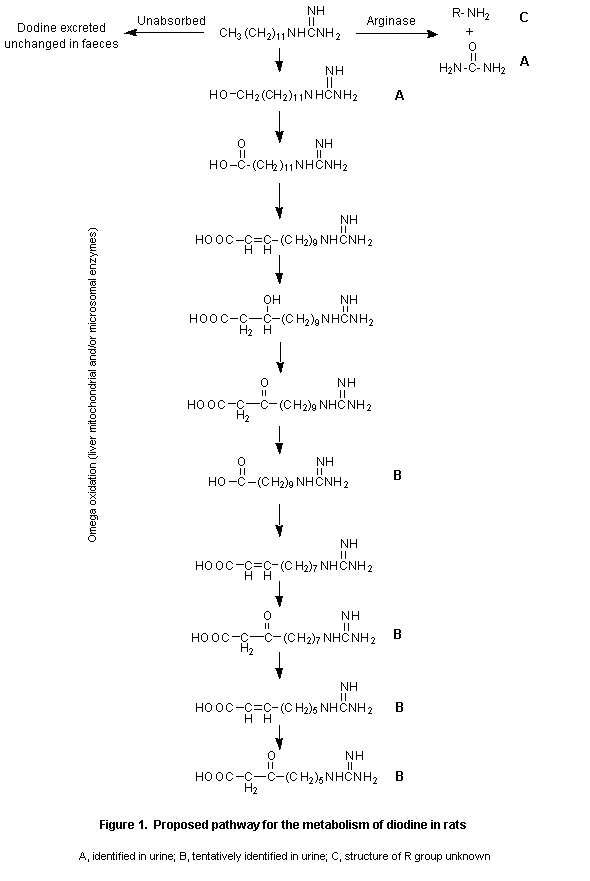
First draft prepared by
Virginia A. Dobozy
United States Environmental Protection Agency
Washington DC, USA
Dodine was first evaluated by the JMPR in 1974, when a temporary ADI of 00.01 mg/kg bw was established on the basis of a NOAEL of 50 ppm (equivalent to1.25 mg/kg bw per day) for effects on the thyroid in a 1-year study in dogs (Annex 1, reference 22). The Meeting at that time required studies of the metabolism of dodine in animals and plants and considered that it would be desirable to have the results of studies of teratogenicity studies in appropriate animal species. Additional data on metabolism in rats were evaluated by the Joint Meeting in 1976. The ADI was maintained at 00.01 mg/kg bw (Annex 1, reference 26). A significant number of studies have since been conducted. Dodine was reviewed by the present Meeting within the periodic review programme of the CCPR.
(a)Absorption, distribution, and excretion
Rats
The absorption, distribution, and excretion of dodine were investigated in groups of five male and five female Sprague-Dawley rats after a single dose of 40 or 400 mg/kg bw of [14C- guanidine]dodine (radiochemical purity, 99%) or 40 mg/kg bw per day of unlabelled dodine for 14 days followed by a single oral dose of 40 mg/kg bw of radiolabelled dodine on day 15. In a preliminary study, one male and one female Sprague-Dawley rat were given a single oral dose of 40 or 400 mg/kg bw of [14C]dodine by gavage to determine the extent of excretion of 14C in expired air. The compound could not be given intravenously because it is insoluble in vehicles suitable for administration by that route. Urine, faeces, and expired air were collected 4, 8, 12, 24, 48, and 72 h after dosing. All animals were killed at 72 h. Animals at the low dose eliminated most of the radiolabel in urine and faeces, but elimination of radiolabel was incomplete for those at the high dose. In the main study, therefore, animals were observed for up to 120 h. As less than 1% of the total dose was eliminated in expired air, carbon dioxide and expired air were not collected in the main study.
In the main study, 4145% of the administered dose was excreted in urine and 4860% in faeces of all doses, for a total recovery of administered radiolabel after 120 h of 94102%. Males and females given the low dose singly or repeatedly excreted most of the radiolabel in urine (3944% of the dose) and faeces (5056%) within the first 48 h after dosing, while males and females given the single high dose excreted most of the radiolabel in urine (4041%) and faeces (4246%) by 96 h after dosing. However, by 120 h, the amount eliminated in urine and faeces was similar for all dose groups. Virtually no radiolabel ( 3.4%) was recovered in tissues 120 h after dosing. In general, the amount of radiolabel recovered in tissues was similar in males and females. The largest amounts (expressed as per cent of dose) were found in the gastrointestinal tract (0.21.1%), muscle (0.20.6%), and skin (0.10.2%). None of the tissues contained > 1.1% of the administered dose. The carcass contained 0.60.9% (Reddy et al., 1999).
The metabolism of dodine was extensive, and no unmetabolized parent compound was detected in urine. In general, the metabolic profile was similar in both sexes and at all doses groups. Four metabolites were found in urine. The major metabolite was identified as an alcohol of dodine, hydroxydodecylguanidine, an omega-oxidation product, which accounted for 1124% of the administered dose. Females had slightly larger amounts of this metabolite in urine than did males. Another metabolite was identified as urea and accounted for 3.35.0% of the dose excreted in the urine. A metabolite which accounted for 7.512% of the administered dose was not identified, while a further metabolite was tentatively identified as a mixture of acidic products produced by beta-oxidation of the alkane side-chain of dodine. The last accounted for 3.714% of the administered dose. Incubation of urine samples with hydrolytic enzymes indicated no glucuronide or sulfate conjugates.
In the faecal samples, the parent compound was the major component (3955% of the dose), and slightly larger amounts were excreted in the faeces of animals given the single high dose than in those of animals given the low dose singly or repeatedly (Reddy et al., 1992)
The metabolic pathway of dodine is presented schematically in Figure 1.
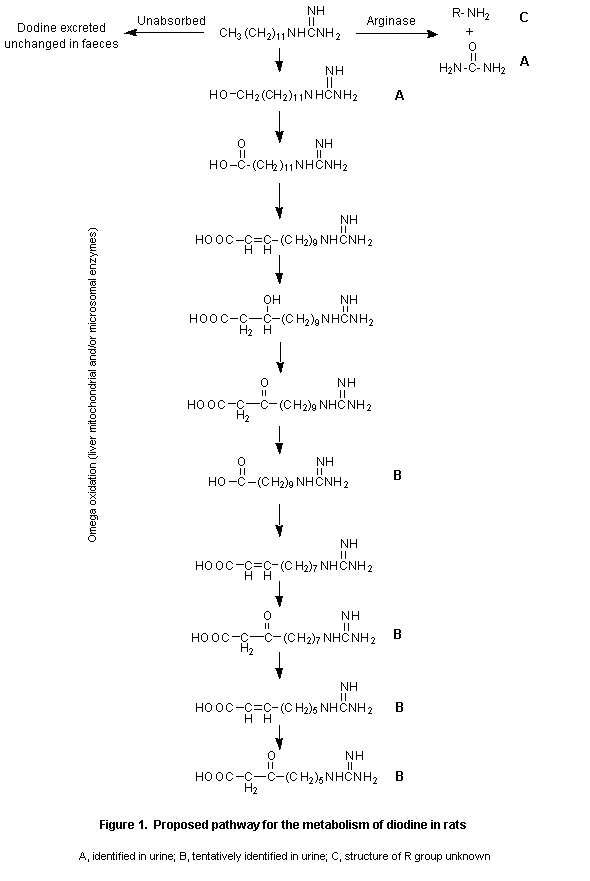
Studies of the acute toxicity of dodine after application orally, dermally, by inhalation, or to the eye or skin are summarized in Table 1. Dodine was slightly toxic to mice and rats when given orally. The clinical signs in rats included abnormal defaecation, various discoloured areas due to discharge or excretion, hypoactivity, prolapsed penis, and impaired muscle coordination. On necropsy, distension of the stomach and/or intestines and abnormal abdominal adhesions were observed. Dodine was not toxic to rats or rabbits when applied dermally, but it was severely toxic to rats exposed by inhalation. The clinical signs included exaggerated breathing, immobility, lethargy, unsteady gait, cold to touch, and piloerection. Dodine was extremely irritating to the eyes and slightly irritating to the skin of rabbits in one study and severely irritating in another study. It was not a skin sensitizer in guinea-pigs.
Table 1. Acute toxicity of dodine
|
Species |
Sex |
Route |
Purity (%) |
LD50 or LC50 |
Reference |
|
Mouse |
Male |
Oral |
97 |
1720 |
Levinskas et al. (1961) |
|
Rat |
Male |
Oral |
97 |
7501540 |
Levinskas et al. (1961), JMPR (1975) |
|
|
Female |
|
|
660 |
|
|
Rat |
Male |
Oral |
NR |
1931 |
Environmental Protection Agency (1987) |
|
|
Female |
|
|
1117 |
|
|
Rat |
Male and female |
Oral |
96.7 |
851 |
Kern (1999a) |
|
Rat |
Male and female |
Dermal |
96.7 |
> 5000 |
Kern (1999b) |
|
Rabbit |
Male and female |
Dermal |
NR |
> 2000 |
Environmental Protection Agency (1987) |
|
Rabbit |
Male and female |
Dermal |
NR |
Severe irritation at 7 days |
Environmental Protection Agency (1987) |
|
Rabbit |
Male and female |
Dermal |
96.7 |
Slightly irritating |
Kern (1999c) |
|
Guinea-pig |
|
Intradermal |
96.7 |
Not sensitizing |
Manciaux (1999) |
|
Rat |
Male |
Inhalation |
100 |
0.47 |
Kenny (1999) |
|
|
Female |
|
|
0.44 |
|
|
Rabbit |
Male |
Ocular |
NR |
Severe corneal opacity at 21 days |
Environmental Protection Agency (1987) |
|
Rabbit |
Male and female |
Ocular |
96.7 |
Severe irritation |
Kern (1999d) |
NR, not reported
(b) Short-term studies of toxicity
Mice
In a range-finding study, dodine (purity, 95.3%) was administered in the diet of groups of five male and five female CD-1 mice at a constant concentration of 0, 100, 250, or 625 ppm for 8 weeks. After three weeks of dosing, the dose of mice at 100 ppm was increased to 1250 ppm, as no toxic effects were observed. The doses of 0, 250, 625, and 1250 ppm were equal to 0, 49, 110, and 230 mg/kg bw per day for males and 0, 61, 150, and 260 mg/kg bw per day for females, respectively. Deaths and clinical signs were monitored once daily, and body weight and food consumption were measured before treatment and weekly during dosing. At necropsy, the heart, kidneys, liver, lungs, and spleen from controls and mice at the high dose were weighed and examined microscopically, with any grossly abnormal tissues.
The death of one female at 1250 ppm could be considered related to treatment as it occurred immediately after the dose of these mice was increased, but the cause of death could not be found at necropsy. Nonspecific findings at necropsy included gross discolouration and microscopic congestion of the lungs and decreased pigment deposition and mild cellular depletion of the spleen than in controls. The body-weight gain of mice for which the dose was increased to 1250 ppm was arrested until week 7. The overall body-weight gain in the 8-week study was decreased by 18% for males and 34% for females. The decrease for females at 250 ppm was considered to be unrelated to treatment because of the lack of a doseresponse relationship. The food consumption of animals at 100/1250 ppm was slightly decreased from week 5. Females at this dose had a statistically significant decrease in absolute spleen weight, but the effect was considered to be the result of the reduced body weight; no microscopic changes were found. All of the males and three of five females at 100/1250 ppm had mild eosinophilia of the liver, but the cause could not be determined even with special stains. The NOAEL was 625 ppm, equal to 110 mg/kg bw per day, on the basis of reduced body-weight gain and eosinophilia in the liver (Mulhern et al., 1988)
Groups of 10 male and 10 female Swiss CR1:CD®-1(ICR)BR mice were given diets containing dodine (purity, 94.1%) at a concentration of 0, 150, 300, 600, 1250, or 2500 ppm, equal to 0, 24, 48, 94, 180, and 350 mg/kg bw per day for males and 0, 31, 60, 120, 220, and 300 mg/kg bw per day for females, respectively, for 13 weeks. Four females at 2500 ppm died during the first 2 weeks of treatment. Generalized deterioration was seen before the death of these animals, consisting of reduced activity, respiratory rate, and body temperature, tremors, generalized paleness or bluish skin colouration, partly closed eyes, dehydration, weakness, and stiffening of the tail. The histological findings consisted of lymphoid atrophy of the spleen and/or lymphoid atrophy and/or necrosis of the thymus. On the basis of the condition of these animals before death and in the absence of similar effects in the surviving animals, these findings were considered to be stress-related. There were no treatment-related ophthalmic changes or abnormal clinical signs, except for apparent stiffening of the tail in 4/10 females at the highest dose.
Significant decreases in body weight (1724%) and in food consumption (3050% lower than controls) were found among males at 2500 ppm and to a lesser extent among those at 1250 ppm, particularly during the first week of treatment. The body weights of females at 2500 ppm were lower than the control values, but the differences were not statistically significant. Overall body-weight gain was decreased in relation to that of controls in males (68%) and females (36%) at 2500 ppm and in males (11%) at 1250 ppm. The food consumption ratios varied among groups, but there was no indication of impaired feed efficiency. Haematological examinations revealed neutrophilia and eosinopenia in males at the high dose. Clinical biochemical analyses revealed significantly elevated blood urea nitrogen concentrations and slight increases in total bilirubin concentration and aspartate aminotransferase activity in a few animals at 2500 ppm. The absolute and relative weights of various organs of males at 2500 ppm were significantly different from those of controls, and females had significantly higher kidney weights at 1250 or 2500 ppm and significantly higher liver weights at 2500 ppm. Gross and histopathological examinations revealed no changes that could be attributed to treatment. The observed variations in blood urea nitrogen concentration and aspartate aminotransferase activity and the increased weights of some organs were considered to be due to the overall nutritional status of the animals. No adverse effects were seen at the other doses. The NOAEL was 600 ppm, equal to 94 mg/kg bw per day, on the basis of decreased body weight and food consumption (Kangas, 1994).
Rats
Dodine (purity, 94.07%) was administered in the diet to groups of 10 male and 10 female Sprague-Dawley rats at a constant concentration of 0, 500, 750, or 1000 ppm, equal to 0, 47, 71, and 87 mg/kg bw per day for males and 0, 50, 72, and 92 mg/kg bw per day for females, respectively, for a minimum of 4 weeks. The end-points evaluated were deaths, clinical signs, body weight, food consumption, haematology and clinical chemistry, and the weights of certain organs. All animals were necropsied grossly, and selected tissues from controls and rats at the high dose were examined histologically.
There were no deaths or treatment-related clinical signs of toxicity during the study. The mean body weights of animals at the two higher doses were significantly decreased, and the mean body-weight gain over the 4-week period was decreased; the differences were statistically significant, except for females at 750 ppm. The mean food consumption was significantly decreased for animals at the highest dose throughout the study and for animals at the intermediate dose in three of the four weeks of dosing. Minor decreases in white blood cell counts in treated males, decreases in alanine aminotransferase activity in all treated animals, and decreases in glucose concentration in males at the high dose were considered not to be toxicologically significant. Differences between the treated and control groups in the absolute weights of several organs were attributed to decreases in body weight. The NOAEL was 500 ppm, equal to 47 mg/kg bw per day, on the basis of decreased body weight, body-weight gain, and food consumption (Batham, 1994a).
Dodine (purity, 98.6%) was administered in the diet to groups of 10 male and 10 female Sprague-Dawley rats at a concentration of 0, 200, or 800 ppm, equal to 0, 18, and 68 mg/kg bw per day for males and 0, 19, and 77 mg/kg bw per day for females, for 28 days. Deaths, clinical signs, body weight, and food consumption were measured during the study. At necropsy, the kidneys and liver were weighed. The organs of the gastrointestinal tract (stomach, duodenum, jejunum, ileum, caecum, colon, rectum, and anus) from controls and rats at the high dose were examined microscopically.
A statistically significant, 57% decrease in mean body weight was found in males at the high dose on days 8 and 15, and body-weight gain was significantly decreased in animals of each sex at this dose during days 1 8. Decreases observed at other times were not statistically significant. Overall (days 128), the decrease was 14% for males and 17% for females. The mean food consumption of males at the high dose was significantly decreased by 810% on days 8 and 15 and was slightly but not significantly lower than that of controls for the remainder of the study. Both the absolute and the relative (to body) weights of the liver were significantly decreased in females at the high dose. No treatment-related macroscopic or microscopic changes were found in the gastrointestinal tract. As the liver was not examined, the toxicological significance of the decreases in liver weight in females at the high dose is uncertain. The NOAEL was 200 ppm, equal to 18 mg/kg bw per day, on the basis of decreased body-weight gain in animals of each sex and decreased food consumption in males (Dange, 1997).
Groups of 10 male and 10 female Sprague-Dawley rats received dodine (purity, 94.07%) by gavage at a dose of 0, 75, 100, or 200 mg/kg bw per day for 4 weeks. A control group of 10 rats of each sex received the vehicle (0.5% methylcellulose) at the same volume (5 ml/kg bw per day) as the treated rats. The treatment of animals at the high dose was terminated owing to the deaths of nine males and six females, and the remaining animals in the group were killed after day 12 of dosing. The deaths of four females at 100 mg/kg bw per day during week 4 of dosing were considered to be related to treatment. One female at 75 mg/kg bw per day was found dead during the first week of dosing. Dose-related clinical signs observed throughout the 4 weeks of treatment included salivation, deteriorating health status, respiratory distress, firm abdomens, pale and/or soft faeces sometimes containing mucoid material, and staining of the head and/or urogenital region.
Decreased body weights, body-weight gains, and food consumption were observed in animals at the two higher doses and occasionally in those at the lowest dose. Significant changes in clinical pathology seen among rats at the high dose during week 2 and in those at the intermediate dose at week 4 consisted of increases in white and red blood cell parameters. Clinical chemistry revealed increased concentrations of blood urea nitrogen, total bilirubin, and phosphorus, increased activity of alanine aminotransferase, decreased concentrations of glucose, total protein, albumin, globulin, and chloride, and decreased activity of alkaline phosphatase. Most of these changes were considered to be related to the severe effect of dodine on the animals food intake, the decreased body-weight gain, and its irritating effect on the digestive tract.
Significant differences in absolute and relative organ weights were seen among males at the intermediate dose (mean absolute weight of liver, lung, and brain) and females at the low and intermediate doses (mean absolute weight of brain). Administration of dodine at 200 mg/kg bw per day for a maximum of 12 days produced macroscopic and microscopic lesions in the stomach, spleen, thymus, adrenal glands, and intestines. Examination of the gastrointestinal tract of animals treated at 75 and 100 mg/kg bw per day showed oedema, mixed-cell infiltration, and hyperplasia of the squamous mucosa of the stomach. Hyperkeratosis, erosion, and ulceration of the forestomach were also noted in animals at 100 mg/kg bw per day. The effect of dodine on liver enzymes was not reflected histopathologically. No NOAEL could be identified (Batham, 1994b).
Dodine (purity, 95%) was administered in the diet to groups of 10 male and 10 female Wistar rats at a concentration of 0, 50, 200, or 800 ppm, equal to 0, 3.6, 14, and 56 mg/kg bw per day for males and 0, 3.9, 15, and 60 mg/kg bw per day for females, respectively, for 90 days. The dose of 400 ppm was raised to 800 ppm on day 7 because no depression of growth was seen. The general condition of the animals was observed daily; body weights were recorded at the beginning of the study and then weekly. Food consumption was measured per cage (five animals) weekly. Haematological, clinical chemical, and urinary end-points were analysed at the end of the study. Ophthalmoscopic examinations were performed before the study was begun and during week 12. At necropsy, selected organs of all animals were weighed, and selected tissues from the controls and rats at the high dose were examined microscopically.
There were no deaths or clinical signs of toxicity in any group. No ophthalmoscopic abnormalities were found. The mean body weights of animals of each sex at the high dose were decreased throughout the study and were significantly different from those of controls at some periods. Overall (days 091), body-weight gain was decreased by 10% in males and 11% in females at the high dose. The mean food consumption of males at the high dose was lower than that of controls throughout the study, although the difference was minor and not statistically significant, but the food consumption of females at the high dose was statistically significantly decreased by 1015% at most times. A statistically significant decrease in mean corpuscular haemoglobin concentration and alterations in the percentages of lymphocytes and neutrophils in animals at the high dose were judged to be toxicologically insignificant. Several significant differences in clinical chemical parameters were also considered not toxicologically significant owing to the small magnitude of the changes or the lack of a doseresponse relationship. Variations in the weights of the kidneys, heart, and testes were not accompanied by microscopic changes in these organs and were also considered not toxicologically significant. The NOAEL was 200 ppm, equal to 14 mg/kg bw per day, on the basis of decreased body weight and body-weight gain in animals of each sex and decreased food consumption by females (Lina et al., 1984).
Dodine (purity, 98%), moistened with deionized water, was applied to the shaved, intact dorsal skin of groups of 10 male and 10 female Crl:CD®IGS(SD)BR rats at a dose of 0, 50, 125, or 200 mg/kg bw per day for a total of 20 applications (6 h/day, 5 days per week for 4 weeks). The doses were selected on the basis of a pilot study in which one male and one female each was dosed at 200, 350, or 500 mg/kg bw per day for 5 consecutive days, with no remarkable clinical signs or effects on body weight. The incidence of dermal irritation was reportedly similar in all groups and included very slight to severe erythema, very slight oedema, desquamation, eschar formation, and blanching. Atonia was observed in one female at the low dose. In the main study, the application site was moved, as necessary, to minimize induction of severe irritation. If irritation was observed before dosing, the material was applied to areas of the skin within the shaved area that were not as severely irritated. Therefore, less than 10% of the total body surface area was treated. Before dosing, physical condition, body weights, and food consumption were assessed. During the study, animals were observed for deaths and moribundity twice daily. Physical examinations were conducted weekly, at which time the application sites were evaluated for evidence of dermal irritation. Body weights and food consumption were measured weekly. Haematological, clinical chemical, and urinary parameters were measured at the end of dosing. Ophthalmic examinations were conducted before treatment and during week 3 of the study. A complete necropsy was conducted on all surviving animals at the end of dosing. Selected organs were weighed, and selected tissues, including treated and untreated skin, were examined microscopically.
All animals survived to the end of the study, and there were no treatment-related clinical signs of toxicity. Dermal irritation was prevalent in the groups at the two higher doses, females being more affected than males, and very slight to severe oedema and erythema were seen at both doses. Desquamation, focal eschar or eschar formation, encrustation, blanching, exfoliation, and fissuring were also reported. Dermal irritation was seen at the low dose, females again being more severely affected. Very slight to slight erythema was observed on all females and one-half of the males; desquamation was observed in all animals. Other findings in this group include oedema, focal eschar or eschar formation, blanching, encrustation, exfoliation, and fissuring. The only finding in the control group was a focal eschar in one female at the end of the study, which was thought to be due to shaving or tape abrasion.
Males at 125 and 200 mg/kg bw per day had significantly decreased body-weight gain (26% and 37%, respectively) during week 1. There were no treatment-related effects on haematological, clinical chemical, or urinary parameters or on ocular findings. At necropsy, scabbing was observed on macroscopic examination of the skin of treated animals in a dose-related manner; most of these animals also had encrustation, eschar, exfoliation, and/or fissuring. One control animal also had scabbing, but no dermal irritation was seen during the study. Sporadic, significant changes in organ weights were judged not to be related to treatment. Microscopic examination of treated skin showed that most animals at the two higher doses had focal or multifocal lesions indicative of varying degrees of irritation. These included ulcers, suppurative inflammation, epidermal hyperplasia, hyperkeratosis, subacute inflammation, inflammatory exudate, and para-keratosis. One female at the low dose had minimal exudate and parakeratosis. The NOAEL for systemic toxicity was 50 mg/kg bw per day, on the basis of decreased body weight (Kern, 1999e).
Dodine (purity, 35%) in distilled water was applied to the shaved, intact dorsal skin of five male and five female Sprague-Dawley rats at a dose of 0, 12, 25, or 50 mg/kg bw per day on 5 days per week for 3 weeks. The doses were selected on the basis of a pilot study in which one male and one female rat were dosed at 20, 100, or 200 mg/kg bw per day for 8 days; dermal irritation occurred at all doses, and very slight erythema and desquamation were observed at the low dose. The severity of these effects was dose-related, and other evidence of dermal irritation (necrosis, eschar, and atonia) was seen at the higher doses. In the main study, animals were observed for deaths and clinical signs of toxicity twice daily. Body weights and food consumption were recorded before treatment and weekly during the study. Haematological and clinical chemical parameters were measured at the end of the study. At necropsy, selected organs were weighed, and selected tissues, including treated and untreated skin, from animals in the control and high-dose groups were examined histologically.
All animals survived to the end of the study. The mean body-weight gain was decreased by 44% on days 1421 in males at 50 mg/kg bw per day; overall (days 021), the body-weight gain was decreased by 9%. There was no effect on food consumption, clinical condition, or organ weights. The only haematological parameter affected was the total white blood cell count, which was increased because of a severe increase in one male at the high dose. Dermal irritation was observed in all treated groups. Males and females at the low dose showed very slight to moderate (one observation) erythema, desquamation, and fissuring. Those at the two higher doses showed a dose-related increase in the incidence and severity of these signs of irritation, with eschar formation, exfoliation, necrosis, and atonia. At necropsy, there were no treatment-related effects on organ weights. Macroscopic examination of the skin showed that most animals at the high dose and fewer at the intermediate dose had evidence of dermal irritation; one animal at the low dose and none in the control group had dermal irritation. Microscopic examination of the treated skin from controls and animals at the high dose showed that most of the latter had lesions, including accumulation of inflammatory cells, hyper- and parakeratosis, squamous-cell hyperplasia, necrosis of the epithelium, ulcers or erosions, and subacute inflammation. The NOAEL for systemic toxicity was 25 mg/kg bw per day, on the basis of decreased body-weight gain (Auletta, 1989).
Dogs
In a range-finding study, groups of two male and two female beagles received gelatin capsules containing dodine (purity, 94.1%) at 12 mg/kg bw per day for 7 days followed by 50 mg/kg bw per day for 5 weeks, 25 mg/kg bw per day for 6 weeks, 6.2 mg/kg bw per day for 3 weeks followed by 60 mg/kg bw per day for a further 2 weeks, or 1.2 mg/kg bw per day for 5 weeks. During the last week of treatment, barium contrast radiography was performed on one dog treated at 1.2 mg/kg bw per day and one treated at 50 mg/kg bw per day to evaluate the effect on gastric emptying. One male at 12 mg/kg bw per day for 7 days and 50 mg/kg bw per day for 4 weeks was killed on day 36 of the study because of excessive weight loss and decreased food intake. The clinical signs observed when the dose was increased to 50 mg/kg bw per day included vomiting, excessive salivation, soft or liquid faeces, dehydration, thinness, weakness, reduced activity, and pale gums. The findings clinically and at gross necropsy were consistent with dehydration and a catabolic condition. Although the animal had had poor food consumption, its stomach contained a large amount of undigested food. Excessive salivation and vomiting were seen in most dogs treated at 25 mg/kg bw per day or more. Vomiting was also seen in females at 12 mg/kg bw per day during week 1; as the dose of these animals was then increased to 50 mg/kg bw per day, the findings at week 1 are difficult to interpret. Liquid faeces were observed on occasion when dogs were treated at 25 mg/kg bw per day or more; one dog each at 1.2 and 12 mg/kg bw per day was also affected. Treatment at 50 or 60 mg/kg bw per day had adverse effects on body weight and food consumption. Reduced food intake and body-weight loss were also observed in one dog at 25 mg/kg bw per day. At the two higher doses, the total protein concentration was decreased, due to decreases in albumin and globulin concentrations. As the albumin:globulin ratios were within the expected physiological range, the observed changes in total protein were considered to be related to the nutritional status of these dogs. Elevated blood urea nitrogen concentrations were seen on occasion in some dogs treated with dodine at 25, 50, or 60 mg/kg bw per day. There were no effects on haematological or urinary end-points or on organ weights.
At necropsy, undigested food was found in the stomachs of all dogs treated at 50 or 60 mg/kg bw per day and in one dog at 25 mg/kg bw per day. These animals also had dark areas, foci, and/or discolouration of the stomach and/or duodenum. Barium contrast radiography showed that the dog at the low dose had a normal gastric emptying time (clearance of opaque material within 2 h), whereas opaque material was still present after 2 h in the stomach of the dog given 50 mg/kg bw per day. No opaque material was present in the stomach of the dog at the high dose after 4 h, although food was still present. No consistent adverse effects were observed, although complete evaluation of the effects of treatment at 12 mg/kg bw per day for more than 1 week is precluded by the change in dose (Smith, 1994).
Dodine (purity, 98.6%) was administered in gelatin capsules to groups of four male and four female beagles at a dose of 0, 2, 10, or 20 mg/kg bw per day for at least 52 weeks. The animals were observed twice daily for morbidity and mortality and once daily for signs of toxicity. Body weights were measured before treatment and then weekly throughout the study. Food consumption was measured weekly. Ophthalmic examinations were conducted before treatment and during week 52 of the study. Haematological, clinical chemical, and urinary end-points were measured before treatment and during weeks 26 and 52 of the study. At the end of the treatment period, the animals were killed and examined grossly and microscopically.
All animals survived to the end of the study, with no treatment-related clinical signs of toxicity. An increased incidence of salivation was observed in animals at the two higher doses, but the frequency was greater before dosing than after, indicating a conditioned reflex. The incidence of emesis was increased in females at the two higher doses, but it too occurred more frequently before dosing. No statistically significant difference was observed in mean body weight, body-weight changes, or food consumption. One female at the intermediate dose and one male and one female at the high dose showed marked weight loss, which was due to reduced food consumption during the first few weeks of the study. Supplemental feeding of basal diet mixed with water and/or canned dog food was initiated during week 2 or 3 to promote eating by these animals and to prevent further weight loss. Basal diet was resumed for the female at the intermediate dose in week 15 and for the male at the high dose in week 8, but the female at the high dose required supplemental feeding throughout the study. No treatment-related effects on haematological, clinical chemical, urinary, or ophthalmic end-points or on organ weights or the findings at gross and microscopic necropsy were observed. The NOAEL was 10 mg/kg bw per day, on the basis of the necessity for supplemental feeding of one male and one female at the higher dose (Trutter, 1996).
The 1-year study in dogs (Levinskas et al., 1961) that was used to establish the previous ADI, with a NOAEL of 50 ppm, equivalent to 1.2 mg/kg bw per day, on the basis of effects on the thyroid, was reevaluated and found to be unacceptable by current standards. Only two animals of each sex per group were tested, and only minimal clinical testing was conducted. Microscopic examinations were performed on only one male and one female control and on two males and two females at the high dose. The thyroid glands of all animals were examined microscopically, and evidence was found that the animals in the study were infected with both internal and external parasites. Thus, the microscopic findings in the thyroid consisted of a shift of the follicular epithelium from a squamous to a predominantly cuboidal variety in animals at the two higher doses. The mean weight of the thyroid of the dodine-treated animals was increased, but there was no relationship between the size of the thyroid and the dose of dodine or the microscopic appearance of the gland.
(c) Long-term studies of toxicity and carcinogenicity
Mice
Dodine (purity, 98.6%) was administered in the diet to groups of 60 male and 60 female Crl:CD-1®(ICR)BR mice at a concentration of 0, 200, 750, or 1500 ppm, equal to 0, 29, 110, and 220 mg/kg bw per day for males and 0, 38, 140, and 280 mg/kg bw per day for females, for 78 weeks. An additional 10 males and 10 females per group were killed at 53 weeks. The doses were selected on the basis of a 13-week study in mice treated in the diet. The animals were checked twice daily for deaths and clinical signs, and were examined weekly in detail, including palpation for masses. Body weight and food consumption were determined on the first day of treatment, then weekly for the first 13 weeks, at least every 4 weeks thereafter, and at termination. Food efficiency, expressed as percentage of body-weight gain/food consumption, was determined for the first 13 weeks. Blood smears were examined at week 53 and at the end of the study and from animals that were killed when moribund. Differential leukocyte counts were not performed because histological examinations indicated that the test material did not induce haematopoietic neoplasia. All mice killed ad interim, when moribund, and at the end of the study underwent a complete gross necropsy. Selected tissues from controls and mice at the high dose killed at the end of the study and from any animal that died or was killed prematurely were weighed and examined microscopically, as were the lungs, liver (with gall-bladder), kidneys, gross lesions, and masses from animals at the two lower doses that were killed at the end of the study.
Females at the high dose were erroneously given doses greater than 1500 ppm on the first day of week 44: Analyses of a composite sample of the diet of these animals for weeks 4144 showed a mean concentration of 9000 ppm of dodine. The food was analysed because of the poor health of the animals and food scattering on the first day the diet was offered during week 44. The report states that the diet was replaced immediately with an appropriately mixed concentration. The error resulted in the death or sacrifice of six females, but no overall increase in mortality rates was observed as compared with controls. The mortality rate of males at this dose (3/70) was significantly decreased when compared with controls (16/70). The percentage survival in all groups was 8497% at 52 weeks and 7393% at 78 weeks. There were no treatment-related clinical signs of toxicity.
Females at 750 ppm had significantly decreased body weight (£ 10%) at some time. Males at this dose showed significant decreases, but only by £ 5%. Females at this dose had significantly decreased overall (178 weeks) mean body-weight gain (20% decrease) and mean food consumption (616%) throughout the study relative to the controls. The mean body weight was significantly decreased in males (£ 10%) and females (£ 15%) at the high dose, and the overall mean body-weight gain was significantly decreased in males (26%) and females (35%). Mean food consumption was significantly decreased in males (at 25/29 intervals) and females (at 17/29 intervals) at the high dose. Overall food efficiency was decreased by 21% in males and 39% in females at the high dose, although the differences were not statistically significant.
At the interim sacrifice, no abnormalities were reported, but microscopic examinations were not performed. The absolute weight of the right kidney and the relative (to body and brain) weight of both kidneys were significantly increased in females at the high dose. At terminal necropsy, a significant increase was found in the relative (to body) weight of the liver and gall-bladder (14% greater than the control value) in males and females at the high dose, and the absolute weight of both kidneys was significantly increased in females at this dose. The relative (to body) weight of both kidneys was significantly increased in females at the two higher doses, and the relative (to brain) weight of both kidneys was significantly increased in females at the high dose. As no macroscopic or microscopic changes were found in the kidneys, these changes in organ weight are of questionable toxicological significance. Significant decreases in the absolute and/or relative weight of one or both adrenal glands were observed sporadically at the interim and terminal necropsies. Since these changes were sporadic and there were no macroscopic or microscopic alterations to the glands, the alterations are of questionable toxicological significance. The NOAEL for toxicity was 200 ppm, equal to 29 mg/kg bw per day, on the basis of decreased body-weight gain and food consumption.
A positive trend was observed in the incidence of hepatocellular adenomas and combined hepatocellular adenomas/carcinomas in females. Although the incidence of adenomas was increased relative to that of the controls (4/60, 6.6%, in treated; 0/60 in controls), the increase was not statistically significant. The incidence of combined hepatocellular adenomas and carcinomas was significantly increased in females at the high dose (5/60, 8.3%, in treated; 0/60 in controls) owing to the incidence of adenomas. No treatment-related increase in the incidence of hepatocellular neoplasms was seen in males. The incidences of tumours in males and females are presented in Table 2. The doses were considered adequate to characterize the carcinogenic potential of dodine. Use of data on controls in another laboratory was considered inappropriate since they were derived from studies conducted much earlier than the present study (Williams, 1998).
Table 2. Incidences of hepatocellular tumours in mice treated with dodine
|
|
Males |
Females |
||||||
|
|
0 |
200 |
750 |
1500 |
0 |
200 |
750 |
1500 |
|
Total examined |
60 |
60 |
60 |
60 |
60 |
58 |
59 |
60 |
|
Adenomaa |
8 |
7 |
9 |
14 |
0 |
1 (1.7) |
1 (1.7) |
4 (6.6) |
|
Carcinoma |
2 |
0 |
3 |
1 |
0 |
1 |
0 |
1 |
|
Adenoma and carcinomaa |
10 |
7 |
12 |
15 |
0 |
2 (3.4) |
1 |
5* (8.3) |
From Williams (1998)
a
Trend test significant at p < 0.05 in females* Significantly different from controls at p < 0.05
Rats
Dodine (purity, 98.6%) was administered in the diet to groups of 60 male and 60 female Sprague-Dawley rats at a concentrations of 0, 200, 400, or 800 ppm, equal to 0, 10, 20, and 42 mg/kg bw per day for males and 0, 13, 26, and 54 mg/kg bw per day for females, respectively, for 106 weeks. Groups of 10 male and 10 female rats were given the same concentrations and killed at 53 weeks. The high dose was selected on the basis of previous studies in which doses greater than 800 ppm caused decreased body-weight gain. The animals were observed twice daily for clinical signs and mortality. They were examined physically twice a month during the first 13 weeks of the study and weekly thereafter. Body weight and food consumption were recorded weekly for the first 13 weeks and once every 4 weeks thereafter. Ophthalmoscopic examinations were performed before treatment and after 1 and 2 years. Haematological and clinical chemical end-points were measured at weeks 26, 52, 78, and 104 and urinary end-points at weeks 25, 51, 79, and 103. At the interim necropsy, all animals were examined grossly, and limited tissues from the controls and animals at the high dose were examined microscopically. A complete complement of tissues from animals killed at the end of the study was examined microscopically.
There was no treatment-related increase in mortality rate. A dose-related increase was found in the number of treated males with clinical signs of toxicity, including no righting reflex (0, 2, 3, and 4 in the four groups, respectively), absent traction reflex (1, 0, 1, and 5), absent grasping reflex (2, 4, 6, and 8), and hunched posture (1, 2, 4, and 7). These data were not analysed statistically. Other clinical signs seen in control and treated animals but with no relation to dose included clonic or tonic convulsions, reduced motor activity, hindlimb oedema, soreness, induration, or limping, hindlimb paralysis, prostration, thinness, and piloerection. Although there was a dose-related increase in the incidence of clinical signs in males, the signs were agonal and did not indicate neurotoxicity. Ten males at the high dose had no righting reflex, tractor reflex, and/or grasping reflex, and all died or became moribund within days of the first sign. The effects were also reported in conjunction with other signs indicative of extreme ill health.
The mean body weights of females at the high dose were significantly reduced (416%) throughout the study. Males at this dose and females at the next lower dose also had significant reductions, but the differences from controls were sporadic and small and were considered not to be toxicologically significant. The mean cumulative body-weight gain was also reduced (by 1325%) throughout the study in females at the high dose. Mean food consumption was significantly decreased in aniamls at this dose, and the frequency and magnitude of the difference from controls were greater in females.
There was no evidence of a treatment-related effect on ophthalmic parameters. Statistically significant changes from control values seen in haematological and clinical chemical parameters were minor, transient, and not dose-dependent; therefore, they were considered not toxicologically significant. Significant differences from control values in urinary parameters were sporadic and highly variable and were considered not toxicologically significant. Significant changes in organ weights and alterations in gross morphological appearance were not dose-dependent, were not accompanied by microscopic findings, and were considered not toxicologically significant. The incidence of ovarian granulosa or theca-cell hyperplasia was increased in females at the high dose when all animals were considered, but the increases were not significant, there was no change in ovarian weight, and there was no increase in the incidence of ovarian granulosa or theca-cell tumours. An increase in the incidence of prostate atrophy in males at the high dose that died or were killed in extremis was not confirmed at terminal sacrifice. The microscopic findings in females and males were considered to be incidental. The NOAEL for toxicity was 400 ppm, equal to 20 mg/kg bw per day, on the basis of decreased body weight, body-weight gain, and food consumption.
There was no treatment-related increase in the incidence of neoplasms at the interim sacrifice. The incidence of focal thyroid C-cell hyperplasia in all animals that were necropsied was 6/66 (9%) in the controls and 7/62 (11%) in rats at the high dose. No pairwise increase was found in the incidence of thyroid C-cell adenomas or carcinomas when analysed separately, but a dose-dependent increase in the combined incidence of adenomas and carcinomas was found in males, with 23/66 (35%) in controls, 21/52 (40%) at the low dose, 27/60 (45%) at the intermediate dose, and 33/62 (53%) at the high dose (Table 3). The combined incidence in all treated males exceeded the mean of the data for 223 controls in the same laboratory used in three studies between June 1995 and October 1996; however, the incidence in the control group was also high in comparison with past control data. No treatment-related increase in tumour incidence was found in females. The high dose given to females was adequate to test the potential carcinogenicity of dodine, on the basis of decreased body weight and body-weight gain, but the high dose given to males was considered to be marginally adequate. Although body weight was statistically significantly decreased during the study, the magnitude of the difference from controls was small (57%); a 14% decrease in body-weight gain occurred in weeks 013 (Dange, 1998).
Table 3. Per cent incidences of thyroid C-cell tumours in male rats treated with dodine
|
|
Dose (ppm) |
|
|||
|
|
0 |
200 |
400 |
800 |
Historical controls (mean, range) |
|
Total no. animals examined |
66 |
52 |
60 |
62 |
223 |
|
Thyroid focal C-cell hyperplasia |
6 (9%) |
5 (10%) |
3 (5%) |
7 (11%) |
24 (2032) |
|
Thyroid C-cell adenoma |
19 (29%) |
20 (38%) |
20 (33%) |
26 (42%) |
29 (2532) |
|
Thyroid C-cell carcinoma |
4 (6%) |
1 (2%) |
7 (12%) |
7 (11%) |
6.7 (5.910) |
|
Thyroid adenoma and carcinoma |
23 (35%) |
21 (40%) |
27 (45%) |
33 (53%) |
33 (2738) |
|
Thyroid hyperplasia, adenoma, and/or carcinoma |
27 (41%) |
26 (50%) |
27 (45%) |
31 (50%) |
49 (3957) |
From Dange (1998)
The results of tests for the genotoxicity of dodine are shown in Table 4.
Table 4. Results of tests for the genotoxicity of dodine
|
End-point |
Test object |
Concentration/Dose |
Purity (%) |
Results |
Reference |
|
In vitro |
|||||
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
05.0 ΅g/plate |
95 |
Negativea,b |
Willems (1981) |
|
Gene mutation |
Chinese hamster ovary cells, Hprt locus |
020 ΅g/ml without S9 activation |
98 |
Negativea,c |
Davis (1985) |
|
|
|
035 ΅g/ml with S9 activation |
|
|
|
|
Chromosomal aberration |
Human lymphocytes |
0.3710 ΅g/ml without S9 activation |
98 |
Negativea,d |
Wilmer (1985) |
|
|
|
0.5615 ΅g/ml with S9 activation |
|
|
|
|
In vivo |
|||||
|
Micronucleus formation |
Swiss random mice |
500 mg/kg bw once orally |
98 |
Negativee |
Willems (1985) |
|
Micronucleus formation |
ICR mice |
100, 200, or 400 mg/kg bw once orally |
94 |
Negativef |
Murli (1992) |
a
With and without metabolic activation (S9)b
Positive controls, sodium azide (0.5 ΅g/0.1 ml water per plate) for strains TA1535 and TA100 without S9 activation, hycanthone methanesulfonate (12.5 ΅g/0.1 ml water per plate) for strains TA1537, TA1538, and TA98 without S9 activation, and 2-aminoanthracene (0.5 ΅g/0.1 ml DMSO per plate) with S9 activation, gave expected positive results.c
Positive controls, ethyl methanesulfonate (no S9, 0.4 ΅l/ml) and N-nitrosodimethylamine (with S9, 2 ΅l/ml and 5 ΅l/ml), gave expected positive results.d
Positive controls, methylmethanesulfonate (no S9, 30 ΅g/ml) and cyclophosphamide (with S9, 20 ΅g/ml), gave expected positive results.e
Positive control, mitomycin C (1.5 mg in 20 ml saline/kg bw) by intraperitoneal injection, gave expected positive results.f
Positive control, cyclophosphamide (80 mg/kg bw), gave expected positive results.Rats
Dodine (purity, 98.6%) was administered continuously in the diet to groups of 30 male and 30 female CD Sprague-Dawley rats at a concentration of 0, 200, 400, or 800 ppm, equal to 0, 13, 26, and 53 mg/kg bw per day for F0 males and 0, 18, 35, and 68 mg/kg bw per day for F0 females, and 0, 15, 30, and 63 mg/kg bw per day for F1 males and 0, 19, 39, and 77 mg/kg bw per day for F1 females. Administration to the F0 animals began when they were about 7 weeks of age (up to 10 weeks before mating) and continued throughout gestation and lactation. On day 4 after birth, the litters were reduced to a maximum of eight pups, with four of each sex when possible. Discarded pups and pups that died or were killed underwent a complete necropsy. At weaning, 10 pups of each sex per group from the F1 and F2 litters were randomly selected and necropsied. Thirty F1 pups of each sex per group selected to produce the F2 generation were exposed to the same dose as their parents from day 21 after birth. F1 animals were given dodine for 10 weeks before mating to produce the F2 litters. All surviving F0 and F1 adult males were killed after mating; all females were killed after weaning of their respective litters. Females that did not deliver a litter were necropsied after day 26 of gestation. All parental animals were subjected to a complete external and internal examination post mortem.
No treatment-related changes in mortality rate or clinical signs were found in the F0 or F1 adults. Significant decreases in mean body weight were observed in male F0 parents at the high dose before and during mating and in females at this dose before mating and during gestation and lactation, although the differences from control were usually < 10%. Significant decreases in mean body weight were observed in F1 males at the high dose before and during mating (1319%) and in females at this dose before mating (1215%), during gestation (1214%), and during lactation (815%). Females at the intermediate dose also showed decreases (46%) before mating and during gestation and lactation. Significant decreases in mean body-weight gain occurred in F0 females at the high dose before mating (1436%) and in males at this dose before and during mating (1332%). F1 females at the high dose showed significant decreases in mean body-weight gain before mating (916%) and during gestation (1120%). Males at this dose had significant decreases (1015%) before and during mating. Decreases in food consumption were observed in F0 males and females at the high dose before mating and in F0 females during lactation (1019%). Food consumption was decreased in F1 females at the high dose before mating (918%) and during gestation (1217%) and lactation (1220%) and in males at this dose before mating (614%).
There were no treatment-related effects on reproductive parameters or on the mortality rate, clinical signs, or findings at necropsy in F1 and F2 litters. The mean body weights of F1 and F2 pups at the high dose were decreased by day 4 after birth (before and after culling) through day 21 (by 717% for F1 and 817% for F2) and in females on days 721 after birth (918%). The NOAEL for parental toxicity was 400 ppm, equal to 26 mg/kg bw per day, on the basis of decreased mean body weights, body-weight gains, and food consumption. The NOAEL for reproductive toxicity was 800 ppm, equal to 53 mg/kg bw per day, the highest dose tested. The NOAEL for toxicity to offspring was 400 ppm, equal to 26 mg/kg bw per day, on the basis of decreased body weights of the F1 and F2 pups (Henwood, 1996).
Rats
Dodine (purity, 95.3%) was administered by oral gavage to 25 mated Sprague-Dawley rats at a dose of 0, 10, 45, or 90 mg/kg bw per day on days 616 of gestation. The day on which sperm was detected in a vaginal lavage or on which a copulatory plug was present was considered day 0. The animals were observed twice daily for deaths and clinical signs of toxicity. Body weights were recorded on days 0, 6, 9, 13, 17, and 20 of gestation. Food consumption was measured daily from day 4. On day 20 of gestation, the fetuses were removed surgically, and reproductive parameters were assessed. After an external examination, about 50% of the fetuses from each litter were fixed and examined for visceral abnormalities, then stained for skeletal examination. The remaining fetuses were fixed and examined for soft-tissue abnormalities.
No deaths occurred during the study. Excessive salivation was reported in three rats at the high dose. Several animals at the two higher doses had redbrown stained fur around the mouth, but the toxicological significance of these findings is unclear. The mean body-weight gain of animals at the high dose was significantly reduced on days 69 of gestation (weight loss of 1 g, with a weight gain of 15 g in controls) and on days 617 (by 20%). The weight gain was also decreased (by 43%) in animals at the intermediate dose on days 69 of gestation, but the difference from controls was not significant; overall (days 617 of gestation), weight gain was not affected. The mean food consumption of animals at the high dose was reduced throughout treatment, but the differences were not statistically significant. There was no evidence of a treatment-related effect on reproductive or developmental parameters. The NOAEL for maternal toxicity was 10 mg/kg bw per day, on the basis of decreased mean body-weight gain. The NOAEL for develop-mental toxicity was 90 mg/kg bw per day, the highest dose tested (Hazelden & Wilson, 1989).
Rabbits
Dodine (purity, 95.3%) was administered by oral gavage to 16 or 20 (high dose only) pregnant New Zealand white rabbits at a dose of 0, 10, 40, or 80 mg/kg bw per day on days 618 of gestation. The rabbits were in day 1, 2, or 3 of gestation when received from the supplier. The groups were observed for deaths and clinical signs of toxicity daily. Body weights were recorded on days 6, 9, 12, 15, 19, 22, 26, and 29 of gestation, and food consumption was measured daily from day 4. The fetuses were removed surgically on day 29. After gross examination, two or three live fetuses from each litter were fixed in methylated ethanol and examined for visceral anomalies; the cranium was sectioned once for observation of the brain, and the eviscerated carcass was stained for skeletal defects. The remaining fetuses were fixed with Bouin solution and underwent whole-body dissection. The study was conducted in compliance with GLP standards, and a signed and dated statement of quality assurance was attached.
Several deaths occurred during the study. One rabbit at the intermediate dose was found dead on day 8 of gestation due to a dosing accident. Three does at the high dose died or were killed before the end of the study: Two had breathing difficulties and had reddened or dark areas of the lung lobes at necropsy. As both had struggled during dosing, a gavage accident may have caused the clinical findings. The other animal was killed on day 15 of gestation as it was in poor condition. An additional two animals at the high dose aborted their litters on day 2021 of gestation. The last three animals had reduced food consumption and body-weight loss (1014% of the weight at day 6 of gestation) before death. Necropsy showed fluid-filled intestines. The animal killed in poor condition had pyloric mucosa covered with mucus-like material and irregular white areas. One animal each in the control and low-dose groups also aborted. There was no treatment-related effect on mean body weight or body-weight gain when animals that aborted were excluded from the analysis. The only other evidence of maternal toxicity was a decrease (23%) in the food consumption of does at the high dose during dosing (days 618 of gestation). There was no evidence of a treatment-related effect on developmental parameters.
The NOAEL for maternal toxicity was 40 mg/kg bw per day, on the basis of decreased mean food consumption during treatment. The death of a female at 80 mg/kg bw per day was possibly related to treatment. The NOAEL for developmental toxicity was 80 mg/kg bw per day, the highest dose tested (Hazelden & McCay, 1989)
(f) Special study: Gut motility
A mechanistic study was performed to assess the gut motility of rats after continuous dietary administration of dodine. Groups of 10 male and 10 female rats were given dodine (purity, 98.6%) in the diet at a concentration of 0, 200, or 800 ppm for 7 or 28 days, when five animals of each sex per dose were killed. On the day of necropsy, animals received 2.5 ml of a charcoal suspension by oral gavage, were observed for 12 h, and were then killed. The gastrointestinal tract was removed and extended to its full length to measure the distance between the pyloric sphincter and the proximal and distal traces of charcoal. In addition, the lengths of the major portions in which the charcoal was located along the gastrointestinal tract were measured, and the intensity of the charcoal in the major portions was evaluated visually. There were no deaths or treatment-related clinical signs. The body weights and body-weight gains of animals at 800 ppm were slightly lower than those of the controls. Food intake was affected during the beginning of the study. The location of charcoal along the gastrointestinal tract was not different in the treated animals as compared with the controls (Dange, 1994).
The Meeting was aware of three studies on workers in chemical plants where dust and liquid pesticides were produced in Poland, which have been reported in the medical literature (Sliwinski et al., 1991; Kossmann et al., 1997, 1999).
The absorption, distribution, and excretion of radiolabelled dodine were investigated in rats given low (40 mg/kg bw) single and repeated doses and a high dose (400 mg/kg bw). Less than 50% of the administered dose was absorbed. By 120 h after dosing, the amount of the administered dose excreted in urine (4145%) and faeces (4860%) was similar in all groups. Most of the radiolabel in urine and faeces was excreted within the first 48 h by the group at the low dose and by 96 h by those at the high dose. Little radiolabel was recovered in the tissues at 120 h, and 3.4% of the administered dose was retained. In general, the recovery of radiolabel was similar in males and females.
Dodine was extensively metabolized, and no unmetabolized parent compound was detected in urine. The metabolic profile was similar in the two sexes and at all doses. Four metabolites were identified in urine. The major one was hydroxydodecylguanidine, an omega-oxidation product, which accounted for 1124% of the administered dose. The minor metabolite was identified as urea, whereas the other two were not clearly identified. In faecal samples, the parent compound was identified as the major component (3955%).
Dodine was slightly toxic in mice and rats given single oral doses. In male mice, the LD50 was 1700 mg/kg bw. In rats, the LD50 values were 7501900 and 6601100 mg/kg bw in males and females, respectively. The compound was moderately toxic when given by inhalation; the LC50 was 0.47 and 0.44 mg/L for males and females, respectively. Dodine was not toxic after single dermal administration; the LD50 value in rabbits and rats was > 2000 and > 5000 mg/kg bw, respectively. It was a severe ocular and dermal irritant, but it is not a dermal sensitizer. WHO (1999) has classified dodine as slightly hazardous.
In short- and long-term studies of toxicity in rodents, rabbits, and dogs, the most consistently observed effects were decreased body weight and body-weight gain, which were frequently accompanied by decreased food consumption. The NOAELs for these parameters were similar in the short- and long-term studies and between species. Other toxic effects were reported only rarely in these studies.
In an 8-week study of toxicity mice at a dietary concentration of 0, 100, 250, or 625 ppm, in which the 100-ppm dose was increased to 1250 ppm after 3 weeks, one death possibly related to treatment, decreased body-weight gain, and cytoplasmic eosinophilia in hepatocytes were observed at 1250 ppm, equal to 230 mg/kg bw per day, the highest dose tested. The NOAEL was 625 ppm, equal to 110 mg/kg bw per day. In a 90-day study of toxicity in mice at a dietary concentration of 0, 150, 300, 600, 1250, or 2500 ppm, four of five females at 2500 ppm died during the first 2 weeks of treatment. Decreased body weight, body-weight gain, and food consumption were observed at 1250 ppm, equal to 180 mg/kg bw per day. The NOAEL was 600 ppm, equal to 94 mg/kg bw per day. In a 28-day study of toxicity in rats at a dietary concentration of 0, 500, 750, or 1000 ppm, decreased body weight, body-weight gain, and food consumption were observed at 750 ppm, equal to 71 mg/kg bw per day. The NOAEL was 500 ppm, equal to 47 mg/kg bw per day. In another 28-day study in rats at a dietary concentration of 0, 200, or 800 ppm, decreased body weight, body-weight gain, and food consumption were reported at 800 ppm, equal to 68 mg/kg bw per day. The NOAEL was 200 ppm, equal to 18 mg/kg bw per day. In a 4-week study in rats given a dose of 0, 75, 100, or 200 mg/kg bw per day by oral gavage, an increased mortality rate, clinical signs of toxicity, decreased body weight, body-weight gain, and food consumption, and histological alterations in the gastrointestinal tract (oedema, mixed-cell infiltration, and hyperplasia of the squamous mucosa of the stomach) were reported at 75 mg/kg bw per day. A NOAEL was not identified. In a 90-day study of toxicity in rats at a dietary concentration of 0, 50, 200, or 800 ppm, decreased body weight and body-weight gain were observed at 800 ppm, equal to 56 mg/kg bw per day. The NOAEL was 200 ppm, equal to 14 mg/kg bw per day. In a 5-week range-finding study, dogs that received dodine in gelatin capsules at increasing doses of 1.260 mg/kg bw per day showed clinical signs of toxicity (salivation, vomiting, liquid faeces), decreased body weight and food consumption, and abnormal gross necroscopic changes in the gastrointestinal tract (undigested food in the stomach and discolouration of the gastric mucosa of one dog) at 25 mg/kg bw per day. No consistent adverse effects were observed after treatment with dodine at doses up to 12 mg/kg bw per day for 1 week, although complete evaluation of this dose and duration was precluded by the increase of the dose to 50 mg/kg bw per day for the next 5 weeks. In a 1-year study of toxicity in dogs given capsules containing a dose of 0, 2, 10, or 20 mg/kg bw per day, decreased food intake by two animals at 20 mg/kg bw per day, which required supplemental feedings for the entire study, was the only adverse effect observed. In this study, salivation and emesis before and after dosing were reported in both treated and control animals, the incidence being higher with the two higher doses of dodine. These findings were considered to be toxicologically insignificant because there was no evidence of alterations in the gastrointestinal tract at necropsy, either macroscopically or microscopically. The NOAEL was 10 mg/kg bw per day.
In a study of mechanism of action, rats given up to 800 ppm of dodine in the diet for 7 or 28 days and then a charcoal suspension showed no evidence of altered gastrointestinal motility. Delayed gastric emptying, as measured by barium contrast radiography, was observed in one dog at 50 mg/kg bw per day in a 5-week range-finding study.
Studies of dermal toxicity in rats of 21 and 28 days duration showed that dodine is severely irritating at a dose as low as 12 mg/kg bw per day. There was some evidence that dermal application at a dose as low as 50 mg/kg bw per day caused systemic toxicity (decreased body weight and body-weight gain), but the severe dermal irritation may have contributed to these findings.
In a 78-week study of carcinogenicity in mice at a dietary concentration of 0, 200, 750, or 1500 ppm, the only evidence of toxicity was decreased body-weight gain and food consumption at 750 ppm, equal to 110 mg/kg bw per day. The study was complicated by the inadvertent mis-dosing of females at 1500 ppm with approximately 9000 ppm of dodine during weeks 4144. The NOAEL for toxicity was 200 ppm, equal to 29 mg/kg bw per day. A positive trend in the incidence of hepatocellular adenomas was observed in females and a statistically nonsignificant increase in the incidence of hepatocellular adenomas in females at 750 ppm. The high dose was considered adequate for testing the carcinogenic potential of dodine in mice. No pertinent data on historical controls were available. The Meeting concluded that the increased incidence of hepatocellular tumours was not relevant for human risk assessment because only benign tumours (adenomas) were observed, they occurred at a dose that exceeded the maximum tolerated dose, and they were reported in only one sex.
In a long-term study of toxicity and carcinogenicity in rats at a dietary concentration of 0, 200, 400, or 800 ppm, the only evidence of toxicity was decreased body weight, body-weight gain, and food consumption at 800 ppm, equal to 42 mg/kg bw per day. The NOAEL was 400 ppm, equal to 20 mg/kg bw per day. There was a statistically nonsignificant increase in the incidence of combined thyroid C-cell adenomas and carcinomas in males at 800 ppm, and the incidence in all treated males exceeded the mean and upper limit of the range for historical controls. However, the incidence in the concurrent control group also exceeded the mean of the historical controls. The high dose was considered marginally adequate for testing the carcinogenic potential of the chemical. The Meeting concluded that the increased incidence of thyroid C-cell adenomas and carcinomas was not relevant for human risk assessment because there was no statistically significant increase in the incidence of the tumours, they occurred in only one sex, and there was no clear doseresponse relationship in the increased incidence of benign (adenomas) and malignant (carcinomas) tumours.
No evidence of genotoxicity was found in vivo or in vitro. The Meeting concluded that dodine is unlikely to be genotoxic.
In view of the lack of genotoxicity and the finding of tumours only at concentrations at which dodine was clearly toxic, the Meeting concluded that the compound is unlikely to pose a carcinogenic risk to humans.
There was no evidence that dodine is a developmental toxicant. The only possible evidence of reproductive toxicity was a decrease in the body weight of offspring in a two-generation study of reproductive toxicity in rats, in which maternal toxicity was observed at the same dose. In a two-generation study in rats at a dietary concentration of 0, 200, 400, or 800 ppm, decreased body weight, body-weight gain, and food consumption were observed in both the parental and F1 generations at 800 ppm, equal to 53 mg/kg bw per day. There was no evidence of a treatment-related effect on reproductive parameters. The offspring of both F1 and F2 generations had decreased mean body weights at postnatal day 4 and through postnatal day 21 at a dose of 800 ppm. The NOAEL for toxicity to parents and offspring was 400 ppm (equal to 26 mg/kg bw per day). In a study of developmental toxicity in rats given a dose of 0, 10, 45, or 90 mg/kg bw per day by gavage, decreased body-weight gain was observed in maternal animals at 45 mg/kg bw per day. The NOAEL for maternal toxicity was 10 mg/kg bw per day. There was no evidence of developmental toxicity at 90 mg/kg bw per day. In a study of developmental toxicity in rabbits given a dose of 0, 10, 40, or 80 mg/kg bw per day by gavage, the evidence of maternal toxicity consisted of a possibly treatment-related death and decreased food consumption at 80 mg/kg bw per day. The NOAEL for maternal toxicity was 40 mg/kg bw per day. There was no evidence of developmental toxicity at 80 mg/kg bw per day.
The Meeting concluded that the existing database was adequate to characterize the potential hazard of dodine to fetuses, infants, and children. There was no evidence that offspring are more sensitive after pre- or postnatal exposure to dodine than are adults in the same experiment.
The Meeting established an ADI of 00.1 mg/kg bw for dodine on the basis of the NOAEL of 10 mg/kg bw per day in the 1-year study in dogs, supported by an identical NOAEL for maternal toxicity in the study of developmental toxicity in rats and applying a safety factor of 100. The 1-year study in dogs, which was used to establish the previous ADI (NOAEL, 50 ppm, equivalent to 1.25 mg/kg bw per day, on the basis of effects on the thyroid), was re-evaluated and found to be unacceptable by current standards.
The Meeting established an acute RfD of 0.2 mg/kg bw on the basis of the absence of toxicity of a single dose of 20 mg/kg bw per day in the 1-year study in dogs and applying a safety factor of 100.
Levels relevant for risk assessment
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
78-week study of toxicity and carcinogenicitya |
Toxicity |
200 ppm, equal to 29 mg/kg bw per day |
750 ppm, equal to 110 mg/kg bw per day |
|
|
|
Carcinogenicity |
1500 ppm, equal to 225 mg/kg bw per dayb |
|
|
Rat |
104-week study of toxicity and carcinogenicitya |
Toxicity |
400 ppm, equal to 20 mg/kg bw per day |
800 ppm, equal to 42 mg/kg bw per day |
|
|
|
Carcinogenicity |
> 800 ppm, equal to 42 mg/kg bw per dayb |
|
|
|
Two-generation study of reproductive toxicitya |
Parental toxicity |
400 ppm, equal to 26 mg/kg bw per day |
800 ppm, equal to 53 mg/kg bw per day |
|
|
|
Reproductive toxicity |
800 ppm, equal to 53 mg/kg bw per dayb |
|
|
|
|
Pup toxicity |
400 ppm, equal to 26 mg/kg bw per day |
800 ppm, equal to 53 mg/kg bw per day |
|
|
Developmental toxicityc |
Maternal toxicity |
10 mg/kg bw per day |
45 mg/kg bw per day |
|
|
|
Embryo- and fetotoxicity |
90 mg/kg bw per dayb |
|
|
Rabbit |
Developmental toxicityc |
Maternal toxicity |
40 mg/kg bw per day |
80 mg/kg bw per day |
|
|
|
Embryo- and fetotoxicity |
80 mg/kg bw per dayb |
|
|
Dog |
1-year study of toxicityd |
Toxicity |
10 mg/kg bw per day |
20 mg/kg bw per day |
a
Dietary administrationb
Highest dose testedc
Gavaged
CapsuleEstimate of acceptable daily intake for humans
00.1 mg/kg bw
Estimate of acute reference dose
0.2 mg/kg bw
Studies that would provide information useful for further evaluation of the compound
Observations in humans
Summary of critical end-points
|
Absorption, distribution, excretion and metabolism in mammals |
|
|
Rate and extent of oral absorption, rats |
Less than 50% absorbed; 4145% excreted in urine; 4860% excreted in faeces |
|
Dermal absorption |
No studies |
|
Distribution |
Largest amounts in gastrointestinal tract, muscle, and skin; no tissues contained > 1.1% of administered dose |
|
Potential for accumulation |
Unknown |
|
Rate and extent of excretion |
Most of single and repeated low dose (40 mg/kg bw) eliminated within 48 h; single high dose (400 mg/kg bw) eliminated within 120 h |
|
Metabolism in animals |
Extensive; four metabolites in urine; major metabolite is hydroxydodecylguanidine |
|
Toxicologically significant compounds |
Parent; significance of metabolites unknown |
|
Acute toxicity |
|
|
Rats, LD 50 |
Males: 7501900 mg/kg bw; females: 6601100 mg/kg bw |
|
Rats, LD 50, dermal |
> 5000 mg/kg bw |
|
Rats, LC 50 , inhalation |
Males: 0.47 mg/l; females: 0.44 mg/l |
|
Rabbits, dermal irritation |
Severe dermal irritant |
|
Rabbits, ocular irritation |
Severe ocular irritant |
|
Guinea-pigs, dermal sensitization |
Not sensitizing |
|
Short-term toxicity |
|
|
Target/critical effect |
Decreased body weight and food consumption |
|
Lowest relevant oral NOAEL, dogs |
10 mg/kg bw per day |
|
Lowest relevant dermal NOAEL, rats |
25 mg/kg bw per day (decreased body weight evidence of possible systemic effects) |
|
Lowest relevant inhalation NOAEC |
Not determined |
|
Genotoxicity |
Unlikely to be genotoxic |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Reduced body weight and food consumption |
|
Lowest relevant NOAEL, rats |
20 mg/kg bw per day (toxicity and carcinogenicity) |
|
Carcinogenicity |
Increased incidence of heptatocellular tumours in mice and thyroid C-cell tumours in rats judged to be irrelevant to human risk assessment |
|
Reproductive toxicity |
|
|
Reproduction target/critical effect, rats |
Decreased body weight of offspring |
|
Lowest relevant reproductive NOAEL, rats |
26 mg/kg bw per day |
|
Developmental target/critical effect |
None observed |
|
Lowest relevant developmental NOAEL, rabbits |
80 mg/kg bw per day |
|
Neurotoxicity/Delayed neurotoxicity |
No evidence of neurotoxicity |
|
Medical data |
No relevant data |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
00.1 mg/kg bw |
1-year study in dogs |
100 |
|
Acute RfD |
0.2 mg/kg bw |
1-year study in dogs |
100 |
Auletta, C.S. (1989) A 21-day dermal toxicity study in rats with CT-334-87, study No. 4932-88. Unpublished report prepared by Bio/Dynamics Inc. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Batham, P. (1994a) A 4-week oral (diet) toxicity study of dodecylguanidine acetate (dodine) in the albino rat, study No. 84569. Unpublished report prepared by Bio-research Laboratories Ltd, Senneville, Quebec, Canada. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Batham, P. (1994b) A 4-week oral (gavage) toxicity study of dodecylguanidine acetate (dodine) in the albino rat, study No. 84568. Unpublished report prepared by Bio-research Laboratories Ltd, Senneville, Quebec, Canada. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Dange, M. (1994) Dodecylguanidine acetate (dodine) assessment of gut motility following dietary administration of dodine in the rat by study SA 94453. Unpublished report prepared by Centre de Recherche Rhône-Poulenc Agrochimie, Sophia Antipolis, France. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Dange, M. (1997) Dodecylguanidine acetate (dodine) 28-day toxicity study in the rat by dietary administration, study SA 94448. Unpublished report prepared by Centre de Recherche Rhône-Poulenc Agrochimie, Sophia Antipolis, France. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Dange, M. (1998) Chronic toxicity and carcinogenicity study of dodecylguanidine acetate (dodine) in the Sprague-Dawley rat by dietary administration, study SA 95083. Unpublished report prepared by Centre de Recherche Rhône-Poulenc Agrochimie, Sophia Antipolis, France. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Davis, P.B. (1985) An investigation into the possible induction of point mutations at the HGPRT-locus of Chinese hamster ovary cells by dodine, study TNO No. R 85/105. Unpublished report prepared by TNO. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Environmental Protection Agency (1987) Guidance for the Reregistration of Pesticide Products Containing Dodine as the Active Ingredient, Washington DC, USA.
Hazelden, K.P. & McCay, C. (1989) Dodine, teratogenicity study in rabbits. Unpublished report IRI No. 5861 prepared by Inveresk Research International Ltd, Scotland. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Hazelden, K.P. & Wilson, J.A. (1989) Dodine, teratogenicity study in rats. Unpublished report IRI No. 5965 prepared by Inveresk Research International Ltd, Scotland. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Henwood, S.M. (1996) Dodine: Two generation reproduction study with in rats, study No. HWI 6224-218. Unpublished report prepared by Corning Hazleton USA. Submitted to WHO by Rhône-Poulenc Agro, Lyon, France.
Kangas, B.A. (1994) A 13-week dietary toxicity study of dodecylguanidine acetate (dodine) in the albino mouse, study No. 84582. Unpublished report prepared by Bio-research Laboratories Ltd, Senneville, Quebec, Canada. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Kenny, T.J., (1999) Dodine technical acute (four-hour) inhalation study in rats, study RPN 605/992051. Unpublished report prepared by Huntingdon Life Sciences Ltd, Huntingdon United Kingdom. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Kern, T.G. (1999a) Acute oral toxicity study of dodine technical material in albino rats, study No. WIL-21130. Unpublished report prepared by WIL Research Laboratories, Ashland, Ohio, USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Kern, T.G. (1999b) Acute dermal toxicity study of dodine technical material in albino rats, study No. WIL-21131. Unpublished report prepared by WIL Research Laboratories, Ashland, Ohio, USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Kern, T.G. (1999c) Acute dermal irritation study of dodine technical material in albino rats, study No. WIL-21132. Unpublished report prepared by WIL Research Laboratories, Ashland, Ohio, USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Kern T.G. (1999d) Acute eye irritation study of dodine technical material in albino rats, study No. WIL-21134. Unpublished report prepared by WIL Research Laboratories, Ashland, Ohio, USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Kern, T.G. (1999e) A 28-day dermal toxicity study of dodine technical material in rats, study No. WIL-21140. Unpublished report prepared by WIL Research Laboratories, Ashland, Ohio, USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Kossmann, S., Konieczny, B. & Hoffmann, A. (1997) The role of respiratory muscles in the impairment of the respiratory system function in the workers of a chemical plant division producing pesticides. Przegl. Lek., 54, 702706.
Kossmann, S., Pierzchala, W. & Hefczyc, J. (1999) Bronchial hyperreactivity in chemical plant workers employed in the production of dust pesticides. Wiad. Lek., 52, 2529.
Levinskas, G.J., Vidone, L.B., OGrady, J.J. & Shaffer, C.B. (1961) Acute and chronic toxicity of dodine (n-dodecylguanidine acetate). Toxicol. Appl. Pharmacol., 3, 127142.
Lina, B.A.R., Til, H.P., Falke, H.E., Beems, R.B. & Hollander, V.M.H. (1984) Sub-chronic (90-day) oral toxicity study with dodine in rats, study No. TNO V 83.130/220623 Unpublished report prepared by TNO. Submitted to WHO by Rhône-Poulenc Agro, Lyon, France
Manciaux, X. (1999) Dodine: Skin sensitization in guinea-pig, study No. 17473 TSG. Unpublished report prepared by Centre International de Toxicologie, Miserey, France. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Mulhern, M., Perry, C.J. & Snodgrass, E. (1988) Dodine: 8 week dietary dose range finding study in mice, IRI project 436893. Unpublished report No. 5411 prepared by Inveresk Research International Ltd, Scotland. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Murli, H. (1992) Mutagenicity test on dodecylguanidine acetate technical, in vivo mammalian micronucleus assay, study No. HWA 14710-0-455. Unpublished report prepared by Hazleton USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Reddy, V., Litle L. & Murrill, E. (1992) Disposition and metabolism of 14C-labeled dodine in rats (preliminary and definitive study), MRI project No.. 9938-F. Unpublished report prepared by Midwest Research Institute, Kansas City, Missouri, USA. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Smith, S.Y. (1994) An oral capsule range-finding study of dodecylguanidine acetate (dodine) in the beagle dog, study No. 84796. Unpublished report prepared by Bio-research Laboratories Ltd, Senneville, Quebec, Canada. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Trutter, J.A. (1996) Dodine: 52-week toxicity study in dogs, study CHV 656-192. Unpublished report prepared by Corning Hazleton USA. Submitted to WHO by Rhône-Poulenc Agro, Lyon, France.
WHO (1999) Recommended classification of pesticides by hazard and guidelines to classification 19981999 (WHO/PCS/98.21/Rev.1), Geneva, International Programme on Chemical Safety (www.who.int/pcs/pcs-act.htm).
Willems, M.I. (1981) Evaluation of dodine tech. 95% for mutagenic activity in the Ames test, study TNO No. V 81.102/210064-7. Unpublished report prepared by TNO. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France
Willems, M.I. (1985) Examination of dodine in the micronucleus test, study TNO No. V 85.198/ 250208. Unpublished report prepared by TNO. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
Williams, K.D. (1998) 78-week dietary oncogenicity study with dodine in mice, study No. 6224-220. Unpublished report prepared by Corning Hazleton USA. Submitted to WHO by Rhône-Poulenc Agro, Lyon, France.
Wilmer, J.W.G.M. (1985) Chromosome analysis of cultured human lymphocytes treated in vitro with dodine, study TNO No. V 85.164/250209. Unpublished report prepared by TNO. Submitted to WHO by Rhône-Poulenc Agrochimie, Lyon, France.
See Also: Toxicological Abbreviations Dodine (WHO Pesticide Residues Series 4) Dodine (Pesticide residues in food: 1976 evaluations) Dodine (Pesticide residues in food: 1977 evaluations)