
CYCLOXYDIM
First draft prepared by M. Watson
Ministry of Agriculture, Fisheries and Food
Harpenden, Hertfordshire, United Kingdom
EXPLANATION
Cycloxydim is a systemic cyclohexanedione herbicide used for
the control of grass weeds in many agricultural and horticultural
broad-leaved crops. Cycloxydim was reviewed for the first time by
the present Meeting.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOLOGICAL DATA
Biochemical aspects
Absorption, distribution, and excretion
Rats
Radiolabelled (4,6-14C) cycloxydim was administered as both
acid and sodium salt in toxicokinetic studies. Five Sprague-Dawley
rats per sex were given single oral doses via stomach tube of 10 or
300 mg/kg bw in PEG 400 for the acid and similar groups were given
10 mg/kg bw of the sodium salt as an aqueous solution. In order to
investigate enterohepatic circulation these experiments were
repeated using groups of 3 males and 3 females in bile duct
cannulated rats. The effects of repeated dosing were investigated in
groups of 5 rats/sex given single radiolabelled doses of 10 mg/kg
bw/days acid after 14 previous daily doses of unlabelled material at
10 mg/kg bw/day and in groups of 3 rats of each sex given 7 daily
doses of 10 or 300 mg/kg bw/day radiolabelled acid. Furthermore, 5
rats per sex were given a single intravenous dose of 10 mg/kg bw
radiolabelled sodium salt (Hawkins et al., 1986).
Both the acid and sodium salt were well absorbed and almost
completely excreted within 5 days after dosing. Elimination
proceeded predominantly via the urine with 74 to 86% of the applied
radioactivity being excreted within 5 days. Faeces contained
approximately 12 to 25% of the applied radiolabel. No volatile
radiolabel was detected in expired air and less than 1% of the
administered dose was retained in the body. In rats with cannulated
bile ducts, 55 to 66% of the dose was excreted into the bile within
48 h regardless of the dose level or type of formulation. In the
same time period, renal excretion amounted to 23 to 37%, and faecal
excretion to < 3%. Therefore, enterohepatic recirculation occurred
in rats and almost all radioactivity excreted in faeces of intact
rats would have been excreted via the bile. It may also be concluded
that both excretion and bioavailability of the two types of
formulation are comparable. The sodium salt seemed to be more
rapidly absorbed after oral administration, but an influence of the
different type of carrier cannot be excluded.
After oral administration of 300 mg/kg bw to rats, normalised
areas under plasma radiolabel concentration against time curves
(AUCs) were approximately twice as high as after administration of
10 mg/kg bw. This indicates a non-linear relationship between dose
level and AUC. Following multiple oral dosing for 7 days, at
10 mg/kg bw/day, normalized AUC figures were double compared to
those figures obtained after single oral administration of 10 mg/kg
bw. Following both single and multiple oral administration of
radiolabelled cycloxydim the highest residue concentrations were
detected in liver and kidneys. The level of residues in the various
tissues was not influenced by single oral administration of either
acid or sodium salt. Compared to the plasma levels, multiple dosing
did not result in essential accumulation of radiolabel.
Radiolabelled cycloxydim was applied to shaved skin areas of
Sprague-Dawley rats in nominal doses of 0.014, 0.14 or 1.4 mg/cm2
(corresponding nominally to 0.9, 9 or 90 mg/kg bw) at a constant
dose volume of approximately 1.3 ml/kg to an area of approximately
12 cm2 on the back. The duration of treatment at the various dose
levels was 0.5, 1, 2, 4 and 10 h with additional groups of animals
treated for 10 h and sacrificed at 72 h (collection of excreta for
62 hours after removal of dermal dose). The results indicated that
within 10 h up to 36%, 36% and 24% of radiolabel is absorbed through
the skin at dose levels of 0.014, 0.14 and 1.4 mg/cm2
respectively. After oral doses of 10 mg/kg bw a mean of 89.5% was
found to be absorbed within the 10 h after treatment. With the
exception of untreated skin, mean quantities of radiolabel in
tissues and plasma of all dermal dose groups were at all sacrifice
times at or below 1% of the applied dose. Unexpected high mean
quantities of radiolabel were found consistently in untreated skin.
The design of experiments and additional tests provided no evidence
that cross-contamination from treated skin to untreated skin
occurred. From data presented in this study it was concluded that
dermal doses of 0.9, 9.0 and 90 mg/kg bw (0.014, 0.14, 1.4 mg/cm2)
could be considered equivalent to oral doses of approximately 0.3,
3.0 and 30 mg/kg bw, respectively, for the purpose of toxicological
evaluation assuming that the distribution and metabolism of a dermal
dose was the same as an oral dose (Hoffmann et al., 1989).
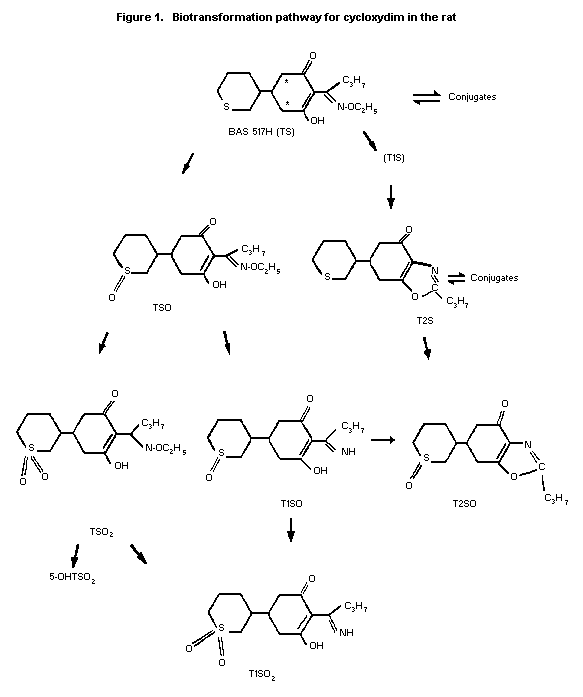
Biotransformation
Rats
Metabolism of cycloxydim in rats was largely unaffected by dose
levels or dosage form (free acid or sodium salt). In all cases the
major metabolite in urine was the sulfoxide (TSO). The next most
important metabolite was T1SO resulting from N-de-ethoxylation of
TSO. Other less important metabolites in urine were the sulfones of
T1SO and T2SO (Beckmann rearranged product of T1SO). The identities
of these major metabolites were confirmed by mass spectrometry.
Minor components in some urine samples corresponded
chromatographically to the sulfone of TSO and unchanged cycloxydim.
The presence of minor metabolites hydroxylated at the 5-position of
the cyclohexone ring of cycloxydim was indicated after oxidation and
methylation of a urine sample. The patterns of radioactive
components in bile and tissue residues were generally similar to the
pattern in urine. A postulated biotransformation pathway for
cycloxydim in the rat is shown in Figure 1.
Toxicological studies
Acute toxicity studies
The results of acute toxicity studies on cycloxydim are
summarised in Table 1. Deaths generally occurred 1-2 days after
dosing and the non-specific signs of toxicity were characterized by
reversibility within the observation period. The gross-pathological
examination of the animals did not show any consistent
substance-induced changes.
Table 1: Acute toxicity of cycloxydim (acid)
Species Sex Route LD50 LD50 Reference
mg/kg bw mg/l
Rat M+F Oral approx. 5000 Hildebrand & Kirsch (1987)
Rat M Oral 4420 Kirsch & Kieczka (1984a)
F 3830
Mouse M+F Oral >5000 Kirsch & Kieczka (1985a)
Rat M+F Dermal >2000 Kirsch & Kieczka (1984b)
Rat M+F Inh >5.28 Klimisch, et al. (1985)
Rat M ip 2370 Kirsch & Kieczka (1985b)
F 1943
Short-term toxicity studies
Subacute toxicity studies with rats were carried out for 4
weeks and 3 months. Mice were used as a second rodent species for 4
weeks. Cycloxydim (as the acid or sodium salt) could not be
administered to rodents via the feed because of insufficient
stability nor was it possible to administer cycloxydim as the acid
in the drinking water on account of its low solubility. After only
one day the active ingredient concentration in the mixture of feed
and test substance was only about 78% of the initial value, and
after 8 days only about 50% was detected. Therefore, following
demonstration of adequate stability by chemical analysis, the test
substance was administered as the sodium salt via the drinking water
in all short- and long-term investigations in rodents. In dogs the
test substance could be administered as sodium salt in the feed
since the mixture was prepared freshly each day shortly before
feeding, and sufficient stability could be verified analytically for
the short period until feed was consumed completely (food was
generally consumed within one hour, at which time the analyzed
concentration was 91% of intended). Altogether, 3 feeding studies
were carried out with beagle dogs for 4 weeks, 3 months and 1 year.
In all studies, the data in ppm or mg/kg bw refer to cycloxydim as
acid rather than sodium salt.
Mice
In a 4-week range-finding study, groups of 10 B6C3F1 mice
per sex were administered doses of 0, 300, 1000, 3000 or 9000 ppm
cycloxydim as the sodium salt (purity 94.8%) via the drinking water.
Drinking water consumption was reduced, in a dose dependent fashion,
up to a maximum of 50%, whereas feed consumption and body-weight
gain were decreased only at 9000 ppm (approximately 14%). The
clinical chemistry examinations revealed reduced cholesterol values,
compared to controls, at a dose of 3000 ppm and above and increased
urea values in both sexes of the highest dose group. At the end of
the study, an increase in the relative liver weight was observed in
the males of all dose groups. The histopathological examination
showed hydropic vacuolar degeneration of hepatocytes only at the
highest dose level (Kuhborth et al., 1986a).
Since a NOAEL could not be determined in the previous study, a
further range-finding study was carried out in mice of the same
strain using a similar experimental design and doses of 0, 30, 100,
300 or 900 ppm. The absolute and relative liver weights of the males
were increased at doses of 100 ppm and above. Reduced drinking water
consumption (4-9%) was observed in the females at doses of 300 ppm
and above, but feed consumption or body-weight gain were not
affected even at 900 ppm. Clinical chemistry values and other organ
weights were not changed by the test substance at any dose level. No
histopathological examinations were carried out in this study since
no histopathological findings were detected in the first range-
finding study in the mouse within a similar dose range. The NOAEL
was difficult to establish since the liver weight increase occurred
without any other indications of toxicity, such as changes in
clinical chemistry parameters or gross-pathological or
histopathological findings. Assuming that the organ weight change
was indicative of toxicity the NOAEL was 30 ppm in males and 100 ppm
in females, equal to 7 mg/kg bw/day in males and 28 mg/kg bw/day in
females, respectively (Kuhborth et al., 1986b).
Rats
In a dose range-finding study, groups of 5 Wistar rats per sex
were administered cycloxydim as the sodium salt (purity 92.8%) for
28 days via the drinking water at levels of 0, 300, 1000, 3000 or
9000 ppm. At the lowest two dose levels the only difference from
controls was a reduction in drinking water intake. On account of the
presence of test substance in the drinking water, the isolated
finding of reduced drinking water consumption is regarded as a
problem of palatability rather than as a sign of toxicity of the
test substance. Reduced feed consumption mainly in the females
(approximately 15%), reduced drinking water consumption in general
(approximately 20-28%) and a slight reduction in body-weight gain
(5-12%) in the females were observed at 3000 ppm. Slightly increased
urea levels in the blood were detected in the clinical chemistry
examinations at 3000 ppm, along with increased relative liver
weights in the females and increased relative kidney weights in the
males, but there was no correlation with the histopathological
evaluation. The highest dose of 9000 ppm showed pronounced toxicity,
which was evident in the form of poor general state, reduced feed
consumption (20-50%), reduced drinking water consumption (42-69%)
and reduced body-weight gain of more than 20% compared to the
control. The clinical chemistry examination revealed slightly
increased urea and sodium values in both sexes, increased
cholesterol and chloride values in the serum of the males and
lowering of the triglyceride levels and alkaline phosphatase
activity in both sexes compared to controls. Investigative,
analytical studies confirmed that an apparent bilirubinaemia was due
to test material or metabolite in serum interfering with the routine
analytical methodology. Among the relative organ weights, the
increased liver and kidney weights were particularly noticeable. No
histopathological correlation was found at this dose level either.
The changes in the clinical chemistry parameters described were
probably due to the reduced feed and drinking water consumption and
the resulting depression of body-weight gain. One female rat
receiving 9000 ppm died after 11 days of treatment. Pathological
investigations revealed heart and lung lesions which were not
considered to be treatment-related. The NOAEL was 1000 ppm, equal to
100 mg/kg bw/day, with the liver and kidneys being regarded as
possible target organs (Kuhborth et al., 1986c).
Groups of 10 Wistar rats/sex were administered 0, 30, 100, 300,
900 or 2700 ppm of cycloxydim as the sodium salt (purity 94.8%) in
the drinking water for 13 weeks. A further 10 animals per sex were
added to the control group and the groups receiving 900 or 2700 ppm
and were maintained for a 6-week withdrawal period after completion
of treatment. The animals tolerated 30, 100 and 300 ppm without any
substance-induced effect. Signs of slight toxicity were observed at
900 ppm. In the clinical chemistry examinations, the creatinine
values were increased in the females and plasma ALAT activities were
increased in both sexes. Plasma ALP activity was reduced in the
males. Body-weight and feed consumption were unaffected, while the
drinking water consumption of the females was reduced by a maximum
of 17%. At the highest dose level of 2700 ppm a reduction in feed
consumption (11%) and drinking water consumption (a maximum of 35%)
were recorded. The body-weight of the males was reduced by 8%
compared with the control group. The creatinine, urea and
cholesterol values of the females and the plasma ALAT activity of
both sexes were increased, while the activity of alkaline
phosphatase in the plasma was reduced. There were no
treatment-related changes in organ weight, gross-pathology or
histopathological findings at any dose level. All changes described
showed a tendency to reversibility during the 6-week withdrawal
period. The NOAEL was 300 ppm, equal to 25 mg/kg bw/day (Kuhborth
et al., 1986d).
 Dogs
In a range-finding study, 2 beagle dogs per sex and dose were
given 0, 300, 1200, 3600 or 10 800 ppm as the sodium salt of
cycloxydim (purity 94.8%) in their feed for 4 weeks (equal to doses
of 0, 10, 40, 120 or 360 mg/kg bw/day). At the highest dose level,
the feed consumption of the females was occasionally reduced, while
body-weight remained unaffected. The plasma ALP activity and the
plasma cholinesterase activity were increased in the males. On
several occasions of the red blood count were marginally reduced.
The relative liver weight was increased compared with the untreated
control and histopathological examination showed enlargement of
hepatocytes. The findings were less pronounced at a dose level of
120 mg/kg bw/day. The only treatment-related findings at this dose
were increased plasma ALP in the males and a tendency to an
increased relative liver weight. The NOAEL in this study was 40
mg/kg bw/day (Hellwig et al., 1985).
Groups of 4 beagle dogs/sex/dose were used for a subchronic
feeding study in dogs over 13 weeks using doses of 0, 60, 300, 1500
or 7500 ppm as the sodium salt (purity 94.8%). No findings related
to the test substance administered were obtained at the 3 lowest
dose levels but toxicity was pronounced at 7500 ppm. However, there
were no clinical changes and body-weight gain remained unaffected by
treatment. The clinical chemistry examination revealed increased
serum activities of ALP and a reduced albumin concentration. The
females had increased globulin values in the serum, while the sodium
level in the blood was reduced in the males. Among the haemato-
logical changes, the reduced erythrocyte counts and presence of
Heinz bodies were of note. However, the number of reticulocytes and
platelets was increased suggesting a compensatory reaction in the
bone marrow. In the males, the MCH and MCV were also increased. Of
the organ weights, the absolute and relative liver weights were
above those of the untreated control in both sexes. The bile was
reddish and histopathological examination revealed an enlargement of
hepatocytes. The NOAEL was 1500 ppm, equal to 50 mg/kg bw/day
(Hellwig et al., 1986).
In a 12-month feeding study, groups of 6 beagle dogs/sex were
given doses of 0, 400, 1600 or 6400 ppm cycloxydim (acid) as sodium
salt (purity 93.9%). The lowest dose of 400 ppm did not lead to any
substance-induced changes. At 1600 ppm, the number of Heinz bodies
was increased in both sexes, indicating a disturbance in the
haemoglobin metabolism. Male animals showed an increased activity of
ALP in plasma and a lowering of the albumin level. The absolute and
relative liver weights of these animals were significantly
increased, but no corresponding histopathological changes were
observed. This finding was also detected in both sexes of the
highest dose group and some further clinical chemistry parameters
were also changed. There was an increase in plasma ALP and a
lowering of the albumin and protein concentrations (in the males
only). The haematological examinations showed signs of slight
anaemia with a compensatory bone marrow reaction. These findings
were manifest in the females in the form of haemosiderosis of
Kupffer's cells in the liver as a histopathological correlation. The
NOAEL was 400 ppm in the diet, equal to 20 mg/kg bw/day (Hellwig et
al., 1988a).
Long-term toxicity/carcinogenicity studies
Mice
In a 24-month study in B6C3F1 mice, cycloxydim was
administered as the sodium salt (purity 93.9%) in the drinking water
at doses of 0, 10, 20, 60 or 240 ppm to groups of 50 mice/sex
(controls 100 mice/sex). There were no clinical findings which were
induced by the test substance. Body-weight gain, food and water
intake and the incidence of mortality remained unaffected by
treatment. The histopathological examination at the end of the study
did not reveal any changes related to the test substance. The
tumours that occurred corresponded to those of the spontaneous range
of the animal strain used so that no carcinogenic potential of the
test substance was apparent, up to a dose level of 240 ppm, equal to
32 mg/kg bw/day (Kuhborth et al., 1988c).
Rats
In a study to determine chronic toxicity, groups of 20 Wistar
rats per sex/dose were administered 0, 100, 400, 1600 or 2700 ppm
of cycloxydim as sodium salt (purity 93.9%) via the drinking water
for 18 months. At the highest dose of 2700 ppm, reduced food and
drinking water consumption were observed in both sexes together with
a decrease in body-weight gain of up to 21% compared with the
control during the major part of the study period. Doses of 400 and
1600 ppm also led to reduced body-weight gain compared with the
control, but drinking water consumption and feed consumption (males
only) were reduced only at 1600 ppm. In the clinical chemistry
examinations, a lowering of the triglyceride level was observed at
doses of 400 ppm and above. The organ weight determinations and the
gross pathological and histopathological examination did not reveal
any treatment-related findings. Therefore, the NOAEL in this study
was 100 ppm, equal to 7 mg/kg bw/day (Kuhborth et al., 1988a).
In a second study carried out simultaneously to clarify
possible carcinogenicity, groups of 50 Wistar rats/sex were given
cycloxydim as the sodium salt (purity 93.9%) in the drinking water
for 24 months at doses of 0, 100, 400 or 1600 ppm. As in the 18-
month study, a reduction in the drinking water consumption and body-
weight by up to 18% was observed in both sexes at 1600 ppm, but feed
consumption was not impaired in this study. The clinical chemistry
examinations revealed a lowering of the triglyceride level in the
females at 1600 ppm. The body-weight reduction (both sexes) and
lowering of the triglyceride level (females) were also observed at a
dose level of 400 ppm. The determination of organ weights at the end
of the study showed a lowering of the absolute and relative liver
weights at the high-dose, but there was no corresponding
morphological change. No findings related to the test substance
administered were obtained in the gross pathological or
histopathological examinations. There was no indication of any
disturbance of the spontaneous tumour profile of the rat strain
used. Under the given test conditions, cycloxydim has no
carcinogenic potential in Wistar rats. The NOAEL with regard to
clinical or clinical chemistry parameters and organ weight changes
was 100 ppm, the same as that of the 18-month toxicity study
(Kuhborth et al., 1988b).
Reproduction study
Rats
Investigations of reproductive toxicity were carried out in
Wistar rats in a multi-generation study in which 2 litters were used
in the first generation and 1 litter in the second generation.
Groups of 24 animals per sex were adminis-tered cycloxydim as the
sodium salt (purity 93.9%) via the drinking water at doses of 0,
100, 400 or 1600 ppm (expressed as acid) for a period of 70 days
prior to mating and then throughout gestation and lactation.
The highest dose of 1600 ppm, equal to 150 mg/kg bw/day caused
systemic toxicity in the parental animals (reduced food and drinking
water consumption and retarded body-weight gain). The number of all
pups (live and stillborn) at parturition was slightly reduced and
the development of the pups was retarded. Pup mortality was also
increased at this dose level. At the mid-dose (400 ppm), there were
only indications of systemic toxicity in the parental animals in the
form of reduced food and drinking water consumption as well as
temporary retardation of body-weight gain. There was no effect on
the number of pups or on pup mortality at this dose. Accordingly,
the NOAEL was 100 ppm, equal to 10 mg/kg bw/day for parental
toxicity and 400 ppm, equal to 38 mg/kg bw/day for reproductive
performance (Hellwig et al., 1988b).
Special studies on embryo/fetotoxicity
Rats
Cycloxydim as the sodium salt (purity 93.9%) was administered
to 23 or 24 pregnant rats per test group by gavage from days 6 to 15
after mating at doses of 0, 100, 200 or 400 mg/kg bw/day. On day 20
of gestation the dams were sacrificed, and the fetuses were examined
after caesarean section. At 100 and 200 mg/kg bw/day no signs of
maternal toxicity were observed but body-weight gain and food
consumption were reduced at the highest dose level at the beginning
of test substance administration compared with the untreated
control. The number of implant-ations and resorptions and the
fertility rate were similar to those of the control at every dose
level. Fetotoxic effects were observed in the range of maternal
toxicity; they were characterized by reduced fetal body-weight and
retardation of ossification of the skeletons of the fetuses.
Furthermore, an increased incidence of vertebral column changes in
the fetuses, which were classified as anomalies, was detected at the
highest dose level. These were mainly changes in thoracic vertebrae,
which had a dumbell-shaped or bipartite appearance. One out of 309
fetuses at 200 mg/kg bw/day and 1/295 fetuses at 400 mg/kg bw/day
had anal/tail defects (Hellwig & Hildebrand, 1987a).
In a second study, maternal toxicity after administration of
the sodium salt (purity 93.9%) was investigated in more detail.
Groups of 25 female Wistar rats were administered cycloxydim at
doses of 0, 200, 400, 600 or 800 mg/kg bw/day by gavage from days 6
to 15 of gestation. The dams were sacrificed on day 20 of gestation.
After caesarean section the fetuses were evaluated only to a limited
extent (weight and sex determinations and gross-pathological
examination) since the main aim of the study was to determine the
exact maternal toxicity threshold. Pronounced maternal toxicity
which, inter alia, was manifest in the form of clearly retarded
body-weight gain together with reduced food consumption was observed
at a dose level of 800 mg/kg bw/day. The general state of health was
poor, 5 dams showing vaginal haemorrhages. RBC parameters
(haemoglobin content, erythrocyte count, haematocrit) were reduced,
and the number of reticulocytes was increased compared with the
untreated control indicating a compensatory reaction of the bone
marrow. These findings were also obtained at 600 and 400 mg/kg
bw/day although they were less pronounced. The NOAEL regarding
maternal toxicity was 200 mg/kg bw/day, confirming the result of the
first study. Fetal weight was reduced at 600 and 800 mg/kg bw/day
and the gross-pathological examination revealed missing or
incomplete tail in some cases together with anal atresia in 3 of 270
fetuses (in 3 out of 22 litters) at 800 mg/kg bw/day and in 2 of 296
fetuses (in 2 out of 24 litters) at 600 mg/kg bw/day (Hellwig et
al., 1987).
A third study in Wistar rats was carried out to investigate
whether the changes in the vertebrae which occurred in the first
prenatal study also persisted postnatally. Two groups, each of 60
pregnant females, were given cycloxydim (as the sodium salt, purity
93.9%) by daily oral gavage, between days 6 and 15 of pregnancy at
doses of 0 or 400 mg/kg bw/day. Twenty-five dams from each group
were sacrificed on day 20 of pregnancy and their fetuses were
examined after caesarean section. The remaining dams were allowed to
litter and rear their pups. The litters of a further 10 dams from
each group were sacrificed on day 7 post-partum and all other
litters were sacrificed on day 21 after parturition and examined in
detail. In the dams sacrificed on day 20 of gestation there were
indications of the beginning of maternal toxicity characterised by
retarded body-weight gain and reduced feed consumption. The weight
of the fetuses was reduced. Four fetuses (out of 286) from 3/22
litters had tail defects. The incidence of retarded ossification of
the skeletons was clearly higher compared with the untreated
control. As in the first prenatal toxicity study dumbell-shaped or
bipartite thoracic vertebrae occurred to an increased extent
compared with the control. The incidence of the changes in the
thoracic vertebrae of the pups sacrificed 7 or 21 days after birth
was clearly reduced compared with the prenatal investigations
although these findings, which also occur spontaneously without
relation to the test substance, were not completely reversible
either in the untreated control or in the animals treated.
Furthermore, perinatal mortality was slightly increased at 400 mg/kg
bw/day compared to controls. This finding was due to an increased
number of dead pups on the day of birth whereas the survival index
of the pups sacrificed 7 days after birth remained unaffected
(Hellwig & Hildebrand, 1987b).
In addition to the in vivo studies, an in vitro study was
carried out with rat embryos (whole embryo culture technique).
Cycloxydim (acid) and the main metabolite (TSO) were investigated in
an experiment in which embryos of Wistar rats which were 9.5 days
old were incubated in the culture medium with the test substance or
solvent control (DMSO) for 48 h. The concentrations were 300 œg/ml
for cycloxydim and 150 œg/ml for the TSO metabolite. Concentrations
were based on results of in vivo teratogenicity studies in the rat
and on investigations into the kinetics and metabolism in the same
animal species. After incubation, the embryos were evaluated under a
stereo-microscope and subsequently examined histopathologically for
possible substance-induced changes. No abnormal morphological
development was induced either by cycloxydim or by its TSO
metabolite in the embryos under the test conditions chosen. These
results clearly demonstrate that a direct embryotoxic effect was not
seen in vitro at this very sensitive development stage of the main
organs (Neubert et al., 1987)
Rabbits
Groups of 14 or 15 pregnant Himalayan rabbits were administered
cycloxydim as the sodium salt (purity 93.9%) by daily gavage from
days 6 to 18 after artificial insemination at doses of 0, 100, 200
or 400 mg/kg bw/day. On day 29 of gestation, the dams were
sacrificed and the fetuses were examined in detail after caesarean
section. At a dose of 400 mg/kg bw/day, the dams showed a
retardation of body-weight gain together with reduced food
consumption primarily during the treatment period compared with the
untreated control group. This finding was also observed after
administration of 200 mg/kg bw/day although it was less pronounced.
No signs of maternal toxicity were observed at 100 mg/kg bw/day.
There were no indications of an embryotoxic effect of the test
substance in the fetuses, except at 400 mg/kg bw/day, where there
was a significant reduction in percentage live fetuses and an
increase in percentage dead implants. The number of implantations
and resorptions, the conception rate and the implantation loss were
otherwise unaffected by treatment. Minor inter-group differences
remained within the historical control range. The variations,
anomalies and retardations that occurred were in the range of
historical control data for all test groups (Merkle & Hildebrand,
1985).
In addition to routine X-ray examination, the skeletons of the
control and highest dose group were additionally stained to look for
abnormalities of the vertebrae. These investigations did not
indicate any substance-induced changes of the vertebrae, as had
occurred in rats at a similar dose level (Hellwig, 1986).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
cycloxydim. The results are summarised in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on skin and eye irritation and sensitization
The skin irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). About 0.5 ml of undiluted
test substance was applied to the clipped, intact dorsal skin of 3
animals per sex for 4 h under a semi-occlusive dressing. Very slight
erythema in 4/6 animals, within 1 hour of patch removal, which was
completely reversible within 2 days, was the only finding that was
obtained (Kirsch & Kieczka, 1984c).
The eye irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). 0.1 Ml of the undiluted test
substance was applied to the eyes of 3 male and 3 female animals.
Slight conjunctival irritation (redness with lacrimation), which was
seen in all animals one hour after treatment, and which was
completely reversible within 8 days, was the only finding that was
observed. Neither cornea nor iris showed any changes related to the
test substance administered (Kirsch & Kieczka, 1984d).
The sensitizing potential of the active ingredient was
investigated in guinea-pigs (Pirbright White) in a maximization test
in which 10 animals were used in each of the two control groups and
20 animals in the test group. The two challenges carried out after
intradermal induction with the test substance did not reveal any
skin changes in the animals of the test group indicating that there
was no sensitizing potential of cycloxydim under the test conditions
chosen (Kirsch & Kieczka, 1985c).
Observations in humans
No information was available.
Table 2: Results of in vitro genotoxicity assays on cycloxydim
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium 20-15000 µg/plate 93.9% Negative Gelbke & Engelhardt (1985a)
TA98,TA100, (Na salt)
TA1535,TA1537
Ames test S. typhimurium 20-5000 µg/plate NK Negative Gelbke & Engelhardt (1984)
TA98, TA100, (acid)
TA1535, TA1537,
TA 1538
Lymphoma forward Mouse L5178Y 1.75-20 µg/ml 93.9% Weak, positive den Boer & Hoorn (1985a)
mutation assay cells (Na salt) at cytotoxic
concentrations
HGPRT forward CHO cells 5-40 mg/ml 93.9% Negative Gelbke & Engelhardt (1986)
mutation assay (Na salt)
HGPRT forward CHO cells 0.215-21.5 mg/ml 93.9% Negative den Boer & Hoorn (1985b)
mutation assay (Na salt)
Cytogenetics CHO cells 500-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985a)
mutation assay (acid) in absence of
activation
Cytogenetics CHO cells 2000-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985b)
mutation assay (Na salt) in absence of
activation
UDS Rat hepatocytes 100-2000 µg/ml NK Negative Cifone & Myhr (1985)
(acid)
UDS Rat hepatocytes 0.9-90.6 µg/ml NK Negative Cifone & Brusick (1985)
(Na salt)
NK: not known
Table 3: Results of in vivo genotoxicity assays on cycloxydim
Test system Test object Dose Purity Results Reference
Micronucleus NMRI mice 225, 450, 900 93.9% Negative Gelbke & Engelhardt (1985b)
test mg/kg bw
Micronucleus Chinese 500, 1700, 5000 NK Negative Taalman & Hoorn (1987)
test hamsters mg/kg bw
NK: not known
COMMENTS
Cycloxydim was extensively absorbed after oral administration
to rats and almost completely excreted within 5 days of dosing.
Elimination proceeded predominantly via the urine (74-86% of applied
dose), with lower levels in the faeces (12-25% of the applied dose).
Less than 1% of an administered dose was retained in the body.
Studies confirmed that enterohepatic circulation occurred in rats.
The metabolism of cycloxydim has been investigated in rats and
a biotransformation pathway has been proposed. The major metabolite
was the sulfoxide, and the pattern of metabolites was similar in
urine, bile and tissue residues.
Cycloxydim has low acute oral toxicity. WHO has classified
cycloxydim as unlikely to present acute hazard in normal use (WHO,
1992).
Owing to instability in dietary admixture the sodium salt of
cycloxydim was administered in the drinking-water in repeat-dose
rodent studies. In dogs, sufficient dietary stability was
demonstrated, and dietary administration of cycloxydim was therefore
used.
In mice, two 4-week dose range-finding studies were conducted,
followed by a 24-month long-term study. In the dose range-finding
studies, employing concentrations between 30 and 9000 ppm, reduced
food and water intake, and reduced body-weight gain were seen at
higher doses, together with increased liver weight and hydropic
vacuolar hepatocyte degeneration. The NOAEL was equal to 7 mg/kg
bw/day in males and 28 mg/kg bw/day in females, the lower NOAEL in
males resulting from liver weight increase in the absence of any
histopatho-logical change. In the long-term study, no treatment-
related effects were seen, up to the highest dose tested of 32 mg/kg
bw/day, although liver weights were not measured.
In rats a 4-week dose range-finding study was followed by a 13-
week study, an 18-month toxicity study and a 24-month
carcinogenicity study. Findings were generally similar to those
observed in mice, the liver being identified as the only target
organ of note, although no histopathological changes were seen at
doses up to 900 mg/kg bw/day. The NOAELs were 100 mg/kg bw/day over
4 weeks, 25 mg/kg bw/day over 13 weeks and 7 mg/kg bw/day over 18/24
months, based on reduced body-weight gain at 25 mg/kg bw/day and
above.
Cycloxydim was not carcinogenic in rats or mice.
In dogs, a 4-week dose range-finding study was followed by a
13-week and a 12-month study. In the dose range-finding study (at
doses up to 360 mg/kg bw/day) the liver and the red blood cells were
identified as target organs and the NOAEL was 40 mg/kg bw/day. In
the longer-term dog studies, the NOAELs were 50 mg/kg bw/day over 13
weeks and 20 mg/kg bw/day over 52 weeks. Target organs were the
liver and red blood cells. A marginal anaemia was seen, along with a
compensatory bone marrow response, but no serious treatment-related
histopathological effects were noted at 80 or 300 mg/kg bw/day.
In a multi-generation reproduction study in rats, the NOAELs
were about 10 mg/kg bw/day for parental toxicity and 38 mg/kg bw/day
for reproductive performance, pup mortality being slightly increased
at 150 mg/kg bw/day.
Teratogenicity studies have been carried out with cycloxydim in
rats and rabbits. In the rat teratogenicity studies the NOAEL for
maternal toxicity was 200 mg/kg bw/day. Fetotoxicity was seen at
maternally toxic doses, together with findings which may be
considered indicative of a teratogenic potential (missing/incomplete
tail and anal atresia at 600 and 800 mg/kg bw/day and vertebral
anomalies at 400 mg/kg bw/day). Although the vertebral anomalies
were not completely reversible post-natally (up to 21 days after
birth), in vitro embryo culture studies demonstrated that
cycloxydim did not show any direct embryotoxic effects. In the
rabbit teratogenicity study the NOAEL for maternal toxicity was 100
mg/kg bw/day. There was no indication of fetotoxicity in rabbits,
even in the presence of maternal toxicity and no indication of any
treatment-related vertebral anomalies, even when a special
examination was conducted to look specifically for such changes.
After reviewing the available genotoxicity data, the Meeting
concluded that cycloxydim and its sodium salt were not genotoxic.
An ADI was allocated based upon the NOAEL from the long-term
rat study in rats, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: > 240 ppm in the drinking water, equal to > 32
mg/kg bw/day (two-year study)
Rat: 100 ppm in the drinking-water, equal to 7 mg/kg
bw/day (18- and 24-month studies)
100 ppm in the drinking-water, equal to 10 mg/kg
bw/day (multi-generation study)
200 mg/kg bw/day (teratogenicity study, maternal and
fetal toxicity)
Dog: 400 ppm in the diet, equal to 20 mg/kg bw/day (one-
year study)
Rabbit: 100 mg/kg bw/day (teratogenicity study, maternal
toxicity).
Estimate of acceptable daily intake for humans:
0-0.07 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
den Boer, W.C. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (Na salt) in the mouse lymphoma forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
den Boer, W.C. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in the CHO HGPRT forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Reg. No. 172 999 in
the in vitro primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics (Hazleton), Kensington,
USA, submitted to WHO by BASF AG.
Cifone, M.A. & Brusick, D.J. (1985) Evaluation of BAS 517 H Na salt
in the in vitro rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished report from Litton Bionetics (Hazleton),
Kensington, USA, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1984) Study of Reg. No. 172 999 in
the Ames test (standard plate test with Salmonella typhimurium).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Gelbke, H.P. & Engelhardt, G. (1985a) Study of Reg. No. 172 999 Na
salt in the Ames test (standard plate test with Salmonella
typhimurium). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1985b) Cytogenetic investigations in
NMRI mice after a single oral administration of Reg. No. 172 999, Na
salt - Micronucleus test. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1986) Point mutation test carried out
on CHO cells (HGPRT locus) with Reg. No. 172 999. Unpublished report
from BASF Aktiengesellschaft, Departmont of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hawkins, D.R., Kirkpatrick, D., Finn, C.M., Till, C.P., Dean, G.M.,
Biggs, S.R. and & Whitby, B.R. (1986). The biokinetics and
metabolism of 14C-BAS 517 H in the rat. Unpublished report from
Huntingdon Research Centre Ltd., Huntingdon, England, submitted to
WHO by BASF AG.
Hellwig, J. (1986) Amendment to the determination of the prenatal
toxicity of Reg. No. 172 999 Na salt in rabbits after oral
administration (stomach tube). Unpublished report from BASF
Aktiengesellschaft. Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987a) Study of the prenatal toxicity
of Reg. No. 172 999 Na salt in rats after oral administration
(gavage). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987b) Study of the pre-, peri- and
postnatal toxicity of Reg. No. 172 999 Na salt in rats after oral
administration (gavage). Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology. D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1985)
Study of the toxicity of Reg. No. 172 999 Na salt in beagles after
4-week administration in the diet (range-finding study). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1986)
Study of Reg. No. 172 999 Na salt in beagles after 3-month
administration in the diet (range-finding study). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Gembardt, Chr. & Hildebrand B. (1987)
Study of the prenatal toxicity of Reg. No. 172 999 Na salt in rats
after oral administration (gavage) with special attention to
maternal toxicity. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1988a)
Study of the toxicity of Reg. No. 172 999 Na salt in purebred beagle
dogs: administration over 12 months via the diet. Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Freisberg, K.O., & Hildebrand, B. (1988b) Reproduction
study with Reg. No. 172 999 Na salt in rats: continuous
administration with the drinking water over 2 generations (2 litters
in the first and one litter in the second generation). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hildebrand, B. & Kirsch, P. (1987). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hoffmann, H.D., Gelbke, H.P., & Hildebrand, B. (1989) Study of the
dermal absorption of BAS 517H sodium salt in rats. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984a). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984b). Acute dermal toxicity of Reg. No.
172 999 in the rat based on OECD and EPA (FIFRA). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984c). Acute dermal
irritation/corrosivity of Reg. No. 172 999 to the intact dorsal skin
of the white rabbit based on OECD and EPA (FIFRA). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984d). Acute irritation of Reg. No. 172
999 to the eye of the white rabbit based on OECD and EPA (FIFRA).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kirsch, P. & Kieczka, H. (1985a). Acute oral toxicity on the mouse
based on OECD of Reg No. 172 999. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985b). Acute intraperitoneal toxicity of
Reg. No. 172 999 in the rat. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985c). Sensitizing effect of Reg. No. 172
999 in the guinea pig maximization test. Unpublished report from
BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Klimisch, H-J., Gelke, H-P., & Freisberg, K.O. (1985). Acute
inhalation toxicity, 4 hours (rat) liquid aerosol study of Reg. No.
172 999 tested as sodium salt. Unpublished report from BASF
Aktiengesellschift, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986a) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (1st range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986b) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (2nd range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986c) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 28-day administration with the drinking water (range-finding
study). Unpublished report from BASF Aktiengesellschaft, Department
of Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by
BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986d) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 3-month administration via the drinking water and a 6-week
observation period. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988a) Study of the toxicity of Reg. No. 172 999 Na
salt in rats: administration in the drinking water over 18 months.
Unpublished report from BASF Aktiengeselischaft, Department of
Toxicology. D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988b) Study on the toxicity of Reg. No. 172 999
Na-salt in rats: administration via the drinking water over 24
months. Unpublished report from BASF Aktiengeselischaft, Department
of Toxicology, D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1988c) Study on the toxicity of Reg. No. 172 999 Na-salt in mice:
administration in the drinking water over 24 months. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Merkle, J. & Hildebrand B. (1985) Pretnatal toxicity of Reg. No. 172
999 Na salt in rabbits after oral administration (stomach tube).
Unpublished report from BASF Aktiengesellchaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Neubert, D., Klug, S. & Merker, H-J. (1987) Effect of cycloxydim on
embryonic development in vitro. Unpublished report from Institute
for Toxicology and Embryopharmacology, Freie Universität, Berlin,
submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (acid) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1987) Cytogenetic investigations
in Chinese hamsters after a single oral administration of Reg. No.
172 999, Na salt - micronucleus test. Unpublished report from Litton
Bionetics (Hazleton), Veenendaal, Netherlands, submitted to WHO by
BASF AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Dogs
In a range-finding study, 2 beagle dogs per sex and dose were
given 0, 300, 1200, 3600 or 10 800 ppm as the sodium salt of
cycloxydim (purity 94.8%) in their feed for 4 weeks (equal to doses
of 0, 10, 40, 120 or 360 mg/kg bw/day). At the highest dose level,
the feed consumption of the females was occasionally reduced, while
body-weight remained unaffected. The plasma ALP activity and the
plasma cholinesterase activity were increased in the males. On
several occasions of the red blood count were marginally reduced.
The relative liver weight was increased compared with the untreated
control and histopathological examination showed enlargement of
hepatocytes. The findings were less pronounced at a dose level of
120 mg/kg bw/day. The only treatment-related findings at this dose
were increased plasma ALP in the males and a tendency to an
increased relative liver weight. The NOAEL in this study was 40
mg/kg bw/day (Hellwig et al., 1985).
Groups of 4 beagle dogs/sex/dose were used for a subchronic
feeding study in dogs over 13 weeks using doses of 0, 60, 300, 1500
or 7500 ppm as the sodium salt (purity 94.8%). No findings related
to the test substance administered were obtained at the 3 lowest
dose levels but toxicity was pronounced at 7500 ppm. However, there
were no clinical changes and body-weight gain remained unaffected by
treatment. The clinical chemistry examination revealed increased
serum activities of ALP and a reduced albumin concentration. The
females had increased globulin values in the serum, while the sodium
level in the blood was reduced in the males. Among the haemato-
logical changes, the reduced erythrocyte counts and presence of
Heinz bodies were of note. However, the number of reticulocytes and
platelets was increased suggesting a compensatory reaction in the
bone marrow. In the males, the MCH and MCV were also increased. Of
the organ weights, the absolute and relative liver weights were
above those of the untreated control in both sexes. The bile was
reddish and histopathological examination revealed an enlargement of
hepatocytes. The NOAEL was 1500 ppm, equal to 50 mg/kg bw/day
(Hellwig et al., 1986).
In a 12-month feeding study, groups of 6 beagle dogs/sex were
given doses of 0, 400, 1600 or 6400 ppm cycloxydim (acid) as sodium
salt (purity 93.9%). The lowest dose of 400 ppm did not lead to any
substance-induced changes. At 1600 ppm, the number of Heinz bodies
was increased in both sexes, indicating a disturbance in the
haemoglobin metabolism. Male animals showed an increased activity of
ALP in plasma and a lowering of the albumin level. The absolute and
relative liver weights of these animals were significantly
increased, but no corresponding histopathological changes were
observed. This finding was also detected in both sexes of the
highest dose group and some further clinical chemistry parameters
were also changed. There was an increase in plasma ALP and a
lowering of the albumin and protein concentrations (in the males
only). The haematological examinations showed signs of slight
anaemia with a compensatory bone marrow reaction. These findings
were manifest in the females in the form of haemosiderosis of
Kupffer's cells in the liver as a histopathological correlation. The
NOAEL was 400 ppm in the diet, equal to 20 mg/kg bw/day (Hellwig et
al., 1988a).
Long-term toxicity/carcinogenicity studies
Mice
In a 24-month study in B6C3F1 mice, cycloxydim was
administered as the sodium salt (purity 93.9%) in the drinking water
at doses of 0, 10, 20, 60 or 240 ppm to groups of 50 mice/sex
(controls 100 mice/sex). There were no clinical findings which were
induced by the test substance. Body-weight gain, food and water
intake and the incidence of mortality remained unaffected by
treatment. The histopathological examination at the end of the study
did not reveal any changes related to the test substance. The
tumours that occurred corresponded to those of the spontaneous range
of the animal strain used so that no carcinogenic potential of the
test substance was apparent, up to a dose level of 240 ppm, equal to
32 mg/kg bw/day (Kuhborth et al., 1988c).
Rats
In a study to determine chronic toxicity, groups of 20 Wistar
rats per sex/dose were administered 0, 100, 400, 1600 or 2700 ppm
of cycloxydim as sodium salt (purity 93.9%) via the drinking water
for 18 months. At the highest dose of 2700 ppm, reduced food and
drinking water consumption were observed in both sexes together with
a decrease in body-weight gain of up to 21% compared with the
control during the major part of the study period. Doses of 400 and
1600 ppm also led to reduced body-weight gain compared with the
control, but drinking water consumption and feed consumption (males
only) were reduced only at 1600 ppm. In the clinical chemistry
examinations, a lowering of the triglyceride level was observed at
doses of 400 ppm and above. The organ weight determinations and the
gross pathological and histopathological examination did not reveal
any treatment-related findings. Therefore, the NOAEL in this study
was 100 ppm, equal to 7 mg/kg bw/day (Kuhborth et al., 1988a).
In a second study carried out simultaneously to clarify
possible carcinogenicity, groups of 50 Wistar rats/sex were given
cycloxydim as the sodium salt (purity 93.9%) in the drinking water
for 24 months at doses of 0, 100, 400 or 1600 ppm. As in the 18-
month study, a reduction in the drinking water consumption and body-
weight by up to 18% was observed in both sexes at 1600 ppm, but feed
consumption was not impaired in this study. The clinical chemistry
examinations revealed a lowering of the triglyceride level in the
females at 1600 ppm. The body-weight reduction (both sexes) and
lowering of the triglyceride level (females) were also observed at a
dose level of 400 ppm. The determination of organ weights at the end
of the study showed a lowering of the absolute and relative liver
weights at the high-dose, but there was no corresponding
morphological change. No findings related to the test substance
administered were obtained in the gross pathological or
histopathological examinations. There was no indication of any
disturbance of the spontaneous tumour profile of the rat strain
used. Under the given test conditions, cycloxydim has no
carcinogenic potential in Wistar rats. The NOAEL with regard to
clinical or clinical chemistry parameters and organ weight changes
was 100 ppm, the same as that of the 18-month toxicity study
(Kuhborth et al., 1988b).
Reproduction study
Rats
Investigations of reproductive toxicity were carried out in
Wistar rats in a multi-generation study in which 2 litters were used
in the first generation and 1 litter in the second generation.
Groups of 24 animals per sex were adminis-tered cycloxydim as the
sodium salt (purity 93.9%) via the drinking water at doses of 0,
100, 400 or 1600 ppm (expressed as acid) for a period of 70 days
prior to mating and then throughout gestation and lactation.
The highest dose of 1600 ppm, equal to 150 mg/kg bw/day caused
systemic toxicity in the parental animals (reduced food and drinking
water consumption and retarded body-weight gain). The number of all
pups (live and stillborn) at parturition was slightly reduced and
the development of the pups was retarded. Pup mortality was also
increased at this dose level. At the mid-dose (400 ppm), there were
only indications of systemic toxicity in the parental animals in the
form of reduced food and drinking water consumption as well as
temporary retardation of body-weight gain. There was no effect on
the number of pups or on pup mortality at this dose. Accordingly,
the NOAEL was 100 ppm, equal to 10 mg/kg bw/day for parental
toxicity and 400 ppm, equal to 38 mg/kg bw/day for reproductive
performance (Hellwig et al., 1988b).
Special studies on embryo/fetotoxicity
Rats
Cycloxydim as the sodium salt (purity 93.9%) was administered
to 23 or 24 pregnant rats per test group by gavage from days 6 to 15
after mating at doses of 0, 100, 200 or 400 mg/kg bw/day. On day 20
of gestation the dams were sacrificed, and the fetuses were examined
after caesarean section. At 100 and 200 mg/kg bw/day no signs of
maternal toxicity were observed but body-weight gain and food
consumption were reduced at the highest dose level at the beginning
of test substance administration compared with the untreated
control. The number of implant-ations and resorptions and the
fertility rate were similar to those of the control at every dose
level. Fetotoxic effects were observed in the range of maternal
toxicity; they were characterized by reduced fetal body-weight and
retardation of ossification of the skeletons of the fetuses.
Furthermore, an increased incidence of vertebral column changes in
the fetuses, which were classified as anomalies, was detected at the
highest dose level. These were mainly changes in thoracic vertebrae,
which had a dumbell-shaped or bipartite appearance. One out of 309
fetuses at 200 mg/kg bw/day and 1/295 fetuses at 400 mg/kg bw/day
had anal/tail defects (Hellwig & Hildebrand, 1987a).
In a second study, maternal toxicity after administration of
the sodium salt (purity 93.9%) was investigated in more detail.
Groups of 25 female Wistar rats were administered cycloxydim at
doses of 0, 200, 400, 600 or 800 mg/kg bw/day by gavage from days 6
to 15 of gestation. The dams were sacrificed on day 20 of gestation.
After caesarean section the fetuses were evaluated only to a limited
extent (weight and sex determinations and gross-pathological
examination) since the main aim of the study was to determine the
exact maternal toxicity threshold. Pronounced maternal toxicity
which, inter alia, was manifest in the form of clearly retarded
body-weight gain together with reduced food consumption was observed
at a dose level of 800 mg/kg bw/day. The general state of health was
poor, 5 dams showing vaginal haemorrhages. RBC parameters
(haemoglobin content, erythrocyte count, haematocrit) were reduced,
and the number of reticulocytes was increased compared with the
untreated control indicating a compensatory reaction of the bone
marrow. These findings were also obtained at 600 and 400 mg/kg
bw/day although they were less pronounced. The NOAEL regarding
maternal toxicity was 200 mg/kg bw/day, confirming the result of the
first study. Fetal weight was reduced at 600 and 800 mg/kg bw/day
and the gross-pathological examination revealed missing or
incomplete tail in some cases together with anal atresia in 3 of 270
fetuses (in 3 out of 22 litters) at 800 mg/kg bw/day and in 2 of 296
fetuses (in 2 out of 24 litters) at 600 mg/kg bw/day (Hellwig et
al., 1987).
A third study in Wistar rats was carried out to investigate
whether the changes in the vertebrae which occurred in the first
prenatal study also persisted postnatally. Two groups, each of 60
pregnant females, were given cycloxydim (as the sodium salt, purity
93.9%) by daily oral gavage, between days 6 and 15 of pregnancy at
doses of 0 or 400 mg/kg bw/day. Twenty-five dams from each group
were sacrificed on day 20 of pregnancy and their fetuses were
examined after caesarean section. The remaining dams were allowed to
litter and rear their pups. The litters of a further 10 dams from
each group were sacrificed on day 7 post-partum and all other
litters were sacrificed on day 21 after parturition and examined in
detail. In the dams sacrificed on day 20 of gestation there were
indications of the beginning of maternal toxicity characterised by
retarded body-weight gain and reduced feed consumption. The weight
of the fetuses was reduced. Four fetuses (out of 286) from 3/22
litters had tail defects. The incidence of retarded ossification of
the skeletons was clearly higher compared with the untreated
control. As in the first prenatal toxicity study dumbell-shaped or
bipartite thoracic vertebrae occurred to an increased extent
compared with the control. The incidence of the changes in the
thoracic vertebrae of the pups sacrificed 7 or 21 days after birth
was clearly reduced compared with the prenatal investigations
although these findings, which also occur spontaneously without
relation to the test substance, were not completely reversible
either in the untreated control or in the animals treated.
Furthermore, perinatal mortality was slightly increased at 400 mg/kg
bw/day compared to controls. This finding was due to an increased
number of dead pups on the day of birth whereas the survival index
of the pups sacrificed 7 days after birth remained unaffected
(Hellwig & Hildebrand, 1987b).
In addition to the in vivo studies, an in vitro study was
carried out with rat embryos (whole embryo culture technique).
Cycloxydim (acid) and the main metabolite (TSO) were investigated in
an experiment in which embryos of Wistar rats which were 9.5 days
old were incubated in the culture medium with the test substance or
solvent control (DMSO) for 48 h. The concentrations were 300 œg/ml
for cycloxydim and 150 œg/ml for the TSO metabolite. Concentrations
were based on results of in vivo teratogenicity studies in the rat
and on investigations into the kinetics and metabolism in the same
animal species. After incubation, the embryos were evaluated under a
stereo-microscope and subsequently examined histopathologically for
possible substance-induced changes. No abnormal morphological
development was induced either by cycloxydim or by its TSO
metabolite in the embryos under the test conditions chosen. These
results clearly demonstrate that a direct embryotoxic effect was not
seen in vitro at this very sensitive development stage of the main
organs (Neubert et al., 1987)
Rabbits
Groups of 14 or 15 pregnant Himalayan rabbits were administered
cycloxydim as the sodium salt (purity 93.9%) by daily gavage from
days 6 to 18 after artificial insemination at doses of 0, 100, 200
or 400 mg/kg bw/day. On day 29 of gestation, the dams were
sacrificed and the fetuses were examined in detail after caesarean
section. At a dose of 400 mg/kg bw/day, the dams showed a
retardation of body-weight gain together with reduced food
consumption primarily during the treatment period compared with the
untreated control group. This finding was also observed after
administration of 200 mg/kg bw/day although it was less pronounced.
No signs of maternal toxicity were observed at 100 mg/kg bw/day.
There were no indications of an embryotoxic effect of the test
substance in the fetuses, except at 400 mg/kg bw/day, where there
was a significant reduction in percentage live fetuses and an
increase in percentage dead implants. The number of implantations
and resorptions, the conception rate and the implantation loss were
otherwise unaffected by treatment. Minor inter-group differences
remained within the historical control range. The variations,
anomalies and retardations that occurred were in the range of
historical control data for all test groups (Merkle & Hildebrand,
1985).
In addition to routine X-ray examination, the skeletons of the
control and highest dose group were additionally stained to look for
abnormalities of the vertebrae. These investigations did not
indicate any substance-induced changes of the vertebrae, as had
occurred in rats at a similar dose level (Hellwig, 1986).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
cycloxydim. The results are summarised in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on skin and eye irritation and sensitization
The skin irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). About 0.5 ml of undiluted
test substance was applied to the clipped, intact dorsal skin of 3
animals per sex for 4 h under a semi-occlusive dressing. Very slight
erythema in 4/6 animals, within 1 hour of patch removal, which was
completely reversible within 2 days, was the only finding that was
obtained (Kirsch & Kieczka, 1984c).
The eye irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). 0.1 Ml of the undiluted test
substance was applied to the eyes of 3 male and 3 female animals.
Slight conjunctival irritation (redness with lacrimation), which was
seen in all animals one hour after treatment, and which was
completely reversible within 8 days, was the only finding that was
observed. Neither cornea nor iris showed any changes related to the
test substance administered (Kirsch & Kieczka, 1984d).
The sensitizing potential of the active ingredient was
investigated in guinea-pigs (Pirbright White) in a maximization test
in which 10 animals were used in each of the two control groups and
20 animals in the test group. The two challenges carried out after
intradermal induction with the test substance did not reveal any
skin changes in the animals of the test group indicating that there
was no sensitizing potential of cycloxydim under the test conditions
chosen (Kirsch & Kieczka, 1985c).
Observations in humans
No information was available.
Table 2: Results of in vitro genotoxicity assays on cycloxydim
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium 20-15000 µg/plate 93.9% Negative Gelbke & Engelhardt (1985a)
TA98,TA100, (Na salt)
TA1535,TA1537
Ames test S. typhimurium 20-5000 µg/plate NK Negative Gelbke & Engelhardt (1984)
TA98, TA100, (acid)
TA1535, TA1537,
TA 1538
Lymphoma forward Mouse L5178Y 1.75-20 µg/ml 93.9% Weak, positive den Boer & Hoorn (1985a)
mutation assay cells (Na salt) at cytotoxic
concentrations
HGPRT forward CHO cells 5-40 mg/ml 93.9% Negative Gelbke & Engelhardt (1986)
mutation assay (Na salt)
HGPRT forward CHO cells 0.215-21.5 mg/ml 93.9% Negative den Boer & Hoorn (1985b)
mutation assay (Na salt)
Cytogenetics CHO cells 500-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985a)
mutation assay (acid) in absence of
activation
Cytogenetics CHO cells 2000-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985b)
mutation assay (Na salt) in absence of
activation
UDS Rat hepatocytes 100-2000 µg/ml NK Negative Cifone & Myhr (1985)
(acid)
UDS Rat hepatocytes 0.9-90.6 µg/ml NK Negative Cifone & Brusick (1985)
(Na salt)
NK: not known
Table 3: Results of in vivo genotoxicity assays on cycloxydim
Test system Test object Dose Purity Results Reference
Micronucleus NMRI mice 225, 450, 900 93.9% Negative Gelbke & Engelhardt (1985b)
test mg/kg bw
Micronucleus Chinese 500, 1700, 5000 NK Negative Taalman & Hoorn (1987)
test hamsters mg/kg bw
NK: not known
COMMENTS
Cycloxydim was extensively absorbed after oral administration
to rats and almost completely excreted within 5 days of dosing.
Elimination proceeded predominantly via the urine (74-86% of applied
dose), with lower levels in the faeces (12-25% of the applied dose).
Less than 1% of an administered dose was retained in the body.
Studies confirmed that enterohepatic circulation occurred in rats.
The metabolism of cycloxydim has been investigated in rats and
a biotransformation pathway has been proposed. The major metabolite
was the sulfoxide, and the pattern of metabolites was similar in
urine, bile and tissue residues.
Cycloxydim has low acute oral toxicity. WHO has classified
cycloxydim as unlikely to present acute hazard in normal use (WHO,
1992).
Owing to instability in dietary admixture the sodium salt of
cycloxydim was administered in the drinking-water in repeat-dose
rodent studies. In dogs, sufficient dietary stability was
demonstrated, and dietary administration of cycloxydim was therefore
used.
In mice, two 4-week dose range-finding studies were conducted,
followed by a 24-month long-term study. In the dose range-finding
studies, employing concentrations between 30 and 9000 ppm, reduced
food and water intake, and reduced body-weight gain were seen at
higher doses, together with increased liver weight and hydropic
vacuolar hepatocyte degeneration. The NOAEL was equal to 7 mg/kg
bw/day in males and 28 mg/kg bw/day in females, the lower NOAEL in
males resulting from liver weight increase in the absence of any
histopatho-logical change. In the long-term study, no treatment-
related effects were seen, up to the highest dose tested of 32 mg/kg
bw/day, although liver weights were not measured.
In rats a 4-week dose range-finding study was followed by a 13-
week study, an 18-month toxicity study and a 24-month
carcinogenicity study. Findings were generally similar to those
observed in mice, the liver being identified as the only target
organ of note, although no histopathological changes were seen at
doses up to 900 mg/kg bw/day. The NOAELs were 100 mg/kg bw/day over
4 weeks, 25 mg/kg bw/day over 13 weeks and 7 mg/kg bw/day over 18/24
months, based on reduced body-weight gain at 25 mg/kg bw/day and
above.
Cycloxydim was not carcinogenic in rats or mice.
In dogs, a 4-week dose range-finding study was followed by a
13-week and a 12-month study. In the dose range-finding study (at
doses up to 360 mg/kg bw/day) the liver and the red blood cells were
identified as target organs and the NOAEL was 40 mg/kg bw/day. In
the longer-term dog studies, the NOAELs were 50 mg/kg bw/day over 13
weeks and 20 mg/kg bw/day over 52 weeks. Target organs were the
liver and red blood cells. A marginal anaemia was seen, along with a
compensatory bone marrow response, but no serious treatment-related
histopathological effects were noted at 80 or 300 mg/kg bw/day.
In a multi-generation reproduction study in rats, the NOAELs
were about 10 mg/kg bw/day for parental toxicity and 38 mg/kg bw/day
for reproductive performance, pup mortality being slightly increased
at 150 mg/kg bw/day.
Teratogenicity studies have been carried out with cycloxydim in
rats and rabbits. In the rat teratogenicity studies the NOAEL for
maternal toxicity was 200 mg/kg bw/day. Fetotoxicity was seen at
maternally toxic doses, together with findings which may be
considered indicative of a teratogenic potential (missing/incomplete
tail and anal atresia at 600 and 800 mg/kg bw/day and vertebral
anomalies at 400 mg/kg bw/day). Although the vertebral anomalies
were not completely reversible post-natally (up to 21 days after
birth), in vitro embryo culture studies demonstrated that
cycloxydim did not show any direct embryotoxic effects. In the
rabbit teratogenicity study the NOAEL for maternal toxicity was 100
mg/kg bw/day. There was no indication of fetotoxicity in rabbits,
even in the presence of maternal toxicity and no indication of any
treatment-related vertebral anomalies, even when a special
examination was conducted to look specifically for such changes.
After reviewing the available genotoxicity data, the Meeting
concluded that cycloxydim and its sodium salt were not genotoxic.
An ADI was allocated based upon the NOAEL from the long-term
rat study in rats, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: > 240 ppm in the drinking water, equal to > 32
mg/kg bw/day (two-year study)
Rat: 100 ppm in the drinking-water, equal to 7 mg/kg
bw/day (18- and 24-month studies)
100 ppm in the drinking-water, equal to 10 mg/kg
bw/day (multi-generation study)
200 mg/kg bw/day (teratogenicity study, maternal and
fetal toxicity)
Dog: 400 ppm in the diet, equal to 20 mg/kg bw/day (one-
year study)
Rabbit: 100 mg/kg bw/day (teratogenicity study, maternal
toxicity).
Estimate of acceptable daily intake for humans:
0-0.07 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
den Boer, W.C. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (Na salt) in the mouse lymphoma forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
den Boer, W.C. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in the CHO HGPRT forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Reg. No. 172 999 in
the in vitro primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics (Hazleton), Kensington,
USA, submitted to WHO by BASF AG.
Cifone, M.A. & Brusick, D.J. (1985) Evaluation of BAS 517 H Na salt
in the in vitro rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished report from Litton Bionetics (Hazleton),
Kensington, USA, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1984) Study of Reg. No. 172 999 in
the Ames test (standard plate test with Salmonella typhimurium).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Gelbke, H.P. & Engelhardt, G. (1985a) Study of Reg. No. 172 999 Na
salt in the Ames test (standard plate test with Salmonella
typhimurium). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1985b) Cytogenetic investigations in
NMRI mice after a single oral administration of Reg. No. 172 999, Na
salt - Micronucleus test. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1986) Point mutation test carried out
on CHO cells (HGPRT locus) with Reg. No. 172 999. Unpublished report
from BASF Aktiengesellschaft, Departmont of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hawkins, D.R., Kirkpatrick, D., Finn, C.M., Till, C.P., Dean, G.M.,
Biggs, S.R. and & Whitby, B.R. (1986). The biokinetics and
metabolism of 14C-BAS 517 H in the rat. Unpublished report from
Huntingdon Research Centre Ltd., Huntingdon, England, submitted to
WHO by BASF AG.
Hellwig, J. (1986) Amendment to the determination of the prenatal
toxicity of Reg. No. 172 999 Na salt in rabbits after oral
administration (stomach tube). Unpublished report from BASF
Aktiengesellschaft. Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987a) Study of the prenatal toxicity
of Reg. No. 172 999 Na salt in rats after oral administration
(gavage). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987b) Study of the pre-, peri- and
postnatal toxicity of Reg. No. 172 999 Na salt in rats after oral
administration (gavage). Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology. D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1985)
Study of the toxicity of Reg. No. 172 999 Na salt in beagles after
4-week administration in the diet (range-finding study). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1986)
Study of Reg. No. 172 999 Na salt in beagles after 3-month
administration in the diet (range-finding study). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Gembardt, Chr. & Hildebrand B. (1987)
Study of the prenatal toxicity of Reg. No. 172 999 Na salt in rats
after oral administration (gavage) with special attention to
maternal toxicity. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1988a)
Study of the toxicity of Reg. No. 172 999 Na salt in purebred beagle
dogs: administration over 12 months via the diet. Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Freisberg, K.O., & Hildebrand, B. (1988b) Reproduction
study with Reg. No. 172 999 Na salt in rats: continuous
administration with the drinking water over 2 generations (2 litters
in the first and one litter in the second generation). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hildebrand, B. & Kirsch, P. (1987). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hoffmann, H.D., Gelbke, H.P., & Hildebrand, B. (1989) Study of the
dermal absorption of BAS 517H sodium salt in rats. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984a). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984b). Acute dermal toxicity of Reg. No.
172 999 in the rat based on OECD and EPA (FIFRA). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984c). Acute dermal
irritation/corrosivity of Reg. No. 172 999 to the intact dorsal skin
of the white rabbit based on OECD and EPA (FIFRA). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984d). Acute irritation of Reg. No. 172
999 to the eye of the white rabbit based on OECD and EPA (FIFRA).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kirsch, P. & Kieczka, H. (1985a). Acute oral toxicity on the mouse
based on OECD of Reg No. 172 999. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985b). Acute intraperitoneal toxicity of
Reg. No. 172 999 in the rat. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985c). Sensitizing effect of Reg. No. 172
999 in the guinea pig maximization test. Unpublished report from
BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Klimisch, H-J., Gelke, H-P., & Freisberg, K.O. (1985). Acute
inhalation toxicity, 4 hours (rat) liquid aerosol study of Reg. No.
172 999 tested as sodium salt. Unpublished report from BASF
Aktiengesellschift, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986a) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (1st range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986b) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (2nd range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986c) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 28-day administration with the drinking water (range-finding
study). Unpublished report from BASF Aktiengesellschaft, Department
of Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by
BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986d) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 3-month administration via the drinking water and a 6-week
observation period. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988a) Study of the toxicity of Reg. No. 172 999 Na
salt in rats: administration in the drinking water over 18 months.
Unpublished report from BASF Aktiengeselischaft, Department of
Toxicology. D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988b) Study on the toxicity of Reg. No. 172 999
Na-salt in rats: administration via the drinking water over 24
months. Unpublished report from BASF Aktiengeselischaft, Department
of Toxicology, D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1988c) Study on the toxicity of Reg. No. 172 999 Na-salt in mice:
administration in the drinking water over 24 months. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Merkle, J. & Hildebrand B. (1985) Pretnatal toxicity of Reg. No. 172
999 Na salt in rabbits after oral administration (stomach tube).
Unpublished report from BASF Aktiengesellchaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Neubert, D., Klug, S. & Merker, H-J. (1987) Effect of cycloxydim on
embryonic development in vitro. Unpublished report from Institute
for Toxicology and Embryopharmacology, Freie Universität, Berlin,
submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (acid) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1987) Cytogenetic investigations
in Chinese hamsters after a single oral administration of Reg. No.
172 999, Na salt - micronucleus test. Unpublished report from Litton
Bionetics (Hazleton), Veenendaal, Netherlands, submitted to WHO by
BASF AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
 Dogs
In a range-finding study, 2 beagle dogs per sex and dose were
given 0, 300, 1200, 3600 or 10 800 ppm as the sodium salt of
cycloxydim (purity 94.8%) in their feed for 4 weeks (equal to doses
of 0, 10, 40, 120 or 360 mg/kg bw/day). At the highest dose level,
the feed consumption of the females was occasionally reduced, while
body-weight remained unaffected. The plasma ALP activity and the
plasma cholinesterase activity were increased in the males. On
several occasions of the red blood count were marginally reduced.
The relative liver weight was increased compared with the untreated
control and histopathological examination showed enlargement of
hepatocytes. The findings were less pronounced at a dose level of
120 mg/kg bw/day. The only treatment-related findings at this dose
were increased plasma ALP in the males and a tendency to an
increased relative liver weight. The NOAEL in this study was 40
mg/kg bw/day (Hellwig et al., 1985).
Groups of 4 beagle dogs/sex/dose were used for a subchronic
feeding study in dogs over 13 weeks using doses of 0, 60, 300, 1500
or 7500 ppm as the sodium salt (purity 94.8%). No findings related
to the test substance administered were obtained at the 3 lowest
dose levels but toxicity was pronounced at 7500 ppm. However, there
were no clinical changes and body-weight gain remained unaffected by
treatment. The clinical chemistry examination revealed increased
serum activities of ALP and a reduced albumin concentration. The
females had increased globulin values in the serum, while the sodium
level in the blood was reduced in the males. Among the haemato-
logical changes, the reduced erythrocyte counts and presence of
Heinz bodies were of note. However, the number of reticulocytes and
platelets was increased suggesting a compensatory reaction in the
bone marrow. In the males, the MCH and MCV were also increased. Of
the organ weights, the absolute and relative liver weights were
above those of the untreated control in both sexes. The bile was
reddish and histopathological examination revealed an enlargement of
hepatocytes. The NOAEL was 1500 ppm, equal to 50 mg/kg bw/day
(Hellwig et al., 1986).
In a 12-month feeding study, groups of 6 beagle dogs/sex were
given doses of 0, 400, 1600 or 6400 ppm cycloxydim (acid) as sodium
salt (purity 93.9%). The lowest dose of 400 ppm did not lead to any
substance-induced changes. At 1600 ppm, the number of Heinz bodies
was increased in both sexes, indicating a disturbance in the
haemoglobin metabolism. Male animals showed an increased activity of
ALP in plasma and a lowering of the albumin level. The absolute and
relative liver weights of these animals were significantly
increased, but no corresponding histopathological changes were
observed. This finding was also detected in both sexes of the
highest dose group and some further clinical chemistry parameters
were also changed. There was an increase in plasma ALP and a
lowering of the albumin and protein concentrations (in the males
only). The haematological examinations showed signs of slight
anaemia with a compensatory bone marrow reaction. These findings
were manifest in the females in the form of haemosiderosis of
Kupffer's cells in the liver as a histopathological correlation. The
NOAEL was 400 ppm in the diet, equal to 20 mg/kg bw/day (Hellwig et
al., 1988a).
Long-term toxicity/carcinogenicity studies
Mice
In a 24-month study in B6C3F1 mice, cycloxydim was
administered as the sodium salt (purity 93.9%) in the drinking water
at doses of 0, 10, 20, 60 or 240 ppm to groups of 50 mice/sex
(controls 100 mice/sex). There were no clinical findings which were
induced by the test substance. Body-weight gain, food and water
intake and the incidence of mortality remained unaffected by
treatment. The histopathological examination at the end of the study
did not reveal any changes related to the test substance. The
tumours that occurred corresponded to those of the spontaneous range
of the animal strain used so that no carcinogenic potential of the
test substance was apparent, up to a dose level of 240 ppm, equal to
32 mg/kg bw/day (Kuhborth et al., 1988c).
Rats
In a study to determine chronic toxicity, groups of 20 Wistar
rats per sex/dose were administered 0, 100, 400, 1600 or 2700 ppm
of cycloxydim as sodium salt (purity 93.9%) via the drinking water
for 18 months. At the highest dose of 2700 ppm, reduced food and
drinking water consumption were observed in both sexes together with
a decrease in body-weight gain of up to 21% compared with the
control during the major part of the study period. Doses of 400 and
1600 ppm also led to reduced body-weight gain compared with the
control, but drinking water consumption and feed consumption (males
only) were reduced only at 1600 ppm. In the clinical chemistry
examinations, a lowering of the triglyceride level was observed at
doses of 400 ppm and above. The organ weight determinations and the
gross pathological and histopathological examination did not reveal
any treatment-related findings. Therefore, the NOAEL in this study
was 100 ppm, equal to 7 mg/kg bw/day (Kuhborth et al., 1988a).
In a second study carried out simultaneously to clarify
possible carcinogenicity, groups of 50 Wistar rats/sex were given
cycloxydim as the sodium salt (purity 93.9%) in the drinking water
for 24 months at doses of 0, 100, 400 or 1600 ppm. As in the 18-
month study, a reduction in the drinking water consumption and body-
weight by up to 18% was observed in both sexes at 1600 ppm, but feed
consumption was not impaired in this study. The clinical chemistry
examinations revealed a lowering of the triglyceride level in the
females at 1600 ppm. The body-weight reduction (both sexes) and
lowering of the triglyceride level (females) were also observed at a
dose level of 400 ppm. The determination of organ weights at the end
of the study showed a lowering of the absolute and relative liver
weights at the high-dose, but there was no corresponding
morphological change. No findings related to the test substance
administered were obtained in the gross pathological or
histopathological examinations. There was no indication of any
disturbance of the spontaneous tumour profile of the rat strain
used. Under the given test conditions, cycloxydim has no
carcinogenic potential in Wistar rats. The NOAEL with regard to
clinical or clinical chemistry parameters and organ weight changes
was 100 ppm, the same as that of the 18-month toxicity study
(Kuhborth et al., 1988b).
Reproduction study
Rats
Investigations of reproductive toxicity were carried out in
Wistar rats in a multi-generation study in which 2 litters were used
in the first generation and 1 litter in the second generation.
Groups of 24 animals per sex were adminis-tered cycloxydim as the
sodium salt (purity 93.9%) via the drinking water at doses of 0,
100, 400 or 1600 ppm (expressed as acid) for a period of 70 days
prior to mating and then throughout gestation and lactation.
The highest dose of 1600 ppm, equal to 150 mg/kg bw/day caused
systemic toxicity in the parental animals (reduced food and drinking
water consumption and retarded body-weight gain). The number of all
pups (live and stillborn) at parturition was slightly reduced and
the development of the pups was retarded. Pup mortality was also
increased at this dose level. At the mid-dose (400 ppm), there were
only indications of systemic toxicity in the parental animals in the
form of reduced food and drinking water consumption as well as
temporary retardation of body-weight gain. There was no effect on
the number of pups or on pup mortality at this dose. Accordingly,
the NOAEL was 100 ppm, equal to 10 mg/kg bw/day for parental
toxicity and 400 ppm, equal to 38 mg/kg bw/day for reproductive
performance (Hellwig et al., 1988b).
Special studies on embryo/fetotoxicity
Rats
Cycloxydim as the sodium salt (purity 93.9%) was administered
to 23 or 24 pregnant rats per test group by gavage from days 6 to 15
after mating at doses of 0, 100, 200 or 400 mg/kg bw/day. On day 20
of gestation the dams were sacrificed, and the fetuses were examined
after caesarean section. At 100 and 200 mg/kg bw/day no signs of
maternal toxicity were observed but body-weight gain and food
consumption were reduced at the highest dose level at the beginning
of test substance administration compared with the untreated
control. The number of implant-ations and resorptions and the
fertility rate were similar to those of the control at every dose
level. Fetotoxic effects were observed in the range of maternal
toxicity; they were characterized by reduced fetal body-weight and
retardation of ossification of the skeletons of the fetuses.
Furthermore, an increased incidence of vertebral column changes in
the fetuses, which were classified as anomalies, was detected at the
highest dose level. These were mainly changes in thoracic vertebrae,
which had a dumbell-shaped or bipartite appearance. One out of 309
fetuses at 200 mg/kg bw/day and 1/295 fetuses at 400 mg/kg bw/day
had anal/tail defects (Hellwig & Hildebrand, 1987a).
In a second study, maternal toxicity after administration of
the sodium salt (purity 93.9%) was investigated in more detail.
Groups of 25 female Wistar rats were administered cycloxydim at
doses of 0, 200, 400, 600 or 800 mg/kg bw/day by gavage from days 6
to 15 of gestation. The dams were sacrificed on day 20 of gestation.
After caesarean section the fetuses were evaluated only to a limited
extent (weight and sex determinations and gross-pathological
examination) since the main aim of the study was to determine the
exact maternal toxicity threshold. Pronounced maternal toxicity
which, inter alia, was manifest in the form of clearly retarded
body-weight gain together with reduced food consumption was observed
at a dose level of 800 mg/kg bw/day. The general state of health was
poor, 5 dams showing vaginal haemorrhages. RBC parameters
(haemoglobin content, erythrocyte count, haematocrit) were reduced,
and the number of reticulocytes was increased compared with the
untreated control indicating a compensatory reaction of the bone
marrow. These findings were also obtained at 600 and 400 mg/kg
bw/day although they were less pronounced. The NOAEL regarding
maternal toxicity was 200 mg/kg bw/day, confirming the result of the
first study. Fetal weight was reduced at 600 and 800 mg/kg bw/day
and the gross-pathological examination revealed missing or
incomplete tail in some cases together with anal atresia in 3 of 270
fetuses (in 3 out of 22 litters) at 800 mg/kg bw/day and in 2 of 296
fetuses (in 2 out of 24 litters) at 600 mg/kg bw/day (Hellwig et
al., 1987).
A third study in Wistar rats was carried out to investigate
whether the changes in the vertebrae which occurred in the first
prenatal study also persisted postnatally. Two groups, each of 60
pregnant females, were given cycloxydim (as the sodium salt, purity
93.9%) by daily oral gavage, between days 6 and 15 of pregnancy at
doses of 0 or 400 mg/kg bw/day. Twenty-five dams from each group
were sacrificed on day 20 of pregnancy and their fetuses were
examined after caesarean section. The remaining dams were allowed to
litter and rear their pups. The litters of a further 10 dams from
each group were sacrificed on day 7 post-partum and all other
litters were sacrificed on day 21 after parturition and examined in
detail. In the dams sacrificed on day 20 of gestation there were
indications of the beginning of maternal toxicity characterised by
retarded body-weight gain and reduced feed consumption. The weight
of the fetuses was reduced. Four fetuses (out of 286) from 3/22
litters had tail defects. The incidence of retarded ossification of
the skeletons was clearly higher compared with the untreated
control. As in the first prenatal toxicity study dumbell-shaped or
bipartite thoracic vertebrae occurred to an increased extent
compared with the control. The incidence of the changes in the
thoracic vertebrae of the pups sacrificed 7 or 21 days after birth
was clearly reduced compared with the prenatal investigations
although these findings, which also occur spontaneously without
relation to the test substance, were not completely reversible
either in the untreated control or in the animals treated.
Furthermore, perinatal mortality was slightly increased at 400 mg/kg
bw/day compared to controls. This finding was due to an increased
number of dead pups on the day of birth whereas the survival index
of the pups sacrificed 7 days after birth remained unaffected
(Hellwig & Hildebrand, 1987b).
In addition to the in vivo studies, an in vitro study was
carried out with rat embryos (whole embryo culture technique).
Cycloxydim (acid) and the main metabolite (TSO) were investigated in
an experiment in which embryos of Wistar rats which were 9.5 days
old were incubated in the culture medium with the test substance or
solvent control (DMSO) for 48 h. The concentrations were 300 œg/ml
for cycloxydim and 150 œg/ml for the TSO metabolite. Concentrations
were based on results of in vivo teratogenicity studies in the rat
and on investigations into the kinetics and metabolism in the same
animal species. After incubation, the embryos were evaluated under a
stereo-microscope and subsequently examined histopathologically for
possible substance-induced changes. No abnormal morphological
development was induced either by cycloxydim or by its TSO
metabolite in the embryos under the test conditions chosen. These
results clearly demonstrate that a direct embryotoxic effect was not
seen in vitro at this very sensitive development stage of the main
organs (Neubert et al., 1987)
Rabbits
Groups of 14 or 15 pregnant Himalayan rabbits were administered
cycloxydim as the sodium salt (purity 93.9%) by daily gavage from
days 6 to 18 after artificial insemination at doses of 0, 100, 200
or 400 mg/kg bw/day. On day 29 of gestation, the dams were
sacrificed and the fetuses were examined in detail after caesarean
section. At a dose of 400 mg/kg bw/day, the dams showed a
retardation of body-weight gain together with reduced food
consumption primarily during the treatment period compared with the
untreated control group. This finding was also observed after
administration of 200 mg/kg bw/day although it was less pronounced.
No signs of maternal toxicity were observed at 100 mg/kg bw/day.
There were no indications of an embryotoxic effect of the test
substance in the fetuses, except at 400 mg/kg bw/day, where there
was a significant reduction in percentage live fetuses and an
increase in percentage dead implants. The number of implantations
and resorptions, the conception rate and the implantation loss were
otherwise unaffected by treatment. Minor inter-group differences
remained within the historical control range. The variations,
anomalies and retardations that occurred were in the range of
historical control data for all test groups (Merkle & Hildebrand,
1985).
In addition to routine X-ray examination, the skeletons of the
control and highest dose group were additionally stained to look for
abnormalities of the vertebrae. These investigations did not
indicate any substance-induced changes of the vertebrae, as had
occurred in rats at a similar dose level (Hellwig, 1986).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
cycloxydim. The results are summarised in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on skin and eye irritation and sensitization
The skin irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). About 0.5 ml of undiluted
test substance was applied to the clipped, intact dorsal skin of 3
animals per sex for 4 h under a semi-occlusive dressing. Very slight
erythema in 4/6 animals, within 1 hour of patch removal, which was
completely reversible within 2 days, was the only finding that was
obtained (Kirsch & Kieczka, 1984c).
The eye irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). 0.1 Ml of the undiluted test
substance was applied to the eyes of 3 male and 3 female animals.
Slight conjunctival irritation (redness with lacrimation), which was
seen in all animals one hour after treatment, and which was
completely reversible within 8 days, was the only finding that was
observed. Neither cornea nor iris showed any changes related to the
test substance administered (Kirsch & Kieczka, 1984d).
The sensitizing potential of the active ingredient was
investigated in guinea-pigs (Pirbright White) in a maximization test
in which 10 animals were used in each of the two control groups and
20 animals in the test group. The two challenges carried out after
intradermal induction with the test substance did not reveal any
skin changes in the animals of the test group indicating that there
was no sensitizing potential of cycloxydim under the test conditions
chosen (Kirsch & Kieczka, 1985c).
Observations in humans
No information was available.
Table 2: Results of in vitro genotoxicity assays on cycloxydim
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium 20-15000 µg/plate 93.9% Negative Gelbke & Engelhardt (1985a)
TA98,TA100, (Na salt)
TA1535,TA1537
Ames test S. typhimurium 20-5000 µg/plate NK Negative Gelbke & Engelhardt (1984)
TA98, TA100, (acid)
TA1535, TA1537,
TA 1538
Lymphoma forward Mouse L5178Y 1.75-20 µg/ml 93.9% Weak, positive den Boer & Hoorn (1985a)
mutation assay cells (Na salt) at cytotoxic
concentrations
HGPRT forward CHO cells 5-40 mg/ml 93.9% Negative Gelbke & Engelhardt (1986)
mutation assay (Na salt)
HGPRT forward CHO cells 0.215-21.5 mg/ml 93.9% Negative den Boer & Hoorn (1985b)
mutation assay (Na salt)
Cytogenetics CHO cells 500-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985a)
mutation assay (acid) in absence of
activation
Cytogenetics CHO cells 2000-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985b)
mutation assay (Na salt) in absence of
activation
UDS Rat hepatocytes 100-2000 µg/ml NK Negative Cifone & Myhr (1985)
(acid)
UDS Rat hepatocytes 0.9-90.6 µg/ml NK Negative Cifone & Brusick (1985)
(Na salt)
NK: not known
Table 3: Results of in vivo genotoxicity assays on cycloxydim
Test system Test object Dose Purity Results Reference
Micronucleus NMRI mice 225, 450, 900 93.9% Negative Gelbke & Engelhardt (1985b)
test mg/kg bw
Micronucleus Chinese 500, 1700, 5000 NK Negative Taalman & Hoorn (1987)
test hamsters mg/kg bw
NK: not known
COMMENTS
Cycloxydim was extensively absorbed after oral administration
to rats and almost completely excreted within 5 days of dosing.
Elimination proceeded predominantly via the urine (74-86% of applied
dose), with lower levels in the faeces (12-25% of the applied dose).
Less than 1% of an administered dose was retained in the body.
Studies confirmed that enterohepatic circulation occurred in rats.
The metabolism of cycloxydim has been investigated in rats and
a biotransformation pathway has been proposed. The major metabolite
was the sulfoxide, and the pattern of metabolites was similar in
urine, bile and tissue residues.
Cycloxydim has low acute oral toxicity. WHO has classified
cycloxydim as unlikely to present acute hazard in normal use (WHO,
1992).
Owing to instability in dietary admixture the sodium salt of
cycloxydim was administered in the drinking-water in repeat-dose
rodent studies. In dogs, sufficient dietary stability was
demonstrated, and dietary administration of cycloxydim was therefore
used.
In mice, two 4-week dose range-finding studies were conducted,
followed by a 24-month long-term study. In the dose range-finding
studies, employing concentrations between 30 and 9000 ppm, reduced
food and water intake, and reduced body-weight gain were seen at
higher doses, together with increased liver weight and hydropic
vacuolar hepatocyte degeneration. The NOAEL was equal to 7 mg/kg
bw/day in males and 28 mg/kg bw/day in females, the lower NOAEL in
males resulting from liver weight increase in the absence of any
histopatho-logical change. In the long-term study, no treatment-
related effects were seen, up to the highest dose tested of 32 mg/kg
bw/day, although liver weights were not measured.
In rats a 4-week dose range-finding study was followed by a 13-
week study, an 18-month toxicity study and a 24-month
carcinogenicity study. Findings were generally similar to those
observed in mice, the liver being identified as the only target
organ of note, although no histopathological changes were seen at
doses up to 900 mg/kg bw/day. The NOAELs were 100 mg/kg bw/day over
4 weeks, 25 mg/kg bw/day over 13 weeks and 7 mg/kg bw/day over 18/24
months, based on reduced body-weight gain at 25 mg/kg bw/day and
above.
Cycloxydim was not carcinogenic in rats or mice.
In dogs, a 4-week dose range-finding study was followed by a
13-week and a 12-month study. In the dose range-finding study (at
doses up to 360 mg/kg bw/day) the liver and the red blood cells were
identified as target organs and the NOAEL was 40 mg/kg bw/day. In
the longer-term dog studies, the NOAELs were 50 mg/kg bw/day over 13
weeks and 20 mg/kg bw/day over 52 weeks. Target organs were the
liver and red blood cells. A marginal anaemia was seen, along with a
compensatory bone marrow response, but no serious treatment-related
histopathological effects were noted at 80 or 300 mg/kg bw/day.
In a multi-generation reproduction study in rats, the NOAELs
were about 10 mg/kg bw/day for parental toxicity and 38 mg/kg bw/day
for reproductive performance, pup mortality being slightly increased
at 150 mg/kg bw/day.
Teratogenicity studies have been carried out with cycloxydim in
rats and rabbits. In the rat teratogenicity studies the NOAEL for
maternal toxicity was 200 mg/kg bw/day. Fetotoxicity was seen at
maternally toxic doses, together with findings which may be
considered indicative of a teratogenic potential (missing/incomplete
tail and anal atresia at 600 and 800 mg/kg bw/day and vertebral
anomalies at 400 mg/kg bw/day). Although the vertebral anomalies
were not completely reversible post-natally (up to 21 days after
birth), in vitro embryo culture studies demonstrated that
cycloxydim did not show any direct embryotoxic effects. In the
rabbit teratogenicity study the NOAEL for maternal toxicity was 100
mg/kg bw/day. There was no indication of fetotoxicity in rabbits,
even in the presence of maternal toxicity and no indication of any
treatment-related vertebral anomalies, even when a special
examination was conducted to look specifically for such changes.
After reviewing the available genotoxicity data, the Meeting
concluded that cycloxydim and its sodium salt were not genotoxic.
An ADI was allocated based upon the NOAEL from the long-term
rat study in rats, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: > 240 ppm in the drinking water, equal to > 32
mg/kg bw/day (two-year study)
Rat: 100 ppm in the drinking-water, equal to 7 mg/kg
bw/day (18- and 24-month studies)
100 ppm in the drinking-water, equal to 10 mg/kg
bw/day (multi-generation study)
200 mg/kg bw/day (teratogenicity study, maternal and
fetal toxicity)
Dog: 400 ppm in the diet, equal to 20 mg/kg bw/day (one-
year study)
Rabbit: 100 mg/kg bw/day (teratogenicity study, maternal
toxicity).
Estimate of acceptable daily intake for humans:
0-0.07 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
den Boer, W.C. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (Na salt) in the mouse lymphoma forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
den Boer, W.C. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in the CHO HGPRT forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Reg. No. 172 999 in
the in vitro primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics (Hazleton), Kensington,
USA, submitted to WHO by BASF AG.
Cifone, M.A. & Brusick, D.J. (1985) Evaluation of BAS 517 H Na salt
in the in vitro rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished report from Litton Bionetics (Hazleton),
Kensington, USA, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1984) Study of Reg. No. 172 999 in
the Ames test (standard plate test with Salmonella typhimurium).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Gelbke, H.P. & Engelhardt, G. (1985a) Study of Reg. No. 172 999 Na
salt in the Ames test (standard plate test with Salmonella
typhimurium). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1985b) Cytogenetic investigations in
NMRI mice after a single oral administration of Reg. No. 172 999, Na
salt - Micronucleus test. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1986) Point mutation test carried out
on CHO cells (HGPRT locus) with Reg. No. 172 999. Unpublished report
from BASF Aktiengesellschaft, Departmont of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hawkins, D.R., Kirkpatrick, D., Finn, C.M., Till, C.P., Dean, G.M.,
Biggs, S.R. and & Whitby, B.R. (1986). The biokinetics and
metabolism of 14C-BAS 517 H in the rat. Unpublished report from
Huntingdon Research Centre Ltd., Huntingdon, England, submitted to
WHO by BASF AG.
Hellwig, J. (1986) Amendment to the determination of the prenatal
toxicity of Reg. No. 172 999 Na salt in rabbits after oral
administration (stomach tube). Unpublished report from BASF
Aktiengesellschaft. Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987a) Study of the prenatal toxicity
of Reg. No. 172 999 Na salt in rats after oral administration
(gavage). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987b) Study of the pre-, peri- and
postnatal toxicity of Reg. No. 172 999 Na salt in rats after oral
administration (gavage). Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology. D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1985)
Study of the toxicity of Reg. No. 172 999 Na salt in beagles after
4-week administration in the diet (range-finding study). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1986)
Study of Reg. No. 172 999 Na salt in beagles after 3-month
administration in the diet (range-finding study). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Gembardt, Chr. & Hildebrand B. (1987)
Study of the prenatal toxicity of Reg. No. 172 999 Na salt in rats
after oral administration (gavage) with special attention to
maternal toxicity. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1988a)
Study of the toxicity of Reg. No. 172 999 Na salt in purebred beagle
dogs: administration over 12 months via the diet. Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Freisberg, K.O., & Hildebrand, B. (1988b) Reproduction
study with Reg. No. 172 999 Na salt in rats: continuous
administration with the drinking water over 2 generations (2 litters
in the first and one litter in the second generation). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hildebrand, B. & Kirsch, P. (1987). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hoffmann, H.D., Gelbke, H.P., & Hildebrand, B. (1989) Study of the
dermal absorption of BAS 517H sodium salt in rats. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984a). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984b). Acute dermal toxicity of Reg. No.
172 999 in the rat based on OECD and EPA (FIFRA). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984c). Acute dermal
irritation/corrosivity of Reg. No. 172 999 to the intact dorsal skin
of the white rabbit based on OECD and EPA (FIFRA). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984d). Acute irritation of Reg. No. 172
999 to the eye of the white rabbit based on OECD and EPA (FIFRA).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kirsch, P. & Kieczka, H. (1985a). Acute oral toxicity on the mouse
based on OECD of Reg No. 172 999. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985b). Acute intraperitoneal toxicity of
Reg. No. 172 999 in the rat. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985c). Sensitizing effect of Reg. No. 172
999 in the guinea pig maximization test. Unpublished report from
BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Klimisch, H-J., Gelke, H-P., & Freisberg, K.O. (1985). Acute
inhalation toxicity, 4 hours (rat) liquid aerosol study of Reg. No.
172 999 tested as sodium salt. Unpublished report from BASF
Aktiengesellschift, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986a) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (1st range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986b) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (2nd range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986c) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 28-day administration with the drinking water (range-finding
study). Unpublished report from BASF Aktiengesellschaft, Department
of Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by
BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986d) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 3-month administration via the drinking water and a 6-week
observation period. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988a) Study of the toxicity of Reg. No. 172 999 Na
salt in rats: administration in the drinking water over 18 months.
Unpublished report from BASF Aktiengeselischaft, Department of
Toxicology. D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988b) Study on the toxicity of Reg. No. 172 999
Na-salt in rats: administration via the drinking water over 24
months. Unpublished report from BASF Aktiengeselischaft, Department
of Toxicology, D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1988c) Study on the toxicity of Reg. No. 172 999 Na-salt in mice:
administration in the drinking water over 24 months. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Merkle, J. & Hildebrand B. (1985) Pretnatal toxicity of Reg. No. 172
999 Na salt in rabbits after oral administration (stomach tube).
Unpublished report from BASF Aktiengesellchaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Neubert, D., Klug, S. & Merker, H-J. (1987) Effect of cycloxydim on
embryonic development in vitro. Unpublished report from Institute
for Toxicology and Embryopharmacology, Freie Universität, Berlin,
submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (acid) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1987) Cytogenetic investigations
in Chinese hamsters after a single oral administration of Reg. No.
172 999, Na salt - micronucleus test. Unpublished report from Litton
Bionetics (Hazleton), Veenendaal, Netherlands, submitted to WHO by
BASF AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
Dogs
In a range-finding study, 2 beagle dogs per sex and dose were
given 0, 300, 1200, 3600 or 10 800 ppm as the sodium salt of
cycloxydim (purity 94.8%) in their feed for 4 weeks (equal to doses
of 0, 10, 40, 120 or 360 mg/kg bw/day). At the highest dose level,
the feed consumption of the females was occasionally reduced, while
body-weight remained unaffected. The plasma ALP activity and the
plasma cholinesterase activity were increased in the males. On
several occasions of the red blood count were marginally reduced.
The relative liver weight was increased compared with the untreated
control and histopathological examination showed enlargement of
hepatocytes. The findings were less pronounced at a dose level of
120 mg/kg bw/day. The only treatment-related findings at this dose
were increased plasma ALP in the males and a tendency to an
increased relative liver weight. The NOAEL in this study was 40
mg/kg bw/day (Hellwig et al., 1985).
Groups of 4 beagle dogs/sex/dose were used for a subchronic
feeding study in dogs over 13 weeks using doses of 0, 60, 300, 1500
or 7500 ppm as the sodium salt (purity 94.8%). No findings related
to the test substance administered were obtained at the 3 lowest
dose levels but toxicity was pronounced at 7500 ppm. However, there
were no clinical changes and body-weight gain remained unaffected by
treatment. The clinical chemistry examination revealed increased
serum activities of ALP and a reduced albumin concentration. The
females had increased globulin values in the serum, while the sodium
level in the blood was reduced in the males. Among the haemato-
logical changes, the reduced erythrocyte counts and presence of
Heinz bodies were of note. However, the number of reticulocytes and
platelets was increased suggesting a compensatory reaction in the
bone marrow. In the males, the MCH and MCV were also increased. Of
the organ weights, the absolute and relative liver weights were
above those of the untreated control in both sexes. The bile was
reddish and histopathological examination revealed an enlargement of
hepatocytes. The NOAEL was 1500 ppm, equal to 50 mg/kg bw/day
(Hellwig et al., 1986).
In a 12-month feeding study, groups of 6 beagle dogs/sex were
given doses of 0, 400, 1600 or 6400 ppm cycloxydim (acid) as sodium
salt (purity 93.9%). The lowest dose of 400 ppm did not lead to any
substance-induced changes. At 1600 ppm, the number of Heinz bodies
was increased in both sexes, indicating a disturbance in the
haemoglobin metabolism. Male animals showed an increased activity of
ALP in plasma and a lowering of the albumin level. The absolute and
relative liver weights of these animals were significantly
increased, but no corresponding histopathological changes were
observed. This finding was also detected in both sexes of the
highest dose group and some further clinical chemistry parameters
were also changed. There was an increase in plasma ALP and a
lowering of the albumin and protein concentrations (in the males
only). The haematological examinations showed signs of slight
anaemia with a compensatory bone marrow reaction. These findings
were manifest in the females in the form of haemosiderosis of
Kupffer's cells in the liver as a histopathological correlation. The
NOAEL was 400 ppm in the diet, equal to 20 mg/kg bw/day (Hellwig et
al., 1988a).
Long-term toxicity/carcinogenicity studies
Mice
In a 24-month study in B6C3F1 mice, cycloxydim was
administered as the sodium salt (purity 93.9%) in the drinking water
at doses of 0, 10, 20, 60 or 240 ppm to groups of 50 mice/sex
(controls 100 mice/sex). There were no clinical findings which were
induced by the test substance. Body-weight gain, food and water
intake and the incidence of mortality remained unaffected by
treatment. The histopathological examination at the end of the study
did not reveal any changes related to the test substance. The
tumours that occurred corresponded to those of the spontaneous range
of the animal strain used so that no carcinogenic potential of the
test substance was apparent, up to a dose level of 240 ppm, equal to
32 mg/kg bw/day (Kuhborth et al., 1988c).
Rats
In a study to determine chronic toxicity, groups of 20 Wistar
rats per sex/dose were administered 0, 100, 400, 1600 or 2700 ppm
of cycloxydim as sodium salt (purity 93.9%) via the drinking water
for 18 months. At the highest dose of 2700 ppm, reduced food and
drinking water consumption were observed in both sexes together with
a decrease in body-weight gain of up to 21% compared with the
control during the major part of the study period. Doses of 400 and
1600 ppm also led to reduced body-weight gain compared with the
control, but drinking water consumption and feed consumption (males
only) were reduced only at 1600 ppm. In the clinical chemistry
examinations, a lowering of the triglyceride level was observed at
doses of 400 ppm and above. The organ weight determinations and the
gross pathological and histopathological examination did not reveal
any treatment-related findings. Therefore, the NOAEL in this study
was 100 ppm, equal to 7 mg/kg bw/day (Kuhborth et al., 1988a).
In a second study carried out simultaneously to clarify
possible carcinogenicity, groups of 50 Wistar rats/sex were given
cycloxydim as the sodium salt (purity 93.9%) in the drinking water
for 24 months at doses of 0, 100, 400 or 1600 ppm. As in the 18-
month study, a reduction in the drinking water consumption and body-
weight by up to 18% was observed in both sexes at 1600 ppm, but feed
consumption was not impaired in this study. The clinical chemistry
examinations revealed a lowering of the triglyceride level in the
females at 1600 ppm. The body-weight reduction (both sexes) and
lowering of the triglyceride level (females) were also observed at a
dose level of 400 ppm. The determination of organ weights at the end
of the study showed a lowering of the absolute and relative liver
weights at the high-dose, but there was no corresponding
morphological change. No findings related to the test substance
administered were obtained in the gross pathological or
histopathological examinations. There was no indication of any
disturbance of the spontaneous tumour profile of the rat strain
used. Under the given test conditions, cycloxydim has no
carcinogenic potential in Wistar rats. The NOAEL with regard to
clinical or clinical chemistry parameters and organ weight changes
was 100 ppm, the same as that of the 18-month toxicity study
(Kuhborth et al., 1988b).
Reproduction study
Rats
Investigations of reproductive toxicity were carried out in
Wistar rats in a multi-generation study in which 2 litters were used
in the first generation and 1 litter in the second generation.
Groups of 24 animals per sex were adminis-tered cycloxydim as the
sodium salt (purity 93.9%) via the drinking water at doses of 0,
100, 400 or 1600 ppm (expressed as acid) for a period of 70 days
prior to mating and then throughout gestation and lactation.
The highest dose of 1600 ppm, equal to 150 mg/kg bw/day caused
systemic toxicity in the parental animals (reduced food and drinking
water consumption and retarded body-weight gain). The number of all
pups (live and stillborn) at parturition was slightly reduced and
the development of the pups was retarded. Pup mortality was also
increased at this dose level. At the mid-dose (400 ppm), there were
only indications of systemic toxicity in the parental animals in the
form of reduced food and drinking water consumption as well as
temporary retardation of body-weight gain. There was no effect on
the number of pups or on pup mortality at this dose. Accordingly,
the NOAEL was 100 ppm, equal to 10 mg/kg bw/day for parental
toxicity and 400 ppm, equal to 38 mg/kg bw/day for reproductive
performance (Hellwig et al., 1988b).
Special studies on embryo/fetotoxicity
Rats
Cycloxydim as the sodium salt (purity 93.9%) was administered
to 23 or 24 pregnant rats per test group by gavage from days 6 to 15
after mating at doses of 0, 100, 200 or 400 mg/kg bw/day. On day 20
of gestation the dams were sacrificed, and the fetuses were examined
after caesarean section. At 100 and 200 mg/kg bw/day no signs of
maternal toxicity were observed but body-weight gain and food
consumption were reduced at the highest dose level at the beginning
of test substance administration compared with the untreated
control. The number of implant-ations and resorptions and the
fertility rate were similar to those of the control at every dose
level. Fetotoxic effects were observed in the range of maternal
toxicity; they were characterized by reduced fetal body-weight and
retardation of ossification of the skeletons of the fetuses.
Furthermore, an increased incidence of vertebral column changes in
the fetuses, which were classified as anomalies, was detected at the
highest dose level. These were mainly changes in thoracic vertebrae,
which had a dumbell-shaped or bipartite appearance. One out of 309
fetuses at 200 mg/kg bw/day and 1/295 fetuses at 400 mg/kg bw/day
had anal/tail defects (Hellwig & Hildebrand, 1987a).
In a second study, maternal toxicity after administration of
the sodium salt (purity 93.9%) was investigated in more detail.
Groups of 25 female Wistar rats were administered cycloxydim at
doses of 0, 200, 400, 600 or 800 mg/kg bw/day by gavage from days 6
to 15 of gestation. The dams were sacrificed on day 20 of gestation.
After caesarean section the fetuses were evaluated only to a limited
extent (weight and sex determinations and gross-pathological
examination) since the main aim of the study was to determine the
exact maternal toxicity threshold. Pronounced maternal toxicity
which, inter alia, was manifest in the form of clearly retarded
body-weight gain together with reduced food consumption was observed
at a dose level of 800 mg/kg bw/day. The general state of health was
poor, 5 dams showing vaginal haemorrhages. RBC parameters
(haemoglobin content, erythrocyte count, haematocrit) were reduced,
and the number of reticulocytes was increased compared with the
untreated control indicating a compensatory reaction of the bone
marrow. These findings were also obtained at 600 and 400 mg/kg
bw/day although they were less pronounced. The NOAEL regarding
maternal toxicity was 200 mg/kg bw/day, confirming the result of the
first study. Fetal weight was reduced at 600 and 800 mg/kg bw/day
and the gross-pathological examination revealed missing or
incomplete tail in some cases together with anal atresia in 3 of 270
fetuses (in 3 out of 22 litters) at 800 mg/kg bw/day and in 2 of 296
fetuses (in 2 out of 24 litters) at 600 mg/kg bw/day (Hellwig et
al., 1987).
A third study in Wistar rats was carried out to investigate
whether the changes in the vertebrae which occurred in the first
prenatal study also persisted postnatally. Two groups, each of 60
pregnant females, were given cycloxydim (as the sodium salt, purity
93.9%) by daily oral gavage, between days 6 and 15 of pregnancy at
doses of 0 or 400 mg/kg bw/day. Twenty-five dams from each group
were sacrificed on day 20 of pregnancy and their fetuses were
examined after caesarean section. The remaining dams were allowed to
litter and rear their pups. The litters of a further 10 dams from
each group were sacrificed on day 7 post-partum and all other
litters were sacrificed on day 21 after parturition and examined in
detail. In the dams sacrificed on day 20 of gestation there were
indications of the beginning of maternal toxicity characterised by
retarded body-weight gain and reduced feed consumption. The weight
of the fetuses was reduced. Four fetuses (out of 286) from 3/22
litters had tail defects. The incidence of retarded ossification of
the skeletons was clearly higher compared with the untreated
control. As in the first prenatal toxicity study dumbell-shaped or
bipartite thoracic vertebrae occurred to an increased extent
compared with the control. The incidence of the changes in the
thoracic vertebrae of the pups sacrificed 7 or 21 days after birth
was clearly reduced compared with the prenatal investigations
although these findings, which also occur spontaneously without
relation to the test substance, were not completely reversible
either in the untreated control or in the animals treated.
Furthermore, perinatal mortality was slightly increased at 400 mg/kg
bw/day compared to controls. This finding was due to an increased
number of dead pups on the day of birth whereas the survival index
of the pups sacrificed 7 days after birth remained unaffected
(Hellwig & Hildebrand, 1987b).
In addition to the in vivo studies, an in vitro study was
carried out with rat embryos (whole embryo culture technique).
Cycloxydim (acid) and the main metabolite (TSO) were investigated in
an experiment in which embryos of Wistar rats which were 9.5 days
old were incubated in the culture medium with the test substance or
solvent control (DMSO) for 48 h. The concentrations were 300 œg/ml
for cycloxydim and 150 œg/ml for the TSO metabolite. Concentrations
were based on results of in vivo teratogenicity studies in the rat
and on investigations into the kinetics and metabolism in the same
animal species. After incubation, the embryos were evaluated under a
stereo-microscope and subsequently examined histopathologically for
possible substance-induced changes. No abnormal morphological
development was induced either by cycloxydim or by its TSO
metabolite in the embryos under the test conditions chosen. These
results clearly demonstrate that a direct embryotoxic effect was not
seen in vitro at this very sensitive development stage of the main
organs (Neubert et al., 1987)
Rabbits
Groups of 14 or 15 pregnant Himalayan rabbits were administered
cycloxydim as the sodium salt (purity 93.9%) by daily gavage from
days 6 to 18 after artificial insemination at doses of 0, 100, 200
or 400 mg/kg bw/day. On day 29 of gestation, the dams were
sacrificed and the fetuses were examined in detail after caesarean
section. At a dose of 400 mg/kg bw/day, the dams showed a
retardation of body-weight gain together with reduced food
consumption primarily during the treatment period compared with the
untreated control group. This finding was also observed after
administration of 200 mg/kg bw/day although it was less pronounced.
No signs of maternal toxicity were observed at 100 mg/kg bw/day.
There were no indications of an embryotoxic effect of the test
substance in the fetuses, except at 400 mg/kg bw/day, where there
was a significant reduction in percentage live fetuses and an
increase in percentage dead implants. The number of implantations
and resorptions, the conception rate and the implantation loss were
otherwise unaffected by treatment. Minor inter-group differences
remained within the historical control range. The variations,
anomalies and retardations that occurred were in the range of
historical control data for all test groups (Merkle & Hildebrand,
1985).
In addition to routine X-ray examination, the skeletons of the
control and highest dose group were additionally stained to look for
abnormalities of the vertebrae. These investigations did not
indicate any substance-induced changes of the vertebrae, as had
occurred in rats at a similar dose level (Hellwig, 1986).
Special studies on genotoxicity
A number of genotoxicity tests have been carried out with
cycloxydim. The results are summarised in Table 2 (in vitro) and
Table 3 (in vivo).
Special studies on skin and eye irritation and sensitization
The skin irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). About 0.5 ml of undiluted
test substance was applied to the clipped, intact dorsal skin of 3
animals per sex for 4 h under a semi-occlusive dressing. Very slight
erythema in 4/6 animals, within 1 hour of patch removal, which was
completely reversible within 2 days, was the only finding that was
obtained (Kirsch & Kieczka, 1984c).
The eye irritation potential of cycloxydim (acid) was
investigated in rabbits (White Vienna). 0.1 Ml of the undiluted test
substance was applied to the eyes of 3 male and 3 female animals.
Slight conjunctival irritation (redness with lacrimation), which was
seen in all animals one hour after treatment, and which was
completely reversible within 8 days, was the only finding that was
observed. Neither cornea nor iris showed any changes related to the
test substance administered (Kirsch & Kieczka, 1984d).
The sensitizing potential of the active ingredient was
investigated in guinea-pigs (Pirbright White) in a maximization test
in which 10 animals were used in each of the two control groups and
20 animals in the test group. The two challenges carried out after
intradermal induction with the test substance did not reveal any
skin changes in the animals of the test group indicating that there
was no sensitizing potential of cycloxydim under the test conditions
chosen (Kirsch & Kieczka, 1985c).
Observations in humans
No information was available.
Table 2: Results of in vitro genotoxicity assays on cycloxydim
Test system Test object Concentration Purity Results Reference
Ames test S. typhimurium 20-15000 µg/plate 93.9% Negative Gelbke & Engelhardt (1985a)
TA98,TA100, (Na salt)
TA1535,TA1537
Ames test S. typhimurium 20-5000 µg/plate NK Negative Gelbke & Engelhardt (1984)
TA98, TA100, (acid)
TA1535, TA1537,
TA 1538
Lymphoma forward Mouse L5178Y 1.75-20 µg/ml 93.9% Weak, positive den Boer & Hoorn (1985a)
mutation assay cells (Na salt) at cytotoxic
concentrations
HGPRT forward CHO cells 5-40 mg/ml 93.9% Negative Gelbke & Engelhardt (1986)
mutation assay (Na salt)
HGPRT forward CHO cells 0.215-21.5 mg/ml 93.9% Negative den Boer & Hoorn (1985b)
mutation assay (Na salt)
Cytogenetics CHO cells 500-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985a)
mutation assay (acid) in absence of
activation
Cytogenetics CHO cells 2000-5000 µg/ml NK Weak, positive Taalman & Hoorn (1985b)
mutation assay (Na salt) in absence of
activation
UDS Rat hepatocytes 100-2000 µg/ml NK Negative Cifone & Myhr (1985)
(acid)
UDS Rat hepatocytes 0.9-90.6 µg/ml NK Negative Cifone & Brusick (1985)
(Na salt)
NK: not known
Table 3: Results of in vivo genotoxicity assays on cycloxydim
Test system Test object Dose Purity Results Reference
Micronucleus NMRI mice 225, 450, 900 93.9% Negative Gelbke & Engelhardt (1985b)
test mg/kg bw
Micronucleus Chinese 500, 1700, 5000 NK Negative Taalman & Hoorn (1987)
test hamsters mg/kg bw
NK: not known
COMMENTS
Cycloxydim was extensively absorbed after oral administration
to rats and almost completely excreted within 5 days of dosing.
Elimination proceeded predominantly via the urine (74-86% of applied
dose), with lower levels in the faeces (12-25% of the applied dose).
Less than 1% of an administered dose was retained in the body.
Studies confirmed that enterohepatic circulation occurred in rats.
The metabolism of cycloxydim has been investigated in rats and
a biotransformation pathway has been proposed. The major metabolite
was the sulfoxide, and the pattern of metabolites was similar in
urine, bile and tissue residues.
Cycloxydim has low acute oral toxicity. WHO has classified
cycloxydim as unlikely to present acute hazard in normal use (WHO,
1992).
Owing to instability in dietary admixture the sodium salt of
cycloxydim was administered in the drinking-water in repeat-dose
rodent studies. In dogs, sufficient dietary stability was
demonstrated, and dietary administration of cycloxydim was therefore
used.
In mice, two 4-week dose range-finding studies were conducted,
followed by a 24-month long-term study. In the dose range-finding
studies, employing concentrations between 30 and 9000 ppm, reduced
food and water intake, and reduced body-weight gain were seen at
higher doses, together with increased liver weight and hydropic
vacuolar hepatocyte degeneration. The NOAEL was equal to 7 mg/kg
bw/day in males and 28 mg/kg bw/day in females, the lower NOAEL in
males resulting from liver weight increase in the absence of any
histopatho-logical change. In the long-term study, no treatment-
related effects were seen, up to the highest dose tested of 32 mg/kg
bw/day, although liver weights were not measured.
In rats a 4-week dose range-finding study was followed by a 13-
week study, an 18-month toxicity study and a 24-month
carcinogenicity study. Findings were generally similar to those
observed in mice, the liver being identified as the only target
organ of note, although no histopathological changes were seen at
doses up to 900 mg/kg bw/day. The NOAELs were 100 mg/kg bw/day over
4 weeks, 25 mg/kg bw/day over 13 weeks and 7 mg/kg bw/day over 18/24
months, based on reduced body-weight gain at 25 mg/kg bw/day and
above.
Cycloxydim was not carcinogenic in rats or mice.
In dogs, a 4-week dose range-finding study was followed by a
13-week and a 12-month study. In the dose range-finding study (at
doses up to 360 mg/kg bw/day) the liver and the red blood cells were
identified as target organs and the NOAEL was 40 mg/kg bw/day. In
the longer-term dog studies, the NOAELs were 50 mg/kg bw/day over 13
weeks and 20 mg/kg bw/day over 52 weeks. Target organs were the
liver and red blood cells. A marginal anaemia was seen, along with a
compensatory bone marrow response, but no serious treatment-related
histopathological effects were noted at 80 or 300 mg/kg bw/day.
In a multi-generation reproduction study in rats, the NOAELs
were about 10 mg/kg bw/day for parental toxicity and 38 mg/kg bw/day
for reproductive performance, pup mortality being slightly increased
at 150 mg/kg bw/day.
Teratogenicity studies have been carried out with cycloxydim in
rats and rabbits. In the rat teratogenicity studies the NOAEL for
maternal toxicity was 200 mg/kg bw/day. Fetotoxicity was seen at
maternally toxic doses, together with findings which may be
considered indicative of a teratogenic potential (missing/incomplete
tail and anal atresia at 600 and 800 mg/kg bw/day and vertebral
anomalies at 400 mg/kg bw/day). Although the vertebral anomalies
were not completely reversible post-natally (up to 21 days after
birth), in vitro embryo culture studies demonstrated that
cycloxydim did not show any direct embryotoxic effects. In the
rabbit teratogenicity study the NOAEL for maternal toxicity was 100
mg/kg bw/day. There was no indication of fetotoxicity in rabbits,
even in the presence of maternal toxicity and no indication of any
treatment-related vertebral anomalies, even when a special
examination was conducted to look specifically for such changes.
After reviewing the available genotoxicity data, the Meeting
concluded that cycloxydim and its sodium salt were not genotoxic.
An ADI was allocated based upon the NOAEL from the long-term
rat study in rats, using a safety factor of 100.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Mouse: > 240 ppm in the drinking water, equal to > 32
mg/kg bw/day (two-year study)
Rat: 100 ppm in the drinking-water, equal to 7 mg/kg
bw/day (18- and 24-month studies)
100 ppm in the drinking-water, equal to 10 mg/kg
bw/day (multi-generation study)
200 mg/kg bw/day (teratogenicity study, maternal and
fetal toxicity)
Dog: 400 ppm in the diet, equal to 20 mg/kg bw/day (one-
year study)
Rabbit: 100 mg/kg bw/day (teratogenicity study, maternal
toxicity).
Estimate of acceptable daily intake for humans:
0-0.07 mg/kg bw
Studies which will provide information valuable in the continued
evaluation of the compound
Observations in humans.
REFERENCES
den Boer, W.C. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (Na salt) in the mouse lymphoma forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
den Boer, W.C. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in the CHO HGPRT forward mutation assay.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Cifone, M.A. & Myhr, B.C. (1985) Evaluation of Reg. No. 172 999 in
the in vitro primary hepatocyte unscheduled DNA synthesis assay.
Unpublished report from Litton Bionetics (Hazleton), Kensington,
USA, submitted to WHO by BASF AG.
Cifone, M.A. & Brusick, D.J. (1985) Evaluation of BAS 517 H Na salt
in the in vitro rat primary hepatocyte unscheduled DNA synthesis
assay. Unpublished report from Litton Bionetics (Hazleton),
Kensington, USA, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1984) Study of Reg. No. 172 999 in
the Ames test (standard plate test with Salmonella typhimurium).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Gelbke, H.P. & Engelhardt, G. (1985a) Study of Reg. No. 172 999 Na
salt in the Ames test (standard plate test with Salmonella
typhimurium). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1985b) Cytogenetic investigations in
NMRI mice after a single oral administration of Reg. No. 172 999, Na
salt - Micronucleus test. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Gelbke, H.P. & Engelhardt, G. (1986) Point mutation test carried out
on CHO cells (HGPRT locus) with Reg. No. 172 999. Unpublished report
from BASF Aktiengesellschaft, Departmont of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hawkins, D.R., Kirkpatrick, D., Finn, C.M., Till, C.P., Dean, G.M.,
Biggs, S.R. and & Whitby, B.R. (1986). The biokinetics and
metabolism of 14C-BAS 517 H in the rat. Unpublished report from
Huntingdon Research Centre Ltd., Huntingdon, England, submitted to
WHO by BASF AG.
Hellwig, J. (1986) Amendment to the determination of the prenatal
toxicity of Reg. No. 172 999 Na salt in rabbits after oral
administration (stomach tube). Unpublished report from BASF
Aktiengesellschaft. Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987a) Study of the prenatal toxicity
of Reg. No. 172 999 Na salt in rats after oral administration
(gavage). Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J. & Hildebrand, B. (1987b) Study of the pre-, peri- and
postnatal toxicity of Reg. No. 172 999 Na salt in rats after oral
administration (gavage). Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology. D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1985)
Study of the toxicity of Reg. No. 172 999 Na salt in beagles after
4-week administration in the diet (range-finding study). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1986)
Study of Reg. No. 172 999 Na salt in beagles after 3-month
administration in the diet (range-finding study). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Deckardt, K., Gembardt, Chr. & Hildebrand B. (1987)
Study of the prenatal toxicity of Reg. No. 172 999 Na salt in rats
after oral administration (gavage) with special attention to
maternal toxicity. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hellwig, J., Deckardt, K., Freisberg, K.O., & Hildebrand, B. (1988a)
Study of the toxicity of Reg. No. 172 999 Na salt in purebred beagle
dogs: administration over 12 months via the diet. Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hellwig, J., Freisberg, K.O., & Hildebrand, B. (1988b) Reproduction
study with Reg. No. 172 999 Na salt in rats: continuous
administration with the drinking water over 2 generations (2 litters
in the first and one litter in the second generation). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Hildebrand, B. & Kirsch, P. (1987). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Hoffmann, H.D., Gelbke, H.P., & Hildebrand, B. (1989) Study of the
dermal absorption of BAS 517H sodium salt in rats. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984a). Acute oral toxicity of Reg. No.
172 999 in the rat. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984b). Acute dermal toxicity of Reg. No.
172 999 in the rat based on OECD and EPA (FIFRA). Unpublished report
from BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984c). Acute dermal
irritation/corrosivity of Reg. No. 172 999 to the intact dorsal skin
of the white rabbit based on OECD and EPA (FIFRA). Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1984d). Acute irritation of Reg. No. 172
999 to the eye of the white rabbit based on OECD and EPA (FIFRA).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kirsch, P. & Kieczka, H. (1985a). Acute oral toxicity on the mouse
based on OECD of Reg No. 172 999. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985b). Acute intraperitoneal toxicity of
Reg. No. 172 999 in the rat. Unpublished report from BASF
Aktiengesellschaft, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kirsch, P. & Kieczka, H. (1985c). Sensitizing effect of Reg. No. 172
999 in the guinea pig maximization test. Unpublished report from
BASF Aktiengesellschaft, Department of Toxicology, D-6700
Ludwigshafen, Germany, submitted to WHO by BASF AG.
Klimisch, H-J., Gelke, H-P., & Freisberg, K.O. (1985). Acute
inhalation toxicity, 4 hours (rat) liquid aerosol study of Reg. No.
172 999 tested as sodium salt. Unpublished report from BASF
Aktiengesellschift, Department of Toxicology, D-6700 Ludwigshafen,
Germany, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986a) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (1st range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., & Hildebrand, B. (1986b) Study of the
toxicity of Reg. No. 172 999 Na salt in mice after 4-week
administration via the drinking water (2nd range-finding study).
Unpublished report from BASF Aktiengesellschaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986c) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 28-day administration with the drinking water (range-finding
study). Unpublished report from BASF Aktiengesellschaft, Department
of Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by
BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1986d) Study of the toxicity of Reg. No. 172 999 Na salt in rats
after 3-month administration via the drinking water and a 6-week
observation period. Unpublished report from BASF Aktiengesellschaft,
Department of Toxicology, D-6700 Ludwigshafen, Germany, submitted to
WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988a) Study of the toxicity of Reg. No. 172 999 Na
salt in rats: administration in the drinking water over 18 months.
Unpublished report from BASF Aktiengeselischaft, Department of
Toxicology. D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., Schilling, K., &
Hildebrand, B. (1988b) Study on the toxicity of Reg. No. 172 999
Na-salt in rats: administration via the drinking water over 24
months. Unpublished report from BASF Aktiengeselischaft, Department
of Toxicology, D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Kuhborth, B., Deckardt, K., Freisberg, K.O., & Hildebrand, B.
(1988c) Study on the toxicity of Reg. No. 172 999 Na-salt in mice:
administration in the drinking water over 24 months. Unpublished
report from BASF Aktiengesellschaft, Department of Toxicology,
D-6700 Ludwigshafen, submitted to WHO by BASF AG.
Merkle, J. & Hildebrand B. (1985) Pretnatal toxicity of Reg. No. 172
999 Na salt in rabbits after oral administration (stomach tube).
Unpublished report from BASF Aktiengesellchaft, Department of
Toxicology, D-6700 Ludwigshafen, Germany, submitted to WHO by BASF
AG.
Neubert, D., Klug, S. & Merker, H-J. (1987) Effect of cycloxydim on
embryonic development in vitro. Unpublished report from Institute
for Toxicology and Embryopharmacology, Freie Universität, Berlin,
submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985a) Mutagenicity evaluation of
BAS 517 H (acid) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1985b) Mutagenicity evaluation of
BAS 517 H (Na salt) in an in vitro cytogenotic assay measuring
chromosome aberration frequencies in Chinese hamster ovary cells.
Unpublished report from Litton Bionetics (Hazleton), Veenendaal,
Netherlands, submitted to WHO by BASF AG.
Taalman, R.D.F.M. & Hoorn, A.J.W. (1987) Cytogenetic investigations
in Chinese hamsters after a single oral administration of Reg. No.
172 999, Na salt - micronucleus test. Unpublished report from Litton
Bionetics (Hazleton), Veenendaal, Netherlands, submitted to WHO by
BASF AG.
WHO (1992). The WHO recommended classification of pesticides by
hazard and guidelines to classification 1992-1993 (WHO/PCS/92.14).
Available from the International Programme on Chemical Safety, World
Health Organization, Geneva, Switzerland.
