
This report contains the collective views of an international group of experts and does not necessarily represent the decisions or the stated policy of the United Nations Environment Programme, the International Labour Organization, or the World Health Organization.
Concise International Chemical Assessment Document 60
CHLOROBENZENES OTHER THAN HEXACHLOROBENZENE:
ENVIRONMENTAL ASPECTS
First draft prepared by H.M. Malcolm, P.D. Howe, and S. Dobson, Centre for Ecology
and Hydrology, Monks Wood, United Kingdom
Published under the joint sponsorship of the United Nations Environment Programme, the International Labour Organization, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2004
The International Programme on Chemical Safety (IPCS), established in 1980, is a joint venture of the United Nations Environment Programme (UNEP), the International Labour Organization (ILO), and the World Health Organization (WHO). The overall objectives of the IPCS are to establish the scientific basis for assessment of the risk to human health and the environment from exposure to chemicals, through international peer review processes, as a prerequisite for the promotion of chemical safety, and to provide technical assistance in strengthening national capacities for the sound management of chemicals.
The Inter-Organization Programme for the Sound Management of Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and Agriculture Organization of the United Nations, WHO, the United Nations Industrial Development Organization, the United Nations Institute for Training and Research, and the Organisation for Economic Co-operation and Development (Participating Organizations), following recommendations made by the 1992 UN Conference on Environment and Development to strengthen cooperation and increase coordination in the field of chemical safety. The purpose of the IOMC is to promote coordination of the policies and activities pursued by the Participating Organizations, jointly or separately, to achieve the sound management of chemicals in relation to human health and the environment.
WHO Library Cataloguing-in-Publication Data
Chlorobenzenes other than hexachlorobenzene : environmental aspects.
(Concise international chemical assessment document ; 60)
1.Chlorobenzenes 2.Risk assessment 3.Environmental exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153060 X (LC/NLM Classification: QV 633)
ISSN 1020-6167
©World Health Organization 2004
All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: bookorders@who.int). Requests for permission to reproduce or translate WHO publications — whether for sale or for noncommercial distribution — should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: permissions@who.int).
The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement.
The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters.
The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.
Risk assessment activities of the International Programme on Chemical Safety, including the production of Concise International Chemical Assessment Documents, are supported financially by the Department of Health and Department for Environment, Food & Rural Affairs, UK, Environmental Protection Agency, Food and Drug Administration, and National Institute of Environmental Health Sciences, USA, European Commission, German Federal Ministry of Environment, Nature Conservation and Nuclear Safety, Health Canada, Japanese Ministry of Health, Labour and Welfare, and the Swiss Agency for Environment, Forests and Landscape.
Technically and linguistically edited by Marla Sheffer, Ottawa, Canada, and printed by Wissenchaftliche Verlagsgesellschaft mbH, Stuttgart, Germany
|
5.0 ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION |
Concise International Chemical Assessment Documents (CICADs) are the latest in a family of publications from the International Programme on Chemical Safety (IPCS) — a cooperative programme of the World Health Organization (WHO), the International Labour Organization (ILO), and the United Nations Environment Programme (UNEP). CICADs join the Environmental Health Criteria documents (EHCs) as authoritative documents on the risk assessment of chemicals.
International Chemical Safety Cards on the relevant chemical(s) are attached at the end of the CICAD, to provide the reader with concise information on the protection of human health and on emergency action. They are produced in a separate peer-reviewed procedure at IPCS. They may be complemented by information from IPCS Poison Information Monographs (PIM), similarly produced separately from the CICAD process.
CICADs are concise documents that provide summaries of the relevant scientific information concerning the potential effects of chemicals upon human health and/or the environment. They are usually based on selected national or regional evaluation documents or on existing EHCs. Before acceptance for publication as CICADs by IPCS, these documents undergo extensive peer review by internationally selected experts to ensure their completeness, accuracy in the way in which the original data are represented, and the validity of the conclusions drawn.
The primary objective of CICADs is characterization of hazard and dose–response from exposure to a chemical. CICADs are not a summary of all available data on a particular chemical; rather, they include only that information considered critical for characterization of the risk posed by the chemical. The critical studies are, however, presented in sufficient detail to support the conclusions drawn. For additional information, the reader should consult the identified source documents upon which the CICAD has been based.
Risks to human health and the environment will vary considerably depending upon the type and extent of exposure. Responsible authorities are strongly encouraged to characterize risk on the basis of locally measured or predicted exposure scenarios. To assist the reader, examples of exposure estimation and risk characterization are provided in CICADs, whenever possible. These examples cannot be considered as representing all possible exposure situations, but are provided as guidance only. The reader is referred to EHC 170.1
While every effort is made to ensure that CICADs represent the current status of knowledge, new information is being developed constantly. Unless otherwise stated, CICADs are based on a search of the scientific literature to the date shown in the executive summary. In the event that a reader becomes aware of new information that would change the conclusions drawn in a CICAD, the reader is requested to contact IPCS to inform it of the new information.
Procedures
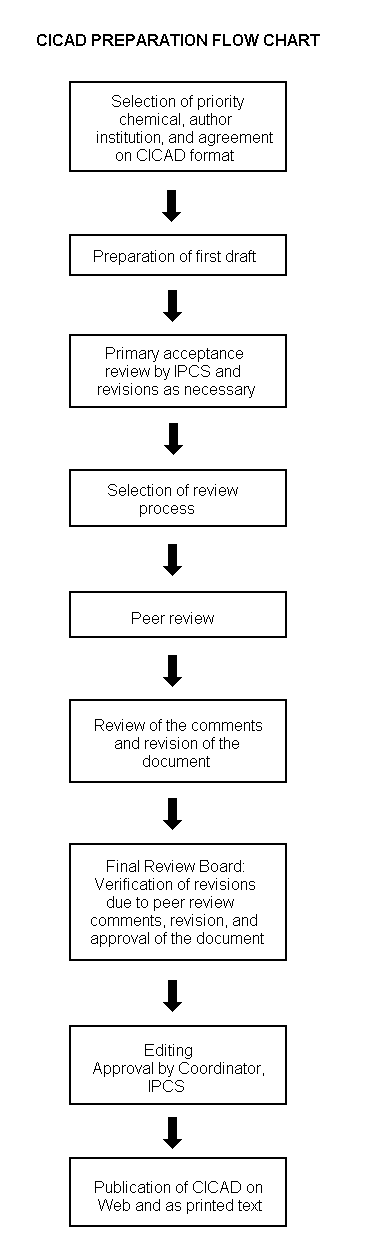
The flow chart on page 2 shows the procedures followed to produce a CICAD. These procedures are designed to take advantage of the expertise that exists around the world — expertise that is required to produce the high-quality evaluations of toxicological, exposure, and other data that are necessary for assessing risks to human health and/or the environment. The IPCS Risk Assessment Steering Group advises the Coordinator, IPCS, on the selection of chemicals for an IPCS risk assessment based on the following criteria:
Thus, it is typical of a priority chemical that

|
Advice from Risk Assessment Steering Group Criteria of priority:
Thus, it is typical of a priority chemical that
Special emphasis is placed on avoiding duplication of effort by WHO and other international organizations. A prerequisite of the production of a CICAD is the availability of a recent high-quality national/regional risk assessment document = source document. The source document and the CICAD may be produced in parallel. If the source document does not contain an environmental section, this may be produced de novo, provided it is not controversial. If no source document is available, IPCS may produce a de novo risk assessment document if the cost is justified. Depending on the complexity and extent of controversy of the issues involved, the steering group may advise on different levels of peer review:
|
The Steering Group will also advise IPCS on the appropriate form of the document (i.e., a standard CICAD or a de novo CICAD) and which institution bears the responsibility of the document production, as well as on the type and extent of the international peer review.
The first draft is usually based on an existing national, regional, or international review. When no appropriate source document is available, a CICAD may be produced de novo. Authors of the first draft are usually, but not necessarily, from the institution that developed the original review. A standard outline has been developed to encourage consistency in form. The first draft undergoes primary review by IPCS to ensure that it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists known for their particular expertise and by scientists selected from an international roster compiled by IPCS through recommendations from IPCS national Contact Points and from IPCS Participating Institutions. Adequate time is allowed for the selected experts to undertake a thorough review. Authors are required to take reviewers’ comments into account and revise their draft, if necessary. The resulting second draft is submitted to a Final Review Board together with the reviewers’ comments. At any stage in the international review process, a consultative group may be necessary to address specific areas of the science. When a CICAD is prepared de novo, a consultative group is normally convened.
The CICAD Final Review Board has several important functions:
Board members serve in their personal capacity, not as representatives of any organization, government, or industry. They are selected because of their expertise in human and environmental toxicology or because of their experience in the regulation of chemicals. Boards are chosen according to the range of expertise required for a meeting and the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who participate in the preparation of a CICAD are required to declare any real or potential conflict of interest in relation to the subjects under discussion at any stage of the process. Representatives of nongovernmental organizations may be invited to observe the proceedings of the Final Review Board. Observers may participate in Board discussions only at the invitation of the Chairperson, and they may not participate in the final decision-making process.
This CICAD on chlorobenzenes other than hexachlorobenzene (environmental aspects) is an update of Environmental Health Criteria (EHC) 128, Chlorobenzenes other than hexachlorobenzene (IPCS, 1991a). Information on the fate and levels of chlorobenzenes was also obtained from Agency for Toxic Substances and Disease Registry reports on chlorobenzene (ATSDR, 1990) and 1,4-dichlorobenzene (ATSDR, 1998). A further literature search was performed up to December 2002 to identify any additional information published since these reviews were completed. Information on the peer review of the source document is presented in Appendix 1. Information on the peer review of this CICAD is presented in Appendix 2. This CICAD was approved as an international assessment at a meeting of the Final Review Board, held in Varna, Bulgaria, on 8–11 September 2003. Participants at the Final Review Board meeting are listed in Appendix 3. The International Chemical Safety Cards for a number of different chlorobenzenes (ICSC 0037, 0344, 0531, 0642, 0676, 1049, 1066, 1095, 1222), produced by the International Programme on Chemical Safety (IPCS, 2000, 2003a–h), have also been reproduced in this document. This CICAD concentrates on environmental aspects because there have been no significant changes to the human health assessment since publication of the EHC (IPCS, 1991a).
Chlorinated benzenes are a group of cyclic aromatic compounds in which one or more hydrogen atoms of the benzene ring have been replaced by a chlorine atom. Chlorobenzenes are used mainly as intermediates in the synthesis of pesticides and other chemicals. 1,4-Dichlorobenzene (1,4-DCB) is used in space deodorants and as a moth repellent. The higher chlorinated benzenes (trichlorobenzenes, 1,2,3,4-tetrachlorobenzene [1,2,3,4-TeCB], and pentachlorobenzene [PeCB]) have been used as components of dielectric fluids.
Natural sources of chlorobenzenes in the environment have not been identified. Chlorobenzenes are released to the environment during manufacture or use as intermediates in the production of other chemicals. They will also be released during the disposal of chlorobenzene products, such as from incinerators and hazardous waste sites. Monochlorobenzene (MCB) is released directly to the environment due to its use as a pesticide carrier. Chlorobenzenes used as deodorizers, fumigants, degreasers, insecticides, herbicides, and defoliants will also be released to the environment as a direct result of their application.
Their physicochemical properties suggest that chlorobenzenes released to the environment are likely to volatilize to the atmosphere. Removal of chlorobenzenes from the atmosphere will occur primarily via reactions with hydroxyl radicals to produce nitrochlorobenzene, chlorophenol, and aliphatic dicarbonyl products, which are further removed by photolysis or reaction with hydroxyl radicals. Chlorobenzenes released into the aquatic environment will be redistributed preferentially to the air and to sediment (particularly organically rich sediments). Chlorobenzenes in aqueous solutions could, in theory, undergo photochemical reductive dechlorination, although studies have been performed only under artificial conditions that were not representative of temperate regions. The most important factor affecting the behaviour and fate of chlorobenzenes in soil is sorption. Adsorption–desorption processes in soil affect the rate of volatilization and leaching and the availability of chemicals to microbial and chemical degradation or uptake by plants or other organisms.
Chlorobenzenes in various substrates, including soil, sediment, and sewage sludge, may be degraded by microorganisms. The major mechanism of aerobic degradation is via oxidative dechlorination, leading to the formation of hydroxylated aromatic compounds (mainly catechols), which undergo ring fission and subsequent mineralization to carbon dioxide and water. The less chlorinated benzenes are more readily degraded than the higher chlorinated ones.
The bioaccumulation of chlorobenzenes by aquatic organisms is determined by their relative water and lipid solubilities (thus reflecting the octanol/water partition coefficients) and the number of chlorine substitutions. Uptake from water increases with increasing chlorination and increasing temperature.
Concentrations of chlorobenzenes (MCB, dichlorobenzenes, and trichlorobenzenes) have been reported in ambient air, with mean concentrations in the order of 0.1 µg/m3 and maximum levels (at hazardous waste sites) of up to 100 µg/m3. Concentrations of chlorobenzenes in surface waters are generally in the ng/litre to µg/litre range, with maximum concentrations up to 0.2 mg/litre in areas close to industrial sources. Levels of chlorobenzenes in industrial wastewaters may be higher and vary according to the nature of the processes used. Chlorobenzene levels in uncontaminated soils are generally less than 0.4 mg/kg for dichlorobenzene congeners and less than 0.1 mg/kg for other chlorobenzene congeners. Levels of chlorobenzenes in sediments are generally in the ng/kg to µg/kg range, although levels in the mg/kg range have been reported in samples from industrial areas.
In general, aquatic toxicity increases with the degree of chlorination of the benzene ring. Seventy-two-hour EC50s for green algae range from 5280 µg/litre for 1,3-DCB to 200 000 µg/litre for MCB; similarly, 48-h EC50s for diatoms range from 8 to 235 000 µg/litre. For freshwater invertebrates, 48-h EC50s range from 10 µg/litre for PeCB to >530 000 µg/litre for 1,2,4,5-TeCB. Ninety-six-hour LC50s for fish range from 135 µg/litre for PeCB to 21 000 µg/litre for 1,2,4-trichlorobenzene (1,2,4-TCB). Chronic no-observed-effect concentrations (NOECs) for freshwater invertebrates range from 32 µg/litre for PeCB to 19 000 µg/litre for MCB; in fish, NOECs range from 18 µg/litre for PeCB to 8500 µg/litre for MCB.
Few data are available on the effects of chlorobenzenes on terrestrial systems. LC50 values for plants grown hydroponically or in soil ranged from 0.028 to 9.3 mg/litre and from 1 to >1000 mg/kg soil, respectively. LC50 values for the earthworms Eisenia andrei and Lumbricus rubellus ranged from 0.22 µmol/litre (pore water) for PeCB to 4281 µmol/litre for MCB.
The risk of chlorinated benzenes causing harm to aquatic organisms is low. Risk factors comparing chronic toxicity values with concentrations measured in the environment were generally below 1, with the exception of some compounds that had higher risk factors, with a maximum value of 200. The highest risk factors were derived using old data from point sources and are therefore unrepresentative of the whole environment, especially when the likelihood of evaporation is considered. There were inadequate data to perform a risk assessment for terrestrial species.
Chlorinated benzenes are a group of cyclic aromatic compounds in which one or more hydrogen atoms of the benzene ring have been replaced by a chlorine atom. The generic molecular formula is C6H6–nCln, where n = 1–6. There are 12 different chlorinated benzenes: monochlorobenzene (MCB), dichlorobenzene (DCB) (three isomers), trichlorobenzene (TCB) (three isomers), tetrachlorobenzene (TeCB) (three isomers), pentachlorobenzene (PeCB), and hexachlorobenzene. Hexachlorobenzene is reviewed in a separate EHC (IPCS, 1997) and is therefore not covered by this CICAD.
The identity of chlorobenzenes and their physical and chemical properties are presented in Table 1. MCB, 1,2-DCB, 1,3-DCB, and 1,2,4-TCB are colourless liquids, while all other congeners are white crystalline solids at room temperature. In general, the solubility of chlorobenzenes in water is low (decreasing with increasing chlorination), flammability is low, the octanol/water partition coefficients are moderate to high (increasing with increasing chlorination), and vapour pressures are low to moderate (decreasing with increasing chlorination) (IPCS, 1991a).
Table 1: Physicochemical properties of chlorobenzenes.a
|
Chlorinated benzene |
Abbreviation |
CAS No. |
Molecular formula |
Relative molecular mass |
Melting point (°C) |
Boiling pointb |
Vapour pressure at 25 °C (Pa) |
Aqueous solubility at 25 °C (mg/litre) |
Henry’s law constant (kPa·m3/mol) |
Log octanol/ water partition coefficient (Kow) |
Soil sorption coefficient (Koc) |
|
Monochlorobenzene |
MCB |
|
C6H5Cl |
112.6 |
−45.6 |
132.0 |
1665 |
293 |
0.377 |
2.98 |
466 |
|
1,2-Dichlorobenzene |
1,2-DCB |
|
C6H4Cl2 |
147.0 |
−17.0 |
180.5 |
197 |
91.1 |
0.198 |
3.38 |
987 |
|
1,3-Dichlorobenzene |
1,3-DCB |
|
C6H4Cl2 |
147.0 |
−24.7 |
173.0 |
269 |
123 |
0.366 |
3.48 |
1070 |
|
1,4-Dichlorobenzene |
1,4-DCB |
|
C6H4Cl2 |
147.0 |
53.1 |
174.0 |
90 |
30.9 |
0.160 |
3.38 |
1470 |
|
1,2,3-Trichlorobenzene |
1,2,3-TCB |
|
C6H3Cl3 |
181.5 |
53.5 |
218.5 |
17.3 |
12.2 |
0.306 |
4.04 |
3680 |
|
1,2,4-Trichlorobenzene |
1,2,4-TCB |
|
C6H3Cl3 |
181.5 |
17.0 |
213.5 |
45.3 |
45.3 |
0.439 |
3.98 |
2670 |
|
1,3,5-Trichlorobenzene |
1,3,5-TCB |
|
C6H3Cl3 |
181.5 |
63.5 |
208.0 |
24.0 |
3.99 |
0.233 |
4.02 |
NAc |
|
1,2,3,4-Tetrachlorobenzene |
1,2,3,4-TeCB |
|
C6H2Cl4 |
215.9 |
47.5 |
254.0 |
5.2 |
12.1 |
0.261 |
4.55 |
NA |
|
1,2,3,5-Tetrachlorobenzene |
1,2,3,5-TeCB |
|
C6H2Cl4 |
215.9 |
54.5 |
246.0 |
9.8 |
2.81 |
0.593 |
4.65 |
8560 |
|
1,2,4,5-Tetrachlorobenzene |
1,2,4,5-TeCB |
|
C6H2Cl4 |
215.9 |
139.5 |
243.6 |
0.72 |
2.16 |
0.261 |
4.51 |
6990 |
|
Pentachlorobenzene |
PeCB |
|
C6HCl5 |
250.3 |
86.0 |
277.0 |
133d |
0.83 |
0.977 |
5.03 |
58 700 |
|
a |
From IPCS (1991a). |
|
b |
Calculated at atmospheric pressure (101.3 kPa), except for 1,3,5-TCB, which was at 93.5 kPa. |
|
c |
NA = not available. |
|
d |
Calculated at 98 °C. |
The analytical technique of choice for the determination of chlorobenzenes in environmental samples is gas chromatography (GC). However, the methods of collection and preparation of samples for GC analysis vary considerably, depending on the medium and the laboratory. Capillary columns with different stationary phases are frequently used to separate compounds. Detection occurs via the use of a flame ionization detector (FID), electron capture detector (ECD), or mass spectrometric (MS) detector (IPCS, 1991a).
Tenax-GC resins have commonly been used as adsorbents for the air sampling of chlorobenzenes (Krost et al., 1982; Pellizzari et al., 1982), although XAD resins have also been used (Langhorst & Nestrick, 1979). Air pollutants collected on Tenax-GC resins can be desorbed directly onto the GC column by heating the tube with sorbent. XAD resins can be extracted with solvents, an aliquot of which can then be injected into a GC. Detection limits in the 1970s ranged from 0.7 µg/m3 for MCB to 0.9 µg/m3 for PeCB (Langhorst & Nestrick, 1979); however, much lower detection limits have been achieved more recently using ECD (0.5 pg/m3 for PeCB to 1.8 pg/m3 for 1,2,4,5-TeCB) (Hermanson et al., 1997).
Solvent extraction is a simple and effective technique for recovering chlorobenzenes from water samples. Hexane, pentane, and a 1:1 mixture of cyclohexane and diethyl ether have been identified as suitable extraction solvents for these compounds (Oliver & Bothen, 1980; Piet et al., 1980; Otson & Williams, 1981; Meharg et al., 2000). Alternatively, preconcentration of the chlorobenzenes on organic resins, such as Chromosorb 102 and Tenax-GC, is also effective; detection limits using Chromosorb 102 were reported to range from 0.5 µg/litre for MCB to 0.01 ng/litre for PeCB (Oliver & Bothen, 1980; Pankow & Isabelle, 1982). The purge-and-trap method has also been used to concentrate the volatile halogenated benzenes before analysis using GC (Jungclaus et al., 1978; Pereira & Hughes, 1980; Otson & Williams, 1982; Huybrechts et al., 2000; Martinez et al., 2002). Detection limits of 0.1–0.2 µg/litre for MCB and various dichlorobenzene isomers were achieved using FID and Hall electrolyte conductivity detectors (Otson & Williams, 1982), 0.08 µg/litre for 1,2,4-TCB using ECD (Martinez et al., 2002), and 0.76–20 ng/litre for di- and trichlorobenzenes using MS (Huybrechts et al., 2000). More recently, alternative extraction techniques such as headspace solid-phase microextraction with GC-MS have achieved detection limits for individual chlorobenzene isomers ranging from 4 to 6 ng/litre (He et al., 2000); however, it should be noted that analytical techniques using simple solvent extraction and GC-MS can now attain detection limits ranging from 5 pg/litre for 1,2,3- and 1,3,5-TCB to 15 pg/litre for PeCB (Meharg et al., 2000).
The extraction of chlorobenzenes from aquatic sediments, sewage sludges, or soil can be achieved by solvent or Soxhlet extraction (Oliver & Bothen, 1982; Lopez-Avila et al., 1983; Onuska & Terry, 1985; Wang & Jones, 1991; Wang et al., 1992). Solvents commonly used are acetone and/or hexane. Other extraction methods, such as sonication, saponification, and supercritical fluid extraction, have been used to extract sediment-bound chlorobenzenes, but were found to be less efficient than Soxhlet extraction (Prytula & Pavlostathis, 1996). The extract is generally dried using sodium sulfate, followed by cleanup on a Florisil column before GC analysis with ECD, with detection limits of 1500 µg/kg for MCB and lower detection limits ranging from 1.5 µg/kg for dichlorobenzenes to 0.05 µg/kg for PeCB (Oliver & Bothen, 1982; Onuska & Terry, 1985; Wang & Jones, 1991; Wang et al., 1992). Alternatively, headspace solid-phase microextraction with GC–ion trap MS has been found to reproduce detection limits of 0.03–0.1 µg/kg for 1,2,3-TCB, 1,2,3,4-TeCB, and PeCB in soil (Santos et al., 1997).
For the detection of chlorobenzenes in biota samples, solvent or Soxhlet extraction with subsequent cleanup on Florisil columns and GC analysis with ECD have commonly been used (Lunde & Ofstad, 1976; Kuehl et al., 1980; Oliver & Bothen, 1982; Muir et al., 1992; Gebauer & Weseloh, 1993; Cobb et al., 1994; Jan et al., 1994; Wade et al., 1998). Detection limits of 1500 µg/kg for MCB and lower detection limits ranging from 5 µg/kg for dichlorobenzenes to 0.02 µg/kg for PeCB have been reported (Oliver & Bothen, 1982; Cobb et al., 1994). Vacuum extraction and the direct purge-and-trap method have also been used to quantify levels of MCB in fish tissue (Hiatt, 1981).
Natural sources of chlorobenzenes in the environment have not been identified. However, 1,2,3,4-TeCB has been identified in the oil of marsh grass, although it is not known whether this was formed naturally (Miles et al., 1973).
Chlorobenzenes are released to the environment from sites where they are either manufactured or used as intermediates in the production of other chemicals. They will also be released during the disposal of chlorobenzene products, such as from incinerators (IPCS, 1991a) and hazardous waste sites (ATSDR, 1998). Chlorobenzenes are a product of incomplete combustion and may therefore be released to the environment from waste incinerators. Chlorobenzenes may be formed from the metabolic breakdown of lindane in higher organisms and from its physical breakdown under extreme environmental conditions (IPCS, 1991b).
Releases of some chlorobenzene compounds to the environment in the USA in 2001, as recorded in the US Toxics Release Inventory (TRI), are listed in Table 2. These data do not form a comprehensive list, as only certain types of industrial facility are required to register in the TRI (ATSDR, 1998). There is a paucity of data on the quantity of chlorobenzenes released to the environment in other parts of the world, although some production and consumption data are available. Approximately 15 000 tonnes of 1,4-DCB were produced in and/or imported into the European Union in 1994 (EC, 2001). Total production of MCB, 1,2-DCB, and 1,4-DCB in Japan in 1998 was 26 351 tonnes (Chemical Daily Company, 1999), with 9073 tonnes imported in 1998 and 8310 tonnes imported in 1999 (Chemical Daily Company, 2000).
Table 2: Total releases of chlorobenzenes in the USA during 2001.a
|
Releases (tonnes) |
||||||
|
MCB |
1,2-DCB |
1,3-DCB |
1,4-DCB |
1,2,4-TCB |
PeCB |
|
|
Total emissions to air |
314 |
56 |
0.50 |
37 |
43.92 |
0.03 |
|
Surface water discharges |
0.3 |
0.38 |
0.26 |
0.51 |
0.04 |
0.06 |
|
Releases to land |
0.01 |
0.00 |
0.00 |
0.00 |
3.5 |
1.07 |
|
Total on-site releases |
362 |
59 |
0.76 |
42 |
49 |
1.16 |
|
Total off-site releases |
2.5 |
0.52 |
0.46 |
0.69 |
4.2 |
0.09 |
a From US EPA (2003).
Some uses of chlorobenzenes, including uses as deodorizers, fumigants, degreasers, insecticides, herbicides, and defoliants, will result in direct releases to the environment.
MCB will be released directly to the environment due to its use as a pesticide carrier (Meek et al., 1994c). MCB is used as a solvent carrier for pesticides (29 000 kg per annum in Canada), in the manufacture of rubber polymers (20 000 kg per annum in Canada), and as a carrier for textile dyes (1000 kg per annum in Canada) (Mackay et al., 1996). Fifty per cent of the MCB used in Canada is released to the environment; 80% is emitted to the atmosphere, 10% to water, and 10% to soil, giving releases of 20 000, 2500, and 2500 tonnes, respectively, per year (Mackay et al., 1996). MCB is used in the production of phenol and nitrochlorobenzene (ortho and para isomers), in the formulation of herbicides, to produce additional chlorobenzenes, and as a solvent in the manufacture of adhesives, paints, resins, dyestuffs, and drugs (Grosjean, 1991). MCB is used in the manufacture of diphenyl oxide, phenylphenol, silicone resin, and other halogenated organics (ATSDR, 1990).
1,2-DCB is used primarily in the automotive and metal industries as a solvent for the removal of carbon and degreasing of metal parts (Meek et al., 1994a). 1,2-DCB is used in the synthesis of organic chemicals such as toluene diisocyanate (Grosjean, 1991).
1,4-DCB is used in air fresheners, urinal deodorants, and moth and bird repellents (Meek et al., 1994b; EC, 2001). All of these uses release 1,4-DCB to the environment, principally the atmosphere. 1,4-DCB is also used as an intermediate in the production of other chemicals, including polyphenylene sulfide resins (Grosjean, 1991) and 1,2,4-TCB (ATSDR, 1998). Minor uses of 1,4-DCB include its use in the control of tree-boring insects, ants, and blue mould in tobacco seedbeds (ATSDR, 1998).
Trichlorobenzenes, especially 1,2,4-TCB, are used as dye carriers, degreasing solvents, oil additives, and dielectric fluids and in the formulation of pesticides (Grosjean, 1991). The use of trichlorobenzenes is restricted to mainly 1,2,4-TCB, which is used as a chemical intermediate and an industrial solvent (Giddings et al., 1994c). 1,2,4-TCB was formerly used as a degreasing agent, in septic tanks, and in drain cleaners, wood preservatives, and abrasive formulations (EC, 2003).
Tetrachlorobenzenes and pentachlorobenzenes may be released to the environment from the spillage of dielectric fluids (Giddings et al., 1994a,b). 1,2,3,4-TeCB is used as a component in dielectric fluids (IPCS, 1991a). 1,2,4,5-TeCB is used as an intermediate in the manufacture of herbicides and defoliants. It is also used as an insecticide, as a moisture-resistant impregnant, in electrical insulation, and in packing protection (IPCS, 1991a). PeCB was formerly used in a pesticide to combat oyster drills (small snails that eat oysters). It has also been used as an intermediate (IPCS, 1991a).
Their physicochemical properties suggest that chlorobenzenes released to the environment are likely to be volatilized to the atmosphere. The Henry’s law constants measured for chlorobenzenes suggest that they are readily volatilized, especially from aquatic systems with long residence times, such as large lakes and oceans (Ten Hulscher et al., 1992). However, chlorobenzenes released to water may also be adsorbed onto sediment, especially if it is rich in organic matter. Volatilization from soil is also likely, although, depending on the characteristics of the soil, there may also be sorption to soil.
The majority of chlorobenzenes added to soil, as either sewage sludge or spiked samples, were volatilized, with biodegradation and abiotic degradation insignificant compared with the amount volatilized (Wang & Jones, 1994a). Volatilization occurred by two-step first-order processes, with high rates of volatilization during an initial step, followed by a second, much slower step, which was presumably controlled by the rate of desorption of the compound from soil. Half-lives for loss of chlorobenzenes ranged from 13.0 to 219 days for sewage sludge applications and from 10.6 to 103 days for spiked samples. Half-lives increased with increasing chlorination and were also higher in sludge-amended soil than in the spiked samples. The half-lives for volatilization of MCB and 1,2-DCB from soil were 2.1 and 4.0 days, respectively. Initial soil concentrations were 100 mg/kg dry weight (Anderson et al., 1991). Transient geochemical conditions can significantly alter the extent of removal. Robertson (1994) studied the fate of a dichlorobenzene mixture (containing 74% 1,2-DCB, 11% 1,3-DCB, and 15% 1,4-DCB) released to sub-surface soil in effluent from a septic system. High dichlorobenzene concentrations were found in the aerobic unsaturated zone (below the septic system) where dichlorobenzene had a residence time of 60 days. The migration of dichlorobenzene to the water table was attenuated by this zone.
The most important factor affecting the behaviour and fate of chlorobenzenes in soil is sorption. Adsorption–desorption processes in soil affect the rate of volatilization and leaching and the availability of chemicals to microbial and chemical degradation or uptake by plants or other organisms (Wang & Jones, 1994a). The soil sorption coefficients for chlorobenzenes range from 466 to 58 700 (Table 1) and generally increase with increasing chlorination (IPCS, 1991a; Schrap et al., 1994). Sorption of chlorobenzenes to soil is affected by many parameters, and it increases with increasing organic matter content (Barber et al., 1992; Faschan et al., 1993).
The adsorption of 1,2,4-TCB to soil was found to decrease with increasing soil depth (Njoroge et al., 1998). These depth-related changes were attributed to changes in composition, texture, and accessibility of the soil organic matter. At deeper levels, extractable organic matter was increasingly dominated by fulvic acids. The higher fulvic:humic acid ratio in deep soil reflects an inceasing hydrophilicity of the soil organic matter. Abundance of iron oxide and size of clay particles also increase with depth.
Sorption of chlorobenzenes is also affected by soil moisture, with reduced sorption to wet soil (Chiou & Shoup, 1985; Thibaud et al., 1993). Adsorption of 1,2,4-TCB to soil was reduced following the addition of sodium dodecyl sulfate, a surfactant that frequently occurs in sewage sludge disposed of onto land (DiVincenzo & Dentel, 1996). Desorption occurred only when the sodium dodecyl sulfate concentration exceeded the critical micelle concentration. However, increased adsorption of MCB to soil was reported following the addition of the surfactant hexadecyltrimethylamminium (HDTMA) (Sheng et al., 1998). Adsorption on the HDTMA phase was 80–160 times higher than sorption on natural organic matter. The sorption of 1,4-DCB by aquifer materials with a low organic carbon content was enhanced in the presence of tetrachloroethene (Brusseau, 1991). The enhanced sorption was suggested to arise from tetrachloroethene increasing the organic carbon content of the sorbent.
Desorption of 1,3-DCB from a silty soil to deionized water had an initial fast labile phase, followed by a slow phase (Lee et al., 2002). An average of 60% of the initial concentration was desorbed. The first-order rate constant was 0.022–0.038 per hour for the labile phase and 4.1 × 10–5 to 7.8 × 10–4 per hour for the slow desorption phase. Single-step batch tests showed that desorption of chlorobenzenes from sediment was slow, with less than 0.5% of 1,2,4,5-TeCB and PeCB desorbed within 62 days. Desorption of 1,2,4-TCB was significantly higher than that of other compounds, with 3% desorbed within 62 days (Gess & Pavlostathis, 1997).
MCB adsorbed onto marine sediment reached equilibrium within 3 h (Zhao et al., 2001). Equilibrium took the same time in natural seawater, artificial seawater, and deionized water. Adsorption occurred via the surface and micropores of sediment and could be described by either the Freundlich or Langmuir model. Adsorption was not affected by temperature (18, 25, or 30 °C), although the saturate adsorption amount decreased at higher temperatures. Adsorption isotherms and the saturate adsorption amounts were higher in natural seawater than in artificial seawater and deionized water. Adsorption of 1,2,4,5-TeCB on sandy aquifer solids took up to hundreds of days to reach equilibrium (Ball & Roberts, 1991). Distribution coefficients were greatest in the size fraction with the largest grains.
Mean (± SD) suspended sediment/water partition coefficients (log Koc) for chlorobenzenes measured in Ise Bay, Japan, were 3.47 ± 0.74 (1,3-DCB), 3.69 ± 0.48 (1,2-DCB), 3.61 ± 0.39 (1,2,3-TCB), 3.86 ± 0.40 (1,2,4-TCB), 3.55 ± 0.47 (1,3,5-TCB), 4.39 ± 0.33 (1,2,3,4-TeCB), 3.94 ± 0.33 (1,2,3,5-TeCB and 1,2,4,5-TeCB), and 4.59 ± 0.41 (PeCB) (Masunaga et al., 1996b). Concentrations of chlorobenzenes in water and adsorbed onto suspended sediment were compared. None of the chlorobenzenes gave a clear adsorbed level distribution pattern, and the correlation between soluble and adsorbed chlorobenzenes was weak.
The fate of MCB, 1,2-DCB, and 1,2,4-TCB in wastewater applied to soil was examined in a microcosm experiment (Piwoni et al., 1986). Initial concentrations of MCB, 1,2-DCB, and 1,2,4-TCB in the wastewater were 1.9–3.1, 2.4, and 0.72 µmol/litre, respectively. The proportions of MCB and 1,2-DCB volatilized were 14% and 21%, respectively, and it was assumed that 84% and 79%, respectively, were degraded, giving concentrations in the volume effluent of 9 ± 10% of the original concentration. Volatility of 1,2,4-TCB was not measured, but it was assumed to be approximately 89%, as <0.7% of the original concentration remained in the effluent.
The half-life for dichlorobenzene (all isomers) in a septic groundwater system was 15 days (Robertson, 1994). The site included a 2-m-thick, sandy aerobic unsaturated zone. This loss was due to a combination of volatilization and aerobic biodegradation. Biodegradation occurred after an initial lag phase and was most likely for 1,3-DCB and 1,4-DCB. Dichlorobenzene in the anaerobic zone was not readily biodegraded.
Octanol/air partition coefficients (log Koa) measured for chlorobenzenes at 25 °C were 4.36 (1,2-DCB), 5.19 (1,2,3-TCB), 5.64 (1,2,3,4-TeCB), 5.63 (1,2,4,5-TeCB), and 6.27 (PeCB) (Harner & Mackay, 1995). Octanol/air partition coefficients determined partitioning from the atmosphere to vegetation, soils, and possibly aerosols.
Microcosm experiments suggested that 1,2-DCB in soil was not taken up by grass (Holcus lanatus) roots, although some foliar adsorption of dichlorobenzene volatilized from soil was reported (Wilson & Meharg, 1999). A root concentration factor of 19 litres/kg has been reported for 1,2,4-TCB (Dietz & Schnoor, 2001). From these data, it can be assumed that tri- and/or tetrachlorinated benzenes have the potential to be taken up by plants.
Removal of chlorobenzenes from the atmosphere will occur primarily via reactions with hydroxyl radicals to produce nitrochlorobenzene, chlorophenol, and aliphatic dicarbonyl products, which are further removed by photolysis or reaction with hydroxyl radicals. Photolysis and reactions with ozone or nitrate radicals are of negligible importance (Grosjean, 1991). Rate constants for reactions with hydroxyl radicals (in cm3/s per molecule) were calculated to be 8.8 × 10–13 (MCB), 4.0 × 10–13 (1,2-DCB), 7.2 × 10–13 (1,3-DCB), 4.3 × 10–13 (1,4-DCB), 6.0 × 10–13 (1,2,3-TCB), and 5.65 × 10–13 (1,2,4-TCB) (Atkinson et al., 1985; Klöpffer et al., 1986; Dilling et al., 1988; Arnts et al., 1989). A rate constant for reaction of MCB with ozone was calculated to be <5 × 10–21 cm3/s per molecule. Assuming 24-h average hydroxyl radical and ozone concentrations of 1 × 106 and 7.2 × 1011 molecules/cm3, tropospheric half-lives for MCB were calculated to be 13 days for reactions with hydroxyl radicals and >8.8 years for reactions with ozone (Atkinson et al., 1985). Tropospheric half-lives for 1,4-DCB and 1,2,4-TCB reacting with hydroxyl radicals were calculated to be 33.4 and 26.7 days, respectively (Klöpffer et al., 1988).
1,2,4-TCB in the atmosphere may be degraded via direct photolysis, although this route of degradation is minor, due to the poor spectral overlap between the solar spectrum and the adsorption spectrum of 1,2,4-TCB. The maximum photolysis rate for 1,2,4-TCB in summer at midday under clear skies was 0.03% per hour (Bunce et al., 1989).
Chlorobenzenes in aqueous solutions may undergo photochemical reductive dechlorination. PeCB was degraded to tetrachlorobenzenes, which in turn were photodegraded to trichlorobenzenes, dichlorobenzenes, MCB, and, ultimately, phenol, benzene, and hydrogen chloride (Chu & Jafvert, 1994). These reactions were reported following exposure to 253.7-nm monochromatic ultraviolet lamps. The rate of photodegradation increased in the presence of surfactants. In addition to the main reductive pathway of photodechlorination, minor pathways, including photochlorination, photohydrolysis, and photoisomerization, also occurred. 1,2,3,5-TeCB was photolysed to 1,2,4-TCB or 1,3,5-TCB in the presence of an acetone sensitizer (Choudhry & Hutzinger, 1984). Photochemical reactions in the absence of a sensitizer transformed tetrachlorobenzenes into other isomers and also produced some chlorobenzenes with greater chlorination than the original tetrachlorobenzene compound. The rate constant for reaction of 1,2,4-TCB with hydroxyl radicals in an acidic solution was 6.0 ± 0.3 × 109 per mol/litre per second (Gallard & De Laat, 2001). 1,4-DCB in aqueous solution was photodegraded to 4-chlorophenol, hydroquinone, hydroxybenzoquinone, and 2,5-dichlorophenol (Meunier et al., 2001). The formation of 2,5-dichlorophenol demonstrates hydroxylation without dechlorination. Photodegradation of MCB in aqueous solutions has been reported under both aerobic and anaerobic conditions and at pHs ranging from <1 to <12 (Tissot et al., 1983, 1984; Dilmeghani & Zahir, 2001). Degradation followed first-order kinetics, with rate constants ranging from 1.8 × 10–4 to 6.4 × 10–4 per second for anaerobic and oxygen-saturated conditions, respectively (Dilmeghani & Zahir, 2001). The rate of degradation was an order of magnitude higher with ultraviolet and hydrogen peroxide or hydrogen peroxide–ozone compared with ultraviolet alone.
The half-lives for photolytic degradation of MCB and 1,2,4-TCB in surface water, simulating summer conditions at 40 degrees latitude, were 170 and 450 years, respectively (Dulin et al., 1986).
Chlorobenzenes in various substrates, including soil, sediment, and sewage sludge, can be degraded by microorganisms. The major mechanism of aerobic degradation is via oxidative dechlorination, usually initiated by dioxygenative hydroxylation, leading to the formation of hydroxylated aromatic compounds (mainly catechols), which undergo ring fission and subsequent mineralization to carbon dioxide and water. The less chlorinated benzenes are more readily degraded than the higher chlorinated ones (IPCS, 1991a). Biodegradation under anerobic conditions has also been reported, although this occurs at a slower rate than aerobic biodegradation.
Chlorobenzene-degrading bacteria isolated from aerobic environments include Burkholderia (previously known as Pseudomonas) species (strains JS150, P51, JS6, PS12, and PS14) (Pettigrew et al., 1991; Sander et al., 1991; Van der Meer et al., 1991, 1997; Nishino et al., 1994; Beil et al., 1997; Meckenstock et al., 1998), Alcaligenes species (strains A175 and OBB65) (De Bont et al., 1986; Schraa et al., 1986), Escherichia hermanii (Kiernicka et al., 1999), Nitrosomonas europaea (Keener & Arp, 1994), Mycobacterium vaccae, and Rhodococcus species (strain R22) (Fairlee et al., 1997).
The degradative abilities of these bacteria vary, with some organisms exhibiting a lag or adaptation period prior to degradation. Some can degrade several chlorobenzenes (Brunsbach & Reineke, 1994), whereas others are compound-specific (Reineke & Knackmuss, 1984; Brunsbach & Reineke, 1994; Keener & Arp, 1994). For some, degradation occurs only in the presence of other sources of carbon and energy, whereas others are able to use chlorobenzenes as their sole carbon and energy source (Van der Meer et al., 1987). Genetic analysis has shown that these bacteria contain a novel combination of previously existing genes — genes for aromatic ring dioxygenase and dihydrodiol dehydrogenase — and other genes for a chlorocatechol oxidative pathway.
Degradation is also dependent upon the initial chlorinated benzene concentrations. Degradation will occur only if the initial concentration is below the toxic threshold, although bacteria that have previously been exposed to MCB have the ability to degrade higher concentrations than those that did not have prior exposure. For example, concentrations of MCB greater than 2.5 mmol/litre (282 mg/litre) were found to be toxic to Pseudomonas sp. strain RHO1 cells. Cells that had previously been exposed to MCB demonstrated toxicity at concentrations greater than 3.5 mmol/litre (394 mg/litre) (Fritz et al., 1992).
MCB and 1,2,4-TCB were degraded by bacteria isolated from solids sampled from pristine aquifers (Swindoll et al., 1988). Degradation followed first-order rate constants, with Vmax values of 0.38–2.71 ng/g per hour for 1,2,4-TCB. Degradation of MCB was not saturated; therefore, Vmax could not be calculated.
A consortium of Gram-negative and Gram-positive bacteria isolated from groundwater and soil contaminated with MCB was able to mineralize 54% of a 2.23 µmol/litre solution via the modified ortho pathway within 7 days in the presence of nutrients. Degradation also occurred without added nutrients, although at a slower rate (Nishino et al., 1992).
Degradation of 1,4-DCB occurred at similar rates under aerobic or anaerobic conditions and was enhanced in mixtures with high sludge content (which reduced overall oxygen) (Gejlsbjerg et al., 2001). Mineralization occurred after a lag phase of 30 days. After inoculation for 2 months, mineralization was 12.4% in the sludge and 21.6% in the 1:20 sludge:soil mixture. The authors concluded that mineralization was probably occurring in the aerobic layers of the sludge–soil mixtures, as mineralization did not occur in sludge in the absence of molecular oxygen.
A consortium of bacteria isolated from Rhine sediment was able to degrade PeCB, 1,2,3,4-TeCB, 1,2,3,5-TeCB, 1,2,4,5-TeCB, and 1,2,3-TCB via reductive dechlorination in the presence of lactate, glucose, ethanol, or isopropanol as the electron donor (Holliger et al., 1992). PeCB was degraded to 1,3,5-TCB, while 1,2,3,4-TeCB and 1,2,4,5-TeCB were degraded to 1,2,4-TCB. Chlorobenzenes that were not dechlorinated during the 4-week incubation included 1,2,4-TCB, 1,3,5-TCB, and all isomers of dichlorobenzene. Other studies have reported complete mineralization of some higher chlorinated compounds. Two Pseudomonas strains (PS12 and PS14) isolated from the soil of an industrial waste deposit were able to mineralize various chlorobenzenes, including MCB, all three dichlorobenzenes, 1,2,4-TCB, and 1,2,4,5-TeCB (strain PS14 only). 1,2,4-TCB and 1,2,4,5-TeCB were degraded via dioxygenation of the aromatic ring, producing 3,4,6-trichlorocatechol. Subsequent ortho cleavage, catalysed by a Type II catechol 1,2-dioxygenase, produced 2,3,5-trichloromuconate, which was degraded via the tricarboxylic acid pathway (Sander et al., 1991).
Degradation of 1,2-DCB and 1,4-DCB within a mixture of organic compounds was reported in a 149-day batch microcosm using sediment and groundwater obtained from various sampling sites of an aquifer (Nielsen & Christensen, 1994). The initial concentrations were approximately 120 µg/litre. Within an average of 82 days, 78.3% of 1,4-DCB and 81.0% of 1,2-DCB were degraded. The lag phases were 4.9 and 4.5 days for 1,4-DCB and 1,2-DCB, respectively. In the Organisation for Economic Co-operation and Development (OECD) closed bottle test, 67% of an initial 1,4-DCB concentration of 1.9 mg/litre was mineralized after 28 days, indicating that 1,4-DCB is readily degradable (Topping, 1987).
Bartholomew & Pfaender (1983) calculated degradation rates for MCB and 1,2,4-TCB at different sites of a river system during different seasons. Rates of degradation of MCB and 1,2,4-TCB were reported to decrease over the freshwater to estuarine to marine gradient. Vmax values for MCB degradation during May and September were 13–14 ng/litre per hour for fresh water, 4.9–10 ng/litre per hour for estuarine water, and <1–1.7 ng/litre per hour for marine water. Vmax values were <1 ng/litre per hour at all three sites in February. The corresponding values for degradation of 1,2,4-TCB in May and July were <1–7.5 ng/litre per hour for fresh water, <1–7.9 ng/litre per hour for estuarine water, and <1–2.3 ng/litre per hour for marine water.
In controlled lysimeter experiments, 80% of 1,2,4,5-TeCB in soils and liquid cultures was mineralized by the bacterial strains Isphingomonas sp. strains HH69 and RW1 and Pseudomonas sp. strain PS14 within a few days (Figge et al., 1993). Degradation was not increased in the presence of additional energy sources such as peptone, triolein, and glucose. Degradation did not occur in acidic soils (pH < 4).
Biodegradation of chlorobenzenes has also been reported in several studies under anaerobic conditions, including methanogenic and sulfate-reducing conditions. As with aerobic degradation, degradability varies between organisms. Under anaerobic conditions, degradation is limited to dechlorination, with no breakdown of the aromatic structure.
Anaerobic degradation of chlorobenzenes has been reported in river sediment (Masunaga et al., 1996a; Susarla et al., 1996). Dechlorination occurred without a lag period, with half-lives ranging from 17 to 433 days. The main pathway for PeCB dechlorination was via 1,2,4,5-TeCB, 1,2,4-TCB, 1,4-DCB, and MCB. A minor pathway, via 1,2,3,4-TeCB, 1,2,3-TCB, 1,2-DCB, and MCB, was also observed. MCB was stable under anaerobic conditions. The preferences for dechlorination were two adjacent chlorine atoms, followed by one chlorine on an adjacent carbon, followed by no chlorine on the adjacent carbon. Other studies have reported similar anaerobic biodegradation (Beurskens et al., 1991; Ramanand et al., 1993; Susarla et al., 1997). Nowak et al. (1996) reported anaerobic degradation of all chlorobenzenes, including MCB, to benzene.
In anaerobic sewage sludge, PeCB was dechlorinated to 1,2,3,4-TeCB and 1,2,3,5-TeCB, which were degraded to 1,2,4-TCB, 1,2,3-TCB, and 1,3,5-TCB, and then 1,2-DCB and 1,3-DCB (Yuan et al., 1999). Sequential dechlorination occurred within a substrate concentra-tion range of 2–50 mg/litre, but was slower at concentrations greater than 50 mg/litre. Dechlorination rates were highest under methanogenic conditions (0.30 mg/litre per day), with slower rates under sulfate-reducing (0.12 mg/litre per day) and denitrifying conditions (0.08 mg/litre per day). The rate of dechlorination of 1,2,3-TCB by anaerobic sediment ranged from 15 to 35 pmol/ml wet sediment per day (Yonezawa et al., 1994).
Some studies have shown chlorobenzenes to be resistant to anaerobic biodegradation. Nielsen et al. (1995) reported no biodegradation of 1,2-DCB or 1,4-DCB in anaerobic landfill leachate collected from four different sites at distances ranging from 2 to 350 m from the landfill. The governing reactions, which varied at each site, included methanogenesis, iron(III) reduction, nitrate reduction, and manganese(IV) reduction. Dichlorobenzenes have been reported to persist for at least 20 years in an aquifer that had been contaminated with rapid-infiltration sewage disposal (Barber, 1988). 1,2,3,5-TeCB and 1,3,5-TCB were resistant to degradation by soil slurry microorganisms that could degrade PeCB, 1,2,3,4-TeCB, and 1,2,4-TCB (Ramanand et al., 1993).
The bioaccumulation of chlorobenzenes by aquatic organisms is determined by their relative water and lipid solubility (thus reflecting the octanol/water partition coefficients) and the number of chlorine substitutions. Uptake from water increases with increasing chlorination (Könemann & Van Leeuwen, 1980; Oliver & Niimi, 1983; Sabljic, 1987; Koelmans & Jimenez, 1994; Wang et al., 1997) and with increasing temperature (Koelmans & Jimenez, 1994).
Mean bioconcentration factors (BCFs) (dry weight) for phytoplankton increased from 4700 for 1,2,3-TCB at 4.5 °C to 26 000 for PeCB at 38.6 °C (Koelmans & Jimenez, 1994). Wang et al. (1997) found significant differences in the accumulation of chlorobenzenes by different marine algal species, with BCFs (dry weight) ranging from 600 to 3000 for 1,2,3,4-TeCB and from 1000 to 6000 for PeCB.
BCFs ranging from 270 for 1,2-DCB to 20 000 for PeCB were reported for laboratory studies on rainbow trout (Oncorhynchus mykiss) (Oliver & Niimi, 1983). BCFs for a variety of fish species ranged from 7000 to 24 000 (lipid weight) for 1,2,4-TCB, with a positive correlation between bioaccumulation and lipid content (Geyer et al., 1985). Galassi & Calamari (1983) found BCFs (lipid weight) ranging from 4000 to 22 000 for 1,2,3- and 1,2,4-TCB in rainbow trout, with newly hatched fish accumulating 2–4 times the amount found in eyed eggs or young fish (alevins). Qiao et al. (2000) report that gill uptake of 1,2,4-TCB and PeCB could account for 98% of the body burden. Uptake of trichlorobenzenes, tetrachlorobenzenes, and PeCB was significantly reduced by the presence of suspended particles (Schrap & Opperhuizen, 1990). However, PeCB was found to be readily desorbed from sediments with a low organic carbon content and subsequently accumulated by fish via the gills (Qiao & Farrell, 1996). The rate of elimination of chlorobenzenes decreases with increasing chlorination (Melancon & Lech, 1985; De Boer et al., 1994). Elimination half-lives for dichlorobenzenes to PeCB in laboratory-exposed fish ranged from 0.05 to 1.6 days (Melancon & Lech, 1985). However, for eels (Anguilla anguilla) transferred from a contaminated lake to a "clean" lake, elimination half-lives of >300 days were reported for tetrachlorobenzenes and PeCB (De Boer et al., 1994). Sijm & Van der Linde (1995) calculated elimination rate constants and predicted elimination half-lives for 1,2,3-TCB to be 40 days in small fish, such as guppies (Poecilia reticulata), and >5 years in larger and/or fatty fish.
The coefficient of adsorption onto sediment influences the uptake into terrestrial plants and sediment-living aquatic invertebrates; the degree of chlorination is also correlated with uptake (Knezovich & Harrison, 1988; IPCS, 1991a). Under non-equilibrium conditions, BCFs for chironomid midge larvae exposed to sediment-bound chlorobenzenes were 5, 29, and 225 for MCB, 1,2-DCB, and 1,2,4-TCB, respectively. BCFs were best correlated with the concentrations of the chlorobenzenes in the interstitial water (Knezovich & Harrison, 1988).
The tri- and tetrachlorinated benzenes may be taken up by plants, as indicated by the root concentration factor of 19 litres/kg reported for 1,2,4-TCB (Dietz & Schnoor, 2001).
However, the prediction of BCFs is more difficult for terrestrial plants than for aquatic organisms because of the complex nature of the root soil interface combined with gaseous uptake by aerial parts (Scheunert et al., 1994). Topp et al. (1986) compared the uptake of chlorobenzenes by plants from the soil and via the air in closed, aerated laboratory systems. A negative correlation was demonstrated between the BCF and the soil adsorption coefficient (based on soil organic matter content) for the uptake into the roots of barley. The adsorption of chlorobenzenes onto soil organic matter increased with increasing chlorination. However, expression of uptake in barley roots in relation to the soil interstitial water concentration of the chlorobenzenes produced a positive correlation between the BCF and the octanol/water partition coefficients. Higher chlorinated chlorobenzenes, therefore, are most readily taken up by the plant roots when they are available in soil interstitial water. This will occur particularly in sandy soils with low organic matter content. In a later study, Topp et al. (1989) found that after growth in soil containing 2 µg each of 1,2,4-TCB and PeCB per kg dry weight, harvested barley grain contained 73 and 82 µg/plant, respectively. The concentrations in the dry grain were 0.05 and 0.06 mg/kg for 1,2,4-TCB and PeCB, respectively. In further studies on soybeans (Glycine max), linear correlations were found between equilibrium tissue/water coefficients, the octanol/water partition coefficient, and measured lipid content (Tam et al., 1996). The bioconcentration of chlorobenzenes into excised soybean (Glycine max) roots increased exponentially with increasing octanol/water partition coefficient (Kraaij & Connell, 1997). Wang & Jones (1994b) concluded that the total amount of chlorobenzenes taken up by carrots grown in sewage sludge-amended and spiked soils was low (<1%) compared with other loss pathways from the soil, principally volatilization.
Belfroid et al. (1994) calculated BCFs for earthworms (Eisenia andrei) of 104 and 156 for 1,2,3,4-TeCB and PeCB in soil; BCFs based on interstitial water were 67 000 and 307 000, respectively, and were found to be similar to BCFs found for worms exposed in water alone (Belfroid et al., 1993). BCFs for earthworms exposed via water show a clear increase in uptake of chlorobenzenes with increasing chlorination, and steady-state concentrations are reached within 5 days (Belfroid et al., 1993). Elimination rate constants reveal that chlorobenzene loss decreases with increasing chlorination. A monophasic elimination curve was observed in water, whereas biphasic elimination was found in the presence of soil (Belfroid et al., 1993); elimination rates in soil experiments were significantly increased by the addition of organic matter (Belfroid & Sijm, 1998). Feeding studies have revealed that earthworms can also take up chlorobenzenes via food. In studies with field-contaminated soil, steady-state concentrations in worms were much lower than in laboratory studies, suggesting decreased bioavailability of chlorobenzenes (Belfroid et al., 1995).
Chlorobenzene (MCB, dichlorobenzenes, and trichlorobenzenes) concentrations have previously been reported in ambient air, with mean concentrations in the order of 0.1 µg/m3 and maximum levels of up to 100 µg/m3 at hazardous waste sites (IPCS, 1991a). Popp et al. (2000) measured tetrachlorobenzenes and PeCB in air sampled from two industrially contaminated sites and a reference site in Germany in 1998. Mean gas-phase concentrations of tetrachlorobenzenes and PeCB at the contaminated sites ranged from 5.7 to 30.9 pg/m3 and from 10.2 to 28 pg/m3, respectively. Mean concentrations at the control site ranged from 6.4 to 10.6 pg/m3. Particulate-bound chlorobenzenes accounted for 1.9% of the total concentrations. A low proportion of particulate-bound chlorobenzenes was also reported in air sampled from the Bering and Chukchi seas in 1993 (Strachan et al., 2001). Mean gas-phase concentrations for the Bering Sea were 1.1, 4.0, and 6.6 pg/m3 for 1,2,3-TCB, 1,2,3,4-TeCB, and PeCB, respectively, and for the Chukchi Sea, 2.8, 10, and 14 pg/m3, respectively. Mean chlorobenzene concentrations at four sites throughout Michigan, USA (1992–1994), ranged from 22 to 30 pg/m3 for 1,2,4,5-TeCB, from 40 to 53 pg/m3 for 1,2,3,4-TeCB, and from 35 to 69 pg/m3 for PeCB (Hermanson et al., 1997). Annual mean concentrations for southern Ontario, Canada (1988–1989), were >5.3 pg/m3 for 1,2,3,4-TeCB and >8.0 pg/m3 for PeCB (Hoff et al., 1992). Higher concentrations have been reported in close proximity to pollution sources. A concentration of 5 µg/m3 for tri- and tetrachlorobenzenes was found within 200 m of an electro-industrial plant in Slovenia (Jan et al., 1994). Seasonal variations in the concentrations of 1,4-DCB in ambient air have also been reported, with concentrations increasing with increasing temperature (Hanai et al., 1985).
Chlorobenzenes have also been detected in rainwater, their presence presumably being due to transfer from the ambient air. Concentrations of all three dichlorobenzene isomers and 1,2,4-TCB in rainwater were less than 10 ng/litre at selected sites in Oregon and California, USA (Pankow et al., 1983). In the United Kingdom, 1,4-DCB was detected in rainwater at a mean concentration of 10 ± 5 ng/litre (Fielding et al., 1981).
In 12 sewage sludges in the United Kingdom, the concentrations of chlorobenzenes ranged from <0.01 mg/kg dry weight for PeCB to 40.2 mg/kg dry weight for 1,3-DCB, with a general reduction in concentration with increased chlorine substitution (Rogers et al., 1989). Further sampling of United Kingdom sewage sludges revealed chlorobenzene concentrations ranging from 35 100 to 192 000 mg/kg dry weight for MCB, from 13 to 4110 mg/kg for dichlorobenzenes, from 2 to 1070 mg/kg for trichlorobenzenes, from 0.2 to 101 mg/kg for tetrachlorobenzenes, and from 2 to 37 mg/kg for PeCB (Wang & Jones, 1994c). Analysis of archived sludge samples showed that concentrations of 1,4-DCB increased over the period 1942–1961, whereas other chlorobenzenes increased in concentration only from 1954 onwards (Wang et al., 1992).
Data on levels of the lower chlorinated benzenes (MCB, dichlorobenzenes, and trichlorobenzenes) in wastewater indicate that MCB is detected the most often and at the highest concentrations, occasionally exceeding 1 mg/litre. Chlorobenzene concentrations in US wastewater have been reported to range from 11 to 6400 µg/litre for MCB, from 10 to 860 µg/litre for dichlorobenzenes, and from 12 to 607 µg/litre for trichlorobenzenes (IPCS, 1991a).
Concentrations of chlorobenzenes in surface waters are generally in the ng/litre to µg/litre range, with maximum concentrations up to 0.2 mg/litre in areas close to industrial sources (IPCS, 1991a). Mean concentrations of dissolved chlorobenzenes in the Bering and Chukchi seas ranged from 3 to 10 pg/litre for 1,2,3-TCB, from 15 to 36 pg/litre for 1,2,3,4-TeCB, and from 9 to 36 pg/litre for PeCB (Strachan et al., 2001). Higher chlorobenzene levels have been detected in coastal waters and estuaries, with Dutch coastal waters containing mean concentrations ranging from 9 to 117 ng/litre for dichlorobenzenes and from 0.7 to 1.6 ng/litre for trichlorobenzenes (Van de Meent et al., 1986) and Japanese coastal waters containing mean dissolved concentrations ranging from 24.3 ng/litre for 1,3-DCB to 0.25 ng/litre for tetrachlorobenzenes (Masunaga et al., 1996b). Waters of the Scheldt estuary (The Netherlands) contained chlorobenzene concentrations ranging from <130 to 315 ng/litre for dichlorobenzenes, from <25 to 320 ng/litre for trichlorobenzenes, and from <45 to 135 ng/litre for tetrachlorobenzenes (Van Zoest & Van Eck, 1991); more recent sampling revealed MCB concentrations ranging from 5 to 31.5 ng/litre (Huybrechts et al., 2000). Chlorobenzene concentrations of up to 500 ng/litre have been reported for MCB in the Tees Estuary, United Kingdom (Law et al., 1991), and for 1,3-DCB in Yokkaichi Port, Ise Bay, Japan, during 1988 (Masunaga et al., 1991a). Mean chlorobenzene concentrations in the Forth Estuary, United Kingdom, during 1987 ranged from <0.1 to 790 ng/litre for dichlorobenzenes, from 4 to 5500 ng/litre for trichlorobenzenes, from <0.04 to 20 ng/litre for tetrachlorobenzenes, and from <0.01 to 40 ng/litre for PeCB. The predominant isomers detected were 1,2,3- and 1,2,4-TCB, and these were found near industrial effluent discharges (Rogers et al., 1989; Harper et al., 1992). Further studies in 1990 revealed 1,2,3- and 1,2,4-TCB concentrations ranging up to 51 and 84 ng/litre, respectively (Harper et al., 1992).
The highest chlorobenzene concentrations in surface waters have been reported for river waters in heavily populated and/or industrialized areas. Mean concentrations in the river Besos, Spain, were 260 ng/litre for MCB, 600 ng/litre for 1,4-DCB, 5000 ng/litre for 1,2-DCB and 1,3-DCB, 1100 ng/litre for 1,2,3-TCB, and 8100 ng/litre for 1,2,4-TCB (Gomez Belinchon et al., 1991). Concentrations of MCB and 1,4-DCB ranging from non-detected to >10 µg/litre have been reported for both compounds in water from the Ohio River (US EPA, 1985). Elder et al. (1981) reported trichlorobenzene concentrations (isomer not specified) ranging from 0.1 to 8 µg/litre in water from Niagara Falls, New York, USA. Corresponding concentrations of tetrachlorobenzene ranged from 0.1 to 200 µg/litre. Concentrations of PeCB in water sampled from the Great Lakes ranged from not detected to 0.0006 µg/litre (Oliver & Nicol, 1982). Concentrations in water sampled from the rivers and estuary of Osaka (a major urban area of Japan) ranged from 0.2 to 30 µg/litre for MCB, from 0.17 to 130 µg/litre for 1,4-DCB, from 0.2 to 10 µg/litre for 1,2-DCB, from 0.16 to 0.35 µg/litre for 1,2,4-TCB, and from 0.18 to 0.30 µg/litre for 1,2,3-TCB (Yamamoto et al., 1997).
Mean chlorobenzene concentrations in sediment from the Bering and Chukchi seas ranged from 0.02 to 0.41 µg/kg for 1,2,3-TCB, from 0.08 to 0.87 µg/kg for 1,2,3,4-TeCB, and from 0.33 to 0.4 µg/kg for PeCB (Strachan et al., 2001). Mean concentrations in coastal sediments from Ise Bay, Japan, were 4.8 µg/kg for 1,2,4-TCB, 2.3 µg/kg for 1,2-DCB, 1.9 µg/kg for 1,3-DCB, and <0.15 µg/kg for 1,3,5-TCB, tetrachlorobenzenes, and PeCB (Masunaga et al., 1991b). Lee & Fang (1997) reported mean values for the Tsen-wen estuary, Taiwan, of 3.2 µg/kg for 1,2-DCB, 20.7 µg/kg for 1,3-DCB, and 11.2 µg/kg for 1,2,4-TCB.
Lake Garda (Italy) contained mean sediment PeCB concentrations of 0.2 µg/kg dry weight (Bossi et al., 1992), whereas Lake Superior (Hamilton Harbour, Canada) contained levels ranging from 3.6 µg/kg for PeCB to 80 µg/kg for 1,4-DCB (Onuska & Terry, 1985). Sediment samples from the river Elbe, Germany, ranged from 30 to 740 µg/kg dry weight for MCB, from 20 to 1060 µg/kg for dichlorobenzenes (1,2- and 1,4-DCB), from 1 to 115 µg/kg for trichlorobenzenes (1,2,3- and 1,2,4-TCB), from 1 to 27 µg/kg for tetrachlorobenzenes, and from 1 to 14 µg/kg for PeCB (Götz et al., 1993), whereas samples from the river Rhine contained concentrations ranging from 40 to 240 µg/kg dry weight for dichlorobenzenes, from <10 to 20 µg/kg for trichlorobenzenes, and from <0.5 to 2 µg/kg for PeCB (Alberti, 1983).
Chlorobenzene levels in uncontaminated soils are generally less than 0.4 mg/kg for dichlorobenzene congeners and less than 0.1 mg/kg for other chlorobenzene congeners (Wang et al., 1995). Multiple applications of sewage sludge can increase the chlorobenzene content in sludge-amended soil compared with control soils. However, Wang et al. (1995) found that most chlorobenzenes disappear rapidly on cessation of sludge application, with around 10% remaining 30 years later. They found that 1,4-DCB levels increased significantly in United Kingdom soils during the 1960s to a maximum mean value in 1967 of 10 mg/kg in control soils and 16.6 mg/kg in sludge-amended soils. Analysis of subsoil from a former pesticide factory in Germany showed that tetrachlorobenzenes and PeCB were dominant in the upper soil layers (up to 1.9 m), accounting for 80% of chlorobenzenes, with 1,2,3,4-TeCB and PeCB accounting for 44% and 24%, respectively. At depths between 1.9 and 5.5 m, trichlorobenzenes were more dominant, accounting for 60%, with 1,2,4-TCB accounting for 37% (Feidieker et al., 1994). Total chlorobenzene concentrations ranged from 1.5 to 18 400 mg/kg.
Mean chlorobenzene concentrations in bivalves from US coastal waters ranged from <0.25 to 28.2 µg/kg dry weight for 1,2,4,5-TeCB, from <0.25 to 10 µg/kg for 1,2,3,4-TeCB, and from <0.25 to 13.3 µg/kg for PeCB (Wade et al., 1998). Aquatic insects from a variety of Canadian sites contained mean PeCB concentrations ranging from <0.49 to 21.4 µg/kg dry weight (Ciborowski & Corkum, 1988). Concentrations in freshwater and marine fish from contaminated areas range from 0.1 to 50 µg/kg wet weight, with higher chlorinated compounds generally present at the highest concentrations (IPCS, 1991a). The eggs of fish-eating birds contained mean PeCB levels of 1.2 and 4.4 µg/kg from two sites in Puget Sound, USA (Cobb et al., 1994). Waterfowl from Lake Ontario, Canada, contained mean chlorobenzene concentrations ranging from 0.3 to 1.7 µg/kg wet weight for 1,2,3,4-TeCB and from 0.65 to 33.4 µg/kg for 1,2,4,5-TeCB (Gebauer & Weseloh, 1993). Mean concentrations in Arctic marine mammal blubber ranged from 1 to 9.7 µg/kg wet weight for 1,2,3,4-TeCB and from 16.8 to 20.2 µg/kg for PeCB (Muir et al., 1992; Weis & Muir, 1997).
The acute toxicity of chlorobenzenes to aquatic organisms is presented in Table 3. Forty-eight-hour EC50s for diatoms range from 8 to 235 000 µg/litre. For freshwater invertebrates, 48-h EC50s range from 10 µg/litre for PeCB to >530 000 µg/litre for 1,2,4,5-TeCB. Ninety-six-hour LC50s for fish range from 135 for PeCB in the freshwater guppy (Poecilia reticulata) to 21 000 µg/litre for 1,2,4-TCB in the saltwater sheepshead minnow (Cyprinodon variegatus).
Table 3: Acute toxicity of chlorobenzenes to aquatic species.
|
Organism |
End-point |
Chlorobenzene |
Test conditionsa |
Concentration (µg/litre) |
Reference |
|
|
Microorganisms — Saltwater |
||||||
|
Diatom (Cyclotella meneghiniana) |
48-h EC50 (DNA measurement) |
MCB |
M |
235 740 |
Figueroa & Simmons (1991) |
|
|
1,2-DCB |
M |
23 330 |
Figueroa & Simmons (1991) |
|||
|
1,3-DCB |
M |
51 880 |
Figueroa & Simmons (1991) |
|||
|
1,4-DCB |
M |
34 300 |
Figueroa & Simmons (1991) |
|||
|
1,2,3-TCB |
M |
6420 |
Figueroa & Simmons (1991) |
|||
|
1,2,4-TCB |
M |
2830 |
Figueroa & Simmons (1991) |
|||
|
1,3,5-TCB |
M |
590 |
Figueroa & Simmons (1991) |
|||
|
1,2,3,5-TeCB |
M |
1370 |
Figueroa & Simmons (1991) |
|||
|
1,2,3,4-TeCB |
M |
1390 |
Figueroa & Simmons (1991) |
|||
|
1,2,4,5-TeCB |
M |
270 |
Figueroa & Simmons (1991) |
|||
|
PeCB |
M |
8 |
Figueroa & Simmons (1991) |
|||
|
Invertebrates — Freshwater |
||||||
|
Water flea (Daphnia magna) |
48-h EC50/LC50 |
MCB |
C M |
585.52 |
Rose et al. (1998) |
|
|
MCB |
C N |
12 900–17 300b |
Cowgill et al. (1985) |
|||
|
MCB |
C N |
5810 |
Abernethy et al. (1986) |
|||
|
MCB |
C N |
86 000 |
LeBlanc (1980) |
|||
|
1,2-DCB |
C N |
2352 |
Abernethy et al. (1986) |
|||
|
1,2-DCB |
N |
740–2200c |
Canton et al. (1985) |
|||
|
1,2-DCB |
C M |
4200–7400 |
Richter et al. (1983) |
|||
|
1,2-DCB |
C N |
2400 |
LeBlanc (1980) |
|||
|
1,3-DCB |
N |
1200–6800c |
Canton et al. (1985) |
|||
|
1,3-DCB |
N |
10 500–13 500 |
Gersich et al. (1986) |
|||
|
1,3-DCB |
C N |
28 000 |
LeBlanc (1980) |
|||
|
1,4-DCB |
N |
700–2200c |
Canton et al. (1985) |
|||
|
1,4-DCB |
C N |
11 000 |
LeBlanc (1980) |
|||
|
1,2,3-TCB |
C N |
1452 |
Abernethy et al. (1986) |
|||
|
1,2,4-TCB |
C M |
1700–2100 |
Richter et al. (1983) |
|||
|
1,2,4-TCB |
C N |
50 000 |
LeBlanc (1980) |
|||
|
1,2,4,5-TeCB |
C N |
>530 000 |
LeBlanc (1980) |
|||
|
PeCB |
C N |
300 |
Abernethy et al. (1986) |
|||
|
Water flea (Ceriodaphnia dubia) |
48-h EC50/LC50 |
MCB |
M C |
7900–47 000 |
Rose et al. (1998) |
|
|
MCB |
C N |
8900–11 100d |
Cowgill et al. (1985) |
|||
|
1,2-DCB |
M C |
661.5 |
Rose et al. (1998) |
|||
|
1,2,4-TCB |
M C |
308 |
Rose et al. (1998) |
|||
|
1,2,3,4-TeCB |
M C |
130 |
Rose et al. (1998) |
|||
|
PeCB |
M C |
10 |
Rose et al. (1998) |
|||
|
Midge (Tanytarsus dissimilis) |
48-h LC50 |
1,2-DCB |
2300–11 800 |
Call et al. (1979) |
||
|
1,4-DCB |
13 000 |
Call et al. (1983) |
||||
|
Midge (Chironomus thummi) |
48-h LC50 |
1,4-DCB |
C M |
1200 |
Roghair et al. (1994) |
|
|
1,2,3-TCB |
C M |
1700 |
Roghair et al. (1994) |
|||
|
1,2,3,4-TeCB |
C M |
540–730 |
Roghair et al. (1994) |
|||
|
PeCB |
C M |
230–320 |
Roghair et al. (1994) |
|||
|
Invertebrates — Saltwater |
||||||
|
Fleshy prawn (Penaeus chinensis) |
96-h LC50 |
MCB |
1720 |
Yin & Lu (1993) |
||
|
Crab (Portunus pelagicus) |
96-h EC50 (growth) |
MCB |
C N |
748 |
Mortimer & Connell (1994) |
|
|
1,4-DCB |
C N |
201 |
Mortimer & Connell (1994) |
|||
|
1,2,3-TCB |
C N |
173 |
Mortimer & Connell (1994) |
|||
|
1,2,3,4-TeCB |
C N |
410 |
Mortimer & Connell (1994) |
|||
|
PeCB |
C N |
87 |
Mortimer & Connell (1994) |
|||
|
Grass shrimp (Palaemonetes pugio) |
96-h LC50 |
1,2-DCB |
M |
9400 |
Curtis et al. (1979) |
|
|
1,4-DCB |
M |
69 000 |
Curtis et al. (1979) |
|||
|
1,2,4-TCB |
M |
540 |
Clark et al. (1987) |
|||
|
Opossum shrimp (Americamysis bahia) |
96-h LC50 |
1,3-DCB |
2850 |
US EPA (1978) |
||
|
1,2,4-TCB |
450 |
US EPA (1978) |
||||
|
1,2,4,5-TeCB |
1480 |
US EPA (1978) |
||||
|
PeCB |
160 |
US EPA (1978) |
||||
|
Fish — Freshwater |
||||||
|
Rainbow trout (Oncorhynchus mykiss) |
96-h LC50 |
MCB |
M |
4700 |
Dalich et al. (1982) |
|
|
1,2-DCB |
1520–1580 |
Call et al. (1979) |
||||
|
1,4-DCB |
880 |
Mayer & Ellersieck (1986) |
||||
|
1,4-DCB |
1120 |
Call et al. (1983) |
||||
|
1,2,4-TCB |
1530 |
Call et al. (1983) |
||||
|
1,2,4,5-TeCB |
N |
1200–10 000e |
Van Leeuwen et al. (1985) |
|||
|
PeCB |
190 |
Call et al. (1979) |
||||
|
16-day LC50 |
MCB |
C N |
90 |
Birge et al. (1979a) |
||
|
14-day LC50 |
1,4-DCB |
C M |
800 |
Calamari et al. (1983) |
||
|
Fathead minnow (Pimephales promelas) |
96-h LC50 |
MCB |
C M |
7700 |
Marchini et al. (1993) |
|
|
MCB |
M |
16 900 |
Geiger et al. (1990) |
|||
|
1,2-DCB |
M |
6027 |
Sijm et al. (1993) |
|||
|
1,3-DCB |
M |
7800 |
Carlson & Kosian (1987) |
|||
|
1,4-DCB |
M |
4200 |
Carlson & Kosian (1987) |
|||
|
1,2,4-TCB |
M |
2760 |
Carlson & Kosian (1987) |
|||
|
1,2,4-TCB |
M |
2990 |
Geiger et al. (1990) |
|||
|
1,2,3,4-TeCB |
M |
1100 |
Carlson & Kosian (1987) |
|||
|
1,2,4,5-TeCB |
89–460 |
Brooke (1991) |
||||
|
Goldfish (Carassius auratus) |
96-h LC50 |
MCB |
C N |
2370–3480f |
Birge et al. (1979a) |
|
|
Guppy (Poecilia reticulata) |
96-h LC50 |
1,2,3-TCB |
M |
348 |
Van Hoogen & Opperhuizen (1988) |
|
|
1,2,3-TCB |
M |
365 |
Van Hoogen & Opperhuizen (1988) |
|||
|
PeCB |
M |
135 |
Van Hoogen & Opperhuizen (1988) |
|||
|
14-day LC50 |
MCB |
C N |
24 964 |
Könemann (1981) |
||
|
1,2-DCB |
C N |
5852 |
Könemann (1981) |
|||
|
1,3-DCB |
C N |
7367 |
Könemann (1981) |
|||
|
1,4-DCB |
C N |
3957 |
Könemann (1981) |
|||
|
1,2,4-TCB |
C N |
2393 |
Könemann (1981) |
|||
|
1,3,5-TCB |
C N |
3302 |
Könemann (1981) |
|||
|
14-day LC50 |
1,2,4,5-TeCB |
C N |
305 |
Könemann (1981) |
||
|
PeCB |
C N |
177 |
Könemann (1981) |
|||
|
Mosquitofish (Gambusia affinis) |
96-h LC50 |
1,2,3-TCB |
C M |
2196 |
Chaisuksant et al. (1998) |
|
|
96-h LC50 |
PeCB |
C M |
200 |
Chaisuksant et al. (1998) |
||
|
Largemouth bass (Micropterus salmoides) |
7.5-day LC50 |
MCB |
C M |
50–60g |
Birge et al. (1979b) |
|
|
Zebrafish (Brachydanio rerio) |
28-day LC50 |
MCB |
M |
10 300 |
Van Leeuwen et al. (1990) |
|
|
7- to 28-day NOEC |
MCB |
M |
8500 |
Van Leeuwen et al. (1990) |
||
|
14- to 28-day NOEC |
1,4-DCB |
M |
2100 |
Van Leeuwen et al. (1990) |
||
|
28-day LC50 |
1,2,3-TCB |
M |
990 |
Van Leeuwen et al. (1990) |
||
|
14- to 28-day NOEC |
1,2,3-TCB |
M |
450 |
Van Leeuwen et al. (1990) |
||
|
14- to 28-day NOEC |
1,2,4-TCB |
M |
450 |
Van Leeuwen et al. (1990) |
||
|
28-day LC50 |
1,2,3,4-TeCB |
M |
410 |
Van Leeuwen et al. (1990) |
||
|
7- to 21-day NOEC |
1,2,3,4-TeCB |
M |
310 |
Van Leeuwen et al. (1990) |
||
|
7- to 28-day NOEC |
PeCB |
M |
110 |
Van Leeuwen et al. (1990) |
||
|
Fish — Saltwater |
||||||
|
Dover sole (Solea solea) |
96-h LC50 |
MCB |
C M |
5821 |
Furay & Smith (1995) |
|
|
European flounder (Platichthys flesus) |
96-h LC50 |
MCB |
C M |
6609 |
Furay & Smith (1995) |
|
|
1,2-DCB |
C M |
4616 |
Furay & Smith (1995) |
|||
|
1,2,4-TCB |
C M |
8585 |
Furay & Smith (1995) |
|||
|
Sheepshead minnow (Cyprinodon variegatus) |
96-h LC50 |
MCB |
N |
10 000 |
Heitmuller et al. (1981) |
|
|
1,2-DCB |
N |
9700 |
Heitmuller et al. (1981) |
|||
|
1,3-DCB |
N |
7800 |
Heitmuller et al. (1981) |
|||
|
1,4-DCB |
N |
7400 |
Heitmuller et al. (1981) |
|||
|
1,2,4-TCB |
N |
21 000 |
Heitmuller et al. (1981) |
|||
|
1,2,3,5-TeCB |
N |
3700 |
Heitmuller et al. (1981) |
|||
|
1,2,4,5-TeCB |
N |
800 |
Heitmuller et al. (1981) |
|||
|
1,2,4,5-TeCB |
M |
330 |
Ward et al. (1981) |
|||
|
PeCB |
N |
800 |
Heitmuller et al. (1981) |
|||
|
Sheepshead minnow (Cyprinodon variegatus) |
28-day NOEC (growth) |
PeCB |
18–86h |
Hansen & Cripe (1991) |
||
|
28-day NOEC (survival) |
PeCB |
19–120h |
Hansen & Cripe (1991) |
|||
|
a |
C = test carried out in a closed exposure system; M = measured exposure concentration; N = nominal exposure concentration. |
|
b |
Range of values for differences in temperature: 12 900 refers to 20 °C, 17 300 refers to 24 °C. |
|
c |
Range indicated difference between EC50 (lower value) and LC50 (upper value). |
|
d |
Range of values for differences in temperature: 8900 refers to 20 °C, 11 100 refers to 24 °C. |
|
e |
Range of values for different life stages: 10 000 refers to all egg stages and sac fry; 1200 refers to early fry. |
|
f |
Range of values for differences in water hardness: 2370 refers to 200 mg calcium carbonate/litre; 3480 refers to 50 mg calcium carbonate/litre. |
|
g |
Range of values for differences in water hardness: 50 refers to 50 mg calcium carbonate/litre; 60 refers to 200 mg calcium carbonate/litre. |
|
h |
Range of values due to interlaboratory comparisons. |
Chronic toxicity of chlorobenzenes to aquatic organisms is presented in Table 4. Seventy-two-hour EC50s for green algae range from 5280 µg/litre for 1,3-DCB to 200 000 µg/litre for MCB. Chronic NOECs for freshwater invertebrates range from 32 µg/litre (PeCB) to 19 000 µg/litre (MCB) for reproduction and from <1400 to 3890 µg/litre (MCB) for survival. In fish, NOECs range from 18 µg/litre for PeCB to 8500 µg/litre for MCB. The data in this table include the standardized ecotoxicological tests, although any species shown to be more sensitive to the effects of chlorobenzenes have also been included. Care must be taken when interpreting these data, as chlorobenzenes may volatilize from the test system (with the lower chlorinated compounds generally more volatile), thus reducing actual exposure.
Table 4: Chronic toxicity of chlorobenzenes to aquatic organisms.
|
Organism |
End-point |
Chlorobenzene |
Test conditionsa |
Concentration (µg/litre) |
Reference |
|
Microorganisms — Freshwater |
|||||
|
Green algae (Pseudokirchneriella subcapitata)b |
72-h EC50 (population) |
MCB |
N |
202 000 |
US EPA (1978) |
|
1,2-DCB |
N |
76 100 |
US EPA (1978) |
||
|
1,3-DCB |
N |
5280 |
US EPA (1978) |
||
|
1,4-DCB |
N |
77 500 |
US EPA (1978) |
||
|
1,2,4-TCB |
N |
21 700 |
US EPA (1978) |
||
|
1,2,3,5-TeCB |
N |
14 700 |
US EPA (1978) |
||
|
PeCB |
N |
13 000 |
US EPA (1978) |
||
|
Invertebrates — Freshwater |
|||||
|
Water flea (Daphnia magna) |
10-day LC50 |
MCB |
C N |
16 000 |
Cowgill & Milazzo (1991) |
|
9- to 11-day NOEC (survival) |
MCB |
C N |
<1400 |
Cowgill & Milazzo (1991) |
|
|
9- to 11-day EC50 (reproduction) |
MCB |
C N |
15 000–19 000c |
Cowgill & Milazzo (1991) |
|
|
9- to 11-day NOEC (reproduction) |
MCB |
C N |
6500–11 000d |
Cowgill & Milazzo (1991) |
|
|
Four-brood EC50 (reproduction) |
MCB |
M |
1912 |
De Wolf et al. (1988) |
|
|
Four-brood NOEC (reproduction) |
MCB |
M |
1004 |
De Wolf et al. (1988) |
|
|
14-day EC50 (reproduction) |
1,2-DCB |
C M |
550 |
Calamari et al. (1983) |
|
|
16-day EC50 (reproduction) |
1,3-DCB |
M |
1400 |
Hermens et al. (1984) |
|
|
14-day EC50 (reproduction) |
1,4-DCB |
C M |
930 |
Calamari et al. (1983) |
|
|
14-day EC50 (reproduction) |
1,2,3-TCB |
C M |
200 |
Calamari et al. (1983) |
|
|
Four-brood EC50 (reproduction) |
1,2,4-TCB |
M |
330 |
De Wolf et al. (1988) |
|
|
Four-brood NOEC (reproduction) |
1,2,4-TCB |
M |
182 |
De Wolf et al. (1988) |
|
|
14-day EC50 (reproduction) |
1,2,4-TCB |
C M |
450 |
Calamari et al. (1983) |
|
|
Four-brood EC50 (reproduction) |
1,2,3,4-TeCB |
M |
90 |
De Wolf et al. (1988) |
|
|
Four-brood NOEC (reproduction) |
1,2,3,4-TeCB |
M |
55 |
De Wolf et al. (1988) |
|
|
Four-brood EC50 (reproduction) |
PeCB |
M |
61 |
De Wolf et al. (1988) |
|
|
Four-brood NOEC (reproduction) |
PeCB |
M |
32 |
De Wolf et al. (1988) |
|
|
Water flea (Ceriodaphnia dubia) |
7-day LC50 |
MCB |
C N |
24 000 |
Cowgill & Milazzo (1991) |
|
7- to 10-day NOEC (survival) |
MCB |
C N |
3890 |
Cowgill & Milazzo (1991) |
|
|
7- to 10-day EC50 (reproduction) |
MCB |
C N |
14 000–26 000e |
Cowgill & Milazzo (1991) |
|
|
7- to 10-day NOEC (reproduction) |
MCB |
C N |
12 000–19 000f |
Cowgill & Milazzo (1991) |
|
|
Invertebrates — Saltwater |
|||||
|
Crab (Portunus pelagicus) |
40-day NOEC (growth) |
MCB |
C N |
125 |
Mortimer & Connell (1995) |
|
1,4-DCB |
C N |
31 |
Mortimer & Connell (1995) |
||
|
1,2,3-TCB |
C N |
25 |
Mortimer & Connell (1995) |
||
|
1,2,3,4-TeCB |
C N |
17 |
Mortimer & Connell (1995) |
||
|
PeCB |
C N |
5 |
Mortimer & Connell (1995) |
||
|
Fish — Freshwater |
|||||
|
Rainbow trout (Oncorhynchus mykiss) |
85-day LOEC (growth) |
1,2,4-TCB |
M |
36.3–90.75 |
Hodson et al. (1991) |
|
85-day LOEC (survival) |
1,2,4-TCB |
M |
454–853 |
Hodson et al. (1991) |
|
|
Fish — Saltwater |
|||||
|
Sheepshead minnow (Cyprinodon variegatus) |
28-day NOEC (growth) |
PeCB |
18–86g |
Hansen & Cripe (1991) |
|
|
28-day NOEC (survival) |
PeCB |
19–120g |
Hansen & Cripe (1991) |
||
|
a |
C = test carried out in a closed exposure system; M = measured exposure concentration; N = nominal exposure concentration. |
|
b |
Previously known as Selenastrum capricornutum. |
|
c |
Range of EC50 values refers to different measurements of reproduction. EC50 values for progeny, broods, and mean brood size were 15 000, 19 000, and 16 000 µg/litre, respectively. |
|
d |
Range of NOEC values refers to different measurements of reproduction. NOEC values for progeny, broods, and mean brood size were 11 000, 11 000, and 6500 µg/litre, respectively. |
|
e |
Range of EC50 values refers to different measurements of reproduction. EC50 values for progeny, broods, and mean brood size were 14 000, 26 000, and 22 000 µg/litre, respectively. |
|
f |
Range of NOEC values refers to different measurements of reproduction. NOEC values for progeny, broods, and mean brood size were 19 000, 19 000, and 12 000 µg/litre, respectively. |
|
g |
Range of values due to interlaboratory comparisons. |
Concentrations of MCB greater than 2.5 mmol/litre (282 mg/litre) were found to be toxic to Pseudomonas sp. strain RHO1 cells. Cells that had previously been exposed to MCB demonstrated toxicity at concentrations greater than 3.5 mmol/litre (394 mg/litre) (Fritz et al., 1992). Figueroa & Simmons (1991) measured the effect of chlorobenzenes on diatom (Cyclotella meneghiniana) DNA. DNA quantification was used as a biological indicator of cellular biomass. Toxicity increased with increasing chlorination and was related to physicochemical and structural properties. Toxicity was explained in terms of partitioning between the aqueous and biological phases.
The acute toxicity of MCB to daphnids (Daphnia magna and Ceriodaphnia dubia) was higher at 20 °C than at 24 °C. Forty-eight-hour LC50 values for Daphnia magna and Ceriodaphnia dubia were 12 900 and 8900 µg/litre at 20 °C and 17 300 and 11 100 µg/litre at 24 °C, respectively (Cowgill et al., 1985). Rose et al. (1998) reported that Ceriodaphnia dubia was approximately 4 times more sensitive to chlorobenzenes than Daphnia magna. Richter et al. (1983) compared the acute 48-h LC50 values and chronic 28-day NOEC values of 1,3-DCB and 1,2,4-TCB for Daphnia magna. The ratio of acute:chronic toxicity in Daphnia was calculated to be 5.1 and 3.0 for 1,3-DCB and 1,2,4-TCB, respectively. Both the number of young produced per adult and the length of adults as expressions of chronic toxicity were equally sensitive for determining statistically significant effects (Richter et al., 1983; De Wolf et al., 1988). The toxicity of chlorobenzenes to midge (Chironomus riparius) larvae increased with increasing chlorination. Ratios of LC50:NOEC values were 13, 5.0, 2.3–6.6, and 2.4 for 1,4-DCB, 1,2,3-TCB, 1,2,3,4-TeCB, and PeCB, respectively (Roghair et al., 1994). The toxicity of chlorobenzenes to sand crab (Portunus pelagicus) increased during the moulting stage. Toxicity also increased with increasing chlorination and octanol/water partition coefficient (Mortimer & Connell, 1994).
The NOEC, with reference to effects on survival and embryo hatchability for zebrafish (Brachydanio rerio) eggs exposed to MCB, 1,4-DCB, 1,2,3-TCB, 1,2,3,4-TeCB, or PeCB, was reported to be the same, regardless of whether the exposure period was 7, 14, 21, or 28 days (Van Leeuwen et al., 1985). As the test solutions were replaced 3 times per week, the identical NOECs are unlikely to be due to evaporation of the chlorobenzenes. The effect of dissolved oxygen on the toxicity of 1,2,4-TCB to fathead minnows (Pimephales promelas) was examined by Carlson (1987). Fish were exposed to 1,2,4-TCB concentrations of up to 920 µg/litre at 4.5, 5.6, or 8.1 mg dissolved oxygen per litre. At all dissolved oxygen concentrations, no effects on survival or growth were reported at 1,2,4-TCB concentrations up to 500 µg/litre. Reduced survival and mean body weight were reported in fish exposed to 920 µg/litre, with toxicity increased in fish from the low dissolved oxygen group. Van Hoogen & Opperhuizen (1988) calculated the chlorobenzene concentration in guppy (Poecilia reticulata) tissues following lethality in acute toxicity experiments. Lethal concentrations were the same for 1,2,3-TCB, 1,2,3,4-TeCB, and PeCB, at 2.0–2.5 mmol/kg fish. The toxicity of chlorobenzenes to flounder (Platichthys flesus) and sole (Solea solea) increased with increasing chlorination and was similar in each species (Furay & Smith, 1995). LC50 values for sheepshead minnow (Cyprinodon variegatus) exposed to nominal concentrations of chlorinated benzenes were similar for 48-h, 72-h, and 96-h exposure times. This was especially true for MCB and dichlorobenzenes, suggesting volatilization of the test substance (Heitmuller et al., 1981).
The toxicity of chlorobenzenes to a terrestrial bacterium modified with the lux gene (Pseudomonas fluorescens) increased with increasing chlorination. Toxicity also increased with increasing molecular symmetry. EC50 values for inhibited bioluminescence ranged from 0.57 to 118.5 mg/litre for 10 chlorobenzene congeners (Boyd et al., 1998).
EC50 values for growth of lettuce (Lactuca sativa) exposed to chlorobenzenes ranged from 2 to 1000 mg/kg soil in a 7-day test and from 1 to >1000 mg/kg soil in a 14-day test. Toxicity increased with increasing chlorination, up to tetrachlorobenzenes. PeCB was less toxic than all congeners, with the exception of MCB (Hulzebos et al., 1993). LC50 values expressed as the test solution ranged from 0.028 to 9.3 mg/litre.
The toxicity of chlorobenzenes to earthworms is presented in Table 5. LC50 values for the earthworms Eisenia andrei and Lumbricus rubellus range from 75 mg/kg soil for tetrachlorobenzene (isomer not specified) to 1107 mg/kg soil for MCB; however, when the results are expressed as pore water concentrations, the values range from 0.22 µmol/litre for PeCB to 4281 µmol/litre for MCB. Two other species tested appear to be more sensitive, with LC50 values of 0.0016–0.0018 mg/kg soil for 1,2,4-TCB; however, the soil type and pore water concentrations were not given, making a direct comparison with the other earthworm tests difficult.
Table 5: Toxicity of chlorobenzenes to earthworms.
|
Species |
End-point |
Mediuma |
Chlorobenzeneb |
Concentration in soil (mg/kg) |
Concentration in pore water (µmol/litre) |
Reference |
|
Eisenia andrei |
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
MCB |
240 |
1453 |
Van Gestel et al. (1991) |
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
MCB |
446 |
797 |
Van Gestel et al. (1991) |
|
|
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
DCB* |
128 |
121 |
Van Gestel et al. (1991) |
|
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
DCB* |
229 |
347 |
Van Gestel et al. (1991) |
|
|
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
TeCB* |
75 |
1.6 |
Van Gestel et al. (1991) |
|
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
TeCB* |
233 |
1.2 |
Van Gestel et al. (1991) |
|
|
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
PeCB |
134 |
0.47 |
Van Gestel et al. (1991) |
|
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
PeCB |
238 |
0.25 |
Van Gestel et al. (1991) |
|
|
Lumbricus rubellus |
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
MCB |
547 |
4281 |
Van Gestel et al. (1991) |
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
MCB |
1107 |
2243 |
Van Gestel et al. (1991) |
|
|
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
DCB* |
184 |
178 |
Van Gestel et al. (1991) |
|
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
DCB* |
615 |
1556 |
Van Gestel et al. (1991) |
|
|
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
TeCB* |
112 |
2.3 |
Van Gestel et al. (1991) |
|
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
TeCB* |
201 |
1.1 |
Van Gestel et al. (1991) |
|
|
LC50 |
Sandy soil (3.7% OM, pH 4.8) |
PeCB |
115 |
0.43 |
Van Gestel et al. (1991) |
|
|
LC50 |
Artificial soil (8.1% OM, pH 5.9) |
PeCB |
201 |
0.22 |
Van Gestel et al. (1991) |
|
|
Eudrilus eugeniae |
LC50 |
Soil |
1,2,4-TCB |
0.0016 |
Callahan et al. (1994) |
|
|
Allolobaphora tuberculata |
LC50 |
Soil |
1,2,4-TCB |
0.0018 |
Callahan et al. (1994) |
a OM = organic matter.
b Asterisk (*) indicates isomer not specified.
There is a paucity of data on the toxicity of chlorobenzenes to other terrestrial organisms.
Chlorobenzenes are released to the environment during manufacture or use as an intermediate in the production of other chemicals, as a solvent, or as a degreasing agent. Some chlorobenzenes will be released directly to the environment as a result of their use as carriers for pesticides or as room deodorizers.
Chlorobenzenes released to the environment are likely to be volatilized to the atmosphere, although sorption to soils and sediment may also occur. Chlorobenzenes in the atmosphere will be degraded via photochemical oxidation reactions with hydroxyl radicals. Chlorobenzenes in the aquatic and terrestrial environment will be biodegraded, although they may persist under anaerobic conditions.
Very wide ranges of acute toxicity values have been reported for all types of aquatic organisms exposed to the various chlorobenzene congeners. These are summarized in Figure 1. Care must be taken when interpreting these data, as chlorobenzenes may volatilize from the test system (with the lower chlorinated compounds generally more volatile), thus reducing actual exposure. EC50 values for microorganisms and invertebrates range from 8 to 235 000 µg/litre and from 10 to >530 000 µg/litre, respectively. LC50 values for fish range from 135 µg/litre upwards. The toxicity of chlorobenzenes to aquatic organisms increases with increasing chlorination, increasing by over an order of magnitude over the chlorination range (Figure 2). This is partly due to increased uptake and bioaccumulation of higher chlorinated compounds.
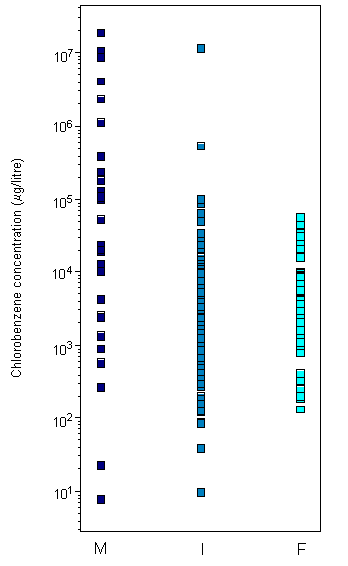
Fig. 1: Acute toxicity of chlorobenzenes (all congeners) to microorganisms (M),
invertebrates (I), and fish (F) (data are from Table 3)
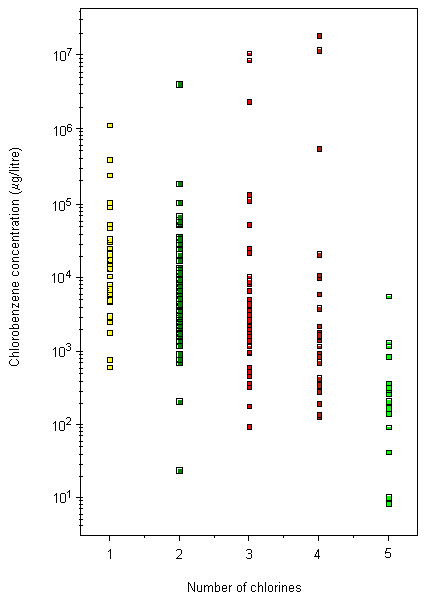
Fig. 2: Acute toxicity of chlorobenzenes to all organisms
related to the chlorination of the congeners
Chronic toxicity tests are available for freshwater organisms (algae, invertebrates, and fish). Lowest NOECs, together with the organism and end-point, are presented in Table 6. Long-term studies of marine organisms reporting NOEC values are available for an invertebrate and a fish species.
Table 6: Calculation of risk factors for aquatic organisms.
|
MCB |
DCBs |
TCBs |
TeCBs |
PeCB |
|
|
Freshwater |
|||||
|
Lowest freshwater chronic NOEC (µg/litre) |
1004 (daphnid reproduction) |
550 (daphnid reproduction)a |
182 (daphnid reproduction) |
55 (daphnid reproduction) |
32 (daphnid reproduction) |
|
Freshwater PNECb (µg/litre) |
20 |
10 |
4 |
1 |
0.6 |
|
Highest measured concentration in fresh water (µg/litre)c |
>10 |
>10 |
8.1 |
200 |
0.0006 |
|
Risk factord (concentration in fresh water/PNEC) |
0.5 |
1 |
2 |
200 |
0.001 |
|
Marine |
|||||
|
Lowest marine chronic NOEC (µg/litre) |
125 (crab growth) |
31 (crab growth) |
25 (crab growth) |
17 (crab growth) |
5 (crab growth) |
|
Marine/estuarine PNECb (µg/litre) |
3 |
0.6 |
0.5 |
0.3 |
0.1 |
|
Highest measured concentration in seawater (µg/litre)c |
0.5 |
0.8 |
5.5 |
0.02 |
0.04 |
|
Risk factord (concentration in seawater/PNEC) |
0.2 |
1 |
10 |
0.07 |
0.4 |
|
a |
Fourteen-day EC50 value used in the absence of a suitable NOEC. |
|
b |
PNEC is calculated by dividing the lowest chronic NOEC by an uncertainty factor of 50 and rounding the calculated value to one significant figure. |
|
c |
Concentration values were the highest mean values reported in individual studies. Data from Yamamoto et al. (1997) were not included in this risk assessment, as it was unclear whether the water samples analysed were fresh, estuarine, or marine. Where data were reported as >10 µg/litre, a value of 10 µg/litre was used to calculate the risk factor. |
|
d |
Risk factors are rounded to one significant figure. |
For compounds studied as extensively as the chlorobenzenes in fresh water, an uncertainty factor of 10 could normally be applied to generate a predicted no-effect concentration (PNEC) for aquatic organisms. However, the lack of chronic NOEC data for algae requires the conservative application of an uncertainty factor of 50. This has been done for both the freshwater and marine data, and results are presented in Table 6.
The concentrations of chlorobenzenes measured in surface waters are presented in Figure 3. This figure includes data points from some additional studies that are not discussed in the text. However, the concentrations in these existing studies fall within the range of concentrations that are discussed in the text. In the 1970s, MCB and dichlorobenzene concentrations in fresh waters ranged up to 10 µg/litre, and trichlorobenzene concentrations ranged up to 1 µg/litre (IPCS, 1991a). Chlorobenzene concentrations measured since the late 1980s show a maximum of 0.6 µg/litre for MCB, a maximum of 130 µg/litre for dichlorobenzenes, and a maximum of 10 µg/litre for trichlorobenzenes (Figure 3). Tetrachlorobenzene concentrations in fresh water show a maximum of 200 µg/litre, and PeCB, 0.0006 µg/litre; these are older measurements (Figure 3).

Fig. 3: Concentrations of chlorobenzene congeners in surface waters
Lowest chronic NOECs, PNECs, highest measured water concentrations, and risk factors are summarized in Table 6. Risk factors at, below, or substantially below 1 are generated for all chlorinated benzene congeners with the exception of trichlorobenzenes and tetrachlorobenzenes. Factors of 2 and 10 for trichlorobenzenes in fresh water and seawater, respectively, and a factor of 200 for tetrachlorobenzene in fresh water indicate some risk, particularly for trichlorobenzenes in seawater and tetrachlorobenzenes in fresh water, with the use of the precautionary uncertainty factor of 50.
The data set on freshwater concentrations of chlorobenzenes is limited, and the high risk factors for trichlorobenzenes and tetrachlorobenzenes come from monitoring studies carried out during the early to mid-1980s. The trichlorobenzene concentrations were reported in a single study of Spanish rivers in industrial areas conducted in the mid-1980s. The highest reported concentrations come from one small river with a low flow rate. The highest tetrachlorobenzene concentrations were reported from areas near dump sites. No follow-up monitoring is available for either of these sites. The only conclusion that can be drawn is that under these exceptional circumstances, concentrations of trichlorobenzenes can exceed those likely to produce long-term, but not acute, toxic effects; it can be presumed that these concentrations would arise from point sources. More generally, measured levels of trichlorobenzenes are substantially lower than this, and risk factors would be below 1.
There are more extensive measurements of chlorobenzenes in estuaries and coastal waters. The risk factors in estuarine water exceeding 1 relate to point sources from industrial plants pre-1989. Follow-up monitoring is available for these plants following control of release and/or replacement of chlorobenzenes with alternatives. These more recent data indicate trichlorobenzene concentrations between 1 and 2 orders of magnitude lower. As with fresh water, uncontrolled point source release of trichlorobenzenes will lead to high local risk to organisms.
Terrestrial data, both toxicity studies and measured levels in soil, are inadequate to perform a risk assessment.
Chlorinated benzenes were reviewed by the World Health Organization in 1991 (IPCS, 1991a).
Abernethy S, Bobra AM, Shiu WY, Wells PG, Mackay D (1986) Acute lethal toxicity of hydrocarbons and chlorinated hydrocarbons to two planktonic crustaceans. The key role of organism–water partitioning. Aquatic Toxicology, 8:163–174.
Alberti J (1983) Organic contaminants in river sediments. Vom Wasser, 61:149–154.
Anderson TA, Beauchamp JJ, Walton BT (1991) Organic chemicals in the environment. Fate of volatile and semivolatile organic chemicals in soils: Abiotic versus biotic losses. Journal of Environmental Quality, 20(2):420–424.
Arnts RR, Seila RL, Bufalini JJ (1989) Determination of room temperature OH rate constants for acetylene, ethylene dichloride, ethylene dibromide, p-dichlorobenzene and carbon disulfide. Journal of the Air and Waste Management Association, 39(4):453–460.
Atkinson R, Aschmann SM, Winer AM, Pitts JN Jr (1985) Atmospheric gas phase loss processes for chlorobenzene, benzotrifluoride, and 4-chlorobenzotrifluoride, and generalization of predictive techniques for atmospheric lifetimes of aromatic compounds. Archives of Environmental Contamination and Toxicology, 14(4):417–425.
ATSDR (1990) Toxicological profile for chlorobenzene. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
ATSDR (1998) Toxicological profile for 1,4-dichlorobenzene (update). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
Ball WP, Roberts PV (1991) Long-term sorption of halogenated organic chemicals by aquifer material. 1. Equilibrium. Environmental Science and Technology, 25(7):1223–1237.
Barber LB II (1988) Dichlorobenzene in ground water: Evidence for long-term persistence. Ground Water, 26(6):696–732.
Barber LB II, Thurman EM, Runnells DR (1992) Geochemical heterogeneity in a sand and gravel aquifer: Effect of sediment mineralogy and particle size on the sorption of chlorobenzenes. Journal of Contaminant Hydrology, 9(1–2):35–54.
Bartholomew GW, Pfaender FK (1983) Influence of spatial and temporal variations on organic pollutant biodegradation rates in an estuarine environment. Applied Environmental Microbiology, 45(1):103–109.
Beil S, Happe B, Timmis KN, Pieper DH (1997) Genetic and biochemical characterization of the broad spectrum chlorobenzene dioxygenase from Burkholderia sp. strain PS12: dechlorination of 1,2,4,5-tetrachlorobenzene. European Journal of Biochemistry, 247(1):190–199.
Belfroid AC, Sijm DTH (1998) Influence of soil organic matter content on elimination rates of hydrophobic compounds in the earthworm: Possible causes and consequences. Chemosphere, 37(7):1221–1234.
Belfroid A, Van Wezel A, Sikkenk M, Van Gestel K, Seinen W, Hermens J (1993) The toxicokinetic behavior of chlorobenzenes in earthworms (Eisenia andrei): Experiments in water. Ecotoxicology and Environmental Safety, 25(2):154–165.
Belfroid A, Sikkenk M, Seinen W, Van Gestel K, Hermens J (1994) The toxicokinetic behavior of chlorobenzenes in earthworm (Eisenia andrei) experiments in soil. Environmental Toxicology and Chemistry, 13(1):93–99.
Belfroid A, Van den Berg M, Seinen W, Hermens J, Van Gestel K (1995) Uptake, bioavailability and elimination of hydrophobic compounds in earthworms (Eisenia andrei) in field-contaminated soil. Environmental Toxicology and Chemistry, 14(4):605–612.
Beurskens JEM, Stams AJM, Zehnder AJB, Bachmann A (1991) Relative biochemical reactivity of three hexachlorocyclohexane isomers. Ecotoxicology and Environmental Safety, 21(2):128–136.
Birge WJ, Black JA, Bruser DM (1979a) Toxicity of organic chemicals to embryo-larval stages of fish. Washington, DC, US Environmental Protection Agency (Report No. EPA 560/11-79-007).
Birge WJ, Black JA, Hudson JE, Bruser DM (1979b) Embryo-larval toxicity tests with organic compounds. In: Marking LL, Kimerle RA, eds. Aquatic toxicology. Philadelphia, PA, American Society for Testing and Materials, pp. 131–147.
Bossi R, Larsen B, Premazzi G (1992) Polychlorinated biphenyl congeners and other chlorinated hydrocarbons in bottom sediment cores of Lake Garda (Italy). The Science of the Total Environment, 121:77–93.
Boyd EM, Meharg AA, Wright J, Killham K (1998) Toxicity of chlorobenzenes to a lux-marked terrestrial bacterium, Pseudomonas fluorescens. Environmental Toxicology and Chemistry, 17(11):2134–2140.
Brooke LT (1991) Results of freshwater exposures with the chemicals atrazine, biphenyl, butachlor, carbaryl, carbazole, dibenzofuran, 3,3-dichlorodibenzidine, dichlorvos. Superior, WI, University of Wisconsin – Superior, Center for Lake Superior Environmental Studies.
Brunsbach FR, Reineke W (1994) Degradation of chlorobenzenes in soil slurry by a specialized organism. Applied Microbiology and Biotechnology, 42(2–3):415–420.
Brusseau ML (1991) Cooperative sorption of organic chemicals in systems composed of low organic carbon aquifer materials. Environmental Science and Technology, 25(10):1747–1752.
Bunce NJ, Landers JP, Langshaw JA, Nakai JS (1989) An assessment of the importance of direct solar degradation of some simple chlorinated benzenes and biphenyls in the vapor phase. Environmental Science and Technology, 23(2):213–218.
Calamari D, Galassi S, Setti F, Vighi M (1983) Toxicity of selected chlorobenzenes to aquatic organisms. Chemosphere, 12(2):253–262.
Call DJ, Brooke LT, Ahmad N (1979) Toxicity, bioconcentration and metabolism of selected chemicals in aquatic organisms. Third quarterly report to EPA. Superior, WI, University of Wisconsin – Superior (Cooperative Agreement No. CR 806864020).
Call DJ, Brooke LT, Ahmad N, Richter JE (1983) Toxicity and metabolism studies with EPA priority pollutants and related chemicals in freshwater organisms. Washington, DC, US Environmental Protection Agency (Report No. EPA 600/3-83-095).
Callahan CA, Shirazi MA, Neuhauser EF (1994) Comparative toxicity of chemicals to earthworms. Environmental Toxicology and Chemistry, 13(2):291–298.
Canton JH, Slooff W, Kool HJ, Struys J, Gouw TJM, Wegman RCC, Piet GJ (1985) Toxicity, biodegradability and accumulation of a number of Cl/N-containing compounds for classification and establishing water quality criteria. Regulatory Toxicology and Pharmacology, 5:123–131.
Carlson AR (1987) Effects of lowered dissolved oxygen concentration on the toxicity of 1,2,4-trichlorobenzene to fathead minnows. Bulletin of Environmental Contamination and Toxicology, 38(4):667–673.
Carlson AR, Kosian PA (1987) Toxicity of chlorinated benzenes to fathead minnows (Pimephales promelas). Archives of Environmental Contamination and Toxicology, 16(2):129–135.
Chaisuksant Y, Yu Q, Connell DW (1998) Effects of halobenzenes on growth rate of fish (Gambusia affinis). Ecotoxicology and Environmental Safety, 39(2):120–130.
Chemical Daily Company (1999) [1999 annual of chemical industry.] Tokyo, The Chemical Daily Company Limited, p. 193 (in Japanese).
Chemical Daily Company (2000) [2000 annual of chemical industry.] Tokyo, The Chemical Daily Company Limited, p. 718 (in Japanese).
Chiou CT, Shoup TD (1985) Soil sorption of organic vapors and effects of humidity on sorptive mechanism and capacity. Environmental Science and Technology, 19(12):1196–1200.
Choudhry GG, Hutzinger O (1984) Acetone-sensitized and nonsensitized photolyses of tetra-, penta-, and hexachlorobenzenes in acetonitrile–water mixtures: Photoisomerization and formation of several products including polychlorobiphenyls. Environmental Science and Technology, 18(4):235–241.
Chu W, Jafvert CT (1994) Photodechlorination of polychlorobenzene congeners in surfactant micelle solutions. Environmental Science and Technology, 28(13):2415–2422.
Ciborowski JJH, Corkum LD (1988) Organic contaminants in adult aquatic insects of the St. Clair and Detroit rivers, Ontario, Canada. Journal of Great Lakes Research, 14(2):148–156.
Clark JR, Patrick JM, Moore JC, Lores EM (1987) Waterborne and sediment-source toxicities of six organic chemicals to grass shrimp (Palaemonetes pugio) and amphioxus (Branchiostoma caribaeum). Archives of Environmental Contamination and Toxicology, 16:401–407.
Cobb GP, Norman DM, Kendall RJ (1994) Organochlorine contaminant assessment in great blue herons using traditional and nonlethal monitoring techniques. Environmental Pollution, 83(3):299–309.
Cowgill UM, Milazzo DP (1991) The sensitivity of Ceriodaphnia dubia and Daphnia magna to seven chemicals utilizing the three-brood test. Archives of Environmental Contamination and Toxicology, 20(2):211–217.
Cowgill UM, Takahashi IT, Applegath SL (1985) A comparison of the effect of four benchmark chemicals on Daphnia magna and Ceriodaphnia dubia-affinis tested at two different temperatures. Environmental Toxicology and Chemistry, 4:415–422.
Curtis MW, Copeland TL, Ward CH (1979) Acute toxicity of 12 industrial chemicals to freshwater and saltwater organisms. Water Research, 13:137–141.
Dalich GM, Larson RE, Gingerich WH (1982) Acute and chronic toxicity studies with monochlorobenzene in rainbow trout. Aquatic Toxicology, 2:127–142.
De Boer J, Van der Valk F, Kerkhoff MAT, Hagel P, Brinkman UAT (1994) 8-year study on the elimination of PCBs and other organochlorine compounds from eel (Anguilla anguilla) under natural conditions. Environmental Science and Technology, 28(13):2242–2248.
De Bont JAM, Vorage M, Hartmans S, Van Den Tweel WJJ (1986) Microbial degradation of 1,3-dichlorobenzene. Applied Environmental Microbiology, 52(4):677–680.
De Wolf W, Canton JH, Deneer JW, Wegman RCC, Hermens JLM (1988) Quantitative structure–activity relationships and mixture-toxicity studies of alcohols and chlorohydrocarbons: Reproducibility of effects on growth and reproduction of Daphnia magna. Aquatic Toxicology, 12(1):39–49.
Dietz A, Schnoor JL (2001) Advances in phytoremediation. Environmental Health Perspectives, 109(Suppl. 1):163–168.
Dilling WL, Gonsior SJ, Boggs GU, Mendoza CG (1988) Organic photochemistry. 20. A method for estimating gas-phase rate constants for reactions of hydroxyl radicals with organic compounds from their relative rates of reaction with hydrogen peroxide under photolysis in 1,1,2-trichlorotrifluoroethane solution. Environmental Science and Technology, 22(12):1447–1453.
Dilmeghani M, Zahir KO (2001) Kinetics and mechanism of chlorobenzene degradation in aqueous samples using advanced oxidation processes. Journal of Environmental Quality, 30(6):2062–2070.
DiVincenzo JP, Dentel SK (1996) Sorption–desorption of 1,2,4-trichlorobenzene on soil: Anionic surfactant and cationic polyelectrolyte effects. Journal of Environmental Quality, 25(6):1193–1202.
Dulin D, Drossman H, Mill T (1986) Products and quantum yields for photolysis of chloroaromatics in water. Environmental Science and Technology, 20(1):72–77.
EC (2001) 1,4-Dichlorobenzene risk assessment. Ispra, European Commission (Report R001-0105-ENV-HH).
EC (2003) 1,2,4-Trichlorobenzene summary risk assessment report. Ispra, European Commission, Joint Research Centre (Special Publication I.02.157).
Elder VA, Proctor BL, Hites RA (1981) Organic compounds found near dump sites in Niagara Falls, New York. Environmental Science and Technology, 15(10):1237–1243.
Fairlee JR, Burback BL, Perry JJ (1997) Biodegradation of groundwater pollutants by a combined culture of Mycobacterium vaccae and a Rhodococcus sp. Canadian Journal of Microbiology, 43(9):841–846.
Faschan A, Tittlebaum M, Cartledge F (1993) Nonionic organic partitioning onto organoclays. Hazardous Waste & Hazardous Materials, 10(3):313–322.
Feidieker D, Kampfer P, Dott W (1994) Microbiological and chemical evaluation of a site contaminated with chlorinated aromatic compounds and hexachlorocyclohexanes. FEMS Microbiology Ecology, 15(3–4):265–278.
Fielding M, Gibson TM, James HA (1981) Levels of trichloroethylene and p-dichlorobenzene in groundwaters. Environmental Technology Letters, 2(12):545–550.
Figge K, Metzdorf U, Nevermann J, Schmiese J, Keskin M, Fortnagel P, Wittich R-M (1993) Bakterielle mineralisierung von dibenzofuran, dibenzo-p-dioxin und 1,2,4,5-tetrachlorobenzol in Böden. Umweltwissenschaften und Schadstoff-Forschung Zeitschrift für Umweltchemie und Ökotoxikologie, 5(3):122–130.
Figueroa IDC, Simmons MS (1991) Structure–activity relationships of chlorobenzenes using DNA measurement as a toxicity parameter in algae. Environmental Toxicology and Chemistry, 10(3):323–329.
Fritz H, Reineke W, Schmidt E (1992) Toxicity of chlorobenzene on Pseudomonas sp. strain RHO1, a chlorobenzene-degrading strain. Biodegradation, 2:165–170.
Furay VJ, Smith S (1995) Toxicity and QSAR of chlorobenzenes in two species of benthic flatfish, flounder (Platichthys flesus L.) and sole (Solea solea L.). Bulletin of Environmental Contamination and Toxicology, 54(1):36–42.
Galassi S, Calamari D (1983) Toxicokinetics of 1,2,3 and 1,2,4 trichlorobenzenes in early life stages of Salmo gairdneri. Chemosphere, 12(11–12):1599–1603.
Gallard H, De Laat J (2001) Kinetics of oxidation of chlorobenzenes and phenyl-ureas by Fe(II)/H2O2 and Fe(III)/H2O2. Evidence of reduction and oxidation reactions of intermediates by Fe(II) or Fe(III). Chemosphere, 42(4):405–413.
Gebauer MB, Weseloh DV (1993) Accumulation of organic contaminants in sentinel mallards utilizing confined disposal facilities at Hamilton Harbour, Lake Ontario, Canada. Archives of Environmental Contamination and Toxicology, 25(2):234–243.
Geiger DL, Brooke LT, Call DJ, eds. (1990) Acute toxicities of organic chemicals to fathead minnows (Pimephales promelas). Vol. 5. Superior, WI, University of Wisconsin – Superior, Center for Lake Superior Environmental Studies.
Gejlsbjerg B, Klinge C, Madsen T (2001) Mineralization of organic contaminants in sludge–soil mixtures. Environmental Toxicology and Chemistry, 20(4):698–705.
Gersich FM, Blanchard FA, Applegath SL, Park CN (1986) The precision of daphnid (Daphnia magna Straus, 1820) static acute toxicity tests. Archives of Environmental Contamination and Toxicology, 15(6):741–749.
Gess P, Pavlostathis SG (1997) Desorption of chlorinated organic compounds from a contaminated estuarine sediment. Environmental Toxicology and Chemistry, 16(8):1598–1605.
Geyer H, Scheunert I, Korte F (1985) Relationship between the lipid content of fish and their bioconcentration potential of 1,2,4-trichlorobenzene. Chemosphere, 14(5):545–555.
Giddings M, Meek ME, Gomes R (1994a) Pentachlorobenzene: Evaluation of risks to health from environmental exposure in Canada. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis and Ecotoxicology Reviews, 12(2):435–441.
Giddings M, Meek ME, Gomes R (1994b) Tetrachlorobenzenes: Evaluation of risks to health from environmental exposure in Canada. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis and Ecotoxicology Reviews, 12(2):473–481.
Giddings M, Meek ME, Gomes R (1994c) Trichlorobenzenes: Evaluation of risks to health from environmental exposure in Canada. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis and Ecotoxicology Reviews, 12(2):517–525.
Gomez Belinchon JI, Grimalt JO, Albaiges J (1991) Volatile organic compounds in two polluted rivers in Barcelona (Catalonia, Spain). Water Research, 25(5):577–589.
Götz R, Friesel P, Roch K, Papke O, Ball M, Lis A (1993) Polychlorinated p-dioxins (PCDDs), dibenzofurans (PCDFs), and other chlorinated compounds in the river Elbe: Results on bottom sediments and fresh sediments collected in sedimentation chambers. Chemosphere, 27(1–3):105–111.
Grosjean D (1991) Atmospheric fate of toxic aromatic compounds. The Science of the Total Environment, 100:367–414.
Hanai Y, Katou T, Jimma T, Nomura K (1985) [The movement of p-dichlorobenzene in the atmosphere.] Yokohama Kokuritsu Daigaku Kankyo Kagaku Kenkyu Senta Kiyo, 12:31–39 (in Japanese).
Hansen DJ, Cripe GM (1991) Interlaboratory comparisons of the early life stage toxicity test using sheepshead minnows (Cyprinodon variegatus). In: Mayes MA, Barron MG, eds. Aquatic toxicology and risk assessment. Vol. 14. Philadelphia, PA, American Society for Testing and Materials (Special Technical Publication No. 1124).
Harner T, Mackay D (1995) Measurement of octanol–air partition coefficients for chlorobenzenes, PCBs, and DDT. Environmental Science and Technology, 29(6):1599–1606.
Harper DJ, Ridgeway IM, Leatherland TM (1992) Concentrations of hexachlorobenzene, trichlorobenzenes and chloroform in the waters of the Forth Estuary, Scotland. Marine Pollution Bulletin, 24(5):244–249.
He Y, Wang Y, Lee HK (2000) Trace analysis of ten chlorinated benzenes in water by headspace solid-phase microextraction. Journal of Chromatography A, 874(1):149–154.
Heitmuller PT, Hollister TA, Parrish PR (1981) Acute toxicity of 54 industrial chemicals to sheepshead minnows (Cyprinodon variegatus). Bulletin of Environmental Contamination and Toxicology, 27:596–604.
Hermanson MH, Monosmith CL, Donnelly-Kelleher MT (1997) Seasonal and spatial trends of certain chlorobenzene isomers in the Michigan atmosphere. Atmospheric Environment, 31(4):567–573.
Hermens J, Canton H, Steyger N, Wegman R (1984) Joint effects of a mixture of 14 chemicals on mortality and inhibition of reproduction of Daphnia magna. Aquatic Toxicology, 5:315–322.
Hiatt MH (1981) Analysis of fish and sediment for volatile priority pollutants. Analytical Chemistry, 53(9):1541–1543.
Hodson PV, Parisella B, Blunt B, Gray B, Kaiser KLE (1991) Quantitative structure–activity relationships for chronic toxicity of phenol, p-phenol, 2,4-dichlorophenol, pentachlorophenol, p-nitrophenol. Ottawa, Ontario, Fisheries and Oceans Canada (Canadian Technical Report of Fisheries and Aquatic Sciences No. 1784).
Hoff RM, Muir DCG, Grift NP (1992) Annual cycle of polychlorinated biphenyls and organohalogen pesticides in air in southern Ontario. 1. Air concentration data. Environmental Science and Technology, 26(2):266–275.
Holliger C, Schraa G, Stams AJM, Zehnder AJB (1992) Enrichment and properties of an anaerobic mixed culture reductively dechlorinating 1,2,3-trichlorobenzene to 1,3-dichlorobenzene. Applied Environmental Microbiology, 58(5):1636–1644.
Hulzebos EM, Adema DMM, Dirven-Van Breemen EM, Henzen L, Van Dis WA, Herbold HA, Hoekstra JA, Baerselman R, Van Gestel CAM (1993) Phytotoxicity studies with Lactuca sativa in soil and nutrient solution. Environmental Toxicology and Chemistry, 12:1079–1094.
Huybrechts T, Dewulf J, Moerman O, Van Langenhove H (2000) Evaluation of purge-and-trap–high-resolution gas chromatography–mass spectrometry for the determination of 27 volatile organic compounds in marine water at the ng l–1 concentration level. Journal of Chromatography A, 893(2):367–382.
IPCS (1991a) Chlorobenzenes other than hexachlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 128).
IPCS (1991b) Lindane. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 124).
IPCS (1997) Hexachlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 195).
IPCS (2000) 1,3-Dichlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1095).
IPCS (2003a) 1,4-Dichlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0037).
IPCS (2003b) 1,3,5-Trichlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0344).
IPCS (2003c) Pentachlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0531).
IPCS (2003d) Chlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0642).
IPCS (2003e) 1,2,4,5-Tetrachlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 0676).
IPCS (2003f) 1,2,4-Trichlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1049).
IPCS (2003g) 1,2-Dichlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1066).
IPCS (2003h) 1,2,3-Trichlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (International Chemical Safety Card 1222).
Jan J, Zupancic Kralj L, Kralj B, Marsel J (1994) The influence of exposure time and transportation routes on the pattern of organochlorines in plants from a polluted region. Chemosphere, 29(8):1603–1610.
Jungclaus GA, Lopez-Avila V, Hites RA (1978) Organic compounds in an industrial wastewater: a case study of their environmental impact. Environmental Science and Technology, 12(1):88–96.
Keener WK, Arp DJ (1994) Transformations of aromatic compounds by Nitrosomonas europaea. Applied Environmental Microbiology, 60(6):1914–1920.
Kiernicka J, Seignez C, Peringer P (1999) Escherichia hermanii — A new bacterial strain for chlorobenzene degradation. Letters in Applied Microbiology, 28(1):27–30.
Klöpffer W, Frank R, Kohl EG, Haag F (1986) Quantitative registration of photochemical transformation processes in the troposphere. Chemiker-Zeitung, 110(2):57–61.
Klöpffer W, Haag F, Kohl EG, Frank R (1988) Testing of the abiotic degradation of chemicals in the atmosphere: The smog chamber approach. Ecotoxicology and Environmental Safety, 15(3):298–319.
Knezovich JP, Harrison FL (1988) The bioavailability of sediment-sorbed chlorobenzenes to larvae of the midge, Chironomus decorus. Ecotoxicology and Environmental Safety, 15:226–241.
Koelmans AA, Jimenez CS (1994) Temperature dependency of chlorobenzene bioaccumulation in phytoplankton. Chemosphere, 28(12):2041–2048.
Könemann H (1981) Quantitative structure–activity relationships in fish toxicity studies. Part 1. Relationship for 50 industrial pollutants. Toxicology, 19:209–221.
Könemann H, Van Leeuwen K (1980) Toxicokinetics in fish: accumulation and elimination of six chlorobenzenes by guppies. Chemosphere, 9:3–19.
Kraaij H, Connell DW (1997) Bioconcentration and uptake kinetics of chlorobenzenes in soy-bean roots. Chemosphere, 34(12):2607–2620.
Krost KJ, Pellizzari ED, Walburn SG, Hubbard SA (1982) Collection and analysis of hazardous organic emissions. Analytical Chemistry, 54:810–817.
Kuehl DW, Leonard EN, Welch KJ, Veith JD (1980) Identification of hazardous chemicals in fish from the Ashtabula River, Ohio, and Wabash River, Indiana. Journal of the Association of Official Analytical Chemists, 63(6):1238–1244.
Langhorst ML, Nestrick TJ (1979) Determination of chlorobenzenes in air and biological samples by gas chromatography with photoionization detection. Analytical Chemistry, 51(12):2018–2025.
Law RJ, Fileman TW, Matthiessen P (1991) Phthalate esters and other industrial organic chemicals in the North and Irish seas. Water Science and Technology, 24(10):127–134.
LeBlanc GA (1980) Acute toxicity of priority pollutants to water flea (Daphnia magna). Bulletin of Environmental Contamination and Toxicology, 24:684–691.
Lee CL, Fang MD (1997) Sources and distribution of chlorobenzenes and hexachlorobutadiene in surficial sediments along the coast of southwestern Taiwan. Chemosphere, 35(9):2039–2050.
Lee S, Kommalapati RR, Valsaraj KT, Pardue JH, Constant WD (2002) Rate-limited desorption of volatile organic compounds from soils and implications for the remediation of a Louisiana Superfund site. Environmental Monitoring and Assessment, 75(1):87–105.
Lopez-Avila V, Northcutt R, Onstot J, Wickham M, Billets S (1983) Determination of 51 priority organic compounds after extraction from standard reference material. Analytical Chemistry, 55(6):881–889.
Lunde G, Ofstad EB (1976) Determination of fat-soluble chlorinated compounds in fish. Zeitschrift für Analytische Chemie, 282:395–399.
Mackay D, Di Guardo A, Paterson S, Kicsi G, Cowan CE, Kane DM (1996) Assessment of chemical fate in the environment using evaluative, regional and local-scale models: Illustrative application to chlorobenzene and linear alkylbenzene sulfonates. Environmental Toxicology and Chemistry, 15(9):1638–1648.
Marchini S, Hoglund MD, Borderius SJ, Tosato ML (1993) Comparison of the susceptibility of daphnids and fish to benzene derivatives. The Science of the Total Environment (1993 Suppl.):799–808.
Martinez E, Llobet I, Lacorte S, Viana P, Barcelo D (2002) Patterns and levels of halogenated volatile compounds in Portuguese surface waters. Journal of Environmental Monitoring, 4(2):253–257.
Masunaga S, Urushigawa Y, Yonezawa Y (1991a) The behaviour of chlorobenzenes in Ise Bay, estimated from their concentrations in various environmental media. Water Research, 25(3):289–297.
Masunaga S, Yonezawa Y, Urushigawa Y (1991b) The distribution of chlorobenzenes in the bottom sediments of Ise Bay. Water Research, 25(3):275–288.
Masunaga S, Susarla S, Yonezawa Y (1996a) Dechlorination of chlorobenzenes in anaerobic estuarine sediment. Water Science and Technology, 33(6):173–180.
Masunaga S, Urushigawa Y, Yonezawa Y, Fukui M (1996b) Partitioning of chlorobenzenes between suspended particulates and water in coastal waters. Journal of Environmental Science and Health, Part A, Environmental Science and Engineering & Toxic and Hazardous Substance Control, 31(4):887–903.
Mayer FL, Ellersieck MR (1986) 1,4-Dichlorobenzene. In: Manual of acute toxicity: interpretation and data base for 410 chemicals and 66 species of freshwater animals. Washington, DC, US Department of the Interior, Fish and Wildlife Service, 505 pp.
Meckenstock R, Steinle P, Van der Meer JR, Snozzi M (1998) Quantification of bacterial mRNA involved in degradation of 1,2,4-trichlorobenzene by Pseudomonas sp. strain P51 from liquid culture and from river sediment by reverse transcriptase PCR (RT/PCR). FEMS Microbiology Letters, 167(2):123–129.
Meek ME, Giddings M, Gomes R (1994a) 1,2-Dichlorobenzene: Evaluation of risks to health from environmental exposure in Canada. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis and Ecotoxicology Reviews, 12(2):269–275.
Meek ME, Giddings M, Gomes R (1994b) 1,4-Dichlorobenzene: Evaluation of risks to health from environmental exposure in Canada. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis and Ecotoxicology Reviews, 12(2):277–285.
Meek ME, Giddings M, Gomes R (1994c) Monochlorobenzene: Evaluation of risks to health from environmental exposure in Canada. Journal of Environmental Science and Health, Part C, Environmental Carcinogenesis and Ecotoxicology Reviews, 12(2):409–415.
Meharg AA, Wright J, Osborn D (2000) Chlorobenzenes in rivers draining industrial catchments. The Science of the Total Environment, 251/252:243–253.
Melancon MJ, Lech JJ (1985) The uptake, distribution and elimination of di-, tri-, tetra-, and pentachlorobenzene in rainbow trout. Federation Proceedings, 44(3):614.
Meunier L, Pilichowski JF, Boule P (2001) Photochemical behaviour of 1,4-dichlorobenzene in aqueous solution. Canadian Journal of Chemistry, 79(7):1179–1186.
Miles DH, Mody NV, Minyard JP (1973) Constituents of marsh grass: survey of the essential oils in Juncus roemerianus. Phytochemistry, 12:1399–1404.
Mortimer MR, Connell DW (1994) Critical internal and aqueous lethal concentrations of chlorobenzenes with the crab Portunus pelagicus (L). Ecotoxicology and Environmental Safety, 28(3):298–312.
Mortimer MR, Connell DW (1995) Effect of exposure to chlorobenzenes on growth rates of the crab Portunus pelagicus (L). Ecotoxicology and Environmental Safety, 29(8):1881–1886.
Muir DCG, Ford CA, Grift NP, Stewart REA, Bidleman TF (1992) Organochlorine contaminants in narwhal (Monodon monoceros) from the Canadian Arctic. Environmental Pollution, 75(3):307–316.
Nielsen PH, Christensen TH (1994) Variability of biological degradation of aromatic hydrocarbons in an aerobic aquifer determined by laboratory batch experiments. Journal of Contaminant Hydrology, 15(4):305–320.
Nielsen PH, Bjarnadottir H, Winter PL, Christensen TH (1995) In situ and laboratory studies of the fate of specific organic compounds in an anaerobic landfill leachate plume. 2. Fate of aromatic and chlorinated aliphatic compounds. Journal of Contaminant Hydrology, 20(1–2):51–66.
Nishino SF, Spain JC, Belcher LA, Litchfield CD (1992) Chlorobenzene degradation by bacteria isolated from contaminated groundwater. Applied Environmental Microbiology, 58(5):1719–1726.
Nishino SF, Spain JC, Pettigrew CA (1994) Biodegradation of chlorobenzene by indigenous bacteria. Environmental Toxicology and Chemistry, 13(6):871–877.
Njoroge BNK, Ball WP, Cherry RS (1998) Sorption of 1,2,4-trichlorobenzene and tetrachloroethene within an authigenic soil profile: Changes in Koc with soil depth. Journal of Contaminant Hydrology, 29(4):347–377.
Nowak J, Kirsch NH, Hegemann W, Stan HJ (1996) Total reductive dechlorination of chlorobenzenes to benzene by a methanogenic mixed culture enriched from Saale river sediment. Applied Microbiology and Biotechnology, 45(5):700–709.
Oliver BG, Bothen KD (1980) Determination of chlorobenzenes in water by capillary gas chromatography. Analytical Chemistry, 52:2066–2069.
Oliver BG, Bothen KD (1982) Extraction and clean-up procedures for measuring chlorobenzenes in sediments and fish by capillary gas chromatography. International Journal of Environmental and Analytical Chemistry, 12:131–139.
Oliver BG, Nicol KD (1982) Chlorobenzenes in sediments, water, and selected fish from lakes Superior, Huron, Erie, and Ontario. Environmental Science and Technology, 16:532–536.
Oliver BG, Niimi AJ (1983) Bioconcentration of chlorobenzenes from water by rainbow trout: Correlations with partition coefficients and environmental residues. Environmental Science and Technology, 17(5):287–291.
Onuska FI, Terry KA (1985) Determination of chlorinated benzenes in bottom sediment samples by WCOT column gas chromatography. Analytical Chemistry, 57(4):801–805.
Otson R, Williams DT (1981) Evaluation of a liquid–liquid extraction technique for water pollutants. Journal of Chromatography, 212:187–197.
Otson R, Williams DT (1982) Headspace chromatographic determination of water pollutants. Analytical Chemistry, 54(6):942–946.
Pankow JF, Isabelle LM (1982) Adsorption–thermal desorption as a method for the determination of low levels of aqueous organics. Journal of Chromatography, 237:25–39.
Pankow JF, Isabelle LM, Asher WE, Kristensen TJ, Peterson ME (1983) Organic compounds in Los Angeles and Portland rain: identities, concentrations, and operative scavenging mechanisms. In: Pruppacher HR, Semonin RG, Slinen WGN, eds. Precipitation scavenging, dry deposition and resuspensions: Proceedings of the 4th International Conference, Santa Monica, California, 29 November – 3 December 1982. New York, NY, Elsevier, pp. 403–415.
Pellizzari ED, Hartwell TD, Harris BSH, Waddell RD, Whitaker DA, Erikson MD (1982) Purgeable organic compounds in mother’s milk. Bulletin of Environmental Contamination and Toxicology, 28:322–328.
Pereira WE, Hughes BA (1980) Determination of selected volatile organic priority polllutants in water by computerized spectrometry. Journal of the American Water Works Association, 72(4):220–230.
Pettigrew CA, Haigler BE, Spain JC (1991) Simultaneous biodegradation of chlorobenzene and toluene by a Pseudomonas strain. Applied Environmental Microbiology, 57(1):157–162.
Piet GJ, Slingerland P, Bijlsma GH, Morra C (1980) Fast quantitative analysis of a wide variety of halogenated compounds in surface-, drinking-, and ground-water. Environmental Science Research, 16:69–80.
Piwoni MD, Wilson JT, Walters DM, Wilson BH, Engfield CG (1986) Behavior of organic pollutants during rapid-infiltration of wastewater into soil: I. Processes, definition, and characterization using a microcosm. Hazardous Waste & Hazardous Materials, 3(1):43–55.
Popp P, Bruggemann L, Keil P, Thuss U, Weiss H (2000) Chlorobenzenes and hexachlorocyclohexanes (HCHs) in the atmosphere of Bitterfeld and Leipzig (Germany). Chemosphere, 41(6):849–855.
Prytula MT, Pavlostathis SG (1996) Extraction of sediment-bound chlorinated organic compounds: Implications on fate and hazard assessment. Water Science and Technology, 33(6):247–254.
Qiao P, Farrell AP (1996) Uptake of hydrophobic xenobiotics by fish in water laden with sediments from the Fraser River. Environmental Toxicology and Chemistry, 15(9):1555–1563.
Qiao P, Gobas F, Farrell AP (2000) Relative contributions of aqueous and dietary uptake of hydrophobic chemicals to the body burden in juvenile rainbow trout. Archives of Environmental Contamination and Toxicology, 39(3):369–377.
Ramanand K, Balba MT, Duffy J (1993) Reductive dehalogenation of chlorinated benzenes and toluenes under methanogenic conditions. Applied Environmental Microbiology, 59(10):3266–3272.
Reineke W, Knackmuss HJ (1984) Microbial metabolism of haloaromatics: isolation and properties of a chlorobenzene-degrading bacterium. Applied Environmental Microbiology, 47(2):395–402.
Richter JE, Peterson SF, Kleiner CF (1983) Acute and chronic toxicity of some chlorinated benzenes, chlorinated ethanes, and tetrachloroethylene to Daphnia magna. Archives of Environmental Contamination and Toxicology, 12(6):679–684.
Robertson WD (1994) Chemical fate and transport in a domestic septic system: Site description and attenuation of dichlorobenzene. Environmental Toxicology and Chemistry, 13(2):183–191.
Rogers HR, Campbell JA, Crathorne B, Dobbs RA (1989) The occurrence of chlorobenzenes and permethrins in twelve U.K. sewage sudges. Water Research, 23(7):913–921.
Roghair CJ, Buijze A, Yedema ESE, Hermens JLM (1994) A QSAR for base-line toxicity to the midge Chironomus riparius. Chemosphere, 28(5):989–997.
Rose RM, Warne MSJ, Lim RP (1998) Quantitative structure–activity relationships and volume fraction analysis for nonpolar narcotic chemicals to the Australian cladoceran Ceriodaphnia dubia. Archives of Environmental Contamination and Toxicology, 34(3):248–252.
Sabljic A (1987) The prediction of fish bioconcentration factors of organic pollutants from the molecular connectivity model. Zeitschrift für die Gesamte Hygiene und Ihre Grenzgebiete, 33(10):493–496.
Sander P, Wittich RM, Fortnagel P, Wilkes H, Francke W (1991) Degradation of 1,2,4-trichloro- and 1,2,4,5-tetrachlorobenzene by Pseudomonas strains. Applied Environmental Microbiology, 57(5):1430–1440.
Santos FJ, Sarrion MN, Galceran MT (1997) Analysis of chlorobenzenes in soils by headspace solid-phase microextraction and gas chromatography–ion trap mass spectrometry. Journal of Chromatography A, 771(1–2):181–189.
Scheunert I, Topp E, Attar A, Korte F (1994) Uptake pathways of chlorobenzenes in plants and their correlation with n-octanol/ water partition coefficients. Ecotoxicology and Environmental Safety, 27(1):90–104.
Schraa G, Boone ML, Jetten MSM, Van Neerven ARW, Colberg PJ, Zehnder AJB (1986) Degradation of 1,4-dichlorobenzene by Alcaligenes sp. strain A175. Applied Environmental Microbiology, 52(6):1374–1381.
Schrap SM, Opperhuizen A (1990) Relationship between bioavailability and hydrophobicity: Reduction of the uptake of organic chemicals by fish due to the sorption on particles. Environmental Toxicology and Chemistry, 9(6):715–724.
Schrap SM, De Vries PJ, Opperhuizen A (1994) Experimental problems in determining sorption coefficients of organic chemicals; an example for chlorobenzenes. Chemosphere, 28(5):931–945.
Sheng G, Wang X, Wu S, Boyd SA (1998) Enhanced sorption of organic contaminants by smectitic soils modified with a cationic surfactant. Journal of Environmental Quality, 27(4):806–814.
Sijm D, Van der Linde A (1995) Size-dependent bioconcentration kinetics of hydrophobic organic chemicals in fish based on diffusive mass transfer and allometric relationships. Environmental Science and Technology, 29(11):2769–2777.
Sijm D, Schipper M, Opperhuizen A (1993) Toxicokinetics of halogenated benzenes in fish: Lethal body burden as a toxicological end point. Environmental Toxicology and Chemistry, 12(6):1117–1127.
Strachan WMJ, Burniston DA, Williamson M, Bohdanowicz H (2001) Spatial differences in persistent organochlorine pollutant concentrations between the Bering and Chukchi seas (1993). Marine Pollution Bulletin, 43(1–6):132–142.
Susarla S, Masunaga S, Yonezawa Y (1996) Reductive dechlorination pathways of chloro organics under anaerobic conditions. Water Science and Technology, 34(5–6):489–494.
Susarla S, Masunaga S, Yonezawa Y (1997) Kinetics of the sequential dechlorination of chloroorganics in an anaerobic sediment. Bulletin of Environmental Contamination and Toxicology, 58(2):227–233.
Swindoll CM, Aelion CM, Dobbins DC, Jiang O, Long SC, Pfaender FK (1988) Aerobic biodegradation of natural and xenobiotic organic compounds by subsurface microbial communities. Environmental Toxicology and Chemistry, 7(4):291–299.
Tam DD, Shiu WY, Qiang K, Mackay D (1996) Uptake of chlorobenzenes by tissues of the soybean plant: Equilibria and kinetics. Environmental Toxicology and Chemistry, 15(4):489–494.
Ten Hulscher TEM, Van der Velde LE, Bruggeman WA (1992) Temperature dependence of Henry’s law constants for selected chlorobenzenes, polychlorinated biphenyls and polycyclic aromatic hydrocarbons. Environmental Toxicology and Chemistry, 11(11):1595–1603.
Thibaud C, Erkey C, Akgerman A (1993) Investigation of the effect of moisture on the sorption and desorption of chlorobenzene and toluene from soil. Environmental Science and Technology, 27(12):2373–2380.
Tissot A, Boule P, Lemaire J (1983) Photochemistry and the environment. V. Photohydrolysis of chlorobenzene in dilute aqueous solution. Chemosphere, 12(6):859–872.
Tissot A, Boule P, Lemaire J (1984) Photochemistry and environment. VII. The photohydrolysis of chlorobenzene. Studies of the excited state involved. Chemosphere, 13(3):381–389.
Topp E, Scheunert I, Attar A, Korte F (1986) Factors affecting the uptake of 14C-labelled organic chemicals by plants from soil. Ecotoxicology and Environmental Safety, 11:219–228.
Topp E, Scheunert I, Korte F (1989) Kinetics of the uptake of 14C-labelled chlorinated benzenes from soil by plants. Ecotoxicology and Environmental Safety, 17(2):157–166.
Topping B (1987) The biodegradability of para-dichlorobenzene and its behaviour in model activated sludge plants. Water Research, 21(3):295–300.
US EPA (1978) In-depth studies on health and environmental impact of selected water pollutants. Washington, DC, US Environmental Protection Agency (Report No. EPA 68-04-4646).
US EPA (1985) Health assessment document for chlorinated benzenes. Final report. Washington, DC, US Environmental Protection Agency, Office of Health and Environmental Assessment (Report No. EPA/600/8-84/015F).
US EPA (2003) Toxics release inventory. US Environmental Protection Agency. Available at http://www.epa.gov/tri/.
Van de Meent D, Den Hollander HA, Pool WG, Vredenbregt MJ, Vanoers H, Degreef E, Luijten JA (1986) Organic micropollutants in Dutch coastal waters. Water Science and Technology, 18(4–5):73–81.
Van der Meer JR (1997) Evolution of novel metabolic pathways for the degradation of chloroaromatic compounds. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 71(1–2):159–178.
Van der Meer JR, Roelofsen W, Schraa G, Zehnder AJB (1987) Degradation of low concentrations of dichlorobenzenes and 1,2,4-trichlorobenzene by Pseudomonas sp. strain P51 in nonsterile soil columns. FEMS Microbiology Ecology, 45(6):333–341.
Van der Meer JR, Van Neerven ARW, De Vries EJ, De Vos WM, Zehnder AJB (1991) Cloning and characterization of plasmid-encoded genes for the degradation of 1,2-dichloro-, 1,4-dichloro-, and 1,2,4-trichlorobenzene of Pseudomonas sp. strain P51. Journal of Bacteriology, 173(1):6–15.
Van Gestel CAM, Ma W, Smit CE (1991) Development of QSARs in terrestrial ecotoxicology: Earthworm toxicity and soil sorption of chlorophenols, chlorobenzenes and dichloroaniline. The Science of the Total Environment, 109–110:589–604.
Van Hoogen G, Opperhuizen A (1988) Toxicokinetics of chlorobenzenes in fish. Environmental Toxicology and Chemistry, 7(3):213–219.
Van Leeuwen CJ, Griffioen PS, Vergouw WHA, Maas Diepeveen JL (1985) Differences in susceptibility of early life stages of rainbow trout (Salmo gairdneri) to environmental pollutants. Aquatic Toxicology, 7(1–2):59–78.
Van Leeuwen CJ, Adema DMM, Hermens J (1990) Quantitative structure–activity relationships for fish early life stage toxicity. Aquatic Toxicology, 16(4):321–334.
Van Zoest R, Van Eck GTM (1991) Occurrence and behaviour of several groups of organic micropollutants in the Scheldt Estuary. The Science of the Total Environment, 103(1):57–71.
Wade TL, Sericano JL, Gardinali PR, Wolff G, Chambers L (1998) NOAA’s "Mussel Watch" Project: Current use organic compounds in bivalves. Marine Pollution Bulletin, 37(1–2):20–26.
Wang MJ, Jones KC (1991) Analysis of chlorobenzenes in sewage sludge by capillary gas chromatography. Chemosphere, 23(5):677–691.
Wang MJ, Jones KC (1994a) Behaviour and fate of chlorobenzenes (CBs) introduced into soil–plant systems by sewage sludge application: A review. Chemosphere, 28(7):1325–1360.
Wang MJ, Jones KC (1994b) Uptake of chlorobenzenes by carrots from spiked and sewage sludge-amended soil. Environmental Science and Technology, 28(7):1260–1267.
Wang MJ, Jones KC (1994c) The chlorobenzene content of contemporary U.K. sewage sludges. Chemosphere, 28(6):1201–1210.
Wang MJ, McGrath SP, Jones KC (1992) The chlorobenzene content of archived sewage sludges. The Science of the Total Environment, 121:159–175.
Wang MJ, McGrath SP, Jones KC (1995) Chlorobenzenes in field soil with a history of multiple sewage sludge applications. Environmental Science and Technology, 29(2):356–362.
Wang X, Ma Y, Yu W, Geyer HJ (1997) Two-compartment thermodynamic model for bioconcentration of hydrophobic organic chemicals by alga: Quantitative relationship between bioconcentration factor and surface area of marine algae or octanol/water partition coefficient. Chemosphere, 35(8):1781–1797.
Ward GS, Parrish PR, Rigby RA (1981) Early life stage toxicity tests with saltwater fish: effects of eight chemicals on survival, growth and development of sheepshead minnows (Cyprinodon variegatus). Journal of Toxicology and Environmental Health, 8:225–240.
Weis IM, Muir DCG (1997) Geographical variation of persistent organochlorine concentrations in blubber of ringed seal (Phoca hispida) from the Canadian Arctic: Univariate and multivariate approaches. Environmental Pollution, 96(3):321–333.
Wilson SC, Meharg AA (1999) Investigation of organic xenobiotic transfers, partitioning and processing in air–soil–plant systems using a microcosm apparatus. Part I: Microcosm development. Chemosphere, 38(12):2885–2896.
Yamamoto K, Fukushima M, Kakutani N, Kuroda K (1997) Volatile organic compounds in urban rivers and their estuaries in Osaka, Japan. Environmental Pollution, 95(1):135–143.
Yin H, Lu J (1993) Toxic effect of two organic toxicants on Penaeus chinensis. Haiyang Kexue (Marine Science), 1:56–62.
Yonezawa Y, Fukui M, Masunaga S, Urushigawa Y (1994) Dechlorination of 1,2,4-trichlorobenzene in the sediment of Ise Bay. Chemosphere, 28(12):2179–2184.
Yuan SY, Su CJ, Chang BV (1999) Microbial dechlorination of hexachlorobenzene in anaerobic sewage sludge. Chemosphere, 38(5):1015–1023.
Zhao XK, Yang GP, Wu P, Li NH (2001) Study on adsorption of chlorobenzene on marine sediment. Journal of Colloid and Interface Science, 243(2):273–279.
IPCS (1991a) Chlorobenzenes other than hexachlorobenzene. Geneva, World Health Organization, International Programme on Chemical Safety (Environmental Health Criteria 128)
A WHO Task Group on Environmental Health Criteria for Chlorobenzenes Other than Hexachlorobenzene met at the Institut d’Hygiène et d’Epidémiologie, Brussels, Belgium, from 25 to 29 June 1990. The Task Group reviewed and revised the draft criteria document and made an evaluation of the risks for human health and the environment from exposure to chlorobenzenes other than hexachlorobenzene. The drafts of this document were prepared by Ms M.E. Meek and Ms M.J. Giddings, Health Protection Branch, Health and Welfare Canada, Ottawa, Canada. Dr G.C. Becking, IPCS Interregional Research Unit, WHO, Research Triangle Park, NC, USA, was responsible for the overall scientific content of the document, and Mrs M.O. Head, Oxford, England, for the editing. Extensive comments were received from Dr U. Schlottmann, Federal Ministry of the Environment, Germany (chemistry and environmental effects), and Dr R. Fielder, Department of Health, United Kingdom (effects on experimental animals), during the initial review of the document. Dr S. Dobson, Co-Chairman of the Task Group, and Dr P.E.T. Douben made significant contributions and revisions of the draft document during the meeting, particularly the sections dealing with environmental effects.
This CICAD was prepared with reference to the above source document. Additional information from other national assessments was also included:
ATSDR (1990) Toxicological profile for chlorobenzene. Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
ATSDR (1998) Toxicological profile for 1,4-dichlorobenzene (update). Atlanta, GA, US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry.
For more information on these ATSDR assessments, contact:
Agency for Toxic Substances and Disease Registry
Division of Toxicology
1600 Clifton Road NE, Mailstop E-29
Atlanta, GA 30333
USA
Telephone: 1-888-422-8737
Fax: 404-498-0057
The draft CICAD on chlorobenzenes other than hexachlorobenzene (environmental aspects) was sent for review to institutions and organizations identified by IPCS after contact with IPCS national Contact Points and Participating Institutions, as well as to identified experts. Comments were received from:
M. Baril, Institut de Recherche en Santé et en Sécurité du Travail, Montreal, Canada
R. Benson, Drinking Water Program, US Environmental Protection Agency, Denver, CO, USA
P. Copestake, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
I. Desi, University of Szeged, Szeged, Hungary
E. Frantik, National Institute of Public Health, Prague, Czech Republic
A. Juhasz, University of South Australia, Mawson Lakes, Australia
U. Kierdorf, Justus-Liebig-University of Giessen, Giessen, Germany
S. Schmidt, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
Varna, Bulgaria
8–11 September 2003
Members
Dr I. Benchev, Sofia, Bulgaria
Dr R. Chhabra, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA
Dr C. De Rosa, Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention, Atlanta, GA, USA
Dr S. Dobson, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr G. Dura, National Institute of Environment, József Fodor Public Health Centre, Budapest, Hungary
Dr L. Fishbein, Fairfax, VA, USA
Dr H. Gibb, National Center for Environmental Assessment, US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Risk Assessment, Berlin, Germany
Mr P. Howe, Centre for Ecology and Hydrology, Monks Wood, Abbots Ripton, Huntingdon, Cambridgeshire, United Kingdom
Dr S. Ishimitsu, Division of Safety Information on Drug, Food and Chemicals, National Institute of Hygienic Sciences, Tokyo, Japan
Dr D. Kanungo, Central Insecticides Board, Directorate of Plant Protection, Quarantine & Storage, Ministry of Agriculture, Haryana, India
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Experimental Medicine, Hanover, Germany
Ms B. Meek, Environmental Health Directorate, Health Canada, Ottawa, Ontario, Canada
Dr T. Morita, Division of Safety Information on Drug, Food and Chemicals, National Institute of Hygienic Sciences, Tokyo, Japan
Mr F.K. Muchiri, Directorate of Occupational Health and Safety Services, Nairobi, Kenya
Dr L. Olsen, Biological Monitoring & Health Assessment Branch, Division of Applied Research & Technology, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr N. Rizov, National Center of Hygiene, Medical Ecology and Nutrition, Sofia, Bulgaria
Dr P. Schulte, Education and Information Division, National Institute for Occupational Safety and Health, Cincinnati, OH, USA
Dr J. Sekizawa, Faculty of Integrated Arts and Sciences, Tokushima University, Tokushima, Japan
Dr F. Petrova Simeonova, Sofia, Bulgaria
Dr S. Soliman, Faculty of Agriculture, Alexandria University, El Shatby, Alexandria, Egypt
Dr J. Stauber, CSIRO Energy Technology, Centre for Advanced Analytical Chemistry, Bangor, NSW, Australia
Mr P. Watts, Toxicology Advice & Consulting Ltd, Surrey, United Kingdom
Ms D. Willcocks, National Industrial Chemicals Notification and Assessment Scheme, Sydney, NSW, Australia
Dr K. Ziegler-Skylakakis, European Commission, Luxembourg
Observers
Dr S. Jacobi, Degussa AG, Fine Chemicals, Hanau-Wolfgang, Germany
Mr M. Southern, Shell International Petroleum Company Ltd, London, United Kingdom
Dr W. ten Berge, DSM, Heerlen, The Netherlands
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
Mr T. Ehara, International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland
|
ATSDR |
Agency for Toxic Substances and Disease Registry (USA) |
|
BCF |
bioconcentration factor |
|
CICAD |
Concise International Chemical Assessment Document |
|
DCB |
dichlorobenzene |
|
EC50 |
median effective concentration |
|
ECD |
electron capture detector |
|
EHC |
Environmental Health Criteria |
|
FID |
flame ionization detector |
|
GC |
gas chromatography |
|
HDTMA |
hexadecyltrimethylamminium |
|
ICSC |
International Chemical Safety Card |
|
ILO |
International Labour Organization |
|
IPCS |
International Programme on Chemical Safety |
|
Koa |
octanol/air partition coefficient |
|
Koc |
soil sorption coefficient; suspended sediment/water partition coefficient |
|
Kow |
octanol/water partition coefficient |
|
LC50 |
median lethal concentration |
|
LOEC |
lowest-observed-effect concentration |
|
MCB |
monochlorobenzene |
|
MS |
mass spectrometry |
|
NOEC |
no-observed-effect concentration |
|
OECD |
Organisation for Economic Co-operation and Development |
|
PeCB |
pentachlorobenzene |
|
PIM |
Poison Information Monograph |
|
PNEC |
predicted no-effect concentration |
|
SD |
standard deviation |
|
TCB |
trichlorobenzene |
|
TeCB |
tetrachlorobenzene |
|
TRI |
Toxics Release Inventory (USA) |
|
UNEP |
United Nations Environment Programme |
|
USA |
United States of America |
|
Vmax |
maximum rate of reaction |
|
WHO |
World Health Organization |
1,2,3-TRICHLOROBENZENE ICSC:1222
1,2,4-TRICHLOROBENZENE ICSC:1049
1,3,5-TRICHLOROBENZENE ICSC:0344
1,2,4,5-TETRACHLOROBENZENE ICSC:0676
Le présent CICAD consacré aux chlorobenzènes autres que l’hexachlorobenzène (aspects environnementaux) est une mise à jour du No 128 de la série Critères d’hygiène de l’environnement (CHE) intitulé Chlorobenzènes autres que l’hexachlorobenzène (IPCS, 1991a). Des renseignements sur le devenir et la concentration des chlorobenzènes ont également été tirés de rapports sur le chlorobenzène (ATSDR, 1990) et le 1,4-dichlorobenzène (ATSDR, 1998) publiés par l’Agency for Toxic Substances and Disease Registry. On a poursuivi le dépouillement de la littérature jusqu’en décembre 2002 à la recherche de références complémentaires qui auraient été publiés postérieusement à ces mises au point. L’appendice 1 donne des informations sur la nature de l’examen par des pairs du document initial. Des renseignements sur l’examen par des pairs du présent CICAD sont donnés à l’appendice 2. Ce CICAD a été adopté en tant qu’évaluation internationale lors de la réunion du Comité d’évaluation finale qui s’est tenue à Varna (Bulgarie) du 8 au 11 septembre 2003. La liste des participants à cette réunion figure à l’appendice 3. Les fiches internationales sur la sécurité chimique de divers chorobenzènes (ICSC 0037, 0344, 0531, 0642, 0676, 1049, 1066, 1095, 1222), établies par le Programme international sur la sécurité chimique (IPCS, 2000, 2003a-h), sont également reproduites dans le présent document. Ce CICAD porte principalement sur les aspects environnementaux de ces composés car depuis la publication du CHE (IPCS, 1991a), l’évaluation des risques pour la santé humaine n’a pas sensiblement changé.
Les dérivés chlorés du benzène ou chlorobenzènes constituent un groupe de composés aromatiques cycliques dans lesquels un atome de chlore est substitué à un ou plusieurs atomes d’hydrogène du noyau benzénique. On les utilise principalement comme intermédiaires dans la synthèse de pesticides ou d’autres produits chimiques. Le 1,4-dichlorobenzène (1,4-DCB) est utilisé comme désodorisant d’ambiance et comme antimites. Les dérivés plus substitués (comme les trichlorobenzènes, le 1,2,3,4-tétrachlorobenzène [1,2,3,4-TeCB] et le pentachlorobenzène [PeCB]) ont été utilisés comme fluides diélectriques.
On n’a pas découvert de sources naturelles de chlorobenzènes. Des chlorobenzènes sont rejetés dans l’environnement lors de leur préparation ou lorsqu’on les utilise comme intermédiaires pour la synthèse d’autres produits. Ils passent également dans l’environnement lorsque des déchets qui en contiennent sont éliminés, par exemple par incinération ou décharge sur des sites mal sécurisés. Le monochlorobenzène (MCB) est rejeté directement dans le milieu ambiant lors de son utilisation comme charge dans certains pesticides. Le passage dans l’environnement des chlorobenzènes utilisés comme désodorisants, fumigants, dégraissants, insecticides, herbicides et défoliants est également la conséquence directe de leur utilisation.
Compte tenu de leurs propriétés physicochimiques, les chlorobenzènes rejetés dans l’environnement se vaporisent vraisemblablement dans l’atmosphère. Ils s’en éliminent ensuite principalement après avoir réagi sur les radicaux hydroxyles pour donner du nitrochlorobenzène, du chlorophénol et des dérivés aliphatiques dicarbonylés, à leur tour éliminés par photolyse ou réaction avec ces mêmes radicaux hydroxyles. Les chlorobenzènes qui passent dans l’environnement aquatique se redistribuent préférentiellement dans l’air et dans les sédiments (notamment les sédiments riches en matières organiques). En solution aqueuse, les chlorobenzènes pourraient théoriquement subir une déchloration réductrice par voie photochimique, mais cette possibilité a été étudiée dans des conditions artificielles qui n’étaient pas représentatives des régions tempérées. Dans le sol, c’est le phénomène de sorption qui constitue le facteur le plus important dont dépendent le devenir et le comportement des chlorobenzènes. Les processus de sorption-désorption qui se déroulent dans le sol conditionnent la vitesse d’évaporation et de lessivage de ces produits, de même que leur faculté de subir une décomposition chimique ou microbienne ou encore d’être fixés par des végétaux ou d’autres organismes.
Dans les divers substrats où ils sont présents - sol, sédiments ou boues d’égout -, les chlorobenzènes sont susceptibles de subir une biodégradation microbienne. Le principal mécanisme de la décomposition anaérobie comporte une déchloration oxydative conduisant à la formation de composés aromatiques hydroxylés (essentiellement des catéchols) suivie de l’ouverture du cycle et d’une minéralisation en dioxyde de carbone et eau. Les chlorobenzènes les moins substitués sont plus facilement dégradés que les autres.
La bioaccumulation de chlorobenzènes par les organismes aquatiques dépend de l’hydro- et de la liposolubilité relative de ces produits (qui correspond au coefficient de partage octanol/eau) ainsi que du degré de substitution. Ces composés sont d’autant mieux captés dans l’eau qu’ils sont plus substitués et que la température est plus élevée.
On a décelé la présence de chlorobenzènes (MCB, dichlorobenzènes et trichlorobenzènes) dans l’air ambiant à une concentration moyenne de 0,1 µg/m3, avec des maxima pouvant atteindre 100 µg/m3 dans des sites dangereux. Dans les eaux de surface, la concentration des chlorobenzènes oscille généralement entre des valeurs de l’ordre du nanogramme ou du microgramme par litre, avec des maxima allant jusqu’à 0,2 mg/litre à proximité des sites industriels. La teneur en chlorobenzènes des eaux usées industrielles est sans doute plus élevée et varie selon les procédés industriels mis en oeuvre. Dans les sols non contaminés, la concentration des différents dichlorobenzènes est généralement inférieure à 0,4 mg/kg et celle des autres chlorobenzènes inférieure à 0,1 mg/kg. Dans les sédiments, les teneurs se situent habituellement dans une gamme de valeurs allant du ng/kg au µg/kg, encore que des valeurs de l’ordre du mg/kg aient été observées dans des échantillons prélevés sur des sites industriels.
D’une façon générale, la toxicité pour les organismes aquatiques augmente avec le degré de chloration du noyau benzénique. Les valeurs de la CE50 à 72 h pour les algues vertes vont de 5280 µg/litre dans le cas du 1,3-DCB à 200 000 µg/litre dans le cas du MCB; de même, la CE50 à 48 h pour les diatomées va de 8 à 235 000 µg/litre. En ce qui concerne les invertébrés d’eau douce, les valeurs de la CE50 à 48 h sont comprises entre 10 µg/litre dans le cas du PeCB et >530 000 µg/litre dans le cas du 1,2,4,5-TeCB. Pour les poissons, les valeurs de la CL50 à 96 h vont de 135 µg/litre dans le cas du PeCB à 21 000 µg/litre dans le cas du 1,2,4-trichlorobenzène (1,2,4-TCB). Les concentrations chroniques sans effet observable (NOEC) sur les invertébrés d’eau douce oscillent entre 32 µg/litre dans le cas du PeCB et 19 000 µg/litre dans le cas du MCB; pour les poissons, les valeurs de la NOEC vont de 18 µg/litre avec le PeCB à 8 500 µg/litre avec le MCB.
On ne possède guère de données concernant les effets des chlorobenzènes sur les organismes terrestres. Pour les plantes en cultures hydroponiques ou en cultures sur sol, on a trouvé des valeurs de la CL50 allant respectivement de 0,028 à 9,3 mg/litre et de 1 à plus de 1000 mg/kg de sol. Pour les lombrics Eisenia andrei et Lumbricus rubellus, on a mesuré des valeurs de la CL50 qui sont comprises entre 0,22 µmol/litre (eau des pores) dans le cas du PeCB et 4281 µmol/litre dans le cas du MCB.
Il y a peu de chances que les chlorobenzènes aient des effets nocifs sur les organismes aquatiques. Les facteurs de risque obtenus en comparant les valeurs de la toxicité chronique aux concentrations mesurées dans l’environnement sont généralement inférieurs à 1, sauf pour quelques composés pour lesquels ils sont plus élevés, avec la valeur maximum de 200. Les facteurs de risque les plus élevés ont été obtenus en utilisant d’anciennes données portant sur des sources ponctuelles et ne sont donc pas représentatifs de l’environnement dans son ensemble, notamment si l’on tient compte de l’évaporation. On ne disposait pas de données suffisantes pour évaluer le risque auquel sont exposées les espèces terrestres.
Este CICAD sobre los clorobencenos distintos del hexaclorobenceno (aspectos ecológicos) es una actualización del Nş 128 de los Criterios de Salud Ambiental (EHC), Chlorobenzenes other than hexachlorobenzene (IPCS, 1991a). También se obtuvo información sobre el destino y las concentraciones de los clorobencenos a partir de los informes de la Agencia para el Registro de Sustancias Tóxicas y Enfermedades sobre el clorobenceno (ATSDR, 1990) y el 1,4-diclorobenceno (ATSDR, 1998). Se realizó asimismo una búsqueda bibliográfica hasta diciembre de 2002 para identificar cualquier información que se hubiera publicado después de la terminación de dichos informes. La información sobre el examen colegiado del documento original se presenta en el apéndice 1. La información sobre el examen colegiado de este CICAD aparece en el apéndice 2. Este CICAD se aprobó como evaluación internacional en una reunión de la Junta de Evaluación Final celebrada en Varna (Bulgaria) del 8 al 11 de septiembre de 2003. La lista de participantes en esta reunión figura en el apéndice 3. También se reproducen en este documento las Fichas internacionales de seguridad química para varios clorobencenos distintos (ICSC 0037, 0344, 0531, 0642, 0676, 1049, 1066, 1095, 1222), preparadas por el Programa Internacional de Seguridad de las Sustancias Químicas (IPCS, 2000, 2003a-h). Este CICAD se concentra en los aspectos ecológicos porque no se han registrado cambios significativos para la salud humana desde la publicación de los Criterios de Salud Ambiental (IPCS, 1991a).
Los bencenos clorados son un grupo de compuestos aromáticos cíclicos en los cuales se han sustituido uno o más átomos de hidrógeno del anillo de benceno por un átomo de cloro. Los clorobencenos se utilizan principalmente como intermediarios en la síntesis de plaguicidas y otros productos químicos. El 1,4-diclorobenceno (1,4-DCB) se utiliza en desodorantes ambientales y como repelente de la polilla. Los bencenos más clorados (triclorobencenos, 1,2,3,4-tetraclorobenceno [1,2,3,4-TeCB] y pentaclorobenceno [PeCB]) se han utilizado como componentes de fluidos dieléctricos.
No se han identificado fuentes naturales de clorobencenos en el medio ambiente. Los clorobencenos se liberan en el medio ambiente durante su fabricación o su utilización como intermediarios en la producción de otras sustancias químicas. También hay emisiones durante la eliminación de productos de clorobenceno, por ejemplo en los incineradores y en los vertederos de desechos peligrosos. El monoclorobenceno (MCB) se libera directamente en el medio ambiente debido a su utilización como excipiente de plaguicidas. Los clorobencenos que se utilizan como desodorantes, fumigantes, desengrasantes, insecticidas, herbicidas y defoliantes también se liberan en el medio ambiente como resultado directo de su aplicación.
Sus propiedades fisicoquímicas parecen indicar que es probable que los clorobencenos liberados en el medio ambiente se volatilicen en la atmósfera. La eliminación de la atmósfera se produce fundamentalmente por medio de su reacción con radicales hidroxilo para formar nitroclorobenceno, clorofenol y dicarbonilos alifáticos, que posteriormente se degradan por fotolisis o por reacción con radicales hidroxilo. Los clorobencenos liberados en el medio acuático se redistribuyen preferentemente entre el aire y los sedimentos (sobre todo los que tienen abundante materia orgánica). En teoría, los clorobencenos en soluciones acuosas podrían sufrir una decloración fotoquímica reductora, aunque sólo se han realizado estudios en condiciones artificiales que no eran representativas de las regiones templadas. El factor más importante que afecta al comportamiento y el destino de los clorobencenos en el suelo es la sorción. Los procesos de adsorción y desorción en el suelo afectan a la tasa de volatilización y lixiviación y a la disponibilidad de sustancias químicas para la degradación microbiana y química o para la absorción por las plantas u otros organismos.
Los microorganismos pueden degradar los clorobencenos en distintos sustratos, por ejemplo el suelo, los sedimentos y los lodos cloacales. El principal mecanismo de degradación aerobia es la decloración oxidativa, que da lugar a la formación de compuestos aromáticos hidroxilados (sobre todo catecoles), cuyo anillo se rompe y se produce la mineralización posterior hasta dióxido de carbono y agua. Los bencenos menos clorados se degradan más fácilmente que los más clorados.
La bioacumulación de clorobencenos por los organismos acuáticos depende de su solubilidad relativa en el agua y los lípidos (reflejando de esta manera los coeficientes de reparto octanol/agua) y del número de sustituciones de cloro. La absorción a partir del agua aumenta con el grado de cloración y la temperatura.
Se ha notificado la presencia de clorobencenos (MCB, diclorobencenos y triclorobencenos) en el aire exterior, con concentraciones medias del orden de 0,1 µg/m3 y máximas (en vertederos de desechos peligrosos) de hasta 100 µg/m3. Las concentraciones de clorobencenos en las aguas superficiales suelen estar en la escala de ng/l a µg/l, con concentraciones máximas de hasta 0,2 mg/l en zonas próximas a fuentes industriales. Las concentraciones de clorobencenos en las aguas residuales industriales pueden ser más elevadas y variar en función del tipo de proceso utilizado. Sus concentraciones en suelos no contaminados son en general inferiores a 0,4 mg/kg de diclorobencenos y inferiores a 0,1 mg/kg para otros clorobencenos. Las concentraciones de clorobencenos en los alimentos suelen estar en la escala de ng/kg a µg/kg, aunque se ha informado de concentraciones del orden de mg/kg en muestras procedentes de zonas industriales.
En general, la toxicidad en el medio acuático aumenta con el grado de cloración del anillo de benceno. La CE50 a las 72 h para las algas verdes oscila entre 5280 µg/l para el 1,3-DCB y 200 000 µg/litro para el MCB; de igual forma, la CE50 a las 48 h para las diatomeas varía entre 8 y 235 000 µg/l. En los invertebrados de agua dulce, la CE50 a las 48 h oscila entre 10 µg/l para el PeCB y > 530 000 µg/l para el 1,2,4,5-TeCB. La CL50 a las 96 h para los peces varía entre 135 µg/l para el PeCB y 21 000 µg/l para el 1,2,4-triclorobenceno (1,2,4-TCB). Las concentraciones sin efectos crónicos observados (NOEC) para los invertebrados de agua dulce oscilan entre 32 µg/l para el PeCB y 19 000 µg/l para el MCB; en los peces, las NOEC varían entre 18 µg/l para el PeCB y 8500 µg/l para el MCB.
Hay pocos datos disponibles sobre los efectos de los clorobencenos en los sistemas terrestres. Los valores de las CL50 para las plantas de cultivos hidropónicos y cultivadas en el suelo oscilan entre 0,028 y 9,3 mg/l y entre 1 y > 1000 mg/kg de suelo, respectivamente. Los valores de las CL50 para las lombrices Eisenia andrei y Lumbricus rubellus varían entre 0,22 µmoles/l (agua intersticial) para el PeCB y 4281 µmoles/l para el MCB.
El riesgo de que los bencenos clorados provoquen daños en los organismos acuáticos es bajo. Los factores de riesgo comparando los valores de la toxicidad crónica con las concentraciones medidas en el medio ambiente son en general inferiores a 1, salvo algunos compuestos que tenían factores de riesgo más altos, con un valor máximo de 200. Los factores de riesgo más elevados se obtuvieron utilizando antiguos datos de fuentes puntuales, por lo que no son representativos del medio en su conjunto, en particular cuando se tiene en cuenta la probabilidad de evaporación. No se dispuso de datos suficientes para realizar una evaluación del riesgo en las especies terrestres.
ENDNOTES:
See Also:
Toxicological Abbreviations