
PESTICIDE RESIDUES IN FOOD - 1997
Sponsored jointly by FAO and WHO
with the support of the International Programme
on Chemical Safety (IPCS)
TOXICOLOGICAL AND ENVIRONMENTAL
EVALUATIONS 1994
Joint meeting of the
FAO Panel of Experts on Pesticide Residues
in Food and the Environment
and the
WHO Core Assessment Group
Lyon 22 September - 1 October 1997
The summaries and evaluations contained in this book are, in most
cases, based on unpublished proprietary data submitted for the purpose
of the JMPR assessment. A registration authority should not grant a
registration on the basis of an evaluation unless it has first
received authorization for such use from the owner who submitted the
data for JMPR review or has received the data on which the summaries
are based, either from the owner of the data or from a second party
that has obtained permission from the owner of the data for this
purpose.
TRIFORINE
First draft prepared by
D.B. McGregor,
International Agency for Research on Cancer, Lyon, France
Explanation
Evaluation for acceptable daily intake
Biochemical aspects
Absorption, distribution, and excretion
Metabolism
Effects on enzymes
Toxicological studies
Acute toxicity
Short-term toxicity
Long-term toxicity and carcinogenicity
Genotoxicity
Reproductive toxicity
Multigeneration reproductive toxicity
Developmental toxicity
Special studies
Dermal and ocular irritation and dermal
sensitization
Mode of action
Interactions with nitroso compounds
Haemolytic anaemia
Observations in humans
Comments
Toxicological evaluation
References
Explanation
Triforine, a fungicide, was evaluated toxicologically by the 1977 JMPR
(Annex 1, reference 28), when no ADI was allocated, and again in 1978
(Annex I, reference 30), when more toxicological data were made
available and an ADI of 0-0.02 mg/kg bw was established. Subsequently,
further data have been provided, which were reviewed by the present
Meeting within the CCPR periodic review programme.
Evaluation for acceptable daily intake
1. Biochemical aspects
(a) Absorption, distribution, and excretion
Triforine labelled with either 3H in the piperazine ring or with 14C
in the side chains was administered orally to male FW-49 rats at doses
of 9-200 mg/kg bw (see Table 1) in studies that allowed some aspects
of their metabolism and disposition to be compared. Kinetic studies by
intravenous administration were impractical because of the low
solubility of triforine in water.
Table 1. Experiments with labelled triforine
No. Experiment Label Dose No. of
rats
Triforine Radioactivity
(mg/kg bw) (µCi/rat)
1 Blood level 3H ring 11.5 40.8 9 M
2a Urinary and faecal excretion 3H ring 11.5 36.4 10 M
2b 14C side-chain 15.0 38.0 10 M
3a Biliary excretion 3H ring 9.0 22.5 4 M
3b 14C side-chain 9.0 38.5 2 M
4a Urinary and faecal excretion 3H ring 25, 50, 100, 200 9.2 8 M
4b 14C side-chain 50, 100 3.2 4 M
5 Urinary, pulmonary and faecal 14C ring 10 2 M + 2 F
excretion, pilot
6 Urinary, pulmonary and faecal 14C side-chain 10 5 M + 5 F
excretion
7 Urinary, pulmonary and faecal 14C side-chain 1000 5 M + 5 F
excretion
8 Urinary, pulmonary and faecal 14C side-chain 10 × 15 daysa 5 M + 5 F
excretion
From Darda (1977); Hawkins et al. (1992)
a 10 × 14 days unlabelled followed by 10 × 1 day labelled
After dosing with [piperazine-3H]-triforine (experiment 1), the
maximal blood concentrations of radioactivity were found after 4 h and
represented 1.3% of the dose. After 96 h, 0.3% of the dose remained in
blood. In experiment 2, 74% of the tritium was eliminated in the urine
and 17% in the faeces within 24 h. During the subsequent four days,
only small amounts were excreted: 3.2% in urine and 1.2% in faeces. A
total of 1.9% of the administered radiolabel was excreted in the urine
as tritiated water within six days. After dosing with 14C-triforine
(experiment 2b), 53% of the dose was eliminated in urine and 39% in
faeces within the first 24 h. There was little further excretion over
next 24-72 h. The different patterns of excretion of radiolabel after
dosing with the two forms of triforine may indicate that metabolism
occurs at the side chains; however, see the results of experiments 5,
6, and 7, below. After treatment with higher doses of triforine
(experiments 4a and 4b), the excretion of radiolabel during the first
48 h was comparable to that at lower doses, urinary excretion
accounting for a mean of 73 % of the tritium and 55% of the 14C
label. Biliary excretion (experiments 3a and 3b) indicated that
maximum excretion (2.4% of the dose) occurred at 3 h and that a mean
of 19% was excreted over the first 30 h. Analysis by thin-layer
chromatography showed that the biliary residue after administration of
the 14C label consisted of four main components, whereas with the H
label only two of these components were present, corresponding to the
two major urinary metabolites, one of which was identified as
N-[2,2,2-trichloro-1-(piperazin-1-yl)ethyl]formamide (Darda, 1977).
Its occurrence confirms the metabolism of triforine by elimination of
one side chain (Boehringer Sohn, 1974a, b).
The absorption and disposition of 14C-triforine suspended in 5%
aqueous sodium carboxymethylcellulose was studied in male and female
CD Sprague-Dawley-derived rats at doses of 10 mg/kg bw, as a single
dose and after treatment with unlabelled triforine for 14 days, and
1000 mg/kg bw as a single radiolabelled dose (experiment 5). Both
side-chain and ring-labelled 14C-triforine were available, but
piperazine ring-labelled 14C-triforine was used only in a pilot
experiment in which a single dose of 10 mg/kg bw was administered to
two male and two female rats. In this experiment, the mean proportions
of the administered dose excreted during 120 h were: urine, 77% in
males and 82% in females; faeces, 18% in males and 19% in females;
expired air, 3.3% in males and 1.5% in females; less than 3% remained
in the carcass. Most of the radiolabel (73% in males and 76% in
females) was excreted in the urine within 0-24 h.
In the main study with side-chain-labelled 14C-triforine, in which
single doses of 10 mg/kg bw were given to five male and five female
rats (experiment 6), the mean proportions of the administered dose
excreted during 120 h were: urine, 78% in males and 79% in females;
faeces, 12% in males and 14% in females; expired air, 5.2% in males
and 6.0% in females. Less than 3% remained in the carcass. Most of the
radiolabel (75% in males and females) was excreted in the urine within
0-24 h.
The excretion profile was radically different in experiment 7, in
which side-chain-labelled 14C-triforine was administered at a single
dose of 1000 mg/kg bw. The mean proportions of the administered dose
excreted during 120 h were: urine, 11% in males and 19% in females;
faeces, 85% in males and 77% in females; expired air, 0.9% in males
and 1.6% in females. Only about 0.5% remained in the carcass. Most of
the urinary radiolabel (7.7% in males and 12% in females) was excreted
within 6-48 h. The delayed urinary excretion (in comparison with
experiment 6) probably reflects absorption limited by the dissolution
rate. More than 90% of the radiolabel recovered from the faeces over
0-72 h was associated with side-chain-labelled 14C-triforine and
presumably represented unabsorbed material.
The effect of repeated low doses on the excretion of
side-chain-labelled 14C-triforine was studied in experiment 8. The
mean proportions of the administered dose excreted during 120 h were:
urine, 71% in males and 74% in females; faeces, 17% in males and 15%
in females; expired air, 6.3% in males and 6.8% in females. About 2%
remained in the carcass. Most of the radiolabel (68% in males and 69%
in females) was excreted in the urine within 0-24 h. Thus, in
comparison with experiments 5 and 6, neither the position of the label
nor the dosing schedule significantly affected the overall pattern of
14C excretion The experiments also show no important sex-specific
differences. Seven days after administration, only low residual
quantities of triforine were found in the tissues; blood, liver,
kidney, and skin contained the largest amount of the administered dose
(about 2.4%).
These experiments indicate that > 80% of a daily dose of 10 mg/kg bw
triforine is absorbed by male and female rats, whereas after a single
dose of 1000 mg/kg bw absorption was about 10% in males and 20% in
females.
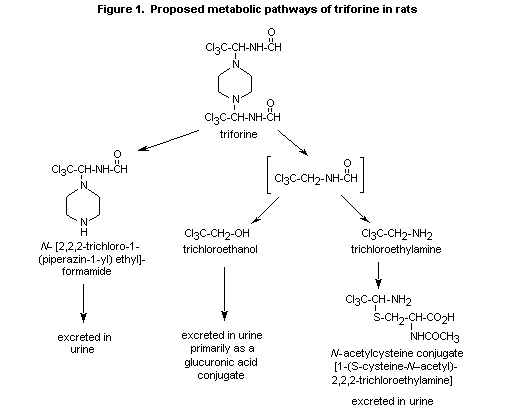
(b) Metabolism
Triforine is rapidly metabolized and excreted in rats; unchanged
compound accounts for only 0-5 % of the dose (Hawkins et al., 1992).
Substantial quantities of unchanged triforine were recovered only from
faeces (Boehringer Sohn, 1974a,b).
The first metabolite to be identified was N-[2,2,2-trichloro-1-
(piperazin-1-yl)ethyl]-formamide, which is formed by the cleavage of
an entire side chain (Darda, 1977). In later metabolic studies with
14C labelling in the piperazine ring and aliphatic side chain
(Hawkins et al., 1992), triforine underwent virtually complete
metabolism after administration as a single oral dose of 10 mg/kg bw.
N-[2,2,2-Trichloro-1-(piperazin-1-yl)ethyl]formamide, the major
radiolabelled urinary component in rats receiving [piperazine
14C]-triforine, accounted for 46 53% of the dose over 0-24; however,
in rats receiving side-chain-labelled 14C-triforine, the proportion
was reduced to 24-27% after a single 10 mg/kg bw dose and 21-24% after
repeated doses. It was excreted as the glucuronide. The side-chain
metabolite trichloroethanol and its glucuronide represented 18-21% of
the dose. Another side-chain metabolite occurring in the urine was the
N-acetylcysteine conjugate of 2,2,2-trichloroethylamine, which
represented 13-15% of the administered dose. In faeces collected from
female rats over 0-48 h, 3.6% of the single 10 mg/kg bw dose and 3.4%
of the repeated doses was present as N-[2,2,2-trichloro-1-
(piperazin-1-yl)ethyl]formamide. This metabolite was not detected in
the faeces of rats receiving 1000 mg/kg bw. Very little unchanged
triforine (0-1%) was detected in the faeces of rats given the low
dose, whereas it represented 70-80% of the dose in rats given 1000
mg/kg bw (Hawkins et al., 1992). This result suggests that absorption
of triforine is a saturable process, unless there is extensive biliary
excretion at the high dose.
A scheme of the metabolic pathways of triforine is presented in Figure
1.
(c) Effects on enzymes
The potential effects of triforine on hepatic xenobiotic metabolizing
enzymes were studied by administering the compound for 28 days to six
male and six female, 35-day-old Sprague-Dawley rats at a dietary
concentration of 20 000 ppm, equal to 1957 mg/kg bw per day in males
and 2094 mg/kg bw per day in females, and to eight male and eight
female, 42-day-old CD-1 mice at a concentration of 7000 ppm, equal to
1555 mg/kg bw per day in males and 1998 mg/kg bw per day in females.
Control groups of equal size were included in the study; the positive
controls received diets containing 500 ppm sodium phenobarbital.
Relative liver weights were increased in male (23%) and female (26%)
rats and male (12%) and female (16%) mice. There were no major
differences between the relevant control and treated groups with
regard to hepatic homogenate protein or DNA content or in
cyanide-insensitive palmitoyl-CoA oxidation activity (as a measure of
peroxisomal fatty-acid oxidizing enzyme activity and peroxisomal
proliferation). Hepatic microsomal fractions were prepared to measure
the activities of 7-ethoxyresorufin- O-deethylase (CYP1A subfamily
marker), 7-pentoxyresorufin- O-depentylase (CYP2B subfamily
marker),erythromycin- N-demethylase (CYP3A subfamily marker), and
lauric acid 11- and 12-hydroxylases (CYP4A subfamily markers). The
microsomal cytochrome P450 content was slightly reduced in male (14%)
and female (13%) rats and slightly increased only in male (28%) mice
fed triforine, 7-Ethoxyresorufin- O-deethylase activity was not
affected in mice but was reduced in both male (54%) and female (60%)
rats. 7-Pentoxy-resorufin- O-depentylase activity was not affected in
any group, while erythromycin- N-demethylase activity was increased
only in male rats (52%) and mice (51%). Lauric acid 12-hydroxylase
activity was not affected in male rats or female mice, while there was
a 36% reduction in female rats and a 30% increase in male mice
(Robbins, 1994).
 2. Toxicological studies
(a) Acute toxicity
The acute toxicity of triforine has been tested by administration by
various routes in mice, rats, and dogs (Table 2). The only reported
observations were reduced activity, swaying, or dyspnoea. There were
no deaths, and no substance-induced changes in organs were detected
post mortem.
(b) Short-term toxicity
Mice
Four groups of five male and five female NMRI mice were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 200, 1000, or 5000 ppm for four weeks, equal to
0, 39, 200, or 980 mg/kg bw per day for males and 0, 45, 240, or 1300
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption; the
apparently increased food consumption by females at 5000 ppm was
possibly due to greater spillage. At the end of the study, males at
5000 ppm had 8% less body-weight gain than controls. Haematological
changes included decreased erythrocyte count (by 8-11%, p < 0.05),
haemoglobin concentration (by 7-9%, p < 0.05), and packed cell volume
(by 6-8% p < 0.05) and increased polychromatic erythrocytes in
animals of each sex at 5000 ppm. Males at this dose also had
moderately increased reticulocyte counts and decreased leukocyte
counts. A significant decrease in mean erythrocyte count was also
observed in males at 1000 ppm, although most of the values fell within
the range for the concurrent control group. Males and females at 5000
ppm showed increased absolute and relative weights of the spleen, and
females had increased relative liver weights (by about 16%,
p < 0.01). There were no gross or microscopic changes. The NOAEL
was 1000 ppm, equal to 196 mg/kg bw per day, on the basis of
haematological changes in animals of each sex, slightly reduced
body-weight gain in males, and increased relative liver weight in
females at 5000 ppm (Tenneker et al., 1988).
Groups of 10 CD-1 mice of each sex were given technical-grade
triforine (purity, 99.1%) in the diet at concentrations of 0 or 7000
ppm for 13 weeks, equal to 1000-1600 mg/kg bw per day in males and
1900-2500 mg/kg bw per day in females. No deaths or clinical signs of
toxicity were recorded, and there were no effects on food consumption
or body-weight gain. The haematological changes included a slight
reduction in erythrocyte numbers, haemoglobin concentration, and
haematocrit in animals of each sex. The absolute weights of the spleen
were increased by 38% in males and 56% in females, and that of the
liver was increased by 17% in males; the relative liver and spleen
weights were increased in animals of each sex ( p < 0.01). There
were no gross pathological findings; tissues were not examined
microscopically, as the study was designed to select concentrations
for use in a long-term study of toxicity and carcinogenicity (Atkinson
et al., 1991a).
Table 2 Acute toxicity of triforine
Species, strain Route of Sex LD50L/C50 Length of Reference
administration (mg/kg bw observation
or mg/L) (days)
Rat, Wistar Oral F, M > 16 000 14 Frohberg et al. (1973)
Rat, Wistar Oral F, M > 5 000 15 Ullman et al. (1986a)
Rat, Wistar Dermal F, M > 10 000 14 Frohberg et al. (1973)
Rat, Wistar Dermal F, M > 2 000 15 Ulman et al. (1990)
Rat, Sprague-Dawley Inhalation (1 h) F, M > 4.5 14 Bullock & Narcisse (1973)
Rat, Wistar Inhalation (4 h) F, M > 5.1 15 Ullman et al. (1986b)
Mouse (strain not stated) Oral F, M > 6 000 7 Muacevic (1968)
Mouse, NMRI Oral F, M > 5 000 15 Ullman et al. (1986c)
Dog (breed not stated) Oral F, M > 2 000 28 Muacevic (1969)
Rats
Groups of five male and five female Wistar rats were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 500, 2500, or 12 500 ppm for four weeks, equal to
0, 50, 240, or 1200 mg/kg bw per day for males and 0, 49, 230, or 1200
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption.
Body-weight gain was reduced by about 15% in males by the end of the
study ( p < 0.05), whereas them were no consistent, treatment-
related effects in females. Haematological changes indicative of
slight anaemia were observed. In rats at 12 500 ppm, slight decreases
in mean cell haemoglobin concentration ( p < 0.01) and mean cell
volume were seen in females ( p < 0.05) and increases in
reticulocyte ( p < 0.01) and polychromatic erythrocyte counts in
animals of each sex. Increased proportions of circulating immature
cells were also seen in rats at 2500 ppm, and the increase was
significant in males ( p < 0.01). Prothrombin time was decreased in
females at 12 500 ppm. At this dose, a slight increase in total
protein (by about 6%, p < 0.05) was seen in animals of each sex and
a slight increase in cholesterol content (57%, p < 0.01) in
females. Urine volume was increased in females at this dose. Changes
in organ weight were restricted to rats at 12 500 ppm. Absolute spleen
weights were increased in animals of each sex ( p < 0.05), as were
the absolute weights of the liver, thyroid, and kidney in females.
Increased relative weights were observed for liver ( p < 0.01) and
spleen ( p < 0.05) in animals of each sex and for thyroid in males
( p < 0.05). There were no treatment-related gross pathological
findings; the treatment-related microscopic changes were slight or
moderate haemosiderin deposition in the spleens of males and females
at 12 500 ppm and females at 2500 ppm; two females at 500 ppm also had
increased haemosiderin deposition. No NOAEL was identified (Tenneker
et al., 1989).
Groups of 15 male and 15 female Wistar FW-49 rats (25 at the high
dose) were given triforine (purity not stated) in the diet at
concentrations of 0, 2500, 7000, or 20 000 ppm for 13 weeks. Ten rats
of each sex at 20 000 ppm were observed for six weeks after the end of
treatment. Haematological, clinical chemical, and extended
histopathological investigations were performed on 10 rats of each sex
at each dose before treatment and after 6, 13, and 19 weeks.
Reversible reductions in erythrocyte count and in haemoglobin and
haematocrit values were seen in all treated groups. These results were
interpreted as a sign of a slight haemolytic anaemia and correlated
with haemosiderin deposition in liver, spleen, kidney, lung, and
heart, which increased in proportion to the dose administered. No
NOAEL was identified (Stötzer et al., 1971a).
As there was no no-effect level in the previous study, another study
was performed in which groups of 25 male and 25 female Wistar FW-49
rats were given triforine (purity not stated) in the diet at
concentrations of 0, 100, or 500 ppm for 13 weeks, equivalent to 5 or
25 mg/kg bw per day. No treatment-related effects were observed. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day, as no effects
were observed at 500 ppm, the highest dose tested (Stttzer et al.,
1971b).
Groups of 10 male and 10 female Sprague-Dawley rats were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0 or 20 000 ppm for 13 weeks, equal to 1300 mg/kg bw
per day. No deaths or clinical signs of toxicity were recorded, and
there were no effects on food consumption or body-weight gain. The
haematological changes included statistically significant but slight
reductions in mean cell haemoglobin concentration, by 1.4% in males
and 2.2% in females, and reduced erythrocyte numbers (by 4-9%) and
increased mean cell volume (by 3.5%) in females. Increases in the
absolute ( p < 0.05) and relative ( p < 0.01) weights of the liver
and spleen were observed in animals of each sex. There were no gross
pathological findings; the tissues were not examined microscopically
(Atkinson et al., 1991b).
Groups of 10 male and 10 female FW-49 rats were given triforine
(purity not stated) in the diet at concentrations of 0, 25, 120, 620,
or 3100 ppm for six months, equivalent to 0, 1.3, 6, 31, or 160 mg/kg
bw per day. Reductions in erythrocyte and haematocrit values and
increases in reticulocyte numbers were observed in rats at 620 and
3100 ppm. No consistent, dose-related variations were found in serum
chemical or urinary parameters. Post-mortem examination did not reveal
any treatment-related changes, but increased liver weights were found
in females at all doses, the relative weights being increased by 27%
in those at 25 ppm, 19% at 120 ppm, 40% at 620 ppm, and 49% at 3100
ppm (no statistical analysis reported). No such increases were
observed in males. Increased haemosiderin deposition was found in the
livers and spleens of male rats at 620 and 3100 ppm and females at
3100 ppm. No treatment-related histopathological changes were seen in
the livers of either male or female rats at 25 or 120 ppm. The NOAEL
was 120 ppm, equivalent to 6 mg/kg bw per day, on the basis of reduced
erythrocyte numbers and packed cell volume at 620 ppm (Stötzer et al.,
1972).
Groups of 10 male and 10 female Sprague-Dawley rats were given topical
applications on shaved intact, occluded skin of a 0, 0.5, or 1.5%
aqueous dilution of a 20% triforine emulsion, equivalent to 0, 10, or
30 mg/kg bw triforine, for 4 h per day daily for 21 days. Five males
and five females per group were followed up for an additional 21 days.
Temporary slight reddening and swelling occurred in the covered skin
areas in all groups, including the controls. No local or systemic
substance-related reactions were observed (Leuschner et al., 1972).
Groups of seven Fischer 344 rats of each sex received technical-grade
triforine in corn oil (3 ml/kg bw) on a shaven area of the back at
doses of 0, 110, 350, or 1100 mg/kg bw per day on five days per week
for three weeks. After each application, the treated area was covered
with gauze and bandage for 6 h, then washed and dried. No deaths or
treatment-related dermal changes were observed, and there were no
effects on food consumption or body-weight gain attributable to
treatment. All animals, including the controls, lost some weight,
particularly during the first week; this response was attributed to
the bandaging procedure. No significant, treatment-related variations
in organ weights or haematological end-points was seen, and them were
no gross or microscopic pathological findings. Female rats at 350 or
1100 mg/kg bw showed statistically significant increases in serum
cholesterol (13% and 22%, respectively), triglycerides (32 and 40%,
respectively), and bilimbin (27 and 13%, respectively); a 25% decrease
in serum alkaline phosphatase activity was seen in females at 110
mg/kg bw. In males, serum alkaline phosphatase activity was reduced by
15% at 350 mg/kg bw and by 21% at 1 100 mg/kg bw; in rats at 1100
mg/kg bw, total serum protein was increased by 5.7% and serum albumin
by 3.8%. Since the changes in bilirubin, cholesterol, and triglyceride
contents were not accompanied by histopathological changes in the
liver and were confined to a single sex, their biological significance
is unclear. The increases in alkaline phosphatase activity may reflect
a toxicological effect, but the decreases observed in this study are
not usually considered to be toxicologically significant. The NOAEL
was 1100 mg/kg bw per day, the highest dose tested, as no toxicity was
observed (Fokkema, 1992).
Dogs
Groups of four male and four female beagle dogs were given triforine
(purity not stated) in the diet at concentrations of 0, 3500, 10 000,
or 30 000 ppm (two groups) for 13 weeks. The supplementary group at 30
000 ppm was observed for an additional six weeks without treatment.
These concentrations were equal to 0, 83, 230, 690, or 710 mg/kg bw
per day for males and 0, 85,240,730, or 720 mg/kg bw per day for
females. Signs of haemolytic anaemia-reduced erythrocyte counts
( p < 0.05) and haemoglobin concentration ( p < 0.05), and,
occasionally, also in haemocrit--were observed at all doses by week
13, but were first observed in week 6 in dogs at 10 000 or 30 000 ppm
( p < 0.01). These effects were accompanied by a consistent increase
in the number of reticulocytes in dogs at 10 000 and 30 000 ppm. All
of the values returned to normal in the group at 30 000 ppm within
three weeks of observation. Serum chemistry in weeks 6 and 13 showed
slight increases in alkaline phosphatase activity and bilirubin and
cholersterol concentrations in all treated groups, but these values
also returned to normal in dogs at 30 000 ppm within three weeks.
Urinalysis and ophthalmoscopy showed no differences between the
groups. Fine, drop-like fatty infiltration of the myocardial fibres
were seen in 0, 5, 1, 5, and 1 dogs in the five groups, respectively,
and fatty accumulation in the liver in 0, 3, 0, 4, and 0 dogs. The
fatty infiltration appeared to be reversible, since it was no longer
detected in the dogs observed for six weeks. Treatment-related
siderosis in Kupffer cells in the liver showed a clear tendency
towards reversibility in the animals allowed to recover. There was no
NOAEL (Stötzer et al., 1971c).
Groups of four beagle dogs of each sex were given triforine in the
diet at concentrations of 0, 100,600, or 3500 ppm for 13 weeks, equal
to 0, 3.6, 22, or 120 mg/kg bw per day for males and females together.
The haematological findings in the dogs at 3500 ppm in the previous
study were essentially confirmed. Furthermore, siderosis was detected
in the spleen, liver, and bone marrow after administration of 600 ppm,
and the weight of the spleen was increased in the dogs at 3500 ppm.
The NOAEL was 100 ppm, equal to 3.6 mg/kg, on the basis of
haemosiderin deposits in the liver and bone marrow at 600 ppm
(Leuschner et al., 1971).
Groups of four male and four female beagle dogs were given
technical-grade triforine (purity not stated) in the diet at
concentrations of 0, 10, 40, 100, or 1000 ppm for two years, equal to
0, 0.23, 0.93, 2.4, or 22 mg/kg bw per day for males and 0, 0.25,
0.99, 2.6, or 24 mg/kg bw per day for females. Samples were taken for
haematology, blood chemistry, and urinalysis during weeks 6, 13, 26,
52, 78, and 104. One male dog at 100 ppm group died of acute
pneumonia. There were no other deaths, and there were no
treatment-related signs of toxicity, changes in food consumption,
changes in body-weight gain, or ophthalmoscopic findings.
Haematological changes in the dogs at 1000 ppm group included
increased mean cell volume in males in week 13 (12%, p < 0.01) and
females in week 26 (3.5%, p < 0.05) and decreased mean cell
haemoglobin concentration in males at week t 3 (3.5 %, p < 0.01).
The other changes were either not statistically significant or were
inconsistent with respect to time interval, sex, or dose. Examination
of femoral bone-marrow smears at termination showed a shift in the
granulopoietic:erythropoietic ratio towards erythropoiesis in two
males and three females at 1000 ppm. The erythropoietic mitotic index
was also increased in one female in this group. No treatment-related
changes were observed on blood chemistry or urinalysis. The absolute
and relative organ weights were comparable in all groups, and there
were no treatment-related gross pathological findings.
Microscopically, haemosiderin deposition of moderate severity was
observed in Kupffer cells in four dogs at 1000 ppm and one control.
Haemosiderosis of the bone marrow was also observed in two dogs at
1000 ppm. The NOAEL was 100 ppm, equal to 2.4 mg/kg bw per day, on the
basis of haematological changes, increased erythropoiesis, and
haemosiderin deposition in the liver and bone marrow in animals of
each sex at 1000 ppm (von Sandersleben et al., 1974; Goburdhun &
Greenough, 1990; Greenough, 1994).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 40 NMRI mice of each sex were given triforine (purity not
stated) in the diet at concentrations of 0, 30, 150, or 750 ppm for 81
weeks, equal to 0, 4.7, 24, or 120 mg/kg bw for males and 0, 5.2, 28,
and 140 mg/kg bw for females. Mortality at 81 weeks was 32% of control
males, 20% of those at 30 ppm, 35% at 150 ppm, and 50% at 750 ppm, and
30% of control females, 40% of those at 30 ppm, 26% at 150 ppm, and
33% at 750 ppm. Survival time, clinical symptoms, body-weight gain,
and food consumption were not affected by treatment. As morphological
findings were described only if they were considered to be relevant to
an evaluation of the carcinogenic effects of the substance, no NOAEL
was identified. Treatment did not increase the total number of
neoplasms (mainly lymphocytic leukaemias and lymphosarcomas), and the
latent periods remained unchanged. The frequencies of specific, less
common tumours in the treated groups were usually not higher than in
the concurrent controls or in the literature (Hofmann et al., 1975).
Four groups of 50 CD-1 mice of each sex were given technical-grade
triforine (purity, 98.9%) in the diet at concentrations of 0, 70, 700,
or 7000 ppm for 105 weeks, equal to 0, 11, 120, or 1200 mg/kg bw per
day for males and 0, 16, 160, or 1600 mg/kg per day for females. Blood
samples were collected for analysis at weeks 52, 77, and 103. The
mortality at 105 weeks was 14% of control males, 19% of those at 70
ppm, 35% at 700 ppm, and 28% at 7000 ppm; for females, 27% of controls
were dead at this time and 27% of those at 70 ppm, 32% at 700 ppm, and
30% at 7000 ppm. The mortality rate in the control group of males was
considered to be unusually low for CD-1 mice at 105 weeks. There were
no treatment-related clinical signs of toxicity or changes in food
consumption. Body-weight gain at the end of the study was reduced by
11% in males at 700 ppm and 16% in those at 7000 ppm. No significant
changes in body-weight gain were seen in females. No haematological
changes were observed. The absolute and relative weights of the liver
were increased by 21% ( p < 0.01) in females at 7000 ppm. At
autopsy, thickening or enlargement of the large intestine was observed
in males that died before the end of the study in the groups receiving
700 ppm (17%) or 7000 ppm (36%), whereas none was seen in the
controls. The large intestine was ulcerated, and inflammation was
observed microscopically, predominantly in male mice that died before
the end of the study after receiving 700 ppm (23%) or 7000 ppm (21%).
The overall occurrence of these findings was none in male controls, 6%
at 70 ppm, 16% at 700 ppm, and 12% at 7000 ppm.
The incidences of hepatocellular adenoma were 9/50 in control males,
12/50 at 70 ppm, 8/50 at 700 ppm, and 8/50 at 7000 ppm; the incidences
of hepatocellular carcinoma were 5/50 in control males, 7/50 at 70
ppm, 8/50 at 700 ppm, and 10/50 at 7000 ppm. There were no significant
differences by either Fisher's exact test or the Cochrane-Armitage
test for trend, for adenomas or carcinomas independently or combined
(Fisher's exact test for comparison of carcinomas in males at 0 and
7000 ppm gave p = 0.263). All of the incidences of liver tumours
were within the ranges found in a compilation of five control groups
of male CD-1 mice in the same laboratory: adenomas, 0-32%; carcinomas,
0-21%. Females had no increase in the incidences of liver tumours. The
incidences of alveolar or bronchiolar adenomas in males were 14/50 in
controls, 13/50 at 70 ppm, 8/50 at 700 ppm, and 17/50 at 7000 ppm, and
the incidences of alveolar or bronchiolar carcinomas were 5/50 in
controls, 2/50 at 70 ppm, 4/50 at 700 ppm, and 7/50 at 7000 ppm. These
differences were not statistically significant. The incidences of
alveolar or bronchiolar adenomas in females were 5/50 in controls,
6/50 at 70 ppm, 7/50 at 700 ppm, and 21/50 at 7000 ppm (Fisher's exact
test, p < 0.001), and the incidences of alveolar or bronchiolar
carcinomas were 1/50 in controls, 2/50 at 70 ppm, 1/50 at 700 ppm, and
6/49 at 7000 ppm (Fisher's exact test, p = 0.059). The incidences of
lung tumours were beyond the ranges observed in five other control
groups of female CD- 1 mice at this laboratory: adenomas, 4-18%;
carcinomas, 0-8% The Meeting concluded that triforine increased the
incidence of alveolar or bronchiolar adenomas fit female mice. The
NOAEL for carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day,
on the basis of an increased incidence of adenomas and/or carcinomas
in females at 7000 ppm. The NOAEL for toxicity was 70 ppm, equal to 11
mg/kg bw per day, on the basis of a slight decrease in body-weight
gain and changes in the large intestine of males fed 700 ppm (Heath et
al., 1992).
Rats
Groups of 35 Wistar (CHBB: THOM-SPF) rats of each sex, with 50 of each
sex in the group of controls and that at the high dose, were given
triforine (purity, 96.6%) in the diet at concentrations of 0, 25,
125,625, or 3120 ppm for two years, equivalent to 0, 1.3, 6.3, 31, or
160 mg/kg per day. Samples were taken for haematological and blood
chemical investigations during weeks 0, 6, 13, 26, 52, 78, and 104
from 20 male and female rats per group. Urine was analysed before and
at the end of the study in 10 animals of each sex from the control
group and that at the highest dose. All animals were examined post
mortem. Complete histopathological examinations were performed at
the end of the study on 20 rats of each sex from the control group and
that at the highest dose, on 15 rats of each sex from the other
groups, on rats that died before the end of the study, and on all
tumour-bearing rats. Mortality, clinical signs, body-weight
development, and parameters of clinical chemisstry and the urinalyses
were not affected by the treatment. Temporary reductions in the number
of erythrocytes ( p < 0.01 for males) and haematocrit values
( p< 0.01 for males) and increased numbers of mticulocytes (39% in
males, 29% in females) were seen in rots at 3125 ppm during week 6,
the variations being more pronounced among male rats. There were no
significant differences in organ weights. Haemosiderosis was observed
in the spleens of both control and treated rats, and the frequency and
intensity of the condition did not differ significantly between the
groups. The sinuses of the adrenals of females in all groups showed
cavernous dilatation, and some were thrombosed. These changes occurred
more frequently in treated than in control animals, but they are
common findings in Wistar rats. No significant difference in tumour
incidence between the groups was found. The NOAEL was 625 ppm,
equivalent to 31 mg/kg bw per day, on the basis of signs of anaemia at
3120 ppm (Hill, 1974).
Groups of 70 Sprague-Dawley rats of each sex were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0, 200, 2000, or 20 000 ppm for two years, equal to
an average of 0, 10, 100, or 1000 mg/kg bw per day for males and 0,
13, 140, or 1400 mg/kg bw per day for females. Twenty rats of each sex
per group were killed at one year and the survivors at two years.
Blood and urine samples were collected for analysis at weeks 26
(females) or 28 (males), 52, and 104. Mortality at two years among the
remaining 50 animals in each group was 25% of control males, 24% of
those at 200 ppm, 19% at 2000 ppm, and 19% at 20 000 ppm and 22% of
control females, 20% of those at 200 ppm, 21% of those at 2000 ppm,,
and 23% of those at 20 000 ppm. There were no treatment-related
clinical signs of toxicity and no changes on ophthalmoscopy or
urinalysis. There were no significant differences in food or water
consumption and only slight differences in body weight between the
groups at the end of the study.
A number of findings could be attributed to the treatment with
triforine. At one year, the body weights of females were moderately
but not statistically significantly lower than those of controls, by
11% in those at 200 ppm, 13% at 2000 ppm, and 21% at 20 000 ppm.
Haemoglobin concentrations were 4-6% lower in male rats at 2000 ppm
( p < 0.05) and 20 000 ppm ( p < 0.01 ) at weeks 28 and 51 and in
those at 200 ppm at week 51. Although the changes were consistent in
males, in female rats the haemoglobin concentrations were slightly and
nonsignificantly lower only at 20 000 ppm at week 26. The mean
corpuscular haemoglobin count was 2% lower in both male and female
rats at 20 000 ppm at weeks 28 and 26, respectively, but not at week
51. The values in the control group were, however, clearly higher than
normally expected for rats of this age, sex, and strain in this
laboratory. Changes in blood chemistry in the group at 20 000 ppm
included slightly raised concentrations of sodium in males at week 28,
of calcium in males at week 28 and females at week 104 (by 4%,
p < 0.01 ), of cholesterol in females at week 104 (by 39%,
p < 0.01), and of total protein in males at week 104 (by 9%,
p < 0.01 ), and a slightly lower concentration of glucose in males
at week 52. females at 2000 ppm also had raised calcium levels at week
104. There were no gross pathological findings. Liver weights were
increased at week 52 in males (11%) and females (15%) at 20 000 ppm
and in all females at week 104; both absolute and relative liver
weights were increased in females at week 104, by 18% in those at 200
ppm ( p < 0.05), 24% at 2000 ppm ( p < 0.01), and 43 % at 20 000
ppm ( p < 0.001 ). Although not significant, the relative weights of
the liver were increased in males, by 19% at 200 ppm, 11% at 2000 ppm,
and 18% at 20 000 ppm. The absence of a dose-related response in the
males calls into question the biological significance of the response
in females at the low dose. Significant, dose-related increases in
absolute and relative spleen weights were seen in females at 2000 and
20 000 ppm that were killed at one year. The relative spleen weights
were also increased in females at 20 000 ppm at week 104. The
increased weights of the liver and spleen correlated with
histopathological findings (described below) and are considered to be
related to treatment. Increased kidney weights were observed at week
52, but only in females, by 9% in those at 200 ppm, 12% at 2000 ppm,
and 17% at 20 000 ppm. Adrenal weights were reduced by 13-15% in all
treated males but were increased by 35% in females at 2000 ppm and by
40% in those at 20 000 ppm. Microscopic examination showed increased
deposition of haemosiderin in the spleen of females at 2000 and 20 000
ppm at two years, in males at these doses at one year, and in females
at 20 000 ppm at one year. The incidence of haemosiderin deposition in
Kupffer cells and macrophages of the liver was increased in male and
female rats at 2000 and 20 000 ppm at one year. The severity was also
increased in these groups of males but only in females at 2000 ppm. In
the latter group, there was an increased incidence of pale-cell foci
in males and bile-duct hyperplasia in females. There were no
treatment-related changes in the proportions of tumour-bearing animals
or in the incidence of any particular tumour type. The Meeting
concluded that triforine is not carcinogenic to rats. The NOAEL was
200 ppm, equal to 10 mg/kg bw per day, on the basis of slight
reductions in body-weight gain in males, haematological changes in
animals of each sex, increased absolute and relative weights of the
spleen in females at one year and of the liver at two years, and
haemosiderin deposition in the spleens of males and females at one
year and in the livers of females after one year at 2000 ppm (Everett
et al., 1992; Perry et at., 1992).
(d) Genotoxicity
Triforine has been tested for genetic damaging activity in an adequate
range of assays, most of which were conducted to currently acceptable
standards (see Table 3 for salient features and references). Mutations
were not induced by triforine in either bacteria or at the hprt
locus of cultured mammalian cell lines, and excision repairable DNA
damage was not increased in treated primary cultures of rat
hepatocytes. Gene conversion was not induced in either yeast or fungal
cells. The potential of triforine for inducing chromosomal damage was
tested in mammalian cell lines in vitro by examining treated
metaphase cells for gross aberrations and in vivo by examining
polychromatic erythrocytes taken from the bone marrow of mice treated
orally for an increase in the proportion of micronucleated cells.
There was no indication of activity in vitro. In one test, an
increase in the proportion of micronucleated cells was observed in
groups of five female mice sampled 48 h after dosing (controls: 0.4 ±
0.55/103; treated with 5000 µg/kg bw: 2.4 ± 1.14/103) but not at 24
or 72 h and not in male mice at any sampling time. The increase was
not confirmed when the study was partially repeated with female mice
sampled only 48 h after dosing (controls: 1.6 ± 1.14/103; treated with
5000 µg/kg bw: 1.2 ± 1.10/103). The Meeting concluded that triforine
is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female CHBB-THOM rats were given triforine
(purity not stated) in the diet at concentrations of 0, 100, 500, or
2500 ppm in a three-generation study in which the rats were mated to
produce two litters in each generation. The only sign of toxicity in
parent rats and their offspring was temporarily, slightly reduced
body-weight gain of male rats, particularly at the high dose.
Table 3. Genetic activity of triforine
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium 100 µg/plate NR Negativea Rohrborn (1977)
TA 100, TA1535
Reverse mutation S. typhimurium 5000 µg/plate NR Negativea Moriya et al. (1983)
TA98, TA 100,
TA1535, TA1537,
TA1538, E. coli
WP2hcr
Reverse mutation S. typhimurium 5000 µg/plate 99.9 Negativea Kramer (1985)
TA98, TA 100,
TA1535, TA1537,
TA1538
Gene conversion, Saccharomycess 1000 µg/ml NR Negativea De Bertoldi et al. (1980)
adc2, trp5 loci cerevisiae D4
Gene conversion, Aspergillus 1000 µg/ml NR Negativeb De Bertoldi et al. (1980)
pabaA locus nidulans
Unscheduled Rat hepatocyte 63 µg/ml 99.8 Negativeb Proudlock et al. (1993)
DNA synthesis primary culture
Cell mutation, Chinese hamster 50 µg/ml NR Negativea Miltenburger (1984)
hprt locus V79 cells
Cell mutation, Chinese hamster 200 µg/ml 99.8 Negativea Adams et al. (1993)
hprt locus ovary cells
Table 3. (continued)
Test system Test object Concentration/ Purity Results References
dose (%)
Chromosomal Chinese hamster 400 µg/ml 99.8 Negativea Brooks & Wiggins (1994)
aberration ovary cells (24 and
48 h sampling)
Chromosomal Chinese hamster 50 µg/ml NR Negativea Miltenburger (1985)
aberration V79 cells (7, 18,
and 28 h sampling)
In vivo
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Weakly Guenard (1984a)
induction marrow (24, 48, by gavage positive
and 72 h sampling)
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Negative Guenard (1984b)
induction (females only, by gavage
48 h sampling)
NR, not reported
a With and without metabolic activation
b Without metabolic activation
Reproductive performance, duration of pregnancy, malformation
frequency, and postnatal development were unaffected by treatment.
When the F3b rats were subjected to post-mortem and histopathological
examinations, no treatment-related change was observed. Since this
study has been superseded by one at higher doses, a full description
is not given; however, the dietary concentration of 2500 ppm had no
adverse effect on reproduction (Niggeschulze et al., 1974).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were given technical-grade triforine (purity,
99.1%) in the diet at concentrations of 0, 1250, 5000, or 20 000 ppm.
The parental (FA) rats received the diets for 10 weeks before mating
and throughout mating, gestataon, and lactation. These concentrations
were equal to 0, 100, 410, or 1600 mg/kg bw per day for males and 0,
100, 400, and 1600 mg/kg per day for females during the premating and
mating periods; for F0 females, they were equal to 0, 97, 400, or
1600 mg/kg bw per day during gestation and 0, 150, 650, or 2900 mg/kg
bw per day during lactation. All F1 rats were weaned onto the same
diets as their parents and continued on this treatment until they were
six to seven weeks old, when they were killedś The only death occurred
in a male rat in the control group. No signs of toxicity were noted.
Food consumption was slightly reduced in the group at 20 000 ppm,
among males during weeks 1-10 and among females during weeks 1-3.
Body-weight gain deficits at these doses during these periods resulted
in a reduction in body weight of about 11% in males throughout the
experiment (17 weeks), whereas females had recovered by the end of the
premating period. The relative liver weights of F0 males and females
at 5000 and 20 000 ppm were increased, and females at these doses also
had increased absolute weights of both liver and spleen. No gross
pathological changes were found at autopsy of the F0 rats; no
histological examinations were performed. Treatment did not affect
mating performance, fertility, duration of gestation, litter size, or
F1 pup survival, and no clinical signs of toxicity were seen among
the F1 pups. From lactation day 14, the body-weight gain of male and
female pups was reduced by 7% in those at 5000 ppm and 11 and 10%,
respectively, at 20 000 ppm. These reductions persisted after weaning
(day 24) and were present at six weeks in males and five weeks in
females. The overall depression in mean weight gain after weaning was
11% for males and 4% for females at 5000 ppm and 19% for males and 10%
for females at 20 000 ppm. Food consumption after weaning was also
reduced at this dose, by 15% in males and 12% in females. There were
no gross pathological findings at autopsy of the F1 rats. The effects
observed at the dietary concentration of 20 000 ppm triforine were
considered not to contraindicate its use as the high dose in a
two-generation study. The NOAEL for reproductive toxicity was 1000 ppm
(McCay & Hazelden, 1990).
Groups of 29 male and 28 female Sprague-Dawley rats were given
technical-grade triforine (purity, 98.9-99.1%) in the diet at
concentrations of 0, 500, 3000, or 20 000 ppm in a two-generation
study. The parental (F0) rats received the diets for 10 weeks before
mating and throughout mating, gestation, and lactation. At weaning of
the F1 litters, pups were selected to provide the F2 generation
litters and continued on the same diets as had been offered to their
parents. The study was completed after weaning of the F2 rats. The
dietary concentrations were equal to 0, 38, 230, or 1500 mg/kg bw per
day for F0 males and 0, 48, 290, or 1900 mg/kg per day for F0
females during the premating and mating periods. For F0 females,
these concentrations were equal to 0, 41,240, or 1700 mg/kg bw per day
during gestation and 0, 63, 380, or 2600 mg/kg bw per day during
lactation. The dietary concentrations of 0,500, 3000, and 20 000 ppm
were equal to 0, 40, 280, and 2000 mg/kg bw per day for F2 males and
0, 61,360, and 2500 mg/kg, per day for F1 females during the
premating and mating periods. For F1 females, these concentrations
were equal to 0, 40,230, and 1800 mg/kg bw per day during gestation
and 0, 65, 420, and 2900 mg/kg bw per day during lactation. Pups were
not culled during the study.
One F0 male and one F0 female at 500 ppm died during the study, and
one F1 female at 20 000 ppm was killed prematurely because of
dystocia. None of these deaths was related to treatment. No clinical
signs of toxicity related to triforine were noted in either the F0 or
F1 generations There were slight reductions in food consumption among
F0 rats at 20 000 ppm and F0 females at 3000 ppm during the first
week of treatment. A decrease of 9% was observed in the body-weight
gain of F0 females, but not males, at 500 ppm during the premating
period, at the beginning of which there had been a 3% body-weight gain
in these rats. Slight deficits in body-weight gain were seen
throughout the treatment period for F0 males at 20 000 ppm, resulting
in an overall reduction in body-weight gain of 9%. During the
premating period, F0 females show body-weight gain deficits of 19% at
3000 ppm and 20% at 20 000 ppm. Overall weight gain was decreased by
9% in F0 females at 20 000 ppm during gestation, whereas there was no
deficit in these rats during, lactation. Food consumption was reduced
among F1 males and females at 3000 ppm (10%) and 20 000 ppm (11 and
12%, respectively), mostly during the first three weeks after weaning.
Corresponding deficits in body-weight gain were seen during this
period, of 9% in males and females at 3000 ppm and 13% in males and 9%
in females at 20 000 ppm. Between weeks 6 and 23, the deficits in body
weight were 5% for males and 10% for females at 20 000 ppm. There were
no effects on the body-weight gain of F1 females at any dose during
gestation or lactation.
There were no gross pathological findings attributable to treatment.
Kidney weights were significantly higher (5-9%), in F0 males and
females at 3000 and 20 000 ppm and in F1 males at 20 000 ppm than in
controls. Liver weights were significantly higher (11-45% and
increasing with dose) in F0 females, F1 males, and F1 females at
3000 and 20 000 ppm; a marginal increase in liver weight was also seen
in F1 males at 500 ppm (6.4%, p < (0.05). Significant increases
were observed in the weight of the spleen in F1 males at 20 000 ppm
(16%) and F1 females at 3000 (20%) and 20 000 ppm (42%). Triforine
treatment did not affect mating performance, fertility, duration of
gestation, gestation index, postimplantation loss, litter size, or pup
survival in either the F0 or F1 generation.
The clinical signs of toxicity in pups that could be attributed to
treatment included a reduction in the mean weights of F1 and F2 pups
at 20 000 ppm from day 4 of lactation, so that, by day 21, the body
weights were reduced by 17% for F1 males, 18% for F1 females, 20%
for F2 males, and 19% for F2 females. Decreases were also observed
in F1 pups at 3000 ppm on days 14-21 of lactation, by 9% in males and
8% in females.
The liver, kidney, spleen, and thyroid of parental F0 and F1 rats at
all doses and a number of other organs of parental F0 and F1 rats at
0 and 20 000 ppm were examined histologically. Dose-related increases
in haemosiderin deposition in the spleen, accompanied by dose-related
increases in extramedullary haematopoeisis and in spleen weight were
observed. There was also a dose-related increase in the severity of
spontaneous nephropathy in males and nephrocalcinosis in females.
Small increases in kidney weight were observed in this study,
particularly in the F0 generation, but with no change at 500 ppm.
Liver size increased with dose and was accompanied by sporadic
centrilobular hepatocyte hypertrophy. The hepatic changes were
regarded by the authors as an adaptive response to increased metabolic
activity. The thyroids of female rats of both generations at 20 000
ppm had the histological appearance of very active secreting glands,
consisting of numerous very small acini with little or no lumina,
scant stored secretion, and lined by cuboidal or columnar epithelium
with pale, foamy cytoplasm. The severity of these effects did not
appear to be increased in the F1 generation. The NOAEL for
reproductive toxicity was 20 000 ppm, equal to 1500 mg/kg per day, the
highest dose tested. The NOAEL for parental toxicity and growth and
development of the offspring was 500 ppm, equal to 40 mg/kg bw per
day, on the basis of decreased food consumption in F0 females, F1
males, and F1 females, decreased body-weight gain in F0 females, F1
males, and F1 females, decreased F1 pup weight, and increased
relative spleen weight in F1 females at 3000 ppm (McCay & Hazelden,
2992; Hazelden & Aitken, 1992; Hazelden, 1994).
(ii) Developmental toxicity
Rats
Groups of 20 inseminated female Sprague-Dawley rats were given 0, 100,
400, 800, or 1600 mg/kg bw per day triforine (purity not stated)
suspended in 1% carboxymethylcellulose by gavage on days 6-15 of
pregnancy. The rates of resorption and variation were increased in
animals at 1600 mg/kg bw per day. Furthermore, the number of fetuses
was reduced, dur to significantly increased postimplantation loss at
this dose. Because the frequency of variations was also slightly
increased at 800 mg/kg bw per day, the NOAEL for fetotoxicity was 400
mg/kg bw per day. Although toxic effects were observed at 800 and 1600
mg/kg bw per day, there were no signs of a teratogenic effect
(Leuschner, 1972).
In a preliminary study, groups of eight inseminated Sprague-Dawley
rats were given triforine (purity, >97%) by garage at doses of 250,
500, or 1000 mg/kg bw per day on days 6-15 of gestation. There were no
deaths or treatment-related clinical signs of toxicity. Mean body
weight was slightly reduced on gestation days 9-12 in animals at 500
and 1000 mg/kg bw per day, but overall body-weight gain in the four
groups was comparable throughout treatment. No gross pathological
changes were found in fetuses removed on day 20 of gestation. No
reproductive parameters were affected by treatment, and them were no
indications of treatment-related embryotoxicity or teratogenicity
(Fuchs, 1992).
Groups of 30 inseminated Sprague-Dawley rats were given triforine
(purity not stated) by garage at doses of 200, 500, or 1000 mg/kg bw
per day on days 6-15 of gestation. There were no deaths or
treatment-related clinical signs of toxicity. Food consumption was
reduced in animals at 1000 mg/kg bw per day, but this was not
accompanied by a significant decrease in body-weight gain. No gross
pathological changes were seen in fetuses removed on day 20 of
gestation. No reproductive parameters were affected by treatment, and
there were no indications of treatment-related embryotoxicity or
teratogenicity. The NOAEL for maternal and developmental toxicity was
1000 mg/kg bw per day (Fuchs, 1993a).
Rabbits
Groups of 15 inseminated Himalayan rabbits were given technical-grade
triforine (purity not stated) in 0.5% carboxymethylcellulose by gavage
on days 6-18 of gestation at doses of 0, 5, 25, or 125 mg/kg bw per
day. Them were no deaths or treatment-related clinical signs of
toxicity. Dose-related decreases in food consumption were observed in
rabbits at 25 and 125 mg/kg bw per day on days 7-14 of treatment. Food
consumption was also reduced in the animals at 5 mg/kg bw per day, but
this had no effect on body-weight gain and was considered to be
toxicologically insignificant. For most of the treatment period, body
weight was reduced in rabbits at 25 and 125 mg/kg bw per day, as a
result of reductions during gestation days 6-9. Towards the end of
treatment, these groups regained weight. The does were killed and
their fetuses removed on day 29 of gestation. No gross pathological
findings were observed. No reproductive parameters were affected by
treatment, and them were no indications of treatment-related
embryotoxicity or fetotoxicity at any dose. The NOAEL for maternal
toxicity was 5 mg/kg bw per day, on the basis of decreased food
consumption and body weight at 25 mg/kg bw per day. The NOAEL for
fetal effects was 125 mg/kg bw per day, the highest dose tested
(Gleich et al., 1981).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in 0.5%
carboxymethylcellulose by garage on days 6-18 of gestation at doses of
0, 6, 30, or 150 mg/kg bw per day. There were no deaths or
treatment-related clinical signs of toxicity. Decreased food
consumption were observed in does at 150 mg/kg bw per day on days
12-18, although no statistically significant reduction in body-weight
gain was observed. The does were killed and their fetuses removed on
day 28 of gestation. No gross pathological findings were observed. No
reproductive parameters were affected by treatment, and there were no
indications of treatment-related embryotoxicity or fetotoxicity at any
dose. The NOAEL for maternal toxicity was 30 mg/kg bw per day, on the
basis of decreased food consumption at 150 mg/kg bw per day. The NOAEL
for fetal effects was 150 mg/kg bw per day, the highest dose tested
(Müller, 1989).
Since maternal effects were minimal in the previous study, triforine
was tested at higher doses. In a preliminary study, four groups of
eight inseminated New Zealand rabbits were given technical-grade
triforine (purity not stated) in distilled water by gavage on days
6-18 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw per day.
There were no deaths or treatment-related clinical signs of toxicity.
Body-weight gain was reduced in all treated groups, mainly due to
body-weight losses on days 6-9 of gestation; the reduction (64%) was
statistically significant in does at 1000 mg/kg bw per day. At the end
of treatment, the body-weight gain in the treated groups exceeded that
of the controls. When the does were killed and their fetuses removed
on day 28 of gestation, no gross pathological changes were found, and
reproductive parameters were not affected. The group mean fetal
weights were slightly reduced at 500 and 1000 mg/kg bw per day. There
were no indications of teratogenicity at any dose (Müller, 1991).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in distilled water by
gavage on days 6-18 of gestation at doses of 0 or 1000 mg/kg bw per
day. There were no deaths or treatment-related clinical signs of
toxicity. Reductions in food consumption and body-weight gain were
observed in does at 1000 mg/kg bw per day, mainly due to body-weight
losses on days 6-9 of gestation. At the end of treatment, food
consumption and body-weight gain in the treated group were comparable
to those of the control group. The does were killed and their fetuses
removed on day 28 of gestation. No gross pathological changes were
observed, and reproductive parameters were not affected by treatment.
The group mean fetal weights were slightly reduced, and this
indication of fetotoxicity was accompanied by reduced ossification of
the carpals and/or tarsals, sternum, and pelvis. There were no
indications of teratogenicity at any dose. No NOAEL for maternal
toxicity was identified (Fuchs, 1993b).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Triforine, moistened with water and left on the shaven skin of
Sprague-Dawley rats for 24 h, was tolerated with no irritation up to a
dose of 10 000 mg/kg bw (Frohberg et al., 1973; Ullmann et al., 1990).
When 0.5 g of moistened triforine (purity, 98.1%) was applied to
shaven skin areas of three male and three female New Zealand whim
rabbits and left on the skin for 4 h under a semi-occlusive bandage,
no erythema or oedema occurred within the 72-h follow-up (Ullmann &
Porricello, 1988a).
No reactions occurred in mucosa or eyes of New Zealand white rabbits
after insertion of 0.1 g triforine into the conjunctival sac (Frohberg
et al., 1973). When triforine (purity, 98.1%) was inserted into the
conjunctival sac of three male and three female New Zealand whim
rabbits, all rabbits showed mild local reactions (reddening, chemosis,
discharge) 1 h after application. The reaction disappeared almost
entirely within 24 h, and only one female still had minor conjunctival
reddening (mean cumulative score, 0.17). No changes were observed in
the eyes with a slit-lamp ophthalmoscope 48 and 72 h after
application. No systemic clinical reactions occurred during the
testing period, and body weight was not affected. The overall
irritation score was 0.83 out of a maximum attainable score of 13
(Ullmann & Porricello, 1988b).
In a Maurer optimization test involving aggravation of testing
conditions by additional injection of Freund's adjuvant in groups of
12 male and 12 female Dunkin-Hartley albino guinea-pigs, triforine
(purity, 99.9%) showed no sensitizing potential after epidermal and
intradermal challenge (Ullmann & Suter, 1984).
(ii) Mode of action
Triforine is regarded as an inhibitor of sterol biosynthesis; in
particular, it inhibits microsomal sterol demethylation, as was shown
by means of gas chromatographic-mass spectrometric measurements of
sterol accumulation in Neurospora crassa and Aspergillus
fumigatus. Because triforine has only weak or no antifungal
properties in vitro, metabolic activation can be assumed (Langcake
et al., 1983).
(iii) Interactions with nitroso compounds
Groups of 25 or 50 male and 25 or 50 female Swiss mice were given
0.05% sodium nitrate in drinking-water, triforine suspended in water
at 300 mg/kg bw by garage twice each week, or a combination of the two
treatments for up to 180 days. Tumour incidences were compared with
those in a control group of 184 males and 117 females. Triforine alone
did not increase the number of tumours; however, the combination
increased the frequencies of lymphomas (including thymoma) and of
epithelial adenomas and carcinomas of the gastrointestinal tract, the
lung, and, in males, the liver. Incubation of triforine with 4%
acidified sodium nitrite solution for 24 h formed dinitrosopiperazine,
which is known to be carcinogenic and was suspected to be the
substance that induced the tumours (Börzsönyi et al., 1978).
(iv) Haemolytic anaemia
The main toxic effect observed in the experiments with triforine was
anaemia, which is a reduction in the concentration of haemoglobin in
the blood below the normal range for the species studied and is
usually accompanied by a reduction in the number of erythrocytes. This
condition occurs when the rate of erythyrocyte production is
outstripped by the rate of erythrocyte destruction. In many cases of
anaemia, there is both a reduced rate of production and an increased
rate of destruction. Nevertheless, because of the greater importance
of one or other of these processes, anaemias are classified as (i)
those involving excessive loss (post-haemorrhagic anaemia) or
destruction (haemolytic anaemias) of erythrocytes and (ii) those
involving failure of production of erythrocytes, diminished production
with bone-marrow hyperplasia being dyserythropoietic anaemia and
diminished production with marrow hypoplasia being hypoplastic or
aplastic anaemia.
Haemolytic anaemia in adult experimental animals can result from an
immune reaction in which the antibody is directed against the chemical
or chemical-plasma protein complex, which can bind to the erythrocyte
cell surface; alternatively, the chemical induces formation of an
antibody which cross-reacts with a normal erythrocytic antigen. In the
first case, haemolysis ceases immediately when exposure to the
chemical ceases; in the second case, evidence of an immune reaction
against erythrocytes may persist for some months after exposure to the
chemical has ceased.
Many chemicals cause haemolysis, e.g. phenylhydrazine, lead, and
arsenic and copper compounds; lead may also interfere with haemoglobin
synthesis. Hypoplastic and aplastic anaemias can be produced by a
number of chemicals, including cytostatic drugs, which generally
depress the bone marrow. In addition, there may be idiosyncratic
reactions to certain drugs, e.g. chloramphenicol, sulfonamides,
phenylbutazone, and other antirheumatic and antithyroid drugs.
Aplastic anaemia can also be induced by some hair dyes and industrial
chemicals, such as benzene.
The effect of triforine is mild, occurs in all or many exposed animals
(depending on the dose), and is characterized by haemosiderin
deposition in several organs, no bone-marrow depression, the entry of
increased numbers of immature cells into peripheral circulation, and
the absence of evidence of immunotoxicty. At least in dogs, there is
actually a (presumably) compensatory increase in erythropoiesis. Of
the three common circumstances in which haemolytic anaemia is induced
by chemicals, two, therefore, are unlikely to be important: an immune
response and increased sensitivity of erythrocytes to oxidative stress
because of glutathione depletion, since glutathione is not
significantly involved in triforine metabolism. The third, oxidative
haemolysis in animals: with normally functioning erythrocytes, would
appear to be the most likely mechanism and one which would probably
cease as soon as exposure ceased. This appears to be the situation in
triforine-exposed rats and dogs. Also, there is no evidence of
methaemoglobin formation or of increased numbers of Heinz
body-containing cells or deformed erythrocytes in the circulation.
3. Observations in humans
Medical reports from three companies that synthesize triforine were
available. Boehringer Ingelheim, Germany, examined 12 workers
repeatedly between 1970 and 1983 and reported no haematological or
blood chemical results considered to be related to exposure to
triforine (Celamerck, 1983a,b). The industrial medical service of E.
Merck Co., Darmstadt, Germany, found no adverse systemic, dermal, or
mucosal effects of exposure to triforine among production workers
during routine examinations performed in conformity with the
requirements of the German Chemical Trade Union (Merck, 1984). No
adverse effects were observed during triforine production at Shell
Agrar GmbH in Spain between 1987 and 1993 (Toubes, 1993).
Comments
A battery of tests for acute toxicity with technical-grade triforine
showed that it is slightly hazardous by both the oral and dermal
routes, with respective LD50 values of > 5000 and > 2000 mg/kg bw.
The LC in rats exposed by inhalation was > 5.1 mg/L. Triforine was
not irritating or sensitizing to the skin of rodents and was minimally
irritating to the eyes of rabbits. WHO has classified triforine as
unlikely to present an acute hazard in normal use (WHO, 1996).
Dietary administration of technical-grade triforine for 4 or 13 weeks
showed that the haematopoietic system is a target, as indicated by
mild haemolytic anaemia with associated secondary effects in the
spleen and liver. Similar results were found in two-year studies of
toxicity in mice, rats, and dogs, in which the haematological changes
were accompanied by increased weights of the spleen and liver. In
addition, in a 105-week dietary study in mice, changes in the large
intestine characterized by thickening, enlargement, inflammation, and
ulceration were observed; in a two-year dietary study in dogs,
increased erythropoiesis and increased haemosiderin deposition were
found in the liver and bone marrow. In a four-week dietary study in
rats at 0, 500, 2500, or 12 500 ppm, there was no NOAEL. In a
four-week study in mice at 0, 200, 1000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 200.mg/kg bw per day. The NOAEL in a 13-week study
in rats at 0, 100, or 500 ppm was 500 ppm, equivalent to 25 mg/kg bw
per day. In a six-month study in rats fed diets giving 0, 25, 120,
620, or 3100 ppm, the NOAEL was 120 ppm, equivalent to 6 mg/kg bw per
day. The two-year dietary studies in rats (0, 200, 2000, or 20 000
ppm), mice (0, 70, 700, or 7000 ppm), and dogs (0, 10, 40, 100, or
1000 ppm) showed NOAELs of 200 ppm, equal to 10 mg/kg bw per day, 70
ppm, equal to 11 mg/kg bw per day, and 100 ppm, equal to 2.4 mg/kg bw
per day, respectively.
The major toxic effect observed in the experiments summarized above
was anaemia. The effect was mild, occurring in all or many of the
exposed animals (depending on the dose); the effects were reversible
in rats and dogs and were characterized by haemosiderin deposition in
several organs, the absence of evidence of bone-marrow depression,
entry of increased numbers of immature cells into the peripheral
circulation, and the absence of effects on organs of the immune
system. In dogs, there was actually an increase in erythropoiesis.
Oxidative haemolysis in animals with normally functioning erythrocytes
would appear to be the most likely mechanism and one that would cease
as soon as exposure ceased. Also, there was no evidence of
methaemoglobin formation or of any increase in Heinz body-containing
cells or deformed erythrocytes in the circulation.
No genotoxic activity was observed in an adequate battery of tests for
mutagenicity and clastogenicity in vitro and in vivo. The Meeting
concluded that triforine is not genotoxic.
The results of the studies critical to derivation of an ADI are shown
below; the list does not include an 81-week study in mice treated
orally, which was considered inadequate for evaluation since
histopathological examination was limited to lesions that were judged
at autopsy to be neoplastic.
No carcinogenic effect was observed in rats given dietary
concentrations of 0, 25, 125,625, or 3120 ppm in one study and 0, 200,
2000, or 20 000 ppm in another. Triforine increased the incidence of
pulmonary tumours in female mice given 7000 ppm; the NOAEL for
carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day. The
Meeting concluded that the murine response involves an unidentified
nongenotoxic mechanism and that the carcinogenic activity seen in mice
is unlikely to be indicative or a human carcinogenic risk at the
expected levels of exposure to triforine.
Triforine at dietary concentrations of 0, 500, 3000, or 20 000 ppm did
not affect reproductive performance in rats over the course of a
two-generation study, the NOAEL being the highest dose tested, 20 000
ppm, equal to 1500 mg/kg bw per day. The NOAEL for parental toxicity
and for the growth and development of the offspring was 500 ppm, equal
to 40 mg/kg bw per day, on the basis of decreased food consumption,
body-weight gain, and F1 pup weight and increased relative spleen
weight at the next highest dose of 3000 ppm.
In a study of developmental toxicity in rabbits given oral doses of 0,
5, 25, or 125 mg/kg bw per day, the maternal NOAEL was 5 mg/kg bw per
day, on the basis of decreased food consumption and body weight at 25
mg/kg bw per day; the NOAEL for developmental toxicity was 125 mg/kg
bw per day. In a later study of rabbits given oral doses of 0, 6, 30,
or 150 mg/kg bw per day, the NOAEL for maternal toxicity was 30 mg/kg
bw per day, on the basis of decreased food consumption and body-weight
gain at 150 mg/kg bw per day; the NOAEL for developmental toxicity was
150 mg/kg bw per day. In two subsequent studies in which triforine was
given orally to rabbits at doses of 0, 250, 500, or 1000 mg/kg bw per
day and 0 or 1000 mg/kg bw per day, maternal and embryotoxicity (as
shown by reduced fetal weight) occurred at 1000 mg/kg bw per day.
Decreased fetal weights and delayed ossification were observed in a
study of developmental toxicity in rabbits at the maternally toxic
dose of 1000 mg/kg bw per day. Thus, developmental toxicity in rabbits
occurred only at doses that were also maternally toxic. A study of
developmental toxicity in rats given triforine orally at doses of 0,
200, 500, or 1000 mg/kg bw per day did not show adverse effects in
either dams or fetuses at doses up to 1000 mg/kg bw per day. The
Meeting concluded that triforine has no specific developmental or
reproductive toxicity.
Monitoring of workers in three manufacturing plants did not reveal any
health effects that might be associated with exposure to triforine.
An ADI of 0-0.02 mg/kg bw was established on the basis of the NOAEL of
2.4 mg/kg bw per day in the two-year study of toxicity in dogs, with a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 70 ppm, equal to 11 mg/kg bw per day (105-week study of
toxicity and carcinogenicity)
Rat: 200 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (parental and
fetal toxicity in a study of reproductive toxicity)
1000 mg/kg bw per day (study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 100 ppm, equal to 2.4 mg/kg bw per day (two-year study
of toxicity)
Estimate of acceptable daily intake
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adams, K., Ransome, S., Anderson, A. & Dawe, I.S. (1993) Chinese
hamster ovary/hprt locus assay: Triforine. Unpublished report from
Huntingdon Research Centre Ltd. (Study No. SLL282/931168). Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Atkinson, C., Perry, C.J. & Hudson, P. (1991 a) Triforine: 13-Week
dietary maximum tolerated dose study in mice. Unpublished report from
Inveresk Research International (Report No. 5875). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Toxicological criteria for estimation of guidance values for dietary and non-dietary exposure to triforine
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral, single dose, rat LD50 > 5000 mg/kg bw
(17 days) Dermal, single dose, rat LD50 > 2000 mg/kg bw
Inhalation (4 h), rat LC50 5.1 mg/L
Dermal, irritation, rabbit Not irritating
Ocural, irritation, rabbit Minimally irritating
Dermal, irritation, rat Not irritating
Dermal, sensitization, guinea-pig Non-sensitizing
Medium-term Dermal, 3 weeks, rat NOAEL = 1100 mg/kg bw per day (highest dose tested)
(1-26 weeks) Oral, 13 weeks, dog NOAEL = 3.6 mg/kg bw per day: haemosiderin deposition
Oral, two-generation, reproductive oxicity, rat NOAEL = 1500 mg/kg bw per day: reproductive toxicity;
NOAEL = 49 mg/kg bw per day: parental and offspring
toxicity
Oral, developmental toxicity, rabbit NOAEL = 5 mg/kg bw per day: maternal toxicity;
NOAEL = 125 mg/kg bw per day: fetal and developmental
toxicity
Long-term Oral, 2 years, dog NOAEL = 2.4 mg/kg bw per day: haematological changes;
(> 1 year) haemosiderin deposition; haematopoiesis
Atkinson, C., Perry, C.J. & Hudson, P .(1991b) Triforine: 13-Week
dietary maximum tolerated dose study in rats. Unpublished report from
Inveresk Research International (Report No. 5879). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Boehringer Sohn (1974a) The pharmacokinetics of the systemic fungicide
triforine (W 524) in the rat. Unpublished report dated 13 October
1974, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Boehringer Sohn (1974b) Excretion and metabolism of triforine (W 524)
in the rat. Unpublished report dated 8 March 1974, Ingelheim, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Börzsönyi, M., Pintér, A., Török, G. & Surján, A. (1978) Formation and
biological effect of N-nitroso compound from piperazine pesticide
triforine. In: Walker, E.A., Castegnaro, M., Griciute, L. & Lyle,
R.E., eds, Environmental Aspects of N-Nitroso Compounds (IARC
Scientific Publications No. 19), Lyon, IARC, pp. 477484
Brooks, T.M. & Wiggins, D.E. (1994) Triforine: In vitro chromosome
studies using cultured Chinese hamster ovary (CHO) cells. Unpublished
report from Shell Research Ltd (Technical Service Report No.
SBTR.93.065). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Bullock, C.H. & Narcisse, J.K. (1973) The acute inhalation toxicity of
Cela W 524 tTechnical. Unpublished report from Chevron, San Francisco,
California, USA. Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Celamerck (1983a) [Occupational medical examination of chemical
workers involved in synthesis operating in the production of
triforine.] Unpublished report dated 22 December 1983, Ingelheim,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in
German).
Celamerck (1983b) [Evaluative position paper on the estimation of risk
potential after application of Saprol on green areas that are to be
used as playing-fields or recreational areas.] (ZN 02092). Unpublished
report dated 27 October 1983, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA (in German).
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci., 8, 193-202.
De Bertoldi, M., Griselli, M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by means of gene conversion in
Saccharomyces cerevisiae and Aspergillus nidulans. Environ.
Mutag., 2, 359-370.
Everett, D.J., Perry, C.J., Hudson, P. & Mulhern, M. (1992) Triforine:
52 Week dietary toxicity study in rats. Unpublished report from
Inveresk Research International Ltd (Report No. 7500). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Fokkema, G.N. (1992) Triforine (SAPROL): A 21 day dermal toxicity
study in rats. Unpublished report from Hazleton UK (Report No.
SBTR.91.011 ). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Frohberg, H., von Eberstein, M. & Weisse, G. (1973) Triforine (W524)
Trial for acute toxicity in rats after oral administration and dermal
application and for primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt (Report No. 4/36/73). Submitted to WHO
by American Cyanamid, Princeton, NJ, USA.
Fuchs, A. (1992) Triforine: Preliminary oral (gavage) embryotoxicity
study in the rat. Unpublished report from Hazleton Deutschland GmbH
(Report No. 1031-121-005). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Fuchs, A. (1993a) Triforine: Oral (gavage) teratogenicity study in the
rat. Unpublished report from Hazleton Deutschland GmbH (Report No
1032-121-006). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Fuchs, A. (1993b) Triforine: Oral (garage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Report No.
1098-121-004). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Goburdhun, R. & Greenough, R.J. (1990) W-524-XX (Triforine), 104 week
oral toxicity study in dogs. Unpublished report from Inveresk Research
International (Report No. 5893). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Greenough, R.J. (1994) Triforine registration standard: Response to
review of 104 week dog study. Unpublished report from Inveresk
Research International Ltd (Report No. 5893). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984a) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 026256). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984b) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 031037). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hawkins, D.R., Wood, S.G., John, B.A., Iqbal, S., Cheng, K.N. &
Kitmitto, A. (1992) 14- C-Triforine: Metabolic fate in Sprague-Dawley
rats. Unpublished report from Huntingdon Research Centre (Report
HRC/SLL 203/920212). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Hazelden, K.P. & Aitken, R. (1992) Triforine: Two generation
reproduction study in rats (supplementary histological report).
Unpublished report from Inveresk Research International Ltd (Report
No. 7874). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Hazelden, K.P. (1994) Triforine registration standard; Response to
review of two-generation reproduction study in rats. Unpublished
report from Inveresk Research International Ltd (Report No. 11125).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Heath, J., Mulhern, M., Perry, C.J. & Henderson, W. (1992a) Triforine:
105 Week dietary carcinogenicity study in mice. Unpublished report
from Inveresk Research International (Report No. 7746). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Hill, K. (197,i) Chronic toxicity tests with the compound W-524-XX in
rats using oral administration, duration 2 years. Unpublished report
from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hofmann, A., Oelrich, I., Sumi, N. & Weisse, G. (1975) W-524
(triforine), 81-weeks carcinogenicity study in mice (substance
administered in the food). Unpublished report from E. Merck,
Darmstadt, Germany. Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Kramer, P.-J. (1985) Triforine techn. T 12756, in vitro assessment for
mutagenic potential in bacteria with and without addition of a
metabolising system. Unpublished report from E. Merck, Darmstadt,
Germany (Study No. T-12 756). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Langcake, P., Kuhn, P.J. & Wade, M. (1983) The mode of action of
systemic fungicides. In: Hudson, D.H. & Roberts, T.R., eds,
Progress in Pesticide Biochemistry and Toxicology, Vol. 3, pp.
1-109.
Leuschner, F. (1972) The effect of W-524, lot 1, on pregnant rats and
their fetuses, following oral administration. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W. (1971
) 13 Weeks oral toxicity study in beagle dogs with W-524. Unpublished
report from Laboratorium für Pharmakologie und Toxikologie, Hamburg;,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W.
(1972) 21-Day toxicity tests with the compounds W-524 in
Sprague-Dawley rats using dermal application. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1990) Triforine dose range finding
reproductive assay in rats. Unpublished report from Inveresk Research
International (Report No. 7073). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1991 ) Triforine, two generation
reproduction study in rats. Unpublished report from Inveresk Research
International (Report No. 7368). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Merck (1984) [Triforine: Medical/occupational health position paper.]
Unpublished report dated 28 March 1984, Darmstadt, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA (in German).
Miltenburger, H.G. (1984) Triforine tech., test report of study LMP
76A (mutations affecting the hypoxanthine-guanine phosphoribosyl
transferase locus in V79 cells: HGPRT-test). Unpublished report from
LMP, Darmstadt, Germany (Study No. LMP 076A). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Miltenburger, H.G. (1985) Triforine tech., test report of study LMP
136 (chromosome aberrations in cells of Chinese hamster cell line
V79). Unpublished report from LMP Darmstadt, Germany (Study No. LMP
136). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Muacevic, G. (1968) Pharmacological investigation, acute oral
toxicity, W-524, male and female albino mice. Unpublished report from
Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by Celamerck
GmbH & Co., Ingelheim/Rhine, Germany.
Muacevic, G. (1969) Pharmacological investigation, acute oral
tolerance in dogs. Unpublished report from Boehringer Sohn, Ingelheim,
Germany. Submitted to WHO by Celamerck GmbH & Co., Ingelheim/Rhine,
Germany.
Müller, W. (1989) Triforine: Oral (gavage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Project No.
460/29). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Müller, W. ( 1991 ) Triforine: Preliminary oral (gavage)
embryotoxicity study in the rabbit. Unpublished report from Hazleton
Deutschland GmbH (Report No. 951-121-003). Submitted to WHO by
American Cyanamid, Princeton, NI, USA.
Niggeschulze, A., Hill, K. & Stötzer, H. (1974) Three generation study
with the fungicide W-524 in rats. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Perry, C.J., Mulhern, M. & Finch, L (1992b) Triforine: 104 Week
dietary carcinogenicity study in rats, incorporating 52 week toxicity
study. Unpublished report from Inveresk Research International (Report
No. 7745). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Proudlock, R.J., Stocker, K.J., Howard, W.R., Anderson, A. & Dawe,
I.S. (1993) Triforine: in vitro DNA repair test using rat hepatocytes.
Unpublished report from Huntingdon Research Centre Ltd (Study Report
No. SLL 281/931152). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Robbins, M.C. (1994) An investigation of the effect of triforine on
rat and mouse hepatic xenobiotic metabolising enzymes. Unpublished
report from BIBRA Toxicology International (Report No. 1356/2/ 2/94).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Röhrborn, G. (1977) [Scientific comment on the mutagenicity of
triforine using the liver microsome test of Ames.] Unpublished report.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in German).
von Sandersleben, J.H., Herbst, M., Weisse, I., Frölke, W., Gutnard,
J. & Stötzer, H. (1974) Chronic toxicity test of the substance
W-524-XX on beagles, oral application, over 104 weeks. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J. & Tiler,
T. (1971a) Testing of the subacute toxicity of the substance W-524 in
rats following oral administration. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1971b) Testing of the subacute toxicity of the
substance W-524 in rats following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke, W., Guénard, J.
& von Sandersleben, J. (1971c) Testing of the subacute toxicity of the
substance W-524 in dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1972) Chronic toxicity studies with the compound
W-524-XX in rats using oral administration; duration 26 weeks.
Unpublished report from Boehringer Seth, Ingelheim, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Toubes, G.P. (1993) Medical certificate, Malgrat de Mer. Unpublished
report. Note of 6.4.1993 from Dr Inchaurrondo to Dr Dammermann, Shell
Agrar GmbH. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1988d) 4-Week toxicity
(feeding) study with triforine techn, in the mouse. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
097751). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1989) 4-Week toxicity
(feeding) study with triforine technical in the rat. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 097740). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Ullmann, L. & Porricello, T. (1988a) Primary skin irritation study
with triforine technical in rabbits (4-hour semi-occlusive
application). Unpublished report from Research & Consulting Co.,
Itingen, Switzerland (Project No. 213300). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Ullmann, L. & Porricello, T. (1988b) Primary eye irritation study with
triforine technical in rabbits. Unpublished report from Research &
Consulting Co., Itingen, Switzerland (Project No. 213311). Submitted
to WHO by American Cyanamid, Princeton, NJ. USA.
Ullmann, L. & Surer, B. (1984) Delayed contact hypersensitivity to
triforine technical in albino guinea pigs, the Maurer optimization
test. Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No, 33276). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Pappritz, G. (1986a) Acute oral
toxicity study with triforine technical in rats. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
076151). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L. Sacher, R., Mohler, H. & Vogel, O. (1986b) 4-Hour acute
inhalation toxicity study with triforine technical in rats.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 076162). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Vogel, O. (1986c) Acute oral
toxicity study with triforine technical in mice. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
077433). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L., Althaus, P., Janiak, T. & Vogel, D. (1990) Acute dermal
toxicity study with triforine techn. (SAG 102) in rats. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 33276, 278774). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of triforine has been tested by administration by
various routes in mice, rats, and dogs (Table 2). The only reported
observations were reduced activity, swaying, or dyspnoea. There were
no deaths, and no substance-induced changes in organs were detected
post mortem.
(b) Short-term toxicity
Mice
Four groups of five male and five female NMRI mice were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 200, 1000, or 5000 ppm for four weeks, equal to
0, 39, 200, or 980 mg/kg bw per day for males and 0, 45, 240, or 1300
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption; the
apparently increased food consumption by females at 5000 ppm was
possibly due to greater spillage. At the end of the study, males at
5000 ppm had 8% less body-weight gain than controls. Haematological
changes included decreased erythrocyte count (by 8-11%, p < 0.05),
haemoglobin concentration (by 7-9%, p < 0.05), and packed cell volume
(by 6-8% p < 0.05) and increased polychromatic erythrocytes in
animals of each sex at 5000 ppm. Males at this dose also had
moderately increased reticulocyte counts and decreased leukocyte
counts. A significant decrease in mean erythrocyte count was also
observed in males at 1000 ppm, although most of the values fell within
the range for the concurrent control group. Males and females at 5000
ppm showed increased absolute and relative weights of the spleen, and
females had increased relative liver weights (by about 16%,
p < 0.01). There were no gross or microscopic changes. The NOAEL
was 1000 ppm, equal to 196 mg/kg bw per day, on the basis of
haematological changes in animals of each sex, slightly reduced
body-weight gain in males, and increased relative liver weight in
females at 5000 ppm (Tenneker et al., 1988).
Groups of 10 CD-1 mice of each sex were given technical-grade
triforine (purity, 99.1%) in the diet at concentrations of 0 or 7000
ppm for 13 weeks, equal to 1000-1600 mg/kg bw per day in males and
1900-2500 mg/kg bw per day in females. No deaths or clinical signs of
toxicity were recorded, and there were no effects on food consumption
or body-weight gain. The haematological changes included a slight
reduction in erythrocyte numbers, haemoglobin concentration, and
haematocrit in animals of each sex. The absolute weights of the spleen
were increased by 38% in males and 56% in females, and that of the
liver was increased by 17% in males; the relative liver and spleen
weights were increased in animals of each sex ( p < 0.01). There
were no gross pathological findings; tissues were not examined
microscopically, as the study was designed to select concentrations
for use in a long-term study of toxicity and carcinogenicity (Atkinson
et al., 1991a).
Table 2 Acute toxicity of triforine
Species, strain Route of Sex LD50L/C50 Length of Reference
administration (mg/kg bw observation
or mg/L) (days)
Rat, Wistar Oral F, M > 16 000 14 Frohberg et al. (1973)
Rat, Wistar Oral F, M > 5 000 15 Ullman et al. (1986a)
Rat, Wistar Dermal F, M > 10 000 14 Frohberg et al. (1973)
Rat, Wistar Dermal F, M > 2 000 15 Ulman et al. (1990)
Rat, Sprague-Dawley Inhalation (1 h) F, M > 4.5 14 Bullock & Narcisse (1973)
Rat, Wistar Inhalation (4 h) F, M > 5.1 15 Ullman et al. (1986b)
Mouse (strain not stated) Oral F, M > 6 000 7 Muacevic (1968)
Mouse, NMRI Oral F, M > 5 000 15 Ullman et al. (1986c)
Dog (breed not stated) Oral F, M > 2 000 28 Muacevic (1969)
Rats
Groups of five male and five female Wistar rats were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 500, 2500, or 12 500 ppm for four weeks, equal to
0, 50, 240, or 1200 mg/kg bw per day for males and 0, 49, 230, or 1200
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption.
Body-weight gain was reduced by about 15% in males by the end of the
study ( p < 0.05), whereas them were no consistent, treatment-
related effects in females. Haematological changes indicative of
slight anaemia were observed. In rats at 12 500 ppm, slight decreases
in mean cell haemoglobin concentration ( p < 0.01) and mean cell
volume were seen in females ( p < 0.05) and increases in
reticulocyte ( p < 0.01) and polychromatic erythrocyte counts in
animals of each sex. Increased proportions of circulating immature
cells were also seen in rats at 2500 ppm, and the increase was
significant in males ( p < 0.01). Prothrombin time was decreased in
females at 12 500 ppm. At this dose, a slight increase in total
protein (by about 6%, p < 0.05) was seen in animals of each sex and
a slight increase in cholesterol content (57%, p < 0.01) in
females. Urine volume was increased in females at this dose. Changes
in organ weight were restricted to rats at 12 500 ppm. Absolute spleen
weights were increased in animals of each sex ( p < 0.05), as were
the absolute weights of the liver, thyroid, and kidney in females.
Increased relative weights were observed for liver ( p < 0.01) and
spleen ( p < 0.05) in animals of each sex and for thyroid in males
( p < 0.05). There were no treatment-related gross pathological
findings; the treatment-related microscopic changes were slight or
moderate haemosiderin deposition in the spleens of males and females
at 12 500 ppm and females at 2500 ppm; two females at 500 ppm also had
increased haemosiderin deposition. No NOAEL was identified (Tenneker
et al., 1989).
Groups of 15 male and 15 female Wistar FW-49 rats (25 at the high
dose) were given triforine (purity not stated) in the diet at
concentrations of 0, 2500, 7000, or 20 000 ppm for 13 weeks. Ten rats
of each sex at 20 000 ppm were observed for six weeks after the end of
treatment. Haematological, clinical chemical, and extended
histopathological investigations were performed on 10 rats of each sex
at each dose before treatment and after 6, 13, and 19 weeks.
Reversible reductions in erythrocyte count and in haemoglobin and
haematocrit values were seen in all treated groups. These results were
interpreted as a sign of a slight haemolytic anaemia and correlated
with haemosiderin deposition in liver, spleen, kidney, lung, and
heart, which increased in proportion to the dose administered. No
NOAEL was identified (Stötzer et al., 1971a).
As there was no no-effect level in the previous study, another study
was performed in which groups of 25 male and 25 female Wistar FW-49
rats were given triforine (purity not stated) in the diet at
concentrations of 0, 100, or 500 ppm for 13 weeks, equivalent to 5 or
25 mg/kg bw per day. No treatment-related effects were observed. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day, as no effects
were observed at 500 ppm, the highest dose tested (Stttzer et al.,
1971b).
Groups of 10 male and 10 female Sprague-Dawley rats were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0 or 20 000 ppm for 13 weeks, equal to 1300 mg/kg bw
per day. No deaths or clinical signs of toxicity were recorded, and
there were no effects on food consumption or body-weight gain. The
haematological changes included statistically significant but slight
reductions in mean cell haemoglobin concentration, by 1.4% in males
and 2.2% in females, and reduced erythrocyte numbers (by 4-9%) and
increased mean cell volume (by 3.5%) in females. Increases in the
absolute ( p < 0.05) and relative ( p < 0.01) weights of the liver
and spleen were observed in animals of each sex. There were no gross
pathological findings; the tissues were not examined microscopically
(Atkinson et al., 1991b).
Groups of 10 male and 10 female FW-49 rats were given triforine
(purity not stated) in the diet at concentrations of 0, 25, 120, 620,
or 3100 ppm for six months, equivalent to 0, 1.3, 6, 31, or 160 mg/kg
bw per day. Reductions in erythrocyte and haematocrit values and
increases in reticulocyte numbers were observed in rats at 620 and
3100 ppm. No consistent, dose-related variations were found in serum
chemical or urinary parameters. Post-mortem examination did not reveal
any treatment-related changes, but increased liver weights were found
in females at all doses, the relative weights being increased by 27%
in those at 25 ppm, 19% at 120 ppm, 40% at 620 ppm, and 49% at 3100
ppm (no statistical analysis reported). No such increases were
observed in males. Increased haemosiderin deposition was found in the
livers and spleens of male rats at 620 and 3100 ppm and females at
3100 ppm. No treatment-related histopathological changes were seen in
the livers of either male or female rats at 25 or 120 ppm. The NOAEL
was 120 ppm, equivalent to 6 mg/kg bw per day, on the basis of reduced
erythrocyte numbers and packed cell volume at 620 ppm (Stötzer et al.,
1972).
Groups of 10 male and 10 female Sprague-Dawley rats were given topical
applications on shaved intact, occluded skin of a 0, 0.5, or 1.5%
aqueous dilution of a 20% triforine emulsion, equivalent to 0, 10, or
30 mg/kg bw triforine, for 4 h per day daily for 21 days. Five males
and five females per group were followed up for an additional 21 days.
Temporary slight reddening and swelling occurred in the covered skin
areas in all groups, including the controls. No local or systemic
substance-related reactions were observed (Leuschner et al., 1972).
Groups of seven Fischer 344 rats of each sex received technical-grade
triforine in corn oil (3 ml/kg bw) on a shaven area of the back at
doses of 0, 110, 350, or 1100 mg/kg bw per day on five days per week
for three weeks. After each application, the treated area was covered
with gauze and bandage for 6 h, then washed and dried. No deaths or
treatment-related dermal changes were observed, and there were no
effects on food consumption or body-weight gain attributable to
treatment. All animals, including the controls, lost some weight,
particularly during the first week; this response was attributed to
the bandaging procedure. No significant, treatment-related variations
in organ weights or haematological end-points was seen, and them were
no gross or microscopic pathological findings. Female rats at 350 or
1100 mg/kg bw showed statistically significant increases in serum
cholesterol (13% and 22%, respectively), triglycerides (32 and 40%,
respectively), and bilimbin (27 and 13%, respectively); a 25% decrease
in serum alkaline phosphatase activity was seen in females at 110
mg/kg bw. In males, serum alkaline phosphatase activity was reduced by
15% at 350 mg/kg bw and by 21% at 1 100 mg/kg bw; in rats at 1100
mg/kg bw, total serum protein was increased by 5.7% and serum albumin
by 3.8%. Since the changes in bilirubin, cholesterol, and triglyceride
contents were not accompanied by histopathological changes in the
liver and were confined to a single sex, their biological significance
is unclear. The increases in alkaline phosphatase activity may reflect
a toxicological effect, but the decreases observed in this study are
not usually considered to be toxicologically significant. The NOAEL
was 1100 mg/kg bw per day, the highest dose tested, as no toxicity was
observed (Fokkema, 1992).
Dogs
Groups of four male and four female beagle dogs were given triforine
(purity not stated) in the diet at concentrations of 0, 3500, 10 000,
or 30 000 ppm (two groups) for 13 weeks. The supplementary group at 30
000 ppm was observed for an additional six weeks without treatment.
These concentrations were equal to 0, 83, 230, 690, or 710 mg/kg bw
per day for males and 0, 85,240,730, or 720 mg/kg bw per day for
females. Signs of haemolytic anaemia-reduced erythrocyte counts
( p < 0.05) and haemoglobin concentration ( p < 0.05), and,
occasionally, also in haemocrit--were observed at all doses by week
13, but were first observed in week 6 in dogs at 10 000 or 30 000 ppm
( p < 0.01). These effects were accompanied by a consistent increase
in the number of reticulocytes in dogs at 10 000 and 30 000 ppm. All
of the values returned to normal in the group at 30 000 ppm within
three weeks of observation. Serum chemistry in weeks 6 and 13 showed
slight increases in alkaline phosphatase activity and bilirubin and
cholersterol concentrations in all treated groups, but these values
also returned to normal in dogs at 30 000 ppm within three weeks.
Urinalysis and ophthalmoscopy showed no differences between the
groups. Fine, drop-like fatty infiltration of the myocardial fibres
were seen in 0, 5, 1, 5, and 1 dogs in the five groups, respectively,
and fatty accumulation in the liver in 0, 3, 0, 4, and 0 dogs. The
fatty infiltration appeared to be reversible, since it was no longer
detected in the dogs observed for six weeks. Treatment-related
siderosis in Kupffer cells in the liver showed a clear tendency
towards reversibility in the animals allowed to recover. There was no
NOAEL (Stötzer et al., 1971c).
Groups of four beagle dogs of each sex were given triforine in the
diet at concentrations of 0, 100,600, or 3500 ppm for 13 weeks, equal
to 0, 3.6, 22, or 120 mg/kg bw per day for males and females together.
The haematological findings in the dogs at 3500 ppm in the previous
study were essentially confirmed. Furthermore, siderosis was detected
in the spleen, liver, and bone marrow after administration of 600 ppm,
and the weight of the spleen was increased in the dogs at 3500 ppm.
The NOAEL was 100 ppm, equal to 3.6 mg/kg, on the basis of
haemosiderin deposits in the liver and bone marrow at 600 ppm
(Leuschner et al., 1971).
Groups of four male and four female beagle dogs were given
technical-grade triforine (purity not stated) in the diet at
concentrations of 0, 10, 40, 100, or 1000 ppm for two years, equal to
0, 0.23, 0.93, 2.4, or 22 mg/kg bw per day for males and 0, 0.25,
0.99, 2.6, or 24 mg/kg bw per day for females. Samples were taken for
haematology, blood chemistry, and urinalysis during weeks 6, 13, 26,
52, 78, and 104. One male dog at 100 ppm group died of acute
pneumonia. There were no other deaths, and there were no
treatment-related signs of toxicity, changes in food consumption,
changes in body-weight gain, or ophthalmoscopic findings.
Haematological changes in the dogs at 1000 ppm group included
increased mean cell volume in males in week 13 (12%, p < 0.01) and
females in week 26 (3.5%, p < 0.05) and decreased mean cell
haemoglobin concentration in males at week t 3 (3.5 %, p < 0.01).
The other changes were either not statistically significant or were
inconsistent with respect to time interval, sex, or dose. Examination
of femoral bone-marrow smears at termination showed a shift in the
granulopoietic:erythropoietic ratio towards erythropoiesis in two
males and three females at 1000 ppm. The erythropoietic mitotic index
was also increased in one female in this group. No treatment-related
changes were observed on blood chemistry or urinalysis. The absolute
and relative organ weights were comparable in all groups, and there
were no treatment-related gross pathological findings.
Microscopically, haemosiderin deposition of moderate severity was
observed in Kupffer cells in four dogs at 1000 ppm and one control.
Haemosiderosis of the bone marrow was also observed in two dogs at
1000 ppm. The NOAEL was 100 ppm, equal to 2.4 mg/kg bw per day, on the
basis of haematological changes, increased erythropoiesis, and
haemosiderin deposition in the liver and bone marrow in animals of
each sex at 1000 ppm (von Sandersleben et al., 1974; Goburdhun &
Greenough, 1990; Greenough, 1994).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 40 NMRI mice of each sex were given triforine (purity not
stated) in the diet at concentrations of 0, 30, 150, or 750 ppm for 81
weeks, equal to 0, 4.7, 24, or 120 mg/kg bw for males and 0, 5.2, 28,
and 140 mg/kg bw for females. Mortality at 81 weeks was 32% of control
males, 20% of those at 30 ppm, 35% at 150 ppm, and 50% at 750 ppm, and
30% of control females, 40% of those at 30 ppm, 26% at 150 ppm, and
33% at 750 ppm. Survival time, clinical symptoms, body-weight gain,
and food consumption were not affected by treatment. As morphological
findings were described only if they were considered to be relevant to
an evaluation of the carcinogenic effects of the substance, no NOAEL
was identified. Treatment did not increase the total number of
neoplasms (mainly lymphocytic leukaemias and lymphosarcomas), and the
latent periods remained unchanged. The frequencies of specific, less
common tumours in the treated groups were usually not higher than in
the concurrent controls or in the literature (Hofmann et al., 1975).
Four groups of 50 CD-1 mice of each sex were given technical-grade
triforine (purity, 98.9%) in the diet at concentrations of 0, 70, 700,
or 7000 ppm for 105 weeks, equal to 0, 11, 120, or 1200 mg/kg bw per
day for males and 0, 16, 160, or 1600 mg/kg per day for females. Blood
samples were collected for analysis at weeks 52, 77, and 103. The
mortality at 105 weeks was 14% of control males, 19% of those at 70
ppm, 35% at 700 ppm, and 28% at 7000 ppm; for females, 27% of controls
were dead at this time and 27% of those at 70 ppm, 32% at 700 ppm, and
30% at 7000 ppm. The mortality rate in the control group of males was
considered to be unusually low for CD-1 mice at 105 weeks. There were
no treatment-related clinical signs of toxicity or changes in food
consumption. Body-weight gain at the end of the study was reduced by
11% in males at 700 ppm and 16% in those at 7000 ppm. No significant
changes in body-weight gain were seen in females. No haematological
changes were observed. The absolute and relative weights of the liver
were increased by 21% ( p < 0.01) in females at 7000 ppm. At
autopsy, thickening or enlargement of the large intestine was observed
in males that died before the end of the study in the groups receiving
700 ppm (17%) or 7000 ppm (36%), whereas none was seen in the
controls. The large intestine was ulcerated, and inflammation was
observed microscopically, predominantly in male mice that died before
the end of the study after receiving 700 ppm (23%) or 7000 ppm (21%).
The overall occurrence of these findings was none in male controls, 6%
at 70 ppm, 16% at 700 ppm, and 12% at 7000 ppm.
The incidences of hepatocellular adenoma were 9/50 in control males,
12/50 at 70 ppm, 8/50 at 700 ppm, and 8/50 at 7000 ppm; the incidences
of hepatocellular carcinoma were 5/50 in control males, 7/50 at 70
ppm, 8/50 at 700 ppm, and 10/50 at 7000 ppm. There were no significant
differences by either Fisher's exact test or the Cochrane-Armitage
test for trend, for adenomas or carcinomas independently or combined
(Fisher's exact test for comparison of carcinomas in males at 0 and
7000 ppm gave p = 0.263). All of the incidences of liver tumours
were within the ranges found in a compilation of five control groups
of male CD-1 mice in the same laboratory: adenomas, 0-32%; carcinomas,
0-21%. Females had no increase in the incidences of liver tumours. The
incidences of alveolar or bronchiolar adenomas in males were 14/50 in
controls, 13/50 at 70 ppm, 8/50 at 700 ppm, and 17/50 at 7000 ppm, and
the incidences of alveolar or bronchiolar carcinomas were 5/50 in
controls, 2/50 at 70 ppm, 4/50 at 700 ppm, and 7/50 at 7000 ppm. These
differences were not statistically significant. The incidences of
alveolar or bronchiolar adenomas in females were 5/50 in controls,
6/50 at 70 ppm, 7/50 at 700 ppm, and 21/50 at 7000 ppm (Fisher's exact
test, p < 0.001), and the incidences of alveolar or bronchiolar
carcinomas were 1/50 in controls, 2/50 at 70 ppm, 1/50 at 700 ppm, and
6/49 at 7000 ppm (Fisher's exact test, p = 0.059). The incidences of
lung tumours were beyond the ranges observed in five other control
groups of female CD- 1 mice at this laboratory: adenomas, 4-18%;
carcinomas, 0-8% The Meeting concluded that triforine increased the
incidence of alveolar or bronchiolar adenomas fit female mice. The
NOAEL for carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day,
on the basis of an increased incidence of adenomas and/or carcinomas
in females at 7000 ppm. The NOAEL for toxicity was 70 ppm, equal to 11
mg/kg bw per day, on the basis of a slight decrease in body-weight
gain and changes in the large intestine of males fed 700 ppm (Heath et
al., 1992).
Rats
Groups of 35 Wistar (CHBB: THOM-SPF) rats of each sex, with 50 of each
sex in the group of controls and that at the high dose, were given
triforine (purity, 96.6%) in the diet at concentrations of 0, 25,
125,625, or 3120 ppm for two years, equivalent to 0, 1.3, 6.3, 31, or
160 mg/kg per day. Samples were taken for haematological and blood
chemical investigations during weeks 0, 6, 13, 26, 52, 78, and 104
from 20 male and female rats per group. Urine was analysed before and
at the end of the study in 10 animals of each sex from the control
group and that at the highest dose. All animals were examined post
mortem. Complete histopathological examinations were performed at
the end of the study on 20 rats of each sex from the control group and
that at the highest dose, on 15 rats of each sex from the other
groups, on rats that died before the end of the study, and on all
tumour-bearing rats. Mortality, clinical signs, body-weight
development, and parameters of clinical chemisstry and the urinalyses
were not affected by the treatment. Temporary reductions in the number
of erythrocytes ( p < 0.01 for males) and haematocrit values
( p< 0.01 for males) and increased numbers of mticulocytes (39% in
males, 29% in females) were seen in rots at 3125 ppm during week 6,
the variations being more pronounced among male rats. There were no
significant differences in organ weights. Haemosiderosis was observed
in the spleens of both control and treated rats, and the frequency and
intensity of the condition did not differ significantly between the
groups. The sinuses of the adrenals of females in all groups showed
cavernous dilatation, and some were thrombosed. These changes occurred
more frequently in treated than in control animals, but they are
common findings in Wistar rats. No significant difference in tumour
incidence between the groups was found. The NOAEL was 625 ppm,
equivalent to 31 mg/kg bw per day, on the basis of signs of anaemia at
3120 ppm (Hill, 1974).
Groups of 70 Sprague-Dawley rats of each sex were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0, 200, 2000, or 20 000 ppm for two years, equal to
an average of 0, 10, 100, or 1000 mg/kg bw per day for males and 0,
13, 140, or 1400 mg/kg bw per day for females. Twenty rats of each sex
per group were killed at one year and the survivors at two years.
Blood and urine samples were collected for analysis at weeks 26
(females) or 28 (males), 52, and 104. Mortality at two years among the
remaining 50 animals in each group was 25% of control males, 24% of
those at 200 ppm, 19% at 2000 ppm, and 19% at 20 000 ppm and 22% of
control females, 20% of those at 200 ppm, 21% of those at 2000 ppm,,
and 23% of those at 20 000 ppm. There were no treatment-related
clinical signs of toxicity and no changes on ophthalmoscopy or
urinalysis. There were no significant differences in food or water
consumption and only slight differences in body weight between the
groups at the end of the study.
A number of findings could be attributed to the treatment with
triforine. At one year, the body weights of females were moderately
but not statistically significantly lower than those of controls, by
11% in those at 200 ppm, 13% at 2000 ppm, and 21% at 20 000 ppm.
Haemoglobin concentrations were 4-6% lower in male rats at 2000 ppm
( p < 0.05) and 20 000 ppm ( p < 0.01 ) at weeks 28 and 51 and in
those at 200 ppm at week 51. Although the changes were consistent in
males, in female rats the haemoglobin concentrations were slightly and
nonsignificantly lower only at 20 000 ppm at week 26. The mean
corpuscular haemoglobin count was 2% lower in both male and female
rats at 20 000 ppm at weeks 28 and 26, respectively, but not at week
51. The values in the control group were, however, clearly higher than
normally expected for rats of this age, sex, and strain in this
laboratory. Changes in blood chemistry in the group at 20 000 ppm
included slightly raised concentrations of sodium in males at week 28,
of calcium in males at week 28 and females at week 104 (by 4%,
p < 0.01 ), of cholesterol in females at week 104 (by 39%,
p < 0.01), and of total protein in males at week 104 (by 9%,
p < 0.01 ), and a slightly lower concentration of glucose in males
at week 52. females at 2000 ppm also had raised calcium levels at week
104. There were no gross pathological findings. Liver weights were
increased at week 52 in males (11%) and females (15%) at 20 000 ppm
and in all females at week 104; both absolute and relative liver
weights were increased in females at week 104, by 18% in those at 200
ppm ( p < 0.05), 24% at 2000 ppm ( p < 0.01), and 43 % at 20 000
ppm ( p < 0.001 ). Although not significant, the relative weights of
the liver were increased in males, by 19% at 200 ppm, 11% at 2000 ppm,
and 18% at 20 000 ppm. The absence of a dose-related response in the
males calls into question the biological significance of the response
in females at the low dose. Significant, dose-related increases in
absolute and relative spleen weights were seen in females at 2000 and
20 000 ppm that were killed at one year. The relative spleen weights
were also increased in females at 20 000 ppm at week 104. The
increased weights of the liver and spleen correlated with
histopathological findings (described below) and are considered to be
related to treatment. Increased kidney weights were observed at week
52, but only in females, by 9% in those at 200 ppm, 12% at 2000 ppm,
and 17% at 20 000 ppm. Adrenal weights were reduced by 13-15% in all
treated males but were increased by 35% in females at 2000 ppm and by
40% in those at 20 000 ppm. Microscopic examination showed increased
deposition of haemosiderin in the spleen of females at 2000 and 20 000
ppm at two years, in males at these doses at one year, and in females
at 20 000 ppm at one year. The incidence of haemosiderin deposition in
Kupffer cells and macrophages of the liver was increased in male and
female rats at 2000 and 20 000 ppm at one year. The severity was also
increased in these groups of males but only in females at 2000 ppm. In
the latter group, there was an increased incidence of pale-cell foci
in males and bile-duct hyperplasia in females. There were no
treatment-related changes in the proportions of tumour-bearing animals
or in the incidence of any particular tumour type. The Meeting
concluded that triforine is not carcinogenic to rats. The NOAEL was
200 ppm, equal to 10 mg/kg bw per day, on the basis of slight
reductions in body-weight gain in males, haematological changes in
animals of each sex, increased absolute and relative weights of the
spleen in females at one year and of the liver at two years, and
haemosiderin deposition in the spleens of males and females at one
year and in the livers of females after one year at 2000 ppm (Everett
et al., 1992; Perry et at., 1992).
(d) Genotoxicity
Triforine has been tested for genetic damaging activity in an adequate
range of assays, most of which were conducted to currently acceptable
standards (see Table 3 for salient features and references). Mutations
were not induced by triforine in either bacteria or at the hprt
locus of cultured mammalian cell lines, and excision repairable DNA
damage was not increased in treated primary cultures of rat
hepatocytes. Gene conversion was not induced in either yeast or fungal
cells. The potential of triforine for inducing chromosomal damage was
tested in mammalian cell lines in vitro by examining treated
metaphase cells for gross aberrations and in vivo by examining
polychromatic erythrocytes taken from the bone marrow of mice treated
orally for an increase in the proportion of micronucleated cells.
There was no indication of activity in vitro. In one test, an
increase in the proportion of micronucleated cells was observed in
groups of five female mice sampled 48 h after dosing (controls: 0.4 ±
0.55/103; treated with 5000 µg/kg bw: 2.4 ± 1.14/103) but not at 24
or 72 h and not in male mice at any sampling time. The increase was
not confirmed when the study was partially repeated with female mice
sampled only 48 h after dosing (controls: 1.6 ± 1.14/103; treated with
5000 µg/kg bw: 1.2 ± 1.10/103). The Meeting concluded that triforine
is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female CHBB-THOM rats were given triforine
(purity not stated) in the diet at concentrations of 0, 100, 500, or
2500 ppm in a three-generation study in which the rats were mated to
produce two litters in each generation. The only sign of toxicity in
parent rats and their offspring was temporarily, slightly reduced
body-weight gain of male rats, particularly at the high dose.
Table 3. Genetic activity of triforine
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium 100 µg/plate NR Negativea Rohrborn (1977)
TA 100, TA1535
Reverse mutation S. typhimurium 5000 µg/plate NR Negativea Moriya et al. (1983)
TA98, TA 100,
TA1535, TA1537,
TA1538, E. coli
WP2hcr
Reverse mutation S. typhimurium 5000 µg/plate 99.9 Negativea Kramer (1985)
TA98, TA 100,
TA1535, TA1537,
TA1538
Gene conversion, Saccharomycess 1000 µg/ml NR Negativea De Bertoldi et al. (1980)
adc2, trp5 loci cerevisiae D4
Gene conversion, Aspergillus 1000 µg/ml NR Negativeb De Bertoldi et al. (1980)
pabaA locus nidulans
Unscheduled Rat hepatocyte 63 µg/ml 99.8 Negativeb Proudlock et al. (1993)
DNA synthesis primary culture
Cell mutation, Chinese hamster 50 µg/ml NR Negativea Miltenburger (1984)
hprt locus V79 cells
Cell mutation, Chinese hamster 200 µg/ml 99.8 Negativea Adams et al. (1993)
hprt locus ovary cells
Table 3. (continued)
Test system Test object Concentration/ Purity Results References
dose (%)
Chromosomal Chinese hamster 400 µg/ml 99.8 Negativea Brooks & Wiggins (1994)
aberration ovary cells (24 and
48 h sampling)
Chromosomal Chinese hamster 50 µg/ml NR Negativea Miltenburger (1985)
aberration V79 cells (7, 18,
and 28 h sampling)
In vivo
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Weakly Guenard (1984a)
induction marrow (24, 48, by gavage positive
and 72 h sampling)
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Negative Guenard (1984b)
induction (females only, by gavage
48 h sampling)
NR, not reported
a With and without metabolic activation
b Without metabolic activation
Reproductive performance, duration of pregnancy, malformation
frequency, and postnatal development were unaffected by treatment.
When the F3b rats were subjected to post-mortem and histopathological
examinations, no treatment-related change was observed. Since this
study has been superseded by one at higher doses, a full description
is not given; however, the dietary concentration of 2500 ppm had no
adverse effect on reproduction (Niggeschulze et al., 1974).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were given technical-grade triforine (purity,
99.1%) in the diet at concentrations of 0, 1250, 5000, or 20 000 ppm.
The parental (FA) rats received the diets for 10 weeks before mating
and throughout mating, gestataon, and lactation. These concentrations
were equal to 0, 100, 410, or 1600 mg/kg bw per day for males and 0,
100, 400, and 1600 mg/kg per day for females during the premating and
mating periods; for F0 females, they were equal to 0, 97, 400, or
1600 mg/kg bw per day during gestation and 0, 150, 650, or 2900 mg/kg
bw per day during lactation. All F1 rats were weaned onto the same
diets as their parents and continued on this treatment until they were
six to seven weeks old, when they were killedś The only death occurred
in a male rat in the control group. No signs of toxicity were noted.
Food consumption was slightly reduced in the group at 20 000 ppm,
among males during weeks 1-10 and among females during weeks 1-3.
Body-weight gain deficits at these doses during these periods resulted
in a reduction in body weight of about 11% in males throughout the
experiment (17 weeks), whereas females had recovered by the end of the
premating period. The relative liver weights of F0 males and females
at 5000 and 20 000 ppm were increased, and females at these doses also
had increased absolute weights of both liver and spleen. No gross
pathological changes were found at autopsy of the F0 rats; no
histological examinations were performed. Treatment did not affect
mating performance, fertility, duration of gestation, litter size, or
F1 pup survival, and no clinical signs of toxicity were seen among
the F1 pups. From lactation day 14, the body-weight gain of male and
female pups was reduced by 7% in those at 5000 ppm and 11 and 10%,
respectively, at 20 000 ppm. These reductions persisted after weaning
(day 24) and were present at six weeks in males and five weeks in
females. The overall depression in mean weight gain after weaning was
11% for males and 4% for females at 5000 ppm and 19% for males and 10%
for females at 20 000 ppm. Food consumption after weaning was also
reduced at this dose, by 15% in males and 12% in females. There were
no gross pathological findings at autopsy of the F1 rats. The effects
observed at the dietary concentration of 20 000 ppm triforine were
considered not to contraindicate its use as the high dose in a
two-generation study. The NOAEL for reproductive toxicity was 1000 ppm
(McCay & Hazelden, 1990).
Groups of 29 male and 28 female Sprague-Dawley rats were given
technical-grade triforine (purity, 98.9-99.1%) in the diet at
concentrations of 0, 500, 3000, or 20 000 ppm in a two-generation
study. The parental (F0) rats received the diets for 10 weeks before
mating and throughout mating, gestation, and lactation. At weaning of
the F1 litters, pups were selected to provide the F2 generation
litters and continued on the same diets as had been offered to their
parents. The study was completed after weaning of the F2 rats. The
dietary concentrations were equal to 0, 38, 230, or 1500 mg/kg bw per
day for F0 males and 0, 48, 290, or 1900 mg/kg per day for F0
females during the premating and mating periods. For F0 females,
these concentrations were equal to 0, 41,240, or 1700 mg/kg bw per day
during gestation and 0, 63, 380, or 2600 mg/kg bw per day during
lactation. The dietary concentrations of 0,500, 3000, and 20 000 ppm
were equal to 0, 40, 280, and 2000 mg/kg bw per day for F2 males and
0, 61,360, and 2500 mg/kg, per day for F1 females during the
premating and mating periods. For F1 females, these concentrations
were equal to 0, 40,230, and 1800 mg/kg bw per day during gestation
and 0, 65, 420, and 2900 mg/kg bw per day during lactation. Pups were
not culled during the study.
One F0 male and one F0 female at 500 ppm died during the study, and
one F1 female at 20 000 ppm was killed prematurely because of
dystocia. None of these deaths was related to treatment. No clinical
signs of toxicity related to triforine were noted in either the F0 or
F1 generations There were slight reductions in food consumption among
F0 rats at 20 000 ppm and F0 females at 3000 ppm during the first
week of treatment. A decrease of 9% was observed in the body-weight
gain of F0 females, but not males, at 500 ppm during the premating
period, at the beginning of which there had been a 3% body-weight gain
in these rats. Slight deficits in body-weight gain were seen
throughout the treatment period for F0 males at 20 000 ppm, resulting
in an overall reduction in body-weight gain of 9%. During the
premating period, F0 females show body-weight gain deficits of 19% at
3000 ppm and 20% at 20 000 ppm. Overall weight gain was decreased by
9% in F0 females at 20 000 ppm during gestation, whereas there was no
deficit in these rats during, lactation. Food consumption was reduced
among F1 males and females at 3000 ppm (10%) and 20 000 ppm (11 and
12%, respectively), mostly during the first three weeks after weaning.
Corresponding deficits in body-weight gain were seen during this
period, of 9% in males and females at 3000 ppm and 13% in males and 9%
in females at 20 000 ppm. Between weeks 6 and 23, the deficits in body
weight were 5% for males and 10% for females at 20 000 ppm. There were
no effects on the body-weight gain of F1 females at any dose during
gestation or lactation.
There were no gross pathological findings attributable to treatment.
Kidney weights were significantly higher (5-9%), in F0 males and
females at 3000 and 20 000 ppm and in F1 males at 20 000 ppm than in
controls. Liver weights were significantly higher (11-45% and
increasing with dose) in F0 females, F1 males, and F1 females at
3000 and 20 000 ppm; a marginal increase in liver weight was also seen
in F1 males at 500 ppm (6.4%, p < (0.05). Significant increases
were observed in the weight of the spleen in F1 males at 20 000 ppm
(16%) and F1 females at 3000 (20%) and 20 000 ppm (42%). Triforine
treatment did not affect mating performance, fertility, duration of
gestation, gestation index, postimplantation loss, litter size, or pup
survival in either the F0 or F1 generation.
The clinical signs of toxicity in pups that could be attributed to
treatment included a reduction in the mean weights of F1 and F2 pups
at 20 000 ppm from day 4 of lactation, so that, by day 21, the body
weights were reduced by 17% for F1 males, 18% for F1 females, 20%
for F2 males, and 19% for F2 females. Decreases were also observed
in F1 pups at 3000 ppm on days 14-21 of lactation, by 9% in males and
8% in females.
The liver, kidney, spleen, and thyroid of parental F0 and F1 rats at
all doses and a number of other organs of parental F0 and F1 rats at
0 and 20 000 ppm were examined histologically. Dose-related increases
in haemosiderin deposition in the spleen, accompanied by dose-related
increases in extramedullary haematopoeisis and in spleen weight were
observed. There was also a dose-related increase in the severity of
spontaneous nephropathy in males and nephrocalcinosis in females.
Small increases in kidney weight were observed in this study,
particularly in the F0 generation, but with no change at 500 ppm.
Liver size increased with dose and was accompanied by sporadic
centrilobular hepatocyte hypertrophy. The hepatic changes were
regarded by the authors as an adaptive response to increased metabolic
activity. The thyroids of female rats of both generations at 20 000
ppm had the histological appearance of very active secreting glands,
consisting of numerous very small acini with little or no lumina,
scant stored secretion, and lined by cuboidal or columnar epithelium
with pale, foamy cytoplasm. The severity of these effects did not
appear to be increased in the F1 generation. The NOAEL for
reproductive toxicity was 20 000 ppm, equal to 1500 mg/kg per day, the
highest dose tested. The NOAEL for parental toxicity and growth and
development of the offspring was 500 ppm, equal to 40 mg/kg bw per
day, on the basis of decreased food consumption in F0 females, F1
males, and F1 females, decreased body-weight gain in F0 females, F1
males, and F1 females, decreased F1 pup weight, and increased
relative spleen weight in F1 females at 3000 ppm (McCay & Hazelden,
2992; Hazelden & Aitken, 1992; Hazelden, 1994).
(ii) Developmental toxicity
Rats
Groups of 20 inseminated female Sprague-Dawley rats were given 0, 100,
400, 800, or 1600 mg/kg bw per day triforine (purity not stated)
suspended in 1% carboxymethylcellulose by gavage on days 6-15 of
pregnancy. The rates of resorption and variation were increased in
animals at 1600 mg/kg bw per day. Furthermore, the number of fetuses
was reduced, dur to significantly increased postimplantation loss at
this dose. Because the frequency of variations was also slightly
increased at 800 mg/kg bw per day, the NOAEL for fetotoxicity was 400
mg/kg bw per day. Although toxic effects were observed at 800 and 1600
mg/kg bw per day, there were no signs of a teratogenic effect
(Leuschner, 1972).
In a preliminary study, groups of eight inseminated Sprague-Dawley
rats were given triforine (purity, >97%) by garage at doses of 250,
500, or 1000 mg/kg bw per day on days 6-15 of gestation. There were no
deaths or treatment-related clinical signs of toxicity. Mean body
weight was slightly reduced on gestation days 9-12 in animals at 500
and 1000 mg/kg bw per day, but overall body-weight gain in the four
groups was comparable throughout treatment. No gross pathological
changes were found in fetuses removed on day 20 of gestation. No
reproductive parameters were affected by treatment, and them were no
indications of treatment-related embryotoxicity or teratogenicity
(Fuchs, 1992).
Groups of 30 inseminated Sprague-Dawley rats were given triforine
(purity not stated) by garage at doses of 200, 500, or 1000 mg/kg bw
per day on days 6-15 of gestation. There were no deaths or
treatment-related clinical signs of toxicity. Food consumption was
reduced in animals at 1000 mg/kg bw per day, but this was not
accompanied by a significant decrease in body-weight gain. No gross
pathological changes were seen in fetuses removed on day 20 of
gestation. No reproductive parameters were affected by treatment, and
there were no indications of treatment-related embryotoxicity or
teratogenicity. The NOAEL for maternal and developmental toxicity was
1000 mg/kg bw per day (Fuchs, 1993a).
Rabbits
Groups of 15 inseminated Himalayan rabbits were given technical-grade
triforine (purity not stated) in 0.5% carboxymethylcellulose by gavage
on days 6-18 of gestation at doses of 0, 5, 25, or 125 mg/kg bw per
day. Them were no deaths or treatment-related clinical signs of
toxicity. Dose-related decreases in food consumption were observed in
rabbits at 25 and 125 mg/kg bw per day on days 7-14 of treatment. Food
consumption was also reduced in the animals at 5 mg/kg bw per day, but
this had no effect on body-weight gain and was considered to be
toxicologically insignificant. For most of the treatment period, body
weight was reduced in rabbits at 25 and 125 mg/kg bw per day, as a
result of reductions during gestation days 6-9. Towards the end of
treatment, these groups regained weight. The does were killed and
their fetuses removed on day 29 of gestation. No gross pathological
findings were observed. No reproductive parameters were affected by
treatment, and them were no indications of treatment-related
embryotoxicity or fetotoxicity at any dose. The NOAEL for maternal
toxicity was 5 mg/kg bw per day, on the basis of decreased food
consumption and body weight at 25 mg/kg bw per day. The NOAEL for
fetal effects was 125 mg/kg bw per day, the highest dose tested
(Gleich et al., 1981).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in 0.5%
carboxymethylcellulose by garage on days 6-18 of gestation at doses of
0, 6, 30, or 150 mg/kg bw per day. There were no deaths or
treatment-related clinical signs of toxicity. Decreased food
consumption were observed in does at 150 mg/kg bw per day on days
12-18, although no statistically significant reduction in body-weight
gain was observed. The does were killed and their fetuses removed on
day 28 of gestation. No gross pathological findings were observed. No
reproductive parameters were affected by treatment, and there were no
indications of treatment-related embryotoxicity or fetotoxicity at any
dose. The NOAEL for maternal toxicity was 30 mg/kg bw per day, on the
basis of decreased food consumption at 150 mg/kg bw per day. The NOAEL
for fetal effects was 150 mg/kg bw per day, the highest dose tested
(Müller, 1989).
Since maternal effects were minimal in the previous study, triforine
was tested at higher doses. In a preliminary study, four groups of
eight inseminated New Zealand rabbits were given technical-grade
triforine (purity not stated) in distilled water by gavage on days
6-18 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw per day.
There were no deaths or treatment-related clinical signs of toxicity.
Body-weight gain was reduced in all treated groups, mainly due to
body-weight losses on days 6-9 of gestation; the reduction (64%) was
statistically significant in does at 1000 mg/kg bw per day. At the end
of treatment, the body-weight gain in the treated groups exceeded that
of the controls. When the does were killed and their fetuses removed
on day 28 of gestation, no gross pathological changes were found, and
reproductive parameters were not affected. The group mean fetal
weights were slightly reduced at 500 and 1000 mg/kg bw per day. There
were no indications of teratogenicity at any dose (Müller, 1991).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in distilled water by
gavage on days 6-18 of gestation at doses of 0 or 1000 mg/kg bw per
day. There were no deaths or treatment-related clinical signs of
toxicity. Reductions in food consumption and body-weight gain were
observed in does at 1000 mg/kg bw per day, mainly due to body-weight
losses on days 6-9 of gestation. At the end of treatment, food
consumption and body-weight gain in the treated group were comparable
to those of the control group. The does were killed and their fetuses
removed on day 28 of gestation. No gross pathological changes were
observed, and reproductive parameters were not affected by treatment.
The group mean fetal weights were slightly reduced, and this
indication of fetotoxicity was accompanied by reduced ossification of
the carpals and/or tarsals, sternum, and pelvis. There were no
indications of teratogenicity at any dose. No NOAEL for maternal
toxicity was identified (Fuchs, 1993b).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Triforine, moistened with water and left on the shaven skin of
Sprague-Dawley rats for 24 h, was tolerated with no irritation up to a
dose of 10 000 mg/kg bw (Frohberg et al., 1973; Ullmann et al., 1990).
When 0.5 g of moistened triforine (purity, 98.1%) was applied to
shaven skin areas of three male and three female New Zealand whim
rabbits and left on the skin for 4 h under a semi-occlusive bandage,
no erythema or oedema occurred within the 72-h follow-up (Ullmann &
Porricello, 1988a).
No reactions occurred in mucosa or eyes of New Zealand white rabbits
after insertion of 0.1 g triforine into the conjunctival sac (Frohberg
et al., 1973). When triforine (purity, 98.1%) was inserted into the
conjunctival sac of three male and three female New Zealand whim
rabbits, all rabbits showed mild local reactions (reddening, chemosis,
discharge) 1 h after application. The reaction disappeared almost
entirely within 24 h, and only one female still had minor conjunctival
reddening (mean cumulative score, 0.17). No changes were observed in
the eyes with a slit-lamp ophthalmoscope 48 and 72 h after
application. No systemic clinical reactions occurred during the
testing period, and body weight was not affected. The overall
irritation score was 0.83 out of a maximum attainable score of 13
(Ullmann & Porricello, 1988b).
In a Maurer optimization test involving aggravation of testing
conditions by additional injection of Freund's adjuvant in groups of
12 male and 12 female Dunkin-Hartley albino guinea-pigs, triforine
(purity, 99.9%) showed no sensitizing potential after epidermal and
intradermal challenge (Ullmann & Suter, 1984).
(ii) Mode of action
Triforine is regarded as an inhibitor of sterol biosynthesis; in
particular, it inhibits microsomal sterol demethylation, as was shown
by means of gas chromatographic-mass spectrometric measurements of
sterol accumulation in Neurospora crassa and Aspergillus
fumigatus. Because triforine has only weak or no antifungal
properties in vitro, metabolic activation can be assumed (Langcake
et al., 1983).
(iii) Interactions with nitroso compounds
Groups of 25 or 50 male and 25 or 50 female Swiss mice were given
0.05% sodium nitrate in drinking-water, triforine suspended in water
at 300 mg/kg bw by garage twice each week, or a combination of the two
treatments for up to 180 days. Tumour incidences were compared with
those in a control group of 184 males and 117 females. Triforine alone
did not increase the number of tumours; however, the combination
increased the frequencies of lymphomas (including thymoma) and of
epithelial adenomas and carcinomas of the gastrointestinal tract, the
lung, and, in males, the liver. Incubation of triforine with 4%
acidified sodium nitrite solution for 24 h formed dinitrosopiperazine,
which is known to be carcinogenic and was suspected to be the
substance that induced the tumours (Börzsönyi et al., 1978).
(iv) Haemolytic anaemia
The main toxic effect observed in the experiments with triforine was
anaemia, which is a reduction in the concentration of haemoglobin in
the blood below the normal range for the species studied and is
usually accompanied by a reduction in the number of erythrocytes. This
condition occurs when the rate of erythyrocyte production is
outstripped by the rate of erythrocyte destruction. In many cases of
anaemia, there is both a reduced rate of production and an increased
rate of destruction. Nevertheless, because of the greater importance
of one or other of these processes, anaemias are classified as (i)
those involving excessive loss (post-haemorrhagic anaemia) or
destruction (haemolytic anaemias) of erythrocytes and (ii) those
involving failure of production of erythrocytes, diminished production
with bone-marrow hyperplasia being dyserythropoietic anaemia and
diminished production with marrow hypoplasia being hypoplastic or
aplastic anaemia.
Haemolytic anaemia in adult experimental animals can result from an
immune reaction in which the antibody is directed against the chemical
or chemical-plasma protein complex, which can bind to the erythrocyte
cell surface; alternatively, the chemical induces formation of an
antibody which cross-reacts with a normal erythrocytic antigen. In the
first case, haemolysis ceases immediately when exposure to the
chemical ceases; in the second case, evidence of an immune reaction
against erythrocytes may persist for some months after exposure to the
chemical has ceased.
Many chemicals cause haemolysis, e.g. phenylhydrazine, lead, and
arsenic and copper compounds; lead may also interfere with haemoglobin
synthesis. Hypoplastic and aplastic anaemias can be produced by a
number of chemicals, including cytostatic drugs, which generally
depress the bone marrow. In addition, there may be idiosyncratic
reactions to certain drugs, e.g. chloramphenicol, sulfonamides,
phenylbutazone, and other antirheumatic and antithyroid drugs.
Aplastic anaemia can also be induced by some hair dyes and industrial
chemicals, such as benzene.
The effect of triforine is mild, occurs in all or many exposed animals
(depending on the dose), and is characterized by haemosiderin
deposition in several organs, no bone-marrow depression, the entry of
increased numbers of immature cells into peripheral circulation, and
the absence of evidence of immunotoxicty. At least in dogs, there is
actually a (presumably) compensatory increase in erythropoiesis. Of
the three common circumstances in which haemolytic anaemia is induced
by chemicals, two, therefore, are unlikely to be important: an immune
response and increased sensitivity of erythrocytes to oxidative stress
because of glutathione depletion, since glutathione is not
significantly involved in triforine metabolism. The third, oxidative
haemolysis in animals: with normally functioning erythrocytes, would
appear to be the most likely mechanism and one which would probably
cease as soon as exposure ceased. This appears to be the situation in
triforine-exposed rats and dogs. Also, there is no evidence of
methaemoglobin formation or of increased numbers of Heinz
body-containing cells or deformed erythrocytes in the circulation.
3. Observations in humans
Medical reports from three companies that synthesize triforine were
available. Boehringer Ingelheim, Germany, examined 12 workers
repeatedly between 1970 and 1983 and reported no haematological or
blood chemical results considered to be related to exposure to
triforine (Celamerck, 1983a,b). The industrial medical service of E.
Merck Co., Darmstadt, Germany, found no adverse systemic, dermal, or
mucosal effects of exposure to triforine among production workers
during routine examinations performed in conformity with the
requirements of the German Chemical Trade Union (Merck, 1984). No
adverse effects were observed during triforine production at Shell
Agrar GmbH in Spain between 1987 and 1993 (Toubes, 1993).
Comments
A battery of tests for acute toxicity with technical-grade triforine
showed that it is slightly hazardous by both the oral and dermal
routes, with respective LD50 values of > 5000 and > 2000 mg/kg bw.
The LC in rats exposed by inhalation was > 5.1 mg/L. Triforine was
not irritating or sensitizing to the skin of rodents and was minimally
irritating to the eyes of rabbits. WHO has classified triforine as
unlikely to present an acute hazard in normal use (WHO, 1996).
Dietary administration of technical-grade triforine for 4 or 13 weeks
showed that the haematopoietic system is a target, as indicated by
mild haemolytic anaemia with associated secondary effects in the
spleen and liver. Similar results were found in two-year studies of
toxicity in mice, rats, and dogs, in which the haematological changes
were accompanied by increased weights of the spleen and liver. In
addition, in a 105-week dietary study in mice, changes in the large
intestine characterized by thickening, enlargement, inflammation, and
ulceration were observed; in a two-year dietary study in dogs,
increased erythropoiesis and increased haemosiderin deposition were
found in the liver and bone marrow. In a four-week dietary study in
rats at 0, 500, 2500, or 12 500 ppm, there was no NOAEL. In a
four-week study in mice at 0, 200, 1000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 200.mg/kg bw per day. The NOAEL in a 13-week study
in rats at 0, 100, or 500 ppm was 500 ppm, equivalent to 25 mg/kg bw
per day. In a six-month study in rats fed diets giving 0, 25, 120,
620, or 3100 ppm, the NOAEL was 120 ppm, equivalent to 6 mg/kg bw per
day. The two-year dietary studies in rats (0, 200, 2000, or 20 000
ppm), mice (0, 70, 700, or 7000 ppm), and dogs (0, 10, 40, 100, or
1000 ppm) showed NOAELs of 200 ppm, equal to 10 mg/kg bw per day, 70
ppm, equal to 11 mg/kg bw per day, and 100 ppm, equal to 2.4 mg/kg bw
per day, respectively.
The major toxic effect observed in the experiments summarized above
was anaemia. The effect was mild, occurring in all or many of the
exposed animals (depending on the dose); the effects were reversible
in rats and dogs and were characterized by haemosiderin deposition in
several organs, the absence of evidence of bone-marrow depression,
entry of increased numbers of immature cells into the peripheral
circulation, and the absence of effects on organs of the immune
system. In dogs, there was actually an increase in erythropoiesis.
Oxidative haemolysis in animals with normally functioning erythrocytes
would appear to be the most likely mechanism and one that would cease
as soon as exposure ceased. Also, there was no evidence of
methaemoglobin formation or of any increase in Heinz body-containing
cells or deformed erythrocytes in the circulation.
No genotoxic activity was observed in an adequate battery of tests for
mutagenicity and clastogenicity in vitro and in vivo. The Meeting
concluded that triforine is not genotoxic.
The results of the studies critical to derivation of an ADI are shown
below; the list does not include an 81-week study in mice treated
orally, which was considered inadequate for evaluation since
histopathological examination was limited to lesions that were judged
at autopsy to be neoplastic.
No carcinogenic effect was observed in rats given dietary
concentrations of 0, 25, 125,625, or 3120 ppm in one study and 0, 200,
2000, or 20 000 ppm in another. Triforine increased the incidence of
pulmonary tumours in female mice given 7000 ppm; the NOAEL for
carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day. The
Meeting concluded that the murine response involves an unidentified
nongenotoxic mechanism and that the carcinogenic activity seen in mice
is unlikely to be indicative or a human carcinogenic risk at the
expected levels of exposure to triforine.
Triforine at dietary concentrations of 0, 500, 3000, or 20 000 ppm did
not affect reproductive performance in rats over the course of a
two-generation study, the NOAEL being the highest dose tested, 20 000
ppm, equal to 1500 mg/kg bw per day. The NOAEL for parental toxicity
and for the growth and development of the offspring was 500 ppm, equal
to 40 mg/kg bw per day, on the basis of decreased food consumption,
body-weight gain, and F1 pup weight and increased relative spleen
weight at the next highest dose of 3000 ppm.
In a study of developmental toxicity in rabbits given oral doses of 0,
5, 25, or 125 mg/kg bw per day, the maternal NOAEL was 5 mg/kg bw per
day, on the basis of decreased food consumption and body weight at 25
mg/kg bw per day; the NOAEL for developmental toxicity was 125 mg/kg
bw per day. In a later study of rabbits given oral doses of 0, 6, 30,
or 150 mg/kg bw per day, the NOAEL for maternal toxicity was 30 mg/kg
bw per day, on the basis of decreased food consumption and body-weight
gain at 150 mg/kg bw per day; the NOAEL for developmental toxicity was
150 mg/kg bw per day. In two subsequent studies in which triforine was
given orally to rabbits at doses of 0, 250, 500, or 1000 mg/kg bw per
day and 0 or 1000 mg/kg bw per day, maternal and embryotoxicity (as
shown by reduced fetal weight) occurred at 1000 mg/kg bw per day.
Decreased fetal weights and delayed ossification were observed in a
study of developmental toxicity in rabbits at the maternally toxic
dose of 1000 mg/kg bw per day. Thus, developmental toxicity in rabbits
occurred only at doses that were also maternally toxic. A study of
developmental toxicity in rats given triforine orally at doses of 0,
200, 500, or 1000 mg/kg bw per day did not show adverse effects in
either dams or fetuses at doses up to 1000 mg/kg bw per day. The
Meeting concluded that triforine has no specific developmental or
reproductive toxicity.
Monitoring of workers in three manufacturing plants did not reveal any
health effects that might be associated with exposure to triforine.
An ADI of 0-0.02 mg/kg bw was established on the basis of the NOAEL of
2.4 mg/kg bw per day in the two-year study of toxicity in dogs, with a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 70 ppm, equal to 11 mg/kg bw per day (105-week study of
toxicity and carcinogenicity)
Rat: 200 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (parental and
fetal toxicity in a study of reproductive toxicity)
1000 mg/kg bw per day (study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 100 ppm, equal to 2.4 mg/kg bw per day (two-year study
of toxicity)
Estimate of acceptable daily intake
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adams, K., Ransome, S., Anderson, A. & Dawe, I.S. (1993) Chinese
hamster ovary/hprt locus assay: Triforine. Unpublished report from
Huntingdon Research Centre Ltd. (Study No. SLL282/931168). Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Atkinson, C., Perry, C.J. & Hudson, P. (1991 a) Triforine: 13-Week
dietary maximum tolerated dose study in mice. Unpublished report from
Inveresk Research International (Report No. 5875). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Toxicological criteria for estimation of guidance values for dietary and non-dietary exposure to triforine
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral, single dose, rat LD50 > 5000 mg/kg bw
(17 days) Dermal, single dose, rat LD50 > 2000 mg/kg bw
Inhalation (4 h), rat LC50 5.1 mg/L
Dermal, irritation, rabbit Not irritating
Ocural, irritation, rabbit Minimally irritating
Dermal, irritation, rat Not irritating
Dermal, sensitization, guinea-pig Non-sensitizing
Medium-term Dermal, 3 weeks, rat NOAEL = 1100 mg/kg bw per day (highest dose tested)
(1-26 weeks) Oral, 13 weeks, dog NOAEL = 3.6 mg/kg bw per day: haemosiderin deposition
Oral, two-generation, reproductive oxicity, rat NOAEL = 1500 mg/kg bw per day: reproductive toxicity;
NOAEL = 49 mg/kg bw per day: parental and offspring
toxicity
Oral, developmental toxicity, rabbit NOAEL = 5 mg/kg bw per day: maternal toxicity;
NOAEL = 125 mg/kg bw per day: fetal and developmental
toxicity
Long-term Oral, 2 years, dog NOAEL = 2.4 mg/kg bw per day: haematological changes;
(> 1 year) haemosiderin deposition; haematopoiesis
Atkinson, C., Perry, C.J. & Hudson, P .(1991b) Triforine: 13-Week
dietary maximum tolerated dose study in rats. Unpublished report from
Inveresk Research International (Report No. 5879). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Boehringer Sohn (1974a) The pharmacokinetics of the systemic fungicide
triforine (W 524) in the rat. Unpublished report dated 13 October
1974, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Boehringer Sohn (1974b) Excretion and metabolism of triforine (W 524)
in the rat. Unpublished report dated 8 March 1974, Ingelheim, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Börzsönyi, M., Pintér, A., Török, G. & Surján, A. (1978) Formation and
biological effect of N-nitroso compound from piperazine pesticide
triforine. In: Walker, E.A., Castegnaro, M., Griciute, L. & Lyle,
R.E., eds, Environmental Aspects of N-Nitroso Compounds (IARC
Scientific Publications No. 19), Lyon, IARC, pp. 477484
Brooks, T.M. & Wiggins, D.E. (1994) Triforine: In vitro chromosome
studies using cultured Chinese hamster ovary (CHO) cells. Unpublished
report from Shell Research Ltd (Technical Service Report No.
SBTR.93.065). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Bullock, C.H. & Narcisse, J.K. (1973) The acute inhalation toxicity of
Cela W 524 tTechnical. Unpublished report from Chevron, San Francisco,
California, USA. Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Celamerck (1983a) [Occupational medical examination of chemical
workers involved in synthesis operating in the production of
triforine.] Unpublished report dated 22 December 1983, Ingelheim,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in
German).
Celamerck (1983b) [Evaluative position paper on the estimation of risk
potential after application of Saprol on green areas that are to be
used as playing-fields or recreational areas.] (ZN 02092). Unpublished
report dated 27 October 1983, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA (in German).
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci., 8, 193-202.
De Bertoldi, M., Griselli, M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by means of gene conversion in
Saccharomyces cerevisiae and Aspergillus nidulans. Environ.
Mutag., 2, 359-370.
Everett, D.J., Perry, C.J., Hudson, P. & Mulhern, M. (1992) Triforine:
52 Week dietary toxicity study in rats. Unpublished report from
Inveresk Research International Ltd (Report No. 7500). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Fokkema, G.N. (1992) Triforine (SAPROL): A 21 day dermal toxicity
study in rats. Unpublished report from Hazleton UK (Report No.
SBTR.91.011 ). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Frohberg, H., von Eberstein, M. & Weisse, G. (1973) Triforine (W524)
Trial for acute toxicity in rats after oral administration and dermal
application and for primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt (Report No. 4/36/73). Submitted to WHO
by American Cyanamid, Princeton, NJ, USA.
Fuchs, A. (1992) Triforine: Preliminary oral (gavage) embryotoxicity
study in the rat. Unpublished report from Hazleton Deutschland GmbH
(Report No. 1031-121-005). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Fuchs, A. (1993a) Triforine: Oral (gavage) teratogenicity study in the
rat. Unpublished report from Hazleton Deutschland GmbH (Report No
1032-121-006). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Fuchs, A. (1993b) Triforine: Oral (garage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Report No.
1098-121-004). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Goburdhun, R. & Greenough, R.J. (1990) W-524-XX (Triforine), 104 week
oral toxicity study in dogs. Unpublished report from Inveresk Research
International (Report No. 5893). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Greenough, R.J. (1994) Triforine registration standard: Response to
review of 104 week dog study. Unpublished report from Inveresk
Research International Ltd (Report No. 5893). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984a) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 026256). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984b) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 031037). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hawkins, D.R., Wood, S.G., John, B.A., Iqbal, S., Cheng, K.N. &
Kitmitto, A. (1992) 14- C-Triforine: Metabolic fate in Sprague-Dawley
rats. Unpublished report from Huntingdon Research Centre (Report
HRC/SLL 203/920212). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Hazelden, K.P. & Aitken, R. (1992) Triforine: Two generation
reproduction study in rats (supplementary histological report).
Unpublished report from Inveresk Research International Ltd (Report
No. 7874). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Hazelden, K.P. (1994) Triforine registration standard; Response to
review of two-generation reproduction study in rats. Unpublished
report from Inveresk Research International Ltd (Report No. 11125).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Heath, J., Mulhern, M., Perry, C.J. & Henderson, W. (1992a) Triforine:
105 Week dietary carcinogenicity study in mice. Unpublished report
from Inveresk Research International (Report No. 7746). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Hill, K. (197,i) Chronic toxicity tests with the compound W-524-XX in
rats using oral administration, duration 2 years. Unpublished report
from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hofmann, A., Oelrich, I., Sumi, N. & Weisse, G. (1975) W-524
(triforine), 81-weeks carcinogenicity study in mice (substance
administered in the food). Unpublished report from E. Merck,
Darmstadt, Germany. Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Kramer, P.-J. (1985) Triforine techn. T 12756, in vitro assessment for
mutagenic potential in bacteria with and without addition of a
metabolising system. Unpublished report from E. Merck, Darmstadt,
Germany (Study No. T-12 756). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Langcake, P., Kuhn, P.J. & Wade, M. (1983) The mode of action of
systemic fungicides. In: Hudson, D.H. & Roberts, T.R., eds,
Progress in Pesticide Biochemistry and Toxicology, Vol. 3, pp.
1-109.
Leuschner, F. (1972) The effect of W-524, lot 1, on pregnant rats and
their fetuses, following oral administration. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W. (1971
) 13 Weeks oral toxicity study in beagle dogs with W-524. Unpublished
report from Laboratorium für Pharmakologie und Toxikologie, Hamburg;,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W.
(1972) 21-Day toxicity tests with the compounds W-524 in
Sprague-Dawley rats using dermal application. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1990) Triforine dose range finding
reproductive assay in rats. Unpublished report from Inveresk Research
International (Report No. 7073). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1991 ) Triforine, two generation
reproduction study in rats. Unpublished report from Inveresk Research
International (Report No. 7368). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Merck (1984) [Triforine: Medical/occupational health position paper.]
Unpublished report dated 28 March 1984, Darmstadt, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA (in German).
Miltenburger, H.G. (1984) Triforine tech., test report of study LMP
76A (mutations affecting the hypoxanthine-guanine phosphoribosyl
transferase locus in V79 cells: HGPRT-test). Unpublished report from
LMP, Darmstadt, Germany (Study No. LMP 076A). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Miltenburger, H.G. (1985) Triforine tech., test report of study LMP
136 (chromosome aberrations in cells of Chinese hamster cell line
V79). Unpublished report from LMP Darmstadt, Germany (Study No. LMP
136). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Muacevic, G. (1968) Pharmacological investigation, acute oral
toxicity, W-524, male and female albino mice. Unpublished report from
Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by Celamerck
GmbH & Co., Ingelheim/Rhine, Germany.
Muacevic, G. (1969) Pharmacological investigation, acute oral
tolerance in dogs. Unpublished report from Boehringer Sohn, Ingelheim,
Germany. Submitted to WHO by Celamerck GmbH & Co., Ingelheim/Rhine,
Germany.
Müller, W. (1989) Triforine: Oral (gavage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Project No.
460/29). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Müller, W. ( 1991 ) Triforine: Preliminary oral (gavage)
embryotoxicity study in the rabbit. Unpublished report from Hazleton
Deutschland GmbH (Report No. 951-121-003). Submitted to WHO by
American Cyanamid, Princeton, NI, USA.
Niggeschulze, A., Hill, K. & Stötzer, H. (1974) Three generation study
with the fungicide W-524 in rats. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Perry, C.J., Mulhern, M. & Finch, L (1992b) Triforine: 104 Week
dietary carcinogenicity study in rats, incorporating 52 week toxicity
study. Unpublished report from Inveresk Research International (Report
No. 7745). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Proudlock, R.J., Stocker, K.J., Howard, W.R., Anderson, A. & Dawe,
I.S. (1993) Triforine: in vitro DNA repair test using rat hepatocytes.
Unpublished report from Huntingdon Research Centre Ltd (Study Report
No. SLL 281/931152). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Robbins, M.C. (1994) An investigation of the effect of triforine on
rat and mouse hepatic xenobiotic metabolising enzymes. Unpublished
report from BIBRA Toxicology International (Report No. 1356/2/ 2/94).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Röhrborn, G. (1977) [Scientific comment on the mutagenicity of
triforine using the liver microsome test of Ames.] Unpublished report.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in German).
von Sandersleben, J.H., Herbst, M., Weisse, I., Frölke, W., Gutnard,
J. & Stötzer, H. (1974) Chronic toxicity test of the substance
W-524-XX on beagles, oral application, over 104 weeks. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J. & Tiler,
T. (1971a) Testing of the subacute toxicity of the substance W-524 in
rats following oral administration. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1971b) Testing of the subacute toxicity of the
substance W-524 in rats following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke, W., Guénard, J.
& von Sandersleben, J. (1971c) Testing of the subacute toxicity of the
substance W-524 in dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1972) Chronic toxicity studies with the compound
W-524-XX in rats using oral administration; duration 26 weeks.
Unpublished report from Boehringer Seth, Ingelheim, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Toubes, G.P. (1993) Medical certificate, Malgrat de Mer. Unpublished
report. Note of 6.4.1993 from Dr Inchaurrondo to Dr Dammermann, Shell
Agrar GmbH. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1988d) 4-Week toxicity
(feeding) study with triforine techn, in the mouse. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
097751). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1989) 4-Week toxicity
(feeding) study with triforine technical in the rat. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 097740). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Ullmann, L. & Porricello, T. (1988a) Primary skin irritation study
with triforine technical in rabbits (4-hour semi-occlusive
application). Unpublished report from Research & Consulting Co.,
Itingen, Switzerland (Project No. 213300). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Ullmann, L. & Porricello, T. (1988b) Primary eye irritation study with
triforine technical in rabbits. Unpublished report from Research &
Consulting Co., Itingen, Switzerland (Project No. 213311). Submitted
to WHO by American Cyanamid, Princeton, NJ. USA.
Ullmann, L. & Surer, B. (1984) Delayed contact hypersensitivity to
triforine technical in albino guinea pigs, the Maurer optimization
test. Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No, 33276). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Pappritz, G. (1986a) Acute oral
toxicity study with triforine technical in rats. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
076151). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L. Sacher, R., Mohler, H. & Vogel, O. (1986b) 4-Hour acute
inhalation toxicity study with triforine technical in rats.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 076162). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Vogel, O. (1986c) Acute oral
toxicity study with triforine technical in mice. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
077433). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L., Althaus, P., Janiak, T. & Vogel, D. (1990) Acute dermal
toxicity study with triforine techn. (SAG 102) in rats. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 33276, 278774). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
 2. Toxicological studies
(a) Acute toxicity
The acute toxicity of triforine has been tested by administration by
various routes in mice, rats, and dogs (Table 2). The only reported
observations were reduced activity, swaying, or dyspnoea. There were
no deaths, and no substance-induced changes in organs were detected
post mortem.
(b) Short-term toxicity
Mice
Four groups of five male and five female NMRI mice were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 200, 1000, or 5000 ppm for four weeks, equal to
0, 39, 200, or 980 mg/kg bw per day for males and 0, 45, 240, or 1300
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption; the
apparently increased food consumption by females at 5000 ppm was
possibly due to greater spillage. At the end of the study, males at
5000 ppm had 8% less body-weight gain than controls. Haematological
changes included decreased erythrocyte count (by 8-11%, p < 0.05),
haemoglobin concentration (by 7-9%, p < 0.05), and packed cell volume
(by 6-8% p < 0.05) and increased polychromatic erythrocytes in
animals of each sex at 5000 ppm. Males at this dose also had
moderately increased reticulocyte counts and decreased leukocyte
counts. A significant decrease in mean erythrocyte count was also
observed in males at 1000 ppm, although most of the values fell within
the range for the concurrent control group. Males and females at 5000
ppm showed increased absolute and relative weights of the spleen, and
females had increased relative liver weights (by about 16%,
p < 0.01). There were no gross or microscopic changes. The NOAEL
was 1000 ppm, equal to 196 mg/kg bw per day, on the basis of
haematological changes in animals of each sex, slightly reduced
body-weight gain in males, and increased relative liver weight in
females at 5000 ppm (Tenneker et al., 1988).
Groups of 10 CD-1 mice of each sex were given technical-grade
triforine (purity, 99.1%) in the diet at concentrations of 0 or 7000
ppm for 13 weeks, equal to 1000-1600 mg/kg bw per day in males and
1900-2500 mg/kg bw per day in females. No deaths or clinical signs of
toxicity were recorded, and there were no effects on food consumption
or body-weight gain. The haematological changes included a slight
reduction in erythrocyte numbers, haemoglobin concentration, and
haematocrit in animals of each sex. The absolute weights of the spleen
were increased by 38% in males and 56% in females, and that of the
liver was increased by 17% in males; the relative liver and spleen
weights were increased in animals of each sex ( p < 0.01). There
were no gross pathological findings; tissues were not examined
microscopically, as the study was designed to select concentrations
for use in a long-term study of toxicity and carcinogenicity (Atkinson
et al., 1991a).
Table 2 Acute toxicity of triforine
Species, strain Route of Sex LD50L/C50 Length of Reference
administration (mg/kg bw observation
or mg/L) (days)
Rat, Wistar Oral F, M > 16 000 14 Frohberg et al. (1973)
Rat, Wistar Oral F, M > 5 000 15 Ullman et al. (1986a)
Rat, Wistar Dermal F, M > 10 000 14 Frohberg et al. (1973)
Rat, Wistar Dermal F, M > 2 000 15 Ulman et al. (1990)
Rat, Sprague-Dawley Inhalation (1 h) F, M > 4.5 14 Bullock & Narcisse (1973)
Rat, Wistar Inhalation (4 h) F, M > 5.1 15 Ullman et al. (1986b)
Mouse (strain not stated) Oral F, M > 6 000 7 Muacevic (1968)
Mouse, NMRI Oral F, M > 5 000 15 Ullman et al. (1986c)
Dog (breed not stated) Oral F, M > 2 000 28 Muacevic (1969)
Rats
Groups of five male and five female Wistar rats were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 500, 2500, or 12 500 ppm for four weeks, equal to
0, 50, 240, or 1200 mg/kg bw per day for males and 0, 49, 230, or 1200
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption.
Body-weight gain was reduced by about 15% in males by the end of the
study ( p < 0.05), whereas them were no consistent, treatment-
related effects in females. Haematological changes indicative of
slight anaemia were observed. In rats at 12 500 ppm, slight decreases
in mean cell haemoglobin concentration ( p < 0.01) and mean cell
volume were seen in females ( p < 0.05) and increases in
reticulocyte ( p < 0.01) and polychromatic erythrocyte counts in
animals of each sex. Increased proportions of circulating immature
cells were also seen in rats at 2500 ppm, and the increase was
significant in males ( p < 0.01). Prothrombin time was decreased in
females at 12 500 ppm. At this dose, a slight increase in total
protein (by about 6%, p < 0.05) was seen in animals of each sex and
a slight increase in cholesterol content (57%, p < 0.01) in
females. Urine volume was increased in females at this dose. Changes
in organ weight were restricted to rats at 12 500 ppm. Absolute spleen
weights were increased in animals of each sex ( p < 0.05), as were
the absolute weights of the liver, thyroid, and kidney in females.
Increased relative weights were observed for liver ( p < 0.01) and
spleen ( p < 0.05) in animals of each sex and for thyroid in males
( p < 0.05). There were no treatment-related gross pathological
findings; the treatment-related microscopic changes were slight or
moderate haemosiderin deposition in the spleens of males and females
at 12 500 ppm and females at 2500 ppm; two females at 500 ppm also had
increased haemosiderin deposition. No NOAEL was identified (Tenneker
et al., 1989).
Groups of 15 male and 15 female Wistar FW-49 rats (25 at the high
dose) were given triforine (purity not stated) in the diet at
concentrations of 0, 2500, 7000, or 20 000 ppm for 13 weeks. Ten rats
of each sex at 20 000 ppm were observed for six weeks after the end of
treatment. Haematological, clinical chemical, and extended
histopathological investigations were performed on 10 rats of each sex
at each dose before treatment and after 6, 13, and 19 weeks.
Reversible reductions in erythrocyte count and in haemoglobin and
haematocrit values were seen in all treated groups. These results were
interpreted as a sign of a slight haemolytic anaemia and correlated
with haemosiderin deposition in liver, spleen, kidney, lung, and
heart, which increased in proportion to the dose administered. No
NOAEL was identified (Stötzer et al., 1971a).
As there was no no-effect level in the previous study, another study
was performed in which groups of 25 male and 25 female Wistar FW-49
rats were given triforine (purity not stated) in the diet at
concentrations of 0, 100, or 500 ppm for 13 weeks, equivalent to 5 or
25 mg/kg bw per day. No treatment-related effects were observed. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day, as no effects
were observed at 500 ppm, the highest dose tested (Stttzer et al.,
1971b).
Groups of 10 male and 10 female Sprague-Dawley rats were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0 or 20 000 ppm for 13 weeks, equal to 1300 mg/kg bw
per day. No deaths or clinical signs of toxicity were recorded, and
there were no effects on food consumption or body-weight gain. The
haematological changes included statistically significant but slight
reductions in mean cell haemoglobin concentration, by 1.4% in males
and 2.2% in females, and reduced erythrocyte numbers (by 4-9%) and
increased mean cell volume (by 3.5%) in females. Increases in the
absolute ( p < 0.05) and relative ( p < 0.01) weights of the liver
and spleen were observed in animals of each sex. There were no gross
pathological findings; the tissues were not examined microscopically
(Atkinson et al., 1991b).
Groups of 10 male and 10 female FW-49 rats were given triforine
(purity not stated) in the diet at concentrations of 0, 25, 120, 620,
or 3100 ppm for six months, equivalent to 0, 1.3, 6, 31, or 160 mg/kg
bw per day. Reductions in erythrocyte and haematocrit values and
increases in reticulocyte numbers were observed in rats at 620 and
3100 ppm. No consistent, dose-related variations were found in serum
chemical or urinary parameters. Post-mortem examination did not reveal
any treatment-related changes, but increased liver weights were found
in females at all doses, the relative weights being increased by 27%
in those at 25 ppm, 19% at 120 ppm, 40% at 620 ppm, and 49% at 3100
ppm (no statistical analysis reported). No such increases were
observed in males. Increased haemosiderin deposition was found in the
livers and spleens of male rats at 620 and 3100 ppm and females at
3100 ppm. No treatment-related histopathological changes were seen in
the livers of either male or female rats at 25 or 120 ppm. The NOAEL
was 120 ppm, equivalent to 6 mg/kg bw per day, on the basis of reduced
erythrocyte numbers and packed cell volume at 620 ppm (Stötzer et al.,
1972).
Groups of 10 male and 10 female Sprague-Dawley rats were given topical
applications on shaved intact, occluded skin of a 0, 0.5, or 1.5%
aqueous dilution of a 20% triforine emulsion, equivalent to 0, 10, or
30 mg/kg bw triforine, for 4 h per day daily for 21 days. Five males
and five females per group were followed up for an additional 21 days.
Temporary slight reddening and swelling occurred in the covered skin
areas in all groups, including the controls. No local or systemic
substance-related reactions were observed (Leuschner et al., 1972).
Groups of seven Fischer 344 rats of each sex received technical-grade
triforine in corn oil (3 ml/kg bw) on a shaven area of the back at
doses of 0, 110, 350, or 1100 mg/kg bw per day on five days per week
for three weeks. After each application, the treated area was covered
with gauze and bandage for 6 h, then washed and dried. No deaths or
treatment-related dermal changes were observed, and there were no
effects on food consumption or body-weight gain attributable to
treatment. All animals, including the controls, lost some weight,
particularly during the first week; this response was attributed to
the bandaging procedure. No significant, treatment-related variations
in organ weights or haematological end-points was seen, and them were
no gross or microscopic pathological findings. Female rats at 350 or
1100 mg/kg bw showed statistically significant increases in serum
cholesterol (13% and 22%, respectively), triglycerides (32 and 40%,
respectively), and bilimbin (27 and 13%, respectively); a 25% decrease
in serum alkaline phosphatase activity was seen in females at 110
mg/kg bw. In males, serum alkaline phosphatase activity was reduced by
15% at 350 mg/kg bw and by 21% at 1 100 mg/kg bw; in rats at 1100
mg/kg bw, total serum protein was increased by 5.7% and serum albumin
by 3.8%. Since the changes in bilirubin, cholesterol, and triglyceride
contents were not accompanied by histopathological changes in the
liver and were confined to a single sex, their biological significance
is unclear. The increases in alkaline phosphatase activity may reflect
a toxicological effect, but the decreases observed in this study are
not usually considered to be toxicologically significant. The NOAEL
was 1100 mg/kg bw per day, the highest dose tested, as no toxicity was
observed (Fokkema, 1992).
Dogs
Groups of four male and four female beagle dogs were given triforine
(purity not stated) in the diet at concentrations of 0, 3500, 10 000,
or 30 000 ppm (two groups) for 13 weeks. The supplementary group at 30
000 ppm was observed for an additional six weeks without treatment.
These concentrations were equal to 0, 83, 230, 690, or 710 mg/kg bw
per day for males and 0, 85,240,730, or 720 mg/kg bw per day for
females. Signs of haemolytic anaemia-reduced erythrocyte counts
( p < 0.05) and haemoglobin concentration ( p < 0.05), and,
occasionally, also in haemocrit--were observed at all doses by week
13, but were first observed in week 6 in dogs at 10 000 or 30 000 ppm
( p < 0.01). These effects were accompanied by a consistent increase
in the number of reticulocytes in dogs at 10 000 and 30 000 ppm. All
of the values returned to normal in the group at 30 000 ppm within
three weeks of observation. Serum chemistry in weeks 6 and 13 showed
slight increases in alkaline phosphatase activity and bilirubin and
cholersterol concentrations in all treated groups, but these values
also returned to normal in dogs at 30 000 ppm within three weeks.
Urinalysis and ophthalmoscopy showed no differences between the
groups. Fine, drop-like fatty infiltration of the myocardial fibres
were seen in 0, 5, 1, 5, and 1 dogs in the five groups, respectively,
and fatty accumulation in the liver in 0, 3, 0, 4, and 0 dogs. The
fatty infiltration appeared to be reversible, since it was no longer
detected in the dogs observed for six weeks. Treatment-related
siderosis in Kupffer cells in the liver showed a clear tendency
towards reversibility in the animals allowed to recover. There was no
NOAEL (Stötzer et al., 1971c).
Groups of four beagle dogs of each sex were given triforine in the
diet at concentrations of 0, 100,600, or 3500 ppm for 13 weeks, equal
to 0, 3.6, 22, or 120 mg/kg bw per day for males and females together.
The haematological findings in the dogs at 3500 ppm in the previous
study were essentially confirmed. Furthermore, siderosis was detected
in the spleen, liver, and bone marrow after administration of 600 ppm,
and the weight of the spleen was increased in the dogs at 3500 ppm.
The NOAEL was 100 ppm, equal to 3.6 mg/kg, on the basis of
haemosiderin deposits in the liver and bone marrow at 600 ppm
(Leuschner et al., 1971).
Groups of four male and four female beagle dogs were given
technical-grade triforine (purity not stated) in the diet at
concentrations of 0, 10, 40, 100, or 1000 ppm for two years, equal to
0, 0.23, 0.93, 2.4, or 22 mg/kg bw per day for males and 0, 0.25,
0.99, 2.6, or 24 mg/kg bw per day for females. Samples were taken for
haematology, blood chemistry, and urinalysis during weeks 6, 13, 26,
52, 78, and 104. One male dog at 100 ppm group died of acute
pneumonia. There were no other deaths, and there were no
treatment-related signs of toxicity, changes in food consumption,
changes in body-weight gain, or ophthalmoscopic findings.
Haematological changes in the dogs at 1000 ppm group included
increased mean cell volume in males in week 13 (12%, p < 0.01) and
females in week 26 (3.5%, p < 0.05) and decreased mean cell
haemoglobin concentration in males at week t 3 (3.5 %, p < 0.01).
The other changes were either not statistically significant or were
inconsistent with respect to time interval, sex, or dose. Examination
of femoral bone-marrow smears at termination showed a shift in the
granulopoietic:erythropoietic ratio towards erythropoiesis in two
males and three females at 1000 ppm. The erythropoietic mitotic index
was also increased in one female in this group. No treatment-related
changes were observed on blood chemistry or urinalysis. The absolute
and relative organ weights were comparable in all groups, and there
were no treatment-related gross pathological findings.
Microscopically, haemosiderin deposition of moderate severity was
observed in Kupffer cells in four dogs at 1000 ppm and one control.
Haemosiderosis of the bone marrow was also observed in two dogs at
1000 ppm. The NOAEL was 100 ppm, equal to 2.4 mg/kg bw per day, on the
basis of haematological changes, increased erythropoiesis, and
haemosiderin deposition in the liver and bone marrow in animals of
each sex at 1000 ppm (von Sandersleben et al., 1974; Goburdhun &
Greenough, 1990; Greenough, 1994).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 40 NMRI mice of each sex were given triforine (purity not
stated) in the diet at concentrations of 0, 30, 150, or 750 ppm for 81
weeks, equal to 0, 4.7, 24, or 120 mg/kg bw for males and 0, 5.2, 28,
and 140 mg/kg bw for females. Mortality at 81 weeks was 32% of control
males, 20% of those at 30 ppm, 35% at 150 ppm, and 50% at 750 ppm, and
30% of control females, 40% of those at 30 ppm, 26% at 150 ppm, and
33% at 750 ppm. Survival time, clinical symptoms, body-weight gain,
and food consumption were not affected by treatment. As morphological
findings were described only if they were considered to be relevant to
an evaluation of the carcinogenic effects of the substance, no NOAEL
was identified. Treatment did not increase the total number of
neoplasms (mainly lymphocytic leukaemias and lymphosarcomas), and the
latent periods remained unchanged. The frequencies of specific, less
common tumours in the treated groups were usually not higher than in
the concurrent controls or in the literature (Hofmann et al., 1975).
Four groups of 50 CD-1 mice of each sex were given technical-grade
triforine (purity, 98.9%) in the diet at concentrations of 0, 70, 700,
or 7000 ppm for 105 weeks, equal to 0, 11, 120, or 1200 mg/kg bw per
day for males and 0, 16, 160, or 1600 mg/kg per day for females. Blood
samples were collected for analysis at weeks 52, 77, and 103. The
mortality at 105 weeks was 14% of control males, 19% of those at 70
ppm, 35% at 700 ppm, and 28% at 7000 ppm; for females, 27% of controls
were dead at this time and 27% of those at 70 ppm, 32% at 700 ppm, and
30% at 7000 ppm. The mortality rate in the control group of males was
considered to be unusually low for CD-1 mice at 105 weeks. There were
no treatment-related clinical signs of toxicity or changes in food
consumption. Body-weight gain at the end of the study was reduced by
11% in males at 700 ppm and 16% in those at 7000 ppm. No significant
changes in body-weight gain were seen in females. No haematological
changes were observed. The absolute and relative weights of the liver
were increased by 21% ( p < 0.01) in females at 7000 ppm. At
autopsy, thickening or enlargement of the large intestine was observed
in males that died before the end of the study in the groups receiving
700 ppm (17%) or 7000 ppm (36%), whereas none was seen in the
controls. The large intestine was ulcerated, and inflammation was
observed microscopically, predominantly in male mice that died before
the end of the study after receiving 700 ppm (23%) or 7000 ppm (21%).
The overall occurrence of these findings was none in male controls, 6%
at 70 ppm, 16% at 700 ppm, and 12% at 7000 ppm.
The incidences of hepatocellular adenoma were 9/50 in control males,
12/50 at 70 ppm, 8/50 at 700 ppm, and 8/50 at 7000 ppm; the incidences
of hepatocellular carcinoma were 5/50 in control males, 7/50 at 70
ppm, 8/50 at 700 ppm, and 10/50 at 7000 ppm. There were no significant
differences by either Fisher's exact test or the Cochrane-Armitage
test for trend, for adenomas or carcinomas independently or combined
(Fisher's exact test for comparison of carcinomas in males at 0 and
7000 ppm gave p = 0.263). All of the incidences of liver tumours
were within the ranges found in a compilation of five control groups
of male CD-1 mice in the same laboratory: adenomas, 0-32%; carcinomas,
0-21%. Females had no increase in the incidences of liver tumours. The
incidences of alveolar or bronchiolar adenomas in males were 14/50 in
controls, 13/50 at 70 ppm, 8/50 at 700 ppm, and 17/50 at 7000 ppm, and
the incidences of alveolar or bronchiolar carcinomas were 5/50 in
controls, 2/50 at 70 ppm, 4/50 at 700 ppm, and 7/50 at 7000 ppm. These
differences were not statistically significant. The incidences of
alveolar or bronchiolar adenomas in females were 5/50 in controls,
6/50 at 70 ppm, 7/50 at 700 ppm, and 21/50 at 7000 ppm (Fisher's exact
test, p < 0.001), and the incidences of alveolar or bronchiolar
carcinomas were 1/50 in controls, 2/50 at 70 ppm, 1/50 at 700 ppm, and
6/49 at 7000 ppm (Fisher's exact test, p = 0.059). The incidences of
lung tumours were beyond the ranges observed in five other control
groups of female CD- 1 mice at this laboratory: adenomas, 4-18%;
carcinomas, 0-8% The Meeting concluded that triforine increased the
incidence of alveolar or bronchiolar adenomas fit female mice. The
NOAEL for carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day,
on the basis of an increased incidence of adenomas and/or carcinomas
in females at 7000 ppm. The NOAEL for toxicity was 70 ppm, equal to 11
mg/kg bw per day, on the basis of a slight decrease in body-weight
gain and changes in the large intestine of males fed 700 ppm (Heath et
al., 1992).
Rats
Groups of 35 Wistar (CHBB: THOM-SPF) rats of each sex, with 50 of each
sex in the group of controls and that at the high dose, were given
triforine (purity, 96.6%) in the diet at concentrations of 0, 25,
125,625, or 3120 ppm for two years, equivalent to 0, 1.3, 6.3, 31, or
160 mg/kg per day. Samples were taken for haematological and blood
chemical investigations during weeks 0, 6, 13, 26, 52, 78, and 104
from 20 male and female rats per group. Urine was analysed before and
at the end of the study in 10 animals of each sex from the control
group and that at the highest dose. All animals were examined post
mortem. Complete histopathological examinations were performed at
the end of the study on 20 rats of each sex from the control group and
that at the highest dose, on 15 rats of each sex from the other
groups, on rats that died before the end of the study, and on all
tumour-bearing rats. Mortality, clinical signs, body-weight
development, and parameters of clinical chemisstry and the urinalyses
were not affected by the treatment. Temporary reductions in the number
of erythrocytes ( p < 0.01 for males) and haematocrit values
( p< 0.01 for males) and increased numbers of mticulocytes (39% in
males, 29% in females) were seen in rots at 3125 ppm during week 6,
the variations being more pronounced among male rats. There were no
significant differences in organ weights. Haemosiderosis was observed
in the spleens of both control and treated rats, and the frequency and
intensity of the condition did not differ significantly between the
groups. The sinuses of the adrenals of females in all groups showed
cavernous dilatation, and some were thrombosed. These changes occurred
more frequently in treated than in control animals, but they are
common findings in Wistar rats. No significant difference in tumour
incidence between the groups was found. The NOAEL was 625 ppm,
equivalent to 31 mg/kg bw per day, on the basis of signs of anaemia at
3120 ppm (Hill, 1974).
Groups of 70 Sprague-Dawley rats of each sex were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0, 200, 2000, or 20 000 ppm for two years, equal to
an average of 0, 10, 100, or 1000 mg/kg bw per day for males and 0,
13, 140, or 1400 mg/kg bw per day for females. Twenty rats of each sex
per group were killed at one year and the survivors at two years.
Blood and urine samples were collected for analysis at weeks 26
(females) or 28 (males), 52, and 104. Mortality at two years among the
remaining 50 animals in each group was 25% of control males, 24% of
those at 200 ppm, 19% at 2000 ppm, and 19% at 20 000 ppm and 22% of
control females, 20% of those at 200 ppm, 21% of those at 2000 ppm,,
and 23% of those at 20 000 ppm. There were no treatment-related
clinical signs of toxicity and no changes on ophthalmoscopy or
urinalysis. There were no significant differences in food or water
consumption and only slight differences in body weight between the
groups at the end of the study.
A number of findings could be attributed to the treatment with
triforine. At one year, the body weights of females were moderately
but not statistically significantly lower than those of controls, by
11% in those at 200 ppm, 13% at 2000 ppm, and 21% at 20 000 ppm.
Haemoglobin concentrations were 4-6% lower in male rats at 2000 ppm
( p < 0.05) and 20 000 ppm ( p < 0.01 ) at weeks 28 and 51 and in
those at 200 ppm at week 51. Although the changes were consistent in
males, in female rats the haemoglobin concentrations were slightly and
nonsignificantly lower only at 20 000 ppm at week 26. The mean
corpuscular haemoglobin count was 2% lower in both male and female
rats at 20 000 ppm at weeks 28 and 26, respectively, but not at week
51. The values in the control group were, however, clearly higher than
normally expected for rats of this age, sex, and strain in this
laboratory. Changes in blood chemistry in the group at 20 000 ppm
included slightly raised concentrations of sodium in males at week 28,
of calcium in males at week 28 and females at week 104 (by 4%,
p < 0.01 ), of cholesterol in females at week 104 (by 39%,
p < 0.01), and of total protein in males at week 104 (by 9%,
p < 0.01 ), and a slightly lower concentration of glucose in males
at week 52. females at 2000 ppm also had raised calcium levels at week
104. There were no gross pathological findings. Liver weights were
increased at week 52 in males (11%) and females (15%) at 20 000 ppm
and in all females at week 104; both absolute and relative liver
weights were increased in females at week 104, by 18% in those at 200
ppm ( p < 0.05), 24% at 2000 ppm ( p < 0.01), and 43 % at 20 000
ppm ( p < 0.001 ). Although not significant, the relative weights of
the liver were increased in males, by 19% at 200 ppm, 11% at 2000 ppm,
and 18% at 20 000 ppm. The absence of a dose-related response in the
males calls into question the biological significance of the response
in females at the low dose. Significant, dose-related increases in
absolute and relative spleen weights were seen in females at 2000 and
20 000 ppm that were killed at one year. The relative spleen weights
were also increased in females at 20 000 ppm at week 104. The
increased weights of the liver and spleen correlated with
histopathological findings (described below) and are considered to be
related to treatment. Increased kidney weights were observed at week
52, but only in females, by 9% in those at 200 ppm, 12% at 2000 ppm,
and 17% at 20 000 ppm. Adrenal weights were reduced by 13-15% in all
treated males but were increased by 35% in females at 2000 ppm and by
40% in those at 20 000 ppm. Microscopic examination showed increased
deposition of haemosiderin in the spleen of females at 2000 and 20 000
ppm at two years, in males at these doses at one year, and in females
at 20 000 ppm at one year. The incidence of haemosiderin deposition in
Kupffer cells and macrophages of the liver was increased in male and
female rats at 2000 and 20 000 ppm at one year. The severity was also
increased in these groups of males but only in females at 2000 ppm. In
the latter group, there was an increased incidence of pale-cell foci
in males and bile-duct hyperplasia in females. There were no
treatment-related changes in the proportions of tumour-bearing animals
or in the incidence of any particular tumour type. The Meeting
concluded that triforine is not carcinogenic to rats. The NOAEL was
200 ppm, equal to 10 mg/kg bw per day, on the basis of slight
reductions in body-weight gain in males, haematological changes in
animals of each sex, increased absolute and relative weights of the
spleen in females at one year and of the liver at two years, and
haemosiderin deposition in the spleens of males and females at one
year and in the livers of females after one year at 2000 ppm (Everett
et al., 1992; Perry et at., 1992).
(d) Genotoxicity
Triforine has been tested for genetic damaging activity in an adequate
range of assays, most of which were conducted to currently acceptable
standards (see Table 3 for salient features and references). Mutations
were not induced by triforine in either bacteria or at the hprt
locus of cultured mammalian cell lines, and excision repairable DNA
damage was not increased in treated primary cultures of rat
hepatocytes. Gene conversion was not induced in either yeast or fungal
cells. The potential of triforine for inducing chromosomal damage was
tested in mammalian cell lines in vitro by examining treated
metaphase cells for gross aberrations and in vivo by examining
polychromatic erythrocytes taken from the bone marrow of mice treated
orally for an increase in the proportion of micronucleated cells.
There was no indication of activity in vitro. In one test, an
increase in the proportion of micronucleated cells was observed in
groups of five female mice sampled 48 h after dosing (controls: 0.4 ±
0.55/103; treated with 5000 µg/kg bw: 2.4 ± 1.14/103) but not at 24
or 72 h and not in male mice at any sampling time. The increase was
not confirmed when the study was partially repeated with female mice
sampled only 48 h after dosing (controls: 1.6 ± 1.14/103; treated with
5000 µg/kg bw: 1.2 ± 1.10/103). The Meeting concluded that triforine
is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female CHBB-THOM rats were given triforine
(purity not stated) in the diet at concentrations of 0, 100, 500, or
2500 ppm in a three-generation study in which the rats were mated to
produce two litters in each generation. The only sign of toxicity in
parent rats and their offspring was temporarily, slightly reduced
body-weight gain of male rats, particularly at the high dose.
Table 3. Genetic activity of triforine
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium 100 µg/plate NR Negativea Rohrborn (1977)
TA 100, TA1535
Reverse mutation S. typhimurium 5000 µg/plate NR Negativea Moriya et al. (1983)
TA98, TA 100,
TA1535, TA1537,
TA1538, E. coli
WP2hcr
Reverse mutation S. typhimurium 5000 µg/plate 99.9 Negativea Kramer (1985)
TA98, TA 100,
TA1535, TA1537,
TA1538
Gene conversion, Saccharomycess 1000 µg/ml NR Negativea De Bertoldi et al. (1980)
adc2, trp5 loci cerevisiae D4
Gene conversion, Aspergillus 1000 µg/ml NR Negativeb De Bertoldi et al. (1980)
pabaA locus nidulans
Unscheduled Rat hepatocyte 63 µg/ml 99.8 Negativeb Proudlock et al. (1993)
DNA synthesis primary culture
Cell mutation, Chinese hamster 50 µg/ml NR Negativea Miltenburger (1984)
hprt locus V79 cells
Cell mutation, Chinese hamster 200 µg/ml 99.8 Negativea Adams et al. (1993)
hprt locus ovary cells
Table 3. (continued)
Test system Test object Concentration/ Purity Results References
dose (%)
Chromosomal Chinese hamster 400 µg/ml 99.8 Negativea Brooks & Wiggins (1994)
aberration ovary cells (24 and
48 h sampling)
Chromosomal Chinese hamster 50 µg/ml NR Negativea Miltenburger (1985)
aberration V79 cells (7, 18,
and 28 h sampling)
In vivo
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Weakly Guenard (1984a)
induction marrow (24, 48, by gavage positive
and 72 h sampling)
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Negative Guenard (1984b)
induction (females only, by gavage
48 h sampling)
NR, not reported
a With and without metabolic activation
b Without metabolic activation
Reproductive performance, duration of pregnancy, malformation
frequency, and postnatal development were unaffected by treatment.
When the F3b rats were subjected to post-mortem and histopathological
examinations, no treatment-related change was observed. Since this
study has been superseded by one at higher doses, a full description
is not given; however, the dietary concentration of 2500 ppm had no
adverse effect on reproduction (Niggeschulze et al., 1974).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were given technical-grade triforine (purity,
99.1%) in the diet at concentrations of 0, 1250, 5000, or 20 000 ppm.
The parental (FA) rats received the diets for 10 weeks before mating
and throughout mating, gestataon, and lactation. These concentrations
were equal to 0, 100, 410, or 1600 mg/kg bw per day for males and 0,
100, 400, and 1600 mg/kg per day for females during the premating and
mating periods; for F0 females, they were equal to 0, 97, 400, or
1600 mg/kg bw per day during gestation and 0, 150, 650, or 2900 mg/kg
bw per day during lactation. All F1 rats were weaned onto the same
diets as their parents and continued on this treatment until they were
six to seven weeks old, when they were killedś The only death occurred
in a male rat in the control group. No signs of toxicity were noted.
Food consumption was slightly reduced in the group at 20 000 ppm,
among males during weeks 1-10 and among females during weeks 1-3.
Body-weight gain deficits at these doses during these periods resulted
in a reduction in body weight of about 11% in males throughout the
experiment (17 weeks), whereas females had recovered by the end of the
premating period. The relative liver weights of F0 males and females
at 5000 and 20 000 ppm were increased, and females at these doses also
had increased absolute weights of both liver and spleen. No gross
pathological changes were found at autopsy of the F0 rats; no
histological examinations were performed. Treatment did not affect
mating performance, fertility, duration of gestation, litter size, or
F1 pup survival, and no clinical signs of toxicity were seen among
the F1 pups. From lactation day 14, the body-weight gain of male and
female pups was reduced by 7% in those at 5000 ppm and 11 and 10%,
respectively, at 20 000 ppm. These reductions persisted after weaning
(day 24) and were present at six weeks in males and five weeks in
females. The overall depression in mean weight gain after weaning was
11% for males and 4% for females at 5000 ppm and 19% for males and 10%
for females at 20 000 ppm. Food consumption after weaning was also
reduced at this dose, by 15% in males and 12% in females. There were
no gross pathological findings at autopsy of the F1 rats. The effects
observed at the dietary concentration of 20 000 ppm triforine were
considered not to contraindicate its use as the high dose in a
two-generation study. The NOAEL for reproductive toxicity was 1000 ppm
(McCay & Hazelden, 1990).
Groups of 29 male and 28 female Sprague-Dawley rats were given
technical-grade triforine (purity, 98.9-99.1%) in the diet at
concentrations of 0, 500, 3000, or 20 000 ppm in a two-generation
study. The parental (F0) rats received the diets for 10 weeks before
mating and throughout mating, gestation, and lactation. At weaning of
the F1 litters, pups were selected to provide the F2 generation
litters and continued on the same diets as had been offered to their
parents. The study was completed after weaning of the F2 rats. The
dietary concentrations were equal to 0, 38, 230, or 1500 mg/kg bw per
day for F0 males and 0, 48, 290, or 1900 mg/kg per day for F0
females during the premating and mating periods. For F0 females,
these concentrations were equal to 0, 41,240, or 1700 mg/kg bw per day
during gestation and 0, 63, 380, or 2600 mg/kg bw per day during
lactation. The dietary concentrations of 0,500, 3000, and 20 000 ppm
were equal to 0, 40, 280, and 2000 mg/kg bw per day for F2 males and
0, 61,360, and 2500 mg/kg, per day for F1 females during the
premating and mating periods. For F1 females, these concentrations
were equal to 0, 40,230, and 1800 mg/kg bw per day during gestation
and 0, 65, 420, and 2900 mg/kg bw per day during lactation. Pups were
not culled during the study.
One F0 male and one F0 female at 500 ppm died during the study, and
one F1 female at 20 000 ppm was killed prematurely because of
dystocia. None of these deaths was related to treatment. No clinical
signs of toxicity related to triforine were noted in either the F0 or
F1 generations There were slight reductions in food consumption among
F0 rats at 20 000 ppm and F0 females at 3000 ppm during the first
week of treatment. A decrease of 9% was observed in the body-weight
gain of F0 females, but not males, at 500 ppm during the premating
period, at the beginning of which there had been a 3% body-weight gain
in these rats. Slight deficits in body-weight gain were seen
throughout the treatment period for F0 males at 20 000 ppm, resulting
in an overall reduction in body-weight gain of 9%. During the
premating period, F0 females show body-weight gain deficits of 19% at
3000 ppm and 20% at 20 000 ppm. Overall weight gain was decreased by
9% in F0 females at 20 000 ppm during gestation, whereas there was no
deficit in these rats during, lactation. Food consumption was reduced
among F1 males and females at 3000 ppm (10%) and 20 000 ppm (11 and
12%, respectively), mostly during the first three weeks after weaning.
Corresponding deficits in body-weight gain were seen during this
period, of 9% in males and females at 3000 ppm and 13% in males and 9%
in females at 20 000 ppm. Between weeks 6 and 23, the deficits in body
weight were 5% for males and 10% for females at 20 000 ppm. There were
no effects on the body-weight gain of F1 females at any dose during
gestation or lactation.
There were no gross pathological findings attributable to treatment.
Kidney weights were significantly higher (5-9%), in F0 males and
females at 3000 and 20 000 ppm and in F1 males at 20 000 ppm than in
controls. Liver weights were significantly higher (11-45% and
increasing with dose) in F0 females, F1 males, and F1 females at
3000 and 20 000 ppm; a marginal increase in liver weight was also seen
in F1 males at 500 ppm (6.4%, p < (0.05). Significant increases
were observed in the weight of the spleen in F1 males at 20 000 ppm
(16%) and F1 females at 3000 (20%) and 20 000 ppm (42%). Triforine
treatment did not affect mating performance, fertility, duration of
gestation, gestation index, postimplantation loss, litter size, or pup
survival in either the F0 or F1 generation.
The clinical signs of toxicity in pups that could be attributed to
treatment included a reduction in the mean weights of F1 and F2 pups
at 20 000 ppm from day 4 of lactation, so that, by day 21, the body
weights were reduced by 17% for F1 males, 18% for F1 females, 20%
for F2 males, and 19% for F2 females. Decreases were also observed
in F1 pups at 3000 ppm on days 14-21 of lactation, by 9% in males and
8% in females.
The liver, kidney, spleen, and thyroid of parental F0 and F1 rats at
all doses and a number of other organs of parental F0 and F1 rats at
0 and 20 000 ppm were examined histologically. Dose-related increases
in haemosiderin deposition in the spleen, accompanied by dose-related
increases in extramedullary haematopoeisis and in spleen weight were
observed. There was also a dose-related increase in the severity of
spontaneous nephropathy in males and nephrocalcinosis in females.
Small increases in kidney weight were observed in this study,
particularly in the F0 generation, but with no change at 500 ppm.
Liver size increased with dose and was accompanied by sporadic
centrilobular hepatocyte hypertrophy. The hepatic changes were
regarded by the authors as an adaptive response to increased metabolic
activity. The thyroids of female rats of both generations at 20 000
ppm had the histological appearance of very active secreting glands,
consisting of numerous very small acini with little or no lumina,
scant stored secretion, and lined by cuboidal or columnar epithelium
with pale, foamy cytoplasm. The severity of these effects did not
appear to be increased in the F1 generation. The NOAEL for
reproductive toxicity was 20 000 ppm, equal to 1500 mg/kg per day, the
highest dose tested. The NOAEL for parental toxicity and growth and
development of the offspring was 500 ppm, equal to 40 mg/kg bw per
day, on the basis of decreased food consumption in F0 females, F1
males, and F1 females, decreased body-weight gain in F0 females, F1
males, and F1 females, decreased F1 pup weight, and increased
relative spleen weight in F1 females at 3000 ppm (McCay & Hazelden,
2992; Hazelden & Aitken, 1992; Hazelden, 1994).
(ii) Developmental toxicity
Rats
Groups of 20 inseminated female Sprague-Dawley rats were given 0, 100,
400, 800, or 1600 mg/kg bw per day triforine (purity not stated)
suspended in 1% carboxymethylcellulose by gavage on days 6-15 of
pregnancy. The rates of resorption and variation were increased in
animals at 1600 mg/kg bw per day. Furthermore, the number of fetuses
was reduced, dur to significantly increased postimplantation loss at
this dose. Because the frequency of variations was also slightly
increased at 800 mg/kg bw per day, the NOAEL for fetotoxicity was 400
mg/kg bw per day. Although toxic effects were observed at 800 and 1600
mg/kg bw per day, there were no signs of a teratogenic effect
(Leuschner, 1972).
In a preliminary study, groups of eight inseminated Sprague-Dawley
rats were given triforine (purity, >97%) by garage at doses of 250,
500, or 1000 mg/kg bw per day on days 6-15 of gestation. There were no
deaths or treatment-related clinical signs of toxicity. Mean body
weight was slightly reduced on gestation days 9-12 in animals at 500
and 1000 mg/kg bw per day, but overall body-weight gain in the four
groups was comparable throughout treatment. No gross pathological
changes were found in fetuses removed on day 20 of gestation. No
reproductive parameters were affected by treatment, and them were no
indications of treatment-related embryotoxicity or teratogenicity
(Fuchs, 1992).
Groups of 30 inseminated Sprague-Dawley rats were given triforine
(purity not stated) by garage at doses of 200, 500, or 1000 mg/kg bw
per day on days 6-15 of gestation. There were no deaths or
treatment-related clinical signs of toxicity. Food consumption was
reduced in animals at 1000 mg/kg bw per day, but this was not
accompanied by a significant decrease in body-weight gain. No gross
pathological changes were seen in fetuses removed on day 20 of
gestation. No reproductive parameters were affected by treatment, and
there were no indications of treatment-related embryotoxicity or
teratogenicity. The NOAEL for maternal and developmental toxicity was
1000 mg/kg bw per day (Fuchs, 1993a).
Rabbits
Groups of 15 inseminated Himalayan rabbits were given technical-grade
triforine (purity not stated) in 0.5% carboxymethylcellulose by gavage
on days 6-18 of gestation at doses of 0, 5, 25, or 125 mg/kg bw per
day. Them were no deaths or treatment-related clinical signs of
toxicity. Dose-related decreases in food consumption were observed in
rabbits at 25 and 125 mg/kg bw per day on days 7-14 of treatment. Food
consumption was also reduced in the animals at 5 mg/kg bw per day, but
this had no effect on body-weight gain and was considered to be
toxicologically insignificant. For most of the treatment period, body
weight was reduced in rabbits at 25 and 125 mg/kg bw per day, as a
result of reductions during gestation days 6-9. Towards the end of
treatment, these groups regained weight. The does were killed and
their fetuses removed on day 29 of gestation. No gross pathological
findings were observed. No reproductive parameters were affected by
treatment, and them were no indications of treatment-related
embryotoxicity or fetotoxicity at any dose. The NOAEL for maternal
toxicity was 5 mg/kg bw per day, on the basis of decreased food
consumption and body weight at 25 mg/kg bw per day. The NOAEL for
fetal effects was 125 mg/kg bw per day, the highest dose tested
(Gleich et al., 1981).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in 0.5%
carboxymethylcellulose by garage on days 6-18 of gestation at doses of
0, 6, 30, or 150 mg/kg bw per day. There were no deaths or
treatment-related clinical signs of toxicity. Decreased food
consumption were observed in does at 150 mg/kg bw per day on days
12-18, although no statistically significant reduction in body-weight
gain was observed. The does were killed and their fetuses removed on
day 28 of gestation. No gross pathological findings were observed. No
reproductive parameters were affected by treatment, and there were no
indications of treatment-related embryotoxicity or fetotoxicity at any
dose. The NOAEL for maternal toxicity was 30 mg/kg bw per day, on the
basis of decreased food consumption at 150 mg/kg bw per day. The NOAEL
for fetal effects was 150 mg/kg bw per day, the highest dose tested
(Müller, 1989).
Since maternal effects were minimal in the previous study, triforine
was tested at higher doses. In a preliminary study, four groups of
eight inseminated New Zealand rabbits were given technical-grade
triforine (purity not stated) in distilled water by gavage on days
6-18 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw per day.
There were no deaths or treatment-related clinical signs of toxicity.
Body-weight gain was reduced in all treated groups, mainly due to
body-weight losses on days 6-9 of gestation; the reduction (64%) was
statistically significant in does at 1000 mg/kg bw per day. At the end
of treatment, the body-weight gain in the treated groups exceeded that
of the controls. When the does were killed and their fetuses removed
on day 28 of gestation, no gross pathological changes were found, and
reproductive parameters were not affected. The group mean fetal
weights were slightly reduced at 500 and 1000 mg/kg bw per day. There
were no indications of teratogenicity at any dose (Müller, 1991).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in distilled water by
gavage on days 6-18 of gestation at doses of 0 or 1000 mg/kg bw per
day. There were no deaths or treatment-related clinical signs of
toxicity. Reductions in food consumption and body-weight gain were
observed in does at 1000 mg/kg bw per day, mainly due to body-weight
losses on days 6-9 of gestation. At the end of treatment, food
consumption and body-weight gain in the treated group were comparable
to those of the control group. The does were killed and their fetuses
removed on day 28 of gestation. No gross pathological changes were
observed, and reproductive parameters were not affected by treatment.
The group mean fetal weights were slightly reduced, and this
indication of fetotoxicity was accompanied by reduced ossification of
the carpals and/or tarsals, sternum, and pelvis. There were no
indications of teratogenicity at any dose. No NOAEL for maternal
toxicity was identified (Fuchs, 1993b).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Triforine, moistened with water and left on the shaven skin of
Sprague-Dawley rats for 24 h, was tolerated with no irritation up to a
dose of 10 000 mg/kg bw (Frohberg et al., 1973; Ullmann et al., 1990).
When 0.5 g of moistened triforine (purity, 98.1%) was applied to
shaven skin areas of three male and three female New Zealand whim
rabbits and left on the skin for 4 h under a semi-occlusive bandage,
no erythema or oedema occurred within the 72-h follow-up (Ullmann &
Porricello, 1988a).
No reactions occurred in mucosa or eyes of New Zealand white rabbits
after insertion of 0.1 g triforine into the conjunctival sac (Frohberg
et al., 1973). When triforine (purity, 98.1%) was inserted into the
conjunctival sac of three male and three female New Zealand whim
rabbits, all rabbits showed mild local reactions (reddening, chemosis,
discharge) 1 h after application. The reaction disappeared almost
entirely within 24 h, and only one female still had minor conjunctival
reddening (mean cumulative score, 0.17). No changes were observed in
the eyes with a slit-lamp ophthalmoscope 48 and 72 h after
application. No systemic clinical reactions occurred during the
testing period, and body weight was not affected. The overall
irritation score was 0.83 out of a maximum attainable score of 13
(Ullmann & Porricello, 1988b).
In a Maurer optimization test involving aggravation of testing
conditions by additional injection of Freund's adjuvant in groups of
12 male and 12 female Dunkin-Hartley albino guinea-pigs, triforine
(purity, 99.9%) showed no sensitizing potential after epidermal and
intradermal challenge (Ullmann & Suter, 1984).
(ii) Mode of action
Triforine is regarded as an inhibitor of sterol biosynthesis; in
particular, it inhibits microsomal sterol demethylation, as was shown
by means of gas chromatographic-mass spectrometric measurements of
sterol accumulation in Neurospora crassa and Aspergillus
fumigatus. Because triforine has only weak or no antifungal
properties in vitro, metabolic activation can be assumed (Langcake
et al., 1983).
(iii) Interactions with nitroso compounds
Groups of 25 or 50 male and 25 or 50 female Swiss mice were given
0.05% sodium nitrate in drinking-water, triforine suspended in water
at 300 mg/kg bw by garage twice each week, or a combination of the two
treatments for up to 180 days. Tumour incidences were compared with
those in a control group of 184 males and 117 females. Triforine alone
did not increase the number of tumours; however, the combination
increased the frequencies of lymphomas (including thymoma) and of
epithelial adenomas and carcinomas of the gastrointestinal tract, the
lung, and, in males, the liver. Incubation of triforine with 4%
acidified sodium nitrite solution for 24 h formed dinitrosopiperazine,
which is known to be carcinogenic and was suspected to be the
substance that induced the tumours (Börzsönyi et al., 1978).
(iv) Haemolytic anaemia
The main toxic effect observed in the experiments with triforine was
anaemia, which is a reduction in the concentration of haemoglobin in
the blood below the normal range for the species studied and is
usually accompanied by a reduction in the number of erythrocytes. This
condition occurs when the rate of erythyrocyte production is
outstripped by the rate of erythrocyte destruction. In many cases of
anaemia, there is both a reduced rate of production and an increased
rate of destruction. Nevertheless, because of the greater importance
of one or other of these processes, anaemias are classified as (i)
those involving excessive loss (post-haemorrhagic anaemia) or
destruction (haemolytic anaemias) of erythrocytes and (ii) those
involving failure of production of erythrocytes, diminished production
with bone-marrow hyperplasia being dyserythropoietic anaemia and
diminished production with marrow hypoplasia being hypoplastic or
aplastic anaemia.
Haemolytic anaemia in adult experimental animals can result from an
immune reaction in which the antibody is directed against the chemical
or chemical-plasma protein complex, which can bind to the erythrocyte
cell surface; alternatively, the chemical induces formation of an
antibody which cross-reacts with a normal erythrocytic antigen. In the
first case, haemolysis ceases immediately when exposure to the
chemical ceases; in the second case, evidence of an immune reaction
against erythrocytes may persist for some months after exposure to the
chemical has ceased.
Many chemicals cause haemolysis, e.g. phenylhydrazine, lead, and
arsenic and copper compounds; lead may also interfere with haemoglobin
synthesis. Hypoplastic and aplastic anaemias can be produced by a
number of chemicals, including cytostatic drugs, which generally
depress the bone marrow. In addition, there may be idiosyncratic
reactions to certain drugs, e.g. chloramphenicol, sulfonamides,
phenylbutazone, and other antirheumatic and antithyroid drugs.
Aplastic anaemia can also be induced by some hair dyes and industrial
chemicals, such as benzene.
The effect of triforine is mild, occurs in all or many exposed animals
(depending on the dose), and is characterized by haemosiderin
deposition in several organs, no bone-marrow depression, the entry of
increased numbers of immature cells into peripheral circulation, and
the absence of evidence of immunotoxicty. At least in dogs, there is
actually a (presumably) compensatory increase in erythropoiesis. Of
the three common circumstances in which haemolytic anaemia is induced
by chemicals, two, therefore, are unlikely to be important: an immune
response and increased sensitivity of erythrocytes to oxidative stress
because of glutathione depletion, since glutathione is not
significantly involved in triforine metabolism. The third, oxidative
haemolysis in animals: with normally functioning erythrocytes, would
appear to be the most likely mechanism and one which would probably
cease as soon as exposure ceased. This appears to be the situation in
triforine-exposed rats and dogs. Also, there is no evidence of
methaemoglobin formation or of increased numbers of Heinz
body-containing cells or deformed erythrocytes in the circulation.
3. Observations in humans
Medical reports from three companies that synthesize triforine were
available. Boehringer Ingelheim, Germany, examined 12 workers
repeatedly between 1970 and 1983 and reported no haematological or
blood chemical results considered to be related to exposure to
triforine (Celamerck, 1983a,b). The industrial medical service of E.
Merck Co., Darmstadt, Germany, found no adverse systemic, dermal, or
mucosal effects of exposure to triforine among production workers
during routine examinations performed in conformity with the
requirements of the German Chemical Trade Union (Merck, 1984). No
adverse effects were observed during triforine production at Shell
Agrar GmbH in Spain between 1987 and 1993 (Toubes, 1993).
Comments
A battery of tests for acute toxicity with technical-grade triforine
showed that it is slightly hazardous by both the oral and dermal
routes, with respective LD50 values of > 5000 and > 2000 mg/kg bw.
The LC in rats exposed by inhalation was > 5.1 mg/L. Triforine was
not irritating or sensitizing to the skin of rodents and was minimally
irritating to the eyes of rabbits. WHO has classified triforine as
unlikely to present an acute hazard in normal use (WHO, 1996).
Dietary administration of technical-grade triforine for 4 or 13 weeks
showed that the haematopoietic system is a target, as indicated by
mild haemolytic anaemia with associated secondary effects in the
spleen and liver. Similar results were found in two-year studies of
toxicity in mice, rats, and dogs, in which the haematological changes
were accompanied by increased weights of the spleen and liver. In
addition, in a 105-week dietary study in mice, changes in the large
intestine characterized by thickening, enlargement, inflammation, and
ulceration were observed; in a two-year dietary study in dogs,
increased erythropoiesis and increased haemosiderin deposition were
found in the liver and bone marrow. In a four-week dietary study in
rats at 0, 500, 2500, or 12 500 ppm, there was no NOAEL. In a
four-week study in mice at 0, 200, 1000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 200.mg/kg bw per day. The NOAEL in a 13-week study
in rats at 0, 100, or 500 ppm was 500 ppm, equivalent to 25 mg/kg bw
per day. In a six-month study in rats fed diets giving 0, 25, 120,
620, or 3100 ppm, the NOAEL was 120 ppm, equivalent to 6 mg/kg bw per
day. The two-year dietary studies in rats (0, 200, 2000, or 20 000
ppm), mice (0, 70, 700, or 7000 ppm), and dogs (0, 10, 40, 100, or
1000 ppm) showed NOAELs of 200 ppm, equal to 10 mg/kg bw per day, 70
ppm, equal to 11 mg/kg bw per day, and 100 ppm, equal to 2.4 mg/kg bw
per day, respectively.
The major toxic effect observed in the experiments summarized above
was anaemia. The effect was mild, occurring in all or many of the
exposed animals (depending on the dose); the effects were reversible
in rats and dogs and were characterized by haemosiderin deposition in
several organs, the absence of evidence of bone-marrow depression,
entry of increased numbers of immature cells into the peripheral
circulation, and the absence of effects on organs of the immune
system. In dogs, there was actually an increase in erythropoiesis.
Oxidative haemolysis in animals with normally functioning erythrocytes
would appear to be the most likely mechanism and one that would cease
as soon as exposure ceased. Also, there was no evidence of
methaemoglobin formation or of any increase in Heinz body-containing
cells or deformed erythrocytes in the circulation.
No genotoxic activity was observed in an adequate battery of tests for
mutagenicity and clastogenicity in vitro and in vivo. The Meeting
concluded that triforine is not genotoxic.
The results of the studies critical to derivation of an ADI are shown
below; the list does not include an 81-week study in mice treated
orally, which was considered inadequate for evaluation since
histopathological examination was limited to lesions that were judged
at autopsy to be neoplastic.
No carcinogenic effect was observed in rats given dietary
concentrations of 0, 25, 125,625, or 3120 ppm in one study and 0, 200,
2000, or 20 000 ppm in another. Triforine increased the incidence of
pulmonary tumours in female mice given 7000 ppm; the NOAEL for
carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day. The
Meeting concluded that the murine response involves an unidentified
nongenotoxic mechanism and that the carcinogenic activity seen in mice
is unlikely to be indicative or a human carcinogenic risk at the
expected levels of exposure to triforine.
Triforine at dietary concentrations of 0, 500, 3000, or 20 000 ppm did
not affect reproductive performance in rats over the course of a
two-generation study, the NOAEL being the highest dose tested, 20 000
ppm, equal to 1500 mg/kg bw per day. The NOAEL for parental toxicity
and for the growth and development of the offspring was 500 ppm, equal
to 40 mg/kg bw per day, on the basis of decreased food consumption,
body-weight gain, and F1 pup weight and increased relative spleen
weight at the next highest dose of 3000 ppm.
In a study of developmental toxicity in rabbits given oral doses of 0,
5, 25, or 125 mg/kg bw per day, the maternal NOAEL was 5 mg/kg bw per
day, on the basis of decreased food consumption and body weight at 25
mg/kg bw per day; the NOAEL for developmental toxicity was 125 mg/kg
bw per day. In a later study of rabbits given oral doses of 0, 6, 30,
or 150 mg/kg bw per day, the NOAEL for maternal toxicity was 30 mg/kg
bw per day, on the basis of decreased food consumption and body-weight
gain at 150 mg/kg bw per day; the NOAEL for developmental toxicity was
150 mg/kg bw per day. In two subsequent studies in which triforine was
given orally to rabbits at doses of 0, 250, 500, or 1000 mg/kg bw per
day and 0 or 1000 mg/kg bw per day, maternal and embryotoxicity (as
shown by reduced fetal weight) occurred at 1000 mg/kg bw per day.
Decreased fetal weights and delayed ossification were observed in a
study of developmental toxicity in rabbits at the maternally toxic
dose of 1000 mg/kg bw per day. Thus, developmental toxicity in rabbits
occurred only at doses that were also maternally toxic. A study of
developmental toxicity in rats given triforine orally at doses of 0,
200, 500, or 1000 mg/kg bw per day did not show adverse effects in
either dams or fetuses at doses up to 1000 mg/kg bw per day. The
Meeting concluded that triforine has no specific developmental or
reproductive toxicity.
Monitoring of workers in three manufacturing plants did not reveal any
health effects that might be associated with exposure to triforine.
An ADI of 0-0.02 mg/kg bw was established on the basis of the NOAEL of
2.4 mg/kg bw per day in the two-year study of toxicity in dogs, with a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 70 ppm, equal to 11 mg/kg bw per day (105-week study of
toxicity and carcinogenicity)
Rat: 200 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (parental and
fetal toxicity in a study of reproductive toxicity)
1000 mg/kg bw per day (study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 100 ppm, equal to 2.4 mg/kg bw per day (two-year study
of toxicity)
Estimate of acceptable daily intake
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adams, K., Ransome, S., Anderson, A. & Dawe, I.S. (1993) Chinese
hamster ovary/hprt locus assay: Triforine. Unpublished report from
Huntingdon Research Centre Ltd. (Study No. SLL282/931168). Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Atkinson, C., Perry, C.J. & Hudson, P. (1991 a) Triforine: 13-Week
dietary maximum tolerated dose study in mice. Unpublished report from
Inveresk Research International (Report No. 5875). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Toxicological criteria for estimation of guidance values for dietary and non-dietary exposure to triforine
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral, single dose, rat LD50 > 5000 mg/kg bw
(17 days) Dermal, single dose, rat LD50 > 2000 mg/kg bw
Inhalation (4 h), rat LC50 5.1 mg/L
Dermal, irritation, rabbit Not irritating
Ocural, irritation, rabbit Minimally irritating
Dermal, irritation, rat Not irritating
Dermal, sensitization, guinea-pig Non-sensitizing
Medium-term Dermal, 3 weeks, rat NOAEL = 1100 mg/kg bw per day (highest dose tested)
(1-26 weeks) Oral, 13 weeks, dog NOAEL = 3.6 mg/kg bw per day: haemosiderin deposition
Oral, two-generation, reproductive oxicity, rat NOAEL = 1500 mg/kg bw per day: reproductive toxicity;
NOAEL = 49 mg/kg bw per day: parental and offspring
toxicity
Oral, developmental toxicity, rabbit NOAEL = 5 mg/kg bw per day: maternal toxicity;
NOAEL = 125 mg/kg bw per day: fetal and developmental
toxicity
Long-term Oral, 2 years, dog NOAEL = 2.4 mg/kg bw per day: haematological changes;
(> 1 year) haemosiderin deposition; haematopoiesis
Atkinson, C., Perry, C.J. & Hudson, P .(1991b) Triforine: 13-Week
dietary maximum tolerated dose study in rats. Unpublished report from
Inveresk Research International (Report No. 5879). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Boehringer Sohn (1974a) The pharmacokinetics of the systemic fungicide
triforine (W 524) in the rat. Unpublished report dated 13 October
1974, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Boehringer Sohn (1974b) Excretion and metabolism of triforine (W 524)
in the rat. Unpublished report dated 8 March 1974, Ingelheim, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Börzsönyi, M., Pintér, A., Török, G. & Surján, A. (1978) Formation and
biological effect of N-nitroso compound from piperazine pesticide
triforine. In: Walker, E.A., Castegnaro, M., Griciute, L. & Lyle,
R.E., eds, Environmental Aspects of N-Nitroso Compounds (IARC
Scientific Publications No. 19), Lyon, IARC, pp. 477484
Brooks, T.M. & Wiggins, D.E. (1994) Triforine: In vitro chromosome
studies using cultured Chinese hamster ovary (CHO) cells. Unpublished
report from Shell Research Ltd (Technical Service Report No.
SBTR.93.065). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Bullock, C.H. & Narcisse, J.K. (1973) The acute inhalation toxicity of
Cela W 524 tTechnical. Unpublished report from Chevron, San Francisco,
California, USA. Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Celamerck (1983a) [Occupational medical examination of chemical
workers involved in synthesis operating in the production of
triforine.] Unpublished report dated 22 December 1983, Ingelheim,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in
German).
Celamerck (1983b) [Evaluative position paper on the estimation of risk
potential after application of Saprol on green areas that are to be
used as playing-fields or recreational areas.] (ZN 02092). Unpublished
report dated 27 October 1983, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA (in German).
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci., 8, 193-202.
De Bertoldi, M., Griselli, M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by means of gene conversion in
Saccharomyces cerevisiae and Aspergillus nidulans. Environ.
Mutag., 2, 359-370.
Everett, D.J., Perry, C.J., Hudson, P. & Mulhern, M. (1992) Triforine:
52 Week dietary toxicity study in rats. Unpublished report from
Inveresk Research International Ltd (Report No. 7500). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Fokkema, G.N. (1992) Triforine (SAPROL): A 21 day dermal toxicity
study in rats. Unpublished report from Hazleton UK (Report No.
SBTR.91.011 ). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Frohberg, H., von Eberstein, M. & Weisse, G. (1973) Triforine (W524)
Trial for acute toxicity in rats after oral administration and dermal
application and for primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt (Report No. 4/36/73). Submitted to WHO
by American Cyanamid, Princeton, NJ, USA.
Fuchs, A. (1992) Triforine: Preliminary oral (gavage) embryotoxicity
study in the rat. Unpublished report from Hazleton Deutschland GmbH
(Report No. 1031-121-005). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Fuchs, A. (1993a) Triforine: Oral (gavage) teratogenicity study in the
rat. Unpublished report from Hazleton Deutschland GmbH (Report No
1032-121-006). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Fuchs, A. (1993b) Triforine: Oral (garage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Report No.
1098-121-004). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Goburdhun, R. & Greenough, R.J. (1990) W-524-XX (Triforine), 104 week
oral toxicity study in dogs. Unpublished report from Inveresk Research
International (Report No. 5893). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Greenough, R.J. (1994) Triforine registration standard: Response to
review of 104 week dog study. Unpublished report from Inveresk
Research International Ltd (Report No. 5893). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984a) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 026256). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984b) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 031037). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hawkins, D.R., Wood, S.G., John, B.A., Iqbal, S., Cheng, K.N. &
Kitmitto, A. (1992) 14- C-Triforine: Metabolic fate in Sprague-Dawley
rats. Unpublished report from Huntingdon Research Centre (Report
HRC/SLL 203/920212). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Hazelden, K.P. & Aitken, R. (1992) Triforine: Two generation
reproduction study in rats (supplementary histological report).
Unpublished report from Inveresk Research International Ltd (Report
No. 7874). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Hazelden, K.P. (1994) Triforine registration standard; Response to
review of two-generation reproduction study in rats. Unpublished
report from Inveresk Research International Ltd (Report No. 11125).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Heath, J., Mulhern, M., Perry, C.J. & Henderson, W. (1992a) Triforine:
105 Week dietary carcinogenicity study in mice. Unpublished report
from Inveresk Research International (Report No. 7746). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Hill, K. (197,i) Chronic toxicity tests with the compound W-524-XX in
rats using oral administration, duration 2 years. Unpublished report
from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hofmann, A., Oelrich, I., Sumi, N. & Weisse, G. (1975) W-524
(triforine), 81-weeks carcinogenicity study in mice (substance
administered in the food). Unpublished report from E. Merck,
Darmstadt, Germany. Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Kramer, P.-J. (1985) Triforine techn. T 12756, in vitro assessment for
mutagenic potential in bacteria with and without addition of a
metabolising system. Unpublished report from E. Merck, Darmstadt,
Germany (Study No. T-12 756). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Langcake, P., Kuhn, P.J. & Wade, M. (1983) The mode of action of
systemic fungicides. In: Hudson, D.H. & Roberts, T.R., eds,
Progress in Pesticide Biochemistry and Toxicology, Vol. 3, pp.
1-109.
Leuschner, F. (1972) The effect of W-524, lot 1, on pregnant rats and
their fetuses, following oral administration. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W. (1971
) 13 Weeks oral toxicity study in beagle dogs with W-524. Unpublished
report from Laboratorium für Pharmakologie und Toxikologie, Hamburg;,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W.
(1972) 21-Day toxicity tests with the compounds W-524 in
Sprague-Dawley rats using dermal application. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1990) Triforine dose range finding
reproductive assay in rats. Unpublished report from Inveresk Research
International (Report No. 7073). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1991 ) Triforine, two generation
reproduction study in rats. Unpublished report from Inveresk Research
International (Report No. 7368). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Merck (1984) [Triforine: Medical/occupational health position paper.]
Unpublished report dated 28 March 1984, Darmstadt, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA (in German).
Miltenburger, H.G. (1984) Triforine tech., test report of study LMP
76A (mutations affecting the hypoxanthine-guanine phosphoribosyl
transferase locus in V79 cells: HGPRT-test). Unpublished report from
LMP, Darmstadt, Germany (Study No. LMP 076A). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Miltenburger, H.G. (1985) Triforine tech., test report of study LMP
136 (chromosome aberrations in cells of Chinese hamster cell line
V79). Unpublished report from LMP Darmstadt, Germany (Study No. LMP
136). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Muacevic, G. (1968) Pharmacological investigation, acute oral
toxicity, W-524, male and female albino mice. Unpublished report from
Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by Celamerck
GmbH & Co., Ingelheim/Rhine, Germany.
Muacevic, G. (1969) Pharmacological investigation, acute oral
tolerance in dogs. Unpublished report from Boehringer Sohn, Ingelheim,
Germany. Submitted to WHO by Celamerck GmbH & Co., Ingelheim/Rhine,
Germany.
Müller, W. (1989) Triforine: Oral (gavage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Project No.
460/29). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Müller, W. ( 1991 ) Triforine: Preliminary oral (gavage)
embryotoxicity study in the rabbit. Unpublished report from Hazleton
Deutschland GmbH (Report No. 951-121-003). Submitted to WHO by
American Cyanamid, Princeton, NI, USA.
Niggeschulze, A., Hill, K. & Stötzer, H. (1974) Three generation study
with the fungicide W-524 in rats. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Perry, C.J., Mulhern, M. & Finch, L (1992b) Triforine: 104 Week
dietary carcinogenicity study in rats, incorporating 52 week toxicity
study. Unpublished report from Inveresk Research International (Report
No. 7745). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Proudlock, R.J., Stocker, K.J., Howard, W.R., Anderson, A. & Dawe,
I.S. (1993) Triforine: in vitro DNA repair test using rat hepatocytes.
Unpublished report from Huntingdon Research Centre Ltd (Study Report
No. SLL 281/931152). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Robbins, M.C. (1994) An investigation of the effect of triforine on
rat and mouse hepatic xenobiotic metabolising enzymes. Unpublished
report from BIBRA Toxicology International (Report No. 1356/2/ 2/94).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Röhrborn, G. (1977) [Scientific comment on the mutagenicity of
triforine using the liver microsome test of Ames.] Unpublished report.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in German).
von Sandersleben, J.H., Herbst, M., Weisse, I., Frölke, W., Gutnard,
J. & Stötzer, H. (1974) Chronic toxicity test of the substance
W-524-XX on beagles, oral application, over 104 weeks. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J. & Tiler,
T. (1971a) Testing of the subacute toxicity of the substance W-524 in
rats following oral administration. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1971b) Testing of the subacute toxicity of the
substance W-524 in rats following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke, W., Guénard, J.
& von Sandersleben, J. (1971c) Testing of the subacute toxicity of the
substance W-524 in dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1972) Chronic toxicity studies with the compound
W-524-XX in rats using oral administration; duration 26 weeks.
Unpublished report from Boehringer Seth, Ingelheim, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Toubes, G.P. (1993) Medical certificate, Malgrat de Mer. Unpublished
report. Note of 6.4.1993 from Dr Inchaurrondo to Dr Dammermann, Shell
Agrar GmbH. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1988d) 4-Week toxicity
(feeding) study with triforine techn, in the mouse. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
097751). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1989) 4-Week toxicity
(feeding) study with triforine technical in the rat. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 097740). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Ullmann, L. & Porricello, T. (1988a) Primary skin irritation study
with triforine technical in rabbits (4-hour semi-occlusive
application). Unpublished report from Research & Consulting Co.,
Itingen, Switzerland (Project No. 213300). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Ullmann, L. & Porricello, T. (1988b) Primary eye irritation study with
triforine technical in rabbits. Unpublished report from Research &
Consulting Co., Itingen, Switzerland (Project No. 213311). Submitted
to WHO by American Cyanamid, Princeton, NJ. USA.
Ullmann, L. & Surer, B. (1984) Delayed contact hypersensitivity to
triforine technical in albino guinea pigs, the Maurer optimization
test. Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No, 33276). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Pappritz, G. (1986a) Acute oral
toxicity study with triforine technical in rats. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
076151). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L. Sacher, R., Mohler, H. & Vogel, O. (1986b) 4-Hour acute
inhalation toxicity study with triforine technical in rats.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 076162). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Vogel, O. (1986c) Acute oral
toxicity study with triforine technical in mice. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
077433). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L., Althaus, P., Janiak, T. & Vogel, D. (1990) Acute dermal
toxicity study with triforine techn. (SAG 102) in rats. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 33276, 278774). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
2. Toxicological studies
(a) Acute toxicity
The acute toxicity of triforine has been tested by administration by
various routes in mice, rats, and dogs (Table 2). The only reported
observations were reduced activity, swaying, or dyspnoea. There were
no deaths, and no substance-induced changes in organs were detected
post mortem.
(b) Short-term toxicity
Mice
Four groups of five male and five female NMRI mice were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 200, 1000, or 5000 ppm for four weeks, equal to
0, 39, 200, or 980 mg/kg bw per day for males and 0, 45, 240, or 1300
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption; the
apparently increased food consumption by females at 5000 ppm was
possibly due to greater spillage. At the end of the study, males at
5000 ppm had 8% less body-weight gain than controls. Haematological
changes included decreased erythrocyte count (by 8-11%, p < 0.05),
haemoglobin concentration (by 7-9%, p < 0.05), and packed cell volume
(by 6-8% p < 0.05) and increased polychromatic erythrocytes in
animals of each sex at 5000 ppm. Males at this dose also had
moderately increased reticulocyte counts and decreased leukocyte
counts. A significant decrease in mean erythrocyte count was also
observed in males at 1000 ppm, although most of the values fell within
the range for the concurrent control group. Males and females at 5000
ppm showed increased absolute and relative weights of the spleen, and
females had increased relative liver weights (by about 16%,
p < 0.01). There were no gross or microscopic changes. The NOAEL
was 1000 ppm, equal to 196 mg/kg bw per day, on the basis of
haematological changes in animals of each sex, slightly reduced
body-weight gain in males, and increased relative liver weight in
females at 5000 ppm (Tenneker et al., 1988).
Groups of 10 CD-1 mice of each sex were given technical-grade
triforine (purity, 99.1%) in the diet at concentrations of 0 or 7000
ppm for 13 weeks, equal to 1000-1600 mg/kg bw per day in males and
1900-2500 mg/kg bw per day in females. No deaths or clinical signs of
toxicity were recorded, and there were no effects on food consumption
or body-weight gain. The haematological changes included a slight
reduction in erythrocyte numbers, haemoglobin concentration, and
haematocrit in animals of each sex. The absolute weights of the spleen
were increased by 38% in males and 56% in females, and that of the
liver was increased by 17% in males; the relative liver and spleen
weights were increased in animals of each sex ( p < 0.01). There
were no gross pathological findings; tissues were not examined
microscopically, as the study was designed to select concentrations
for use in a long-term study of toxicity and carcinogenicity (Atkinson
et al., 1991a).
Table 2 Acute toxicity of triforine
Species, strain Route of Sex LD50L/C50 Length of Reference
administration (mg/kg bw observation
or mg/L) (days)
Rat, Wistar Oral F, M > 16 000 14 Frohberg et al. (1973)
Rat, Wistar Oral F, M > 5 000 15 Ullman et al. (1986a)
Rat, Wistar Dermal F, M > 10 000 14 Frohberg et al. (1973)
Rat, Wistar Dermal F, M > 2 000 15 Ulman et al. (1990)
Rat, Sprague-Dawley Inhalation (1 h) F, M > 4.5 14 Bullock & Narcisse (1973)
Rat, Wistar Inhalation (4 h) F, M > 5.1 15 Ullman et al. (1986b)
Mouse (strain not stated) Oral F, M > 6 000 7 Muacevic (1968)
Mouse, NMRI Oral F, M > 5 000 15 Ullman et al. (1986c)
Dog (breed not stated) Oral F, M > 2 000 28 Muacevic (1969)
Rats
Groups of five male and five female Wistar rats were given
technical-grade triforine (purity, 98.1%) in the diet at
concentrations of 0, 500, 2500, or 12 500 ppm for four weeks, equal to
0, 50, 240, or 1200 mg/kg bw per day for males and 0, 49, 230, or 1200
mg/kg bw per day for females. No deaths or clinical signs of toxicity
were recorded, and there were no effects on food consumption.
Body-weight gain was reduced by about 15% in males by the end of the
study ( p < 0.05), whereas them were no consistent, treatment-
related effects in females. Haematological changes indicative of
slight anaemia were observed. In rats at 12 500 ppm, slight decreases
in mean cell haemoglobin concentration ( p < 0.01) and mean cell
volume were seen in females ( p < 0.05) and increases in
reticulocyte ( p < 0.01) and polychromatic erythrocyte counts in
animals of each sex. Increased proportions of circulating immature
cells were also seen in rats at 2500 ppm, and the increase was
significant in males ( p < 0.01). Prothrombin time was decreased in
females at 12 500 ppm. At this dose, a slight increase in total
protein (by about 6%, p < 0.05) was seen in animals of each sex and
a slight increase in cholesterol content (57%, p < 0.01) in
females. Urine volume was increased in females at this dose. Changes
in organ weight were restricted to rats at 12 500 ppm. Absolute spleen
weights were increased in animals of each sex ( p < 0.05), as were
the absolute weights of the liver, thyroid, and kidney in females.
Increased relative weights were observed for liver ( p < 0.01) and
spleen ( p < 0.05) in animals of each sex and for thyroid in males
( p < 0.05). There were no treatment-related gross pathological
findings; the treatment-related microscopic changes were slight or
moderate haemosiderin deposition in the spleens of males and females
at 12 500 ppm and females at 2500 ppm; two females at 500 ppm also had
increased haemosiderin deposition. No NOAEL was identified (Tenneker
et al., 1989).
Groups of 15 male and 15 female Wistar FW-49 rats (25 at the high
dose) were given triforine (purity not stated) in the diet at
concentrations of 0, 2500, 7000, or 20 000 ppm for 13 weeks. Ten rats
of each sex at 20 000 ppm were observed for six weeks after the end of
treatment. Haematological, clinical chemical, and extended
histopathological investigations were performed on 10 rats of each sex
at each dose before treatment and after 6, 13, and 19 weeks.
Reversible reductions in erythrocyte count and in haemoglobin and
haematocrit values were seen in all treated groups. These results were
interpreted as a sign of a slight haemolytic anaemia and correlated
with haemosiderin deposition in liver, spleen, kidney, lung, and
heart, which increased in proportion to the dose administered. No
NOAEL was identified (Stötzer et al., 1971a).
As there was no no-effect level in the previous study, another study
was performed in which groups of 25 male and 25 female Wistar FW-49
rats were given triforine (purity not stated) in the diet at
concentrations of 0, 100, or 500 ppm for 13 weeks, equivalent to 5 or
25 mg/kg bw per day. No treatment-related effects were observed. The
NOAEL was 500 ppm, equivalent to 25 mg/kg bw per day, as no effects
were observed at 500 ppm, the highest dose tested (Stttzer et al.,
1971b).
Groups of 10 male and 10 female Sprague-Dawley rats were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0 or 20 000 ppm for 13 weeks, equal to 1300 mg/kg bw
per day. No deaths or clinical signs of toxicity were recorded, and
there were no effects on food consumption or body-weight gain. The
haematological changes included statistically significant but slight
reductions in mean cell haemoglobin concentration, by 1.4% in males
and 2.2% in females, and reduced erythrocyte numbers (by 4-9%) and
increased mean cell volume (by 3.5%) in females. Increases in the
absolute ( p < 0.05) and relative ( p < 0.01) weights of the liver
and spleen were observed in animals of each sex. There were no gross
pathological findings; the tissues were not examined microscopically
(Atkinson et al., 1991b).
Groups of 10 male and 10 female FW-49 rats were given triforine
(purity not stated) in the diet at concentrations of 0, 25, 120, 620,
or 3100 ppm for six months, equivalent to 0, 1.3, 6, 31, or 160 mg/kg
bw per day. Reductions in erythrocyte and haematocrit values and
increases in reticulocyte numbers were observed in rats at 620 and
3100 ppm. No consistent, dose-related variations were found in serum
chemical or urinary parameters. Post-mortem examination did not reveal
any treatment-related changes, but increased liver weights were found
in females at all doses, the relative weights being increased by 27%
in those at 25 ppm, 19% at 120 ppm, 40% at 620 ppm, and 49% at 3100
ppm (no statistical analysis reported). No such increases were
observed in males. Increased haemosiderin deposition was found in the
livers and spleens of male rats at 620 and 3100 ppm and females at
3100 ppm. No treatment-related histopathological changes were seen in
the livers of either male or female rats at 25 or 120 ppm. The NOAEL
was 120 ppm, equivalent to 6 mg/kg bw per day, on the basis of reduced
erythrocyte numbers and packed cell volume at 620 ppm (Stötzer et al.,
1972).
Groups of 10 male and 10 female Sprague-Dawley rats were given topical
applications on shaved intact, occluded skin of a 0, 0.5, or 1.5%
aqueous dilution of a 20% triforine emulsion, equivalent to 0, 10, or
30 mg/kg bw triforine, for 4 h per day daily for 21 days. Five males
and five females per group were followed up for an additional 21 days.
Temporary slight reddening and swelling occurred in the covered skin
areas in all groups, including the controls. No local or systemic
substance-related reactions were observed (Leuschner et al., 1972).
Groups of seven Fischer 344 rats of each sex received technical-grade
triforine in corn oil (3 ml/kg bw) on a shaven area of the back at
doses of 0, 110, 350, or 1100 mg/kg bw per day on five days per week
for three weeks. After each application, the treated area was covered
with gauze and bandage for 6 h, then washed and dried. No deaths or
treatment-related dermal changes were observed, and there were no
effects on food consumption or body-weight gain attributable to
treatment. All animals, including the controls, lost some weight,
particularly during the first week; this response was attributed to
the bandaging procedure. No significant, treatment-related variations
in organ weights or haematological end-points was seen, and them were
no gross or microscopic pathological findings. Female rats at 350 or
1100 mg/kg bw showed statistically significant increases in serum
cholesterol (13% and 22%, respectively), triglycerides (32 and 40%,
respectively), and bilimbin (27 and 13%, respectively); a 25% decrease
in serum alkaline phosphatase activity was seen in females at 110
mg/kg bw. In males, serum alkaline phosphatase activity was reduced by
15% at 350 mg/kg bw and by 21% at 1 100 mg/kg bw; in rats at 1100
mg/kg bw, total serum protein was increased by 5.7% and serum albumin
by 3.8%. Since the changes in bilirubin, cholesterol, and triglyceride
contents were not accompanied by histopathological changes in the
liver and were confined to a single sex, their biological significance
is unclear. The increases in alkaline phosphatase activity may reflect
a toxicological effect, but the decreases observed in this study are
not usually considered to be toxicologically significant. The NOAEL
was 1100 mg/kg bw per day, the highest dose tested, as no toxicity was
observed (Fokkema, 1992).
Dogs
Groups of four male and four female beagle dogs were given triforine
(purity not stated) in the diet at concentrations of 0, 3500, 10 000,
or 30 000 ppm (two groups) for 13 weeks. The supplementary group at 30
000 ppm was observed for an additional six weeks without treatment.
These concentrations were equal to 0, 83, 230, 690, or 710 mg/kg bw
per day for males and 0, 85,240,730, or 720 mg/kg bw per day for
females. Signs of haemolytic anaemia-reduced erythrocyte counts
( p < 0.05) and haemoglobin concentration ( p < 0.05), and,
occasionally, also in haemocrit--were observed at all doses by week
13, but were first observed in week 6 in dogs at 10 000 or 30 000 ppm
( p < 0.01). These effects were accompanied by a consistent increase
in the number of reticulocytes in dogs at 10 000 and 30 000 ppm. All
of the values returned to normal in the group at 30 000 ppm within
three weeks of observation. Serum chemistry in weeks 6 and 13 showed
slight increases in alkaline phosphatase activity and bilirubin and
cholersterol concentrations in all treated groups, but these values
also returned to normal in dogs at 30 000 ppm within three weeks.
Urinalysis and ophthalmoscopy showed no differences between the
groups. Fine, drop-like fatty infiltration of the myocardial fibres
were seen in 0, 5, 1, 5, and 1 dogs in the five groups, respectively,
and fatty accumulation in the liver in 0, 3, 0, 4, and 0 dogs. The
fatty infiltration appeared to be reversible, since it was no longer
detected in the dogs observed for six weeks. Treatment-related
siderosis in Kupffer cells in the liver showed a clear tendency
towards reversibility in the animals allowed to recover. There was no
NOAEL (Stötzer et al., 1971c).
Groups of four beagle dogs of each sex were given triforine in the
diet at concentrations of 0, 100,600, or 3500 ppm for 13 weeks, equal
to 0, 3.6, 22, or 120 mg/kg bw per day for males and females together.
The haematological findings in the dogs at 3500 ppm in the previous
study were essentially confirmed. Furthermore, siderosis was detected
in the spleen, liver, and bone marrow after administration of 600 ppm,
and the weight of the spleen was increased in the dogs at 3500 ppm.
The NOAEL was 100 ppm, equal to 3.6 mg/kg, on the basis of
haemosiderin deposits in the liver and bone marrow at 600 ppm
(Leuschner et al., 1971).
Groups of four male and four female beagle dogs were given
technical-grade triforine (purity not stated) in the diet at
concentrations of 0, 10, 40, 100, or 1000 ppm for two years, equal to
0, 0.23, 0.93, 2.4, or 22 mg/kg bw per day for males and 0, 0.25,
0.99, 2.6, or 24 mg/kg bw per day for females. Samples were taken for
haematology, blood chemistry, and urinalysis during weeks 6, 13, 26,
52, 78, and 104. One male dog at 100 ppm group died of acute
pneumonia. There were no other deaths, and there were no
treatment-related signs of toxicity, changes in food consumption,
changes in body-weight gain, or ophthalmoscopic findings.
Haematological changes in the dogs at 1000 ppm group included
increased mean cell volume in males in week 13 (12%, p < 0.01) and
females in week 26 (3.5%, p < 0.05) and decreased mean cell
haemoglobin concentration in males at week t 3 (3.5 %, p < 0.01).
The other changes were either not statistically significant or were
inconsistent with respect to time interval, sex, or dose. Examination
of femoral bone-marrow smears at termination showed a shift in the
granulopoietic:erythropoietic ratio towards erythropoiesis in two
males and three females at 1000 ppm. The erythropoietic mitotic index
was also increased in one female in this group. No treatment-related
changes were observed on blood chemistry or urinalysis. The absolute
and relative organ weights were comparable in all groups, and there
were no treatment-related gross pathological findings.
Microscopically, haemosiderin deposition of moderate severity was
observed in Kupffer cells in four dogs at 1000 ppm and one control.
Haemosiderosis of the bone marrow was also observed in two dogs at
1000 ppm. The NOAEL was 100 ppm, equal to 2.4 mg/kg bw per day, on the
basis of haematological changes, increased erythropoiesis, and
haemosiderin deposition in the liver and bone marrow in animals of
each sex at 1000 ppm (von Sandersleben et al., 1974; Goburdhun &
Greenough, 1990; Greenough, 1994).
(c) Long-term toxicity and carcinogenicity
Mice
Groups of 40 NMRI mice of each sex were given triforine (purity not
stated) in the diet at concentrations of 0, 30, 150, or 750 ppm for 81
weeks, equal to 0, 4.7, 24, or 120 mg/kg bw for males and 0, 5.2, 28,
and 140 mg/kg bw for females. Mortality at 81 weeks was 32% of control
males, 20% of those at 30 ppm, 35% at 150 ppm, and 50% at 750 ppm, and
30% of control females, 40% of those at 30 ppm, 26% at 150 ppm, and
33% at 750 ppm. Survival time, clinical symptoms, body-weight gain,
and food consumption were not affected by treatment. As morphological
findings were described only if they were considered to be relevant to
an evaluation of the carcinogenic effects of the substance, no NOAEL
was identified. Treatment did not increase the total number of
neoplasms (mainly lymphocytic leukaemias and lymphosarcomas), and the
latent periods remained unchanged. The frequencies of specific, less
common tumours in the treated groups were usually not higher than in
the concurrent controls or in the literature (Hofmann et al., 1975).
Four groups of 50 CD-1 mice of each sex were given technical-grade
triforine (purity, 98.9%) in the diet at concentrations of 0, 70, 700,
or 7000 ppm for 105 weeks, equal to 0, 11, 120, or 1200 mg/kg bw per
day for males and 0, 16, 160, or 1600 mg/kg per day for females. Blood
samples were collected for analysis at weeks 52, 77, and 103. The
mortality at 105 weeks was 14% of control males, 19% of those at 70
ppm, 35% at 700 ppm, and 28% at 7000 ppm; for females, 27% of controls
were dead at this time and 27% of those at 70 ppm, 32% at 700 ppm, and
30% at 7000 ppm. The mortality rate in the control group of males was
considered to be unusually low for CD-1 mice at 105 weeks. There were
no treatment-related clinical signs of toxicity or changes in food
consumption. Body-weight gain at the end of the study was reduced by
11% in males at 700 ppm and 16% in those at 7000 ppm. No significant
changes in body-weight gain were seen in females. No haematological
changes were observed. The absolute and relative weights of the liver
were increased by 21% ( p < 0.01) in females at 7000 ppm. At
autopsy, thickening or enlargement of the large intestine was observed
in males that died before the end of the study in the groups receiving
700 ppm (17%) or 7000 ppm (36%), whereas none was seen in the
controls. The large intestine was ulcerated, and inflammation was
observed microscopically, predominantly in male mice that died before
the end of the study after receiving 700 ppm (23%) or 7000 ppm (21%).
The overall occurrence of these findings was none in male controls, 6%
at 70 ppm, 16% at 700 ppm, and 12% at 7000 ppm.
The incidences of hepatocellular adenoma were 9/50 in control males,
12/50 at 70 ppm, 8/50 at 700 ppm, and 8/50 at 7000 ppm; the incidences
of hepatocellular carcinoma were 5/50 in control males, 7/50 at 70
ppm, 8/50 at 700 ppm, and 10/50 at 7000 ppm. There were no significant
differences by either Fisher's exact test or the Cochrane-Armitage
test for trend, for adenomas or carcinomas independently or combined
(Fisher's exact test for comparison of carcinomas in males at 0 and
7000 ppm gave p = 0.263). All of the incidences of liver tumours
were within the ranges found in a compilation of five control groups
of male CD-1 mice in the same laboratory: adenomas, 0-32%; carcinomas,
0-21%. Females had no increase in the incidences of liver tumours. The
incidences of alveolar or bronchiolar adenomas in males were 14/50 in
controls, 13/50 at 70 ppm, 8/50 at 700 ppm, and 17/50 at 7000 ppm, and
the incidences of alveolar or bronchiolar carcinomas were 5/50 in
controls, 2/50 at 70 ppm, 4/50 at 700 ppm, and 7/50 at 7000 ppm. These
differences were not statistically significant. The incidences of
alveolar or bronchiolar adenomas in females were 5/50 in controls,
6/50 at 70 ppm, 7/50 at 700 ppm, and 21/50 at 7000 ppm (Fisher's exact
test, p < 0.001), and the incidences of alveolar or bronchiolar
carcinomas were 1/50 in controls, 2/50 at 70 ppm, 1/50 at 700 ppm, and
6/49 at 7000 ppm (Fisher's exact test, p = 0.059). The incidences of
lung tumours were beyond the ranges observed in five other control
groups of female CD- 1 mice at this laboratory: adenomas, 4-18%;
carcinomas, 0-8% The Meeting concluded that triforine increased the
incidence of alveolar or bronchiolar adenomas fit female mice. The
NOAEL for carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day,
on the basis of an increased incidence of adenomas and/or carcinomas
in females at 7000 ppm. The NOAEL for toxicity was 70 ppm, equal to 11
mg/kg bw per day, on the basis of a slight decrease in body-weight
gain and changes in the large intestine of males fed 700 ppm (Heath et
al., 1992).
Rats
Groups of 35 Wistar (CHBB: THOM-SPF) rats of each sex, with 50 of each
sex in the group of controls and that at the high dose, were given
triforine (purity, 96.6%) in the diet at concentrations of 0, 25,
125,625, or 3120 ppm for two years, equivalent to 0, 1.3, 6.3, 31, or
160 mg/kg per day. Samples were taken for haematological and blood
chemical investigations during weeks 0, 6, 13, 26, 52, 78, and 104
from 20 male and female rats per group. Urine was analysed before and
at the end of the study in 10 animals of each sex from the control
group and that at the highest dose. All animals were examined post
mortem. Complete histopathological examinations were performed at
the end of the study on 20 rats of each sex from the control group and
that at the highest dose, on 15 rats of each sex from the other
groups, on rats that died before the end of the study, and on all
tumour-bearing rats. Mortality, clinical signs, body-weight
development, and parameters of clinical chemisstry and the urinalyses
were not affected by the treatment. Temporary reductions in the number
of erythrocytes ( p < 0.01 for males) and haematocrit values
( p< 0.01 for males) and increased numbers of mticulocytes (39% in
males, 29% in females) were seen in rots at 3125 ppm during week 6,
the variations being more pronounced among male rats. There were no
significant differences in organ weights. Haemosiderosis was observed
in the spleens of both control and treated rats, and the frequency and
intensity of the condition did not differ significantly between the
groups. The sinuses of the adrenals of females in all groups showed
cavernous dilatation, and some were thrombosed. These changes occurred
more frequently in treated than in control animals, but they are
common findings in Wistar rats. No significant difference in tumour
incidence between the groups was found. The NOAEL was 625 ppm,
equivalent to 31 mg/kg bw per day, on the basis of signs of anaemia at
3120 ppm (Hill, 1974).
Groups of 70 Sprague-Dawley rats of each sex were given
technical-grade triforine (purity, 99.1%) in the diet at
concentrations of 0, 200, 2000, or 20 000 ppm for two years, equal to
an average of 0, 10, 100, or 1000 mg/kg bw per day for males and 0,
13, 140, or 1400 mg/kg bw per day for females. Twenty rats of each sex
per group were killed at one year and the survivors at two years.
Blood and urine samples were collected for analysis at weeks 26
(females) or 28 (males), 52, and 104. Mortality at two years among the
remaining 50 animals in each group was 25% of control males, 24% of
those at 200 ppm, 19% at 2000 ppm, and 19% at 20 000 ppm and 22% of
control females, 20% of those at 200 ppm, 21% of those at 2000 ppm,,
and 23% of those at 20 000 ppm. There were no treatment-related
clinical signs of toxicity and no changes on ophthalmoscopy or
urinalysis. There were no significant differences in food or water
consumption and only slight differences in body weight between the
groups at the end of the study.
A number of findings could be attributed to the treatment with
triforine. At one year, the body weights of females were moderately
but not statistically significantly lower than those of controls, by
11% in those at 200 ppm, 13% at 2000 ppm, and 21% at 20 000 ppm.
Haemoglobin concentrations were 4-6% lower in male rats at 2000 ppm
( p < 0.05) and 20 000 ppm ( p < 0.01 ) at weeks 28 and 51 and in
those at 200 ppm at week 51. Although the changes were consistent in
males, in female rats the haemoglobin concentrations were slightly and
nonsignificantly lower only at 20 000 ppm at week 26. The mean
corpuscular haemoglobin count was 2% lower in both male and female
rats at 20 000 ppm at weeks 28 and 26, respectively, but not at week
51. The values in the control group were, however, clearly higher than
normally expected for rats of this age, sex, and strain in this
laboratory. Changes in blood chemistry in the group at 20 000 ppm
included slightly raised concentrations of sodium in males at week 28,
of calcium in males at week 28 and females at week 104 (by 4%,
p < 0.01 ), of cholesterol in females at week 104 (by 39%,
p < 0.01), and of total protein in males at week 104 (by 9%,
p < 0.01 ), and a slightly lower concentration of glucose in males
at week 52. females at 2000 ppm also had raised calcium levels at week
104. There were no gross pathological findings. Liver weights were
increased at week 52 in males (11%) and females (15%) at 20 000 ppm
and in all females at week 104; both absolute and relative liver
weights were increased in females at week 104, by 18% in those at 200
ppm ( p < 0.05), 24% at 2000 ppm ( p < 0.01), and 43 % at 20 000
ppm ( p < 0.001 ). Although not significant, the relative weights of
the liver were increased in males, by 19% at 200 ppm, 11% at 2000 ppm,
and 18% at 20 000 ppm. The absence of a dose-related response in the
males calls into question the biological significance of the response
in females at the low dose. Significant, dose-related increases in
absolute and relative spleen weights were seen in females at 2000 and
20 000 ppm that were killed at one year. The relative spleen weights
were also increased in females at 20 000 ppm at week 104. The
increased weights of the liver and spleen correlated with
histopathological findings (described below) and are considered to be
related to treatment. Increased kidney weights were observed at week
52, but only in females, by 9% in those at 200 ppm, 12% at 2000 ppm,
and 17% at 20 000 ppm. Adrenal weights were reduced by 13-15% in all
treated males but were increased by 35% in females at 2000 ppm and by
40% in those at 20 000 ppm. Microscopic examination showed increased
deposition of haemosiderin in the spleen of females at 2000 and 20 000
ppm at two years, in males at these doses at one year, and in females
at 20 000 ppm at one year. The incidence of haemosiderin deposition in
Kupffer cells and macrophages of the liver was increased in male and
female rats at 2000 and 20 000 ppm at one year. The severity was also
increased in these groups of males but only in females at 2000 ppm. In
the latter group, there was an increased incidence of pale-cell foci
in males and bile-duct hyperplasia in females. There were no
treatment-related changes in the proportions of tumour-bearing animals
or in the incidence of any particular tumour type. The Meeting
concluded that triforine is not carcinogenic to rats. The NOAEL was
200 ppm, equal to 10 mg/kg bw per day, on the basis of slight
reductions in body-weight gain in males, haematological changes in
animals of each sex, increased absolute and relative weights of the
spleen in females at one year and of the liver at two years, and
haemosiderin deposition in the spleens of males and females at one
year and in the livers of females after one year at 2000 ppm (Everett
et al., 1992; Perry et at., 1992).
(d) Genotoxicity
Triforine has been tested for genetic damaging activity in an adequate
range of assays, most of which were conducted to currently acceptable
standards (see Table 3 for salient features and references). Mutations
were not induced by triforine in either bacteria or at the hprt
locus of cultured mammalian cell lines, and excision repairable DNA
damage was not increased in treated primary cultures of rat
hepatocytes. Gene conversion was not induced in either yeast or fungal
cells. The potential of triforine for inducing chromosomal damage was
tested in mammalian cell lines in vitro by examining treated
metaphase cells for gross aberrations and in vivo by examining
polychromatic erythrocytes taken from the bone marrow of mice treated
orally for an increase in the proportion of micronucleated cells.
There was no indication of activity in vitro. In one test, an
increase in the proportion of micronucleated cells was observed in
groups of five female mice sampled 48 h after dosing (controls: 0.4 ±
0.55/103; treated with 5000 µg/kg bw: 2.4 ± 1.14/103) but not at 24
or 72 h and not in male mice at any sampling time. The increase was
not confirmed when the study was partially repeated with female mice
sampled only 48 h after dosing (controls: 1.6 ± 1.14/103; treated with
5000 µg/kg bw: 1.2 ± 1.10/103). The Meeting concluded that triforine
is not genotoxic.
(e) Reproductive toxicity
(i) Multigeneration reproductive toxicity
Rats
Groups of 10 male and 20 female CHBB-THOM rats were given triforine
(purity not stated) in the diet at concentrations of 0, 100, 500, or
2500 ppm in a three-generation study in which the rats were mated to
produce two litters in each generation. The only sign of toxicity in
parent rats and their offspring was temporarily, slightly reduced
body-weight gain of male rats, particularly at the high dose.
Table 3. Genetic activity of triforine
Test system Test object Concentration/ Purity Results References
dose (%)
In vitro
Reverse mutation S. typhimurium 100 µg/plate NR Negativea Rohrborn (1977)
TA 100, TA1535
Reverse mutation S. typhimurium 5000 µg/plate NR Negativea Moriya et al. (1983)
TA98, TA 100,
TA1535, TA1537,
TA1538, E. coli
WP2hcr
Reverse mutation S. typhimurium 5000 µg/plate 99.9 Negativea Kramer (1985)
TA98, TA 100,
TA1535, TA1537,
TA1538
Gene conversion, Saccharomycess 1000 µg/ml NR Negativea De Bertoldi et al. (1980)
adc2, trp5 loci cerevisiae D4
Gene conversion, Aspergillus 1000 µg/ml NR Negativeb De Bertoldi et al. (1980)
pabaA locus nidulans
Unscheduled Rat hepatocyte 63 µg/ml 99.8 Negativeb Proudlock et al. (1993)
DNA synthesis primary culture
Cell mutation, Chinese hamster 50 µg/ml NR Negativea Miltenburger (1984)
hprt locus V79 cells
Cell mutation, Chinese hamster 200 µg/ml 99.8 Negativea Adams et al. (1993)
hprt locus ovary cells
Table 3. (continued)
Test system Test object Concentration/ Purity Results References
dose (%)
Chromosomal Chinese hamster 400 µg/ml 99.8 Negativea Brooks & Wiggins (1994)
aberration ovary cells (24 and
48 h sampling)
Chromosomal Chinese hamster 50 µg/ml NR Negativea Miltenburger (1985)
aberration V79 cells (7, 18,
and 28 h sampling)
In vivo
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Weakly Guenard (1984a)
induction marrow (24, 48, by gavage positive
and 72 h sampling)
Micronucleus Mouse bone 5000 mg/kg bw 98.8 Negative Guenard (1984b)
induction (females only, by gavage
48 h sampling)
NR, not reported
a With and without metabolic activation
b Without metabolic activation
Reproductive performance, duration of pregnancy, malformation
frequency, and postnatal development were unaffected by treatment.
When the F3b rats were subjected to post-mortem and histopathological
examinations, no treatment-related change was observed. Since this
study has been superseded by one at higher doses, a full description
is not given; however, the dietary concentration of 2500 ppm had no
adverse effect on reproduction (Niggeschulze et al., 1974).
In a range-finding study, groups of 10 male and 10 female
Sprague-Dawley rats were given technical-grade triforine (purity,
99.1%) in the diet at concentrations of 0, 1250, 5000, or 20 000 ppm.
The parental (FA) rats received the diets for 10 weeks before mating
and throughout mating, gestataon, and lactation. These concentrations
were equal to 0, 100, 410, or 1600 mg/kg bw per day for males and 0,
100, 400, and 1600 mg/kg per day for females during the premating and
mating periods; for F0 females, they were equal to 0, 97, 400, or
1600 mg/kg bw per day during gestation and 0, 150, 650, or 2900 mg/kg
bw per day during lactation. All F1 rats were weaned onto the same
diets as their parents and continued on this treatment until they were
six to seven weeks old, when they were killedś The only death occurred
in a male rat in the control group. No signs of toxicity were noted.
Food consumption was slightly reduced in the group at 20 000 ppm,
among males during weeks 1-10 and among females during weeks 1-3.
Body-weight gain deficits at these doses during these periods resulted
in a reduction in body weight of about 11% in males throughout the
experiment (17 weeks), whereas females had recovered by the end of the
premating period. The relative liver weights of F0 males and females
at 5000 and 20 000 ppm were increased, and females at these doses also
had increased absolute weights of both liver and spleen. No gross
pathological changes were found at autopsy of the F0 rats; no
histological examinations were performed. Treatment did not affect
mating performance, fertility, duration of gestation, litter size, or
F1 pup survival, and no clinical signs of toxicity were seen among
the F1 pups. From lactation day 14, the body-weight gain of male and
female pups was reduced by 7% in those at 5000 ppm and 11 and 10%,
respectively, at 20 000 ppm. These reductions persisted after weaning
(day 24) and were present at six weeks in males and five weeks in
females. The overall depression in mean weight gain after weaning was
11% for males and 4% for females at 5000 ppm and 19% for males and 10%
for females at 20 000 ppm. Food consumption after weaning was also
reduced at this dose, by 15% in males and 12% in females. There were
no gross pathological findings at autopsy of the F1 rats. The effects
observed at the dietary concentration of 20 000 ppm triforine were
considered not to contraindicate its use as the high dose in a
two-generation study. The NOAEL for reproductive toxicity was 1000 ppm
(McCay & Hazelden, 1990).
Groups of 29 male and 28 female Sprague-Dawley rats were given
technical-grade triforine (purity, 98.9-99.1%) in the diet at
concentrations of 0, 500, 3000, or 20 000 ppm in a two-generation
study. The parental (F0) rats received the diets for 10 weeks before
mating and throughout mating, gestation, and lactation. At weaning of
the F1 litters, pups were selected to provide the F2 generation
litters and continued on the same diets as had been offered to their
parents. The study was completed after weaning of the F2 rats. The
dietary concentrations were equal to 0, 38, 230, or 1500 mg/kg bw per
day for F0 males and 0, 48, 290, or 1900 mg/kg per day for F0
females during the premating and mating periods. For F0 females,
these concentrations were equal to 0, 41,240, or 1700 mg/kg bw per day
during gestation and 0, 63, 380, or 2600 mg/kg bw per day during
lactation. The dietary concentrations of 0,500, 3000, and 20 000 ppm
were equal to 0, 40, 280, and 2000 mg/kg bw per day for F2 males and
0, 61,360, and 2500 mg/kg, per day for F1 females during the
premating and mating periods. For F1 females, these concentrations
were equal to 0, 40,230, and 1800 mg/kg bw per day during gestation
and 0, 65, 420, and 2900 mg/kg bw per day during lactation. Pups were
not culled during the study.
One F0 male and one F0 female at 500 ppm died during the study, and
one F1 female at 20 000 ppm was killed prematurely because of
dystocia. None of these deaths was related to treatment. No clinical
signs of toxicity related to triforine were noted in either the F0 or
F1 generations There were slight reductions in food consumption among
F0 rats at 20 000 ppm and F0 females at 3000 ppm during the first
week of treatment. A decrease of 9% was observed in the body-weight
gain of F0 females, but not males, at 500 ppm during the premating
period, at the beginning of which there had been a 3% body-weight gain
in these rats. Slight deficits in body-weight gain were seen
throughout the treatment period for F0 males at 20 000 ppm, resulting
in an overall reduction in body-weight gain of 9%. During the
premating period, F0 females show body-weight gain deficits of 19% at
3000 ppm and 20% at 20 000 ppm. Overall weight gain was decreased by
9% in F0 females at 20 000 ppm during gestation, whereas there was no
deficit in these rats during, lactation. Food consumption was reduced
among F1 males and females at 3000 ppm (10%) and 20 000 ppm (11 and
12%, respectively), mostly during the first three weeks after weaning.
Corresponding deficits in body-weight gain were seen during this
period, of 9% in males and females at 3000 ppm and 13% in males and 9%
in females at 20 000 ppm. Between weeks 6 and 23, the deficits in body
weight were 5% for males and 10% for females at 20 000 ppm. There were
no effects on the body-weight gain of F1 females at any dose during
gestation or lactation.
There were no gross pathological findings attributable to treatment.
Kidney weights were significantly higher (5-9%), in F0 males and
females at 3000 and 20 000 ppm and in F1 males at 20 000 ppm than in
controls. Liver weights were significantly higher (11-45% and
increasing with dose) in F0 females, F1 males, and F1 females at
3000 and 20 000 ppm; a marginal increase in liver weight was also seen
in F1 males at 500 ppm (6.4%, p < (0.05). Significant increases
were observed in the weight of the spleen in F1 males at 20 000 ppm
(16%) and F1 females at 3000 (20%) and 20 000 ppm (42%). Triforine
treatment did not affect mating performance, fertility, duration of
gestation, gestation index, postimplantation loss, litter size, or pup
survival in either the F0 or F1 generation.
The clinical signs of toxicity in pups that could be attributed to
treatment included a reduction in the mean weights of F1 and F2 pups
at 20 000 ppm from day 4 of lactation, so that, by day 21, the body
weights were reduced by 17% for F1 males, 18% for F1 females, 20%
for F2 males, and 19% for F2 females. Decreases were also observed
in F1 pups at 3000 ppm on days 14-21 of lactation, by 9% in males and
8% in females.
The liver, kidney, spleen, and thyroid of parental F0 and F1 rats at
all doses and a number of other organs of parental F0 and F1 rats at
0 and 20 000 ppm were examined histologically. Dose-related increases
in haemosiderin deposition in the spleen, accompanied by dose-related
increases in extramedullary haematopoeisis and in spleen weight were
observed. There was also a dose-related increase in the severity of
spontaneous nephropathy in males and nephrocalcinosis in females.
Small increases in kidney weight were observed in this study,
particularly in the F0 generation, but with no change at 500 ppm.
Liver size increased with dose and was accompanied by sporadic
centrilobular hepatocyte hypertrophy. The hepatic changes were
regarded by the authors as an adaptive response to increased metabolic
activity. The thyroids of female rats of both generations at 20 000
ppm had the histological appearance of very active secreting glands,
consisting of numerous very small acini with little or no lumina,
scant stored secretion, and lined by cuboidal or columnar epithelium
with pale, foamy cytoplasm. The severity of these effects did not
appear to be increased in the F1 generation. The NOAEL for
reproductive toxicity was 20 000 ppm, equal to 1500 mg/kg per day, the
highest dose tested. The NOAEL for parental toxicity and growth and
development of the offspring was 500 ppm, equal to 40 mg/kg bw per
day, on the basis of decreased food consumption in F0 females, F1
males, and F1 females, decreased body-weight gain in F0 females, F1
males, and F1 females, decreased F1 pup weight, and increased
relative spleen weight in F1 females at 3000 ppm (McCay & Hazelden,
2992; Hazelden & Aitken, 1992; Hazelden, 1994).
(ii) Developmental toxicity
Rats
Groups of 20 inseminated female Sprague-Dawley rats were given 0, 100,
400, 800, or 1600 mg/kg bw per day triforine (purity not stated)
suspended in 1% carboxymethylcellulose by gavage on days 6-15 of
pregnancy. The rates of resorption and variation were increased in
animals at 1600 mg/kg bw per day. Furthermore, the number of fetuses
was reduced, dur to significantly increased postimplantation loss at
this dose. Because the frequency of variations was also slightly
increased at 800 mg/kg bw per day, the NOAEL for fetotoxicity was 400
mg/kg bw per day. Although toxic effects were observed at 800 and 1600
mg/kg bw per day, there were no signs of a teratogenic effect
(Leuschner, 1972).
In a preliminary study, groups of eight inseminated Sprague-Dawley
rats were given triforine (purity, >97%) by garage at doses of 250,
500, or 1000 mg/kg bw per day on days 6-15 of gestation. There were no
deaths or treatment-related clinical signs of toxicity. Mean body
weight was slightly reduced on gestation days 9-12 in animals at 500
and 1000 mg/kg bw per day, but overall body-weight gain in the four
groups was comparable throughout treatment. No gross pathological
changes were found in fetuses removed on day 20 of gestation. No
reproductive parameters were affected by treatment, and them were no
indications of treatment-related embryotoxicity or teratogenicity
(Fuchs, 1992).
Groups of 30 inseminated Sprague-Dawley rats were given triforine
(purity not stated) by garage at doses of 200, 500, or 1000 mg/kg bw
per day on days 6-15 of gestation. There were no deaths or
treatment-related clinical signs of toxicity. Food consumption was
reduced in animals at 1000 mg/kg bw per day, but this was not
accompanied by a significant decrease in body-weight gain. No gross
pathological changes were seen in fetuses removed on day 20 of
gestation. No reproductive parameters were affected by treatment, and
there were no indications of treatment-related embryotoxicity or
teratogenicity. The NOAEL for maternal and developmental toxicity was
1000 mg/kg bw per day (Fuchs, 1993a).
Rabbits
Groups of 15 inseminated Himalayan rabbits were given technical-grade
triforine (purity not stated) in 0.5% carboxymethylcellulose by gavage
on days 6-18 of gestation at doses of 0, 5, 25, or 125 mg/kg bw per
day. Them were no deaths or treatment-related clinical signs of
toxicity. Dose-related decreases in food consumption were observed in
rabbits at 25 and 125 mg/kg bw per day on days 7-14 of treatment. Food
consumption was also reduced in the animals at 5 mg/kg bw per day, but
this had no effect on body-weight gain and was considered to be
toxicologically insignificant. For most of the treatment period, body
weight was reduced in rabbits at 25 and 125 mg/kg bw per day, as a
result of reductions during gestation days 6-9. Towards the end of
treatment, these groups regained weight. The does were killed and
their fetuses removed on day 29 of gestation. No gross pathological
findings were observed. No reproductive parameters were affected by
treatment, and them were no indications of treatment-related
embryotoxicity or fetotoxicity at any dose. The NOAEL for maternal
toxicity was 5 mg/kg bw per day, on the basis of decreased food
consumption and body weight at 25 mg/kg bw per day. The NOAEL for
fetal effects was 125 mg/kg bw per day, the highest dose tested
(Gleich et al., 1981).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in 0.5%
carboxymethylcellulose by garage on days 6-18 of gestation at doses of
0, 6, 30, or 150 mg/kg bw per day. There were no deaths or
treatment-related clinical signs of toxicity. Decreased food
consumption were observed in does at 150 mg/kg bw per day on days
12-18, although no statistically significant reduction in body-weight
gain was observed. The does were killed and their fetuses removed on
day 28 of gestation. No gross pathological findings were observed. No
reproductive parameters were affected by treatment, and there were no
indications of treatment-related embryotoxicity or fetotoxicity at any
dose. The NOAEL for maternal toxicity was 30 mg/kg bw per day, on the
basis of decreased food consumption at 150 mg/kg bw per day. The NOAEL
for fetal effects was 150 mg/kg bw per day, the highest dose tested
(Müller, 1989).
Since maternal effects were minimal in the previous study, triforine
was tested at higher doses. In a preliminary study, four groups of
eight inseminated New Zealand rabbits were given technical-grade
triforine (purity not stated) in distilled water by gavage on days
6-18 of gestation at doses of 0, 250, 500, or 1000 mg/kg bw per day.
There were no deaths or treatment-related clinical signs of toxicity.
Body-weight gain was reduced in all treated groups, mainly due to
body-weight losses on days 6-9 of gestation; the reduction (64%) was
statistically significant in does at 1000 mg/kg bw per day. At the end
of treatment, the body-weight gain in the treated groups exceeded that
of the controls. When the does were killed and their fetuses removed
on day 28 of gestation, no gross pathological changes were found, and
reproductive parameters were not affected. The group mean fetal
weights were slightly reduced at 500 and 1000 mg/kg bw per day. There
were no indications of teratogenicity at any dose (Müller, 1991).
Groups of 18 inseminated New Zealand rabbits were given
technical-grade triforine (purity not stated) in distilled water by
gavage on days 6-18 of gestation at doses of 0 or 1000 mg/kg bw per
day. There were no deaths or treatment-related clinical signs of
toxicity. Reductions in food consumption and body-weight gain were
observed in does at 1000 mg/kg bw per day, mainly due to body-weight
losses on days 6-9 of gestation. At the end of treatment, food
consumption and body-weight gain in the treated group were comparable
to those of the control group. The does were killed and their fetuses
removed on day 28 of gestation. No gross pathological changes were
observed, and reproductive parameters were not affected by treatment.
The group mean fetal weights were slightly reduced, and this
indication of fetotoxicity was accompanied by reduced ossification of
the carpals and/or tarsals, sternum, and pelvis. There were no
indications of teratogenicity at any dose. No NOAEL for maternal
toxicity was identified (Fuchs, 1993b).
(f) Special studies
(i) Dermal and ocular irritation and dermal sensitization
Triforine, moistened with water and left on the shaven skin of
Sprague-Dawley rats for 24 h, was tolerated with no irritation up to a
dose of 10 000 mg/kg bw (Frohberg et al., 1973; Ullmann et al., 1990).
When 0.5 g of moistened triforine (purity, 98.1%) was applied to
shaven skin areas of three male and three female New Zealand whim
rabbits and left on the skin for 4 h under a semi-occlusive bandage,
no erythema or oedema occurred within the 72-h follow-up (Ullmann &
Porricello, 1988a).
No reactions occurred in mucosa or eyes of New Zealand white rabbits
after insertion of 0.1 g triforine into the conjunctival sac (Frohberg
et al., 1973). When triforine (purity, 98.1%) was inserted into the
conjunctival sac of three male and three female New Zealand whim
rabbits, all rabbits showed mild local reactions (reddening, chemosis,
discharge) 1 h after application. The reaction disappeared almost
entirely within 24 h, and only one female still had minor conjunctival
reddening (mean cumulative score, 0.17). No changes were observed in
the eyes with a slit-lamp ophthalmoscope 48 and 72 h after
application. No systemic clinical reactions occurred during the
testing period, and body weight was not affected. The overall
irritation score was 0.83 out of a maximum attainable score of 13
(Ullmann & Porricello, 1988b).
In a Maurer optimization test involving aggravation of testing
conditions by additional injection of Freund's adjuvant in groups of
12 male and 12 female Dunkin-Hartley albino guinea-pigs, triforine
(purity, 99.9%) showed no sensitizing potential after epidermal and
intradermal challenge (Ullmann & Suter, 1984).
(ii) Mode of action
Triforine is regarded as an inhibitor of sterol biosynthesis; in
particular, it inhibits microsomal sterol demethylation, as was shown
by means of gas chromatographic-mass spectrometric measurements of
sterol accumulation in Neurospora crassa and Aspergillus
fumigatus. Because triforine has only weak or no antifungal
properties in vitro, metabolic activation can be assumed (Langcake
et al., 1983).
(iii) Interactions with nitroso compounds
Groups of 25 or 50 male and 25 or 50 female Swiss mice were given
0.05% sodium nitrate in drinking-water, triforine suspended in water
at 300 mg/kg bw by garage twice each week, or a combination of the two
treatments for up to 180 days. Tumour incidences were compared with
those in a control group of 184 males and 117 females. Triforine alone
did not increase the number of tumours; however, the combination
increased the frequencies of lymphomas (including thymoma) and of
epithelial adenomas and carcinomas of the gastrointestinal tract, the
lung, and, in males, the liver. Incubation of triforine with 4%
acidified sodium nitrite solution for 24 h formed dinitrosopiperazine,
which is known to be carcinogenic and was suspected to be the
substance that induced the tumours (Börzsönyi et al., 1978).
(iv) Haemolytic anaemia
The main toxic effect observed in the experiments with triforine was
anaemia, which is a reduction in the concentration of haemoglobin in
the blood below the normal range for the species studied and is
usually accompanied by a reduction in the number of erythrocytes. This
condition occurs when the rate of erythyrocyte production is
outstripped by the rate of erythrocyte destruction. In many cases of
anaemia, there is both a reduced rate of production and an increased
rate of destruction. Nevertheless, because of the greater importance
of one or other of these processes, anaemias are classified as (i)
those involving excessive loss (post-haemorrhagic anaemia) or
destruction (haemolytic anaemias) of erythrocytes and (ii) those
involving failure of production of erythrocytes, diminished production
with bone-marrow hyperplasia being dyserythropoietic anaemia and
diminished production with marrow hypoplasia being hypoplastic or
aplastic anaemia.
Haemolytic anaemia in adult experimental animals can result from an
immune reaction in which the antibody is directed against the chemical
or chemical-plasma protein complex, which can bind to the erythrocyte
cell surface; alternatively, the chemical induces formation of an
antibody which cross-reacts with a normal erythrocytic antigen. In the
first case, haemolysis ceases immediately when exposure to the
chemical ceases; in the second case, evidence of an immune reaction
against erythrocytes may persist for some months after exposure to the
chemical has ceased.
Many chemicals cause haemolysis, e.g. phenylhydrazine, lead, and
arsenic and copper compounds; lead may also interfere with haemoglobin
synthesis. Hypoplastic and aplastic anaemias can be produced by a
number of chemicals, including cytostatic drugs, which generally
depress the bone marrow. In addition, there may be idiosyncratic
reactions to certain drugs, e.g. chloramphenicol, sulfonamides,
phenylbutazone, and other antirheumatic and antithyroid drugs.
Aplastic anaemia can also be induced by some hair dyes and industrial
chemicals, such as benzene.
The effect of triforine is mild, occurs in all or many exposed animals
(depending on the dose), and is characterized by haemosiderin
deposition in several organs, no bone-marrow depression, the entry of
increased numbers of immature cells into peripheral circulation, and
the absence of evidence of immunotoxicty. At least in dogs, there is
actually a (presumably) compensatory increase in erythropoiesis. Of
the three common circumstances in which haemolytic anaemia is induced
by chemicals, two, therefore, are unlikely to be important: an immune
response and increased sensitivity of erythrocytes to oxidative stress
because of glutathione depletion, since glutathione is not
significantly involved in triforine metabolism. The third, oxidative
haemolysis in animals: with normally functioning erythrocytes, would
appear to be the most likely mechanism and one which would probably
cease as soon as exposure ceased. This appears to be the situation in
triforine-exposed rats and dogs. Also, there is no evidence of
methaemoglobin formation or of increased numbers of Heinz
body-containing cells or deformed erythrocytes in the circulation.
3. Observations in humans
Medical reports from three companies that synthesize triforine were
available. Boehringer Ingelheim, Germany, examined 12 workers
repeatedly between 1970 and 1983 and reported no haematological or
blood chemical results considered to be related to exposure to
triforine (Celamerck, 1983a,b). The industrial medical service of E.
Merck Co., Darmstadt, Germany, found no adverse systemic, dermal, or
mucosal effects of exposure to triforine among production workers
during routine examinations performed in conformity with the
requirements of the German Chemical Trade Union (Merck, 1984). No
adverse effects were observed during triforine production at Shell
Agrar GmbH in Spain between 1987 and 1993 (Toubes, 1993).
Comments
A battery of tests for acute toxicity with technical-grade triforine
showed that it is slightly hazardous by both the oral and dermal
routes, with respective LD50 values of > 5000 and > 2000 mg/kg bw.
The LC in rats exposed by inhalation was > 5.1 mg/L. Triforine was
not irritating or sensitizing to the skin of rodents and was minimally
irritating to the eyes of rabbits. WHO has classified triforine as
unlikely to present an acute hazard in normal use (WHO, 1996).
Dietary administration of technical-grade triforine for 4 or 13 weeks
showed that the haematopoietic system is a target, as indicated by
mild haemolytic anaemia with associated secondary effects in the
spleen and liver. Similar results were found in two-year studies of
toxicity in mice, rats, and dogs, in which the haematological changes
were accompanied by increased weights of the spleen and liver. In
addition, in a 105-week dietary study in mice, changes in the large
intestine characterized by thickening, enlargement, inflammation, and
ulceration were observed; in a two-year dietary study in dogs,
increased erythropoiesis and increased haemosiderin deposition were
found in the liver and bone marrow. In a four-week dietary study in
rats at 0, 500, 2500, or 12 500 ppm, there was no NOAEL. In a
four-week study in mice at 0, 200, 1000, or 5000 ppm, the NOAEL was
1000 ppm, equal to 200.mg/kg bw per day. The NOAEL in a 13-week study
in rats at 0, 100, or 500 ppm was 500 ppm, equivalent to 25 mg/kg bw
per day. In a six-month study in rats fed diets giving 0, 25, 120,
620, or 3100 ppm, the NOAEL was 120 ppm, equivalent to 6 mg/kg bw per
day. The two-year dietary studies in rats (0, 200, 2000, or 20 000
ppm), mice (0, 70, 700, or 7000 ppm), and dogs (0, 10, 40, 100, or
1000 ppm) showed NOAELs of 200 ppm, equal to 10 mg/kg bw per day, 70
ppm, equal to 11 mg/kg bw per day, and 100 ppm, equal to 2.4 mg/kg bw
per day, respectively.
The major toxic effect observed in the experiments summarized above
was anaemia. The effect was mild, occurring in all or many of the
exposed animals (depending on the dose); the effects were reversible
in rats and dogs and were characterized by haemosiderin deposition in
several organs, the absence of evidence of bone-marrow depression,
entry of increased numbers of immature cells into the peripheral
circulation, and the absence of effects on organs of the immune
system. In dogs, there was actually an increase in erythropoiesis.
Oxidative haemolysis in animals with normally functioning erythrocytes
would appear to be the most likely mechanism and one that would cease
as soon as exposure ceased. Also, there was no evidence of
methaemoglobin formation or of any increase in Heinz body-containing
cells or deformed erythrocytes in the circulation.
No genotoxic activity was observed in an adequate battery of tests for
mutagenicity and clastogenicity in vitro and in vivo. The Meeting
concluded that triforine is not genotoxic.
The results of the studies critical to derivation of an ADI are shown
below; the list does not include an 81-week study in mice treated
orally, which was considered inadequate for evaluation since
histopathological examination was limited to lesions that were judged
at autopsy to be neoplastic.
No carcinogenic effect was observed in rats given dietary
concentrations of 0, 25, 125,625, or 3120 ppm in one study and 0, 200,
2000, or 20 000 ppm in another. Triforine increased the incidence of
pulmonary tumours in female mice given 7000 ppm; the NOAEL for
carcinogenicity was 700 ppm, equal to 160 mg/kg bw per day. The
Meeting concluded that the murine response involves an unidentified
nongenotoxic mechanism and that the carcinogenic activity seen in mice
is unlikely to be indicative or a human carcinogenic risk at the
expected levels of exposure to triforine.
Triforine at dietary concentrations of 0, 500, 3000, or 20 000 ppm did
not affect reproductive performance in rats over the course of a
two-generation study, the NOAEL being the highest dose tested, 20 000
ppm, equal to 1500 mg/kg bw per day. The NOAEL for parental toxicity
and for the growth and development of the offspring was 500 ppm, equal
to 40 mg/kg bw per day, on the basis of decreased food consumption,
body-weight gain, and F1 pup weight and increased relative spleen
weight at the next highest dose of 3000 ppm.
In a study of developmental toxicity in rabbits given oral doses of 0,
5, 25, or 125 mg/kg bw per day, the maternal NOAEL was 5 mg/kg bw per
day, on the basis of decreased food consumption and body weight at 25
mg/kg bw per day; the NOAEL for developmental toxicity was 125 mg/kg
bw per day. In a later study of rabbits given oral doses of 0, 6, 30,
or 150 mg/kg bw per day, the NOAEL for maternal toxicity was 30 mg/kg
bw per day, on the basis of decreased food consumption and body-weight
gain at 150 mg/kg bw per day; the NOAEL for developmental toxicity was
150 mg/kg bw per day. In two subsequent studies in which triforine was
given orally to rabbits at doses of 0, 250, 500, or 1000 mg/kg bw per
day and 0 or 1000 mg/kg bw per day, maternal and embryotoxicity (as
shown by reduced fetal weight) occurred at 1000 mg/kg bw per day.
Decreased fetal weights and delayed ossification were observed in a
study of developmental toxicity in rabbits at the maternally toxic
dose of 1000 mg/kg bw per day. Thus, developmental toxicity in rabbits
occurred only at doses that were also maternally toxic. A study of
developmental toxicity in rats given triforine orally at doses of 0,
200, 500, or 1000 mg/kg bw per day did not show adverse effects in
either dams or fetuses at doses up to 1000 mg/kg bw per day. The
Meeting concluded that triforine has no specific developmental or
reproductive toxicity.
Monitoring of workers in three manufacturing plants did not reveal any
health effects that might be associated with exposure to triforine.
An ADI of 0-0.02 mg/kg bw was established on the basis of the NOAEL of
2.4 mg/kg bw per day in the two-year study of toxicity in dogs, with a
safety factor of 100.
Toxicological evaluation
Levels that cause no toxic effect
Mouse: 70 ppm, equal to 11 mg/kg bw per day (105-week study of
toxicity and carcinogenicity)
Rat: 200 ppm, equal to 10 mg/kg bw per day (two-year study
of toxicity and carcinogenicity)
500 ppm, equal to 40 mg/kg bw per day (parental and
fetal toxicity in a study of reproductive toxicity)
1000 mg/kg bw per day (study of developmental toxicity)
Rabbit: 5 mg/kg bw per day (maternal toxicity in a study of
developmental toxicity)
Dog: 100 ppm, equal to 2.4 mg/kg bw per day (two-year study
of toxicity)
Estimate of acceptable daily intake
0-0.02 mg/kg bw
Studies that would provide information useful for continued
evaluation of the compound
Further observations in humans
References
Adams, K., Ransome, S., Anderson, A. & Dawe, I.S. (1993) Chinese
hamster ovary/hprt locus assay: Triforine. Unpublished report from
Huntingdon Research Centre Ltd. (Study No. SLL282/931168). Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Atkinson, C., Perry, C.J. & Hudson, P. (1991 a) Triforine: 13-Week
dietary maximum tolerated dose study in mice. Unpublished report from
Inveresk Research International (Report No. 5875). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Toxicological criteria for estimation of guidance values for dietary and non-dietary exposure to triforine
Human exposure Relevant route, study type, species Results, remarks
Short-term Oral, single dose, rat LD50 > 5000 mg/kg bw
(17 days) Dermal, single dose, rat LD50 > 2000 mg/kg bw
Inhalation (4 h), rat LC50 5.1 mg/L
Dermal, irritation, rabbit Not irritating
Ocural, irritation, rabbit Minimally irritating
Dermal, irritation, rat Not irritating
Dermal, sensitization, guinea-pig Non-sensitizing
Medium-term Dermal, 3 weeks, rat NOAEL = 1100 mg/kg bw per day (highest dose tested)
(1-26 weeks) Oral, 13 weeks, dog NOAEL = 3.6 mg/kg bw per day: haemosiderin deposition
Oral, two-generation, reproductive oxicity, rat NOAEL = 1500 mg/kg bw per day: reproductive toxicity;
NOAEL = 49 mg/kg bw per day: parental and offspring
toxicity
Oral, developmental toxicity, rabbit NOAEL = 5 mg/kg bw per day: maternal toxicity;
NOAEL = 125 mg/kg bw per day: fetal and developmental
toxicity
Long-term Oral, 2 years, dog NOAEL = 2.4 mg/kg bw per day: haematological changes;
(> 1 year) haemosiderin deposition; haematopoiesis
Atkinson, C., Perry, C.J. & Hudson, P .(1991b) Triforine: 13-Week
dietary maximum tolerated dose study in rats. Unpublished report from
Inveresk Research International (Report No. 5879). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Boehringer Sohn (1974a) The pharmacokinetics of the systemic fungicide
triforine (W 524) in the rat. Unpublished report dated 13 October
1974, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Boehringer Sohn (1974b) Excretion and metabolism of triforine (W 524)
in the rat. Unpublished report dated 8 March 1974, Ingelheim, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Börzsönyi, M., Pintér, A., Török, G. & Surján, A. (1978) Formation and
biological effect of N-nitroso compound from piperazine pesticide
triforine. In: Walker, E.A., Castegnaro, M., Griciute, L. & Lyle,
R.E., eds, Environmental Aspects of N-Nitroso Compounds (IARC
Scientific Publications No. 19), Lyon, IARC, pp. 477484
Brooks, T.M. & Wiggins, D.E. (1994) Triforine: In vitro chromosome
studies using cultured Chinese hamster ovary (CHO) cells. Unpublished
report from Shell Research Ltd (Technical Service Report No.
SBTR.93.065). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Bullock, C.H. & Narcisse, J.K. (1973) The acute inhalation toxicity of
Cela W 524 tTechnical. Unpublished report from Chevron, San Francisco,
California, USA. Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Celamerck (1983a) [Occupational medical examination of chemical
workers involved in synthesis operating in the production of
triforine.] Unpublished report dated 22 December 1983, Ingelheim,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in
German).
Celamerck (1983b) [Evaluative position paper on the estimation of risk
potential after application of Saprol on green areas that are to be
used as playing-fields or recreational areas.] (ZN 02092). Unpublished
report dated 27 October 1983, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA (in German).
Darda, S. (1977) Absorption, metabolism and excretion of the fungicide
triforine in the rat. Pestic. Sci., 8, 193-202.
De Bertoldi, M., Griselli, M., Giovannetti, M. & Barale, R. (1980)
Mutagenicity of pesticides evaluated by means of gene conversion in
Saccharomyces cerevisiae and Aspergillus nidulans. Environ.
Mutag., 2, 359-370.
Everett, D.J., Perry, C.J., Hudson, P. & Mulhern, M. (1992) Triforine:
52 Week dietary toxicity study in rats. Unpublished report from
Inveresk Research International Ltd (Report No. 7500). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Fokkema, G.N. (1992) Triforine (SAPROL): A 21 day dermal toxicity
study in rats. Unpublished report from Hazleton UK (Report No.
SBTR.91.011 ). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Frohberg, H., von Eberstein, M. & Weisse, G. (1973) Triforine (W524)
Trial for acute toxicity in rats after oral administration and dermal
application and for primary mucosal irritation in rabbits. Unpublished
report from E. Merck, Darmstadt (Report No. 4/36/73). Submitted to WHO
by American Cyanamid, Princeton, NJ, USA.
Fuchs, A. (1992) Triforine: Preliminary oral (gavage) embryotoxicity
study in the rat. Unpublished report from Hazleton Deutschland GmbH
(Report No. 1031-121-005). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Fuchs, A. (1993a) Triforine: Oral (gavage) teratogenicity study in the
rat. Unpublished report from Hazleton Deutschland GmbH (Report No
1032-121-006). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Fuchs, A. (1993b) Triforine: Oral (garage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Report No.
1098-121-004). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Goburdhun, R. & Greenough, R.J. (1990) W-524-XX (Triforine), 104 week
oral toxicity study in dogs. Unpublished report from Inveresk Research
International (Report No. 5893). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Greenough, R.J. (1994) Triforine registration standard: Response to
review of 104 week dog study. Unpublished report from Inveresk
Research International Ltd (Report No. 5893). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984a) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 026256). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Guernard, J. (1984b) Mouse micronucleus assay with triforine.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 031037). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hawkins, D.R., Wood, S.G., John, B.A., Iqbal, S., Cheng, K.N. &
Kitmitto, A. (1992) 14- C-Triforine: Metabolic fate in Sprague-Dawley
rats. Unpublished report from Huntingdon Research Centre (Report
HRC/SLL 203/920212). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Hazelden, K.P. & Aitken, R. (1992) Triforine: Two generation
reproduction study in rats (supplementary histological report).
Unpublished report from Inveresk Research International Ltd (Report
No. 7874). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Hazelden, K.P. (1994) Triforine registration standard; Response to
review of two-generation reproduction study in rats. Unpublished
report from Inveresk Research International Ltd (Report No. 11125).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Heath, J., Mulhern, M., Perry, C.J. & Henderson, W. (1992a) Triforine:
105 Week dietary carcinogenicity study in mice. Unpublished report
from Inveresk Research International (Report No. 7746). Submitted to
WHO by American Cyanamid, Princeton, NJ, USA.
Hill, K. (197,i) Chronic toxicity tests with the compound W-524-XX in
rats using oral administration, duration 2 years. Unpublished report
from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Hofmann, A., Oelrich, I., Sumi, N. & Weisse, G. (1975) W-524
(triforine), 81-weeks carcinogenicity study in mice (substance
administered in the food). Unpublished report from E. Merck,
Darmstadt, Germany. Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Kramer, P.-J. (1985) Triforine techn. T 12756, in vitro assessment for
mutagenic potential in bacteria with and without addition of a
metabolising system. Unpublished report from E. Merck, Darmstadt,
Germany (Study No. T-12 756). Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Langcake, P., Kuhn, P.J. & Wade, M. (1983) The mode of action of
systemic fungicides. In: Hudson, D.H. & Roberts, T.R., eds,
Progress in Pesticide Biochemistry and Toxicology, Vol. 3, pp.
1-109.
Leuschner, F. (1972) The effect of W-524, lot 1, on pregnant rats and
their fetuses, following oral administration. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W. (1971
) 13 Weeks oral toxicity study in beagle dogs with W-524. Unpublished
report from Laboratorium für Pharmakologie und Toxikologie, Hamburg;,
Germany. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Leuschner, F., Leuschner, A., Schwerdtfeser, W. & Dontenwill, W.
(1972) 21-Day toxicity tests with the compounds W-524 in
Sprague-Dawley rats using dermal application. Unpublished report from
Laboratorium für Pharmakologie und Toxikologie, Hamburg, Germany.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1990) Triforine dose range finding
reproductive assay in rats. Unpublished report from Inveresk Research
International (Report No. 7073). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
McCay, C. & Hazelden, K.P. (1991 ) Triforine, two generation
reproduction study in rats. Unpublished report from Inveresk Research
International (Report No. 7368). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Merck (1984) [Triforine: Medical/occupational health position paper.]
Unpublished report dated 28 March 1984, Darmstadt, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA (in German).
Miltenburger, H.G. (1984) Triforine tech., test report of study LMP
76A (mutations affecting the hypoxanthine-guanine phosphoribosyl
transferase locus in V79 cells: HGPRT-test). Unpublished report from
LMP, Darmstadt, Germany (Study No. LMP 076A). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Miltenburger, H.G. (1985) Triforine tech., test report of study LMP
136 (chromosome aberrations in cells of Chinese hamster cell line
V79). Unpublished report from LMP Darmstadt, Germany (Study No. LMP
136). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Moriya, M., Ohta, T., Watanabe, K., Miyazawa, T., Kato, K. & Shirasu,
Y. (1983) Further mutagenicity studies on pesticides in bacterial
reversion assay systems. Mutat. Res., 116, 185-216.
Muacevic, G. (1968) Pharmacological investigation, acute oral
toxicity, W-524, male and female albino mice. Unpublished report from
Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by Celamerck
GmbH & Co., Ingelheim/Rhine, Germany.
Muacevic, G. (1969) Pharmacological investigation, acute oral
tolerance in dogs. Unpublished report from Boehringer Sohn, Ingelheim,
Germany. Submitted to WHO by Celamerck GmbH & Co., Ingelheim/Rhine,
Germany.
Müller, W. (1989) Triforine: Oral (gavage) teratogenicity study in the
rabbit. Unpublished report from Hazleton Deutschland GmbH (Project No.
460/29). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Müller, W. ( 1991 ) Triforine: Preliminary oral (gavage)
embryotoxicity study in the rabbit. Unpublished report from Hazleton
Deutschland GmbH (Report No. 951-121-003). Submitted to WHO by
American Cyanamid, Princeton, NI, USA.
Niggeschulze, A., Hill, K. & Stötzer, H. (1974) Three generation study
with the fungicide W-524 in rats. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Perry, C.J., Mulhern, M. & Finch, L (1992b) Triforine: 104 Week
dietary carcinogenicity study in rats, incorporating 52 week toxicity
study. Unpublished report from Inveresk Research International (Report
No. 7745). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Proudlock, R.J., Stocker, K.J., Howard, W.R., Anderson, A. & Dawe,
I.S. (1993) Triforine: in vitro DNA repair test using rat hepatocytes.
Unpublished report from Huntingdon Research Centre Ltd (Study Report
No. SLL 281/931152). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
Robbins, M.C. (1994) An investigation of the effect of triforine on
rat and mouse hepatic xenobiotic metabolising enzymes. Unpublished
report from BIBRA Toxicology International (Report No. 1356/2/ 2/94).
Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Röhrborn, G. (1977) [Scientific comment on the mutagenicity of
triforine using the liver microsome test of Ames.] Unpublished report.
Submitted to WHO by American Cyanamid, Princeton, NJ, USA (in German).
von Sandersleben, J.H., Herbst, M., Weisse, I., Frölke, W., Gutnard,
J. & Stötzer, H. (1974) Chronic toxicity test of the substance
W-524-XX on beagles, oral application, over 104 weeks. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Guénard, J. & Tiler,
T. (1971a) Testing of the subacute toxicity of the substance W-524 in
rats following oral administration. Unpublished report from Boehringer
Sohn, Ingelheim, Germany. Submitted to WHO by American Cyanamid,
Princeton, NJ, USA.
Stötzer, H., Herbst, M., Köllmer, H., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1971b) Testing of the subacute toxicity of the
substance W-524 in rats following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Herbst, M., Ganz, H., Weisse, I., Frölke, W., Guénard, J.
& von Sandersleben, J. (1971c) Testing of the subacute toxicity of the
substance W-524 in dogs following oral administration. Unpublished
report from Boehringer Sohn, Ingelheim, Germany. Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Stötzer, H., Köllmer, H., Herbst, M., Weisse, I., Frölke, W., Guénard,
J. & Tilov, T. (1972) Chronic toxicity studies with the compound
W-524-XX in rats using oral administration; duration 26 weeks.
Unpublished report from Boehringer Seth, Ingelheim, Germany. Submitted
to WHO by American Cyanamid, Princeton, NJ, USA.
Toubes, G.P. (1993) Medical certificate, Malgrat de Mer. Unpublished
report. Note of 6.4.1993 from Dr Inchaurrondo to Dr Dammermann, Shell
Agrar GmbH. Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1988d) 4-Week toxicity
(feeding) study with triforine techn, in the mouse. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
097751). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Tenneker, H., Stucki, H.P., Luetkemeier, H., Vogel, O., Terrier, C.,
Pappritz, G., Mladenovic, P. & Janiak, T. (1989) 4-Week toxicity
(feeding) study with triforine technical in the rat. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 097740). Submitted to WHO by American Cyanamid, Princeton, NJ,
USA.
Ullmann, L. & Porricello, T. (1988a) Primary skin irritation study
with triforine technical in rabbits (4-hour semi-occlusive
application). Unpublished report from Research & Consulting Co.,
Itingen, Switzerland (Project No. 213300). Submitted to WHO by
American Cyanamid, Princeton, NJ, USA.
Ullmann, L. & Porricello, T. (1988b) Primary eye irritation study with
triforine technical in rabbits. Unpublished report from Research &
Consulting Co., Itingen, Switzerland (Project No. 213311). Submitted
to WHO by American Cyanamid, Princeton, NJ. USA.
Ullmann, L. & Surer, B. (1984) Delayed contact hypersensitivity to
triforine technical in albino guinea pigs, the Maurer optimization
test. Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No, 33276). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Pappritz, G. (1986a) Acute oral
toxicity study with triforine technical in rats. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
076151). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L. Sacher, R., Mohler, H. & Vogel, O. (1986b) 4-Hour acute
inhalation toxicity study with triforine technical in rats.
Unpublished report from Research & Consulting Co., Itingen,
Switzerland (Project No. 076162). Submitted to WHO by American
Cyanamid, Princeton, NJ, USA.
Ullmann, L., Sacher, R., Mohler, H. & Vogel, O. (1986c) Acute oral
toxicity study with triforine technical in mice. Unpublished report
from Research & Consulting Co., Itingen, Switzerland (Project No.
077433). Submitted to WHO by American Cyanamid, Princeton, NJ, USA.
Ullmann, L., Althaus, P., Janiak, T. & Vogel, D. (1990) Acute dermal
toxicity study with triforine techn. (SAG 102) in rats. Unpublished
report from Research & Consulting Co., Itingen, Switzerland (Project
No. 33276, 278774). Submitted to WHO by American Cyanamid, Princeton,
NJ, USA.
WHO (1996) The WHO Recommended Classification of Pesticides by
Hazard and Guidelines to Classification 1996-1997 (WHO/PCS/96.3),
Geneva, International Programme on Chemical Safety.
