
PESTICIDE RESIDUES IN FOOD - 1984
Sponsored jointly by FAO and WHO
EVALUATIONS 1984
The monographs
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 24 September - 3 October 1984
Food and Agriculture Organization of the United Nations
Rome 1985
METHOPRENE
IDENTITY
Chemical Name: isopropyl (E,E)-(RS)-11-methoxy-3,7,11-
trimethyldodeca-2,4-dienoate.
Structural formula:
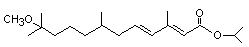 Synonyms: ALTOSID, APEX, DIACON, KABAT, DIANEX, PRECOR.
(All registered Trade Names) ZR-515; ENT-70460;
CAS No: 114-26-1
Other information on identity and properties:
Molecular formula C19H34O3
Molecular weight: 310
Physical form: Pale yellow liquid
B.P.: 100° at 0.05 mm Hg
Vapour pressure: 2.37 × 10-5 mm Hg at 25°C
1.60 × 10-4 mm Hg at 40°C
Solubility: all organic solvents-infinity
water - 1.39 mg/l.
Specific gravity: 0.9261 g/cc at 20°C
Octanol/water
partition coefficient: 10,000
Cis-2 : trans-2 ratio: 8:92
Purity: methoprene - 92-95%
related isomers - 5-2%
solvent - 1-2%
unidentified - 2-1%
Stability: extremely stable in sterile aqueous
solutions over wide range. Readily
biodegraded by common bacteria. Rapidly
degraded in solution or in thin films by
sunlight and UV light. Identity of
degradation products known. (Quistad
et al., 1975b).
Formulations: Several emulsifiable formulations for
agricultural, stored product and public
health use; solution formulation for
treating cured tobacco; fogging solution
for public health use; emulsifiable
formulations for addition to feed
supplements; slow release formulation
for use in mosquito abatement;
emulsifiable formulation for protection
of houseplants.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methoprene is an insect growth regulator (IGR), being a synthetic
analogue of the insect juvenile hormone (Henrick et al., 1973).
Unlike conventional insecticides, which act as direct poisons,
methoprene acts by disrupting the morphological development of
insects. After contact, inhalation or ingestion the insect grows and
pupates normally, but the pupa dies in the pupal stage. As a result,
methoprene is not generally used in pre-harvest situations when a
quick kill of insect pests is required.
Methoprene has found widespread use in mushroom culture to
prevent the emergence of adult sciarid flies, where it is applied to
compost or casing at the rate of about 100 g/100 sq.m. This
application represents about 12% of methoprene use. A commonly used
treatment is 175 ml of 650 g/l emulsifiable concentrate per 100 sq.m.,
equivalent to 113 g a.i./100 sq.m. One or two applications are used.
Special slow-release formulations are used to control mosquito
breeding in floodwater sites, rice cultivations, storm drains, ponds,
water treatment works etc. (Schaefer and Wilder, 1973; Mura and
Takahashi, 1973).
Methoprene is formulated into feed supplements for cattle to
control adult hornfly breeding in cattle manure. The rate is
equivalent to 0.016 mg/kg body weight daily. This, together with
mosquito control represents about 50% of current use.
Methoprene is marketed as a 65% emulsifiable concentrate
formulation for use as a space spray for control of pests in food
handling and storage establishments. It is applied as an aqueous spray
or via mechanical foggers at a rate of 10 ml per 1,000 m2.
Post-harvest treatments
Methoprene has proved effective in the control of numerous pests
of stored products such as peanuts where it is used at the rate of
10g/tonne against most moth and beetle pests. It is being tested on
cocoa beans and cereal grains as a 657g/l emulsifiable concentrate
formulation. This is applied at a rate of 15ml/tonne of stored
commodity, equivalent to 10g a.i./tonne, in either water or mineral
oil.
Trial work indicates that methoprene will control most insect
pests affecting stored grain though it is significantly less effective
against Sitophilus spp. which include three of the major pests of
maize, rice and wheat. It may have to be combined with a conventional
insecticide where these species are a problem.
Methoprene has been used for more than five years for treating
stored tobacco. The cured tobacco is treated, before packing, at the
rate of 200g methoprene per tonne of tobacco. It is used for treating
food-handling and tobacco-processing establishments to prevent insect
breeding and development. A fogging formulation is used to control
pre-adult fleas in premises.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in stored agricultural commodities
Peanuts. During the period 1979-1981 the manufacturers, in
conjunction with the US Department of Agriculture, undertook trials of
methoprene for protecting peanuts against stored-product insect pests
(Miller 1981). The methoprene was added as the peanuts were being
placed in storage silos. Normally peanuts are stored for a period from
a few months to a year.
Treatment rates were 0, 4, 10, 25 and 100 mg/kg. Nuts (kernels)
and hulls were analysed separately. Residues at the proposed use rate
of 10 mg/kg on the day of treatment were below the limit of
determination (0.05 mg/kg) in kernels with wrappers, 0.05-0.64 mg/kg
in kernels without hulls (split hulls) and 14-20 mg/kg in hulls. After
278 days storage the kernels with wrappers contained 0.13-0.38 mg/kg
(mean of 11 samples 0.22 mg/kg), kernels without hulls (split hulls)
0.38-2.1 mg/kg and hulls 8.3-14.0 mg/kg.
Peanuts treated at exaggerated rates contained residues
proportionally higher than those treated at the proposed use rate.
Maize. Eight studies were carried out on stored shelled maize
(Miller, 1983a). Random samples from bulk lots treated at rates of 10,
15, 20 and 23.5 mg/kg were drawn at intervals up to 13 months after
treatment. At all application rates, residues showed no significant
losses with time, even at 200 days after treatment. It is noteworthy
that at no stage did the residues determined by analysis reflect the
amount of active ingredient actually applied to the grain, and in most
trials ranged between 40% and 70% of it. It is difficult to see
whether this was due to failure to deposit the methoprene on grain,
loss from the grain surface immediately after treatment, loss from the
samples between collection and analysis, or failure to recover residue
from the substrate.
The analytical method included an internal standard which was
added in the blender. Recovery of the internal standard was always
high (80-110%), implying validity of the method and analytical
procedures. However, there is no explanation of the apparent absence
of methoprene. Nevertheless the residue levels found throughout the
storage period were similar to, or higher than, those reported for the
initial sample, indicating that little degradation had occurred during
storage.
Wheat. Data from two studies (Miller, 1983b) were available. Stored
wheat was treated with methoprene at 10 and 20 mg/kg and sampled 3,
35, 95 and 150 days after treatment. Methoprene residues recovered by
analysis were about one-half of the treatment rate but did not show
any decline up to 150 days after treatment. Residues from the 10mg/kg
treatment were 5.2 mg/kg after 150 days. An explanation for this
discrepancy is needed.
In a laboratory study at the University of California using GLC
analysis, Mian and Mula (1983) found that when methoprene was applied
at rates of 1, 5 and 10 mg/kg to wheat (13.5% moisture content) stored
at 27 ± 1°C, losses were 61, 66 and 62% respectively over a period of
12 months. The results of analyses carried out at six times within the
12-month period are given in Table 1.
These data seem more reliable than those reported by Miller
(1983b), since the main initial recovery of insect growth regulator was
99% of that applied. Thus it can be accepted that the loss over 12
months is of the order of 60% of the amount applied.
Rowlands (1976), determined the rate of breakdown at 20°C during
10 weeks storage in the dark, using radiolabelled methoprene with
three samples of wheat: fresh wheat of 19.1% moisture content, old
wheat of 12% moisture content and old wheat of 18% moisture content.
Table 2 (adapted from Rowlands, 1976) indicates that the breakdown was
quite rapid at the high moisture levels (half-life of three weeks at
18-19% moisture) and was about half as fast on grain with 12% moisture
(half-life six weeks).
Table 1. Residues of methoprene in treated wheat grain at
various post-treatment intervals 1
Residue, mg/kg
Interval
Application rate, mg/kg
1 5 10
0 0.99a 4.97a 9.92a
1 wk 0.99a 4.60a 9.39a
(0) (7) (5)
1 mo 0.94a 3.93a 6.62b
(5) (21) (33)
4 mo 0.82a 3.49ab 5.53bc
(17) (30) (44)
8 mo 0.80a 1.78bc 4.07c
(19) (64) (59)
12 mo 0.38b 1.71c 3.73c
(61) (66) (62)
1 Mean of four analyses. Means followed by the same letters
in a column are no significantly different from one another
(Duncan's multiple range test. P = 0.05). Values in
parentheses below each mean represent percent loss in
residues at indicated post-treatment interval.
Table 2. Breakdown of methoprene on stored wheat grains at 20°C*
Residue, mg/kg found in grain
Storage time
in weeks Fresh wheat Old wheat Old wheat
(19%) (12%) (18%)
0 5.6 9.8 9.6
1 7.2 9.2 7.7
2 5.5 7.9 6.3
3 4.4 7.6 5.0
4 3.4 7.0 4.3
5 2.2 6.2 3.2
6 1.4 5.1 2.1
7 0.5 4.2 1.4
8 0.2 3.2 0.5
9 0.1 2.4 0.4
10 nil 1.5 0.2
* Results are means from triplicate 25g samples, treated at
10 mg/kg (approx.)
Cocoa beans. In two trials carried out in Brazil, methoprene was
applied to cocoa beans at rates of 1, 2.5, 5.0, 10.0 and 15 mg/kg. The
beans were sampled on the day of treatment and after 50 days storage
at 27°C. Less than half of the applied methoprene was recovered by
analysis but the analytical results on samples taken after 50 days in
storage were not significantly different from those on the initial
samples (Miller, 1983c).
Residues in mushrooms
Data from 7 studies were available (Miller 1978), reflecting both
compost and casing treatment at 0.2 to 4.6 times the recommended
application rates. Samples were collected at 0, 9 and 35 days after
application. When less than 2.5 times the recommended combined
treatment rates were used, the residues were undetectable
(<0.05 mg/kg). At 2.5 and 4.6 times the recommended application rate,
residues ranged from 0.17 mg/kg to 150 mg/kg. The high values were due
to contamination with compost or peat moss "casing" which was shown to
contain methoprene at hundreds of mg/kg. Subsequent samples were
rinsed with cold water to remove the contamination. Most samples
examined in these later experiments contained no methoprene or the
residues were less than 0.1 mg/kg (Miller, 1978).
Dust samples from food-handling establishments were analysed in
six studies (Miller, 1984). Isolated areas were treated with
0.6g a.i./1000 sq.m. (the food being covered according to label
direction before application). Samples were collected in petri dishes
located in appropriate areas of the treated spaces 0, 30, 60 and 90
days after treatment. Residues expressed as mg/kg dust were high owing
to the small amount of dust collected in the dishes. Consequently, the
total quantity of methoprene collected is a more realistic value to
consider when evaluating the results.
Immediately after application methoprene residues were high,
ranging from 26 micrograms to 191 mg. However within 30 days they
declined significantly and ranged from 0.5 to 24 mg. Although there is
some variation in the results, within 60 days the majority of the
residues were below the level of detection (0.5 micrograms).
Residues in forage and field crops
Methoprene is marketed for the control of floodwater mosquitoes
as a 10% slow-release encapsulated aqueous solution (Schaefer and
Wilder, 1973). Since treated areas include pastures, rice fields and
marshlands, residues might occur on certain food crops. Consequently,
Zoecon conducted a series of studies on rice, legumes and forage crops
to analyse for possible methoprene residues which could enter the food
chain through ingestion by livestock.
In a study on rice, methoprene was applied prior to "heading"
(Miller, 1974a). The crop was then grown to harvest. Samples were
collected at 45, 62 and 71 days after application, resulting in a
built-in pre-harvest interval of two months. No detectable residues
(<0.05 mg/kg) were found in the rice grains, hulls or straw of any of
the samples.
In nine studies on pasture legumes and forage crops (Miller,
1974b) pasture land was treated at rates of 9, 11, 23 and 45 g/ha. The
highest treatment rate is four times the highest recommended rate.
Samples were collected 0, 1, 2, 3, 5, 7, 9, 11 and 365 days after
application. Residue levels indicated that methoprene is rapidly
degraded. By day 3, the residues from the highest treatment ranged
from 0.12 to 0.30 mg/kg.
FATE OF RESIDUES
In animals
Numerous studies have been conducted to determine the fate of
methoprene in livestock because of the commercial use in animal feeds
to suppress the breeding of flies in manure.
Quistad et al. (1974b, 1975a) administered [5-14C] methoprene
to a steer which was slaughtered two weeks later. Samples of fat,
muscle, liver, lung, blood and bile were analysed for radioactive
residues. No primary methoprene metabolites could be characterised but
the majority of the tissue radioactivity was positively identified as
cholesterol. A total of 72% of the bile radioactivity was contributed
by cholesterol, cholic acid and deoxycholic acid. Radioactivity from
metabolised methoprene was associated with protein and cholesteryl
esters of fatty acids.
A metabolic balance study was conducted on a 338 kg Jersey cow
dosed with 207 mg of [5-14C] methoprene by capsule (Chamberlain
et al 1975b). This is 40 times the proposed dose for fly control.
The study proceeded for 7 days after dosing. It was found that 16.4%
of the radioactivity was excreted as CO2 between 0 and 170 hours,
with peak excretion after a little under 24 hours. A total of 30.3% of
the applied dose was eliminated via the faeces within about 50 hours
and 19.7% via the urine in 30 hours. By the end of the experiment 7.6%
of the radiolabel had been excreted via the milk. Radioactivity was
measured in 30 tissue samples after the cow was sacrificed 7 days
after dosing. The tissues contained a total of 20% of the applied
dose, intestine and contents contributing 5.96% and fat 4.59%. The
organs of metabolism, lung, liver and kidney, contributed a further
1.68%. The presence of radiocarbon in the tissues was attributed to
steroidal derivatives in which labelled acetate from the methoprene
was anabolically incorporated into natural body constituents.
The results of the above two studies and another on a guinea pig
were compared by Chamberlain et al. (1975a). It was reported that a
rather large percentage of the radiolabel was incorporated in the
tissues and respired by the animals. A small proportion was
incorporated into free primary metabolites, a greater amount into
glucuronides and a considerable proportion within polar components,
possibly complex conjugates or biochemicals. Methoprene was not found
in the urine, but accounted for approximately 40% of the radiolabel in
the faeces. This explains the activity of the administered methoprene
against fly larvae developing in dung pats. The formation of
conjugates and complex metabolites was more extensive in the steer
than the guinea pig. Comprehensive information was provided on the
radiolabel profiles in tissues, organs and other components of the
animals.
On the basis of detailed biochemical analysis of tissues and
organs from the above three studies Quistad et al. (1975c)
elucidated the metabolism by the cow to natural products in milk and
blood. Only a trace of methoprene (0.015/l) and no detectable primary
metabolites occurred in milk.
Quistad et al. (1976a) studied the metabolism of methoprene in
leghorn chickens give single oral doses of [5-14C] methoprene.
Residual radioactivity was found in tissues and eggs. Although a high
initial dose (59 mg/kg)resulted in residues of methoprene in muscle
(0.01 mg/kg), fat (2.13 mg/kg) and egg yolk (8.03 mg/kg), these
represented only 39% and 2% of the total radiolabel in fat and egg
yolk respectively. Radiolabelled natural products were by far the main
14C residues in tissues and eggs, particularly at the lower dose of
0.6 mg/kg where cholesterol and normal fatty acids contributed 8% and
71% of the total radiolabel in egg yolk.
When chickens were given feed treated with methoprene at 0, 25,
50 and 100 ppm for 14-63 days, residues in poultry meat and eggs were
less than 0.1 mg/kg (Zoecon, 1973).
Quistad et al. (1976b) studied methoprene metabolism in
bluegill fish in a dynamic flow-through system and a model aquatic
ecosystem. The fish in the flow-through system acquired moderate
residues of largely unmetabolised methoprene when continuously exposed
to about 30 times the anticipated environmental levels of methoprene,
but these were 93-95% eliminated within two weeks when the fish were
transferred to flowing uncontaminated water. In the model aquatic
ecosystem, the fish accumulated 14C, but the radioactivity was almost
exclusively in radiolabelled natural products, including cholesterol,
free fatty acids, glycerides and protein. Less than 0.1% of the total
radioactivity could be attributed to methoprene or its primary
metabolites.
In plants
Methoprene is rapidly degraded in plants with a half-life of
about 2 days in alfalfa and about 0.5 days in rice. The major
metabolic pathways include ester hydrolysis, O-demethylation and
oxidative scissions of the 4-ene double bond, producing primary
metabolites which are present in relatively low yields (Figure 1)
(Quistad et al. 1974a). These low amounts suggest that subsequent
degradation or conjugation occurs, and in fact the recovery of polar
metabolites as well as the loss of radioactivity through
volatilization are evidence of additional metabolism. In addition,
unextractable residues suggest the extensive degradation of methoprene
and incorporation into natural cellular products. The metabolic fate
of methoprene in alfalfa and rice is shown in Figure 1. Metabolism
resulted in five primary non-polar products isolated from the plant
(ZR-669, ZR-725, ZR-724, ZR-1945 and ZR-1602, see Figure 1). The
combined yield of these products was 1.4% of the applied dose after 7
days from alfalfa and 2.1% after 3 days from rice. The most abundant
non-polar metabolite was ZR-1564 (13% from rice, 2% from alfalfa)
which was isolated from vapours and not found within the plant. The
main aglycones after enzymic cleavage of alfalfa conjugates were
ZR-725 (2.2%), ZR-1945 (0.8%), and ZR-724 (7.4%). The major aglycone
from rice was ZR-1945 (1.2%) with a small amount of ZR-1602 (0.3%).
After 30 days, only 1% of the applied dose remained in alfalfa as
unmetabolised methoprene and 0.4% remained in rice after 15 days. The
initial rapid loss of methoprene and radiolabel from plants was
attributed to evaporation of methoprene and 7-methoxycitronellal
(ZR-1564) on the evidence from supporting studies of methoprene
evaporation from glass plates and of compounds volatilized from leaf
surfaces.
10-3H-methoprene painted on the leaf surface or injected into
the stem of the dwarf Lima bean plant was found not to be
translocated. When injected into the stem, the parent compound was
partially metabolised to ZR-724, ZR-725 and a polar conjugate,
possibly the glycoside. Topical application resulted in more rapid
metabolism to the same metabolites after 24 hours incubation
(Schooley, 1974).
Rowlands (1976) studied the uptake and metabolism of methoprene
and two other IGRs by stored wheat grains. He applied the
radiolabelled compounds topically to individual grains and to small
samples of wheat in glass jars. The wheat contained 19.5% moisture and
was kept at 20°C in the dark. The methoprene penetrated rapidly from
both vapour and topical treatments, but the quantities sorbed differed
between the two routes. Two days after treatment most of the intact
methoprene was found in the aleurone layers, much less in the germ and
virtually none in the endo-sperm proper or outer seedcoat. The rate of
degradation is indicated in Table 2 above. Degradation is promoted by
grain moisture, wheat with 19% moisture giving a half-life of 2-3
weeks, 18% moisture 3-4 weeks and 12% moisture 6-7 weeks.
Mian and Mullar (1983) determined the residues of methoprene on
wheat over a 12-month storage period. Details are given above
("Residues resulting from supervised trials").
In soil
Schooley et al. (1975b) studied the degradation of methoprene
in soil as a function of time under various conditions. On aerobic
sandy loam methoprene showed an initial half-life of about 10 days at
a surface treatment rate of 1 kg/ha; decomposition was much slower on
autoclaved soil. Only small amounts of non-polar metabolites were
isolated, including the hydroxy ester resulting from O-demethylation
(0.7% of the applied dose). Over 50% of the applied dose was converted
to CO2. Radioactivity from [5-14C] methoprene was incorporated into
humic acid, fulvic acid, and humin fractions of sandy loams. These
studies indicate rapid and extensive breakdown of methoprene in soils.
Leaching experiments with radiolabelled methoprene were performed
to determine the mobility of the chemical in different soils. The four
agricultural soils used were sand, sandy loam, silt loam and clay
loam. Experiments with soil columns and soil thin-layer plates
revealed that no significant leaching of methoprene occurred with the
four soils tests (Zoecon, 1984b).
Quistad and Staiger (1974a) determined the amount and nature of
bound residues in soil. They found that in sandy loam soil treated
with [5-14C] methoprene humic acids (3%) and fulvic acids (8.4%) were
most abundant after 30 days. The total recovery of the label from
treated soil was essentially quantitative.
Quistad and Staiger (1974b) determined the partition between
water and soil using a 0.01 mg/l solution of [5-14C] methoprene
agitated with sandy loam soil. After thorough mixing for 15 minutes,
87% of the applied dose was bound to the soil.
Staiger et al. (1974a) grew wheat plants in soil that had been
treated 6 months previously to determine the uptake by a second crop
grown in methoprene-contaminated soil. After treatment of the soil at
the rate of 1 kg/ha, less than 0.006% of the administered label was
taken up in wheat six months after dosing. This uptake is about
one-tenth of that observed when soil was treated with radiolabelled
methoprene at the time seeds were planted.
Staiger et al. (1974b) determined the uptake of methoprene by
wheat plants when wheat seeds were planted in sandy loam and
radiolabelled methoprene was incorporated at the rate of 1 kg/ha at
the seed level and in the soil at a depth of 9 cm below the seed. The
wheat was harvested 11 days after planting and assayed after total
combustion. Uptake was found to be 0.09% of the applied radioactivity
when the IGR was inserted at the level of the seed and 0.04% of the
radioactivity when it was incorporated 9 cm below the seeds. It appears
that very little methoprene or its metabolites are translocated into
wheat from soil.
In water
Sterile aqueous solutions of methoprene (0.5 mg/l), buffered at
various pH values, were found to be extremely stable to hydrolysis
over four weeks at 20°C in the dark. No degradation was seen for the
duration of the experiment in sterile water buffered at pH 7 and 9,
and similar stability was observed in pH 5 buffer for 21 days. However
the pH 5 buffer became non-sterile between the 21- and 30-day
observations, and 59% degradation occurred between days 21 and 30.
(Schooley and Bergot, 1975a).
Schaefer and Dupras (1973) studied the persistence of methoprene
in water. They found that residues were greatly affected by sunlight
and temperature. The methoprene tended to remain on the water surface
and its distribution was affected by wind. The half-life in field
water was only about two hours. A slow-release formulation showed
almost no detectable residues after 24 hours, although biological
activity persisted for several days.
Schooley and Bergot (1974) measured the dissipation of methoprene
in pond water and sewage at dose rates of 0.001 and 0.01 mg/l. A
half-life of 30-45 hours was observed in pond water, with a 60-70 hour
half-life in sewage. This significantly longer half-life in sewage may
have been due to the fact that the primary sewage sample had been
standing in a closed bottle for 30 days in the laboratory, while the
pond water had been collected fresh on the day of the experiment.
On standing for thirteen days in unsterilised pond water
containing suspended sediment in full summer sunlight at a
concentration of 0.66 mg/l, methoprene was metabolised and/or
photodegraded mainly to one product, ZR-1945 methoxycitronellic acid
(Schooley et al. 1975a). This is one of many products formed by the
combined action of oxygen and sunlight on thin films of methoprene on
glass plates. ZR-724, ZR-725 and ZR-669 (See Figure 1) were either not
present or present in very low concentrations.
A 3-day study was conducted on methoprene in non-sterile pond
water at ambient temperature in direct sunlight (Schooley and Bergot,
1975b). Three primary products, ZR-724, ZR-725, ZR-669 were
identified. Each compound, as well as the recovered methoprene, was
found to be an approximately 1:1 mixture of cis-2 and trans-2
isomers, in contrast to the 94% trans-2 methoprene which was
applied. This conversion has been shown in other studies to be ready
photoisomerization rather than an enzymic process.
The biodegradation of (2E)-[10-3H] methoprene was studied in
pond water containing unknown micro-organisms (Schooley et al
1975a). The methoprene showed a half-life of approximately 30 hrs at
0.001 mg/l and 40 hr at 0.01 mg/l. Incubation of the same preparation
for 3 days at 0.42 mg/l generated three primary metabolites, the
result of ester hydrolysis and/or O-demethylation. These metabolites
and the recovered methoprene were photoequilibrium mixtures of 2-ene
isomers. In another incubation experiment with (2E)-[5-14C]
methoprene at 0.66 mg/l in a pond water sample with presumably
different microflora, a completely different metabolic profile was
observed. The main and only identifiable metabolite, resulting from
oxidative scission of the 4-ene double bond, was 7-methoxycitronellic
acid (29% of the applied dose).
In processing and cooking
Peanuts. Peanuts from one of the trials Miller (1981) (see
"Residues resulting from supervised trials") with residues ranging
from 0.18 to 1.21 mg/kg at the time of treatment, were processed into
peanut butter and peanut oil. The peanut butter was reported to
contain less than 0.05 mg/kg of methoprene, indistinguishable from
untreated controls. The peanut oil, however, contained 0.25 mg/kg
(0.20-0.29) of methoprene. These results do not appear to be
consistent.
A trial was carried out in Australia (Zoecon 1984c) in which
methoprene was applied to wheat at the rate of 10 mg/kg. After storage
for two weeks the wheat was milled through an experimental Buhler mill
to provide bran, pollard and white flour. A separate milling produced
wholemeal. The residues found, and the proportion of each fraction in
the first milling, are shown in Table 3.
A separate trial carried out at Kansas State University (Zoecon,
1984d) involved treatment of two lots of wheat with methoprene at the
rate of 10 mg/kg followed, 9 days later, by milling through a Ross
Walking Flour Mill. A separate milling through a pin mill produced
wholemeal.
The white and wholemeal flours were used to prepare bread. The
results of analysis are shown in Table 4.
METHODS OF RESIDUE ANALYSIS
Residues of methoprene have been determined in waters, soils,
plants, stored grain and corn, milk, eggs, fish, shellfish, poultry
and cattle tissues, blood, urine and faeces. Residues in all samples
are extracted by high-speed blending with acetonitrile followed by
vacuum filtration. Fatty extracts are subjected to cold-temperature
precipitation and filtration to remove fat. Petroleum ether
partitioning, Florisil and neutral alumina chromatography are used for
clean-up. The concentrated eluates are analyzed by GLC on columns of
differing polarity, using flame ionization detectors. Confirmation of
identity by additional GLC and mass fragmentography (MF). The lower
limits of determination are: soils, blood and urine, 0.001 mg/kg;
forage grasses, forage legumes and rice foliage, 0.005 mg/kg; milk,
eggs, stored grain and corn kernels, fish, shellfish, poultry and
cattle tissues and faeces, 0.01 mg/kg. Limits of determination and
recoveries were validated by analysis of laboratory and field samples
fortified with 14C-methoprene (Miller et al., 1975).
Table 3. Methoprene residues in wheat fractions Zoecon, 1984c)
Fraction % wt./wt/ of each Methoprene (mg/kg)
fraction (excluding
wholemeal)
Wheat 8.9
Wholemeal flour 6.7
White flour 74 1.8
Bran 23 11.4
Pollard 3 17.7
An internal standard was used in the analysis.
Table 4 Methoprene residues in wheat fractions (Zoecon, 1984d)
Fraction % mt/wt of each fraction Methoprene (mg/kg)
(excluding wholemeal)
Trial Trial Trial Trial
Wheat (whole) 5.0 5.7(Note 1)
White flour 70.2 70.1 2.8 2.8
Wholemeal flour 4.1 5.5
Bran 14.1 14.4 8.7 10.2
Shorts ) 12.7 12.4 9.6 9.6
pollard
Red dog ) 2.4 2.5 9.5 11.0
Germ 0.6 0.6 8.7 10.7
White bread (33% moisture) 0.41
Wholemeal bread (33% moisture) 0.88
Note 1 It appears that a significant proportion of the original
deposit (10 mg/kg) was lost, possibly by evaporation, between
application and analysis. Spiked samples analysed at the same
time yielded the results expected.
Mian and Mulla (1983) describe a method derived from that of
Dunham and Miller (1978) which they applied successfully to studying
the persistence of methoprene in stored wheat. This involved Florisil
and Alumina chromatography with petroleum ether followed by GLC/FID on
a column of 3% OV-101 on 100-120 mesh Chromosorb W (acid-washed,
dimethyldichlorosilane treated), conditioned at 350°C. The column and
injector temperatures were 190°C for retention times of 8 min for
methoprene and 12 min for n-docosane used as an internal standard. The
detector temperature was 350°C. The limit of determination was
reported to be 0.01 mg/kg and the recovery of 10, 5 and 1 mg/kg in
grain extracts ranged between 80 and 100%.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
No information was available.
NATIONAL MAXIMUM RESIDUE LIMITS
The meeting was advised that the following national maximum
residue limits had been established in the USA.
Meat of cattle, goats, hogs, sheep,
poultry and horses 0.1 mg/kg
Fat of cattle, goats, hogs, sheep,
poultry and horses 0.3 mg/kg
Meat by-products of cattle, goats, hogs,
sheep, poultry and horses 0.1 mg/kg
Milk fat 0.05 mg/kg
Eggs 0.05 mg/kg
Peanuts 2.0 mg/kg
Mushrooms 1.0 mg/kg
A petition has been presented for tolerances of 10 mg/kg on
barley, buckwheat, corn, millet, oats, rice, rye, sorghum, sunflower
and wheat. In addition a substantial increase has been proposed in the
tolerances for indirect residues, derived from animal feeds, in meat,
fat and meat by-products of cattle, goats, hogs, sheep, poultry and
horses and in eggs and milk fat.
The meeting was not aware of similar action by any other national
authority.
APPRAISAL
Methoprene is an insect growth regulator. It is an analogue of
the insect juvenile hormone. After contact, inhalation or ingestion it
interferes with the normal process of insect development preventing
the emergency of adults from pupae or larvae.
So far there have been no practical pre-harvest uses of
methoprene on crop plants and it appears unlikely that such uses will
develop. There is an important application for the control of sciarid
flies in mushroom culture and as a feed supplement for cattle to
prevent the breeding of hornflies in cattle manure. Methoprene is
applied to water to prevent the breeding of mosquitoes.
The most important application currently under development is the
use of methoprene to prevent the breeding of stored-product insect
pests in tobacco, peanuts, cocoa beans and a wide range of stored
commodities. There are good prospects for use in stored grain to
inhibit the development of stored-product insects. Extensive studies
on these uses are at an advanced stage in several countries.
Methoprene formulations are stable under normal storage
conditions but are rapidly degraded by light. Sterile aqueous
solutions are stable over a wide pH range but non-sterile solutions
are rapidly biodegraded.
When used for the control of insects in stored products
methoprene is applied alone at the rate of 10 mg/kg or in conjunction
with conventional insecticides at much lower rates (1-2 mg/kg).
Although the residues studies available at this stage are not
extensive it would appear that there is relatively little degradation
on dry stored commodities over many months. Degradation is promoted by
moisture and probably also by temperature.
In numerous field studies on peanuts, maize, cocoa beans and
wheat, investigators have failed to recover more than half of the
methoprene applied. This difficulty was not encountered in one small
scale laboratory study at the University of California. This suggests
a pronounced tendency for the methoprene to evaporate with the water
used to apply the insect growth regulator or a fundamental weakness in
the trial technique. This feature is currently receiving further
study.
The meeting received studies from Australia and the USA designed
to determine the distribution of methoprene in grain and the effect of
processing and cooking on the residues. These enabled the meeting to
propose temporary maximum residue limits.
One study with the labelled compound applied to individual wheat
grains indicated rapid penetration. A study on peanuts indicated that
by far the greater proportion of the deposit remained on the hulls and
wrappers and was not taken up by the kernel. However, allowance must
be made for the fact that a proportion of the kernels lose their
shells. When the peanut kernels were processed into peanut butter the
methoprene disappeared but significant amounts were detected in peanut
oil. These studies should be repeated.
When methoprene is applied to mushroom compost or casing at rates
which will control sciarid flies there are no detectable residues in
mushrooms. However, it is necessary to wash the peat moss casing from
the mushrooms before analysis to prevent anomalous results.
Methoprene would be suitable for the treatment of surfaces and
structures to prevent insect pests breeding in food storage and
preparation areas. Several studies indicated that such treatments
would not lead to significant residues in foodstuffs.
Studies have been conducted with cattle, poultry and fish to
elucidate the metabolic processes and determine whether residues occur
when methoprene is administered to livestock directly for fly control,
or in their feed from cereal grain and milling products, or when the
compound is used for mosquito control. All available information
points to the rapid degradation of the parent compound and
incorporation of fragments into natural body components but the
meeting, recognizing that it would not be practical to withdraw
treated feed before slaughter, recommended temporary MRLs for
methoprene residues in foods of animal origin to cover the feeding of
treated grain and its milling products.
Methoprene is rapidly metabolised in plants, yielding products
that are further degraded to normal plant building materials. When
applied to soil, most of the dose is converted rapidly to CO2. There
is no significant leaching of methoprene from treated soil and no
uptake by plants. There appears to be no likelihood of methoprene
being taken up by rice, forage or other crops growing in water treated
for mosquito control.
Methoprene residues may be determined by extraction with
acetonitrile followed by clean-up on Florisil and alumina columns and
quantitation by GLC with a flame ionization detector. The method
appears suitable for regulatory purposes. The limit of determination
ranges from 0.001 mg/kg to 0.01 mg/kg.
The meeting was aware that MRLs had been established for a range
of commodities in the USA and that others were under consideration.
RECOMMENDATIONS
The meeting recognized that there were, as yet, only a limited
number of registrations for the use of methoprene but that potentially
important developments might not proceed in the absence of appropriate
maximum residue limits. To assist these further developments and to
cater for existing uses, the following temporary MRLs are recommended
to apply to residues of methoprene resulting from the direct
application of methoprene or the feeding of methoprene-treated
commodities. They apply to methoprene only.
Commodity MRL (mg/kg)
Cereal grains 10
Bran 20
Flour (wholemeal) 10
Flour (white) 5
Carcase meat 0.2 (in the carcase fat)
Meat byproducts 0.1
Eggs 0.05
Milk 0.002
Peanuts 2
Mushrooms 1
FURTHER WORK OR INFORMATION
Required (before maximum residue limits can be confirmed):
1. Information from studies (now in progress) in which methoprene is
used for the control of pests of stored grain.
2. Studies to define the rate of degradation of methoprene in stored
products under a range of conditions.
3. Further information on the fate of methoprene residues in cereal
grains and other stored products as a result of processing and
cooking.
4. Information from current trials with stored dried lentils, beans,
dried fruit, cocoa beans, coffee beans, spices and oilseeds to
define the treatment necessary, the rate of degradation and the
fate of residues in processing and cooking.
REFERENCES
Chamberlain, W.F., Hunt, L.W.M., Hopkins, D.E., Gingrich, A.R.,
1975a Miller, J.A. and Gilbert, B.N. Absorption, excretion, and
metabolism of methoprene by a guinea pig, a steer and a cow.
J. Agric. Food Chem., 23(4):736-742.
Chamberlain, W.F., Hunt, L.M., Hopkins, D.E., Miller, J.A., Gingrich,
1975b A.R. and Gilbert, B.N. A balance study with 14C-labelled
methoprene in a dairy cow. Official report from US
Department of Agriculture, Kerrville, apparently
incorporated in previous reference.
Dunham, L.L. and Miller, W.W. Methoprene in Analytical Methods for
1978 Pesticides and Plant Growth Regulators (G. Zweig ed).
10:95-109. Academic Press Inc., New York.
Henrick, C.A., Staal, G.B. and Siddal, J.B. Alkyl 3,7,11-trimethyl-2,
1973 4-dodecadenoates, a new class of potent insect growth
regulators with juvenile hormone activity. J. Agr. Food
Chem., 21(3):354-359.
Mian, L.S. and Mulla, M.S. Persistence of three IGRs in stored wheat.
1983 J. Econ. 1983 Entomol., 76(3):622-625.
Miller, W.W. Analyses of indirect methoprene residues in rice. Report
1974a No. RM-026. Zoecon Corporation.
Miller, W.W. Analysis of methoprene residues in legumes and forage
1974b crops. Zoecon Corporation, Report No. E-48-B-73.
Miller, W.W. Analysis of methoprene residues in mushrooms. Zoecon
1978 Corporation, Report No. E-7-P-78.
Miller, W.W. Analysis of methoprene residues in stored peanuts and
1981 peanut hulls. Zoecon Corporation, Report No. SE-GR 340-79.
Miller, W.W. Analysis of methoprene residue in stored shelled corn.
1983a Zoecon Corporation, Reports Nos. RM-1054, RM-0159 and RM-
0167.
Miller, W.W. Analysis of methoprene residue in wheat grain. Zeocon
1983b Corporation, Report No. R7-0130.
Miller, W.W. Analysis of methoprene residues in cocoa beans. Zoecon
1983c Corporation, Reports Nos. RM-0168, RM-0169.
Miller, W.W. Methoprene residue analysis of dust samples in food
1984 handling establishments. Zoecon Corporation, Reports Nos.
RM-0177, RM-0090, RM-0178, RM-0075 and RM-0132.
Miller, W.W., Wilkins, J.S. and Durham, L.A. Determination of Altosid
1975 insect growth regulator in waters, soils, plants, and
animals by gas-liquid chromatography. J. Assoc. Offic. Anal.
Chem., 58:10-18.
Mura, T. and Takahashi, R.M. Insect development inhibitors. 3. Effects
1973 on non-target aquatic organisms. J. Econ. Entomol.,
66(4):917-922.
Quistad, G.B. and Staiger, L. Determination of bound residues and
1974a material balance for Altosid IGR treated soil. Zoecon
Corporation, Report No. 3760-12-01-74.
Quistad, G.B. and Staiger, L. Partition of Altosid between water and
1974b soil. Zoecon Corporation, Report No. 3760-12-03-74.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1974a degradation of the insect growth regulator methoprene. I.
Metabolism by alfalfa and rice. J. Agric. Food Chem., 22(4):
582-589.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Cholesterol and bile
1974b acids via acetate from the insect juvenile growth hormone
analogue, methoprene. Life Sciences, 15(10):1797-1804.
Quistad, G.B., Staiger, L.E., Bergot, B.J. and Schooley, D.A.
1975a Environmental degradation of the insect growth regulator
methoprene. VII. Bovine metabolism to cholesterol and
related natural products. J. Agric. Food Chem., 213:743-749.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975b degradation of the insect growth regulator, methoprene. III
Photodecomposition. J. Agric. Food Chem., 23:299-303.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975c degradation of the insect growth regulator methoprene. VIII.
Bovine metabolism to natural products in milk and blood. J.
Agric. Food Chem., 23:750.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1976a degradation of the insect growth regulator methoprene X.
Chicken metabolism. J. Agric. Food Chem., 24:644-648.
Quistad, G.B., Schooley, D.A., Staiger, L.E., Bergot, B.J. Sleight,
1976b B.H. and Macek, L.K. Environmental degradation of the insect
growth regulator methoprene. IX. Metabolism by bluegill
fish. Pestic. Biochem. Physiol., 6:523-529.
Rowlands, D.G. The uptake and metabolism by stored wheat grains of an
1976 insect juvenile hormone and two insect hormone mimics. J.
Stored Prod. Res., 12:35-41.
Schaefer, C.H. and Dupras, E.F. Insect developmental inhibitors. 4.
1973 Persistence of ZR-515 in water. J. Econ. Entomol., 66:923.
Schaeffer, CH. and Wilder, W.H. Insect development inhibitors. 2.
1973 Effects on target mosquito species. J. Econ. Entomol.,
66(4): 913-916.
Schooley, D.A. Metabolism of 3H-methoprene by the dwarf lima bean
1974 plant. Zoecon Corporation, Report No. 3760-12-02-74.
Schooley, D.A. and Bergot, B.J. 10-3H-methoprene dissipation rates in
1974 pond water and sewage. Zoecon Corporation, report no.
3760-12-06-74.
Schooley, D.A. and Bergot B.J. Hydrolytic stability of radiolabelled
1975a methoprene. Zoecon Corporation, Report No.
Schooley, D.A. and Bergot, B.J. 3-day metabolism study of Altosid in
1975b non-sterile water. Zoecon Corporation Report No.
Schooley, D.A., Bergot, B.J., Durham, L.L., and Siddall, J.B.
1975a Environmental degradation of the insect growth regulator,
methoprene. II. Metabolism by aquatic micro-organisms. J.
Agr. Food Chem., 23(2):293-298.
Schooley, D.A., Creswell, K.M., Staiger, L.E. and Quistad, G.B.
1975b Environmental degradation of the insect growth regulator
methoprene. IV. Soil metabolism. J. Agric. Food Chem.,
23(3): 369-373.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-update of
1974a methoprene and its metabolites by a second crop of wheat
grown in treated soil. Zoecon Corporation, Report No.
3760-12-08-74.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-translocation of
1974b methoprene and its metabolites from soil into water. Zoecon
Corporation, Report No. 3760-12-04-74.
Zoecon Methoprene residues in chickens - Report submitted to FAO for
1984a consideration by JMPR. Zoecon Corporation.
Zoecon Altosid leaching studies - Report submitted to FAO for
1984b consideration by JMPR - Zoecon Corporation.
Zoecon Methoprene distribution in Australian wheat fractionation.
1984c Report RM-0248, 17 September 1984. Zoecon Corporation.
Zoecon Methoprene distribution in Kansas State University. Wheat
1984d fractonation study. Report RM-0250, 18 September 1984.
Zoecon Corporation.
Synonyms: ALTOSID, APEX, DIACON, KABAT, DIANEX, PRECOR.
(All registered Trade Names) ZR-515; ENT-70460;
CAS No: 114-26-1
Other information on identity and properties:
Molecular formula C19H34O3
Molecular weight: 310
Physical form: Pale yellow liquid
B.P.: 100° at 0.05 mm Hg
Vapour pressure: 2.37 × 10-5 mm Hg at 25°C
1.60 × 10-4 mm Hg at 40°C
Solubility: all organic solvents-infinity
water - 1.39 mg/l.
Specific gravity: 0.9261 g/cc at 20°C
Octanol/water
partition coefficient: 10,000
Cis-2 : trans-2 ratio: 8:92
Purity: methoprene - 92-95%
related isomers - 5-2%
solvent - 1-2%
unidentified - 2-1%
Stability: extremely stable in sterile aqueous
solutions over wide range. Readily
biodegraded by common bacteria. Rapidly
degraded in solution or in thin films by
sunlight and UV light. Identity of
degradation products known. (Quistad
et al., 1975b).
Formulations: Several emulsifiable formulations for
agricultural, stored product and public
health use; solution formulation for
treating cured tobacco; fogging solution
for public health use; emulsifiable
formulations for addition to feed
supplements; slow release formulation
for use in mosquito abatement;
emulsifiable formulation for protection
of houseplants.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methoprene is an insect growth regulator (IGR), being a synthetic
analogue of the insect juvenile hormone (Henrick et al., 1973).
Unlike conventional insecticides, which act as direct poisons,
methoprene acts by disrupting the morphological development of
insects. After contact, inhalation or ingestion the insect grows and
pupates normally, but the pupa dies in the pupal stage. As a result,
methoprene is not generally used in pre-harvest situations when a
quick kill of insect pests is required.
Methoprene has found widespread use in mushroom culture to
prevent the emergence of adult sciarid flies, where it is applied to
compost or casing at the rate of about 100 g/100 sq.m. This
application represents about 12% of methoprene use. A commonly used
treatment is 175 ml of 650 g/l emulsifiable concentrate per 100 sq.m.,
equivalent to 113 g a.i./100 sq.m. One or two applications are used.
Special slow-release formulations are used to control mosquito
breeding in floodwater sites, rice cultivations, storm drains, ponds,
water treatment works etc. (Schaefer and Wilder, 1973; Mura and
Takahashi, 1973).
Methoprene is formulated into feed supplements for cattle to
control adult hornfly breeding in cattle manure. The rate is
equivalent to 0.016 mg/kg body weight daily. This, together with
mosquito control represents about 50% of current use.
Methoprene is marketed as a 65% emulsifiable concentrate
formulation for use as a space spray for control of pests in food
handling and storage establishments. It is applied as an aqueous spray
or via mechanical foggers at a rate of 10 ml per 1,000 m2.
Post-harvest treatments
Methoprene has proved effective in the control of numerous pests
of stored products such as peanuts where it is used at the rate of
10g/tonne against most moth and beetle pests. It is being tested on
cocoa beans and cereal grains as a 657g/l emulsifiable concentrate
formulation. This is applied at a rate of 15ml/tonne of stored
commodity, equivalent to 10g a.i./tonne, in either water or mineral
oil.
Trial work indicates that methoprene will control most insect
pests affecting stored grain though it is significantly less effective
against Sitophilus spp. which include three of the major pests of
maize, rice and wheat. It may have to be combined with a conventional
insecticide where these species are a problem.
Methoprene has been used for more than five years for treating
stored tobacco. The cured tobacco is treated, before packing, at the
rate of 200g methoprene per tonne of tobacco. It is used for treating
food-handling and tobacco-processing establishments to prevent insect
breeding and development. A fogging formulation is used to control
pre-adult fleas in premises.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in stored agricultural commodities
Peanuts. During the period 1979-1981 the manufacturers, in
conjunction with the US Department of Agriculture, undertook trials of
methoprene for protecting peanuts against stored-product insect pests
(Miller 1981). The methoprene was added as the peanuts were being
placed in storage silos. Normally peanuts are stored for a period from
a few months to a year.
Treatment rates were 0, 4, 10, 25 and 100 mg/kg. Nuts (kernels)
and hulls were analysed separately. Residues at the proposed use rate
of 10 mg/kg on the day of treatment were below the limit of
determination (0.05 mg/kg) in kernels with wrappers, 0.05-0.64 mg/kg
in kernels without hulls (split hulls) and 14-20 mg/kg in hulls. After
278 days storage the kernels with wrappers contained 0.13-0.38 mg/kg
(mean of 11 samples 0.22 mg/kg), kernels without hulls (split hulls)
0.38-2.1 mg/kg and hulls 8.3-14.0 mg/kg.
Peanuts treated at exaggerated rates contained residues
proportionally higher than those treated at the proposed use rate.
Maize. Eight studies were carried out on stored shelled maize
(Miller, 1983a). Random samples from bulk lots treated at rates of 10,
15, 20 and 23.5 mg/kg were drawn at intervals up to 13 months after
treatment. At all application rates, residues showed no significant
losses with time, even at 200 days after treatment. It is noteworthy
that at no stage did the residues determined by analysis reflect the
amount of active ingredient actually applied to the grain, and in most
trials ranged between 40% and 70% of it. It is difficult to see
whether this was due to failure to deposit the methoprene on grain,
loss from the grain surface immediately after treatment, loss from the
samples between collection and analysis, or failure to recover residue
from the substrate.
The analytical method included an internal standard which was
added in the blender. Recovery of the internal standard was always
high (80-110%), implying validity of the method and analytical
procedures. However, there is no explanation of the apparent absence
of methoprene. Nevertheless the residue levels found throughout the
storage period were similar to, or higher than, those reported for the
initial sample, indicating that little degradation had occurred during
storage.
Wheat. Data from two studies (Miller, 1983b) were available. Stored
wheat was treated with methoprene at 10 and 20 mg/kg and sampled 3,
35, 95 and 150 days after treatment. Methoprene residues recovered by
analysis were about one-half of the treatment rate but did not show
any decline up to 150 days after treatment. Residues from the 10mg/kg
treatment were 5.2 mg/kg after 150 days. An explanation for this
discrepancy is needed.
In a laboratory study at the University of California using GLC
analysis, Mian and Mula (1983) found that when methoprene was applied
at rates of 1, 5 and 10 mg/kg to wheat (13.5% moisture content) stored
at 27 ± 1°C, losses were 61, 66 and 62% respectively over a period of
12 months. The results of analyses carried out at six times within the
12-month period are given in Table 1.
These data seem more reliable than those reported by Miller
(1983b), since the main initial recovery of insect growth regulator was
99% of that applied. Thus it can be accepted that the loss over 12
months is of the order of 60% of the amount applied.
Rowlands (1976), determined the rate of breakdown at 20°C during
10 weeks storage in the dark, using radiolabelled methoprene with
three samples of wheat: fresh wheat of 19.1% moisture content, old
wheat of 12% moisture content and old wheat of 18% moisture content.
Table 2 (adapted from Rowlands, 1976) indicates that the breakdown was
quite rapid at the high moisture levels (half-life of three weeks at
18-19% moisture) and was about half as fast on grain with 12% moisture
(half-life six weeks).
Table 1. Residues of methoprene in treated wheat grain at
various post-treatment intervals 1
Residue, mg/kg
Interval
Application rate, mg/kg
1 5 10
0 0.99a 4.97a 9.92a
1 wk 0.99a 4.60a 9.39a
(0) (7) (5)
1 mo 0.94a 3.93a 6.62b
(5) (21) (33)
4 mo 0.82a 3.49ab 5.53bc
(17) (30) (44)
8 mo 0.80a 1.78bc 4.07c
(19) (64) (59)
12 mo 0.38b 1.71c 3.73c
(61) (66) (62)
1 Mean of four analyses. Means followed by the same letters
in a column are no significantly different from one another
(Duncan's multiple range test. P = 0.05). Values in
parentheses below each mean represent percent loss in
residues at indicated post-treatment interval.
Table 2. Breakdown of methoprene on stored wheat grains at 20°C*
Residue, mg/kg found in grain
Storage time
in weeks Fresh wheat Old wheat Old wheat
(19%) (12%) (18%)
0 5.6 9.8 9.6
1 7.2 9.2 7.7
2 5.5 7.9 6.3
3 4.4 7.6 5.0
4 3.4 7.0 4.3
5 2.2 6.2 3.2
6 1.4 5.1 2.1
7 0.5 4.2 1.4
8 0.2 3.2 0.5
9 0.1 2.4 0.4
10 nil 1.5 0.2
* Results are means from triplicate 25g samples, treated at
10 mg/kg (approx.)
Cocoa beans. In two trials carried out in Brazil, methoprene was
applied to cocoa beans at rates of 1, 2.5, 5.0, 10.0 and 15 mg/kg. The
beans were sampled on the day of treatment and after 50 days storage
at 27°C. Less than half of the applied methoprene was recovered by
analysis but the analytical results on samples taken after 50 days in
storage were not significantly different from those on the initial
samples (Miller, 1983c).
Residues in mushrooms
Data from 7 studies were available (Miller 1978), reflecting both
compost and casing treatment at 0.2 to 4.6 times the recommended
application rates. Samples were collected at 0, 9 and 35 days after
application. When less than 2.5 times the recommended combined
treatment rates were used, the residues were undetectable
(<0.05 mg/kg). At 2.5 and 4.6 times the recommended application rate,
residues ranged from 0.17 mg/kg to 150 mg/kg. The high values were due
to contamination with compost or peat moss "casing" which was shown to
contain methoprene at hundreds of mg/kg. Subsequent samples were
rinsed with cold water to remove the contamination. Most samples
examined in these later experiments contained no methoprene or the
residues were less than 0.1 mg/kg (Miller, 1978).
Dust samples from food-handling establishments were analysed in
six studies (Miller, 1984). Isolated areas were treated with
0.6g a.i./1000 sq.m. (the food being covered according to label
direction before application). Samples were collected in petri dishes
located in appropriate areas of the treated spaces 0, 30, 60 and 90
days after treatment. Residues expressed as mg/kg dust were high owing
to the small amount of dust collected in the dishes. Consequently, the
total quantity of methoprene collected is a more realistic value to
consider when evaluating the results.
Immediately after application methoprene residues were high,
ranging from 26 micrograms to 191 mg. However within 30 days they
declined significantly and ranged from 0.5 to 24 mg. Although there is
some variation in the results, within 60 days the majority of the
residues were below the level of detection (0.5 micrograms).
Residues in forage and field crops
Methoprene is marketed for the control of floodwater mosquitoes
as a 10% slow-release encapsulated aqueous solution (Schaefer and
Wilder, 1973). Since treated areas include pastures, rice fields and
marshlands, residues might occur on certain food crops. Consequently,
Zoecon conducted a series of studies on rice, legumes and forage crops
to analyse for possible methoprene residues which could enter the food
chain through ingestion by livestock.
In a study on rice, methoprene was applied prior to "heading"
(Miller, 1974a). The crop was then grown to harvest. Samples were
collected at 45, 62 and 71 days after application, resulting in a
built-in pre-harvest interval of two months. No detectable residues
(<0.05 mg/kg) were found in the rice grains, hulls or straw of any of
the samples.
In nine studies on pasture legumes and forage crops (Miller,
1974b) pasture land was treated at rates of 9, 11, 23 and 45 g/ha. The
highest treatment rate is four times the highest recommended rate.
Samples were collected 0, 1, 2, 3, 5, 7, 9, 11 and 365 days after
application. Residue levels indicated that methoprene is rapidly
degraded. By day 3, the residues from the highest treatment ranged
from 0.12 to 0.30 mg/kg.
FATE OF RESIDUES
In animals
Numerous studies have been conducted to determine the fate of
methoprene in livestock because of the commercial use in animal feeds
to suppress the breeding of flies in manure.
Quistad et al. (1974b, 1975a) administered [5-14C] methoprene
to a steer which was slaughtered two weeks later. Samples of fat,
muscle, liver, lung, blood and bile were analysed for radioactive
residues. No primary methoprene metabolites could be characterised but
the majority of the tissue radioactivity was positively identified as
cholesterol. A total of 72% of the bile radioactivity was contributed
by cholesterol, cholic acid and deoxycholic acid. Radioactivity from
metabolised methoprene was associated with protein and cholesteryl
esters of fatty acids.
A metabolic balance study was conducted on a 338 kg Jersey cow
dosed with 207 mg of [5-14C] methoprene by capsule (Chamberlain
et al 1975b). This is 40 times the proposed dose for fly control.
The study proceeded for 7 days after dosing. It was found that 16.4%
of the radioactivity was excreted as CO2 between 0 and 170 hours,
with peak excretion after a little under 24 hours. A total of 30.3% of
the applied dose was eliminated via the faeces within about 50 hours
and 19.7% via the urine in 30 hours. By the end of the experiment 7.6%
of the radiolabel had been excreted via the milk. Radioactivity was
measured in 30 tissue samples after the cow was sacrificed 7 days
after dosing. The tissues contained a total of 20% of the applied
dose, intestine and contents contributing 5.96% and fat 4.59%. The
organs of metabolism, lung, liver and kidney, contributed a further
1.68%. The presence of radiocarbon in the tissues was attributed to
steroidal derivatives in which labelled acetate from the methoprene
was anabolically incorporated into natural body constituents.
The results of the above two studies and another on a guinea pig
were compared by Chamberlain et al. (1975a). It was reported that a
rather large percentage of the radiolabel was incorporated in the
tissues and respired by the animals. A small proportion was
incorporated into free primary metabolites, a greater amount into
glucuronides and a considerable proportion within polar components,
possibly complex conjugates or biochemicals. Methoprene was not found
in the urine, but accounted for approximately 40% of the radiolabel in
the faeces. This explains the activity of the administered methoprene
against fly larvae developing in dung pats. The formation of
conjugates and complex metabolites was more extensive in the steer
than the guinea pig. Comprehensive information was provided on the
radiolabel profiles in tissues, organs and other components of the
animals.
On the basis of detailed biochemical analysis of tissues and
organs from the above three studies Quistad et al. (1975c)
elucidated the metabolism by the cow to natural products in milk and
blood. Only a trace of methoprene (0.015/l) and no detectable primary
metabolites occurred in milk.
Quistad et al. (1976a) studied the metabolism of methoprene in
leghorn chickens give single oral doses of [5-14C] methoprene.
Residual radioactivity was found in tissues and eggs. Although a high
initial dose (59 mg/kg)resulted in residues of methoprene in muscle
(0.01 mg/kg), fat (2.13 mg/kg) and egg yolk (8.03 mg/kg), these
represented only 39% and 2% of the total radiolabel in fat and egg
yolk respectively. Radiolabelled natural products were by far the main
14C residues in tissues and eggs, particularly at the lower dose of
0.6 mg/kg where cholesterol and normal fatty acids contributed 8% and
71% of the total radiolabel in egg yolk.
When chickens were given feed treated with methoprene at 0, 25,
50 and 100 ppm for 14-63 days, residues in poultry meat and eggs were
less than 0.1 mg/kg (Zoecon, 1973).
Quistad et al. (1976b) studied methoprene metabolism in
bluegill fish in a dynamic flow-through system and a model aquatic
ecosystem. The fish in the flow-through system acquired moderate
residues of largely unmetabolised methoprene when continuously exposed
to about 30 times the anticipated environmental levels of methoprene,
but these were 93-95% eliminated within two weeks when the fish were
transferred to flowing uncontaminated water. In the model aquatic
ecosystem, the fish accumulated 14C, but the radioactivity was almost
exclusively in radiolabelled natural products, including cholesterol,
free fatty acids, glycerides and protein. Less than 0.1% of the total
radioactivity could be attributed to methoprene or its primary
metabolites.
In plants
Methoprene is rapidly degraded in plants with a half-life of
about 2 days in alfalfa and about 0.5 days in rice. The major
metabolic pathways include ester hydrolysis, O-demethylation and
oxidative scissions of the 4-ene double bond, producing primary
metabolites which are present in relatively low yields (Figure 1)
(Quistad et al. 1974a). These low amounts suggest that subsequent
degradation or conjugation occurs, and in fact the recovery of polar
metabolites as well as the loss of radioactivity through
volatilization are evidence of additional metabolism. In addition,
unextractable residues suggest the extensive degradation of methoprene
and incorporation into natural cellular products. The metabolic fate
of methoprene in alfalfa and rice is shown in Figure 1. Metabolism
resulted in five primary non-polar products isolated from the plant
(ZR-669, ZR-725, ZR-724, ZR-1945 and ZR-1602, see Figure 1). The
combined yield of these products was 1.4% of the applied dose after 7
days from alfalfa and 2.1% after 3 days from rice. The most abundant
non-polar metabolite was ZR-1564 (13% from rice, 2% from alfalfa)
which was isolated from vapours and not found within the plant. The
main aglycones after enzymic cleavage of alfalfa conjugates were
ZR-725 (2.2%), ZR-1945 (0.8%), and ZR-724 (7.4%). The major aglycone
from rice was ZR-1945 (1.2%) with a small amount of ZR-1602 (0.3%).
After 30 days, only 1% of the applied dose remained in alfalfa as
unmetabolised methoprene and 0.4% remained in rice after 15 days. The
initial rapid loss of methoprene and radiolabel from plants was
attributed to evaporation of methoprene and 7-methoxycitronellal
(ZR-1564) on the evidence from supporting studies of methoprene
evaporation from glass plates and of compounds volatilized from leaf
surfaces.
10-3H-methoprene painted on the leaf surface or injected into
the stem of the dwarf Lima bean plant was found not to be
translocated. When injected into the stem, the parent compound was
partially metabolised to ZR-724, ZR-725 and a polar conjugate,
possibly the glycoside. Topical application resulted in more rapid
metabolism to the same metabolites after 24 hours incubation
(Schooley, 1974).
Rowlands (1976) studied the uptake and metabolism of methoprene
and two other IGRs by stored wheat grains. He applied the
radiolabelled compounds topically to individual grains and to small
samples of wheat in glass jars. The wheat contained 19.5% moisture and
was kept at 20°C in the dark. The methoprene penetrated rapidly from
both vapour and topical treatments, but the quantities sorbed differed
between the two routes. Two days after treatment most of the intact
methoprene was found in the aleurone layers, much less in the germ and
virtually none in the endo-sperm proper or outer seedcoat. The rate of
degradation is indicated in Table 2 above. Degradation is promoted by
grain moisture, wheat with 19% moisture giving a half-life of 2-3
weeks, 18% moisture 3-4 weeks and 12% moisture 6-7 weeks.
Mian and Mullar (1983) determined the residues of methoprene on
wheat over a 12-month storage period. Details are given above
("Residues resulting from supervised trials").
In soil
Schooley et al. (1975b) studied the degradation of methoprene
in soil as a function of time under various conditions. On aerobic
sandy loam methoprene showed an initial half-life of about 10 days at
a surface treatment rate of 1 kg/ha; decomposition was much slower on
autoclaved soil. Only small amounts of non-polar metabolites were
isolated, including the hydroxy ester resulting from O-demethylation
(0.7% of the applied dose). Over 50% of the applied dose was converted
to CO2. Radioactivity from [5-14C] methoprene was incorporated into
humic acid, fulvic acid, and humin fractions of sandy loams. These
studies indicate rapid and extensive breakdown of methoprene in soils.
Leaching experiments with radiolabelled methoprene were performed
to determine the mobility of the chemical in different soils. The four
agricultural soils used were sand, sandy loam, silt loam and clay
loam. Experiments with soil columns and soil thin-layer plates
revealed that no significant leaching of methoprene occurred with the
four soils tests (Zoecon, 1984b).
Quistad and Staiger (1974a) determined the amount and nature of
bound residues in soil. They found that in sandy loam soil treated
with [5-14C] methoprene humic acids (3%) and fulvic acids (8.4%) were
most abundant after 30 days. The total recovery of the label from
treated soil was essentially quantitative.
Quistad and Staiger (1974b) determined the partition between
water and soil using a 0.01 mg/l solution of [5-14C] methoprene
agitated with sandy loam soil. After thorough mixing for 15 minutes,
87% of the applied dose was bound to the soil.
Staiger et al. (1974a) grew wheat plants in soil that had been
treated 6 months previously to determine the uptake by a second crop
grown in methoprene-contaminated soil. After treatment of the soil at
the rate of 1 kg/ha, less than 0.006% of the administered label was
taken up in wheat six months after dosing. This uptake is about
one-tenth of that observed when soil was treated with radiolabelled
methoprene at the time seeds were planted.
Staiger et al. (1974b) determined the uptake of methoprene by
wheat plants when wheat seeds were planted in sandy loam and
radiolabelled methoprene was incorporated at the rate of 1 kg/ha at
the seed level and in the soil at a depth of 9 cm below the seed. The
wheat was harvested 11 days after planting and assayed after total
combustion. Uptake was found to be 0.09% of the applied radioactivity
when the IGR was inserted at the level of the seed and 0.04% of the
radioactivity when it was incorporated 9 cm below the seeds. It appears
that very little methoprene or its metabolites are translocated into
wheat from soil.
In water
Sterile aqueous solutions of methoprene (0.5 mg/l), buffered at
various pH values, were found to be extremely stable to hydrolysis
over four weeks at 20°C in the dark. No degradation was seen for the
duration of the experiment in sterile water buffered at pH 7 and 9,
and similar stability was observed in pH 5 buffer for 21 days. However
the pH 5 buffer became non-sterile between the 21- and 30-day
observations, and 59% degradation occurred between days 21 and 30.
(Schooley and Bergot, 1975a).
Schaefer and Dupras (1973) studied the persistence of methoprene
in water. They found that residues were greatly affected by sunlight
and temperature. The methoprene tended to remain on the water surface
and its distribution was affected by wind. The half-life in field
water was only about two hours. A slow-release formulation showed
almost no detectable residues after 24 hours, although biological
activity persisted for several days.
Schooley and Bergot (1974) measured the dissipation of methoprene
in pond water and sewage at dose rates of 0.001 and 0.01 mg/l. A
half-life of 30-45 hours was observed in pond water, with a 60-70 hour
half-life in sewage. This significantly longer half-life in sewage may
have been due to the fact that the primary sewage sample had been
standing in a closed bottle for 30 days in the laboratory, while the
pond water had been collected fresh on the day of the experiment.
On standing for thirteen days in unsterilised pond water
containing suspended sediment in full summer sunlight at a
concentration of 0.66 mg/l, methoprene was metabolised and/or
photodegraded mainly to one product, ZR-1945 methoxycitronellic acid
(Schooley et al. 1975a). This is one of many products formed by the
combined action of oxygen and sunlight on thin films of methoprene on
glass plates. ZR-724, ZR-725 and ZR-669 (See Figure 1) were either not
present or present in very low concentrations.
A 3-day study was conducted on methoprene in non-sterile pond
water at ambient temperature in direct sunlight (Schooley and Bergot,
1975b). Three primary products, ZR-724, ZR-725, ZR-669 were
identified. Each compound, as well as the recovered methoprene, was
found to be an approximately 1:1 mixture of cis-2 and trans-2
isomers, in contrast to the 94% trans-2 methoprene which was
applied. This conversion has been shown in other studies to be ready
photoisomerization rather than an enzymic process.
The biodegradation of (2E)-[10-3H] methoprene was studied in
pond water containing unknown micro-organisms (Schooley et al
1975a). The methoprene showed a half-life of approximately 30 hrs at
0.001 mg/l and 40 hr at 0.01 mg/l. Incubation of the same preparation
for 3 days at 0.42 mg/l generated three primary metabolites, the
result of ester hydrolysis and/or O-demethylation. These metabolites
and the recovered methoprene were photoequilibrium mixtures of 2-ene
isomers. In another incubation experiment with (2E)-[5-14C]
methoprene at 0.66 mg/l in a pond water sample with presumably
different microflora, a completely different metabolic profile was
observed. The main and only identifiable metabolite, resulting from
oxidative scission of the 4-ene double bond, was 7-methoxycitronellic
acid (29% of the applied dose).
In processing and cooking
Peanuts. Peanuts from one of the trials Miller (1981) (see
"Residues resulting from supervised trials") with residues ranging
from 0.18 to 1.21 mg/kg at the time of treatment, were processed into
peanut butter and peanut oil. The peanut butter was reported to
contain less than 0.05 mg/kg of methoprene, indistinguishable from
untreated controls. The peanut oil, however, contained 0.25 mg/kg
(0.20-0.29) of methoprene. These results do not appear to be
consistent.
A trial was carried out in Australia (Zoecon 1984c) in which
methoprene was applied to wheat at the rate of 10 mg/kg. After storage
for two weeks the wheat was milled through an experimental Buhler mill
to provide bran, pollard and white flour. A separate milling produced
wholemeal. The residues found, and the proportion of each fraction in
the first milling, are shown in Table 3.
A separate trial carried out at Kansas State University (Zoecon,
1984d) involved treatment of two lots of wheat with methoprene at the
rate of 10 mg/kg followed, 9 days later, by milling through a Ross
Walking Flour Mill. A separate milling through a pin mill produced
wholemeal.
The white and wholemeal flours were used to prepare bread. The
results of analysis are shown in Table 4.
METHODS OF RESIDUE ANALYSIS
Residues of methoprene have been determined in waters, soils,
plants, stored grain and corn, milk, eggs, fish, shellfish, poultry
and cattle tissues, blood, urine and faeces. Residues in all samples
are extracted by high-speed blending with acetonitrile followed by
vacuum filtration. Fatty extracts are subjected to cold-temperature
precipitation and filtration to remove fat. Petroleum ether
partitioning, Florisil and neutral alumina chromatography are used for
clean-up. The concentrated eluates are analyzed by GLC on columns of
differing polarity, using flame ionization detectors. Confirmation of
identity by additional GLC and mass fragmentography (MF). The lower
limits of determination are: soils, blood and urine, 0.001 mg/kg;
forage grasses, forage legumes and rice foliage, 0.005 mg/kg; milk,
eggs, stored grain and corn kernels, fish, shellfish, poultry and
cattle tissues and faeces, 0.01 mg/kg. Limits of determination and
recoveries were validated by analysis of laboratory and field samples
fortified with 14C-methoprene (Miller et al., 1975).
Table 3. Methoprene residues in wheat fractions Zoecon, 1984c)
Fraction % wt./wt/ of each Methoprene (mg/kg)
fraction (excluding
wholemeal)
Wheat 8.9
Wholemeal flour 6.7
White flour 74 1.8
Bran 23 11.4
Pollard 3 17.7
An internal standard was used in the analysis.
Table 4 Methoprene residues in wheat fractions (Zoecon, 1984d)
Fraction % mt/wt of each fraction Methoprene (mg/kg)
(excluding wholemeal)
Trial Trial Trial Trial
Wheat (whole) 5.0 5.7(Note 1)
White flour 70.2 70.1 2.8 2.8
Wholemeal flour 4.1 5.5
Bran 14.1 14.4 8.7 10.2
Shorts ) 12.7 12.4 9.6 9.6
pollard
Red dog ) 2.4 2.5 9.5 11.0
Germ 0.6 0.6 8.7 10.7
White bread (33% moisture) 0.41
Wholemeal bread (33% moisture) 0.88
Note 1 It appears that a significant proportion of the original
deposit (10 mg/kg) was lost, possibly by evaporation, between
application and analysis. Spiked samples analysed at the same
time yielded the results expected.
Mian and Mulla (1983) describe a method derived from that of
Dunham and Miller (1978) which they applied successfully to studying
the persistence of methoprene in stored wheat. This involved Florisil
and Alumina chromatography with petroleum ether followed by GLC/FID on
a column of 3% OV-101 on 100-120 mesh Chromosorb W (acid-washed,
dimethyldichlorosilane treated), conditioned at 350°C. The column and
injector temperatures were 190°C for retention times of 8 min for
methoprene and 12 min for n-docosane used as an internal standard. The
detector temperature was 350°C. The limit of determination was
reported to be 0.01 mg/kg and the recovery of 10, 5 and 1 mg/kg in
grain extracts ranged between 80 and 100%.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
No information was available.
NATIONAL MAXIMUM RESIDUE LIMITS
The meeting was advised that the following national maximum
residue limits had been established in the USA.
Meat of cattle, goats, hogs, sheep,
poultry and horses 0.1 mg/kg
Fat of cattle, goats, hogs, sheep,
poultry and horses 0.3 mg/kg
Meat by-products of cattle, goats, hogs,
sheep, poultry and horses 0.1 mg/kg
Milk fat 0.05 mg/kg
Eggs 0.05 mg/kg
Peanuts 2.0 mg/kg
Mushrooms 1.0 mg/kg
A petition has been presented for tolerances of 10 mg/kg on
barley, buckwheat, corn, millet, oats, rice, rye, sorghum, sunflower
and wheat. In addition a substantial increase has been proposed in the
tolerances for indirect residues, derived from animal feeds, in meat,
fat and meat by-products of cattle, goats, hogs, sheep, poultry and
horses and in eggs and milk fat.
The meeting was not aware of similar action by any other national
authority.
APPRAISAL
Methoprene is an insect growth regulator. It is an analogue of
the insect juvenile hormone. After contact, inhalation or ingestion it
interferes with the normal process of insect development preventing
the emergency of adults from pupae or larvae.
So far there have been no practical pre-harvest uses of
methoprene on crop plants and it appears unlikely that such uses will
develop. There is an important application for the control of sciarid
flies in mushroom culture and as a feed supplement for cattle to
prevent the breeding of hornflies in cattle manure. Methoprene is
applied to water to prevent the breeding of mosquitoes.
The most important application currently under development is the
use of methoprene to prevent the breeding of stored-product insect
pests in tobacco, peanuts, cocoa beans and a wide range of stored
commodities. There are good prospects for use in stored grain to
inhibit the development of stored-product insects. Extensive studies
on these uses are at an advanced stage in several countries.
Methoprene formulations are stable under normal storage
conditions but are rapidly degraded by light. Sterile aqueous
solutions are stable over a wide pH range but non-sterile solutions
are rapidly biodegraded.
When used for the control of insects in stored products
methoprene is applied alone at the rate of 10 mg/kg or in conjunction
with conventional insecticides at much lower rates (1-2 mg/kg).
Although the residues studies available at this stage are not
extensive it would appear that there is relatively little degradation
on dry stored commodities over many months. Degradation is promoted by
moisture and probably also by temperature.
In numerous field studies on peanuts, maize, cocoa beans and
wheat, investigators have failed to recover more than half of the
methoprene applied. This difficulty was not encountered in one small
scale laboratory study at the University of California. This suggests
a pronounced tendency for the methoprene to evaporate with the water
used to apply the insect growth regulator or a fundamental weakness in
the trial technique. This feature is currently receiving further
study.
The meeting received studies from Australia and the USA designed
to determine the distribution of methoprene in grain and the effect of
processing and cooking on the residues. These enabled the meeting to
propose temporary maximum residue limits.
One study with the labelled compound applied to individual wheat
grains indicated rapid penetration. A study on peanuts indicated that
by far the greater proportion of the deposit remained on the hulls and
wrappers and was not taken up by the kernel. However, allowance must
be made for the fact that a proportion of the kernels lose their
shells. When the peanut kernels were processed into peanut butter the
methoprene disappeared but significant amounts were detected in peanut
oil. These studies should be repeated.
When methoprene is applied to mushroom compost or casing at rates
which will control sciarid flies there are no detectable residues in
mushrooms. However, it is necessary to wash the peat moss casing from
the mushrooms before analysis to prevent anomalous results.
Methoprene would be suitable for the treatment of surfaces and
structures to prevent insect pests breeding in food storage and
preparation areas. Several studies indicated that such treatments
would not lead to significant residues in foodstuffs.
Studies have been conducted with cattle, poultry and fish to
elucidate the metabolic processes and determine whether residues occur
when methoprene is administered to livestock directly for fly control,
or in their feed from cereal grain and milling products, or when the
compound is used for mosquito control. All available information
points to the rapid degradation of the parent compound and
incorporation of fragments into natural body components but the
meeting, recognizing that it would not be practical to withdraw
treated feed before slaughter, recommended temporary MRLs for
methoprene residues in foods of animal origin to cover the feeding of
treated grain and its milling products.
Methoprene is rapidly metabolised in plants, yielding products
that are further degraded to normal plant building materials. When
applied to soil, most of the dose is converted rapidly to CO2. There
is no significant leaching of methoprene from treated soil and no
uptake by plants. There appears to be no likelihood of methoprene
being taken up by rice, forage or other crops growing in water treated
for mosquito control.
Methoprene residues may be determined by extraction with
acetonitrile followed by clean-up on Florisil and alumina columns and
quantitation by GLC with a flame ionization detector. The method
appears suitable for regulatory purposes. The limit of determination
ranges from 0.001 mg/kg to 0.01 mg/kg.
The meeting was aware that MRLs had been established for a range
of commodities in the USA and that others were under consideration.
RECOMMENDATIONS
The meeting recognized that there were, as yet, only a limited
number of registrations for the use of methoprene but that potentially
important developments might not proceed in the absence of appropriate
maximum residue limits. To assist these further developments and to
cater for existing uses, the following temporary MRLs are recommended
to apply to residues of methoprene resulting from the direct
application of methoprene or the feeding of methoprene-treated
commodities. They apply to methoprene only.
Commodity MRL (mg/kg)
Cereal grains 10
Bran 20
Flour (wholemeal) 10
Flour (white) 5
Carcase meat 0.2 (in the carcase fat)
Meat byproducts 0.1
Eggs 0.05
Milk 0.002
Peanuts 2
Mushrooms 1
FURTHER WORK OR INFORMATION
Required (before maximum residue limits can be confirmed):
1. Information from studies (now in progress) in which methoprene is
used for the control of pests of stored grain.
2. Studies to define the rate of degradation of methoprene in stored
products under a range of conditions.
3. Further information on the fate of methoprene residues in cereal
grains and other stored products as a result of processing and
cooking.
4. Information from current trials with stored dried lentils, beans,
dried fruit, cocoa beans, coffee beans, spices and oilseeds to
define the treatment necessary, the rate of degradation and the
fate of residues in processing and cooking.
REFERENCES
Chamberlain, W.F., Hunt, L.W.M., Hopkins, D.E., Gingrich, A.R.,
1975a Miller, J.A. and Gilbert, B.N. Absorption, excretion, and
metabolism of methoprene by a guinea pig, a steer and a cow.
J. Agric. Food Chem., 23(4):736-742.
Chamberlain, W.F., Hunt, L.M., Hopkins, D.E., Miller, J.A., Gingrich,
1975b A.R. and Gilbert, B.N. A balance study with 14C-labelled
methoprene in a dairy cow. Official report from US
Department of Agriculture, Kerrville, apparently
incorporated in previous reference.
Dunham, L.L. and Miller, W.W. Methoprene in Analytical Methods for
1978 Pesticides and Plant Growth Regulators (G. Zweig ed).
10:95-109. Academic Press Inc., New York.
Henrick, C.A., Staal, G.B. and Siddal, J.B. Alkyl 3,7,11-trimethyl-2,
1973 4-dodecadenoates, a new class of potent insect growth
regulators with juvenile hormone activity. J. Agr. Food
Chem., 21(3):354-359.
Mian, L.S. and Mulla, M.S. Persistence of three IGRs in stored wheat.
1983 J. Econ. 1983 Entomol., 76(3):622-625.
Miller, W.W. Analyses of indirect methoprene residues in rice. Report
1974a No. RM-026. Zoecon Corporation.
Miller, W.W. Analysis of methoprene residues in legumes and forage
1974b crops. Zoecon Corporation, Report No. E-48-B-73.
Miller, W.W. Analysis of methoprene residues in mushrooms. Zoecon
1978 Corporation, Report No. E-7-P-78.
Miller, W.W. Analysis of methoprene residues in stored peanuts and
1981 peanut hulls. Zoecon Corporation, Report No. SE-GR 340-79.
Miller, W.W. Analysis of methoprene residue in stored shelled corn.
1983a Zoecon Corporation, Reports Nos. RM-1054, RM-0159 and RM-
0167.
Miller, W.W. Analysis of methoprene residue in wheat grain. Zeocon
1983b Corporation, Report No. R7-0130.
Miller, W.W. Analysis of methoprene residues in cocoa beans. Zoecon
1983c Corporation, Reports Nos. RM-0168, RM-0169.
Miller, W.W. Methoprene residue analysis of dust samples in food
1984 handling establishments. Zoecon Corporation, Reports Nos.
RM-0177, RM-0090, RM-0178, RM-0075 and RM-0132.
Miller, W.W., Wilkins, J.S. and Durham, L.A. Determination of Altosid
1975 insect growth regulator in waters, soils, plants, and
animals by gas-liquid chromatography. J. Assoc. Offic. Anal.
Chem., 58:10-18.
Mura, T. and Takahashi, R.M. Insect development inhibitors. 3. Effects
1973 on non-target aquatic organisms. J. Econ. Entomol.,
66(4):917-922.
Quistad, G.B. and Staiger, L. Determination of bound residues and
1974a material balance for Altosid IGR treated soil. Zoecon
Corporation, Report No. 3760-12-01-74.
Quistad, G.B. and Staiger, L. Partition of Altosid between water and
1974b soil. Zoecon Corporation, Report No. 3760-12-03-74.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1974a degradation of the insect growth regulator methoprene. I.
Metabolism by alfalfa and rice. J. Agric. Food Chem., 22(4):
582-589.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Cholesterol and bile
1974b acids via acetate from the insect juvenile growth hormone
analogue, methoprene. Life Sciences, 15(10):1797-1804.
Quistad, G.B., Staiger, L.E., Bergot, B.J. and Schooley, D.A.
1975a Environmental degradation of the insect growth regulator
methoprene. VII. Bovine metabolism to cholesterol and
related natural products. J. Agric. Food Chem., 213:743-749.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975b degradation of the insect growth regulator, methoprene. III
Photodecomposition. J. Agric. Food Chem., 23:299-303.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975c degradation of the insect growth regulator methoprene. VIII.
Bovine metabolism to natural products in milk and blood. J.
Agric. Food Chem., 23:750.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1976a degradation of the insect growth regulator methoprene X.
Chicken metabolism. J. Agric. Food Chem., 24:644-648.
Quistad, G.B., Schooley, D.A., Staiger, L.E., Bergot, B.J. Sleight,
1976b B.H. and Macek, L.K. Environmental degradation of the insect
growth regulator methoprene. IX. Metabolism by bluegill
fish. Pestic. Biochem. Physiol., 6:523-529.
Rowlands, D.G. The uptake and metabolism by stored wheat grains of an
1976 insect juvenile hormone and two insect hormone mimics. J.
Stored Prod. Res., 12:35-41.
Schaefer, C.H. and Dupras, E.F. Insect developmental inhibitors. 4.
1973 Persistence of ZR-515 in water. J. Econ. Entomol., 66:923.
Schaeffer, CH. and Wilder, W.H. Insect development inhibitors. 2.
1973 Effects on target mosquito species. J. Econ. Entomol.,
66(4): 913-916.
Schooley, D.A. Metabolism of 3H-methoprene by the dwarf lima bean
1974 plant. Zoecon Corporation, Report No. 3760-12-02-74.
Schooley, D.A. and Bergot, B.J. 10-3H-methoprene dissipation rates in
1974 pond water and sewage. Zoecon Corporation, report no.
3760-12-06-74.
Schooley, D.A. and Bergot B.J. Hydrolytic stability of radiolabelled
1975a methoprene. Zoecon Corporation, Report No.
Schooley, D.A. and Bergot, B.J. 3-day metabolism study of Altosid in
1975b non-sterile water. Zoecon Corporation Report No.
Schooley, D.A., Bergot, B.J., Durham, L.L., and Siddall, J.B.
1975a Environmental degradation of the insect growth regulator,
methoprene. II. Metabolism by aquatic micro-organisms. J.
Agr. Food Chem., 23(2):293-298.
Schooley, D.A., Creswell, K.M., Staiger, L.E. and Quistad, G.B.
1975b Environmental degradation of the insect growth regulator
methoprene. IV. Soil metabolism. J. Agric. Food Chem.,
23(3): 369-373.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-update of
1974a methoprene and its metabolites by a second crop of wheat
grown in treated soil. Zoecon Corporation, Report No.
3760-12-08-74.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-translocation of
1974b methoprene and its metabolites from soil into water. Zoecon
Corporation, Report No. 3760-12-04-74.
Zoecon Methoprene residues in chickens - Report submitted to FAO for
1984a consideration by JMPR. Zoecon Corporation.
Zoecon Altosid leaching studies - Report submitted to FAO for
1984b consideration by JMPR - Zoecon Corporation.
Zoecon Methoprene distribution in Australian wheat fractionation.
1984c Report RM-0248, 17 September 1984. Zoecon Corporation.
Zoecon Methoprene distribution in Kansas State University. Wheat
1984d fractonation study. Report RM-0250, 18 September 1984.
Zoecon Corporation.
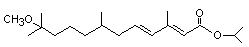 Synonyms: ALTOSID, APEX, DIACON, KABAT, DIANEX, PRECOR.
(All registered Trade Names) ZR-515; ENT-70460;
CAS No: 114-26-1
Other information on identity and properties:
Molecular formula C19H34O3
Molecular weight: 310
Physical form: Pale yellow liquid
B.P.: 100° at 0.05 mm Hg
Vapour pressure: 2.37 × 10-5 mm Hg at 25°C
1.60 × 10-4 mm Hg at 40°C
Solubility: all organic solvents-infinity
water - 1.39 mg/l.
Specific gravity: 0.9261 g/cc at 20°C
Octanol/water
partition coefficient: 10,000
Cis-2 : trans-2 ratio: 8:92
Purity: methoprene - 92-95%
related isomers - 5-2%
solvent - 1-2%
unidentified - 2-1%
Stability: extremely stable in sterile aqueous
solutions over wide range. Readily
biodegraded by common bacteria. Rapidly
degraded in solution or in thin films by
sunlight and UV light. Identity of
degradation products known. (Quistad
et al., 1975b).
Formulations: Several emulsifiable formulations for
agricultural, stored product and public
health use; solution formulation for
treating cured tobacco; fogging solution
for public health use; emulsifiable
formulations for addition to feed
supplements; slow release formulation
for use in mosquito abatement;
emulsifiable formulation for protection
of houseplants.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methoprene is an insect growth regulator (IGR), being a synthetic
analogue of the insect juvenile hormone (Henrick et al., 1973).
Unlike conventional insecticides, which act as direct poisons,
methoprene acts by disrupting the morphological development of
insects. After contact, inhalation or ingestion the insect grows and
pupates normally, but the pupa dies in the pupal stage. As a result,
methoprene is not generally used in pre-harvest situations when a
quick kill of insect pests is required.
Methoprene has found widespread use in mushroom culture to
prevent the emergence of adult sciarid flies, where it is applied to
compost or casing at the rate of about 100 g/100 sq.m. This
application represents about 12% of methoprene use. A commonly used
treatment is 175 ml of 650 g/l emulsifiable concentrate per 100 sq.m.,
equivalent to 113 g a.i./100 sq.m. One or two applications are used.
Special slow-release formulations are used to control mosquito
breeding in floodwater sites, rice cultivations, storm drains, ponds,
water treatment works etc. (Schaefer and Wilder, 1973; Mura and
Takahashi, 1973).
Methoprene is formulated into feed supplements for cattle to
control adult hornfly breeding in cattle manure. The rate is
equivalent to 0.016 mg/kg body weight daily. This, together with
mosquito control represents about 50% of current use.
Methoprene is marketed as a 65% emulsifiable concentrate
formulation for use as a space spray for control of pests in food
handling and storage establishments. It is applied as an aqueous spray
or via mechanical foggers at a rate of 10 ml per 1,000 m2.
Post-harvest treatments
Methoprene has proved effective in the control of numerous pests
of stored products such as peanuts where it is used at the rate of
10g/tonne against most moth and beetle pests. It is being tested on
cocoa beans and cereal grains as a 657g/l emulsifiable concentrate
formulation. This is applied at a rate of 15ml/tonne of stored
commodity, equivalent to 10g a.i./tonne, in either water or mineral
oil.
Trial work indicates that methoprene will control most insect
pests affecting stored grain though it is significantly less effective
against Sitophilus spp. which include three of the major pests of
maize, rice and wheat. It may have to be combined with a conventional
insecticide where these species are a problem.
Methoprene has been used for more than five years for treating
stored tobacco. The cured tobacco is treated, before packing, at the
rate of 200g methoprene per tonne of tobacco. It is used for treating
food-handling and tobacco-processing establishments to prevent insect
breeding and development. A fogging formulation is used to control
pre-adult fleas in premises.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in stored agricultural commodities
Peanuts. During the period 1979-1981 the manufacturers, in
conjunction with the US Department of Agriculture, undertook trials of
methoprene for protecting peanuts against stored-product insect pests
(Miller 1981). The methoprene was added as the peanuts were being
placed in storage silos. Normally peanuts are stored for a period from
a few months to a year.
Treatment rates were 0, 4, 10, 25 and 100 mg/kg. Nuts (kernels)
and hulls were analysed separately. Residues at the proposed use rate
of 10 mg/kg on the day of treatment were below the limit of
determination (0.05 mg/kg) in kernels with wrappers, 0.05-0.64 mg/kg
in kernels without hulls (split hulls) and 14-20 mg/kg in hulls. After
278 days storage the kernels with wrappers contained 0.13-0.38 mg/kg
(mean of 11 samples 0.22 mg/kg), kernels without hulls (split hulls)
0.38-2.1 mg/kg and hulls 8.3-14.0 mg/kg.
Peanuts treated at exaggerated rates contained residues
proportionally higher than those treated at the proposed use rate.
Maize. Eight studies were carried out on stored shelled maize
(Miller, 1983a). Random samples from bulk lots treated at rates of 10,
15, 20 and 23.5 mg/kg were drawn at intervals up to 13 months after
treatment. At all application rates, residues showed no significant
losses with time, even at 200 days after treatment. It is noteworthy
that at no stage did the residues determined by analysis reflect the
amount of active ingredient actually applied to the grain, and in most
trials ranged between 40% and 70% of it. It is difficult to see
whether this was due to failure to deposit the methoprene on grain,
loss from the grain surface immediately after treatment, loss from the
samples between collection and analysis, or failure to recover residue
from the substrate.
The analytical method included an internal standard which was
added in the blender. Recovery of the internal standard was always
high (80-110%), implying validity of the method and analytical
procedures. However, there is no explanation of the apparent absence
of methoprene. Nevertheless the residue levels found throughout the
storage period were similar to, or higher than, those reported for the
initial sample, indicating that little degradation had occurred during
storage.
Wheat. Data from two studies (Miller, 1983b) were available. Stored
wheat was treated with methoprene at 10 and 20 mg/kg and sampled 3,
35, 95 and 150 days after treatment. Methoprene residues recovered by
analysis were about one-half of the treatment rate but did not show
any decline up to 150 days after treatment. Residues from the 10mg/kg
treatment were 5.2 mg/kg after 150 days. An explanation for this
discrepancy is needed.
In a laboratory study at the University of California using GLC
analysis, Mian and Mula (1983) found that when methoprene was applied
at rates of 1, 5 and 10 mg/kg to wheat (13.5% moisture content) stored
at 27 ± 1°C, losses were 61, 66 and 62% respectively over a period of
12 months. The results of analyses carried out at six times within the
12-month period are given in Table 1.
These data seem more reliable than those reported by Miller
(1983b), since the main initial recovery of insect growth regulator was
99% of that applied. Thus it can be accepted that the loss over 12
months is of the order of 60% of the amount applied.
Rowlands (1976), determined the rate of breakdown at 20°C during
10 weeks storage in the dark, using radiolabelled methoprene with
three samples of wheat: fresh wheat of 19.1% moisture content, old
wheat of 12% moisture content and old wheat of 18% moisture content.
Table 2 (adapted from Rowlands, 1976) indicates that the breakdown was
quite rapid at the high moisture levels (half-life of three weeks at
18-19% moisture) and was about half as fast on grain with 12% moisture
(half-life six weeks).
Table 1. Residues of methoprene in treated wheat grain at
various post-treatment intervals 1
Residue, mg/kg
Interval
Application rate, mg/kg
1 5 10
0 0.99a 4.97a 9.92a
1 wk 0.99a 4.60a 9.39a
(0) (7) (5)
1 mo 0.94a 3.93a 6.62b
(5) (21) (33)
4 mo 0.82a 3.49ab 5.53bc
(17) (30) (44)
8 mo 0.80a 1.78bc 4.07c
(19) (64) (59)
12 mo 0.38b 1.71c 3.73c
(61) (66) (62)
1 Mean of four analyses. Means followed by the same letters
in a column are no significantly different from one another
(Duncan's multiple range test. P = 0.05). Values in
parentheses below each mean represent percent loss in
residues at indicated post-treatment interval.
Table 2. Breakdown of methoprene on stored wheat grains at 20°C*
Residue, mg/kg found in grain
Storage time
in weeks Fresh wheat Old wheat Old wheat
(19%) (12%) (18%)
0 5.6 9.8 9.6
1 7.2 9.2 7.7
2 5.5 7.9 6.3
3 4.4 7.6 5.0
4 3.4 7.0 4.3
5 2.2 6.2 3.2
6 1.4 5.1 2.1
7 0.5 4.2 1.4
8 0.2 3.2 0.5
9 0.1 2.4 0.4
10 nil 1.5 0.2
* Results are means from triplicate 25g samples, treated at
10 mg/kg (approx.)
Cocoa beans. In two trials carried out in Brazil, methoprene was
applied to cocoa beans at rates of 1, 2.5, 5.0, 10.0 and 15 mg/kg. The
beans were sampled on the day of treatment and after 50 days storage
at 27°C. Less than half of the applied methoprene was recovered by
analysis but the analytical results on samples taken after 50 days in
storage were not significantly different from those on the initial
samples (Miller, 1983c).
Residues in mushrooms
Data from 7 studies were available (Miller 1978), reflecting both
compost and casing treatment at 0.2 to 4.6 times the recommended
application rates. Samples were collected at 0, 9 and 35 days after
application. When less than 2.5 times the recommended combined
treatment rates were used, the residues were undetectable
(<0.05 mg/kg). At 2.5 and 4.6 times the recommended application rate,
residues ranged from 0.17 mg/kg to 150 mg/kg. The high values were due
to contamination with compost or peat moss "casing" which was shown to
contain methoprene at hundreds of mg/kg. Subsequent samples were
rinsed with cold water to remove the contamination. Most samples
examined in these later experiments contained no methoprene or the
residues were less than 0.1 mg/kg (Miller, 1978).
Dust samples from food-handling establishments were analysed in
six studies (Miller, 1984). Isolated areas were treated with
0.6g a.i./1000 sq.m. (the food being covered according to label
direction before application). Samples were collected in petri dishes
located in appropriate areas of the treated spaces 0, 30, 60 and 90
days after treatment. Residues expressed as mg/kg dust were high owing
to the small amount of dust collected in the dishes. Consequently, the
total quantity of methoprene collected is a more realistic value to
consider when evaluating the results.
Immediately after application methoprene residues were high,
ranging from 26 micrograms to 191 mg. However within 30 days they
declined significantly and ranged from 0.5 to 24 mg. Although there is
some variation in the results, within 60 days the majority of the
residues were below the level of detection (0.5 micrograms).
Residues in forage and field crops
Methoprene is marketed for the control of floodwater mosquitoes
as a 10% slow-release encapsulated aqueous solution (Schaefer and
Wilder, 1973). Since treated areas include pastures, rice fields and
marshlands, residues might occur on certain food crops. Consequently,
Zoecon conducted a series of studies on rice, legumes and forage crops
to analyse for possible methoprene residues which could enter the food
chain through ingestion by livestock.
In a study on rice, methoprene was applied prior to "heading"
(Miller, 1974a). The crop was then grown to harvest. Samples were
collected at 45, 62 and 71 days after application, resulting in a
built-in pre-harvest interval of two months. No detectable residues
(<0.05 mg/kg) were found in the rice grains, hulls or straw of any of
the samples.
In nine studies on pasture legumes and forage crops (Miller,
1974b) pasture land was treated at rates of 9, 11, 23 and 45 g/ha. The
highest treatment rate is four times the highest recommended rate.
Samples were collected 0, 1, 2, 3, 5, 7, 9, 11 and 365 days after
application. Residue levels indicated that methoprene is rapidly
degraded. By day 3, the residues from the highest treatment ranged
from 0.12 to 0.30 mg/kg.
FATE OF RESIDUES
In animals
Numerous studies have been conducted to determine the fate of
methoprene in livestock because of the commercial use in animal feeds
to suppress the breeding of flies in manure.
Quistad et al. (1974b, 1975a) administered [5-14C] methoprene
to a steer which was slaughtered two weeks later. Samples of fat,
muscle, liver, lung, blood and bile were analysed for radioactive
residues. No primary methoprene metabolites could be characterised but
the majority of the tissue radioactivity was positively identified as
cholesterol. A total of 72% of the bile radioactivity was contributed
by cholesterol, cholic acid and deoxycholic acid. Radioactivity from
metabolised methoprene was associated with protein and cholesteryl
esters of fatty acids.
A metabolic balance study was conducted on a 338 kg Jersey cow
dosed with 207 mg of [5-14C] methoprene by capsule (Chamberlain
et al 1975b). This is 40 times the proposed dose for fly control.
The study proceeded for 7 days after dosing. It was found that 16.4%
of the radioactivity was excreted as CO2 between 0 and 170 hours,
with peak excretion after a little under 24 hours. A total of 30.3% of
the applied dose was eliminated via the faeces within about 50 hours
and 19.7% via the urine in 30 hours. By the end of the experiment 7.6%
of the radiolabel had been excreted via the milk. Radioactivity was
measured in 30 tissue samples after the cow was sacrificed 7 days
after dosing. The tissues contained a total of 20% of the applied
dose, intestine and contents contributing 5.96% and fat 4.59%. The
organs of metabolism, lung, liver and kidney, contributed a further
1.68%. The presence of radiocarbon in the tissues was attributed to
steroidal derivatives in which labelled acetate from the methoprene
was anabolically incorporated into natural body constituents.
The results of the above two studies and another on a guinea pig
were compared by Chamberlain et al. (1975a). It was reported that a
rather large percentage of the radiolabel was incorporated in the
tissues and respired by the animals. A small proportion was
incorporated into free primary metabolites, a greater amount into
glucuronides and a considerable proportion within polar components,
possibly complex conjugates or biochemicals. Methoprene was not found
in the urine, but accounted for approximately 40% of the radiolabel in
the faeces. This explains the activity of the administered methoprene
against fly larvae developing in dung pats. The formation of
conjugates and complex metabolites was more extensive in the steer
than the guinea pig. Comprehensive information was provided on the
radiolabel profiles in tissues, organs and other components of the
animals.
On the basis of detailed biochemical analysis of tissues and
organs from the above three studies Quistad et al. (1975c)
elucidated the metabolism by the cow to natural products in milk and
blood. Only a trace of methoprene (0.015/l) and no detectable primary
metabolites occurred in milk.
Quistad et al. (1976a) studied the metabolism of methoprene in
leghorn chickens give single oral doses of [5-14C] methoprene.
Residual radioactivity was found in tissues and eggs. Although a high
initial dose (59 mg/kg)resulted in residues of methoprene in muscle
(0.01 mg/kg), fat (2.13 mg/kg) and egg yolk (8.03 mg/kg), these
represented only 39% and 2% of the total radiolabel in fat and egg
yolk respectively. Radiolabelled natural products were by far the main
14C residues in tissues and eggs, particularly at the lower dose of
0.6 mg/kg where cholesterol and normal fatty acids contributed 8% and
71% of the total radiolabel in egg yolk.
When chickens were given feed treated with methoprene at 0, 25,
50 and 100 ppm for 14-63 days, residues in poultry meat and eggs were
less than 0.1 mg/kg (Zoecon, 1973).
Quistad et al. (1976b) studied methoprene metabolism in
bluegill fish in a dynamic flow-through system and a model aquatic
ecosystem. The fish in the flow-through system acquired moderate
residues of largely unmetabolised methoprene when continuously exposed
to about 30 times the anticipated environmental levels of methoprene,
but these were 93-95% eliminated within two weeks when the fish were
transferred to flowing uncontaminated water. In the model aquatic
ecosystem, the fish accumulated 14C, but the radioactivity was almost
exclusively in radiolabelled natural products, including cholesterol,
free fatty acids, glycerides and protein. Less than 0.1% of the total
radioactivity could be attributed to methoprene or its primary
metabolites.
In plants
Methoprene is rapidly degraded in plants with a half-life of
about 2 days in alfalfa and about 0.5 days in rice. The major
metabolic pathways include ester hydrolysis, O-demethylation and
oxidative scissions of the 4-ene double bond, producing primary
metabolites which are present in relatively low yields (Figure 1)
(Quistad et al. 1974a). These low amounts suggest that subsequent
degradation or conjugation occurs, and in fact the recovery of polar
metabolites as well as the loss of radioactivity through
volatilization are evidence of additional metabolism. In addition,
unextractable residues suggest the extensive degradation of methoprene
and incorporation into natural cellular products. The metabolic fate
of methoprene in alfalfa and rice is shown in Figure 1. Metabolism
resulted in five primary non-polar products isolated from the plant
(ZR-669, ZR-725, ZR-724, ZR-1945 and ZR-1602, see Figure 1). The
combined yield of these products was 1.4% of the applied dose after 7
days from alfalfa and 2.1% after 3 days from rice. The most abundant
non-polar metabolite was ZR-1564 (13% from rice, 2% from alfalfa)
which was isolated from vapours and not found within the plant. The
main aglycones after enzymic cleavage of alfalfa conjugates were
ZR-725 (2.2%), ZR-1945 (0.8%), and ZR-724 (7.4%). The major aglycone
from rice was ZR-1945 (1.2%) with a small amount of ZR-1602 (0.3%).
After 30 days, only 1% of the applied dose remained in alfalfa as
unmetabolised methoprene and 0.4% remained in rice after 15 days. The
initial rapid loss of methoprene and radiolabel from plants was
attributed to evaporation of methoprene and 7-methoxycitronellal
(ZR-1564) on the evidence from supporting studies of methoprene
evaporation from glass plates and of compounds volatilized from leaf
surfaces.
10-3H-methoprene painted on the leaf surface or injected into
the stem of the dwarf Lima bean plant was found not to be
translocated. When injected into the stem, the parent compound was
partially metabolised to ZR-724, ZR-725 and a polar conjugate,
possibly the glycoside. Topical application resulted in more rapid
metabolism to the same metabolites after 24 hours incubation
(Schooley, 1974).
Rowlands (1976) studied the uptake and metabolism of methoprene
and two other IGRs by stored wheat grains. He applied the
radiolabelled compounds topically to individual grains and to small
samples of wheat in glass jars. The wheat contained 19.5% moisture and
was kept at 20°C in the dark. The methoprene penetrated rapidly from
both vapour and topical treatments, but the quantities sorbed differed
between the two routes. Two days after treatment most of the intact
methoprene was found in the aleurone layers, much less in the germ and
virtually none in the endo-sperm proper or outer seedcoat. The rate of
degradation is indicated in Table 2 above. Degradation is promoted by
grain moisture, wheat with 19% moisture giving a half-life of 2-3
weeks, 18% moisture 3-4 weeks and 12% moisture 6-7 weeks.
Mian and Mullar (1983) determined the residues of methoprene on
wheat over a 12-month storage period. Details are given above
("Residues resulting from supervised trials").
In soil
Schooley et al. (1975b) studied the degradation of methoprene
in soil as a function of time under various conditions. On aerobic
sandy loam methoprene showed an initial half-life of about 10 days at
a surface treatment rate of 1 kg/ha; decomposition was much slower on
autoclaved soil. Only small amounts of non-polar metabolites were
isolated, including the hydroxy ester resulting from O-demethylation
(0.7% of the applied dose). Over 50% of the applied dose was converted
to CO2. Radioactivity from [5-14C] methoprene was incorporated into
humic acid, fulvic acid, and humin fractions of sandy loams. These
studies indicate rapid and extensive breakdown of methoprene in soils.
Leaching experiments with radiolabelled methoprene were performed
to determine the mobility of the chemical in different soils. The four
agricultural soils used were sand, sandy loam, silt loam and clay
loam. Experiments with soil columns and soil thin-layer plates
revealed that no significant leaching of methoprene occurred with the
four soils tests (Zoecon, 1984b).
Quistad and Staiger (1974a) determined the amount and nature of
bound residues in soil. They found that in sandy loam soil treated
with [5-14C] methoprene humic acids (3%) and fulvic acids (8.4%) were
most abundant after 30 days. The total recovery of the label from
treated soil was essentially quantitative.
Quistad and Staiger (1974b) determined the partition between
water and soil using a 0.01 mg/l solution of [5-14C] methoprene
agitated with sandy loam soil. After thorough mixing for 15 minutes,
87% of the applied dose was bound to the soil.
Staiger et al. (1974a) grew wheat plants in soil that had been
treated 6 months previously to determine the uptake by a second crop
grown in methoprene-contaminated soil. After treatment of the soil at
the rate of 1 kg/ha, less than 0.006% of the administered label was
taken up in wheat six months after dosing. This uptake is about
one-tenth of that observed when soil was treated with radiolabelled
methoprene at the time seeds were planted.
Staiger et al. (1974b) determined the uptake of methoprene by
wheat plants when wheat seeds were planted in sandy loam and
radiolabelled methoprene was incorporated at the rate of 1 kg/ha at
the seed level and in the soil at a depth of 9 cm below the seed. The
wheat was harvested 11 days after planting and assayed after total
combustion. Uptake was found to be 0.09% of the applied radioactivity
when the IGR was inserted at the level of the seed and 0.04% of the
radioactivity when it was incorporated 9 cm below the seeds. It appears
that very little methoprene or its metabolites are translocated into
wheat from soil.
In water
Sterile aqueous solutions of methoprene (0.5 mg/l), buffered at
various pH values, were found to be extremely stable to hydrolysis
over four weeks at 20°C in the dark. No degradation was seen for the
duration of the experiment in sterile water buffered at pH 7 and 9,
and similar stability was observed in pH 5 buffer for 21 days. However
the pH 5 buffer became non-sterile between the 21- and 30-day
observations, and 59% degradation occurred between days 21 and 30.
(Schooley and Bergot, 1975a).
Schaefer and Dupras (1973) studied the persistence of methoprene
in water. They found that residues were greatly affected by sunlight
and temperature. The methoprene tended to remain on the water surface
and its distribution was affected by wind. The half-life in field
water was only about two hours. A slow-release formulation showed
almost no detectable residues after 24 hours, although biological
activity persisted for several days.
Schooley and Bergot (1974) measured the dissipation of methoprene
in pond water and sewage at dose rates of 0.001 and 0.01 mg/l. A
half-life of 30-45 hours was observed in pond water, with a 60-70 hour
half-life in sewage. This significantly longer half-life in sewage may
have been due to the fact that the primary sewage sample had been
standing in a closed bottle for 30 days in the laboratory, while the
pond water had been collected fresh on the day of the experiment.
On standing for thirteen days in unsterilised pond water
containing suspended sediment in full summer sunlight at a
concentration of 0.66 mg/l, methoprene was metabolised and/or
photodegraded mainly to one product, ZR-1945 methoxycitronellic acid
(Schooley et al. 1975a). This is one of many products formed by the
combined action of oxygen and sunlight on thin films of methoprene on
glass plates. ZR-724, ZR-725 and ZR-669 (See Figure 1) were either not
present or present in very low concentrations.
A 3-day study was conducted on methoprene in non-sterile pond
water at ambient temperature in direct sunlight (Schooley and Bergot,
1975b). Three primary products, ZR-724, ZR-725, ZR-669 were
identified. Each compound, as well as the recovered methoprene, was
found to be an approximately 1:1 mixture of cis-2 and trans-2
isomers, in contrast to the 94% trans-2 methoprene which was
applied. This conversion has been shown in other studies to be ready
photoisomerization rather than an enzymic process.
The biodegradation of (2E)-[10-3H] methoprene was studied in
pond water containing unknown micro-organisms (Schooley et al
1975a). The methoprene showed a half-life of approximately 30 hrs at
0.001 mg/l and 40 hr at 0.01 mg/l. Incubation of the same preparation
for 3 days at 0.42 mg/l generated three primary metabolites, the
result of ester hydrolysis and/or O-demethylation. These metabolites
and the recovered methoprene were photoequilibrium mixtures of 2-ene
isomers. In another incubation experiment with (2E)-[5-14C]
methoprene at 0.66 mg/l in a pond water sample with presumably
different microflora, a completely different metabolic profile was
observed. The main and only identifiable metabolite, resulting from
oxidative scission of the 4-ene double bond, was 7-methoxycitronellic
acid (29% of the applied dose).
In processing and cooking
Peanuts. Peanuts from one of the trials Miller (1981) (see
"Residues resulting from supervised trials") with residues ranging
from 0.18 to 1.21 mg/kg at the time of treatment, were processed into
peanut butter and peanut oil. The peanut butter was reported to
contain less than 0.05 mg/kg of methoprene, indistinguishable from
untreated controls. The peanut oil, however, contained 0.25 mg/kg
(0.20-0.29) of methoprene. These results do not appear to be
consistent.
A trial was carried out in Australia (Zoecon 1984c) in which
methoprene was applied to wheat at the rate of 10 mg/kg. After storage
for two weeks the wheat was milled through an experimental Buhler mill
to provide bran, pollard and white flour. A separate milling produced
wholemeal. The residues found, and the proportion of each fraction in
the first milling, are shown in Table 3.
A separate trial carried out at Kansas State University (Zoecon,
1984d) involved treatment of two lots of wheat with methoprene at the
rate of 10 mg/kg followed, 9 days later, by milling through a Ross
Walking Flour Mill. A separate milling through a pin mill produced
wholemeal.
The white and wholemeal flours were used to prepare bread. The
results of analysis are shown in Table 4.
METHODS OF RESIDUE ANALYSIS
Residues of methoprene have been determined in waters, soils,
plants, stored grain and corn, milk, eggs, fish, shellfish, poultry
and cattle tissues, blood, urine and faeces. Residues in all samples
are extracted by high-speed blending with acetonitrile followed by
vacuum filtration. Fatty extracts are subjected to cold-temperature
precipitation and filtration to remove fat. Petroleum ether
partitioning, Florisil and neutral alumina chromatography are used for
clean-up. The concentrated eluates are analyzed by GLC on columns of
differing polarity, using flame ionization detectors. Confirmation of
identity by additional GLC and mass fragmentography (MF). The lower
limits of determination are: soils, blood and urine, 0.001 mg/kg;
forage grasses, forage legumes and rice foliage, 0.005 mg/kg; milk,
eggs, stored grain and corn kernels, fish, shellfish, poultry and
cattle tissues and faeces, 0.01 mg/kg. Limits of determination and
recoveries were validated by analysis of laboratory and field samples
fortified with 14C-methoprene (Miller et al., 1975).
Table 3. Methoprene residues in wheat fractions Zoecon, 1984c)
Fraction % wt./wt/ of each Methoprene (mg/kg)
fraction (excluding
wholemeal)
Wheat 8.9
Wholemeal flour 6.7
White flour 74 1.8
Bran 23 11.4
Pollard 3 17.7
An internal standard was used in the analysis.
Table 4 Methoprene residues in wheat fractions (Zoecon, 1984d)
Fraction % mt/wt of each fraction Methoprene (mg/kg)
(excluding wholemeal)
Trial Trial Trial Trial
Wheat (whole) 5.0 5.7(Note 1)
White flour 70.2 70.1 2.8 2.8
Wholemeal flour 4.1 5.5
Bran 14.1 14.4 8.7 10.2
Shorts ) 12.7 12.4 9.6 9.6
pollard
Red dog ) 2.4 2.5 9.5 11.0
Germ 0.6 0.6 8.7 10.7
White bread (33% moisture) 0.41
Wholemeal bread (33% moisture) 0.88
Note 1 It appears that a significant proportion of the original
deposit (10 mg/kg) was lost, possibly by evaporation, between
application and analysis. Spiked samples analysed at the same
time yielded the results expected.
Mian and Mulla (1983) describe a method derived from that of
Dunham and Miller (1978) which they applied successfully to studying
the persistence of methoprene in stored wheat. This involved Florisil
and Alumina chromatography with petroleum ether followed by GLC/FID on
a column of 3% OV-101 on 100-120 mesh Chromosorb W (acid-washed,
dimethyldichlorosilane treated), conditioned at 350°C. The column and
injector temperatures were 190°C for retention times of 8 min for
methoprene and 12 min for n-docosane used as an internal standard. The
detector temperature was 350°C. The limit of determination was
reported to be 0.01 mg/kg and the recovery of 10, 5 and 1 mg/kg in
grain extracts ranged between 80 and 100%.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
No information was available.
NATIONAL MAXIMUM RESIDUE LIMITS
The meeting was advised that the following national maximum
residue limits had been established in the USA.
Meat of cattle, goats, hogs, sheep,
poultry and horses 0.1 mg/kg
Fat of cattle, goats, hogs, sheep,
poultry and horses 0.3 mg/kg
Meat by-products of cattle, goats, hogs,
sheep, poultry and horses 0.1 mg/kg
Milk fat 0.05 mg/kg
Eggs 0.05 mg/kg
Peanuts 2.0 mg/kg
Mushrooms 1.0 mg/kg
A petition has been presented for tolerances of 10 mg/kg on
barley, buckwheat, corn, millet, oats, rice, rye, sorghum, sunflower
and wheat. In addition a substantial increase has been proposed in the
tolerances for indirect residues, derived from animal feeds, in meat,
fat and meat by-products of cattle, goats, hogs, sheep, poultry and
horses and in eggs and milk fat.
The meeting was not aware of similar action by any other national
authority.
APPRAISAL
Methoprene is an insect growth regulator. It is an analogue of
the insect juvenile hormone. After contact, inhalation or ingestion it
interferes with the normal process of insect development preventing
the emergency of adults from pupae or larvae.
So far there have been no practical pre-harvest uses of
methoprene on crop plants and it appears unlikely that such uses will
develop. There is an important application for the control of sciarid
flies in mushroom culture and as a feed supplement for cattle to
prevent the breeding of hornflies in cattle manure. Methoprene is
applied to water to prevent the breeding of mosquitoes.
The most important application currently under development is the
use of methoprene to prevent the breeding of stored-product insect
pests in tobacco, peanuts, cocoa beans and a wide range of stored
commodities. There are good prospects for use in stored grain to
inhibit the development of stored-product insects. Extensive studies
on these uses are at an advanced stage in several countries.
Methoprene formulations are stable under normal storage
conditions but are rapidly degraded by light. Sterile aqueous
solutions are stable over a wide pH range but non-sterile solutions
are rapidly biodegraded.
When used for the control of insects in stored products
methoprene is applied alone at the rate of 10 mg/kg or in conjunction
with conventional insecticides at much lower rates (1-2 mg/kg).
Although the residues studies available at this stage are not
extensive it would appear that there is relatively little degradation
on dry stored commodities over many months. Degradation is promoted by
moisture and probably also by temperature.
In numerous field studies on peanuts, maize, cocoa beans and
wheat, investigators have failed to recover more than half of the
methoprene applied. This difficulty was not encountered in one small
scale laboratory study at the University of California. This suggests
a pronounced tendency for the methoprene to evaporate with the water
used to apply the insect growth regulator or a fundamental weakness in
the trial technique. This feature is currently receiving further
study.
The meeting received studies from Australia and the USA designed
to determine the distribution of methoprene in grain and the effect of
processing and cooking on the residues. These enabled the meeting to
propose temporary maximum residue limits.
One study with the labelled compound applied to individual wheat
grains indicated rapid penetration. A study on peanuts indicated that
by far the greater proportion of the deposit remained on the hulls and
wrappers and was not taken up by the kernel. However, allowance must
be made for the fact that a proportion of the kernels lose their
shells. When the peanut kernels were processed into peanut butter the
methoprene disappeared but significant amounts were detected in peanut
oil. These studies should be repeated.
When methoprene is applied to mushroom compost or casing at rates
which will control sciarid flies there are no detectable residues in
mushrooms. However, it is necessary to wash the peat moss casing from
the mushrooms before analysis to prevent anomalous results.
Methoprene would be suitable for the treatment of surfaces and
structures to prevent insect pests breeding in food storage and
preparation areas. Several studies indicated that such treatments
would not lead to significant residues in foodstuffs.
Studies have been conducted with cattle, poultry and fish to
elucidate the metabolic processes and determine whether residues occur
when methoprene is administered to livestock directly for fly control,
or in their feed from cereal grain and milling products, or when the
compound is used for mosquito control. All available information
points to the rapid degradation of the parent compound and
incorporation of fragments into natural body components but the
meeting, recognizing that it would not be practical to withdraw
treated feed before slaughter, recommended temporary MRLs for
methoprene residues in foods of animal origin to cover the feeding of
treated grain and its milling products.
Methoprene is rapidly metabolised in plants, yielding products
that are further degraded to normal plant building materials. When
applied to soil, most of the dose is converted rapidly to CO2. There
is no significant leaching of methoprene from treated soil and no
uptake by plants. There appears to be no likelihood of methoprene
being taken up by rice, forage or other crops growing in water treated
for mosquito control.
Methoprene residues may be determined by extraction with
acetonitrile followed by clean-up on Florisil and alumina columns and
quantitation by GLC with a flame ionization detector. The method
appears suitable for regulatory purposes. The limit of determination
ranges from 0.001 mg/kg to 0.01 mg/kg.
The meeting was aware that MRLs had been established for a range
of commodities in the USA and that others were under consideration.
RECOMMENDATIONS
The meeting recognized that there were, as yet, only a limited
number of registrations for the use of methoprene but that potentially
important developments might not proceed in the absence of appropriate
maximum residue limits. To assist these further developments and to
cater for existing uses, the following temporary MRLs are recommended
to apply to residues of methoprene resulting from the direct
application of methoprene or the feeding of methoprene-treated
commodities. They apply to methoprene only.
Commodity MRL (mg/kg)
Cereal grains 10
Bran 20
Flour (wholemeal) 10
Flour (white) 5
Carcase meat 0.2 (in the carcase fat)
Meat byproducts 0.1
Eggs 0.05
Milk 0.002
Peanuts 2
Mushrooms 1
FURTHER WORK OR INFORMATION
Required (before maximum residue limits can be confirmed):
1. Information from studies (now in progress) in which methoprene is
used for the control of pests of stored grain.
2. Studies to define the rate of degradation of methoprene in stored
products under a range of conditions.
3. Further information on the fate of methoprene residues in cereal
grains and other stored products as a result of processing and
cooking.
4. Information from current trials with stored dried lentils, beans,
dried fruit, cocoa beans, coffee beans, spices and oilseeds to
define the treatment necessary, the rate of degradation and the
fate of residues in processing and cooking.
REFERENCES
Chamberlain, W.F., Hunt, L.W.M., Hopkins, D.E., Gingrich, A.R.,
1975a Miller, J.A. and Gilbert, B.N. Absorption, excretion, and
metabolism of methoprene by a guinea pig, a steer and a cow.
J. Agric. Food Chem., 23(4):736-742.
Chamberlain, W.F., Hunt, L.M., Hopkins, D.E., Miller, J.A., Gingrich,
1975b A.R. and Gilbert, B.N. A balance study with 14C-labelled
methoprene in a dairy cow. Official report from US
Department of Agriculture, Kerrville, apparently
incorporated in previous reference.
Dunham, L.L. and Miller, W.W. Methoprene in Analytical Methods for
1978 Pesticides and Plant Growth Regulators (G. Zweig ed).
10:95-109. Academic Press Inc., New York.
Henrick, C.A., Staal, G.B. and Siddal, J.B. Alkyl 3,7,11-trimethyl-2,
1973 4-dodecadenoates, a new class of potent insect growth
regulators with juvenile hormone activity. J. Agr. Food
Chem., 21(3):354-359.
Mian, L.S. and Mulla, M.S. Persistence of three IGRs in stored wheat.
1983 J. Econ. 1983 Entomol., 76(3):622-625.
Miller, W.W. Analyses of indirect methoprene residues in rice. Report
1974a No. RM-026. Zoecon Corporation.
Miller, W.W. Analysis of methoprene residues in legumes and forage
1974b crops. Zoecon Corporation, Report No. E-48-B-73.
Miller, W.W. Analysis of methoprene residues in mushrooms. Zoecon
1978 Corporation, Report No. E-7-P-78.
Miller, W.W. Analysis of methoprene residues in stored peanuts and
1981 peanut hulls. Zoecon Corporation, Report No. SE-GR 340-79.
Miller, W.W. Analysis of methoprene residue in stored shelled corn.
1983a Zoecon Corporation, Reports Nos. RM-1054, RM-0159 and RM-
0167.
Miller, W.W. Analysis of methoprene residue in wheat grain. Zeocon
1983b Corporation, Report No. R7-0130.
Miller, W.W. Analysis of methoprene residues in cocoa beans. Zoecon
1983c Corporation, Reports Nos. RM-0168, RM-0169.
Miller, W.W. Methoprene residue analysis of dust samples in food
1984 handling establishments. Zoecon Corporation, Reports Nos.
RM-0177, RM-0090, RM-0178, RM-0075 and RM-0132.
Miller, W.W., Wilkins, J.S. and Durham, L.A. Determination of Altosid
1975 insect growth regulator in waters, soils, plants, and
animals by gas-liquid chromatography. J. Assoc. Offic. Anal.
Chem., 58:10-18.
Mura, T. and Takahashi, R.M. Insect development inhibitors. 3. Effects
1973 on non-target aquatic organisms. J. Econ. Entomol.,
66(4):917-922.
Quistad, G.B. and Staiger, L. Determination of bound residues and
1974a material balance for Altosid IGR treated soil. Zoecon
Corporation, Report No. 3760-12-01-74.
Quistad, G.B. and Staiger, L. Partition of Altosid between water and
1974b soil. Zoecon Corporation, Report No. 3760-12-03-74.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1974a degradation of the insect growth regulator methoprene. I.
Metabolism by alfalfa and rice. J. Agric. Food Chem., 22(4):
582-589.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Cholesterol and bile
1974b acids via acetate from the insect juvenile growth hormone
analogue, methoprene. Life Sciences, 15(10):1797-1804.
Quistad, G.B., Staiger, L.E., Bergot, B.J. and Schooley, D.A.
1975a Environmental degradation of the insect growth regulator
methoprene. VII. Bovine metabolism to cholesterol and
related natural products. J. Agric. Food Chem., 213:743-749.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975b degradation of the insect growth regulator, methoprene. III
Photodecomposition. J. Agric. Food Chem., 23:299-303.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975c degradation of the insect growth regulator methoprene. VIII.
Bovine metabolism to natural products in milk and blood. J.
Agric. Food Chem., 23:750.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1976a degradation of the insect growth regulator methoprene X.
Chicken metabolism. J. Agric. Food Chem., 24:644-648.
Quistad, G.B., Schooley, D.A., Staiger, L.E., Bergot, B.J. Sleight,
1976b B.H. and Macek, L.K. Environmental degradation of the insect
growth regulator methoprene. IX. Metabolism by bluegill
fish. Pestic. Biochem. Physiol., 6:523-529.
Rowlands, D.G. The uptake and metabolism by stored wheat grains of an
1976 insect juvenile hormone and two insect hormone mimics. J.
Stored Prod. Res., 12:35-41.
Schaefer, C.H. and Dupras, E.F. Insect developmental inhibitors. 4.
1973 Persistence of ZR-515 in water. J. Econ. Entomol., 66:923.
Schaeffer, CH. and Wilder, W.H. Insect development inhibitors. 2.
1973 Effects on target mosquito species. J. Econ. Entomol.,
66(4): 913-916.
Schooley, D.A. Metabolism of 3H-methoprene by the dwarf lima bean
1974 plant. Zoecon Corporation, Report No. 3760-12-02-74.
Schooley, D.A. and Bergot, B.J. 10-3H-methoprene dissipation rates in
1974 pond water and sewage. Zoecon Corporation, report no.
3760-12-06-74.
Schooley, D.A. and Bergot B.J. Hydrolytic stability of radiolabelled
1975a methoprene. Zoecon Corporation, Report No.
Schooley, D.A. and Bergot, B.J. 3-day metabolism study of Altosid in
1975b non-sterile water. Zoecon Corporation Report No.
Schooley, D.A., Bergot, B.J., Durham, L.L., and Siddall, J.B.
1975a Environmental degradation of the insect growth regulator,
methoprene. II. Metabolism by aquatic micro-organisms. J.
Agr. Food Chem., 23(2):293-298.
Schooley, D.A., Creswell, K.M., Staiger, L.E. and Quistad, G.B.
1975b Environmental degradation of the insect growth regulator
methoprene. IV. Soil metabolism. J. Agric. Food Chem.,
23(3): 369-373.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-update of
1974a methoprene and its metabolites by a second crop of wheat
grown in treated soil. Zoecon Corporation, Report No.
3760-12-08-74.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-translocation of
1974b methoprene and its metabolites from soil into water. Zoecon
Corporation, Report No. 3760-12-04-74.
Zoecon Methoprene residues in chickens - Report submitted to FAO for
1984a consideration by JMPR. Zoecon Corporation.
Zoecon Altosid leaching studies - Report submitted to FAO for
1984b consideration by JMPR - Zoecon Corporation.
Zoecon Methoprene distribution in Australian wheat fractionation.
1984c Report RM-0248, 17 September 1984. Zoecon Corporation.
Zoecon Methoprene distribution in Kansas State University. Wheat
1984d fractonation study. Report RM-0250, 18 September 1984.
Zoecon Corporation.
Synonyms: ALTOSID, APEX, DIACON, KABAT, DIANEX, PRECOR.
(All registered Trade Names) ZR-515; ENT-70460;
CAS No: 114-26-1
Other information on identity and properties:
Molecular formula C19H34O3
Molecular weight: 310
Physical form: Pale yellow liquid
B.P.: 100° at 0.05 mm Hg
Vapour pressure: 2.37 × 10-5 mm Hg at 25°C
1.60 × 10-4 mm Hg at 40°C
Solubility: all organic solvents-infinity
water - 1.39 mg/l.
Specific gravity: 0.9261 g/cc at 20°C
Octanol/water
partition coefficient: 10,000
Cis-2 : trans-2 ratio: 8:92
Purity: methoprene - 92-95%
related isomers - 5-2%
solvent - 1-2%
unidentified - 2-1%
Stability: extremely stable in sterile aqueous
solutions over wide range. Readily
biodegraded by common bacteria. Rapidly
degraded in solution or in thin films by
sunlight and UV light. Identity of
degradation products known. (Quistad
et al., 1975b).
Formulations: Several emulsifiable formulations for
agricultural, stored product and public
health use; solution formulation for
treating cured tobacco; fogging solution
for public health use; emulsifiable
formulations for addition to feed
supplements; slow release formulation
for use in mosquito abatement;
emulsifiable formulation for protection
of houseplants.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Methoprene is an insect growth regulator (IGR), being a synthetic
analogue of the insect juvenile hormone (Henrick et al., 1973).
Unlike conventional insecticides, which act as direct poisons,
methoprene acts by disrupting the morphological development of
insects. After contact, inhalation or ingestion the insect grows and
pupates normally, but the pupa dies in the pupal stage. As a result,
methoprene is not generally used in pre-harvest situations when a
quick kill of insect pests is required.
Methoprene has found widespread use in mushroom culture to
prevent the emergence of adult sciarid flies, where it is applied to
compost or casing at the rate of about 100 g/100 sq.m. This
application represents about 12% of methoprene use. A commonly used
treatment is 175 ml of 650 g/l emulsifiable concentrate per 100 sq.m.,
equivalent to 113 g a.i./100 sq.m. One or two applications are used.
Special slow-release formulations are used to control mosquito
breeding in floodwater sites, rice cultivations, storm drains, ponds,
water treatment works etc. (Schaefer and Wilder, 1973; Mura and
Takahashi, 1973).
Methoprene is formulated into feed supplements for cattle to
control adult hornfly breeding in cattle manure. The rate is
equivalent to 0.016 mg/kg body weight daily. This, together with
mosquito control represents about 50% of current use.
Methoprene is marketed as a 65% emulsifiable concentrate
formulation for use as a space spray for control of pests in food
handling and storage establishments. It is applied as an aqueous spray
or via mechanical foggers at a rate of 10 ml per 1,000 m2.
Post-harvest treatments
Methoprene has proved effective in the control of numerous pests
of stored products such as peanuts where it is used at the rate of
10g/tonne against most moth and beetle pests. It is being tested on
cocoa beans and cereal grains as a 657g/l emulsifiable concentrate
formulation. This is applied at a rate of 15ml/tonne of stored
commodity, equivalent to 10g a.i./tonne, in either water or mineral
oil.
Trial work indicates that methoprene will control most insect
pests affecting stored grain though it is significantly less effective
against Sitophilus spp. which include three of the major pests of
maize, rice and wheat. It may have to be combined with a conventional
insecticide where these species are a problem.
Methoprene has been used for more than five years for treating
stored tobacco. The cured tobacco is treated, before packing, at the
rate of 200g methoprene per tonne of tobacco. It is used for treating
food-handling and tobacco-processing establishments to prevent insect
breeding and development. A fogging formulation is used to control
pre-adult fleas in premises.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Residues in stored agricultural commodities
Peanuts. During the period 1979-1981 the manufacturers, in
conjunction with the US Department of Agriculture, undertook trials of
methoprene for protecting peanuts against stored-product insect pests
(Miller 1981). The methoprene was added as the peanuts were being
placed in storage silos. Normally peanuts are stored for a period from
a few months to a year.
Treatment rates were 0, 4, 10, 25 and 100 mg/kg. Nuts (kernels)
and hulls were analysed separately. Residues at the proposed use rate
of 10 mg/kg on the day of treatment were below the limit of
determination (0.05 mg/kg) in kernels with wrappers, 0.05-0.64 mg/kg
in kernels without hulls (split hulls) and 14-20 mg/kg in hulls. After
278 days storage the kernels with wrappers contained 0.13-0.38 mg/kg
(mean of 11 samples 0.22 mg/kg), kernels without hulls (split hulls)
0.38-2.1 mg/kg and hulls 8.3-14.0 mg/kg.
Peanuts treated at exaggerated rates contained residues
proportionally higher than those treated at the proposed use rate.
Maize. Eight studies were carried out on stored shelled maize
(Miller, 1983a). Random samples from bulk lots treated at rates of 10,
15, 20 and 23.5 mg/kg were drawn at intervals up to 13 months after
treatment. At all application rates, residues showed no significant
losses with time, even at 200 days after treatment. It is noteworthy
that at no stage did the residues determined by analysis reflect the
amount of active ingredient actually applied to the grain, and in most
trials ranged between 40% and 70% of it. It is difficult to see
whether this was due to failure to deposit the methoprene on grain,
loss from the grain surface immediately after treatment, loss from the
samples between collection and analysis, or failure to recover residue
from the substrate.
The analytical method included an internal standard which was
added in the blender. Recovery of the internal standard was always
high (80-110%), implying validity of the method and analytical
procedures. However, there is no explanation of the apparent absence
of methoprene. Nevertheless the residue levels found throughout the
storage period were similar to, or higher than, those reported for the
initial sample, indicating that little degradation had occurred during
storage.
Wheat. Data from two studies (Miller, 1983b) were available. Stored
wheat was treated with methoprene at 10 and 20 mg/kg and sampled 3,
35, 95 and 150 days after treatment. Methoprene residues recovered by
analysis were about one-half of the treatment rate but did not show
any decline up to 150 days after treatment. Residues from the 10mg/kg
treatment were 5.2 mg/kg after 150 days. An explanation for this
discrepancy is needed.
In a laboratory study at the University of California using GLC
analysis, Mian and Mula (1983) found that when methoprene was applied
at rates of 1, 5 and 10 mg/kg to wheat (13.5% moisture content) stored
at 27 ± 1°C, losses were 61, 66 and 62% respectively over a period of
12 months. The results of analyses carried out at six times within the
12-month period are given in Table 1.
These data seem more reliable than those reported by Miller
(1983b), since the main initial recovery of insect growth regulator was
99% of that applied. Thus it can be accepted that the loss over 12
months is of the order of 60% of the amount applied.
Rowlands (1976), determined the rate of breakdown at 20°C during
10 weeks storage in the dark, using radiolabelled methoprene with
three samples of wheat: fresh wheat of 19.1% moisture content, old
wheat of 12% moisture content and old wheat of 18% moisture content.
Table 2 (adapted from Rowlands, 1976) indicates that the breakdown was
quite rapid at the high moisture levels (half-life of three weeks at
18-19% moisture) and was about half as fast on grain with 12% moisture
(half-life six weeks).
Table 1. Residues of methoprene in treated wheat grain at
various post-treatment intervals 1
Residue, mg/kg
Interval
Application rate, mg/kg
1 5 10
0 0.99a 4.97a 9.92a
1 wk 0.99a 4.60a 9.39a
(0) (7) (5)
1 mo 0.94a 3.93a 6.62b
(5) (21) (33)
4 mo 0.82a 3.49ab 5.53bc
(17) (30) (44)
8 mo 0.80a 1.78bc 4.07c
(19) (64) (59)
12 mo 0.38b 1.71c 3.73c
(61) (66) (62)
1 Mean of four analyses. Means followed by the same letters
in a column are no significantly different from one another
(Duncan's multiple range test. P = 0.05). Values in
parentheses below each mean represent percent loss in
residues at indicated post-treatment interval.
Table 2. Breakdown of methoprene on stored wheat grains at 20°C*
Residue, mg/kg found in grain
Storage time
in weeks Fresh wheat Old wheat Old wheat
(19%) (12%) (18%)
0 5.6 9.8 9.6
1 7.2 9.2 7.7
2 5.5 7.9 6.3
3 4.4 7.6 5.0
4 3.4 7.0 4.3
5 2.2 6.2 3.2
6 1.4 5.1 2.1
7 0.5 4.2 1.4
8 0.2 3.2 0.5
9 0.1 2.4 0.4
10 nil 1.5 0.2
* Results are means from triplicate 25g samples, treated at
10 mg/kg (approx.)
Cocoa beans. In two trials carried out in Brazil, methoprene was
applied to cocoa beans at rates of 1, 2.5, 5.0, 10.0 and 15 mg/kg. The
beans were sampled on the day of treatment and after 50 days storage
at 27°C. Less than half of the applied methoprene was recovered by
analysis but the analytical results on samples taken after 50 days in
storage were not significantly different from those on the initial
samples (Miller, 1983c).
Residues in mushrooms
Data from 7 studies were available (Miller 1978), reflecting both
compost and casing treatment at 0.2 to 4.6 times the recommended
application rates. Samples were collected at 0, 9 and 35 days after
application. When less than 2.5 times the recommended combined
treatment rates were used, the residues were undetectable
(<0.05 mg/kg). At 2.5 and 4.6 times the recommended application rate,
residues ranged from 0.17 mg/kg to 150 mg/kg. The high values were due
to contamination with compost or peat moss "casing" which was shown to
contain methoprene at hundreds of mg/kg. Subsequent samples were
rinsed with cold water to remove the contamination. Most samples
examined in these later experiments contained no methoprene or the
residues were less than 0.1 mg/kg (Miller, 1978).
Dust samples from food-handling establishments were analysed in
six studies (Miller, 1984). Isolated areas were treated with
0.6g a.i./1000 sq.m. (the food being covered according to label
direction before application). Samples were collected in petri dishes
located in appropriate areas of the treated spaces 0, 30, 60 and 90
days after treatment. Residues expressed as mg/kg dust were high owing
to the small amount of dust collected in the dishes. Consequently, the
total quantity of methoprene collected is a more realistic value to
consider when evaluating the results.
Immediately after application methoprene residues were high,
ranging from 26 micrograms to 191 mg. However within 30 days they
declined significantly and ranged from 0.5 to 24 mg. Although there is
some variation in the results, within 60 days the majority of the
residues were below the level of detection (0.5 micrograms).
Residues in forage and field crops
Methoprene is marketed for the control of floodwater mosquitoes
as a 10% slow-release encapsulated aqueous solution (Schaefer and
Wilder, 1973). Since treated areas include pastures, rice fields and
marshlands, residues might occur on certain food crops. Consequently,
Zoecon conducted a series of studies on rice, legumes and forage crops
to analyse for possible methoprene residues which could enter the food
chain through ingestion by livestock.
In a study on rice, methoprene was applied prior to "heading"
(Miller, 1974a). The crop was then grown to harvest. Samples were
collected at 45, 62 and 71 days after application, resulting in a
built-in pre-harvest interval of two months. No detectable residues
(<0.05 mg/kg) were found in the rice grains, hulls or straw of any of
the samples.
In nine studies on pasture legumes and forage crops (Miller,
1974b) pasture land was treated at rates of 9, 11, 23 and 45 g/ha. The
highest treatment rate is four times the highest recommended rate.
Samples were collected 0, 1, 2, 3, 5, 7, 9, 11 and 365 days after
application. Residue levels indicated that methoprene is rapidly
degraded. By day 3, the residues from the highest treatment ranged
from 0.12 to 0.30 mg/kg.
FATE OF RESIDUES
In animals
Numerous studies have been conducted to determine the fate of
methoprene in livestock because of the commercial use in animal feeds
to suppress the breeding of flies in manure.
Quistad et al. (1974b, 1975a) administered [5-14C] methoprene
to a steer which was slaughtered two weeks later. Samples of fat,
muscle, liver, lung, blood and bile were analysed for radioactive
residues. No primary methoprene metabolites could be characterised but
the majority of the tissue radioactivity was positively identified as
cholesterol. A total of 72% of the bile radioactivity was contributed
by cholesterol, cholic acid and deoxycholic acid. Radioactivity from
metabolised methoprene was associated with protein and cholesteryl
esters of fatty acids.
A metabolic balance study was conducted on a 338 kg Jersey cow
dosed with 207 mg of [5-14C] methoprene by capsule (Chamberlain
et al 1975b). This is 40 times the proposed dose for fly control.
The study proceeded for 7 days after dosing. It was found that 16.4%
of the radioactivity was excreted as CO2 between 0 and 170 hours,
with peak excretion after a little under 24 hours. A total of 30.3% of
the applied dose was eliminated via the faeces within about 50 hours
and 19.7% via the urine in 30 hours. By the end of the experiment 7.6%
of the radiolabel had been excreted via the milk. Radioactivity was
measured in 30 tissue samples after the cow was sacrificed 7 days
after dosing. The tissues contained a total of 20% of the applied
dose, intestine and contents contributing 5.96% and fat 4.59%. The
organs of metabolism, lung, liver and kidney, contributed a further
1.68%. The presence of radiocarbon in the tissues was attributed to
steroidal derivatives in which labelled acetate from the methoprene
was anabolically incorporated into natural body constituents.
The results of the above two studies and another on a guinea pig
were compared by Chamberlain et al. (1975a). It was reported that a
rather large percentage of the radiolabel was incorporated in the
tissues and respired by the animals. A small proportion was
incorporated into free primary metabolites, a greater amount into
glucuronides and a considerable proportion within polar components,
possibly complex conjugates or biochemicals. Methoprene was not found
in the urine, but accounted for approximately 40% of the radiolabel in
the faeces. This explains the activity of the administered methoprene
against fly larvae developing in dung pats. The formation of
conjugates and complex metabolites was more extensive in the steer
than the guinea pig. Comprehensive information was provided on the
radiolabel profiles in tissues, organs and other components of the
animals.
On the basis of detailed biochemical analysis of tissues and
organs from the above three studies Quistad et al. (1975c)
elucidated the metabolism by the cow to natural products in milk and
blood. Only a trace of methoprene (0.015/l) and no detectable primary
metabolites occurred in milk.
Quistad et al. (1976a) studied the metabolism of methoprene in
leghorn chickens give single oral doses of [5-14C] methoprene.
Residual radioactivity was found in tissues and eggs. Although a high
initial dose (59 mg/kg)resulted in residues of methoprene in muscle
(0.01 mg/kg), fat (2.13 mg/kg) and egg yolk (8.03 mg/kg), these
represented only 39% and 2% of the total radiolabel in fat and egg
yolk respectively. Radiolabelled natural products were by far the main
14C residues in tissues and eggs, particularly at the lower dose of
0.6 mg/kg where cholesterol and normal fatty acids contributed 8% and
71% of the total radiolabel in egg yolk.
When chickens were given feed treated with methoprene at 0, 25,
50 and 100 ppm for 14-63 days, residues in poultry meat and eggs were
less than 0.1 mg/kg (Zoecon, 1973).
Quistad et al. (1976b) studied methoprene metabolism in
bluegill fish in a dynamic flow-through system and a model aquatic
ecosystem. The fish in the flow-through system acquired moderate
residues of largely unmetabolised methoprene when continuously exposed
to about 30 times the anticipated environmental levels of methoprene,
but these were 93-95% eliminated within two weeks when the fish were
transferred to flowing uncontaminated water. In the model aquatic
ecosystem, the fish accumulated 14C, but the radioactivity was almost
exclusively in radiolabelled natural products, including cholesterol,
free fatty acids, glycerides and protein. Less than 0.1% of the total
radioactivity could be attributed to methoprene or its primary
metabolites.
In plants
Methoprene is rapidly degraded in plants with a half-life of
about 2 days in alfalfa and about 0.5 days in rice. The major
metabolic pathways include ester hydrolysis, O-demethylation and
oxidative scissions of the 4-ene double bond, producing primary
metabolites which are present in relatively low yields (Figure 1)
(Quistad et al. 1974a). These low amounts suggest that subsequent
degradation or conjugation occurs, and in fact the recovery of polar
metabolites as well as the loss of radioactivity through
volatilization are evidence of additional metabolism. In addition,
unextractable residues suggest the extensive degradation of methoprene
and incorporation into natural cellular products. The metabolic fate
of methoprene in alfalfa and rice is shown in Figure 1. Metabolism
resulted in five primary non-polar products isolated from the plant
(ZR-669, ZR-725, ZR-724, ZR-1945 and ZR-1602, see Figure 1). The
combined yield of these products was 1.4% of the applied dose after 7
days from alfalfa and 2.1% after 3 days from rice. The most abundant
non-polar metabolite was ZR-1564 (13% from rice, 2% from alfalfa)
which was isolated from vapours and not found within the plant. The
main aglycones after enzymic cleavage of alfalfa conjugates were
ZR-725 (2.2%), ZR-1945 (0.8%), and ZR-724 (7.4%). The major aglycone
from rice was ZR-1945 (1.2%) with a small amount of ZR-1602 (0.3%).
After 30 days, only 1% of the applied dose remained in alfalfa as
unmetabolised methoprene and 0.4% remained in rice after 15 days. The
initial rapid loss of methoprene and radiolabel from plants was
attributed to evaporation of methoprene and 7-methoxycitronellal
(ZR-1564) on the evidence from supporting studies of methoprene
evaporation from glass plates and of compounds volatilized from leaf
surfaces.
10-3H-methoprene painted on the leaf surface or injected into
the stem of the dwarf Lima bean plant was found not to be
translocated. When injected into the stem, the parent compound was
partially metabolised to ZR-724, ZR-725 and a polar conjugate,
possibly the glycoside. Topical application resulted in more rapid
metabolism to the same metabolites after 24 hours incubation
(Schooley, 1974).
Rowlands (1976) studied the uptake and metabolism of methoprene
and two other IGRs by stored wheat grains. He applied the
radiolabelled compounds topically to individual grains and to small
samples of wheat in glass jars. The wheat contained 19.5% moisture and
was kept at 20°C in the dark. The methoprene penetrated rapidly from
both vapour and topical treatments, but the quantities sorbed differed
between the two routes. Two days after treatment most of the intact
methoprene was found in the aleurone layers, much less in the germ and
virtually none in the endo-sperm proper or outer seedcoat. The rate of
degradation is indicated in Table 2 above. Degradation is promoted by
grain moisture, wheat with 19% moisture giving a half-life of 2-3
weeks, 18% moisture 3-4 weeks and 12% moisture 6-7 weeks.
Mian and Mullar (1983) determined the residues of methoprene on
wheat over a 12-month storage period. Details are given above
("Residues resulting from supervised trials").
In soil
Schooley et al. (1975b) studied the degradation of methoprene
in soil as a function of time under various conditions. On aerobic
sandy loam methoprene showed an initial half-life of about 10 days at
a surface treatment rate of 1 kg/ha; decomposition was much slower on
autoclaved soil. Only small amounts of non-polar metabolites were
isolated, including the hydroxy ester resulting from O-demethylation
(0.7% of the applied dose). Over 50% of the applied dose was converted
to CO2. Radioactivity from [5-14C] methoprene was incorporated into
humic acid, fulvic acid, and humin fractions of sandy loams. These
studies indicate rapid and extensive breakdown of methoprene in soils.
Leaching experiments with radiolabelled methoprene were performed
to determine the mobility of the chemical in different soils. The four
agricultural soils used were sand, sandy loam, silt loam and clay
loam. Experiments with soil columns and soil thin-layer plates
revealed that no significant leaching of methoprene occurred with the
four soils tests (Zoecon, 1984b).
Quistad and Staiger (1974a) determined the amount and nature of
bound residues in soil. They found that in sandy loam soil treated
with [5-14C] methoprene humic acids (3%) and fulvic acids (8.4%) were
most abundant after 30 days. The total recovery of the label from
treated soil was essentially quantitative.
Quistad and Staiger (1974b) determined the partition between
water and soil using a 0.01 mg/l solution of [5-14C] methoprene
agitated with sandy loam soil. After thorough mixing for 15 minutes,
87% of the applied dose was bound to the soil.
Staiger et al. (1974a) grew wheat plants in soil that had been
treated 6 months previously to determine the uptake by a second crop
grown in methoprene-contaminated soil. After treatment of the soil at
the rate of 1 kg/ha, less than 0.006% of the administered label was
taken up in wheat six months after dosing. This uptake is about
one-tenth of that observed when soil was treated with radiolabelled
methoprene at the time seeds were planted.
Staiger et al. (1974b) determined the uptake of methoprene by
wheat plants when wheat seeds were planted in sandy loam and
radiolabelled methoprene was incorporated at the rate of 1 kg/ha at
the seed level and in the soil at a depth of 9 cm below the seed. The
wheat was harvested 11 days after planting and assayed after total
combustion. Uptake was found to be 0.09% of the applied radioactivity
when the IGR was inserted at the level of the seed and 0.04% of the
radioactivity when it was incorporated 9 cm below the seeds. It appears
that very little methoprene or its metabolites are translocated into
wheat from soil.
In water
Sterile aqueous solutions of methoprene (0.5 mg/l), buffered at
various pH values, were found to be extremely stable to hydrolysis
over four weeks at 20°C in the dark. No degradation was seen for the
duration of the experiment in sterile water buffered at pH 7 and 9,
and similar stability was observed in pH 5 buffer for 21 days. However
the pH 5 buffer became non-sterile between the 21- and 30-day
observations, and 59% degradation occurred between days 21 and 30.
(Schooley and Bergot, 1975a).
Schaefer and Dupras (1973) studied the persistence of methoprene
in water. They found that residues were greatly affected by sunlight
and temperature. The methoprene tended to remain on the water surface
and its distribution was affected by wind. The half-life in field
water was only about two hours. A slow-release formulation showed
almost no detectable residues after 24 hours, although biological
activity persisted for several days.
Schooley and Bergot (1974) measured the dissipation of methoprene
in pond water and sewage at dose rates of 0.001 and 0.01 mg/l. A
half-life of 30-45 hours was observed in pond water, with a 60-70 hour
half-life in sewage. This significantly longer half-life in sewage may
have been due to the fact that the primary sewage sample had been
standing in a closed bottle for 30 days in the laboratory, while the
pond water had been collected fresh on the day of the experiment.
On standing for thirteen days in unsterilised pond water
containing suspended sediment in full summer sunlight at a
concentration of 0.66 mg/l, methoprene was metabolised and/or
photodegraded mainly to one product, ZR-1945 methoxycitronellic acid
(Schooley et al. 1975a). This is one of many products formed by the
combined action of oxygen and sunlight on thin films of methoprene on
glass plates. ZR-724, ZR-725 and ZR-669 (See Figure 1) were either not
present or present in very low concentrations.
A 3-day study was conducted on methoprene in non-sterile pond
water at ambient temperature in direct sunlight (Schooley and Bergot,
1975b). Three primary products, ZR-724, ZR-725, ZR-669 were
identified. Each compound, as well as the recovered methoprene, was
found to be an approximately 1:1 mixture of cis-2 and trans-2
isomers, in contrast to the 94% trans-2 methoprene which was
applied. This conversion has been shown in other studies to be ready
photoisomerization rather than an enzymic process.
The biodegradation of (2E)-[10-3H] methoprene was studied in
pond water containing unknown micro-organisms (Schooley et al
1975a). The methoprene showed a half-life of approximately 30 hrs at
0.001 mg/l and 40 hr at 0.01 mg/l. Incubation of the same preparation
for 3 days at 0.42 mg/l generated three primary metabolites, the
result of ester hydrolysis and/or O-demethylation. These metabolites
and the recovered methoprene were photoequilibrium mixtures of 2-ene
isomers. In another incubation experiment with (2E)-[5-14C]
methoprene at 0.66 mg/l in a pond water sample with presumably
different microflora, a completely different metabolic profile was
observed. The main and only identifiable metabolite, resulting from
oxidative scission of the 4-ene double bond, was 7-methoxycitronellic
acid (29% of the applied dose).
In processing and cooking
Peanuts. Peanuts from one of the trials Miller (1981) (see
"Residues resulting from supervised trials") with residues ranging
from 0.18 to 1.21 mg/kg at the time of treatment, were processed into
peanut butter and peanut oil. The peanut butter was reported to
contain less than 0.05 mg/kg of methoprene, indistinguishable from
untreated controls. The peanut oil, however, contained 0.25 mg/kg
(0.20-0.29) of methoprene. These results do not appear to be
consistent.
A trial was carried out in Australia (Zoecon 1984c) in which
methoprene was applied to wheat at the rate of 10 mg/kg. After storage
for two weeks the wheat was milled through an experimental Buhler mill
to provide bran, pollard and white flour. A separate milling produced
wholemeal. The residues found, and the proportion of each fraction in
the first milling, are shown in Table 3.
A separate trial carried out at Kansas State University (Zoecon,
1984d) involved treatment of two lots of wheat with methoprene at the
rate of 10 mg/kg followed, 9 days later, by milling through a Ross
Walking Flour Mill. A separate milling through a pin mill produced
wholemeal.
The white and wholemeal flours were used to prepare bread. The
results of analysis are shown in Table 4.
METHODS OF RESIDUE ANALYSIS
Residues of methoprene have been determined in waters, soils,
plants, stored grain and corn, milk, eggs, fish, shellfish, poultry
and cattle tissues, blood, urine and faeces. Residues in all samples
are extracted by high-speed blending with acetonitrile followed by
vacuum filtration. Fatty extracts are subjected to cold-temperature
precipitation and filtration to remove fat. Petroleum ether
partitioning, Florisil and neutral alumina chromatography are used for
clean-up. The concentrated eluates are analyzed by GLC on columns of
differing polarity, using flame ionization detectors. Confirmation of
identity by additional GLC and mass fragmentography (MF). The lower
limits of determination are: soils, blood and urine, 0.001 mg/kg;
forage grasses, forage legumes and rice foliage, 0.005 mg/kg; milk,
eggs, stored grain and corn kernels, fish, shellfish, poultry and
cattle tissues and faeces, 0.01 mg/kg. Limits of determination and
recoveries were validated by analysis of laboratory and field samples
fortified with 14C-methoprene (Miller et al., 1975).
Table 3. Methoprene residues in wheat fractions Zoecon, 1984c)
Fraction % wt./wt/ of each Methoprene (mg/kg)
fraction (excluding
wholemeal)
Wheat 8.9
Wholemeal flour 6.7
White flour 74 1.8
Bran 23 11.4
Pollard 3 17.7
An internal standard was used in the analysis.
Table 4 Methoprene residues in wheat fractions (Zoecon, 1984d)
Fraction % mt/wt of each fraction Methoprene (mg/kg)
(excluding wholemeal)
Trial Trial Trial Trial
Wheat (whole) 5.0 5.7(Note 1)
White flour 70.2 70.1 2.8 2.8
Wholemeal flour 4.1 5.5
Bran 14.1 14.4 8.7 10.2
Shorts ) 12.7 12.4 9.6 9.6
pollard
Red dog ) 2.4 2.5 9.5 11.0
Germ 0.6 0.6 8.7 10.7
White bread (33% moisture) 0.41
Wholemeal bread (33% moisture) 0.88
Note 1 It appears that a significant proportion of the original
deposit (10 mg/kg) was lost, possibly by evaporation, between
application and analysis. Spiked samples analysed at the same
time yielded the results expected.
Mian and Mulla (1983) describe a method derived from that of
Dunham and Miller (1978) which they applied successfully to studying
the persistence of methoprene in stored wheat. This involved Florisil
and Alumina chromatography with petroleum ether followed by GLC/FID on
a column of 3% OV-101 on 100-120 mesh Chromosorb W (acid-washed,
dimethyldichlorosilane treated), conditioned at 350°C. The column and
injector temperatures were 190°C for retention times of 8 min for
methoprene and 12 min for n-docosane used as an internal standard. The
detector temperature was 350°C. The limit of determination was
reported to be 0.01 mg/kg and the recovery of 10, 5 and 1 mg/kg in
grain extracts ranged between 80 and 100%.
RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
No information was available.
NATIONAL MAXIMUM RESIDUE LIMITS
The meeting was advised that the following national maximum
residue limits had been established in the USA.
Meat of cattle, goats, hogs, sheep,
poultry and horses 0.1 mg/kg
Fat of cattle, goats, hogs, sheep,
poultry and horses 0.3 mg/kg
Meat by-products of cattle, goats, hogs,
sheep, poultry and horses 0.1 mg/kg
Milk fat 0.05 mg/kg
Eggs 0.05 mg/kg
Peanuts 2.0 mg/kg
Mushrooms 1.0 mg/kg
A petition has been presented for tolerances of 10 mg/kg on
barley, buckwheat, corn, millet, oats, rice, rye, sorghum, sunflower
and wheat. In addition a substantial increase has been proposed in the
tolerances for indirect residues, derived from animal feeds, in meat,
fat and meat by-products of cattle, goats, hogs, sheep, poultry and
horses and in eggs and milk fat.
The meeting was not aware of similar action by any other national
authority.
APPRAISAL
Methoprene is an insect growth regulator. It is an analogue of
the insect juvenile hormone. After contact, inhalation or ingestion it
interferes with the normal process of insect development preventing
the emergency of adults from pupae or larvae.
So far there have been no practical pre-harvest uses of
methoprene on crop plants and it appears unlikely that such uses will
develop. There is an important application for the control of sciarid
flies in mushroom culture and as a feed supplement for cattle to
prevent the breeding of hornflies in cattle manure. Methoprene is
applied to water to prevent the breeding of mosquitoes.
The most important application currently under development is the
use of methoprene to prevent the breeding of stored-product insect
pests in tobacco, peanuts, cocoa beans and a wide range of stored
commodities. There are good prospects for use in stored grain to
inhibit the development of stored-product insects. Extensive studies
on these uses are at an advanced stage in several countries.
Methoprene formulations are stable under normal storage
conditions but are rapidly degraded by light. Sterile aqueous
solutions are stable over a wide pH range but non-sterile solutions
are rapidly biodegraded.
When used for the control of insects in stored products
methoprene is applied alone at the rate of 10 mg/kg or in conjunction
with conventional insecticides at much lower rates (1-2 mg/kg).
Although the residues studies available at this stage are not
extensive it would appear that there is relatively little degradation
on dry stored commodities over many months. Degradation is promoted by
moisture and probably also by temperature.
In numerous field studies on peanuts, maize, cocoa beans and
wheat, investigators have failed to recover more than half of the
methoprene applied. This difficulty was not encountered in one small
scale laboratory study at the University of California. This suggests
a pronounced tendency for the methoprene to evaporate with the water
used to apply the insect growth regulator or a fundamental weakness in
the trial technique. This feature is currently receiving further
study.
The meeting received studies from Australia and the USA designed
to determine the distribution of methoprene in grain and the effect of
processing and cooking on the residues. These enabled the meeting to
propose temporary maximum residue limits.
One study with the labelled compound applied to individual wheat
grains indicated rapid penetration. A study on peanuts indicated that
by far the greater proportion of the deposit remained on the hulls and
wrappers and was not taken up by the kernel. However, allowance must
be made for the fact that a proportion of the kernels lose their
shells. When the peanut kernels were processed into peanut butter the
methoprene disappeared but significant amounts were detected in peanut
oil. These studies should be repeated.
When methoprene is applied to mushroom compost or casing at rates
which will control sciarid flies there are no detectable residues in
mushrooms. However, it is necessary to wash the peat moss casing from
the mushrooms before analysis to prevent anomalous results.
Methoprene would be suitable for the treatment of surfaces and
structures to prevent insect pests breeding in food storage and
preparation areas. Several studies indicated that such treatments
would not lead to significant residues in foodstuffs.
Studies have been conducted with cattle, poultry and fish to
elucidate the metabolic processes and determine whether residues occur
when methoprene is administered to livestock directly for fly control,
or in their feed from cereal grain and milling products, or when the
compound is used for mosquito control. All available information
points to the rapid degradation of the parent compound and
incorporation of fragments into natural body components but the
meeting, recognizing that it would not be practical to withdraw
treated feed before slaughter, recommended temporary MRLs for
methoprene residues in foods of animal origin to cover the feeding of
treated grain and its milling products.
Methoprene is rapidly metabolised in plants, yielding products
that are further degraded to normal plant building materials. When
applied to soil, most of the dose is converted rapidly to CO2. There
is no significant leaching of methoprene from treated soil and no
uptake by plants. There appears to be no likelihood of methoprene
being taken up by rice, forage or other crops growing in water treated
for mosquito control.
Methoprene residues may be determined by extraction with
acetonitrile followed by clean-up on Florisil and alumina columns and
quantitation by GLC with a flame ionization detector. The method
appears suitable for regulatory purposes. The limit of determination
ranges from 0.001 mg/kg to 0.01 mg/kg.
The meeting was aware that MRLs had been established for a range
of commodities in the USA and that others were under consideration.
RECOMMENDATIONS
The meeting recognized that there were, as yet, only a limited
number of registrations for the use of methoprene but that potentially
important developments might not proceed in the absence of appropriate
maximum residue limits. To assist these further developments and to
cater for existing uses, the following temporary MRLs are recommended
to apply to residues of methoprene resulting from the direct
application of methoprene or the feeding of methoprene-treated
commodities. They apply to methoprene only.
Commodity MRL (mg/kg)
Cereal grains 10
Bran 20
Flour (wholemeal) 10
Flour (white) 5
Carcase meat 0.2 (in the carcase fat)
Meat byproducts 0.1
Eggs 0.05
Milk 0.002
Peanuts 2
Mushrooms 1
FURTHER WORK OR INFORMATION
Required (before maximum residue limits can be confirmed):
1. Information from studies (now in progress) in which methoprene is
used for the control of pests of stored grain.
2. Studies to define the rate of degradation of methoprene in stored
products under a range of conditions.
3. Further information on the fate of methoprene residues in cereal
grains and other stored products as a result of processing and
cooking.
4. Information from current trials with stored dried lentils, beans,
dried fruit, cocoa beans, coffee beans, spices and oilseeds to
define the treatment necessary, the rate of degradation and the
fate of residues in processing and cooking.
REFERENCES
Chamberlain, W.F., Hunt, L.W.M., Hopkins, D.E., Gingrich, A.R.,
1975a Miller, J.A. and Gilbert, B.N. Absorption, excretion, and
metabolism of methoprene by a guinea pig, a steer and a cow.
J. Agric. Food Chem., 23(4):736-742.
Chamberlain, W.F., Hunt, L.M., Hopkins, D.E., Miller, J.A., Gingrich,
1975b A.R. and Gilbert, B.N. A balance study with 14C-labelled
methoprene in a dairy cow. Official report from US
Department of Agriculture, Kerrville, apparently
incorporated in previous reference.
Dunham, L.L. and Miller, W.W. Methoprene in Analytical Methods for
1978 Pesticides and Plant Growth Regulators (G. Zweig ed).
10:95-109. Academic Press Inc., New York.
Henrick, C.A., Staal, G.B. and Siddal, J.B. Alkyl 3,7,11-trimethyl-2,
1973 4-dodecadenoates, a new class of potent insect growth
regulators with juvenile hormone activity. J. Agr. Food
Chem., 21(3):354-359.
Mian, L.S. and Mulla, M.S. Persistence of three IGRs in stored wheat.
1983 J. Econ. 1983 Entomol., 76(3):622-625.
Miller, W.W. Analyses of indirect methoprene residues in rice. Report
1974a No. RM-026. Zoecon Corporation.
Miller, W.W. Analysis of methoprene residues in legumes and forage
1974b crops. Zoecon Corporation, Report No. E-48-B-73.
Miller, W.W. Analysis of methoprene residues in mushrooms. Zoecon
1978 Corporation, Report No. E-7-P-78.
Miller, W.W. Analysis of methoprene residues in stored peanuts and
1981 peanut hulls. Zoecon Corporation, Report No. SE-GR 340-79.
Miller, W.W. Analysis of methoprene residue in stored shelled corn.
1983a Zoecon Corporation, Reports Nos. RM-1054, RM-0159 and RM-
0167.
Miller, W.W. Analysis of methoprene residue in wheat grain. Zeocon
1983b Corporation, Report No. R7-0130.
Miller, W.W. Analysis of methoprene residues in cocoa beans. Zoecon
1983c Corporation, Reports Nos. RM-0168, RM-0169.
Miller, W.W. Methoprene residue analysis of dust samples in food
1984 handling establishments. Zoecon Corporation, Reports Nos.
RM-0177, RM-0090, RM-0178, RM-0075 and RM-0132.
Miller, W.W., Wilkins, J.S. and Durham, L.A. Determination of Altosid
1975 insect growth regulator in waters, soils, plants, and
animals by gas-liquid chromatography. J. Assoc. Offic. Anal.
Chem., 58:10-18.
Mura, T. and Takahashi, R.M. Insect development inhibitors. 3. Effects
1973 on non-target aquatic organisms. J. Econ. Entomol.,
66(4):917-922.
Quistad, G.B. and Staiger, L. Determination of bound residues and
1974a material balance for Altosid IGR treated soil. Zoecon
Corporation, Report No. 3760-12-01-74.
Quistad, G.B. and Staiger, L. Partition of Altosid between water and
1974b soil. Zoecon Corporation, Report No. 3760-12-03-74.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1974a degradation of the insect growth regulator methoprene. I.
Metabolism by alfalfa and rice. J. Agric. Food Chem., 22(4):
582-589.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Cholesterol and bile
1974b acids via acetate from the insect juvenile growth hormone
analogue, methoprene. Life Sciences, 15(10):1797-1804.
Quistad, G.B., Staiger, L.E., Bergot, B.J. and Schooley, D.A.
1975a Environmental degradation of the insect growth regulator
methoprene. VII. Bovine metabolism to cholesterol and
related natural products. J. Agric. Food Chem., 213:743-749.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975b degradation of the insect growth regulator, methoprene. III
Photodecomposition. J. Agric. Food Chem., 23:299-303.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1975c degradation of the insect growth regulator methoprene. VIII.
Bovine metabolism to natural products in milk and blood. J.
Agric. Food Chem., 23:750.
Quistad, G.B., Staiger, L.E. and Schooley, D.A. Environmental
1976a degradation of the insect growth regulator methoprene X.
Chicken metabolism. J. Agric. Food Chem., 24:644-648.
Quistad, G.B., Schooley, D.A., Staiger, L.E., Bergot, B.J. Sleight,
1976b B.H. and Macek, L.K. Environmental degradation of the insect
growth regulator methoprene. IX. Metabolism by bluegill
fish. Pestic. Biochem. Physiol., 6:523-529.
Rowlands, D.G. The uptake and metabolism by stored wheat grains of an
1976 insect juvenile hormone and two insect hormone mimics. J.
Stored Prod. Res., 12:35-41.
Schaefer, C.H. and Dupras, E.F. Insect developmental inhibitors. 4.
1973 Persistence of ZR-515 in water. J. Econ. Entomol., 66:923.
Schaeffer, CH. and Wilder, W.H. Insect development inhibitors. 2.
1973 Effects on target mosquito species. J. Econ. Entomol.,
66(4): 913-916.
Schooley, D.A. Metabolism of 3H-methoprene by the dwarf lima bean
1974 plant. Zoecon Corporation, Report No. 3760-12-02-74.
Schooley, D.A. and Bergot, B.J. 10-3H-methoprene dissipation rates in
1974 pond water and sewage. Zoecon Corporation, report no.
3760-12-06-74.
Schooley, D.A. and Bergot B.J. Hydrolytic stability of radiolabelled
1975a methoprene. Zoecon Corporation, Report No.
Schooley, D.A. and Bergot, B.J. 3-day metabolism study of Altosid in
1975b non-sterile water. Zoecon Corporation Report No.
Schooley, D.A., Bergot, B.J., Durham, L.L., and Siddall, J.B.
1975a Environmental degradation of the insect growth regulator,
methoprene. II. Metabolism by aquatic micro-organisms. J.
Agr. Food Chem., 23(2):293-298.
Schooley, D.A., Creswell, K.M., Staiger, L.E. and Quistad, G.B.
1975b Environmental degradation of the insect growth regulator
methoprene. IV. Soil metabolism. J. Agric. Food Chem.,
23(3): 369-373.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-update of
1974a methoprene and its metabolites by a second crop of wheat
grown in treated soil. Zoecon Corporation, Report No.
3760-12-08-74.
Staiger, L.E., Quistad, G.B. and Schooley, D.A. Non-translocation of
1974b methoprene and its metabolites from soil into water. Zoecon
Corporation, Report No. 3760-12-04-74.
Zoecon Methoprene residues in chickens - Report submitted to FAO for
1984a consideration by JMPR. Zoecon Corporation.
Zoecon Altosid leaching studies - Report submitted to FAO for
1984b consideration by JMPR - Zoecon Corporation.
Zoecon Methoprene distribution in Australian wheat fractionation.
1984c Report RM-0248, 17 September 1984. Zoecon Corporation.
Zoecon Methoprene distribution in Kansas State University. Wheat
1984d fractonation study. Report RM-0250, 18 September 1984.
Zoecon Corporation.
