and haemosiderin deposition in lymph
nodes. The man was successfully treated by gastrectomy (Schielke,
1987).
Two cases of suspected exposure to acrolein have been reported.
The death of two young boys who inhaled smoke from an overheated
frier for approximately 2 h was thought to be related to acrolein
exposure, although other chemicals might also have been involved.
One of the boys was found dead, while the other suffered from acute
respiratory failure. Following oxygen therapy, the second boy died
due to asphyxia. At autopsy a massive cellular desquamation of the
bronchial lining was observed. The tracheal and bronchial lumina
were filled with debris and the lungs showed multiple infarcts
(Gosselin et al., 1979).
Four female factory workers operating a machine for cutting and
sealing polyethylene bags and a fifth sitting next to the machine
complained of a burning sensation in the eyes, a feeling of dryness
and irritation in the nose and throat, and itching and irritation of
the skin of the face, neck and forearms. These complaints were
related to the smoke developed. The presence of formaldehyde and
"acrolein and/or other aldehydes" in the smoke was suspected and
confirmed. During heavy smoke exposure, itching eruptions developed
on exposed skin. Drowsiness and headache was also experienced. All
symptoms were reversible (Hovding, 1969).
8.1.2 Controlled experiments
8.1.2.1 Vapour exposure
Several studies with volunteers have been conducted with the
object of establing thresholds for odour perception and recognition
and for effects on the eyes, nose, respiratory tract, and nervous
system. The results of these studies are summarized in Table 11. The
exposure period was up to 60 min. In most cases the concentration
of acrolein was determined colorimetrically, although a few reports
did not include a description of the analytical method (Plotnikova,
1957; Sinkuvene, 1970; Harada, 1977). Sinkuvene (1970) reported the
threshold for changes in the electrical activity of the brain
cortex, as measured by electro-encephalography, to be 0.05 mg/m3.
However, this result cannot be evaluated since experimental data
were not provided. The odour perception threshold for sensitive
people was 0.07 mg/m3.
In studies by Weber-Tschopp et al. (1977), groups of human
volunteer students of both sexes were exposed either for 60 min to
acrolein at a concentration of 0.69 mg/m3 or to gradually
increasing acrolein concentrations from 0 up to 1.37 mg/m3 over
35 min followed by a 5-min exposure to 1.37 mg/m3. In further
experiments with side-stream cigarette smoke instead of pure
acrolein vapour, it was noted that the effects of pure acrolein
vapour were small compared to those produced by side-stream smoke
with the same acrolein vapour concentration. It was concluded that
acrolein was only to a minor extent responsible for the effects
observed (Weber-Tschopp et al., 1976). It must be noted, however,
that a significant part of the acrolein in side-stream cigarette
smoke may be associated with particulate matter (Ayer & Yeager,
1982) and would not have been measured. This may have resulted in an
underestimation of the acrolein concentration in the smoke. Many of
the studies considered in this section are old and the analytical
techniques are often poorly described; the absolute figures reported
may, therefore, be suspect.
8.1.2.2 Dermal exposure
In an investigation into irritant dermatitis possibly caused by
contaminants present in diallylglycol carbonate monomer, patch tests
were conducted with acrolein in ethanol at concentrations of 0.01,
0.1, 1, and 10% on groups of 8, 10, 48, and 20 volunteers,
respectively. At 1%, six positive reactions were recorded, four
cases of serious oedema with bullae and two of erythema. At 10%,
all subjects showed positive reactions with bullae, necrosis,
inflammatory cell infiltrate, and papillary oedema (Lacroix et al.,
1976).
Table 11. Thresholds for acute effects of acrolein on humans
Concentration Exposure Effect Reference
(mg/m3) period (min)
0.05 changes in electrical activity of brain cortex Sinkuvene (1970)
0.07 odour perception by most sensitive individuals Sinkuvene (1970)
0.13 5 no or medium subjective eye irritation Darley et al. (1960)a
0.21 5 increased incidence of subjective eye irritation Weber-Tschopp et al. (1977)b
0.34 10 increased incidence of subjective nasal irritation Weber-Tschopp et al. (1977)b
0.34 30 time-related increase in eye-blink frequency van Eick (1977)a
0.39 10 increased incidence of subjective annoyance Weber-Tschopp et al. (1977)b
0.48 odour recognition Leonardos et al. (1969)
0.59 15 increase in eye-blink frequency Weber-Tschopp et al. (1977)b
0.6 10 increase in sensitivity to light Plotnikova (1957)
0.69 40 decrease in respiratory rate; increased incidence of Weber-Tschopp et al. (1977)c
subjective general irritation of eyes, nose, and neck
0.69 10 increase in eye-blink frequency Weber-Tschopp et al. (1977)c
1 3 slight subjective conjunctival irritation Plotnikova (1957)
1 3 stinging sensation in nose Plotnikova (1957)
1.1 5 increased incidence of subjective eye irritation Stephens et al. (1961)a
1.1 5 increase in tear volume, pH,and lysozyme activity Harada (1977)
1.37 35 decrease in respiratory rate Weber-Tschopp et al. (1977)b
1.5 3 pneumographic changes in rhythm and amplitude of Plotnikova (1957)
respiratory movements
Table 11 (contd).
Concentration Exposure Effect Reference
(mg/m3) period (min)
1.7 3 reflex action on optical chronaxy Plotnikova (1957)
1.88 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 20 seconds
2.80 extreme subjective irritation of all exposed mucosae; Sim & Pattle (1957)
lacrimation within 5 seconds
3 5 medium to severe subjective eye irritation Darley et al. (1960)a
4 2-3 acute subjective conjunctival and nasal irritation; Plotnikova (1957)
painful sensation in nasopharyngeal region
a exposure of eyes only
b exposure to gradually increasing concentrations up to 1.37 mg/m3
c exposure to a fixed concentration
8.2 Long-term exposure
No data are available on the long-term exposure of humans to
acrolein.
9. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
9.1 Aquatic organisms
A summary of acute aquatic toxicity data is presented in
Table 12. In most of these studies, the amount of acrolein added was
reported but the concentrations present were not measured. In these
cases, the actual concentrations may have been lower than the
nominal ones in view of the volatility of the substance and its
hydration rate (see section 4.2).
One of the studies in Table 12 (Lorz et al., 1979) is a
comparatively detailed examination of the acute toxicity of acrolein
to Coho salmon. Within 144 h of exposure to 0.075 mg/litre or more,
all fish died. In surviving fish the activity of gill
Na+,K+-ATPase (EC 3.6.1.37) and the tolerance to subsequent
sea-water exposure were not affected at concentrations up to
0.05 mg/litre. A histological examination of the gills, kidneys, and
liver at 0, 0.05, and 0.1 mg/litre revealed concentration-dependent
adverse effects.
A 3-generation 64-day test with the crustacean Daphnia magna
was conducted in a flow-through open system with well water at
20 °C, a pH between 7.0 and 7.3, a dissolved oxygen concentration of
7.5 mg/litre, and a water hardness of 35 mg CaCO3/litre. The
highest concentration that did not result in mortality was
0.0169 mg/litre (acrolein concentrations were measured in this
study). Survival was reduced at levels of 0.0336 mg/litre or more,
but the number of young per female was not affected even at the
highest concentration tested, 0.0427 mg/litre (Macek et al.,
1976).
Macek et al. (1976) also reported on a 60-day test with
fathead minnow (Pimephales promelas) in a flow-through open system
with well water at 25 °C, a pH between 6.6 and 6.8, a dissolved
oxygen concentration of 8.2 mg/litre, and a water hardness of 32 mg
CaCO3/litre. The highest concentration without adverse effects
was 0.0114 mg/litre (acrolein concentrations were measured in this
study). At 0.0417 mg/litre, there was increased mortality among
offspring. No adverse effects were found on survival and mortality
of adults, number of spawnings and number of eggs per female, number
of eggs per spawn, length of offspring, or hatchability.
It is clear from Table 12 why acrolein is also used as an
algicide, slimicide, and molluscicide.
Table 12. Acute aquatic toxicity of acrolein
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Fresh water
alga Enteromorpha 25 stat, closed 24 h 50% inhibition 1.8c Fritz-
intestinalis of photosynthesis Sheridan
(1982)
alga Cladophora 25 stat, closed 24 h 50% inhibition 1.00c
glomerata of photosynthesis
alga Anabaena 25 stat, closed 24 h 50% inhibition 0.69c
of photosynthesis
bacterium Proteus 37 7.0 stat, closed 2 h 50% growth 0.02 Brown &
vulgaris reduction Fowler
(1967)
bacterium Pseudomonas 25 7.0 stat, closed 16 h TT 0.21 Bringmann
putida & Kuhn
(1977)
protozoan Entosiphon 25 6.9 stat, closed 72 h TT 0.85 Bringmann
sulcatum (1978)
protozoan Chilomonas 20 6.9 stat, closed 48 h TT 1.7 Bringmann
paramecium et al.
(1980)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
protozoan Uronema 25 6.8 stat, closed 20 h TT 0.44 Bringmann
parduczi & Kuhn
(1980)
mollusc snail 21-25 flow, open 48 h 99-100% 20-25 Unrau et
(Bulinus mortality al. (1965)d
truncatus)
mollusc snail stat, open 3 h 100% mortality 10 Ferguson
(Biomphalaria 24 h 10% mortality 1.25 et al.
glabrata), eggs (1961)
mollusc snail (Biomphalaria stat, open 24 h 98% mortality 10 Ferguson
glabrata), adults 24 h 35% mortality 2.5 et al.
(1961)
crustacean water flea 20 7.0- 7.5 35 stat, open 48 h LC50 0.057 Macek et
(Daphnia magna) 7.3 al. (1976)
crustacean water flea 22 7.0- 154 stat, closed 48 h EC50f 0.093 Randall &
(Daphnia magna) 8.2 Knopp (1980)
crustacean water flea 22 7.4- 6-9 173 stat, closed 48 h LC50 0.083 LeBlanc
(Daphnia magna) 9.4 (1980)
fish harlequin fish 20 7.2 20 flow, open 48 h LC50 0.06 Alabaster
(Rasbora (1969)
heteromorpha)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
fish fathead minnow 25 6.6- 8.2 32 flow, open 144 h LC50 0.084 Macek et
(Pimephales 6.8 al.
promelas) (1976)e
fish golden orfe 20 7-8 > 5 200-300 stat 48 h LC50 0.25 & Juhnke &
(Leuciscus idus 2.5 Ludemann
melanotus) (1978)
fish goldfish 20 6-8 > 4 108 stat, open 24 h LC50 < 0.08 Bridie
(Carassius et al.
auratus) (1979)c e
fish Bluegill 21-23 6.5- 10- 32-48 stat, closed 96 h LC50 0.09 Buccafusco
sunfish 7.9 0.3 et al.
(Leopomis (1981)
macrochirus)
fish Coho salmon 10 7.4- > 10 100 stat, open 96 h LC50 0.068 Lorz et al.
(Oncorhynchus 7.6 (1979)g
kisutch)
Table 12 (contd).
Organism Species Temperature pH Dissolved Hardness Stat/flow Exposure Parameterb Concentration Reference
(°C) O2 (mg/ (mg CaCO3 open/ period (mg/litre)
litre) per litre) closeda
Marine
mollusc common mussel 15 stat, closed 6 h 40% mortality 0.6 Rijstenbil
(Mytilus edulis) 6 h 70% mortality 1.0 & van Galen
8 h 70% detached 0.57 (1981)e h
mussels
a static or flow-through test, open or closed system
b TT = toxic threshold for inhibition of cell multiplication
c exposure to Magnacide-H (92% acrolein, 8% inert ingredients)
d field study, resurgence of snails was delayed by 8 to 12 months
e analysis for acrolein was reported
f the effect was complete immobilization
g static-renewal test
h static-renewal test (1.6% salinity)
9.2 Terrestrial organisms
9.2.1 Birds
The LD50 for the adult starling (Sturnus vulgaris) was
reported to be > 100 mg/kg body weight. The birds were observed for
7 days after dosing, but only two birds per dose were tested
(Schafer, 1972).
9.2.2 Plants
Acrolein is used as biocide, particularly to control aquatic
plants such as Elodea canadensis, Vallisneria spiralis
(ribbonweed), and Potamogeton tricarinatus (floating pondweed).
In Australia, a maximum concentration of about 15 mg/litre over a
period not exceeding a few hours has been imposed. In the USA,
acrolein is injected into larger channels over longer periods at low
concentrations (approximately 0.1 mg/litre over 48 h) (Bowmer &
Sainty, 1977). It has been shown that the dosage of acrolein
required for control, as defined by the product of time and
concentration required for 80% reduction in biomass, is independent
of the separate values of concentration and time, provided that the
concentration exceeds 0.1 mg/litre and the dosage exceeds 2 mg/litre
per h. In tank experiments, the minimum dosages required for 80%
control of ribbonweed and floating pondweed were about 4 and
26 mg/litre per h, respectively (Bowmer & Sainty, 1977). The
effective dosage (> 80% kill) for Elodea canadensis was 8 to
10 mg/litre per h (Van Overbeek et al., 1959; Bowmer & Smith,
1984). Sublethal concentrations of acrolein stimulated the growth
of Elodea (Bowmer & Smith, 1984).
Elongation of pollen tubes of lily seeds (Lilium longiflorum)
was inhibited completely after a 5-h exposure to acrolein vapour at
a measured concentration of 0.91 mg/m3, a temperature of 28 °C,
and a relative humidity of 60%. A 10% inhibition was found after
1 h (Masaru er al., 1976).
The nature and extent of adverse effects on various crops grown
in soil irrigated by acrolein-treated water have been investigated
in two studies. Acrolein concentrations varied between 15 and
50 mg/litre of supply water. Most furrow-irrigated crops, including
beans, clover, corn, and millet, did not show any damage.
Significant damage to foliage was observed in cotton at acrolein
concentrations of 25 mg/litre or more, but there was no evidence of
chronic or residual phytotoxicity. Slight damage to the foliage of
cucumbers and tomatoes was observed at 40 mg/litre. Vegetable
seedlings in contact with treated water were damaged even at the
lowest concentrations used (Unrau et al., 1965; Ferguson et al.,
1965).
10. EVALUATION OF HUMAN HEALTH RISKS AND EFFECTS ON THE
ENVIRONMENT
10.1 Evaluation of human health risks
10.1.1 Exposure
Exposure of the general population to acrolein occurs mainly
via air. Exposure via water would only be significant in cases of
ingestion of, or skin contact with, acrolein deliberately applied as
a biocide to irrigation water. Oral exposure to acrolein may also
occur via alcoholic beverages or heated foodstuffs (chapters 3 and
5).
In urban areas, average levels of up to 15 µg/m3 and maximum
levels of up to 32 µg/m3 have been measured away from industrial
sources. Near industries and close to the exhaust pipes of vehicles,
engines, and combustion appliances, levels ten to one hundred times
higher may occur. Extremely high levels of acrolein in the mg/m3
range can be found as a result of fires (section 5.2.1).
Major indoor sources of acrolein are combustion appliances and
tobacco smoking (section 3.2.4). Levels of acrolein in smoke from
indoor open fires for cooking or heating purposes have not been
reported. Smoking one cigarette per m3 of room-space in 10-13 min
has been shown to lead to acrolein vapour concentrations of
450-840 µg/m3 (section 5.2.1). Recent occupational exposure levels
of acrolein in the air at sites of its production or processing are
not available. Workplace levels of over 1000 µg/m3 have been
reported in situations involving the heating of organic materials
(section 5.3).
In summary, the main source of exposure of the general
population to acrolein is via tobacco smoke. General environmental
pollution by vehicle exhaust and the smoke of burning organic
materials is the next most important source.
10.1.2 Health effects
Owing to the reactivity of acrolein, retention at the site of
entry into the body, usually the respiratory tract, is high
(section 6.1). Primary pathological findings are limited
principally to these sites (sections 7 and 8). Any acrolein absorbed
is liable to react directly with protein and non-protein sulfhydryl
groups or with primary and secondary amines such as those found in
proteins and nucleic acids (sections 6.2 and 7.3). Acrolein may also
be metabolized to mercapturic acids, acrylic acid, glycidaldehyde or
glyceraldehyde (section 6.3). Evidence for the last three
metabolites has only been obtained in vitro.
Acrolein is a cytotoxic agent (section 7.1.5) highly toxic to
experimental animals and man following acute exposure via different
routes (sections 7.1.1 and 8.1.1). The vapour is very irritating to
the eyes and the respiratory tract. Liquid acrolein is a corrosive
substance. The no-observed-adverse-effect level for irritant
dermatitis from ethanolic acrolein was found to be 0.1% (section
8.1.2.2). The odour perception threshold for the most sensitive
individuals is reported to be 0.07 mg/m3 (8.1.2.1). Experiments
with human volunteers show a lowest-observed-adverse-effect level of
0.13 mg/m3, at which level eyes may become irritated after 5 min.
In addition to irritation of the eyes, changes in respiratory tract
function are evident at or above 0.7 mg/m3 (40-min exposure)
(section 8.1.2.1). At higher concentrations, degeneration of the
respiratory epithelium and irritation of all exposed mucosa develop.
Oedematous changes in the tracheal and bronchial mucosa and
bronchial obstruction can be expected after very high exposure to
acrolein vapour (section 8.1).
There are no human toxicological data from long-term exposure
to acrolein. The toxicity from exposure to acrolein vapour has been
relatively well investigated in several animal studies for exposure
periods of up to 52 weeks (section 7.2). Both respiratory tract
function and histopathological effects have been observed at
0.5-0.8 mg/m3 (continuous exposure). Toxicological effects in the
respiratory tract have been documented in most animal species
exposed repeatedly to acrolein concentrations of 1.6-3.2 mg/m3 or
more, and mortality has occurred following exposure to
concentrations above 9 mg/m3. There is limited evidence that
acrolein can depress pulmonary host defenses in mice and rats.
Acrolein can induce teratogenic and embryotoxic effects if
administered directly into the amnion. However, the fact that no
effect was found in rabbits injected intravenously with 3 mg/kg
suggests that human exposure to acrolein is unlikely to affect the
developing embryo (section 7.5).
Acrolein has been shown to interact with DNA and RNA in vitro
and to inhibit their synthesis both in vivo and in vitro. In
vitro, it induces gene mutations in bacteria and fungi and sister
chromatid exchanges in mammalian cells (section 7.6). There is
inadequate evidence to allow the mutagenic potential in humans to be
assessed reliably.
One long-term drinking-water study with rats (130 weeks) and
two inhalation tests, one with hamsters (81 weeks) and the other
with rats (40 or 70 weeks), failed to demonstrate carcinogenic or
clear co-carcinogenic effects of acrolein (section 7.7). Due to the
shortcomings of the tests used, acrolein cannot be considered to
have been adequately tested for carcinogenicity and no conclusions
concerning its carcinogenicity are possible.
The threshold levels of acrolein that cause irritation and
health effects are 0.07 mg/m3 for odour perception, 0.13 mg/m3
for eye irritation, 0.3 mg/m3 for nasal irritation and eye
blinking, and 0.7 mg/m3 for decreased respiratory rate. Since
the level of acrolein rarely exceeds 0.030-0.040 µg/m3 in polluted
urban air or smoke-filled restaurants, acrolein alone is unlikely to
reach annoyance or harmful levels in normal circumstances. Provided
that acrolein concentrations are maintained below 0.05 mg/m3, most
of the population will be spared from any known annoyance or health
effects. However, in polluted urban areas and smoke-filled rooms,
acrolein is present in combination with other irritating aldehydes,
and control of acrolein alone is not sufficient to prevent annoyance
or harmful effects.
10.2 Evaluation of effects on the environment
Acrolein is released into the environment during production of
the compound itself and its derivatives, in processes involving
incomplete combustion and/or pyrolysis of organic substances, by
photochemical oxidation of specific air pollutants, and through
biocidal use, spills, and waste disposal (chapter 3).
Degradation in the atmosphere begins mainly by reaction with
hydroxyl radicals. The calculated atmospheric residence time is
approximately one day (section 4.2). Photolysis does not occur to a
significant degree (section 4.2.1). In natural water, acrolein
dissipates fairly rapidly as a result of catalysed hydration,
reactions with organic material, and volatilization (sections 4.2
and 4.3). Acrolein has a low soil adsorption potential
(section 4.1). Aerobic and anaerobic biodegradation of the compound
has been reported, although its toxicity to microorganisms may
prevent biodegradation (section 4.3.1). Based on its physical and
chemical properties, bioaccumulation would not be expected to occur
(section 4.3.2). It can be concluded that acrolein is unlikely to
persist in any environmental compartment.
Acrolein is very toxic to aquatic organisms. Acute EC50 or
LC50 values for various species range between 0.02 and
2.5 mg/litre. The 60-day NOAEL for fish (fathead minnow) is
0.0114 mg/litre (section 9.1).
In view of the high toxicity of acrolein to aquatic organisms,
the substance presents a risk to aquatic life at or near sites of
industrial discharges, spills, and biocidal use.
11. FURTHER RESEARCH
a) Human exposure characteristics should be further evaluated.
This applies to exposure due to environmental and
occupational air, as well as to intake from food and beverages.
b) These evaluations should include determinations of other
chemicals that occur with acrolein and that interact or have
biological effects similar to those due to acrolein exposure.
c) The most important target organ for airborne acrolein exposure
is the respiratory system. Therefore, further studies
including epidemiological studies should focus on this system
and particularly on the occupational environment. Possible
decreases in host resistance to respiratory infections should
be investigated.
d) The uptake of acrolein in the different parts of the
respiratory system should be examined further. The metabolism
and excretion of acrolein, as well as of its metabolites from
the respiratory system, should be given high priority as there
is an almost total lack of information about these processes.
e) The efficacy of sulfhydryl compounds, such as N-acetylcysteine
or 2-mercaptoethylsulfonic acid sodium salt (MESNA) as
antidotes for acrolein poisoning should be evaluated.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
Evidence for the potential carcinogenicity of acrolein has been
evaluated by the International Agency for Research on Cancer (IARC,
1979, 1985, 1987). The evidence for carcinogenicity was considered
to be inadequate both in animals and in humans. Thus no evaluation
could be made of the carcinogenicity of acrolein to humans.
Regulatory standards established by national bodies in various
countries and the EEC are summarized in the data profile of the
International Register of Potentially Toxic Chemicals (IRPTC, 1990)
and are tabulated in the Health and Safety Guide for Acrolein (WHO,
1991).
REFERENCES
ABERNETHY, D.J., FRAZELLE, J.H., & BOREIKO, C.J. (1983) Relative
cytotoxicity and transforming potential of respiratory irritants in
the C3H/10T1/2 cell transformation system. Environ. Mutagen., 5:
419 (abstract).
ALABASTER, J.S. (1969) Survival of fish in 164 herbicides,
insecticides, fungicides, wetting agents, and miscellaneous
substances. Int. Pest Control, 11: 29-35.
ALARCON, R.A. (1968) Fluorometric determination of acrolein and
related compounds with m-aminophenol. Anal. Chem., 40: 1704-1708.
ALARCON, R.A. (1972) Acrolein, a component of a universal
cell-growth regulatory system: a theory. J theor. Biol., 37:
159-167.
ALARCON, R.A. (1976) Formation of acrolein from various amino-acids
and polyamines under degradation at 100 °C. Environ. Res., 12:
317-326.
ALTSHULLER, A.P. & BUFALINI, J.J. (1965) Photochemical aspects of
air pollution: a review. Photochem. Photobiol., 4: 97-146.
ALTSHULLER, A.P. & MCPHERSON, S.P. (1963) Spectrophotometric
analysis of aldehydes in the Los Angeles atmosphere. J. Air Pollut.
Control Assoc., 13: 109-111.
ANDERSEN, K.J., LEIGHTY, E.G., & TAKAHASHI, M.T. (1972) Evaluation
of herbicides for possible mutagenic properties. J. agric. food
Chem., 20: 649-656.
ANDERSSON, K., HALLGREN, C., LEVIN, J.-O., & NILSSON, C.-A. (1981)
Solid chemosorbent for sampling sub-ppm levels of acrolein and
glutaraldehyde in air. Chemosphere, 10: 275-280.
ARANYI, C., O'SHEA, W.J., GRAHAM, J.A., & MILLER, F.J. (1986) The
effects of inhalation of organic chemical air contaminants on murine
lung host defenses. Fundam. appl. Toxicol., 6: 713-720.
ARTHO, A. & KOCH, R. (1969) [The concentration of acrolein and
hydrogen cyanide in cigarette smoke]. Mitt. Lebensm. Hyg. (Bern),
60: 379-388 (in German).
ASTRY, C. & JAKAB, G.J. (1983) The effects of acrolein exposure on
pulmonary antibacterial defenses. Toxicol. appl. Pharmacol., 67:
49-54.
ATKINSON, R., ASCHMANN, S.M., WINER, A.M., & PITTS, J.N. (1981) Rate
constants for the gas-phase reactions of O3 with a series of
carbonyls at 296 K. Int. J. chem. Kinet., 13: 1133-1150.
ATKINSON, R., ASCHMANN, S.M., & PITTS, J.N. (1983) Kinetics of the
gas-phase reactions of OH radicals with a series of
alpha,ß-unsaturated carbonyls at 299 + 2 K. Int. J. chem. Kinet.,
15: 75-81.
ATKINSON, R., ASCHMANN, S.M., & GOODMAN, M.A. (1987) Kinetics of the
gas-phase reactions of NO3 radicals with a series of alkynes,
haloalkenes, and alpha,ß-unsaturated aldehydes. Int. J. chem.
Kinet., 19: 299-307.
AU, W., SOKOVA, O.I., KOPNIN, B., & ARRIGHI, F.E. (1980) Cytogenetic
toxicity of cyclophosphamide and its metabolites in vitro.
Cytogenet. cell Genet., 26: 108-116.
AYER, H.E. & YEAGER, D.W. (1982) Irritants in cigarette smoke
plumes. Am. J. public Health, 72: 1283-1285.
BABIUK, C., STEINHAGEN, W.H., & BARROW, C.S. (1985) Sensory
irritation response to inhaled aldehydes after formaldehyde
pretreatment. Toxicol. appl. Pharmacol., 79: 143-149.
BAKER, R.R., DYMOND, H.F., & SHILLABEER, P.K. (1984) Determination
of alpha,ß-unsaturated compounds formed by a burning cigarette.
Anal. Proc. (Lond.), 21: 135-137.
BALLANTYNE, B., DODD, D.E., PRITTS, I.M., NACHREIMER, D.J., &
FOWLER, E.H. (1989) Acute vapour inhalation toxicity of acrolein
and its influence as a trace contaminent in
2-methoxy-3,4-dihydro-2H-pyran. Hum. Toxicol., 8: 229-235.
BARROWS, M.E., PETROCELLI, S.R., MACEK, K.J., & CARROLL, J.J. (1980)
Bioconcentration and elimination of selected water pollutants by
bluegill sunfish (Lepomis macrochirus). In: Proceedings of the
1978 Symposium on dynamics, exposure and hazard assessment of toxic
chemicals, Ann Arbor, Michigan, Ann Arbor Science, pp. 379-392.
BARTLEY, T.R. & GANGSTAD, E.O. (1974) Environmental aspects of
aquatic plant control. J. Irrig. Drain. Div., 100: 231-244.
BASU, A.K. & MARNETT, L.J. (1984) Molecular requirements for the
mutagenicity of malondialdehyde and related acroleins. Cancer Res.,
44: 2848-2854.
BEAUCHAMP, R.O., ANDJELKOVICH, D.A., KLIGERMAN, A.D., MORGAN, K.T.,
& HECK, H. d'A. (1985) A critical review of the literature on
acrolein toxicity. CRC crit. Rev. Toxicol., 14: 309-380.
BEELEY, J.M., CROW, J., JONES, J.G., MINTY, B., LYNCH, R.D., &
PRYCE, D.P. (1986) Mortality and lung histopathology after
inhalation lung injury. The effect of corticosteroids. Am. Rev.
respir. Dis., 133: 191-196.
BEN-DYKE, R., SANDERSON, D.M., & NOAKES, D.N. (1970) Acute toxicity
data for pesticides. World Rev. Pest. Control, 9: 119-127.
BENEDICT, R.C. & STEDMAN, R.L. (1969) Composition studies on
tobacco. XXXVII. Inhibition of lactic, alcohol and
glucose-6-phosphate dehydrogenases by cigarette smoke and components
thereof. Tob. Sci., 13: 166-168.
BERRIGAN, M.J., GURTOO, H.L., SHARMA, S.D., STRUCK, R.F., &
MARINELLO, A.J. (1980) Protection by N-acetylcysteine of
cyclophosphamide metabolism - related in vivo depression of mixed
function oxygenase activity and in vitro denaturation of
cytochrome P-450. Biochem. biophys. Res. Commun., 93: 797-803.
BIGNOZZI, C.A., CHIORBOLI, C., MALDOTTI, A., & CARASSITI, V. (1980).
Atmospheric reactivity of 1,3-butadiene - nitrogen monoxide and
acrolein - nitrogen monoxide systems. Ann. Chim. (Rome), 70:
453-461.
BOETTNER, E.A. & BALL, G.L. (1980). Thermal degradation products
from PVC film in food-wrapping operations. Am. Ind. Hyg. Assoc. J.,
41: 513-522. BOHMANN, J.-J. (1985) [Aging behaviour of beer.]
Monatsschr. Brauwiss., 4: 175-180 (in German).
BOOR, P.J. & ANSARI, G.A.S. (1986) High-performance liquid
chromatographic method for quantitation of acrolein in biological
samples. J. Chromatogr., 375: 159-164.
BOULEY, G., DUBREUIL, A., GODIN, J., & BOUDENE, C. (1975) Effets,
chez le rat, d'une faible dose d'acroléine inhalée en continu. Eur.
J. Toxicol., 8: 291-297.
BOWMER, K.H. & HIGGINS, M.L. (1976) Some aspects of the persistence
and fate of acrolein herbicide in water. Arch.environ. Contam.
Toxicol., 5: 87-96.
BOWMER, K.H. & SAINTY, G.R. (1977) Management of aquatic plants with
acrolein. J. aquat. Plant Manage., 15: 40-46.
BOWMER, K.H. & SMITH, G.H. (1984) Herbicides for injection into
flowing water: acrolein and endothal-amine. Weed Res., 24: 201-211.
BOWMER, K.H., LANG, A.R.G., HIGGINS, M.L., PILLAY, A.R., & TCHAN,
Y.T. (1974) Loss of acrolein from water by volatilization and
degradation. Weed Res., 14: 325-328.
BOYLAND, E. & CHASSEAUD, L.F. (1967) Enzyme-catalysed conjugations
of glutathione with unsaturated compounds. Biochem. J., 104:
95-102.
BRIDGES, R.B., KRAAL, J.H., HUANG, L.J.T., & CHANCELLOR, M.B. (1977)
Effects of cigarette smoke components on in vitro chemotaxis of
human polymorphonuclear leukocytes. Infect. Immun., 16: 240-248.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979a) BOD and COD of
some petrochemicals. Water Res., 13: 627-630.
BRIDIE, A.L., WOLFF, C.J.M., & WINTER, M. (1979b) The acute toxicity
of some petrochemicals to goldfish. Water Res., 13: 623-626.
BRINGMANN, G. (1978) [Determination of the harmful biological action
of water-endangering substances on protozoa; I. Bacteria fed
flagellates.] Z. Wasser-Abwasser Forsch., 11: 210-215 (in German).
BRINGMANN, G. & KUHN, R. (1977) [Limiting values of the harmful
action of water-endangering substances on bacteria (Pseudomonas
putida) and green algae (Scenedesmus quadricauda) in the cell
multiplication inhibition test.] Z. Wasser-Abwasser Forsch., 10:
87-98 (in German).
BRINGMANN, G. & KUHN, R. (1980) [Determination of the harmful
biological action of water-endangering substances on protozoa; II.
Bacteria fed ciliates.] Z. Wasser-Abwasser Forsch., 13: 26-31 (in
German).
BRINGMANN, G., KUHN, R., & WINTER, A. (1980) [Determination of the
harmful biological action of water-endangering substances on
protozoa; III Saprozoic flagellates.] Z. Wasser-Abwasser Forsch.,
13: 170-173 (in German).
BROWN, P.W. & FOWLER, C.A. (1967) The toxicity of tobacco smoke
solutions to Proteus vulgaris. Beitr. Tabakforsch., 4: 78-83.
BUCCAFUSCO, R.J., ELLS, S.J., & LEBLANC, G.A. (1981) Acute toxicity
of priority pollutants to Bluegill (Lepomis macrochirus). Bull.
environ. Contam. Toxicol., 26: 446-452.
BUCKLEY, L.A., JIANG, X.Z., JAMES, R.A., MORGAN, K.T., & BARROW,
C.S. (1984) Respiratory tract lesions induced by sensory irritants
at the RD50 concentration. Toxicol. appl. Pharmacol., 74:
417-429.
CAMPBELL, D.N. & MOORE, R.H. (1979) The quantitative determination
of acrylonitrile, acrolein, acetonitrile and acetone in workplace
air. Am. Ind. Hyg. Assoc. J., 40: 904-909.
CAMPBELL, K.I., GEORGE, E.L., & WASHINGTON, I.S. (1981) Enhanced
susceptibility to infection in mice after exposure to dilute exhaust
from light duty diesel engines. Environ. Int., 5: 377-382.
CANTONI, C., BIANCHI, M.A., RENON, P., & CALCINARDI, C. (1969)
[Bacterial and chemical alterations during souring in salted pork.]
Atti. Soc. Ital. Sci. Vet., 23: 752-756 (in Italian).
CARPENTER, C.P., SMYTH, H.F., POZZANI, U.C. (1949) The assay of
acute vapor toxicity, and the grading and interpretation of results
on 96 compounds. J. ind. Hyg. Toxicol., 31: 343-346.
CATILINA, P., THIEBLOT, L., & CHAMPEIX, J. (1966) Lesions
respiratoires expérimentales par inhalation d'acroléine chez le rat.
Arch. Mal. prof. Méd. Trav. Sécur. soc. (Paris), 27: 857-867.
CHAMPEIX, J., COURTIAL, L., PERCHE, E., & CATILINA, P. (1966)
Broncho-pneumopathie aiguë par vapeurs d'acroléine. Arch. Mal.
prof. Méd. Trav. Sécur. Soc., 27: 794-796.
CHAVIAMO, A.H., GILL, W.B., RUGGIERO, U.J., & VERMEULEN, C.W. (1985)
Experimental cytoran cystitis and prevention by acetylcysteine. J.
Urol., 134: 598-600.
CHHIBBER, G. & GILANI, S.H. (1986) Acrolein and embryogenesis: an
experimental study. Environ. Res., 39: 44-49.
CHOU, W.L., SPEECE, R.E., & SIDDIQI, R.H. (1978) Acclimation and
degradation of petrochemical wastewater components by methane
fermentation. Biotechnol. Bioeng. Symp., 8: 391-414.
CHRAIBER, L.B., SOSNOVSKY, S.I., TATARKIN, Y.N., & VINNIKOVA, L.I.
(1964) [Air pollution with acrolein vapors in expeller and forepress
shops of butter and fat mills of Uzbekistan.] Gig. Tr. prof.
Zabol., 1(11): 49-50 (in Russian).
CHUNG, F.-L., YOUNG, R., & HECHT, S.S. (1984) Formation of cyclic
1,N2-propanodeoxyguanosine adducts in DNA upon reaction with
acrolein or crotonaldehyde. Cancer Res., 44: 990-995.
CLAUSSEN, U., HELLMANN, W., & PACHE, G. (1980) The embryotoxicity of
the cyclophosphamide metabolite acrolein in rabbits, tested in vivo
by i.v. injection and by the yolk-sac method. Drug Res., 30:
2080-2083.
COHEN, I.R. & ALTSHULLER, A.P. (1961) A new spectrophotometric
method for the determination of acrolein in combustion gases and in
the atmosphere. Anal. Chem., 33: 726-733.
COOMBER, J.W. & PITTS, J.N. (1969) Molecular structure and
photochemical reactivity. XII. The vapor-phase photochemistry of
acrolein at 3130 A. J. Am. Chem. Soc., 91: 547-550.
COOPER, K.O., WITMER, C.M., & WITZ, G. (1987) Inhibition of
microsomal cytochrome c reductase activity by a series of
alpha,ß-unsaturated aldehydes. Biochem. Pharmacol., 36: 627-631.
COSTA, D.L., KUTZMAN, R.S., LEHMANN, J.R., & DREW, R.T. (1986)
Altered lung function and structure in the rat after subchronic
exposure to acrolein. Am. Rev. respir. Dis., 133: 286-291.
COX, R., GOORHA, S., & IRVING, C.C. (1988) Inhibition of DNA
methylase activity by acrolein. Carcinogenesis, 9: 463-465.
CRANE, C.R., SANDERS, D.C., ENDECOTT, B.R., & ABBOTT, J.K. (1986)
Inhalation toxicology: VII. Times to incapacitiation and death for
rats exposed continuously to atmospheric acrolein vapor, Washington,
D.C., Federal Aviation Administration, Office of Aviation Medicine
(DOT/FAA/AM-86/5)
CROOK, T.R., SOUHAMI, R.L., & MCLEAN, A.E.M. (1986a) Cytotoxicity,
DNA cross-linking, and single strand breaks induced by activated
cyclophosphamide and acrolein in human leukemia cells. Cancer Res.,
46: 5029-5034.
CROOK, T.R., SOUHAMI, R.L., WHYMAN, G.D., & MCLEAN, A.E.M. (1986b)
Glutathione depletion as a determinant of sensitivity of human
leukemia cells to cyclophosphamide. Cancer Res., 46: 5035-5038.
CURREN, R.D., YANG, L.L., CONKLIN, P.M., GRAFSTROM, R.C., & HARRIS,
C.C. (1988) Mutagenesis of xeroderma pigmentosum fibroblasts by
acrolein. Mutat. Res., 209: 17-22.
DAHLGREN, S.E., DALEN, H., & DALHAMM, T. (1972) Ultrastructural
observations on chemically induced inflammation in guinea pig
trachea. Virchows Arch. Abt. B Zellpathol., 11: 211-223.
DALHAMN, T. & ROSENGREN, A. (1971) Effect of different aldehydes on
tracheal mucosa. Arch. Otolarygnol., 83: 496-500.
DARLEY, E.F., MIDDLETON, J.T., & GARBER, M.J. (1960) Plant damage
and eye irritation from ozone-hydrocarbon reactions. J. agric. food
Chem., 8: 483-485.
DAVIS, T.R.A., BATTISTA, S.P., & KENSLER, C.J. (1967) Mechanism of
respiratory effects during exposure of guinea pigs to irritants.
Arch. environ. Health, 15: 412-419.
DAWSON, J.R., NORBECK, K., ANUNDI, I., & MOLDEUS, P. (1984) The
effectiveness of N-acetylcysteine in isolated hepatocytes, against
the toxicity of paracetamol, acrolein, and paraquat. Arch.
Toxicol., 55: 11-15.
DEBETHIZY, J.D., UDINSKY, J.R., SCRIBNER, H.E., & FREDERICK, C.B.
(1987) The disposition and metabolism of acrylic acid and ethyl
acrylate in male Sprague-Dawley rats. Fundam. appl. Pharmacol., 8:
549-561.
DESCROIX, H. (1972) Etude complémentaire des interactions entre
acroléine et cytidine-monophosphate. C. R. Acad. Sci. Paris, D274:
2362-2365.
DGEP (1988) Unpublished Review of literature data on acrolein,
Leidschendam, The Netherlands, Ministry of Housing, Physical
Planning and Environment, Directorate-General of Environmental
Protection.
DIAZ MAROT, A., GASSIOT MATAS, M., & FERRER MARTIN, M. (1983)
[Stripping and high performance liquid chromatography in the
analysis of carbonyl compounds in beers at ppb level.] Afinidad,
40: 21-24 (in Spanish).
DORE, M. & MONTALDO, C. (1984) [Study on the conjungation in vitro
of allyl alcohol and its metabolites with reduced glutathione.]
Boll. Soc. Ital. Biol. Sper., 60: 1497-1501 (in Italian).
DRAMINSKI, W., EDER, E., & HENSCHLER, D. (1983) A new pathway of
acrolein metabolism in rats. Arch. Toxicol., 52: 243-247.
EASLEY, D.M., KLEOPFER, R.D., & CARASEA, A.M. (1981) Gas
chromatographic-mass spectrometric determination of volatile organic
compounds in fish. J. Assoc. Off. Anal. Chem., 64: 653-656.
EDNEY, E.O., MITCHELL, S., & BUFALINI, J.J. (1982) Atmospheric
chemistry of several toxic compounds, Research Triangle Park, North
Carolina, US Environmental Protection Agency, Atmospheric Chemistry
and Physics Division, Environmental Sciences Research Laboratory,
Office of Research and Development (EPA-600/3-82-092, PB 83-146340).
EDNEY, E.O., KLEINDIENST, T.E., & CORSE, E.W. (1986a) Room
temperature rate constants for the reaction of OH with selected
chlorinated and oxygenated hydrocarbons. Int. J. chem. Kinet., 18:
1355-1371.
EDNEY, E.O., SHEPSON, P.B., KLEINDIENST, T.E. & CORSE, E.W. (1986b)
The photooxidation of allyl chloride. Int. J. chem. Kinet., 18:
597-608.
EGLE, J.L. (1972) Retention of inhaled formaldehyde,
propionaldehyde, and acrolein in the dog. Arch. environ. Health,
25: 119-124.
EGLE, J.L. & HUDGINS, P.M. (1974) Dose-dependent sympathomimetic and
cardioinhibitory effects of acrolein and formaldehyde in the
anesthetized rat. Toxicol. appl. Pharmacol., 28: 358-366.
ELLENBERGER, J. & MOHN, G.R. (1976) Comparative mutagenicity testing
of cyclphosphamide and some of its metabolites. Mutat. Res., 37:
120 (abstract).
ELLENBERGER, J. & MOHN, G.R. (1977) Mutagenic activity of major
mammalian metabolites of cyclophosphamide toward several genes of
Escherichia coli. J. Toxicol. environ. Health, 3: 637-650.
EPSTEIN, S.S. & SHAFNER H. (1968) Chemical mutagens in the human
environment. Nature (Lond.), 219: 385-387.
ERICKSON, L.C., RAMONAS, L.M., ZAHARKO, D.S., & KOHN, K.W. (1980)
Cytotoxicity and DNA cross-linking activity of
4-sulfidocyclophosphamides in mouse leukemia cells in vitro.
Cancer Res., 40: 4216-4220.
ESTERBAUER, H., ZOLLNER, H., & SCHOLZ, N. (1975) Reaction of
glutathione with conjugated carbonyls. Z. Naturforsch., 30:
466-473.
FACCHINI, M.C., CHIAVARI, G., & FUZZI, S. (1986) An improved HPLC
method for carbonyl compound speciation in the atmospheric liquid
phase. Chemosphere, 15: 667-674.
FERGUSON, F.F., RICHARDS, C.S., & PALMER, J.R. (1961) Control of
Australorbis glabratus by acrolein in Puerto Rico. Public Health
Rep., 76: 461-468.
FERGUSON, F.F., DAWOOD, I.K., & BLONDEAU, R. (1965) Preliminary
field trials of acrolein in the Sudan. Bull. World Health Organ.,
32: 243-248.
FERON, V.J. & KRUYSSE, A. (1977) Effects of exposure to acrolein
vapor in hamsters simultaneously treated with benzo [a]pyrene or
diethylnitrosamine. J. Toxicol. environ. Health, 3: 379-394.
FERON, V.J., KRUYSSE, A., TIL, H.P., & IMMEL, H.R. (1978) Repeated
exposure to acrolein vapour: subacute studies in hamsters, rats and
rabbits. Toxicology, 9: 47-57.
FISCHER, T., WEBER, A., & GRANDJEAN, E. (1978) [Air pollution due to
tobacco smoke in restaurants.] Int. Arch. occup. environ. Health,
41: 267-280 (in German).
FLEER, R. & BRENDEL, M. (1982) Toxicity, interstrand cross-links and
DNA fragmentation induced by `activated' cyclophosphamide in yeast:
comparative studies on 4-hydroperoxy-cyclophosphamide, its
monofunctional analogue, acrolein, phosphoramide mustard, and
non-nitrogen mustard. Chem.-biol. Interact., 39: 1-15.
FLORIN, I., RUTBERG, L., CURVALL, M., & ENZELL, C.R. (1980)
Screening of tobacco smoke constituents for mutagenicity using the
Ames' test. Toxicology, 18; 219-232.
FOILES, P.G., AKERKAR, S.A., & CHUNG, F.-L. (1989) Application of an
immunoassay for cyclic acrolein deoxyguanosine adducts to assess
their formation in DNA of Salmonella typhimurium under conditions
of mutation induction by acrolein. Carcinogenesis, 10: 87-90.
FRACCHIA, M.F., SCHUETTE, F.J., & MUELLER, P.K. (1967) A method for
sampling and determination of organic carbonyl compounds in
automobile exhaust. Environ. Sci. Technol., 1: 915-922.
FRITZ-SHERIDAN, R.P. (1982) Impact of the herbicide Magnacide-H
(2-propenal) on algae. Bull. environ. Contam. Toxicol., 28:
245-249.
GALLOWAY, S.M., ARMSTRONG, M.J., REUBEN, C., COLMAN, S., BROWN, B.,
CANNON, C., BLOOM, A.D., NAKAMURA, F., AHMED, M., DUK, S., RIMPO,
J., MARGOLIN, B.H., RESNICK, M.A., ANDERSON, B., & ZEIGER, E. (1987)
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. mol.
Mutagen., 10(supplement): 1-175.
GAN, J.C. & ANSARI, G.A.S. (1987) Plausible mechanism of
inactivation of plasma alpha1-proteinase inhibitor by acrolein.
Res. Commun. chem. Pathol. Pharmacol., 55: 419-422.
GIUSTI, D.M., CONWAY, R.A., & LAWSON, C.T. (1974) Activated carbon
adsorption of petrochemicals. J. Water Pollut. Control Fed., 46:
947-965.
GOSSELIN, B., WATTEL, F., CHOPIN, C., DEGAND, P., FRUCHART, J.C.,
VAN DER LOO, D., & CRASQUIN, O. (1979) Intoxication aiguë par
l'acroléine, une observation. Nouv. Presse méd., 8: 2469-2472.
GRAEDEL, T.E., FARROW, L.A., & WEBER, T.A. (1976) Kinetic studies of
the photochemistry of the urban atmosphere. Atmos. Environ., 10:
1095-1116.
GRAFSTROM, R.C., CURREN, R.D., YANG, L.L., & HARRIS, C.C. (1986)
Aldehyde-induced inhibition of DNA repair and potentiation of
N-nitrosocompound-induced mutagenesis in cultured human cells.
Prog. clin. biol. Res., 209A: 255-264.
GRAFSTROM, R.C., DYPBUKT, J.M., WILLEY, J.C., SUNDQVIST, K., EDMAN,
C., ATZORI, L., & HARRIS, C.C. (1988) Pathobiological effects of
acrolein in cultured human bronchial epithelial cells. Cancer Res.,
48: 1717-1721.
GREENHOFF, K. & WHEELER, R.E. (1981) Analysis of beer carbonyls at
the part per billion level by combined liquid chromatography and
high pressure liquid chromatography. J. Inst. Brew., 86: 35-41.
GREY, T.C. & SHRIMPTON, D.H. (1966) Volatile components of raw
chicken breast muscle. Br. poult. Sci., 8: 23-33.
GROSJEAN, D. & WRIGHT ,B. (1983) Carbonyls in urban fog, ice fog,
cloudwater and rainwater. Atmos. Environ., 17: 2093-2096.
GUERIN, M.R., OLERICH, G., & HORTON, A.D. (1974) Routine gas
chromatographic component profiling of cigarette smoke for the
identification of biologically significant constituents. J.
chromatogr. Sci., 12: 385-391.
GUICHERIT, R. & SCHULTING, F.L. (1985) The occurrence of organic
chemicals in the atmosphere of The Netherlands. Sci. total
Environ., 43: 193-219.
GUILLERM, R., BADRE, R., & HEE, J. (1967a) Détermination du seuil
d'action des polluants irritants de l'atmosphère - Comparaison de
deux méthodes. Ann. occup. Hyg., 10: 127-133.
GUILLERM, R., SAINDELLE, A., FALTOT, P., & HEE, J. (1967b) Action de
la fumée de cigarette et de quelques-uns de ses constituants sur les
resistances ventilatoires chez le cobaye. Arch. int. Pharmacodyn.
Ther., 167: 101-114.
GURTOO, H.L., HIPKENS, J.H., & SHARMA, S.D. (1981a) Role of
glutathione in the metabolism-dependent toxicity and chemotherapy of
cyclophosphamide. Cancer Res., 41: 3584-3591.
GURTOO, H.L., MARINELLO, A.J., STRUCK, R.F., PAUL, B., & DAHMS, R.P.
(1981b) Studies on the mechanism of denaturation of cytochrome P-450
by cyclophosphamide and its metabolites. J. biol. Chem., 256:
11691-11701.
GUSEV, M.I., SVECHNIKOVA, A.I., DRONOV, I.S., GREBENSKOVA, M.D., &
GOLOVINA, A.I. (1966) [Substantiation of the daily average maximum
permissible concentration of acrolein in the atmosphere.] Gig. i
Sanit., 31: 9-13 (in Russian).
HAENEN, G.R.M.M., VERMEULEN, N.P.E., TAI TIN TSOI, J.N.L., RAGETLI,
H.M.N., TIMMERMAN, H., & BAST, A. (1988) Activation of the
microsomal glutathione- S-transferase dependent protection against
lipid peroxidation by acrolein. Biochem. Pharmacol., 37: 1933-1938.
HALES, B.F. (1982) Comparison of the mutagenicity and teratogenicity
of cyclophosphamide and its active metabolites,
4-hydroxycyclophosphamide, phosphoramide mustard, and acrolein.
Cancer Res., 42: 3016-3021.
HALES, C.A., BARKIN, P.W., JUNG, W., TRAUTMAN, E., LAMBORGHINI, D.,
HERRIG, N., & BURKE, J. (1988) Synthetic smoke with acrolein but not
HCL produces pulmonary edema. J. appl. Physiol., 64: 1121-1133.
HALL, R.H., & STERN, E.S. (1950) Acid-catalysed hydration of
acraldehyde. Kinetics of the reaction and isolation of
ß-hydroxypropaldehyde. J. Chem. Soc., 1950: 490-498.
HARADA, M. (1977) [Biochemical analysis of tear fluid in
photochemical smog.] Nippon Ganka Gakkai Zasshi, 81: 275-286 (in
Japanese).
HARKE, H.-P., BAARS, A., FRAHM, B., PETERS, H., & SCHULTZ, C. (1972)
[On the problem of passive smoking. Relation between the
concentration of smoke constituents in the air of several large
rooms and the number of smoked cigarettes and time.] Int. Arch.
Arbeitsmed., 29: 323-339 (in German).
HAWLEY, G.G. (1981) The condensed chemical dictionary, 10th ed.,
New York, Van Nostrand Reinhold Co.
HAWORTH, S., LAWLOR, T., MORTELMANS, K., SPECK, W., & ZEIGER, E.
(1983) Salmonella mutagenicity test results for 250 chemicals.
Environ. Mutagen., Suppl. 1: 3-142.
HAYASE, F., CHUNG, T.-Y., & KATO, H. (1984) Changes of volatile
components of tomato fruits during ripening. Food Chem., 14:
113-124.
HEMMINKI, K., FALCK, K., & VAINIO, H. (1980) Comparison of
alkylation rates and mutagenicity of directly acting industrial and
laboratory chemicals. Arch. Toxicol., 46: 277-285.
HESS, L.G., KURTZ, A.N., & STANTON, D.B. (1978) Acrolein and
derivatives. In: Kirk, R.E. & Othmer, D.F. ed. Encyclopedia of
chemical technology, 3rd ed., New York, Wiley Interscience, Vol 1:
pp. 277-297.
HODGE, H.C. & STERNER, J.H. (1943) Tabulation of toxicity classes.
Ind. Hyg. Q., 10: 93-96.
HOFFMAN, C., BASTIAN, H., WIEDENMANN, M., DEININGER, C., & EDER, E.
(1989) Detection of acrolein congener-DNA adducts isolated from
cellular systems. Arch. Toxicol., Suppl. 13: 219-223.
HOFFMANN, D., BRUNNEMANN, K.D., GORI, G.B., & WYNDER, E.L. (1975) On
the carcinogenicity of marijuana smoke. Recent Adv. Phytochem., 9:
63-81.
HOLMBERG, B. & MALMFORS, T. (1974) The cytotoxicity of some organic
solvents. Environ. Res., 7: 183-192.
HOSHIKA, Y. & TAKATA, Y. (1976) Gas chromatographic separation of
carbonyl compounds as their 2,4-dinitrophenylhydrazones using glass
capillary columns. J. Chromatogr., 120: 379-389.
HOVDING, G. (1969) Occupational dermatitis from pyrolysis products
of polythene. Acta dermatovenereol, 49: 147-149.
HRDLICKA, J. & KUCA, J. (1965) The changes of carbonyl compounds in
the heat-processing of meat. Poult. Sci., 44: 7-31.
HUGOD, C., HAWKINS, L.H., & ASTRUP, P. (1978) Exposure of passive
smokers to tobacco smoke constituents. Int. Arch. occup. environ.
Health, 42: 21-29.
IARC (1979) Some monomers, plastics and synthetic elastomers, and
acrolein, Lyons, International Agency for Research on Cancer, pp.
479-495 (IARC Monographs on the Evaluation of the Carcinogenic Risk
of Chemicals to Man, Vol. 19).
IARC (1985) Allyl compounds, aldehydes, epoxides and peroxides,
Lyons, International Agency for Research on Cancer, pp. 133-161
(IARC Monographs on the Evaluation of the Carcinogenic Risk of
Chemicals to Man, Vol. 36).
IRPTC (1985) Treatment and disposal methods for waste chemicals,
Geneva, International Register of Potentially Toxic Chemicals,
United Nations Environment Programme.
IRPTC (1990) Data profile on acrolein, Geneva, International
Register of Potentially Toxic Chemicals, United Nations Environment
Programme.
IVANETICH, K.M., LUCAS, S., MARSH, J.A., ZIMAN, M.R., KATZ, I.D., &
BRADSHAW, J.J. (1978) Organic compounds - Their interaction with and
degradation of hepatic microsomal drug-metabolizing enzymes in
vitro. Drug Metab. Disp., 6: 218-225.
IZARD, C. (1973) Effets de l'acroléine sur la division cellulaire,
le cycle et la synthèse de l'ADN, chez Vicia faba. C. R. Acad.
Sci. Paris Ser. D, 276: 1745-1747.
IZARD, C. & LIBERMANN, C. (1978) Acrolein. Mutat. Res., 47:
115-138.
IZMEROV, N.F., ed. (1984) Acrolein. Moscow, Centre of International
Projects, GKNT, 15 pp (IRPTC Scientific Reviews of Soviet Literature
on Toxicity and Hazards of Chemicals 50).
JACOBS, W.A. & KISSINGER, P.T. (1982) Determination of carbonyl 2,4-
dinitrophenylhydrazones by liquid chromatography/electrochemistry.
J. liq. Chromatogr., 5: 669-676.
JAKAB, G.J. (1977) Adverse effect of a cigarette smoke component,
acrolein, on pulmonary defenses and on viral-bacterial interactions
in the lung. Am. Rev. respir. Dis., 115: 33-38.
JERMINI, C., WEBER, A., & GRANDJEAN, E. (1976) [Quantitative
determination of several gas-phase components of side-stream smoke
of cigarettes in indoor air as contribution towards the problem of
passive smoking.] Int. Arch. occup. environ. Health, 36: 169-181
(in German).
JONSSON, A. & BERG, S. (1983) Determination of low-molecular-weight
oxygenated hydrocarbons in ambient air by cryogradient sampling and
two-dimensional gas chromatography. J. Chromatogr., 279: 307-322.
JOUSSERANDOT, P., GREMAIN, J., & DU BOISTESSELIN, R. (1981)
L'utilisation de méthodes histologiques pour la démonstration de
l'activite anti-inflammatoire de la fusafungine. Sem. Hop. Paris,
57: 143-150.
JUHNKE, I. & LUDEMANN, D. (1978) [Results of examination of 200
chemical compounds for acute toxicity towards fish by means of the
goldfish test.] Z. Wasser-Abwasser Forsch., 11: 161-164 (in
German).
KANE, L.E. & ALARIE, Y. (1977) Sensory irritation to formaldehyde
and acrolein during single and repeated exposures in mice. Am. Ind.
Hyg. Assoc. J., 38: 509-521.
KANE, L.E. & ALARIE, Y. (1978) Evaluation of sensory irritation from
acrolein-formaldehyde mixtures. Am Ind. Hyg. Assoc. J., 39:
270-274.
KANE, L.E. & ALARIE, Y. (1979) Interactions of sulfur dioxide and
acrolein as sensory irritants. Toxicol. appl. Pharmacol., 48:
305-315.
KANKAANPAA, J., ELOVAARA, E., HEMMINKI, K., & VAINIO, H. (1979)
Embryotoxicity of acrolein, acrylonitrile and acrylamide in
developing chick embryos. Toxicol. Lett., 4: 93-96.
KANTEMIROVA, A.E. (1975) [The disease incidence rate, including
temporal disability of workers engaged in the production of acrolein
and methylmercaptopropione (MMP) aldehyde.] Trans. Volgograd med.
Inst., 26(4): 79-85 (in Russian).
KANTEMIROVA, A.E. (1977) [The disease incidence rate, involving
temporal disability of workers engaged in the production of acrolein
and MMP aldehyde.] Trans. Volgograd med. Inst., 27(5): 32-33 (in
Russian).
KATZ, M. (1977) Methods of air sampling and analysis, 2nd ed.,
Washington, DC, American Public Health Association.
KAYE, C.M. (1973) Biosynthesis of mercapturic acids from allyl
alcohol, allyl esters and acrolein. Biochem. J., 134: 1093-1101.
KERR, J.A. & SHEPPARD (1981) Kinetics of the reactions of hydroxyl
radicals with aldehydes studied under atmospheric conditions.
Environ. Sci. Technol., 15: 960-963.
KHUDOLEY, V.V., MIZGIREV, I.V., & PLISS, G.B. (1986) [Evaluation of
mutagenic activity of carcinogens and other chemical agents with
Salmonella typhimurium assays.] Vopr. Onkol., 32: 73-80 (in
Russian).
KILBURN, K.H. & MCKENZIE, W.N. (1978) Leukocyte recruitment to
airways by aldehyde-carbon combinations that mimic cigarette smoke.
Lab. Invest., 38: 134-142.
KIMES, B.W. & MORRIS, D.R. (1971) Inhibition of nucleic acid and
protein synthesis in Escherichia coli by oxidized polyamines and
acrolein. Biochim. Biophys. Acta, 228: 235-244.
KISSEL, C.L., BRADY, J.L., GUERRA, A.M., PAU, J.K., ROCKIE, B.A., &
CASERIO, F.F. (1978) Analysis of acrolein in aged aqueous media.
Comparison of various analytical methods with bioassays. J. agric.
food Chem., 26: 1338-1343.
KLOCHKOVSKII, S.P., LUKASHENKO, R.D., PODVYSOTSKII, K.S., &
KAGRAMANYAN, N.P. (1981) [Acrolein and formaldehyde content in the
air of quarries.] Bezoppasnost. Tr. Prom-St. 1981(12):38 (in
Russian).
KOERKER, R.L., BERLIN, A.J., & SCHNEIDER, F.H. (1976) The
cytotoxicity of short-chain alcohols and aldehydes in cultured
neuroblastoma cells. Toxicol. appl. Pharmacol., 37: 281-288.
KORHONEN, A., HEMMINKI, K., & VAINIO, H. (1983) Embryotoxic effects
of acrolein, methylacrylates, guanidines and resorcinol on three day
chicken embryos. Acta pharmacol. toxicol., 52: 95-99.
KRILL, R.M. & SONZOGNI, W.C. (1986) Chemical monitoring of
Wisconsin's groundwater. J. Am. Water Works Assoc., 78: 70-75
KROKAN, H., GRAFSTROM, R.C., SUNDQVIST, K., ESTERBAUER, H., &
HARRIS, C.C. (1985) Cytotoxicity, thiol depletion and inhibition of
O6-methylguanine-DNA methyltransferase by various aldehydes in
cultured human bronchial fibroblasts. Carcinogenesis, 6: 1755-1759.
KROST, K.J., PELLIZZARI, E.D., WALBURN, S.G., & HUBBARD, S.A. (1982)
Collection and analysis of hazardous organic emissions. Anal.
Chem., 54: 810-817.
KRUYSSE, A. (1971) Acute inhalation toxicity of acrolein in
hamsters, Zeist, The Netherlands, Central Institute for Nutrition
and Food Research, TNO (Report No. R3516).
KU, R.H. & BILLINGS, R.E. (1986) The role of mitochondrial
glutathione and cellular protein sulfhydryls in formaldehyde
toxicity in glutathione-depleted rat hepatocytes. Arch. Biochem.
Biophys., 247: 183-189.
KUBINSKI, H., GUTZKE, G.E., & KUBINSKI, Z.O. (1981) DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
KUTZMAN, R.S., MEYER, G.-J., & WOLF, A.P. (1982) The biodistribution
and metabolic fate of [11C]-acrylic acid in the rat after acute
inhalation exposure or stomach intubation. J. Toxicol. environ.
Health, 10: 969-979.
KUTZMAN, R.S., WEHNER, R.W., & HABER, S.B. (1984) Selected responses
of hypertension sensitive and resistant rats to inhaled acrolein.
Toxicology, 31: 53-65.
KUTZMAN, R.S., POPENOE, E.A., SCHMAELER, M., & DREW, R.T. (1985)
Changes in rat lung structure and composition as a result of
subchronic exposure to acrolein. Toxicology, 34: 139-151.
KUWATA, K., UEBORI, M., YAMASAKI, H., KUGE, Y., & KISO, Y. (1983)
Determination of aliphatic aldehydes in air by liquid
chromatography. Anal. Chem., 55: 2013-2016.
LACROIX, M., BURCKEL, H., FOUSSEREAU, J., GROSSHANS, E., CAVELIER,
C., LIMASSET, J.C., DUCOS, P., GRADINSKI, D., & DUPRAT, P. (1976)
Irritant dermatitis from diallylglycol carbonate monomer in the
optical industry. Contact dermatitis, 2: 183-195.
LAM, C.-W., CASANOVA, M., & HECK, H.D'A. (1985) Depletion of nasal
mucosal glutathione by acrolein and enhancement of
formaldehyde-induced DNA-protein cross-linking by simultaneous
exposure to acrolein. Arch. Toxicol., 58: 67-71.
LEACH, P.W., LENG, L.J., BELLAR, T.A., SIGSBY, J.E., & ALTSHULLER,
A.P. (1964) Effects of HC/NOx ratios on irradiated auto exhaust,
part II. J. Air Pollut. Control Assoc., 14: 176-183.
LEACH, C.L., HATOUM, N.S., RATAJCZAK, H.V., & GERHART, J.M. (1987)
The pathologic and immunologic effects of inhaled acrolein in rats.
Toxicol. Lett., 39: 189-198.
LEBLANC, G.A. (1980) Acute toxicity of priority pollutants to water
flea (Daphnia magna). Bull. environ. Contam. Toxicol., 24:
684-691.
LE BOUFFANT, L., MARTIN, J.C., DANIEL, H., HENIN, J.P., & NORMAND,
C. (1980) Action of intensive cigarette smoke inhalations on the rat
lung. Role of particulate and gaseous cofactors. J. Natl Cancer
Inst., 64: 273-281.
LEFFINGWELL, C.M. & LOW, R.B. (1979) Cigarette smoke components and
alveolar macrophage protein synthesis. Arch. environ. Health, 34:
97-102.
LENCREROT, P., PARFAIT, A., & JOURET, C. (1984) Rôle des
corynebactéries dans la production d'acroléine (2-propenal) dans les
rhums. Ind. aliment. agric., 101: 763-765.
LEONARDOS, G., KENDALL, D., & BARNARD, N. (1969) Odor threshold
determinations of 53 odorant chemicals. J. Air Pollut. Control
Assoc., 19: 91-95.
LEUCHTENBERGER, C., SCHUMACHER, M., & HADIMANN, T. (1968) Further
cytological and cytochemical studies on the biological significance
of the gas phase of fresh cigarette smoke. Z. Präventivmed., 13:
130-141.
LEVAGGI, D.A. & FELDSTEIN, M. (1970) The determination of
formaldehyde, acrolein, and low molecular weight aldehydes in
industrial emissions on a single collection sample. J. Air Pollut.
Control Assoc., 20: 312-313.
LIES, K.-H., POSTULKA, A., GRING, H., & HARTUNG, A. (1986) Aldehyde
emissions from passenger cars. Staub-Reinhalt. Luft, 46: 136-139.
LIJINSKY, W. & ANDREWS, A.W. (1980) Mutagenicity of vinyl compounds
in Salmonella typhimurium. Teratogen. Carcinogen. Mutagen., 1:
259-267.
LIJINSKI, W. & REUBER, M.D. (1987) Chronic carcinogenesis studies of
acrolein and related compounds. Toxicol. ind. Health, 3: 337-345.
LIPARI, F. & SWARIN, S.J. (1982) Determination of formaldehyde and
other aldehydes in automobile exhaust with an improved
2,4-dinitrophenylhydrazine method. J. Chromatogr., 247: 297-306.
LIPARI, F., DASCH, J.M., & SCRUGGS, W.F. (1984) Aldehyde emissions
from wood-burning fireplaces. Environ. Sci. Technol., 18: 326-330.
LIU, Y. & TAI, H.-H. (1985) Inactivation of pulmonary NAD-dependent
15-hydroxyprostaglandin dehydrogenase by acrolein. Biochem.
Pharmacol., 34: 4275-4278.
LOQUET, C., TOUSSAINT, G., & LETALAER, J.Y. (1981) Studies on
mutagenic constituents of apple brandy and various alcoholic
beverages collected in Western France, a high incidence area for
oesophageal cancer. Mutat. Res., 88: 155-164.
LORZ, H.W., GLENN, S., WILLIAMS, R.H., KUNKEL, C.M., NORRIS, L.A., &
LOPER, B.R. (1979) Effects of selected herbicides on Smolting
Salmon, Corvallis, Oregon, USA Environmental Protection Agency,
Environment Research Laboratory Corvallis, Office of Research and
Development (Report EPA-600/3-79-071).
LOW, E.S., LOW, R.B., & GREEN, G.M. (1977) Correlated effects of
cigarette smoke components on alveolar macrophage adenosine
triphosphatase activity and phagocytosis. Am. Rev. respir. Dis.,
115: 963-970.
LUTZ, D., EDER, E., NEUDECKER, T., & HENSCHLER, D. (1982)
Structure-mutagenicity relationship in alpha,ß-unsaturated
carbonylic compounds and their corresponding allylic alcohols.
Mutat. Res., 93: 305-315.
LYMAN, W.J., REEHL, W.F., & ROSENBLATT, D.H. (1982) Handbook of
chemical property estimation methods, New York, McGraw-Hill Book
Company.
LYON, J.P., JENKINS, L.J., JONES, R.A., COON, R.A., & SIEGEL, J.
(1970) Repeated and continuous exposure of laboratory animals to
acrolein. Toxicol. appl. Pharmacol., 17: 726-732.
MACEK, K.J., LINDBERG, M.A., SAUTER, S., BUXTON, K.S., & COSTA, P.A.
(1976) Toxicity of four pesticides to water fleas and fathead
minnows, Duluth, Minnesota, US Environmental Protection Agency,
Environmental Research Laboratory, Office of Research and
Development, (Ecological Research Series EPA-600/3-76-099).
MCNULTY, M.J., HECK, H.D'A., & CASANOVA-SCHMITZ, M. (1984) Depletion
of glutathione in rat respiratory mucosa by inhaled acrolein. Fed.
Proc., 43: 575 (abstract).
MAGIN, D.F. (1980) Gas chromatography of simple monocarbonyls in
cigarette whole smoke as the benzyloxime derivatives. J.
Chromatogr., 202: 255-261.
MALDOTTI, A., CHIORBOLI, C., BIGNOZZI, C.A., BARTOCCI, C., &
CARASSITI, V. (1980) Photooxidation of 1,3-butadiene containing
systems: rate constant determination for the reaction of acrolein
with OH radicals. Int. J. chem. Kinet., 12: 905-913.
MANITA, M.D. & GOLDBERG, E.K. (1970) [Spectrophotometric analysis of
airborne acrolein using the semithiocarbazide reagent.] Gig. i
Sanit., 35(5): 63-65 (in Russian).
MANNING, D.L., MASKARINEC, M.P., JENKINS, R.A., & MARSHALL, A.H.
(1983) High performance liquid chromatographic determination of
selected gas phase carbonyl in tobacco smoke. J. Assoc. Off. Anal.
Chem., 66: 8-12.
MARANO, F. & DEMESTERE, M. (1976) Ultrastructural autoradiographic
study of the intracellular fixation of 3H-acrolein. Experientia
(Basel), 32: 501-503.
MARANO, F. & PUISEUX-DAO, S. (1982) Acrolein and cell cycle.
Toxicol. Lett., 14: 143-149.
MARINELLO, A.J., GURTOO, H.L., STRUCK, R.F., & PAUL, B. (1978)
Denaturation of cytochrome P-450 by cyclophosphamide metabolites.
Biochem. Biophys. Res. Commun., 83: 1347-1353.
MARINELLO, A.J., BERRIGAN, M.J., STRUCK, R.F., GUENGERICH, F.P., &
GURTOO, H.L. (1981) Inhibition of NADPH-cytochrome P450 reductase by
cyclophosphamide and its metabolites. Biochem. biophys. Res.
Commun., 99: 399-406.
MARINELLO, A.J., BANSAL, S.K., PAUL, B., KOSER, P.L., LOVE, J.,
STRUCK, R.F., & GURTOO, H.L. (1984) Metabolism and binding of
cyclophosphamide and its metabolite acrolein to rat hepatic
microsomal cytochrome P-450. Cancer Res., 44: 4615-4621.
MARNETT, L.J., HURD, H.K., HOLLSTEIN, M.C., LEVIN, D.E., ESTERBAUER,
H., & AMES, B.N. (1985) Naturally occurring carbonyl compounds are
mutagens in Salmonella tester strain TA 104. Mutat. Res., 148:
25-34.
MASARU, N., SYOZO, F., & SABURO, K. (1976) Effects of exposure to
various injurious gases on germination of lily pollen. Environ.
Pollut., 11: 181-187.
MASEK, V. (1972) [Aldehydes in the air at working places in coal and
pitch coking plants.] Staub-Reinhalt. Luft, 32: 335-336 (in
German).
MASLOWSKA, J. & BAZYLAK, G. (1985) Thermofractographic determination
of monocarbonyl compounds in animal waste fats used as feed fat.
Anim. Feed Sci. Technol., 13: 227-236.
METTIER, S.R., BOYER, H.K., HINE, C.H., & MCEWEN, W.K. (1960) A
study of the effects of air pollutants on the eye. Am. Med. Assoc.
Arch. ind. Health, 21: 13-18.
MILLS, D.E., BAUGH, W.D., & CONNER, H.A. (1954) Studies on the
formation of acrolein in distillery mashes. Appl. Microbiol., 2:
9-13.
MIRKES, P.E., GREENAWAY, J.C., ROGERS, J.G., & BRUNDRETT, R.B.
(1984) Role of acrolein in cyclophosphamide teratogenicity in rat
embryos in vitro. Toxicol. appl. Pharmacol., 72: 281-291.
MIYAMOTO, Y. (1986) Eye and respiratory irritants in jet engine
exhaust. Aviat. Space environ. Med., 57: 1104-1108.
MORIKAWA, T. (1976) Acrolein, formaldehyde, and volatile fatty acids
from smoldering combustion. J. Combust. Toxicol., 3: 135-150.
MORIKAWA, T. & YANAI, E. (1986) Toxic gases evolution from
air-controlled fires in a semi-full scale room. J. Fire Sci., 4:
299-314.
MOULE, Y. & FRAYSSINET, C. (1971) Effects of acrolein on
transcription in vitro. Fed. Eur. Biochem. Soc. Lett., 16:
216-218.
MULDERS, E.J. & DHONT, J.H. (1972) The odour of white bread. III.
Identification of volatile carbony compounds and fatty acids. Z.
Lebensm. Unters. Forsch., 150: 228-232.
MUNSCH, N. & FRAYSSINET, C. (1971) Action de l'acroléine sur les
syntheses d'acides nucléiques in vivo. Biochimie, 53: 243-248.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1973) Effects of
acrolein on DNA synthesis in vitro. Fed. Eur. Biochem. Soc.
Lett., 30: 286-289.
MUNSCH, N., MARANO, F., & FRAYSSINET, C. (1974a) Incorporation
d'acroleine 3H dans le foie du rat et chez Dunaliella bioculata.
Biochimie, 56: 1433-1436.
MUNSCH, N., DE RECONDO, A.-M., & FRAYSSINET, C. (1974b) In vitro
binding of 3H-acrolein to regenerating rat liver DNA polymerase.
Experientia, 30: 1234-1236.
MURPHY, S.D. (1965) Mechanism of the effect of acrolein on rat liver
enzymes. Toxicol. appl. Pharmacol., 7: 833-843.
MURPHY, S.D. & PORTER, S. (1966) Effects of toxic chemicals on some
adaptive liver enzymes, liver glycogen, and blood glucose in fasted
rats. Biochem. Pharmacol., 15: 1665-1676.
MURPHY, S.D., KLINGSHIRN, D.A., & ULRICH, C.E. (1963) Respiratory
response of guinea pigs during acrolein inhalation and its
modification by drugs. J. Pharmacol. exp. Ther., 141: 79-83.
MURPHY, S.D., DAVIS, H.V., & ZARATZIAN, V.L. (1964) Biochemical
effects in rats from irritating air contaminants. Toxicol. appl.
Pharmacol., 6: 520-528.
NATUSCH, D.F.S. (1978) Potentially carcinogenic species emitted to
the atmosphere by fossil-fueled power plants. Environ. Health
Perspect., 22: 79-90.
NIELSEN, G.D., BAKBO, J.C., & HOLST, E. (1984) Sensory irritation
and pulmonary irritation by airborne allyl acetate, allyl alcohol,
and allyl ether compared to acrolein. Acta pharmacol. toxicol., 54:
292-298.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1986) Determination of
micro amounts of acrolein in air by gas chromatography. J.
Chromatogr., 370: 327-332.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987a) Gas chromatographic
determination of acrolein in rain water using bromination of
O-methyloxime. Analyst, 112: 45-48.
NISHIKAWA, H., HAYAKAWA, T., & SAKAI, T. (1987b) Determination of
acrolein and crotonaldehyde in automobile exhaust gas by gas
chromatography with electron-capture detection. Analyst, 112:
859-862.
OBERDORFER, P.E. (1971) The determination of aldehydes in automobile
exhaust gas. In: Vehicle emissions, Part III, Society of Automotive
Engineers Prog. Tech., Vol. 14, pp. 32-42.
OHARA, T., SATO, T., SHIMUZU, N., PRESCHER, G., SCHWIND, H., &
WEIBURG, O. (1987) Acrolein and methacrolein. In: Encyclopedia of
chemical technology, Deerfield Beech, Florida, Ullmann Verlag
Chemie.
O'LOUGHLIN, E.M. & BOWMER, K.H. (1975) Dilution and decay of aquatic
herbicides in flowing channels. J. Hydrol., 26: 217-235.
OLSON, K.L. & SWARIN, S.J. (1985) Determination of aldehydes and
ketones by derivatization and liquid chromatography-mass
spectrometry. J. Chromatogr., 333: 337-347.
OSBORNE, A.D., PITTS, J.N., & DARLEY, E.F. (1962) On the stability
of acrolein towards photooxidation in the near ultra-violet. Int.
J. Air Water Pollut., 6: 1-3.
PATEL, J.M., WOOD, J.C., & LEIBMAN, K.C. (1980) The
biotransformation of allyl alcohol and acrolein in rat liver and
lung preparations. Drug Metab. Disposal, 8: 305-308.
PATEL, J.M., ORTIZ, E., KOLMSTETTER, C., & LEIBMAN, K.C. (1984)
Selective inactivation of rat lung and liver microsomal
NADPH-cytochrome c reductase by acrolein. Drug Metab. Disposal, 12:
460-463.
PEREZ, J.M., LIPARI, F., & SEIZINGER, D.E. (1984) Cooperative
development of analytical methods for diesel emissions and
particulates - solvent extractables, aldehydes and sulfate methods.
In: Diesel exhaust emissions, Society of Automotive Engineers, pp.
95-114 (Special Publication No. 578).
PETTERSSON, B., CURVALL, M., & ENZELL, C.R. (1980) Effects of
tobacco smoke compounds on the noradrenaline induced oxidative
metabolism in isolated brown fat cells. Toxicology, 18: 1-15.
PFAFFLI, P. (1982) III. Industrial hygiene measurements. Scand. J
Work Environ. Health, 8(suppl. 2): 27-43.
PHILIPPIN, C., GILGEN, A., & GRANDJEAN, E. (1970) Etude
toxicologique et physiologique de l'acroléine chez la souris. Int.
Arch. Arbeitsmed., 26: 281-305.
PILOTTI, A., ANCKER, K., ARRHENIUS, E., & ENZELL, C. (1975) Effects
of tobacco and tobacco smoke constituents on cell multiplication in
vitro. Toxicology, 5: 49-62.
PLOTNIKOVA, M.M. (1957) [Data on hygienic evaluation of acrolein as
a pollution of the atmosphere.] Gig. i Sanit., 22(6): 10-15 (in
Russian).
POSTEL, W. & ADAM, L. (1983) [Gas chromatographic characterization
of raspberry brandy.] Dtsch. Lebensm. Rundschau, 79: 117-122 (in
German).
POTTS, W.J., LEDERER, T.S., & QUAST, J.F. (1978) A study of the
inhalation toxicity of smoke produced upon pyrolysis and combustion
of polyethylene foams. Part I. Laboratory studies. J. Combust.
Toxicol., 5: 408-433.
PRESSMAN, D. & LUCAS, H.J. (1942) Hydration of unsaturated
compounds. XI. Acrolein and acrylic acid. J. Am. Chem. Soc., 64:
1953-1957.
PROTSENKO, G.A., TRUBILKO, V.I., & SAVCHENKOV, V.A. (1973) Working
conditions when metals to which primer has been applied are welded
evaluated from the health and hygiene aspect. Avt. Svarka, 2:
65-68.
RANDALL, T.L. & KNOPP, P.V. (1980) Detoxification of specific
organic substances by wet oxidation. J. Water Pollut. Control Fed.,
52: 2117-2130.
RAPOPORT, I.A. (1948) [Mutations under the influence of unsaturated
aldehydes.] Dokl. Akad. Nauk. SSSR, 16: 713-715 (in Russian).
RATHKAMP, G., TSO, T.C., & HOFFMANN, D. (1973) Chemical studies on
tobacco smoke. XX: Smoke analysis of cigarettes made from bright
tobaccos differing in variety and stalk positions. Beitr.
Tabakforsch., 7: 179-189.
RENZETTI, N.A. & BRYAN, R.J. (1961) Atmospheric sampling for
aldehydes and eye irritation in Los Angeles smog. J. Air Pollut.
Control Assoc., 11: 421-424, 427.
RICHTER, M. & ERFURTH, I. (1979) [On the gas chromatographic
determination of acrolein in its original state in the main stream
smoke of cigarettes.] Ber. Inst. Tabaksforsch. 26: 36-45 (in
German).
RICKERT, W.S., ROBINSON, J.C., & YOUNG, J.C. (1980) Estmating the
hazards of "less hazardous" cigarettes. I. Tar, nicotine, carbon
monoxide, acrolein, hydrogen cyanide, and total aldehyde deliveries
of Canadian cigarettes. J. Toxicol. environ. Health, 6: 351-365.
RIETZ, B. (1985) Determination of three aldehydes in the air of
working environments. Anal. Lett., 18(A19): 2369-2379.
RIJSTENBIL, J.W. & VAN GALEN, G.C. (1981) Chemical control of mussel
settlement in a cooling water system using acrolein. Environ,
Pollut., A25: 187-195.
RIKANS, L.E. (1987) The oxidation of acrolein by rat liver aldehyde
dehydrogenases. Relation to allyl alcohol hepatoxicity. Metab.
Disposal, 15: 356-362.
ROSENTHALER, L. & VEGEZZI, G. (1955) [Acrolein in spirits.] Z.
Lebensm. Unters. Forsch., 102: 117-123 (in German).
RYLANDER, R. (1973) Toxicity of cigarette smoke components: free
lung cell response in acute exposures. Am. Rev. respir. Dis., 108:
1279-1282.
SAITO, T., TAKASHINA, T., YANAGISAWA, S., & SHIRAI, T. (1983)
[Determination of trace low molecular weight aliphatic compounds in
auto exhaust by gas chromatography with a glass capillary column.]
Bunseki Kagaku, 32: 33-38 (in Japanese).
SAKATA, T., SMITH, R.A., GARLAND, E.M., & COHEN, S.M. (1989) Rat
urinary bladder epithelial lesions induced by acrolein. J. environ.
Pathol. Toxicol., 9: 159-170.
SALAMAN, M.H. & ROE, F.J.C. (1956) Further tests for tumour-
initiating activity: N,N-di-(2-chloroethyl)-p-aminophenylbutyric
acid (CB1348) as an initiator of skin tumour formation in the mouse.
Br. J. Cancer, 10: 363-378.
SALEM, H. & CULLUMBINE, H. (1960) Inhalation toxicities of some
aldehydes. Toxicol. appl. Pharmacol., 2: 183-187.
SCHAFER, E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds. Toxicol. appl.
Pharmacol., 21: 315-330.
SCHIELKE, D.-J. (1987) [Gastrectomy following a rare caustic
lesion.] Chirurg, 58: 50-52 (in German).
SCHMID, B.P., GOULDING, E., KITCHIN, K., & SANYAL, M.K. (1981)
Assessment of the teratogenic potential of acrolein and
cyclophosphamide in a rat embryo culture system. Toxicology, 22:
235-243.
SCHUCK, E.A. & RENZETTI, N.A. (1960) Eye irritants formed during
photo-oxidation of hydrocarbons in the presence of oxides of
nitrogen. J. Air Pollut. Control Assoc., 10: 389-392.
SCHUTTE, N.P. (1977) Hazard evaluation and technical assistance
report No. TA 77-11: Xomed Company, Cincinnati, Ohio, Cincinnati,
Ohio, USA, National Institute for Occupational Safety and Health
(NIOSH-TR-TA-77-11, PB 278834).
SEIZINGER, D.E. & DIMITRIADES, B. (1972) Oxygenates in exhaust from
simple hydrocarbon fuels. J. Air Pollut. Control Assoc., 22: 47-51.
SERTH, R.W., TIERNEY, D.R., & HUGHES, T.W. (1978) Source
assessment: acrylic acid manufacture, Cincinnati, Ohio, US
Environmental Protection Agency, Industrial Environmental Research
Laboratory (Report EPA-600/2-78-004w).
SHAPIRO, R., SODUM, R.S., EVERETT, D.W., & KUNDU, S.K. (1986)
Reactions of nucleosides with glyoxal and acrolein. In: The role of
cyclic nucleic acid adducts in carcinogenesis and mutagenesis,
Proceedings of a meeting organized by the IARC and co-sponsored by
the US National Cancer Institute, and the Lawrence Berkely
Laboratory at the University of California, Lyon, 17-19 September,
Lyon, International Agency for Research on Cancer, pp. 165-173 (IARC
Scientific Publications No. 70).
SHERWOOD, R.L., LEACH, C.L., HATOUM, N.S., & ARANYI, C. (1986)
Effects of acrolein on macrophage functions in rats. Toxicol.
Lett., 32: 41-49.
SHIMOMURA, M., YOSHIMATSU, F., & MATSUMOTO, F. (1971) [Fish odour of
cooked horse mackerel.] Kaseigaku Zasshi, 2: 106-112 (in Japanese).
SIM, V.M. & PATTLE, R.E. (1957) Effect of possible smog irritants on
human subjects. J. Am. Med. Assoc., 165: 1908-1913.
SINKUVENE, D. (1970) [Hygienic assessment of acrolein as an
atmospheric pollutant.] Gig. i Sanit., 35(3): 6-10 (in Russian).
SKOG, E. (1950) A toxicological investigation of lower aliphatic
aldehydes. I. Toxicity of formaldehyde, acetaldehyde,
propionaldehyde and butyraldehyde, as well as of acrolein and
crotonaldehyde. Acta pharmacol., 6: 299-318.
SLOTT, V.L. & HALES, B.F. (1985) Teratogenicity and embryolethality
of acrolein and structurally related compounds in rats. Teratology,
32: 65-72.
SLOTT, V.L. & HALES, B.F. (1986) The embryolethality and
teratogenicity of acrolein in cultured rat embryos. Teratology, 34:
155-163.
SLOTT, V.L. & HALES, B.F. (1987a) Enhancement of the embryotoxicity
of acrolein, but not phosphoramide mustard, by glutathione depletion
in rat embryos in vitro. Biochem. Pharmacol., 36: 2019-2025.
SLOTT, V.L. & HALES, B.F. (1987b) Protection of rat embryos in
culture against the embryotoxicity of acrolein using exogenous
glutathione., Biochem. Pharmacol., 36: 2087-2194.
SMITH, R.A., COHEN, S.M., & LAWSON, T.A. (1990) Acrolein
mutagenicity in the V79 assay. Carcinogenesis, 11: 497-498.
SMYTH, H.F., CARPENTER, C.P., & WEIL, C.S. (1951) Range-finding
toxicity data: list IV. Arch. ind. Hyg. occup. Med., 4: 119-122.
SMYTHE, R.J. & KARASEK, F.W. (1973) The analysis of diesel engine
exhaust for low-molecular-weight carbonyl compounds. J.
Chromatogr., 86: 228-231.
SNYDER, J.M., FRANKEL, E.N., & SELKE, E. (1985) Capillary gas
chromatographic analyses of headspace volatiles from vegetable oils.
J. Am. Oil Chem. Soc., 6: 1675-1679.
SPONHOLZ, W.-R. (1982) [Analysis and occurrence of aldehydes in
wines.] Z. Lebensm. Unters. Forsch., 174: 458-462 (in German).
SPRINCE, H., PARKER, C.M., & SMITH, G.G. (1979) Comparison of
protection by L-ascorbic acid, L-cysteine, and adrenergic-blocking
agents against acetaldehyde, acrolein, and formaldehyde toxicity:
implications in smoking. Agents Actions, 9: 407-414.
STACK, V.T. (1957) Toxicity of alpha,ß-unsaturated carbonyl
compounds to microorganisms. Ind. eng. Chem., 49: 913-917.
STAHLMANN, R., BLUTH, U., & NEUBERT, D. (1985) Effects of the
cyclophosphamide metabolite acrolein in mammalian limb bud cultures.
Arch. Toxicol., 57: 163-167.
STEINHAGEN, W.H. & BARROW, C.S. (1984) Sensory irritation
structure-activity study of inhaled aldehydes in B6C3F1 and
Swiss-Webster mice. Toxicol. appl. Pharmacol., 72: 495-503.
STEPHENS, E.R., DARLEY, E.F., TAYLOR, O.C., & SCOTT, W.E. (1961)
Photochemical reaction products in air pollution. Int. J. Air Water
Pollut., 4: 79-100.
SUBDEN, R.E., KRIZUS, A., & AKHTAR, M. (1986) Mutagen content of
Canadian apple eau-de-vie. Can. Inst. Food Sci. Technol. J., 19:
134-136.
SUZUKI, Y. & IMAI, S. (1982) Determination of traces of gaseous
acrolein by collection on molecular sieves and fluorimetry with
o-aminobiphenyl. Anal. Chim. Acta, 136: 155-162.
SWARIN, S.J. & LIPARI, F. (1983) Determination of formaldehyde and
other aldehydes by high performance liquid chromatography with
fluorescence detection. J. liq. Chromatogr., 6: 425-444.
SZOT, R.J. & MURPHY, S.D. (1971) Relationships between cyclic
variations in adrenocortical secretory activity in rats and the
adrenocortical response to toxic chemical stress. Environ. Res.,
4: 530-538.
TABAK, H.H., QUAVE, S.A., MASHNI, C.E., & BARTH, E.F. (1981)
Biodegradability studies with organic priority pollutant compounds.
J. Water Pollut. Control Fed., 53: 1503-1517.
TAJIMA, M., KIDA, K., & FUJIMAKI, M. (1967) Effect of gamma
irradiation on volatile compounds from cooked potato. Agric. biol.
Chem., 31: 935-938.
TAKEUCHI, K. & IBUSUKI, T. (1986) Heterogeneous photochemical
reactions of a propylene-nitrogen dioxide-metal oxide-dry air
system. Atmos. Environ., 20: 1155-1160.
TEJADA, S.B. (1986) Evaluation of silica gel cartridges coated in
situ with acidified 2,4-dinitrophenylhydrazine for sampling
aldehydes and ketones in air. Int. J. environ. anal. Chem., 26:
167-185.
TESTA, A. & JOIGNY, C. (1972) Dosage par chromatographie en phase
gazeuse de l'acroléine et d'autres composés alpha,ß-insaturés de la
phase gazeuse de la fumée de cigarette. Ann. Serv. Exploit. ind.
Tab. Allumettes - Div. Etud. Equip., 10: 67-81.
TORAASON, M., LUKEN, M.E., BREITENSTEIN, M., KRUEGER, J.A., &
BIAGINI, R.E. (1989) Comparative toxicity of allylamine and acrolein
in cultured myocytes and fibroblasts from neonatal rat heart.
Toxicology, 56: 107-117.
TREITMAN, R.D., BURGESS, W.A., & GOLD, A. (1980) Air contaminants
encountered by firefighters. Am. Ind. Hyg. Assoc. J., 41: 796-802.
TURUK-PCHELINA, Z.F. (1960) [Acrolein releases into the air while
cooking.] Gig. i Sanit., 25(5): 96-97 (in Russian).
UMANO, K. & SHIBAMOTO, T. (1987) Analysis of headspace volatiles
from overheated beef fat. J. agric. food Chem., 35: 14-18.
UNRAU, G.O., FAROOQ, M., DAWOOD, I.K., MIGUEL, L.C., & DAZO, B.C.
(1965) Field trials in Egypt with acrolein herbicide-molluscicide.
World Health Organ. Bull., 32: 249-260.
US-NIOSH (1984) NIOSH manual of analytical methods, 3rd ed.,
Cincinnati, Ohio, National Institute for Occupational Safety and
Health (DHHS (NIOSH) Publication No. 84-100. Method 2501).
VAN EICK, A.J. (1977) [The effect of acrolein in air on the eye
blinking frequency of man and guinea pig], Rijswijk, The
Netherlands, Technical and Physical Research, Medical Biological
Laboratory, TNO-MBL, (Report No. A76/K/098) (in Dutch).
VAN OVERBEEK, J., HUGHES, W.J., & BLONDEAU, R. (1959) Acrolein for
the control of water weeds and disease-carrying water snails.
Science, 129: 335-336.
VEITH, G.D., MACEK, K.J., PETROCELLI, S.R., & CARROL, J. (1980) An
evaluation of using partition coefficients and water solubility to
estimate bioconcentration factors for organic chemicals in fish.
Aquat. Toxicol.: 116-129.
VOLKOVA, Z.A. & BAGDINOV, Z.M. (1969) [Industrial hygiene problems
in vulcanization processes of rubber production]. Gig. i Sanit.,
34: 33-40 (in Russian).
VOROB'EVA, A.I., VOLKOTRUB, L.P., FEOKTISTOVA, N.F., BOBIN, V.I.,
SHESTAKOVA, N.A., & USHAKOVA, N.S. (1982) [Characteristics of
atmospheric pollution from enameled wire manufacturing plants.]
Gig. i Sanit., 1982(6): 66-67 (in Russian).
WARHOLM, M., HOLMBERG, B., & MALMFORS, T. (1984) The cytotoxicity of
some organic solvents. Environ. Res., 7: 183-192.
WATANABE, T. & AVIADO, D.M. (1974) Functional and biochemical
effects on the lung following inhalation of cigarette smoke and
constituents. II. Skatole, acrolein, and acetaldehyde. Toxicol.
appl. Pharmacol. 30: 201-209.
WEBER-TSCHOPP, A., FISCHER, T., & GRANDJEAN, E. (1976) [Objective
and subjective physiological effects of passive smoking.] Int. Arch.
occup. environ. Health, 37: 277-288 (in German).
WEBER-TSCHOPP, A., FISCHER, T., GIERER, R., & GRANDJEAN, E. (1977)
[Experimental irritation by acrolein in human beings.] Z.
Arbeitswiss., 32: 166-171 (in German).
WHITEHOUSE, M.W., BECK, F.W.J., DROGE, M.M., & STRUCK, R.F. (1974)
Lymphocyte deactivation by (potential immunosuppressant) alkylating
metabolites of cyclophosphamide. Agents Actions, 4: 117-123.
WHO (1991) Health and Safety Guide 67: Acrolein, Geneva, World
Health Organization.
WILMER, J.L., EREXSON, G.L., & KLIGERMAN, A.D. (1986) Attenuation of
cytogenetic damage by 2-mercaptoethanesulphonate in cultured human
lymphocytes exposed to cyclophosphamide and its reactive
metabolites. Cancer Res., 46: 203-210.
WILTON, D.C. (1976) Acrolein, an irreversible active-site-directed
inhibitor of deoxyribose 5-phosphate aldolase? Biochem. J., 153:
495-497.
WITZ, G., LAWRIE, N.J., AMORUSO, M.A., & GOLDSTEIN, B.D. (1987)
Inhibition by reactive aldehydes of superoxide anion radical
production from stimulated polymorphonuclear leukocytes and
pulmonary alveolar macrophages. Biochem. Pharmacol., 36: 721-726.
WRABETZ, E., PETER, G., & HOHORST, H.J. (1980) Does acrolein
contribute to the cytotoxicity of cyclophosphamide? J. Cancer Res.,
98: 119-126.
ZIMMERING, S., MASON, J.M., VALENCIA, R., & WOODRUFF, R. (1985)
Chemical mutagenesis testing in Drosophila. II. Results of 20
coded compounds tested for the National Toxicology Program.
Environ. Mutagen., 7: 87-100.
ZITTING, A. & HEINONEN, T. (1980) Decrease of reduced glutathione in
isolated rat hepatocytes caused by acrolein, acrylonitril, and the
thermal degradation products of styrene copolymers. Toxicology, 17:
333-341.
ZOLLNER, H. (1973) Inhibition of some mitochondrial functions by
acrolein and methylvinylketone. Biochem. Pharmacol., 22: 1171-1178.
ZORIN, V.M. (1966) [Acrolein-induced air pollution.] Zdravookhr.
Belorussii, 1966(7): 43-44 (in Russian).
RESUME
L'acroléine est un liquide volatil extrêmement inflammable dont
l'odeur, âcre et suffocante, est très désagréable. C'est un composé
très réactif.
En 1975, on estime que la production mondiale d'acroléine en
tant que telle était de 59 000 tonnes. On en produit et consomme
encore davantage comme intermédiaire pour la synthèse de l'acide
acrylique et de ses esters.
On dispose d'un certain nombre de méthodes d'analyse pour la
recherche et le dosage de l'acroléine dans divers milieux. On a fait
état de limites inférieures de détection de l'ordre de 0,1 µg/m3
dans l'air (chromatographie en phase gazeuse/spectrométrie de
masse), de 0,1 µg/l dans l'eau (chromatographie liquide à haute
pression), de 2,8 µg/litre dans les milieux biologiques
(fluorimétrie), de 590 µg/kg dans le poisson (chromatographie en
phase gazeuse/spectrométrie de masse) et de 1,4 µg/m3 dans les gaz
d'échappement (chromatographie liquide à haute pression).
On a trouvé de l'acroléine dans certains produits d'origine
végétale et animale et notamment dans des denrées alimentaires et
des boissons. L'acroléine est utilisée principalement comme
intermédiaire en synthèse organique, mais également comme produit
biocide en milieu aquatique.
Des émissions d'acroléine peuvent se produire sur les lieux de
production ou d'utilisation. Elles peuvent être importantes dans
l'air à la suite de la combustion ou de la pyrolyse incomplète de
produits organiques tels que combustibles, polymères de synthèse,
dans certains aliments et le tabac. L'acroléine peut représenter
jusqu'à 3-10 % des aldéhydes totaux présents dans les gaz
d'échappement des véhicules à moteur. La consommation d'une
cigarette fournit de 3 à 228 µg d'acroléine. L'acroléine est un
produit d'oxydation photochimique de certains polluants organiques
de l'atmosphère.
La population générale est essentiellement exposée par
l'intermédiaire de l'air. Une exposition peut également se produire
par voie orale par suite de la consommation de boissons alcoolisées
ou de denrées alimentaires chauffées.
On a mesuré dans l'air des villes des concentrations moyennes
d'acroléine atteignant environ 15 µg/m3 avec des maxima allant
jusqu'à 32 µg/m3. A proximité d'installations industrielles et de
pots d'échappement, des concentrations dix à cent fois plus élevées
sont possibles. Les incendies peuvent donner naissance à des teneurs
très élevées d'acroléine, de l'ordre du mg/m3 d'air. A
l'intérieur des habitations, on a observé que la consommation d'une
cigarette par m3 d'air dans un local en l'espace de 10 à
13 minutes produisait des concentrations en vapeurs d'acroléine de
l'ordre de 450 à 840 µg/m3. Sur les lieux de travail, on a signalé
des teneurs dépassant 100 µg/m3 dans des cas où l'on élevait la
température de certains produits organiques, par exemple lors du
chauffage ou du soudage de ces substances.
Dans l'atmosphère, l'acroléine est dégradée par réaction avec
les radicaux hydroxyles. Sa durée de séjour dans l'atmosphère est de
l'ordre d'une journée. Dans les eaux de surface, l'acroléine se
dissipe en quelques jours. Elle est faiblement adsorbée aux
particules du sol. On a fait état de dégradation aérobie et
anaérobie, encore que la toxicité du composé pour les
micro-organismes puisse faire obstacle à sa biodégradation. Compte
tenu des propriétés physiques et chimiques de l'acroléine, il ne
semble pas que cette substance ait une tendance à la
bioaccumulation.
L'acroléine est extrêmement toxique pour les organismes
aquatiques. Pour les bactéries, les algues, les crustacés et les
poissons, sa toxicité aiguë, estimée d'après les valeurs de la CE50
et de la CL50, se situe entre 0,02 et 2,5 mg/litre, les bactéries
étant l'espèce la plus sensible. Pour le poisson, on a fixé à
0,0114 mg/litre la dose sans effet nocif observable à 60 jours.
L'acroléine détruit efficacement les végétaux aquatiques à des doses
comprises entre 4 et 26 mg/litre.h. A partir de 15 mg/litre, on
observe des effets nocifs sur les cultures irriguées au moyen d'eau
traitée à l'acroléine.
Chez l'homme et l'animal, l'acroléine reste confinée sur son
site d'exposition en raison de sa réactivité et les observations
pathologiques sont également limitées à ce site. Chez des chiens
exposés à des doses de 400 à 600 mg/m3, on a observé un taux de
rétention de 80 à 85 % au niveau des voies respiratoires.
L'acroléine réagit directement sur les groupements sulfhydryles
protéiques et non protéiques ainsi que sur les amines primaires et
secondaires. Elle peut également être métabolisée en acide
mercapturique, en acide acrylique, en glycidaldéhyde ou en
glycéraldéhyde. Les trois derniers métabolites n'ont été observés
qu' in vitro.
L'acroléine est un agent cytotoxique. Sa cytotoxicité s'observe
in vitro dès 0,1 mg/litre. Elle est extrêmement toxique pour les
animaux de laboratoire et l'homme, à la suite d'une seule exposition
quelle qu'en soit la voie. Sa vapeur est irritante pour l'oeil et
les muqueuses respiratoires. Le liquide est corrosif et on a
constaté qu'en solution éthanolique le seuil d'apparition d'une
dermatite d'irritation était de 0,1%. L'expérimentation sur des
volontaires humains exposés à des vapeurs d'acroléine a permis de
fixer à 0,13 mg/m3 la dose la plus faible produisant des effets
nocifs observables; à cette dose, une irritation des yeux se produit
en l'espace de cinq minutes. En outre, les effets au niveau des
voies respiratoires deviennent évidents à partir de 0,7 mg/m3. Une
seule exposition à des doses plus élevées entraîne une
dégénérescence de l'épithélium respiratoire, des séquelles
inflammatoires et une perturbation de la fonction respiratoire.
On a étudié sur des rats, des chiens, des cobayes et des singes
les effets toxicologiques de l'inhalation continue d'acroléine à des
concentrations de 0,5 à 4,1 mg/m3. Des effets histopathologiques
et des effets sur la fonction respiratoire ont été observés chez les
animaux exposés à des teneurs supérieures ou égales à 0,5 mg/m3
pendant 90 jours.
On a étudié sur divers animaux de laboratoire les effets
toxicologiques d'expositions répétées par la voie respiratoire à des
vapeurs d'acroléine, à des concentrations allant de 0,39 mg/m3 à
11,2 mg/m3. La durée de l'exposition allait de cinq jours à
52 semaines. En général, on a fait état chez la plupart des espèces
exposées huit heures par jour à des concentrations de 1,6 mg/m3 ou
davantage, d'une réduction du gain de poids, d'une diminution de la
fonction respiratoire et de modifications pathologiques au niveau du
nez, des voies respiratoires supérieures et des poumons. Parmi les
modifications anatomopathologiques figuraient une inflammation, une
métaplasie et une hyperplasie des voies respiratoires. On a observé
une mortalité importante après expositions répétées à des vapeurs
d'acroléine à des concentrations dépassant 9,07 mg/m3. Chez
l'animal d'expérience, on a montré que l'acroléine provoquait une
déplétion du glutathion tissulaire in vivo et une inhibition des
enzymes in vitro par réaction sur les groupements sulfhydryles au
niveau des sites actifs. Il existe quelques données selon lesquelles
l'acroléine est susceptible d'amoindrir les défenses pulmonaires de
l'hôte chez la souris et le rat.
L'acroléine peut produire des effets tératogènes et
embryotoxiques lorsqu'on l'introduit directement dans l'amnios.
Toutefois, l'absence d'effets chez des lapins à qui elle avait été
injectée par voie intraveineuse à la dose de 3 mg/kg incite à penser
que l'exposition de l'homme à l'acroléine ne devrait pas avoir
d'effet nocif sur le développement de l'embryon.
On a montré que l'acroléine interagissait avec les acides
nucléiques in vitro et en inhibait la synthèse tant in vitro
qu' in vivo. Sans avoir besoin d'être activée, elle produit des
mutations géniques chez les bactéries et les champignons et induit
des échanges entre chromatides soeurs dans les cellules
mammaliennes. Dans tous les cas, ces effets se sont produits dans un
intervalle de dose extrêmement limité qui était fonction de la
réactivité, de la volatilité et de la cytotoxicité de l'acroléine.
Une épreuve de mutation létale dominante chez la souris a donné des
résultats négatifs. Les données disponibles montrent que l'acroléine
est faiblement mutagène pour certains champignons et bactéries et
certaines cultures de cellules mammaliennes.
Des hamsters ont été exposés pendant 52 semaines à des vapeurs
d'acroléine à la dose de 9,2 mg/m3, 7 heures par jour et 5 jours
par semaine, puis ont été placés en observation pendant les 29
semaines suivantes; aucune tumeur n'a été observée. En exposant ces
hamsters dans les mêmes conditions à des vapeurs d'acroléine et
pendant la même durée avec, en outre, des doses intra-trachéennes
hebdomadaires de benzo[a]pyrène ou des doses sous-cutanées une fois
toutes les trois semaines de diéthylnitrosamine, on n'a pas non plus
observé d'effets co-cancérogènes bien nets attribuables à
l'acroléine. Des rats exposés par voie orale à de l'acroléine dans
leur eau de boisson à des doses comprises entre 5 et 50 mg/kg par kg
de poids corporel (quotidiennement, cinq jours par semaine pendant
100 à 124 semaines) n'ont pas présenté de tumeur. En raison du
caractère limité de toutes ces épreuves, on estime que les données
qui permettraient d'évaluer la cancérogénicité de l'acroléine chez
l'animal d'expérience sont encore insuffisantes. De ce fait, il est
impossible pour l'instant d'évaluer la cancérogénicité de
l'acroléine pour l'homme.
Les différents seuils de concentration auxquels apparaissent
les différents effets de l'acroléine sont les suivants : perception
d'une odeur, 0,007 mg/m3, irritation oculaire, 0,3 mg/m3,
irritation du nez et clignement des yeux, 0,03 mg/m3, réduction de
la fréquence respiratoire, 0,7 mg/m3. Comme la concentration de
l'acroléine dépasse rarement 0,03 mg/m3 dans l'air des villes,
elle n'est pas susceptible de constituer une nuisance dans les
circonstances normales.
Du fait de sa forte toxicité pour les organismes aquatiques,
l'acroléine présente un danger pour la faune et la flore aquatique à
proximité ou sur les sites de décharge de déchets industriels, en
cas de déversements et là où l'on utilise ce produit comme biocide.
1. RESUMEN
La acroleína es un líquido volátil, sumamente inflamable, con
un olor pungente, asfixiante y desagradable. Se trata de un
compuesto muy reactivo.
La producción mundial de acroleína aislada se calculó en 59 000
toneladas en 1975. Se produce y consume una cantidad aún mayor de
acroleína como intermediaria en la síntesis de ácido acrílico y sus
ésteres.
Se dispone de métodos analíticos para determinar la acroleína
presente en diversos medios. Los límites mínimos de detección que
se han comunicado son 0,1 µg/m3 de aire (cromatografía
gaseosa/spectrometría de masas), 0,1 µg/litro de agua (cromatografía
líquida a alta presión), 2,8 µg/litro de medio biológico
(fluorimetría), 590 µg/kg en peces (cromatografía
gaseosa/espectrometría de masas), y 1,4 µg/m3 de gases de escape
(cromatografía líquida a alta presión).
La acroleína se ha detectado en algunos vegetales y animales,
inclusive en alimentos y bebidas. La sustancia se usa
principalmente como intermediaria en la síntesis química aunque
también como biocida acuático.
Pueden producirse emisiones de acroleína en sus lugares de
producción o de uso. Las emisiones importantes a la atmósfera se
deben a la combustión incompleta o la pirólisis de materiales
orgánicos como ser combustibles, polímeros sintéticos, alimentos y
tabaco. La acroleína puede representar el 3-10% de los aldehídos
totales presentes en los escapes de automóviles. El humo de un
cigarrillo libera 3-228 µg de acroleína. La acroleína es uno de los
productos de la oxidación fotoquímica de ciertos contaminantes
orgánicos de la atmósfera.
La exposición de la población general se produce principalmente
por el aire. La exposición por vía oral puede producirse por el
consumo de bebidas alcohólicas o alimentos calentados.
En la atmósfera urbana se han medido niveles promedio de
acroleína de hasta unos 15 µg/m3 y niveles máximos de hasta
32 µg/m3. En las cercanías de las industrias y junto a los caños
de escape pueden registrarse niveles entre 10 y 100 veces
superiores. Como resultado de incendios pueden hallarse niveles
sumamente elevados en el aire, del orden de mg/m3. En el aire
cerrado de interiores, el consumo de un cigarrillo por m3 de
volumen de la habitación produjo en 10-13 minutos concentraciones de
vapor de acroleína de 450-840 µg/m3. En el medio ambiente laboral
se han detectado niveles de más de 1000 µg/m3 en situaciones que
entrañaban aumento de temperatura de materiales orgánicos, por
ejemplo durante la soldadura o el calentamiento.
La acroleína se degrada en la atmósfera por reacción con
radicales hidroxilo. El tiempo de persistencia en la atmósfera es
de aproximadamente un día. En aguas de superficie, la acroleína se
disipa en pocos días. Tiene un bajo potencial de adsorción en el
suelo. Se ha observado su degradación en condiciones aerobias y
anaerobias, si bien la toxicidad del compuesto para los
microorganismos puede impedir la biodegradación. En vista de sus
propiedades físicas y químicas, es improbable que se produzca
bioacumulación de acroleína.
La acroleína es sumamente tóxica para los organismos acuáticos.
Los valores de la CE50 y la CL50 correspondientes a bacterias,
algas, crustáceos y peces se encuentran entre 0,02 y 2,5 mg/litro,
siendo las bacterias los organismos más sensibles. En peces se ha
determinado que el nivel sin observación de efectos adversos (NOAEL)
a 60 días es de 0,0114 mg/litro. Se ha conseguido combatir
eficazmente los vegetales acuáticos con dosis de acroleína
comprendidas entre 4 y 26 mg/litro.h. Se han observado efectos
adversos en cultivos que crecen en suelos irrigados con agua tratada
con acroleína en concentraciones de 15 mg/litro o más.
En el animale y en el ser humano la reactividad de la acroleína
limita efectivamente la sustancia al lugar de exposición; los
hallazgos patológicos se limitan asimismo a esos lugares. En el
tracto respiratorio de perros expuestos a 400-600 mg/m3 se
encontró una retención del 80-85% de acroleína. La acroleína
reacciona directamente con los grupos sulfhidrilo contenidos en
radicales proteicos o no proteicos y con aminas primarias y
secundarias. También puede ser metabolizado a ácidos mercaptúricos,
ácido acrílico, glicidaldehído o gliceraldehído. Estos tres últimos
metabolitos sólo se han encontrado in vitro.
La acroleína es un agente citotóxico. Se ha observado
citotoxicidad in vitro con niveles de solamente 0,1 mg/litro. La
sustancia es sumamente tóxica para los animales de experimentación y
el ser humano tras una exposición única por diferentes vías. El
vapor es irritante para los ojos y el tracto respiratorio. En
estado líquido es corrosiva. Con respecto a la dermatitis
irritante, se encontró que el NOAEL de la acroleína etanólica era de
0,1%. Los experimentos con voluntarios humanos expuestos a vapores
de acroleína mostraron un nivel mínimo de observación de efectos
(LOAEL) de 0,13 mg/m3, dosis con la que los ojos pueden irritarse
al cabo de cinco minutos. Además, se observan efectos en el tracto
respiratorio a partir de 0,7 mg/m3. Con exposiciones aisladas a
niveles más altos, aparecen: degeneración del epitelio respiratorio,
secuelas inflamatorias y trastorno de la función respiratoria.
Los efectos toxicológicos de la exposición por inhalación
continua de concentraciones comprendidas entre 0,5 y 4,1 mg/m3 se
han estudiado en la rata, el perro, el cobayo y el mono. Se
observaron efectos sobre la función respiratoria y trastornos
histopatológicos cuando se expuso a los animales a niveles de
acroleína de 0,5 mg/m3 o más, durante 90 días.
Los efectos toxicológicos de la inhalación repetida de vapores
de acroleína en concentraciones comprendidas entre 0,39 mg/m3 y
11,2 mg/m3 se han estudiado en diversos animales de laboratorio.
Las duraciones de la exposición variaron entre 5 días y hasta 52
semanas. En general, se han documentado: reducción de la
adquisición de peso corporal, disminución de la función pulmonar y
cambios patológicos en la nariz, las vías aéreas superiores y los
pulmones en la mayoría de las especies expuestas a concentraciones
de 1,6 mg/m3 o más, durante 8 h/día. Entre los cambios
patológicos se observaron inflamación, metaplasia e hiperplasia del
tracto respiratorio. Se ha observado un nivel significativo de
mortalidad tras la exposición repetida a concentraciones de vapor de
acroleína superiores a 9,07 mg/m3. En animales de
experimentación, se ha demostrado que la acroleína agota el
glutatión tisular y que in vitro inhibe enzimas reaccionando con
los grupos sulfhidrilo de los sitios activos. Hay limitada
evidencia de que la acroleína pueda deprimir las defensas pulmonares
en el ratón y la rata.
La acroleína puede inducir efectos teratogénicos y
embriotóxicos si se administra directamente en el amnios. No
obstante, el hecho de que no se observaran efectos en ratones a los
que se inyectó 3 mg/kg por vía intravenosa sugiere que la exposición
humana a la acroleína tiene pocas probabilidades de afectar al
embrión en desarrollo.
Se ha demostrado que la acroleína interacciona con los ácidos
nucleicos in vitro y que inhibe su síntesis tanto in vitro como
in vivo. Sin activación, indujo mutaciones génicas en bacterias y
hongos y provocó intercambios de cromátidas hermanas en células de
mamíferos. En todos los casos esos efectos se produjeron en un
margen muy reducido de concentraciones, limitado por la reactividad,
la volatilidad y la citotoxicidad de la acroleína. Un ensayo de
letalidad dominante en ratones dio resultado negativo. Los datos
disponibles muestran que la acroleína es un mutágeno débil para
ciertas bacterias, hongos y cultivo celular de mamífero.
No se encontraron tumores en hámsters expuestos durante 52
semanas a vapores de acroleína con una concentración de 9,2 mg/m3
durante 7 h/día, 5 días a la semana, y observados durante 29 semanas
más. Cuando se expusieron hámsters a vapores de acroleína en las
mismas condiciones durante 52 semanas y, además, a dosis
intratraqueales de benzo[a]pireno semanalmente o a dosis subcutáneas
de dietilnitrosamina una vez cada tres semanas, no se observó una
acción cocarcinogénica clara de la acroleína. La exposición de
ratas por vía oral a la acroleína en el agua de bebida, en dosis
comprendidas entre 5 y 50 mg/kg de peso corporal al día
(5 días/semana durante 104-124 semanas) no indujo tumores. Dado el
carácter limitado de todos esos ensayos, se considera que no se
dispone de datos suficientes para determinar la carcinogenicidad de
la acroleína en los animales de experimentación. En consecuencia,
se considera asimismo imposible evaluar la carcinogenicidad de la
acroleína para el ser humano.
Los umbrales de acroleína que causan irritación y efectos en la
salud son 0,07 mg/m3 en el caso de la percepción del olor,
0,13 mg/m3 en la irritación ocular, 0,3 mg/m3 en la irritación
nasal y el parpadeo, y 0,7 mg/m3 en la disminución del ritmo
respiratorio. Puesto que el nivel de acroleína raras veces supera
los 0,03 mg/m3 en el aire urbano, es poco probable que alcance
niveles molestos o nocivos en circunstancias normales.
En vista de la elevada toxicidad de la acroleína para los
organismos acuáticos, la sustancia representa un riesgo para la vida
acuática en las proximidades de las zonas donde se producen vertidos
y escapes industriales, y en los lugares donde se usa como biocida.
