
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
CONCISE INTERNATIONAL CHEMICAL ASSESSMENT DOCUMENT NO. 25
CHLORAL HYDRATE
INTER-ORGANIZATION PROGRAMME FOR THE SOUND MANAGEMENT OF CHEMICALS
A cooperative agreement among UNEP, ILO, FAO, WHO, UNIDO, UNITAR and
OECD
This report contains the collective views of an international group of
experts and does not necessarily represent the decisions or the stated
policy of the United Nations Environment Programme, the International
Labour Organization, or the World Health Organization.
First draft prepared by Dr R. Benson, Region VIII, Environmental
Protection Agency, Denver, CO, USA
Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organization, and the
World Health Organization, and produced within the framework of the
Inter-Organization Programme for the Sound Management of Chemicals.
World Health Organization
Geneva, 2000
The International Programme on Chemical Safety (IPCS),
established in 1980, is a joint venture of the United Nations
Environment Programme (UNEP), the International Labour Organization
(ILO), and the World Health Organization (WHO). The overall objectives
of the IPCS are to establish the scientific basis for assessment of
the risk to human health and the environment from exposure to
chemicals, through international peer review processes, as a
prerequisite for the promotion of chemical safety, and to provide
technical assistance in strengthening national capacities for the
sound management of chemicals.
The Inter-Organization Programme for the Sound Management of
Chemicals (IOMC) was established in 1995 by UNEP, ILO, the Food and
Agriculture Organization of the United Nations, WHO, the United
Nations Industrial Development Organization, the United Nations
Institute for Training and Research, and the Organisation for Economic
Co-operation and Development (Participating Organizations), following
recommendations made by the 1992 UN Conference on Environment and
Development to strengthen cooperation and increase coordination in the
field of chemical safety. The purpose of the IOMC is to promote
coordination of the policies and activities pursued by the
Participating Organizations, jointly or separately, to achieve the
sound management of chemicals in relation to human health and the
environment.
WHO Library Cataloguing-in-Publication Data
Chloral hydrate.
(Concise international chemical assessment document ; 25)
1.Chloral hydrate - toxicity 2.Risk assessment
3.Environmental exposure
I.International Programme on Chemical Safety II.Series
ISBN 92 4 153025 1 (NLM Classification: QV 85)
ISSN 1020-6167
The World Health Organization welcomes requests for permission to
reproduce or translate its publications, in part or in full.
Applications and enquiries should be addressed to the Office of
Publications, World Health Organization, Geneva, Switzerland, which
will be glad to provide the latest information on any changes made to
the text, plans for new editions, and reprints and translations
already available.
(c) World Health Organization 2000
Publications of the World Health Organization enjoy copyright
protection in accordance with the provisions of Protocol 2 of the
Universal Copyright Convention. All rights reserved.
The designations employed and the presentation of the material in
this publication do not imply the expression of any opinion whatsoever
on the part of the Secretariat of the World Health Organization
concerning the legal status of any country, territory, city, or area
or of its authorities, or concerning the delimitation of its frontiers
or boundaries.
The mention of specific companies or of certain manufacturers'
products does not imply that they are endorsed or recommended by the
World Health Organization in preference to others of a similar nature
that are not mentioned. Errors and omissions excepted, the names of
proprietary products are distinguished by initial capital letters.
The Federal Ministry for the Environment, Nature Conservation and
Nuclear Safety, Germany, provided financial support for the printing
of this publication.
TABLE OF CONTENTS
FOREWORD
1. EXECUTIVE SUMMARY
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
3. ANALYTICAL METHODS
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1. Environmental levels
6.2. Human exposure
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS AND HUMANS
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1. Single exposure
8.1.1. Oral
8.1.2. Inhalation
8.2. Irritation and sensitization
8.3. Short-term exposure
8.4. Long-term exposure
8.4.1. Subchronic exposure
8.4.2. Chronic exposure and carcinogenicity
8.5. Genotoxicity and related end-points
8.5.1. Genotoxicity
8.5.2. Cell proliferation
8.5.3. Oncogene activation
8.5.4. Free radicals and DNA adduct formation
8.5.5. Cell communication
8.5.6. Peroxisome proliferation
8.6. Reproductive and developmental toxicity
8.7. Immunological and neurological effects
9. EFFECTS ON HUMANS
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
11. EFFECTS EVALUATION
11.1. Evaluation of health effects
11.1.1. Hazard identification and dose-response assessment
11.1.2. Criteria for setting tolerable intakes or guidance values for chloral hydrate
11.1.3. Sample risk characterization
11.2. Evaluation of environmental effects
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
REFERENCES
APPENDIX 1 -- TOXICOKINETICS
APPENDIX 2 -- CALCULATION OF BENCHMARK DOSE FOR TUMOUR INCIDENCE
APPENDIX 3 -- SOURCE DOCUMENT
APPENDIX 4 -- CICAD PEER REVIEW
APPENDIX 5 -- CICAD FINAL REVIEW BOARD
APPENDIX 6 -- INTERNATIONAL CHEMICAL SAFETY CARD
RÉSUMÉ D'ORIENTATION
RESUMEN DE ORIENTACI²N
FOREWORD
Concise International Chemical Assessment Documents (CICADs) are
the latest in a family of publications from the International
Programme on Chemical Safety (IPCS) -- a cooperative programme of the
World Health Organization (WHO), the International Labour Organization
(ILO), and the United Nations Environment Programme (UNEP). CICADs
join the Environmental Health Criteria documents (EHCs) as
authoritative documents on the risk assessment of chemicals.
CICADs are concise documents that provide summaries of the
relevant scientific information concerning the potential effects of
chemicals upon human health and/or the environment. They are based on
selected national or regional evaluation documents or on existing
EHCs. Before acceptance for publication as CICADs by IPCS, these
documents undergo extensive peer review by internationally selected
experts to ensure their completeness, accuracy in the way in which the
original data are represented, and the validity of the conclusions
drawn.
The primary objective of CICADs is characterization of hazard and
dose-response from exposure to a chemical. CICADs are not a summary of
all available data on a particular chemical; rather, they include only
that information considered critical for characterization of the risk
posed by the chemical. The critical studies are, however, presented in
sufficient detail to support the conclusions drawn. For additional
information, the reader should consult the identified source documents
upon which the CICAD has been based.
Risks to human health and the environment will vary considerably
depending upon the type and extent of exposure. Responsible
authorities are strongly encouraged to characterize risk on the basis
of locally measured or predicted exposure scenarios. To assist the
reader, examples of exposure estimation and risk characterization are
provided in CICADs, whenever possible. These examples cannot be
considered as representing all possible exposure situations, but are
provided as guidance only. The reader is referred to EHC 1701 for
advice on the derivation of health-based tolerable intakes and
guidance values.
1 International Programme on Chemical Safety (1994)
Assessing human health risks of chemicals: deriviation of guidance
values for health-based exposure limits. Geneva, World Health
Organization (Environmental Health Criteria 170).
While every effort is made to ensure that CICADs represent the
current status of knowledge, new information is being developed
constantly. Unless otherwise stated, CICADs are based on a search of
the scientific literature to the date shown in the executive summary.
In the event that a reader becomes aware of new information that would
change the conclusions drawn in a CICAD, the reader is requested to
contact IPCS to inform it of the new information.
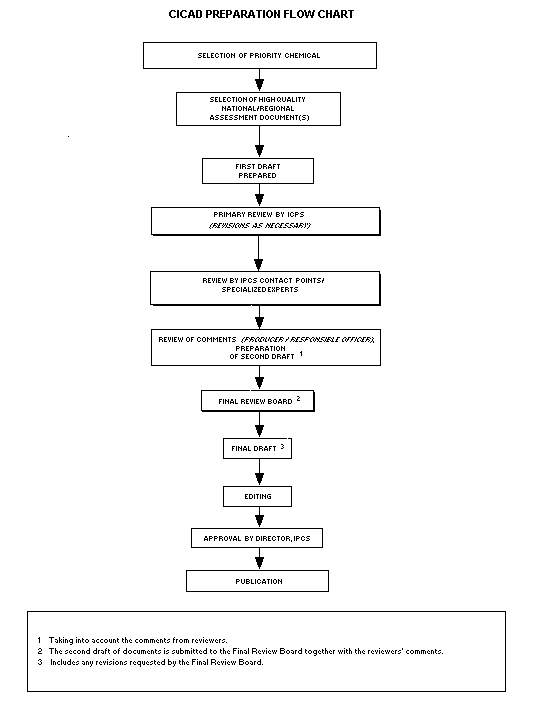
Procedures
The flow chart shows the procedures followed to produce a CICAD.
These procedures are designed to take advantage of the expertise that
exists around the world -- expertise that is required to produce the
high-quality evaluations of toxicological, exposure, and other data
that are necessary for assessing risks to human health and/or the
environment.
The first draft is based on an existing national, regional, or
international review. Authors of the first draft are usually, but not
necessarily, from the institution that developed the original review.
A standard outline has been developed to encourage consistency in
form. The first draft undergoes primary review by IPCS to ensure that
it meets the specified criteria for CICADs.
The second stage involves international peer review by scientists
known for their particular expertise and by scientists selected from
an international roster compiled by IPCS through recommendations from
IPCS national Contact Points and from IPCS Participating Institutions.
Adequate time is allowed for the selected experts to undertake a
thorough review. Authors are required to take reviewers' comments into
account and revise their draft, if necessary. The resulting second
draft is submitted to a Final Review Board together with the
reviewers' comments.
The CICAD Final Review Board has several important functions:
- to ensure that each CICAD has been subjected to an appropriate
and thorough peer review;
- to verify that the peer reviewers' comments have been addressed
appropriately;
- to provide guidance to those responsible for the preparation of
CICADs on how to resolve any remaining issues if, in the opinion
of the Board, the author has not adequately addressed all
comments of the reviewers; and
- to approve CICADs as international assessments.
Board members serve in their personal capacity, not as representatives
of any organization, government, or industry. They are selected
because of their expertise in human and environmental toxicology or
because of their experience in the regulation of chemicals. Boards are
chosen according to the range of expertise required for a meeting and
the need for balanced geographic representation.
Board members, authors, reviewers, consultants, and advisers who
participate in the preparation of a CICAD are required to declare any
real or potential conflict of interest in relation to the subjects
under discussion at any stage of the process. Representatives of
nongovernmental organizations may be invited to observe the
proceedings of the Final Review Board. Observers may participate in
Board discussions only at the invitation of the Chairperson, and they
may not participate in the final decision-making process.
 1. EXECUTIVE SUMMARY
This CICAD on chloral hydrate was prepared by the US
Environmental Protection Agency (EPA) and is based on the US EPA's
Toxicological review on chloral hydrate (US EPA, 2000). Scientific
literature identified as of March 1999 was included. Information on
the nature of the review processes and the availability of the source
document is presented in Appendix 3. Information on the peer review of
this CICAD is presented in Appendix 4. This CICAD was approved as an
international assessment at a meeting of the Final Review Board, held
in Sydney, Australia, on 21-24 November 1999. Participants at the
Final Review Board meeting are listed in Appendix 5. The International
Chemical Safety Card (ICSC 0234) for chloral hydrate, produced by the
International Programme on Chemical Safety, has been reproduced in
Appendix 6 (IPCS, 1993).
Chloral hydrate (CAS No. 302-17-0) is synthesized by the
chlorination of ethanol. It is used in human and veterinary medicine
as a sedative and hypnotic drug. The anhydrous chemical, chloral
(CAS No. 75-87-6), is used as an intermediate in the synthesis of
DDT, methoxychlor, naled, trichlorfon, dichlorvos, and
trichloroacetic acid.
The major route of exposure of the general public is from
drinking-water, as chloral hydrate is formed when drinking-water is
disinfected with chlorine. A typical concentration of chloral hydrate
in a public water supply in the USA is 5 µg/litre. Since chloral
hydrate is a metabolite of trichloroethylene and tetrachloroethylene,
people will be exposed to chloral hydrate if they are exposed to these
chemicals. The public will be exposed to the metabolites of chloral
hydrate, trichloroacetic acid and dichloroacetic acid, as these
chemicals are also formed when drinking-water is disinfected with
chlorine. In its use as a sedative for people, the usual clinical dose
is 250 mg, 3 times a day (equivalent to 10.7 mg/kg body weight per
day). The metabolite trichloroethanol is responsible for the
pharmacological effect. No quantitative information is available from
occupational exposure.
Chloral hydrate is irritating to the skin and mucous membranes
and often causes gastric distress, nausea, and vomiting at the
recommended clinical dose. An acute overdose produces (in order of
progression) ataxia, lethargy, deep coma, respiratory depression,
hypotension, and cardiac arrhythmia. There is some evidence of hepatic
injury in people surviving near-lethal, acute overdoses, but no
convincing evidence that hepatic injury results from the recommended
clinical dose. Several studies of the clinical use of chloral hydrate
show a low incidence of minor side-effects. Despite its long use in
human medicine, there is no published information on toxicity in
controlled studies in humans following extended exposure.
Chloral hydrate is completely absorbed and rapidly metabolized
following oral administration. The major metabolites are
trichloroethanol and its glucuronide and trichloroacetic acid. Some
data suggest that a small amount of dichloroacetic acid may be formed.
In humans, the half-life of trichloroethanol and its glucuronide is
about 8 h; the half-life of trichloroacetic acid is about 4 days. Some
data suggest that the half-life of trichloroethanol is increased
several-fold in pre-term and full-term infants compared with toddlers
and adults. The major route of excretion of the metabolites of chloral
hydrate is elimination in the urine. Chloral hydrate and its
metabolites have been found in milk from women treated with chloral
hydrate. The concentration of these chemicals, however, is too low to
cause a pharmacological effect in the nursing infant.
Acute administration of chloral hydrate to mice causes loss of
coordination (ataxia) at about the same exposure as in humans for the
same effect. A 90-day study in mice shows no evidence of behavioural
changes or other neurotoxicity. Chronic studies in rats and mice show
no evidence of behavioural changes and no evidence of
histopathological changes in nervous tissue. A slight decrement in
humoral immunity was observed following exposure of mice for 90 days.
Chloral hydrate has been tested for developmental effects in rats and
mice. No structural abnormalities were observed. In a
neurodevelopmental study in mice, there was a slight effect in passive
avoidance learning. Although chloral hydrate has not been tested in a
two-generation reproduction study, the data on reproductive
performance and on effects on sperm and oocytes do not suggest that
reproductive toxicity is likely to be a critical effect. In addition,
no histopathological effects are observed in reproductive organs of
rodents in subchronic or chronic studies. All of the studies in
laboratory animals show non-cancer health effects at an exposure far
in excess of the exposure that is effective for sedation in humans.
There are no carcinogenicity data from humans. Two bioassays in
rats show no increase in tumours at any site. Three separate bioassays
in male mice show an increased incidence of liver tumours. The most
definitive of these studies shows an increased incidence and
multiplicity of liver tumours at each of three exposures. These data
provide suggestive evidence of carcinogenicity in male mice but are
not considered appropriate for conducting a human health risk
assessment with a linear response at low exposure.1
1 In a National Toxicology Program carcinogenicity bioassay in mice
that became available after the Final Review Board meeting, males
had an increased incidence of hepatic tumours, and females had a
low increased incidence of pituitary adenomas that was of borderline
statistical significance.
There is an extensive database on genetic toxicity. A variety of
results show that chloral hydrate is a weak gene mutagen and
clastogen. Chloral hydrate induces aneuploidy in a wide variety of
cell types. These latter effects are thought to arise by disruption of
the spindle apparatus. High concentrations of chloral hydrate are
required to cause observable effects. Although these data suggest that
genotoxicity may play a role in the toxicity of chloral hydrate, the
data indicate that these effects require concentrations that are
unlikely to occur under physiological conditions at the exposures
typically encountered in the environment. Some likely candidates for
the induction of liver tumours in male mice include the formation of
DNA adducts caused by free radicals generated by the metabolism of
chloral hydrate by cytochrome P450 2E1 (CYP2E1) and through
cytotoxicity leading to compensatory hyperplasia.
The tolerable intake for non-cancer health effects of 0.1 mg/kg
body weight per day was estimated from the
lowest-observed-adverse-effect level (LOAEL) for sedation in humans of
10.7 mg/kg body weight per day using a total uncertainty factor of
100.
Only limited data are available on environmental effects.
Methanotrophs can convert chloral hydrate to trichloroethanol and
trichloroacetic acid. Chloral hydrate also undergoes abiotic
degradation under some conditions. Limited data are available on the
inhibition of growth of bacteria, algae, and protozoa and
developmental effects in sea urchins. Insufficient data are available
with which to assess the risk to the environment from chloral hydrate.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Chloral hydrate (CAS No. 302-17-0) is synthesized by the
chlorination of ethanol. The structural formula is given in section 7.
The CAS name is 2,2,2-trichloro-1,1-ethanediol. Synonyms include
chloral monohydrate, trichloroacetaldehyde hydrate,
trichloroacetaldehyde monohydrate, and
1,1,1-trichloro-2,2-dihydroxyethane. The relative molecular mass is
165.42; the solubility in water is 8.3 g/ml; the octanol/water
partition coefficient (log Kow) is 0.99; and the vapour pressure
is 2 kPa at 25°C. The chemical and physical properties of chloral
hydrate are summarized in the International Chemical Safety Card
included in this document (Appendix 6).
Chloral (CAS No. 75-87-6) is the anhydrous form of the chemical.
The conversion from chloral to chloral hydrate occurs spontaneously
when chloral is placed in aqueous media.
3. ANALYTICAL METHODS
A method for the determination of trace amounts of chloral
hydrate in environmental samples is available. Carbonyl compounds are
converted to their 2,4-dinitrophenylhydrazone derivatives, separated
with high-performance liquid chromatography, and detected by
ultraviolet absorbance (Fung & Grosjean, 1981). The lowest
quantifiable limit for a variety of carbonyls ranges from 1 to 6 ng.
Chloral hydrate and its metabolites (trichloroethanol,
trichloroethanol glucuronide, and trichloroacetic acid) can be
determined in rat liver homogenates using headspace gas chromatography
and electron capture detection (Koppen & Dalgaard, 1988). The
detection limits are 0.06 µg/ml for trichloroethanol and
trichloroethanol glucuronide and 0.02 µg/ml for chloral hydrate and
trichloroacetic acid. A comparable method for the determination of
these chemicals in blood and urine is also available (Breimer et al.,
1974). The detection limits are 0.5 µg/ml for chloral hydrate and
trichloroethanol and 0.1 µg/ml for trichloroacetic acid.
Chloral hydrate and its metabolites can be measured in biological
samples after conversion to the methyl esters and separation and
detection with gas chromatography/mass spectrometry (Yan et al.,
1999). The range for measurement is between 0.12 and 7.83 µmol/litre
(equivalent to about 20-1290 µg/litre).
A method for determining trichloroethanol in plasma for use in a
clinical laboratory with liquid chromatography has also been developed
(Gupta, 1990). The method is useful for determining trichloroethanol
in plasma in the pharmacologically active range (up to 12 mg/litre)
and in the acutely toxic range (about 100 mg/litre). The method takes
about 2 h to complete.
A spectrophotometric method for the determination of chloral
hydrate in commercial drug products is based on the reaction of
quinaldine ethyl iodide with chloral hydrate to produce a stable blue
cyanine dye with an absorption maximum at about 605 nm
(Helrich, 1990).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Chloral hydrate is not known to occur as a natural product. The
major route of human exposure to chloral hydrate is from
drinking-water. Chloral hydrate and its metabolites, trichloroacetic
acid and dichloroacetic acid, are formed as by-products when water is
disinfected with chlorine. The carbon is derived from natural organic
matter (humic and fulvic substances) in the source water. The amount
of chloral hydrate formed depends on the concentration of humic and
fulvic substances and the conditions of chlorination. Additional
chloral hydrate can be formed if water containing chlorine is mixed
with food containing humic and fulvic acids (Wu et al., 1998). Chloral
hydrate is also a metabolite of trichloroethylene and
tetrachloroethylene. Humans will be exposed to chloral hydrate if they
are exposed to these chemicals. Chloral hydrate has been widely used
as a sedative and hypnotic drug in adult and pediatric medicine.
Chloral is used as an intermediate in the synthesis of the
insecticides DDT, methoxychlor, naled, trichlorfon, and dichlorvos and
the herbicide trichloroacetic acid (IARC, 1995).
Chlorate hydrate could be released to the environment from
wastewater treatment facilities, from the manufacture of
pharmaceutical-grade chloral hydrate, and from the waste stream during
the manufacture of insecticides and herbicides that use chloral as an
intermediate.
In the USA, production of chloral hydrate/chloral was estimated
at 590 tonnes in 1975, and imports were estimated at 47 tonnes in
1986 (HSDB, 1999). Production of chloral hydrate/chloral by Member
States of the European Union was estimated at 2500 tonnes in 1984
(IARC, 1995).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Newman & Wackett (1991) reported the transformation of chloral
hydrate to trichloroethanol and trichloroacetic acid by methanotrophic
bacteria. These investigators also reported the abiotic breakdown of
chloral hydrate to chloroform and formic acid. No detectable breakdown
occurred at pH 7.0 and 30°C for 24 h. At pH 9.0 and 60°C, the
half-time for breakdown was 16 min.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
No information is available.
6.2 Human exposure
The major route of exposure to chloral hydrate is from
chlorinated drinking-water. A typical concentration of chloral hydrate
in a public water supply in the USA is 5 µg/litre (US EPA, 1994). More
than 200 million people in the USA are routinely exposed to chloral
hydrate from this route. Assuming water consumption of 2 litres per
day and a body weight of 70 kg, the exposure is 0.14 µg/kg body weight
per day. Additional exposure could result from inhalation of aerolized
water during showering. As these water droplets are typically not
small enough to penetrate deep in the lung, they are deposited in the
upper airways. Thus, the water droplets are an additional source of
oral exposure to chloral hydrate. Some chloral hydrate from water used
for showering/bathing would also be absorbed through the skin.
Quantitative data on these additional sources of exposure are not
available.
Simpson & Hayes (1998) reported the occurrence of chloral hydrate
in the drinking-water of seven cities in Australia. The reported range
was 0.2-19 µg/litre.
When chloral hydrate is used in clinical medicine, the
recommended dose for an adult as a sedative is 250 mg, 3 times a day
(equivalent to 10.7 mg/kg body weight per day); the recommended dose
as a hypnotic drug is 500-1000 mg (equivalent to 7.1-14.3 mg/kg body
weight) (Goodman & Gilman, 1985). The recommended dose for a child
undergoing a medical or dental procedure is 50-100 mg/kg body weight
(Badalaty et al., 1990; Fox et al., 1990). A child is typically given
a higher dose than an adult because a deeper level of sedation is
desired to obtain better cooperation from the child during the medical
or dental procedure. There is no evidence that a child is less
sensitive than an adult to the sedative effects of chloral hydrate.
No quantitative information is available from occupational
exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Chloral hydrate is completely absorbed following oral
administration; no information is available on dermal absorption.
Qualitatively similar metabolism occurs in mice, rats, dogs, Japanese
medaka (Oryzias latipes), and humans (Marshall & Owens, 1954; Owens
& Marshall, 1955; Breimer, 1977; Gosselin et al., 1981; Goodman &
Gilman, 1985; Hobara et al., 1986, 1987a,b, 1988a,b; Reimche et al.,
1989; Gorecki et al., 1990; Hindmarsh et al., 1991; Mayers et al.,
1991; Abbas et al., 1996; Lipscomb et al., 1996, 1998; Abbas & Fisher,
1997; Henderson et al., 1997; Stenner et al., 1997, 1998;
Beland et al., 1998; Elfarra et al., 1998; Fisher et al., 1998;
Merdink et al., 1998, 1999; Greenberg et al., 1999). The metabolic
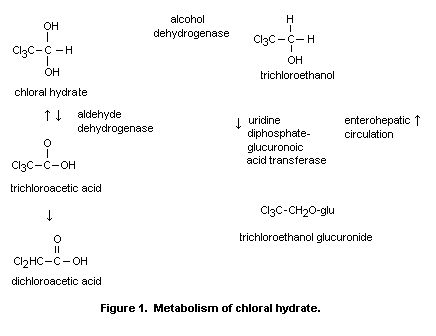
pathway is shown in Figure 1.
Chloral hydrate is rapidly metabolized in both hepatic and
extrahepatic tissues to trichloroethanol and trichloroacetic acid. The
alcohol dehydrogenase responsible for reducing it to trichloroethanol
is located in both liver and erythrocytes. A portion of the
trichloroethanol produced is conjugated with glucuronic acid. The
majority of the trichloroethanol glucuronide is excreted in the urine.
A portion of the trichloroethanol glucuronide is secreted into the
bile and is subject to enterohepatic circulation. Oxidation of chloral
hydrate to trichloracetic acid occurs primarily in the liver and
kidney via an aldehyde dehydrogenase using nicotinamide adenine
dinucleotide (NAD) as a cofactor. The major route of excretion of the
metabolites of chloral hydrate is elimination in the urine. Chloral
hydrate and its metabolites have been found in milk from women treated
with chloral hydrate (Bernstine et al., 1954). The concentration of
these chemicals, however, is too low to cause a pharmacological effect
in the nursing infant (HSDB, 1999).
In mice and rats, 8% of the administered dose of chloral hydrate
is directly eliminated in urine, 15% is converted to trichloroacetic
acid (including the contribution from enterohepatic circulation), and
77% is converted to trichloroethanol (Beland et al., 1998). In humans,
92% of the administered dose of chloral hydrate is converted to
trichloroethanol, and 8% is converted directly to trichloroacetic
acid; additional trichloroacetic acid is formed during enterohepatic
circulation of trichloroethanol, such that 35% of the initial dose of
chloral hydrate is converted to trichloroacetic acid (Allen & Fisher,
1993).
1. EXECUTIVE SUMMARY
This CICAD on chloral hydrate was prepared by the US
Environmental Protection Agency (EPA) and is based on the US EPA's
Toxicological review on chloral hydrate (US EPA, 2000). Scientific
literature identified as of March 1999 was included. Information on
the nature of the review processes and the availability of the source
document is presented in Appendix 3. Information on the peer review of
this CICAD is presented in Appendix 4. This CICAD was approved as an
international assessment at a meeting of the Final Review Board, held
in Sydney, Australia, on 21-24 November 1999. Participants at the
Final Review Board meeting are listed in Appendix 5. The International
Chemical Safety Card (ICSC 0234) for chloral hydrate, produced by the
International Programme on Chemical Safety, has been reproduced in
Appendix 6 (IPCS, 1993).
Chloral hydrate (CAS No. 302-17-0) is synthesized by the
chlorination of ethanol. It is used in human and veterinary medicine
as a sedative and hypnotic drug. The anhydrous chemical, chloral
(CAS No. 75-87-6), is used as an intermediate in the synthesis of
DDT, methoxychlor, naled, trichlorfon, dichlorvos, and
trichloroacetic acid.
The major route of exposure of the general public is from
drinking-water, as chloral hydrate is formed when drinking-water is
disinfected with chlorine. A typical concentration of chloral hydrate
in a public water supply in the USA is 5 µg/litre. Since chloral
hydrate is a metabolite of trichloroethylene and tetrachloroethylene,
people will be exposed to chloral hydrate if they are exposed to these
chemicals. The public will be exposed to the metabolites of chloral
hydrate, trichloroacetic acid and dichloroacetic acid, as these
chemicals are also formed when drinking-water is disinfected with
chlorine. In its use as a sedative for people, the usual clinical dose
is 250 mg, 3 times a day (equivalent to 10.7 mg/kg body weight per
day). The metabolite trichloroethanol is responsible for the
pharmacological effect. No quantitative information is available from
occupational exposure.
Chloral hydrate is irritating to the skin and mucous membranes
and often causes gastric distress, nausea, and vomiting at the
recommended clinical dose. An acute overdose produces (in order of
progression) ataxia, lethargy, deep coma, respiratory depression,
hypotension, and cardiac arrhythmia. There is some evidence of hepatic
injury in people surviving near-lethal, acute overdoses, but no
convincing evidence that hepatic injury results from the recommended
clinical dose. Several studies of the clinical use of chloral hydrate
show a low incidence of minor side-effects. Despite its long use in
human medicine, there is no published information on toxicity in
controlled studies in humans following extended exposure.
Chloral hydrate is completely absorbed and rapidly metabolized
following oral administration. The major metabolites are
trichloroethanol and its glucuronide and trichloroacetic acid. Some
data suggest that a small amount of dichloroacetic acid may be formed.
In humans, the half-life of trichloroethanol and its glucuronide is
about 8 h; the half-life of trichloroacetic acid is about 4 days. Some
data suggest that the half-life of trichloroethanol is increased
several-fold in pre-term and full-term infants compared with toddlers
and adults. The major route of excretion of the metabolites of chloral
hydrate is elimination in the urine. Chloral hydrate and its
metabolites have been found in milk from women treated with chloral
hydrate. The concentration of these chemicals, however, is too low to
cause a pharmacological effect in the nursing infant.
Acute administration of chloral hydrate to mice causes loss of
coordination (ataxia) at about the same exposure as in humans for the
same effect. A 90-day study in mice shows no evidence of behavioural
changes or other neurotoxicity. Chronic studies in rats and mice show
no evidence of behavioural changes and no evidence of
histopathological changes in nervous tissue. A slight decrement in
humoral immunity was observed following exposure of mice for 90 days.
Chloral hydrate has been tested for developmental effects in rats and
mice. No structural abnormalities were observed. In a
neurodevelopmental study in mice, there was a slight effect in passive
avoidance learning. Although chloral hydrate has not been tested in a
two-generation reproduction study, the data on reproductive
performance and on effects on sperm and oocytes do not suggest that
reproductive toxicity is likely to be a critical effect. In addition,
no histopathological effects are observed in reproductive organs of
rodents in subchronic or chronic studies. All of the studies in
laboratory animals show non-cancer health effects at an exposure far
in excess of the exposure that is effective for sedation in humans.
There are no carcinogenicity data from humans. Two bioassays in
rats show no increase in tumours at any site. Three separate bioassays
in male mice show an increased incidence of liver tumours. The most
definitive of these studies shows an increased incidence and
multiplicity of liver tumours at each of three exposures. These data
provide suggestive evidence of carcinogenicity in male mice but are
not considered appropriate for conducting a human health risk
assessment with a linear response at low exposure.1
1 In a National Toxicology Program carcinogenicity bioassay in mice
that became available after the Final Review Board meeting, males
had an increased incidence of hepatic tumours, and females had a
low increased incidence of pituitary adenomas that was of borderline
statistical significance.
There is an extensive database on genetic toxicity. A variety of
results show that chloral hydrate is a weak gene mutagen and
clastogen. Chloral hydrate induces aneuploidy in a wide variety of
cell types. These latter effects are thought to arise by disruption of
the spindle apparatus. High concentrations of chloral hydrate are
required to cause observable effects. Although these data suggest that
genotoxicity may play a role in the toxicity of chloral hydrate, the
data indicate that these effects require concentrations that are
unlikely to occur under physiological conditions at the exposures
typically encountered in the environment. Some likely candidates for
the induction of liver tumours in male mice include the formation of
DNA adducts caused by free radicals generated by the metabolism of
chloral hydrate by cytochrome P450 2E1 (CYP2E1) and through
cytotoxicity leading to compensatory hyperplasia.
The tolerable intake for non-cancer health effects of 0.1 mg/kg
body weight per day was estimated from the
lowest-observed-adverse-effect level (LOAEL) for sedation in humans of
10.7 mg/kg body weight per day using a total uncertainty factor of
100.
Only limited data are available on environmental effects.
Methanotrophs can convert chloral hydrate to trichloroethanol and
trichloroacetic acid. Chloral hydrate also undergoes abiotic
degradation under some conditions. Limited data are available on the
inhibition of growth of bacteria, algae, and protozoa and
developmental effects in sea urchins. Insufficient data are available
with which to assess the risk to the environment from chloral hydrate.
2. IDENTITY AND PHYSICAL/CHEMICAL PROPERTIES
Chloral hydrate (CAS No. 302-17-0) is synthesized by the
chlorination of ethanol. The structural formula is given in section 7.
The CAS name is 2,2,2-trichloro-1,1-ethanediol. Synonyms include
chloral monohydrate, trichloroacetaldehyde hydrate,
trichloroacetaldehyde monohydrate, and
1,1,1-trichloro-2,2-dihydroxyethane. The relative molecular mass is
165.42; the solubility in water is 8.3 g/ml; the octanol/water
partition coefficient (log Kow) is 0.99; and the vapour pressure
is 2 kPa at 25°C. The chemical and physical properties of chloral
hydrate are summarized in the International Chemical Safety Card
included in this document (Appendix 6).
Chloral (CAS No. 75-87-6) is the anhydrous form of the chemical.
The conversion from chloral to chloral hydrate occurs spontaneously
when chloral is placed in aqueous media.
3. ANALYTICAL METHODS
A method for the determination of trace amounts of chloral
hydrate in environmental samples is available. Carbonyl compounds are
converted to their 2,4-dinitrophenylhydrazone derivatives, separated
with high-performance liquid chromatography, and detected by
ultraviolet absorbance (Fung & Grosjean, 1981). The lowest
quantifiable limit for a variety of carbonyls ranges from 1 to 6 ng.
Chloral hydrate and its metabolites (trichloroethanol,
trichloroethanol glucuronide, and trichloroacetic acid) can be
determined in rat liver homogenates using headspace gas chromatography
and electron capture detection (Koppen & Dalgaard, 1988). The
detection limits are 0.06 µg/ml for trichloroethanol and
trichloroethanol glucuronide and 0.02 µg/ml for chloral hydrate and
trichloroacetic acid. A comparable method for the determination of
these chemicals in blood and urine is also available (Breimer et al.,
1974). The detection limits are 0.5 µg/ml for chloral hydrate and
trichloroethanol and 0.1 µg/ml for trichloroacetic acid.
Chloral hydrate and its metabolites can be measured in biological
samples after conversion to the methyl esters and separation and
detection with gas chromatography/mass spectrometry (Yan et al.,
1999). The range for measurement is between 0.12 and 7.83 µmol/litre
(equivalent to about 20-1290 µg/litre).
A method for determining trichloroethanol in plasma for use in a
clinical laboratory with liquid chromatography has also been developed
(Gupta, 1990). The method is useful for determining trichloroethanol
in plasma in the pharmacologically active range (up to 12 mg/litre)
and in the acutely toxic range (about 100 mg/litre). The method takes
about 2 h to complete.
A spectrophotometric method for the determination of chloral
hydrate in commercial drug products is based on the reaction of
quinaldine ethyl iodide with chloral hydrate to produce a stable blue
cyanine dye with an absorption maximum at about 605 nm
(Helrich, 1990).
4. SOURCES OF HUMAN AND ENVIRONMENTAL EXPOSURE
Chloral hydrate is not known to occur as a natural product. The
major route of human exposure to chloral hydrate is from
drinking-water. Chloral hydrate and its metabolites, trichloroacetic
acid and dichloroacetic acid, are formed as by-products when water is
disinfected with chlorine. The carbon is derived from natural organic
matter (humic and fulvic substances) in the source water. The amount
of chloral hydrate formed depends on the concentration of humic and
fulvic substances and the conditions of chlorination. Additional
chloral hydrate can be formed if water containing chlorine is mixed
with food containing humic and fulvic acids (Wu et al., 1998). Chloral
hydrate is also a metabolite of trichloroethylene and
tetrachloroethylene. Humans will be exposed to chloral hydrate if they
are exposed to these chemicals. Chloral hydrate has been widely used
as a sedative and hypnotic drug in adult and pediatric medicine.
Chloral is used as an intermediate in the synthesis of the
insecticides DDT, methoxychlor, naled, trichlorfon, and dichlorvos and
the herbicide trichloroacetic acid (IARC, 1995).
Chlorate hydrate could be released to the environment from
wastewater treatment facilities, from the manufacture of
pharmaceutical-grade chloral hydrate, and from the waste stream during
the manufacture of insecticides and herbicides that use chloral as an
intermediate.
In the USA, production of chloral hydrate/chloral was estimated
at 590 tonnes in 1975, and imports were estimated at 47 tonnes in
1986 (HSDB, 1999). Production of chloral hydrate/chloral by Member
States of the European Union was estimated at 2500 tonnes in 1984
(IARC, 1995).
5. ENVIRONMENTAL TRANSPORT, DISTRIBUTION, AND TRANSFORMATION
Newman & Wackett (1991) reported the transformation of chloral
hydrate to trichloroethanol and trichloroacetic acid by methanotrophic
bacteria. These investigators also reported the abiotic breakdown of
chloral hydrate to chloroform and formic acid. No detectable breakdown
occurred at pH 7.0 and 30°C for 24 h. At pH 9.0 and 60°C, the
half-time for breakdown was 16 min.
6. ENVIRONMENTAL LEVELS AND HUMAN EXPOSURE
6.1 Environmental levels
No information is available.
6.2 Human exposure
The major route of exposure to chloral hydrate is from
chlorinated drinking-water. A typical concentration of chloral hydrate
in a public water supply in the USA is 5 µg/litre (US EPA, 1994). More
than 200 million people in the USA are routinely exposed to chloral
hydrate from this route. Assuming water consumption of 2 litres per
day and a body weight of 70 kg, the exposure is 0.14 µg/kg body weight
per day. Additional exposure could result from inhalation of aerolized
water during showering. As these water droplets are typically not
small enough to penetrate deep in the lung, they are deposited in the
upper airways. Thus, the water droplets are an additional source of
oral exposure to chloral hydrate. Some chloral hydrate from water used
for showering/bathing would also be absorbed through the skin.
Quantitative data on these additional sources of exposure are not
available.
Simpson & Hayes (1998) reported the occurrence of chloral hydrate
in the drinking-water of seven cities in Australia. The reported range
was 0.2-19 µg/litre.
When chloral hydrate is used in clinical medicine, the
recommended dose for an adult as a sedative is 250 mg, 3 times a day
(equivalent to 10.7 mg/kg body weight per day); the recommended dose
as a hypnotic drug is 500-1000 mg (equivalent to 7.1-14.3 mg/kg body
weight) (Goodman & Gilman, 1985). The recommended dose for a child
undergoing a medical or dental procedure is 50-100 mg/kg body weight
(Badalaty et al., 1990; Fox et al., 1990). A child is typically given
a higher dose than an adult because a deeper level of sedation is
desired to obtain better cooperation from the child during the medical
or dental procedure. There is no evidence that a child is less
sensitive than an adult to the sedative effects of chloral hydrate.
No quantitative information is available from occupational
exposure.
7. COMPARATIVE KINETICS AND METABOLISM IN LABORATORY ANIMALS
AND HUMANS
Chloral hydrate is completely absorbed following oral
administration; no information is available on dermal absorption.
Qualitatively similar metabolism occurs in mice, rats, dogs, Japanese
medaka (Oryzias latipes), and humans (Marshall & Owens, 1954; Owens
& Marshall, 1955; Breimer, 1977; Gosselin et al., 1981; Goodman &
Gilman, 1985; Hobara et al., 1986, 1987a,b, 1988a,b; Reimche et al.,
1989; Gorecki et al., 1990; Hindmarsh et al., 1991; Mayers et al.,
1991; Abbas et al., 1996; Lipscomb et al., 1996, 1998; Abbas & Fisher,
1997; Henderson et al., 1997; Stenner et al., 1997, 1998;
Beland et al., 1998; Elfarra et al., 1998; Fisher et al., 1998;
Merdink et al., 1998, 1999; Greenberg et al., 1999). The metabolic
pathway is shown in Figure 1.
Chloral hydrate is rapidly metabolized in both hepatic and
extrahepatic tissues to trichloroethanol and trichloroacetic acid. The
alcohol dehydrogenase responsible for reducing it to trichloroethanol
is located in both liver and erythrocytes. A portion of the
trichloroethanol produced is conjugated with glucuronic acid. The
majority of the trichloroethanol glucuronide is excreted in the urine.
A portion of the trichloroethanol glucuronide is secreted into the
bile and is subject to enterohepatic circulation. Oxidation of chloral
hydrate to trichloracetic acid occurs primarily in the liver and
kidney via an aldehyde dehydrogenase using nicotinamide adenine
dinucleotide (NAD) as a cofactor. The major route of excretion of the
metabolites of chloral hydrate is elimination in the urine. Chloral
hydrate and its metabolites have been found in milk from women treated
with chloral hydrate (Bernstine et al., 1954). The concentration of
these chemicals, however, is too low to cause a pharmacological effect
in the nursing infant (HSDB, 1999).
In mice and rats, 8% of the administered dose of chloral hydrate
is directly eliminated in urine, 15% is converted to trichloroacetic
acid (including the contribution from enterohepatic circulation), and
77% is converted to trichloroethanol (Beland et al., 1998). In humans,
92% of the administered dose of chloral hydrate is converted to
trichloroethanol, and 8% is converted directly to trichloroacetic
acid; additional trichloroacetic acid is formed during enterohepatic
circulation of trichloroethanol, such that 35% of the initial dose of
chloral hydrate is converted to trichloroacetic acid (Allen & Fisher,
1993).
 Although earlier reports claimed the detection of substantial
quantities of dichloroacetic acid in blood in studies with rodents
(Abbas et al., 1996), data show that the dichloroacetic acid is most
likely formed by an acid-catalysed dechlorination of trichloroacetic
acid in the presence of reduced haemoglobin (Ketcha et al., 1996).
Recent experimental data and pharmacokinetic model simulations in
rodents suggest that dichloroacetic acid occurs only as a short-lived
metabolite in the liver and is rapidly converted to two-carbon,
non-chlorinated metabolites and carbon dioxide, with the chlorine
atoms entering the chloride pool (Merdink et al., 1998). Using a
different extraction procedure less likely to induce the artefactual
formation of dichloroacetic acid, Henderson et al. (1997) showed the
presence of dichloroacetic acid in children treated with chloral
hydrate in a clinic.
Breimer (1977) administered an aqueous solution of chloral
hydrate to five human volunteers. Each volunteer received a single
oral dose of 15 mg/kg body weight. Chloral hydrate could not be
detected in the plasma even at the first sampling time of 10 min.
A method with a limit of detection of 0.5 mg/litre was used.
Trichloroethanol and trichloroethanol glucuronide reached peak
concentrations 20-60 min after administration of chloral hydrate. The
maximum concentration of trichloroethanol in the plasma was about
5 mg/litre. The average half-lives of trichloroethanol and
trichloroethanol glucuronide were 8 h (range 7-9.5 h) and 6.7 h (range
6-8 h), respectively. The half-life of trichloroacetic acid was about
4 days. Zimmermann et al. (1998) administered a single dose of 250 mg
chloral hydrate in drinking-water to 18 healthy male volunteers
(20-28 years of age). Chloral hydrate, trichloroethanol, and
trichloroacetic acid were measured in plasma. Chloral hydrate could be
detected 8-60 min after dosing in only some of the plasma samples.
The measured concentration of chloral hydrate was not reported, but
the limit of detection was stated as 0.1 mg/litre. The maximum plasma
concentration of trichloroethanol of 3 mg/litre was achieved 0.67 h
after dosing, and the maximum plasma concentration of trichloroacetic
acid of 8 mg/litre was achieved 32 h after dosing. The terminal
half-life was 9.3-10.2 h for trichloroethanol and 89-94 h for
trichloroacetic acid.
Two toxicokinetic models are available for chloral hydrate in
rats and mice (Abbas et al., 1996; Beland et al., 1998). Beland et al.
(1998) treated rats and mice with chloral hydrate by gavage with 1 or
12 doses using 50 or 200 mg/kg body weight per dose. The maximum
levels of chloral hydrate, trichloroethanol, and trichloroethanol
glucuronide in the plasma were observed at the initial sampling time
of 0.25 h. The half-life of chloral hydrate in the plasma was
approximately 3 min. The half-lives of trichloroethanol and
trichloroethanol glucuronide in the plasma were approximately 5 and
7 min, respectively. Trichloroacetic acid was the major metabolite
found in the plasma, with the maximum level being reached 1-6 h after
dosing. The half-life of trichloroacetic acid in the plasma was
approximately 8-11 h. Comparable values were obtained for rats.
Estimates of the concentrations of trichloroacetic acid and
trichloroethanol at steady state under various exposure conditions are
in Appendix 1.
Several studies have investigated the age dependence of the
metabolism of chloral hydrate (Reimche et al., 1989; Gorecki et al.,
1990; Hindmarsh et al., 1991; Mayers et al., 1991). These studies were
conducted in critically ill patients in neonatal and paediatric
intensive care units and may not be representative of a population of
healthy infants. The half-lives for trichloroethanol and its
glucuronide were increased several-fold in pre-term and full-term
infants compared with toddlers and adults. The half-lives for
trichloroethanol in toddlers and adults were similar. These
age-related differences likely are the result of the immaturity of
hepatic metabolism, particularly glucuronidation, and decreased
glomerular filtration.
Kaplan et al. (1967) investigated the effect of ethanol
consumption on the metabolism of chloral hydrate in adults. Subjects
ingested doses of ethanol (880 mg/kg body weight), chloral hydrate
(9-14 mg/kg body weight), or both. In subjects consuming both ethanol
and chloral hydrate, blood trichloroethanol levels rose more rapidly
and reached higher values than in subjects consuming chloral hydrate
only. Ethanol promotes the formation of trichloroethanol because the
oxidation of ethanol provides NADH used for the reduction of chloral
hydrate (Watanabe et al., 1998).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
Sanders et al. (1982) studied the acute toxicity of chloral
hydrate in CD-1 mice. Groups of eight male and eight female mice were
given chloral hydrate by gavage in distilled water at 300, 600, 900,
1200, 1500, or 1800 mg/kg body weight. No deaths occurred at 900 mg/kg
body weight or below in either sex. The calculated LD50 for females
was 1265 mg/kg body weight and for males was 1442 mg/kg body weight.
Effects were seen within 10 min of dosing. The mice became sedated at
300 mg/kg body weight. At 600 and 900 mg/kg body weight, the animals
became lethargic and exhibited loss of righting reflex. Respiration
was markedly inhibited at 1200, 1500, and 1800 mg/kg body weight.
Inhibition of respiration appeared to be the immediate cause of death.
Most deaths occurred within 4 h at 1800 mg/kg body weight. At 1200 and
1500 mg/kg body weight, some deaths occurred after 4 h, with all
deaths occurring within 24 h.
Goldenthal (1971) reported an oral LD50 in rats of 480 mg/kg
body weight.
8.1.2 Inhalation
Odum et al. (1992) exposed four female CD-1 mice to chloral for
6 h at a concentration of 100 ppm (603 mg/m3). This exposure induced
deep anaesthesia. The mice recovered normally after the exposure
stopped. The effects in the lung included vacuolization of clara
cells, alveolar necrosis, desquamination of the epithelium, and
alveolar oedema. The lung to body weight ratio increased 1.5-fold,
most likely due to the alveolar oedema.
8.2 Irritation and sensitization
There are no studies of irritation or sensitization in laboratory
animals.
8.3 Short-term exposure
Sanders et al. (1982) studied the short-term toxicity of chloral
hydrate in mice. Groups of male CD-1 mice were given chloral hydrate
by gavage in distilled water at 14.4 or 144 mg/kg body weight per day
for 14 days. No significant effect on body weight was observed. No
changes in internal organs were noted from a gross examination. Groups
of 11-12 mice were evaluated for several toxicological parameters. No
significant effects on haematological or serum biochemical parameters
were noted. There was a statistically significant (P < 0.05)
increase in liver weight (17%) and a decrease in spleen weight (27%)
at the high exposure. The no-observed-adverse-effect level (NOAEL) in
this study is 14.4 mg/kg body weight per day; the LOAEL is 144 mg/kg
body weight per day. The increase in liver weight, but not the
decrease in spleen weight, was confirmed in a subsequent 90-day study
by the same researchers.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Sanders et al. (1982) administered chloral hydrate in
drinking-water to CD-1 mice at 70 or 700 mg/litre (equivalent to 16 or
160 mg/kg body weight per day) for 90 days. In males, hepatomegaly (an
increase in weight of 20% and 34% at the low and high exposure,
respectively) and microsome proliferation (no increase in total
microsomal protein, increase in cytochrome b5 of 26% and 40%,
increase in aminopyrine N-demethylase of 28% and 20%, and increase
in aniline hydroxylase of 24% and 30% at the low and high exposures,
respectively, when reported as mg of protein per mg of total liver
protein) were observed. There were no biologically significant changes
in serum enzymes. Hepatomegaly was not seen in females, but there were
changes in hepatic microsomal parameters (increase in total microsomal
protein of 10%, increase in aniline hydroxylase of 23%, and decrease
in cytochrome b5 of 12% when reported as mg of protein per mg of
total liver protein), but only at the high exposure. No other
significant toxicological changes were observed. Based on hepatomegaly
and changes in microsomal parameters in males at the high exposure,
this study identifies a LOAEL of 160 mg/kg body weight per day and a
NOAEL of 16 mg/kg body weight per day.
Daniel et al. (1992b) exposed male and female Sprague-Dawley rats
(10 per sex per dose) for 90 days to chloral hydrate in drinking-water
at a concentration of 300, 600, 1200, or 2400 mg/litre (equivalent to
an exposure of 24, 48, 96, or 168 mg/kg body weight per day in males
and 33, 72, 132, or 288 mg/kg body weight per day in females). The
tissues of animals from the high-exposure group and liver sections
from all treated males were examined histopathologically. No mortality
occurred in any groups prior to sacrifice. Organ weights, including
liver weight, and clinical chemistry values in treated animals were
only sporadically or inconsistently different from control animal
values. Focal hepatocellular necrosis was observed in 2 of 10 males in
each of the groups exposed to 96 and 168 mg/kg body weight per day.
The necrotic lesion was minimal at 96 mg/kg body weight per day and
was significantly more severe at 168 mg/kg body weight per day.
Necrotic lesions were not reported in any treated females or in any
control animals. While serum enzymes were generally increased in
treated animals, dramatic increases were reported in males in the
168 mg/kg body weight per day group; mean aspartate aminotransferase,
alanine aminotransferase, and lactate dehydrogenase levels in this
group were elevated 89%, 54%, and 127% above the corresponding control
values, respectively.
8.4.2 Chronic exposure and carcinogenicity
Rijhsinghani et al. (1986) evaluated carcinogenic effects in male
mice (C57BL × C3HF1). Groups of 15-day-old mice received chloral
hydrate by gavage in distilled water at 0, 5, or 10 mg/kg body weight
(26, 15, and 14 mice per group, respectively). Animals were sacrificed
when moribund or at week 78, at week 88, or between weeks 89 and 92.
Livers were examined histopathologically using light and electron
microscopy. In mice sacrificed 48-92 weeks after treatment, the
incidence of hepatic nodules (adenomas or trabecular carcinomas) was
3/9 and 6/8 for animals from the 5 and 10 mg/kg body weight per day
dose groups, respectively, compared with 2/19 in controls. The
increase in tumours was statistically significant (P < 0.05) only
in the 10 mg/kg body weight group.1
Daniel et al. (1992a) exposed 40 male B6C3F1 mice for 104 weeks
to drinking-water containing chloral hydrate at 1 g/litre (equivalent
to 166 mg/kg body weight per day). Untreated control animals (23 in
one group and 10 in a second group) received distilled water. Interim
sacrifices were conducted at 30 and 60 weeks of exposure (five animals
per group at each sacrifice interval). Complete necropsy and
microscopic examination were performed. There were no significant
treatment-related effects on survival or body weight. There were no
changes in spleen, kidney, or testis weights or histopathological
changes in any tissue except the liver. The toxicity in the liver was
characterized by increased absolute liver weight and liver to body
weight ratio at all three sacrifice intervals. At week 104, liver
weight was 37% higher than in controls, and liver to body weight ratio
was 42% higher than in controls. Hepatocellular necrosis was noted in
10/24 (42%) treated animals; other pathological changes of mild
severity reported in the livers of treated animals included
cytoplasmic vacuolization, cytomegaly, and cytoplasmic alteration. The
prevalence of liver tumours at terminal sacrifice was statistically
significantly (P < 0.05) increased over controls, with
hepatocellular carcinomas in 11/24 and hepatocellular adenomas in
7/24 animals; for carcinomas and adenomas combined, the prevalence was
17/24. In control animals, carcinomas, adenomas, and carcinomas and
adenomas (combined) occurred in 2/20, 1/20, and 3/20, respectively.
At the 60-week sacrifice, there were 2/5 treated animals with
hepatocellular carcinomas, compared with 0/5 controls. No carcinomas,
adenomas, or hyperplastic nodules were reported in animals sacrificed
at week 30.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity bioassay became available. In this study, an
up to 5 times higher single dose of chloral hydrate than that used
in the Rijhsinghani et al. (1986) study administered to male or
female B6C3F1 mice failed to induce tumours in any organ
(NTP, 2000a).
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male B6C3F1 mice. Mice were administered chloral
hydrate in drinking-water for 104 weeks. Mice (72 in each group) had a
mean exposure of 0, 13.5, 65, or 146.6 mg/kg body weight per day.
There was no change in water consumption, survival, behaviour, body
weight, or organ weights at any exposure. There was no evidence of
hepatocellular necrosis at any exposure and only minimal changes in
the levels of serum enzymes. This study identifies a NOAEL for
non-cancer effects in mice of 146.6 mg/kg body weight per day (the
highest exposure tested). There was no increase in the prevalence of
neoplasia at sites other than the liver. Although the background
response in this study is higher than normal for this strain of mice,
the mice showed an increase in proliferative lesions in the liver
(hyperplasia, adenoma, carcinoma, and combined adenoma and carcinoma)
at all exposures. These data are summarized in Table 1. The calculated
effective dose for a 10% tumour incidence (ED10) is 1.98 mg/kg body
weight per day, and its 95% lower confidence limit (LED10) is
1.09 mg/kg body weight per day (see Appendix 2).
Leuschner & Beuscher (1998) conducted a chronic bioassay for
carcinogenicity in Sprague-Dawley rats. Chloral hydrate was
administered in drinking-water for 124 weeks (males) and 128 weeks
(females). The rats (50 males and 50 females in each group) had an
exposure of 15, 45, or 135 mg/kg body weight per day. There was no
effect on survival, appearance, behaviour, body weight, food and water
consumption, or organ weights. There was no evidence of an increased
incidence of tumours in any organ. Histopathological examination
revealed an increased incidence of hepatocellular hypertrophy at the
highest exposure in males only (11% in controls versus 28% at the
highest exposure; P < 0.01). This finding, graded as minimal to
slight in severity, was characterized by a diffuse liver cell
enlargement with slightly eosinophilic cytoplasm and was considered by
the authors as a first sign of toxicity. The type, incidence, and
severity of other non-neoplastic lesions were not increased in treated
animals compared with controls. Based on the evidence of minimal
toxicity in the liver, which is of doubtful biological significance,
this study establishes a NOAEL of 45 mg/kg body weight per day and a
LOAEL of 135 mg/kg body weight per day.
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male F344 rats. Rats were administered chloral
hydrate in drinking-water for 104 weeks. Rats (78 in each group) had a
mean daily exposure of 0, 7.4, 37.4, or 162.6 mg/kg body weight per
day. There was no change in water consumption, survival, behaviour,
body weight, or organ weights at any exposure. There was no indication
of liver toxicity at any exposure as shown by the lack of liver
necrosis, lack of hyperplasia, no increase in mitotic index, and only
minimal changes in the levels of serum enzymes. There was no increase
at any exposure in the prevalence or multiplicity of hepatocellular
neoplasia or neoplasia at any other site. This study identifies a
NOAEL of 162.6 mg/kg body weight per day (the highest exposure
tested).1
Two of the metabolites of chloral hydrate, trichloroacetic acid
and dichloroacetic acid, have been shown to cause liver tumours in
rodents. For example, trichloroacetic acid in drinking-water induced
liver tumours in male and female mice when the exposure exceeded 200
mg/kg body weight per day (Herren-Freund et al., 1987; Bull et al.,
1990; Pereira, 1996). There was no evidence of increased
carcinogenicity, however, when male rats were exposed to
trichloroacetic acid at 360 mg/kg body weight per day (DeAngelo et
al., 1997). Dichloroacetic acid in drinking-water induced liver
tumours in male and female mice when the exposure exceeded 160 mg/kg
body weight per day (Herren-Freund et al., 1987; Bull et al., 1990;
DeAngelo et al., 1991; Daniel et al., 1992a; Ferreira-Gonzalez et al.,
1995; Pereira, 1996). Dichloroacetic acid also induced liver tumours
in male rats when the exposure exceeded 40 mg/kg body weight per day
(Richmond et al., 1995; DeAngelo et al., 1996).
A number of studies have shown that trichloroethylene is toxic to
the mouse lung bronchiolar epithelium, causing a highly specific
lesion to the clara cells of mice. Short-term exposure causes
vacuolization of the clara cells; long-term exposure causes pulmonary
adenomas and adenocarcinomas (Odum et al., 1992; Green et al., 1997).
These effects are thought to be due to the accumulation of chloral
within the clara cells. Trichloroethylene is efficiently metabolized
to chloral, but the major pathway from chloral to trichloroethanol and
its glucuronide is blocked, leading to an accumulation of chloral and
the observed toxicity.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity assay became available. In this study,
lifetime gavage administration of chloral hydrate at similar dose
levels induced hepatocellular tumours in male B6C3F1 mice and a low
frequency of pituitary hyperplasia and adenomas in females that was
of borderline statistical significance (NTP, 2000b).
Table 1: Prevalence and multiplicity of hepatocellular proliferative lesions in mice at 104 weeks.a
Treatment group Number examinedc Hyperplasia Adenoma Carcinoma Adenoma
(mg/kg body + carcinoma
weight per day)b
0 42 7.1d 21.4d 54.8d 64.3d
0.07 ± 0.04e 0.21 ± 0.06e 0.74 ± 0.12e 0.95 ± 0.12e
13.5 46 32.6f 43.5f 54.3 78.6f
0.41 ± 0.10f 0.65 ± 0.12f 0.72 ± 0.11 1.37 ± 0.16f
65 39 33.3f 51.3f 59.0 79.5f
0.38 ± 0.09f 0.95 ± 0.18f 1.03 ± 0.19 1.97 ± 0.23f
146.6 32 37.5f 50.0f 84.4f 90.6f
0.41 ± 0.10f 0.72 ± 0.15f 1.31 ± 0.17f 2.03 ± 0.25f
a From George et al. (2000).
b Time-weighted mean daily dose.
c Animals surviving longer than 78 weeks.
d Prevalence (percentage of animals with at least one lesion).
e Multiplicity (number of lesions per animal ± SEM).
f Statistically different from the control value, P < 0.05.
8.5 Genotoxicity and related end-points
8.5.1 Genotoxicity
There is an extensive database on the genotoxicity of chloral
hydrate and its metabolites. A complete summary of these results is
provided in US EPA (2000).
Chloral hydrate did not induce mutation in most strains of
Salmonella typhimurium, but did in some studies with S. typhimurium
TA100 and in a single study with S. typhimurium TA104. The latter
response was inhibited by free-radical scavengers alpha-tocopherol and
menadione (Ni et al., 1994).
Chloral hydrate did not induce mitotic crossing-over in
Aspergillus nidulans in the absence of metabolic activation. Chloral
hydrate caused weak induction of meiotic recombination in the presence
of metabolic activation and gene conversion in the absence of
metabolic activation in Saccharomyces cerevisiae. It did not induce
reverse mutation in S. cerevisiae. Chloral hydrate clearly induced
aneuploidy in various fungi in the absence of metabolic activation.
Chloral hydrate induced somatic and germ cell mutations in
Drosophila melanogaster.
Chloral hydrate did not produce DNA-protein cross-links in rat
liver nuclei, DNA single-strand breaks/alkaline-labile sites in
primary hepatocytes in vitro, or DNA repair in Escherichia coli.
One study showed induction of single-strand breaks in liver DNA of
both rats and mice treated in vivo; another study in both species
using higher concentrations of chloral hydrate found no such effect.
Chloral hydrate was weakly mutagenic, but did not induce
micronuclei in mouse lymphoma cells in vitro. Chloral hydrate
increased the frequency of micronuclei in Chinese hamster cell lines.
Although a single study suggested that chloral hydrate induces
chromosomal aberrations in Chinese hamster CHED cells in vitro, the
micronuclei produced probably contained whole chromosomes and not
chromosome fragments, as the micronuclei could all be labelled with
antikinetochore antibodies.
In kangaroo rat kidney epithelial cells, chloral hydrate
inhibited spindle elongation and broke down mitotic microtubuli,
although it did not inhibit pole-to-pole movement of chromosomes. It
produced multipolar spindles, chromosomal dislocation from the mitotic
spindle, and a total lack of mitotic spindles in Chinese hamster
DON:Wg.3h cells.
Chloral hydrate weakly induced sister chromatid exchange in
cultures of human lymphocytes. It induced micronuclei, aneuploidy,
C-mitosis, and polyploidy in human lymphocytes in vitro. Micronuclei
were induced in studies with human whole blood cultures but not in one
study with isolated lymphocytes. The differences seen in the
micronucleus test have been attributed to differences between whole
blood and purified lymphocyte cultures (Vian et al., 1995), but this
hypothesis has not been tested.
Chloral hydrate increased the frequency of chromosomal
aberrations in mouse bone marrow, spermatogonia, and primary and
secondary spermatocytes, but not in oocytes, after in vivo
treatment. Chloral hydrate induced chromosomal aberrations in mouse
bone marrow erythrocytes after treatment in vivo. In one of these
studies, the use of antikinetochore antibodies suggested induction of
micronuclei containing both whole chromosomes and fragments. Chloral
hydrate induced micronuclei in the spermatids of mice treated in vivo
in some studies. Chloral hydrate induced aneuploidy in the bone
marrow of mice treated in vivo. It increased the rate of aneuploidy
in mouse secondary spermatocytes. It did not produce polyploidy in
bone marrow, oocytes, or gonosomal or autosomal univalents in primary
spermatocytes of mice treated in vivo. Chloral hydrate, however,
induced polyploidy and meiotic delay when a synchronized population of
mouse oocytes was exposed in vitro prior to the resumption of
maturation.
Trichloroethanol, a reduction product of chloral hydrate, did not
induce lambda prophage in E. coli or mutation in S. typhimurium
TA100. Trichloroethanol caused spindle aberrations when mouse oocytes
were treated in vitro.
Trichloroacetic acid did not induce lambda prophage in E. coli
and was not mutagenic to S. typhimurium in the presence or absence
of metabolic activation. Trichloroacetic acid was weakly positive in
the mouse lymphoma assay with metabolic activation. Trichloroacetic
acid also did not induce chromosomal damage in human lymphocytes or
micronuclei in bone marrow in vitro. It is unclear whether
trichloroacetic acid can induce chromosomal damage in vivo, because
some studies have been positive and others negative.
Dichloroacetic acid did not induce differential toxicity in DNA
repair-deficient strains of S. typhimurium but did induce lambda
prophage in E. coli. Dichloroacetic acid gave equivocal results for
gene mutation in S. typhimurium TA100 and TA98. Dichloroacetic acid
was weakly mutagenic in the in vitro mouse lymphoma assay and
induced chromosomal aberrations but not micronuclei or aneuploidy in
that test system. Dichloroacetic acid induced micronuclei in mouse
polychromatic erythrocytes in vivo and mutations at the lacI locus
in the transgenic B6C3F1 mouse (the Big Blue Mouse) in vivo at an
exposure that induces liver tumours in male mice. It is unclear
whether dichloroacetic acid can induce primary DNA damage, as some
assays are positive and others negative.
8.5.2 Cell proliferation
Rijhsinghani et al. (1986) evaluated the acute effects of chloral
hydrate on liver cell proliferation in 15-day-old male mice (C57BL ×
C3HF1). Mice were given 0, 5, or 10 mg chloral hydrate/kg body
weight by gavage in distilled water (9, 10, and 6 mice per group,
respectively) and sacrificed after 24 h. Cell proliferation was
evaluated by calculating the mitotic index (number of mitoses per 100
nuclei) from liver sections. The mitotic index in liver cells was
significantly increased (0.9235) in mice receiving 5 mg/kg body weight
when compared with the control value (0.3382), and elevated (0.7433)
(although not statistically significantly) in mice receiving 10 mg/kg
body weight. Hepatic necrosis was not observed in mice from either
treatment group at autopsy.
As part of the chronic bioassay for carcinogenicity, George et
al. (2000) evaluated hepatocyte proliferation in male F344 rats and
male B6C3F1 mice. Exposures are given in section 8.4.2. Five days
prior to sacrifice at 13, 26, 52, or 72 weeks in rats and 26, 52, or
78 weeks in mice, animals were given bromodeoxyuridine. Labelled
nuclei were identified by chromogen pigment over the nuclei, and the
labelling index was calculated. Outside of the areas with tumours in
the liver of mice, there was no significant evidence of increased
hepatocyte proliferation in rats or mice.
8.5.3 Oncogene activation
Velazquez (1994) investigated the induction of H- ras
proto-oncogene mutations in mice. DNA from normal liver and tumour
tissue was obtained from male B6C3F1 mice administered 1 g chloral
hydrate/litre (166 mg/kg body weight per day) in drinking-water for
2 years. H- ras mutations were present in one out of seven (14%)
tumours. The spectrum of mutations was the same as that of spontaneous
liver tumours. Based on these data, it is unlikely that H- ras
activation is a mechanism of carcinogenicity relevant to chloral
hydrate.
8.5.4 Free radicals and DNA adduct formation
Ni et al. (1994, 1995, 1996) studied the metabolism of chloral
hydrate in an in vitro system using microsomes from male B6C3F1
mice. The metabolism of chloral hydrate generated free radicals as
detected by electron spin resonance spectroscopy and caused endogenous
lipid peroxidation, resulting in the production of malondialdehyde,
formaldehyde, and acetaldehyde, all of which are known to produce
liver tumours in rodents. Trichloroacetic acid and trichloroethanol
also produced free radicals and induced lipid peroxidation when tested
in this system. The authors speculated that the free radicals were
Cl3CCO2Ê. and/or Cl3CÊ. Incubation of chloral hydrate,
trichloroethanol, or trichloroacetic acid in the presence of
microsomes and calf thymus DNA resulted in the formation of a
malondialdehyde-modified DNA adduct. This research group further
showed that chloral hydrate induced an increase in mutations at the
hprt and tk loci in transgenic human lymphoblastoid cells
containing CYP2E1. In contrast, when the parental cell line lacking
CYP2E1 was treated with the same concentration of chloral hydrate, no
mutations were found at either locus. These data implicate CYP2E1 as
the primary cytochrome subfamily involved in the metabolism of chloral
hydrate to reactive intermediates.
8.5.5 Cell communication
The effects of 1-, 4-, 6-, 24-, 48-, and 168-h exposures to
chloral hydrate (0, 1, 5, or 10 mmol/litre) on gap junction
intercellular communication in Clone 9 cell cultures (normal rat
hepatocytes) were reported by Benane et al. (1996). No differences in
intercellular communication were seen between the groups treated with
1 mmol/litre at 1, 4, and 6 h of exposure and controls, as measured by
a dye transfer protocol. There were significant differences between
all other groups and the controls. The shortest exposure time and
lowest exposure concentration that reduced dye transfer significantly
were in the group treated with 1 mmol/litre for 24 h.
8.5.6 Peroxisome proliferation
As part of the chronic bioassay for carcinogenicity in mice,
George et al. (2000) found no evidence of peroxisome proliferation
using cyanide-insensitive palmitoyl CoA oxidase in the livers of male
mice treated with chloral hydrate for 26 weeks.
8.6 Reproductive and developmental toxicity
Klinefelter et al. (1995) evaluated effects on sperm morphology
and motility in F344 rats administered chloral hydrate in
drinking-water for 52 weeks at levels of 0, 55, or 188 mg/kg body
weight per day. The researchers examined cauda epididymal sperm motion
parameters and testicular and epididymal histopathology. Chloral
hydrate did not cause any visible systemic toxicity and had no effects
on epididymal or testicular histopathology. However, the percentage of
motile sperm was significantly decreased (P < 0.01) from 68% in
controls to 58% in rats exposed to 188 mg/kg body weight per day. The
percentage of progressively motile sperm was also significantly
decreased (P < 0.01) from 63% in controls to 53% in this group. In
addition, the frequency distribution of the average straight-line
velocities of sperm at this exposure was significantly shifted
(P < 0.01) to the lower ranges when compared with controls. In this
study, the NOAEL is 55 mg/kg body weight per day; the LOAEL is 188
mg/kg body weight per day.
Kallman et al. (1984) exposed male and female CD-1 mice to
chloral hydrate in drinking-water at 21.3 or 204.8 mg/kg body weight
per day. Animals were exposed for 3 weeks prior to breeding. Exposure
of females (5 per group) continued during gestation and until pups
were weaned at 21 days of age. No gross malformations were noted, and
no significant effects were observed in duration of gestation, number
of pups delivered, pup weight, or number of stillborn pups. All pups
(15 per group) showed the same rate of development and level of
performance on several neurobehavioural tests, except that pups
exposed to 204.8 mg/kg body weight per day when tested at 23 days of
age showed impaired retention of passive avoidance learning on both
the 1-h and 24-h retention tests (P < 0.05). This study identified
a NOAEL for neurodevelopmental toxicity of 21.3 mg/kg body weight per
day and a LOAEL of 204.8 mg/kg body weight per day based on the
impairment in passive avoidance learning. This study also identifies a
NOAEL for reproductive and other developmental effects of 204.8 mg/kg
body weight per day (the highest exposure tested).
Johnson et al. (1998) tested the potential for chloral hydrate to
cause developmental toxicity in Sprague-Dawley rats. Chloral hydrate
was administered in drinking-water to 20 rats from gestational day 1
to gestational day 22 at an average exposure of 151 mg/kg body weight
per day. Control animals were given distilled water. There was no
evidence of maternal toxicity, no change in the number of implantation
or resorption sites, no change in the number of live or dead fetuses,
no change in placental or fetal weight, no change in crown-rump
length, and no increase in the incidence of morphological changes. A
detailed examination found no evidence of cardiac anomalies. Based on
this study, the NOAEL for developmental toxicity is 151 mg/kg body
weight per day (the highest exposure tested).
Johnson et al. (1998) also tested the potential for
trichloroethanol and trichloroacetic acid to cause developmental
toxicity in Sprague-Dawley rats. The protocol was identical to the
study with chloral hydrate. Trichloroethanol was administered to 10
rats at an average exposure of 153 mg/kg body weight per day. No
evidence of developmental toxicity was found. In contrast, when
trichloroacetic acid was administered to 11 rats at an average
exposure of 291 mg/kg body weight per day, developmental toxicity was
observed. The effects included statistically significant (P < 0.05)
increases in average resorptions, in average implantations, and in
cardiac anomalies. Although the specific cardiac anomalies found were
different, the results with trichloroacetic acid are generally
consistent with those reported by Smith et al. (1989), who observed
adverse developmental effects from trichloroacetic acid at an exposure
of 330 mg/kg body weight per day and above.
Saillenfait et al. (1995) tested the potential of chloral hydrate
to cause developmental toxicity using a rat whole-embryo culture
system. Embryos (20 per dose) from Sprague-Dawley rats were explanted
on gestational day 10 and exposed to chloral hydrate at a
concentration of 0, 0.5, 1, 1.5, 2, or 2.5 mmol/litre (equivalent to
0, 83, 165, 248, 331, or 414 mg/litre) for 46 h. At 2.5 mmol/litre,
all embryos died. No lethality was seen at lower exposures. Chloral
hydrate caused concentration-dependent decreases in growth and
differentiation and increases in the incidence of morphologically
abnormal embryos. No effects were observed in any parameter at
0.5 mmol/litre. Decreases in crown-rump length, somite
(embryonic segment) number, and the protein or DNA content of embryos
were seen at 1 mmol/litre and above. At 1, 1.5, and 2 mmol chloral
hydrate/litre, respectively, 18%, 68%, and 100% of embryos were
malformed. Brain, eye, and ear malformations were the most prominent
effects at these concentrations. Abnormalities in the trunk and
pericardial dilation also occurred at 2 mmol/litre. In this in vitro
test system, chloral hydrate was a slightly more potent teratogen than
trichloroacetic acid or dichloroacetic acid.
Although chloral hydrate did not cause meiotic delay in the
oocytes of adult mice when administered at the time of resumption of
maturation induced by hormones (Mailhes & Marchetti, 1994), it did
cause adverse effects in vitro when a synchronized population of
oocytes was exposed prior to resumption of maturation
(Eichenlaub-Ritter & Betzendahl, 1995; Eichenlaub-Ritter et al.,
1996). In this test system, chloral hydrate induced lagging of
chromosomes during telophase I, inhibited spindle elongation in
anaphase B, and caused chromosome displacement from the spindle
equator in metaphase I and II. Oocytes became irreversibly arrested in
maturation when exposed to chloral hydrate prior to resumption of
maturation or when chloral hydrate was present during the first or
second 8 h of maturation. Spindle aberrations were observed when
oocytes were treated with trichloroethanol (Eichenlaub-Ritter et al.,
1996).
8.7 Immunological and neurological effects
Kauffmann et al. (1982) administered chloral hydrate by gavage in
distilled water at 14.4 or 144 mg/kg body weight per day to groups of
11-12 male CD-1 mice for 14 days. No effects on humoral or
cell-mediated immunity were detected at either exposure.
Kauffmann et al. (1982) administered chloral hydrate to male and
female CD-1 mice in drinking-water at 70 or 700 mg/litre (equivalent
to 16 or 160 mg/kg body weight per day) for 90 days. Humoral immunity
was assessed by the number of splenic antibody-forming cells produced
against sheep red blood cells (12 mice in the control group and 8 mice
in the exposed groups) and haemagglutination titres (20-21 mice in the
control group and 13-16 mice in the exposed groups).
Cell-mediated immunity was assessed by delayed-type hypersensitivity
to sheep red blood cells (17-20 mice in the control group and
15-16 mice in the exposed groups). Lymphocyte response was assessed
using a T-cell mitogen (Con A) and a B-cell mitogen (LPS)
(17-22 animals in the control group and 13-16 mice in the exposed
groups). In males, no effects were detected in either humoral or
cell-mediated immunity at either exposure. No effects on cell-mediated
immunity were noted in females at either exposure. In females, both
exposures resulted in a statistically significant decrease
(P < 0.05) in humoral immune function (36% and 40% at the
low and high exposures, respectively) when expressed as
antibody-forming cells per spleen. The decrease, however, was
statistically significant only at the higher exposure when
expressed as antibody-forming cells per million spleen cells
(a 32% decrease). There was no effect on haemagglutination titres
or on spleen cell response to the B-cell mitogen at either exposure.
The decrease in antibody-forming cells per million spleen cells at
the higher exposure in female mice is regarded as an adverse
response in this study. Accordingly, the NOAEL for immunotoxicity is
16 mg/kg body weight per day; the LOAEL is 160 mg/kg body weight
per day.
Kallman et al. (1984) administered chloral hydrate by gavage in
distilled water at 50, 100, 200, 300, or 400 mg/kg body weight to
groups of 12 male CD-1 mice. All doses resulted in the rapid onset of
ataxia, with an ED50 (maximal effect seen in 50% of animals) of
84.2 mg/kg body weight at 5 min (the time of maximal effect). Animals
recovered within 2-3 h. No delayed changes in muscular coordination
were detectable when the mice were tested 24 h after treatment.
Kallman et al. (1984) evaluated behavioural toxicity in groups of
12 male CD-1 mice administered chloral hydrate by gavage in distilled
water at 14.4 or 144 mg/kg body weight per day for 14 days. When
measured 24-48 h after exposure was terminated, no significant effects
on body weight, motor activity, physical appearance, behaviour,
muscular coordination, or endurance were observed.
Kallman et al. (1984) exposed groups of 12 male CD-1 mice to
drinking-water containing chloral hydrate at a concentration of 70 or
700 mg/litre (equivalent to 16 or 160 mg/kg body weight per day) for
90 days. When measured 24 h after exposure was terminated, no
treatment-related effects on mortality, body weight, physical
appearance, behaviour, locomotor activity, learning in repetitive
tests of coordination, response to painful stimuli, strength,
endurance, or passive avoidance learning were observed. Both exposures
resulted in a decrease of about 1°C in mean body temperature
(P < 0.05). Because of the lack of increased effect with a 10-fold
increase in exposure and because hypothermia as a side-effect of
chloral hydrate or from an overdose of chloral hydrate has not been
reported in humans, the decrease in body temperature is not considered
an adverse effect. This study identifies a NOAEL for neurobehavioural
toxicity of 160 mg/kg body weight per day (the highest exposure
tested).
A condensation product of tryptamine and chloral hydrate,
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline (TaClo), has been
found in the blood of elderly patients administered chloral hydrate
for 2-7 days (Bringmann et al., 1999). This metabolite initiated a
slowly progressive neurodegeneration when administered to rats in a
subchronic study (Gerlach et al., 1998). There is, however, no
evidence of neurodegeneration in the chronic studies with chloral
hydrate in rats and mice.
9. EFFECTS ON HUMANS
Chloral hydrate has been widely used as a sedative and hypnotic
drug in humans. Trichloroethanol is responsible for the
pharmacological activity (Marshall & Owens, 1954; Breimer, 1977;
Goodman & Gilman, 1985). Exposure information is discussed in section
6.2.
Chloral hydrate is irritating to the skin and mucous membranes
and often causes gastric distress, nausea, and vomiting at recommended
doses. There are no reports of sensitization in humans. Overdoses
produce (in order of progression) ataxia, lethargy, deep coma,
respiratory depression, hypotension, and cardiac arrhythmias. The
life-threatening effects are from severe respiratory depression,
hypotension, and cardiac arrhythmias. For some representative case
reports, see Marshall (1977), Anyebuno & Rosenfeld (1991), Ludwigs et
al. (1996), and Sing et al. (1996). A potentially life-threatening
oral dose for humans is approximately 10 g (143 mg/kg body weight),
although death has been reported from as little as 4 g, and some
individuals have survived ingesting 30 g or more. Extended use of
chloral hydrate may result in development of tolerance to the
pharmacological effect and physical dependence on or addiction to
chloral hydrate.
Shapiro et al. (1969) reviewed the medical records of 1618
patients who had received chloral hydrate at 1 g (213 patients, 13%),
0.5 g (1345 patients, 83%), or various other doses (60 patients, 4%).
Adverse reactions were reported in 38 patients (2.3%). Of these
patients, 4 received 1 g, 1 received 0.75 g, and 33 received 0.5 g.
Reported adverse reactions included gastrointestinal symptoms in 10
patients, central nervous system (CNS) depression in 20 patients, skin
rash in 5 patients, prolonged prothrombin time in 1 patient, and
bradycardia in 1 patient. In all patients, the side-effects
disappeared when chloral hydrate therapy was stopped. There was no
evidence of association between adverse side-effects and age, weight,
or sex.
Miller & Greenblatt (1979) reviewed the medical records of 5435
hospital patients who received chloral hydrate at a dose of either
0.5 g (about 7-8 mg/kg body weight) or 1 g (about 14-16 mg/kg body
weight). Adverse reactions were noted in 119 cases (2.2%).
CNS depression was most common (58 patients, or 1.1%), with minor
sensitivity reactions, including rash, pruritus, fever, and
eosinophilia, second most common (19 patients, or 0.35%). Other
adverse reactions included gastrointestinal disturbances (0.28%) and
CNS excitement (0.22%). Three individuals (0.05%) were judged to have
life-threatening reactions involving CNS depression, asterixis
(involuntary jerking movements), or hypotension. The data show that
adverse reactions involving the CNS become more frequent with
increasing dosage in patients older than 50 years, in patients who
died during hospitalization, in patients who received concurrently
benzodiazepine anti-anxiety drugs, and in patients with elevated
levels of blood urea nitrogen.
Greenberg et al. (1991) reported various side-effects experienced
by children receiving chloral hydrate sedation in preparation for
computer tomography (CT) procedures. In a "high-dose" group, composed
of 295 children (average age 2.18 years) who received a single dose of
80-100 mg/kg body weight and a maximum total dose of 2 g, adverse
reactions occurred in 23 of the patients (7%) and included vomiting
(14 patients), hyperactivity (5 patients), and respiratory symptoms,
such as wheezing and secretion aspiration (4 patients). Cardiac
monitoring did not reveal any abnormalities or arrhythmias in any of
the children. A second "lower-dose" cohort of 111 children (average
age 1.9 years) received 40-75 mg chloral hydrate/kg body weight. These
patients received the lower dose because of existing liver or renal
impairment, respiratory insufficiency, or CNS depression. There were
no adverse side-effects or complications reported in this group.
Children with severe liver or renal disease or affected by severe
CNS depression were not treated with chloral hydrate.
Lambert et al. (1990) conducted a retrospective analysis of
hospital medical records to investigate a possible link between
chloral hydrate administration and direct hyperbilirubinaemia (DHB) in
neonates following prolonged administration of chloral hydrate
(25-50 mg/kg body weight for up to 20 days). Direct bilirubin is a
measure of the free, unconjugated bilirubin in the serum. In the first
study, the DHB was of unknown etiology in 10 of the 14 newborns with
DHB; all 10 of these DHB patients had received chloral hydrate. In the
second study, among 44 newborns who had received chloral hydrate,
10 patients who developed DHB had received a mean cumulative dose of
1035 mg/kg body weight. In contrast, 34 patients whose direct
bilirubin levels were within normal ranges received a mean cumulative
dose of 183 mg/kg body weight. The total bilirubin levels (free plus
conjugated) were the same in both groups and within the normal range.
Kaplan et al. (1967) investigated whether ethanol ingestion
increased the effects of chloral hydrate. Five male volunteers
weighing 70-107 kg consumed ethanol (880 mg/kg body weight), chloral
hydrate (1 g, 9-14 mg/kg body weight), or both. Blood pressure and
cardiac rate did not vary significantly among treatments. In the
presence of ethanol, the concentration of trichloroethanol in the
blood rose more rapidly and reached a higher value, but the rate of
depletion was not significantly changed. The increase in the
concentration of trichloroethanol was not sufficient to produce a
marked enhancement of the hypnotic effect. The volunteers reported
symptoms (drowsiness, dizziness, blurred vision) and their severity
during the 6-h observation period. At all time points, the rank order
of effects was ethanol plus chloral hydrate > ethanol > chloral
hydrate.
No long-term studies of chloral hydrate in humans were located.
Chloral hydrate is addictive and is a controlled substance (Schedule
IV) in the USA.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Some data are available from cell multiplication inhibition tests
(toxic thresholds) in bacteria, algae, and protozoa. These data are
summarized in Table 2.
Schatten & Chakrabarti (1998) showed that chloral hydrate at 0.1%
(only concentration tested) causes alteration of centrosomal material
and abnormal microtubule configurations in California sea urchins
(Strongylocentrotus purpuratus and Lytechinus pictus). Chakrabarti
et al. (1998) also showed that chloral hydrate at 4 mmol/litre (660
mg/litre, only concentration tested) induced ciliary loss in the early
embryo phase of Lytechinus pictus. Exposure in this study was for 30
h at the blastula stage (14 h after fertilization).
Table 2: Effects of chloral hydrate on bacteria, algae, and protozoa.
Test system Effect Reference
Bacteria 16-h EC3 at 1.6 mg/litre Bringmann & Kuehn,
(Pseudomonas putida) 1980a
Green alga 7-day EC3 at 2.8 mg/litre Bringmann & Kuehn,
(Scenedesmus 1980a
quadricaudata)
Blue-green alga 8-day EC3 at 78 mg/litre Bringmann & Kuehn,
(cyanobacterium) 1976
(Microcystis
aeruginosa)
Protozoan 72-h EC5 at 79 mg/litre Bringmann & Kuehn,
(Enterosiphon sulcatum) 1980a
Protozoan EC5 at 86 mg/litre Bringmann & Kuehn,
(Uronema parduczi) 1980b
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Chloral hydrate has been extensively used as a sedative and
hypnotic drug in human and veterinary medicine. The metabolite
trichloroethanol is responsible for the pharmacological effect.
Chloral hydrate is irritating to the skin and mucous membranes and
often causes gastric distress, nausea, and vomiting at recommended
doses. Acute overdoses produce (in order of progression) ataxia,
lethargy, deep coma, respiratory depression, hypotension, and cardiac
arrhythmias. There is some evidence of hepatic injury in people
surviving near-lethal, acute overdoses, but no convincing evidence
that hepatic injury results from the recommended clinical dose.
Despite its long use in human medicine, there is no published
information on toxicity in controlled studies in humans following
extended exposure.
Chloral hydrate is completely absorbed and rapidly metabolized
following oral administration. The major metabolites are
trichloroethanol and its glucuronide and trichloroacetic acid. Some
data suggest that a small amount of dichloroacetic acid may be formed.
In humans, the half-life of trichloroethanol and its glucuronide is
about 8 h; the half-life of trichloroacetic acid is about 4 days. Some
data suggest that the half-life of trichloroethanol is increased
several-fold in pre-term and full-term infants compared with toddlers
and adults. The major route of excretion of the metabolites of chloral
hydrate is elimination in the urine. Chloral hydrate and its
metabolites have been found in milk from women treated with chloral
hydrate. The concentration of these chemicals, however, is too low to
cause a pharmacological effect in the nursing infant.
Acute administration of chloral hydrate to mice causes loss of
coordination (ataxia) at about the same exposure as in humans for the
same effect. A 90-day study in mice shows no evidence of behavioural
changes or other neurotoxicity. Chronic studies in rats and mice show
no evidence of behavioural changes and no evidence of
histopathological changes in nervous tissue. These studies used an
exposure approximately 15 times the recommended clinical dose in
humans. There is some evidence of mild liver toxicity following
chronic exposure in rats and mice. A slight decrement in humoral
immunity was observed in female mice following exposure for 90 days.
The antibody-forming cell response is considered an excellent
indicator of the status of humoral immunity because of the complex
cellular cooperation required to produce antibody and because the
number of cells that produce antibody can be quantified. A depression
in the number of these cells is considered an adverse response because
the production of antibodies is important to the defence strategy of
the organism. However, the quantitative relationship between the
depression in antibody-forming cells in the spleen and the
concentration of circulating antibody is unknown. In this study,
because there was no depression in circulating antibodies measured by
the haemagglutination titre, there might be no significant depression
in the ability of the host to mount a protective antibody response.
Chloral hydrate has been tested for developmental effects in rats and
mice. No structural abnormalities were observed. A slight effect was
observed in mice in passive avoidance learning when dams were exposed
prior to breeding, during gestation, and during nursing and pups were
tested at 23 days of age. Although chloral hydrate has not been tested
in a two-generation reproduction study, the data on reproductive
performance and on effects on sperm and oocytes do not suggest that
reproductive toxicity is likely to be a critical effect. In addition,
no histopathological effects are observed in reproductive organs of
rodents in subchronic or chronic studies. Some in vitro data,
however, suggest that chloral hydrate administered to young female
children might have a latent effect on fertility. All of the studies
in laboratory animals show non-cancer health effects at an exposure
far in excess of the exposure that is effective for sedation in
humans. A complete summary of the exposure-response data is presented
in Table 3.
Simultaneous ingestion of ethanol and chloral hydrate increases
the sedative effects and side-effects of chloral hydrate. The
mechanism is the increase in the concentration of the
pharmacologically active metabolite, trichloroethanol, in the presence
of ethanol. Chronic users of ethanol are, therefore, somewhat more
sensitive to the adverse effects of chloral hydrate.
Because of the immaturity of hepatic metabolism, particularly the
glucuronidation pathway, and decreased glomerular filtration in
infants, the half-life of trichloroethanol is longer in pre-term and
full-term infants. This group is therefore somewhat more sensitive to
the adverse effects of chloral hydrate. Toddlers and adults are likely
to show similar sensitivity to chloral hydrate.
Although male laboratory rodents seem to be more sensitive than
female laboratory rodents to hepatic effects, there is no evidence of
a gender effect in humans with respect to the sedative effects or
side-effects of chloral hydrate at the recommended clinical dose.
There are no carcinogenicity data from humans. Two bioassays in
rats show no increase in tumours at any site. These studies were
limited, because only minimal toxicity was observed in the livers of
the rats in these bioassays. In one study, only slight hypertrophy was
observed at the highest exposure; in the other study, no effects were
observed at the highest exposure. No data are available in female
mice. There are three separate bioassays showing an increased
incidence of liver tumours in male mice. One study, conducted in a
very limited number of animals, showed an increase in tumours
Table 3: Summary of non-neoplastic effects.
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Human 1 day, 3 doses Sedation - 10.7 Goodman & Gilman, 1985
Rat 90 days Mild liver necrosis 96 168 Daniel et al., 1992b
and increase in
serum enzymes
Rat 104 weeks - 162.6 - George et al., 2000
Rat 124 weeks Liver hypertrophy 45 135 Leuschner & Beuscher, 1998
Rat 52 weeks Sperm motility 55 188 Klinefelter et al., 1995
Rat gestation Development 151 - Johnson et al., 1998
days 1-22
Mouse 14 days Increased liver weight 14.4 144 Sanders et al., 1982
Mouse 90 days Increased liver weight 16 160 Sanders et al., 1982
Mouse 104 weeks Increased liver weight - 166a Daniel et al., 1992a
and necrosis
Mouse 104 weeks - 146.6b - George et al., 2000
Mouse 3 weeks Reproduction and 204.8 - Kallman et al., 1984
pre-breeding development
and during
gestation
Mouse Pre-breeding, Passive avoidance 21.3 204.8 Kallman et al., 1984
gestation, and learning in pups
nursing
Table 3 (cont'd)
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Mouse 1 day Ataxia - 50 Kallman et al., 1984
Mouse 14 days Neurobehaviour 144 - Kallman et al., 1984
Mouse 90 days Neurobehaviour 160 - Kallman et al., 1984
Mouse 14 days Immunotoxicity 144 - Kauffmann et al., 1982
Mouse 90 days Humoral immunity 16 160 Kauffmann et al., 1982
a Tumours at 166 mg/kg body weight per day.
b Hyperplasia and tumours at 13.5, 65, and 146.6 mg/kg body weight per day.
following a single exposure. The second study tested only one exposure
level but used an adequate number of animals. The third study shows an
increase in incidence and multiplicity of liver tumours at each of
three exposures. There are no data identifying a lesion that is a
precursor to the tumours. The strain of mice used has a very high
spontaneous incidence of liver tumours. Two of the metabolites of
chloral hydrate, trichloroacetic acid and dichloroacetic acid, have
been shown to cause liver tumours in rodents. Trichloroacetic acid
causes liver tumours only in mice. Dichloroacetic acid causes tumours
in both rats and mice.1
Chloral hydrate has been extensively studied as a genotoxic
agent. Chloral hydrate was positive in some bacterial mutation tests,
indicating that it may be capable of inducing point mutations. It was
also positive in the mouse lymphoma assay for mutations at the tk
locus. Chloral hydrate also induced somatic and germ cell mutations
in D. melanogaster. Some data also show chloral hydrate to be a very
weak clastogen in mammalian cells.
Chloral hydrate has been shown to induce aneuploidy in a variety
of cells, including S. cerevisiae, A. nidulans, Chinese hamster
embryonic fibroblasts, Chinese hamster primary cell lines LUC2 and
DON:Wg3h, human peripheral blood lymphocytes, mouse spermatocytes, and
mouse spermatids. Because there is a mixture of positive and negative
in vivo data, with no reason to weigh some studies more than others,
it is not clear whether chloral hydrate is capable of inducing genetic
damage in vivo. Additional in vivo studies using standard
protocols would help clarify the relevance of genetic damage to a
human health risk assessment.
The effects on aneuploidy are thought to arise via disruption of
the mitotic spindle structure or function by inhibition of tubulin
and/or microtubule-associated proteins; both substances are components
of the spindle apparatus. Some data also suggest that chloral hydrate
may act on the spindle apparatus through an increase in the
concentration of intracellular free calcium.
1 In a National Toxicology Program carcinogenicity bioassay that
became available after the Final Review Board meeting, a
carcinogenic effect was not observed after a single dose of chloral
hydrate; after lifetime exposure, males had an increased incidence
of hepatic tumours, and females had a low increased incidence of
pituitary adenomas that was of borderline statistical significance.
Several other mechanisms may play a role in the induction of
tumours in the liver of male mice. There is no convincing evidence
that chloral hydrate causes direct damage to DNA. In vitro studies
with chloral hydrate, trichloroethanol, and trichloroacetic acid and
mouse microsomes, however, show lipid peroxidation and the formation
of covalently bound DNA adducts. These effects appear to be mediated
by the formation of free radicals by CYP2E1. Another possibility is
cytotoxicity leading to compensatory hyperplasia. A single treatment
of mice with chloral hydrate caused an increase in the mitotic index
in liver cells. The increased cell division is hypothesized to either
provide additional opportunities for errors in DNA replication or
allow initiated cells to progress to a tumour. Another potentially
contributing mechanism of carcinogenesis is disruption of
intercellular communication, which has been shown in one experiment to
be influenced by chloral hydrate.
The mechanism of chloral hydrate-induced carcinogenicity in male
mice is unclear. Two mechanisms that appear ruled out are H- ras
proto-oncogene activation and peroxisome proliferation.
Although there is suggestive evidence of carcinogenicity in male
mice, the weight of evidence is not sufficient to consider tumour
induction as the critical effect.
11.1.2 Criteria for setting tolerable intakes or guidance values for
chloral hydrate
The effect that occurs at the lowest exposure is mild sedation in
humans. As this effect would not be intended or desirable in the
general population outside of the clinical setting, this response is
considered an adverse effect and is used to derive the tolerable
intake.
Acute gavage exposure in mice shows neurological effects (ataxia)
at about the same exposure for the comparable effect in humans. A
subchronic study in mice using sensitive tests for neurobehavioural
changes found none. Chronic studies in rats and mice show no evidence
of neurobehavioural changes and no evidence of histopathological
changes in nervous tissue. As with other chlorinated chemicals, there
is some evidence of carcinogenic effects in the liver of male mice
following chronic exposure.
Although the tolerable intake derived from the pharmacologically
active dose in humans is an acute tolerable intake, keeping the
exposure below this level will also be protective for any non-cancer
health effect from chronic exposure. Therefore, it is appropriate to
use the acute tolerable intake as the chronic tolerable intake as
well.
No data are available to determine a NOAEL in humans. The
recommended clinical dose for sedation in adults is 250 mg, taken 3
times a day (Goodman & Gilman, 1985). A low incidence of side-effects
also occurs at this exposure. The LOAEL is 10.7 mg/kg body weight per
day (assuming a 70-kg body weight). The pharmacokinetic information
shows that chloral hydrate and the pharmacologically active
metabolite, trichloroethanol, will not bioaccumulate.
The tolerable intake (IPCS, 1994) of 0.1 mg/kg body weight per
day was derived from the LOAEL of 10.7 mg/kg body weight per day using
a total uncertainty factor of 100. An uncertainty factor of 10 was
used to extrapolate from a LOAEL to a NOAEL, and an uncertainty factor
of 10 was used for intraspecies variability. An uncertainty factor for
chronic duration was not used. Chloral hydrate and the active
metabolite, trichloroethanol, do not bioaccumulate. The half-life of
chloral hydrate is a few minutes, and the half-life of
trichloroethanol is a few hours. Therefore, an enhanced effect from
continuous, daily exposure is not possible. Finally, there is
information from clinical use that long-term exposure to chloral
hydrate results in tolerance to the sedative effect. Developmental
toxicity, including developmental neurotoxicity, and immunotoxicity
are not critical effects. Although there is no two-generation
reproduction study, an uncertainty factor for database limitations is
not needed, as there is evidence from several studies that
reproductive toxicity is not likely to be a critical effect.
There are no inhalation studies adequate for setting a guidance
value or tolerable intake.
There are data in male mice showing that chloral hydrate causes
tumours in the liver. It is not known whether this response is
relevant for humans.
11.1.3 Sample risk characterization
The quantitative estimate of human risk for non-cancer effects is
based on the recommended clinical dose for sedation in humans and the
minor incidence of side-effects at this dose. The tolerable intake is
0.1 mg/kg body weight per day. This is 1% of the recommended single
dose for sedation in humans.
Although there is suggestive evidence of formation of tumours in
the liver of male mice and there are some data showing genotoxicity,
the mode of action for the formation of tumours is not known. It is
also not known whether this response is relevant for humans.
Millions of people are exposed to chloral hydrate on a daily
basis because it is formed during the disinfection of drinking-water
with chlorine. The typical concentration in a public water supply in
the USA is 5 µg/litre. Assuming a water consumption of 2 litres
per day and a body weight of 70 kg, the exposure is 0.14 µg/kg body
weight per day. This exposure is approximately 700 times lower than
the tolerable intake.
11.2 Evaluation of environmental effects
Insufficient data are available with which to assess the risk to
the environment from chloral hydrate.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1995) evaluated the carcinogenicity data for chloral
hydrate. It was concluded that there is inadequate evidence in humans
and limited evidence in experimental animals for the carcinogenicity
of chloral hydrate. Chloral hydrate is therefore not classifiable as
to its carcinogenicity to humans (Group 3).
IPCS (2000) recently evaluated the toxicological data on water
disinfectants and disinfectant by-products, including chloral hydrate.
Considering the dose level of 16 mg/kg body weight per day in the
90-day study in mice (Sanders et al., 1982; see section 8.4.1) as a
LOAEL (rather than as a NOAEL, as was done in the present document)
and using an uncertainty factor of 10 for intra- and interspecies
extrapolation and another factor of 10 for the use of a LOAEL rather
than a NOAEL, the Task Group calculated a tolerable daily intake (TDI)
for chloral hydrate of 16 µg/kg body weight per day. (As the present
document considered the increase in liver weight at 16 mg/kg body
weight to be a NOAEL rather than a LOAEL, the tolerable intake derived
from the studies among humans was lower, as discussed in section
11.1.)
REFERENCES
Abbas R, Fisher JW (1997) A physiologically based pharmacokinetic
model for trichloroethylene, and its metabolites, chloral hydrate,
trichloroacetate, dichloroacetate, trichloroethanol, and
trichloroethanol glucuronide in B6C3F1 mice. Toxicology and
applied pharmacology, 137:15-30.
Abbas R, Seckel CS, Kidney JK, Fisher JW (1996) Pharmacokinetic
analysis of chloral hydrate and its metabolism in B6C3F1 mice.
Drug metabolism and disposition, 24:1340-1346. See also Erratum,
Drug metabolism and disposition, 25:1449 (1997).
Allen BC, Fisher JW (1993) Pharmacokinetic modeling of
trichloroethylene and trichloroacetic acid in humans. Risk analysis,
13:71-86.
Anyebuno MA, Rosenfeld CR (1991) Chloral hydrate toxicity in a term
infant. Developments in pharmacological therapy, 17:116-120.
Badalaty MM, Houpt MI, Koenigsberg SR, Maxwell KC, Desjardins PJ
(1990) A comparison of chloral hydrate and diazepam sedation in young
children. Pediatric dentistry, 12:33-37.
Beland FA, Schmitt TC, Fullerton NF, Young JF (1998) Metabolism of
chloral hydrate in mice and rats after single and multiple doses.
Journal of toxicology and environmental health, 54:209-226.
Benane SG, Blackman CF, House DE (1996) Effect of perchloroethylene
and its metabolites on intercellular communication in clone 9 rat
liver cells. Journal of toxicology and environmental health,
48:427-437.
Bernstine JB, Meyer AE, Bernstine RL (1954) Maternal blood and breast
milk estimation following the administration of chloral hydrate during
the puerperium. Journal of obstetrics and gynaecology of the British
Commonwealth, 63:228-231.
Breimer DD (1977) Clinical pharmacokinetics of hypnotics. Clinical
pharmacokinetics, 2:93-109.
Breimer DD, Ketelaars HCJ, Van Rossum JM (1974) Gas chromatographic
determination of chloral hydrate, trichloroethanol, and
trichloroacetic acid in blood and urine employing head-space analysis.
Journal of chromatography, 88:55-63.
Bringmann G, Kuehn R (1976) Vergleichende Befunde der Schadwirkung
wassergerfahrden der Stoffe gegen Bakterien (Pseudomonas putida) und
Blaualgen (Microcystis aeruginosa). Gas- und Wasserfach:
Wasser/Abwasser, 117:410-413.
Bringmann G, Kuehn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in cell
multiplication inhibition tests. Water research, 14:231-241.
Bringmann G, Kuehn R (1980b) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen II Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, God R, Fähr S, Feines D, Fornadi K, Fornadi F (1999)
Identification of the dopaminergic neurotoxin
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline in human blood after
intake of the hypnotic chloral hydrate. Analytical biochemistry,
270:167-175.
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver
tumor induction in B6C3F1 mice by dichloroacetate and
trichloroacetate. Toxicology, 63:341-359.
Chakrabarti A, Schatten H, Mitchell KD, Crosser M, Taylor M (1998)
Chloral hydrate alters the organization of the ciliary basal apparatus
and cell organelles in sea urchin embryos. Cell and tissue research,
293:453-462.
Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP (1992a)
Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and
dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 19:159-168.
Daniel FB, Robinson M, Stober JA, Page NP, Olson GR (1992b) Ninety-day
toxicity study of chloral hydrate in the Sprague-Dawley rat. Drug and
chemical toxicology, 15:217-232.
DeAngelo AB, Daniel FB, Stober JA, Olson GR (1991) The carcinogenicity
of dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 16:337-347.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1996) The carcinogenicity
of dichloroacetic acid in the male Fischer 344 rat. Toxicology,
114:207-221.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1997) The failure of
monochloroacetic acid and trichloroacetic acid administered in the
drinking water to produce liver cancer in male F344/N rats. Journal
of toxicology and environmental health, 52:425-445.
Eichenlaub-Ritter U, Betzendahl I (1995) Chloral hydrate induced
spindle aberrations, metaphase I arrest and aneuploidy in mouse
oocytes. Mutagenesis, 10:477-486.
Eichenlaub-Ritter U, Baart E, Yin H, Betzendahl I (1996) Mechanisms of
spontaneous and chemically-induced aneuploidy in mammalian oogenesis:
Basis of sex specific differences in response to aneugens and the
necessity for further tests. Mutation research, 372:274-294.
Elfarra AA, Krause RJ, Last AR, Lash LH, Parker JC (1998) Species- and
sex-related differences in metabolism of trichloroethylene to yield
chloral and trichloroethanol in mouse, rat, and human liver
microsomes. Drug metabolism and disposition, 26:779-785.
Ferreira-Gonzalez A, DeAngelo AB, Nasim S, Garrett CT (1995) Ras
oncogene activation during hepatocarcinogenesis in B6C3F1 male mice
by dichloroacetic and trichloroacetic acid. Carcinogenesis,
16:495-500.
Fisher JW, Mahle D, Abbas R (1998) A human physiologically based
pharmacokinetic model for trichloroethylene and its metabolites:
trichloroacetic acid and free trichloroethanol. Toxicology and
applied pharmacology, 152:339-359.
Fox BE, O'Brien CO, Kangas KJ, Murphree AL, Wright KW (1990) Use of
high dose chloral hydrate for ophthalmic exams in children: A
retrospective review of 302 cases. Journal of pediatric ophthalmology
and strabismus, 27:242-244.
Fung K, Grosjean D (1981) Determination of nanogram amounts of
carbonyls as 2,4-dinitrophenylhydrazones by high-performance liquid
chromatography. Analytical chemistry, 53:168-171.
George MH, Kilburn S, Moore T, DeAngelo AB (2000) The carcinogenicity
of chloral hydrate administered in the drinking water to the male
B6C3F1 mouse and F344/N rat. Toxicologic pathology (in press).
Gerlach M, Xiao A-Y, Heim C, Lan J, God R, Feines D, Bringmann G,
Riederer P, Sontag K-H (1998)
1-Trichloromethyl-1,2,3,4-tetrahydro-ß-carboline increases
extracellular serotonin and stimulates hydroxyl radical production in
rats. Neuroscience letters, 257:17-20.
Goldenthal EI (1971) A compilation of LD50 values in newborn and
adult animals. Toxicology and applied pharmacology, 18:185-207.
Goodman LS, Gilman A (1985) The pharmacological basis of
therapeutics, 7th ed. New York, NY, The Macmillan Co.
Gorecki DKJ, Hindmarsh KW, Hall CA, Mayers DJ, Sankaran K (1990)
Determination of chloral hydrate metabolism in adult and neonate
biological fluids after single-dose administration.
Journal of chromatography, 528:333-341.
Gosselin RE, Smith RP, Hodge HC (1981) Clinical toxicology of
commercial products. Baltimore, MD, Williams & Wilkins.
Green T, Mainwaring GW, Foster JR (1997) Trichloroethylene-induced
mouse lung tumors: Studies of the mode of action and comparisons
between species. Fundamental and applied toxicology, 37:125-130.
Greenberg MS, Burton GA Jr, Fisher FW (1999) Physiologically based
pharmacokinetic modeling of inhaled trichloroethylene and its
oxidative metabolites in B6C3F1 mice. Toxicology and applied
pharmacology, 154:264-278.
Greenberg SB, Faerber EN, Aspinall CL (1991) High dose chloral hydrate
sedation for children undergoing CT. Journal of computer assisted
tomography, 15:467-469.
Gupta RN (1990) Determination of trichloroethanol, the active
metabolite of chloral hydrate, in plasma by liquid chromatography.
Journal of chromatography, 500:655-659.
HSDB (1999) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
Helrich K, ed. (1990) Official methods of analysis of the Association
of Official Analytical Chemists, 15th ed. Vol. 1. Arlington, VA,
Association of Official Analytical Chemists, p. 562.
Henderson GN, Yan Z, James MO, Davydova N, Stacpoole PW (1997)
Kinetics and metabolism of chloral hydrate in children: identification
of dichloroacetate as a metabolite. Biochemical and biophysical
research communications, 235:695-698.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The
carcinogenicity of trichloroethylene and its metabolites,
trichloroacetic acid and dichloroacetic acid, in mouse liver.
Toxicology and applied pharmacology, 90:183-189.
Hindmarsh KW, Gorecki DKJ, Sankaran K, Mayers DJ (1991) Chloral
hydrate administration to neonates: Potential toxicological
implications. Canadian Society of Forensic Science, 24:239-245.
Hobara T, Kobayashi H, Kawamoto T, Sato T, Iwamoto S, Hirota S, Sakai
T (1986) Biliary excretion of trichloroethylene and its metabolites in
dogs. Toxicology letters, 32:119-122.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1987a) The
cholecystohepatic circulation of trichloroethylene and its metabolites
in dogs. Toxicology, 44:283-295.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Hirota S, Sakai T
(1987b) Extrahepatic metabolism of chloral hydrate, trichloroethanol,
and trichloroacetic acid in dogs. Pharmacology and toxicology,
61:58-62.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988a)
Intestinal absorption of chloral hydrate, free trichloroethanol, and
trichloroacetic acid in dogs. Pharmacology and toxicology,
62:250-258.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988b) The
absorption of trichloroethylene and its metabolites from the urinary
bladder of anesthetized dogs. Toxicology, 48:141-153.
IARC (1995) Chloral and chloral hydrate. IARC (International Agency
for Research on Cancer) monographs, 63:245-269.
IPCS (1993) International Chemical Safety Card -- Chloral hydrate.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0234).
IPCS (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
IPCS (2000) Disinfectants and disinfectant by-products. Geneva,
World Health Organization (Environmental Health Criteria 216).
Johnson PD, Dawson BV, Goldberg SJ (1998) Cardiac teratogenicity of
trichloroethylene metabolites. Journal of the American College of
Cardiology, 32:540-545.
Kallman MJ, Kaempf GL, Balster RL (1984) Behavioral toxicity of
chloral in mice: An approach to evaluation. Neurobehavioral
toxicology and teratology, 6:137-146.
Kaplan HL, Forney RB, Hughes FW, Jain NC, Crim D (1967) Chloral
hydrate and alcohol metabolism in human subjects. Journal of forensic
sciences, 12:295-304.
Kauffmann BM, White KL, Sanders VM, Douglas KA, Sain LE, Borzelleca
JF, Munson AE (1982) Humoral and cell-mediated immune status in mice
exposed to chloral hydrate. Environmental health perspectives,
44:147-151.
Ketcha MM, Stevens DK, Warren DA, Bishop CT, Brashear WT (1996)
Conversion of trichloroacetic acid to dichloroacetic acid in
biological samples. Journal of analytical toxicology, 20:236-241.
Klinefelter GR, Suarez JD, Roberts NL (1995) Preliminary screening
test for the potential of drinking water disinfectant by-products to
alter male reproduction. Reproductive toxicology, 9:571-578.
Koppen B, Dalgaard L (1988) Determination of trichloroethylene
metabolites in rat liver homogenates using headspace gas
chromatography. Journal of chromatography, 442:325-332.
Lambert GH, Muraskas J, Anderson CL, Myers TF (1990) Direct
hyperbilirubinemia associated with chloral hydrate administration in
the newborn. Pediatrics, 86:277-281.
Leuschner J, Beuscher N (1998) Studies on the mutagenic and
carcinogenic potential of chloral hydrate. Arzneimittel-Forschung
drug research, 48:961-968.
Lipscomb JC, Mahle DA, Brashear WT, Garrett CM (1996) A species
comparison of chloral hydrate metabolism in blood and liver.
Biochemical and biophysical research communications, 227:340-350.
Lipscomb JC, Confer PD, Miller MR, Stamm SC, Snawder JE, Candiera SM
(1998) Metabolism of trichloroethylene and chloral hydrate by the
Japanese medaka (Oryzias latipes) in vitro. Environmental
toxicology and chemistry, 17:325-332.
Ludwigs U, Divino-Fiiho JC, Magnusson N (1996) Suicidal chloral
hydrate poisoning. Journal of clinical toxicology, 344:97-99.
Mailhes JB, Marchetti F (1994) Chemically induced aneuploidy in
mammalian oocytes. Mutation research, 320:87-111.
Marshall AJ (1977) Cardiac arrhythmias caused by chloral hydrate.
British medical journal, 2:994.
Marshall EK, Owens AH (1954) Absorption, excretion and metabolic fate
of chloral hydrate and trichloroethanol. Bulletin of the Johns
Hopkins Hospital, 95:1-18.
Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DKJ, Kasian GF (1991)
Chloral hydrate disposition following single-dose administration to
critically ill neonates and children. Developments in pharmacological
therapy, 16:71-77.
Merdink JL, Conzalez-Leon A, Bull RJ, Schultz IR (1998) The extent of
dichloroacetate formation from trichloroethylene, chloral hydrate,
trichloroacetate, and trichloroethanol in B6C3F1 mice. Toxicological
sciences, 45:33-41.
Merdink JL, Stenner RD, Stevens DK, Parker JC, Bull RJ (1999) Effect
of enterohepatic circulation on the pharmacokinetics of chloral
hydrate and its metabolites in F344 rats. Journal of toxicology and
environmental health, 56:357-368.
Miller RR, Greenblatt DJ (1979) Clinical effects of chloral hydrate in
hospitalized medical patients. Journal of clinical pharmacology,
19:669-674.
Newman LM, Wackett LP (1991) Fate of 2,2,2-trichloroacetaldehyde
(chloral hydrate) produced during trichloroethylene oxidation by
methanotrophs. Applied and environmental microbiology, 57:2399-2402.
Ni Y-C, Wong T-Y, Kadlubar FF, Fu PP (1994) Hepatic metabolism of
chloral hydrate to free-radical(s) and induction of lipid
peroxidation. Biochemical and biophysical research communications,
204:937-943.
Ni Y-C, Kadlubar FF, Fu FF (1995) Formation of
malondialdehyde-modified 2'-deoxyguanosinyl adduct from metabolism of
chloral hydrate by mouse liver microsomes. Biochemical and
biophysical research communications, 205:1110-1117.
Ni Y-C, Wong T-Y, Lloyd RV, Heinze TM, Shelton S, Casciano D, Kadlubar
FF, Fu PP (1996) Mouse liver microsomal metabolism of chloral hydrate,
trichloroacetic acid, and trichloroethanol leading to induction of
lipid peroxidation via a free radical mechanism. Drug metabolism and
disposition, 24:81-90.
NTP (2000a) Toxicology and carcinogenesis studies of chloral hydrate
in B6C3F1 mice (gavage studies). Research Triangle Park, NC,
National Institutes of Health, National Toxicology Program (NTP TR
502).
NTP (2000b) Toxicology and carcinogenesis studies of chloral hydrate
(ad libitum and dietary controlled) in male B6C3F1 mice (gavage
study). Research Triangle Park, NC, National Institutes of Health,
National Toxicology Program (NTP TR 503).
Odum J, Foster JR, Green T (1992) A mechanism for the development of
clara cell lesions in the mouse lung after exposure to
trichloroethylene. Chemico-biological interactions, 83:135-153.
Owens AH, Marshall EK (1955) Further studies on the metabolic fate of
chloral hydrate and trichloroethanol. Bulletin of the Johns Hopkins
Hospital, 97:320-326.
Pereira MA (1996) Carcinogenic activity of dichloroacetic acid and
trichloroacetic acid in the liver of female B6C3F1 mice.
Fundamental and applied toxicology, 31:192-199.
Reimche LD, Sankaran K, Hindmarsh KW, Kasian GF, Gorecki DKJ, Tan L
(1989) Chloral hydrate sedation in neonates and infants -- clinical
and pharmacologic considerations. Developments in pharmacological
therapy, 12:57-64.
Richmond RE, Carter JH, Carter HW, Daniel FB, DeAngelo AB (1995)
Immunohistochemical analysis of dichloroacetic acid (DCA)-induced
hepatocarcinogenesis in male Fischer (F344) rats. Cancer letters,
92:67-76.
Rijhsinghani KS, Abrahams C, Swerdlow MA, Rao KVN, Ghose T (1986)
Induction of neoplastic lesions in the livers of C57BL × C3HF1 mice
by chloral hydrate. Cancer detection and prevention, 9:279-288.
Saillenfait AM, Langonne I, Sabate JP (1995) Developmental toxicity of
trichloroethylene, tetrachloroethylene and four of their metabolites
in rat whole embryo culture. Archives of toxicology, 70:71-82.
Sanders VM, Kauffman BM, White KL, Douglas KA, Barnes DW, Sain LE,
Bradshaw TJ, Borzelleca JF, Munson AE (1982) Toxicology of chloral
hydrate in the mouse. Environmental health perspectives, 44:137-146.
Schatten H, Chakrabarti A (1998) Centrosome structure and function is
altered by chloral hydrate and diazepam during the first reproductive
cell cycles in sea urchin eggs. European journal of cell biology,
75:9-20.
Shapiro S, Stone D, Lewis GP, Jick H (1969) Clinical effects of
hypnotics. II. An epidemiological study. Journal of the American
Medical Association, 209:2016-2020.
Simpson KL, Hayes KP (1998) Drinking water disinfection by-products:
an Australian perspective. Water research, 32:1522-1528.
Sing K, Erickson T, Amitai Y, Hryhorczuk D (1996) Chloral hydrate
toxicity from oral and intravenous administration. Journal of
toxicology and clinical toxicology, 34:101-106.
Smith MK, Randall JL, Read EJ, Stober JA (1989) Teratogenic activity
of trichloroacetic acid in the rat. Teratology, 40:445-451.
Stenner RD, Merdink JL, Stevens DK, Springer DL, Bull RJ (1997)
Enterohepatic recirculation of trichloroethanol glucuronide as a
significant source of trichloroacetic acid. Drug metabolism and
disposition, 25:529-535.
Stenner RD, Merdink JL, Fisher JW, Bull RJ (1998)
Physiologically-based pharmacokinetic model for trichloroethylene
considering enterohepatic recirculation of major metabolites.
Risk analysis, 18:261-269.
US EPA (1994) National primary drinking water regulations;
disinfectants and disinfection byproducts; proposed rule. US
Environmental Protection Agency. Federal register, 59:38668-38829.
US EPA (2000) Toxicological review on chloral hydrate. Available
from US Environmental Protection Agency's Risk Assessment Hotline
[513-569-7254 (phone), 513-569-7159 (fax), rih.iris@epa.gov (e-mail
address), or www.epa.gov/iris (Website)].
Velazquez SF (1994) Activation of the H-ras oncogene by drinking
water disinfection by-products. Report submitted under the US
Environmental Protection Agency's Small Grant Program. April 1994. 43
pp. (NTIS/PB95-200515).
Vian L, Van Hummelen P, Bichet N, Gouy D, Kirsch-Volders M (1995)
Evaluation of hydroquinone and chloral hydrate on the in vitro
micronucleus test on isolated lymphocytes. Mutation research,
334:1-7.
Watanabe M, Takano T, Nakamura K (1998) Effect of ethanol on the
metabolism of trichloroethylene in the perfused rat liver. Journal of
toxicology and environmental health, 55:297-305.
Wu WW, Chadik PA, Davis WM, Powell DH, Delfino JJ (1998) Disinfection
byproduct formation from the preparation of instant tea. Journal of
agricultural and food chemistry, 46:3272-3279.
Yan Z, Henderson GN, James MO, Stacpoole P (1999) Determination of
chloral hydrate in human plasma by gas chromatography-mass
spectrometry. Journal of pharmaceutical and biomedical analysis,
19:309-318.
Zimmermann T, Wehling M, Schulz HU (1998) Untersuchungen zur relativen
Bioverfugbarkeit und Pharmakokinetik von Chloralhydrat und seinen
Metaboliten. [The relative bioavailability and pharmacokinetics of
chloral hydrate and its metabolites.] Arzneimittelforschung,
48:5-12.
APPENDIX 1 -- TOXICOKINETICS
This toxicokinetic analysis is used to estimate the steady-state
concentrations of trichloroacetic acid (TCA) and trichloroethanol
(TCEOH) in mice and humans using a one-compartment model, assuming
that the absorption of chloral hydrate (CH) from the gastrointestinal
tract and its metabolism in the blood are very rapid compared with the
rate of elimination of TCA and TCEOH. This assumption is supported by
the data of Beland et al. (1998) in mice and Breimer (1977) and
Zimmermann et al. (1998) in humans.
Beland et al. (1998) indicated that 15% of the dose of chloral
hydrate is converted directly to TCA and 77% is converted to TCEOH. In
humans, Allen & Fisher (1993) estimated that 8% of a dose of chloral
hydrate is converted directly to TCA and 92% is converted to TCEOH.
Additional TCA is formed from TCEOH. The total TCA formed in humans is
approximately 35% of the dose of chloral hydrate.
Estimation of TCA concentration in mice at steady state at the
clinically recommended dose for humans:
[TCA]ss-blood = PKo/ VKel = 2.5 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 3.0 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.15
(Beland et al., 1998)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.321 litre/kg
(Beland et al., 1998)
* Kel is the first-order elimination constant for TCA =
0.0819/h (Beland et al., 1998)
* PC is the liver/blood partition coefficient = 1.18 (Abbas &
Fisher, 1997)
Estimation of TCA concentration in humans at steady state at the
clinically recommended dose:
[TCA]ss-blood = PKo/ VKel = 55 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 36 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA = 0.028/h
(Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCA concentration in humans at steady state at the
tolerable intake:
[TCA]ss-blood = PKo/ VKel = 1.8 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 1.2 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA =
0.0078/h (Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCEOH concentration in mice at steady state at 166 mg/kg
body weight per day:
[TCEOH]ss-blood = PKo/ VKel = 0.6 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.77
(Beland et al., 1998)
* Ko is the dosing rate for CH = 166 mg/kg body weight per day,
equivalent to 6.917 mg/kg body weight per hour
* V is the volume of distribution = 1 litre/kg (cited in
Beland et al., 1998)
* Kel is the first-order elimination constant for TCEOH =
9.24/h (Beland et al., 1998)
Chloral hydrate at 160 mg/kg body weight per day was the highest
exposure used in the 90-day neurobehavioural study by Kallman et al.
(1984); chloral hydrate at 166 mg/kg body weight per day was the
highest exposure used in the 104-week bioassay of Daniel et al.
(1992a). These exposures are a NOAEL for sedation in mice.
Estimation of TCEOH concentration in humans at steady state at the
clinically recommended dose:
[TCEOH]ss-blood = PKo/ VKel = 4.7 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
Estimation of TCEOH concentration in humans at steady state at the
tolerable intake:
[TCEOH]ss-blood = PKo/ VKel = 0.04 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
APPENDIX 2 -- CALCULATION OF BENCHMARK DOSE FOR
TUMOUR INCIDENCE
The Benchmark Dose (ED) for tumour incidence was derived from the
incidence of adenoma plus carcinoma as reported by George et al.
(2000). The human equivalent dose was calculated using (body
weight)3/4, assuming a human body weight of 70 kg and a mouse body
weight of 0.035 kg. EPA Benchmark Dose Software was used to calculate
the ED and its lower 95% confidence limit (LED) corresponding to a 10%
increase in extra risk for tumour prevalence with the multistage
model.
Multistage Model, Version Number 1.1.0b
The form of the probability function is:
P[response] =
(1 - background ) × [1 - (e - beta 1 × dose 1 - beta 2 × dose 2) ]
The parameter betas are restricted to be positive.
Dependent variable = Incidence
Independent variable = Dose
Total number of observations = 4
Total number of records with missing values = 0
Total number of parameters in model = 3
Total number of specified parameters = 0
Degree of polynomial = 2
Maximum number of iterations = 250
Relative function convergence has been set to 2.220 45e-16
Parameter convergence has been set to 1.490 12e-8
Default initial parameter values
Background = 0.698 863
Beta(1) = 0.043 897
Beta(2) = 0.000 400 241
Parameter estimates
Variable Estimate Standard error
Background 0.691 141 0.073 072 3
Beta(1) 0.053 218 1 0.084 548 3
Beta(2) 0 0.004 035 19
Asymptotic correlation matrix of parameter estimates
Background Beta(1) Beta(2)
Background 1 -0.6319 0.5007
Beta(1) -0.6319 1 -0.9507
Beta(2) 0.5007 -0.9507 1
Analysis of deviance table
Model Log(likelihood) Deviance DF P-value
Full model -81.2046
Fitted model -81.922 1.434 7 2 0.230 999
Reduced mode -85.0504 6.256 83 2 0.043 787
Goodness of fit analysis
Administered dose Human equivalent Estimated Expected Observed Size
(mg/kg body weight dose probability
per day) (mg/kg body weight
per day)
0 0 0.6911 29.028 27 42
13.5 2.0000 0.7223 33.227 36 46
65 9.7 0.8157 31.812 31 39
146.6 21.9 0.9037 28.919 29 32
Chi-square = 1.41; DF = 2; P-value = 0.4949.
Benchmark dose computation
Specified effect 0.100 000
Risk type Extra risk
Confidence level 0.950 000
ED 1.979 786
LED 1.090 1
APPENDIX 3 -- SOURCE DOCUMENT
US Environmental Protection Agency (2000):
Toxicological review on chloral hydrate
Copies of the document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (phone)
513-569-7159 (fax)
rih.iris@epa.gov (e-mail address)
www.epa.gov/iris (Website)
This document was prepared by R. Benson, Region VIII, Denver, CO.
The document and summary information on the Integrated Risk
Information System (IRIS) have received peer review both by EPA
scientists and by independent scientists external to EPA. Subsequent
to external review and incorporation of comments, this assessment has
undergone an Agency-wide review process whereby the IRIS Program
Manager has achieved a consensus approval among the Office of Research
and Development; Office of Air and Radiation; Office of Prevention,
Pesticides, and Toxic Substances; Office of Solid Waste and Emergency
Response; Office of Water; Office of Policy, Planning, and Evaluation;
and the Regional Offices.
Internal EPA reviewers:
National Center for Environmental Assessment, Washington, DC
J. Cogliano
C. Siegel Scott
V. Vu
National Health and Environmental Effects Research Laboratory,
Research Triangle Park, NC
A. DeAngelo
R. Luebke
Office of Water, Washington, DC
A. Bathija
External peer reviewers:
P.E. Brubaker, Private Consultant
J.W. Fisher, Operational Toxicology Branch, Wright-Patterson
Air Force Base
C.C. Willhite, Department of Toxic Substances Control,
State of California
APPENDIX 4 -- CICAD PEER REVIEW
The draft CICAD on chloral hydrate was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS National Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Centre of Industrial Hygiene and Occupational Diseases, Czech
Republic
Department of Health, London, United Kingdom
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Berlin, Germany
Fraunhofer Institute for Toxicology and Aerosol Research,
Hannover, Germany
GSF Forschungszentrum für Umwelt und Gesundheit, GmbH,
Oberschleissheim, Germany
Health and Safety Executive, Bootle, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Beijing, People's Republic of China
National Center for Environmental Assessment, US Environmental
Protection Agency, Washington, DC, USA
National Center for Toxicological Research, US Food and Drug
Administration, Jefferson, AK, USA
National Chemicals Inspectorate, Solna, Sweden
National Industrial Chemicals Notification and Assessment Scheme
(NICNAS), Sydney, Australia
National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, Research Triangle Park, NC, USA
University of Bielefeld, Bielefeld, Germany
APPENDIX 5 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment,
US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry,
Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment,
US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 6 -- INTERNATIONAL CHEMICAL SAFETY CARD
CHLORAL HYDRATE ICSC: 0234
October 1999
CAS# 302-17-0 Trichloroacetaldehyde monohydrate
RTECS# FM8750000 2,2,2-Trichloro-1,1-ethanediol
UN# 2811 C2H3Cl3O2/Cl3CCH(OH)2
EC# 605-014-00-6
Molecular mass: 165.4
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Not combustible. In case of fire in the
Gives off irritating surroundings, all
or toxic fumes extinguishing agents allowed.
(or gases) in a
fire.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with
water.
EXPOSURE PREVENT DISPERSION
OF DUST!
Inhalation Confusion. Local exhaust Fresh air, rest. Artificial
Drowsiness. or breathing respiration if indicated. Refer
Nausea. protection. for medical attention.
Unconsciousness.
Skin Redness. Protective gloves. Rinse skin with plenty
of water or shower.
Eyes Redness. Safety spectacles or First rinse with plenty
eye protection in of water for several minutes
combination with (remove contact lenses if
breathing protection easily possible), then take
if powder. to a doctor.
Ingestion Abdominal pain. Do not eat, drink, or Rinse mouth. Give a slurry of
Vomiting (further smoke during work. activated charcoal in water
see inhalation). Wash hands before to drink. Refer for medical
eating. attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into containers; Do not transport with food and feedstuffs.
if appropriate, moisten first to prevent EU Classification
dusting. Carefully collect remainder, then Symbol: T
remove to safe place. R: 25-36/38
(Extra personal protection: P3 filter S: (1/2-)25-45
respirator for toxic particles). UN Classification
UN Hazard Class: 6.1
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-61G12b Separated from strong bases,
food and feedstuffs.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
TRANSPARENT, COLOURLESS CRYSTALS, WITH The substance can be absorbed
CHARACTERISTIC ODOUR. into the body by inhalation of
its aerosol and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating A harmful contamination of the air will be
producing toxic and corrosive fumes including reached rather slowly on evaporation of this
hydrogen chloride. Reacts with strong bases substance at 20°C.
producing chloroform.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. The substance irritates the eyes, the skin
and the respiratory tract. The substance
may cause effects on the central nervous
system, cardiovascular system, liver and
kidneys, resulting in lowering of
consciousness, cardiac disorders and impaired
functions. Exposure at high levels may result
in unconsciousness.
PHYSICAL PROPERTIES
Boiling Point: 97°C
Melting Point: 57-60°C
Density: 1.9 g/cm3
Solubility in water: very good
Octanol/water partition coefficient as log Pow: 0.99
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should
be given to water organisms.
NOTES
Use of alcoholic beverages enhances the harmful effect.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Le présent CICAD relatif à l'hydrate de chloral a été préparé par
l'Environmental Protection Agency des États-Unis (EPA) sur la base
d'un de ses documents intitulé Toxicological review on chloral
hydrate (US EPA, 2000). Les données qu'il contient proviennent d'un
dépouillement de la littérature scientifique jusqu'en mars 1999. On
trouvera à l'appendice 3 des renseignements sur la manière dont
l'étude bibliographique a été effectuée et sur les sources de données
disponibles. L'appendice 4 donne des indications sur les modalités
d'examen du présent CICAD par des pairs. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors d'une réunion du Comité
d'évaluation finale qui s'est tenue à Sydney (Australie) du 21 au 24
novembre 1999. La liste des participants à cette réunion figure à
l'appendice 5. La Fiche internationale sur la sécurité chimique (ICSC
0234) de l'hydrate de chloral établie par le Programme international
sur la sécurité chimique est reproduite à l'appendice 6 (IPCS, 1993).
La synthèse de l'hydrate de chloral (No CAS 302-17-0) s'effectue
par chloration de l'éthanol. On l'utilise en médécine humaine et
vétérinaire comme sédatif et hypnotique. Le chloral, qui en est la
forme anhydre (No CAS 75-87-6) est utilisé comme intermédiaire dans la
synthèse du DDT, du méthoxychlore, du naled, du trichlorfon, du
dichlorvos et de l'acide trichloracétique.
La principale voie d'exposition de la population générale est
l'eau de boisson, car il se forme de l'hydrate de chloral lors de la
désinfection de l'eau par le chlore. Aux États-Unis, la concentration
habituelle d'hydrate de chloral dans l'eau des réseaux publics de
distribution est de 5 µg/litre. Comme ce composé est un métabolite du
trichloréthylène et du tétrachloréthylène, la population se trouve
exposée à l'hydrate de chloral si elle l'est à ces deux composés. Par
ailleurs, il y a également exposition à deux métabolites de l'hydrate
de chloral, les acides dichlor- et trichloracétique, du fait que ces
deux composés se forment également dans l'eau de consommation lors de
sa désinfection par le chlore. Lorsque l'hydrate de chloral est
utilisé comme sédatif, la dose habituelle est de 250 mg trois fois par
jour (soit l'équivalent de 10,7 mg/kg de poids corporel par jour).
C'est un métabolite, le trichloréthanol, qui est responsable de
l'effet pharmacologique. On ne dispose d'aucune donnée quantitative
sur l'exposition professionnelle.
L'hydrate de chloral est irritant pour la peau et les muqueuses
et il provoque souvent des troubles gastriques, des nausées et des
vomissements lorsqu'on l'utilise à la dose recommandée dans la
pratique clinique. Une surdose aiguë entraîne progressivement ataxie,
léthargie, coma profond, dépression respiratoire, hypotension et
arrythmie cardiaque. On a trouvé des signes de lésions hépatiques chez
des sujets ayant échappé de peu à la mort par intoxication aiguë due
une surdose, mais rien ne prouve par contre de façon convaincante qu'à
la dose clinique recommandée, le composé entraîne des lésions
hépatiques. Plusieurs études portant sur l'utilisation clinique de
l'hydrate de chloral ont mis en évidence des effets secondaires
mineurs et peu fréquents. Bien que ce produit soit utilisé depuis
longtemps en médecine, aucune étude toxicologique contrôlée sur des
sujets humains n'a été publiée.
L'hydrate de chloral est intégralement absorbé et rapidement
métabolisé après administration par la voie orale. Ses principaux
métabolites sont le trichloréthanol et son glucuronide ainsi que
l'acide trichloracétique. D'après certaines données, il pourrait se
former également un peu d'acide dichloracétique. Chez l'Homme, la
demi-vie du trichloréthanol et de son glucuronide est d'environ 8 h;
celle de l'acide trichloracétique est à peu près égale à 4 jours. Un
certain nombre de données incitent à penser que la demi-vie du
trichloréthanol est plus de deux fois plus longue chez les prématurés
et les nouveau-nés à terme que chez les enfants en bas âge et les
adultes. La principale voie d'excrétion des métabolites de l'hydrate
de chloral est la voie urinaire. On peut le retrouver, accompagné de
ses métabolites, dans le lait de mères traitées par ce produit.
Toutefois leur concentration est trop faible pour avoir des effets
pharmacologiques chez les nourrissons alimentés au sein.
Administré à des souris, le composé provoque une perte de
coordination (ataxie) à une dose comparable à celle qui produit le
même effet chez l'Homme. Une étude de 90 jours sur des souris n'a
révélé aucun signe d'altération du comportement ni de neurotoxicité.
Des études au long cours sur des rats et des souris n'ont pas non plus
permis de constater d'anomalies comportementales ni de modifications
histopathologiques touchant les tissus nerveux. Après exposition de
souris pendant 90 jours, on a observé une légère diminution de
l'immunité humorale. D'autres études n'ont mis en évidence aucun effet
sur le développement des souris et des rats. Aucune anomalie
structurale n'a été relevée. Une étude consacrée à l'action de
l'hydrate de chloral sur le développement nerveux de la souris n'a mis
en évidence qu'un léger effet sur l'apprentissage de l'évitement
passif. Le composé n'a pas fait l'objet d'études de toxicité génésique
sur deux générations, mais les données dont on dispose sur l'activité
génésique des animaux et les effets sur les spermatozoïdes et les
ovocytes ne permettent pas de penser que l'hydrate de chloral puisse
avoir des effets majeurs sur la reproduction. Par ailleurs, les études
chroniques et subchroniques effectuées sur des rongeurs n'ont pas mis
en évidence d'effets histopathologiques au niveau de l'appareil
reproducteur. Toutes les études effectuées sur des animaux de
laboratoire mettent en évidence un certain nombre d'effets, mais à
l'exclusion de tout effet cancérogène et à des doses qui sont très
supérieures à celle qui provoque la sédation chez l'Homme.
En ce qui concerne l'Homme, on ne possède aucune donnée de
cancérogénicité. Deux tests biologiques effectués sur le rat ne
révèlent aucune augmentation de la fréquence des tumeurs, quelle que
soit la localisation. Par contre, dans trois autres tests distincts
effectués sur des souris mâles, on constate une augmentation de
l'incidence des tumeurs hépatiques. Celle de ces études dont le
caractère est le plus définitif indique une augmentation de
l'incidence et de la multiplicité des tumeurs pour chacune des trois
doses utilisées. Ces données semblent indiquer que le produit est
cancérogène chez la souris mâle mais on estime qu'elles ne permettent
pas d'évaluer le risque pour l'Homme avec une réponse linéaire aux
faibles doses.1
Il existe une importante base de données sur les effets
génotoxiques. Divers résultats indiquent que l'hydrate de chloral est
faiblement mutagène et clastogène. Il provoque une aneuploïdie chez
des cellules très diverses. On pense que cet effet est dû à
destruction de l'appareil fusorial. Des concentrations élevées sont
nécessaires pour que ces effets soient observables. Même si ces
résultats donnent à penser que la toxicité de l'hydrate de chloral
s'exerce notamment au niveau des gènes, ils montrent également que ces
effets ne se produisent qu'à des concentrations qui ont peu de chances
d'exister dans les conditions physiologiques, compte tenu de
l'exposition habituelle à ce produit dans l'environnement. La
formation des tumeurs hépatiques chez la souris mâle peut s'expliquer
par la formation d'adduits de l'ADN avec des radicaux libres produits
lors de la métabolisation de l'hydrate de chloral par les enzymes du
cytochrome P450 2E1 (CYP 2E1) ou par une cytotoxicité conduisant à une
hyperplasie compensatoire.
La dose journalière tolérable pour les effets non cancérogènes a
été estimée à 0,1 mg/kg pc à partir de la dose la plus faible
produisant un effet sédatif observable chez l'Homme (LOAEL), dose qui
est égale à 10,7 mg/kg par jour, avec un facteur d'incertitude de 100.
On ne possède que des données limitées sur les effets
environnementaux. Les méthanotrophes sont capables de transformer
l'hydrate de chloral en trichloréthanol et en acide trichloracétique.
Le composé subit également une dégradation abiotique dans certaines
conditions. On dispose de données limitées sur l'inhibition de la
croissance des bactéries, des algues et des protozoaires. Des
résultats sont également disponibles concernant l'effet du composé sur
le développement des oursins. On ne possède pas assez de données pour
pouvoir évaluer le risque que l'hydrate de chloral représente pour
l'environnement.
1 Un test biologique effectué dans le cadre du Programme national de
toxicologie et dont les résultats n'ont été connus qu'après la
réunion du Comité d'évaluation finale, a montré que l'incidence des
tumeurs hépatiques était en augmentation chez les souris mâles et
que chez les femelles, il y avait une faible augmentation des
adénomes hypophysaires, augmentation dont la signification
statistique était limite.
RESUMEN DE ORIENTACI²N
Este CICAD sobre el hidrato de cloral, preparado por la Agencia
para la Protección del Medio Ambiente (EPA), se basó en el
Examen toxicológico sobre el hidrato de cloral de la EPA de los
Estados Unidos (US EPA, 2000). Se incluyó la bibliografía científica
localizada hasta marzo de 1999. La información relativa al carácter de
los procesos de examen y a la disponibilidad del documento original
figura en el apéndice 3. La información sobre el examen colegiado de
este CICAD se presenta en el apéndice 4. Este CICAD se aprobó como
evaluación internacional en una reunión de la Junta de Evaluación
Final celebrada en Sydney, Australia, los días 21-24 de noviembre de
1999. En el apéndice 5 figura la lista de participantes en esta
reunión. La Ficha internacional de seguridad química (ICSC 0234) para
el hidrato de cloral, preparada por el Programa Internacional de
Seguridad de la Sustancias Químicas, se reproduce en el apéndice 6
(IPCS, 1993).
El hidrato de cloral (CAS No 302-17-0) se sintetiza mediante la
cloración de etanol. Se utiliza en la medicina humana y veterinaria
como sedante e hipnótico. El cloral (CAS No 75-87-6), producto
químico anhidro, se utiliza como intermediario en la síntesis de DDT,
metoxicloro, naled, triclorfon, diclorvos y ácido tricloroacético.
La vía principal de exposición del público general es el agua de
bebida, puesto que al desinfectar dicha agua con cloro se forma
hidrato de cloral. La concentración normal de hidrato de cloral en el
sistema público de abastecimiento de agua de los Estados Unidos es 5
µg/litro. Debido a que el hidrato de cloral es un metabolito del
tricloroetileno y el tetracloroetileno, el público estará expuesto al
hidrato de cloral si lo está a estos productos químicos. La población
está expuesta a los ácidos tricloroacético y dicloroacético,
metabolitos del hidrato de cloral, porque también se forman cuando se
desinfecta el agua de bebida con cloro. En su uso como sedante humano,
la dosis clínica normal es de 250 mg tres veces al día (equivalente a
10,7 mg/kg de peso corporal al día). El metabolito tricloroetanol es
el responsable del efecto farmacológico. No se dispone de información
cuantitativa relativa a la exposición ocupacional.
El hidrato de cloral es irritante de la piel y las membranas
mucosas y con frecuencia provoca trastornos gástricos, náuseas y
vómitos con la dosis clínica recomendada. Una sobredosis aguda produce
(en orden de progresión) ataxia, letargo, coma profundo, depresión
respiratoria, hipotensión y arritmia cardíaca. Hay algunas pruebas de
lesiones hepáticas en personas que sobreviven a sobredosis agudas casi
letales, pero no hay pruebas convincentes de que se produzcan tales
lesiones con la dosis clínica recomendada. En varios estudios sobre el
uso clínico del hidrato de cloral se ha puesto de manifiesto una
incidencia baja de efectos secundarios menores. A pesar de utilizarse
desde hace mucho tiempo en la medicina humana, no hay información
publicada sobre la toxicidad en estudios controlados realizados con
personas después de una exposición prolongada.
Tras la administración oral, el hidrato de cloral se absorbe
completamente y se metaboliza con rapidez. Los principales metabolitos
son el tricloroetanol y su glucurónido y el ácido tricloroacético.
Algunos datos parecen indicar que se puede formar una pequeña cantidad
de ácido dicloroacético. En el ser humano, la semivida del
tricloroetanol y su glucurónido es de unas ocho horas; la semivida del
ácido tricloroacético es de alrededor de cuatro días. Algunos datos
indican que la semivida del tricloroetanol aumenta varias veces en los
niños prematuros y los nacidos a término en comparación con los niños
que empiezan a caminar y los adultos. La vía principal de excreción de
los metabolitos del hidrato de cloral es la orina. Se han detectado
hidrato de cloral y sus metabolitos en la leche de mujeres tratadas
con este producto. Sin embargo, su concentración es demasiado baja
para provocar un efecto farmacológico en los niños lactantes.
La administración aguda de hidrato de cloral a ratones provoca la
pérdida de la coordinación (ataxia) con una exposición prácticamente
semejante a la de las personas para el mismo efecto. En un estudio de
90 días en ratones no se obtuvieron pruebas de cambios de
comportamiento u otros signos de neurotoxicidad. En estudios crónicos
con ratas y ratones no se detectaron cambios de comportamiento ni
cambios histopatológicos en el tejido nervioso. Tras la exposición de
ratones durante 90 días al hidrato de cloral se observó una ligera
disminución en la inmunidad humoral. Se han realizado pruebas con
hidrato de cloral para estudiar sus efectos en el desarrollo de ratas
y ratones. No se observaron anomalías estructurales. En un estudio del
neurodesarrollo en ratones, se observó un ligero efecto en el
aprendizaje de la evitación pasiva. Aunque no se ha realizado ningún
estudio de reproducción de dos generaciones con hidrato de cloral, los
datos sobre el rendimiento reproductivo y sobre sus efectos en el
esperma y los oocitos no indican que haya probabilidad de que la
toxicidad reproductiva sea un efecto crítico. Además, en estudios
subcrónicos o crónicos no se observaron efectos histopatológicos en
los órganos reproductores de roedores. En todos los estudios
realizados con animales de laboratorio se detectaron efectos en la
salud distintos del cáncer con una exposición muy superior a la eficaz
para la sedación humana.
No hay datos de carcinogenicidad en el ser humano. En dos
biovaloraciones en ratas no se observó un aumento de tumores en
ninguna parte. En tres biovaloraciones separadas en ratones machos se
detectó un aumento de la incidencia de tumores hepáticos. El más
definitivo de estos estudios demostró una mayor incidencia y
multiplicidad de tumores hepáticos en cada una de las tres
exposiciones. Estos datos parecen indicar la existencia de
carcinogenicidad en ratones machos, pero no se consideran adecuados
para realizar una evaluación del riesgo en la salud humana con una
respuesta lineal para una exposición baja.1
Hay una amplia base de datos sobre la toxicidad genética.
Diversos resultados ponen de manifiesto que el hidrato de cloral tiene
una actividad mutagénica de los genes y clastogénica débil. El hidrato
de cloral induce aneuploidía en una gran variedad de tipos de células.
Se considera que estos últimos efectos se deben a una perturbación del
huso acromático. Se necesitan concentraciones altas de hidrato de
cloral para provocar efectos observables. Aunque estos datos parecen
indicar que la genotoxicidad puede desempeñar una función en la
toxicidad del hidrato de cloral, también ponen de manifiesto que estos
efectos requieren concentraciones que no es probable que se alcancen
en condiciones fisiológicas con las exposiciones que se producen
normalmente a partir del medio ambiente. Algunos mecanismos probables
para la inducción de tumores hepáticos en ratones machos son la
formación de aductos de ADN mediante radicales libres generados en el
metabolismo del hidrato de cloral en el citocromo P450 2E1 (CYP2E1) y
la citotoxicidad que da lugar a una hiperplasia compensatoria.
Se estimó una ingesta tolerable para los efectos en la salud
distintos del cáncer de 0,1 mg/kg de peso corporal al día a partir de
la concentración más baja con efectos adversos observados (LOAEL) para
la sedación en las personas de 10,7 mg/kg, utilizando un factor de
incertidumbre total de 100.
Sólo se dispone de datos limitados sobre los efectos en el medio
ambiente. Los organismos metanotróficos pueden convertir el hidrato de
cloral en tricloroetanol y ácido tricloroacético. El hidrato de cloral
experimenta asimismo degradación abiótica en algunas condiciones. Hay
datos limitados sobre la inhibición del crecimiento de bacterias,
algas y protozoos y sobre los efectos en el desarrollo de los erizos
de mar. No hay datos disponibles suficientes que permitan evaluar el
riesgo para el medio ambiente derivado del hidrato de cloral.
1 En una biovaloración de la carcinogenicidad en ratones del
Programa Nacional de Toxicología, disponible después de la reunión
de la Junta de Evaluación Final, los machos presentaban una mayor
incidencia de tumores hepáticos y las hembras un pequeño aumento
de la incidencia de adenomas hipofisiarios, en el límite de la
significación estadística.
Although earlier reports claimed the detection of substantial
quantities of dichloroacetic acid in blood in studies with rodents
(Abbas et al., 1996), data show that the dichloroacetic acid is most
likely formed by an acid-catalysed dechlorination of trichloroacetic
acid in the presence of reduced haemoglobin (Ketcha et al., 1996).
Recent experimental data and pharmacokinetic model simulations in
rodents suggest that dichloroacetic acid occurs only as a short-lived
metabolite in the liver and is rapidly converted to two-carbon,
non-chlorinated metabolites and carbon dioxide, with the chlorine
atoms entering the chloride pool (Merdink et al., 1998). Using a
different extraction procedure less likely to induce the artefactual
formation of dichloroacetic acid, Henderson et al. (1997) showed the
presence of dichloroacetic acid in children treated with chloral
hydrate in a clinic.
Breimer (1977) administered an aqueous solution of chloral
hydrate to five human volunteers. Each volunteer received a single
oral dose of 15 mg/kg body weight. Chloral hydrate could not be
detected in the plasma even at the first sampling time of 10 min.
A method with a limit of detection of 0.5 mg/litre was used.
Trichloroethanol and trichloroethanol glucuronide reached peak
concentrations 20-60 min after administration of chloral hydrate. The
maximum concentration of trichloroethanol in the plasma was about
5 mg/litre. The average half-lives of trichloroethanol and
trichloroethanol glucuronide were 8 h (range 7-9.5 h) and 6.7 h (range
6-8 h), respectively. The half-life of trichloroacetic acid was about
4 days. Zimmermann et al. (1998) administered a single dose of 250 mg
chloral hydrate in drinking-water to 18 healthy male volunteers
(20-28 years of age). Chloral hydrate, trichloroethanol, and
trichloroacetic acid were measured in plasma. Chloral hydrate could be
detected 8-60 min after dosing in only some of the plasma samples.
The measured concentration of chloral hydrate was not reported, but
the limit of detection was stated as 0.1 mg/litre. The maximum plasma
concentration of trichloroethanol of 3 mg/litre was achieved 0.67 h
after dosing, and the maximum plasma concentration of trichloroacetic
acid of 8 mg/litre was achieved 32 h after dosing. The terminal
half-life was 9.3-10.2 h for trichloroethanol and 89-94 h for
trichloroacetic acid.
Two toxicokinetic models are available for chloral hydrate in
rats and mice (Abbas et al., 1996; Beland et al., 1998). Beland et al.
(1998) treated rats and mice with chloral hydrate by gavage with 1 or
12 doses using 50 or 200 mg/kg body weight per dose. The maximum
levels of chloral hydrate, trichloroethanol, and trichloroethanol
glucuronide in the plasma were observed at the initial sampling time
of 0.25 h. The half-life of chloral hydrate in the plasma was
approximately 3 min. The half-lives of trichloroethanol and
trichloroethanol glucuronide in the plasma were approximately 5 and
7 min, respectively. Trichloroacetic acid was the major metabolite
found in the plasma, with the maximum level being reached 1-6 h after
dosing. The half-life of trichloroacetic acid in the plasma was
approximately 8-11 h. Comparable values were obtained for rats.
Estimates of the concentrations of trichloroacetic acid and
trichloroethanol at steady state under various exposure conditions are
in Appendix 1.
Several studies have investigated the age dependence of the
metabolism of chloral hydrate (Reimche et al., 1989; Gorecki et al.,
1990; Hindmarsh et al., 1991; Mayers et al., 1991). These studies were
conducted in critically ill patients in neonatal and paediatric
intensive care units and may not be representative of a population of
healthy infants. The half-lives for trichloroethanol and its
glucuronide were increased several-fold in pre-term and full-term
infants compared with toddlers and adults. The half-lives for
trichloroethanol in toddlers and adults were similar. These
age-related differences likely are the result of the immaturity of
hepatic metabolism, particularly glucuronidation, and decreased
glomerular filtration.
Kaplan et al. (1967) investigated the effect of ethanol
consumption on the metabolism of chloral hydrate in adults. Subjects
ingested doses of ethanol (880 mg/kg body weight), chloral hydrate
(9-14 mg/kg body weight), or both. In subjects consuming both ethanol
and chloral hydrate, blood trichloroethanol levels rose more rapidly
and reached higher values than in subjects consuming chloral hydrate
only. Ethanol promotes the formation of trichloroethanol because the
oxidation of ethanol provides NADH used for the reduction of chloral
hydrate (Watanabe et al., 1998).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
Sanders et al. (1982) studied the acute toxicity of chloral
hydrate in CD-1 mice. Groups of eight male and eight female mice were
given chloral hydrate by gavage in distilled water at 300, 600, 900,
1200, 1500, or 1800 mg/kg body weight. No deaths occurred at 900 mg/kg
body weight or below in either sex. The calculated LD50 for females
was 1265 mg/kg body weight and for males was 1442 mg/kg body weight.
Effects were seen within 10 min of dosing. The mice became sedated at
300 mg/kg body weight. At 600 and 900 mg/kg body weight, the animals
became lethargic and exhibited loss of righting reflex. Respiration
was markedly inhibited at 1200, 1500, and 1800 mg/kg body weight.
Inhibition of respiration appeared to be the immediate cause of death.
Most deaths occurred within 4 h at 1800 mg/kg body weight. At 1200 and
1500 mg/kg body weight, some deaths occurred after 4 h, with all
deaths occurring within 24 h.
Goldenthal (1971) reported an oral LD50 in rats of 480 mg/kg
body weight.
8.1.2 Inhalation
Odum et al. (1992) exposed four female CD-1 mice to chloral for
6 h at a concentration of 100 ppm (603 mg/m3). This exposure induced
deep anaesthesia. The mice recovered normally after the exposure
stopped. The effects in the lung included vacuolization of clara
cells, alveolar necrosis, desquamination of the epithelium, and
alveolar oedema. The lung to body weight ratio increased 1.5-fold,
most likely due to the alveolar oedema.
8.2 Irritation and sensitization
There are no studies of irritation or sensitization in laboratory
animals.
8.3 Short-term exposure
Sanders et al. (1982) studied the short-term toxicity of chloral
hydrate in mice. Groups of male CD-1 mice were given chloral hydrate
by gavage in distilled water at 14.4 or 144 mg/kg body weight per day
for 14 days. No significant effect on body weight was observed. No
changes in internal organs were noted from a gross examination. Groups
of 11-12 mice were evaluated for several toxicological parameters. No
significant effects on haematological or serum biochemical parameters
were noted. There was a statistically significant (P < 0.05)
increase in liver weight (17%) and a decrease in spleen weight (27%)
at the high exposure. The no-observed-adverse-effect level (NOAEL) in
this study is 14.4 mg/kg body weight per day; the LOAEL is 144 mg/kg
body weight per day. The increase in liver weight, but not the
decrease in spleen weight, was confirmed in a subsequent 90-day study
by the same researchers.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Sanders et al. (1982) administered chloral hydrate in
drinking-water to CD-1 mice at 70 or 700 mg/litre (equivalent to 16 or
160 mg/kg body weight per day) for 90 days. In males, hepatomegaly (an
increase in weight of 20% and 34% at the low and high exposure,
respectively) and microsome proliferation (no increase in total
microsomal protein, increase in cytochrome b5 of 26% and 40%,
increase in aminopyrine N-demethylase of 28% and 20%, and increase
in aniline hydroxylase of 24% and 30% at the low and high exposures,
respectively, when reported as mg of protein per mg of total liver
protein) were observed. There were no biologically significant changes
in serum enzymes. Hepatomegaly was not seen in females, but there were
changes in hepatic microsomal parameters (increase in total microsomal
protein of 10%, increase in aniline hydroxylase of 23%, and decrease
in cytochrome b5 of 12% when reported as mg of protein per mg of
total liver protein), but only at the high exposure. No other
significant toxicological changes were observed. Based on hepatomegaly
and changes in microsomal parameters in males at the high exposure,
this study identifies a LOAEL of 160 mg/kg body weight per day and a
NOAEL of 16 mg/kg body weight per day.
Daniel et al. (1992b) exposed male and female Sprague-Dawley rats
(10 per sex per dose) for 90 days to chloral hydrate in drinking-water
at a concentration of 300, 600, 1200, or 2400 mg/litre (equivalent to
an exposure of 24, 48, 96, or 168 mg/kg body weight per day in males
and 33, 72, 132, or 288 mg/kg body weight per day in females). The
tissues of animals from the high-exposure group and liver sections
from all treated males were examined histopathologically. No mortality
occurred in any groups prior to sacrifice. Organ weights, including
liver weight, and clinical chemistry values in treated animals were
only sporadically or inconsistently different from control animal
values. Focal hepatocellular necrosis was observed in 2 of 10 males in
each of the groups exposed to 96 and 168 mg/kg body weight per day.
The necrotic lesion was minimal at 96 mg/kg body weight per day and
was significantly more severe at 168 mg/kg body weight per day.
Necrotic lesions were not reported in any treated females or in any
control animals. While serum enzymes were generally increased in
treated animals, dramatic increases were reported in males in the
168 mg/kg body weight per day group; mean aspartate aminotransferase,
alanine aminotransferase, and lactate dehydrogenase levels in this
group were elevated 89%, 54%, and 127% above the corresponding control
values, respectively.
8.4.2 Chronic exposure and carcinogenicity
Rijhsinghani et al. (1986) evaluated carcinogenic effects in male
mice (C57BL × C3HF1). Groups of 15-day-old mice received chloral
hydrate by gavage in distilled water at 0, 5, or 10 mg/kg body weight
(26, 15, and 14 mice per group, respectively). Animals were sacrificed
when moribund or at week 78, at week 88, or between weeks 89 and 92.
Livers were examined histopathologically using light and electron
microscopy. In mice sacrificed 48-92 weeks after treatment, the
incidence of hepatic nodules (adenomas or trabecular carcinomas) was
3/9 and 6/8 for animals from the 5 and 10 mg/kg body weight per day
dose groups, respectively, compared with 2/19 in controls. The
increase in tumours was statistically significant (P < 0.05) only
in the 10 mg/kg body weight group.1
Daniel et al. (1992a) exposed 40 male B6C3F1 mice for 104 weeks
to drinking-water containing chloral hydrate at 1 g/litre (equivalent
to 166 mg/kg body weight per day). Untreated control animals (23 in
one group and 10 in a second group) received distilled water. Interim
sacrifices were conducted at 30 and 60 weeks of exposure (five animals
per group at each sacrifice interval). Complete necropsy and
microscopic examination were performed. There were no significant
treatment-related effects on survival or body weight. There were no
changes in spleen, kidney, or testis weights or histopathological
changes in any tissue except the liver. The toxicity in the liver was
characterized by increased absolute liver weight and liver to body
weight ratio at all three sacrifice intervals. At week 104, liver
weight was 37% higher than in controls, and liver to body weight ratio
was 42% higher than in controls. Hepatocellular necrosis was noted in
10/24 (42%) treated animals; other pathological changes of mild
severity reported in the livers of treated animals included
cytoplasmic vacuolization, cytomegaly, and cytoplasmic alteration. The
prevalence of liver tumours at terminal sacrifice was statistically
significantly (P < 0.05) increased over controls, with
hepatocellular carcinomas in 11/24 and hepatocellular adenomas in
7/24 animals; for carcinomas and adenomas combined, the prevalence was
17/24. In control animals, carcinomas, adenomas, and carcinomas and
adenomas (combined) occurred in 2/20, 1/20, and 3/20, respectively.
At the 60-week sacrifice, there were 2/5 treated animals with
hepatocellular carcinomas, compared with 0/5 controls. No carcinomas,
adenomas, or hyperplastic nodules were reported in animals sacrificed
at week 30.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity bioassay became available. In this study, an
up to 5 times higher single dose of chloral hydrate than that used
in the Rijhsinghani et al. (1986) study administered to male or
female B6C3F1 mice failed to induce tumours in any organ
(NTP, 2000a).
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male B6C3F1 mice. Mice were administered chloral
hydrate in drinking-water for 104 weeks. Mice (72 in each group) had a
mean exposure of 0, 13.5, 65, or 146.6 mg/kg body weight per day.
There was no change in water consumption, survival, behaviour, body
weight, or organ weights at any exposure. There was no evidence of
hepatocellular necrosis at any exposure and only minimal changes in
the levels of serum enzymes. This study identifies a NOAEL for
non-cancer effects in mice of 146.6 mg/kg body weight per day (the
highest exposure tested). There was no increase in the prevalence of
neoplasia at sites other than the liver. Although the background
response in this study is higher than normal for this strain of mice,
the mice showed an increase in proliferative lesions in the liver
(hyperplasia, adenoma, carcinoma, and combined adenoma and carcinoma)
at all exposures. These data are summarized in Table 1. The calculated
effective dose for a 10% tumour incidence (ED10) is 1.98 mg/kg body
weight per day, and its 95% lower confidence limit (LED10) is
1.09 mg/kg body weight per day (see Appendix 2).
Leuschner & Beuscher (1998) conducted a chronic bioassay for
carcinogenicity in Sprague-Dawley rats. Chloral hydrate was
administered in drinking-water for 124 weeks (males) and 128 weeks
(females). The rats (50 males and 50 females in each group) had an
exposure of 15, 45, or 135 mg/kg body weight per day. There was no
effect on survival, appearance, behaviour, body weight, food and water
consumption, or organ weights. There was no evidence of an increased
incidence of tumours in any organ. Histopathological examination
revealed an increased incidence of hepatocellular hypertrophy at the
highest exposure in males only (11% in controls versus 28% at the
highest exposure; P < 0.01). This finding, graded as minimal to
slight in severity, was characterized by a diffuse liver cell
enlargement with slightly eosinophilic cytoplasm and was considered by
the authors as a first sign of toxicity. The type, incidence, and
severity of other non-neoplastic lesions were not increased in treated
animals compared with controls. Based on the evidence of minimal
toxicity in the liver, which is of doubtful biological significance,
this study establishes a NOAEL of 45 mg/kg body weight per day and a
LOAEL of 135 mg/kg body weight per day.
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male F344 rats. Rats were administered chloral
hydrate in drinking-water for 104 weeks. Rats (78 in each group) had a
mean daily exposure of 0, 7.4, 37.4, or 162.6 mg/kg body weight per
day. There was no change in water consumption, survival, behaviour,
body weight, or organ weights at any exposure. There was no indication
of liver toxicity at any exposure as shown by the lack of liver
necrosis, lack of hyperplasia, no increase in mitotic index, and only
minimal changes in the levels of serum enzymes. There was no increase
at any exposure in the prevalence or multiplicity of hepatocellular
neoplasia or neoplasia at any other site. This study identifies a
NOAEL of 162.6 mg/kg body weight per day (the highest exposure
tested).1
Two of the metabolites of chloral hydrate, trichloroacetic acid
and dichloroacetic acid, have been shown to cause liver tumours in
rodents. For example, trichloroacetic acid in drinking-water induced
liver tumours in male and female mice when the exposure exceeded 200
mg/kg body weight per day (Herren-Freund et al., 1987; Bull et al.,
1990; Pereira, 1996). There was no evidence of increased
carcinogenicity, however, when male rats were exposed to
trichloroacetic acid at 360 mg/kg body weight per day (DeAngelo et
al., 1997). Dichloroacetic acid in drinking-water induced liver
tumours in male and female mice when the exposure exceeded 160 mg/kg
body weight per day (Herren-Freund et al., 1987; Bull et al., 1990;
DeAngelo et al., 1991; Daniel et al., 1992a; Ferreira-Gonzalez et al.,
1995; Pereira, 1996). Dichloroacetic acid also induced liver tumours
in male rats when the exposure exceeded 40 mg/kg body weight per day
(Richmond et al., 1995; DeAngelo et al., 1996).
A number of studies have shown that trichloroethylene is toxic to
the mouse lung bronchiolar epithelium, causing a highly specific
lesion to the clara cells of mice. Short-term exposure causes
vacuolization of the clara cells; long-term exposure causes pulmonary
adenomas and adenocarcinomas (Odum et al., 1992; Green et al., 1997).
These effects are thought to be due to the accumulation of chloral
within the clara cells. Trichloroethylene is efficiently metabolized
to chloral, but the major pathway from chloral to trichloroethanol and
its glucuronide is blocked, leading to an accumulation of chloral and
the observed toxicity.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity assay became available. In this study,
lifetime gavage administration of chloral hydrate at similar dose
levels induced hepatocellular tumours in male B6C3F1 mice and a low
frequency of pituitary hyperplasia and adenomas in females that was
of borderline statistical significance (NTP, 2000b).
Table 1: Prevalence and multiplicity of hepatocellular proliferative lesions in mice at 104 weeks.a
Treatment group Number examinedc Hyperplasia Adenoma Carcinoma Adenoma
(mg/kg body + carcinoma
weight per day)b
0 42 7.1d 21.4d 54.8d 64.3d
0.07 ± 0.04e 0.21 ± 0.06e 0.74 ± 0.12e 0.95 ± 0.12e
13.5 46 32.6f 43.5f 54.3 78.6f
0.41 ± 0.10f 0.65 ± 0.12f 0.72 ± 0.11 1.37 ± 0.16f
65 39 33.3f 51.3f 59.0 79.5f
0.38 ± 0.09f 0.95 ± 0.18f 1.03 ± 0.19 1.97 ± 0.23f
146.6 32 37.5f 50.0f 84.4f 90.6f
0.41 ± 0.10f 0.72 ± 0.15f 1.31 ± 0.17f 2.03 ± 0.25f
a From George et al. (2000).
b Time-weighted mean daily dose.
c Animals surviving longer than 78 weeks.
d Prevalence (percentage of animals with at least one lesion).
e Multiplicity (number of lesions per animal ± SEM).
f Statistically different from the control value, P < 0.05.
8.5 Genotoxicity and related end-points
8.5.1 Genotoxicity
There is an extensive database on the genotoxicity of chloral
hydrate and its metabolites. A complete summary of these results is
provided in US EPA (2000).
Chloral hydrate did not induce mutation in most strains of
Salmonella typhimurium, but did in some studies with S. typhimurium
TA100 and in a single study with S. typhimurium TA104. The latter
response was inhibited by free-radical scavengers alpha-tocopherol and
menadione (Ni et al., 1994).
Chloral hydrate did not induce mitotic crossing-over in
Aspergillus nidulans in the absence of metabolic activation. Chloral
hydrate caused weak induction of meiotic recombination in the presence
of metabolic activation and gene conversion in the absence of
metabolic activation in Saccharomyces cerevisiae. It did not induce
reverse mutation in S. cerevisiae. Chloral hydrate clearly induced
aneuploidy in various fungi in the absence of metabolic activation.
Chloral hydrate induced somatic and germ cell mutations in
Drosophila melanogaster.
Chloral hydrate did not produce DNA-protein cross-links in rat
liver nuclei, DNA single-strand breaks/alkaline-labile sites in
primary hepatocytes in vitro, or DNA repair in Escherichia coli.
One study showed induction of single-strand breaks in liver DNA of
both rats and mice treated in vivo; another study in both species
using higher concentrations of chloral hydrate found no such effect.
Chloral hydrate was weakly mutagenic, but did not induce
micronuclei in mouse lymphoma cells in vitro. Chloral hydrate
increased the frequency of micronuclei in Chinese hamster cell lines.
Although a single study suggested that chloral hydrate induces
chromosomal aberrations in Chinese hamster CHED cells in vitro, the
micronuclei produced probably contained whole chromosomes and not
chromosome fragments, as the micronuclei could all be labelled with
antikinetochore antibodies.
In kangaroo rat kidney epithelial cells, chloral hydrate
inhibited spindle elongation and broke down mitotic microtubuli,
although it did not inhibit pole-to-pole movement of chromosomes. It
produced multipolar spindles, chromosomal dislocation from the mitotic
spindle, and a total lack of mitotic spindles in Chinese hamster
DON:Wg.3h cells.
Chloral hydrate weakly induced sister chromatid exchange in
cultures of human lymphocytes. It induced micronuclei, aneuploidy,
C-mitosis, and polyploidy in human lymphocytes in vitro. Micronuclei
were induced in studies with human whole blood cultures but not in one
study with isolated lymphocytes. The differences seen in the
micronucleus test have been attributed to differences between whole
blood and purified lymphocyte cultures (Vian et al., 1995), but this
hypothesis has not been tested.
Chloral hydrate increased the frequency of chromosomal
aberrations in mouse bone marrow, spermatogonia, and primary and
secondary spermatocytes, but not in oocytes, after in vivo
treatment. Chloral hydrate induced chromosomal aberrations in mouse
bone marrow erythrocytes after treatment in vivo. In one of these
studies, the use of antikinetochore antibodies suggested induction of
micronuclei containing both whole chromosomes and fragments. Chloral
hydrate induced micronuclei in the spermatids of mice treated in vivo
in some studies. Chloral hydrate induced aneuploidy in the bone
marrow of mice treated in vivo. It increased the rate of aneuploidy
in mouse secondary spermatocytes. It did not produce polyploidy in
bone marrow, oocytes, or gonosomal or autosomal univalents in primary
spermatocytes of mice treated in vivo. Chloral hydrate, however,
induced polyploidy and meiotic delay when a synchronized population of
mouse oocytes was exposed in vitro prior to the resumption of
maturation.
Trichloroethanol, a reduction product of chloral hydrate, did not
induce lambda prophage in E. coli or mutation in S. typhimurium
TA100. Trichloroethanol caused spindle aberrations when mouse oocytes
were treated in vitro.
Trichloroacetic acid did not induce lambda prophage in E. coli
and was not mutagenic to S. typhimurium in the presence or absence
of metabolic activation. Trichloroacetic acid was weakly positive in
the mouse lymphoma assay with metabolic activation. Trichloroacetic
acid also did not induce chromosomal damage in human lymphocytes or
micronuclei in bone marrow in vitro. It is unclear whether
trichloroacetic acid can induce chromosomal damage in vivo, because
some studies have been positive and others negative.
Dichloroacetic acid did not induce differential toxicity in DNA
repair-deficient strains of S. typhimurium but did induce lambda
prophage in E. coli. Dichloroacetic acid gave equivocal results for
gene mutation in S. typhimurium TA100 and TA98. Dichloroacetic acid
was weakly mutagenic in the in vitro mouse lymphoma assay and
induced chromosomal aberrations but not micronuclei or aneuploidy in
that test system. Dichloroacetic acid induced micronuclei in mouse
polychromatic erythrocytes in vivo and mutations at the lacI locus
in the transgenic B6C3F1 mouse (the Big Blue Mouse) in vivo at an
exposure that induces liver tumours in male mice. It is unclear
whether dichloroacetic acid can induce primary DNA damage, as some
assays are positive and others negative.
8.5.2 Cell proliferation
Rijhsinghani et al. (1986) evaluated the acute effects of chloral
hydrate on liver cell proliferation in 15-day-old male mice (C57BL ×
C3HF1). Mice were given 0, 5, or 10 mg chloral hydrate/kg body
weight by gavage in distilled water (9, 10, and 6 mice per group,
respectively) and sacrificed after 24 h. Cell proliferation was
evaluated by calculating the mitotic index (number of mitoses per 100
nuclei) from liver sections. The mitotic index in liver cells was
significantly increased (0.9235) in mice receiving 5 mg/kg body weight
when compared with the control value (0.3382), and elevated (0.7433)
(although not statistically significantly) in mice receiving 10 mg/kg
body weight. Hepatic necrosis was not observed in mice from either
treatment group at autopsy.
As part of the chronic bioassay for carcinogenicity, George et
al. (2000) evaluated hepatocyte proliferation in male F344 rats and
male B6C3F1 mice. Exposures are given in section 8.4.2. Five days
prior to sacrifice at 13, 26, 52, or 72 weeks in rats and 26, 52, or
78 weeks in mice, animals were given bromodeoxyuridine. Labelled
nuclei were identified by chromogen pigment over the nuclei, and the
labelling index was calculated. Outside of the areas with tumours in
the liver of mice, there was no significant evidence of increased
hepatocyte proliferation in rats or mice.
8.5.3 Oncogene activation
Velazquez (1994) investigated the induction of H- ras
proto-oncogene mutations in mice. DNA from normal liver and tumour
tissue was obtained from male B6C3F1 mice administered 1 g chloral
hydrate/litre (166 mg/kg body weight per day) in drinking-water for
2 years. H- ras mutations were present in one out of seven (14%)
tumours. The spectrum of mutations was the same as that of spontaneous
liver tumours. Based on these data, it is unlikely that H- ras
activation is a mechanism of carcinogenicity relevant to chloral
hydrate.
8.5.4 Free radicals and DNA adduct formation
Ni et al. (1994, 1995, 1996) studied the metabolism of chloral
hydrate in an in vitro system using microsomes from male B6C3F1
mice. The metabolism of chloral hydrate generated free radicals as
detected by electron spin resonance spectroscopy and caused endogenous
lipid peroxidation, resulting in the production of malondialdehyde,
formaldehyde, and acetaldehyde, all of which are known to produce
liver tumours in rodents. Trichloroacetic acid and trichloroethanol
also produced free radicals and induced lipid peroxidation when tested
in this system. The authors speculated that the free radicals were
Cl3CCO2Ê. and/or Cl3CÊ. Incubation of chloral hydrate,
trichloroethanol, or trichloroacetic acid in the presence of
microsomes and calf thymus DNA resulted in the formation of a
malondialdehyde-modified DNA adduct. This research group further
showed that chloral hydrate induced an increase in mutations at the
hprt and tk loci in transgenic human lymphoblastoid cells
containing CYP2E1. In contrast, when the parental cell line lacking
CYP2E1 was treated with the same concentration of chloral hydrate, no
mutations were found at either locus. These data implicate CYP2E1 as
the primary cytochrome subfamily involved in the metabolism of chloral
hydrate to reactive intermediates.
8.5.5 Cell communication
The effects of 1-, 4-, 6-, 24-, 48-, and 168-h exposures to
chloral hydrate (0, 1, 5, or 10 mmol/litre) on gap junction
intercellular communication in Clone 9 cell cultures (normal rat
hepatocytes) were reported by Benane et al. (1996). No differences in
intercellular communication were seen between the groups treated with
1 mmol/litre at 1, 4, and 6 h of exposure and controls, as measured by
a dye transfer protocol. There were significant differences between
all other groups and the controls. The shortest exposure time and
lowest exposure concentration that reduced dye transfer significantly
were in the group treated with 1 mmol/litre for 24 h.
8.5.6 Peroxisome proliferation
As part of the chronic bioassay for carcinogenicity in mice,
George et al. (2000) found no evidence of peroxisome proliferation
using cyanide-insensitive palmitoyl CoA oxidase in the livers of male
mice treated with chloral hydrate for 26 weeks.
8.6 Reproductive and developmental toxicity
Klinefelter et al. (1995) evaluated effects on sperm morphology
and motility in F344 rats administered chloral hydrate in
drinking-water for 52 weeks at levels of 0, 55, or 188 mg/kg body
weight per day. The researchers examined cauda epididymal sperm motion
parameters and testicular and epididymal histopathology. Chloral
hydrate did not cause any visible systemic toxicity and had no effects
on epididymal or testicular histopathology. However, the percentage of
motile sperm was significantly decreased (P < 0.01) from 68% in
controls to 58% in rats exposed to 188 mg/kg body weight per day. The
percentage of progressively motile sperm was also significantly
decreased (P < 0.01) from 63% in controls to 53% in this group. In
addition, the frequency distribution of the average straight-line
velocities of sperm at this exposure was significantly shifted
(P < 0.01) to the lower ranges when compared with controls. In this
study, the NOAEL is 55 mg/kg body weight per day; the LOAEL is 188
mg/kg body weight per day.
Kallman et al. (1984) exposed male and female CD-1 mice to
chloral hydrate in drinking-water at 21.3 or 204.8 mg/kg body weight
per day. Animals were exposed for 3 weeks prior to breeding. Exposure
of females (5 per group) continued during gestation and until pups
were weaned at 21 days of age. No gross malformations were noted, and
no significant effects were observed in duration of gestation, number
of pups delivered, pup weight, or number of stillborn pups. All pups
(15 per group) showed the same rate of development and level of
performance on several neurobehavioural tests, except that pups
exposed to 204.8 mg/kg body weight per day when tested at 23 days of
age showed impaired retention of passive avoidance learning on both
the 1-h and 24-h retention tests (P < 0.05). This study identified
a NOAEL for neurodevelopmental toxicity of 21.3 mg/kg body weight per
day and a LOAEL of 204.8 mg/kg body weight per day based on the
impairment in passive avoidance learning. This study also identifies a
NOAEL for reproductive and other developmental effects of 204.8 mg/kg
body weight per day (the highest exposure tested).
Johnson et al. (1998) tested the potential for chloral hydrate to
cause developmental toxicity in Sprague-Dawley rats. Chloral hydrate
was administered in drinking-water to 20 rats from gestational day 1
to gestational day 22 at an average exposure of 151 mg/kg body weight
per day. Control animals were given distilled water. There was no
evidence of maternal toxicity, no change in the number of implantation
or resorption sites, no change in the number of live or dead fetuses,
no change in placental or fetal weight, no change in crown-rump
length, and no increase in the incidence of morphological changes. A
detailed examination found no evidence of cardiac anomalies. Based on
this study, the NOAEL for developmental toxicity is 151 mg/kg body
weight per day (the highest exposure tested).
Johnson et al. (1998) also tested the potential for
trichloroethanol and trichloroacetic acid to cause developmental
toxicity in Sprague-Dawley rats. The protocol was identical to the
study with chloral hydrate. Trichloroethanol was administered to 10
rats at an average exposure of 153 mg/kg body weight per day. No
evidence of developmental toxicity was found. In contrast, when
trichloroacetic acid was administered to 11 rats at an average
exposure of 291 mg/kg body weight per day, developmental toxicity was
observed. The effects included statistically significant (P < 0.05)
increases in average resorptions, in average implantations, and in
cardiac anomalies. Although the specific cardiac anomalies found were
different, the results with trichloroacetic acid are generally
consistent with those reported by Smith et al. (1989), who observed
adverse developmental effects from trichloroacetic acid at an exposure
of 330 mg/kg body weight per day and above.
Saillenfait et al. (1995) tested the potential of chloral hydrate
to cause developmental toxicity using a rat whole-embryo culture
system. Embryos (20 per dose) from Sprague-Dawley rats were explanted
on gestational day 10 and exposed to chloral hydrate at a
concentration of 0, 0.5, 1, 1.5, 2, or 2.5 mmol/litre (equivalent to
0, 83, 165, 248, 331, or 414 mg/litre) for 46 h. At 2.5 mmol/litre,
all embryos died. No lethality was seen at lower exposures. Chloral
hydrate caused concentration-dependent decreases in growth and
differentiation and increases in the incidence of morphologically
abnormal embryos. No effects were observed in any parameter at
0.5 mmol/litre. Decreases in crown-rump length, somite
(embryonic segment) number, and the protein or DNA content of embryos
were seen at 1 mmol/litre and above. At 1, 1.5, and 2 mmol chloral
hydrate/litre, respectively, 18%, 68%, and 100% of embryos were
malformed. Brain, eye, and ear malformations were the most prominent
effects at these concentrations. Abnormalities in the trunk and
pericardial dilation also occurred at 2 mmol/litre. In this in vitro
test system, chloral hydrate was a slightly more potent teratogen than
trichloroacetic acid or dichloroacetic acid.
Although chloral hydrate did not cause meiotic delay in the
oocytes of adult mice when administered at the time of resumption of
maturation induced by hormones (Mailhes & Marchetti, 1994), it did
cause adverse effects in vitro when a synchronized population of
oocytes was exposed prior to resumption of maturation
(Eichenlaub-Ritter & Betzendahl, 1995; Eichenlaub-Ritter et al.,
1996). In this test system, chloral hydrate induced lagging of
chromosomes during telophase I, inhibited spindle elongation in
anaphase B, and caused chromosome displacement from the spindle
equator in metaphase I and II. Oocytes became irreversibly arrested in
maturation when exposed to chloral hydrate prior to resumption of
maturation or when chloral hydrate was present during the first or
second 8 h of maturation. Spindle aberrations were observed when
oocytes were treated with trichloroethanol (Eichenlaub-Ritter et al.,
1996).
8.7 Immunological and neurological effects
Kauffmann et al. (1982) administered chloral hydrate by gavage in
distilled water at 14.4 or 144 mg/kg body weight per day to groups of
11-12 male CD-1 mice for 14 days. No effects on humoral or
cell-mediated immunity were detected at either exposure.
Kauffmann et al. (1982) administered chloral hydrate to male and
female CD-1 mice in drinking-water at 70 or 700 mg/litre (equivalent
to 16 or 160 mg/kg body weight per day) for 90 days. Humoral immunity
was assessed by the number of splenic antibody-forming cells produced
against sheep red blood cells (12 mice in the control group and 8 mice
in the exposed groups) and haemagglutination titres (20-21 mice in the
control group and 13-16 mice in the exposed groups).
Cell-mediated immunity was assessed by delayed-type hypersensitivity
to sheep red blood cells (17-20 mice in the control group and
15-16 mice in the exposed groups). Lymphocyte response was assessed
using a T-cell mitogen (Con A) and a B-cell mitogen (LPS)
(17-22 animals in the control group and 13-16 mice in the exposed
groups). In males, no effects were detected in either humoral or
cell-mediated immunity at either exposure. No effects on cell-mediated
immunity were noted in females at either exposure. In females, both
exposures resulted in a statistically significant decrease
(P < 0.05) in humoral immune function (36% and 40% at the
low and high exposures, respectively) when expressed as
antibody-forming cells per spleen. The decrease, however, was
statistically significant only at the higher exposure when
expressed as antibody-forming cells per million spleen cells
(a 32% decrease). There was no effect on haemagglutination titres
or on spleen cell response to the B-cell mitogen at either exposure.
The decrease in antibody-forming cells per million spleen cells at
the higher exposure in female mice is regarded as an adverse
response in this study. Accordingly, the NOAEL for immunotoxicity is
16 mg/kg body weight per day; the LOAEL is 160 mg/kg body weight
per day.
Kallman et al. (1984) administered chloral hydrate by gavage in
distilled water at 50, 100, 200, 300, or 400 mg/kg body weight to
groups of 12 male CD-1 mice. All doses resulted in the rapid onset of
ataxia, with an ED50 (maximal effect seen in 50% of animals) of
84.2 mg/kg body weight at 5 min (the time of maximal effect). Animals
recovered within 2-3 h. No delayed changes in muscular coordination
were detectable when the mice were tested 24 h after treatment.
Kallman et al. (1984) evaluated behavioural toxicity in groups of
12 male CD-1 mice administered chloral hydrate by gavage in distilled
water at 14.4 or 144 mg/kg body weight per day for 14 days. When
measured 24-48 h after exposure was terminated, no significant effects
on body weight, motor activity, physical appearance, behaviour,
muscular coordination, or endurance were observed.
Kallman et al. (1984) exposed groups of 12 male CD-1 mice to
drinking-water containing chloral hydrate at a concentration of 70 or
700 mg/litre (equivalent to 16 or 160 mg/kg body weight per day) for
90 days. When measured 24 h after exposure was terminated, no
treatment-related effects on mortality, body weight, physical
appearance, behaviour, locomotor activity, learning in repetitive
tests of coordination, response to painful stimuli, strength,
endurance, or passive avoidance learning were observed. Both exposures
resulted in a decrease of about 1°C in mean body temperature
(P < 0.05). Because of the lack of increased effect with a 10-fold
increase in exposure and because hypothermia as a side-effect of
chloral hydrate or from an overdose of chloral hydrate has not been
reported in humans, the decrease in body temperature is not considered
an adverse effect. This study identifies a NOAEL for neurobehavioural
toxicity of 160 mg/kg body weight per day (the highest exposure
tested).
A condensation product of tryptamine and chloral hydrate,
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline (TaClo), has been
found in the blood of elderly patients administered chloral hydrate
for 2-7 days (Bringmann et al., 1999). This metabolite initiated a
slowly progressive neurodegeneration when administered to rats in a
subchronic study (Gerlach et al., 1998). There is, however, no
evidence of neurodegeneration in the chronic studies with chloral
hydrate in rats and mice.
9. EFFECTS ON HUMANS
Chloral hydrate has been widely used as a sedative and hypnotic
drug in humans. Trichloroethanol is responsible for the
pharmacological activity (Marshall & Owens, 1954; Breimer, 1977;
Goodman & Gilman, 1985). Exposure information is discussed in section
6.2.
Chloral hydrate is irritating to the skin and mucous membranes
and often causes gastric distress, nausea, and vomiting at recommended
doses. There are no reports of sensitization in humans. Overdoses
produce (in order of progression) ataxia, lethargy, deep coma,
respiratory depression, hypotension, and cardiac arrhythmias. The
life-threatening effects are from severe respiratory depression,
hypotension, and cardiac arrhythmias. For some representative case
reports, see Marshall (1977), Anyebuno & Rosenfeld (1991), Ludwigs et
al. (1996), and Sing et al. (1996). A potentially life-threatening
oral dose for humans is approximately 10 g (143 mg/kg body weight),
although death has been reported from as little as 4 g, and some
individuals have survived ingesting 30 g or more. Extended use of
chloral hydrate may result in development of tolerance to the
pharmacological effect and physical dependence on or addiction to
chloral hydrate.
Shapiro et al. (1969) reviewed the medical records of 1618
patients who had received chloral hydrate at 1 g (213 patients, 13%),
0.5 g (1345 patients, 83%), or various other doses (60 patients, 4%).
Adverse reactions were reported in 38 patients (2.3%). Of these
patients, 4 received 1 g, 1 received 0.75 g, and 33 received 0.5 g.
Reported adverse reactions included gastrointestinal symptoms in 10
patients, central nervous system (CNS) depression in 20 patients, skin
rash in 5 patients, prolonged prothrombin time in 1 patient, and
bradycardia in 1 patient. In all patients, the side-effects
disappeared when chloral hydrate therapy was stopped. There was no
evidence of association between adverse side-effects and age, weight,
or sex.
Miller & Greenblatt (1979) reviewed the medical records of 5435
hospital patients who received chloral hydrate at a dose of either
0.5 g (about 7-8 mg/kg body weight) or 1 g (about 14-16 mg/kg body
weight). Adverse reactions were noted in 119 cases (2.2%).
CNS depression was most common (58 patients, or 1.1%), with minor
sensitivity reactions, including rash, pruritus, fever, and
eosinophilia, second most common (19 patients, or 0.35%). Other
adverse reactions included gastrointestinal disturbances (0.28%) and
CNS excitement (0.22%). Three individuals (0.05%) were judged to have
life-threatening reactions involving CNS depression, asterixis
(involuntary jerking movements), or hypotension. The data show that
adverse reactions involving the CNS become more frequent with
increasing dosage in patients older than 50 years, in patients who
died during hospitalization, in patients who received concurrently
benzodiazepine anti-anxiety drugs, and in patients with elevated
levels of blood urea nitrogen.
Greenberg et al. (1991) reported various side-effects experienced
by children receiving chloral hydrate sedation in preparation for
computer tomography (CT) procedures. In a "high-dose" group, composed
of 295 children (average age 2.18 years) who received a single dose of
80-100 mg/kg body weight and a maximum total dose of 2 g, adverse
reactions occurred in 23 of the patients (7%) and included vomiting
(14 patients), hyperactivity (5 patients), and respiratory symptoms,
such as wheezing and secretion aspiration (4 patients). Cardiac
monitoring did not reveal any abnormalities or arrhythmias in any of
the children. A second "lower-dose" cohort of 111 children (average
age 1.9 years) received 40-75 mg chloral hydrate/kg body weight. These
patients received the lower dose because of existing liver or renal
impairment, respiratory insufficiency, or CNS depression. There were
no adverse side-effects or complications reported in this group.
Children with severe liver or renal disease or affected by severe
CNS depression were not treated with chloral hydrate.
Lambert et al. (1990) conducted a retrospective analysis of
hospital medical records to investigate a possible link between
chloral hydrate administration and direct hyperbilirubinaemia (DHB) in
neonates following prolonged administration of chloral hydrate
(25-50 mg/kg body weight for up to 20 days). Direct bilirubin is a
measure of the free, unconjugated bilirubin in the serum. In the first
study, the DHB was of unknown etiology in 10 of the 14 newborns with
DHB; all 10 of these DHB patients had received chloral hydrate. In the
second study, among 44 newborns who had received chloral hydrate,
10 patients who developed DHB had received a mean cumulative dose of
1035 mg/kg body weight. In contrast, 34 patients whose direct
bilirubin levels were within normal ranges received a mean cumulative
dose of 183 mg/kg body weight. The total bilirubin levels (free plus
conjugated) were the same in both groups and within the normal range.
Kaplan et al. (1967) investigated whether ethanol ingestion
increased the effects of chloral hydrate. Five male volunteers
weighing 70-107 kg consumed ethanol (880 mg/kg body weight), chloral
hydrate (1 g, 9-14 mg/kg body weight), or both. Blood pressure and
cardiac rate did not vary significantly among treatments. In the
presence of ethanol, the concentration of trichloroethanol in the
blood rose more rapidly and reached a higher value, but the rate of
depletion was not significantly changed. The increase in the
concentration of trichloroethanol was not sufficient to produce a
marked enhancement of the hypnotic effect. The volunteers reported
symptoms (drowsiness, dizziness, blurred vision) and their severity
during the 6-h observation period. At all time points, the rank order
of effects was ethanol plus chloral hydrate > ethanol > chloral
hydrate.
No long-term studies of chloral hydrate in humans were located.
Chloral hydrate is addictive and is a controlled substance (Schedule
IV) in the USA.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Some data are available from cell multiplication inhibition tests
(toxic thresholds) in bacteria, algae, and protozoa. These data are
summarized in Table 2.
Schatten & Chakrabarti (1998) showed that chloral hydrate at 0.1%
(only concentration tested) causes alteration of centrosomal material
and abnormal microtubule configurations in California sea urchins
(Strongylocentrotus purpuratus and Lytechinus pictus). Chakrabarti
et al. (1998) also showed that chloral hydrate at 4 mmol/litre (660
mg/litre, only concentration tested) induced ciliary loss in the early
embryo phase of Lytechinus pictus. Exposure in this study was for 30
h at the blastula stage (14 h after fertilization).
Table 2: Effects of chloral hydrate on bacteria, algae, and protozoa.
Test system Effect Reference
Bacteria 16-h EC3 at 1.6 mg/litre Bringmann & Kuehn,
(Pseudomonas putida) 1980a
Green alga 7-day EC3 at 2.8 mg/litre Bringmann & Kuehn,
(Scenedesmus 1980a
quadricaudata)
Blue-green alga 8-day EC3 at 78 mg/litre Bringmann & Kuehn,
(cyanobacterium) 1976
(Microcystis
aeruginosa)
Protozoan 72-h EC5 at 79 mg/litre Bringmann & Kuehn,
(Enterosiphon sulcatum) 1980a
Protozoan EC5 at 86 mg/litre Bringmann & Kuehn,
(Uronema parduczi) 1980b
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Chloral hydrate has been extensively used as a sedative and
hypnotic drug in human and veterinary medicine. The metabolite
trichloroethanol is responsible for the pharmacological effect.
Chloral hydrate is irritating to the skin and mucous membranes and
often causes gastric distress, nausea, and vomiting at recommended
doses. Acute overdoses produce (in order of progression) ataxia,
lethargy, deep coma, respiratory depression, hypotension, and cardiac
arrhythmias. There is some evidence of hepatic injury in people
surviving near-lethal, acute overdoses, but no convincing evidence
that hepatic injury results from the recommended clinical dose.
Despite its long use in human medicine, there is no published
information on toxicity in controlled studies in humans following
extended exposure.
Chloral hydrate is completely absorbed and rapidly metabolized
following oral administration. The major metabolites are
trichloroethanol and its glucuronide and trichloroacetic acid. Some
data suggest that a small amount of dichloroacetic acid may be formed.
In humans, the half-life of trichloroethanol and its glucuronide is
about 8 h; the half-life of trichloroacetic acid is about 4 days. Some
data suggest that the half-life of trichloroethanol is increased
several-fold in pre-term and full-term infants compared with toddlers
and adults. The major route of excretion of the metabolites of chloral
hydrate is elimination in the urine. Chloral hydrate and its
metabolites have been found in milk from women treated with chloral
hydrate. The concentration of these chemicals, however, is too low to
cause a pharmacological effect in the nursing infant.
Acute administration of chloral hydrate to mice causes loss of
coordination (ataxia) at about the same exposure as in humans for the
same effect. A 90-day study in mice shows no evidence of behavioural
changes or other neurotoxicity. Chronic studies in rats and mice show
no evidence of behavioural changes and no evidence of
histopathological changes in nervous tissue. These studies used an
exposure approximately 15 times the recommended clinical dose in
humans. There is some evidence of mild liver toxicity following
chronic exposure in rats and mice. A slight decrement in humoral
immunity was observed in female mice following exposure for 90 days.
The antibody-forming cell response is considered an excellent
indicator of the status of humoral immunity because of the complex
cellular cooperation required to produce antibody and because the
number of cells that produce antibody can be quantified. A depression
in the number of these cells is considered an adverse response because
the production of antibodies is important to the defence strategy of
the organism. However, the quantitative relationship between the
depression in antibody-forming cells in the spleen and the
concentration of circulating antibody is unknown. In this study,
because there was no depression in circulating antibodies measured by
the haemagglutination titre, there might be no significant depression
in the ability of the host to mount a protective antibody response.
Chloral hydrate has been tested for developmental effects in rats and
mice. No structural abnormalities were observed. A slight effect was
observed in mice in passive avoidance learning when dams were exposed
prior to breeding, during gestation, and during nursing and pups were
tested at 23 days of age. Although chloral hydrate has not been tested
in a two-generation reproduction study, the data on reproductive
performance and on effects on sperm and oocytes do not suggest that
reproductive toxicity is likely to be a critical effect. In addition,
no histopathological effects are observed in reproductive organs of
rodents in subchronic or chronic studies. Some in vitro data,
however, suggest that chloral hydrate administered to young female
children might have a latent effect on fertility. All of the studies
in laboratory animals show non-cancer health effects at an exposure
far in excess of the exposure that is effective for sedation in
humans. A complete summary of the exposure-response data is presented
in Table 3.
Simultaneous ingestion of ethanol and chloral hydrate increases
the sedative effects and side-effects of chloral hydrate. The
mechanism is the increase in the concentration of the
pharmacologically active metabolite, trichloroethanol, in the presence
of ethanol. Chronic users of ethanol are, therefore, somewhat more
sensitive to the adverse effects of chloral hydrate.
Because of the immaturity of hepatic metabolism, particularly the
glucuronidation pathway, and decreased glomerular filtration in
infants, the half-life of trichloroethanol is longer in pre-term and
full-term infants. This group is therefore somewhat more sensitive to
the adverse effects of chloral hydrate. Toddlers and adults are likely
to show similar sensitivity to chloral hydrate.
Although male laboratory rodents seem to be more sensitive than
female laboratory rodents to hepatic effects, there is no evidence of
a gender effect in humans with respect to the sedative effects or
side-effects of chloral hydrate at the recommended clinical dose.
There are no carcinogenicity data from humans. Two bioassays in
rats show no increase in tumours at any site. These studies were
limited, because only minimal toxicity was observed in the livers of
the rats in these bioassays. In one study, only slight hypertrophy was
observed at the highest exposure; in the other study, no effects were
observed at the highest exposure. No data are available in female
mice. There are three separate bioassays showing an increased
incidence of liver tumours in male mice. One study, conducted in a
very limited number of animals, showed an increase in tumours
Table 3: Summary of non-neoplastic effects.
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Human 1 day, 3 doses Sedation - 10.7 Goodman & Gilman, 1985
Rat 90 days Mild liver necrosis 96 168 Daniel et al., 1992b
and increase in
serum enzymes
Rat 104 weeks - 162.6 - George et al., 2000
Rat 124 weeks Liver hypertrophy 45 135 Leuschner & Beuscher, 1998
Rat 52 weeks Sperm motility 55 188 Klinefelter et al., 1995
Rat gestation Development 151 - Johnson et al., 1998
days 1-22
Mouse 14 days Increased liver weight 14.4 144 Sanders et al., 1982
Mouse 90 days Increased liver weight 16 160 Sanders et al., 1982
Mouse 104 weeks Increased liver weight - 166a Daniel et al., 1992a
and necrosis
Mouse 104 weeks - 146.6b - George et al., 2000
Mouse 3 weeks Reproduction and 204.8 - Kallman et al., 1984
pre-breeding development
and during
gestation
Mouse Pre-breeding, Passive avoidance 21.3 204.8 Kallman et al., 1984
gestation, and learning in pups
nursing
Table 3 (cont'd)
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Mouse 1 day Ataxia - 50 Kallman et al., 1984
Mouse 14 days Neurobehaviour 144 - Kallman et al., 1984
Mouse 90 days Neurobehaviour 160 - Kallman et al., 1984
Mouse 14 days Immunotoxicity 144 - Kauffmann et al., 1982
Mouse 90 days Humoral immunity 16 160 Kauffmann et al., 1982
a Tumours at 166 mg/kg body weight per day.
b Hyperplasia and tumours at 13.5, 65, and 146.6 mg/kg body weight per day.
following a single exposure. The second study tested only one exposure
level but used an adequate number of animals. The third study shows an
increase in incidence and multiplicity of liver tumours at each of
three exposures. There are no data identifying a lesion that is a
precursor to the tumours. The strain of mice used has a very high
spontaneous incidence of liver tumours. Two of the metabolites of
chloral hydrate, trichloroacetic acid and dichloroacetic acid, have
been shown to cause liver tumours in rodents. Trichloroacetic acid
causes liver tumours only in mice. Dichloroacetic acid causes tumours
in both rats and mice.1
Chloral hydrate has been extensively studied as a genotoxic
agent. Chloral hydrate was positive in some bacterial mutation tests,
indicating that it may be capable of inducing point mutations. It was
also positive in the mouse lymphoma assay for mutations at the tk
locus. Chloral hydrate also induced somatic and germ cell mutations
in D. melanogaster. Some data also show chloral hydrate to be a very
weak clastogen in mammalian cells.
Chloral hydrate has been shown to induce aneuploidy in a variety
of cells, including S. cerevisiae, A. nidulans, Chinese hamster
embryonic fibroblasts, Chinese hamster primary cell lines LUC2 and
DON:Wg3h, human peripheral blood lymphocytes, mouse spermatocytes, and
mouse spermatids. Because there is a mixture of positive and negative
in vivo data, with no reason to weigh some studies more than others,
it is not clear whether chloral hydrate is capable of inducing genetic
damage in vivo. Additional in vivo studies using standard
protocols would help clarify the relevance of genetic damage to a
human health risk assessment.
The effects on aneuploidy are thought to arise via disruption of
the mitotic spindle structure or function by inhibition of tubulin
and/or microtubule-associated proteins; both substances are components
of the spindle apparatus. Some data also suggest that chloral hydrate
may act on the spindle apparatus through an increase in the
concentration of intracellular free calcium.
1 In a National Toxicology Program carcinogenicity bioassay that
became available after the Final Review Board meeting, a
carcinogenic effect was not observed after a single dose of chloral
hydrate; after lifetime exposure, males had an increased incidence
of hepatic tumours, and females had a low increased incidence of
pituitary adenomas that was of borderline statistical significance.
Several other mechanisms may play a role in the induction of
tumours in the liver of male mice. There is no convincing evidence
that chloral hydrate causes direct damage to DNA. In vitro studies
with chloral hydrate, trichloroethanol, and trichloroacetic acid and
mouse microsomes, however, show lipid peroxidation and the formation
of covalently bound DNA adducts. These effects appear to be mediated
by the formation of free radicals by CYP2E1. Another possibility is
cytotoxicity leading to compensatory hyperplasia. A single treatment
of mice with chloral hydrate caused an increase in the mitotic index
in liver cells. The increased cell division is hypothesized to either
provide additional opportunities for errors in DNA replication or
allow initiated cells to progress to a tumour. Another potentially
contributing mechanism of carcinogenesis is disruption of
intercellular communication, which has been shown in one experiment to
be influenced by chloral hydrate.
The mechanism of chloral hydrate-induced carcinogenicity in male
mice is unclear. Two mechanisms that appear ruled out are H- ras
proto-oncogene activation and peroxisome proliferation.
Although there is suggestive evidence of carcinogenicity in male
mice, the weight of evidence is not sufficient to consider tumour
induction as the critical effect.
11.1.2 Criteria for setting tolerable intakes or guidance values for
chloral hydrate
The effect that occurs at the lowest exposure is mild sedation in
humans. As this effect would not be intended or desirable in the
general population outside of the clinical setting, this response is
considered an adverse effect and is used to derive the tolerable
intake.
Acute gavage exposure in mice shows neurological effects (ataxia)
at about the same exposure for the comparable effect in humans. A
subchronic study in mice using sensitive tests for neurobehavioural
changes found none. Chronic studies in rats and mice show no evidence
of neurobehavioural changes and no evidence of histopathological
changes in nervous tissue. As with other chlorinated chemicals, there
is some evidence of carcinogenic effects in the liver of male mice
following chronic exposure.
Although the tolerable intake derived from the pharmacologically
active dose in humans is an acute tolerable intake, keeping the
exposure below this level will also be protective for any non-cancer
health effect from chronic exposure. Therefore, it is appropriate to
use the acute tolerable intake as the chronic tolerable intake as
well.
No data are available to determine a NOAEL in humans. The
recommended clinical dose for sedation in adults is 250 mg, taken 3
times a day (Goodman & Gilman, 1985). A low incidence of side-effects
also occurs at this exposure. The LOAEL is 10.7 mg/kg body weight per
day (assuming a 70-kg body weight). The pharmacokinetic information
shows that chloral hydrate and the pharmacologically active
metabolite, trichloroethanol, will not bioaccumulate.
The tolerable intake (IPCS, 1994) of 0.1 mg/kg body weight per
day was derived from the LOAEL of 10.7 mg/kg body weight per day using
a total uncertainty factor of 100. An uncertainty factor of 10 was
used to extrapolate from a LOAEL to a NOAEL, and an uncertainty factor
of 10 was used for intraspecies variability. An uncertainty factor for
chronic duration was not used. Chloral hydrate and the active
metabolite, trichloroethanol, do not bioaccumulate. The half-life of
chloral hydrate is a few minutes, and the half-life of
trichloroethanol is a few hours. Therefore, an enhanced effect from
continuous, daily exposure is not possible. Finally, there is
information from clinical use that long-term exposure to chloral
hydrate results in tolerance to the sedative effect. Developmental
toxicity, including developmental neurotoxicity, and immunotoxicity
are not critical effects. Although there is no two-generation
reproduction study, an uncertainty factor for database limitations is
not needed, as there is evidence from several studies that
reproductive toxicity is not likely to be a critical effect.
There are no inhalation studies adequate for setting a guidance
value or tolerable intake.
There are data in male mice showing that chloral hydrate causes
tumours in the liver. It is not known whether this response is
relevant for humans.
11.1.3 Sample risk characterization
The quantitative estimate of human risk for non-cancer effects is
based on the recommended clinical dose for sedation in humans and the
minor incidence of side-effects at this dose. The tolerable intake is
0.1 mg/kg body weight per day. This is 1% of the recommended single
dose for sedation in humans.
Although there is suggestive evidence of formation of tumours in
the liver of male mice and there are some data showing genotoxicity,
the mode of action for the formation of tumours is not known. It is
also not known whether this response is relevant for humans.
Millions of people are exposed to chloral hydrate on a daily
basis because it is formed during the disinfection of drinking-water
with chlorine. The typical concentration in a public water supply in
the USA is 5 µg/litre. Assuming a water consumption of 2 litres
per day and a body weight of 70 kg, the exposure is 0.14 µg/kg body
weight per day. This exposure is approximately 700 times lower than
the tolerable intake.
11.2 Evaluation of environmental effects
Insufficient data are available with which to assess the risk to
the environment from chloral hydrate.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1995) evaluated the carcinogenicity data for chloral
hydrate. It was concluded that there is inadequate evidence in humans
and limited evidence in experimental animals for the carcinogenicity
of chloral hydrate. Chloral hydrate is therefore not classifiable as
to its carcinogenicity to humans (Group 3).
IPCS (2000) recently evaluated the toxicological data on water
disinfectants and disinfectant by-products, including chloral hydrate.
Considering the dose level of 16 mg/kg body weight per day in the
90-day study in mice (Sanders et al., 1982; see section 8.4.1) as a
LOAEL (rather than as a NOAEL, as was done in the present document)
and using an uncertainty factor of 10 for intra- and interspecies
extrapolation and another factor of 10 for the use of a LOAEL rather
than a NOAEL, the Task Group calculated a tolerable daily intake (TDI)
for chloral hydrate of 16 µg/kg body weight per day. (As the present
document considered the increase in liver weight at 16 mg/kg body
weight to be a NOAEL rather than a LOAEL, the tolerable intake derived
from the studies among humans was lower, as discussed in section
11.1.)
REFERENCES
Abbas R, Fisher JW (1997) A physiologically based pharmacokinetic
model for trichloroethylene, and its metabolites, chloral hydrate,
trichloroacetate, dichloroacetate, trichloroethanol, and
trichloroethanol glucuronide in B6C3F1 mice. Toxicology and
applied pharmacology, 137:15-30.
Abbas R, Seckel CS, Kidney JK, Fisher JW (1996) Pharmacokinetic
analysis of chloral hydrate and its metabolism in B6C3F1 mice.
Drug metabolism and disposition, 24:1340-1346. See also Erratum,
Drug metabolism and disposition, 25:1449 (1997).
Allen BC, Fisher JW (1993) Pharmacokinetic modeling of
trichloroethylene and trichloroacetic acid in humans. Risk analysis,
13:71-86.
Anyebuno MA, Rosenfeld CR (1991) Chloral hydrate toxicity in a term
infant. Developments in pharmacological therapy, 17:116-120.
Badalaty MM, Houpt MI, Koenigsberg SR, Maxwell KC, Desjardins PJ
(1990) A comparison of chloral hydrate and diazepam sedation in young
children. Pediatric dentistry, 12:33-37.
Beland FA, Schmitt TC, Fullerton NF, Young JF (1998) Metabolism of
chloral hydrate in mice and rats after single and multiple doses.
Journal of toxicology and environmental health, 54:209-226.
Benane SG, Blackman CF, House DE (1996) Effect of perchloroethylene
and its metabolites on intercellular communication in clone 9 rat
liver cells. Journal of toxicology and environmental health,
48:427-437.
Bernstine JB, Meyer AE, Bernstine RL (1954) Maternal blood and breast
milk estimation following the administration of chloral hydrate during
the puerperium. Journal of obstetrics and gynaecology of the British
Commonwealth, 63:228-231.
Breimer DD (1977) Clinical pharmacokinetics of hypnotics. Clinical
pharmacokinetics, 2:93-109.
Breimer DD, Ketelaars HCJ, Van Rossum JM (1974) Gas chromatographic
determination of chloral hydrate, trichloroethanol, and
trichloroacetic acid in blood and urine employing head-space analysis.
Journal of chromatography, 88:55-63.
Bringmann G, Kuehn R (1976) Vergleichende Befunde der Schadwirkung
wassergerfahrden der Stoffe gegen Bakterien (Pseudomonas putida) und
Blaualgen (Microcystis aeruginosa). Gas- und Wasserfach:
Wasser/Abwasser, 117:410-413.
Bringmann G, Kuehn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in cell
multiplication inhibition tests. Water research, 14:231-241.
Bringmann G, Kuehn R (1980b) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen II Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, God R, Fähr S, Feines D, Fornadi K, Fornadi F (1999)
Identification of the dopaminergic neurotoxin
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline in human blood after
intake of the hypnotic chloral hydrate. Analytical biochemistry,
270:167-175.
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver
tumor induction in B6C3F1 mice by dichloroacetate and
trichloroacetate. Toxicology, 63:341-359.
Chakrabarti A, Schatten H, Mitchell KD, Crosser M, Taylor M (1998)
Chloral hydrate alters the organization of the ciliary basal apparatus
and cell organelles in sea urchin embryos. Cell and tissue research,
293:453-462.
Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP (1992a)
Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and
dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 19:159-168.
Daniel FB, Robinson M, Stober JA, Page NP, Olson GR (1992b) Ninety-day
toxicity study of chloral hydrate in the Sprague-Dawley rat. Drug and
chemical toxicology, 15:217-232.
DeAngelo AB, Daniel FB, Stober JA, Olson GR (1991) The carcinogenicity
of dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 16:337-347.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1996) The carcinogenicity
of dichloroacetic acid in the male Fischer 344 rat. Toxicology,
114:207-221.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1997) The failure of
monochloroacetic acid and trichloroacetic acid administered in the
drinking water to produce liver cancer in male F344/N rats. Journal
of toxicology and environmental health, 52:425-445.
Eichenlaub-Ritter U, Betzendahl I (1995) Chloral hydrate induced
spindle aberrations, metaphase I arrest and aneuploidy in mouse
oocytes. Mutagenesis, 10:477-486.
Eichenlaub-Ritter U, Baart E, Yin H, Betzendahl I (1996) Mechanisms of
spontaneous and chemically-induced aneuploidy in mammalian oogenesis:
Basis of sex specific differences in response to aneugens and the
necessity for further tests. Mutation research, 372:274-294.
Elfarra AA, Krause RJ, Last AR, Lash LH, Parker JC (1998) Species- and
sex-related differences in metabolism of trichloroethylene to yield
chloral and trichloroethanol in mouse, rat, and human liver
microsomes. Drug metabolism and disposition, 26:779-785.
Ferreira-Gonzalez A, DeAngelo AB, Nasim S, Garrett CT (1995) Ras
oncogene activation during hepatocarcinogenesis in B6C3F1 male mice
by dichloroacetic and trichloroacetic acid. Carcinogenesis,
16:495-500.
Fisher JW, Mahle D, Abbas R (1998) A human physiologically based
pharmacokinetic model for trichloroethylene and its metabolites:
trichloroacetic acid and free trichloroethanol. Toxicology and
applied pharmacology, 152:339-359.
Fox BE, O'Brien CO, Kangas KJ, Murphree AL, Wright KW (1990) Use of
high dose chloral hydrate for ophthalmic exams in children: A
retrospective review of 302 cases. Journal of pediatric ophthalmology
and strabismus, 27:242-244.
Fung K, Grosjean D (1981) Determination of nanogram amounts of
carbonyls as 2,4-dinitrophenylhydrazones by high-performance liquid
chromatography. Analytical chemistry, 53:168-171.
George MH, Kilburn S, Moore T, DeAngelo AB (2000) The carcinogenicity
of chloral hydrate administered in the drinking water to the male
B6C3F1 mouse and F344/N rat. Toxicologic pathology (in press).
Gerlach M, Xiao A-Y, Heim C, Lan J, God R, Feines D, Bringmann G,
Riederer P, Sontag K-H (1998)
1-Trichloromethyl-1,2,3,4-tetrahydro-ß-carboline increases
extracellular serotonin and stimulates hydroxyl radical production in
rats. Neuroscience letters, 257:17-20.
Goldenthal EI (1971) A compilation of LD50 values in newborn and
adult animals. Toxicology and applied pharmacology, 18:185-207.
Goodman LS, Gilman A (1985) The pharmacological basis of
therapeutics, 7th ed. New York, NY, The Macmillan Co.
Gorecki DKJ, Hindmarsh KW, Hall CA, Mayers DJ, Sankaran K (1990)
Determination of chloral hydrate metabolism in adult and neonate
biological fluids after single-dose administration.
Journal of chromatography, 528:333-341.
Gosselin RE, Smith RP, Hodge HC (1981) Clinical toxicology of
commercial products. Baltimore, MD, Williams & Wilkins.
Green T, Mainwaring GW, Foster JR (1997) Trichloroethylene-induced
mouse lung tumors: Studies of the mode of action and comparisons
between species. Fundamental and applied toxicology, 37:125-130.
Greenberg MS, Burton GA Jr, Fisher FW (1999) Physiologically based
pharmacokinetic modeling of inhaled trichloroethylene and its
oxidative metabolites in B6C3F1 mice. Toxicology and applied
pharmacology, 154:264-278.
Greenberg SB, Faerber EN, Aspinall CL (1991) High dose chloral hydrate
sedation for children undergoing CT. Journal of computer assisted
tomography, 15:467-469.
Gupta RN (1990) Determination of trichloroethanol, the active
metabolite of chloral hydrate, in plasma by liquid chromatography.
Journal of chromatography, 500:655-659.
HSDB (1999) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
Helrich K, ed. (1990) Official methods of analysis of the Association
of Official Analytical Chemists, 15th ed. Vol. 1. Arlington, VA,
Association of Official Analytical Chemists, p. 562.
Henderson GN, Yan Z, James MO, Davydova N, Stacpoole PW (1997)
Kinetics and metabolism of chloral hydrate in children: identification
of dichloroacetate as a metabolite. Biochemical and biophysical
research communications, 235:695-698.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The
carcinogenicity of trichloroethylene and its metabolites,
trichloroacetic acid and dichloroacetic acid, in mouse liver.
Toxicology and applied pharmacology, 90:183-189.
Hindmarsh KW, Gorecki DKJ, Sankaran K, Mayers DJ (1991) Chloral
hydrate administration to neonates: Potential toxicological
implications. Canadian Society of Forensic Science, 24:239-245.
Hobara T, Kobayashi H, Kawamoto T, Sato T, Iwamoto S, Hirota S, Sakai
T (1986) Biliary excretion of trichloroethylene and its metabolites in
dogs. Toxicology letters, 32:119-122.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1987a) The
cholecystohepatic circulation of trichloroethylene and its metabolites
in dogs. Toxicology, 44:283-295.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Hirota S, Sakai T
(1987b) Extrahepatic metabolism of chloral hydrate, trichloroethanol,
and trichloroacetic acid in dogs. Pharmacology and toxicology,
61:58-62.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988a)
Intestinal absorption of chloral hydrate, free trichloroethanol, and
trichloroacetic acid in dogs. Pharmacology and toxicology,
62:250-258.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988b) The
absorption of trichloroethylene and its metabolites from the urinary
bladder of anesthetized dogs. Toxicology, 48:141-153.
IARC (1995) Chloral and chloral hydrate. IARC (International Agency
for Research on Cancer) monographs, 63:245-269.
IPCS (1993) International Chemical Safety Card -- Chloral hydrate.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0234).
IPCS (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
IPCS (2000) Disinfectants and disinfectant by-products. Geneva,
World Health Organization (Environmental Health Criteria 216).
Johnson PD, Dawson BV, Goldberg SJ (1998) Cardiac teratogenicity of
trichloroethylene metabolites. Journal of the American College of
Cardiology, 32:540-545.
Kallman MJ, Kaempf GL, Balster RL (1984) Behavioral toxicity of
chloral in mice: An approach to evaluation. Neurobehavioral
toxicology and teratology, 6:137-146.
Kaplan HL, Forney RB, Hughes FW, Jain NC, Crim D (1967) Chloral
hydrate and alcohol metabolism in human subjects. Journal of forensic
sciences, 12:295-304.
Kauffmann BM, White KL, Sanders VM, Douglas KA, Sain LE, Borzelleca
JF, Munson AE (1982) Humoral and cell-mediated immune status in mice
exposed to chloral hydrate. Environmental health perspectives,
44:147-151.
Ketcha MM, Stevens DK, Warren DA, Bishop CT, Brashear WT (1996)
Conversion of trichloroacetic acid to dichloroacetic acid in
biological samples. Journal of analytical toxicology, 20:236-241.
Klinefelter GR, Suarez JD, Roberts NL (1995) Preliminary screening
test for the potential of drinking water disinfectant by-products to
alter male reproduction. Reproductive toxicology, 9:571-578.
Koppen B, Dalgaard L (1988) Determination of trichloroethylene
metabolites in rat liver homogenates using headspace gas
chromatography. Journal of chromatography, 442:325-332.
Lambert GH, Muraskas J, Anderson CL, Myers TF (1990) Direct
hyperbilirubinemia associated with chloral hydrate administration in
the newborn. Pediatrics, 86:277-281.
Leuschner J, Beuscher N (1998) Studies on the mutagenic and
carcinogenic potential of chloral hydrate. Arzneimittel-Forschung
drug research, 48:961-968.
Lipscomb JC, Mahle DA, Brashear WT, Garrett CM (1996) A species
comparison of chloral hydrate metabolism in blood and liver.
Biochemical and biophysical research communications, 227:340-350.
Lipscomb JC, Confer PD, Miller MR, Stamm SC, Snawder JE, Candiera SM
(1998) Metabolism of trichloroethylene and chloral hydrate by the
Japanese medaka (Oryzias latipes) in vitro. Environmental
toxicology and chemistry, 17:325-332.
Ludwigs U, Divino-Fiiho JC, Magnusson N (1996) Suicidal chloral
hydrate poisoning. Journal of clinical toxicology, 344:97-99.
Mailhes JB, Marchetti F (1994) Chemically induced aneuploidy in
mammalian oocytes. Mutation research, 320:87-111.
Marshall AJ (1977) Cardiac arrhythmias caused by chloral hydrate.
British medical journal, 2:994.
Marshall EK, Owens AH (1954) Absorption, excretion and metabolic fate
of chloral hydrate and trichloroethanol. Bulletin of the Johns
Hopkins Hospital, 95:1-18.
Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DKJ, Kasian GF (1991)
Chloral hydrate disposition following single-dose administration to
critically ill neonates and children. Developments in pharmacological
therapy, 16:71-77.
Merdink JL, Conzalez-Leon A, Bull RJ, Schultz IR (1998) The extent of
dichloroacetate formation from trichloroethylene, chloral hydrate,
trichloroacetate, and trichloroethanol in B6C3F1 mice. Toxicological
sciences, 45:33-41.
Merdink JL, Stenner RD, Stevens DK, Parker JC, Bull RJ (1999) Effect
of enterohepatic circulation on the pharmacokinetics of chloral
hydrate and its metabolites in F344 rats. Journal of toxicology and
environmental health, 56:357-368.
Miller RR, Greenblatt DJ (1979) Clinical effects of chloral hydrate in
hospitalized medical patients. Journal of clinical pharmacology,
19:669-674.
Newman LM, Wackett LP (1991) Fate of 2,2,2-trichloroacetaldehyde
(chloral hydrate) produced during trichloroethylene oxidation by
methanotrophs. Applied and environmental microbiology, 57:2399-2402.
Ni Y-C, Wong T-Y, Kadlubar FF, Fu PP (1994) Hepatic metabolism of
chloral hydrate to free-radical(s) and induction of lipid
peroxidation. Biochemical and biophysical research communications,
204:937-943.
Ni Y-C, Kadlubar FF, Fu FF (1995) Formation of
malondialdehyde-modified 2'-deoxyguanosinyl adduct from metabolism of
chloral hydrate by mouse liver microsomes. Biochemical and
biophysical research communications, 205:1110-1117.
Ni Y-C, Wong T-Y, Lloyd RV, Heinze TM, Shelton S, Casciano D, Kadlubar
FF, Fu PP (1996) Mouse liver microsomal metabolism of chloral hydrate,
trichloroacetic acid, and trichloroethanol leading to induction of
lipid peroxidation via a free radical mechanism. Drug metabolism and
disposition, 24:81-90.
NTP (2000a) Toxicology and carcinogenesis studies of chloral hydrate
in B6C3F1 mice (gavage studies). Research Triangle Park, NC,
National Institutes of Health, National Toxicology Program (NTP TR
502).
NTP (2000b) Toxicology and carcinogenesis studies of chloral hydrate
(ad libitum and dietary controlled) in male B6C3F1 mice (gavage
study). Research Triangle Park, NC, National Institutes of Health,
National Toxicology Program (NTP TR 503).
Odum J, Foster JR, Green T (1992) A mechanism for the development of
clara cell lesions in the mouse lung after exposure to
trichloroethylene. Chemico-biological interactions, 83:135-153.
Owens AH, Marshall EK (1955) Further studies on the metabolic fate of
chloral hydrate and trichloroethanol. Bulletin of the Johns Hopkins
Hospital, 97:320-326.
Pereira MA (1996) Carcinogenic activity of dichloroacetic acid and
trichloroacetic acid in the liver of female B6C3F1 mice.
Fundamental and applied toxicology, 31:192-199.
Reimche LD, Sankaran K, Hindmarsh KW, Kasian GF, Gorecki DKJ, Tan L
(1989) Chloral hydrate sedation in neonates and infants -- clinical
and pharmacologic considerations. Developments in pharmacological
therapy, 12:57-64.
Richmond RE, Carter JH, Carter HW, Daniel FB, DeAngelo AB (1995)
Immunohistochemical analysis of dichloroacetic acid (DCA)-induced
hepatocarcinogenesis in male Fischer (F344) rats. Cancer letters,
92:67-76.
Rijhsinghani KS, Abrahams C, Swerdlow MA, Rao KVN, Ghose T (1986)
Induction of neoplastic lesions in the livers of C57BL × C3HF1 mice
by chloral hydrate. Cancer detection and prevention, 9:279-288.
Saillenfait AM, Langonne I, Sabate JP (1995) Developmental toxicity of
trichloroethylene, tetrachloroethylene and four of their metabolites
in rat whole embryo culture. Archives of toxicology, 70:71-82.
Sanders VM, Kauffman BM, White KL, Douglas KA, Barnes DW, Sain LE,
Bradshaw TJ, Borzelleca JF, Munson AE (1982) Toxicology of chloral
hydrate in the mouse. Environmental health perspectives, 44:137-146.
Schatten H, Chakrabarti A (1998) Centrosome structure and function is
altered by chloral hydrate and diazepam during the first reproductive
cell cycles in sea urchin eggs. European journal of cell biology,
75:9-20.
Shapiro S, Stone D, Lewis GP, Jick H (1969) Clinical effects of
hypnotics. II. An epidemiological study. Journal of the American
Medical Association, 209:2016-2020.
Simpson KL, Hayes KP (1998) Drinking water disinfection by-products:
an Australian perspective. Water research, 32:1522-1528.
Sing K, Erickson T, Amitai Y, Hryhorczuk D (1996) Chloral hydrate
toxicity from oral and intravenous administration. Journal of
toxicology and clinical toxicology, 34:101-106.
Smith MK, Randall JL, Read EJ, Stober JA (1989) Teratogenic activity
of trichloroacetic acid in the rat. Teratology, 40:445-451.
Stenner RD, Merdink JL, Stevens DK, Springer DL, Bull RJ (1997)
Enterohepatic recirculation of trichloroethanol glucuronide as a
significant source of trichloroacetic acid. Drug metabolism and
disposition, 25:529-535.
Stenner RD, Merdink JL, Fisher JW, Bull RJ (1998)
Physiologically-based pharmacokinetic model for trichloroethylene
considering enterohepatic recirculation of major metabolites.
Risk analysis, 18:261-269.
US EPA (1994) National primary drinking water regulations;
disinfectants and disinfection byproducts; proposed rule. US
Environmental Protection Agency. Federal register, 59:38668-38829.
US EPA (2000) Toxicological review on chloral hydrate. Available
from US Environmental Protection Agency's Risk Assessment Hotline
[513-569-7254 (phone), 513-569-7159 (fax), rih.iris@epa.gov (e-mail
address), or www.epa.gov/iris (Website)].
Velazquez SF (1994) Activation of the H-ras oncogene by drinking
water disinfection by-products. Report submitted under the US
Environmental Protection Agency's Small Grant Program. April 1994. 43
pp. (NTIS/PB95-200515).
Vian L, Van Hummelen P, Bichet N, Gouy D, Kirsch-Volders M (1995)
Evaluation of hydroquinone and chloral hydrate on the in vitro
micronucleus test on isolated lymphocytes. Mutation research,
334:1-7.
Watanabe M, Takano T, Nakamura K (1998) Effect of ethanol on the
metabolism of trichloroethylene in the perfused rat liver. Journal of
toxicology and environmental health, 55:297-305.
Wu WW, Chadik PA, Davis WM, Powell DH, Delfino JJ (1998) Disinfection
byproduct formation from the preparation of instant tea. Journal of
agricultural and food chemistry, 46:3272-3279.
Yan Z, Henderson GN, James MO, Stacpoole P (1999) Determination of
chloral hydrate in human plasma by gas chromatography-mass
spectrometry. Journal of pharmaceutical and biomedical analysis,
19:309-318.
Zimmermann T, Wehling M, Schulz HU (1998) Untersuchungen zur relativen
Bioverfugbarkeit und Pharmakokinetik von Chloralhydrat und seinen
Metaboliten. [The relative bioavailability and pharmacokinetics of
chloral hydrate and its metabolites.] Arzneimittelforschung,
48:5-12.
APPENDIX 1 -- TOXICOKINETICS
This toxicokinetic analysis is used to estimate the steady-state
concentrations of trichloroacetic acid (TCA) and trichloroethanol
(TCEOH) in mice and humans using a one-compartment model, assuming
that the absorption of chloral hydrate (CH) from the gastrointestinal
tract and its metabolism in the blood are very rapid compared with the
rate of elimination of TCA and TCEOH. This assumption is supported by
the data of Beland et al. (1998) in mice and Breimer (1977) and
Zimmermann et al. (1998) in humans.
Beland et al. (1998) indicated that 15% of the dose of chloral
hydrate is converted directly to TCA and 77% is converted to TCEOH. In
humans, Allen & Fisher (1993) estimated that 8% of a dose of chloral
hydrate is converted directly to TCA and 92% is converted to TCEOH.
Additional TCA is formed from TCEOH. The total TCA formed in humans is
approximately 35% of the dose of chloral hydrate.
Estimation of TCA concentration in mice at steady state at the
clinically recommended dose for humans:
[TCA]ss-blood = PKo/ VKel = 2.5 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 3.0 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.15
(Beland et al., 1998)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.321 litre/kg
(Beland et al., 1998)
* Kel is the first-order elimination constant for TCA =
0.0819/h (Beland et al., 1998)
* PC is the liver/blood partition coefficient = 1.18 (Abbas &
Fisher, 1997)
Estimation of TCA concentration in humans at steady state at the
clinically recommended dose:
[TCA]ss-blood = PKo/ VKel = 55 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 36 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA = 0.028/h
(Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCA concentration in humans at steady state at the
tolerable intake:
[TCA]ss-blood = PKo/ VKel = 1.8 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 1.2 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA =
0.0078/h (Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCEOH concentration in mice at steady state at 166 mg/kg
body weight per day:
[TCEOH]ss-blood = PKo/ VKel = 0.6 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.77
(Beland et al., 1998)
* Ko is the dosing rate for CH = 166 mg/kg body weight per day,
equivalent to 6.917 mg/kg body weight per hour
* V is the volume of distribution = 1 litre/kg (cited in
Beland et al., 1998)
* Kel is the first-order elimination constant for TCEOH =
9.24/h (Beland et al., 1998)
Chloral hydrate at 160 mg/kg body weight per day was the highest
exposure used in the 90-day neurobehavioural study by Kallman et al.
(1984); chloral hydrate at 166 mg/kg body weight per day was the
highest exposure used in the 104-week bioassay of Daniel et al.
(1992a). These exposures are a NOAEL for sedation in mice.
Estimation of TCEOH concentration in humans at steady state at the
clinically recommended dose:
[TCEOH]ss-blood = PKo/ VKel = 4.7 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
Estimation of TCEOH concentration in humans at steady state at the
tolerable intake:
[TCEOH]ss-blood = PKo/ VKel = 0.04 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
APPENDIX 2 -- CALCULATION OF BENCHMARK DOSE FOR
TUMOUR INCIDENCE
The Benchmark Dose (ED) for tumour incidence was derived from the
incidence of adenoma plus carcinoma as reported by George et al.
(2000). The human equivalent dose was calculated using (body
weight)3/4, assuming a human body weight of 70 kg and a mouse body
weight of 0.035 kg. EPA Benchmark Dose Software was used to calculate
the ED and its lower 95% confidence limit (LED) corresponding to a 10%
increase in extra risk for tumour prevalence with the multistage
model.
Multistage Model, Version Number 1.1.0b
The form of the probability function is:
P[response] =
(1 - background ) × [1 - (e - beta 1 × dose 1 - beta 2 × dose 2) ]
The parameter betas are restricted to be positive.
Dependent variable = Incidence
Independent variable = Dose
Total number of observations = 4
Total number of records with missing values = 0
Total number of parameters in model = 3
Total number of specified parameters = 0
Degree of polynomial = 2
Maximum number of iterations = 250
Relative function convergence has been set to 2.220 45e-16
Parameter convergence has been set to 1.490 12e-8
Default initial parameter values
Background = 0.698 863
Beta(1) = 0.043 897
Beta(2) = 0.000 400 241
Parameter estimates
Variable Estimate Standard error
Background 0.691 141 0.073 072 3
Beta(1) 0.053 218 1 0.084 548 3
Beta(2) 0 0.004 035 19
Asymptotic correlation matrix of parameter estimates
Background Beta(1) Beta(2)
Background 1 -0.6319 0.5007
Beta(1) -0.6319 1 -0.9507
Beta(2) 0.5007 -0.9507 1
Analysis of deviance table
Model Log(likelihood) Deviance DF P-value
Full model -81.2046
Fitted model -81.922 1.434 7 2 0.230 999
Reduced mode -85.0504 6.256 83 2 0.043 787
Goodness of fit analysis
Administered dose Human equivalent Estimated Expected Observed Size
(mg/kg body weight dose probability
per day) (mg/kg body weight
per day)
0 0 0.6911 29.028 27 42
13.5 2.0000 0.7223 33.227 36 46
65 9.7 0.8157 31.812 31 39
146.6 21.9 0.9037 28.919 29 32
Chi-square = 1.41; DF = 2; P-value = 0.4949.
Benchmark dose computation
Specified effect 0.100 000
Risk type Extra risk
Confidence level 0.950 000
ED 1.979 786
LED 1.090 1
APPENDIX 3 -- SOURCE DOCUMENT
US Environmental Protection Agency (2000):
Toxicological review on chloral hydrate
Copies of the document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (phone)
513-569-7159 (fax)
rih.iris@epa.gov (e-mail address)
www.epa.gov/iris (Website)
This document was prepared by R. Benson, Region VIII, Denver, CO.
The document and summary information on the Integrated Risk
Information System (IRIS) have received peer review both by EPA
scientists and by independent scientists external to EPA. Subsequent
to external review and incorporation of comments, this assessment has
undergone an Agency-wide review process whereby the IRIS Program
Manager has achieved a consensus approval among the Office of Research
and Development; Office of Air and Radiation; Office of Prevention,
Pesticides, and Toxic Substances; Office of Solid Waste and Emergency
Response; Office of Water; Office of Policy, Planning, and Evaluation;
and the Regional Offices.
Internal EPA reviewers:
National Center for Environmental Assessment, Washington, DC
J. Cogliano
C. Siegel Scott
V. Vu
National Health and Environmental Effects Research Laboratory,
Research Triangle Park, NC
A. DeAngelo
R. Luebke
Office of Water, Washington, DC
A. Bathija
External peer reviewers:
P.E. Brubaker, Private Consultant
J.W. Fisher, Operational Toxicology Branch, Wright-Patterson
Air Force Base
C.C. Willhite, Department of Toxic Substances Control,
State of California
APPENDIX 4 -- CICAD PEER REVIEW
The draft CICAD on chloral hydrate was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS National Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Centre of Industrial Hygiene and Occupational Diseases, Czech
Republic
Department of Health, London, United Kingdom
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Berlin, Germany
Fraunhofer Institute for Toxicology and Aerosol Research,
Hannover, Germany
GSF Forschungszentrum für Umwelt und Gesundheit, GmbH,
Oberschleissheim, Germany
Health and Safety Executive, Bootle, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Beijing, People's Republic of China
National Center for Environmental Assessment, US Environmental
Protection Agency, Washington, DC, USA
National Center for Toxicological Research, US Food and Drug
Administration, Jefferson, AK, USA
National Chemicals Inspectorate, Solna, Sweden
National Industrial Chemicals Notification and Assessment Scheme
(NICNAS), Sydney, Australia
National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, Research Triangle Park, NC, USA
University of Bielefeld, Bielefeld, Germany
APPENDIX 5 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment,
US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry,
Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment,
US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 6 -- INTERNATIONAL CHEMICAL SAFETY CARD
CHLORAL HYDRATE ICSC: 0234
October 1999
CAS# 302-17-0 Trichloroacetaldehyde monohydrate
RTECS# FM8750000 2,2,2-Trichloro-1,1-ethanediol
UN# 2811 C2H3Cl3O2/Cl3CCH(OH)2
EC# 605-014-00-6
Molecular mass: 165.4
TYPES OF HAZARD ACUTE HAZARDS/ PREVENTION FIRST AID / FIRE
/ EXPOSURE SYMPTOMS FIGHTING
FIRE Not combustible. In case of fire in the
Gives off irritating surroundings, all
or toxic fumes extinguishing agents allowed.
(or gases) in a
fire.
EXPLOSION In case of fire: keep drums,
etc., cool by spraying with
water.
EXPOSURE PREVENT DISPERSION
OF DUST!
Inhalation Confusion. Local exhaust Fresh air, rest. Artificial
Drowsiness. or breathing respiration if indicated. Refer
Nausea. protection. for medical attention.
Unconsciousness.
Skin Redness. Protective gloves. Rinse skin with plenty
of water or shower.
Eyes Redness. Safety spectacles or First rinse with plenty
eye protection in of water for several minutes
combination with (remove contact lenses if
breathing protection easily possible), then take
if powder. to a doctor.
Ingestion Abdominal pain. Do not eat, drink, or Rinse mouth. Give a slurry of
Vomiting (further smoke during work. activated charcoal in water
see inhalation). Wash hands before to drink. Refer for medical
eating. attention.
SPILLAGE DISPOSAL PACKAGING & LABELLING
Sweep spilled substance into containers; Do not transport with food and feedstuffs.
if appropriate, moisten first to prevent EU Classification
dusting. Carefully collect remainder, then Symbol: T
remove to safe place. R: 25-36/38
(Extra personal protection: P3 filter S: (1/2-)25-45
respirator for toxic particles). UN Classification
UN Hazard Class: 6.1
EMERGENCY RESPONSE STORAGE
Transport Emergency Card: TEC (R)-61G12b Separated from strong bases,
food and feedstuffs.
IMPORTANT DATA
PHYSICAL STATE; APPEARANCE: ROUTES OF EXPOSURE:
TRANSPARENT, COLOURLESS CRYSTALS, WITH The substance can be absorbed
CHARACTERISTIC ODOUR. into the body by inhalation of
its aerosol and by ingestion.
CHEMICAL DANGERS: INHALATION RISK:
The substance decomposes on heating A harmful contamination of the air will be
producing toxic and corrosive fumes including reached rather slowly on evaporation of this
hydrogen chloride. Reacts with strong bases substance at 20°C.
producing chloroform.
OCCUPATIONAL EXPOSURE LIMITS: EFFECTS OF LONG-TERM OR REPEATED EXPOSURE:
TLV not established. The substance irritates the eyes, the skin
and the respiratory tract. The substance
may cause effects on the central nervous
system, cardiovascular system, liver and
kidneys, resulting in lowering of
consciousness, cardiac disorders and impaired
functions. Exposure at high levels may result
in unconsciousness.
PHYSICAL PROPERTIES
Boiling Point: 97°C
Melting Point: 57-60°C
Density: 1.9 g/cm3
Solubility in water: very good
Octanol/water partition coefficient as log Pow: 0.99
ENVIRONMENTAL DATA
This substance may be hazardous to the environment; special attention should
be given to water organisms.
NOTES
Use of alcoholic beverages enhances the harmful effect.
ADDITIONAL INFORMATION
LEGAL NOTICE Neither the CEC nor the IPCS nor any person acting on
behalf of the CEC or the IPCS is responsible for the use
which might be made of this information.
RÉSUMÉ D'ORIENTATION
Le présent CICAD relatif à l'hydrate de chloral a été préparé par
l'Environmental Protection Agency des États-Unis (EPA) sur la base
d'un de ses documents intitulé Toxicological review on chloral
hydrate (US EPA, 2000). Les données qu'il contient proviennent d'un
dépouillement de la littérature scientifique jusqu'en mars 1999. On
trouvera à l'appendice 3 des renseignements sur la manière dont
l'étude bibliographique a été effectuée et sur les sources de données
disponibles. L'appendice 4 donne des indications sur les modalités
d'examen du présent CICAD par des pairs. Ce CICAD a été approuvé en
tant qu'évaluation internationale lors d'une réunion du Comité
d'évaluation finale qui s'est tenue à Sydney (Australie) du 21 au 24
novembre 1999. La liste des participants à cette réunion figure à
l'appendice 5. La Fiche internationale sur la sécurité chimique (ICSC
0234) de l'hydrate de chloral établie par le Programme international
sur la sécurité chimique est reproduite à l'appendice 6 (IPCS, 1993).
La synthèse de l'hydrate de chloral (No CAS 302-17-0) s'effectue
par chloration de l'éthanol. On l'utilise en médécine humaine et
vétérinaire comme sédatif et hypnotique. Le chloral, qui en est la
forme anhydre (No CAS 75-87-6) est utilisé comme intermédiaire dans la
synthèse du DDT, du méthoxychlore, du naled, du trichlorfon, du
dichlorvos et de l'acide trichloracétique.
La principale voie d'exposition de la population générale est
l'eau de boisson, car il se forme de l'hydrate de chloral lors de la
désinfection de l'eau par le chlore. Aux États-Unis, la concentration
habituelle d'hydrate de chloral dans l'eau des réseaux publics de
distribution est de 5 µg/litre. Comme ce composé est un métabolite du
trichloréthylène et du tétrachloréthylène, la population se trouve
exposée à l'hydrate de chloral si elle l'est à ces deux composés. Par
ailleurs, il y a également exposition à deux métabolites de l'hydrate
de chloral, les acides dichlor- et trichloracétique, du fait que ces
deux composés se forment également dans l'eau de consommation lors de
sa désinfection par le chlore. Lorsque l'hydrate de chloral est
utilisé comme sédatif, la dose habituelle est de 250 mg trois fois par
jour (soit l'équivalent de 10,7 mg/kg de poids corporel par jour).
C'est un métabolite, le trichloréthanol, qui est responsable de
l'effet pharmacologique. On ne dispose d'aucune donnée quantitative
sur l'exposition professionnelle.
L'hydrate de chloral est irritant pour la peau et les muqueuses
et il provoque souvent des troubles gastriques, des nausées et des
vomissements lorsqu'on l'utilise à la dose recommandée dans la
pratique clinique. Une surdose aiguë entraîne progressivement ataxie,
léthargie, coma profond, dépression respiratoire, hypotension et
arrythmie cardiaque. On a trouvé des signes de lésions hépatiques chez
des sujets ayant échappé de peu à la mort par intoxication aiguë due
une surdose, mais rien ne prouve par contre de façon convaincante qu'à
la dose clinique recommandée, le composé entraîne des lésions
hépatiques. Plusieurs études portant sur l'utilisation clinique de
l'hydrate de chloral ont mis en évidence des effets secondaires
mineurs et peu fréquents. Bien que ce produit soit utilisé depuis
longtemps en médecine, aucune étude toxicologique contrôlée sur des
sujets humains n'a été publiée.
L'hydrate de chloral est intégralement absorbé et rapidement
métabolisé après administration par la voie orale. Ses principaux
métabolites sont le trichloréthanol et son glucuronide ainsi que
l'acide trichloracétique. D'après certaines données, il pourrait se
former également un peu d'acide dichloracétique. Chez l'Homme, la
demi-vie du trichloréthanol et de son glucuronide est d'environ 8 h;
celle de l'acide trichloracétique est à peu près égale à 4 jours. Un
certain nombre de données incitent à penser que la demi-vie du
trichloréthanol est plus de deux fois plus longue chez les prématurés
et les nouveau-nés à terme que chez les enfants en bas âge et les
adultes. La principale voie d'excrétion des métabolites de l'hydrate
de chloral est la voie urinaire. On peut le retrouver, accompagné de
ses métabolites, dans le lait de mères traitées par ce produit.
Toutefois leur concentration est trop faible pour avoir des effets
pharmacologiques chez les nourrissons alimentés au sein.
Administré à des souris, le composé provoque une perte de
coordination (ataxie) à une dose comparable à celle qui produit le
même effet chez l'Homme. Une étude de 90 jours sur des souris n'a
révélé aucun signe d'altération du comportement ni de neurotoxicité.
Des études au long cours sur des rats et des souris n'ont pas non plus
permis de constater d'anomalies comportementales ni de modifications
histopathologiques touchant les tissus nerveux. Après exposition de
souris pendant 90 jours, on a observé une légère diminution de
l'immunité humorale. D'autres études n'ont mis en évidence aucun effet
sur le développement des souris et des rats. Aucune anomalie
structurale n'a été relevée. Une étude consacrée à l'action de
l'hydrate de chloral sur le développement nerveux de la souris n'a mis
en évidence qu'un léger effet sur l'apprentissage de l'évitement
passif. Le composé n'a pas fait l'objet d'études de toxicité génésique
sur deux générations, mais les données dont on dispose sur l'activité
génésique des animaux et les effets sur les spermatozoïdes et les
ovocytes ne permettent pas de penser que l'hydrate de chloral puisse
avoir des effets majeurs sur la reproduction. Par ailleurs, les études
chroniques et subchroniques effectuées sur des rongeurs n'ont pas mis
en évidence d'effets histopathologiques au niveau de l'appareil
reproducteur. Toutes les études effectuées sur des animaux de
laboratoire mettent en évidence un certain nombre d'effets, mais à
l'exclusion de tout effet cancérogène et à des doses qui sont très
supérieures à celle qui provoque la sédation chez l'Homme.
En ce qui concerne l'Homme, on ne possède aucune donnée de
cancérogénicité. Deux tests biologiques effectués sur le rat ne
révèlent aucune augmentation de la fréquence des tumeurs, quelle que
soit la localisation. Par contre, dans trois autres tests distincts
effectués sur des souris mâles, on constate une augmentation de
l'incidence des tumeurs hépatiques. Celle de ces études dont le
caractère est le plus définitif indique une augmentation de
l'incidence et de la multiplicité des tumeurs pour chacune des trois
doses utilisées. Ces données semblent indiquer que le produit est
cancérogène chez la souris mâle mais on estime qu'elles ne permettent
pas d'évaluer le risque pour l'Homme avec une réponse linéaire aux
faibles doses.1
Il existe une importante base de données sur les effets
génotoxiques. Divers résultats indiquent que l'hydrate de chloral est
faiblement mutagène et clastogène. Il provoque une aneuploïdie chez
des cellules très diverses. On pense que cet effet est dû à
destruction de l'appareil fusorial. Des concentrations élevées sont
nécessaires pour que ces effets soient observables. Même si ces
résultats donnent à penser que la toxicité de l'hydrate de chloral
s'exerce notamment au niveau des gènes, ils montrent également que ces
effets ne se produisent qu'à des concentrations qui ont peu de chances
d'exister dans les conditions physiologiques, compte tenu de
l'exposition habituelle à ce produit dans l'environnement. La
formation des tumeurs hépatiques chez la souris mâle peut s'expliquer
par la formation d'adduits de l'ADN avec des radicaux libres produits
lors de la métabolisation de l'hydrate de chloral par les enzymes du
cytochrome P450 2E1 (CYP 2E1) ou par une cytotoxicité conduisant à une
hyperplasie compensatoire.
La dose journalière tolérable pour les effets non cancérogènes a
été estimée à 0,1 mg/kg pc à partir de la dose la plus faible
produisant un effet sédatif observable chez l'Homme (LOAEL), dose qui
est égale à 10,7 mg/kg par jour, avec un facteur d'incertitude de 100.
On ne possède que des données limitées sur les effets
environnementaux. Les méthanotrophes sont capables de transformer
l'hydrate de chloral en trichloréthanol et en acide trichloracétique.
Le composé subit également une dégradation abiotique dans certaines
conditions. On dispose de données limitées sur l'inhibition de la
croissance des bactéries, des algues et des protozoaires. Des
résultats sont également disponibles concernant l'effet du composé sur
le développement des oursins. On ne possède pas assez de données pour
pouvoir évaluer le risque que l'hydrate de chloral représente pour
l'environnement.
1 Un test biologique effectué dans le cadre du Programme national de
toxicologie et dont les résultats n'ont été connus qu'après la
réunion du Comité d'évaluation finale, a montré que l'incidence des
tumeurs hépatiques était en augmentation chez les souris mâles et
que chez les femelles, il y avait une faible augmentation des
adénomes hypophysaires, augmentation dont la signification
statistique était limite.
RESUMEN DE ORIENTACI²N
Este CICAD sobre el hidrato de cloral, preparado por la Agencia
para la Protección del Medio Ambiente (EPA), se basó en el
Examen toxicológico sobre el hidrato de cloral de la EPA de los
Estados Unidos (US EPA, 2000). Se incluyó la bibliografía científica
localizada hasta marzo de 1999. La información relativa al carácter de
los procesos de examen y a la disponibilidad del documento original
figura en el apéndice 3. La información sobre el examen colegiado de
este CICAD se presenta en el apéndice 4. Este CICAD se aprobó como
evaluación internacional en una reunión de la Junta de Evaluación
Final celebrada en Sydney, Australia, los días 21-24 de noviembre de
1999. En el apéndice 5 figura la lista de participantes en esta
reunión. La Ficha internacional de seguridad química (ICSC 0234) para
el hidrato de cloral, preparada por el Programa Internacional de
Seguridad de la Sustancias Químicas, se reproduce en el apéndice 6
(IPCS, 1993).
El hidrato de cloral (CAS No 302-17-0) se sintetiza mediante la
cloración de etanol. Se utiliza en la medicina humana y veterinaria
como sedante e hipnótico. El cloral (CAS No 75-87-6), producto
químico anhidro, se utiliza como intermediario en la síntesis de DDT,
metoxicloro, naled, triclorfon, diclorvos y ácido tricloroacético.
La vía principal de exposición del público general es el agua de
bebida, puesto que al desinfectar dicha agua con cloro se forma
hidrato de cloral. La concentración normal de hidrato de cloral en el
sistema público de abastecimiento de agua de los Estados Unidos es 5
µg/litro. Debido a que el hidrato de cloral es un metabolito del
tricloroetileno y el tetracloroetileno, el público estará expuesto al
hidrato de cloral si lo está a estos productos químicos. La población
está expuesta a los ácidos tricloroacético y dicloroacético,
metabolitos del hidrato de cloral, porque también se forman cuando se
desinfecta el agua de bebida con cloro. En su uso como sedante humano,
la dosis clínica normal es de 250 mg tres veces al día (equivalente a
10,7 mg/kg de peso corporal al día). El metabolito tricloroetanol es
el responsable del efecto farmacológico. No se dispone de información
cuantitativa relativa a la exposición ocupacional.
El hidrato de cloral es irritante de la piel y las membranas
mucosas y con frecuencia provoca trastornos gástricos, náuseas y
vómitos con la dosis clínica recomendada. Una sobredosis aguda produce
(en orden de progresión) ataxia, letargo, coma profundo, depresión
respiratoria, hipotensión y arritmia cardíaca. Hay algunas pruebas de
lesiones hepáticas en personas que sobreviven a sobredosis agudas casi
letales, pero no hay pruebas convincentes de que se produzcan tales
lesiones con la dosis clínica recomendada. En varios estudios sobre el
uso clínico del hidrato de cloral se ha puesto de manifiesto una
incidencia baja de efectos secundarios menores. A pesar de utilizarse
desde hace mucho tiempo en la medicina humana, no hay información
publicada sobre la toxicidad en estudios controlados realizados con
personas después de una exposición prolongada.
Tras la administración oral, el hidrato de cloral se absorbe
completamente y se metaboliza con rapidez. Los principales metabolitos
son el tricloroetanol y su glucurónido y el ácido tricloroacético.
Algunos datos parecen indicar que se puede formar una pequeña cantidad
de ácido dicloroacético. En el ser humano, la semivida del
tricloroetanol y su glucurónido es de unas ocho horas; la semivida del
ácido tricloroacético es de alrededor de cuatro días. Algunos datos
indican que la semivida del tricloroetanol aumenta varias veces en los
niños prematuros y los nacidos a término en comparación con los niños
que empiezan a caminar y los adultos. La vía principal de excreción de
los metabolitos del hidrato de cloral es la orina. Se han detectado
hidrato de cloral y sus metabolitos en la leche de mujeres tratadas
con este producto. Sin embargo, su concentración es demasiado baja
para provocar un efecto farmacológico en los niños lactantes.
La administración aguda de hidrato de cloral a ratones provoca la
pérdida de la coordinación (ataxia) con una exposición prácticamente
semejante a la de las personas para el mismo efecto. En un estudio de
90 días en ratones no se obtuvieron pruebas de cambios de
comportamiento u otros signos de neurotoxicidad. En estudios crónicos
con ratas y ratones no se detectaron cambios de comportamiento ni
cambios histopatológicos en el tejido nervioso. Tras la exposición de
ratones durante 90 días al hidrato de cloral se observó una ligera
disminución en la inmunidad humoral. Se han realizado pruebas con
hidrato de cloral para estudiar sus efectos en el desarrollo de ratas
y ratones. No se observaron anomalías estructurales. En un estudio del
neurodesarrollo en ratones, se observó un ligero efecto en el
aprendizaje de la evitación pasiva. Aunque no se ha realizado ningún
estudio de reproducción de dos generaciones con hidrato de cloral, los
datos sobre el rendimiento reproductivo y sobre sus efectos en el
esperma y los oocitos no indican que haya probabilidad de que la
toxicidad reproductiva sea un efecto crítico. Además, en estudios
subcrónicos o crónicos no se observaron efectos histopatológicos en
los órganos reproductores de roedores. En todos los estudios
realizados con animales de laboratorio se detectaron efectos en la
salud distintos del cáncer con una exposición muy superior a la eficaz
para la sedación humana.
No hay datos de carcinogenicidad en el ser humano. En dos
biovaloraciones en ratas no se observó un aumento de tumores en
ninguna parte. En tres biovaloraciones separadas en ratones machos se
detectó un aumento de la incidencia de tumores hepáticos. El más
definitivo de estos estudios demostró una mayor incidencia y
multiplicidad de tumores hepáticos en cada una de las tres
exposiciones. Estos datos parecen indicar la existencia de
carcinogenicidad en ratones machos, pero no se consideran adecuados
para realizar una evaluación del riesgo en la salud humana con una
respuesta lineal para una exposición baja.1
Hay una amplia base de datos sobre la toxicidad genética.
Diversos resultados ponen de manifiesto que el hidrato de cloral tiene
una actividad mutagénica de los genes y clastogénica débil. El hidrato
de cloral induce aneuploidía en una gran variedad de tipos de células.
Se considera que estos últimos efectos se deben a una perturbación del
huso acromático. Se necesitan concentraciones altas de hidrato de
cloral para provocar efectos observables. Aunque estos datos parecen
indicar que la genotoxicidad puede desempeñar una función en la
toxicidad del hidrato de cloral, también ponen de manifiesto que estos
efectos requieren concentraciones que no es probable que se alcancen
en condiciones fisiológicas con las exposiciones que se producen
normalmente a partir del medio ambiente. Algunos mecanismos probables
para la inducción de tumores hepáticos en ratones machos son la
formación de aductos de ADN mediante radicales libres generados en el
metabolismo del hidrato de cloral en el citocromo P450 2E1 (CYP2E1) y
la citotoxicidad que da lugar a una hiperplasia compensatoria.
Se estimó una ingesta tolerable para los efectos en la salud
distintos del cáncer de 0,1 mg/kg de peso corporal al día a partir de
la concentración más baja con efectos adversos observados (LOAEL) para
la sedación en las personas de 10,7 mg/kg, utilizando un factor de
incertidumbre total de 100.
Sólo se dispone de datos limitados sobre los efectos en el medio
ambiente. Los organismos metanotróficos pueden convertir el hidrato de
cloral en tricloroetanol y ácido tricloroacético. El hidrato de cloral
experimenta asimismo degradación abiótica en algunas condiciones. Hay
datos limitados sobre la inhibición del crecimiento de bacterias,
algas y protozoos y sobre los efectos en el desarrollo de los erizos
de mar. No hay datos disponibles suficientes que permitan evaluar el
riesgo para el medio ambiente derivado del hidrato de cloral.
1 En una biovaloración de la carcinogenicidad en ratones del
Programa Nacional de Toxicología, disponible después de la reunión
de la Junta de Evaluación Final, los machos presentaban una mayor
incidencia de tumores hepáticos y las hembras un pequeño aumento
de la incidencia de adenomas hipofisiarios, en el límite de la
significación estadística.
 1. EXECUTIVE SUMMARY
This CICAD on chloral hydrate was prepared by the US
Environmental Protection Agency (EPA) and is based on the US EPA's
Toxicological review on chloral hydrate (US EPA, 2000). Scientific
literature identified as of March 1999 was included. Information on
the nature of the review processes and the availability of the source
document is presented in Appendix 3. Information on the peer review of
this CICAD is presented in Appendix 4. This CICAD was approved as an
international assessment at a meeting of the Final Review Board, held
in Sydney, Australia, on 21-24 November 1999. Participants at the
Final Review Board meeting are listed in Appendix 5. The International
Chemical Safety Card (ICSC 0234) for chloral hydrate, produced by the
International Programme on Chemical Safety, has been reproduced in
Appendix 6 (IPCS, 1993).
Chloral hydrate (CAS No.
1. EXECUTIVE SUMMARY
This CICAD on chloral hydrate was prepared by the US
Environmental Protection Agency (EPA) and is based on the US EPA's
Toxicological review on chloral hydrate (US EPA, 2000). Scientific
literature identified as of March 1999 was included. Information on
the nature of the review processes and the availability of the source
document is presented in Appendix 3. Information on the peer review of
this CICAD is presented in Appendix 4. This CICAD was approved as an
international assessment at a meeting of the Final Review Board, held
in Sydney, Australia, on 21-24 November 1999. Participants at the
Final Review Board meeting are listed in Appendix 5. The International
Chemical Safety Card (ICSC 0234) for chloral hydrate, produced by the
International Programme on Chemical Safety, has been reproduced in
Appendix 6 (IPCS, 1993).
Chloral hydrate (CAS No.  Although earlier reports claimed the detection of substantial
quantities of dichloroacetic acid in blood in studies with rodents
(Abbas et al., 1996), data show that the dichloroacetic acid is most
likely formed by an acid-catalysed dechlorination of trichloroacetic
acid in the presence of reduced haemoglobin (Ketcha et al., 1996).
Recent experimental data and pharmacokinetic model simulations in
rodents suggest that dichloroacetic acid occurs only as a short-lived
metabolite in the liver and is rapidly converted to two-carbon,
non-chlorinated metabolites and carbon dioxide, with the chlorine
atoms entering the chloride pool (Merdink et al., 1998). Using a
different extraction procedure less likely to induce the artefactual
formation of dichloroacetic acid, Henderson et al. (1997) showed the
presence of dichloroacetic acid in children treated with chloral
hydrate in a clinic.
Breimer (1977) administered an aqueous solution of chloral
hydrate to five human volunteers. Each volunteer received a single
oral dose of 15 mg/kg body weight. Chloral hydrate could not be
detected in the plasma even at the first sampling time of 10 min.
A method with a limit of detection of 0.5 mg/litre was used.
Trichloroethanol and trichloroethanol glucuronide reached peak
concentrations 20-60 min after administration of chloral hydrate. The
maximum concentration of trichloroethanol in the plasma was about
5 mg/litre. The average half-lives of trichloroethanol and
trichloroethanol glucuronide were 8 h (range 7-9.5 h) and 6.7 h (range
6-8 h), respectively. The half-life of trichloroacetic acid was about
4 days. Zimmermann et al. (1998) administered a single dose of 250 mg
chloral hydrate in drinking-water to 18 healthy male volunteers
(20-28 years of age). Chloral hydrate, trichloroethanol, and
trichloroacetic acid were measured in plasma. Chloral hydrate could be
detected 8-60 min after dosing in only some of the plasma samples.
The measured concentration of chloral hydrate was not reported, but
the limit of detection was stated as 0.1 mg/litre. The maximum plasma
concentration of trichloroethanol of 3 mg/litre was achieved 0.67 h
after dosing, and the maximum plasma concentration of trichloroacetic
acid of 8 mg/litre was achieved 32 h after dosing. The terminal
half-life was 9.3-10.2 h for trichloroethanol and 89-94 h for
trichloroacetic acid.
Two toxicokinetic models are available for chloral hydrate in
rats and mice (Abbas et al., 1996; Beland et al., 1998). Beland et al.
(1998) treated rats and mice with chloral hydrate by gavage with 1 or
12 doses using 50 or 200 mg/kg body weight per dose. The maximum
levels of chloral hydrate, trichloroethanol, and trichloroethanol
glucuronide in the plasma were observed at the initial sampling time
of 0.25 h. The half-life of chloral hydrate in the plasma was
approximately 3 min. The half-lives of trichloroethanol and
trichloroethanol glucuronide in the plasma were approximately 5 and
7 min, respectively. Trichloroacetic acid was the major metabolite
found in the plasma, with the maximum level being reached 1-6 h after
dosing. The half-life of trichloroacetic acid in the plasma was
approximately 8-11 h. Comparable values were obtained for rats.
Estimates of the concentrations of trichloroacetic acid and
trichloroethanol at steady state under various exposure conditions are
in Appendix 1.
Several studies have investigated the age dependence of the
metabolism of chloral hydrate (Reimche et al., 1989; Gorecki et al.,
1990; Hindmarsh et al., 1991; Mayers et al., 1991). These studies were
conducted in critically ill patients in neonatal and paediatric
intensive care units and may not be representative of a population of
healthy infants. The half-lives for trichloroethanol and its
glucuronide were increased several-fold in pre-term and full-term
infants compared with toddlers and adults. The half-lives for
trichloroethanol in toddlers and adults were similar. These
age-related differences likely are the result of the immaturity of
hepatic metabolism, particularly glucuronidation, and decreased
glomerular filtration.
Kaplan et al. (1967) investigated the effect of ethanol
consumption on the metabolism of chloral hydrate in adults. Subjects
ingested doses of ethanol (880 mg/kg body weight), chloral hydrate
(9-14 mg/kg body weight), or both. In subjects consuming both ethanol
and chloral hydrate, blood trichloroethanol levels rose more rapidly
and reached higher values than in subjects consuming chloral hydrate
only. Ethanol promotes the formation of trichloroethanol because the
oxidation of ethanol provides NADH used for the reduction of chloral
hydrate (Watanabe et al., 1998).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
Sanders et al. (1982) studied the acute toxicity of chloral
hydrate in CD-1 mice. Groups of eight male and eight female mice were
given chloral hydrate by gavage in distilled water at 300, 600, 900,
1200, 1500, or 1800 mg/kg body weight. No deaths occurred at 900 mg/kg
body weight or below in either sex. The calculated LD50 for females
was 1265 mg/kg body weight and for males was 1442 mg/kg body weight.
Effects were seen within 10 min of dosing. The mice became sedated at
300 mg/kg body weight. At 600 and 900 mg/kg body weight, the animals
became lethargic and exhibited loss of righting reflex. Respiration
was markedly inhibited at 1200, 1500, and 1800 mg/kg body weight.
Inhibition of respiration appeared to be the immediate cause of death.
Most deaths occurred within 4 h at 1800 mg/kg body weight. At 1200 and
1500 mg/kg body weight, some deaths occurred after 4 h, with all
deaths occurring within 24 h.
Goldenthal (1971) reported an oral LD50 in rats of 480 mg/kg
body weight.
8.1.2 Inhalation
Odum et al. (1992) exposed four female CD-1 mice to chloral for
6 h at a concentration of 100 ppm (603 mg/m3). This exposure induced
deep anaesthesia. The mice recovered normally after the exposure
stopped. The effects in the lung included vacuolization of clara
cells, alveolar necrosis, desquamination of the epithelium, and
alveolar oedema. The lung to body weight ratio increased 1.5-fold,
most likely due to the alveolar oedema.
8.2 Irritation and sensitization
There are no studies of irritation or sensitization in laboratory
animals.
8.3 Short-term exposure
Sanders et al. (1982) studied the short-term toxicity of chloral
hydrate in mice. Groups of male CD-1 mice were given chloral hydrate
by gavage in distilled water at 14.4 or 144 mg/kg body weight per day
for 14 days. No significant effect on body weight was observed. No
changes in internal organs were noted from a gross examination. Groups
of 11-12 mice were evaluated for several toxicological parameters. No
significant effects on haematological or serum biochemical parameters
were noted. There was a statistically significant (P < 0.05)
increase in liver weight (17%) and a decrease in spleen weight (27%)
at the high exposure. The no-observed-adverse-effect level (NOAEL) in
this study is 14.4 mg/kg body weight per day; the LOAEL is 144 mg/kg
body weight per day. The increase in liver weight, but not the
decrease in spleen weight, was confirmed in a subsequent 90-day study
by the same researchers.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Sanders et al. (1982) administered chloral hydrate in
drinking-water to CD-1 mice at 70 or 700 mg/litre (equivalent to 16 or
160 mg/kg body weight per day) for 90 days. In males, hepatomegaly (an
increase in weight of 20% and 34% at the low and high exposure,
respectively) and microsome proliferation (no increase in total
microsomal protein, increase in cytochrome b5 of 26% and 40%,
increase in aminopyrine N-demethylase of 28% and 20%, and increase
in aniline hydroxylase of 24% and 30% at the low and high exposures,
respectively, when reported as mg of protein per mg of total liver
protein) were observed. There were no biologically significant changes
in serum enzymes. Hepatomegaly was not seen in females, but there were
changes in hepatic microsomal parameters (increase in total microsomal
protein of 10%, increase in aniline hydroxylase of 23%, and decrease
in cytochrome b5 of 12% when reported as mg of protein per mg of
total liver protein), but only at the high exposure. No other
significant toxicological changes were observed. Based on hepatomegaly
and changes in microsomal parameters in males at the high exposure,
this study identifies a LOAEL of 160 mg/kg body weight per day and a
NOAEL of 16 mg/kg body weight per day.
Daniel et al. (1992b) exposed male and female Sprague-Dawley rats
(10 per sex per dose) for 90 days to chloral hydrate in drinking-water
at a concentration of 300, 600, 1200, or 2400 mg/litre (equivalent to
an exposure of 24, 48, 96, or 168 mg/kg body weight per day in males
and 33, 72, 132, or 288 mg/kg body weight per day in females). The
tissues of animals from the high-exposure group and liver sections
from all treated males were examined histopathologically. No mortality
occurred in any groups prior to sacrifice. Organ weights, including
liver weight, and clinical chemistry values in treated animals were
only sporadically or inconsistently different from control animal
values. Focal hepatocellular necrosis was observed in 2 of 10 males in
each of the groups exposed to 96 and 168 mg/kg body weight per day.
The necrotic lesion was minimal at 96 mg/kg body weight per day and
was significantly more severe at 168 mg/kg body weight per day.
Necrotic lesions were not reported in any treated females or in any
control animals. While serum enzymes were generally increased in
treated animals, dramatic increases were reported in males in the
168 mg/kg body weight per day group; mean aspartate aminotransferase,
alanine aminotransferase, and lactate dehydrogenase levels in this
group were elevated 89%, 54%, and 127% above the corresponding control
values, respectively.
8.4.2 Chronic exposure and carcinogenicity
Rijhsinghani et al. (1986) evaluated carcinogenic effects in male
mice (C57BL × C3HF1). Groups of 15-day-old mice received chloral
hydrate by gavage in distilled water at 0, 5, or 10 mg/kg body weight
(26, 15, and 14 mice per group, respectively). Animals were sacrificed
when moribund or at week 78, at week 88, or between weeks 89 and 92.
Livers were examined histopathologically using light and electron
microscopy. In mice sacrificed 48-92 weeks after treatment, the
incidence of hepatic nodules (adenomas or trabecular carcinomas) was
3/9 and 6/8 for animals from the 5 and 10 mg/kg body weight per day
dose groups, respectively, compared with 2/19 in controls. The
increase in tumours was statistically significant (P < 0.05) only
in the 10 mg/kg body weight group.1
Daniel et al. (1992a) exposed 40 male B6C3F1 mice for 104 weeks
to drinking-water containing chloral hydrate at 1 g/litre (equivalent
to 166 mg/kg body weight per day). Untreated control animals (23 in
one group and 10 in a second group) received distilled water. Interim
sacrifices were conducted at 30 and 60 weeks of exposure (five animals
per group at each sacrifice interval). Complete necropsy and
microscopic examination were performed. There were no significant
treatment-related effects on survival or body weight. There were no
changes in spleen, kidney, or testis weights or histopathological
changes in any tissue except the liver. The toxicity in the liver was
characterized by increased absolute liver weight and liver to body
weight ratio at all three sacrifice intervals. At week 104, liver
weight was 37% higher than in controls, and liver to body weight ratio
was 42% higher than in controls. Hepatocellular necrosis was noted in
10/24 (42%) treated animals; other pathological changes of mild
severity reported in the livers of treated animals included
cytoplasmic vacuolization, cytomegaly, and cytoplasmic alteration. The
prevalence of liver tumours at terminal sacrifice was statistically
significantly (P < 0.05) increased over controls, with
hepatocellular carcinomas in 11/24 and hepatocellular adenomas in
7/24 animals; for carcinomas and adenomas combined, the prevalence was
17/24. In control animals, carcinomas, adenomas, and carcinomas and
adenomas (combined) occurred in 2/20, 1/20, and 3/20, respectively.
At the 60-week sacrifice, there were 2/5 treated animals with
hepatocellular carcinomas, compared with 0/5 controls. No carcinomas,
adenomas, or hyperplastic nodules were reported in animals sacrificed
at week 30.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity bioassay became available. In this study, an
up to 5 times higher single dose of chloral hydrate than that used
in the Rijhsinghani et al. (1986) study administered to male or
female B6C3F1 mice failed to induce tumours in any organ
(NTP, 2000a).
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male B6C3F1 mice. Mice were administered chloral
hydrate in drinking-water for 104 weeks. Mice (72 in each group) had a
mean exposure of 0, 13.5, 65, or 146.6 mg/kg body weight per day.
There was no change in water consumption, survival, behaviour, body
weight, or organ weights at any exposure. There was no evidence of
hepatocellular necrosis at any exposure and only minimal changes in
the levels of serum enzymes. This study identifies a NOAEL for
non-cancer effects in mice of 146.6 mg/kg body weight per day (the
highest exposure tested). There was no increase in the prevalence of
neoplasia at sites other than the liver. Although the background
response in this study is higher than normal for this strain of mice,
the mice showed an increase in proliferative lesions in the liver
(hyperplasia, adenoma, carcinoma, and combined adenoma and carcinoma)
at all exposures. These data are summarized in Table 1. The calculated
effective dose for a 10% tumour incidence (ED10) is 1.98 mg/kg body
weight per day, and its 95% lower confidence limit (LED10) is
1.09 mg/kg body weight per day (see Appendix 2).
Leuschner & Beuscher (1998) conducted a chronic bioassay for
carcinogenicity in Sprague-Dawley rats. Chloral hydrate was
administered in drinking-water for 124 weeks (males) and 128 weeks
(females). The rats (50 males and 50 females in each group) had an
exposure of 15, 45, or 135 mg/kg body weight per day. There was no
effect on survival, appearance, behaviour, body weight, food and water
consumption, or organ weights. There was no evidence of an increased
incidence of tumours in any organ. Histopathological examination
revealed an increased incidence of hepatocellular hypertrophy at the
highest exposure in males only (11% in controls versus 28% at the
highest exposure; P < 0.01). This finding, graded as minimal to
slight in severity, was characterized by a diffuse liver cell
enlargement with slightly eosinophilic cytoplasm and was considered by
the authors as a first sign of toxicity. The type, incidence, and
severity of other non-neoplastic lesions were not increased in treated
animals compared with controls. Based on the evidence of minimal
toxicity in the liver, which is of doubtful biological significance,
this study establishes a NOAEL of 45 mg/kg body weight per day and a
LOAEL of 135 mg/kg body weight per day.
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male F344 rats. Rats were administered chloral
hydrate in drinking-water for 104 weeks. Rats (78 in each group) had a
mean daily exposure of 0, 7.4, 37.4, or 162.6 mg/kg body weight per
day. There was no change in water consumption, survival, behaviour,
body weight, or organ weights at any exposure. There was no indication
of liver toxicity at any exposure as shown by the lack of liver
necrosis, lack of hyperplasia, no increase in mitotic index, and only
minimal changes in the levels of serum enzymes. There was no increase
at any exposure in the prevalence or multiplicity of hepatocellular
neoplasia or neoplasia at any other site. This study identifies a
NOAEL of 162.6 mg/kg body weight per day (the highest exposure
tested).1
Two of the metabolites of chloral hydrate, trichloroacetic acid
and dichloroacetic acid, have been shown to cause liver tumours in
rodents. For example, trichloroacetic acid in drinking-water induced
liver tumours in male and female mice when the exposure exceeded 200
mg/kg body weight per day (Herren-Freund et al., 1987; Bull et al.,
1990; Pereira, 1996). There was no evidence of increased
carcinogenicity, however, when male rats were exposed to
trichloroacetic acid at 360 mg/kg body weight per day (DeAngelo et
al., 1997). Dichloroacetic acid in drinking-water induced liver
tumours in male and female mice when the exposure exceeded 160 mg/kg
body weight per day (Herren-Freund et al., 1987; Bull et al., 1990;
DeAngelo et al., 1991; Daniel et al., 1992a; Ferreira-Gonzalez et al.,
1995; Pereira, 1996). Dichloroacetic acid also induced liver tumours
in male rats when the exposure exceeded 40 mg/kg body weight per day
(Richmond et al., 1995; DeAngelo et al., 1996).
A number of studies have shown that trichloroethylene is toxic to
the mouse lung bronchiolar epithelium, causing a highly specific
lesion to the clara cells of mice. Short-term exposure causes
vacuolization of the clara cells; long-term exposure causes pulmonary
adenomas and adenocarcinomas (Odum et al., 1992; Green et al., 1997).
These effects are thought to be due to the accumulation of chloral
within the clara cells. Trichloroethylene is efficiently metabolized
to chloral, but the major pathway from chloral to trichloroethanol and
its glucuronide is blocked, leading to an accumulation of chloral and
the observed toxicity.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity assay became available. In this study,
lifetime gavage administration of chloral hydrate at similar dose
levels induced hepatocellular tumours in male B6C3F1 mice and a low
frequency of pituitary hyperplasia and adenomas in females that was
of borderline statistical significance (NTP, 2000b).
Table 1: Prevalence and multiplicity of hepatocellular proliferative lesions in mice at 104 weeks.a
Treatment group Number examinedc Hyperplasia Adenoma Carcinoma Adenoma
(mg/kg body + carcinoma
weight per day)b
0 42 7.1d 21.4d 54.8d 64.3d
0.07 ± 0.04e 0.21 ± 0.06e 0.74 ± 0.12e 0.95 ± 0.12e
13.5 46 32.6f 43.5f 54.3 78.6f
0.41 ± 0.10f 0.65 ± 0.12f 0.72 ± 0.11 1.37 ± 0.16f
65 39 33.3f 51.3f 59.0 79.5f
0.38 ± 0.09f 0.95 ± 0.18f 1.03 ± 0.19 1.97 ± 0.23f
146.6 32 37.5f 50.0f 84.4f 90.6f
0.41 ± 0.10f 0.72 ± 0.15f 1.31 ± 0.17f 2.03 ± 0.25f
a From George et al. (2000).
b Time-weighted mean daily dose.
c Animals surviving longer than 78 weeks.
d Prevalence (percentage of animals with at least one lesion).
e Multiplicity (number of lesions per animal ± SEM).
f Statistically different from the control value, P < 0.05.
8.5 Genotoxicity and related end-points
8.5.1 Genotoxicity
There is an extensive database on the genotoxicity of chloral
hydrate and its metabolites. A complete summary of these results is
provided in US EPA (2000).
Chloral hydrate did not induce mutation in most strains of
Salmonella typhimurium, but did in some studies with S. typhimurium
TA100 and in a single study with S. typhimurium TA104. The latter
response was inhibited by free-radical scavengers alpha-tocopherol and
menadione (Ni et al., 1994).
Chloral hydrate did not induce mitotic crossing-over in
Aspergillus nidulans in the absence of metabolic activation. Chloral
hydrate caused weak induction of meiotic recombination in the presence
of metabolic activation and gene conversion in the absence of
metabolic activation in Saccharomyces cerevisiae. It did not induce
reverse mutation in S. cerevisiae. Chloral hydrate clearly induced
aneuploidy in various fungi in the absence of metabolic activation.
Chloral hydrate induced somatic and germ cell mutations in
Drosophila melanogaster.
Chloral hydrate did not produce DNA-protein cross-links in rat
liver nuclei, DNA single-strand breaks/alkaline-labile sites in
primary hepatocytes in vitro, or DNA repair in Escherichia coli.
One study showed induction of single-strand breaks in liver DNA of
both rats and mice treated in vivo; another study in both species
using higher concentrations of chloral hydrate found no such effect.
Chloral hydrate was weakly mutagenic, but did not induce
micronuclei in mouse lymphoma cells in vitro. Chloral hydrate
increased the frequency of micronuclei in Chinese hamster cell lines.
Although a single study suggested that chloral hydrate induces
chromosomal aberrations in Chinese hamster CHED cells in vitro, the
micronuclei produced probably contained whole chromosomes and not
chromosome fragments, as the micronuclei could all be labelled with
antikinetochore antibodies.
In kangaroo rat kidney epithelial cells, chloral hydrate
inhibited spindle elongation and broke down mitotic microtubuli,
although it did not inhibit pole-to-pole movement of chromosomes. It
produced multipolar spindles, chromosomal dislocation from the mitotic
spindle, and a total lack of mitotic spindles in Chinese hamster
DON:Wg.3h cells.
Chloral hydrate weakly induced sister chromatid exchange in
cultures of human lymphocytes. It induced micronuclei, aneuploidy,
C-mitosis, and polyploidy in human lymphocytes in vitro. Micronuclei
were induced in studies with human whole blood cultures but not in one
study with isolated lymphocytes. The differences seen in the
micronucleus test have been attributed to differences between whole
blood and purified lymphocyte cultures (Vian et al., 1995), but this
hypothesis has not been tested.
Chloral hydrate increased the frequency of chromosomal
aberrations in mouse bone marrow, spermatogonia, and primary and
secondary spermatocytes, but not in oocytes, after in vivo
treatment. Chloral hydrate induced chromosomal aberrations in mouse
bone marrow erythrocytes after treatment in vivo. In one of these
studies, the use of antikinetochore antibodies suggested induction of
micronuclei containing both whole chromosomes and fragments. Chloral
hydrate induced micronuclei in the spermatids of mice treated in vivo
in some studies. Chloral hydrate induced aneuploidy in the bone
marrow of mice treated in vivo. It increased the rate of aneuploidy
in mouse secondary spermatocytes. It did not produce polyploidy in
bone marrow, oocytes, or gonosomal or autosomal univalents in primary
spermatocytes of mice treated in vivo. Chloral hydrate, however,
induced polyploidy and meiotic delay when a synchronized population of
mouse oocytes was exposed in vitro prior to the resumption of
maturation.
Trichloroethanol, a reduction product of chloral hydrate, did not
induce lambda prophage in E. coli or mutation in S. typhimurium
TA100. Trichloroethanol caused spindle aberrations when mouse oocytes
were treated in vitro.
Trichloroacetic acid did not induce lambda prophage in E. coli
and was not mutagenic to S. typhimurium in the presence or absence
of metabolic activation. Trichloroacetic acid was weakly positive in
the mouse lymphoma assay with metabolic activation. Trichloroacetic
acid also did not induce chromosomal damage in human lymphocytes or
micronuclei in bone marrow in vitro. It is unclear whether
trichloroacetic acid can induce chromosomal damage in vivo, because
some studies have been positive and others negative.
Dichloroacetic acid did not induce differential toxicity in DNA
repair-deficient strains of S. typhimurium but did induce lambda
prophage in E. coli. Dichloroacetic acid gave equivocal results for
gene mutation in S. typhimurium TA100 and TA98. Dichloroacetic acid
was weakly mutagenic in the in vitro mouse lymphoma assay and
induced chromosomal aberrations but not micronuclei or aneuploidy in
that test system. Dichloroacetic acid induced micronuclei in mouse
polychromatic erythrocytes in vivo and mutations at the lacI locus
in the transgenic B6C3F1 mouse (the Big Blue Mouse) in vivo at an
exposure that induces liver tumours in male mice. It is unclear
whether dichloroacetic acid can induce primary DNA damage, as some
assays are positive and others negative.
8.5.2 Cell proliferation
Rijhsinghani et al. (1986) evaluated the acute effects of chloral
hydrate on liver cell proliferation in 15-day-old male mice (C57BL ×
C3HF1). Mice were given 0, 5, or 10 mg chloral hydrate/kg body
weight by gavage in distilled water (9, 10, and 6 mice per group,
respectively) and sacrificed after 24 h. Cell proliferation was
evaluated by calculating the mitotic index (number of mitoses per 100
nuclei) from liver sections. The mitotic index in liver cells was
significantly increased (0.9235) in mice receiving 5 mg/kg body weight
when compared with the control value (0.3382), and elevated (0.7433)
(although not statistically significantly) in mice receiving 10 mg/kg
body weight. Hepatic necrosis was not observed in mice from either
treatment group at autopsy.
As part of the chronic bioassay for carcinogenicity, George et
al. (2000) evaluated hepatocyte proliferation in male F344 rats and
male B6C3F1 mice. Exposures are given in section 8.4.2. Five days
prior to sacrifice at 13, 26, 52, or 72 weeks in rats and 26, 52, or
78 weeks in mice, animals were given bromodeoxyuridine. Labelled
nuclei were identified by chromogen pigment over the nuclei, and the
labelling index was calculated. Outside of the areas with tumours in
the liver of mice, there was no significant evidence of increased
hepatocyte proliferation in rats or mice.
8.5.3 Oncogene activation
Velazquez (1994) investigated the induction of H- ras
proto-oncogene mutations in mice. DNA from normal liver and tumour
tissue was obtained from male B6C3F1 mice administered 1 g chloral
hydrate/litre (166 mg/kg body weight per day) in drinking-water for
2 years. H- ras mutations were present in one out of seven (14%)
tumours. The spectrum of mutations was the same as that of spontaneous
liver tumours. Based on these data, it is unlikely that H- ras
activation is a mechanism of carcinogenicity relevant to chloral
hydrate.
8.5.4 Free radicals and DNA adduct formation
Ni et al. (1994, 1995, 1996) studied the metabolism of chloral
hydrate in an in vitro system using microsomes from male B6C3F1
mice. The metabolism of chloral hydrate generated free radicals as
detected by electron spin resonance spectroscopy and caused endogenous
lipid peroxidation, resulting in the production of malondialdehyde,
formaldehyde, and acetaldehyde, all of which are known to produce
liver tumours in rodents. Trichloroacetic acid and trichloroethanol
also produced free radicals and induced lipid peroxidation when tested
in this system. The authors speculated that the free radicals were
Cl3CCO2Ê. and/or Cl3CÊ. Incubation of chloral hydrate,
trichloroethanol, or trichloroacetic acid in the presence of
microsomes and calf thymus DNA resulted in the formation of a
malondialdehyde-modified DNA adduct. This research group further
showed that chloral hydrate induced an increase in mutations at the
hprt and tk loci in transgenic human lymphoblastoid cells
containing CYP2E1. In contrast, when the parental cell line lacking
CYP2E1 was treated with the same concentration of chloral hydrate, no
mutations were found at either locus. These data implicate CYP2E1 as
the primary cytochrome subfamily involved in the metabolism of chloral
hydrate to reactive intermediates.
8.5.5 Cell communication
The effects of 1-, 4-, 6-, 24-, 48-, and 168-h exposures to
chloral hydrate (0, 1, 5, or 10 mmol/litre) on gap junction
intercellular communication in Clone 9 cell cultures (normal rat
hepatocytes) were reported by Benane et al. (1996). No differences in
intercellular communication were seen between the groups treated with
1 mmol/litre at 1, 4, and 6 h of exposure and controls, as measured by
a dye transfer protocol. There were significant differences between
all other groups and the controls. The shortest exposure time and
lowest exposure concentration that reduced dye transfer significantly
were in the group treated with 1 mmol/litre for 24 h.
8.5.6 Peroxisome proliferation
As part of the chronic bioassay for carcinogenicity in mice,
George et al. (2000) found no evidence of peroxisome proliferation
using cyanide-insensitive palmitoyl CoA oxidase in the livers of male
mice treated with chloral hydrate for 26 weeks.
8.6 Reproductive and developmental toxicity
Klinefelter et al. (1995) evaluated effects on sperm morphology
and motility in F344 rats administered chloral hydrate in
drinking-water for 52 weeks at levels of 0, 55, or 188 mg/kg body
weight per day. The researchers examined cauda epididymal sperm motion
parameters and testicular and epididymal histopathology. Chloral
hydrate did not cause any visible systemic toxicity and had no effects
on epididymal or testicular histopathology. However, the percentage of
motile sperm was significantly decreased (P < 0.01) from 68% in
controls to 58% in rats exposed to 188 mg/kg body weight per day. The
percentage of progressively motile sperm was also significantly
decreased (P < 0.01) from 63% in controls to 53% in this group. In
addition, the frequency distribution of the average straight-line
velocities of sperm at this exposure was significantly shifted
(P < 0.01) to the lower ranges when compared with controls. In this
study, the NOAEL is 55 mg/kg body weight per day; the LOAEL is 188
mg/kg body weight per day.
Kallman et al. (1984) exposed male and female CD-1 mice to
chloral hydrate in drinking-water at 21.3 or 204.8 mg/kg body weight
per day. Animals were exposed for 3 weeks prior to breeding. Exposure
of females (5 per group) continued during gestation and until pups
were weaned at 21 days of age. No gross malformations were noted, and
no significant effects were observed in duration of gestation, number
of pups delivered, pup weight, or number of stillborn pups. All pups
(15 per group) showed the same rate of development and level of
performance on several neurobehavioural tests, except that pups
exposed to 204.8 mg/kg body weight per day when tested at 23 days of
age showed impaired retention of passive avoidance learning on both
the 1-h and 24-h retention tests (P < 0.05). This study identified
a NOAEL for neurodevelopmental toxicity of 21.3 mg/kg body weight per
day and a LOAEL of 204.8 mg/kg body weight per day based on the
impairment in passive avoidance learning. This study also identifies a
NOAEL for reproductive and other developmental effects of 204.8 mg/kg
body weight per day (the highest exposure tested).
Johnson et al. (1998) tested the potential for chloral hydrate to
cause developmental toxicity in Sprague-Dawley rats. Chloral hydrate
was administered in drinking-water to 20 rats from gestational day 1
to gestational day 22 at an average exposure of 151 mg/kg body weight
per day. Control animals were given distilled water. There was no
evidence of maternal toxicity, no change in the number of implantation
or resorption sites, no change in the number of live or dead fetuses,
no change in placental or fetal weight, no change in crown-rump
length, and no increase in the incidence of morphological changes. A
detailed examination found no evidence of cardiac anomalies. Based on
this study, the NOAEL for developmental toxicity is 151 mg/kg body
weight per day (the highest exposure tested).
Johnson et al. (1998) also tested the potential for
trichloroethanol and trichloroacetic acid to cause developmental
toxicity in Sprague-Dawley rats. The protocol was identical to the
study with chloral hydrate. Trichloroethanol was administered to 10
rats at an average exposure of 153 mg/kg body weight per day. No
evidence of developmental toxicity was found. In contrast, when
trichloroacetic acid was administered to 11 rats at an average
exposure of 291 mg/kg body weight per day, developmental toxicity was
observed. The effects included statistically significant (P < 0.05)
increases in average resorptions, in average implantations, and in
cardiac anomalies. Although the specific cardiac anomalies found were
different, the results with trichloroacetic acid are generally
consistent with those reported by Smith et al. (1989), who observed
adverse developmental effects from trichloroacetic acid at an exposure
of 330 mg/kg body weight per day and above.
Saillenfait et al. (1995) tested the potential of chloral hydrate
to cause developmental toxicity using a rat whole-embryo culture
system. Embryos (20 per dose) from Sprague-Dawley rats were explanted
on gestational day 10 and exposed to chloral hydrate at a
concentration of 0, 0.5, 1, 1.5, 2, or 2.5 mmol/litre (equivalent to
0, 83, 165, 248, 331, or 414 mg/litre) for 46 h. At 2.5 mmol/litre,
all embryos died. No lethality was seen at lower exposures. Chloral
hydrate caused concentration-dependent decreases in growth and
differentiation and increases in the incidence of morphologically
abnormal embryos. No effects were observed in any parameter at
0.5 mmol/litre. Decreases in crown-rump length, somite
(embryonic segment) number, and the protein or DNA content of embryos
were seen at 1 mmol/litre and above. At 1, 1.5, and 2 mmol chloral
hydrate/litre, respectively, 18%, 68%, and 100% of embryos were
malformed. Brain, eye, and ear malformations were the most prominent
effects at these concentrations. Abnormalities in the trunk and
pericardial dilation also occurred at 2 mmol/litre. In this in vitro
test system, chloral hydrate was a slightly more potent teratogen than
trichloroacetic acid or dichloroacetic acid.
Although chloral hydrate did not cause meiotic delay in the
oocytes of adult mice when administered at the time of resumption of
maturation induced by hormones (Mailhes & Marchetti, 1994), it did
cause adverse effects in vitro when a synchronized population of
oocytes was exposed prior to resumption of maturation
(Eichenlaub-Ritter & Betzendahl, 1995; Eichenlaub-Ritter et al.,
1996). In this test system, chloral hydrate induced lagging of
chromosomes during telophase I, inhibited spindle elongation in
anaphase B, and caused chromosome displacement from the spindle
equator in metaphase I and II. Oocytes became irreversibly arrested in
maturation when exposed to chloral hydrate prior to resumption of
maturation or when chloral hydrate was present during the first or
second 8 h of maturation. Spindle aberrations were observed when
oocytes were treated with trichloroethanol (Eichenlaub-Ritter et al.,
1996).
8.7 Immunological and neurological effects
Kauffmann et al. (1982) administered chloral hydrate by gavage in
distilled water at 14.4 or 144 mg/kg body weight per day to groups of
11-12 male CD-1 mice for 14 days. No effects on humoral or
cell-mediated immunity were detected at either exposure.
Kauffmann et al. (1982) administered chloral hydrate to male and
female CD-1 mice in drinking-water at 70 or 700 mg/litre (equivalent
to 16 or 160 mg/kg body weight per day) for 90 days. Humoral immunity
was assessed by the number of splenic antibody-forming cells produced
against sheep red blood cells (12 mice in the control group and 8 mice
in the exposed groups) and haemagglutination titres (20-21 mice in the
control group and 13-16 mice in the exposed groups).
Cell-mediated immunity was assessed by delayed-type hypersensitivity
to sheep red blood cells (17-20 mice in the control group and
15-16 mice in the exposed groups). Lymphocyte response was assessed
using a T-cell mitogen (Con A) and a B-cell mitogen (LPS)
(17-22 animals in the control group and 13-16 mice in the exposed
groups). In males, no effects were detected in either humoral or
cell-mediated immunity at either exposure. No effects on cell-mediated
immunity were noted in females at either exposure. In females, both
exposures resulted in a statistically significant decrease
(P < 0.05) in humoral immune function (36% and 40% at the
low and high exposures, respectively) when expressed as
antibody-forming cells per spleen. The decrease, however, was
statistically significant only at the higher exposure when
expressed as antibody-forming cells per million spleen cells
(a 32% decrease). There was no effect on haemagglutination titres
or on spleen cell response to the B-cell mitogen at either exposure.
The decrease in antibody-forming cells per million spleen cells at
the higher exposure in female mice is regarded as an adverse
response in this study. Accordingly, the NOAEL for immunotoxicity is
16 mg/kg body weight per day; the LOAEL is 160 mg/kg body weight
per day.
Kallman et al. (1984) administered chloral hydrate by gavage in
distilled water at 50, 100, 200, 300, or 400 mg/kg body weight to
groups of 12 male CD-1 mice. All doses resulted in the rapid onset of
ataxia, with an ED50 (maximal effect seen in 50% of animals) of
84.2 mg/kg body weight at 5 min (the time of maximal effect). Animals
recovered within 2-3 h. No delayed changes in muscular coordination
were detectable when the mice were tested 24 h after treatment.
Kallman et al. (1984) evaluated behavioural toxicity in groups of
12 male CD-1 mice administered chloral hydrate by gavage in distilled
water at 14.4 or 144 mg/kg body weight per day for 14 days. When
measured 24-48 h after exposure was terminated, no significant effects
on body weight, motor activity, physical appearance, behaviour,
muscular coordination, or endurance were observed.
Kallman et al. (1984) exposed groups of 12 male CD-1 mice to
drinking-water containing chloral hydrate at a concentration of 70 or
700 mg/litre (equivalent to 16 or 160 mg/kg body weight per day) for
90 days. When measured 24 h after exposure was terminated, no
treatment-related effects on mortality, body weight, physical
appearance, behaviour, locomotor activity, learning in repetitive
tests of coordination, response to painful stimuli, strength,
endurance, or passive avoidance learning were observed. Both exposures
resulted in a decrease of about 1°C in mean body temperature
(P < 0.05). Because of the lack of increased effect with a 10-fold
increase in exposure and because hypothermia as a side-effect of
chloral hydrate or from an overdose of chloral hydrate has not been
reported in humans, the decrease in body temperature is not considered
an adverse effect. This study identifies a NOAEL for neurobehavioural
toxicity of 160 mg/kg body weight per day (the highest exposure
tested).
A condensation product of tryptamine and chloral hydrate,
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline (TaClo), has been
found in the blood of elderly patients administered chloral hydrate
for 2-7 days (Bringmann et al., 1999). This metabolite initiated a
slowly progressive neurodegeneration when administered to rats in a
subchronic study (Gerlach et al., 1998). There is, however, no
evidence of neurodegeneration in the chronic studies with chloral
hydrate in rats and mice.
9. EFFECTS ON HUMANS
Chloral hydrate has been widely used as a sedative and hypnotic
drug in humans. Trichloroethanol is responsible for the
pharmacological activity (Marshall & Owens, 1954; Breimer, 1977;
Goodman & Gilman, 1985). Exposure information is discussed in section
6.2.
Chloral hydrate is irritating to the skin and mucous membranes
and often causes gastric distress, nausea, and vomiting at recommended
doses. There are no reports of sensitization in humans. Overdoses
produce (in order of progression) ataxia, lethargy, deep coma,
respiratory depression, hypotension, and cardiac arrhythmias. The
life-threatening effects are from severe respiratory depression,
hypotension, and cardiac arrhythmias. For some representative case
reports, see Marshall (1977), Anyebuno & Rosenfeld (1991), Ludwigs et
al. (1996), and Sing et al. (1996). A potentially life-threatening
oral dose for humans is approximately 10 g (143 mg/kg body weight),
although death has been reported from as little as 4 g, and some
individuals have survived ingesting 30 g or more. Extended use of
chloral hydrate may result in development of tolerance to the
pharmacological effect and physical dependence on or addiction to
chloral hydrate.
Shapiro et al. (1969) reviewed the medical records of 1618
patients who had received chloral hydrate at 1 g (213 patients, 13%),
0.5 g (1345 patients, 83%), or various other doses (60 patients, 4%).
Adverse reactions were reported in 38 patients (2.3%). Of these
patients, 4 received 1 g, 1 received 0.75 g, and 33 received 0.5 g.
Reported adverse reactions included gastrointestinal symptoms in 10
patients, central nervous system (CNS) depression in 20 patients, skin
rash in 5 patients, prolonged prothrombin time in 1 patient, and
bradycardia in 1 patient. In all patients, the side-effects
disappeared when chloral hydrate therapy was stopped. There was no
evidence of association between adverse side-effects and age, weight,
or sex.
Miller & Greenblatt (1979) reviewed the medical records of 5435
hospital patients who received chloral hydrate at a dose of either
0.5 g (about 7-8 mg/kg body weight) or 1 g (about 14-16 mg/kg body
weight). Adverse reactions were noted in 119 cases (2.2%).
CNS depression was most common (58 patients, or 1.1%), with minor
sensitivity reactions, including rash, pruritus, fever, and
eosinophilia, second most common (19 patients, or 0.35%). Other
adverse reactions included gastrointestinal disturbances (0.28%) and
CNS excitement (0.22%). Three individuals (0.05%) were judged to have
life-threatening reactions involving CNS depression, asterixis
(involuntary jerking movements), or hypotension. The data show that
adverse reactions involving the CNS become more frequent with
increasing dosage in patients older than 50 years, in patients who
died during hospitalization, in patients who received concurrently
benzodiazepine anti-anxiety drugs, and in patients with elevated
levels of blood urea nitrogen.
Greenberg et al. (1991) reported various side-effects experienced
by children receiving chloral hydrate sedation in preparation for
computer tomography (CT) procedures. In a "high-dose" group, composed
of 295 children (average age 2.18 years) who received a single dose of
80-100 mg/kg body weight and a maximum total dose of 2 g, adverse
reactions occurred in 23 of the patients (7%) and included vomiting
(14 patients), hyperactivity (5 patients), and respiratory symptoms,
such as wheezing and secretion aspiration (4 patients). Cardiac
monitoring did not reveal any abnormalities or arrhythmias in any of
the children. A second "lower-dose" cohort of 111 children (average
age 1.9 years) received 40-75 mg chloral hydrate/kg body weight. These
patients received the lower dose because of existing liver or renal
impairment, respiratory insufficiency, or CNS depression. There were
no adverse side-effects or complications reported in this group.
Children with severe liver or renal disease or affected by severe
CNS depression were not treated with chloral hydrate.
Lambert et al. (1990) conducted a retrospective analysis of
hospital medical records to investigate a possible link between
chloral hydrate administration and direct hyperbilirubinaemia (DHB) in
neonates following prolonged administration of chloral hydrate
(25-50 mg/kg body weight for up to 20 days). Direct bilirubin is a
measure of the free, unconjugated bilirubin in the serum. In the first
study, the DHB was of unknown etiology in 10 of the 14 newborns with
DHB; all 10 of these DHB patients had received chloral hydrate. In the
second study, among 44 newborns who had received chloral hydrate,
10 patients who developed DHB had received a mean cumulative dose of
1035 mg/kg body weight. In contrast, 34 patients whose direct
bilirubin levels were within normal ranges received a mean cumulative
dose of 183 mg/kg body weight. The total bilirubin levels (free plus
conjugated) were the same in both groups and within the normal range.
Kaplan et al. (1967) investigated whether ethanol ingestion
increased the effects of chloral hydrate. Five male volunteers
weighing 70-107 kg consumed ethanol (880 mg/kg body weight), chloral
hydrate (1 g, 9-14 mg/kg body weight), or both. Blood pressure and
cardiac rate did not vary significantly among treatments. In the
presence of ethanol, the concentration of trichloroethanol in the
blood rose more rapidly and reached a higher value, but the rate of
depletion was not significantly changed. The increase in the
concentration of trichloroethanol was not sufficient to produce a
marked enhancement of the hypnotic effect. The volunteers reported
symptoms (drowsiness, dizziness, blurred vision) and their severity
during the 6-h observation period. At all time points, the rank order
of effects was ethanol plus chloral hydrate > ethanol > chloral
hydrate.
No long-term studies of chloral hydrate in humans were located.
Chloral hydrate is addictive and is a controlled substance (Schedule
IV) in the USA.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Some data are available from cell multiplication inhibition tests
(toxic thresholds) in bacteria, algae, and protozoa. These data are
summarized in Table 2.
Schatten & Chakrabarti (1998) showed that chloral hydrate at 0.1%
(only concentration tested) causes alteration of centrosomal material
and abnormal microtubule configurations in California sea urchins
(Strongylocentrotus purpuratus and Lytechinus pictus). Chakrabarti
et al. (1998) also showed that chloral hydrate at 4 mmol/litre (660
mg/litre, only concentration tested) induced ciliary loss in the early
embryo phase of Lytechinus pictus. Exposure in this study was for 30
h at the blastula stage (14 h after fertilization).
Table 2: Effects of chloral hydrate on bacteria, algae, and protozoa.
Test system Effect Reference
Bacteria 16-h EC3 at 1.6 mg/litre Bringmann & Kuehn,
(Pseudomonas putida) 1980a
Green alga 7-day EC3 at 2.8 mg/litre Bringmann & Kuehn,
(Scenedesmus 1980a
quadricaudata)
Blue-green alga 8-day EC3 at 78 mg/litre Bringmann & Kuehn,
(cyanobacterium) 1976
(Microcystis
aeruginosa)
Protozoan 72-h EC5 at 79 mg/litre Bringmann & Kuehn,
(Enterosiphon sulcatum) 1980a
Protozoan EC5 at 86 mg/litre Bringmann & Kuehn,
(Uronema parduczi) 1980b
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Chloral hydrate has been extensively used as a sedative and
hypnotic drug in human and veterinary medicine. The metabolite
trichloroethanol is responsible for the pharmacological effect.
Chloral hydrate is irritating to the skin and mucous membranes and
often causes gastric distress, nausea, and vomiting at recommended
doses. Acute overdoses produce (in order of progression) ataxia,
lethargy, deep coma, respiratory depression, hypotension, and cardiac
arrhythmias. There is some evidence of hepatic injury in people
surviving near-lethal, acute overdoses, but no convincing evidence
that hepatic injury results from the recommended clinical dose.
Despite its long use in human medicine, there is no published
information on toxicity in controlled studies in humans following
extended exposure.
Chloral hydrate is completely absorbed and rapidly metabolized
following oral administration. The major metabolites are
trichloroethanol and its glucuronide and trichloroacetic acid. Some
data suggest that a small amount of dichloroacetic acid may be formed.
In humans, the half-life of trichloroethanol and its glucuronide is
about 8 h; the half-life of trichloroacetic acid is about 4 days. Some
data suggest that the half-life of trichloroethanol is increased
several-fold in pre-term and full-term infants compared with toddlers
and adults. The major route of excretion of the metabolites of chloral
hydrate is elimination in the urine. Chloral hydrate and its
metabolites have been found in milk from women treated with chloral
hydrate. The concentration of these chemicals, however, is too low to
cause a pharmacological effect in the nursing infant.
Acute administration of chloral hydrate to mice causes loss of
coordination (ataxia) at about the same exposure as in humans for the
same effect. A 90-day study in mice shows no evidence of behavioural
changes or other neurotoxicity. Chronic studies in rats and mice show
no evidence of behavioural changes and no evidence of
histopathological changes in nervous tissue. These studies used an
exposure approximately 15 times the recommended clinical dose in
humans. There is some evidence of mild liver toxicity following
chronic exposure in rats and mice. A slight decrement in humoral
immunity was observed in female mice following exposure for 90 days.
The antibody-forming cell response is considered an excellent
indicator of the status of humoral immunity because of the complex
cellular cooperation required to produce antibody and because the
number of cells that produce antibody can be quantified. A depression
in the number of these cells is considered an adverse response because
the production of antibodies is important to the defence strategy of
the organism. However, the quantitative relationship between the
depression in antibody-forming cells in the spleen and the
concentration of circulating antibody is unknown. In this study,
because there was no depression in circulating antibodies measured by
the haemagglutination titre, there might be no significant depression
in the ability of the host to mount a protective antibody response.
Chloral hydrate has been tested for developmental effects in rats and
mice. No structural abnormalities were observed. A slight effect was
observed in mice in passive avoidance learning when dams were exposed
prior to breeding, during gestation, and during nursing and pups were
tested at 23 days of age. Although chloral hydrate has not been tested
in a two-generation reproduction study, the data on reproductive
performance and on effects on sperm and oocytes do not suggest that
reproductive toxicity is likely to be a critical effect. In addition,
no histopathological effects are observed in reproductive organs of
rodents in subchronic or chronic studies. Some in vitro data,
however, suggest that chloral hydrate administered to young female
children might have a latent effect on fertility. All of the studies
in laboratory animals show non-cancer health effects at an exposure
far in excess of the exposure that is effective for sedation in
humans. A complete summary of the exposure-response data is presented
in Table 3.
Simultaneous ingestion of ethanol and chloral hydrate increases
the sedative effects and side-effects of chloral hydrate. The
mechanism is the increase in the concentration of the
pharmacologically active metabolite, trichloroethanol, in the presence
of ethanol. Chronic users of ethanol are, therefore, somewhat more
sensitive to the adverse effects of chloral hydrate.
Because of the immaturity of hepatic metabolism, particularly the
glucuronidation pathway, and decreased glomerular filtration in
infants, the half-life of trichloroethanol is longer in pre-term and
full-term infants. This group is therefore somewhat more sensitive to
the adverse effects of chloral hydrate. Toddlers and adults are likely
to show similar sensitivity to chloral hydrate.
Although male laboratory rodents seem to be more sensitive than
female laboratory rodents to hepatic effects, there is no evidence of
a gender effect in humans with respect to the sedative effects or
side-effects of chloral hydrate at the recommended clinical dose.
There are no carcinogenicity data from humans. Two bioassays in
rats show no increase in tumours at any site. These studies were
limited, because only minimal toxicity was observed in the livers of
the rats in these bioassays. In one study, only slight hypertrophy was
observed at the highest exposure; in the other study, no effects were
observed at the highest exposure. No data are available in female
mice. There are three separate bioassays showing an increased
incidence of liver tumours in male mice. One study, conducted in a
very limited number of animals, showed an increase in tumours
Table 3: Summary of non-neoplastic effects.
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Human 1 day, 3 doses Sedation - 10.7 Goodman & Gilman, 1985
Rat 90 days Mild liver necrosis 96 168 Daniel et al., 1992b
and increase in
serum enzymes
Rat 104 weeks - 162.6 - George et al., 2000
Rat 124 weeks Liver hypertrophy 45 135 Leuschner & Beuscher, 1998
Rat 52 weeks Sperm motility 55 188 Klinefelter et al., 1995
Rat gestation Development 151 - Johnson et al., 1998
days 1-22
Mouse 14 days Increased liver weight 14.4 144 Sanders et al., 1982
Mouse 90 days Increased liver weight 16 160 Sanders et al., 1982
Mouse 104 weeks Increased liver weight - 166a Daniel et al., 1992a
and necrosis
Mouse 104 weeks - 146.6b - George et al., 2000
Mouse 3 weeks Reproduction and 204.8 - Kallman et al., 1984
pre-breeding development
and during
gestation
Mouse Pre-breeding, Passive avoidance 21.3 204.8 Kallman et al., 1984
gestation, and learning in pups
nursing
Table 3 (cont'd)
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Mouse 1 day Ataxia - 50 Kallman et al., 1984
Mouse 14 days Neurobehaviour 144 - Kallman et al., 1984
Mouse 90 days Neurobehaviour 160 - Kallman et al., 1984
Mouse 14 days Immunotoxicity 144 - Kauffmann et al., 1982
Mouse 90 days Humoral immunity 16 160 Kauffmann et al., 1982
a Tumours at 166 mg/kg body weight per day.
b Hyperplasia and tumours at 13.5, 65, and 146.6 mg/kg body weight per day.
following a single exposure. The second study tested only one exposure
level but used an adequate number of animals. The third study shows an
increase in incidence and multiplicity of liver tumours at each of
three exposures. There are no data identifying a lesion that is a
precursor to the tumours. The strain of mice used has a very high
spontaneous incidence of liver tumours. Two of the metabolites of
chloral hydrate, trichloroacetic acid and dichloroacetic acid, have
been shown to cause liver tumours in rodents. Trichloroacetic acid
causes liver tumours only in mice. Dichloroacetic acid causes tumours
in both rats and mice.1
Chloral hydrate has been extensively studied as a genotoxic
agent. Chloral hydrate was positive in some bacterial mutation tests,
indicating that it may be capable of inducing point mutations. It was
also positive in the mouse lymphoma assay for mutations at the tk
locus. Chloral hydrate also induced somatic and germ cell mutations
in D. melanogaster. Some data also show chloral hydrate to be a very
weak clastogen in mammalian cells.
Chloral hydrate has been shown to induce aneuploidy in a variety
of cells, including S. cerevisiae, A. nidulans, Chinese hamster
embryonic fibroblasts, Chinese hamster primary cell lines LUC2 and
DON:Wg3h, human peripheral blood lymphocytes, mouse spermatocytes, and
mouse spermatids. Because there is a mixture of positive and negative
in vivo data, with no reason to weigh some studies more than others,
it is not clear whether chloral hydrate is capable of inducing genetic
damage in vivo. Additional in vivo studies using standard
protocols would help clarify the relevance of genetic damage to a
human health risk assessment.
The effects on aneuploidy are thought to arise via disruption of
the mitotic spindle structure or function by inhibition of tubulin
and/or microtubule-associated proteins; both substances are components
of the spindle apparatus. Some data also suggest that chloral hydrate
may act on the spindle apparatus through an increase in the
concentration of intracellular free calcium.
1 In a National Toxicology Program carcinogenicity bioassay that
became available after the Final Review Board meeting, a
carcinogenic effect was not observed after a single dose of chloral
hydrate; after lifetime exposure, males had an increased incidence
of hepatic tumours, and females had a low increased incidence of
pituitary adenomas that was of borderline statistical significance.
Several other mechanisms may play a role in the induction of
tumours in the liver of male mice. There is no convincing evidence
that chloral hydrate causes direct damage to DNA. In vitro studies
with chloral hydrate, trichloroethanol, and trichloroacetic acid and
mouse microsomes, however, show lipid peroxidation and the formation
of covalently bound DNA adducts. These effects appear to be mediated
by the formation of free radicals by CYP2E1. Another possibility is
cytotoxicity leading to compensatory hyperplasia. A single treatment
of mice with chloral hydrate caused an increase in the mitotic index
in liver cells. The increased cell division is hypothesized to either
provide additional opportunities for errors in DNA replication or
allow initiated cells to progress to a tumour. Another potentially
contributing mechanism of carcinogenesis is disruption of
intercellular communication, which has been shown in one experiment to
be influenced by chloral hydrate.
The mechanism of chloral hydrate-induced carcinogenicity in male
mice is unclear. Two mechanisms that appear ruled out are H- ras
proto-oncogene activation and peroxisome proliferation.
Although there is suggestive evidence of carcinogenicity in male
mice, the weight of evidence is not sufficient to consider tumour
induction as the critical effect.
11.1.2 Criteria for setting tolerable intakes or guidance values for
chloral hydrate
The effect that occurs at the lowest exposure is mild sedation in
humans. As this effect would not be intended or desirable in the
general population outside of the clinical setting, this response is
considered an adverse effect and is used to derive the tolerable
intake.
Acute gavage exposure in mice shows neurological effects (ataxia)
at about the same exposure for the comparable effect in humans. A
subchronic study in mice using sensitive tests for neurobehavioural
changes found none. Chronic studies in rats and mice show no evidence
of neurobehavioural changes and no evidence of histopathological
changes in nervous tissue. As with other chlorinated chemicals, there
is some evidence of carcinogenic effects in the liver of male mice
following chronic exposure.
Although the tolerable intake derived from the pharmacologically
active dose in humans is an acute tolerable intake, keeping the
exposure below this level will also be protective for any non-cancer
health effect from chronic exposure. Therefore, it is appropriate to
use the acute tolerable intake as the chronic tolerable intake as
well.
No data are available to determine a NOAEL in humans. The
recommended clinical dose for sedation in adults is 250 mg, taken 3
times a day (Goodman & Gilman, 1985). A low incidence of side-effects
also occurs at this exposure. The LOAEL is 10.7 mg/kg body weight per
day (assuming a 70-kg body weight). The pharmacokinetic information
shows that chloral hydrate and the pharmacologically active
metabolite, trichloroethanol, will not bioaccumulate.
The tolerable intake (IPCS, 1994) of 0.1 mg/kg body weight per
day was derived from the LOAEL of 10.7 mg/kg body weight per day using
a total uncertainty factor of 100. An uncertainty factor of 10 was
used to extrapolate from a LOAEL to a NOAEL, and an uncertainty factor
of 10 was used for intraspecies variability. An uncertainty factor for
chronic duration was not used. Chloral hydrate and the active
metabolite, trichloroethanol, do not bioaccumulate. The half-life of
chloral hydrate is a few minutes, and the half-life of
trichloroethanol is a few hours. Therefore, an enhanced effect from
continuous, daily exposure is not possible. Finally, there is
information from clinical use that long-term exposure to chloral
hydrate results in tolerance to the sedative effect. Developmental
toxicity, including developmental neurotoxicity, and immunotoxicity
are not critical effects. Although there is no two-generation
reproduction study, an uncertainty factor for database limitations is
not needed, as there is evidence from several studies that
reproductive toxicity is not likely to be a critical effect.
There are no inhalation studies adequate for setting a guidance
value or tolerable intake.
There are data in male mice showing that chloral hydrate causes
tumours in the liver. It is not known whether this response is
relevant for humans.
11.1.3 Sample risk characterization
The quantitative estimate of human risk for non-cancer effects is
based on the recommended clinical dose for sedation in humans and the
minor incidence of side-effects at this dose. The tolerable intake is
0.1 mg/kg body weight per day. This is 1% of the recommended single
dose for sedation in humans.
Although there is suggestive evidence of formation of tumours in
the liver of male mice and there are some data showing genotoxicity,
the mode of action for the formation of tumours is not known. It is
also not known whether this response is relevant for humans.
Millions of people are exposed to chloral hydrate on a daily
basis because it is formed during the disinfection of drinking-water
with chlorine. The typical concentration in a public water supply in
the USA is 5 µg/litre. Assuming a water consumption of 2 litres
per day and a body weight of 70 kg, the exposure is 0.14 µg/kg body
weight per day. This exposure is approximately 700 times lower than
the tolerable intake.
11.2 Evaluation of environmental effects
Insufficient data are available with which to assess the risk to
the environment from chloral hydrate.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1995) evaluated the carcinogenicity data for chloral
hydrate. It was concluded that there is inadequate evidence in humans
and limited evidence in experimental animals for the carcinogenicity
of chloral hydrate. Chloral hydrate is therefore not classifiable as
to its carcinogenicity to humans (Group 3).
IPCS (2000) recently evaluated the toxicological data on water
disinfectants and disinfectant by-products, including chloral hydrate.
Considering the dose level of 16 mg/kg body weight per day in the
90-day study in mice (Sanders et al., 1982; see section 8.4.1) as a
LOAEL (rather than as a NOAEL, as was done in the present document)
and using an uncertainty factor of 10 for intra- and interspecies
extrapolation and another factor of 10 for the use of a LOAEL rather
than a NOAEL, the Task Group calculated a tolerable daily intake (TDI)
for chloral hydrate of 16 µg/kg body weight per day. (As the present
document considered the increase in liver weight at 16 mg/kg body
weight to be a NOAEL rather than a LOAEL, the tolerable intake derived
from the studies among humans was lower, as discussed in section
11.1.)
REFERENCES
Abbas R, Fisher JW (1997) A physiologically based pharmacokinetic
model for trichloroethylene, and its metabolites, chloral hydrate,
trichloroacetate, dichloroacetate, trichloroethanol, and
trichloroethanol glucuronide in B6C3F1 mice. Toxicology and
applied pharmacology, 137:15-30.
Abbas R, Seckel CS, Kidney JK, Fisher JW (1996) Pharmacokinetic
analysis of chloral hydrate and its metabolism in B6C3F1 mice.
Drug metabolism and disposition, 24:1340-1346. See also Erratum,
Drug metabolism and disposition, 25:1449 (1997).
Allen BC, Fisher JW (1993) Pharmacokinetic modeling of
trichloroethylene and trichloroacetic acid in humans. Risk analysis,
13:71-86.
Anyebuno MA, Rosenfeld CR (1991) Chloral hydrate toxicity in a term
infant. Developments in pharmacological therapy, 17:116-120.
Badalaty MM, Houpt MI, Koenigsberg SR, Maxwell KC, Desjardins PJ
(1990) A comparison of chloral hydrate and diazepam sedation in young
children. Pediatric dentistry, 12:33-37.
Beland FA, Schmitt TC, Fullerton NF, Young JF (1998) Metabolism of
chloral hydrate in mice and rats after single and multiple doses.
Journal of toxicology and environmental health, 54:209-226.
Benane SG, Blackman CF, House DE (1996) Effect of perchloroethylene
and its metabolites on intercellular communication in clone 9 rat
liver cells. Journal of toxicology and environmental health,
48:427-437.
Bernstine JB, Meyer AE, Bernstine RL (1954) Maternal blood and breast
milk estimation following the administration of chloral hydrate during
the puerperium. Journal of obstetrics and gynaecology of the British
Commonwealth, 63:228-231.
Breimer DD (1977) Clinical pharmacokinetics of hypnotics. Clinical
pharmacokinetics, 2:93-109.
Breimer DD, Ketelaars HCJ, Van Rossum JM (1974) Gas chromatographic
determination of chloral hydrate, trichloroethanol, and
trichloroacetic acid in blood and urine employing head-space analysis.
Journal of chromatography, 88:55-63.
Bringmann G, Kuehn R (1976) Vergleichende Befunde der Schadwirkung
wassergerfahrden der Stoffe gegen Bakterien (Pseudomonas putida) und
Blaualgen (Microcystis aeruginosa). Gas- und Wasserfach:
Wasser/Abwasser, 117:410-413.
Bringmann G, Kuehn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in cell
multiplication inhibition tests. Water research, 14:231-241.
Bringmann G, Kuehn R (1980b) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen II Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, God R, Fähr S, Feines D, Fornadi K, Fornadi F (1999)
Identification of the dopaminergic neurotoxin
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline in human blood after
intake of the hypnotic chloral hydrate. Analytical biochemistry,
270:167-175.
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver
tumor induction in B6C3F1 mice by dichloroacetate and
trichloroacetate. Toxicology, 63:341-359.
Chakrabarti A, Schatten H, Mitchell KD, Crosser M, Taylor M (1998)
Chloral hydrate alters the organization of the ciliary basal apparatus
and cell organelles in sea urchin embryos. Cell and tissue research,
293:453-462.
Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP (1992a)
Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and
dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 19:159-168.
Daniel FB, Robinson M, Stober JA, Page NP, Olson GR (1992b) Ninety-day
toxicity study of chloral hydrate in the Sprague-Dawley rat. Drug and
chemical toxicology, 15:217-232.
DeAngelo AB, Daniel FB, Stober JA, Olson GR (1991) The carcinogenicity
of dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 16:337-347.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1996) The carcinogenicity
of dichloroacetic acid in the male Fischer 344 rat. Toxicology,
114:207-221.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1997) The failure of
monochloroacetic acid and trichloroacetic acid administered in the
drinking water to produce liver cancer in male F344/N rats. Journal
of toxicology and environmental health, 52:425-445.
Eichenlaub-Ritter U, Betzendahl I (1995) Chloral hydrate induced
spindle aberrations, metaphase I arrest and aneuploidy in mouse
oocytes. Mutagenesis, 10:477-486.
Eichenlaub-Ritter U, Baart E, Yin H, Betzendahl I (1996) Mechanisms of
spontaneous and chemically-induced aneuploidy in mammalian oogenesis:
Basis of sex specific differences in response to aneugens and the
necessity for further tests. Mutation research, 372:274-294.
Elfarra AA, Krause RJ, Last AR, Lash LH, Parker JC (1998) Species- and
sex-related differences in metabolism of trichloroethylene to yield
chloral and trichloroethanol in mouse, rat, and human liver
microsomes. Drug metabolism and disposition, 26:779-785.
Ferreira-Gonzalez A, DeAngelo AB, Nasim S, Garrett CT (1995) Ras
oncogene activation during hepatocarcinogenesis in B6C3F1 male mice
by dichloroacetic and trichloroacetic acid. Carcinogenesis,
16:495-500.
Fisher JW, Mahle D, Abbas R (1998) A human physiologically based
pharmacokinetic model for trichloroethylene and its metabolites:
trichloroacetic acid and free trichloroethanol. Toxicology and
applied pharmacology, 152:339-359.
Fox BE, O'Brien CO, Kangas KJ, Murphree AL, Wright KW (1990) Use of
high dose chloral hydrate for ophthalmic exams in children: A
retrospective review of 302 cases. Journal of pediatric ophthalmology
and strabismus, 27:242-244.
Fung K, Grosjean D (1981) Determination of nanogram amounts of
carbonyls as 2,4-dinitrophenylhydrazones by high-performance liquid
chromatography. Analytical chemistry, 53:168-171.
George MH, Kilburn S, Moore T, DeAngelo AB (2000) The carcinogenicity
of chloral hydrate administered in the drinking water to the male
B6C3F1 mouse and F344/N rat. Toxicologic pathology (in press).
Gerlach M, Xiao A-Y, Heim C, Lan J, God R, Feines D, Bringmann G,
Riederer P, Sontag K-H (1998)
1-Trichloromethyl-1,2,3,4-tetrahydro-ß-carboline increases
extracellular serotonin and stimulates hydroxyl radical production in
rats. Neuroscience letters, 257:17-20.
Goldenthal EI (1971) A compilation of LD50 values in newborn and
adult animals. Toxicology and applied pharmacology, 18:185-207.
Goodman LS, Gilman A (1985) The pharmacological basis of
therapeutics, 7th ed. New York, NY, The Macmillan Co.
Gorecki DKJ, Hindmarsh KW, Hall CA, Mayers DJ, Sankaran K (1990)
Determination of chloral hydrate metabolism in adult and neonate
biological fluids after single-dose administration.
Journal of chromatography, 528:333-341.
Gosselin RE, Smith RP, Hodge HC (1981) Clinical toxicology of
commercial products. Baltimore, MD, Williams & Wilkins.
Green T, Mainwaring GW, Foster JR (1997) Trichloroethylene-induced
mouse lung tumors: Studies of the mode of action and comparisons
between species. Fundamental and applied toxicology, 37:125-130.
Greenberg MS, Burton GA Jr, Fisher FW (1999) Physiologically based
pharmacokinetic modeling of inhaled trichloroethylene and its
oxidative metabolites in B6C3F1 mice. Toxicology and applied
pharmacology, 154:264-278.
Greenberg SB, Faerber EN, Aspinall CL (1991) High dose chloral hydrate
sedation for children undergoing CT. Journal of computer assisted
tomography, 15:467-469.
Gupta RN (1990) Determination of trichloroethanol, the active
metabolite of chloral hydrate, in plasma by liquid chromatography.
Journal of chromatography, 500:655-659.
HSDB (1999) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
Helrich K, ed. (1990) Official methods of analysis of the Association
of Official Analytical Chemists, 15th ed. Vol. 1. Arlington, VA,
Association of Official Analytical Chemists, p. 562.
Henderson GN, Yan Z, James MO, Davydova N, Stacpoole PW (1997)
Kinetics and metabolism of chloral hydrate in children: identification
of dichloroacetate as a metabolite. Biochemical and biophysical
research communications, 235:695-698.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The
carcinogenicity of trichloroethylene and its metabolites,
trichloroacetic acid and dichloroacetic acid, in mouse liver.
Toxicology and applied pharmacology, 90:183-189.
Hindmarsh KW, Gorecki DKJ, Sankaran K, Mayers DJ (1991) Chloral
hydrate administration to neonates: Potential toxicological
implications. Canadian Society of Forensic Science, 24:239-245.
Hobara T, Kobayashi H, Kawamoto T, Sato T, Iwamoto S, Hirota S, Sakai
T (1986) Biliary excretion of trichloroethylene and its metabolites in
dogs. Toxicology letters, 32:119-122.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1987a) The
cholecystohepatic circulation of trichloroethylene and its metabolites
in dogs. Toxicology, 44:283-295.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Hirota S, Sakai T
(1987b) Extrahepatic metabolism of chloral hydrate, trichloroethanol,
and trichloroacetic acid in dogs. Pharmacology and toxicology,
61:58-62.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988a)
Intestinal absorption of chloral hydrate, free trichloroethanol, and
trichloroacetic acid in dogs. Pharmacology and toxicology,
62:250-258.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988b) The
absorption of trichloroethylene and its metabolites from the urinary
bladder of anesthetized dogs. Toxicology, 48:141-153.
IARC (1995) Chloral and chloral hydrate. IARC (International Agency
for Research on Cancer) monographs, 63:245-269.
IPCS (1993) International Chemical Safety Card -- Chloral hydrate.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0234).
IPCS (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
IPCS (2000) Disinfectants and disinfectant by-products. Geneva,
World Health Organization (Environmental Health Criteria 216).
Johnson PD, Dawson BV, Goldberg SJ (1998) Cardiac teratogenicity of
trichloroethylene metabolites. Journal of the American College of
Cardiology, 32:540-545.
Kallman MJ, Kaempf GL, Balster RL (1984) Behavioral toxicity of
chloral in mice: An approach to evaluation. Neurobehavioral
toxicology and teratology, 6:137-146.
Kaplan HL, Forney RB, Hughes FW, Jain NC, Crim D (1967) Chloral
hydrate and alcohol metabolism in human subjects. Journal of forensic
sciences, 12:295-304.
Kauffmann BM, White KL, Sanders VM, Douglas KA, Sain LE, Borzelleca
JF, Munson AE (1982) Humoral and cell-mediated immune status in mice
exposed to chloral hydrate. Environmental health perspectives,
44:147-151.
Ketcha MM, Stevens DK, Warren DA, Bishop CT, Brashear WT (1996)
Conversion of trichloroacetic acid to dichloroacetic acid in
biological samples. Journal of analytical toxicology, 20:236-241.
Klinefelter GR, Suarez JD, Roberts NL (1995) Preliminary screening
test for the potential of drinking water disinfectant by-products to
alter male reproduction. Reproductive toxicology, 9:571-578.
Koppen B, Dalgaard L (1988) Determination of trichloroethylene
metabolites in rat liver homogenates using headspace gas
chromatography. Journal of chromatography, 442:325-332.
Lambert GH, Muraskas J, Anderson CL, Myers TF (1990) Direct
hyperbilirubinemia associated with chloral hydrate administration in
the newborn. Pediatrics, 86:277-281.
Leuschner J, Beuscher N (1998) Studies on the mutagenic and
carcinogenic potential of chloral hydrate. Arzneimittel-Forschung
drug research, 48:961-968.
Lipscomb JC, Mahle DA, Brashear WT, Garrett CM (1996) A species
comparison of chloral hydrate metabolism in blood and liver.
Biochemical and biophysical research communications, 227:340-350.
Lipscomb JC, Confer PD, Miller MR, Stamm SC, Snawder JE, Candiera SM
(1998) Metabolism of trichloroethylene and chloral hydrate by the
Japanese medaka (Oryzias latipes) in vitro. Environmental
toxicology and chemistry, 17:325-332.
Ludwigs U, Divino-Fiiho JC, Magnusson N (1996) Suicidal chloral
hydrate poisoning. Journal of clinical toxicology, 344:97-99.
Mailhes JB, Marchetti F (1994) Chemically induced aneuploidy in
mammalian oocytes. Mutation research, 320:87-111.
Marshall AJ (1977) Cardiac arrhythmias caused by chloral hydrate.
British medical journal, 2:994.
Marshall EK, Owens AH (1954) Absorption, excretion and metabolic fate
of chloral hydrate and trichloroethanol. Bulletin of the Johns
Hopkins Hospital, 95:1-18.
Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DKJ, Kasian GF (1991)
Chloral hydrate disposition following single-dose administration to
critically ill neonates and children. Developments in pharmacological
therapy, 16:71-77.
Merdink JL, Conzalez-Leon A, Bull RJ, Schultz IR (1998) The extent of
dichloroacetate formation from trichloroethylene, chloral hydrate,
trichloroacetate, and trichloroethanol in B6C3F1 mice. Toxicological
sciences, 45:33-41.
Merdink JL, Stenner RD, Stevens DK, Parker JC, Bull RJ (1999) Effect
of enterohepatic circulation on the pharmacokinetics of chloral
hydrate and its metabolites in F344 rats. Journal of toxicology and
environmental health, 56:357-368.
Miller RR, Greenblatt DJ (1979) Clinical effects of chloral hydrate in
hospitalized medical patients. Journal of clinical pharmacology,
19:669-674.
Newman LM, Wackett LP (1991) Fate of 2,2,2-trichloroacetaldehyde
(chloral hydrate) produced during trichloroethylene oxidation by
methanotrophs. Applied and environmental microbiology, 57:2399-2402.
Ni Y-C, Wong T-Y, Kadlubar FF, Fu PP (1994) Hepatic metabolism of
chloral hydrate to free-radical(s) and induction of lipid
peroxidation. Biochemical and biophysical research communications,
204:937-943.
Ni Y-C, Kadlubar FF, Fu FF (1995) Formation of
malondialdehyde-modified 2'-deoxyguanosinyl adduct from metabolism of
chloral hydrate by mouse liver microsomes. Biochemical and
biophysical research communications, 205:1110-1117.
Ni Y-C, Wong T-Y, Lloyd RV, Heinze TM, Shelton S, Casciano D, Kadlubar
FF, Fu PP (1996) Mouse liver microsomal metabolism of chloral hydrate,
trichloroacetic acid, and trichloroethanol leading to induction of
lipid peroxidation via a free radical mechanism. Drug metabolism and
disposition, 24:81-90.
NTP (2000a) Toxicology and carcinogenesis studies of chloral hydrate
in B6C3F1 mice (gavage studies). Research Triangle Park, NC,
National Institutes of Health, National Toxicology Program (NTP TR
502).
NTP (2000b) Toxicology and carcinogenesis studies of chloral hydrate
(ad libitum and dietary controlled) in male B6C3F1 mice (gavage
study). Research Triangle Park, NC, National Institutes of Health,
National Toxicology Program (NTP TR 503).
Odum J, Foster JR, Green T (1992) A mechanism for the development of
clara cell lesions in the mouse lung after exposure to
trichloroethylene. Chemico-biological interactions, 83:135-153.
Owens AH, Marshall EK (1955) Further studies on the metabolic fate of
chloral hydrate and trichloroethanol. Bulletin of the Johns Hopkins
Hospital, 97:320-326.
Pereira MA (1996) Carcinogenic activity of dichloroacetic acid and
trichloroacetic acid in the liver of female B6C3F1 mice.
Fundamental and applied toxicology, 31:192-199.
Reimche LD, Sankaran K, Hindmarsh KW, Kasian GF, Gorecki DKJ, Tan L
(1989) Chloral hydrate sedation in neonates and infants -- clinical
and pharmacologic considerations. Developments in pharmacological
therapy, 12:57-64.
Richmond RE, Carter JH, Carter HW, Daniel FB, DeAngelo AB (1995)
Immunohistochemical analysis of dichloroacetic acid (DCA)-induced
hepatocarcinogenesis in male Fischer (F344) rats. Cancer letters,
92:67-76.
Rijhsinghani KS, Abrahams C, Swerdlow MA, Rao KVN, Ghose T (1986)
Induction of neoplastic lesions in the livers of C57BL × C3HF1 mice
by chloral hydrate. Cancer detection and prevention, 9:279-288.
Saillenfait AM, Langonne I, Sabate JP (1995) Developmental toxicity of
trichloroethylene, tetrachloroethylene and four of their metabolites
in rat whole embryo culture. Archives of toxicology, 70:71-82.
Sanders VM, Kauffman BM, White KL, Douglas KA, Barnes DW, Sain LE,
Bradshaw TJ, Borzelleca JF, Munson AE (1982) Toxicology of chloral
hydrate in the mouse. Environmental health perspectives, 44:137-146.
Schatten H, Chakrabarti A (1998) Centrosome structure and function is
altered by chloral hydrate and diazepam during the first reproductive
cell cycles in sea urchin eggs. European journal of cell biology,
75:9-20.
Shapiro S, Stone D, Lewis GP, Jick H (1969) Clinical effects of
hypnotics. II. An epidemiological study. Journal of the American
Medical Association, 209:2016-2020.
Simpson KL, Hayes KP (1998) Drinking water disinfection by-products:
an Australian perspective. Water research, 32:1522-1528.
Sing K, Erickson T, Amitai Y, Hryhorczuk D (1996) Chloral hydrate
toxicity from oral and intravenous administration. Journal of
toxicology and clinical toxicology, 34:101-106.
Smith MK, Randall JL, Read EJ, Stober JA (1989) Teratogenic activity
of trichloroacetic acid in the rat. Teratology, 40:445-451.
Stenner RD, Merdink JL, Stevens DK, Springer DL, Bull RJ (1997)
Enterohepatic recirculation of trichloroethanol glucuronide as a
significant source of trichloroacetic acid. Drug metabolism and
disposition, 25:529-535.
Stenner RD, Merdink JL, Fisher JW, Bull RJ (1998)
Physiologically-based pharmacokinetic model for trichloroethylene
considering enterohepatic recirculation of major metabolites.
Risk analysis, 18:261-269.
US EPA (1994) National primary drinking water regulations;
disinfectants and disinfection byproducts; proposed rule. US
Environmental Protection Agency. Federal register, 59:38668-38829.
US EPA (2000) Toxicological review on chloral hydrate. Available
from US Environmental Protection Agency's Risk Assessment Hotline
[513-569-7254 (phone), 513-569-7159 (fax), rih.iris@epa.gov (e-mail
address), or www.epa.gov/iris (Website)].
Velazquez SF (1994) Activation of the H-ras oncogene by drinking
water disinfection by-products. Report submitted under the US
Environmental Protection Agency's Small Grant Program. April 1994. 43
pp. (NTIS/PB95-200515).
Vian L, Van Hummelen P, Bichet N, Gouy D, Kirsch-Volders M (1995)
Evaluation of hydroquinone and chloral hydrate on the in vitro
micronucleus test on isolated lymphocytes. Mutation research,
334:1-7.
Watanabe M, Takano T, Nakamura K (1998) Effect of ethanol on the
metabolism of trichloroethylene in the perfused rat liver. Journal of
toxicology and environmental health, 55:297-305.
Wu WW, Chadik PA, Davis WM, Powell DH, Delfino JJ (1998) Disinfection
byproduct formation from the preparation of instant tea. Journal of
agricultural and food chemistry, 46:3272-3279.
Yan Z, Henderson GN, James MO, Stacpoole P (1999) Determination of
chloral hydrate in human plasma by gas chromatography-mass
spectrometry. Journal of pharmaceutical and biomedical analysis,
19:309-318.
Zimmermann T, Wehling M, Schulz HU (1998) Untersuchungen zur relativen
Bioverfugbarkeit und Pharmakokinetik von Chloralhydrat und seinen
Metaboliten. [The relative bioavailability and pharmacokinetics of
chloral hydrate and its metabolites.] Arzneimittelforschung,
48:5-12.
APPENDIX 1 -- TOXICOKINETICS
This toxicokinetic analysis is used to estimate the steady-state
concentrations of trichloroacetic acid (TCA) and trichloroethanol
(TCEOH) in mice and humans using a one-compartment model, assuming
that the absorption of chloral hydrate (CH) from the gastrointestinal
tract and its metabolism in the blood are very rapid compared with the
rate of elimination of TCA and TCEOH. This assumption is supported by
the data of Beland et al. (1998) in mice and Breimer (1977) and
Zimmermann et al. (1998) in humans.
Beland et al. (1998) indicated that 15% of the dose of chloral
hydrate is converted directly to TCA and 77% is converted to TCEOH. In
humans, Allen & Fisher (1993) estimated that 8% of a dose of chloral
hydrate is converted directly to TCA and 92% is converted to TCEOH.
Additional TCA is formed from TCEOH. The total TCA formed in humans is
approximately 35% of the dose of chloral hydrate.
Estimation of TCA concentration in mice at steady state at the
clinically recommended dose for humans:
[TCA]ss-blood = PKo/ VKel = 2.5 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 3.0 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.15
(Beland et al., 1998)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.321 litre/kg
(Beland et al., 1998)
* Kel is the first-order elimination constant for TCA =
0.0819/h (Beland et al., 1998)
* PC is the liver/blood partition coefficient = 1.18 (Abbas &
Fisher, 1997)
Estimation of TCA concentration in humans at steady state at the
clinically recommended dose:
[TCA]ss-blood = PKo/ VKel = 55 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 36 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA = 0.028/h
(Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCA concentration in humans at steady state at the
tolerable intake:
[TCA]ss-blood = PKo/ VKel = 1.8 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 1.2 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA =
0.0078/h (Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCEOH concentration in mice at steady state at 166 mg/kg
body weight per day:
[TCEOH]ss-blood = PKo/ VKel = 0.6 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.77
(Beland et al., 1998)
* Ko is the dosing rate for CH = 166 mg/kg body weight per day,
equivalent to 6.917 mg/kg body weight per hour
* V is the volume of distribution = 1 litre/kg (cited in
Beland et al., 1998)
* Kel is the first-order elimination constant for TCEOH =
9.24/h (Beland et al., 1998)
Chloral hydrate at 160 mg/kg body weight per day was the highest
exposure used in the 90-day neurobehavioural study by Kallman et al.
(1984); chloral hydrate at 166 mg/kg body weight per day was the
highest exposure used in the 104-week bioassay of Daniel et al.
(1992a). These exposures are a NOAEL for sedation in mice.
Estimation of TCEOH concentration in humans at steady state at the
clinically recommended dose:
[TCEOH]ss-blood = PKo/ VKel = 4.7 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
Estimation of TCEOH concentration in humans at steady state at the
tolerable intake:
[TCEOH]ss-blood = PKo/ VKel = 0.04 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
APPENDIX 2 -- CALCULATION OF BENCHMARK DOSE FOR
TUMOUR INCIDENCE
The Benchmark Dose (ED) for tumour incidence was derived from the
incidence of adenoma plus carcinoma as reported by George et al.
(2000). The human equivalent dose was calculated using (body
weight)3/4, assuming a human body weight of 70 kg and a mouse body
weight of 0.035 kg. EPA Benchmark Dose Software was used to calculate
the ED and its lower 95% confidence limit (LED) corresponding to a 10%
increase in extra risk for tumour prevalence with the multistage
model.
Multistage Model, Version Number 1.1.0b
The form of the probability function is:
P[response] =
(1 - background ) × [1 - (e - beta 1 × dose 1 - beta 2 × dose 2) ]
The parameter betas are restricted to be positive.
Dependent variable = Incidence
Independent variable = Dose
Total number of observations = 4
Total number of records with missing values = 0
Total number of parameters in model = 3
Total number of specified parameters = 0
Degree of polynomial = 2
Maximum number of iterations = 250
Relative function convergence has been set to 2.220 45e-16
Parameter convergence has been set to 1.490 12e-8
Default initial parameter values
Background = 0.698 863
Beta(1) = 0.043 897
Beta(2) = 0.000 400 241
Parameter estimates
Variable Estimate Standard error
Background 0.691 141 0.073 072 3
Beta(1) 0.053 218 1 0.084 548 3
Beta(2) 0 0.004 035 19
Asymptotic correlation matrix of parameter estimates
Background Beta(1) Beta(2)
Background 1 -0.6319 0.5007
Beta(1) -0.6319 1 -0.9507
Beta(2) 0.5007 -0.9507 1
Analysis of deviance table
Model Log(likelihood) Deviance DF P-value
Full model -81.2046
Fitted model -81.922 1.434 7 2 0.230 999
Reduced mode -85.0504 6.256 83 2 0.043 787
Goodness of fit analysis
Administered dose Human equivalent Estimated Expected Observed Size
(mg/kg body weight dose probability
per day) (mg/kg body weight
per day)
0 0 0.6911 29.028 27 42
13.5 2.0000 0.7223 33.227 36 46
65 9.7 0.8157 31.812 31 39
146.6 21.9 0.9037 28.919 29 32
Chi-square = 1.41; DF = 2; P-value = 0.4949.
Benchmark dose computation
Specified effect 0.100 000
Risk type Extra risk
Confidence level 0.950 000
ED 1.979 786
LED 1.090 1
APPENDIX 3 -- SOURCE DOCUMENT
US Environmental Protection Agency (2000):
Toxicological review on chloral hydrate
Copies of the document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (phone)
513-569-7159 (fax)
rih.iris@epa.gov (e-mail address)
www.epa.gov/iris (Website)
This document was prepared by R. Benson, Region VIII, Denver, CO.
The document and summary information on the Integrated Risk
Information System (IRIS) have received peer review both by EPA
scientists and by independent scientists external to EPA. Subsequent
to external review and incorporation of comments, this assessment has
undergone an Agency-wide review process whereby the IRIS Program
Manager has achieved a consensus approval among the Office of Research
and Development; Office of Air and Radiation; Office of Prevention,
Pesticides, and Toxic Substances; Office of Solid Waste and Emergency
Response; Office of Water; Office of Policy, Planning, and Evaluation;
and the Regional Offices.
Internal EPA reviewers:
National Center for Environmental Assessment, Washington, DC
J. Cogliano
C. Siegel Scott
V. Vu
National Health and Environmental Effects Research Laboratory,
Research Triangle Park, NC
A. DeAngelo
R. Luebke
Office of Water, Washington, DC
A. Bathija
External peer reviewers:
P.E. Brubaker, Private Consultant
J.W. Fisher, Operational Toxicology Branch, Wright-Patterson
Air Force Base
C.C. Willhite, Department of Toxic Substances Control,
State of California
APPENDIX 4 -- CICAD PEER REVIEW
The draft CICAD on chloral hydrate was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS National Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Centre of Industrial Hygiene and Occupational Diseases, Czech
Republic
Department of Health, London, United Kingdom
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Berlin, Germany
Fraunhofer Institute for Toxicology and Aerosol Research,
Hannover, Germany
GSF Forschungszentrum für Umwelt und Gesundheit, GmbH,
Oberschleissheim, Germany
Health and Safety Executive, Bootle, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Beijing, People's Republic of China
National Center for Environmental Assessment, US Environmental
Protection Agency, Washington, DC, USA
National Center for Toxicological Research, US Food and Drug
Administration, Jefferson, AK, USA
National Chemicals Inspectorate, Solna, Sweden
National Industrial Chemicals Notification and Assessment Scheme
(NICNAS), Sydney, Australia
National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, Research Triangle Park, NC, USA
University of Bielefeld, Bielefeld, Germany
APPENDIX 5 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment,
US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry,
Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment,
US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 6 -- INTERNATIONAL CHEMICAL SAFETY CARD
CHLORAL HYDRATE ICSC: 0234
October 1999
CAS#
Although earlier reports claimed the detection of substantial
quantities of dichloroacetic acid in blood in studies with rodents
(Abbas et al., 1996), data show that the dichloroacetic acid is most
likely formed by an acid-catalysed dechlorination of trichloroacetic
acid in the presence of reduced haemoglobin (Ketcha et al., 1996).
Recent experimental data and pharmacokinetic model simulations in
rodents suggest that dichloroacetic acid occurs only as a short-lived
metabolite in the liver and is rapidly converted to two-carbon,
non-chlorinated metabolites and carbon dioxide, with the chlorine
atoms entering the chloride pool (Merdink et al., 1998). Using a
different extraction procedure less likely to induce the artefactual
formation of dichloroacetic acid, Henderson et al. (1997) showed the
presence of dichloroacetic acid in children treated with chloral
hydrate in a clinic.
Breimer (1977) administered an aqueous solution of chloral
hydrate to five human volunteers. Each volunteer received a single
oral dose of 15 mg/kg body weight. Chloral hydrate could not be
detected in the plasma even at the first sampling time of 10 min.
A method with a limit of detection of 0.5 mg/litre was used.
Trichloroethanol and trichloroethanol glucuronide reached peak
concentrations 20-60 min after administration of chloral hydrate. The
maximum concentration of trichloroethanol in the plasma was about
5 mg/litre. The average half-lives of trichloroethanol and
trichloroethanol glucuronide were 8 h (range 7-9.5 h) and 6.7 h (range
6-8 h), respectively. The half-life of trichloroacetic acid was about
4 days. Zimmermann et al. (1998) administered a single dose of 250 mg
chloral hydrate in drinking-water to 18 healthy male volunteers
(20-28 years of age). Chloral hydrate, trichloroethanol, and
trichloroacetic acid were measured in plasma. Chloral hydrate could be
detected 8-60 min after dosing in only some of the plasma samples.
The measured concentration of chloral hydrate was not reported, but
the limit of detection was stated as 0.1 mg/litre. The maximum plasma
concentration of trichloroethanol of 3 mg/litre was achieved 0.67 h
after dosing, and the maximum plasma concentration of trichloroacetic
acid of 8 mg/litre was achieved 32 h after dosing. The terminal
half-life was 9.3-10.2 h for trichloroethanol and 89-94 h for
trichloroacetic acid.
Two toxicokinetic models are available for chloral hydrate in
rats and mice (Abbas et al., 1996; Beland et al., 1998). Beland et al.
(1998) treated rats and mice with chloral hydrate by gavage with 1 or
12 doses using 50 or 200 mg/kg body weight per dose. The maximum
levels of chloral hydrate, trichloroethanol, and trichloroethanol
glucuronide in the plasma were observed at the initial sampling time
of 0.25 h. The half-life of chloral hydrate in the plasma was
approximately 3 min. The half-lives of trichloroethanol and
trichloroethanol glucuronide in the plasma were approximately 5 and
7 min, respectively. Trichloroacetic acid was the major metabolite
found in the plasma, with the maximum level being reached 1-6 h after
dosing. The half-life of trichloroacetic acid in the plasma was
approximately 8-11 h. Comparable values were obtained for rats.
Estimates of the concentrations of trichloroacetic acid and
trichloroethanol at steady state under various exposure conditions are
in Appendix 1.
Several studies have investigated the age dependence of the
metabolism of chloral hydrate (Reimche et al., 1989; Gorecki et al.,
1990; Hindmarsh et al., 1991; Mayers et al., 1991). These studies were
conducted in critically ill patients in neonatal and paediatric
intensive care units and may not be representative of a population of
healthy infants. The half-lives for trichloroethanol and its
glucuronide were increased several-fold in pre-term and full-term
infants compared with toddlers and adults. The half-lives for
trichloroethanol in toddlers and adults were similar. These
age-related differences likely are the result of the immaturity of
hepatic metabolism, particularly glucuronidation, and decreased
glomerular filtration.
Kaplan et al. (1967) investigated the effect of ethanol
consumption on the metabolism of chloral hydrate in adults. Subjects
ingested doses of ethanol (880 mg/kg body weight), chloral hydrate
(9-14 mg/kg body weight), or both. In subjects consuming both ethanol
and chloral hydrate, blood trichloroethanol levels rose more rapidly
and reached higher values than in subjects consuming chloral hydrate
only. Ethanol promotes the formation of trichloroethanol because the
oxidation of ethanol provides NADH used for the reduction of chloral
hydrate (Watanabe et al., 1998).
8. EFFECTS ON LABORATORY MAMMALS AND IN VITRO TEST SYSTEMS
8.1 Single exposure
8.1.1 Oral
Sanders et al. (1982) studied the acute toxicity of chloral
hydrate in CD-1 mice. Groups of eight male and eight female mice were
given chloral hydrate by gavage in distilled water at 300, 600, 900,
1200, 1500, or 1800 mg/kg body weight. No deaths occurred at 900 mg/kg
body weight or below in either sex. The calculated LD50 for females
was 1265 mg/kg body weight and for males was 1442 mg/kg body weight.
Effects were seen within 10 min of dosing. The mice became sedated at
300 mg/kg body weight. At 600 and 900 mg/kg body weight, the animals
became lethargic and exhibited loss of righting reflex. Respiration
was markedly inhibited at 1200, 1500, and 1800 mg/kg body weight.
Inhibition of respiration appeared to be the immediate cause of death.
Most deaths occurred within 4 h at 1800 mg/kg body weight. At 1200 and
1500 mg/kg body weight, some deaths occurred after 4 h, with all
deaths occurring within 24 h.
Goldenthal (1971) reported an oral LD50 in rats of 480 mg/kg
body weight.
8.1.2 Inhalation
Odum et al. (1992) exposed four female CD-1 mice to chloral for
6 h at a concentration of 100 ppm (603 mg/m3). This exposure induced
deep anaesthesia. The mice recovered normally after the exposure
stopped. The effects in the lung included vacuolization of clara
cells, alveolar necrosis, desquamination of the epithelium, and
alveolar oedema. The lung to body weight ratio increased 1.5-fold,
most likely due to the alveolar oedema.
8.2 Irritation and sensitization
There are no studies of irritation or sensitization in laboratory
animals.
8.3 Short-term exposure
Sanders et al. (1982) studied the short-term toxicity of chloral
hydrate in mice. Groups of male CD-1 mice were given chloral hydrate
by gavage in distilled water at 14.4 or 144 mg/kg body weight per day
for 14 days. No significant effect on body weight was observed. No
changes in internal organs were noted from a gross examination. Groups
of 11-12 mice were evaluated for several toxicological parameters. No
significant effects on haematological or serum biochemical parameters
were noted. There was a statistically significant (P < 0.05)
increase in liver weight (17%) and a decrease in spleen weight (27%)
at the high exposure. The no-observed-adverse-effect level (NOAEL) in
this study is 14.4 mg/kg body weight per day; the LOAEL is 144 mg/kg
body weight per day. The increase in liver weight, but not the
decrease in spleen weight, was confirmed in a subsequent 90-day study
by the same researchers.
8.4 Long-term exposure
8.4.1 Subchronic exposure
Sanders et al. (1982) administered chloral hydrate in
drinking-water to CD-1 mice at 70 or 700 mg/litre (equivalent to 16 or
160 mg/kg body weight per day) for 90 days. In males, hepatomegaly (an
increase in weight of 20% and 34% at the low and high exposure,
respectively) and microsome proliferation (no increase in total
microsomal protein, increase in cytochrome b5 of 26% and 40%,
increase in aminopyrine N-demethylase of 28% and 20%, and increase
in aniline hydroxylase of 24% and 30% at the low and high exposures,
respectively, when reported as mg of protein per mg of total liver
protein) were observed. There were no biologically significant changes
in serum enzymes. Hepatomegaly was not seen in females, but there were
changes in hepatic microsomal parameters (increase in total microsomal
protein of 10%, increase in aniline hydroxylase of 23%, and decrease
in cytochrome b5 of 12% when reported as mg of protein per mg of
total liver protein), but only at the high exposure. No other
significant toxicological changes were observed. Based on hepatomegaly
and changes in microsomal parameters in males at the high exposure,
this study identifies a LOAEL of 160 mg/kg body weight per day and a
NOAEL of 16 mg/kg body weight per day.
Daniel et al. (1992b) exposed male and female Sprague-Dawley rats
(10 per sex per dose) for 90 days to chloral hydrate in drinking-water
at a concentration of 300, 600, 1200, or 2400 mg/litre (equivalent to
an exposure of 24, 48, 96, or 168 mg/kg body weight per day in males
and 33, 72, 132, or 288 mg/kg body weight per day in females). The
tissues of animals from the high-exposure group and liver sections
from all treated males were examined histopathologically. No mortality
occurred in any groups prior to sacrifice. Organ weights, including
liver weight, and clinical chemistry values in treated animals were
only sporadically or inconsistently different from control animal
values. Focal hepatocellular necrosis was observed in 2 of 10 males in
each of the groups exposed to 96 and 168 mg/kg body weight per day.
The necrotic lesion was minimal at 96 mg/kg body weight per day and
was significantly more severe at 168 mg/kg body weight per day.
Necrotic lesions were not reported in any treated females or in any
control animals. While serum enzymes were generally increased in
treated animals, dramatic increases were reported in males in the
168 mg/kg body weight per day group; mean aspartate aminotransferase,
alanine aminotransferase, and lactate dehydrogenase levels in this
group were elevated 89%, 54%, and 127% above the corresponding control
values, respectively.
8.4.2 Chronic exposure and carcinogenicity
Rijhsinghani et al. (1986) evaluated carcinogenic effects in male
mice (C57BL × C3HF1). Groups of 15-day-old mice received chloral
hydrate by gavage in distilled water at 0, 5, or 10 mg/kg body weight
(26, 15, and 14 mice per group, respectively). Animals were sacrificed
when moribund or at week 78, at week 88, or between weeks 89 and 92.
Livers were examined histopathologically using light and electron
microscopy. In mice sacrificed 48-92 weeks after treatment, the
incidence of hepatic nodules (adenomas or trabecular carcinomas) was
3/9 and 6/8 for animals from the 5 and 10 mg/kg body weight per day
dose groups, respectively, compared with 2/19 in controls. The
increase in tumours was statistically significant (P < 0.05) only
in the 10 mg/kg body weight group.1
Daniel et al. (1992a) exposed 40 male B6C3F1 mice for 104 weeks
to drinking-water containing chloral hydrate at 1 g/litre (equivalent
to 166 mg/kg body weight per day). Untreated control animals (23 in
one group and 10 in a second group) received distilled water. Interim
sacrifices were conducted at 30 and 60 weeks of exposure (five animals
per group at each sacrifice interval). Complete necropsy and
microscopic examination were performed. There were no significant
treatment-related effects on survival or body weight. There were no
changes in spleen, kidney, or testis weights or histopathological
changes in any tissue except the liver. The toxicity in the liver was
characterized by increased absolute liver weight and liver to body
weight ratio at all three sacrifice intervals. At week 104, liver
weight was 37% higher than in controls, and liver to body weight ratio
was 42% higher than in controls. Hepatocellular necrosis was noted in
10/24 (42%) treated animals; other pathological changes of mild
severity reported in the livers of treated animals included
cytoplasmic vacuolization, cytomegaly, and cytoplasmic alteration. The
prevalence of liver tumours at terminal sacrifice was statistically
significantly (P < 0.05) increased over controls, with
hepatocellular carcinomas in 11/24 and hepatocellular adenomas in
7/24 animals; for carcinomas and adenomas combined, the prevalence was
17/24. In control animals, carcinomas, adenomas, and carcinomas and
adenomas (combined) occurred in 2/20, 1/20, and 3/20, respectively.
At the 60-week sacrifice, there were 2/5 treated animals with
hepatocellular carcinomas, compared with 0/5 controls. No carcinomas,
adenomas, or hyperplastic nodules were reported in animals sacrificed
at week 30.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity bioassay became available. In this study, an
up to 5 times higher single dose of chloral hydrate than that used
in the Rijhsinghani et al. (1986) study administered to male or
female B6C3F1 mice failed to induce tumours in any organ
(NTP, 2000a).
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male B6C3F1 mice. Mice were administered chloral
hydrate in drinking-water for 104 weeks. Mice (72 in each group) had a
mean exposure of 0, 13.5, 65, or 146.6 mg/kg body weight per day.
There was no change in water consumption, survival, behaviour, body
weight, or organ weights at any exposure. There was no evidence of
hepatocellular necrosis at any exposure and only minimal changes in
the levels of serum enzymes. This study identifies a NOAEL for
non-cancer effects in mice of 146.6 mg/kg body weight per day (the
highest exposure tested). There was no increase in the prevalence of
neoplasia at sites other than the liver. Although the background
response in this study is higher than normal for this strain of mice,
the mice showed an increase in proliferative lesions in the liver
(hyperplasia, adenoma, carcinoma, and combined adenoma and carcinoma)
at all exposures. These data are summarized in Table 1. The calculated
effective dose for a 10% tumour incidence (ED10) is 1.98 mg/kg body
weight per day, and its 95% lower confidence limit (LED10) is
1.09 mg/kg body weight per day (see Appendix 2).
Leuschner & Beuscher (1998) conducted a chronic bioassay for
carcinogenicity in Sprague-Dawley rats. Chloral hydrate was
administered in drinking-water for 124 weeks (males) and 128 weeks
(females). The rats (50 males and 50 females in each group) had an
exposure of 15, 45, or 135 mg/kg body weight per day. There was no
effect on survival, appearance, behaviour, body weight, food and water
consumption, or organ weights. There was no evidence of an increased
incidence of tumours in any organ. Histopathological examination
revealed an increased incidence of hepatocellular hypertrophy at the
highest exposure in males only (11% in controls versus 28% at the
highest exposure; P < 0.01). This finding, graded as minimal to
slight in severity, was characterized by a diffuse liver cell
enlargement with slightly eosinophilic cytoplasm and was considered by
the authors as a first sign of toxicity. The type, incidence, and
severity of other non-neoplastic lesions were not increased in treated
animals compared with controls. Based on the evidence of minimal
toxicity in the liver, which is of doubtful biological significance,
this study establishes a NOAEL of 45 mg/kg body weight per day and a
LOAEL of 135 mg/kg body weight per day.
George et al. (2000) conducted a chronic bioassay for
carcinogenicity in male F344 rats. Rats were administered chloral
hydrate in drinking-water for 104 weeks. Rats (78 in each group) had a
mean daily exposure of 0, 7.4, 37.4, or 162.6 mg/kg body weight per
day. There was no change in water consumption, survival, behaviour,
body weight, or organ weights at any exposure. There was no indication
of liver toxicity at any exposure as shown by the lack of liver
necrosis, lack of hyperplasia, no increase in mitotic index, and only
minimal changes in the levels of serum enzymes. There was no increase
at any exposure in the prevalence or multiplicity of hepatocellular
neoplasia or neoplasia at any other site. This study identifies a
NOAEL of 162.6 mg/kg body weight per day (the highest exposure
tested).1
Two of the metabolites of chloral hydrate, trichloroacetic acid
and dichloroacetic acid, have been shown to cause liver tumours in
rodents. For example, trichloroacetic acid in drinking-water induced
liver tumours in male and female mice when the exposure exceeded 200
mg/kg body weight per day (Herren-Freund et al., 1987; Bull et al.,
1990; Pereira, 1996). There was no evidence of increased
carcinogenicity, however, when male rats were exposed to
trichloroacetic acid at 360 mg/kg body weight per day (DeAngelo et
al., 1997). Dichloroacetic acid in drinking-water induced liver
tumours in male and female mice when the exposure exceeded 160 mg/kg
body weight per day (Herren-Freund et al., 1987; Bull et al., 1990;
DeAngelo et al., 1991; Daniel et al., 1992a; Ferreira-Gonzalez et al.,
1995; Pereira, 1996). Dichloroacetic acid also induced liver tumours
in male rats when the exposure exceeded 40 mg/kg body weight per day
(Richmond et al., 1995; DeAngelo et al., 1996).
A number of studies have shown that trichloroethylene is toxic to
the mouse lung bronchiolar epithelium, causing a highly specific
lesion to the clara cells of mice. Short-term exposure causes
vacuolization of the clara cells; long-term exposure causes pulmonary
adenomas and adenocarcinomas (Odum et al., 1992; Green et al., 1997).
These effects are thought to be due to the accumulation of chloral
within the clara cells. Trichloroethylene is efficiently metabolized
to chloral, but the major pathway from chloral to trichloroethanol and
its glucuronide is blocked, leading to an accumulation of chloral and
the observed toxicity.
1 After the Final Review Board meeting, a National Toxicology
Program carcinogenicity assay became available. In this study,
lifetime gavage administration of chloral hydrate at similar dose
levels induced hepatocellular tumours in male B6C3F1 mice and a low
frequency of pituitary hyperplasia and adenomas in females that was
of borderline statistical significance (NTP, 2000b).
Table 1: Prevalence and multiplicity of hepatocellular proliferative lesions in mice at 104 weeks.a
Treatment group Number examinedc Hyperplasia Adenoma Carcinoma Adenoma
(mg/kg body + carcinoma
weight per day)b
0 42 7.1d 21.4d 54.8d 64.3d
0.07 ± 0.04e 0.21 ± 0.06e 0.74 ± 0.12e 0.95 ± 0.12e
13.5 46 32.6f 43.5f 54.3 78.6f
0.41 ± 0.10f 0.65 ± 0.12f 0.72 ± 0.11 1.37 ± 0.16f
65 39 33.3f 51.3f 59.0 79.5f
0.38 ± 0.09f 0.95 ± 0.18f 1.03 ± 0.19 1.97 ± 0.23f
146.6 32 37.5f 50.0f 84.4f 90.6f
0.41 ± 0.10f 0.72 ± 0.15f 1.31 ± 0.17f 2.03 ± 0.25f
a From George et al. (2000).
b Time-weighted mean daily dose.
c Animals surviving longer than 78 weeks.
d Prevalence (percentage of animals with at least one lesion).
e Multiplicity (number of lesions per animal ± SEM).
f Statistically different from the control value, P < 0.05.
8.5 Genotoxicity and related end-points
8.5.1 Genotoxicity
There is an extensive database on the genotoxicity of chloral
hydrate and its metabolites. A complete summary of these results is
provided in US EPA (2000).
Chloral hydrate did not induce mutation in most strains of
Salmonella typhimurium, but did in some studies with S. typhimurium
TA100 and in a single study with S. typhimurium TA104. The latter
response was inhibited by free-radical scavengers alpha-tocopherol and
menadione (Ni et al., 1994).
Chloral hydrate did not induce mitotic crossing-over in
Aspergillus nidulans in the absence of metabolic activation. Chloral
hydrate caused weak induction of meiotic recombination in the presence
of metabolic activation and gene conversion in the absence of
metabolic activation in Saccharomyces cerevisiae. It did not induce
reverse mutation in S. cerevisiae. Chloral hydrate clearly induced
aneuploidy in various fungi in the absence of metabolic activation.
Chloral hydrate induced somatic and germ cell mutations in
Drosophila melanogaster.
Chloral hydrate did not produce DNA-protein cross-links in rat
liver nuclei, DNA single-strand breaks/alkaline-labile sites in
primary hepatocytes in vitro, or DNA repair in Escherichia coli.
One study showed induction of single-strand breaks in liver DNA of
both rats and mice treated in vivo; another study in both species
using higher concentrations of chloral hydrate found no such effect.
Chloral hydrate was weakly mutagenic, but did not induce
micronuclei in mouse lymphoma cells in vitro. Chloral hydrate
increased the frequency of micronuclei in Chinese hamster cell lines.
Although a single study suggested that chloral hydrate induces
chromosomal aberrations in Chinese hamster CHED cells in vitro, the
micronuclei produced probably contained whole chromosomes and not
chromosome fragments, as the micronuclei could all be labelled with
antikinetochore antibodies.
In kangaroo rat kidney epithelial cells, chloral hydrate
inhibited spindle elongation and broke down mitotic microtubuli,
although it did not inhibit pole-to-pole movement of chromosomes. It
produced multipolar spindles, chromosomal dislocation from the mitotic
spindle, and a total lack of mitotic spindles in Chinese hamster
DON:Wg.3h cells.
Chloral hydrate weakly induced sister chromatid exchange in
cultures of human lymphocytes. It induced micronuclei, aneuploidy,
C-mitosis, and polyploidy in human lymphocytes in vitro. Micronuclei
were induced in studies with human whole blood cultures but not in one
study with isolated lymphocytes. The differences seen in the
micronucleus test have been attributed to differences between whole
blood and purified lymphocyte cultures (Vian et al., 1995), but this
hypothesis has not been tested.
Chloral hydrate increased the frequency of chromosomal
aberrations in mouse bone marrow, spermatogonia, and primary and
secondary spermatocytes, but not in oocytes, after in vivo
treatment. Chloral hydrate induced chromosomal aberrations in mouse
bone marrow erythrocytes after treatment in vivo. In one of these
studies, the use of antikinetochore antibodies suggested induction of
micronuclei containing both whole chromosomes and fragments. Chloral
hydrate induced micronuclei in the spermatids of mice treated in vivo
in some studies. Chloral hydrate induced aneuploidy in the bone
marrow of mice treated in vivo. It increased the rate of aneuploidy
in mouse secondary spermatocytes. It did not produce polyploidy in
bone marrow, oocytes, or gonosomal or autosomal univalents in primary
spermatocytes of mice treated in vivo. Chloral hydrate, however,
induced polyploidy and meiotic delay when a synchronized population of
mouse oocytes was exposed in vitro prior to the resumption of
maturation.
Trichloroethanol, a reduction product of chloral hydrate, did not
induce lambda prophage in E. coli or mutation in S. typhimurium
TA100. Trichloroethanol caused spindle aberrations when mouse oocytes
were treated in vitro.
Trichloroacetic acid did not induce lambda prophage in E. coli
and was not mutagenic to S. typhimurium in the presence or absence
of metabolic activation. Trichloroacetic acid was weakly positive in
the mouse lymphoma assay with metabolic activation. Trichloroacetic
acid also did not induce chromosomal damage in human lymphocytes or
micronuclei in bone marrow in vitro. It is unclear whether
trichloroacetic acid can induce chromosomal damage in vivo, because
some studies have been positive and others negative.
Dichloroacetic acid did not induce differential toxicity in DNA
repair-deficient strains of S. typhimurium but did induce lambda
prophage in E. coli. Dichloroacetic acid gave equivocal results for
gene mutation in S. typhimurium TA100 and TA98. Dichloroacetic acid
was weakly mutagenic in the in vitro mouse lymphoma assay and
induced chromosomal aberrations but not micronuclei or aneuploidy in
that test system. Dichloroacetic acid induced micronuclei in mouse
polychromatic erythrocytes in vivo and mutations at the lacI locus
in the transgenic B6C3F1 mouse (the Big Blue Mouse) in vivo at an
exposure that induces liver tumours in male mice. It is unclear
whether dichloroacetic acid can induce primary DNA damage, as some
assays are positive and others negative.
8.5.2 Cell proliferation
Rijhsinghani et al. (1986) evaluated the acute effects of chloral
hydrate on liver cell proliferation in 15-day-old male mice (C57BL ×
C3HF1). Mice were given 0, 5, or 10 mg chloral hydrate/kg body
weight by gavage in distilled water (9, 10, and 6 mice per group,
respectively) and sacrificed after 24 h. Cell proliferation was
evaluated by calculating the mitotic index (number of mitoses per 100
nuclei) from liver sections. The mitotic index in liver cells was
significantly increased (0.9235) in mice receiving 5 mg/kg body weight
when compared with the control value (0.3382), and elevated (0.7433)
(although not statistically significantly) in mice receiving 10 mg/kg
body weight. Hepatic necrosis was not observed in mice from either
treatment group at autopsy.
As part of the chronic bioassay for carcinogenicity, George et
al. (2000) evaluated hepatocyte proliferation in male F344 rats and
male B6C3F1 mice. Exposures are given in section 8.4.2. Five days
prior to sacrifice at 13, 26, 52, or 72 weeks in rats and 26, 52, or
78 weeks in mice, animals were given bromodeoxyuridine. Labelled
nuclei were identified by chromogen pigment over the nuclei, and the
labelling index was calculated. Outside of the areas with tumours in
the liver of mice, there was no significant evidence of increased
hepatocyte proliferation in rats or mice.
8.5.3 Oncogene activation
Velazquez (1994) investigated the induction of H- ras
proto-oncogene mutations in mice. DNA from normal liver and tumour
tissue was obtained from male B6C3F1 mice administered 1 g chloral
hydrate/litre (166 mg/kg body weight per day) in drinking-water for
2 years. H- ras mutations were present in one out of seven (14%)
tumours. The spectrum of mutations was the same as that of spontaneous
liver tumours. Based on these data, it is unlikely that H- ras
activation is a mechanism of carcinogenicity relevant to chloral
hydrate.
8.5.4 Free radicals and DNA adduct formation
Ni et al. (1994, 1995, 1996) studied the metabolism of chloral
hydrate in an in vitro system using microsomes from male B6C3F1
mice. The metabolism of chloral hydrate generated free radicals as
detected by electron spin resonance spectroscopy and caused endogenous
lipid peroxidation, resulting in the production of malondialdehyde,
formaldehyde, and acetaldehyde, all of which are known to produce
liver tumours in rodents. Trichloroacetic acid and trichloroethanol
also produced free radicals and induced lipid peroxidation when tested
in this system. The authors speculated that the free radicals were
Cl3CCO2Ê. and/or Cl3CÊ. Incubation of chloral hydrate,
trichloroethanol, or trichloroacetic acid in the presence of
microsomes and calf thymus DNA resulted in the formation of a
malondialdehyde-modified DNA adduct. This research group further
showed that chloral hydrate induced an increase in mutations at the
hprt and tk loci in transgenic human lymphoblastoid cells
containing CYP2E1. In contrast, when the parental cell line lacking
CYP2E1 was treated with the same concentration of chloral hydrate, no
mutations were found at either locus. These data implicate CYP2E1 as
the primary cytochrome subfamily involved in the metabolism of chloral
hydrate to reactive intermediates.
8.5.5 Cell communication
The effects of 1-, 4-, 6-, 24-, 48-, and 168-h exposures to
chloral hydrate (0, 1, 5, or 10 mmol/litre) on gap junction
intercellular communication in Clone 9 cell cultures (normal rat
hepatocytes) were reported by Benane et al. (1996). No differences in
intercellular communication were seen between the groups treated with
1 mmol/litre at 1, 4, and 6 h of exposure and controls, as measured by
a dye transfer protocol. There were significant differences between
all other groups and the controls. The shortest exposure time and
lowest exposure concentration that reduced dye transfer significantly
were in the group treated with 1 mmol/litre for 24 h.
8.5.6 Peroxisome proliferation
As part of the chronic bioassay for carcinogenicity in mice,
George et al. (2000) found no evidence of peroxisome proliferation
using cyanide-insensitive palmitoyl CoA oxidase in the livers of male
mice treated with chloral hydrate for 26 weeks.
8.6 Reproductive and developmental toxicity
Klinefelter et al. (1995) evaluated effects on sperm morphology
and motility in F344 rats administered chloral hydrate in
drinking-water for 52 weeks at levels of 0, 55, or 188 mg/kg body
weight per day. The researchers examined cauda epididymal sperm motion
parameters and testicular and epididymal histopathology. Chloral
hydrate did not cause any visible systemic toxicity and had no effects
on epididymal or testicular histopathology. However, the percentage of
motile sperm was significantly decreased (P < 0.01) from 68% in
controls to 58% in rats exposed to 188 mg/kg body weight per day. The
percentage of progressively motile sperm was also significantly
decreased (P < 0.01) from 63% in controls to 53% in this group. In
addition, the frequency distribution of the average straight-line
velocities of sperm at this exposure was significantly shifted
(P < 0.01) to the lower ranges when compared with controls. In this
study, the NOAEL is 55 mg/kg body weight per day; the LOAEL is 188
mg/kg body weight per day.
Kallman et al. (1984) exposed male and female CD-1 mice to
chloral hydrate in drinking-water at 21.3 or 204.8 mg/kg body weight
per day. Animals were exposed for 3 weeks prior to breeding. Exposure
of females (5 per group) continued during gestation and until pups
were weaned at 21 days of age. No gross malformations were noted, and
no significant effects were observed in duration of gestation, number
of pups delivered, pup weight, or number of stillborn pups. All pups
(15 per group) showed the same rate of development and level of
performance on several neurobehavioural tests, except that pups
exposed to 204.8 mg/kg body weight per day when tested at 23 days of
age showed impaired retention of passive avoidance learning on both
the 1-h and 24-h retention tests (P < 0.05). This study identified
a NOAEL for neurodevelopmental toxicity of 21.3 mg/kg body weight per
day and a LOAEL of 204.8 mg/kg body weight per day based on the
impairment in passive avoidance learning. This study also identifies a
NOAEL for reproductive and other developmental effects of 204.8 mg/kg
body weight per day (the highest exposure tested).
Johnson et al. (1998) tested the potential for chloral hydrate to
cause developmental toxicity in Sprague-Dawley rats. Chloral hydrate
was administered in drinking-water to 20 rats from gestational day 1
to gestational day 22 at an average exposure of 151 mg/kg body weight
per day. Control animals were given distilled water. There was no
evidence of maternal toxicity, no change in the number of implantation
or resorption sites, no change in the number of live or dead fetuses,
no change in placental or fetal weight, no change in crown-rump
length, and no increase in the incidence of morphological changes. A
detailed examination found no evidence of cardiac anomalies. Based on
this study, the NOAEL for developmental toxicity is 151 mg/kg body
weight per day (the highest exposure tested).
Johnson et al. (1998) also tested the potential for
trichloroethanol and trichloroacetic acid to cause developmental
toxicity in Sprague-Dawley rats. The protocol was identical to the
study with chloral hydrate. Trichloroethanol was administered to 10
rats at an average exposure of 153 mg/kg body weight per day. No
evidence of developmental toxicity was found. In contrast, when
trichloroacetic acid was administered to 11 rats at an average
exposure of 291 mg/kg body weight per day, developmental toxicity was
observed. The effects included statistically significant (P < 0.05)
increases in average resorptions, in average implantations, and in
cardiac anomalies. Although the specific cardiac anomalies found were
different, the results with trichloroacetic acid are generally
consistent with those reported by Smith et al. (1989), who observed
adverse developmental effects from trichloroacetic acid at an exposure
of 330 mg/kg body weight per day and above.
Saillenfait et al. (1995) tested the potential of chloral hydrate
to cause developmental toxicity using a rat whole-embryo culture
system. Embryos (20 per dose) from Sprague-Dawley rats were explanted
on gestational day 10 and exposed to chloral hydrate at a
concentration of 0, 0.5, 1, 1.5, 2, or 2.5 mmol/litre (equivalent to
0, 83, 165, 248, 331, or 414 mg/litre) for 46 h. At 2.5 mmol/litre,
all embryos died. No lethality was seen at lower exposures. Chloral
hydrate caused concentration-dependent decreases in growth and
differentiation and increases in the incidence of morphologically
abnormal embryos. No effects were observed in any parameter at
0.5 mmol/litre. Decreases in crown-rump length, somite
(embryonic segment) number, and the protein or DNA content of embryos
were seen at 1 mmol/litre and above. At 1, 1.5, and 2 mmol chloral
hydrate/litre, respectively, 18%, 68%, and 100% of embryos were
malformed. Brain, eye, and ear malformations were the most prominent
effects at these concentrations. Abnormalities in the trunk and
pericardial dilation also occurred at 2 mmol/litre. In this in vitro
test system, chloral hydrate was a slightly more potent teratogen than
trichloroacetic acid or dichloroacetic acid.
Although chloral hydrate did not cause meiotic delay in the
oocytes of adult mice when administered at the time of resumption of
maturation induced by hormones (Mailhes & Marchetti, 1994), it did
cause adverse effects in vitro when a synchronized population of
oocytes was exposed prior to resumption of maturation
(Eichenlaub-Ritter & Betzendahl, 1995; Eichenlaub-Ritter et al.,
1996). In this test system, chloral hydrate induced lagging of
chromosomes during telophase I, inhibited spindle elongation in
anaphase B, and caused chromosome displacement from the spindle
equator in metaphase I and II. Oocytes became irreversibly arrested in
maturation when exposed to chloral hydrate prior to resumption of
maturation or when chloral hydrate was present during the first or
second 8 h of maturation. Spindle aberrations were observed when
oocytes were treated with trichloroethanol (Eichenlaub-Ritter et al.,
1996).
8.7 Immunological and neurological effects
Kauffmann et al. (1982) administered chloral hydrate by gavage in
distilled water at 14.4 or 144 mg/kg body weight per day to groups of
11-12 male CD-1 mice for 14 days. No effects on humoral or
cell-mediated immunity were detected at either exposure.
Kauffmann et al. (1982) administered chloral hydrate to male and
female CD-1 mice in drinking-water at 70 or 700 mg/litre (equivalent
to 16 or 160 mg/kg body weight per day) for 90 days. Humoral immunity
was assessed by the number of splenic antibody-forming cells produced
against sheep red blood cells (12 mice in the control group and 8 mice
in the exposed groups) and haemagglutination titres (20-21 mice in the
control group and 13-16 mice in the exposed groups).
Cell-mediated immunity was assessed by delayed-type hypersensitivity
to sheep red blood cells (17-20 mice in the control group and
15-16 mice in the exposed groups). Lymphocyte response was assessed
using a T-cell mitogen (Con A) and a B-cell mitogen (LPS)
(17-22 animals in the control group and 13-16 mice in the exposed
groups). In males, no effects were detected in either humoral or
cell-mediated immunity at either exposure. No effects on cell-mediated
immunity were noted in females at either exposure. In females, both
exposures resulted in a statistically significant decrease
(P < 0.05) in humoral immune function (36% and 40% at the
low and high exposures, respectively) when expressed as
antibody-forming cells per spleen. The decrease, however, was
statistically significant only at the higher exposure when
expressed as antibody-forming cells per million spleen cells
(a 32% decrease). There was no effect on haemagglutination titres
or on spleen cell response to the B-cell mitogen at either exposure.
The decrease in antibody-forming cells per million spleen cells at
the higher exposure in female mice is regarded as an adverse
response in this study. Accordingly, the NOAEL for immunotoxicity is
16 mg/kg body weight per day; the LOAEL is 160 mg/kg body weight
per day.
Kallman et al. (1984) administered chloral hydrate by gavage in
distilled water at 50, 100, 200, 300, or 400 mg/kg body weight to
groups of 12 male CD-1 mice. All doses resulted in the rapid onset of
ataxia, with an ED50 (maximal effect seen in 50% of animals) of
84.2 mg/kg body weight at 5 min (the time of maximal effect). Animals
recovered within 2-3 h. No delayed changes in muscular coordination
were detectable when the mice were tested 24 h after treatment.
Kallman et al. (1984) evaluated behavioural toxicity in groups of
12 male CD-1 mice administered chloral hydrate by gavage in distilled
water at 14.4 or 144 mg/kg body weight per day for 14 days. When
measured 24-48 h after exposure was terminated, no significant effects
on body weight, motor activity, physical appearance, behaviour,
muscular coordination, or endurance were observed.
Kallman et al. (1984) exposed groups of 12 male CD-1 mice to
drinking-water containing chloral hydrate at a concentration of 70 or
700 mg/litre (equivalent to 16 or 160 mg/kg body weight per day) for
90 days. When measured 24 h after exposure was terminated, no
treatment-related effects on mortality, body weight, physical
appearance, behaviour, locomotor activity, learning in repetitive
tests of coordination, response to painful stimuli, strength,
endurance, or passive avoidance learning were observed. Both exposures
resulted in a decrease of about 1°C in mean body temperature
(P < 0.05). Because of the lack of increased effect with a 10-fold
increase in exposure and because hypothermia as a side-effect of
chloral hydrate or from an overdose of chloral hydrate has not been
reported in humans, the decrease in body temperature is not considered
an adverse effect. This study identifies a NOAEL for neurobehavioural
toxicity of 160 mg/kg body weight per day (the highest exposure
tested).
A condensation product of tryptamine and chloral hydrate,
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline (TaClo), has been
found in the blood of elderly patients administered chloral hydrate
for 2-7 days (Bringmann et al., 1999). This metabolite initiated a
slowly progressive neurodegeneration when administered to rats in a
subchronic study (Gerlach et al., 1998). There is, however, no
evidence of neurodegeneration in the chronic studies with chloral
hydrate in rats and mice.
9. EFFECTS ON HUMANS
Chloral hydrate has been widely used as a sedative and hypnotic
drug in humans. Trichloroethanol is responsible for the
pharmacological activity (Marshall & Owens, 1954; Breimer, 1977;
Goodman & Gilman, 1985). Exposure information is discussed in section
6.2.
Chloral hydrate is irritating to the skin and mucous membranes
and often causes gastric distress, nausea, and vomiting at recommended
doses. There are no reports of sensitization in humans. Overdoses
produce (in order of progression) ataxia, lethargy, deep coma,
respiratory depression, hypotension, and cardiac arrhythmias. The
life-threatening effects are from severe respiratory depression,
hypotension, and cardiac arrhythmias. For some representative case
reports, see Marshall (1977), Anyebuno & Rosenfeld (1991), Ludwigs et
al. (1996), and Sing et al. (1996). A potentially life-threatening
oral dose for humans is approximately 10 g (143 mg/kg body weight),
although death has been reported from as little as 4 g, and some
individuals have survived ingesting 30 g or more. Extended use of
chloral hydrate may result in development of tolerance to the
pharmacological effect and physical dependence on or addiction to
chloral hydrate.
Shapiro et al. (1969) reviewed the medical records of 1618
patients who had received chloral hydrate at 1 g (213 patients, 13%),
0.5 g (1345 patients, 83%), or various other doses (60 patients, 4%).
Adverse reactions were reported in 38 patients (2.3%). Of these
patients, 4 received 1 g, 1 received 0.75 g, and 33 received 0.5 g.
Reported adverse reactions included gastrointestinal symptoms in 10
patients, central nervous system (CNS) depression in 20 patients, skin
rash in 5 patients, prolonged prothrombin time in 1 patient, and
bradycardia in 1 patient. In all patients, the side-effects
disappeared when chloral hydrate therapy was stopped. There was no
evidence of association between adverse side-effects and age, weight,
or sex.
Miller & Greenblatt (1979) reviewed the medical records of 5435
hospital patients who received chloral hydrate at a dose of either
0.5 g (about 7-8 mg/kg body weight) or 1 g (about 14-16 mg/kg body
weight). Adverse reactions were noted in 119 cases (2.2%).
CNS depression was most common (58 patients, or 1.1%), with minor
sensitivity reactions, including rash, pruritus, fever, and
eosinophilia, second most common (19 patients, or 0.35%). Other
adverse reactions included gastrointestinal disturbances (0.28%) and
CNS excitement (0.22%). Three individuals (0.05%) were judged to have
life-threatening reactions involving CNS depression, asterixis
(involuntary jerking movements), or hypotension. The data show that
adverse reactions involving the CNS become more frequent with
increasing dosage in patients older than 50 years, in patients who
died during hospitalization, in patients who received concurrently
benzodiazepine anti-anxiety drugs, and in patients with elevated
levels of blood urea nitrogen.
Greenberg et al. (1991) reported various side-effects experienced
by children receiving chloral hydrate sedation in preparation for
computer tomography (CT) procedures. In a "high-dose" group, composed
of 295 children (average age 2.18 years) who received a single dose of
80-100 mg/kg body weight and a maximum total dose of 2 g, adverse
reactions occurred in 23 of the patients (7%) and included vomiting
(14 patients), hyperactivity (5 patients), and respiratory symptoms,
such as wheezing and secretion aspiration (4 patients). Cardiac
monitoring did not reveal any abnormalities or arrhythmias in any of
the children. A second "lower-dose" cohort of 111 children (average
age 1.9 years) received 40-75 mg chloral hydrate/kg body weight. These
patients received the lower dose because of existing liver or renal
impairment, respiratory insufficiency, or CNS depression. There were
no adverse side-effects or complications reported in this group.
Children with severe liver or renal disease or affected by severe
CNS depression were not treated with chloral hydrate.
Lambert et al. (1990) conducted a retrospective analysis of
hospital medical records to investigate a possible link between
chloral hydrate administration and direct hyperbilirubinaemia (DHB) in
neonates following prolonged administration of chloral hydrate
(25-50 mg/kg body weight for up to 20 days). Direct bilirubin is a
measure of the free, unconjugated bilirubin in the serum. In the first
study, the DHB was of unknown etiology in 10 of the 14 newborns with
DHB; all 10 of these DHB patients had received chloral hydrate. In the
second study, among 44 newborns who had received chloral hydrate,
10 patients who developed DHB had received a mean cumulative dose of
1035 mg/kg body weight. In contrast, 34 patients whose direct
bilirubin levels were within normal ranges received a mean cumulative
dose of 183 mg/kg body weight. The total bilirubin levels (free plus
conjugated) were the same in both groups and within the normal range.
Kaplan et al. (1967) investigated whether ethanol ingestion
increased the effects of chloral hydrate. Five male volunteers
weighing 70-107 kg consumed ethanol (880 mg/kg body weight), chloral
hydrate (1 g, 9-14 mg/kg body weight), or both. Blood pressure and
cardiac rate did not vary significantly among treatments. In the
presence of ethanol, the concentration of trichloroethanol in the
blood rose more rapidly and reached a higher value, but the rate of
depletion was not significantly changed. The increase in the
concentration of trichloroethanol was not sufficient to produce a
marked enhancement of the hypnotic effect. The volunteers reported
symptoms (drowsiness, dizziness, blurred vision) and their severity
during the 6-h observation period. At all time points, the rank order
of effects was ethanol plus chloral hydrate > ethanol > chloral
hydrate.
No long-term studies of chloral hydrate in humans were located.
Chloral hydrate is addictive and is a controlled substance (Schedule
IV) in the USA.
10. EFFECTS ON OTHER ORGANISMS IN THE LABORATORY AND FIELD
Some data are available from cell multiplication inhibition tests
(toxic thresholds) in bacteria, algae, and protozoa. These data are
summarized in Table 2.
Schatten & Chakrabarti (1998) showed that chloral hydrate at 0.1%
(only concentration tested) causes alteration of centrosomal material
and abnormal microtubule configurations in California sea urchins
(Strongylocentrotus purpuratus and Lytechinus pictus). Chakrabarti
et al. (1998) also showed that chloral hydrate at 4 mmol/litre (660
mg/litre, only concentration tested) induced ciliary loss in the early
embryo phase of Lytechinus pictus. Exposure in this study was for 30
h at the blastula stage (14 h after fertilization).
Table 2: Effects of chloral hydrate on bacteria, algae, and protozoa.
Test system Effect Reference
Bacteria 16-h EC3 at 1.6 mg/litre Bringmann & Kuehn,
(Pseudomonas putida) 1980a
Green alga 7-day EC3 at 2.8 mg/litre Bringmann & Kuehn,
(Scenedesmus 1980a
quadricaudata)
Blue-green alga 8-day EC3 at 78 mg/litre Bringmann & Kuehn,
(cyanobacterium) 1976
(Microcystis
aeruginosa)
Protozoan 72-h EC5 at 79 mg/litre Bringmann & Kuehn,
(Enterosiphon sulcatum) 1980a
Protozoan EC5 at 86 mg/litre Bringmann & Kuehn,
(Uronema parduczi) 1980b
11. EFFECTS EVALUATION
11.1 Evaluation of health effects
11.1.1 Hazard identification and dose-response assessment
Chloral hydrate has been extensively used as a sedative and
hypnotic drug in human and veterinary medicine. The metabolite
trichloroethanol is responsible for the pharmacological effect.
Chloral hydrate is irritating to the skin and mucous membranes and
often causes gastric distress, nausea, and vomiting at recommended
doses. Acute overdoses produce (in order of progression) ataxia,
lethargy, deep coma, respiratory depression, hypotension, and cardiac
arrhythmias. There is some evidence of hepatic injury in people
surviving near-lethal, acute overdoses, but no convincing evidence
that hepatic injury results from the recommended clinical dose.
Despite its long use in human medicine, there is no published
information on toxicity in controlled studies in humans following
extended exposure.
Chloral hydrate is completely absorbed and rapidly metabolized
following oral administration. The major metabolites are
trichloroethanol and its glucuronide and trichloroacetic acid. Some
data suggest that a small amount of dichloroacetic acid may be formed.
In humans, the half-life of trichloroethanol and its glucuronide is
about 8 h; the half-life of trichloroacetic acid is about 4 days. Some
data suggest that the half-life of trichloroethanol is increased
several-fold in pre-term and full-term infants compared with toddlers
and adults. The major route of excretion of the metabolites of chloral
hydrate is elimination in the urine. Chloral hydrate and its
metabolites have been found in milk from women treated with chloral
hydrate. The concentration of these chemicals, however, is too low to
cause a pharmacological effect in the nursing infant.
Acute administration of chloral hydrate to mice causes loss of
coordination (ataxia) at about the same exposure as in humans for the
same effect. A 90-day study in mice shows no evidence of behavioural
changes or other neurotoxicity. Chronic studies in rats and mice show
no evidence of behavioural changes and no evidence of
histopathological changes in nervous tissue. These studies used an
exposure approximately 15 times the recommended clinical dose in
humans. There is some evidence of mild liver toxicity following
chronic exposure in rats and mice. A slight decrement in humoral
immunity was observed in female mice following exposure for 90 days.
The antibody-forming cell response is considered an excellent
indicator of the status of humoral immunity because of the complex
cellular cooperation required to produce antibody and because the
number of cells that produce antibody can be quantified. A depression
in the number of these cells is considered an adverse response because
the production of antibodies is important to the defence strategy of
the organism. However, the quantitative relationship between the
depression in antibody-forming cells in the spleen and the
concentration of circulating antibody is unknown. In this study,
because there was no depression in circulating antibodies measured by
the haemagglutination titre, there might be no significant depression
in the ability of the host to mount a protective antibody response.
Chloral hydrate has been tested for developmental effects in rats and
mice. No structural abnormalities were observed. A slight effect was
observed in mice in passive avoidance learning when dams were exposed
prior to breeding, during gestation, and during nursing and pups were
tested at 23 days of age. Although chloral hydrate has not been tested
in a two-generation reproduction study, the data on reproductive
performance and on effects on sperm and oocytes do not suggest that
reproductive toxicity is likely to be a critical effect. In addition,
no histopathological effects are observed in reproductive organs of
rodents in subchronic or chronic studies. Some in vitro data,
however, suggest that chloral hydrate administered to young female
children might have a latent effect on fertility. All of the studies
in laboratory animals show non-cancer health effects at an exposure
far in excess of the exposure that is effective for sedation in
humans. A complete summary of the exposure-response data is presented
in Table 3.
Simultaneous ingestion of ethanol and chloral hydrate increases
the sedative effects and side-effects of chloral hydrate. The
mechanism is the increase in the concentration of the
pharmacologically active metabolite, trichloroethanol, in the presence
of ethanol. Chronic users of ethanol are, therefore, somewhat more
sensitive to the adverse effects of chloral hydrate.
Because of the immaturity of hepatic metabolism, particularly the
glucuronidation pathway, and decreased glomerular filtration in
infants, the half-life of trichloroethanol is longer in pre-term and
full-term infants. This group is therefore somewhat more sensitive to
the adverse effects of chloral hydrate. Toddlers and adults are likely
to show similar sensitivity to chloral hydrate.
Although male laboratory rodents seem to be more sensitive than
female laboratory rodents to hepatic effects, there is no evidence of
a gender effect in humans with respect to the sedative effects or
side-effects of chloral hydrate at the recommended clinical dose.
There are no carcinogenicity data from humans. Two bioassays in
rats show no increase in tumours at any site. These studies were
limited, because only minimal toxicity was observed in the livers of
the rats in these bioassays. In one study, only slight hypertrophy was
observed at the highest exposure; in the other study, no effects were
observed at the highest exposure. No data are available in female
mice. There are three separate bioassays showing an increased
incidence of liver tumours in male mice. One study, conducted in a
very limited number of animals, showed an increase in tumours
Table 3: Summary of non-neoplastic effects.
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Human 1 day, 3 doses Sedation - 10.7 Goodman & Gilman, 1985
Rat 90 days Mild liver necrosis 96 168 Daniel et al., 1992b
and increase in
serum enzymes
Rat 104 weeks - 162.6 - George et al., 2000
Rat 124 weeks Liver hypertrophy 45 135 Leuschner & Beuscher, 1998
Rat 52 weeks Sperm motility 55 188 Klinefelter et al., 1995
Rat gestation Development 151 - Johnson et al., 1998
days 1-22
Mouse 14 days Increased liver weight 14.4 144 Sanders et al., 1982
Mouse 90 days Increased liver weight 16 160 Sanders et al., 1982
Mouse 104 weeks Increased liver weight - 166a Daniel et al., 1992a
and necrosis
Mouse 104 weeks - 146.6b - George et al., 2000
Mouse 3 weeks Reproduction and 204.8 - Kallman et al., 1984
pre-breeding development
and during
gestation
Mouse Pre-breeding, Passive avoidance 21.3 204.8 Kallman et al., 1984
gestation, and learning in pups
nursing
Table 3 (cont'd)
Species Duration End-point NOAEL LOAEL Reference
(mg/kg body (mg/kg body
weight per weight per
day) day)
Mouse 1 day Ataxia - 50 Kallman et al., 1984
Mouse 14 days Neurobehaviour 144 - Kallman et al., 1984
Mouse 90 days Neurobehaviour 160 - Kallman et al., 1984
Mouse 14 days Immunotoxicity 144 - Kauffmann et al., 1982
Mouse 90 days Humoral immunity 16 160 Kauffmann et al., 1982
a Tumours at 166 mg/kg body weight per day.
b Hyperplasia and tumours at 13.5, 65, and 146.6 mg/kg body weight per day.
following a single exposure. The second study tested only one exposure
level but used an adequate number of animals. The third study shows an
increase in incidence and multiplicity of liver tumours at each of
three exposures. There are no data identifying a lesion that is a
precursor to the tumours. The strain of mice used has a very high
spontaneous incidence of liver tumours. Two of the metabolites of
chloral hydrate, trichloroacetic acid and dichloroacetic acid, have
been shown to cause liver tumours in rodents. Trichloroacetic acid
causes liver tumours only in mice. Dichloroacetic acid causes tumours
in both rats and mice.1
Chloral hydrate has been extensively studied as a genotoxic
agent. Chloral hydrate was positive in some bacterial mutation tests,
indicating that it may be capable of inducing point mutations. It was
also positive in the mouse lymphoma assay for mutations at the tk
locus. Chloral hydrate also induced somatic and germ cell mutations
in D. melanogaster. Some data also show chloral hydrate to be a very
weak clastogen in mammalian cells.
Chloral hydrate has been shown to induce aneuploidy in a variety
of cells, including S. cerevisiae, A. nidulans, Chinese hamster
embryonic fibroblasts, Chinese hamster primary cell lines LUC2 and
DON:Wg3h, human peripheral blood lymphocytes, mouse spermatocytes, and
mouse spermatids. Because there is a mixture of positive and negative
in vivo data, with no reason to weigh some studies more than others,
it is not clear whether chloral hydrate is capable of inducing genetic
damage in vivo. Additional in vivo studies using standard
protocols would help clarify the relevance of genetic damage to a
human health risk assessment.
The effects on aneuploidy are thought to arise via disruption of
the mitotic spindle structure or function by inhibition of tubulin
and/or microtubule-associated proteins; both substances are components
of the spindle apparatus. Some data also suggest that chloral hydrate
may act on the spindle apparatus through an increase in the
concentration of intracellular free calcium.
1 In a National Toxicology Program carcinogenicity bioassay that
became available after the Final Review Board meeting, a
carcinogenic effect was not observed after a single dose of chloral
hydrate; after lifetime exposure, males had an increased incidence
of hepatic tumours, and females had a low increased incidence of
pituitary adenomas that was of borderline statistical significance.
Several other mechanisms may play a role in the induction of
tumours in the liver of male mice. There is no convincing evidence
that chloral hydrate causes direct damage to DNA. In vitro studies
with chloral hydrate, trichloroethanol, and trichloroacetic acid and
mouse microsomes, however, show lipid peroxidation and the formation
of covalently bound DNA adducts. These effects appear to be mediated
by the formation of free radicals by CYP2E1. Another possibility is
cytotoxicity leading to compensatory hyperplasia. A single treatment
of mice with chloral hydrate caused an increase in the mitotic index
in liver cells. The increased cell division is hypothesized to either
provide additional opportunities for errors in DNA replication or
allow initiated cells to progress to a tumour. Another potentially
contributing mechanism of carcinogenesis is disruption of
intercellular communication, which has been shown in one experiment to
be influenced by chloral hydrate.
The mechanism of chloral hydrate-induced carcinogenicity in male
mice is unclear. Two mechanisms that appear ruled out are H- ras
proto-oncogene activation and peroxisome proliferation.
Although there is suggestive evidence of carcinogenicity in male
mice, the weight of evidence is not sufficient to consider tumour
induction as the critical effect.
11.1.2 Criteria for setting tolerable intakes or guidance values for
chloral hydrate
The effect that occurs at the lowest exposure is mild sedation in
humans. As this effect would not be intended or desirable in the
general population outside of the clinical setting, this response is
considered an adverse effect and is used to derive the tolerable
intake.
Acute gavage exposure in mice shows neurological effects (ataxia)
at about the same exposure for the comparable effect in humans. A
subchronic study in mice using sensitive tests for neurobehavioural
changes found none. Chronic studies in rats and mice show no evidence
of neurobehavioural changes and no evidence of histopathological
changes in nervous tissue. As with other chlorinated chemicals, there
is some evidence of carcinogenic effects in the liver of male mice
following chronic exposure.
Although the tolerable intake derived from the pharmacologically
active dose in humans is an acute tolerable intake, keeping the
exposure below this level will also be protective for any non-cancer
health effect from chronic exposure. Therefore, it is appropriate to
use the acute tolerable intake as the chronic tolerable intake as
well.
No data are available to determine a NOAEL in humans. The
recommended clinical dose for sedation in adults is 250 mg, taken 3
times a day (Goodman & Gilman, 1985). A low incidence of side-effects
also occurs at this exposure. The LOAEL is 10.7 mg/kg body weight per
day (assuming a 70-kg body weight). The pharmacokinetic information
shows that chloral hydrate and the pharmacologically active
metabolite, trichloroethanol, will not bioaccumulate.
The tolerable intake (IPCS, 1994) of 0.1 mg/kg body weight per
day was derived from the LOAEL of 10.7 mg/kg body weight per day using
a total uncertainty factor of 100. An uncertainty factor of 10 was
used to extrapolate from a LOAEL to a NOAEL, and an uncertainty factor
of 10 was used for intraspecies variability. An uncertainty factor for
chronic duration was not used. Chloral hydrate and the active
metabolite, trichloroethanol, do not bioaccumulate. The half-life of
chloral hydrate is a few minutes, and the half-life of
trichloroethanol is a few hours. Therefore, an enhanced effect from
continuous, daily exposure is not possible. Finally, there is
information from clinical use that long-term exposure to chloral
hydrate results in tolerance to the sedative effect. Developmental
toxicity, including developmental neurotoxicity, and immunotoxicity
are not critical effects. Although there is no two-generation
reproduction study, an uncertainty factor for database limitations is
not needed, as there is evidence from several studies that
reproductive toxicity is not likely to be a critical effect.
There are no inhalation studies adequate for setting a guidance
value or tolerable intake.
There are data in male mice showing that chloral hydrate causes
tumours in the liver. It is not known whether this response is
relevant for humans.
11.1.3 Sample risk characterization
The quantitative estimate of human risk for non-cancer effects is
based on the recommended clinical dose for sedation in humans and the
minor incidence of side-effects at this dose. The tolerable intake is
0.1 mg/kg body weight per day. This is 1% of the recommended single
dose for sedation in humans.
Although there is suggestive evidence of formation of tumours in
the liver of male mice and there are some data showing genotoxicity,
the mode of action for the formation of tumours is not known. It is
also not known whether this response is relevant for humans.
Millions of people are exposed to chloral hydrate on a daily
basis because it is formed during the disinfection of drinking-water
with chlorine. The typical concentration in a public water supply in
the USA is 5 µg/litre. Assuming a water consumption of 2 litres
per day and a body weight of 70 kg, the exposure is 0.14 µg/kg body
weight per day. This exposure is approximately 700 times lower than
the tolerable intake.
11.2 Evaluation of environmental effects
Insufficient data are available with which to assess the risk to
the environment from chloral hydrate.
12. PREVIOUS EVALUATIONS BY INTERNATIONAL BODIES
IARC (1995) evaluated the carcinogenicity data for chloral
hydrate. It was concluded that there is inadequate evidence in humans
and limited evidence in experimental animals for the carcinogenicity
of chloral hydrate. Chloral hydrate is therefore not classifiable as
to its carcinogenicity to humans (Group 3).
IPCS (2000) recently evaluated the toxicological data on water
disinfectants and disinfectant by-products, including chloral hydrate.
Considering the dose level of 16 mg/kg body weight per day in the
90-day study in mice (Sanders et al., 1982; see section 8.4.1) as a
LOAEL (rather than as a NOAEL, as was done in the present document)
and using an uncertainty factor of 10 for intra- and interspecies
extrapolation and another factor of 10 for the use of a LOAEL rather
than a NOAEL, the Task Group calculated a tolerable daily intake (TDI)
for chloral hydrate of 16 µg/kg body weight per day. (As the present
document considered the increase in liver weight at 16 mg/kg body
weight to be a NOAEL rather than a LOAEL, the tolerable intake derived
from the studies among humans was lower, as discussed in section
11.1.)
REFERENCES
Abbas R, Fisher JW (1997) A physiologically based pharmacokinetic
model for trichloroethylene, and its metabolites, chloral hydrate,
trichloroacetate, dichloroacetate, trichloroethanol, and
trichloroethanol glucuronide in B6C3F1 mice. Toxicology and
applied pharmacology, 137:15-30.
Abbas R, Seckel CS, Kidney JK, Fisher JW (1996) Pharmacokinetic
analysis of chloral hydrate and its metabolism in B6C3F1 mice.
Drug metabolism and disposition, 24:1340-1346. See also Erratum,
Drug metabolism and disposition, 25:1449 (1997).
Allen BC, Fisher JW (1993) Pharmacokinetic modeling of
trichloroethylene and trichloroacetic acid in humans. Risk analysis,
13:71-86.
Anyebuno MA, Rosenfeld CR (1991) Chloral hydrate toxicity in a term
infant. Developments in pharmacological therapy, 17:116-120.
Badalaty MM, Houpt MI, Koenigsberg SR, Maxwell KC, Desjardins PJ
(1990) A comparison of chloral hydrate and diazepam sedation in young
children. Pediatric dentistry, 12:33-37.
Beland FA, Schmitt TC, Fullerton NF, Young JF (1998) Metabolism of
chloral hydrate in mice and rats after single and multiple doses.
Journal of toxicology and environmental health, 54:209-226.
Benane SG, Blackman CF, House DE (1996) Effect of perchloroethylene
and its metabolites on intercellular communication in clone 9 rat
liver cells. Journal of toxicology and environmental health,
48:427-437.
Bernstine JB, Meyer AE, Bernstine RL (1954) Maternal blood and breast
milk estimation following the administration of chloral hydrate during
the puerperium. Journal of obstetrics and gynaecology of the British
Commonwealth, 63:228-231.
Breimer DD (1977) Clinical pharmacokinetics of hypnotics. Clinical
pharmacokinetics, 2:93-109.
Breimer DD, Ketelaars HCJ, Van Rossum JM (1974) Gas chromatographic
determination of chloral hydrate, trichloroethanol, and
trichloroacetic acid in blood and urine employing head-space analysis.
Journal of chromatography, 88:55-63.
Bringmann G, Kuehn R (1976) Vergleichende Befunde der Schadwirkung
wassergerfahrden der Stoffe gegen Bakterien (Pseudomonas putida) und
Blaualgen (Microcystis aeruginosa). Gas- und Wasserfach:
Wasser/Abwasser, 117:410-413.
Bringmann G, Kuehn R (1980a) Comparison of the toxicity thresholds of
water pollutants to bacteria, algae and protozoa in cell
multiplication inhibition tests. Water research, 14:231-241.
Bringmann G, Kuehn R (1980b) Bestimmung der biologischen Schadwirkung
wassergefahrdender Stoffe gegen Protozoen II Bakterienfressende
Ciliaten. Zeitschrift für Wasser und Abwasser Forschung, 1:26-31.
Bringmann G, God R, Fähr S, Feines D, Fornadi K, Fornadi F (1999)
Identification of the dopaminergic neurotoxin
1-trichloromethyl-1,2,3,4-tetrahydro-ß-carboline in human blood after
intake of the hypnotic chloral hydrate. Analytical biochemistry,
270:167-175.
Bull RJ, Sanchez IM, Nelson MA, Larson JL, Lansing AJ (1990) Liver
tumor induction in B6C3F1 mice by dichloroacetate and
trichloroacetate. Toxicology, 63:341-359.
Chakrabarti A, Schatten H, Mitchell KD, Crosser M, Taylor M (1998)
Chloral hydrate alters the organization of the ciliary basal apparatus
and cell organelles in sea urchin embryos. Cell and tissue research,
293:453-462.
Daniel FB, DeAngelo AB, Stober JA, Olson GR, Page NP (1992a)
Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and
dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 19:159-168.
Daniel FB, Robinson M, Stober JA, Page NP, Olson GR (1992b) Ninety-day
toxicity study of chloral hydrate in the Sprague-Dawley rat. Drug and
chemical toxicology, 15:217-232.
DeAngelo AB, Daniel FB, Stober JA, Olson GR (1991) The carcinogenicity
of dichloroacetic acid in the male B6C3F1 mouse. Fundamental and
applied toxicology, 16:337-347.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1996) The carcinogenicity
of dichloroacetic acid in the male Fischer 344 rat. Toxicology,
114:207-221.
DeAngelo AB, Daniel FB, Most BM, Olson GR (1997) The failure of
monochloroacetic acid and trichloroacetic acid administered in the
drinking water to produce liver cancer in male F344/N rats. Journal
of toxicology and environmental health, 52:425-445.
Eichenlaub-Ritter U, Betzendahl I (1995) Chloral hydrate induced
spindle aberrations, metaphase I arrest and aneuploidy in mouse
oocytes. Mutagenesis, 10:477-486.
Eichenlaub-Ritter U, Baart E, Yin H, Betzendahl I (1996) Mechanisms of
spontaneous and chemically-induced aneuploidy in mammalian oogenesis:
Basis of sex specific differences in response to aneugens and the
necessity for further tests. Mutation research, 372:274-294.
Elfarra AA, Krause RJ, Last AR, Lash LH, Parker JC (1998) Species- and
sex-related differences in metabolism of trichloroethylene to yield
chloral and trichloroethanol in mouse, rat, and human liver
microsomes. Drug metabolism and disposition, 26:779-785.
Ferreira-Gonzalez A, DeAngelo AB, Nasim S, Garrett CT (1995) Ras
oncogene activation during hepatocarcinogenesis in B6C3F1 male mice
by dichloroacetic and trichloroacetic acid. Carcinogenesis,
16:495-500.
Fisher JW, Mahle D, Abbas R (1998) A human physiologically based
pharmacokinetic model for trichloroethylene and its metabolites:
trichloroacetic acid and free trichloroethanol. Toxicology and
applied pharmacology, 152:339-359.
Fox BE, O'Brien CO, Kangas KJ, Murphree AL, Wright KW (1990) Use of
high dose chloral hydrate for ophthalmic exams in children: A
retrospective review of 302 cases. Journal of pediatric ophthalmology
and strabismus, 27:242-244.
Fung K, Grosjean D (1981) Determination of nanogram amounts of
carbonyls as 2,4-dinitrophenylhydrazones by high-performance liquid
chromatography. Analytical chemistry, 53:168-171.
George MH, Kilburn S, Moore T, DeAngelo AB (2000) The carcinogenicity
of chloral hydrate administered in the drinking water to the male
B6C3F1 mouse and F344/N rat. Toxicologic pathology (in press).
Gerlach M, Xiao A-Y, Heim C, Lan J, God R, Feines D, Bringmann G,
Riederer P, Sontag K-H (1998)
1-Trichloromethyl-1,2,3,4-tetrahydro-ß-carboline increases
extracellular serotonin and stimulates hydroxyl radical production in
rats. Neuroscience letters, 257:17-20.
Goldenthal EI (1971) A compilation of LD50 values in newborn and
adult animals. Toxicology and applied pharmacology, 18:185-207.
Goodman LS, Gilman A (1985) The pharmacological basis of
therapeutics, 7th ed. New York, NY, The Macmillan Co.
Gorecki DKJ, Hindmarsh KW, Hall CA, Mayers DJ, Sankaran K (1990)
Determination of chloral hydrate metabolism in adult and neonate
biological fluids after single-dose administration.
Journal of chromatography, 528:333-341.
Gosselin RE, Smith RP, Hodge HC (1981) Clinical toxicology of
commercial products. Baltimore, MD, Williams & Wilkins.
Green T, Mainwaring GW, Foster JR (1997) Trichloroethylene-induced
mouse lung tumors: Studies of the mode of action and comparisons
between species. Fundamental and applied toxicology, 37:125-130.
Greenberg MS, Burton GA Jr, Fisher FW (1999) Physiologically based
pharmacokinetic modeling of inhaled trichloroethylene and its
oxidative metabolites in B6C3F1 mice. Toxicology and applied
pharmacology, 154:264-278.
Greenberg SB, Faerber EN, Aspinall CL (1991) High dose chloral hydrate
sedation for children undergoing CT. Journal of computer assisted
tomography, 15:467-469.
Gupta RN (1990) Determination of trichloroethanol, the active
metabolite of chloral hydrate, in plasma by liquid chromatography.
Journal of chromatography, 500:655-659.
HSDB (1999) Hazardous substances data bank. Micromedex Inc. (CD-ROM
version).
Helrich K, ed. (1990) Official methods of analysis of the Association
of Official Analytical Chemists, 15th ed. Vol. 1. Arlington, VA,
Association of Official Analytical Chemists, p. 562.
Henderson GN, Yan Z, James MO, Davydova N, Stacpoole PW (1997)
Kinetics and metabolism of chloral hydrate in children: identification
of dichloroacetate as a metabolite. Biochemical and biophysical
research communications, 235:695-698.
Herren-Freund SL, Pereira MA, Khoury MD, Olson G (1987) The
carcinogenicity of trichloroethylene and its metabolites,
trichloroacetic acid and dichloroacetic acid, in mouse liver.
Toxicology and applied pharmacology, 90:183-189.
Hindmarsh KW, Gorecki DKJ, Sankaran K, Mayers DJ (1991) Chloral
hydrate administration to neonates: Potential toxicological
implications. Canadian Society of Forensic Science, 24:239-245.
Hobara T, Kobayashi H, Kawamoto T, Sato T, Iwamoto S, Hirota S, Sakai
T (1986) Biliary excretion of trichloroethylene and its metabolites in
dogs. Toxicology letters, 32:119-122.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1987a) The
cholecystohepatic circulation of trichloroethylene and its metabolites
in dogs. Toxicology, 44:283-295.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Hirota S, Sakai T
(1987b) Extrahepatic metabolism of chloral hydrate, trichloroethanol,
and trichloroacetic acid in dogs. Pharmacology and toxicology,
61:58-62.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988a)
Intestinal absorption of chloral hydrate, free trichloroethanol, and
trichloroacetic acid in dogs. Pharmacology and toxicology,
62:250-258.
Hobara T, Kobayashi H, Kawamoto T, Iwamoto S, Sakai T (1988b) The
absorption of trichloroethylene and its metabolites from the urinary
bladder of anesthetized dogs. Toxicology, 48:141-153.
IARC (1995) Chloral and chloral hydrate. IARC (International Agency
for Research on Cancer) monographs, 63:245-269.
IPCS (1993) International Chemical Safety Card -- Chloral hydrate.
Geneva, World Health Organization, International Programme on
Chemical Safety (ICSC 0234).
IPCS (1994) Assessing human health risks of chemicals: derivation of
guidance values for health-based exposure limits. Geneva, World
Health Organization, International Programme on Chemical Safety
(Environmental Health Criteria 170).
IPCS (2000) Disinfectants and disinfectant by-products. Geneva,
World Health Organization (Environmental Health Criteria 216).
Johnson PD, Dawson BV, Goldberg SJ (1998) Cardiac teratogenicity of
trichloroethylene metabolites. Journal of the American College of
Cardiology, 32:540-545.
Kallman MJ, Kaempf GL, Balster RL (1984) Behavioral toxicity of
chloral in mice: An approach to evaluation. Neurobehavioral
toxicology and teratology, 6:137-146.
Kaplan HL, Forney RB, Hughes FW, Jain NC, Crim D (1967) Chloral
hydrate and alcohol metabolism in human subjects. Journal of forensic
sciences, 12:295-304.
Kauffmann BM, White KL, Sanders VM, Douglas KA, Sain LE, Borzelleca
JF, Munson AE (1982) Humoral and cell-mediated immune status in mice
exposed to chloral hydrate. Environmental health perspectives,
44:147-151.
Ketcha MM, Stevens DK, Warren DA, Bishop CT, Brashear WT (1996)
Conversion of trichloroacetic acid to dichloroacetic acid in
biological samples. Journal of analytical toxicology, 20:236-241.
Klinefelter GR, Suarez JD, Roberts NL (1995) Preliminary screening
test for the potential of drinking water disinfectant by-products to
alter male reproduction. Reproductive toxicology, 9:571-578.
Koppen B, Dalgaard L (1988) Determination of trichloroethylene
metabolites in rat liver homogenates using headspace gas
chromatography. Journal of chromatography, 442:325-332.
Lambert GH, Muraskas J, Anderson CL, Myers TF (1990) Direct
hyperbilirubinemia associated with chloral hydrate administration in
the newborn. Pediatrics, 86:277-281.
Leuschner J, Beuscher N (1998) Studies on the mutagenic and
carcinogenic potential of chloral hydrate. Arzneimittel-Forschung
drug research, 48:961-968.
Lipscomb JC, Mahle DA, Brashear WT, Garrett CM (1996) A species
comparison of chloral hydrate metabolism in blood and liver.
Biochemical and biophysical research communications, 227:340-350.
Lipscomb JC, Confer PD, Miller MR, Stamm SC, Snawder JE, Candiera SM
(1998) Metabolism of trichloroethylene and chloral hydrate by the
Japanese medaka (Oryzias latipes) in vitro. Environmental
toxicology and chemistry, 17:325-332.
Ludwigs U, Divino-Fiiho JC, Magnusson N (1996) Suicidal chloral
hydrate poisoning. Journal of clinical toxicology, 344:97-99.
Mailhes JB, Marchetti F (1994) Chemically induced aneuploidy in
mammalian oocytes. Mutation research, 320:87-111.
Marshall AJ (1977) Cardiac arrhythmias caused by chloral hydrate.
British medical journal, 2:994.
Marshall EK, Owens AH (1954) Absorption, excretion and metabolic fate
of chloral hydrate and trichloroethanol. Bulletin of the Johns
Hopkins Hospital, 95:1-18.
Mayers DJ, Hindmarsh KW, Sankaran K, Gorecki DKJ, Kasian GF (1991)
Chloral hydrate disposition following single-dose administration to
critically ill neonates and children. Developments in pharmacological
therapy, 16:71-77.
Merdink JL, Conzalez-Leon A, Bull RJ, Schultz IR (1998) The extent of
dichloroacetate formation from trichloroethylene, chloral hydrate,
trichloroacetate, and trichloroethanol in B6C3F1 mice. Toxicological
sciences, 45:33-41.
Merdink JL, Stenner RD, Stevens DK, Parker JC, Bull RJ (1999) Effect
of enterohepatic circulation on the pharmacokinetics of chloral
hydrate and its metabolites in F344 rats. Journal of toxicology and
environmental health, 56:357-368.
Miller RR, Greenblatt DJ (1979) Clinical effects of chloral hydrate in
hospitalized medical patients. Journal of clinical pharmacology,
19:669-674.
Newman LM, Wackett LP (1991) Fate of 2,2,2-trichloroacetaldehyde
(chloral hydrate) produced during trichloroethylene oxidation by
methanotrophs. Applied and environmental microbiology, 57:2399-2402.
Ni Y-C, Wong T-Y, Kadlubar FF, Fu PP (1994) Hepatic metabolism of
chloral hydrate to free-radical(s) and induction of lipid
peroxidation. Biochemical and biophysical research communications,
204:937-943.
Ni Y-C, Kadlubar FF, Fu FF (1995) Formation of
malondialdehyde-modified 2'-deoxyguanosinyl adduct from metabolism of
chloral hydrate by mouse liver microsomes. Biochemical and
biophysical research communications, 205:1110-1117.
Ni Y-C, Wong T-Y, Lloyd RV, Heinze TM, Shelton S, Casciano D, Kadlubar
FF, Fu PP (1996) Mouse liver microsomal metabolism of chloral hydrate,
trichloroacetic acid, and trichloroethanol leading to induction of
lipid peroxidation via a free radical mechanism. Drug metabolism and
disposition, 24:81-90.
NTP (2000a) Toxicology and carcinogenesis studies of chloral hydrate
in B6C3F1 mice (gavage studies). Research Triangle Park, NC,
National Institutes of Health, National Toxicology Program (NTP TR
502).
NTP (2000b) Toxicology and carcinogenesis studies of chloral hydrate
(ad libitum and dietary controlled) in male B6C3F1 mice (gavage
study). Research Triangle Park, NC, National Institutes of Health,
National Toxicology Program (NTP TR 503).
Odum J, Foster JR, Green T (1992) A mechanism for the development of
clara cell lesions in the mouse lung after exposure to
trichloroethylene. Chemico-biological interactions, 83:135-153.
Owens AH, Marshall EK (1955) Further studies on the metabolic fate of
chloral hydrate and trichloroethanol. Bulletin of the Johns Hopkins
Hospital, 97:320-326.
Pereira MA (1996) Carcinogenic activity of dichloroacetic acid and
trichloroacetic acid in the liver of female B6C3F1 mice.
Fundamental and applied toxicology, 31:192-199.
Reimche LD, Sankaran K, Hindmarsh KW, Kasian GF, Gorecki DKJ, Tan L
(1989) Chloral hydrate sedation in neonates and infants -- clinical
and pharmacologic considerations. Developments in pharmacological
therapy, 12:57-64.
Richmond RE, Carter JH, Carter HW, Daniel FB, DeAngelo AB (1995)
Immunohistochemical analysis of dichloroacetic acid (DCA)-induced
hepatocarcinogenesis in male Fischer (F344) rats. Cancer letters,
92:67-76.
Rijhsinghani KS, Abrahams C, Swerdlow MA, Rao KVN, Ghose T (1986)
Induction of neoplastic lesions in the livers of C57BL × C3HF1 mice
by chloral hydrate. Cancer detection and prevention, 9:279-288.
Saillenfait AM, Langonne I, Sabate JP (1995) Developmental toxicity of
trichloroethylene, tetrachloroethylene and four of their metabolites
in rat whole embryo culture. Archives of toxicology, 70:71-82.
Sanders VM, Kauffman BM, White KL, Douglas KA, Barnes DW, Sain LE,
Bradshaw TJ, Borzelleca JF, Munson AE (1982) Toxicology of chloral
hydrate in the mouse. Environmental health perspectives, 44:137-146.
Schatten H, Chakrabarti A (1998) Centrosome structure and function is
altered by chloral hydrate and diazepam during the first reproductive
cell cycles in sea urchin eggs. European journal of cell biology,
75:9-20.
Shapiro S, Stone D, Lewis GP, Jick H (1969) Clinical effects of
hypnotics. II. An epidemiological study. Journal of the American
Medical Association, 209:2016-2020.
Simpson KL, Hayes KP (1998) Drinking water disinfection by-products:
an Australian perspective. Water research, 32:1522-1528.
Sing K, Erickson T, Amitai Y, Hryhorczuk D (1996) Chloral hydrate
toxicity from oral and intravenous administration. Journal of
toxicology and clinical toxicology, 34:101-106.
Smith MK, Randall JL, Read EJ, Stober JA (1989) Teratogenic activity
of trichloroacetic acid in the rat. Teratology, 40:445-451.
Stenner RD, Merdink JL, Stevens DK, Springer DL, Bull RJ (1997)
Enterohepatic recirculation of trichloroethanol glucuronide as a
significant source of trichloroacetic acid. Drug metabolism and
disposition, 25:529-535.
Stenner RD, Merdink JL, Fisher JW, Bull RJ (1998)
Physiologically-based pharmacokinetic model for trichloroethylene
considering enterohepatic recirculation of major metabolites.
Risk analysis, 18:261-269.
US EPA (1994) National primary drinking water regulations;
disinfectants and disinfection byproducts; proposed rule. US
Environmental Protection Agency. Federal register, 59:38668-38829.
US EPA (2000) Toxicological review on chloral hydrate. Available
from US Environmental Protection Agency's Risk Assessment Hotline
[513-569-7254 (phone), 513-569-7159 (fax), rih.iris@epa.gov (e-mail
address), or www.epa.gov/iris (Website)].
Velazquez SF (1994) Activation of the H-ras oncogene by drinking
water disinfection by-products. Report submitted under the US
Environmental Protection Agency's Small Grant Program. April 1994. 43
pp. (NTIS/PB95-200515).
Vian L, Van Hummelen P, Bichet N, Gouy D, Kirsch-Volders M (1995)
Evaluation of hydroquinone and chloral hydrate on the in vitro
micronucleus test on isolated lymphocytes. Mutation research,
334:1-7.
Watanabe M, Takano T, Nakamura K (1998) Effect of ethanol on the
metabolism of trichloroethylene in the perfused rat liver. Journal of
toxicology and environmental health, 55:297-305.
Wu WW, Chadik PA, Davis WM, Powell DH, Delfino JJ (1998) Disinfection
byproduct formation from the preparation of instant tea. Journal of
agricultural and food chemistry, 46:3272-3279.
Yan Z, Henderson GN, James MO, Stacpoole P (1999) Determination of
chloral hydrate in human plasma by gas chromatography-mass
spectrometry. Journal of pharmaceutical and biomedical analysis,
19:309-318.
Zimmermann T, Wehling M, Schulz HU (1998) Untersuchungen zur relativen
Bioverfugbarkeit und Pharmakokinetik von Chloralhydrat und seinen
Metaboliten. [The relative bioavailability and pharmacokinetics of
chloral hydrate and its metabolites.] Arzneimittelforschung,
48:5-12.
APPENDIX 1 -- TOXICOKINETICS
This toxicokinetic analysis is used to estimate the steady-state
concentrations of trichloroacetic acid (TCA) and trichloroethanol
(TCEOH) in mice and humans using a one-compartment model, assuming
that the absorption of chloral hydrate (CH) from the gastrointestinal
tract and its metabolism in the blood are very rapid compared with the
rate of elimination of TCA and TCEOH. This assumption is supported by
the data of Beland et al. (1998) in mice and Breimer (1977) and
Zimmermann et al. (1998) in humans.
Beland et al. (1998) indicated that 15% of the dose of chloral
hydrate is converted directly to TCA and 77% is converted to TCEOH. In
humans, Allen & Fisher (1993) estimated that 8% of a dose of chloral
hydrate is converted directly to TCA and 92% is converted to TCEOH.
Additional TCA is formed from TCEOH. The total TCA formed in humans is
approximately 35% of the dose of chloral hydrate.
Estimation of TCA concentration in mice at steady state at the
clinically recommended dose for humans:
[TCA]ss-blood = PKo/ VKel = 2.5 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 3.0 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.15
(Beland et al., 1998)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.321 litre/kg
(Beland et al., 1998)
* Kel is the first-order elimination constant for TCA =
0.0819/h (Beland et al., 1998)
* PC is the liver/blood partition coefficient = 1.18 (Abbas &
Fisher, 1997)
Estimation of TCA concentration in humans at steady state at the
clinically recommended dose:
[TCA]ss-blood = PKo/ VKel = 55 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 36 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA = 0.028/h
(Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCA concentration in humans at steady state at the
tolerable intake:
[TCA]ss-blood = PKo/ VKel = 1.8 mg/litre
[TCA]ss-liver = [TCA]ss-blood × PC = 1.2 mg/litre
where:
* P is the proportion of CH converted to TCA = 0.35 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution = 0.102 litre/kg (Allen &
Fisher, 1993)
* Kel is the first-order elimination constant for TCA =
0.0078/h (Allen & Fisher, 1993)
* PC is the liver/blood partition coefficient = 0.66
(Fisher et al., 1998)
Estimation of TCEOH concentration in mice at steady state at 166 mg/kg
body weight per day:
[TCEOH]ss-blood = PKo/ VKel = 0.6 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.77
(Beland et al., 1998)
* Ko is the dosing rate for CH = 166 mg/kg body weight per day,
equivalent to 6.917 mg/kg body weight per hour
* V is the volume of distribution = 1 litre/kg (cited in
Beland et al., 1998)
* Kel is the first-order elimination constant for TCEOH =
9.24/h (Beland et al., 1998)
Chloral hydrate at 160 mg/kg body weight per day was the highest
exposure used in the 90-day neurobehavioural study by Kallman et al.
(1984); chloral hydrate at 166 mg/kg body weight per day was the
highest exposure used in the 104-week bioassay of Daniel et al.
(1992a). These exposures are a NOAEL for sedation in mice.
Estimation of TCEOH concentration in humans at steady state at the
clinically recommended dose:
[TCEOH]ss-blood = PKo/ VKel = 4.7 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 10.7 mg/kg body weight per
day, equivalent to 0.446 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
Estimation of TCEOH concentration in humans at steady state at the
tolerable intake:
[TCEOH]ss-blood = PKo/ VKel = 0.04 mg/litre
where:
* P is the proportion of CH converted to TCEOH = 0.92 (Allen &
Fisher, 1993)
* Ko is the dosing rate for CH = 0.1 mg/kg body weight per day,
equivalent to 0.004 mg/kg body weight per hour
* V is the volume of distribution -- not available, assumed
1 litre/kg
* Kel is the first-order elimination constant for TCEOH =
0.087/h (Breimer, 1977)
APPENDIX 2 -- CALCULATION OF BENCHMARK DOSE FOR
TUMOUR INCIDENCE
The Benchmark Dose (ED) for tumour incidence was derived from the
incidence of adenoma plus carcinoma as reported by George et al.
(2000). The human equivalent dose was calculated using (body
weight)3/4, assuming a human body weight of 70 kg and a mouse body
weight of 0.035 kg. EPA Benchmark Dose Software was used to calculate
the ED and its lower 95% confidence limit (LED) corresponding to a 10%
increase in extra risk for tumour prevalence with the multistage
model.
Multistage Model, Version Number 1.1.0b
The form of the probability function is:
P[response] =
(1 - background ) × [1 - (e - beta 1 × dose 1 - beta 2 × dose 2) ]
The parameter betas are restricted to be positive.
Dependent variable = Incidence
Independent variable = Dose
Total number of observations = 4
Total number of records with missing values = 0
Total number of parameters in model = 3
Total number of specified parameters = 0
Degree of polynomial = 2
Maximum number of iterations = 250
Relative function convergence has been set to 2.220 45e-16
Parameter convergence has been set to 1.490 12e-8
Default initial parameter values
Background = 0.698 863
Beta(1) = 0.043 897
Beta(2) = 0.000 400 241
Parameter estimates
Variable Estimate Standard error
Background 0.691 141 0.073 072 3
Beta(1) 0.053 218 1 0.084 548 3
Beta(2) 0 0.004 035 19
Asymptotic correlation matrix of parameter estimates
Background Beta(1) Beta(2)
Background 1 -0.6319 0.5007
Beta(1) -0.6319 1 -0.9507
Beta(2) 0.5007 -0.9507 1
Analysis of deviance table
Model Log(likelihood) Deviance DF P-value
Full model -81.2046
Fitted model -81.922 1.434 7 2 0.230 999
Reduced mode -85.0504 6.256 83 2 0.043 787
Goodness of fit analysis
Administered dose Human equivalent Estimated Expected Observed Size
(mg/kg body weight dose probability
per day) (mg/kg body weight
per day)
0 0 0.6911 29.028 27 42
13.5 2.0000 0.7223 33.227 36 46
65 9.7 0.8157 31.812 31 39
146.6 21.9 0.9037 28.919 29 32
Chi-square = 1.41; DF = 2; P-value = 0.4949.
Benchmark dose computation
Specified effect 0.100 000
Risk type Extra risk
Confidence level 0.950 000
ED 1.979 786
LED 1.090 1
APPENDIX 3 -- SOURCE DOCUMENT
US Environmental Protection Agency (2000):
Toxicological review on chloral hydrate
Copies of the document may be obtained from:
EPA Risk Assessment Hotline
513-569-7254 (phone)
513-569-7159 (fax)
rih.iris@epa.gov (e-mail address)
www.epa.gov/iris (Website)
This document was prepared by R. Benson, Region VIII, Denver, CO.
The document and summary information on the Integrated Risk
Information System (IRIS) have received peer review both by EPA
scientists and by independent scientists external to EPA. Subsequent
to external review and incorporation of comments, this assessment has
undergone an Agency-wide review process whereby the IRIS Program
Manager has achieved a consensus approval among the Office of Research
and Development; Office of Air and Radiation; Office of Prevention,
Pesticides, and Toxic Substances; Office of Solid Waste and Emergency
Response; Office of Water; Office of Policy, Planning, and Evaluation;
and the Regional Offices.
Internal EPA reviewers:
National Center for Environmental Assessment, Washington, DC
J. Cogliano
C. Siegel Scott
V. Vu
National Health and Environmental Effects Research Laboratory,
Research Triangle Park, NC
A. DeAngelo
R. Luebke
Office of Water, Washington, DC
A. Bathija
External peer reviewers:
P.E. Brubaker, Private Consultant
J.W. Fisher, Operational Toxicology Branch, Wright-Patterson
Air Force Base
C.C. Willhite, Department of Toxic Substances Control,
State of California
APPENDIX 4 -- CICAD PEER REVIEW
The draft CICAD on chloral hydrate was sent for review to
institutions and organizations identified by IPCS after contact with
IPCS National Contact Points and Participating Institutions, as well
as to identified experts. Comments were received from:
Centre of Industrial Hygiene and Occupational Diseases, Czech
Republic
Department of Health, London, United Kingdom
Federal Institute for Health Protection of Consumers and
Veterinary Medicine, Berlin, Germany
Fraunhofer Institute for Toxicology and Aerosol Research,
Hannover, Germany
GSF Forschungszentrum für Umwelt und Gesundheit, GmbH,
Oberschleissheim, Germany
Health and Safety Executive, Bootle, United Kingdom
Institut de Recherche en Santé et en Sécurité du Travail du
Québec, Montreal, Canada
Institute of Occupational Medicine, Chinese Academy of Preventive
Medicine, Beijing, People's Republic of China
National Center for Environmental Assessment, US Environmental
Protection Agency, Washington, DC, USA
National Center for Toxicological Research, US Food and Drug
Administration, Jefferson, AK, USA
National Chemicals Inspectorate, Solna, Sweden
National Industrial Chemicals Notification and Assessment Scheme
(NICNAS), Sydney, Australia
National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
National Institute of Environmental Health Sciences, National
Institutes of Health, Research Triangle Park, NC, USA
University of Bielefeld, Bielefeld, Germany
APPENDIX 5 -- CICAD FINAL REVIEW BOARD
Sydney, Australia, 21-24 November 1999
Members
Dr R. Benson, Drinking Water Program, US Environmental Protection
Agency, Region VIII, Denver, CO, USA
Dr T. Berzins, National Chemicals Inspectorate (KEMI), Solna, Sweden
Dr R.M. Bruce, National Center for Environmental Assessment,
US Environmental Protection Agency, Cincinnati, OH, USA
Mr R. Cary, Health and Safety Executive, Merseyside, United Kingdom
Dr R.S. Chhabra, National Institute of Environmental Health Sciences,
National Institutes of Health, Research Triangle Park, NC, USA
Dr S. Chou, Agency for Toxic Substances and Disease Registry,
Atlanta, GA, USA
Dr S. Dobson, Institute of Terrestrial Ecology, Monks Wood,
Cambridgeshire, United Kingdom
Dr H. Gibb, National Center for Environmental Assessment,
US Environmental Protection Agency, Washington, DC, USA
Dr R.F. Hertel, Federal Institute for Health Protection of Consumers
and Veterinary Medicine, Berlin, Germany
Dr J. Kielhorn, Fraunhofer Institute for Toxicology and Aerosol
Research, Hannover, Germany
Dr S. Kristensen, National Occupational Health and Safety Commission
(Worksafe), Sydney, NSW, Australia
Mr C. Lee-Steere, Environment Australia, Canberra, ACT, Australia
Ms M. Meek, Environmental Health Directorate, Health Canada, Ottawa,
Ontario, Canada
Ms F. Rice, National Institute for Occupational Safety and Health,
Cincinnati, OH, USA
Dr J. Sekizawa, National Institute of Health Sciences, Tokyo, Japan
Dr D. Willcocks, National Industrial Chemicals Notification and
Assessment Scheme (NICNAS), Sydney, NSW, Australia (Chairperson)
Professor P. Yao, Institute of Occupational Medicine, Chinese Academy
of Preventive Medicine, Beijing, People's Republic of China
Observers
Mr P. Howe, Institute of Terrestrial Ecology, Huntingdon,
Cambridgeshire, United Kingdom
Dr K. Ziegler-Skylakakis, GSF-Forschungszentrum für Umwelt und
Gesundheit, GmbH, Oberschleissheim, Germany
Secretariat
Dr A. Aitio, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
Ms M. Godden, Health and Safety Executive, Bootle, Merseyside, United
Kingdom
Dr M. Younes, International Programme on Chemical Safety, World Health
Organization, Geneva, Switzerland
APPENDIX 6 -- INTERNATIONAL CHEMICAL SAFETY CARD
CHLORAL HYDRATE ICSC: 0234
October 1999
CAS# 