
BIORESMETHRIN JMPR 1975
IDENTITY
Chemical name
5-benzyl-3-furylmethyl(+)-trans-chrysanthemate
Synonyms
5-benzyl-3-furylmethyl(+)-trans-2,2-dimethyl-3-(2-methyl
propenyl) cyclopropane carboxylate
5-benzyl-3-furylmethyl(+)-trans-chrysanthemum monocarboxylate
2-benzyl-4-furylmethyl(+)-trans-chrysanthemate
NRDC 107; Bio NRDC 104; Biorestrin; Biobenzyl furoline, 95H66; RU
1.484; SBP 1390. (Resmethrin is the name applied to the racemic
(+)-cis,trans-mixture. Also known as NRDC 104, SEP 1384).
Structural formula
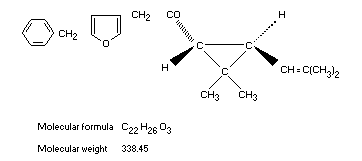 Molecular formula C22H26O3
Molecular weight 338.45
Other information on identity and properties
Description : A viscous yellow liquid which on
crystallization forms an off-white solid
Specific gravity: 1.050 at 20°C
Flash point : 125°C (open cup)
Optical rotation: D20-5 to -8° : measurement on a 10% solution
in ethanol
Solubility : Soluble in most organic solvents but
substantially insoluble in water
Saponification
value : 160-175 mg KOH/gm
Stability : Bioresmethrin is sensitive to light but its
photo stability is greater than that of
pyrethrins. Bioresmethrin is stable to
temperatures met under most normal storage
conditions.
Bioresmethrin spray deposits appear subject
to atmospheric oxidation but any adverse
effect appears to be reduced by the
introduction of antioxidants including
piperonyl butoxide and butylated
hydroxyanisole.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bioresmethrin is one of the most effective broad spectrum
insecticides currently available. It exhibits a high order of
insecticidal activity, which when coupled with its excellent
toxicological properties, makes it potentially one of the safest and
most useful insecticides now being produced.
In addition to its insecticidal properties, bioresmethrin has a
good knock-down performance against flying insects, particularly when
this is compared with the organochlorine or organophosphorus
insecticides.
Bioresmethrin, at low concentrations, is an effective killing
agent against most insect pests affecting households, industrial
premises, public health, food storage and livestock production.
Efficacy against many insect pests of food crops and ornamentals has
been demonstrated.
The principal uses to which bioresmethrin is currently being put
are:
(a) in household aerosols and sprays formulated in combination
with pyrethrum, bioallethrin, tetramethrin and piperonyl
butoxide;
(b) as an insecticide for the control of pests in food premises;
(c) as a general purpose broad spectrum insecticide; and
(d) in grain disinfestation and protection.
Bioresmethrin has been tested in conjunction with a number of
well established synergists. Unlike natural pyrethrum, there is only a
minimal increase in performance against flies, mosquitos and
cockroaches when piperonyl butoxide is added to bioresmethrin.
However, in the case of a number of the more important insect pests of
stored products, bioresmethrin is synergized to a significant extent
with piperonyl butoxide, the factor of synergism ranging from four- to
ninefold.
Bioresmethrin shows good promise of becoming a major insecticide
for use in the control of stored product pests. It combines a high
potency, good persistence, and excellent safety features.
Treatment of plants
Although a number of trials have been reported where
bioresmethrin has given satisfactory results against a wide variety of
insect and mite pests of crops and ornamentals (Cooper McDougall and
Robertson, 1971, 1975; Cantu et al., 1970) there appears as yet only
limited commercial acceptance of bioresmethrin as a horticultural
insecticide. This reflects not only the state of development, but the
comparatively high cost and the relatively short residual life of
bioresmethrin spray deposits. There are however, many obvious
applications which will no doubt reach commercial development within
the next two to three years.
Non-crop situations
Many commercial uses have been developed for bioresmethrin in
non-crop situations including the control of flies, mosquitos and
other flying pests in households, pests of institutions and food
storage, aircraft disinfestation, animal-house treatments and similar
situations where aerosols, knockdown sprays and residual sprays are
conventionally used. In addition, bioresmethrin has been used in
thermal fog generators and in mosquito coils. None of these uses are
likely to give rise to significant residues in food or water, or to
cause significant intake by the general public.
Post-harvest application
Following the demonstration of outstanding activity against many
insect pests of stored products, numerous authors have reported
studies on the effect and usefulness of bioresmethrin for the control
of stored product pests and as a grain protectant (Ardley, 1973 and
1975; Ardley and Desmarchelier, 1974; Cooper McDougall and Robertson,
1971; Lloyd, 1973; Rowlands, 1975; Bengston et al., 1975a, 1975b).
Ardley (1975) showed that of many pyrethroid and organophosphorus
insecticides and insecticide combinations evaluated in silo trials,
the best product on a cost/efficiency basis was 4 ppm bioresmethrin
plus 20 ppm piperonyl butoxide. After 12 months, the treated grain
controlled all species and strains exposed in bioassay. A lower
efficiency was observed in the control of confused flour beetle
(Tribolium confusum).
The problem of resistance to malathion, dichlorvos and lindane
which is developing in stored product insects in almost all countries
has caused intensive examination of alternative protectant chemicals,
Lloyd (1973) in examining the toxicity of pyrethrins and five
synthetic pyrethroids to susceptible and pyrethrin-resistant strains
of stored product pests reported bioresmethrin to have up to 16 times
more toxicity than that of pyrethrins. He showed that piperonyl
butoxide synergized all the compounds against the various strains.
When synergized, bioresmethrin was again the most potent of the
synthetic compounds. L'Hoste et al. (1969) showed that bioresmethrin
deteriorated less rapidly than malathion when admixed with grain.
In Australia, the occurrence of malathion-resistant strains of
Rhyzopertha dominica is a severe threat to the grain export
industry. Malathion was never entirely satisfactory for the control of
this species, which shows a high degree of tolerance to all
organophosphorus materials that could possibly be used for the
protection of stored grains. The efficiency of bioresmethrin or
bioresmethrin/piperonyl butoxide combinations in controlling this
species is therefore important.
Bengston et al. (1975b) have shown clearly the high potency of
bioresmethrin against Rhyzopertha dominica which makes it an ideal
insecticide to complement such organophosphorus grain protectants as
malathion, pirimiphos-methyl and chlorpyrifos-methyl.
Table 1, from the work of Ardley and Desmarchelier (1974), shows
the efficiency of bioresmethrin/piperonyl butoxide combinations
against both susceptible and resistant strains of the more important
grain pests.
TABLE 1. Summary of Grain Protectant Trials, 1967-1974
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
1967 2 ppm 11-1/4 11.4 20.6 100 100 0 0 - 0 0 -
resmethrin + 20 ppm
pbo;2 2 ppm
pyrethrins + 20 10 11.5 20.7 85 100 1 0 - 0 0 -
ppm pbo
1968 1 ppm 4 approx. <10.0 29.0 99 100 0 1 0 0 0 0 0
bio-resmethrin + 10 or
ppm pbo; 0.5 ppm equal
to
bioresmethrin + 4 " <10.0 28.4 100 100 40 1 0 0 4 0 0
5 ppm pbo; 2 ppm
pyrethrins 3 " <10.0 20.6 95 100 20 0 0 2 10 10 1
+20 ppm pbo
1969 2 ppm 8 10.9 19.2 75 100 40 0 0 0 50 10 9
bio-resmethrin + 0.2
ppm antioxidant;
12 ppm malathion 8 10.8 20.7 100 80 90 23 0 0 0 1 0
* **
1973 4 ppm
bio-resmethrin 12 10.2 23.9 5 100 <8 - 0 0 63 0 -
8 ppm
bio-resmethrin 12 10.7 24.0 40 100 <15 - 0 0 3 1 -
12 ppm
bio-resmethrin 12 10.5 24.4 95 100 <38 - 2 0 0 0 -
TABLE 1. (cont'd)
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
4 ppm
bio-resmethrin +
20 ppm pbo 12 10.5 23.5 100 100 <10 - 1 0 0 0 -
8 ppm
bio-resmethrin + 20
ppm pbo 12 10.5 23.6 100 100 <9 - 0 0 1 1 -
12 ppm malathion 12 10.4 23.9 95 0 <8 - 3 1 0 6 -
control, on
clean wheat 12 10.5 23.5 4 5 - - 0 4 376 0 -
1 14 day period.
2 pbo = piperonyl butoxide.
3 Sgs = Sitophilus granarius )malathion
Rds = Rhyzopertha dominica )susceptible
Tcs = Tribolium castaneum ) strains.
*S. oryzae in all 1973/74 trials.
**T. castaneum assays discontinued after nine months.
Carter et al. (1975) have evaluated a range of synthetic
pyrethroid insecticides against susceptible and resistant strains of
stored product pests and have concluded that synergized bioresmethrin
was the most suitable pyrethroid and that it was of value in
controlling organophosphorus resistant beetles.
For reasons of economy, to increase the spectrum of effect
against the many different pests encountered in stored products and to
reduce the possibility of selecting resistant strains, it is proposed
that bioresmethrin be combined with one of the organophosphorus grain
protectants. There is already a good deal of evidence (Abernathy et
al., 1973; Jao and Casida, 1974) that organophosphorus compounds
inhibit the esterases responsible for the hydrolysis of bioresmethrin
in living systems.
When used for grain protection, bioresmethrin is applied with
piperonyl butoxide in the form of an emulsion which has been diluted
with water to a concentration which, when applied to bulk grain at the
rate of 1 litre per tonne, will deposit a known amount of
bioresmethrin evenly on the grain. The amount applied ranges from 1 to
4 ppm. The amount of piperonyl butoxide applied concurrently would
range from 5 to 20 mg/kg.
Treatment of animals
Bioresmethrin is suitable for the control of flies on cattle and
pigs but no information was available to indicate whether such uses
had yet been approved by registration authorities.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Fruit and vegetable crops
Only preliminary results are available to show the fate of
bioresmethrin on tomatoes (Buick and Flanagan, 1973) and on cucumbers
(Buick and Flanagan, 1974; Cooper McDougall and Robertson, 1975).
In these studies, radio-labelled bioresmethrin emulsified in
water was applied to tomato fruits (growing in a laboratory) at a rate
sufficient to deposit 1.25 mg/kg of bioresmethrin on the tomatoes. The
deposit applied to the growing tomatoes was estimated by determining
the total radioactivity after allowing the deposit to dry for one
hour. The fruit was harvested at 6, 12, 24, 48 and 72 hours after
application and the amount of bioresmethrin in the flesh, skin and on
the skin surface was measured after washing the fruit for two minutes
in benzene. The degradation products were removed from the benzene
solution by passing through alumina. The distribution of bioresmethrin
in and on tomatoes is shown in Table 2. The results are expressed as a
percentage of the total radioactivity applied.
TABLE 2. Distribution of bioresmethrin in tomatoes
Hours 1 6 12 24 48 72
Flesh 0.2 0.2 0.2 0.2 0.2 0.2
Skin 1.75 2.45 3.2 1.95 4.65 4.65
Benzene wash 92.5 39.0 50.0 54.5 22.5 15.5
TOTAL 94.25 41.45 53.2 56.45 27.15 20.15
From the table, it is evident that over 90% of the bioresmethrin
will be recovered by simply washing with benzene if the fruit is
harvested within 24 hours of spraying. If harvested later than this,
then up to 25% of the remaining insecticide may be held in the skin
three days after treatment but this will represent less than 5% of
that originally applied. The amount of bioresmethrin found in the
flesh is insignificant.
In the case of cucumbers (Buick and Flanagan, 1974) bioresmethrin
was applied as an emulsion to the surface of cucumbers growing in a
darkened laboratory equivalent to approximately 1 mg/kg of fruit. The
total radioactivity and radioactivity due to intact bioresmethrin was
determined on the surface, in the skin and in the flesh of cucumbers
harvested 1, 6, 12, 24, 48 and 72 hours after application.
The results indicate that although most of the radioactivity
applied is retained, the bioresmethrin is substantially degraded
within an hour of application and more slowly thereafter (24% survival
after one hour, 10% after 72 hours). This contrasts with the results
of the tomato experiments in which the bioresmethrin was found to
degrade more slowly. A simple washing of the intact fruit with benzene
removes at least 85% of the surviving bioresmethrin at any time up to
three days after treatment, whether or not the fruit is stored in the
dark before analysis. The results of this work are summarized in Table
3.
TABLE 3. Distribution of undegraded bioresmethrin in cucumbers
Bioresmethrin, mean of four results (from stored and unstored vegetables)
shown as % of "concentration applied", after interval (hours)
Fraction 1 6 12 24 48 72
Flesh <0.1 <0.1 <0.1 <0.1 <0.1 <0.1
Skin 0.8 1.6 0.7 1.4 0.8 1.2
Wash 23.3 14.7 8.1 9.8 7.0 8.8
TOTAL 24.1 16.3 8.8 11.2 7.8 10.0
FATE OF RESIDUES
On plants
Rosen (1972) showed that resmethrin and its alcohol moiety
photodecompose to many unidentified products at a rate which varies
with the supporting surface. Residues exposed to sunlight on silica
gel degrade more rapidly than residues on glass and different products
are formed. The author postulates that plant and soil surfaces will
show greater differences. Photodecomposition of bioresmethrin was
studied intensively, although under limited experimental conditions,
by using phenyl-14C alcohol and carboxy-14C acid preparations (Ueda
et al., 1974). After irradiation of the radioactive resmethrin on a
silica gel plate with a sunlamp for seven hours, 88% of the
radioactivity was recovered, 5% of which was the intact resmethrin.
Forty-three per cent. of the recovered radioactivity was identified
and of this 33% and 10% respectively were ester and non-ester
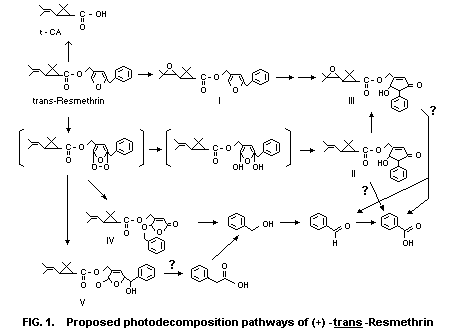
products. Figure 1 illustrates the proposed, although somewhat
speculative, photodecomposition pathways. The photodecomposition
product in largest amount (approximately one-fifth of the applied
radioactivity) was 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-chrysanthemate (II in Figure 1), followed by
trans-chrysanthemic acid and an unidentified ester (about 5% of
each). Four other oxidized esters with a modified alcohol moiety
accumulated slowly, but not to a high level (less than 3%). They are
the R and S isomers of 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-epoxychrysanthemate (III),
2-benzyloxy-5-oxo-2,5-dihydro-3-furylmethyl trans-chrysanthemic acid (V).
A minute amount of 2-benzyloxy-5-oxo- 2,5-dihydro-3-furylmethyl
trans-chrysanthemate (IV) was also detected. The R and S isomers
of trans-epoxyresmethrin (I) were minor products and did not
accumulate.
Thus, the initial pathways are of three types:
(1) oxidation of the furan ring to give a cyclic ozonide-type
peroxide intermediate (II);
(2) epoxidation of the isobutenyl double bond; and
(3) cleavage at the ester linkage to give chrysanthemic acid.
Ester bond cleavage would yield 5-benzyl-3-furylmethanol, but
none was found. 5-benzyl-3-furoic acid, alpha-(4-carboxyl-2-furyl)
benzyl alcohol and 5-benzoyl-3-furoic acid were not detected either.
This indicates that a more complex cleavage mechanism is involved or
that 5-benzyl-3-furylmethanol is not sufficiently stable to
accumulate. Benzyl alcohol, benzoic acid and phenylacetic acid (less
than 2% of each) were formed and tended to increase by extensive
oxidation. The unpleasant odour of photodecomposed bioresmethrin
appears to be due, at least in part, to the photochemical formation of
phenylacetic acid.
Following irradiation by sunlight, no appreciable changes in the
deco position pattern were observed except an increase of compound V.
A 0.14 mg/l aqueous solution of bioresmethrin irradiated with a
sunlamp yielded a larger amount of the R isomer of
trans-epoxyresmethrin (5.2% of the total radioactivity following 60
minutes' irradiation) and trans-chrysanthemic acid (11%). No
information was available on degradation studies carried out under
other conditions.
Andrews (1974) reports studies which indicate that when
bioresmethrin is applied to a forest environment by helicopter in the
form of an oil solution, there is a rapid disappearance of residues
from forest foliage and the surface of ponds. When 50 and 150 grams of
bioresmethrin in five litres of mineral oil was applied, it was
possible to determine residues of even the lower rate on foliage of
several forest species. Following application of the higher rate,
approximately 1 ppm of bioresmethrin was deposited on the foliage of
trees. Three days after application, approximately 0.3 mg/kg of
bioresmethrin could be detected on the foliage of willows but not on
other species. After seven days the deposit on willow foliage was at
the limit of determination (0.05 mg/kg). Although bioresmethrin
applied at the rate of 50 g/ha was toxic to most of the aquatic
insects in the first hour after application, no trace of bioresmethrin
could be found in any samples of pond water. Bioresmethrin was
detected in control samples containing 10 micrograms of bioresmethrin
per 100 ml of tap water.
Molecular formula C22H26O3
Molecular weight 338.45
Other information on identity and properties
Description : A viscous yellow liquid which on
crystallization forms an off-white solid
Specific gravity: 1.050 at 20°C
Flash point : 125°C (open cup)
Optical rotation: D20-5 to -8° : measurement on a 10% solution
in ethanol
Solubility : Soluble in most organic solvents but
substantially insoluble in water
Saponification
value : 160-175 mg KOH/gm
Stability : Bioresmethrin is sensitive to light but its
photo stability is greater than that of
pyrethrins. Bioresmethrin is stable to
temperatures met under most normal storage
conditions.
Bioresmethrin spray deposits appear subject
to atmospheric oxidation but any adverse
effect appears to be reduced by the
introduction of antioxidants including
piperonyl butoxide and butylated
hydroxyanisole.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bioresmethrin is one of the most effective broad spectrum
insecticides currently available. It exhibits a high order of
insecticidal activity, which when coupled with its excellent
toxicological properties, makes it potentially one of the safest and
most useful insecticides now being produced.
In addition to its insecticidal properties, bioresmethrin has a
good knock-down performance against flying insects, particularly when
this is compared with the organochlorine or organophosphorus
insecticides.
Bioresmethrin, at low concentrations, is an effective killing
agent against most insect pests affecting households, industrial
premises, public health, food storage and livestock production.
Efficacy against many insect pests of food crops and ornamentals has
been demonstrated.
The principal uses to which bioresmethrin is currently being put
are:
(a) in household aerosols and sprays formulated in combination
with pyrethrum, bioallethrin, tetramethrin and piperonyl
butoxide;
(b) as an insecticide for the control of pests in food premises;
(c) as a general purpose broad spectrum insecticide; and
(d) in grain disinfestation and protection.
Bioresmethrin has been tested in conjunction with a number of
well established synergists. Unlike natural pyrethrum, there is only a
minimal increase in performance against flies, mosquitos and
cockroaches when piperonyl butoxide is added to bioresmethrin.
However, in the case of a number of the more important insect pests of
stored products, bioresmethrin is synergized to a significant extent
with piperonyl butoxide, the factor of synergism ranging from four- to
ninefold.
Bioresmethrin shows good promise of becoming a major insecticide
for use in the control of stored product pests. It combines a high
potency, good persistence, and excellent safety features.
Treatment of plants
Although a number of trials have been reported where
bioresmethrin has given satisfactory results against a wide variety of
insect and mite pests of crops and ornamentals (Cooper McDougall and
Robertson, 1971, 1975; Cantu et al., 1970) there appears as yet only
limited commercial acceptance of bioresmethrin as a horticultural
insecticide. This reflects not only the state of development, but the
comparatively high cost and the relatively short residual life of
bioresmethrin spray deposits. There are however, many obvious
applications which will no doubt reach commercial development within
the next two to three years.
Non-crop situations
Many commercial uses have been developed for bioresmethrin in
non-crop situations including the control of flies, mosquitos and
other flying pests in households, pests of institutions and food
storage, aircraft disinfestation, animal-house treatments and similar
situations where aerosols, knockdown sprays and residual sprays are
conventionally used. In addition, bioresmethrin has been used in
thermal fog generators and in mosquito coils. None of these uses are
likely to give rise to significant residues in food or water, or to
cause significant intake by the general public.
Post-harvest application
Following the demonstration of outstanding activity against many
insect pests of stored products, numerous authors have reported
studies on the effect and usefulness of bioresmethrin for the control
of stored product pests and as a grain protectant (Ardley, 1973 and
1975; Ardley and Desmarchelier, 1974; Cooper McDougall and Robertson,
1971; Lloyd, 1973; Rowlands, 1975; Bengston et al., 1975a, 1975b).
Ardley (1975) showed that of many pyrethroid and organophosphorus
insecticides and insecticide combinations evaluated in silo trials,
the best product on a cost/efficiency basis was 4 ppm bioresmethrin
plus 20 ppm piperonyl butoxide. After 12 months, the treated grain
controlled all species and strains exposed in bioassay. A lower
efficiency was observed in the control of confused flour beetle
(Tribolium confusum).
The problem of resistance to malathion, dichlorvos and lindane
which is developing in stored product insects in almost all countries
has caused intensive examination of alternative protectant chemicals,
Lloyd (1973) in examining the toxicity of pyrethrins and five
synthetic pyrethroids to susceptible and pyrethrin-resistant strains
of stored product pests reported bioresmethrin to have up to 16 times
more toxicity than that of pyrethrins. He showed that piperonyl
butoxide synergized all the compounds against the various strains.
When synergized, bioresmethrin was again the most potent of the
synthetic compounds. L'Hoste et al. (1969) showed that bioresmethrin
deteriorated less rapidly than malathion when admixed with grain.
In Australia, the occurrence of malathion-resistant strains of
Rhyzopertha dominica is a severe threat to the grain export
industry. Malathion was never entirely satisfactory for the control of
this species, which shows a high degree of tolerance to all
organophosphorus materials that could possibly be used for the
protection of stored grains. The efficiency of bioresmethrin or
bioresmethrin/piperonyl butoxide combinations in controlling this
species is therefore important.
Bengston et al. (1975b) have shown clearly the high potency of
bioresmethrin against Rhyzopertha dominica which makes it an ideal
insecticide to complement such organophosphorus grain protectants as
malathion, pirimiphos-methyl and chlorpyrifos-methyl.
Table 1, from the work of Ardley and Desmarchelier (1974), shows
the efficiency of bioresmethrin/piperonyl butoxide combinations
against both susceptible and resistant strains of the more important
grain pests.
TABLE 1. Summary of Grain Protectant Trials, 1967-1974
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
1967 2 ppm 11-1/4 11.4 20.6 100 100 0 0 - 0 0 -
resmethrin + 20 ppm
pbo;2 2 ppm
pyrethrins + 20 10 11.5 20.7 85 100 1 0 - 0 0 -
ppm pbo
1968 1 ppm 4 approx. <10.0 29.0 99 100 0 1 0 0 0 0 0
bio-resmethrin + 10 or
ppm pbo; 0.5 ppm equal
to
bioresmethrin + 4 " <10.0 28.4 100 100 40 1 0 0 4 0 0
5 ppm pbo; 2 ppm
pyrethrins 3 " <10.0 20.6 95 100 20 0 0 2 10 10 1
+20 ppm pbo
1969 2 ppm 8 10.9 19.2 75 100 40 0 0 0 50 10 9
bio-resmethrin + 0.2
ppm antioxidant;
12 ppm malathion 8 10.8 20.7 100 80 90 23 0 0 0 1 0
* **
1973 4 ppm
bio-resmethrin 12 10.2 23.9 5 100 <8 - 0 0 63 0 -
8 ppm
bio-resmethrin 12 10.7 24.0 40 100 <15 - 0 0 3 1 -
12 ppm
bio-resmethrin 12 10.5 24.4 95 100 <38 - 2 0 0 0 -
TABLE 1. (cont'd)
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
4 ppm
bio-resmethrin +
20 ppm pbo 12 10.5 23.5 100 100 <10 - 1 0 0 0 -
8 ppm
bio-resmethrin + 20
ppm pbo 12 10.5 23.6 100 100 <9 - 0 0 1 1 -
12 ppm malathion 12 10.4 23.9 95 0 <8 - 3 1 0 6 -
control, on
clean wheat 12 10.5 23.5 4 5 - - 0 4 376 0 -
1 14 day period.
2 pbo = piperonyl butoxide.
3 Sgs = Sitophilus granarius )malathion
Rds = Rhyzopertha dominica )susceptible
Tcs = Tribolium castaneum ) strains.
*S. oryzae in all 1973/74 trials.
**T. castaneum assays discontinued after nine months.
Carter et al. (1975) have evaluated a range of synthetic
pyrethroid insecticides against susceptible and resistant strains of
stored product pests and have concluded that synergized bioresmethrin
was the most suitable pyrethroid and that it was of value in
controlling organophosphorus resistant beetles.
For reasons of economy, to increase the spectrum of effect
against the many different pests encountered in stored products and to
reduce the possibility of selecting resistant strains, it is proposed
that bioresmethrin be combined with one of the organophosphorus grain
protectants. There is already a good deal of evidence (Abernathy et
al., 1973; Jao and Casida, 1974) that organophosphorus compounds
inhibit the esterases responsible for the hydrolysis of bioresmethrin
in living systems.
When used for grain protection, bioresmethrin is applied with
piperonyl butoxide in the form of an emulsion which has been diluted
with water to a concentration which, when applied to bulk grain at the
rate of 1 litre per tonne, will deposit a known amount of
bioresmethrin evenly on the grain. The amount applied ranges from 1 to
4 ppm. The amount of piperonyl butoxide applied concurrently would
range from 5 to 20 mg/kg.
Treatment of animals
Bioresmethrin is suitable for the control of flies on cattle and
pigs but no information was available to indicate whether such uses
had yet been approved by registration authorities.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Fruit and vegetable crops
Only preliminary results are available to show the fate of
bioresmethrin on tomatoes (Buick and Flanagan, 1973) and on cucumbers
(Buick and Flanagan, 1974; Cooper McDougall and Robertson, 1975).
In these studies, radio-labelled bioresmethrin emulsified in
water was applied to tomato fruits (growing in a laboratory) at a rate
sufficient to deposit 1.25 mg/kg of bioresmethrin on the tomatoes. The
deposit applied to the growing tomatoes was estimated by determining
the total radioactivity after allowing the deposit to dry for one
hour. The fruit was harvested at 6, 12, 24, 48 and 72 hours after
application and the amount of bioresmethrin in the flesh, skin and on
the skin surface was measured after washing the fruit for two minutes
in benzene. The degradation products were removed from the benzene
solution by passing through alumina. The distribution of bioresmethrin
in and on tomatoes is shown in Table 2. The results are expressed as a
percentage of the total radioactivity applied.
TABLE 2. Distribution of bioresmethrin in tomatoes
Hours 1 6 12 24 48 72
Flesh 0.2 0.2 0.2 0.2 0.2 0.2
Skin 1.75 2.45 3.2 1.95 4.65 4.65
Benzene wash 92.5 39.0 50.0 54.5 22.5 15.5
TOTAL 94.25 41.45 53.2 56.45 27.15 20.15
From the table, it is evident that over 90% of the bioresmethrin
will be recovered by simply washing with benzene if the fruit is
harvested within 24 hours of spraying. If harvested later than this,
then up to 25% of the remaining insecticide may be held in the skin
three days after treatment but this will represent less than 5% of
that originally applied. The amount of bioresmethrin found in the
flesh is insignificant.
In the case of cucumbers (Buick and Flanagan, 1974) bioresmethrin
was applied as an emulsion to the surface of cucumbers growing in a
darkened laboratory equivalent to approximately 1 mg/kg of fruit. The
total radioactivity and radioactivity due to intact bioresmethrin was
determined on the surface, in the skin and in the flesh of cucumbers
harvested 1, 6, 12, 24, 48 and 72 hours after application.
The results indicate that although most of the radioactivity
applied is retained, the bioresmethrin is substantially degraded
within an hour of application and more slowly thereafter (24% survival
after one hour, 10% after 72 hours). This contrasts with the results
of the tomato experiments in which the bioresmethrin was found to
degrade more slowly. A simple washing of the intact fruit with benzene
removes at least 85% of the surviving bioresmethrin at any time up to
three days after treatment, whether or not the fruit is stored in the
dark before analysis. The results of this work are summarized in Table
3.
TABLE 3. Distribution of undegraded bioresmethrin in cucumbers
Bioresmethrin, mean of four results (from stored and unstored vegetables)
shown as % of "concentration applied", after interval (hours)
Fraction 1 6 12 24 48 72
Flesh <0.1 <0.1 <0.1 <0.1 <0.1 <0.1
Skin 0.8 1.6 0.7 1.4 0.8 1.2
Wash 23.3 14.7 8.1 9.8 7.0 8.8
TOTAL 24.1 16.3 8.8 11.2 7.8 10.0
FATE OF RESIDUES
On plants
Rosen (1972) showed that resmethrin and its alcohol moiety
photodecompose to many unidentified products at a rate which varies
with the supporting surface. Residues exposed to sunlight on silica
gel degrade more rapidly than residues on glass and different products
are formed. The author postulates that plant and soil surfaces will
show greater differences. Photodecomposition of bioresmethrin was
studied intensively, although under limited experimental conditions,
by using phenyl-14C alcohol and carboxy-14C acid preparations (Ueda
et al., 1974). After irradiation of the radioactive resmethrin on a
silica gel plate with a sunlamp for seven hours, 88% of the
radioactivity was recovered, 5% of which was the intact resmethrin.
Forty-three per cent. of the recovered radioactivity was identified
and of this 33% and 10% respectively were ester and non-ester
products. Figure 1 illustrates the proposed, although somewhat
speculative, photodecomposition pathways. The photodecomposition
product in largest amount (approximately one-fifth of the applied
radioactivity) was 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-chrysanthemate (II in Figure 1), followed by
trans-chrysanthemic acid and an unidentified ester (about 5% of
each). Four other oxidized esters with a modified alcohol moiety
accumulated slowly, but not to a high level (less than 3%). They are
the R and S isomers of 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-epoxychrysanthemate (III),
2-benzyloxy-5-oxo-2,5-dihydro-3-furylmethyl trans-chrysanthemic acid (V).
A minute amount of 2-benzyloxy-5-oxo- 2,5-dihydro-3-furylmethyl
trans-chrysanthemate (IV) was also detected. The R and S isomers
of trans-epoxyresmethrin (I) were minor products and did not
accumulate.
Thus, the initial pathways are of three types:
(1) oxidation of the furan ring to give a cyclic ozonide-type
peroxide intermediate (II);
(2) epoxidation of the isobutenyl double bond; and
(3) cleavage at the ester linkage to give chrysanthemic acid.
Ester bond cleavage would yield 5-benzyl-3-furylmethanol, but
none was found. 5-benzyl-3-furoic acid, alpha-(4-carboxyl-2-furyl)
benzyl alcohol and 5-benzoyl-3-furoic acid were not detected either.
This indicates that a more complex cleavage mechanism is involved or
that 5-benzyl-3-furylmethanol is not sufficiently stable to
accumulate. Benzyl alcohol, benzoic acid and phenylacetic acid (less
than 2% of each) were formed and tended to increase by extensive
oxidation. The unpleasant odour of photodecomposed bioresmethrin
appears to be due, at least in part, to the photochemical formation of
phenylacetic acid.
Following irradiation by sunlight, no appreciable changes in the
deco position pattern were observed except an increase of compound V.
A 0.14 mg/l aqueous solution of bioresmethrin irradiated with a
sunlamp yielded a larger amount of the R isomer of
trans-epoxyresmethrin (5.2% of the total radioactivity following 60
minutes' irradiation) and trans-chrysanthemic acid (11%). No
information was available on degradation studies carried out under
other conditions.
Andrews (1974) reports studies which indicate that when
bioresmethrin is applied to a forest environment by helicopter in the
form of an oil solution, there is a rapid disappearance of residues
from forest foliage and the surface of ponds. When 50 and 150 grams of
bioresmethrin in five litres of mineral oil was applied, it was
possible to determine residues of even the lower rate on foliage of
several forest species. Following application of the higher rate,
approximately 1 ppm of bioresmethrin was deposited on the foliage of
trees. Three days after application, approximately 0.3 mg/kg of
bioresmethrin could be detected on the foliage of willows but not on
other species. After seven days the deposit on willow foliage was at
the limit of determination (0.05 mg/kg). Although bioresmethrin
applied at the rate of 50 g/ha was toxic to most of the aquatic
insects in the first hour after application, no trace of bioresmethrin
could be found in any samples of pond water. Bioresmethrin was
detected in control samples containing 10 micrograms of bioresmethrin
per 100 ml of tap water.
 Extensive studies now in progress in Australia are designed to
determine the effective life of bioresmethrin deposits on grain stored
under a wide variety of conditions of temperature, moisture and
aeration. Preliminary results indicate that temperature has a distinct
bearing on the residual life of the treatment and that grain
temperatures of 30-35°C reduce the half-life to approximately 8-10
weeks.
Desmarchelier (1975b) has found that grain treated with
bioresmethrin and subjected to bioassay with Rhyzopertha dominica,
shows a consistent loss of insecticidal effect which is directly
dependent on temperature. The "half-life" shown by these studies is as
follows:
Storage temperature Half-life
20°C >20 weeks
25°C 12 weeks
30°C 10 weeks
35°C 8 weeks
These studies are being confirmed by chemical analysis.
Desmarchelier (1975a) who is studying the fate of bioresmethrin
in various grains stored under a range of temperature and moisture
conditions reports preliminary results after seven weeks of a 12-month
storage programme. The results in Table 4 indicate that piperonyl
butoxide has a pronounced influence on stability even at 35°C.
Preliminary calculations indicate a half-life of 30 weeks at 30°C in
the presence of 10 times the amount of piperonyl butoxide.
Studies currently in progress in Australia (Bengston et al.,
1975) in which bioresmethrin has been used with primiphos-methyl to
treat bulk wheat held in commercial silos, both aerated and
non-aerated, indicate that the bioresmethrin deposit remains stable
for long periods. Wheat treated with a nominal 4 mg/kg bioresmethrin
was found to contain 3.1 mg/kg when analysed a few days later. This
wheat was maintained in an unaerated silo at 24°C and in an aerated
silo at 14°C (lower temperature due to aeration). Six weeks after
treatment the residue level was found to be 2.2 mg/kg (unaerated) and
2.4 mg/kg (aerated) At the end of six months, the bioresmethrin
residue had declined in only 1.1 mg/kg (unaerated) and 1.9 mg/kg
(aerated).
FATE OF RESIDUES
In animals
Extensive studies of the metabolism of bioresmethrin in animals
have been carried out by many authors including the following:
Abernathy and Casida (1973); Abernathy et al. (1973a); Farebrother
(1973); Foote et al. (1967); Jao and Casida (1974); Miyamoto (1975);
Miyamoto et al. (1971); Miyamoto et al. (1974); Suzuki and Miyamoto
(1974); Ueda et al. (1975a,b); Weeks et al. (1972).
Most of these studies have been carried out on laboratory
animals, mainly rats. Radioactivity measurements and radioautographs
indicate that the compound is rapidly absorbed from the intestinal
tract and distributed into various tissues where only a negligible
amount of intact bioresmethrin was found. However, the radioactivity
was excreted rather slowly and it took three weeks to recover all the
radioactivity in the excrete (36% in urine and 64% in faeces).
Neither urine nor faeces contained intact bioresmethrin or the
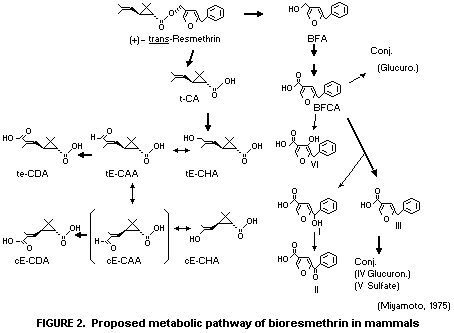
ester metabolites. The predominant urinary metabolite being
5-benzyl-3-furoic acid amounting to approximately one-third of the
radiocarbon recovered. A proposed metabolic pathway of bioresmethrin
is shown in Figure 2.
TABLE 4. Bioresmethrin residues in wheat stored at various temperatures
Moisture Bioresmethrin residue, mg/kg, after
content Initial storage at:
of grain bioresmethrin
Treatment % level 10°C 20°C 25°C 30°C 35°C
Bioresmethrin on
wheat 11.9 3.3 2.7 2.4 2.1 1.9 1.9
Bioresmethrin +
piperonyl
butoxide +
antioxidant
1/10/1 on wheat 11.9 3.3 - - - 2.9 -
Bioresmethrin on
wheat 10.2 2.8 - - 2.3 - 2.2
Bioresmethrin on
husked rice 12.0 3.3 2.9 2.3 - 1.5 -
Bioresmethrin +
piperonyl
butoxide (1/10)
on husked rice 12.0 3.3 - 3.1 - 2.9 -
Bioresmethrin on
polished rice 13.0 2.8 - - 0.8 - 0.8
Bioresmethrin +
piperonyl
butoxide (1/10)
on polished rice 13.0 2.8 - - 1.7 - 1.4
Extensive studies now in progress in Australia are designed to
determine the effective life of bioresmethrin deposits on grain stored
under a wide variety of conditions of temperature, moisture and
aeration. Preliminary results indicate that temperature has a distinct
bearing on the residual life of the treatment and that grain
temperatures of 30-35°C reduce the half-life to approximately 8-10
weeks.
Desmarchelier (1975b) has found that grain treated with
bioresmethrin and subjected to bioassay with Rhyzopertha dominica,
shows a consistent loss of insecticidal effect which is directly
dependent on temperature. The "half-life" shown by these studies is as
follows:
Storage temperature Half-life
20°C >20 weeks
25°C 12 weeks
30°C 10 weeks
35°C 8 weeks
These studies are being confirmed by chemical analysis.
Desmarchelier (1975a) who is studying the fate of bioresmethrin
in various grains stored under a range of temperature and moisture
conditions reports preliminary results after seven weeks of a 12-month
storage programme. The results in Table 4 indicate that piperonyl
butoxide has a pronounced influence on stability even at 35°C.
Preliminary calculations indicate a half-life of 30 weeks at 30°C in
the presence of 10 times the amount of piperonyl butoxide.
Studies currently in progress in Australia (Bengston et al.,
1975) in which bioresmethrin has been used with primiphos-methyl to
treat bulk wheat held in commercial silos, both aerated and
non-aerated, indicate that the bioresmethrin deposit remains stable
for long periods. Wheat treated with a nominal 4 mg/kg bioresmethrin
was found to contain 3.1 mg/kg when analysed a few days later. This
wheat was maintained in an unaerated silo at 24°C and in an aerated
silo at 14°C (lower temperature due to aeration). Six weeks after
treatment the residue level was found to be 2.2 mg/kg (unaerated) and
2.4 mg/kg (aerated) At the end of six months, the bioresmethrin
residue had declined in only 1.1 mg/kg (unaerated) and 1.9 mg/kg
(aerated).
FATE OF RESIDUES
In animals
Extensive studies of the metabolism of bioresmethrin in animals
have been carried out by many authors including the following:
Abernathy and Casida (1973); Abernathy et al. (1973a); Farebrother
(1973); Foote et al. (1967); Jao and Casida (1974); Miyamoto (1975);
Miyamoto et al. (1971); Miyamoto et al. (1974); Suzuki and Miyamoto
(1974); Ueda et al. (1975a,b); Weeks et al. (1972).
Most of these studies have been carried out on laboratory
animals, mainly rats. Radioactivity measurements and radioautographs
indicate that the compound is rapidly absorbed from the intestinal
tract and distributed into various tissues where only a negligible
amount of intact bioresmethrin was found. However, the radioactivity
was excreted rather slowly and it took three weeks to recover all the
radioactivity in the excrete (36% in urine and 64% in faeces).
Neither urine nor faeces contained intact bioresmethrin or the
ester metabolites. The predominant urinary metabolite being
5-benzyl-3-furoic acid amounting to approximately one-third of the
radiocarbon recovered. A proposed metabolic pathway of bioresmethrin
is shown in Figure 2.
TABLE 4. Bioresmethrin residues in wheat stored at various temperatures
Moisture Bioresmethrin residue, mg/kg, after
content Initial storage at:
of grain bioresmethrin
Treatment % level 10°C 20°C 25°C 30°C 35°C
Bioresmethrin on
wheat 11.9 3.3 2.7 2.4 2.1 1.9 1.9
Bioresmethrin +
piperonyl
butoxide +
antioxidant
1/10/1 on wheat 11.9 3.3 - - - 2.9 -
Bioresmethrin on
wheat 10.2 2.8 - - 2.3 - 2.2
Bioresmethrin on
husked rice 12.0 3.3 2.9 2.3 - 1.5 -
Bioresmethrin +
piperonyl
butoxide (1/10)
on husked rice 12.0 3.3 - 3.1 - 2.9 -
Bioresmethrin on
polished rice 13.0 2.8 - - 0.8 - 0.8
Bioresmethrin +
piperonyl
butoxide (1/10)
on polished rice 13.0 2.8 - - 1.7 - 1.4
 Farebrother (1973) in a whole body autoradiographical study in
rats showed that bioresmethrin was absorbed through the gut and widely
distributed in the body two hours after dosing. At six hours the
distribution was similar but the concentrations were increased,
particularly in fatty tissues. At 24 hours most tissues showed greatly
reduced activity but the concentration in fatty tissue remained high.
No studies appear to have been carried out to determine the level
or nature of the metabolites in animal fat or other tissues. No
studies are yet available to show the fate of bioresmethrin when fed
to livestock or poultry.
Wheat
Ardley (1975) reports trials in which wheat treated with
bioresmethrin at varying rates with and without piperonyl butoxide,
was subjected to standard milling and baking tests.
The grain, when milled gave 25% bran, 7% pollard (shorts) and 68%
flour. The flour was converted into bread. All loaves were
satisfactory. No taint or odour was evident during dough processing or
in the loaves fresh from the oven.
The milling fractions and the bread were analysed. The nature of
the chemical treatment and the results of analysis are provided in the
following table. Analyses were carried out by two laboratories and the
results were in good agreement.
TABLE 5. Residues of bioresmethrin and piperonyl butoxide in milling
fractions and bread
Recovered residue (i) bioresmethrin (ii) piperonyl butoxide (ppm)
(i) (ii)
Protectant1
treatment bran pollard flour bread bran pollard flour bread
a 0.3 4.0 4.0 nil 21.0 10.0 2.0 2.0
b 0.3 1.0 1.4 nil 0.5 0.3 nil nil
c 0.5 1.0 0.5 nil 11.0 8.0 10.0 nil
d 1.1 1.2 0.5 nil 2.0 2.0 2.0 nil
e nil nil nil nil nil nil nil nil
1 a = 4 mg/kg bioresmethrin + 20 mg/kg piperonyl butoxide
b = 2 " " alone
c = 4 " " + 10 mg/kg piperonyl butoxide +
4 mg/kg anti-oxidant
d = 2 " " + 2 mg/kg piperonyl butoxide +
10 mg/kg anti-oxidant + 4 mg/kg
fenitrothion.
In a subsequent study Desmarchelier ( 1975b) took wheat which had
been treated six weeks (A) and seven months (B) previously and
subjected it to standard milling procedures for the preparation of
wholemeal and white flour. The following results indicate the
distribution and fate of the bioresmethrin:
Sample A B
Interval since treated 6 weeks 7 months
Residue in wheat 2.9 mg/kg 1.9 mg/kg
bran 5.2 " 1.7 "
pollard (shorts) 0.7 " -
white flour * *
wholemeal bread 1.0 " 0.6 "
white bread ND ND
limit of determination = 0.05 mg/kg
* Interference led to poor recoveries during assay of flour.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Bioresmethrin has not yet been widely used on food crops or for
the treatment of stored grain and therefore no information is
available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The analytical methods for resmethrin have been reviewed by Brown
(1973) and also by Murano et al. (1971), Murano (1972) and
Desmarchelier (1975). The methods include ultra-violet absorption
spectrophotometry, colorimetry, TLC and GLC. SE-30, DC200 ‰ QFI and DEGS
have been used as stationary phases for the GLC determination of
resmethrin in crops after suitable clean-up procedures. Brown (1973)
proposes a residue analytical method for wheat, wheat flour and corn
meal in which resmethrin is extracted with hexane, partitioned between
hexane and acetonitrile, cleaned up on an Alumina column, and
determined by GLC.
The difficulty in the residue analysis of resmethrin, which would
apply equally to the optical isomer bioresmethrin, seems to lie in the
intrinsically low sensitivity to the compound of electron capture or
flame ionization detectors. The limit of detection ranges at best from
0.1 to 0.05 µg, equivalent in practice to a residue level of about
0.1 mg/kg.
To overcome the drawback, the component chrysanthemic acid is
quantitatively condensed with 2,4-dichlorobenzyl chloride to yield
2,4-dichlorobenzyl chrysanthemate, which can be determined by electron
capture (Miyamoto, 1975), It is reported that this modification allows
as little as 0.01 ng of the derivative or approximately 0.01 ng of
bioresmethrin to be analysed. Up to 100 µg of bioresmethrin is
hydrolyzed in 10% methanolic potassium hydroxide for one hour at 60°C.
Chrysanthemic acid is extracted with chloroform at pH2. The chloroform
is evaporated in vacuo and the residue dissolved in 5 ml acetone and
warmed at 60°C for 120 min with the addition of 0.1 ml of
N,N-dimethylformamide and one drop each of triethylamine and
2,4-dichlorobenzyl chloride. The reaction mixture is passed through a
Florisil column and eluted with benzene. 2,4-dichlorobenzyl
chrysanthemate is chromatographed under the following conditions.
Liquid phase Epon-1001 (1%) and QF-1 (1%) on Chromosorb B (100 to 120
mesh); temperature of oven and detector, 200°C and of injection port,
240°C; carrier nitrogen, 1.0 kg/cm›. Retention time of
2,4-dichlorobenzyl chrysanthemate about 4-1/2 min. The overall
recovery in the range 1 to 100 ng of resmethrin is at least 80%, often
more than 90%. Resmethrin residues of the order of 1 µg/kg can
therefore be determined.
Desmarchelier (1975a,b) reports a colorimetric method based an
the procedure of Screiber and McClellan (1954) and McClellan (1964)
originally designed for the analysis of pyrethrum and based on the
analysis of chrysanthemic acid. The author examines the applicability
of this procedure to residue analysis of five pyrethroid derivatives
of chrysanthemic acid on six commodities and extends it to derivatives
of pyrethric acid, for example pyrethrin II. In addition, a TLC
procedure is described that allows positive identification of
derivatives of chrysanthemic acid in a one-step process. In the case
of bioresmethrin, results obtained by these two procedures on aged
residues on wheat were compared with results obtained by two
procedures specific to bioresmethrin. Appreciable differences between
results occurred only at the limit of determination. In the method of
Desmarchelier, petroleum spirit (b.p. 30-40°C) was added to whole
grain (100 g) and left overnight. The supernatant was decanted, the
grain covered with solvent and extracted for a further hour and this
procedure repeated once more. Methanol, acetone and 10% acetone in
petroleum spirit appear equally suitable as solvents.
Although none of the metabolites or photo-products have been
reported to be analysed in the actual samples, various techniques and
procedures for separation as well as identification of the possible
terminal residues of bioresmethrin are described in a number of papers
including those of Miyamoto et al. (1971); Ueda et al. (1974, 1975a
and b); Abernathy (1973); Brown (1973) and Murano (1972).
Simonartis and Coil (1975) have developed a GLC method for the
determination of resmethrin in corn, corn meal, flour and wheat. it
involves extraction with pentane, transfer to acetonitrile, Florisil
clean-up using 3% ethyl acetate in perthane followed by GLC using
flame conization detector. The method is sensitive to 0.2 ppm with
better than 85% recovery and good reproductivity.
NATIONAL TOLERANCES
The information available to the Meeting suggests that no
national tolerances have yet been established.
APPRAISAL
Bioresmethrin is a broad spectrum pyrethroid insecticide with
contact action and pronounced knockdown effect. It shows only limited
biological persistence on plant surfaces and is readily degraded by
sunlight but gives long-lasting control of insect pests on inert
surfaces and retains its biological activity when applied to stored
agricultural commodities, including raw grain and nuts.
The Meeting had only limited information concerning the use and
performance of this material on crops pre-harvest but extensive data
were available on the use, performance and fate of bioresmethrin on a
variety of grains. The recommended rate of application to raw cereals
is 1 to 4 mg/kg. The biological half-life on grain ranges from eight
weeks at 35°C to more than 20 weeks at 20°C.
Data available suggest that when bioresmethrin is applied to
wheat, the deposit is not confined to the seed coat but penetrates to
the endosperm so that a substantial proportion of the amount applied
is found in flour. Standard milling techniques which remove most of
the seed coat, produce a bran which contains considerably less
bioresmethrin than does the whole grain or the flour. Virtually all of
the bioresmethrin is destroyed when bread prepared from such flour is
cooked.
Extensive information is available on the biological degradation
and metabolism of bioresmethrin including the nature of the
metabolites formed under a wide variety of conditions. The initial
step in the metabolism of bioresmethrin is hydrolysis at the ester
linkage, yielding 5-benzyl-3-furylmethanol and chrysanthemic acid.
Several GLC methods of analysis suitable for the determination of
residues of bioresmethrin and metabolites containing chrysanthemic
acid in plant materials are available. The limit of determination
ranges from 0.1 to 0.001 mg/kg. In addition there are several
colorimetric methods based an the determination of chrysanthemic acid
which can be used in conjunction with a TLC procedure for identifying
the pyrethroid residue involved. No national tolerances have yet been
determined.
In proposing maximum residue limits for bioresmethrin on raw
grain, milled cereal products and foods prepared therefrom, careful
consideration has been given to the fact that this insecticide is to
be used as a grain protectant, that a certain concentration must be
present in the grain to control infestations and prevent damage to
stored products and that the compound is moderately stable under
storage conditions. Under practical conditions of grain handling and
storage, there will always be a natural variation in the level of the
deposit resulting from a fluctuation in the flow of grain and
insecticide. it is therefore not possible to fix the maximum limit on
the minimum necessary to control insect pests. At the present time, it
is anticipated that bioresmethrin will be used in conjunction with one
or more of the organophosphorus grain protectants whose effect is
complementary and possibly synergistic. Under such conditions the
amount of bioresmethrin used will be at the lower range of the
proposed rates. However, there will be situations where bioresmethrin
must be used alone at the maximum of the proposed rate and due
allowance must be made for the amplitude of the variations in
concentration which are inevitable and the problems of sampling and
analysis involved.
RECOMMENDATIONS
The following guideline levels are recommended as limits which
need not be exceeded when bioresmethrin is used according to good
agricultural practices in the following commodities:
Commodity Maximum residue limit, mg/kg
Raw grain 5
Milled products from grain 5
Cooked cereal products, including 0.05*
bread
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Full toxicological data.
DESIRED
1. Further information on the level and fate of bioresmethrin
on different classes of raw grains.
2. Information on residues from supervised trials on other
stored commodities, including nuts, peanuts, lentils, dried
fruit and dried vegetables,
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in
food at the point of consumption following the use of
bioresmethrin for the control of various stored-product
pests.
5. Improved procedures for the determination of bioresmethrin
residues in fruit and vegetables as well as stored products.
REFERENCES
Abernathy, C. O. and Casida, J. E. (1973) Pyrethroid insecticides:
esterase cleavage in relation to selective toxicity. Science, 179:
1235-1236.
Abernathy, C. O., Ueda, K., Engel, J. L., Gaughan, L. C. and Casida,
J. E. (1973a) Substrate specificity and toxicological significance of
pyrethroid hydrolyzing esterases of mouse liver microsomes. Pesticide
Biochemistry and Physiology, 3: 300-311.
Andrews, T. L. (1974) Resmethrin Residues in foliage after aerial
application. Pesticide Monitoring Journal, 8(1):50-52.
Ardley, J. H. (1973) Further field evaluation of bioresmethrin as a
potential replacement for malathion in grain protection. Wellcome
Australia Ltd Report TD/5/73/Kl-36.
Ardley, J. H. (1975) Use and application of resmethrin and
bioresmethrin as potential grain protectants. Paper submitted to
Journal of Stored Products Research (May 1975)
Ardley, J. H. and Desmarchelier, J. M. (1974) Investigations into the
use of resmethrin and bioresmethrin as potential grain protectants.
Proceedings of the First International Working Conference on Stored
Product Entomology, Savannah, Georgia, USA.
Ardley, J. H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat. Report of Wellcome Australia Ltd, 27
August 1975.
Bengston, M., Cooper, L. M. and Holton, F. J. (1975b) A comparison of
bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as grain
protectants against malathion-resistant insects in wheat. Submitted
for publication, Queensland Journal of Agricultural and Animal
Sciences.
Bengston, M., Connell M., Desmarchelier, J., Snelson, J. and Sticka,
R. (1975a) Report of field trials with grain protectants. To be
published.
Brooks, I. C., Hans, J., Blumenthal, R. R. and Davis, B. S. (1969) SBP
1382, a new synthetic pyrethroid. Soap Chem. Spec., 45:62.
Brown, B. B. (1973) In "Analytical methods for pesticides and growth
regulators". Edited by G. Zweig (Academic Press), p. 441.
Buick, A. R. and Flanagan, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes. Wellcome Foundation Report HEGH 73/1.
Buick, A. R. and Flanagan, P. (1974) The fate of bioresmethrin on
cucumbers. Wellcome Foundation Report HEGH 74/1.
Cantu, E. and Wolfenbarger, D. A. (1970) Toxicity tests of three
pyrethroids to several insect pests of cotton. J. econ. Ent., 63(4):
1373-1374
Carter, S. W., Chadwick, P. R. and Wickham, J. C. (1975) Comparative
observations on the activity of pyrethroids against some susceptible
and resistant stored products insects. Submitted for publication.
Chesher, B. C. and Malone, J. C. (1970) Acute toxicity tests with NRDC
107 in hens. Wellcome Foundation Report B 236-70.
Chesher, B. C. and Malone, B. C. (1970) Sensitisation study with NRDC
107 on guinea pigs. Wellcome Foundation Report B 198-70.
Chesher, B. C. and Malone, J. C. (1970) Ocular irritancy of NRDC 107
in rabbits. Wellcome Foundation Report B 217-70.
Chesher, B. C. and Malone, J. C. (1971) Toxicity to dogs of NRDC 107
(continuation). Wellcome Foundation Report No. B 27-71.
Chesher, B. C. and Malone, J. C. (1971) Acute toxicity of NRDC 107 by
the intravenous route. Wellcome Foundation Report B 329-71.
Colas, R. (1970) Toxicité NRDC 107 et NRDC 104 sur poissons. Report
from Procida, 9 September 1970.
Cooper McDougall and Robertson. (1971) Bioresmethrin Technical Data
BRM 1-8.
Cooper McDougall and Robertson Ltd. (1975) Bioresmethrin for
agricultural use. Technical Bulletin, 41 pages.
Desmarchelier, J. M. (1975a) Analysis of pyrethroids on grains (In
press). CSIRO Division of Entomology, P.O. Box 1700, Canberra City,
Australia.
Desmarchelier, J. M. (1975b) Results of studies to determine fate of
bioresmethrin on stored grain. CSIRO Stored Grain Research Laboratory,
Canberra, Australia (Personal communication).
Elliott, M. (1969) Structural requirements for pyrethrum-like
activity. Chemistry and Industry, 14 June 1969, 776-781.
Elliott, M., Farnham, A. W., Janes, N. F., Needham, P. H. and Pearson,
B. C. (1967) 5-benzyl-3-furfuryl methyl chrysanthemate - a new potent
insecticide. Nature, 213(5075):493.
Elliott, M., Janes, N. F. and Pearson, B. C. (1971) The pyrethrins and
related compounds, Part XIII. Insecticidal methyl -, alkenyl- and
benzyl-substituted furfuryl and furylmethyl chrysanthemates. Pestic.
Sci., 2:243.
Elliott, W., Janes, N. F. and Spanner, J. A. (1973) The pyrethrins and
related compounds. Preparation of bioresmethrin, Pestic. Sci.,
4:677-681.
Fales, J. H., Bodensten, O. F., Waters, R. M., Fields, E. S. and Hall,
R. P. (1971) Insecticidal Evaluation of SBP-1390 (bioresmethrin). Soap
Chem. Spec., September 1971, pp. 54-62, 73.
Farebrother, D. A. NRDC 107. (1973) Whole body radioautographal study
in male albino rats. Wellcome Foundation Report HEFH 73-1.
Flanagan, F. & Poll, G. S. (1970) Synthetic pyrethroids - estimation
of cis and trans isomers. Cooper Technical Bureau Series B Report
93/70, 21 May 1970.
Foote, C. S., Wuesthoff, M. T., Wexler, S., Burnstain, I. G., Denny,
R., Schenck, G. O. and Schulte-Elte, K.-H. (1967) Tetrahedron,
23:2583.
Ford, M. G. and Pert, D. R. (1974) Time/Dose/Response relationships of
pyrethroids insecticides with special reference to knockdown.
Pesticide Science, 5:635-641
Fujimoto, K., Itaya, N., Okuno, Y., Kadota, T. and Yamaguchi, T.
(1973) A new insecticidal pyrethroid ester. Agri. Biol. Chem.,
37(1):2681-2682.
Glomot, R. (Undated) Etude de la toxicité chronique de 3 semaines chez
le rat du RU11484 (NRDC 107) Roussel Uclaf Rapport.
Glomot, R. and Chevalier, B. (1969) Etude de la Toxicité argue du
RU11484 (NRDC 107) Roussel Uclaf Rapport Toxico 11386.
Jao, L. T. and Casida, J. E. (1974) Pestic. Biochem. Physiol., 4:456
L'Hoste, J., Balloy, J. and Rauch, F. (1969) La protection des blés
contre Sitophilus granarius. Congr. Pl. Prot. Org. by CIA and CITA,
Milan 1969, No. 22, 6 pp.
L'Hoste, J., Martel, J. and Rauch, F. (1969) A new insecticide
5-benzyl 3-furfuryl methyl d-trans ethanochrysanthémate. Proc. 5th
Br. Insectic. Fungic. Conf., pp. 554-557.
L'Hoste, J. and Rauch, F. (1969) Remarques sur quelques
chrysanthémates insecticides de synthèse. Revue de zoologie Agricole
et Appliqués, Deuxième Trimestre, Nos. 4-6, pp. 53-66.
L'Hoste, J. and Rauch, F. (1969) Sur les propriétés insecticides du
d-trans ethanochrysanthémate de benzyl-5 furylméthyle-3. Conte
Rendu Acad. Sc., Paris 368, 3218-3220 (30 juin 1969)
Lloyd, C. J. (1973) The toxicity of pyrethrins and five synthetic
pyrethroids to Tribolium castaneum and susceptible and pyrethrins
resistant Sitophilus granarius. J. Stored Prod. Res., 9:77-92
Malone, J. C. and Chesher, B. C. (1970) Toxicity to dogs of NRDC 107.
Wellcome Foundation Ltd - Report No. B 204-70.
Malone, J. C. and Chesher, B. C. (1970) Toxicity of bioallethrin/NRDC
107 crawling insect killer to canaries and budgerigars. Wellcome
Foundation Report No. B 211-70.
Malone, J. C. (1971) NRDC 107, 3 month oral toxicity study in beagle
dogs. Wellcome Foundation Report No. B 109-71.
Miyamoto, J. (1975) Terminal residues of bioresmethrin. Submission to
IUPAC Terminal Residues Commission, Madrid, September 1975.
Miyamoto, J., Nishida, T. and Ueda , K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furyl methyl-dl-trans-chrysanthemate in the
rat. Pesticide Biochemistry and Physiology, 1:293, 306.
Miyamoto, J., Suzuki, T. and Nakae, C. (1974) Pestic. Biochem., 4:438
Murano, A. (1972) Agr. Biol. Chem., 36:2203
Murano, J., Fujiwara, S., Horiba, M. and Miyamoto, J. (1971) Agr.
Biol. Chem., 35(8):1200-1207.
Noel, P. R. B., Rivett, K. F., Chesterman, H., Street, A. E. and
Spicer, E. J. F. (1971) Oral toxicity study in Beagle dogs (repeated
dosage for 3 months), Huntington Research Centre Report 3900/71/58, 76
pp.
Okuno, J., Fujimoto, K., Kadota, T., Miyamoto, J. and Hamuro, K.
(1969) Insecticidal activity of a new synthetic pyrethroidal compound,
5-benzyl-3-furyl-methyl dl-cis, trans chrysanthemate (NRDC - 104,
Chrysron), Botyu-Kagaku, 34:157.
Rosen, J. D. (1972) In "Environmental Toxicology of Pesticides",
Matsumura, F., Bosh, G. M. and Misato, T. Academic Press N.Y., p. 438.
Rowlands, D. (1975) Breakdown of bioresmethrin on wheat. Note prepared
by Pest Infestation Control Laboratory - Ministry of Agriculture,
Fisheries and Food, Slough, United Kingdom.
Simonartis, R. A. and Coil, R. S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour and wheat. J.
AOAC, 58(5):1032-1035.
Smith, F. F. and Boswell, A. L. Toxicity of micronised dusts to some
glasshouse insects. J. econ. Ent., 65(5):1466-1468.
Sullivan, J. W. N. et al. (1972) Gas propelled aerosols and micronised
dusts for control of insects in aircraft 7 - Effectiveness against
pests of agriculture. J. econ. Ent., 65(5):1462-1466.
Suzuki, T. and Miyamoto, J. (1974) Pestic. Biochem. Physiol., 4:86.
Tooby, T. E. (1973) Toxicity of the synthetic pyrethroid NRDC 107 to
fish. Ministry of Agriculture, Fisheries and Food, Salmon and
Freshwater Fisheries Laboratory Report FGB 29092.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1974) Photodecomposition
of resmethrin and related pyrethroids. J. Agr. Food Chem.,
22(2):212-220.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975a) Metabolism of
(+)-trans and (+)-cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975b) Pesticide Biochem.
Physiol., 5:280
Verschoyle R. D. and Barnes, J. M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. Physiol., 2(3):308-311.
Waldron, M. M. (1969) Foetal toxicity study - 95 H 56 - given orally
in the rabbit. Wellcome Foundation Report, Path 51.
Wallwork, L., Chesher, B. C. and Malone, J. C. (1970) Toxicity of NRDC
107 to laboratory animals. Wellcome Foundation Report B 199-70.
Wallwork, L. M., Clampitt, R. B. and Malone, J. C. (1971) NRDC 107,
rat oral 91 day toxicity study. Wellcome Foundation Report B 119-71.
Wallwork, L. M. and Malone, J. C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Wellcome
Foundation Report B 47-71.
Wallwork, L. M. and Malone, J. C. (1972) Bioresmethrin - Preliminary
24 hour rat inhalation study. Wellcome Foundation Report B 290-72.
Weeks, M. H., Leland, T. M., Boldt, R. E. and Mellick, P. W. (1972)
Toxicological evaluation of pyrethroid insecticide SBP 1382. US Army
Environmental Hygienic Agency Special Study No. 51-127-71/72.
Wellcome. 1973(ca.) Summary of trial results. Agricultural uses of
bioresmethrin. Wellcome Research Laboratories, Berkhamsted, 29 pp.
Winney, R. (1973) The biological performance of synthetic pyrethroids.
Pyrethrum Post., 12(1):2-11.
Farebrother (1973) in a whole body autoradiographical study in
rats showed that bioresmethrin was absorbed through the gut and widely
distributed in the body two hours after dosing. At six hours the
distribution was similar but the concentrations were increased,
particularly in fatty tissues. At 24 hours most tissues showed greatly
reduced activity but the concentration in fatty tissue remained high.
No studies appear to have been carried out to determine the level
or nature of the metabolites in animal fat or other tissues. No
studies are yet available to show the fate of bioresmethrin when fed
to livestock or poultry.
Wheat
Ardley (1975) reports trials in which wheat treated with
bioresmethrin at varying rates with and without piperonyl butoxide,
was subjected to standard milling and baking tests.
The grain, when milled gave 25% bran, 7% pollard (shorts) and 68%
flour. The flour was converted into bread. All loaves were
satisfactory. No taint or odour was evident during dough processing or
in the loaves fresh from the oven.
The milling fractions and the bread were analysed. The nature of
the chemical treatment and the results of analysis are provided in the
following table. Analyses were carried out by two laboratories and the
results were in good agreement.
TABLE 5. Residues of bioresmethrin and piperonyl butoxide in milling
fractions and bread
Recovered residue (i) bioresmethrin (ii) piperonyl butoxide (ppm)
(i) (ii)
Protectant1
treatment bran pollard flour bread bran pollard flour bread
a 0.3 4.0 4.0 nil 21.0 10.0 2.0 2.0
b 0.3 1.0 1.4 nil 0.5 0.3 nil nil
c 0.5 1.0 0.5 nil 11.0 8.0 10.0 nil
d 1.1 1.2 0.5 nil 2.0 2.0 2.0 nil
e nil nil nil nil nil nil nil nil
1 a = 4 mg/kg bioresmethrin + 20 mg/kg piperonyl butoxide
b = 2 " " alone
c = 4 " " + 10 mg/kg piperonyl butoxide +
4 mg/kg anti-oxidant
d = 2 " " + 2 mg/kg piperonyl butoxide +
10 mg/kg anti-oxidant + 4 mg/kg
fenitrothion.
In a subsequent study Desmarchelier ( 1975b) took wheat which had
been treated six weeks (A) and seven months (B) previously and
subjected it to standard milling procedures for the preparation of
wholemeal and white flour. The following results indicate the
distribution and fate of the bioresmethrin:
Sample A B
Interval since treated 6 weeks 7 months
Residue in wheat 2.9 mg/kg 1.9 mg/kg
bran 5.2 " 1.7 "
pollard (shorts) 0.7 " -
white flour * *
wholemeal bread 1.0 " 0.6 "
white bread ND ND
limit of determination = 0.05 mg/kg
* Interference led to poor recoveries during assay of flour.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Bioresmethrin has not yet been widely used on food crops or for
the treatment of stored grain and therefore no information is
available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The analytical methods for resmethrin have been reviewed by Brown
(1973) and also by Murano et al. (1971), Murano (1972) and
Desmarchelier (1975). The methods include ultra-violet absorption
spectrophotometry, colorimetry, TLC and GLC. SE-30, DC200 ‰ QFI and DEGS
have been used as stationary phases for the GLC determination of
resmethrin in crops after suitable clean-up procedures. Brown (1973)
proposes a residue analytical method for wheat, wheat flour and corn
meal in which resmethrin is extracted with hexane, partitioned between
hexane and acetonitrile, cleaned up on an Alumina column, and
determined by GLC.
The difficulty in the residue analysis of resmethrin, which would
apply equally to the optical isomer bioresmethrin, seems to lie in the
intrinsically low sensitivity to the compound of electron capture or
flame ionization detectors. The limit of detection ranges at best from
0.1 to 0.05 µg, equivalent in practice to a residue level of about
0.1 mg/kg.
To overcome the drawback, the component chrysanthemic acid is
quantitatively condensed with 2,4-dichlorobenzyl chloride to yield
2,4-dichlorobenzyl chrysanthemate, which can be determined by electron
capture (Miyamoto, 1975), It is reported that this modification allows
as little as 0.01 ng of the derivative or approximately 0.01 ng of
bioresmethrin to be analysed. Up to 100 µg of bioresmethrin is
hydrolyzed in 10% methanolic potassium hydroxide for one hour at 60°C.
Chrysanthemic acid is extracted with chloroform at pH2. The chloroform
is evaporated in vacuo and the residue dissolved in 5 ml acetone and
warmed at 60°C for 120 min with the addition of 0.1 ml of
N,N-dimethylformamide and one drop each of triethylamine and
2,4-dichlorobenzyl chloride. The reaction mixture is passed through a
Florisil column and eluted with benzene. 2,4-dichlorobenzyl
chrysanthemate is chromatographed under the following conditions.
Liquid phase Epon-1001 (1%) and QF-1 (1%) on Chromosorb B (100 to 120
mesh); temperature of oven and detector, 200°C and of injection port,
240°C; carrier nitrogen, 1.0 kg/cm›. Retention time of
2,4-dichlorobenzyl chrysanthemate about 4-1/2 min. The overall
recovery in the range 1 to 100 ng of resmethrin is at least 80%, often
more than 90%. Resmethrin residues of the order of 1 µg/kg can
therefore be determined.
Desmarchelier (1975a,b) reports a colorimetric method based an
the procedure of Screiber and McClellan (1954) and McClellan (1964)
originally designed for the analysis of pyrethrum and based on the
analysis of chrysanthemic acid. The author examines the applicability
of this procedure to residue analysis of five pyrethroid derivatives
of chrysanthemic acid on six commodities and extends it to derivatives
of pyrethric acid, for example pyrethrin II. In addition, a TLC
procedure is described that allows positive identification of
derivatives of chrysanthemic acid in a one-step process. In the case
of bioresmethrin, results obtained by these two procedures on aged
residues on wheat were compared with results obtained by two
procedures specific to bioresmethrin. Appreciable differences between
results occurred only at the limit of determination. In the method of
Desmarchelier, petroleum spirit (b.p. 30-40°C) was added to whole
grain (100 g) and left overnight. The supernatant was decanted, the
grain covered with solvent and extracted for a further hour and this
procedure repeated once more. Methanol, acetone and 10% acetone in
petroleum spirit appear equally suitable as solvents.
Although none of the metabolites or photo-products have been
reported to be analysed in the actual samples, various techniques and
procedures for separation as well as identification of the possible
terminal residues of bioresmethrin are described in a number of papers
including those of Miyamoto et al. (1971); Ueda et al. (1974, 1975a
and b); Abernathy (1973); Brown (1973) and Murano (1972).
Simonartis and Coil (1975) have developed a GLC method for the
determination of resmethrin in corn, corn meal, flour and wheat. it
involves extraction with pentane, transfer to acetonitrile, Florisil
clean-up using 3% ethyl acetate in perthane followed by GLC using
flame conization detector. The method is sensitive to 0.2 ppm with
better than 85% recovery and good reproductivity.
NATIONAL TOLERANCES
The information available to the Meeting suggests that no
national tolerances have yet been established.
APPRAISAL
Bioresmethrin is a broad spectrum pyrethroid insecticide with
contact action and pronounced knockdown effect. It shows only limited
biological persistence on plant surfaces and is readily degraded by
sunlight but gives long-lasting control of insect pests on inert
surfaces and retains its biological activity when applied to stored
agricultural commodities, including raw grain and nuts.
The Meeting had only limited information concerning the use and
performance of this material on crops pre-harvest but extensive data
were available on the use, performance and fate of bioresmethrin on a
variety of grains. The recommended rate of application to raw cereals
is 1 to 4 mg/kg. The biological half-life on grain ranges from eight
weeks at 35°C to more than 20 weeks at 20°C.
Data available suggest that when bioresmethrin is applied to
wheat, the deposit is not confined to the seed coat but penetrates to
the endosperm so that a substantial proportion of the amount applied
is found in flour. Standard milling techniques which remove most of
the seed coat, produce a bran which contains considerably less
bioresmethrin than does the whole grain or the flour. Virtually all of
the bioresmethrin is destroyed when bread prepared from such flour is
cooked.
Extensive information is available on the biological degradation
and metabolism of bioresmethrin including the nature of the
metabolites formed under a wide variety of conditions. The initial
step in the metabolism of bioresmethrin is hydrolysis at the ester
linkage, yielding 5-benzyl-3-furylmethanol and chrysanthemic acid.
Several GLC methods of analysis suitable for the determination of
residues of bioresmethrin and metabolites containing chrysanthemic
acid in plant materials are available. The limit of determination
ranges from 0.1 to 0.001 mg/kg. In addition there are several
colorimetric methods based an the determination of chrysanthemic acid
which can be used in conjunction with a TLC procedure for identifying
the pyrethroid residue involved. No national tolerances have yet been
determined.
In proposing maximum residue limits for bioresmethrin on raw
grain, milled cereal products and foods prepared therefrom, careful
consideration has been given to the fact that this insecticide is to
be used as a grain protectant, that a certain concentration must be
present in the grain to control infestations and prevent damage to
stored products and that the compound is moderately stable under
storage conditions. Under practical conditions of grain handling and
storage, there will always be a natural variation in the level of the
deposit resulting from a fluctuation in the flow of grain and
insecticide. it is therefore not possible to fix the maximum limit on
the minimum necessary to control insect pests. At the present time, it
is anticipated that bioresmethrin will be used in conjunction with one
or more of the organophosphorus grain protectants whose effect is
complementary and possibly synergistic. Under such conditions the
amount of bioresmethrin used will be at the lower range of the
proposed rates. However, there will be situations where bioresmethrin
must be used alone at the maximum of the proposed rate and due
allowance must be made for the amplitude of the variations in
concentration which are inevitable and the problems of sampling and
analysis involved.
RECOMMENDATIONS
The following guideline levels are recommended as limits which
need not be exceeded when bioresmethrin is used according to good
agricultural practices in the following commodities:
Commodity Maximum residue limit, mg/kg
Raw grain 5
Milled products from grain 5
Cooked cereal products, including 0.05*
bread
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Full toxicological data.
DESIRED
1. Further information on the level and fate of bioresmethrin
on different classes of raw grains.
2. Information on residues from supervised trials on other
stored commodities, including nuts, peanuts, lentils, dried
fruit and dried vegetables,
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in
food at the point of consumption following the use of
bioresmethrin for the control of various stored-product
pests.
5. Improved procedures for the determination of bioresmethrin
residues in fruit and vegetables as well as stored products.
REFERENCES
Abernathy, C. O. and Casida, J. E. (1973) Pyrethroid insecticides:
esterase cleavage in relation to selective toxicity. Science, 179:
1235-1236.
Abernathy, C. O., Ueda, K., Engel, J. L., Gaughan, L. C. and Casida,
J. E. (1973a) Substrate specificity and toxicological significance of
pyrethroid hydrolyzing esterases of mouse liver microsomes. Pesticide
Biochemistry and Physiology, 3: 300-311.
Andrews, T. L. (1974) Resmethrin Residues in foliage after aerial
application. Pesticide Monitoring Journal, 8(1):50-52.
Ardley, J. H. (1973) Further field evaluation of bioresmethrin as a
potential replacement for malathion in grain protection. Wellcome
Australia Ltd Report TD/5/73/Kl-36.
Ardley, J. H. (1975) Use and application of resmethrin and
bioresmethrin as potential grain protectants. Paper submitted to
Journal of Stored Products Research (May 1975)
Ardley, J. H. and Desmarchelier, J. M. (1974) Investigations into the
use of resmethrin and bioresmethrin as potential grain protectants.
Proceedings of the First International Working Conference on Stored
Product Entomology, Savannah, Georgia, USA.
Ardley, J. H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat. Report of Wellcome Australia Ltd, 27
August 1975.
Bengston, M., Cooper, L. M. and Holton, F. J. (1975b) A comparison of
bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as grain
protectants against malathion-resistant insects in wheat. Submitted
for publication, Queensland Journal of Agricultural and Animal
Sciences.
Bengston, M., Connell M., Desmarchelier, J., Snelson, J. and Sticka,
R. (1975a) Report of field trials with grain protectants. To be
published.
Brooks, I. C., Hans, J., Blumenthal, R. R. and Davis, B. S. (1969) SBP
1382, a new synthetic pyrethroid. Soap Chem. Spec., 45:62.
Brown, B. B. (1973) In "Analytical methods for pesticides and growth
regulators". Edited by G. Zweig (Academic Press), p. 441.
Buick, A. R. and Flanagan, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes. Wellcome Foundation Report HEGH 73/1.
Buick, A. R. and Flanagan, P. (1974) The fate of bioresmethrin on
cucumbers. Wellcome Foundation Report HEGH 74/1.
Cantu, E. and Wolfenbarger, D. A. (1970) Toxicity tests of three
pyrethroids to several insect pests of cotton. J. econ. Ent., 63(4):
1373-1374
Carter, S. W., Chadwick, P. R. and Wickham, J. C. (1975) Comparative
observations on the activity of pyrethroids against some susceptible
and resistant stored products insects. Submitted for publication.
Chesher, B. C. and Malone, J. C. (1970) Acute toxicity tests with NRDC
107 in hens. Wellcome Foundation Report B 236-70.
Chesher, B. C. and Malone, B. C. (1970) Sensitisation study with NRDC
107 on guinea pigs. Wellcome Foundation Report B 198-70.
Chesher, B. C. and Malone, J. C. (1970) Ocular irritancy of NRDC 107
in rabbits. Wellcome Foundation Report B 217-70.
Chesher, B. C. and Malone, J. C. (1971) Toxicity to dogs of NRDC 107
(continuation). Wellcome Foundation Report No. B 27-71.
Chesher, B. C. and Malone, J. C. (1971) Acute toxicity of NRDC 107 by
the intravenous route. Wellcome Foundation Report B 329-71.
Colas, R. (1970) Toxicité NRDC 107 et NRDC 104 sur poissons. Report
from Procida, 9 September 1970.
Cooper McDougall and Robertson. (1971) Bioresmethrin Technical Data
BRM 1-8.
Cooper McDougall and Robertson Ltd. (1975) Bioresmethrin for
agricultural use. Technical Bulletin, 41 pages.
Desmarchelier, J. M. (1975a) Analysis of pyrethroids on grains (In
press). CSIRO Division of Entomology, P.O. Box 1700, Canberra City,
Australia.
Desmarchelier, J. M. (1975b) Results of studies to determine fate of
bioresmethrin on stored grain. CSIRO Stored Grain Research Laboratory,
Canberra, Australia (Personal communication).
Elliott, M. (1969) Structural requirements for pyrethrum-like
activity. Chemistry and Industry, 14 June 1969, 776-781.
Elliott, M., Farnham, A. W., Janes, N. F., Needham, P. H. and Pearson,
B. C. (1967) 5-benzyl-3-furfuryl methyl chrysanthemate - a new potent
insecticide. Nature, 213(5075):493.
Elliott, M., Janes, N. F. and Pearson, B. C. (1971) The pyrethrins and
related compounds, Part XIII. Insecticidal methyl -, alkenyl- and
benzyl-substituted furfuryl and furylmethyl chrysanthemates. Pestic.
Sci., 2:243.
Elliott, W., Janes, N. F. and Spanner, J. A. (1973) The pyrethrins and
related compounds. Preparation of bioresmethrin, Pestic. Sci.,
4:677-681.
Fales, J. H., Bodensten, O. F., Waters, R. M., Fields, E. S. and Hall,
R. P. (1971) Insecticidal Evaluation of SBP-1390 (bioresmethrin). Soap
Chem. Spec., September 1971, pp. 54-62, 73.
Farebrother, D. A. NRDC 107. (1973) Whole body radioautographal study
in male albino rats. Wellcome Foundation Report HEFH 73-1.
Flanagan, F. & Poll, G. S. (1970) Synthetic pyrethroids - estimation
of cis and trans isomers. Cooper Technical Bureau Series B Report
93/70, 21 May 1970.
Foote, C. S., Wuesthoff, M. T., Wexler, S., Burnstain, I. G., Denny,
R., Schenck, G. O. and Schulte-Elte, K.-H. (1967) Tetrahedron,
23:2583.
Ford, M. G. and Pert, D. R. (1974) Time/Dose/Response relationships of
pyrethroids insecticides with special reference to knockdown.
Pesticide Science, 5:635-641
Fujimoto, K., Itaya, N., Okuno, Y., Kadota, T. and Yamaguchi, T.
(1973) A new insecticidal pyrethroid ester. Agri. Biol. Chem.,
37(1):2681-2682.
Glomot, R. (Undated) Etude de la toxicité chronique de 3 semaines chez
le rat du RU11484 (NRDC 107) Roussel Uclaf Rapport.
Glomot, R. and Chevalier, B. (1969) Etude de la Toxicité argue du
RU11484 (NRDC 107) Roussel Uclaf Rapport Toxico 11386.
Jao, L. T. and Casida, J. E. (1974) Pestic. Biochem. Physiol., 4:456
L'Hoste, J., Balloy, J. and Rauch, F. (1969) La protection des blés
contre Sitophilus granarius. Congr. Pl. Prot. Org. by CIA and CITA,
Milan 1969, No. 22, 6 pp.
L'Hoste, J., Martel, J. and Rauch, F. (1969) A new insecticide
5-benzyl 3-furfuryl methyl d-trans ethanochrysanthémate. Proc. 5th
Br. Insectic. Fungic. Conf., pp. 554-557.
L'Hoste, J. and Rauch, F. (1969) Remarques sur quelques
chrysanthémates insecticides de synthèse. Revue de zoologie Agricole
et Appliqués, Deuxième Trimestre, Nos. 4-6, pp. 53-66.
L'Hoste, J. and Rauch, F. (1969) Sur les propriétés insecticides du
d-trans ethanochrysanthémate de benzyl-5 furylméthyle-3. Conte
Rendu Acad. Sc., Paris 368, 3218-3220 (30 juin 1969)
Lloyd, C. J. (1973) The toxicity of pyrethrins and five synthetic
pyrethroids to Tribolium castaneum and susceptible and pyrethrins
resistant Sitophilus granarius. J. Stored Prod. Res., 9:77-92
Malone, J. C. and Chesher, B. C. (1970) Toxicity to dogs of NRDC 107.
Wellcome Foundation Ltd - Report No. B 204-70.
Malone, J. C. and Chesher, B. C. (1970) Toxicity of bioallethrin/NRDC
107 crawling insect killer to canaries and budgerigars. Wellcome
Foundation Report No. B 211-70.
Malone, J. C. (1971) NRDC 107, 3 month oral toxicity study in beagle
dogs. Wellcome Foundation Report No. B 109-71.
Miyamoto, J. (1975) Terminal residues of bioresmethrin. Submission to
IUPAC Terminal Residues Commission, Madrid, September 1975.
Miyamoto, J., Nishida, T. and Ueda , K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furyl methyl-dl-trans-chrysanthemate in the
rat. Pesticide Biochemistry and Physiology, 1:293, 306.
Miyamoto, J., Suzuki, T. and Nakae, C. (1974) Pestic. Biochem., 4:438
Murano, A. (1972) Agr. Biol. Chem., 36:2203
Murano, J., Fujiwara, S., Horiba, M. and Miyamoto, J. (1971) Agr.
Biol. Chem., 35(8):1200-1207.
Noel, P. R. B., Rivett, K. F., Chesterman, H., Street, A. E. and
Spicer, E. J. F. (1971) Oral toxicity study in Beagle dogs (repeated
dosage for 3 months), Huntington Research Centre Report 3900/71/58, 76
pp.
Okuno, J., Fujimoto, K., Kadota, T., Miyamoto, J. and Hamuro, K.
(1969) Insecticidal activity of a new synthetic pyrethroidal compound,
5-benzyl-3-furyl-methyl dl-cis, trans chrysanthemate (NRDC - 104,
Chrysron), Botyu-Kagaku, 34:157.
Rosen, J. D. (1972) In "Environmental Toxicology of Pesticides",
Matsumura, F., Bosh, G. M. and Misato, T. Academic Press N.Y., p. 438.
Rowlands, D. (1975) Breakdown of bioresmethrin on wheat. Note prepared
by Pest Infestation Control Laboratory - Ministry of Agriculture,
Fisheries and Food, Slough, United Kingdom.
Simonartis, R. A. and Coil, R. S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour and wheat. J.
AOAC, 58(5):1032-1035.
Smith, F. F. and Boswell, A. L. Toxicity of micronised dusts to some
glasshouse insects. J. econ. Ent., 65(5):1466-1468.
Sullivan, J. W. N. et al. (1972) Gas propelled aerosols and micronised
dusts for control of insects in aircraft 7 - Effectiveness against
pests of agriculture. J. econ. Ent., 65(5):1462-1466.
Suzuki, T. and Miyamoto, J. (1974) Pestic. Biochem. Physiol., 4:86.
Tooby, T. E. (1973) Toxicity of the synthetic pyrethroid NRDC 107 to
fish. Ministry of Agriculture, Fisheries and Food, Salmon and
Freshwater Fisheries Laboratory Report FGB 29092.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1974) Photodecomposition
of resmethrin and related pyrethroids. J. Agr. Food Chem.,
22(2):212-220.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975a) Metabolism of
(+)-trans and (+)-cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975b) Pesticide Biochem.
Physiol., 5:280
Verschoyle R. D. and Barnes, J. M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. Physiol., 2(3):308-311.
Waldron, M. M. (1969) Foetal toxicity study - 95 H 56 - given orally
in the rabbit. Wellcome Foundation Report, Path 51.
Wallwork, L., Chesher, B. C. and Malone, J. C. (1970) Toxicity of NRDC
107 to laboratory animals. Wellcome Foundation Report B 199-70.
Wallwork, L. M., Clampitt, R. B. and Malone, J. C. (1971) NRDC 107,
rat oral 91 day toxicity study. Wellcome Foundation Report B 119-71.
Wallwork, L. M. and Malone, J. C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Wellcome
Foundation Report B 47-71.
Wallwork, L. M. and Malone, J. C. (1972) Bioresmethrin - Preliminary
24 hour rat inhalation study. Wellcome Foundation Report B 290-72.
Weeks, M. H., Leland, T. M., Boldt, R. E. and Mellick, P. W. (1972)
Toxicological evaluation of pyrethroid insecticide SBP 1382. US Army
Environmental Hygienic Agency Special Study No. 51-127-71/72.
Wellcome. 1973(ca.) Summary of trial results. Agricultural uses of
bioresmethrin. Wellcome Research Laboratories, Berkhamsted, 29 pp.
Winney, R. (1973) The biological performance of synthetic pyrethroids.
Pyrethrum Post., 12(1):2-11.
 Molecular formula C22H26O3
Molecular weight 338.45
Other information on identity and properties
Description : A viscous yellow liquid which on
crystallization forms an off-white solid
Specific gravity: 1.050 at 20°C
Flash point : 125°C (open cup)
Optical rotation: D20-5 to -8° : measurement on a 10% solution
in ethanol
Solubility : Soluble in most organic solvents but
substantially insoluble in water
Saponification
value : 160-175 mg KOH/gm
Stability : Bioresmethrin is sensitive to light but its
photo stability is greater than that of
pyrethrins. Bioresmethrin is stable to
temperatures met under most normal storage
conditions.
Bioresmethrin spray deposits appear subject
to atmospheric oxidation but any adverse
effect appears to be reduced by the
introduction of antioxidants including
piperonyl butoxide and butylated
hydroxyanisole.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bioresmethrin is one of the most effective broad spectrum
insecticides currently available. It exhibits a high order of
insecticidal activity, which when coupled with its excellent
toxicological properties, makes it potentially one of the safest and
most useful insecticides now being produced.
In addition to its insecticidal properties, bioresmethrin has a
good knock-down performance against flying insects, particularly when
this is compared with the organochlorine or organophosphorus
insecticides.
Bioresmethrin, at low concentrations, is an effective killing
agent against most insect pests affecting households, industrial
premises, public health, food storage and livestock production.
Efficacy against many insect pests of food crops and ornamentals has
been demonstrated.
The principal uses to which bioresmethrin is currently being put
are:
(a) in household aerosols and sprays formulated in combination
with pyrethrum, bioallethrin, tetramethrin and piperonyl
butoxide;
(b) as an insecticide for the control of pests in food premises;
(c) as a general purpose broad spectrum insecticide; and
(d) in grain disinfestation and protection.
Bioresmethrin has been tested in conjunction with a number of
well established synergists. Unlike natural pyrethrum, there is only a
minimal increase in performance against flies, mosquitos and
cockroaches when piperonyl butoxide is added to bioresmethrin.
However, in the case of a number of the more important insect pests of
stored products, bioresmethrin is synergized to a significant extent
with piperonyl butoxide, the factor of synergism ranging from four- to
ninefold.
Bioresmethrin shows good promise of becoming a major insecticide
for use in the control of stored product pests. It combines a high
potency, good persistence, and excellent safety features.
Treatment of plants
Although a number of trials have been reported where
bioresmethrin has given satisfactory results against a wide variety of
insect and mite pests of crops and ornamentals (Cooper McDougall and
Robertson, 1971, 1975; Cantu et al., 1970) there appears as yet only
limited commercial acceptance of bioresmethrin as a horticultural
insecticide. This reflects not only the state of development, but the
comparatively high cost and the relatively short residual life of
bioresmethrin spray deposits. There are however, many obvious
applications which will no doubt reach commercial development within
the next two to three years.
Non-crop situations
Many commercial uses have been developed for bioresmethrin in
non-crop situations including the control of flies, mosquitos and
other flying pests in households, pests of institutions and food
storage, aircraft disinfestation, animal-house treatments and similar
situations where aerosols, knockdown sprays and residual sprays are
conventionally used. In addition, bioresmethrin has been used in
thermal fog generators and in mosquito coils. None of these uses are
likely to give rise to significant residues in food or water, or to
cause significant intake by the general public.
Post-harvest application
Following the demonstration of outstanding activity against many
insect pests of stored products, numerous authors have reported
studies on the effect and usefulness of bioresmethrin for the control
of stored product pests and as a grain protectant (Ardley, 1973 and
1975; Ardley and Desmarchelier, 1974; Cooper McDougall and Robertson,
1971; Lloyd, 1973; Rowlands, 1975; Bengston et al., 1975a, 1975b).
Ardley (1975) showed that of many pyrethroid and organophosphorus
insecticides and insecticide combinations evaluated in silo trials,
the best product on a cost/efficiency basis was 4 ppm bioresmethrin
plus 20 ppm piperonyl butoxide. After 12 months, the treated grain
controlled all species and strains exposed in bioassay. A lower
efficiency was observed in the control of confused flour beetle
(Tribolium confusum).
The problem of resistance to malathion, dichlorvos and lindane
which is developing in stored product insects in almost all countries
has caused intensive examination of alternative protectant chemicals,
Lloyd (1973) in examining the toxicity of pyrethrins and five
synthetic pyrethroids to susceptible and pyrethrin-resistant strains
of stored product pests reported bioresmethrin to have up to 16 times
more toxicity than that of pyrethrins. He showed that piperonyl
butoxide synergized all the compounds against the various strains.
When synergized, bioresmethrin was again the most potent of the
synthetic compounds. L'Hoste et al. (1969) showed that bioresmethrin
deteriorated less rapidly than malathion when admixed with grain.
In Australia, the occurrence of malathion-resistant strains of
Rhyzopertha dominica is a severe threat to the grain export
industry. Malathion was never entirely satisfactory for the control of
this species, which shows a high degree of tolerance to all
organophosphorus materials that could possibly be used for the
protection of stored grains. The efficiency of bioresmethrin or
bioresmethrin/piperonyl butoxide combinations in controlling this
species is therefore important.
Bengston et al. (1975b) have shown clearly the high potency of
bioresmethrin against Rhyzopertha dominica which makes it an ideal
insecticide to complement such organophosphorus grain protectants as
malathion, pirimiphos-methyl and chlorpyrifos-methyl.
Table 1, from the work of Ardley and Desmarchelier (1974), shows
the efficiency of bioresmethrin/piperonyl butoxide combinations
against both susceptible and resistant strains of the more important
grain pests.
TABLE 1. Summary of Grain Protectant Trials, 1967-1974
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
1967 2 ppm 11-1/4 11.4 20.6 100 100 0 0 - 0 0 -
resmethrin + 20 ppm
pbo;2 2 ppm
pyrethrins + 20 10 11.5 20.7 85 100 1 0 - 0 0 -
ppm pbo
1968 1 ppm 4 approx. <10.0 29.0 99 100 0 1 0 0 0 0 0
bio-resmethrin + 10 or
ppm pbo; 0.5 ppm equal
to
bioresmethrin + 4 " <10.0 28.4 100 100 40 1 0 0 4 0 0
5 ppm pbo; 2 ppm
pyrethrins 3 " <10.0 20.6 95 100 20 0 0 2 10 10 1
+20 ppm pbo
1969 2 ppm 8 10.9 19.2 75 100 40 0 0 0 50 10 9
bio-resmethrin + 0.2
ppm antioxidant;
12 ppm malathion 8 10.8 20.7 100 80 90 23 0 0 0 1 0
* **
1973 4 ppm
bio-resmethrin 12 10.2 23.9 5 100 <8 - 0 0 63 0 -
8 ppm
bio-resmethrin 12 10.7 24.0 40 100 <15 - 0 0 3 1 -
12 ppm
bio-resmethrin 12 10.5 24.4 95 100 <38 - 2 0 0 0 -
TABLE 1. (cont'd)
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
4 ppm
bio-resmethrin +
20 ppm pbo 12 10.5 23.5 100 100 <10 - 1 0 0 0 -
8 ppm
bio-resmethrin + 20
ppm pbo 12 10.5 23.6 100 100 <9 - 0 0 1 1 -
12 ppm malathion 12 10.4 23.9 95 0 <8 - 3 1 0 6 -
control, on
clean wheat 12 10.5 23.5 4 5 - - 0 4 376 0 -
1 14 day period.
2 pbo = piperonyl butoxide.
3 Sgs = Sitophilus granarius )malathion
Rds = Rhyzopertha dominica )susceptible
Tcs = Tribolium castaneum ) strains.
*S. oryzae in all 1973/74 trials.
**T. castaneum assays discontinued after nine months.
Carter et al. (1975) have evaluated a range of synthetic
pyrethroid insecticides against susceptible and resistant strains of
stored product pests and have concluded that synergized bioresmethrin
was the most suitable pyrethroid and that it was of value in
controlling organophosphorus resistant beetles.
For reasons of economy, to increase the spectrum of effect
against the many different pests encountered in stored products and to
reduce the possibility of selecting resistant strains, it is proposed
that bioresmethrin be combined with one of the organophosphorus grain
protectants. There is already a good deal of evidence (Abernathy et
al., 1973; Jao and Casida, 1974) that organophosphorus compounds
inhibit the esterases responsible for the hydrolysis of bioresmethrin
in living systems.
When used for grain protection, bioresmethrin is applied with
piperonyl butoxide in the form of an emulsion which has been diluted
with water to a concentration which, when applied to bulk grain at the
rate of 1 litre per tonne, will deposit a known amount of
bioresmethrin evenly on the grain. The amount applied ranges from 1 to
4 ppm. The amount of piperonyl butoxide applied concurrently would
range from 5 to 20 mg/kg.
Treatment of animals
Bioresmethrin is suitable for the control of flies on cattle and
pigs but no information was available to indicate whether such uses
had yet been approved by registration authorities.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Fruit and vegetable crops
Only preliminary results are available to show the fate of
bioresmethrin on tomatoes (Buick and Flanagan, 1973) and on cucumbers
(Buick and Flanagan, 1974; Cooper McDougall and Robertson, 1975).
In these studies, radio-labelled bioresmethrin emulsified in
water was applied to tomato fruits (growing in a laboratory) at a rate
sufficient to deposit 1.25 mg/kg of bioresmethrin on the tomatoes. The
deposit applied to the growing tomatoes was estimated by determining
the total radioactivity after allowing the deposit to dry for one
hour. The fruit was harvested at 6, 12, 24, 48 and 72 hours after
application and the amount of bioresmethrin in the flesh, skin and on
the skin surface was measured after washing the fruit for two minutes
in benzene. The degradation products were removed from the benzene
solution by passing through alumina. The distribution of bioresmethrin
in and on tomatoes is shown in Table 2. The results are expressed as a
percentage of the total radioactivity applied.
TABLE 2. Distribution of bioresmethrin in tomatoes
Hours 1 6 12 24 48 72
Flesh 0.2 0.2 0.2 0.2 0.2 0.2
Skin 1.75 2.45 3.2 1.95 4.65 4.65
Benzene wash 92.5 39.0 50.0 54.5 22.5 15.5
TOTAL 94.25 41.45 53.2 56.45 27.15 20.15
From the table, it is evident that over 90% of the bioresmethrin
will be recovered by simply washing with benzene if the fruit is
harvested within 24 hours of spraying. If harvested later than this,
then up to 25% of the remaining insecticide may be held in the skin
three days after treatment but this will represent less than 5% of
that originally applied. The amount of bioresmethrin found in the
flesh is insignificant.
In the case of cucumbers (Buick and Flanagan, 1974) bioresmethrin
was applied as an emulsion to the surface of cucumbers growing in a
darkened laboratory equivalent to approximately 1 mg/kg of fruit. The
total radioactivity and radioactivity due to intact bioresmethrin was
determined on the surface, in the skin and in the flesh of cucumbers
harvested 1, 6, 12, 24, 48 and 72 hours after application.
The results indicate that although most of the radioactivity
applied is retained, the bioresmethrin is substantially degraded
within an hour of application and more slowly thereafter (24% survival
after one hour, 10% after 72 hours). This contrasts with the results
of the tomato experiments in which the bioresmethrin was found to
degrade more slowly. A simple washing of the intact fruit with benzene
removes at least 85% of the surviving bioresmethrin at any time up to
three days after treatment, whether or not the fruit is stored in the
dark before analysis. The results of this work are summarized in Table
3.
TABLE 3. Distribution of undegraded bioresmethrin in cucumbers
Bioresmethrin, mean of four results (from stored and unstored vegetables)
shown as % of "concentration applied", after interval (hours)
Fraction 1 6 12 24 48 72
Flesh <0.1 <0.1 <0.1 <0.1 <0.1 <0.1
Skin 0.8 1.6 0.7 1.4 0.8 1.2
Wash 23.3 14.7 8.1 9.8 7.0 8.8
TOTAL 24.1 16.3 8.8 11.2 7.8 10.0
FATE OF RESIDUES
On plants
Rosen (1972) showed that resmethrin and its alcohol moiety
photodecompose to many unidentified products at a rate which varies
with the supporting surface. Residues exposed to sunlight on silica
gel degrade more rapidly than residues on glass and different products
are formed. The author postulates that plant and soil surfaces will
show greater differences. Photodecomposition of bioresmethrin was
studied intensively, although under limited experimental conditions,
by using phenyl-14C alcohol and carboxy-14C acid preparations (Ueda
et al., 1974). After irradiation of the radioactive resmethrin on a
silica gel plate with a sunlamp for seven hours, 88% of the
radioactivity was recovered, 5% of which was the intact resmethrin.
Forty-three per cent. of the recovered radioactivity was identified
and of this 33% and 10% respectively were ester and non-ester
products. Figure 1 illustrates the proposed, although somewhat
speculative, photodecomposition pathways. The photodecomposition
product in largest amount (approximately one-fifth of the applied
radioactivity) was 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-chrysanthemate (II in Figure 1), followed by
trans-chrysanthemic acid and an unidentified ester (about 5% of
each). Four other oxidized esters with a modified alcohol moiety
accumulated slowly, but not to a high level (less than 3%). They are
the R and S isomers of 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-epoxychrysanthemate (III),
2-benzyloxy-5-oxo-2,5-dihydro-3-furylmethyl trans-chrysanthemic acid (V).
A minute amount of 2-benzyloxy-5-oxo- 2,5-dihydro-3-furylmethyl
trans-chrysanthemate (IV) was also detected. The R and S isomers
of trans-epoxyresmethrin (I) were minor products and did not
accumulate.
Thus, the initial pathways are of three types:
(1) oxidation of the furan ring to give a cyclic ozonide-type
peroxide intermediate (II);
(2) epoxidation of the isobutenyl double bond; and
(3) cleavage at the ester linkage to give chrysanthemic acid.
Ester bond cleavage would yield 5-benzyl-3-furylmethanol, but
none was found. 5-benzyl-3-furoic acid, alpha-(4-carboxyl-2-furyl)
benzyl alcohol and 5-benzoyl-3-furoic acid were not detected either.
This indicates that a more complex cleavage mechanism is involved or
that 5-benzyl-3-furylmethanol is not sufficiently stable to
accumulate. Benzyl alcohol, benzoic acid and phenylacetic acid (less
than 2% of each) were formed and tended to increase by extensive
oxidation. The unpleasant odour of photodecomposed bioresmethrin
appears to be due, at least in part, to the photochemical formation of
phenylacetic acid.
Following irradiation by sunlight, no appreciable changes in the
deco position pattern were observed except an increase of compound V.
A 0.14 mg/l aqueous solution of bioresmethrin irradiated with a
sunlamp yielded a larger amount of the R isomer of
trans-epoxyresmethrin (5.2% of the total radioactivity following 60
minutes' irradiation) and trans-chrysanthemic acid (11%). No
information was available on degradation studies carried out under
other conditions.
Andrews (1974) reports studies which indicate that when
bioresmethrin is applied to a forest environment by helicopter in the
form of an oil solution, there is a rapid disappearance of residues
from forest foliage and the surface of ponds. When 50 and 150 grams of
bioresmethrin in five litres of mineral oil was applied, it was
possible to determine residues of even the lower rate on foliage of
several forest species. Following application of the higher rate,
approximately 1 ppm of bioresmethrin was deposited on the foliage of
trees. Three days after application, approximately 0.3 mg/kg of
bioresmethrin could be detected on the foliage of willows but not on
other species. After seven days the deposit on willow foliage was at
the limit of determination (0.05 mg/kg). Although bioresmethrin
applied at the rate of 50 g/ha was toxic to most of the aquatic
insects in the first hour after application, no trace of bioresmethrin
could be found in any samples of pond water. Bioresmethrin was
detected in control samples containing 10 micrograms of bioresmethrin
per 100 ml of tap water.
Molecular formula C22H26O3
Molecular weight 338.45
Other information on identity and properties
Description : A viscous yellow liquid which on
crystallization forms an off-white solid
Specific gravity: 1.050 at 20°C
Flash point : 125°C (open cup)
Optical rotation: D20-5 to -8° : measurement on a 10% solution
in ethanol
Solubility : Soluble in most organic solvents but
substantially insoluble in water
Saponification
value : 160-175 mg KOH/gm
Stability : Bioresmethrin is sensitive to light but its
photo stability is greater than that of
pyrethrins. Bioresmethrin is stable to
temperatures met under most normal storage
conditions.
Bioresmethrin spray deposits appear subject
to atmospheric oxidation but any adverse
effect appears to be reduced by the
introduction of antioxidants including
piperonyl butoxide and butylated
hydroxyanisole.
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Bioresmethrin is one of the most effective broad spectrum
insecticides currently available. It exhibits a high order of
insecticidal activity, which when coupled with its excellent
toxicological properties, makes it potentially one of the safest and
most useful insecticides now being produced.
In addition to its insecticidal properties, bioresmethrin has a
good knock-down performance against flying insects, particularly when
this is compared with the organochlorine or organophosphorus
insecticides.
Bioresmethrin, at low concentrations, is an effective killing
agent against most insect pests affecting households, industrial
premises, public health, food storage and livestock production.
Efficacy against many insect pests of food crops and ornamentals has
been demonstrated.
The principal uses to which bioresmethrin is currently being put
are:
(a) in household aerosols and sprays formulated in combination
with pyrethrum, bioallethrin, tetramethrin and piperonyl
butoxide;
(b) as an insecticide for the control of pests in food premises;
(c) as a general purpose broad spectrum insecticide; and
(d) in grain disinfestation and protection.
Bioresmethrin has been tested in conjunction with a number of
well established synergists. Unlike natural pyrethrum, there is only a
minimal increase in performance against flies, mosquitos and
cockroaches when piperonyl butoxide is added to bioresmethrin.
However, in the case of a number of the more important insect pests of
stored products, bioresmethrin is synergized to a significant extent
with piperonyl butoxide, the factor of synergism ranging from four- to
ninefold.
Bioresmethrin shows good promise of becoming a major insecticide
for use in the control of stored product pests. It combines a high
potency, good persistence, and excellent safety features.
Treatment of plants
Although a number of trials have been reported where
bioresmethrin has given satisfactory results against a wide variety of
insect and mite pests of crops and ornamentals (Cooper McDougall and
Robertson, 1971, 1975; Cantu et al., 1970) there appears as yet only
limited commercial acceptance of bioresmethrin as a horticultural
insecticide. This reflects not only the state of development, but the
comparatively high cost and the relatively short residual life of
bioresmethrin spray deposits. There are however, many obvious
applications which will no doubt reach commercial development within
the next two to three years.
Non-crop situations
Many commercial uses have been developed for bioresmethrin in
non-crop situations including the control of flies, mosquitos and
other flying pests in households, pests of institutions and food
storage, aircraft disinfestation, animal-house treatments and similar
situations where aerosols, knockdown sprays and residual sprays are
conventionally used. In addition, bioresmethrin has been used in
thermal fog generators and in mosquito coils. None of these uses are
likely to give rise to significant residues in food or water, or to
cause significant intake by the general public.
Post-harvest application
Following the demonstration of outstanding activity against many
insect pests of stored products, numerous authors have reported
studies on the effect and usefulness of bioresmethrin for the control
of stored product pests and as a grain protectant (Ardley, 1973 and
1975; Ardley and Desmarchelier, 1974; Cooper McDougall and Robertson,
1971; Lloyd, 1973; Rowlands, 1975; Bengston et al., 1975a, 1975b).
Ardley (1975) showed that of many pyrethroid and organophosphorus
insecticides and insecticide combinations evaluated in silo trials,
the best product on a cost/efficiency basis was 4 ppm bioresmethrin
plus 20 ppm piperonyl butoxide. After 12 months, the treated grain
controlled all species and strains exposed in bioassay. A lower
efficiency was observed in the control of confused flour beetle
(Tribolium confusum).
The problem of resistance to malathion, dichlorvos and lindane
which is developing in stored product insects in almost all countries
has caused intensive examination of alternative protectant chemicals,
Lloyd (1973) in examining the toxicity of pyrethrins and five
synthetic pyrethroids to susceptible and pyrethrin-resistant strains
of stored product pests reported bioresmethrin to have up to 16 times
more toxicity than that of pyrethrins. He showed that piperonyl
butoxide synergized all the compounds against the various strains.
When synergized, bioresmethrin was again the most potent of the
synthetic compounds. L'Hoste et al. (1969) showed that bioresmethrin
deteriorated less rapidly than malathion when admixed with grain.
In Australia, the occurrence of malathion-resistant strains of
Rhyzopertha dominica is a severe threat to the grain export
industry. Malathion was never entirely satisfactory for the control of
this species, which shows a high degree of tolerance to all
organophosphorus materials that could possibly be used for the
protection of stored grains. The efficiency of bioresmethrin or
bioresmethrin/piperonyl butoxide combinations in controlling this
species is therefore important.
Bengston et al. (1975b) have shown clearly the high potency of
bioresmethrin against Rhyzopertha dominica which makes it an ideal
insecticide to complement such organophosphorus grain protectants as
malathion, pirimiphos-methyl and chlorpyrifos-methyl.
Table 1, from the work of Ardley and Desmarchelier (1974), shows
the efficiency of bioresmethrin/piperonyl butoxide combinations
against both susceptible and resistant strains of the more important
grain pests.
TABLE 1. Summary of Grain Protectant Trials, 1967-1974
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
1967 2 ppm 11-1/4 11.4 20.6 100 100 0 0 - 0 0 -
resmethrin + 20 ppm
pbo;2 2 ppm
pyrethrins + 20 10 11.5 20.7 85 100 1 0 - 0 0 -
ppm pbo
1968 1 ppm 4 approx. <10.0 29.0 99 100 0 1 0 0 0 0 0
bio-resmethrin + 10 or
ppm pbo; 0.5 ppm equal
to
bioresmethrin + 4 " <10.0 28.4 100 100 40 1 0 0 4 0 0
5 ppm pbo; 2 ppm
pyrethrins 3 " <10.0 20.6 95 100 20 0 0 2 10 10 1
+20 ppm pbo
1969 2 ppm 8 10.9 19.2 75 100 40 0 0 0 50 10 9
bio-resmethrin + 0.2
ppm antioxidant;
12 ppm malathion 8 10.8 20.7 100 80 90 23 0 0 0 1 0
* **
1973 4 ppm
bio-resmethrin 12 10.2 23.9 5 100 <8 - 0 0 63 0 -
8 ppm
bio-resmethrin 12 10.7 24.0 40 100 <15 - 0 0 3 1 -
12 ppm
bio-resmethrin 12 10.5 24.4 95 100 <38 - 2 0 0 0 -
TABLE 1. (cont'd)
Storage details Final laboratory assay1
Grain
holding Grain Grain Bioassay3 F1 generation (actual numbers)3
Protectant2 period moisture temperature %mortality Dead Live
treatment (months) (% average) (°C average) Sgs Rds Tcs Sgs Rds Tcs Sgs Rds Tcs
4 ppm
bio-resmethrin +
20 ppm pbo 12 10.5 23.5 100 100 <10 - 1 0 0 0 -
8 ppm
bio-resmethrin + 20
ppm pbo 12 10.5 23.6 100 100 <9 - 0 0 1 1 -
12 ppm malathion 12 10.4 23.9 95 0 <8 - 3 1 0 6 -
control, on
clean wheat 12 10.5 23.5 4 5 - - 0 4 376 0 -
1 14 day period.
2 pbo = piperonyl butoxide.
3 Sgs = Sitophilus granarius )malathion
Rds = Rhyzopertha dominica )susceptible
Tcs = Tribolium castaneum ) strains.
*S. oryzae in all 1973/74 trials.
**T. castaneum assays discontinued after nine months.
Carter et al. (1975) have evaluated a range of synthetic
pyrethroid insecticides against susceptible and resistant strains of
stored product pests and have concluded that synergized bioresmethrin
was the most suitable pyrethroid and that it was of value in
controlling organophosphorus resistant beetles.
For reasons of economy, to increase the spectrum of effect
against the many different pests encountered in stored products and to
reduce the possibility of selecting resistant strains, it is proposed
that bioresmethrin be combined with one of the organophosphorus grain
protectants. There is already a good deal of evidence (Abernathy et
al., 1973; Jao and Casida, 1974) that organophosphorus compounds
inhibit the esterases responsible for the hydrolysis of bioresmethrin
in living systems.
When used for grain protection, bioresmethrin is applied with
piperonyl butoxide in the form of an emulsion which has been diluted
with water to a concentration which, when applied to bulk grain at the
rate of 1 litre per tonne, will deposit a known amount of
bioresmethrin evenly on the grain. The amount applied ranges from 1 to
4 ppm. The amount of piperonyl butoxide applied concurrently would
range from 5 to 20 mg/kg.
Treatment of animals
Bioresmethrin is suitable for the control of flies on cattle and
pigs but no information was available to indicate whether such uses
had yet been approved by registration authorities.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Fruit and vegetable crops
Only preliminary results are available to show the fate of
bioresmethrin on tomatoes (Buick and Flanagan, 1973) and on cucumbers
(Buick and Flanagan, 1974; Cooper McDougall and Robertson, 1975).
In these studies, radio-labelled bioresmethrin emulsified in
water was applied to tomato fruits (growing in a laboratory) at a rate
sufficient to deposit 1.25 mg/kg of bioresmethrin on the tomatoes. The
deposit applied to the growing tomatoes was estimated by determining
the total radioactivity after allowing the deposit to dry for one
hour. The fruit was harvested at 6, 12, 24, 48 and 72 hours after
application and the amount of bioresmethrin in the flesh, skin and on
the skin surface was measured after washing the fruit for two minutes
in benzene. The degradation products were removed from the benzene
solution by passing through alumina. The distribution of bioresmethrin
in and on tomatoes is shown in Table 2. The results are expressed as a
percentage of the total radioactivity applied.
TABLE 2. Distribution of bioresmethrin in tomatoes
Hours 1 6 12 24 48 72
Flesh 0.2 0.2 0.2 0.2 0.2 0.2
Skin 1.75 2.45 3.2 1.95 4.65 4.65
Benzene wash 92.5 39.0 50.0 54.5 22.5 15.5
TOTAL 94.25 41.45 53.2 56.45 27.15 20.15
From the table, it is evident that over 90% of the bioresmethrin
will be recovered by simply washing with benzene if the fruit is
harvested within 24 hours of spraying. If harvested later than this,
then up to 25% of the remaining insecticide may be held in the skin
three days after treatment but this will represent less than 5% of
that originally applied. The amount of bioresmethrin found in the
flesh is insignificant.
In the case of cucumbers (Buick and Flanagan, 1974) bioresmethrin
was applied as an emulsion to the surface of cucumbers growing in a
darkened laboratory equivalent to approximately 1 mg/kg of fruit. The
total radioactivity and radioactivity due to intact bioresmethrin was
determined on the surface, in the skin and in the flesh of cucumbers
harvested 1, 6, 12, 24, 48 and 72 hours after application.
The results indicate that although most of the radioactivity
applied is retained, the bioresmethrin is substantially degraded
within an hour of application and more slowly thereafter (24% survival
after one hour, 10% after 72 hours). This contrasts with the results
of the tomato experiments in which the bioresmethrin was found to
degrade more slowly. A simple washing of the intact fruit with benzene
removes at least 85% of the surviving bioresmethrin at any time up to
three days after treatment, whether or not the fruit is stored in the
dark before analysis. The results of this work are summarized in Table
3.
TABLE 3. Distribution of undegraded bioresmethrin in cucumbers
Bioresmethrin, mean of four results (from stored and unstored vegetables)
shown as % of "concentration applied", after interval (hours)
Fraction 1 6 12 24 48 72
Flesh <0.1 <0.1 <0.1 <0.1 <0.1 <0.1
Skin 0.8 1.6 0.7 1.4 0.8 1.2
Wash 23.3 14.7 8.1 9.8 7.0 8.8
TOTAL 24.1 16.3 8.8 11.2 7.8 10.0
FATE OF RESIDUES
On plants
Rosen (1972) showed that resmethrin and its alcohol moiety
photodecompose to many unidentified products at a rate which varies
with the supporting surface. Residues exposed to sunlight on silica
gel degrade more rapidly than residues on glass and different products
are formed. The author postulates that plant and soil surfaces will
show greater differences. Photodecomposition of bioresmethrin was
studied intensively, although under limited experimental conditions,
by using phenyl-14C alcohol and carboxy-14C acid preparations (Ueda
et al., 1974). After irradiation of the radioactive resmethrin on a
silica gel plate with a sunlamp for seven hours, 88% of the
radioactivity was recovered, 5% of which was the intact resmethrin.
Forty-three per cent. of the recovered radioactivity was identified
and of this 33% and 10% respectively were ester and non-ester
products. Figure 1 illustrates the proposed, although somewhat
speculative, photodecomposition pathways. The photodecomposition
product in largest amount (approximately one-fifth of the applied
radioactivity) was 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-chrysanthemate (II in Figure 1), followed by
trans-chrysanthemic acid and an unidentified ester (about 5% of
each). Four other oxidized esters with a modified alcohol moiety
accumulated slowly, but not to a high level (less than 3%). They are
the R and S isomers of 5-hydroxy-3-oxo-4-phenyl-1-cyclopentenylmethyl
trans-epoxychrysanthemate (III),
2-benzyloxy-5-oxo-2,5-dihydro-3-furylmethyl trans-chrysanthemic acid (V).
A minute amount of 2-benzyloxy-5-oxo- 2,5-dihydro-3-furylmethyl
trans-chrysanthemate (IV) was also detected. The R and S isomers
of trans-epoxyresmethrin (I) were minor products and did not
accumulate.
Thus, the initial pathways are of three types:
(1) oxidation of the furan ring to give a cyclic ozonide-type
peroxide intermediate (II);
(2) epoxidation of the isobutenyl double bond; and
(3) cleavage at the ester linkage to give chrysanthemic acid.
Ester bond cleavage would yield 5-benzyl-3-furylmethanol, but
none was found. 5-benzyl-3-furoic acid, alpha-(4-carboxyl-2-furyl)
benzyl alcohol and 5-benzoyl-3-furoic acid were not detected either.
This indicates that a more complex cleavage mechanism is involved or
that 5-benzyl-3-furylmethanol is not sufficiently stable to
accumulate. Benzyl alcohol, benzoic acid and phenylacetic acid (less
than 2% of each) were formed and tended to increase by extensive
oxidation. The unpleasant odour of photodecomposed bioresmethrin
appears to be due, at least in part, to the photochemical formation of
phenylacetic acid.
Following irradiation by sunlight, no appreciable changes in the
deco position pattern were observed except an increase of compound V.
A 0.14 mg/l aqueous solution of bioresmethrin irradiated with a
sunlamp yielded a larger amount of the R isomer of
trans-epoxyresmethrin (5.2% of the total radioactivity following 60
minutes' irradiation) and trans-chrysanthemic acid (11%). No
information was available on degradation studies carried out under
other conditions.
Andrews (1974) reports studies which indicate that when
bioresmethrin is applied to a forest environment by helicopter in the
form of an oil solution, there is a rapid disappearance of residues
from forest foliage and the surface of ponds. When 50 and 150 grams of
bioresmethrin in five litres of mineral oil was applied, it was
possible to determine residues of even the lower rate on foliage of
several forest species. Following application of the higher rate,
approximately 1 ppm of bioresmethrin was deposited on the foliage of
trees. Three days after application, approximately 0.3 mg/kg of
bioresmethrin could be detected on the foliage of willows but not on
other species. After seven days the deposit on willow foliage was at
the limit of determination (0.05 mg/kg). Although bioresmethrin
applied at the rate of 50 g/ha was toxic to most of the aquatic
insects in the first hour after application, no trace of bioresmethrin
could be found in any samples of pond water. Bioresmethrin was
detected in control samples containing 10 micrograms of bioresmethrin
per 100 ml of tap water.
 Extensive studies now in progress in Australia are designed to
determine the effective life of bioresmethrin deposits on grain stored
under a wide variety of conditions of temperature, moisture and
aeration. Preliminary results indicate that temperature has a distinct
bearing on the residual life of the treatment and that grain
temperatures of 30-35°C reduce the half-life to approximately 8-10
weeks.
Desmarchelier (1975b) has found that grain treated with
bioresmethrin and subjected to bioassay with Rhyzopertha dominica,
shows a consistent loss of insecticidal effect which is directly
dependent on temperature. The "half-life" shown by these studies is as
follows:
Storage temperature Half-life
20°C >20 weeks
25°C 12 weeks
30°C 10 weeks
35°C 8 weeks
These studies are being confirmed by chemical analysis.
Desmarchelier (1975a) who is studying the fate of bioresmethrin
in various grains stored under a range of temperature and moisture
conditions reports preliminary results after seven weeks of a 12-month
storage programme. The results in Table 4 indicate that piperonyl
butoxide has a pronounced influence on stability even at 35°C.
Preliminary calculations indicate a half-life of 30 weeks at 30°C in
the presence of 10 times the amount of piperonyl butoxide.
Studies currently in progress in Australia (Bengston et al.,
1975) in which bioresmethrin has been used with primiphos-methyl to
treat bulk wheat held in commercial silos, both aerated and
non-aerated, indicate that the bioresmethrin deposit remains stable
for long periods. Wheat treated with a nominal 4 mg/kg bioresmethrin
was found to contain 3.1 mg/kg when analysed a few days later. This
wheat was maintained in an unaerated silo at 24°C and in an aerated
silo at 14°C (lower temperature due to aeration). Six weeks after
treatment the residue level was found to be 2.2 mg/kg (unaerated) and
2.4 mg/kg (aerated) At the end of six months, the bioresmethrin
residue had declined in only 1.1 mg/kg (unaerated) and 1.9 mg/kg
(aerated).
FATE OF RESIDUES
In animals
Extensive studies of the metabolism of bioresmethrin in animals
have been carried out by many authors including the following:
Abernathy and Casida (1973); Abernathy et al. (1973a); Farebrother
(1973); Foote et al. (1967); Jao and Casida (1974); Miyamoto (1975);
Miyamoto et al. (1971); Miyamoto et al. (1974); Suzuki and Miyamoto
(1974); Ueda et al. (1975a,b); Weeks et al. (1972).
Most of these studies have been carried out on laboratory
animals, mainly rats. Radioactivity measurements and radioautographs
indicate that the compound is rapidly absorbed from the intestinal
tract and distributed into various tissues where only a negligible
amount of intact bioresmethrin was found. However, the radioactivity
was excreted rather slowly and it took three weeks to recover all the
radioactivity in the excrete (36% in urine and 64% in faeces).
Neither urine nor faeces contained intact bioresmethrin or the
ester metabolites. The predominant urinary metabolite being
5-benzyl-3-furoic acid amounting to approximately one-third of the
radiocarbon recovered. A proposed metabolic pathway of bioresmethrin
is shown in Figure 2.
TABLE 4. Bioresmethrin residues in wheat stored at various temperatures
Moisture Bioresmethrin residue, mg/kg, after
content Initial storage at:
of grain bioresmethrin
Treatment % level 10°C 20°C 25°C 30°C 35°C
Bioresmethrin on
wheat 11.9 3.3 2.7 2.4 2.1 1.9 1.9
Bioresmethrin +
piperonyl
butoxide +
antioxidant
1/10/1 on wheat 11.9 3.3 - - - 2.9 -
Bioresmethrin on
wheat 10.2 2.8 - - 2.3 - 2.2
Bioresmethrin on
husked rice 12.0 3.3 2.9 2.3 - 1.5 -
Bioresmethrin +
piperonyl
butoxide (1/10)
on husked rice 12.0 3.3 - 3.1 - 2.9 -
Bioresmethrin on
polished rice 13.0 2.8 - - 0.8 - 0.8
Bioresmethrin +
piperonyl
butoxide (1/10)
on polished rice 13.0 2.8 - - 1.7 - 1.4
Extensive studies now in progress in Australia are designed to
determine the effective life of bioresmethrin deposits on grain stored
under a wide variety of conditions of temperature, moisture and
aeration. Preliminary results indicate that temperature has a distinct
bearing on the residual life of the treatment and that grain
temperatures of 30-35°C reduce the half-life to approximately 8-10
weeks.
Desmarchelier (1975b) has found that grain treated with
bioresmethrin and subjected to bioassay with Rhyzopertha dominica,
shows a consistent loss of insecticidal effect which is directly
dependent on temperature. The "half-life" shown by these studies is as
follows:
Storage temperature Half-life
20°C >20 weeks
25°C 12 weeks
30°C 10 weeks
35°C 8 weeks
These studies are being confirmed by chemical analysis.
Desmarchelier (1975a) who is studying the fate of bioresmethrin
in various grains stored under a range of temperature and moisture
conditions reports preliminary results after seven weeks of a 12-month
storage programme. The results in Table 4 indicate that piperonyl
butoxide has a pronounced influence on stability even at 35°C.
Preliminary calculations indicate a half-life of 30 weeks at 30°C in
the presence of 10 times the amount of piperonyl butoxide.
Studies currently in progress in Australia (Bengston et al.,
1975) in which bioresmethrin has been used with primiphos-methyl to
treat bulk wheat held in commercial silos, both aerated and
non-aerated, indicate that the bioresmethrin deposit remains stable
for long periods. Wheat treated with a nominal 4 mg/kg bioresmethrin
was found to contain 3.1 mg/kg when analysed a few days later. This
wheat was maintained in an unaerated silo at 24°C and in an aerated
silo at 14°C (lower temperature due to aeration). Six weeks after
treatment the residue level was found to be 2.2 mg/kg (unaerated) and
2.4 mg/kg (aerated) At the end of six months, the bioresmethrin
residue had declined in only 1.1 mg/kg (unaerated) and 1.9 mg/kg
(aerated).
FATE OF RESIDUES
In animals
Extensive studies of the metabolism of bioresmethrin in animals
have been carried out by many authors including the following:
Abernathy and Casida (1973); Abernathy et al. (1973a); Farebrother
(1973); Foote et al. (1967); Jao and Casida (1974); Miyamoto (1975);
Miyamoto et al. (1971); Miyamoto et al. (1974); Suzuki and Miyamoto
(1974); Ueda et al. (1975a,b); Weeks et al. (1972).
Most of these studies have been carried out on laboratory
animals, mainly rats. Radioactivity measurements and radioautographs
indicate that the compound is rapidly absorbed from the intestinal
tract and distributed into various tissues where only a negligible
amount of intact bioresmethrin was found. However, the radioactivity
was excreted rather slowly and it took three weeks to recover all the
radioactivity in the excrete (36% in urine and 64% in faeces).
Neither urine nor faeces contained intact bioresmethrin or the
ester metabolites. The predominant urinary metabolite being
5-benzyl-3-furoic acid amounting to approximately one-third of the
radiocarbon recovered. A proposed metabolic pathway of bioresmethrin
is shown in Figure 2.
TABLE 4. Bioresmethrin residues in wheat stored at various temperatures
Moisture Bioresmethrin residue, mg/kg, after
content Initial storage at:
of grain bioresmethrin
Treatment % level 10°C 20°C 25°C 30°C 35°C
Bioresmethrin on
wheat 11.9 3.3 2.7 2.4 2.1 1.9 1.9
Bioresmethrin +
piperonyl
butoxide +
antioxidant
1/10/1 on wheat 11.9 3.3 - - - 2.9 -
Bioresmethrin on
wheat 10.2 2.8 - - 2.3 - 2.2
Bioresmethrin on
husked rice 12.0 3.3 2.9 2.3 - 1.5 -
Bioresmethrin +
piperonyl
butoxide (1/10)
on husked rice 12.0 3.3 - 3.1 - 2.9 -
Bioresmethrin on
polished rice 13.0 2.8 - - 0.8 - 0.8
Bioresmethrin +
piperonyl
butoxide (1/10)
on polished rice 13.0 2.8 - - 1.7 - 1.4
 Farebrother (1973) in a whole body autoradiographical study in
rats showed that bioresmethrin was absorbed through the gut and widely
distributed in the body two hours after dosing. At six hours the
distribution was similar but the concentrations were increased,
particularly in fatty tissues. At 24 hours most tissues showed greatly
reduced activity but the concentration in fatty tissue remained high.
No studies appear to have been carried out to determine the level
or nature of the metabolites in animal fat or other tissues. No
studies are yet available to show the fate of bioresmethrin when fed
to livestock or poultry.
Wheat
Ardley (1975) reports trials in which wheat treated with
bioresmethrin at varying rates with and without piperonyl butoxide,
was subjected to standard milling and baking tests.
The grain, when milled gave 25% bran, 7% pollard (shorts) and 68%
flour. The flour was converted into bread. All loaves were
satisfactory. No taint or odour was evident during dough processing or
in the loaves fresh from the oven.
The milling fractions and the bread were analysed. The nature of
the chemical treatment and the results of analysis are provided in the
following table. Analyses were carried out by two laboratories and the
results were in good agreement.
TABLE 5. Residues of bioresmethrin and piperonyl butoxide in milling
fractions and bread
Recovered residue (i) bioresmethrin (ii) piperonyl butoxide (ppm)
(i) (ii)
Protectant1
treatment bran pollard flour bread bran pollard flour bread
a 0.3 4.0 4.0 nil 21.0 10.0 2.0 2.0
b 0.3 1.0 1.4 nil 0.5 0.3 nil nil
c 0.5 1.0 0.5 nil 11.0 8.0 10.0 nil
d 1.1 1.2 0.5 nil 2.0 2.0 2.0 nil
e nil nil nil nil nil nil nil nil
1 a = 4 mg/kg bioresmethrin + 20 mg/kg piperonyl butoxide
b = 2 " " alone
c = 4 " " + 10 mg/kg piperonyl butoxide +
4 mg/kg anti-oxidant
d = 2 " " + 2 mg/kg piperonyl butoxide +
10 mg/kg anti-oxidant + 4 mg/kg
fenitrothion.
In a subsequent study Desmarchelier ( 1975b) took wheat which had
been treated six weeks (A) and seven months (B) previously and
subjected it to standard milling procedures for the preparation of
wholemeal and white flour. The following results indicate the
distribution and fate of the bioresmethrin:
Sample A B
Interval since treated 6 weeks 7 months
Residue in wheat 2.9 mg/kg 1.9 mg/kg
bran 5.2 " 1.7 "
pollard (shorts) 0.7 " -
white flour * *
wholemeal bread 1.0 " 0.6 "
white bread ND ND
limit of determination = 0.05 mg/kg
* Interference led to poor recoveries during assay of flour.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Bioresmethrin has not yet been widely used on food crops or for
the treatment of stored grain and therefore no information is
available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The analytical methods for resmethrin have been reviewed by Brown
(1973) and also by Murano et al. (1971), Murano (1972) and
Desmarchelier (1975). The methods include ultra-violet absorption
spectrophotometry, colorimetry, TLC and GLC. SE-30, DC200 ‰ QFI and DEGS
have been used as stationary phases for the GLC determination of
resmethrin in crops after suitable clean-up procedures. Brown (1973)
proposes a residue analytical method for wheat, wheat flour and corn
meal in which resmethrin is extracted with hexane, partitioned between
hexane and acetonitrile, cleaned up on an Alumina column, and
determined by GLC.
The difficulty in the residue analysis of resmethrin, which would
apply equally to the optical isomer bioresmethrin, seems to lie in the
intrinsically low sensitivity to the compound of electron capture or
flame ionization detectors. The limit of detection ranges at best from
0.1 to 0.05 µg, equivalent in practice to a residue level of about
0.1 mg/kg.
To overcome the drawback, the component chrysanthemic acid is
quantitatively condensed with 2,4-dichlorobenzyl chloride to yield
2,4-dichlorobenzyl chrysanthemate, which can be determined by electron
capture (Miyamoto, 1975), It is reported that this modification allows
as little as 0.01 ng of the derivative or approximately 0.01 ng of
bioresmethrin to be analysed. Up to 100 µg of bioresmethrin is
hydrolyzed in 10% methanolic potassium hydroxide for one hour at 60°C.
Chrysanthemic acid is extracted with chloroform at pH2. The chloroform
is evaporated in vacuo and the residue dissolved in 5 ml acetone and
warmed at 60°C for 120 min with the addition of 0.1 ml of
N,N-dimethylformamide and one drop each of triethylamine and
2,4-dichlorobenzyl chloride. The reaction mixture is passed through a
Florisil column and eluted with benzene. 2,4-dichlorobenzyl
chrysanthemate is chromatographed under the following conditions.
Liquid phase Epon-1001 (1%) and QF-1 (1%) on Chromosorb B (100 to 120
mesh); temperature of oven and detector, 200°C and of injection port,
240°C; carrier nitrogen, 1.0 kg/cm›. Retention time of
2,4-dichlorobenzyl chrysanthemate about 4-1/2 min. The overall
recovery in the range 1 to 100 ng of resmethrin is at least 80%, often
more than 90%. Resmethrin residues of the order of 1 µg/kg can
therefore be determined.
Desmarchelier (1975a,b) reports a colorimetric method based an
the procedure of Screiber and McClellan (1954) and McClellan (1964)
originally designed for the analysis of pyrethrum and based on the
analysis of chrysanthemic acid. The author examines the applicability
of this procedure to residue analysis of five pyrethroid derivatives
of chrysanthemic acid on six commodities and extends it to derivatives
of pyrethric acid, for example pyrethrin II. In addition, a TLC
procedure is described that allows positive identification of
derivatives of chrysanthemic acid in a one-step process. In the case
of bioresmethrin, results obtained by these two procedures on aged
residues on wheat were compared with results obtained by two
procedures specific to bioresmethrin. Appreciable differences between
results occurred only at the limit of determination. In the method of
Desmarchelier, petroleum spirit (b.p. 30-40°C) was added to whole
grain (100 g) and left overnight. The supernatant was decanted, the
grain covered with solvent and extracted for a further hour and this
procedure repeated once more. Methanol, acetone and 10% acetone in
petroleum spirit appear equally suitable as solvents.
Although none of the metabolites or photo-products have been
reported to be analysed in the actual samples, various techniques and
procedures for separation as well as identification of the possible
terminal residues of bioresmethrin are described in a number of papers
including those of Miyamoto et al. (1971); Ueda et al. (1974, 1975a
and b); Abernathy (1973); Brown (1973) and Murano (1972).
Simonartis and Coil (1975) have developed a GLC method for the
determination of resmethrin in corn, corn meal, flour and wheat. it
involves extraction with pentane, transfer to acetonitrile, Florisil
clean-up using 3% ethyl acetate in perthane followed by GLC using
flame conization detector. The method is sensitive to 0.2 ppm with
better than 85% recovery and good reproductivity.
NATIONAL TOLERANCES
The information available to the Meeting suggests that no
national tolerances have yet been established.
APPRAISAL
Bioresmethrin is a broad spectrum pyrethroid insecticide with
contact action and pronounced knockdown effect. It shows only limited
biological persistence on plant surfaces and is readily degraded by
sunlight but gives long-lasting control of insect pests on inert
surfaces and retains its biological activity when applied to stored
agricultural commodities, including raw grain and nuts.
The Meeting had only limited information concerning the use and
performance of this material on crops pre-harvest but extensive data
were available on the use, performance and fate of bioresmethrin on a
variety of grains. The recommended rate of application to raw cereals
is 1 to 4 mg/kg. The biological half-life on grain ranges from eight
weeks at 35°C to more than 20 weeks at 20°C.
Data available suggest that when bioresmethrin is applied to
wheat, the deposit is not confined to the seed coat but penetrates to
the endosperm so that a substantial proportion of the amount applied
is found in flour. Standard milling techniques which remove most of
the seed coat, produce a bran which contains considerably less
bioresmethrin than does the whole grain or the flour. Virtually all of
the bioresmethrin is destroyed when bread prepared from such flour is
cooked.
Extensive information is available on the biological degradation
and metabolism of bioresmethrin including the nature of the
metabolites formed under a wide variety of conditions. The initial
step in the metabolism of bioresmethrin is hydrolysis at the ester
linkage, yielding 5-benzyl-3-furylmethanol and chrysanthemic acid.
Several GLC methods of analysis suitable for the determination of
residues of bioresmethrin and metabolites containing chrysanthemic
acid in plant materials are available. The limit of determination
ranges from 0.1 to 0.001 mg/kg. In addition there are several
colorimetric methods based an the determination of chrysanthemic acid
which can be used in conjunction with a TLC procedure for identifying
the pyrethroid residue involved. No national tolerances have yet been
determined.
In proposing maximum residue limits for bioresmethrin on raw
grain, milled cereal products and foods prepared therefrom, careful
consideration has been given to the fact that this insecticide is to
be used as a grain protectant, that a certain concentration must be
present in the grain to control infestations and prevent damage to
stored products and that the compound is moderately stable under
storage conditions. Under practical conditions of grain handling and
storage, there will always be a natural variation in the level of the
deposit resulting from a fluctuation in the flow of grain and
insecticide. it is therefore not possible to fix the maximum limit on
the minimum necessary to control insect pests. At the present time, it
is anticipated that bioresmethrin will be used in conjunction with one
or more of the organophosphorus grain protectants whose effect is
complementary and possibly synergistic. Under such conditions the
amount of bioresmethrin used will be at the lower range of the
proposed rates. However, there will be situations where bioresmethrin
must be used alone at the maximum of the proposed rate and due
allowance must be made for the amplitude of the variations in
concentration which are inevitable and the problems of sampling and
analysis involved.
RECOMMENDATIONS
The following guideline levels are recommended as limits which
need not be exceeded when bioresmethrin is used according to good
agricultural practices in the following commodities:
Commodity Maximum residue limit, mg/kg
Raw grain 5
Milled products from grain 5
Cooked cereal products, including 0.05*
bread
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Full toxicological data.
DESIRED
1. Further information on the level and fate of bioresmethrin
on different classes of raw grains.
2. Information on residues from supervised trials on other
stored commodities, including nuts, peanuts, lentils, dried
fruit and dried vegetables,
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in
food at the point of consumption following the use of
bioresmethrin for the control of various stored-product
pests.
5. Improved procedures for the determination of bioresmethrin
residues in fruit and vegetables as well as stored products.
REFERENCES
Abernathy, C. O. and Casida, J. E. (1973) Pyrethroid insecticides:
esterase cleavage in relation to selective toxicity. Science, 179:
1235-1236.
Abernathy, C. O., Ueda, K., Engel, J. L., Gaughan, L. C. and Casida,
J. E. (1973a) Substrate specificity and toxicological significance of
pyrethroid hydrolyzing esterases of mouse liver microsomes. Pesticide
Biochemistry and Physiology, 3: 300-311.
Andrews, T. L. (1974) Resmethrin Residues in foliage after aerial
application. Pesticide Monitoring Journal, 8(1):50-52.
Ardley, J. H. (1973) Further field evaluation of bioresmethrin as a
potential replacement for malathion in grain protection. Wellcome
Australia Ltd Report TD/5/73/Kl-36.
Ardley, J. H. (1975) Use and application of resmethrin and
bioresmethrin as potential grain protectants. Paper submitted to
Journal of Stored Products Research (May 1975)
Ardley, J. H. and Desmarchelier, J. M. (1974) Investigations into the
use of resmethrin and bioresmethrin as potential grain protectants.
Proceedings of the First International Working Conference on Stored
Product Entomology, Savannah, Georgia, USA.
Ardley, J. H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat. Report of Wellcome Australia Ltd, 27
August 1975.
Bengston, M., Cooper, L. M. and Holton, F. J. (1975b) A comparison of
bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as grain
protectants against malathion-resistant insects in wheat. Submitted
for publication, Queensland Journal of Agricultural and Animal
Sciences.
Bengston, M., Connell M., Desmarchelier, J., Snelson, J. and Sticka,
R. (1975a) Report of field trials with grain protectants. To be
published.
Brooks, I. C., Hans, J., Blumenthal, R. R. and Davis, B. S. (1969) SBP
1382, a new synthetic pyrethroid. Soap Chem. Spec., 45:62.
Brown, B. B. (1973) In "Analytical methods for pesticides and growth
regulators". Edited by G. Zweig (Academic Press), p. 441.
Buick, A. R. and Flanagan, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes. Wellcome Foundation Report HEGH 73/1.
Buick, A. R. and Flanagan, P. (1974) The fate of bioresmethrin on
cucumbers. Wellcome Foundation Report HEGH 74/1.
Cantu, E. and Wolfenbarger, D. A. (1970) Toxicity tests of three
pyrethroids to several insect pests of cotton. J. econ. Ent., 63(4):
1373-1374
Carter, S. W., Chadwick, P. R. and Wickham, J. C. (1975) Comparative
observations on the activity of pyrethroids against some susceptible
and resistant stored products insects. Submitted for publication.
Chesher, B. C. and Malone, J. C. (1970) Acute toxicity tests with NRDC
107 in hens. Wellcome Foundation Report B 236-70.
Chesher, B. C. and Malone, B. C. (1970) Sensitisation study with NRDC
107 on guinea pigs. Wellcome Foundation Report B 198-70.
Chesher, B. C. and Malone, J. C. (1970) Ocular irritancy of NRDC 107
in rabbits. Wellcome Foundation Report B 217-70.
Chesher, B. C. and Malone, J. C. (1971) Toxicity to dogs of NRDC 107
(continuation). Wellcome Foundation Report No. B 27-71.
Chesher, B. C. and Malone, J. C. (1971) Acute toxicity of NRDC 107 by
the intravenous route. Wellcome Foundation Report B 329-71.
Colas, R. (1970) Toxicité NRDC 107 et NRDC 104 sur poissons. Report
from Procida, 9 September 1970.
Cooper McDougall and Robertson. (1971) Bioresmethrin Technical Data
BRM 1-8.
Cooper McDougall and Robertson Ltd. (1975) Bioresmethrin for
agricultural use. Technical Bulletin, 41 pages.
Desmarchelier, J. M. (1975a) Analysis of pyrethroids on grains (In
press). CSIRO Division of Entomology, P.O. Box 1700, Canberra City,
Australia.
Desmarchelier, J. M. (1975b) Results of studies to determine fate of
bioresmethrin on stored grain. CSIRO Stored Grain Research Laboratory,
Canberra, Australia (Personal communication).
Elliott, M. (1969) Structural requirements for pyrethrum-like
activity. Chemistry and Industry, 14 June 1969, 776-781.
Elliott, M., Farnham, A. W., Janes, N. F., Needham, P. H. and Pearson,
B. C. (1967) 5-benzyl-3-furfuryl methyl chrysanthemate - a new potent
insecticide. Nature, 213(5075):493.
Elliott, M., Janes, N. F. and Pearson, B. C. (1971) The pyrethrins and
related compounds, Part XIII. Insecticidal methyl -, alkenyl- and
benzyl-substituted furfuryl and furylmethyl chrysanthemates. Pestic.
Sci., 2:243.
Elliott, W., Janes, N. F. and Spanner, J. A. (1973) The pyrethrins and
related compounds. Preparation of bioresmethrin, Pestic. Sci.,
4:677-681.
Fales, J. H., Bodensten, O. F., Waters, R. M., Fields, E. S. and Hall,
R. P. (1971) Insecticidal Evaluation of SBP-1390 (bioresmethrin). Soap
Chem. Spec., September 1971, pp. 54-62, 73.
Farebrother, D. A. NRDC 107. (1973) Whole body radioautographal study
in male albino rats. Wellcome Foundation Report HEFH 73-1.
Flanagan, F. & Poll, G. S. (1970) Synthetic pyrethroids - estimation
of cis and trans isomers. Cooper Technical Bureau Series B Report
93/70, 21 May 1970.
Foote, C. S., Wuesthoff, M. T., Wexler, S., Burnstain, I. G., Denny,
R., Schenck, G. O. and Schulte-Elte, K.-H. (1967) Tetrahedron,
23:2583.
Ford, M. G. and Pert, D. R. (1974) Time/Dose/Response relationships of
pyrethroids insecticides with special reference to knockdown.
Pesticide Science, 5:635-641
Fujimoto, K., Itaya, N., Okuno, Y., Kadota, T. and Yamaguchi, T.
(1973) A new insecticidal pyrethroid ester. Agri. Biol. Chem.,
37(1):2681-2682.
Glomot, R. (Undated) Etude de la toxicité chronique de 3 semaines chez
le rat du RU11484 (NRDC 107) Roussel Uclaf Rapport.
Glomot, R. and Chevalier, B. (1969) Etude de la Toxicité argue du
RU11484 (NRDC 107) Roussel Uclaf Rapport Toxico 11386.
Jao, L. T. and Casida, J. E. (1974) Pestic. Biochem. Physiol., 4:456
L'Hoste, J., Balloy, J. and Rauch, F. (1969) La protection des blés
contre Sitophilus granarius. Congr. Pl. Prot. Org. by CIA and CITA,
Milan 1969, No. 22, 6 pp.
L'Hoste, J., Martel, J. and Rauch, F. (1969) A new insecticide
5-benzyl 3-furfuryl methyl d-trans ethanochrysanthémate. Proc. 5th
Br. Insectic. Fungic. Conf., pp. 554-557.
L'Hoste, J. and Rauch, F. (1969) Remarques sur quelques
chrysanthémates insecticides de synthèse. Revue de zoologie Agricole
et Appliqués, Deuxième Trimestre, Nos. 4-6, pp. 53-66.
L'Hoste, J. and Rauch, F. (1969) Sur les propriétés insecticides du
d-trans ethanochrysanthémate de benzyl-5 furylméthyle-3. Conte
Rendu Acad. Sc., Paris 368, 3218-3220 (30 juin 1969)
Lloyd, C. J. (1973) The toxicity of pyrethrins and five synthetic
pyrethroids to Tribolium castaneum and susceptible and pyrethrins
resistant Sitophilus granarius. J. Stored Prod. Res., 9:77-92
Malone, J. C. and Chesher, B. C. (1970) Toxicity to dogs of NRDC 107.
Wellcome Foundation Ltd - Report No. B 204-70.
Malone, J. C. and Chesher, B. C. (1970) Toxicity of bioallethrin/NRDC
107 crawling insect killer to canaries and budgerigars. Wellcome
Foundation Report No. B 211-70.
Malone, J. C. (1971) NRDC 107, 3 month oral toxicity study in beagle
dogs. Wellcome Foundation Report No. B 109-71.
Miyamoto, J. (1975) Terminal residues of bioresmethrin. Submission to
IUPAC Terminal Residues Commission, Madrid, September 1975.
Miyamoto, J., Nishida, T. and Ueda , K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furyl methyl-dl-trans-chrysanthemate in the
rat. Pesticide Biochemistry and Physiology, 1:293, 306.
Miyamoto, J., Suzuki, T. and Nakae, C. (1974) Pestic. Biochem., 4:438
Murano, A. (1972) Agr. Biol. Chem., 36:2203
Murano, J., Fujiwara, S., Horiba, M. and Miyamoto, J. (1971) Agr.
Biol. Chem., 35(8):1200-1207.
Noel, P. R. B., Rivett, K. F., Chesterman, H., Street, A. E. and
Spicer, E. J. F. (1971) Oral toxicity study in Beagle dogs (repeated
dosage for 3 months), Huntington Research Centre Report 3900/71/58, 76
pp.
Okuno, J., Fujimoto, K., Kadota, T., Miyamoto, J. and Hamuro, K.
(1969) Insecticidal activity of a new synthetic pyrethroidal compound,
5-benzyl-3-furyl-methyl dl-cis, trans chrysanthemate (NRDC - 104,
Chrysron), Botyu-Kagaku, 34:157.
Rosen, J. D. (1972) In "Environmental Toxicology of Pesticides",
Matsumura, F., Bosh, G. M. and Misato, T. Academic Press N.Y., p. 438.
Rowlands, D. (1975) Breakdown of bioresmethrin on wheat. Note prepared
by Pest Infestation Control Laboratory - Ministry of Agriculture,
Fisheries and Food, Slough, United Kingdom.
Simonartis, R. A. and Coil, R. S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour and wheat. J.
AOAC, 58(5):1032-1035.
Smith, F. F. and Boswell, A. L. Toxicity of micronised dusts to some
glasshouse insects. J. econ. Ent., 65(5):1466-1468.
Sullivan, J. W. N. et al. (1972) Gas propelled aerosols and micronised
dusts for control of insects in aircraft 7 - Effectiveness against
pests of agriculture. J. econ. Ent., 65(5):1462-1466.
Suzuki, T. and Miyamoto, J. (1974) Pestic. Biochem. Physiol., 4:86.
Tooby, T. E. (1973) Toxicity of the synthetic pyrethroid NRDC 107 to
fish. Ministry of Agriculture, Fisheries and Food, Salmon and
Freshwater Fisheries Laboratory Report FGB 29092.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1974) Photodecomposition
of resmethrin and related pyrethroids. J. Agr. Food Chem.,
22(2):212-220.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975a) Metabolism of
(+)-trans and (+)-cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975b) Pesticide Biochem.
Physiol., 5:280
Verschoyle R. D. and Barnes, J. M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. Physiol., 2(3):308-311.
Waldron, M. M. (1969) Foetal toxicity study - 95 H 56 - given orally
in the rabbit. Wellcome Foundation Report, Path 51.
Wallwork, L., Chesher, B. C. and Malone, J. C. (1970) Toxicity of NRDC
107 to laboratory animals. Wellcome Foundation Report B 199-70.
Wallwork, L. M., Clampitt, R. B. and Malone, J. C. (1971) NRDC 107,
rat oral 91 day toxicity study. Wellcome Foundation Report B 119-71.
Wallwork, L. M. and Malone, J. C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Wellcome
Foundation Report B 47-71.
Wallwork, L. M. and Malone, J. C. (1972) Bioresmethrin - Preliminary
24 hour rat inhalation study. Wellcome Foundation Report B 290-72.
Weeks, M. H., Leland, T. M., Boldt, R. E. and Mellick, P. W. (1972)
Toxicological evaluation of pyrethroid insecticide SBP 1382. US Army
Environmental Hygienic Agency Special Study No. 51-127-71/72.
Wellcome. 1973(ca.) Summary of trial results. Agricultural uses of
bioresmethrin. Wellcome Research Laboratories, Berkhamsted, 29 pp.
Winney, R. (1973) The biological performance of synthetic pyrethroids.
Pyrethrum Post., 12(1):2-11.
Farebrother (1973) in a whole body autoradiographical study in
rats showed that bioresmethrin was absorbed through the gut and widely
distributed in the body two hours after dosing. At six hours the
distribution was similar but the concentrations were increased,
particularly in fatty tissues. At 24 hours most tissues showed greatly
reduced activity but the concentration in fatty tissue remained high.
No studies appear to have been carried out to determine the level
or nature of the metabolites in animal fat or other tissues. No
studies are yet available to show the fate of bioresmethrin when fed
to livestock or poultry.
Wheat
Ardley (1975) reports trials in which wheat treated with
bioresmethrin at varying rates with and without piperonyl butoxide,
was subjected to standard milling and baking tests.
The grain, when milled gave 25% bran, 7% pollard (shorts) and 68%
flour. The flour was converted into bread. All loaves were
satisfactory. No taint or odour was evident during dough processing or
in the loaves fresh from the oven.
The milling fractions and the bread were analysed. The nature of
the chemical treatment and the results of analysis are provided in the
following table. Analyses were carried out by two laboratories and the
results were in good agreement.
TABLE 5. Residues of bioresmethrin and piperonyl butoxide in milling
fractions and bread
Recovered residue (i) bioresmethrin (ii) piperonyl butoxide (ppm)
(i) (ii)
Protectant1
treatment bran pollard flour bread bran pollard flour bread
a 0.3 4.0 4.0 nil 21.0 10.0 2.0 2.0
b 0.3 1.0 1.4 nil 0.5 0.3 nil nil
c 0.5 1.0 0.5 nil 11.0 8.0 10.0 nil
d 1.1 1.2 0.5 nil 2.0 2.0 2.0 nil
e nil nil nil nil nil nil nil nil
1 a = 4 mg/kg bioresmethrin + 20 mg/kg piperonyl butoxide
b = 2 " " alone
c = 4 " " + 10 mg/kg piperonyl butoxide +
4 mg/kg anti-oxidant
d = 2 " " + 2 mg/kg piperonyl butoxide +
10 mg/kg anti-oxidant + 4 mg/kg
fenitrothion.
In a subsequent study Desmarchelier ( 1975b) took wheat which had
been treated six weeks (A) and seven months (B) previously and
subjected it to standard milling procedures for the preparation of
wholemeal and white flour. The following results indicate the
distribution and fate of the bioresmethrin:
Sample A B
Interval since treated 6 weeks 7 months
Residue in wheat 2.9 mg/kg 1.9 mg/kg
bran 5.2 " 1.7 "
pollard (shorts) 0.7 " -
white flour * *
wholemeal bread 1.0 " 0.6 "
white bread ND ND
limit of determination = 0.05 mg/kg
* Interference led to poor recoveries during assay of flour.
EVIDENCE OF RESIDUES IN FOOD IN COMMERCE OR AT CONSUMPTION
Bioresmethrin has not yet been widely used on food crops or for
the treatment of stored grain and therefore no information is
available on residues in food moving in commerce.
METHODS OF RESIDUE ANALYSIS
The analytical methods for resmethrin have been reviewed by Brown
(1973) and also by Murano et al. (1971), Murano (1972) and
Desmarchelier (1975). The methods include ultra-violet absorption
spectrophotometry, colorimetry, TLC and GLC. SE-30, DC200 ‰ QFI and DEGS
have been used as stationary phases for the GLC determination of
resmethrin in crops after suitable clean-up procedures. Brown (1973)
proposes a residue analytical method for wheat, wheat flour and corn
meal in which resmethrin is extracted with hexane, partitioned between
hexane and acetonitrile, cleaned up on an Alumina column, and
determined by GLC.
The difficulty in the residue analysis of resmethrin, which would
apply equally to the optical isomer bioresmethrin, seems to lie in the
intrinsically low sensitivity to the compound of electron capture or
flame ionization detectors. The limit of detection ranges at best from
0.1 to 0.05 µg, equivalent in practice to a residue level of about
0.1 mg/kg.
To overcome the drawback, the component chrysanthemic acid is
quantitatively condensed with 2,4-dichlorobenzyl chloride to yield
2,4-dichlorobenzyl chrysanthemate, which can be determined by electron
capture (Miyamoto, 1975), It is reported that this modification allows
as little as 0.01 ng of the derivative or approximately 0.01 ng of
bioresmethrin to be analysed. Up to 100 µg of bioresmethrin is
hydrolyzed in 10% methanolic potassium hydroxide for one hour at 60°C.
Chrysanthemic acid is extracted with chloroform at pH2. The chloroform
is evaporated in vacuo and the residue dissolved in 5 ml acetone and
warmed at 60°C for 120 min with the addition of 0.1 ml of
N,N-dimethylformamide and one drop each of triethylamine and
2,4-dichlorobenzyl chloride. The reaction mixture is passed through a
Florisil column and eluted with benzene. 2,4-dichlorobenzyl
chrysanthemate is chromatographed under the following conditions.
Liquid phase Epon-1001 (1%) and QF-1 (1%) on Chromosorb B (100 to 120
mesh); temperature of oven and detector, 200°C and of injection port,
240°C; carrier nitrogen, 1.0 kg/cm›. Retention time of
2,4-dichlorobenzyl chrysanthemate about 4-1/2 min. The overall
recovery in the range 1 to 100 ng of resmethrin is at least 80%, often
more than 90%. Resmethrin residues of the order of 1 µg/kg can
therefore be determined.
Desmarchelier (1975a,b) reports a colorimetric method based an
the procedure of Screiber and McClellan (1954) and McClellan (1964)
originally designed for the analysis of pyrethrum and based on the
analysis of chrysanthemic acid. The author examines the applicability
of this procedure to residue analysis of five pyrethroid derivatives
of chrysanthemic acid on six commodities and extends it to derivatives
of pyrethric acid, for example pyrethrin II. In addition, a TLC
procedure is described that allows positive identification of
derivatives of chrysanthemic acid in a one-step process. In the case
of bioresmethrin, results obtained by these two procedures on aged
residues on wheat were compared with results obtained by two
procedures specific to bioresmethrin. Appreciable differences between
results occurred only at the limit of determination. In the method of
Desmarchelier, petroleum spirit (b.p. 30-40°C) was added to whole
grain (100 g) and left overnight. The supernatant was decanted, the
grain covered with solvent and extracted for a further hour and this
procedure repeated once more. Methanol, acetone and 10% acetone in
petroleum spirit appear equally suitable as solvents.
Although none of the metabolites or photo-products have been
reported to be analysed in the actual samples, various techniques and
procedures for separation as well as identification of the possible
terminal residues of bioresmethrin are described in a number of papers
including those of Miyamoto et al. (1971); Ueda et al. (1974, 1975a
and b); Abernathy (1973); Brown (1973) and Murano (1972).
Simonartis and Coil (1975) have developed a GLC method for the
determination of resmethrin in corn, corn meal, flour and wheat. it
involves extraction with pentane, transfer to acetonitrile, Florisil
clean-up using 3% ethyl acetate in perthane followed by GLC using
flame conization detector. The method is sensitive to 0.2 ppm with
better than 85% recovery and good reproductivity.
NATIONAL TOLERANCES
The information available to the Meeting suggests that no
national tolerances have yet been established.
APPRAISAL
Bioresmethrin is a broad spectrum pyrethroid insecticide with
contact action and pronounced knockdown effect. It shows only limited
biological persistence on plant surfaces and is readily degraded by
sunlight but gives long-lasting control of insect pests on inert
surfaces and retains its biological activity when applied to stored
agricultural commodities, including raw grain and nuts.
The Meeting had only limited information concerning the use and
performance of this material on crops pre-harvest but extensive data
were available on the use, performance and fate of bioresmethrin on a
variety of grains. The recommended rate of application to raw cereals
is 1 to 4 mg/kg. The biological half-life on grain ranges from eight
weeks at 35°C to more than 20 weeks at 20°C.
Data available suggest that when bioresmethrin is applied to
wheat, the deposit is not confined to the seed coat but penetrates to
the endosperm so that a substantial proportion of the amount applied
is found in flour. Standard milling techniques which remove most of
the seed coat, produce a bran which contains considerably less
bioresmethrin than does the whole grain or the flour. Virtually all of
the bioresmethrin is destroyed when bread prepared from such flour is
cooked.
Extensive information is available on the biological degradation
and metabolism of bioresmethrin including the nature of the
metabolites formed under a wide variety of conditions. The initial
step in the metabolism of bioresmethrin is hydrolysis at the ester
linkage, yielding 5-benzyl-3-furylmethanol and chrysanthemic acid.
Several GLC methods of analysis suitable for the determination of
residues of bioresmethrin and metabolites containing chrysanthemic
acid in plant materials are available. The limit of determination
ranges from 0.1 to 0.001 mg/kg. In addition there are several
colorimetric methods based an the determination of chrysanthemic acid
which can be used in conjunction with a TLC procedure for identifying
the pyrethroid residue involved. No national tolerances have yet been
determined.
In proposing maximum residue limits for bioresmethrin on raw
grain, milled cereal products and foods prepared therefrom, careful
consideration has been given to the fact that this insecticide is to
be used as a grain protectant, that a certain concentration must be
present in the grain to control infestations and prevent damage to
stored products and that the compound is moderately stable under
storage conditions. Under practical conditions of grain handling and
storage, there will always be a natural variation in the level of the
deposit resulting from a fluctuation in the flow of grain and
insecticide. it is therefore not possible to fix the maximum limit on
the minimum necessary to control insect pests. At the present time, it
is anticipated that bioresmethrin will be used in conjunction with one
or more of the organophosphorus grain protectants whose effect is
complementary and possibly synergistic. Under such conditions the
amount of bioresmethrin used will be at the lower range of the
proposed rates. However, there will be situations where bioresmethrin
must be used alone at the maximum of the proposed rate and due
allowance must be made for the amplitude of the variations in
concentration which are inevitable and the problems of sampling and
analysis involved.
RECOMMENDATIONS
The following guideline levels are recommended as limits which
need not be exceeded when bioresmethrin is used according to good
agricultural practices in the following commodities:
Commodity Maximum residue limit, mg/kg
Raw grain 5
Milled products from grain 5
Cooked cereal products, including 0.05*
bread
* At or about the limit of determination.
FURTHER WORK OR INFORMATION
REQUIRED (before an acceptable daily intake can be allocated)
1. Full toxicological data.
DESIRED
1. Further information on the level and fate of bioresmethrin
on different classes of raw grains.
2. Information on residues from supervised trials on other
stored commodities, including nuts, peanuts, lentils, dried
fruit and dried vegetables,
3. Information on residues in fruit and vegetables following
approved uses.
4. Further information on the level and fate of residues in
food at the point of consumption following the use of
bioresmethrin for the control of various stored-product
pests.
5. Improved procedures for the determination of bioresmethrin
residues in fruit and vegetables as well as stored products.
REFERENCES
Abernathy, C. O. and Casida, J. E. (1973) Pyrethroid insecticides:
esterase cleavage in relation to selective toxicity. Science, 179:
1235-1236.
Abernathy, C. O., Ueda, K., Engel, J. L., Gaughan, L. C. and Casida,
J. E. (1973a) Substrate specificity and toxicological significance of
pyrethroid hydrolyzing esterases of mouse liver microsomes. Pesticide
Biochemistry and Physiology, 3: 300-311.
Andrews, T. L. (1974) Resmethrin Residues in foliage after aerial
application. Pesticide Monitoring Journal, 8(1):50-52.
Ardley, J. H. (1973) Further field evaluation of bioresmethrin as a
potential replacement for malathion in grain protection. Wellcome
Australia Ltd Report TD/5/73/Kl-36.
Ardley, J. H. (1975) Use and application of resmethrin and
bioresmethrin as potential grain protectants. Paper submitted to
Journal of Stored Products Research (May 1975)
Ardley, J. H. and Desmarchelier, J. M. (1974) Investigations into the
use of resmethrin and bioresmethrin as potential grain protectants.
Proceedings of the First International Working Conference on Stored
Product Entomology, Savannah, Georgia, USA.
Ardley, J. H. (1975) Report of milling and baking trials with
bioresmethrin treated wheat. Report of Wellcome Australia Ltd, 27
August 1975.
Bengston, M., Cooper, L. M. and Holton, F. J. (1975b) A comparison of
bioresmethrin, chlorpyrifos-methyl and pirimiphos-methyl as grain
protectants against malathion-resistant insects in wheat. Submitted
for publication, Queensland Journal of Agricultural and Animal
Sciences.
Bengston, M., Connell M., Desmarchelier, J., Snelson, J. and Sticka,
R. (1975a) Report of field trials with grain protectants. To be
published.
Brooks, I. C., Hans, J., Blumenthal, R. R. and Davis, B. S. (1969) SBP
1382, a new synthetic pyrethroid. Soap Chem. Spec., 45:62.
Brown, B. B. (1973) In "Analytical methods for pesticides and growth
regulators". Edited by G. Zweig (Academic Press), p. 441.
Buick, A. R. and Flanagan, P. (1973) The fate of NRDC 107
(bioresmethrin) on tomatoes. Wellcome Foundation Report HEGH 73/1.
Buick, A. R. and Flanagan, P. (1974) The fate of bioresmethrin on
cucumbers. Wellcome Foundation Report HEGH 74/1.
Cantu, E. and Wolfenbarger, D. A. (1970) Toxicity tests of three
pyrethroids to several insect pests of cotton. J. econ. Ent., 63(4):
1373-1374
Carter, S. W., Chadwick, P. R. and Wickham, J. C. (1975) Comparative
observations on the activity of pyrethroids against some susceptible
and resistant stored products insects. Submitted for publication.
Chesher, B. C. and Malone, J. C. (1970) Acute toxicity tests with NRDC
107 in hens. Wellcome Foundation Report B 236-70.
Chesher, B. C. and Malone, B. C. (1970) Sensitisation study with NRDC
107 on guinea pigs. Wellcome Foundation Report B 198-70.
Chesher, B. C. and Malone, J. C. (1970) Ocular irritancy of NRDC 107
in rabbits. Wellcome Foundation Report B 217-70.
Chesher, B. C. and Malone, J. C. (1971) Toxicity to dogs of NRDC 107
(continuation). Wellcome Foundation Report No. B 27-71.
Chesher, B. C. and Malone, J. C. (1971) Acute toxicity of NRDC 107 by
the intravenous route. Wellcome Foundation Report B 329-71.
Colas, R. (1970) Toxicité NRDC 107 et NRDC 104 sur poissons. Report
from Procida, 9 September 1970.
Cooper McDougall and Robertson. (1971) Bioresmethrin Technical Data
BRM 1-8.
Cooper McDougall and Robertson Ltd. (1975) Bioresmethrin for
agricultural use. Technical Bulletin, 41 pages.
Desmarchelier, J. M. (1975a) Analysis of pyrethroids on grains (In
press). CSIRO Division of Entomology, P.O. Box 1700, Canberra City,
Australia.
Desmarchelier, J. M. (1975b) Results of studies to determine fate of
bioresmethrin on stored grain. CSIRO Stored Grain Research Laboratory,
Canberra, Australia (Personal communication).
Elliott, M. (1969) Structural requirements for pyrethrum-like
activity. Chemistry and Industry, 14 June 1969, 776-781.
Elliott, M., Farnham, A. W., Janes, N. F., Needham, P. H. and Pearson,
B. C. (1967) 5-benzyl-3-furfuryl methyl chrysanthemate - a new potent
insecticide. Nature, 213(5075):493.
Elliott, M., Janes, N. F. and Pearson, B. C. (1971) The pyrethrins and
related compounds, Part XIII. Insecticidal methyl -, alkenyl- and
benzyl-substituted furfuryl and furylmethyl chrysanthemates. Pestic.
Sci., 2:243.
Elliott, W., Janes, N. F. and Spanner, J. A. (1973) The pyrethrins and
related compounds. Preparation of bioresmethrin, Pestic. Sci.,
4:677-681.
Fales, J. H., Bodensten, O. F., Waters, R. M., Fields, E. S. and Hall,
R. P. (1971) Insecticidal Evaluation of SBP-1390 (bioresmethrin). Soap
Chem. Spec., September 1971, pp. 54-62, 73.
Farebrother, D. A. NRDC 107. (1973) Whole body radioautographal study
in male albino rats. Wellcome Foundation Report HEFH 73-1.
Flanagan, F. & Poll, G. S. (1970) Synthetic pyrethroids - estimation
of cis and trans isomers. Cooper Technical Bureau Series B Report
93/70, 21 May 1970.
Foote, C. S., Wuesthoff, M. T., Wexler, S., Burnstain, I. G., Denny,
R., Schenck, G. O. and Schulte-Elte, K.-H. (1967) Tetrahedron,
23:2583.
Ford, M. G. and Pert, D. R. (1974) Time/Dose/Response relationships of
pyrethroids insecticides with special reference to knockdown.
Pesticide Science, 5:635-641
Fujimoto, K., Itaya, N., Okuno, Y., Kadota, T. and Yamaguchi, T.
(1973) A new insecticidal pyrethroid ester. Agri. Biol. Chem.,
37(1):2681-2682.
Glomot, R. (Undated) Etude de la toxicité chronique de 3 semaines chez
le rat du RU11484 (NRDC 107) Roussel Uclaf Rapport.
Glomot, R. and Chevalier, B. (1969) Etude de la Toxicité argue du
RU11484 (NRDC 107) Roussel Uclaf Rapport Toxico 11386.
Jao, L. T. and Casida, J. E. (1974) Pestic. Biochem. Physiol., 4:456
L'Hoste, J., Balloy, J. and Rauch, F. (1969) La protection des blés
contre Sitophilus granarius. Congr. Pl. Prot. Org. by CIA and CITA,
Milan 1969, No. 22, 6 pp.
L'Hoste, J., Martel, J. and Rauch, F. (1969) A new insecticide
5-benzyl 3-furfuryl methyl d-trans ethanochrysanthémate. Proc. 5th
Br. Insectic. Fungic. Conf., pp. 554-557.
L'Hoste, J. and Rauch, F. (1969) Remarques sur quelques
chrysanthémates insecticides de synthèse. Revue de zoologie Agricole
et Appliqués, Deuxième Trimestre, Nos. 4-6, pp. 53-66.
L'Hoste, J. and Rauch, F. (1969) Sur les propriétés insecticides du
d-trans ethanochrysanthémate de benzyl-5 furylméthyle-3. Conte
Rendu Acad. Sc., Paris 368, 3218-3220 (30 juin 1969)
Lloyd, C. J. (1973) The toxicity of pyrethrins and five synthetic
pyrethroids to Tribolium castaneum and susceptible and pyrethrins
resistant Sitophilus granarius. J. Stored Prod. Res., 9:77-92
Malone, J. C. and Chesher, B. C. (1970) Toxicity to dogs of NRDC 107.
Wellcome Foundation Ltd - Report No. B 204-70.
Malone, J. C. and Chesher, B. C. (1970) Toxicity of bioallethrin/NRDC
107 crawling insect killer to canaries and budgerigars. Wellcome
Foundation Report No. B 211-70.
Malone, J. C. (1971) NRDC 107, 3 month oral toxicity study in beagle
dogs. Wellcome Foundation Report No. B 109-71.
Miyamoto, J. (1975) Terminal residues of bioresmethrin. Submission to
IUPAC Terminal Residues Commission, Madrid, September 1975.
Miyamoto, J., Nishida, T. and Ueda , K. (1971) Metabolic fate of
resmethrin, 5-benzyl-3-furyl methyl-dl-trans-chrysanthemate in the
rat. Pesticide Biochemistry and Physiology, 1:293, 306.
Miyamoto, J., Suzuki, T. and Nakae, C. (1974) Pestic. Biochem., 4:438
Murano, A. (1972) Agr. Biol. Chem., 36:2203
Murano, J., Fujiwara, S., Horiba, M. and Miyamoto, J. (1971) Agr.
Biol. Chem., 35(8):1200-1207.
Noel, P. R. B., Rivett, K. F., Chesterman, H., Street, A. E. and
Spicer, E. J. F. (1971) Oral toxicity study in Beagle dogs (repeated
dosage for 3 months), Huntington Research Centre Report 3900/71/58, 76
pp.
Okuno, J., Fujimoto, K., Kadota, T., Miyamoto, J. and Hamuro, K.
(1969) Insecticidal activity of a new synthetic pyrethroidal compound,
5-benzyl-3-furyl-methyl dl-cis, trans chrysanthemate (NRDC - 104,
Chrysron), Botyu-Kagaku, 34:157.
Rosen, J. D. (1972) In "Environmental Toxicology of Pesticides",
Matsumura, F., Bosh, G. M. and Misato, T. Academic Press N.Y., p. 438.
Rowlands, D. (1975) Breakdown of bioresmethrin on wheat. Note prepared
by Pest Infestation Control Laboratory - Ministry of Agriculture,
Fisheries and Food, Slough, United Kingdom.
Simonartis, R. A. and Coil, R. S. (1975) Gas-liquid chromatographic
determination of resmethrin in corn, cornmeal, flour and wheat. J.
AOAC, 58(5):1032-1035.
Smith, F. F. and Boswell, A. L. Toxicity of micronised dusts to some
glasshouse insects. J. econ. Ent., 65(5):1466-1468.
Sullivan, J. W. N. et al. (1972) Gas propelled aerosols and micronised
dusts for control of insects in aircraft 7 - Effectiveness against
pests of agriculture. J. econ. Ent., 65(5):1462-1466.
Suzuki, T. and Miyamoto, J. (1974) Pestic. Biochem. Physiol., 4:86.
Tooby, T. E. (1973) Toxicity of the synthetic pyrethroid NRDC 107 to
fish. Ministry of Agriculture, Fisheries and Food, Salmon and
Freshwater Fisheries Laboratory Report FGB 29092.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1974) Photodecomposition
of resmethrin and related pyrethroids. J. Agr. Food Chem.,
22(2):212-220.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975a) Metabolism of
(+)-trans and (+)-cis resmethrin in rats. J. Agr. Food Chem.,
23(1): 106-115.
Ueda, K., Gaughan, L. C. and Casida, J. E. (1975b) Pesticide Biochem.
Physiol., 5:280
Verschoyle R. D. and Barnes, J. M. (1972) Toxicity of natural and
synthetic pyrethrins/rats. Pesticide Biochem. Physiol., 2(3):308-311.
Waldron, M. M. (1969) Foetal toxicity study - 95 H 56 - given orally
in the rabbit. Wellcome Foundation Report, Path 51.
Wallwork, L., Chesher, B. C. and Malone, J. C. (1970) Toxicity of NRDC
107 to laboratory animals. Wellcome Foundation Report B 199-70.
Wallwork, L. M., Clampitt, R. B. and Malone, J. C. (1971) NRDC 107,
rat oral 91 day toxicity study. Wellcome Foundation Report B 119-71.
Wallwork, L. M. and Malone, J. C. (1971) Potentiation toxicity studies
with NRDC 107, bioallethrin and piperonyl butoxide. Wellcome
Foundation Report B 47-71.
Wallwork, L. M. and Malone, J. C. (1972) Bioresmethrin - Preliminary
24 hour rat inhalation study. Wellcome Foundation Report B 290-72.
Weeks, M. H., Leland, T. M., Boldt, R. E. and Mellick, P. W. (1972)
Toxicological evaluation of pyrethroid insecticide SBP 1382. US Army
Environmental Hygienic Agency Special Study No. 51-127-71/72.
Wellcome. 1973(ca.) Summary of trial results. Agricultural uses of
bioresmethrin. Wellcome Research Laboratories, Berkhamsted, 29 pp.
Winney, R. (1973) The biological performance of synthetic pyrethroids.
Pyrethrum Post., 12(1):2-11.
