
FAO Meeting Report No. PL/1965/10/1
WHO/Food Add./27.65
EVALUATION OF THE TOXICITY OF PESTICIDE RESIDUES IN FOOD
The content of this document is the result of the deliberations of the
Joint Meeting of the FAO Committee on Pesticides in Agriculture and
the WHO Expert Committee on Pesticide Residues, which met in Rome,
15-22 March 19651
Food and Agriculture Organization of the United Nations
World Health Organization
1965
1 Report of the second joint meeting of the FAO Committee on
Pesticides in Agriculture and the WHO Expert Committee on Pesticide
Residues, FAO Meeting Report No. PL/1965/10; WHO/Food Add./26.65
HEPTACHLOR
Chemical name
1,4,5,6,7,10,10-heptachloro-4,7,8,9-tetrahydro-4,
7-methyleneindene, or 1,4,5,6,7,8,8-heptachloro-3a,4,7,7a-
tetrahydro-4, 7-methanoindene.
Empirical formula
C10H5Cl7
Structural formula
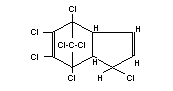 BIOLOGICAL DATA
Biochemical aspects
In plants and soil heptachlor is converted to its epoxide which
is more persistent on plants than the parent heptachlor (Gannon &
Bigger, 1958; Gannon & Decker, 1958).
Heptachlor is also readily transformed in the animal body into
heptachlor epoxide, which is stored in the fat of dogs and rats. Some
storage occurs in the liver, kidney and muscle, but none in the brain.
In dogs, after feeding heptachlor in a concentration of 1-3 mg/kg
body-weight the metabolite was found (Davidow & Radomski, 1953). When
heptachlor was fed at high dosage levels to dogs, small amounts of
unmetabolized heptachlor were also found in the fat of dogs but this
was not the case in the rat regardless of the level fed.
In the rat the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm
storage occurred in females but not in males and 0.1 ppm no storage
occurred in either sex. Female rats accumulate about 6 times as much
epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days the epoxide level in the milk rose to a maximum of 1.8 ppm
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at a 5 ppm and 2 at a 10 ppm dally intake). Three days
after feeding commenced, the minimum levels of heptachlor epoxide in
the milk were 0.26 and 0.34 ppm in the 2 cows fed 5 ppm and 0.23, and
0.65 ppm in the 2 cows fed 10 ppm; the maximum levels after 15 days'
feeding were 0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively
(Storherr et al., 1960).
Disturbance of the acid-base balance seem to be of great
significance in the mechanism of action of heptachlor (Spynu &
Osetrov, 1960).
Acute toxicity
Heptachlor
Animal Route LD50 mg/kg References
body-weight
Rat, male Oral 60-169* Negherbon, 1959; Velsicol, 1959
Mouse Oral 68 Negherbon, 1959
Guinea-pig Oral 116 Negherbon, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 mg/kg References
body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide
in the mouse are given as 40 and 100 mg/kg body-weight respectively
(Negherbon, 1959). In the rabbit the lethal dose of epoxide is 5-10
mg/kg body-weight (Velsicol, 1959).
The acute effects are neurotoxic disturbances like those
observed with other chlorinated hydrocarbons.
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide
(up to 60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleolim cytoplasm fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla (Stemmler & Hamdi, 1963).
Electron microscopic studies demonstrated an increase of rough and
smooth endoplasmic reticulum (Stemmler & Hamdi, 1963).
Dog. Three dogs given heptachlor epoxide orally in dosages of
2, 4 and 8 mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively.
One mg/kg given in the same way to 2 dogs resulted in the death
of one animal after 42 weeks, but the other survived for 52 weeks.
This animal showed only a slight growth inhibition. Doses of 0.25 and
0.5 mg/kg body-weight did not produce any sign of illness during 52
weeks (Velsicol, 1959).
When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor
epoxide were given to groups of 5 dogs (2 males and 3 females ranging
from 23 to 27 weeks of age) for 60 weeks. No deaths attributable to
heptachlor epoxide occurred. The weights of the male dogs tended to
be inversely proportional to the concentration of the compound in the
diet. The female dogs had normal weights. The liver weights of all
the animals were increased in proportion to the concentration of
heptachlor epoxide in the diet, and degenerative liver changes were
seen at 2.5 ppm and above (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed
diets containing 5, 10, 20, 40, 80, 160, and 300 ppm of heptachlor
epoxide for 2 years. Concentrations of 80 ppm or higher resulted in
100% mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver-weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females)(Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10,
20 and 40 ppm for 2 years showed significant increases in mortality
only in females at 40 ppm. Liver-weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
In another experiment which has already been considered1 CFN
rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5, 7.5
and 10 ppm. More details are now available about the tumour incidence
in untreated controls as well as the distribution or tumours among the
different groups. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34%) and 13/24 (54%) in
the control males and females respectively; it was 65/111 (58%) and
92/114 (80%) in the experimental males and females respectively.
Again, many tumours were located in endocrine organs. Liver tumours
were observed in 7 males and 12 females in the experimental groups
only (over-all incidence 19/225 (8.4%), but only two of them were
malignant (Kettering Laboratory, 1959).
Finally, in another experiment heptachlor dissolved in ethanol
was added to the diet of CF rats as 1.5, 3, 5, 7 and 10 ppm for 110
weeks. Each group, as well as an untreated control, included 40
animals (20 of each sex). Mortality was comparable in all groups. The
number of tumour-bearing animals was 16/40 at 0 ppm, 9/40 at 1.5 ppm,
13/40 at 3 ppm, 12/40 at 5 ppm, 15/40 at 7 ppm and 12/40 at 10 ppm.
Most tumours were found in the pituitary and other endocrine organs.
No liver tumours were recorded. No preferential tumour site in any
particular group was observed but all the 4 thyroid tumours observed
were in the 7 and 10 ppm groups (Witherup et al., 1955).
In some other experiments, the continuous exposure of rats to
doses of over 7 ppm of either heptachlor or its epoxide increased the
mortality during the suckling period. In others, 10 ppm fed to 3
generations of rats showed no adverse effects on reproductive
capacity, probability of survival or growth (Witherup et al., 1955;
Kettering Laboratory, 1959).
Comments on experimental studies reported
It is well established that heptachlor and its epoxide accumulate
in body fat and persist them for long periods. The formation of
epoxide is relatively rapid in the animal, plants and in the soil.
1 Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues. FAO
Meeting Report No. PL/1963/13 and WHO/Food Add./23 (1964).
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the 2
substances.
A sex difference in toxicity has been observed, particularly in
the rat, females accumulating heptachlor and its epoxide more than
males. The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to
explain. A possible relation with tumour formation has not been
demonstrated, while evidence has been presented that the change is
reversible.
Some suspicion of carcinogenicity can arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful. Nevertheless, until new data are
available from other long-term experiments involving other strains of
rats or other species a definite statement is not possible.
EVALUATION
From the data presented an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming every
effort should be made to see that the intake of heptachlor for man is
kept at the lowest possible level.
Further work required
As the residue in the crop may contain the epoxide, the
experiments should be done with this compound also. A maximum
no-effect level should be determined in more than one species.
Additional long-term toxicity studies in rats and other species are
required.
REFERENCES
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely. R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
The Kettering Laboratory (1959) University of Cincinnati
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S.,15, 47
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Radomski, J. L. & Davidow B. (1953) J. Pharmacol. exp. Ther., 107,
266
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1960) Chem. Abstr. 54, 13435
Stemmler, K. L. & Hamdi, E. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Stemmler, K. L. & Jolley, W. P. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S. et al. (1955) Data submitted by the Kettering Laboratory,
University of Cincinnati
BIOLOGICAL DATA
Biochemical aspects
In plants and soil heptachlor is converted to its epoxide which
is more persistent on plants than the parent heptachlor (Gannon &
Bigger, 1958; Gannon & Decker, 1958).
Heptachlor is also readily transformed in the animal body into
heptachlor epoxide, which is stored in the fat of dogs and rats. Some
storage occurs in the liver, kidney and muscle, but none in the brain.
In dogs, after feeding heptachlor in a concentration of 1-3 mg/kg
body-weight the metabolite was found (Davidow & Radomski, 1953). When
heptachlor was fed at high dosage levels to dogs, small amounts of
unmetabolized heptachlor were also found in the fat of dogs but this
was not the case in the rat regardless of the level fed.
In the rat the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm
storage occurred in females but not in males and 0.1 ppm no storage
occurred in either sex. Female rats accumulate about 6 times as much
epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days the epoxide level in the milk rose to a maximum of 1.8 ppm
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at a 5 ppm and 2 at a 10 ppm dally intake). Three days
after feeding commenced, the minimum levels of heptachlor epoxide in
the milk were 0.26 and 0.34 ppm in the 2 cows fed 5 ppm and 0.23, and
0.65 ppm in the 2 cows fed 10 ppm; the maximum levels after 15 days'
feeding were 0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively
(Storherr et al., 1960).
Disturbance of the acid-base balance seem to be of great
significance in the mechanism of action of heptachlor (Spynu &
Osetrov, 1960).
Acute toxicity
Heptachlor
Animal Route LD50 mg/kg References
body-weight
Rat, male Oral 60-169* Negherbon, 1959; Velsicol, 1959
Mouse Oral 68 Negherbon, 1959
Guinea-pig Oral 116 Negherbon, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 mg/kg References
body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide
in the mouse are given as 40 and 100 mg/kg body-weight respectively
(Negherbon, 1959). In the rabbit the lethal dose of epoxide is 5-10
mg/kg body-weight (Velsicol, 1959).
The acute effects are neurotoxic disturbances like those
observed with other chlorinated hydrocarbons.
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide
(up to 60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleolim cytoplasm fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla (Stemmler & Hamdi, 1963).
Electron microscopic studies demonstrated an increase of rough and
smooth endoplasmic reticulum (Stemmler & Hamdi, 1963).
Dog. Three dogs given heptachlor epoxide orally in dosages of
2, 4 and 8 mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively.
One mg/kg given in the same way to 2 dogs resulted in the death
of one animal after 42 weeks, but the other survived for 52 weeks.
This animal showed only a slight growth inhibition. Doses of 0.25 and
0.5 mg/kg body-weight did not produce any sign of illness during 52
weeks (Velsicol, 1959).
When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor
epoxide were given to groups of 5 dogs (2 males and 3 females ranging
from 23 to 27 weeks of age) for 60 weeks. No deaths attributable to
heptachlor epoxide occurred. The weights of the male dogs tended to
be inversely proportional to the concentration of the compound in the
diet. The female dogs had normal weights. The liver weights of all
the animals were increased in proportion to the concentration of
heptachlor epoxide in the diet, and degenerative liver changes were
seen at 2.5 ppm and above (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed
diets containing 5, 10, 20, 40, 80, 160, and 300 ppm of heptachlor
epoxide for 2 years. Concentrations of 80 ppm or higher resulted in
100% mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver-weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females)(Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10,
20 and 40 ppm for 2 years showed significant increases in mortality
only in females at 40 ppm. Liver-weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
In another experiment which has already been considered1 CFN
rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5, 7.5
and 10 ppm. More details are now available about the tumour incidence
in untreated controls as well as the distribution or tumours among the
different groups. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34%) and 13/24 (54%) in
the control males and females respectively; it was 65/111 (58%) and
92/114 (80%) in the experimental males and females respectively.
Again, many tumours were located in endocrine organs. Liver tumours
were observed in 7 males and 12 females in the experimental groups
only (over-all incidence 19/225 (8.4%), but only two of them were
malignant (Kettering Laboratory, 1959).
Finally, in another experiment heptachlor dissolved in ethanol
was added to the diet of CF rats as 1.5, 3, 5, 7 and 10 ppm for 110
weeks. Each group, as well as an untreated control, included 40
animals (20 of each sex). Mortality was comparable in all groups. The
number of tumour-bearing animals was 16/40 at 0 ppm, 9/40 at 1.5 ppm,
13/40 at 3 ppm, 12/40 at 5 ppm, 15/40 at 7 ppm and 12/40 at 10 ppm.
Most tumours were found in the pituitary and other endocrine organs.
No liver tumours were recorded. No preferential tumour site in any
particular group was observed but all the 4 thyroid tumours observed
were in the 7 and 10 ppm groups (Witherup et al., 1955).
In some other experiments, the continuous exposure of rats to
doses of over 7 ppm of either heptachlor or its epoxide increased the
mortality during the suckling period. In others, 10 ppm fed to 3
generations of rats showed no adverse effects on reproductive
capacity, probability of survival or growth (Witherup et al., 1955;
Kettering Laboratory, 1959).
Comments on experimental studies reported
It is well established that heptachlor and its epoxide accumulate
in body fat and persist them for long periods. The formation of
epoxide is relatively rapid in the animal, plants and in the soil.
1 Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues. FAO
Meeting Report No. PL/1963/13 and WHO/Food Add./23 (1964).
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the 2
substances.
A sex difference in toxicity has been observed, particularly in
the rat, females accumulating heptachlor and its epoxide more than
males. The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to
explain. A possible relation with tumour formation has not been
demonstrated, while evidence has been presented that the change is
reversible.
Some suspicion of carcinogenicity can arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful. Nevertheless, until new data are
available from other long-term experiments involving other strains of
rats or other species a definite statement is not possible.
EVALUATION
From the data presented an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming every
effort should be made to see that the intake of heptachlor for man is
kept at the lowest possible level.
Further work required
As the residue in the crop may contain the epoxide, the
experiments should be done with this compound also. A maximum
no-effect level should be determined in more than one species.
Additional long-term toxicity studies in rats and other species are
required.
REFERENCES
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely. R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
The Kettering Laboratory (1959) University of Cincinnati
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S.,15, 47
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Radomski, J. L. & Davidow B. (1953) J. Pharmacol. exp. Ther., 107,
266
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1960) Chem. Abstr. 54, 13435
Stemmler, K. L. & Hamdi, E. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Stemmler, K. L. & Jolley, W. P. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S. et al. (1955) Data submitted by the Kettering Laboratory,
University of Cincinnati
 BIOLOGICAL DATA
Biochemical aspects
In plants and soil heptachlor is converted to its epoxide which
is more persistent on plants than the parent heptachlor (Gannon &
Bigger, 1958; Gannon & Decker, 1958).
Heptachlor is also readily transformed in the animal body into
heptachlor epoxide, which is stored in the fat of dogs and rats. Some
storage occurs in the liver, kidney and muscle, but none in the brain.
In dogs, after feeding heptachlor in a concentration of 1-3 mg/kg
body-weight the metabolite was found (Davidow & Radomski, 1953). When
heptachlor was fed at high dosage levels to dogs, small amounts of
unmetabolized heptachlor were also found in the fat of dogs but this
was not the case in the rat regardless of the level fed.
In the rat the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm
storage occurred in females but not in males and 0.1 ppm no storage
occurred in either sex. Female rats accumulate about 6 times as much
epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days the epoxide level in the milk rose to a maximum of 1.8 ppm
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at a 5 ppm and 2 at a 10 ppm dally intake). Three days
after feeding commenced, the minimum levels of heptachlor epoxide in
the milk were 0.26 and 0.34 ppm in the 2 cows fed 5 ppm and 0.23, and
0.65 ppm in the 2 cows fed 10 ppm; the maximum levels after 15 days'
feeding were 0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively
(Storherr et al., 1960).
Disturbance of the acid-base balance seem to be of great
significance in the mechanism of action of heptachlor (Spynu &
Osetrov, 1960).
Acute toxicity
Heptachlor
Animal Route LD50 mg/kg References
body-weight
Rat, male Oral 60-169* Negherbon, 1959; Velsicol, 1959
Mouse Oral 68 Negherbon, 1959
Guinea-pig Oral 116 Negherbon, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 mg/kg References
body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide
in the mouse are given as 40 and 100 mg/kg body-weight respectively
(Negherbon, 1959). In the rabbit the lethal dose of epoxide is 5-10
mg/kg body-weight (Velsicol, 1959).
The acute effects are neurotoxic disturbances like those
observed with other chlorinated hydrocarbons.
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide
(up to 60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleolim cytoplasm fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla (Stemmler & Hamdi, 1963).
Electron microscopic studies demonstrated an increase of rough and
smooth endoplasmic reticulum (Stemmler & Hamdi, 1963).
Dog. Three dogs given heptachlor epoxide orally in dosages of
2, 4 and 8 mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively.
One mg/kg given in the same way to 2 dogs resulted in the death
of one animal after 42 weeks, but the other survived for 52 weeks.
This animal showed only a slight growth inhibition. Doses of 0.25 and
0.5 mg/kg body-weight did not produce any sign of illness during 52
weeks (Velsicol, 1959).
When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor
epoxide were given to groups of 5 dogs (2 males and 3 females ranging
from 23 to 27 weeks of age) for 60 weeks. No deaths attributable to
heptachlor epoxide occurred. The weights of the male dogs tended to
be inversely proportional to the concentration of the compound in the
diet. The female dogs had normal weights. The liver weights of all
the animals were increased in proportion to the concentration of
heptachlor epoxide in the diet, and degenerative liver changes were
seen at 2.5 ppm and above (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed
diets containing 5, 10, 20, 40, 80, 160, and 300 ppm of heptachlor
epoxide for 2 years. Concentrations of 80 ppm or higher resulted in
100% mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver-weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females)(Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10,
20 and 40 ppm for 2 years showed significant increases in mortality
only in females at 40 ppm. Liver-weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
In another experiment which has already been considered1 CFN
rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5, 7.5
and 10 ppm. More details are now available about the tumour incidence
in untreated controls as well as the distribution or tumours among the
different groups. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34%) and 13/24 (54%) in
the control males and females respectively; it was 65/111 (58%) and
92/114 (80%) in the experimental males and females respectively.
Again, many tumours were located in endocrine organs. Liver tumours
were observed in 7 males and 12 females in the experimental groups
only (over-all incidence 19/225 (8.4%), but only two of them were
malignant (Kettering Laboratory, 1959).
Finally, in another experiment heptachlor dissolved in ethanol
was added to the diet of CF rats as 1.5, 3, 5, 7 and 10 ppm for 110
weeks. Each group, as well as an untreated control, included 40
animals (20 of each sex). Mortality was comparable in all groups. The
number of tumour-bearing animals was 16/40 at 0 ppm, 9/40 at 1.5 ppm,
13/40 at 3 ppm, 12/40 at 5 ppm, 15/40 at 7 ppm and 12/40 at 10 ppm.
Most tumours were found in the pituitary and other endocrine organs.
No liver tumours were recorded. No preferential tumour site in any
particular group was observed but all the 4 thyroid tumours observed
were in the 7 and 10 ppm groups (Witherup et al., 1955).
In some other experiments, the continuous exposure of rats to
doses of over 7 ppm of either heptachlor or its epoxide increased the
mortality during the suckling period. In others, 10 ppm fed to 3
generations of rats showed no adverse effects on reproductive
capacity, probability of survival or growth (Witherup et al., 1955;
Kettering Laboratory, 1959).
Comments on experimental studies reported
It is well established that heptachlor and its epoxide accumulate
in body fat and persist them for long periods. The formation of
epoxide is relatively rapid in the animal, plants and in the soil.
1 Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues. FAO
Meeting Report No. PL/1963/13 and WHO/Food Add./23 (1964).
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the 2
substances.
A sex difference in toxicity has been observed, particularly in
the rat, females accumulating heptachlor and its epoxide more than
males. The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to
explain. A possible relation with tumour formation has not been
demonstrated, while evidence has been presented that the change is
reversible.
Some suspicion of carcinogenicity can arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful. Nevertheless, until new data are
available from other long-term experiments involving other strains of
rats or other species a definite statement is not possible.
EVALUATION
From the data presented an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming every
effort should be made to see that the intake of heptachlor for man is
kept at the lowest possible level.
Further work required
As the residue in the crop may contain the epoxide, the
experiments should be done with this compound also. A maximum
no-effect level should be determined in more than one species.
Additional long-term toxicity studies in rats and other species are
required.
REFERENCES
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely. R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
The Kettering Laboratory (1959) University of Cincinnati
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S.,15, 47
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Radomski, J. L. & Davidow B. (1953) J. Pharmacol. exp. Ther., 107,
266
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1960) Chem. Abstr. 54, 13435
Stemmler, K. L. & Hamdi, E. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Stemmler, K. L. & Jolley, W. P. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S. et al. (1955) Data submitted by the Kettering Laboratory,
University of Cincinnati
BIOLOGICAL DATA
Biochemical aspects
In plants and soil heptachlor is converted to its epoxide which
is more persistent on plants than the parent heptachlor (Gannon &
Bigger, 1958; Gannon & Decker, 1958).
Heptachlor is also readily transformed in the animal body into
heptachlor epoxide, which is stored in the fat of dogs and rats. Some
storage occurs in the liver, kidney and muscle, but none in the brain.
In dogs, after feeding heptachlor in a concentration of 1-3 mg/kg
body-weight the metabolite was found (Davidow & Radomski, 1953). When
heptachlor was fed at high dosage levels to dogs, small amounts of
unmetabolized heptachlor were also found in the fat of dogs but this
was not the case in the rat regardless of the level fed.
In the rat the maximum amount of the metabolite appeared after
feeding 1, 5, 15 and 30 ppm heptachlor for two weeks. At 0.3 ppm
storage occurred in females but not in males and 0.1 ppm no storage
occurred in either sex. Female rats accumulate about 6 times as much
epoxide as males. Twelve weeks were required for complete
disappearance of the metabolite from the fat after discontinuing
heptachlor feeding (Radomski & Davidow, 1953).
When cows were fed 3 mg/kg body-weight (in corn oil) daily for 14
days the epoxide level in the milk rose to a maximum of 1.8 ppm
equivalent to 44 ppm in butter fat. Residues could be detected in milk
51 days after feeding ceased (Davidow et al., 1953). Mixtures of
heptachlor and heptachlor epoxide were fed daily to 4 dairy cows for
20 days (2 at a 5 ppm and 2 at a 10 ppm dally intake). Three days
after feeding commenced, the minimum levels of heptachlor epoxide in
the milk were 0.26 and 0.34 ppm in the 2 cows fed 5 ppm and 0.23, and
0.65 ppm in the 2 cows fed 10 ppm; the maximum levels after 15 days'
feeding were 0.63 and 0.80 ppm, and 1.51 and 1.66 ppm respectively
(Storherr et al., 1960).
Disturbance of the acid-base balance seem to be of great
significance in the mechanism of action of heptachlor (Spynu &
Osetrov, 1960).
Acute toxicity
Heptachlor
Animal Route LD50 mg/kg References
body-weight
Rat, male Oral 60-169* Negherbon, 1959; Velsicol, 1959
Mouse Oral 68 Negherbon, 1959
Guinea-pig Oral 116 Negherbon, 1959
Chick Oral 63 Sherman & Ross, 1961
Heptachlor epoxide
Animal Route LD50 mg/kg References
body-weight
Rat Oral 34-88* Velsicol Corporation, 1959
Mouse, male Oral 32-48 Velsicol Corporation, 1959
* Sex differences.
Intravenous lethal doses for heptachlor and heptachlor epoxide
in the mouse are given as 40 and 100 mg/kg body-weight respectively
(Negherbon, 1959). In the rabbit the lethal dose of epoxide is 5-10
mg/kg body-weight (Velsicol, 1959).
The acute effects are neurotoxic disturbances like those
observed with other chlorinated hydrocarbons.
Short-term studies
Rat. The addition of heptachlor (up to 45 ppm) or its epoxide
(up to 60 ppm) or both to diet of rats for 140 days produced liver
microscopical changes, i.e. enlarged centrolobular cells showing big
nuclei with prominent nucleolim cytoplasm fat accumulation and
occasional aggregation of granules (Stemmer & Jolley, 1963). In an
experiment involving 269 rats it was demonstrated that these changes
regress after withdrawal of the pesticide. It has also been suggested
that these changes are produced indirectly, through the autonomic
nervous system and the adrenal medulla (Stemmler & Hamdi, 1963).
Electron microscopic studies demonstrated an increase of rough and
smooth endoplasmic reticulum (Stemmler & Hamdi, 1963).
Dog. Three dogs given heptachlor epoxide orally in dosages of
2, 4 and 8 mg/kg a day for 5 days a week died after 22, 10 and 3 weeks
respectively.
One mg/kg given in the same way to 2 dogs resulted in the death
of one animal after 42 weeks, but the other survived for 52 weeks.
This animal showed only a slight growth inhibition. Doses of 0.25 and
0.5 mg/kg body-weight did not produce any sign of illness during 52
weeks (Velsicol, 1959).
When heptachlor was fed orally, dissolved in corn oil, to
groups of 2 and 4 dogs at levels of 5 mg/kg and 1 mg/kg body-weight
respectively, all the animals at the higher dose level died within 21
days. At the lower dose level 3 out of 4 dogs died within 424 days but
one was living at 455 days. No pathological data are available from
this experiment (Lehman, 1952).
Diets containing 0.5, 2.5, 5.0 and 7.5 ppm of heptachlor
epoxide were given to groups of 5 dogs (2 males and 3 females ranging
from 23 to 27 weeks of age) for 60 weeks. No deaths attributable to
heptachlor epoxide occurred. The weights of the male dogs tended to
be inversely proportional to the concentration of the compound in the
diet. The female dogs had normal weights. The liver weights of all
the animals were increased in proportion to the concentration of
heptachlor epoxide in the diet, and degenerative liver changes were
seen at 2.5 ppm and above (Velsicol, 1959).
Long-term studies
Rat. Groups of rats (usually 10 males or 10 females) were fed
diets containing 5, 10, 20, 40, 80, 160, and 300 ppm of heptachlor
epoxide for 2 years. Concentrations of 80 ppm or higher resulted in
100% mortality in 2-20 weeks. All the female animals given 40 ppm died
within a period of 54 weeks. This concentration had no effect on the
mortality of the male animals up to 104 weeks. Diets containing 20 ppm
or less produced no signs of illness in male or female rats during a
2-year period but an increase in liver-weight was observed in diets
containing more than 10 ppm (males) and 5 ppm (females)(Velsicol,
1959).
Groups of 20 rats of strain CFW fed heptachlor epoxide at 10,
20 and 40 ppm for 2 years showed significant increases in mortality
only in females at 40 ppm. Liver-weights in the females were slightly
increased. Tumour incidence was lower in the experimental groups than
in the controls and was independent of the content of heptachlor
epoxide in the diet (Velsicol, 1959).
In another experiment which has already been considered1 CFN
rats were fed heptachlor epoxide in concentrations of 0.5, 2.5, 5, 7.5
and 10 ppm. More details are now available about the tumour incidence
in untreated controls as well as the distribution or tumours among the
different groups. No differences have been observed among the five
experimental groups and their results can be considered together. The
incidence of tumour-bearing animals was 8/23 (34%) and 13/24 (54%) in
the control males and females respectively; it was 65/111 (58%) and
92/114 (80%) in the experimental males and females respectively.
Again, many tumours were located in endocrine organs. Liver tumours
were observed in 7 males and 12 females in the experimental groups
only (over-all incidence 19/225 (8.4%), but only two of them were
malignant (Kettering Laboratory, 1959).
Finally, in another experiment heptachlor dissolved in ethanol
was added to the diet of CF rats as 1.5, 3, 5, 7 and 10 ppm for 110
weeks. Each group, as well as an untreated control, included 40
animals (20 of each sex). Mortality was comparable in all groups. The
number of tumour-bearing animals was 16/40 at 0 ppm, 9/40 at 1.5 ppm,
13/40 at 3 ppm, 12/40 at 5 ppm, 15/40 at 7 ppm and 12/40 at 10 ppm.
Most tumours were found in the pituitary and other endocrine organs.
No liver tumours were recorded. No preferential tumour site in any
particular group was observed but all the 4 thyroid tumours observed
were in the 7 and 10 ppm groups (Witherup et al., 1955).
In some other experiments, the continuous exposure of rats to
doses of over 7 ppm of either heptachlor or its epoxide increased the
mortality during the suckling period. In others, 10 ppm fed to 3
generations of rats showed no adverse effects on reproductive
capacity, probability of survival or growth (Witherup et al., 1955;
Kettering Laboratory, 1959).
Comments on experimental studies reported
It is well established that heptachlor and its epoxide accumulate
in body fat and persist them for long periods. The formation of
epoxide is relatively rapid in the animal, plants and in the soil.
1 Report of a joint meeting of the FAO Committee on Pesticides in
Agriculture and the WHO Expert Committee on Pesticide Residues. FAO
Meeting Report No. PL/1963/13 and WHO/Food Add./23 (1964).
The epoxide is more persistent than the parent compound and
calculations of toxicity should perhaps be based on the data obtained
with the epoxide rather than heptachlor, particularly since most
reports indicate that the epoxide is the more toxic of the 2
substances.
A sex difference in toxicity has been observed, particularly in
the rat, females accumulating heptachlor and its epoxide more than
males. The toxic dose for female rats is lower than that for males.
The changes observed in the liver cells are difficult to
explain. A possible relation with tumour formation has not been
demonstrated, while evidence has been presented that the change is
reversible.
Some suspicion of carcinogenicity can arise from one of the
long-term experiments with heptachlor epoxide, since in both males and
females the total number of tumour-bearing animals was higher than in
the controls and liver tumours were only found in the experimental
group. However, the interpretation of this finding is debatable: in
the first place it concerns only one experiment among several
long-term studies. In addition to this, the over-all incidence of
liver tumours was low, most of them were histologically benign and no
dose-response relationship was observed among the different
experimental groups. For these reasons the carcinogenicity of
heptachlor epoxide appears doubtful. Nevertheless, until new data are
available from other long-term experiments involving other strains of
rats or other species a definite statement is not possible.
EVALUATION
From the data presented an acceptable daily intake for man
cannot be estimated. Until further evidence is forthcoming every
effort should be made to see that the intake of heptachlor for man is
kept at the lowest possible level.
Further work required
As the residue in the crop may contain the epoxide, the
experiments should be done with this compound also. A maximum
no-effect level should be determined in more than one species.
Additional long-term toxicity studies in rats and other species are
required.
REFERENCES
Davidow, B. & Radomski, J. L. (1953) J. Pharmacol. exp. Ther.,
107, 259
Davidow, B., Radomski, J. L. & Ely. R. (1953) Science, 118, 383
Gannon, N. & Bigger, J. H. (1958) J. econ. Ent., 51, 1
Gannon, N. & Decker, G. C. (1958) J. econ. Ent., 51, 3
The Kettering Laboratory (1959) University of Cincinnati
Lehman, A. J. (1952) Quart. Bull. Assoc. Food and Drug Officials
U.S.,15, 47
Negherbon, W. O. (1959) Handbook of Toxicology, Philadelphia and
London, Saunders, vol. III
Radomski, J. L. & Davidow B. (1953) J. Pharmacol. exp. Ther., 107,
266
Sherman, M. & Ross, E. (1961) Toxicol. Appl. Pharmacol., 3, 521
Spynu, E. I. & Osetrov, V. I. (1960) Chem. Abstr. 54, 13435
Stemmler, K. L. & Hamdi, E. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Stemmler, K. L. & Jolley, W. P. (1963) Data submitted by the Kettering
Laboratory, University of Cincinnati
Storherr, R. W., Tighe, J. F. & Sykes, J. F. (1960) J. Assoc. Offic.
Agr. Chemists, 43, 731
Velsicol Corporation (1959) Unpublished report
Witherup, S. et al. (1955) Data submitted by the Kettering Laboratory,
University of Cincinnati
