
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
ENVIRONMENTAL HEALTH CRITERIA 14
ULTRAVIOLET RADIATION
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of either the World Health Organization or the United Nations
Environment Programme.
Published under the joint sponsorship of the United Nations
Environment Programme, the World Health Organization and the
International Radiation Protection Association
World Health Organization Geneva, 1979
ISBN 92 4 154074 5
(c) World Health Organization 1979
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. For rights of reproduction or
translation of WHO publications, in part or in toto, application
should be made to the Office of Publications, World Health
Organization, Geneva, Switzerland. The World Health Organization
welcomes such applications.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city or area or
of its authorities, or concerning the delimitation of its frontiers or
boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
CONTENTS
ENVIRONMENTAL HEALTH CRITERIA FOR ULTRAVIOLET RADIATION
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1. Summary
1.2. Recommendations for further studies
1.2.1. Measurement of ultraviolet radiation from
natural and artificial sources
1.2.1.1 Measurement devices
1.2.1.2 Monitoring of natural sources
1.2.1.3 Monitoring of artificial sources
1.2.1.4 Development of personal monitoring devices
1.2.1.5 Improvement of high intensity sources
1.2.2. Effects of UV-B, UV-A, and visible light on
cells and their constituents
1.2.3. The relationship between ultraviolet radiation
and skin cancer
1.2.4. Epidemiological studies of skin cancer and
ultraviolet radiation deficiency in man
1.2.4.1 Non-melanoma skin cancer
1.2.4.2 Malignant melanoma
1.2.4.3 Identification of populations with
an increased risk of skin cancer
1.2.4.4 UVR deficiency
1.2.5. Studies of the interaction of ultraviolet radiation
and environmental chemicals
1.2.6. Studies of beneficial effects
1.2.7. Control measures and protection
1.2.7.1 Control measures
1.2.7.2 Sunscreen preparations
1.2.7.3 Behavioural modifications
2. PROPERTIES AND MEASUREMENT OF ULTRAVIOLET RADIATION
2.1. Sources
2.1.1. Solar radiation -- the biologically active UVR
spectrum
2.1.1.1 Influence of stratospheric constituents
2.1.1.2 Influence of clouds, haze, and smog
2.1.1.3 Amount of sea level solar ultraviolet
radiation in the biologically active
UVR spectrum
2.1.2. Artificial sources
2.1.2.1 Gas discharge arcs
2.1.2.2 Fluorescent lamps
2.1.2.3 Carbon arcs
2.1.2.4 Quartz halogen lamps
2.1.2.5 Oxyacetylene, oxyhydrogen, and plasma
torches
2.2. Detection and measurement of ultraviolet radiation
2.2.1. Units and conversion factors
2.2.2. Chemical and biological detectors
2.2.2.1 Photographic plates
2.2.2.2 Chemical methods
2.2.2.3 Biological detectors
2.2.3. Physical detectors
2.2.3.1 Radiometric devices
2.2.3.2 Photoelectric devices
2.2.4. Measuring devices
3. BIOLOGICAL EFFECTS OF ULTRAVIOLET RADIATION ON UNICELLULAR
ORGANISMS, MAMMALIAN CELLS AND TISSUE, AND INVERTEBRATES
3.1. Introduction
3.1.1. Absorption spectra
3.1.2. Evaluation of administered and absorbed doses
3.1.3. Action spectra
3.2. The molecular basis of the effects of ultraviolet
radiation on living matter
3.2.1. Molecular lesions in DNA
3.2.2. Consequences of photolesions
3.2.3. Repair of UVR-induced lesions
3.2.3.1 Prereplication repair
3.2.3.2 Repair during or after replication
3.2.3.3 SOS repair
3.3. Bacteria and yeasts
3.3.1. Effects on bacterial cell constituents
and macromolecular synthesis
3.3.2. Sublethal effects
3.3.3. Effects of ultraviolet radiation of wavelengths
longer than 280 nm
3.3.4. Genetic factors in photosensitivity
3.3.5. Repair of photolesions in bacteria
3.3.6. Yeasts
3.4. Protozoa
3.5. Effects on mammalian cells in culture
3.5.1. Sublethal effects
3.5.2. Effects of UV-A
3.5.3. Lesions produced in DNA
3.5.3.1 Pyrimidine dimers
3.5.3.2 DNA-protein cross-links
3.5.4. The consequences of photolesions in mammalian cells
3.5.4.1 Inhibition of DNA synthesis
3.5.4.2 Chromosome aberrations and mutagenic
effects
3.5.5. The repair of photolesions
3.5.5.1 Photoreactivation
3.5.5.2 Excision repair
3.5.5.3 Repair during or after replication
3.5.5.4 SOS repair
3.5.6. Effects on cell-virus relationships
3.5.6.1 Sensitivity to vital infection
3.5.6.2 Viral transformation
3.5.6.3 Activation of viruses
3.6. Effects on invertebrates
3.6.1. Effects on eggs and embryos of invertebrates
3.6.2. Effects on insects
3.7. Modification of the effects of ultraviolet radiation by
chemical agents
3.7.1. Halogenated analogues
3.7.2. Caffeine
3.7.3. Furocoumarins
3.7.4. Other photosensitizing agents
3.7.5. Protection by carotene
3.8. Conclusions
4. THE BIOLOGICAL ACTION OF ULTRAVIOLET RADIATION ON VERTEBRATE
ANIMALS
4.1. General aspects
4.2. Acute reactions in skin
4.2.1. Epidermal changes
4.2.2. Erythema and inflammation
4.2.3. Tanning
4.3. Acute changes in the eye
4.3.1. Photokeratitis and photoconjunctivitis
4.3.2. Cataracts
4.4. Effects of long-term exposure of skin to UVR
4.4.1. UVR-induced mutagenesis and carcinogenesis
4.4.1.1 Mutagenesis
4.4.1.2 Mechanism of UVR carcinogenesis
4.4.1.3 Tumour types
4.4.2. Species-specificity
4.4.3. Ultraviolet radiation as an initiating agent
4.5. Interactions between ultraviolet radiation and chemicals
4.5.1. Chemically-enhanced photocarcinogenesis
4.5.2. Interaction between light and chemical carcinogens
4.5.3. UVR-induced carcinogen formation
4.6. Physical and quantitative aspects of ultraviolet irradiation
in animal studies
4.6.1. Carcinogenic action spectrum
4.6.2. Dose-response relationships
4.6.3. Physical factors influencing UVR carcinogenesis
4.7. The immune response to rumour induction
5. EFFECTS OF ULTRAVIOLET RADIATION ON MAN
5.1. Beneficial effects
5.2. Induction of erythema in human skin
5.2.1. Action spectra of human skin erythema
5.3. Natural protection against erythema-inducing ultraviolet
radiation
5.3.1. Melanin (see also section 4.2.3)
5.3.2. Thickening of the stratum corneum
5.4. Solar elastosis and other dermal effects of ultraviolet
radiation (see also section 4.2)
5.5. Ultraviolet radiation and skin cancer in man (see also
section 4.2)
5.5.1. Anatomical distribution of skin cancer
5.5.2. Occupation and skin cancer
5.5.3. Genetics and skin cancer
5.5.4. Geographical distribution of non-melanoma skin
cancer
5.5.5. Dose-response relationship for skin cancer (see also
section 4.5.2)
5.5.6. Mortality from skin cancer
5.5.7. Malignant melanoma
5.6. Phototoxic and photoallergic diseases
5.6.1. Phototoxicity
5.6.2. Photoallergy
5.7. Pterygium and cancer of the eye
6. EVALUATION OF HEALTH RISKS TO MAN
6.1. The significance and extent of different environmental
sources of ultraviolet radiation and pathways
of exposure
6.2. Types of biological effects and their significance
for human health
6.3. The risk associated with combined exposure
with other agents
6.4. The population at risk -- geographical distribution,
genetic influences, and occupation
6.5. The reliability and range of known dose-effect and dose-
response curves
6.5.1. Dose-effect curves for acute skin erythema
6.5.2. Averages and limits, minimal and slightly more
than minimal erythema doses
6.5.3. The "erythema range" effects
6.5.4. Dose-response curves for keratoconjunctivitis
6.5.5. Dose-response relationship for photocarcinogenesis
7. GUIDELINES FOR HEALTH PROTECTION
7.1. Range of exposure limits
7.1.1. Exposure to solar ultraviolet radiation
7.1.2. Occupational exposure to artificial
ultraviolet radiation
7.1.3. Exposure of general population to artificial
ultraviolet radiation
7.1.4. Measurement of natural and artificial
ultraviolet radiation
7.2. Health effects of solar ultraviolet radiation
in the general population
7.3. UVR deficiency and its prevention
7.3.1. Insolation and UV irradiation of built-up areas
7.3.2. Sunbathing and air-bathing in the prevention
of UVR deficiency
7.3.3. Artificial ultraviolet radiation in the prevention
of UVR deficiency
7.4. Protection against ultraviolet radiation
7.4.1. Sunscreen preparations
7.4.2. Clothing
7.4.3. Behavioural conformity with environment
7.4.4. Occupational protection
8. REFERENCES
NOTE TO READERS OF THE CRITERIA DOCUMENTS
While every effort has been made to present information in the
criteria documents as accurately as possible without unduly delaying
their publication, mistakes might have occurred and are likely to
occur in the future. In the interest of all users of the environmental
health criteria documents, readers are kindly requested to communicate
any errors found to the Division of Environmental Health, World Health
Organization, Geneva, Switzerland, in order that they may be included
in corrigenda which will appear in subsequent volumes.
In addition, experts in any particular field dealt with in the
criteria documents are kindly requested to make available to the WHO
Secretariat any important published information that may have
inadvertently been omitted and which may change the evaluation of
health risks from exposure to the environmental agent under
examination, so that the information may be considered in the event of
updating and re-evaluation of the conclusions contained in the
criteria documents.
WHO TASK GROUP ON ENVIRONMENTAL HEALTH CRITERIA FOR ULTRAVIOLET
RADIATION
Members
Dr J. Chavaudra, Institut Gustave Roussy, Villejuif, France
Dr M. Faber, Finsen Laboratory, Finsen Institute, Copenhagen, Denmark
a (Chairman)
Dr C. Fröhlich, World Radiation Center, Davos, Switzerland
Dr Y. I. Prokopenko, Sysin Institute of General & Community Medicine,
Moscow, USSR (Vice Chairman)
Dr Y. Skreb, Institute of Medical Research & Occupational Health,
Zagreb, Yugoslavia
Professor F. Stenbäck, Department of Pathology, University of Kuopio,
Kuopio, Finland
Professor F. Urbach, Temple University School of Medicine,
Philadelphia, PA, USA (Rapporteur)
Professor M. Wassermann, Department of Occupational Health, Hadassah
Medical School, The Hebrew University, Jerusalem, Israel
Representatives of other organizations
Dr R. D. Bojkov, Atmospheric Sciences Division, World Meteorological
Organization, Geneva, Switzerland
Mr M. Malone, Instruments & Observing Techniques Branch, Research &
Development Department, World Meteorological Organization,
Geneva, Switzerland
Secretariat
Dr E. Komarov, Environmental Health Criteria & Standards, Division of
Environmental Health, WHO, Geneva, Switzerland (Secretary)
Dr V. B. Vouk, Environmental Health Criteria & Standards, Division of
Environmental Health, WHO, Geneva, Switzerland
a Also representing the Committee on Non-Ionizing Radiation of the
International Radiation Protection Association
ENVIRONMENTAL HEALTH CRITERIA FOR ULTRAVIOLET RADIATION
A WHO Task Group on Environmental Health Criteria for Ultraviolet
Radiation met in Geneva from 30 October to 3 November 1978. Dr V.
Vouk, Manager, Health Criteria and Standards, Division of
Environmental Health opened the meeting on behalf of the
Director-General. The Task Group reviewed and revised the third draft
criteria document and made an evaluation of the health risks from
exposure to ultraviolet radiation (UVR).
The first draft was prepared by Professor F. Urbach of the Temple
University School of Medicine, Philadelphia, PA, USA on the basis of
reviews prepared by Dr Y. Skreb of the Institute of Medical Research
and Occupational Health, Zagreb, Yugoslavia, Professor F. Stenbäck of
the Department of Pathology, University of Kuopio, Kuopio, Finland,
and the Sysin Institute of General and Community Medicine, USSR. The
second and third drafts were prepared taking into account comments
received from the national focal points and from the United Nations
Environmental Programme (UNEP), the International Labour Organisation
(ILO), the World Meteorological Organization (WMO), and the
International Atomic Energy Agency (IAEA).
The collaboration of these national institutions, international
organizations, WHO collaborating centres, and individual experts is
gratefully acknowledged. The Secretariat wishes to thank, in
particular, Professor Urbach for his help in all phases of preparation
of the document and Dr M. Faber of the Finsen Institute, Copenhagen,
Denmark, who assisted the Secretariat in the final editing of the
document.
This document is based primarily on original publications listed
in the reference section together with several recent reviews of the
health aspects of UVR including publications by Urbach (1969),
Fitzpatrick et al. (1974), and Forbes et al. (1978).
Details of the WHO Environmental Health Criteria Programme
including some of the terms frequently used in the documents may be
found in the introduction to the Environmental Health Criteria
Programme published together with the environmental health criteria
document on mercury (Environmental Health Criteria 1 -- Mercury, World
Health Organization, Geneva, 1976), and now available as a reprint.
1. SUMMARY AND RECOMMENDATIONS FOR FURTHER STUDIES
1.1 Summary
Exposure to ultraviolet radiation (UVR) occurs from both natural
and artificial sources. The sun is the principal natural source. The
known effects of UVR on man may be beneficial or detrimental,
depending on a number of circumstances.
Artificial UVR sources are widely used in industry and, because
of the germicidal properties of certain portions of the UVR spectrum,
they are also used in hospitals, biological laboratories, and schools.
UVR is extensively used for therapeutic purposes, as in the prevention
of vitamin D deficiency, the treatment of skin diseases, and for
cosmetic purposes. Artificial UVR sources are available as consumer
products.
The migration of people between areas of different UVR exposure,
whether for occupational or recreational reasons gives rise to
unforseen exposures.
UVR can be classified into UV-A, UV-B, and UV-C regions.
Wavelengths in the UV-C region (200-280 nm) cause unpleasant, but
usually not serious effects on the skin and eye. Although UV-C is very
efficiently absorbed by nucleic acids, the overlying dead layers of
skin absorb the radiation to such a degree that there is only mild
erythema and, usually, no late sequelae, even after repeated
exposures. Since solar UVR below 290 nm is effectively absorbed by
stratospheric ozone, no such radiation reaches living organisms from
natural sources.
Most observed biological effects of UV-B radiation (280-320 nm)
are extremely detrimental to living organisms. However, living
organisms are usually protected from excessive solar UV-B radiation by
feathers, fur, or pigments that absorb the radiation before it reaches
sensitive physiological targets. Other means of protection include
behavioural patterns and the ability to tolerate certain UV-B
radiation injury because of molecular and other repair mechanisms.
Much less is known about the biological effects of UV-A radiation
(320-400 nm). It can augment the biological effects of UV-B, and doses
of UV-A, which, alone, do not show any biological effect, can, in the
presence of certain chemical agents, result in injury to tissues
(phototoxicity, photoallergy, enhancement of photocarcinogenesis).
Beneficial effects: It is now generally acknowledged that a
long period of UVR deficiency may have a harmful effect on the human
body. The best known manifestation of "UVR deficiency" is the
development of vitamin D deficiency and rickets in children because of
a disturbance in the phosphorus and calcium metabolism. The resultant
effect on the bone-forming processes is accompanied by a sharp
reduction in the defensive powers of the body, making it particularly
vulnerable to many diseases. Appropriate measures to increase UV-B
exposure by improving the architectural features of buildings
(orientation of windows, use of UV-B transmitting window glass), the
use of sun and sun-and-air bathing (solaria), and the development of
artificial UVR sources and installations (photaria) have been shown to
correct and prevent disease states due to UVR deficiency. In
fair-skinned people, all the beneficial effects can be obtained with
daily suberythemal doses.
Harmful effects: These may be acute or chronic, and involve
primarily the eyes and skin. The acute effects of UVR on the eyes
consist of the development of photokeratitis and photoconjunctivitis,
which are unpleasant but usually reversible and easily prevented by
appropriate eyewear. Acute effects on the skin consist of solar
erythema, "sunburn", which, if severe enough, may result in blistering
and destruction of the surface of the skin with secondary infection
and systemic effects, similar to a first or second degree heat burn.
The skin has natural, adaptive protective mechanisms consisting of
increased production of the skin pigment melanin, and thickening of
the outer horny layer.
Chronic effects on the eye consist of the development of
pterygium and squamous cell cancer of the conjunctiva and perhaps
cataracts. Chronic skin changes due to UVR consist of "aging" (solar
elastosis) and the induction of premalignant changes (actinic
keratoses) and malignant skin tumours (non-melanoma and melanoma skin
cancers). The evidence for a causal association of UV-B radiation with
these chronic changes, particularly with skin cancer induction, is
reviewed in detail.
Additional harmful effects (phototoxicity, photoallergy, and
enhanced photocarcinogenesis) are produced by the interaction of UVR
and a variety of environmental and medicinal chemicals. This results
in acute and chronic skin changes caused by UVR of wavelengths which
are not normally of an injurious nature.
Ranges of exposure limits for solar UVR are described in
section 7.1.
The following criteria for occupational exposure levels in work
places have been proposed:
(a) for the UV spectral region of 315-400 nm, total irradiance on
unprotected skin or eyes, based on either measurement or output data,
should not exceed 10.0 W/m2 for periods of more than 100 seconds,
and, for exposure times of 1000 seconds or less, the total radiant
energy should not exceed 1.0 X 104 J/m2;
(b) for the UV spectral region of 200-315 nm, total irradiance
incident on unprotected skin or eyes, based on either measurement or
output data, should not exceed 1.0 W/m2 of energy equivalent to the
effective irradiance relative to a 270 nm monochromatic source for 8 h
of exposure per day. (Details of the calculation and interpretation of
"effective irradiance" are given in section 6.
This exposure limit is applicable for acute effects only. The
extent to which it must be changed when long-term effects are taken
into account is unknown, because of lack of information concerning the
dose-effect relationships in human skin carcinogenesis.
It must be recognized that significant nonoccupational exposure
to UVR occurs from exposure to sunlight, particularly during the
summer months, and throughout the year in the tropics. Thus, exposure
limits for the general population are difficult to recommend.
The use of artificial UVR of appropriate wavelengths in
suberythemal doses is also proposed for prophylactic purposes in
populations living in UVR-deficient areas of the world, and for
workers employed in workplaces without natural illumination.
Finally, the document describes existing protection and control
measures such as the containment of UVR sources, and methods for
personal protection including the use of sunscreen preparations,
clothing, transparent material for eye and skin protection, and
behavioural modifications.
1.2 Recommendations for Further Studies
The following recommendations pertain to information needed for
the adequate evaluation of health risks and the establishment of
appropriate protective measures and guidelines.
1.2.1 Measurement of ultraviolet radiation from natural and
artificial sources
1.2.1.1 Measurement devices
Instruments are needed that can integrate incident UVR from 200
to 320 nm according to the "effectiveness action spectrum" for skin
and eye proposed in section 6, in order to enforce the proposed
standard for occupational exposure to UVR. Such instruments do not
exist at present.
The design and accuracy of instruments for measuring UV-A
(320-400 nm) should be improved.
Models for the evaluation of the effective absorbed dose in the
critical cells of the skin must be developed taking spectral
efficiency, pigmentation, skin thickness, and other relevant factors
into consideration.
1.2.1.2 Monitoring of natural sources
Accurate and continuous measurement of the UVR reaching the earth
from the sun and sky (direct and global) is necessary to:
(a) establish baseline levels;
(b) establish the range of natural existing variation;
(c) monitor persistent changes resulting from various causes
(e.g., pollution); and
(d) establish, more reliably, the relationship between the status
of the stratospheric ozone layer and effective UVR for various
biological systems.
Measurements of the spectral distribution of solar UVR should be
continued. A network of integrating UV-B meters should be established.
Regular observations carried out in many areas of the world with
identical instruments for long periods (a minimum of one complete
sunspot cycle) are needed to obtain information on UVR climatology. Of
particular importance are measurements north of latitude 55° and in
the region of the tropics.
An instrument capable of measuring UVR of wavelengths shorter
than 290 nm should be developed, since such wavelengths can reach the
earth, if the stratospheric ozone layer is compromised.
1.2.1.3 Monitoring of artificial sources
Environmental monitoring of UVR sources is necessary to recognize
and control direct and stray radiation. Wherever chemical substances
are handled, the monitoring should cover the whole of the UVR
spectrum.
1.2.1.4 Development of personal monitoring devices
Population studies using personal monitoring devices for UV-B
radiation are needed to determine the fraction of the daily natural UV
dose received by persons at risk either from UVR deficiency or excess,
or from occupational exposure. The daily amount of UVR received by
human skin must vary greatly with occupation, behaviour, and local
climatic and environmental conditions. Little is known about these
factors and this seriously interferes with the interpretation of
existing data on the relationship between UVR and the development of
skin cancer and of chronic skin and eye damage. Thus, the development
of personal UVR monitoring devices is of the utmost priority.
1.2.1.5 Improvement of high intensity sources
One major problem in applying data from field measurements of UVR
to the projection of changes in the incidence of skin cancer is the
uncertainty of the shape of the action spectrum for skin carcino-
genesis. Although the general direction and approximate limits of this
action spectrum seem to parallel those for skin erythema, the fine
structure of the carcinogenesis action spectrum is not known. The main
reason for this is the lack of high intensity, narrow band, UV sources
capable of irradiating relatively large areas (e.g., even the surface
of one mouse). Improved high intensity, large-area solar simulators
for chronic (1-2 year) animal studies are also urgently required.
1.2.2 Effects of UV-B, UV-A, and visible light on cells and their
constituents
Most of the information on the chemical and biological effects of
UVR comes from experiments using UV-C (particularly 254 nm) radiation
not normally found in sunlight reaching the earth's surface. There are
recent studies showing direct and indirect effects on cells and
cellular constituents of UV-B, UV-A, and visible light that differ
considerably from those of UV-C. Thus, the chemical and biological
effects of the wavelengths of UVR found in sunlight should be studied.
There is much evidence that visible light can, under different
conditions, either help cells to repair UVR-induced damage or can
potentiate the detrimental effects of UVR. Thus, to better understand
the effects of sunlight on man and his environment, experiments should
be performed using natural sunlight or artificial lamps with
well-known continuous spectra.
1.2.3 The relationship between ultraviolet radiation and skin
cancer
(a) A study is required of the effects of the interaction between
UV-B and the rest of the solar spectrum in relation to DNA repair,
malignant transformation, and skin tumour development.
(b) Cellular genetics should be studied in relation to
differences in UVR sensitivity, and defects in DNA repair.
(c) Investigation is needed of the influence of change in the
dose-rate of UV-B on skin carcinogenesis. Preliminary experiments show
that protracting the delivery of a dose of UVR significantly increases
skin carcinogenesis.
(d) It is recommended that the effect of varying intervals
between UVR exposures during carcinogenesis experiments should be
studied in detail.
(e) Studies are required to develop additional animal models,
particularly for the study of the experimental induction of malignant
melanoma.
1.2.4 Epidemiological studies of skin cancer melanoma and UVR
deficiency in man
1.2.4.1 Non-melanoma skin cancer
Since incidence data are extremely difficult to obtain
accurately, prevalence data over a wide span of latitudes should be
obtained first. Areas of study should be separated by at least 500 km
north-south over a latitude span reaching beyond the most populated
areas. The effect of altitude needs to be investigated. Data should
include age at which the first tumour appears, sex, occupation, skin
phenotype, and estimate of solar UV-B dose, obtained by personal
dosimeters. It is of the utmost importance that all these studies be
performed with a unified protocol, so that valid comparisons can be
made. Promising areas for such studies are Australia (particularly
Queensland), Finland, Scandinavia, South Africa, Yugoslavia, southern
USSR, and the USA.
1.2.4.2 Malignant melanoma
While much less common than non-melanoma skin cancer, malignant
melanoma is a serious cancer with a survival rate similar to that of
cancer of the breast. The relationship of malignant melanoma to UVR
may be less obvious than that of non-melanoma skin cancer, and more
detailed studies of the epidemiology, anatomical distribution, and
associated factors of this severe skin cancer are urgently needed.
1.2.4.3 Identification of populations with an increased risk of skin
cancer
Existing population studies on the prevalence or incidence of
skin cancer suggest, very strongly, that persons with certain
phenotypes such as fair skin, light eyes, and freckles, who burn
easily and tan poorly, are at higher risk of developing skin cancer
than others, and that this is a genetic trait (Celts).
Efforts should be made to develop simple screening methods for
the identification of the most susceptible members of the population.
1.2.4.4 UVR deficiency
A study of the extent and distribution of the health effects of
UVR deficiency is needed. The existing epidemiological studies in the
USSR should be extended to other populations.
1.2.5 Studies of the interaction of ultraviolet radiation
and environmental chemicals
Too little is known about the mechanisms of interaction of UVR
and environmental chemical agents on biological systems. Many widely
distributed natural or artificial chemicals (pesticides, halocarbons,
etc.) can be altered by UVR, resulting in photoproducts that may be
less or more biologically effective than the parent compound.
Furthermore, many chemicals can be activated by UVR in situ in
biological systems and this activation may elicit a biological effect
which neither the chemical nor the radiation alone exhibits
(Psoralens).
Studies of the chemical, physical, and biological interaction of
light and chemicals on biological systems at the subcellular,
cellular, organ, and whole organism levels are much needed. Methods
should also be developed to predict the extent and type of the
photo-injury caused by such agents.
An international registry and notification of photobiologically-
effective agents would speed identification of such agents. This is
particularly important for manufacturers and users of solaria for
human use, now widely produced in various parts of the world.
1.2.6 Studies of beneficial effects
In the early photobiological literature, claims were made,
supported by few data, of beneficial effects of UVR (other than
Vitamin D production and subsequent effects on mineral metabolism). In
recent years, scientists in the USSR have placed particular emphasis
on studies of the primary mechanisms of beneficial UVR effects. More
detailed comparable studies, particularly in man, under carefully
controlled conditions, need to be carried out to determine the
importance of such effects on man.
1.2.7 Control measures and protection
1.2.7.1 Control measures
Known UVR-emitting artificial sources should be clearly
identified by appropriate hazard labels. Where possible, such sources
should be housed in protective enclosures and equipped with
appropriate safety devices including those necessary for eye and skin
protection.
Appropriate information concerning the spectral composition,
intensity, and handling of such sources should be provided. Licensing
of high intensity sources is recommended.
1.2.7.2 Sunscreen preparations
Existing sunscreen preparations differ widely in their
effectiveness, cosmetic acceptability, and usefulness. Studies are
needed to find new UVR absorbers, particularly in the UV-A region.
Vehicles for application need to be improved to make such preparations
resistant to wash-off and to ensure simple methods for the application
of a sufficiently thick and even film to the skin.
Methods for the uniform testing of sunscreens for effectiveness
need to be developed and should be standardized and accepted on an
international basis. Search for a systemically effective sunscreen is
needed.
1.2.7.3 Behavioural modifications
It is essential to educate the general population and workers
concerning the profound importance of sunlight and the possibilities
of either UVR deprivation or of acute and chronic UVR injury. It is
also important to overcome the lack of respect for the biological
effects of sunlight, simply because sunlight is ubiquitous, and the
concept that, if something is natural, it must be totally beneficial
and safe.
2. PROPERTIES AND MEASUREMENT OF ULTRAVIOLET RADIATION
2.1 Sources
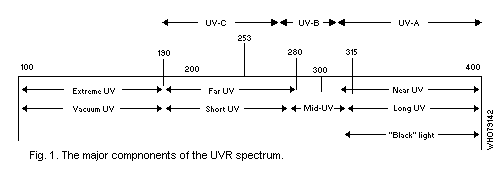
The UVR spectrum may be divided into three major components which
induce significantly different biological effects: UV-A -- wavelengths
from 400 nm to 320 nm (synonyms: long wave UVR, near UVR, black
light); UV-B -- 320 to 280 nm (synonyms: middle UVR, "sunburn"
radiation); UV-C -- 280 nm to 200 nm (synonyms: short wave UVR, far
UVR, germicidal radiation). Wavelengths below 200 nm are of little
biological significance, since radiation in this region ("vacuum UVR")
is absorbed in very short pathlengths in air (Fig. 1).
2.1.1 Solar radiation -- the biologically active UVR spectrum
The sun, being essentially a very hot black body radiator, emits
radiation within a wide range of wavelengths. The relative intensities
of UV and visible radiation that reach the earth's surface depend, to
a considerable extent, on attenuation by the atmosphere because of
absorption and scattering. Below 320 nm, the intensity of UVR falls
very rapidly because of absorption by stratospheric ozone. Virtually
no radiation below 288 nm reaches the earth's surface. Thus, most
known biological effects of solar radiation are confined to the
extreme short end of the terrestrial solar spectrum and involve not
more than about 1.5% of the total solar energy reaching the earth.
 UVR is not only present in the direct solar beam, but also
reaches the earth's surface as diffuse radiation, the solar UVR being
scattered within the atmosphere. Under hazy and cloudy conditions this
component can be very important.
2.1.1.1 Influence of stratospheric constituents
The solar UVR flux that reaches the surface of the earth is a
function of the solar spectral irradiance at the upper surface of the
atmosphere and the absorption and scattering of UVR by the atmosphere.
In the stratosphere, the spectral irradiance of the sun is mainly
absorbed by ozone. Molecular ozone has a strong absorption band in the
UVR centred at 250 nm and extending beyond 350 nm. The absorption
coefficient falls off rapidly with wavelength and the attenuation of
the incident solar flux is, therefore, a strong function of
wavelength. In order to determine the impact on man of a change in the
amount of ozone, these solar irradiances must be weighted with a
suitable response function for human skin.
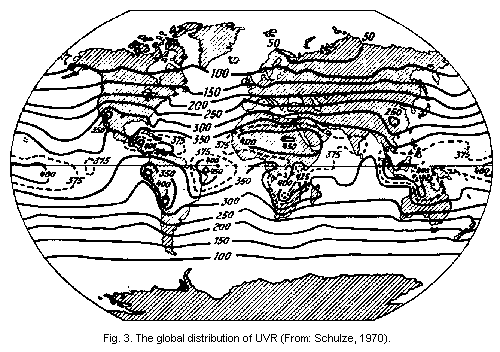
The percentage increase in erythema-producing UVR is
approximately 2 times the percentage decrease in ozone (Schulze,
1970).
2.1.1.2 Influence of clouds, haze, and smog
In addition to the absorption of the incident solar irradiance by
ozone there is also molecular scattering by air and aerosols;
reflection, scattering, and attenuation by clouds, haze, and smog near
the ground; and reflection from the ground. The computation of the
direct and diffuse radiation that impinges on a surface near the
ground is well understood in theory, but difficult to carry out in
practice, particularly if clouds, haze, and pollution are present
(Belinsky & Andrienko, 1974; Green et al., 1975).
The major factors affecting the amount of UVR in the 290-320 nm
range that will reach the earth's surface are solar elevation (thus,
season and latitude), and the type and amount of cloud cover and
aerosols. The importance of atmospheric conditions and their
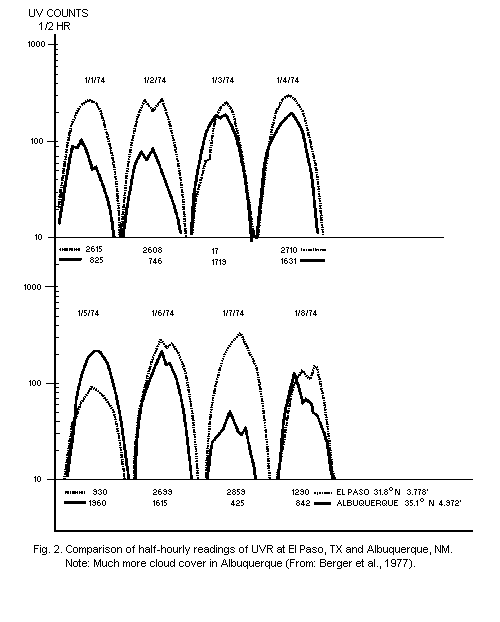
variability from hour to hour and from day to day have been measured
by Bener (1972) and Berger et al. (1975) (Fig. 2). Data on the effect
of clouds, haze, and albedo have also been published by the Moscow
State University group (Garadza, 1974).
UVR is not only present in the direct solar beam, but also
reaches the earth's surface as diffuse radiation, the solar UVR being
scattered within the atmosphere. Under hazy and cloudy conditions this
component can be very important.
2.1.1.1 Influence of stratospheric constituents
The solar UVR flux that reaches the surface of the earth is a
function of the solar spectral irradiance at the upper surface of the
atmosphere and the absorption and scattering of UVR by the atmosphere.
In the stratosphere, the spectral irradiance of the sun is mainly
absorbed by ozone. Molecular ozone has a strong absorption band in the
UVR centred at 250 nm and extending beyond 350 nm. The absorption
coefficient falls off rapidly with wavelength and the attenuation of
the incident solar flux is, therefore, a strong function of
wavelength. In order to determine the impact on man of a change in the
amount of ozone, these solar irradiances must be weighted with a
suitable response function for human skin.
The percentage increase in erythema-producing UVR is
approximately 2 times the percentage decrease in ozone (Schulze,
1970).
2.1.1.2 Influence of clouds, haze, and smog
In addition to the absorption of the incident solar irradiance by
ozone there is also molecular scattering by air and aerosols;
reflection, scattering, and attenuation by clouds, haze, and smog near
the ground; and reflection from the ground. The computation of the
direct and diffuse radiation that impinges on a surface near the
ground is well understood in theory, but difficult to carry out in
practice, particularly if clouds, haze, and pollution are present
(Belinsky & Andrienko, 1974; Green et al., 1975).
The major factors affecting the amount of UVR in the 290-320 nm
range that will reach the earth's surface are solar elevation (thus,
season and latitude), and the type and amount of cloud cover and
aerosols. The importance of atmospheric conditions and their
variability from hour to hour and from day to day have been measured
by Bener (1972) and Berger et al. (1975) (Fig. 2). Data on the effect
of clouds, haze, and albedo have also been published by the Moscow
State University group (Garadza, 1974).
 2.1.1.3 Amount of sea level solar ultraviolet radiation in the
biologically active UVR spectrum
The spectroradiometric measurements of global and sky UVR
performed by Belinskij & Garadza (1962), Belinskij et al. (1968),
Garadza (1965, 1967), Garadza & Nezval, (1971), Bener (1972), and
Belinsky & Adrienko (1974) have served as the standards for all modern
calculations, model systems, and comparisons with other measuring
devices (Green et al., 1976). The global distribution of UVR has been
illustrated by Schulze (1970) (Fig. 3).
2.1.1.3 Amount of sea level solar ultraviolet radiation in the
biologically active UVR spectrum
The spectroradiometric measurements of global and sky UVR
performed by Belinskij & Garadza (1962), Belinskij et al. (1968),
Garadza (1965, 1967), Garadza & Nezval, (1971), Bener (1972), and
Belinsky & Adrienko (1974) have served as the standards for all modern
calculations, model systems, and comparisons with other measuring
devices (Green et al., 1976). The global distribution of UVR has been
illustrated by Schulze (1970) (Fig. 3).
 Long-term results of actual measurements with an analogue
integrating UVR dosimeter, having an action spectrum similar to that
of skin erythema, are also available (Scotto et al., 1976). From these
measurements, it is apparent that there are daily fluctuations,
throughout the year, at each location. Weekly patterns are less
erratic, and seasonal variations depend on the latitude. The solar UVR
that causes skin erythema reaches a maximum intensity between 10h00
and 14h00. About 60% of the daily dose reaches the ground between
10h00 and 14h00, and 80% between 9h00 and 15h00 (Fig. 2). Similar
observations over a shorter period of time, using an instrument based
on a filtered selenium photocell, have given virtually the same
results (Garadza, 1965).
2.1.2 Artificial sources
Any material heated to temperatures exceeding 2500 K begins to
emit UVR. For practical purposes, sources emitting significant amounts
of biologically effective UVR can be classified into confined gas
discharge arcs, fluorescent lamps, and incandescent sources. It should
be noted that any UVR source emitting intense radiation below 260 nm
will produce ozone, which has to be removed to prevent any health
hazard.
2.1.2.1 Gas discharge arcs
The optical spectra produced by arcs depend on the nature of the
gas molecules through which the current discharge takes place, its
pressure, and the electrical conditions in the discharge.
Direct gas discharge arcs are widely used for the generation of
UVR. Generally, they differ from each other in such respects as the
type of gas, pressure, starting mechanisms, lamp shape, reflector
systems, electrodes, etc. (Andreev et al., 1975). As there is such a
wide variety of these arcs, it is not possible to describe them all.
However, the most important types are listed below.
Low pressure mercury arcs. These vapour lamps emit several
narrow UV bands. Most of the emitted power is of a wavelength of
253.7 nm, which is near the maximum for germicidal effectiveness,
hence its usefulness in the control of microorganisms.
High pressure mercury arcs. These vapour arcs (operating at
20-100 atmospheres, i.e., 200-1000 kPa, and usually encased in quartz
envelopes) emit much broader and more intense bands of UVR than low
pressure mercury arcs at wavelengths of 254, 297, 303, 313 and 365 nm.
They are extensively used in industry in photochemical reactors and in
printing. Other uses include phototherapy of skin diseases (Meyer &
Seitz, 1949; Nilender & Gavanin, 1971).
High pressure xenon arcs. These arc lamps may operate at very
high internal pressures, and have the advantage that the spectral
distribution of their radiant output shows a continuum similar to that
of the sun above the stratosphere. Their output is quite constant over
long periods of time, and they are available in a variety of
configurations. The major problem with xenon arcs is their very high
emission in the near infrared region (Gavrilova et al., 1975). Their
uses are similar to those of mercury arcs.
Flash tubes. Another form of gas discharge arc that produces
UVR is usually referred to as the flash tube. The gas within a flash
tube is excited and/or ionized, when a capacitor is discharged, to
pass an avalanche of fast electrons through the gas between the
electrodes. Depending on the gas used, i.e., xenon, krypton, argon,
neon, etc., different optical spectra are produced.
2.1.2.2 Fluorescent lamps
A fluorescent lamp contains an electric arc discharge source.
UVR, generated at high efficiency by mercury vapour in an inert gas at
low pressure, activates a coating of fluorescent material (phosphor)
on the inner surface of a glass tube. The phosphor simply acts as a
"transformer", converting shorter wavelength UVR into longer
wavelength radiation, i.e., UV and/or visible light. The spectral
characteristics, which depend on the phosphor used, vary with the gas
pressure in the lamp, and the temperature at the coldest point in the
lamp.
"Fluorescent Sun" (FS) type UVR emitters. "Fluorescent Sun"
type emitters contain a phosphor that emits more than half of its
radiant output at wavelengths shorter than 340 nm. In general, the
range of UVR emitted is from 275 to 380 nm, but the maximum is located
at 313 nm. Thus, this light source is extremely effective in producing
suntan, sunburn, and, at least in animals, cutaneous cancer. The
linear configuration of fluorescent lamps has a distinct advantage for
any application where a uniform irradiation field of considerable size
is required. The disadvantage of these lamps is that there is very
much less energy output per unit area compared with compact mercury or
xenon high pressure arc sources (Sozin, 1975).
"Black Light" (BL) type UVR emitters. The "Black Light" type
UVR emitters are very similar in construction to the FS lamps, except
that the phosphor used emits radiation ranging from 300 to 410 nm,
with a maximum in the 350-365 nm region. Usually, BL lamps emit less
than 0.1% of their total UVR in the less than 320 nm (i.e.,
biologically most effective) region. Their primary use is for
producing fluorescence in a variety of paints and inks. In recent
years, such BL lamps have been used together with photoactive drugs
such as 8-methoxypsoralen in phototherapy of skin diseases (Parrish et
al., 1974).
2.1.2.3 Carbon arcs
The arc is due to an electric discharge between two carbon
electrodes in air at atmospheric pressure. The output of radiation
from a carbon arc increases with increase in arc current and its more
or less continuous shape depends, in part, on the type of metal added
to the electrodes. The use of these arcs has been limited, because of
gaseous waste products that require adequate venting, and the
maintenance problems that result from consumable electrodes.
2.1.2.4 Quartz halogen lamps
These are tungsten filament lamps, enclosed in a quartz envelope,
filled with a small amount of a halogen gas, usually iodine or
bromine. This allows operation at much higher temperatures without
deposition of the metal on the envelope. Such lamps, which may operate
at temperatures up to 3500 K or more, are stable and very intense.
Their UVR output is mainly in the region above 330 nm, and their
primary use is in illumination and as reference standard lamps.
2.1.2.5 Oxyacetylene, oxyhydrogen, and plasma torches
Oil, coal, and gas flames normally operate below 2000 K and,
thus, emit virtually no UVR. Oxyacetylene and oxyhydrogen flames burn
at a much higher temperature and solids heated by these two flames may
radiate UVR.
Welding produces UVR in broad bands, which often appear as a
continuous spectrum. The intensities of the various bands depend on
many factors including the materials from which the electrodes are
made, the discharge current, and the gases surrounding the arc. Arc
welding is a common cause of UVR eye and skin damage.
The plasma torch can produce temperatures of over 6000 K (the
temperature at the surface of the sun) and intense UVR can result.
Exposure to radiation from plasma torches can result in
keratoconjunctivitis and sunburn, if eyes and skin are not protected.
2.2 Detection and Measurement of Ultraviolet Radiation
The measurement of UVR differs from that of visible radiation in
that the eye cannot be used directly as a detecting instrument. Thus,
other means for detection must be used, which can either be based on a
physical principle, or on a chemical or biological reaction. The
physical detectors are mainly used to measure instantaneous
irradiance, whereas the chemical and biological detectors are normally
used to determine radiant exposure (dose).a
2.2.1 Units and conversion factors
Table 1 describes terms frequently used in radiometric
techniques, and Table 2 gives a simple scheme for conversion between
commonly used irradiance units.
In addition to these energy units, units of biological effect,
based on interactions between UVR and living organisms, have been
proposed. The oldest of these is the Finsen. More recently, a system
of bactericidal units for evaluating UVR on the basis of its
disinfectant effect and a system of erythema units for evaluating UVR
on the basis of its beneficial effects on man have been described
(Lazarev & Sokolov, 1971, 1974). In order to allow for intercomparison
with other measurement units, the bactericidal and erythema quantities
are now expressed in SI units (Sokolov, 1975, 1976).
2.2.2 Chemical and biological detectors
2.2.2.1 Photographic plates
The photographic plate is the usual detector in UV spectroscopy.
The degree of blackening of the plate is a measure of radiation
intensity. The measurement is made photometrically by means of some
form of densitometer. Under carefully controlled conditions of
exposure and development, this method is capable of a high degree of
accuracy.
Ordinary photographic emulsions are sensitive in the region of
280 to 500 nm.
a Throughout the document, the term dose refers to the action
spectrum weighted radiant exposure.
2.2.2.2 Chemical methods
Chemicals which undergo some measurable change on exposure to UVR
can be used for the measurement of radiant exposure. These methods are
relatively simple, but are slow and require laborious analysis. They
are sensitive to temperature and to small amounts of impurities. The
most widely used detector has been the acetone-methylene blue
reaction. A more accurate actinometer is based on the rate of
photochemical decomposition of oxalic acid in the presence of uranyl
acetate. A system based on the photolysis of iron (III) oxalate is
more sensitive (Meyer & Seitz, 1949; Koller, 1965). Recently, chemical
dosimeters with action spectra similar to that of human skin erythema
have been reported by Zweig & Henderson (1976). Challoner et el.,
(1976) have used change in the coloration of a plastic film for this
purpose.
2.2.2.3 Biological detectors
The human skin has been used as a UVR dosimeter in an indirect
fashion (Robertson, 1975) and some work using microorganisms as a UVR
dosimeter has been reported (Latarjet, 1977; Billen & Green, 1975).
2.2.3 Physical detectors
Physical detectors have been reviewed by Koller (1965) and Kiefer
(1971).
2.2.3.1 Radiometric devices
These radiation detectors depend for their response on the
heating effect of radiation. The change of temperature due to heating
can, for example, be detected with a thermopile or a resistance
thermometer, that is a bolometer. Their spectral response is normally
quite constant over a wide range of wavelengths. Because these sensors
detect energy, they are not very sensitive to UVR and are mainly used
for standardization.
Table 1. Some basic radiometric terminology
Term SI International Definition Comments and Synonyms
units symbol
Wavelength nm, µm lambda Nanometer = 10-9 metre (also
called "millimicron", mµ;
µm, micrometer, micron = 10-6
metre
Radiant energy J Qe 1 joule = 1 watt second
Radiant flux W phi, Pe dQe Rate of radiant energy delivery
dt ("radiant power"). mW= 10-3W
µW = 10-6 W
Radiant intensity W/sr Ie dPe Describes the radiant flux emitted
d omega by the source Into a given solid
angle (solid angle expressed In
steradlana).
Irradiance W/m2 Ee dPe In photobiology, has been express
dA in W/cm2, mW/cm2 or µW/cm3.
Radiant flux arriving over
a given area. Note Implied
dependence of irradiance on the
angle of the area being irradiated,
relative to a beam. In a collimated,
uniform beam, the irradiance
Ee on a planar surface
varies directly with cos theta, where
theta = angle of incidence from normal
to the surface ("dose-rate"
"intensity", see section 2.2.1-1).
Table 1 (contd)
Term SI International Definition Comments and Synonyms
units symbol
Radiant exposure J/m2 He Ee x t Has been expressed as J/cm2 or
mJ/cm2. ("exposure dose",
"dose", see section 2.2.1).
Note: The subscript "e" serves to distinguish radiometric quantities from photometric quantities,
which have a "v" subscript The "e" subscript is often dropped when only radiometric
terms are used.
t = exposure in seconds
Table 2. Conversion between irradiance units
W/m2 mW/cm2 µW/cm2
1 W/m2 = 1 0.1 100
1 mW/cm2 = 10 1 103
1 µW/cm2 = 0.01 10-3 1
1 erg/cm2 * s = 10-3 10-4 0.1
1 erg/m2 * s 10-7 10-8 10-5
Similar conversions hold for radiant exposure units, if watts (W) are
replaced by joules (J) in the table.
2.2.3.2 Photoelectric devices
These are detectors based on a quantum effect such as the
production of electrons by absorbed photons. Their sensitivity varies
inherently with the energy of the photon (the wavelength of the
radiation). The place and width of the spectral response band depends
on the detector material. In general, these detectors are much more
sensitive than radiometric sensors.
Photomultiplyers, photovoltaic cells, and some, semiconductors
can be used for detecting UVR.
2.2.4 Measuring devices
In order to perform specific UVR measurements, the detector will
normally be placed behind some wavelength selective device such as a
bandpass filter or a monochromator.
It is very important that particular attention is paid to the
form of the spectral response, as this determines how the result can
be used.
For instance, the results from an instrument with a response
according to the skin erythema will not be valuable for atmospheric
research, or other biological effects, e.g., vision. This is because
most of the detail of the spectral information is lost by integration
over a specific action spectrum curve. On the other hand, high
resolution data from a spectroradiometric device can be integrated
afterwards for various uses. However, such measurements are much more
complicated and expensive. Thus, there will always be a conflict
between the information really needed and the amount of effort needed
to acquire the data.
Analogue integrating dosimeters are designed to simulate the
action curve of a particular process, such as skin erythema
(Robertson, 1969; Berger et al., 1975; Lazarev et al., 1975; Sivilova
et al., 1975). Measurements with such a sensor are applicable only to
biological responses with the same, or very similar, action spectra.
3. BIOLOGICAL EFFECTS OF ULTRAVIOLET RADIATION ON UNICELLULAR
ORGANISMS, MAMMALIAN CELLS AND TISSUE, AND INVERTEBRATES
3.1 Introduction
All photobiological responses to UVR and visible radiation are
dependent on the energy of the incident photons, with a maximum
response at a fairly well-defined photon energy within a limited
range, and a "threshold" beyond which the lower photon energies are
very much less effective. This is mainly because of the ability of
biologically important molecules to absorb appropriate photons, since
without such absorption no effect is possible.
The biological effectiveness of a beam of radiation depends on
the photon flux and on the relative efficiency of the photon energy to
produce a particular biological effect. When the beam contains photons
with a range of energies, it is assumed that the overall effect is
equal to the sum of all the individual contributions determined by the
product of the intensity at each photon energy and its relative
biological efficiency. The most spectacular photobiological effects,
other than vision, involve photons of energy greater than about 3.9 eV
(wavelength less than 320 nm). There are, however, some processes that
operate on photons with energy between 4 and 3 eV and even less. When
the effectiveness of a beam is to be evaluated, it is essential that
the relative efficiency of all photon energies (or wavelengths) be
known and allowed for, by comparing the appropriate action or response
spectrum with the intensity spectrum of the beam.
3.1.1 Absorption spectra
Absorption of photons of UVR by a molecule results in the
conversion of radiant energy into rotation-vibrational energy, and a
change in the electronic configuration inside the molecule. In the
ground state, most of the molecules are in a singlet state and
absorption of light causes a transition into an excited singlet state
from which they may pass into the excited triplet state of lower
energy. For many molecules this metastable state is chemically
reactive. However, the opposite is true for oxygen.
The light-absorbing capacity of a molecule depends not only on
the electronic configuration of the molecule but also on the
possibility of higher energy states (Smith & Hanawalt, 1969). The
absorption spectrum of a given substance is the quantitative
description of its capacity for the absorption of photons in a
particular range of electromagnetic frequencies.
Among the components of living matter, only the unsaturated
organic compounds should be taken into consideration, since others
show negligible absorption (at least above 200 nm). The effects on
water need not be dealt with, since it has practically no absorption
above 185 nm (Jagger, 1969).
Chromophores are the chemical groupings of a molecule that can
absorb photons. Molecular constituents that contain conjugated double
bonds freely absorb energy in the UV region. Benzene rings with one or
two atoms of nitrogen show high absorption in the UV-B. Porphyrins,
some steroids, and long-chain compounds such as carotene show good
absorption in the UV-A.
In the nucleic acids, the absorption takes place in the purines
and pyrimidines which absorb at 260 nm.
As far as proteins are concerned, tyrosine, tryptophane, and
peptide bonds are the major chromophores. Absorption of UV photons by
a protein is roughly equivalent to the sum of the absorption by its
constituent amino acids. The absorption peak is usually located at
280 nm.
3.1.2 Evaluation of administered and absorbed doses
While it is easy to measure the dose administered, the dose
absorbed depends on: the composition of the medium, the constituents
of which absorb differently in the UVR; the thickness of the medium;
and on the heterogeneity of the cell material itself including the
thickness of the cell layer, the distribution of the intracellular
organelles and pigments, and the structure and configuration of the
molecules at the time of irradiation (Jagger, 1967). As with
microorganisms, most of the work on this subject has been done using
the low pressure mercury arc, but more reports are now appearing in
relation to UV-A and UV-B.
In the UV-C, cells absorb in the nucleic-acid bland with a peak
at 260 nm. Absorption between 270 and 290 nm with a peak at 280 nm
corresponds to absorption by proteins (amino acids). In the UV-A and
the UV-B, absorption varies considerably, depending on the quantity of
absorbing intracellular molecules (porphyrins, haemoglobin,
cytochrome, carotene, etc.) found in the natural state in certain
cells.
3.1.3 Action spectra
An action spectrum indicates the wavelengths that are most
capable of producing a given effect. Comparison of an action spectrum
with an absorption spectrum of certain constituents of an irradiated
substance or cell often makes it possible to identify the component
responsible for the effect obtained.
In more complex systems, where secondary reactions enter into the
effect measured, the identities of the absorption and action spectra
become less definite, and the conclusions to be drawn, much less
certain.
In bacteria for example, the curve of effectiveness of the UV
wavelengths will peak at 265 nm and is similar to the absorption
spectrum of nucleic acids. It may be deduced that the main target is
DNA (Smith & Hanawalt, 1969).
3.2 The Molecular Basis of the Effects of Ultraviolet Radiation on
Living Matter
3.2.1 Molecular lesions in DNA
Deoxyribonucleic acid (DNA) is one of the most important target
molecules for photobiological effects. DNA can be represented as a
double-stranded helix built up of purine and pyrimidine bases, held
together by sugar and phosphate groups. If the features of the DNA
macromolecule and the universality of the cell structure of living
organisms in which DNA represents the genetic heritage are considered,
it can be anticipated that any lesion inflicted on DNA, however
slight, may have serious repercussions. A lesion in a cell genome is
always serious, because, in genera], the genome exists only in one
copy in the cell concerned, whereas a lesion in a protein, even of the
same magnitude, may remain undetected because there are many copies of
the proteins. The latter is also true of ribonucleic acids (RNA).
Most studies have been performed with low pressure mercury arcs
emitting primarily UVR of 254 nm. Excellent reviews of this subject
include those by Setlow (1968), Latarjet (1972), and Smith (1974).
The effect of UVR is above all destructive. The most common
changes produced in DNA are damage to the bases and to the
polynucleotide chains. Damage to the bases may be unimolecular or
bimolecular. Since pyrimidine bases are ten times more sensitive to
UVR than purine bases, the only unimolecular reaction discussed will
be the formation of pyrimidine hydrates.
Bimolecular reactions are very numerous. They may occur between
two bases, or between a base and another molecule. The most important
effect is the formation of dimer compounds, particularly thymine
dimers. Thymine dimers were demonstrated by Beukers & Berends (1960)
in frozen solutions of irradiated pyrimidine (a special orientation of
the bases being necessary before the dimers could be formed). The
dimer brings about a twisting of the secondary helical structure of
DNA and causes local denaturation. New biochemical methods have made
it possible to detect dimers in vivo in all types of irradiated
cells studied. The number of dimers has been shown to be proportional
to the dose of UVR and to vary with wavelength with a peak at 280 nm.
While the production of dimers has been shown to be directly linked to
the harmful effects of UVR on biological material, it is not the only
serious lesion produced in DNA by UVR.
Dimers are normally produced by UV-B but can also be formed after
exposure to UV-A (Pollard, 1974) and after photosensitization
reactions (Lamola & Yamane, 1967). Product additions to DNA bases are
very numerous (Smith, 1974). Cross-links between DNA bases and
proteins are generally formed after exposure to very high doses of
UV-A (Varghese, 1973). They also occur following exposure to UV-A in
the presence of photosensitizers such as acridine.
Following exposure to UV-A, numerous addition products are formed
with photosensitizing agents including the aromatic ketones,
acetophenone, and benzophenone (Helene & Charlier, 1971), or the
furocoumarins (Chandra, 1972). Polynucleotide chain breaks represent
another type of lesion that may occur in DNA. RNA, the structure of
which is similar to that of DNA, can be directly affected by UVR, but
since the biosynthesis is a continuous process and RNA exists in
multiple copies, very high doses of UVR are needed before such lesions
have any serious repercussions. UVR also produces dimers in RNA (Huang
& Gordon, 1973).
The proteins that make up the bulk of the cell may sustain damage
to the secondary or tertiary structures. Breaks may occur in peptide
chains or bonds or cross-links (Smith, 1974).
3.2.2 Consequences of photolesions
The distortion produced in the DNA-molecule prevents it from
carrying out its functions, i.e., transcription and replication may be
blocked. These lesions can be recognized by repair enzymes or may act
as a signal for other biological processes to intervene. They may
result in cell death, genetic recombination, mutagenesis, or even
carcinogenesis.
Inhibition of DNA synthesis by UVR has long been known to occur
(Kelner, 1953) and has been shown to be a sensitive parameter for
evaluating the effects of UVR (Smith & Hanawalt, 1969). The restarting
of DNA synthesis after a more or less long delay shows that
photolesions can be repaired and these mechanisms are of greatest
importance. Synthesis and transcription of RNA may also be blocked
(Sauerbier, 1976).
3.2.3 Repair of UVR-induced lesions
The existence of several distinct repair mechanisms that operate
in almost all cells but vary considerably in their respective
effectiveness has been demonstrated in in vivo studies of
UVR-induced DNA lesions. The importance and complexity of the repair
processes has been described in numerous reviews and in a book by
Hanawalt & Setlow (1975).
3.2.3.1 Prereplication repair
Photoreactivation. Photoreactivitation was the first discovered
and most primitive mode of repair. As long ago as 1949, both Kelner
and Dulbecco noted that certain bacteria contained a so-called
"photoreactivating" enzyme. The enzyme has been shown to recognize the
dimer and to bind to DNA in the dark. In a wet medium and with
exposure to visible light or long-wave UVR (330 to 550 nm), which
provides the energy needed for the reaction, the enzyme monomerizes
the dimer by breaking the cyclobutane linkages, thus restoring the
molecule to its original state. It is the only repair mechanism in
which a primary UVR lesion can be chemically reversed and where repair
is completed in a single enzymatic stage (Rupert, 1975). This mode of
repair has been demonstrated in all living organisms including
mammals.
Excision repair. Unlike photoreactivation, this repair process
does not require light. It takes place through recognition of the
lesion by complex enzyme mechanisms. This repair process is not
specific for UVR-induced dimers. Similar lesions caused by nitrogen
mustard, 4-nitroquinoline, mitomycin C, nitrous acid, ionizing
radiation etc., can be repaired by the same process.
Following the formation of a dimer, DNA is incised at the bases
near the dimer by a specific endonuclease. The DNA segment bearing the
dimer is then eliminated by an exonuclease and the gap thus created is
filled by local synthesis of DNA, the intact homologous strand serving
as a template. The continuity of the strand is re-established by a
ligase that welds the ends of the resynthesized portion to the
undamaged continuous segment (Boyce & Howard-Flanders, 1964; Pettijohn
& Hanawalt, 1964; Setlow & Carrier, 1964). This type of repair has
been demonstrated in all living organisms including mammals.
3.2.3.2 Repair during or after replication
This type of repair is not initiated by enzymatic recognition of
the lesion. In this case, replication does occur but the lesion is
ignored or bypassed. There remains a gap in the DNA on the side
opposite to the damaged region which can be filled by a process of
"recombination". Homologous DNA molecules with photolesions can use a
process for the exchange of intact genetic material to weld together
the undamaged segments and restore a normal DNA molecule (Rupp &
Howard-Flanders, 1968).
This mode of repair has also been observed in various types of
cells. However the operational capacity of such repair processes is
different from that of photoreactivation. These processes may become
more easily saturated and, above certain UVR doses, the proportion of
unrepaired lesions increases considerably.
3.2.3.3 SOS repair
Since repair yields are never complete, the residual lesions may
then cause errors such as mutations, the frequency of which seems to
depend on the effectiveness of the repair systems. Witkin (1969) has
analyzed the processes of error-prone repair in bacteria, which Radman
(1975) named SOS repair.
It occurs in phage and bacterial DNA which still contains lesions
such as dimers. These can block the normal operation of other repair
processes. The presence of these lesions represents an SOS signal for
the triggering of certain repair mechanisms that are normally
repressed. Replication is then effected by fraudulent incorporation of
bases that cause mutations.
3.3 Bacteria and Yeasts
Most of the principles of molecular photobiology have been
established as a result of work on Escherichia coli and its numerous
mutants, and on the specific phages that infect it. However, it must
be remembered that these results may not, in all cases, be valid for
mammalian cells.
3.3.1 Effects on bacterial cell constituents and macromolecular
synthesis
Apart from chromosomes, structures containing membrane-bound DNA
and structures containing RNA are the main targets for UVR-induced
lesions, which are reflected in alterations in the templates needed
for macromolecular syntheses. DNA synthesis is first blocked, at least
for a time. The blockage is photoreversible. Changes in other
biochemical constituents of varying importance may occur (Jacobson &
Yatvi, 1976).
3.3.2 Sublethal effects
Functional alteration is shown by a slowing-down of the growth
rate of bacteria, which may also grow abnormally without dividing into
filaments.
Survival is evaluated on the basis of counting the colonies that
can be formed by surviving cells. This method is one of the most
sensitive and most commonly used for evaluation of the biological
effects of UVR at the cellular level.
3.3.3 Effects of ultraviolet radiation of wavelengths longer
than 280 nm
Effects produced by UVR longer than 280 nm can be divided into
those specific to these wavelengths and those that are similar to the
effects of UV-C. The increase in complexity of the mechanisms that
come into play at these wavelengths has been demonstrated by Mills et
al., (1975) and Jagger (1976).
A cell irradiated at 254 nm is said to tolerate 5´ times as many
dimers as one irradiated at 365 nm. To kill E.coli, a dose of UV-A
one thousand times greater than that of UV-C or UV-B is required
(Tyrell, 1973).
A review by Jagger (1976), which emphasizes the complexity of the
processes induced by UV wavelengths other than 254 nm, affords a
glimpse of the difficulties that will be encountered in interpreting
results relating to eukaryotic cells.
3.3.4 Genetic factors in photosensitivity
The UVR resistance or UVR sensitivity of the various bacterial
mutants depends on the genetic make-up of the species concerned. The
UVR doses that have to be used to produce the same effect, and the
number of dimers, the induction and excision rate of which are
responsible for this sensitivity, vary considerably (Hill, 1958;
Setlow & Duggan, 1964; Lewis & Kumta, 1972). The enzymes responsible
for the repairs of the lesions are also genetically coded as are the
enzymes responsible for regulating the expression of the repair
enzymes (Hanawalt & Setlow, 1975).
3.3.5 Repair of photolesions in bacteria
All the modes of repair described in section 3.2 are applicable
to, and were mainly discovered in, bacteria. As long ago as 1963,
Setlow & Setlow determined, inter alia, the photoreactivation
mechanisms demonstrating that monomerization was related to the
decrease in the number of photolesions.
3.3.6 Yeasts
Yeasts are microorganisms but are nevertheless eukaryotic cells.
Resnick (1969), Fabre (1971), Cox & Game (1974), and Haynes (1975),
among others, have obtained a great variety of mutants with different
photosensitivities. These mutants differ from the wild type with
regard to UV lethality, mutagenesis, and recombination. Genetic
analysis has shown that several recessive genes are involved in the
control of these responses in a way that is already much more complex
than that found in bacteria. Moustacchi et al. (1975) have reviewed
the specific aspects of repair mechanisms in yeasts as a model for
eukaryotic cell systems.
3.4 Protozoa
Protozoa, including the ciliata paramecium, tetrahymena, and
blepharisma, and amoebae have proved very useful as tools for studies
on the biological effects of UVR (Skreb et al., 1972; Whitson, 1972).
3.5 Effects on Mammalian Cells in Culture
An attempt has been made to apply the knowledge of the mechanisms
of photolesion formation and repair discovered in microorganisms to a
model more similar to the human body; namely established strains of
mammalian cell cultures. The strains most commonly used are HeLa
(human cells of cancerous origin), mouse L fibroblasts and Chinese
hamster cells.
The ability of a surviving cell to form a colony (Lee & Puck,
1960) and the numerous parameters used in radiobiology (Elkind &
Whitmore, 1967) have been widely used to study the effects of UVR on
this material. Among the numerous works on this subject, reference has
often been made to the review paper by Rauth (1970).
3.5.1 Sublethal effects
The most marked effects of UVR on cells are death, mutagenesis,
and malignant transformation. Sublethal effects include various
degrees of inhibition of growth and colony-forming ability. At low
doses, the growth of L fibroblasts is merely slowed down. Higher
doses, however, bring about lysis of the cells (Djordjevic & Tolmach,
1967), although the value of lysis as a parameter is doubtful. All
cell strains give a similar response.
In a study of the colony-forming ability of synchronized and then
irradiated cells, Djordjevic & Tolmach (1967) and Han & Sinclair
(1969, 1971) found that cells were quite resistant at the beginning of
G1 but that sensitivity began to increase at the end of G1 reaching
a peak in the middle of the S phase of DNA synthesis. Thereafter,
sensitivity gradually decreased. In G2, the situation varied
according to the type of cell. However, all authors agree that peak
sensitivity occurs from the beginning to the middle of the S phase of
the cell cycle.
3.5.2 Effects of UV-A
Studies of the effects of UV-A on bacteria have made it possible
to elucidate, at least in part, the complex phenomena that occur in
mammalian cells. Wang et al. (1974) showed that cells can be damaged
by UV-A or visible light (300-420 nm with a peak at 365 nm). The
shoulder of the inactivation curve changes according to cell density,
but the curves remain similar according to the origin of the strain.
Evaluation of viability with trypan blue stain showed that, after
exposure to 2 X 104 J/m2, 99% of human cells, 90% of mouse cells,
and 50% of hamster cells were destroyed.
The fact that survival depends on the density of the cell
population suggests that perhaps some of the effects of UV-A are
indirect. This is supported by the observation of Wang (1975) that the
medium in which cells have been irradiated is toxic. Wang et al.,
(1974) have also shown that riboflavin may cause considerable
photosensitization of cells exposed to UV-A.
3.5.3 Lesions produced in DNA
The lesions produced by UVR in mammalian cellular DNA may be
divided into two categories that are not mutually exclusive, i.e.,
those that prevent replication processes and those that permit
replication processes, but with considerable error.
3.5.3.1 Pyrimidine dimers
It is thought that, as in other types of cells already mentioned,
the formation of pyrimidine dimers results in the essential lesion
that causes most of the effects observed. However, not all the
observed effects can be attributed to dimers and other photoproducts
should not be neglected.
3.5.3.2 DNA-protein cross-links
As demonstrated some time ago (Smith & Hanawalt, 1969),
DNA-protein cross-links are to be expected in view of the
configuration of the DNA molecule, with its backbone folded back on
itself and its association with chromosomal proteins.
DNA-protein cross-links may also help to kill cells by preventing
the switching-on of repair processes (Todd & Han, 1976).
3.5.4 The consequences of photolesions in mammalian cells
3.5.4.1 Inhibition of DNA synthesis
As in microorganisms, the major effect in mammalian cells is a
more or less marked and long-lasting inhibition of DNA synthesis. The
rate of synthesis can be estimated directly by incorporating tritiated
thymidine into the DNA.
3.5.4.2 Chromosome aberrations and mutagenic effects
As regards the effects of UVR on the chromosomes of mammalian
cells, several types of lesions were described long ago including
breaks and rearrangement. A review has been published by Rauth (1970).
These lesions have been studied (among others) in Chinese hamster
cells and human lymphocytes. Chromosome lesions are generally produced
by low doses of UVR. Their production is enzyme-dependent and is
related to repair mechanisms (Bender et al., 1973). They do not
necessarily appear after the first division (Parrington, 1972). They
can be photoreactivated and, therefore, are probably produced by
pyrimidine dimers (Griggs & Bender, 1973). Rommelaere et al., (1973)
discovered another widespread type of lesion in the form of
sister-chromatid exchanges, the frequency of which increased greatly
after UV irradiation. This parameter has been shown to be extremely
sensitive (Ikushima & Wolff, 1974) and, thus, valuable in detecting
very low doses of UVR, although it is not specific to that agent.
UV and X-ray irradiation exert a synergistic effect on the
frequency of chromosome breaks in human lymphocytes (Holmberg &
Jonasson, 1974).
3.5.5 The repair of photolesions
Animal cells in culture also possess the ability to repair
photolesions in DNA. While the lesions are comparable to those
observed in bacteria, and have already been described, there are some
differences in the repair mechanisms (Hanawalt & Setlow, 1975).
3.5.5.1 Photoreactivation
It was believed that photoreactivation was absent from the cells
of placental mammals. However, Sutherland (1974), using appropriate
biochemical techniques, isolated an enzyme in human leukocytes with
properties similar to those of the photoreactivating enzyme. In
mammals, this enzyme is probably not expressed or is masked by other
more effective repair mechanisms. Its presence in other tissues --
nervous, hepatic, and renal -- which are never exposed to light,
suggests that it may have other functions.
3.5.5.2 Excision repair
The number of dimers formed in human cells increases linearly
with the dose of UVR, if the cells are fixed immediately after
irradiation (Cleaver & Trosko, 1969). After a variable lapse of time,
cells begin to excise their dimers in the way already described
(Cleaver et al., 1972). Edenberg & Hanawalt (1973) showed that, four
hours after irradiation with 0.2 J/m2, about 50% of the dimers had
been excised. The proportion varied between 30 and 90% depending on
the type of cell.
Repair replication had already been detected by autoradiography
and shown to be unscheduled DNA synthesis which differed from the
synthesis of normal replication (Rasmussen & Painter, 1964).
Excision repair has been detected in rodents even though the
efficiency is very low.
3.5.5.3 Repair during or after replication
Numerous authors have shown by various biochemical methods that
synthesis of DNA of low relative molecular mass takes place
immediately after irradiation and that, a short time afterwards, this
newly-formed DNA is of normal mass (Painter, 1975).
The dimers prevent replication from being carried out
continuously. Replication probably occurs between the dimers that have
not yet been excised, leaving gaps in the new strands opposite each
dimer of the parental strand (Lehmann, 1972). The mechanism by which
replication fills the gaps has not yet been properly elucidated, nor
has the degree to which the replication is error-free. It is probable
that part, at least, of the repair is inaccurate as a result of errors
of replication opposite a lesion. Depending on the degree of accuracy,
either repair is total or a lesion persists that may lead to death,
mutation, or carcinogenesis.
3.5.5.4 SOS repair
As will be seen later, in mammalian cells, the repair systems of
the host cell may play a part in repairing an irradiated virus
developing in the cell.
3.5.6. Effects on cell-virus relationships
3.5.6.1 Sensitivity to vital infection
Infection with herpes virus is enhanced in mammalian cells
exposed to UV-A (Mills et al., 1975). Relatively low exposures
increase infectivity by 20-30% and it remains high for several days.
3.5.6.2 Vital transformation
UV-C increases the rate of transformation of mouse and hamster
cells by various viruses (Lytle et al., 1970). However, it does not
induce direct transformation in all cell species. Certain
photosensitizing agents enhance this viral transformation (Casto,
1973).
3.5.6.3 Activation of viruses
Since some mammalian cells harbour viruses that normally remain
latent, their activation or induction might transform them and endow
them with the characteristics of cancer cells. UV-C exposure of rat or
hamster cells, transformed by certain oncogenic viruses, can activate
the production of virus particles (Hellman et al., 1974), whereas
irradiation of normal cells can activate tumour viruses of the
leukemia-leukosis type (Lytle, 1971).
However, there is nothing to show that malignant transformation
necessarily involves the development of an oncogenic virus.
3.6 Effects on Invertebrates
Since the invertebrates are extremely heterogeneous, it is very
difficult to draw general conclusions, and the data on the response of
certain invertebrates will be given without such conclusions.
3.6.1 Effects on eggs and embryos of invertebrates
Hamilton (1973) emphasized the value of invertebrate eggs for
radiation studies. Since UVR has low penetration, only very
transparent small eggs can be totally irradiated. The others must be
stripped or dechorionated by physical or chemical means. The whole egg
or part of an egg has been irradiated with UVR mainly to destroy
certain cells, in order to watch how development proceeds in their
absence and thus deduce the role they play. Some doses, while not
preventing segmentation, arrest the onset of differentiation at the
sensitive stage represented by the end of blastulation and the
beginning of gastrulation.
Wavelengths of 225-313 nm have caused appreciable delays in
development. Undivided eggs have been shown to be highly sensitive to
UVR (Hsiao, 1975). There has been very little work using UV-A.
In conclusion, it may be said that, in general, eggs are well
protected against the harmful effects of UVR. If they are directly
exposed, they respond to UV irradiation by means of the mechanisms
already described. From the time when the cells begin to
differentiate, the quality and intensity of the photolesions and their
repair depend on the stage of differentiation of the embryo. If it is
not an advanced stage, regulatory mechanisms sometimes enable
photolesions produced at an early stage, when the cells are still
totipotential, to be eliminated naturally.
3.6.2 Effects on insects
Insects comprise a major part of ecosystems. UVR may act on the
vital processes of the insects in different ways and these have been
summarized by Hsiao (1975).
From the large numbers of papers that have been written, a first
conclusion is that UV-B is perceived by numerous insects. Several
diurnal and nocturnal species show positive phototropism towards UVR.
Most of the UV-A lamps used to trap insects have an emission spectrum
with a peak at 360 nm corresponding to the peak sensitivity of the UV
receptors in the insects. However, the peak differs slightly for each
species.
Solar UVR has been found to modify the biological clock in
insects and other arthropods.
Photoreactivation and other types of repair have been
demonstrated in insects.
3.7 Modification of the Effects of Ultraviolet Radiation by Chemical
Agents
Numerous chemical agents increase the photochemical reactivity of
nucleic acids. They act in various ways, by becoming incorporated in
the nucleic acids, or by forming various complexes with them that
increase their absorption or reactivity. They can also absorb UVR
directly and transfer the energy to nucleic acids.
Various physical factors may change the intensity of irradiation
and the efficiency of the repair systems.
The way in which irradiation is carried out, ie., whether it is
continuous or fractionated, total or partial is also important in the
evaluation of the final results.
3.7.1 Halogenated analogues
Incorporation of 5-bromouracil (5-BrU) and 5-bromodeoxyuridine
(5-BUdR) sensitizes both viruses and cells to UVR. A very detailed
review has been made by Hutchinson (1973).
3.7.2 Caffeine
Caffeine is a trimethylxanthine that acts by inhibiting the
repair systems. At doses not themselves toxic, caffeine considerably
increases the effects of UVR.
The absence of any effect of caffeine on excision repair suggests
that it acts by interfering with a post-replication system (Cleaver &
Thomas, 1969).
3.7.3 Furocoumarins
Photosensitizers are becoming increasingly important among agents
that modify the effects of UVR on biological systems.
Furocoumarins are natural products isolated from plants.
Important applications in therapy have led to thorough studies of
their mode of action at the molecular level (Chandra, 1972; Pathak et
al., 1974).
Musajo et al., (1974) have shown, in vitro, that furocoumarins
exert photosensitizing effects following irradiation at wavelengths of
320-400 nm. These effects result from the formation of certain
addition products with DNA and particularly from the linking of the
furocoumarin molecule with the pyrimide bases. These products may
cause breaks in the molecule and thus prevent if from carrying out its
functions. Oxidation of amino acids may also occur in proteins.
The so-called 'linear' furocoumarins such as psoralen react with
native DNA in the presence of UVR to make, monofunctional and
bifunctional addition products. The latter cause cross-linking between
the DNA strands. Certain so-called 'angular' furocoumarins (angelicin)
can only give rise to monofunctional addition products.
These addition products cause lesions that can be repaired by the
excision repair mechanism. Monofunctional addition products seem to be
easier to repair than bifunctional products (Chandra et al., 1976).
Depending on their molecular structure, not all the furocoumarins
show the same degree of photosensitization. Ben Hur & Elkind (1973)
demonstrated that, following exposure of hamster cells to UV-A, 11% of
the addition products were formed between the complementary strands.
During incubation in the dark, 90% of the cross-links gradually
disappeared from the DNA.
These reactions have found applications in the treatment of
certain skin diseases, particularly psoriasis.
3.7.4 Other photosensitizing agents
Charlier & Helene (1972) carried out an in vitro analysis of
the photochemical reactions of benzophenone and acetophenone with
purine and pyrimide derivatives in aqueous solution during irradiation
with light between 400 and 600 nm. Dimers and chain breaks were the
essential lesions.
The effects of light on bacteria in the presence of
photosensitizing chemicals such as toluidine blue and acridine yellow
were compared by Harrison (1967). The four sorts of lesions observed
-- lack of colony-forming capacity, DNA lesions and mutations, changes
in cell permeability, and enzyme inactivation -- were similar in both
cases.
Rauth & Domon (1973) studied the mechanisms of photosensitization
in animal cells in cultures with 1-cyclohexyl-3(2-morpholinyl-4-ethyl)
carbodiimide metho- p-toluene sulfonate (CMEC), which binds
preferentially to partially denatured regions of DNA after irradiation
at 254 nm, thus inhibiting replication and increasing lethality.
3.7.5 Protection by carotene
Since the work of Cohen-Bazire & Stanier (1958), some
investigations have established the protective role of carotene
against the photodynamic effects of light, in very special cases.
Since the energy level of carotenes is very low, they can accept, by
transfer, the energy of sensitizers transformed into the triplet state
and even the energy of oxygen transformed into the singlet state by
exposure to light.
Mutant strains of bacteria and fungi lacking carotenoids have
proved much more sensitive to photodynamic effects than normal
strains, but this effect is only valid for wavelengths shorter than
those that carotenes absorb (Mathews & Krinsky, 1965).
3.8 Conclusions
Knowledge of the molecular basis for the biological effects of
UVR has emphasized the importance of the photolesions produced in DNA
and the effectiveness of the enzymatic stages of repair, both of which
depend on the genetic make-up of the organism concerned.
Studies of the sensitivity of mutants, on one hand, and of
factors able to modify the effects of irradiation, on the other, will,
perhaps, make it possible to strengthen repair systems and to increase
the resistance of organisms to UVR.
The great differences observed make it difficult to lay down
protection standards.
4. THE BIOLOGICAL ACTION OF ULTRAVIOLET RADIATION ON VERTEBRATE
ANIMALS
4.1 General Aspects
The anatomical structure of the skin of vertebrate animals
resembles that of man in many respects. The surface stratum corneum is
important in affecting the penetration of UVR (Fig. 4), the
thicknesses of the stratum spinosum and stratum granulosum also affect
the relative amounts of UVR reaching the dermis. Other constituents of
the skin, e.g., the melanocyte population, the Langerhans cells,
vascular structures, as well as cutaneous innervation vary in amount
and distribution from species to species, but remain basically the
same.
Long-term results of actual measurements with an analogue
integrating UVR dosimeter, having an action spectrum similar to that
of skin erythema, are also available (Scotto et al., 1976). From these
measurements, it is apparent that there are daily fluctuations,
throughout the year, at each location. Weekly patterns are less
erratic, and seasonal variations depend on the latitude. The solar UVR
that causes skin erythema reaches a maximum intensity between 10h00
and 14h00. About 60% of the daily dose reaches the ground between
10h00 and 14h00, and 80% between 9h00 and 15h00 (Fig. 2). Similar
observations over a shorter period of time, using an instrument based
on a filtered selenium photocell, have given virtually the same
results (Garadza, 1965).
2.1.2 Artificial sources
Any material heated to temperatures exceeding 2500 K begins to
emit UVR. For practical purposes, sources emitting significant amounts
of biologically effective UVR can be classified into confined gas
discharge arcs, fluorescent lamps, and incandescent sources. It should
be noted that any UVR source emitting intense radiation below 260 nm
will produce ozone, which has to be removed to prevent any health
hazard.
2.1.2.1 Gas discharge arcs
The optical spectra produced by arcs depend on the nature of the
gas molecules through which the current discharge takes place, its
pressure, and the electrical conditions in the discharge.
Direct gas discharge arcs are widely used for the generation of
UVR. Generally, they differ from each other in such respects as the
type of gas, pressure, starting mechanisms, lamp shape, reflector
systems, electrodes, etc. (Andreev et al., 1975). As there is such a
wide variety of these arcs, it is not possible to describe them all.
However, the most important types are listed below.
Low pressure mercury arcs. These vapour lamps emit several
narrow UV bands. Most of the emitted power is of a wavelength of
253.7 nm, which is near the maximum for germicidal effectiveness,
hence its usefulness in the control of microorganisms.
High pressure mercury arcs. These vapour arcs (operating at
20-100 atmospheres, i.e., 200-1000 kPa, and usually encased in quartz
envelopes) emit much broader and more intense bands of UVR than low
pressure mercury arcs at wavelengths of 254, 297, 303, 313 and 365 nm.
They are extensively used in industry in photochemical reactors and in
printing. Other uses include phototherapy of skin diseases (Meyer &
Seitz, 1949; Nilender & Gavanin, 1971).
High pressure xenon arcs. These arc lamps may operate at very
high internal pressures, and have the advantage that the spectral
distribution of their radiant output shows a continuum similar to that
of the sun above the stratosphere. Their output is quite constant over
long periods of time, and they are available in a variety of
configurations. The major problem with xenon arcs is their very high
emission in the near infrared region (Gavrilova et al., 1975). Their
uses are similar to those of mercury arcs.
Flash tubes. Another form of gas discharge arc that produces
UVR is usually referred to as the flash tube. The gas within a flash
tube is excited and/or ionized, when a capacitor is discharged, to
pass an avalanche of fast electrons through the gas between the
electrodes. Depending on the gas used, i.e., xenon, krypton, argon,
neon, etc., different optical spectra are produced.
2.1.2.2 Fluorescent lamps
A fluorescent lamp contains an electric arc discharge source.
UVR, generated at high efficiency by mercury vapour in an inert gas at
low pressure, activates a coating of fluorescent material (phosphor)
on the inner surface of a glass tube. The phosphor simply acts as a
"transformer", converting shorter wavelength UVR into longer
wavelength radiation, i.e., UV and/or visible light. The spectral
characteristics, which depend on the phosphor used, vary with the gas
pressure in the lamp, and the temperature at the coldest point in the
lamp.
"Fluorescent Sun" (FS) type UVR emitters. "Fluorescent Sun"
type emitters contain a phosphor that emits more than half of its
radiant output at wavelengths shorter than 340 nm. In general, the
range of UVR emitted is from 275 to 380 nm, but the maximum is located
at 313 nm. Thus, this light source is extremely effective in producing
suntan, sunburn, and, at least in animals, cutaneous cancer. The
linear configuration of fluorescent lamps has a distinct advantage for
any application where a uniform irradiation field of considerable size
is required. The disadvantage of these lamps is that there is very
much less energy output per unit area compared with compact mercury or
xenon high pressure arc sources (Sozin, 1975).
"Black Light" (BL) type UVR emitters. The "Black Light" type
UVR emitters are very similar in construction to the FS lamps, except
that the phosphor used emits radiation ranging from 300 to 410 nm,
with a maximum in the 350-365 nm region. Usually, BL lamps emit less
than 0.1% of their total UVR in the less than 320 nm (i.e.,
biologically most effective) region. Their primary use is for
producing fluorescence in a variety of paints and inks. In recent
years, such BL lamps have been used together with photoactive drugs
such as 8-methoxypsoralen in phototherapy of skin diseases (Parrish et
al., 1974).
2.1.2.3 Carbon arcs
The arc is due to an electric discharge between two carbon
electrodes in air at atmospheric pressure. The output of radiation
from a carbon arc increases with increase in arc current and its more
or less continuous shape depends, in part, on the type of metal added
to the electrodes. The use of these arcs has been limited, because of
gaseous waste products that require adequate venting, and the
maintenance problems that result from consumable electrodes.
2.1.2.4 Quartz halogen lamps
These are tungsten filament lamps, enclosed in a quartz envelope,
filled with a small amount of a halogen gas, usually iodine or
bromine. This allows operation at much higher temperatures without
deposition of the metal on the envelope. Such lamps, which may operate
at temperatures up to 3500 K or more, are stable and very intense.
Their UVR output is mainly in the region above 330 nm, and their
primary use is in illumination and as reference standard lamps.
2.1.2.5 Oxyacetylene, oxyhydrogen, and plasma torches
Oil, coal, and gas flames normally operate below 2000 K and,
thus, emit virtually no UVR. Oxyacetylene and oxyhydrogen flames burn
at a much higher temperature and solids heated by these two flames may
radiate UVR.
Welding produces UVR in broad bands, which often appear as a
continuous spectrum. The intensities of the various bands depend on
many factors including the materials from which the electrodes are
made, the discharge current, and the gases surrounding the arc. Arc
welding is a common cause of UVR eye and skin damage.
The plasma torch can produce temperatures of over 6000 K (the
temperature at the surface of the sun) and intense UVR can result.
Exposure to radiation from plasma torches can result in
keratoconjunctivitis and sunburn, if eyes and skin are not protected.
2.2 Detection and Measurement of Ultraviolet Radiation
The measurement of UVR differs from that of visible radiation in
that the eye cannot be used directly as a detecting instrument. Thus,
other means for detection must be used, which can either be based on a
physical principle, or on a chemical or biological reaction. The
physical detectors are mainly used to measure instantaneous
irradiance, whereas the chemical and biological detectors are normally
used to determine radiant exposure (dose).a
2.2.1 Units and conversion factors
Table 1 describes terms frequently used in radiometric
techniques, and Table 2 gives a simple scheme for conversion between
commonly used irradiance units.
In addition to these energy units, units of biological effect,
based on interactions between UVR and living organisms, have been
proposed. The oldest of these is the Finsen. More recently, a system
of bactericidal units for evaluating UVR on the basis of its
disinfectant effect and a system of erythema units for evaluating UVR
on the basis of its beneficial effects on man have been described
(Lazarev & Sokolov, 1971, 1974). In order to allow for intercomparison
with other measurement units, the bactericidal and erythema quantities
are now expressed in SI units (Sokolov, 1975, 1976).
2.2.2 Chemical and biological detectors
2.2.2.1 Photographic plates
The photographic plate is the usual detector in UV spectroscopy.
The degree of blackening of the plate is a measure of radiation
intensity. The measurement is made photometrically by means of some
form of densitometer. Under carefully controlled conditions of
exposure and development, this method is capable of a high degree of
accuracy.
Ordinary photographic emulsions are sensitive in the region of
280 to 500 nm.
a Throughout the document, the term dose refers to the action
spectrum weighted radiant exposure.
2.2.2.2 Chemical methods
Chemicals which undergo some measurable change on exposure to UVR
can be used for the measurement of radiant exposure. These methods are
relatively simple, but are slow and require laborious analysis. They
are sensitive to temperature and to small amounts of impurities. The
most widely used detector has been the acetone-methylene blue
reaction. A more accurate actinometer is based on the rate of
photochemical decomposition of oxalic acid in the presence of uranyl
acetate. A system based on the photolysis of iron (III) oxalate is
more sensitive (Meyer & Seitz, 1949; Koller, 1965). Recently, chemical
dosimeters with action spectra similar to that of human skin erythema
have been reported by Zweig & Henderson (1976). Challoner et el.,
(1976) have used change in the coloration of a plastic film for this
purpose.
2.2.2.3 Biological detectors
The human skin has been used as a UVR dosimeter in an indirect
fashion (Robertson, 1975) and some work using microorganisms as a UVR
dosimeter has been reported (Latarjet, 1977; Billen & Green, 1975).
2.2.3 Physical detectors
Physical detectors have been reviewed by Koller (1965) and Kiefer
(1971).
2.2.3.1 Radiometric devices
These radiation detectors depend for their response on the
heating effect of radiation. The change of temperature due to heating
can, for example, be detected with a thermopile or a resistance
thermometer, that is a bolometer. Their spectral response is normally
quite constant over a wide range of wavelengths. Because these sensors
detect energy, they are not very sensitive to UVR and are mainly used
for standardization.
Table 1. Some basic radiometric terminology
Term SI International Definition Comments and Synonyms
units symbol
Wavelength nm, µm lambda Nanometer = 10-9 metre (also
called "millimicron", mµ;
µm, micrometer, micron = 10-6
metre
Radiant energy J Qe 1 joule = 1 watt second
Radiant flux W phi, Pe dQe Rate of radiant energy delivery
dt ("radiant power"). mW= 10-3W
µW = 10-6 W
Radiant intensity W/sr Ie dPe Describes the radiant flux emitted
d omega by the source Into a given solid
angle (solid angle expressed In
steradlana).
Irradiance W/m2 Ee dPe In photobiology, has been express
dA in W/cm2, mW/cm2 or µW/cm3.
Radiant flux arriving over
a given area. Note Implied
dependence of irradiance on the
angle of the area being irradiated,
relative to a beam. In a collimated,
uniform beam, the irradiance
Ee on a planar surface
varies directly with cos theta, where
theta = angle of incidence from normal
to the surface ("dose-rate"
"intensity", see section 2.2.1-1).
Table 1 (contd)
Term SI International Definition Comments and Synonyms
units symbol
Radiant exposure J/m2 He Ee x t Has been expressed as J/cm2 or
mJ/cm2. ("exposure dose",
"dose", see section 2.2.1).
Note: The subscript "e" serves to distinguish radiometric quantities from photometric quantities,
which have a "v" subscript The "e" subscript is often dropped when only radiometric
terms are used.
t = exposure in seconds
Table 2. Conversion between irradiance units
W/m2 mW/cm2 µW/cm2
1 W/m2 = 1 0.1 100
1 mW/cm2 = 10 1 103
1 µW/cm2 = 0.01 10-3 1
1 erg/cm2 * s = 10-3 10-4 0.1
1 erg/m2 * s 10-7 10-8 10-5
Similar conversions hold for radiant exposure units, if watts (W) are
replaced by joules (J) in the table.
2.2.3.2 Photoelectric devices
These are detectors based on a quantum effect such as the
production of electrons by absorbed photons. Their sensitivity varies
inherently with the energy of the photon (the wavelength of the
radiation). The place and width of the spectral response band depends
on the detector material. In general, these detectors are much more
sensitive than radiometric sensors.
Photomultiplyers, photovoltaic cells, and some, semiconductors
can be used for detecting UVR.
2.2.4 Measuring devices
In order to perform specific UVR measurements, the detector will
normally be placed behind some wavelength selective device such as a
bandpass filter or a monochromator.
It is very important that particular attention is paid to the
form of the spectral response, as this determines how the result can
be used.
For instance, the results from an instrument with a response
according to the skin erythema will not be valuable for atmospheric
research, or other biological effects, e.g., vision. This is because
most of the detail of the spectral information is lost by integration
over a specific action spectrum curve. On the other hand, high
resolution data from a spectroradiometric device can be integrated
afterwards for various uses. However, such measurements are much more
complicated and expensive. Thus, there will always be a conflict
between the information really needed and the amount of effort needed
to acquire the data.
Analogue integrating dosimeters are designed to simulate the
action curve of a particular process, such as skin erythema
(Robertson, 1969; Berger et al., 1975; Lazarev et al., 1975; Sivilova
et al., 1975). Measurements with such a sensor are applicable only to
biological responses with the same, or very similar, action spectra.
3. BIOLOGICAL EFFECTS OF ULTRAVIOLET RADIATION ON UNICELLULAR
ORGANISMS, MAMMALIAN CELLS AND TISSUE, AND INVERTEBRATES
3.1 Introduction
All photobiological responses to UVR and visible radiation are
dependent on the energy of the incident photons, with a maximum
response at a fairly well-defined photon energy within a limited
range, and a "threshold" beyond which the lower photon energies are
very much less effective. This is mainly because of the ability of
biologically important molecules to absorb appropriate photons, since
without such absorption no effect is possible.
The biological effectiveness of a beam of radiation depends on
the photon flux and on the relative efficiency of the photon energy to
produce a particular biological effect. When the beam contains photons
with a range of energies, it is assumed that the overall effect is
equal to the sum of all the individual contributions determined by the
product of the intensity at each photon energy and its relative
biological efficiency. The most spectacular photobiological effects,
other than vision, involve photons of energy greater than about 3.9 eV
(wavelength less than 320 nm). There are, however, some processes that
operate on photons with energy between 4 and 3 eV and even less. When
the effectiveness of a beam is to be evaluated, it is essential that
the relative efficiency of all photon energies (or wavelengths) be
known and allowed for, by comparing the appropriate action or response
spectrum with the intensity spectrum of the beam.
3.1.1 Absorption spectra
Absorption of photons of UVR by a molecule results in the
conversion of radiant energy into rotation-vibrational energy, and a
change in the electronic configuration inside the molecule. In the
ground state, most of the molecules are in a singlet state and
absorption of light causes a transition into an excited singlet state
from which they may pass into the excited triplet state of lower
energy. For many molecules this metastable state is chemically
reactive. However, the opposite is true for oxygen.
The light-absorbing capacity of a molecule depends not only on
the electronic configuration of the molecule but also on the
possibility of higher energy states (Smith & Hanawalt, 1969). The
absorption spectrum of a given substance is the quantitative
description of its capacity for the absorption of photons in a
particular range of electromagnetic frequencies.
Among the components of living matter, only the unsaturated
organic compounds should be taken into consideration, since others
show negligible absorption (at least above 200 nm). The effects on
water need not be dealt with, since it has practically no absorption
above 185 nm (Jagger, 1969).
Chromophores are the chemical groupings of a molecule that can
absorb photons. Molecular constituents that contain conjugated double
bonds freely absorb energy in the UV region. Benzene rings with one or
two atoms of nitrogen show high absorption in the UV-B. Porphyrins,
some steroids, and long-chain compounds such as carotene show good
absorption in the UV-A.
In the nucleic acids, the absorption takes place in the purines
and pyrimidines which absorb at 260 nm.
As far as proteins are concerned, tyrosine, tryptophane, and
peptide bonds are the major chromophores. Absorption of UV photons by
a protein is roughly equivalent to the sum of the absorption by its
constituent amino acids. The absorption peak is usually located at
280 nm.
3.1.2 Evaluation of administered and absorbed doses
While it is easy to measure the dose administered, the dose
absorbed depends on: the composition of the medium, the constituents
of which absorb differently in the UVR; the thickness of the medium;
and on the heterogeneity of the cell material itself including the
thickness of the cell layer, the distribution of the intracellular
organelles and pigments, and the structure and configuration of the
molecules at the time of irradiation (Jagger, 1967). As with
microorganisms, most of the work on this subject has been done using
the low pressure mercury arc, but more reports are now appearing in
relation to UV-A and UV-B.
In the UV-C, cells absorb in the nucleic-acid bland with a peak
at 260 nm. Absorption between 270 and 290 nm with a peak at 280 nm
corresponds to absorption by proteins (amino acids). In the UV-A and
the UV-B, absorption varies considerably, depending on the quantity of
absorbing intracellular molecules (porphyrins, haemoglobin,
cytochrome, carotene, etc.) found in the natural state in certain
cells.
3.1.3 Action spectra
An action spectrum indicates the wavelengths that are most
capable of producing a given effect. Comparison of an action spectrum
with an absorption spectrum of certain constituents of an irradiated
substance or cell often makes it possible to identify the component
responsible for the effect obtained.
In more complex systems, where secondary reactions enter into the
effect measured, the identities of the absorption and action spectra
become less definite, and the conclusions to be drawn, much less
certain.
In bacteria for example, the curve of effectiveness of the UV
wavelengths will peak at 265 nm and is similar to the absorption
spectrum of nucleic acids. It may be deduced that the main target is
DNA (Smith & Hanawalt, 1969).
3.2 The Molecular Basis of the Effects of Ultraviolet Radiation on
Living Matter
3.2.1 Molecular lesions in DNA
Deoxyribonucleic acid (DNA) is one of the most important target
molecules for photobiological effects. DNA can be represented as a
double-stranded helix built up of purine and pyrimidine bases, held
together by sugar and phosphate groups. If the features of the DNA
macromolecule and the universality of the cell structure of living
organisms in which DNA represents the genetic heritage are considered,
it can be anticipated that any lesion inflicted on DNA, however
slight, may have serious repercussions. A lesion in a cell genome is
always serious, because, in genera], the genome exists only in one
copy in the cell concerned, whereas a lesion in a protein, even of the
same magnitude, may remain undetected because there are many copies of
the proteins. The latter is also true of ribonucleic acids (RNA).
Most studies have been performed with low pressure mercury arcs
emitting primarily UVR of 254 nm. Excellent reviews of this subject
include those by Setlow (1968), Latarjet (1972), and Smith (1974).
The effect of UVR is above all destructive. The most common
changes produced in DNA are damage to the bases and to the
polynucleotide chains. Damage to the bases may be unimolecular or
bimolecular. Since pyrimidine bases are ten times more sensitive to
UVR than purine bases, the only unimolecular reaction discussed will
be the formation of pyrimidine hydrates.
Bimolecular reactions are very numerous. They may occur between
two bases, or between a base and another molecule. The most important
effect is the formation of dimer compounds, particularly thymine
dimers. Thymine dimers were demonstrated by Beukers & Berends (1960)
in frozen solutions of irradiated pyrimidine (a special orientation of
the bases being necessary before the dimers could be formed). The
dimer brings about a twisting of the secondary helical structure of
DNA and causes local denaturation. New biochemical methods have made
it possible to detect dimers in vivo in all types of irradiated
cells studied. The number of dimers has been shown to be proportional
to the dose of UVR and to vary with wavelength with a peak at 280 nm.
While the production of dimers has been shown to be directly linked to
the harmful effects of UVR on biological material, it is not the only
serious lesion produced in DNA by UVR.
Dimers are normally produced by UV-B but can also be formed after
exposure to UV-A (Pollard, 1974) and after photosensitization
reactions (Lamola & Yamane, 1967). Product additions to DNA bases are
very numerous (Smith, 1974). Cross-links between DNA bases and
proteins are generally formed after exposure to very high doses of
UV-A (Varghese, 1973). They also occur following exposure to UV-A in
the presence of photosensitizers such as acridine.
Following exposure to UV-A, numerous addition products are formed
with photosensitizing agents including the aromatic ketones,
acetophenone, and benzophenone (Helene & Charlier, 1971), or the
furocoumarins (Chandra, 1972). Polynucleotide chain breaks represent
another type of lesion that may occur in DNA. RNA, the structure of
which is similar to that of DNA, can be directly affected by UVR, but
since the biosynthesis is a continuous process and RNA exists in
multiple copies, very high doses of UVR are needed before such lesions
have any serious repercussions. UVR also produces dimers in RNA (Huang
& Gordon, 1973).
The proteins that make up the bulk of the cell may sustain damage
to the secondary or tertiary structures. Breaks may occur in peptide
chains or bonds or cross-links (Smith, 1974).
3.2.2 Consequences of photolesions
The distortion produced in the DNA-molecule prevents it from
carrying out its functions, i.e., transcription and replication may be
blocked. These lesions can be recognized by repair enzymes or may act
as a signal for other biological processes to intervene. They may
result in cell death, genetic recombination, mutagenesis, or even
carcinogenesis.
Inhibition of DNA synthesis by UVR has long been known to occur
(Kelner, 1953) and has been shown to be a sensitive parameter for
evaluating the effects of UVR (Smith & Hanawalt, 1969). The restarting
of DNA synthesis after a more or less long delay shows that
photolesions can be repaired and these mechanisms are of greatest
importance. Synthesis and transcription of RNA may also be blocked
(Sauerbier, 1976).
3.2.3 Repair of UVR-induced lesions
The existence of several distinct repair mechanisms that operate
in almost all cells but vary considerably in their respective
effectiveness has been demonstrated in in vivo studies of
UVR-induced DNA lesions. The importance and complexity of the repair
processes has been described in numerous reviews and in a book by
Hanawalt & Setlow (1975).
3.2.3.1 Prereplication repair
Photoreactivation. Photoreactivitation was the first discovered
and most primitive mode of repair. As long ago as 1949, both Kelner
and Dulbecco noted that certain bacteria contained a so-called
"photoreactivating" enzyme. The enzyme has been shown to recognize the
dimer and to bind to DNA in the dark. In a wet medium and with
exposure to visible light or long-wave UVR (330 to 550 nm), which
provides the energy needed for the reaction, the enzyme monomerizes
the dimer by breaking the cyclobutane linkages, thus restoring the
molecule to its original state. It is the only repair mechanism in
which a primary UVR lesion can be chemically reversed and where repair
is completed in a single enzymatic stage (Rupert, 1975). This mode of
repair has been demonstrated in all living organisms including
mammals.
Excision repair. Unlike photoreactivation, this repair process
does not require light. It takes place through recognition of the
lesion by complex enzyme mechanisms. This repair process is not
specific for UVR-induced dimers. Similar lesions caused by nitrogen
mustard, 4-nitroquinoline, mitomycin C, nitrous acid, ionizing
radiation etc., can be repaired by the same process.
Following the formation of a dimer, DNA is incised at the bases
near the dimer by a specific endonuclease. The DNA segment bearing the
dimer is then eliminated by an exonuclease and the gap thus created is
filled by local synthesis of DNA, the intact homologous strand serving
as a template. The continuity of the strand is re-established by a
ligase that welds the ends of the resynthesized portion to the
undamaged continuous segment (Boyce & Howard-Flanders, 1964; Pettijohn
& Hanawalt, 1964; Setlow & Carrier, 1964). This type of repair has
been demonstrated in all living organisms including mammals.
3.2.3.2 Repair during or after replication
This type of repair is not initiated by enzymatic recognition of
the lesion. In this case, replication does occur but the lesion is
ignored or bypassed. There remains a gap in the DNA on the side
opposite to the damaged region which can be filled by a process of
"recombination". Homologous DNA molecules with photolesions can use a
process for the exchange of intact genetic material to weld together
the undamaged segments and restore a normal DNA molecule (Rupp &
Howard-Flanders, 1968).
This mode of repair has also been observed in various types of
cells. However the operational capacity of such repair processes is
different from that of photoreactivation. These processes may become
more easily saturated and, above certain UVR doses, the proportion of
unrepaired lesions increases considerably.
3.2.3.3 SOS repair
Since repair yields are never complete, the residual lesions may
then cause errors such as mutations, the frequency of which seems to
depend on the effectiveness of the repair systems. Witkin (1969) has
analyzed the processes of error-prone repair in bacteria, which Radman
(1975) named SOS repair.
It occurs in phage and bacterial DNA which still contains lesions
such as dimers. These can block the normal operation of other repair
processes. The presence of these lesions represents an SOS signal for
the triggering of certain repair mechanisms that are normally
repressed. Replication is then effected by fraudulent incorporation of
bases that cause mutations.
3.3 Bacteria and Yeasts
Most of the principles of molecular photobiology have been
established as a result of work on Escherichia coli and its numerous
mutants, and on the specific phages that infect it. However, it must
be remembered that these results may not, in all cases, be valid for
mammalian cells.
3.3.1 Effects on bacterial cell constituents and macromolecular
synthesis
Apart from chromosomes, structures containing membrane-bound DNA
and structures containing RNA are the main targets for UVR-induced
lesions, which are reflected in alterations in the templates needed
for macromolecular syntheses. DNA synthesis is first blocked, at least
for a time. The blockage is photoreversible. Changes in other
biochemical constituents of varying importance may occur (Jacobson &
Yatvi, 1976).
3.3.2 Sublethal effects
Functional alteration is shown by a slowing-down of the growth
rate of bacteria, which may also grow abnormally without dividing into
filaments.
Survival is evaluated on the basis of counting the colonies that
can be formed by surviving cells. This method is one of the most
sensitive and most commonly used for evaluation of the biological
effects of UVR at the cellular level.
3.3.3 Effects of ultraviolet radiation of wavelengths longer
than 280 nm
Effects produced by UVR longer than 280 nm can be divided into
those specific to these wavelengths and those that are similar to the
effects of UV-C. The increase in complexity of the mechanisms that
come into play at these wavelengths has been demonstrated by Mills et
al., (1975) and Jagger (1976).
A cell irradiated at 254 nm is said to tolerate 5´ times as many
dimers as one irradiated at 365 nm. To kill E.coli, a dose of UV-A
one thousand times greater than that of UV-C or UV-B is required
(Tyrell, 1973).
A review by Jagger (1976), which emphasizes the complexity of the
processes induced by UV wavelengths other than 254 nm, affords a
glimpse of the difficulties that will be encountered in interpreting
results relating to eukaryotic cells.
3.3.4 Genetic factors in photosensitivity
The UVR resistance or UVR sensitivity of the various bacterial
mutants depends on the genetic make-up of the species concerned. The
UVR doses that have to be used to produce the same effect, and the
number of dimers, the induction and excision rate of which are
responsible for this sensitivity, vary considerably (Hill, 1958;
Setlow & Duggan, 1964; Lewis & Kumta, 1972). The enzymes responsible
for the repairs of the lesions are also genetically coded as are the
enzymes responsible for regulating the expression of the repair
enzymes (Hanawalt & Setlow, 1975).
3.3.5 Repair of photolesions in bacteria
All the modes of repair described in section 3.2 are applicable
to, and were mainly discovered in, bacteria. As long ago as 1963,
Setlow & Setlow determined, inter alia, the photoreactivation
mechanisms demonstrating that monomerization was related to the
decrease in the number of photolesions.
3.3.6 Yeasts
Yeasts are microorganisms but are nevertheless eukaryotic cells.
Resnick (1969), Fabre (1971), Cox & Game (1974), and Haynes (1975),
among others, have obtained a great variety of mutants with different
photosensitivities. These mutants differ from the wild type with
regard to UV lethality, mutagenesis, and recombination. Genetic
analysis has shown that several recessive genes are involved in the
control of these responses in a way that is already much more complex
than that found in bacteria. Moustacchi et al. (1975) have reviewed
the specific aspects of repair mechanisms in yeasts as a model for
eukaryotic cell systems.
3.4 Protozoa
Protozoa, including the ciliata paramecium, tetrahymena, and
blepharisma, and amoebae have proved very useful as tools for studies
on the biological effects of UVR (Skreb et al., 1972; Whitson, 1972).
3.5 Effects on Mammalian Cells in Culture
An attempt has been made to apply the knowledge of the mechanisms
of photolesion formation and repair discovered in microorganisms to a
model more similar to the human body; namely established strains of
mammalian cell cultures. The strains most commonly used are HeLa
(human cells of cancerous origin), mouse L fibroblasts and Chinese
hamster cells.
The ability of a surviving cell to form a colony (Lee & Puck,
1960) and the numerous parameters used in radiobiology (Elkind &
Whitmore, 1967) have been widely used to study the effects of UVR on
this material. Among the numerous works on this subject, reference has
often been made to the review paper by Rauth (1970).
3.5.1 Sublethal effects
The most marked effects of UVR on cells are death, mutagenesis,
and malignant transformation. Sublethal effects include various
degrees of inhibition of growth and colony-forming ability. At low
doses, the growth of L fibroblasts is merely slowed down. Higher
doses, however, bring about lysis of the cells (Djordjevic & Tolmach,
1967), although the value of lysis as a parameter is doubtful. All
cell strains give a similar response.
In a study of the colony-forming ability of synchronized and then
irradiated cells, Djordjevic & Tolmach (1967) and Han & Sinclair
(1969, 1971) found that cells were quite resistant at the beginning of
G1 but that sensitivity began to increase at the end of G1 reaching
a peak in the middle of the S phase of DNA synthesis. Thereafter,
sensitivity gradually decreased. In G2, the situation varied
according to the type of cell. However, all authors agree that peak
sensitivity occurs from the beginning to the middle of the S phase of
the cell cycle.
3.5.2 Effects of UV-A
Studies of the effects of UV-A on bacteria have made it possible
to elucidate, at least in part, the complex phenomena that occur in
mammalian cells. Wang et al. (1974) showed that cells can be damaged
by UV-A or visible light (300-420 nm with a peak at 365 nm). The
shoulder of the inactivation curve changes according to cell density,
but the curves remain similar according to the origin of the strain.
Evaluation of viability with trypan blue stain showed that, after
exposure to 2 X 104 J/m2, 99% of human cells, 90% of mouse cells,
and 50% of hamster cells were destroyed.
The fact that survival depends on the density of the cell
population suggests that perhaps some of the effects of UV-A are
indirect. This is supported by the observation of Wang (1975) that the
medium in which cells have been irradiated is toxic. Wang et al.,
(1974) have also shown that riboflavin may cause considerable
photosensitization of cells exposed to UV-A.
3.5.3 Lesions produced in DNA
The lesions produced by UVR in mammalian cellular DNA may be
divided into two categories that are not mutually exclusive, i.e.,
those that prevent replication processes and those that permit
replication processes, but with considerable error.
3.5.3.1 Pyrimidine dimers
It is thought that, as in other types of cells already mentioned,
the formation of pyrimidine dimers results in the essential lesion
that causes most of the effects observed. However, not all the
observed effects can be attributed to dimers and other photoproducts
should not be neglected.
3.5.3.2 DNA-protein cross-links
As demonstrated some time ago (Smith & Hanawalt, 1969),
DNA-protein cross-links are to be expected in view of the
configuration of the DNA molecule, with its backbone folded back on
itself and its association with chromosomal proteins.
DNA-protein cross-links may also help to kill cells by preventing
the switching-on of repair processes (Todd & Han, 1976).
3.5.4 The consequences of photolesions in mammalian cells
3.5.4.1 Inhibition of DNA synthesis
As in microorganisms, the major effect in mammalian cells is a
more or less marked and long-lasting inhibition of DNA synthesis. The
rate of synthesis can be estimated directly by incorporating tritiated
thymidine into the DNA.
3.5.4.2 Chromosome aberrations and mutagenic effects
As regards the effects of UVR on the chromosomes of mammalian
cells, several types of lesions were described long ago including
breaks and rearrangement. A review has been published by Rauth (1970).
These lesions have been studied (among others) in Chinese hamster
cells and human lymphocytes. Chromosome lesions are generally produced
by low doses of UVR. Their production is enzyme-dependent and is
related to repair mechanisms (Bender et al., 1973). They do not
necessarily appear after the first division (Parrington, 1972). They
can be photoreactivated and, therefore, are probably produced by
pyrimidine dimers (Griggs & Bender, 1973). Rommelaere et al., (1973)
discovered another widespread type of lesion in the form of
sister-chromatid exchanges, the frequency of which increased greatly
after UV irradiation. This parameter has been shown to be extremely
sensitive (Ikushima & Wolff, 1974) and, thus, valuable in detecting
very low doses of UVR, although it is not specific to that agent.
UV and X-ray irradiation exert a synergistic effect on the
frequency of chromosome breaks in human lymphocytes (Holmberg &
Jonasson, 1974).
3.5.5 The repair of photolesions
Animal cells in culture also possess the ability to repair
photolesions in DNA. While the lesions are comparable to those
observed in bacteria, and have already been described, there are some
differences in the repair mechanisms (Hanawalt & Setlow, 1975).
3.5.5.1 Photoreactivation
It was believed that photoreactivation was absent from the cells
of placental mammals. However, Sutherland (1974), using appropriate
biochemical techniques, isolated an enzyme in human leukocytes with
properties similar to those of the photoreactivating enzyme. In
mammals, this enzyme is probably not expressed or is masked by other
more effective repair mechanisms. Its presence in other tissues --
nervous, hepatic, and renal -- which are never exposed to light,
suggests that it may have other functions.
3.5.5.2 Excision repair
The number of dimers formed in human cells increases linearly
with the dose of UVR, if the cells are fixed immediately after
irradiation (Cleaver & Trosko, 1969). After a variable lapse of time,
cells begin to excise their dimers in the way already described
(Cleaver et al., 1972). Edenberg & Hanawalt (1973) showed that, four
hours after irradiation with 0.2 J/m2, about 50% of the dimers had
been excised. The proportion varied between 30 and 90% depending on
the type of cell.
Repair replication had already been detected by autoradiography
and shown to be unscheduled DNA synthesis which differed from the
synthesis of normal replication (Rasmussen & Painter, 1964).
Excision repair has been detected in rodents even though the
efficiency is very low.
3.5.5.3 Repair during or after replication
Numerous authors have shown by various biochemical methods that
synthesis of DNA of low relative molecular mass takes place
immediately after irradiation and that, a short time afterwards, this
newly-formed DNA is of normal mass (Painter, 1975).
The dimers prevent replication from being carried out
continuously. Replication probably occurs between the dimers that have
not yet been excised, leaving gaps in the new strands opposite each
dimer of the parental strand (Lehmann, 1972). The mechanism by which
replication fills the gaps has not yet been properly elucidated, nor
has the degree to which the replication is error-free. It is probable
that part, at least, of the repair is inaccurate as a result of errors
of replication opposite a lesion. Depending on the degree of accuracy,
either repair is total or a lesion persists that may lead to death,
mutation, or carcinogenesis.
3.5.5.4 SOS repair
As will be seen later, in mammalian cells, the repair systems of
the host cell may play a part in repairing an irradiated virus
developing in the cell.
3.5.6. Effects on cell-virus relationships
3.5.6.1 Sensitivity to vital infection
Infection with herpes virus is enhanced in mammalian cells
exposed to UV-A (Mills et al., 1975). Relatively low exposures
increase infectivity by 20-30% and it remains high for several days.
3.5.6.2 Vital transformation
UV-C increases the rate of transformation of mouse and hamster
cells by various viruses (Lytle et al., 1970). However, it does not
induce direct transformation in all cell species. Certain
photosensitizing agents enhance this viral transformation (Casto,
1973).
3.5.6.3 Activation of viruses
Since some mammalian cells harbour viruses that normally remain
latent, their activation or induction might transform them and endow
them with the characteristics of cancer cells. UV-C exposure of rat or
hamster cells, transformed by certain oncogenic viruses, can activate
the production of virus particles (Hellman et al., 1974), whereas
irradiation of normal cells can activate tumour viruses of the
leukemia-leukosis type (Lytle, 1971).
However, there is nothing to show that malignant transformation
necessarily involves the development of an oncogenic virus.
3.6 Effects on Invertebrates
Since the invertebrates are extremely heterogeneous, it is very
difficult to draw general conclusions, and the data on the response of
certain invertebrates will be given without such conclusions.
3.6.1 Effects on eggs and embryos of invertebrates
Hamilton (1973) emphasized the value of invertebrate eggs for
radiation studies. Since UVR has low penetration, only very
transparent small eggs can be totally irradiated. The others must be
stripped or dechorionated by physical or chemical means. The whole egg
or part of an egg has been irradiated with UVR mainly to destroy
certain cells, in order to watch how development proceeds in their
absence and thus deduce the role they play. Some doses, while not
preventing segmentation, arrest the onset of differentiation at the
sensitive stage represented by the end of blastulation and the
beginning of gastrulation.
Wavelengths of 225-313 nm have caused appreciable delays in
development. Undivided eggs have been shown to be highly sensitive to
UVR (Hsiao, 1975). There has been very little work using UV-A.
In conclusion, it may be said that, in general, eggs are well
protected against the harmful effects of UVR. If they are directly
exposed, they respond to UV irradiation by means of the mechanisms
already described. From the time when the cells begin to
differentiate, the quality and intensity of the photolesions and their
repair depend on the stage of differentiation of the embryo. If it is
not an advanced stage, regulatory mechanisms sometimes enable
photolesions produced at an early stage, when the cells are still
totipotential, to be eliminated naturally.
3.6.2 Effects on insects
Insects comprise a major part of ecosystems. UVR may act on the
vital processes of the insects in different ways and these have been
summarized by Hsiao (1975).
From the large numbers of papers that have been written, a first
conclusion is that UV-B is perceived by numerous insects. Several
diurnal and nocturnal species show positive phototropism towards UVR.
Most of the UV-A lamps used to trap insects have an emission spectrum
with a peak at 360 nm corresponding to the peak sensitivity of the UV
receptors in the insects. However, the peak differs slightly for each
species.
Solar UVR has been found to modify the biological clock in
insects and other arthropods.
Photoreactivation and other types of repair have been
demonstrated in insects.
3.7 Modification of the Effects of Ultraviolet Radiation by Chemical
Agents
Numerous chemical agents increase the photochemical reactivity of
nucleic acids. They act in various ways, by becoming incorporated in
the nucleic acids, or by forming various complexes with them that
increase their absorption or reactivity. They can also absorb UVR
directly and transfer the energy to nucleic acids.
Various physical factors may change the intensity of irradiation
and the efficiency of the repair systems.
The way in which irradiation is carried out, ie., whether it is
continuous or fractionated, total or partial is also important in the
evaluation of the final results.
3.7.1 Halogenated analogues
Incorporation of 5-bromouracil (5-BrU) and 5-bromodeoxyuridine
(5-BUdR) sensitizes both viruses and cells to UVR. A very detailed
review has been made by Hutchinson (1973).
3.7.2 Caffeine
Caffeine is a trimethylxanthine that acts by inhibiting the
repair systems. At doses not themselves toxic, caffeine considerably
increases the effects of UVR.
The absence of any effect of caffeine on excision repair suggests
that it acts by interfering with a post-replication system (Cleaver &
Thomas, 1969).
3.7.3 Furocoumarins
Photosensitizers are becoming increasingly important among agents
that modify the effects of UVR on biological systems.
Furocoumarins are natural products isolated from plants.
Important applications in therapy have led to thorough studies of
their mode of action at the molecular level (Chandra, 1972; Pathak et
al., 1974).
Musajo et al., (1974) have shown, in vitro, that furocoumarins
exert photosensitizing effects following irradiation at wavelengths of
320-400 nm. These effects result from the formation of certain
addition products with DNA and particularly from the linking of the
furocoumarin molecule with the pyrimide bases. These products may
cause breaks in the molecule and thus prevent if from carrying out its
functions. Oxidation of amino acids may also occur in proteins.
The so-called 'linear' furocoumarins such as psoralen react with
native DNA in the presence of UVR to make, monofunctional and
bifunctional addition products. The latter cause cross-linking between
the DNA strands. Certain so-called 'angular' furocoumarins (angelicin)
can only give rise to monofunctional addition products.
These addition products cause lesions that can be repaired by the
excision repair mechanism. Monofunctional addition products seem to be
easier to repair than bifunctional products (Chandra et al., 1976).
Depending on their molecular structure, not all the furocoumarins
show the same degree of photosensitization. Ben Hur & Elkind (1973)
demonstrated that, following exposure of hamster cells to UV-A, 11% of
the addition products were formed between the complementary strands.
During incubation in the dark, 90% of the cross-links gradually
disappeared from the DNA.
These reactions have found applications in the treatment of
certain skin diseases, particularly psoriasis.
3.7.4 Other photosensitizing agents
Charlier & Helene (1972) carried out an in vitro analysis of
the photochemical reactions of benzophenone and acetophenone with
purine and pyrimide derivatives in aqueous solution during irradiation
with light between 400 and 600 nm. Dimers and chain breaks were the
essential lesions.
The effects of light on bacteria in the presence of
photosensitizing chemicals such as toluidine blue and acridine yellow
were compared by Harrison (1967). The four sorts of lesions observed
-- lack of colony-forming capacity, DNA lesions and mutations, changes
in cell permeability, and enzyme inactivation -- were similar in both
cases.
Rauth & Domon (1973) studied the mechanisms of photosensitization
in animal cells in cultures with 1-cyclohexyl-3(2-morpholinyl-4-ethyl)
carbodiimide metho- p-toluene sulfonate (CMEC), which binds
preferentially to partially denatured regions of DNA after irradiation
at 254 nm, thus inhibiting replication and increasing lethality.
3.7.5 Protection by carotene
Since the work of Cohen-Bazire & Stanier (1958), some
investigations have established the protective role of carotene
against the photodynamic effects of light, in very special cases.
Since the energy level of carotenes is very low, they can accept, by
transfer, the energy of sensitizers transformed into the triplet state
and even the energy of oxygen transformed into the singlet state by
exposure to light.
Mutant strains of bacteria and fungi lacking carotenoids have
proved much more sensitive to photodynamic effects than normal
strains, but this effect is only valid for wavelengths shorter than
those that carotenes absorb (Mathews & Krinsky, 1965).
3.8 Conclusions
Knowledge of the molecular basis for the biological effects of
UVR has emphasized the importance of the photolesions produced in DNA
and the effectiveness of the enzymatic stages of repair, both of which
depend on the genetic make-up of the organism concerned.
Studies of the sensitivity of mutants, on one hand, and of
factors able to modify the effects of irradiation, on the other, will,
perhaps, make it possible to strengthen repair systems and to increase
the resistance of organisms to UVR.
The great differences observed make it difficult to lay down
protection standards.
4. THE BIOLOGICAL ACTION OF ULTRAVIOLET RADIATION ON VERTEBRATE
ANIMALS
4.1 General Aspects
The anatomical structure of the skin of vertebrate animals
resembles that of man in many respects. The surface stratum corneum is
important in affecting the penetration of UVR (Fig. 4), the
thicknesses of the stratum spinosum and stratum granulosum also affect
the relative amounts of UVR reaching the dermis. Other constituents of
the skin, e.g., the melanocyte population, the Langerhans cells,
vascular structures, as well as cutaneous innervation vary in amount
and distribution from species to species, but remain basically the
same.
 4.2 Acute Reactions in the Skin
4.2.1 Epidermal changes
Histological investigations of the early effects of UV
irradiation on animal skin show the changes that occur in the
epidermal cells particularly well.
Twenty-four hours after a single irradiation of skin with an
unfiltered mercury vapour lamp at doses between four and eight times
the minimal erythema dose, distinct signs of cell injury can be seen
including vacuolation of the cell cytoplasm and increased or decreased
density of the nucleus (Stenbäck, 1975a).
One distinctive feature, observed in both man (Daniels et al.,
1961) and experimental animals (Woodcock & Magnus, 1976) 24 hours
after exposure, is the presence of "sunburn cells" (SBC), scattered
diffusely throughout the superficial epidermis. These cells are
characterized by a densely-staining, glassy, homogeneous cytoplasm and
pyknotic nuclei. The appearance is similar to that of an individual
cell undergoing keratinization and thus, the process is regarded as a
form of individual cell shrinkage necrosis or dyskeratosis.
Following these early effects, hyperplasia, induced by radiation,
is observed (Blum et al., 1959; Stenbäck, 1975a). An increase in
mitosis indicates the onset of hyperplasia, which begins after about
two days.
Quantitative measurements have been made of the following aspects
of the effects of single doses of UVR on mouse skin (Blum et al.,
1959): (a) incidence of mitotic figures; (b) number of epidermal cells
per unit area; (c) epidermal thickness; (d) dermal thickness; (e) mean
nuclear diameter; and (f) number of groups of mitotic figures. All the
aspects measured changed in a similar fashion quantitatively following
irradiation; they increased rapidly at first, reaching a maximum and
then fell gradually towards normal. Soffen & Blum (1961) found a
gradual increase in epidermal thickness reaching a maximum between 8
and 14 days. These changes represent repair of the cell injury caused
by UVR; at the same time, the hyperplasia protects against further UV
irradiation.
For a long time, it was believed that thickening of the horny
layer was the essential adaptive change to UV irradiation in the
environment. The idea now prevails that melanin granules in the horny
layer and the Malpighian layer accumulate to form a protective screen
which is not only significant, but possibly even more important for
light protection than the thickness of the horny layer alone.
4.2.2 Erythema and inflammation
Sunburn (UVR erythema) and suntanning are the visible signs of UV
injury to the skin and the repair of such injury. UV erythema is
evidence of an inflammatory reaction to radiation, and appears after a
latent period of a few hours.
Erythema caused by UVR is confined to the exposed areas and
reflects blood vessel dilation and increased blood flow in the dermis.
It is often assumed that the initial photochemical reaction is in the
epidermis, where photon absorption by keratinocytes may lead to
liberation of intracellular substances that diffuse into the papillary
dermis to cause vasodilation. This diffusion theory is supported by
the existence of a latent period between exposure and erythema, and by
the fact that much of the radiant energy of the erythemogenic
wavelength region is absorbed by the epidermis. However, there may
also be direct injury to the vascular endothelium or to other sites in
the dermis.
Most studies of the mechanism of "sunburn" have used artificial
sources of UV-B or sources in which the spectral distribution is such
that UV-B is assumed to provide the major erythemogenic influence. In
experimental animals, the vascular response to UVR is biphasic. A
transient immediate vasopermeability is followed, after a latent
period of 2-8 hours, by a delayed, prolonged, increased,
vasopermeability and vasodilation. In some animal models, the initial
vasopermeability is accompanied by a faint erythema, which may begin
during exposure. This immediate effect has been attributed to
histamine release, possibly due to a direct effect of photons on
dermal mast cells. There is evidence that serotonin may also play some
role. In rats and guineapigs, serotonin and histamine antagonists
suppress the immediate phase of UVR vascular responses. In vivo
studies of human skin have shown the transient appearance of kinins
within minutes of UV irradiation. Kinin was not found after the onset
of delayed erythema.
The mediators of the delayed phase of UVR-induced vascular
response have been difficult to define. Antihistamines do not suppress
the delayed erythema phase of the vascular response to UVR in
guineapigs, rats, or man. Kinins have been stated to be either absent
or not elevated. Delayed erythema was not suppressed by various
inhibitors of proteases, plasminogen activitors, or kallikrein.
Serotonin has been found in urine following exposure to UVR, but the
significance of this finding is not clear.
More recently, prostaglandins, a group of long-chain fatty acids
with vasoactive properties, have been implicated as possible mediators
of the delayed phase of erythema. Prostaglandins are produced in human
and animal skin (prostaglandin groups E and F), intradermal injection
of prostaglandins produces erythema (PGE mainly, PGF group are much
less active), and furthermore the production of prostaglandins
increases following UVR exposure. Indomethacin is a potent inhibitor
of the conversion of arachnidonic acid to active prostaglandin, a
reaction catalyzed by the enzyme prostaglandin synthase. Topical
indemethacin produces a profound and prolonged blanching of
UV-B-induced, delayed erythema in both human subjects and guineapigs.
In man, intradermal indomethacin has been shown to consistently
decrease erythema due to UV-B but not all areas of erythema caused by
irradiation with UV-C; these effects appear to be due to the
inhibition of prostaglandin synthase.
It has been suggested that the complex reaction known as
"sunburn" may result from the release of hydrolytic enzymes and
possibly other substances by lysosomes within keratinocytes.
Histochemical studies of human skin exposed to UVR were
consistent with the theory that specific damage to lysosomal membranes
caused partial to complete rupture and release of enzymes.
The release of lysosomal enzymes may not only lead to damage of
the keratinocytes but may also release enzymes, vasodilator
substances, or subsequently formed cell-breakdown products into the
dermis, where they may directly or indirectly lead to erythema. It has
been suggested that the immediate erythema caused by UV irradiation
results from disruption of lysosomes of endothelial cells with release
of chemical mediators. The delayed erythema could then result from
secondary diffusion of proteinases from the epidermis, following
lysosomal rupture. It is also possible that direct photon damage to
the lysosomes of mast cells of the dermis or endothelial cells of
dermal blood vessels may play a role in the delayed erythema of
sunburn.
Multiple chromophores may exist in skin, and irradiation probably
leads to activation of a complicated cascade of mediators, the final
endpoint of which is erythema. Multiple pathways may exist. It is also
possible that photons have direct effects on blood vessels or nerves.
UVR causes dilation of isolated exposed dermal blood vessels. Dermal
proteins may be directly changed by radiation (Magnus, 1977).
4.2.3 Tanning
In addition to erythema, another consequence of exposure to UVR
is the pigmentation of the skin generally known as "tanning". This
becomes noticeable about 48 h after exposure and increases gradually
for several days. Tanning is, in part, due to migration of the pigment
melanin already present in the basal cells to the more superficial
layers of the skin where it has a greater effect on the appearance of
the reflected light. It is also partly due to the formation of new
pigment.
It is generally accepted that repeated irradiation with UVR
induces increases in the population of melanocytes in the epidermis of
man and experimental animals. With regard to the mechanism of this
phenomenon, the following processes or possibilities have been
considered: (a) increased division of melanocytes; (b) activation of
pigment formation in amelanogenic melanocytes: (c) migration of dermal
melanocytes into the epidermis; (d) various combinations of these
processes (Quevedo et al., 1965). That division of melanocytes occurs
has been shown in murine skin by Quevedo et al. (1963). Further,
published reports have provided evidence in support of the occurrence
of activation of amelogenic melanocytes in man (Mishima & Widlan,
1967) and experimental animals (Reynolds, 1954; Quevedo & Smith, 1963;
Miyazaki et al., 1968; Sato, 1971). However, the relative contribution
of each process remains to be established. In a study using tritiated
thymidine, it was demonstrated that approximately 1.1% of
dopa-positive epidermal melanocytes were labelled in hairless mice
receiving 9 daily exposures to UVR (Sato & Kawada, 1972). However, an
attempt to trace the labelled melanocytes using split epidermal sheets
was unsuccessful.
4.3 Acute Changes in the Eye
4.3.1 Photokeratitis and photoconjunctivitis
Although more energetic than the visible portion of the
electromagnetic spectrum, UVR cannot be detected by the visual
receptors in mammals, including man, because of absorption by the
ocular media. Thus, exposure to UVR may result in ocular damage before
the recipient is aware of the potential danger. Many cases of
keratitis of the cornea and cataracts of the lens have been reported
due to exposure to UVR produced by welding arcs, high-pressure pulsed
lamps, and the reflection of solar radiation from snow and sand.
Extensive reviews of the literature on the biological effects of
UVR have been compiled by Verhoeff et al. (1916) and Buchanan et al.
(1960). Verhoeff and his colleagues included all the, then available,
research data in their report and formulated some of the basic
hypotheses regarding ocular damage caused by UVR. Research on
threshold values and destructive and repair processes was summarized
by Duke-Elder (1926). Buschke et al. (1945) stressed the destructive
effects of UVR on the corneal epithelial cell nuclei, the loss of
epithelial adhesion to Bowman's membrane, and the inhibiting effects
of UVR on the healing process.
In studies by Cogan & Kinsey (1946), a monochromator was used to
evaluate the sensitivity of the cornea to individual spectral lines.
Their work, which was, for the most part, carried out with one to four
rabbits, provides the most reliable quantitative data on damaging
threshold values of UV energy of individual wavelengths. They
established a long wavelength limit of between 306 and 326 nm and a
threshold of 152 J/m2 at 288 nm for the rabbit.
Ordinary clinical photokeratitis is characterized by a period of
latency that tends to vary inversely with the severity of exposure.
The latent period may be as short as 30 min or as long as 24 h, but is
usually 6-12 h. Conjunctivitis follows, often accompanied by erythema
of facial skin and eyelids. There is a sensation of a foreign body or
"sand" in the eyes and various degrees of photophobia, lacrimation,
and blepharospasm. The importance of these acute symptoms lies in the
fact that the individual is visually incapacitated for 6-24 h and that
the ocular system, unlike the skin, does not develop tolerance to
repeated exposure to UVR. Almost all discomfort disappears within
48 h, and exposure rarely results in permanent damage.
Pitts & Kay (1969) and Pitts (1970) sought to establish the
experimental threshold for photokeratitis in rabbits and primates,
including man. Rabbits and monkeys showed a maximum sensitivity to UVR
at 270 nm. The radiant exposure threshold for man at 270 nm was
50J/m2 compared with 110 J/m2 for the rabbit and 60 J/m2
for the monkey.
The UVR incident on the eye is absorbed in turn by the cornea,
the aqueous humor, the lens, and the vitreous humor before reaching
the retina. Absorption is greater in the lens than in the cornea, and
is least in the aqueous humor. Below 300 nm, most of the UVR is
absorbed in the cornea and aqueous humor and very little penetrates as
far as the lens.
As a result of observations at above-threshold intensities, it
was felt that the reaction of the cornea to exposure to UVR wavebands
of 220 to 250 nm was different from that to wavebands of 250 to
310 nm. For exposures below 250 nm, signs and symptoms occurred soon after
exposure, and symptoms always returned to normal approximately 14 h
later. For exposure above 250 nm, symptoms did not generally occur
until 9 to 11 h after exposure, and visual acuity remained below
normal for 24 h after exposure. The differences observed were
attributed to the differences in the absorption of the different
wavebands. The shorter wavebands are absorbed in the outer corneal
epithelial layers, which undergo rapid change, whereas the longer
wavebands are absorbed in the deeper epithelial layers which show
delayed changes because these cells are more viable. Thus, the lesions
produced by shorter wavelengths are rapidly repaired while, at the
longer wavelengths, there is a delayed and more serious response
(Pitts & Cullen, 1977).
4.3.2 Cataracts
A cataract is a partial or complete loss of transparency of the
crystalline lens or its capsule. The wavelengths that affect the lens
appear to be in the same area as those that are most effective in
producing erythema on human skin.
Bachem (1956), using a filtered UVR system, concluded that
exposure to repeated high doses of longer UVR wavelengths could cause
cataracts through cumulative effects. He reported that the action
spectrum for cataracts began abruptly between 293 and 297 nm, reached
a peak near 297 nm, and fell abruptly near 313 nm. Minimal effects
existed through the remainder of the near UVR. In both the rabbit and
guineapig, reversible lenticular "blurring" occurred 5 to 10 days
after exposure. With repeated excessive exposures to the 297-355 nm
wavebands, irreversible lenticular opacities occurred after a latent
period of between 2.5 and 15 months.
Bachem (1956) concluded that since daylight does not contain any
UV-C or far infrared, and since both the visible and near infrared are
freely transmitted by the ocular media, it would appear that UV-B and
A are responsible for cataracts.
The chemical effects of UV-A exposure on tryptophan were studied
by Zigman et al. (1973) using human crystalline lenses. They found
that exposure of tryptophan to UV-A led to the formation of chromatic
photoproducts which bound to the lens proteins, altered their colour
and changed the solubility. Human lens material without added
tryptophan did not show chromatic changes on exposure to UV-A, until
48 h after exposure. Tryptophan showed an excitation wavelength at
278 nm and a fluorescent emission at 330 nm. However, following
exposure to UV-A, tryptophan showed an additional 360 nm excitation
and 440 nm fluorescence similar to that found in the brunescent human
cataract lenses. The UV irradiance for these studies was 30-50 W/m2
at 365 nm and exposures were made for at least several hours. These
exposure levels exceed those expected from sunlight for the same
period of time.
Thus, exposure of the eye to UV-A, for sufficient periods of
time, with an irradiance comparable to the irradiance level of
sunlight may interfere with the synthesis of lens proteins, catalyse
insoluble lens protein, and may result in chromatic changes in the
lens. While the basic mechanisms remain to be found, the evidence
clearly demonstrates that both in vitro and in vivo exposure to
UV-A can enhance cataractous changes in the crystalline lens.
Recently, Pitts & Cullen (1977) have studied the effects of the
300 nm-400 nm wavelength range on the rabbit eye in vivo. The
criteria used to determine corneal damage were epithelial debris,
epithelial stippling, epithelial granules, epithelial haze, epithelial
exfoliation, stromal haze, stromal opacities, and endothelial
disturbances. Anterior chamber signs included flare and cells. The
crystalline lens criteria were subcapsular opacities, capsular and
stromal haze, stromal opacities, and increased prominence of the
anterior suture. Criteria for the iris were the presence of the
anterior chamber signs, changes in clarity of the iris stroma, and
sluggish pupillary response.
Tables 3 and 4 show data for corneal and lenticular damage in the
290 nm-400 nm wavelength range for the rabbit. Limited data above
300 nm for the human eye do not allow a detailed comparison; however,
the human corneal threshold was considerably below that for either
the rabbit or the nonhuman primate.
Table 3. UV threshold data for the rabbit cornea and lensa
Threshold radiant exposure
Wavelength Threshold radiant exposure (J/m2) permanent damage (J/m2)
(nm)
corneal lens time to lens time for permanent
threshold threshold disappear threshold damage to appear
295 200 7500 24 h 10 000 2 h
300 500 1500 3 days 5000 24 h
305 700 3000 7 days 5000 24 h
310 550 7500 2 weeks 15 000 24 h
315 22 500 45 000 1 week 60 000 24 h
320 75 000 > 80 000 -- -- --
335 109 000 > 150 000 -- -- --
365 > 250 000 > 250 000 -- -- --
a From: Pitts & Cullen (1977).
Table 4. Permanent lenticular opacitiesa
Wavelength Radiant exposure Appearance of Permanenceb of
(nm) (J/m2) lens opacities lens opacities
300 5000 24 h permanent
305 5000 24 h permanent
310 15 000 24 h permanent
315 60 000 24 h permanent
a From: Pitts & Cullen (1977).
b Lenticular opacities present one month after exposure.
Phototoxic psoralen derivatives have been used recently in the
treatment of certain dermatological conditions. Such compounds caused
UV-A-induced corneal opacities and cataracts in mice (Griffin, 1959;
Koch, 1967).
Research on the effects of psoralens on the production of
cataracts in man needs to be pursued. As more and more people are
subjected to dermatological treatment using UV photosensitizing
compounds, the role of these compounds and their effects on the ocular
system should be defined.
4.4 Effects of Long-Term Exposure of Skin to UVR
UV irradiation induces an inflammatory response and ulceration in
both the epidermis and the dermis, the latter being infiltrated with
leukocytes in the region of the lesions, and to a much lesser extent
between them. These lesions ulcerate and the epidermis may disappear
for a time in the centre. However, peripherally there is particularly
active hyperplasia. The basal membrane (between the epidermis and
dermis) may disappear for a time in the regions of these "open"
lesions. Between the lesions, the infiltration of leukocytes is
relatively slight.
Injury to the epidermis and dermis, brought about by long-term
exposure to UVR leads to dermal alteration, fibrosis, and elastosis,
as well as to epidermal atrophy. However, experimental production of
cutaneous elastotic changes in animals by artificial UV irradiation
has only been reported rarely. Using histochemical methods, Sams et
al. (1964) demonstrated focal dermal elastosis in mice after prolonged
exposure to artificial UVR. UVR-induced changes in connective tissue
were also seen in rat skin by Nakamura & Johnson (1968).
4.4.1 UVR-induced mutagenesis and carcinogenesis
4.4.1.1 Mutagenesis
The nature of the effects of UVR on DNA, described earlier, shows
the importance and the specificity of the lesions in genetic material
and focuses interest on the mutations that might result in the various
cell types studied. The production of such mutations has best been
demonstrated in bacteria.
Among many others, Grossman (1968), Eisenstark (1971), and Witkin
(1976) have proposed, analysed, and verified some complex mechanisms
that control UVR-induced mutagenesis. It will be recalled also that,
according to the results obtained by Radman (1975), most of the
UVR-induced mutations would appear to be errors introduced in the DNA
during error-prone SOS repair.
Mammalian cells in culture have proved to be very suitable
material for the study of UVR mutagenesis (Kao & Puck, 1969). There is
quite good agreement between the dose of UVR and the proportion of
mutants (Bridges & Huckle, 1970).
Fox (1974) has shown that caffeine can reduce the rate of
mutations induced by UVR in cultures of rodent cells by inhibiting a
process of error-prone repair.
Isolation and study of UVR sensitive mutants in animal cells have
found remarkable applications in the study of various human xeroderma
pigmentosum mutants (Cleaver, 1973). However, such mutations in
mammalian germinal cells are not possible, because the cells are
located below the depths of penetration of UVR.
4.4.1.2 Mechanism of UVR carcinogenesis
In order to understand the possible mechanisms of UVR
carcinogenesis, it has been necessary to study the formation and
excision of pyrimidine directs, unscheduled DNA synthesis, and all the
data valid for the range from bacteria to mammalian cells.
It is now generally believed that UVR carcinogenesis results from
a succession of events originating in a photolesion of the genetic
material.
From the numerous studies that have led to en elucidation of some
of these mechanisms, it emerges that faulty DNA repair can increase
the frequency of carcinogenesis in the following ways: by causing
alterations in DNA, which also find expression in an increased
frequency of chromosome aberrations and a rise in mutation rate; by
increasing the rate of transformation of normal cells into cancer
cells; and by facilitating the expression of latent oncogenic viruses
able to trigger cancerous growth (Setlow, 1973; Trosko & Chu, 1973).
Errors induced by DNA repair during the initiation phase of
carcinogenesis seem to be the most likely mechanism leading to UVR
cancers.
4.4.1.3 Tumour types
Epidermal tumours. The first visible step in UVR-induced
epidermal tumour formation in animal skin consists of cell
proliferation, i.e., an increase in the number of squamous cells and
cell layers, which gradually become papillomatous in character
(Stenbäck, 1978). This is accompanied by an increase in cellular
atypia, nuclear enlargement, hyperchromatism, indentation, and
prominence of nucleoli. This basically proliferative response is
frequently replaced by a dysplastic progression showing a solar
keratosis-like pattern with cellular pleomorphism, occasionally with
pseudo-epitheliomatous hyperplasia-like features, which ultimately
invade the dermis. The tumours first seen are acanthomatous papillomas
(trichoepitheliomas), with a predominantly epithelial component or
fibropapillomas in which the tumour is composed of a fibrous stroma
covered by squamous epithelium.
The malignant tumours that ultimately develop are squamous cell
carcinomas of different types including: solid keratin containing
tumours; moderately differentiated, individually keratinizing tumours
with distinct intercellular bridges; and less-differentiated,
non-keratinizing spindle cell tumours, in which ultrastructural
analysis reveals squamous cell patterns.
Keratoacanthomas, i.e., proliferating epithelium on a cup-shaped
base, are relatively more frequent in animals of different species
receiving large doses of UVR than in animals treated with chemical
carcinogens.
Epidermal tumours are easily induced by different agents in mouse
skin (Stenbäck, 1969). Winkelman et al. (1960) reported the production
of squamous cell carcinomas on the backs of hairless mice exposed to
UVR. Further studies established that carcinomas could be induced in
this animal almost to the exclusion of sarcoma formation (Epstein &
Epstein, 1963). In early studies, epithelial tumours were reported in
both rats and mice (Findlay, 1930; Herlitz et al., 1930; Putschar &
Holz, 1930), but in later studies the deeper lying dermal tumour
response to UVR predominated. No mention of skin sarcomas was made,
however, in the studies of Beard et al. (1936) on albino rats in which
12 animals exhibited 9 carcinomas of the external ear, 6 sarcomas of
the eye, and 1 carcinoma of the skin of the nose.
The difference in the distribution of tumour types, with
sarcomatous growth predominating in haired mice and carcinomas in man
(Urbach, 1969) may, in part, be explained by the difference in
penetration of UVR, a greater amount reaching the dermis in mice
(Kirby-Smith et al., 1942; Everett et al., 1966).
Dermal tumours. Another type of neoplastic progression seen in
mice, particularly after intensive treatment with large doses of UVR
over a short time period, consists of ulceration, scarring, and the
subsequent formation of dermal tumours. These tumours begin as
aggregates of regularly built, elongated cells with small monomorphic
nuclei. Epithelial proliferation is occasionally observed as a
secondary phenomenon. The tumours rarely extend grossly through the
surface. In the early stages, they appear to be papillomas, although
they consist entirely of fibroblastic cells. Some tumours are
remarkably acellular, with a prominent fibrillary pattern. The tumours
are composed of large, polymorphic cells with prominent nuclei.
Ultrastructural analysis shows the predominant cellular components --
a dark cell type, with hyperchromatic nuclei and scanty cytoplasm, and
a light cell type, with large nuclei and abundant cytoplasmic
ribosomes (Stenbäck, 1975a). The same cell types are also seen in
malignant tumours, in which the cellular polymorphism frequently is
considerable, with nuclear atypism and enlargement, numerous nucleoli
and a generally disorganized arrangement. Sarcoma induction is partly
species-specific, as these tumours were not seen in UV-irradiated
Syrian golden hamsters (Stenbäck, 1975b) nor were they seen in
hairless mice (Epstein & Epstein, 1963) or guineapigs, susceptible to
chemical sarcoma induction (Stenbäck, 1969, 1975b).
An infrequent neoplastic alteration in several animal species is
the vascular tumour (Stenbäck, 1975b). This begins as a proliferation
of dilated vascular spaces with regular endothelial lining. Rarely,
the endothelium proliferates to the point of forming angiosarcomas, or
invasive tumours composed of large, atypical cells arranged in a
nodular pattern.
The role of the dermis in epidermal rumour formation has been
emphasized by numerous investigators (Orr, 1938; Mackie & McGovern,
1958). A proliferation of elastic tissue was induced experimentally in
mice, by Sams et al. (1964), through repeated exposure to UVR.
Similarly, Magnus & Johnson (1965) stimulated formation of elastotic
tissue, following early destruction of elastic fibres, with radiation
of 300 nm from a monochromatic source. Nakamura & Johnson (1968)
reported that dermal elastic tissue proliferation occurred in albino
rats after chronic irradiation with UVR, only after discontinuation of
the exposure. It was postulated by Johnson et al. (1968) that this
change was the result of photochemically-induced alterations in
fibroblast function, rather than the degradation of normal elastic
fibres. In support of this concept, Epstein et al. (1969) noted that
unscheduled DNA synthesis occurred in connective tissue cells of the
upper dermis within minutes of exposure to UVR shorter than 320 nm,
demonstrating a direct effect of UVR on dermal fibroblasts.
Because of its frequent association with skin cancer formation,
actinic elastosis has been considered to play an important role in
tumour development. However, Sams and his co-workers (1964) and Graham
& Helwig (1965) demonstrated that actinic elastosis was not essential
for the development of epidermal malignancies. Furthermore, the
experimental production of elastosis in animals has not been
associated with cancer formation, nor has UVR-induced experimental
cancer depended on the presence of this change (Epstein & Epstein,
1963).
Adnexal tumour formation is not as common in UVR-treated animals
(Stenbäck, 1975) as in, for example, carcinogen-treated rats
(Zackheim, 1964; Stenbäck, 1969). Hyperplasia and cystic
disorganization of hair follicle walls is very common, but rarely
progresses to grossly visible neoplasia. Trichoepitheliomas with
barely visible follicular arrangements are rarely seen. Even more
uncommon are hamartomatous tumours, hair follicle-derived
trichofolliculomas, and sebaceous gland tumours. Sebaceous gland
epitheliomas and carcinomas are even rarer. In a study in 1930,
Putschar & Holtz reported only a very small number of basal cell
carcinomas in rats.
Pigmented tumours. Studies on the induction of pigmented
tumours with UVR have been less successful. Benign, dermal melanocytic
lesions, or blue nevi, have been observed in hairless mice exposed to
UVR (Epstein et al., 1967). They were grossly seen as papules, 2-20 mm
in diameter, histologically composed of tightly arranged, heavily
pigment-laden polyhedral cells. Subcutaneous accumulation of pigmented
cells has also been seen in pigmented animal strains, as well as in
the skin of the Syrian golden hamster (Stenbäck, 1976). Such tumours
were possibly spontaneous, as the incidence was very low -- only
around 4% -- and unrelated to treatment. They were composed of
polyhedral or spindle-like cells arranged in a whorl-like pattern
beginning as hyperplasia of perifollicular melanocytes, before
spreading both laterally and deeply in the dermis. These tumours do
not show junctional activity; they do not metastasize and rarely kill
the host.
Melanomas, the type of greatest interest from an epidemiological
standpoint, are rarely seen in animals. Benign melanocytomas are
easily induced by treatment with chemical carcinogens as shown by
Shubik et al. (1960) and Rappaport et al. (1961). Fortner et al.
(1961) reported spontaneous melanomas in hamsters, similar to those in
man. However, the sensitivity of these animals to UVR is not known.
The effect of pigment, in general, in animal models has received
little attention. Freeman & Knox (1964a) induced melanocytoma in 67%
of a pigmented strain of rats. The tumours had an average latency
period of 193 days. In albino rats there was an 8% tumour incidence
with a 283-day latency period.
4.4.2 Species-specificity
Three specific factors -- pigment, hair, and thickness of the
stratum corneum -- have been found to alter susceptibility to tumour
induction. It was found that pigmented mice required significantly
more radiation to induce tumours than albino animals. Hair offered
even greater protection (Blum et al., 1959), and thus the hairless rat
appeared to be a likely subject for tumour induction studies. However,
the results of Hueper's (1941) extensive studies indicated that this
animal was, in fact, quite resistant to UV penetration because of its
thick stratum corneum. Since pigment, hair, and the stratum corneum
were limiting factors, the ears of albino mice and rats became the
traditional test sites for experimental production of cancer by UV
irradiation. A remarkable amount of quantitative data has been
accumulated using this system. The usual tumour produced in this
tissue was a sarcoma (Roffo, 1934; Grady et al., 1943b). Thus, the
albino ear model could not be used for evaluating qualitative changes
associated with epidermal carcinogenesis, which is the primary process
induced by UVR in human skin.
Winkelman and his co-workers (1960) reported the production of
squamous cell carcinomas on the backs of hairless mice exposed to UVR.
Further studies established that carcinomas could be induced in this
animal almost to the exclusion of sarcomas (Epstein & Epstein, 1963;
Epstein, 1965). In addition, UVR-induced pigmented tumours were
reported in pigmented hairless mice (Epstein et al., 1967). Thus, the
hairless mouse has provided a model for both the qualitative and
quantitative examination of the carcinogenicity of UVR.
Though penetration of UVR appears to be of obvious importance,
other factors also influence the type of growth induced by UVR. Grady
et al. (1943 a) found that the size of individual doses did not have
any effect on the carcinoma/sarcoma ratio in the albino, hairy mouse
but that reduced intervals between exposures increased the number of
epidermal carcinomas. These findings suggest that various tissues
respond differently to the carcinogenic effects of UVR (Stenbäck,
1975a). In part, this may be associated with differences in
penetration of various wavelengths of UVR. Furthermore, there are
great species differences in the repair capability of cells.
4.4.3 Ultraviolet radiation as an initiating agent
The two-stage concept for skin tumour formation proposed by
Berenblum & Shubik (1949), supposed formation of dormant tumour cells
by a single application of a carcinogen. These latent tumour cells
were provoked by the subsequent application of a promoter to form
visible tumours. In his studies on the induction of skin cancer by
exposure to UVR, Blum (1969) indicated that the process was continuous
beginning with the first exposure and progressing to ultimate tumour
formation. Blum's conclusions were based on experiments in which he
(Blum et al., 1943) and Rusch et al. (1941) could not produce tumours,
unless exposures were carried out over a minimum of 2 ´ months,
regardless of the amount of energy used. Blum's experiments suggested
that, with shorter exposure periods, tumour formation was not
accelerated enough to become visible within the lifetime of the
experimental animal. Epstein & Roth (1968), using a single exposure to
UVR as an initiatior and treatment with croton oil as a promotor,
concluded that croton oil stimulated rumour formation, the
characteristics of which were established by initial exposure to UVR.
The results of these studies were significantly different from those
encountered when a chemical carcinogen was used as the initiator
(Stenbäck, 1969).
4.5 Interactions between Ultraviolet Radiation and Chemicals
4.5.1 Chemically-enhanced photocarcinogenesis
An equally significant problem concerns photo-induced
carcinogenesis following the application to the skin of agents which
are phototoxic, but not in themselves carcinogenic.
A portion of the sunlight spectrum is carcinogenic, even in the
absence of an exogenous photosensitizer. At the current rate of
introduction of new compounds into the environment, it has become
increasingly important to determine whether a readily demonstrable
property, such as phototoxicity, can be used to predict compounds or
treatment regimes that could enhance photocarcinogenesis.
Concepts of chemical interaction with UVR-photocarcinogenesis are
of recent origin. Blum (1969) and Emmett (1973) reviewed a number of
reports dealing with the influence of phototoxic substances on
photocarcinogenesis. The results frequently appeared to be in
disagreement, a situation possibly reflecting differences in
technique, including solvent, routes of administration, light sources,
criteria for tumour recognition, and in statistical evaluation (Blum,
1969). In addition, characteristics of some compounds (toxicity,
carcinogencity, instability) rendered their interactions with light
complex, and their analysis difficult.
The relative enhancing effects on photocarcinogenesis of two
widely recognized photoactive compounds 8-methoxypsoralen, (8-MOP) and
anthracene were studied by Forbes et al. (1976). Both compounds were
phototoxic, but only the 8-MOP solutions markedly enhanced
photocarcinogenesis. Thus, the ability of a chemical to induce
phototoxicity is not always sufficient to augment
photo-carcinogenesis.
4.5.2 Interaction between light and chemical carcinogens
The fact that UVR can alter several phenanthrene carcinogens
photochemically has been known for some time. The studies of Davies et
al. (1972 a, b) showed that the carcinogenicity of 7,12
dimethylbenz(a)anthracene (DMBA) was reduced by light according to the
demonstrable photochemical lability of the compound. There was also
evidence that an additional time-dependent factor could influence this
effect. Thus, it appears that, at least in the case of DMBA-treated
animals, light may contribute in two opposing ways: (a) by
degradation of the carcinogen to non-carcinogenic products and (b)
by stimulating a phototoxic response that appears to coincide with a
relatively increased tumour yield.
Depending on the wavelengths of the UVR used, carcinogens can be
photodegraded to a less carcinogenic compound, or can induce
phototoxicity which may augment carcinogenesis or cause such a severe
local phototoxic reaction that the epithelial skin cells are nearly
all destroyed. Thus, either enhancement or inhibition of skin
carcinogenesis may occur, depending upon the carcinogen and the
wavelength of the light source used.
4.5.3 UVR-induced carcinogen formation
The photochemical conversion of sterols to carcinogenic
substances has been proposed as a potential explanation for the
cancer-causing effects of light upon skin (Black & Douglas, 1973). It
has recently been demonstrated, in vitro, that one such compound,
cholesterol-5a-oxide, which possesses carcinogenic properties
(Bishoff, 1969), is formed in human skin exposed to UVR (Black & Lo,
1971).
4.6 Physical and Quantitative Aspects of Ultraviolet Irradiation in
Animal Studies
4.6.1 Carcinogenic action spectrum
Determination of the effective wavelengths or "action spectrum"
is one of the primary objectives in the study of photobiological
responses. However, data are not available for the action spectrum of
UVR-induced cancer formation. The paucity of this information for one
of the most extensively studied photobiological reactions is due to a
number of factors, including the large number of potential
wavelengths, the considerable number of animals necessary and the
length of time (a matter of many months or years) required for
exposure to each wavelength, the difficulties in immobilizing
experimental animals, and the need for an especially good
monochromator with practically no stray light contamination. Though
the complete curve of the carcinogenic spectrum is not known, certain
aspects have been determined by less sophisticated methods. Roffo
(1934) reported that windowglass filtration eliminated the
carcinogenic effects of sunlight on white rats. Thus the offending
rays of the sun would be found approximately between 290-320 nm. A
number of investigators using mercury arc and fluorescent sun lamps
with filters have confirmed that, under their experimental conditions,
320 nm represented the longer wavelength limit for cancer formation
(Griffin et al., 1955; Blum, 1969). Furthermore, carcinogenic
responses have been produced by radiation as short at 230.2 nm (Roffo,
1934) and skin cancer has long been known to be induced by UV-C and
UV-B. Thus the action spectrum appears to include wavelengths between
230 and 320 nm but wavelengths between 290 and 320 nm have been shown
to have significantly greater carcinogenic effects than UVR shorter
than 260 nm (Rusch et al., 1941; Blum, 1943; Blum & Lippincott, 1943;
Kelner & Taft, 1956; Tung et al., 1971).
Freeman (1975) performed a series of experiments to provide more
specific comparative data by testing the hypothesis that the action
spectrum for carcinogenesis parallelled that for erythema. In these
studies, squamous cell carcinomas developed at approximately the same
rate and frequency, when UVR exposure was proportional to that for
erythema, with a decreasing potency from 300 to 320 nm. No tumours
occurred in mice exposed to 290 nm. These cancer-producing wavelengths
are also responsible for the normal phototoxic sunburn reaction.
Longer UV and visible light are neither erythema-producing nor
carcinogenic under ordinary conditions.
It cannot be assumed that the action spectra for human skin
erythema and mouse skin photocarcinogenesis are, similar, unless a
common chromophore or action mechanism is involved. Setlow (1974)
proposed that the common denominator was the action spectrum for
affecting DNA. Making some allowance for the skin transmission of UVR,
he showed that the shapes of action spectra for DNA, erythema, and
possibly skin cancer production were similar and could be made to
coincide.
4.6.2 Dose-response relationships
The second law of photochemistry (the law of reciprocity of
Bunsen & Roscoe) states that photochemical action depends only on the
product of the light intensity and the duration of exposure. This law,
however, holds only for primary photochemical action, and cannot be
applied to secondary reactions. Since the biological endpoints that
can be observed, such as erythema, pigmentation, skin cancer
production, etc., are certainly indirect effects, and since we still
know little about the primary photochemical reactions that underlie
them, it is not surprising that "reciprocity"' holds only for some of
the effects studied.
In the first quantitative photocarcinogenesis experiments ever
performed, Blum (1969) found that, within relatively narrow limits
(approximate factor of 5), differences in dose, intensity, or interval
between doses did not alter the shape or slope of tumour incidence
curves, but only their positions on the log-time axis. Blum, however,
was careful to point out that this was only true, as long as the
experimental conditions remained the same until the time the tumours
appeared.
With the accumulated data, he surmised that UVR-induced cancer
formation was a continuous process that began with the initial
exposure and that the appearance of tumours within the lifetime of the
animal depended on sufficient acceleration of the growth process.
In the majority of studies on photocarcinogenesis, fixed doses of
UVR have been given at a fixed dose rate, and the interval between
doses altered, but in increments of at least 24 hours. Such
experiments, while very valuable, are far removed from the conditions
found in nature under which human skin is exposed. Man is exposed to a
relatively low UVR flux that varies with time of day, season, and
environmental conditions, such as cloud cover, and also during the
exposure period.
Two recent animal experiments have shown that both varying the
UVR dose increment and varying the dose-rate while the daily dose
remains constant, affects UVR-induced skin carcinogenesis.
In the first experiment, groups of hairless mice were exposed to
doses of UVR from a bank of "Fluorescent Sun" (FS) lamps known to
produce skin cancer in these animals. Equal doses of UVR were
delivered in periods of 5 minutes, 50 minutes, or 500 minutes. Thus,
while the doses (given 5 times weekly) were the same, the flux varied
by a factor of 10 or 100. Striking differences in both tumour
development time and tumour yield were noted. The animals given the
total UVR dose in 5 minutes developed tumours later and in smaller
numbers than those given the same total dose in 50 or 500 minutes
(Forbes, personal communication). Thus, protracting the UVR dose over
longer time periods resulted in a striking increase in the
carcinogenic effects of the radiation.
In another experiment, mice were exposed to UVR doses per day
differing by a factor of two. As Blum had found previously, the lower
daily dose resulted in the delayed onset of first tumours without
significantly changing the shape of the response curve (Forbes, 1978).
4.6.3 Physical factors influencing UVR carcinogenesis
Although the tumour-promoting properties of such physical factors
as freezing, scalding, and wounding have been described for chemical
carcinogenesis systems, little information is available about the
effects of these factors on UVR-induced cancer formation. Bain & Rusch
(1943) reported that increasing the temperature to 35-38°C accelerated
the tumour growth rate. The stimulating effects of heat on UVR
carcinogenesis were confirmed by Freeman & Knox (1964b). Heat also
enhanced the acute injury response to UVR.
Temperature does not affect the photochemical reactions that
follow UV irradiation, but it does affect many of the biochemical
reactions that follow the initial photochemical change (Blum, 1941,
1969). Although it is known that heat adversely affects
photosensitivity (Lipson & Baldes, 1960), and other phenomena of light
injury (Bovie & Klein, 1919; Hill & Eidenow, 1923), and that heat
alters the effects of X-ray (Carlson & Jackson, 1959), the influence
of heat on burns produced by sunlight or UVR has rarely been
considered (Freeman & Knox, 1964b).
Other studies have shown that high winds and high humidity
significantly increase tumour incidence (Zilov, 1971; Owens et al.,
1977).
4.7 The Immune Response to Tumour Induction
A number of studies have shown that the immune status of the host
and tumour induction are potentially interactive processes. Chemical
carcinogens cause alterations of the host immune-response, the type
and extent of which depend on the rumour-inducing agent (Curtis,
1975). UVR also profoundly affects immunological reactivity,
particularly the immune response to skin tumours induced by UVR.
Studies leading to this conclusion were prompted by an observation by
Kripke (1974) that tumours induced by UVR in C3Hf mice were highly
antigenic and are usually immunologically rejected when transplanted
to normal, nonirradiated syngeneic recipients. This raised the
question as to why these tumours were able to grow progressively in
their primary host without succumbing to immunological rejection. In
an extensive series of experiments, Kripke & Fisher (1976) found that
pretreatment of mice with UVR for periods of time too short to induce
skin tumours made them unable to reject transplants of UVR-induced
tumours, even though such transplants were immunologically rejected by
unexposed animals. This indicates that UVR-exposed mice are
systemically altered in a way that prevents immunological rejection of
highly antigenic UVR-induced tumours.
Similarly, inability of unexposed secondary hosts to reject
UVR-induced tumours after transfer of lymphoid cells from UVR-treated
mice has been established, and demonstrates the immunological nature
of the systemic alteration in the UVR-treated mice (Fisher & Kripke,
1977). Furthermore, the failure of lymphoid cells from UVR-exposed
mice to react against UVR-induced tumours is due to the presence of
suppresser T lymphocytes in the lymphoid organs of UVR-treated
animals. In spite of their inability to reject highly antigenic
UVR-induced tumours, UVR-exposed mice respond normally to most other
antigens (Kripke, et al., 1977; Norbury et al., 1977). The one
exception is that UVR-treated mice have a transient defect in antigen
processing in the skin, which is reflected in their inability to
develop contact hypersensitivity reactions (Jessup et al., 1978).
The finding that a selective immunological defect precedes the
appearance of UVR-induced primary tumours suggests that the immune
system might control early UVR-induced skin cancers and that tumours
ultimately appear because of this interference by UVR with host
defence mechanisms.
The carcinogenic action of polycyclic hydrocarbons has been
associated with their immunosuppressive action (Stenbäck, 1969).
Immunodeficiency states and immunosuppression therapy are both
associated with an increased rumour incidence. Immunosuppressive
agents, such as antilymphocyte serum, enhance both chemically- and
UVR-induced tumour formation (Nathanson et al., 1973, 1976).
5. EFFECTS OF ULTRAVIOLET RADIATION ON MAN
5.1 Beneficial Effects
In addition to the direct effects on the skin, UVR produces a
number of systemic effects. It has the capacity to increase the tonus
of the sympathico-adrenal system, enhances mitochondrial and
microsomal enzyme activity and the non-specific immunity level, and
increases the secretion of a number of hormones (Tung, 1976).
Systolic and diastolic blood pressures are reduced before sunburn
appears and may even be reduced with exposures so mild that no visible
erythema is produced (Aitken, 1937). Blood pressure gradually falls
for 24 hours, and lowered pressure may persist for several days.
Studies have demonstrated that the exercise tolerance of children
receiving UVR through the winter is greater than that of control
groups not receiving radiation (Ronge, 1948; Zilov, 1971).
Other changes that have been attributed to UVR exposure include
reduction in serum cholesterol, increase in the glucose tolerance
curve, and decrease in serum tyrosine (Kameneckaja & Mitrofanova,
1975).
Seasonal changes in various diseases are often considered to be
evidence of UVR effects, but there are many other climatic variables
that change with the season, including temperature and daylength.
Blood volume, blood content of the skin, blood flow in the skin, and
hydration of the skin due to sweating vary with seasonal adaptation.
Thus few changes in disease patterns can be attributed to the effects
of UVR alone.
Data have been obtained indicating that the body's tolerance
towards exposure to chemical substances such as nitrites, benzpyrene,
carcinogens etc, which produce general toxic, carcinogenic, and
allergenic effects depends, to some extent, on the degree of exposure
to UVR (Gabovic et al., 1975; Prokopenko & Zabulueva, 1975;
Prokopenko, 1976). Prophylactic treatment with UVR preceding specific
immunization reduces the risk of vaccination allergy and helps to
increase the effectiveness of the immunization (Talanova et al.,
1975).
Where sizeable populations live in far northern areas, it is now
generally acknowledged that a long period of UVR deficit may have a
harmful effect on the human body. Numerous investigations indicate
that lack of exposure to solar UVR can lead to the development of a
pathological condition known as "UVR deficiency" or "light
starvation". The most frequent manifestation of this disease condition
is a disturbance in mineral metabolism and the development of Vitamin
D deficiency and rickets in children, accompanied by a sharp reduction
in the defensive powers of the body, making it particularly vulnerable
to unfavourable environmental factors. The development of UVR
deficiency is confirmed by data from a survey conducted, mainly among
children, in different photoclimatic zones of the USSR (Belikova et
al., 1975; Dancig, 1975), and from a survey of ships' crews working in
the north and in the tropics.
Vitamin D. Sunbathing is popular, and there is a widespread
feeling that "sunlight is good for you", but the physiological
benefits that presumably underlie the feeling of well-being have not
been adequately explained or studied.
The only thoroughly established beneficial effect of UVR on the
skin is the conversion of 7-dehydrocholesterol to Vitamin D3. Several
investigators have helped to promote the understanding of the
mechanisms of Vitamin D production and its metabolism and functioning.
It has long been noted that prolonged limitation or complete
absence of exposure of the human skin to solar UVR makes natural
activation of Vitamin D impossible and is an important factor in the
spread among the population of "chronic latent D avitaminosis"
reflected in widespread rickets and dental caries. Data from the
previously mentioned survey by Belikova et al. (1975) indicate that,
despite an overall reduction in the incidence of rickets and in its
severity, it is still frequent among young children in the north of
the USSR. Morbidity indices for rickets at latitude 65°N are 2.5-3
times as high as at latitude 45°N.
Comparative investigations among healthy children aged 3-6 years
in the central zone of the USSR and beyond the Polar Circle have also
confirmed that disturbances in mineral metabolism in children in the
far north occur earlier and are more marked (Talanova & Zabalueva,
1972).
In this connection, it is of some interest that Vitamin D
deficiency may have a direct effect on the pathogenesis of dental
caries (Dancig, 1974).
The problems associated with UVR deficiency are frequently
enhanced by social factors and by degree of pigmentation (Loomis,
1970).
Phototherapy. Some information on the beneficial effects of UVR
comes from the past and present use of sunlight and UVR in medical
treatment.
In the pre-antibiotic era, several forms of skin tuberculosis and
skin infections were treated with UVR. At present, UVR treatment in
medicine is largely confined to treating skin diseases, such as
psoriasis, acne, atopic dermatitis, and recurrent boils. There have
been reports that UVR, administered in gradually increasing doses, has
been helpful in the treatment of both chronic pneumonia (Boguckij et
al., 1975), and rheumatic diseases in childhood (Karacevceva, 1971).
5.2 Induction of Erythema in Human Skin
Erythema solare, or more commonly "sunburn", consists, in its
mildest form, of a reddening of the skin that appears 1-6 hours after
exposure to erythemogenic UVR and gradually fades in 1-3 days. In its
more severe forms, sunburn results in inflammation, blistering, and
peeling of the skin; it is followed by tanning of the skin, which
becomes noticeable within 2 or 3 days of irradiation.
The amount of radiation required to produce solar erythema
provides a convenient measure of UVR dosage. The actual amount of
energy required varies with the wavelength of the radiation, since
some regions of the spectrum are more effective than others. Because
of large variations from one individual to another, as well as
variations between different parts of the body, an "erythema unit"
cannot be determined with the same accuracy as physical units or even
units of visual luminosity. There are also appreciable variations in a
given individual from time to time, depending upon such factors as
physical condition and previous exposure. The methods for evaluating
erythema introduce additional variables. There is a latent period
after exposure before reddening of the skin is observable. Thus, the
length of time after exposure that the observation is made will affect
the results. In spite of these limitations, a "sunburn unit" (SU),
based on the effect of solar UVR on the average untanned human white
skin, provides a useful method of rating and comparing various sources
of UVR.
The use of the SU (Lazarev & Sokolov, 1971) is based upon the
applicability of the Bunsen-Roscoe law of reciprocity (see section
4.6.2). Over a reasonable range of exposure times, skin erythema
depends on the total UVR dose, but is independent of exposure rate and
duration (Seidl, 1969).
Monochromatic sources of radiation are not generally considered,
but the erythemal effectiveness depends upon the sum of the effects of
those wavelengths that are present. In the case of a line spectrum,
the total effect is calculated by adding the weighted intensities of
the various lines of the source, the weighting depending on the action
spectrum in use.
By adopting this method, the erythemal equivalent of any
particular distribution of radiant energy may be calculated and
expressed as the equivalent amount of energy at a particular
wavelength (e.g., 296.7 nm) that would produce the same erythemal
effect as the given heterogenous radiation (section 6).
Numerous factors contribute to the complexity of the erythema
response (skin temperature, sweating, dose-rate, etc., section 6). In
applying the reciprocity law to polychromatic radiation, the fact that
the observed differences in biological effects of different
wavelengths may introduce inaccuracies must be considered.
5.2.1 Action spectra of human skin erythema
Hausser & Vahle (1927) reported the first precise determination
of the action spectrum for the erytherna of human skin; a double peak
was shown with maxima at about 250 and 297 nm and a minimum at about
280 nm. In these and related studies, the skin of several individuals
was exposed to UVR from a mercury arc passed through a double-quartz-
prism monochromator, and the influence of wavelength, exposure time
and rate of exposure upon the nature, degree, and course of erythema
was examined. Similar action spectra were published by Coblentz et al.
(1932), Lukiesh et al. (1939), and Magnus (1977) (Fig. 5).
5.3 Natural Protection against Erythema-inducing Ultraviolet Radiation
5.3.1 Melanin (see also section 4.2.3)
Skin tanning during and following sun exposure is one of the
major protective devices of the skin against further damage by UVR.
The UVR range from 290 to 320 nm produces sunburn and subsequent new
pigment formation. UVR in the range of 320 to 400 nm produces little
erythema, except at very high doses, but may produce immediate pigment
darkening and other increases in melanin pigment in those who have
this capacity.
4.2 Acute Reactions in the Skin
4.2.1 Epidermal changes
Histological investigations of the early effects of UV
irradiation on animal skin show the changes that occur in the
epidermal cells particularly well.
Twenty-four hours after a single irradiation of skin with an
unfiltered mercury vapour lamp at doses between four and eight times
the minimal erythema dose, distinct signs of cell injury can be seen
including vacuolation of the cell cytoplasm and increased or decreased
density of the nucleus (Stenbäck, 1975a).
One distinctive feature, observed in both man (Daniels et al.,
1961) and experimental animals (Woodcock & Magnus, 1976) 24 hours
after exposure, is the presence of "sunburn cells" (SBC), scattered
diffusely throughout the superficial epidermis. These cells are
characterized by a densely-staining, glassy, homogeneous cytoplasm and
pyknotic nuclei. The appearance is similar to that of an individual
cell undergoing keratinization and thus, the process is regarded as a
form of individual cell shrinkage necrosis or dyskeratosis.
Following these early effects, hyperplasia, induced by radiation,
is observed (Blum et al., 1959; Stenbäck, 1975a). An increase in
mitosis indicates the onset of hyperplasia, which begins after about
two days.
Quantitative measurements have been made of the following aspects
of the effects of single doses of UVR on mouse skin (Blum et al.,
1959): (a) incidence of mitotic figures; (b) number of epidermal cells
per unit area; (c) epidermal thickness; (d) dermal thickness; (e) mean
nuclear diameter; and (f) number of groups of mitotic figures. All the
aspects measured changed in a similar fashion quantitatively following
irradiation; they increased rapidly at first, reaching a maximum and
then fell gradually towards normal. Soffen & Blum (1961) found a
gradual increase in epidermal thickness reaching a maximum between 8
and 14 days. These changes represent repair of the cell injury caused
by UVR; at the same time, the hyperplasia protects against further UV
irradiation.
For a long time, it was believed that thickening of the horny
layer was the essential adaptive change to UV irradiation in the
environment. The idea now prevails that melanin granules in the horny
layer and the Malpighian layer accumulate to form a protective screen
which is not only significant, but possibly even more important for
light protection than the thickness of the horny layer alone.
4.2.2 Erythema and inflammation
Sunburn (UVR erythema) and suntanning are the visible signs of UV
injury to the skin and the repair of such injury. UV erythema is
evidence of an inflammatory reaction to radiation, and appears after a
latent period of a few hours.
Erythema caused by UVR is confined to the exposed areas and
reflects blood vessel dilation and increased blood flow in the dermis.
It is often assumed that the initial photochemical reaction is in the
epidermis, where photon absorption by keratinocytes may lead to
liberation of intracellular substances that diffuse into the papillary
dermis to cause vasodilation. This diffusion theory is supported by
the existence of a latent period between exposure and erythema, and by
the fact that much of the radiant energy of the erythemogenic
wavelength region is absorbed by the epidermis. However, there may
also be direct injury to the vascular endothelium or to other sites in
the dermis.
Most studies of the mechanism of "sunburn" have used artificial
sources of UV-B or sources in which the spectral distribution is such
that UV-B is assumed to provide the major erythemogenic influence. In
experimental animals, the vascular response to UVR is biphasic. A
transient immediate vasopermeability is followed, after a latent
period of 2-8 hours, by a delayed, prolonged, increased,
vasopermeability and vasodilation. In some animal models, the initial
vasopermeability is accompanied by a faint erythema, which may begin
during exposure. This immediate effect has been attributed to
histamine release, possibly due to a direct effect of photons on
dermal mast cells. There is evidence that serotonin may also play some
role. In rats and guineapigs, serotonin and histamine antagonists
suppress the immediate phase of UVR vascular responses. In vivo
studies of human skin have shown the transient appearance of kinins
within minutes of UV irradiation. Kinin was not found after the onset
of delayed erythema.
The mediators of the delayed phase of UVR-induced vascular
response have been difficult to define. Antihistamines do not suppress
the delayed erythema phase of the vascular response to UVR in
guineapigs, rats, or man. Kinins have been stated to be either absent
or not elevated. Delayed erythema was not suppressed by various
inhibitors of proteases, plasminogen activitors, or kallikrein.
Serotonin has been found in urine following exposure to UVR, but the
significance of this finding is not clear.
More recently, prostaglandins, a group of long-chain fatty acids
with vasoactive properties, have been implicated as possible mediators
of the delayed phase of erythema. Prostaglandins are produced in human
and animal skin (prostaglandin groups E and F), intradermal injection
of prostaglandins produces erythema (PGE mainly, PGF group are much
less active), and furthermore the production of prostaglandins
increases following UVR exposure. Indomethacin is a potent inhibitor
of the conversion of arachnidonic acid to active prostaglandin, a
reaction catalyzed by the enzyme prostaglandin synthase. Topical
indemethacin produces a profound and prolonged blanching of
UV-B-induced, delayed erythema in both human subjects and guineapigs.
In man, intradermal indomethacin has been shown to consistently
decrease erythema due to UV-B but not all areas of erythema caused by
irradiation with UV-C; these effects appear to be due to the
inhibition of prostaglandin synthase.
It has been suggested that the complex reaction known as
"sunburn" may result from the release of hydrolytic enzymes and
possibly other substances by lysosomes within keratinocytes.
Histochemical studies of human skin exposed to UVR were
consistent with the theory that specific damage to lysosomal membranes
caused partial to complete rupture and release of enzymes.
The release of lysosomal enzymes may not only lead to damage of
the keratinocytes but may also release enzymes, vasodilator
substances, or subsequently formed cell-breakdown products into the
dermis, where they may directly or indirectly lead to erythema. It has
been suggested that the immediate erythema caused by UV irradiation
results from disruption of lysosomes of endothelial cells with release
of chemical mediators. The delayed erythema could then result from
secondary diffusion of proteinases from the epidermis, following
lysosomal rupture. It is also possible that direct photon damage to
the lysosomes of mast cells of the dermis or endothelial cells of
dermal blood vessels may play a role in the delayed erythema of
sunburn.
Multiple chromophores may exist in skin, and irradiation probably
leads to activation of a complicated cascade of mediators, the final
endpoint of which is erythema. Multiple pathways may exist. It is also
possible that photons have direct effects on blood vessels or nerves.
UVR causes dilation of isolated exposed dermal blood vessels. Dermal
proteins may be directly changed by radiation (Magnus, 1977).
4.2.3 Tanning
In addition to erythema, another consequence of exposure to UVR
is the pigmentation of the skin generally known as "tanning". This
becomes noticeable about 48 h after exposure and increases gradually
for several days. Tanning is, in part, due to migration of the pigment
melanin already present in the basal cells to the more superficial
layers of the skin where it has a greater effect on the appearance of
the reflected light. It is also partly due to the formation of new
pigment.
It is generally accepted that repeated irradiation with UVR
induces increases in the population of melanocytes in the epidermis of
man and experimental animals. With regard to the mechanism of this
phenomenon, the following processes or possibilities have been
considered: (a) increased division of melanocytes; (b) activation of
pigment formation in amelanogenic melanocytes: (c) migration of dermal
melanocytes into the epidermis; (d) various combinations of these
processes (Quevedo et al., 1965). That division of melanocytes occurs
has been shown in murine skin by Quevedo et al. (1963). Further,
published reports have provided evidence in support of the occurrence
of activation of amelogenic melanocytes in man (Mishima & Widlan,
1967) and experimental animals (Reynolds, 1954; Quevedo & Smith, 1963;
Miyazaki et al., 1968; Sato, 1971). However, the relative contribution
of each process remains to be established. In a study using tritiated
thymidine, it was demonstrated that approximately 1.1% of
dopa-positive epidermal melanocytes were labelled in hairless mice
receiving 9 daily exposures to UVR (Sato & Kawada, 1972). However, an
attempt to trace the labelled melanocytes using split epidermal sheets
was unsuccessful.
4.3 Acute Changes in the Eye
4.3.1 Photokeratitis and photoconjunctivitis
Although more energetic than the visible portion of the
electromagnetic spectrum, UVR cannot be detected by the visual
receptors in mammals, including man, because of absorption by the
ocular media. Thus, exposure to UVR may result in ocular damage before
the recipient is aware of the potential danger. Many cases of
keratitis of the cornea and cataracts of the lens have been reported
due to exposure to UVR produced by welding arcs, high-pressure pulsed
lamps, and the reflection of solar radiation from snow and sand.
Extensive reviews of the literature on the biological effects of
UVR have been compiled by Verhoeff et al. (1916) and Buchanan et al.
(1960). Verhoeff and his colleagues included all the, then available,
research data in their report and formulated some of the basic
hypotheses regarding ocular damage caused by UVR. Research on
threshold values and destructive and repair processes was summarized
by Duke-Elder (1926). Buschke et al. (1945) stressed the destructive
effects of UVR on the corneal epithelial cell nuclei, the loss of
epithelial adhesion to Bowman's membrane, and the inhibiting effects
of UVR on the healing process.
In studies by Cogan & Kinsey (1946), a monochromator was used to
evaluate the sensitivity of the cornea to individual spectral lines.
Their work, which was, for the most part, carried out with one to four
rabbits, provides the most reliable quantitative data on damaging
threshold values of UV energy of individual wavelengths. They
established a long wavelength limit of between 306 and 326 nm and a
threshold of 152 J/m2 at 288 nm for the rabbit.
Ordinary clinical photokeratitis is characterized by a period of
latency that tends to vary inversely with the severity of exposure.
The latent period may be as short as 30 min or as long as 24 h, but is
usually 6-12 h. Conjunctivitis follows, often accompanied by erythema
of facial skin and eyelids. There is a sensation of a foreign body or
"sand" in the eyes and various degrees of photophobia, lacrimation,
and blepharospasm. The importance of these acute symptoms lies in the
fact that the individual is visually incapacitated for 6-24 h and that
the ocular system, unlike the skin, does not develop tolerance to
repeated exposure to UVR. Almost all discomfort disappears within
48 h, and exposure rarely results in permanent damage.
Pitts & Kay (1969) and Pitts (1970) sought to establish the
experimental threshold for photokeratitis in rabbits and primates,
including man. Rabbits and monkeys showed a maximum sensitivity to UVR
at 270 nm. The radiant exposure threshold for man at 270 nm was
50J/m2 compared with 110 J/m2 for the rabbit and 60 J/m2
for the monkey.
The UVR incident on the eye is absorbed in turn by the cornea,
the aqueous humor, the lens, and the vitreous humor before reaching
the retina. Absorption is greater in the lens than in the cornea, and
is least in the aqueous humor. Below 300 nm, most of the UVR is
absorbed in the cornea and aqueous humor and very little penetrates as
far as the lens.
As a result of observations at above-threshold intensities, it
was felt that the reaction of the cornea to exposure to UVR wavebands
of 220 to 250 nm was different from that to wavebands of 250 to
310 nm. For exposures below 250 nm, signs and symptoms occurred soon after
exposure, and symptoms always returned to normal approximately 14 h
later. For exposure above 250 nm, symptoms did not generally occur
until 9 to 11 h after exposure, and visual acuity remained below
normal for 24 h after exposure. The differences observed were
attributed to the differences in the absorption of the different
wavebands. The shorter wavebands are absorbed in the outer corneal
epithelial layers, which undergo rapid change, whereas the longer
wavebands are absorbed in the deeper epithelial layers which show
delayed changes because these cells are more viable. Thus, the lesions
produced by shorter wavelengths are rapidly repaired while, at the
longer wavelengths, there is a delayed and more serious response
(Pitts & Cullen, 1977).
4.3.2 Cataracts
A cataract is a partial or complete loss of transparency of the
crystalline lens or its capsule. The wavelengths that affect the lens
appear to be in the same area as those that are most effective in
producing erythema on human skin.
Bachem (1956), using a filtered UVR system, concluded that
exposure to repeated high doses of longer UVR wavelengths could cause
cataracts through cumulative effects. He reported that the action
spectrum for cataracts began abruptly between 293 and 297 nm, reached
a peak near 297 nm, and fell abruptly near 313 nm. Minimal effects
existed through the remainder of the near UVR. In both the rabbit and
guineapig, reversible lenticular "blurring" occurred 5 to 10 days
after exposure. With repeated excessive exposures to the 297-355 nm
wavebands, irreversible lenticular opacities occurred after a latent
period of between 2.5 and 15 months.
Bachem (1956) concluded that since daylight does not contain any
UV-C or far infrared, and since both the visible and near infrared are
freely transmitted by the ocular media, it would appear that UV-B and
A are responsible for cataracts.
The chemical effects of UV-A exposure on tryptophan were studied
by Zigman et al. (1973) using human crystalline lenses. They found
that exposure of tryptophan to UV-A led to the formation of chromatic
photoproducts which bound to the lens proteins, altered their colour
and changed the solubility. Human lens material without added
tryptophan did not show chromatic changes on exposure to UV-A, until
48 h after exposure. Tryptophan showed an excitation wavelength at
278 nm and a fluorescent emission at 330 nm. However, following
exposure to UV-A, tryptophan showed an additional 360 nm excitation
and 440 nm fluorescence similar to that found in the brunescent human
cataract lenses. The UV irradiance for these studies was 30-50 W/m2
at 365 nm and exposures were made for at least several hours. These
exposure levels exceed those expected from sunlight for the same
period of time.
Thus, exposure of the eye to UV-A, for sufficient periods of
time, with an irradiance comparable to the irradiance level of
sunlight may interfere with the synthesis of lens proteins, catalyse
insoluble lens protein, and may result in chromatic changes in the
lens. While the basic mechanisms remain to be found, the evidence
clearly demonstrates that both in vitro and in vivo exposure to
UV-A can enhance cataractous changes in the crystalline lens.
Recently, Pitts & Cullen (1977) have studied the effects of the
300 nm-400 nm wavelength range on the rabbit eye in vivo. The
criteria used to determine corneal damage were epithelial debris,
epithelial stippling, epithelial granules, epithelial haze, epithelial
exfoliation, stromal haze, stromal opacities, and endothelial
disturbances. Anterior chamber signs included flare and cells. The
crystalline lens criteria were subcapsular opacities, capsular and
stromal haze, stromal opacities, and increased prominence of the
anterior suture. Criteria for the iris were the presence of the
anterior chamber signs, changes in clarity of the iris stroma, and
sluggish pupillary response.
Tables 3 and 4 show data for corneal and lenticular damage in the
290 nm-400 nm wavelength range for the rabbit. Limited data above
300 nm for the human eye do not allow a detailed comparison; however,
the human corneal threshold was considerably below that for either
the rabbit or the nonhuman primate.
Table 3. UV threshold data for the rabbit cornea and lensa
Threshold radiant exposure
Wavelength Threshold radiant exposure (J/m2) permanent damage (J/m2)
(nm)
corneal lens time to lens time for permanent
threshold threshold disappear threshold damage to appear
295 200 7500 24 h 10 000 2 h
300 500 1500 3 days 5000 24 h
305 700 3000 7 days 5000 24 h
310 550 7500 2 weeks 15 000 24 h
315 22 500 45 000 1 week 60 000 24 h
320 75 000 > 80 000 -- -- --
335 109 000 > 150 000 -- -- --
365 > 250 000 > 250 000 -- -- --
a From: Pitts & Cullen (1977).
Table 4. Permanent lenticular opacitiesa
Wavelength Radiant exposure Appearance of Permanenceb of
(nm) (J/m2) lens opacities lens opacities
300 5000 24 h permanent
305 5000 24 h permanent
310 15 000 24 h permanent
315 60 000 24 h permanent
a From: Pitts & Cullen (1977).
b Lenticular opacities present one month after exposure.
Phototoxic psoralen derivatives have been used recently in the
treatment of certain dermatological conditions. Such compounds caused
UV-A-induced corneal opacities and cataracts in mice (Griffin, 1959;
Koch, 1967).
Research on the effects of psoralens on the production of
cataracts in man needs to be pursued. As more and more people are
subjected to dermatological treatment using UV photosensitizing
compounds, the role of these compounds and their effects on the ocular
system should be defined.
4.4 Effects of Long-Term Exposure of Skin to UVR
UV irradiation induces an inflammatory response and ulceration in
both the epidermis and the dermis, the latter being infiltrated with
leukocytes in the region of the lesions, and to a much lesser extent
between them. These lesions ulcerate and the epidermis may disappear
for a time in the centre. However, peripherally there is particularly
active hyperplasia. The basal membrane (between the epidermis and
dermis) may disappear for a time in the regions of these "open"
lesions. Between the lesions, the infiltration of leukocytes is
relatively slight.
Injury to the epidermis and dermis, brought about by long-term
exposure to UVR leads to dermal alteration, fibrosis, and elastosis,
as well as to epidermal atrophy. However, experimental production of
cutaneous elastotic changes in animals by artificial UV irradiation
has only been reported rarely. Using histochemical methods, Sams et
al. (1964) demonstrated focal dermal elastosis in mice after prolonged
exposure to artificial UVR. UVR-induced changes in connective tissue
were also seen in rat skin by Nakamura & Johnson (1968).
4.4.1 UVR-induced mutagenesis and carcinogenesis
4.4.1.1 Mutagenesis
The nature of the effects of UVR on DNA, described earlier, shows
the importance and the specificity of the lesions in genetic material
and focuses interest on the mutations that might result in the various
cell types studied. The production of such mutations has best been
demonstrated in bacteria.
Among many others, Grossman (1968), Eisenstark (1971), and Witkin
(1976) have proposed, analysed, and verified some complex mechanisms
that control UVR-induced mutagenesis. It will be recalled also that,
according to the results obtained by Radman (1975), most of the
UVR-induced mutations would appear to be errors introduced in the DNA
during error-prone SOS repair.
Mammalian cells in culture have proved to be very suitable
material for the study of UVR mutagenesis (Kao & Puck, 1969). There is
quite good agreement between the dose of UVR and the proportion of
mutants (Bridges & Huckle, 1970).
Fox (1974) has shown that caffeine can reduce the rate of
mutations induced by UVR in cultures of rodent cells by inhibiting a
process of error-prone repair.
Isolation and study of UVR sensitive mutants in animal cells have
found remarkable applications in the study of various human xeroderma
pigmentosum mutants (Cleaver, 1973). However, such mutations in
mammalian germinal cells are not possible, because the cells are
located below the depths of penetration of UVR.
4.4.1.2 Mechanism of UVR carcinogenesis
In order to understand the possible mechanisms of UVR
carcinogenesis, it has been necessary to study the formation and
excision of pyrimidine directs, unscheduled DNA synthesis, and all the
data valid for the range from bacteria to mammalian cells.
It is now generally believed that UVR carcinogenesis results from
a succession of events originating in a photolesion of the genetic
material.
From the numerous studies that have led to en elucidation of some
of these mechanisms, it emerges that faulty DNA repair can increase
the frequency of carcinogenesis in the following ways: by causing
alterations in DNA, which also find expression in an increased
frequency of chromosome aberrations and a rise in mutation rate; by
increasing the rate of transformation of normal cells into cancer
cells; and by facilitating the expression of latent oncogenic viruses
able to trigger cancerous growth (Setlow, 1973; Trosko & Chu, 1973).
Errors induced by DNA repair during the initiation phase of
carcinogenesis seem to be the most likely mechanism leading to UVR
cancers.
4.4.1.3 Tumour types
Epidermal tumours. The first visible step in UVR-induced
epidermal tumour formation in animal skin consists of cell
proliferation, i.e., an increase in the number of squamous cells and
cell layers, which gradually become papillomatous in character
(Stenbäck, 1978). This is accompanied by an increase in cellular
atypia, nuclear enlargement, hyperchromatism, indentation, and
prominence of nucleoli. This basically proliferative response is
frequently replaced by a dysplastic progression showing a solar
keratosis-like pattern with cellular pleomorphism, occasionally with
pseudo-epitheliomatous hyperplasia-like features, which ultimately
invade the dermis. The tumours first seen are acanthomatous papillomas
(trichoepitheliomas), with a predominantly epithelial component or
fibropapillomas in which the tumour is composed of a fibrous stroma
covered by squamous epithelium.
The malignant tumours that ultimately develop are squamous cell
carcinomas of different types including: solid keratin containing
tumours; moderately differentiated, individually keratinizing tumours
with distinct intercellular bridges; and less-differentiated,
non-keratinizing spindle cell tumours, in which ultrastructural
analysis reveals squamous cell patterns.
Keratoacanthomas, i.e., proliferating epithelium on a cup-shaped
base, are relatively more frequent in animals of different species
receiving large doses of UVR than in animals treated with chemical
carcinogens.
Epidermal tumours are easily induced by different agents in mouse
skin (Stenbäck, 1969). Winkelman et al. (1960) reported the production
of squamous cell carcinomas on the backs of hairless mice exposed to
UVR. Further studies established that carcinomas could be induced in
this animal almost to the exclusion of sarcoma formation (Epstein &
Epstein, 1963). In early studies, epithelial tumours were reported in
both rats and mice (Findlay, 1930; Herlitz et al., 1930; Putschar &
Holz, 1930), but in later studies the deeper lying dermal tumour
response to UVR predominated. No mention of skin sarcomas was made,
however, in the studies of Beard et al. (1936) on albino rats in which
12 animals exhibited 9 carcinomas of the external ear, 6 sarcomas of
the eye, and 1 carcinoma of the skin of the nose.
The difference in the distribution of tumour types, with
sarcomatous growth predominating in haired mice and carcinomas in man
(Urbach, 1969) may, in part, be explained by the difference in
penetration of UVR, a greater amount reaching the dermis in mice
(Kirby-Smith et al., 1942; Everett et al., 1966).
Dermal tumours. Another type of neoplastic progression seen in
mice, particularly after intensive treatment with large doses of UVR
over a short time period, consists of ulceration, scarring, and the
subsequent formation of dermal tumours. These tumours begin as
aggregates of regularly built, elongated cells with small monomorphic
nuclei. Epithelial proliferation is occasionally observed as a
secondary phenomenon. The tumours rarely extend grossly through the
surface. In the early stages, they appear to be papillomas, although
they consist entirely of fibroblastic cells. Some tumours are
remarkably acellular, with a prominent fibrillary pattern. The tumours
are composed of large, polymorphic cells with prominent nuclei.
Ultrastructural analysis shows the predominant cellular components --
a dark cell type, with hyperchromatic nuclei and scanty cytoplasm, and
a light cell type, with large nuclei and abundant cytoplasmic
ribosomes (Stenbäck, 1975a). The same cell types are also seen in
malignant tumours, in which the cellular polymorphism frequently is
considerable, with nuclear atypism and enlargement, numerous nucleoli
and a generally disorganized arrangement. Sarcoma induction is partly
species-specific, as these tumours were not seen in UV-irradiated
Syrian golden hamsters (Stenbäck, 1975b) nor were they seen in
hairless mice (Epstein & Epstein, 1963) or guineapigs, susceptible to
chemical sarcoma induction (Stenbäck, 1969, 1975b).
An infrequent neoplastic alteration in several animal species is
the vascular tumour (Stenbäck, 1975b). This begins as a proliferation
of dilated vascular spaces with regular endothelial lining. Rarely,
the endothelium proliferates to the point of forming angiosarcomas, or
invasive tumours composed of large, atypical cells arranged in a
nodular pattern.
The role of the dermis in epidermal rumour formation has been
emphasized by numerous investigators (Orr, 1938; Mackie & McGovern,
1958). A proliferation of elastic tissue was induced experimentally in
mice, by Sams et al. (1964), through repeated exposure to UVR.
Similarly, Magnus & Johnson (1965) stimulated formation of elastotic
tissue, following early destruction of elastic fibres, with radiation
of 300 nm from a monochromatic source. Nakamura & Johnson (1968)
reported that dermal elastic tissue proliferation occurred in albino
rats after chronic irradiation with UVR, only after discontinuation of
the exposure. It was postulated by Johnson et al. (1968) that this
change was the result of photochemically-induced alterations in
fibroblast function, rather than the degradation of normal elastic
fibres. In support of this concept, Epstein et al. (1969) noted that
unscheduled DNA synthesis occurred in connective tissue cells of the
upper dermis within minutes of exposure to UVR shorter than 320 nm,
demonstrating a direct effect of UVR on dermal fibroblasts.
Because of its frequent association with skin cancer formation,
actinic elastosis has been considered to play an important role in
tumour development. However, Sams and his co-workers (1964) and Graham
& Helwig (1965) demonstrated that actinic elastosis was not essential
for the development of epidermal malignancies. Furthermore, the
experimental production of elastosis in animals has not been
associated with cancer formation, nor has UVR-induced experimental
cancer depended on the presence of this change (Epstein & Epstein,
1963).
Adnexal tumour formation is not as common in UVR-treated animals
(Stenbäck, 1975) as in, for example, carcinogen-treated rats
(Zackheim, 1964; Stenbäck, 1969). Hyperplasia and cystic
disorganization of hair follicle walls is very common, but rarely
progresses to grossly visible neoplasia. Trichoepitheliomas with
barely visible follicular arrangements are rarely seen. Even more
uncommon are hamartomatous tumours, hair follicle-derived
trichofolliculomas, and sebaceous gland tumours. Sebaceous gland
epitheliomas and carcinomas are even rarer. In a study in 1930,
Putschar & Holtz reported only a very small number of basal cell
carcinomas in rats.
Pigmented tumours. Studies on the induction of pigmented
tumours with UVR have been less successful. Benign, dermal melanocytic
lesions, or blue nevi, have been observed in hairless mice exposed to
UVR (Epstein et al., 1967). They were grossly seen as papules, 2-20 mm
in diameter, histologically composed of tightly arranged, heavily
pigment-laden polyhedral cells. Subcutaneous accumulation of pigmented
cells has also been seen in pigmented animal strains, as well as in
the skin of the Syrian golden hamster (Stenbäck, 1976). Such tumours
were possibly spontaneous, as the incidence was very low -- only
around 4% -- and unrelated to treatment. They were composed of
polyhedral or spindle-like cells arranged in a whorl-like pattern
beginning as hyperplasia of perifollicular melanocytes, before
spreading both laterally and deeply in the dermis. These tumours do
not show junctional activity; they do not metastasize and rarely kill
the host.
Melanomas, the type of greatest interest from an epidemiological
standpoint, are rarely seen in animals. Benign melanocytomas are
easily induced by treatment with chemical carcinogens as shown by
Shubik et al. (1960) and Rappaport et al. (1961). Fortner et al.
(1961) reported spontaneous melanomas in hamsters, similar to those in
man. However, the sensitivity of these animals to UVR is not known.
The effect of pigment, in general, in animal models has received
little attention. Freeman & Knox (1964a) induced melanocytoma in 67%
of a pigmented strain of rats. The tumours had an average latency
period of 193 days. In albino rats there was an 8% tumour incidence
with a 283-day latency period.
4.4.2 Species-specificity
Three specific factors -- pigment, hair, and thickness of the
stratum corneum -- have been found to alter susceptibility to tumour
induction. It was found that pigmented mice required significantly
more radiation to induce tumours than albino animals. Hair offered
even greater protection (Blum et al., 1959), and thus the hairless rat
appeared to be a likely subject for tumour induction studies. However,
the results of Hueper's (1941) extensive studies indicated that this
animal was, in fact, quite resistant to UV penetration because of its
thick stratum corneum. Since pigment, hair, and the stratum corneum
were limiting factors, the ears of albino mice and rats became the
traditional test sites for experimental production of cancer by UV
irradiation. A remarkable amount of quantitative data has been
accumulated using this system. The usual tumour produced in this
tissue was a sarcoma (Roffo, 1934; Grady et al., 1943b). Thus, the
albino ear model could not be used for evaluating qualitative changes
associated with epidermal carcinogenesis, which is the primary process
induced by UVR in human skin.
Winkelman and his co-workers (1960) reported the production of
squamous cell carcinomas on the backs of hairless mice exposed to UVR.
Further studies established that carcinomas could be induced in this
animal almost to the exclusion of sarcomas (Epstein & Epstein, 1963;
Epstein, 1965). In addition, UVR-induced pigmented tumours were
reported in pigmented hairless mice (Epstein et al., 1967). Thus, the
hairless mouse has provided a model for both the qualitative and
quantitative examination of the carcinogenicity of UVR.
Though penetration of UVR appears to be of obvious importance,
other factors also influence the type of growth induced by UVR. Grady
et al. (1943 a) found that the size of individual doses did not have
any effect on the carcinoma/sarcoma ratio in the albino, hairy mouse
but that reduced intervals between exposures increased the number of
epidermal carcinomas. These findings suggest that various tissues
respond differently to the carcinogenic effects of UVR (Stenbäck,
1975a). In part, this may be associated with differences in
penetration of various wavelengths of UVR. Furthermore, there are
great species differences in the repair capability of cells.
4.4.3 Ultraviolet radiation as an initiating agent
The two-stage concept for skin tumour formation proposed by
Berenblum & Shubik (1949), supposed formation of dormant tumour cells
by a single application of a carcinogen. These latent tumour cells
were provoked by the subsequent application of a promoter to form
visible tumours. In his studies on the induction of skin cancer by
exposure to UVR, Blum (1969) indicated that the process was continuous
beginning with the first exposure and progressing to ultimate tumour
formation. Blum's conclusions were based on experiments in which he
(Blum et al., 1943) and Rusch et al. (1941) could not produce tumours,
unless exposures were carried out over a minimum of 2 ´ months,
regardless of the amount of energy used. Blum's experiments suggested
that, with shorter exposure periods, tumour formation was not
accelerated enough to become visible within the lifetime of the
experimental animal. Epstein & Roth (1968), using a single exposure to
UVR as an initiatior and treatment with croton oil as a promotor,
concluded that croton oil stimulated rumour formation, the
characteristics of which were established by initial exposure to UVR.
The results of these studies were significantly different from those
encountered when a chemical carcinogen was used as the initiator
(Stenbäck, 1969).
4.5 Interactions between Ultraviolet Radiation and Chemicals
4.5.1 Chemically-enhanced photocarcinogenesis
An equally significant problem concerns photo-induced
carcinogenesis following the application to the skin of agents which
are phototoxic, but not in themselves carcinogenic.
A portion of the sunlight spectrum is carcinogenic, even in the
absence of an exogenous photosensitizer. At the current rate of
introduction of new compounds into the environment, it has become
increasingly important to determine whether a readily demonstrable
property, such as phototoxicity, can be used to predict compounds or
treatment regimes that could enhance photocarcinogenesis.
Concepts of chemical interaction with UVR-photocarcinogenesis are
of recent origin. Blum (1969) and Emmett (1973) reviewed a number of
reports dealing with the influence of phototoxic substances on
photocarcinogenesis. The results frequently appeared to be in
disagreement, a situation possibly reflecting differences in
technique, including solvent, routes of administration, light sources,
criteria for tumour recognition, and in statistical evaluation (Blum,
1969). In addition, characteristics of some compounds (toxicity,
carcinogencity, instability) rendered their interactions with light
complex, and their analysis difficult.
The relative enhancing effects on photocarcinogenesis of two
widely recognized photoactive compounds 8-methoxypsoralen, (8-MOP) and
anthracene were studied by Forbes et al. (1976). Both compounds were
phototoxic, but only the 8-MOP solutions markedly enhanced
photocarcinogenesis. Thus, the ability of a chemical to induce
phototoxicity is not always sufficient to augment
photo-carcinogenesis.
4.5.2 Interaction between light and chemical carcinogens
The fact that UVR can alter several phenanthrene carcinogens
photochemically has been known for some time. The studies of Davies et
al. (1972 a, b) showed that the carcinogenicity of 7,12
dimethylbenz(a)anthracene (DMBA) was reduced by light according to the
demonstrable photochemical lability of the compound. There was also
evidence that an additional time-dependent factor could influence this
effect. Thus, it appears that, at least in the case of DMBA-treated
animals, light may contribute in two opposing ways: (a) by
degradation of the carcinogen to non-carcinogenic products and (b)
by stimulating a phototoxic response that appears to coincide with a
relatively increased tumour yield.
Depending on the wavelengths of the UVR used, carcinogens can be
photodegraded to a less carcinogenic compound, or can induce
phototoxicity which may augment carcinogenesis or cause such a severe
local phototoxic reaction that the epithelial skin cells are nearly
all destroyed. Thus, either enhancement or inhibition of skin
carcinogenesis may occur, depending upon the carcinogen and the
wavelength of the light source used.
4.5.3 UVR-induced carcinogen formation
The photochemical conversion of sterols to carcinogenic
substances has been proposed as a potential explanation for the
cancer-causing effects of light upon skin (Black & Douglas, 1973). It
has recently been demonstrated, in vitro, that one such compound,
cholesterol-5a-oxide, which possesses carcinogenic properties
(Bishoff, 1969), is formed in human skin exposed to UVR (Black & Lo,
1971).
4.6 Physical and Quantitative Aspects of Ultraviolet Irradiation in
Animal Studies
4.6.1 Carcinogenic action spectrum
Determination of the effective wavelengths or "action spectrum"
is one of the primary objectives in the study of photobiological
responses. However, data are not available for the action spectrum of
UVR-induced cancer formation. The paucity of this information for one
of the most extensively studied photobiological reactions is due to a
number of factors, including the large number of potential
wavelengths, the considerable number of animals necessary and the
length of time (a matter of many months or years) required for
exposure to each wavelength, the difficulties in immobilizing
experimental animals, and the need for an especially good
monochromator with practically no stray light contamination. Though
the complete curve of the carcinogenic spectrum is not known, certain
aspects have been determined by less sophisticated methods. Roffo
(1934) reported that windowglass filtration eliminated the
carcinogenic effects of sunlight on white rats. Thus the offending
rays of the sun would be found approximately between 290-320 nm. A
number of investigators using mercury arc and fluorescent sun lamps
with filters have confirmed that, under their experimental conditions,
320 nm represented the longer wavelength limit for cancer formation
(Griffin et al., 1955; Blum, 1969). Furthermore, carcinogenic
responses have been produced by radiation as short at 230.2 nm (Roffo,
1934) and skin cancer has long been known to be induced by UV-C and
UV-B. Thus the action spectrum appears to include wavelengths between
230 and 320 nm but wavelengths between 290 and 320 nm have been shown
to have significantly greater carcinogenic effects than UVR shorter
than 260 nm (Rusch et al., 1941; Blum, 1943; Blum & Lippincott, 1943;
Kelner & Taft, 1956; Tung et al., 1971).
Freeman (1975) performed a series of experiments to provide more
specific comparative data by testing the hypothesis that the action
spectrum for carcinogenesis parallelled that for erythema. In these
studies, squamous cell carcinomas developed at approximately the same
rate and frequency, when UVR exposure was proportional to that for
erythema, with a decreasing potency from 300 to 320 nm. No tumours
occurred in mice exposed to 290 nm. These cancer-producing wavelengths
are also responsible for the normal phototoxic sunburn reaction.
Longer UV and visible light are neither erythema-producing nor
carcinogenic under ordinary conditions.
It cannot be assumed that the action spectra for human skin
erythema and mouse skin photocarcinogenesis are, similar, unless a
common chromophore or action mechanism is involved. Setlow (1974)
proposed that the common denominator was the action spectrum for
affecting DNA. Making some allowance for the skin transmission of UVR,
he showed that the shapes of action spectra for DNA, erythema, and
possibly skin cancer production were similar and could be made to
coincide.
4.6.2 Dose-response relationships
The second law of photochemistry (the law of reciprocity of
Bunsen & Roscoe) states that photochemical action depends only on the
product of the light intensity and the duration of exposure. This law,
however, holds only for primary photochemical action, and cannot be
applied to secondary reactions. Since the biological endpoints that
can be observed, such as erythema, pigmentation, skin cancer
production, etc., are certainly indirect effects, and since we still
know little about the primary photochemical reactions that underlie
them, it is not surprising that "reciprocity"' holds only for some of
the effects studied.
In the first quantitative photocarcinogenesis experiments ever
performed, Blum (1969) found that, within relatively narrow limits
(approximate factor of 5), differences in dose, intensity, or interval
between doses did not alter the shape or slope of tumour incidence
curves, but only their positions on the log-time axis. Blum, however,
was careful to point out that this was only true, as long as the
experimental conditions remained the same until the time the tumours
appeared.
With the accumulated data, he surmised that UVR-induced cancer
formation was a continuous process that began with the initial
exposure and that the appearance of tumours within the lifetime of the
animal depended on sufficient acceleration of the growth process.
In the majority of studies on photocarcinogenesis, fixed doses of
UVR have been given at a fixed dose rate, and the interval between
doses altered, but in increments of at least 24 hours. Such
experiments, while very valuable, are far removed from the conditions
found in nature under which human skin is exposed. Man is exposed to a
relatively low UVR flux that varies with time of day, season, and
environmental conditions, such as cloud cover, and also during the
exposure period.
Two recent animal experiments have shown that both varying the
UVR dose increment and varying the dose-rate while the daily dose
remains constant, affects UVR-induced skin carcinogenesis.
In the first experiment, groups of hairless mice were exposed to
doses of UVR from a bank of "Fluorescent Sun" (FS) lamps known to
produce skin cancer in these animals. Equal doses of UVR were
delivered in periods of 5 minutes, 50 minutes, or 500 minutes. Thus,
while the doses (given 5 times weekly) were the same, the flux varied
by a factor of 10 or 100. Striking differences in both tumour
development time and tumour yield were noted. The animals given the
total UVR dose in 5 minutes developed tumours later and in smaller
numbers than those given the same total dose in 50 or 500 minutes
(Forbes, personal communication). Thus, protracting the UVR dose over
longer time periods resulted in a striking increase in the
carcinogenic effects of the radiation.
In another experiment, mice were exposed to UVR doses per day
differing by a factor of two. As Blum had found previously, the lower
daily dose resulted in the delayed onset of first tumours without
significantly changing the shape of the response curve (Forbes, 1978).
4.6.3 Physical factors influencing UVR carcinogenesis
Although the tumour-promoting properties of such physical factors
as freezing, scalding, and wounding have been described for chemical
carcinogenesis systems, little information is available about the
effects of these factors on UVR-induced cancer formation. Bain & Rusch
(1943) reported that increasing the temperature to 35-38°C accelerated
the tumour growth rate. The stimulating effects of heat on UVR
carcinogenesis were confirmed by Freeman & Knox (1964b). Heat also
enhanced the acute injury response to UVR.
Temperature does not affect the photochemical reactions that
follow UV irradiation, but it does affect many of the biochemical
reactions that follow the initial photochemical change (Blum, 1941,
1969). Although it is known that heat adversely affects
photosensitivity (Lipson & Baldes, 1960), and other phenomena of light
injury (Bovie & Klein, 1919; Hill & Eidenow, 1923), and that heat
alters the effects of X-ray (Carlson & Jackson, 1959), the influence
of heat on burns produced by sunlight or UVR has rarely been
considered (Freeman & Knox, 1964b).
Other studies have shown that high winds and high humidity
significantly increase tumour incidence (Zilov, 1971; Owens et al.,
1977).
4.7 The Immune Response to Tumour Induction
A number of studies have shown that the immune status of the host
and tumour induction are potentially interactive processes. Chemical
carcinogens cause alterations of the host immune-response, the type
and extent of which depend on the rumour-inducing agent (Curtis,
1975). UVR also profoundly affects immunological reactivity,
particularly the immune response to skin tumours induced by UVR.
Studies leading to this conclusion were prompted by an observation by
Kripke (1974) that tumours induced by UVR in C3Hf mice were highly
antigenic and are usually immunologically rejected when transplanted
to normal, nonirradiated syngeneic recipients. This raised the
question as to why these tumours were able to grow progressively in
their primary host without succumbing to immunological rejection. In
an extensive series of experiments, Kripke & Fisher (1976) found that
pretreatment of mice with UVR for periods of time too short to induce
skin tumours made them unable to reject transplants of UVR-induced
tumours, even though such transplants were immunologically rejected by
unexposed animals. This indicates that UVR-exposed mice are
systemically altered in a way that prevents immunological rejection of
highly antigenic UVR-induced tumours.
Similarly, inability of unexposed secondary hosts to reject
UVR-induced tumours after transfer of lymphoid cells from UVR-treated
mice has been established, and demonstrates the immunological nature
of the systemic alteration in the UVR-treated mice (Fisher & Kripke,
1977). Furthermore, the failure of lymphoid cells from UVR-exposed
mice to react against UVR-induced tumours is due to the presence of
suppresser T lymphocytes in the lymphoid organs of UVR-treated
animals. In spite of their inability to reject highly antigenic
UVR-induced tumours, UVR-exposed mice respond normally to most other
antigens (Kripke, et al., 1977; Norbury et al., 1977). The one
exception is that UVR-treated mice have a transient defect in antigen
processing in the skin, which is reflected in their inability to
develop contact hypersensitivity reactions (Jessup et al., 1978).
The finding that a selective immunological defect precedes the
appearance of UVR-induced primary tumours suggests that the immune
system might control early UVR-induced skin cancers and that tumours
ultimately appear because of this interference by UVR with host
defence mechanisms.
The carcinogenic action of polycyclic hydrocarbons has been
associated with their immunosuppressive action (Stenbäck, 1969).
Immunodeficiency states and immunosuppression therapy are both
associated with an increased rumour incidence. Immunosuppressive
agents, such as antilymphocyte serum, enhance both chemically- and
UVR-induced tumour formation (Nathanson et al., 1973, 1976).
5. EFFECTS OF ULTRAVIOLET RADIATION ON MAN
5.1 Beneficial Effects
In addition to the direct effects on the skin, UVR produces a
number of systemic effects. It has the capacity to increase the tonus
of the sympathico-adrenal system, enhances mitochondrial and
microsomal enzyme activity and the non-specific immunity level, and
increases the secretion of a number of hormones (Tung, 1976).
Systolic and diastolic blood pressures are reduced before sunburn
appears and may even be reduced with exposures so mild that no visible
erythema is produced (Aitken, 1937). Blood pressure gradually falls
for 24 hours, and lowered pressure may persist for several days.
Studies have demonstrated that the exercise tolerance of children
receiving UVR through the winter is greater than that of control
groups not receiving radiation (Ronge, 1948; Zilov, 1971).
Other changes that have been attributed to UVR exposure include
reduction in serum cholesterol, increase in the glucose tolerance
curve, and decrease in serum tyrosine (Kameneckaja & Mitrofanova,
1975).
Seasonal changes in various diseases are often considered to be
evidence of UVR effects, but there are many other climatic variables
that change with the season, including temperature and daylength.
Blood volume, blood content of the skin, blood flow in the skin, and
hydration of the skin due to sweating vary with seasonal adaptation.
Thus few changes in disease patterns can be attributed to the effects
of UVR alone.
Data have been obtained indicating that the body's tolerance
towards exposure to chemical substances such as nitrites, benzpyrene,
carcinogens etc, which produce general toxic, carcinogenic, and
allergenic effects depends, to some extent, on the degree of exposure
to UVR (Gabovic et al., 1975; Prokopenko & Zabulueva, 1975;
Prokopenko, 1976). Prophylactic treatment with UVR preceding specific
immunization reduces the risk of vaccination allergy and helps to
increase the effectiveness of the immunization (Talanova et al.,
1975).
Where sizeable populations live in far northern areas, it is now
generally acknowledged that a long period of UVR deficit may have a
harmful effect on the human body. Numerous investigations indicate
that lack of exposure to solar UVR can lead to the development of a
pathological condition known as "UVR deficiency" or "light
starvation". The most frequent manifestation of this disease condition
is a disturbance in mineral metabolism and the development of Vitamin
D deficiency and rickets in children, accompanied by a sharp reduction
in the defensive powers of the body, making it particularly vulnerable
to unfavourable environmental factors. The development of UVR
deficiency is confirmed by data from a survey conducted, mainly among
children, in different photoclimatic zones of the USSR (Belikova et
al., 1975; Dancig, 1975), and from a survey of ships' crews working in
the north and in the tropics.
Vitamin D. Sunbathing is popular, and there is a widespread
feeling that "sunlight is good for you", but the physiological
benefits that presumably underlie the feeling of well-being have not
been adequately explained or studied.
The only thoroughly established beneficial effect of UVR on the
skin is the conversion of 7-dehydrocholesterol to Vitamin D3. Several
investigators have helped to promote the understanding of the
mechanisms of Vitamin D production and its metabolism and functioning.
It has long been noted that prolonged limitation or complete
absence of exposure of the human skin to solar UVR makes natural
activation of Vitamin D impossible and is an important factor in the
spread among the population of "chronic latent D avitaminosis"
reflected in widespread rickets and dental caries. Data from the
previously mentioned survey by Belikova et al. (1975) indicate that,
despite an overall reduction in the incidence of rickets and in its
severity, it is still frequent among young children in the north of
the USSR. Morbidity indices for rickets at latitude 65°N are 2.5-3
times as high as at latitude 45°N.
Comparative investigations among healthy children aged 3-6 years
in the central zone of the USSR and beyond the Polar Circle have also
confirmed that disturbances in mineral metabolism in children in the
far north occur earlier and are more marked (Talanova & Zabalueva,
1972).
In this connection, it is of some interest that Vitamin D
deficiency may have a direct effect on the pathogenesis of dental
caries (Dancig, 1974).
The problems associated with UVR deficiency are frequently
enhanced by social factors and by degree of pigmentation (Loomis,
1970).
Phototherapy. Some information on the beneficial effects of UVR
comes from the past and present use of sunlight and UVR in medical
treatment.
In the pre-antibiotic era, several forms of skin tuberculosis and
skin infections were treated with UVR. At present, UVR treatment in
medicine is largely confined to treating skin diseases, such as
psoriasis, acne, atopic dermatitis, and recurrent boils. There have
been reports that UVR, administered in gradually increasing doses, has
been helpful in the treatment of both chronic pneumonia (Boguckij et
al., 1975), and rheumatic diseases in childhood (Karacevceva, 1971).
5.2 Induction of Erythema in Human Skin
Erythema solare, or more commonly "sunburn", consists, in its
mildest form, of a reddening of the skin that appears 1-6 hours after
exposure to erythemogenic UVR and gradually fades in 1-3 days. In its
more severe forms, sunburn results in inflammation, blistering, and
peeling of the skin; it is followed by tanning of the skin, which
becomes noticeable within 2 or 3 days of irradiation.
The amount of radiation required to produce solar erythema
provides a convenient measure of UVR dosage. The actual amount of
energy required varies with the wavelength of the radiation, since
some regions of the spectrum are more effective than others. Because
of large variations from one individual to another, as well as
variations between different parts of the body, an "erythema unit"
cannot be determined with the same accuracy as physical units or even
units of visual luminosity. There are also appreciable variations in a
given individual from time to time, depending upon such factors as
physical condition and previous exposure. The methods for evaluating
erythema introduce additional variables. There is a latent period
after exposure before reddening of the skin is observable. Thus, the
length of time after exposure that the observation is made will affect
the results. In spite of these limitations, a "sunburn unit" (SU),
based on the effect of solar UVR on the average untanned human white
skin, provides a useful method of rating and comparing various sources
of UVR.
The use of the SU (Lazarev & Sokolov, 1971) is based upon the
applicability of the Bunsen-Roscoe law of reciprocity (see section
4.6.2). Over a reasonable range of exposure times, skin erythema
depends on the total UVR dose, but is independent of exposure rate and
duration (Seidl, 1969).
Monochromatic sources of radiation are not generally considered,
but the erythemal effectiveness depends upon the sum of the effects of
those wavelengths that are present. In the case of a line spectrum,
the total effect is calculated by adding the weighted intensities of
the various lines of the source, the weighting depending on the action
spectrum in use.
By adopting this method, the erythemal equivalent of any
particular distribution of radiant energy may be calculated and
expressed as the equivalent amount of energy at a particular
wavelength (e.g., 296.7 nm) that would produce the same erythemal
effect as the given heterogenous radiation (section 6).
Numerous factors contribute to the complexity of the erythema
response (skin temperature, sweating, dose-rate, etc., section 6). In
applying the reciprocity law to polychromatic radiation, the fact that
the observed differences in biological effects of different
wavelengths may introduce inaccuracies must be considered.
5.2.1 Action spectra of human skin erythema
Hausser & Vahle (1927) reported the first precise determination
of the action spectrum for the erytherna of human skin; a double peak
was shown with maxima at about 250 and 297 nm and a minimum at about
280 nm. In these and related studies, the skin of several individuals
was exposed to UVR from a mercury arc passed through a double-quartz-
prism monochromator, and the influence of wavelength, exposure time
and rate of exposure upon the nature, degree, and course of erythema
was examined. Similar action spectra were published by Coblentz et al.
(1932), Lukiesh et al. (1939), and Magnus (1977) (Fig. 5).
5.3 Natural Protection against Erythema-inducing Ultraviolet Radiation
5.3.1 Melanin (see also section 4.2.3)
Skin tanning during and following sun exposure is one of the
major protective devices of the skin against further damage by UVR.
The UVR range from 290 to 320 nm produces sunburn and subsequent new
pigment formation. UVR in the range of 320 to 400 nm produces little
erythema, except at very high doses, but may produce immediate pigment
darkening and other increases in melanin pigment in those who have
this capacity.
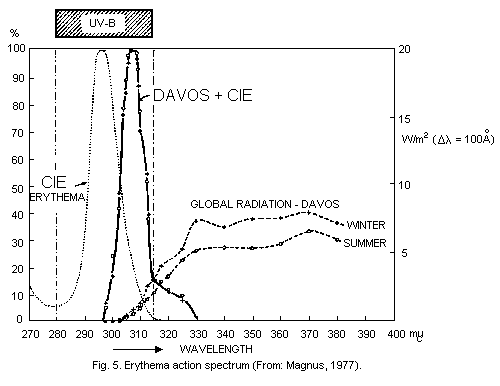 Constitutional skin colour in man is the "baseline" colour that
develops in the absence of exposure to solar radiation or other
environmental influences and results from genetically determined
levels of melanin pigmentation. Facultative skin colour or "tan" is
the increase in melanin pigmentation above the constitutional level
which is induced by UVR or by pituitary hormones such as the
melanocyte-stimulating or the adrenocorticotropic hormones. The
facultative tanning (darkening) response induced by solar irradiation
can be divided into immediate tanning (IT), which occurs within
minutes of exposure to sunlight, and delayed tanning (DT), which
becomes evident several days after exposure (for review see
Fitzpatrick et al., 1974).
Immediate tanning (IT). IT, also called immediate pigment
darkening (IPD), is best seen in pigmented individuals. In general,
the darker the unexposed, baseline, inherited colouration, the greater
the ability to exhibit IT.
Within 5-10 min of exposure to the noonday sun, a gradual
darkening is noticed, which is confined to exposed skin. If exposure
continues, darkening increases until it reaches a maximum after about
1 h of irradiation. The colour ranges from light brown to dark brown
or, in the more deeply pigmented races, from grey-brown to black.
Brief exposures to sunlight may lead to slight to moderate IT, which
begins to fade within 30 min of the end of exposure and is scarcely
visible after 3-8 h. Prolonged exposure to sunlight or high-intensity
artificial long wave UVR sources can lead to striking IT that lasts
longer than 36 hours. IT is optimally produced by both long wave UVR
and visible light.
Delayed tanning (DT). DT, also referred to as "true
melanogenesis", becomes visible 72 hours after exposure to UVR,
although electron microscope studies have shown ultrastructural
evidence of the formation of new melanosomes and melanin much earlier.
The major action spectrum of DT is the same as that for sunburn but DT
can also follow exposure to UV-C and UV-A and shorter wavelength
visible light.
Exposure to UVR can also modify the pattern of distribution of
melanosomes in keratinocytes. In Mongoloid peoples, repeated UVR
exposure results in a predominance of non-aggregated single
melanosomes. Interpretation of variations in melanosome packaging
within the epidermis must take into account the history of the
previous exposure of individuals to solar radiation and other factors.
Once present, melanin acts as a neutral density filter and
decreases the amounts of UVR that can reach tile lower layer of the
skin containing viable keratinocytes or penetrate into the dermis to
strike blood vessels. As constitutional or facultative pigmentation
increases, the dose of UVR required to produce erythema increases.
5.3.2 Thickening of the stratum corneum
Thickening and brownish discoloration of the stratum corneum of
light-exposed areas of human skin are noted after exposure to UVR.
While a protective effect unquestionably exists, it is of relatively
less practical importance than the protection afforded by melanin
pigmentation.
5.4 Solar Elastosis and Other Dermal Effects of Ultraviolet Radiation
(See also section 4.2)
Sunlight may have many effects on the skin, and one of the most
important both clinically and cosmetically is aging. Gross changes in
actinically damaged skin are a dry, coarse, leathery appearance,
laxity with wrinkling, and various pigmentary changes. Frequently, in
elderly and even in some relatively young fair-skinned adults, there
is a striking difference between light-exposed regions and those
protected by clothing. A weather-beaten farmer often appears
considerably older than a clerk of comparable age. Black skin has
natural protection because of its high melanin content and elderly
Negroes often appear deceptively young (Silverstone & Searle, 1970).
It now seems clear that collagen degeneration in the dermis is
independent of age and is determined simply by the cumulative amount
of injury from UVR. This depends on the degree and frequency of
exposure and the extent of natural (and artificial) protection
afforded to the patient's skin by the thickening of the stratum
corneum, melanin pigment, clothing, or chemical sunscreens.
The visible cutaneous changes usually interpreted as "aging" are
apparently due, to a large extent, to chronic exposure to sunlight.
5.5 Ultraviolet Radiation and Skin Cancer in Man
(See also Section 4.2)
Classical evidence supporting the role of sunlight, particularly
of UVR, as a causal factor in human skin cancer can mainly be
summarized in the form of six associations of skin cancer (Urbach et
al., 1972):
(a) Association with exposed areas of the skin. Among
white-skinned people, skin cancers occur most frequently on the parts
of the body most exposed to sunlight -- the head, neck, arms, and
hands, and the legs of women.
(b) Association with protection against UVR. Among races with
dark skin, in which pigment filters UVR, there is very little skin
cancer and the disease does not occur predominantly in areas of the
skin exposed to the sun. Sunburn and skin cancer arise in the same
tissue, and UVR is known to cause sunburn. It appears that those who
are more susceptible to skin cancer sunburn more easily. White-skinned
people of Celtic origin are more susceptible to both skin cancer and
sunburn while those of Latin origin are less susceptible.
(c) Association with the amount of exposure to the sun. Among
fair-skinned people, there appears to be a greater prevalence of skin
cancer in those who spend more time outdoors.
(d) Association with the intensity of solar exposure. The
incidence of skin cancer among white-skinned people increases with
increasing proximity to the equator and thus with increasing solar
radiation and intensity of UVR.
(e) Association with UVR in laboratory studies. Skin cancer can
be produced in mice with repeated doses of UVR in the same spectral
range that produces sunburn in the human skin.
(f) Association with insufficient ability to repair DNA damaged
by UVR. Those with the recessive disease xeroderma pigmentosum, who
have a defect in DNA repair, develop skin cancer prematurely. Such
persons are photosensitive, and develop tumours, induced by exposure
to solar UVR. They frequently die of skin cancer before reaching adult
life.
5.5.1 Anatomical distribution of skin cancer
Numerous studies have shown that, in fair-skinned people, skin
cancers arise primarily on sites exposed to sunlight. It has been
demonstrated that about 90% of all basal cell carcinomas and more than
half of all squamous cell carcinomas occur on the head and neck. The
majority of those squamous cell cancers not occurring on the head and
neck are found on the hands and forearms; the ears of females are
markedly protected by hair (Silverstone & Searle, 1970; Swanbeck &
Hillstrom, 1971).
Comparing the sites of non-melanoma cancers with studies made of
the geometry of insolation of the head and neck areas, it becomes
clear that two thirds of all basal cell carcinomas occur on the skin
sites receiving the highest doses of UVR, and that virtually all
squamous cell carcinomas occur at these sites (Urbach et al., 1972).
The anatomical distribution of malignant melanoma, a less common
but more deadly form of skin cancer, suggests a less striking
association with UVR exposure. However, there has been a considerable
increase in the prevalence of this type of cancer on the legs of women
during the past 25 years and a special form of melanoma, lentigo
maligna, almost always arises on the face.
5.5.2 Occupation and skin cancer
As has been pointed out previously, surveys of the incidence of
skin cancer other than malignant melanoma are, generally, not very
reliable. Consequently, data concerning the relationship between
occupation and skin cancer incidence are also scarce. From the studies
carried out in Queensland, Australia, and in Galway, Ireland, the most
reliable sources, it appears that those in outdoor occupations are the
most highly exposed and, therefore, at the highest risk. Thus farmers,
fishermen, sailors, and others such as road workers, roofers,
policemen, and postmen, have a higher incidence of skin cancer than
office and factory workers (Swanbeck & Hillstörm, 1971; Gordon &
Silverstone, 1976).
5.5.3 Genetics and skin cancer
In several carefully controlled studies comparing patients with
non-melanoma and melanoma skin cancer to age-sex matched controls from
the same populations, a distinct association was found between skin
cancer and light coloured eyes, fair complexion, light hair colour,
poor ability to tan, ease of sunburning, and a history of repeated
severe sunburn. Furthermore, whenever looked for, there was a higher
prevalence of Celtic stock among skin cancer patients (Silverstone &
Searle, 1970; Urbach et al., 1972). Xeroderma pigmentosum (XP) is a
hereditary skin disease in man. Studies on cells from the patients
have supplied the most decisive arguments regarding the relationships
between photolesion repair and carcinogenesis. Persons suffering from
this disease show abnormal pigmentation and a high incidence of skin
cancers triggered off by exposure to the solar UVR. Cleaver (1973),
who was the first to draw attention to the possible causes of the
disease, has written a critical review of the main biochemical and
genetic studies which reveal the extreme complexity, but also the
ingenuity and logic of the molecular processes that play a part in the
development of the disease.
In general, it has proved possible to establish a correlation
between the level of DNA repair and the seriousness of the symptoms in
XP patients.
Numerous and more complex studies have shown that the various
degrees of UVR sensitivity observed in XP patients correspond to
different sensitivity mutants. Using techniques such as cell fusion
and complementation, several groups have been distinguished that are
characterized by various defects in the repair mechanisms (Kraemer et
al., 1975). Some mutants show normal excision but no repair synthesis
(Lehmann et al., 1975). In others, repair replication would seem to be
normal, but chain breaks appear more slowly than in normal cells.
Caffeine emphasizes these differences still further (Fornace et al.,
1976).
Although the mechanisms are far from completely elucidated, they
show the considerable value of this kind of genetic disorder, in which
the problems of the photolesions and their repair and of
carcinogenesis are intimately linked.
5.5.4 Geographical distribution of non-melanoma skin cancer
Incidence data for skin cancer, other than melanoma, must be
treated with considerable reserve. Many cancer registries do not
register non-melanoma skin cancer at all, and those that do are
uniformly incomplete, since most of these tumours are treated in
physicians' offices and either not reported at all or reported without
histological verification.
A survey of the recorded geographical distribution of
non-melanoma skin cancer has been made by Cutchis (1978) (Fig. 6 and
7). From the data of Scotto et al. (1974), it appears that in Iowa,
USA, for instance, the incidence of skin cancer in males rose from
61.4/100 000 in 1950 to 174/100 000 in 1972. This approximately 3-fold
increase is also noticeable in all areas in Texas, with the exception
of El Paso, where the incidence rate actually decreased, and Houston,
where the increase was apparently only 2-fold. The Texas data
(MacDonald, 1976) showed that incidence rates increased with
descending latitude, but not in a stepwise fashion. In the most recent
5-year period (1962-1966), the rate for El Paso was 183/100 000 and
that for San Antonio (1° further south) was 147.35/100 000. In San
Antonio, a large part of the permanent population consisted of retired
military personnel and their families. Most of these people had spent
much less time in the sunny south than the permanent population in El
Paso. The highest incidence rate was found at Corpus Christi at
latitude 28°N (371/100 000). This was significantly greater than the
incidence at Harlingen (284.5/100 000), at latitude 26°N. At Corpus
Christi, there was another factor involved besides exposure, i.e., a
great preponderance of Celtic inhabitants whose forebears migrated to
that area about a century ago. Thus the disproportionately high
incidence of skin cancer in Corpus Christi could be due to a
combination of intense insolation and a very susceptible population, a
situation similar to that existing in Queensland, Australia.
These findings demonstrate how important the confounding social
factors can be in evaluating skin cancer statistics in relation to UVR
exposure.
Europe. In Europe, just as in the USA and Australia, a marked
north-south gradient of non-melanoma skin cancer exists.
In 1976, Waterhouse et al. reported skin cancer incidence rates
for males and females as follows: Sweden -- 10.6 and 6.5/100 000;
Denmark -- 33.4 and 24.5; Federal Republic of Germany -- 7.3 and 4.6;
UK -- 40.3 and 21.4; and Yogoslavia -- 23.2 and 22.8. Other reports
suggest incidences in the German Democratic Republic of 43.9 and
40.3/100 000 (Herold & Berndt, 1968), and in Bulgaria, of 42.2 for
rural and 25.0 for urban people (Anchev et al., 1968).
Constitutional skin colour in man is the "baseline" colour that
develops in the absence of exposure to solar radiation or other
environmental influences and results from genetically determined
levels of melanin pigmentation. Facultative skin colour or "tan" is
the increase in melanin pigmentation above the constitutional level
which is induced by UVR or by pituitary hormones such as the
melanocyte-stimulating or the adrenocorticotropic hormones. The
facultative tanning (darkening) response induced by solar irradiation
can be divided into immediate tanning (IT), which occurs within
minutes of exposure to sunlight, and delayed tanning (DT), which
becomes evident several days after exposure (for review see
Fitzpatrick et al., 1974).
Immediate tanning (IT). IT, also called immediate pigment
darkening (IPD), is best seen in pigmented individuals. In general,
the darker the unexposed, baseline, inherited colouration, the greater
the ability to exhibit IT.
Within 5-10 min of exposure to the noonday sun, a gradual
darkening is noticed, which is confined to exposed skin. If exposure
continues, darkening increases until it reaches a maximum after about
1 h of irradiation. The colour ranges from light brown to dark brown
or, in the more deeply pigmented races, from grey-brown to black.
Brief exposures to sunlight may lead to slight to moderate IT, which
begins to fade within 30 min of the end of exposure and is scarcely
visible after 3-8 h. Prolonged exposure to sunlight or high-intensity
artificial long wave UVR sources can lead to striking IT that lasts
longer than 36 hours. IT is optimally produced by both long wave UVR
and visible light.
Delayed tanning (DT). DT, also referred to as "true
melanogenesis", becomes visible 72 hours after exposure to UVR,
although electron microscope studies have shown ultrastructural
evidence of the formation of new melanosomes and melanin much earlier.
The major action spectrum of DT is the same as that for sunburn but DT
can also follow exposure to UV-C and UV-A and shorter wavelength
visible light.
Exposure to UVR can also modify the pattern of distribution of
melanosomes in keratinocytes. In Mongoloid peoples, repeated UVR
exposure results in a predominance of non-aggregated single
melanosomes. Interpretation of variations in melanosome packaging
within the epidermis must take into account the history of the
previous exposure of individuals to solar radiation and other factors.
Once present, melanin acts as a neutral density filter and
decreases the amounts of UVR that can reach tile lower layer of the
skin containing viable keratinocytes or penetrate into the dermis to
strike blood vessels. As constitutional or facultative pigmentation
increases, the dose of UVR required to produce erythema increases.
5.3.2 Thickening of the stratum corneum
Thickening and brownish discoloration of the stratum corneum of
light-exposed areas of human skin are noted after exposure to UVR.
While a protective effect unquestionably exists, it is of relatively
less practical importance than the protection afforded by melanin
pigmentation.
5.4 Solar Elastosis and Other Dermal Effects of Ultraviolet Radiation
(See also section 4.2)
Sunlight may have many effects on the skin, and one of the most
important both clinically and cosmetically is aging. Gross changes in
actinically damaged skin are a dry, coarse, leathery appearance,
laxity with wrinkling, and various pigmentary changes. Frequently, in
elderly and even in some relatively young fair-skinned adults, there
is a striking difference between light-exposed regions and those
protected by clothing. A weather-beaten farmer often appears
considerably older than a clerk of comparable age. Black skin has
natural protection because of its high melanin content and elderly
Negroes often appear deceptively young (Silverstone & Searle, 1970).
It now seems clear that collagen degeneration in the dermis is
independent of age and is determined simply by the cumulative amount
of injury from UVR. This depends on the degree and frequency of
exposure and the extent of natural (and artificial) protection
afforded to the patient's skin by the thickening of the stratum
corneum, melanin pigment, clothing, or chemical sunscreens.
The visible cutaneous changes usually interpreted as "aging" are
apparently due, to a large extent, to chronic exposure to sunlight.
5.5 Ultraviolet Radiation and Skin Cancer in Man
(See also Section 4.2)
Classical evidence supporting the role of sunlight, particularly
of UVR, as a causal factor in human skin cancer can mainly be
summarized in the form of six associations of skin cancer (Urbach et
al., 1972):
(a) Association with exposed areas of the skin. Among
white-skinned people, skin cancers occur most frequently on the parts
of the body most exposed to sunlight -- the head, neck, arms, and
hands, and the legs of women.
(b) Association with protection against UVR. Among races with
dark skin, in which pigment filters UVR, there is very little skin
cancer and the disease does not occur predominantly in areas of the
skin exposed to the sun. Sunburn and skin cancer arise in the same
tissue, and UVR is known to cause sunburn. It appears that those who
are more susceptible to skin cancer sunburn more easily. White-skinned
people of Celtic origin are more susceptible to both skin cancer and
sunburn while those of Latin origin are less susceptible.
(c) Association with the amount of exposure to the sun. Among
fair-skinned people, there appears to be a greater prevalence of skin
cancer in those who spend more time outdoors.
(d) Association with the intensity of solar exposure. The
incidence of skin cancer among white-skinned people increases with
increasing proximity to the equator and thus with increasing solar
radiation and intensity of UVR.
(e) Association with UVR in laboratory studies. Skin cancer can
be produced in mice with repeated doses of UVR in the same spectral
range that produces sunburn in the human skin.
(f) Association with insufficient ability to repair DNA damaged
by UVR. Those with the recessive disease xeroderma pigmentosum, who
have a defect in DNA repair, develop skin cancer prematurely. Such
persons are photosensitive, and develop tumours, induced by exposure
to solar UVR. They frequently die of skin cancer before reaching adult
life.
5.5.1 Anatomical distribution of skin cancer
Numerous studies have shown that, in fair-skinned people, skin
cancers arise primarily on sites exposed to sunlight. It has been
demonstrated that about 90% of all basal cell carcinomas and more than
half of all squamous cell carcinomas occur on the head and neck. The
majority of those squamous cell cancers not occurring on the head and
neck are found on the hands and forearms; the ears of females are
markedly protected by hair (Silverstone & Searle, 1970; Swanbeck &
Hillstrom, 1971).
Comparing the sites of non-melanoma cancers with studies made of
the geometry of insolation of the head and neck areas, it becomes
clear that two thirds of all basal cell carcinomas occur on the skin
sites receiving the highest doses of UVR, and that virtually all
squamous cell carcinomas occur at these sites (Urbach et al., 1972).
The anatomical distribution of malignant melanoma, a less common
but more deadly form of skin cancer, suggests a less striking
association with UVR exposure. However, there has been a considerable
increase in the prevalence of this type of cancer on the legs of women
during the past 25 years and a special form of melanoma, lentigo
maligna, almost always arises on the face.
5.5.2 Occupation and skin cancer
As has been pointed out previously, surveys of the incidence of
skin cancer other than malignant melanoma are, generally, not very
reliable. Consequently, data concerning the relationship between
occupation and skin cancer incidence are also scarce. From the studies
carried out in Queensland, Australia, and in Galway, Ireland, the most
reliable sources, it appears that those in outdoor occupations are the
most highly exposed and, therefore, at the highest risk. Thus farmers,
fishermen, sailors, and others such as road workers, roofers,
policemen, and postmen, have a higher incidence of skin cancer than
office and factory workers (Swanbeck & Hillstörm, 1971; Gordon &
Silverstone, 1976).
5.5.3 Genetics and skin cancer
In several carefully controlled studies comparing patients with
non-melanoma and melanoma skin cancer to age-sex matched controls from
the same populations, a distinct association was found between skin
cancer and light coloured eyes, fair complexion, light hair colour,
poor ability to tan, ease of sunburning, and a history of repeated
severe sunburn. Furthermore, whenever looked for, there was a higher
prevalence of Celtic stock among skin cancer patients (Silverstone &
Searle, 1970; Urbach et al., 1972). Xeroderma pigmentosum (XP) is a
hereditary skin disease in man. Studies on cells from the patients
have supplied the most decisive arguments regarding the relationships
between photolesion repair and carcinogenesis. Persons suffering from
this disease show abnormal pigmentation and a high incidence of skin
cancers triggered off by exposure to the solar UVR. Cleaver (1973),
who was the first to draw attention to the possible causes of the
disease, has written a critical review of the main biochemical and
genetic studies which reveal the extreme complexity, but also the
ingenuity and logic of the molecular processes that play a part in the
development of the disease.
In general, it has proved possible to establish a correlation
between the level of DNA repair and the seriousness of the symptoms in
XP patients.
Numerous and more complex studies have shown that the various
degrees of UVR sensitivity observed in XP patients correspond to
different sensitivity mutants. Using techniques such as cell fusion
and complementation, several groups have been distinguished that are
characterized by various defects in the repair mechanisms (Kraemer et
al., 1975). Some mutants show normal excision but no repair synthesis
(Lehmann et al., 1975). In others, repair replication would seem to be
normal, but chain breaks appear more slowly than in normal cells.
Caffeine emphasizes these differences still further (Fornace et al.,
1976).
Although the mechanisms are far from completely elucidated, they
show the considerable value of this kind of genetic disorder, in which
the problems of the photolesions and their repair and of
carcinogenesis are intimately linked.
5.5.4 Geographical distribution of non-melanoma skin cancer
Incidence data for skin cancer, other than melanoma, must be
treated with considerable reserve. Many cancer registries do not
register non-melanoma skin cancer at all, and those that do are
uniformly incomplete, since most of these tumours are treated in
physicians' offices and either not reported at all or reported without
histological verification.
A survey of the recorded geographical distribution of
non-melanoma skin cancer has been made by Cutchis (1978) (Fig. 6 and
7). From the data of Scotto et al. (1974), it appears that in Iowa,
USA, for instance, the incidence of skin cancer in males rose from
61.4/100 000 in 1950 to 174/100 000 in 1972. This approximately 3-fold
increase is also noticeable in all areas in Texas, with the exception
of El Paso, where the incidence rate actually decreased, and Houston,
where the increase was apparently only 2-fold. The Texas data
(MacDonald, 1976) showed that incidence rates increased with
descending latitude, but not in a stepwise fashion. In the most recent
5-year period (1962-1966), the rate for El Paso was 183/100 000 and
that for San Antonio (1° further south) was 147.35/100 000. In San
Antonio, a large part of the permanent population consisted of retired
military personnel and their families. Most of these people had spent
much less time in the sunny south than the permanent population in El
Paso. The highest incidence rate was found at Corpus Christi at
latitude 28°N (371/100 000). This was significantly greater than the
incidence at Harlingen (284.5/100 000), at latitude 26°N. At Corpus
Christi, there was another factor involved besides exposure, i.e., a
great preponderance of Celtic inhabitants whose forebears migrated to
that area about a century ago. Thus the disproportionately high
incidence of skin cancer in Corpus Christi could be due to a
combination of intense insolation and a very susceptible population, a
situation similar to that existing in Queensland, Australia.
These findings demonstrate how important the confounding social
factors can be in evaluating skin cancer statistics in relation to UVR
exposure.
Europe. In Europe, just as in the USA and Australia, a marked
north-south gradient of non-melanoma skin cancer exists.
In 1976, Waterhouse et al. reported skin cancer incidence rates
for males and females as follows: Sweden -- 10.6 and 6.5/100 000;
Denmark -- 33.4 and 24.5; Federal Republic of Germany -- 7.3 and 4.6;
UK -- 40.3 and 21.4; and Yogoslavia -- 23.2 and 22.8. Other reports
suggest incidences in the German Democratic Republic of 43.9 and
40.3/100 000 (Herold & Berndt, 1968), and in Bulgaria, of 42.2 for
rural and 25.0 for urban people (Anchev et al., 1968).
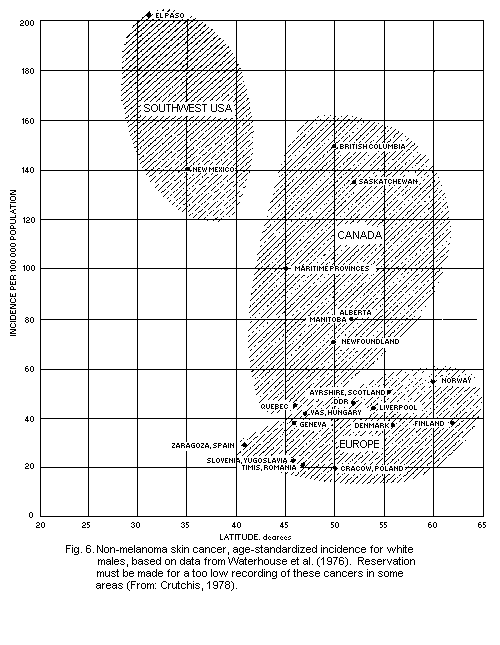
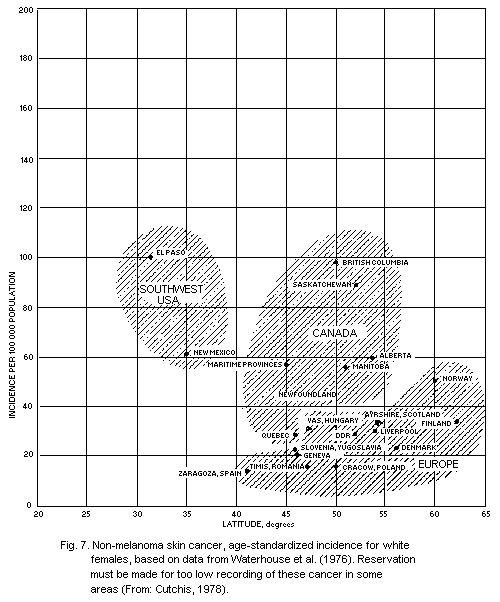 In the USSR, skin cancer morbidity increases from north to south.
According to these data, skin cancer represents 15-26% of the total
cancer morbidity in the south, and 9-14° in the north (Caklin, 1974).
Africa. Information concerning skin cancer in Africa, gathered
from various sources, but mainly from the reports of Oettle (1962) and
Davies et al. (1965), show that the native Africans have extremely low
rates of both non-melanoma and melanoma skin cancer.
The incidence rates in the Johannesburg Bantus and in Uganda are
of the order of 1-2/100 000 for non-melanoma skin cancer (almost all
squamous cell carcinomas of the lower extremities) and 0.4-0.6/100 000
for malignant melanoma (almost all located on the foot) (Oettle,
1962). A more recent study in the Sudan shows a somewhat higher
incidence rate, particularly for squamous cell carcinomas (Malik et
al., 1974).
The exceptions are albino Negroes, who are extremely prone to the
development of skin cancer. In South Africa, albinism is quite common
among the Bantus. Oettle (1962) estimated a crude annual incidence
rate of 579/100 000 for male and 408/100 000 for female albino Bantus
for squamous cell carcinomas of the skin. Interestingly, the rates for
basal cell carcinoma were recorded as only 36/100 000 for both sexes
combined, as only 1 case was found.
India. Incidence data for skin cancer in India are not
available. However, it is clear that the majority of skin cancers seen
in hospitals occur in fair-skinned people. Among native indians,
special types of squamous cell carcinomas of the skin are found such
as the Kangri, Dhoti, and Chutta cancers, which are presumably due to
extreme heat, smoke, and chronic friction (Mulay, 1962).
China and Japan. While incidence figures are again not
available, it is clear that skin cancer is uncommon in the Province of
Taiwan, China, and Japan. This is also borne out by a reversal of the
usual basal cell to squamous cell carcinoma ratio. Squamous cell
carcinomas are 2-3 times more common than basal cell cancers, and may
arise at the site of premalignant skin changes such as burns, chronic
trauma, or secondary to arsenic ingestion (Miyaji, 1962; Yeh, 1962).
Australia and New Zealand. Probably the best skin cancer
surveys of recent years have been carried out in Australia,
particularly in Queensland, by Gordon & Silverstone (1976). Their
values for the incidence of skin cancer in various parts of Australia
are reported in Table 5.
Table 5. Skin cancer in Australiaa
Annual incidence per 100 000 population
Male Female
State
Age Age
Crude standardized Crude standardized
Victoria (40°S) 68.5 66.6 50.5 38.5
Queensland 265.1 265.1 174 155.8
Brisbane (28°S) 242 172
Townsville (19°S) 466 300
a From: Gordon & Silverstone (1976).
Comparable demographic data in those areas of the world that are
warm and sunny and to which people from northern Europe including the
United Kingdom have migrated are given in Table 6.
Table 6. Examples of high incidence of skin cancer. Annual incidence
per 100 000 population by sexa
Region Male Female Latitude
S.W. England 28 15 53°N
South Africa -- Cape Whites 133 72 35°--25°S
Texas (non-Latin) 168 106 28°--32°N
Queensland (Whites) 265 156 28°--10°S
a From: Gordon & Silverstone (1976).
Skin cancer tends to appear at a much earlier age in the
Queensland population than in populations living further away from the
equator.
If the distribution of annual solar UVR (Green et al., 1975) is
plotted against the incidence of skin cancer on a global basis, it can
be shown that skin cancer incidence doubles for every 10° decrease in
latitude. The Australian data would fit this concept, particularly in
Queensland, where the difference in incidence between Brisbane and
Townsville is about two to one and the difference in latitude about
9°.
Not as much work on skin cancers has been done in New Zealand as
in Australia; however good estimates of skin cancer incidence have
been reported by Eastcott (1962). These are: 113/100 000 for basal
cell carcinoma, 38/100 000 for squamous cell carcinoma; and
5.5/100 000 for malignant melanoma. These rates are considerable
lower than those for Australia, but New Zealand is much further from
the equator and thus receives less UVR.
5.5.5 Dose-response relationship for skin cancer (see also
section 4.5.2)
Although a correlation between the incidence of skin malignancy
and solar UVR levels has not yet been established with great accuracy,
it has been possible to demonstrate a correlation between latitude and
skin cancer incidence (Gordon & Silverstone (1976), Fig. 8). Some
confounding factors have been obtained from animal experiments.
In the USSR, skin cancer morbidity increases from north to south.
According to these data, skin cancer represents 15-26% of the total
cancer morbidity in the south, and 9-14° in the north (Caklin, 1974).
Africa. Information concerning skin cancer in Africa, gathered
from various sources, but mainly from the reports of Oettle (1962) and
Davies et al. (1965), show that the native Africans have extremely low
rates of both non-melanoma and melanoma skin cancer.
The incidence rates in the Johannesburg Bantus and in Uganda are
of the order of 1-2/100 000 for non-melanoma skin cancer (almost all
squamous cell carcinomas of the lower extremities) and 0.4-0.6/100 000
for malignant melanoma (almost all located on the foot) (Oettle,
1962). A more recent study in the Sudan shows a somewhat higher
incidence rate, particularly for squamous cell carcinomas (Malik et
al., 1974).
The exceptions are albino Negroes, who are extremely prone to the
development of skin cancer. In South Africa, albinism is quite common
among the Bantus. Oettle (1962) estimated a crude annual incidence
rate of 579/100 000 for male and 408/100 000 for female albino Bantus
for squamous cell carcinomas of the skin. Interestingly, the rates for
basal cell carcinoma were recorded as only 36/100 000 for both sexes
combined, as only 1 case was found.
India. Incidence data for skin cancer in India are not
available. However, it is clear that the majority of skin cancers seen
in hospitals occur in fair-skinned people. Among native indians,
special types of squamous cell carcinomas of the skin are found such
as the Kangri, Dhoti, and Chutta cancers, which are presumably due to
extreme heat, smoke, and chronic friction (Mulay, 1962).
China and Japan. While incidence figures are again not
available, it is clear that skin cancer is uncommon in the Province of
Taiwan, China, and Japan. This is also borne out by a reversal of the
usual basal cell to squamous cell carcinoma ratio. Squamous cell
carcinomas are 2-3 times more common than basal cell cancers, and may
arise at the site of premalignant skin changes such as burns, chronic
trauma, or secondary to arsenic ingestion (Miyaji, 1962; Yeh, 1962).
Australia and New Zealand. Probably the best skin cancer
surveys of recent years have been carried out in Australia,
particularly in Queensland, by Gordon & Silverstone (1976). Their
values for the incidence of skin cancer in various parts of Australia
are reported in Table 5.
Table 5. Skin cancer in Australiaa
Annual incidence per 100 000 population
Male Female
State
Age Age
Crude standardized Crude standardized
Victoria (40°S) 68.5 66.6 50.5 38.5
Queensland 265.1 265.1 174 155.8
Brisbane (28°S) 242 172
Townsville (19°S) 466 300
a From: Gordon & Silverstone (1976).
Comparable demographic data in those areas of the world that are
warm and sunny and to which people from northern Europe including the
United Kingdom have migrated are given in Table 6.
Table 6. Examples of high incidence of skin cancer. Annual incidence
per 100 000 population by sexa
Region Male Female Latitude
S.W. England 28 15 53°N
South Africa -- Cape Whites 133 72 35°--25°S
Texas (non-Latin) 168 106 28°--32°N
Queensland (Whites) 265 156 28°--10°S
a From: Gordon & Silverstone (1976).
Skin cancer tends to appear at a much earlier age in the
Queensland population than in populations living further away from the
equator.
If the distribution of annual solar UVR (Green et al., 1975) is
plotted against the incidence of skin cancer on a global basis, it can
be shown that skin cancer incidence doubles for every 10° decrease in
latitude. The Australian data would fit this concept, particularly in
Queensland, where the difference in incidence between Brisbane and
Townsville is about two to one and the difference in latitude about
9°.
Not as much work on skin cancers has been done in New Zealand as
in Australia; however good estimates of skin cancer incidence have
been reported by Eastcott (1962). These are: 113/100 000 for basal
cell carcinoma, 38/100 000 for squamous cell carcinoma; and
5.5/100 000 for malignant melanoma. These rates are considerable
lower than those for Australia, but New Zealand is much further from
the equator and thus receives less UVR.
5.5.5 Dose-response relationship for skin cancer (see also
section 4.5.2)
Although a correlation between the incidence of skin malignancy
and solar UVR levels has not yet been established with great accuracy,
it has been possible to demonstrate a correlation between latitude and
skin cancer incidence (Gordon & Silverstone (1976), Fig. 8). Some
confounding factors have been obtained from animal experiments.
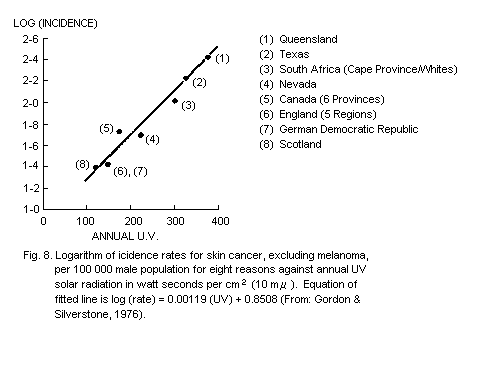 There are data that indicate that physical and chemical factors
may attenuate or intensify the carcinogenic effect of UVR
(Sviderskaja, 1971). Chronic exposure of animals to small doses of
ionizing radiation reduces resistance to the carcinogenic effects of
UVR, increasing the incidence and reducing the length of the latent
period. UV-B radiation has variable effects on the growth of
transplanted and chemically induced tumours. UV-B radiation can affect
the resistance of the body to tumour formation, increasing it with use
of sub-erythemal doses and considerably reducing it with large doses
(Dancig et al., 1975). These data, although obtained in animal systems
only, may be of great significance for human health, since the
resistance of the body to exposure to various harmful factors in the
environment operates against a certain background of natural UVR.
5.5.6 Mortality from skin cancer
In contrast to malignant melanoma, where mortality in most series
stiff exceeds 40%, the fatality rate in non-melanoma skin cancer is as
low as 1%. However, it would be incorrect to try to draw conclusions
from disease specific mortality rates.
5.5.7 Malignant melanoma
Both the incidence and the mortality rates of malignant melanoma
are rising rapidly in all countries in which they have been studied,
with mortality rates doubling in a 10-15 year period (Elwood & Lee,
1975).
The incidence varies from less than 1/100 000 in Japan, Nigeria,
and India to as high as 24/100 000 in Queensland, Australia. The rise
in incidence and mortality has been much greater in younger people
than in those over 65 years of age, implying that a causal mechanism
operates from an early age. The increase has been greater at certain
body sites and thus the site distribution has changed. The most
striking increase in incidence has been on the lower limbs in females
and the trunk in men (MacGovern, 1977).
In countries where such data are available, there is a distinct
association between latitude of residence and development of malignant
melanoma (Lancaster, 1956; Magnus, 1973; Movshovitz & Modan, 1973;
Elwood et al., 1974). The closer to the equator and the longer the
residence in countries with high insolation, the higher, in general,
is the incidence of malignant melanoma.
However, while there is usually a demonstrable latitude gradient
within a country, the latitude association in much less marked than
that for non-melanoma skin cancer. For instance, incidence rates for
malignant melanoma in Norway and Sweden are much higher than in
England and France both of which are much further south (Cutchis,
1978, Fig. 6 and 7).
Basal cell and squamous cell carcinoma, which are considered to
be due primarily to chronic UV-B exposure, are commonest on the areas
of skin exposed to sunlight, occur at a later age than malignant
melanoma, and are strikingly associated with severe solar damage to
the skin. In contrast, malignant melanoma occur more often on the
trunk of men and legs of women than non-melanoma skin cancer, and only
one type, the lentigo maligna melanoma (comprising not more than 15%
of all melanoma), is associated with histological UVR-induced skin
damage (McGovern & Mackie, 1959).
Thus, it appears that non-melanoma and melanoma skin cancers are
related to UVR exposure in different ways. Table 7 lists the
similarities and differences between these two types of tumours.
Parallel increases in melanoma and non-melanoma skin cancer cannot be
expected, as the latent period for malignant melanoma is apparently
much shorter than that for non-melanoma skin cancer. Reasons suggested
for the differences between the two types include: the greater
sensitivity of melanocytes to UVR (McGovern, 1977); the presence of
some secondarily produced circulating factor (Lee & Merrill, 1970);
and intermittent overdoses of UVR (Fears et al., 1977).
In the absence of an experimental animal model, and with the
present state of knowledge, it must be assumed that there is some
association between UVR and the development of malignant melanoma.
Thus any additional UVR exposure of susceptible individuals may
increase the risk of development of this very serious malignant skin
tumour.
5.6 Phototoxic and Photoallergic Diseases
5.6.1 Phototoxicity
Light-induced damage to the skin that does not depend on an
allergic mechanism may be considered phototoxic. Theoretically, these
reactions will occur in everyone, if the skin is exposed to enough
light energy of the proper wavelengths and if enough molecules that
will absorb these wavelengths are present. The radiation must
penetrate to the absorbing molecules for the reaction to occur.
Clinically, phototoxic reactions are usually characterized by erythema
(and at times oedema) occurring from a few minutes to several hours
after exposure, followed by hyperpigmentation and desquamation. The
sunburn reaction is the classical example of response to a phototoxic
effect (Ippen, 1969).
Table 7. Comparison of epidemiological factors in the etiology of malignant melanoma and
non-melanoma skin cancer
Factor Malignant melanoma Non-melanoma skin cancer
Latitude of residence Increases linearly within Increases geometrically with
countries, Incidence not latitude.
strictly related to latitude
globally.
Age of onset 3rd and 4th decade most 6th to 8th decade most common.
common.
Sex Moderate preponderance for Great preponderance for males.
females.
Anatomical distribution: Back, anterior torso, upper Head and neck (particularly ears
Male, white extremity, head, and neck. and lip), upper back, hand,
upper extremity.
Anatomical distribution: Back, lower leg, upper Head and neck (ears and lower
female white extremity, head, and neck. lip, spared), hands, upper
extremity, anterior chest.
Anatomical distribution: Soles, mucous membranes Anterior lower extremities, other
Black and oriental palms, nail bed. (all sites (all sites rare).
rare).
Racial (genetic) factors Celtic background, Scandinavians Celtic background, Scandinavians.
Rare in pigmented races. Rare in pigmented races.
Table 7 (contd).
Factor Malignant melanoma Non-melanoma skin cancer
Possible etiological Genetic (xeroderma pigmentosum Genetic (XP)
factors XP, B-K mole) Physical (UVR, X-ray)
Physical (UVR, trauma) Chemical (arsenic, coal tar).
Chemical (PCBsa, alpha-DOPA)
Developmental (nevi)
a PCBs = polychlorinated biphenyls.
From the clinical point of view, the erythema produced by various
phototoxic agents differs greatly in type of onset and type of
reaction; the ability of the agents to elicit pigmentation also
varies.
In contrast to the usual acute solar erythema, which begins after
a latent period of a few hours, peaks at 24 h, and subsides in a few
days to be replaced by moderate melanin pigmentation, the erythema due
to photodynamic compounds appears immediately after or during
radiation, may be associated with striking wheal formation, and
disappears in 3-6 h. Pigmentation is usually minimal.
The erythema due to furocoumarins (8-MOP) begins later than that
caused by solar radiation, peaks at 48-72 h, may persist for days, and
is followed by very intense pigmentation.
Despite a considerable amount of investigation, the mechanisms by
which phototoxic responses occur are not well understood. In the case
of the exogenous photosensitizer, either the molecule alone or a
complex of the chemical and cellular organelles becomes excited by the
absorption of light; triplet states and free radicals, or both, may be
formed.
Certain dyes and chemicals such as methylene blue, acriflavine,
rose bengal, and porphyrins produce photochemical effects on living
and non-living substrates only in the presence of oxygen. The
photo-dynamically active substance becomes excited and forms a triplet
state or a free radical. The excited chemical may also form peroxides
and then oxidize the substrate. Other possibilities include passing
the energy from the excited chemical to the biological substrate,
which then becomes oxidized, or the activated chemical may be able to
accept electrons, resulting in the oxidation of the substrate. After
excitation, the photosensitizing molecules return to the ground state
and are structurally unchanged.
Photosensitizing compounds may be endogenous, i.e., formed in the
body, usually by abnormal metabolism (e.g., porphyrins), or exogenous,
i.e., contacted externally or given as medication.
Exogenous photosensitizers may reach the skin by topical or
systemic routes and the reactions may be phototoxic or photoallergic
in nature. The action spectra for most of the phototoxic agents that
may cause skin disorders in man lie in the long-wavelength UVR range
(320-400 nm).
Contact photosensitizers include: cosmetics such as perfumes,
colognes, after-shave lotions (essential oils and psoralens),
lipsticks (fluorescein derivatives), creams, and hair preparations
(coal tar derivatives); and plants that cause phytophotodermatitis
such as Persian limes, pink rot-infested celery, many members of the
Umbelliferae and Rutaceae orders. These problems are primarily due to
psoralen compounds and therapeutic agents including phenothiazines and
sulfonamides (usually used therapeutically), halogenated
salicylanilides, sunscreens, and blankophores (usually photoallergic).
Systemic photosensitizers include: thiazide diuretics;
antibacterial sulfonamides; sulfonylurea antidiabetic drugs;
phenothiazines (especially chlorpromazine); and antibiotics
(especially dimethylchlortetracycline).
A large number of other drugs may occasionally induce
photosensitivity.
5.6.2 Photoallergy
Photoallergy can be defined as an acquired altered capacity of
the skin to react to light energy alone or in the presence of a
photosensitizer (Harber & Baer, 1969).
In photoallergic reactions, the photosensitizer leads to the
formation of the photohapten, which binds (covalently) with a suitable
carrier molecule to form the complete photoantigen. The carrier may be
a protein, polypeptide, mucopolypeptide, mucopolysaccharide, or other
macromolecule present in the skin. Once developed, photoallergy can
apparently occur with light energy alone, but presumably small
quantitites of the photoantigen are still present in the skin and
involve a circulating antibody or a cell-mediated response. In
contrast to phototoxicity, photoallergy is uncommon and is
characterized clinically by such reactions as immediate urticaria or
delayed papular or eczematous responses similar to contact dermatitis.
The hallmark of a sunlight-induced reaction, whether toxic or
allergic, is the distribution of the eruption. The exposed areas of
the face, neck, upper extremities, and, in women, the anterior surface
of the legs and the proximal, dorsal areas of the feet are mainly
involved. Exposure while driving may accentuate the eruption on the
side of the face and arm adjacent to the window. The upper eyelids,
subnasal and submental areas, flexural aspects of the wrists, and the
antecubital fossae tend to be spared. Clothing generally provides
protection, but reactions can be produced by penetration of UVR
especially through the light fabrics worn in summer.
The most common photoallergens are: 3,5-dibromosalicylanilide
(3,5 DBS); 4,5 dibromosalicylanilide (4,5 DBS); tribromosalicylanilide
(TBS); hexachlorophene; bithionol; and trichlorcarbanilide.
5.7 Pterygium and Cancer of the Eye
While detailed epidemiological evidence does not seem to exist,
there is a clinical impression among competent ophthalmologists that a
latitude gradient exists for the development of pterygium of the eye,
a benign hyperplasia of the bulbar conjunctiva which may eventually
interfere with vision by growing over the pupil (Dolezova, 1976).
Epidermoid carcinoma of the bulbar conjunctiva is a rare neoplasm
which appears with increased frequency in people living in the tropics
or subtropics (Afghanistan, Colombia, Ethiopia, Haiti, Malawi, Middle
East, Nigeria, Pakistan, Senegal, South Africa, and Uganda). It has
also been reported in cattle in the same region. Such tumours of the
eye can be induced in experimental animals with artificial UVR. Early
lesions, which are exophytic and tend to be papillary, are often
accompanied by basophilic degeneration of subepithelial collagen and
chronic inflammation.
The tumours are moderately to poorly differentiated keratinizing
epidermoid carcinomas.
In contrast to carcinoma of the skin, carcinoma of the eye is
more common in dark-skinned people, probably because of much greater
UVR exposure, and lack of pigment in the conjunctiva.
6. EVALUATION OF HEALTH RISKS TO MAN
6.1 The Significance and Extent of Different Environmental Sources of
Ultraviolet Radiation and Pathways of Exposure
The major health risks from natural UVR arise from chronic,
excessive, and unwise exposure to solar radiation. Section 2.1.1 of
this document describes in detail the wavelengths and quantities of
solar UVR that reach the earth and factors affecting it.
Briefly, depending on latitude and stratospheric ozone
concentration, the shortest wavelength measured (at noon, near the
equator) in solar radiation is about 290 nm. In most regions of earth,
the lower cut-off limit is at about 295 nm. The spectral composition
and radiation intensity of solar UVR is greatly influenced by
latitude, season, time of day (i.e., angle of the sun), cloud cover,
and the albedo of the surface. About two thirds of the skin-erythema-
producing solar UVR reaches the earth between 10h00 and 14h00.
In man, the extent of human exposure to solar UVR varies with
posture. In the upright position, esentially only portions of the
head, back of the neck, shoulders, forearms, and hands are exposed. In
addition, the skin of the thighs and upper arms may be heavily
exposed in some occupations such as driving a tractor. Exposure also
varies with time of day and local weather conditions; clothing
(wearing of hats, short- or long-sleeved shirts, shorts, etc.); work
and social habits; and ground albedo (snow, ice, and sand being the
only effective reflectors).
The maximum amount of solar UVR to which an individual could be
exposed in one day, represents about 25 minimal erythema skin doses,
i.e., about 7500 J/m2 of radiation equivalent to the skin erythema
effect of 297 nm monochromatic UVR.
Occupational exposure from artificial sources is either
inadvertent, when the sources produce UVR as a by-product, or
deliberate, when sources are designed to generate UVR to use its
properties. Depending on the characteristics of the source (section
2.1), the spectral composition of the emitted UVR can contain
wavelengths in the UV-A, UV-B, and UV-C regions.
Some industrial processes in which UV energy is a by-product are
welding, plasma torch operations, photoelectric scanning, and hot
metal operations. Because of the germicidal properties of certain
portions of the UV spectrum, artificial sources are used in hospitals,
biological laboratories, schools, and industry. Other common
applications are illumination, advertising, crime detection, chemical
synthesis and analysis, photoengraving, food, water, and air
sterilization, vitamin production, and medical diagnosis. Many other
occupations are listed in Table 8. New sources, such as UV lasers and
fluorescent panels, are being developed.
Table 8. Occupations potentially associated with UVR exposure
aircraft workers furnace workers oil field workers
barbers gardeners pipeline workers
bath attendants gas mantle workers plasma torch operators
brick layers glass blowers printers railroad track workers
burners, metal glass furnace workers ranchers
cattleman hairdressers road workers
construction workers herders seamen
cutters, metal iron workers skimmers, glass
drug makers lifeguards steel mill workers
electricians lithographers stockmen
farmers metal casting inspectors stokers
fisherman miners, open pit tobacco irradiators
food irradiators nurses vitamin D preparation makers
foundry workers welders
The amount of UVR exposure from artificial sources depends on the
spectral composition, radiant intensity, distance from source,
shielding, etc., and must de determined for individual conditions.
6.2 Types of Biological Effects and Their Significance for Human
Health
Since UVR penetrates essentially only into the skin and eyes of
man, the deleterious effects on these organs are of the greatest
importance. The acute and chronic effects of UVR are described in
detail in sections 4 and 5. Also of concern, however, are the
deleterious effects of "UVR deficiency" which can occur at latitudes
of about 60° (section 7.2).
Acute effects of UVR in the 250-320 nm wavelength range consist
of reddening, swelling, and blistering of the skin, occurring 3-24 h
after exposure, followed in 3-6 days by the production of melanin
("suntan"), in those capable of producing this pigment.
Acute effects on the eye consist of painful keratoconjunctivitis,
which recedes in 36-48 h.
After many years of repeated UVR exposure, the skin of
susceptible individuals becomes leathery, wrinkled, and discoloured
("aging changes") and skin cancer may develop (sections 5.4 and 5.5).
The degree to which these changes develop depends not only on the UVR
dose, but also, to a large extent, on the genetic background, and
particularly on the ability of the skin to pigment. For this reason,
"aging" changes and skin cancer are very much less common in
genetically heavily pigmented individuals.
The development of "aging" changes is irreversible, and presents
a major cosmetic (and thus psychological) problem, particularly for
women.
While skin cancer, with the exception of malignant melanoma, is
rarely fatal, it constitutes a social burden in terms of loss of work,
and medical expenses.
6.3 The Risk Associated with Combined Exposure with Other Agents
The interaction of UVR of various wavelengths, particularly UV-A
(320-400 nm), with natural and artificial chemical agents may result
in a variety of deleterious effect not elicited by UVR or the chemical
agents alone.
Among the most common of these effects are the phenomena of
phototoxicity, photoallergy, and chemically enhanced
photocarcinogenesis (sections 4.5 and 5.6). Fortunately, the actual
risk from such photobiological responses is small, as yet.
Of the phototoxic agents, the psoralens (furocoumarins) occur in
the rind of most citrus fruit, and in many green leafy plants. Contact
occurs most often in fruit pickers, others involved in the citrus
industry, and through the use of bergamot-containing perfumes. The
phototoxic reactions simulate sunburn. Acute skin and eye
phototoxicity are frequent, and sometimes serious, problems in workers
handling tars, such as roofers and road workers. Similar findings have
been made in creosote workers (Emmett, 1977a). Chronic phototoxicity,
and perhaps enhancement of carcinogenicity can also be induced by coal
tar products and phenanthrene carcinogen-containing materials. At risk
are roofers, road workers, and those in the tar and pitch-using
industries. The extent of augmentation of photocarcinogenesis in man
by this route is not known.
The introduction of man-made photoactive chemicals into the
environment is increasing. Serious, although small, outbreaks of
photoallergic reactions have been reported caused by soap additives
(halogenated salicylanilides), antibiotics (bithionol, griseofulvin),
drugs (chlortetracycline, thiazides, chlorpromazine), and, most
recently, compounds deliberately used in photochemical processes, such
as printing inks (Emmett, 1977b).
As yet, the risk of such reactions occurring is very small, but
with the continued introduction of new chemicals into the environment,
it is bound to increase.
6.4 The Population at Risk -- Geographical Distribution, Genetic
Influences and Occupation
As far as exposure to solar UVR is concerned, virtually the whole
of the world's population is at some risk. However, the degree of risk
varies greatly.
Of primary importance is the geographical distribution of the
population. Between latitudes 30° and 50°, the intensity and amount
(dose) of erythema-effective (and presumably carcinogenic) solar UVR
increases linearly with latitude towards the equator. This increase,
however, is modified by such conditions as cloud cover, the presence
of aerosols and "smog", the altitude above sea level (approximately a
15% increase in UVR for each 1000 metres elevation), and the degree of
obstruction of the sky by mountains, buildings, trees, etc.
Another factor of importance is genetic. Constitutional skin
pigmentation acts as a highly protective factor against the
deleterious effects of UVR on skin. Consequently, at least as far as
advanced "aging" changes and skin cancer are concerned, only subjects
with minimal or slight constitutional pigmentation are at risk. More
than two thirds of the world's population is more or less dark skinned
and has little chance of developing such UVR-induced changes. However,
pale skinned human beings living in tropical climates run a very high
risk of developing skin cancer. The highest incidence of solar skin
neoplasms exists in Queensland, Australia, where the combination of a
primarily Northern European and Celtic population and a latitude near
the equator results in a greater risk from occupational and social
outdoor exposure. In contrast, little skin cancer is found in the
native populations of tropical Africa, although latitude and exposure
are similar.
Table 9 shows the best available estimate of the number of
workers at risk from industrial exposure to various sources of UVR in
the USA. Data from other countries were not available to the Task
Group.
Table 9. Number of workers exposed to UVR (estimate from
Chicago Metropolitan Survey extrapolated to the
population of the USA)
Manufacturing
standard industrial classifications 19-39 211 000
Transportation and communication
standard industrial classifications 40-49 49 000
Wholesale, miscellaneous retail, service stations
standard industrial classifications 50, 55, 59 17 000
Services
standard industrial classifications 79-89 41 000
Total 320 000
As already pointed out, workers in many occupations are exposed
to various levels of artificial UVR.
6.5 The Reliability and Range of Known Dose-Effect and Dose-Response
Curves
6.5.1 Dose-effect curves for acute skin erythema
Erythema due to 254 nm radiation appears within 3-4 h of
exposure, reaches a peak between 8 and 12 h, and begins to subside
markedly by 24 h. Even at its peak intensity, the colour is a pale
pink-red, and it is very difficult to be certain of the minimal
erythema dose. At very low doses (of the order of less than 50 J/m2),
a weak effect can be recognized, which is clearly (because of the
shape and reasonably sharp borders) produced by the radiation.
However, it is not clear that this represents true erythema; the
colour is yellowish brown and seems to be extremely superficial,
almost on the surface of the skin. As reported by Hausser, even five
times the minimal erythema dose does not produce any severe erythema
at a wavelength of 254 nm. In contrast, with the minimal erythema dose
(MED) for wavelengths from 280-313 nm, the erythema is quite sharply
defined. The erythema produced by wavelengths 297, 303, and 313 nm is
deep red-purple and peaks in 24-28 h. It persists for 3-5 days and
imperceptibly changes into pigmentation.
6.5.2 Averages and limits, minimal, and slightly more than minimal
erythema doses
The great effect of time after irradiation and of choice of
degree of redness on the "action spectrum" of human skin is shown in
Fig. 9 and Table 10. Preliminary experiments suggest that the true
sensitivity peak lies between 290 and 294 nm. From 297 nm on, there
appears to be remarkably good agreement between most published
figures. The disagreement at shorter wavelengths is clearly because of
differences in time of evaluation, the difficulties inherent in the
delineation of "minimal erythema", and possible differences in skin
thickness.
Furthermore, if an erythema grade slightly above minimal is used
as a reference point, the resultant action spectrum closely approaches
that originally described by Hausser & Vahle (Fig. 10).
Table 10. Averages and limits (J/m2) for minimal erythema doses
(MED) read at 8 and 24 h and for a "moderate" erythema dose
read at 24 h (30 R). All radiation given at the second
exit slit, bandwidth 2.16 nm.a
nm MED 8 h MED 24 h 30 R 24 h
(J/m2) (J/m2) (J/m2)
254 63 (35-84) 100 (60-170) 194 (100-400)
280 140 (50-240) 220 (120-336) 320 (230-480)
297 140 (60-240) 140 (60-240) 140 (70-200)
303 390 (280-480) 410 (340-480) 450 (400-480)
313 6320 (4500-7700) 6800 (5400-7700) 7240
(5400-7700)
a From: Berger et al. (1967).
There are data that indicate that physical and chemical factors
may attenuate or intensify the carcinogenic effect of UVR
(Sviderskaja, 1971). Chronic exposure of animals to small doses of
ionizing radiation reduces resistance to the carcinogenic effects of
UVR, increasing the incidence and reducing the length of the latent
period. UV-B radiation has variable effects on the growth of
transplanted and chemically induced tumours. UV-B radiation can affect
the resistance of the body to tumour formation, increasing it with use
of sub-erythemal doses and considerably reducing it with large doses
(Dancig et al., 1975). These data, although obtained in animal systems
only, may be of great significance for human health, since the
resistance of the body to exposure to various harmful factors in the
environment operates against a certain background of natural UVR.
5.5.6 Mortality from skin cancer
In contrast to malignant melanoma, where mortality in most series
stiff exceeds 40%, the fatality rate in non-melanoma skin cancer is as
low as 1%. However, it would be incorrect to try to draw conclusions
from disease specific mortality rates.
5.5.7 Malignant melanoma
Both the incidence and the mortality rates of malignant melanoma
are rising rapidly in all countries in which they have been studied,
with mortality rates doubling in a 10-15 year period (Elwood & Lee,
1975).
The incidence varies from less than 1/100 000 in Japan, Nigeria,
and India to as high as 24/100 000 in Queensland, Australia. The rise
in incidence and mortality has been much greater in younger people
than in those over 65 years of age, implying that a causal mechanism
operates from an early age. The increase has been greater at certain
body sites and thus the site distribution has changed. The most
striking increase in incidence has been on the lower limbs in females
and the trunk in men (MacGovern, 1977).
In countries where such data are available, there is a distinct
association between latitude of residence and development of malignant
melanoma (Lancaster, 1956; Magnus, 1973; Movshovitz & Modan, 1973;
Elwood et al., 1974). The closer to the equator and the longer the
residence in countries with high insolation, the higher, in general,
is the incidence of malignant melanoma.
However, while there is usually a demonstrable latitude gradient
within a country, the latitude association in much less marked than
that for non-melanoma skin cancer. For instance, incidence rates for
malignant melanoma in Norway and Sweden are much higher than in
England and France both of which are much further south (Cutchis,
1978, Fig. 6 and 7).
Basal cell and squamous cell carcinoma, which are considered to
be due primarily to chronic UV-B exposure, are commonest on the areas
of skin exposed to sunlight, occur at a later age than malignant
melanoma, and are strikingly associated with severe solar damage to
the skin. In contrast, malignant melanoma occur more often on the
trunk of men and legs of women than non-melanoma skin cancer, and only
one type, the lentigo maligna melanoma (comprising not more than 15%
of all melanoma), is associated with histological UVR-induced skin
damage (McGovern & Mackie, 1959).
Thus, it appears that non-melanoma and melanoma skin cancers are
related to UVR exposure in different ways. Table 7 lists the
similarities and differences between these two types of tumours.
Parallel increases in melanoma and non-melanoma skin cancer cannot be
expected, as the latent period for malignant melanoma is apparently
much shorter than that for non-melanoma skin cancer. Reasons suggested
for the differences between the two types include: the greater
sensitivity of melanocytes to UVR (McGovern, 1977); the presence of
some secondarily produced circulating factor (Lee & Merrill, 1970);
and intermittent overdoses of UVR (Fears et al., 1977).
In the absence of an experimental animal model, and with the
present state of knowledge, it must be assumed that there is some
association between UVR and the development of malignant melanoma.
Thus any additional UVR exposure of susceptible individuals may
increase the risk of development of this very serious malignant skin
tumour.
5.6 Phototoxic and Photoallergic Diseases
5.6.1 Phototoxicity
Light-induced damage to the skin that does not depend on an
allergic mechanism may be considered phototoxic. Theoretically, these
reactions will occur in everyone, if the skin is exposed to enough
light energy of the proper wavelengths and if enough molecules that
will absorb these wavelengths are present. The radiation must
penetrate to the absorbing molecules for the reaction to occur.
Clinically, phototoxic reactions are usually characterized by erythema
(and at times oedema) occurring from a few minutes to several hours
after exposure, followed by hyperpigmentation and desquamation. The
sunburn reaction is the classical example of response to a phototoxic
effect (Ippen, 1969).
Table 7. Comparison of epidemiological factors in the etiology of malignant melanoma and
non-melanoma skin cancer
Factor Malignant melanoma Non-melanoma skin cancer
Latitude of residence Increases linearly within Increases geometrically with
countries, Incidence not latitude.
strictly related to latitude
globally.
Age of onset 3rd and 4th decade most 6th to 8th decade most common.
common.
Sex Moderate preponderance for Great preponderance for males.
females.
Anatomical distribution: Back, anterior torso, upper Head and neck (particularly ears
Male, white extremity, head, and neck. and lip), upper back, hand,
upper extremity.
Anatomical distribution: Back, lower leg, upper Head and neck (ears and lower
female white extremity, head, and neck. lip, spared), hands, upper
extremity, anterior chest.
Anatomical distribution: Soles, mucous membranes Anterior lower extremities, other
Black and oriental palms, nail bed. (all sites (all sites rare).
rare).
Racial (genetic) factors Celtic background, Scandinavians Celtic background, Scandinavians.
Rare in pigmented races. Rare in pigmented races.
Table 7 (contd).
Factor Malignant melanoma Non-melanoma skin cancer
Possible etiological Genetic (xeroderma pigmentosum Genetic (XP)
factors XP, B-K mole) Physical (UVR, X-ray)
Physical (UVR, trauma) Chemical (arsenic, coal tar).
Chemical (PCBsa, alpha-DOPA)
Developmental (nevi)
a PCBs = polychlorinated biphenyls.
From the clinical point of view, the erythema produced by various
phototoxic agents differs greatly in type of onset and type of
reaction; the ability of the agents to elicit pigmentation also
varies.
In contrast to the usual acute solar erythema, which begins after
a latent period of a few hours, peaks at 24 h, and subsides in a few
days to be replaced by moderate melanin pigmentation, the erythema due
to photodynamic compounds appears immediately after or during
radiation, may be associated with striking wheal formation, and
disappears in 3-6 h. Pigmentation is usually minimal.
The erythema due to furocoumarins (8-MOP) begins later than that
caused by solar radiation, peaks at 48-72 h, may persist for days, and
is followed by very intense pigmentation.
Despite a considerable amount of investigation, the mechanisms by
which phototoxic responses occur are not well understood. In the case
of the exogenous photosensitizer, either the molecule alone or a
complex of the chemical and cellular organelles becomes excited by the
absorption of light; triplet states and free radicals, or both, may be
formed.
Certain dyes and chemicals such as methylene blue, acriflavine,
rose bengal, and porphyrins produce photochemical effects on living
and non-living substrates only in the presence of oxygen. The
photo-dynamically active substance becomes excited and forms a triplet
state or a free radical. The excited chemical may also form peroxides
and then oxidize the substrate. Other possibilities include passing
the energy from the excited chemical to the biological substrate,
which then becomes oxidized, or the activated chemical may be able to
accept electrons, resulting in the oxidation of the substrate. After
excitation, the photosensitizing molecules return to the ground state
and are structurally unchanged.
Photosensitizing compounds may be endogenous, i.e., formed in the
body, usually by abnormal metabolism (e.g., porphyrins), or exogenous,
i.e., contacted externally or given as medication.
Exogenous photosensitizers may reach the skin by topical or
systemic routes and the reactions may be phototoxic or photoallergic
in nature. The action spectra for most of the phototoxic agents that
may cause skin disorders in man lie in the long-wavelength UVR range
(320-400 nm).
Contact photosensitizers include: cosmetics such as perfumes,
colognes, after-shave lotions (essential oils and psoralens),
lipsticks (fluorescein derivatives), creams, and hair preparations
(coal tar derivatives); and plants that cause phytophotodermatitis
such as Persian limes, pink rot-infested celery, many members of the
Umbelliferae and Rutaceae orders. These problems are primarily due to
psoralen compounds and therapeutic agents including phenothiazines and
sulfonamides (usually used therapeutically), halogenated
salicylanilides, sunscreens, and blankophores (usually photoallergic).
Systemic photosensitizers include: thiazide diuretics;
antibacterial sulfonamides; sulfonylurea antidiabetic drugs;
phenothiazines (especially chlorpromazine); and antibiotics
(especially dimethylchlortetracycline).
A large number of other drugs may occasionally induce
photosensitivity.
5.6.2 Photoallergy
Photoallergy can be defined as an acquired altered capacity of
the skin to react to light energy alone or in the presence of a
photosensitizer (Harber & Baer, 1969).
In photoallergic reactions, the photosensitizer leads to the
formation of the photohapten, which binds (covalently) with a suitable
carrier molecule to form the complete photoantigen. The carrier may be
a protein, polypeptide, mucopolypeptide, mucopolysaccharide, or other
macromolecule present in the skin. Once developed, photoallergy can
apparently occur with light energy alone, but presumably small
quantitites of the photoantigen are still present in the skin and
involve a circulating antibody or a cell-mediated response. In
contrast to phototoxicity, photoallergy is uncommon and is
characterized clinically by such reactions as immediate urticaria or
delayed papular or eczematous responses similar to contact dermatitis.
The hallmark of a sunlight-induced reaction, whether toxic or
allergic, is the distribution of the eruption. The exposed areas of
the face, neck, upper extremities, and, in women, the anterior surface
of the legs and the proximal, dorsal areas of the feet are mainly
involved. Exposure while driving may accentuate the eruption on the
side of the face and arm adjacent to the window. The upper eyelids,
subnasal and submental areas, flexural aspects of the wrists, and the
antecubital fossae tend to be spared. Clothing generally provides
protection, but reactions can be produced by penetration of UVR
especially through the light fabrics worn in summer.
The most common photoallergens are: 3,5-dibromosalicylanilide
(3,5 DBS); 4,5 dibromosalicylanilide (4,5 DBS); tribromosalicylanilide
(TBS); hexachlorophene; bithionol; and trichlorcarbanilide.
5.7 Pterygium and Cancer of the Eye
While detailed epidemiological evidence does not seem to exist,
there is a clinical impression among competent ophthalmologists that a
latitude gradient exists for the development of pterygium of the eye,
a benign hyperplasia of the bulbar conjunctiva which may eventually
interfere with vision by growing over the pupil (Dolezova, 1976).
Epidermoid carcinoma of the bulbar conjunctiva is a rare neoplasm
which appears with increased frequency in people living in the tropics
or subtropics (Afghanistan, Colombia, Ethiopia, Haiti, Malawi, Middle
East, Nigeria, Pakistan, Senegal, South Africa, and Uganda). It has
also been reported in cattle in the same region. Such tumours of the
eye can be induced in experimental animals with artificial UVR. Early
lesions, which are exophytic and tend to be papillary, are often
accompanied by basophilic degeneration of subepithelial collagen and
chronic inflammation.
The tumours are moderately to poorly differentiated keratinizing
epidermoid carcinomas.
In contrast to carcinoma of the skin, carcinoma of the eye is
more common in dark-skinned people, probably because of much greater
UVR exposure, and lack of pigment in the conjunctiva.
6. EVALUATION OF HEALTH RISKS TO MAN
6.1 The Significance and Extent of Different Environmental Sources of
Ultraviolet Radiation and Pathways of Exposure
The major health risks from natural UVR arise from chronic,
excessive, and unwise exposure to solar radiation. Section 2.1.1 of
this document describes in detail the wavelengths and quantities of
solar UVR that reach the earth and factors affecting it.
Briefly, depending on latitude and stratospheric ozone
concentration, the shortest wavelength measured (at noon, near the
equator) in solar radiation is about 290 nm. In most regions of earth,
the lower cut-off limit is at about 295 nm. The spectral composition
and radiation intensity of solar UVR is greatly influenced by
latitude, season, time of day (i.e., angle of the sun), cloud cover,
and the albedo of the surface. About two thirds of the skin-erythema-
producing solar UVR reaches the earth between 10h00 and 14h00.
In man, the extent of human exposure to solar UVR varies with
posture. In the upright position, esentially only portions of the
head, back of the neck, shoulders, forearms, and hands are exposed. In
addition, the skin of the thighs and upper arms may be heavily
exposed in some occupations such as driving a tractor. Exposure also
varies with time of day and local weather conditions; clothing
(wearing of hats, short- or long-sleeved shirts, shorts, etc.); work
and social habits; and ground albedo (snow, ice, and sand being the
only effective reflectors).
The maximum amount of solar UVR to which an individual could be
exposed in one day, represents about 25 minimal erythema skin doses,
i.e., about 7500 J/m2 of radiation equivalent to the skin erythema
effect of 297 nm monochromatic UVR.
Occupational exposure from artificial sources is either
inadvertent, when the sources produce UVR as a by-product, or
deliberate, when sources are designed to generate UVR to use its
properties. Depending on the characteristics of the source (section
2.1), the spectral composition of the emitted UVR can contain
wavelengths in the UV-A, UV-B, and UV-C regions.
Some industrial processes in which UV energy is a by-product are
welding, plasma torch operations, photoelectric scanning, and hot
metal operations. Because of the germicidal properties of certain
portions of the UV spectrum, artificial sources are used in hospitals,
biological laboratories, schools, and industry. Other common
applications are illumination, advertising, crime detection, chemical
synthesis and analysis, photoengraving, food, water, and air
sterilization, vitamin production, and medical diagnosis. Many other
occupations are listed in Table 8. New sources, such as UV lasers and
fluorescent panels, are being developed.
Table 8. Occupations potentially associated with UVR exposure
aircraft workers furnace workers oil field workers
barbers gardeners pipeline workers
bath attendants gas mantle workers plasma torch operators
brick layers glass blowers printers railroad track workers
burners, metal glass furnace workers ranchers
cattleman hairdressers road workers
construction workers herders seamen
cutters, metal iron workers skimmers, glass
drug makers lifeguards steel mill workers
electricians lithographers stockmen
farmers metal casting inspectors stokers
fisherman miners, open pit tobacco irradiators
food irradiators nurses vitamin D preparation makers
foundry workers welders
The amount of UVR exposure from artificial sources depends on the
spectral composition, radiant intensity, distance from source,
shielding, etc., and must de determined for individual conditions.
6.2 Types of Biological Effects and Their Significance for Human
Health
Since UVR penetrates essentially only into the skin and eyes of
man, the deleterious effects on these organs are of the greatest
importance. The acute and chronic effects of UVR are described in
detail in sections 4 and 5. Also of concern, however, are the
deleterious effects of "UVR deficiency" which can occur at latitudes
of about 60° (section 7.2).
Acute effects of UVR in the 250-320 nm wavelength range consist
of reddening, swelling, and blistering of the skin, occurring 3-24 h
after exposure, followed in 3-6 days by the production of melanin
("suntan"), in those capable of producing this pigment.
Acute effects on the eye consist of painful keratoconjunctivitis,
which recedes in 36-48 h.
After many years of repeated UVR exposure, the skin of
susceptible individuals becomes leathery, wrinkled, and discoloured
("aging changes") and skin cancer may develop (sections 5.4 and 5.5).
The degree to which these changes develop depends not only on the UVR
dose, but also, to a large extent, on the genetic background, and
particularly on the ability of the skin to pigment. For this reason,
"aging" changes and skin cancer are very much less common in
genetically heavily pigmented individuals.
The development of "aging" changes is irreversible, and presents
a major cosmetic (and thus psychological) problem, particularly for
women.
While skin cancer, with the exception of malignant melanoma, is
rarely fatal, it constitutes a social burden in terms of loss of work,
and medical expenses.
6.3 The Risk Associated with Combined Exposure with Other Agents
The interaction of UVR of various wavelengths, particularly UV-A
(320-400 nm), with natural and artificial chemical agents may result
in a variety of deleterious effect not elicited by UVR or the chemical
agents alone.
Among the most common of these effects are the phenomena of
phototoxicity, photoallergy, and chemically enhanced
photocarcinogenesis (sections 4.5 and 5.6). Fortunately, the actual
risk from such photobiological responses is small, as yet.
Of the phototoxic agents, the psoralens (furocoumarins) occur in
the rind of most citrus fruit, and in many green leafy plants. Contact
occurs most often in fruit pickers, others involved in the citrus
industry, and through the use of bergamot-containing perfumes. The
phototoxic reactions simulate sunburn. Acute skin and eye
phototoxicity are frequent, and sometimes serious, problems in workers
handling tars, such as roofers and road workers. Similar findings have
been made in creosote workers (Emmett, 1977a). Chronic phototoxicity,
and perhaps enhancement of carcinogenicity can also be induced by coal
tar products and phenanthrene carcinogen-containing materials. At risk
are roofers, road workers, and those in the tar and pitch-using
industries. The extent of augmentation of photocarcinogenesis in man
by this route is not known.
The introduction of man-made photoactive chemicals into the
environment is increasing. Serious, although small, outbreaks of
photoallergic reactions have been reported caused by soap additives
(halogenated salicylanilides), antibiotics (bithionol, griseofulvin),
drugs (chlortetracycline, thiazides, chlorpromazine), and, most
recently, compounds deliberately used in photochemical processes, such
as printing inks (Emmett, 1977b).
As yet, the risk of such reactions occurring is very small, but
with the continued introduction of new chemicals into the environment,
it is bound to increase.
6.4 The Population at Risk -- Geographical Distribution, Genetic
Influences and Occupation
As far as exposure to solar UVR is concerned, virtually the whole
of the world's population is at some risk. However, the degree of risk
varies greatly.
Of primary importance is the geographical distribution of the
population. Between latitudes 30° and 50°, the intensity and amount
(dose) of erythema-effective (and presumably carcinogenic) solar UVR
increases linearly with latitude towards the equator. This increase,
however, is modified by such conditions as cloud cover, the presence
of aerosols and "smog", the altitude above sea level (approximately a
15% increase in UVR for each 1000 metres elevation), and the degree of
obstruction of the sky by mountains, buildings, trees, etc.
Another factor of importance is genetic. Constitutional skin
pigmentation acts as a highly protective factor against the
deleterious effects of UVR on skin. Consequently, at least as far as
advanced "aging" changes and skin cancer are concerned, only subjects
with minimal or slight constitutional pigmentation are at risk. More
than two thirds of the world's population is more or less dark skinned
and has little chance of developing such UVR-induced changes. However,
pale skinned human beings living in tropical climates run a very high
risk of developing skin cancer. The highest incidence of solar skin
neoplasms exists in Queensland, Australia, where the combination of a
primarily Northern European and Celtic population and a latitude near
the equator results in a greater risk from occupational and social
outdoor exposure. In contrast, little skin cancer is found in the
native populations of tropical Africa, although latitude and exposure
are similar.
Table 9 shows the best available estimate of the number of
workers at risk from industrial exposure to various sources of UVR in
the USA. Data from other countries were not available to the Task
Group.
Table 9. Number of workers exposed to UVR (estimate from
Chicago Metropolitan Survey extrapolated to the
population of the USA)
Manufacturing
standard industrial classifications 19-39 211 000
Transportation and communication
standard industrial classifications 40-49 49 000
Wholesale, miscellaneous retail, service stations
standard industrial classifications 50, 55, 59 17 000
Services
standard industrial classifications 79-89 41 000
Total 320 000
As already pointed out, workers in many occupations are exposed
to various levels of artificial UVR.
6.5 The Reliability and Range of Known Dose-Effect and Dose-Response
Curves
6.5.1 Dose-effect curves for acute skin erythema
Erythema due to 254 nm radiation appears within 3-4 h of
exposure, reaches a peak between 8 and 12 h, and begins to subside
markedly by 24 h. Even at its peak intensity, the colour is a pale
pink-red, and it is very difficult to be certain of the minimal
erythema dose. At very low doses (of the order of less than 50 J/m2),
a weak effect can be recognized, which is clearly (because of the
shape and reasonably sharp borders) produced by the radiation.
However, it is not clear that this represents true erythema; the
colour is yellowish brown and seems to be extremely superficial,
almost on the surface of the skin. As reported by Hausser, even five
times the minimal erythema dose does not produce any severe erythema
at a wavelength of 254 nm. In contrast, with the minimal erythema dose
(MED) for wavelengths from 280-313 nm, the erythema is quite sharply
defined. The erythema produced by wavelengths 297, 303, and 313 nm is
deep red-purple and peaks in 24-28 h. It persists for 3-5 days and
imperceptibly changes into pigmentation.
6.5.2 Averages and limits, minimal, and slightly more than minimal
erythema doses
The great effect of time after irradiation and of choice of
degree of redness on the "action spectrum" of human skin is shown in
Fig. 9 and Table 10. Preliminary experiments suggest that the true
sensitivity peak lies between 290 and 294 nm. From 297 nm on, there
appears to be remarkably good agreement between most published
figures. The disagreement at shorter wavelengths is clearly because of
differences in time of evaluation, the difficulties inherent in the
delineation of "minimal erythema", and possible differences in skin
thickness.
Furthermore, if an erythema grade slightly above minimal is used
as a reference point, the resultant action spectrum closely approaches
that originally described by Hausser & Vahle (Fig. 10).
Table 10. Averages and limits (J/m2) for minimal erythema doses
(MED) read at 8 and 24 h and for a "moderate" erythema dose
read at 24 h (30 R). All radiation given at the second
exit slit, bandwidth 2.16 nm.a
nm MED 8 h MED 24 h 30 R 24 h
(J/m2) (J/m2) (J/m2)
254 63 (35-84) 100 (60-170) 194 (100-400)
280 140 (50-240) 220 (120-336) 320 (230-480)
297 140 (60-240) 140 (60-240) 140 (70-200)
303 390 (280-480) 410 (340-480) 450 (400-480)
313 6320 (4500-7700) 6800 (5400-7700) 7240
(5400-7700)
a From: Berger et al. (1967).
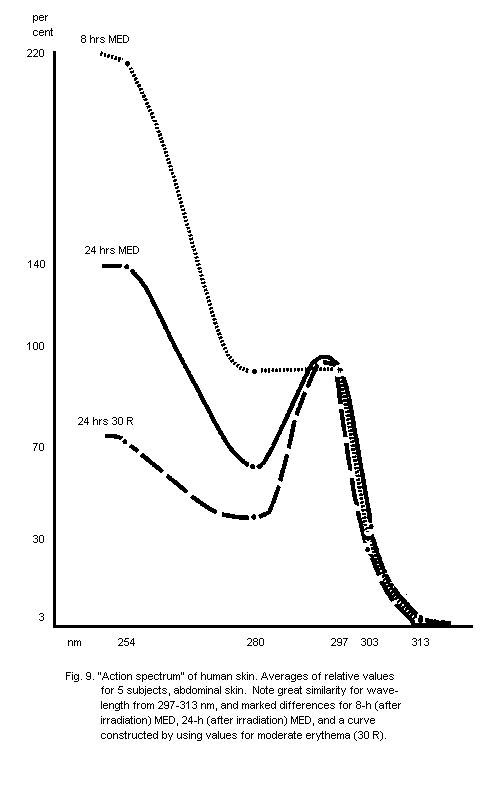
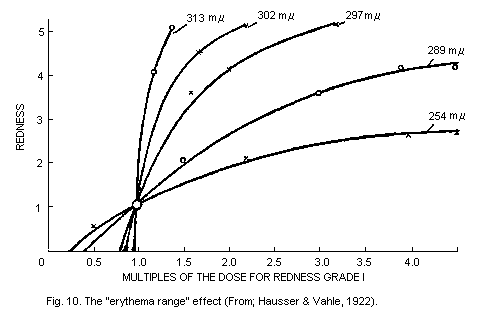 6.5.3 The "erythema range" effect
One of the important observations of Hausser & Vahle (1922) was
that there appeared to be a significant difference between the doses
needed to produce slight and maximal erytherna at different
wavelengths. This concept is of great potential significance for the
prevention of sunburn and the understanding of diseases due to light.
In Fig. 10, data are plotted from an experiment designed by Hausser to
test this effect, which show that 5 times the minimal erytherna dose
produces much less than the maximal erytherna at 254 nm, while 2.5
times the minimal erythema dose produces maximal erytherna at 303 nm.
While it takes much more energy to produce any erytherna at 303 nm
than at 254 nm, not much more than the threshold dose produces a
significant burn.
These observations are of great importance because they show that
the acute biological effects of several wavelengths in the UV-B
proceed at different time scales, and have very different
dose-response relationships. Thus, the usual assumptions that it is
realistic to weight the effectiveness of different wavelengths in a
continuous UVR source (such as the sun) by an action spectrum obtained
for a threshold effect with a monochromator is not correct, nor is it
appropriate to consider the effect of these wavelengths to be
biologically equivalent.
Unfortunately, in the absence of knowledge of chromophores or
biological mechanisms, there is no better way of comparing the
effectiveness of sources of different UVR composition than the present
method of calculating the skin erytherna effectiveness of UV-B.
As in all photobiological cutaneous effects, pigmentation plays a
major role in the sensitivity of the skin to UVR. Heavily pigmented
people are 10-20 times less sensitive than untanned fair-skinned
people. As far as is known, the action spectra for erythema are
similar for all races, differing only in the amount of energy
necessary to produce threshold effects.
6.5.4 Dose-response curves for keratoconjunctivitis
These are essentially similar to those for erythema, except that
the peak of the action spectrum is located at 270 nm. This is most
likely because of the absence of a filtering stratum corneum and
because the conjunctiva is not pigmented. The peak of 270 nm is
therefore used for the normalization of the dose limits of broad band
sources.
6.5.5 Dose-response relationship for photocarcinogenesis
Most of the existing evidence is consistent with the concepts
that UV-induced photodamage to skin is the main causal factor in the
development of skin cancer, that the development of skin cancer is a
stochastic effect, and that there is no threshold (sections 4.3 and
5.5).
Thus, a relationship should exist between skin cancer incidence
and accumulated dose, using a sensitivity function. In mice, the
quantitative relationship between UVR dose and the production of skin
cancer has been thoroughly explored; tumour incidence in this
experimental model is proportional to the square root of the number of
doses, the dose size, and the interval between doses.
In man, there is ample evidence that a latitude gradient exists
for the incidence of skin cancer in sensitive, fair-skinned
populations, and that this gradient is non-linear. There is obviously
a relationship between latitude and the intensity of solar radiation,
and this gradient is exaggerated in the UVR portion of the spectrum.
Although the shape of this relationship is relatively complex and
depends on a number of variables, for a range of mid-latitudes
(30°-50°), both the theoretical form and that obtained from actual
field measurements closely approximate a straight line. It is
generally accepted that latitude-related climatic and environmental
conditions and behavioural effects must modify the UVR dose actually
reaching a population. The factors involved, and their magnitude are
largely speculative.
A series of mathematical models relating human skin cancer
incidence to solar UVR has been proposed at various times in the past
few years, because of concern about alteration of the stratospheric
ozone layer (Green et al., 1978; Rundel & Nachtwey, 1978). While the
uncertainties are very great, the best accepted model data suggest
that a 5% increase in erythema-effective solar UVR may result in a 15%
(range 7.5-25%) increase in skin cancer in a susceptible population
after about 60 years, when a steady state has been reached. As far as
the relative risk is concerned in susceptible white-skinned
populations, people in older age groups, with fair skin that sunburns
easily and with a high life-time solar exposure, run 10-20 times more
risk of developing skin cancer than their contemporaries, who tan
easily and have a low life-time solar exposure (Vitaliano, 1978).
Incidences of nonmelanoma skin cancer as high as 350/100 000
population per year have been observed in elderly, white-skinned males
in Queensland, Australia, and Texas, USA. Populations with
constitutionally, heavily pigmented skin run only a minimum risk of
developing skin cancer in their lifetime.
7. GUIDELINES FOR HEALTH PROTECTION
The development of criteria for both upper and lower limits of
exposure to either natural or artificial UVR is extremely difficult
because of such problems as:
(a) the variation in both the acute and chronic effects of UVR
of different wave lengths;
(b) the considerable differences in the spectral composition of
light from different sources and particularly of sunlight at different
latitudes;
(c) the great differences in cutaneous sensitivity to UVR due
to genetic, environmental, and adaptive effects, and the considerable
variation in sensitivity in the same person at different times; and
(d) the difficulty of differentiating between the necessary
dose of UVR compatible with the upkeep of life, and the lowest dose
that results in serious detrimental effects.
7.1 Range of Exposure Limits
7.1.1 Exposure to solar ultraviolet radiation
As described in section 6, two thirds of the daily amount of
solar UV-B radiation reaches the earth between 10h00 and 14h00. Thus,
exposure should be reduced to a miminum during these hours. After a
period of acclimatization, most people can tolerate several hours of
outdoor exposure in the morning and afternoon, but shielding in the
near noon hours should always be considered. In general, exposure
during this very important period of acclimatization should not exceed
4 minimal erythema doses per day without some protection (either
clothing or sunscreens, section 7.3). This would correspond to 1 h of
exposure in the tropics. A dose of 1/8 MED per day appears to be
sufficient to prevent UVR deficiency.
7.1.2 Occupational exposure to artificial ultraviolet radiation
No internationally agreed limits exist at this time for
occupational exposure to UVR, which take into consideration the acute
effects and the risk of late cancer development. So far, the only
limits available, which could be used in the preparation of
guidelines, have been proposed by the National Institute of
Occupational Safety and Health in the USA (NIOSH, 1975).
The limits are based on the minimal erythema dose (MED) and the
minimal photokeratitic dose, which means that only acute effects have
been taken into consideration. For the UVR 200-315 nm region, it is
stated that radiant exposure in any 8-h period must not exceed the
values given in Table 11. For the wavelength range 315-400 nm, the
total irradiance on unprotected skin or eye must not exceed 10 W/m2
for periods exceeding 103 seconds. For radiant exposure of shorter
durations, it should not exceed 10 000 J/m2. The limit for 315-400 nm
is probably much too low and could be revised to a higher level.
Table 11. Total permissible 8-h doses and relative spectral
effectiveness of some selected monochromatic
wavelengthsa
Wavelength Permissible 8-h Relative spectral
(nm) dose (J/m2) effectiveness
(S lambda)b
200 1000 0.03
210 400 0.075
220 250 0.12
230 160 0.19
240 100 0.30
250 70 0.43
254 60 0.50
280 46 0.65
270 30 1.00
280 34 0.88
290 47 0.84
300 100 0.30
305 500 0.06
310 2000 0.015
315 10 000 0.003
a "From: NIOSH (1975).
Table 12. Maximum permissible exposure times for selected
values of Ieffa
Duration of exposure Effective irradiance,
per day Ieff (W/m2)b
8 h 1.0
4 h 2.0
2 h 4.0
1 h 8.0
30 min 17.0
15 min 33.0
10 min 50.0
5 min 100.0
1 min 500.0
30 sec 1000.0
a From: NIOSH (1975).
b Effective irradiance = action spectrum weighted irradiance.
However, these values only apply to sources emitting essentially
monochromatic UVR. The maximum permissible exposure for a broad band
source should be calculated by summing up the relative contributions
from all its spectral components, each being weighted by the relative
spectral effect SL, as given in Table 12. In addition, these
guidelines for determining exposure limits should not be used for
photosensitive individuals.
So far, none of the guidelines has included an evaluation of the
carcinogenic risk, as neither the action spectrum nor the
dose-response curve is known for man. It is hoped that this will be
obtained by comparison of cancer incidence and UV irradiance as
measured by the Robertson-Berger method.
7.1.3 Exposure of the general population to artificial ultraviolet
radiation
The exposure of the general population to artificial UVR is
primarily for hygienic and for cosmetic purposes. The use of UVR for
health purposes is discussed in sections 7.2 and 7.3.
The use of UVR for cosmetic purposes generally involves a
requirement for the development of skin pigmentation. For this reason,
it is unrealistic to follow the occupational standards, since they
would not allow the desired effects to be obtained.
Doses of UVR sufficient to produce slight erythema in 24 hours
are usually adequate for cosmetic purposes. Significant overdoses and
repeated UVR exposures for prolonged periods should be avoided. The
eyes must be properly shielded during each exposure. The risk of the
development of skin cancer due to chronic exposure to UVR for cosmetic
purposes is not known.
A number of devices available to the general population emit
significant amounts of UVR, or will do so if the protective envelope
of the device is damaged, and may cause acute UVR-induced eye and skin
damage. Such devices include Wood's lights, sterilizing and
ozone-producing lamps, and high power mercury and xenon arc lamps used
for illumination.
7.1.4 Measurement of natural and artificial ultraviolet radiation
Measurement of solar UVR involves serious difficulties, because
of the need for accurate spectral discrimination at the shortest end
of the solar spectrum. This is necessary because of the considerable
variations in the biological effectiveness of UVR shorter than 320 nm.
Few really practical, accurate, stray light-free spectroradiometers
have been developed so far, for use in the middle UVR region (UV-B)
and the use of action spectrum weighted, integrating analogue UVR
meters has been found to be much more practical.
The few practical prototypes of integrating chemical UVR
dosimeters that have been developed are still in the experimental
stage. If they can be perfected, such personal UVR dosimeters would be
of the greatest use (Challoner et al., 1976; Davis et al., 1976).
Basically, the same problems pertain to monitoring of artificial
UVR, although measurements are somewhat easier in a laboratory
setting.
The exposure criteria recommended in section 7.1.2 have not been
put to practical use, because it is still not technologically possible
to measure UVR adequately for compliance.
Thus, working practices are recommended for the control of
exposure in situations where sufficient measurement or emission data
are not available. A frequently practiced method of protection
consists of adequate containement of UVR-producing light sources.
7.2 Health Effects of Solar Ultraviolet Radiation in the General
Population
Two aspects of health protection in the general population are of
interest. One is the prevention of UVR deficiency that can occur in
populations living near the north and south poles (generally at
latitudes of 60° or more). The other deals with the protection of
pale-skinned people from excessive UVR in subtropical and tropical
areas (generally between latitudes 35° N and 35° S).
The zoning of the USSR for UVR is particularly interesting from a
health point of view (Belinskij, 1971). In the "UVR-deficit zone" it
appears essential to use irradiation from artificial sources, during
certain months, to compensate for UVR deficiency. In the "UV comfort
zone", artificial irradiation is unnecessary. In the "UVR excess
zone", it is essential to undertake measures for protection against
solar UVR, in order to avoid skin cancer. It has also been proposed
that, in order to prevent skin tumours among town dwellers in whom
light starvation has caused depigmentation of the skin, preventive UVR
should be carried out to increase skin pigmentation.
7.3 UVR Deficiency and its Prevention
7.3.1 Insolation and UV irradiation of built-up areas
It is well known that solar energy entering a room illuminates,
warms, dries, and particularly important, disinfects it, thus having a
beneficial, physical and psychological effect. The sanitation
standards and regulations for the insolation of dwellings and public
buildings and built-up areas, which are in force at present in the
USSR, are based on the requirement that premises should receive three
hours' uninterrupted insolation on cloudless days during the period 22
March-22 September and that protection should be given to limit the
thermal effect of solar radiation in the southern regions, below
latitude 55°N (State Standards, 1976b).
This health requirement is evident from a number of reviews
(Dancig, 1971; Galanin & Pirkin, 1971; Aleksandrov et al., 1975).
Solar UVR has a particularly valuable health-effect in that, inter
alia, it speeds up the processes of environmental selfpurification
(Belikova, 1960; Gluscenko et al., 1975). Therefore, town planning
tasks should include the rational use of UVR from the sun and sky
(Davidson, 1970; Gusev & Dunaev, 1971).
Since window glass cuts off the most biologically active
component of natural UVR, research to increase the UVR transparency of
window glass has been of particular concern to health workers in the
most northern latitudes (Belikova, 1964).
7.3.2 Sunbathing and air-bathing in the prevention of UVR deficiency
In addition to measures ensuring the maximum possible penetration
of natural UVR into working and dwelling places, prevention of UVR
deficiency can also be ensured by organizing solaria, beaches, and
sports areas attached to children's establishments (kindergartens and
pioneer camps), and to factories and mills. A schedule for the UV
irradiation of children and adults, for sun and sun-and-air baths at
different geographical latitudes, at different seasons of the year,
and at different times of the day has been developed (Generalov,
1971). At present, the effects of such solar radiation schedules
cannot be extrapolated to potential late effects.
7.3.3 Artificial ultraviolet radiation in the prevention of UVR
deficiency
Effective principles for the use of artificial UVR in the
prevention of UVR deficiency and "light starvation" in man have been
developed in the USSR. The recommended levels consist of a daily dose
of UV-B of between 0.125 and 0.75 of the threshold erytherna dose
(State Standards, 1964). The use of prophylactic UV irradiation has
been shown to be quite effective in workers in industries lacking
natural light (Bocenkova, 1971) and particularly effective in children
of preschool and school age (Ronge, 1948; Zilov, 1971).
Regulations have been drawn up for the planning and operation of
artificial UV irradiation devices in industrial enterprises (State
Standards, 1976b).
7.4 Protection against Ultraviolet Radiation
The whole of the world population has a potential for developing
skin cancer, the risk depending on the intensity and degree of
exposure to solar UVR during the life span. If all current exposure to
solar UVR could be significantly reduced, the incidence of skin cancer
would eventually decrease greatly.
Health and appearance can be adequately catered for by exposing
only part of the body for less than half an hour per day at or near
noon, except at latitudes north of 60° N.
7.4.1 Sunscreen preparations
Sunscreen preparations are usually classified as chemical or
physical agents. The former include para-aminobenzoic acid and its
esters, cinnamates, and benzophenones, all of which act by absorbing
radiation, which is dissipated as radiation of lower energy. Physical
agents act as simple physical barriers, reflecting, blocking, or
scattering light. They include titanium dioxide, talc, and zinc oxide.
Mainly because of cosmetic objections, the physical barriers are not
often used in sunscreen formulations (Robertson, 1972).
The principle of covering the skin by spreading a layer of
reliable UV absorber on the surface has proved a popular one.
Providing that the thickness of the layer applied is adequate and that
it adheres to the skin, it gives protection to the wearer under all
conditions.
Details of the kind of chemicals and bases used are beyond the
scope of this report.
7.4.2 Clothing
Covering the skin with clothing gives a sense of security that is
often misleading. The most frequent sites for skin cancer are those
that face upward. Thus, a hat is essential for adequate protection of
the forehead, scalp, and tops of ears. However, generally, it gives
only partial protection for the nose, less for the lower face, and
none for the hands and arms.
Body coverings worn in hot climates are generally not complete
absorbers. The average white shirt worn by men may transmit 20% of
UVR, while lighter weaves favoured by women may allow 50% of UVR to
reach the shoulders. Fairly complete clothing cover is more tolerable
in a dry atmosphere than a humid, coastal, tropical environment.
7.4.3 Behavioural conformity with the environment
When unexpectedly detained in the sun, protective procedures
available include facing in a variety of directions, hanging the head
and shielding the head with the hands or with a handkerchief. Shadows
should be used whenever possible, including one's own shadow. When
standing in the shadow of a building, only rays from about half the
sky are received, that is, about one quarter of the harmful intensity
of full daylight. This is certainly useful protection, but fair skin
will still burn in about one hour; in three hours, a painful burn may
be initiated, although the sufferer has not been in direct sunlight at
all.
The dominant factor in the daily erythemal exposure is the angle
of the sun above the horizon. This varies with latitude and with time
of year. Thus, maximum exposure occurs, when the sun is almost
directly overhead. The simplest means of protection is to take shelter
around the middle of the day, especially in the summer months.
At least one third of the whole day's UVR exposure occurs in the
hour before and the hour after noon; half the day's exposure, enough
for a very disabling sunburn, occurs during the 3-hour period around
noon. If shelter is taken between 10h00 and 14h00, only one third of
the day's exposure remains. Outside the period from 9h00 to 15h00,
only one sixth remains, permitting considerable outdoor activity even
for subjects with the most sensitive skin types.
7.4.4 Occupational protection
With the exception of medically prescribed doses, exposure of
both the eyes and skin to UVR should be kept to a minimum. In order to
protect persons in the vicinity of artificial UVR, the following
precautions are recommended:
(a) Whenever possible, prevention of excessive exposure of the
eyes and skin should be ensured by proper engineering design of
UVR-emitting installations and suitable enclosures, so that any UVR is
either adequately contained or sufficiently attenuated.
(b) When, for justifiable reasons, such containment is not
possible, protection should be afforded by providing close-fitting
goggles and/or face shields accompanied, if necessary, by suitable
UVR-opaque clothing and gloves to cover the skin.
(c) Adequate and appropriate instruction should be given, to
any person liable to be excessively exposed to UVR, concerning the
hazards involved and the precautions to be observed to avoid excessive
exposure.
(d) For artificial UVR sources that do not emit significant
visible light, a visible or audible warning signal may be required to
show when the UVR is being emitted.
(e) Powerful short wavelength UVR sources may generate ozone.
This additional hazard should be avoided by providing either adequate
ventilation or an adequate ozone removal system in the workplace.
REFERENCES
AITKEN, R. (1937) Ultraviolet radiation treatment of preeclamptic
toxemia. Br. J. Phys. Med., 12: 45-47.
ALEKSANDROV, V. N. & ALEKSANDROVA, A. I. (1975) [The insolation and UV
climate of housing in the far north.] Gig. i Sanit. 5: 16 (in
Russian).
ANCHEV, N., POPOV, I., MONOV N., & OUZOUNOV, N. (1968) Spreading of
malignant neoplasms in the People's Republic of Bulgaria.
Neoplasma, 15: 451-468.
ANDREEV, M. G., VASIL'EV B. D., ZAHAR'EVSKIJ, A. V., & HOROKORIN, T.
(1975) [Artificial sources of UV radiation with biological
effect.] In: [The biological effect of UV radiation.] Moscow,
Nauka, pp. 221-225 (in Russian).
BACHEM, A. (1956) Ophthalmic ultraviolet action spectra. Am. J.
Ophthalmol., 41: 969-975.
BAIN, J. & RUSCH, H. P. (1943) Carcinogenesis with ultraviolet
radiation of wave length 2, 800-3, 400 A. Cancer Res.,
3: 425-430.
BEARD, H. H., BOGGESS, T. S., & VON HAAM, E. (1936) Experimental
production of malignant tumors in the albino rat by means of
ultraviolet rays. Am. J. Cancer, 27: 257-266.
BELIKOVA, V. K. (1964) [Evaluation from the hygienic standpoint of
experimental samples of a window glass for residential and public
buildings.] Gig. i. Sanit., 1: 21-28 (in Russian).
BELIKOVA, V. K. (1966) [Natural ultraviolet radiation and its
bactericidal importance.] Moscow, Medicina, pp. 322-326 (in
Russian).
BELIKOVA, V. K., ZABALUEVA, A. P., & GALPERIN, E. L. (1975)
[Evaluation from the standpoint of hygiene of the zoning of the
territory of the U.S.S.R. in respect to UV radiation levels.] In:
[The biological effects of UV radiation.] Moscow, Nauka, pp.
161-165 (in Russian).
BELINSKIJ, V. A. (1971) [Zoning of the territory of the USSR in
respect to the natural UV radiation.] In: [Meteorology and
climatology.] Issue 2, Moscow, Publishing House of the Moscow
Branch of the Geographical Society, (in Russian).
BELINSKIJ, B. A. & ANDRIENKO, L. M. (1974) [A simplified radiation
model of the atmosphere in the UV spectral region.] In:
[Radiation, processes in the atmosphere and on the earth's
surface.] Leningrad, Gidrometeoizdat, pp. 273-276, (in
Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1963) [Ultraviolet climate of the
USSR.] In [Proceedings of the All-Union Scientific
Meteorological Meeting.] Leningrad, Gidrometeozidat,
pp. 244-252 (in Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1962) [Scattered UV-radiation in the
Eastern Pamirs.] In: [Scientific Communications of the Institute
of Geology and Geography of the Academy of Sciences of the
Lithuanian SSR.] Vilanus, Vil. XIII, pp. 283-287 (in Russian).
BELINSKIJ, V. A., GARADZA, M.P., MEZENNAJA, L. M., & NEZVAL, E. I.
(1968) [Sun and sky ultraviolet radiation.] Moscow, Moscow
University Press (in Russian).
BENDER, M. A., GRIGGS, H. G., & WALKER, P. L. (1973) Mechanisms of
chromosomal aberration production. I. Aberration induction by
ultraviolet light. Mutat. Res., 20: 387-402.
BENER, P. (1972) Approximate values of intensity of natural
ultraviolet radiation for different amounts of atmospheric
ozone. London, European Research Office, United States Army.
(Contract DAJA 36-68-C-1017).
BEN-HUR, E. & ELKIND, M. M. (1973) DNA cross-linking in Chinese
hamster cells exposed to near ultraviolet light in the presence
of 4,5',8-trimethylpsoralen. Biochim. Biophys. Acta,
331: 181-193.
BERENBLUM, I. & SHUBIK, P. (1947) The role of croton oil applications,
associated with a single painting of a carcinogen, in tumor
induction of the mouse's skin. Br. J. Cancer, 1: 379-382.
BERENBLUM, I. & SHUBIK, P. (1949) An experimental study of the
initiating stage of carcinogenesis, and a re-examination of the
somatic cell mutation theory of cancer. Br. J. Cancer,
3: 109-118.
BERGER, D., URBACH, F., & DAVIES, R. E. (1967) The action spectrum of
erythema induced by ultraviolet radiation. Proceedings of the
13th International Congress of Dermatology, Berlin, Springer
Verlag, pp. 1112-1117.
BERGER, D., ROBERTSON, D. F. & DAVIES R. E. (1975) Field measurements
of biologically effective UV radiation. In: Impacts of climatic
change on the biosphere, Springfield, VA, National Technical
Information Service, Part I, Chapter 2, pp. 233-264 (Appendix D,
CIAP Monograph No. 5).
BEUKERS R. & BERENDS, W. (1960) Isolation and identification of the
irradiation product of thymine. Biochim. Biophys. Acta,
41: 550-551.
BILLEN, D. & GREEN, A. E. S. (1975) Comparison of germicidal activity
of sunlight with the response of a sunburning ultraviolet meter.
In: Impacts of climatic change on the biosphere, Springfield,
VA, National Technical Information Service, Part 1, Chapter 2,
pp. 299-305 (Appendix F, CIAP Monograph No. 5).
BISCHOFF, F. (1969) Carcinogenic effects of steroids. In: Paoletti, &
Kritchevsky, ed. Advances in lipid research. New York, Academic
Press, Vol. 7, pp. 165-244.
BLACK, H. S. & CHAN, J. T. (1976) Etiologic related studies of
ultraviolet light-mediated carcinogenesis. Oncology,
33: 119-122.
BLACK, H. S. & DOUGLAS, D. R. (1973) Formation of a carcinogen of
natural origin in the etiology of UV-carcinogenesis. Cancer
Res., 33: 2094-2096.
BLACK, H. S. & LO, W. B. (1971) Formation of a carcinogen in human
skin irradiated with ultraviolet light. Nature (Lond.),
234: 306-308.
BLACK, H. S., CHAN, J. T., & BROWN, G. E. (1978) Effects of dietary
constituents on ultraviolet light-mediated carcinogenesis.
Cancer Res., 38: 1384-1387.
BLUM, H. F. (1941) Sunlight and cancer of the skin. J. Natl Cancer
Inst., 1: 397-431.
BLUM, H. F. (1943) Wavelength dependence of tumor induction by
ultraviolet radiation. J. Natl Cancer Inst., 3: 533-537.
BLUM, H. F. (1969) Quantitative aspects of cancer induction by
ultraviolet light: Including a revised model. In: Urbach, F., ed.
The biological effects of ultraviolet radiation, Oxford,
Pergamon Press.
BLUM, H. F. & LIPPINCOTT, S. W. (1943) Carcinogenic effectiveness of
ultraviolet radiation of wavelength 2537A. J. Natl Cancer Inst.,
3: 211-216.
BLUM, H. F., GRADY, H. G., & KIRBY-SMITH, J. S. (1943) Relationships
between dosage and rate of tumor induction by ultraviolet
radiation. J. Natl Cancer Inst., 3: 91-97.
BLUM, H. F., BUTLER, E. G., DAILEY, T. H. DAUBE, J. R., MAWE, R. C., &
SOFFEN, G. W. (1959) Irradiation of mouse skin with single doses
of ultraviolet light. J. Natl Cancer Inst., 22: 979-993.
BOCENKOVA, T. D. (1971) [The application of prophylactic
UV-irradiation to workers in buildings of a new type.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 245-251 (in
Russian).
BOGUCKIJ, B. V., KAPELEVA, A. I., BOKSA, V. G., LESINSKAJ, N. P.,
CIBIREVA, E. M, KOSTIN, N. F., & EMELKIN, V. I. (1975)
[Continuous and pulsed ultraviolet irradiation in combined
treatment of protracted and chronic pneumonia.] In:
[The biological effect of UV-radiation.] Moscow, Nauka,
pp. 126-127 (in Russian).
BOVIE, W. T. & KLEIN, A. (1919) Sensitization to heat due to exposure
to light of short wavelengths. J. Gen. Physiol., 1: 331-336.
BOYCE, R. P. & HOWARD-FLANDERS, P. (1964) Release of ultraviolet
light-induced thymine dimers from DNA in E. coli K-12. Proc.
Natl Acad. Sci., 51: 293-300.
BRIDGES, B. A. & HUCKLE, J. (1970) Mutagenesis of cultured mammalian
cells by X-radiation and ultraviolet light. Mutat. Res.
10: 141-151.
BUCHANAN, A. R., HELM, H. C., & STILSON, D. W. (1960) Biomedical
effects of exposure to electromagnetic radiation.
I. Ultraviolet. Dayton, OH, Wright-Patterson Air Force Base,
pp. 60-376 (WADD Technical Report).
BUSCHKE, W., FRIEDENWALD, J. S., & MOSES, S. G. (1945) Effects of
ultraviolet irradiation on corneal epithelium: Mitosis, nuclear
fragmentation, post-traumatic cell movements, loss of tissue
cohesion. J. Cell. Comp. Physiol., 26: 147-164.
CAKLIN, A. V. (1974) [The epidemiology of malignant neoplasms.] In:
[Handbook on oncology.] Moscow, Medicina, pp. 26-27 (in
Russian).
CARLSON, L. D. & JACKSON, B. H. (1959) Combined effects of ionizing
radiation and high temperature on longevity of Sprague-Dawley
rat. Radiat. Res., 11: 509-519.
CASTO, B.C. (1973) Enhancement of adenovirus transformation by
treatment of hamster cells with ultraviolet irradiation, DNA base
analogs, and dibenz(a,h,)anthracene. Cancer Res., 33: 402-407.
CHALLONER, A. V. J., CORLESS, D., DAVIS, A., DEANE, G. H. W., DIFFERY,
B. L., GUPTA, S. P., & MAGNUS, I. A. (1976) Personnel monitoring
of exposure to ultraviolet radiation. Clin. exp. Dermatol.,
1: 175-179.
CHANDRA, P. (1972) Photodynamic action: A valuable tool in molecular
biology. Res. org. biol. med. chem., 3 (1): 232-258.
CHANDRA, P., RODIGHIERO, G., PALIKCIOGLU, S., & BISWAS, R. K. (1976)
Nucleic acid modification by furocoumarins and light: Some
biomedical implications. In: Jung, E., ed. Photochemotherapie,
Stuttgart, F. K. Schattauer Verlag, pp. 25-32.
CHARLIER, M. & HELENE, C. (1972) Photochemical reactions of aromatic
ketones with nucleic acids and their components I-. Purine and
pyrimidine bases and nucleosides. Photochem. Photobiol.,
15: 71-87.
CLEAVER, J. E. (1973) Xeroderma pigmentosum- progress and regress.
J. invest. Dermatol., 60: 374-380.
CLEAVER, J. E. & THOMAS, G. H. (1969) Single-strand interruptions in
DNA and the effects of caffeine in Chinese hamster cells
irradiated with ultraviolet light. Biochem. biophys. Res. Comm.
26: 203-208.
CLEAVER, J. E., THOMAS, G. H., TROSKO, J. E., & LETT, J. T. (1972)
Excision repair (dimer excision, strand breakage and repair
replication) in primary cultures of eukaryotic (bovine) cells.
Exp. cell Res., 74: 67-80.
CLEAVER, J. E. & TROSKO, J. E. (1969) DNA-degradation productions from
mammalian cells irradiated with ultraviolet light. Int. J.
radiat. Biol., 15: 411-424.
COBLENTZ, W. W., STAIR, R., & HOGUE, J. M. (1932) The spectral
erythemic reaction of the untanned human skin to UV radiation.
Bur. Stand. J. Res., 8: 541-547.
COGAN, D. G. & KINSEY, V. E. (1946) Action spectrum of keratitis
produced by ultraviolet radiation. Arch. Ophthalmol.,
35: 670-677.
COHEN-BAZIRE, G. & STANIER, R. Y. (1958) Inhibition of carotenoid
synthesis in photosynthetic bacteria. Nature (Lond.),
181: 250-252.
COX, B. & GAME, J. (1974) Repair systems in Saccharomyces. Mutat.
Res., 26: 257-264.
CURTIS, G. L. (1975) Initiation-promotion skin carcinogenesis and
immunological competence. Proc. Soc. Exp. Med., 150 (1):
pp. 61-64.
CUTCHIS, P. (1978) On the linkage of solar ultraviolet radiation to
skin cancer. Washington, DC, Federal Aviation Administration,
Office of Environmental Quality, pp. 1342 (Institute for Defense
Analysis Paper).
DANCIG, N.M. (1971) [Lighting hygiene and insolation of buildings and
built up areas in towns.] In: [Questions of housing hygiene and
of curative power and preventive medicine.] Moscow, A. N. Sysin
Institute, pp. 13-19 (in Russian).
DANCIG, I. N. (1974) [Dental caries and UV deficiency.]
Stimatologija, 1: 11-13 (in Russian).
DANCIG, N.M. (1975) [The hygienic basis for preventing light sarvation
among ships' crews.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 168-171 (in Russian).
DANCIG, N. M., ZABALNEVA, A. P., & PROKOPENKO, Ju.I. (1975) [The
importance of the protective effect of UV radiation in the
development of the tumoral process under experimental
conditions.] In: [The biological effects of UV radiation.]
Moscow, Nauka, pp. 137-142 (in Russian).
DANIELS, F. J., BROPHY, D., & LOWITS, W. C. (1961) Histochemical
response of human skin following ultraviolet radiation.
J. invest. Dermatol., 37: 351-357.
DAVIDSON, B. M. (1970) Relative evaluation of the insolation resources
of flats in a residential "microdistrict". Svetotekhnika,
7; 13-15.
DAVIES, J. N. P., KNOWLDEN, J., & WILSON, B. A. (1965) Incidence rates
of cancer in Kyandondo County, Uganda, 1954-1960. J. Natl Cancer
Inst., 35: 789-821.
DAVIES, R. E., DODGE, H. A., & AUSTIN, W. A. (1972a) Carcinogenicity
of DMBA under various light sources. Proceedings of the IX
Congress on Photobiology, pp. 247.
DAVIES, R. E., DODGE, H. A., & DESCHIELDS, L. H. (1972b) Alteration of
the carcinogenic activity of DMBA by light. Proc. Am. Assoc.
Cancer Res., 13: 14.
DAVIS, A., DEANE, G. H., & DIFFEY, B. L. (1976) Possible dosimeter for
ultraviolet radiation. Nature (Lond.), 261: 169-170.
DJORDJEVIC, B. & TOLMACH, L. J. (1967) Responses of synchronous
populations of HeLa cells to ultraviolet irradiation at selected
stages of the generation cycle. Radiat. Res., 32: 327-346.
DOLEZOVA, V. (1976) To the question of the geographic distribution of
pterygium. Rev. int. Trach., 53: 55-64.
DULBECCO, R. (i949) Reactivation of ultraviolet inactivated
bacteriophage by visible light. Nature (Lond.), 163: 949-950.
DUKE-ELDER, W. S. (1926) The pathological action of light upon the
eye. I. Action of the outer eye: Photophthalmia. Lancet,
1: 1137-1141.
EASTCOTT, D. F. (1962) Epidemiology of skin cancer in New Zealand. In:
Urbach, F., ed. The biology of cutaneous cancer, Washington,
DC, US Government Printing Office, pp. 141-152 (Monograph No. 10,
National Cancer Institute).
EDENBERG, H. J. & HANAWALT, P. C. (1973) The timecourse of DNA repair
replication in ultraviolet irradiated HeLa cells. Biochim.
Biophys. Acta, 324: 206-217.
EISENSTARK, A. (1971) Mutagenic and lethal effects of visible and
near-ultraviolet light on bacterial cells. Advances in Genetics,
16: 167-198.
ELKIND, M. M. & WHITMORE, G. F. (1967) The radiobiology of cultured
mammalian cells, New York, Gordon & Breach.
ELWOOD, J. M., LEE, J. A. H., WALTER, S. D., MO, T., & GREEN, A. E. S.
(1974) Relationship of melanoma and other skin cancer mortality
to latitude and ultraviolet radiation in the United States and
Canada. Int. J. Epidemiol. 3: 325-332.
ELWOOD, J. M. & LEE, J. A. H. (1975) Recent data on the epidemiology
of malignant melanoma. Semin. Oncol., 2 (2): 149-154.
EMMETT, E. A. (1973) Ultraviolet radiation as a cause of skin tumors.
CRC Crit. Rev. Toxicol., 2: 211.
EMMETT, E. A. (1977a) Phototoxic keratoconjunctivitis from coal tar
pitch volatiles. Science, 198: 841-842.
EMMETT, E. A. (1977b) Phototoxicity occurring during the manufacture
of ultraviolet cured ink. Arch. Dermatol., 113: 770-775.
EPSTEIN, J. H. (1965) Comparison of the carcinogenic and
co-carcinogenic effects of ultraviolet light on hairless mice.
J. Natl Cancer Inst., 34: 741-745.
EPSTEIN, J. H. & EPSTEIN, W. L. (1963) A study of tumor types produced
by ultraviolet light in hairless and hairy mice. J. invest.
Dermatol., 41: 463-473.
EPSTEIN, J. H., EPSTEIN, W. L., & NAKAI, T. (1967) Production of
melanomas from DMBA-induced «Blue Nevi» in hairless mice with
ultraviolet light. J. Natl Cancer Inst., 38: 18-22.
EPSTEIN, H. J., FUKUYAMA, K., & DOBSON, R. (1969) Ultraviolet light
carcinogenesis. In: Urbach, F., ed. The biological effects of
ultraviolet radiation, Oxford, Pergamon Press.
EVERETT, M. A., YEARGERS, E., SAYRE, R. M., & OLSON, R. L. (1966)
Penetration of epidermis by ultraviolet rays. Photochem.
Photobiol., 5: 533-542.
FABRE, F. (1971) A UV-supersensitive mutant in the yeast
Schizosaccharomyces pombe. Molec. Gen. Genet., 110: 134-143.
FEARS, T. R., SCOTTO, J., & SCHNEIDERMAN, M. A. (1977) Mathematical
models of age and ultraviolet effects on the incidence of skin
cancer among whites in the United States. Am. J. Epidemiol.,
105: 420-427.
FINDLAY, G. M. (1930) Cutaneous papillomata in the rat following
exposure to ultraviolet light. Lancet, pp. 1229-1231.
FISHER, M. S. & KRIPKE, M. L. (1977) Systemic alteration induced in
mice by ultraviolet light irradiation and its relationship to
ultraviolet carcinogenesis. Proc. Natl Acad. Sci., 74
(4): 1688-1692.
FITZPATRICK, T. B., PATHAK, M. A., HARBER, L. C., SEIJI, M., & KUKITA,
A. (1974) Sunlight and Man, Tokyo, University of Tokyo Press.
FORBES, P. D. (1978) Experimental UV photocarcinogenesis. In:
International Conference on Ultraviolet Carcinogenesis.
Washington, DC, US Government Printing Office pp. 31-38
(Monograph No. 50, National Cancer Institute).
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. I. Fluorescent whitening agents and
short-wave UVR. Food Cosmet. Toxicol., 13: 335-337.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. II. Fluorescent whitening agents and
simulated solar UVR. Food Cosmet. Toxicol., 13: 339-342.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photo-carcinogenesis. III. Simulation of exposure to sunlight and
fluorescent whitening Agents. Food Cosmet. Toxicol.,
13: 343-345.
FORBES, P. D., DAVIES, R. E., & URBACH, F. (1976) Phototoxicity and
photocarcinogenesis: Comparative effects of anthracene and
8-methoxypsoralen in the skin of mice. Food Cosmet. Toxicol.,
14: 243.
FORNACE, A. J., KOHN, K. W., & KANN, H. E. (1976) DNA single-strand
breaks during repair of UV damage in human fibroblasts and
abnormalities of repair in xeroderma pigmentosum. Proc. Natl
Acad. Sci., 73: 39-43.
FORTNER, J. G., MAKY, A. G., & SCHRODT, G. R. (1961) Transplantable
tumors of the Syrian (golden) hamster. I. Tumors of the
alimentary tract, endocrine glands and melanomas. Cancer Res.,
6 (Part 2): 161-198.
FOX, M. (1974) The effect of post-treatment with caffeine on survival
and UV-induced mutation frequencies in Chinese hamster and mouse
lymphoma cells in vitro. Mutat. Res., 24: 187-204.
FREEMAN, R. G. (1975) Data on the action spectrum for ultraviolet
carcinogenesis. J. Natl Cancer Inst., 55: 1119-1121.
FREEMAN, R. G. & KNOX, J. M. (1964a) Ultraviolet-induced corneal
tumors in different species and strains of animals. J. invest.
Dermatol., 43: 431-436.
FREEMAN, R. G. & KNOX, J. M. (1964b) Influence of temperature on
ultraviolet injury. Arch. Dermatol., 89: 858-864.
GABOVIC, R. E., MINH, A. A., & MOTUZKOV, I. N. (1975) [The effect of
UV radiation on the body's level of tolerance to exposure to
chemicals.] Vestn. Akad. Med. Nauk., 3: 26-37 (in Russian).
GALANIN, N. F. & PIRKIN, V. M. (1971) [The rational utilization of the
sun's radiant energy with a view to preventing ultraviolet
deficiency.] In: [Ultraviolet Radiation.] Moscow, Medicina, pp.
209-212 (in Russian).
GARADZA, M.P. (1965) [The natural UV radiation regime as shown by the
results of measurements at the Moscow State University
Meteorological Observatory.] In: [The climate of a big city.]
Moscow, MGU Publishing House, pp. 186-195 (in Russian).
GARADZA, M. P. (1967) [Direct UV-radiation at different times of
year.] In: [The radiation regime and precipitations in Moscow.]
Moscow, MGU Publishing House, pp. 165-175 (in Russian).
GARADZA, M. P. (1974) [Features of the inflow of UV-radiation under
different cloud condition.] In: [Radiation processes in the
atmosphere and on the earth's surface.] Leningrad,
Cidrometeoizdat, pp. 261-264 (in Russian).
GARADZA, M. P. & NEZVAL', E. I. (1971) [The effect of atmospheric
transparency and cloud on the UV-radiation regime.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 316-321 (in
Russian).
GAVRILOVA, L. I., DOJNIKOV, A. S., & KORCAGINA, T. N. (1975) [UV
radiation from arc and intermittent xenon lamps.] In:
[The biological effect of UV radiation] Moscow, Nauka, pp.
226-229 (in Russian).
GENERALOV, A. A. (1971) [The ultraviolet irradiation regime for
children and adults taking sun-baths and light and air baths at
various latitudes.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 203-207 (in Russian).
GLUSCENKO, A. G., ARTJUSENCO, I. S., & BARANOVA, M. A. (1975) [Health
indicators for children of crèche age in the ultraviolet climatic
conditions of Kiev.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 171-174 (in Russian).
GORDON, D. & SILVERSTONE, H. (1976) Worldwide epidemiology of
premalignant and malignant cutaneous lesions. In: Cancer of the
skin, Philadelphia, W. B. Saunders Co., pp. 405-434.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943a) Types of
tumors induced by ultraviolet radiation and factors influencing
their relative incidence. J. Natl Cancer Inst., 3: 371-378.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943b) Pathology of
tumors of the external ear in mice induced by ultraviolet
radiation. J. Natl Cancer Inst., 2: 269-275.
GRAHAM, J. H. & HELWIG, E. B. (1965) In: Montagna, W. & Dobson, R. L.,
ed. Advances in biology of the skin, New York, Macmillan
(Pergamon), Vol. II, pp. 277-327.
GREEN, A. E. S., ed. (1960) The middle ultraviolet: Its science and
technology, New York, John Wiley and Sons, Inc.
GREEN, A. E. S. & HEIDINGER, R. A. (1978) Models relating ultraviolet
light and non-melanoma skin cancer incidence. Photochem.
Photobiol., 28: 283-291.
GREEN, A. E. S., SAWADA, T., & SHETTLE, E. P. (1975) The middle
ultraviolet reaching ground. In: Impacts of climatic change on
the biosphere. Part 1, Chapter 2, pp. 29-49 (CIAP Monograph
No. 5).
GREEN, A. E. S., FINDLEY, G. B., Jr, KLENK, K. F., WILSON, W. M., &
MO, T. (1976) The ultraviolet dose dependence of non-melanoma
skin cancer incidence. Photochem. Photobiol., 24: 353-362.
GRIFFIN, A. C. (1959) Methoxsalen in ultraviolet carcinogenesis in the
mouse. J. Invest. Dermatol, 32: 367-372.
GRIFFIN, A. C., DOLMAN, V. S., ROHLKE, E. B., ROUVART, P., & TATUM, E.
L. (1955) The effect of visible light on the carcinogenicity of
ultraviolet light. Cancer Res., 15: 523.
GRIGGS, H. C. & BENDER, M. A. (1973) Photoreactivation of ultraviolet
induced chromosomal aberrations. Science, 179: 86-88.
GROSSMAN, L. (1968) Studies on mutagenesis induced in vitro.
Photochem. Photobiol, 7: 727-735.
GUSEV, N. M. & DUNAEV, B. A. (1971) [The utilization of ultraviolet
radiation in building.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 218-221 (in Russian).
HAMILTON, L. (1973) The influence of the cell cycle on the radiation
response of early embryos. In: Balls, M. & Billet, F. F., ed.
The cell cycle in development and differentiation, Cambridge,
Cambridge University Press, pp. 229-247.
HAN, A. & SINCLAIR, W. K. (1969) Sensitivity of synchronized Chinese
hamster cells to ultraviolet light. Biophys. J., 9: 1171-1192.
HAN, A., SINCLAIR, W. K., & YU, C. K. (1971) Ultraviolet-light-induced
division delay in synchronized Chinese hamster cells.
Biophys. J., 11: 540-549.
HANAWALT, P. C. & SETLOW, R. B., ed. (1975) Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 827.
HARBER, L. C. & BAER, R. L. (1969) Classification and characteristics
of photoallergy. In: Urbach, F., ed. The biologic effects of
ultraviolet radiation, Oxford, Pergamon Press, pp. 519-526.
HARRISON, A. P. (1967) Survival of bacteria. Harmful effects of light,
with some comparisons with other adverse physical agents. Annual
Rev. Microbiol., 21: 143-156.
HAUSSER, K. W. & VAHLE, W. (1922) [The dependence of light induced
erythema and pigment formation upon the frequency (or wavelength)
of the inducing radiation.] Strahlentherapia, 13: 41-71 (in
German).
HAUSSER, K. W. & VAHLE, W. (1927) [Sunburn and suntan.] Wiss
Veröffent. Siemens-Konzern, 6: 101 (in German).
HAYNES, R. H. (1975) DNA repair and the genetic control of
radio-sensitivity in yeast. In: Hanawalt, P. C. & Setlow, R. B.,
ed. Molecular mechanisms for repair of DNA, New York & London,
Plenum Press, pp. 529-540.
HÉLENE, C. & CHARLIER, M. (1971) Photosensitized reactions in nucleic
acids. Photosensitized formation and splitting of pyrimidine
dimers. Biochimie, 53: (11-12): 1175-1180.
HELLMAN, K. B., HAYNES, K. F., & BOCKSTAHLER, L. E. (1974)
Radiation-enhanced survival of a human virus in normal and
malignant rat cells (37788). Proc. Soc. Exp. Biol. Med.,
145: 255-262.
HERLITZ, C. W., JUNDELL, I., & WAHLGREN, F. (1930) [Malignant tumours
in white mice caused by ultraviolet irradiation.] Acta Paediat.,
10: 333-347 (in German).
HEROLD, H. J. & BERNDT, H. (1968) Cancer incidence in the German
Democratic Republic. Selected tables. Neoplasma, 15: 517-522.
HILL, R. H. (1958) A radiation-sensitive mutant of E. coli. Biochim.
Biophys. Acta, 30: 636-637.
HILL, L. & EIDENOW, A. (1923) Biological action of light: I. The
influence of temperature. Proc. R. Soc. (Biol.), 95: 163-180.
HOLMBERG, M. & JONASSON, J. (1974) Synergistic effect of X-ray and UV
irradiation on the frequency of chromosome breakage in human
lymphocytes. Mutat. Res., 23: 213-221.
HSIAO, D. (1975) Impacts of climatic change on the biosphere.
Springfield, Virginia. National Technical Information Service
(CIAP Monograph No. 5).
HUANG, C. W. & GORDON, M.P. (1973) Formation of cyclobutane-type
pyrimidine dimers in RNA by sunlight. Int. J. radiat. Biol.,
23: 527-529.
HUEPER, W. C. (1941) Cutaneous neoplastic responses elicited by
ultraviolet rays in hairless rats and in their haired litter
mates. Cancer Res., 1: 402-406.
HUTCHINSON, F. (1973) The lesions produced by ultraviolet light in DNA
containing 5-bromouracil. Quart. Rev. Biophys. 6: 201-246.
IKUSHIMA, T. & WOLFF, S. (1974) UV-induced chromatid aberrations in
cultured Chinese Hamster cells after one, two or three rounds of
DNA replication. Mutat. Res., 22: 193-201.
IPPEN, H. (1969) Mechanisms of photopathological reactions. In:
Urbach, F., ed. The biologic effects of ultraviolet radiation,
Oxford, Pergamon Press, pp. 513-518.
JACOBSON, A. F. & YATVIN, M. B. (1976) Changes in the phospholipid
composition of E. coli following X and UV-irradiation. Radiat.
Res., 66: 247-266.
JAGGER, J. (1967) Introduction to research in ultraviolet
photobiology, Englewood Cliffs, N J, Prentice-Hall, Inc.,
pp. 164.
JAGGER, J. (1969) Photoreactivation. In: Hollaender, A., ed.
Radiation protection and recovery, New York, Pergamon Press,
pp. 352-377.
JAGGER, J. (1976) Effects of near-ultraviolet radiation on
microorganisms. Photochem. Photobiol., 23: 451-454.
JESSUP, J. M., HANNA, N., PALASZYNSKI, E., & KRIPKE, M. L. (1978)
Mechanisms of depressed reactivity to dinitrochlorobenzene and
ultraviolet-induced tumours during ultraviolet carcinogenesis in
BALB/c mice. Cell. Immunol., 38: 105-115.
JOHNSON, B. E. (1968) Ultraviolet radiation and lysosomes in skin.
Nature (Lond.), 219: 1258-1259.
JOHNSON, B. E., DANIELS, F., & MAGNUS, I. A. (1968) Response of human
skin to ultraviolet light. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV.
JUNG, E. G. (1976) [Photochemotherapy, background, techniques and
complications.] Stuttgart -- New York, F. K. Schattaver Verlag
(in German).
JUNG, E. G., BOHNERT, E., & ERBS, G. (1971) Wavelength dependence of
UV-induced alterations of epidermal cells in hairless albino
mice. Arch. Derm. Forsch., 241: 284-291.
KAMENECKAJA, T. M. & MITROFANOVA, G. F. (1975) [The condition of
certain neurohumoral regulatory systems following UV radiation at
different wavelengths.] In: [The biological effects of UV
radiation.] Moscow, Nauka, (in Russian).
KAO, F. & PUCK, T. T. (1969) Genetics of somatic mammalian cells. IX.
Quantitation of mutagenesis by physical and chemical agents.
J. Cell. Physiol., 74: 245-248.
KARACEVCEVA, T. V. (1971) [The role on UV radiation as part of modern
methods of treating and preventing rheumatism in children.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 154-158 (in
Russian).
KELNER, A. (1949) Photoreactivation of ultraviolet-irradiated
Escherichia coli with special reference to close-reduction
principle and to ultraviolet-induced mutation. J. Bacteriol.,
58: 511-522.
KELNER, A. (1953) Growth, respiration, and nucleic acid synthesis in
ultraviolet-irradiated and in photoreactivated E. coli.
J. Bacteriol., 65: 252-262.
KELNER, A. & TAFT, E. B. (1956) The influence of photoreactivating
light on the type and frequence of tumors induced by ultraviolet
radiation. Cancer Res., 16: 860-866.
KIEFER, J. (1971) The importance of cellular energy metabolism for the
sparing effect of dose fractionation witch electrons and
ultraviolet light. Int. J. radiat. Biol., 20: 325-336.
KIRBY-SMITH, J. S., BLUM, H. F., & GRADY, H. G. (1942) Penetration of
ultraviolet radiation into skin, as a factor in carcinogenesis.
J. Natl Cancer Inst., 2: 403-412.
KOCH, H. R. (1967) [Photochemotherapy and cataract formation.]
Dermatosen im Beruf und Umwelt., 26; 162-165 (in German).
KOLLER, L. R. (1965) Ultraviolet radiation, 2nd ed., New York, John
Wiley and Sons.
KRAEMER, K. H., DE WEERD-KASTELEIN, E. A., ROBBINS, J. H., KEIJZER,
W., BARRETT, S. F., PETINGA, R. A., & BOOTSMA, D. (1975) Five
complementation groups in xeroderma pigmentosum. Mutat. Res.,
33: 327-340.
KRIPKE, M. L. (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J. Natl Cancer Inst., 53: 1333-1336.
KRIPKE, M. L. (1976) Target organ for a systemic effect of ultraviolet
radiation. Photochem. Photobiol., 24: 599-600.
KRIPKE, M. L. (1977) Latency, histology, and antigenicity of tumors
induced by ultraviolet light in three inbred mouse strains.
Cancer Res., 37: 1395-1400.
KRIPKE, M. L. & FISHER, M. S. (1976) Effect of UV light on the host
response to UV-induced tumirs. Proceedings of the 4th Annual
Meeting, American Society of Photobiology, Denver, CO, February
16-20.
KRIPKE, M. L. & FISHER, M. S. (1976) Immunologic parameters of
ultraviolet carcinogenesis. J. Natl Cancer Inst., 57: 211-215.
KRIPKE, M. L., LOFGREEN, J. S., BEARD, J., JESSUP, J. M., & FISHER, M.
S. (1977) In vivo immune responses of mice during
carcinogenesis by ultraviolet irradiation. J. Natl Cancer Inst.,
59: 1227-1230.
LABORDUS, V. (1970) The effect of ultraviolet light on developing eggs
of Lymnaea stagnails (mollusca, pulmonata) I, II, III. Proc.
K. Ned. Akad. Wet. Amsterdam, C, 366-493.
LAMOLA, A. A. & YAMANE, T. (1967) Sensitized photodimerization of
thymine in DNA. Proc. Natl Acad. Sci., 58: 443-446.
LANCASTER, H. O. (1956) Some geographical aspects of the mortality
from melanoma in Europeans. Med. J. Aust., 1: 1082-1087.
LATARJET, R. (1972) Interaction of radiation energy with nucleic
acids. Curr. Top. Radiat. Res. Q., 8: 1-38.
LATARJET, R. & ZAJDELA, F. (1974) Effet inhibitcur de la caféine sur
l'induction de cancers cutanés par les rayons ultraviolet chez la
souris. C. R. Acad. Sci. (Paris), 277: 1073-1076.
LAZAREV, D. N. & SOKOLOV, M. V. (1971) [The measurement of ultraviolet
radiation in units of effect.] In: [UV Radiation.] Moscow,
Medicina, pp. 328-333 (in Russian).
LAZAREV, D. N. & SOKOLOV, M. V. (1974) [Ultraviolet radiation.
Quantities and units. Terms and definitions. Technical
material for guidance.] Puscino, Institute of Biophysics of
the Academy of Sciences of the USSR, pp. 1-8 (in Russian).
LAZAREV, D. M., BESSALOVA, E. S., BALINA, G. P., & ERMILOVA, M. G.
(1975) [A UV biological photometer.] In: [The biological effects
of UV radiation.] Moscow, Nauka, pp. 244-246 (in Russian).
LEE, J. A. H. & MERRILL, J. M. (1970) Sunlight and the aetiology of
malignant melanoma: A Synthesis. Med. J. Aust., 2: 840-841.
LEE, H. H. & PUCK, T. T. (1960) The action of ultraviolet radiation on
mammalian cells as studied by single-cell technique. Radiat.
Res., 12: 340-348.
LEHMANN, A. R. (1972) Postreplication repair of DNA in
ultraviolet-irradiated mammalian cells. Mol. Biol.,
66: 319-337.
LEHMANN, A. R., KIRK-BELL, S., ARLETT, C. F., PATERSON, M. C., LOHMAN,
P. H. M., WEERD-KASTELEIN de, E. A., & BOOTSMA, D. (1975)
Xeroderma pigmentosum cells with normal levels of excision repair
have a defect in DNA synthesis after UV irradiation. Proc. Natl
Acad. Sci., 72: 219-223.
LEWIS, N. F. & KUMTA, U.S. (1972) Evidence for extreme UV resistance
of Micrococcus sp. NCTC 10785. Biochem. biophys. Res. Commun.,
47: 1100-1105.
LIPSON, R. L. & BALDES, E. J. (1960) Photosensitivity and heat. Arch.
Dermatol., 82: 517-520.
LOOMIS, W. F. (1970) Rickets. Sci. Am., 233: 76-91.
LUKIESH, M., HOLLADAY, L. L., & TAYLOR, A. H. (1939) Erythemal and
tanning effectiveness of UV energy. Gen. Electr. Rev.,
42: 274-278.
LYTLE, C. D. (1971) Host-cell reactivation in mammalian cells. I.
Survival or ultraviolet-irradiated herpes virus in different
cell-lines. Int. J. radiat. Biol., 19: 329-337.
LYTLE, C. D., HELLMAN, K. B., & TELLES, N. C. (1970) Enhancement of
viral transformation by ultraviolet light. Int. J. radiat.
Biol., 18: 397-300.
MACDONALD, E. J. (1976) Epidemiology of skin cancer 1975. In:
Neoplasms of the skin and malignant melanoma, Chicago, Year
Book Publishers, Inc., pp. 27-42.
MACKIE, B. S. & MCGOVERN, V. J. (1958) The mechanism of solar
carcinogenesis: A study of the role of collagen degeneration of
the dermis in the production of skin cancer. Arch. Dermatol.
Syph., 78: 218-244.
MAGNUS, I. A. & JOHNSON, B. E. (1965) Cited by Johnson, B. E.,
Daniels, F. Jr, & Magnus, I. A. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV,
pp. 139-202.
MAGNUS, K. (1973) Incidence of malignant melanoma of the skin in
Norway, 1955-1970. Cancer, 32: 1275-1286.
MAGNUS, K. (1977) Incidence of malignant melanoma of the skin in the
five nordic countries: significance of solar radiation. Int. J.
Cancer, 20: 477-485.
MALIK, M. O. A., MIDAYTALLA, A., DAOUC, E. H., & EL HASSAN, A. M.
(1974) Superficial cancer in the Sudan. A study of 1225 primary
malignant superficial tumours, Br. J. Cancer, 30: 355-364.
MATHEWS, M. M. & KRINSKY, N. (1965) The relationship between
carotenoid pigments and resistance to radiation in
non-photosynthetic bacteria. Photochem. Photobiol., 4: 813-817.
MCGOVERN, V. J. (1977) Epidemiological aspects of melanoma: A review.
Pathology, 9: 233-241.
MCGOVERN, V. J. & MACKIE, B. S. (1959) The relationship of solar
radiation to melanoblastoma. Aust. N. Z. J. Surg., 28: 257-262.
MEYER, A. E. H. & SEITZ, E. O. (1949) [Ultraviolet Radiation.]
Berlin, Walter de Gruyter & Co. (in German).
MILLS, L. F., LYTLE, C. D., ANDERSEN, F. A., HELLMAN, K. B., &
BOCKSTAHLER, L. H. (1975) A review of biological effects and
potential risks associated with ultraviolet radiation as used in
dentistry. pp. 1-27 (DHEW Publication (FDA) 76-8021).
MISHIMA, Y. & WIDLAN, S. (1967) Enzymatically active and inactive
melanocyte populations and ultraviolet irradiation: Combined
dopapremelanin reaction and electron microscopy. J. invest.
Dermatol., 49: 273.
MIYAJI, T. (1962) Skin cancer in Japan. A nationwide 5 year survey.
In: Urbach, F., ed. The biology of cutaneous cancer,
Washington, DC, US Government Printing Office, pp. 515-70
(Monograph No. 10, National Cancer Institute).
MIYAZAKI, H., KAWADA, A., TAKAKI, Y., SATO, T., & MASUTANI, M. (1968)
Influence of ultraviolet light on pigmentation of hairless mouse
epidermis. Jap. J. Dermatol., Ser. B, 78: 605.
MORENO, F. (1969) Contribution à l'étude de la létalité et de la
stérilité induites chez Drosophita melanogaster par irradiation
UV des cellules polaires de l'oeuf. I. Action létale, II. Action
stérilisante. Int. J. radiat. Biol., 16: 441-465.
MOUSTACCHI, E., WATERS, R., HEUDE, M., & CHANET, R. (1975 The present
status of DNA repair mechanisms in UV irradiated yeast taken as a
model eukaryotic system. In: Nygaard, O., Adler, H., & Sinclair,
W., ed. Radiation research: Biomedical, chemical and physical
perspectives, New York, Academic Press, pp. 632-650.
MOVSHOVITZ, M. & MODAN, B. (1973) Role of sun exposure in the etiology
of malignant melanoma: Epidemiologic inference. J. Natl Cancer
Inst., 51: 777-779.
MULAY, D. M. (1962) Skin Cancer in India. In: Urbach, F., ed.
The biology of cutaneous Cancer, Washington, DC, US Government
Printing Office, pp. 215-224 (Monograph No. 10, National Cancer
Institute).
MURPHY, T. M. (1975a) Inactivation of tobacco mosaic virus ribonucleic
acid by near- and middle-ultraviolet light: Sensitization by
sulfanilamide and chlortetracycline. Biochem. biophys. Res.
Commun., 65: 1108-1114.
MURPHY, T. M. (1975b) Effects of UV-B radiation on nucleic acids. In:
Impacts of climatic changes on the biosphere, pp. 19-40 (CIAP
Monograph No. 5).
MUSAJO, L., RODIGHIERO, G., CAPORALE, G., DALL'ACQUA, F., MARCIANI,
S., BORDIN, F., BACCICHETTI, F., & BEVILACQUA, R. (1974)
Photoreactions between skin-photosensitizing furocoumarins and
nucleic acids. In: Fitzpatrick, T. B., Pathak, M. A., Harber, L.
C., Seiji, M., & Kukita, A., ed. Sunlight and Man, Tokyo,
University of Tokyo Press, pp. 369-387.
NAKAMURA, K. & JOHNSON, W. C. (1968) Ultraviolet light-induced
connective tissues changes in rat skin: A histopathologic and
histochemical study. J. invest. Dermatol., 51: 253-258.
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1973) UV
photocarcinogenesis: Modification by anti-lymphocytic serum or
6-marcaptopurine. Proc. Am. Assoc. Cancer Res., 14: 46
(Abstract 182).
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1976) Modification of
photocarcinogenesis by two immunosuppressive agents. Cancer
Lett., 1: 243-247.
NILENDER, R. A. & GAVANIN, V. A. (1971) [Artificial sources of
ultraviolet radiation.] In: [UV Radiation.] Moscow, Medicina,
pp. 348-352 (in Russian).
NIOSH (1975) Ultraviolet transfer standard detectors and evaluation
and calibration of NIOSH UV hazard monitor. Cincinnati, OH, US
Department of Health, Education, and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and
Health, Division of Laboratories and Criteria Development, 12 pp. (HEW
Publication No. (NIOSH) 75-131).
NORBURY, K. C., KRIPKE, M. L., & BUDMEN, M. B. (1977) In vitro
reactivity of macrophages and lymphocytes from
ultraviolet-irradiated mice. J. Natl Cancer Inst.,
59: 1231-1235.
OETTLE, A. G. (1962) Skin cancer in Africa. In: Urbach, F, ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 197-214 (Monograph No. 10, National Cancer
Institute).
ORR, J. W. (1938) The changes antecedent to tumor formation during the
treatment of mouse skin with carcinogenic hydrocarbons.
J. Pathol. Bacteriol., 46: 495-515.
OWENS, D. W., KNOX, J. M., HUDSON, H. T., RUDOLPH, A. H., & TROLL, D.
(1977) Influence of wind on chronic ultraviolet light-induced
carcinogenesis. Br. J. Dermatol., 97: 285.
PAINTER, R. B. (1975) Repair in mammalian cells: Overview. In:
Hanawalt, P. C. & Setlow, R. B., ed. Molecular mechamisms for
repair of DNA, New York & London, Plenum Press, pp. 595-600.
PARRINGTON, J. M. (1972) Ultraviolet-induced chromosome aberration and
mitotic delay in human fibroblast cells. Cytogenetics,
11: 117-131.
PARRISH, J. A., FITZPATRICK, T. B., TANNENBAUM, L., & PATHAK, M. A.
(1974) Photochemotherapy of psoriasis with oral methoxsalen and
long wave ultraviolet light. New Engl. J. Med. 291: 1207-1212.
PATHAK, M. A., KRAMER, D. M., & FITZPATRICK, T. B. (1974) Photobiology
and photochemistry of furocoumarins (psoralens). In: Fitzpatrick,
T. B., Pathak, M. A., Harber, L. C., Seiji, M., & Kukita, A., ed.
Sunlight and Man, Tokyo, University of Tokyo Press,
pp. 335-368.
PETTIJOHN, D. & HANAWALT, P. (1964) Evidence for repair-replication of
ultraviolet damaged DNA in bacteria. J. mol. Biol., 9: 395-410.
PITTS, D. G. (1970) A comparative study of the effects of ultraviolet
radiation on the eye. Am. J. Optom., 47: 535-546.
PITTS, D. G. & CULLEN, A. P. (1977) Ocular ultraviolet effects from
300 nm to 400 nm: A preliminary report. Cincinatti, OH, US
Department of Health, Education and Welfare, National Institute
of Occupational Safety and Health, Division of Biomedical and
Behavioral Science (NIOSH Contract CDC-99-74-12).
PITTS, D. G. & KAY, K. R. (1969) The photo-ophthalmic threshold for
the rabbit. Am. J. Optom., 46: 561-572.
POLLARD, E. C. (1974) Cellular and molecular effects of solar
ultraviolet radiation. Photochem. Photobiol., 20: 301-308.
PROKOPENKO, Ju, I. (1976) [Some data on the mechanisms of protective
effect of UV-radiation.] Gig. i Sanit., 1: 100-102 (in
Russian).
PROKOPENKO, Ju, I. & ZABALUEVA, A. P. (1975) [Activation of
sanogenesis mechanisms with the help of UV-irradiation.] In:
[The biological effect of UV-radiation,] Moscow, Nauka,
pp. 156-159 (in Russian).
PUTSCHAR, W. & HOLTZ, F. (1930) [Induction of skin cancer in rats by
prolonged ultraviolet irradiation.] Z. Krebsforsch.,
23: 219-232 (in German).
QUEVEDO, W. C. Jr & SMITH, J. A. (1963) Studies on radiation-induced
tanning of skin. Ann. N. Y. Acad. Sci., 100: 364.
QUEVEDO, W. C. Jr, BRENNER, R. M., & KECHIJIAN, P. (1963) Melanocyte
performance during radiation induced tanning of murine skin.
Proceedings of the XVI International Congress on Zoology, 2: 306.
QUEVEDO, W. C. Jr, SZABO, G., VIRKS, J., & SINESI, S. J. (1965)
Melanocyte populations in UV-irradiated human skin. J. invest.
Dermatol., 45: 295.
RADMAN, M. (1975) SOS repair hypothesis: Phenomenology of an inducible
DNA repair which is accompanied by mutagenesis. In: Hanawalt, P.
C. & Setlow, R. B., ed. Molecular mechanisms for repair of DNA,
New York & London, Plenum Press, pp. 355-367.
RAPPAPORT, H., PIETRA, G., & SHUBIK, P. (1961) The induction of
melanotic tumors resembling cellular blue nevi in the Syrian
white hamster by cutaneous application of 7,
12-Dimethylbenz(a)anthracene. Cancer Res., 21: 661-666.
RASMUSSEN, R. E. & PAINTER, R. B. (1964) Evidence for repair of
ultraviolet damaged deoxyribonucleic acid in cultured mammal
cells. Nature (Lond.), 203: 1360-1362.
RAUTH, A. M. (1970) Effects of ultraviolet light on mammalian cells in
culture. Curr. Top. radiat. Res., 6: 195-248.
RAUTH, A. M. & DOMON, M. (1973) Potentiation of ultraviolet light
damage in mouse L cells by 1-cyclohexyl-3-(2-morpholinyl-4-et
carbodiimide metho-p-toluene sulphonate (CMEC). Int. J. radiat.
Biol., 24: 189-198.
RESNICK, M. A. (1969) Genetic control of radiation sensitivity in
Saccharomyces cerevisiae. Genetics, 62: 519-531.
REYNOLDS, J. (1954) The epidermal melanocytes of mice. J. Anat.,
88: 45.
ROBERTSON, D. F. (1969) Long term field measurements of erythemally
effective natural ultraviolet radiation. In: Urbach, F., ed.
The biologic effects of ultraviolet radiation, Pergamon
Press, Oxford, pp. 433-436.
ROBERTSON, D. F. (1972) Solar ultraviolet radiation in relation to
human sunburn and skin cancer. Thesis, Australia, University of
Queensland.
ROBERTSON, D. F. (1975) Calculated sunburn responses, In: Impacts of
climatic change on the biosphere. Springfield, Virginia,
National Technical Information Service, Part 1, Chapter 2,
Appendix J. (CIAP Monograph 5).
ROFFO, A. H. (1934) Cancer et soleil. Carcinomes et sarcomes provoqués
par l'action du soleil in toto. Bull. Assoc. Fr. Etud. Cancer,
53: 59.
ROMMELAERE, J., SUSSKIND, M., & ERRERA, M. (1973) Chromosome and
chromatid exchanges in Chinese hamster cells. Chromosoma,
41: 243-257.
RUNDEL, R. D. & NACHTWEY, D. S. (1978) Skin cancer and ultraviolet
radiation. Photochem. Photobiol., 28: 345-356.
RONGE, H. E. (1948) Ultraviolet irradiation with artificial
illumination Acta Phys. Scand., 15 (Suppl. 49): 1-191.
RUPERT, C. S. (1975) Enzymatic photoreactivation: Overview. In:
Hanawalt, P. C. & SETLOW, R. B. ed. Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 73-87.
RUPP, W. D. & HOWARD-FLANDERS, P, (1968) Discontinuities in the DNA
synthesized in an excision-defective strain of E. coli
following ultraviolet irradiation. J. Mol. Biol., 31: 291-304.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1941) Carcinogenesis by
ultraviolet rays with reference to wavelength and energy. Arch.
Pathol., 371: 135-148.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1942) Nonadditive effect
of UV light and other carcinogenic procedures. Cancer Res.,
2: 183-188.
RUSTAD, R. C. (1972) Techniques for the analysis of radiation-induced
mitotic delay in synchronously dividing sea urchin eggs. In:
Whitson, G. L., ed. Concepts in radiation cell biology, New
York & London, Academic Press, pp. 153-181.
SAMS, W. M. Jr, SMITH, J. G., & BURK, P. G. (1964) The experimental
production of elastosis with ultraviolet light. J. invest.
Dermatol., 43: 467.
SATO, T. (1971) Pigmentation induced by ultraviolet irradiation in
hairless mice with particular references to epidermal dendritic
cells. Jap. J. Dermatol. Ser. A, 81: 488.
SATO, T. & KAWADA, A. (1972) Mitotic activity of hairless mouse
epidermal melanocytes: Its role in the increase of melanocytes
during ultraviolet radiation. J. invest. Dermatol.,
58: 392-395.
SATO, T. & KAEADA, A. (1972) Uptake of tritiated thymidine by
epidermal melanocytes of hairless mice during ultraviolet light
radiation. J. invest. Dermatol., 58: 71.
SAUERBIER, W. (1976) UV damage at the transcriptional level. Advances
in radiation biology, 6: 50-106.
SAYENKO, A. J. (1968) A method for studying morbidity from
precancerous conditions and the question as to frequency of their
occurrence on the territory of the Kirghiz Soviet Socialist
Republic. Neoplasma, 15: 565-571.
SCHULZE, R. (1970) [Global Radiation Climate.] Wiss. Forschungsber.,
72: 220 pp. (in German).
SCOTTO, J., KOPF, A. W., & URBACH, F. (1974) Non-melanoma skin cancer
among Caucasians in four areas of the United States. Cancer,
34: 1333-1338.
SCOTTO, J., FEARS, T. R., & GORI, G. B. (1976) Measurements of
ultraviolet radiation in the United States and comparisons with
skin cancer data, National Cancer Institute (DHEW
No. (NIH) 76-1029).
SEIDL, E. (1969) Blitzgerät therapy. In: Urbach, F., ed. The biologic
effects of ultraviolet radiation, Oxford, Pergamon Press,
pp. 663-672.
SETLOW, R. B. (1968) The photochemistry, photobiology, and repair of
polynucleotides. Prog. N. A. Res. Mol. Biol., 8: 257-295.
SETLOW, R. B. (1973) The relevance of photobiological repair.
An. Acad. Bras. Ciencas, 45: 215-220.
SETLOW, R. B. (1974) The wavelengths in sunlight effective in
producing skin cancer: A theoretical analysis. Proc. Natl Acad.
Sci., 71: 3363-3366.
SETLOW, R. B. & CARRIER, W. L. (1964) The disappearance of thymine
dimers from DNA: An error-correcting mechanism. Proc. Natl Acad.
Sci., 51: 226-231. p. 34.
SETLOW, J. K. & DUGGAN, D. E. (1964) The resistance of Micrococcus
radiodurans to ultraviolet radiation. Biochim. Biophys. Acta,
87: 664-668.
SETLOW, J. K. & SETLOW, R. B. (1963) Nature of the photoreactivability
ultraviolet lesion in deoxyribonucleic acid. Nature (Lond.),
197: 560-562. p 36.
SHUBIK, P., PIETRA, G., & DELLA PORTA, G. (1960) Studies of skin
carcinogenesis in the Syrian golden hamster. Cancer Res.,
20: 1.
SILVERSTONE, H. & SEARLE, J. H. A. (1970) The epidemiology of skin
cancer in Queensland: The influence of phenotype and environment.
Br. J. Cancer, 24: 235-252.
SKREB, Y., HORVAT, D., & EGER, M. (1972) Modifications of
radiosensitivity in nucleate and anucleate amoeba fragments. In:
Bonotto, S., Goutier, R., Kirchmann, R., & Maisin, J.-R., ed.
Biology and radiobiology of anucleate systems I. New York,
Academic Press, pp. 67-82.
SMITH, K. C. (1974) The cellular repair of radiation damage. In:
Fitzpatrick, T. B., Pathak, M. A., Harber, L. C., Seiji M., &
Kukita, A., ed. Sunlight and Man, Tokyo, University of Tokyo
Press, pp. 67-77.
SMITH, K. C. (1975) The radiation-induced addition of proteins and
other molecules to nucleic acids. In: Wang, S. Y., ed.
Photochemistry and photobiology of nucleic acids, New York,
Academic Press, Vol. II, pp. 187-218.
SMITH, K. C. & HANAWALT, P. C. (1969) Molecular photobiology.
Inactivation and recovery, New York & London, Academic Press.
SOFFEN, G. A. & BLUM, H. F. (1961) Quantitative measurements of cell
changes in mouse skin following a single dose of ultraviolet
light. J. cell. comp. Physiol., 58: 81-96.
SOKOLOV, M. V. (1975) [Standardization of UV photometry.] In:
[The biological effects of ultraviolet radiation.] Moscow,
Nauka, pp. 232-235 (in Russian).
SOKOLOV, M. V. (1976) [Topical questions of UV biophotometry.]
Svetotecnika, 2: 20-22 (in Russian).
SOZIN, D. S. (1975) [Low pressure fluorescent lamps with combined
radiation.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 225-226 (in Russian).
SPIKES, J. D., KRIPKE, M. L., CONNOR, R. J., & EICHWALD, E. J. (1977)
Time of appearance and histology of tumors induced in the dorsal
skin of C3Hf mice by ultraviolet radiation from a mercury arc
lamp. J. Natl Cancer Inst. 59: 1637-1643.
STATE STANDARDS (1964) [Recommendations for the prevention of
ultraviolet deficiency.] Moscow Nauka, 50 pp. (in Russian).
STATE STANDARDS (1976a) [Regulations for the design and operation of
artificial UV-irradiation installations in industrial
undertakings.] In: [Regulations on the design of electrical
engineering installations for industry.] Energija 6: pp. 30-39
(in Russian).
STATE STANDARDS (1976b) [USSR building norms and regulations.]
Strijizdat, Moscow, p. 20 (SNIP 11-60-75) (in Russian).
STENBÄCK, F. (1969) Promotion in the morphogenesis of chemically
inducible skin tumors. Acta path. microbiol. Scand. Suppl.,
208: 1-116.
STENBÄCK, F. (1975a) Cellular injury and cell proliferation in skin
carcinogenesis by UV light. Oncology, : 31: 61-65.
STENBÄCK, F. (1975b) Species-specific neoplastic progression by
ultraviolet light on the skin of rats, guinea pigs, hamsters and
mice. Oncology, 31: 209-225.
STENBÄCK, F. (1975) Ultraviolet light irradiation as initiating agent
in skin tumor formation by the two-stage method. Europ. J.
Cancer, 11: 241-246.
STENBÄCK, F. (1978) Life history and histopathology of ultraviolet
light-induced skin tumors. In: International Conference on
Ultraviolet Carcinogenesis. Washington, DC, US Government
Printing Office, pp. 57-70 (Monograph No. 50, National Cancer
Institute).
SUTHERLAND, B. M. (1974) Photoreactivating enzyme from human
leukocytes. Nature (Lond.), 109-112.
SVIDERSKAJA, T. A. (1971) [Some experimental data on the conditions
accompanying an intensification of blastomogenic effect.] In:
[UV Radiation.] Moscow, Medicina, pp. 260-264 (in Russian).
SWANBECK, G. & HILLSTRöM, L. (1971) Analysis of etiological factors of
squamous cell skin cancer of different locations. Acta
Dermatovener, 51: 151-156.
TALANOVA, I. K. & ZABALUEVA, A. P. (1972) [Evaluation of the extent to
which children are provided with UV radiation in middle and
northern latitudes.] In: [Questions of experimental and clinical
spa treatment and physiotherapy.] 20: 323-329 (in Russian).
TALANOVA, I. K., KARACEVCEVA, T. V., & IVANOV, V. G. (1975) [The
combination of physical methods of prophylaxis and specific
immunization in children.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 116-120 (in Russian).
TODD, P. & HAN, A. (1976) Ultraviolet light induced
DNA-to-protein-cross-linking in mammalian cells. In: Smith, K.
C., ed. Aging, carcinogenesis and radiation-biology: The role of
nucleic acid addition reactions, New York & London, Plenum
Press, pp. 83-104.
TROSKO, J. E. & CHU, E. H. (1973) Inhibition of repair of UV-damaged
DNA by caffeine and mutation induction in Chinese hamster cells.
Chem. Biol. Interactions, 6: 317-332.
TYRRELL, R. M. (1973) Induction of pyrimidine dimers in bacterial DNA
by 365 nm radiation Photochem. Photobiol., 17: 69-73.
URBACH, F. (1969) The biologic effect of ultraviolet radiation.
Oxford, Pergamon Press, pp. 704.
URBACH, F., ROSE, D. B., & BONNEM, M. (1972) Genetic and environmental
interactions in skin carcinogenesis. In: Environment and skin
cancer, Baltimore, Maryland, Williams & Wilkins Co.,
pp. 355-371.
VARGHESE, A. J. (1973) Properties of photoaddition products of thymine
and cysteine. Biochemistry, 12 (14): 2725-2730.
VERHOEFF, F. H., BELL, L., & WALKER, C. B. (1916) The pathological
effects of radiant energy on the eye: An experimental
investigation with a systematic review of the (literature. Proc.
Am. Acad. Arts Sci., 51: 630-818.
VITALIANO, P. P. (1978) The use of logistic regression for modelling
risk factors in the development of nonmelanoma skin cancer.
Am. J. Epidemiol., 108: 402-414.
WANG, R. J. (1975) Lethal effect of »daylights» fluorescent light on
human cells in tissue-culture medium. Photochem. Photobiol.,
21: 373-375.
WANG, R. J., STOIEN, J. D., & LANDA, F. (1974) Lethal effect of
near-ultraviolet irradiation on mammalian cells in culture.
Nature (Lond.), 247: 43-45.
WATERHOUSE, J., MUIR, C., CORREA, P., & POWELL, J. (1976) Cancer
incidence in five continents, Lyons, IARC (Vol. III).
WHITSON, G. L. (1972) Radiation-induced biochemical changes in
protozoa. In: Whitson, G. L., ed. Concepts in radiation cell
biology, New York, Academic Press, pp. 123-152.
WINKELMAN, R. K., BALDES, E. J., & ZOLLMAN, P. E. (1960) Squamous cell
tumors induced in hairless mice with ultraviolet light.
J. invest. Dermatol., 34: 131-138.
WINKELMANN, R. K., ZOLLMAN, P. E., & BALDES, E. J. (1965) Squamous
cell carcinoma produced by ultraviolet light in hairless mice.
J. invest. Dermatol., 40: 217-224.
WITKIN, E. M. (1969) Ultraviolet-induced mutation and DNA repair.
Ann. Rev. Genet., 3: 525-552.
WITKIN, E. M. (1976) Ultraviolet mutagenesis and inducible DNA repair
in Escherichia coli. Bacteriol. Rev., 40: 869-907.
WOODCOCK, A. & MAGNUS, I. A. (1976) The sunburn cell on mouse skin:
Preliminary quantitative studies on its production. Br. J.
Dermatol., 95: 459-468.
YEH, S. (1962) Relative incidence of skin cancer in Chinese in Taiwan:
With special reference to arsenical cancer. In: Urbach, F., ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 81-108 (Monograph No. 10, National Cancer
Institute).
ZACKHEIM, H. S. (1964) Comparative cutaneous carcinogenesis in the
rat. Differential response to the application of anthramine,
methylcholanthrene, and dimethylbenzanthacene. Oncology,
17: 236-246.
ZIGMAN, S., SCHULTZ, J. B., & YULO, T. (1973) Possible roles of near
UV light in the cataractous process. Exp. eye Res.,
15: 201-208.
ZILOV, JU.D. (1971) [The prophylactic irradiation of children and
adolscents living in different climatic zones of the Soviet
Union.] In: [Ultraviolet radiation.] Moscow, Medicina,
pp. 237-241 (in Russian).
ZIVILOVA, N. D., ISTJUFEEV, V. A., SMIRNOV, E. S., & LEVITIN Ju.S.
(1975) [The UFM-meter and the UFB-72 laktmeter.] In: [The
biological effect of UV-radiation.] Moscow, Nauka, pp. 247-249
(in Russian).
ZWEIG, A. & HENDERSON, W. A. Jr (1976) A photochemical mid-ultraviolet
dosimeter. Photochem. Photobiol., 24: 543-549.
6.5.3 The "erythema range" effect
One of the important observations of Hausser & Vahle (1922) was
that there appeared to be a significant difference between the doses
needed to produce slight and maximal erytherna at different
wavelengths. This concept is of great potential significance for the
prevention of sunburn and the understanding of diseases due to light.
In Fig. 10, data are plotted from an experiment designed by Hausser to
test this effect, which show that 5 times the minimal erytherna dose
produces much less than the maximal erytherna at 254 nm, while 2.5
times the minimal erythema dose produces maximal erytherna at 303 nm.
While it takes much more energy to produce any erytherna at 303 nm
than at 254 nm, not much more than the threshold dose produces a
significant burn.
These observations are of great importance because they show that
the acute biological effects of several wavelengths in the UV-B
proceed at different time scales, and have very different
dose-response relationships. Thus, the usual assumptions that it is
realistic to weight the effectiveness of different wavelengths in a
continuous UVR source (such as the sun) by an action spectrum obtained
for a threshold effect with a monochromator is not correct, nor is it
appropriate to consider the effect of these wavelengths to be
biologically equivalent.
Unfortunately, in the absence of knowledge of chromophores or
biological mechanisms, there is no better way of comparing the
effectiveness of sources of different UVR composition than the present
method of calculating the skin erytherna effectiveness of UV-B.
As in all photobiological cutaneous effects, pigmentation plays a
major role in the sensitivity of the skin to UVR. Heavily pigmented
people are 10-20 times less sensitive than untanned fair-skinned
people. As far as is known, the action spectra for erythema are
similar for all races, differing only in the amount of energy
necessary to produce threshold effects.
6.5.4 Dose-response curves for keratoconjunctivitis
These are essentially similar to those for erythema, except that
the peak of the action spectrum is located at 270 nm. This is most
likely because of the absence of a filtering stratum corneum and
because the conjunctiva is not pigmented. The peak of 270 nm is
therefore used for the normalization of the dose limits of broad band
sources.
6.5.5 Dose-response relationship for photocarcinogenesis
Most of the existing evidence is consistent with the concepts
that UV-induced photodamage to skin is the main causal factor in the
development of skin cancer, that the development of skin cancer is a
stochastic effect, and that there is no threshold (sections 4.3 and
5.5).
Thus, a relationship should exist between skin cancer incidence
and accumulated dose, using a sensitivity function. In mice, the
quantitative relationship between UVR dose and the production of skin
cancer has been thoroughly explored; tumour incidence in this
experimental model is proportional to the square root of the number of
doses, the dose size, and the interval between doses.
In man, there is ample evidence that a latitude gradient exists
for the incidence of skin cancer in sensitive, fair-skinned
populations, and that this gradient is non-linear. There is obviously
a relationship between latitude and the intensity of solar radiation,
and this gradient is exaggerated in the UVR portion of the spectrum.
Although the shape of this relationship is relatively complex and
depends on a number of variables, for a range of mid-latitudes
(30°-50°), both the theoretical form and that obtained from actual
field measurements closely approximate a straight line. It is
generally accepted that latitude-related climatic and environmental
conditions and behavioural effects must modify the UVR dose actually
reaching a population. The factors involved, and their magnitude are
largely speculative.
A series of mathematical models relating human skin cancer
incidence to solar UVR has been proposed at various times in the past
few years, because of concern about alteration of the stratospheric
ozone layer (Green et al., 1978; Rundel & Nachtwey, 1978). While the
uncertainties are very great, the best accepted model data suggest
that a 5% increase in erythema-effective solar UVR may result in a 15%
(range 7.5-25%) increase in skin cancer in a susceptible population
after about 60 years, when a steady state has been reached. As far as
the relative risk is concerned in susceptible white-skinned
populations, people in older age groups, with fair skin that sunburns
easily and with a high life-time solar exposure, run 10-20 times more
risk of developing skin cancer than their contemporaries, who tan
easily and have a low life-time solar exposure (Vitaliano, 1978).
Incidences of nonmelanoma skin cancer as high as 350/100 000
population per year have been observed in elderly, white-skinned males
in Queensland, Australia, and Texas, USA. Populations with
constitutionally, heavily pigmented skin run only a minimum risk of
developing skin cancer in their lifetime.
7. GUIDELINES FOR HEALTH PROTECTION
The development of criteria for both upper and lower limits of
exposure to either natural or artificial UVR is extremely difficult
because of such problems as:
(a) the variation in both the acute and chronic effects of UVR
of different wave lengths;
(b) the considerable differences in the spectral composition of
light from different sources and particularly of sunlight at different
latitudes;
(c) the great differences in cutaneous sensitivity to UVR due
to genetic, environmental, and adaptive effects, and the considerable
variation in sensitivity in the same person at different times; and
(d) the difficulty of differentiating between the necessary
dose of UVR compatible with the upkeep of life, and the lowest dose
that results in serious detrimental effects.
7.1 Range of Exposure Limits
7.1.1 Exposure to solar ultraviolet radiation
As described in section 6, two thirds of the daily amount of
solar UV-B radiation reaches the earth between 10h00 and 14h00. Thus,
exposure should be reduced to a miminum during these hours. After a
period of acclimatization, most people can tolerate several hours of
outdoor exposure in the morning and afternoon, but shielding in the
near noon hours should always be considered. In general, exposure
during this very important period of acclimatization should not exceed
4 minimal erythema doses per day without some protection (either
clothing or sunscreens, section 7.3). This would correspond to 1 h of
exposure in the tropics. A dose of 1/8 MED per day appears to be
sufficient to prevent UVR deficiency.
7.1.2 Occupational exposure to artificial ultraviolet radiation
No internationally agreed limits exist at this time for
occupational exposure to UVR, which take into consideration the acute
effects and the risk of late cancer development. So far, the only
limits available, which could be used in the preparation of
guidelines, have been proposed by the National Institute of
Occupational Safety and Health in the USA (NIOSH, 1975).
The limits are based on the minimal erythema dose (MED) and the
minimal photokeratitic dose, which means that only acute effects have
been taken into consideration. For the UVR 200-315 nm region, it is
stated that radiant exposure in any 8-h period must not exceed the
values given in Table 11. For the wavelength range 315-400 nm, the
total irradiance on unprotected skin or eye must not exceed 10 W/m2
for periods exceeding 103 seconds. For radiant exposure of shorter
durations, it should not exceed 10 000 J/m2. The limit for 315-400 nm
is probably much too low and could be revised to a higher level.
Table 11. Total permissible 8-h doses and relative spectral
effectiveness of some selected monochromatic
wavelengthsa
Wavelength Permissible 8-h Relative spectral
(nm) dose (J/m2) effectiveness
(S lambda)b
200 1000 0.03
210 400 0.075
220 250 0.12
230 160 0.19
240 100 0.30
250 70 0.43
254 60 0.50
280 46 0.65
270 30 1.00
280 34 0.88
290 47 0.84
300 100 0.30
305 500 0.06
310 2000 0.015
315 10 000 0.003
a "From: NIOSH (1975).
Table 12. Maximum permissible exposure times for selected
values of Ieffa
Duration of exposure Effective irradiance,
per day Ieff (W/m2)b
8 h 1.0
4 h 2.0
2 h 4.0
1 h 8.0
30 min 17.0
15 min 33.0
10 min 50.0
5 min 100.0
1 min 500.0
30 sec 1000.0
a From: NIOSH (1975).
b Effective irradiance = action spectrum weighted irradiance.
However, these values only apply to sources emitting essentially
monochromatic UVR. The maximum permissible exposure for a broad band
source should be calculated by summing up the relative contributions
from all its spectral components, each being weighted by the relative
spectral effect SL, as given in Table 12. In addition, these
guidelines for determining exposure limits should not be used for
photosensitive individuals.
So far, none of the guidelines has included an evaluation of the
carcinogenic risk, as neither the action spectrum nor the
dose-response curve is known for man. It is hoped that this will be
obtained by comparison of cancer incidence and UV irradiance as
measured by the Robertson-Berger method.
7.1.3 Exposure of the general population to artificial ultraviolet
radiation
The exposure of the general population to artificial UVR is
primarily for hygienic and for cosmetic purposes. The use of UVR for
health purposes is discussed in sections 7.2 and 7.3.
The use of UVR for cosmetic purposes generally involves a
requirement for the development of skin pigmentation. For this reason,
it is unrealistic to follow the occupational standards, since they
would not allow the desired effects to be obtained.
Doses of UVR sufficient to produce slight erythema in 24 hours
are usually adequate for cosmetic purposes. Significant overdoses and
repeated UVR exposures for prolonged periods should be avoided. The
eyes must be properly shielded during each exposure. The risk of the
development of skin cancer due to chronic exposure to UVR for cosmetic
purposes is not known.
A number of devices available to the general population emit
significant amounts of UVR, or will do so if the protective envelope
of the device is damaged, and may cause acute UVR-induced eye and skin
damage. Such devices include Wood's lights, sterilizing and
ozone-producing lamps, and high power mercury and xenon arc lamps used
for illumination.
7.1.4 Measurement of natural and artificial ultraviolet radiation
Measurement of solar UVR involves serious difficulties, because
of the need for accurate spectral discrimination at the shortest end
of the solar spectrum. This is necessary because of the considerable
variations in the biological effectiveness of UVR shorter than 320 nm.
Few really practical, accurate, stray light-free spectroradiometers
have been developed so far, for use in the middle UVR region (UV-B)
and the use of action spectrum weighted, integrating analogue UVR
meters has been found to be much more practical.
The few practical prototypes of integrating chemical UVR
dosimeters that have been developed are still in the experimental
stage. If they can be perfected, such personal UVR dosimeters would be
of the greatest use (Challoner et al., 1976; Davis et al., 1976).
Basically, the same problems pertain to monitoring of artificial
UVR, although measurements are somewhat easier in a laboratory
setting.
The exposure criteria recommended in section 7.1.2 have not been
put to practical use, because it is still not technologically possible
to measure UVR adequately for compliance.
Thus, working practices are recommended for the control of
exposure in situations where sufficient measurement or emission data
are not available. A frequently practiced method of protection
consists of adequate containement of UVR-producing light sources.
7.2 Health Effects of Solar Ultraviolet Radiation in the General
Population
Two aspects of health protection in the general population are of
interest. One is the prevention of UVR deficiency that can occur in
populations living near the north and south poles (generally at
latitudes of 60° or more). The other deals with the protection of
pale-skinned people from excessive UVR in subtropical and tropical
areas (generally between latitudes 35° N and 35° S).
The zoning of the USSR for UVR is particularly interesting from a
health point of view (Belinskij, 1971). In the "UVR-deficit zone" it
appears essential to use irradiation from artificial sources, during
certain months, to compensate for UVR deficiency. In the "UV comfort
zone", artificial irradiation is unnecessary. In the "UVR excess
zone", it is essential to undertake measures for protection against
solar UVR, in order to avoid skin cancer. It has also been proposed
that, in order to prevent skin tumours among town dwellers in whom
light starvation has caused depigmentation of the skin, preventive UVR
should be carried out to increase skin pigmentation.
7.3 UVR Deficiency and its Prevention
7.3.1 Insolation and UV irradiation of built-up areas
It is well known that solar energy entering a room illuminates,
warms, dries, and particularly important, disinfects it, thus having a
beneficial, physical and psychological effect. The sanitation
standards and regulations for the insolation of dwellings and public
buildings and built-up areas, which are in force at present in the
USSR, are based on the requirement that premises should receive three
hours' uninterrupted insolation on cloudless days during the period 22
March-22 September and that protection should be given to limit the
thermal effect of solar radiation in the southern regions, below
latitude 55°N (State Standards, 1976b).
This health requirement is evident from a number of reviews
(Dancig, 1971; Galanin & Pirkin, 1971; Aleksandrov et al., 1975).
Solar UVR has a particularly valuable health-effect in that, inter
alia, it speeds up the processes of environmental selfpurification
(Belikova, 1960; Gluscenko et al., 1975). Therefore, town planning
tasks should include the rational use of UVR from the sun and sky
(Davidson, 1970; Gusev & Dunaev, 1971).
Since window glass cuts off the most biologically active
component of natural UVR, research to increase the UVR transparency of
window glass has been of particular concern to health workers in the
most northern latitudes (Belikova, 1964).
7.3.2 Sunbathing and air-bathing in the prevention of UVR deficiency
In addition to measures ensuring the maximum possible penetration
of natural UVR into working and dwelling places, prevention of UVR
deficiency can also be ensured by organizing solaria, beaches, and
sports areas attached to children's establishments (kindergartens and
pioneer camps), and to factories and mills. A schedule for the UV
irradiation of children and adults, for sun and sun-and-air baths at
different geographical latitudes, at different seasons of the year,
and at different times of the day has been developed (Generalov,
1971). At present, the effects of such solar radiation schedules
cannot be extrapolated to potential late effects.
7.3.3 Artificial ultraviolet radiation in the prevention of UVR
deficiency
Effective principles for the use of artificial UVR in the
prevention of UVR deficiency and "light starvation" in man have been
developed in the USSR. The recommended levels consist of a daily dose
of UV-B of between 0.125 and 0.75 of the threshold erytherna dose
(State Standards, 1964). The use of prophylactic UV irradiation has
been shown to be quite effective in workers in industries lacking
natural light (Bocenkova, 1971) and particularly effective in children
of preschool and school age (Ronge, 1948; Zilov, 1971).
Regulations have been drawn up for the planning and operation of
artificial UV irradiation devices in industrial enterprises (State
Standards, 1976b).
7.4 Protection against Ultraviolet Radiation
The whole of the world population has a potential for developing
skin cancer, the risk depending on the intensity and degree of
exposure to solar UVR during the life span. If all current exposure to
solar UVR could be significantly reduced, the incidence of skin cancer
would eventually decrease greatly.
Health and appearance can be adequately catered for by exposing
only part of the body for less than half an hour per day at or near
noon, except at latitudes north of 60° N.
7.4.1 Sunscreen preparations
Sunscreen preparations are usually classified as chemical or
physical agents. The former include para-aminobenzoic acid and its
esters, cinnamates, and benzophenones, all of which act by absorbing
radiation, which is dissipated as radiation of lower energy. Physical
agents act as simple physical barriers, reflecting, blocking, or
scattering light. They include titanium dioxide, talc, and zinc oxide.
Mainly because of cosmetic objections, the physical barriers are not
often used in sunscreen formulations (Robertson, 1972).
The principle of covering the skin by spreading a layer of
reliable UV absorber on the surface has proved a popular one.
Providing that the thickness of the layer applied is adequate and that
it adheres to the skin, it gives protection to the wearer under all
conditions.
Details of the kind of chemicals and bases used are beyond the
scope of this report.
7.4.2 Clothing
Covering the skin with clothing gives a sense of security that is
often misleading. The most frequent sites for skin cancer are those
that face upward. Thus, a hat is essential for adequate protection of
the forehead, scalp, and tops of ears. However, generally, it gives
only partial protection for the nose, less for the lower face, and
none for the hands and arms.
Body coverings worn in hot climates are generally not complete
absorbers. The average white shirt worn by men may transmit 20% of
UVR, while lighter weaves favoured by women may allow 50% of UVR to
reach the shoulders. Fairly complete clothing cover is more tolerable
in a dry atmosphere than a humid, coastal, tropical environment.
7.4.3 Behavioural conformity with the environment
When unexpectedly detained in the sun, protective procedures
available include facing in a variety of directions, hanging the head
and shielding the head with the hands or with a handkerchief. Shadows
should be used whenever possible, including one's own shadow. When
standing in the shadow of a building, only rays from about half the
sky are received, that is, about one quarter of the harmful intensity
of full daylight. This is certainly useful protection, but fair skin
will still burn in about one hour; in three hours, a painful burn may
be initiated, although the sufferer has not been in direct sunlight at
all.
The dominant factor in the daily erythemal exposure is the angle
of the sun above the horizon. This varies with latitude and with time
of year. Thus, maximum exposure occurs, when the sun is almost
directly overhead. The simplest means of protection is to take shelter
around the middle of the day, especially in the summer months.
At least one third of the whole day's UVR exposure occurs in the
hour before and the hour after noon; half the day's exposure, enough
for a very disabling sunburn, occurs during the 3-hour period around
noon. If shelter is taken between 10h00 and 14h00, only one third of
the day's exposure remains. Outside the period from 9h00 to 15h00,
only one sixth remains, permitting considerable outdoor activity even
for subjects with the most sensitive skin types.
7.4.4 Occupational protection
With the exception of medically prescribed doses, exposure of
both the eyes and skin to UVR should be kept to a minimum. In order to
protect persons in the vicinity of artificial UVR, the following
precautions are recommended:
(a) Whenever possible, prevention of excessive exposure of the
eyes and skin should be ensured by proper engineering design of
UVR-emitting installations and suitable enclosures, so that any UVR is
either adequately contained or sufficiently attenuated.
(b) When, for justifiable reasons, such containment is not
possible, protection should be afforded by providing close-fitting
goggles and/or face shields accompanied, if necessary, by suitable
UVR-opaque clothing and gloves to cover the skin.
(c) Adequate and appropriate instruction should be given, to
any person liable to be excessively exposed to UVR, concerning the
hazards involved and the precautions to be observed to avoid excessive
exposure.
(d) For artificial UVR sources that do not emit significant
visible light, a visible or audible warning signal may be required to
show when the UVR is being emitted.
(e) Powerful short wavelength UVR sources may generate ozone.
This additional hazard should be avoided by providing either adequate
ventilation or an adequate ozone removal system in the workplace.
REFERENCES
AITKEN, R. (1937) Ultraviolet radiation treatment of preeclamptic
toxemia. Br. J. Phys. Med., 12: 45-47.
ALEKSANDROV, V. N. & ALEKSANDROVA, A. I. (1975) [The insolation and UV
climate of housing in the far north.] Gig. i Sanit. 5: 16 (in
Russian).
ANCHEV, N., POPOV, I., MONOV N., & OUZOUNOV, N. (1968) Spreading of
malignant neoplasms in the People's Republic of Bulgaria.
Neoplasma, 15: 451-468.
ANDREEV, M. G., VASIL'EV B. D., ZAHAR'EVSKIJ, A. V., & HOROKORIN, T.
(1975) [Artificial sources of UV radiation with biological
effect.] In: [The biological effect of UV radiation.] Moscow,
Nauka, pp. 221-225 (in Russian).
BACHEM, A. (1956) Ophthalmic ultraviolet action spectra. Am. J.
Ophthalmol., 41: 969-975.
BAIN, J. & RUSCH, H. P. (1943) Carcinogenesis with ultraviolet
radiation of wave length 2, 800-3, 400 A. Cancer Res.,
3: 425-430.
BEARD, H. H., BOGGESS, T. S., & VON HAAM, E. (1936) Experimental
production of malignant tumors in the albino rat by means of
ultraviolet rays. Am. J. Cancer, 27: 257-266.
BELIKOVA, V. K. (1964) [Evaluation from the hygienic standpoint of
experimental samples of a window glass for residential and public
buildings.] Gig. i. Sanit., 1: 21-28 (in Russian).
BELIKOVA, V. K. (1966) [Natural ultraviolet radiation and its
bactericidal importance.] Moscow, Medicina, pp. 322-326 (in
Russian).
BELIKOVA, V. K., ZABALUEVA, A. P., & GALPERIN, E. L. (1975)
[Evaluation from the standpoint of hygiene of the zoning of the
territory of the U.S.S.R. in respect to UV radiation levels.] In:
[The biological effects of UV radiation.] Moscow, Nauka, pp.
161-165 (in Russian).
BELINSKIJ, V. A. (1971) [Zoning of the territory of the USSR in
respect to the natural UV radiation.] In: [Meteorology and
climatology.] Issue 2, Moscow, Publishing House of the Moscow
Branch of the Geographical Society, (in Russian).
BELINSKIJ, B. A. & ANDRIENKO, L. M. (1974) [A simplified radiation
model of the atmosphere in the UV spectral region.] In:
[Radiation, processes in the atmosphere and on the earth's
surface.] Leningrad, Gidrometeoizdat, pp. 273-276, (in
Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1963) [Ultraviolet climate of the
USSR.] In [Proceedings of the All-Union Scientific
Meteorological Meeting.] Leningrad, Gidrometeozidat,
pp. 244-252 (in Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1962) [Scattered UV-radiation in the
Eastern Pamirs.] In: [Scientific Communications of the Institute
of Geology and Geography of the Academy of Sciences of the
Lithuanian SSR.] Vilanus, Vil. XIII, pp. 283-287 (in Russian).
BELINSKIJ, V. A., GARADZA, M.P., MEZENNAJA, L. M., & NEZVAL, E. I.
(1968) [Sun and sky ultraviolet radiation.] Moscow, Moscow
University Press (in Russian).
BENDER, M. A., GRIGGS, H. G., & WALKER, P. L. (1973) Mechanisms of
chromosomal aberration production. I. Aberration induction by
ultraviolet light. Mutat. Res., 20: 387-402.
BENER, P. (1972) Approximate values of intensity of natural
ultraviolet radiation for different amounts of atmospheric
ozone. London, European Research Office, United States Army.
(Contract DAJA 36-68-C-1017).
BEN-HUR, E. & ELKIND, M. M. (1973) DNA cross-linking in Chinese
hamster cells exposed to near ultraviolet light in the presence
of 4,5',8-trimethylpsoralen. Biochim. Biophys. Acta,
331: 181-193.
BERENBLUM, I. & SHUBIK, P. (1947) The role of croton oil applications,
associated with a single painting of a carcinogen, in tumor
induction of the mouse's skin. Br. J. Cancer, 1: 379-382.
BERENBLUM, I. & SHUBIK, P. (1949) An experimental study of the
initiating stage of carcinogenesis, and a re-examination of the
somatic cell mutation theory of cancer. Br. J. Cancer,
3: 109-118.
BERGER, D., URBACH, F., & DAVIES, R. E. (1967) The action spectrum of
erythema induced by ultraviolet radiation. Proceedings of the
13th International Congress of Dermatology, Berlin, Springer
Verlag, pp. 1112-1117.
BERGER, D., ROBERTSON, D. F. & DAVIES R. E. (1975) Field measurements
of biologically effective UV radiation. In: Impacts of climatic
change on the biosphere, Springfield, VA, National Technical
Information Service, Part I, Chapter 2, pp. 233-264 (Appendix D,
CIAP Monograph No. 5).
BEUKERS R. & BERENDS, W. (1960) Isolation and identification of the
irradiation product of thymine. Biochim. Biophys. Acta,
41: 550-551.
BILLEN, D. & GREEN, A. E. S. (1975) Comparison of germicidal activity
of sunlight with the response of a sunburning ultraviolet meter.
In: Impacts of climatic change on the biosphere, Springfield,
VA, National Technical Information Service, Part 1, Chapter 2,
pp. 299-305 (Appendix F, CIAP Monograph No. 5).
BISCHOFF, F. (1969) Carcinogenic effects of steroids. In: Paoletti, &
Kritchevsky, ed. Advances in lipid research. New York, Academic
Press, Vol. 7, pp. 165-244.
BLACK, H. S. & CHAN, J. T. (1976) Etiologic related studies of
ultraviolet light-mediated carcinogenesis. Oncology,
33: 119-122.
BLACK, H. S. & DOUGLAS, D. R. (1973) Formation of a carcinogen of
natural origin in the etiology of UV-carcinogenesis. Cancer
Res., 33: 2094-2096.
BLACK, H. S. & LO, W. B. (1971) Formation of a carcinogen in human
skin irradiated with ultraviolet light. Nature (Lond.),
234: 306-308.
BLACK, H. S., CHAN, J. T., & BROWN, G. E. (1978) Effects of dietary
constituents on ultraviolet light-mediated carcinogenesis.
Cancer Res., 38: 1384-1387.
BLUM, H. F. (1941) Sunlight and cancer of the skin. J. Natl Cancer
Inst., 1: 397-431.
BLUM, H. F. (1943) Wavelength dependence of tumor induction by
ultraviolet radiation. J. Natl Cancer Inst., 3: 533-537.
BLUM, H. F. (1969) Quantitative aspects of cancer induction by
ultraviolet light: Including a revised model. In: Urbach, F., ed.
The biological effects of ultraviolet radiation, Oxford,
Pergamon Press.
BLUM, H. F. & LIPPINCOTT, S. W. (1943) Carcinogenic effectiveness of
ultraviolet radiation of wavelength 2537A. J. Natl Cancer Inst.,
3: 211-216.
BLUM, H. F., GRADY, H. G., & KIRBY-SMITH, J. S. (1943) Relationships
between dosage and rate of tumor induction by ultraviolet
radiation. J. Natl Cancer Inst., 3: 91-97.
BLUM, H. F., BUTLER, E. G., DAILEY, T. H. DAUBE, J. R., MAWE, R. C., &
SOFFEN, G. W. (1959) Irradiation of mouse skin with single doses
of ultraviolet light. J. Natl Cancer Inst., 22: 979-993.
BOCENKOVA, T. D. (1971) [The application of prophylactic
UV-irradiation to workers in buildings of a new type.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 245-251 (in
Russian).
BOGUCKIJ, B. V., KAPELEVA, A. I., BOKSA, V. G., LESINSKAJ, N. P.,
CIBIREVA, E. M, KOSTIN, N. F., & EMELKIN, V. I. (1975)
[Continuous and pulsed ultraviolet irradiation in combined
treatment of protracted and chronic pneumonia.] In:
[The biological effect of UV-radiation.] Moscow, Nauka,
pp. 126-127 (in Russian).
BOVIE, W. T. & KLEIN, A. (1919) Sensitization to heat due to exposure
to light of short wavelengths. J. Gen. Physiol., 1: 331-336.
BOYCE, R. P. & HOWARD-FLANDERS, P. (1964) Release of ultraviolet
light-induced thymine dimers from DNA in E. coli K-12. Proc.
Natl Acad. Sci., 51: 293-300.
BRIDGES, B. A. & HUCKLE, J. (1970) Mutagenesis of cultured mammalian
cells by X-radiation and ultraviolet light. Mutat. Res.
10: 141-151.
BUCHANAN, A. R., HELM, H. C., & STILSON, D. W. (1960) Biomedical
effects of exposure to electromagnetic radiation.
I. Ultraviolet. Dayton, OH, Wright-Patterson Air Force Base,
pp. 60-376 (WADD Technical Report).
BUSCHKE, W., FRIEDENWALD, J. S., & MOSES, S. G. (1945) Effects of
ultraviolet irradiation on corneal epithelium: Mitosis, nuclear
fragmentation, post-traumatic cell movements, loss of tissue
cohesion. J. Cell. Comp. Physiol., 26: 147-164.
CAKLIN, A. V. (1974) [The epidemiology of malignant neoplasms.] In:
[Handbook on oncology.] Moscow, Medicina, pp. 26-27 (in
Russian).
CARLSON, L. D. & JACKSON, B. H. (1959) Combined effects of ionizing
radiation and high temperature on longevity of Sprague-Dawley
rat. Radiat. Res., 11: 509-519.
CASTO, B.C. (1973) Enhancement of adenovirus transformation by
treatment of hamster cells with ultraviolet irradiation, DNA base
analogs, and dibenz(a,h,)anthracene. Cancer Res., 33: 402-407.
CHALLONER, A. V. J., CORLESS, D., DAVIS, A., DEANE, G. H. W., DIFFERY,
B. L., GUPTA, S. P., & MAGNUS, I. A. (1976) Personnel monitoring
of exposure to ultraviolet radiation. Clin. exp. Dermatol.,
1: 175-179.
CHANDRA, P. (1972) Photodynamic action: A valuable tool in molecular
biology. Res. org. biol. med. chem., 3 (1): 232-258.
CHANDRA, P., RODIGHIERO, G., PALIKCIOGLU, S., & BISWAS, R. K. (1976)
Nucleic acid modification by furocoumarins and light: Some
biomedical implications. In: Jung, E., ed. Photochemotherapie,
Stuttgart, F. K. Schattauer Verlag, pp. 25-32.
CHARLIER, M. & HELENE, C. (1972) Photochemical reactions of aromatic
ketones with nucleic acids and their components I-. Purine and
pyrimidine bases and nucleosides. Photochem. Photobiol.,
15: 71-87.
CLEAVER, J. E. (1973) Xeroderma pigmentosum- progress and regress.
J. invest. Dermatol., 60: 374-380.
CLEAVER, J. E. & THOMAS, G. H. (1969) Single-strand interruptions in
DNA and the effects of caffeine in Chinese hamster cells
irradiated with ultraviolet light. Biochem. biophys. Res. Comm.
26: 203-208.
CLEAVER, J. E., THOMAS, G. H., TROSKO, J. E., & LETT, J. T. (1972)
Excision repair (dimer excision, strand breakage and repair
replication) in primary cultures of eukaryotic (bovine) cells.
Exp. cell Res., 74: 67-80.
CLEAVER, J. E. & TROSKO, J. E. (1969) DNA-degradation productions from
mammalian cells irradiated with ultraviolet light. Int. J.
radiat. Biol., 15: 411-424.
COBLENTZ, W. W., STAIR, R., & HOGUE, J. M. (1932) The spectral
erythemic reaction of the untanned human skin to UV radiation.
Bur. Stand. J. Res., 8: 541-547.
COGAN, D. G. & KINSEY, V. E. (1946) Action spectrum of keratitis
produced by ultraviolet radiation. Arch. Ophthalmol.,
35: 670-677.
COHEN-BAZIRE, G. & STANIER, R. Y. (1958) Inhibition of carotenoid
synthesis in photosynthetic bacteria. Nature (Lond.),
181: 250-252.
COX, B. & GAME, J. (1974) Repair systems in Saccharomyces. Mutat.
Res., 26: 257-264.
CURTIS, G. L. (1975) Initiation-promotion skin carcinogenesis and
immunological competence. Proc. Soc. Exp. Med., 150 (1):
pp. 61-64.
CUTCHIS, P. (1978) On the linkage of solar ultraviolet radiation to
skin cancer. Washington, DC, Federal Aviation Administration,
Office of Environmental Quality, pp. 1342 (Institute for Defense
Analysis Paper).
DANCIG, N.M. (1971) [Lighting hygiene and insolation of buildings and
built up areas in towns.] In: [Questions of housing hygiene and
of curative power and preventive medicine.] Moscow, A. N. Sysin
Institute, pp. 13-19 (in Russian).
DANCIG, I. N. (1974) [Dental caries and UV deficiency.]
Stimatologija, 1: 11-13 (in Russian).
DANCIG, N.M. (1975) [The hygienic basis for preventing light sarvation
among ships' crews.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 168-171 (in Russian).
DANCIG, N. M., ZABALNEVA, A. P., & PROKOPENKO, Ju.I. (1975) [The
importance of the protective effect of UV radiation in the
development of the tumoral process under experimental
conditions.] In: [The biological effects of UV radiation.]
Moscow, Nauka, pp. 137-142 (in Russian).
DANIELS, F. J., BROPHY, D., & LOWITS, W. C. (1961) Histochemical
response of human skin following ultraviolet radiation.
J. invest. Dermatol., 37: 351-357.
DAVIDSON, B. M. (1970) Relative evaluation of the insolation resources
of flats in a residential "microdistrict". Svetotekhnika,
7; 13-15.
DAVIES, J. N. P., KNOWLDEN, J., & WILSON, B. A. (1965) Incidence rates
of cancer in Kyandondo County, Uganda, 1954-1960. J. Natl Cancer
Inst., 35: 789-821.
DAVIES, R. E., DODGE, H. A., & AUSTIN, W. A. (1972a) Carcinogenicity
of DMBA under various light sources. Proceedings of the IX
Congress on Photobiology, pp. 247.
DAVIES, R. E., DODGE, H. A., & DESCHIELDS, L. H. (1972b) Alteration of
the carcinogenic activity of DMBA by light. Proc. Am. Assoc.
Cancer Res., 13: 14.
DAVIS, A., DEANE, G. H., & DIFFEY, B. L. (1976) Possible dosimeter for
ultraviolet radiation. Nature (Lond.), 261: 169-170.
DJORDJEVIC, B. & TOLMACH, L. J. (1967) Responses of synchronous
populations of HeLa cells to ultraviolet irradiation at selected
stages of the generation cycle. Radiat. Res., 32: 327-346.
DOLEZOVA, V. (1976) To the question of the geographic distribution of
pterygium. Rev. int. Trach., 53: 55-64.
DULBECCO, R. (i949) Reactivation of ultraviolet inactivated
bacteriophage by visible light. Nature (Lond.), 163: 949-950.
DUKE-ELDER, W. S. (1926) The pathological action of light upon the
eye. I. Action of the outer eye: Photophthalmia. Lancet,
1: 1137-1141.
EASTCOTT, D. F. (1962) Epidemiology of skin cancer in New Zealand. In:
Urbach, F., ed. The biology of cutaneous cancer, Washington,
DC, US Government Printing Office, pp. 141-152 (Monograph No. 10,
National Cancer Institute).
EDENBERG, H. J. & HANAWALT, P. C. (1973) The timecourse of DNA repair
replication in ultraviolet irradiated HeLa cells. Biochim.
Biophys. Acta, 324: 206-217.
EISENSTARK, A. (1971) Mutagenic and lethal effects of visible and
near-ultraviolet light on bacterial cells. Advances in Genetics,
16: 167-198.
ELKIND, M. M. & WHITMORE, G. F. (1967) The radiobiology of cultured
mammalian cells, New York, Gordon & Breach.
ELWOOD, J. M., LEE, J. A. H., WALTER, S. D., MO, T., & GREEN, A. E. S.
(1974) Relationship of melanoma and other skin cancer mortality
to latitude and ultraviolet radiation in the United States and
Canada. Int. J. Epidemiol. 3: 325-332.
ELWOOD, J. M. & LEE, J. A. H. (1975) Recent data on the epidemiology
of malignant melanoma. Semin. Oncol., 2 (2): 149-154.
EMMETT, E. A. (1973) Ultraviolet radiation as a cause of skin tumors.
CRC Crit. Rev. Toxicol., 2: 211.
EMMETT, E. A. (1977a) Phototoxic keratoconjunctivitis from coal tar
pitch volatiles. Science, 198: 841-842.
EMMETT, E. A. (1977b) Phototoxicity occurring during the manufacture
of ultraviolet cured ink. Arch. Dermatol., 113: 770-775.
EPSTEIN, J. H. (1965) Comparison of the carcinogenic and
co-carcinogenic effects of ultraviolet light on hairless mice.
J. Natl Cancer Inst., 34: 741-745.
EPSTEIN, J. H. & EPSTEIN, W. L. (1963) A study of tumor types produced
by ultraviolet light in hairless and hairy mice. J. invest.
Dermatol., 41: 463-473.
EPSTEIN, J. H., EPSTEIN, W. L., & NAKAI, T. (1967) Production of
melanomas from DMBA-induced «Blue Nevi» in hairless mice with
ultraviolet light. J. Natl Cancer Inst., 38: 18-22.
EPSTEIN, H. J., FUKUYAMA, K., & DOBSON, R. (1969) Ultraviolet light
carcinogenesis. In: Urbach, F., ed. The biological effects of
ultraviolet radiation, Oxford, Pergamon Press.
EVERETT, M. A., YEARGERS, E., SAYRE, R. M., & OLSON, R. L. (1966)
Penetration of epidermis by ultraviolet rays. Photochem.
Photobiol., 5: 533-542.
FABRE, F. (1971) A UV-supersensitive mutant in the yeast
Schizosaccharomyces pombe. Molec. Gen. Genet., 110: 134-143.
FEARS, T. R., SCOTTO, J., & SCHNEIDERMAN, M. A. (1977) Mathematical
models of age and ultraviolet effects on the incidence of skin
cancer among whites in the United States. Am. J. Epidemiol.,
105: 420-427.
FINDLAY, G. M. (1930) Cutaneous papillomata in the rat following
exposure to ultraviolet light. Lancet, pp. 1229-1231.
FISHER, M. S. & KRIPKE, M. L. (1977) Systemic alteration induced in
mice by ultraviolet light irradiation and its relationship to
ultraviolet carcinogenesis. Proc. Natl Acad. Sci., 74
(4): 1688-1692.
FITZPATRICK, T. B., PATHAK, M. A., HARBER, L. C., SEIJI, M., & KUKITA,
A. (1974) Sunlight and Man, Tokyo, University of Tokyo Press.
FORBES, P. D. (1978) Experimental UV photocarcinogenesis. In:
International Conference on Ultraviolet Carcinogenesis.
Washington, DC, US Government Printing Office pp. 31-38
(Monograph No. 50, National Cancer Institute).
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. I. Fluorescent whitening agents and
short-wave UVR. Food Cosmet. Toxicol., 13: 335-337.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. II. Fluorescent whitening agents and
simulated solar UVR. Food Cosmet. Toxicol., 13: 339-342.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photo-carcinogenesis. III. Simulation of exposure to sunlight and
fluorescent whitening Agents. Food Cosmet. Toxicol.,
13: 343-345.
FORBES, P. D., DAVIES, R. E., & URBACH, F. (1976) Phototoxicity and
photocarcinogenesis: Comparative effects of anthracene and
8-methoxypsoralen in the skin of mice. Food Cosmet. Toxicol.,
14: 243.
FORNACE, A. J., KOHN, K. W., & KANN, H. E. (1976) DNA single-strand
breaks during repair of UV damage in human fibroblasts and
abnormalities of repair in xeroderma pigmentosum. Proc. Natl
Acad. Sci., 73: 39-43.
FORTNER, J. G., MAKY, A. G., & SCHRODT, G. R. (1961) Transplantable
tumors of the Syrian (golden) hamster. I. Tumors of the
alimentary tract, endocrine glands and melanomas. Cancer Res.,
6 (Part 2): 161-198.
FOX, M. (1974) The effect of post-treatment with caffeine on survival
and UV-induced mutation frequencies in Chinese hamster and mouse
lymphoma cells in vitro. Mutat. Res., 24: 187-204.
FREEMAN, R. G. (1975) Data on the action spectrum for ultraviolet
carcinogenesis. J. Natl Cancer Inst., 55: 1119-1121.
FREEMAN, R. G. & KNOX, J. M. (1964a) Ultraviolet-induced corneal
tumors in different species and strains of animals. J. invest.
Dermatol., 43: 431-436.
FREEMAN, R. G. & KNOX, J. M. (1964b) Influence of temperature on
ultraviolet injury. Arch. Dermatol., 89: 858-864.
GABOVIC, R. E., MINH, A. A., & MOTUZKOV, I. N. (1975) [The effect of
UV radiation on the body's level of tolerance to exposure to
chemicals.] Vestn. Akad. Med. Nauk., 3: 26-37 (in Russian).
GALANIN, N. F. & PIRKIN, V. M. (1971) [The rational utilization of the
sun's radiant energy with a view to preventing ultraviolet
deficiency.] In: [Ultraviolet Radiation.] Moscow, Medicina, pp.
209-212 (in Russian).
GARADZA, M.P. (1965) [The natural UV radiation regime as shown by the
results of measurements at the Moscow State University
Meteorological Observatory.] In: [The climate of a big city.]
Moscow, MGU Publishing House, pp. 186-195 (in Russian).
GARADZA, M. P. (1967) [Direct UV-radiation at different times of
year.] In: [The radiation regime and precipitations in Moscow.]
Moscow, MGU Publishing House, pp. 165-175 (in Russian).
GARADZA, M. P. (1974) [Features of the inflow of UV-radiation under
different cloud condition.] In: [Radiation processes in the
atmosphere and on the earth's surface.] Leningrad,
Cidrometeoizdat, pp. 261-264 (in Russian).
GARADZA, M. P. & NEZVAL', E. I. (1971) [The effect of atmospheric
transparency and cloud on the UV-radiation regime.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 316-321 (in
Russian).
GAVRILOVA, L. I., DOJNIKOV, A. S., & KORCAGINA, T. N. (1975) [UV
radiation from arc and intermittent xenon lamps.] In:
[The biological effect of UV radiation] Moscow, Nauka, pp.
226-229 (in Russian).
GENERALOV, A. A. (1971) [The ultraviolet irradiation regime for
children and adults taking sun-baths and light and air baths at
various latitudes.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 203-207 (in Russian).
GLUSCENKO, A. G., ARTJUSENCO, I. S., & BARANOVA, M. A. (1975) [Health
indicators for children of crèche age in the ultraviolet climatic
conditions of Kiev.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 171-174 (in Russian).
GORDON, D. & SILVERSTONE, H. (1976) Worldwide epidemiology of
premalignant and malignant cutaneous lesions. In: Cancer of the
skin, Philadelphia, W. B. Saunders Co., pp. 405-434.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943a) Types of
tumors induced by ultraviolet radiation and factors influencing
their relative incidence. J. Natl Cancer Inst., 3: 371-378.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943b) Pathology of
tumors of the external ear in mice induced by ultraviolet
radiation. J. Natl Cancer Inst., 2: 269-275.
GRAHAM, J. H. & HELWIG, E. B. (1965) In: Montagna, W. & Dobson, R. L.,
ed. Advances in biology of the skin, New York, Macmillan
(Pergamon), Vol. II, pp. 277-327.
GREEN, A. E. S., ed. (1960) The middle ultraviolet: Its science and
technology, New York, John Wiley and Sons, Inc.
GREEN, A. E. S. & HEIDINGER, R. A. (1978) Models relating ultraviolet
light and non-melanoma skin cancer incidence. Photochem.
Photobiol., 28: 283-291.
GREEN, A. E. S., SAWADA, T., & SHETTLE, E. P. (1975) The middle
ultraviolet reaching ground. In: Impacts of climatic change on
the biosphere. Part 1, Chapter 2, pp. 29-49 (CIAP Monograph
No. 5).
GREEN, A. E. S., FINDLEY, G. B., Jr, KLENK, K. F., WILSON, W. M., &
MO, T. (1976) The ultraviolet dose dependence of non-melanoma
skin cancer incidence. Photochem. Photobiol., 24: 353-362.
GRIFFIN, A. C. (1959) Methoxsalen in ultraviolet carcinogenesis in the
mouse. J. Invest. Dermatol, 32: 367-372.
GRIFFIN, A. C., DOLMAN, V. S., ROHLKE, E. B., ROUVART, P., & TATUM, E.
L. (1955) The effect of visible light on the carcinogenicity of
ultraviolet light. Cancer Res., 15: 523.
GRIGGS, H. C. & BENDER, M. A. (1973) Photoreactivation of ultraviolet
induced chromosomal aberrations. Science, 179: 86-88.
GROSSMAN, L. (1968) Studies on mutagenesis induced in vitro.
Photochem. Photobiol, 7: 727-735.
GUSEV, N. M. & DUNAEV, B. A. (1971) [The utilization of ultraviolet
radiation in building.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 218-221 (in Russian).
HAMILTON, L. (1973) The influence of the cell cycle on the radiation
response of early embryos. In: Balls, M. & Billet, F. F., ed.
The cell cycle in development and differentiation, Cambridge,
Cambridge University Press, pp. 229-247.
HAN, A. & SINCLAIR, W. K. (1969) Sensitivity of synchronized Chinese
hamster cells to ultraviolet light. Biophys. J., 9: 1171-1192.
HAN, A., SINCLAIR, W. K., & YU, C. K. (1971) Ultraviolet-light-induced
division delay in synchronized Chinese hamster cells.
Biophys. J., 11: 540-549.
HANAWALT, P. C. & SETLOW, R. B., ed. (1975) Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 827.
HARBER, L. C. & BAER, R. L. (1969) Classification and characteristics
of photoallergy. In: Urbach, F., ed. The biologic effects of
ultraviolet radiation, Oxford, Pergamon Press, pp. 519-526.
HARRISON, A. P. (1967) Survival of bacteria. Harmful effects of light,
with some comparisons with other adverse physical agents. Annual
Rev. Microbiol., 21: 143-156.
HAUSSER, K. W. & VAHLE, W. (1922) [The dependence of light induced
erythema and pigment formation upon the frequency (or wavelength)
of the inducing radiation.] Strahlentherapia, 13: 41-71 (in
German).
HAUSSER, K. W. & VAHLE, W. (1927) [Sunburn and suntan.] Wiss
Veröffent. Siemens-Konzern, 6: 101 (in German).
HAYNES, R. H. (1975) DNA repair and the genetic control of
radio-sensitivity in yeast. In: Hanawalt, P. C. & Setlow, R. B.,
ed. Molecular mechanisms for repair of DNA, New York & London,
Plenum Press, pp. 529-540.
HÉLENE, C. & CHARLIER, M. (1971) Photosensitized reactions in nucleic
acids. Photosensitized formation and splitting of pyrimidine
dimers. Biochimie, 53: (11-12): 1175-1180.
HELLMAN, K. B., HAYNES, K. F., & BOCKSTAHLER, L. E. (1974)
Radiation-enhanced survival of a human virus in normal and
malignant rat cells (37788). Proc. Soc. Exp. Biol. Med.,
145: 255-262.
HERLITZ, C. W., JUNDELL, I., & WAHLGREN, F. (1930) [Malignant tumours
in white mice caused by ultraviolet irradiation.] Acta Paediat.,
10: 333-347 (in German).
HEROLD, H. J. & BERNDT, H. (1968) Cancer incidence in the German
Democratic Republic. Selected tables. Neoplasma, 15: 517-522.
HILL, R. H. (1958) A radiation-sensitive mutant of E. coli. Biochim.
Biophys. Acta, 30: 636-637.
HILL, L. & EIDENOW, A. (1923) Biological action of light: I. The
influence of temperature. Proc. R. Soc. (Biol.), 95: 163-180.
HOLMBERG, M. & JONASSON, J. (1974) Synergistic effect of X-ray and UV
irradiation on the frequency of chromosome breakage in human
lymphocytes. Mutat. Res., 23: 213-221.
HSIAO, D. (1975) Impacts of climatic change on the biosphere.
Springfield, Virginia. National Technical Information Service
(CIAP Monograph No. 5).
HUANG, C. W. & GORDON, M.P. (1973) Formation of cyclobutane-type
pyrimidine dimers in RNA by sunlight. Int. J. radiat. Biol.,
23: 527-529.
HUEPER, W. C. (1941) Cutaneous neoplastic responses elicited by
ultraviolet rays in hairless rats and in their haired litter
mates. Cancer Res., 1: 402-406.
HUTCHINSON, F. (1973) The lesions produced by ultraviolet light in DNA
containing 5-bromouracil. Quart. Rev. Biophys. 6: 201-246.
IKUSHIMA, T. & WOLFF, S. (1974) UV-induced chromatid aberrations in
cultured Chinese Hamster cells after one, two or three rounds of
DNA replication. Mutat. Res., 22: 193-201.
IPPEN, H. (1969) Mechanisms of photopathological reactions. In:
Urbach, F., ed. The biologic effects of ultraviolet radiation,
Oxford, Pergamon Press, pp. 513-518.
JACOBSON, A. F. & YATVIN, M. B. (1976) Changes in the phospholipid
composition of E. coli following X and UV-irradiation. Radiat.
Res., 66: 247-266.
JAGGER, J. (1967) Introduction to research in ultraviolet
photobiology, Englewood Cliffs, N J, Prentice-Hall, Inc.,
pp. 164.
JAGGER, J. (1969) Photoreactivation. In: Hollaender, A., ed.
Radiation protection and recovery, New York, Pergamon Press,
pp. 352-377.
JAGGER, J. (1976) Effects of near-ultraviolet radiation on
microorganisms. Photochem. Photobiol., 23: 451-454.
JESSUP, J. M., HANNA, N., PALASZYNSKI, E., & KRIPKE, M. L. (1978)
Mechanisms of depressed reactivity to dinitrochlorobenzene and
ultraviolet-induced tumours during ultraviolet carcinogenesis in
BALB/c mice. Cell. Immunol., 38: 105-115.
JOHNSON, B. E. (1968) Ultraviolet radiation and lysosomes in skin.
Nature (Lond.), 219: 1258-1259.
JOHNSON, B. E., DANIELS, F., & MAGNUS, I. A. (1968) Response of human
skin to ultraviolet light. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV.
JUNG, E. G. (1976) [Photochemotherapy, background, techniques and
complications.] Stuttgart -- New York, F. K. Schattaver Verlag
(in German).
JUNG, E. G., BOHNERT, E., & ERBS, G. (1971) Wavelength dependence of
UV-induced alterations of epidermal cells in hairless albino
mice. Arch. Derm. Forsch., 241: 284-291.
KAMENECKAJA, T. M. & MITROFANOVA, G. F. (1975) [The condition of
certain neurohumoral regulatory systems following UV radiation at
different wavelengths.] In: [The biological effects of UV
radiation.] Moscow, Nauka, (in Russian).
KAO, F. & PUCK, T. T. (1969) Genetics of somatic mammalian cells. IX.
Quantitation of mutagenesis by physical and chemical agents.
J. Cell. Physiol., 74: 245-248.
KARACEVCEVA, T. V. (1971) [The role on UV radiation as part of modern
methods of treating and preventing rheumatism in children.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 154-158 (in
Russian).
KELNER, A. (1949) Photoreactivation of ultraviolet-irradiated
Escherichia coli with special reference to close-reduction
principle and to ultraviolet-induced mutation. J. Bacteriol.,
58: 511-522.
KELNER, A. (1953) Growth, respiration, and nucleic acid synthesis in
ultraviolet-irradiated and in photoreactivated E. coli.
J. Bacteriol., 65: 252-262.
KELNER, A. & TAFT, E. B. (1956) The influence of photoreactivating
light on the type and frequence of tumors induced by ultraviolet
radiation. Cancer Res., 16: 860-866.
KIEFER, J. (1971) The importance of cellular energy metabolism for the
sparing effect of dose fractionation witch electrons and
ultraviolet light. Int. J. radiat. Biol., 20: 325-336.
KIRBY-SMITH, J. S., BLUM, H. F., & GRADY, H. G. (1942) Penetration of
ultraviolet radiation into skin, as a factor in carcinogenesis.
J. Natl Cancer Inst., 2: 403-412.
KOCH, H. R. (1967) [Photochemotherapy and cataract formation.]
Dermatosen im Beruf und Umwelt., 26; 162-165 (in German).
KOLLER, L. R. (1965) Ultraviolet radiation, 2nd ed., New York, John
Wiley and Sons.
KRAEMER, K. H., DE WEERD-KASTELEIN, E. A., ROBBINS, J. H., KEIJZER,
W., BARRETT, S. F., PETINGA, R. A., & BOOTSMA, D. (1975) Five
complementation groups in xeroderma pigmentosum. Mutat. Res.,
33: 327-340.
KRIPKE, M. L. (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J. Natl Cancer Inst., 53: 1333-1336.
KRIPKE, M. L. (1976) Target organ for a systemic effect of ultraviolet
radiation. Photochem. Photobiol., 24: 599-600.
KRIPKE, M. L. (1977) Latency, histology, and antigenicity of tumors
induced by ultraviolet light in three inbred mouse strains.
Cancer Res., 37: 1395-1400.
KRIPKE, M. L. & FISHER, M. S. (1976) Effect of UV light on the host
response to UV-induced tumirs. Proceedings of the 4th Annual
Meeting, American Society of Photobiology, Denver, CO, February
16-20.
KRIPKE, M. L. & FISHER, M. S. (1976) Immunologic parameters of
ultraviolet carcinogenesis. J. Natl Cancer Inst., 57: 211-215.
KRIPKE, M. L., LOFGREEN, J. S., BEARD, J., JESSUP, J. M., & FISHER, M.
S. (1977) In vivo immune responses of mice during
carcinogenesis by ultraviolet irradiation. J. Natl Cancer Inst.,
59: 1227-1230.
LABORDUS, V. (1970) The effect of ultraviolet light on developing eggs
of Lymnaea stagnails (mollusca, pulmonata) I, II, III. Proc.
K. Ned. Akad. Wet. Amsterdam, C, 366-493.
LAMOLA, A. A. & YAMANE, T. (1967) Sensitized photodimerization of
thymine in DNA. Proc. Natl Acad. Sci., 58: 443-446.
LANCASTER, H. O. (1956) Some geographical aspects of the mortality
from melanoma in Europeans. Med. J. Aust., 1: 1082-1087.
LATARJET, R. (1972) Interaction of radiation energy with nucleic
acids. Curr. Top. Radiat. Res. Q., 8: 1-38.
LATARJET, R. & ZAJDELA, F. (1974) Effet inhibitcur de la caféine sur
l'induction de cancers cutanés par les rayons ultraviolet chez la
souris. C. R. Acad. Sci. (Paris), 277: 1073-1076.
LAZAREV, D. N. & SOKOLOV, M. V. (1971) [The measurement of ultraviolet
radiation in units of effect.] In: [UV Radiation.] Moscow,
Medicina, pp. 328-333 (in Russian).
LAZAREV, D. N. & SOKOLOV, M. V. (1974) [Ultraviolet radiation.
Quantities and units. Terms and definitions. Technical
material for guidance.] Puscino, Institute of Biophysics of
the Academy of Sciences of the USSR, pp. 1-8 (in Russian).
LAZAREV, D. M., BESSALOVA, E. S., BALINA, G. P., & ERMILOVA, M. G.
(1975) [A UV biological photometer.] In: [The biological effects
of UV radiation.] Moscow, Nauka, pp. 244-246 (in Russian).
LEE, J. A. H. & MERRILL, J. M. (1970) Sunlight and the aetiology of
malignant melanoma: A Synthesis. Med. J. Aust., 2: 840-841.
LEE, H. H. & PUCK, T. T. (1960) The action of ultraviolet radiation on
mammalian cells as studied by single-cell technique. Radiat.
Res., 12: 340-348.
LEHMANN, A. R. (1972) Postreplication repair of DNA in
ultraviolet-irradiated mammalian cells. Mol. Biol.,
66: 319-337.
LEHMANN, A. R., KIRK-BELL, S., ARLETT, C. F., PATERSON, M. C., LOHMAN,
P. H. M., WEERD-KASTELEIN de, E. A., & BOOTSMA, D. (1975)
Xeroderma pigmentosum cells with normal levels of excision repair
have a defect in DNA synthesis after UV irradiation. Proc. Natl
Acad. Sci., 72: 219-223.
LEWIS, N. F. & KUMTA, U.S. (1972) Evidence for extreme UV resistance
of Micrococcus sp. NCTC 10785. Biochem. biophys. Res. Commun.,
47: 1100-1105.
LIPSON, R. L. & BALDES, E. J. (1960) Photosensitivity and heat. Arch.
Dermatol., 82: 517-520.
LOOMIS, W. F. (1970) Rickets. Sci. Am., 233: 76-91.
LUKIESH, M., HOLLADAY, L. L., & TAYLOR, A. H. (1939) Erythemal and
tanning effectiveness of UV energy. Gen. Electr. Rev.,
42: 274-278.
LYTLE, C. D. (1971) Host-cell reactivation in mammalian cells. I.
Survival or ultraviolet-irradiated herpes virus in different
cell-lines. Int. J. radiat. Biol., 19: 329-337.
LYTLE, C. D., HELLMAN, K. B., & TELLES, N. C. (1970) Enhancement of
viral transformation by ultraviolet light. Int. J. radiat.
Biol., 18: 397-300.
MACDONALD, E. J. (1976) Epidemiology of skin cancer 1975. In:
Neoplasms of the skin and malignant melanoma, Chicago, Year
Book Publishers, Inc., pp. 27-42.
MACKIE, B. S. & MCGOVERN, V. J. (1958) The mechanism of solar
carcinogenesis: A study of the role of collagen degeneration of
the dermis in the production of skin cancer. Arch. Dermatol.
Syph., 78: 218-244.
MAGNUS, I. A. & JOHNSON, B. E. (1965) Cited by Johnson, B. E.,
Daniels, F. Jr, & Magnus, I. A. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV,
pp. 139-202.
MAGNUS, K. (1973) Incidence of malignant melanoma of the skin in
Norway, 1955-1970. Cancer, 32: 1275-1286.
MAGNUS, K. (1977) Incidence of malignant melanoma of the skin in the
five nordic countries: significance of solar radiation. Int. J.
Cancer, 20: 477-485.
MALIK, M. O. A., MIDAYTALLA, A., DAOUC, E. H., & EL HASSAN, A. M.
(1974) Superficial cancer in the Sudan. A study of 1225 primary
malignant superficial tumours, Br. J. Cancer, 30: 355-364.
MATHEWS, M. M. & KRINSKY, N. (1965) The relationship between
carotenoid pigments and resistance to radiation in
non-photosynthetic bacteria. Photochem. Photobiol., 4: 813-817.
MCGOVERN, V. J. (1977) Epidemiological aspects of melanoma: A review.
Pathology, 9: 233-241.
MCGOVERN, V. J. & MACKIE, B. S. (1959) The relationship of solar
radiation to melanoblastoma. Aust. N. Z. J. Surg., 28: 257-262.
MEYER, A. E. H. & SEITZ, E. O. (1949) [Ultraviolet Radiation.]
Berlin, Walter de Gruyter & Co. (in German).
MILLS, L. F., LYTLE, C. D., ANDERSEN, F. A., HELLMAN, K. B., &
BOCKSTAHLER, L. H. (1975) A review of biological effects and
potential risks associated with ultraviolet radiation as used in
dentistry. pp. 1-27 (DHEW Publication (FDA) 76-8021).
MISHIMA, Y. & WIDLAN, S. (1967) Enzymatically active and inactive
melanocyte populations and ultraviolet irradiation: Combined
dopapremelanin reaction and electron microscopy. J. invest.
Dermatol., 49: 273.
MIYAJI, T. (1962) Skin cancer in Japan. A nationwide 5 year survey.
In: Urbach, F., ed. The biology of cutaneous cancer,
Washington, DC, US Government Printing Office, pp. 515-70
(Monograph No. 10, National Cancer Institute).
MIYAZAKI, H., KAWADA, A., TAKAKI, Y., SATO, T., & MASUTANI, M. (1968)
Influence of ultraviolet light on pigmentation of hairless mouse
epidermis. Jap. J. Dermatol., Ser. B, 78: 605.
MORENO, F. (1969) Contribution à l'étude de la létalité et de la
stérilité induites chez Drosophita melanogaster par irradiation
UV des cellules polaires de l'oeuf. I. Action létale, II. Action
stérilisante. Int. J. radiat. Biol., 16: 441-465.
MOUSTACCHI, E., WATERS, R., HEUDE, M., & CHANET, R. (1975 The present
status of DNA repair mechanisms in UV irradiated yeast taken as a
model eukaryotic system. In: Nygaard, O., Adler, H., & Sinclair,
W., ed. Radiation research: Biomedical, chemical and physical
perspectives, New York, Academic Press, pp. 632-650.
MOVSHOVITZ, M. & MODAN, B. (1973) Role of sun exposure in the etiology
of malignant melanoma: Epidemiologic inference. J. Natl Cancer
Inst., 51: 777-779.
MULAY, D. M. (1962) Skin Cancer in India. In: Urbach, F., ed.
The biology of cutaneous Cancer, Washington, DC, US Government
Printing Office, pp. 215-224 (Monograph No. 10, National Cancer
Institute).
MURPHY, T. M. (1975a) Inactivation of tobacco mosaic virus ribonucleic
acid by near- and middle-ultraviolet light: Sensitization by
sulfanilamide and chlortetracycline. Biochem. biophys. Res.
Commun., 65: 1108-1114.
MURPHY, T. M. (1975b) Effects of UV-B radiation on nucleic acids. In:
Impacts of climatic changes on the biosphere, pp. 19-40 (CIAP
Monograph No. 5).
MUSAJO, L., RODIGHIERO, G., CAPORALE, G., DALL'ACQUA, F., MARCIANI,
S., BORDIN, F., BACCICHETTI, F., & BEVILACQUA, R. (1974)
Photoreactions between skin-photosensitizing furocoumarins and
nucleic acids. In: Fitzpatrick, T. B., Pathak, M. A., Harber, L.
C., Seiji, M., & Kukita, A., ed. Sunlight and Man, Tokyo,
University of Tokyo Press, pp. 369-387.
NAKAMURA, K. & JOHNSON, W. C. (1968) Ultraviolet light-induced
connective tissues changes in rat skin: A histopathologic and
histochemical study. J. invest. Dermatol., 51: 253-258.
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1973) UV
photocarcinogenesis: Modification by anti-lymphocytic serum or
6-marcaptopurine. Proc. Am. Assoc. Cancer Res., 14: 46
(Abstract 182).
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1976) Modification of
photocarcinogenesis by two immunosuppressive agents. Cancer
Lett., 1: 243-247.
NILENDER, R. A. & GAVANIN, V. A. (1971) [Artificial sources of
ultraviolet radiation.] In: [UV Radiation.] Moscow, Medicina,
pp. 348-352 (in Russian).
NIOSH (1975) Ultraviolet transfer standard detectors and evaluation
and calibration of NIOSH UV hazard monitor. Cincinnati, OH, US
Department of Health, Education, and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and
Health, Division of Laboratories and Criteria Development, 12 pp. (HEW
Publication No. (NIOSH) 75-131).
NORBURY, K. C., KRIPKE, M. L., & BUDMEN, M. B. (1977) In vitro
reactivity of macrophages and lymphocytes from
ultraviolet-irradiated mice. J. Natl Cancer Inst.,
59: 1231-1235.
OETTLE, A. G. (1962) Skin cancer in Africa. In: Urbach, F, ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 197-214 (Monograph No. 10, National Cancer
Institute).
ORR, J. W. (1938) The changes antecedent to tumor formation during the
treatment of mouse skin with carcinogenic hydrocarbons.
J. Pathol. Bacteriol., 46: 495-515.
OWENS, D. W., KNOX, J. M., HUDSON, H. T., RUDOLPH, A. H., & TROLL, D.
(1977) Influence of wind on chronic ultraviolet light-induced
carcinogenesis. Br. J. Dermatol., 97: 285.
PAINTER, R. B. (1975) Repair in mammalian cells: Overview. In:
Hanawalt, P. C. & Setlow, R. B., ed. Molecular mechamisms for
repair of DNA, New York & London, Plenum Press, pp. 595-600.
PARRINGTON, J. M. (1972) Ultraviolet-induced chromosome aberration and
mitotic delay in human fibroblast cells. Cytogenetics,
11: 117-131.
PARRISH, J. A., FITZPATRICK, T. B., TANNENBAUM, L., & PATHAK, M. A.
(1974) Photochemotherapy of psoriasis with oral methoxsalen and
long wave ultraviolet light. New Engl. J. Med. 291: 1207-1212.
PATHAK, M. A., KRAMER, D. M., & FITZPATRICK, T. B. (1974) Photobiology
and photochemistry of furocoumarins (psoralens). In: Fitzpatrick,
T. B., Pathak, M. A., Harber, L. C., Seiji, M., & Kukita, A., ed.
Sunlight and Man, Tokyo, University of Tokyo Press,
pp. 335-368.
PETTIJOHN, D. & HANAWALT, P. (1964) Evidence for repair-replication of
ultraviolet damaged DNA in bacteria. J. mol. Biol., 9: 395-410.
PITTS, D. G. (1970) A comparative study of the effects of ultraviolet
radiation on the eye. Am. J. Optom., 47: 535-546.
PITTS, D. G. & CULLEN, A. P. (1977) Ocular ultraviolet effects from
300 nm to 400 nm: A preliminary report. Cincinatti, OH, US
Department of Health, Education and Welfare, National Institute
of Occupational Safety and Health, Division of Biomedical and
Behavioral Science (NIOSH Contract CDC-99-74-12).
PITTS, D. G. & KAY, K. R. (1969) The photo-ophthalmic threshold for
the rabbit. Am. J. Optom., 46: 561-572.
POLLARD, E. C. (1974) Cellular and molecular effects of solar
ultraviolet radiation. Photochem. Photobiol., 20: 301-308.
PROKOPENKO, Ju, I. (1976) [Some data on the mechanisms of protective
effect of UV-radiation.] Gig. i Sanit., 1: 100-102 (in
Russian).
PROKOPENKO, Ju, I. & ZABALUEVA, A. P. (1975) [Activation of
sanogenesis mechanisms with the help of UV-irradiation.] In:
[The biological effect of UV-radiation,] Moscow, Nauka,
pp. 156-159 (in Russian).
PUTSCHAR, W. & HOLTZ, F. (1930) [Induction of skin cancer in rats by
prolonged ultraviolet irradiation.] Z. Krebsforsch.,
23: 219-232 (in German).
QUEVEDO, W. C. Jr & SMITH, J. A. (1963) Studies on radiation-induced
tanning of skin. Ann. N. Y. Acad. Sci., 100: 364.
QUEVEDO, W. C. Jr, BRENNER, R. M., & KECHIJIAN, P. (1963) Melanocyte
performance during radiation induced tanning of murine skin.
Proceedings of the XVI International Congress on Zoology, 2: 306.
QUEVEDO, W. C. Jr, SZABO, G., VIRKS, J., & SINESI, S. J. (1965)
Melanocyte populations in UV-irradiated human skin. J. invest.
Dermatol., 45: 295.
RADMAN, M. (1975) SOS repair hypothesis: Phenomenology of an inducible
DNA repair which is accompanied by mutagenesis. In: Hanawalt, P.
C. & Setlow, R. B., ed. Molecular mechanisms for repair of DNA,
New York & London, Plenum Press, pp. 355-367.
RAPPAPORT, H., PIETRA, G., & SHUBIK, P. (1961) The induction of
melanotic tumors resembling cellular blue nevi in the Syrian
white hamster by cutaneous application of 7,
12-Dimethylbenz(a)anthracene. Cancer Res., 21: 661-666.
RASMUSSEN, R. E. & PAINTER, R. B. (1964) Evidence for repair of
ultraviolet damaged deoxyribonucleic acid in cultured mammal
cells. Nature (Lond.), 203: 1360-1362.
RAUTH, A. M. (1970) Effects of ultraviolet light on mammalian cells in
culture. Curr. Top. radiat. Res., 6: 195-248.
RAUTH, A. M. & DOMON, M. (1973) Potentiation of ultraviolet light
damage in mouse L cells by 1-cyclohexyl-3-(2-morpholinyl-4-et
carbodiimide metho-p-toluene sulphonate (CMEC). Int. J. radiat.
Biol., 24: 189-198.
RESNICK, M. A. (1969) Genetic control of radiation sensitivity in
Saccharomyces cerevisiae. Genetics, 62: 519-531.
REYNOLDS, J. (1954) The epidermal melanocytes of mice. J. Anat.,
88: 45.
ROBERTSON, D. F. (1969) Long term field measurements of erythemally
effective natural ultraviolet radiation. In: Urbach, F., ed.
The biologic effects of ultraviolet radiation, Pergamon
Press, Oxford, pp. 433-436.
ROBERTSON, D. F. (1972) Solar ultraviolet radiation in relation to
human sunburn and skin cancer. Thesis, Australia, University of
Queensland.
ROBERTSON, D. F. (1975) Calculated sunburn responses, In: Impacts of
climatic change on the biosphere. Springfield, Virginia,
National Technical Information Service, Part 1, Chapter 2,
Appendix J. (CIAP Monograph 5).
ROFFO, A. H. (1934) Cancer et soleil. Carcinomes et sarcomes provoqués
par l'action du soleil in toto. Bull. Assoc. Fr. Etud. Cancer,
53: 59.
ROMMELAERE, J., SUSSKIND, M., & ERRERA, M. (1973) Chromosome and
chromatid exchanges in Chinese hamster cells. Chromosoma,
41: 243-257.
RUNDEL, R. D. & NACHTWEY, D. S. (1978) Skin cancer and ultraviolet
radiation. Photochem. Photobiol., 28: 345-356.
RONGE, H. E. (1948) Ultraviolet irradiation with artificial
illumination Acta Phys. Scand., 15 (Suppl. 49): 1-191.
RUPERT, C. S. (1975) Enzymatic photoreactivation: Overview. In:
Hanawalt, P. C. & SETLOW, R. B. ed. Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 73-87.
RUPP, W. D. & HOWARD-FLANDERS, P, (1968) Discontinuities in the DNA
synthesized in an excision-defective strain of E. coli
following ultraviolet irradiation. J. Mol. Biol., 31: 291-304.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1941) Carcinogenesis by
ultraviolet rays with reference to wavelength and energy. Arch.
Pathol., 371: 135-148.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1942) Nonadditive effect
of UV light and other carcinogenic procedures. Cancer Res.,
2: 183-188.
RUSTAD, R. C. (1972) Techniques for the analysis of radiation-induced
mitotic delay in synchronously dividing sea urchin eggs. In:
Whitson, G. L., ed. Concepts in radiation cell biology, New
York & London, Academic Press, pp. 153-181.
SAMS, W. M. Jr, SMITH, J. G., & BURK, P. G. (1964) The experimental
production of elastosis with ultraviolet light. J. invest.
Dermatol., 43: 467.
SATO, T. (1971) Pigmentation induced by ultraviolet irradiation in
hairless mice with particular references to epidermal dendritic
cells. Jap. J. Dermatol. Ser. A, 81: 488.
SATO, T. & KAWADA, A. (1972) Mitotic activity of hairless mouse
epidermal melanocytes: Its role in the increase of melanocytes
during ultraviolet radiation. J. invest. Dermatol.,
58: 392-395.
SATO, T. & KAEADA, A. (1972) Uptake of tritiated thymidine by
epidermal melanocytes of hairless mice during ultraviolet light
radiation. J. invest. Dermatol., 58: 71.
SAUERBIER, W. (1976) UV damage at the transcriptional level. Advances
in radiation biology, 6: 50-106.
SAYENKO, A. J. (1968) A method for studying morbidity from
precancerous conditions and the question as to frequency of their
occurrence on the territory of the Kirghiz Soviet Socialist
Republic. Neoplasma, 15: 565-571.
SCHULZE, R. (1970) [Global Radiation Climate.] Wiss. Forschungsber.,
72: 220 pp. (in German).
SCOTTO, J., KOPF, A. W., & URBACH, F. (1974) Non-melanoma skin cancer
among Caucasians in four areas of the United States. Cancer,
34: 1333-1338.
SCOTTO, J., FEARS, T. R., & GORI, G. B. (1976) Measurements of
ultraviolet radiation in the United States and comparisons with
skin cancer data, National Cancer Institute (DHEW
No. (NIH) 76-1029).
SEIDL, E. (1969) Blitzgerät therapy. In: Urbach, F., ed. The biologic
effects of ultraviolet radiation, Oxford, Pergamon Press,
pp. 663-672.
SETLOW, R. B. (1968) The photochemistry, photobiology, and repair of
polynucleotides. Prog. N. A. Res. Mol. Biol., 8: 257-295.
SETLOW, R. B. (1973) The relevance of photobiological repair.
An. Acad. Bras. Ciencas, 45: 215-220.
SETLOW, R. B. (1974) The wavelengths in sunlight effective in
producing skin cancer: A theoretical analysis. Proc. Natl Acad.
Sci., 71: 3363-3366.
SETLOW, R. B. & CARRIER, W. L. (1964) The disappearance of thymine
dimers from DNA: An error-correcting mechanism. Proc. Natl Acad.
Sci., 51: 226-231. p. 34.
SETLOW, J. K. & DUGGAN, D. E. (1964) The resistance of Micrococcus
radiodurans to ultraviolet radiation. Biochim. Biophys. Acta,
87: 664-668.
SETLOW, J. K. & SETLOW, R. B. (1963) Nature of the photoreactivability
ultraviolet lesion in deoxyribonucleic acid. Nature (Lond.),
197: 560-562. p 36.
SHUBIK, P., PIETRA, G., & DELLA PORTA, G. (1960) Studies of skin
carcinogenesis in the Syrian golden hamster. Cancer Res.,
20: 1.
SILVERSTONE, H. & SEARLE, J. H. A. (1970) The epidemiology of skin
cancer in Queensland: The influence of phenotype and environment.
Br. J. Cancer, 24: 235-252.
SKREB, Y., HORVAT, D., & EGER, M. (1972) Modifications of
radiosensitivity in nucleate and anucleate amoeba fragments. In:
Bonotto, S., Goutier, R., Kirchmann, R., & Maisin, J.-R., ed.
Biology and radiobiology of anucleate systems I. New York,
Academic Press, pp. 67-82.
SMITH, K. C. (1974) The cellular repair of radiation damage. In:
Fitzpatrick, T. B., Pathak, M. A., Harber, L. C., Seiji M., &
Kukita, A., ed. Sunlight and Man, Tokyo, University of Tokyo
Press, pp. 67-77.
SMITH, K. C. (1975) The radiation-induced addition of proteins and
other molecules to nucleic acids. In: Wang, S. Y., ed.
Photochemistry and photobiology of nucleic acids, New York,
Academic Press, Vol. II, pp. 187-218.
SMITH, K. C. & HANAWALT, P. C. (1969) Molecular photobiology.
Inactivation and recovery, New York & London, Academic Press.
SOFFEN, G. A. & BLUM, H. F. (1961) Quantitative measurements of cell
changes in mouse skin following a single dose of ultraviolet
light. J. cell. comp. Physiol., 58: 81-96.
SOKOLOV, M. V. (1975) [Standardization of UV photometry.] In:
[The biological effects of ultraviolet radiation.] Moscow,
Nauka, pp. 232-235 (in Russian).
SOKOLOV, M. V. (1976) [Topical questions of UV biophotometry.]
Svetotecnika, 2: 20-22 (in Russian).
SOZIN, D. S. (1975) [Low pressure fluorescent lamps with combined
radiation.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 225-226 (in Russian).
SPIKES, J. D., KRIPKE, M. L., CONNOR, R. J., & EICHWALD, E. J. (1977)
Time of appearance and histology of tumors induced in the dorsal
skin of C3Hf mice by ultraviolet radiation from a mercury arc
lamp. J. Natl Cancer Inst. 59: 1637-1643.
STATE STANDARDS (1964) [Recommendations for the prevention of
ultraviolet deficiency.] Moscow Nauka, 50 pp. (in Russian).
STATE STANDARDS (1976a) [Regulations for the design and operation of
artificial UV-irradiation installations in industrial
undertakings.] In: [Regulations on the design of electrical
engineering installations for industry.] Energija 6: pp. 30-39
(in Russian).
STATE STANDARDS (1976b) [USSR building norms and regulations.]
Strijizdat, Moscow, p. 20 (SNIP 11-60-75) (in Russian).
STENBÄCK, F. (1969) Promotion in the morphogenesis of chemically
inducible skin tumors. Acta path. microbiol. Scand. Suppl.,
208: 1-116.
STENBÄCK, F. (1975a) Cellular injury and cell proliferation in skin
carcinogenesis by UV light. Oncology, : 31: 61-65.
STENBÄCK, F. (1975b) Species-specific neoplastic progression by
ultraviolet light on the skin of rats, guinea pigs, hamsters and
mice. Oncology, 31: 209-225.
STENBÄCK, F. (1975) Ultraviolet light irradiation as initiating agent
in skin tumor formation by the two-stage method. Europ. J.
Cancer, 11: 241-246.
STENBÄCK, F. (1978) Life history and histopathology of ultraviolet
light-induced skin tumors. In: International Conference on
Ultraviolet Carcinogenesis. Washington, DC, US Government
Printing Office, pp. 57-70 (Monograph No. 50, National Cancer
Institute).
SUTHERLAND, B. M. (1974) Photoreactivating enzyme from human
leukocytes. Nature (Lond.), 109-112.
SVIDERSKAJA, T. A. (1971) [Some experimental data on the conditions
accompanying an intensification of blastomogenic effect.] In:
[UV Radiation.] Moscow, Medicina, pp. 260-264 (in Russian).
SWANBECK, G. & HILLSTRöM, L. (1971) Analysis of etiological factors of
squamous cell skin cancer of different locations. Acta
Dermatovener, 51: 151-156.
TALANOVA, I. K. & ZABALUEVA, A. P. (1972) [Evaluation of the extent to
which children are provided with UV radiation in middle and
northern latitudes.] In: [Questions of experimental and clinical
spa treatment and physiotherapy.] 20: 323-329 (in Russian).
TALANOVA, I. K., KARACEVCEVA, T. V., & IVANOV, V. G. (1975) [The
combination of physical methods of prophylaxis and specific
immunization in children.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 116-120 (in Russian).
TODD, P. & HAN, A. (1976) Ultraviolet light induced
DNA-to-protein-cross-linking in mammalian cells. In: Smith, K.
C., ed. Aging, carcinogenesis and radiation-biology: The role of
nucleic acid addition reactions, New York & London, Plenum
Press, pp. 83-104.
TROSKO, J. E. & CHU, E. H. (1973) Inhibition of repair of UV-damaged
DNA by caffeine and mutation induction in Chinese hamster cells.
Chem. Biol. Interactions, 6: 317-332.
TYRRELL, R. M. (1973) Induction of pyrimidine dimers in bacterial DNA
by 365 nm radiation Photochem. Photobiol., 17: 69-73.
URBACH, F. (1969) The biologic effect of ultraviolet radiation.
Oxford, Pergamon Press, pp. 704.
URBACH, F., ROSE, D. B., & BONNEM, M. (1972) Genetic and environmental
interactions in skin carcinogenesis. In: Environment and skin
cancer, Baltimore, Maryland, Williams & Wilkins Co.,
pp. 355-371.
VARGHESE, A. J. (1973) Properties of photoaddition products of thymine
and cysteine. Biochemistry, 12 (14): 2725-2730.
VERHOEFF, F. H., BELL, L., & WALKER, C. B. (1916) The pathological
effects of radiant energy on the eye: An experimental
investigation with a systematic review of the (literature. Proc.
Am. Acad. Arts Sci., 51: 630-818.
VITALIANO, P. P. (1978) The use of logistic regression for modelling
risk factors in the development of nonmelanoma skin cancer.
Am. J. Epidemiol., 108: 402-414.
WANG, R. J. (1975) Lethal effect of »daylights» fluorescent light on
human cells in tissue-culture medium. Photochem. Photobiol.,
21: 373-375.
WANG, R. J., STOIEN, J. D., & LANDA, F. (1974) Lethal effect of
near-ultraviolet irradiation on mammalian cells in culture.
Nature (Lond.), 247: 43-45.
WATERHOUSE, J., MUIR, C., CORREA, P., & POWELL, J. (1976) Cancer
incidence in five continents, Lyons, IARC (Vol. III).
WHITSON, G. L. (1972) Radiation-induced biochemical changes in
protozoa. In: Whitson, G. L., ed. Concepts in radiation cell
biology, New York, Academic Press, pp. 123-152.
WINKELMAN, R. K., BALDES, E. J., & ZOLLMAN, P. E. (1960) Squamous cell
tumors induced in hairless mice with ultraviolet light.
J. invest. Dermatol., 34: 131-138.
WINKELMANN, R. K., ZOLLMAN, P. E., & BALDES, E. J. (1965) Squamous
cell carcinoma produced by ultraviolet light in hairless mice.
J. invest. Dermatol., 40: 217-224.
WITKIN, E. M. (1969) Ultraviolet-induced mutation and DNA repair.
Ann. Rev. Genet., 3: 525-552.
WITKIN, E. M. (1976) Ultraviolet mutagenesis and inducible DNA repair
in Escherichia coli. Bacteriol. Rev., 40: 869-907.
WOODCOCK, A. & MAGNUS, I. A. (1976) The sunburn cell on mouse skin:
Preliminary quantitative studies on its production. Br. J.
Dermatol., 95: 459-468.
YEH, S. (1962) Relative incidence of skin cancer in Chinese in Taiwan:
With special reference to arsenical cancer. In: Urbach, F., ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 81-108 (Monograph No. 10, National Cancer
Institute).
ZACKHEIM, H. S. (1964) Comparative cutaneous carcinogenesis in the
rat. Differential response to the application of anthramine,
methylcholanthrene, and dimethylbenzanthacene. Oncology,
17: 236-246.
ZIGMAN, S., SCHULTZ, J. B., & YULO, T. (1973) Possible roles of near
UV light in the cataractous process. Exp. eye Res.,
15: 201-208.
ZILOV, JU.D. (1971) [The prophylactic irradiation of children and
adolscents living in different climatic zones of the Soviet
Union.] In: [Ultraviolet radiation.] Moscow, Medicina,
pp. 237-241 (in Russian).
ZIVILOVA, N. D., ISTJUFEEV, V. A., SMIRNOV, E. S., & LEVITIN Ju.S.
(1975) [The UFM-meter and the UFB-72 laktmeter.] In: [The
biological effect of UV-radiation.] Moscow, Nauka, pp. 247-249
(in Russian).
ZWEIG, A. & HENDERSON, W. A. Jr (1976) A photochemical mid-ultraviolet
dosimeter. Photochem. Photobiol., 24: 543-549.
 UVR is not only present in the direct solar beam, but also
reaches the earth's surface as diffuse radiation, the solar UVR being
scattered within the atmosphere. Under hazy and cloudy conditions this
component can be very important.
2.1.1.1 Influence of stratospheric constituents
The solar UVR flux that reaches the surface of the earth is a
function of the solar spectral irradiance at the upper surface of the
atmosphere and the absorption and scattering of UVR by the atmosphere.
In the stratosphere, the spectral irradiance of the sun is mainly
absorbed by ozone. Molecular ozone has a strong absorption band in the
UVR centred at 250 nm and extending beyond 350 nm. The absorption
coefficient falls off rapidly with wavelength and the attenuation of
the incident solar flux is, therefore, a strong function of
wavelength. In order to determine the impact on man of a change in the
amount of ozone, these solar irradiances must be weighted with a
suitable response function for human skin.
The percentage increase in erythema-producing UVR is
approximately 2 times the percentage decrease in ozone (Schulze,
1970).
2.1.1.2 Influence of clouds, haze, and smog
In addition to the absorption of the incident solar irradiance by
ozone there is also molecular scattering by air and aerosols;
reflection, scattering, and attenuation by clouds, haze, and smog near
the ground; and reflection from the ground. The computation of the
direct and diffuse radiation that impinges on a surface near the
ground is well understood in theory, but difficult to carry out in
practice, particularly if clouds, haze, and pollution are present
(Belinsky & Andrienko, 1974; Green et al., 1975).
The major factors affecting the amount of UVR in the 290-320 nm
range that will reach the earth's surface are solar elevation (thus,
season and latitude), and the type and amount of cloud cover and
aerosols. The importance of atmospheric conditions and their
variability from hour to hour and from day to day have been measured
by Bener (1972) and Berger et al. (1975) (Fig. 2). Data on the effect
of clouds, haze, and albedo have also been published by the Moscow
State University group (Garadza, 1974).
UVR is not only present in the direct solar beam, but also
reaches the earth's surface as diffuse radiation, the solar UVR being
scattered within the atmosphere. Under hazy and cloudy conditions this
component can be very important.
2.1.1.1 Influence of stratospheric constituents
The solar UVR flux that reaches the surface of the earth is a
function of the solar spectral irradiance at the upper surface of the
atmosphere and the absorption and scattering of UVR by the atmosphere.
In the stratosphere, the spectral irradiance of the sun is mainly
absorbed by ozone. Molecular ozone has a strong absorption band in the
UVR centred at 250 nm and extending beyond 350 nm. The absorption
coefficient falls off rapidly with wavelength and the attenuation of
the incident solar flux is, therefore, a strong function of
wavelength. In order to determine the impact on man of a change in the
amount of ozone, these solar irradiances must be weighted with a
suitable response function for human skin.
The percentage increase in erythema-producing UVR is
approximately 2 times the percentage decrease in ozone (Schulze,
1970).
2.1.1.2 Influence of clouds, haze, and smog
In addition to the absorption of the incident solar irradiance by
ozone there is also molecular scattering by air and aerosols;
reflection, scattering, and attenuation by clouds, haze, and smog near
the ground; and reflection from the ground. The computation of the
direct and diffuse radiation that impinges on a surface near the
ground is well understood in theory, but difficult to carry out in
practice, particularly if clouds, haze, and pollution are present
(Belinsky & Andrienko, 1974; Green et al., 1975).
The major factors affecting the amount of UVR in the 290-320 nm
range that will reach the earth's surface are solar elevation (thus,
season and latitude), and the type and amount of cloud cover and
aerosols. The importance of atmospheric conditions and their
variability from hour to hour and from day to day have been measured
by Bener (1972) and Berger et al. (1975) (Fig. 2). Data on the effect
of clouds, haze, and albedo have also been published by the Moscow
State University group (Garadza, 1974).
 2.1.1.3 Amount of sea level solar ultraviolet radiation in the
biologically active UVR spectrum
The spectroradiometric measurements of global and sky UVR
performed by Belinskij & Garadza (1962), Belinskij et al. (1968),
Garadza (1965, 1967), Garadza & Nezval, (1971), Bener (1972), and
Belinsky & Adrienko (1974) have served as the standards for all modern
calculations, model systems, and comparisons with other measuring
devices (Green et al., 1976). The global distribution of UVR has been
illustrated by Schulze (1970) (Fig. 3).
2.1.1.3 Amount of sea level solar ultraviolet radiation in the
biologically active UVR spectrum
The spectroradiometric measurements of global and sky UVR
performed by Belinskij & Garadza (1962), Belinskij et al. (1968),
Garadza (1965, 1967), Garadza & Nezval, (1971), Bener (1972), and
Belinsky & Adrienko (1974) have served as the standards for all modern
calculations, model systems, and comparisons with other measuring
devices (Green et al., 1976). The global distribution of UVR has been
illustrated by Schulze (1970) (Fig. 3).
 Long-term results of actual measurements with an analogue
integrating UVR dosimeter, having an action spectrum similar to that
of skin erythema, are also available (Scotto et al., 1976). From these
measurements, it is apparent that there are daily fluctuations,
throughout the year, at each location. Weekly patterns are less
erratic, and seasonal variations depend on the latitude. The solar UVR
that causes skin erythema reaches a maximum intensity between 10h00
and 14h00. About 60% of the daily dose reaches the ground between
10h00 and 14h00, and 80% between 9h00 and 15h00 (Fig. 2). Similar
observations over a shorter period of time, using an instrument based
on a filtered selenium photocell, have given virtually the same
results (Garadza, 1965).
2.1.2 Artificial sources
Any material heated to temperatures exceeding 2500 K begins to
emit UVR. For practical purposes, sources emitting significant amounts
of biologically effective UVR can be classified into confined gas
discharge arcs, fluorescent lamps, and incandescent sources. It should
be noted that any UVR source emitting intense radiation below 260 nm
will produce ozone, which has to be removed to prevent any health
hazard.
2.1.2.1 Gas discharge arcs
The optical spectra produced by arcs depend on the nature of the
gas molecules through which the current discharge takes place, its
pressure, and the electrical conditions in the discharge.
Direct gas discharge arcs are widely used for the generation of
UVR. Generally, they differ from each other in such respects as the
type of gas, pressure, starting mechanisms, lamp shape, reflector
systems, electrodes, etc. (Andreev et al., 1975). As there is such a
wide variety of these arcs, it is not possible to describe them all.
However, the most important types are listed below.
Low pressure mercury arcs. These vapour lamps emit several
narrow UV bands. Most of the emitted power is of a wavelength of
253.7 nm, which is near the maximum for germicidal effectiveness,
hence its usefulness in the control of microorganisms.
High pressure mercury arcs. These vapour arcs (operating at
20-100 atmospheres, i.e., 200-1000 kPa, and usually encased in quartz
envelopes) emit much broader and more intense bands of UVR than low
pressure mercury arcs at wavelengths of 254, 297, 303, 313 and 365 nm.
They are extensively used in industry in photochemical reactors and in
printing. Other uses include phototherapy of skin diseases (Meyer &
Seitz, 1949; Nilender & Gavanin, 1971).
High pressure xenon arcs. These arc lamps may operate at very
high internal pressures, and have the advantage that the spectral
distribution of their radiant output shows a continuum similar to that
of the sun above the stratosphere. Their output is quite constant over
long periods of time, and they are available in a variety of
configurations. The major problem with xenon arcs is their very high
emission in the near infrared region (Gavrilova et al., 1975). Their
uses are similar to those of mercury arcs.
Flash tubes. Another form of gas discharge arc that produces
UVR is usually referred to as the flash tube. The gas within a flash
tube is excited and/or ionized, when a capacitor is discharged, to
pass an avalanche of fast electrons through the gas between the
electrodes. Depending on the gas used, i.e., xenon, krypton, argon,
neon, etc., different optical spectra are produced.
2.1.2.2 Fluorescent lamps
A fluorescent lamp contains an electric arc discharge source.
UVR, generated at high efficiency by mercury vapour in an inert gas at
low pressure, activates a coating of fluorescent material (phosphor)
on the inner surface of a glass tube. The phosphor simply acts as a
"transformer", converting shorter wavelength UVR into longer
wavelength radiation, i.e., UV and/or visible light. The spectral
characteristics, which depend on the phosphor used, vary with the gas
pressure in the lamp, and the temperature at the coldest point in the
lamp.
"Fluorescent Sun" (FS) type UVR emitters. "Fluorescent Sun"
type emitters contain a phosphor that emits more than half of its
radiant output at wavelengths shorter than 340 nm. In general, the
range of UVR emitted is from 275 to 380 nm, but the maximum is located
at 313 nm. Thus, this light source is extremely effective in producing
suntan, sunburn, and, at least in animals, cutaneous cancer. The
linear configuration of fluorescent lamps has a distinct advantage for
any application where a uniform irradiation field of considerable size
is required. The disadvantage of these lamps is that there is very
much less energy output per unit area compared with compact mercury or
xenon high pressure arc sources (Sozin, 1975).
"Black Light" (BL) type UVR emitters. The "Black Light" type
UVR emitters are very similar in construction to the FS lamps, except
that the phosphor used emits radiation ranging from 300 to 410 nm,
with a maximum in the 350-365 nm region. Usually, BL lamps emit less
than 0.1% of their total UVR in the less than 320 nm (i.e.,
biologically most effective) region. Their primary use is for
producing fluorescence in a variety of paints and inks. In recent
years, such BL lamps have been used together with photoactive drugs
such as 8-methoxypsoralen in phototherapy of skin diseases (Parrish et
al., 1974).
2.1.2.3 Carbon arcs
The arc is due to an electric discharge between two carbon
electrodes in air at atmospheric pressure. The output of radiation
from a carbon arc increases with increase in arc current and its more
or less continuous shape depends, in part, on the type of metal added
to the electrodes. The use of these arcs has been limited, because of
gaseous waste products that require adequate venting, and the
maintenance problems that result from consumable electrodes.
2.1.2.4 Quartz halogen lamps
These are tungsten filament lamps, enclosed in a quartz envelope,
filled with a small amount of a halogen gas, usually iodine or
bromine. This allows operation at much higher temperatures without
deposition of the metal on the envelope. Such lamps, which may operate
at temperatures up to 3500 K or more, are stable and very intense.
Their UVR output is mainly in the region above 330 nm, and their
primary use is in illumination and as reference standard lamps.
2.1.2.5 Oxyacetylene, oxyhydrogen, and plasma torches
Oil, coal, and gas flames normally operate below 2000 K and,
thus, emit virtually no UVR. Oxyacetylene and oxyhydrogen flames burn
at a much higher temperature and solids heated by these two flames may
radiate UVR.
Welding produces UVR in broad bands, which often appear as a
continuous spectrum. The intensities of the various bands depend on
many factors including the materials from which the electrodes are
made, the discharge current, and the gases surrounding the arc. Arc
welding is a common cause of UVR eye and skin damage.
The plasma torch can produce temperatures of over 6000 K (the
temperature at the surface of the sun) and intense UVR can result.
Exposure to radiation from plasma torches can result in
keratoconjunctivitis and sunburn, if eyes and skin are not protected.
2.2 Detection and Measurement of Ultraviolet Radiation
The measurement of UVR differs from that of visible radiation in
that the eye cannot be used directly as a detecting instrument. Thus,
other means for detection must be used, which can either be based on a
physical principle, or on a chemical or biological reaction. The
physical detectors are mainly used to measure instantaneous
irradiance, whereas the chemical and biological detectors are normally
used to determine radiant exposure (dose).a
2.2.1 Units and conversion factors
Table 1 describes terms frequently used in radiometric
techniques, and Table 2 gives a simple scheme for conversion between
commonly used irradiance units.
In addition to these energy units, units of biological effect,
based on interactions between UVR and living organisms, have been
proposed. The oldest of these is the Finsen. More recently, a system
of bactericidal units for evaluating UVR on the basis of its
disinfectant effect and a system of erythema units for evaluating UVR
on the basis of its beneficial effects on man have been described
(Lazarev & Sokolov, 1971, 1974). In order to allow for intercomparison
with other measurement units, the bactericidal and erythema quantities
are now expressed in SI units (Sokolov, 1975, 1976).
2.2.2 Chemical and biological detectors
2.2.2.1 Photographic plates
The photographic plate is the usual detector in UV spectroscopy.
The degree of blackening of the plate is a measure of radiation
intensity. The measurement is made photometrically by means of some
form of densitometer. Under carefully controlled conditions of
exposure and development, this method is capable of a high degree of
accuracy.
Ordinary photographic emulsions are sensitive in the region of
280 to 500 nm.
a Throughout the document, the term dose refers to the action
spectrum weighted radiant exposure.
2.2.2.2 Chemical methods
Chemicals which undergo some measurable change on exposure to UVR
can be used for the measurement of radiant exposure. These methods are
relatively simple, but are slow and require laborious analysis. They
are sensitive to temperature and to small amounts of impurities. The
most widely used detector has been the acetone-methylene blue
reaction. A more accurate actinometer is based on the rate of
photochemical decomposition of oxalic acid in the presence of uranyl
acetate. A system based on the photolysis of iron (III) oxalate is
more sensitive (Meyer & Seitz, 1949; Koller, 1965). Recently, chemical
dosimeters with action spectra similar to that of human skin erythema
have been reported by Zweig & Henderson (1976). Challoner et el.,
(1976) have used change in the coloration of a plastic film for this
purpose.
2.2.2.3 Biological detectors
The human skin has been used as a UVR dosimeter in an indirect
fashion (Robertson, 1975) and some work using microorganisms as a UVR
dosimeter has been reported (Latarjet, 1977; Billen & Green, 1975).
2.2.3 Physical detectors
Physical detectors have been reviewed by Koller (1965) and Kiefer
(1971).
2.2.3.1 Radiometric devices
These radiation detectors depend for their response on the
heating effect of radiation. The change of temperature due to heating
can, for example, be detected with a thermopile or a resistance
thermometer, that is a bolometer. Their spectral response is normally
quite constant over a wide range of wavelengths. Because these sensors
detect energy, they are not very sensitive to UVR and are mainly used
for standardization.
Table 1. Some basic radiometric terminology
Term SI International Definition Comments and Synonyms
units symbol
Wavelength nm, µm lambda Nanometer = 10-9 metre (also
called "millimicron", mµ;
µm, micrometer, micron = 10-6
metre
Radiant energy J Qe 1 joule = 1 watt second
Radiant flux W phi, Pe dQe Rate of radiant energy delivery
dt ("radiant power"). mW= 10-3W
µW = 10-6 W
Radiant intensity W/sr Ie dPe Describes the radiant flux emitted
d omega by the source Into a given solid
angle (solid angle expressed In
steradlana).
Irradiance W/m2 Ee dPe In photobiology, has been express
dA in W/cm2, mW/cm2 or µW/cm3.
Radiant flux arriving over
a given area. Note Implied
dependence of irradiance on the
angle of the area being irradiated,
relative to a beam. In a collimated,
uniform beam, the irradiance
Ee on a planar surface
varies directly with cos theta, where
theta = angle of incidence from normal
to the surface ("dose-rate"
"intensity", see section 2.2.1-1).
Table 1 (contd)
Term SI International Definition Comments and Synonyms
units symbol
Radiant exposure J/m2 He Ee x t Has been expressed as J/cm2 or
mJ/cm2. ("exposure dose",
"dose", see section 2.2.1).
Note: The subscript "e" serves to distinguish radiometric quantities from photometric quantities,
which have a "v" subscript The "e" subscript is often dropped when only radiometric
terms are used.
t = exposure in seconds
Table 2. Conversion between irradiance units
W/m2 mW/cm2 µW/cm2
1 W/m2 = 1 0.1 100
1 mW/cm2 = 10 1 103
1 µW/cm2 = 0.01 10-3 1
1 erg/cm2 * s = 10-3 10-4 0.1
1 erg/m2 * s 10-7 10-8 10-5
Similar conversions hold for radiant exposure units, if watts (W) are
replaced by joules (J) in the table.
2.2.3.2 Photoelectric devices
These are detectors based on a quantum effect such as the
production of electrons by absorbed photons. Their sensitivity varies
inherently with the energy of the photon (the wavelength of the
radiation). The place and width of the spectral response band depends
on the detector material. In general, these detectors are much more
sensitive than radiometric sensors.
Photomultiplyers, photovoltaic cells, and some, semiconductors
can be used for detecting UVR.
2.2.4 Measuring devices
In order to perform specific UVR measurements, the detector will
normally be placed behind some wavelength selective device such as a
bandpass filter or a monochromator.
It is very important that particular attention is paid to the
form of the spectral response, as this determines how the result can
be used.
For instance, the results from an instrument with a response
according to the skin erythema will not be valuable for atmospheric
research, or other biological effects, e.g., vision. This is because
most of the detail of the spectral information is lost by integration
over a specific action spectrum curve. On the other hand, high
resolution data from a spectroradiometric device can be integrated
afterwards for various uses. However, such measurements are much more
complicated and expensive. Thus, there will always be a conflict
between the information really needed and the amount of effort needed
to acquire the data.
Analogue integrating dosimeters are designed to simulate the
action curve of a particular process, such as skin erythema
(Robertson, 1969; Berger et al., 1975; Lazarev et al., 1975; Sivilova
et al., 1975). Measurements with such a sensor are applicable only to
biological responses with the same, or very similar, action spectra.
3. BIOLOGICAL EFFECTS OF ULTRAVIOLET RADIATION ON UNICELLULAR
ORGANISMS, MAMMALIAN CELLS AND TISSUE, AND INVERTEBRATES
3.1 Introduction
All photobiological responses to UVR and visible radiation are
dependent on the energy of the incident photons, with a maximum
response at a fairly well-defined photon energy within a limited
range, and a "threshold" beyond which the lower photon energies are
very much less effective. This is mainly because of the ability of
biologically important molecules to absorb appropriate photons, since
without such absorption no effect is possible.
The biological effectiveness of a beam of radiation depends on
the photon flux and on the relative efficiency of the photon energy to
produce a particular biological effect. When the beam contains photons
with a range of energies, it is assumed that the overall effect is
equal to the sum of all the individual contributions determined by the
product of the intensity at each photon energy and its relative
biological efficiency. The most spectacular photobiological effects,
other than vision, involve photons of energy greater than about 3.9 eV
(wavelength less than 320 nm). There are, however, some processes that
operate on photons with energy between 4 and 3 eV and even less. When
the effectiveness of a beam is to be evaluated, it is essential that
the relative efficiency of all photon energies (or wavelengths) be
known and allowed for, by comparing the appropriate action or response
spectrum with the intensity spectrum of the beam.
3.1.1 Absorption spectra
Absorption of photons of UVR by a molecule results in the
conversion of radiant energy into rotation-vibrational energy, and a
change in the electronic configuration inside the molecule. In the
ground state, most of the molecules are in a singlet state and
absorption of light causes a transition into an excited singlet state
from which they may pass into the excited triplet state of lower
energy. For many molecules this metastable state is chemically
reactive. However, the opposite is true for oxygen.
The light-absorbing capacity of a molecule depends not only on
the electronic configuration of the molecule but also on the
possibility of higher energy states (Smith & Hanawalt, 1969). The
absorption spectrum of a given substance is the quantitative
description of its capacity for the absorption of photons in a
particular range of electromagnetic frequencies.
Among the components of living matter, only the unsaturated
organic compounds should be taken into consideration, since others
show negligible absorption (at least above 200 nm). The effects on
water need not be dealt with, since it has practically no absorption
above 185 nm (Jagger, 1969).
Chromophores are the chemical groupings of a molecule that can
absorb photons. Molecular constituents that contain conjugated double
bonds freely absorb energy in the UV region. Benzene rings with one or
two atoms of nitrogen show high absorption in the UV-B. Porphyrins,
some steroids, and long-chain compounds such as carotene show good
absorption in the UV-A.
In the nucleic acids, the absorption takes place in the purines
and pyrimidines which absorb at 260 nm.
As far as proteins are concerned, tyrosine, tryptophane, and
peptide bonds are the major chromophores. Absorption of UV photons by
a protein is roughly equivalent to the sum of the absorption by its
constituent amino acids. The absorption peak is usually located at
280 nm.
3.1.2 Evaluation of administered and absorbed doses
While it is easy to measure the dose administered, the dose
absorbed depends on: the composition of the medium, the constituents
of which absorb differently in the UVR; the thickness of the medium;
and on the heterogeneity of the cell material itself including the
thickness of the cell layer, the distribution of the intracellular
organelles and pigments, and the structure and configuration of the
molecules at the time of irradiation (Jagger, 1967). As with
microorganisms, most of the work on this subject has been done using
the low pressure mercury arc, but more reports are now appearing in
relation to UV-A and UV-B.
In the UV-C, cells absorb in the nucleic-acid bland with a peak
at 260 nm. Absorption between 270 and 290 nm with a peak at 280 nm
corresponds to absorption by proteins (amino acids). In the UV-A and
the UV-B, absorption varies considerably, depending on the quantity of
absorbing intracellular molecules (porphyrins, haemoglobin,
cytochrome, carotene, etc.) found in the natural state in certain
cells.
3.1.3 Action spectra
An action spectrum indicates the wavelengths that are most
capable of producing a given effect. Comparison of an action spectrum
with an absorption spectrum of certain constituents of an irradiated
substance or cell often makes it possible to identify the component
responsible for the effect obtained.
In more complex systems, where secondary reactions enter into the
effect measured, the identities of the absorption and action spectra
become less definite, and the conclusions to be drawn, much less
certain.
In bacteria for example, the curve of effectiveness of the UV
wavelengths will peak at 265 nm and is similar to the absorption
spectrum of nucleic acids. It may be deduced that the main target is
DNA (Smith & Hanawalt, 1969).
3.2 The Molecular Basis of the Effects of Ultraviolet Radiation on
Living Matter
3.2.1 Molecular lesions in DNA
Deoxyribonucleic acid (DNA) is one of the most important target
molecules for photobiological effects. DNA can be represented as a
double-stranded helix built up of purine and pyrimidine bases, held
together by sugar and phosphate groups. If the features of the DNA
macromolecule and the universality of the cell structure of living
organisms in which DNA represents the genetic heritage are considered,
it can be anticipated that any lesion inflicted on DNA, however
slight, may have serious repercussions. A lesion in a cell genome is
always serious, because, in genera], the genome exists only in one
copy in the cell concerned, whereas a lesion in a protein, even of the
same magnitude, may remain undetected because there are many copies of
the proteins. The latter is also true of ribonucleic acids (RNA).
Most studies have been performed with low pressure mercury arcs
emitting primarily UVR of 254 nm. Excellent reviews of this subject
include those by Setlow (1968), Latarjet (1972), and Smith (1974).
The effect of UVR is above all destructive. The most common
changes produced in DNA are damage to the bases and to the
polynucleotide chains. Damage to the bases may be unimolecular or
bimolecular. Since pyrimidine bases are ten times more sensitive to
UVR than purine bases, the only unimolecular reaction discussed will
be the formation of pyrimidine hydrates.
Bimolecular reactions are very numerous. They may occur between
two bases, or between a base and another molecule. The most important
effect is the formation of dimer compounds, particularly thymine
dimers. Thymine dimers were demonstrated by Beukers & Berends (1960)
in frozen solutions of irradiated pyrimidine (a special orientation of
the bases being necessary before the dimers could be formed). The
dimer brings about a twisting of the secondary helical structure of
DNA and causes local denaturation. New biochemical methods have made
it possible to detect dimers in vivo in all types of irradiated
cells studied. The number of dimers has been shown to be proportional
to the dose of UVR and to vary with wavelength with a peak at 280 nm.
While the production of dimers has been shown to be directly linked to
the harmful effects of UVR on biological material, it is not the only
serious lesion produced in DNA by UVR.
Dimers are normally produced by UV-B but can also be formed after
exposure to UV-A (Pollard, 1974) and after photosensitization
reactions (Lamola & Yamane, 1967). Product additions to DNA bases are
very numerous (Smith, 1974). Cross-links between DNA bases and
proteins are generally formed after exposure to very high doses of
UV-A (Varghese, 1973). They also occur following exposure to UV-A in
the presence of photosensitizers such as acridine.
Following exposure to UV-A, numerous addition products are formed
with photosensitizing agents including the aromatic ketones,
acetophenone, and benzophenone (Helene & Charlier, 1971), or the
furocoumarins (Chandra, 1972). Polynucleotide chain breaks represent
another type of lesion that may occur in DNA. RNA, the structure of
which is similar to that of DNA, can be directly affected by UVR, but
since the biosynthesis is a continuous process and RNA exists in
multiple copies, very high doses of UVR are needed before such lesions
have any serious repercussions. UVR also produces dimers in RNA (Huang
& Gordon, 1973).
The proteins that make up the bulk of the cell may sustain damage
to the secondary or tertiary structures. Breaks may occur in peptide
chains or bonds or cross-links (Smith, 1974).
3.2.2 Consequences of photolesions
The distortion produced in the DNA-molecule prevents it from
carrying out its functions, i.e., transcription and replication may be
blocked. These lesions can be recognized by repair enzymes or may act
as a signal for other biological processes to intervene. They may
result in cell death, genetic recombination, mutagenesis, or even
carcinogenesis.
Inhibition of DNA synthesis by UVR has long been known to occur
(Kelner, 1953) and has been shown to be a sensitive parameter for
evaluating the effects of UVR (Smith & Hanawalt, 1969). The restarting
of DNA synthesis after a more or less long delay shows that
photolesions can be repaired and these mechanisms are of greatest
importance. Synthesis and transcription of RNA may also be blocked
(Sauerbier, 1976).
3.2.3 Repair of UVR-induced lesions
The existence of several distinct repair mechanisms that operate
in almost all cells but vary considerably in their respective
effectiveness has been demonstrated in in vivo studies of
UVR-induced DNA lesions. The importance and complexity of the repair
processes has been described in numerous reviews and in a book by
Hanawalt & Setlow (1975).
3.2.3.1 Prereplication repair
Photoreactivation. Photoreactivitation was the first discovered
and most primitive mode of repair. As long ago as 1949, both Kelner
and Dulbecco noted that certain bacteria contained a so-called
"photoreactivating" enzyme. The enzyme has been shown to recognize the
dimer and to bind to DNA in the dark. In a wet medium and with
exposure to visible light or long-wave UVR (330 to 550 nm), which
provides the energy needed for the reaction, the enzyme monomerizes
the dimer by breaking the cyclobutane linkages, thus restoring the
molecule to its original state. It is the only repair mechanism in
which a primary UVR lesion can be chemically reversed and where repair
is completed in a single enzymatic stage (Rupert, 1975). This mode of
repair has been demonstrated in all living organisms including
mammals.
Excision repair. Unlike photoreactivation, this repair process
does not require light. It takes place through recognition of the
lesion by complex enzyme mechanisms. This repair process is not
specific for UVR-induced dimers. Similar lesions caused by nitrogen
mustard, 4-nitroquinoline, mitomycin C, nitrous acid, ionizing
radiation etc., can be repaired by the same process.
Following the formation of a dimer, DNA is incised at the bases
near the dimer by a specific endonuclease. The DNA segment bearing the
dimer is then eliminated by an exonuclease and the gap thus created is
filled by local synthesis of DNA, the intact homologous strand serving
as a template. The continuity of the strand is re-established by a
ligase that welds the ends of the resynthesized portion to the
undamaged continuous segment (Boyce & Howard-Flanders, 1964; Pettijohn
& Hanawalt, 1964; Setlow & Carrier, 1964). This type of repair has
been demonstrated in all living organisms including mammals.
3.2.3.2 Repair during or after replication
This type of repair is not initiated by enzymatic recognition of
the lesion. In this case, replication does occur but the lesion is
ignored or bypassed. There remains a gap in the DNA on the side
opposite to the damaged region which can be filled by a process of
"recombination". Homologous DNA molecules with photolesions can use a
process for the exchange of intact genetic material to weld together
the undamaged segments and restore a normal DNA molecule (Rupp &
Howard-Flanders, 1968).
This mode of repair has also been observed in various types of
cells. However the operational capacity of such repair processes is
different from that of photoreactivation. These processes may become
more easily saturated and, above certain UVR doses, the proportion of
unrepaired lesions increases considerably.
3.2.3.3 SOS repair
Since repair yields are never complete, the residual lesions may
then cause errors such as mutations, the frequency of which seems to
depend on the effectiveness of the repair systems. Witkin (1969) has
analyzed the processes of error-prone repair in bacteria, which Radman
(1975) named SOS repair.
It occurs in phage and bacterial DNA which still contains lesions
such as dimers. These can block the normal operation of other repair
processes. The presence of these lesions represents an SOS signal for
the triggering of certain repair mechanisms that are normally
repressed. Replication is then effected by fraudulent incorporation of
bases that cause mutations.
3.3 Bacteria and Yeasts
Most of the principles of molecular photobiology have been
established as a result of work on Escherichia coli and its numerous
mutants, and on the specific phages that infect it. However, it must
be remembered that these results may not, in all cases, be valid for
mammalian cells.
3.3.1 Effects on bacterial cell constituents and macromolecular
synthesis
Apart from chromosomes, structures containing membrane-bound DNA
and structures containing RNA are the main targets for UVR-induced
lesions, which are reflected in alterations in the templates needed
for macromolecular syntheses. DNA synthesis is first blocked, at least
for a time. The blockage is photoreversible. Changes in other
biochemical constituents of varying importance may occur (Jacobson &
Yatvi, 1976).
3.3.2 Sublethal effects
Functional alteration is shown by a slowing-down of the growth
rate of bacteria, which may also grow abnormally without dividing into
filaments.
Survival is evaluated on the basis of counting the colonies that
can be formed by surviving cells. This method is one of the most
sensitive and most commonly used for evaluation of the biological
effects of UVR at the cellular level.
3.3.3 Effects of ultraviolet radiation of wavelengths longer
than 280 nm
Effects produced by UVR longer than 280 nm can be divided into
those specific to these wavelengths and those that are similar to the
effects of UV-C. The increase in complexity of the mechanisms that
come into play at these wavelengths has been demonstrated by Mills et
al., (1975) and Jagger (1976).
A cell irradiated at 254 nm is said to tolerate 5´ times as many
dimers as one irradiated at 365 nm. To kill E.coli, a dose of UV-A
one thousand times greater than that of UV-C or UV-B is required
(Tyrell, 1973).
A review by Jagger (1976), which emphasizes the complexity of the
processes induced by UV wavelengths other than 254 nm, affords a
glimpse of the difficulties that will be encountered in interpreting
results relating to eukaryotic cells.
3.3.4 Genetic factors in photosensitivity
The UVR resistance or UVR sensitivity of the various bacterial
mutants depends on the genetic make-up of the species concerned. The
UVR doses that have to be used to produce the same effect, and the
number of dimers, the induction and excision rate of which are
responsible for this sensitivity, vary considerably (Hill, 1958;
Setlow & Duggan, 1964; Lewis & Kumta, 1972). The enzymes responsible
for the repairs of the lesions are also genetically coded as are the
enzymes responsible for regulating the expression of the repair
enzymes (Hanawalt & Setlow, 1975).
3.3.5 Repair of photolesions in bacteria
All the modes of repair described in section 3.2 are applicable
to, and were mainly discovered in, bacteria. As long ago as 1963,
Setlow & Setlow determined, inter alia, the photoreactivation
mechanisms demonstrating that monomerization was related to the
decrease in the number of photolesions.
3.3.6 Yeasts
Yeasts are microorganisms but are nevertheless eukaryotic cells.
Resnick (1969), Fabre (1971), Cox & Game (1974), and Haynes (1975),
among others, have obtained a great variety of mutants with different
photosensitivities. These mutants differ from the wild type with
regard to UV lethality, mutagenesis, and recombination. Genetic
analysis has shown that several recessive genes are involved in the
control of these responses in a way that is already much more complex
than that found in bacteria. Moustacchi et al. (1975) have reviewed
the specific aspects of repair mechanisms in yeasts as a model for
eukaryotic cell systems.
3.4 Protozoa
Protozoa, including the ciliata paramecium, tetrahymena, and
blepharisma, and amoebae have proved very useful as tools for studies
on the biological effects of UVR (Skreb et al., 1972; Whitson, 1972).
3.5 Effects on Mammalian Cells in Culture
An attempt has been made to apply the knowledge of the mechanisms
of photolesion formation and repair discovered in microorganisms to a
model more similar to the human body; namely established strains of
mammalian cell cultures. The strains most commonly used are HeLa
(human cells of cancerous origin), mouse L fibroblasts and Chinese
hamster cells.
The ability of a surviving cell to form a colony (Lee & Puck,
1960) and the numerous parameters used in radiobiology (Elkind &
Whitmore, 1967) have been widely used to study the effects of UVR on
this material. Among the numerous works on this subject, reference has
often been made to the review paper by Rauth (1970).
3.5.1 Sublethal effects
The most marked effects of UVR on cells are death, mutagenesis,
and malignant transformation. Sublethal effects include various
degrees of inhibition of growth and colony-forming ability. At low
doses, the growth of L fibroblasts is merely slowed down. Higher
doses, however, bring about lysis of the cells (Djordjevic & Tolmach,
1967), although the value of lysis as a parameter is doubtful. All
cell strains give a similar response.
In a study of the colony-forming ability of synchronized and then
irradiated cells, Djordjevic & Tolmach (1967) and Han & Sinclair
(1969, 1971) found that cells were quite resistant at the beginning of
G1 but that sensitivity began to increase at the end of G1 reaching
a peak in the middle of the S phase of DNA synthesis. Thereafter,
sensitivity gradually decreased. In G2, the situation varied
according to the type of cell. However, all authors agree that peak
sensitivity occurs from the beginning to the middle of the S phase of
the cell cycle.
3.5.2 Effects of UV-A
Studies of the effects of UV-A on bacteria have made it possible
to elucidate, at least in part, the complex phenomena that occur in
mammalian cells. Wang et al. (1974) showed that cells can be damaged
by UV-A or visible light (300-420 nm with a peak at 365 nm). The
shoulder of the inactivation curve changes according to cell density,
but the curves remain similar according to the origin of the strain.
Evaluation of viability with trypan blue stain showed that, after
exposure to 2 X 104 J/m2, 99% of human cells, 90% of mouse cells,
and 50% of hamster cells were destroyed.
The fact that survival depends on the density of the cell
population suggests that perhaps some of the effects of UV-A are
indirect. This is supported by the observation of Wang (1975) that the
medium in which cells have been irradiated is toxic. Wang et al.,
(1974) have also shown that riboflavin may cause considerable
photosensitization of cells exposed to UV-A.
3.5.3 Lesions produced in DNA
The lesions produced by UVR in mammalian cellular DNA may be
divided into two categories that are not mutually exclusive, i.e.,
those that prevent replication processes and those that permit
replication processes, but with considerable error.
3.5.3.1 Pyrimidine dimers
It is thought that, as in other types of cells already mentioned,
the formation of pyrimidine dimers results in the essential lesion
that causes most of the effects observed. However, not all the
observed effects can be attributed to dimers and other photoproducts
should not be neglected.
3.5.3.2 DNA-protein cross-links
As demonstrated some time ago (Smith & Hanawalt, 1969),
DNA-protein cross-links are to be expected in view of the
configuration of the DNA molecule, with its backbone folded back on
itself and its association with chromosomal proteins.
DNA-protein cross-links may also help to kill cells by preventing
the switching-on of repair processes (Todd & Han, 1976).
3.5.4 The consequences of photolesions in mammalian cells
3.5.4.1 Inhibition of DNA synthesis
As in microorganisms, the major effect in mammalian cells is a
more or less marked and long-lasting inhibition of DNA synthesis. The
rate of synthesis can be estimated directly by incorporating tritiated
thymidine into the DNA.
3.5.4.2 Chromosome aberrations and mutagenic effects
As regards the effects of UVR on the chromosomes of mammalian
cells, several types of lesions were described long ago including
breaks and rearrangement. A review has been published by Rauth (1970).
These lesions have been studied (among others) in Chinese hamster
cells and human lymphocytes. Chromosome lesions are generally produced
by low doses of UVR. Their production is enzyme-dependent and is
related to repair mechanisms (Bender et al., 1973). They do not
necessarily appear after the first division (Parrington, 1972). They
can be photoreactivated and, therefore, are probably produced by
pyrimidine dimers (Griggs & Bender, 1973). Rommelaere et al., (1973)
discovered another widespread type of lesion in the form of
sister-chromatid exchanges, the frequency of which increased greatly
after UV irradiation. This parameter has been shown to be extremely
sensitive (Ikushima & Wolff, 1974) and, thus, valuable in detecting
very low doses of UVR, although it is not specific to that agent.
UV and X-ray irradiation exert a synergistic effect on the
frequency of chromosome breaks in human lymphocytes (Holmberg &
Jonasson, 1974).
3.5.5 The repair of photolesions
Animal cells in culture also possess the ability to repair
photolesions in DNA. While the lesions are comparable to those
observed in bacteria, and have already been described, there are some
differences in the repair mechanisms (Hanawalt & Setlow, 1975).
3.5.5.1 Photoreactivation
It was believed that photoreactivation was absent from the cells
of placental mammals. However, Sutherland (1974), using appropriate
biochemical techniques, isolated an enzyme in human leukocytes with
properties similar to those of the photoreactivating enzyme. In
mammals, this enzyme is probably not expressed or is masked by other
more effective repair mechanisms. Its presence in other tissues --
nervous, hepatic, and renal -- which are never exposed to light,
suggests that it may have other functions.
3.5.5.2 Excision repair
The number of dimers formed in human cells increases linearly
with the dose of UVR, if the cells are fixed immediately after
irradiation (Cleaver & Trosko, 1969). After a variable lapse of time,
cells begin to excise their dimers in the way already described
(Cleaver et al., 1972). Edenberg & Hanawalt (1973) showed that, four
hours after irradiation with 0.2 J/m2, about 50% of the dimers had
been excised. The proportion varied between 30 and 90% depending on
the type of cell.
Repair replication had already been detected by autoradiography
and shown to be unscheduled DNA synthesis which differed from the
synthesis of normal replication (Rasmussen & Painter, 1964).
Excision repair has been detected in rodents even though the
efficiency is very low.
3.5.5.3 Repair during or after replication
Numerous authors have shown by various biochemical methods that
synthesis of DNA of low relative molecular mass takes place
immediately after irradiation and that, a short time afterwards, this
newly-formed DNA is of normal mass (Painter, 1975).
The dimers prevent replication from being carried out
continuously. Replication probably occurs between the dimers that have
not yet been excised, leaving gaps in the new strands opposite each
dimer of the parental strand (Lehmann, 1972). The mechanism by which
replication fills the gaps has not yet been properly elucidated, nor
has the degree to which the replication is error-free. It is probable
that part, at least, of the repair is inaccurate as a result of errors
of replication opposite a lesion. Depending on the degree of accuracy,
either repair is total or a lesion persists that may lead to death,
mutation, or carcinogenesis.
3.5.5.4 SOS repair
As will be seen later, in mammalian cells, the repair systems of
the host cell may play a part in repairing an irradiated virus
developing in the cell.
3.5.6. Effects on cell-virus relationships
3.5.6.1 Sensitivity to vital infection
Infection with herpes virus is enhanced in mammalian cells
exposed to UV-A (Mills et al., 1975). Relatively low exposures
increase infectivity by 20-30% and it remains high for several days.
3.5.6.2 Vital transformation
UV-C increases the rate of transformation of mouse and hamster
cells by various viruses (Lytle et al., 1970). However, it does not
induce direct transformation in all cell species. Certain
photosensitizing agents enhance this viral transformation (Casto,
1973).
3.5.6.3 Activation of viruses
Since some mammalian cells harbour viruses that normally remain
latent, their activation or induction might transform them and endow
them with the characteristics of cancer cells. UV-C exposure of rat or
hamster cells, transformed by certain oncogenic viruses, can activate
the production of virus particles (Hellman et al., 1974), whereas
irradiation of normal cells can activate tumour viruses of the
leukemia-leukosis type (Lytle, 1971).
However, there is nothing to show that malignant transformation
necessarily involves the development of an oncogenic virus.
3.6 Effects on Invertebrates
Since the invertebrates are extremely heterogeneous, it is very
difficult to draw general conclusions, and the data on the response of
certain invertebrates will be given without such conclusions.
3.6.1 Effects on eggs and embryos of invertebrates
Hamilton (1973) emphasized the value of invertebrate eggs for
radiation studies. Since UVR has low penetration, only very
transparent small eggs can be totally irradiated. The others must be
stripped or dechorionated by physical or chemical means. The whole egg
or part of an egg has been irradiated with UVR mainly to destroy
certain cells, in order to watch how development proceeds in their
absence and thus deduce the role they play. Some doses, while not
preventing segmentation, arrest the onset of differentiation at the
sensitive stage represented by the end of blastulation and the
beginning of gastrulation.
Wavelengths of 225-313 nm have caused appreciable delays in
development. Undivided eggs have been shown to be highly sensitive to
UVR (Hsiao, 1975). There has been very little work using UV-A.
In conclusion, it may be said that, in general, eggs are well
protected against the harmful effects of UVR. If they are directly
exposed, they respond to UV irradiation by means of the mechanisms
already described. From the time when the cells begin to
differentiate, the quality and intensity of the photolesions and their
repair depend on the stage of differentiation of the embryo. If it is
not an advanced stage, regulatory mechanisms sometimes enable
photolesions produced at an early stage, when the cells are still
totipotential, to be eliminated naturally.
3.6.2 Effects on insects
Insects comprise a major part of ecosystems. UVR may act on the
vital processes of the insects in different ways and these have been
summarized by Hsiao (1975).
From the large numbers of papers that have been written, a first
conclusion is that UV-B is perceived by numerous insects. Several
diurnal and nocturnal species show positive phototropism towards UVR.
Most of the UV-A lamps used to trap insects have an emission spectrum
with a peak at 360 nm corresponding to the peak sensitivity of the UV
receptors in the insects. However, the peak differs slightly for each
species.
Solar UVR has been found to modify the biological clock in
insects and other arthropods.
Photoreactivation and other types of repair have been
demonstrated in insects.
3.7 Modification of the Effects of Ultraviolet Radiation by Chemical
Agents
Numerous chemical agents increase the photochemical reactivity of
nucleic acids. They act in various ways, by becoming incorporated in
the nucleic acids, or by forming various complexes with them that
increase their absorption or reactivity. They can also absorb UVR
directly and transfer the energy to nucleic acids.
Various physical factors may change the intensity of irradiation
and the efficiency of the repair systems.
The way in which irradiation is carried out, ie., whether it is
continuous or fractionated, total or partial is also important in the
evaluation of the final results.
3.7.1 Halogenated analogues
Incorporation of 5-bromouracil (5-BrU) and 5-bromodeoxyuridine
(5-BUdR) sensitizes both viruses and cells to UVR. A very detailed
review has been made by Hutchinson (1973).
3.7.2 Caffeine
Caffeine is a trimethylxanthine that acts by inhibiting the
repair systems. At doses not themselves toxic, caffeine considerably
increases the effects of UVR.
The absence of any effect of caffeine on excision repair suggests
that it acts by interfering with a post-replication system (Cleaver &
Thomas, 1969).
3.7.3 Furocoumarins
Photosensitizers are becoming increasingly important among agents
that modify the effects of UVR on biological systems.
Furocoumarins are natural products isolated from plants.
Important applications in therapy have led to thorough studies of
their mode of action at the molecular level (Chandra, 1972; Pathak et
al., 1974).
Musajo et al., (1974) have shown, in vitro, that furocoumarins
exert photosensitizing effects following irradiation at wavelengths of
320-400 nm. These effects result from the formation of certain
addition products with DNA and particularly from the linking of the
furocoumarin molecule with the pyrimide bases. These products may
cause breaks in the molecule and thus prevent if from carrying out its
functions. Oxidation of amino acids may also occur in proteins.
The so-called 'linear' furocoumarins such as psoralen react with
native DNA in the presence of UVR to make, monofunctional and
bifunctional addition products. The latter cause cross-linking between
the DNA strands. Certain so-called 'angular' furocoumarins (angelicin)
can only give rise to monofunctional addition products.
These addition products cause lesions that can be repaired by the
excision repair mechanism. Monofunctional addition products seem to be
easier to repair than bifunctional products (Chandra et al., 1976).
Depending on their molecular structure, not all the furocoumarins
show the same degree of photosensitization. Ben Hur & Elkind (1973)
demonstrated that, following exposure of hamster cells to UV-A, 11% of
the addition products were formed between the complementary strands.
During incubation in the dark, 90% of the cross-links gradually
disappeared from the DNA.
These reactions have found applications in the treatment of
certain skin diseases, particularly psoriasis.
3.7.4 Other photosensitizing agents
Charlier & Helene (1972) carried out an in vitro analysis of
the photochemical reactions of benzophenone and acetophenone with
purine and pyrimide derivatives in aqueous solution during irradiation
with light between 400 and 600 nm. Dimers and chain breaks were the
essential lesions.
The effects of light on bacteria in the presence of
photosensitizing chemicals such as toluidine blue and acridine yellow
were compared by Harrison (1967). The four sorts of lesions observed
-- lack of colony-forming capacity, DNA lesions and mutations, changes
in cell permeability, and enzyme inactivation -- were similar in both
cases.
Rauth & Domon (1973) studied the mechanisms of photosensitization
in animal cells in cultures with 1-cyclohexyl-3(2-morpholinyl-4-ethyl)
carbodiimide metho- p-toluene sulfonate (CMEC), which binds
preferentially to partially denatured regions of DNA after irradiation
at 254 nm, thus inhibiting replication and increasing lethality.
3.7.5 Protection by carotene
Since the work of Cohen-Bazire & Stanier (1958), some
investigations have established the protective role of carotene
against the photodynamic effects of light, in very special cases.
Since the energy level of carotenes is very low, they can accept, by
transfer, the energy of sensitizers transformed into the triplet state
and even the energy of oxygen transformed into the singlet state by
exposure to light.
Mutant strains of bacteria and fungi lacking carotenoids have
proved much more sensitive to photodynamic effects than normal
strains, but this effect is only valid for wavelengths shorter than
those that carotenes absorb (Mathews & Krinsky, 1965).
3.8 Conclusions
Knowledge of the molecular basis for the biological effects of
UVR has emphasized the importance of the photolesions produced in DNA
and the effectiveness of the enzymatic stages of repair, both of which
depend on the genetic make-up of the organism concerned.
Studies of the sensitivity of mutants, on one hand, and of
factors able to modify the effects of irradiation, on the other, will,
perhaps, make it possible to strengthen repair systems and to increase
the resistance of organisms to UVR.
The great differences observed make it difficult to lay down
protection standards.
4. THE BIOLOGICAL ACTION OF ULTRAVIOLET RADIATION ON VERTEBRATE
ANIMALS
4.1 General Aspects
The anatomical structure of the skin of vertebrate animals
resembles that of man in many respects. The surface stratum corneum is
important in affecting the penetration of UVR (Fig. 4), the
thicknesses of the stratum spinosum and stratum granulosum also affect
the relative amounts of UVR reaching the dermis. Other constituents of
the skin, e.g., the melanocyte population, the Langerhans cells,
vascular structures, as well as cutaneous innervation vary in amount
and distribution from species to species, but remain basically the
same.
Long-term results of actual measurements with an analogue
integrating UVR dosimeter, having an action spectrum similar to that
of skin erythema, are also available (Scotto et al., 1976). From these
measurements, it is apparent that there are daily fluctuations,
throughout the year, at each location. Weekly patterns are less
erratic, and seasonal variations depend on the latitude. The solar UVR
that causes skin erythema reaches a maximum intensity between 10h00
and 14h00. About 60% of the daily dose reaches the ground between
10h00 and 14h00, and 80% between 9h00 and 15h00 (Fig. 2). Similar
observations over a shorter period of time, using an instrument based
on a filtered selenium photocell, have given virtually the same
results (Garadza, 1965).
2.1.2 Artificial sources
Any material heated to temperatures exceeding 2500 K begins to
emit UVR. For practical purposes, sources emitting significant amounts
of biologically effective UVR can be classified into confined gas
discharge arcs, fluorescent lamps, and incandescent sources. It should
be noted that any UVR source emitting intense radiation below 260 nm
will produce ozone, which has to be removed to prevent any health
hazard.
2.1.2.1 Gas discharge arcs
The optical spectra produced by arcs depend on the nature of the
gas molecules through which the current discharge takes place, its
pressure, and the electrical conditions in the discharge.
Direct gas discharge arcs are widely used for the generation of
UVR. Generally, they differ from each other in such respects as the
type of gas, pressure, starting mechanisms, lamp shape, reflector
systems, electrodes, etc. (Andreev et al., 1975). As there is such a
wide variety of these arcs, it is not possible to describe them all.
However, the most important types are listed below.
Low pressure mercury arcs. These vapour lamps emit several
narrow UV bands. Most of the emitted power is of a wavelength of
253.7 nm, which is near the maximum for germicidal effectiveness,
hence its usefulness in the control of microorganisms.
High pressure mercury arcs. These vapour arcs (operating at
20-100 atmospheres, i.e., 200-1000 kPa, and usually encased in quartz
envelopes) emit much broader and more intense bands of UVR than low
pressure mercury arcs at wavelengths of 254, 297, 303, 313 and 365 nm.
They are extensively used in industry in photochemical reactors and in
printing. Other uses include phototherapy of skin diseases (Meyer &
Seitz, 1949; Nilender & Gavanin, 1971).
High pressure xenon arcs. These arc lamps may operate at very
high internal pressures, and have the advantage that the spectral
distribution of their radiant output shows a continuum similar to that
of the sun above the stratosphere. Their output is quite constant over
long periods of time, and they are available in a variety of
configurations. The major problem with xenon arcs is their very high
emission in the near infrared region (Gavrilova et al., 1975). Their
uses are similar to those of mercury arcs.
Flash tubes. Another form of gas discharge arc that produces
UVR is usually referred to as the flash tube. The gas within a flash
tube is excited and/or ionized, when a capacitor is discharged, to
pass an avalanche of fast electrons through the gas between the
electrodes. Depending on the gas used, i.e., xenon, krypton, argon,
neon, etc., different optical spectra are produced.
2.1.2.2 Fluorescent lamps
A fluorescent lamp contains an electric arc discharge source.
UVR, generated at high efficiency by mercury vapour in an inert gas at
low pressure, activates a coating of fluorescent material (phosphor)
on the inner surface of a glass tube. The phosphor simply acts as a
"transformer", converting shorter wavelength UVR into longer
wavelength radiation, i.e., UV and/or visible light. The spectral
characteristics, which depend on the phosphor used, vary with the gas
pressure in the lamp, and the temperature at the coldest point in the
lamp.
"Fluorescent Sun" (FS) type UVR emitters. "Fluorescent Sun"
type emitters contain a phosphor that emits more than half of its
radiant output at wavelengths shorter than 340 nm. In general, the
range of UVR emitted is from 275 to 380 nm, but the maximum is located
at 313 nm. Thus, this light source is extremely effective in producing
suntan, sunburn, and, at least in animals, cutaneous cancer. The
linear configuration of fluorescent lamps has a distinct advantage for
any application where a uniform irradiation field of considerable size
is required. The disadvantage of these lamps is that there is very
much less energy output per unit area compared with compact mercury or
xenon high pressure arc sources (Sozin, 1975).
"Black Light" (BL) type UVR emitters. The "Black Light" type
UVR emitters are very similar in construction to the FS lamps, except
that the phosphor used emits radiation ranging from 300 to 410 nm,
with a maximum in the 350-365 nm region. Usually, BL lamps emit less
than 0.1% of their total UVR in the less than 320 nm (i.e.,
biologically most effective) region. Their primary use is for
producing fluorescence in a variety of paints and inks. In recent
years, such BL lamps have been used together with photoactive drugs
such as 8-methoxypsoralen in phototherapy of skin diseases (Parrish et
al., 1974).
2.1.2.3 Carbon arcs
The arc is due to an electric discharge between two carbon
electrodes in air at atmospheric pressure. The output of radiation
from a carbon arc increases with increase in arc current and its more
or less continuous shape depends, in part, on the type of metal added
to the electrodes. The use of these arcs has been limited, because of
gaseous waste products that require adequate venting, and the
maintenance problems that result from consumable electrodes.
2.1.2.4 Quartz halogen lamps
These are tungsten filament lamps, enclosed in a quartz envelope,
filled with a small amount of a halogen gas, usually iodine or
bromine. This allows operation at much higher temperatures without
deposition of the metal on the envelope. Such lamps, which may operate
at temperatures up to 3500 K or more, are stable and very intense.
Their UVR output is mainly in the region above 330 nm, and their
primary use is in illumination and as reference standard lamps.
2.1.2.5 Oxyacetylene, oxyhydrogen, and plasma torches
Oil, coal, and gas flames normally operate below 2000 K and,
thus, emit virtually no UVR. Oxyacetylene and oxyhydrogen flames burn
at a much higher temperature and solids heated by these two flames may
radiate UVR.
Welding produces UVR in broad bands, which often appear as a
continuous spectrum. The intensities of the various bands depend on
many factors including the materials from which the electrodes are
made, the discharge current, and the gases surrounding the arc. Arc
welding is a common cause of UVR eye and skin damage.
The plasma torch can produce temperatures of over 6000 K (the
temperature at the surface of the sun) and intense UVR can result.
Exposure to radiation from plasma torches can result in
keratoconjunctivitis and sunburn, if eyes and skin are not protected.
2.2 Detection and Measurement of Ultraviolet Radiation
The measurement of UVR differs from that of visible radiation in
that the eye cannot be used directly as a detecting instrument. Thus,
other means for detection must be used, which can either be based on a
physical principle, or on a chemical or biological reaction. The
physical detectors are mainly used to measure instantaneous
irradiance, whereas the chemical and biological detectors are normally
used to determine radiant exposure (dose).a
2.2.1 Units and conversion factors
Table 1 describes terms frequently used in radiometric
techniques, and Table 2 gives a simple scheme for conversion between
commonly used irradiance units.
In addition to these energy units, units of biological effect,
based on interactions between UVR and living organisms, have been
proposed. The oldest of these is the Finsen. More recently, a system
of bactericidal units for evaluating UVR on the basis of its
disinfectant effect and a system of erythema units for evaluating UVR
on the basis of its beneficial effects on man have been described
(Lazarev & Sokolov, 1971, 1974). In order to allow for intercomparison
with other measurement units, the bactericidal and erythema quantities
are now expressed in SI units (Sokolov, 1975, 1976).
2.2.2 Chemical and biological detectors
2.2.2.1 Photographic plates
The photographic plate is the usual detector in UV spectroscopy.
The degree of blackening of the plate is a measure of radiation
intensity. The measurement is made photometrically by means of some
form of densitometer. Under carefully controlled conditions of
exposure and development, this method is capable of a high degree of
accuracy.
Ordinary photographic emulsions are sensitive in the region of
280 to 500 nm.
a Throughout the document, the term dose refers to the action
spectrum weighted radiant exposure.
2.2.2.2 Chemical methods
Chemicals which undergo some measurable change on exposure to UVR
can be used for the measurement of radiant exposure. These methods are
relatively simple, but are slow and require laborious analysis. They
are sensitive to temperature and to small amounts of impurities. The
most widely used detector has been the acetone-methylene blue
reaction. A more accurate actinometer is based on the rate of
photochemical decomposition of oxalic acid in the presence of uranyl
acetate. A system based on the photolysis of iron (III) oxalate is
more sensitive (Meyer & Seitz, 1949; Koller, 1965). Recently, chemical
dosimeters with action spectra similar to that of human skin erythema
have been reported by Zweig & Henderson (1976). Challoner et el.,
(1976) have used change in the coloration of a plastic film for this
purpose.
2.2.2.3 Biological detectors
The human skin has been used as a UVR dosimeter in an indirect
fashion (Robertson, 1975) and some work using microorganisms as a UVR
dosimeter has been reported (Latarjet, 1977; Billen & Green, 1975).
2.2.3 Physical detectors
Physical detectors have been reviewed by Koller (1965) and Kiefer
(1971).
2.2.3.1 Radiometric devices
These radiation detectors depend for their response on the
heating effect of radiation. The change of temperature due to heating
can, for example, be detected with a thermopile or a resistance
thermometer, that is a bolometer. Their spectral response is normally
quite constant over a wide range of wavelengths. Because these sensors
detect energy, they are not very sensitive to UVR and are mainly used
for standardization.
Table 1. Some basic radiometric terminology
Term SI International Definition Comments and Synonyms
units symbol
Wavelength nm, µm lambda Nanometer = 10-9 metre (also
called "millimicron", mµ;
µm, micrometer, micron = 10-6
metre
Radiant energy J Qe 1 joule = 1 watt second
Radiant flux W phi, Pe dQe Rate of radiant energy delivery
dt ("radiant power"). mW= 10-3W
µW = 10-6 W
Radiant intensity W/sr Ie dPe Describes the radiant flux emitted
d omega by the source Into a given solid
angle (solid angle expressed In
steradlana).
Irradiance W/m2 Ee dPe In photobiology, has been express
dA in W/cm2, mW/cm2 or µW/cm3.
Radiant flux arriving over
a given area. Note Implied
dependence of irradiance on the
angle of the area being irradiated,
relative to a beam. In a collimated,
uniform beam, the irradiance
Ee on a planar surface
varies directly with cos theta, where
theta = angle of incidence from normal
to the surface ("dose-rate"
"intensity", see section 2.2.1-1).
Table 1 (contd)
Term SI International Definition Comments and Synonyms
units symbol
Radiant exposure J/m2 He Ee x t Has been expressed as J/cm2 or
mJ/cm2. ("exposure dose",
"dose", see section 2.2.1).
Note: The subscript "e" serves to distinguish radiometric quantities from photometric quantities,
which have a "v" subscript The "e" subscript is often dropped when only radiometric
terms are used.
t = exposure in seconds
Table 2. Conversion between irradiance units
W/m2 mW/cm2 µW/cm2
1 W/m2 = 1 0.1 100
1 mW/cm2 = 10 1 103
1 µW/cm2 = 0.01 10-3 1
1 erg/cm2 * s = 10-3 10-4 0.1
1 erg/m2 * s 10-7 10-8 10-5
Similar conversions hold for radiant exposure units, if watts (W) are
replaced by joules (J) in the table.
2.2.3.2 Photoelectric devices
These are detectors based on a quantum effect such as the
production of electrons by absorbed photons. Their sensitivity varies
inherently with the energy of the photon (the wavelength of the
radiation). The place and width of the spectral response band depends
on the detector material. In general, these detectors are much more
sensitive than radiometric sensors.
Photomultiplyers, photovoltaic cells, and some, semiconductors
can be used for detecting UVR.
2.2.4 Measuring devices
In order to perform specific UVR measurements, the detector will
normally be placed behind some wavelength selective device such as a
bandpass filter or a monochromator.
It is very important that particular attention is paid to the
form of the spectral response, as this determines how the result can
be used.
For instance, the results from an instrument with a response
according to the skin erythema will not be valuable for atmospheric
research, or other biological effects, e.g., vision. This is because
most of the detail of the spectral information is lost by integration
over a specific action spectrum curve. On the other hand, high
resolution data from a spectroradiometric device can be integrated
afterwards for various uses. However, such measurements are much more
complicated and expensive. Thus, there will always be a conflict
between the information really needed and the amount of effort needed
to acquire the data.
Analogue integrating dosimeters are designed to simulate the
action curve of a particular process, such as skin erythema
(Robertson, 1969; Berger et al., 1975; Lazarev et al., 1975; Sivilova
et al., 1975). Measurements with such a sensor are applicable only to
biological responses with the same, or very similar, action spectra.
3. BIOLOGICAL EFFECTS OF ULTRAVIOLET RADIATION ON UNICELLULAR
ORGANISMS, MAMMALIAN CELLS AND TISSUE, AND INVERTEBRATES
3.1 Introduction
All photobiological responses to UVR and visible radiation are
dependent on the energy of the incident photons, with a maximum
response at a fairly well-defined photon energy within a limited
range, and a "threshold" beyond which the lower photon energies are
very much less effective. This is mainly because of the ability of
biologically important molecules to absorb appropriate photons, since
without such absorption no effect is possible.
The biological effectiveness of a beam of radiation depends on
the photon flux and on the relative efficiency of the photon energy to
produce a particular biological effect. When the beam contains photons
with a range of energies, it is assumed that the overall effect is
equal to the sum of all the individual contributions determined by the
product of the intensity at each photon energy and its relative
biological efficiency. The most spectacular photobiological effects,
other than vision, involve photons of energy greater than about 3.9 eV
(wavelength less than 320 nm). There are, however, some processes that
operate on photons with energy between 4 and 3 eV and even less. When
the effectiveness of a beam is to be evaluated, it is essential that
the relative efficiency of all photon energies (or wavelengths) be
known and allowed for, by comparing the appropriate action or response
spectrum with the intensity spectrum of the beam.
3.1.1 Absorption spectra
Absorption of photons of UVR by a molecule results in the
conversion of radiant energy into rotation-vibrational energy, and a
change in the electronic configuration inside the molecule. In the
ground state, most of the molecules are in a singlet state and
absorption of light causes a transition into an excited singlet state
from which they may pass into the excited triplet state of lower
energy. For many molecules this metastable state is chemically
reactive. However, the opposite is true for oxygen.
The light-absorbing capacity of a molecule depends not only on
the electronic configuration of the molecule but also on the
possibility of higher energy states (Smith & Hanawalt, 1969). The
absorption spectrum of a given substance is the quantitative
description of its capacity for the absorption of photons in a
particular range of electromagnetic frequencies.
Among the components of living matter, only the unsaturated
organic compounds should be taken into consideration, since others
show negligible absorption (at least above 200 nm). The effects on
water need not be dealt with, since it has practically no absorption
above 185 nm (Jagger, 1969).
Chromophores are the chemical groupings of a molecule that can
absorb photons. Molecular constituents that contain conjugated double
bonds freely absorb energy in the UV region. Benzene rings with one or
two atoms of nitrogen show high absorption in the UV-B. Porphyrins,
some steroids, and long-chain compounds such as carotene show good
absorption in the UV-A.
In the nucleic acids, the absorption takes place in the purines
and pyrimidines which absorb at 260 nm.
As far as proteins are concerned, tyrosine, tryptophane, and
peptide bonds are the major chromophores. Absorption of UV photons by
a protein is roughly equivalent to the sum of the absorption by its
constituent amino acids. The absorption peak is usually located at
280 nm.
3.1.2 Evaluation of administered and absorbed doses
While it is easy to measure the dose administered, the dose
absorbed depends on: the composition of the medium, the constituents
of which absorb differently in the UVR; the thickness of the medium;
and on the heterogeneity of the cell material itself including the
thickness of the cell layer, the distribution of the intracellular
organelles and pigments, and the structure and configuration of the
molecules at the time of irradiation (Jagger, 1967). As with
microorganisms, most of the work on this subject has been done using
the low pressure mercury arc, but more reports are now appearing in
relation to UV-A and UV-B.
In the UV-C, cells absorb in the nucleic-acid bland with a peak
at 260 nm. Absorption between 270 and 290 nm with a peak at 280 nm
corresponds to absorption by proteins (amino acids). In the UV-A and
the UV-B, absorption varies considerably, depending on the quantity of
absorbing intracellular molecules (porphyrins, haemoglobin,
cytochrome, carotene, etc.) found in the natural state in certain
cells.
3.1.3 Action spectra
An action spectrum indicates the wavelengths that are most
capable of producing a given effect. Comparison of an action spectrum
with an absorption spectrum of certain constituents of an irradiated
substance or cell often makes it possible to identify the component
responsible for the effect obtained.
In more complex systems, where secondary reactions enter into the
effect measured, the identities of the absorption and action spectra
become less definite, and the conclusions to be drawn, much less
certain.
In bacteria for example, the curve of effectiveness of the UV
wavelengths will peak at 265 nm and is similar to the absorption
spectrum of nucleic acids. It may be deduced that the main target is
DNA (Smith & Hanawalt, 1969).
3.2 The Molecular Basis of the Effects of Ultraviolet Radiation on
Living Matter
3.2.1 Molecular lesions in DNA
Deoxyribonucleic acid (DNA) is one of the most important target
molecules for photobiological effects. DNA can be represented as a
double-stranded helix built up of purine and pyrimidine bases, held
together by sugar and phosphate groups. If the features of the DNA
macromolecule and the universality of the cell structure of living
organisms in which DNA represents the genetic heritage are considered,
it can be anticipated that any lesion inflicted on DNA, however
slight, may have serious repercussions. A lesion in a cell genome is
always serious, because, in genera], the genome exists only in one
copy in the cell concerned, whereas a lesion in a protein, even of the
same magnitude, may remain undetected because there are many copies of
the proteins. The latter is also true of ribonucleic acids (RNA).
Most studies have been performed with low pressure mercury arcs
emitting primarily UVR of 254 nm. Excellent reviews of this subject
include those by Setlow (1968), Latarjet (1972), and Smith (1974).
The effect of UVR is above all destructive. The most common
changes produced in DNA are damage to the bases and to the
polynucleotide chains. Damage to the bases may be unimolecular or
bimolecular. Since pyrimidine bases are ten times more sensitive to
UVR than purine bases, the only unimolecular reaction discussed will
be the formation of pyrimidine hydrates.
Bimolecular reactions are very numerous. They may occur between
two bases, or between a base and another molecule. The most important
effect is the formation of dimer compounds, particularly thymine
dimers. Thymine dimers were demonstrated by Beukers & Berends (1960)
in frozen solutions of irradiated pyrimidine (a special orientation of
the bases being necessary before the dimers could be formed). The
dimer brings about a twisting of the secondary helical structure of
DNA and causes local denaturation. New biochemical methods have made
it possible to detect dimers in vivo in all types of irradiated
cells studied. The number of dimers has been shown to be proportional
to the dose of UVR and to vary with wavelength with a peak at 280 nm.
While the production of dimers has been shown to be directly linked to
the harmful effects of UVR on biological material, it is not the only
serious lesion produced in DNA by UVR.
Dimers are normally produced by UV-B but can also be formed after
exposure to UV-A (Pollard, 1974) and after photosensitization
reactions (Lamola & Yamane, 1967). Product additions to DNA bases are
very numerous (Smith, 1974). Cross-links between DNA bases and
proteins are generally formed after exposure to very high doses of
UV-A (Varghese, 1973). They also occur following exposure to UV-A in
the presence of photosensitizers such as acridine.
Following exposure to UV-A, numerous addition products are formed
with photosensitizing agents including the aromatic ketones,
acetophenone, and benzophenone (Helene & Charlier, 1971), or the
furocoumarins (Chandra, 1972). Polynucleotide chain breaks represent
another type of lesion that may occur in DNA. RNA, the structure of
which is similar to that of DNA, can be directly affected by UVR, but
since the biosynthesis is a continuous process and RNA exists in
multiple copies, very high doses of UVR are needed before such lesions
have any serious repercussions. UVR also produces dimers in RNA (Huang
& Gordon, 1973).
The proteins that make up the bulk of the cell may sustain damage
to the secondary or tertiary structures. Breaks may occur in peptide
chains or bonds or cross-links (Smith, 1974).
3.2.2 Consequences of photolesions
The distortion produced in the DNA-molecule prevents it from
carrying out its functions, i.e., transcription and replication may be
blocked. These lesions can be recognized by repair enzymes or may act
as a signal for other biological processes to intervene. They may
result in cell death, genetic recombination, mutagenesis, or even
carcinogenesis.
Inhibition of DNA synthesis by UVR has long been known to occur
(Kelner, 1953) and has been shown to be a sensitive parameter for
evaluating the effects of UVR (Smith & Hanawalt, 1969). The restarting
of DNA synthesis after a more or less long delay shows that
photolesions can be repaired and these mechanisms are of greatest
importance. Synthesis and transcription of RNA may also be blocked
(Sauerbier, 1976).
3.2.3 Repair of UVR-induced lesions
The existence of several distinct repair mechanisms that operate
in almost all cells but vary considerably in their respective
effectiveness has been demonstrated in in vivo studies of
UVR-induced DNA lesions. The importance and complexity of the repair
processes has been described in numerous reviews and in a book by
Hanawalt & Setlow (1975).
3.2.3.1 Prereplication repair
Photoreactivation. Photoreactivitation was the first discovered
and most primitive mode of repair. As long ago as 1949, both Kelner
and Dulbecco noted that certain bacteria contained a so-called
"photoreactivating" enzyme. The enzyme has been shown to recognize the
dimer and to bind to DNA in the dark. In a wet medium and with
exposure to visible light or long-wave UVR (330 to 550 nm), which
provides the energy needed for the reaction, the enzyme monomerizes
the dimer by breaking the cyclobutane linkages, thus restoring the
molecule to its original state. It is the only repair mechanism in
which a primary UVR lesion can be chemically reversed and where repair
is completed in a single enzymatic stage (Rupert, 1975). This mode of
repair has been demonstrated in all living organisms including
mammals.
Excision repair. Unlike photoreactivation, this repair process
does not require light. It takes place through recognition of the
lesion by complex enzyme mechanisms. This repair process is not
specific for UVR-induced dimers. Similar lesions caused by nitrogen
mustard, 4-nitroquinoline, mitomycin C, nitrous acid, ionizing
radiation etc., can be repaired by the same process.
Following the formation of a dimer, DNA is incised at the bases
near the dimer by a specific endonuclease. The DNA segment bearing the
dimer is then eliminated by an exonuclease and the gap thus created is
filled by local synthesis of DNA, the intact homologous strand serving
as a template. The continuity of the strand is re-established by a
ligase that welds the ends of the resynthesized portion to the
undamaged continuous segment (Boyce & Howard-Flanders, 1964; Pettijohn
& Hanawalt, 1964; Setlow & Carrier, 1964). This type of repair has
been demonstrated in all living organisms including mammals.
3.2.3.2 Repair during or after replication
This type of repair is not initiated by enzymatic recognition of
the lesion. In this case, replication does occur but the lesion is
ignored or bypassed. There remains a gap in the DNA on the side
opposite to the damaged region which can be filled by a process of
"recombination". Homologous DNA molecules with photolesions can use a
process for the exchange of intact genetic material to weld together
the undamaged segments and restore a normal DNA molecule (Rupp &
Howard-Flanders, 1968).
This mode of repair has also been observed in various types of
cells. However the operational capacity of such repair processes is
different from that of photoreactivation. These processes may become
more easily saturated and, above certain UVR doses, the proportion of
unrepaired lesions increases considerably.
3.2.3.3 SOS repair
Since repair yields are never complete, the residual lesions may
then cause errors such as mutations, the frequency of which seems to
depend on the effectiveness of the repair systems. Witkin (1969) has
analyzed the processes of error-prone repair in bacteria, which Radman
(1975) named SOS repair.
It occurs in phage and bacterial DNA which still contains lesions
such as dimers. These can block the normal operation of other repair
processes. The presence of these lesions represents an SOS signal for
the triggering of certain repair mechanisms that are normally
repressed. Replication is then effected by fraudulent incorporation of
bases that cause mutations.
3.3 Bacteria and Yeasts
Most of the principles of molecular photobiology have been
established as a result of work on Escherichia coli and its numerous
mutants, and on the specific phages that infect it. However, it must
be remembered that these results may not, in all cases, be valid for
mammalian cells.
3.3.1 Effects on bacterial cell constituents and macromolecular
synthesis
Apart from chromosomes, structures containing membrane-bound DNA
and structures containing RNA are the main targets for UVR-induced
lesions, which are reflected in alterations in the templates needed
for macromolecular syntheses. DNA synthesis is first blocked, at least
for a time. The blockage is photoreversible. Changes in other
biochemical constituents of varying importance may occur (Jacobson &
Yatvi, 1976).
3.3.2 Sublethal effects
Functional alteration is shown by a slowing-down of the growth
rate of bacteria, which may also grow abnormally without dividing into
filaments.
Survival is evaluated on the basis of counting the colonies that
can be formed by surviving cells. This method is one of the most
sensitive and most commonly used for evaluation of the biological
effects of UVR at the cellular level.
3.3.3 Effects of ultraviolet radiation of wavelengths longer
than 280 nm
Effects produced by UVR longer than 280 nm can be divided into
those specific to these wavelengths and those that are similar to the
effects of UV-C. The increase in complexity of the mechanisms that
come into play at these wavelengths has been demonstrated by Mills et
al., (1975) and Jagger (1976).
A cell irradiated at 254 nm is said to tolerate 5´ times as many
dimers as one irradiated at 365 nm. To kill E.coli, a dose of UV-A
one thousand times greater than that of UV-C or UV-B is required
(Tyrell, 1973).
A review by Jagger (1976), which emphasizes the complexity of the
processes induced by UV wavelengths other than 254 nm, affords a
glimpse of the difficulties that will be encountered in interpreting
results relating to eukaryotic cells.
3.3.4 Genetic factors in photosensitivity
The UVR resistance or UVR sensitivity of the various bacterial
mutants depends on the genetic make-up of the species concerned. The
UVR doses that have to be used to produce the same effect, and the
number of dimers, the induction and excision rate of which are
responsible for this sensitivity, vary considerably (Hill, 1958;
Setlow & Duggan, 1964; Lewis & Kumta, 1972). The enzymes responsible
for the repairs of the lesions are also genetically coded as are the
enzymes responsible for regulating the expression of the repair
enzymes (Hanawalt & Setlow, 1975).
3.3.5 Repair of photolesions in bacteria
All the modes of repair described in section 3.2 are applicable
to, and were mainly discovered in, bacteria. As long ago as 1963,
Setlow & Setlow determined, inter alia, the photoreactivation
mechanisms demonstrating that monomerization was related to the
decrease in the number of photolesions.
3.3.6 Yeasts
Yeasts are microorganisms but are nevertheless eukaryotic cells.
Resnick (1969), Fabre (1971), Cox & Game (1974), and Haynes (1975),
among others, have obtained a great variety of mutants with different
photosensitivities. These mutants differ from the wild type with
regard to UV lethality, mutagenesis, and recombination. Genetic
analysis has shown that several recessive genes are involved in the
control of these responses in a way that is already much more complex
than that found in bacteria. Moustacchi et al. (1975) have reviewed
the specific aspects of repair mechanisms in yeasts as a model for
eukaryotic cell systems.
3.4 Protozoa
Protozoa, including the ciliata paramecium, tetrahymena, and
blepharisma, and amoebae have proved very useful as tools for studies
on the biological effects of UVR (Skreb et al., 1972; Whitson, 1972).
3.5 Effects on Mammalian Cells in Culture
An attempt has been made to apply the knowledge of the mechanisms
of photolesion formation and repair discovered in microorganisms to a
model more similar to the human body; namely established strains of
mammalian cell cultures. The strains most commonly used are HeLa
(human cells of cancerous origin), mouse L fibroblasts and Chinese
hamster cells.
The ability of a surviving cell to form a colony (Lee & Puck,
1960) and the numerous parameters used in radiobiology (Elkind &
Whitmore, 1967) have been widely used to study the effects of UVR on
this material. Among the numerous works on this subject, reference has
often been made to the review paper by Rauth (1970).
3.5.1 Sublethal effects
The most marked effects of UVR on cells are death, mutagenesis,
and malignant transformation. Sublethal effects include various
degrees of inhibition of growth and colony-forming ability. At low
doses, the growth of L fibroblasts is merely slowed down. Higher
doses, however, bring about lysis of the cells (Djordjevic & Tolmach,
1967), although the value of lysis as a parameter is doubtful. All
cell strains give a similar response.
In a study of the colony-forming ability of synchronized and then
irradiated cells, Djordjevic & Tolmach (1967) and Han & Sinclair
(1969, 1971) found that cells were quite resistant at the beginning of
G1 but that sensitivity began to increase at the end of G1 reaching
a peak in the middle of the S phase of DNA synthesis. Thereafter,
sensitivity gradually decreased. In G2, the situation varied
according to the type of cell. However, all authors agree that peak
sensitivity occurs from the beginning to the middle of the S phase of
the cell cycle.
3.5.2 Effects of UV-A
Studies of the effects of UV-A on bacteria have made it possible
to elucidate, at least in part, the complex phenomena that occur in
mammalian cells. Wang et al. (1974) showed that cells can be damaged
by UV-A or visible light (300-420 nm with a peak at 365 nm). The
shoulder of the inactivation curve changes according to cell density,
but the curves remain similar according to the origin of the strain.
Evaluation of viability with trypan blue stain showed that, after
exposure to 2 X 104 J/m2, 99% of human cells, 90% of mouse cells,
and 50% of hamster cells were destroyed.
The fact that survival depends on the density of the cell
population suggests that perhaps some of the effects of UV-A are
indirect. This is supported by the observation of Wang (1975) that the
medium in which cells have been irradiated is toxic. Wang et al.,
(1974) have also shown that riboflavin may cause considerable
photosensitization of cells exposed to UV-A.
3.5.3 Lesions produced in DNA
The lesions produced by UVR in mammalian cellular DNA may be
divided into two categories that are not mutually exclusive, i.e.,
those that prevent replication processes and those that permit
replication processes, but with considerable error.
3.5.3.1 Pyrimidine dimers
It is thought that, as in other types of cells already mentioned,
the formation of pyrimidine dimers results in the essential lesion
that causes most of the effects observed. However, not all the
observed effects can be attributed to dimers and other photoproducts
should not be neglected.
3.5.3.2 DNA-protein cross-links
As demonstrated some time ago (Smith & Hanawalt, 1969),
DNA-protein cross-links are to be expected in view of the
configuration of the DNA molecule, with its backbone folded back on
itself and its association with chromosomal proteins.
DNA-protein cross-links may also help to kill cells by preventing
the switching-on of repair processes (Todd & Han, 1976).
3.5.4 The consequences of photolesions in mammalian cells
3.5.4.1 Inhibition of DNA synthesis
As in microorganisms, the major effect in mammalian cells is a
more or less marked and long-lasting inhibition of DNA synthesis. The
rate of synthesis can be estimated directly by incorporating tritiated
thymidine into the DNA.
3.5.4.2 Chromosome aberrations and mutagenic effects
As regards the effects of UVR on the chromosomes of mammalian
cells, several types of lesions were described long ago including
breaks and rearrangement. A review has been published by Rauth (1970).
These lesions have been studied (among others) in Chinese hamster
cells and human lymphocytes. Chromosome lesions are generally produced
by low doses of UVR. Their production is enzyme-dependent and is
related to repair mechanisms (Bender et al., 1973). They do not
necessarily appear after the first division (Parrington, 1972). They
can be photoreactivated and, therefore, are probably produced by
pyrimidine dimers (Griggs & Bender, 1973). Rommelaere et al., (1973)
discovered another widespread type of lesion in the form of
sister-chromatid exchanges, the frequency of which increased greatly
after UV irradiation. This parameter has been shown to be extremely
sensitive (Ikushima & Wolff, 1974) and, thus, valuable in detecting
very low doses of UVR, although it is not specific to that agent.
UV and X-ray irradiation exert a synergistic effect on the
frequency of chromosome breaks in human lymphocytes (Holmberg &
Jonasson, 1974).
3.5.5 The repair of photolesions
Animal cells in culture also possess the ability to repair
photolesions in DNA. While the lesions are comparable to those
observed in bacteria, and have already been described, there are some
differences in the repair mechanisms (Hanawalt & Setlow, 1975).
3.5.5.1 Photoreactivation
It was believed that photoreactivation was absent from the cells
of placental mammals. However, Sutherland (1974), using appropriate
biochemical techniques, isolated an enzyme in human leukocytes with
properties similar to those of the photoreactivating enzyme. In
mammals, this enzyme is probably not expressed or is masked by other
more effective repair mechanisms. Its presence in other tissues --
nervous, hepatic, and renal -- which are never exposed to light,
suggests that it may have other functions.
3.5.5.2 Excision repair
The number of dimers formed in human cells increases linearly
with the dose of UVR, if the cells are fixed immediately after
irradiation (Cleaver & Trosko, 1969). After a variable lapse of time,
cells begin to excise their dimers in the way already described
(Cleaver et al., 1972). Edenberg & Hanawalt (1973) showed that, four
hours after irradiation with 0.2 J/m2, about 50% of the dimers had
been excised. The proportion varied between 30 and 90% depending on
the type of cell.
Repair replication had already been detected by autoradiography
and shown to be unscheduled DNA synthesis which differed from the
synthesis of normal replication (Rasmussen & Painter, 1964).
Excision repair has been detected in rodents even though the
efficiency is very low.
3.5.5.3 Repair during or after replication
Numerous authors have shown by various biochemical methods that
synthesis of DNA of low relative molecular mass takes place
immediately after irradiation and that, a short time afterwards, this
newly-formed DNA is of normal mass (Painter, 1975).
The dimers prevent replication from being carried out
continuously. Replication probably occurs between the dimers that have
not yet been excised, leaving gaps in the new strands opposite each
dimer of the parental strand (Lehmann, 1972). The mechanism by which
replication fills the gaps has not yet been properly elucidated, nor
has the degree to which the replication is error-free. It is probable
that part, at least, of the repair is inaccurate as a result of errors
of replication opposite a lesion. Depending on the degree of accuracy,
either repair is total or a lesion persists that may lead to death,
mutation, or carcinogenesis.
3.5.5.4 SOS repair
As will be seen later, in mammalian cells, the repair systems of
the host cell may play a part in repairing an irradiated virus
developing in the cell.
3.5.6. Effects on cell-virus relationships
3.5.6.1 Sensitivity to vital infection
Infection with herpes virus is enhanced in mammalian cells
exposed to UV-A (Mills et al., 1975). Relatively low exposures
increase infectivity by 20-30% and it remains high for several days.
3.5.6.2 Vital transformation
UV-C increases the rate of transformation of mouse and hamster
cells by various viruses (Lytle et al., 1970). However, it does not
induce direct transformation in all cell species. Certain
photosensitizing agents enhance this viral transformation (Casto,
1973).
3.5.6.3 Activation of viruses
Since some mammalian cells harbour viruses that normally remain
latent, their activation or induction might transform them and endow
them with the characteristics of cancer cells. UV-C exposure of rat or
hamster cells, transformed by certain oncogenic viruses, can activate
the production of virus particles (Hellman et al., 1974), whereas
irradiation of normal cells can activate tumour viruses of the
leukemia-leukosis type (Lytle, 1971).
However, there is nothing to show that malignant transformation
necessarily involves the development of an oncogenic virus.
3.6 Effects on Invertebrates
Since the invertebrates are extremely heterogeneous, it is very
difficult to draw general conclusions, and the data on the response of
certain invertebrates will be given without such conclusions.
3.6.1 Effects on eggs and embryos of invertebrates
Hamilton (1973) emphasized the value of invertebrate eggs for
radiation studies. Since UVR has low penetration, only very
transparent small eggs can be totally irradiated. The others must be
stripped or dechorionated by physical or chemical means. The whole egg
or part of an egg has been irradiated with UVR mainly to destroy
certain cells, in order to watch how development proceeds in their
absence and thus deduce the role they play. Some doses, while not
preventing segmentation, arrest the onset of differentiation at the
sensitive stage represented by the end of blastulation and the
beginning of gastrulation.
Wavelengths of 225-313 nm have caused appreciable delays in
development. Undivided eggs have been shown to be highly sensitive to
UVR (Hsiao, 1975). There has been very little work using UV-A.
In conclusion, it may be said that, in general, eggs are well
protected against the harmful effects of UVR. If they are directly
exposed, they respond to UV irradiation by means of the mechanisms
already described. From the time when the cells begin to
differentiate, the quality and intensity of the photolesions and their
repair depend on the stage of differentiation of the embryo. If it is
not an advanced stage, regulatory mechanisms sometimes enable
photolesions produced at an early stage, when the cells are still
totipotential, to be eliminated naturally.
3.6.2 Effects on insects
Insects comprise a major part of ecosystems. UVR may act on the
vital processes of the insects in different ways and these have been
summarized by Hsiao (1975).
From the large numbers of papers that have been written, a first
conclusion is that UV-B is perceived by numerous insects. Several
diurnal and nocturnal species show positive phototropism towards UVR.
Most of the UV-A lamps used to trap insects have an emission spectrum
with a peak at 360 nm corresponding to the peak sensitivity of the UV
receptors in the insects. However, the peak differs slightly for each
species.
Solar UVR has been found to modify the biological clock in
insects and other arthropods.
Photoreactivation and other types of repair have been
demonstrated in insects.
3.7 Modification of the Effects of Ultraviolet Radiation by Chemical
Agents
Numerous chemical agents increase the photochemical reactivity of
nucleic acids. They act in various ways, by becoming incorporated in
the nucleic acids, or by forming various complexes with them that
increase their absorption or reactivity. They can also absorb UVR
directly and transfer the energy to nucleic acids.
Various physical factors may change the intensity of irradiation
and the efficiency of the repair systems.
The way in which irradiation is carried out, ie., whether it is
continuous or fractionated, total or partial is also important in the
evaluation of the final results.
3.7.1 Halogenated analogues
Incorporation of 5-bromouracil (5-BrU) and 5-bromodeoxyuridine
(5-BUdR) sensitizes both viruses and cells to UVR. A very detailed
review has been made by Hutchinson (1973).
3.7.2 Caffeine
Caffeine is a trimethylxanthine that acts by inhibiting the
repair systems. At doses not themselves toxic, caffeine considerably
increases the effects of UVR.
The absence of any effect of caffeine on excision repair suggests
that it acts by interfering with a post-replication system (Cleaver &
Thomas, 1969).
3.7.3 Furocoumarins
Photosensitizers are becoming increasingly important among agents
that modify the effects of UVR on biological systems.
Furocoumarins are natural products isolated from plants.
Important applications in therapy have led to thorough studies of
their mode of action at the molecular level (Chandra, 1972; Pathak et
al., 1974).
Musajo et al., (1974) have shown, in vitro, that furocoumarins
exert photosensitizing effects following irradiation at wavelengths of
320-400 nm. These effects result from the formation of certain
addition products with DNA and particularly from the linking of the
furocoumarin molecule with the pyrimide bases. These products may
cause breaks in the molecule and thus prevent if from carrying out its
functions. Oxidation of amino acids may also occur in proteins.
The so-called 'linear' furocoumarins such as psoralen react with
native DNA in the presence of UVR to make, monofunctional and
bifunctional addition products. The latter cause cross-linking between
the DNA strands. Certain so-called 'angular' furocoumarins (angelicin)
can only give rise to monofunctional addition products.
These addition products cause lesions that can be repaired by the
excision repair mechanism. Monofunctional addition products seem to be
easier to repair than bifunctional products (Chandra et al., 1976).
Depending on their molecular structure, not all the furocoumarins
show the same degree of photosensitization. Ben Hur & Elkind (1973)
demonstrated that, following exposure of hamster cells to UV-A, 11% of
the addition products were formed between the complementary strands.
During incubation in the dark, 90% of the cross-links gradually
disappeared from the DNA.
These reactions have found applications in the treatment of
certain skin diseases, particularly psoriasis.
3.7.4 Other photosensitizing agents
Charlier & Helene (1972) carried out an in vitro analysis of
the photochemical reactions of benzophenone and acetophenone with
purine and pyrimide derivatives in aqueous solution during irradiation
with light between 400 and 600 nm. Dimers and chain breaks were the
essential lesions.
The effects of light on bacteria in the presence of
photosensitizing chemicals such as toluidine blue and acridine yellow
were compared by Harrison (1967). The four sorts of lesions observed
-- lack of colony-forming capacity, DNA lesions and mutations, changes
in cell permeability, and enzyme inactivation -- were similar in both
cases.
Rauth & Domon (1973) studied the mechanisms of photosensitization
in animal cells in cultures with 1-cyclohexyl-3(2-morpholinyl-4-ethyl)
carbodiimide metho- p-toluene sulfonate (CMEC), which binds
preferentially to partially denatured regions of DNA after irradiation
at 254 nm, thus inhibiting replication and increasing lethality.
3.7.5 Protection by carotene
Since the work of Cohen-Bazire & Stanier (1958), some
investigations have established the protective role of carotene
against the photodynamic effects of light, in very special cases.
Since the energy level of carotenes is very low, they can accept, by
transfer, the energy of sensitizers transformed into the triplet state
and even the energy of oxygen transformed into the singlet state by
exposure to light.
Mutant strains of bacteria and fungi lacking carotenoids have
proved much more sensitive to photodynamic effects than normal
strains, but this effect is only valid for wavelengths shorter than
those that carotenes absorb (Mathews & Krinsky, 1965).
3.8 Conclusions
Knowledge of the molecular basis for the biological effects of
UVR has emphasized the importance of the photolesions produced in DNA
and the effectiveness of the enzymatic stages of repair, both of which
depend on the genetic make-up of the organism concerned.
Studies of the sensitivity of mutants, on one hand, and of
factors able to modify the effects of irradiation, on the other, will,
perhaps, make it possible to strengthen repair systems and to increase
the resistance of organisms to UVR.
The great differences observed make it difficult to lay down
protection standards.
4. THE BIOLOGICAL ACTION OF ULTRAVIOLET RADIATION ON VERTEBRATE
ANIMALS
4.1 General Aspects
The anatomical structure of the skin of vertebrate animals
resembles that of man in many respects. The surface stratum corneum is
important in affecting the penetration of UVR (Fig. 4), the
thicknesses of the stratum spinosum and stratum granulosum also affect
the relative amounts of UVR reaching the dermis. Other constituents of
the skin, e.g., the melanocyte population, the Langerhans cells,
vascular structures, as well as cutaneous innervation vary in amount
and distribution from species to species, but remain basically the
same.
 4.2 Acute Reactions in the Skin
4.2.1 Epidermal changes
Histological investigations of the early effects of UV
irradiation on animal skin show the changes that occur in the
epidermal cells particularly well.
Twenty-four hours after a single irradiation of skin with an
unfiltered mercury vapour lamp at doses between four and eight times
the minimal erythema dose, distinct signs of cell injury can be seen
including vacuolation of the cell cytoplasm and increased or decreased
density of the nucleus (Stenbäck, 1975a).
One distinctive feature, observed in both man (Daniels et al.,
1961) and experimental animals (Woodcock & Magnus, 1976) 24 hours
after exposure, is the presence of "sunburn cells" (SBC), scattered
diffusely throughout the superficial epidermis. These cells are
characterized by a densely-staining, glassy, homogeneous cytoplasm and
pyknotic nuclei. The appearance is similar to that of an individual
cell undergoing keratinization and thus, the process is regarded as a
form of individual cell shrinkage necrosis or dyskeratosis.
Following these early effects, hyperplasia, induced by radiation,
is observed (Blum et al., 1959; Stenbäck, 1975a). An increase in
mitosis indicates the onset of hyperplasia, which begins after about
two days.
Quantitative measurements have been made of the following aspects
of the effects of single doses of UVR on mouse skin (Blum et al.,
1959): (a) incidence of mitotic figures; (b) number of epidermal cells
per unit area; (c) epidermal thickness; (d) dermal thickness; (e) mean
nuclear diameter; and (f) number of groups of mitotic figures. All the
aspects measured changed in a similar fashion quantitatively following
irradiation; they increased rapidly at first, reaching a maximum and
then fell gradually towards normal. Soffen & Blum (1961) found a
gradual increase in epidermal thickness reaching a maximum between 8
and 14 days. These changes represent repair of the cell injury caused
by UVR; at the same time, the hyperplasia protects against further UV
irradiation.
For a long time, it was believed that thickening of the horny
layer was the essential adaptive change to UV irradiation in the
environment. The idea now prevails that melanin granules in the horny
layer and the Malpighian layer accumulate to form a protective screen
which is not only significant, but possibly even more important for
light protection than the thickness of the horny layer alone.
4.2.2 Erythema and inflammation
Sunburn (UVR erythema) and suntanning are the visible signs of UV
injury to the skin and the repair of such injury. UV erythema is
evidence of an inflammatory reaction to radiation, and appears after a
latent period of a few hours.
Erythema caused by UVR is confined to the exposed areas and
reflects blood vessel dilation and increased blood flow in the dermis.
It is often assumed that the initial photochemical reaction is in the
epidermis, where photon absorption by keratinocytes may lead to
liberation of intracellular substances that diffuse into the papillary
dermis to cause vasodilation. This diffusion theory is supported by
the existence of a latent period between exposure and erythema, and by
the fact that much of the radiant energy of the erythemogenic
wavelength region is absorbed by the epidermis. However, there may
also be direct injury to the vascular endothelium or to other sites in
the dermis.
Most studies of the mechanism of "sunburn" have used artificial
sources of UV-B or sources in which the spectral distribution is such
that UV-B is assumed to provide the major erythemogenic influence. In
experimental animals, the vascular response to UVR is biphasic. A
transient immediate vasopermeability is followed, after a latent
period of 2-8 hours, by a delayed, prolonged, increased,
vasopermeability and vasodilation. In some animal models, the initial
vasopermeability is accompanied by a faint erythema, which may begin
during exposure. This immediate effect has been attributed to
histamine release, possibly due to a direct effect of photons on
dermal mast cells. There is evidence that serotonin may also play some
role. In rats and guineapigs, serotonin and histamine antagonists
suppress the immediate phase of UVR vascular responses. In vivo
studies of human skin have shown the transient appearance of kinins
within minutes of UV irradiation. Kinin was not found after the onset
of delayed erythema.
The mediators of the delayed phase of UVR-induced vascular
response have been difficult to define. Antihistamines do not suppress
the delayed erythema phase of the vascular response to UVR in
guineapigs, rats, or man. Kinins have been stated to be either absent
or not elevated. Delayed erythema was not suppressed by various
inhibitors of proteases, plasminogen activitors, or kallikrein.
Serotonin has been found in urine following exposure to UVR, but the
significance of this finding is not clear.
More recently, prostaglandins, a group of long-chain fatty acids
with vasoactive properties, have been implicated as possible mediators
of the delayed phase of erythema. Prostaglandins are produced in human
and animal skin (prostaglandin groups E and F), intradermal injection
of prostaglandins produces erythema (PGE mainly, PGF group are much
less active), and furthermore the production of prostaglandins
increases following UVR exposure. Indomethacin is a potent inhibitor
of the conversion of arachnidonic acid to active prostaglandin, a
reaction catalyzed by the enzyme prostaglandin synthase. Topical
indemethacin produces a profound and prolonged blanching of
UV-B-induced, delayed erythema in both human subjects and guineapigs.
In man, intradermal indomethacin has been shown to consistently
decrease erythema due to UV-B but not all areas of erythema caused by
irradiation with UV-C; these effects appear to be due to the
inhibition of prostaglandin synthase.
It has been suggested that the complex reaction known as
"sunburn" may result from the release of hydrolytic enzymes and
possibly other substances by lysosomes within keratinocytes.
Histochemical studies of human skin exposed to UVR were
consistent with the theory that specific damage to lysosomal membranes
caused partial to complete rupture and release of enzymes.
The release of lysosomal enzymes may not only lead to damage of
the keratinocytes but may also release enzymes, vasodilator
substances, or subsequently formed cell-breakdown products into the
dermis, where they may directly or indirectly lead to erythema. It has
been suggested that the immediate erythema caused by UV irradiation
results from disruption of lysosomes of endothelial cells with release
of chemical mediators. The delayed erythema could then result from
secondary diffusion of proteinases from the epidermis, following
lysosomal rupture. It is also possible that direct photon damage to
the lysosomes of mast cells of the dermis or endothelial cells of
dermal blood vessels may play a role in the delayed erythema of
sunburn.
Multiple chromophores may exist in skin, and irradiation probably
leads to activation of a complicated cascade of mediators, the final
endpoint of which is erythema. Multiple pathways may exist. It is also
possible that photons have direct effects on blood vessels or nerves.
UVR causes dilation of isolated exposed dermal blood vessels. Dermal
proteins may be directly changed by radiation (Magnus, 1977).
4.2.3 Tanning
In addition to erythema, another consequence of exposure to UVR
is the pigmentation of the skin generally known as "tanning". This
becomes noticeable about 48 h after exposure and increases gradually
for several days. Tanning is, in part, due to migration of the pigment
melanin already present in the basal cells to the more superficial
layers of the skin where it has a greater effect on the appearance of
the reflected light. It is also partly due to the formation of new
pigment.
It is generally accepted that repeated irradiation with UVR
induces increases in the population of melanocytes in the epidermis of
man and experimental animals. With regard to the mechanism of this
phenomenon, the following processes or possibilities have been
considered: (a) increased division of melanocytes; (b) activation of
pigment formation in amelanogenic melanocytes: (c) migration of dermal
melanocytes into the epidermis; (d) various combinations of these
processes (Quevedo et al., 1965). That division of melanocytes occurs
has been shown in murine skin by Quevedo et al. (1963). Further,
published reports have provided evidence in support of the occurrence
of activation of amelogenic melanocytes in man (Mishima & Widlan,
1967) and experimental animals (Reynolds, 1954; Quevedo & Smith, 1963;
Miyazaki et al., 1968; Sato, 1971). However, the relative contribution
of each process remains to be established. In a study using tritiated
thymidine, it was demonstrated that approximately 1.1% of
dopa-positive epidermal melanocytes were labelled in hairless mice
receiving 9 daily exposures to UVR (Sato & Kawada, 1972). However, an
attempt to trace the labelled melanocytes using split epidermal sheets
was unsuccessful.
4.3 Acute Changes in the Eye
4.3.1 Photokeratitis and photoconjunctivitis
Although more energetic than the visible portion of the
electromagnetic spectrum, UVR cannot be detected by the visual
receptors in mammals, including man, because of absorption by the
ocular media. Thus, exposure to UVR may result in ocular damage before
the recipient is aware of the potential danger. Many cases of
keratitis of the cornea and cataracts of the lens have been reported
due to exposure to UVR produced by welding arcs, high-pressure pulsed
lamps, and the reflection of solar radiation from snow and sand.
Extensive reviews of the literature on the biological effects of
UVR have been compiled by Verhoeff et al. (1916) and Buchanan et al.
(1960). Verhoeff and his colleagues included all the, then available,
research data in their report and formulated some of the basic
hypotheses regarding ocular damage caused by UVR. Research on
threshold values and destructive and repair processes was summarized
by Duke-Elder (1926). Buschke et al. (1945) stressed the destructive
effects of UVR on the corneal epithelial cell nuclei, the loss of
epithelial adhesion to Bowman's membrane, and the inhibiting effects
of UVR on the healing process.
In studies by Cogan & Kinsey (1946), a monochromator was used to
evaluate the sensitivity of the cornea to individual spectral lines.
Their work, which was, for the most part, carried out with one to four
rabbits, provides the most reliable quantitative data on damaging
threshold values of UV energy of individual wavelengths. They
established a long wavelength limit of between 306 and 326 nm and a
threshold of 152 J/m2 at 288 nm for the rabbit.
Ordinary clinical photokeratitis is characterized by a period of
latency that tends to vary inversely with the severity of exposure.
The latent period may be as short as 30 min or as long as 24 h, but is
usually 6-12 h. Conjunctivitis follows, often accompanied by erythema
of facial skin and eyelids. There is a sensation of a foreign body or
"sand" in the eyes and various degrees of photophobia, lacrimation,
and blepharospasm. The importance of these acute symptoms lies in the
fact that the individual is visually incapacitated for 6-24 h and that
the ocular system, unlike the skin, does not develop tolerance to
repeated exposure to UVR. Almost all discomfort disappears within
48 h, and exposure rarely results in permanent damage.
Pitts & Kay (1969) and Pitts (1970) sought to establish the
experimental threshold for photokeratitis in rabbits and primates,
including man. Rabbits and monkeys showed a maximum sensitivity to UVR
at 270 nm. The radiant exposure threshold for man at 270 nm was
50J/m2 compared with 110 J/m2 for the rabbit and 60 J/m2
for the monkey.
The UVR incident on the eye is absorbed in turn by the cornea,
the aqueous humor, the lens, and the vitreous humor before reaching
the retina. Absorption is greater in the lens than in the cornea, and
is least in the aqueous humor. Below 300 nm, most of the UVR is
absorbed in the cornea and aqueous humor and very little penetrates as
far as the lens.
As a result of observations at above-threshold intensities, it
was felt that the reaction of the cornea to exposure to UVR wavebands
of 220 to 250 nm was different from that to wavebands of 250 to
310 nm. For exposures below 250 nm, signs and symptoms occurred soon after
exposure, and symptoms always returned to normal approximately 14 h
later. For exposure above 250 nm, symptoms did not generally occur
until 9 to 11 h after exposure, and visual acuity remained below
normal for 24 h after exposure. The differences observed were
attributed to the differences in the absorption of the different
wavebands. The shorter wavebands are absorbed in the outer corneal
epithelial layers, which undergo rapid change, whereas the longer
wavebands are absorbed in the deeper epithelial layers which show
delayed changes because these cells are more viable. Thus, the lesions
produced by shorter wavelengths are rapidly repaired while, at the
longer wavelengths, there is a delayed and more serious response
(Pitts & Cullen, 1977).
4.3.2 Cataracts
A cataract is a partial or complete loss of transparency of the
crystalline lens or its capsule. The wavelengths that affect the lens
appear to be in the same area as those that are most effective in
producing erythema on human skin.
Bachem (1956), using a filtered UVR system, concluded that
exposure to repeated high doses of longer UVR wavelengths could cause
cataracts through cumulative effects. He reported that the action
spectrum for cataracts began abruptly between 293 and 297 nm, reached
a peak near 297 nm, and fell abruptly near 313 nm. Minimal effects
existed through the remainder of the near UVR. In both the rabbit and
guineapig, reversible lenticular "blurring" occurred 5 to 10 days
after exposure. With repeated excessive exposures to the 297-355 nm
wavebands, irreversible lenticular opacities occurred after a latent
period of between 2.5 and 15 months.
Bachem (1956) concluded that since daylight does not contain any
UV-C or far infrared, and since both the visible and near infrared are
freely transmitted by the ocular media, it would appear that UV-B and
A are responsible for cataracts.
The chemical effects of UV-A exposure on tryptophan were studied
by Zigman et al. (1973) using human crystalline lenses. They found
that exposure of tryptophan to UV-A led to the formation of chromatic
photoproducts which bound to the lens proteins, altered their colour
and changed the solubility. Human lens material without added
tryptophan did not show chromatic changes on exposure to UV-A, until
48 h after exposure. Tryptophan showed an excitation wavelength at
278 nm and a fluorescent emission at 330 nm. However, following
exposure to UV-A, tryptophan showed an additional 360 nm excitation
and 440 nm fluorescence similar to that found in the brunescent human
cataract lenses. The UV irradiance for these studies was 30-50 W/m2
at 365 nm and exposures were made for at least several hours. These
exposure levels exceed those expected from sunlight for the same
period of time.
Thus, exposure of the eye to UV-A, for sufficient periods of
time, with an irradiance comparable to the irradiance level of
sunlight may interfere with the synthesis of lens proteins, catalyse
insoluble lens protein, and may result in chromatic changes in the
lens. While the basic mechanisms remain to be found, the evidence
clearly demonstrates that both in vitro and in vivo exposure to
UV-A can enhance cataractous changes in the crystalline lens.
Recently, Pitts & Cullen (1977) have studied the effects of the
300 nm-400 nm wavelength range on the rabbit eye in vivo. The
criteria used to determine corneal damage were epithelial debris,
epithelial stippling, epithelial granules, epithelial haze, epithelial
exfoliation, stromal haze, stromal opacities, and endothelial
disturbances. Anterior chamber signs included flare and cells. The
crystalline lens criteria were subcapsular opacities, capsular and
stromal haze, stromal opacities, and increased prominence of the
anterior suture. Criteria for the iris were the presence of the
anterior chamber signs, changes in clarity of the iris stroma, and
sluggish pupillary response.
Tables 3 and 4 show data for corneal and lenticular damage in the
290 nm-400 nm wavelength range for the rabbit. Limited data above
300 nm for the human eye do not allow a detailed comparison; however,
the human corneal threshold was considerably below that for either
the rabbit or the nonhuman primate.
Table 3. UV threshold data for the rabbit cornea and lensa
Threshold radiant exposure
Wavelength Threshold radiant exposure (J/m2) permanent damage (J/m2)
(nm)
corneal lens time to lens time for permanent
threshold threshold disappear threshold damage to appear
295 200 7500 24 h 10 000 2 h
300 500 1500 3 days 5000 24 h
305 700 3000 7 days 5000 24 h
310 550 7500 2 weeks 15 000 24 h
315 22 500 45 000 1 week 60 000 24 h
320 75 000 > 80 000 -- -- --
335 109 000 > 150 000 -- -- --
365 > 250 000 > 250 000 -- -- --
a From: Pitts & Cullen (1977).
Table 4. Permanent lenticular opacitiesa
Wavelength Radiant exposure Appearance of Permanenceb of
(nm) (J/m2) lens opacities lens opacities
300 5000 24 h permanent
305 5000 24 h permanent
310 15 000 24 h permanent
315 60 000 24 h permanent
a From: Pitts & Cullen (1977).
b Lenticular opacities present one month after exposure.
Phototoxic psoralen derivatives have been used recently in the
treatment of certain dermatological conditions. Such compounds caused
UV-A-induced corneal opacities and cataracts in mice (Griffin, 1959;
Koch, 1967).
Research on the effects of psoralens on the production of
cataracts in man needs to be pursued. As more and more people are
subjected to dermatological treatment using UV photosensitizing
compounds, the role of these compounds and their effects on the ocular
system should be defined.
4.4 Effects of Long-Term Exposure of Skin to UVR
UV irradiation induces an inflammatory response and ulceration in
both the epidermis and the dermis, the latter being infiltrated with
leukocytes in the region of the lesions, and to a much lesser extent
between them. These lesions ulcerate and the epidermis may disappear
for a time in the centre. However, peripherally there is particularly
active hyperplasia. The basal membrane (between the epidermis and
dermis) may disappear for a time in the regions of these "open"
lesions. Between the lesions, the infiltration of leukocytes is
relatively slight.
Injury to the epidermis and dermis, brought about by long-term
exposure to UVR leads to dermal alteration, fibrosis, and elastosis,
as well as to epidermal atrophy. However, experimental production of
cutaneous elastotic changes in animals by artificial UV irradiation
has only been reported rarely. Using histochemical methods, Sams et
al. (1964) demonstrated focal dermal elastosis in mice after prolonged
exposure to artificial UVR. UVR-induced changes in connective tissue
were also seen in rat skin by Nakamura & Johnson (1968).
4.4.1 UVR-induced mutagenesis and carcinogenesis
4.4.1.1 Mutagenesis
The nature of the effects of UVR on DNA, described earlier, shows
the importance and the specificity of the lesions in genetic material
and focuses interest on the mutations that might result in the various
cell types studied. The production of such mutations has best been
demonstrated in bacteria.
Among many others, Grossman (1968), Eisenstark (1971), and Witkin
(1976) have proposed, analysed, and verified some complex mechanisms
that control UVR-induced mutagenesis. It will be recalled also that,
according to the results obtained by Radman (1975), most of the
UVR-induced mutations would appear to be errors introduced in the DNA
during error-prone SOS repair.
Mammalian cells in culture have proved to be very suitable
material for the study of UVR mutagenesis (Kao & Puck, 1969). There is
quite good agreement between the dose of UVR and the proportion of
mutants (Bridges & Huckle, 1970).
Fox (1974) has shown that caffeine can reduce the rate of
mutations induced by UVR in cultures of rodent cells by inhibiting a
process of error-prone repair.
Isolation and study of UVR sensitive mutants in animal cells have
found remarkable applications in the study of various human xeroderma
pigmentosum mutants (Cleaver, 1973). However, such mutations in
mammalian germinal cells are not possible, because the cells are
located below the depths of penetration of UVR.
4.4.1.2 Mechanism of UVR carcinogenesis
In order to understand the possible mechanisms of UVR
carcinogenesis, it has been necessary to study the formation and
excision of pyrimidine directs, unscheduled DNA synthesis, and all the
data valid for the range from bacteria to mammalian cells.
It is now generally believed that UVR carcinogenesis results from
a succession of events originating in a photolesion of the genetic
material.
From the numerous studies that have led to en elucidation of some
of these mechanisms, it emerges that faulty DNA repair can increase
the frequency of carcinogenesis in the following ways: by causing
alterations in DNA, which also find expression in an increased
frequency of chromosome aberrations and a rise in mutation rate; by
increasing the rate of transformation of normal cells into cancer
cells; and by facilitating the expression of latent oncogenic viruses
able to trigger cancerous growth (Setlow, 1973; Trosko & Chu, 1973).
Errors induced by DNA repair during the initiation phase of
carcinogenesis seem to be the most likely mechanism leading to UVR
cancers.
4.4.1.3 Tumour types
Epidermal tumours. The first visible step in UVR-induced
epidermal tumour formation in animal skin consists of cell
proliferation, i.e., an increase in the number of squamous cells and
cell layers, which gradually become papillomatous in character
(Stenbäck, 1978). This is accompanied by an increase in cellular
atypia, nuclear enlargement, hyperchromatism, indentation, and
prominence of nucleoli. This basically proliferative response is
frequently replaced by a dysplastic progression showing a solar
keratosis-like pattern with cellular pleomorphism, occasionally with
pseudo-epitheliomatous hyperplasia-like features, which ultimately
invade the dermis. The tumours first seen are acanthomatous papillomas
(trichoepitheliomas), with a predominantly epithelial component or
fibropapillomas in which the tumour is composed of a fibrous stroma
covered by squamous epithelium.
The malignant tumours that ultimately develop are squamous cell
carcinomas of different types including: solid keratin containing
tumours; moderately differentiated, individually keratinizing tumours
with distinct intercellular bridges; and less-differentiated,
non-keratinizing spindle cell tumours, in which ultrastructural
analysis reveals squamous cell patterns.
Keratoacanthomas, i.e., proliferating epithelium on a cup-shaped
base, are relatively more frequent in animals of different species
receiving large doses of UVR than in animals treated with chemical
carcinogens.
Epidermal tumours are easily induced by different agents in mouse
skin (Stenbäck, 1969). Winkelman et al. (1960) reported the production
of squamous cell carcinomas on the backs of hairless mice exposed to
UVR. Further studies established that carcinomas could be induced in
this animal almost to the exclusion of sarcoma formation (Epstein &
Epstein, 1963). In early studies, epithelial tumours were reported in
both rats and mice (Findlay, 1930; Herlitz et al., 1930; Putschar &
Holz, 1930), but in later studies the deeper lying dermal tumour
response to UVR predominated. No mention of skin sarcomas was made,
however, in the studies of Beard et al. (1936) on albino rats in which
12 animals exhibited 9 carcinomas of the external ear, 6 sarcomas of
the eye, and 1 carcinoma of the skin of the nose.
The difference in the distribution of tumour types, with
sarcomatous growth predominating in haired mice and carcinomas in man
(Urbach, 1969) may, in part, be explained by the difference in
penetration of UVR, a greater amount reaching the dermis in mice
(Kirby-Smith et al., 1942; Everett et al., 1966).
Dermal tumours. Another type of neoplastic progression seen in
mice, particularly after intensive treatment with large doses of UVR
over a short time period, consists of ulceration, scarring, and the
subsequent formation of dermal tumours. These tumours begin as
aggregates of regularly built, elongated cells with small monomorphic
nuclei. Epithelial proliferation is occasionally observed as a
secondary phenomenon. The tumours rarely extend grossly through the
surface. In the early stages, they appear to be papillomas, although
they consist entirely of fibroblastic cells. Some tumours are
remarkably acellular, with a prominent fibrillary pattern. The tumours
are composed of large, polymorphic cells with prominent nuclei.
Ultrastructural analysis shows the predominant cellular components --
a dark cell type, with hyperchromatic nuclei and scanty cytoplasm, and
a light cell type, with large nuclei and abundant cytoplasmic
ribosomes (Stenbäck, 1975a). The same cell types are also seen in
malignant tumours, in which the cellular polymorphism frequently is
considerable, with nuclear atypism and enlargement, numerous nucleoli
and a generally disorganized arrangement. Sarcoma induction is partly
species-specific, as these tumours were not seen in UV-irradiated
Syrian golden hamsters (Stenbäck, 1975b) nor were they seen in
hairless mice (Epstein & Epstein, 1963) or guineapigs, susceptible to
chemical sarcoma induction (Stenbäck, 1969, 1975b).
An infrequent neoplastic alteration in several animal species is
the vascular tumour (Stenbäck, 1975b). This begins as a proliferation
of dilated vascular spaces with regular endothelial lining. Rarely,
the endothelium proliferates to the point of forming angiosarcomas, or
invasive tumours composed of large, atypical cells arranged in a
nodular pattern.
The role of the dermis in epidermal rumour formation has been
emphasized by numerous investigators (Orr, 1938; Mackie & McGovern,
1958). A proliferation of elastic tissue was induced experimentally in
mice, by Sams et al. (1964), through repeated exposure to UVR.
Similarly, Magnus & Johnson (1965) stimulated formation of elastotic
tissue, following early destruction of elastic fibres, with radiation
of 300 nm from a monochromatic source. Nakamura & Johnson (1968)
reported that dermal elastic tissue proliferation occurred in albino
rats after chronic irradiation with UVR, only after discontinuation of
the exposure. It was postulated by Johnson et al. (1968) that this
change was the result of photochemically-induced alterations in
fibroblast function, rather than the degradation of normal elastic
fibres. In support of this concept, Epstein et al. (1969) noted that
unscheduled DNA synthesis occurred in connective tissue cells of the
upper dermis within minutes of exposure to UVR shorter than 320 nm,
demonstrating a direct effect of UVR on dermal fibroblasts.
Because of its frequent association with skin cancer formation,
actinic elastosis has been considered to play an important role in
tumour development. However, Sams and his co-workers (1964) and Graham
& Helwig (1965) demonstrated that actinic elastosis was not essential
for the development of epidermal malignancies. Furthermore, the
experimental production of elastosis in animals has not been
associated with cancer formation, nor has UVR-induced experimental
cancer depended on the presence of this change (Epstein & Epstein,
1963).
Adnexal tumour formation is not as common in UVR-treated animals
(Stenbäck, 1975) as in, for example, carcinogen-treated rats
(Zackheim, 1964; Stenbäck, 1969). Hyperplasia and cystic
disorganization of hair follicle walls is very common, but rarely
progresses to grossly visible neoplasia. Trichoepitheliomas with
barely visible follicular arrangements are rarely seen. Even more
uncommon are hamartomatous tumours, hair follicle-derived
trichofolliculomas, and sebaceous gland tumours. Sebaceous gland
epitheliomas and carcinomas are even rarer. In a study in 1930,
Putschar & Holtz reported only a very small number of basal cell
carcinomas in rats.
Pigmented tumours. Studies on the induction of pigmented
tumours with UVR have been less successful. Benign, dermal melanocytic
lesions, or blue nevi, have been observed in hairless mice exposed to
UVR (Epstein et al., 1967). They were grossly seen as papules, 2-20 mm
in diameter, histologically composed of tightly arranged, heavily
pigment-laden polyhedral cells. Subcutaneous accumulation of pigmented
cells has also been seen in pigmented animal strains, as well as in
the skin of the Syrian golden hamster (Stenbäck, 1976). Such tumours
were possibly spontaneous, as the incidence was very low -- only
around 4% -- and unrelated to treatment. They were composed of
polyhedral or spindle-like cells arranged in a whorl-like pattern
beginning as hyperplasia of perifollicular melanocytes, before
spreading both laterally and deeply in the dermis. These tumours do
not show junctional activity; they do not metastasize and rarely kill
the host.
Melanomas, the type of greatest interest from an epidemiological
standpoint, are rarely seen in animals. Benign melanocytomas are
easily induced by treatment with chemical carcinogens as shown by
Shubik et al. (1960) and Rappaport et al. (1961). Fortner et al.
(1961) reported spontaneous melanomas in hamsters, similar to those in
man. However, the sensitivity of these animals to UVR is not known.
The effect of pigment, in general, in animal models has received
little attention. Freeman & Knox (1964a) induced melanocytoma in 67%
of a pigmented strain of rats. The tumours had an average latency
period of 193 days. In albino rats there was an 8% tumour incidence
with a 283-day latency period.
4.4.2 Species-specificity
Three specific factors -- pigment, hair, and thickness of the
stratum corneum -- have been found to alter susceptibility to tumour
induction. It was found that pigmented mice required significantly
more radiation to induce tumours than albino animals. Hair offered
even greater protection (Blum et al., 1959), and thus the hairless rat
appeared to be a likely subject for tumour induction studies. However,
the results of Hueper's (1941) extensive studies indicated that this
animal was, in fact, quite resistant to UV penetration because of its
thick stratum corneum. Since pigment, hair, and the stratum corneum
were limiting factors, the ears of albino mice and rats became the
traditional test sites for experimental production of cancer by UV
irradiation. A remarkable amount of quantitative data has been
accumulated using this system. The usual tumour produced in this
tissue was a sarcoma (Roffo, 1934; Grady et al., 1943b). Thus, the
albino ear model could not be used for evaluating qualitative changes
associated with epidermal carcinogenesis, which is the primary process
induced by UVR in human skin.
Winkelman and his co-workers (1960) reported the production of
squamous cell carcinomas on the backs of hairless mice exposed to UVR.
Further studies established that carcinomas could be induced in this
animal almost to the exclusion of sarcomas (Epstein & Epstein, 1963;
Epstein, 1965). In addition, UVR-induced pigmented tumours were
reported in pigmented hairless mice (Epstein et al., 1967). Thus, the
hairless mouse has provided a model for both the qualitative and
quantitative examination of the carcinogenicity of UVR.
Though penetration of UVR appears to be of obvious importance,
other factors also influence the type of growth induced by UVR. Grady
et al. (1943 a) found that the size of individual doses did not have
any effect on the carcinoma/sarcoma ratio in the albino, hairy mouse
but that reduced intervals between exposures increased the number of
epidermal carcinomas. These findings suggest that various tissues
respond differently to the carcinogenic effects of UVR (Stenbäck,
1975a). In part, this may be associated with differences in
penetration of various wavelengths of UVR. Furthermore, there are
great species differences in the repair capability of cells.
4.4.3 Ultraviolet radiation as an initiating agent
The two-stage concept for skin tumour formation proposed by
Berenblum & Shubik (1949), supposed formation of dormant tumour cells
by a single application of a carcinogen. These latent tumour cells
were provoked by the subsequent application of a promoter to form
visible tumours. In his studies on the induction of skin cancer by
exposure to UVR, Blum (1969) indicated that the process was continuous
beginning with the first exposure and progressing to ultimate tumour
formation. Blum's conclusions were based on experiments in which he
(Blum et al., 1943) and Rusch et al. (1941) could not produce tumours,
unless exposures were carried out over a minimum of 2 ´ months,
regardless of the amount of energy used. Blum's experiments suggested
that, with shorter exposure periods, tumour formation was not
accelerated enough to become visible within the lifetime of the
experimental animal. Epstein & Roth (1968), using a single exposure to
UVR as an initiatior and treatment with croton oil as a promotor,
concluded that croton oil stimulated rumour formation, the
characteristics of which were established by initial exposure to UVR.
The results of these studies were significantly different from those
encountered when a chemical carcinogen was used as the initiator
(Stenbäck, 1969).
4.5 Interactions between Ultraviolet Radiation and Chemicals
4.5.1 Chemically-enhanced photocarcinogenesis
An equally significant problem concerns photo-induced
carcinogenesis following the application to the skin of agents which
are phototoxic, but not in themselves carcinogenic.
A portion of the sunlight spectrum is carcinogenic, even in the
absence of an exogenous photosensitizer. At the current rate of
introduction of new compounds into the environment, it has become
increasingly important to determine whether a readily demonstrable
property, such as phototoxicity, can be used to predict compounds or
treatment regimes that could enhance photocarcinogenesis.
Concepts of chemical interaction with UVR-photocarcinogenesis are
of recent origin. Blum (1969) and Emmett (1973) reviewed a number of
reports dealing with the influence of phototoxic substances on
photocarcinogenesis. The results frequently appeared to be in
disagreement, a situation possibly reflecting differences in
technique, including solvent, routes of administration, light sources,
criteria for tumour recognition, and in statistical evaluation (Blum,
1969). In addition, characteristics of some compounds (toxicity,
carcinogencity, instability) rendered their interactions with light
complex, and their analysis difficult.
The relative enhancing effects on photocarcinogenesis of two
widely recognized photoactive compounds 8-methoxypsoralen, (8-MOP) and
anthracene were studied by Forbes et al. (1976). Both compounds were
phototoxic, but only the 8-MOP solutions markedly enhanced
photocarcinogenesis. Thus, the ability of a chemical to induce
phototoxicity is not always sufficient to augment
photo-carcinogenesis.
4.5.2 Interaction between light and chemical carcinogens
The fact that UVR can alter several phenanthrene carcinogens
photochemically has been known for some time. The studies of Davies et
al. (1972 a, b) showed that the carcinogenicity of 7,12
dimethylbenz(a)anthracene (DMBA) was reduced by light according to the
demonstrable photochemical lability of the compound. There was also
evidence that an additional time-dependent factor could influence this
effect. Thus, it appears that, at least in the case of DMBA-treated
animals, light may contribute in two opposing ways: (a) by
degradation of the carcinogen to non-carcinogenic products and (b)
by stimulating a phototoxic response that appears to coincide with a
relatively increased tumour yield.
Depending on the wavelengths of the UVR used, carcinogens can be
photodegraded to a less carcinogenic compound, or can induce
phototoxicity which may augment carcinogenesis or cause such a severe
local phototoxic reaction that the epithelial skin cells are nearly
all destroyed. Thus, either enhancement or inhibition of skin
carcinogenesis may occur, depending upon the carcinogen and the
wavelength of the light source used.
4.5.3 UVR-induced carcinogen formation
The photochemical conversion of sterols to carcinogenic
substances has been proposed as a potential explanation for the
cancer-causing effects of light upon skin (Black & Douglas, 1973). It
has recently been demonstrated, in vitro, that one such compound,
cholesterol-5a-oxide, which possesses carcinogenic properties
(Bishoff, 1969), is formed in human skin exposed to UVR (Black & Lo,
1971).
4.6 Physical and Quantitative Aspects of Ultraviolet Irradiation in
Animal Studies
4.6.1 Carcinogenic action spectrum
Determination of the effective wavelengths or "action spectrum"
is one of the primary objectives in the study of photobiological
responses. However, data are not available for the action spectrum of
UVR-induced cancer formation. The paucity of this information for one
of the most extensively studied photobiological reactions is due to a
number of factors, including the large number of potential
wavelengths, the considerable number of animals necessary and the
length of time (a matter of many months or years) required for
exposure to each wavelength, the difficulties in immobilizing
experimental animals, and the need for an especially good
monochromator with practically no stray light contamination. Though
the complete curve of the carcinogenic spectrum is not known, certain
aspects have been determined by less sophisticated methods. Roffo
(1934) reported that windowglass filtration eliminated the
carcinogenic effects of sunlight on white rats. Thus the offending
rays of the sun would be found approximately between 290-320 nm. A
number of investigators using mercury arc and fluorescent sun lamps
with filters have confirmed that, under their experimental conditions,
320 nm represented the longer wavelength limit for cancer formation
(Griffin et al., 1955; Blum, 1969). Furthermore, carcinogenic
responses have been produced by radiation as short at 230.2 nm (Roffo,
1934) and skin cancer has long been known to be induced by UV-C and
UV-B. Thus the action spectrum appears to include wavelengths between
230 and 320 nm but wavelengths between 290 and 320 nm have been shown
to have significantly greater carcinogenic effects than UVR shorter
than 260 nm (Rusch et al., 1941; Blum, 1943; Blum & Lippincott, 1943;
Kelner & Taft, 1956; Tung et al., 1971).
Freeman (1975) performed a series of experiments to provide more
specific comparative data by testing the hypothesis that the action
spectrum for carcinogenesis parallelled that for erythema. In these
studies, squamous cell carcinomas developed at approximately the same
rate and frequency, when UVR exposure was proportional to that for
erythema, with a decreasing potency from 300 to 320 nm. No tumours
occurred in mice exposed to 290 nm. These cancer-producing wavelengths
are also responsible for the normal phototoxic sunburn reaction.
Longer UV and visible light are neither erythema-producing nor
carcinogenic under ordinary conditions.
It cannot be assumed that the action spectra for human skin
erythema and mouse skin photocarcinogenesis are, similar, unless a
common chromophore or action mechanism is involved. Setlow (1974)
proposed that the common denominator was the action spectrum for
affecting DNA. Making some allowance for the skin transmission of UVR,
he showed that the shapes of action spectra for DNA, erythema, and
possibly skin cancer production were similar and could be made to
coincide.
4.6.2 Dose-response relationships
The second law of photochemistry (the law of reciprocity of
Bunsen & Roscoe) states that photochemical action depends only on the
product of the light intensity and the duration of exposure. This law,
however, holds only for primary photochemical action, and cannot be
applied to secondary reactions. Since the biological endpoints that
can be observed, such as erythema, pigmentation, skin cancer
production, etc., are certainly indirect effects, and since we still
know little about the primary photochemical reactions that underlie
them, it is not surprising that "reciprocity"' holds only for some of
the effects studied.
In the first quantitative photocarcinogenesis experiments ever
performed, Blum (1969) found that, within relatively narrow limits
(approximate factor of 5), differences in dose, intensity, or interval
between doses did not alter the shape or slope of tumour incidence
curves, but only their positions on the log-time axis. Blum, however,
was careful to point out that this was only true, as long as the
experimental conditions remained the same until the time the tumours
appeared.
With the accumulated data, he surmised that UVR-induced cancer
formation was a continuous process that began with the initial
exposure and that the appearance of tumours within the lifetime of the
animal depended on sufficient acceleration of the growth process.
In the majority of studies on photocarcinogenesis, fixed doses of
UVR have been given at a fixed dose rate, and the interval between
doses altered, but in increments of at least 24 hours. Such
experiments, while very valuable, are far removed from the conditions
found in nature under which human skin is exposed. Man is exposed to a
relatively low UVR flux that varies with time of day, season, and
environmental conditions, such as cloud cover, and also during the
exposure period.
Two recent animal experiments have shown that both varying the
UVR dose increment and varying the dose-rate while the daily dose
remains constant, affects UVR-induced skin carcinogenesis.
In the first experiment, groups of hairless mice were exposed to
doses of UVR from a bank of "Fluorescent Sun" (FS) lamps known to
produce skin cancer in these animals. Equal doses of UVR were
delivered in periods of 5 minutes, 50 minutes, or 500 minutes. Thus,
while the doses (given 5 times weekly) were the same, the flux varied
by a factor of 10 or 100. Striking differences in both tumour
development time and tumour yield were noted. The animals given the
total UVR dose in 5 minutes developed tumours later and in smaller
numbers than those given the same total dose in 50 or 500 minutes
(Forbes, personal communication). Thus, protracting the UVR dose over
longer time periods resulted in a striking increase in the
carcinogenic effects of the radiation.
In another experiment, mice were exposed to UVR doses per day
differing by a factor of two. As Blum had found previously, the lower
daily dose resulted in the delayed onset of first tumours without
significantly changing the shape of the response curve (Forbes, 1978).
4.6.3 Physical factors influencing UVR carcinogenesis
Although the tumour-promoting properties of such physical factors
as freezing, scalding, and wounding have been described for chemical
carcinogenesis systems, little information is available about the
effects of these factors on UVR-induced cancer formation. Bain & Rusch
(1943) reported that increasing the temperature to 35-38°C accelerated
the tumour growth rate. The stimulating effects of heat on UVR
carcinogenesis were confirmed by Freeman & Knox (1964b). Heat also
enhanced the acute injury response to UVR.
Temperature does not affect the photochemical reactions that
follow UV irradiation, but it does affect many of the biochemical
reactions that follow the initial photochemical change (Blum, 1941,
1969). Although it is known that heat adversely affects
photosensitivity (Lipson & Baldes, 1960), and other phenomena of light
injury (Bovie & Klein, 1919; Hill & Eidenow, 1923), and that heat
alters the effects of X-ray (Carlson & Jackson, 1959), the influence
of heat on burns produced by sunlight or UVR has rarely been
considered (Freeman & Knox, 1964b).
Other studies have shown that high winds and high humidity
significantly increase tumour incidence (Zilov, 1971; Owens et al.,
1977).
4.7 The Immune Response to Tumour Induction
A number of studies have shown that the immune status of the host
and tumour induction are potentially interactive processes. Chemical
carcinogens cause alterations of the host immune-response, the type
and extent of which depend on the rumour-inducing agent (Curtis,
1975). UVR also profoundly affects immunological reactivity,
particularly the immune response to skin tumours induced by UVR.
Studies leading to this conclusion were prompted by an observation by
Kripke (1974) that tumours induced by UVR in C3Hf mice were highly
antigenic and are usually immunologically rejected when transplanted
to normal, nonirradiated syngeneic recipients. This raised the
question as to why these tumours were able to grow progressively in
their primary host without succumbing to immunological rejection. In
an extensive series of experiments, Kripke & Fisher (1976) found that
pretreatment of mice with UVR for periods of time too short to induce
skin tumours made them unable to reject transplants of UVR-induced
tumours, even though such transplants were immunologically rejected by
unexposed animals. This indicates that UVR-exposed mice are
systemically altered in a way that prevents immunological rejection of
highly antigenic UVR-induced tumours.
Similarly, inability of unexposed secondary hosts to reject
UVR-induced tumours after transfer of lymphoid cells from UVR-treated
mice has been established, and demonstrates the immunological nature
of the systemic alteration in the UVR-treated mice (Fisher & Kripke,
1977). Furthermore, the failure of lymphoid cells from UVR-exposed
mice to react against UVR-induced tumours is due to the presence of
suppresser T lymphocytes in the lymphoid organs of UVR-treated
animals. In spite of their inability to reject highly antigenic
UVR-induced tumours, UVR-exposed mice respond normally to most other
antigens (Kripke, et al., 1977; Norbury et al., 1977). The one
exception is that UVR-treated mice have a transient defect in antigen
processing in the skin, which is reflected in their inability to
develop contact hypersensitivity reactions (Jessup et al., 1978).
The finding that a selective immunological defect precedes the
appearance of UVR-induced primary tumours suggests that the immune
system might control early UVR-induced skin cancers and that tumours
ultimately appear because of this interference by UVR with host
defence mechanisms.
The carcinogenic action of polycyclic hydrocarbons has been
associated with their immunosuppressive action (Stenbäck, 1969).
Immunodeficiency states and immunosuppression therapy are both
associated with an increased rumour incidence. Immunosuppressive
agents, such as antilymphocyte serum, enhance both chemically- and
UVR-induced tumour formation (Nathanson et al., 1973, 1976).
5. EFFECTS OF ULTRAVIOLET RADIATION ON MAN
5.1 Beneficial Effects
In addition to the direct effects on the skin, UVR produces a
number of systemic effects. It has the capacity to increase the tonus
of the sympathico-adrenal system, enhances mitochondrial and
microsomal enzyme activity and the non-specific immunity level, and
increases the secretion of a number of hormones (Tung, 1976).
Systolic and diastolic blood pressures are reduced before sunburn
appears and may even be reduced with exposures so mild that no visible
erythema is produced (Aitken, 1937). Blood pressure gradually falls
for 24 hours, and lowered pressure may persist for several days.
Studies have demonstrated that the exercise tolerance of children
receiving UVR through the winter is greater than that of control
groups not receiving radiation (Ronge, 1948; Zilov, 1971).
Other changes that have been attributed to UVR exposure include
reduction in serum cholesterol, increase in the glucose tolerance
curve, and decrease in serum tyrosine (Kameneckaja & Mitrofanova,
1975).
Seasonal changes in various diseases are often considered to be
evidence of UVR effects, but there are many other climatic variables
that change with the season, including temperature and daylength.
Blood volume, blood content of the skin, blood flow in the skin, and
hydration of the skin due to sweating vary with seasonal adaptation.
Thus few changes in disease patterns can be attributed to the effects
of UVR alone.
Data have been obtained indicating that the body's tolerance
towards exposure to chemical substances such as nitrites, benzpyrene,
carcinogens etc, which produce general toxic, carcinogenic, and
allergenic effects depends, to some extent, on the degree of exposure
to UVR (Gabovic et al., 1975; Prokopenko & Zabulueva, 1975;
Prokopenko, 1976). Prophylactic treatment with UVR preceding specific
immunization reduces the risk of vaccination allergy and helps to
increase the effectiveness of the immunization (Talanova et al.,
1975).
Where sizeable populations live in far northern areas, it is now
generally acknowledged that a long period of UVR deficit may have a
harmful effect on the human body. Numerous investigations indicate
that lack of exposure to solar UVR can lead to the development of a
pathological condition known as "UVR deficiency" or "light
starvation". The most frequent manifestation of this disease condition
is a disturbance in mineral metabolism and the development of Vitamin
D deficiency and rickets in children, accompanied by a sharp reduction
in the defensive powers of the body, making it particularly vulnerable
to unfavourable environmental factors. The development of UVR
deficiency is confirmed by data from a survey conducted, mainly among
children, in different photoclimatic zones of the USSR (Belikova et
al., 1975; Dancig, 1975), and from a survey of ships' crews working in
the north and in the tropics.
Vitamin D. Sunbathing is popular, and there is a widespread
feeling that "sunlight is good for you", but the physiological
benefits that presumably underlie the feeling of well-being have not
been adequately explained or studied.
The only thoroughly established beneficial effect of UVR on the
skin is the conversion of 7-dehydrocholesterol to Vitamin D3. Several
investigators have helped to promote the understanding of the
mechanisms of Vitamin D production and its metabolism and functioning.
It has long been noted that prolonged limitation or complete
absence of exposure of the human skin to solar UVR makes natural
activation of Vitamin D impossible and is an important factor in the
spread among the population of "chronic latent D avitaminosis"
reflected in widespread rickets and dental caries. Data from the
previously mentioned survey by Belikova et al. (1975) indicate that,
despite an overall reduction in the incidence of rickets and in its
severity, it is still frequent among young children in the north of
the USSR. Morbidity indices for rickets at latitude 65°N are 2.5-3
times as high as at latitude 45°N.
Comparative investigations among healthy children aged 3-6 years
in the central zone of the USSR and beyond the Polar Circle have also
confirmed that disturbances in mineral metabolism in children in the
far north occur earlier and are more marked (Talanova & Zabalueva,
1972).
In this connection, it is of some interest that Vitamin D
deficiency may have a direct effect on the pathogenesis of dental
caries (Dancig, 1974).
The problems associated with UVR deficiency are frequently
enhanced by social factors and by degree of pigmentation (Loomis,
1970).
Phototherapy. Some information on the beneficial effects of UVR
comes from the past and present use of sunlight and UVR in medical
treatment.
In the pre-antibiotic era, several forms of skin tuberculosis and
skin infections were treated with UVR. At present, UVR treatment in
medicine is largely confined to treating skin diseases, such as
psoriasis, acne, atopic dermatitis, and recurrent boils. There have
been reports that UVR, administered in gradually increasing doses, has
been helpful in the treatment of both chronic pneumonia (Boguckij et
al., 1975), and rheumatic diseases in childhood (Karacevceva, 1971).
5.2 Induction of Erythema in Human Skin
Erythema solare, or more commonly "sunburn", consists, in its
mildest form, of a reddening of the skin that appears 1-6 hours after
exposure to erythemogenic UVR and gradually fades in 1-3 days. In its
more severe forms, sunburn results in inflammation, blistering, and
peeling of the skin; it is followed by tanning of the skin, which
becomes noticeable within 2 or 3 days of irradiation.
The amount of radiation required to produce solar erythema
provides a convenient measure of UVR dosage. The actual amount of
energy required varies with the wavelength of the radiation, since
some regions of the spectrum are more effective than others. Because
of large variations from one individual to another, as well as
variations between different parts of the body, an "erythema unit"
cannot be determined with the same accuracy as physical units or even
units of visual luminosity. There are also appreciable variations in a
given individual from time to time, depending upon such factors as
physical condition and previous exposure. The methods for evaluating
erythema introduce additional variables. There is a latent period
after exposure before reddening of the skin is observable. Thus, the
length of time after exposure that the observation is made will affect
the results. In spite of these limitations, a "sunburn unit" (SU),
based on the effect of solar UVR on the average untanned human white
skin, provides a useful method of rating and comparing various sources
of UVR.
The use of the SU (Lazarev & Sokolov, 1971) is based upon the
applicability of the Bunsen-Roscoe law of reciprocity (see section
4.6.2). Over a reasonable range of exposure times, skin erythema
depends on the total UVR dose, but is independent of exposure rate and
duration (Seidl, 1969).
Monochromatic sources of radiation are not generally considered,
but the erythemal effectiveness depends upon the sum of the effects of
those wavelengths that are present. In the case of a line spectrum,
the total effect is calculated by adding the weighted intensities of
the various lines of the source, the weighting depending on the action
spectrum in use.
By adopting this method, the erythemal equivalent of any
particular distribution of radiant energy may be calculated and
expressed as the equivalent amount of energy at a particular
wavelength (e.g., 296.7 nm) that would produce the same erythemal
effect as the given heterogenous radiation (section 6).
Numerous factors contribute to the complexity of the erythema
response (skin temperature, sweating, dose-rate, etc., section 6). In
applying the reciprocity law to polychromatic radiation, the fact that
the observed differences in biological effects of different
wavelengths may introduce inaccuracies must be considered.
5.2.1 Action spectra of human skin erythema
Hausser & Vahle (1927) reported the first precise determination
of the action spectrum for the erytherna of human skin; a double peak
was shown with maxima at about 250 and 297 nm and a minimum at about
280 nm. In these and related studies, the skin of several individuals
was exposed to UVR from a mercury arc passed through a double-quartz-
prism monochromator, and the influence of wavelength, exposure time
and rate of exposure upon the nature, degree, and course of erythema
was examined. Similar action spectra were published by Coblentz et al.
(1932), Lukiesh et al. (1939), and Magnus (1977) (Fig. 5).
5.3 Natural Protection against Erythema-inducing Ultraviolet Radiation
5.3.1 Melanin (see also section 4.2.3)
Skin tanning during and following sun exposure is one of the
major protective devices of the skin against further damage by UVR.
The UVR range from 290 to 320 nm produces sunburn and subsequent new
pigment formation. UVR in the range of 320 to 400 nm produces little
erythema, except at very high doses, but may produce immediate pigment
darkening and other increases in melanin pigment in those who have
this capacity.
4.2 Acute Reactions in the Skin
4.2.1 Epidermal changes
Histological investigations of the early effects of UV
irradiation on animal skin show the changes that occur in the
epidermal cells particularly well.
Twenty-four hours after a single irradiation of skin with an
unfiltered mercury vapour lamp at doses between four and eight times
the minimal erythema dose, distinct signs of cell injury can be seen
including vacuolation of the cell cytoplasm and increased or decreased
density of the nucleus (Stenbäck, 1975a).
One distinctive feature, observed in both man (Daniels et al.,
1961) and experimental animals (Woodcock & Magnus, 1976) 24 hours
after exposure, is the presence of "sunburn cells" (SBC), scattered
diffusely throughout the superficial epidermis. These cells are
characterized by a densely-staining, glassy, homogeneous cytoplasm and
pyknotic nuclei. The appearance is similar to that of an individual
cell undergoing keratinization and thus, the process is regarded as a
form of individual cell shrinkage necrosis or dyskeratosis.
Following these early effects, hyperplasia, induced by radiation,
is observed (Blum et al., 1959; Stenbäck, 1975a). An increase in
mitosis indicates the onset of hyperplasia, which begins after about
two days.
Quantitative measurements have been made of the following aspects
of the effects of single doses of UVR on mouse skin (Blum et al.,
1959): (a) incidence of mitotic figures; (b) number of epidermal cells
per unit area; (c) epidermal thickness; (d) dermal thickness; (e) mean
nuclear diameter; and (f) number of groups of mitotic figures. All the
aspects measured changed in a similar fashion quantitatively following
irradiation; they increased rapidly at first, reaching a maximum and
then fell gradually towards normal. Soffen & Blum (1961) found a
gradual increase in epidermal thickness reaching a maximum between 8
and 14 days. These changes represent repair of the cell injury caused
by UVR; at the same time, the hyperplasia protects against further UV
irradiation.
For a long time, it was believed that thickening of the horny
layer was the essential adaptive change to UV irradiation in the
environment. The idea now prevails that melanin granules in the horny
layer and the Malpighian layer accumulate to form a protective screen
which is not only significant, but possibly even more important for
light protection than the thickness of the horny layer alone.
4.2.2 Erythema and inflammation
Sunburn (UVR erythema) and suntanning are the visible signs of UV
injury to the skin and the repair of such injury. UV erythema is
evidence of an inflammatory reaction to radiation, and appears after a
latent period of a few hours.
Erythema caused by UVR is confined to the exposed areas and
reflects blood vessel dilation and increased blood flow in the dermis.
It is often assumed that the initial photochemical reaction is in the
epidermis, where photon absorption by keratinocytes may lead to
liberation of intracellular substances that diffuse into the papillary
dermis to cause vasodilation. This diffusion theory is supported by
the existence of a latent period between exposure and erythema, and by
the fact that much of the radiant energy of the erythemogenic
wavelength region is absorbed by the epidermis. However, there may
also be direct injury to the vascular endothelium or to other sites in
the dermis.
Most studies of the mechanism of "sunburn" have used artificial
sources of UV-B or sources in which the spectral distribution is such
that UV-B is assumed to provide the major erythemogenic influence. In
experimental animals, the vascular response to UVR is biphasic. A
transient immediate vasopermeability is followed, after a latent
period of 2-8 hours, by a delayed, prolonged, increased,
vasopermeability and vasodilation. In some animal models, the initial
vasopermeability is accompanied by a faint erythema, which may begin
during exposure. This immediate effect has been attributed to
histamine release, possibly due to a direct effect of photons on
dermal mast cells. There is evidence that serotonin may also play some
role. In rats and guineapigs, serotonin and histamine antagonists
suppress the immediate phase of UVR vascular responses. In vivo
studies of human skin have shown the transient appearance of kinins
within minutes of UV irradiation. Kinin was not found after the onset
of delayed erythema.
The mediators of the delayed phase of UVR-induced vascular
response have been difficult to define. Antihistamines do not suppress
the delayed erythema phase of the vascular response to UVR in
guineapigs, rats, or man. Kinins have been stated to be either absent
or not elevated. Delayed erythema was not suppressed by various
inhibitors of proteases, plasminogen activitors, or kallikrein.
Serotonin has been found in urine following exposure to UVR, but the
significance of this finding is not clear.
More recently, prostaglandins, a group of long-chain fatty acids
with vasoactive properties, have been implicated as possible mediators
of the delayed phase of erythema. Prostaglandins are produced in human
and animal skin (prostaglandin groups E and F), intradermal injection
of prostaglandins produces erythema (PGE mainly, PGF group are much
less active), and furthermore the production of prostaglandins
increases following UVR exposure. Indomethacin is a potent inhibitor
of the conversion of arachnidonic acid to active prostaglandin, a
reaction catalyzed by the enzyme prostaglandin synthase. Topical
indemethacin produces a profound and prolonged blanching of
UV-B-induced, delayed erythema in both human subjects and guineapigs.
In man, intradermal indomethacin has been shown to consistently
decrease erythema due to UV-B but not all areas of erythema caused by
irradiation with UV-C; these effects appear to be due to the
inhibition of prostaglandin synthase.
It has been suggested that the complex reaction known as
"sunburn" may result from the release of hydrolytic enzymes and
possibly other substances by lysosomes within keratinocytes.
Histochemical studies of human skin exposed to UVR were
consistent with the theory that specific damage to lysosomal membranes
caused partial to complete rupture and release of enzymes.
The release of lysosomal enzymes may not only lead to damage of
the keratinocytes but may also release enzymes, vasodilator
substances, or subsequently formed cell-breakdown products into the
dermis, where they may directly or indirectly lead to erythema. It has
been suggested that the immediate erythema caused by UV irradiation
results from disruption of lysosomes of endothelial cells with release
of chemical mediators. The delayed erythema could then result from
secondary diffusion of proteinases from the epidermis, following
lysosomal rupture. It is also possible that direct photon damage to
the lysosomes of mast cells of the dermis or endothelial cells of
dermal blood vessels may play a role in the delayed erythema of
sunburn.
Multiple chromophores may exist in skin, and irradiation probably
leads to activation of a complicated cascade of mediators, the final
endpoint of which is erythema. Multiple pathways may exist. It is also
possible that photons have direct effects on blood vessels or nerves.
UVR causes dilation of isolated exposed dermal blood vessels. Dermal
proteins may be directly changed by radiation (Magnus, 1977).
4.2.3 Tanning
In addition to erythema, another consequence of exposure to UVR
is the pigmentation of the skin generally known as "tanning". This
becomes noticeable about 48 h after exposure and increases gradually
for several days. Tanning is, in part, due to migration of the pigment
melanin already present in the basal cells to the more superficial
layers of the skin where it has a greater effect on the appearance of
the reflected light. It is also partly due to the formation of new
pigment.
It is generally accepted that repeated irradiation with UVR
induces increases in the population of melanocytes in the epidermis of
man and experimental animals. With regard to the mechanism of this
phenomenon, the following processes or possibilities have been
considered: (a) increased division of melanocytes; (b) activation of
pigment formation in amelanogenic melanocytes: (c) migration of dermal
melanocytes into the epidermis; (d) various combinations of these
processes (Quevedo et al., 1965). That division of melanocytes occurs
has been shown in murine skin by Quevedo et al. (1963). Further,
published reports have provided evidence in support of the occurrence
of activation of amelogenic melanocytes in man (Mishima & Widlan,
1967) and experimental animals (Reynolds, 1954; Quevedo & Smith, 1963;
Miyazaki et al., 1968; Sato, 1971). However, the relative contribution
of each process remains to be established. In a study using tritiated
thymidine, it was demonstrated that approximately 1.1% of
dopa-positive epidermal melanocytes were labelled in hairless mice
receiving 9 daily exposures to UVR (Sato & Kawada, 1972). However, an
attempt to trace the labelled melanocytes using split epidermal sheets
was unsuccessful.
4.3 Acute Changes in the Eye
4.3.1 Photokeratitis and photoconjunctivitis
Although more energetic than the visible portion of the
electromagnetic spectrum, UVR cannot be detected by the visual
receptors in mammals, including man, because of absorption by the
ocular media. Thus, exposure to UVR may result in ocular damage before
the recipient is aware of the potential danger. Many cases of
keratitis of the cornea and cataracts of the lens have been reported
due to exposure to UVR produced by welding arcs, high-pressure pulsed
lamps, and the reflection of solar radiation from snow and sand.
Extensive reviews of the literature on the biological effects of
UVR have been compiled by Verhoeff et al. (1916) and Buchanan et al.
(1960). Verhoeff and his colleagues included all the, then available,
research data in their report and formulated some of the basic
hypotheses regarding ocular damage caused by UVR. Research on
threshold values and destructive and repair processes was summarized
by Duke-Elder (1926). Buschke et al. (1945) stressed the destructive
effects of UVR on the corneal epithelial cell nuclei, the loss of
epithelial adhesion to Bowman's membrane, and the inhibiting effects
of UVR on the healing process.
In studies by Cogan & Kinsey (1946), a monochromator was used to
evaluate the sensitivity of the cornea to individual spectral lines.
Their work, which was, for the most part, carried out with one to four
rabbits, provides the most reliable quantitative data on damaging
threshold values of UV energy of individual wavelengths. They
established a long wavelength limit of between 306 and 326 nm and a
threshold of 152 J/m2 at 288 nm for the rabbit.
Ordinary clinical photokeratitis is characterized by a period of
latency that tends to vary inversely with the severity of exposure.
The latent period may be as short as 30 min or as long as 24 h, but is
usually 6-12 h. Conjunctivitis follows, often accompanied by erythema
of facial skin and eyelids. There is a sensation of a foreign body or
"sand" in the eyes and various degrees of photophobia, lacrimation,
and blepharospasm. The importance of these acute symptoms lies in the
fact that the individual is visually incapacitated for 6-24 h and that
the ocular system, unlike the skin, does not develop tolerance to
repeated exposure to UVR. Almost all discomfort disappears within
48 h, and exposure rarely results in permanent damage.
Pitts & Kay (1969) and Pitts (1970) sought to establish the
experimental threshold for photokeratitis in rabbits and primates,
including man. Rabbits and monkeys showed a maximum sensitivity to UVR
at 270 nm. The radiant exposure threshold for man at 270 nm was
50J/m2 compared with 110 J/m2 for the rabbit and 60 J/m2
for the monkey.
The UVR incident on the eye is absorbed in turn by the cornea,
the aqueous humor, the lens, and the vitreous humor before reaching
the retina. Absorption is greater in the lens than in the cornea, and
is least in the aqueous humor. Below 300 nm, most of the UVR is
absorbed in the cornea and aqueous humor and very little penetrates as
far as the lens.
As a result of observations at above-threshold intensities, it
was felt that the reaction of the cornea to exposure to UVR wavebands
of 220 to 250 nm was different from that to wavebands of 250 to
310 nm. For exposures below 250 nm, signs and symptoms occurred soon after
exposure, and symptoms always returned to normal approximately 14 h
later. For exposure above 250 nm, symptoms did not generally occur
until 9 to 11 h after exposure, and visual acuity remained below
normal for 24 h after exposure. The differences observed were
attributed to the differences in the absorption of the different
wavebands. The shorter wavebands are absorbed in the outer corneal
epithelial layers, which undergo rapid change, whereas the longer
wavebands are absorbed in the deeper epithelial layers which show
delayed changes because these cells are more viable. Thus, the lesions
produced by shorter wavelengths are rapidly repaired while, at the
longer wavelengths, there is a delayed and more serious response
(Pitts & Cullen, 1977).
4.3.2 Cataracts
A cataract is a partial or complete loss of transparency of the
crystalline lens or its capsule. The wavelengths that affect the lens
appear to be in the same area as those that are most effective in
producing erythema on human skin.
Bachem (1956), using a filtered UVR system, concluded that
exposure to repeated high doses of longer UVR wavelengths could cause
cataracts through cumulative effects. He reported that the action
spectrum for cataracts began abruptly between 293 and 297 nm, reached
a peak near 297 nm, and fell abruptly near 313 nm. Minimal effects
existed through the remainder of the near UVR. In both the rabbit and
guineapig, reversible lenticular "blurring" occurred 5 to 10 days
after exposure. With repeated excessive exposures to the 297-355 nm
wavebands, irreversible lenticular opacities occurred after a latent
period of between 2.5 and 15 months.
Bachem (1956) concluded that since daylight does not contain any
UV-C or far infrared, and since both the visible and near infrared are
freely transmitted by the ocular media, it would appear that UV-B and
A are responsible for cataracts.
The chemical effects of UV-A exposure on tryptophan were studied
by Zigman et al. (1973) using human crystalline lenses. They found
that exposure of tryptophan to UV-A led to the formation of chromatic
photoproducts which bound to the lens proteins, altered their colour
and changed the solubility. Human lens material without added
tryptophan did not show chromatic changes on exposure to UV-A, until
48 h after exposure. Tryptophan showed an excitation wavelength at
278 nm and a fluorescent emission at 330 nm. However, following
exposure to UV-A, tryptophan showed an additional 360 nm excitation
and 440 nm fluorescence similar to that found in the brunescent human
cataract lenses. The UV irradiance for these studies was 30-50 W/m2
at 365 nm and exposures were made for at least several hours. These
exposure levels exceed those expected from sunlight for the same
period of time.
Thus, exposure of the eye to UV-A, for sufficient periods of
time, with an irradiance comparable to the irradiance level of
sunlight may interfere with the synthesis of lens proteins, catalyse
insoluble lens protein, and may result in chromatic changes in the
lens. While the basic mechanisms remain to be found, the evidence
clearly demonstrates that both in vitro and in vivo exposure to
UV-A can enhance cataractous changes in the crystalline lens.
Recently, Pitts & Cullen (1977) have studied the effects of the
300 nm-400 nm wavelength range on the rabbit eye in vivo. The
criteria used to determine corneal damage were epithelial debris,
epithelial stippling, epithelial granules, epithelial haze, epithelial
exfoliation, stromal haze, stromal opacities, and endothelial
disturbances. Anterior chamber signs included flare and cells. The
crystalline lens criteria were subcapsular opacities, capsular and
stromal haze, stromal opacities, and increased prominence of the
anterior suture. Criteria for the iris were the presence of the
anterior chamber signs, changes in clarity of the iris stroma, and
sluggish pupillary response.
Tables 3 and 4 show data for corneal and lenticular damage in the
290 nm-400 nm wavelength range for the rabbit. Limited data above
300 nm for the human eye do not allow a detailed comparison; however,
the human corneal threshold was considerably below that for either
the rabbit or the nonhuman primate.
Table 3. UV threshold data for the rabbit cornea and lensa
Threshold radiant exposure
Wavelength Threshold radiant exposure (J/m2) permanent damage (J/m2)
(nm)
corneal lens time to lens time for permanent
threshold threshold disappear threshold damage to appear
295 200 7500 24 h 10 000 2 h
300 500 1500 3 days 5000 24 h
305 700 3000 7 days 5000 24 h
310 550 7500 2 weeks 15 000 24 h
315 22 500 45 000 1 week 60 000 24 h
320 75 000 > 80 000 -- -- --
335 109 000 > 150 000 -- -- --
365 > 250 000 > 250 000 -- -- --
a From: Pitts & Cullen (1977).
Table 4. Permanent lenticular opacitiesa
Wavelength Radiant exposure Appearance of Permanenceb of
(nm) (J/m2) lens opacities lens opacities
300 5000 24 h permanent
305 5000 24 h permanent
310 15 000 24 h permanent
315 60 000 24 h permanent
a From: Pitts & Cullen (1977).
b Lenticular opacities present one month after exposure.
Phototoxic psoralen derivatives have been used recently in the
treatment of certain dermatological conditions. Such compounds caused
UV-A-induced corneal opacities and cataracts in mice (Griffin, 1959;
Koch, 1967).
Research on the effects of psoralens on the production of
cataracts in man needs to be pursued. As more and more people are
subjected to dermatological treatment using UV photosensitizing
compounds, the role of these compounds and their effects on the ocular
system should be defined.
4.4 Effects of Long-Term Exposure of Skin to UVR
UV irradiation induces an inflammatory response and ulceration in
both the epidermis and the dermis, the latter being infiltrated with
leukocytes in the region of the lesions, and to a much lesser extent
between them. These lesions ulcerate and the epidermis may disappear
for a time in the centre. However, peripherally there is particularly
active hyperplasia. The basal membrane (between the epidermis and
dermis) may disappear for a time in the regions of these "open"
lesions. Between the lesions, the infiltration of leukocytes is
relatively slight.
Injury to the epidermis and dermis, brought about by long-term
exposure to UVR leads to dermal alteration, fibrosis, and elastosis,
as well as to epidermal atrophy. However, experimental production of
cutaneous elastotic changes in animals by artificial UV irradiation
has only been reported rarely. Using histochemical methods, Sams et
al. (1964) demonstrated focal dermal elastosis in mice after prolonged
exposure to artificial UVR. UVR-induced changes in connective tissue
were also seen in rat skin by Nakamura & Johnson (1968).
4.4.1 UVR-induced mutagenesis and carcinogenesis
4.4.1.1 Mutagenesis
The nature of the effects of UVR on DNA, described earlier, shows
the importance and the specificity of the lesions in genetic material
and focuses interest on the mutations that might result in the various
cell types studied. The production of such mutations has best been
demonstrated in bacteria.
Among many others, Grossman (1968), Eisenstark (1971), and Witkin
(1976) have proposed, analysed, and verified some complex mechanisms
that control UVR-induced mutagenesis. It will be recalled also that,
according to the results obtained by Radman (1975), most of the
UVR-induced mutations would appear to be errors introduced in the DNA
during error-prone SOS repair.
Mammalian cells in culture have proved to be very suitable
material for the study of UVR mutagenesis (Kao & Puck, 1969). There is
quite good agreement between the dose of UVR and the proportion of
mutants (Bridges & Huckle, 1970).
Fox (1974) has shown that caffeine can reduce the rate of
mutations induced by UVR in cultures of rodent cells by inhibiting a
process of error-prone repair.
Isolation and study of UVR sensitive mutants in animal cells have
found remarkable applications in the study of various human xeroderma
pigmentosum mutants (Cleaver, 1973). However, such mutations in
mammalian germinal cells are not possible, because the cells are
located below the depths of penetration of UVR.
4.4.1.2 Mechanism of UVR carcinogenesis
In order to understand the possible mechanisms of UVR
carcinogenesis, it has been necessary to study the formation and
excision of pyrimidine directs, unscheduled DNA synthesis, and all the
data valid for the range from bacteria to mammalian cells.
It is now generally believed that UVR carcinogenesis results from
a succession of events originating in a photolesion of the genetic
material.
From the numerous studies that have led to en elucidation of some
of these mechanisms, it emerges that faulty DNA repair can increase
the frequency of carcinogenesis in the following ways: by causing
alterations in DNA, which also find expression in an increased
frequency of chromosome aberrations and a rise in mutation rate; by
increasing the rate of transformation of normal cells into cancer
cells; and by facilitating the expression of latent oncogenic viruses
able to trigger cancerous growth (Setlow, 1973; Trosko & Chu, 1973).
Errors induced by DNA repair during the initiation phase of
carcinogenesis seem to be the most likely mechanism leading to UVR
cancers.
4.4.1.3 Tumour types
Epidermal tumours. The first visible step in UVR-induced
epidermal tumour formation in animal skin consists of cell
proliferation, i.e., an increase in the number of squamous cells and
cell layers, which gradually become papillomatous in character
(Stenbäck, 1978). This is accompanied by an increase in cellular
atypia, nuclear enlargement, hyperchromatism, indentation, and
prominence of nucleoli. This basically proliferative response is
frequently replaced by a dysplastic progression showing a solar
keratosis-like pattern with cellular pleomorphism, occasionally with
pseudo-epitheliomatous hyperplasia-like features, which ultimately
invade the dermis. The tumours first seen are acanthomatous papillomas
(trichoepitheliomas), with a predominantly epithelial component or
fibropapillomas in which the tumour is composed of a fibrous stroma
covered by squamous epithelium.
The malignant tumours that ultimately develop are squamous cell
carcinomas of different types including: solid keratin containing
tumours; moderately differentiated, individually keratinizing tumours
with distinct intercellular bridges; and less-differentiated,
non-keratinizing spindle cell tumours, in which ultrastructural
analysis reveals squamous cell patterns.
Keratoacanthomas, i.e., proliferating epithelium on a cup-shaped
base, are relatively more frequent in animals of different species
receiving large doses of UVR than in animals treated with chemical
carcinogens.
Epidermal tumours are easily induced by different agents in mouse
skin (Stenbäck, 1969). Winkelman et al. (1960) reported the production
of squamous cell carcinomas on the backs of hairless mice exposed to
UVR. Further studies established that carcinomas could be induced in
this animal almost to the exclusion of sarcoma formation (Epstein &
Epstein, 1963). In early studies, epithelial tumours were reported in
both rats and mice (Findlay, 1930; Herlitz et al., 1930; Putschar &
Holz, 1930), but in later studies the deeper lying dermal tumour
response to UVR predominated. No mention of skin sarcomas was made,
however, in the studies of Beard et al. (1936) on albino rats in which
12 animals exhibited 9 carcinomas of the external ear, 6 sarcomas of
the eye, and 1 carcinoma of the skin of the nose.
The difference in the distribution of tumour types, with
sarcomatous growth predominating in haired mice and carcinomas in man
(Urbach, 1969) may, in part, be explained by the difference in
penetration of UVR, a greater amount reaching the dermis in mice
(Kirby-Smith et al., 1942; Everett et al., 1966).
Dermal tumours. Another type of neoplastic progression seen in
mice, particularly after intensive treatment with large doses of UVR
over a short time period, consists of ulceration, scarring, and the
subsequent formation of dermal tumours. These tumours begin as
aggregates of regularly built, elongated cells with small monomorphic
nuclei. Epithelial proliferation is occasionally observed as a
secondary phenomenon. The tumours rarely extend grossly through the
surface. In the early stages, they appear to be papillomas, although
they consist entirely of fibroblastic cells. Some tumours are
remarkably acellular, with a prominent fibrillary pattern. The tumours
are composed of large, polymorphic cells with prominent nuclei.
Ultrastructural analysis shows the predominant cellular components --
a dark cell type, with hyperchromatic nuclei and scanty cytoplasm, and
a light cell type, with large nuclei and abundant cytoplasmic
ribosomes (Stenbäck, 1975a). The same cell types are also seen in
malignant tumours, in which the cellular polymorphism frequently is
considerable, with nuclear atypism and enlargement, numerous nucleoli
and a generally disorganized arrangement. Sarcoma induction is partly
species-specific, as these tumours were not seen in UV-irradiated
Syrian golden hamsters (Stenbäck, 1975b) nor were they seen in
hairless mice (Epstein & Epstein, 1963) or guineapigs, susceptible to
chemical sarcoma induction (Stenbäck, 1969, 1975b).
An infrequent neoplastic alteration in several animal species is
the vascular tumour (Stenbäck, 1975b). This begins as a proliferation
of dilated vascular spaces with regular endothelial lining. Rarely,
the endothelium proliferates to the point of forming angiosarcomas, or
invasive tumours composed of large, atypical cells arranged in a
nodular pattern.
The role of the dermis in epidermal rumour formation has been
emphasized by numerous investigators (Orr, 1938; Mackie & McGovern,
1958). A proliferation of elastic tissue was induced experimentally in
mice, by Sams et al. (1964), through repeated exposure to UVR.
Similarly, Magnus & Johnson (1965) stimulated formation of elastotic
tissue, following early destruction of elastic fibres, with radiation
of 300 nm from a monochromatic source. Nakamura & Johnson (1968)
reported that dermal elastic tissue proliferation occurred in albino
rats after chronic irradiation with UVR, only after discontinuation of
the exposure. It was postulated by Johnson et al. (1968) that this
change was the result of photochemically-induced alterations in
fibroblast function, rather than the degradation of normal elastic
fibres. In support of this concept, Epstein et al. (1969) noted that
unscheduled DNA synthesis occurred in connective tissue cells of the
upper dermis within minutes of exposure to UVR shorter than 320 nm,
demonstrating a direct effect of UVR on dermal fibroblasts.
Because of its frequent association with skin cancer formation,
actinic elastosis has been considered to play an important role in
tumour development. However, Sams and his co-workers (1964) and Graham
& Helwig (1965) demonstrated that actinic elastosis was not essential
for the development of epidermal malignancies. Furthermore, the
experimental production of elastosis in animals has not been
associated with cancer formation, nor has UVR-induced experimental
cancer depended on the presence of this change (Epstein & Epstein,
1963).
Adnexal tumour formation is not as common in UVR-treated animals
(Stenbäck, 1975) as in, for example, carcinogen-treated rats
(Zackheim, 1964; Stenbäck, 1969). Hyperplasia and cystic
disorganization of hair follicle walls is very common, but rarely
progresses to grossly visible neoplasia. Trichoepitheliomas with
barely visible follicular arrangements are rarely seen. Even more
uncommon are hamartomatous tumours, hair follicle-derived
trichofolliculomas, and sebaceous gland tumours. Sebaceous gland
epitheliomas and carcinomas are even rarer. In a study in 1930,
Putschar & Holtz reported only a very small number of basal cell
carcinomas in rats.
Pigmented tumours. Studies on the induction of pigmented
tumours with UVR have been less successful. Benign, dermal melanocytic
lesions, or blue nevi, have been observed in hairless mice exposed to
UVR (Epstein et al., 1967). They were grossly seen as papules, 2-20 mm
in diameter, histologically composed of tightly arranged, heavily
pigment-laden polyhedral cells. Subcutaneous accumulation of pigmented
cells has also been seen in pigmented animal strains, as well as in
the skin of the Syrian golden hamster (Stenbäck, 1976). Such tumours
were possibly spontaneous, as the incidence was very low -- only
around 4% -- and unrelated to treatment. They were composed of
polyhedral or spindle-like cells arranged in a whorl-like pattern
beginning as hyperplasia of perifollicular melanocytes, before
spreading both laterally and deeply in the dermis. These tumours do
not show junctional activity; they do not metastasize and rarely kill
the host.
Melanomas, the type of greatest interest from an epidemiological
standpoint, are rarely seen in animals. Benign melanocytomas are
easily induced by treatment with chemical carcinogens as shown by
Shubik et al. (1960) and Rappaport et al. (1961). Fortner et al.
(1961) reported spontaneous melanomas in hamsters, similar to those in
man. However, the sensitivity of these animals to UVR is not known.
The effect of pigment, in general, in animal models has received
little attention. Freeman & Knox (1964a) induced melanocytoma in 67%
of a pigmented strain of rats. The tumours had an average latency
period of 193 days. In albino rats there was an 8% tumour incidence
with a 283-day latency period.
4.4.2 Species-specificity
Three specific factors -- pigment, hair, and thickness of the
stratum corneum -- have been found to alter susceptibility to tumour
induction. It was found that pigmented mice required significantly
more radiation to induce tumours than albino animals. Hair offered
even greater protection (Blum et al., 1959), and thus the hairless rat
appeared to be a likely subject for tumour induction studies. However,
the results of Hueper's (1941) extensive studies indicated that this
animal was, in fact, quite resistant to UV penetration because of its
thick stratum corneum. Since pigment, hair, and the stratum corneum
were limiting factors, the ears of albino mice and rats became the
traditional test sites for experimental production of cancer by UV
irradiation. A remarkable amount of quantitative data has been
accumulated using this system. The usual tumour produced in this
tissue was a sarcoma (Roffo, 1934; Grady et al., 1943b). Thus, the
albino ear model could not be used for evaluating qualitative changes
associated with epidermal carcinogenesis, which is the primary process
induced by UVR in human skin.
Winkelman and his co-workers (1960) reported the production of
squamous cell carcinomas on the backs of hairless mice exposed to UVR.
Further studies established that carcinomas could be induced in this
animal almost to the exclusion of sarcomas (Epstein & Epstein, 1963;
Epstein, 1965). In addition, UVR-induced pigmented tumours were
reported in pigmented hairless mice (Epstein et al., 1967). Thus, the
hairless mouse has provided a model for both the qualitative and
quantitative examination of the carcinogenicity of UVR.
Though penetration of UVR appears to be of obvious importance,
other factors also influence the type of growth induced by UVR. Grady
et al. (1943 a) found that the size of individual doses did not have
any effect on the carcinoma/sarcoma ratio in the albino, hairy mouse
but that reduced intervals between exposures increased the number of
epidermal carcinomas. These findings suggest that various tissues
respond differently to the carcinogenic effects of UVR (Stenbäck,
1975a). In part, this may be associated with differences in
penetration of various wavelengths of UVR. Furthermore, there are
great species differences in the repair capability of cells.
4.4.3 Ultraviolet radiation as an initiating agent
The two-stage concept for skin tumour formation proposed by
Berenblum & Shubik (1949), supposed formation of dormant tumour cells
by a single application of a carcinogen. These latent tumour cells
were provoked by the subsequent application of a promoter to form
visible tumours. In his studies on the induction of skin cancer by
exposure to UVR, Blum (1969) indicated that the process was continuous
beginning with the first exposure and progressing to ultimate tumour
formation. Blum's conclusions were based on experiments in which he
(Blum et al., 1943) and Rusch et al. (1941) could not produce tumours,
unless exposures were carried out over a minimum of 2 ´ months,
regardless of the amount of energy used. Blum's experiments suggested
that, with shorter exposure periods, tumour formation was not
accelerated enough to become visible within the lifetime of the
experimental animal. Epstein & Roth (1968), using a single exposure to
UVR as an initiatior and treatment with croton oil as a promotor,
concluded that croton oil stimulated rumour formation, the
characteristics of which were established by initial exposure to UVR.
The results of these studies were significantly different from those
encountered when a chemical carcinogen was used as the initiator
(Stenbäck, 1969).
4.5 Interactions between Ultraviolet Radiation and Chemicals
4.5.1 Chemically-enhanced photocarcinogenesis
An equally significant problem concerns photo-induced
carcinogenesis following the application to the skin of agents which
are phototoxic, but not in themselves carcinogenic.
A portion of the sunlight spectrum is carcinogenic, even in the
absence of an exogenous photosensitizer. At the current rate of
introduction of new compounds into the environment, it has become
increasingly important to determine whether a readily demonstrable
property, such as phototoxicity, can be used to predict compounds or
treatment regimes that could enhance photocarcinogenesis.
Concepts of chemical interaction with UVR-photocarcinogenesis are
of recent origin. Blum (1969) and Emmett (1973) reviewed a number of
reports dealing with the influence of phototoxic substances on
photocarcinogenesis. The results frequently appeared to be in
disagreement, a situation possibly reflecting differences in
technique, including solvent, routes of administration, light sources,
criteria for tumour recognition, and in statistical evaluation (Blum,
1969). In addition, characteristics of some compounds (toxicity,
carcinogencity, instability) rendered their interactions with light
complex, and their analysis difficult.
The relative enhancing effects on photocarcinogenesis of two
widely recognized photoactive compounds 8-methoxypsoralen, (8-MOP) and
anthracene were studied by Forbes et al. (1976). Both compounds were
phototoxic, but only the 8-MOP solutions markedly enhanced
photocarcinogenesis. Thus, the ability of a chemical to induce
phototoxicity is not always sufficient to augment
photo-carcinogenesis.
4.5.2 Interaction between light and chemical carcinogens
The fact that UVR can alter several phenanthrene carcinogens
photochemically has been known for some time. The studies of Davies et
al. (1972 a, b) showed that the carcinogenicity of 7,12
dimethylbenz(a)anthracene (DMBA) was reduced by light according to the
demonstrable photochemical lability of the compound. There was also
evidence that an additional time-dependent factor could influence this
effect. Thus, it appears that, at least in the case of DMBA-treated
animals, light may contribute in two opposing ways: (a) by
degradation of the carcinogen to non-carcinogenic products and (b)
by stimulating a phototoxic response that appears to coincide with a
relatively increased tumour yield.
Depending on the wavelengths of the UVR used, carcinogens can be
photodegraded to a less carcinogenic compound, or can induce
phototoxicity which may augment carcinogenesis or cause such a severe
local phototoxic reaction that the epithelial skin cells are nearly
all destroyed. Thus, either enhancement or inhibition of skin
carcinogenesis may occur, depending upon the carcinogen and the
wavelength of the light source used.
4.5.3 UVR-induced carcinogen formation
The photochemical conversion of sterols to carcinogenic
substances has been proposed as a potential explanation for the
cancer-causing effects of light upon skin (Black & Douglas, 1973). It
has recently been demonstrated, in vitro, that one such compound,
cholesterol-5a-oxide, which possesses carcinogenic properties
(Bishoff, 1969), is formed in human skin exposed to UVR (Black & Lo,
1971).
4.6 Physical and Quantitative Aspects of Ultraviolet Irradiation in
Animal Studies
4.6.1 Carcinogenic action spectrum
Determination of the effective wavelengths or "action spectrum"
is one of the primary objectives in the study of photobiological
responses. However, data are not available for the action spectrum of
UVR-induced cancer formation. The paucity of this information for one
of the most extensively studied photobiological reactions is due to a
number of factors, including the large number of potential
wavelengths, the considerable number of animals necessary and the
length of time (a matter of many months or years) required for
exposure to each wavelength, the difficulties in immobilizing
experimental animals, and the need for an especially good
monochromator with practically no stray light contamination. Though
the complete curve of the carcinogenic spectrum is not known, certain
aspects have been determined by less sophisticated methods. Roffo
(1934) reported that windowglass filtration eliminated the
carcinogenic effects of sunlight on white rats. Thus the offending
rays of the sun would be found approximately between 290-320 nm. A
number of investigators using mercury arc and fluorescent sun lamps
with filters have confirmed that, under their experimental conditions,
320 nm represented the longer wavelength limit for cancer formation
(Griffin et al., 1955; Blum, 1969). Furthermore, carcinogenic
responses have been produced by radiation as short at 230.2 nm (Roffo,
1934) and skin cancer has long been known to be induced by UV-C and
UV-B. Thus the action spectrum appears to include wavelengths between
230 and 320 nm but wavelengths between 290 and 320 nm have been shown
to have significantly greater carcinogenic effects than UVR shorter
than 260 nm (Rusch et al., 1941; Blum, 1943; Blum & Lippincott, 1943;
Kelner & Taft, 1956; Tung et al., 1971).
Freeman (1975) performed a series of experiments to provide more
specific comparative data by testing the hypothesis that the action
spectrum for carcinogenesis parallelled that for erythema. In these
studies, squamous cell carcinomas developed at approximately the same
rate and frequency, when UVR exposure was proportional to that for
erythema, with a decreasing potency from 300 to 320 nm. No tumours
occurred in mice exposed to 290 nm. These cancer-producing wavelengths
are also responsible for the normal phototoxic sunburn reaction.
Longer UV and visible light are neither erythema-producing nor
carcinogenic under ordinary conditions.
It cannot be assumed that the action spectra for human skin
erythema and mouse skin photocarcinogenesis are, similar, unless a
common chromophore or action mechanism is involved. Setlow (1974)
proposed that the common denominator was the action spectrum for
affecting DNA. Making some allowance for the skin transmission of UVR,
he showed that the shapes of action spectra for DNA, erythema, and
possibly skin cancer production were similar and could be made to
coincide.
4.6.2 Dose-response relationships
The second law of photochemistry (the law of reciprocity of
Bunsen & Roscoe) states that photochemical action depends only on the
product of the light intensity and the duration of exposure. This law,
however, holds only for primary photochemical action, and cannot be
applied to secondary reactions. Since the biological endpoints that
can be observed, such as erythema, pigmentation, skin cancer
production, etc., are certainly indirect effects, and since we still
know little about the primary photochemical reactions that underlie
them, it is not surprising that "reciprocity"' holds only for some of
the effects studied.
In the first quantitative photocarcinogenesis experiments ever
performed, Blum (1969) found that, within relatively narrow limits
(approximate factor of 5), differences in dose, intensity, or interval
between doses did not alter the shape or slope of tumour incidence
curves, but only their positions on the log-time axis. Blum, however,
was careful to point out that this was only true, as long as the
experimental conditions remained the same until the time the tumours
appeared.
With the accumulated data, he surmised that UVR-induced cancer
formation was a continuous process that began with the initial
exposure and that the appearance of tumours within the lifetime of the
animal depended on sufficient acceleration of the growth process.
In the majority of studies on photocarcinogenesis, fixed doses of
UVR have been given at a fixed dose rate, and the interval between
doses altered, but in increments of at least 24 hours. Such
experiments, while very valuable, are far removed from the conditions
found in nature under which human skin is exposed. Man is exposed to a
relatively low UVR flux that varies with time of day, season, and
environmental conditions, such as cloud cover, and also during the
exposure period.
Two recent animal experiments have shown that both varying the
UVR dose increment and varying the dose-rate while the daily dose
remains constant, affects UVR-induced skin carcinogenesis.
In the first experiment, groups of hairless mice were exposed to
doses of UVR from a bank of "Fluorescent Sun" (FS) lamps known to
produce skin cancer in these animals. Equal doses of UVR were
delivered in periods of 5 minutes, 50 minutes, or 500 minutes. Thus,
while the doses (given 5 times weekly) were the same, the flux varied
by a factor of 10 or 100. Striking differences in both tumour
development time and tumour yield were noted. The animals given the
total UVR dose in 5 minutes developed tumours later and in smaller
numbers than those given the same total dose in 50 or 500 minutes
(Forbes, personal communication). Thus, protracting the UVR dose over
longer time periods resulted in a striking increase in the
carcinogenic effects of the radiation.
In another experiment, mice were exposed to UVR doses per day
differing by a factor of two. As Blum had found previously, the lower
daily dose resulted in the delayed onset of first tumours without
significantly changing the shape of the response curve (Forbes, 1978).
4.6.3 Physical factors influencing UVR carcinogenesis
Although the tumour-promoting properties of such physical factors
as freezing, scalding, and wounding have been described for chemical
carcinogenesis systems, little information is available about the
effects of these factors on UVR-induced cancer formation. Bain & Rusch
(1943) reported that increasing the temperature to 35-38°C accelerated
the tumour growth rate. The stimulating effects of heat on UVR
carcinogenesis were confirmed by Freeman & Knox (1964b). Heat also
enhanced the acute injury response to UVR.
Temperature does not affect the photochemical reactions that
follow UV irradiation, but it does affect many of the biochemical
reactions that follow the initial photochemical change (Blum, 1941,
1969). Although it is known that heat adversely affects
photosensitivity (Lipson & Baldes, 1960), and other phenomena of light
injury (Bovie & Klein, 1919; Hill & Eidenow, 1923), and that heat
alters the effects of X-ray (Carlson & Jackson, 1959), the influence
of heat on burns produced by sunlight or UVR has rarely been
considered (Freeman & Knox, 1964b).
Other studies have shown that high winds and high humidity
significantly increase tumour incidence (Zilov, 1971; Owens et al.,
1977).
4.7 The Immune Response to Tumour Induction
A number of studies have shown that the immune status of the host
and tumour induction are potentially interactive processes. Chemical
carcinogens cause alterations of the host immune-response, the type
and extent of which depend on the rumour-inducing agent (Curtis,
1975). UVR also profoundly affects immunological reactivity,
particularly the immune response to skin tumours induced by UVR.
Studies leading to this conclusion were prompted by an observation by
Kripke (1974) that tumours induced by UVR in C3Hf mice were highly
antigenic and are usually immunologically rejected when transplanted
to normal, nonirradiated syngeneic recipients. This raised the
question as to why these tumours were able to grow progressively in
their primary host without succumbing to immunological rejection. In
an extensive series of experiments, Kripke & Fisher (1976) found that
pretreatment of mice with UVR for periods of time too short to induce
skin tumours made them unable to reject transplants of UVR-induced
tumours, even though such transplants were immunologically rejected by
unexposed animals. This indicates that UVR-exposed mice are
systemically altered in a way that prevents immunological rejection of
highly antigenic UVR-induced tumours.
Similarly, inability of unexposed secondary hosts to reject
UVR-induced tumours after transfer of lymphoid cells from UVR-treated
mice has been established, and demonstrates the immunological nature
of the systemic alteration in the UVR-treated mice (Fisher & Kripke,
1977). Furthermore, the failure of lymphoid cells from UVR-exposed
mice to react against UVR-induced tumours is due to the presence of
suppresser T lymphocytes in the lymphoid organs of UVR-treated
animals. In spite of their inability to reject highly antigenic
UVR-induced tumours, UVR-exposed mice respond normally to most other
antigens (Kripke, et al., 1977; Norbury et al., 1977). The one
exception is that UVR-treated mice have a transient defect in antigen
processing in the skin, which is reflected in their inability to
develop contact hypersensitivity reactions (Jessup et al., 1978).
The finding that a selective immunological defect precedes the
appearance of UVR-induced primary tumours suggests that the immune
system might control early UVR-induced skin cancers and that tumours
ultimately appear because of this interference by UVR with host
defence mechanisms.
The carcinogenic action of polycyclic hydrocarbons has been
associated with their immunosuppressive action (Stenbäck, 1969).
Immunodeficiency states and immunosuppression therapy are both
associated with an increased rumour incidence. Immunosuppressive
agents, such as antilymphocyte serum, enhance both chemically- and
UVR-induced tumour formation (Nathanson et al., 1973, 1976).
5. EFFECTS OF ULTRAVIOLET RADIATION ON MAN
5.1 Beneficial Effects
In addition to the direct effects on the skin, UVR produces a
number of systemic effects. It has the capacity to increase the tonus
of the sympathico-adrenal system, enhances mitochondrial and
microsomal enzyme activity and the non-specific immunity level, and
increases the secretion of a number of hormones (Tung, 1976).
Systolic and diastolic blood pressures are reduced before sunburn
appears and may even be reduced with exposures so mild that no visible
erythema is produced (Aitken, 1937). Blood pressure gradually falls
for 24 hours, and lowered pressure may persist for several days.
Studies have demonstrated that the exercise tolerance of children
receiving UVR through the winter is greater than that of control
groups not receiving radiation (Ronge, 1948; Zilov, 1971).
Other changes that have been attributed to UVR exposure include
reduction in serum cholesterol, increase in the glucose tolerance
curve, and decrease in serum tyrosine (Kameneckaja & Mitrofanova,
1975).
Seasonal changes in various diseases are often considered to be
evidence of UVR effects, but there are many other climatic variables
that change with the season, including temperature and daylength.
Blood volume, blood content of the skin, blood flow in the skin, and
hydration of the skin due to sweating vary with seasonal adaptation.
Thus few changes in disease patterns can be attributed to the effects
of UVR alone.
Data have been obtained indicating that the body's tolerance
towards exposure to chemical substances such as nitrites, benzpyrene,
carcinogens etc, which produce general toxic, carcinogenic, and
allergenic effects depends, to some extent, on the degree of exposure
to UVR (Gabovic et al., 1975; Prokopenko & Zabulueva, 1975;
Prokopenko, 1976). Prophylactic treatment with UVR preceding specific
immunization reduces the risk of vaccination allergy and helps to
increase the effectiveness of the immunization (Talanova et al.,
1975).
Where sizeable populations live in far northern areas, it is now
generally acknowledged that a long period of UVR deficit may have a
harmful effect on the human body. Numerous investigations indicate
that lack of exposure to solar UVR can lead to the development of a
pathological condition known as "UVR deficiency" or "light
starvation". The most frequent manifestation of this disease condition
is a disturbance in mineral metabolism and the development of Vitamin
D deficiency and rickets in children, accompanied by a sharp reduction
in the defensive powers of the body, making it particularly vulnerable
to unfavourable environmental factors. The development of UVR
deficiency is confirmed by data from a survey conducted, mainly among
children, in different photoclimatic zones of the USSR (Belikova et
al., 1975; Dancig, 1975), and from a survey of ships' crews working in
the north and in the tropics.
Vitamin D. Sunbathing is popular, and there is a widespread
feeling that "sunlight is good for you", but the physiological
benefits that presumably underlie the feeling of well-being have not
been adequately explained or studied.
The only thoroughly established beneficial effect of UVR on the
skin is the conversion of 7-dehydrocholesterol to Vitamin D3. Several
investigators have helped to promote the understanding of the
mechanisms of Vitamin D production and its metabolism and functioning.
It has long been noted that prolonged limitation or complete
absence of exposure of the human skin to solar UVR makes natural
activation of Vitamin D impossible and is an important factor in the
spread among the population of "chronic latent D avitaminosis"
reflected in widespread rickets and dental caries. Data from the
previously mentioned survey by Belikova et al. (1975) indicate that,
despite an overall reduction in the incidence of rickets and in its
severity, it is still frequent among young children in the north of
the USSR. Morbidity indices for rickets at latitude 65°N are 2.5-3
times as high as at latitude 45°N.
Comparative investigations among healthy children aged 3-6 years
in the central zone of the USSR and beyond the Polar Circle have also
confirmed that disturbances in mineral metabolism in children in the
far north occur earlier and are more marked (Talanova & Zabalueva,
1972).
In this connection, it is of some interest that Vitamin D
deficiency may have a direct effect on the pathogenesis of dental
caries (Dancig, 1974).
The problems associated with UVR deficiency are frequently
enhanced by social factors and by degree of pigmentation (Loomis,
1970).
Phototherapy. Some information on the beneficial effects of UVR
comes from the past and present use of sunlight and UVR in medical
treatment.
In the pre-antibiotic era, several forms of skin tuberculosis and
skin infections were treated with UVR. At present, UVR treatment in
medicine is largely confined to treating skin diseases, such as
psoriasis, acne, atopic dermatitis, and recurrent boils. There have
been reports that UVR, administered in gradually increasing doses, has
been helpful in the treatment of both chronic pneumonia (Boguckij et
al., 1975), and rheumatic diseases in childhood (Karacevceva, 1971).
5.2 Induction of Erythema in Human Skin
Erythema solare, or more commonly "sunburn", consists, in its
mildest form, of a reddening of the skin that appears 1-6 hours after
exposure to erythemogenic UVR and gradually fades in 1-3 days. In its
more severe forms, sunburn results in inflammation, blistering, and
peeling of the skin; it is followed by tanning of the skin, which
becomes noticeable within 2 or 3 days of irradiation.
The amount of radiation required to produce solar erythema
provides a convenient measure of UVR dosage. The actual amount of
energy required varies with the wavelength of the radiation, since
some regions of the spectrum are more effective than others. Because
of large variations from one individual to another, as well as
variations between different parts of the body, an "erythema unit"
cannot be determined with the same accuracy as physical units or even
units of visual luminosity. There are also appreciable variations in a
given individual from time to time, depending upon such factors as
physical condition and previous exposure. The methods for evaluating
erythema introduce additional variables. There is a latent period
after exposure before reddening of the skin is observable. Thus, the
length of time after exposure that the observation is made will affect
the results. In spite of these limitations, a "sunburn unit" (SU),
based on the effect of solar UVR on the average untanned human white
skin, provides a useful method of rating and comparing various sources
of UVR.
The use of the SU (Lazarev & Sokolov, 1971) is based upon the
applicability of the Bunsen-Roscoe law of reciprocity (see section
4.6.2). Over a reasonable range of exposure times, skin erythema
depends on the total UVR dose, but is independent of exposure rate and
duration (Seidl, 1969).
Monochromatic sources of radiation are not generally considered,
but the erythemal effectiveness depends upon the sum of the effects of
those wavelengths that are present. In the case of a line spectrum,
the total effect is calculated by adding the weighted intensities of
the various lines of the source, the weighting depending on the action
spectrum in use.
By adopting this method, the erythemal equivalent of any
particular distribution of radiant energy may be calculated and
expressed as the equivalent amount of energy at a particular
wavelength (e.g., 296.7 nm) that would produce the same erythemal
effect as the given heterogenous radiation (section 6).
Numerous factors contribute to the complexity of the erythema
response (skin temperature, sweating, dose-rate, etc., section 6). In
applying the reciprocity law to polychromatic radiation, the fact that
the observed differences in biological effects of different
wavelengths may introduce inaccuracies must be considered.
5.2.1 Action spectra of human skin erythema
Hausser & Vahle (1927) reported the first precise determination
of the action spectrum for the erytherna of human skin; a double peak
was shown with maxima at about 250 and 297 nm and a minimum at about
280 nm. In these and related studies, the skin of several individuals
was exposed to UVR from a mercury arc passed through a double-quartz-
prism monochromator, and the influence of wavelength, exposure time
and rate of exposure upon the nature, degree, and course of erythema
was examined. Similar action spectra were published by Coblentz et al.
(1932), Lukiesh et al. (1939), and Magnus (1977) (Fig. 5).
5.3 Natural Protection against Erythema-inducing Ultraviolet Radiation
5.3.1 Melanin (see also section 4.2.3)
Skin tanning during and following sun exposure is one of the
major protective devices of the skin against further damage by UVR.
The UVR range from 290 to 320 nm produces sunburn and subsequent new
pigment formation. UVR in the range of 320 to 400 nm produces little
erythema, except at very high doses, but may produce immediate pigment
darkening and other increases in melanin pigment in those who have
this capacity.
 Constitutional skin colour in man is the "baseline" colour that
develops in the absence of exposure to solar radiation or other
environmental influences and results from genetically determined
levels of melanin pigmentation. Facultative skin colour or "tan" is
the increase in melanin pigmentation above the constitutional level
which is induced by UVR or by pituitary hormones such as the
melanocyte-stimulating or the adrenocorticotropic hormones. The
facultative tanning (darkening) response induced by solar irradiation
can be divided into immediate tanning (IT), which occurs within
minutes of exposure to sunlight, and delayed tanning (DT), which
becomes evident several days after exposure (for review see
Fitzpatrick et al., 1974).
Immediate tanning (IT). IT, also called immediate pigment
darkening (IPD), is best seen in pigmented individuals. In general,
the darker the unexposed, baseline, inherited colouration, the greater
the ability to exhibit IT.
Within 5-10 min of exposure to the noonday sun, a gradual
darkening is noticed, which is confined to exposed skin. If exposure
continues, darkening increases until it reaches a maximum after about
1 h of irradiation. The colour ranges from light brown to dark brown
or, in the more deeply pigmented races, from grey-brown to black.
Brief exposures to sunlight may lead to slight to moderate IT, which
begins to fade within 30 min of the end of exposure and is scarcely
visible after 3-8 h. Prolonged exposure to sunlight or high-intensity
artificial long wave UVR sources can lead to striking IT that lasts
longer than 36 hours. IT is optimally produced by both long wave UVR
and visible light.
Delayed tanning (DT). DT, also referred to as "true
melanogenesis", becomes visible 72 hours after exposure to UVR,
although electron microscope studies have shown ultrastructural
evidence of the formation of new melanosomes and melanin much earlier.
The major action spectrum of DT is the same as that for sunburn but DT
can also follow exposure to UV-C and UV-A and shorter wavelength
visible light.
Exposure to UVR can also modify the pattern of distribution of
melanosomes in keratinocytes. In Mongoloid peoples, repeated UVR
exposure results in a predominance of non-aggregated single
melanosomes. Interpretation of variations in melanosome packaging
within the epidermis must take into account the history of the
previous exposure of individuals to solar radiation and other factors.
Once present, melanin acts as a neutral density filter and
decreases the amounts of UVR that can reach tile lower layer of the
skin containing viable keratinocytes or penetrate into the dermis to
strike blood vessels. As constitutional or facultative pigmentation
increases, the dose of UVR required to produce erythema increases.
5.3.2 Thickening of the stratum corneum
Thickening and brownish discoloration of the stratum corneum of
light-exposed areas of human skin are noted after exposure to UVR.
While a protective effect unquestionably exists, it is of relatively
less practical importance than the protection afforded by melanin
pigmentation.
5.4 Solar Elastosis and Other Dermal Effects of Ultraviolet Radiation
(See also section 4.2)
Sunlight may have many effects on the skin, and one of the most
important both clinically and cosmetically is aging. Gross changes in
actinically damaged skin are a dry, coarse, leathery appearance,
laxity with wrinkling, and various pigmentary changes. Frequently, in
elderly and even in some relatively young fair-skinned adults, there
is a striking difference between light-exposed regions and those
protected by clothing. A weather-beaten farmer often appears
considerably older than a clerk of comparable age. Black skin has
natural protection because of its high melanin content and elderly
Negroes often appear deceptively young (Silverstone & Searle, 1970).
It now seems clear that collagen degeneration in the dermis is
independent of age and is determined simply by the cumulative amount
of injury from UVR. This depends on the degree and frequency of
exposure and the extent of natural (and artificial) protection
afforded to the patient's skin by the thickening of the stratum
corneum, melanin pigment, clothing, or chemical sunscreens.
The visible cutaneous changes usually interpreted as "aging" are
apparently due, to a large extent, to chronic exposure to sunlight.
5.5 Ultraviolet Radiation and Skin Cancer in Man
(See also Section 4.2)
Classical evidence supporting the role of sunlight, particularly
of UVR, as a causal factor in human skin cancer can mainly be
summarized in the form of six associations of skin cancer (Urbach et
al., 1972):
(a) Association with exposed areas of the skin. Among
white-skinned people, skin cancers occur most frequently on the parts
of the body most exposed to sunlight -- the head, neck, arms, and
hands, and the legs of women.
(b) Association with protection against UVR. Among races with
dark skin, in which pigment filters UVR, there is very little skin
cancer and the disease does not occur predominantly in areas of the
skin exposed to the sun. Sunburn and skin cancer arise in the same
tissue, and UVR is known to cause sunburn. It appears that those who
are more susceptible to skin cancer sunburn more easily. White-skinned
people of Celtic origin are more susceptible to both skin cancer and
sunburn while those of Latin origin are less susceptible.
(c) Association with the amount of exposure to the sun. Among
fair-skinned people, there appears to be a greater prevalence of skin
cancer in those who spend more time outdoors.
(d) Association with the intensity of solar exposure. The
incidence of skin cancer among white-skinned people increases with
increasing proximity to the equator and thus with increasing solar
radiation and intensity of UVR.
(e) Association with UVR in laboratory studies. Skin cancer can
be produced in mice with repeated doses of UVR in the same spectral
range that produces sunburn in the human skin.
(f) Association with insufficient ability to repair DNA damaged
by UVR. Those with the recessive disease xeroderma pigmentosum, who
have a defect in DNA repair, develop skin cancer prematurely. Such
persons are photosensitive, and develop tumours, induced by exposure
to solar UVR. They frequently die of skin cancer before reaching adult
life.
5.5.1 Anatomical distribution of skin cancer
Numerous studies have shown that, in fair-skinned people, skin
cancers arise primarily on sites exposed to sunlight. It has been
demonstrated that about 90% of all basal cell carcinomas and more than
half of all squamous cell carcinomas occur on the head and neck. The
majority of those squamous cell cancers not occurring on the head and
neck are found on the hands and forearms; the ears of females are
markedly protected by hair (Silverstone & Searle, 1970; Swanbeck &
Hillstrom, 1971).
Comparing the sites of non-melanoma cancers with studies made of
the geometry of insolation of the head and neck areas, it becomes
clear that two thirds of all basal cell carcinomas occur on the skin
sites receiving the highest doses of UVR, and that virtually all
squamous cell carcinomas occur at these sites (Urbach et al., 1972).
The anatomical distribution of malignant melanoma, a less common
but more deadly form of skin cancer, suggests a less striking
association with UVR exposure. However, there has been a considerable
increase in the prevalence of this type of cancer on the legs of women
during the past 25 years and a special form of melanoma, lentigo
maligna, almost always arises on the face.
5.5.2 Occupation and skin cancer
As has been pointed out previously, surveys of the incidence of
skin cancer other than malignant melanoma are, generally, not very
reliable. Consequently, data concerning the relationship between
occupation and skin cancer incidence are also scarce. From the studies
carried out in Queensland, Australia, and in Galway, Ireland, the most
reliable sources, it appears that those in outdoor occupations are the
most highly exposed and, therefore, at the highest risk. Thus farmers,
fishermen, sailors, and others such as road workers, roofers,
policemen, and postmen, have a higher incidence of skin cancer than
office and factory workers (Swanbeck & Hillstörm, 1971; Gordon &
Silverstone, 1976).
5.5.3 Genetics and skin cancer
In several carefully controlled studies comparing patients with
non-melanoma and melanoma skin cancer to age-sex matched controls from
the same populations, a distinct association was found between skin
cancer and light coloured eyes, fair complexion, light hair colour,
poor ability to tan, ease of sunburning, and a history of repeated
severe sunburn. Furthermore, whenever looked for, there was a higher
prevalence of Celtic stock among skin cancer patients (Silverstone &
Searle, 1970; Urbach et al., 1972). Xeroderma pigmentosum (XP) is a
hereditary skin disease in man. Studies on cells from the patients
have supplied the most decisive arguments regarding the relationships
between photolesion repair and carcinogenesis. Persons suffering from
this disease show abnormal pigmentation and a high incidence of skin
cancers triggered off by exposure to the solar UVR. Cleaver (1973),
who was the first to draw attention to the possible causes of the
disease, has written a critical review of the main biochemical and
genetic studies which reveal the extreme complexity, but also the
ingenuity and logic of the molecular processes that play a part in the
development of the disease.
In general, it has proved possible to establish a correlation
between the level of DNA repair and the seriousness of the symptoms in
XP patients.
Numerous and more complex studies have shown that the various
degrees of UVR sensitivity observed in XP patients correspond to
different sensitivity mutants. Using techniques such as cell fusion
and complementation, several groups have been distinguished that are
characterized by various defects in the repair mechanisms (Kraemer et
al., 1975). Some mutants show normal excision but no repair synthesis
(Lehmann et al., 1975). In others, repair replication would seem to be
normal, but chain breaks appear more slowly than in normal cells.
Caffeine emphasizes these differences still further (Fornace et al.,
1976).
Although the mechanisms are far from completely elucidated, they
show the considerable value of this kind of genetic disorder, in which
the problems of the photolesions and their repair and of
carcinogenesis are intimately linked.
5.5.4 Geographical distribution of non-melanoma skin cancer
Incidence data for skin cancer, other than melanoma, must be
treated with considerable reserve. Many cancer registries do not
register non-melanoma skin cancer at all, and those that do are
uniformly incomplete, since most of these tumours are treated in
physicians' offices and either not reported at all or reported without
histological verification.
A survey of the recorded geographical distribution of
non-melanoma skin cancer has been made by Cutchis (1978) (Fig. 6 and
7). From the data of Scotto et al. (1974), it appears that in Iowa,
USA, for instance, the incidence of skin cancer in males rose from
61.4/100 000 in 1950 to 174/100 000 in 1972. This approximately 3-fold
increase is also noticeable in all areas in Texas, with the exception
of El Paso, where the incidence rate actually decreased, and Houston,
where the increase was apparently only 2-fold. The Texas data
(MacDonald, 1976) showed that incidence rates increased with
descending latitude, but not in a stepwise fashion. In the most recent
5-year period (1962-1966), the rate for El Paso was 183/100 000 and
that for San Antonio (1° further south) was 147.35/100 000. In San
Antonio, a large part of the permanent population consisted of retired
military personnel and their families. Most of these people had spent
much less time in the sunny south than the permanent population in El
Paso. The highest incidence rate was found at Corpus Christi at
latitude 28°N (371/100 000). This was significantly greater than the
incidence at Harlingen (284.5/100 000), at latitude 26°N. At Corpus
Christi, there was another factor involved besides exposure, i.e., a
great preponderance of Celtic inhabitants whose forebears migrated to
that area about a century ago. Thus the disproportionately high
incidence of skin cancer in Corpus Christi could be due to a
combination of intense insolation and a very susceptible population, a
situation similar to that existing in Queensland, Australia.
These findings demonstrate how important the confounding social
factors can be in evaluating skin cancer statistics in relation to UVR
exposure.
Europe. In Europe, just as in the USA and Australia, a marked
north-south gradient of non-melanoma skin cancer exists.
In 1976, Waterhouse et al. reported skin cancer incidence rates
for males and females as follows: Sweden -- 10.6 and 6.5/100 000;
Denmark -- 33.4 and 24.5; Federal Republic of Germany -- 7.3 and 4.6;
UK -- 40.3 and 21.4; and Yogoslavia -- 23.2 and 22.8. Other reports
suggest incidences in the German Democratic Republic of 43.9 and
40.3/100 000 (Herold & Berndt, 1968), and in Bulgaria, of 42.2 for
rural and 25.0 for urban people (Anchev et al., 1968).
Constitutional skin colour in man is the "baseline" colour that
develops in the absence of exposure to solar radiation or other
environmental influences and results from genetically determined
levels of melanin pigmentation. Facultative skin colour or "tan" is
the increase in melanin pigmentation above the constitutional level
which is induced by UVR or by pituitary hormones such as the
melanocyte-stimulating or the adrenocorticotropic hormones. The
facultative tanning (darkening) response induced by solar irradiation
can be divided into immediate tanning (IT), which occurs within
minutes of exposure to sunlight, and delayed tanning (DT), which
becomes evident several days after exposure (for review see
Fitzpatrick et al., 1974).
Immediate tanning (IT). IT, also called immediate pigment
darkening (IPD), is best seen in pigmented individuals. In general,
the darker the unexposed, baseline, inherited colouration, the greater
the ability to exhibit IT.
Within 5-10 min of exposure to the noonday sun, a gradual
darkening is noticed, which is confined to exposed skin. If exposure
continues, darkening increases until it reaches a maximum after about
1 h of irradiation. The colour ranges from light brown to dark brown
or, in the more deeply pigmented races, from grey-brown to black.
Brief exposures to sunlight may lead to slight to moderate IT, which
begins to fade within 30 min of the end of exposure and is scarcely
visible after 3-8 h. Prolonged exposure to sunlight or high-intensity
artificial long wave UVR sources can lead to striking IT that lasts
longer than 36 hours. IT is optimally produced by both long wave UVR
and visible light.
Delayed tanning (DT). DT, also referred to as "true
melanogenesis", becomes visible 72 hours after exposure to UVR,
although electron microscope studies have shown ultrastructural
evidence of the formation of new melanosomes and melanin much earlier.
The major action spectrum of DT is the same as that for sunburn but DT
can also follow exposure to UV-C and UV-A and shorter wavelength
visible light.
Exposure to UVR can also modify the pattern of distribution of
melanosomes in keratinocytes. In Mongoloid peoples, repeated UVR
exposure results in a predominance of non-aggregated single
melanosomes. Interpretation of variations in melanosome packaging
within the epidermis must take into account the history of the
previous exposure of individuals to solar radiation and other factors.
Once present, melanin acts as a neutral density filter and
decreases the amounts of UVR that can reach tile lower layer of the
skin containing viable keratinocytes or penetrate into the dermis to
strike blood vessels. As constitutional or facultative pigmentation
increases, the dose of UVR required to produce erythema increases.
5.3.2 Thickening of the stratum corneum
Thickening and brownish discoloration of the stratum corneum of
light-exposed areas of human skin are noted after exposure to UVR.
While a protective effect unquestionably exists, it is of relatively
less practical importance than the protection afforded by melanin
pigmentation.
5.4 Solar Elastosis and Other Dermal Effects of Ultraviolet Radiation
(See also section 4.2)
Sunlight may have many effects on the skin, and one of the most
important both clinically and cosmetically is aging. Gross changes in
actinically damaged skin are a dry, coarse, leathery appearance,
laxity with wrinkling, and various pigmentary changes. Frequently, in
elderly and even in some relatively young fair-skinned adults, there
is a striking difference between light-exposed regions and those
protected by clothing. A weather-beaten farmer often appears
considerably older than a clerk of comparable age. Black skin has
natural protection because of its high melanin content and elderly
Negroes often appear deceptively young (Silverstone & Searle, 1970).
It now seems clear that collagen degeneration in the dermis is
independent of age and is determined simply by the cumulative amount
of injury from UVR. This depends on the degree and frequency of
exposure and the extent of natural (and artificial) protection
afforded to the patient's skin by the thickening of the stratum
corneum, melanin pigment, clothing, or chemical sunscreens.
The visible cutaneous changes usually interpreted as "aging" are
apparently due, to a large extent, to chronic exposure to sunlight.
5.5 Ultraviolet Radiation and Skin Cancer in Man
(See also Section 4.2)
Classical evidence supporting the role of sunlight, particularly
of UVR, as a causal factor in human skin cancer can mainly be
summarized in the form of six associations of skin cancer (Urbach et
al., 1972):
(a) Association with exposed areas of the skin. Among
white-skinned people, skin cancers occur most frequently on the parts
of the body most exposed to sunlight -- the head, neck, arms, and
hands, and the legs of women.
(b) Association with protection against UVR. Among races with
dark skin, in which pigment filters UVR, there is very little skin
cancer and the disease does not occur predominantly in areas of the
skin exposed to the sun. Sunburn and skin cancer arise in the same
tissue, and UVR is known to cause sunburn. It appears that those who
are more susceptible to skin cancer sunburn more easily. White-skinned
people of Celtic origin are more susceptible to both skin cancer and
sunburn while those of Latin origin are less susceptible.
(c) Association with the amount of exposure to the sun. Among
fair-skinned people, there appears to be a greater prevalence of skin
cancer in those who spend more time outdoors.
(d) Association with the intensity of solar exposure. The
incidence of skin cancer among white-skinned people increases with
increasing proximity to the equator and thus with increasing solar
radiation and intensity of UVR.
(e) Association with UVR in laboratory studies. Skin cancer can
be produced in mice with repeated doses of UVR in the same spectral
range that produces sunburn in the human skin.
(f) Association with insufficient ability to repair DNA damaged
by UVR. Those with the recessive disease xeroderma pigmentosum, who
have a defect in DNA repair, develop skin cancer prematurely. Such
persons are photosensitive, and develop tumours, induced by exposure
to solar UVR. They frequently die of skin cancer before reaching adult
life.
5.5.1 Anatomical distribution of skin cancer
Numerous studies have shown that, in fair-skinned people, skin
cancers arise primarily on sites exposed to sunlight. It has been
demonstrated that about 90% of all basal cell carcinomas and more than
half of all squamous cell carcinomas occur on the head and neck. The
majority of those squamous cell cancers not occurring on the head and
neck are found on the hands and forearms; the ears of females are
markedly protected by hair (Silverstone & Searle, 1970; Swanbeck &
Hillstrom, 1971).
Comparing the sites of non-melanoma cancers with studies made of
the geometry of insolation of the head and neck areas, it becomes
clear that two thirds of all basal cell carcinomas occur on the skin
sites receiving the highest doses of UVR, and that virtually all
squamous cell carcinomas occur at these sites (Urbach et al., 1972).
The anatomical distribution of malignant melanoma, a less common
but more deadly form of skin cancer, suggests a less striking
association with UVR exposure. However, there has been a considerable
increase in the prevalence of this type of cancer on the legs of women
during the past 25 years and a special form of melanoma, lentigo
maligna, almost always arises on the face.
5.5.2 Occupation and skin cancer
As has been pointed out previously, surveys of the incidence of
skin cancer other than malignant melanoma are, generally, not very
reliable. Consequently, data concerning the relationship between
occupation and skin cancer incidence are also scarce. From the studies
carried out in Queensland, Australia, and in Galway, Ireland, the most
reliable sources, it appears that those in outdoor occupations are the
most highly exposed and, therefore, at the highest risk. Thus farmers,
fishermen, sailors, and others such as road workers, roofers,
policemen, and postmen, have a higher incidence of skin cancer than
office and factory workers (Swanbeck & Hillstörm, 1971; Gordon &
Silverstone, 1976).
5.5.3 Genetics and skin cancer
In several carefully controlled studies comparing patients with
non-melanoma and melanoma skin cancer to age-sex matched controls from
the same populations, a distinct association was found between skin
cancer and light coloured eyes, fair complexion, light hair colour,
poor ability to tan, ease of sunburning, and a history of repeated
severe sunburn. Furthermore, whenever looked for, there was a higher
prevalence of Celtic stock among skin cancer patients (Silverstone &
Searle, 1970; Urbach et al., 1972). Xeroderma pigmentosum (XP) is a
hereditary skin disease in man. Studies on cells from the patients
have supplied the most decisive arguments regarding the relationships
between photolesion repair and carcinogenesis. Persons suffering from
this disease show abnormal pigmentation and a high incidence of skin
cancers triggered off by exposure to the solar UVR. Cleaver (1973),
who was the first to draw attention to the possible causes of the
disease, has written a critical review of the main biochemical and
genetic studies which reveal the extreme complexity, but also the
ingenuity and logic of the molecular processes that play a part in the
development of the disease.
In general, it has proved possible to establish a correlation
between the level of DNA repair and the seriousness of the symptoms in
XP patients.
Numerous and more complex studies have shown that the various
degrees of UVR sensitivity observed in XP patients correspond to
different sensitivity mutants. Using techniques such as cell fusion
and complementation, several groups have been distinguished that are
characterized by various defects in the repair mechanisms (Kraemer et
al., 1975). Some mutants show normal excision but no repair synthesis
(Lehmann et al., 1975). In others, repair replication would seem to be
normal, but chain breaks appear more slowly than in normal cells.
Caffeine emphasizes these differences still further (Fornace et al.,
1976).
Although the mechanisms are far from completely elucidated, they
show the considerable value of this kind of genetic disorder, in which
the problems of the photolesions and their repair and of
carcinogenesis are intimately linked.
5.5.4 Geographical distribution of non-melanoma skin cancer
Incidence data for skin cancer, other than melanoma, must be
treated with considerable reserve. Many cancer registries do not
register non-melanoma skin cancer at all, and those that do are
uniformly incomplete, since most of these tumours are treated in
physicians' offices and either not reported at all or reported without
histological verification.
A survey of the recorded geographical distribution of
non-melanoma skin cancer has been made by Cutchis (1978) (Fig. 6 and
7). From the data of Scotto et al. (1974), it appears that in Iowa,
USA, for instance, the incidence of skin cancer in males rose from
61.4/100 000 in 1950 to 174/100 000 in 1972. This approximately 3-fold
increase is also noticeable in all areas in Texas, with the exception
of El Paso, where the incidence rate actually decreased, and Houston,
where the increase was apparently only 2-fold. The Texas data
(MacDonald, 1976) showed that incidence rates increased with
descending latitude, but not in a stepwise fashion. In the most recent
5-year period (1962-1966), the rate for El Paso was 183/100 000 and
that for San Antonio (1° further south) was 147.35/100 000. In San
Antonio, a large part of the permanent population consisted of retired
military personnel and their families. Most of these people had spent
much less time in the sunny south than the permanent population in El
Paso. The highest incidence rate was found at Corpus Christi at
latitude 28°N (371/100 000). This was significantly greater than the
incidence at Harlingen (284.5/100 000), at latitude 26°N. At Corpus
Christi, there was another factor involved besides exposure, i.e., a
great preponderance of Celtic inhabitants whose forebears migrated to
that area about a century ago. Thus the disproportionately high
incidence of skin cancer in Corpus Christi could be due to a
combination of intense insolation and a very susceptible population, a
situation similar to that existing in Queensland, Australia.
These findings demonstrate how important the confounding social
factors can be in evaluating skin cancer statistics in relation to UVR
exposure.
Europe. In Europe, just as in the USA and Australia, a marked
north-south gradient of non-melanoma skin cancer exists.
In 1976, Waterhouse et al. reported skin cancer incidence rates
for males and females as follows: Sweden -- 10.6 and 6.5/100 000;
Denmark -- 33.4 and 24.5; Federal Republic of Germany -- 7.3 and 4.6;
UK -- 40.3 and 21.4; and Yogoslavia -- 23.2 and 22.8. Other reports
suggest incidences in the German Democratic Republic of 43.9 and
40.3/100 000 (Herold & Berndt, 1968), and in Bulgaria, of 42.2 for
rural and 25.0 for urban people (Anchev et al., 1968).

 In the USSR, skin cancer morbidity increases from north to south.
According to these data, skin cancer represents 15-26% of the total
cancer morbidity in the south, and 9-14° in the north (Caklin, 1974).
Africa. Information concerning skin cancer in Africa, gathered
from various sources, but mainly from the reports of Oettle (1962) and
Davies et al. (1965), show that the native Africans have extremely low
rates of both non-melanoma and melanoma skin cancer.
The incidence rates in the Johannesburg Bantus and in Uganda are
of the order of 1-2/100 000 for non-melanoma skin cancer (almost all
squamous cell carcinomas of the lower extremities) and 0.4-0.6/100 000
for malignant melanoma (almost all located on the foot) (Oettle,
1962). A more recent study in the Sudan shows a somewhat higher
incidence rate, particularly for squamous cell carcinomas (Malik et
al., 1974).
The exceptions are albino Negroes, who are extremely prone to the
development of skin cancer. In South Africa, albinism is quite common
among the Bantus. Oettle (1962) estimated a crude annual incidence
rate of 579/100 000 for male and 408/100 000 for female albino Bantus
for squamous cell carcinomas of the skin. Interestingly, the rates for
basal cell carcinoma were recorded as only 36/100 000 for both sexes
combined, as only 1 case was found.
India. Incidence data for skin cancer in India are not
available. However, it is clear that the majority of skin cancers seen
in hospitals occur in fair-skinned people. Among native indians,
special types of squamous cell carcinomas of the skin are found such
as the Kangri, Dhoti, and Chutta cancers, which are presumably due to
extreme heat, smoke, and chronic friction (Mulay, 1962).
China and Japan. While incidence figures are again not
available, it is clear that skin cancer is uncommon in the Province of
Taiwan, China, and Japan. This is also borne out by a reversal of the
usual basal cell to squamous cell carcinoma ratio. Squamous cell
carcinomas are 2-3 times more common than basal cell cancers, and may
arise at the site of premalignant skin changes such as burns, chronic
trauma, or secondary to arsenic ingestion (Miyaji, 1962; Yeh, 1962).
Australia and New Zealand. Probably the best skin cancer
surveys of recent years have been carried out in Australia,
particularly in Queensland, by Gordon & Silverstone (1976). Their
values for the incidence of skin cancer in various parts of Australia
are reported in Table 5.
Table 5. Skin cancer in Australiaa
Annual incidence per 100 000 population
Male Female
State
Age Age
Crude standardized Crude standardized
Victoria (40°S) 68.5 66.6 50.5 38.5
Queensland 265.1 265.1 174 155.8
Brisbane (28°S) 242 172
Townsville (19°S) 466 300
a From: Gordon & Silverstone (1976).
Comparable demographic data in those areas of the world that are
warm and sunny and to which people from northern Europe including the
United Kingdom have migrated are given in Table 6.
Table 6. Examples of high incidence of skin cancer. Annual incidence
per 100 000 population by sexa
Region Male Female Latitude
S.W. England 28 15 53°N
South Africa -- Cape Whites 133 72 35°--25°S
Texas (non-Latin) 168 106 28°--32°N
Queensland (Whites) 265 156 28°--10°S
a From: Gordon & Silverstone (1976).
Skin cancer tends to appear at a much earlier age in the
Queensland population than in populations living further away from the
equator.
If the distribution of annual solar UVR (Green et al., 1975) is
plotted against the incidence of skin cancer on a global basis, it can
be shown that skin cancer incidence doubles for every 10° decrease in
latitude. The Australian data would fit this concept, particularly in
Queensland, where the difference in incidence between Brisbane and
Townsville is about two to one and the difference in latitude about
9°.
Not as much work on skin cancers has been done in New Zealand as
in Australia; however good estimates of skin cancer incidence have
been reported by Eastcott (1962). These are: 113/100 000 for basal
cell carcinoma, 38/100 000 for squamous cell carcinoma; and
5.5/100 000 for malignant melanoma. These rates are considerable
lower than those for Australia, but New Zealand is much further from
the equator and thus receives less UVR.
5.5.5 Dose-response relationship for skin cancer (see also
section 4.5.2)
Although a correlation between the incidence of skin malignancy
and solar UVR levels has not yet been established with great accuracy,
it has been possible to demonstrate a correlation between latitude and
skin cancer incidence (Gordon & Silverstone (1976), Fig. 8). Some
confounding factors have been obtained from animal experiments.
In the USSR, skin cancer morbidity increases from north to south.
According to these data, skin cancer represents 15-26% of the total
cancer morbidity in the south, and 9-14° in the north (Caklin, 1974).
Africa. Information concerning skin cancer in Africa, gathered
from various sources, but mainly from the reports of Oettle (1962) and
Davies et al. (1965), show that the native Africans have extremely low
rates of both non-melanoma and melanoma skin cancer.
The incidence rates in the Johannesburg Bantus and in Uganda are
of the order of 1-2/100 000 for non-melanoma skin cancer (almost all
squamous cell carcinomas of the lower extremities) and 0.4-0.6/100 000
for malignant melanoma (almost all located on the foot) (Oettle,
1962). A more recent study in the Sudan shows a somewhat higher
incidence rate, particularly for squamous cell carcinomas (Malik et
al., 1974).
The exceptions are albino Negroes, who are extremely prone to the
development of skin cancer. In South Africa, albinism is quite common
among the Bantus. Oettle (1962) estimated a crude annual incidence
rate of 579/100 000 for male and 408/100 000 for female albino Bantus
for squamous cell carcinomas of the skin. Interestingly, the rates for
basal cell carcinoma were recorded as only 36/100 000 for both sexes
combined, as only 1 case was found.
India. Incidence data for skin cancer in India are not
available. However, it is clear that the majority of skin cancers seen
in hospitals occur in fair-skinned people. Among native indians,
special types of squamous cell carcinomas of the skin are found such
as the Kangri, Dhoti, and Chutta cancers, which are presumably due to
extreme heat, smoke, and chronic friction (Mulay, 1962).
China and Japan. While incidence figures are again not
available, it is clear that skin cancer is uncommon in the Province of
Taiwan, China, and Japan. This is also borne out by a reversal of the
usual basal cell to squamous cell carcinoma ratio. Squamous cell
carcinomas are 2-3 times more common than basal cell cancers, and may
arise at the site of premalignant skin changes such as burns, chronic
trauma, or secondary to arsenic ingestion (Miyaji, 1962; Yeh, 1962).
Australia and New Zealand. Probably the best skin cancer
surveys of recent years have been carried out in Australia,
particularly in Queensland, by Gordon & Silverstone (1976). Their
values for the incidence of skin cancer in various parts of Australia
are reported in Table 5.
Table 5. Skin cancer in Australiaa
Annual incidence per 100 000 population
Male Female
State
Age Age
Crude standardized Crude standardized
Victoria (40°S) 68.5 66.6 50.5 38.5
Queensland 265.1 265.1 174 155.8
Brisbane (28°S) 242 172
Townsville (19°S) 466 300
a From: Gordon & Silverstone (1976).
Comparable demographic data in those areas of the world that are
warm and sunny and to which people from northern Europe including the
United Kingdom have migrated are given in Table 6.
Table 6. Examples of high incidence of skin cancer. Annual incidence
per 100 000 population by sexa
Region Male Female Latitude
S.W. England 28 15 53°N
South Africa -- Cape Whites 133 72 35°--25°S
Texas (non-Latin) 168 106 28°--32°N
Queensland (Whites) 265 156 28°--10°S
a From: Gordon & Silverstone (1976).
Skin cancer tends to appear at a much earlier age in the
Queensland population than in populations living further away from the
equator.
If the distribution of annual solar UVR (Green et al., 1975) is
plotted against the incidence of skin cancer on a global basis, it can
be shown that skin cancer incidence doubles for every 10° decrease in
latitude. The Australian data would fit this concept, particularly in
Queensland, where the difference in incidence between Brisbane and
Townsville is about two to one and the difference in latitude about
9°.
Not as much work on skin cancers has been done in New Zealand as
in Australia; however good estimates of skin cancer incidence have
been reported by Eastcott (1962). These are: 113/100 000 for basal
cell carcinoma, 38/100 000 for squamous cell carcinoma; and
5.5/100 000 for malignant melanoma. These rates are considerable
lower than those for Australia, but New Zealand is much further from
the equator and thus receives less UVR.
5.5.5 Dose-response relationship for skin cancer (see also
section 4.5.2)
Although a correlation between the incidence of skin malignancy
and solar UVR levels has not yet been established with great accuracy,
it has been possible to demonstrate a correlation between latitude and
skin cancer incidence (Gordon & Silverstone (1976), Fig. 8). Some
confounding factors have been obtained from animal experiments.
 There are data that indicate that physical and chemical factors
may attenuate or intensify the carcinogenic effect of UVR
(Sviderskaja, 1971). Chronic exposure of animals to small doses of
ionizing radiation reduces resistance to the carcinogenic effects of
UVR, increasing the incidence and reducing the length of the latent
period. UV-B radiation has variable effects on the growth of
transplanted and chemically induced tumours. UV-B radiation can affect
the resistance of the body to tumour formation, increasing it with use
of sub-erythemal doses and considerably reducing it with large doses
(Dancig et al., 1975). These data, although obtained in animal systems
only, may be of great significance for human health, since the
resistance of the body to exposure to various harmful factors in the
environment operates against a certain background of natural UVR.
5.5.6 Mortality from skin cancer
In contrast to malignant melanoma, where mortality in most series
stiff exceeds 40%, the fatality rate in non-melanoma skin cancer is as
low as 1%. However, it would be incorrect to try to draw conclusions
from disease specific mortality rates.
5.5.7 Malignant melanoma
Both the incidence and the mortality rates of malignant melanoma
are rising rapidly in all countries in which they have been studied,
with mortality rates doubling in a 10-15 year period (Elwood & Lee,
1975).
The incidence varies from less than 1/100 000 in Japan, Nigeria,
and India to as high as 24/100 000 in Queensland, Australia. The rise
in incidence and mortality has been much greater in younger people
than in those over 65 years of age, implying that a causal mechanism
operates from an early age. The increase has been greater at certain
body sites and thus the site distribution has changed. The most
striking increase in incidence has been on the lower limbs in females
and the trunk in men (MacGovern, 1977).
In countries where such data are available, there is a distinct
association between latitude of residence and development of malignant
melanoma (Lancaster, 1956; Magnus, 1973; Movshovitz & Modan, 1973;
Elwood et al., 1974). The closer to the equator and the longer the
residence in countries with high insolation, the higher, in general,
is the incidence of malignant melanoma.
However, while there is usually a demonstrable latitude gradient
within a country, the latitude association in much less marked than
that for non-melanoma skin cancer. For instance, incidence rates for
malignant melanoma in Norway and Sweden are much higher than in
England and France both of which are much further south (Cutchis,
1978, Fig. 6 and 7).
Basal cell and squamous cell carcinoma, which are considered to
be due primarily to chronic UV-B exposure, are commonest on the areas
of skin exposed to sunlight, occur at a later age than malignant
melanoma, and are strikingly associated with severe solar damage to
the skin. In contrast, malignant melanoma occur more often on the
trunk of men and legs of women than non-melanoma skin cancer, and only
one type, the lentigo maligna melanoma (comprising not more than 15%
of all melanoma), is associated with histological UVR-induced skin
damage (McGovern & Mackie, 1959).
Thus, it appears that non-melanoma and melanoma skin cancers are
related to UVR exposure in different ways. Table 7 lists the
similarities and differences between these two types of tumours.
Parallel increases in melanoma and non-melanoma skin cancer cannot be
expected, as the latent period for malignant melanoma is apparently
much shorter than that for non-melanoma skin cancer. Reasons suggested
for the differences between the two types include: the greater
sensitivity of melanocytes to UVR (McGovern, 1977); the presence of
some secondarily produced circulating factor (Lee & Merrill, 1970);
and intermittent overdoses of UVR (Fears et al., 1977).
In the absence of an experimental animal model, and with the
present state of knowledge, it must be assumed that there is some
association between UVR and the development of malignant melanoma.
Thus any additional UVR exposure of susceptible individuals may
increase the risk of development of this very serious malignant skin
tumour.
5.6 Phototoxic and Photoallergic Diseases
5.6.1 Phototoxicity
Light-induced damage to the skin that does not depend on an
allergic mechanism may be considered phototoxic. Theoretically, these
reactions will occur in everyone, if the skin is exposed to enough
light energy of the proper wavelengths and if enough molecules that
will absorb these wavelengths are present. The radiation must
penetrate to the absorbing molecules for the reaction to occur.
Clinically, phototoxic reactions are usually characterized by erythema
(and at times oedema) occurring from a few minutes to several hours
after exposure, followed by hyperpigmentation and desquamation. The
sunburn reaction is the classical example of response to a phototoxic
effect (Ippen, 1969).
Table 7. Comparison of epidemiological factors in the etiology of malignant melanoma and
non-melanoma skin cancer
Factor Malignant melanoma Non-melanoma skin cancer
Latitude of residence Increases linearly within Increases geometrically with
countries, Incidence not latitude.
strictly related to latitude
globally.
Age of onset 3rd and 4th decade most 6th to 8th decade most common.
common.
Sex Moderate preponderance for Great preponderance for males.
females.
Anatomical distribution: Back, anterior torso, upper Head and neck (particularly ears
Male, white extremity, head, and neck. and lip), upper back, hand,
upper extremity.
Anatomical distribution: Back, lower leg, upper Head and neck (ears and lower
female white extremity, head, and neck. lip, spared), hands, upper
extremity, anterior chest.
Anatomical distribution: Soles, mucous membranes Anterior lower extremities, other
Black and oriental palms, nail bed. (all sites (all sites rare).
rare).
Racial (genetic) factors Celtic background, Scandinavians Celtic background, Scandinavians.
Rare in pigmented races. Rare in pigmented races.
Table 7 (contd).
Factor Malignant melanoma Non-melanoma skin cancer
Possible etiological Genetic (xeroderma pigmentosum Genetic (XP)
factors XP, B-K mole) Physical (UVR, X-ray)
Physical (UVR, trauma) Chemical (arsenic, coal tar).
Chemical (PCBsa, alpha-DOPA)
Developmental (nevi)
a PCBs = polychlorinated biphenyls.
From the clinical point of view, the erythema produced by various
phototoxic agents differs greatly in type of onset and type of
reaction; the ability of the agents to elicit pigmentation also
varies.
In contrast to the usual acute solar erythema, which begins after
a latent period of a few hours, peaks at 24 h, and subsides in a few
days to be replaced by moderate melanin pigmentation, the erythema due
to photodynamic compounds appears immediately after or during
radiation, may be associated with striking wheal formation, and
disappears in 3-6 h. Pigmentation is usually minimal.
The erythema due to furocoumarins (8-MOP) begins later than that
caused by solar radiation, peaks at 48-72 h, may persist for days, and
is followed by very intense pigmentation.
Despite a considerable amount of investigation, the mechanisms by
which phototoxic responses occur are not well understood. In the case
of the exogenous photosensitizer, either the molecule alone or a
complex of the chemical and cellular organelles becomes excited by the
absorption of light; triplet states and free radicals, or both, may be
formed.
Certain dyes and chemicals such as methylene blue, acriflavine,
rose bengal, and porphyrins produce photochemical effects on living
and non-living substrates only in the presence of oxygen. The
photo-dynamically active substance becomes excited and forms a triplet
state or a free radical. The excited chemical may also form peroxides
and then oxidize the substrate. Other possibilities include passing
the energy from the excited chemical to the biological substrate,
which then becomes oxidized, or the activated chemical may be able to
accept electrons, resulting in the oxidation of the substrate. After
excitation, the photosensitizing molecules return to the ground state
and are structurally unchanged.
Photosensitizing compounds may be endogenous, i.e., formed in the
body, usually by abnormal metabolism (e.g., porphyrins), or exogenous,
i.e., contacted externally or given as medication.
Exogenous photosensitizers may reach the skin by topical or
systemic routes and the reactions may be phototoxic or photoallergic
in nature. The action spectra for most of the phototoxic agents that
may cause skin disorders in man lie in the long-wavelength UVR range
(320-400 nm).
Contact photosensitizers include: cosmetics such as perfumes,
colognes, after-shave lotions (essential oils and psoralens),
lipsticks (fluorescein derivatives), creams, and hair preparations
(coal tar derivatives); and plants that cause phytophotodermatitis
such as Persian limes, pink rot-infested celery, many members of the
Umbelliferae and Rutaceae orders. These problems are primarily due to
psoralen compounds and therapeutic agents including phenothiazines and
sulfonamides (usually used therapeutically), halogenated
salicylanilides, sunscreens, and blankophores (usually photoallergic).
Systemic photosensitizers include: thiazide diuretics;
antibacterial sulfonamides; sulfonylurea antidiabetic drugs;
phenothiazines (especially chlorpromazine); and antibiotics
(especially dimethylchlortetracycline).
A large number of other drugs may occasionally induce
photosensitivity.
5.6.2 Photoallergy
Photoallergy can be defined as an acquired altered capacity of
the skin to react to light energy alone or in the presence of a
photosensitizer (Harber & Baer, 1969).
In photoallergic reactions, the photosensitizer leads to the
formation of the photohapten, which binds (covalently) with a suitable
carrier molecule to form the complete photoantigen. The carrier may be
a protein, polypeptide, mucopolypeptide, mucopolysaccharide, or other
macromolecule present in the skin. Once developed, photoallergy can
apparently occur with light energy alone, but presumably small
quantitites of the photoantigen are still present in the skin and
involve a circulating antibody or a cell-mediated response. In
contrast to phototoxicity, photoallergy is uncommon and is
characterized clinically by such reactions as immediate urticaria or
delayed papular or eczematous responses similar to contact dermatitis.
The hallmark of a sunlight-induced reaction, whether toxic or
allergic, is the distribution of the eruption. The exposed areas of
the face, neck, upper extremities, and, in women, the anterior surface
of the legs and the proximal, dorsal areas of the feet are mainly
involved. Exposure while driving may accentuate the eruption on the
side of the face and arm adjacent to the window. The upper eyelids,
subnasal and submental areas, flexural aspects of the wrists, and the
antecubital fossae tend to be spared. Clothing generally provides
protection, but reactions can be produced by penetration of UVR
especially through the light fabrics worn in summer.
The most common photoallergens are: 3,5-dibromosalicylanilide
(3,5 DBS); 4,5 dibromosalicylanilide (4,5 DBS); tribromosalicylanilide
(TBS); hexachlorophene; bithionol; and trichlorcarbanilide.
5.7 Pterygium and Cancer of the Eye
While detailed epidemiological evidence does not seem to exist,
there is a clinical impression among competent ophthalmologists that a
latitude gradient exists for the development of pterygium of the eye,
a benign hyperplasia of the bulbar conjunctiva which may eventually
interfere with vision by growing over the pupil (Dolezova, 1976).
Epidermoid carcinoma of the bulbar conjunctiva is a rare neoplasm
which appears with increased frequency in people living in the tropics
or subtropics (Afghanistan, Colombia, Ethiopia, Haiti, Malawi, Middle
East, Nigeria, Pakistan, Senegal, South Africa, and Uganda). It has
also been reported in cattle in the same region. Such tumours of the
eye can be induced in experimental animals with artificial UVR. Early
lesions, which are exophytic and tend to be papillary, are often
accompanied by basophilic degeneration of subepithelial collagen and
chronic inflammation.
The tumours are moderately to poorly differentiated keratinizing
epidermoid carcinomas.
In contrast to carcinoma of the skin, carcinoma of the eye is
more common in dark-skinned people, probably because of much greater
UVR exposure, and lack of pigment in the conjunctiva.
6. EVALUATION OF HEALTH RISKS TO MAN
6.1 The Significance and Extent of Different Environmental Sources of
Ultraviolet Radiation and Pathways of Exposure
The major health risks from natural UVR arise from chronic,
excessive, and unwise exposure to solar radiation. Section 2.1.1 of
this document describes in detail the wavelengths and quantities of
solar UVR that reach the earth and factors affecting it.
Briefly, depending on latitude and stratospheric ozone
concentration, the shortest wavelength measured (at noon, near the
equator) in solar radiation is about 290 nm. In most regions of earth,
the lower cut-off limit is at about 295 nm. The spectral composition
and radiation intensity of solar UVR is greatly influenced by
latitude, season, time of day (i.e., angle of the sun), cloud cover,
and the albedo of the surface. About two thirds of the skin-erythema-
producing solar UVR reaches the earth between 10h00 and 14h00.
In man, the extent of human exposure to solar UVR varies with
posture. In the upright position, esentially only portions of the
head, back of the neck, shoulders, forearms, and hands are exposed. In
addition, the skin of the thighs and upper arms may be heavily
exposed in some occupations such as driving a tractor. Exposure also
varies with time of day and local weather conditions; clothing
(wearing of hats, short- or long-sleeved shirts, shorts, etc.); work
and social habits; and ground albedo (snow, ice, and sand being the
only effective reflectors).
The maximum amount of solar UVR to which an individual could be
exposed in one day, represents about 25 minimal erythema skin doses,
i.e., about 7500 J/m2 of radiation equivalent to the skin erythema
effect of 297 nm monochromatic UVR.
Occupational exposure from artificial sources is either
inadvertent, when the sources produce UVR as a by-product, or
deliberate, when sources are designed to generate UVR to use its
properties. Depending on the characteristics of the source (section
2.1), the spectral composition of the emitted UVR can contain
wavelengths in the UV-A, UV-B, and UV-C regions.
Some industrial processes in which UV energy is a by-product are
welding, plasma torch operations, photoelectric scanning, and hot
metal operations. Because of the germicidal properties of certain
portions of the UV spectrum, artificial sources are used in hospitals,
biological laboratories, schools, and industry. Other common
applications are illumination, advertising, crime detection, chemical
synthesis and analysis, photoengraving, food, water, and air
sterilization, vitamin production, and medical diagnosis. Many other
occupations are listed in Table 8. New sources, such as UV lasers and
fluorescent panels, are being developed.
Table 8. Occupations potentially associated with UVR exposure
aircraft workers furnace workers oil field workers
barbers gardeners pipeline workers
bath attendants gas mantle workers plasma torch operators
brick layers glass blowers printers railroad track workers
burners, metal glass furnace workers ranchers
cattleman hairdressers road workers
construction workers herders seamen
cutters, metal iron workers skimmers, glass
drug makers lifeguards steel mill workers
electricians lithographers stockmen
farmers metal casting inspectors stokers
fisherman miners, open pit tobacco irradiators
food irradiators nurses vitamin D preparation makers
foundry workers welders
The amount of UVR exposure from artificial sources depends on the
spectral composition, radiant intensity, distance from source,
shielding, etc., and must de determined for individual conditions.
6.2 Types of Biological Effects and Their Significance for Human
Health
Since UVR penetrates essentially only into the skin and eyes of
man, the deleterious effects on these organs are of the greatest
importance. The acute and chronic effects of UVR are described in
detail in sections 4 and 5. Also of concern, however, are the
deleterious effects of "UVR deficiency" which can occur at latitudes
of about 60° (section 7.2).
Acute effects of UVR in the 250-320 nm wavelength range consist
of reddening, swelling, and blistering of the skin, occurring 3-24 h
after exposure, followed in 3-6 days by the production of melanin
("suntan"), in those capable of producing this pigment.
Acute effects on the eye consist of painful keratoconjunctivitis,
which recedes in 36-48 h.
After many years of repeated UVR exposure, the skin of
susceptible individuals becomes leathery, wrinkled, and discoloured
("aging changes") and skin cancer may develop (sections 5.4 and 5.5).
The degree to which these changes develop depends not only on the UVR
dose, but also, to a large extent, on the genetic background, and
particularly on the ability of the skin to pigment. For this reason,
"aging" changes and skin cancer are very much less common in
genetically heavily pigmented individuals.
The development of "aging" changes is irreversible, and presents
a major cosmetic (and thus psychological) problem, particularly for
women.
While skin cancer, with the exception of malignant melanoma, is
rarely fatal, it constitutes a social burden in terms of loss of work,
and medical expenses.
6.3 The Risk Associated with Combined Exposure with Other Agents
The interaction of UVR of various wavelengths, particularly UV-A
(320-400 nm), with natural and artificial chemical agents may result
in a variety of deleterious effect not elicited by UVR or the chemical
agents alone.
Among the most common of these effects are the phenomena of
phototoxicity, photoallergy, and chemically enhanced
photocarcinogenesis (sections 4.5 and 5.6). Fortunately, the actual
risk from such photobiological responses is small, as yet.
Of the phototoxic agents, the psoralens (furocoumarins) occur in
the rind of most citrus fruit, and in many green leafy plants. Contact
occurs most often in fruit pickers, others involved in the citrus
industry, and through the use of bergamot-containing perfumes. The
phototoxic reactions simulate sunburn. Acute skin and eye
phototoxicity are frequent, and sometimes serious, problems in workers
handling tars, such as roofers and road workers. Similar findings have
been made in creosote workers (Emmett, 1977a). Chronic phototoxicity,
and perhaps enhancement of carcinogenicity can also be induced by coal
tar products and phenanthrene carcinogen-containing materials. At risk
are roofers, road workers, and those in the tar and pitch-using
industries. The extent of augmentation of photocarcinogenesis in man
by this route is not known.
The introduction of man-made photoactive chemicals into the
environment is increasing. Serious, although small, outbreaks of
photoallergic reactions have been reported caused by soap additives
(halogenated salicylanilides), antibiotics (bithionol, griseofulvin),
drugs (chlortetracycline, thiazides, chlorpromazine), and, most
recently, compounds deliberately used in photochemical processes, such
as printing inks (Emmett, 1977b).
As yet, the risk of such reactions occurring is very small, but
with the continued introduction of new chemicals into the environment,
it is bound to increase.
6.4 The Population at Risk -- Geographical Distribution, Genetic
Influences and Occupation
As far as exposure to solar UVR is concerned, virtually the whole
of the world's population is at some risk. However, the degree of risk
varies greatly.
Of primary importance is the geographical distribution of the
population. Between latitudes 30° and 50°, the intensity and amount
(dose) of erythema-effective (and presumably carcinogenic) solar UVR
increases linearly with latitude towards the equator. This increase,
however, is modified by such conditions as cloud cover, the presence
of aerosols and "smog", the altitude above sea level (approximately a
15% increase in UVR for each 1000 metres elevation), and the degree of
obstruction of the sky by mountains, buildings, trees, etc.
Another factor of importance is genetic. Constitutional skin
pigmentation acts as a highly protective factor against the
deleterious effects of UVR on skin. Consequently, at least as far as
advanced "aging" changes and skin cancer are concerned, only subjects
with minimal or slight constitutional pigmentation are at risk. More
than two thirds of the world's population is more or less dark skinned
and has little chance of developing such UVR-induced changes. However,
pale skinned human beings living in tropical climates run a very high
risk of developing skin cancer. The highest incidence of solar skin
neoplasms exists in Queensland, Australia, where the combination of a
primarily Northern European and Celtic population and a latitude near
the equator results in a greater risk from occupational and social
outdoor exposure. In contrast, little skin cancer is found in the
native populations of tropical Africa, although latitude and exposure
are similar.
Table 9 shows the best available estimate of the number of
workers at risk from industrial exposure to various sources of UVR in
the USA. Data from other countries were not available to the Task
Group.
Table 9. Number of workers exposed to UVR (estimate from
Chicago Metropolitan Survey extrapolated to the
population of the USA)
Manufacturing
standard industrial classifications 19-39 211 000
Transportation and communication
standard industrial classifications 40-49 49 000
Wholesale, miscellaneous retail, service stations
standard industrial classifications 50, 55, 59 17 000
Services
standard industrial classifications 79-89 41 000
Total 320 000
As already pointed out, workers in many occupations are exposed
to various levels of artificial UVR.
6.5 The Reliability and Range of Known Dose-Effect and Dose-Response
Curves
6.5.1 Dose-effect curves for acute skin erythema
Erythema due to 254 nm radiation appears within 3-4 h of
exposure, reaches a peak between 8 and 12 h, and begins to subside
markedly by 24 h. Even at its peak intensity, the colour is a pale
pink-red, and it is very difficult to be certain of the minimal
erythema dose. At very low doses (of the order of less than 50 J/m2),
a weak effect can be recognized, which is clearly (because of the
shape and reasonably sharp borders) produced by the radiation.
However, it is not clear that this represents true erythema; the
colour is yellowish brown and seems to be extremely superficial,
almost on the surface of the skin. As reported by Hausser, even five
times the minimal erythema dose does not produce any severe erythema
at a wavelength of 254 nm. In contrast, with the minimal erythema dose
(MED) for wavelengths from 280-313 nm, the erythema is quite sharply
defined. The erythema produced by wavelengths 297, 303, and 313 nm is
deep red-purple and peaks in 24-28 h. It persists for 3-5 days and
imperceptibly changes into pigmentation.
6.5.2 Averages and limits, minimal, and slightly more than minimal
erythema doses
The great effect of time after irradiation and of choice of
degree of redness on the "action spectrum" of human skin is shown in
Fig. 9 and Table 10. Preliminary experiments suggest that the true
sensitivity peak lies between 290 and 294 nm. From 297 nm on, there
appears to be remarkably good agreement between most published
figures. The disagreement at shorter wavelengths is clearly because of
differences in time of evaluation, the difficulties inherent in the
delineation of "minimal erythema", and possible differences in skin
thickness.
Furthermore, if an erythema grade slightly above minimal is used
as a reference point, the resultant action spectrum closely approaches
that originally described by Hausser & Vahle (Fig. 10).
Table 10. Averages and limits (J/m2) for minimal erythema doses
(MED) read at 8 and 24 h and for a "moderate" erythema dose
read at 24 h (30 R). All radiation given at the second
exit slit, bandwidth 2.16 nm.a
nm MED 8 h MED 24 h 30 R 24 h
(J/m2) (J/m2) (J/m2)
254 63 (35-84) 100 (60-170) 194 (100-400)
280 140 (50-240) 220 (120-336) 320 (230-480)
297 140 (60-240) 140 (60-240) 140 (70-200)
303 390 (280-480) 410 (340-480) 450 (400-480)
313 6320 (4500-7700) 6800 (5400-7700) 7240
(5400-7700)
a From: Berger et al. (1967).
There are data that indicate that physical and chemical factors
may attenuate or intensify the carcinogenic effect of UVR
(Sviderskaja, 1971). Chronic exposure of animals to small doses of
ionizing radiation reduces resistance to the carcinogenic effects of
UVR, increasing the incidence and reducing the length of the latent
period. UV-B radiation has variable effects on the growth of
transplanted and chemically induced tumours. UV-B radiation can affect
the resistance of the body to tumour formation, increasing it with use
of sub-erythemal doses and considerably reducing it with large doses
(Dancig et al., 1975). These data, although obtained in animal systems
only, may be of great significance for human health, since the
resistance of the body to exposure to various harmful factors in the
environment operates against a certain background of natural UVR.
5.5.6 Mortality from skin cancer
In contrast to malignant melanoma, where mortality in most series
stiff exceeds 40%, the fatality rate in non-melanoma skin cancer is as
low as 1%. However, it would be incorrect to try to draw conclusions
from disease specific mortality rates.
5.5.7 Malignant melanoma
Both the incidence and the mortality rates of malignant melanoma
are rising rapidly in all countries in which they have been studied,
with mortality rates doubling in a 10-15 year period (Elwood & Lee,
1975).
The incidence varies from less than 1/100 000 in Japan, Nigeria,
and India to as high as 24/100 000 in Queensland, Australia. The rise
in incidence and mortality has been much greater in younger people
than in those over 65 years of age, implying that a causal mechanism
operates from an early age. The increase has been greater at certain
body sites and thus the site distribution has changed. The most
striking increase in incidence has been on the lower limbs in females
and the trunk in men (MacGovern, 1977).
In countries where such data are available, there is a distinct
association between latitude of residence and development of malignant
melanoma (Lancaster, 1956; Magnus, 1973; Movshovitz & Modan, 1973;
Elwood et al., 1974). The closer to the equator and the longer the
residence in countries with high insolation, the higher, in general,
is the incidence of malignant melanoma.
However, while there is usually a demonstrable latitude gradient
within a country, the latitude association in much less marked than
that for non-melanoma skin cancer. For instance, incidence rates for
malignant melanoma in Norway and Sweden are much higher than in
England and France both of which are much further south (Cutchis,
1978, Fig. 6 and 7).
Basal cell and squamous cell carcinoma, which are considered to
be due primarily to chronic UV-B exposure, are commonest on the areas
of skin exposed to sunlight, occur at a later age than malignant
melanoma, and are strikingly associated with severe solar damage to
the skin. In contrast, malignant melanoma occur more often on the
trunk of men and legs of women than non-melanoma skin cancer, and only
one type, the lentigo maligna melanoma (comprising not more than 15%
of all melanoma), is associated with histological UVR-induced skin
damage (McGovern & Mackie, 1959).
Thus, it appears that non-melanoma and melanoma skin cancers are
related to UVR exposure in different ways. Table 7 lists the
similarities and differences between these two types of tumours.
Parallel increases in melanoma and non-melanoma skin cancer cannot be
expected, as the latent period for malignant melanoma is apparently
much shorter than that for non-melanoma skin cancer. Reasons suggested
for the differences between the two types include: the greater
sensitivity of melanocytes to UVR (McGovern, 1977); the presence of
some secondarily produced circulating factor (Lee & Merrill, 1970);
and intermittent overdoses of UVR (Fears et al., 1977).
In the absence of an experimental animal model, and with the
present state of knowledge, it must be assumed that there is some
association between UVR and the development of malignant melanoma.
Thus any additional UVR exposure of susceptible individuals may
increase the risk of development of this very serious malignant skin
tumour.
5.6 Phototoxic and Photoallergic Diseases
5.6.1 Phototoxicity
Light-induced damage to the skin that does not depend on an
allergic mechanism may be considered phototoxic. Theoretically, these
reactions will occur in everyone, if the skin is exposed to enough
light energy of the proper wavelengths and if enough molecules that
will absorb these wavelengths are present. The radiation must
penetrate to the absorbing molecules for the reaction to occur.
Clinically, phototoxic reactions are usually characterized by erythema
(and at times oedema) occurring from a few minutes to several hours
after exposure, followed by hyperpigmentation and desquamation. The
sunburn reaction is the classical example of response to a phototoxic
effect (Ippen, 1969).
Table 7. Comparison of epidemiological factors in the etiology of malignant melanoma and
non-melanoma skin cancer
Factor Malignant melanoma Non-melanoma skin cancer
Latitude of residence Increases linearly within Increases geometrically with
countries, Incidence not latitude.
strictly related to latitude
globally.
Age of onset 3rd and 4th decade most 6th to 8th decade most common.
common.
Sex Moderate preponderance for Great preponderance for males.
females.
Anatomical distribution: Back, anterior torso, upper Head and neck (particularly ears
Male, white extremity, head, and neck. and lip), upper back, hand,
upper extremity.
Anatomical distribution: Back, lower leg, upper Head and neck (ears and lower
female white extremity, head, and neck. lip, spared), hands, upper
extremity, anterior chest.
Anatomical distribution: Soles, mucous membranes Anterior lower extremities, other
Black and oriental palms, nail bed. (all sites (all sites rare).
rare).
Racial (genetic) factors Celtic background, Scandinavians Celtic background, Scandinavians.
Rare in pigmented races. Rare in pigmented races.
Table 7 (contd).
Factor Malignant melanoma Non-melanoma skin cancer
Possible etiological Genetic (xeroderma pigmentosum Genetic (XP)
factors XP, B-K mole) Physical (UVR, X-ray)
Physical (UVR, trauma) Chemical (arsenic, coal tar).
Chemical (PCBsa, alpha-DOPA)
Developmental (nevi)
a PCBs = polychlorinated biphenyls.
From the clinical point of view, the erythema produced by various
phototoxic agents differs greatly in type of onset and type of
reaction; the ability of the agents to elicit pigmentation also
varies.
In contrast to the usual acute solar erythema, which begins after
a latent period of a few hours, peaks at 24 h, and subsides in a few
days to be replaced by moderate melanin pigmentation, the erythema due
to photodynamic compounds appears immediately after or during
radiation, may be associated with striking wheal formation, and
disappears in 3-6 h. Pigmentation is usually minimal.
The erythema due to furocoumarins (8-MOP) begins later than that
caused by solar radiation, peaks at 48-72 h, may persist for days, and
is followed by very intense pigmentation.
Despite a considerable amount of investigation, the mechanisms by
which phototoxic responses occur are not well understood. In the case
of the exogenous photosensitizer, either the molecule alone or a
complex of the chemical and cellular organelles becomes excited by the
absorption of light; triplet states and free radicals, or both, may be
formed.
Certain dyes and chemicals such as methylene blue, acriflavine,
rose bengal, and porphyrins produce photochemical effects on living
and non-living substrates only in the presence of oxygen. The
photo-dynamically active substance becomes excited and forms a triplet
state or a free radical. The excited chemical may also form peroxides
and then oxidize the substrate. Other possibilities include passing
the energy from the excited chemical to the biological substrate,
which then becomes oxidized, or the activated chemical may be able to
accept electrons, resulting in the oxidation of the substrate. After
excitation, the photosensitizing molecules return to the ground state
and are structurally unchanged.
Photosensitizing compounds may be endogenous, i.e., formed in the
body, usually by abnormal metabolism (e.g., porphyrins), or exogenous,
i.e., contacted externally or given as medication.
Exogenous photosensitizers may reach the skin by topical or
systemic routes and the reactions may be phototoxic or photoallergic
in nature. The action spectra for most of the phototoxic agents that
may cause skin disorders in man lie in the long-wavelength UVR range
(320-400 nm).
Contact photosensitizers include: cosmetics such as perfumes,
colognes, after-shave lotions (essential oils and psoralens),
lipsticks (fluorescein derivatives), creams, and hair preparations
(coal tar derivatives); and plants that cause phytophotodermatitis
such as Persian limes, pink rot-infested celery, many members of the
Umbelliferae and Rutaceae orders. These problems are primarily due to
psoralen compounds and therapeutic agents including phenothiazines and
sulfonamides (usually used therapeutically), halogenated
salicylanilides, sunscreens, and blankophores (usually photoallergic).
Systemic photosensitizers include: thiazide diuretics;
antibacterial sulfonamides; sulfonylurea antidiabetic drugs;
phenothiazines (especially chlorpromazine); and antibiotics
(especially dimethylchlortetracycline).
A large number of other drugs may occasionally induce
photosensitivity.
5.6.2 Photoallergy
Photoallergy can be defined as an acquired altered capacity of
the skin to react to light energy alone or in the presence of a
photosensitizer (Harber & Baer, 1969).
In photoallergic reactions, the photosensitizer leads to the
formation of the photohapten, which binds (covalently) with a suitable
carrier molecule to form the complete photoantigen. The carrier may be
a protein, polypeptide, mucopolypeptide, mucopolysaccharide, or other
macromolecule present in the skin. Once developed, photoallergy can
apparently occur with light energy alone, but presumably small
quantitites of the photoantigen are still present in the skin and
involve a circulating antibody or a cell-mediated response. In
contrast to phototoxicity, photoallergy is uncommon and is
characterized clinically by such reactions as immediate urticaria or
delayed papular or eczematous responses similar to contact dermatitis.
The hallmark of a sunlight-induced reaction, whether toxic or
allergic, is the distribution of the eruption. The exposed areas of
the face, neck, upper extremities, and, in women, the anterior surface
of the legs and the proximal, dorsal areas of the feet are mainly
involved. Exposure while driving may accentuate the eruption on the
side of the face and arm adjacent to the window. The upper eyelids,
subnasal and submental areas, flexural aspects of the wrists, and the
antecubital fossae tend to be spared. Clothing generally provides
protection, but reactions can be produced by penetration of UVR
especially through the light fabrics worn in summer.
The most common photoallergens are: 3,5-dibromosalicylanilide
(3,5 DBS); 4,5 dibromosalicylanilide (4,5 DBS); tribromosalicylanilide
(TBS); hexachlorophene; bithionol; and trichlorcarbanilide.
5.7 Pterygium and Cancer of the Eye
While detailed epidemiological evidence does not seem to exist,
there is a clinical impression among competent ophthalmologists that a
latitude gradient exists for the development of pterygium of the eye,
a benign hyperplasia of the bulbar conjunctiva which may eventually
interfere with vision by growing over the pupil (Dolezova, 1976).
Epidermoid carcinoma of the bulbar conjunctiva is a rare neoplasm
which appears with increased frequency in people living in the tropics
or subtropics (Afghanistan, Colombia, Ethiopia, Haiti, Malawi, Middle
East, Nigeria, Pakistan, Senegal, South Africa, and Uganda). It has
also been reported in cattle in the same region. Such tumours of the
eye can be induced in experimental animals with artificial UVR. Early
lesions, which are exophytic and tend to be papillary, are often
accompanied by basophilic degeneration of subepithelial collagen and
chronic inflammation.
The tumours are moderately to poorly differentiated keratinizing
epidermoid carcinomas.
In contrast to carcinoma of the skin, carcinoma of the eye is
more common in dark-skinned people, probably because of much greater
UVR exposure, and lack of pigment in the conjunctiva.
6. EVALUATION OF HEALTH RISKS TO MAN
6.1 The Significance and Extent of Different Environmental Sources of
Ultraviolet Radiation and Pathways of Exposure
The major health risks from natural UVR arise from chronic,
excessive, and unwise exposure to solar radiation. Section 2.1.1 of
this document describes in detail the wavelengths and quantities of
solar UVR that reach the earth and factors affecting it.
Briefly, depending on latitude and stratospheric ozone
concentration, the shortest wavelength measured (at noon, near the
equator) in solar radiation is about 290 nm. In most regions of earth,
the lower cut-off limit is at about 295 nm. The spectral composition
and radiation intensity of solar UVR is greatly influenced by
latitude, season, time of day (i.e., angle of the sun), cloud cover,
and the albedo of the surface. About two thirds of the skin-erythema-
producing solar UVR reaches the earth between 10h00 and 14h00.
In man, the extent of human exposure to solar UVR varies with
posture. In the upright position, esentially only portions of the
head, back of the neck, shoulders, forearms, and hands are exposed. In
addition, the skin of the thighs and upper arms may be heavily
exposed in some occupations such as driving a tractor. Exposure also
varies with time of day and local weather conditions; clothing
(wearing of hats, short- or long-sleeved shirts, shorts, etc.); work
and social habits; and ground albedo (snow, ice, and sand being the
only effective reflectors).
The maximum amount of solar UVR to which an individual could be
exposed in one day, represents about 25 minimal erythema skin doses,
i.e., about 7500 J/m2 of radiation equivalent to the skin erythema
effect of 297 nm monochromatic UVR.
Occupational exposure from artificial sources is either
inadvertent, when the sources produce UVR as a by-product, or
deliberate, when sources are designed to generate UVR to use its
properties. Depending on the characteristics of the source (section
2.1), the spectral composition of the emitted UVR can contain
wavelengths in the UV-A, UV-B, and UV-C regions.
Some industrial processes in which UV energy is a by-product are
welding, plasma torch operations, photoelectric scanning, and hot
metal operations. Because of the germicidal properties of certain
portions of the UV spectrum, artificial sources are used in hospitals,
biological laboratories, schools, and industry. Other common
applications are illumination, advertising, crime detection, chemical
synthesis and analysis, photoengraving, food, water, and air
sterilization, vitamin production, and medical diagnosis. Many other
occupations are listed in Table 8. New sources, such as UV lasers and
fluorescent panels, are being developed.
Table 8. Occupations potentially associated with UVR exposure
aircraft workers furnace workers oil field workers
barbers gardeners pipeline workers
bath attendants gas mantle workers plasma torch operators
brick layers glass blowers printers railroad track workers
burners, metal glass furnace workers ranchers
cattleman hairdressers road workers
construction workers herders seamen
cutters, metal iron workers skimmers, glass
drug makers lifeguards steel mill workers
electricians lithographers stockmen
farmers metal casting inspectors stokers
fisherman miners, open pit tobacco irradiators
food irradiators nurses vitamin D preparation makers
foundry workers welders
The amount of UVR exposure from artificial sources depends on the
spectral composition, radiant intensity, distance from source,
shielding, etc., and must de determined for individual conditions.
6.2 Types of Biological Effects and Their Significance for Human
Health
Since UVR penetrates essentially only into the skin and eyes of
man, the deleterious effects on these organs are of the greatest
importance. The acute and chronic effects of UVR are described in
detail in sections 4 and 5. Also of concern, however, are the
deleterious effects of "UVR deficiency" which can occur at latitudes
of about 60° (section 7.2).
Acute effects of UVR in the 250-320 nm wavelength range consist
of reddening, swelling, and blistering of the skin, occurring 3-24 h
after exposure, followed in 3-6 days by the production of melanin
("suntan"), in those capable of producing this pigment.
Acute effects on the eye consist of painful keratoconjunctivitis,
which recedes in 36-48 h.
After many years of repeated UVR exposure, the skin of
susceptible individuals becomes leathery, wrinkled, and discoloured
("aging changes") and skin cancer may develop (sections 5.4 and 5.5).
The degree to which these changes develop depends not only on the UVR
dose, but also, to a large extent, on the genetic background, and
particularly on the ability of the skin to pigment. For this reason,
"aging" changes and skin cancer are very much less common in
genetically heavily pigmented individuals.
The development of "aging" changes is irreversible, and presents
a major cosmetic (and thus psychological) problem, particularly for
women.
While skin cancer, with the exception of malignant melanoma, is
rarely fatal, it constitutes a social burden in terms of loss of work,
and medical expenses.
6.3 The Risk Associated with Combined Exposure with Other Agents
The interaction of UVR of various wavelengths, particularly UV-A
(320-400 nm), with natural and artificial chemical agents may result
in a variety of deleterious effect not elicited by UVR or the chemical
agents alone.
Among the most common of these effects are the phenomena of
phototoxicity, photoallergy, and chemically enhanced
photocarcinogenesis (sections 4.5 and 5.6). Fortunately, the actual
risk from such photobiological responses is small, as yet.
Of the phototoxic agents, the psoralens (furocoumarins) occur in
the rind of most citrus fruit, and in many green leafy plants. Contact
occurs most often in fruit pickers, others involved in the citrus
industry, and through the use of bergamot-containing perfumes. The
phototoxic reactions simulate sunburn. Acute skin and eye
phototoxicity are frequent, and sometimes serious, problems in workers
handling tars, such as roofers and road workers. Similar findings have
been made in creosote workers (Emmett, 1977a). Chronic phototoxicity,
and perhaps enhancement of carcinogenicity can also be induced by coal
tar products and phenanthrene carcinogen-containing materials. At risk
are roofers, road workers, and those in the tar and pitch-using
industries. The extent of augmentation of photocarcinogenesis in man
by this route is not known.
The introduction of man-made photoactive chemicals into the
environment is increasing. Serious, although small, outbreaks of
photoallergic reactions have been reported caused by soap additives
(halogenated salicylanilides), antibiotics (bithionol, griseofulvin),
drugs (chlortetracycline, thiazides, chlorpromazine), and, most
recently, compounds deliberately used in photochemical processes, such
as printing inks (Emmett, 1977b).
As yet, the risk of such reactions occurring is very small, but
with the continued introduction of new chemicals into the environment,
it is bound to increase.
6.4 The Population at Risk -- Geographical Distribution, Genetic
Influences and Occupation
As far as exposure to solar UVR is concerned, virtually the whole
of the world's population is at some risk. However, the degree of risk
varies greatly.
Of primary importance is the geographical distribution of the
population. Between latitudes 30° and 50°, the intensity and amount
(dose) of erythema-effective (and presumably carcinogenic) solar UVR
increases linearly with latitude towards the equator. This increase,
however, is modified by such conditions as cloud cover, the presence
of aerosols and "smog", the altitude above sea level (approximately a
15% increase in UVR for each 1000 metres elevation), and the degree of
obstruction of the sky by mountains, buildings, trees, etc.
Another factor of importance is genetic. Constitutional skin
pigmentation acts as a highly protective factor against the
deleterious effects of UVR on skin. Consequently, at least as far as
advanced "aging" changes and skin cancer are concerned, only subjects
with minimal or slight constitutional pigmentation are at risk. More
than two thirds of the world's population is more or less dark skinned
and has little chance of developing such UVR-induced changes. However,
pale skinned human beings living in tropical climates run a very high
risk of developing skin cancer. The highest incidence of solar skin
neoplasms exists in Queensland, Australia, where the combination of a
primarily Northern European and Celtic population and a latitude near
the equator results in a greater risk from occupational and social
outdoor exposure. In contrast, little skin cancer is found in the
native populations of tropical Africa, although latitude and exposure
are similar.
Table 9 shows the best available estimate of the number of
workers at risk from industrial exposure to various sources of UVR in
the USA. Data from other countries were not available to the Task
Group.
Table 9. Number of workers exposed to UVR (estimate from
Chicago Metropolitan Survey extrapolated to the
population of the USA)
Manufacturing
standard industrial classifications 19-39 211 000
Transportation and communication
standard industrial classifications 40-49 49 000
Wholesale, miscellaneous retail, service stations
standard industrial classifications 50, 55, 59 17 000
Services
standard industrial classifications 79-89 41 000
Total 320 000
As already pointed out, workers in many occupations are exposed
to various levels of artificial UVR.
6.5 The Reliability and Range of Known Dose-Effect and Dose-Response
Curves
6.5.1 Dose-effect curves for acute skin erythema
Erythema due to 254 nm radiation appears within 3-4 h of
exposure, reaches a peak between 8 and 12 h, and begins to subside
markedly by 24 h. Even at its peak intensity, the colour is a pale
pink-red, and it is very difficult to be certain of the minimal
erythema dose. At very low doses (of the order of less than 50 J/m2),
a weak effect can be recognized, which is clearly (because of the
shape and reasonably sharp borders) produced by the radiation.
However, it is not clear that this represents true erythema; the
colour is yellowish brown and seems to be extremely superficial,
almost on the surface of the skin. As reported by Hausser, even five
times the minimal erythema dose does not produce any severe erythema
at a wavelength of 254 nm. In contrast, with the minimal erythema dose
(MED) for wavelengths from 280-313 nm, the erythema is quite sharply
defined. The erythema produced by wavelengths 297, 303, and 313 nm is
deep red-purple and peaks in 24-28 h. It persists for 3-5 days and
imperceptibly changes into pigmentation.
6.5.2 Averages and limits, minimal, and slightly more than minimal
erythema doses
The great effect of time after irradiation and of choice of
degree of redness on the "action spectrum" of human skin is shown in
Fig. 9 and Table 10. Preliminary experiments suggest that the true
sensitivity peak lies between 290 and 294 nm. From 297 nm on, there
appears to be remarkably good agreement between most published
figures. The disagreement at shorter wavelengths is clearly because of
differences in time of evaluation, the difficulties inherent in the
delineation of "minimal erythema", and possible differences in skin
thickness.
Furthermore, if an erythema grade slightly above minimal is used
as a reference point, the resultant action spectrum closely approaches
that originally described by Hausser & Vahle (Fig. 10).
Table 10. Averages and limits (J/m2) for minimal erythema doses
(MED) read at 8 and 24 h and for a "moderate" erythema dose
read at 24 h (30 R). All radiation given at the second
exit slit, bandwidth 2.16 nm.a
nm MED 8 h MED 24 h 30 R 24 h
(J/m2) (J/m2) (J/m2)
254 63 (35-84) 100 (60-170) 194 (100-400)
280 140 (50-240) 220 (120-336) 320 (230-480)
297 140 (60-240) 140 (60-240) 140 (70-200)
303 390 (280-480) 410 (340-480) 450 (400-480)
313 6320 (4500-7700) 6800 (5400-7700) 7240
(5400-7700)
a From: Berger et al. (1967).

 6.5.3 The "erythema range" effect
One of the important observations of Hausser & Vahle (1922) was
that there appeared to be a significant difference between the doses
needed to produce slight and maximal erytherna at different
wavelengths. This concept is of great potential significance for the
prevention of sunburn and the understanding of diseases due to light.
In Fig. 10, data are plotted from an experiment designed by Hausser to
test this effect, which show that 5 times the minimal erytherna dose
produces much less than the maximal erytherna at 254 nm, while 2.5
times the minimal erythema dose produces maximal erytherna at 303 nm.
While it takes much more energy to produce any erytherna at 303 nm
than at 254 nm, not much more than the threshold dose produces a
significant burn.
These observations are of great importance because they show that
the acute biological effects of several wavelengths in the UV-B
proceed at different time scales, and have very different
dose-response relationships. Thus, the usual assumptions that it is
realistic to weight the effectiveness of different wavelengths in a
continuous UVR source (such as the sun) by an action spectrum obtained
for a threshold effect with a monochromator is not correct, nor is it
appropriate to consider the effect of these wavelengths to be
biologically equivalent.
Unfortunately, in the absence of knowledge of chromophores or
biological mechanisms, there is no better way of comparing the
effectiveness of sources of different UVR composition than the present
method of calculating the skin erytherna effectiveness of UV-B.
As in all photobiological cutaneous effects, pigmentation plays a
major role in the sensitivity of the skin to UVR. Heavily pigmented
people are 10-20 times less sensitive than untanned fair-skinned
people. As far as is known, the action spectra for erythema are
similar for all races, differing only in the amount of energy
necessary to produce threshold effects.
6.5.4 Dose-response curves for keratoconjunctivitis
These are essentially similar to those for erythema, except that
the peak of the action spectrum is located at 270 nm. This is most
likely because of the absence of a filtering stratum corneum and
because the conjunctiva is not pigmented. The peak of 270 nm is
therefore used for the normalization of the dose limits of broad band
sources.
6.5.5 Dose-response relationship for photocarcinogenesis
Most of the existing evidence is consistent with the concepts
that UV-induced photodamage to skin is the main causal factor in the
development of skin cancer, that the development of skin cancer is a
stochastic effect, and that there is no threshold (sections 4.3 and
5.5).
Thus, a relationship should exist between skin cancer incidence
and accumulated dose, using a sensitivity function. In mice, the
quantitative relationship between UVR dose and the production of skin
cancer has been thoroughly explored; tumour incidence in this
experimental model is proportional to the square root of the number of
doses, the dose size, and the interval between doses.
In man, there is ample evidence that a latitude gradient exists
for the incidence of skin cancer in sensitive, fair-skinned
populations, and that this gradient is non-linear. There is obviously
a relationship between latitude and the intensity of solar radiation,
and this gradient is exaggerated in the UVR portion of the spectrum.
Although the shape of this relationship is relatively complex and
depends on a number of variables, for a range of mid-latitudes
(30°-50°), both the theoretical form and that obtained from actual
field measurements closely approximate a straight line. It is
generally accepted that latitude-related climatic and environmental
conditions and behavioural effects must modify the UVR dose actually
reaching a population. The factors involved, and their magnitude are
largely speculative.
A series of mathematical models relating human skin cancer
incidence to solar UVR has been proposed at various times in the past
few years, because of concern about alteration of the stratospheric
ozone layer (Green et al., 1978; Rundel & Nachtwey, 1978). While the
uncertainties are very great, the best accepted model data suggest
that a 5% increase in erythema-effective solar UVR may result in a 15%
(range 7.5-25%) increase in skin cancer in a susceptible population
after about 60 years, when a steady state has been reached. As far as
the relative risk is concerned in susceptible white-skinned
populations, people in older age groups, with fair skin that sunburns
easily and with a high life-time solar exposure, run 10-20 times more
risk of developing skin cancer than their contemporaries, who tan
easily and have a low life-time solar exposure (Vitaliano, 1978).
Incidences of nonmelanoma skin cancer as high as 350/100 000
population per year have been observed in elderly, white-skinned males
in Queensland, Australia, and Texas, USA. Populations with
constitutionally, heavily pigmented skin run only a minimum risk of
developing skin cancer in their lifetime.
7. GUIDELINES FOR HEALTH PROTECTION
The development of criteria for both upper and lower limits of
exposure to either natural or artificial UVR is extremely difficult
because of such problems as:
(a) the variation in both the acute and chronic effects of UVR
of different wave lengths;
(b) the considerable differences in the spectral composition of
light from different sources and particularly of sunlight at different
latitudes;
(c) the great differences in cutaneous sensitivity to UVR due
to genetic, environmental, and adaptive effects, and the considerable
variation in sensitivity in the same person at different times; and
(d) the difficulty of differentiating between the necessary
dose of UVR compatible with the upkeep of life, and the lowest dose
that results in serious detrimental effects.
7.1 Range of Exposure Limits
7.1.1 Exposure to solar ultraviolet radiation
As described in section 6, two thirds of the daily amount of
solar UV-B radiation reaches the earth between 10h00 and 14h00. Thus,
exposure should be reduced to a miminum during these hours. After a
period of acclimatization, most people can tolerate several hours of
outdoor exposure in the morning and afternoon, but shielding in the
near noon hours should always be considered. In general, exposure
during this very important period of acclimatization should not exceed
4 minimal erythema doses per day without some protection (either
clothing or sunscreens, section 7.3). This would correspond to 1 h of
exposure in the tropics. A dose of 1/8 MED per day appears to be
sufficient to prevent UVR deficiency.
7.1.2 Occupational exposure to artificial ultraviolet radiation
No internationally agreed limits exist at this time for
occupational exposure to UVR, which take into consideration the acute
effects and the risk of late cancer development. So far, the only
limits available, which could be used in the preparation of
guidelines, have been proposed by the National Institute of
Occupational Safety and Health in the USA (NIOSH, 1975).
The limits are based on the minimal erythema dose (MED) and the
minimal photokeratitic dose, which means that only acute effects have
been taken into consideration. For the UVR 200-315 nm region, it is
stated that radiant exposure in any 8-h period must not exceed the
values given in Table 11. For the wavelength range 315-400 nm, the
total irradiance on unprotected skin or eye must not exceed 10 W/m2
for periods exceeding 103 seconds. For radiant exposure of shorter
durations, it should not exceed 10 000 J/m2. The limit for 315-400 nm
is probably much too low and could be revised to a higher level.
Table 11. Total permissible 8-h doses and relative spectral
effectiveness of some selected monochromatic
wavelengthsa
Wavelength Permissible 8-h Relative spectral
(nm) dose (J/m2) effectiveness
(S lambda)b
200 1000 0.03
210 400 0.075
220 250 0.12
230 160 0.19
240 100 0.30
250 70 0.43
254 60 0.50
280 46 0.65
270 30 1.00
280 34 0.88
290 47 0.84
300 100 0.30
305 500 0.06
310 2000 0.015
315 10 000 0.003
a "From: NIOSH (1975).
Table 12. Maximum permissible exposure times for selected
values of Ieffa
Duration of exposure Effective irradiance,
per day Ieff (W/m2)b
8 h 1.0
4 h 2.0
2 h 4.0
1 h 8.0
30 min 17.0
15 min 33.0
10 min 50.0
5 min 100.0
1 min 500.0
30 sec 1000.0
a From: NIOSH (1975).
b Effective irradiance = action spectrum weighted irradiance.
However, these values only apply to sources emitting essentially
monochromatic UVR. The maximum permissible exposure for a broad band
source should be calculated by summing up the relative contributions
from all its spectral components, each being weighted by the relative
spectral effect SL, as given in Table 12. In addition, these
guidelines for determining exposure limits should not be used for
photosensitive individuals.
So far, none of the guidelines has included an evaluation of the
carcinogenic risk, as neither the action spectrum nor the
dose-response curve is known for man. It is hoped that this will be
obtained by comparison of cancer incidence and UV irradiance as
measured by the Robertson-Berger method.
7.1.3 Exposure of the general population to artificial ultraviolet
radiation
The exposure of the general population to artificial UVR is
primarily for hygienic and for cosmetic purposes. The use of UVR for
health purposes is discussed in sections 7.2 and 7.3.
The use of UVR for cosmetic purposes generally involves a
requirement for the development of skin pigmentation. For this reason,
it is unrealistic to follow the occupational standards, since they
would not allow the desired effects to be obtained.
Doses of UVR sufficient to produce slight erythema in 24 hours
are usually adequate for cosmetic purposes. Significant overdoses and
repeated UVR exposures for prolonged periods should be avoided. The
eyes must be properly shielded during each exposure. The risk of the
development of skin cancer due to chronic exposure to UVR for cosmetic
purposes is not known.
A number of devices available to the general population emit
significant amounts of UVR, or will do so if the protective envelope
of the device is damaged, and may cause acute UVR-induced eye and skin
damage. Such devices include Wood's lights, sterilizing and
ozone-producing lamps, and high power mercury and xenon arc lamps used
for illumination.
7.1.4 Measurement of natural and artificial ultraviolet radiation
Measurement of solar UVR involves serious difficulties, because
of the need for accurate spectral discrimination at the shortest end
of the solar spectrum. This is necessary because of the considerable
variations in the biological effectiveness of UVR shorter than 320 nm.
Few really practical, accurate, stray light-free spectroradiometers
have been developed so far, for use in the middle UVR region (UV-B)
and the use of action spectrum weighted, integrating analogue UVR
meters has been found to be much more practical.
The few practical prototypes of integrating chemical UVR
dosimeters that have been developed are still in the experimental
stage. If they can be perfected, such personal UVR dosimeters would be
of the greatest use (Challoner et al., 1976; Davis et al., 1976).
Basically, the same problems pertain to monitoring of artificial
UVR, although measurements are somewhat easier in a laboratory
setting.
The exposure criteria recommended in section 7.1.2 have not been
put to practical use, because it is still not technologically possible
to measure UVR adequately for compliance.
Thus, working practices are recommended for the control of
exposure in situations where sufficient measurement or emission data
are not available. A frequently practiced method of protection
consists of adequate containement of UVR-producing light sources.
7.2 Health Effects of Solar Ultraviolet Radiation in the General
Population
Two aspects of health protection in the general population are of
interest. One is the prevention of UVR deficiency that can occur in
populations living near the north and south poles (generally at
latitudes of 60° or more). The other deals with the protection of
pale-skinned people from excessive UVR in subtropical and tropical
areas (generally between latitudes 35° N and 35° S).
The zoning of the USSR for UVR is particularly interesting from a
health point of view (Belinskij, 1971). In the "UVR-deficit zone" it
appears essential to use irradiation from artificial sources, during
certain months, to compensate for UVR deficiency. In the "UV comfort
zone", artificial irradiation is unnecessary. In the "UVR excess
zone", it is essential to undertake measures for protection against
solar UVR, in order to avoid skin cancer. It has also been proposed
that, in order to prevent skin tumours among town dwellers in whom
light starvation has caused depigmentation of the skin, preventive UVR
should be carried out to increase skin pigmentation.
7.3 UVR Deficiency and its Prevention
7.3.1 Insolation and UV irradiation of built-up areas
It is well known that solar energy entering a room illuminates,
warms, dries, and particularly important, disinfects it, thus having a
beneficial, physical and psychological effect. The sanitation
standards and regulations for the insolation of dwellings and public
buildings and built-up areas, which are in force at present in the
USSR, are based on the requirement that premises should receive three
hours' uninterrupted insolation on cloudless days during the period 22
March-22 September and that protection should be given to limit the
thermal effect of solar radiation in the southern regions, below
latitude 55°N (State Standards, 1976b).
This health requirement is evident from a number of reviews
(Dancig, 1971; Galanin & Pirkin, 1971; Aleksandrov et al., 1975).
Solar UVR has a particularly valuable health-effect in that, inter
alia, it speeds up the processes of environmental selfpurification
(Belikova, 1960; Gluscenko et al., 1975). Therefore, town planning
tasks should include the rational use of UVR from the sun and sky
(Davidson, 1970; Gusev & Dunaev, 1971).
Since window glass cuts off the most biologically active
component of natural UVR, research to increase the UVR transparency of
window glass has been of particular concern to health workers in the
most northern latitudes (Belikova, 1964).
7.3.2 Sunbathing and air-bathing in the prevention of UVR deficiency
In addition to measures ensuring the maximum possible penetration
of natural UVR into working and dwelling places, prevention of UVR
deficiency can also be ensured by organizing solaria, beaches, and
sports areas attached to children's establishments (kindergartens and
pioneer camps), and to factories and mills. A schedule for the UV
irradiation of children and adults, for sun and sun-and-air baths at
different geographical latitudes, at different seasons of the year,
and at different times of the day has been developed (Generalov,
1971). At present, the effects of such solar radiation schedules
cannot be extrapolated to potential late effects.
7.3.3 Artificial ultraviolet radiation in the prevention of UVR
deficiency
Effective principles for the use of artificial UVR in the
prevention of UVR deficiency and "light starvation" in man have been
developed in the USSR. The recommended levels consist of a daily dose
of UV-B of between 0.125 and 0.75 of the threshold erytherna dose
(State Standards, 1964). The use of prophylactic UV irradiation has
been shown to be quite effective in workers in industries lacking
natural light (Bocenkova, 1971) and particularly effective in children
of preschool and school age (Ronge, 1948; Zilov, 1971).
Regulations have been drawn up for the planning and operation of
artificial UV irradiation devices in industrial enterprises (State
Standards, 1976b).
7.4 Protection against Ultraviolet Radiation
The whole of the world population has a potential for developing
skin cancer, the risk depending on the intensity and degree of
exposure to solar UVR during the life span. If all current exposure to
solar UVR could be significantly reduced, the incidence of skin cancer
would eventually decrease greatly.
Health and appearance can be adequately catered for by exposing
only part of the body for less than half an hour per day at or near
noon, except at latitudes north of 60° N.
7.4.1 Sunscreen preparations
Sunscreen preparations are usually classified as chemical or
physical agents. The former include para-aminobenzoic acid and its
esters, cinnamates, and benzophenones, all of which act by absorbing
radiation, which is dissipated as radiation of lower energy. Physical
agents act as simple physical barriers, reflecting, blocking, or
scattering light. They include titanium dioxide, talc, and zinc oxide.
Mainly because of cosmetic objections, the physical barriers are not
often used in sunscreen formulations (Robertson, 1972).
The principle of covering the skin by spreading a layer of
reliable UV absorber on the surface has proved a popular one.
Providing that the thickness of the layer applied is adequate and that
it adheres to the skin, it gives protection to the wearer under all
conditions.
Details of the kind of chemicals and bases used are beyond the
scope of this report.
7.4.2 Clothing
Covering the skin with clothing gives a sense of security that is
often misleading. The most frequent sites for skin cancer are those
that face upward. Thus, a hat is essential for adequate protection of
the forehead, scalp, and tops of ears. However, generally, it gives
only partial protection for the nose, less for the lower face, and
none for the hands and arms.
Body coverings worn in hot climates are generally not complete
absorbers. The average white shirt worn by men may transmit 20% of
UVR, while lighter weaves favoured by women may allow 50% of UVR to
reach the shoulders. Fairly complete clothing cover is more tolerable
in a dry atmosphere than a humid, coastal, tropical environment.
7.4.3 Behavioural conformity with the environment
When unexpectedly detained in the sun, protective procedures
available include facing in a variety of directions, hanging the head
and shielding the head with the hands or with a handkerchief. Shadows
should be used whenever possible, including one's own shadow. When
standing in the shadow of a building, only rays from about half the
sky are received, that is, about one quarter of the harmful intensity
of full daylight. This is certainly useful protection, but fair skin
will still burn in about one hour; in three hours, a painful burn may
be initiated, although the sufferer has not been in direct sunlight at
all.
The dominant factor in the daily erythemal exposure is the angle
of the sun above the horizon. This varies with latitude and with time
of year. Thus, maximum exposure occurs, when the sun is almost
directly overhead. The simplest means of protection is to take shelter
around the middle of the day, especially in the summer months.
At least one third of the whole day's UVR exposure occurs in the
hour before and the hour after noon; half the day's exposure, enough
for a very disabling sunburn, occurs during the 3-hour period around
noon. If shelter is taken between 10h00 and 14h00, only one third of
the day's exposure remains. Outside the period from 9h00 to 15h00,
only one sixth remains, permitting considerable outdoor activity even
for subjects with the most sensitive skin types.
7.4.4 Occupational protection
With the exception of medically prescribed doses, exposure of
both the eyes and skin to UVR should be kept to a minimum. In order to
protect persons in the vicinity of artificial UVR, the following
precautions are recommended:
(a) Whenever possible, prevention of excessive exposure of the
eyes and skin should be ensured by proper engineering design of
UVR-emitting installations and suitable enclosures, so that any UVR is
either adequately contained or sufficiently attenuated.
(b) When, for justifiable reasons, such containment is not
possible, protection should be afforded by providing close-fitting
goggles and/or face shields accompanied, if necessary, by suitable
UVR-opaque clothing and gloves to cover the skin.
(c) Adequate and appropriate instruction should be given, to
any person liable to be excessively exposed to UVR, concerning the
hazards involved and the precautions to be observed to avoid excessive
exposure.
(d) For artificial UVR sources that do not emit significant
visible light, a visible or audible warning signal may be required to
show when the UVR is being emitted.
(e) Powerful short wavelength UVR sources may generate ozone.
This additional hazard should be avoided by providing either adequate
ventilation or an adequate ozone removal system in the workplace.
REFERENCES
AITKEN, R. (1937) Ultraviolet radiation treatment of preeclamptic
toxemia. Br. J. Phys. Med., 12: 45-47.
ALEKSANDROV, V. N. & ALEKSANDROVA, A. I. (1975) [The insolation and UV
climate of housing in the far north.] Gig. i Sanit. 5: 16 (in
Russian).
ANCHEV, N., POPOV, I., MONOV N., & OUZOUNOV, N. (1968) Spreading of
malignant neoplasms in the People's Republic of Bulgaria.
Neoplasma, 15: 451-468.
ANDREEV, M. G., VASIL'EV B. D., ZAHAR'EVSKIJ, A. V., & HOROKORIN, T.
(1975) [Artificial sources of UV radiation with biological
effect.] In: [The biological effect of UV radiation.] Moscow,
Nauka, pp. 221-225 (in Russian).
BACHEM, A. (1956) Ophthalmic ultraviolet action spectra. Am. J.
Ophthalmol., 41: 969-975.
BAIN, J. & RUSCH, H. P. (1943) Carcinogenesis with ultraviolet
radiation of wave length 2, 800-3, 400 A. Cancer Res.,
3: 425-430.
BEARD, H. H., BOGGESS, T. S., & VON HAAM, E. (1936) Experimental
production of malignant tumors in the albino rat by means of
ultraviolet rays. Am. J. Cancer, 27: 257-266.
BELIKOVA, V. K. (1964) [Evaluation from the hygienic standpoint of
experimental samples of a window glass for residential and public
buildings.] Gig. i. Sanit., 1: 21-28 (in Russian).
BELIKOVA, V. K. (1966) [Natural ultraviolet radiation and its
bactericidal importance.] Moscow, Medicina, pp. 322-326 (in
Russian).
BELIKOVA, V. K., ZABALUEVA, A. P., & GALPERIN, E. L. (1975)
[Evaluation from the standpoint of hygiene of the zoning of the
territory of the U.S.S.R. in respect to UV radiation levels.] In:
[The biological effects of UV radiation.] Moscow, Nauka, pp.
161-165 (in Russian).
BELINSKIJ, V. A. (1971) [Zoning of the territory of the USSR in
respect to the natural UV radiation.] In: [Meteorology and
climatology.] Issue 2, Moscow, Publishing House of the Moscow
Branch of the Geographical Society, (in Russian).
BELINSKIJ, B. A. & ANDRIENKO, L. M. (1974) [A simplified radiation
model of the atmosphere in the UV spectral region.] In:
[Radiation, processes in the atmosphere and on the earth's
surface.] Leningrad, Gidrometeoizdat, pp. 273-276, (in
Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1963) [Ultraviolet climate of the
USSR.] In [Proceedings of the All-Union Scientific
Meteorological Meeting.] Leningrad, Gidrometeozidat,
pp. 244-252 (in Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1962) [Scattered UV-radiation in the
Eastern Pamirs.] In: [Scientific Communications of the Institute
of Geology and Geography of the Academy of Sciences of the
Lithuanian SSR.] Vilanus, Vil. XIII, pp. 283-287 (in Russian).
BELINSKIJ, V. A., GARADZA, M.P., MEZENNAJA, L. M., & NEZVAL, E. I.
(1968) [Sun and sky ultraviolet radiation.] Moscow, Moscow
University Press (in Russian).
BENDER, M. A., GRIGGS, H. G., & WALKER, P. L. (1973) Mechanisms of
chromosomal aberration production. I. Aberration induction by
ultraviolet light. Mutat. Res., 20: 387-402.
BENER, P. (1972) Approximate values of intensity of natural
ultraviolet radiation for different amounts of atmospheric
ozone. London, European Research Office, United States Army.
(Contract DAJA 36-68-C-1017).
BEN-HUR, E. & ELKIND, M. M. (1973) DNA cross-linking in Chinese
hamster cells exposed to near ultraviolet light in the presence
of 4,5',8-trimethylpsoralen. Biochim. Biophys. Acta,
331: 181-193.
BERENBLUM, I. & SHUBIK, P. (1947) The role of croton oil applications,
associated with a single painting of a carcinogen, in tumor
induction of the mouse's skin. Br. J. Cancer, 1: 379-382.
BERENBLUM, I. & SHUBIK, P. (1949) An experimental study of the
initiating stage of carcinogenesis, and a re-examination of the
somatic cell mutation theory of cancer. Br. J. Cancer,
3: 109-118.
BERGER, D., URBACH, F., & DAVIES, R. E. (1967) The action spectrum of
erythema induced by ultraviolet radiation. Proceedings of the
13th International Congress of Dermatology, Berlin, Springer
Verlag, pp. 1112-1117.
BERGER, D., ROBERTSON, D. F. & DAVIES R. E. (1975) Field measurements
of biologically effective UV radiation. In: Impacts of climatic
change on the biosphere, Springfield, VA, National Technical
Information Service, Part I, Chapter 2, pp. 233-264 (Appendix D,
CIAP Monograph No. 5).
BEUKERS R. & BERENDS, W. (1960) Isolation and identification of the
irradiation product of thymine. Biochim. Biophys. Acta,
41: 550-551.
BILLEN, D. & GREEN, A. E. S. (1975) Comparison of germicidal activity
of sunlight with the response of a sunburning ultraviolet meter.
In: Impacts of climatic change on the biosphere, Springfield,
VA, National Technical Information Service, Part 1, Chapter 2,
pp. 299-305 (Appendix F, CIAP Monograph No. 5).
BISCHOFF, F. (1969) Carcinogenic effects of steroids. In: Paoletti, &
Kritchevsky, ed. Advances in lipid research. New York, Academic
Press, Vol. 7, pp. 165-244.
BLACK, H. S. & CHAN, J. T. (1976) Etiologic related studies of
ultraviolet light-mediated carcinogenesis. Oncology,
33: 119-122.
BLACK, H. S. & DOUGLAS, D. R. (1973) Formation of a carcinogen of
natural origin in the etiology of UV-carcinogenesis. Cancer
Res., 33: 2094-2096.
BLACK, H. S. & LO, W. B. (1971) Formation of a carcinogen in human
skin irradiated with ultraviolet light. Nature (Lond.),
234: 306-308.
BLACK, H. S., CHAN, J. T., & BROWN, G. E. (1978) Effects of dietary
constituents on ultraviolet light-mediated carcinogenesis.
Cancer Res., 38: 1384-1387.
BLUM, H. F. (1941) Sunlight and cancer of the skin. J. Natl Cancer
Inst., 1: 397-431.
BLUM, H. F. (1943) Wavelength dependence of tumor induction by
ultraviolet radiation. J. Natl Cancer Inst., 3: 533-537.
BLUM, H. F. (1969) Quantitative aspects of cancer induction by
ultraviolet light: Including a revised model. In: Urbach, F., ed.
The biological effects of ultraviolet radiation, Oxford,
Pergamon Press.
BLUM, H. F. & LIPPINCOTT, S. W. (1943) Carcinogenic effectiveness of
ultraviolet radiation of wavelength 2537A. J. Natl Cancer Inst.,
3: 211-216.
BLUM, H. F., GRADY, H. G., & KIRBY-SMITH, J. S. (1943) Relationships
between dosage and rate of tumor induction by ultraviolet
radiation. J. Natl Cancer Inst., 3: 91-97.
BLUM, H. F., BUTLER, E. G., DAILEY, T. H. DAUBE, J. R., MAWE, R. C., &
SOFFEN, G. W. (1959) Irradiation of mouse skin with single doses
of ultraviolet light. J. Natl Cancer Inst., 22: 979-993.
BOCENKOVA, T. D. (1971) [The application of prophylactic
UV-irradiation to workers in buildings of a new type.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 245-251 (in
Russian).
BOGUCKIJ, B. V., KAPELEVA, A. I., BOKSA, V. G., LESINSKAJ, N. P.,
CIBIREVA, E. M, KOSTIN, N. F., & EMELKIN, V. I. (1975)
[Continuous and pulsed ultraviolet irradiation in combined
treatment of protracted and chronic pneumonia.] In:
[The biological effect of UV-radiation.] Moscow, Nauka,
pp. 126-127 (in Russian).
BOVIE, W. T. & KLEIN, A. (1919) Sensitization to heat due to exposure
to light of short wavelengths. J. Gen. Physiol., 1: 331-336.
BOYCE, R. P. & HOWARD-FLANDERS, P. (1964) Release of ultraviolet
light-induced thymine dimers from DNA in E. coli K-12. Proc.
Natl Acad. Sci., 51: 293-300.
BRIDGES, B. A. & HUCKLE, J. (1970) Mutagenesis of cultured mammalian
cells by X-radiation and ultraviolet light. Mutat. Res.
10: 141-151.
BUCHANAN, A. R., HELM, H. C., & STILSON, D. W. (1960) Biomedical
effects of exposure to electromagnetic radiation.
I. Ultraviolet. Dayton, OH, Wright-Patterson Air Force Base,
pp. 60-376 (WADD Technical Report).
BUSCHKE, W., FRIEDENWALD, J. S., & MOSES, S. G. (1945) Effects of
ultraviolet irradiation on corneal epithelium: Mitosis, nuclear
fragmentation, post-traumatic cell movements, loss of tissue
cohesion. J. Cell. Comp. Physiol., 26: 147-164.
CAKLIN, A. V. (1974) [The epidemiology of malignant neoplasms.] In:
[Handbook on oncology.] Moscow, Medicina, pp. 26-27 (in
Russian).
CARLSON, L. D. & JACKSON, B. H. (1959) Combined effects of ionizing
radiation and high temperature on longevity of Sprague-Dawley
rat. Radiat. Res., 11: 509-519.
CASTO, B.C. (1973) Enhancement of adenovirus transformation by
treatment of hamster cells with ultraviolet irradiation, DNA base
analogs, and dibenz(a,h,)anthracene. Cancer Res., 33: 402-407.
CHALLONER, A. V. J., CORLESS, D., DAVIS, A., DEANE, G. H. W., DIFFERY,
B. L., GUPTA, S. P., & MAGNUS, I. A. (1976) Personnel monitoring
of exposure to ultraviolet radiation. Clin. exp. Dermatol.,
1: 175-179.
CHANDRA, P. (1972) Photodynamic action: A valuable tool in molecular
biology. Res. org. biol. med. chem., 3 (1): 232-258.
CHANDRA, P., RODIGHIERO, G., PALIKCIOGLU, S., & BISWAS, R. K. (1976)
Nucleic acid modification by furocoumarins and light: Some
biomedical implications. In: Jung, E., ed. Photochemotherapie,
Stuttgart, F. K. Schattauer Verlag, pp. 25-32.
CHARLIER, M. & HELENE, C. (1972) Photochemical reactions of aromatic
ketones with nucleic acids and their components I-. Purine and
pyrimidine bases and nucleosides. Photochem. Photobiol.,
15: 71-87.
CLEAVER, J. E. (1973) Xeroderma pigmentosum- progress and regress.
J. invest. Dermatol., 60: 374-380.
CLEAVER, J. E. & THOMAS, G. H. (1969) Single-strand interruptions in
DNA and the effects of caffeine in Chinese hamster cells
irradiated with ultraviolet light. Biochem. biophys. Res. Comm.
26: 203-208.
CLEAVER, J. E., THOMAS, G. H., TROSKO, J. E., & LETT, J. T. (1972)
Excision repair (dimer excision, strand breakage and repair
replication) in primary cultures of eukaryotic (bovine) cells.
Exp. cell Res., 74: 67-80.
CLEAVER, J. E. & TROSKO, J. E. (1969) DNA-degradation productions from
mammalian cells irradiated with ultraviolet light. Int. J.
radiat. Biol., 15: 411-424.
COBLENTZ, W. W., STAIR, R., & HOGUE, J. M. (1932) The spectral
erythemic reaction of the untanned human skin to UV radiation.
Bur. Stand. J. Res., 8: 541-547.
COGAN, D. G. & KINSEY, V. E. (1946) Action spectrum of keratitis
produced by ultraviolet radiation. Arch. Ophthalmol.,
35: 670-677.
COHEN-BAZIRE, G. & STANIER, R. Y. (1958) Inhibition of carotenoid
synthesis in photosynthetic bacteria. Nature (Lond.),
181: 250-252.
COX, B. & GAME, J. (1974) Repair systems in Saccharomyces. Mutat.
Res., 26: 257-264.
CURTIS, G. L. (1975) Initiation-promotion skin carcinogenesis and
immunological competence. Proc. Soc. Exp. Med., 150 (1):
pp. 61-64.
CUTCHIS, P. (1978) On the linkage of solar ultraviolet radiation to
skin cancer. Washington, DC, Federal Aviation Administration,
Office of Environmental Quality, pp. 1342 (Institute for Defense
Analysis Paper).
DANCIG, N.M. (1971) [Lighting hygiene and insolation of buildings and
built up areas in towns.] In: [Questions of housing hygiene and
of curative power and preventive medicine.] Moscow, A. N. Sysin
Institute, pp. 13-19 (in Russian).
DANCIG, I. N. (1974) [Dental caries and UV deficiency.]
Stimatologija, 1: 11-13 (in Russian).
DANCIG, N.M. (1975) [The hygienic basis for preventing light sarvation
among ships' crews.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 168-171 (in Russian).
DANCIG, N. M., ZABALNEVA, A. P., & PROKOPENKO, Ju.I. (1975) [The
importance of the protective effect of UV radiation in the
development of the tumoral process under experimental
conditions.] In: [The biological effects of UV radiation.]
Moscow, Nauka, pp. 137-142 (in Russian).
DANIELS, F. J., BROPHY, D., & LOWITS, W. C. (1961) Histochemical
response of human skin following ultraviolet radiation.
J. invest. Dermatol., 37: 351-357.
DAVIDSON, B. M. (1970) Relative evaluation of the insolation resources
of flats in a residential "microdistrict". Svetotekhnika,
7; 13-15.
DAVIES, J. N. P., KNOWLDEN, J., & WILSON, B. A. (1965) Incidence rates
of cancer in Kyandondo County, Uganda, 1954-1960. J. Natl Cancer
Inst., 35: 789-821.
DAVIES, R. E., DODGE, H. A., & AUSTIN, W. A. (1972a) Carcinogenicity
of DMBA under various light sources. Proceedings of the IX
Congress on Photobiology, pp. 247.
DAVIES, R. E., DODGE, H. A., & DESCHIELDS, L. H. (1972b) Alteration of
the carcinogenic activity of DMBA by light. Proc. Am. Assoc.
Cancer Res., 13: 14.
DAVIS, A., DEANE, G. H., & DIFFEY, B. L. (1976) Possible dosimeter for
ultraviolet radiation. Nature (Lond.), 261: 169-170.
DJORDJEVIC, B. & TOLMACH, L. J. (1967) Responses of synchronous
populations of HeLa cells to ultraviolet irradiation at selected
stages of the generation cycle. Radiat. Res., 32: 327-346.
DOLEZOVA, V. (1976) To the question of the geographic distribution of
pterygium. Rev. int. Trach., 53: 55-64.
DULBECCO, R. (i949) Reactivation of ultraviolet inactivated
bacteriophage by visible light. Nature (Lond.), 163: 949-950.
DUKE-ELDER, W. S. (1926) The pathological action of light upon the
eye. I. Action of the outer eye: Photophthalmia. Lancet,
1: 1137-1141.
EASTCOTT, D. F. (1962) Epidemiology of skin cancer in New Zealand. In:
Urbach, F., ed. The biology of cutaneous cancer, Washington,
DC, US Government Printing Office, pp. 141-152 (Monograph No. 10,
National Cancer Institute).
EDENBERG, H. J. & HANAWALT, P. C. (1973) The timecourse of DNA repair
replication in ultraviolet irradiated HeLa cells. Biochim.
Biophys. Acta, 324: 206-217.
EISENSTARK, A. (1971) Mutagenic and lethal effects of visible and
near-ultraviolet light on bacterial cells. Advances in Genetics,
16: 167-198.
ELKIND, M. M. & WHITMORE, G. F. (1967) The radiobiology of cultured
mammalian cells, New York, Gordon & Breach.
ELWOOD, J. M., LEE, J. A. H., WALTER, S. D., MO, T., & GREEN, A. E. S.
(1974) Relationship of melanoma and other skin cancer mortality
to latitude and ultraviolet radiation in the United States and
Canada. Int. J. Epidemiol. 3: 325-332.
ELWOOD, J. M. & LEE, J. A. H. (1975) Recent data on the epidemiology
of malignant melanoma. Semin. Oncol., 2 (2): 149-154.
EMMETT, E. A. (1973) Ultraviolet radiation as a cause of skin tumors.
CRC Crit. Rev. Toxicol., 2: 211.
EMMETT, E. A. (1977a) Phototoxic keratoconjunctivitis from coal tar
pitch volatiles. Science, 198: 841-842.
EMMETT, E. A. (1977b) Phototoxicity occurring during the manufacture
of ultraviolet cured ink. Arch. Dermatol., 113: 770-775.
EPSTEIN, J. H. (1965) Comparison of the carcinogenic and
co-carcinogenic effects of ultraviolet light on hairless mice.
J. Natl Cancer Inst., 34: 741-745.
EPSTEIN, J. H. & EPSTEIN, W. L. (1963) A study of tumor types produced
by ultraviolet light in hairless and hairy mice. J. invest.
Dermatol., 41: 463-473.
EPSTEIN, J. H., EPSTEIN, W. L., & NAKAI, T. (1967) Production of
melanomas from DMBA-induced «Blue Nevi» in hairless mice with
ultraviolet light. J. Natl Cancer Inst., 38: 18-22.
EPSTEIN, H. J., FUKUYAMA, K., & DOBSON, R. (1969) Ultraviolet light
carcinogenesis. In: Urbach, F., ed. The biological effects of
ultraviolet radiation, Oxford, Pergamon Press.
EVERETT, M. A., YEARGERS, E., SAYRE, R. M., & OLSON, R. L. (1966)
Penetration of epidermis by ultraviolet rays. Photochem.
Photobiol., 5: 533-542.
FABRE, F. (1971) A UV-supersensitive mutant in the yeast
Schizosaccharomyces pombe. Molec. Gen. Genet., 110: 134-143.
FEARS, T. R., SCOTTO, J., & SCHNEIDERMAN, M. A. (1977) Mathematical
models of age and ultraviolet effects on the incidence of skin
cancer among whites in the United States. Am. J. Epidemiol.,
105: 420-427.
FINDLAY, G. M. (1930) Cutaneous papillomata in the rat following
exposure to ultraviolet light. Lancet, pp. 1229-1231.
FISHER, M. S. & KRIPKE, M. L. (1977) Systemic alteration induced in
mice by ultraviolet light irradiation and its relationship to
ultraviolet carcinogenesis. Proc. Natl Acad. Sci., 74
(4): 1688-1692.
FITZPATRICK, T. B., PATHAK, M. A., HARBER, L. C., SEIJI, M., & KUKITA,
A. (1974) Sunlight and Man, Tokyo, University of Tokyo Press.
FORBES, P. D. (1978) Experimental UV photocarcinogenesis. In:
International Conference on Ultraviolet Carcinogenesis.
Washington, DC, US Government Printing Office pp. 31-38
(Monograph No. 50, National Cancer Institute).
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. I. Fluorescent whitening agents and
short-wave UVR. Food Cosmet. Toxicol., 13: 335-337.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. II. Fluorescent whitening agents and
simulated solar UVR. Food Cosmet. Toxicol., 13: 339-342.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photo-carcinogenesis. III. Simulation of exposure to sunlight and
fluorescent whitening Agents. Food Cosmet. Toxicol.,
13: 343-345.
FORBES, P. D., DAVIES, R. E., & URBACH, F. (1976) Phototoxicity and
photocarcinogenesis: Comparative effects of anthracene and
8-methoxypsoralen in the skin of mice. Food Cosmet. Toxicol.,
14: 243.
FORNACE, A. J., KOHN, K. W., & KANN, H. E. (1976) DNA single-strand
breaks during repair of UV damage in human fibroblasts and
abnormalities of repair in xeroderma pigmentosum. Proc. Natl
Acad. Sci., 73: 39-43.
FORTNER, J. G., MAKY, A. G., & SCHRODT, G. R. (1961) Transplantable
tumors of the Syrian (golden) hamster. I. Tumors of the
alimentary tract, endocrine glands and melanomas. Cancer Res.,
6 (Part 2): 161-198.
FOX, M. (1974) The effect of post-treatment with caffeine on survival
and UV-induced mutation frequencies in Chinese hamster and mouse
lymphoma cells in vitro. Mutat. Res., 24: 187-204.
FREEMAN, R. G. (1975) Data on the action spectrum for ultraviolet
carcinogenesis. J. Natl Cancer Inst., 55: 1119-1121.
FREEMAN, R. G. & KNOX, J. M. (1964a) Ultraviolet-induced corneal
tumors in different species and strains of animals. J. invest.
Dermatol., 43: 431-436.
FREEMAN, R. G. & KNOX, J. M. (1964b) Influence of temperature on
ultraviolet injury. Arch. Dermatol., 89: 858-864.
GABOVIC, R. E., MINH, A. A., & MOTUZKOV, I. N. (1975) [The effect of
UV radiation on the body's level of tolerance to exposure to
chemicals.] Vestn. Akad. Med. Nauk., 3: 26-37 (in Russian).
GALANIN, N. F. & PIRKIN, V. M. (1971) [The rational utilization of the
sun's radiant energy with a view to preventing ultraviolet
deficiency.] In: [Ultraviolet Radiation.] Moscow, Medicina, pp.
209-212 (in Russian).
GARADZA, M.P. (1965) [The natural UV radiation regime as shown by the
results of measurements at the Moscow State University
Meteorological Observatory.] In: [The climate of a big city.]
Moscow, MGU Publishing House, pp. 186-195 (in Russian).
GARADZA, M. P. (1967) [Direct UV-radiation at different times of
year.] In: [The radiation regime and precipitations in Moscow.]
Moscow, MGU Publishing House, pp. 165-175 (in Russian).
GARADZA, M. P. (1974) [Features of the inflow of UV-radiation under
different cloud condition.] In: [Radiation processes in the
atmosphere and on the earth's surface.] Leningrad,
Cidrometeoizdat, pp. 261-264 (in Russian).
GARADZA, M. P. & NEZVAL', E. I. (1971) [The effect of atmospheric
transparency and cloud on the UV-radiation regime.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 316-321 (in
Russian).
GAVRILOVA, L. I., DOJNIKOV, A. S., & KORCAGINA, T. N. (1975) [UV
radiation from arc and intermittent xenon lamps.] In:
[The biological effect of UV radiation] Moscow, Nauka, pp.
226-229 (in Russian).
GENERALOV, A. A. (1971) [The ultraviolet irradiation regime for
children and adults taking sun-baths and light and air baths at
various latitudes.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 203-207 (in Russian).
GLUSCENKO, A. G., ARTJUSENCO, I. S., & BARANOVA, M. A. (1975) [Health
indicators for children of crèche age in the ultraviolet climatic
conditions of Kiev.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 171-174 (in Russian).
GORDON, D. & SILVERSTONE, H. (1976) Worldwide epidemiology of
premalignant and malignant cutaneous lesions. In: Cancer of the
skin, Philadelphia, W. B. Saunders Co., pp. 405-434.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943a) Types of
tumors induced by ultraviolet radiation and factors influencing
their relative incidence. J. Natl Cancer Inst., 3: 371-378.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943b) Pathology of
tumors of the external ear in mice induced by ultraviolet
radiation. J. Natl Cancer Inst., 2: 269-275.
GRAHAM, J. H. & HELWIG, E. B. (1965) In: Montagna, W. & Dobson, R. L.,
ed. Advances in biology of the skin, New York, Macmillan
(Pergamon), Vol. II, pp. 277-327.
GREEN, A. E. S., ed. (1960) The middle ultraviolet: Its science and
technology, New York, John Wiley and Sons, Inc.
GREEN, A. E. S. & HEIDINGER, R. A. (1978) Models relating ultraviolet
light and non-melanoma skin cancer incidence. Photochem.
Photobiol., 28: 283-291.
GREEN, A. E. S., SAWADA, T., & SHETTLE, E. P. (1975) The middle
ultraviolet reaching ground. In: Impacts of climatic change on
the biosphere. Part 1, Chapter 2, pp. 29-49 (CIAP Monograph
No. 5).
GREEN, A. E. S., FINDLEY, G. B., Jr, KLENK, K. F., WILSON, W. M., &
MO, T. (1976) The ultraviolet dose dependence of non-melanoma
skin cancer incidence. Photochem. Photobiol., 24: 353-362.
GRIFFIN, A. C. (1959) Methoxsalen in ultraviolet carcinogenesis in the
mouse. J. Invest. Dermatol, 32: 367-372.
GRIFFIN, A. C., DOLMAN, V. S., ROHLKE, E. B., ROUVART, P., & TATUM, E.
L. (1955) The effect of visible light on the carcinogenicity of
ultraviolet light. Cancer Res., 15: 523.
GRIGGS, H. C. & BENDER, M. A. (1973) Photoreactivation of ultraviolet
induced chromosomal aberrations. Science, 179: 86-88.
GROSSMAN, L. (1968) Studies on mutagenesis induced in vitro.
Photochem. Photobiol, 7: 727-735.
GUSEV, N. M. & DUNAEV, B. A. (1971) [The utilization of ultraviolet
radiation in building.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 218-221 (in Russian).
HAMILTON, L. (1973) The influence of the cell cycle on the radiation
response of early embryos. In: Balls, M. & Billet, F. F., ed.
The cell cycle in development and differentiation, Cambridge,
Cambridge University Press, pp. 229-247.
HAN, A. & SINCLAIR, W. K. (1969) Sensitivity of synchronized Chinese
hamster cells to ultraviolet light. Biophys. J., 9: 1171-1192.
HAN, A., SINCLAIR, W. K., & YU, C. K. (1971) Ultraviolet-light-induced
division delay in synchronized Chinese hamster cells.
Biophys. J., 11: 540-549.
HANAWALT, P. C. & SETLOW, R. B., ed. (1975) Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 827.
HARBER, L. C. & BAER, R. L. (1969) Classification and characteristics
of photoallergy. In: Urbach, F., ed. The biologic effects of
ultraviolet radiation, Oxford, Pergamon Press, pp. 519-526.
HARRISON, A. P. (1967) Survival of bacteria. Harmful effects of light,
with some comparisons with other adverse physical agents. Annual
Rev. Microbiol., 21: 143-156.
HAUSSER, K. W. & VAHLE, W. (1922) [The dependence of light induced
erythema and pigment formation upon the frequency (or wavelength)
of the inducing radiation.] Strahlentherapia, 13: 41-71 (in
German).
HAUSSER, K. W. & VAHLE, W. (1927) [Sunburn and suntan.] Wiss
Veröffent. Siemens-Konzern, 6: 101 (in German).
HAYNES, R. H. (1975) DNA repair and the genetic control of
radio-sensitivity in yeast. In: Hanawalt, P. C. & Setlow, R. B.,
ed. Molecular mechanisms for repair of DNA, New York & London,
Plenum Press, pp. 529-540.
HÉLENE, C. & CHARLIER, M. (1971) Photosensitized reactions in nucleic
acids. Photosensitized formation and splitting of pyrimidine
dimers. Biochimie, 53: (11-12): 1175-1180.
HELLMAN, K. B., HAYNES, K. F., & BOCKSTAHLER, L. E. (1974)
Radiation-enhanced survival of a human virus in normal and
malignant rat cells (37788). Proc. Soc. Exp. Biol. Med.,
145: 255-262.
HERLITZ, C. W., JUNDELL, I., & WAHLGREN, F. (1930) [Malignant tumours
in white mice caused by ultraviolet irradiation.] Acta Paediat.,
10: 333-347 (in German).
HEROLD, H. J. & BERNDT, H. (1968) Cancer incidence in the German
Democratic Republic. Selected tables. Neoplasma, 15: 517-522.
HILL, R. H. (1958) A radiation-sensitive mutant of E. coli. Biochim.
Biophys. Acta, 30: 636-637.
HILL, L. & EIDENOW, A. (1923) Biological action of light: I. The
influence of temperature. Proc. R. Soc. (Biol.), 95: 163-180.
HOLMBERG, M. & JONASSON, J. (1974) Synergistic effect of X-ray and UV
irradiation on the frequency of chromosome breakage in human
lymphocytes. Mutat. Res., 23: 213-221.
HSIAO, D. (1975) Impacts of climatic change on the biosphere.
Springfield, Virginia. National Technical Information Service
(CIAP Monograph No. 5).
HUANG, C. W. & GORDON, M.P. (1973) Formation of cyclobutane-type
pyrimidine dimers in RNA by sunlight. Int. J. radiat. Biol.,
23: 527-529.
HUEPER, W. C. (1941) Cutaneous neoplastic responses elicited by
ultraviolet rays in hairless rats and in their haired litter
mates. Cancer Res., 1: 402-406.
HUTCHINSON, F. (1973) The lesions produced by ultraviolet light in DNA
containing 5-bromouracil. Quart. Rev. Biophys. 6: 201-246.
IKUSHIMA, T. & WOLFF, S. (1974) UV-induced chromatid aberrations in
cultured Chinese Hamster cells after one, two or three rounds of
DNA replication. Mutat. Res., 22: 193-201.
IPPEN, H. (1969) Mechanisms of photopathological reactions. In:
Urbach, F., ed. The biologic effects of ultraviolet radiation,
Oxford, Pergamon Press, pp. 513-518.
JACOBSON, A. F. & YATVIN, M. B. (1976) Changes in the phospholipid
composition of E. coli following X and UV-irradiation. Radiat.
Res., 66: 247-266.
JAGGER, J. (1967) Introduction to research in ultraviolet
photobiology, Englewood Cliffs, N J, Prentice-Hall, Inc.,
pp. 164.
JAGGER, J. (1969) Photoreactivation. In: Hollaender, A., ed.
Radiation protection and recovery, New York, Pergamon Press,
pp. 352-377.
JAGGER, J. (1976) Effects of near-ultraviolet radiation on
microorganisms. Photochem. Photobiol., 23: 451-454.
JESSUP, J. M., HANNA, N., PALASZYNSKI, E., & KRIPKE, M. L. (1978)
Mechanisms of depressed reactivity to dinitrochlorobenzene and
ultraviolet-induced tumours during ultraviolet carcinogenesis in
BALB/c mice. Cell. Immunol., 38: 105-115.
JOHNSON, B. E. (1968) Ultraviolet radiation and lysosomes in skin.
Nature (Lond.), 219: 1258-1259.
JOHNSON, B. E., DANIELS, F., & MAGNUS, I. A. (1968) Response of human
skin to ultraviolet light. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV.
JUNG, E. G. (1976) [Photochemotherapy, background, techniques and
complications.] Stuttgart -- New York, F. K. Schattaver Verlag
(in German).
JUNG, E. G., BOHNERT, E., & ERBS, G. (1971) Wavelength dependence of
UV-induced alterations of epidermal cells in hairless albino
mice. Arch. Derm. Forsch., 241: 284-291.
KAMENECKAJA, T. M. & MITROFANOVA, G. F. (1975) [The condition of
certain neurohumoral regulatory systems following UV radiation at
different wavelengths.] In: [The biological effects of UV
radiation.] Moscow, Nauka, (in Russian).
KAO, F. & PUCK, T. T. (1969) Genetics of somatic mammalian cells. IX.
Quantitation of mutagenesis by physical and chemical agents.
J. Cell. Physiol., 74: 245-248.
KARACEVCEVA, T. V. (1971) [The role on UV radiation as part of modern
methods of treating and preventing rheumatism in children.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 154-158 (in
Russian).
KELNER, A. (1949) Photoreactivation of ultraviolet-irradiated
Escherichia coli with special reference to close-reduction
principle and to ultraviolet-induced mutation. J. Bacteriol.,
58: 511-522.
KELNER, A. (1953) Growth, respiration, and nucleic acid synthesis in
ultraviolet-irradiated and in photoreactivated E. coli.
J. Bacteriol., 65: 252-262.
KELNER, A. & TAFT, E. B. (1956) The influence of photoreactivating
light on the type and frequence of tumors induced by ultraviolet
radiation. Cancer Res., 16: 860-866.
KIEFER, J. (1971) The importance of cellular energy metabolism for the
sparing effect of dose fractionation witch electrons and
ultraviolet light. Int. J. radiat. Biol., 20: 325-336.
KIRBY-SMITH, J. S., BLUM, H. F., & GRADY, H. G. (1942) Penetration of
ultraviolet radiation into skin, as a factor in carcinogenesis.
J. Natl Cancer Inst., 2: 403-412.
KOCH, H. R. (1967) [Photochemotherapy and cataract formation.]
Dermatosen im Beruf und Umwelt., 26; 162-165 (in German).
KOLLER, L. R. (1965) Ultraviolet radiation, 2nd ed., New York, John
Wiley and Sons.
KRAEMER, K. H., DE WEERD-KASTELEIN, E. A., ROBBINS, J. H., KEIJZER,
W., BARRETT, S. F., PETINGA, R. A., & BOOTSMA, D. (1975) Five
complementation groups in xeroderma pigmentosum. Mutat. Res.,
33: 327-340.
KRIPKE, M. L. (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J. Natl Cancer Inst., 53: 1333-1336.
KRIPKE, M. L. (1976) Target organ for a systemic effect of ultraviolet
radiation. Photochem. Photobiol., 24: 599-600.
KRIPKE, M. L. (1977) Latency, histology, and antigenicity of tumors
induced by ultraviolet light in three inbred mouse strains.
Cancer Res., 37: 1395-1400.
KRIPKE, M. L. & FISHER, M. S. (1976) Effect of UV light on the host
response to UV-induced tumirs. Proceedings of the 4th Annual
Meeting, American Society of Photobiology, Denver, CO, February
16-20.
KRIPKE, M. L. & FISHER, M. S. (1976) Immunologic parameters of
ultraviolet carcinogenesis. J. Natl Cancer Inst., 57: 211-215.
KRIPKE, M. L., LOFGREEN, J. S., BEARD, J., JESSUP, J. M., & FISHER, M.
S. (1977) In vivo immune responses of mice during
carcinogenesis by ultraviolet irradiation. J. Natl Cancer Inst.,
59: 1227-1230.
LABORDUS, V. (1970) The effect of ultraviolet light on developing eggs
of Lymnaea stagnails (mollusca, pulmonata) I, II, III. Proc.
K. Ned. Akad. Wet. Amsterdam, C, 366-493.
LAMOLA, A. A. & YAMANE, T. (1967) Sensitized photodimerization of
thymine in DNA. Proc. Natl Acad. Sci., 58: 443-446.
LANCASTER, H. O. (1956) Some geographical aspects of the mortality
from melanoma in Europeans. Med. J. Aust., 1: 1082-1087.
LATARJET, R. (1972) Interaction of radiation energy with nucleic
acids. Curr. Top. Radiat. Res. Q., 8: 1-38.
LATARJET, R. & ZAJDELA, F. (1974) Effet inhibitcur de la caféine sur
l'induction de cancers cutanés par les rayons ultraviolet chez la
souris. C. R. Acad. Sci. (Paris), 277: 1073-1076.
LAZAREV, D. N. & SOKOLOV, M. V. (1971) [The measurement of ultraviolet
radiation in units of effect.] In: [UV Radiation.] Moscow,
Medicina, pp. 328-333 (in Russian).
LAZAREV, D. N. & SOKOLOV, M. V. (1974) [Ultraviolet radiation.
Quantities and units. Terms and definitions. Technical
material for guidance.] Puscino, Institute of Biophysics of
the Academy of Sciences of the USSR, pp. 1-8 (in Russian).
LAZAREV, D. M., BESSALOVA, E. S., BALINA, G. P., & ERMILOVA, M. G.
(1975) [A UV biological photometer.] In: [The biological effects
of UV radiation.] Moscow, Nauka, pp. 244-246 (in Russian).
LEE, J. A. H. & MERRILL, J. M. (1970) Sunlight and the aetiology of
malignant melanoma: A Synthesis. Med. J. Aust., 2: 840-841.
LEE, H. H. & PUCK, T. T. (1960) The action of ultraviolet radiation on
mammalian cells as studied by single-cell technique. Radiat.
Res., 12: 340-348.
LEHMANN, A. R. (1972) Postreplication repair of DNA in
ultraviolet-irradiated mammalian cells. Mol. Biol.,
66: 319-337.
LEHMANN, A. R., KIRK-BELL, S., ARLETT, C. F., PATERSON, M. C., LOHMAN,
P. H. M., WEERD-KASTELEIN de, E. A., & BOOTSMA, D. (1975)
Xeroderma pigmentosum cells with normal levels of excision repair
have a defect in DNA synthesis after UV irradiation. Proc. Natl
Acad. Sci., 72: 219-223.
LEWIS, N. F. & KUMTA, U.S. (1972) Evidence for extreme UV resistance
of Micrococcus sp. NCTC 10785. Biochem. biophys. Res. Commun.,
47: 1100-1105.
LIPSON, R. L. & BALDES, E. J. (1960) Photosensitivity and heat. Arch.
Dermatol., 82: 517-520.
LOOMIS, W. F. (1970) Rickets. Sci. Am., 233: 76-91.
LUKIESH, M., HOLLADAY, L. L., & TAYLOR, A. H. (1939) Erythemal and
tanning effectiveness of UV energy. Gen. Electr. Rev.,
42: 274-278.
LYTLE, C. D. (1971) Host-cell reactivation in mammalian cells. I.
Survival or ultraviolet-irradiated herpes virus in different
cell-lines. Int. J. radiat. Biol., 19: 329-337.
LYTLE, C. D., HELLMAN, K. B., & TELLES, N. C. (1970) Enhancement of
viral transformation by ultraviolet light. Int. J. radiat.
Biol., 18: 397-300.
MACDONALD, E. J. (1976) Epidemiology of skin cancer 1975. In:
Neoplasms of the skin and malignant melanoma, Chicago, Year
Book Publishers, Inc., pp. 27-42.
MACKIE, B. S. & MCGOVERN, V. J. (1958) The mechanism of solar
carcinogenesis: A study of the role of collagen degeneration of
the dermis in the production of skin cancer. Arch. Dermatol.
Syph., 78: 218-244.
MAGNUS, I. A. & JOHNSON, B. E. (1965) Cited by Johnson, B. E.,
Daniels, F. Jr, & Magnus, I. A. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV,
pp. 139-202.
MAGNUS, K. (1973) Incidence of malignant melanoma of the skin in
Norway, 1955-1970. Cancer, 32: 1275-1286.
MAGNUS, K. (1977) Incidence of malignant melanoma of the skin in the
five nordic countries: significance of solar radiation. Int. J.
Cancer, 20: 477-485.
MALIK, M. O. A., MIDAYTALLA, A., DAOUC, E. H., & EL HASSAN, A. M.
(1974) Superficial cancer in the Sudan. A study of 1225 primary
malignant superficial tumours, Br. J. Cancer, 30: 355-364.
MATHEWS, M. M. & KRINSKY, N. (1965) The relationship between
carotenoid pigments and resistance to radiation in
non-photosynthetic bacteria. Photochem. Photobiol., 4: 813-817.
MCGOVERN, V. J. (1977) Epidemiological aspects of melanoma: A review.
Pathology, 9: 233-241.
MCGOVERN, V. J. & MACKIE, B. S. (1959) The relationship of solar
radiation to melanoblastoma. Aust. N. Z. J. Surg., 28: 257-262.
MEYER, A. E. H. & SEITZ, E. O. (1949) [Ultraviolet Radiation.]
Berlin, Walter de Gruyter & Co. (in German).
MILLS, L. F., LYTLE, C. D., ANDERSEN, F. A., HELLMAN, K. B., &
BOCKSTAHLER, L. H. (1975) A review of biological effects and
potential risks associated with ultraviolet radiation as used in
dentistry. pp. 1-27 (DHEW Publication (FDA) 76-8021).
MISHIMA, Y. & WIDLAN, S. (1967) Enzymatically active and inactive
melanocyte populations and ultraviolet irradiation: Combined
dopapremelanin reaction and electron microscopy. J. invest.
Dermatol., 49: 273.
MIYAJI, T. (1962) Skin cancer in Japan. A nationwide 5 year survey.
In: Urbach, F., ed. The biology of cutaneous cancer,
Washington, DC, US Government Printing Office, pp. 515-70
(Monograph No. 10, National Cancer Institute).
MIYAZAKI, H., KAWADA, A., TAKAKI, Y., SATO, T., & MASUTANI, M. (1968)
Influence of ultraviolet light on pigmentation of hairless mouse
epidermis. Jap. J. Dermatol., Ser. B, 78: 605.
MORENO, F. (1969) Contribution à l'étude de la létalité et de la
stérilité induites chez Drosophita melanogaster par irradiation
UV des cellules polaires de l'oeuf. I. Action létale, II. Action
stérilisante. Int. J. radiat. Biol., 16: 441-465.
MOUSTACCHI, E., WATERS, R., HEUDE, M., & CHANET, R. (1975 The present
status of DNA repair mechanisms in UV irradiated yeast taken as a
model eukaryotic system. In: Nygaard, O., Adler, H., & Sinclair,
W., ed. Radiation research: Biomedical, chemical and physical
perspectives, New York, Academic Press, pp. 632-650.
MOVSHOVITZ, M. & MODAN, B. (1973) Role of sun exposure in the etiology
of malignant melanoma: Epidemiologic inference. J. Natl Cancer
Inst., 51: 777-779.
MULAY, D. M. (1962) Skin Cancer in India. In: Urbach, F., ed.
The biology of cutaneous Cancer, Washington, DC, US Government
Printing Office, pp. 215-224 (Monograph No. 10, National Cancer
Institute).
MURPHY, T. M. (1975a) Inactivation of tobacco mosaic virus ribonucleic
acid by near- and middle-ultraviolet light: Sensitization by
sulfanilamide and chlortetracycline. Biochem. biophys. Res.
Commun., 65: 1108-1114.
MURPHY, T. M. (1975b) Effects of UV-B radiation on nucleic acids. In:
Impacts of climatic changes on the biosphere, pp. 19-40 (CIAP
Monograph No. 5).
MUSAJO, L., RODIGHIERO, G., CAPORALE, G., DALL'ACQUA, F., MARCIANI,
S., BORDIN, F., BACCICHETTI, F., & BEVILACQUA, R. (1974)
Photoreactions between skin-photosensitizing furocoumarins and
nucleic acids. In: Fitzpatrick, T. B., Pathak, M. A., Harber, L.
C., Seiji, M., & Kukita, A., ed. Sunlight and Man, Tokyo,
University of Tokyo Press, pp. 369-387.
NAKAMURA, K. & JOHNSON, W. C. (1968) Ultraviolet light-induced
connective tissues changes in rat skin: A histopathologic and
histochemical study. J. invest. Dermatol., 51: 253-258.
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1973) UV
photocarcinogenesis: Modification by anti-lymphocytic serum or
6-marcaptopurine. Proc. Am. Assoc. Cancer Res., 14: 46
(Abstract 182).
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1976) Modification of
photocarcinogenesis by two immunosuppressive agents. Cancer
Lett., 1: 243-247.
NILENDER, R. A. & GAVANIN, V. A. (1971) [Artificial sources of
ultraviolet radiation.] In: [UV Radiation.] Moscow, Medicina,
pp. 348-352 (in Russian).
NIOSH (1975) Ultraviolet transfer standard detectors and evaluation
and calibration of NIOSH UV hazard monitor. Cincinnati, OH, US
Department of Health, Education, and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and
Health, Division of Laboratories and Criteria Development, 12 pp. (HEW
Publication No. (NIOSH) 75-131).
NORBURY, K. C., KRIPKE, M. L., & BUDMEN, M. B. (1977) In vitro
reactivity of macrophages and lymphocytes from
ultraviolet-irradiated mice. J. Natl Cancer Inst.,
59: 1231-1235.
OETTLE, A. G. (1962) Skin cancer in Africa. In: Urbach, F, ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 197-214 (Monograph No. 10, National Cancer
Institute).
ORR, J. W. (1938) The changes antecedent to tumor formation during the
treatment of mouse skin with carcinogenic hydrocarbons.
J. Pathol. Bacteriol., 46: 495-515.
OWENS, D. W., KNOX, J. M., HUDSON, H. T., RUDOLPH, A. H., & TROLL, D.
(1977) Influence of wind on chronic ultraviolet light-induced
carcinogenesis. Br. J. Dermatol., 97: 285.
PAINTER, R. B. (1975) Repair in mammalian cells: Overview. In:
Hanawalt, P. C. & Setlow, R. B., ed. Molecular mechamisms for
repair of DNA, New York & London, Plenum Press, pp. 595-600.
PARRINGTON, J. M. (1972) Ultraviolet-induced chromosome aberration and
mitotic delay in human fibroblast cells. Cytogenetics,
11: 117-131.
PARRISH, J. A., FITZPATRICK, T. B., TANNENBAUM, L., & PATHAK, M. A.
(1974) Photochemotherapy of psoriasis with oral methoxsalen and
long wave ultraviolet light. New Engl. J. Med. 291: 1207-1212.
PATHAK, M. A., KRAMER, D. M., & FITZPATRICK, T. B. (1974) Photobiology
and photochemistry of furocoumarins (psoralens). In: Fitzpatrick,
T. B., Pathak, M. A., Harber, L. C., Seiji, M., & Kukita, A., ed.
Sunlight and Man, Tokyo, University of Tokyo Press,
pp. 335-368.
PETTIJOHN, D. & HANAWALT, P. (1964) Evidence for repair-replication of
ultraviolet damaged DNA in bacteria. J. mol. Biol., 9: 395-410.
PITTS, D. G. (1970) A comparative study of the effects of ultraviolet
radiation on the eye. Am. J. Optom., 47: 535-546.
PITTS, D. G. & CULLEN, A. P. (1977) Ocular ultraviolet effects from
300 nm to 400 nm: A preliminary report. Cincinatti, OH, US
Department of Health, Education and Welfare, National Institute
of Occupational Safety and Health, Division of Biomedical and
Behavioral Science (NIOSH Contract CDC-99-74-12).
PITTS, D. G. & KAY, K. R. (1969) The photo-ophthalmic threshold for
the rabbit. Am. J. Optom., 46: 561-572.
POLLARD, E. C. (1974) Cellular and molecular effects of solar
ultraviolet radiation. Photochem. Photobiol., 20: 301-308.
PROKOPENKO, Ju, I. (1976) [Some data on the mechanisms of protective
effect of UV-radiation.] Gig. i Sanit., 1: 100-102 (in
Russian).
PROKOPENKO, Ju, I. & ZABALUEVA, A. P. (1975) [Activation of
sanogenesis mechanisms with the help of UV-irradiation.] In:
[The biological effect of UV-radiation,] Moscow, Nauka,
pp. 156-159 (in Russian).
PUTSCHAR, W. & HOLTZ, F. (1930) [Induction of skin cancer in rats by
prolonged ultraviolet irradiation.] Z. Krebsforsch.,
23: 219-232 (in German).
QUEVEDO, W. C. Jr & SMITH, J. A. (1963) Studies on radiation-induced
tanning of skin. Ann. N. Y. Acad. Sci., 100: 364.
QUEVEDO, W. C. Jr, BRENNER, R. M., & KECHIJIAN, P. (1963) Melanocyte
performance during radiation induced tanning of murine skin.
Proceedings of the XVI International Congress on Zoology, 2: 306.
QUEVEDO, W. C. Jr, SZABO, G., VIRKS, J., & SINESI, S. J. (1965)
Melanocyte populations in UV-irradiated human skin. J. invest.
Dermatol., 45: 295.
RADMAN, M. (1975) SOS repair hypothesis: Phenomenology of an inducible
DNA repair which is accompanied by mutagenesis. In: Hanawalt, P.
C. & Setlow, R. B., ed. Molecular mechanisms for repair of DNA,
New York & London, Plenum Press, pp. 355-367.
RAPPAPORT, H., PIETRA, G., & SHUBIK, P. (1961) The induction of
melanotic tumors resembling cellular blue nevi in the Syrian
white hamster by cutaneous application of 7,
12-Dimethylbenz(a)anthracene. Cancer Res., 21: 661-666.
RASMUSSEN, R. E. & PAINTER, R. B. (1964) Evidence for repair of
ultraviolet damaged deoxyribonucleic acid in cultured mammal
cells. Nature (Lond.), 203: 1360-1362.
RAUTH, A. M. (1970) Effects of ultraviolet light on mammalian cells in
culture. Curr. Top. radiat. Res., 6: 195-248.
RAUTH, A. M. & DOMON, M. (1973) Potentiation of ultraviolet light
damage in mouse L cells by 1-cyclohexyl-3-(2-morpholinyl-4-et
carbodiimide metho-p-toluene sulphonate (CMEC). Int. J. radiat.
Biol., 24: 189-198.
RESNICK, M. A. (1969) Genetic control of radiation sensitivity in
Saccharomyces cerevisiae. Genetics, 62: 519-531.
REYNOLDS, J. (1954) The epidermal melanocytes of mice. J. Anat.,
88: 45.
ROBERTSON, D. F. (1969) Long term field measurements of erythemally
effective natural ultraviolet radiation. In: Urbach, F., ed.
The biologic effects of ultraviolet radiation, Pergamon
Press, Oxford, pp. 433-436.
ROBERTSON, D. F. (1972) Solar ultraviolet radiation in relation to
human sunburn and skin cancer. Thesis, Australia, University of
Queensland.
ROBERTSON, D. F. (1975) Calculated sunburn responses, In: Impacts of
climatic change on the biosphere. Springfield, Virginia,
National Technical Information Service, Part 1, Chapter 2,
Appendix J. (CIAP Monograph 5).
ROFFO, A. H. (1934) Cancer et soleil. Carcinomes et sarcomes provoqués
par l'action du soleil in toto. Bull. Assoc. Fr. Etud. Cancer,
53: 59.
ROMMELAERE, J., SUSSKIND, M., & ERRERA, M. (1973) Chromosome and
chromatid exchanges in Chinese hamster cells. Chromosoma,
41: 243-257.
RUNDEL, R. D. & NACHTWEY, D. S. (1978) Skin cancer and ultraviolet
radiation. Photochem. Photobiol., 28: 345-356.
RONGE, H. E. (1948) Ultraviolet irradiation with artificial
illumination Acta Phys. Scand., 15 (Suppl. 49): 1-191.
RUPERT, C. S. (1975) Enzymatic photoreactivation: Overview. In:
Hanawalt, P. C. & SETLOW, R. B. ed. Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 73-87.
RUPP, W. D. & HOWARD-FLANDERS, P, (1968) Discontinuities in the DNA
synthesized in an excision-defective strain of E. coli
following ultraviolet irradiation. J. Mol. Biol., 31: 291-304.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1941) Carcinogenesis by
ultraviolet rays with reference to wavelength and energy. Arch.
Pathol., 371: 135-148.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1942) Nonadditive effect
of UV light and other carcinogenic procedures. Cancer Res.,
2: 183-188.
RUSTAD, R. C. (1972) Techniques for the analysis of radiation-induced
mitotic delay in synchronously dividing sea urchin eggs. In:
Whitson, G. L., ed. Concepts in radiation cell biology, New
York & London, Academic Press, pp. 153-181.
SAMS, W. M. Jr, SMITH, J. G., & BURK, P. G. (1964) The experimental
production of elastosis with ultraviolet light. J. invest.
Dermatol., 43: 467.
SATO, T. (1971) Pigmentation induced by ultraviolet irradiation in
hairless mice with particular references to epidermal dendritic
cells. Jap. J. Dermatol. Ser. A, 81: 488.
SATO, T. & KAWADA, A. (1972) Mitotic activity of hairless mouse
epidermal melanocytes: Its role in the increase of melanocytes
during ultraviolet radiation. J. invest. Dermatol.,
58: 392-395.
SATO, T. & KAEADA, A. (1972) Uptake of tritiated thymidine by
epidermal melanocytes of hairless mice during ultraviolet light
radiation. J. invest. Dermatol., 58: 71.
SAUERBIER, W. (1976) UV damage at the transcriptional level. Advances
in radiation biology, 6: 50-106.
SAYENKO, A. J. (1968) A method for studying morbidity from
precancerous conditions and the question as to frequency of their
occurrence on the territory of the Kirghiz Soviet Socialist
Republic. Neoplasma, 15: 565-571.
SCHULZE, R. (1970) [Global Radiation Climate.] Wiss. Forschungsber.,
72: 220 pp. (in German).
SCOTTO, J., KOPF, A. W., & URBACH, F. (1974) Non-melanoma skin cancer
among Caucasians in four areas of the United States. Cancer,
34: 1333-1338.
SCOTTO, J., FEARS, T. R., & GORI, G. B. (1976) Measurements of
ultraviolet radiation in the United States and comparisons with
skin cancer data, National Cancer Institute (DHEW
No. (NIH) 76-1029).
SEIDL, E. (1969) Blitzgerät therapy. In: Urbach, F., ed. The biologic
effects of ultraviolet radiation, Oxford, Pergamon Press,
pp. 663-672.
SETLOW, R. B. (1968) The photochemistry, photobiology, and repair of
polynucleotides. Prog. N. A. Res. Mol. Biol., 8: 257-295.
SETLOW, R. B. (1973) The relevance of photobiological repair.
An. Acad. Bras. Ciencas, 45: 215-220.
SETLOW, R. B. (1974) The wavelengths in sunlight effective in
producing skin cancer: A theoretical analysis. Proc. Natl Acad.
Sci., 71: 3363-3366.
SETLOW, R. B. & CARRIER, W. L. (1964) The disappearance of thymine
dimers from DNA: An error-correcting mechanism. Proc. Natl Acad.
Sci., 51: 226-231. p. 34.
SETLOW, J. K. & DUGGAN, D. E. (1964) The resistance of Micrococcus
radiodurans to ultraviolet radiation. Biochim. Biophys. Acta,
87: 664-668.
SETLOW, J. K. & SETLOW, R. B. (1963) Nature of the photoreactivability
ultraviolet lesion in deoxyribonucleic acid. Nature (Lond.),
197: 560-562. p 36.
SHUBIK, P., PIETRA, G., & DELLA PORTA, G. (1960) Studies of skin
carcinogenesis in the Syrian golden hamster. Cancer Res.,
20: 1.
SILVERSTONE, H. & SEARLE, J. H. A. (1970) The epidemiology of skin
cancer in Queensland: The influence of phenotype and environment.
Br. J. Cancer, 24: 235-252.
SKREB, Y., HORVAT, D., & EGER, M. (1972) Modifications of
radiosensitivity in nucleate and anucleate amoeba fragments. In:
Bonotto, S., Goutier, R., Kirchmann, R., & Maisin, J.-R., ed.
Biology and radiobiology of anucleate systems I. New York,
Academic Press, pp. 67-82.
SMITH, K. C. (1974) The cellular repair of radiation damage. In:
Fitzpatrick, T. B., Pathak, M. A., Harber, L. C., Seiji M., &
Kukita, A., ed. Sunlight and Man, Tokyo, University of Tokyo
Press, pp. 67-77.
SMITH, K. C. (1975) The radiation-induced addition of proteins and
other molecules to nucleic acids. In: Wang, S. Y., ed.
Photochemistry and photobiology of nucleic acids, New York,
Academic Press, Vol. II, pp. 187-218.
SMITH, K. C. & HANAWALT, P. C. (1969) Molecular photobiology.
Inactivation and recovery, New York & London, Academic Press.
SOFFEN, G. A. & BLUM, H. F. (1961) Quantitative measurements of cell
changes in mouse skin following a single dose of ultraviolet
light. J. cell. comp. Physiol., 58: 81-96.
SOKOLOV, M. V. (1975) [Standardization of UV photometry.] In:
[The biological effects of ultraviolet radiation.] Moscow,
Nauka, pp. 232-235 (in Russian).
SOKOLOV, M. V. (1976) [Topical questions of UV biophotometry.]
Svetotecnika, 2: 20-22 (in Russian).
SOZIN, D. S. (1975) [Low pressure fluorescent lamps with combined
radiation.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 225-226 (in Russian).
SPIKES, J. D., KRIPKE, M. L., CONNOR, R. J., & EICHWALD, E. J. (1977)
Time of appearance and histology of tumors induced in the dorsal
skin of C3Hf mice by ultraviolet radiation from a mercury arc
lamp. J. Natl Cancer Inst. 59: 1637-1643.
STATE STANDARDS (1964) [Recommendations for the prevention of
ultraviolet deficiency.] Moscow Nauka, 50 pp. (in Russian).
STATE STANDARDS (1976a) [Regulations for the design and operation of
artificial UV-irradiation installations in industrial
undertakings.] In: [Regulations on the design of electrical
engineering installations for industry.] Energija 6: pp. 30-39
(in Russian).
STATE STANDARDS (1976b) [USSR building norms and regulations.]
Strijizdat, Moscow, p. 20 (SNIP 11-60-75) (in Russian).
STENBÄCK, F. (1969) Promotion in the morphogenesis of chemically
inducible skin tumors. Acta path. microbiol. Scand. Suppl.,
208: 1-116.
STENBÄCK, F. (1975a) Cellular injury and cell proliferation in skin
carcinogenesis by UV light. Oncology, : 31: 61-65.
STENBÄCK, F. (1975b) Species-specific neoplastic progression by
ultraviolet light on the skin of rats, guinea pigs, hamsters and
mice. Oncology, 31: 209-225.
STENBÄCK, F. (1975) Ultraviolet light irradiation as initiating agent
in skin tumor formation by the two-stage method. Europ. J.
Cancer, 11: 241-246.
STENBÄCK, F. (1978) Life history and histopathology of ultraviolet
light-induced skin tumors. In: International Conference on
Ultraviolet Carcinogenesis. Washington, DC, US Government
Printing Office, pp. 57-70 (Monograph No. 50, National Cancer
Institute).
SUTHERLAND, B. M. (1974) Photoreactivating enzyme from human
leukocytes. Nature (Lond.), 109-112.
SVIDERSKAJA, T. A. (1971) [Some experimental data on the conditions
accompanying an intensification of blastomogenic effect.] In:
[UV Radiation.] Moscow, Medicina, pp. 260-264 (in Russian).
SWANBECK, G. & HILLSTRöM, L. (1971) Analysis of etiological factors of
squamous cell skin cancer of different locations. Acta
Dermatovener, 51: 151-156.
TALANOVA, I. K. & ZABALUEVA, A. P. (1972) [Evaluation of the extent to
which children are provided with UV radiation in middle and
northern latitudes.] In: [Questions of experimental and clinical
spa treatment and physiotherapy.] 20: 323-329 (in Russian).
TALANOVA, I. K., KARACEVCEVA, T. V., & IVANOV, V. G. (1975) [The
combination of physical methods of prophylaxis and specific
immunization in children.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 116-120 (in Russian).
TODD, P. & HAN, A. (1976) Ultraviolet light induced
DNA-to-protein-cross-linking in mammalian cells. In: Smith, K.
C., ed. Aging, carcinogenesis and radiation-biology: The role of
nucleic acid addition reactions, New York & London, Plenum
Press, pp. 83-104.
TROSKO, J. E. & CHU, E. H. (1973) Inhibition of repair of UV-damaged
DNA by caffeine and mutation induction in Chinese hamster cells.
Chem. Biol. Interactions, 6: 317-332.
TYRRELL, R. M. (1973) Induction of pyrimidine dimers in bacterial DNA
by 365 nm radiation Photochem. Photobiol., 17: 69-73.
URBACH, F. (1969) The biologic effect of ultraviolet radiation.
Oxford, Pergamon Press, pp. 704.
URBACH, F., ROSE, D. B., & BONNEM, M. (1972) Genetic and environmental
interactions in skin carcinogenesis. In: Environment and skin
cancer, Baltimore, Maryland, Williams & Wilkins Co.,
pp. 355-371.
VARGHESE, A. J. (1973) Properties of photoaddition products of thymine
and cysteine. Biochemistry, 12 (14): 2725-2730.
VERHOEFF, F. H., BELL, L., & WALKER, C. B. (1916) The pathological
effects of radiant energy on the eye: An experimental
investigation with a systematic review of the (literature. Proc.
Am. Acad. Arts Sci., 51: 630-818.
VITALIANO, P. P. (1978) The use of logistic regression for modelling
risk factors in the development of nonmelanoma skin cancer.
Am. J. Epidemiol., 108: 402-414.
WANG, R. J. (1975) Lethal effect of »daylights» fluorescent light on
human cells in tissue-culture medium. Photochem. Photobiol.,
21: 373-375.
WANG, R. J., STOIEN, J. D., & LANDA, F. (1974) Lethal effect of
near-ultraviolet irradiation on mammalian cells in culture.
Nature (Lond.), 247: 43-45.
WATERHOUSE, J., MUIR, C., CORREA, P., & POWELL, J. (1976) Cancer
incidence in five continents, Lyons, IARC (Vol. III).
WHITSON, G. L. (1972) Radiation-induced biochemical changes in
protozoa. In: Whitson, G. L., ed. Concepts in radiation cell
biology, New York, Academic Press, pp. 123-152.
WINKELMAN, R. K., BALDES, E. J., & ZOLLMAN, P. E. (1960) Squamous cell
tumors induced in hairless mice with ultraviolet light.
J. invest. Dermatol., 34: 131-138.
WINKELMANN, R. K., ZOLLMAN, P. E., & BALDES, E. J. (1965) Squamous
cell carcinoma produced by ultraviolet light in hairless mice.
J. invest. Dermatol., 40: 217-224.
WITKIN, E. M. (1969) Ultraviolet-induced mutation and DNA repair.
Ann. Rev. Genet., 3: 525-552.
WITKIN, E. M. (1976) Ultraviolet mutagenesis and inducible DNA repair
in Escherichia coli. Bacteriol. Rev., 40: 869-907.
WOODCOCK, A. & MAGNUS, I. A. (1976) The sunburn cell on mouse skin:
Preliminary quantitative studies on its production. Br. J.
Dermatol., 95: 459-468.
YEH, S. (1962) Relative incidence of skin cancer in Chinese in Taiwan:
With special reference to arsenical cancer. In: Urbach, F., ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 81-108 (Monograph No. 10, National Cancer
Institute).
ZACKHEIM, H. S. (1964) Comparative cutaneous carcinogenesis in the
rat. Differential response to the application of anthramine,
methylcholanthrene, and dimethylbenzanthacene. Oncology,
17: 236-246.
ZIGMAN, S., SCHULTZ, J. B., & YULO, T. (1973) Possible roles of near
UV light in the cataractous process. Exp. eye Res.,
15: 201-208.
ZILOV, JU.D. (1971) [The prophylactic irradiation of children and
adolscents living in different climatic zones of the Soviet
Union.] In: [Ultraviolet radiation.] Moscow, Medicina,
pp. 237-241 (in Russian).
ZIVILOVA, N. D., ISTJUFEEV, V. A., SMIRNOV, E. S., & LEVITIN Ju.S.
(1975) [The UFM-meter and the UFB-72 laktmeter.] In: [The
biological effect of UV-radiation.] Moscow, Nauka, pp. 247-249
(in Russian).
ZWEIG, A. & HENDERSON, W. A. Jr (1976) A photochemical mid-ultraviolet
dosimeter. Photochem. Photobiol., 24: 543-549.
6.5.3 The "erythema range" effect
One of the important observations of Hausser & Vahle (1922) was
that there appeared to be a significant difference between the doses
needed to produce slight and maximal erytherna at different
wavelengths. This concept is of great potential significance for the
prevention of sunburn and the understanding of diseases due to light.
In Fig. 10, data are plotted from an experiment designed by Hausser to
test this effect, which show that 5 times the minimal erytherna dose
produces much less than the maximal erytherna at 254 nm, while 2.5
times the minimal erythema dose produces maximal erytherna at 303 nm.
While it takes much more energy to produce any erytherna at 303 nm
than at 254 nm, not much more than the threshold dose produces a
significant burn.
These observations are of great importance because they show that
the acute biological effects of several wavelengths in the UV-B
proceed at different time scales, and have very different
dose-response relationships. Thus, the usual assumptions that it is
realistic to weight the effectiveness of different wavelengths in a
continuous UVR source (such as the sun) by an action spectrum obtained
for a threshold effect with a monochromator is not correct, nor is it
appropriate to consider the effect of these wavelengths to be
biologically equivalent.
Unfortunately, in the absence of knowledge of chromophores or
biological mechanisms, there is no better way of comparing the
effectiveness of sources of different UVR composition than the present
method of calculating the skin erytherna effectiveness of UV-B.
As in all photobiological cutaneous effects, pigmentation plays a
major role in the sensitivity of the skin to UVR. Heavily pigmented
people are 10-20 times less sensitive than untanned fair-skinned
people. As far as is known, the action spectra for erythema are
similar for all races, differing only in the amount of energy
necessary to produce threshold effects.
6.5.4 Dose-response curves for keratoconjunctivitis
These are essentially similar to those for erythema, except that
the peak of the action spectrum is located at 270 nm. This is most
likely because of the absence of a filtering stratum corneum and
because the conjunctiva is not pigmented. The peak of 270 nm is
therefore used for the normalization of the dose limits of broad band
sources.
6.5.5 Dose-response relationship for photocarcinogenesis
Most of the existing evidence is consistent with the concepts
that UV-induced photodamage to skin is the main causal factor in the
development of skin cancer, that the development of skin cancer is a
stochastic effect, and that there is no threshold (sections 4.3 and
5.5).
Thus, a relationship should exist between skin cancer incidence
and accumulated dose, using a sensitivity function. In mice, the
quantitative relationship between UVR dose and the production of skin
cancer has been thoroughly explored; tumour incidence in this
experimental model is proportional to the square root of the number of
doses, the dose size, and the interval between doses.
In man, there is ample evidence that a latitude gradient exists
for the incidence of skin cancer in sensitive, fair-skinned
populations, and that this gradient is non-linear. There is obviously
a relationship between latitude and the intensity of solar radiation,
and this gradient is exaggerated in the UVR portion of the spectrum.
Although the shape of this relationship is relatively complex and
depends on a number of variables, for a range of mid-latitudes
(30°-50°), both the theoretical form and that obtained from actual
field measurements closely approximate a straight line. It is
generally accepted that latitude-related climatic and environmental
conditions and behavioural effects must modify the UVR dose actually
reaching a population. The factors involved, and their magnitude are
largely speculative.
A series of mathematical models relating human skin cancer
incidence to solar UVR has been proposed at various times in the past
few years, because of concern about alteration of the stratospheric
ozone layer (Green et al., 1978; Rundel & Nachtwey, 1978). While the
uncertainties are very great, the best accepted model data suggest
that a 5% increase in erythema-effective solar UVR may result in a 15%
(range 7.5-25%) increase in skin cancer in a susceptible population
after about 60 years, when a steady state has been reached. As far as
the relative risk is concerned in susceptible white-skinned
populations, people in older age groups, with fair skin that sunburns
easily and with a high life-time solar exposure, run 10-20 times more
risk of developing skin cancer than their contemporaries, who tan
easily and have a low life-time solar exposure (Vitaliano, 1978).
Incidences of nonmelanoma skin cancer as high as 350/100 000
population per year have been observed in elderly, white-skinned males
in Queensland, Australia, and Texas, USA. Populations with
constitutionally, heavily pigmented skin run only a minimum risk of
developing skin cancer in their lifetime.
7. GUIDELINES FOR HEALTH PROTECTION
The development of criteria for both upper and lower limits of
exposure to either natural or artificial UVR is extremely difficult
because of such problems as:
(a) the variation in both the acute and chronic effects of UVR
of different wave lengths;
(b) the considerable differences in the spectral composition of
light from different sources and particularly of sunlight at different
latitudes;
(c) the great differences in cutaneous sensitivity to UVR due
to genetic, environmental, and adaptive effects, and the considerable
variation in sensitivity in the same person at different times; and
(d) the difficulty of differentiating between the necessary
dose of UVR compatible with the upkeep of life, and the lowest dose
that results in serious detrimental effects.
7.1 Range of Exposure Limits
7.1.1 Exposure to solar ultraviolet radiation
As described in section 6, two thirds of the daily amount of
solar UV-B radiation reaches the earth between 10h00 and 14h00. Thus,
exposure should be reduced to a miminum during these hours. After a
period of acclimatization, most people can tolerate several hours of
outdoor exposure in the morning and afternoon, but shielding in the
near noon hours should always be considered. In general, exposure
during this very important period of acclimatization should not exceed
4 minimal erythema doses per day without some protection (either
clothing or sunscreens, section 7.3). This would correspond to 1 h of
exposure in the tropics. A dose of 1/8 MED per day appears to be
sufficient to prevent UVR deficiency.
7.1.2 Occupational exposure to artificial ultraviolet radiation
No internationally agreed limits exist at this time for
occupational exposure to UVR, which take into consideration the acute
effects and the risk of late cancer development. So far, the only
limits available, which could be used in the preparation of
guidelines, have been proposed by the National Institute of
Occupational Safety and Health in the USA (NIOSH, 1975).
The limits are based on the minimal erythema dose (MED) and the
minimal photokeratitic dose, which means that only acute effects have
been taken into consideration. For the UVR 200-315 nm region, it is
stated that radiant exposure in any 8-h period must not exceed the
values given in Table 11. For the wavelength range 315-400 nm, the
total irradiance on unprotected skin or eye must not exceed 10 W/m2
for periods exceeding 103 seconds. For radiant exposure of shorter
durations, it should not exceed 10 000 J/m2. The limit for 315-400 nm
is probably much too low and could be revised to a higher level.
Table 11. Total permissible 8-h doses and relative spectral
effectiveness of some selected monochromatic
wavelengthsa
Wavelength Permissible 8-h Relative spectral
(nm) dose (J/m2) effectiveness
(S lambda)b
200 1000 0.03
210 400 0.075
220 250 0.12
230 160 0.19
240 100 0.30
250 70 0.43
254 60 0.50
280 46 0.65
270 30 1.00
280 34 0.88
290 47 0.84
300 100 0.30
305 500 0.06
310 2000 0.015
315 10 000 0.003
a "From: NIOSH (1975).
Table 12. Maximum permissible exposure times for selected
values of Ieffa
Duration of exposure Effective irradiance,
per day Ieff (W/m2)b
8 h 1.0
4 h 2.0
2 h 4.0
1 h 8.0
30 min 17.0
15 min 33.0
10 min 50.0
5 min 100.0
1 min 500.0
30 sec 1000.0
a From: NIOSH (1975).
b Effective irradiance = action spectrum weighted irradiance.
However, these values only apply to sources emitting essentially
monochromatic UVR. The maximum permissible exposure for a broad band
source should be calculated by summing up the relative contributions
from all its spectral components, each being weighted by the relative
spectral effect SL, as given in Table 12. In addition, these
guidelines for determining exposure limits should not be used for
photosensitive individuals.
So far, none of the guidelines has included an evaluation of the
carcinogenic risk, as neither the action spectrum nor the
dose-response curve is known for man. It is hoped that this will be
obtained by comparison of cancer incidence and UV irradiance as
measured by the Robertson-Berger method.
7.1.3 Exposure of the general population to artificial ultraviolet
radiation
The exposure of the general population to artificial UVR is
primarily for hygienic and for cosmetic purposes. The use of UVR for
health purposes is discussed in sections 7.2 and 7.3.
The use of UVR for cosmetic purposes generally involves a
requirement for the development of skin pigmentation. For this reason,
it is unrealistic to follow the occupational standards, since they
would not allow the desired effects to be obtained.
Doses of UVR sufficient to produce slight erythema in 24 hours
are usually adequate for cosmetic purposes. Significant overdoses and
repeated UVR exposures for prolonged periods should be avoided. The
eyes must be properly shielded during each exposure. The risk of the
development of skin cancer due to chronic exposure to UVR for cosmetic
purposes is not known.
A number of devices available to the general population emit
significant amounts of UVR, or will do so if the protective envelope
of the device is damaged, and may cause acute UVR-induced eye and skin
damage. Such devices include Wood's lights, sterilizing and
ozone-producing lamps, and high power mercury and xenon arc lamps used
for illumination.
7.1.4 Measurement of natural and artificial ultraviolet radiation
Measurement of solar UVR involves serious difficulties, because
of the need for accurate spectral discrimination at the shortest end
of the solar spectrum. This is necessary because of the considerable
variations in the biological effectiveness of UVR shorter than 320 nm.
Few really practical, accurate, stray light-free spectroradiometers
have been developed so far, for use in the middle UVR region (UV-B)
and the use of action spectrum weighted, integrating analogue UVR
meters has been found to be much more practical.
The few practical prototypes of integrating chemical UVR
dosimeters that have been developed are still in the experimental
stage. If they can be perfected, such personal UVR dosimeters would be
of the greatest use (Challoner et al., 1976; Davis et al., 1976).
Basically, the same problems pertain to monitoring of artificial
UVR, although measurements are somewhat easier in a laboratory
setting.
The exposure criteria recommended in section 7.1.2 have not been
put to practical use, because it is still not technologically possible
to measure UVR adequately for compliance.
Thus, working practices are recommended for the control of
exposure in situations where sufficient measurement or emission data
are not available. A frequently practiced method of protection
consists of adequate containement of UVR-producing light sources.
7.2 Health Effects of Solar Ultraviolet Radiation in the General
Population
Two aspects of health protection in the general population are of
interest. One is the prevention of UVR deficiency that can occur in
populations living near the north and south poles (generally at
latitudes of 60° or more). The other deals with the protection of
pale-skinned people from excessive UVR in subtropical and tropical
areas (generally between latitudes 35° N and 35° S).
The zoning of the USSR for UVR is particularly interesting from a
health point of view (Belinskij, 1971). In the "UVR-deficit zone" it
appears essential to use irradiation from artificial sources, during
certain months, to compensate for UVR deficiency. In the "UV comfort
zone", artificial irradiation is unnecessary. In the "UVR excess
zone", it is essential to undertake measures for protection against
solar UVR, in order to avoid skin cancer. It has also been proposed
that, in order to prevent skin tumours among town dwellers in whom
light starvation has caused depigmentation of the skin, preventive UVR
should be carried out to increase skin pigmentation.
7.3 UVR Deficiency and its Prevention
7.3.1 Insolation and UV irradiation of built-up areas
It is well known that solar energy entering a room illuminates,
warms, dries, and particularly important, disinfects it, thus having a
beneficial, physical and psychological effect. The sanitation
standards and regulations for the insolation of dwellings and public
buildings and built-up areas, which are in force at present in the
USSR, are based on the requirement that premises should receive three
hours' uninterrupted insolation on cloudless days during the period 22
March-22 September and that protection should be given to limit the
thermal effect of solar radiation in the southern regions, below
latitude 55°N (State Standards, 1976b).
This health requirement is evident from a number of reviews
(Dancig, 1971; Galanin & Pirkin, 1971; Aleksandrov et al., 1975).
Solar UVR has a particularly valuable health-effect in that, inter
alia, it speeds up the processes of environmental selfpurification
(Belikova, 1960; Gluscenko et al., 1975). Therefore, town planning
tasks should include the rational use of UVR from the sun and sky
(Davidson, 1970; Gusev & Dunaev, 1971).
Since window glass cuts off the most biologically active
component of natural UVR, research to increase the UVR transparency of
window glass has been of particular concern to health workers in the
most northern latitudes (Belikova, 1964).
7.3.2 Sunbathing and air-bathing in the prevention of UVR deficiency
In addition to measures ensuring the maximum possible penetration
of natural UVR into working and dwelling places, prevention of UVR
deficiency can also be ensured by organizing solaria, beaches, and
sports areas attached to children's establishments (kindergartens and
pioneer camps), and to factories and mills. A schedule for the UV
irradiation of children and adults, for sun and sun-and-air baths at
different geographical latitudes, at different seasons of the year,
and at different times of the day has been developed (Generalov,
1971). At present, the effects of such solar radiation schedules
cannot be extrapolated to potential late effects.
7.3.3 Artificial ultraviolet radiation in the prevention of UVR
deficiency
Effective principles for the use of artificial UVR in the
prevention of UVR deficiency and "light starvation" in man have been
developed in the USSR. The recommended levels consist of a daily dose
of UV-B of between 0.125 and 0.75 of the threshold erytherna dose
(State Standards, 1964). The use of prophylactic UV irradiation has
been shown to be quite effective in workers in industries lacking
natural light (Bocenkova, 1971) and particularly effective in children
of preschool and school age (Ronge, 1948; Zilov, 1971).
Regulations have been drawn up for the planning and operation of
artificial UV irradiation devices in industrial enterprises (State
Standards, 1976b).
7.4 Protection against Ultraviolet Radiation
The whole of the world population has a potential for developing
skin cancer, the risk depending on the intensity and degree of
exposure to solar UVR during the life span. If all current exposure to
solar UVR could be significantly reduced, the incidence of skin cancer
would eventually decrease greatly.
Health and appearance can be adequately catered for by exposing
only part of the body for less than half an hour per day at or near
noon, except at latitudes north of 60° N.
7.4.1 Sunscreen preparations
Sunscreen preparations are usually classified as chemical or
physical agents. The former include para-aminobenzoic acid and its
esters, cinnamates, and benzophenones, all of which act by absorbing
radiation, which is dissipated as radiation of lower energy. Physical
agents act as simple physical barriers, reflecting, blocking, or
scattering light. They include titanium dioxide, talc, and zinc oxide.
Mainly because of cosmetic objections, the physical barriers are not
often used in sunscreen formulations (Robertson, 1972).
The principle of covering the skin by spreading a layer of
reliable UV absorber on the surface has proved a popular one.
Providing that the thickness of the layer applied is adequate and that
it adheres to the skin, it gives protection to the wearer under all
conditions.
Details of the kind of chemicals and bases used are beyond the
scope of this report.
7.4.2 Clothing
Covering the skin with clothing gives a sense of security that is
often misleading. The most frequent sites for skin cancer are those
that face upward. Thus, a hat is essential for adequate protection of
the forehead, scalp, and tops of ears. However, generally, it gives
only partial protection for the nose, less for the lower face, and
none for the hands and arms.
Body coverings worn in hot climates are generally not complete
absorbers. The average white shirt worn by men may transmit 20% of
UVR, while lighter weaves favoured by women may allow 50% of UVR to
reach the shoulders. Fairly complete clothing cover is more tolerable
in a dry atmosphere than a humid, coastal, tropical environment.
7.4.3 Behavioural conformity with the environment
When unexpectedly detained in the sun, protective procedures
available include facing in a variety of directions, hanging the head
and shielding the head with the hands or with a handkerchief. Shadows
should be used whenever possible, including one's own shadow. When
standing in the shadow of a building, only rays from about half the
sky are received, that is, about one quarter of the harmful intensity
of full daylight. This is certainly useful protection, but fair skin
will still burn in about one hour; in three hours, a painful burn may
be initiated, although the sufferer has not been in direct sunlight at
all.
The dominant factor in the daily erythemal exposure is the angle
of the sun above the horizon. This varies with latitude and with time
of year. Thus, maximum exposure occurs, when the sun is almost
directly overhead. The simplest means of protection is to take shelter
around the middle of the day, especially in the summer months.
At least one third of the whole day's UVR exposure occurs in the
hour before and the hour after noon; half the day's exposure, enough
for a very disabling sunburn, occurs during the 3-hour period around
noon. If shelter is taken between 10h00 and 14h00, only one third of
the day's exposure remains. Outside the period from 9h00 to 15h00,
only one sixth remains, permitting considerable outdoor activity even
for subjects with the most sensitive skin types.
7.4.4 Occupational protection
With the exception of medically prescribed doses, exposure of
both the eyes and skin to UVR should be kept to a minimum. In order to
protect persons in the vicinity of artificial UVR, the following
precautions are recommended:
(a) Whenever possible, prevention of excessive exposure of the
eyes and skin should be ensured by proper engineering design of
UVR-emitting installations and suitable enclosures, so that any UVR is
either adequately contained or sufficiently attenuated.
(b) When, for justifiable reasons, such containment is not
possible, protection should be afforded by providing close-fitting
goggles and/or face shields accompanied, if necessary, by suitable
UVR-opaque clothing and gloves to cover the skin.
(c) Adequate and appropriate instruction should be given, to
any person liable to be excessively exposed to UVR, concerning the
hazards involved and the precautions to be observed to avoid excessive
exposure.
(d) For artificial UVR sources that do not emit significant
visible light, a visible or audible warning signal may be required to
show when the UVR is being emitted.
(e) Powerful short wavelength UVR sources may generate ozone.
This additional hazard should be avoided by providing either adequate
ventilation or an adequate ozone removal system in the workplace.
REFERENCES
AITKEN, R. (1937) Ultraviolet radiation treatment of preeclamptic
toxemia. Br. J. Phys. Med., 12: 45-47.
ALEKSANDROV, V. N. & ALEKSANDROVA, A. I. (1975) [The insolation and UV
climate of housing in the far north.] Gig. i Sanit. 5: 16 (in
Russian).
ANCHEV, N., POPOV, I., MONOV N., & OUZOUNOV, N. (1968) Spreading of
malignant neoplasms in the People's Republic of Bulgaria.
Neoplasma, 15: 451-468.
ANDREEV, M. G., VASIL'EV B. D., ZAHAR'EVSKIJ, A. V., & HOROKORIN, T.
(1975) [Artificial sources of UV radiation with biological
effect.] In: [The biological effect of UV radiation.] Moscow,
Nauka, pp. 221-225 (in Russian).
BACHEM, A. (1956) Ophthalmic ultraviolet action spectra. Am. J.
Ophthalmol., 41: 969-975.
BAIN, J. & RUSCH, H. P. (1943) Carcinogenesis with ultraviolet
radiation of wave length 2, 800-3, 400 A. Cancer Res.,
3: 425-430.
BEARD, H. H., BOGGESS, T. S., & VON HAAM, E. (1936) Experimental
production of malignant tumors in the albino rat by means of
ultraviolet rays. Am. J. Cancer, 27: 257-266.
BELIKOVA, V. K. (1964) [Evaluation from the hygienic standpoint of
experimental samples of a window glass for residential and public
buildings.] Gig. i. Sanit., 1: 21-28 (in Russian).
BELIKOVA, V. K. (1966) [Natural ultraviolet radiation and its
bactericidal importance.] Moscow, Medicina, pp. 322-326 (in
Russian).
BELIKOVA, V. K., ZABALUEVA, A. P., & GALPERIN, E. L. (1975)
[Evaluation from the standpoint of hygiene of the zoning of the
territory of the U.S.S.R. in respect to UV radiation levels.] In:
[The biological effects of UV radiation.] Moscow, Nauka, pp.
161-165 (in Russian).
BELINSKIJ, V. A. (1971) [Zoning of the territory of the USSR in
respect to the natural UV radiation.] In: [Meteorology and
climatology.] Issue 2, Moscow, Publishing House of the Moscow
Branch of the Geographical Society, (in Russian).
BELINSKIJ, B. A. & ANDRIENKO, L. M. (1974) [A simplified radiation
model of the atmosphere in the UV spectral region.] In:
[Radiation, processes in the atmosphere and on the earth's
surface.] Leningrad, Gidrometeoizdat, pp. 273-276, (in
Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1963) [Ultraviolet climate of the
USSR.] In [Proceedings of the All-Union Scientific
Meteorological Meeting.] Leningrad, Gidrometeozidat,
pp. 244-252 (in Russian).
BELINSKIJ, V. A. & GARADZA, M.P. (1962) [Scattered UV-radiation in the
Eastern Pamirs.] In: [Scientific Communications of the Institute
of Geology and Geography of the Academy of Sciences of the
Lithuanian SSR.] Vilanus, Vil. XIII, pp. 283-287 (in Russian).
BELINSKIJ, V. A., GARADZA, M.P., MEZENNAJA, L. M., & NEZVAL, E. I.
(1968) [Sun and sky ultraviolet radiation.] Moscow, Moscow
University Press (in Russian).
BENDER, M. A., GRIGGS, H. G., & WALKER, P. L. (1973) Mechanisms of
chromosomal aberration production. I. Aberration induction by
ultraviolet light. Mutat. Res., 20: 387-402.
BENER, P. (1972) Approximate values of intensity of natural
ultraviolet radiation for different amounts of atmospheric
ozone. London, European Research Office, United States Army.
(Contract DAJA 36-68-C-1017).
BEN-HUR, E. & ELKIND, M. M. (1973) DNA cross-linking in Chinese
hamster cells exposed to near ultraviolet light in the presence
of 4,5',8-trimethylpsoralen. Biochim. Biophys. Acta,
331: 181-193.
BERENBLUM, I. & SHUBIK, P. (1947) The role of croton oil applications,
associated with a single painting of a carcinogen, in tumor
induction of the mouse's skin. Br. J. Cancer, 1: 379-382.
BERENBLUM, I. & SHUBIK, P. (1949) An experimental study of the
initiating stage of carcinogenesis, and a re-examination of the
somatic cell mutation theory of cancer. Br. J. Cancer,
3: 109-118.
BERGER, D., URBACH, F., & DAVIES, R. E. (1967) The action spectrum of
erythema induced by ultraviolet radiation. Proceedings of the
13th International Congress of Dermatology, Berlin, Springer
Verlag, pp. 1112-1117.
BERGER, D., ROBERTSON, D. F. & DAVIES R. E. (1975) Field measurements
of biologically effective UV radiation. In: Impacts of climatic
change on the biosphere, Springfield, VA, National Technical
Information Service, Part I, Chapter 2, pp. 233-264 (Appendix D,
CIAP Monograph No. 5).
BEUKERS R. & BERENDS, W. (1960) Isolation and identification of the
irradiation product of thymine. Biochim. Biophys. Acta,
41: 550-551.
BILLEN, D. & GREEN, A. E. S. (1975) Comparison of germicidal activity
of sunlight with the response of a sunburning ultraviolet meter.
In: Impacts of climatic change on the biosphere, Springfield,
VA, National Technical Information Service, Part 1, Chapter 2,
pp. 299-305 (Appendix F, CIAP Monograph No. 5).
BISCHOFF, F. (1969) Carcinogenic effects of steroids. In: Paoletti, &
Kritchevsky, ed. Advances in lipid research. New York, Academic
Press, Vol. 7, pp. 165-244.
BLACK, H. S. & CHAN, J. T. (1976) Etiologic related studies of
ultraviolet light-mediated carcinogenesis. Oncology,
33: 119-122.
BLACK, H. S. & DOUGLAS, D. R. (1973) Formation of a carcinogen of
natural origin in the etiology of UV-carcinogenesis. Cancer
Res., 33: 2094-2096.
BLACK, H. S. & LO, W. B. (1971) Formation of a carcinogen in human
skin irradiated with ultraviolet light. Nature (Lond.),
234: 306-308.
BLACK, H. S., CHAN, J. T., & BROWN, G. E. (1978) Effects of dietary
constituents on ultraviolet light-mediated carcinogenesis.
Cancer Res., 38: 1384-1387.
BLUM, H. F. (1941) Sunlight and cancer of the skin. J. Natl Cancer
Inst., 1: 397-431.
BLUM, H. F. (1943) Wavelength dependence of tumor induction by
ultraviolet radiation. J. Natl Cancer Inst., 3: 533-537.
BLUM, H. F. (1969) Quantitative aspects of cancer induction by
ultraviolet light: Including a revised model. In: Urbach, F., ed.
The biological effects of ultraviolet radiation, Oxford,
Pergamon Press.
BLUM, H. F. & LIPPINCOTT, S. W. (1943) Carcinogenic effectiveness of
ultraviolet radiation of wavelength 2537A. J. Natl Cancer Inst.,
3: 211-216.
BLUM, H. F., GRADY, H. G., & KIRBY-SMITH, J. S. (1943) Relationships
between dosage and rate of tumor induction by ultraviolet
radiation. J. Natl Cancer Inst., 3: 91-97.
BLUM, H. F., BUTLER, E. G., DAILEY, T. H. DAUBE, J. R., MAWE, R. C., &
SOFFEN, G. W. (1959) Irradiation of mouse skin with single doses
of ultraviolet light. J. Natl Cancer Inst., 22: 979-993.
BOCENKOVA, T. D. (1971) [The application of prophylactic
UV-irradiation to workers in buildings of a new type.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 245-251 (in
Russian).
BOGUCKIJ, B. V., KAPELEVA, A. I., BOKSA, V. G., LESINSKAJ, N. P.,
CIBIREVA, E. M, KOSTIN, N. F., & EMELKIN, V. I. (1975)
[Continuous and pulsed ultraviolet irradiation in combined
treatment of protracted and chronic pneumonia.] In:
[The biological effect of UV-radiation.] Moscow, Nauka,
pp. 126-127 (in Russian).
BOVIE, W. T. & KLEIN, A. (1919) Sensitization to heat due to exposure
to light of short wavelengths. J. Gen. Physiol., 1: 331-336.
BOYCE, R. P. & HOWARD-FLANDERS, P. (1964) Release of ultraviolet
light-induced thymine dimers from DNA in E. coli K-12. Proc.
Natl Acad. Sci., 51: 293-300.
BRIDGES, B. A. & HUCKLE, J. (1970) Mutagenesis of cultured mammalian
cells by X-radiation and ultraviolet light. Mutat. Res.
10: 141-151.
BUCHANAN, A. R., HELM, H. C., & STILSON, D. W. (1960) Biomedical
effects of exposure to electromagnetic radiation.
I. Ultraviolet. Dayton, OH, Wright-Patterson Air Force Base,
pp. 60-376 (WADD Technical Report).
BUSCHKE, W., FRIEDENWALD, J. S., & MOSES, S. G. (1945) Effects of
ultraviolet irradiation on corneal epithelium: Mitosis, nuclear
fragmentation, post-traumatic cell movements, loss of tissue
cohesion. J. Cell. Comp. Physiol., 26: 147-164.
CAKLIN, A. V. (1974) [The epidemiology of malignant neoplasms.] In:
[Handbook on oncology.] Moscow, Medicina, pp. 26-27 (in
Russian).
CARLSON, L. D. & JACKSON, B. H. (1959) Combined effects of ionizing
radiation and high temperature on longevity of Sprague-Dawley
rat. Radiat. Res., 11: 509-519.
CASTO, B.C. (1973) Enhancement of adenovirus transformation by
treatment of hamster cells with ultraviolet irradiation, DNA base
analogs, and dibenz(a,h,)anthracene. Cancer Res., 33: 402-407.
CHALLONER, A. V. J., CORLESS, D., DAVIS, A., DEANE, G. H. W., DIFFERY,
B. L., GUPTA, S. P., & MAGNUS, I. A. (1976) Personnel monitoring
of exposure to ultraviolet radiation. Clin. exp. Dermatol.,
1: 175-179.
CHANDRA, P. (1972) Photodynamic action: A valuable tool in molecular
biology. Res. org. biol. med. chem., 3 (1): 232-258.
CHANDRA, P., RODIGHIERO, G., PALIKCIOGLU, S., & BISWAS, R. K. (1976)
Nucleic acid modification by furocoumarins and light: Some
biomedical implications. In: Jung, E., ed. Photochemotherapie,
Stuttgart, F. K. Schattauer Verlag, pp. 25-32.
CHARLIER, M. & HELENE, C. (1972) Photochemical reactions of aromatic
ketones with nucleic acids and their components I-. Purine and
pyrimidine bases and nucleosides. Photochem. Photobiol.,
15: 71-87.
CLEAVER, J. E. (1973) Xeroderma pigmentosum- progress and regress.
J. invest. Dermatol., 60: 374-380.
CLEAVER, J. E. & THOMAS, G. H. (1969) Single-strand interruptions in
DNA and the effects of caffeine in Chinese hamster cells
irradiated with ultraviolet light. Biochem. biophys. Res. Comm.
26: 203-208.
CLEAVER, J. E., THOMAS, G. H., TROSKO, J. E., & LETT, J. T. (1972)
Excision repair (dimer excision, strand breakage and repair
replication) in primary cultures of eukaryotic (bovine) cells.
Exp. cell Res., 74: 67-80.
CLEAVER, J. E. & TROSKO, J. E. (1969) DNA-degradation productions from
mammalian cells irradiated with ultraviolet light. Int. J.
radiat. Biol., 15: 411-424.
COBLENTZ, W. W., STAIR, R., & HOGUE, J. M. (1932) The spectral
erythemic reaction of the untanned human skin to UV radiation.
Bur. Stand. J. Res., 8: 541-547.
COGAN, D. G. & KINSEY, V. E. (1946) Action spectrum of keratitis
produced by ultraviolet radiation. Arch. Ophthalmol.,
35: 670-677.
COHEN-BAZIRE, G. & STANIER, R. Y. (1958) Inhibition of carotenoid
synthesis in photosynthetic bacteria. Nature (Lond.),
181: 250-252.
COX, B. & GAME, J. (1974) Repair systems in Saccharomyces. Mutat.
Res., 26: 257-264.
CURTIS, G. L. (1975) Initiation-promotion skin carcinogenesis and
immunological competence. Proc. Soc. Exp. Med., 150 (1):
pp. 61-64.
CUTCHIS, P. (1978) On the linkage of solar ultraviolet radiation to
skin cancer. Washington, DC, Federal Aviation Administration,
Office of Environmental Quality, pp. 1342 (Institute for Defense
Analysis Paper).
DANCIG, N.M. (1971) [Lighting hygiene and insolation of buildings and
built up areas in towns.] In: [Questions of housing hygiene and
of curative power and preventive medicine.] Moscow, A. N. Sysin
Institute, pp. 13-19 (in Russian).
DANCIG, I. N. (1974) [Dental caries and UV deficiency.]
Stimatologija, 1: 11-13 (in Russian).
DANCIG, N.M. (1975) [The hygienic basis for preventing light sarvation
among ships' crews.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 168-171 (in Russian).
DANCIG, N. M., ZABALNEVA, A. P., & PROKOPENKO, Ju.I. (1975) [The
importance of the protective effect of UV radiation in the
development of the tumoral process under experimental
conditions.] In: [The biological effects of UV radiation.]
Moscow, Nauka, pp. 137-142 (in Russian).
DANIELS, F. J., BROPHY, D., & LOWITS, W. C. (1961) Histochemical
response of human skin following ultraviolet radiation.
J. invest. Dermatol., 37: 351-357.
DAVIDSON, B. M. (1970) Relative evaluation of the insolation resources
of flats in a residential "microdistrict". Svetotekhnika,
7; 13-15.
DAVIES, J. N. P., KNOWLDEN, J., & WILSON, B. A. (1965) Incidence rates
of cancer in Kyandondo County, Uganda, 1954-1960. J. Natl Cancer
Inst., 35: 789-821.
DAVIES, R. E., DODGE, H. A., & AUSTIN, W. A. (1972a) Carcinogenicity
of DMBA under various light sources. Proceedings of the IX
Congress on Photobiology, pp. 247.
DAVIES, R. E., DODGE, H. A., & DESCHIELDS, L. H. (1972b) Alteration of
the carcinogenic activity of DMBA by light. Proc. Am. Assoc.
Cancer Res., 13: 14.
DAVIS, A., DEANE, G. H., & DIFFEY, B. L. (1976) Possible dosimeter for
ultraviolet radiation. Nature (Lond.), 261: 169-170.
DJORDJEVIC, B. & TOLMACH, L. J. (1967) Responses of synchronous
populations of HeLa cells to ultraviolet irradiation at selected
stages of the generation cycle. Radiat. Res., 32: 327-346.
DOLEZOVA, V. (1976) To the question of the geographic distribution of
pterygium. Rev. int. Trach., 53: 55-64.
DULBECCO, R. (i949) Reactivation of ultraviolet inactivated
bacteriophage by visible light. Nature (Lond.), 163: 949-950.
DUKE-ELDER, W. S. (1926) The pathological action of light upon the
eye. I. Action of the outer eye: Photophthalmia. Lancet,
1: 1137-1141.
EASTCOTT, D. F. (1962) Epidemiology of skin cancer in New Zealand. In:
Urbach, F., ed. The biology of cutaneous cancer, Washington,
DC, US Government Printing Office, pp. 141-152 (Monograph No. 10,
National Cancer Institute).
EDENBERG, H. J. & HANAWALT, P. C. (1973) The timecourse of DNA repair
replication in ultraviolet irradiated HeLa cells. Biochim.
Biophys. Acta, 324: 206-217.
EISENSTARK, A. (1971) Mutagenic and lethal effects of visible and
near-ultraviolet light on bacterial cells. Advances in Genetics,
16: 167-198.
ELKIND, M. M. & WHITMORE, G. F. (1967) The radiobiology of cultured
mammalian cells, New York, Gordon & Breach.
ELWOOD, J. M., LEE, J. A. H., WALTER, S. D., MO, T., & GREEN, A. E. S.
(1974) Relationship of melanoma and other skin cancer mortality
to latitude and ultraviolet radiation in the United States and
Canada. Int. J. Epidemiol. 3: 325-332.
ELWOOD, J. M. & LEE, J. A. H. (1975) Recent data on the epidemiology
of malignant melanoma. Semin. Oncol., 2 (2): 149-154.
EMMETT, E. A. (1973) Ultraviolet radiation as a cause of skin tumors.
CRC Crit. Rev. Toxicol., 2: 211.
EMMETT, E. A. (1977a) Phototoxic keratoconjunctivitis from coal tar
pitch volatiles. Science, 198: 841-842.
EMMETT, E. A. (1977b) Phototoxicity occurring during the manufacture
of ultraviolet cured ink. Arch. Dermatol., 113: 770-775.
EPSTEIN, J. H. (1965) Comparison of the carcinogenic and
co-carcinogenic effects of ultraviolet light on hairless mice.
J. Natl Cancer Inst., 34: 741-745.
EPSTEIN, J. H. & EPSTEIN, W. L. (1963) A study of tumor types produced
by ultraviolet light in hairless and hairy mice. J. invest.
Dermatol., 41: 463-473.
EPSTEIN, J. H., EPSTEIN, W. L., & NAKAI, T. (1967) Production of
melanomas from DMBA-induced «Blue Nevi» in hairless mice with
ultraviolet light. J. Natl Cancer Inst., 38: 18-22.
EPSTEIN, H. J., FUKUYAMA, K., & DOBSON, R. (1969) Ultraviolet light
carcinogenesis. In: Urbach, F., ed. The biological effects of
ultraviolet radiation, Oxford, Pergamon Press.
EVERETT, M. A., YEARGERS, E., SAYRE, R. M., & OLSON, R. L. (1966)
Penetration of epidermis by ultraviolet rays. Photochem.
Photobiol., 5: 533-542.
FABRE, F. (1971) A UV-supersensitive mutant in the yeast
Schizosaccharomyces pombe. Molec. Gen. Genet., 110: 134-143.
FEARS, T. R., SCOTTO, J., & SCHNEIDERMAN, M. A. (1977) Mathematical
models of age and ultraviolet effects on the incidence of skin
cancer among whites in the United States. Am. J. Epidemiol.,
105: 420-427.
FINDLAY, G. M. (1930) Cutaneous papillomata in the rat following
exposure to ultraviolet light. Lancet, pp. 1229-1231.
FISHER, M. S. & KRIPKE, M. L. (1977) Systemic alteration induced in
mice by ultraviolet light irradiation and its relationship to
ultraviolet carcinogenesis. Proc. Natl Acad. Sci., 74
(4): 1688-1692.
FITZPATRICK, T. B., PATHAK, M. A., HARBER, L. C., SEIJI, M., & KUKITA,
A. (1974) Sunlight and Man, Tokyo, University of Tokyo Press.
FORBES, P. D. (1978) Experimental UV photocarcinogenesis. In:
International Conference on Ultraviolet Carcinogenesis.
Washington, DC, US Government Printing Office pp. 31-38
(Monograph No. 50, National Cancer Institute).
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. I. Fluorescent whitening agents and
short-wave UVR. Food Cosmet. Toxicol., 13: 335-337.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photocarcinogenesis. II. Fluorescent whitening agents and
simulated solar UVR. Food Cosmet. Toxicol., 13: 339-342.
FORBES, P. D. & URBACH, F. (1975) Experimental modification of
photo-carcinogenesis. III. Simulation of exposure to sunlight and
fluorescent whitening Agents. Food Cosmet. Toxicol.,
13: 343-345.
FORBES, P. D., DAVIES, R. E., & URBACH, F. (1976) Phototoxicity and
photocarcinogenesis: Comparative effects of anthracene and
8-methoxypsoralen in the skin of mice. Food Cosmet. Toxicol.,
14: 243.
FORNACE, A. J., KOHN, K. W., & KANN, H. E. (1976) DNA single-strand
breaks during repair of UV damage in human fibroblasts and
abnormalities of repair in xeroderma pigmentosum. Proc. Natl
Acad. Sci., 73: 39-43.
FORTNER, J. G., MAKY, A. G., & SCHRODT, G. R. (1961) Transplantable
tumors of the Syrian (golden) hamster. I. Tumors of the
alimentary tract, endocrine glands and melanomas. Cancer Res.,
6 (Part 2): 161-198.
FOX, M. (1974) The effect of post-treatment with caffeine on survival
and UV-induced mutation frequencies in Chinese hamster and mouse
lymphoma cells in vitro. Mutat. Res., 24: 187-204.
FREEMAN, R. G. (1975) Data on the action spectrum for ultraviolet
carcinogenesis. J. Natl Cancer Inst., 55: 1119-1121.
FREEMAN, R. G. & KNOX, J. M. (1964a) Ultraviolet-induced corneal
tumors in different species and strains of animals. J. invest.
Dermatol., 43: 431-436.
FREEMAN, R. G. & KNOX, J. M. (1964b) Influence of temperature on
ultraviolet injury. Arch. Dermatol., 89: 858-864.
GABOVIC, R. E., MINH, A. A., & MOTUZKOV, I. N. (1975) [The effect of
UV radiation on the body's level of tolerance to exposure to
chemicals.] Vestn. Akad. Med. Nauk., 3: 26-37 (in Russian).
GALANIN, N. F. & PIRKIN, V. M. (1971) [The rational utilization of the
sun's radiant energy with a view to preventing ultraviolet
deficiency.] In: [Ultraviolet Radiation.] Moscow, Medicina, pp.
209-212 (in Russian).
GARADZA, M.P. (1965) [The natural UV radiation regime as shown by the
results of measurements at the Moscow State University
Meteorological Observatory.] In: [The climate of a big city.]
Moscow, MGU Publishing House, pp. 186-195 (in Russian).
GARADZA, M. P. (1967) [Direct UV-radiation at different times of
year.] In: [The radiation regime and precipitations in Moscow.]
Moscow, MGU Publishing House, pp. 165-175 (in Russian).
GARADZA, M. P. (1974) [Features of the inflow of UV-radiation under
different cloud condition.] In: [Radiation processes in the
atmosphere and on the earth's surface.] Leningrad,
Cidrometeoizdat, pp. 261-264 (in Russian).
GARADZA, M. P. & NEZVAL', E. I. (1971) [The effect of atmospheric
transparency and cloud on the UV-radiation regime.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 316-321 (in
Russian).
GAVRILOVA, L. I., DOJNIKOV, A. S., & KORCAGINA, T. N. (1975) [UV
radiation from arc and intermittent xenon lamps.] In:
[The biological effect of UV radiation] Moscow, Nauka, pp.
226-229 (in Russian).
GENERALOV, A. A. (1971) [The ultraviolet irradiation regime for
children and adults taking sun-baths and light and air baths at
various latitudes.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 203-207 (in Russian).
GLUSCENKO, A. G., ARTJUSENCO, I. S., & BARANOVA, M. A. (1975) [Health
indicators for children of crèche age in the ultraviolet climatic
conditions of Kiev.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 171-174 (in Russian).
GORDON, D. & SILVERSTONE, H. (1976) Worldwide epidemiology of
premalignant and malignant cutaneous lesions. In: Cancer of the
skin, Philadelphia, W. B. Saunders Co., pp. 405-434.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943a) Types of
tumors induced by ultraviolet radiation and factors influencing
their relative incidence. J. Natl Cancer Inst., 3: 371-378.
GRADY, H. G., BLUM, H. F., & KIRBY-SMITH, J. S. (1943b) Pathology of
tumors of the external ear in mice induced by ultraviolet
radiation. J. Natl Cancer Inst., 2: 269-275.
GRAHAM, J. H. & HELWIG, E. B. (1965) In: Montagna, W. & Dobson, R. L.,
ed. Advances in biology of the skin, New York, Macmillan
(Pergamon), Vol. II, pp. 277-327.
GREEN, A. E. S., ed. (1960) The middle ultraviolet: Its science and
technology, New York, John Wiley and Sons, Inc.
GREEN, A. E. S. & HEIDINGER, R. A. (1978) Models relating ultraviolet
light and non-melanoma skin cancer incidence. Photochem.
Photobiol., 28: 283-291.
GREEN, A. E. S., SAWADA, T., & SHETTLE, E. P. (1975) The middle
ultraviolet reaching ground. In: Impacts of climatic change on
the biosphere. Part 1, Chapter 2, pp. 29-49 (CIAP Monograph
No. 5).
GREEN, A. E. S., FINDLEY, G. B., Jr, KLENK, K. F., WILSON, W. M., &
MO, T. (1976) The ultraviolet dose dependence of non-melanoma
skin cancer incidence. Photochem. Photobiol., 24: 353-362.
GRIFFIN, A. C. (1959) Methoxsalen in ultraviolet carcinogenesis in the
mouse. J. Invest. Dermatol, 32: 367-372.
GRIFFIN, A. C., DOLMAN, V. S., ROHLKE, E. B., ROUVART, P., & TATUM, E.
L. (1955) The effect of visible light on the carcinogenicity of
ultraviolet light. Cancer Res., 15: 523.
GRIGGS, H. C. & BENDER, M. A. (1973) Photoreactivation of ultraviolet
induced chromosomal aberrations. Science, 179: 86-88.
GROSSMAN, L. (1968) Studies on mutagenesis induced in vitro.
Photochem. Photobiol, 7: 727-735.
GUSEV, N. M. & DUNAEV, B. A. (1971) [The utilization of ultraviolet
radiation in building.] In: [Ultraviolet radiation.] Moscow,
Medicina, pp. 218-221 (in Russian).
HAMILTON, L. (1973) The influence of the cell cycle on the radiation
response of early embryos. In: Balls, M. & Billet, F. F., ed.
The cell cycle in development and differentiation, Cambridge,
Cambridge University Press, pp. 229-247.
HAN, A. & SINCLAIR, W. K. (1969) Sensitivity of synchronized Chinese
hamster cells to ultraviolet light. Biophys. J., 9: 1171-1192.
HAN, A., SINCLAIR, W. K., & YU, C. K. (1971) Ultraviolet-light-induced
division delay in synchronized Chinese hamster cells.
Biophys. J., 11: 540-549.
HANAWALT, P. C. & SETLOW, R. B., ed. (1975) Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 827.
HARBER, L. C. & BAER, R. L. (1969) Classification and characteristics
of photoallergy. In: Urbach, F., ed. The biologic effects of
ultraviolet radiation, Oxford, Pergamon Press, pp. 519-526.
HARRISON, A. P. (1967) Survival of bacteria. Harmful effects of light,
with some comparisons with other adverse physical agents. Annual
Rev. Microbiol., 21: 143-156.
HAUSSER, K. W. & VAHLE, W. (1922) [The dependence of light induced
erythema and pigment formation upon the frequency (or wavelength)
of the inducing radiation.] Strahlentherapia, 13: 41-71 (in
German).
HAUSSER, K. W. & VAHLE, W. (1927) [Sunburn and suntan.] Wiss
Veröffent. Siemens-Konzern, 6: 101 (in German).
HAYNES, R. H. (1975) DNA repair and the genetic control of
radio-sensitivity in yeast. In: Hanawalt, P. C. & Setlow, R. B.,
ed. Molecular mechanisms for repair of DNA, New York & London,
Plenum Press, pp. 529-540.
HÉLENE, C. & CHARLIER, M. (1971) Photosensitized reactions in nucleic
acids. Photosensitized formation and splitting of pyrimidine
dimers. Biochimie, 53: (11-12): 1175-1180.
HELLMAN, K. B., HAYNES, K. F., & BOCKSTAHLER, L. E. (1974)
Radiation-enhanced survival of a human virus in normal and
malignant rat cells (37788). Proc. Soc. Exp. Biol. Med.,
145: 255-262.
HERLITZ, C. W., JUNDELL, I., & WAHLGREN, F. (1930) [Malignant tumours
in white mice caused by ultraviolet irradiation.] Acta Paediat.,
10: 333-347 (in German).
HEROLD, H. J. & BERNDT, H. (1968) Cancer incidence in the German
Democratic Republic. Selected tables. Neoplasma, 15: 517-522.
HILL, R. H. (1958) A radiation-sensitive mutant of E. coli. Biochim.
Biophys. Acta, 30: 636-637.
HILL, L. & EIDENOW, A. (1923) Biological action of light: I. The
influence of temperature. Proc. R. Soc. (Biol.), 95: 163-180.
HOLMBERG, M. & JONASSON, J. (1974) Synergistic effect of X-ray and UV
irradiation on the frequency of chromosome breakage in human
lymphocytes. Mutat. Res., 23: 213-221.
HSIAO, D. (1975) Impacts of climatic change on the biosphere.
Springfield, Virginia. National Technical Information Service
(CIAP Monograph No. 5).
HUANG, C. W. & GORDON, M.P. (1973) Formation of cyclobutane-type
pyrimidine dimers in RNA by sunlight. Int. J. radiat. Biol.,
23: 527-529.
HUEPER, W. C. (1941) Cutaneous neoplastic responses elicited by
ultraviolet rays in hairless rats and in their haired litter
mates. Cancer Res., 1: 402-406.
HUTCHINSON, F. (1973) The lesions produced by ultraviolet light in DNA
containing 5-bromouracil. Quart. Rev. Biophys. 6: 201-246.
IKUSHIMA, T. & WOLFF, S. (1974) UV-induced chromatid aberrations in
cultured Chinese Hamster cells after one, two or three rounds of
DNA replication. Mutat. Res., 22: 193-201.
IPPEN, H. (1969) Mechanisms of photopathological reactions. In:
Urbach, F., ed. The biologic effects of ultraviolet radiation,
Oxford, Pergamon Press, pp. 513-518.
JACOBSON, A. F. & YATVIN, M. B. (1976) Changes in the phospholipid
composition of E. coli following X and UV-irradiation. Radiat.
Res., 66: 247-266.
JAGGER, J. (1967) Introduction to research in ultraviolet
photobiology, Englewood Cliffs, N J, Prentice-Hall, Inc.,
pp. 164.
JAGGER, J. (1969) Photoreactivation. In: Hollaender, A., ed.
Radiation protection and recovery, New York, Pergamon Press,
pp. 352-377.
JAGGER, J. (1976) Effects of near-ultraviolet radiation on
microorganisms. Photochem. Photobiol., 23: 451-454.
JESSUP, J. M., HANNA, N., PALASZYNSKI, E., & KRIPKE, M. L. (1978)
Mechanisms of depressed reactivity to dinitrochlorobenzene and
ultraviolet-induced tumours during ultraviolet carcinogenesis in
BALB/c mice. Cell. Immunol., 38: 105-115.
JOHNSON, B. E. (1968) Ultraviolet radiation and lysosomes in skin.
Nature (Lond.), 219: 1258-1259.
JOHNSON, B. E., DANIELS, F., & MAGNUS, I. A. (1968) Response of human
skin to ultraviolet light. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV.
JUNG, E. G. (1976) [Photochemotherapy, background, techniques and
complications.] Stuttgart -- New York, F. K. Schattaver Verlag
(in German).
JUNG, E. G., BOHNERT, E., & ERBS, G. (1971) Wavelength dependence of
UV-induced alterations of epidermal cells in hairless albino
mice. Arch. Derm. Forsch., 241: 284-291.
KAMENECKAJA, T. M. & MITROFANOVA, G. F. (1975) [The condition of
certain neurohumoral regulatory systems following UV radiation at
different wavelengths.] In: [The biological effects of UV
radiation.] Moscow, Nauka, (in Russian).
KAO, F. & PUCK, T. T. (1969) Genetics of somatic mammalian cells. IX.
Quantitation of mutagenesis by physical and chemical agents.
J. Cell. Physiol., 74: 245-248.
KARACEVCEVA, T. V. (1971) [The role on UV radiation as part of modern
methods of treating and preventing rheumatism in children.] In:
[Ultraviolet radiation.] Moscow, Medicina, pp. 154-158 (in
Russian).
KELNER, A. (1949) Photoreactivation of ultraviolet-irradiated
Escherichia coli with special reference to close-reduction
principle and to ultraviolet-induced mutation. J. Bacteriol.,
58: 511-522.
KELNER, A. (1953) Growth, respiration, and nucleic acid synthesis in
ultraviolet-irradiated and in photoreactivated E. coli.
J. Bacteriol., 65: 252-262.
KELNER, A. & TAFT, E. B. (1956) The influence of photoreactivating
light on the type and frequence of tumors induced by ultraviolet
radiation. Cancer Res., 16: 860-866.
KIEFER, J. (1971) The importance of cellular energy metabolism for the
sparing effect of dose fractionation witch electrons and
ultraviolet light. Int. J. radiat. Biol., 20: 325-336.
KIRBY-SMITH, J. S., BLUM, H. F., & GRADY, H. G. (1942) Penetration of
ultraviolet radiation into skin, as a factor in carcinogenesis.
J. Natl Cancer Inst., 2: 403-412.
KOCH, H. R. (1967) [Photochemotherapy and cataract formation.]
Dermatosen im Beruf und Umwelt., 26; 162-165 (in German).
KOLLER, L. R. (1965) Ultraviolet radiation, 2nd ed., New York, John
Wiley and Sons.
KRAEMER, K. H., DE WEERD-KASTELEIN, E. A., ROBBINS, J. H., KEIJZER,
W., BARRETT, S. F., PETINGA, R. A., & BOOTSMA, D. (1975) Five
complementation groups in xeroderma pigmentosum. Mutat. Res.,
33: 327-340.
KRIPKE, M. L. (1974) Antigenicity of murine skin tumors induced by
ultraviolet light. J. Natl Cancer Inst., 53: 1333-1336.
KRIPKE, M. L. (1976) Target organ for a systemic effect of ultraviolet
radiation. Photochem. Photobiol., 24: 599-600.
KRIPKE, M. L. (1977) Latency, histology, and antigenicity of tumors
induced by ultraviolet light in three inbred mouse strains.
Cancer Res., 37: 1395-1400.
KRIPKE, M. L. & FISHER, M. S. (1976) Effect of UV light on the host
response to UV-induced tumirs. Proceedings of the 4th Annual
Meeting, American Society of Photobiology, Denver, CO, February
16-20.
KRIPKE, M. L. & FISHER, M. S. (1976) Immunologic parameters of
ultraviolet carcinogenesis. J. Natl Cancer Inst., 57: 211-215.
KRIPKE, M. L., LOFGREEN, J. S., BEARD, J., JESSUP, J. M., & FISHER, M.
S. (1977) In vivo immune responses of mice during
carcinogenesis by ultraviolet irradiation. J. Natl Cancer Inst.,
59: 1227-1230.
LABORDUS, V. (1970) The effect of ultraviolet light on developing eggs
of Lymnaea stagnails (mollusca, pulmonata) I, II, III. Proc.
K. Ned. Akad. Wet. Amsterdam, C, 366-493.
LAMOLA, A. A. & YAMANE, T. (1967) Sensitized photodimerization of
thymine in DNA. Proc. Natl Acad. Sci., 58: 443-446.
LANCASTER, H. O. (1956) Some geographical aspects of the mortality
from melanoma in Europeans. Med. J. Aust., 1: 1082-1087.
LATARJET, R. (1972) Interaction of radiation energy with nucleic
acids. Curr. Top. Radiat. Res. Q., 8: 1-38.
LATARJET, R. & ZAJDELA, F. (1974) Effet inhibitcur de la caféine sur
l'induction de cancers cutanés par les rayons ultraviolet chez la
souris. C. R. Acad. Sci. (Paris), 277: 1073-1076.
LAZAREV, D. N. & SOKOLOV, M. V. (1971) [The measurement of ultraviolet
radiation in units of effect.] In: [UV Radiation.] Moscow,
Medicina, pp. 328-333 (in Russian).
LAZAREV, D. N. & SOKOLOV, M. V. (1974) [Ultraviolet radiation.
Quantities and units. Terms and definitions. Technical
material for guidance.] Puscino, Institute of Biophysics of
the Academy of Sciences of the USSR, pp. 1-8 (in Russian).
LAZAREV, D. M., BESSALOVA, E. S., BALINA, G. P., & ERMILOVA, M. G.
(1975) [A UV biological photometer.] In: [The biological effects
of UV radiation.] Moscow, Nauka, pp. 244-246 (in Russian).
LEE, J. A. H. & MERRILL, J. M. (1970) Sunlight and the aetiology of
malignant melanoma: A Synthesis. Med. J. Aust., 2: 840-841.
LEE, H. H. & PUCK, T. T. (1960) The action of ultraviolet radiation on
mammalian cells as studied by single-cell technique. Radiat.
Res., 12: 340-348.
LEHMANN, A. R. (1972) Postreplication repair of DNA in
ultraviolet-irradiated mammalian cells. Mol. Biol.,
66: 319-337.
LEHMANN, A. R., KIRK-BELL, S., ARLETT, C. F., PATERSON, M. C., LOHMAN,
P. H. M., WEERD-KASTELEIN de, E. A., & BOOTSMA, D. (1975)
Xeroderma pigmentosum cells with normal levels of excision repair
have a defect in DNA synthesis after UV irradiation. Proc. Natl
Acad. Sci., 72: 219-223.
LEWIS, N. F. & KUMTA, U.S. (1972) Evidence for extreme UV resistance
of Micrococcus sp. NCTC 10785. Biochem. biophys. Res. Commun.,
47: 1100-1105.
LIPSON, R. L. & BALDES, E. J. (1960) Photosensitivity and heat. Arch.
Dermatol., 82: 517-520.
LOOMIS, W. F. (1970) Rickets. Sci. Am., 233: 76-91.
LUKIESH, M., HOLLADAY, L. L., & TAYLOR, A. H. (1939) Erythemal and
tanning effectiveness of UV energy. Gen. Electr. Rev.,
42: 274-278.
LYTLE, C. D. (1971) Host-cell reactivation in mammalian cells. I.
Survival or ultraviolet-irradiated herpes virus in different
cell-lines. Int. J. radiat. Biol., 19: 329-337.
LYTLE, C. D., HELLMAN, K. B., & TELLES, N. C. (1970) Enhancement of
viral transformation by ultraviolet light. Int. J. radiat.
Biol., 18: 397-300.
MACDONALD, E. J. (1976) Epidemiology of skin cancer 1975. In:
Neoplasms of the skin and malignant melanoma, Chicago, Year
Book Publishers, Inc., pp. 27-42.
MACKIE, B. S. & MCGOVERN, V. J. (1958) The mechanism of solar
carcinogenesis: A study of the role of collagen degeneration of
the dermis in the production of skin cancer. Arch. Dermatol.
Syph., 78: 218-244.
MAGNUS, I. A. & JOHNSON, B. E. (1965) Cited by Johnson, B. E.,
Daniels, F. Jr, & Magnus, I. A. In: Giese, A. C., ed.
Photophysiology, New York, Academic Press, Vol. IV,
pp. 139-202.
MAGNUS, K. (1973) Incidence of malignant melanoma of the skin in
Norway, 1955-1970. Cancer, 32: 1275-1286.
MAGNUS, K. (1977) Incidence of malignant melanoma of the skin in the
five nordic countries: significance of solar radiation. Int. J.
Cancer, 20: 477-485.
MALIK, M. O. A., MIDAYTALLA, A., DAOUC, E. H., & EL HASSAN, A. M.
(1974) Superficial cancer in the Sudan. A study of 1225 primary
malignant superficial tumours, Br. J. Cancer, 30: 355-364.
MATHEWS, M. M. & KRINSKY, N. (1965) The relationship between
carotenoid pigments and resistance to radiation in
non-photosynthetic bacteria. Photochem. Photobiol., 4: 813-817.
MCGOVERN, V. J. (1977) Epidemiological aspects of melanoma: A review.
Pathology, 9: 233-241.
MCGOVERN, V. J. & MACKIE, B. S. (1959) The relationship of solar
radiation to melanoblastoma. Aust. N. Z. J. Surg., 28: 257-262.
MEYER, A. E. H. & SEITZ, E. O. (1949) [Ultraviolet Radiation.]
Berlin, Walter de Gruyter & Co. (in German).
MILLS, L. F., LYTLE, C. D., ANDERSEN, F. A., HELLMAN, K. B., &
BOCKSTAHLER, L. H. (1975) A review of biological effects and
potential risks associated with ultraviolet radiation as used in
dentistry. pp. 1-27 (DHEW Publication (FDA) 76-8021).
MISHIMA, Y. & WIDLAN, S. (1967) Enzymatically active and inactive
melanocyte populations and ultraviolet irradiation: Combined
dopapremelanin reaction and electron microscopy. J. invest.
Dermatol., 49: 273.
MIYAJI, T. (1962) Skin cancer in Japan. A nationwide 5 year survey.
In: Urbach, F., ed. The biology of cutaneous cancer,
Washington, DC, US Government Printing Office, pp. 515-70
(Monograph No. 10, National Cancer Institute).
MIYAZAKI, H., KAWADA, A., TAKAKI, Y., SATO, T., & MASUTANI, M. (1968)
Influence of ultraviolet light on pigmentation of hairless mouse
epidermis. Jap. J. Dermatol., Ser. B, 78: 605.
MORENO, F. (1969) Contribution à l'étude de la létalité et de la
stérilité induites chez Drosophita melanogaster par irradiation
UV des cellules polaires de l'oeuf. I. Action létale, II. Action
stérilisante. Int. J. radiat. Biol., 16: 441-465.
MOUSTACCHI, E., WATERS, R., HEUDE, M., & CHANET, R. (1975 The present
status of DNA repair mechanisms in UV irradiated yeast taken as a
model eukaryotic system. In: Nygaard, O., Adler, H., & Sinclair,
W., ed. Radiation research: Biomedical, chemical and physical
perspectives, New York, Academic Press, pp. 632-650.
MOVSHOVITZ, M. & MODAN, B. (1973) Role of sun exposure in the etiology
of malignant melanoma: Epidemiologic inference. J. Natl Cancer
Inst., 51: 777-779.
MULAY, D. M. (1962) Skin Cancer in India. In: Urbach, F., ed.
The biology of cutaneous Cancer, Washington, DC, US Government
Printing Office, pp. 215-224 (Monograph No. 10, National Cancer
Institute).
MURPHY, T. M. (1975a) Inactivation of tobacco mosaic virus ribonucleic
acid by near- and middle-ultraviolet light: Sensitization by
sulfanilamide and chlortetracycline. Biochem. biophys. Res.
Commun., 65: 1108-1114.
MURPHY, T. M. (1975b) Effects of UV-B radiation on nucleic acids. In:
Impacts of climatic changes on the biosphere, pp. 19-40 (CIAP
Monograph No. 5).
MUSAJO, L., RODIGHIERO, G., CAPORALE, G., DALL'ACQUA, F., MARCIANI,
S., BORDIN, F., BACCICHETTI, F., & BEVILACQUA, R. (1974)
Photoreactions between skin-photosensitizing furocoumarins and
nucleic acids. In: Fitzpatrick, T. B., Pathak, M. A., Harber, L.
C., Seiji, M., & Kukita, A., ed. Sunlight and Man, Tokyo,
University of Tokyo Press, pp. 369-387.
NAKAMURA, K. & JOHNSON, W. C. (1968) Ultraviolet light-induced
connective tissues changes in rat skin: A histopathologic and
histochemical study. J. invest. Dermatol., 51: 253-258.
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1973) UV
photocarcinogenesis: Modification by anti-lymphocytic serum or
6-marcaptopurine. Proc. Am. Assoc. Cancer Res., 14: 46
(Abstract 182).
NATHANSON, R. B., FORBES, P. D., & URBACH, F. (1976) Modification of
photocarcinogenesis by two immunosuppressive agents. Cancer
Lett., 1: 243-247.
NILENDER, R. A. & GAVANIN, V. A. (1971) [Artificial sources of
ultraviolet radiation.] In: [UV Radiation.] Moscow, Medicina,
pp. 348-352 (in Russian).
NIOSH (1975) Ultraviolet transfer standard detectors and evaluation
and calibration of NIOSH UV hazard monitor. Cincinnati, OH, US
Department of Health, Education, and Welfare, Public Health
Service, Center for Disease Control, National Institute for
Occupational Safety and
Health, Division of Laboratories and Criteria Development, 12 pp. (HEW
Publication No. (NIOSH) 75-131).
NORBURY, K. C., KRIPKE, M. L., & BUDMEN, M. B. (1977) In vitro
reactivity of macrophages and lymphocytes from
ultraviolet-irradiated mice. J. Natl Cancer Inst.,
59: 1231-1235.
OETTLE, A. G. (1962) Skin cancer in Africa. In: Urbach, F, ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 197-214 (Monograph No. 10, National Cancer
Institute).
ORR, J. W. (1938) The changes antecedent to tumor formation during the
treatment of mouse skin with carcinogenic hydrocarbons.
J. Pathol. Bacteriol., 46: 495-515.
OWENS, D. W., KNOX, J. M., HUDSON, H. T., RUDOLPH, A. H., & TROLL, D.
(1977) Influence of wind on chronic ultraviolet light-induced
carcinogenesis. Br. J. Dermatol., 97: 285.
PAINTER, R. B. (1975) Repair in mammalian cells: Overview. In:
Hanawalt, P. C. & Setlow, R. B., ed. Molecular mechamisms for
repair of DNA, New York & London, Plenum Press, pp. 595-600.
PARRINGTON, J. M. (1972) Ultraviolet-induced chromosome aberration and
mitotic delay in human fibroblast cells. Cytogenetics,
11: 117-131.
PARRISH, J. A., FITZPATRICK, T. B., TANNENBAUM, L., & PATHAK, M. A.
(1974) Photochemotherapy of psoriasis with oral methoxsalen and
long wave ultraviolet light. New Engl. J. Med. 291: 1207-1212.
PATHAK, M. A., KRAMER, D. M., & FITZPATRICK, T. B. (1974) Photobiology
and photochemistry of furocoumarins (psoralens). In: Fitzpatrick,
T. B., Pathak, M. A., Harber, L. C., Seiji, M., & Kukita, A., ed.
Sunlight and Man, Tokyo, University of Tokyo Press,
pp. 335-368.
PETTIJOHN, D. & HANAWALT, P. (1964) Evidence for repair-replication of
ultraviolet damaged DNA in bacteria. J. mol. Biol., 9: 395-410.
PITTS, D. G. (1970) A comparative study of the effects of ultraviolet
radiation on the eye. Am. J. Optom., 47: 535-546.
PITTS, D. G. & CULLEN, A. P. (1977) Ocular ultraviolet effects from
300 nm to 400 nm: A preliminary report. Cincinatti, OH, US
Department of Health, Education and Welfare, National Institute
of Occupational Safety and Health, Division of Biomedical and
Behavioral Science (NIOSH Contract CDC-99-74-12).
PITTS, D. G. & KAY, K. R. (1969) The photo-ophthalmic threshold for
the rabbit. Am. J. Optom., 46: 561-572.
POLLARD, E. C. (1974) Cellular and molecular effects of solar
ultraviolet radiation. Photochem. Photobiol., 20: 301-308.
PROKOPENKO, Ju, I. (1976) [Some data on the mechanisms of protective
effect of UV-radiation.] Gig. i Sanit., 1: 100-102 (in
Russian).
PROKOPENKO, Ju, I. & ZABALUEVA, A. P. (1975) [Activation of
sanogenesis mechanisms with the help of UV-irradiation.] In:
[The biological effect of UV-radiation,] Moscow, Nauka,
pp. 156-159 (in Russian).
PUTSCHAR, W. & HOLTZ, F. (1930) [Induction of skin cancer in rats by
prolonged ultraviolet irradiation.] Z. Krebsforsch.,
23: 219-232 (in German).
QUEVEDO, W. C. Jr & SMITH, J. A. (1963) Studies on radiation-induced
tanning of skin. Ann. N. Y. Acad. Sci., 100: 364.
QUEVEDO, W. C. Jr, BRENNER, R. M., & KECHIJIAN, P. (1963) Melanocyte
performance during radiation induced tanning of murine skin.
Proceedings of the XVI International Congress on Zoology, 2: 306.
QUEVEDO, W. C. Jr, SZABO, G., VIRKS, J., & SINESI, S. J. (1965)
Melanocyte populations in UV-irradiated human skin. J. invest.
Dermatol., 45: 295.
RADMAN, M. (1975) SOS repair hypothesis: Phenomenology of an inducible
DNA repair which is accompanied by mutagenesis. In: Hanawalt, P.
C. & Setlow, R. B., ed. Molecular mechanisms for repair of DNA,
New York & London, Plenum Press, pp. 355-367.
RAPPAPORT, H., PIETRA, G., & SHUBIK, P. (1961) The induction of
melanotic tumors resembling cellular blue nevi in the Syrian
white hamster by cutaneous application of 7,
12-Dimethylbenz(a)anthracene. Cancer Res., 21: 661-666.
RASMUSSEN, R. E. & PAINTER, R. B. (1964) Evidence for repair of
ultraviolet damaged deoxyribonucleic acid in cultured mammal
cells. Nature (Lond.), 203: 1360-1362.
RAUTH, A. M. (1970) Effects of ultraviolet light on mammalian cells in
culture. Curr. Top. radiat. Res., 6: 195-248.
RAUTH, A. M. & DOMON, M. (1973) Potentiation of ultraviolet light
damage in mouse L cells by 1-cyclohexyl-3-(2-morpholinyl-4-et
carbodiimide metho-p-toluene sulphonate (CMEC). Int. J. radiat.
Biol., 24: 189-198.
RESNICK, M. A. (1969) Genetic control of radiation sensitivity in
Saccharomyces cerevisiae. Genetics, 62: 519-531.
REYNOLDS, J. (1954) The epidermal melanocytes of mice. J. Anat.,
88: 45.
ROBERTSON, D. F. (1969) Long term field measurements of erythemally
effective natural ultraviolet radiation. In: Urbach, F., ed.
The biologic effects of ultraviolet radiation, Pergamon
Press, Oxford, pp. 433-436.
ROBERTSON, D. F. (1972) Solar ultraviolet radiation in relation to
human sunburn and skin cancer. Thesis, Australia, University of
Queensland.
ROBERTSON, D. F. (1975) Calculated sunburn responses, In: Impacts of
climatic change on the biosphere. Springfield, Virginia,
National Technical Information Service, Part 1, Chapter 2,
Appendix J. (CIAP Monograph 5).
ROFFO, A. H. (1934) Cancer et soleil. Carcinomes et sarcomes provoqués
par l'action du soleil in toto. Bull. Assoc. Fr. Etud. Cancer,
53: 59.
ROMMELAERE, J., SUSSKIND, M., & ERRERA, M. (1973) Chromosome and
chromatid exchanges in Chinese hamster cells. Chromosoma,
41: 243-257.
RUNDEL, R. D. & NACHTWEY, D. S. (1978) Skin cancer and ultraviolet
radiation. Photochem. Photobiol., 28: 345-356.
RONGE, H. E. (1948) Ultraviolet irradiation with artificial
illumination Acta Phys. Scand., 15 (Suppl. 49): 1-191.
RUPERT, C. S. (1975) Enzymatic photoreactivation: Overview. In:
Hanawalt, P. C. & SETLOW, R. B. ed. Molecular mechanisms for
repair of DNA, New York & London, Plenum Press, pp. 73-87.
RUPP, W. D. & HOWARD-FLANDERS, P, (1968) Discontinuities in the DNA
synthesized in an excision-defective strain of E. coli
following ultraviolet irradiation. J. Mol. Biol., 31: 291-304.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1941) Carcinogenesis by
ultraviolet rays with reference to wavelength and energy. Arch.
Pathol., 371: 135-148.
RUSCH, H. P., KLINE, B. E., & BAUMANN, C. A. (1942) Nonadditive effect
of UV light and other carcinogenic procedures. Cancer Res.,
2: 183-188.
RUSTAD, R. C. (1972) Techniques for the analysis of radiation-induced
mitotic delay in synchronously dividing sea urchin eggs. In:
Whitson, G. L., ed. Concepts in radiation cell biology, New
York & London, Academic Press, pp. 153-181.
SAMS, W. M. Jr, SMITH, J. G., & BURK, P. G. (1964) The experimental
production of elastosis with ultraviolet light. J. invest.
Dermatol., 43: 467.
SATO, T. (1971) Pigmentation induced by ultraviolet irradiation in
hairless mice with particular references to epidermal dendritic
cells. Jap. J. Dermatol. Ser. A, 81: 488.
SATO, T. & KAWADA, A. (1972) Mitotic activity of hairless mouse
epidermal melanocytes: Its role in the increase of melanocytes
during ultraviolet radiation. J. invest. Dermatol.,
58: 392-395.
SATO, T. & KAEADA, A. (1972) Uptake of tritiated thymidine by
epidermal melanocytes of hairless mice during ultraviolet light
radiation. J. invest. Dermatol., 58: 71.
SAUERBIER, W. (1976) UV damage at the transcriptional level. Advances
in radiation biology, 6: 50-106.
SAYENKO, A. J. (1968) A method for studying morbidity from
precancerous conditions and the question as to frequency of their
occurrence on the territory of the Kirghiz Soviet Socialist
Republic. Neoplasma, 15: 565-571.
SCHULZE, R. (1970) [Global Radiation Climate.] Wiss. Forschungsber.,
72: 220 pp. (in German).
SCOTTO, J., KOPF, A. W., & URBACH, F. (1974) Non-melanoma skin cancer
among Caucasians in four areas of the United States. Cancer,
34: 1333-1338.
SCOTTO, J., FEARS, T. R., & GORI, G. B. (1976) Measurements of
ultraviolet radiation in the United States and comparisons with
skin cancer data, National Cancer Institute (DHEW
No. (NIH) 76-1029).
SEIDL, E. (1969) Blitzgerät therapy. In: Urbach, F., ed. The biologic
effects of ultraviolet radiation, Oxford, Pergamon Press,
pp. 663-672.
SETLOW, R. B. (1968) The photochemistry, photobiology, and repair of
polynucleotides. Prog. N. A. Res. Mol. Biol., 8: 257-295.
SETLOW, R. B. (1973) The relevance of photobiological repair.
An. Acad. Bras. Ciencas, 45: 215-220.
SETLOW, R. B. (1974) The wavelengths in sunlight effective in
producing skin cancer: A theoretical analysis. Proc. Natl Acad.
Sci., 71: 3363-3366.
SETLOW, R. B. & CARRIER, W. L. (1964) The disappearance of thymine
dimers from DNA: An error-correcting mechanism. Proc. Natl Acad.
Sci., 51: 226-231. p. 34.
SETLOW, J. K. & DUGGAN, D. E. (1964) The resistance of Micrococcus
radiodurans to ultraviolet radiation. Biochim. Biophys. Acta,
87: 664-668.
SETLOW, J. K. & SETLOW, R. B. (1963) Nature of the photoreactivability
ultraviolet lesion in deoxyribonucleic acid. Nature (Lond.),
197: 560-562. p 36.
SHUBIK, P., PIETRA, G., & DELLA PORTA, G. (1960) Studies of skin
carcinogenesis in the Syrian golden hamster. Cancer Res.,
20: 1.
SILVERSTONE, H. & SEARLE, J. H. A. (1970) The epidemiology of skin
cancer in Queensland: The influence of phenotype and environment.
Br. J. Cancer, 24: 235-252.
SKREB, Y., HORVAT, D., & EGER, M. (1972) Modifications of
radiosensitivity in nucleate and anucleate amoeba fragments. In:
Bonotto, S., Goutier, R., Kirchmann, R., & Maisin, J.-R., ed.
Biology and radiobiology of anucleate systems I. New York,
Academic Press, pp. 67-82.
SMITH, K. C. (1974) The cellular repair of radiation damage. In:
Fitzpatrick, T. B., Pathak, M. A., Harber, L. C., Seiji M., &
Kukita, A., ed. Sunlight and Man, Tokyo, University of Tokyo
Press, pp. 67-77.
SMITH, K. C. (1975) The radiation-induced addition of proteins and
other molecules to nucleic acids. In: Wang, S. Y., ed.
Photochemistry and photobiology of nucleic acids, New York,
Academic Press, Vol. II, pp. 187-218.
SMITH, K. C. & HANAWALT, P. C. (1969) Molecular photobiology.
Inactivation and recovery, New York & London, Academic Press.
SOFFEN, G. A. & BLUM, H. F. (1961) Quantitative measurements of cell
changes in mouse skin following a single dose of ultraviolet
light. J. cell. comp. Physiol., 58: 81-96.
SOKOLOV, M. V. (1975) [Standardization of UV photometry.] In:
[The biological effects of ultraviolet radiation.] Moscow,
Nauka, pp. 232-235 (in Russian).
SOKOLOV, M. V. (1976) [Topical questions of UV biophotometry.]
Svetotecnika, 2: 20-22 (in Russian).
SOZIN, D. S. (1975) [Low pressure fluorescent lamps with combined
radiation.] In: [The biological effects of ultraviolet
radiation.] Moscow, Nauka, pp. 225-226 (in Russian).
SPIKES, J. D., KRIPKE, M. L., CONNOR, R. J., & EICHWALD, E. J. (1977)
Time of appearance and histology of tumors induced in the dorsal
skin of C3Hf mice by ultraviolet radiation from a mercury arc
lamp. J. Natl Cancer Inst. 59: 1637-1643.
STATE STANDARDS (1964) [Recommendations for the prevention of
ultraviolet deficiency.] Moscow Nauka, 50 pp. (in Russian).
STATE STANDARDS (1976a) [Regulations for the design and operation of
artificial UV-irradiation installations in industrial
undertakings.] In: [Regulations on the design of electrical
engineering installations for industry.] Energija 6: pp. 30-39
(in Russian).
STATE STANDARDS (1976b) [USSR building norms and regulations.]
Strijizdat, Moscow, p. 20 (SNIP 11-60-75) (in Russian).
STENBÄCK, F. (1969) Promotion in the morphogenesis of chemically
inducible skin tumors. Acta path. microbiol. Scand. Suppl.,
208: 1-116.
STENBÄCK, F. (1975a) Cellular injury and cell proliferation in skin
carcinogenesis by UV light. Oncology, : 31: 61-65.
STENBÄCK, F. (1975b) Species-specific neoplastic progression by
ultraviolet light on the skin of rats, guinea pigs, hamsters and
mice. Oncology, 31: 209-225.
STENBÄCK, F. (1975) Ultraviolet light irradiation as initiating agent
in skin tumor formation by the two-stage method. Europ. J.
Cancer, 11: 241-246.
STENBÄCK, F. (1978) Life history and histopathology of ultraviolet
light-induced skin tumors. In: International Conference on
Ultraviolet Carcinogenesis. Washington, DC, US Government
Printing Office, pp. 57-70 (Monograph No. 50, National Cancer
Institute).
SUTHERLAND, B. M. (1974) Photoreactivating enzyme from human
leukocytes. Nature (Lond.), 109-112.
SVIDERSKAJA, T. A. (1971) [Some experimental data on the conditions
accompanying an intensification of blastomogenic effect.] In:
[UV Radiation.] Moscow, Medicina, pp. 260-264 (in Russian).
SWANBECK, G. & HILLSTRöM, L. (1971) Analysis of etiological factors of
squamous cell skin cancer of different locations. Acta
Dermatovener, 51: 151-156.
TALANOVA, I. K. & ZABALUEVA, A. P. (1972) [Evaluation of the extent to
which children are provided with UV radiation in middle and
northern latitudes.] In: [Questions of experimental and clinical
spa treatment and physiotherapy.] 20: 323-329 (in Russian).
TALANOVA, I. K., KARACEVCEVA, T. V., & IVANOV, V. G. (1975) [The
combination of physical methods of prophylaxis and specific
immunization in children.] In: [The biological effects of UV
radiation.] Moscow, Nauka, pp. 116-120 (in Russian).
TODD, P. & HAN, A. (1976) Ultraviolet light induced
DNA-to-protein-cross-linking in mammalian cells. In: Smith, K.
C., ed. Aging, carcinogenesis and radiation-biology: The role of
nucleic acid addition reactions, New York & London, Plenum
Press, pp. 83-104.
TROSKO, J. E. & CHU, E. H. (1973) Inhibition of repair of UV-damaged
DNA by caffeine and mutation induction in Chinese hamster cells.
Chem. Biol. Interactions, 6: 317-332.
TYRRELL, R. M. (1973) Induction of pyrimidine dimers in bacterial DNA
by 365 nm radiation Photochem. Photobiol., 17: 69-73.
URBACH, F. (1969) The biologic effect of ultraviolet radiation.
Oxford, Pergamon Press, pp. 704.
URBACH, F., ROSE, D. B., & BONNEM, M. (1972) Genetic and environmental
interactions in skin carcinogenesis. In: Environment and skin
cancer, Baltimore, Maryland, Williams & Wilkins Co.,
pp. 355-371.
VARGHESE, A. J. (1973) Properties of photoaddition products of thymine
and cysteine. Biochemistry, 12 (14): 2725-2730.
VERHOEFF, F. H., BELL, L., & WALKER, C. B. (1916) The pathological
effects of radiant energy on the eye: An experimental
investigation with a systematic review of the (literature. Proc.
Am. Acad. Arts Sci., 51: 630-818.
VITALIANO, P. P. (1978) The use of logistic regression for modelling
risk factors in the development of nonmelanoma skin cancer.
Am. J. Epidemiol., 108: 402-414.
WANG, R. J. (1975) Lethal effect of »daylights» fluorescent light on
human cells in tissue-culture medium. Photochem. Photobiol.,
21: 373-375.
WANG, R. J., STOIEN, J. D., & LANDA, F. (1974) Lethal effect of
near-ultraviolet irradiation on mammalian cells in culture.
Nature (Lond.), 247: 43-45.
WATERHOUSE, J., MUIR, C., CORREA, P., & POWELL, J. (1976) Cancer
incidence in five continents, Lyons, IARC (Vol. III).
WHITSON, G. L. (1972) Radiation-induced biochemical changes in
protozoa. In: Whitson, G. L., ed. Concepts in radiation cell
biology, New York, Academic Press, pp. 123-152.
WINKELMAN, R. K., BALDES, E. J., & ZOLLMAN, P. E. (1960) Squamous cell
tumors induced in hairless mice with ultraviolet light.
J. invest. Dermatol., 34: 131-138.
WINKELMANN, R. K., ZOLLMAN, P. E., & BALDES, E. J. (1965) Squamous
cell carcinoma produced by ultraviolet light in hairless mice.
J. invest. Dermatol., 40: 217-224.
WITKIN, E. M. (1969) Ultraviolet-induced mutation and DNA repair.
Ann. Rev. Genet., 3: 525-552.
WITKIN, E. M. (1976) Ultraviolet mutagenesis and inducible DNA repair
in Escherichia coli. Bacteriol. Rev., 40: 869-907.
WOODCOCK, A. & MAGNUS, I. A. (1976) The sunburn cell on mouse skin:
Preliminary quantitative studies on its production. Br. J.
Dermatol., 95: 459-468.
YEH, S. (1962) Relative incidence of skin cancer in Chinese in Taiwan:
With special reference to arsenical cancer. In: Urbach, F., ed.
The biology of cutaneous cancer, Washington, DC, US Government
Printing Office, pp. 81-108 (Monograph No. 10, National Cancer
Institute).
ZACKHEIM, H. S. (1964) Comparative cutaneous carcinogenesis in the
rat. Differential response to the application of anthramine,
methylcholanthrene, and dimethylbenzanthacene. Oncology,
17: 236-246.
ZIGMAN, S., SCHULTZ, J. B., & YULO, T. (1973) Possible roles of near
UV light in the cataractous process. Exp. eye Res.,
15: 201-208.
ZILOV, JU.D. (1971) [The prophylactic irradiation of children and
adolscents living in different climatic zones of the Soviet
Union.] In: [Ultraviolet radiation.] Moscow, Medicina,
pp. 237-241 (in Russian).
ZIVILOVA, N. D., ISTJUFEEV, V. A., SMIRNOV, E. S., & LEVITIN Ju.S.
(1975) [The UFM-meter and the UFB-72 laktmeter.] In: [The
biological effect of UV-radiation.] Moscow, Nauka, pp. 247-249
(in Russian).
ZWEIG, A. & HENDERSON, W. A. Jr (1976) A photochemical mid-ultraviolet
dosimeter. Photochem. Photobiol., 24: 543-549.
