
AGP:1970/M/12/1
WHO/FOOD ADD/71.42
1970 EVALUATIONS OF SOME PESTICIDE RESIDUES IN FOOD
THE MONOGRAPHS
Issued jointly by FAO and WHO
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Working Party of Experts and the WHO Expert
Group on Pesticide Residues, which met in Rome, 9-16 November, 1970.
FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
WORLD HEALTH ORGANIZATION
Rome, 1971
DICHLORVOS
Explanation
This pesticide was evaluated by the 1965, 1966 and 1967 Joint Meetings
of the FAO Working Party and WHO Expert Committee on Pesticide
Residues (FAO/WHO 1965, 1967, 1968). Since the previous publications,
the results of some additional experimental work have been reported.
This new work in summarized and discussed in the following monograph
addendum.
IDENTITY
Composition of the technical product
Technical dichlorvos contains not loss than 93 percent w/w of
o,o-dimethyl-2, 2-dichlorovinyl phosphate and not more than 7 percent
w/w of related compounds.
EVALUATION FOR ACCEPTABLE DAILY INTAKE
BIOCHEMICAL ASPECTS
The rate of detoxification of dichlorvos appears to vary among
different mammalian species. Based upon studies where dichlorvos was
infused into the jugular vein of animals and the time to mortality
measured, the detoxification rate for dogs, pigs, sheep and monkeys is
3.0, 5.5, 0.9 and 0.4 mg/kg/hr., respectively (Young, 1969).
Studies in vivo and in vitro in the rat, rabbit, goat and cow
using 32P-labelled or unlabelled dichlorvos (Casida et al., 1962;
Hodgson and Casida, 1962) have previously been described, and the
results of these experiments have fairly clearly established the fate
of the phosphorous containing portion of the molecule (FAO/WHO, 1967).
Rats were administered intraperitoneally with 0.5 mg of dichlorvos or
1.7 mg of its metabolite o-demethyldichlorvos. Both compounds were
32P-labelled. Dimethylphosphate was by far the most predominant
urinary metabolite (64.1 percent); methylphosphoric acid and
phosphoric acid accounted for a further 9.2 percent of the
radioactivity and the remaining 1.7 percent was o-demethyldichlorvos.
In the urine from the rats administered o-demethyldichlorvos directly,
21.2 percent was excreted unchanged, but the major metabolites were
methylphosphoric acid and phosphoric acid (43.6 percent) and an
unknown compound (metabolite E), which was chromatographically similar
to an unknown metabolite obtained from rats fed the related
insecticide trichlorphon. This compound was not found after
administration of dichlorvos itself. The authors speculated that the
compound was a conjugate of a non-toxic metabolite with glucuronic
acid, or perhaps sulphuric acid, but this fact was not established.
Based upon the results of this experiment, the cleavage of the vinyl
group appears to greatly exceed o-demethylation (Bull and Ridgeway,
1969).
Three male rats were exposed for one hour to the vapour of dichlorvos
labelled with 14C in the vinyl group. A separate group of three male
rats of the same age were given an oral dose of the same 14C-labelled
dichlorvos. The excretion of the radioactivity of both groups in the
urine, faeces and respired air was measured for four days, and the
identity of the radioactivity in the liver of the inhalation group was
also determined. Metabolism was rapid with both groups, and the rates
and routes of excretion were very similar. The radioactivity in the
livers of the group studied was found to be largely glycine and serine
incorporated into the protein (Blair et al., 1970).
Further information on the metabolism of dichlorvos is given in the
section entitled "Fate of residues - In animals".
The hydrolytic degradation of 32P-labelled dichlorvos was
investigated using postmitochondrial supernatant fluid from rat liver
and reduced glutathione as a methyl group acceptor. It was found that
the rate of hydrolysis of dichlorvos was much greater than was found
in some phosphorothiono insecticides. The monomethyl derivative was
the major hydrolytic product (Palut et al., 1969).
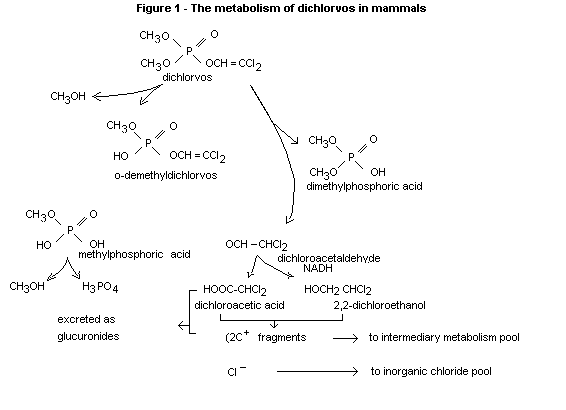
Based upon the experiments described above and those reviewed
previously, the metabolic scheme for dichlorovos in represented is
Fig. 1.
Using purified human serum anticholinesterase, the I50 value for
dichlorvos is 10-6M. An enzyme considered to have similar esteratic
site, namely chymotrypsin, had a value of 10-3M (Arthur and Casida,
1957).
More recently, I50 values for dichlorvos using human plasma and
erythrocyte cholinesterase have been shown to be the same, 4.1 ×
10-6M (Boyer, 1969).
TOXICOLOGICAL STUDIES
Special studies on reproduction
Chicken embryo
When 10 mg of dichlorvos or less were injected into the yolk sac of
fertile eggs prior to incubation, there were no teratogenic effects
(Roger et al., 1964).
When 1 mg dichlorvos was injected in eggs on day four of incubation,
borderline teratogenic signs (shortened body and legs) occurred (Roger
et al., 1969).
 Rat
Reproduction studies in rats fed 0, 0.1, 1,0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965; see FAO/WHO, 1967).
Dichlorvos was administered at unspecified levels intraperitoneally to
female rats on day eleven after insemination. Doses of 20 mg/kg
body-weight killed the animals; 15 mg/kg produced toxic signs and
weight loss. There was no adverse effect on litter size or on the
placenta at 15 mg/kg, but three foetuses from one of four litters
available had omphaloceles, although none were observed in the six
litters from control animals (Kimbrough, 1970).
Rabbit
A total of 168 adult female rabbits were artificially inseminated with
20-30 million viable and mobile rabbit sperm, and on days 6 to 18 of
gestation were administered daily oral doses of 0, 6 or 18 mg/kg
body-weight of dichlorvos by capsule, or 31 mg/kg on days 6 to 11 of
gestation. Foetuses were recovered by Caesarean section. There was
increased incidence of in utero and neonatal toxicity in the top
dose group only, which was of doubtful significance. There were no
teratogenic effects observed, based upon skeletal examination of the
foetuses. Maternal mortality was increased in the 31 mg/kg group
(Carson, 1969).
In a separate experiment, 104 female rabbits were artificially
inseminated with rabbit sperm and administered 0, 3 or 12 mg/kg
body-weight of dichlorvos by capsule on gestation days 6 to 16
inclusive. Other rabbits were given higher doses, but they could not
tolerate levels of 24 mg/kg or higher, and no live litters were
produced from such groups. At 12 mg/kg, there was a significant
reduction in the number of implant sites and foetuses compared to the
control or lower dose group. At 3 mg/kg, the number of implant sites
and foetuses were not lower than the controls. However, one deformed
stillborn foetus was encountered from this group. Liver sections from
parents and foetuses displayed no pathological abnormalities that
could be related to dichlorvos treatment (Vogin, 1969).
Pig
Female pigs were fed dietary levels of 0, 200, 250, 288, 400, 500 or
750 ppm of dichlorvos for up to 37 months. The animals were first
mated after they had received the test diet for six months, and the
study was followed through two generations. Initially, a total of 22
females and one male were used, but after the first generation, four
more males were used in order to prevent in-breeding. The males
received 0, 288 or 400 ppm of dichlorvos in their diets. Dichlorvos
did not affect either the numbers of litters or their size and the
survival in the groups fed dichlorvos. A total of 490 piglets were
examined and none showed anatomical abnormalities (Singh and Rainier,
1966).
Special studies on mutagenicity
Secondary roots of Vicia fabia were treated with varying
concentrations of dichlorvos for one hour. Concentrations down to 1 mM
were mostly lethal, and root tips surviving this and lower
concentrations had a low mitotic index and showed c-mitotic effects,
and chromatid breaks and gaps were observed. In another experiment, a
streptomycin dependent, Sd-4, mutant of E. coli was treated in the
lug phase at cell densities of about 108 cells/ml during one hour.
The LD50 concentration for the bacteria of dichlorvos in the medium
was estimated to be between 10 and 60 mM (2200 ppm and 1.3 percent).
At concentrations of 1-4 mM, which caused no significant killing, an
increased mutation rate was observed when compared with controls and
measured as revertants to streptomycin independency (Löfroth, 1969 a,
1969 b).
Segments of Vapona-strips of various sizes were placed in an inflated
6000 ml plastic bag containing an uncovered petri-dish with
germinating onion seeds. There was no retardation of root growth or
mitotic frequency, but the number of chromosome aberrations, when
scored at anaphase, increased constantly with increasing size of the
Vapona-strip segment. The lowest concentration used was about ten
times as great as one strip in a 1000 ft3 room (Sax and Sax, 1968).
Attention has been drawn to the alkylating property of a number of
alkyl phosphates (Preussman, 1967). For this reason, possible
mutagenic effects have been looked for. Calf thymus deoxyribonucleic
acid (DNA) was incubated with varying concentrations of dichlorvos
solution. In a typical experiment with 2 percent dichlorvos incubated
for 66 hours, there was a yield of 1 percent of N-7-methylguanine from
the available guanine. Similar experiments with deoxyguanosine gave a
3-5 times higher yield of N-7-methylguanine than from DNA (Löfroth,
1970).
Eight mice were injected intraperitoneally with 10-20 mg/kg
body-weight of dichlorvos. After 23 hours, 100 cells of their bone
marrow were examined. The incidences of aberrations were not greater
than observed in four control mice (Hunter, 1970).
A human subject was exposed to 1 mg/m3 of dichlorvos for six hours.
A sample of blood cultured for leucocytes for 72 hours showed no
increase in chromosome aberrations in 100 cells compared to the
pre-exposure values (Hunter, 1970).
No chromosomal aberrations were seen after examination of blood
samples from a family of four female and one male human subjects which
had been exposed to dichlorvos strips. A control group had two male
and three female subjects (Hine, 1970).
Special studies on carcinogenicity
Rat
Rats (24) were injected subcutaneously, once weekly, with trichlorphon
(Dipterex), of which dichlorvos is a known metabolite. Despite a high
(unspecified) dosage and a latent period of almost 800 days, only two
local sarcomas developed at the site of injection. No data on controls
are given. (Preussmann, 1968). In a subsequent publication, Preussmann
et al. (1969) refer to the above experiment as a "negative result,
presumably due to the very rapid metabolism of Dipterex."
Acute toxicity
After direct infusion of dichlorvos into a peripheral vein at various
rates, pigs can survive an LD50 dose each hour for at least nine
hours (page, 1970).
The acute toxicity of dichlorvos has been compared with the
metabolites. The LD50 values in mg/kg body-weight for intraperitoneal
administration to mice are: dichlorvos, 28; o-demethyldichlorvos
sodium salt, 1500; dichloroacetaldehyde, 440; 2,2-dichloroethanol,
890; dichloroacetic acid, 250; sodium dichloroacetate, >3000;
mono- and dimethylphosphoric acid mixture, 1500; sodium
monodimethylphosphoric acid mixture, >3000 (Casida, et al., 1962).
Short-term studies
Comparative studies on inhalation toxicity
Guinea pigs have been exposed for five hours per day for five
consecutive days to a concentration of 130 mg dichlorvos/m3 of air
without visible effects on health. Rats and mice were not visibly
affected by similar exposures to 50 mg/m3. At concentrations above
50 mg/m3, the mice became visibly distressed, and prolonged exposure
to 80 mg/m3 was frequently lethal. Rats were less severely affected
than mice. In all cases, stress reactions were either apparent during
the first hours of exposure or they did not occur at all. No
cholinesterase determinations were made (Stevenson and Blair, 1969).
Three samples of dichlorvos were investigated (82.5 percent pure,
Russian produced, 40% pure, Japanese, and 100% pure from Swiss
origin); the oral LD50 for Russian and Japanese samples was similar
(87 mg/kg body-weight for mice and 65 mg/kg for rats). The Swiss
sample was less toxic: oral LD50, mice: 108 mg/kg. Rabbits were found
to be the most sensitive species (oral LD50, Russian sample: 22.5
mg/kg). Inhalation toxicity was investigated for exposure periods of
four hours, The highest concentration which could be produced (30
mg/m3) was lethal for mice and rats. The LD50 (four hours) for mice
was 13.2 mg/m3 and for rats 14.8 mg/m3. At 5 mg/m3, blood
cholinesterase activity in rat was 69 percent of pre-exposure values.
A level of 0.5 mg/m3 caused no observable effects in blood
cholinesterase. By interpolation, those concentrations of dichlorvos
in air were calculated which would after four hours exposure cause a
50 percent drop in blood cholinesterase activity in rat (I50 = 2.4
mg/m3) or a 25 percent drop (I25 = 1 mg/m3). Rats and rabbits
were exposed for 4 hours/day for a period of four months to an average
dichlorvos vapour concentration of 1 mg/m3 of air. No visible signs
of intoxication occurred. Cholinesterase activity in the serums
erythrocytes, medullary tissue and liver of rats varied throughout the
experiment within the limits of the norm or was slightly depressed.
After four months, the depression of cholinesterase activity in the
medulla averaged 8 percent in the liver, serum and erythrocytes the
depression was 30 percent 22 percent and 24 percent, respectively.
Blood sugar content, blood sugar curves after loading with galactose,
synthesis of hippuric acid after loading with sodium benzoate and
duration of Hexenal induced sleep were not affected. Rabbits exposed
under the same conditions showed 11-30 percent reduction of serum
cholinesterase activity and 28-30 percent reduction of erythrocyte
cholinesterase activity. It was concluded that 1 mg/m3 was the
threshold for rats and rabbits which caused an insignificant and
variable depression of cholinesterase. It was also the threshold for
cats. Rats exposed to an average concentration of 5.2 mg/m3 for 4
hours/day over a period of two months remained visibly healthy. After
15 days, the cholinesterase depression in the medulla was on an
average 22 percent in the serum 58 percent in the erythrocytes 22
percent. The duration of drug induced sleep was increased from 33
minutes to 57 minutes. The blood sugar level was unchanged, but the
rise of the curve after galactose loading was far higher than in the
controls. After 60 days, the blood sugar curve was affected, the
duration of Hexenal induced sleep was the same as in the controls, and
cholinesterase activity in the medulla fell by an average of 31
percent, in the liver by 35.5 percent, in serum by 52 percent and in
erythrocytes by 58 percent. At 8.2 mg/m3 for 45 days, rats lost
appetite and weight, and in some cases trembling of the head and
entire body was noted. Signs of intoxication occurred in some of the
rats and some died (Sasinovich, 1968).
OBSERVATIONS IN MAN
A total of 10 human subjects, in groups of two, ingested pellets of a
slow-release formulation of dichlorvos in daily dosages, doubling
weekly, up to 8 mg/kg body-weight/day for periods up to three weeks;
one group of two received 16 mg/kg/day for 5.5 days. Doses above 1
mg/kg were administered in two equally divided doses per day. The
majority of the clinical side effects occurred after the doses had
reached the 8 mg/kg/day level. Most of the subjective clinical
complaints referred to the gastrointestinal tract, but none of the
studies had to be terminated because of side-effects. The study with
16 mg/kg/day was terminated because of the magnitude of the red blood
cell cholinesterase activity depression. Doses of 1-8 mg/kg/day for up
to 21 days produced an average plasma cholinesterase activity
depression of 70 percent irrespective of dose or duration. Doses of 16
mg/kg/day for 5.5 days produced approximately 90 percent depression of
the plasma cholinesterase activity. Red cell cholinesterase activity
after multiple dosing of 1 mg/kg/day was 11-27 percent depressed.
Doses which reached 8 mg/kg/day caused 45-86 percent depression. At 16
mg/kg/day, depressions of 90 percent were observed. Single doses of
dichlorvos needed to be more than 4 mg/kg in order to cause measurable
red cell cholinesterase depression. At single doses of 8 mg/kg and
higher, maximal plasma cholinesterase inhibition was present and did
not increase at higher dosages. Significant and consistent erythrocyte
cholinesterase inhibition occurred after single doses of 16 mg/kg (36
percent inhibition) and 32 mg/kg (46 percent inhibition). About 50
percent of the dichlorvos was still present in the excreted pellets,
Therefore, the doses absorbed were assumed to be about one half of the
above levels (Hine et al., 1966).
In another study, 12 male subjects ingested 5 mg of dichlorvos daily
in three equal portions with their food until the plasma
cholinesterase fell to 75 percent of the pre-exposure value. The time
required averaged 12 to 20 days. No effect occurred on erythrocyte
cholinesterase. One of the subjects continued the exposure for a total
of 77 days. A questionable erythrocyte cholinesterase depression of
10-15 percent occurred during part of the exposure period, but in the
beginning and at the end of the exposure period, this activity was
normal or slightly above (Hunter, 1970).
Six human subjects were exposed to concentrations of dichlorvos in the
air, the maximum level being 52 mg/m3 for 62 minutes and the maximum
period 240 minutes to 13 mg/m3. No clinical abnormalities were
observed, subjective complaints were only related to the dryness of
the atmosphere, but the plasma cholinesterase activity was decreased;
the extent of the depression was proportional to the product of the
concentration and time of exposure. During some of these exposures, a
questionable depression of erythrocyte cholinesterase occurred, but
the extent of depression was not directly related to the product of
the concentration and time of exposure (Hunter, 1969).
In six homes in Tucson, Arizona, 18 individuals submitted to exposure
to dichlorvos strips and six others served as controls.
In order to maximize exposure, the study was made during the winter
months, and strips were hung in all rooms in the houses at the rate of
1 strip/1000 ft3. Maximum concentrations of 0.1-0.2 mg/m3 occurred
within the first week, levelling off to 0.05-0.10 mg/m3 during the
remainder of the regular exposure period of 28 days. Samples of meals
and beverages were collected at various intervals. During the exposure
period, more than 85 percent of the individual meals contained less
than 0.3 ppm dichlorvos and more than half the meals 0.1 ppm or less.
Physical examination, complete blood counts, blood chemical profiles
and plasma and erythrocyte cholinesterase measurements were performed
at various intervals. Levels of dichlorvos in the food eaten ranged
from 0.1 to 0.3 ppm. There were no clinical abnormalities evident, nor
were there any significant differences in plasma and red-blood cell
cholinesterase, blood count and blood chemical profiles between the
exposure and pre-exposure periods (Shell, 1970a).
A study was made in Italy of the effect of continuous exposure to
insecticide levels of dichlorvos by patients in hospital wards.
Control values for plasma and erythrocyte cholinesterase were
established for 250 healthy male and 100 healthy female adults not
exposed to dichlorvos. The variation in the activity of the
cholinesterases of the individuals when measured at different times,
depending on the time-interval, varied on average between 1.4 and 4.4
percent for the erythrocyte cholinesterase and on average between 5.0
and 14.2 percent for plasma cholinesterase. These were average
variations measured in ten to 20 individuals. In single individuals,
the maximum variation was 7.7 percent for erythrocyte cholinesterase
and 23.2 percent for plasma cholinesterase.
A total of 44 adult male patients with diseases other than liver
diseases were exposed to atmospheric concentrations of dichlorvos
ranging from 0.02 to 0.1 mg/m3 for periods of 3 to 29 days. Another
similar group of 22 male patients was exposed to dichlorvos
concentrations of 0.1 to 0.28 mg/m3 for periods from 3 to 16 days.
Only in the case of five patients exposed 24 hours per day at levels
above 0.1 mg/m3 was there a depression of plasma cholinesterase (35
to 72 percent) below the pre-exposure level. There was no depression
of erythrocyte cholinesterase nor were any symptoms typical of
cholinergic stimulation observed, in any patients. With six other
patients suffering from liver disease exposed in the same room, all
showed a reduction in plasma cholinesterase (25 to 66 percent of
pre-exposure values). Reduction was evident even in patients exposed
at levels below 0.1 mg/m3. These patients, because of their liver
disease, had already before exposure a considerably reduced plasma
cholinesterase activity. However, there was no depression of
erythrocyte cholinesterase nor any symptoms of poisoning, in spite of
the fact that in these patients with liver insufficiency had a 40
percent depression in plasma cholinesterase lasting for one to three
weeks after the removal from exposure. In babies, sick children and
women in labour or postpartum exposed to dichlorvos, a similar
situation to that of sick adults was seen; depression of plasma (but
not erythrocyte) cholinesterase was observed only in some cases when
the dichlorvos level was above 0.1 mg/m3. Again, no symptoms were
seen. Assuming an inhaled volume of 10 m3/24 hours in adults and 1.4
m3/24 hours in children, the authors calculated that a reduction in
plasma cholinesterase for a calculated daily inhalation of 1.7 mg of
dichlorvos could occur in adults with normal liver function and 0.2 mg
for children. They further calculated that the amount of dichlorvos
inhaled which might produce a plasma cholinesterase depression is
about the same for adults and children, viz. 0.028 to 0.030 mg/kg
body-weight/day. In patients with liver malfunction, daily inhalation
intake of 0.34 mg (equivalent to 0.005 to 0.006 mg/kg body-weight/day
was sufficient to produce plasma cholinesterase depression (Cavagna et
al., 1969; Cavagna and Vigliani, 1970).
In two nurseries 22 healthy newborn babies were kept from birth for a
period of five days for 18 hours/day in an atmosphere in which average
dichlorvos concentrations of 0.15 - 0.16 mg/m3 were maintained. Blood
cholinesterase levels were determined at birth and after five days.
There were no effects on the health of the babies nor on the activity
of their plasma or erythrocyte cholinesterase (Cavagna et al., 1970).
In a preliminary study with asthmatic patients, there appeared to be
no subjective worsening of asthma during exposure to dichlorvos at
levels of 0.1 to 0.2 mg/m3 for two consecutive days. In some cases,
an increase in airways resistance and sensitivity to acetylcholine
occurred during and after exposure, but these manifestations may be
related to other factors (e.g. poor ventilation) than to dichlorvos.
Further work in this field is reported to be in progress (Vigliani,
1970).
COMMENT
On the basis of recent and earlier studies the metabolic pattern of
dichlorvos when fed to mammals is now fairly clearly elucidated.
Evidence is presented that cleavage of the dichlorovinyl group
proceeds much more rapidly than o-demethylation. Information on the
exposure of man to varying levels of dichlorvos vapour is available,
but such studies do not include assessment as to whether the
metabolism is the same after inhalation as after oral intake. Data on
the toxicology of the metabolites dichloracetaldehyde and
dichloroethanol are considered pertinent in evaluating the toxicity of
dichlorvos. A long-term study in rats reported in the monograph from
the 1967 Joint Meeting is considered adequate. Feeding studies in rats
and rabbits did not show any indication of a teratogenic effect.
Additional information on feeding dichlorvos to man has become
available, and the no-effect level previously determined in man is
used as a basis for establishing an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In addition to its use on beef and dairy cattle and goats, sheep and
pigs, and in and around buildings which house these animals,
dichlorvos is finding increasing use on fruit and vegetable crops.
Fruit and citrus crops account for more than one third of the
agricultural use. Rice accounts for just over one quarter and field
crops, including vegetables, approximately one quarter of the
agricultural uses. There are minor uses on grapes, glasshouse crops,
mushrooms, tobacco, tea, coffee, cocoa and miscellaneous crops.
Dichlorvos is known to be registered in at least forty eight
countries.
Recently it has been introduced as an anthelmintic for pigs, poultry
and horses, being administered in the form of plastic granules for
this purpose.
Post-harvest treatments
Dichlorvos is being increasingly used for the control of insect
infestation in stored grain. For this purpose it is used as an aerosol
or as impregnated resin strips, which are applied in the overhead
spaces of storage bins. In some countries, dichlorvos preparations
have also been registered for incorporation into stored grain either
as a dust, as a direct spray or as an aqueous emulsion.
Dichlorvos aerosols, automatic dispensers, sprays and resin
impregnated strips are used in many countries for the control of
insect infestations in stores, in transport and in warehouses holding
processed food. Food processing establishments are also treated with
dichlorvos in one of the above forms.
Other uses
As defined in the monograph of the 1967 Joint Meeting (FAO/WHO 1968),
dichlorvos is used extensively in the public health field (homes,
hospitals, etc.). It is also used in aircraft during flight. Trials
are being carried out on its use by aerial application against insect
pests of forests, pastures and field crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown in the open
Table I gives typical results of the analysis for dichlorvos residues
following controlled application to various fruit and vegetable crops
(Ciba 1970).
Crops grown under cover
In glasshouses, the rate of decay of dichlorvos residues is fast, with
a half-life of approximately one day. Table II gives results of
residue trials carried out on crops growing under cover (Ciba 1970).
Livestock and poultry
Trials in the United States demonstrate that the spraying of
dichlorvos on livestock (including cattle, sheep, goats and pigs) and
poultry is unlikely to produce significant residues in meat, milk or
eggs. When dichlorvos was applied as a single pour-on application of 1
percent at the rate of 16.5 ounces per cow, no residues were
TABLE I
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Apples 0.05% ai 1 day 0.05 ChE1 0.05
2 days <0.05
Peaches 0.05% ai 0 day 0.8 ChE 0.02
1 day 0.55 " 0.02
2 days 0.35 " 0.02
Strawberries 0.05% ai 2 days 0.35 ChE 0.02
3 days 0.06 " 0.02
8 days <0.02 " 0.02
Carrots 0.025% al. 28 days <0.05 ChE 0.05
31 days <0.05 " 0.05
Cauliflower 0.05% ai 0 days 0.37 ChE 0.04
5 days <0.04 "
Brussels 0.05% ai 0 days 1.35 ChE 0.04
sprouts 5 days <0.04
Cabbage 0.05% ai 0 days 1.5 ChE 0.05
1 day 0.14
2 days 0.05
3 days <0.05
Spinach 0.05% 0 days ChE 0.05
1 day 1.6
2 days 0.06
3 days <0.05
TABLE I (Cont'd.)
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Lettuce 0.05 0 days ChE 0.05
3 days <0.05 " 0.05
French beans 0.05 0 days 0.3 ChE 0.05
<0.05
Cucumber 0.05 9 days <0.05 ChE 0.05
1 ChE = cholinesterase inhibition method
TABLE II
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Tomato 20 mg/m3 0 days 0.2 ChE1 0.04
1 day 0.06 " 0.04
2 days 0.04 " 0.04
England Lettuce 130 mg/m3 0 days 78 ChE 0.04
1 day 4.9
70 mg/m3 1 day 2.1
2 days 0.4
3 days 0.2
Germany Lettuce 30 mg/m3 0 days 3.4 B.T.2 0.04
1 day 1.8
3 days 0.3
20 mg/m3 0 days 2.4 B.T. 0.04
1 day 0.9
2 days 0.4
3 days 0.16
Switzerland Cucumber 20 mg/m3 0 days 7.3 ChE 0.04
2 days 0.15
U.S.A. Mushroom 130 mg/m3 1 day 0.1 ChE 0.04
TABLE II (Cont'd.)
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
England Mushroom 160 mg/m3 0 days 25 D.N.P.3 0.04
1 day 0.9
3 days 0.27
160 mg/m3 1 day 0.9 D.N.P. 0.04
2 days 0.05
100 mg/m3 0 days 0.3 D.N.P. 0.04
1 day 0.1
1 ChE - cholinesterase inhibition method
2 B.T. - bioassay
3 D.N.P. - 2,4-dinitrophonylhydrazine colorimetric method
detectable at the level of sensitivity of the method (0.1 ppm) in any
of the milk samples collected from 4-5 hours to 5 days, after
treatment (Singh, 1965). In another study (Noetzel, 1964), groups of
Holstein and Guernsey cattle received dermal applications, twice daily
for 28 consecutive days, of 2 ounces of 0.5 percent dichlorvos and 1
percent dichlorvos, respectively. No residues were detected in the
milk (<0.02 ppm). In a similar study (Wisconsin, 1968) in which
dichlorvos was dermally applied to lactating dairy cows at the rate of
1 ounce/cow or 3 ounces/cow of a 1 percent dichlorvos for thirty days,
milk samples from 1 through 7 days and thereafter at 3 day intervals
were analysed for residues of dichlorvos and dichloroacetaldehyde
(DCA). No residues were detectable in any of the samples (<0.05 ppm
dichlorvos, <0.01 ppm DCA).
In other extensive trials (Wisconsin, 1968/1969) Brahma cattle, goats
and sheep were sprayed 3 times to saturation (1 gallon) at 14 day
intervals with 0.025 percent dichlorvos emulsion. No residues of
dichlorvos were detectable at the limits of sensitivity in tissues or
organs (including renal, omental and subcutaneous fat, muscle, liver
and kidney) of cattle (<0.25 ppm), goats (<0.25 ppm) or sheep
(<0.05 ppm).
Ivey and Claborn (1969a) sprayed lactating dairy cows for 31
consecutive days with 2 ounces of a 1 percent dichlorvos insecticide
spray solution. Milk samples were taken at 2 hours, 1, 2, 4, 8, 16, 24
and 31 days. One day after final treatment, the cows were slaughtered
and samples of renal, omental and subcutaneous fat, muscle liver,
kidney and blood were taken. Using a highly sensitive flame
photometric method (Ivey and Claborn, 1969b) with improved clean-up
technique, no residues of dichlorvos were detected in milk (<0.003
ppm) or body tissue (<0.002 ppm).
Pigs were fed daily at the high dosage of 9 450 ppm dichlorvos for 90
days (Singh, 1964). Dichlorvos residues were not detectable (<0.05
ppm) in tissues and organs including spleen, kidney, small intestine,
lung, heart, muscle and liver 0 and 1 day after last treatment.
Analysis of meat, fat, liver and eggs derived from poultry sprayed
with 2 ounces of diluted 2 lb/gal dichlorvos insecticide E.C. (0.295
ppm grams DDVP/bird) showed residues of equal to or less than 0.03 ppm
when samples of tissue were taken 6 hours, 3, 5 and 7 days after
application. Eggs were collected 1, 2, 3, 4, 5, 6 and 7 days after
treatment (Shell, 1970b). Poultry exposed to dichlorvon in the form of
2 percent granules (2 lb/100 square feet or 4 lb/100 square feet), or
to resin strands containing 20 percent dichlorvos (1 foot of strand/1
foot of cage) caused no residues in either tissue or eggs (<0.02 ppm)
(Lancaster, 1962; Loomis and Hodel, 1965; Lancaster, 1963; Shaw, 1964;
Nelson, 1968).
TABLE III
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Singh, Cows, pour on 480 1 Milk2 <0.1
1965
Noetzel, Cows, dermal 60 28×1 Milk <0.02
1964
Wisconsin, Cows, dermal 90 30×1 Milk <0.05
1968
Wisconsin, Cows, dermal 4540 3×14 Fat, muscle <0.25
1968, 1969 Goats, dermal 4540 3×14 Fat, muscle <0.25
Sheep, dermal 4540 3×14 Fat, muscle <0.05
Ivey & Claborn, Cows, dermal 60 31×1 Milk <0.003
1969a Cows, dermal 60 31×1 Fat, muscle, <0.002
etc.
ppm
Singh, Pigs, oral 9450 90×1 All tissues <0.05
1964
g
Shell, Chickens, dermal 0.3 1 All tissues3 <0.03
1970b Chickens, dermal 0.3 1 Eggs4 <0.03
Lancaster, Chickens, vapour - 30×1 All tissues <0.2
1962
TABLE III (Cont'd.)
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Loomis, Chickens, vapour - 30×1 Eggs <0.02
1965
Nelson, Chickens, contact - 28×1 All tissues <0.03
1968 Chickens, contact - 28×1 Eggs <0.05
mg
Pitts, Chickens, oral 8.5 over 10 Meat <0.01
1961 Chickens, oral 8.5 over 10 Eggs <0.04
1 Waiting period Nil unless otherwise indicated
2 Waiting period 4 hours and 5 days
3 Waiting period 6 hours
4 Waiting period 1 day and 7 days
Uses in the protection of stored products
Grain, meals and flour
Many of the published and unpublished reports available on the rates
of loss of dichlorvos from grain, meals and flour are not reliable,
because the investigators have used unsatisfactory methods to recover
any residues of dichlorvos from the treated grain and cereal products
(see comments under Methods of Residue Analysis).
Residues of dichlorvos in flour resulting from the use of resin strips
in a flour mill have been investigated (Somme, 1967). Strips were hung
at the rate of 1/1000 feet3, and samples of flour made from grain
stored in the mill were taken 4 days and 2´ weeks after production had
rebegun. Analysis showed that residues of 0.04 ppm and 0.06 ppm
dichlorvos were present in the flour and that bran samples averaged
0.09 ppm dichlorvos. In another trial (Shell, 1966a) a "Vapona" strip
was hung inside a covered flour bin of 600 ft3 total capacity. A
sample of flour drawn from a conveyer belt taking flour from the
bottom of the bin one week and two weeks after fitting the strip in a
nearly full bin was found to have a dichlorvos residue of 0.03 ppm.
Samples taken after the third week and after the bin had been refilled
with flour, at the end of the fifth week, contained residues no
greater than the limits of detection, Wheatings exposed at a rate of
one "Vapona" strip per bin of 1150 feet3 for ten hours to six weeks
after the strips had been hung also did not contain residues in the
surface layer above the limit of detection (ca. 0.02 ppm), (Shell,
1965a).
Dichlorvos has been successfully used to control Oryzaephilus
surinamensis and Sitophilus granarius (Green and Wilkin, 1968).
The insecticide was sprayed into the air stream from a motorized
knapsack sprayer through to a perforated lance which was inserted into
the grain. Dichlorvos residues were higher on wheat than on barley and
greatest in places where dust and frass had accumulated. When
dichlorvos was injected at 20 ppm, the highest residue found after one
day was 30 ppm in a dusty pocket of wheat, and this fell to 5.3 ppm
after six days.
The range of residues reported for barley were 5.8-24.0 ppm after one
day, falling to 0.9-1.4 ppm after six days. However, the method of
analysis may not have extracted all the dichlorvos from the grain (See
Methods of Residue Analysis).
In a laboratory study Kirkpatrick et al., (1968) demonstrated that
dichlorvos residues of 3.8-8.0 ppm decreased 89 percent and 76
percent, respectively, in 28 days.
An unpublished study was conducted by USDA Plant Pest Control (Padget,
1968). Wheat grain was treated with 4, 6 and 8 ppm of dichlorvos
formulated from a 25% dichlorvos emulsifiable concentrate. Residues on
the wheat were reported to be less than 2 ppm by the third day after
application, and by 14 days all dichlorvos residues were <0.5 ppm.
Details of the analytical method used were not given.
Rice
Shell Chemical Company (1969) conducted an extensive study on rough
rice and rice products treated for rice weevil control. Rough rice,
stored as 45 lb lots were each sprayed with 20 ml of a solution
containing 1.5 percent, 1 percent and 0.5 percent dichlorvos prepared
from a 23.5% E.C. After treatment, the rough rice was mill processed
and the products (brown rice, rice bran, milled rice and rice hulls)
were collected. Residue samples were taken 6 hours, 1, 5, 10, 20 and
30 days after application. Dichlorvos residues in the rough rice one
day after treatment were 7.3, 4.6 and 1.6 ppm for the 1.5 percent, 1
percent and 0.5 percent applications, respectively. After 30 days,
residues for all three treatment rates had dissipated to less than 1.0
ppm. Residues in brown rice and milled rice, after 30 days, were
<0.05 ppm for all treatment rates. Rice bran treated at the highest
rate contained 0.4 ppm and rice hulls 3 ppm dichlorvos after 30 days.
Soybeans
Soybeans stored in open bins were exposed to resin strips for
intervals ranging from 13 to 126 days (Shell, 1970c). Samples were
taken through depths of 0-2" and 2-4" from the surface and the
residues observed are summarized in Table IV.
TABLE IV
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
13 .20 - .06 -
28 .06 - .03 -
42 .11 .07 .08 .03
57 - .08 - .03
70 .07 .08 .04 .03
84 .04 .04 .04 .03
TABLE IV (cont'd)
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
97 .04 .075 .04 .06
111 .05 .04 .04 .04
125 .04 .04 .04 .04
Temperature - 65-96°F
Rel. Humidity - 48-76 percent
A test conducted by USDA, Stored Products Insect Research and
Development Laboratory (Padget, 1969), demonstrated that dichlorvos
residues on soybeans decrease rapidly. Initial residues of 5 ppm
decreased rapidly to 0.5 ppm or less after one week of storage at
80°F. Only trace amounts (0.1 ppm or less) of dichlorvos were found on
the soybeans after the second week of storage.
Coffee beans
Residues in bagged coffee beans were examined (Shell, 1965a) following
storage in a warehouse using resin strips at either one per 1000 cubic
feet or 1 per 2000 cubic feet. Some natural ventilation was allowed
for 9 hours daily.
Samples taken after 15 weeks storage from the surface and interior of
the bags did not contain detectable residues (limit 0.01 ppm), but one
sample exposed at the rate of one strip per 2000 cubic feet did
contain residues of 0.02 ppm, just above the detection limit.
Cocoa beans
In a trial with stacks of bagged cocoa beans carried out in the same
warehouse and under similar conditions (Shell, 1965a) as the above
coffee, residues in a bulked sample of cocoa from the top of the stack
reached 0.04 ppm, although none were detected in comparable samples
from the side of the stack. In a further trial (Shell, 1965a) with
another stored supply of cocoa beans, detectable residues (0.01 ppm)
were not found after 14 weeks exposure. Another trial (Shell, 1965b)
was undertaken in which sacks of cocoa beans were exposed for three
months. Surface samples taken from the top sacks were found to have a
maximum content of 0.04 ppm, whilst sub-surface samples and samples
from the middle of the sack did not contain detectable residues. In a
further trial in which exposure was 8 weeks, the maximum residue found
in surface samples from the top sacks was only 0.02 ppm. This was
mainly in the husks (0.03 ppm); the kernels contained only 0.01 ppm.
In a series of trials in Switzerland (Ciba), dichlorvos was applied to
cocoa beans in silos at rates ranging from 10 ppm through 100 ppm to
500 ppm. After three weeks storage at 15°C, the residues had fallen to
<0.1, 13 and 50 ppm, respectively, on the raw beans. When beans were
then roasted, the residues fell to <0.1, 0.2 and 0.5 ppm,
respectively. In the samples treated at 500 ppm, most of the residue
was in the mash cake with a minimum in the cocoa butter. Further
trials (Ciba) showed that the residues in cocoa beans treated at the
rate of 12 ppm with dichlorvos emulsion fell from 0.8 to 0.09 ppm
during 28 days in storage at 20°C.
Groundnuts
Sacks of bagged groundnuts (Shell, 1966b) were exposed for 82 days in
a warehouse at the recommended rate of one strip/1000 cubic feet. One
residue sample taken from the surface (2 cm) layer of the top sack was
found to contain 0.10 ppm dichlorvos, but two other similar samples
were found to contain only 0.01 ppm dichlorvos. One sample from the
middle of the top sacks contained 0.03 ppm, but in two further samples
no residues were found.
Residues arising from the exposure of foods in processing plants
Plants producing foods not processed further after exposure
In a cheese factory, in Minnesota, strips were placed throughout the
milk receiving and processing areas (Shell, 1964). A monitoring
experiment showed that the finished Cheddar cheese did not contain
detectable dichlorvos and DCA residues (i.e. below 0.03 ppm and 0.01
ppm, respectively). Similar trials in butter and ice cream factories
(Shell, 1965c) showed negligible residues of dichlorvos and DCA in
both products.
In tests carried out in cooperation with the German Public Health
Service in a cheese factory, Lindenberg, Edam and Danish Tilsit cheese
(each containing 45% fat) were exposed for four hours. Cheese samples
were cut into 20 mm thick slices following normal practice, and
exposed for a period of 30 to 60 minutes. Residues, which were
determined in the outermost 1 mm layers, were not detectable (Anon.,
1966).
Strips were hung in a cheese aging room at the rate of one strip/1000
cubic feet. Samples of cheese taken after six and ten weeks of
exposure showed residues of <0.04 to 0.10 ppm (Shell, 1965a). In the
case of milk (Shell, 1965d), whole milk was exposed in open milk
churns for periods of 24 and 48 hours in rooms where fresh strips were
installed at the rate of one strip/1000 cubic feet. None of the
representative samples taken contained measurable amounts of
dichlorvos (detection limit 0.02 ppm).
In a study in Kentucky (Shell, 1965d), strips were installed in a farm
dairy at the rate of one strip/1000 cubic feet. The air was humid and
the temperature about 75°F. The milk samples were taken after being
passed through an open coil cooler during the period 0-14 days after
the strips were hung. Residues of dichlorvos ranged from the limits of
detection to 0.04 ppm.
Plants handling foods which are processed after exposure
Fresh strips were suspended in areas of a plant where grading and
filleting of fish was carried out, and after two days exposure,
residues in the prepared product were not detected (detection limit
0.05 ppm) (Shell, 1965e).
Exposure tests were also carried out in a slaughterhouse (Shell,
1965c). The various products, beef, liver, T-bone steak, hamburger,
beef tongue and heart, pork liver, pork sausage, pork chops, pork
tongue and heart were exposed to dichlorvos resin strips during the
time normally required for processing. None of them showed any
dichlorvos or DCA residues; detection limit was 0.01 ppm. In this
test, a sample of bacon showed a residue of 1.4 ppm dichlorvos; DCA
was not found (detection limit 0.01 ppm). It was subsequently found,
however, that this particular sample had been returned to the cooling
room and was exposed for several days longer than normal. Further,
data cited later (Shell, 1966c) show that residues of dichlorvos are
removed upon cooking; specifically, bacon is cited as an example.
An experiment on similar lines was also conducted in a sausage factory
where strips were installed throughout the processing areas (Anon,
1966). No residues were detected in either meat or finished sausages.
FATE OF RESIDUES
In animals
Page (1970) has reviewed the extensive literature on the metabolism of
dichlorvos and in the same paper has reported on extensive trials
designed to show the metabolic fate and tissue residues of dichlorvos
following oral administration. The results of this work and earlier
studies are summarized in the metabolic chart given under "Biochemical
aspects" (Fig. 1).
A series of experiments have been conducted recently in pigs to
provide further information on the metabolic fate of the dichlorvos
portion of the dichlorvos molecule. In a preliminary study, 1-14C
labelled dichlorvos was infused into the isolated duodenal loop of
anaesthetized male pigs for four hours at the rate of 1 mg/kg
body-weight/hour. Urine, bile and peripheral and portal blood were
collected hourly and analysed for dichlorvos and its previously
reported mammalian metabolites. Dichlorvos, dichloroacetaldehyde and
dichloroacetic acid could not be detected in the blood. "The
concentration of 30 ppm dichlorvos in the intestinal lumen resulted in
less than 0.05 ppm dichlorvos in the blood. Neither could
dichloroacetaldehyde or dichloroacetic acid be detected in the blood.
The level of 2,2 dichloroethanol in the blood rose from 0.2 ppm after
one hour to 0.9 ppm after four hours" whereas o-demethyldichlorvos
occurred only near the limit of detection (0.03 ppm or less). In all
cases, levels of 14-carbon radioactivity in urine, bile and tissue
were higher than the level of 2,2-dichloroethanol, thus in urine the
respective figures were 17 ppm compared to 3 ppm; in bile 12 and 0.5;
in kidney 21 and 1.2; in liver 17 and 0.9 and in muscle 2.2 and 0.6
ppm. The figures indicated that 2,2-dichloroethanol or its conjugates
are not the end products of the metabolism. In other experiments, pigs
were given sustained release pellets of 14C- or 36Cl-labelled
dichlorvos at an oral dose of 40 mg/kg body-weight, and excreta and
tissue were analysed after various intervals. A similar situation to
that found in the infusion experiment occurred. It was concluded that
there was a rapid dechlorination of dichloroacetaldehyde and that the
radioactive carbon enters the metabolic pool possibly via glyoxal
(which compound has been found in in vitro but not in in vivo
studies). No retention in tissue of any chlorine containing organic
compound has been found, and the end product of the chlorine fragment
appears to be chloride ion (Page, 1970).
All the data support the conclusion that when administered orally over
a period of weeks, dichlorvos does not result in detectable toxic
residues in tissues of the parent compound or product-related
metabolites. The results of some of the published trials are
summarized in Table III.
In plants
New data have become available on the chemical degradation of
dichlorvos residues on plants. Careful experiments with various plant
species (cotton, maize, peas, beans) have demonstrated that dichlorvos
is rapidly lost from leaf surfaces by volatilization and by hydrolysis
and, disregarding the fraction of insecticide lost by volatilization
during the first few minutes after application, the half-life of the
compound under laboratory conditions is of the order of a few hours. A
small percentage (0.5-5 percent) of the dichlorvos deposited, appears
to penetrate into the waxy layers of leaf and fruit cuticle where it
persists longer. Dichlorvos taken up by plants from aqueous solutions
by the root system and translocated into aerial parts is less rapidly
lost than insecticide applied to leaf and fruit surfaces, but even
under these conditions the half-life of the compound is no more than
12 hours.
Various measurements have confirmed the rapid disappearance of
dichlorvos from vegetable and fruit crops under practical conditions
(see Table 1). Residues in vegetables were below 0.1 ppm two days
after application and were no longer detectable on the third day.
Dichlorvos was somewhat more persistent in fruits, but the residues
are below 0.1 ppm three days after application.
In vegetable crops or mushrooms grown under cover or in greenhouses,
losses of dichlorvos from leaf and fruit surfaces by volatilization
are less rapid than outdoors. However, on all crops analysed so far,
including such broad leaf vegetables as lettuce, dichlorvos residues
have been below 0.5 ppm two days after treatment and below 0.3 ppm
after three days.
Casida et al. (1962) used 32P-labelling to study the fate of
dichlorvos residues on maize, cotton and peas; a 0.1 percent aqueous
solution of dichlorvos was applied uniformly to the upper leaf
surfaces of each plant. It was found that about half of the dose
volatilized within five minutes. An additional 45 percent was absorbed
within 20 minutes, so that only 5 percent remained on the surface 20
minutes after application.
The surface residue was nonhydrolysed dichlorvos while 70-80 percent
of the absorbed material was organosoluble and was presumably
nonhydrolysed dichlorvos. However, hydrolysis proceeded rapidly.
Ignoring the dichlorvos lost by volatilization during the first five
minutes after treatment, the half-life of the dichlorvos was 1.2 hours
with loss primarily occurring by hydrolysis. In addition to the
volatilization of the dichlorvos, which resulted in the total
dichlorvos equivalent to dropping from 100 per leaf at zero time to 35
per leaf at 30 minutes, the hydrolysis products were also lost from
the treated leaves. Only 14 units remained after 24 hours and 7 units
after 72 hours, despite the fact that no dichlorvos or other
organosoluble derivatives were detected after eight hours.
The same authors carried out experiments with radio-labelled
dichlorvos and showed that, when solutions of the insecticide were
absorbed by the roots of plants, 77 percent of the absorbed dichlorvos
appeared as hydrolysis products in peas and 33 percent in maize and
cotton. Once the plants were removed from the insecticide source, the
hydrolysis of the absorbed dichlorvos followed first order reaction
kinetics, with half-life values for both maize and cotton of 9 hours
and for peas for three hours. Following systemic uptake through the
roots of plants, dichlorvos is hydrolysed rapidly, but the products of
hydrolysis are not lost from the plant during a 40 hour
post-absorption period. Bull and Ridgeway (1969) using 32P labelled
dichlorvos showed that 54 percent of the amount applied was lost
within one hour by volatilization from the treated leaves. The major
metabolite of dichlorvos was dimethyl phosphate; only minor amounts of
o-desmethyldichlorvos were detected.
Casida et al. (1962) carried out studies with mice on the acute
toxicity of dichlorvos and potential hydrolysis products and showed
relatively high toxicity associated only with the parent compound.
In storage and processing
Dichlorvos is readily lost by evaporation from stored products,
including raw grain and cereal products. Except at very low
temperatures, it appears likely that dichlorvos is also hydrolysed to
biologically inactive metabolites.
A study (Shell, 1970d) using a number of materials with abnormally
high dichlorvos residues showed that residues can be almost completely
removed by cooking. Rice containing 4.5 ppm dichlorvos has been shown
to lose 90% of the residues on boiling from 20 to 30 minutes. When
rice containing 19 ppm was similarly treated, 98% of the residue was
destroyed. Samples of flour containing dichlorvos residues in the
range 4.5 to 14 ppm have been found to lose about 90% of the residue
on baking for 10 to 12 minutes at 230°C in the preparation of
biscuits. Boiling the flour with water for 2 minutes, as in the
preparation of gravy, has been shown to decrease residue levels by
97%. Residues in cocoa have been shown to be lost during the
manufacture of cocoa butter. No dichlorvos was found in the fat, the
nibs, liquor or mash cake prepared from beans containing 2.9 ppm
dichlorvos.
Residues in food moving in commerce
Two shiploads of wheat were treated with dichlorvos emulsion
immediately prior to shipment from Australia. The dichlorvos was
applied at a nominal concentration of 6 ppm (found to be 4.6 -5.5
ppm). Upon arrival in the Netherlands, the grain was thoroughly
sampled and all samples were found to contain residues below 2 ppm
(range 0.68-1.59 ppm - mean 0.96 ppm). A major portion of each
consignment was trans-shipped to the United Kingdom,where it was
sampled for analysis on arrival. All samples revealed dichlorvos
residues, but at levels lower than those detected in the Netherlands,
with a range of 0.4-1.3 ppm and a mean of 0.68 ppm. From the results
of analysis prior to loading and at the end of a 7-week voyage, it was
calculated that the half-life of the dichlorvos residues was between
20 and 23 days.
Before the general release of dichlorvos resin strips, many
experiments were carried out to simulate the conditions that would be
encountered in practical usage. Subsequent data which related to food
exposed in shops, restaurants and homes under various practical
conditions where the dichlorvos fumigant strips were used according to
directions, broadly show that results from the simulated experiments
overestimated the residue levels.
Surveys have been carried out in the United Kingdom (17 shops) and in
France (20 shops) to establish the residue level of dichlorvos in
unwrapped foods bought by the public from shops using the strips at
the recommended rate under normal practical conditions. The residues
found are shown in Tables V and VI.
The somewhat higher results obtained in some of the commodities in the
French survey may have been due to the common practice there of
closing the shops completely during the heat of the afternoon.
TABLE V
Residues of dichlorvos (ppm) in food purchased from shops in France
Sampling time : days after hanging strips
7 42 70
Commodity Range Mean Range Mean Range Mean
Bread <0.05-0.10 <0.05 <0.05 <0.05 <0.05-0.06 <0.05
Cakes <0.05-0.24 0.08 <0.05-0.30 0.10 <0.05-0.17 0.07
Apples <0.05-0.14 0.06 <0.05-0.32 0.07 <0.05-0.30 0.06
Lettuce <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Tomatoes <0.05-0.08 <0.05 <0.05 <0.05 <0.05 0.05
Ham <0.05-0.08 0.06 <0.05-0.28 0.06 <0.05-0.09 0.05
Cheese
(cow) <0.05-0.08 0.06 <0.05-0.16 0.06 <0.05-0.15 0.06
Cheese
(goat) <0.05-0.35 0.13 <0.05-0.25 0.08 <0.05-0.30 0.08
A number of surveys have been conducted in the U.S.A. on the residue
levels in foods which had been exposed to resin strips in restaurants.
In the first of two studies conducted in restaurants near Cincinnati,
Ohio, typical restaurant meals were subjected to an exaggerated
exposure of 24 hours to 5-10 day old strips. In a second study, meals
were exposed for 22 hours to strips which were initially 51 days old.
Under the conditions of the first survey, residues were only 0.05-0.15
ppm of apparent dichlorvos: under the second survey, no residues (less
than 0.04) were found. In another investigation carried out in four
different restaurants in New York City under normal operating
conditions, ready to eat meals were sampled as composite samples.
Whole meals were sampled at various stages in the life of the strips,
ranging from 24 hours to 4 weeks. The meals consisted of various
mixtures of meat or fish and vegetables and varied widely, in
composition. The following residues were found:
Restaurant No. of meals sampled Residue range,ppm
A 5 0.04 -0.10
B 5 0.02 -0.11
C 9 0.03 -0.16
D 5 0.03 -0.08
To obtain information on food residues arising from the typical
practice of hanging strips in kitchens, surveys were carried out in
homes in the U.K. and France. Food samples were made up of whole food
and beverage intakes for one day. Each home was sampled the day before
the strips were hung and one week and six weeks later. In the U.K.
study, no residues exceeded 0.09 ppm, and the mean of all samples
taken at one week was 0.034 ppm. The samples at six weeks showed no
residues in excess of 0.05 ppm, with a mean value of 0.034 ppm.
Similar studies in 14 French homes showed no residues in excess of
0.07 ppm, and the mean of all samples taken at one week was 0.024 ppm.
The six week samples showed no residues in excess of 0.03 ppm.
METHODS OF RESIDUE ANALYSIS
Cholinesterase inhibition
A common technique which has been used for routine residue
determinations in commodities of known treatment history is based on
cholinesterase inhibition. Human and animal plasma cholinesterases are
considerably more sensitive to dichlorvos than erythrocyte
cholinesterases, and are therefore particularly suitable for residue
measurements. Out of date stocks of human blood plasma or horse serum
are satisfactory sources of enzyme.
Extraction and clean-up prior to the cholinesterase assay are normally
fairly simple, involving maceration of the material with a solvent of
medium polarity (chloroform, methylene chloride), filtration and
careful evaporation of the organic phase in the presence of water.
More elaborate procedures may be required for plant materials with a
high fat content, each as cocoa beans or groundnuts, and for certain
animal products such as milk, cheese and butter. Steam distillation of
dichlorvos prior to the enzyme assay has been suggested, not only for
removing interference,but also for increasing the specificity of the
residue method. For the enzyme assay proper, any of the common
techniques for measuring cholinesterase activity may be used. The
sensitivity of these methods is 0.05 to 0.1 ppm. Cholinesterase
residue analysis for dichlorvos has recently been automated by using
Technicon Auto-Analyser facilities.
TABLE VI
Residues of dichlorvos (ppm) in food purchased from shope in the UK1
Sampling time : days after hanging strips
No. of No. of 2 28 70
Commodity samples shops
Range Mean2 Range Mean Range Mean
Cooked meat 35 7 <0.05-0.13 <0.05 <0.05-0.10 <0.05 <0.05-0.09 <0.05
Cheese 35 7 <0.05-0.07 <0.05 <0.05-0.12 0.05 <0.05-0.12 <0.05
Apples 49 10 <0.05-0.26 0.08 <0.05-0.54 0.14 <0.05-0.19 0.06
Tomatoes 33 11 All <0.05 <0.05 All <0.05 <0.05 <0.05-0.05 <0.05
Lettuce 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Broad 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Cakes 19 7 <0.05-0.07 <0.05 <0.05-0.05 <0.05 <0.05-0.05 <0.05
1 No residues of DCA (0.03 ppm) were found in any of the samples
2 Mean values are geometric means and are calculated by assuming that samples containing less than the
detectable level (<0.05 ppm) in fact contain <0.025 ppm
Such automated methods permit rapid routine measurements of large
series of samples.
Since other organophosphorus insecticides or carbamates can interfere,
the cholinesterase inhibition method is not suitable for detecting and
measuring dichlorvos in samples of unknown or ill-defined treatment
history. Both thin-layer and paper chromatographic procedures are
available for separating dichlorvos from other
cholinesterase-inhibiting pesticides. In these methods, the
cholinesterase inhibitors are normally revealed by enzymatic spot
tests involving colour reactions of the substrate or its breakdown
products after hydrolysis. It must be remembered that dichlorvos is a
very volatile compound which, upon solvent evaporation, heating,
aeration, etc. is easily lost from vessels or chromatographic support
materials. These procedures are therefore recommended for qualitative
purposes, but great care must be taken when they are used in
quantitative analysis.
Bioassay
The vinegar fly (Drosophila melanogaster) and daphnia (Daphnia
magna) are suitable test organisms for the bioassay of dichlorvos in
plant materials. A specific test, which involves absorption barrier
chromatography and which distinguishes between dichlorvos and other
insecticides, has been described by Sun and Johnson (1963). The
sensitivity of the bioassay methods is about 0.1 ppm.
Colorimetry
Although colorimetric techniques have been developed mainly for
measuring traces in air samples or aqueous solutions, some of them can
be used for the determination of residues in food materials, provided
that the sensitivity required is not better than 0.3-1 ppm. Thus, the
color reaction of dichlorvos with alkaline resorcinol has given
satisfactory results with extracts of animal food commodities. This
method demonstrates not only the parent insecticide, but also
dichloroacetaldehyde, one of its potential hydrolysis products. Acting
on suggestions made by Hodgson and Casida (1962), Hughes (1963)
measured residues of dichlorvos and of dichloroacetaldehyde in air,
using colorimetry, following reaction with alkali and
2,4-dinitrophenylhydrazine. This method can also be used for
determining residues in food commodities. A further colorimetric
procedure, based on formation of an orange-red complex between
dichlorvos and acetone in the presence of alcoholic potassium
hydroxide, has been described by Mitsui.
Gas-liquid chromatography (GLC)
The most specific residue methods currently available rely on the
separation and detection of dichlorvos by gas chromatography. The
sensitivity of these procedures is about equal to that obtained with
the cholinesterase techniques, i.e. 0.03-0.1 ppm. Methods have been
described by El-Refai and Guiffrida who used the sodium thermionic
detector selective to phosphorus, and by Boone (1965) who used a
microcoulometric detection system. Both methods use fairly high column
temperatures, and the possibility of thermal or catalytic
decomposition of dichlorvos on certain column materials must not be
overlooked. Gas chromatographic residue measurements in industrial
laboratories are usually carried out at lower column temperatures
(150°C), and such stationary phases an phenyl diethanolamine succinate
and Reoplex 400 have given satisfactory retention times and
separations from interfering insecticides.
Ivey and Claborn (1969) have published a GLC method for the
determination of dichlorvos in milk, eggs and various body tissues of
cattle and chickens. Using a gas chromatograph equipped with a flame
photometric detector, they were able to detect 0.003 ppm of dichlorvos
in milk and 0.002 ppm in body tissues and eggs. Methylene
dichloridehexane was used to extract milk and hexane was used for fat
and chicken skin. To recover dichlorvos from muscle, blood and eggs
the authors used a preliminary extraction with acetonitrile.
Abbott at al.(1970) report the successful application of the use of
the caesium bromide tipped detector of Hartmann (1966) for the
determination of dichlorvos on a variety of foods in a total diet
survey.
Drager (1968) has published a gas chromatographic method for
determining dichlorvos residues in plants and milk. In this method,
plant material is extracted by macerating it with methanol and water.
The dichlorvos in the extract is partitioned into a mixture of ether
and petroleum ether. The residue is then transferred into ethanol, and
dichlorvos is determined by gas chromatography using the phosphorous
detector.
Minett and Belcher (1969) have developed a method for the
determination of dichlorvos residues in wheat using GLC techniques.
For the extraction of the ground wheat the authors used ethanol.
Many of the published methods and many residue reports indicate that a
variety of non-polar and polar solvents, such as methylene dichloride
were used to extract the dichlorvos residue from the food commodity.
Minett and Belcher (1969) and Elms at al. (1970) have shown that
dichlorvos cannot be recovered quantitatively from plant materials,
especially grain, by the use of solvents even as polar as methylene
dichloride. Water or water-miscible solvents, such as methanol or
ethanol, are absolutely essential for the recovery of dichlorvos
residues from plant materials.
Collaborative studies are currently being carried out in the United
Kingdom on a method of detecting dichlorvos in grain. The procedure
uses GLC with thermionic or flame photometric detection following
extraction with methanol or acetone.
APPRAISAL
Dichlorvos is used extensively for the control of insect pests of
importance in public health, in homes, warehouses, food stores and
transport, stored grain as well as insects attacking domestic animals.
It is also finding increasing use on horticultural and field crops and
is registered in over forty eight countries. Dichlorvos impregnated
resin strips are extensively used to control insect pests, especially
flies, in homes, stores and food processing establishments.
Extensive data on residues in food commodities were available from
many countries in the form of published reports end submissions from
three manufacturers. All data indicate that the level of residues
occurring in raw agricultural commodities is low and that the residue
levels decline rapidly. Due to the comparatively high vapour pressure
of dichlorvos, the applied deposit is quickly lost by volatilization,
but that portion which in absorbed into plant tissues undergoes
hydrolysis to inactive metabolites.
Dichlorvos applied to or fed to domestic animals undergoes rapid
detoxification and degradation in all species examined and is unlikely
to produce significant residues in meat, milk or eggs.
The use of dichlorvos impregnated strips for controlling insects in
warehouses, stores and shops gives rise to detectable residues in
stored or prepared foods. Residues resulting from exposures at rates
much higher than the recommended ones have been shown to decrease very
rapidly on exposure to the atmosphere. They also are readily destroyed
during the preparation of many products for consumption (e.g. by
washing or by cooking).
The proposal that a tolerance in meat and meat products was required
in view of the use of dichlorvos-resin fumigant strips in meat storage
and processing places in some countries (CCPR, 1971) was considered by
the Meeting.
Considerable data were available on the uptake of dichlorvos by many
different foods under varying conditions of storage and exaggerated
exposure to dichlorvos-resin strips, It was recognized that, under
some conditions, residues as high as 0.1 ppm could result but even in
fatty meat products the uptake was only at the surface. Residues
declined during atmospheric exposure and were completely destroyed by
cooking. Meat and meat products were included among the commodities
for which a 0.1 ppm tolerance was recommended. These products were
specially considered because of the request made at the 1970 meeting
of the Codex Committee on Pesticide Residues (CCPR, 1971).
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are for residues likely to be found in raw
commodities at harvest, in stored products shortly after treatment and
in miscellaneous prepared foods exposed during preparation and
storage. Recommendations have been made on broad categories of fruit
and vegetables, because extensive data have indicated that when
dichlorvos is applied to these crops the level of residues shortly
afterwards does not vary significantly on different varieties.
Due to the transient nature of the insecticide, residue levels will
continue to decline during shipment and storage, with a probable half
life of less than one day on fresh fruit, vegetables and miscellaneous
foods. Residues in food at time of consumption have normally been
below the limits of detection by current analytical methods.
Foodstuff Tolerance
Recommended Period on which
recommendation
based
Cocoa beans 5 ppm
Raw grain (wheat, rice,
rye, oats, barley,
maize, sorghum, etc,) 2 ppm
Coffee beans, soybeans,
lentils, peanuts (groundnuts) 2 ppm
Mushrooms 0.5 ppm 2 days
Milled products from raw
grain 0.5 ppm
Fresh vegetables
(excluding lettuce) 0.5 ppm 2 days
Tomatoes 0.5 ppm 1 day and post
harvest
Lettuce 1 ppm 2 days
Fresh fruit (apples, pears,
peaches, strawberries, etc.) 0.1 ppm 2 days
Meat of cattle, sheep,
goats, pigs and poultry 0.05 ppm
Eggs (on shell free
basis) 0.05 ppm
Milk (whole) 0.02 ppm
Miscellaneous food items
not otherwise
specified 001 ppm
As recommended in previous monographs, the content of
dichloroacetaldehyde (DCA) should be reported where known.
Furthermore, the amounts found should be added to those for dichlorvos
when assessing adherence to the tolerances recommended.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Continued observation of the effects of repeated exposure of man
to dichlorvos in order to determine if there is any qualitative
or quantitative differences between the metabolic route following
oral or inhalation intake.
2. Data from additional countries on residues in commodities moving
in international trade.
3. Analytical methods capable of recovering and determining residues
of dichlorvos in foods should be established for regulatory
purposes.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-69.
Pesticide Science, 1 (i):10-13
Anon. (1966) Communication from F.I.D. Nordrhein-Westfalen to Deutsche
Shell Chemie, Frankfurt, dated 20.1.66 (unpublished)
Anon. (1966) Communication from Hamburg Public Health Service to
Deutsche Shell Chemie, Frankfurt, dated 19.7.66 (unpublished)
Arthur, B.W. and Casida, J.E. (1957) Metabolism and selectivity of
o,o-dimethyl 2,2,2-trichloro-1-hydroxyethyl phosphonate and its acetyl
and vinyl derivates. J. Agr. Fd. Chem., 5 : 186-192
Blair, D., Hutson, D.H. and Pickering, B.A. (1970) The comparative
metabolism of (vinyl-14C) Vapona in rats after inhalation exposure
and oral ingestion of the compound. Unpublished draft summary from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Boone, G.H. (1965) Determination of Dibrom and DDVP residues in some
fruit and vegetable crops by microcoulometric gas chromatography. J.
A.O.A.C., 48 : 748-752
Boyer, A.C. (1969) I50 values for the inhibition of human and monkey
blood cholinesterases by dichlorvos. Unpublished technical information
report prepared by Shell Development Co.
Bull, D.L. and Ridgway, R.L. (1969) Metabolism of trichlorfon 1969 in
animals and plants. J. Agr. Fd. Chem., 17 : 837-841
Carson, S. (1969) Teratology studies in rabbits. Unpublished report
from Food and Drug Laboratories Inc., Maspeth, N.Y., prepared for
Shell Chemical Co.
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agr. Fd. Chem., 10 : 370-376
Cavagna, G., Locati, G. and Vigliani, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch.
Environmn. Hlth., 19 : 112-123
Cavagna, G., Locati, G. and Vigliani, E.C. (1970) Exposure of newborn
babies to 'Vapona' insecticide. Europ. J. Toxicol. 3 : 49-57
Cavagna, G. and Vigliani, E.C. (1970) Problèmes d'hygiène et de
sécurité dans l'emploi du Vapona insecticide dans les locaux
domestiques. Med. d. Lavoro, 61 : 409-423
CCPR. (1971) Report of the Fifth Session of the Codex Committee on
Pesticide Residues para. 165. Alinorm 71/24
Ciba, A.G. (1970) Basle - Submission to FAO/WHO Joint Meeting -
Dichlorvos
Drager, G. (1968) Gas chromatographic method for determining
dichlorvos residues in plants and milk. Pflanzenschutz - Nachrichten.,
21 : (3) 373-380
Elms, K.D., Kerr, J.D. and Champ, B.R. (1970) Breakdown of malathion
and dichlorvos mixture applied to wheat. In press
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FAO/WHO. (1967) Evaluation of some pesticide residues in food, FAO
PL:CP/15, WHO/Pood Add./67.32
FAO/WHO. (1968) 1967 Evaluation of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Green, A.A. and Wilkin, D.R. (1968) The control of insects in bagged
grain by the injection of dichlorvos. J. Stored Prod. Res., 5 :
11-19
Hartmann, C.H. (1966) Phosphorus detector for pesticide analysis. Bull
Environm. Contam. and Toxicol., 1 : 159-168
Hine, C.H., Beltran, S., Cavalli, R.D., Keating, J.M., Keating, W.C.
Jr. and Sample, H.G. Jr. (1966) Pre-clinical pharmacologic studies on
dichlorvos in formulation (V-12). Unpublished report from the Hine
Laboratories, Inc., submitted to Shell Chemical Co.
Hine, C.H. (1970) Arizona Vapona (DDVP) human study program.
Chromosomal analysis. Unpublished report from the Hine Laboratories,
Inc., prepared for Shell Chemical Research Development Co.
Hodgson, E. and Casida, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethyl phosphate. J. Agr. Fd.
Chem., 10 : 208-214
Hughes, G.T. (1963) Colorimetric determination of low concentrations
of 2,2-dichlorovinyl dimethyl phosphate in the atmosphere. Analyst,
88 : 318-319
Hunter, C.G. (1969) Report on initial studies of deliberate exposure
to high concentrations of dichlorvos by human subjects. Unpublished
report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Hunter, C.G. (1970) Dichlorvos: inhalational exposures with human
subjects, Part 1. Group research report TLGR. 0061.70
Ivey, M.C. and Claborn, H.V. (1969a) Kerrville, Texas, Laboratory,
Entomology Research Division, ARS, USDA. Results of research on
residues of dichlorvos in milk and body tissue of dairy cows treated
for fly control
Ivey, M.C. and Claborn, H.V. (1969b) GLC determination of dichlorvos
in milk, eggs and various body tissues of cattle and chickens. Journal
of the AOAC, 52 (6) : 1248-1251
Kimbrough, R.D. (1970) Work sited in "Summary of Vapona R
insecticide review", report of a meeting chaired by L. Golberg at
Cincinnati, 9 February 1970
Kirkpatrick, R.L., Harein, P.K. and Cooper, C.V. (1968) Laboratory
tests with dichlorvos applied as a wheat protectant against rice
weevils. J. of Econ. Entomol. 61 : 356-358
Lancaster, J.L. (1962) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lancaster, J.L. (1963) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lofroth, G. (1969a) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Paper presented at
a meeting of the Children's Cancer Research Foundation, Boston, 3
September 1969
Lofroth, G. (1969b) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Unpublished report
reproduced in part in Environmental Mutagen Society Newsletter, No, 2:
p. 21-24
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57 : 393-394
Loomis, E. and Hodel, E. (1965) (in cooperation with Shell Development
Co. Residue project from Rio Linda, California (unpublished)
Minett, W. and Belcher, R. (1969) Determination of malathion and
dichlorvos residues in wheat grains by gas-liquid chromatography. J.
Stored Prod. Res., 5 : 417-421
Modesto home trials with Vapona insecticide strips
Nelson, C.B. (1968) (in cooperation with Shell Chemical Co. Residue
project from Fillmore, California (unpublished)
Noetzel, D. (1964) (in cooperation with Shell Development Co. Residue
project from North Dakota State University (unpublished)
Padget, T.J. (1968) Evaluation of dichlorvos as a grain treatment for
protection against the white-fringed beetle. USDA Plant Pest Control
Laboratory (unpublished)
Padget, T.J. (1969) Communication on the evaluation of dichlorvos as a
grain treatment for protection against the white-fringed beetle
(unpublished)
Page, A.C. (1970) Metabolic fate and tissue residues following oral
administration of dichlorvos. Collection of unpublished reports
prepared and submitted by Shell Chemical Co.
Palut, D., Grzymala, W. and Syrowatka, T. (1969) Investigation of the
metabolism of some organophosphorus insecticides on animal model
systems. II Hydrolytic degradation in vitro (original in Polish),
Roczn. Zak. Hig. (Warsz.), 20 : 551-555
Pitts, C.W. (1961) (in cooperation with Shell Development Co. Residue
project from Kansas State University (unpublished)
Preussmann, R. (1967) Analytical determination of alkylating agents.
Zweite Konferenz über aktuelle Probleme der Tabaksforschung, Freiburg,
p. 92 (cited by Lofroth, 1969, q.v.)
Preussmann, R. (1968) Direct alkylating agents as carcinogens. Fd.
Cosm. Toxicol, 6 : 576-577
Preussmann, R., Schneider, H. and Epple, F. (1969) Untersuchungen zum
Nachweis Alkylierender Agentien. Arzneimittelforschung, 19 :
1059-1073
Roger, J.C., Chambers, H. and Casida, J.E. (1964) Nicotinic acid
analogs: effects on response of chick embryos and hens to
organophosphate toxicants. Science, 144 : 539-540
Roger, J.C., Upshall, D.G. and Casida, J.E. (1969) Structure activity
and metabolism studies on organophosphate teratogens and their
alleviating agents in developing hen eggs with special emphasis on
Bidrin. Biochem. pharmacol., 18 : 373-392
Sasinovich, L.M. (1968) The maximum permissible concentration of DDVP
in the air of the working zone. Gig. i Sanit, 33 (12) 35-39
Sax, K. and Sax, H.J. (1968) Possible mutagenic hazards of some food
additives, beverages and insecticides. Jap. J. Genetics, 43 : 89-94
Shaw, F. (1964) (in cooperation with Shell Development Co. Residue
project from University of Massachusetts (unpublished)
Shell Development Co. (1964) Modesto Project No. RES 64-111
(unpublished)
Shell Research Ltd. (1965a) Woodstock Technical Memo. 165/65
(unpublished)
Shell Research Ltd. (1965b) Woodstock Technical Memo. 159/65
(unpublished)
Shell Development Co. (1965c) Modesto Project No. RES 65-42
(unpublished)
Shell Chemical Company, New York. (1965d) Brief in Support of Vapona
Insecticide Resin Strips. Section VII : 11-12. Submitted to Panel of
U.S.P.H.S. and U.S.D.A., 29.10.65
Shell Development Company. (1965e) Modesto Project No. RES 65-36
(unpublished)
Shell Research Ltd. (1966a) Woodstock Technical Service Note 146/66
(unpublished)
Shell Research Ltd. (1966b) Woodstock Technical Service Note 178/66
(unpublished)
Shell Research Ltd. (1966c) Woodstock Technical Memo 1/66
(unpublished)
Shell Chemical Co. (1967) Petition proposing a tolerance for the food
additive (pesticide chemical) dichlorvos in or on stored food
commodities. Section D-III
Shell Research Ltd. (1968) Woodstock WKGR. 0041.68 (unpublished)
Shell Chemical Co. (1969) Princeton laboratory residue analysis report
project no. PRL 69-62, (unpublished)
Shell Development Co. (1970a) The third Arizona home study. The
quantification of DDVP residues in foods consumed by human volunteers
exposed to "No-Pest" strip insecticide. (unpublished report)
Shell Chemical Co. (1970b) Princeton laboratory residue analysis
report project no. PRL 70-12, (unpublished)
Shell Chemical Co. (1970c) Princeton laboratory residue analysis
report project no. PRL 69-26 (II) (unpublished)
Shell Research Ltd. (1970d) Interim report - Residues of Vapona in
meals prepared in domestic kitchens. Woodstock Laboratory
(unpublished)
Singh, V.K. (1964) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. (1965) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. and Rainier, R.H. (1966) Three year chronic oral toxicity
of formulated dichlorvos in swine with special reference to possible
effects upon fertility and the viability of the offspring of the
animals fed continuously on diets containing the drug. Unpublished
report from Bio/Toxicological Research Associates, Spencerville, Ohio,
prepared for Shell Chemical Co.
Somme, L. (1967) A field trial with dichlorvos vapor for the control
of Ephestia Kühniella Zell (Lepidoptera, Phycitidae) in flour mills.
Reprinted from Journal of Stored Products Research, 4 (3)
Stevenson, D.E. and Blair, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos. Shell
Research Tunstall Report No. TLGR. 0024.69
Sun, Y.P. and Johnson, E.R. (1963) A new bioassay technique with
special reference to the specific bioassay of dichlorvos insecticide.
J. Econ. Entomol., 56 : 635-641
Vigliani, E.C. (1970) Results of recent studies on Vapona. Paper
presented at the 7th Shell Industrial Doctors' Meeting, Strasbourg,
27-29 May 1970, submitted by Shell International Research Ltd.
Vogin, E.E. (1969) Teratological studies with dichlorvos in rabbits.
Unpublished report from Food and Drug Laboratories, Inc., Maspeth,
N.Y., prepared for Shell Chemical Co.
Wisconsin Alumni Research Foundation No. 8030193 (1968) (in
cooperation with Shell Chemical Co. Residue project report
(unpublished).
Wisconsin Alumni Research Foundation (1968/9) (in cooperation with
Shell Chemical Co. Residue project reports on sheep, cattle and goats
(unpublished)
Witherup, S., Caldwell, J.S. Jr. and Hull, L. (1965) The effects
exerted upon the fertility of rats, and upon the viability of their
offspring, by the introduction of Vapona R insecticide into their
diets. Unpublished report from the Kettering Laboratory Cincinnati
Young, R., Jr. (1969) Dichlorvos infusion studies. Unpublished
technical information report prepared by Shell Development Co.
Rat
Reproduction studies in rats fed 0, 0.1, 1,0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965; see FAO/WHO, 1967).
Dichlorvos was administered at unspecified levels intraperitoneally to
female rats on day eleven after insemination. Doses of 20 mg/kg
body-weight killed the animals; 15 mg/kg produced toxic signs and
weight loss. There was no adverse effect on litter size or on the
placenta at 15 mg/kg, but three foetuses from one of four litters
available had omphaloceles, although none were observed in the six
litters from control animals (Kimbrough, 1970).
Rabbit
A total of 168 adult female rabbits were artificially inseminated with
20-30 million viable and mobile rabbit sperm, and on days 6 to 18 of
gestation were administered daily oral doses of 0, 6 or 18 mg/kg
body-weight of dichlorvos by capsule, or 31 mg/kg on days 6 to 11 of
gestation. Foetuses were recovered by Caesarean section. There was
increased incidence of in utero and neonatal toxicity in the top
dose group only, which was of doubtful significance. There were no
teratogenic effects observed, based upon skeletal examination of the
foetuses. Maternal mortality was increased in the 31 mg/kg group
(Carson, 1969).
In a separate experiment, 104 female rabbits were artificially
inseminated with rabbit sperm and administered 0, 3 or 12 mg/kg
body-weight of dichlorvos by capsule on gestation days 6 to 16
inclusive. Other rabbits were given higher doses, but they could not
tolerate levels of 24 mg/kg or higher, and no live litters were
produced from such groups. At 12 mg/kg, there was a significant
reduction in the number of implant sites and foetuses compared to the
control or lower dose group. At 3 mg/kg, the number of implant sites
and foetuses were not lower than the controls. However, one deformed
stillborn foetus was encountered from this group. Liver sections from
parents and foetuses displayed no pathological abnormalities that
could be related to dichlorvos treatment (Vogin, 1969).
Pig
Female pigs were fed dietary levels of 0, 200, 250, 288, 400, 500 or
750 ppm of dichlorvos for up to 37 months. The animals were first
mated after they had received the test diet for six months, and the
study was followed through two generations. Initially, a total of 22
females and one male were used, but after the first generation, four
more males were used in order to prevent in-breeding. The males
received 0, 288 or 400 ppm of dichlorvos in their diets. Dichlorvos
did not affect either the numbers of litters or their size and the
survival in the groups fed dichlorvos. A total of 490 piglets were
examined and none showed anatomical abnormalities (Singh and Rainier,
1966).
Special studies on mutagenicity
Secondary roots of Vicia fabia were treated with varying
concentrations of dichlorvos for one hour. Concentrations down to 1 mM
were mostly lethal, and root tips surviving this and lower
concentrations had a low mitotic index and showed c-mitotic effects,
and chromatid breaks and gaps were observed. In another experiment, a
streptomycin dependent, Sd-4, mutant of E. coli was treated in the
lug phase at cell densities of about 108 cells/ml during one hour.
The LD50 concentration for the bacteria of dichlorvos in the medium
was estimated to be between 10 and 60 mM (2200 ppm and 1.3 percent).
At concentrations of 1-4 mM, which caused no significant killing, an
increased mutation rate was observed when compared with controls and
measured as revertants to streptomycin independency (Löfroth, 1969 a,
1969 b).
Segments of Vapona-strips of various sizes were placed in an inflated
6000 ml plastic bag containing an uncovered petri-dish with
germinating onion seeds. There was no retardation of root growth or
mitotic frequency, but the number of chromosome aberrations, when
scored at anaphase, increased constantly with increasing size of the
Vapona-strip segment. The lowest concentration used was about ten
times as great as one strip in a 1000 ft3 room (Sax and Sax, 1968).
Attention has been drawn to the alkylating property of a number of
alkyl phosphates (Preussman, 1967). For this reason, possible
mutagenic effects have been looked for. Calf thymus deoxyribonucleic
acid (DNA) was incubated with varying concentrations of dichlorvos
solution. In a typical experiment with 2 percent dichlorvos incubated
for 66 hours, there was a yield of 1 percent of N-7-methylguanine from
the available guanine. Similar experiments with deoxyguanosine gave a
3-5 times higher yield of N-7-methylguanine than from DNA (Löfroth,
1970).
Eight mice were injected intraperitoneally with 10-20 mg/kg
body-weight of dichlorvos. After 23 hours, 100 cells of their bone
marrow were examined. The incidences of aberrations were not greater
than observed in four control mice (Hunter, 1970).
A human subject was exposed to 1 mg/m3 of dichlorvos for six hours.
A sample of blood cultured for leucocytes for 72 hours showed no
increase in chromosome aberrations in 100 cells compared to the
pre-exposure values (Hunter, 1970).
No chromosomal aberrations were seen after examination of blood
samples from a family of four female and one male human subjects which
had been exposed to dichlorvos strips. A control group had two male
and three female subjects (Hine, 1970).
Special studies on carcinogenicity
Rat
Rats (24) were injected subcutaneously, once weekly, with trichlorphon
(Dipterex), of which dichlorvos is a known metabolite. Despite a high
(unspecified) dosage and a latent period of almost 800 days, only two
local sarcomas developed at the site of injection. No data on controls
are given. (Preussmann, 1968). In a subsequent publication, Preussmann
et al. (1969) refer to the above experiment as a "negative result,
presumably due to the very rapid metabolism of Dipterex."
Acute toxicity
After direct infusion of dichlorvos into a peripheral vein at various
rates, pigs can survive an LD50 dose each hour for at least nine
hours (page, 1970).
The acute toxicity of dichlorvos has been compared with the
metabolites. The LD50 values in mg/kg body-weight for intraperitoneal
administration to mice are: dichlorvos, 28; o-demethyldichlorvos
sodium salt, 1500; dichloroacetaldehyde, 440; 2,2-dichloroethanol,
890; dichloroacetic acid, 250; sodium dichloroacetate, >3000;
mono- and dimethylphosphoric acid mixture, 1500; sodium
monodimethylphosphoric acid mixture, >3000 (Casida, et al., 1962).
Short-term studies
Comparative studies on inhalation toxicity
Guinea pigs have been exposed for five hours per day for five
consecutive days to a concentration of 130 mg dichlorvos/m3 of air
without visible effects on health. Rats and mice were not visibly
affected by similar exposures to 50 mg/m3. At concentrations above
50 mg/m3, the mice became visibly distressed, and prolonged exposure
to 80 mg/m3 was frequently lethal. Rats were less severely affected
than mice. In all cases, stress reactions were either apparent during
the first hours of exposure or they did not occur at all. No
cholinesterase determinations were made (Stevenson and Blair, 1969).
Three samples of dichlorvos were investigated (82.5 percent pure,
Russian produced, 40% pure, Japanese, and 100% pure from Swiss
origin); the oral LD50 for Russian and Japanese samples was similar
(87 mg/kg body-weight for mice and 65 mg/kg for rats). The Swiss
sample was less toxic: oral LD50, mice: 108 mg/kg. Rabbits were found
to be the most sensitive species (oral LD50, Russian sample: 22.5
mg/kg). Inhalation toxicity was investigated for exposure periods of
four hours, The highest concentration which could be produced (30
mg/m3) was lethal for mice and rats. The LD50 (four hours) for mice
was 13.2 mg/m3 and for rats 14.8 mg/m3. At 5 mg/m3, blood
cholinesterase activity in rat was 69 percent of pre-exposure values.
A level of 0.5 mg/m3 caused no observable effects in blood
cholinesterase. By interpolation, those concentrations of dichlorvos
in air were calculated which would after four hours exposure cause a
50 percent drop in blood cholinesterase activity in rat (I50 = 2.4
mg/m3) or a 25 percent drop (I25 = 1 mg/m3). Rats and rabbits
were exposed for 4 hours/day for a period of four months to an average
dichlorvos vapour concentration of 1 mg/m3 of air. No visible signs
of intoxication occurred. Cholinesterase activity in the serums
erythrocytes, medullary tissue and liver of rats varied throughout the
experiment within the limits of the norm or was slightly depressed.
After four months, the depression of cholinesterase activity in the
medulla averaged 8 percent in the liver, serum and erythrocytes the
depression was 30 percent 22 percent and 24 percent, respectively.
Blood sugar content, blood sugar curves after loading with galactose,
synthesis of hippuric acid after loading with sodium benzoate and
duration of Hexenal induced sleep were not affected. Rabbits exposed
under the same conditions showed 11-30 percent reduction of serum
cholinesterase activity and 28-30 percent reduction of erythrocyte
cholinesterase activity. It was concluded that 1 mg/m3 was the
threshold for rats and rabbits which caused an insignificant and
variable depression of cholinesterase. It was also the threshold for
cats. Rats exposed to an average concentration of 5.2 mg/m3 for 4
hours/day over a period of two months remained visibly healthy. After
15 days, the cholinesterase depression in the medulla was on an
average 22 percent in the serum 58 percent in the erythrocytes 22
percent. The duration of drug induced sleep was increased from 33
minutes to 57 minutes. The blood sugar level was unchanged, but the
rise of the curve after galactose loading was far higher than in the
controls. After 60 days, the blood sugar curve was affected, the
duration of Hexenal induced sleep was the same as in the controls, and
cholinesterase activity in the medulla fell by an average of 31
percent, in the liver by 35.5 percent, in serum by 52 percent and in
erythrocytes by 58 percent. At 8.2 mg/m3 for 45 days, rats lost
appetite and weight, and in some cases trembling of the head and
entire body was noted. Signs of intoxication occurred in some of the
rats and some died (Sasinovich, 1968).
OBSERVATIONS IN MAN
A total of 10 human subjects, in groups of two, ingested pellets of a
slow-release formulation of dichlorvos in daily dosages, doubling
weekly, up to 8 mg/kg body-weight/day for periods up to three weeks;
one group of two received 16 mg/kg/day for 5.5 days. Doses above 1
mg/kg were administered in two equally divided doses per day. The
majority of the clinical side effects occurred after the doses had
reached the 8 mg/kg/day level. Most of the subjective clinical
complaints referred to the gastrointestinal tract, but none of the
studies had to be terminated because of side-effects. The study with
16 mg/kg/day was terminated because of the magnitude of the red blood
cell cholinesterase activity depression. Doses of 1-8 mg/kg/day for up
to 21 days produced an average plasma cholinesterase activity
depression of 70 percent irrespective of dose or duration. Doses of 16
mg/kg/day for 5.5 days produced approximately 90 percent depression of
the plasma cholinesterase activity. Red cell cholinesterase activity
after multiple dosing of 1 mg/kg/day was 11-27 percent depressed.
Doses which reached 8 mg/kg/day caused 45-86 percent depression. At 16
mg/kg/day, depressions of 90 percent were observed. Single doses of
dichlorvos needed to be more than 4 mg/kg in order to cause measurable
red cell cholinesterase depression. At single doses of 8 mg/kg and
higher, maximal plasma cholinesterase inhibition was present and did
not increase at higher dosages. Significant and consistent erythrocyte
cholinesterase inhibition occurred after single doses of 16 mg/kg (36
percent inhibition) and 32 mg/kg (46 percent inhibition). About 50
percent of the dichlorvos was still present in the excreted pellets,
Therefore, the doses absorbed were assumed to be about one half of the
above levels (Hine et al., 1966).
In another study, 12 male subjects ingested 5 mg of dichlorvos daily
in three equal portions with their food until the plasma
cholinesterase fell to 75 percent of the pre-exposure value. The time
required averaged 12 to 20 days. No effect occurred on erythrocyte
cholinesterase. One of the subjects continued the exposure for a total
of 77 days. A questionable erythrocyte cholinesterase depression of
10-15 percent occurred during part of the exposure period, but in the
beginning and at the end of the exposure period, this activity was
normal or slightly above (Hunter, 1970).
Six human subjects were exposed to concentrations of dichlorvos in the
air, the maximum level being 52 mg/m3 for 62 minutes and the maximum
period 240 minutes to 13 mg/m3. No clinical abnormalities were
observed, subjective complaints were only related to the dryness of
the atmosphere, but the plasma cholinesterase activity was decreased;
the extent of the depression was proportional to the product of the
concentration and time of exposure. During some of these exposures, a
questionable depression of erythrocyte cholinesterase occurred, but
the extent of depression was not directly related to the product of
the concentration and time of exposure (Hunter, 1969).
In six homes in Tucson, Arizona, 18 individuals submitted to exposure
to dichlorvos strips and six others served as controls.
In order to maximize exposure, the study was made during the winter
months, and strips were hung in all rooms in the houses at the rate of
1 strip/1000 ft3. Maximum concentrations of 0.1-0.2 mg/m3 occurred
within the first week, levelling off to 0.05-0.10 mg/m3 during the
remainder of the regular exposure period of 28 days. Samples of meals
and beverages were collected at various intervals. During the exposure
period, more than 85 percent of the individual meals contained less
than 0.3 ppm dichlorvos and more than half the meals 0.1 ppm or less.
Physical examination, complete blood counts, blood chemical profiles
and plasma and erythrocyte cholinesterase measurements were performed
at various intervals. Levels of dichlorvos in the food eaten ranged
from 0.1 to 0.3 ppm. There were no clinical abnormalities evident, nor
were there any significant differences in plasma and red-blood cell
cholinesterase, blood count and blood chemical profiles between the
exposure and pre-exposure periods (Shell, 1970a).
A study was made in Italy of the effect of continuous exposure to
insecticide levels of dichlorvos by patients in hospital wards.
Control values for plasma and erythrocyte cholinesterase were
established for 250 healthy male and 100 healthy female adults not
exposed to dichlorvos. The variation in the activity of the
cholinesterases of the individuals when measured at different times,
depending on the time-interval, varied on average between 1.4 and 4.4
percent for the erythrocyte cholinesterase and on average between 5.0
and 14.2 percent for plasma cholinesterase. These were average
variations measured in ten to 20 individuals. In single individuals,
the maximum variation was 7.7 percent for erythrocyte cholinesterase
and 23.2 percent for plasma cholinesterase.
A total of 44 adult male patients with diseases other than liver
diseases were exposed to atmospheric concentrations of dichlorvos
ranging from 0.02 to 0.1 mg/m3 for periods of 3 to 29 days. Another
similar group of 22 male patients was exposed to dichlorvos
concentrations of 0.1 to 0.28 mg/m3 for periods from 3 to 16 days.
Only in the case of five patients exposed 24 hours per day at levels
above 0.1 mg/m3 was there a depression of plasma cholinesterase (35
to 72 percent) below the pre-exposure level. There was no depression
of erythrocyte cholinesterase nor were any symptoms typical of
cholinergic stimulation observed, in any patients. With six other
patients suffering from liver disease exposed in the same room, all
showed a reduction in plasma cholinesterase (25 to 66 percent of
pre-exposure values). Reduction was evident even in patients exposed
at levels below 0.1 mg/m3. These patients, because of their liver
disease, had already before exposure a considerably reduced plasma
cholinesterase activity. However, there was no depression of
erythrocyte cholinesterase nor any symptoms of poisoning, in spite of
the fact that in these patients with liver insufficiency had a 40
percent depression in plasma cholinesterase lasting for one to three
weeks after the removal from exposure. In babies, sick children and
women in labour or postpartum exposed to dichlorvos, a similar
situation to that of sick adults was seen; depression of plasma (but
not erythrocyte) cholinesterase was observed only in some cases when
the dichlorvos level was above 0.1 mg/m3. Again, no symptoms were
seen. Assuming an inhaled volume of 10 m3/24 hours in adults and 1.4
m3/24 hours in children, the authors calculated that a reduction in
plasma cholinesterase for a calculated daily inhalation of 1.7 mg of
dichlorvos could occur in adults with normal liver function and 0.2 mg
for children. They further calculated that the amount of dichlorvos
inhaled which might produce a plasma cholinesterase depression is
about the same for adults and children, viz. 0.028 to 0.030 mg/kg
body-weight/day. In patients with liver malfunction, daily inhalation
intake of 0.34 mg (equivalent to 0.005 to 0.006 mg/kg body-weight/day
was sufficient to produce plasma cholinesterase depression (Cavagna et
al., 1969; Cavagna and Vigliani, 1970).
In two nurseries 22 healthy newborn babies were kept from birth for a
period of five days for 18 hours/day in an atmosphere in which average
dichlorvos concentrations of 0.15 - 0.16 mg/m3 were maintained. Blood
cholinesterase levels were determined at birth and after five days.
There were no effects on the health of the babies nor on the activity
of their plasma or erythrocyte cholinesterase (Cavagna et al., 1970).
In a preliminary study with asthmatic patients, there appeared to be
no subjective worsening of asthma during exposure to dichlorvos at
levels of 0.1 to 0.2 mg/m3 for two consecutive days. In some cases,
an increase in airways resistance and sensitivity to acetylcholine
occurred during and after exposure, but these manifestations may be
related to other factors (e.g. poor ventilation) than to dichlorvos.
Further work in this field is reported to be in progress (Vigliani,
1970).
COMMENT
On the basis of recent and earlier studies the metabolic pattern of
dichlorvos when fed to mammals is now fairly clearly elucidated.
Evidence is presented that cleavage of the dichlorovinyl group
proceeds much more rapidly than o-demethylation. Information on the
exposure of man to varying levels of dichlorvos vapour is available,
but such studies do not include assessment as to whether the
metabolism is the same after inhalation as after oral intake. Data on
the toxicology of the metabolites dichloracetaldehyde and
dichloroethanol are considered pertinent in evaluating the toxicity of
dichlorvos. A long-term study in rats reported in the monograph from
the 1967 Joint Meeting is considered adequate. Feeding studies in rats
and rabbits did not show any indication of a teratogenic effect.
Additional information on feeding dichlorvos to man has become
available, and the no-effect level previously determined in man is
used as a basis for establishing an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In addition to its use on beef and dairy cattle and goats, sheep and
pigs, and in and around buildings which house these animals,
dichlorvos is finding increasing use on fruit and vegetable crops.
Fruit and citrus crops account for more than one third of the
agricultural use. Rice accounts for just over one quarter and field
crops, including vegetables, approximately one quarter of the
agricultural uses. There are minor uses on grapes, glasshouse crops,
mushrooms, tobacco, tea, coffee, cocoa and miscellaneous crops.
Dichlorvos is known to be registered in at least forty eight
countries.
Recently it has been introduced as an anthelmintic for pigs, poultry
and horses, being administered in the form of plastic granules for
this purpose.
Post-harvest treatments
Dichlorvos is being increasingly used for the control of insect
infestation in stored grain. For this purpose it is used as an aerosol
or as impregnated resin strips, which are applied in the overhead
spaces of storage bins. In some countries, dichlorvos preparations
have also been registered for incorporation into stored grain either
as a dust, as a direct spray or as an aqueous emulsion.
Dichlorvos aerosols, automatic dispensers, sprays and resin
impregnated strips are used in many countries for the control of
insect infestations in stores, in transport and in warehouses holding
processed food. Food processing establishments are also treated with
dichlorvos in one of the above forms.
Other uses
As defined in the monograph of the 1967 Joint Meeting (FAO/WHO 1968),
dichlorvos is used extensively in the public health field (homes,
hospitals, etc.). It is also used in aircraft during flight. Trials
are being carried out on its use by aerial application against insect
pests of forests, pastures and field crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown in the open
Table I gives typical results of the analysis for dichlorvos residues
following controlled application to various fruit and vegetable crops
(Ciba 1970).
Crops grown under cover
In glasshouses, the rate of decay of dichlorvos residues is fast, with
a half-life of approximately one day. Table II gives results of
residue trials carried out on crops growing under cover (Ciba 1970).
Livestock and poultry
Trials in the United States demonstrate that the spraying of
dichlorvos on livestock (including cattle, sheep, goats and pigs) and
poultry is unlikely to produce significant residues in meat, milk or
eggs. When dichlorvos was applied as a single pour-on application of 1
percent at the rate of 16.5 ounces per cow, no residues were
TABLE I
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Apples 0.05% ai 1 day 0.05 ChE1 0.05
2 days <0.05
Peaches 0.05% ai 0 day 0.8 ChE 0.02
1 day 0.55 " 0.02
2 days 0.35 " 0.02
Strawberries 0.05% ai 2 days 0.35 ChE 0.02
3 days 0.06 " 0.02
8 days <0.02 " 0.02
Carrots 0.025% al. 28 days <0.05 ChE 0.05
31 days <0.05 " 0.05
Cauliflower 0.05% ai 0 days 0.37 ChE 0.04
5 days <0.04 "
Brussels 0.05% ai 0 days 1.35 ChE 0.04
sprouts 5 days <0.04
Cabbage 0.05% ai 0 days 1.5 ChE 0.05
1 day 0.14
2 days 0.05
3 days <0.05
Spinach 0.05% 0 days ChE 0.05
1 day 1.6
2 days 0.06
3 days <0.05
TABLE I (Cont'd.)
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Lettuce 0.05 0 days ChE 0.05
3 days <0.05 " 0.05
French beans 0.05 0 days 0.3 ChE 0.05
<0.05
Cucumber 0.05 9 days <0.05 ChE 0.05
1 ChE = cholinesterase inhibition method
TABLE II
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Tomato 20 mg/m3 0 days 0.2 ChE1 0.04
1 day 0.06 " 0.04
2 days 0.04 " 0.04
England Lettuce 130 mg/m3 0 days 78 ChE 0.04
1 day 4.9
70 mg/m3 1 day 2.1
2 days 0.4
3 days 0.2
Germany Lettuce 30 mg/m3 0 days 3.4 B.T.2 0.04
1 day 1.8
3 days 0.3
20 mg/m3 0 days 2.4 B.T. 0.04
1 day 0.9
2 days 0.4
3 days 0.16
Switzerland Cucumber 20 mg/m3 0 days 7.3 ChE 0.04
2 days 0.15
U.S.A. Mushroom 130 mg/m3 1 day 0.1 ChE 0.04
TABLE II (Cont'd.)
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
England Mushroom 160 mg/m3 0 days 25 D.N.P.3 0.04
1 day 0.9
3 days 0.27
160 mg/m3 1 day 0.9 D.N.P. 0.04
2 days 0.05
100 mg/m3 0 days 0.3 D.N.P. 0.04
1 day 0.1
1 ChE - cholinesterase inhibition method
2 B.T. - bioassay
3 D.N.P. - 2,4-dinitrophonylhydrazine colorimetric method
detectable at the level of sensitivity of the method (0.1 ppm) in any
of the milk samples collected from 4-5 hours to 5 days, after
treatment (Singh, 1965). In another study (Noetzel, 1964), groups of
Holstein and Guernsey cattle received dermal applications, twice daily
for 28 consecutive days, of 2 ounces of 0.5 percent dichlorvos and 1
percent dichlorvos, respectively. No residues were detected in the
milk (<0.02 ppm). In a similar study (Wisconsin, 1968) in which
dichlorvos was dermally applied to lactating dairy cows at the rate of
1 ounce/cow or 3 ounces/cow of a 1 percent dichlorvos for thirty days,
milk samples from 1 through 7 days and thereafter at 3 day intervals
were analysed for residues of dichlorvos and dichloroacetaldehyde
(DCA). No residues were detectable in any of the samples (<0.05 ppm
dichlorvos, <0.01 ppm DCA).
In other extensive trials (Wisconsin, 1968/1969) Brahma cattle, goats
and sheep were sprayed 3 times to saturation (1 gallon) at 14 day
intervals with 0.025 percent dichlorvos emulsion. No residues of
dichlorvos were detectable at the limits of sensitivity in tissues or
organs (including renal, omental and subcutaneous fat, muscle, liver
and kidney) of cattle (<0.25 ppm), goats (<0.25 ppm) or sheep
(<0.05 ppm).
Ivey and Claborn (1969a) sprayed lactating dairy cows for 31
consecutive days with 2 ounces of a 1 percent dichlorvos insecticide
spray solution. Milk samples were taken at 2 hours, 1, 2, 4, 8, 16, 24
and 31 days. One day after final treatment, the cows were slaughtered
and samples of renal, omental and subcutaneous fat, muscle liver,
kidney and blood were taken. Using a highly sensitive flame
photometric method (Ivey and Claborn, 1969b) with improved clean-up
technique, no residues of dichlorvos were detected in milk (<0.003
ppm) or body tissue (<0.002 ppm).
Pigs were fed daily at the high dosage of 9 450 ppm dichlorvos for 90
days (Singh, 1964). Dichlorvos residues were not detectable (<0.05
ppm) in tissues and organs including spleen, kidney, small intestine,
lung, heart, muscle and liver 0 and 1 day after last treatment.
Analysis of meat, fat, liver and eggs derived from poultry sprayed
with 2 ounces of diluted 2 lb/gal dichlorvos insecticide E.C. (0.295
ppm grams DDVP/bird) showed residues of equal to or less than 0.03 ppm
when samples of tissue were taken 6 hours, 3, 5 and 7 days after
application. Eggs were collected 1, 2, 3, 4, 5, 6 and 7 days after
treatment (Shell, 1970b). Poultry exposed to dichlorvon in the form of
2 percent granules (2 lb/100 square feet or 4 lb/100 square feet), or
to resin strands containing 20 percent dichlorvos (1 foot of strand/1
foot of cage) caused no residues in either tissue or eggs (<0.02 ppm)
(Lancaster, 1962; Loomis and Hodel, 1965; Lancaster, 1963; Shaw, 1964;
Nelson, 1968).
TABLE III
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Singh, Cows, pour on 480 1 Milk2 <0.1
1965
Noetzel, Cows, dermal 60 28×1 Milk <0.02
1964
Wisconsin, Cows, dermal 90 30×1 Milk <0.05
1968
Wisconsin, Cows, dermal 4540 3×14 Fat, muscle <0.25
1968, 1969 Goats, dermal 4540 3×14 Fat, muscle <0.25
Sheep, dermal 4540 3×14 Fat, muscle <0.05
Ivey & Claborn, Cows, dermal 60 31×1 Milk <0.003
1969a Cows, dermal 60 31×1 Fat, muscle, <0.002
etc.
ppm
Singh, Pigs, oral 9450 90×1 All tissues <0.05
1964
g
Shell, Chickens, dermal 0.3 1 All tissues3 <0.03
1970b Chickens, dermal 0.3 1 Eggs4 <0.03
Lancaster, Chickens, vapour - 30×1 All tissues <0.2
1962
TABLE III (Cont'd.)
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Loomis, Chickens, vapour - 30×1 Eggs <0.02
1965
Nelson, Chickens, contact - 28×1 All tissues <0.03
1968 Chickens, contact - 28×1 Eggs <0.05
mg
Pitts, Chickens, oral 8.5 over 10 Meat <0.01
1961 Chickens, oral 8.5 over 10 Eggs <0.04
1 Waiting period Nil unless otherwise indicated
2 Waiting period 4 hours and 5 days
3 Waiting period 6 hours
4 Waiting period 1 day and 7 days
Uses in the protection of stored products
Grain, meals and flour
Many of the published and unpublished reports available on the rates
of loss of dichlorvos from grain, meals and flour are not reliable,
because the investigators have used unsatisfactory methods to recover
any residues of dichlorvos from the treated grain and cereal products
(see comments under Methods of Residue Analysis).
Residues of dichlorvos in flour resulting from the use of resin strips
in a flour mill have been investigated (Somme, 1967). Strips were hung
at the rate of 1/1000 feet3, and samples of flour made from grain
stored in the mill were taken 4 days and 2´ weeks after production had
rebegun. Analysis showed that residues of 0.04 ppm and 0.06 ppm
dichlorvos were present in the flour and that bran samples averaged
0.09 ppm dichlorvos. In another trial (Shell, 1966a) a "Vapona" strip
was hung inside a covered flour bin of 600 ft3 total capacity. A
sample of flour drawn from a conveyer belt taking flour from the
bottom of the bin one week and two weeks after fitting the strip in a
nearly full bin was found to have a dichlorvos residue of 0.03 ppm.
Samples taken after the third week and after the bin had been refilled
with flour, at the end of the fifth week, contained residues no
greater than the limits of detection, Wheatings exposed at a rate of
one "Vapona" strip per bin of 1150 feet3 for ten hours to six weeks
after the strips had been hung also did not contain residues in the
surface layer above the limit of detection (ca. 0.02 ppm), (Shell,
1965a).
Dichlorvos has been successfully used to control Oryzaephilus
surinamensis and Sitophilus granarius (Green and Wilkin, 1968).
The insecticide was sprayed into the air stream from a motorized
knapsack sprayer through to a perforated lance which was inserted into
the grain. Dichlorvos residues were higher on wheat than on barley and
greatest in places where dust and frass had accumulated. When
dichlorvos was injected at 20 ppm, the highest residue found after one
day was 30 ppm in a dusty pocket of wheat, and this fell to 5.3 ppm
after six days.
The range of residues reported for barley were 5.8-24.0 ppm after one
day, falling to 0.9-1.4 ppm after six days. However, the method of
analysis may not have extracted all the dichlorvos from the grain (See
Methods of Residue Analysis).
In a laboratory study Kirkpatrick et al., (1968) demonstrated that
dichlorvos residues of 3.8-8.0 ppm decreased 89 percent and 76
percent, respectively, in 28 days.
An unpublished study was conducted by USDA Plant Pest Control (Padget,
1968). Wheat grain was treated with 4, 6 and 8 ppm of dichlorvos
formulated from a 25% dichlorvos emulsifiable concentrate. Residues on
the wheat were reported to be less than 2 ppm by the third day after
application, and by 14 days all dichlorvos residues were <0.5 ppm.
Details of the analytical method used were not given.
Rice
Shell Chemical Company (1969) conducted an extensive study on rough
rice and rice products treated for rice weevil control. Rough rice,
stored as 45 lb lots were each sprayed with 20 ml of a solution
containing 1.5 percent, 1 percent and 0.5 percent dichlorvos prepared
from a 23.5% E.C. After treatment, the rough rice was mill processed
and the products (brown rice, rice bran, milled rice and rice hulls)
were collected. Residue samples were taken 6 hours, 1, 5, 10, 20 and
30 days after application. Dichlorvos residues in the rough rice one
day after treatment were 7.3, 4.6 and 1.6 ppm for the 1.5 percent, 1
percent and 0.5 percent applications, respectively. After 30 days,
residues for all three treatment rates had dissipated to less than 1.0
ppm. Residues in brown rice and milled rice, after 30 days, were
<0.05 ppm for all treatment rates. Rice bran treated at the highest
rate contained 0.4 ppm and rice hulls 3 ppm dichlorvos after 30 days.
Soybeans
Soybeans stored in open bins were exposed to resin strips for
intervals ranging from 13 to 126 days (Shell, 1970c). Samples were
taken through depths of 0-2" and 2-4" from the surface and the
residues observed are summarized in Table IV.
TABLE IV
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
13 .20 - .06 -
28 .06 - .03 -
42 .11 .07 .08 .03
57 - .08 - .03
70 .07 .08 .04 .03
84 .04 .04 .04 .03
TABLE IV (cont'd)
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
97 .04 .075 .04 .06
111 .05 .04 .04 .04
125 .04 .04 .04 .04
Temperature - 65-96°F
Rel. Humidity - 48-76 percent
A test conducted by USDA, Stored Products Insect Research and
Development Laboratory (Padget, 1969), demonstrated that dichlorvos
residues on soybeans decrease rapidly. Initial residues of 5 ppm
decreased rapidly to 0.5 ppm or less after one week of storage at
80°F. Only trace amounts (0.1 ppm or less) of dichlorvos were found on
the soybeans after the second week of storage.
Coffee beans
Residues in bagged coffee beans were examined (Shell, 1965a) following
storage in a warehouse using resin strips at either one per 1000 cubic
feet or 1 per 2000 cubic feet. Some natural ventilation was allowed
for 9 hours daily.
Samples taken after 15 weeks storage from the surface and interior of
the bags did not contain detectable residues (limit 0.01 ppm), but one
sample exposed at the rate of one strip per 2000 cubic feet did
contain residues of 0.02 ppm, just above the detection limit.
Cocoa beans
In a trial with stacks of bagged cocoa beans carried out in the same
warehouse and under similar conditions (Shell, 1965a) as the above
coffee, residues in a bulked sample of cocoa from the top of the stack
reached 0.04 ppm, although none were detected in comparable samples
from the side of the stack. In a further trial (Shell, 1965a) with
another stored supply of cocoa beans, detectable residues (0.01 ppm)
were not found after 14 weeks exposure. Another trial (Shell, 1965b)
was undertaken in which sacks of cocoa beans were exposed for three
months. Surface samples taken from the top sacks were found to have a
maximum content of 0.04 ppm, whilst sub-surface samples and samples
from the middle of the sack did not contain detectable residues. In a
further trial in which exposure was 8 weeks, the maximum residue found
in surface samples from the top sacks was only 0.02 ppm. This was
mainly in the husks (0.03 ppm); the kernels contained only 0.01 ppm.
In a series of trials in Switzerland (Ciba), dichlorvos was applied to
cocoa beans in silos at rates ranging from 10 ppm through 100 ppm to
500 ppm. After three weeks storage at 15°C, the residues had fallen to
<0.1, 13 and 50 ppm, respectively, on the raw beans. When beans were
then roasted, the residues fell to <0.1, 0.2 and 0.5 ppm,
respectively. In the samples treated at 500 ppm, most of the residue
was in the mash cake with a minimum in the cocoa butter. Further
trials (Ciba) showed that the residues in cocoa beans treated at the
rate of 12 ppm with dichlorvos emulsion fell from 0.8 to 0.09 ppm
during 28 days in storage at 20°C.
Groundnuts
Sacks of bagged groundnuts (Shell, 1966b) were exposed for 82 days in
a warehouse at the recommended rate of one strip/1000 cubic feet. One
residue sample taken from the surface (2 cm) layer of the top sack was
found to contain 0.10 ppm dichlorvos, but two other similar samples
were found to contain only 0.01 ppm dichlorvos. One sample from the
middle of the top sacks contained 0.03 ppm, but in two further samples
no residues were found.
Residues arising from the exposure of foods in processing plants
Plants producing foods not processed further after exposure
In a cheese factory, in Minnesota, strips were placed throughout the
milk receiving and processing areas (Shell, 1964). A monitoring
experiment showed that the finished Cheddar cheese did not contain
detectable dichlorvos and DCA residues (i.e. below 0.03 ppm and 0.01
ppm, respectively). Similar trials in butter and ice cream factories
(Shell, 1965c) showed negligible residues of dichlorvos and DCA in
both products.
In tests carried out in cooperation with the German Public Health
Service in a cheese factory, Lindenberg, Edam and Danish Tilsit cheese
(each containing 45% fat) were exposed for four hours. Cheese samples
were cut into 20 mm thick slices following normal practice, and
exposed for a period of 30 to 60 minutes. Residues, which were
determined in the outermost 1 mm layers, were not detectable (Anon.,
1966).
Strips were hung in a cheese aging room at the rate of one strip/1000
cubic feet. Samples of cheese taken after six and ten weeks of
exposure showed residues of <0.04 to 0.10 ppm (Shell, 1965a). In the
case of milk (Shell, 1965d), whole milk was exposed in open milk
churns for periods of 24 and 48 hours in rooms where fresh strips were
installed at the rate of one strip/1000 cubic feet. None of the
representative samples taken contained measurable amounts of
dichlorvos (detection limit 0.02 ppm).
In a study in Kentucky (Shell, 1965d), strips were installed in a farm
dairy at the rate of one strip/1000 cubic feet. The air was humid and
the temperature about 75°F. The milk samples were taken after being
passed through an open coil cooler during the period 0-14 days after
the strips were hung. Residues of dichlorvos ranged from the limits of
detection to 0.04 ppm.
Plants handling foods which are processed after exposure
Fresh strips were suspended in areas of a plant where grading and
filleting of fish was carried out, and after two days exposure,
residues in the prepared product were not detected (detection limit
0.05 ppm) (Shell, 1965e).
Exposure tests were also carried out in a slaughterhouse (Shell,
1965c). The various products, beef, liver, T-bone steak, hamburger,
beef tongue and heart, pork liver, pork sausage, pork chops, pork
tongue and heart were exposed to dichlorvos resin strips during the
time normally required for processing. None of them showed any
dichlorvos or DCA residues; detection limit was 0.01 ppm. In this
test, a sample of bacon showed a residue of 1.4 ppm dichlorvos; DCA
was not found (detection limit 0.01 ppm). It was subsequently found,
however, that this particular sample had been returned to the cooling
room and was exposed for several days longer than normal. Further,
data cited later (Shell, 1966c) show that residues of dichlorvos are
removed upon cooking; specifically, bacon is cited as an example.
An experiment on similar lines was also conducted in a sausage factory
where strips were installed throughout the processing areas (Anon,
1966). No residues were detected in either meat or finished sausages.
FATE OF RESIDUES
In animals
Page (1970) has reviewed the extensive literature on the metabolism of
dichlorvos and in the same paper has reported on extensive trials
designed to show the metabolic fate and tissue residues of dichlorvos
following oral administration. The results of this work and earlier
studies are summarized in the metabolic chart given under "Biochemical
aspects" (Fig. 1).
A series of experiments have been conducted recently in pigs to
provide further information on the metabolic fate of the dichlorvos
portion of the dichlorvos molecule. In a preliminary study, 1-14C
labelled dichlorvos was infused into the isolated duodenal loop of
anaesthetized male pigs for four hours at the rate of 1 mg/kg
body-weight/hour. Urine, bile and peripheral and portal blood were
collected hourly and analysed for dichlorvos and its previously
reported mammalian metabolites. Dichlorvos, dichloroacetaldehyde and
dichloroacetic acid could not be detected in the blood. "The
concentration of 30 ppm dichlorvos in the intestinal lumen resulted in
less than 0.05 ppm dichlorvos in the blood. Neither could
dichloroacetaldehyde or dichloroacetic acid be detected in the blood.
The level of 2,2 dichloroethanol in the blood rose from 0.2 ppm after
one hour to 0.9 ppm after four hours" whereas o-demethyldichlorvos
occurred only near the limit of detection (0.03 ppm or less). In all
cases, levels of 14-carbon radioactivity in urine, bile and tissue
were higher than the level of 2,2-dichloroethanol, thus in urine the
respective figures were 17 ppm compared to 3 ppm; in bile 12 and 0.5;
in kidney 21 and 1.2; in liver 17 and 0.9 and in muscle 2.2 and 0.6
ppm. The figures indicated that 2,2-dichloroethanol or its conjugates
are not the end products of the metabolism. In other experiments, pigs
were given sustained release pellets of 14C- or 36Cl-labelled
dichlorvos at an oral dose of 40 mg/kg body-weight, and excreta and
tissue were analysed after various intervals. A similar situation to
that found in the infusion experiment occurred. It was concluded that
there was a rapid dechlorination of dichloroacetaldehyde and that the
radioactive carbon enters the metabolic pool possibly via glyoxal
(which compound has been found in in vitro but not in in vivo
studies). No retention in tissue of any chlorine containing organic
compound has been found, and the end product of the chlorine fragment
appears to be chloride ion (Page, 1970).
All the data support the conclusion that when administered orally over
a period of weeks, dichlorvos does not result in detectable toxic
residues in tissues of the parent compound or product-related
metabolites. The results of some of the published trials are
summarized in Table III.
In plants
New data have become available on the chemical degradation of
dichlorvos residues on plants. Careful experiments with various plant
species (cotton, maize, peas, beans) have demonstrated that dichlorvos
is rapidly lost from leaf surfaces by volatilization and by hydrolysis
and, disregarding the fraction of insecticide lost by volatilization
during the first few minutes after application, the half-life of the
compound under laboratory conditions is of the order of a few hours. A
small percentage (0.5-5 percent) of the dichlorvos deposited, appears
to penetrate into the waxy layers of leaf and fruit cuticle where it
persists longer. Dichlorvos taken up by plants from aqueous solutions
by the root system and translocated into aerial parts is less rapidly
lost than insecticide applied to leaf and fruit surfaces, but even
under these conditions the half-life of the compound is no more than
12 hours.
Various measurements have confirmed the rapid disappearance of
dichlorvos from vegetable and fruit crops under practical conditions
(see Table 1). Residues in vegetables were below 0.1 ppm two days
after application and were no longer detectable on the third day.
Dichlorvos was somewhat more persistent in fruits, but the residues
are below 0.1 ppm three days after application.
In vegetable crops or mushrooms grown under cover or in greenhouses,
losses of dichlorvos from leaf and fruit surfaces by volatilization
are less rapid than outdoors. However, on all crops analysed so far,
including such broad leaf vegetables as lettuce, dichlorvos residues
have been below 0.5 ppm two days after treatment and below 0.3 ppm
after three days.
Casida et al. (1962) used 32P-labelling to study the fate of
dichlorvos residues on maize, cotton and peas; a 0.1 percent aqueous
solution of dichlorvos was applied uniformly to the upper leaf
surfaces of each plant. It was found that about half of the dose
volatilized within five minutes. An additional 45 percent was absorbed
within 20 minutes, so that only 5 percent remained on the surface 20
minutes after application.
The surface residue was nonhydrolysed dichlorvos while 70-80 percent
of the absorbed material was organosoluble and was presumably
nonhydrolysed dichlorvos. However, hydrolysis proceeded rapidly.
Ignoring the dichlorvos lost by volatilization during the first five
minutes after treatment, the half-life of the dichlorvos was 1.2 hours
with loss primarily occurring by hydrolysis. In addition to the
volatilization of the dichlorvos, which resulted in the total
dichlorvos equivalent to dropping from 100 per leaf at zero time to 35
per leaf at 30 minutes, the hydrolysis products were also lost from
the treated leaves. Only 14 units remained after 24 hours and 7 units
after 72 hours, despite the fact that no dichlorvos or other
organosoluble derivatives were detected after eight hours.
The same authors carried out experiments with radio-labelled
dichlorvos and showed that, when solutions of the insecticide were
absorbed by the roots of plants, 77 percent of the absorbed dichlorvos
appeared as hydrolysis products in peas and 33 percent in maize and
cotton. Once the plants were removed from the insecticide source, the
hydrolysis of the absorbed dichlorvos followed first order reaction
kinetics, with half-life values for both maize and cotton of 9 hours
and for peas for three hours. Following systemic uptake through the
roots of plants, dichlorvos is hydrolysed rapidly, but the products of
hydrolysis are not lost from the plant during a 40 hour
post-absorption period. Bull and Ridgeway (1969) using 32P labelled
dichlorvos showed that 54 percent of the amount applied was lost
within one hour by volatilization from the treated leaves. The major
metabolite of dichlorvos was dimethyl phosphate; only minor amounts of
o-desmethyldichlorvos were detected.
Casida et al. (1962) carried out studies with mice on the acute
toxicity of dichlorvos and potential hydrolysis products and showed
relatively high toxicity associated only with the parent compound.
In storage and processing
Dichlorvos is readily lost by evaporation from stored products,
including raw grain and cereal products. Except at very low
temperatures, it appears likely that dichlorvos is also hydrolysed to
biologically inactive metabolites.
A study (Shell, 1970d) using a number of materials with abnormally
high dichlorvos residues showed that residues can be almost completely
removed by cooking. Rice containing 4.5 ppm dichlorvos has been shown
to lose 90% of the residues on boiling from 20 to 30 minutes. When
rice containing 19 ppm was similarly treated, 98% of the residue was
destroyed. Samples of flour containing dichlorvos residues in the
range 4.5 to 14 ppm have been found to lose about 90% of the residue
on baking for 10 to 12 minutes at 230°C in the preparation of
biscuits. Boiling the flour with water for 2 minutes, as in the
preparation of gravy, has been shown to decrease residue levels by
97%. Residues in cocoa have been shown to be lost during the
manufacture of cocoa butter. No dichlorvos was found in the fat, the
nibs, liquor or mash cake prepared from beans containing 2.9 ppm
dichlorvos.
Residues in food moving in commerce
Two shiploads of wheat were treated with dichlorvos emulsion
immediately prior to shipment from Australia. The dichlorvos was
applied at a nominal concentration of 6 ppm (found to be 4.6 -5.5
ppm). Upon arrival in the Netherlands, the grain was thoroughly
sampled and all samples were found to contain residues below 2 ppm
(range 0.68-1.59 ppm - mean 0.96 ppm). A major portion of each
consignment was trans-shipped to the United Kingdom,where it was
sampled for analysis on arrival. All samples revealed dichlorvos
residues, but at levels lower than those detected in the Netherlands,
with a range of 0.4-1.3 ppm and a mean of 0.68 ppm. From the results
of analysis prior to loading and at the end of a 7-week voyage, it was
calculated that the half-life of the dichlorvos residues was between
20 and 23 days.
Before the general release of dichlorvos resin strips, many
experiments were carried out to simulate the conditions that would be
encountered in practical usage. Subsequent data which related to food
exposed in shops, restaurants and homes under various practical
conditions where the dichlorvos fumigant strips were used according to
directions, broadly show that results from the simulated experiments
overestimated the residue levels.
Surveys have been carried out in the United Kingdom (17 shops) and in
France (20 shops) to establish the residue level of dichlorvos in
unwrapped foods bought by the public from shops using the strips at
the recommended rate under normal practical conditions. The residues
found are shown in Tables V and VI.
The somewhat higher results obtained in some of the commodities in the
French survey may have been due to the common practice there of
closing the shops completely during the heat of the afternoon.
TABLE V
Residues of dichlorvos (ppm) in food purchased from shops in France
Sampling time : days after hanging strips
7 42 70
Commodity Range Mean Range Mean Range Mean
Bread <0.05-0.10 <0.05 <0.05 <0.05 <0.05-0.06 <0.05
Cakes <0.05-0.24 0.08 <0.05-0.30 0.10 <0.05-0.17 0.07
Apples <0.05-0.14 0.06 <0.05-0.32 0.07 <0.05-0.30 0.06
Lettuce <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Tomatoes <0.05-0.08 <0.05 <0.05 <0.05 <0.05 0.05
Ham <0.05-0.08 0.06 <0.05-0.28 0.06 <0.05-0.09 0.05
Cheese
(cow) <0.05-0.08 0.06 <0.05-0.16 0.06 <0.05-0.15 0.06
Cheese
(goat) <0.05-0.35 0.13 <0.05-0.25 0.08 <0.05-0.30 0.08
A number of surveys have been conducted in the U.S.A. on the residue
levels in foods which had been exposed to resin strips in restaurants.
In the first of two studies conducted in restaurants near Cincinnati,
Ohio, typical restaurant meals were subjected to an exaggerated
exposure of 24 hours to 5-10 day old strips. In a second study, meals
were exposed for 22 hours to strips which were initially 51 days old.
Under the conditions of the first survey, residues were only 0.05-0.15
ppm of apparent dichlorvos: under the second survey, no residues (less
than 0.04) were found. In another investigation carried out in four
different restaurants in New York City under normal operating
conditions, ready to eat meals were sampled as composite samples.
Whole meals were sampled at various stages in the life of the strips,
ranging from 24 hours to 4 weeks. The meals consisted of various
mixtures of meat or fish and vegetables and varied widely, in
composition. The following residues were found:
Restaurant No. of meals sampled Residue range,ppm
A 5 0.04 -0.10
B 5 0.02 -0.11
C 9 0.03 -0.16
D 5 0.03 -0.08
To obtain information on food residues arising from the typical
practice of hanging strips in kitchens, surveys were carried out in
homes in the U.K. and France. Food samples were made up of whole food
and beverage intakes for one day. Each home was sampled the day before
the strips were hung and one week and six weeks later. In the U.K.
study, no residues exceeded 0.09 ppm, and the mean of all samples
taken at one week was 0.034 ppm. The samples at six weeks showed no
residues in excess of 0.05 ppm, with a mean value of 0.034 ppm.
Similar studies in 14 French homes showed no residues in excess of
0.07 ppm, and the mean of all samples taken at one week was 0.024 ppm.
The six week samples showed no residues in excess of 0.03 ppm.
METHODS OF RESIDUE ANALYSIS
Cholinesterase inhibition
A common technique which has been used for routine residue
determinations in commodities of known treatment history is based on
cholinesterase inhibition. Human and animal plasma cholinesterases are
considerably more sensitive to dichlorvos than erythrocyte
cholinesterases, and are therefore particularly suitable for residue
measurements. Out of date stocks of human blood plasma or horse serum
are satisfactory sources of enzyme.
Extraction and clean-up prior to the cholinesterase assay are normally
fairly simple, involving maceration of the material with a solvent of
medium polarity (chloroform, methylene chloride), filtration and
careful evaporation of the organic phase in the presence of water.
More elaborate procedures may be required for plant materials with a
high fat content, each as cocoa beans or groundnuts, and for certain
animal products such as milk, cheese and butter. Steam distillation of
dichlorvos prior to the enzyme assay has been suggested, not only for
removing interference,but also for increasing the specificity of the
residue method. For the enzyme assay proper, any of the common
techniques for measuring cholinesterase activity may be used. The
sensitivity of these methods is 0.05 to 0.1 ppm. Cholinesterase
residue analysis for dichlorvos has recently been automated by using
Technicon Auto-Analyser facilities.
TABLE VI
Residues of dichlorvos (ppm) in food purchased from shope in the UK1
Sampling time : days after hanging strips
No. of No. of 2 28 70
Commodity samples shops
Range Mean2 Range Mean Range Mean
Cooked meat 35 7 <0.05-0.13 <0.05 <0.05-0.10 <0.05 <0.05-0.09 <0.05
Cheese 35 7 <0.05-0.07 <0.05 <0.05-0.12 0.05 <0.05-0.12 <0.05
Apples 49 10 <0.05-0.26 0.08 <0.05-0.54 0.14 <0.05-0.19 0.06
Tomatoes 33 11 All <0.05 <0.05 All <0.05 <0.05 <0.05-0.05 <0.05
Lettuce 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Broad 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Cakes 19 7 <0.05-0.07 <0.05 <0.05-0.05 <0.05 <0.05-0.05 <0.05
1 No residues of DCA (0.03 ppm) were found in any of the samples
2 Mean values are geometric means and are calculated by assuming that samples containing less than the
detectable level (<0.05 ppm) in fact contain <0.025 ppm
Such automated methods permit rapid routine measurements of large
series of samples.
Since other organophosphorus insecticides or carbamates can interfere,
the cholinesterase inhibition method is not suitable for detecting and
measuring dichlorvos in samples of unknown or ill-defined treatment
history. Both thin-layer and paper chromatographic procedures are
available for separating dichlorvos from other
cholinesterase-inhibiting pesticides. In these methods, the
cholinesterase inhibitors are normally revealed by enzymatic spot
tests involving colour reactions of the substrate or its breakdown
products after hydrolysis. It must be remembered that dichlorvos is a
very volatile compound which, upon solvent evaporation, heating,
aeration, etc. is easily lost from vessels or chromatographic support
materials. These procedures are therefore recommended for qualitative
purposes, but great care must be taken when they are used in
quantitative analysis.
Bioassay
The vinegar fly (Drosophila melanogaster) and daphnia (Daphnia
magna) are suitable test organisms for the bioassay of dichlorvos in
plant materials. A specific test, which involves absorption barrier
chromatography and which distinguishes between dichlorvos and other
insecticides, has been described by Sun and Johnson (1963). The
sensitivity of the bioassay methods is about 0.1 ppm.
Colorimetry
Although colorimetric techniques have been developed mainly for
measuring traces in air samples or aqueous solutions, some of them can
be used for the determination of residues in food materials, provided
that the sensitivity required is not better than 0.3-1 ppm. Thus, the
color reaction of dichlorvos with alkaline resorcinol has given
satisfactory results with extracts of animal food commodities. This
method demonstrates not only the parent insecticide, but also
dichloroacetaldehyde, one of its potential hydrolysis products. Acting
on suggestions made by Hodgson and Casida (1962), Hughes (1963)
measured residues of dichlorvos and of dichloroacetaldehyde in air,
using colorimetry, following reaction with alkali and
2,4-dinitrophenylhydrazine. This method can also be used for
determining residues in food commodities. A further colorimetric
procedure, based on formation of an orange-red complex between
dichlorvos and acetone in the presence of alcoholic potassium
hydroxide, has been described by Mitsui.
Gas-liquid chromatography (GLC)
The most specific residue methods currently available rely on the
separation and detection of dichlorvos by gas chromatography. The
sensitivity of these procedures is about equal to that obtained with
the cholinesterase techniques, i.e. 0.03-0.1 ppm. Methods have been
described by El-Refai and Guiffrida who used the sodium thermionic
detector selective to phosphorus, and by Boone (1965) who used a
microcoulometric detection system. Both methods use fairly high column
temperatures, and the possibility of thermal or catalytic
decomposition of dichlorvos on certain column materials must not be
overlooked. Gas chromatographic residue measurements in industrial
laboratories are usually carried out at lower column temperatures
(150°C), and such stationary phases an phenyl diethanolamine succinate
and Reoplex 400 have given satisfactory retention times and
separations from interfering insecticides.
Ivey and Claborn (1969) have published a GLC method for the
determination of dichlorvos in milk, eggs and various body tissues of
cattle and chickens. Using a gas chromatograph equipped with a flame
photometric detector, they were able to detect 0.003 ppm of dichlorvos
in milk and 0.002 ppm in body tissues and eggs. Methylene
dichloridehexane was used to extract milk and hexane was used for fat
and chicken skin. To recover dichlorvos from muscle, blood and eggs
the authors used a preliminary extraction with acetonitrile.
Abbott at al.(1970) report the successful application of the use of
the caesium bromide tipped detector of Hartmann (1966) for the
determination of dichlorvos on a variety of foods in a total diet
survey.
Drager (1968) has published a gas chromatographic method for
determining dichlorvos residues in plants and milk. In this method,
plant material is extracted by macerating it with methanol and water.
The dichlorvos in the extract is partitioned into a mixture of ether
and petroleum ether. The residue is then transferred into ethanol, and
dichlorvos is determined by gas chromatography using the phosphorous
detector.
Minett and Belcher (1969) have developed a method for the
determination of dichlorvos residues in wheat using GLC techniques.
For the extraction of the ground wheat the authors used ethanol.
Many of the published methods and many residue reports indicate that a
variety of non-polar and polar solvents, such as methylene dichloride
were used to extract the dichlorvos residue from the food commodity.
Minett and Belcher (1969) and Elms at al. (1970) have shown that
dichlorvos cannot be recovered quantitatively from plant materials,
especially grain, by the use of solvents even as polar as methylene
dichloride. Water or water-miscible solvents, such as methanol or
ethanol, are absolutely essential for the recovery of dichlorvos
residues from plant materials.
Collaborative studies are currently being carried out in the United
Kingdom on a method of detecting dichlorvos in grain. The procedure
uses GLC with thermionic or flame photometric detection following
extraction with methanol or acetone.
APPRAISAL
Dichlorvos is used extensively for the control of insect pests of
importance in public health, in homes, warehouses, food stores and
transport, stored grain as well as insects attacking domestic animals.
It is also finding increasing use on horticultural and field crops and
is registered in over forty eight countries. Dichlorvos impregnated
resin strips are extensively used to control insect pests, especially
flies, in homes, stores and food processing establishments.
Extensive data on residues in food commodities were available from
many countries in the form of published reports end submissions from
three manufacturers. All data indicate that the level of residues
occurring in raw agricultural commodities is low and that the residue
levels decline rapidly. Due to the comparatively high vapour pressure
of dichlorvos, the applied deposit is quickly lost by volatilization,
but that portion which in absorbed into plant tissues undergoes
hydrolysis to inactive metabolites.
Dichlorvos applied to or fed to domestic animals undergoes rapid
detoxification and degradation in all species examined and is unlikely
to produce significant residues in meat, milk or eggs.
The use of dichlorvos impregnated strips for controlling insects in
warehouses, stores and shops gives rise to detectable residues in
stored or prepared foods. Residues resulting from exposures at rates
much higher than the recommended ones have been shown to decrease very
rapidly on exposure to the atmosphere. They also are readily destroyed
during the preparation of many products for consumption (e.g. by
washing or by cooking).
The proposal that a tolerance in meat and meat products was required
in view of the use of dichlorvos-resin fumigant strips in meat storage
and processing places in some countries (CCPR, 1971) was considered by
the Meeting.
Considerable data were available on the uptake of dichlorvos by many
different foods under varying conditions of storage and exaggerated
exposure to dichlorvos-resin strips, It was recognized that, under
some conditions, residues as high as 0.1 ppm could result but even in
fatty meat products the uptake was only at the surface. Residues
declined during atmospheric exposure and were completely destroyed by
cooking. Meat and meat products were included among the commodities
for which a 0.1 ppm tolerance was recommended. These products were
specially considered because of the request made at the 1970 meeting
of the Codex Committee on Pesticide Residues (CCPR, 1971).
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are for residues likely to be found in raw
commodities at harvest, in stored products shortly after treatment and
in miscellaneous prepared foods exposed during preparation and
storage. Recommendations have been made on broad categories of fruit
and vegetables, because extensive data have indicated that when
dichlorvos is applied to these crops the level of residues shortly
afterwards does not vary significantly on different varieties.
Due to the transient nature of the insecticide, residue levels will
continue to decline during shipment and storage, with a probable half
life of less than one day on fresh fruit, vegetables and miscellaneous
foods. Residues in food at time of consumption have normally been
below the limits of detection by current analytical methods.
Foodstuff Tolerance
Recommended Period on which
recommendation
based
Cocoa beans 5 ppm
Raw grain (wheat, rice,
rye, oats, barley,
maize, sorghum, etc,) 2 ppm
Coffee beans, soybeans,
lentils, peanuts (groundnuts) 2 ppm
Mushrooms 0.5 ppm 2 days
Milled products from raw
grain 0.5 ppm
Fresh vegetables
(excluding lettuce) 0.5 ppm 2 days
Tomatoes 0.5 ppm 1 day and post
harvest
Lettuce 1 ppm 2 days
Fresh fruit (apples, pears,
peaches, strawberries, etc.) 0.1 ppm 2 days
Meat of cattle, sheep,
goats, pigs and poultry 0.05 ppm
Eggs (on shell free
basis) 0.05 ppm
Milk (whole) 0.02 ppm
Miscellaneous food items
not otherwise
specified 001 ppm
As recommended in previous monographs, the content of
dichloroacetaldehyde (DCA) should be reported where known.
Furthermore, the amounts found should be added to those for dichlorvos
when assessing adherence to the tolerances recommended.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Continued observation of the effects of repeated exposure of man
to dichlorvos in order to determine if there is any qualitative
or quantitative differences between the metabolic route following
oral or inhalation intake.
2. Data from additional countries on residues in commodities moving
in international trade.
3. Analytical methods capable of recovering and determining residues
of dichlorvos in foods should be established for regulatory
purposes.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-69.
Pesticide Science, 1 (i):10-13
Anon. (1966) Communication from F.I.D. Nordrhein-Westfalen to Deutsche
Shell Chemie, Frankfurt, dated 20.1.66 (unpublished)
Anon. (1966) Communication from Hamburg Public Health Service to
Deutsche Shell Chemie, Frankfurt, dated 19.7.66 (unpublished)
Arthur, B.W. and Casida, J.E. (1957) Metabolism and selectivity of
o,o-dimethyl 2,2,2-trichloro-1-hydroxyethyl phosphonate and its acetyl
and vinyl derivates. J. Agr. Fd. Chem., 5 : 186-192
Blair, D., Hutson, D.H. and Pickering, B.A. (1970) The comparative
metabolism of (vinyl-14C) Vapona in rats after inhalation exposure
and oral ingestion of the compound. Unpublished draft summary from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Boone, G.H. (1965) Determination of Dibrom and DDVP residues in some
fruit and vegetable crops by microcoulometric gas chromatography. J.
A.O.A.C., 48 : 748-752
Boyer, A.C. (1969) I50 values for the inhibition of human and monkey
blood cholinesterases by dichlorvos. Unpublished technical information
report prepared by Shell Development Co.
Bull, D.L. and Ridgway, R.L. (1969) Metabolism of trichlorfon 1969 in
animals and plants. J. Agr. Fd. Chem., 17 : 837-841
Carson, S. (1969) Teratology studies in rabbits. Unpublished report
from Food and Drug Laboratories Inc., Maspeth, N.Y., prepared for
Shell Chemical Co.
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agr. Fd. Chem., 10 : 370-376
Cavagna, G., Locati, G. and Vigliani, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch.
Environmn. Hlth., 19 : 112-123
Cavagna, G., Locati, G. and Vigliani, E.C. (1970) Exposure of newborn
babies to 'Vapona' insecticide. Europ. J. Toxicol. 3 : 49-57
Cavagna, G. and Vigliani, E.C. (1970) Problèmes d'hygiène et de
sécurité dans l'emploi du Vapona insecticide dans les locaux
domestiques. Med. d. Lavoro, 61 : 409-423
CCPR. (1971) Report of the Fifth Session of the Codex Committee on
Pesticide Residues para. 165. Alinorm 71/24
Ciba, A.G. (1970) Basle - Submission to FAO/WHO Joint Meeting -
Dichlorvos
Drager, G. (1968) Gas chromatographic method for determining
dichlorvos residues in plants and milk. Pflanzenschutz - Nachrichten.,
21 : (3) 373-380
Elms, K.D., Kerr, J.D. and Champ, B.R. (1970) Breakdown of malathion
and dichlorvos mixture applied to wheat. In press
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FAO/WHO. (1967) Evaluation of some pesticide residues in food, FAO
PL:CP/15, WHO/Pood Add./67.32
FAO/WHO. (1968) 1967 Evaluation of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Green, A.A. and Wilkin, D.R. (1968) The control of insects in bagged
grain by the injection of dichlorvos. J. Stored Prod. Res., 5 :
11-19
Hartmann, C.H. (1966) Phosphorus detector for pesticide analysis. Bull
Environm. Contam. and Toxicol., 1 : 159-168
Hine, C.H., Beltran, S., Cavalli, R.D., Keating, J.M., Keating, W.C.
Jr. and Sample, H.G. Jr. (1966) Pre-clinical pharmacologic studies on
dichlorvos in formulation (V-12). Unpublished report from the Hine
Laboratories, Inc., submitted to Shell Chemical Co.
Hine, C.H. (1970) Arizona Vapona (DDVP) human study program.
Chromosomal analysis. Unpublished report from the Hine Laboratories,
Inc., prepared for Shell Chemical Research Development Co.
Hodgson, E. and Casida, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethyl phosphate. J. Agr. Fd.
Chem., 10 : 208-214
Hughes, G.T. (1963) Colorimetric determination of low concentrations
of 2,2-dichlorovinyl dimethyl phosphate in the atmosphere. Analyst,
88 : 318-319
Hunter, C.G. (1969) Report on initial studies of deliberate exposure
to high concentrations of dichlorvos by human subjects. Unpublished
report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Hunter, C.G. (1970) Dichlorvos: inhalational exposures with human
subjects, Part 1. Group research report TLGR. 0061.70
Ivey, M.C. and Claborn, H.V. (1969a) Kerrville, Texas, Laboratory,
Entomology Research Division, ARS, USDA. Results of research on
residues of dichlorvos in milk and body tissue of dairy cows treated
for fly control
Ivey, M.C. and Claborn, H.V. (1969b) GLC determination of dichlorvos
in milk, eggs and various body tissues of cattle and chickens. Journal
of the AOAC, 52 (6) : 1248-1251
Kimbrough, R.D. (1970) Work sited in "Summary of Vapona R
insecticide review", report of a meeting chaired by L. Golberg at
Cincinnati, 9 February 1970
Kirkpatrick, R.L., Harein, P.K. and Cooper, C.V. (1968) Laboratory
tests with dichlorvos applied as a wheat protectant against rice
weevils. J. of Econ. Entomol. 61 : 356-358
Lancaster, J.L. (1962) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lancaster, J.L. (1963) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lofroth, G. (1969a) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Paper presented at
a meeting of the Children's Cancer Research Foundation, Boston, 3
September 1969
Lofroth, G. (1969b) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Unpublished report
reproduced in part in Environmental Mutagen Society Newsletter, No, 2:
p. 21-24
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57 : 393-394
Loomis, E. and Hodel, E. (1965) (in cooperation with Shell Development
Co. Residue project from Rio Linda, California (unpublished)
Minett, W. and Belcher, R. (1969) Determination of malathion and
dichlorvos residues in wheat grains by gas-liquid chromatography. J.
Stored Prod. Res., 5 : 417-421
Modesto home trials with Vapona insecticide strips
Nelson, C.B. (1968) (in cooperation with Shell Chemical Co. Residue
project from Fillmore, California (unpublished)
Noetzel, D. (1964) (in cooperation with Shell Development Co. Residue
project from North Dakota State University (unpublished)
Padget, T.J. (1968) Evaluation of dichlorvos as a grain treatment for
protection against the white-fringed beetle. USDA Plant Pest Control
Laboratory (unpublished)
Padget, T.J. (1969) Communication on the evaluation of dichlorvos as a
grain treatment for protection against the white-fringed beetle
(unpublished)
Page, A.C. (1970) Metabolic fate and tissue residues following oral
administration of dichlorvos. Collection of unpublished reports
prepared and submitted by Shell Chemical Co.
Palut, D., Grzymala, W. and Syrowatka, T. (1969) Investigation of the
metabolism of some organophosphorus insecticides on animal model
systems. II Hydrolytic degradation in vitro (original in Polish),
Roczn. Zak. Hig. (Warsz.), 20 : 551-555
Pitts, C.W. (1961) (in cooperation with Shell Development Co. Residue
project from Kansas State University (unpublished)
Preussmann, R. (1967) Analytical determination of alkylating agents.
Zweite Konferenz über aktuelle Probleme der Tabaksforschung, Freiburg,
p. 92 (cited by Lofroth, 1969, q.v.)
Preussmann, R. (1968) Direct alkylating agents as carcinogens. Fd.
Cosm. Toxicol, 6 : 576-577
Preussmann, R., Schneider, H. and Epple, F. (1969) Untersuchungen zum
Nachweis Alkylierender Agentien. Arzneimittelforschung, 19 :
1059-1073
Roger, J.C., Chambers, H. and Casida, J.E. (1964) Nicotinic acid
analogs: effects on response of chick embryos and hens to
organophosphate toxicants. Science, 144 : 539-540
Roger, J.C., Upshall, D.G. and Casida, J.E. (1969) Structure activity
and metabolism studies on organophosphate teratogens and their
alleviating agents in developing hen eggs with special emphasis on
Bidrin. Biochem. pharmacol., 18 : 373-392
Sasinovich, L.M. (1968) The maximum permissible concentration of DDVP
in the air of the working zone. Gig. i Sanit, 33 (12) 35-39
Sax, K. and Sax, H.J. (1968) Possible mutagenic hazards of some food
additives, beverages and insecticides. Jap. J. Genetics, 43 : 89-94
Shaw, F. (1964) (in cooperation with Shell Development Co. Residue
project from University of Massachusetts (unpublished)
Shell Development Co. (1964) Modesto Project No. RES 64-111
(unpublished)
Shell Research Ltd. (1965a) Woodstock Technical Memo. 165/65
(unpublished)
Shell Research Ltd. (1965b) Woodstock Technical Memo. 159/65
(unpublished)
Shell Development Co. (1965c) Modesto Project No. RES 65-42
(unpublished)
Shell Chemical Company, New York. (1965d) Brief in Support of Vapona
Insecticide Resin Strips. Section VII : 11-12. Submitted to Panel of
U.S.P.H.S. and U.S.D.A., 29.10.65
Shell Development Company. (1965e) Modesto Project No. RES 65-36
(unpublished)
Shell Research Ltd. (1966a) Woodstock Technical Service Note 146/66
(unpublished)
Shell Research Ltd. (1966b) Woodstock Technical Service Note 178/66
(unpublished)
Shell Research Ltd. (1966c) Woodstock Technical Memo 1/66
(unpublished)
Shell Chemical Co. (1967) Petition proposing a tolerance for the food
additive (pesticide chemical) dichlorvos in or on stored food
commodities. Section D-III
Shell Research Ltd. (1968) Woodstock WKGR. 0041.68 (unpublished)
Shell Chemical Co. (1969) Princeton laboratory residue analysis report
project no. PRL 69-62, (unpublished)
Shell Development Co. (1970a) The third Arizona home study. The
quantification of DDVP residues in foods consumed by human volunteers
exposed to "No-Pest" strip insecticide. (unpublished report)
Shell Chemical Co. (1970b) Princeton laboratory residue analysis
report project no. PRL 70-12, (unpublished)
Shell Chemical Co. (1970c) Princeton laboratory residue analysis
report project no. PRL 69-26 (II) (unpublished)
Shell Research Ltd. (1970d) Interim report - Residues of Vapona in
meals prepared in domestic kitchens. Woodstock Laboratory
(unpublished)
Singh, V.K. (1964) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. (1965) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. and Rainier, R.H. (1966) Three year chronic oral toxicity
of formulated dichlorvos in swine with special reference to possible
effects upon fertility and the viability of the offspring of the
animals fed continuously on diets containing the drug. Unpublished
report from Bio/Toxicological Research Associates, Spencerville, Ohio,
prepared for Shell Chemical Co.
Somme, L. (1967) A field trial with dichlorvos vapor for the control
of Ephestia Kühniella Zell (Lepidoptera, Phycitidae) in flour mills.
Reprinted from Journal of Stored Products Research, 4 (3)
Stevenson, D.E. and Blair, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos. Shell
Research Tunstall Report No. TLGR. 0024.69
Sun, Y.P. and Johnson, E.R. (1963) A new bioassay technique with
special reference to the specific bioassay of dichlorvos insecticide.
J. Econ. Entomol., 56 : 635-641
Vigliani, E.C. (1970) Results of recent studies on Vapona. Paper
presented at the 7th Shell Industrial Doctors' Meeting, Strasbourg,
27-29 May 1970, submitted by Shell International Research Ltd.
Vogin, E.E. (1969) Teratological studies with dichlorvos in rabbits.
Unpublished report from Food and Drug Laboratories, Inc., Maspeth,
N.Y., prepared for Shell Chemical Co.
Wisconsin Alumni Research Foundation No. 8030193 (1968) (in
cooperation with Shell Chemical Co. Residue project report
(unpublished).
Wisconsin Alumni Research Foundation (1968/9) (in cooperation with
Shell Chemical Co. Residue project reports on sheep, cattle and goats
(unpublished)
Witherup, S., Caldwell, J.S. Jr. and Hull, L. (1965) The effects
exerted upon the fertility of rats, and upon the viability of their
offspring, by the introduction of Vapona R insecticide into their
diets. Unpublished report from the Kettering Laboratory Cincinnati
Young, R., Jr. (1969) Dichlorvos infusion studies. Unpublished
technical information report prepared by Shell Development Co.
 Rat
Reproduction studies in rats fed 0, 0.1, 1,0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965; see FAO/WHO, 1967).
Dichlorvos was administered at unspecified levels intraperitoneally to
female rats on day eleven after insemination. Doses of 20 mg/kg
body-weight killed the animals; 15 mg/kg produced toxic signs and
weight loss. There was no adverse effect on litter size or on the
placenta at 15 mg/kg, but three foetuses from one of four litters
available had omphaloceles, although none were observed in the six
litters from control animals (Kimbrough, 1970).
Rabbit
A total of 168 adult female rabbits were artificially inseminated with
20-30 million viable and mobile rabbit sperm, and on days 6 to 18 of
gestation were administered daily oral doses of 0, 6 or 18 mg/kg
body-weight of dichlorvos by capsule, or 31 mg/kg on days 6 to 11 of
gestation. Foetuses were recovered by Caesarean section. There was
increased incidence of in utero and neonatal toxicity in the top
dose group only, which was of doubtful significance. There were no
teratogenic effects observed, based upon skeletal examination of the
foetuses. Maternal mortality was increased in the 31 mg/kg group
(Carson, 1969).
In a separate experiment, 104 female rabbits were artificially
inseminated with rabbit sperm and administered 0, 3 or 12 mg/kg
body-weight of dichlorvos by capsule on gestation days 6 to 16
inclusive. Other rabbits were given higher doses, but they could not
tolerate levels of 24 mg/kg or higher, and no live litters were
produced from such groups. At 12 mg/kg, there was a significant
reduction in the number of implant sites and foetuses compared to the
control or lower dose group. At 3 mg/kg, the number of implant sites
and foetuses were not lower than the controls. However, one deformed
stillborn foetus was encountered from this group. Liver sections from
parents and foetuses displayed no pathological abnormalities that
could be related to dichlorvos treatment (Vogin, 1969).
Pig
Female pigs were fed dietary levels of 0, 200, 250, 288, 400, 500 or
750 ppm of dichlorvos for up to 37 months. The animals were first
mated after they had received the test diet for six months, and the
study was followed through two generations. Initially, a total of 22
females and one male were used, but after the first generation, four
more males were used in order to prevent in-breeding. The males
received 0, 288 or 400 ppm of dichlorvos in their diets. Dichlorvos
did not affect either the numbers of litters or their size and the
survival in the groups fed dichlorvos. A total of 490 piglets were
examined and none showed anatomical abnormalities (Singh and Rainier,
1966).
Special studies on mutagenicity
Secondary roots of Vicia fabia were treated with varying
concentrations of dichlorvos for one hour. Concentrations down to 1 mM
were mostly lethal, and root tips surviving this and lower
concentrations had a low mitotic index and showed c-mitotic effects,
and chromatid breaks and gaps were observed. In another experiment, a
streptomycin dependent, Sd-4, mutant of E. coli was treated in the
lug phase at cell densities of about 108 cells/ml during one hour.
The LD50 concentration for the bacteria of dichlorvos in the medium
was estimated to be between 10 and 60 mM (2200 ppm and 1.3 percent).
At concentrations of 1-4 mM, which caused no significant killing, an
increased mutation rate was observed when compared with controls and
measured as revertants to streptomycin independency (Löfroth, 1969 a,
1969 b).
Segments of Vapona-strips of various sizes were placed in an inflated
6000 ml plastic bag containing an uncovered petri-dish with
germinating onion seeds. There was no retardation of root growth or
mitotic frequency, but the number of chromosome aberrations, when
scored at anaphase, increased constantly with increasing size of the
Vapona-strip segment. The lowest concentration used was about ten
times as great as one strip in a 1000 ft3 room (Sax and Sax, 1968).
Attention has been drawn to the alkylating property of a number of
alkyl phosphates (Preussman, 1967). For this reason, possible
mutagenic effects have been looked for. Calf thymus deoxyribonucleic
acid (DNA) was incubated with varying concentrations of dichlorvos
solution. In a typical experiment with 2 percent dichlorvos incubated
for 66 hours, there was a yield of 1 percent of N-7-methylguanine from
the available guanine. Similar experiments with deoxyguanosine gave a
3-5 times higher yield of N-7-methylguanine than from DNA (Löfroth,
1970).
Eight mice were injected intraperitoneally with 10-20 mg/kg
body-weight of dichlorvos. After 23 hours, 100 cells of their bone
marrow were examined. The incidences of aberrations were not greater
than observed in four control mice (Hunter, 1970).
A human subject was exposed to 1 mg/m3 of dichlorvos for six hours.
A sample of blood cultured for leucocytes for 72 hours showed no
increase in chromosome aberrations in 100 cells compared to the
pre-exposure values (Hunter, 1970).
No chromosomal aberrations were seen after examination of blood
samples from a family of four female and one male human subjects which
had been exposed to dichlorvos strips. A control group had two male
and three female subjects (Hine, 1970).
Special studies on carcinogenicity
Rat
Rats (24) were injected subcutaneously, once weekly, with trichlorphon
(Dipterex), of which dichlorvos is a known metabolite. Despite a high
(unspecified) dosage and a latent period of almost 800 days, only two
local sarcomas developed at the site of injection. No data on controls
are given. (Preussmann, 1968). In a subsequent publication, Preussmann
et al. (1969) refer to the above experiment as a "negative result,
presumably due to the very rapid metabolism of Dipterex."
Acute toxicity
After direct infusion of dichlorvos into a peripheral vein at various
rates, pigs can survive an LD50 dose each hour for at least nine
hours (page, 1970).
The acute toxicity of dichlorvos has been compared with the
metabolites. The LD50 values in mg/kg body-weight for intraperitoneal
administration to mice are: dichlorvos, 28; o-demethyldichlorvos
sodium salt, 1500; dichloroacetaldehyde, 440; 2,2-dichloroethanol,
890; dichloroacetic acid, 250; sodium dichloroacetate, >3000;
mono- and dimethylphosphoric acid mixture, 1500; sodium
monodimethylphosphoric acid mixture, >3000 (Casida, et al., 1962).
Short-term studies
Comparative studies on inhalation toxicity
Guinea pigs have been exposed for five hours per day for five
consecutive days to a concentration of 130 mg dichlorvos/m3 of air
without visible effects on health. Rats and mice were not visibly
affected by similar exposures to 50 mg/m3. At concentrations above
50 mg/m3, the mice became visibly distressed, and prolonged exposure
to 80 mg/m3 was frequently lethal. Rats were less severely affected
than mice. In all cases, stress reactions were either apparent during
the first hours of exposure or they did not occur at all. No
cholinesterase determinations were made (Stevenson and Blair, 1969).
Three samples of dichlorvos were investigated (82.5 percent pure,
Russian produced, 40% pure, Japanese, and 100% pure from Swiss
origin); the oral LD50 for Russian and Japanese samples was similar
(87 mg/kg body-weight for mice and 65 mg/kg for rats). The Swiss
sample was less toxic: oral LD50, mice: 108 mg/kg. Rabbits were found
to be the most sensitive species (oral LD50, Russian sample: 22.5
mg/kg). Inhalation toxicity was investigated for exposure periods of
four hours, The highest concentration which could be produced (30
mg/m3) was lethal for mice and rats. The LD50 (four hours) for mice
was 13.2 mg/m3 and for rats 14.8 mg/m3. At 5 mg/m3, blood
cholinesterase activity in rat was 69 percent of pre-exposure values.
A level of 0.5 mg/m3 caused no observable effects in blood
cholinesterase. By interpolation, those concentrations of dichlorvos
in air were calculated which would after four hours exposure cause a
50 percent drop in blood cholinesterase activity in rat (I50 = 2.4
mg/m3) or a 25 percent drop (I25 = 1 mg/m3). Rats and rabbits
were exposed for 4 hours/day for a period of four months to an average
dichlorvos vapour concentration of 1 mg/m3 of air. No visible signs
of intoxication occurred. Cholinesterase activity in the serums
erythrocytes, medullary tissue and liver of rats varied throughout the
experiment within the limits of the norm or was slightly depressed.
After four months, the depression of cholinesterase activity in the
medulla averaged 8 percent in the liver, serum and erythrocytes the
depression was 30 percent 22 percent and 24 percent, respectively.
Blood sugar content, blood sugar curves after loading with galactose,
synthesis of hippuric acid after loading with sodium benzoate and
duration of Hexenal induced sleep were not affected. Rabbits exposed
under the same conditions showed 11-30 percent reduction of serum
cholinesterase activity and 28-30 percent reduction of erythrocyte
cholinesterase activity. It was concluded that 1 mg/m3 was the
threshold for rats and rabbits which caused an insignificant and
variable depression of cholinesterase. It was also the threshold for
cats. Rats exposed to an average concentration of 5.2 mg/m3 for 4
hours/day over a period of two months remained visibly healthy. After
15 days, the cholinesterase depression in the medulla was on an
average 22 percent in the serum 58 percent in the erythrocytes 22
percent. The duration of drug induced sleep was increased from 33
minutes to 57 minutes. The blood sugar level was unchanged, but the
rise of the curve after galactose loading was far higher than in the
controls. After 60 days, the blood sugar curve was affected, the
duration of Hexenal induced sleep was the same as in the controls, and
cholinesterase activity in the medulla fell by an average of 31
percent, in the liver by 35.5 percent, in serum by 52 percent and in
erythrocytes by 58 percent. At 8.2 mg/m3 for 45 days, rats lost
appetite and weight, and in some cases trembling of the head and
entire body was noted. Signs of intoxication occurred in some of the
rats and some died (Sasinovich, 1968).
OBSERVATIONS IN MAN
A total of 10 human subjects, in groups of two, ingested pellets of a
slow-release formulation of dichlorvos in daily dosages, doubling
weekly, up to 8 mg/kg body-weight/day for periods up to three weeks;
one group of two received 16 mg/kg/day for 5.5 days. Doses above 1
mg/kg were administered in two equally divided doses per day. The
majority of the clinical side effects occurred after the doses had
reached the 8 mg/kg/day level. Most of the subjective clinical
complaints referred to the gastrointestinal tract, but none of the
studies had to be terminated because of side-effects. The study with
16 mg/kg/day was terminated because of the magnitude of the red blood
cell cholinesterase activity depression. Doses of 1-8 mg/kg/day for up
to 21 days produced an average plasma cholinesterase activity
depression of 70 percent irrespective of dose or duration. Doses of 16
mg/kg/day for 5.5 days produced approximately 90 percent depression of
the plasma cholinesterase activity. Red cell cholinesterase activity
after multiple dosing of 1 mg/kg/day was 11-27 percent depressed.
Doses which reached 8 mg/kg/day caused 45-86 percent depression. At 16
mg/kg/day, depressions of 90 percent were observed. Single doses of
dichlorvos needed to be more than 4 mg/kg in order to cause measurable
red cell cholinesterase depression. At single doses of 8 mg/kg and
higher, maximal plasma cholinesterase inhibition was present and did
not increase at higher dosages. Significant and consistent erythrocyte
cholinesterase inhibition occurred after single doses of 16 mg/kg (36
percent inhibition) and 32 mg/kg (46 percent inhibition). About 50
percent of the dichlorvos was still present in the excreted pellets,
Therefore, the doses absorbed were assumed to be about one half of the
above levels (Hine et al., 1966).
In another study, 12 male subjects ingested 5 mg of dichlorvos daily
in three equal portions with their food until the plasma
cholinesterase fell to 75 percent of the pre-exposure value. The time
required averaged 12 to 20 days. No effect occurred on erythrocyte
cholinesterase. One of the subjects continued the exposure for a total
of 77 days. A questionable erythrocyte cholinesterase depression of
10-15 percent occurred during part of the exposure period, but in the
beginning and at the end of the exposure period, this activity was
normal or slightly above (Hunter, 1970).
Six human subjects were exposed to concentrations of dichlorvos in the
air, the maximum level being 52 mg/m3 for 62 minutes and the maximum
period 240 minutes to 13 mg/m3. No clinical abnormalities were
observed, subjective complaints were only related to the dryness of
the atmosphere, but the plasma cholinesterase activity was decreased;
the extent of the depression was proportional to the product of the
concentration and time of exposure. During some of these exposures, a
questionable depression of erythrocyte cholinesterase occurred, but
the extent of depression was not directly related to the product of
the concentration and time of exposure (Hunter, 1969).
In six homes in Tucson, Arizona, 18 individuals submitted to exposure
to dichlorvos strips and six others served as controls.
In order to maximize exposure, the study was made during the winter
months, and strips were hung in all rooms in the houses at the rate of
1 strip/1000 ft3. Maximum concentrations of 0.1-0.2 mg/m3 occurred
within the first week, levelling off to 0.05-0.10 mg/m3 during the
remainder of the regular exposure period of 28 days. Samples of meals
and beverages were collected at various intervals. During the exposure
period, more than 85 percent of the individual meals contained less
than 0.3 ppm dichlorvos and more than half the meals 0.1 ppm or less.
Physical examination, complete blood counts, blood chemical profiles
and plasma and erythrocyte cholinesterase measurements were performed
at various intervals. Levels of dichlorvos in the food eaten ranged
from 0.1 to 0.3 ppm. There were no clinical abnormalities evident, nor
were there any significant differences in plasma and red-blood cell
cholinesterase, blood count and blood chemical profiles between the
exposure and pre-exposure periods (Shell, 1970a).
A study was made in Italy of the effect of continuous exposure to
insecticide levels of dichlorvos by patients in hospital wards.
Control values for plasma and erythrocyte cholinesterase were
established for 250 healthy male and 100 healthy female adults not
exposed to dichlorvos. The variation in the activity of the
cholinesterases of the individuals when measured at different times,
depending on the time-interval, varied on average between 1.4 and 4.4
percent for the erythrocyte cholinesterase and on average between 5.0
and 14.2 percent for plasma cholinesterase. These were average
variations measured in ten to 20 individuals. In single individuals,
the maximum variation was 7.7 percent for erythrocyte cholinesterase
and 23.2 percent for plasma cholinesterase.
A total of 44 adult male patients with diseases other than liver
diseases were exposed to atmospheric concentrations of dichlorvos
ranging from 0.02 to 0.1 mg/m3 for periods of 3 to 29 days. Another
similar group of 22 male patients was exposed to dichlorvos
concentrations of 0.1 to 0.28 mg/m3 for periods from 3 to 16 days.
Only in the case of five patients exposed 24 hours per day at levels
above 0.1 mg/m3 was there a depression of plasma cholinesterase (35
to 72 percent) below the pre-exposure level. There was no depression
of erythrocyte cholinesterase nor were any symptoms typical of
cholinergic stimulation observed, in any patients. With six other
patients suffering from liver disease exposed in the same room, all
showed a reduction in plasma cholinesterase (25 to 66 percent of
pre-exposure values). Reduction was evident even in patients exposed
at levels below 0.1 mg/m3. These patients, because of their liver
disease, had already before exposure a considerably reduced plasma
cholinesterase activity. However, there was no depression of
erythrocyte cholinesterase nor any symptoms of poisoning, in spite of
the fact that in these patients with liver insufficiency had a 40
percent depression in plasma cholinesterase lasting for one to three
weeks after the removal from exposure. In babies, sick children and
women in labour or postpartum exposed to dichlorvos, a similar
situation to that of sick adults was seen; depression of plasma (but
not erythrocyte) cholinesterase was observed only in some cases when
the dichlorvos level was above 0.1 mg/m3. Again, no symptoms were
seen. Assuming an inhaled volume of 10 m3/24 hours in adults and 1.4
m3/24 hours in children, the authors calculated that a reduction in
plasma cholinesterase for a calculated daily inhalation of 1.7 mg of
dichlorvos could occur in adults with normal liver function and 0.2 mg
for children. They further calculated that the amount of dichlorvos
inhaled which might produce a plasma cholinesterase depression is
about the same for adults and children, viz. 0.028 to 0.030 mg/kg
body-weight/day. In patients with liver malfunction, daily inhalation
intake of 0.34 mg (equivalent to 0.005 to 0.006 mg/kg body-weight/day
was sufficient to produce plasma cholinesterase depression (Cavagna et
al., 1969; Cavagna and Vigliani, 1970).
In two nurseries 22 healthy newborn babies were kept from birth for a
period of five days for 18 hours/day in an atmosphere in which average
dichlorvos concentrations of 0.15 - 0.16 mg/m3 were maintained. Blood
cholinesterase levels were determined at birth and after five days.
There were no effects on the health of the babies nor on the activity
of their plasma or erythrocyte cholinesterase (Cavagna et al., 1970).
In a preliminary study with asthmatic patients, there appeared to be
no subjective worsening of asthma during exposure to dichlorvos at
levels of 0.1 to 0.2 mg/m3 for two consecutive days. In some cases,
an increase in airways resistance and sensitivity to acetylcholine
occurred during and after exposure, but these manifestations may be
related to other factors (e.g. poor ventilation) than to dichlorvos.
Further work in this field is reported to be in progress (Vigliani,
1970).
COMMENT
On the basis of recent and earlier studies the metabolic pattern of
dichlorvos when fed to mammals is now fairly clearly elucidated.
Evidence is presented that cleavage of the dichlorovinyl group
proceeds much more rapidly than o-demethylation. Information on the
exposure of man to varying levels of dichlorvos vapour is available,
but such studies do not include assessment as to whether the
metabolism is the same after inhalation as after oral intake. Data on
the toxicology of the metabolites dichloracetaldehyde and
dichloroethanol are considered pertinent in evaluating the toxicity of
dichlorvos. A long-term study in rats reported in the monograph from
the 1967 Joint Meeting is considered adequate. Feeding studies in rats
and rabbits did not show any indication of a teratogenic effect.
Additional information on feeding dichlorvos to man has become
available, and the no-effect level previously determined in man is
used as a basis for establishing an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In addition to its use on beef and dairy cattle and goats, sheep and
pigs, and in and around buildings which house these animals,
dichlorvos is finding increasing use on fruit and vegetable crops.
Fruit and citrus crops account for more than one third of the
agricultural use. Rice accounts for just over one quarter and field
crops, including vegetables, approximately one quarter of the
agricultural uses. There are minor uses on grapes, glasshouse crops,
mushrooms, tobacco, tea, coffee, cocoa and miscellaneous crops.
Dichlorvos is known to be registered in at least forty eight
countries.
Recently it has been introduced as an anthelmintic for pigs, poultry
and horses, being administered in the form of plastic granules for
this purpose.
Post-harvest treatments
Dichlorvos is being increasingly used for the control of insect
infestation in stored grain. For this purpose it is used as an aerosol
or as impregnated resin strips, which are applied in the overhead
spaces of storage bins. In some countries, dichlorvos preparations
have also been registered for incorporation into stored grain either
as a dust, as a direct spray or as an aqueous emulsion.
Dichlorvos aerosols, automatic dispensers, sprays and resin
impregnated strips are used in many countries for the control of
insect infestations in stores, in transport and in warehouses holding
processed food. Food processing establishments are also treated with
dichlorvos in one of the above forms.
Other uses
As defined in the monograph of the 1967 Joint Meeting (FAO/WHO 1968),
dichlorvos is used extensively in the public health field (homes,
hospitals, etc.). It is also used in aircraft during flight. Trials
are being carried out on its use by aerial application against insect
pests of forests, pastures and field crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown in the open
Table I gives typical results of the analysis for dichlorvos residues
following controlled application to various fruit and vegetable crops
(Ciba 1970).
Crops grown under cover
In glasshouses, the rate of decay of dichlorvos residues is fast, with
a half-life of approximately one day. Table II gives results of
residue trials carried out on crops growing under cover (Ciba 1970).
Livestock and poultry
Trials in the United States demonstrate that the spraying of
dichlorvos on livestock (including cattle, sheep, goats and pigs) and
poultry is unlikely to produce significant residues in meat, milk or
eggs. When dichlorvos was applied as a single pour-on application of 1
percent at the rate of 16.5 ounces per cow, no residues were
TABLE I
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Apples 0.05% ai 1 day 0.05 ChE1 0.05
2 days <0.05
Peaches 0.05% ai 0 day 0.8 ChE 0.02
1 day 0.55 " 0.02
2 days 0.35 " 0.02
Strawberries 0.05% ai 2 days 0.35 ChE 0.02
3 days 0.06 " 0.02
8 days <0.02 " 0.02
Carrots 0.025% al. 28 days <0.05 ChE 0.05
31 days <0.05 " 0.05
Cauliflower 0.05% ai 0 days 0.37 ChE 0.04
5 days <0.04 "
Brussels 0.05% ai 0 days 1.35 ChE 0.04
sprouts 5 days <0.04
Cabbage 0.05% ai 0 days 1.5 ChE 0.05
1 day 0.14
2 days 0.05
3 days <0.05
Spinach 0.05% 0 days ChE 0.05
1 day 1.6
2 days 0.06
3 days <0.05
TABLE I (Cont'd.)
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Lettuce 0.05 0 days ChE 0.05
3 days <0.05 " 0.05
French beans 0.05 0 days 0.3 ChE 0.05
<0.05
Cucumber 0.05 9 days <0.05 ChE 0.05
1 ChE = cholinesterase inhibition method
TABLE II
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Tomato 20 mg/m3 0 days 0.2 ChE1 0.04
1 day 0.06 " 0.04
2 days 0.04 " 0.04
England Lettuce 130 mg/m3 0 days 78 ChE 0.04
1 day 4.9
70 mg/m3 1 day 2.1
2 days 0.4
3 days 0.2
Germany Lettuce 30 mg/m3 0 days 3.4 B.T.2 0.04
1 day 1.8
3 days 0.3
20 mg/m3 0 days 2.4 B.T. 0.04
1 day 0.9
2 days 0.4
3 days 0.16
Switzerland Cucumber 20 mg/m3 0 days 7.3 ChE 0.04
2 days 0.15
U.S.A. Mushroom 130 mg/m3 1 day 0.1 ChE 0.04
TABLE II (Cont'd.)
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
England Mushroom 160 mg/m3 0 days 25 D.N.P.3 0.04
1 day 0.9
3 days 0.27
160 mg/m3 1 day 0.9 D.N.P. 0.04
2 days 0.05
100 mg/m3 0 days 0.3 D.N.P. 0.04
1 day 0.1
1 ChE - cholinesterase inhibition method
2 B.T. - bioassay
3 D.N.P. - 2,4-dinitrophonylhydrazine colorimetric method
detectable at the level of sensitivity of the method (0.1 ppm) in any
of the milk samples collected from 4-5 hours to 5 days, after
treatment (Singh, 1965). In another study (Noetzel, 1964), groups of
Holstein and Guernsey cattle received dermal applications, twice daily
for 28 consecutive days, of 2 ounces of 0.5 percent dichlorvos and 1
percent dichlorvos, respectively. No residues were detected in the
milk (<0.02 ppm). In a similar study (Wisconsin, 1968) in which
dichlorvos was dermally applied to lactating dairy cows at the rate of
1 ounce/cow or 3 ounces/cow of a 1 percent dichlorvos for thirty days,
milk samples from 1 through 7 days and thereafter at 3 day intervals
were analysed for residues of dichlorvos and dichloroacetaldehyde
(DCA). No residues were detectable in any of the samples (<0.05 ppm
dichlorvos, <0.01 ppm DCA).
In other extensive trials (Wisconsin, 1968/1969) Brahma cattle, goats
and sheep were sprayed 3 times to saturation (1 gallon) at 14 day
intervals with 0.025 percent dichlorvos emulsion. No residues of
dichlorvos were detectable at the limits of sensitivity in tissues or
organs (including renal, omental and subcutaneous fat, muscle, liver
and kidney) of cattle (<0.25 ppm), goats (<0.25 ppm) or sheep
(<0.05 ppm).
Ivey and Claborn (1969a) sprayed lactating dairy cows for 31
consecutive days with 2 ounces of a 1 percent dichlorvos insecticide
spray solution. Milk samples were taken at 2 hours, 1, 2, 4, 8, 16, 24
and 31 days. One day after final treatment, the cows were slaughtered
and samples of renal, omental and subcutaneous fat, muscle liver,
kidney and blood were taken. Using a highly sensitive flame
photometric method (Ivey and Claborn, 1969b) with improved clean-up
technique, no residues of dichlorvos were detected in milk (<0.003
ppm) or body tissue (<0.002 ppm).
Pigs were fed daily at the high dosage of 9 450 ppm dichlorvos for 90
days (Singh, 1964). Dichlorvos residues were not detectable (<0.05
ppm) in tissues and organs including spleen, kidney, small intestine,
lung, heart, muscle and liver 0 and 1 day after last treatment.
Analysis of meat, fat, liver and eggs derived from poultry sprayed
with 2 ounces of diluted 2 lb/gal dichlorvos insecticide E.C. (0.295
ppm grams DDVP/bird) showed residues of equal to or less than 0.03 ppm
when samples of tissue were taken 6 hours, 3, 5 and 7 days after
application. Eggs were collected 1, 2, 3, 4, 5, 6 and 7 days after
treatment (Shell, 1970b). Poultry exposed to dichlorvon in the form of
2 percent granules (2 lb/100 square feet or 4 lb/100 square feet), or
to resin strands containing 20 percent dichlorvos (1 foot of strand/1
foot of cage) caused no residues in either tissue or eggs (<0.02 ppm)
(Lancaster, 1962; Loomis and Hodel, 1965; Lancaster, 1963; Shaw, 1964;
Nelson, 1968).
TABLE III
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Singh, Cows, pour on 480 1 Milk2 <0.1
1965
Noetzel, Cows, dermal 60 28×1 Milk <0.02
1964
Wisconsin, Cows, dermal 90 30×1 Milk <0.05
1968
Wisconsin, Cows, dermal 4540 3×14 Fat, muscle <0.25
1968, 1969 Goats, dermal 4540 3×14 Fat, muscle <0.25
Sheep, dermal 4540 3×14 Fat, muscle <0.05
Ivey & Claborn, Cows, dermal 60 31×1 Milk <0.003
1969a Cows, dermal 60 31×1 Fat, muscle, <0.002
etc.
ppm
Singh, Pigs, oral 9450 90×1 All tissues <0.05
1964
g
Shell, Chickens, dermal 0.3 1 All tissues3 <0.03
1970b Chickens, dermal 0.3 1 Eggs4 <0.03
Lancaster, Chickens, vapour - 30×1 All tissues <0.2
1962
TABLE III (Cont'd.)
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Loomis, Chickens, vapour - 30×1 Eggs <0.02
1965
Nelson, Chickens, contact - 28×1 All tissues <0.03
1968 Chickens, contact - 28×1 Eggs <0.05
mg
Pitts, Chickens, oral 8.5 over 10 Meat <0.01
1961 Chickens, oral 8.5 over 10 Eggs <0.04
1 Waiting period Nil unless otherwise indicated
2 Waiting period 4 hours and 5 days
3 Waiting period 6 hours
4 Waiting period 1 day and 7 days
Uses in the protection of stored products
Grain, meals and flour
Many of the published and unpublished reports available on the rates
of loss of dichlorvos from grain, meals and flour are not reliable,
because the investigators have used unsatisfactory methods to recover
any residues of dichlorvos from the treated grain and cereal products
(see comments under Methods of Residue Analysis).
Residues of dichlorvos in flour resulting from the use of resin strips
in a flour mill have been investigated (Somme, 1967). Strips were hung
at the rate of 1/1000 feet3, and samples of flour made from grain
stored in the mill were taken 4 days and 2´ weeks after production had
rebegun. Analysis showed that residues of 0.04 ppm and 0.06 ppm
dichlorvos were present in the flour and that bran samples averaged
0.09 ppm dichlorvos. In another trial (Shell, 1966a) a "Vapona" strip
was hung inside a covered flour bin of 600 ft3 total capacity. A
sample of flour drawn from a conveyer belt taking flour from the
bottom of the bin one week and two weeks after fitting the strip in a
nearly full bin was found to have a dichlorvos residue of 0.03 ppm.
Samples taken after the third week and after the bin had been refilled
with flour, at the end of the fifth week, contained residues no
greater than the limits of detection, Wheatings exposed at a rate of
one "Vapona" strip per bin of 1150 feet3 for ten hours to six weeks
after the strips had been hung also did not contain residues in the
surface layer above the limit of detection (ca. 0.02 ppm), (Shell,
1965a).
Dichlorvos has been successfully used to control Oryzaephilus
surinamensis and Sitophilus granarius (Green and Wilkin, 1968).
The insecticide was sprayed into the air stream from a motorized
knapsack sprayer through to a perforated lance which was inserted into
the grain. Dichlorvos residues were higher on wheat than on barley and
greatest in places where dust and frass had accumulated. When
dichlorvos was injected at 20 ppm, the highest residue found after one
day was 30 ppm in a dusty pocket of wheat, and this fell to 5.3 ppm
after six days.
The range of residues reported for barley were 5.8-24.0 ppm after one
day, falling to 0.9-1.4 ppm after six days. However, the method of
analysis may not have extracted all the dichlorvos from the grain (See
Methods of Residue Analysis).
In a laboratory study Kirkpatrick et al., (1968) demonstrated that
dichlorvos residues of 3.8-8.0 ppm decreased 89 percent and 76
percent, respectively, in 28 days.
An unpublished study was conducted by USDA Plant Pest Control (Padget,
1968). Wheat grain was treated with 4, 6 and 8 ppm of dichlorvos
formulated from a 25% dichlorvos emulsifiable concentrate. Residues on
the wheat were reported to be less than 2 ppm by the third day after
application, and by 14 days all dichlorvos residues were <0.5 ppm.
Details of the analytical method used were not given.
Rice
Shell Chemical Company (1969) conducted an extensive study on rough
rice and rice products treated for rice weevil control. Rough rice,
stored as 45 lb lots were each sprayed with 20 ml of a solution
containing 1.5 percent, 1 percent and 0.5 percent dichlorvos prepared
from a 23.5% E.C. After treatment, the rough rice was mill processed
and the products (brown rice, rice bran, milled rice and rice hulls)
were collected. Residue samples were taken 6 hours, 1, 5, 10, 20 and
30 days after application. Dichlorvos residues in the rough rice one
day after treatment were 7.3, 4.6 and 1.6 ppm for the 1.5 percent, 1
percent and 0.5 percent applications, respectively. After 30 days,
residues for all three treatment rates had dissipated to less than 1.0
ppm. Residues in brown rice and milled rice, after 30 days, were
<0.05 ppm for all treatment rates. Rice bran treated at the highest
rate contained 0.4 ppm and rice hulls 3 ppm dichlorvos after 30 days.
Soybeans
Soybeans stored in open bins were exposed to resin strips for
intervals ranging from 13 to 126 days (Shell, 1970c). Samples were
taken through depths of 0-2" and 2-4" from the surface and the
residues observed are summarized in Table IV.
TABLE IV
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
13 .20 - .06 -
28 .06 - .03 -
42 .11 .07 .08 .03
57 - .08 - .03
70 .07 .08 .04 .03
84 .04 .04 .04 .03
TABLE IV (cont'd)
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
97 .04 .075 .04 .06
111 .05 .04 .04 .04
125 .04 .04 .04 .04
Temperature - 65-96°F
Rel. Humidity - 48-76 percent
A test conducted by USDA, Stored Products Insect Research and
Development Laboratory (Padget, 1969), demonstrated that dichlorvos
residues on soybeans decrease rapidly. Initial residues of 5 ppm
decreased rapidly to 0.5 ppm or less after one week of storage at
80°F. Only trace amounts (0.1 ppm or less) of dichlorvos were found on
the soybeans after the second week of storage.
Coffee beans
Residues in bagged coffee beans were examined (Shell, 1965a) following
storage in a warehouse using resin strips at either one per 1000 cubic
feet or 1 per 2000 cubic feet. Some natural ventilation was allowed
for 9 hours daily.
Samples taken after 15 weeks storage from the surface and interior of
the bags did not contain detectable residues (limit 0.01 ppm), but one
sample exposed at the rate of one strip per 2000 cubic feet did
contain residues of 0.02 ppm, just above the detection limit.
Cocoa beans
In a trial with stacks of bagged cocoa beans carried out in the same
warehouse and under similar conditions (Shell, 1965a) as the above
coffee, residues in a bulked sample of cocoa from the top of the stack
reached 0.04 ppm, although none were detected in comparable samples
from the side of the stack. In a further trial (Shell, 1965a) with
another stored supply of cocoa beans, detectable residues (0.01 ppm)
were not found after 14 weeks exposure. Another trial (Shell, 1965b)
was undertaken in which sacks of cocoa beans were exposed for three
months. Surface samples taken from the top sacks were found to have a
maximum content of 0.04 ppm, whilst sub-surface samples and samples
from the middle of the sack did not contain detectable residues. In a
further trial in which exposure was 8 weeks, the maximum residue found
in surface samples from the top sacks was only 0.02 ppm. This was
mainly in the husks (0.03 ppm); the kernels contained only 0.01 ppm.
In a series of trials in Switzerland (Ciba), dichlorvos was applied to
cocoa beans in silos at rates ranging from 10 ppm through 100 ppm to
500 ppm. After three weeks storage at 15°C, the residues had fallen to
<0.1, 13 and 50 ppm, respectively, on the raw beans. When beans were
then roasted, the residues fell to <0.1, 0.2 and 0.5 ppm,
respectively. In the samples treated at 500 ppm, most of the residue
was in the mash cake with a minimum in the cocoa butter. Further
trials (Ciba) showed that the residues in cocoa beans treated at the
rate of 12 ppm with dichlorvos emulsion fell from 0.8 to 0.09 ppm
during 28 days in storage at 20°C.
Groundnuts
Sacks of bagged groundnuts (Shell, 1966b) were exposed for 82 days in
a warehouse at the recommended rate of one strip/1000 cubic feet. One
residue sample taken from the surface (2 cm) layer of the top sack was
found to contain 0.10 ppm dichlorvos, but two other similar samples
were found to contain only 0.01 ppm dichlorvos. One sample from the
middle of the top sacks contained 0.03 ppm, but in two further samples
no residues were found.
Residues arising from the exposure of foods in processing plants
Plants producing foods not processed further after exposure
In a cheese factory, in Minnesota, strips were placed throughout the
milk receiving and processing areas (Shell, 1964). A monitoring
experiment showed that the finished Cheddar cheese did not contain
detectable dichlorvos and DCA residues (i.e. below 0.03 ppm and 0.01
ppm, respectively). Similar trials in butter and ice cream factories
(Shell, 1965c) showed negligible residues of dichlorvos and DCA in
both products.
In tests carried out in cooperation with the German Public Health
Service in a cheese factory, Lindenberg, Edam and Danish Tilsit cheese
(each containing 45% fat) were exposed for four hours. Cheese samples
were cut into 20 mm thick slices following normal practice, and
exposed for a period of 30 to 60 minutes. Residues, which were
determined in the outermost 1 mm layers, were not detectable (Anon.,
1966).
Strips were hung in a cheese aging room at the rate of one strip/1000
cubic feet. Samples of cheese taken after six and ten weeks of
exposure showed residues of <0.04 to 0.10 ppm (Shell, 1965a). In the
case of milk (Shell, 1965d), whole milk was exposed in open milk
churns for periods of 24 and 48 hours in rooms where fresh strips were
installed at the rate of one strip/1000 cubic feet. None of the
representative samples taken contained measurable amounts of
dichlorvos (detection limit 0.02 ppm).
In a study in Kentucky (Shell, 1965d), strips were installed in a farm
dairy at the rate of one strip/1000 cubic feet. The air was humid and
the temperature about 75°F. The milk samples were taken after being
passed through an open coil cooler during the period 0-14 days after
the strips were hung. Residues of dichlorvos ranged from the limits of
detection to 0.04 ppm.
Plants handling foods which are processed after exposure
Fresh strips were suspended in areas of a plant where grading and
filleting of fish was carried out, and after two days exposure,
residues in the prepared product were not detected (detection limit
0.05 ppm) (Shell, 1965e).
Exposure tests were also carried out in a slaughterhouse (Shell,
1965c). The various products, beef, liver, T-bone steak, hamburger,
beef tongue and heart, pork liver, pork sausage, pork chops, pork
tongue and heart were exposed to dichlorvos resin strips during the
time normally required for processing. None of them showed any
dichlorvos or DCA residues; detection limit was 0.01 ppm. In this
test, a sample of bacon showed a residue of 1.4 ppm dichlorvos; DCA
was not found (detection limit 0.01 ppm). It was subsequently found,
however, that this particular sample had been returned to the cooling
room and was exposed for several days longer than normal. Further,
data cited later (Shell, 1966c) show that residues of dichlorvos are
removed upon cooking; specifically, bacon is cited as an example.
An experiment on similar lines was also conducted in a sausage factory
where strips were installed throughout the processing areas (Anon,
1966). No residues were detected in either meat or finished sausages.
FATE OF RESIDUES
In animals
Page (1970) has reviewed the extensive literature on the metabolism of
dichlorvos and in the same paper has reported on extensive trials
designed to show the metabolic fate and tissue residues of dichlorvos
following oral administration. The results of this work and earlier
studies are summarized in the metabolic chart given under "Biochemical
aspects" (Fig. 1).
A series of experiments have been conducted recently in pigs to
provide further information on the metabolic fate of the dichlorvos
portion of the dichlorvos molecule. In a preliminary study, 1-14C
labelled dichlorvos was infused into the isolated duodenal loop of
anaesthetized male pigs for four hours at the rate of 1 mg/kg
body-weight/hour. Urine, bile and peripheral and portal blood were
collected hourly and analysed for dichlorvos and its previously
reported mammalian metabolites. Dichlorvos, dichloroacetaldehyde and
dichloroacetic acid could not be detected in the blood. "The
concentration of 30 ppm dichlorvos in the intestinal lumen resulted in
less than 0.05 ppm dichlorvos in the blood. Neither could
dichloroacetaldehyde or dichloroacetic acid be detected in the blood.
The level of 2,2 dichloroethanol in the blood rose from 0.2 ppm after
one hour to 0.9 ppm after four hours" whereas o-demethyldichlorvos
occurred only near the limit of detection (0.03 ppm or less). In all
cases, levels of 14-carbon radioactivity in urine, bile and tissue
were higher than the level of 2,2-dichloroethanol, thus in urine the
respective figures were 17 ppm compared to 3 ppm; in bile 12 and 0.5;
in kidney 21 and 1.2; in liver 17 and 0.9 and in muscle 2.2 and 0.6
ppm. The figures indicated that 2,2-dichloroethanol or its conjugates
are not the end products of the metabolism. In other experiments, pigs
were given sustained release pellets of 14C- or 36Cl-labelled
dichlorvos at an oral dose of 40 mg/kg body-weight, and excreta and
tissue were analysed after various intervals. A similar situation to
that found in the infusion experiment occurred. It was concluded that
there was a rapid dechlorination of dichloroacetaldehyde and that the
radioactive carbon enters the metabolic pool possibly via glyoxal
(which compound has been found in in vitro but not in in vivo
studies). No retention in tissue of any chlorine containing organic
compound has been found, and the end product of the chlorine fragment
appears to be chloride ion (Page, 1970).
All the data support the conclusion that when administered orally over
a period of weeks, dichlorvos does not result in detectable toxic
residues in tissues of the parent compound or product-related
metabolites. The results of some of the published trials are
summarized in Table III.
In plants
New data have become available on the chemical degradation of
dichlorvos residues on plants. Careful experiments with various plant
species (cotton, maize, peas, beans) have demonstrated that dichlorvos
is rapidly lost from leaf surfaces by volatilization and by hydrolysis
and, disregarding the fraction of insecticide lost by volatilization
during the first few minutes after application, the half-life of the
compound under laboratory conditions is of the order of a few hours. A
small percentage (0.5-5 percent) of the dichlorvos deposited, appears
to penetrate into the waxy layers of leaf and fruit cuticle where it
persists longer. Dichlorvos taken up by plants from aqueous solutions
by the root system and translocated into aerial parts is less rapidly
lost than insecticide applied to leaf and fruit surfaces, but even
under these conditions the half-life of the compound is no more than
12 hours.
Various measurements have confirmed the rapid disappearance of
dichlorvos from vegetable and fruit crops under practical conditions
(see Table 1). Residues in vegetables were below 0.1 ppm two days
after application and were no longer detectable on the third day.
Dichlorvos was somewhat more persistent in fruits, but the residues
are below 0.1 ppm three days after application.
In vegetable crops or mushrooms grown under cover or in greenhouses,
losses of dichlorvos from leaf and fruit surfaces by volatilization
are less rapid than outdoors. However, on all crops analysed so far,
including such broad leaf vegetables as lettuce, dichlorvos residues
have been below 0.5 ppm two days after treatment and below 0.3 ppm
after three days.
Casida et al. (1962) used 32P-labelling to study the fate of
dichlorvos residues on maize, cotton and peas; a 0.1 percent aqueous
solution of dichlorvos was applied uniformly to the upper leaf
surfaces of each plant. It was found that about half of the dose
volatilized within five minutes. An additional 45 percent was absorbed
within 20 minutes, so that only 5 percent remained on the surface 20
minutes after application.
The surface residue was nonhydrolysed dichlorvos while 70-80 percent
of the absorbed material was organosoluble and was presumably
nonhydrolysed dichlorvos. However, hydrolysis proceeded rapidly.
Ignoring the dichlorvos lost by volatilization during the first five
minutes after treatment, the half-life of the dichlorvos was 1.2 hours
with loss primarily occurring by hydrolysis. In addition to the
volatilization of the dichlorvos, which resulted in the total
dichlorvos equivalent to dropping from 100 per leaf at zero time to 35
per leaf at 30 minutes, the hydrolysis products were also lost from
the treated leaves. Only 14 units remained after 24 hours and 7 units
after 72 hours, despite the fact that no dichlorvos or other
organosoluble derivatives were detected after eight hours.
The same authors carried out experiments with radio-labelled
dichlorvos and showed that, when solutions of the insecticide were
absorbed by the roots of plants, 77 percent of the absorbed dichlorvos
appeared as hydrolysis products in peas and 33 percent in maize and
cotton. Once the plants were removed from the insecticide source, the
hydrolysis of the absorbed dichlorvos followed first order reaction
kinetics, with half-life values for both maize and cotton of 9 hours
and for peas for three hours. Following systemic uptake through the
roots of plants, dichlorvos is hydrolysed rapidly, but the products of
hydrolysis are not lost from the plant during a 40 hour
post-absorption period. Bull and Ridgeway (1969) using 32P labelled
dichlorvos showed that 54 percent of the amount applied was lost
within one hour by volatilization from the treated leaves. The major
metabolite of dichlorvos was dimethyl phosphate; only minor amounts of
o-desmethyldichlorvos were detected.
Casida et al. (1962) carried out studies with mice on the acute
toxicity of dichlorvos and potential hydrolysis products and showed
relatively high toxicity associated only with the parent compound.
In storage and processing
Dichlorvos is readily lost by evaporation from stored products,
including raw grain and cereal products. Except at very low
temperatures, it appears likely that dichlorvos is also hydrolysed to
biologically inactive metabolites.
A study (Shell, 1970d) using a number of materials with abnormally
high dichlorvos residues showed that residues can be almost completely
removed by cooking. Rice containing 4.5 ppm dichlorvos has been shown
to lose 90% of the residues on boiling from 20 to 30 minutes. When
rice containing 19 ppm was similarly treated, 98% of the residue was
destroyed. Samples of flour containing dichlorvos residues in the
range 4.5 to 14 ppm have been found to lose about 90% of the residue
on baking for 10 to 12 minutes at 230°C in the preparation of
biscuits. Boiling the flour with water for 2 minutes, as in the
preparation of gravy, has been shown to decrease residue levels by
97%. Residues in cocoa have been shown to be lost during the
manufacture of cocoa butter. No dichlorvos was found in the fat, the
nibs, liquor or mash cake prepared from beans containing 2.9 ppm
dichlorvos.
Residues in food moving in commerce
Two shiploads of wheat were treated with dichlorvos emulsion
immediately prior to shipment from Australia. The dichlorvos was
applied at a nominal concentration of 6 ppm (found to be 4.6 -5.5
ppm). Upon arrival in the Netherlands, the grain was thoroughly
sampled and all samples were found to contain residues below 2 ppm
(range 0.68-1.59 ppm - mean 0.96 ppm). A major portion of each
consignment was trans-shipped to the United Kingdom,where it was
sampled for analysis on arrival. All samples revealed dichlorvos
residues, but at levels lower than those detected in the Netherlands,
with a range of 0.4-1.3 ppm and a mean of 0.68 ppm. From the results
of analysis prior to loading and at the end of a 7-week voyage, it was
calculated that the half-life of the dichlorvos residues was between
20 and 23 days.
Before the general release of dichlorvos resin strips, many
experiments were carried out to simulate the conditions that would be
encountered in practical usage. Subsequent data which related to food
exposed in shops, restaurants and homes under various practical
conditions where the dichlorvos fumigant strips were used according to
directions, broadly show that results from the simulated experiments
overestimated the residue levels.
Surveys have been carried out in the United Kingdom (17 shops) and in
France (20 shops) to establish the residue level of dichlorvos in
unwrapped foods bought by the public from shops using the strips at
the recommended rate under normal practical conditions. The residues
found are shown in Tables V and VI.
The somewhat higher results obtained in some of the commodities in the
French survey may have been due to the common practice there of
closing the shops completely during the heat of the afternoon.
TABLE V
Residues of dichlorvos (ppm) in food purchased from shops in France
Sampling time : days after hanging strips
7 42 70
Commodity Range Mean Range Mean Range Mean
Bread <0.05-0.10 <0.05 <0.05 <0.05 <0.05-0.06 <0.05
Cakes <0.05-0.24 0.08 <0.05-0.30 0.10 <0.05-0.17 0.07
Apples <0.05-0.14 0.06 <0.05-0.32 0.07 <0.05-0.30 0.06
Lettuce <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Tomatoes <0.05-0.08 <0.05 <0.05 <0.05 <0.05 0.05
Ham <0.05-0.08 0.06 <0.05-0.28 0.06 <0.05-0.09 0.05
Cheese
(cow) <0.05-0.08 0.06 <0.05-0.16 0.06 <0.05-0.15 0.06
Cheese
(goat) <0.05-0.35 0.13 <0.05-0.25 0.08 <0.05-0.30 0.08
A number of surveys have been conducted in the U.S.A. on the residue
levels in foods which had been exposed to resin strips in restaurants.
In the first of two studies conducted in restaurants near Cincinnati,
Ohio, typical restaurant meals were subjected to an exaggerated
exposure of 24 hours to 5-10 day old strips. In a second study, meals
were exposed for 22 hours to strips which were initially 51 days old.
Under the conditions of the first survey, residues were only 0.05-0.15
ppm of apparent dichlorvos: under the second survey, no residues (less
than 0.04) were found. In another investigation carried out in four
different restaurants in New York City under normal operating
conditions, ready to eat meals were sampled as composite samples.
Whole meals were sampled at various stages in the life of the strips,
ranging from 24 hours to 4 weeks. The meals consisted of various
mixtures of meat or fish and vegetables and varied widely, in
composition. The following residues were found:
Restaurant No. of meals sampled Residue range,ppm
A 5 0.04 -0.10
B 5 0.02 -0.11
C 9 0.03 -0.16
D 5 0.03 -0.08
To obtain information on food residues arising from the typical
practice of hanging strips in kitchens, surveys were carried out in
homes in the U.K. and France. Food samples were made up of whole food
and beverage intakes for one day. Each home was sampled the day before
the strips were hung and one week and six weeks later. In the U.K.
study, no residues exceeded 0.09 ppm, and the mean of all samples
taken at one week was 0.034 ppm. The samples at six weeks showed no
residues in excess of 0.05 ppm, with a mean value of 0.034 ppm.
Similar studies in 14 French homes showed no residues in excess of
0.07 ppm, and the mean of all samples taken at one week was 0.024 ppm.
The six week samples showed no residues in excess of 0.03 ppm.
METHODS OF RESIDUE ANALYSIS
Cholinesterase inhibition
A common technique which has been used for routine residue
determinations in commodities of known treatment history is based on
cholinesterase inhibition. Human and animal plasma cholinesterases are
considerably more sensitive to dichlorvos than erythrocyte
cholinesterases, and are therefore particularly suitable for residue
measurements. Out of date stocks of human blood plasma or horse serum
are satisfactory sources of enzyme.
Extraction and clean-up prior to the cholinesterase assay are normally
fairly simple, involving maceration of the material with a solvent of
medium polarity (chloroform, methylene chloride), filtration and
careful evaporation of the organic phase in the presence of water.
More elaborate procedures may be required for plant materials with a
high fat content, each as cocoa beans or groundnuts, and for certain
animal products such as milk, cheese and butter. Steam distillation of
dichlorvos prior to the enzyme assay has been suggested, not only for
removing interference,but also for increasing the specificity of the
residue method. For the enzyme assay proper, any of the common
techniques for measuring cholinesterase activity may be used. The
sensitivity of these methods is 0.05 to 0.1 ppm. Cholinesterase
residue analysis for dichlorvos has recently been automated by using
Technicon Auto-Analyser facilities.
TABLE VI
Residues of dichlorvos (ppm) in food purchased from shope in the UK1
Sampling time : days after hanging strips
No. of No. of 2 28 70
Commodity samples shops
Range Mean2 Range Mean Range Mean
Cooked meat 35 7 <0.05-0.13 <0.05 <0.05-0.10 <0.05 <0.05-0.09 <0.05
Cheese 35 7 <0.05-0.07 <0.05 <0.05-0.12 0.05 <0.05-0.12 <0.05
Apples 49 10 <0.05-0.26 0.08 <0.05-0.54 0.14 <0.05-0.19 0.06
Tomatoes 33 11 All <0.05 <0.05 All <0.05 <0.05 <0.05-0.05 <0.05
Lettuce 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Broad 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Cakes 19 7 <0.05-0.07 <0.05 <0.05-0.05 <0.05 <0.05-0.05 <0.05
1 No residues of DCA (0.03 ppm) were found in any of the samples
2 Mean values are geometric means and are calculated by assuming that samples containing less than the
detectable level (<0.05 ppm) in fact contain <0.025 ppm
Such automated methods permit rapid routine measurements of large
series of samples.
Since other organophosphorus insecticides or carbamates can interfere,
the cholinesterase inhibition method is not suitable for detecting and
measuring dichlorvos in samples of unknown or ill-defined treatment
history. Both thin-layer and paper chromatographic procedures are
available for separating dichlorvos from other
cholinesterase-inhibiting pesticides. In these methods, the
cholinesterase inhibitors are normally revealed by enzymatic spot
tests involving colour reactions of the substrate or its breakdown
products after hydrolysis. It must be remembered that dichlorvos is a
very volatile compound which, upon solvent evaporation, heating,
aeration, etc. is easily lost from vessels or chromatographic support
materials. These procedures are therefore recommended for qualitative
purposes, but great care must be taken when they are used in
quantitative analysis.
Bioassay
The vinegar fly (Drosophila melanogaster) and daphnia (Daphnia
magna) are suitable test organisms for the bioassay of dichlorvos in
plant materials. A specific test, which involves absorption barrier
chromatography and which distinguishes between dichlorvos and other
insecticides, has been described by Sun and Johnson (1963). The
sensitivity of the bioassay methods is about 0.1 ppm.
Colorimetry
Although colorimetric techniques have been developed mainly for
measuring traces in air samples or aqueous solutions, some of them can
be used for the determination of residues in food materials, provided
that the sensitivity required is not better than 0.3-1 ppm. Thus, the
color reaction of dichlorvos with alkaline resorcinol has given
satisfactory results with extracts of animal food commodities. This
method demonstrates not only the parent insecticide, but also
dichloroacetaldehyde, one of its potential hydrolysis products. Acting
on suggestions made by Hodgson and Casida (1962), Hughes (1963)
measured residues of dichlorvos and of dichloroacetaldehyde in air,
using colorimetry, following reaction with alkali and
2,4-dinitrophenylhydrazine. This method can also be used for
determining residues in food commodities. A further colorimetric
procedure, based on formation of an orange-red complex between
dichlorvos and acetone in the presence of alcoholic potassium
hydroxide, has been described by Mitsui.
Gas-liquid chromatography (GLC)
The most specific residue methods currently available rely on the
separation and detection of dichlorvos by gas chromatography. The
sensitivity of these procedures is about equal to that obtained with
the cholinesterase techniques, i.e. 0.03-0.1 ppm. Methods have been
described by El-Refai and Guiffrida who used the sodium thermionic
detector selective to phosphorus, and by Boone (1965) who used a
microcoulometric detection system. Both methods use fairly high column
temperatures, and the possibility of thermal or catalytic
decomposition of dichlorvos on certain column materials must not be
overlooked. Gas chromatographic residue measurements in industrial
laboratories are usually carried out at lower column temperatures
(150°C), and such stationary phases an phenyl diethanolamine succinate
and Reoplex 400 have given satisfactory retention times and
separations from interfering insecticides.
Ivey and Claborn (1969) have published a GLC method for the
determination of dichlorvos in milk, eggs and various body tissues of
cattle and chickens. Using a gas chromatograph equipped with a flame
photometric detector, they were able to detect 0.003 ppm of dichlorvos
in milk and 0.002 ppm in body tissues and eggs. Methylene
dichloridehexane was used to extract milk and hexane was used for fat
and chicken skin. To recover dichlorvos from muscle, blood and eggs
the authors used a preliminary extraction with acetonitrile.
Abbott at al.(1970) report the successful application of the use of
the caesium bromide tipped detector of Hartmann (1966) for the
determination of dichlorvos on a variety of foods in a total diet
survey.
Drager (1968) has published a gas chromatographic method for
determining dichlorvos residues in plants and milk. In this method,
plant material is extracted by macerating it with methanol and water.
The dichlorvos in the extract is partitioned into a mixture of ether
and petroleum ether. The residue is then transferred into ethanol, and
dichlorvos is determined by gas chromatography using the phosphorous
detector.
Minett and Belcher (1969) have developed a method for the
determination of dichlorvos residues in wheat using GLC techniques.
For the extraction of the ground wheat the authors used ethanol.
Many of the published methods and many residue reports indicate that a
variety of non-polar and polar solvents, such as methylene dichloride
were used to extract the dichlorvos residue from the food commodity.
Minett and Belcher (1969) and Elms at al. (1970) have shown that
dichlorvos cannot be recovered quantitatively from plant materials,
especially grain, by the use of solvents even as polar as methylene
dichloride. Water or water-miscible solvents, such as methanol or
ethanol, are absolutely essential for the recovery of dichlorvos
residues from plant materials.
Collaborative studies are currently being carried out in the United
Kingdom on a method of detecting dichlorvos in grain. The procedure
uses GLC with thermionic or flame photometric detection following
extraction with methanol or acetone.
APPRAISAL
Dichlorvos is used extensively for the control of insect pests of
importance in public health, in homes, warehouses, food stores and
transport, stored grain as well as insects attacking domestic animals.
It is also finding increasing use on horticultural and field crops and
is registered in over forty eight countries. Dichlorvos impregnated
resin strips are extensively used to control insect pests, especially
flies, in homes, stores and food processing establishments.
Extensive data on residues in food commodities were available from
many countries in the form of published reports end submissions from
three manufacturers. All data indicate that the level of residues
occurring in raw agricultural commodities is low and that the residue
levels decline rapidly. Due to the comparatively high vapour pressure
of dichlorvos, the applied deposit is quickly lost by volatilization,
but that portion which in absorbed into plant tissues undergoes
hydrolysis to inactive metabolites.
Dichlorvos applied to or fed to domestic animals undergoes rapid
detoxification and degradation in all species examined and is unlikely
to produce significant residues in meat, milk or eggs.
The use of dichlorvos impregnated strips for controlling insects in
warehouses, stores and shops gives rise to detectable residues in
stored or prepared foods. Residues resulting from exposures at rates
much higher than the recommended ones have been shown to decrease very
rapidly on exposure to the atmosphere. They also are readily destroyed
during the preparation of many products for consumption (e.g. by
washing or by cooking).
The proposal that a tolerance in meat and meat products was required
in view of the use of dichlorvos-resin fumigant strips in meat storage
and processing places in some countries (CCPR, 1971) was considered by
the Meeting.
Considerable data were available on the uptake of dichlorvos by many
different foods under varying conditions of storage and exaggerated
exposure to dichlorvos-resin strips, It was recognized that, under
some conditions, residues as high as 0.1 ppm could result but even in
fatty meat products the uptake was only at the surface. Residues
declined during atmospheric exposure and were completely destroyed by
cooking. Meat and meat products were included among the commodities
for which a 0.1 ppm tolerance was recommended. These products were
specially considered because of the request made at the 1970 meeting
of the Codex Committee on Pesticide Residues (CCPR, 1971).
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are for residues likely to be found in raw
commodities at harvest, in stored products shortly after treatment and
in miscellaneous prepared foods exposed during preparation and
storage. Recommendations have been made on broad categories of fruit
and vegetables, because extensive data have indicated that when
dichlorvos is applied to these crops the level of residues shortly
afterwards does not vary significantly on different varieties.
Due to the transient nature of the insecticide, residue levels will
continue to decline during shipment and storage, with a probable half
life of less than one day on fresh fruit, vegetables and miscellaneous
foods. Residues in food at time of consumption have normally been
below the limits of detection by current analytical methods.
Foodstuff Tolerance
Recommended Period on which
recommendation
based
Cocoa beans 5 ppm
Raw grain (wheat, rice,
rye, oats, barley,
maize, sorghum, etc,) 2 ppm
Coffee beans, soybeans,
lentils, peanuts (groundnuts) 2 ppm
Mushrooms 0.5 ppm 2 days
Milled products from raw
grain 0.5 ppm
Fresh vegetables
(excluding lettuce) 0.5 ppm 2 days
Tomatoes 0.5 ppm 1 day and post
harvest
Lettuce 1 ppm 2 days
Fresh fruit (apples, pears,
peaches, strawberries, etc.) 0.1 ppm 2 days
Meat of cattle, sheep,
goats, pigs and poultry 0.05 ppm
Eggs (on shell free
basis) 0.05 ppm
Milk (whole) 0.02 ppm
Miscellaneous food items
not otherwise
specified 001 ppm
As recommended in previous monographs, the content of
dichloroacetaldehyde (DCA) should be reported where known.
Furthermore, the amounts found should be added to those for dichlorvos
when assessing adherence to the tolerances recommended.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Continued observation of the effects of repeated exposure of man
to dichlorvos in order to determine if there is any qualitative
or quantitative differences between the metabolic route following
oral or inhalation intake.
2. Data from additional countries on residues in commodities moving
in international trade.
3. Analytical methods capable of recovering and determining residues
of dichlorvos in foods should be established for regulatory
purposes.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-69.
Pesticide Science, 1 (i):10-13
Anon. (1966) Communication from F.I.D. Nordrhein-Westfalen to Deutsche
Shell Chemie, Frankfurt, dated 20.1.66 (unpublished)
Anon. (1966) Communication from Hamburg Public Health Service to
Deutsche Shell Chemie, Frankfurt, dated 19.7.66 (unpublished)
Arthur, B.W. and Casida, J.E. (1957) Metabolism and selectivity of
o,o-dimethyl 2,2,2-trichloro-1-hydroxyethyl phosphonate and its acetyl
and vinyl derivates. J. Agr. Fd. Chem., 5 : 186-192
Blair, D., Hutson, D.H. and Pickering, B.A. (1970) The comparative
metabolism of (vinyl-14C) Vapona in rats after inhalation exposure
and oral ingestion of the compound. Unpublished draft summary from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Boone, G.H. (1965) Determination of Dibrom and DDVP residues in some
fruit and vegetable crops by microcoulometric gas chromatography. J.
A.O.A.C., 48 : 748-752
Boyer, A.C. (1969) I50 values for the inhibition of human and monkey
blood cholinesterases by dichlorvos. Unpublished technical information
report prepared by Shell Development Co.
Bull, D.L. and Ridgway, R.L. (1969) Metabolism of trichlorfon 1969 in
animals and plants. J. Agr. Fd. Chem., 17 : 837-841
Carson, S. (1969) Teratology studies in rabbits. Unpublished report
from Food and Drug Laboratories Inc., Maspeth, N.Y., prepared for
Shell Chemical Co.
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agr. Fd. Chem., 10 : 370-376
Cavagna, G., Locati, G. and Vigliani, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch.
Environmn. Hlth., 19 : 112-123
Cavagna, G., Locati, G. and Vigliani, E.C. (1970) Exposure of newborn
babies to 'Vapona' insecticide. Europ. J. Toxicol. 3 : 49-57
Cavagna, G. and Vigliani, E.C. (1970) Problèmes d'hygiène et de
sécurité dans l'emploi du Vapona insecticide dans les locaux
domestiques. Med. d. Lavoro, 61 : 409-423
CCPR. (1971) Report of the Fifth Session of the Codex Committee on
Pesticide Residues para. 165. Alinorm 71/24
Ciba, A.G. (1970) Basle - Submission to FAO/WHO Joint Meeting -
Dichlorvos
Drager, G. (1968) Gas chromatographic method for determining
dichlorvos residues in plants and milk. Pflanzenschutz - Nachrichten.,
21 : (3) 373-380
Elms, K.D., Kerr, J.D. and Champ, B.R. (1970) Breakdown of malathion
and dichlorvos mixture applied to wheat. In press
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FAO/WHO. (1967) Evaluation of some pesticide residues in food, FAO
PL:CP/15, WHO/Pood Add./67.32
FAO/WHO. (1968) 1967 Evaluation of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Green, A.A. and Wilkin, D.R. (1968) The control of insects in bagged
grain by the injection of dichlorvos. J. Stored Prod. Res., 5 :
11-19
Hartmann, C.H. (1966) Phosphorus detector for pesticide analysis. Bull
Environm. Contam. and Toxicol., 1 : 159-168
Hine, C.H., Beltran, S., Cavalli, R.D., Keating, J.M., Keating, W.C.
Jr. and Sample, H.G. Jr. (1966) Pre-clinical pharmacologic studies on
dichlorvos in formulation (V-12). Unpublished report from the Hine
Laboratories, Inc., submitted to Shell Chemical Co.
Hine, C.H. (1970) Arizona Vapona (DDVP) human study program.
Chromosomal analysis. Unpublished report from the Hine Laboratories,
Inc., prepared for Shell Chemical Research Development Co.
Hodgson, E. and Casida, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethyl phosphate. J. Agr. Fd.
Chem., 10 : 208-214
Hughes, G.T. (1963) Colorimetric determination of low concentrations
of 2,2-dichlorovinyl dimethyl phosphate in the atmosphere. Analyst,
88 : 318-319
Hunter, C.G. (1969) Report on initial studies of deliberate exposure
to high concentrations of dichlorvos by human subjects. Unpublished
report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Hunter, C.G. (1970) Dichlorvos: inhalational exposures with human
subjects, Part 1. Group research report TLGR. 0061.70
Ivey, M.C. and Claborn, H.V. (1969a) Kerrville, Texas, Laboratory,
Entomology Research Division, ARS, USDA. Results of research on
residues of dichlorvos in milk and body tissue of dairy cows treated
for fly control
Ivey, M.C. and Claborn, H.V. (1969b) GLC determination of dichlorvos
in milk, eggs and various body tissues of cattle and chickens. Journal
of the AOAC, 52 (6) : 1248-1251
Kimbrough, R.D. (1970) Work sited in "Summary of Vapona R
insecticide review", report of a meeting chaired by L. Golberg at
Cincinnati, 9 February 1970
Kirkpatrick, R.L., Harein, P.K. and Cooper, C.V. (1968) Laboratory
tests with dichlorvos applied as a wheat protectant against rice
weevils. J. of Econ. Entomol. 61 : 356-358
Lancaster, J.L. (1962) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lancaster, J.L. (1963) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lofroth, G. (1969a) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Paper presented at
a meeting of the Children's Cancer Research Foundation, Boston, 3
September 1969
Lofroth, G. (1969b) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Unpublished report
reproduced in part in Environmental Mutagen Society Newsletter, No, 2:
p. 21-24
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57 : 393-394
Loomis, E. and Hodel, E. (1965) (in cooperation with Shell Development
Co. Residue project from Rio Linda, California (unpublished)
Minett, W. and Belcher, R. (1969) Determination of malathion and
dichlorvos residues in wheat grains by gas-liquid chromatography. J.
Stored Prod. Res., 5 : 417-421
Modesto home trials with Vapona insecticide strips
Nelson, C.B. (1968) (in cooperation with Shell Chemical Co. Residue
project from Fillmore, California (unpublished)
Noetzel, D. (1964) (in cooperation with Shell Development Co. Residue
project from North Dakota State University (unpublished)
Padget, T.J. (1968) Evaluation of dichlorvos as a grain treatment for
protection against the white-fringed beetle. USDA Plant Pest Control
Laboratory (unpublished)
Padget, T.J. (1969) Communication on the evaluation of dichlorvos as a
grain treatment for protection against the white-fringed beetle
(unpublished)
Page, A.C. (1970) Metabolic fate and tissue residues following oral
administration of dichlorvos. Collection of unpublished reports
prepared and submitted by Shell Chemical Co.
Palut, D., Grzymala, W. and Syrowatka, T. (1969) Investigation of the
metabolism of some organophosphorus insecticides on animal model
systems. II Hydrolytic degradation in vitro (original in Polish),
Roczn. Zak. Hig. (Warsz.), 20 : 551-555
Pitts, C.W. (1961) (in cooperation with Shell Development Co. Residue
project from Kansas State University (unpublished)
Preussmann, R. (1967) Analytical determination of alkylating agents.
Zweite Konferenz über aktuelle Probleme der Tabaksforschung, Freiburg,
p. 92 (cited by Lofroth, 1969, q.v.)
Preussmann, R. (1968) Direct alkylating agents as carcinogens. Fd.
Cosm. Toxicol, 6 : 576-577
Preussmann, R., Schneider, H. and Epple, F. (1969) Untersuchungen zum
Nachweis Alkylierender Agentien. Arzneimittelforschung, 19 :
1059-1073
Roger, J.C., Chambers, H. and Casida, J.E. (1964) Nicotinic acid
analogs: effects on response of chick embryos and hens to
organophosphate toxicants. Science, 144 : 539-540
Roger, J.C., Upshall, D.G. and Casida, J.E. (1969) Structure activity
and metabolism studies on organophosphate teratogens and their
alleviating agents in developing hen eggs with special emphasis on
Bidrin. Biochem. pharmacol., 18 : 373-392
Sasinovich, L.M. (1968) The maximum permissible concentration of DDVP
in the air of the working zone. Gig. i Sanit, 33 (12) 35-39
Sax, K. and Sax, H.J. (1968) Possible mutagenic hazards of some food
additives, beverages and insecticides. Jap. J. Genetics, 43 : 89-94
Shaw, F. (1964) (in cooperation with Shell Development Co. Residue
project from University of Massachusetts (unpublished)
Shell Development Co. (1964) Modesto Project No. RES 64-111
(unpublished)
Shell Research Ltd. (1965a) Woodstock Technical Memo. 165/65
(unpublished)
Shell Research Ltd. (1965b) Woodstock Technical Memo. 159/65
(unpublished)
Shell Development Co. (1965c) Modesto Project No. RES 65-42
(unpublished)
Shell Chemical Company, New York. (1965d) Brief in Support of Vapona
Insecticide Resin Strips. Section VII : 11-12. Submitted to Panel of
U.S.P.H.S. and U.S.D.A., 29.10.65
Shell Development Company. (1965e) Modesto Project No. RES 65-36
(unpublished)
Shell Research Ltd. (1966a) Woodstock Technical Service Note 146/66
(unpublished)
Shell Research Ltd. (1966b) Woodstock Technical Service Note 178/66
(unpublished)
Shell Research Ltd. (1966c) Woodstock Technical Memo 1/66
(unpublished)
Shell Chemical Co. (1967) Petition proposing a tolerance for the food
additive (pesticide chemical) dichlorvos in or on stored food
commodities. Section D-III
Shell Research Ltd. (1968) Woodstock WKGR. 0041.68 (unpublished)
Shell Chemical Co. (1969) Princeton laboratory residue analysis report
project no. PRL 69-62, (unpublished)
Shell Development Co. (1970a) The third Arizona home study. The
quantification of DDVP residues in foods consumed by human volunteers
exposed to "No-Pest" strip insecticide. (unpublished report)
Shell Chemical Co. (1970b) Princeton laboratory residue analysis
report project no. PRL 70-12, (unpublished)
Shell Chemical Co. (1970c) Princeton laboratory residue analysis
report project no. PRL 69-26 (II) (unpublished)
Shell Research Ltd. (1970d) Interim report - Residues of Vapona in
meals prepared in domestic kitchens. Woodstock Laboratory
(unpublished)
Singh, V.K. (1964) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. (1965) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. and Rainier, R.H. (1966) Three year chronic oral toxicity
of formulated dichlorvos in swine with special reference to possible
effects upon fertility and the viability of the offspring of the
animals fed continuously on diets containing the drug. Unpublished
report from Bio/Toxicological Research Associates, Spencerville, Ohio,
prepared for Shell Chemical Co.
Somme, L. (1967) A field trial with dichlorvos vapor for the control
of Ephestia Kühniella Zell (Lepidoptera, Phycitidae) in flour mills.
Reprinted from Journal of Stored Products Research, 4 (3)
Stevenson, D.E. and Blair, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos. Shell
Research Tunstall Report No. TLGR. 0024.69
Sun, Y.P. and Johnson, E.R. (1963) A new bioassay technique with
special reference to the specific bioassay of dichlorvos insecticide.
J. Econ. Entomol., 56 : 635-641
Vigliani, E.C. (1970) Results of recent studies on Vapona. Paper
presented at the 7th Shell Industrial Doctors' Meeting, Strasbourg,
27-29 May 1970, submitted by Shell International Research Ltd.
Vogin, E.E. (1969) Teratological studies with dichlorvos in rabbits.
Unpublished report from Food and Drug Laboratories, Inc., Maspeth,
N.Y., prepared for Shell Chemical Co.
Wisconsin Alumni Research Foundation No. 8030193 (1968) (in
cooperation with Shell Chemical Co. Residue project report
(unpublished).
Wisconsin Alumni Research Foundation (1968/9) (in cooperation with
Shell Chemical Co. Residue project reports on sheep, cattle and goats
(unpublished)
Witherup, S., Caldwell, J.S. Jr. and Hull, L. (1965) The effects
exerted upon the fertility of rats, and upon the viability of their
offspring, by the introduction of Vapona R insecticide into their
diets. Unpublished report from the Kettering Laboratory Cincinnati
Young, R., Jr. (1969) Dichlorvos infusion studies. Unpublished
technical information report prepared by Shell Development Co.
Rat
Reproduction studies in rats fed 0, 0.1, 1,0, 10.0, 100 or 500 ppm in
the diet through three successive generations did not show any
significant effect on numbers and sizes of litters, survival of young
or growth of young being suckled while dichlorvos was still being fed
to the dams. Dichlorvos appeared to be without teratogenic effect
(Witherup et al., 1965; see FAO/WHO, 1967).
Dichlorvos was administered at unspecified levels intraperitoneally to
female rats on day eleven after insemination. Doses of 20 mg/kg
body-weight killed the animals; 15 mg/kg produced toxic signs and
weight loss. There was no adverse effect on litter size or on the
placenta at 15 mg/kg, but three foetuses from one of four litters
available had omphaloceles, although none were observed in the six
litters from control animals (Kimbrough, 1970).
Rabbit
A total of 168 adult female rabbits were artificially inseminated with
20-30 million viable and mobile rabbit sperm, and on days 6 to 18 of
gestation were administered daily oral doses of 0, 6 or 18 mg/kg
body-weight of dichlorvos by capsule, or 31 mg/kg on days 6 to 11 of
gestation. Foetuses were recovered by Caesarean section. There was
increased incidence of in utero and neonatal toxicity in the top
dose group only, which was of doubtful significance. There were no
teratogenic effects observed, based upon skeletal examination of the
foetuses. Maternal mortality was increased in the 31 mg/kg group
(Carson, 1969).
In a separate experiment, 104 female rabbits were artificially
inseminated with rabbit sperm and administered 0, 3 or 12 mg/kg
body-weight of dichlorvos by capsule on gestation days 6 to 16
inclusive. Other rabbits were given higher doses, but they could not
tolerate levels of 24 mg/kg or higher, and no live litters were
produced from such groups. At 12 mg/kg, there was a significant
reduction in the number of implant sites and foetuses compared to the
control or lower dose group. At 3 mg/kg, the number of implant sites
and foetuses were not lower than the controls. However, one deformed
stillborn foetus was encountered from this group. Liver sections from
parents and foetuses displayed no pathological abnormalities that
could be related to dichlorvos treatment (Vogin, 1969).
Pig
Female pigs were fed dietary levels of 0, 200, 250, 288, 400, 500 or
750 ppm of dichlorvos for up to 37 months. The animals were first
mated after they had received the test diet for six months, and the
study was followed through two generations. Initially, a total of 22
females and one male were used, but after the first generation, four
more males were used in order to prevent in-breeding. The males
received 0, 288 or 400 ppm of dichlorvos in their diets. Dichlorvos
did not affect either the numbers of litters or their size and the
survival in the groups fed dichlorvos. A total of 490 piglets were
examined and none showed anatomical abnormalities (Singh and Rainier,
1966).
Special studies on mutagenicity
Secondary roots of Vicia fabia were treated with varying
concentrations of dichlorvos for one hour. Concentrations down to 1 mM
were mostly lethal, and root tips surviving this and lower
concentrations had a low mitotic index and showed c-mitotic effects,
and chromatid breaks and gaps were observed. In another experiment, a
streptomycin dependent, Sd-4, mutant of E. coli was treated in the
lug phase at cell densities of about 108 cells/ml during one hour.
The LD50 concentration for the bacteria of dichlorvos in the medium
was estimated to be between 10 and 60 mM (2200 ppm and 1.3 percent).
At concentrations of 1-4 mM, which caused no significant killing, an
increased mutation rate was observed when compared with controls and
measured as revertants to streptomycin independency (Löfroth, 1969 a,
1969 b).
Segments of Vapona-strips of various sizes were placed in an inflated
6000 ml plastic bag containing an uncovered petri-dish with
germinating onion seeds. There was no retardation of root growth or
mitotic frequency, but the number of chromosome aberrations, when
scored at anaphase, increased constantly with increasing size of the
Vapona-strip segment. The lowest concentration used was about ten
times as great as one strip in a 1000 ft3 room (Sax and Sax, 1968).
Attention has been drawn to the alkylating property of a number of
alkyl phosphates (Preussman, 1967). For this reason, possible
mutagenic effects have been looked for. Calf thymus deoxyribonucleic
acid (DNA) was incubated with varying concentrations of dichlorvos
solution. In a typical experiment with 2 percent dichlorvos incubated
for 66 hours, there was a yield of 1 percent of N-7-methylguanine from
the available guanine. Similar experiments with deoxyguanosine gave a
3-5 times higher yield of N-7-methylguanine than from DNA (Löfroth,
1970).
Eight mice were injected intraperitoneally with 10-20 mg/kg
body-weight of dichlorvos. After 23 hours, 100 cells of their bone
marrow were examined. The incidences of aberrations were not greater
than observed in four control mice (Hunter, 1970).
A human subject was exposed to 1 mg/m3 of dichlorvos for six hours.
A sample of blood cultured for leucocytes for 72 hours showed no
increase in chromosome aberrations in 100 cells compared to the
pre-exposure values (Hunter, 1970).
No chromosomal aberrations were seen after examination of blood
samples from a family of four female and one male human subjects which
had been exposed to dichlorvos strips. A control group had two male
and three female subjects (Hine, 1970).
Special studies on carcinogenicity
Rat
Rats (24) were injected subcutaneously, once weekly, with trichlorphon
(Dipterex), of which dichlorvos is a known metabolite. Despite a high
(unspecified) dosage and a latent period of almost 800 days, only two
local sarcomas developed at the site of injection. No data on controls
are given. (Preussmann, 1968). In a subsequent publication, Preussmann
et al. (1969) refer to the above experiment as a "negative result,
presumably due to the very rapid metabolism of Dipterex."
Acute toxicity
After direct infusion of dichlorvos into a peripheral vein at various
rates, pigs can survive an LD50 dose each hour for at least nine
hours (page, 1970).
The acute toxicity of dichlorvos has been compared with the
metabolites. The LD50 values in mg/kg body-weight for intraperitoneal
administration to mice are: dichlorvos, 28; o-demethyldichlorvos
sodium salt, 1500; dichloroacetaldehyde, 440; 2,2-dichloroethanol,
890; dichloroacetic acid, 250; sodium dichloroacetate, >3000;
mono- and dimethylphosphoric acid mixture, 1500; sodium
monodimethylphosphoric acid mixture, >3000 (Casida, et al., 1962).
Short-term studies
Comparative studies on inhalation toxicity
Guinea pigs have been exposed for five hours per day for five
consecutive days to a concentration of 130 mg dichlorvos/m3 of air
without visible effects on health. Rats and mice were not visibly
affected by similar exposures to 50 mg/m3. At concentrations above
50 mg/m3, the mice became visibly distressed, and prolonged exposure
to 80 mg/m3 was frequently lethal. Rats were less severely affected
than mice. In all cases, stress reactions were either apparent during
the first hours of exposure or they did not occur at all. No
cholinesterase determinations were made (Stevenson and Blair, 1969).
Three samples of dichlorvos were investigated (82.5 percent pure,
Russian produced, 40% pure, Japanese, and 100% pure from Swiss
origin); the oral LD50 for Russian and Japanese samples was similar
(87 mg/kg body-weight for mice and 65 mg/kg for rats). The Swiss
sample was less toxic: oral LD50, mice: 108 mg/kg. Rabbits were found
to be the most sensitive species (oral LD50, Russian sample: 22.5
mg/kg). Inhalation toxicity was investigated for exposure periods of
four hours, The highest concentration which could be produced (30
mg/m3) was lethal for mice and rats. The LD50 (four hours) for mice
was 13.2 mg/m3 and for rats 14.8 mg/m3. At 5 mg/m3, blood
cholinesterase activity in rat was 69 percent of pre-exposure values.
A level of 0.5 mg/m3 caused no observable effects in blood
cholinesterase. By interpolation, those concentrations of dichlorvos
in air were calculated which would after four hours exposure cause a
50 percent drop in blood cholinesterase activity in rat (I50 = 2.4
mg/m3) or a 25 percent drop (I25 = 1 mg/m3). Rats and rabbits
were exposed for 4 hours/day for a period of four months to an average
dichlorvos vapour concentration of 1 mg/m3 of air. No visible signs
of intoxication occurred. Cholinesterase activity in the serums
erythrocytes, medullary tissue and liver of rats varied throughout the
experiment within the limits of the norm or was slightly depressed.
After four months, the depression of cholinesterase activity in the
medulla averaged 8 percent in the liver, serum and erythrocytes the
depression was 30 percent 22 percent and 24 percent, respectively.
Blood sugar content, blood sugar curves after loading with galactose,
synthesis of hippuric acid after loading with sodium benzoate and
duration of Hexenal induced sleep were not affected. Rabbits exposed
under the same conditions showed 11-30 percent reduction of serum
cholinesterase activity and 28-30 percent reduction of erythrocyte
cholinesterase activity. It was concluded that 1 mg/m3 was the
threshold for rats and rabbits which caused an insignificant and
variable depression of cholinesterase. It was also the threshold for
cats. Rats exposed to an average concentration of 5.2 mg/m3 for 4
hours/day over a period of two months remained visibly healthy. After
15 days, the cholinesterase depression in the medulla was on an
average 22 percent in the serum 58 percent in the erythrocytes 22
percent. The duration of drug induced sleep was increased from 33
minutes to 57 minutes. The blood sugar level was unchanged, but the
rise of the curve after galactose loading was far higher than in the
controls. After 60 days, the blood sugar curve was affected, the
duration of Hexenal induced sleep was the same as in the controls, and
cholinesterase activity in the medulla fell by an average of 31
percent, in the liver by 35.5 percent, in serum by 52 percent and in
erythrocytes by 58 percent. At 8.2 mg/m3 for 45 days, rats lost
appetite and weight, and in some cases trembling of the head and
entire body was noted. Signs of intoxication occurred in some of the
rats and some died (Sasinovich, 1968).
OBSERVATIONS IN MAN
A total of 10 human subjects, in groups of two, ingested pellets of a
slow-release formulation of dichlorvos in daily dosages, doubling
weekly, up to 8 mg/kg body-weight/day for periods up to three weeks;
one group of two received 16 mg/kg/day for 5.5 days. Doses above 1
mg/kg were administered in two equally divided doses per day. The
majority of the clinical side effects occurred after the doses had
reached the 8 mg/kg/day level. Most of the subjective clinical
complaints referred to the gastrointestinal tract, but none of the
studies had to be terminated because of side-effects. The study with
16 mg/kg/day was terminated because of the magnitude of the red blood
cell cholinesterase activity depression. Doses of 1-8 mg/kg/day for up
to 21 days produced an average plasma cholinesterase activity
depression of 70 percent irrespective of dose or duration. Doses of 16
mg/kg/day for 5.5 days produced approximately 90 percent depression of
the plasma cholinesterase activity. Red cell cholinesterase activity
after multiple dosing of 1 mg/kg/day was 11-27 percent depressed.
Doses which reached 8 mg/kg/day caused 45-86 percent depression. At 16
mg/kg/day, depressions of 90 percent were observed. Single doses of
dichlorvos needed to be more than 4 mg/kg in order to cause measurable
red cell cholinesterase depression. At single doses of 8 mg/kg and
higher, maximal plasma cholinesterase inhibition was present and did
not increase at higher dosages. Significant and consistent erythrocyte
cholinesterase inhibition occurred after single doses of 16 mg/kg (36
percent inhibition) and 32 mg/kg (46 percent inhibition). About 50
percent of the dichlorvos was still present in the excreted pellets,
Therefore, the doses absorbed were assumed to be about one half of the
above levels (Hine et al., 1966).
In another study, 12 male subjects ingested 5 mg of dichlorvos daily
in three equal portions with their food until the plasma
cholinesterase fell to 75 percent of the pre-exposure value. The time
required averaged 12 to 20 days. No effect occurred on erythrocyte
cholinesterase. One of the subjects continued the exposure for a total
of 77 days. A questionable erythrocyte cholinesterase depression of
10-15 percent occurred during part of the exposure period, but in the
beginning and at the end of the exposure period, this activity was
normal or slightly above (Hunter, 1970).
Six human subjects were exposed to concentrations of dichlorvos in the
air, the maximum level being 52 mg/m3 for 62 minutes and the maximum
period 240 minutes to 13 mg/m3. No clinical abnormalities were
observed, subjective complaints were only related to the dryness of
the atmosphere, but the plasma cholinesterase activity was decreased;
the extent of the depression was proportional to the product of the
concentration and time of exposure. During some of these exposures, a
questionable depression of erythrocyte cholinesterase occurred, but
the extent of depression was not directly related to the product of
the concentration and time of exposure (Hunter, 1969).
In six homes in Tucson, Arizona, 18 individuals submitted to exposure
to dichlorvos strips and six others served as controls.
In order to maximize exposure, the study was made during the winter
months, and strips were hung in all rooms in the houses at the rate of
1 strip/1000 ft3. Maximum concentrations of 0.1-0.2 mg/m3 occurred
within the first week, levelling off to 0.05-0.10 mg/m3 during the
remainder of the regular exposure period of 28 days. Samples of meals
and beverages were collected at various intervals. During the exposure
period, more than 85 percent of the individual meals contained less
than 0.3 ppm dichlorvos and more than half the meals 0.1 ppm or less.
Physical examination, complete blood counts, blood chemical profiles
and plasma and erythrocyte cholinesterase measurements were performed
at various intervals. Levels of dichlorvos in the food eaten ranged
from 0.1 to 0.3 ppm. There were no clinical abnormalities evident, nor
were there any significant differences in plasma and red-blood cell
cholinesterase, blood count and blood chemical profiles between the
exposure and pre-exposure periods (Shell, 1970a).
A study was made in Italy of the effect of continuous exposure to
insecticide levels of dichlorvos by patients in hospital wards.
Control values for plasma and erythrocyte cholinesterase were
established for 250 healthy male and 100 healthy female adults not
exposed to dichlorvos. The variation in the activity of the
cholinesterases of the individuals when measured at different times,
depending on the time-interval, varied on average between 1.4 and 4.4
percent for the erythrocyte cholinesterase and on average between 5.0
and 14.2 percent for plasma cholinesterase. These were average
variations measured in ten to 20 individuals. In single individuals,
the maximum variation was 7.7 percent for erythrocyte cholinesterase
and 23.2 percent for plasma cholinesterase.
A total of 44 adult male patients with diseases other than liver
diseases were exposed to atmospheric concentrations of dichlorvos
ranging from 0.02 to 0.1 mg/m3 for periods of 3 to 29 days. Another
similar group of 22 male patients was exposed to dichlorvos
concentrations of 0.1 to 0.28 mg/m3 for periods from 3 to 16 days.
Only in the case of five patients exposed 24 hours per day at levels
above 0.1 mg/m3 was there a depression of plasma cholinesterase (35
to 72 percent) below the pre-exposure level. There was no depression
of erythrocyte cholinesterase nor were any symptoms typical of
cholinergic stimulation observed, in any patients. With six other
patients suffering from liver disease exposed in the same room, all
showed a reduction in plasma cholinesterase (25 to 66 percent of
pre-exposure values). Reduction was evident even in patients exposed
at levels below 0.1 mg/m3. These patients, because of their liver
disease, had already before exposure a considerably reduced plasma
cholinesterase activity. However, there was no depression of
erythrocyte cholinesterase nor any symptoms of poisoning, in spite of
the fact that in these patients with liver insufficiency had a 40
percent depression in plasma cholinesterase lasting for one to three
weeks after the removal from exposure. In babies, sick children and
women in labour or postpartum exposed to dichlorvos, a similar
situation to that of sick adults was seen; depression of plasma (but
not erythrocyte) cholinesterase was observed only in some cases when
the dichlorvos level was above 0.1 mg/m3. Again, no symptoms were
seen. Assuming an inhaled volume of 10 m3/24 hours in adults and 1.4
m3/24 hours in children, the authors calculated that a reduction in
plasma cholinesterase for a calculated daily inhalation of 1.7 mg of
dichlorvos could occur in adults with normal liver function and 0.2 mg
for children. They further calculated that the amount of dichlorvos
inhaled which might produce a plasma cholinesterase depression is
about the same for adults and children, viz. 0.028 to 0.030 mg/kg
body-weight/day. In patients with liver malfunction, daily inhalation
intake of 0.34 mg (equivalent to 0.005 to 0.006 mg/kg body-weight/day
was sufficient to produce plasma cholinesterase depression (Cavagna et
al., 1969; Cavagna and Vigliani, 1970).
In two nurseries 22 healthy newborn babies were kept from birth for a
period of five days for 18 hours/day in an atmosphere in which average
dichlorvos concentrations of 0.15 - 0.16 mg/m3 were maintained. Blood
cholinesterase levels were determined at birth and after five days.
There were no effects on the health of the babies nor on the activity
of their plasma or erythrocyte cholinesterase (Cavagna et al., 1970).
In a preliminary study with asthmatic patients, there appeared to be
no subjective worsening of asthma during exposure to dichlorvos at
levels of 0.1 to 0.2 mg/m3 for two consecutive days. In some cases,
an increase in airways resistance and sensitivity to acetylcholine
occurred during and after exposure, but these manifestations may be
related to other factors (e.g. poor ventilation) than to dichlorvos.
Further work in this field is reported to be in progress (Vigliani,
1970).
COMMENT
On the basis of recent and earlier studies the metabolic pattern of
dichlorvos when fed to mammals is now fairly clearly elucidated.
Evidence is presented that cleavage of the dichlorovinyl group
proceeds much more rapidly than o-demethylation. Information on the
exposure of man to varying levels of dichlorvos vapour is available,
but such studies do not include assessment as to whether the
metabolism is the same after inhalation as after oral intake. Data on
the toxicology of the metabolites dichloracetaldehyde and
dichloroethanol are considered pertinent in evaluating the toxicity of
dichlorvos. A long-term study in rats reported in the monograph from
the 1967 Joint Meeting is considered adequate. Feeding studies in rats
and rabbits did not show any indication of a teratogenic effect.
Additional information on feeding dichlorvos to man has become
available, and the no-effect level previously determined in man is
used as a basis for establishing an acceptable daily intake.
TOXICOLOGICAL EVALUATION
Level causing no toxicological effect
Man : 0.033 mg/kg body-weight/day
ESTIMATE OF ACCEPTABLE DAILY INTAKE FOR MAN
0-0.004 mg/kg body-weight
RESIDUES IN FOOD AND THEIR EVALUATION
USE PATTERN
Pre-harvest treatments
In addition to its use on beef and dairy cattle and goats, sheep and
pigs, and in and around buildings which house these animals,
dichlorvos is finding increasing use on fruit and vegetable crops.
Fruit and citrus crops account for more than one third of the
agricultural use. Rice accounts for just over one quarter and field
crops, including vegetables, approximately one quarter of the
agricultural uses. There are minor uses on grapes, glasshouse crops,
mushrooms, tobacco, tea, coffee, cocoa and miscellaneous crops.
Dichlorvos is known to be registered in at least forty eight
countries.
Recently it has been introduced as an anthelmintic for pigs, poultry
and horses, being administered in the form of plastic granules for
this purpose.
Post-harvest treatments
Dichlorvos is being increasingly used for the control of insect
infestation in stored grain. For this purpose it is used as an aerosol
or as impregnated resin strips, which are applied in the overhead
spaces of storage bins. In some countries, dichlorvos preparations
have also been registered for incorporation into stored grain either
as a dust, as a direct spray or as an aqueous emulsion.
Dichlorvos aerosols, automatic dispensers, sprays and resin
impregnated strips are used in many countries for the control of
insect infestations in stores, in transport and in warehouses holding
processed food. Food processing establishments are also treated with
dichlorvos in one of the above forms.
Other uses
As defined in the monograph of the 1967 Joint Meeting (FAO/WHO 1968),
dichlorvos is used extensively in the public health field (homes,
hospitals, etc.). It is also used in aircraft during flight. Trials
are being carried out on its use by aerial application against insect
pests of forests, pastures and field crops.
RESIDUES RESULTING FROM SUPERVISED TRIALS
Crops grown in the open
Table I gives typical results of the analysis for dichlorvos residues
following controlled application to various fruit and vegetable crops
(Ciba 1970).
Crops grown under cover
In glasshouses, the rate of decay of dichlorvos residues is fast, with
a half-life of approximately one day. Table II gives results of
residue trials carried out on crops growing under cover (Ciba 1970).
Livestock and poultry
Trials in the United States demonstrate that the spraying of
dichlorvos on livestock (including cattle, sheep, goats and pigs) and
poultry is unlikely to produce significant residues in meat, milk or
eggs. When dichlorvos was applied as a single pour-on application of 1
percent at the rate of 16.5 ounces per cow, no residues were
TABLE I
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Apples 0.05% ai 1 day 0.05 ChE1 0.05
2 days <0.05
Peaches 0.05% ai 0 day 0.8 ChE 0.02
1 day 0.55 " 0.02
2 days 0.35 " 0.02
Strawberries 0.05% ai 2 days 0.35 ChE 0.02
3 days 0.06 " 0.02
8 days <0.02 " 0.02
Carrots 0.025% al. 28 days <0.05 ChE 0.05
31 days <0.05 " 0.05
Cauliflower 0.05% ai 0 days 0.37 ChE 0.04
5 days <0.04 "
Brussels 0.05% ai 0 days 1.35 ChE 0.04
sprouts 5 days <0.04
Cabbage 0.05% ai 0 days 1.5 ChE 0.05
1 day 0.14
2 days 0.05
3 days <0.05
Spinach 0.05% 0 days ChE 0.05
1 day 1.6
2 days 0.06
3 days <0.05
TABLE I (Cont'd.)
Crops grown in the open
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Lettuce 0.05 0 days ChE 0.05
3 days <0.05 " 0.05
French beans 0.05 0 days 0.3 ChE 0.05
<0.05
Cucumber 0.05 9 days <0.05 ChE 0.05
1 ChE = cholinesterase inhibition method
TABLE II
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
Switzerland Tomato 20 mg/m3 0 days 0.2 ChE1 0.04
1 day 0.06 " 0.04
2 days 0.04 " 0.04
England Lettuce 130 mg/m3 0 days 78 ChE 0.04
1 day 4.9
70 mg/m3 1 day 2.1
2 days 0.4
3 days 0.2
Germany Lettuce 30 mg/m3 0 days 3.4 B.T.2 0.04
1 day 1.8
3 days 0.3
20 mg/m3 0 days 2.4 B.T. 0.04
1 day 0.9
2 days 0.4
3 days 0.16
Switzerland Cucumber 20 mg/m3 0 days 7.3 ChE 0.04
2 days 0.15
U.S.A. Mushroom 130 mg/m3 1 day 0.1 ChE 0.04
TABLE II (Cont'd.)
Crops grown under cover
Residue Analytical Limit of
Country Commodity Rate of Interval ppm Method Detection
Application to Harvest ppm
England Mushroom 160 mg/m3 0 days 25 D.N.P.3 0.04
1 day 0.9
3 days 0.27
160 mg/m3 1 day 0.9 D.N.P. 0.04
2 days 0.05
100 mg/m3 0 days 0.3 D.N.P. 0.04
1 day 0.1
1 ChE - cholinesterase inhibition method
2 B.T. - bioassay
3 D.N.P. - 2,4-dinitrophonylhydrazine colorimetric method
detectable at the level of sensitivity of the method (0.1 ppm) in any
of the milk samples collected from 4-5 hours to 5 days, after
treatment (Singh, 1965). In another study (Noetzel, 1964), groups of
Holstein and Guernsey cattle received dermal applications, twice daily
for 28 consecutive days, of 2 ounces of 0.5 percent dichlorvos and 1
percent dichlorvos, respectively. No residues were detected in the
milk (<0.02 ppm). In a similar study (Wisconsin, 1968) in which
dichlorvos was dermally applied to lactating dairy cows at the rate of
1 ounce/cow or 3 ounces/cow of a 1 percent dichlorvos for thirty days,
milk samples from 1 through 7 days and thereafter at 3 day intervals
were analysed for residues of dichlorvos and dichloroacetaldehyde
(DCA). No residues were detectable in any of the samples (<0.05 ppm
dichlorvos, <0.01 ppm DCA).
In other extensive trials (Wisconsin, 1968/1969) Brahma cattle, goats
and sheep were sprayed 3 times to saturation (1 gallon) at 14 day
intervals with 0.025 percent dichlorvos emulsion. No residues of
dichlorvos were detectable at the limits of sensitivity in tissues or
organs (including renal, omental and subcutaneous fat, muscle, liver
and kidney) of cattle (<0.25 ppm), goats (<0.25 ppm) or sheep
(<0.05 ppm).
Ivey and Claborn (1969a) sprayed lactating dairy cows for 31
consecutive days with 2 ounces of a 1 percent dichlorvos insecticide
spray solution. Milk samples were taken at 2 hours, 1, 2, 4, 8, 16, 24
and 31 days. One day after final treatment, the cows were slaughtered
and samples of renal, omental and subcutaneous fat, muscle liver,
kidney and blood were taken. Using a highly sensitive flame
photometric method (Ivey and Claborn, 1969b) with improved clean-up
technique, no residues of dichlorvos were detected in milk (<0.003
ppm) or body tissue (<0.002 ppm).
Pigs were fed daily at the high dosage of 9 450 ppm dichlorvos for 90
days (Singh, 1964). Dichlorvos residues were not detectable (<0.05
ppm) in tissues and organs including spleen, kidney, small intestine,
lung, heart, muscle and liver 0 and 1 day after last treatment.
Analysis of meat, fat, liver and eggs derived from poultry sprayed
with 2 ounces of diluted 2 lb/gal dichlorvos insecticide E.C. (0.295
ppm grams DDVP/bird) showed residues of equal to or less than 0.03 ppm
when samples of tissue were taken 6 hours, 3, 5 and 7 days after
application. Eggs were collected 1, 2, 3, 4, 5, 6 and 7 days after
treatment (Shell, 1970b). Poultry exposed to dichlorvon in the form of
2 percent granules (2 lb/100 square feet or 4 lb/100 square feet), or
to resin strands containing 20 percent dichlorvos (1 foot of strand/1
foot of cage) caused no residues in either tissue or eggs (<0.02 ppm)
(Lancaster, 1962; Loomis and Hodel, 1965; Lancaster, 1963; Shaw, 1964;
Nelson, 1968).
TABLE III
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Singh, Cows, pour on 480 1 Milk2 <0.1
1965
Noetzel, Cows, dermal 60 28×1 Milk <0.02
1964
Wisconsin, Cows, dermal 90 30×1 Milk <0.05
1968
Wisconsin, Cows, dermal 4540 3×14 Fat, muscle <0.25
1968, 1969 Goats, dermal 4540 3×14 Fat, muscle <0.25
Sheep, dermal 4540 3×14 Fat, muscle <0.05
Ivey & Claborn, Cows, dermal 60 31×1 Milk <0.003
1969a Cows, dermal 60 31×1 Fat, muscle, <0.002
etc.
ppm
Singh, Pigs, oral 9450 90×1 All tissues <0.05
1964
g
Shell, Chickens, dermal 0.3 1 All tissues3 <0.03
1970b Chickens, dermal 0.3 1 Eggs4 <0.03
Lancaster, Chickens, vapour - 30×1 All tissues <0.2
1962
TABLE III (Cont'd.)
Dichlorvos residues following treatment of animals
Reference Animal and Route Quantity Frequency Tissue Residue
Analysed1 ppm
cm3 days
Loomis, Chickens, vapour - 30×1 Eggs <0.02
1965
Nelson, Chickens, contact - 28×1 All tissues <0.03
1968 Chickens, contact - 28×1 Eggs <0.05
mg
Pitts, Chickens, oral 8.5 over 10 Meat <0.01
1961 Chickens, oral 8.5 over 10 Eggs <0.04
1 Waiting period Nil unless otherwise indicated
2 Waiting period 4 hours and 5 days
3 Waiting period 6 hours
4 Waiting period 1 day and 7 days
Uses in the protection of stored products
Grain, meals and flour
Many of the published and unpublished reports available on the rates
of loss of dichlorvos from grain, meals and flour are not reliable,
because the investigators have used unsatisfactory methods to recover
any residues of dichlorvos from the treated grain and cereal products
(see comments under Methods of Residue Analysis).
Residues of dichlorvos in flour resulting from the use of resin strips
in a flour mill have been investigated (Somme, 1967). Strips were hung
at the rate of 1/1000 feet3, and samples of flour made from grain
stored in the mill were taken 4 days and 2´ weeks after production had
rebegun. Analysis showed that residues of 0.04 ppm and 0.06 ppm
dichlorvos were present in the flour and that bran samples averaged
0.09 ppm dichlorvos. In another trial (Shell, 1966a) a "Vapona" strip
was hung inside a covered flour bin of 600 ft3 total capacity. A
sample of flour drawn from a conveyer belt taking flour from the
bottom of the bin one week and two weeks after fitting the strip in a
nearly full bin was found to have a dichlorvos residue of 0.03 ppm.
Samples taken after the third week and after the bin had been refilled
with flour, at the end of the fifth week, contained residues no
greater than the limits of detection, Wheatings exposed at a rate of
one "Vapona" strip per bin of 1150 feet3 for ten hours to six weeks
after the strips had been hung also did not contain residues in the
surface layer above the limit of detection (ca. 0.02 ppm), (Shell,
1965a).
Dichlorvos has been successfully used to control Oryzaephilus
surinamensis and Sitophilus granarius (Green and Wilkin, 1968).
The insecticide was sprayed into the air stream from a motorized
knapsack sprayer through to a perforated lance which was inserted into
the grain. Dichlorvos residues were higher on wheat than on barley and
greatest in places where dust and frass had accumulated. When
dichlorvos was injected at 20 ppm, the highest residue found after one
day was 30 ppm in a dusty pocket of wheat, and this fell to 5.3 ppm
after six days.
The range of residues reported for barley were 5.8-24.0 ppm after one
day, falling to 0.9-1.4 ppm after six days. However, the method of
analysis may not have extracted all the dichlorvos from the grain (See
Methods of Residue Analysis).
In a laboratory study Kirkpatrick et al., (1968) demonstrated that
dichlorvos residues of 3.8-8.0 ppm decreased 89 percent and 76
percent, respectively, in 28 days.
An unpublished study was conducted by USDA Plant Pest Control (Padget,
1968). Wheat grain was treated with 4, 6 and 8 ppm of dichlorvos
formulated from a 25% dichlorvos emulsifiable concentrate. Residues on
the wheat were reported to be less than 2 ppm by the third day after
application, and by 14 days all dichlorvos residues were <0.5 ppm.
Details of the analytical method used were not given.
Rice
Shell Chemical Company (1969) conducted an extensive study on rough
rice and rice products treated for rice weevil control. Rough rice,
stored as 45 lb lots were each sprayed with 20 ml of a solution
containing 1.5 percent, 1 percent and 0.5 percent dichlorvos prepared
from a 23.5% E.C. After treatment, the rough rice was mill processed
and the products (brown rice, rice bran, milled rice and rice hulls)
were collected. Residue samples were taken 6 hours, 1, 5, 10, 20 and
30 days after application. Dichlorvos residues in the rough rice one
day after treatment were 7.3, 4.6 and 1.6 ppm for the 1.5 percent, 1
percent and 0.5 percent applications, respectively. After 30 days,
residues for all three treatment rates had dissipated to less than 1.0
ppm. Residues in brown rice and milled rice, after 30 days, were
<0.05 ppm for all treatment rates. Rice bran treated at the highest
rate contained 0.4 ppm and rice hulls 3 ppm dichlorvos after 30 days.
Soybeans
Soybeans stored in open bins were exposed to resin strips for
intervals ranging from 13 to 126 days (Shell, 1970c). Samples were
taken through depths of 0-2" and 2-4" from the surface and the
residues observed are summarized in Table IV.
TABLE IV
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
13 .20 - .06 -
28 .06 - .03 -
42 .11 .07 .08 .03
57 - .08 - .03
70 .07 .08 .04 .03
84 .04 .04 .04 .03
TABLE IV (cont'd)
Average residues on soybeans after exposure to dichlorvos
0-2 inch depth 2-4 inch depth
Period of Strips per 1000 ft3 Strips per 1000 ft3
Exposure, 1 1.5 1 1.5
Days Residues, ppm Residues, ppm
97 .04 .075 .04 .06
111 .05 .04 .04 .04
125 .04 .04 .04 .04
Temperature - 65-96°F
Rel. Humidity - 48-76 percent
A test conducted by USDA, Stored Products Insect Research and
Development Laboratory (Padget, 1969), demonstrated that dichlorvos
residues on soybeans decrease rapidly. Initial residues of 5 ppm
decreased rapidly to 0.5 ppm or less after one week of storage at
80°F. Only trace amounts (0.1 ppm or less) of dichlorvos were found on
the soybeans after the second week of storage.
Coffee beans
Residues in bagged coffee beans were examined (Shell, 1965a) following
storage in a warehouse using resin strips at either one per 1000 cubic
feet or 1 per 2000 cubic feet. Some natural ventilation was allowed
for 9 hours daily.
Samples taken after 15 weeks storage from the surface and interior of
the bags did not contain detectable residues (limit 0.01 ppm), but one
sample exposed at the rate of one strip per 2000 cubic feet did
contain residues of 0.02 ppm, just above the detection limit.
Cocoa beans
In a trial with stacks of bagged cocoa beans carried out in the same
warehouse and under similar conditions (Shell, 1965a) as the above
coffee, residues in a bulked sample of cocoa from the top of the stack
reached 0.04 ppm, although none were detected in comparable samples
from the side of the stack. In a further trial (Shell, 1965a) with
another stored supply of cocoa beans, detectable residues (0.01 ppm)
were not found after 14 weeks exposure. Another trial (Shell, 1965b)
was undertaken in which sacks of cocoa beans were exposed for three
months. Surface samples taken from the top sacks were found to have a
maximum content of 0.04 ppm, whilst sub-surface samples and samples
from the middle of the sack did not contain detectable residues. In a
further trial in which exposure was 8 weeks, the maximum residue found
in surface samples from the top sacks was only 0.02 ppm. This was
mainly in the husks (0.03 ppm); the kernels contained only 0.01 ppm.
In a series of trials in Switzerland (Ciba), dichlorvos was applied to
cocoa beans in silos at rates ranging from 10 ppm through 100 ppm to
500 ppm. After three weeks storage at 15°C, the residues had fallen to
<0.1, 13 and 50 ppm, respectively, on the raw beans. When beans were
then roasted, the residues fell to <0.1, 0.2 and 0.5 ppm,
respectively. In the samples treated at 500 ppm, most of the residue
was in the mash cake with a minimum in the cocoa butter. Further
trials (Ciba) showed that the residues in cocoa beans treated at the
rate of 12 ppm with dichlorvos emulsion fell from 0.8 to 0.09 ppm
during 28 days in storage at 20°C.
Groundnuts
Sacks of bagged groundnuts (Shell, 1966b) were exposed for 82 days in
a warehouse at the recommended rate of one strip/1000 cubic feet. One
residue sample taken from the surface (2 cm) layer of the top sack was
found to contain 0.10 ppm dichlorvos, but two other similar samples
were found to contain only 0.01 ppm dichlorvos. One sample from the
middle of the top sacks contained 0.03 ppm, but in two further samples
no residues were found.
Residues arising from the exposure of foods in processing plants
Plants producing foods not processed further after exposure
In a cheese factory, in Minnesota, strips were placed throughout the
milk receiving and processing areas (Shell, 1964). A monitoring
experiment showed that the finished Cheddar cheese did not contain
detectable dichlorvos and DCA residues (i.e. below 0.03 ppm and 0.01
ppm, respectively). Similar trials in butter and ice cream factories
(Shell, 1965c) showed negligible residues of dichlorvos and DCA in
both products.
In tests carried out in cooperation with the German Public Health
Service in a cheese factory, Lindenberg, Edam and Danish Tilsit cheese
(each containing 45% fat) were exposed for four hours. Cheese samples
were cut into 20 mm thick slices following normal practice, and
exposed for a period of 30 to 60 minutes. Residues, which were
determined in the outermost 1 mm layers, were not detectable (Anon.,
1966).
Strips were hung in a cheese aging room at the rate of one strip/1000
cubic feet. Samples of cheese taken after six and ten weeks of
exposure showed residues of <0.04 to 0.10 ppm (Shell, 1965a). In the
case of milk (Shell, 1965d), whole milk was exposed in open milk
churns for periods of 24 and 48 hours in rooms where fresh strips were
installed at the rate of one strip/1000 cubic feet. None of the
representative samples taken contained measurable amounts of
dichlorvos (detection limit 0.02 ppm).
In a study in Kentucky (Shell, 1965d), strips were installed in a farm
dairy at the rate of one strip/1000 cubic feet. The air was humid and
the temperature about 75°F. The milk samples were taken after being
passed through an open coil cooler during the period 0-14 days after
the strips were hung. Residues of dichlorvos ranged from the limits of
detection to 0.04 ppm.
Plants handling foods which are processed after exposure
Fresh strips were suspended in areas of a plant where grading and
filleting of fish was carried out, and after two days exposure,
residues in the prepared product were not detected (detection limit
0.05 ppm) (Shell, 1965e).
Exposure tests were also carried out in a slaughterhouse (Shell,
1965c). The various products, beef, liver, T-bone steak, hamburger,
beef tongue and heart, pork liver, pork sausage, pork chops, pork
tongue and heart were exposed to dichlorvos resin strips during the
time normally required for processing. None of them showed any
dichlorvos or DCA residues; detection limit was 0.01 ppm. In this
test, a sample of bacon showed a residue of 1.4 ppm dichlorvos; DCA
was not found (detection limit 0.01 ppm). It was subsequently found,
however, that this particular sample had been returned to the cooling
room and was exposed for several days longer than normal. Further,
data cited later (Shell, 1966c) show that residues of dichlorvos are
removed upon cooking; specifically, bacon is cited as an example.
An experiment on similar lines was also conducted in a sausage factory
where strips were installed throughout the processing areas (Anon,
1966). No residues were detected in either meat or finished sausages.
FATE OF RESIDUES
In animals
Page (1970) has reviewed the extensive literature on the metabolism of
dichlorvos and in the same paper has reported on extensive trials
designed to show the metabolic fate and tissue residues of dichlorvos
following oral administration. The results of this work and earlier
studies are summarized in the metabolic chart given under "Biochemical
aspects" (Fig. 1).
A series of experiments have been conducted recently in pigs to
provide further information on the metabolic fate of the dichlorvos
portion of the dichlorvos molecule. In a preliminary study, 1-14C
labelled dichlorvos was infused into the isolated duodenal loop of
anaesthetized male pigs for four hours at the rate of 1 mg/kg
body-weight/hour. Urine, bile and peripheral and portal blood were
collected hourly and analysed for dichlorvos and its previously
reported mammalian metabolites. Dichlorvos, dichloroacetaldehyde and
dichloroacetic acid could not be detected in the blood. "The
concentration of 30 ppm dichlorvos in the intestinal lumen resulted in
less than 0.05 ppm dichlorvos in the blood. Neither could
dichloroacetaldehyde or dichloroacetic acid be detected in the blood.
The level of 2,2 dichloroethanol in the blood rose from 0.2 ppm after
one hour to 0.9 ppm after four hours" whereas o-demethyldichlorvos
occurred only near the limit of detection (0.03 ppm or less). In all
cases, levels of 14-carbon radioactivity in urine, bile and tissue
were higher than the level of 2,2-dichloroethanol, thus in urine the
respective figures were 17 ppm compared to 3 ppm; in bile 12 and 0.5;
in kidney 21 and 1.2; in liver 17 and 0.9 and in muscle 2.2 and 0.6
ppm. The figures indicated that 2,2-dichloroethanol or its conjugates
are not the end products of the metabolism. In other experiments, pigs
were given sustained release pellets of 14C- or 36Cl-labelled
dichlorvos at an oral dose of 40 mg/kg body-weight, and excreta and
tissue were analysed after various intervals. A similar situation to
that found in the infusion experiment occurred. It was concluded that
there was a rapid dechlorination of dichloroacetaldehyde and that the
radioactive carbon enters the metabolic pool possibly via glyoxal
(which compound has been found in in vitro but not in in vivo
studies). No retention in tissue of any chlorine containing organic
compound has been found, and the end product of the chlorine fragment
appears to be chloride ion (Page, 1970).
All the data support the conclusion that when administered orally over
a period of weeks, dichlorvos does not result in detectable toxic
residues in tissues of the parent compound or product-related
metabolites. The results of some of the published trials are
summarized in Table III.
In plants
New data have become available on the chemical degradation of
dichlorvos residues on plants. Careful experiments with various plant
species (cotton, maize, peas, beans) have demonstrated that dichlorvos
is rapidly lost from leaf surfaces by volatilization and by hydrolysis
and, disregarding the fraction of insecticide lost by volatilization
during the first few minutes after application, the half-life of the
compound under laboratory conditions is of the order of a few hours. A
small percentage (0.5-5 percent) of the dichlorvos deposited, appears
to penetrate into the waxy layers of leaf and fruit cuticle where it
persists longer. Dichlorvos taken up by plants from aqueous solutions
by the root system and translocated into aerial parts is less rapidly
lost than insecticide applied to leaf and fruit surfaces, but even
under these conditions the half-life of the compound is no more than
12 hours.
Various measurements have confirmed the rapid disappearance of
dichlorvos from vegetable and fruit crops under practical conditions
(see Table 1). Residues in vegetables were below 0.1 ppm two days
after application and were no longer detectable on the third day.
Dichlorvos was somewhat more persistent in fruits, but the residues
are below 0.1 ppm three days after application.
In vegetable crops or mushrooms grown under cover or in greenhouses,
losses of dichlorvos from leaf and fruit surfaces by volatilization
are less rapid than outdoors. However, on all crops analysed so far,
including such broad leaf vegetables as lettuce, dichlorvos residues
have been below 0.5 ppm two days after treatment and below 0.3 ppm
after three days.
Casida et al. (1962) used 32P-labelling to study the fate of
dichlorvos residues on maize, cotton and peas; a 0.1 percent aqueous
solution of dichlorvos was applied uniformly to the upper leaf
surfaces of each plant. It was found that about half of the dose
volatilized within five minutes. An additional 45 percent was absorbed
within 20 minutes, so that only 5 percent remained on the surface 20
minutes after application.
The surface residue was nonhydrolysed dichlorvos while 70-80 percent
of the absorbed material was organosoluble and was presumably
nonhydrolysed dichlorvos. However, hydrolysis proceeded rapidly.
Ignoring the dichlorvos lost by volatilization during the first five
minutes after treatment, the half-life of the dichlorvos was 1.2 hours
with loss primarily occurring by hydrolysis. In addition to the
volatilization of the dichlorvos, which resulted in the total
dichlorvos equivalent to dropping from 100 per leaf at zero time to 35
per leaf at 30 minutes, the hydrolysis products were also lost from
the treated leaves. Only 14 units remained after 24 hours and 7 units
after 72 hours, despite the fact that no dichlorvos or other
organosoluble derivatives were detected after eight hours.
The same authors carried out experiments with radio-labelled
dichlorvos and showed that, when solutions of the insecticide were
absorbed by the roots of plants, 77 percent of the absorbed dichlorvos
appeared as hydrolysis products in peas and 33 percent in maize and
cotton. Once the plants were removed from the insecticide source, the
hydrolysis of the absorbed dichlorvos followed first order reaction
kinetics, with half-life values for both maize and cotton of 9 hours
and for peas for three hours. Following systemic uptake through the
roots of plants, dichlorvos is hydrolysed rapidly, but the products of
hydrolysis are not lost from the plant during a 40 hour
post-absorption period. Bull and Ridgeway (1969) using 32P labelled
dichlorvos showed that 54 percent of the amount applied was lost
within one hour by volatilization from the treated leaves. The major
metabolite of dichlorvos was dimethyl phosphate; only minor amounts of
o-desmethyldichlorvos were detected.
Casida et al. (1962) carried out studies with mice on the acute
toxicity of dichlorvos and potential hydrolysis products and showed
relatively high toxicity associated only with the parent compound.
In storage and processing
Dichlorvos is readily lost by evaporation from stored products,
including raw grain and cereal products. Except at very low
temperatures, it appears likely that dichlorvos is also hydrolysed to
biologically inactive metabolites.
A study (Shell, 1970d) using a number of materials with abnormally
high dichlorvos residues showed that residues can be almost completely
removed by cooking. Rice containing 4.5 ppm dichlorvos has been shown
to lose 90% of the residues on boiling from 20 to 30 minutes. When
rice containing 19 ppm was similarly treated, 98% of the residue was
destroyed. Samples of flour containing dichlorvos residues in the
range 4.5 to 14 ppm have been found to lose about 90% of the residue
on baking for 10 to 12 minutes at 230°C in the preparation of
biscuits. Boiling the flour with water for 2 minutes, as in the
preparation of gravy, has been shown to decrease residue levels by
97%. Residues in cocoa have been shown to be lost during the
manufacture of cocoa butter. No dichlorvos was found in the fat, the
nibs, liquor or mash cake prepared from beans containing 2.9 ppm
dichlorvos.
Residues in food moving in commerce
Two shiploads of wheat were treated with dichlorvos emulsion
immediately prior to shipment from Australia. The dichlorvos was
applied at a nominal concentration of 6 ppm (found to be 4.6 -5.5
ppm). Upon arrival in the Netherlands, the grain was thoroughly
sampled and all samples were found to contain residues below 2 ppm
(range 0.68-1.59 ppm - mean 0.96 ppm). A major portion of each
consignment was trans-shipped to the United Kingdom,where it was
sampled for analysis on arrival. All samples revealed dichlorvos
residues, but at levels lower than those detected in the Netherlands,
with a range of 0.4-1.3 ppm and a mean of 0.68 ppm. From the results
of analysis prior to loading and at the end of a 7-week voyage, it was
calculated that the half-life of the dichlorvos residues was between
20 and 23 days.
Before the general release of dichlorvos resin strips, many
experiments were carried out to simulate the conditions that would be
encountered in practical usage. Subsequent data which related to food
exposed in shops, restaurants and homes under various practical
conditions where the dichlorvos fumigant strips were used according to
directions, broadly show that results from the simulated experiments
overestimated the residue levels.
Surveys have been carried out in the United Kingdom (17 shops) and in
France (20 shops) to establish the residue level of dichlorvos in
unwrapped foods bought by the public from shops using the strips at
the recommended rate under normal practical conditions. The residues
found are shown in Tables V and VI.
The somewhat higher results obtained in some of the commodities in the
French survey may have been due to the common practice there of
closing the shops completely during the heat of the afternoon.
TABLE V
Residues of dichlorvos (ppm) in food purchased from shops in France
Sampling time : days after hanging strips
7 42 70
Commodity Range Mean Range Mean Range Mean
Bread <0.05-0.10 <0.05 <0.05 <0.05 <0.05-0.06 <0.05
Cakes <0.05-0.24 0.08 <0.05-0.30 0.10 <0.05-0.17 0.07
Apples <0.05-0.14 0.06 <0.05-0.32 0.07 <0.05-0.30 0.06
Lettuce <0.05 <0.05 <0.05 <0.05 <0.05 <0.05
Tomatoes <0.05-0.08 <0.05 <0.05 <0.05 <0.05 0.05
Ham <0.05-0.08 0.06 <0.05-0.28 0.06 <0.05-0.09 0.05
Cheese
(cow) <0.05-0.08 0.06 <0.05-0.16 0.06 <0.05-0.15 0.06
Cheese
(goat) <0.05-0.35 0.13 <0.05-0.25 0.08 <0.05-0.30 0.08
A number of surveys have been conducted in the U.S.A. on the residue
levels in foods which had been exposed to resin strips in restaurants.
In the first of two studies conducted in restaurants near Cincinnati,
Ohio, typical restaurant meals were subjected to an exaggerated
exposure of 24 hours to 5-10 day old strips. In a second study, meals
were exposed for 22 hours to strips which were initially 51 days old.
Under the conditions of the first survey, residues were only 0.05-0.15
ppm of apparent dichlorvos: under the second survey, no residues (less
than 0.04) were found. In another investigation carried out in four
different restaurants in New York City under normal operating
conditions, ready to eat meals were sampled as composite samples.
Whole meals were sampled at various stages in the life of the strips,
ranging from 24 hours to 4 weeks. The meals consisted of various
mixtures of meat or fish and vegetables and varied widely, in
composition. The following residues were found:
Restaurant No. of meals sampled Residue range,ppm
A 5 0.04 -0.10
B 5 0.02 -0.11
C 9 0.03 -0.16
D 5 0.03 -0.08
To obtain information on food residues arising from the typical
practice of hanging strips in kitchens, surveys were carried out in
homes in the U.K. and France. Food samples were made up of whole food
and beverage intakes for one day. Each home was sampled the day before
the strips were hung and one week and six weeks later. In the U.K.
study, no residues exceeded 0.09 ppm, and the mean of all samples
taken at one week was 0.034 ppm. The samples at six weeks showed no
residues in excess of 0.05 ppm, with a mean value of 0.034 ppm.
Similar studies in 14 French homes showed no residues in excess of
0.07 ppm, and the mean of all samples taken at one week was 0.024 ppm.
The six week samples showed no residues in excess of 0.03 ppm.
METHODS OF RESIDUE ANALYSIS
Cholinesterase inhibition
A common technique which has been used for routine residue
determinations in commodities of known treatment history is based on
cholinesterase inhibition. Human and animal plasma cholinesterases are
considerably more sensitive to dichlorvos than erythrocyte
cholinesterases, and are therefore particularly suitable for residue
measurements. Out of date stocks of human blood plasma or horse serum
are satisfactory sources of enzyme.
Extraction and clean-up prior to the cholinesterase assay are normally
fairly simple, involving maceration of the material with a solvent of
medium polarity (chloroform, methylene chloride), filtration and
careful evaporation of the organic phase in the presence of water.
More elaborate procedures may be required for plant materials with a
high fat content, each as cocoa beans or groundnuts, and for certain
animal products such as milk, cheese and butter. Steam distillation of
dichlorvos prior to the enzyme assay has been suggested, not only for
removing interference,but also for increasing the specificity of the
residue method. For the enzyme assay proper, any of the common
techniques for measuring cholinesterase activity may be used. The
sensitivity of these methods is 0.05 to 0.1 ppm. Cholinesterase
residue analysis for dichlorvos has recently been automated by using
Technicon Auto-Analyser facilities.
TABLE VI
Residues of dichlorvos (ppm) in food purchased from shope in the UK1
Sampling time : days after hanging strips
No. of No. of 2 28 70
Commodity samples shops
Range Mean2 Range Mean Range Mean
Cooked meat 35 7 <0.05-0.13 <0.05 <0.05-0.10 <0.05 <0.05-0.09 <0.05
Cheese 35 7 <0.05-0.07 <0.05 <0.05-0.12 0.05 <0.05-0.12 <0.05
Apples 49 10 <0.05-0.26 0.08 <0.05-0.54 0.14 <0.05-0.19 0.06
Tomatoes 33 11 All <0.05 <0.05 All <0.05 <0.05 <0.05-0.05 <0.05
Lettuce 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Broad 27 9 All <0.05 <0.05 All <0.05 <0.05 All <0.05 <0.05
Cakes 19 7 <0.05-0.07 <0.05 <0.05-0.05 <0.05 <0.05-0.05 <0.05
1 No residues of DCA (0.03 ppm) were found in any of the samples
2 Mean values are geometric means and are calculated by assuming that samples containing less than the
detectable level (<0.05 ppm) in fact contain <0.025 ppm
Such automated methods permit rapid routine measurements of large
series of samples.
Since other organophosphorus insecticides or carbamates can interfere,
the cholinesterase inhibition method is not suitable for detecting and
measuring dichlorvos in samples of unknown or ill-defined treatment
history. Both thin-layer and paper chromatographic procedures are
available for separating dichlorvos from other
cholinesterase-inhibiting pesticides. In these methods, the
cholinesterase inhibitors are normally revealed by enzymatic spot
tests involving colour reactions of the substrate or its breakdown
products after hydrolysis. It must be remembered that dichlorvos is a
very volatile compound which, upon solvent evaporation, heating,
aeration, etc. is easily lost from vessels or chromatographic support
materials. These procedures are therefore recommended for qualitative
purposes, but great care must be taken when they are used in
quantitative analysis.
Bioassay
The vinegar fly (Drosophila melanogaster) and daphnia (Daphnia
magna) are suitable test organisms for the bioassay of dichlorvos in
plant materials. A specific test, which involves absorption barrier
chromatography and which distinguishes between dichlorvos and other
insecticides, has been described by Sun and Johnson (1963). The
sensitivity of the bioassay methods is about 0.1 ppm.
Colorimetry
Although colorimetric techniques have been developed mainly for
measuring traces in air samples or aqueous solutions, some of them can
be used for the determination of residues in food materials, provided
that the sensitivity required is not better than 0.3-1 ppm. Thus, the
color reaction of dichlorvos with alkaline resorcinol has given
satisfactory results with extracts of animal food commodities. This
method demonstrates not only the parent insecticide, but also
dichloroacetaldehyde, one of its potential hydrolysis products. Acting
on suggestions made by Hodgson and Casida (1962), Hughes (1963)
measured residues of dichlorvos and of dichloroacetaldehyde in air,
using colorimetry, following reaction with alkali and
2,4-dinitrophenylhydrazine. This method can also be used for
determining residues in food commodities. A further colorimetric
procedure, based on formation of an orange-red complex between
dichlorvos and acetone in the presence of alcoholic potassium
hydroxide, has been described by Mitsui.
Gas-liquid chromatography (GLC)
The most specific residue methods currently available rely on the
separation and detection of dichlorvos by gas chromatography. The
sensitivity of these procedures is about equal to that obtained with
the cholinesterase techniques, i.e. 0.03-0.1 ppm. Methods have been
described by El-Refai and Guiffrida who used the sodium thermionic
detector selective to phosphorus, and by Boone (1965) who used a
microcoulometric detection system. Both methods use fairly high column
temperatures, and the possibility of thermal or catalytic
decomposition of dichlorvos on certain column materials must not be
overlooked. Gas chromatographic residue measurements in industrial
laboratories are usually carried out at lower column temperatures
(150°C), and such stationary phases an phenyl diethanolamine succinate
and Reoplex 400 have given satisfactory retention times and
separations from interfering insecticides.
Ivey and Claborn (1969) have published a GLC method for the
determination of dichlorvos in milk, eggs and various body tissues of
cattle and chickens. Using a gas chromatograph equipped with a flame
photometric detector, they were able to detect 0.003 ppm of dichlorvos
in milk and 0.002 ppm in body tissues and eggs. Methylene
dichloridehexane was used to extract milk and hexane was used for fat
and chicken skin. To recover dichlorvos from muscle, blood and eggs
the authors used a preliminary extraction with acetonitrile.
Abbott at al.(1970) report the successful application of the use of
the caesium bromide tipped detector of Hartmann (1966) for the
determination of dichlorvos on a variety of foods in a total diet
survey.
Drager (1968) has published a gas chromatographic method for
determining dichlorvos residues in plants and milk. In this method,
plant material is extracted by macerating it with methanol and water.
The dichlorvos in the extract is partitioned into a mixture of ether
and petroleum ether. The residue is then transferred into ethanol, and
dichlorvos is determined by gas chromatography using the phosphorous
detector.
Minett and Belcher (1969) have developed a method for the
determination of dichlorvos residues in wheat using GLC techniques.
For the extraction of the ground wheat the authors used ethanol.
Many of the published methods and many residue reports indicate that a
variety of non-polar and polar solvents, such as methylene dichloride
were used to extract the dichlorvos residue from the food commodity.
Minett and Belcher (1969) and Elms at al. (1970) have shown that
dichlorvos cannot be recovered quantitatively from plant materials,
especially grain, by the use of solvents even as polar as methylene
dichloride. Water or water-miscible solvents, such as methanol or
ethanol, are absolutely essential for the recovery of dichlorvos
residues from plant materials.
Collaborative studies are currently being carried out in the United
Kingdom on a method of detecting dichlorvos in grain. The procedure
uses GLC with thermionic or flame photometric detection following
extraction with methanol or acetone.
APPRAISAL
Dichlorvos is used extensively for the control of insect pests of
importance in public health, in homes, warehouses, food stores and
transport, stored grain as well as insects attacking domestic animals.
It is also finding increasing use on horticultural and field crops and
is registered in over forty eight countries. Dichlorvos impregnated
resin strips are extensively used to control insect pests, especially
flies, in homes, stores and food processing establishments.
Extensive data on residues in food commodities were available from
many countries in the form of published reports end submissions from
three manufacturers. All data indicate that the level of residues
occurring in raw agricultural commodities is low and that the residue
levels decline rapidly. Due to the comparatively high vapour pressure
of dichlorvos, the applied deposit is quickly lost by volatilization,
but that portion which in absorbed into plant tissues undergoes
hydrolysis to inactive metabolites.
Dichlorvos applied to or fed to domestic animals undergoes rapid
detoxification and degradation in all species examined and is unlikely
to produce significant residues in meat, milk or eggs.
The use of dichlorvos impregnated strips for controlling insects in
warehouses, stores and shops gives rise to detectable residues in
stored or prepared foods. Residues resulting from exposures at rates
much higher than the recommended ones have been shown to decrease very
rapidly on exposure to the atmosphere. They also are readily destroyed
during the preparation of many products for consumption (e.g. by
washing or by cooking).
The proposal that a tolerance in meat and meat products was required
in view of the use of dichlorvos-resin fumigant strips in meat storage
and processing places in some countries (CCPR, 1971) was considered by
the Meeting.
Considerable data were available on the uptake of dichlorvos by many
different foods under varying conditions of storage and exaggerated
exposure to dichlorvos-resin strips, It was recognized that, under
some conditions, residues as high as 0.1 ppm could result but even in
fatty meat products the uptake was only at the surface. Residues
declined during atmospheric exposure and were completely destroyed by
cooking. Meat and meat products were included among the commodities
for which a 0.1 ppm tolerance was recommended. These products were
specially considered because of the request made at the 1970 meeting
of the Codex Committee on Pesticide Residues (CCPR, 1971).
RECOMMENDATIONS FOR TOLERANCES
The following tolerances are for residues likely to be found in raw
commodities at harvest, in stored products shortly after treatment and
in miscellaneous prepared foods exposed during preparation and
storage. Recommendations have been made on broad categories of fruit
and vegetables, because extensive data have indicated that when
dichlorvos is applied to these crops the level of residues shortly
afterwards does not vary significantly on different varieties.
Due to the transient nature of the insecticide, residue levels will
continue to decline during shipment and storage, with a probable half
life of less than one day on fresh fruit, vegetables and miscellaneous
foods. Residues in food at time of consumption have normally been
below the limits of detection by current analytical methods.
Foodstuff Tolerance
Recommended Period on which
recommendation
based
Cocoa beans 5 ppm
Raw grain (wheat, rice,
rye, oats, barley,
maize, sorghum, etc,) 2 ppm
Coffee beans, soybeans,
lentils, peanuts (groundnuts) 2 ppm
Mushrooms 0.5 ppm 2 days
Milled products from raw
grain 0.5 ppm
Fresh vegetables
(excluding lettuce) 0.5 ppm 2 days
Tomatoes 0.5 ppm 1 day and post
harvest
Lettuce 1 ppm 2 days
Fresh fruit (apples, pears,
peaches, strawberries, etc.) 0.1 ppm 2 days
Meat of cattle, sheep,
goats, pigs and poultry 0.05 ppm
Eggs (on shell free
basis) 0.05 ppm
Milk (whole) 0.02 ppm
Miscellaneous food items
not otherwise
specified 001 ppm
As recommended in previous monographs, the content of
dichloroacetaldehyde (DCA) should be reported where known.
Furthermore, the amounts found should be added to those for dichlorvos
when assessing adherence to the tolerances recommended.
FURTHER WORK OR INFORMATION
DESIRABLE
1. Continued observation of the effects of repeated exposure of man
to dichlorvos in order to determine if there is any qualitative
or quantitative differences between the metabolic route following
oral or inhalation intake.
2. Data from additional countries on residues in commodities moving
in international trade.
3. Analytical methods capable of recovering and determining residues
of dichlorvos in foods should be established for regulatory
purposes.
REFERENCES
Abbott, D.C., Crisp, S., Tarrant, K.R. and Tatton, J.O'G. (1970)
Pesticide residues in the total diet in England and Wales 1966-69.
Pesticide Science, 1 (i):10-13
Anon. (1966) Communication from F.I.D. Nordrhein-Westfalen to Deutsche
Shell Chemie, Frankfurt, dated 20.1.66 (unpublished)
Anon. (1966) Communication from Hamburg Public Health Service to
Deutsche Shell Chemie, Frankfurt, dated 19.7.66 (unpublished)
Arthur, B.W. and Casida, J.E. (1957) Metabolism and selectivity of
o,o-dimethyl 2,2,2-trichloro-1-hydroxyethyl phosphonate and its acetyl
and vinyl derivates. J. Agr. Fd. Chem., 5 : 186-192
Blair, D., Hutson, D.H. and Pickering, B.A. (1970) The comparative
metabolism of (vinyl-14C) Vapona in rats after inhalation exposure
and oral ingestion of the compound. Unpublished draft summary from the
Tunstall Laboratory, Shell Research Ltd., Sittingbourne
Boone, G.H. (1965) Determination of Dibrom and DDVP residues in some
fruit and vegetable crops by microcoulometric gas chromatography. J.
A.O.A.C., 48 : 748-752
Boyer, A.C. (1969) I50 values for the inhibition of human and monkey
blood cholinesterases by dichlorvos. Unpublished technical information
report prepared by Shell Development Co.
Bull, D.L. and Ridgway, R.L. (1969) Metabolism of trichlorfon 1969 in
animals and plants. J. Agr. Fd. Chem., 17 : 837-841
Carson, S. (1969) Teratology studies in rabbits. Unpublished report
from Food and Drug Laboratories Inc., Maspeth, N.Y., prepared for
Shell Chemical Co.
Casida, J.E., McBride, L. and Niedermeier, R.P. (1962) Metabolism of
2,2-dichlorovinyl dimethyl phosphate in relation to residues in milk
and mammalian tissues. J. Agr. Fd. Chem., 10 : 370-376
Cavagna, G., Locati, G. and Vigliani, E.C. (1969) Clinical effects of
exposure to DDVP (Vapona) insecticide in hospital wards. Arch.
Environmn. Hlth., 19 : 112-123
Cavagna, G., Locati, G. and Vigliani, E.C. (1970) Exposure of newborn
babies to 'Vapona' insecticide. Europ. J. Toxicol. 3 : 49-57
Cavagna, G. and Vigliani, E.C. (1970) Problèmes d'hygiène et de
sécurité dans l'emploi du Vapona insecticide dans les locaux
domestiques. Med. d. Lavoro, 61 : 409-423
CCPR. (1971) Report of the Fifth Session of the Codex Committee on
Pesticide Residues para. 165. Alinorm 71/24
Ciba, A.G. (1970) Basle - Submission to FAO/WHO Joint Meeting -
Dichlorvos
Drager, G. (1968) Gas chromatographic method for determining
dichlorvos residues in plants and milk. Pflanzenschutz - Nachrichten.,
21 : (3) 373-380
Elms, K.D., Kerr, J.D. and Champ, B.R. (1970) Breakdown of malathion
and dichlorvos mixture applied to wheat. In press
FAO/WHO. (1965) Evaluation of the toxicity of pesticide residues in
food. FAO Meeting Report No. PL/1965/10/1; WHO/Food Add./27.65
FAO/WHO. (1967) Evaluation of some pesticide residues in food, FAO
PL:CP/15, WHO/Pood Add./67.32
FAO/WHO. (1968) 1967 Evaluation of some pesticide residues in food.
FAO/PL:1967/M/11/1; WHO/Food Add./68.30
Green, A.A. and Wilkin, D.R. (1968) The control of insects in bagged
grain by the injection of dichlorvos. J. Stored Prod. Res., 5 :
11-19
Hartmann, C.H. (1966) Phosphorus detector for pesticide analysis. Bull
Environm. Contam. and Toxicol., 1 : 159-168
Hine, C.H., Beltran, S., Cavalli, R.D., Keating, J.M., Keating, W.C.
Jr. and Sample, H.G. Jr. (1966) Pre-clinical pharmacologic studies on
dichlorvos in formulation (V-12). Unpublished report from the Hine
Laboratories, Inc., submitted to Shell Chemical Co.
Hine, C.H. (1970) Arizona Vapona (DDVP) human study program.
Chromosomal analysis. Unpublished report from the Hine Laboratories,
Inc., prepared for Shell Chemical Research Development Co.
Hodgson, E. and Casida, J.E. (1962) Mammalian enzymes involved in the
degradation of 2,2-dichlorovinyl dimethyl phosphate. J. Agr. Fd.
Chem., 10 : 208-214
Hughes, G.T. (1963) Colorimetric determination of low concentrations
of 2,2-dichlorovinyl dimethyl phosphate in the atmosphere. Analyst,
88 : 318-319
Hunter, C.G. (1969) Report on initial studies of deliberate exposure
to high concentrations of dichlorvos by human subjects. Unpublished
report from the Tunstall Laboratory, Shell Research Ltd.,
Sittingbourne
Hunter, C.G. (1970) Dichlorvos: inhalational exposures with human
subjects, Part 1. Group research report TLGR. 0061.70
Ivey, M.C. and Claborn, H.V. (1969a) Kerrville, Texas, Laboratory,
Entomology Research Division, ARS, USDA. Results of research on
residues of dichlorvos in milk and body tissue of dairy cows treated
for fly control
Ivey, M.C. and Claborn, H.V. (1969b) GLC determination of dichlorvos
in milk, eggs and various body tissues of cattle and chickens. Journal
of the AOAC, 52 (6) : 1248-1251
Kimbrough, R.D. (1970) Work sited in "Summary of Vapona R
insecticide review", report of a meeting chaired by L. Golberg at
Cincinnati, 9 February 1970
Kirkpatrick, R.L., Harein, P.K. and Cooper, C.V. (1968) Laboratory
tests with dichlorvos applied as a wheat protectant against rice
weevils. J. of Econ. Entomol. 61 : 356-358
Lancaster, J.L. (1962) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lancaster, J.L. (1963) (in cooperation with Shell Development Co.
Residue project from University of Arkansas (unpublished)
Lofroth, G. (1969a) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Paper presented at
a meeting of the Children's Cancer Research Foundation, Boston, 3
September 1969
Lofroth, G. (1969b) Alkylating property of 2,2-dichlorovinyl dimethyl
phosphate (dichlorvos, DDVP): a disregarded hazard. Unpublished report
reproduced in part in Environmental Mutagen Society Newsletter, No, 2:
p. 21-24
Lofroth, G. (1970) Alkylation of DNA by dichlorvos.
Naturwissenschaften, 57 : 393-394
Loomis, E. and Hodel, E. (1965) (in cooperation with Shell Development
Co. Residue project from Rio Linda, California (unpublished)
Minett, W. and Belcher, R. (1969) Determination of malathion and
dichlorvos residues in wheat grains by gas-liquid chromatography. J.
Stored Prod. Res., 5 : 417-421
Modesto home trials with Vapona insecticide strips
Nelson, C.B. (1968) (in cooperation with Shell Chemical Co. Residue
project from Fillmore, California (unpublished)
Noetzel, D. (1964) (in cooperation with Shell Development Co. Residue
project from North Dakota State University (unpublished)
Padget, T.J. (1968) Evaluation of dichlorvos as a grain treatment for
protection against the white-fringed beetle. USDA Plant Pest Control
Laboratory (unpublished)
Padget, T.J. (1969) Communication on the evaluation of dichlorvos as a
grain treatment for protection against the white-fringed beetle
(unpublished)
Page, A.C. (1970) Metabolic fate and tissue residues following oral
administration of dichlorvos. Collection of unpublished reports
prepared and submitted by Shell Chemical Co.
Palut, D., Grzymala, W. and Syrowatka, T. (1969) Investigation of the
metabolism of some organophosphorus insecticides on animal model
systems. II Hydrolytic degradation in vitro (original in Polish),
Roczn. Zak. Hig. (Warsz.), 20 : 551-555
Pitts, C.W. (1961) (in cooperation with Shell Development Co. Residue
project from Kansas State University (unpublished)
Preussmann, R. (1967) Analytical determination of alkylating agents.
Zweite Konferenz über aktuelle Probleme der Tabaksforschung, Freiburg,
p. 92 (cited by Lofroth, 1969, q.v.)
Preussmann, R. (1968) Direct alkylating agents as carcinogens. Fd.
Cosm. Toxicol, 6 : 576-577
Preussmann, R., Schneider, H. and Epple, F. (1969) Untersuchungen zum
Nachweis Alkylierender Agentien. Arzneimittelforschung, 19 :
1059-1073
Roger, J.C., Chambers, H. and Casida, J.E. (1964) Nicotinic acid
analogs: effects on response of chick embryos and hens to
organophosphate toxicants. Science, 144 : 539-540
Roger, J.C., Upshall, D.G. and Casida, J.E. (1969) Structure activity
and metabolism studies on organophosphate teratogens and their
alleviating agents in developing hen eggs with special emphasis on
Bidrin. Biochem. pharmacol., 18 : 373-392
Sasinovich, L.M. (1968) The maximum permissible concentration of DDVP
in the air of the working zone. Gig. i Sanit, 33 (12) 35-39
Sax, K. and Sax, H.J. (1968) Possible mutagenic hazards of some food
additives, beverages and insecticides. Jap. J. Genetics, 43 : 89-94
Shaw, F. (1964) (in cooperation with Shell Development Co. Residue
project from University of Massachusetts (unpublished)
Shell Development Co. (1964) Modesto Project No. RES 64-111
(unpublished)
Shell Research Ltd. (1965a) Woodstock Technical Memo. 165/65
(unpublished)
Shell Research Ltd. (1965b) Woodstock Technical Memo. 159/65
(unpublished)
Shell Development Co. (1965c) Modesto Project No. RES 65-42
(unpublished)
Shell Chemical Company, New York. (1965d) Brief in Support of Vapona
Insecticide Resin Strips. Section VII : 11-12. Submitted to Panel of
U.S.P.H.S. and U.S.D.A., 29.10.65
Shell Development Company. (1965e) Modesto Project No. RES 65-36
(unpublished)
Shell Research Ltd. (1966a) Woodstock Technical Service Note 146/66
(unpublished)
Shell Research Ltd. (1966b) Woodstock Technical Service Note 178/66
(unpublished)
Shell Research Ltd. (1966c) Woodstock Technical Memo 1/66
(unpublished)
Shell Chemical Co. (1967) Petition proposing a tolerance for the food
additive (pesticide chemical) dichlorvos in or on stored food
commodities. Section D-III
Shell Research Ltd. (1968) Woodstock WKGR. 0041.68 (unpublished)
Shell Chemical Co. (1969) Princeton laboratory residue analysis report
project no. PRL 69-62, (unpublished)
Shell Development Co. (1970a) The third Arizona home study. The
quantification of DDVP residues in foods consumed by human volunteers
exposed to "No-Pest" strip insecticide. (unpublished report)
Shell Chemical Co. (1970b) Princeton laboratory residue analysis
report project no. PRL 70-12, (unpublished)
Shell Chemical Co. (1970c) Princeton laboratory residue analysis
report project no. PRL 69-26 (II) (unpublished)
Shell Research Ltd. (1970d) Interim report - Residues of Vapona in
meals prepared in domestic kitchens. Woodstock Laboratory
(unpublished)
Singh, V.K. (1964) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. (1965) (in cooperation with Shell Development Co. Residue
project from Bio/Toxicological Research Associates (unpublished)
Singh, V.K. and Rainier, R.H. (1966) Three year chronic oral toxicity
of formulated dichlorvos in swine with special reference to possible
effects upon fertility and the viability of the offspring of the
animals fed continuously on diets containing the drug. Unpublished
report from Bio/Toxicological Research Associates, Spencerville, Ohio,
prepared for Shell Chemical Co.
Somme, L. (1967) A field trial with dichlorvos vapor for the control
of Ephestia Kühniella Zell (Lepidoptera, Phycitidae) in flour mills.
Reprinted from Journal of Stored Products Research, 4 (3)
Stevenson, D.E. and Blair, D. (1969) A preliminary report on the
inhalation toxicity of high concentrations of dichlorvos. Shell
Research Tunstall Report No. TLGR. 0024.69
Sun, Y.P. and Johnson, E.R. (1963) A new bioassay technique with
special reference to the specific bioassay of dichlorvos insecticide.
J. Econ. Entomol., 56 : 635-641
Vigliani, E.C. (1970) Results of recent studies on Vapona. Paper
presented at the 7th Shell Industrial Doctors' Meeting, Strasbourg,
27-29 May 1970, submitted by Shell International Research Ltd.
Vogin, E.E. (1969) Teratological studies with dichlorvos in rabbits.
Unpublished report from Food and Drug Laboratories, Inc., Maspeth,
N.Y., prepared for Shell Chemical Co.
Wisconsin Alumni Research Foundation No. 8030193 (1968) (in
cooperation with Shell Chemical Co. Residue project report
(unpublished).
Wisconsin Alumni Research Foundation (1968/9) (in cooperation with
Shell Chemical Co. Residue project reports on sheep, cattle and goats
(unpublished)
Witherup, S., Caldwell, J.S. Jr. and Hull, L. (1965) The effects
exerted upon the fertility of rats, and upon the viability of their
offspring, by the introduction of Vapona R insecticide into their
diets. Unpublished report from the Kettering Laboratory Cincinnati
Young, R., Jr. (1969) Dichlorvos infusion studies. Unpublished
technical information report prepared by Shell Development Co.
