
Colchicine
| 1. NAME |
| 1.1 Substance |
| 1.2 Group |
| 1.3 Synonyms |
| 1.4 Identification numbers |
| 1.4.1 CAS number |
| 1.4.2 Other numbers |
| 1.5 Brand names, Trade names |
| 1.6 Manufacturers, Importers |
| 1.7 Presentation, Formulation |
| 2. SUMMARY |
| 2.1 Main risks and target organs |
| 2.2 Summary of clinical effects |
| 2.3 Diagnosis |
| 2.4 First aid measures and management principles |
| 3. PHYSICO-CHEMICAL PROPERTIES |
| 3.1 Origin of the substance |
| 3.2 Chemical structure |
| 3.3 Physical properties |
| 3.3.1 Properties of the substance |
| 3.3.1.1 Colour |
| 3.3.1.2 State/Form |
| 3.3.1.3 Description |
| 3.3.2 Properties of the locally available formulation(s) |
| 3.4 Other characteristics |
| 3.4.1 Shelf-life of the substance |
| 3.4.2 Shelf-life of the locally available formulation(s) |
| 3.4.3 Storage conditions |
| 3.4.4 Bioavailability |
| 3.4.5 Specific properties and composition |
| 4. USES |
| 4.1 Indications |
| 4.1.1 Indications |
| 4.1.2 Description |
| 4.2 Therapeutic dosage |
| 4.2.1 Adults |
| 4.2.2 Children |
| 4.3 Contraindications |
| 5. ROUTES OF ENTRY |
| 5.1 Oral |
| 5.2 Inhalational |
| 5.3 Dermal |
| 5.4 Eye |
| 5.5 Parenteral |
| 5.6 Other |
| 6. KINETICS |
| 6.1 Absorption by route of exposure |
| 6.2 Distribution by route of exposure |
| 6.3 Biological half-life by route of exposure |
| 6.4 Metabolism |
| 6.5 Elimination by route of exposure |
| 7. PHARMACOLOGY AND TOXICOLOGY |
| 7.1 Mode of action |
| 7.1.1 Toxicodynamics |
| 7.1.2 Pharmacodynamics |
| 7.2 Toxicity |
| 7.2.1 Human data |
| 7.2.1.1 Adults |
| 7.2.1.2 Children |
| 7.2.2 Relevant animal data |
| 7.2.3 Relevant in vitro data |
| 7.3 Carcinogenicity |
| 7.4 Teratogenicity |
| 7.5 Mutagenicity |
| 7.6 Interactions |
| 7.7 Main adverse effects |
| 8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS |
| 8.1 Methods |
| 8.2 Therapeutic and toxic concentration |
| 9. CLINICAL EFFECTS |
| 9.1 Acute poisoning |
| 9.1.1 Ingestion |
| 9.1.2 Inhalation |
| 9.1.3 Skin exposure |
| 9.1.4 Eye contact |
| 9.1.5 Parenteral exposure |
| 9.1.6 Other |
| 9.2 Chronic poisoning |
| 9.2.1 Ingestion |
| 9.2.2 Inhalation |
| 9.2.3 Skin contact |
| 9.2.4 Eye contact |
| 9.2.5 Parenteral exposure |
| 9.2.6 Other |
| 9.3 Course, prognosis, cause of death |
| 9.4 Systematic description of clinical effects |
| 9.4.1 Cardiovascular |
| 9.4.2 Respiratory |
| 9.4.3 Neurological |
| 9.4.3.1 Central nervous system (CNS) |
| 9.4.3.2 Peripheral nervous system |
| 9.4.3.3 Autonomic nervous system |
| 9.4.3.4 Skeletal and smooth muscle |
| 9.4.4 Gastrointestinal |
| 9.4.5 Hepatic |
| 9.4.6 Urinary |
| 9.4.6.1 Renal |
| 9.4.6.2 Other |
| 9.4.7 Endocrine and reproductive systems |
| 9.4.8 Dermatological |
| 9.4.9 Eye, ear, nose, throat: local effects |
| 9.4.10 Haematological |
| 9.4.11 Immunological |
| 9.4.12 Metabolic |
| 9.4.12.1 Acid-base disturbances |
| 9.4.12.2 Fluid and electrolyte disturbances |
| 9.4.12.3 Others |
| 9.4.13 Allergic reactions |
| 9.4.14 Other clinical effects |
| 9.4.15 Special risks |
| 9.5 Other |
| 9.6 Summary |
| 10. MANAGEMENT |
| 10.1 General principles |
| 10.2 Relevant laboratory analyses |
| 10.2.1 Sample collection |
| 10.2.2 Biomedical analysis |
| 10.2.3 Toxicological analysis |
| 10.2.4 Other investigations |
| 10.3 Life supportive procedures and symptomatic/specific treatment |
| 10.4 Decontamination |
| 10.5 Elimination |
| 10.6 Antidote treatment |
| 10.6.1 Adults |
| 10.6.2 Children |
| 10.7 Management discussion |
| 11. ILLUSTRATIVE CASES |
| 11.1 Case reports from literature |
| 11.2 Internally extracted data on cases |
| 11.3 Internal cases |
| 12. ADDITIONAL INFORMATION |
| 12.1 Availability of antidotes |
| 12.2 Specific preventive measures |
| 12.3 Other |
| 13. REFERENCES |
| 14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE ADDRESS(ES) |
1. NAME
1.1 Substance
Colchicine (USAN)
(Fleeger,1993)
1.2 Group
ATC classification index
Antigout preparations (M04)
Preparations with no effect on uric acid metabolism (M04AC)
(WHO, 1992)
1.3 Synonyms
Colchicina (Italian)
Colchicin (German)
Colchicum
Colchique (French)
NSC 757
(To be completed by each Centre using local data)
1.4 Identification numbers
1.4.1 CAS number
64-86-8
1.4.2 Other numbers
UPDT 8211
NIOSH/RTECS GH 0700000
NSC 757
1.5 Brand names, Trade names
Colchicine (monocomponent)
Australia Colgout (Protea); Colchicine (Medical
Research); Colcin (Knoll); Coluric (Nelson).
Canada Colchicine (Abbott).
France Colchicine (Houde ISH); Colchineos
(Houde ISH).
�
Germany Colchicum-Dispert (Kali-Chemie).
South Africa Colchicine Houdse (Roussel).
USA Colchicine (Abbott, Barr Lab., Danbury,
Lilly, Rugby, Towne, United Research
Laboratories)
Colchicine plus probenecid
UK ColBenemid (Merck Sharpe & Dohme)
USA ColBenemid (Merck Sharpe & Dohme);
Col-Probencid (Danbury, Goldline, Interstate,
Parmed, United Research Lab.,) Proben-C
(Rugby)
Colchicine associated with other compounds
France Colchimax - contains colchicine,
phenobarbitone and opium tincture
(Houde ISH).
Generic products are also available.
(To be completed by each Centre using local data)
1.6 Manufacturers, Importers
Biogen Laboratories, Columbia, Ethical Pharmaceuticals,
Fisher Scientific, Purepac, Regal Laboratories, Rondex,
Schein, Tourne- Paulsen, Westward, Zenith.
(To be completed by each Centre using local data)
1.7 Presentation, Formulation
Colchicine is available as tablets and, in some
countries, as injectable solutions.
Tablets contain mostly 0.5 to 0.65 mg. Formulations with 1
mg per tablet are also available.
Sterile solutions containing 0.5 mg/ml are also available for
intravenous injection.
(To be completed by each Centre using local data)
�
2. SUMMARY
2.1 Main risks and target organs
Colchicine exerts a multiorgan toxicity. The main toxic
effects are related to the effects of colchicine on cellular
division and account for diarrhoea, bone marrow depression,
alopecia. Other acute effects are hypovolaemia, shock, and
coagulation disturbances, which may lead to death.
2.2 Summary of clinical effects
Toxic manifestations appear after a delay of 2 to 12
hours following ingestion or parenteral administration.
Symptomatology progresses in three stages:
Stage I (Day 1 to 3) gastrointestinal and circulatory phase
Severe gastrointestinal irritation: Nausea, vomiting,
abdominal cramps, severe diarrhoea.
Dehydration, hypovolaemia, shock. Cardiogenic shock may
occur and may result in death within the first 72 hours.
Hypoventilation, acute respiratory distress syndrome.
Stage II (Day 3 to 10) bone marrow aplasia phase
Bone marrow aplasia with agranulocytosis.
Coagulation disorders with diffuse haemorrhages.
Rhabdomyolyis.
Polyneuritis, myopathy.
Acute renal failure.
Infectious complications.
Stage III: (After 10 day) recovery phase
Alopecia.
�
2.3 Diagnosis
Clinical diagnosis is difficult because of the
multiorgan toxicity.
Colchicine levels are not clinically useful.
Note: Biological samples must be stored in airtight
conditions and protected from light.
Monitor the following:
Electrolytes, particularly potassium, calcium.
Acid-base balance.
Full blood count and platelets.
Coagulation profile and fibrin/fibrinogen degradation
products.
Creatinine phosphokinase and transaminases.
2.4 First aid measures and management principles
Patients with colchicine overdose should always be
admitted as soon as possible to an intensive care unit for a
minimum of 48 hours.
Monitor vital signs (ECG, blood pressure, respiration,
central venous pressure), fluid and electrolyte balance, and
blood cells.
Treatment may include the following:
Rehydration, plasma expander infusion, inotropic and
vasopressor drugs,
Artificial ventilation.
Early gastric lavage.
Correction of electrolytes and acid-base disorders.
Early forced diuresis.
Correction of coagulation disorders.
Prevention of infectious complications.
�
3. PHYSICO-CHEMICAL PROPERTIES
3.1 Origin of the substance
Colchicine is an alkaloid of Colchicum autumnale (autumn
crocus, meadow saffron). It was isolated in 1820 by
Pelletier and Caventou. Colchicum is also present in Gloriosa
superba (Glory Lily) (Gooneratne, 1966; Nagaratnam et al.,
1973). For more information see the PIM on Colchicum
autumnale.
The chemical structured was described by Dewar in 1945. The
chemical synthesis was first realised by Woodward.
3.2 Chemical structure
Structural formula
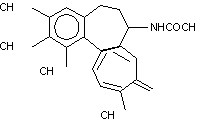 Molecular formula
C22H25NO6
Molecular weight
399.4
Structural Chemical names
(S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo
[alpha] heptalen-7-yl)acetamide.
N-(5,5,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-
oxobenzo[alpha]heptalen-7-yl)acetamide.
(Budavari, 1989; Reynolds, 1993)
Derivatives
Different compounds have been isolated from Colchicum
autumnal. Colchicine has the most important biological
activity which is related to the tropolonic cycle (C).
�
Biological Substance R1 R2 R3 R3
activity name
+++ Colchicine CH3 COCH3 0 OCH3
++ Desacetyl CH3 CH3 0 OCH3
methylcolchicine CH3 H 0 SCH3
++ Desacetylthiocolchicine C6H1105 COCH3 OCH3
++ Colchicoside CH3 H 0 OH
+ Trimethylcolchicinic acid CH3 COCH3 0 0
0 Colchiceine 0H
3.3 Physical properties
3.3.1 Properties of the substance
3.3.1.1 Colour
Pale yellow. It darkens on exposure to light.
3.3.1.2 State/Form
Odourless powder or scales.
3.3.1.3 Description
Melting point 153-157°C
pH of 0.5% solution is 5.9
Freely soluble in alcohol or chloroform,
slightly soluble in ether (1/220) and
insoluble in petroleum ether. Solubility in
water is 1/25.
�
3.3.2 Properties of the locally available formulation(s)
To be completed by each Centre using local
data.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Shelf-life of the substance is three to five years.
3.4.2 Shelf-life of the locally available formulation(s)
To be completed by each Centre using local data.
3.4.3 Storage conditions
Store in airtight conditions and protect from light.
3.4.4 Bioavailability
To be completed by each Centre using local data.
3.4.5 Specific properties and composition
To be completed by each Centre using local data.
4. USES
4.1 Indications
4.1.1 Indications
Not relevant
4.1.2 Description
Gout
Colchicine is used for acute gout attacks to reduce
pain and inflammation. It may be used on long-term
basis to prevent or reduce the frequency of
attacks.
Familial Mediterranean Fever
Colchicine is used on long-term basis to prevent fever
and recurrent polyserositis. Colchicine is effective
in preventing the amyloidosis in this condition.
Behcet's disease
Colchicine has been showed to be effective in the
treatment of articular, cutaneous and mucosal
symptoms.
�
Other
Colchicine has been used in the treatment of
scleroderma and sarcoidosis.
4.2 Therapeutic dosage
4.2.1 Adults
Acute Gout Attack
Oral
1 or 1.3 mg initial dose, followed by 0.5 to 0.65 mg
every 1 to 2 hours (or 1 to 1.3 mg every 2 hours)
until the pain is relieved or nausea and diarrhoea
appear. The total dose should not exceed 10 mg over 3
days.
A course should not be repeated within three days.
Tolerance is usually 4 to 8 mg.
Parenteral
Intravenous injection. Initial dose is 1 to 2 mg.
The total dose is 4 mg in 24 hours or in an
attack.
Prophylaxis of recurrent gout
Oral
0.5 mg to 0.65 mg once weekly or up to three times
daily, depending on the frequency of prior acute
attacks.
(Reynolds 1993)
4.2.2 Children
No dosage is available for use in young
children (Levy, 1977).
A dose of 0.5 mg daily has been used in children with
familial mediterranean fever from the age of 5 years
of age or under, and 1 mg daily for older children
(Reynolds 1993).
�
4.3 Contraindications
Underlying disease.
Pregnancy: There is a risk of foetal chromosomal damage
(Reynolds, 1989).
5. ROUTES OF ENTRY
5.1 Oral
Oral absorption is the most frequent cause of
intoxication.
5.2 Inhalational
Not relevant.
5.3 Dermal
Not relevant.
5.4 Eye
Not relevant.
5.5 Parenteral
Intoxications after parenteral administration are rare,
(Benoit et al., 1974, Jaeger et al., 1980, Liu et al., 1978),
however, the toxic dose appears to be lower than the oral
toxic dose.
Five fatal outcomes after intravenous colchicine: the daily
dose was 3 to 6 mg and the total dose was 9 to 21 mg over 2
to 8 days.(Jaeger et al., 1980)
A fatal bone marrow aplasia in a 70 year-old man after 10 mg
intravenous colchicine over 5 days (Liu et al., 1978).
5.6 Other
Intoxication with multisystemic reactions after
instillation of 50 mg colchicine into the penile urethra for
treatment of condyloma acuminata (Naidus et al., 1977).
�
6. KINETICS
6.1 Absorption by route of exposure
Oral
Rapidly absorbed from the gastro-intestinal tract. Peak
plasma concentration is reached 0.5 to 2 hours after
ingestion (Wallace & Ertel, 1973).
Half time of absorption is 15 minutes (Galliot, 1979).
Absorption may be modified by pH, gastric contents,
intestinal motility (Wallace et al., 1970).
Colchicine is not totally absorbed. There is an important
hepatic first pass effect.
6.2 Distribution by route of exposure
Protein binding is 10 to 20% (Bennett et al., 1980).
Colchicine distributes in a space larger than that of the
body. The apparent volume of distribution is 2.2 L/kg
(Wallace et al., 1970). In severe renal or liver diseases the
volume of distribution is smaller (1.8 L/kg).
Colchicine accumulates in kidney, liver, spleen, gastro-
intestinal wall and leucocytes and is apparently excluded in
heart, brain, skeletal muscle.
Colchicine crosses the placenta and has also been found in
maternal milk.
6.3 Biological half-life by route of exposure
Parenteral
After a single 2 mg intravenous dose the average plasma half-
life is 20 minutes (Wallace et al., 1970). Plasma half-life
is increased in severe renal disease (40 min) and decreased
in severe hepatic disease (9 min) (Wallace et al., 1970).
Oral
After oral administration plasma concentrations reach a peak
within 0.5 to 2 hours and afterwards decrease rapidly within
2 hours (Wallace & Ertel, 1973). The plasma half-life is 60
minutes (Galliot, 1979). Colchicine may remain in tissues for
as long as 10 days.
�
6.4 Metabolism
Colchicine undergoes some hepatic metabolism.
Colchicine is partially deacetylated in the liver (Naidus et
al., 1977). Large amounts of colchicine and of its
metabolites undergo enterohepatic circulation. This may
explain the occurrence of a second plasma peak concentration
observed 5 to 6 hours after ingestion (Galliot, 1979;
Walaszek et al., 1960).
6.5 Elimination by route of exposure
Colchicine is excreted unchanged (10 to 20 percent) or
as metabolites.
Oral
Kidney
Urinary excretion amount to 16 to 47% of an administered dose
(Heaney et al., 1976). 50 to 70% of colchicine is excreted
unchanged and 30 to 50% as metabolites. 20% of the dose
administered is excreted in urine in the first 24 hours and
27.5% in the first 48 hours. Colchicine is detected in urine
up to 7 to 10 days after ingestion. Urinary excretion is
increased in patients with impaired hepatic function (
Wallace et al., 1970).
Bile
10 to 25% of colchicine is excreted in the bile (Heaney et
al., 1976).
Faeces
Large amounts of the drug are excreted in the faeces.
Breast Milk
Colchicine may be eliminated in breast milk (White & White,
1980).
Intravenous
Faeces
After intravenous administration 10 to 56% is excreted in the
faeces within the first 48 hours (Walaczek et al., 1960).
Breast Milk
Colchicine may be eliminated in breast milk.
�
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Colchicine binds to tubulin and this prevents
its polymerization into microtubules. The binding is
reversible and the half-life of the colchicine-tubulin
complex is 36 hours. Colchicine impairs the different
cellular functions of the microtubule: separation of
chromosome pairs during mitosis (because colchicine
arrests mitosis in metaphase), amoeboid movements,
phagocytosis.
Mitosis blockade accounts for diarrhoea, bone marrow
depression and alopecia. Colchicine may have a direct
toxic effect on muscle, peripheral nervous system and
liver. Inhibition of cellular function does not,
however, account for all the organ failures seen in
severe overdose.
7.1.2 Pharmacodynamics
Gout inflammation is initiated by urate
crystals within tissues. The crystals are ingested by
neutrophils but this leads to the release of enzymes
and the destruction of the cells. Chemotactic factors
are released and attract more neutrophils. Colchicine
may act by preventing phagocytosis, the release of
chemotactic factors and the response of
neutrophils.
Colchicine has other properties such as antipyretic
effects, respiratory depression, vasoconstriction and
hypertension.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Oral
Fatalities have been reported after ingestion
of 7 to 12 mg (Stapczynski et al., 1981).
The severity and the mortality rate of the
poisoning is directly related to the dose
ingested (Gaultier & Bismuth, 1978; Bismuth
et al., 1977; Lambert et al., 1981).
�
Dose Symptoms Mortality
absorbed Rate
mg/kg
<0.5 Gastrointestinal symptoms 0%
decrease of coagulation
factors
0.5-0.8 + Bone marrow aplasia 10-50%
+ alopecia
> 0.8 + circulatory failure 100%
Intravenous
Fatal outcomes in five patients who had
received a total dose of 9 to 21 mg over two
to eight days (daily dose 3 to 6 mg) (Jaeger
et al., 1980).
A fatal bone marrow aplasia in a 70-year-old
patient who had received 10 mg of intravenous
colchicine over 5 days (Liu et al.,
1978).
The enhanced toxicity of intravenous
colchicine is probably due to the higher
bioavailability of colchicine after
parenteral administration.
7.2.1.2 Children
Oral
The toxic dose is about 0.1 mg/kg and the
lethal dose is between 0.5 and 0.8 mg/kg
(Besson-Leaud et al., 1977).
�
7.2.2 Relevant animal data
Species Route Effect Dose (mg/kg)
Rat Intravenous LD 50 1.6
Rat Intraperitoneal LD 50 6.1
Rat Subcutaneous LD Lo 4
Mouse Intravenous LD 50 4.13
Mouse Intraperitoneal LD 50 2
Dog Oral LD Lo 0.125
Dog Subcutaneous LD Lo 0.571
Cat Oral LD Lo 0.125
Cat Subcutaneous LD Lo 0.5
Cat Intravenous LD 50 0.25
(RTECS, 1979)
7.2.3 Relevant in vitro data
No data available.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
Colchicine is contraindicated in pregnancy as Down's
syndrome and spontaneous abortion have been reported.
Colchicine should be discontinued three months prior to
conception (Drugdex, 1989).
7.5 Mutagenicity
See section 9.4.7.
�
7.6 Interactions
Menta et al. (1987) reported a case of acute cyclosporin
nephrotoxicity induced by colchicine administration.
Colchicine may interfere with cyclosporin pharmacokinetics by
increasing cyclosporin plasma levels either by enhancing
cyclosporin absorption or by reducing its hepatic
metabolism.
7.7 Main adverse effects
Gastrointestinal symptoms are a common complication of
chronic colchicine therapy. Thus the dose of colchicine is
usually limited by gastrointestinal toxicity.
About 80 per cent of the patients treated with colchicine at
a dose of 3 to 4 mg per day develop gastrointestinal
disturbances (Wallace, 1980).
Fatal outcomes have been reported after intravenous
colchicine therapy (see section 7.2.1.1).
The following adverse reactions have been reported during
colchicine treatment (Dukes, 1983):
Gastrointestinal
Vomiting, diarrhoea, abdominal discomfort, paralytic ileus,
malabsorption syndrome with steatorrhoea.
Haematological
Bone marrow depression with agranulocytosis, acute
myelomonocytic leukaemia, multiple myeloma,
thrombocytopenia.
Neurological
Peripheral neuritis, myopathy, rhabdomyolysis (Riggs et al.,
1986).
Dermatological
Allergic reactions are rare urticaria; oedema may be seen.
Alopecia has been reported after chronic treatment.
Reproductive system
A reversible, complete azoospermia has been reported (Merlin
1972).
�
Metabolic
Colchicine is capable of producing a reversible impairment of
vitamin B12 absorption (Webb et al, 1968). Porphyria cutanea
tarda has been reported (Gossweiler, 1985).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Methods
Colchicine may be analysed in biological fluids by
different methods:
Fluorometric method: Fluorescence of organometallic
(Gallium) complexes (Bourdon & Galliot, 1976).
Radioimmunoassay: (Ertel et al., 1979; Scherrman et al.,
1980).
High performance liquid chromatography: (Jarvie et al, 1979,
Caplan et al., 1980; Harzer, 1984; Lhermitte et al.,
1985).
Liquid chromatography (Thompson, 1985).
8.2 Therapeutic and toxic concentration
Colchicine may be measured in biological fluids but
levels are not useful or necessary for the management of
colchicine poisoning.
Serum/Plasma/Blood
Plasma
Plasma levels lower than 20 ng/ml at the 6th hour in severe
intoxications have been reported (Bourdon & Galliot
1976).
In an overdose with 7.5 mg, plasma levels of 21 ng/ml at the
6th hour and below 5 mg/ml at the 24th hour were noted
(Jarvie et al., 1979).
In severe intoxications plasma levels usually range between
20 to 50 ng/ml during the 24 first hours. After the 24th
hour only small amounts of colchicine (< 20 ng/ml) are
detected in plasma (Bismuth et al., 1977; Lambert et al.,
1981; Jaeger et al., 1985).
Post-mortem serum blood levels of 170 and 240 ng/mL (at the
4th and 8th hour) in 2 heroin addicts following intravenous
injection were reported (Harzer, 1984) .
�
The following plasma levels were noted in an overdose with 31
mg orally: 720, 212, 132 and 120 ng/mL at the 20, 125, 305,
605 minutes respectively (Lhermitte et al., 1985).
Blood
Colchicine levels in blood are higher than those in
plasma.
In an overdose with 20 mg colchicine orally a blood level of
250 ng/ml at the second hour was noted (Caplan et al. 1980).
No colchicine could be detected at the 40th hour.
Urine
Colchicine levels in urine range between 200 and 2500 ng/ml
over the first 24 hours (Bismuth et al., 1977; Lambert et al,
1981, Jaeger et al. 1985).
Information was available on urinary excretion in 5 cases.
Concentrations in urine are 10 to 80 fold higher than those
in plasma. Four to 25 per cent of the dose ingested was
excreted in urine over three to ten days. Excretion was
specially high during the first 24 hours following ingestion.
Colchicine is eliminated in urine up to the tenth day (Jaeger
et al., 1985).
Gastric lavage fluid
In four cases, gastric lavage performed 3 to 6 hours post
ingestion removed 7 to 25 per cent of the dose ingested
(Jaeger et al., 1985).
Diarrhoea
In an overdose with 25 mg colchicine orally, 1.4 mg were
eliminated in diarrhoea on the 2nd day (Jaeger et al.,
1985).
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Toxic manifestations appear after a delay of 2
to 12 hours following ingestion. The delay may be
increased if other drugs decreasing gastro-intestinal
motility have also been ingested (phenobarbitone,
psychotropic drugs, opium derivatives). Symptomatology
progresses in three stages and may include:
�
Stage I (Day 1 to 3) Gastrointestinal and circulatory
phase:
Severe gastrointestinal irritation: Nausea, vomiting,
abdominal cramps, severe diarrhoea.
Dehydration, hypovolemia, shock. Cardiogenic shock
may occur and may result in death within the first 72
hours.
Hypoventilation, acute respiratory distress
syndrome.
Stage II (Day 3 to 10) Bone marrow aplasia phase:
Bone marrow aplasia with agranulocytosis.
Coagulation disorders with diffuse haemorrhages.
Rhabdomyolyis.
Polyneuritis, myopathy.
Acute renal failure.
Infectious complications.
Stage III: (After ten days) Recovery phase:
Alopecia
(Gaultier & Bismuth, 1978, Ellenhorn & Barceloux,
1988, Stapczynski et al., 1981).
9.1.2 Inhalation
Not relevant.
9.1.3 Skin exposure
Not relevant.
9.1.4 Eye contact
Not relevant.
�
9.1.5 Parenteral exposure
The clinical course after intravenous injection
is similar to that observed after ingestion. The time
to onset of symptoms depends on the dose and rate of
injection but gastro-intestinal symptoms usually
appear two to six days after the beginning of
colchicine therapy.
Two cases of lethal overdose after a single
intravenous injection of 30 mg colchicine were
reported; gastro-intestinal symptoms appeared 2 hours
after injection (Michaux et al., 1972) .
9.1.6 Other
An intoxication with multisystemic reactions
after instillation of 50 mg colchicine into the penile
urethra for treatment of condyloma acuminata has been
reported. (Naidus et al., 1977)
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic administration of colchicine may induce
similar toxicity to that seen in acute poisoning:
gastro-intestinal symptoms (vomiting, diarrhoea),
agranulocytosis, aplastic anaemia, myopathy (Gilman et
al., 1985)
9.2.2 Inhalation
No data available.
9.2.3 Skin contact
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
Similar to acute poisoning.
9.2.6 Other
No data available.
�
9.3 Course, prognosis, cause of death
Course
See section 9.1.1.
Prognosis
Prognosis is related to the dose ingested (see section
7.2.1).
Occurrence of cardiogenic shock indicates a poor prognosis
(Sauder et al., 1983).
If the patient has recovered from aplasia and has not
developed acute respiratory distress syndrome or systemic
infectious complications, prognosis is usually good.
Cause of death
At the early stage (day 1 to 3) of the intoxication, death is
due to cardiogenic shock and/or acute respiratory distress
syndrome.
Death due to haemorrhagic or infectious complications may
occur at the stage of bone marrow aplasia (day 3 to
10).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Cardiovascular shock is always present in
severe intoxications. Most deaths result from shock
within the first 72 hours.
Hypotension is usually the result of hypovolaemia due
to gastrointestinal fluid loss. Hypovolaemia with
decreased central venous pressure is initially always
present but some patients may develop cardiogenic
shock. (Sauder et al., 1983; Bismuth & Sebag
1981).
Haemodynamic studies showed two different profiles:
Patients with a hyperkinetic state (increased cardiac
index and decreased systemic vascular resistances);
patients with cardiogenic shock (decreased cardiac
index and increased systemic vascular resistances)
(Sauder et al., 1983). Occurrence of cardiogenic shock
indicates a poor prognosis. Septic shock may occur
during the phase of aplasia.
�
9.4.2 Respiratory
Acute respiratory failure is usually
concomitant of circulatory failure, although Murray et
al 1983, reported a case with ascending paralysis
occurring more than 4 hours post-exposure.
Acute respiratory distress syndrome due to diffuse
interstitial and alveolar oedema has been reported in
severe cases (Hill et al., 1975; Corbin et al., 1989;
Maurizi et al., 1986; Davies et al., 1988; Hobson &
Rankin, 1986).
9.4.3 Neurological
9.4.3.1 Central nervous system (CNS)
In severe cases hypotension and/or
hypoxaemia can lead to confusion, agitation,
and mental depression. Coma and seizures are
observed. Profound coma may be due to
cerebral complications such as
haemorrhages.
9.4.3.2 Peripheral nervous system
Peripheral neuritis, neuromyopathy
and myopathy have been reported (Carr, 1965;
Favarel-Garrigues et al., 1975; Kuncl, 1987;
Bismuth et al., 1977; Mouren et al., 1969;
Kontos, 1962). Ascending paralysis may be
responsible for respiratory failure (Carr,
1965). Polyneuritis usually recovers within
one month (Bismuth et al., 1977).
9.4.3.3 Autonomic nervous system
None described.
9.4.3.4 Skeletal and smooth muscle
Rhabdomyolysis may occur with an
increase in muscle enzymes and myoglobinuria
(Murray et al., 1983; Kontos et al., 1962;
Letellier et al., 1979). A case of
rhabdomyolysis was reported in a 58-year-old
patient treated with 3 mg colchicine daily
over 6 days (Letellier et al., 1979). The
patient developed proximal scapular weakness
with muscle oedema and increase in muscle
enzymes.
�
9.4.4 Gastrointestinal
Acute
Gastrointestinal symptoms develop after a delay of 2
to 12 hours following ingestion and include nausea,
vomiting, abdominal pain and severe diarrhoea.
Usually diarrhoea lasts for 48 hours and may induce
hypovolaemia and electrolyte disturbances.
Gastrointestinal symptoms also occur after colchicine
overdose by the intravenous route. Paralytic ileus
may develop (Heaney et al., 1976).
Gastrointestinal disturbances may be lacking or
decreased if drugs decreasing gastrointestinal
motility (atropine, phenobarbitone, opium tincture)
have also been ingested.
Chronic
Gastrointestinal symptoms are a common feature during
colchicine treatment. Paralytic ileus has been
reported after intravenous colchicine
treatment.
9.4.5 Hepatic
Colchicine may exert direct hepatic toxicity.
Hepatomegaly has been reported. Hepatic damage may
occur in severe poisoning and includes cytolysis and
hepatocellular insufficiency, increase in glutamic
pyruvic transaminase (SGOT) (alanine amino
transferase, ALT) and glutamic oxaloacetic
transaminase (SGOT) (aspartate amino transferase, AST)
and in alkaline phosphatase, a decrease in coagulation
factors. Histological examination has shown necrosis
and steatosis of hepatocytes.
9.4.6 Urinary
9.4.6.1 Renal
No direct nephrotoxic effect has
been reported. Functional renal insufficiency
is usually observed and is secondary to fluid
and electrolytes losses or hypovolaemia.
Acute renal failure may occur following
cardio-vascular or septic shock.
�
9.4.6.2 Other
Proteinuria and haematuria have been
reported.
9.4.7 Endocrine and reproductive systems
Endocrine
Transient diabetes mellitus has been reported by
Hillemand et al. 1977 in a 58-year-old woman after an
overdose with 25 mg.
Inappropriate antidiuretic syndrome has been reported
(Gauthier et al., 1975).
Reproductive
Acute
A case of colchicine poisoning (40 mg) has been
reported in a 18 year old pregnant woman (Lambert et
al., 1981). The patient developed severe poisoning
with coagulopathy, ARDS and abortion on day 7
following ingestion. The patient recovered.
Chronic
A reversible complete azoospermia has been reported in
a 36- year-old man treated with colchicine for gout
(Merlin, 1972). 2 cases of Down's syndrome babies
have been reported (Ehrenfeld et al., 1987). The
obstetric histories of 36 women with familial
Mediterranean fever on long-term colchicine treatment
between 3 and 12 years have been reported (Ehrenfeld
et al., 1987). Seven of 28 pregnancies ended in
miscarriage. 13 women had periods of infertility.
All 16 infants born to mothers who had taken
colchicine during pregnancy were healthy. The authors
do not advise discontinuation of colchicine before
planned pregnancy but recommend amniocentesis for
karyotyping and reassurance.
9.4.8 Dermatological
Acute
Alopecia begins at about the 12th day and is complete
by 3 weeks after ingestion. Hair regrowth begins
after the first month.
�
Cutaneous and subcutaneous haemorrhages are frequent
in severe poisoning. They are due to coagulation
disturbances and may be induced by venous or arterial
punctures.
9.4.9 Eye, ear, nose, throat: local effects
Eye
Subconjunctival haemorrhage may occur.
Ear
Definitive unilateral deafness due to an inner ear
haemorrhage has been observed (personal
experience).
Nose
Nasal haemorrhages may occur especially after local
trauma due to insertion of tracheal or gastric
tubes.
Throat
Stomatitis may also occur (Wallace, 1974; Lambert et
al., 1981).
9.4.10 Haematological
At toxic doses, colchicine induces marked bone
marrow depression.
Leucocytes
At the initial stage a peripheral leucocytosis occurs
frequently. a leucopenia with agranulocytosis begins
at the third day and reaches a maximum at day 5 to 7.
WBC return to normal values at about the 10 to 12th
day.
Erythrocytes
Anaemia is frequent in severe cases and may be due to
different factors:
Hypoplastic anaemia due to bone marrow suppression may
be observed but is rarely important.
Haemolytic anaemia with Heinz body has been rarely
reported (Heaney et al., 1976).
�
Acute intravascular haemolysis with haemoglobinemia
and haemoglobinuria has been observed in 6 severe
cases (Lambert et al., 1981)
Severe anaemia is mostly secondary to multiple diffuse
haemorrhages.
Bleeding diatheses and coagulopathy
A tendency toward bleeding is always present in severe
cases. It appears two to three days following
ingestion and may last for eight to ten days.
Usually the earliest indication of coagulopathy is
persistent bleeding from venous or arterial puncture
sites and subcutaneous haemorrhages. Other types of
bleeding include epistaxis, gingival, conjunctival and
gastrointestinal haemorrhages. Bleeding may be due to
thrombocytopenia or a consumptive coagulopathy.
A consumptive coagulopathy with prolongation of
coagulation time, hypoprothrombinaemia, a decrease in
fibrinogen, elevated fibrin degradation products and
thrombocytopenia is observed in severe intoxication
(Bismuth et al., 1977; Lambert et al., 1981; Crabie et
al.,1970)
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Metabolic acidosis due to diarrhoea
and/or shock may be seen.
9.4.12.2 Fluid and electrolyte disturbances
The gastro-intestinal syndrome
often results in marked dehydration and
hypovolaemia with haemoconcentration and
functional renal failure.
Hypokalaemia due to gastrointestinal losses
is also frequent at the initial stage.
�
Hypocalcaemia may be seen and can persist for
several days. Frayha et al. (1984) reported,
in a 20-year-old girl who had ingested 20 mg,
convulsions and paralytic ileus which were
related to a hypocalcaemia (1.25 mmol/L).
Hypocalcaemia may be due to a direct toxic
effect of colchicine (Heath et al.,
1972).
9.4.12.3 Others
Hyperglycaemia
A 58-year-old woman who ingested 25 mg and
developed transient diabetes mellitus has
been reported (Hillemand et al., 1977).
Hyperlipaemia
A transient hyperlipaemia has been reported
(Hillemand et al. 1977).
Hyperuricaemia
A transient hyperuricaemia has also been
noted (Hillemand et al. 1977).
Hyperthermia-fever
Occurrence of fever may be relate to an
infectious complication, especially during
the stage of aplasia.
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Pregnancy
Two cases of Down's syndrome babies have been
reported. The obstetric histories of 36 women with
familial Mediterranean fever on long-term colchicine
treatment between 3 and 12 years have been reported
(Ehrenfeld et al. 1987). Seven of 28 pregnancies
ended in miscarriage. Thirteen women had periods of
infertility. All 16 infants born to mothers who had
�
taken colchicine during pregnancy were healthy. The
authors do not advise discontinuation of colchicine
before planned pregnancy but recommend amniocentesis
for karyotyping and reassurance.
Breast-feeding
As colchicine is eliminated in the breast milk breast-
feeding should be avoided.
9.5 Other
No data available.
9.6 Summary
Not relevant
10. MANAGEMENT
10.1 General principles
Patients with colchicine overdose should always be
admitted in an intensive care unit. Treatment depends on the
dose ingested, the symptomatology and the delay following
ingestion. It includes gastric lavage, early forced
diuresis, and supportive treatment with correction of the
shock, artificial ventilation, treatment and prevention of
haemorrhagic and infectious complications. Vital signs (ECG,
blood pressure, central venous pressure, respiration) should
be monitored. Be careful about venous and arterial punctures
if there is a severe coagulopathy.
10.2 Relevant laboratory analyses
10.2.1 Sample collection
Blood samples for colchicine should be drawn
in plastic tubes with heparin. Colchicine may be
analysed in whole blood or plasma. Biological samples
(blood, plasma, urine...) should be stored in airtight
conditions and protected from light. Concentrations in
whole blood are markedly higher than those in plasma.
Concentration in urine are 10 to 80 fold higher than
those in plasma.
�
10.2.2 Biomedical analysis
A biochemical profile with glucose, BUN,
electrolytes, creatinine, blood cells, coagulation
parameters, enzymes, and blood gases should be
obtained on admission and repeated every 12 hours.
Samples for bacteriological analysis should be
obtained at the stage of aplasia or when fever
occurs.
10.2.3 Toxicological analysis
Colchicine analysis in biological fluids is
not necessary or useful for the management of the
poisoning.
10.2.4 Other investigations
No other specific investigations are
required.
10.3 Life supportive procedures and symptomatic/specific treatment
Observation and monitoring
Monitor systematically vital signs, ECG, blood pressure and
central venous pressure. Repeated monitoring of central
venous pressure is essential to avoid circulatory overload
during plasma expander infusion.
Insert a venous catheter for rehydration and drug
injection.
If shock is present, insertion of a pulmonary artery (Swan-
Ganz) catheter for monitoring of haemodynamic parameters may
be useful for guiding the treatment.
The patient remains at risk until at least 48 hours after
exposure.
Diarrhoea
Diarrhoea should not be treated because some colchicine is
eliminated in faeces.
Dehydration - Electrolyte disturbances - Acidosis
Give intravenous fluids and electrolytes according to
clinical and biological status. If metabolic acidosis is
present give intravenous bicarbonate. Monitor potassium
levels and blood gases. Maintain adequate urinary output
(>100 ml/hr).
�
Hypotension, shock
Hypotension should be anticipated and treated with adequate
fluid replacement and vasoactive drugs. Monitor blood
pressure. Early institution of hemodynamic monitoring is
very helpful for adequate treatment of shock.
Hypotension and shock are due primarily to hypovolaemia.
Cardiogenic shock may occur.
Plasma expanders
Infuse plasma expander solutions (e.g. modified gelatine
fluids) under control of haemodynamic parameters e.g. central
venous pressure, pulmonary arterial pressure. Very large
amounts of plasma expanders may be necessary: 3 to 4 litres
over 24 hours (personal observation).
Inotropic and vasoconstrictor drugs
If the patient is unresponsive to these measures administer
inotropic and vasoconstrictor drugs e.g. dopamine or
dobutamine in doses sufficient to cause vasoconstriction (10
to 20 mcg/kg/min).
Vasodilators
Vasodilators e.g. glyceryl trinitrate may be useful in the
case of cardiogenic shock with increased systemic arterial
resistance (personal observation).
Respiratory disturbances
Respiratory depression or ARDS should be treated by
artificial ventilation. The early institution of mechanical
ventilation is indicated in patients with severe intoxication
and shock.
Bone Marrow Depression
Isolate the patient if there is evidence of bone marrow
depression. Infusion of white blood cell units is usually not
necessary because aplasia is transient. However, it may be
useful in patients who develop concomitant infection
(Gauthier & Bismuth, 1978).
Coagulation Disorders
Prevent haemorrhagic complications due to local trauma: avoid
insertion of endotracheal tube by the nasal route, avoid
femoral arterial puncture.
�
Coagulation disorders require specific treatment only if
haemorrhages develop. According to biological parameters,
treatment may include infusion of fresh-frozen plasma,
platelet units, fibrinogen and coagulation factors
(PPSB).
Prevention of Infectious Complications
In severe cases with shock and/or aplasia a prophylactic
antibiotic treatment should be given. Prophylactic
antibiotherapy may be directed towards gram positive (e.g.
staphylococcal) and negative bacteria and also anaerobic
bacteria. Preventative treatment for fungal infections should
also be given because fungal septicaemia may develop
(personal observation).
10.4 Decontamination
Gastric Lavage
Early gastric lavage is indicated because it may remove 7 to
25 per cent of the dose ingested if it is performed within 6
hours of ingestion (Jaeger et al., 1985).
Emesis
Emesis may be useful in recent ingestion if there are no
contraindications.
Oral Activated Charcoal
The efficacy of oral activated charcoal has not been
established. However, because colchicine undergoes
enterohepatic circulation, oral activated charcoal may be
indicated: one dose at the end of the gastric lavage and
repeated every 4 to 6 hours.
Cathartics
The usefulness of cathartics has not been established and is
not recommended.
�
10.5 Elimination
Forced Diuresis
Toxicokinetic studies (Jaeger et al., 1985) indicate that
significant amounts of colchicine are eliminated in urine,
especially during the first 24 hours following ingestion.
Thus early forced diuresis should be instituted after
correction of dehydration and/or shock (Jaeger et al., 1985).
Continue forced diuresis until the third or fourth day
provided there are no contraindications.
Haemoperfusion, Haemodialysis
No data about haemoperfusion or haemodialysis clearances have
been reported. However, the low colchicine plasma
concentrations reported in acute poisonings and the large
volume of distribution indicate that haemoperfusion or
haemodialysis is not useful.
10.6 Antidote treatment
10.6.1 Adults
Currently no antidote for colchicine is
available.
10.6.2 Children
No antidote available.
10.7 Management discussion
Gastrointestinal symptoms may be overshadowed if
psychotropic drugs or drugs decreasing gastrointestinal
motility have also been ingested.
Institute prophylactic antibiotic therapy.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
Case report 1
In 69 reported cases of colchicine poisoning (Bismuth et al.,
1977) thirty eight patients (dose ingested <0.5 mg/kg)
developed gastro- intestinal symptoms and coagulation
disturbances; all survived. Twenty patients (dose ingested
0.5 to 0.8 mg/kg) developed bone marrow aplasia: mortality
was 10 per cent. Eleven patients (dose ingested > 0.8 mg/kg)
died within 72 hours from cardiovascular shock.
�
Case report 2
In another reported 22 cases (Lambert et al., 1981),
according to the doses ingested (DI), mortality rates were:
100% for DI > 1 mg/kg; 50% for DI = 0.5 to 0.9 mg/kg; 10%
for DI < 0.5 mg/kg. Clinical features included:
gastrointestinal symptoms (22 cases), leucopenia, aplasia (11
cases), disseminated intravascular coagulation (9 cases),
shock (9 cases), acute respiratory distress syndrome (8
cases), polyneuropathy (4 cases).
Case report 3
Two cases were reported (Gaultier et al., 1969) who developed
inappropriate antidiuresis following ingestion of about 40
mg. Both patients recovered.
Case report 4
A 18-year-old woman developed an acute respiratory distress
syndrome (ARDS) following ingestion of 150 mg. The patient
died at the 42nd hour (Hill et al., 1975).
Case report 5
An acute respiratory distress syndrome was reported in a 17-
year-old woman who had ingested 0.37 mg/kg (Corbin et al.,
1989). Pulmonary capillary wedge pressure was 7 mm Hg. The
patient died (72nd hour), despite mechanical ventilation
(PEEP), from shock any hypoxaemia.
Case report 6
Two cases with ARDS have been reported (Maurizi et al.,
1986). A 25- year-old woman who had ingested 80 mg died on
the 7th day from septic shock with acute renal failure; a 21
year old man who had ingested 15 to 20 mg also developed
aplasia and recovered.
Case report 7
A fatal overdose in a 15-year-old boy who had ingested 18 mg
colchicine and developed cardiovascular shock, ARDS,
metabolic acidosis, hypocalcaemia, hypokalaemia,
hypophosphotaemia, bone marrow suppression and coagulopathy
has been reported (Hobson & Rankin, 1986).
�
Case report 8
Report of an overdose with about 24 mg in a 15 year old girl
(Murray et al., 1983). The clinical picture showed:
* myocardial injury with cardiogenic shock
* ventilatory insufficiency with ARDS
* rhabdomyolysis
* metabolic acidosis
* agranulocytosis
* coagulopathy
* alopecia.
The patient recovered without sequelae.
11.2 Internally extracted data on cases
Jaeger et al. (1980) reported five fatal outcomes in
five patients after intravenous colchicine treatment for
gout. The total dose ranged between 9 to 21 mg administered
over two to eight days. During the treatment the patients
developed gastro-intestinal symptoms and thereafter (on about
the 7th day) agranulocytosis, thrombocytopenia and shock with
acute renal failure. Death occurred between the 7th and 15th
day after beginning of the treatment.
Sauder et al. (1983) performed haemodynamic studies in eight
cases of colchicine poisoning. The doses ingested ranged
between 9 and 160 mg. Haemodynamic studies performed between
the 6th and 72nd hour following ingestion showed:
Hypovolemia in all cases,
A hyperkinetic state with increased cardiac index and
decreased systemic vascular resistance in the 4 patients who
recovered.
Cardiogenic shock with decreased cardiac index and increased
systemic vascular resistances in the 4 patients who died.
An initial decrease of cardiac performance is an index of
severity and poor prognosis.
Jaeger et al, (1985) performed a toxicokinetic study in 5
cases (dose ingested 19 to 60 mg). Plasma concentrations
ranged between 20 and 54 ng/mL during the 24 first hours.
Gastric lavage removed 7.1 to 25% of the dose ingested. In
one case 1.4 mg colchicine was excreted in diarrhoea on the
second day. Four to 25 per cent of the dose ingested was
eliminated in urine over 3 to 10 days.
�
Urinary colchicine excretion was especially high during the
first day following ingestion (2 to 10 per cent of the dose
ingested). Colchicine levels in urine were 10 to 80 times
higher than those in plasma. This study emphasizes the
usefulness of early gastric lavage, of early diuresis and of
colchicine elimination in diarrhoea.
11.3 Internal cases
To be completed by each Centre using local data.
12. ADDITIONAL INFORMATION
12.1 Availability of antidotes
Not relevant
12.2 Specific preventive measures
Not relevant
12.3 Other
Unknown
13. REFERENCES
Bennet WF & Glenn KC (1980) Hypersensitivity of platelets to
thrombin: formation of stable thrombin-receptor complexes and the
role of shape-change. Cell 22(2): 621-627.
Benoit JP, Leveque M, Carli PM (1974) Aplasie médullaire mortelle
au cours d'un bref traitement de colchicine. Rev Méd Dijon, 9:
485-488.
Besson-Leaud M (1977) De quelques intoxications chez l'enfant.
Ann Pédiatr, 24: 363-371.
Bismuth C, Gaultier M, & Conso F (1977). Aplasie médullaire aprés
intoxication aiguë à la colchicine. Nouv Presse Med, 6: 1625-
1629.
Bismuth C & Sebag C (1981) Choc cardiogénique lors d'une
intoxication aigue par la colchicine. Nouv Presse Méd, 10:
1073.
Bourdon R & Galliot M (1976) Dosage de la colchicine dans les
liquides biologiques. Ann Biol Clin, 34: 393-401.
Budavari S ed. (1989) The Merck index, an encyclopedia of
chemicals, drugs, and biologicals, 11th ed. Rahway, New Jersey,
Merck and Co Inc, pp 386- 387.
�
Caplan YH, Orloff KG & Thompson BC (1980) A fatal overdose with
colchicine. J Anal Toxicol 4(3): 153-5
Carr AA (1965) Colchicine toxicity. Arch Intern Med, 115: 29-
33.
Corbin JC, Duval G, Plane M, & Chuet C (1989) Syndrome de
détresse respiratoire aigue de l'adulte au cours d'une
intoxication par la colchicine. Rean Soins Intens Med Urg, 2: 187-
189.
Crabie P., Pollet J., Pebay-Peyroula F. (1970). Etude de l'
hémostase au cours des intoxications aiguës par la colchicine.
J.E.T., 3: 374-385.
Davies H.O., Hyland R.H., Morgan C.D. (1988) Massive overdose of
colchicine. Can Med Assoc J, 138: 335-336.
Drugdex (1989) February issue.
Dukes MNG (1983) Side effects of Drugs. Annual 7, Excerpta
Medica.
Ehrenfeld M, Levy M, Margalioth EJ, Eliakim M (1986) The effects
of long- term colchicine therapy on male fertility in patients
with familial mediterranean fever. Andrologia, 18: 420-426.
Ehrenfeld M, Brzezinski A, Levy M, & Eliakim M (1987) Fertility
and obstetric history in patients with familial mediterranean
fever on long-term colchicine therapy. Br J Obstet Gynaecol, 94:
1186-1191.
Ellenhorn M.J., Barceloux D.G. (1988) Medical Toxicology -
Diagnosis and Treatment of Human poisoning, 1st ed. New York,
Elsevier.
Ertel NH, Mittler JC, Akgun S, & Wallace SL (1976)
Radioimmunoassay for colchicine in plasma and urine. Science, 193:
233-234.
Favarel-Garrigues JC, Bony D, & Poisot D (1975) Intoxications
aiguës par la colchicine. Concours Med, 97: 5183-5197.
Fleeger CA ed. (1993) USAN 1994: USAN and the USP dictionary of
drug names. Rockville, MD, United States Pharmacopeial Convention,
Inc., p 171.
Frayha RA, Tabbara Z, & Berbir N (1984) Acute colchicine poisoning
presenting asymptomatic hypocalcaemia. Br J Rheumatol, 23: 292-
295.
�
Galliot M (1979) Complexes metaliques dérivés de la colchicine.
Application au dosage biologique dans le médicaments et les
liquides biologiques. Thése, Paris, p 134.
Gaultier M, Kanfer A, Bismuth C, Crabie P, & Frejaville JP (1969)
Données actuelles sur l' intoxication aiguë par la colchicine. A
propos de 23 observations. Ann Med Interne, 120: 605-618.
Gaultier M, Bismuth C, Autret A, & Pillon M (1975) Antidiurése
inapproprieée après intoxication aigue par la colchicine. Nouv
Presse Méd, 4: 3132-3134.
Gaultier M & Bismuth C (1978) Intoxication aiguë par la
colchicine. Revue du Praticien 28(57): 4545-4554.
Goodman LS, Gilman AG, Rall TW, & Murad F eds. (1985) Goodman and
Gilman's Pharmacological Basis of Therapeutics. 7th Ed. New York,
Macmillan.
Gooneratne BWM (1966) Massive generalized alopecia after poisoning
by Gloriosa superba. Br Med J, 1(5494): 1023-1024.
Gossweiler B (1985) Kolchizinvergiftung Schweiz. Rundschau Med,
74: 1443-1449.
Heaney D, Derghazarian CB, Pineo GF (1976) Massive colchicine
overdose. A report on the toxicity. Am J Med Sci, 271: 233-
238.
Harzer K (1984)Tödliche Vergiftugh mit Colchicin. Z Rechtsmed, 93:
181-185.
Heath DA, Palmer JS, Aurbach D (1972) The hypocalcaemic action of
colchicine. Endocrinology, 90(6): 1589-1593.
Hill RN, Spragg RG, Wedel MK, & Moser KM (1975) Adult respiratory
distress syndrome associated with colchicine intoxication.
Hillemand B, Joly JP, Membrey-Maheo E, & Ouvry D (1977) Diabète
transitoire avec hyperlipidémie et hyperuricémie régressives au
cours d' une intoxication aiguë par la colchicine. Relation d'un
cas. Ann Med Interne, 128: 379-385.
Hobson CH & Rankin AP (1986) A fatal colchicine overdose. Anaesth
Intensive Care, 14(4): 453-455.
Jaeger A, Simon CH, Tempe JD, Mantz JM, Bismuth C, Conso F,
Mathiot C, Baumelou A, Bavoux F, Jouanjean X, & Toulet R (1980)
Accidents thérapeutiques mortels après colchicine intraveineuse.
Nouvelle Presse Med, 9: 1587.
�
Jaeger A, Galliot M, Zaehringer M, Sauder PH, Bourdon R,
Kopferschmitt J, Jaegle ML, & Mantz JM (1985) Elimination
digestive et excrétion rénale de la colchicine au cours des
intoxications aiguës. In: "Pharmacocinétique appliquée en
réanimation" Monographie de la Société de Réanimation de Langue
Française, Ed Expansion Scientifique, pp 257-262.
Jarvie D, Park J, & Stewart MJ (1979) Estimation of colchicine in
a poisoned patient by using high performance liquid
chromatography. Clin Toxicol, 14: 375-381.
Kontos HA (1962) Myopathy associated with chronic colchicine
toxicity. N Engl J Med, 266: 38.
Kuncl RW (1987) Colchicine myopathy and neuropathy. New Engl J
Med, 316: 1562-1568.
Lambert H, Laprevote-Heully MC, Manel J, Gilgenkrantz S, & Larcan
A (1981) Les intoxications aiguës par la colchicine. A propos de
22 observations. Ann Med Nancy et de l'Est, 20: 891-900.
Letellier PH., Langeard M., Agullo M. (1979). Rhabdomyolyse
secondaire à une série d'injections intra-veineuses de colchicine.
J. Med. Caen, 14, 4: 157-159.
Levy M & Eliakim M (1977) Long-term prophylaxis in familial
Mediterranean fever. Br Med J, 2(6090): 808
Lhermitte M, Bernier JL, Mathieu D, Mathieu-Nolf M, Erb F, &
Roussel P (1985) Colchicine quantitation by high-performance
liquid chromatography in human plasma and urine. J Chromatogr,
342:416-423.
Liu YK, Hymowitz R, & Carroll MG (1978) Marrow aplasia induced by
colchicine. Arthritis, Rheumatism, 21: 731-735.
Maurizi M, Delorme N, Laprevote-Heully MC, Lambert H, & Larcan A
(1986) Syndrome de détresse respiratorie aiguë de l' adulte au
cours des intoxications par la colchicine. Ann Fr Anesth Reanim,
5: 530-532.
Menta R, Rossi E, Guariglia A, David S, & Cambi V (1987)
Reversible acute cyclosporin nephrotoxicity induced by colchicine
administration. Nephrol Dial Transplant 2:380-382.
Merlin HE (1972) Azoospermia caused by colchicine. A case report.
Fertil and Steril. 23: 180-191.
Michaux P, Curtes JP, & Lejeune (1972) Responsabilité médicale et
pharmaceutique à la suite de l' administration intraveineuse d'
une préparation contenant de la colchicine. Méd Lég Dommage Corp,
5: 248-250.
�
Mouren P, Tatossian A, Poiso Y, Giudicelli S, Jouglard J, Dufour
H, & Poyen D (1969) L' intoxication aiguë par la colchicine.
Presse Med, 77, 14: 505-508.
Murray SS, Kramlinger KG, & McMichan JC (1983) Acute toxicity
after excessive ingestion of colchicine. Mayo Clin Proc, 58: 528-
532.
Nadius RM, Rodvien R, & Mielke CH Jr. (1977 Colchicine toxicity -
a multisystem disease. Arch Intern Med, 137: 394-396.
Nagaratnam N, De Silva DP, & De Silva N (1973) Colchicine
poisoning following ingestion of Gloriosa superba tubers. Trop
Geogr Med, 25: 15-17.
Registry OF Toxic Effects OF Chemical Substances (1979) Volume
One. US Department of Health and Human Services. Ed. Richard J.
Lewis Sr. and Rodger L. Tatken.
Reynolds JEF ed.(1989) Martindale, the extra pharmacopoeia, 29th
ed. London, The Pharmaceutical Press. pp 438-439.
Reynolds JEF ed. (1993) Martindale, the extra pharmacopoeia, 30th
ed. London, The Pharmaceutical Press. pp 335-337.
Riggs JE, Schochet SS, Gutmann L, Crosby TW, & Dibartolomeo AG
(1986) Chronic human colchicine neuropathy and myopathy. Arch
Neurol, 43: 521-523.
Sauder PH, Kopferschmitt J, Jaeger A, & Mantz JM (1983)
Haemodynamic studies in eight cases of acute colchicine poisoning.
Human Toxicol, 2: 169-173.
Scherrmann JM, Boudet L, Pontikis R, Nguyen HN, & Fournier E
(1980) A sensitive radioimmunoassay for colchicine. J Pharm
Pharmacol 32(11): 800-802.
Stapczynski JS, Rothstin RJ, Gaye WA (1981) Colchicine overdose:
report of two cases and review of the literature. Ann Emerg Med,
10: 364.
Thompson RD (1985) Liquid chromatographic determination of
colchicine in pharmaceuticals: collaborative study. J Assoc Off
Anal Chem, 68: 1051-1055.
Walaszek EJ, Kocsis JJ, Leroy GV, & Geiling EMK (1960) Studies on
the excretion of radioactive colchicine. Arch Int Pharmacodyn
Ther, 125: 371-382.
Wallace SL & Ertel NH (1970) Occupancy approach to colchicine
dosage. Lancet, 2: 1250-1251.
�
Wallace SL, Omokoku B & Ertel NH (1970) Colchicine plasma levels.
Am J Med, 48: 443-448.
Wallace SL (1974) Colchicine. Senin Arthritis Rheum 3(4): 369-
81
Wallace SL (1980) Colchicine. In: WN Kelley, Ed. Harris, S.
Ruddy, CB. Sledge. Textbook of Rheumatology, Philadelphia, W.B.
Saunders Company, 878-884.
Webb DI (1968) Mechanism of vitamin B12 malabsorption in patients
receiving colchicine. N Engl J Med, 279: 845.
White GJH & White MK (1980) Breast feeding and drugs in human
milk. Vet Human Toxicol, 22: 1-43.
WHO (1992) Anatomical Therapeutic Chemical (ATC) classification
index. Oslo, WHO Collaborating Centre for Drug Statistics
Methodology, p 73.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors A. Jaeger, F. Flesch, Ph. Sauder, J Kopferschmitt
Poison Control Center
Strasbourg
France
Tel: 33-88161144
Fax: 33-88161930
Date 28 March 1989
Peer Review London, United Kingdom, March 1990
Berlin, Germany, October 1995
Molecular formula
C22H25NO6
Molecular weight
399.4
Structural Chemical names
(S)-N-(5,6,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-oxobenzo
[alpha] heptalen-7-yl)acetamide.
N-(5,5,7,9-Tetrahydro-1,2,3,10-tetramethoxy-9-
oxobenzo[alpha]heptalen-7-yl)acetamide.
(Budavari, 1989; Reynolds, 1993)
Derivatives
Different compounds have been isolated from Colchicum
autumnal. Colchicine has the most important biological
activity which is related to the tropolonic cycle (C).
�
Biological Substance R1 R2 R3 R3
activity name
+++ Colchicine CH3 COCH3 0 OCH3
++ Desacetyl CH3 CH3 0 OCH3
methylcolchicine CH3 H 0 SCH3
++ Desacetylthiocolchicine C6H1105 COCH3 OCH3
++ Colchicoside CH3 H 0 OH
+ Trimethylcolchicinic acid CH3 COCH3 0 0
0 Colchiceine 0H
3.3 Physical properties
3.3.1 Properties of the substance
3.3.1.1 Colour
Pale yellow. It darkens on exposure to light.
3.3.1.2 State/Form
Odourless powder or scales.
3.3.1.3 Description
Melting point 153-157°C
pH of 0.5% solution is 5.9
Freely soluble in alcohol or chloroform,
slightly soluble in ether (1/220) and
insoluble in petroleum ether. Solubility in
water is 1/25.
�
3.3.2 Properties of the locally available formulation(s)
To be completed by each Centre using local
data.
3.4 Other characteristics
3.4.1 Shelf-life of the substance
Shelf-life of the substance is three to five years.
3.4.2 Shelf-life of the locally available formulation(s)
To be completed by each Centre using local data.
3.4.3 Storage conditions
Store in airtight conditions and protect from light.
3.4.4 Bioavailability
To be completed by each Centre using local data.
3.4.5 Specific properties and composition
To be completed by each Centre using local data.
4. USES
4.1 Indications
4.1.1 Indications
Not relevant
4.1.2 Description
Gout
Colchicine is used for acute gout attacks to reduce
pain and inflammation. It may be used on long-term
basis to prevent or reduce the frequency of
attacks.
Familial Mediterranean Fever
Colchicine is used on long-term basis to prevent fever
and recurrent polyserositis. Colchicine is effective
in preventing the amyloidosis in this condition.
Behcet's disease
Colchicine has been showed to be effective in the
treatment of articular, cutaneous and mucosal
symptoms.
�
Other
Colchicine has been used in the treatment of
scleroderma and sarcoidosis.
4.2 Therapeutic dosage
4.2.1 Adults
Acute Gout Attack
Oral
1 or 1.3 mg initial dose, followed by 0.5 to 0.65 mg
every 1 to 2 hours (or 1 to 1.3 mg every 2 hours)
until the pain is relieved or nausea and diarrhoea
appear. The total dose should not exceed 10 mg over 3
days.
A course should not be repeated within three days.
Tolerance is usually 4 to 8 mg.
Parenteral
Intravenous injection. Initial dose is 1 to 2 mg.
The total dose is 4 mg in 24 hours or in an
attack.
Prophylaxis of recurrent gout
Oral
0.5 mg to 0.65 mg once weekly or up to three times
daily, depending on the frequency of prior acute
attacks.
(Reynolds 1993)
4.2.2 Children
No dosage is available for use in young
children (Levy, 1977).
A dose of 0.5 mg daily has been used in children with
familial mediterranean fever from the age of 5 years
of age or under, and 1 mg daily for older children
(Reynolds 1993).
�
4.3 Contraindications
Underlying disease.
Pregnancy: There is a risk of foetal chromosomal damage
(Reynolds, 1989).
5. ROUTES OF ENTRY
5.1 Oral
Oral absorption is the most frequent cause of
intoxication.
5.2 Inhalational
Not relevant.
5.3 Dermal
Not relevant.
5.4 Eye
Not relevant.
5.5 Parenteral
Intoxications after parenteral administration are rare,
(Benoit et al., 1974, Jaeger et al., 1980, Liu et al., 1978),
however, the toxic dose appears to be lower than the oral
toxic dose.
Five fatal outcomes after intravenous colchicine: the daily
dose was 3 to 6 mg and the total dose was 9 to 21 mg over 2
to 8 days.(Jaeger et al., 1980)
A fatal bone marrow aplasia in a 70 year-old man after 10 mg
intravenous colchicine over 5 days (Liu et al., 1978).
5.6 Other
Intoxication with multisystemic reactions after
instillation of 50 mg colchicine into the penile urethra for
treatment of condyloma acuminata (Naidus et al., 1977).
�
6. KINETICS
6.1 Absorption by route of exposure
Oral
Rapidly absorbed from the gastro-intestinal tract. Peak
plasma concentration is reached 0.5 to 2 hours after
ingestion (Wallace & Ertel, 1973).
Half time of absorption is 15 minutes (Galliot, 1979).
Absorption may be modified by pH, gastric contents,
intestinal motility (Wallace et al., 1970).
Colchicine is not totally absorbed. There is an important
hepatic first pass effect.
6.2 Distribution by route of exposure
Protein binding is 10 to 20% (Bennett et al., 1980).
Colchicine distributes in a space larger than that of the
body. The apparent volume of distribution is 2.2 L/kg
(Wallace et al., 1970). In severe renal or liver diseases the
volume of distribution is smaller (1.8 L/kg).
Colchicine accumulates in kidney, liver, spleen, gastro-
intestinal wall and leucocytes and is apparently excluded in
heart, brain, skeletal muscle.
Colchicine crosses the placenta and has also been found in
maternal milk.
6.3 Biological half-life by route of exposure
Parenteral
After a single 2 mg intravenous dose the average plasma half-
life is 20 minutes (Wallace et al., 1970). Plasma half-life
is increased in severe renal disease (40 min) and decreased
in severe hepatic disease (9 min) (Wallace et al., 1970).
Oral
After oral administration plasma concentrations reach a peak
within 0.5 to 2 hours and afterwards decrease rapidly within
2 hours (Wallace & Ertel, 1973). The plasma half-life is 60
minutes (Galliot, 1979). Colchicine may remain in tissues for
as long as 10 days.
�
6.4 Metabolism
Colchicine undergoes some hepatic metabolism.
Colchicine is partially deacetylated in the liver (Naidus et
al., 1977). Large amounts of colchicine and of its
metabolites undergo enterohepatic circulation. This may
explain the occurrence of a second plasma peak concentration
observed 5 to 6 hours after ingestion (Galliot, 1979;
Walaszek et al., 1960).
6.5 Elimination by route of exposure
Colchicine is excreted unchanged (10 to 20 percent) or
as metabolites.
Oral
Kidney
Urinary excretion amount to 16 to 47% of an administered dose
(Heaney et al., 1976). 50 to 70% of colchicine is excreted
unchanged and 30 to 50% as metabolites. 20% of the dose
administered is excreted in urine in the first 24 hours and
27.5% in the first 48 hours. Colchicine is detected in urine
up to 7 to 10 days after ingestion. Urinary excretion is
increased in patients with impaired hepatic function (
Wallace et al., 1970).
Bile
10 to 25% of colchicine is excreted in the bile (Heaney et
al., 1976).
Faeces
Large amounts of the drug are excreted in the faeces.
Breast Milk
Colchicine may be eliminated in breast milk (White & White,
1980).
Intravenous
Faeces
After intravenous administration 10 to 56% is excreted in the
faeces within the first 48 hours (Walaczek et al., 1960).
Breast Milk
Colchicine may be eliminated in breast milk.
�
7. PHARMACOLOGY AND TOXICOLOGY
7.1 Mode of action
7.1.1 Toxicodynamics
Colchicine binds to tubulin and this prevents
its polymerization into microtubules. The binding is
reversible and the half-life of the colchicine-tubulin
complex is 36 hours. Colchicine impairs the different
cellular functions of the microtubule: separation of
chromosome pairs during mitosis (because colchicine
arrests mitosis in metaphase), amoeboid movements,
phagocytosis.
Mitosis blockade accounts for diarrhoea, bone marrow
depression and alopecia. Colchicine may have a direct
toxic effect on muscle, peripheral nervous system and
liver. Inhibition of cellular function does not,
however, account for all the organ failures seen in
severe overdose.
7.1.2 Pharmacodynamics
Gout inflammation is initiated by urate
crystals within tissues. The crystals are ingested by
neutrophils but this leads to the release of enzymes
and the destruction of the cells. Chemotactic factors
are released and attract more neutrophils. Colchicine
may act by preventing phagocytosis, the release of
chemotactic factors and the response of
neutrophils.
Colchicine has other properties such as antipyretic
effects, respiratory depression, vasoconstriction and
hypertension.
7.2 Toxicity
7.2.1 Human data
7.2.1.1 Adults
Oral
Fatalities have been reported after ingestion
of 7 to 12 mg (Stapczynski et al., 1981).
The severity and the mortality rate of the
poisoning is directly related to the dose
ingested (Gaultier & Bismuth, 1978; Bismuth
et al., 1977; Lambert et al., 1981).
�
Dose Symptoms Mortality
absorbed Rate
mg/kg
<0.5 Gastrointestinal symptoms 0%
decrease of coagulation
factors
0.5-0.8 + Bone marrow aplasia 10-50%
+ alopecia
> 0.8 + circulatory failure 100%
Intravenous
Fatal outcomes in five patients who had
received a total dose of 9 to 21 mg over two
to eight days (daily dose 3 to 6 mg) (Jaeger
et al., 1980).
A fatal bone marrow aplasia in a 70-year-old
patient who had received 10 mg of intravenous
colchicine over 5 days (Liu et al.,
1978).
The enhanced toxicity of intravenous
colchicine is probably due to the higher
bioavailability of colchicine after
parenteral administration.
7.2.1.2 Children
Oral
The toxic dose is about 0.1 mg/kg and the
lethal dose is between 0.5 and 0.8 mg/kg
(Besson-Leaud et al., 1977).
�
7.2.2 Relevant animal data
Species Route Effect Dose (mg/kg)
Rat Intravenous LD 50 1.6
Rat Intraperitoneal LD 50 6.1
Rat Subcutaneous LD Lo 4
Mouse Intravenous LD 50 4.13
Mouse Intraperitoneal LD 50 2
Dog Oral LD Lo 0.125
Dog Subcutaneous LD Lo 0.571
Cat Oral LD Lo 0.125
Cat Subcutaneous LD Lo 0.5
Cat Intravenous LD 50 0.25
(RTECS, 1979)
7.2.3 Relevant in vitro data
No data available.
7.3 Carcinogenicity
No data available.
7.4 Teratogenicity
Colchicine is contraindicated in pregnancy as Down's
syndrome and spontaneous abortion have been reported.
Colchicine should be discontinued three months prior to
conception (Drugdex, 1989).
7.5 Mutagenicity
See section 9.4.7.
�
7.6 Interactions
Menta et al. (1987) reported a case of acute cyclosporin
nephrotoxicity induced by colchicine administration.
Colchicine may interfere with cyclosporin pharmacokinetics by
increasing cyclosporin plasma levels either by enhancing
cyclosporin absorption or by reducing its hepatic
metabolism.
7.7 Main adverse effects
Gastrointestinal symptoms are a common complication of
chronic colchicine therapy. Thus the dose of colchicine is
usually limited by gastrointestinal toxicity.
About 80 per cent of the patients treated with colchicine at
a dose of 3 to 4 mg per day develop gastrointestinal
disturbances (Wallace, 1980).
Fatal outcomes have been reported after intravenous
colchicine therapy (see section 7.2.1.1).
The following adverse reactions have been reported during
colchicine treatment (Dukes, 1983):
Gastrointestinal
Vomiting, diarrhoea, abdominal discomfort, paralytic ileus,
malabsorption syndrome with steatorrhoea.
Haematological
Bone marrow depression with agranulocytosis, acute
myelomonocytic leukaemia, multiple myeloma,
thrombocytopenia.
Neurological
Peripheral neuritis, myopathy, rhabdomyolysis (Riggs et al.,
1986).
Dermatological
Allergic reactions are rare urticaria; oedema may be seen.
Alopecia has been reported after chronic treatment.
Reproductive system
A reversible, complete azoospermia has been reported (Merlin
1972).
�
Metabolic
Colchicine is capable of producing a reversible impairment of
vitamin B12 absorption (Webb et al, 1968). Porphyria cutanea
tarda has been reported (Gossweiler, 1985).
8. TOXICOLOGICAL ANALYSES AND BIOMEDICAL INVESTIGATIONS
8.1 Methods
Colchicine may be analysed in biological fluids by
different methods:
Fluorometric method: Fluorescence of organometallic
(Gallium) complexes (Bourdon & Galliot, 1976).
Radioimmunoassay: (Ertel et al., 1979; Scherrman et al.,
1980).
High performance liquid chromatography: (Jarvie et al, 1979,
Caplan et al., 1980; Harzer, 1984; Lhermitte et al.,
1985).
Liquid chromatography (Thompson, 1985).
8.2 Therapeutic and toxic concentration
Colchicine may be measured in biological fluids but
levels are not useful or necessary for the management of
colchicine poisoning.
Serum/Plasma/Blood
Plasma
Plasma levels lower than 20 ng/ml at the 6th hour in severe
intoxications have been reported (Bourdon & Galliot
1976).
In an overdose with 7.5 mg, plasma levels of 21 ng/ml at the
6th hour and below 5 mg/ml at the 24th hour were noted
(Jarvie et al., 1979).
In severe intoxications plasma levels usually range between
20 to 50 ng/ml during the 24 first hours. After the 24th
hour only small amounts of colchicine (< 20 ng/ml) are
detected in plasma (Bismuth et al., 1977; Lambert et al.,
1981; Jaeger et al., 1985).
Post-mortem serum blood levels of 170 and 240 ng/mL (at the
4th and 8th hour) in 2 heroin addicts following intravenous
injection were reported (Harzer, 1984) .
�
The following plasma levels were noted in an overdose with 31
mg orally: 720, 212, 132 and 120 ng/mL at the 20, 125, 305,
605 minutes respectively (Lhermitte et al., 1985).
Blood
Colchicine levels in blood are higher than those in
plasma.
In an overdose with 20 mg colchicine orally a blood level of
250 ng/ml at the second hour was noted (Caplan et al. 1980).
No colchicine could be detected at the 40th hour.
Urine
Colchicine levels in urine range between 200 and 2500 ng/ml
over the first 24 hours (Bismuth et al., 1977; Lambert et al,
1981, Jaeger et al. 1985).
Information was available on urinary excretion in 5 cases.
Concentrations in urine are 10 to 80 fold higher than those
in plasma. Four to 25 per cent of the dose ingested was
excreted in urine over three to ten days. Excretion was
specially high during the first 24 hours following ingestion.
Colchicine is eliminated in urine up to the tenth day (Jaeger
et al., 1985).
Gastric lavage fluid
In four cases, gastric lavage performed 3 to 6 hours post
ingestion removed 7 to 25 per cent of the dose ingested
(Jaeger et al., 1985).
Diarrhoea
In an overdose with 25 mg colchicine orally, 1.4 mg were
eliminated in diarrhoea on the 2nd day (Jaeger et al.,
1985).
9. CLINICAL EFFECTS
9.1 Acute poisoning
9.1.1 Ingestion
Toxic manifestations appear after a delay of 2
to 12 hours following ingestion. The delay may be
increased if other drugs decreasing gastro-intestinal
motility have also been ingested (phenobarbitone,
psychotropic drugs, opium derivatives). Symptomatology
progresses in three stages and may include:
�
Stage I (Day 1 to 3) Gastrointestinal and circulatory
phase:
Severe gastrointestinal irritation: Nausea, vomiting,
abdominal cramps, severe diarrhoea.
Dehydration, hypovolemia, shock. Cardiogenic shock
may occur and may result in death within the first 72
hours.
Hypoventilation, acute respiratory distress
syndrome.
Stage II (Day 3 to 10) Bone marrow aplasia phase:
Bone marrow aplasia with agranulocytosis.
Coagulation disorders with diffuse haemorrhages.
Rhabdomyolyis.
Polyneuritis, myopathy.
Acute renal failure.
Infectious complications.
Stage III: (After ten days) Recovery phase:
Alopecia
(Gaultier & Bismuth, 1978, Ellenhorn & Barceloux,
1988, Stapczynski et al., 1981).
9.1.2 Inhalation
Not relevant.
9.1.3 Skin exposure
Not relevant.
9.1.4 Eye contact
Not relevant.
�
9.1.5 Parenteral exposure
The clinical course after intravenous injection
is similar to that observed after ingestion. The time
to onset of symptoms depends on the dose and rate of
injection but gastro-intestinal symptoms usually
appear two to six days after the beginning of
colchicine therapy.
Two cases of lethal overdose after a single
intravenous injection of 30 mg colchicine were
reported; gastro-intestinal symptoms appeared 2 hours
after injection (Michaux et al., 1972) .
9.1.6 Other
An intoxication with multisystemic reactions
after instillation of 50 mg colchicine into the penile
urethra for treatment of condyloma acuminata has been
reported. (Naidus et al., 1977)
9.2 Chronic poisoning
9.2.1 Ingestion
Chronic administration of colchicine may induce
similar toxicity to that seen in acute poisoning:
gastro-intestinal symptoms (vomiting, diarrhoea),
agranulocytosis, aplastic anaemia, myopathy (Gilman et
al., 1985)
9.2.2 Inhalation
No data available.
9.2.3 Skin contact
No data available.
9.2.4 Eye contact
No data available.
9.2.5 Parenteral exposure
Similar to acute poisoning.
9.2.6 Other
No data available.
�
9.3 Course, prognosis, cause of death
Course
See section 9.1.1.
Prognosis
Prognosis is related to the dose ingested (see section
7.2.1).
Occurrence of cardiogenic shock indicates a poor prognosis
(Sauder et al., 1983).
If the patient has recovered from aplasia and has not
developed acute respiratory distress syndrome or systemic
infectious complications, prognosis is usually good.
Cause of death
At the early stage (day 1 to 3) of the intoxication, death is
due to cardiogenic shock and/or acute respiratory distress
syndrome.
Death due to haemorrhagic or infectious complications may
occur at the stage of bone marrow aplasia (day 3 to
10).
9.4 Systematic description of clinical effects
9.4.1 Cardiovascular
Cardiovascular shock is always present in
severe intoxications. Most deaths result from shock
within the first 72 hours.
Hypotension is usually the result of hypovolaemia due
to gastrointestinal fluid loss. Hypovolaemia with
decreased central venous pressure is initially always
present but some patients may develop cardiogenic
shock. (Sauder et al., 1983; Bismuth & Sebag
1981).
Haemodynamic studies showed two different profiles:
Patients with a hyperkinetic state (increased cardiac
index and decreased systemic vascular resistances);
patients with cardiogenic shock (decreased cardiac
index and increased systemic vascular resistances)
(Sauder et al., 1983). Occurrence of cardiogenic shock
indicates a poor prognosis. Septic shock may occur
during the phase of aplasia.
�
9.4.2 Respiratory
Acute respiratory failure is usually
concomitant of circulatory failure, although Murray et
al 1983, reported a case with ascending paralysis
occurring more than 4 hours post-exposure.
Acute respiratory distress syndrome due to diffuse
interstitial and alveolar oedema has been reported in
severe cases (Hill et al., 1975; Corbin et al., 1989;
Maurizi et al., 1986; Davies et al., 1988; Hobson &
Rankin, 1986).
9.4.3 Neurological
9.4.3.1 Central nervous system (CNS)
In severe cases hypotension and/or
hypoxaemia can lead to confusion, agitation,
and mental depression. Coma and seizures are
observed. Profound coma may be due to
cerebral complications such as
haemorrhages.
9.4.3.2 Peripheral nervous system
Peripheral neuritis, neuromyopathy
and myopathy have been reported (Carr, 1965;
Favarel-Garrigues et al., 1975; Kuncl, 1987;
Bismuth et al., 1977; Mouren et al., 1969;
Kontos, 1962). Ascending paralysis may be
responsible for respiratory failure (Carr,
1965). Polyneuritis usually recovers within
one month (Bismuth et al., 1977).
9.4.3.3 Autonomic nervous system
None described.
9.4.3.4 Skeletal and smooth muscle
Rhabdomyolysis may occur with an
increase in muscle enzymes and myoglobinuria
(Murray et al., 1983; Kontos et al., 1962;
Letellier et al., 1979). A case of
rhabdomyolysis was reported in a 58-year-old
patient treated with 3 mg colchicine daily
over 6 days (Letellier et al., 1979). The
patient developed proximal scapular weakness
with muscle oedema and increase in muscle
enzymes.
�
9.4.4 Gastrointestinal
Acute
Gastrointestinal symptoms develop after a delay of 2
to 12 hours following ingestion and include nausea,
vomiting, abdominal pain and severe diarrhoea.
Usually diarrhoea lasts for 48 hours and may induce
hypovolaemia and electrolyte disturbances.
Gastrointestinal symptoms also occur after colchicine
overdose by the intravenous route. Paralytic ileus
may develop (Heaney et al., 1976).
Gastrointestinal disturbances may be lacking or
decreased if drugs decreasing gastrointestinal
motility (atropine, phenobarbitone, opium tincture)
have also been ingested.
Chronic
Gastrointestinal symptoms are a common feature during
colchicine treatment. Paralytic ileus has been
reported after intravenous colchicine
treatment.
9.4.5 Hepatic
Colchicine may exert direct hepatic toxicity.
Hepatomegaly has been reported. Hepatic damage may
occur in severe poisoning and includes cytolysis and
hepatocellular insufficiency, increase in glutamic
pyruvic transaminase (SGOT) (alanine amino
transferase, ALT) and glutamic oxaloacetic
transaminase (SGOT) (aspartate amino transferase, AST)
and in alkaline phosphatase, a decrease in coagulation
factors. Histological examination has shown necrosis
and steatosis of hepatocytes.
9.4.6 Urinary
9.4.6.1 Renal
No direct nephrotoxic effect has
been reported. Functional renal insufficiency
is usually observed and is secondary to fluid
and electrolytes losses or hypovolaemia.
Acute renal failure may occur following
cardio-vascular or septic shock.
�
9.4.6.2 Other
Proteinuria and haematuria have been
reported.
9.4.7 Endocrine and reproductive systems
Endocrine
Transient diabetes mellitus has been reported by
Hillemand et al. 1977 in a 58-year-old woman after an
overdose with 25 mg.
Inappropriate antidiuretic syndrome has been reported
(Gauthier et al., 1975).
Reproductive
Acute
A case of colchicine poisoning (40 mg) has been
reported in a 18 year old pregnant woman (Lambert et
al., 1981). The patient developed severe poisoning
with coagulopathy, ARDS and abortion on day 7
following ingestion. The patient recovered.
Chronic
A reversible complete azoospermia has been reported in
a 36- year-old man treated with colchicine for gout
(Merlin, 1972). 2 cases of Down's syndrome babies
have been reported (Ehrenfeld et al., 1987). The
obstetric histories of 36 women with familial
Mediterranean fever on long-term colchicine treatment
between 3 and 12 years have been reported (Ehrenfeld
et al., 1987). Seven of 28 pregnancies ended in
miscarriage. 13 women had periods of infertility.
All 16 infants born to mothers who had taken
colchicine during pregnancy were healthy. The authors
do not advise discontinuation of colchicine before
planned pregnancy but recommend amniocentesis for
karyotyping and reassurance.
9.4.8 Dermatological
Acute
Alopecia begins at about the 12th day and is complete
by 3 weeks after ingestion. Hair regrowth begins
after the first month.
�
Cutaneous and subcutaneous haemorrhages are frequent
in severe poisoning. They are due to coagulation
disturbances and may be induced by venous or arterial
punctures.
9.4.9 Eye, ear, nose, throat: local effects
Eye
Subconjunctival haemorrhage may occur.
Ear
Definitive unilateral deafness due to an inner ear
haemorrhage has been observed (personal
experience).
Nose
Nasal haemorrhages may occur especially after local
trauma due to insertion of tracheal or gastric
tubes.
Throat
Stomatitis may also occur (Wallace, 1974; Lambert et
al., 1981).
9.4.10 Haematological
At toxic doses, colchicine induces marked bone
marrow depression.
Leucocytes
At the initial stage a peripheral leucocytosis occurs
frequently. a leucopenia with agranulocytosis begins
at the third day and reaches a maximum at day 5 to 7.
WBC return to normal values at about the 10 to 12th
day.
Erythrocytes
Anaemia is frequent in severe cases and may be due to
different factors:
Hypoplastic anaemia due to bone marrow suppression may
be observed but is rarely important.
Haemolytic anaemia with Heinz body has been rarely
reported (Heaney et al., 1976).
�
Acute intravascular haemolysis with haemoglobinemia
and haemoglobinuria has been observed in 6 severe
cases (Lambert et al., 1981)
Severe anaemia is mostly secondary to multiple diffuse
haemorrhages.
Bleeding diatheses and coagulopathy
A tendency toward bleeding is always present in severe
cases. It appears two to three days following
ingestion and may last for eight to ten days.
Usually the earliest indication of coagulopathy is
persistent bleeding from venous or arterial puncture
sites and subcutaneous haemorrhages. Other types of
bleeding include epistaxis, gingival, conjunctival and
gastrointestinal haemorrhages. Bleeding may be due to
thrombocytopenia or a consumptive coagulopathy.
A consumptive coagulopathy with prolongation of
coagulation time, hypoprothrombinaemia, a decrease in
fibrinogen, elevated fibrin degradation products and
thrombocytopenia is observed in severe intoxication
(Bismuth et al., 1977; Lambert et al., 1981; Crabie et
al.,1970)
9.4.11 Immunological
No data available.
9.4.12 Metabolic
9.4.12.1 Acid-base disturbances
Metabolic acidosis due to diarrhoea
and/or shock may be seen.
9.4.12.2 Fluid and electrolyte disturbances
The gastro-intestinal syndrome
often results in marked dehydration and
hypovolaemia with haemoconcentration and
functional renal failure.
Hypokalaemia due to gastrointestinal losses
is also frequent at the initial stage.
�
Hypocalcaemia may be seen and can persist for
several days. Frayha et al. (1984) reported,
in a 20-year-old girl who had ingested 20 mg,
convulsions and paralytic ileus which were
related to a hypocalcaemia (1.25 mmol/L).
Hypocalcaemia may be due to a direct toxic
effect of colchicine (Heath et al.,
1972).
9.4.12.3 Others
Hyperglycaemia
A 58-year-old woman who ingested 25 mg and
developed transient diabetes mellitus has
been reported (Hillemand et al., 1977).
Hyperlipaemia
A transient hyperlipaemia has been reported
(Hillemand et al. 1977).
Hyperuricaemia
A transient hyperuricaemia has also been
noted (Hillemand et al. 1977).
Hyperthermia-fever
Occurrence of fever may be relate to an
infectious complication, especially during
the stage of aplasia.
9.4.13 Allergic reactions
No data available.
9.4.14 Other clinical effects
No data available.
9.4.15 Special risks
Pregnancy
Two cases of Down's syndrome babies have been
reported. The obstetric histories of 36 women with
familial Mediterranean fever on long-term colchicine
treatment between 3 and 12 years have been reported
(Ehrenfeld et al. 1987). Seven of 28 pregnancies
ended in miscarriage. Thirteen women had periods of
infertility. All 16 infants born to mothers who had
�
taken colchicine during pregnancy were healthy. The
authors do not advise discontinuation of colchicine
before planned pregnancy but recommend amniocentesis
for karyotyping and reassurance.
Breast-feeding
As colchicine is eliminated in the breast milk breast-
feeding should be avoided.
9.5 Other
No data available.
9.6 Summary
Not relevant
10. MANAGEMENT
10.1 General principles
Patients with colchicine overdose should always be
admitted in an intensive care unit. Treatment depends on the
dose ingested, the symptomatology and the delay following
ingestion. It includes gastric lavage, early forced
diuresis, and supportive treatment with correction of the
shock, artificial ventilation, treatment and prevention of
haemorrhagic and infectious complications. Vital signs (ECG,
blood pressure, central venous pressure, respiration) should
be monitored. Be careful about venous and arterial punctures
if there is a severe coagulopathy.
10.2 Relevant laboratory analyses
10.2.1 Sample collection
Blood samples for colchicine should be drawn
in plastic tubes with heparin. Colchicine may be
analysed in whole blood or plasma. Biological samples
(blood, plasma, urine...) should be stored in airtight
conditions and protected from light. Concentrations in
whole blood are markedly higher than those in plasma.
Concentration in urine are 10 to 80 fold higher than
those in plasma.
�
10.2.2 Biomedical analysis
A biochemical profile with glucose, BUN,
electrolytes, creatinine, blood cells, coagulation
parameters, enzymes, and blood gases should be
obtained on admission and repeated every 12 hours.
Samples for bacteriological analysis should be
obtained at the stage of aplasia or when fever
occurs.
10.2.3 Toxicological analysis
Colchicine analysis in biological fluids is
not necessary or useful for the management of the
poisoning.
10.2.4 Other investigations
No other specific investigations are
required.
10.3 Life supportive procedures and symptomatic/specific treatment
Observation and monitoring
Monitor systematically vital signs, ECG, blood pressure and
central venous pressure. Repeated monitoring of central
venous pressure is essential to avoid circulatory overload
during plasma expander infusion.
Insert a venous catheter for rehydration and drug
injection.
If shock is present, insertion of a pulmonary artery (Swan-
Ganz) catheter for monitoring of haemodynamic parameters may
be useful for guiding the treatment.
The patient remains at risk until at least 48 hours after
exposure.
Diarrhoea
Diarrhoea should not be treated because some colchicine is
eliminated in faeces.
Dehydration - Electrolyte disturbances - Acidosis
Give intravenous fluids and electrolytes according to
clinical and biological status. If metabolic acidosis is
present give intravenous bicarbonate. Monitor potassium
levels and blood gases. Maintain adequate urinary output
(>100 ml/hr).
�
Hypotension, shock
Hypotension should be anticipated and treated with adequate
fluid replacement and vasoactive drugs. Monitor blood
pressure. Early institution of hemodynamic monitoring is
very helpful for adequate treatment of shock.
Hypotension and shock are due primarily to hypovolaemia.
Cardiogenic shock may occur.
Plasma expanders
Infuse plasma expander solutions (e.g. modified gelatine
fluids) under control of haemodynamic parameters e.g. central
venous pressure, pulmonary arterial pressure. Very large
amounts of plasma expanders may be necessary: 3 to 4 litres
over 24 hours (personal observation).
Inotropic and vasoconstrictor drugs
If the patient is unresponsive to these measures administer
inotropic and vasoconstrictor drugs e.g. dopamine or
dobutamine in doses sufficient to cause vasoconstriction (10
to 20 mcg/kg/min).
Vasodilators
Vasodilators e.g. glyceryl trinitrate may be useful in the
case of cardiogenic shock with increased systemic arterial
resistance (personal observation).
Respiratory disturbances
Respiratory depression or ARDS should be treated by
artificial ventilation. The early institution of mechanical
ventilation is indicated in patients with severe intoxication
and shock.
Bone Marrow Depression
Isolate the patient if there is evidence of bone marrow
depression. Infusion of white blood cell units is usually not
necessary because aplasia is transient. However, it may be
useful in patients who develop concomitant infection
(Gauthier & Bismuth, 1978).
Coagulation Disorders
Prevent haemorrhagic complications due to local trauma: avoid
insertion of endotracheal tube by the nasal route, avoid
femoral arterial puncture.
�
Coagulation disorders require specific treatment only if
haemorrhages develop. According to biological parameters,
treatment may include infusion of fresh-frozen plasma,
platelet units, fibrinogen and coagulation factors
(PPSB).
Prevention of Infectious Complications
In severe cases with shock and/or aplasia a prophylactic
antibiotic treatment should be given. Prophylactic
antibiotherapy may be directed towards gram positive (e.g.
staphylococcal) and negative bacteria and also anaerobic
bacteria. Preventative treatment for fungal infections should
also be given because fungal septicaemia may develop
(personal observation).
10.4 Decontamination
Gastric Lavage
Early gastric lavage is indicated because it may remove 7 to
25 per cent of the dose ingested if it is performed within 6
hours of ingestion (Jaeger et al., 1985).
Emesis
Emesis may be useful in recent ingestion if there are no
contraindications.
Oral Activated Charcoal
The efficacy of oral activated charcoal has not been
established. However, because colchicine undergoes
enterohepatic circulation, oral activated charcoal may be
indicated: one dose at the end of the gastric lavage and
repeated every 4 to 6 hours.
Cathartics
The usefulness of cathartics has not been established and is
not recommended.
�
10.5 Elimination
Forced Diuresis
Toxicokinetic studies (Jaeger et al., 1985) indicate that
significant amounts of colchicine are eliminated in urine,
especially during the first 24 hours following ingestion.
Thus early forced diuresis should be instituted after
correction of dehydration and/or shock (Jaeger et al., 1985).
Continue forced diuresis until the third or fourth day
provided there are no contraindications.
Haemoperfusion, Haemodialysis
No data about haemoperfusion or haemodialysis clearances have
been reported. However, the low colchicine plasma
concentrations reported in acute poisonings and the large
volume of distribution indicate that haemoperfusion or
haemodialysis is not useful.
10.6 Antidote treatment
10.6.1 Adults
Currently no antidote for colchicine is
available.
10.6.2 Children
No antidote available.
10.7 Management discussion
Gastrointestinal symptoms may be overshadowed if
psychotropic drugs or drugs decreasing gastrointestinal
motility have also been ingested.
Institute prophylactic antibiotic therapy.
11. ILLUSTRATIVE CASES
11.1 Case reports from literature
Case report 1
In 69 reported cases of colchicine poisoning (Bismuth et al.,
1977) thirty eight patients (dose ingested <0.5 mg/kg)
developed gastro- intestinal symptoms and coagulation
disturbances; all survived. Twenty patients (dose ingested
0.5 to 0.8 mg/kg) developed bone marrow aplasia: mortality
was 10 per cent. Eleven patients (dose ingested > 0.8 mg/kg)
died within 72 hours from cardiovascular shock.
�
Case report 2
In another reported 22 cases (Lambert et al., 1981),
according to the doses ingested (DI), mortality rates were:
100% for DI > 1 mg/kg; 50% for DI = 0.5 to 0.9 mg/kg; 10%
for DI < 0.5 mg/kg. Clinical features included:
gastrointestinal symptoms (22 cases), leucopenia, aplasia (11
cases), disseminated intravascular coagulation (9 cases),
shock (9 cases), acute respiratory distress syndrome (8
cases), polyneuropathy (4 cases).
Case report 3
Two cases were reported (Gaultier et al., 1969) who developed
inappropriate antidiuresis following ingestion of about 40
mg. Both patients recovered.
Case report 4
A 18-year-old woman developed an acute respiratory distress
syndrome (ARDS) following ingestion of 150 mg. The patient
died at the 42nd hour (Hill et al., 1975).
Case report 5
An acute respiratory distress syndrome was reported in a 17-
year-old woman who had ingested 0.37 mg/kg (Corbin et al.,
1989). Pulmonary capillary wedge pressure was 7 mm Hg. The
patient died (72nd hour), despite mechanical ventilation
(PEEP), from shock any hypoxaemia.
Case report 6
Two cases with ARDS have been reported (Maurizi et al.,
1986). A 25- year-old woman who had ingested 80 mg died on
the 7th day from septic shock with acute renal failure; a 21
year old man who had ingested 15 to 20 mg also developed
aplasia and recovered.
Case report 7
A fatal overdose in a 15-year-old boy who had ingested 18 mg
colchicine and developed cardiovascular shock, ARDS,
metabolic acidosis, hypocalcaemia, hypokalaemia,
hypophosphotaemia, bone marrow suppression and coagulopathy
has been reported (Hobson & Rankin, 1986).
�
Case report 8
Report of an overdose with about 24 mg in a 15 year old girl
(Murray et al., 1983). The clinical picture showed:
* myocardial injury with cardiogenic shock
* ventilatory insufficiency with ARDS
* rhabdomyolysis
* metabolic acidosis
* agranulocytosis
* coagulopathy
* alopecia.
The patient recovered without sequelae.
11.2 Internally extracted data on cases
Jaeger et al. (1980) reported five fatal outcomes in
five patients after intravenous colchicine treatment for
gout. The total dose ranged between 9 to 21 mg administered
over two to eight days. During the treatment the patients
developed gastro-intestinal symptoms and thereafter (on about
the 7th day) agranulocytosis, thrombocytopenia and shock with
acute renal failure. Death occurred between the 7th and 15th
day after beginning of the treatment.
Sauder et al. (1983) performed haemodynamic studies in eight
cases of colchicine poisoning. The doses ingested ranged
between 9 and 160 mg. Haemodynamic studies performed between
the 6th and 72nd hour following ingestion showed:
Hypovolemia in all cases,
A hyperkinetic state with increased cardiac index and
decreased systemic vascular resistance in the 4 patients who
recovered.
Cardiogenic shock with decreased cardiac index and increased
systemic vascular resistances in the 4 patients who died.
An initial decrease of cardiac performance is an index of
severity and poor prognosis.
Jaeger et al, (1985) performed a toxicokinetic study in 5
cases (dose ingested 19 to 60 mg). Plasma concentrations
ranged between 20 and 54 ng/mL during the 24 first hours.
Gastric lavage removed 7.1 to 25% of the dose ingested. In
one case 1.4 mg colchicine was excreted in diarrhoea on the
second day. Four to 25 per cent of the dose ingested was
eliminated in urine over 3 to 10 days.
�
Urinary colchicine excretion was especially high during the
first day following ingestion (2 to 10 per cent of the dose
ingested). Colchicine levels in urine were 10 to 80 times
higher than those in plasma. This study emphasizes the
usefulness of early gastric lavage, of early diuresis and of
colchicine elimination in diarrhoea.
11.3 Internal cases
To be completed by each Centre using local data.
12. ADDITIONAL INFORMATION
12.1 Availability of antidotes
Not relevant
12.2 Specific preventive measures
Not relevant
12.3 Other
Unknown
13. REFERENCES
Bennet WF & Glenn KC (1980) Hypersensitivity of platelets to
thrombin: formation of stable thrombin-receptor complexes and the
role of shape-change. Cell 22(2): 621-627.
Benoit JP, Leveque M, Carli PM (1974) Aplasie médullaire mortelle
au cours d'un bref traitement de colchicine. Rev Méd Dijon, 9:
485-488.
Besson-Leaud M (1977) De quelques intoxications chez l'enfant.
Ann Pédiatr, 24: 363-371.
Bismuth C, Gaultier M, & Conso F (1977). Aplasie médullaire aprés
intoxication aiguë à la colchicine. Nouv Presse Med, 6: 1625-
1629.
Bismuth C & Sebag C (1981) Choc cardiogénique lors d'une
intoxication aigue par la colchicine. Nouv Presse Méd, 10:
1073.
Bourdon R & Galliot M (1976) Dosage de la colchicine dans les
liquides biologiques. Ann Biol Clin, 34: 393-401.
Budavari S ed. (1989) The Merck index, an encyclopedia of
chemicals, drugs, and biologicals, 11th ed. Rahway, New Jersey,
Merck and Co Inc, pp 386- 387.
�
Caplan YH, Orloff KG & Thompson BC (1980) A fatal overdose with
colchicine. J Anal Toxicol 4(3): 153-5
Carr AA (1965) Colchicine toxicity. Arch Intern Med, 115: 29-
33.
Corbin JC, Duval G, Plane M, & Chuet C (1989) Syndrome de
détresse respiratoire aigue de l'adulte au cours d'une
intoxication par la colchicine. Rean Soins Intens Med Urg, 2: 187-
189.
Crabie P., Pollet J., Pebay-Peyroula F. (1970). Etude de l'
hémostase au cours des intoxications aiguës par la colchicine.
J.E.T., 3: 374-385.
Davies H.O., Hyland R.H., Morgan C.D. (1988) Massive overdose of
colchicine. Can Med Assoc J, 138: 335-336.
Drugdex (1989) February issue.
Dukes MNG (1983) Side effects of Drugs. Annual 7, Excerpta
Medica.
Ehrenfeld M, Levy M, Margalioth EJ, Eliakim M (1986) The effects
of long- term colchicine therapy on male fertility in patients
with familial mediterranean fever. Andrologia, 18: 420-426.
Ehrenfeld M, Brzezinski A, Levy M, & Eliakim M (1987) Fertility
and obstetric history in patients with familial mediterranean
fever on long-term colchicine therapy. Br J Obstet Gynaecol, 94:
1186-1191.
Ellenhorn M.J., Barceloux D.G. (1988) Medical Toxicology -
Diagnosis and Treatment of Human poisoning, 1st ed. New York,
Elsevier.
Ertel NH, Mittler JC, Akgun S, & Wallace SL (1976)
Radioimmunoassay for colchicine in plasma and urine. Science, 193:
233-234.
Favarel-Garrigues JC, Bony D, & Poisot D (1975) Intoxications
aiguës par la colchicine. Concours Med, 97: 5183-5197.
Fleeger CA ed. (1993) USAN 1994: USAN and the USP dictionary of
drug names. Rockville, MD, United States Pharmacopeial Convention,
Inc., p 171.
Frayha RA, Tabbara Z, & Berbir N (1984) Acute colchicine poisoning
presenting asymptomatic hypocalcaemia. Br J Rheumatol, 23: 292-
295.
�
Galliot M (1979) Complexes metaliques dérivés de la colchicine.
Application au dosage biologique dans le médicaments et les
liquides biologiques. Thése, Paris, p 134.
Gaultier M, Kanfer A, Bismuth C, Crabie P, & Frejaville JP (1969)
Données actuelles sur l' intoxication aiguë par la colchicine. A
propos de 23 observations. Ann Med Interne, 120: 605-618.
Gaultier M, Bismuth C, Autret A, & Pillon M (1975) Antidiurése
inapproprieée après intoxication aigue par la colchicine. Nouv
Presse Méd, 4: 3132-3134.
Gaultier M & Bismuth C (1978) Intoxication aiguë par la
colchicine. Revue du Praticien 28(57): 4545-4554.
Goodman LS, Gilman AG, Rall TW, & Murad F eds. (1985) Goodman and
Gilman's Pharmacological Basis of Therapeutics. 7th Ed. New York,
Macmillan.
Gooneratne BWM (1966) Massive generalized alopecia after poisoning
by Gloriosa superba. Br Med J, 1(5494): 1023-1024.
Gossweiler B (1985) Kolchizinvergiftung Schweiz. Rundschau Med,
74: 1443-1449.
Heaney D, Derghazarian CB, Pineo GF (1976) Massive colchicine
overdose. A report on the toxicity. Am J Med Sci, 271: 233-
238.
Harzer K (1984)Tödliche Vergiftugh mit Colchicin. Z Rechtsmed, 93:
181-185.
Heath DA, Palmer JS, Aurbach D (1972) The hypocalcaemic action of
colchicine. Endocrinology, 90(6): 1589-1593.
Hill RN, Spragg RG, Wedel MK, & Moser KM (1975) Adult respiratory
distress syndrome associated with colchicine intoxication.
Hillemand B, Joly JP, Membrey-Maheo E, & Ouvry D (1977) Diabète
transitoire avec hyperlipidémie et hyperuricémie régressives au
cours d' une intoxication aiguë par la colchicine. Relation d'un
cas. Ann Med Interne, 128: 379-385.
Hobson CH & Rankin AP (1986) A fatal colchicine overdose. Anaesth
Intensive Care, 14(4): 453-455.
Jaeger A, Simon CH, Tempe JD, Mantz JM, Bismuth C, Conso F,
Mathiot C, Baumelou A, Bavoux F, Jouanjean X, & Toulet R (1980)
Accidents thérapeutiques mortels après colchicine intraveineuse.
Nouvelle Presse Med, 9: 1587.
�
Jaeger A, Galliot M, Zaehringer M, Sauder PH, Bourdon R,
Kopferschmitt J, Jaegle ML, & Mantz JM (1985) Elimination
digestive et excrétion rénale de la colchicine au cours des
intoxications aiguës. In: "Pharmacocinétique appliquée en
réanimation" Monographie de la Société de Réanimation de Langue
Française, Ed Expansion Scientifique, pp 257-262.
Jarvie D, Park J, & Stewart MJ (1979) Estimation of colchicine in
a poisoned patient by using high performance liquid
chromatography. Clin Toxicol, 14: 375-381.
Kontos HA (1962) Myopathy associated with chronic colchicine
toxicity. N Engl J Med, 266: 38.
Kuncl RW (1987) Colchicine myopathy and neuropathy. New Engl J
Med, 316: 1562-1568.
Lambert H, Laprevote-Heully MC, Manel J, Gilgenkrantz S, & Larcan
A (1981) Les intoxications aiguës par la colchicine. A propos de
22 observations. Ann Med Nancy et de l'Est, 20: 891-900.
Letellier PH., Langeard M., Agullo M. (1979). Rhabdomyolyse
secondaire à une série d'injections intra-veineuses de colchicine.
J. Med. Caen, 14, 4: 157-159.
Levy M & Eliakim M (1977) Long-term prophylaxis in familial
Mediterranean fever. Br Med J, 2(6090): 808
Lhermitte M, Bernier JL, Mathieu D, Mathieu-Nolf M, Erb F, &
Roussel P (1985) Colchicine quantitation by high-performance
liquid chromatography in human plasma and urine. J Chromatogr,
342:416-423.
Liu YK, Hymowitz R, & Carroll MG (1978) Marrow aplasia induced by
colchicine. Arthritis, Rheumatism, 21: 731-735.
Maurizi M, Delorme N, Laprevote-Heully MC, Lambert H, & Larcan A
(1986) Syndrome de détresse respiratorie aiguë de l' adulte au
cours des intoxications par la colchicine. Ann Fr Anesth Reanim,
5: 530-532.
Menta R, Rossi E, Guariglia A, David S, & Cambi V (1987)
Reversible acute cyclosporin nephrotoxicity induced by colchicine
administration. Nephrol Dial Transplant 2:380-382.
Merlin HE (1972) Azoospermia caused by colchicine. A case report.
Fertil and Steril. 23: 180-191.
Michaux P, Curtes JP, & Lejeune (1972) Responsabilité médicale et
pharmaceutique à la suite de l' administration intraveineuse d'
une préparation contenant de la colchicine. Méd Lég Dommage Corp,
5: 248-250.
�
Mouren P, Tatossian A, Poiso Y, Giudicelli S, Jouglard J, Dufour
H, & Poyen D (1969) L' intoxication aiguë par la colchicine.
Presse Med, 77, 14: 505-508.
Murray SS, Kramlinger KG, & McMichan JC (1983) Acute toxicity
after excessive ingestion of colchicine. Mayo Clin Proc, 58: 528-
532.
Nadius RM, Rodvien R, & Mielke CH Jr. (1977 Colchicine toxicity -
a multisystem disease. Arch Intern Med, 137: 394-396.
Nagaratnam N, De Silva DP, & De Silva N (1973) Colchicine
poisoning following ingestion of Gloriosa superba tubers. Trop
Geogr Med, 25: 15-17.
Registry OF Toxic Effects OF Chemical Substances (1979) Volume
One. US Department of Health and Human Services. Ed. Richard J.
Lewis Sr. and Rodger L. Tatken.
Reynolds JEF ed.(1989) Martindale, the extra pharmacopoeia, 29th
ed. London, The Pharmaceutical Press. pp 438-439.
Reynolds JEF ed. (1993) Martindale, the extra pharmacopoeia, 30th
ed. London, The Pharmaceutical Press. pp 335-337.
Riggs JE, Schochet SS, Gutmann L, Crosby TW, & Dibartolomeo AG
(1986) Chronic human colchicine neuropathy and myopathy. Arch
Neurol, 43: 521-523.
Sauder PH, Kopferschmitt J, Jaeger A, & Mantz JM (1983)
Haemodynamic studies in eight cases of acute colchicine poisoning.
Human Toxicol, 2: 169-173.
Scherrmann JM, Boudet L, Pontikis R, Nguyen HN, & Fournier E
(1980) A sensitive radioimmunoassay for colchicine. J Pharm
Pharmacol 32(11): 800-802.
Stapczynski JS, Rothstin RJ, Gaye WA (1981) Colchicine overdose:
report of two cases and review of the literature. Ann Emerg Med,
10: 364.
Thompson RD (1985) Liquid chromatographic determination of
colchicine in pharmaceuticals: collaborative study. J Assoc Off
Anal Chem, 68: 1051-1055.
Walaszek EJ, Kocsis JJ, Leroy GV, & Geiling EMK (1960) Studies on
the excretion of radioactive colchicine. Arch Int Pharmacodyn
Ther, 125: 371-382.
Wallace SL & Ertel NH (1970) Occupancy approach to colchicine
dosage. Lancet, 2: 1250-1251.
�
Wallace SL, Omokoku B & Ertel NH (1970) Colchicine plasma levels.
Am J Med, 48: 443-448.
Wallace SL (1974) Colchicine. Senin Arthritis Rheum 3(4): 369-
81
Wallace SL (1980) Colchicine. In: WN Kelley, Ed. Harris, S.
Ruddy, CB. Sledge. Textbook of Rheumatology, Philadelphia, W.B.
Saunders Company, 878-884.
Webb DI (1968) Mechanism of vitamin B12 malabsorption in patients
receiving colchicine. N Engl J Med, 279: 845.
White GJH & White MK (1980) Breast feeding and drugs in human
milk. Vet Human Toxicol, 22: 1-43.
WHO (1992) Anatomical Therapeutic Chemical (ATC) classification
index. Oslo, WHO Collaborating Centre for Drug Statistics
Methodology, p 73.
14. AUTHOR(S), REVIEWER(S), DATE(S) (INCLUDING UPDATES), COMPLETE
ADDRESS(ES)
Authors A. Jaeger, F. Flesch, Ph. Sauder, J Kopferschmitt
Poison Control Center
Strasbourg
France
Tel: 33-88161144
Fax: 33-88161930
Date 28 March 1989
Peer Review London, United Kingdom, March 1990
Berlin, Germany, October 1995
 Molecular formula
Molecular formula
 Molecular formula
Molecular formula