
CARBOPHENOTHION JMPR 1977
Explanation
Carbophenothion was reviewed at the Joint Meeting in 1972 (FAO/WHO,
1973), when further studies to substantiate the marked species
difference in sensitivity to plasma cholinesterase depression and an
adequate reproduction study were required. At the Joint Meeting of
1976 (FAO/WHO, 1977) the temporary ADI was withdrawn since the
additional data required by the Joint Meeting of 1972 had not been
made available.
Part of the additional studies required by the 1972 Meeting have been
received and are reviewed in this monograph addendum. In addition
short-term and lone-term rat and dog studies, in which cholinesterase
activity was determined are re-evaluated.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Excretion via faeces, urine and expired air was studies after oral
administration (2.5 mg/kg) of phenyl-14C carbophenothion to 2 male
and 2 female Simonsen albino rats. Twelve similarly treated rats were
placed in plastic metabolism cages and allowed food and water
ad libitum. Four animals of each group were killed 1, 2, 4 and 8
days after administration of the radiolabelled dose and blood, brain,
adipose tissues, gonad, skin, kidney, liver, lung, muscle, stomach,
intestine and remaining carcass were collected for determination of
14C content.
Within 96 hours after receiving a single oral dose of phenyl-14C
carbophenothion an average of 97.8% of the administered radiocarbon
was excreted in the urine (78.5%) and faeces (19.3%). No radiocarbon
was found in the expired air trap. In all tissues only small amounts
of 14C (<0.1% of the administered dose) were detected 96 hours after
dosing (Hoffman et al., 1976).
Male Simonsen albino rats (200g) were dosed orally by stomach
intubation with 3 mg/kg b.w. of phenyl-14C carbophenothion dissolved
in propylene glycol. The animals were housed in metabolism cages
designed for the separate collection of urine and faeces. Urine was
collected 24 and 48 hours after dosing.
The animals remained healthy and active during the 48-hour collection
period during which time an average of 66% of the administered 14C
was excreted in urine; 14% was present in the ethyl acetate extract,
while virtually all of the 14C in the extracted aqueous phase was
soluble in methanol after lyophilization.
The metabolic study resulted in the following identified urinary
metabolites: 4-chloro-benzenesulphinic acid (46.8%), 4-chlorobenzene
sulphonic acid (5.3%), 4-chlorobenzenethio-sulphuric acid (3.0%),
4-chlorothio-phenyl-S-glucuronide (2 8%), 4-chlorophenyl methyl
sulphone (1.7%), 4-chloro-3-chydroxyphenyl methyl sulphone (23.9%,
both free and conjugated), 4-chlorophenylsulphinylmethyl methyl
sulphone (0.7%), and 4-chlorophenylsulphonylmethyl methyl sulphone
(1.9%). None of the oxygen analogues of carbophenothion were detected
in urine. Of the 13.5% of the metabolites which could not be
completely identified 6.3% was extractable in ethylacetate.
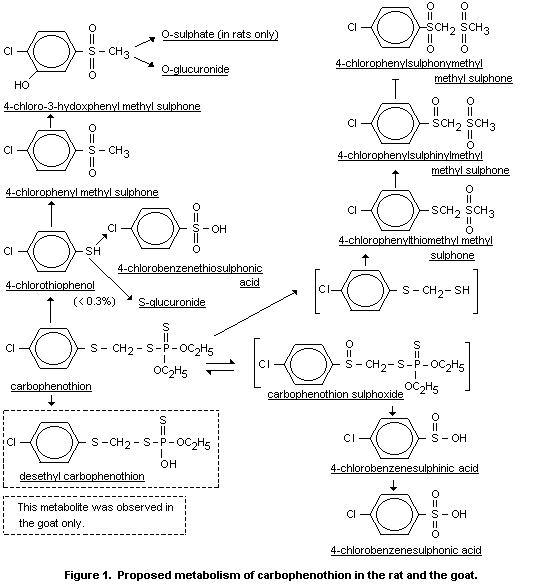
Figure 1, shows the proposed metabolism of carbophenothion in the rat
based on the present results. The metabolism in the rat is similar to
that observed in the goat (De Baun et al., 1976a). The major
degradative route appears to involve sulfoxidation and subsequent
conversion to 4-chlorobenzene sulphinic and 4-chlorobenzene sulphonic
acid. Another significant metabolic pathway involves methylation,
sulfoxidation, and ring-hydroxylation of liberated 4-chlorothiophenol.
The resultant 4-chloro-3-chydroxyphenyl methyl sulphone is converted
in nearly equal proportions to the sulfate and glucuronide conjugates.
Other metabolites including 4-chlorophenylsulphinylmethyl
methylsulphone and 4-chlorophenylsulphonylmethyl methylsulphone
presumably arise from cleavage of the carbophenothion P-S bond,
followed by methylation and sulphoxidation. The results show that
carbophenothion is readily degraded in the rat, primarily to water
soluble products which are excreted in the urine (DeBaun et al.,
1976b, Menn et al., 1976, DeBaun and Menn, 1976).
In an experiment with 4-chlorothiophenol, one of the metabolites of
carbophenothion, 4 male rats were dosed by stomach intubation of 1 ml
of aqueous ring 14C-4-chlorothiophenol. The animals remained active
and healthy throughout the 144-hours study. Complete urine and faecal
excretion of the administered 14C was not achieved until
approximately 6 Days after dosing. No 14C was detected in the expired
air at any interval. The metabolites are shown in figure 1 (DeBaun et
al., 1974).
Carbophenothion sulphoxide, an oxidative metabolite of carbophenothion
is also reduced to carbophenothion by an in vitro system containing
rat liver enzymes, reduced nicotinamide adenine dinucleotide
phosphate, and flavin adenine dinucleotide phosphate. After incubation
for 2 hours 78% unmetabolized carbophenothion and sulphoxide, 1%
carbophenothion sulphone acid and 12% carbophenothion were found
(DeBaun and Menn, 1976).
 Two lactating miniature Mexican goats were used to study the uptake,
distribution, excretion, and metabolic fate of phenyl-14C
carbophenothion in a polygastric animal, after oral application. The
goats were preconditioned with 10 mg unlabelled carbophenothion, twice
daily. After the preconditioning procedure of seven days, the animals
were dosed once with approximately 22 mg carbophenothion/kg b.w. The
goats were sacrificed 1 and 8 days after administration. With the
exception of the digestive system and the central nervous system, no
overt clinical changes were observed after treatment with
carbophenothion. Some diarrhoea and behavioural changes were observed
after carbophenothion administration. At necropsy no detectable
macroscopic lesions due to carbophenothion exposure were noticed.
Eight days after dosing, 82.6% of the administered radiocarbon was
recovered in urine, 15.6% in faeces, 1.4% in cage washes and 1.0% in
milk. The excretion was rapid; 90% of the dose was recovered within 72
hours. There was no evidence of selective storage of 14C in tissues.
Eight days after dosing less than 0.04 mg/kg carbophenothion
equivalents was recovered in tissues and organs. The maximum
concentration of 14C in milk, which occurred in the first 24 hours
after dosing, was 0.7-0.8 mg/kg carbophenothion equivalents. Of this,
0.014 mg/kg was characterized as carbophenothion and no oxidized
carbophenothion metabolites were detected. The remaining 14C in milk
was characterized as detoxication products resulting from cleavage of
the leaving group (e.g. from carbophenothion sulphoxide and
carbophenothion oxon sulphone). In this experiment the urine was also
examined for metabolites of carbophenothion. The proposed metabolism
in the goat based on these results as is shown in Figure 1. In general
the fate of carbophenothion is similar to that observed in rats:
methylation, sulphoxidation and ring-hydroxylation of liberated
4-cholorothiophenol, formation of 4-chlorobenzenesulphinic and
4-chlorobenzenesulphonic acids and the metabolites arising from
cleavage of the carbophenothion P-S bond, followed by methylation and
sulphoxidation of the resultant thiol intermediate. The goat
desalkylates carbophenothion to yield the desethyl derivative (Menn et
al., 1976, DeBaun et al., 1976b).
Eighty-seven percent of the urinary 14C and 88% of the milk 14C was
identified (DeBaun et al., 1976a). After an oral dose of 25 mg/kg body
weight, Greylag and Pink-footed geese died with brain and plasma
cholinesterase inhibition of 90%. The Canada goose showed symptoms
after 2-3 hours, but appeared normal after 8 hours, with less
cholinesterase inhibition than the other two species.
The highest residue levels were found in fat and were considerably
higher than in the other tissues. A range of tissues was also examined
for the presence of carbophenothion metabolites. Oxidative metabolites
were detected in muscle, brain# kidney and liver. Three of these were
tentatively identified as the oxygen analogue and its sulphone and
sulphoxide. The sulphone and sulphoxide of the parent compound
appeared to be present in liver (Stanley at al., 1976).
Effects on enzymes and other biochemical parameters
Eighteen organophosphorus insecticides were fed to 30-day-old female
rats for 1 week at various dietary levels. For each compound the
dietary levels was calculated to produce a 50% inhibition of liver and
serum aliesterases as well as brain, liver and serum cholinesterase.
Inhibition of liver aliesterases was generally found at a much lower
dose level than cholinesterase inhibition. Carbophenothion inhibited
aliesterases in liver by 50% at a dose level of 0.5-2.7 ppm and in
serum at 6.0-9.3 ppm in the diet. For brain, liver and serum
cholinesterase these levels were 17.0, 60.0 and 21.0 ppm respectively
(Su et al., 1971).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three generation (1 litter) reproduction study with 120 rats (10
males and 20 females/group) per generation was carried out.
Two lactating miniature Mexican goats were used to study the uptake,
distribution, excretion, and metabolic fate of phenyl-14C
carbophenothion in a polygastric animal, after oral application. The
goats were preconditioned with 10 mg unlabelled carbophenothion, twice
daily. After the preconditioning procedure of seven days, the animals
were dosed once with approximately 22 mg carbophenothion/kg b.w. The
goats were sacrificed 1 and 8 days after administration. With the
exception of the digestive system and the central nervous system, no
overt clinical changes were observed after treatment with
carbophenothion. Some diarrhoea and behavioural changes were observed
after carbophenothion administration. At necropsy no detectable
macroscopic lesions due to carbophenothion exposure were noticed.
Eight days after dosing, 82.6% of the administered radiocarbon was
recovered in urine, 15.6% in faeces, 1.4% in cage washes and 1.0% in
milk. The excretion was rapid; 90% of the dose was recovered within 72
hours. There was no evidence of selective storage of 14C in tissues.
Eight days after dosing less than 0.04 mg/kg carbophenothion
equivalents was recovered in tissues and organs. The maximum
concentration of 14C in milk, which occurred in the first 24 hours
after dosing, was 0.7-0.8 mg/kg carbophenothion equivalents. Of this,
0.014 mg/kg was characterized as carbophenothion and no oxidized
carbophenothion metabolites were detected. The remaining 14C in milk
was characterized as detoxication products resulting from cleavage of
the leaving group (e.g. from carbophenothion sulphoxide and
carbophenothion oxon sulphone). In this experiment the urine was also
examined for metabolites of carbophenothion. The proposed metabolism
in the goat based on these results as is shown in Figure 1. In general
the fate of carbophenothion is similar to that observed in rats:
methylation, sulphoxidation and ring-hydroxylation of liberated
4-cholorothiophenol, formation of 4-chlorobenzenesulphinic and
4-chlorobenzenesulphonic acids and the metabolites arising from
cleavage of the carbophenothion P-S bond, followed by methylation and
sulphoxidation of the resultant thiol intermediate. The goat
desalkylates carbophenothion to yield the desethyl derivative (Menn et
al., 1976, DeBaun et al., 1976b).
Eighty-seven percent of the urinary 14C and 88% of the milk 14C was
identified (DeBaun et al., 1976a). After an oral dose of 25 mg/kg body
weight, Greylag and Pink-footed geese died with brain and plasma
cholinesterase inhibition of 90%. The Canada goose showed symptoms
after 2-3 hours, but appeared normal after 8 hours, with less
cholinesterase inhibition than the other two species.
The highest residue levels were found in fat and were considerably
higher than in the other tissues. A range of tissues was also examined
for the presence of carbophenothion metabolites. Oxidative metabolites
were detected in muscle, brain# kidney and liver. Three of these were
tentatively identified as the oxygen analogue and its sulphone and
sulphoxide. The sulphone and sulphoxide of the parent compound
appeared to be present in liver (Stanley at al., 1976).
Effects on enzymes and other biochemical parameters
Eighteen organophosphorus insecticides were fed to 30-day-old female
rats for 1 week at various dietary levels. For each compound the
dietary levels was calculated to produce a 50% inhibition of liver and
serum aliesterases as well as brain, liver and serum cholinesterase.
Inhibition of liver aliesterases was generally found at a much lower
dose level than cholinesterase inhibition. Carbophenothion inhibited
aliesterases in liver by 50% at a dose level of 0.5-2.7 ppm and in
serum at 6.0-9.3 ppm in the diet. For brain, liver and serum
cholinesterase these levels were 17.0, 60.0 and 21.0 ppm respectively
(Su et al., 1971).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three generation (1 litter) reproduction study with 120 rats (10
males and 20 females/group) per generation was carried out.
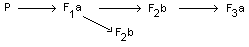 The dietary administered doses were 0, 3, 10 and 30 ppm
carbophenothion (95%, technical). Only the second generation was mated
twice. The foetuses of these litters (F2b) were examined for possible
teratological or embryo-toxicological effects.
The parameters studied were: individual body weight, food consumption
behaviour and observation of physical appearance of the parental
generation, fertility index, the total number of live and still-born
pups, the total weight of live pups per sex at day 1, 7 and 21, the
lactation index, viability index and the individual grossly observable
findings. In the F2b the number of corpora lutea of pregnancy per
ovary, the number and placement of implantation sites, resorption
sites and live and dead foetuses were recorded. The foetus was
examined individually and the weight, crown-rump distance and sex were
determined. Necropsies were performed in 10 males and 10 females of
the F3a generation at an age of 3 weeks. Approximately one-third of
the F2b fetuses from each litter were examined internally,
eviscerated, macerated and stained. The stained skeletons were
examined for degree of ossification and anomalies.
Mean body weights of the 10 and 30 ppm F1a females were significantly
lower during the first half of the prebreeding growth phase.
Significant decreases in the F2a growth period body weight data were
limited to the males of the 10 ppm dose group and to the females of
the 30 ppm dose group (during the first weeks after birth). The
incidence of a hunched appearance was somewhat higher among the
high-dose animals in all generations. No other signs of
compound-induced toxicity were observed during the growth, gestation
or lactation periods. In the F3a live birth, lactation and survival
indices in the 30 ppm group were lower. In addition the lactation
index (0-21) of the F2a generation was also lower than those of the
control group. In all three generations significantly lower mean body
weight of the pups was noted in the 30 ppm dose group, while in the
F1a this effect was found even in the 3 and 10 ppm dose groups.
Except for a higher incidence of treated pups appearing small in size,
generally corresponding to the compound-related weight suppression,
and a not-dose-related dilated pelvis of the kidney and enlarged lymph
nodes found in some animals of the treatment groups, no clinical or
gross pathological signs of toxicity were observed.
The incidences of resorption were somewhat higher and the
corresponding incidences of foetal viability were lower in the 10 and
30 ppm carbophenothion dose groups. A relationship between these
findings and the decreased neonate viability is likely. No significant
teratological effects due to the treatment were noted (Trutter J.A.,
1976).
Special studies on potentiation
In a study in which potentiation of sixteen organophosphorus compounds
with triamiphos was studied no potentiation in LD50 was found for
carbophenothion. (Speyers et al., 1976)
Acute toxicity
TABLE 1. Acute toxicity of carbophenothion
LD50
Species Sex Route (mg/kg) References
Rat M Oral 37 Speyers et al., 1976
F Oral 12 Speyers et al., 1976
Pigeon Oral 35 Jennings et al., 1975
Quail Oral 57 Jennings et al. 1975
Canada goose Oral 29-35 Jennings et al., 1975
Starling Oral 5.6 Shafer, 1972
Redwing Oral 7.5 Shafer, 1972
In addition to the acute toxicity data, the acute toxicity of certain
of the metabolites of carbophenothion to the rat are summarized in
Table 2.
Table 2. Acute toxicity of proposed metabolites and intermediates
of carbophenothion in the rat. (Hoffman et al., 1976)
Compound oral LD50
mg/kg b.w.
desethyl-carbophenothion (sodium salt) >1000
4-chlorobenzenesulphinic acid (sodium salt) > 500
4-chlorobenzenesulphonic acid (sodium salt) > 500
4-chlorobenzene thiosulphate (sodium salt) > 500
4-chlorothiophenol1/ 316
4-chlorophenyl methyl sulphoxide > 500
4-chlorophenyl methyl sulphone > 500
4-chloro-3-hydroxyphenyl methyl sulphone > 500
4-chlorophenylsulphenylmethyl methyl sulphone > 500
1/ The values for 4-chlorothiophenol represents the actual oral LD50.
One calf was given carbophenothion 1 mg/kg body weight by oral
capsule. The animal developed diarrhoea and showed ChE inhibition of
77%. In addition the plasma tocopherol and carotene contents were
lower (Hunt and McCarty, 1972).
OBSERVATIONS IN HUMANS
Seven members of a family became ill after eating tortillas made from
flour contaminated with 0.3% carbophenothion. They all showed
inhibition of serum cholinesterase. The level of cholinesterase
inhibition corresponded to the number of tortillas eaten and the
severity of the illness. The main symptoms were gastro-intestinal
upset (vomiting, diarrhoea), salivation and lacrimation. Four of the
affected people became comatose, but all recovered (Older and Hatcher,
1969).
Values for potential dermal and respiratory exposure and for total
exposure in terms of fraction of toxic doses were determined for 11
different pesticides during orchard spraying. The highest total
exposure was found for carbophenothion and was calculated to be 1.12%
(range 0.26-2.38) of a toxic dose-hour. Dermal exposure was much
greater than respiratory exposure (Wolfe et al., 1972).
COMMENTS
Carbophenothion was previously reviewed and further studies to
substantiate the marked species difference in sensitivity to plasma
cholinesterase depression and an adequate reproduction study were
required.
In 1976 the temporary ADI for humans was withdrawn because the
information previously requested had not been provided. Part of this
information has now been received and has been considered together
with re-evaluation of short-term and long-term rat and dog studies in
which cholinesterase activity was determined.
Present carbophenothion metabolism studies confirm and extend the
studies previously reported. Results show that carbophenothion is
readily degraded both in the rat and the goat, primarily to water
soluble products, which are excreted in the urine.
None of the oxygen analogues of carbophenothion or its sulphoxide and
sulphone were detected. The acute toxicity of the major metabolites of
carbophenothion was considerably lower than that of carbophenothion.
From both the data previously reported and the present data it is
clear that plasma cholinesterase inhibition is the most sensitive
criterion in both short- and long-term studies in rats and dogs. In a
two-year study in dogs 5 ppm or 0.125 mg/kg bw-day was not a no-effect
level with respect to plasma cholinesterase inhibition. In a 90 day
experiment in dogs an effect was found even at 0.04 mg/kg bw/day,
while 0.02 mg/kg bw was a marginal no-effect level in this respect. In
a two-year experiment with rats 5 ppm in the diet (0.25 mg/kg bw/day)
caused an inhibition of RCB-cholinesterase after 13 and 26 weeks.
A three generation reproduction study including a teratological study
revealed a no-effect level of 3 ppm equivalent to 0.15 mg/kg bw. With
respect to the lowest no-effect level this study has no consequence
for the calculation of the acceptable daily intake for humans, the
most sensitive criterion still remaining is the plasma cholinesterase
depression in dogs. An extreme difference in sensitivity between
humans and dog is noticed in the reported cholinesterase depression
studies. There seems to be an indication that 018 mg/kg/ day for 30
days did not result in cholinesterase inhibition in humans. However, 1
mg/kg bw in a calf caused symptoms and severe cholinesterase
inhibition. The Meeting decided to allocate a temporary ADI for humans
at a lower value owing to the marked species difference in sensitivity
to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw
Dog: 0.02 mg/kg bw/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.0002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
No new data were evaluated. Since the Meeting allocated a temporary
ADI, the previously recorded guideline levels were converted to
recommended temporary maximum residue limits.
FURTHER WORK OR INFORMATION
Required (before July 1979)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
Desirable
1. Further elucidation of the nature of the terminal residues on
crops, particularly as regards the reported possibility of the
presence under field conditions of photolysis products.
REFERENCES
FAO/WHO (1973) 1972 evaluations of some pesticide residues in food.
AGP:1972/M/9/1; WHO Pesticide Residues Series, No. 2.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
FAO/APG: 1977/M/5.
De Baun, J.R. and Menn, J.J. (1976) Sulfoxide reduction in relation to
organophosphorus insecticide detoxification. Science, 191, 187-188.
De Baun, J.R., Finley, K.A., Gruwell, L.A. and Menn, JJ. (1976a)
Metabolism of (Phyenyl-14C) carbophenothion in the lactating goat.
Stauffer Chemical Company, Report MRC-B-54, Mountain View, California,
USA. (Unpublished report)
De Baun, J.R., Hoffman, L.J., Rose, J.H. and Menn, J.J. (1976b)
Metabolism of (Phenyl-14C) carbophenothion in the rat: Urinary
metabolite identification. Stauffer Chemical Company, Report MRC-B-61,
Mountain View, California, USA. (Unpublished report)
De Baun, J.R., Rose, J.H. and Menn, J.J. (1974) Metabolism of
4-chloro-(U-14C) thiophenol in the rat. Stauffer Chemical Company,
Report MRC-B-50, Mountain View, California, USA. (Unpublished report)
Hoffman, L.J., Ross, J.H. and Menn J.J. (1976) Metabolism of
(Phenyl-14C carbophenothion in the rat: Blance and tissue residue
study. Stauffer Chemical Company, Report MRC-B-62, Mountain View,
California, USA. (Unpublished report)
Hunt, L.M. and McCarthy, R.T. (1972) Effects of some organophosphorus
insecticides on vitamin E and other blood constituents and on the
apparent inducement of diarrhoea in neonatal calves.
Bull. Environ. Contam. Toxicol. 8: 297-305
Jennings, D.M., Bunyan, P.J., Brown, P.M., Stanley, P.I. and Jones,
F.J.S. (1975) Organophosphorus poisoning: a comparative study of the
toxicity of carbophenothion to the Canada goose, the pigeon and the
Japanese quail. Pestic. Sci. 6: 245-257
Older J.J. and Hatcher, R.L. (1969) Food poisoning caused by
carbophenothion JAMA 209: 1328-1330.
Shafer E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds.
Toxicol. Appl. Pharmacol., 21: 315-330
Speijers, G.J.A., Verschuuren, H.G., Van Logten, MJ. an Van Esch, G.J.
(1976) Onderzoek naar de potentiŽrende werking van 16 organische
fosfor verbindingen en carbaryl. Intern Report N.I.P.H. 36-76 Tox.
(Unpublished report)
Stanley, P.I., Brown, P.M., Martin, A.D., Steed, L.C., Westlake, G.E.,
Howells, L.C. and Machin, A.J. The avian toxicology of carbophenothin.
Pest Infestation Control Laboratory and the Central Veterinary
Laboratory, Ministry of Agriculture, Fisheries and Food. (Unpublished
report)
Su, M.Q., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. Appl. Pharmacol.
20: 241-249
Trutter, J.A. (1976) A three-generation reproduction study in rats
with trithion (technical C). Hazleton Laboratories America, Report
submitted to the World Health Organization, by Stauffer Chemical
Company, Mountain View, California, USA. (Unpublished report)
Wolfe, H.R., Armstron, J.F., Staiff, D.A. and Corner, S.W. (1972)
Exposure of spraymen to pesticides. Arch. Environ. Health 25: 29-31
The dietary administered doses were 0, 3, 10 and 30 ppm
carbophenothion (95%, technical). Only the second generation was mated
twice. The foetuses of these litters (F2b) were examined for possible
teratological or embryo-toxicological effects.
The parameters studied were: individual body weight, food consumption
behaviour and observation of physical appearance of the parental
generation, fertility index, the total number of live and still-born
pups, the total weight of live pups per sex at day 1, 7 and 21, the
lactation index, viability index and the individual grossly observable
findings. In the F2b the number of corpora lutea of pregnancy per
ovary, the number and placement of implantation sites, resorption
sites and live and dead foetuses were recorded. The foetus was
examined individually and the weight, crown-rump distance and sex were
determined. Necropsies were performed in 10 males and 10 females of
the F3a generation at an age of 3 weeks. Approximately one-third of
the F2b fetuses from each litter were examined internally,
eviscerated, macerated and stained. The stained skeletons were
examined for degree of ossification and anomalies.
Mean body weights of the 10 and 30 ppm F1a females were significantly
lower during the first half of the prebreeding growth phase.
Significant decreases in the F2a growth period body weight data were
limited to the males of the 10 ppm dose group and to the females of
the 30 ppm dose group (during the first weeks after birth). The
incidence of a hunched appearance was somewhat higher among the
high-dose animals in all generations. No other signs of
compound-induced toxicity were observed during the growth, gestation
or lactation periods. In the F3a live birth, lactation and survival
indices in the 30 ppm group were lower. In addition the lactation
index (0-21) of the F2a generation was also lower than those of the
control group. In all three generations significantly lower mean body
weight of the pups was noted in the 30 ppm dose group, while in the
F1a this effect was found even in the 3 and 10 ppm dose groups.
Except for a higher incidence of treated pups appearing small in size,
generally corresponding to the compound-related weight suppression,
and a not-dose-related dilated pelvis of the kidney and enlarged lymph
nodes found in some animals of the treatment groups, no clinical or
gross pathological signs of toxicity were observed.
The incidences of resorption were somewhat higher and the
corresponding incidences of foetal viability were lower in the 10 and
30 ppm carbophenothion dose groups. A relationship between these
findings and the decreased neonate viability is likely. No significant
teratological effects due to the treatment were noted (Trutter J.A.,
1976).
Special studies on potentiation
In a study in which potentiation of sixteen organophosphorus compounds
with triamiphos was studied no potentiation in LD50 was found for
carbophenothion. (Speyers et al., 1976)
Acute toxicity
TABLE 1. Acute toxicity of carbophenothion
LD50
Species Sex Route (mg/kg) References
Rat M Oral 37 Speyers et al., 1976
F Oral 12 Speyers et al., 1976
Pigeon Oral 35 Jennings et al., 1975
Quail Oral 57 Jennings et al. 1975
Canada goose Oral 29-35 Jennings et al., 1975
Starling Oral 5.6 Shafer, 1972
Redwing Oral 7.5 Shafer, 1972
In addition to the acute toxicity data, the acute toxicity of certain
of the metabolites of carbophenothion to the rat are summarized in
Table 2.
Table 2. Acute toxicity of proposed metabolites and intermediates
of carbophenothion in the rat. (Hoffman et al., 1976)
Compound oral LD50
mg/kg b.w.
desethyl-carbophenothion (sodium salt) >1000
4-chlorobenzenesulphinic acid (sodium salt) > 500
4-chlorobenzenesulphonic acid (sodium salt) > 500
4-chlorobenzene thiosulphate (sodium salt) > 500
4-chlorothiophenol1/ 316
4-chlorophenyl methyl sulphoxide > 500
4-chlorophenyl methyl sulphone > 500
4-chloro-3-hydroxyphenyl methyl sulphone > 500
4-chlorophenylsulphenylmethyl methyl sulphone > 500
1/ The values for 4-chlorothiophenol represents the actual oral LD50.
One calf was given carbophenothion 1 mg/kg body weight by oral
capsule. The animal developed diarrhoea and showed ChE inhibition of
77%. In addition the plasma tocopherol and carotene contents were
lower (Hunt and McCarty, 1972).
OBSERVATIONS IN HUMANS
Seven members of a family became ill after eating tortillas made from
flour contaminated with 0.3% carbophenothion. They all showed
inhibition of serum cholinesterase. The level of cholinesterase
inhibition corresponded to the number of tortillas eaten and the
severity of the illness. The main symptoms were gastro-intestinal
upset (vomiting, diarrhoea), salivation and lacrimation. Four of the
affected people became comatose, but all recovered (Older and Hatcher,
1969).
Values for potential dermal and respiratory exposure and for total
exposure in terms of fraction of toxic doses were determined for 11
different pesticides during orchard spraying. The highest total
exposure was found for carbophenothion and was calculated to be 1.12%
(range 0.26-2.38) of a toxic dose-hour. Dermal exposure was much
greater than respiratory exposure (Wolfe et al., 1972).
COMMENTS
Carbophenothion was previously reviewed and further studies to
substantiate the marked species difference in sensitivity to plasma
cholinesterase depression and an adequate reproduction study were
required.
In 1976 the temporary ADI for humans was withdrawn because the
information previously requested had not been provided. Part of this
information has now been received and has been considered together
with re-evaluation of short-term and long-term rat and dog studies in
which cholinesterase activity was determined.
Present carbophenothion metabolism studies confirm and extend the
studies previously reported. Results show that carbophenothion is
readily degraded both in the rat and the goat, primarily to water
soluble products, which are excreted in the urine.
None of the oxygen analogues of carbophenothion or its sulphoxide and
sulphone were detected. The acute toxicity of the major metabolites of
carbophenothion was considerably lower than that of carbophenothion.
From both the data previously reported and the present data it is
clear that plasma cholinesterase inhibition is the most sensitive
criterion in both short- and long-term studies in rats and dogs. In a
two-year study in dogs 5 ppm or 0.125 mg/kg bw-day was not a no-effect
level with respect to plasma cholinesterase inhibition. In a 90 day
experiment in dogs an effect was found even at 0.04 mg/kg bw/day,
while 0.02 mg/kg bw was a marginal no-effect level in this respect. In
a two-year experiment with rats 5 ppm in the diet (0.25 mg/kg bw/day)
caused an inhibition of RCB-cholinesterase after 13 and 26 weeks.
A three generation reproduction study including a teratological study
revealed a no-effect level of 3 ppm equivalent to 0.15 mg/kg bw. With
respect to the lowest no-effect level this study has no consequence
for the calculation of the acceptable daily intake for humans, the
most sensitive criterion still remaining is the plasma cholinesterase
depression in dogs. An extreme difference in sensitivity between
humans and dog is noticed in the reported cholinesterase depression
studies. There seems to be an indication that 018 mg/kg/ day for 30
days did not result in cholinesterase inhibition in humans. However, 1
mg/kg bw in a calf caused symptoms and severe cholinesterase
inhibition. The Meeting decided to allocate a temporary ADI for humans
at a lower value owing to the marked species difference in sensitivity
to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw
Dog: 0.02 mg/kg bw/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.0002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
No new data were evaluated. Since the Meeting allocated a temporary
ADI, the previously recorded guideline levels were converted to
recommended temporary maximum residue limits.
FURTHER WORK OR INFORMATION
Required (before July 1979)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
Desirable
1. Further elucidation of the nature of the terminal residues on
crops, particularly as regards the reported possibility of the
presence under field conditions of photolysis products.
REFERENCES
FAO/WHO (1973) 1972 evaluations of some pesticide residues in food.
AGP:1972/M/9/1; WHO Pesticide Residues Series, No. 2.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
FAO/APG: 1977/M/5.
De Baun, J.R. and Menn, J.J. (1976) Sulfoxide reduction in relation to
organophosphorus insecticide detoxification. Science, 191, 187-188.
De Baun, J.R., Finley, K.A., Gruwell, L.A. and Menn, JJ. (1976a)
Metabolism of (Phyenyl-14C) carbophenothion in the lactating goat.
Stauffer Chemical Company, Report MRC-B-54, Mountain View, California,
USA. (Unpublished report)
De Baun, J.R., Hoffman, L.J., Rose, J.H. and Menn, J.J. (1976b)
Metabolism of (Phenyl-14C) carbophenothion in the rat: Urinary
metabolite identification. Stauffer Chemical Company, Report MRC-B-61,
Mountain View, California, USA. (Unpublished report)
De Baun, J.R., Rose, J.H. and Menn, J.J. (1974) Metabolism of
4-chloro-(U-14C) thiophenol in the rat. Stauffer Chemical Company,
Report MRC-B-50, Mountain View, California, USA. (Unpublished report)
Hoffman, L.J., Ross, J.H. and Menn J.J. (1976) Metabolism of
(Phenyl-14C carbophenothion in the rat: Blance and tissue residue
study. Stauffer Chemical Company, Report MRC-B-62, Mountain View,
California, USA. (Unpublished report)
Hunt, L.M. and McCarthy, R.T. (1972) Effects of some organophosphorus
insecticides on vitamin E and other blood constituents and on the
apparent inducement of diarrhoea in neonatal calves.
Bull. Environ. Contam. Toxicol. 8: 297-305
Jennings, D.M., Bunyan, P.J., Brown, P.M., Stanley, P.I. and Jones,
F.J.S. (1975) Organophosphorus poisoning: a comparative study of the
toxicity of carbophenothion to the Canada goose, the pigeon and the
Japanese quail. Pestic. Sci. 6: 245-257
Older J.J. and Hatcher, R.L. (1969) Food poisoning caused by
carbophenothion JAMA 209: 1328-1330.
Shafer E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds.
Toxicol. Appl. Pharmacol., 21: 315-330
Speijers, G.J.A., Verschuuren, H.G., Van Logten, MJ. an Van Esch, G.J.
(1976) Onderzoek naar de potentiŽrende werking van 16 organische
fosfor verbindingen en carbaryl. Intern Report N.I.P.H. 36-76 Tox.
(Unpublished report)
Stanley, P.I., Brown, P.M., Martin, A.D., Steed, L.C., Westlake, G.E.,
Howells, L.C. and Machin, A.J. The avian toxicology of carbophenothin.
Pest Infestation Control Laboratory and the Central Veterinary
Laboratory, Ministry of Agriculture, Fisheries and Food. (Unpublished
report)
Su, M.Q., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. Appl. Pharmacol.
20: 241-249
Trutter, J.A. (1976) A three-generation reproduction study in rats
with trithion (technical C). Hazleton Laboratories America, Report
submitted to the World Health Organization, by Stauffer Chemical
Company, Mountain View, California, USA. (Unpublished report)
Wolfe, H.R., Armstron, J.F., Staiff, D.A. and Corner, S.W. (1972)
Exposure of spraymen to pesticides. Arch. Environ. Health 25: 29-31
 Two lactating miniature Mexican goats were used to study the uptake,
distribution, excretion, and metabolic fate of phenyl-14C
carbophenothion in a polygastric animal, after oral application. The
goats were preconditioned with 10 mg unlabelled carbophenothion, twice
daily. After the preconditioning procedure of seven days, the animals
were dosed once with approximately 22 mg carbophenothion/kg b.w. The
goats were sacrificed 1 and 8 days after administration. With the
exception of the digestive system and the central nervous system, no
overt clinical changes were observed after treatment with
carbophenothion. Some diarrhoea and behavioural changes were observed
after carbophenothion administration. At necropsy no detectable
macroscopic lesions due to carbophenothion exposure were noticed.
Eight days after dosing, 82.6% of the administered radiocarbon was
recovered in urine, 15.6% in faeces, 1.4% in cage washes and 1.0% in
milk. The excretion was rapid; 90% of the dose was recovered within 72
hours. There was no evidence of selective storage of 14C in tissues.
Eight days after dosing less than 0.04 mg/kg carbophenothion
equivalents was recovered in tissues and organs. The maximum
concentration of 14C in milk, which occurred in the first 24 hours
after dosing, was 0.7-0.8 mg/kg carbophenothion equivalents. Of this,
0.014 mg/kg was characterized as carbophenothion and no oxidized
carbophenothion metabolites were detected. The remaining 14C in milk
was characterized as detoxication products resulting from cleavage of
the leaving group (e.g. from carbophenothion sulphoxide and
carbophenothion oxon sulphone). In this experiment the urine was also
examined for metabolites of carbophenothion. The proposed metabolism
in the goat based on these results as is shown in Figure 1. In general
the fate of carbophenothion is similar to that observed in rats:
methylation, sulphoxidation and ring-hydroxylation of liberated
4-cholorothiophenol, formation of 4-chlorobenzenesulphinic and
4-chlorobenzenesulphonic acids and the metabolites arising from
cleavage of the carbophenothion P-S bond, followed by methylation and
sulphoxidation of the resultant thiol intermediate. The goat
desalkylates carbophenothion to yield the desethyl derivative (Menn et
al., 1976, DeBaun et al., 1976b).
Eighty-seven percent of the urinary 14C and 88% of the milk 14C was
identified (DeBaun et al., 1976a). After an oral dose of 25 mg/kg body
weight, Greylag and Pink-footed geese died with brain and plasma
cholinesterase inhibition of 90%. The Canada goose showed symptoms
after 2-3 hours, but appeared normal after 8 hours, with less
cholinesterase inhibition than the other two species.
The highest residue levels were found in fat and were considerably
higher than in the other tissues. A range of tissues was also examined
for the presence of carbophenothion metabolites. Oxidative metabolites
were detected in muscle, brain# kidney and liver. Three of these were
tentatively identified as the oxygen analogue and its sulphone and
sulphoxide. The sulphone and sulphoxide of the parent compound
appeared to be present in liver (Stanley at al., 1976).
Effects on enzymes and other biochemical parameters
Eighteen organophosphorus insecticides were fed to 30-day-old female
rats for 1 week at various dietary levels. For each compound the
dietary levels was calculated to produce a 50% inhibition of liver and
serum aliesterases as well as brain, liver and serum cholinesterase.
Inhibition of liver aliesterases was generally found at a much lower
dose level than cholinesterase inhibition. Carbophenothion inhibited
aliesterases in liver by 50% at a dose level of 0.5-2.7 ppm and in
serum at 6.0-9.3 ppm in the diet. For brain, liver and serum
cholinesterase these levels were 17.0, 60.0 and 21.0 ppm respectively
(Su et al., 1971).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three generation (1 litter) reproduction study with 120 rats (10
males and 20 females/group) per generation was carried out.
Two lactating miniature Mexican goats were used to study the uptake,
distribution, excretion, and metabolic fate of phenyl-14C
carbophenothion in a polygastric animal, after oral application. The
goats were preconditioned with 10 mg unlabelled carbophenothion, twice
daily. After the preconditioning procedure of seven days, the animals
were dosed once with approximately 22 mg carbophenothion/kg b.w. The
goats were sacrificed 1 and 8 days after administration. With the
exception of the digestive system and the central nervous system, no
overt clinical changes were observed after treatment with
carbophenothion. Some diarrhoea and behavioural changes were observed
after carbophenothion administration. At necropsy no detectable
macroscopic lesions due to carbophenothion exposure were noticed.
Eight days after dosing, 82.6% of the administered radiocarbon was
recovered in urine, 15.6% in faeces, 1.4% in cage washes and 1.0% in
milk. The excretion was rapid; 90% of the dose was recovered within 72
hours. There was no evidence of selective storage of 14C in tissues.
Eight days after dosing less than 0.04 mg/kg carbophenothion
equivalents was recovered in tissues and organs. The maximum
concentration of 14C in milk, which occurred in the first 24 hours
after dosing, was 0.7-0.8 mg/kg carbophenothion equivalents. Of this,
0.014 mg/kg was characterized as carbophenothion and no oxidized
carbophenothion metabolites were detected. The remaining 14C in milk
was characterized as detoxication products resulting from cleavage of
the leaving group (e.g. from carbophenothion sulphoxide and
carbophenothion oxon sulphone). In this experiment the urine was also
examined for metabolites of carbophenothion. The proposed metabolism
in the goat based on these results as is shown in Figure 1. In general
the fate of carbophenothion is similar to that observed in rats:
methylation, sulphoxidation and ring-hydroxylation of liberated
4-cholorothiophenol, formation of 4-chlorobenzenesulphinic and
4-chlorobenzenesulphonic acids and the metabolites arising from
cleavage of the carbophenothion P-S bond, followed by methylation and
sulphoxidation of the resultant thiol intermediate. The goat
desalkylates carbophenothion to yield the desethyl derivative (Menn et
al., 1976, DeBaun et al., 1976b).
Eighty-seven percent of the urinary 14C and 88% of the milk 14C was
identified (DeBaun et al., 1976a). After an oral dose of 25 mg/kg body
weight, Greylag and Pink-footed geese died with brain and plasma
cholinesterase inhibition of 90%. The Canada goose showed symptoms
after 2-3 hours, but appeared normal after 8 hours, with less
cholinesterase inhibition than the other two species.
The highest residue levels were found in fat and were considerably
higher than in the other tissues. A range of tissues was also examined
for the presence of carbophenothion metabolites. Oxidative metabolites
were detected in muscle, brain# kidney and liver. Three of these were
tentatively identified as the oxygen analogue and its sulphone and
sulphoxide. The sulphone and sulphoxide of the parent compound
appeared to be present in liver (Stanley at al., 1976).
Effects on enzymes and other biochemical parameters
Eighteen organophosphorus insecticides were fed to 30-day-old female
rats for 1 week at various dietary levels. For each compound the
dietary levels was calculated to produce a 50% inhibition of liver and
serum aliesterases as well as brain, liver and serum cholinesterase.
Inhibition of liver aliesterases was generally found at a much lower
dose level than cholinesterase inhibition. Carbophenothion inhibited
aliesterases in liver by 50% at a dose level of 0.5-2.7 ppm and in
serum at 6.0-9.3 ppm in the diet. For brain, liver and serum
cholinesterase these levels were 17.0, 60.0 and 21.0 ppm respectively
(Su et al., 1971).
TOXICOLOGICAL STUDIES
Special studies on reproduction
A three generation (1 litter) reproduction study with 120 rats (10
males and 20 females/group) per generation was carried out.
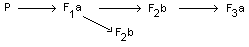 The dietary administered doses were 0, 3, 10 and 30 ppm
carbophenothion (95%, technical). Only the second generation was mated
twice. The foetuses of these litters (F2b) were examined for possible
teratological or embryo-toxicological effects.
The parameters studied were: individual body weight, food consumption
behaviour and observation of physical appearance of the parental
generation, fertility index, the total number of live and still-born
pups, the total weight of live pups per sex at day 1, 7 and 21, the
lactation index, viability index and the individual grossly observable
findings. In the F2b the number of corpora lutea of pregnancy per
ovary, the number and placement of implantation sites, resorption
sites and live and dead foetuses were recorded. The foetus was
examined individually and the weight, crown-rump distance and sex were
determined. Necropsies were performed in 10 males and 10 females of
the F3a generation at an age of 3 weeks. Approximately one-third of
the F2b fetuses from each litter were examined internally,
eviscerated, macerated and stained. The stained skeletons were
examined for degree of ossification and anomalies.
Mean body weights of the 10 and 30 ppm F1a females were significantly
lower during the first half of the prebreeding growth phase.
Significant decreases in the F2a growth period body weight data were
limited to the males of the 10 ppm dose group and to the females of
the 30 ppm dose group (during the first weeks after birth). The
incidence of a hunched appearance was somewhat higher among the
high-dose animals in all generations. No other signs of
compound-induced toxicity were observed during the growth, gestation
or lactation periods. In the F3a live birth, lactation and survival
indices in the 30 ppm group were lower. In addition the lactation
index (0-21) of the F2a generation was also lower than those of the
control group. In all three generations significantly lower mean body
weight of the pups was noted in the 30 ppm dose group, while in the
F1a this effect was found even in the 3 and 10 ppm dose groups.
Except for a higher incidence of treated pups appearing small in size,
generally corresponding to the compound-related weight suppression,
and a not-dose-related dilated pelvis of the kidney and enlarged lymph
nodes found in some animals of the treatment groups, no clinical or
gross pathological signs of toxicity were observed.
The incidences of resorption were somewhat higher and the
corresponding incidences of foetal viability were lower in the 10 and
30 ppm carbophenothion dose groups. A relationship between these
findings and the decreased neonate viability is likely. No significant
teratological effects due to the treatment were noted (Trutter J.A.,
1976).
Special studies on potentiation
In a study in which potentiation of sixteen organophosphorus compounds
with triamiphos was studied no potentiation in LD50 was found for
carbophenothion. (Speyers et al., 1976)
Acute toxicity
TABLE 1. Acute toxicity of carbophenothion
LD50
Species Sex Route (mg/kg) References
Rat M Oral 37 Speyers et al., 1976
F Oral 12 Speyers et al., 1976
Pigeon Oral 35 Jennings et al., 1975
Quail Oral 57 Jennings et al. 1975
Canada goose Oral 29-35 Jennings et al., 1975
Starling Oral 5.6 Shafer, 1972
Redwing Oral 7.5 Shafer, 1972
In addition to the acute toxicity data, the acute toxicity of certain
of the metabolites of carbophenothion to the rat are summarized in
Table 2.
Table 2. Acute toxicity of proposed metabolites and intermediates
of carbophenothion in the rat. (Hoffman et al., 1976)
Compound oral LD50
mg/kg b.w.
desethyl-carbophenothion (sodium salt) >1000
4-chlorobenzenesulphinic acid (sodium salt) > 500
4-chlorobenzenesulphonic acid (sodium salt) > 500
4-chlorobenzene thiosulphate (sodium salt) > 500
4-chlorothiophenol1/ 316
4-chlorophenyl methyl sulphoxide > 500
4-chlorophenyl methyl sulphone > 500
4-chloro-3-hydroxyphenyl methyl sulphone > 500
4-chlorophenylsulphenylmethyl methyl sulphone > 500
1/ The values for 4-chlorothiophenol represents the actual oral LD50.
One calf was given carbophenothion 1 mg/kg body weight by oral
capsule. The animal developed diarrhoea and showed ChE inhibition of
77%. In addition the plasma tocopherol and carotene contents were
lower (Hunt and McCarty, 1972).
OBSERVATIONS IN HUMANS
Seven members of a family became ill after eating tortillas made from
flour contaminated with 0.3% carbophenothion. They all showed
inhibition of serum cholinesterase. The level of cholinesterase
inhibition corresponded to the number of tortillas eaten and the
severity of the illness. The main symptoms were gastro-intestinal
upset (vomiting, diarrhoea), salivation and lacrimation. Four of the
affected people became comatose, but all recovered (Older and Hatcher,
1969).
Values for potential dermal and respiratory exposure and for total
exposure in terms of fraction of toxic doses were determined for 11
different pesticides during orchard spraying. The highest total
exposure was found for carbophenothion and was calculated to be 1.12%
(range 0.26-2.38) of a toxic dose-hour. Dermal exposure was much
greater than respiratory exposure (Wolfe et al., 1972).
COMMENTS
Carbophenothion was previously reviewed and further studies to
substantiate the marked species difference in sensitivity to plasma
cholinesterase depression and an adequate reproduction study were
required.
In 1976 the temporary ADI for humans was withdrawn because the
information previously requested had not been provided. Part of this
information has now been received and has been considered together
with re-evaluation of short-term and long-term rat and dog studies in
which cholinesterase activity was determined.
Present carbophenothion metabolism studies confirm and extend the
studies previously reported. Results show that carbophenothion is
readily degraded both in the rat and the goat, primarily to water
soluble products, which are excreted in the urine.
None of the oxygen analogues of carbophenothion or its sulphoxide and
sulphone were detected. The acute toxicity of the major metabolites of
carbophenothion was considerably lower than that of carbophenothion.
From both the data previously reported and the present data it is
clear that plasma cholinesterase inhibition is the most sensitive
criterion in both short- and long-term studies in rats and dogs. In a
two-year study in dogs 5 ppm or 0.125 mg/kg bw-day was not a no-effect
level with respect to plasma cholinesterase inhibition. In a 90 day
experiment in dogs an effect was found even at 0.04 mg/kg bw/day,
while 0.02 mg/kg bw was a marginal no-effect level in this respect. In
a two-year experiment with rats 5 ppm in the diet (0.25 mg/kg bw/day)
caused an inhibition of RCB-cholinesterase after 13 and 26 weeks.
A three generation reproduction study including a teratological study
revealed a no-effect level of 3 ppm equivalent to 0.15 mg/kg bw. With
respect to the lowest no-effect level this study has no consequence
for the calculation of the acceptable daily intake for humans, the
most sensitive criterion still remaining is the plasma cholinesterase
depression in dogs. An extreme difference in sensitivity between
humans and dog is noticed in the reported cholinesterase depression
studies. There seems to be an indication that 018 mg/kg/ day for 30
days did not result in cholinesterase inhibition in humans. However, 1
mg/kg bw in a calf caused symptoms and severe cholinesterase
inhibition. The Meeting decided to allocate a temporary ADI for humans
at a lower value owing to the marked species difference in sensitivity
to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw
Dog: 0.02 mg/kg bw/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.0002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
No new data were evaluated. Since the Meeting allocated a temporary
ADI, the previously recorded guideline levels were converted to
recommended temporary maximum residue limits.
FURTHER WORK OR INFORMATION
Required (before July 1979)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
Desirable
1. Further elucidation of the nature of the terminal residues on
crops, particularly as regards the reported possibility of the
presence under field conditions of photolysis products.
REFERENCES
FAO/WHO (1973) 1972 evaluations of some pesticide residues in food.
AGP:1972/M/9/1; WHO Pesticide Residues Series, No. 2.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
FAO/APG: 1977/M/5.
De Baun, J.R. and Menn, J.J. (1976) Sulfoxide reduction in relation to
organophosphorus insecticide detoxification. Science, 191, 187-188.
De Baun, J.R., Finley, K.A., Gruwell, L.A. and Menn, JJ. (1976a)
Metabolism of (Phyenyl-14C) carbophenothion in the lactating goat.
Stauffer Chemical Company, Report MRC-B-54, Mountain View, California,
USA. (Unpublished report)
De Baun, J.R., Hoffman, L.J., Rose, J.H. and Menn, J.J. (1976b)
Metabolism of (Phenyl-14C) carbophenothion in the rat: Urinary
metabolite identification. Stauffer Chemical Company, Report MRC-B-61,
Mountain View, California, USA. (Unpublished report)
De Baun, J.R., Rose, J.H. and Menn, J.J. (1974) Metabolism of
4-chloro-(U-14C) thiophenol in the rat. Stauffer Chemical Company,
Report MRC-B-50, Mountain View, California, USA. (Unpublished report)
Hoffman, L.J., Ross, J.H. and Menn J.J. (1976) Metabolism of
(Phenyl-14C carbophenothion in the rat: Blance and tissue residue
study. Stauffer Chemical Company, Report MRC-B-62, Mountain View,
California, USA. (Unpublished report)
Hunt, L.M. and McCarthy, R.T. (1972) Effects of some organophosphorus
insecticides on vitamin E and other blood constituents and on the
apparent inducement of diarrhoea in neonatal calves.
Bull. Environ. Contam. Toxicol. 8: 297-305
Jennings, D.M., Bunyan, P.J., Brown, P.M., Stanley, P.I. and Jones,
F.J.S. (1975) Organophosphorus poisoning: a comparative study of the
toxicity of carbophenothion to the Canada goose, the pigeon and the
Japanese quail. Pestic. Sci. 6: 245-257
Older J.J. and Hatcher, R.L. (1969) Food poisoning caused by
carbophenothion JAMA 209: 1328-1330.
Shafer E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds.
Toxicol. Appl. Pharmacol., 21: 315-330
Speijers, G.J.A., Verschuuren, H.G., Van Logten, MJ. an Van Esch, G.J.
(1976) Onderzoek naar de potentiŽrende werking van 16 organische
fosfor verbindingen en carbaryl. Intern Report N.I.P.H. 36-76 Tox.
(Unpublished report)
Stanley, P.I., Brown, P.M., Martin, A.D., Steed, L.C., Westlake, G.E.,
Howells, L.C. and Machin, A.J. The avian toxicology of carbophenothin.
Pest Infestation Control Laboratory and the Central Veterinary
Laboratory, Ministry of Agriculture, Fisheries and Food. (Unpublished
report)
Su, M.Q., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. Appl. Pharmacol.
20: 241-249
Trutter, J.A. (1976) A three-generation reproduction study in rats
with trithion (technical C). Hazleton Laboratories America, Report
submitted to the World Health Organization, by Stauffer Chemical
Company, Mountain View, California, USA. (Unpublished report)
Wolfe, H.R., Armstron, J.F., Staiff, D.A. and Corner, S.W. (1972)
Exposure of spraymen to pesticides. Arch. Environ. Health 25: 29-31
The dietary administered doses were 0, 3, 10 and 30 ppm
carbophenothion (95%, technical). Only the second generation was mated
twice. The foetuses of these litters (F2b) were examined for possible
teratological or embryo-toxicological effects.
The parameters studied were: individual body weight, food consumption
behaviour and observation of physical appearance of the parental
generation, fertility index, the total number of live and still-born
pups, the total weight of live pups per sex at day 1, 7 and 21, the
lactation index, viability index and the individual grossly observable
findings. In the F2b the number of corpora lutea of pregnancy per
ovary, the number and placement of implantation sites, resorption
sites and live and dead foetuses were recorded. The foetus was
examined individually and the weight, crown-rump distance and sex were
determined. Necropsies were performed in 10 males and 10 females of
the F3a generation at an age of 3 weeks. Approximately one-third of
the F2b fetuses from each litter were examined internally,
eviscerated, macerated and stained. The stained skeletons were
examined for degree of ossification and anomalies.
Mean body weights of the 10 and 30 ppm F1a females were significantly
lower during the first half of the prebreeding growth phase.
Significant decreases in the F2a growth period body weight data were
limited to the males of the 10 ppm dose group and to the females of
the 30 ppm dose group (during the first weeks after birth). The
incidence of a hunched appearance was somewhat higher among the
high-dose animals in all generations. No other signs of
compound-induced toxicity were observed during the growth, gestation
or lactation periods. In the F3a live birth, lactation and survival
indices in the 30 ppm group were lower. In addition the lactation
index (0-21) of the F2a generation was also lower than those of the
control group. In all three generations significantly lower mean body
weight of the pups was noted in the 30 ppm dose group, while in the
F1a this effect was found even in the 3 and 10 ppm dose groups.
Except for a higher incidence of treated pups appearing small in size,
generally corresponding to the compound-related weight suppression,
and a not-dose-related dilated pelvis of the kidney and enlarged lymph
nodes found in some animals of the treatment groups, no clinical or
gross pathological signs of toxicity were observed.
The incidences of resorption were somewhat higher and the
corresponding incidences of foetal viability were lower in the 10 and
30 ppm carbophenothion dose groups. A relationship between these
findings and the decreased neonate viability is likely. No significant
teratological effects due to the treatment were noted (Trutter J.A.,
1976).
Special studies on potentiation
In a study in which potentiation of sixteen organophosphorus compounds
with triamiphos was studied no potentiation in LD50 was found for
carbophenothion. (Speyers et al., 1976)
Acute toxicity
TABLE 1. Acute toxicity of carbophenothion
LD50
Species Sex Route (mg/kg) References
Rat M Oral 37 Speyers et al., 1976
F Oral 12 Speyers et al., 1976
Pigeon Oral 35 Jennings et al., 1975
Quail Oral 57 Jennings et al. 1975
Canada goose Oral 29-35 Jennings et al., 1975
Starling Oral 5.6 Shafer, 1972
Redwing Oral 7.5 Shafer, 1972
In addition to the acute toxicity data, the acute toxicity of certain
of the metabolites of carbophenothion to the rat are summarized in
Table 2.
Table 2. Acute toxicity of proposed metabolites and intermediates
of carbophenothion in the rat. (Hoffman et al., 1976)
Compound oral LD50
mg/kg b.w.
desethyl-carbophenothion (sodium salt) >1000
4-chlorobenzenesulphinic acid (sodium salt) > 500
4-chlorobenzenesulphonic acid (sodium salt) > 500
4-chlorobenzene thiosulphate (sodium salt) > 500
4-chlorothiophenol1/ 316
4-chlorophenyl methyl sulphoxide > 500
4-chlorophenyl methyl sulphone > 500
4-chloro-3-hydroxyphenyl methyl sulphone > 500
4-chlorophenylsulphenylmethyl methyl sulphone > 500
1/ The values for 4-chlorothiophenol represents the actual oral LD50.
One calf was given carbophenothion 1 mg/kg body weight by oral
capsule. The animal developed diarrhoea and showed ChE inhibition of
77%. In addition the plasma tocopherol and carotene contents were
lower (Hunt and McCarty, 1972).
OBSERVATIONS IN HUMANS
Seven members of a family became ill after eating tortillas made from
flour contaminated with 0.3% carbophenothion. They all showed
inhibition of serum cholinesterase. The level of cholinesterase
inhibition corresponded to the number of tortillas eaten and the
severity of the illness. The main symptoms were gastro-intestinal
upset (vomiting, diarrhoea), salivation and lacrimation. Four of the
affected people became comatose, but all recovered (Older and Hatcher,
1969).
Values for potential dermal and respiratory exposure and for total
exposure in terms of fraction of toxic doses were determined for 11
different pesticides during orchard spraying. The highest total
exposure was found for carbophenothion and was calculated to be 1.12%
(range 0.26-2.38) of a toxic dose-hour. Dermal exposure was much
greater than respiratory exposure (Wolfe et al., 1972).
COMMENTS
Carbophenothion was previously reviewed and further studies to
substantiate the marked species difference in sensitivity to plasma
cholinesterase depression and an adequate reproduction study were
required.
In 1976 the temporary ADI for humans was withdrawn because the
information previously requested had not been provided. Part of this
information has now been received and has been considered together
with re-evaluation of short-term and long-term rat and dog studies in
which cholinesterase activity was determined.
Present carbophenothion metabolism studies confirm and extend the
studies previously reported. Results show that carbophenothion is
readily degraded both in the rat and the goat, primarily to water
soluble products, which are excreted in the urine.
None of the oxygen analogues of carbophenothion or its sulphoxide and
sulphone were detected. The acute toxicity of the major metabolites of
carbophenothion was considerably lower than that of carbophenothion.
From both the data previously reported and the present data it is
clear that plasma cholinesterase inhibition is the most sensitive
criterion in both short- and long-term studies in rats and dogs. In a
two-year study in dogs 5 ppm or 0.125 mg/kg bw-day was not a no-effect
level with respect to plasma cholinesterase inhibition. In a 90 day
experiment in dogs an effect was found even at 0.04 mg/kg bw/day,
while 0.02 mg/kg bw was a marginal no-effect level in this respect. In
a two-year experiment with rats 5 ppm in the diet (0.25 mg/kg bw/day)
caused an inhibition of RCB-cholinesterase after 13 and 26 weeks.
A three generation reproduction study including a teratological study
revealed a no-effect level of 3 ppm equivalent to 0.15 mg/kg bw. With
respect to the lowest no-effect level this study has no consequence
for the calculation of the acceptable daily intake for humans, the
most sensitive criterion still remaining is the plasma cholinesterase
depression in dogs. An extreme difference in sensitivity between
humans and dog is noticed in the reported cholinesterase depression
studies. There seems to be an indication that 018 mg/kg/ day for 30
days did not result in cholinesterase inhibition in humans. However, 1
mg/kg bw in a calf caused symptoms and severe cholinesterase
inhibition. The Meeting decided to allocate a temporary ADI for humans
at a lower value owing to the marked species difference in sensitivity
to plasma cholinesterase depression.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Rat: 3 mg/kg in the diet, equivalent to 0.15 mg/kg bw
Dog: 0.02 mg/kg bw/day
ESTIMATE OF TEMPORARY ACCEPTABLE DAILY INTAKE FOR HUMANS
0-0.0002 mg/kg bw
RESIDUES IN FOOD AND THEIR EVALUATION
No new data were evaluated. Since the Meeting allocated a temporary
ADI, the previously recorded guideline levels were converted to
recommended temporary maximum residue limits.
FURTHER WORK OR INFORMATION
Required (before July 1979)
1. Further studies to substantiate the marked species difference in
sensitivity to plasma cholinesterase depression.
Desirable
1. Further elucidation of the nature of the terminal residues on
crops, particularly as regards the reported possibility of the
presence under field conditions of photolysis products.
REFERENCES
FAO/WHO (1973) 1972 evaluations of some pesticide residues in food.
AGP:1972/M/9/1; WHO Pesticide Residues Series, No. 2.
FAO/WHO (1977) 1976 evaluations of some pesticide residues in food.
FAO/APG: 1977/M/5.
De Baun, J.R. and Menn, J.J. (1976) Sulfoxide reduction in relation to
organophosphorus insecticide detoxification. Science, 191, 187-188.
De Baun, J.R., Finley, K.A., Gruwell, L.A. and Menn, JJ. (1976a)
Metabolism of (Phyenyl-14C) carbophenothion in the lactating goat.
Stauffer Chemical Company, Report MRC-B-54, Mountain View, California,
USA. (Unpublished report)
De Baun, J.R., Hoffman, L.J., Rose, J.H. and Menn, J.J. (1976b)
Metabolism of (Phenyl-14C) carbophenothion in the rat: Urinary
metabolite identification. Stauffer Chemical Company, Report MRC-B-61,
Mountain View, California, USA. (Unpublished report)
De Baun, J.R., Rose, J.H. and Menn, J.J. (1974) Metabolism of
4-chloro-(U-14C) thiophenol in the rat. Stauffer Chemical Company,
Report MRC-B-50, Mountain View, California, USA. (Unpublished report)
Hoffman, L.J., Ross, J.H. and Menn J.J. (1976) Metabolism of
(Phenyl-14C carbophenothion in the rat: Blance and tissue residue
study. Stauffer Chemical Company, Report MRC-B-62, Mountain View,
California, USA. (Unpublished report)
Hunt, L.M. and McCarthy, R.T. (1972) Effects of some organophosphorus
insecticides on vitamin E and other blood constituents and on the
apparent inducement of diarrhoea in neonatal calves.
Bull. Environ. Contam. Toxicol. 8: 297-305
Jennings, D.M., Bunyan, P.J., Brown, P.M., Stanley, P.I. and Jones,
F.J.S. (1975) Organophosphorus poisoning: a comparative study of the
toxicity of carbophenothion to the Canada goose, the pigeon and the
Japanese quail. Pestic. Sci. 6: 245-257
Older J.J. and Hatcher, R.L. (1969) Food poisoning caused by
carbophenothion JAMA 209: 1328-1330.
Shafer E.W. (1972) The acute oral toxicity of 369 pesticidal,
pharmaceutical and other chemicals to wild birds.
Toxicol. Appl. Pharmacol., 21: 315-330
Speijers, G.J.A., Verschuuren, H.G., Van Logten, MJ. an Van Esch, G.J.
(1976) Onderzoek naar de potentiŽrende werking van 16 organische
fosfor verbindingen en carbaryl. Intern Report N.I.P.H. 36-76 Tox.
(Unpublished report)
Stanley, P.I., Brown, P.M., Martin, A.D., Steed, L.C., Westlake, G.E.,
Howells, L.C. and Machin, A.J. The avian toxicology of carbophenothin.
Pest Infestation Control Laboratory and the Central Veterinary
Laboratory, Ministry of Agriculture, Fisheries and Food. (Unpublished
report)
Su, M.Q., Kinoshita, F.K., Frawley, J.P. and DuBois, K.P. (1971)
Comparative inhibition of aliesterases and cholinesterase in rats fed
eighteen organophosphorus insecticides. Toxicol. Appl. Pharmacol.
20: 241-249
Trutter, J.A. (1976) A three-generation reproduction study in rats
with trithion (technical C). Hazleton Laboratories America, Report
submitted to the World Health Organization, by Stauffer Chemical
Company, Mountain View, California, USA. (Unpublished report)
Wolfe, H.R., Armstron, J.F., Staiff, D.A. and Corner, S.W. (1972)
Exposure of spraymen to pesticides. Arch. Environ. Health 25: 29-31
