
PESTICIDE RESIDUES IN FOOD - 1982
Sponsored jointly by FAO and WHO
EVALUATIONS 1982
Data and recommendations of the joint meeting
of the FAO Panel of Experts on Pesticide Residues
in Food and the Environment and the
WHO Expert Group on Pesticide Residues
Rome, 23 November - 2 December 1982
Food and Agriculture Organization of the United Nations
Rome 1983
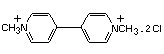
PARAQUAT
 Explanation
Paraquat was evaluated by the Joint Meeting of 1970, 1972 and
1976 (FAO/WHO 1971b, 1973b and 1976).1 An ADI was established
(FAO/WHO 1973b) on the basis of long-term studies conducted by
Industrial Bio-Test Laboratories (IBT), for which no replacement
studies, validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for paraquat was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
A substantial amount of the toxicological data serving as the
basis for the ADI for paraquat are essentially IBT in origin (Baran
and Calanoba 1965; Cervenka et al 1964; Kohn et al 1964; Palazzolo
et al 1964 and 1965). Several replacement studies are in progress.
The data developed by Fletcher et al (1972a, b) and McElligott
(1966) provide information on the reproductive/teratogenic response in
rats/rabbits, respectively, and on the oncogenic response in mice. No
independently obtained validations, replacement studies or other
additional data have been submitted to the JMPR concerning these data.
Considering the evidence available, which demonstrates that paraquat
accumulates in the lung following ingestion, resulting in rapid
progressive fibrosis of the lung, and that renal failure may also
occur, as evidenced by kidney hydropic degeneration, it is recommended
that the present ADI be retained as a temporary ADI, pending receipt
of data validation and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 30 ppm in the diet, equivalent to 1.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw as paraquat dichloride (0-0.0007 mg/kg bw
expressed as paraquat ion).
FURTHER WORK OR INFORMATION
Required (by 1985)
1. A chronic toxicity/carcinogenicity study in the rat.
2. A 12-month oral study in the dog.
3. A multigeneration study in the rat.
Desirable
1. Further observations in humans.
REFERENCES
Baran, J. and Calandra, J.C. Two-year chronic oral toxicity of
1965 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Cervenka, H., Kay, J.H. and Calandra, J.C. Chronic oral toxicity of
1964 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Fletcher, K., Herring, C.M. and Robinson, V.M. Paraquat three
1972a generation reproduction study in rats. Report from ICI
Industrial Hygiene Research Laboratories, submitted to the
World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fletcher, K., Flegg, R. and Kinch, D.A. Paraquat: carcinogenic study
1972b in the mouse. Report from ICI Industrial Hygiene Research
Laboratories, submitted to the World Health Organization
from ICI, Industrial Hygiene Research Laboratories.
(Unpublished)
Kohn, F.E., Kay, J.H. and Calandra, J.C. Two-year chronic oral
1964 toxicity of paraquat-albino rats. Report from Industrial
Bio-Test Laboratories (July 1964) submitted to the World
Health Organization from ICI through Chevron Chemical Co.
(Unpublished)
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. Acute aerosol inhalation
1964 toxicity of paraquat dichloride. Report from Industrial
Bio-Test Laboratories Inc. submitted to the World Health
Organization from ICI (15 April 1964) through Chevron
Chemical Co. (Unpublished)
1965 Sub-acute aerosol inhalation toxicity of paraquat
dichloride. Report from Industrial Bio-Test Laboratories
(January 1965) submitted to the World Health Organization
from ICI Ltd. through Chevron Chemical Co. (Unpublished)
McElligott, T.F. Reproduction in paraquat-treated rabbits. Report from
1966 ICI Industrial Hygiene Research Laboratories, submitted to
the World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Explanation
Paraquat was evaluated by the Joint Meeting of 1970, 1972 and
1976 (FAO/WHO 1971b, 1973b and 1976).1 An ADI was established
(FAO/WHO 1973b) on the basis of long-term studies conducted by
Industrial Bio-Test Laboratories (IBT), for which no replacement
studies, validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for paraquat was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
A substantial amount of the toxicological data serving as the
basis for the ADI for paraquat are essentially IBT in origin (Baran
and Calanoba 1965; Cervenka et al 1964; Kohn et al 1964; Palazzolo
et al 1964 and 1965). Several replacement studies are in progress.
The data developed by Fletcher et al (1972a, b) and McElligott
(1966) provide information on the reproductive/teratogenic response in
rats/rabbits, respectively, and on the oncogenic response in mice. No
independently obtained validations, replacement studies or other
additional data have been submitted to the JMPR concerning these data.
Considering the evidence available, which demonstrates that paraquat
accumulates in the lung following ingestion, resulting in rapid
progressive fibrosis of the lung, and that renal failure may also
occur, as evidenced by kidney hydropic degeneration, it is recommended
that the present ADI be retained as a temporary ADI, pending receipt
of data validation and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 30 ppm in the diet, equivalent to 1.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw as paraquat dichloride (0-0.0007 mg/kg bw
expressed as paraquat ion).
FURTHER WORK OR INFORMATION
Required (by 1985)
1. A chronic toxicity/carcinogenicity study in the rat.
2. A 12-month oral study in the dog.
3. A multigeneration study in the rat.
Desirable
1. Further observations in humans.
REFERENCES
Baran, J. and Calandra, J.C. Two-year chronic oral toxicity of
1965 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Cervenka, H., Kay, J.H. and Calandra, J.C. Chronic oral toxicity of
1964 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Fletcher, K., Herring, C.M. and Robinson, V.M. Paraquat three
1972a generation reproduction study in rats. Report from ICI
Industrial Hygiene Research Laboratories, submitted to the
World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fletcher, K., Flegg, R. and Kinch, D.A. Paraquat: carcinogenic study
1972b in the mouse. Report from ICI Industrial Hygiene Research
Laboratories, submitted to the World Health Organization
from ICI, Industrial Hygiene Research Laboratories.
(Unpublished)
Kohn, F.E., Kay, J.H. and Calandra, J.C. Two-year chronic oral
1964 toxicity of paraquat-albino rats. Report from Industrial
Bio-Test Laboratories (July 1964) submitted to the World
Health Organization from ICI through Chevron Chemical Co.
(Unpublished)
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. Acute aerosol inhalation
1964 toxicity of paraquat dichloride. Report from Industrial
Bio-Test Laboratories Inc. submitted to the World Health
Organization from ICI (15 April 1964) through Chevron
Chemical Co. (Unpublished)
1965 Sub-acute aerosol inhalation toxicity of paraquat
dichloride. Report from Industrial Bio-Test Laboratories
(January 1965) submitted to the World Health Organization
from ICI Ltd. through Chevron Chemical Co. (Unpublished)
McElligott, T.F. Reproduction in paraquat-treated rabbits. Report from
1966 ICI Industrial Hygiene Research Laboratories, submitted to
the World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
 Explanation
Paraquat was evaluated by the Joint Meeting of 1970, 1972 and
1976 (FAO/WHO 1971b, 1973b and 1976).1 An ADI was established
(FAO/WHO 1973b) on the basis of long-term studies conducted by
Industrial Bio-Test Laboratories (IBT), for which no replacement
studies, validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for paraquat was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
A substantial amount of the toxicological data serving as the
basis for the ADI for paraquat are essentially IBT in origin (Baran
and Calanoba 1965; Cervenka et al 1964; Kohn et al 1964; Palazzolo
et al 1964 and 1965). Several replacement studies are in progress.
The data developed by Fletcher et al (1972a, b) and McElligott
(1966) provide information on the reproductive/teratogenic response in
rats/rabbits, respectively, and on the oncogenic response in mice. No
independently obtained validations, replacement studies or other
additional data have been submitted to the JMPR concerning these data.
Considering the evidence available, which demonstrates that paraquat
accumulates in the lung following ingestion, resulting in rapid
progressive fibrosis of the lung, and that renal failure may also
occur, as evidenced by kidney hydropic degeneration, it is recommended
that the present ADI be retained as a temporary ADI, pending receipt
of data validation and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 30 ppm in the diet, equivalent to 1.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw as paraquat dichloride (0-0.0007 mg/kg bw
expressed as paraquat ion).
FURTHER WORK OR INFORMATION
Required (by 1985)
1. A chronic toxicity/carcinogenicity study in the rat.
2. A 12-month oral study in the dog.
3. A multigeneration study in the rat.
Desirable
1. Further observations in humans.
REFERENCES
Baran, J. and Calandra, J.C. Two-year chronic oral toxicity of
1965 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Cervenka, H., Kay, J.H. and Calandra, J.C. Chronic oral toxicity of
1964 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Fletcher, K., Herring, C.M. and Robinson, V.M. Paraquat three
1972a generation reproduction study in rats. Report from ICI
Industrial Hygiene Research Laboratories, submitted to the
World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fletcher, K., Flegg, R. and Kinch, D.A. Paraquat: carcinogenic study
1972b in the mouse. Report from ICI Industrial Hygiene Research
Laboratories, submitted to the World Health Organization
from ICI, Industrial Hygiene Research Laboratories.
(Unpublished)
Kohn, F.E., Kay, J.H. and Calandra, J.C. Two-year chronic oral
1964 toxicity of paraquat-albino rats. Report from Industrial
Bio-Test Laboratories (July 1964) submitted to the World
Health Organization from ICI through Chevron Chemical Co.
(Unpublished)
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. Acute aerosol inhalation
1964 toxicity of paraquat dichloride. Report from Industrial
Bio-Test Laboratories Inc. submitted to the World Health
Organization from ICI (15 April 1964) through Chevron
Chemical Co. (Unpublished)
1965 Sub-acute aerosol inhalation toxicity of paraquat
dichloride. Report from Industrial Bio-Test Laboratories
(January 1965) submitted to the World Health Organization
from ICI Ltd. through Chevron Chemical Co. (Unpublished)
McElligott, T.F. Reproduction in paraquat-treated rabbits. Report from
1966 ICI Industrial Hygiene Research Laboratories, submitted to
the World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Explanation
Paraquat was evaluated by the Joint Meeting of 1970, 1972 and
1976 (FAO/WHO 1971b, 1973b and 1976).1 An ADI was established
(FAO/WHO 1973b) on the basis of long-term studies conducted by
Industrial Bio-Test Laboratories (IBT), for which no replacement
studies, validations or additional data have been submitted to the
JMPR. Accordingly, the ADI for paraquat was re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
COMMENTS
A substantial amount of the toxicological data serving as the
basis for the ADI for paraquat are essentially IBT in origin (Baran
and Calanoba 1965; Cervenka et al 1964; Kohn et al 1964; Palazzolo
et al 1964 and 1965). Several replacement studies are in progress.
The data developed by Fletcher et al (1972a, b) and McElligott
(1966) provide information on the reproductive/teratogenic response in
rats/rabbits, respectively, and on the oncogenic response in mice. No
independently obtained validations, replacement studies or other
additional data have been submitted to the JMPR concerning these data.
Considering the evidence available, which demonstrates that paraquat
accumulates in the lung following ingestion, resulting in rapid
progressive fibrosis of the lung, and that renal failure may also
occur, as evidenced by kidney hydropic degeneration, it is recommended
that the present ADI be retained as a temporary ADI, pending receipt
of data validation and/or additional data.
1 See Annex 2 for WHO and FAO documentation.
TOXICOLOGICAL EVALUATION
Level Causing no Toxicological Effect
Rat : 30 ppm in the diet, equivalent to 1.5 mg/kg bw.
Estimate of Temporary Acceptable Daily Intake for Man
0 - 0.001 mg/kg bw as paraquat dichloride (0-0.0007 mg/kg bw
expressed as paraquat ion).
FURTHER WORK OR INFORMATION
Required (by 1985)
1. A chronic toxicity/carcinogenicity study in the rat.
2. A 12-month oral study in the dog.
3. A multigeneration study in the rat.
Desirable
1. Further observations in humans.
REFERENCES
Baran, J. and Calandra, J.C. Two-year chronic oral toxicity of
1965 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Cervenka, H., Kay, J.H. and Calandra, J.C. Chronic oral toxicity of
1964 paraquat-beagle dogs. Report from Industrial Bio-Test
Laboratories (August 1964) submitted to the World Health
Organization from ICI through Chevron Chemical Co.
(Unpublished)
Fletcher, K., Herring, C.M. and Robinson, V.M. Paraquat three
1972a generation reproduction study in rats. Report from ICI
Industrial Hygiene Research Laboratories, submitted to the
World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
Fletcher, K., Flegg, R. and Kinch, D.A. Paraquat: carcinogenic study
1972b in the mouse. Report from ICI Industrial Hygiene Research
Laboratories, submitted to the World Health Organization
from ICI, Industrial Hygiene Research Laboratories.
(Unpublished)
Kohn, F.E., Kay, J.H. and Calandra, J.C. Two-year chronic oral
1964 toxicity of paraquat-albino rats. Report from Industrial
Bio-Test Laboratories (July 1964) submitted to the World
Health Organization from ICI through Chevron Chemical Co.
(Unpublished)
Palazzolo, R.J., Kay, J.H. and Calandra, J.C. Acute aerosol inhalation
1964 toxicity of paraquat dichloride. Report from Industrial
Bio-Test Laboratories Inc. submitted to the World Health
Organization from ICI (15 April 1964) through Chevron
Chemical Co. (Unpublished)
1965 Sub-acute aerosol inhalation toxicity of paraquat
dichloride. Report from Industrial Bio-Test Laboratories
(January 1965) submitted to the World Health Organization
from ICI Ltd. through Chevron Chemical Co. (Unpublished)
McElligott, T.F. Reproduction in paraquat-treated rabbits. Report from
1966 ICI Industrial Hygiene Research Laboratories, submitted to
the World Health Organization from ICI Industrial Hygiene
Research Laboratories. (Unpublished)
