
Pesticide residues in food - 2002 - Joint FAO/WHO Meeting on Pesticide Residues
First draft prepared by
G. Wolterink and P.H. van Hoeven-Arentzen
Centre for Substances and Risk Assessment, National Institute of Public Health and the Environment, Bilthoven, Netherlands
Oxamyl [N,N-dimethyl-2-methylcarbamoyloxyimino-2-(methylthio)acetamide] is a carbamate insecticide that acts by inhibiting acetylcholinesterase activity. It was evaluated by the JMPR in 1980, 1983, 1984 and 1985 (Annex 1, references 34, 40, 42 and 44). An ADI of 00.03 mg/kg bw was established in 1984 on the basis of a NOAEL of 2.5 mg/kg bw per day in a 2-year feeding study in rats and a NOAEL of 2.5 mg/kg bw per day in a 2-year feeding study in dogs. Oxamyl was evaluated by the present Meeting within the periodic review programme of the Codex Committee on Pesticide Residues. The Meeting reviewed new data on oxamyl-induced neurotoxicity and inhibition of cholinesterase activity in brain, erythrocytes and plasma, which had been reported since the previous evaluations, and relevant data from previous evaluations.
Mice
Four groups of five male Swiss-Webster mice were given an intraperitoneal injection of [14C]oxamyl (purity, > 97%; position of radiolabel not indicated) in saline at a dose of 1.2 mg/kg bw. Urine and faeces were collected from groups of five mice 6, 12, 24, 48, 72 and 96 h after treatment. Excretion was very rapid: by 6 h, 73% of the administered dose had been excreted in urine and 3% in faeces; urinary and faecal excretion were 84% and 5% by 24 h and 89% and 8% after 96 h, respectively. By 96 h, the concentrations of radiolabel (as oxamyl equivalents) in tissues were low, ranging from 11 ng/g in testes to 37 ng/g in liver. Only a summary of the results of this study was provided, and the original data were not available (Chang & Knowles, 1979).
Rats
The kinetics and metabolism of oxamyl were studied in CD rats, according to FIFRA guideline 85-1. In a pilot experiment, one male and one female received [1-14C]oxamyl (purity, 98%) in 0.1 mol/l sodium acetate containing 1% carboxymethylcellulose, at a single oral dose of 1 mg/kg bw. Urine was collected at 06 h, 624 h and daily thereafter up to 168 h. Faeces were collected daily. Excretion of 14CO2 in expired air was assessed over 168 h. At that time, the rats were killed, and the radioactivity in the carcass was measured. Statements of adherence to good laboratory practice (GLP) and quality assurance (QA) were included.
About 84% and 1% of the administered radioactivity were excreted within 24 h in urine and faeces, respectively. After 168 h, a total of 93% (males) and 95% (females) had been excreted in urine, 2% in faeces and 0.40.5% in expired air; 4% of the administered radioactivity remained in the carcass.
In the main study, five male and five female rats received [1-14C]oxamyl (purity, 96%) at a single oral dose of 1 mg/kg bw, and urine and faeces were collected at the same intervals as in the pilot study. The concentration of radioactivity in expired air was not measured. At 168 h, the animals were killed, blood was sampled, and heart, lungs, liver, kidneys, spleen, gastrointestinal tract, brain, ovaries/testes, skin, muscle, fat and bone were dissected. The concentrations of radioactivity in blood, tissues and remaining carcass were measured. Approximately 80% of the administered radioactivity was excreted in urine and 1% in faeces within 24 h. After 168 h, the total urinary excretion was 95%, and faecal excretion was 3%. At sacrifice, the highest concentrations of radioactivity were found in whole blood (0.080.1 ΅g/g, expressed as oxamyl equivalents). The concentrations in heart, liver, kidney, lungs, spleen and gastrointestinal tract ranged from 0.04 to 0.09 ΅g/g. The concentrations in other tissues were < 0.03 ΅g/g. No marked differences in tissue concentrations were found between male and female rats.
In a study with whole-body autoradiography, one male and one female rat received [14C]oxamyl (purity, 96%) at a single oral dose of 1 mg/kg bw. After 168 h, the animals were killed, and sagital sections were cut at six levels. The autoradiograms of both male and female rats showed the highest concentrations of radioactivity in gastric mucosa, stomach content, kidneys, ureter, blood, hair follicles, liver and lung. The authors considered that the radioactivity on the fur was the result of urinary contamination, and the Meeting endorsed that view.
Male rats received a single oral dose of 2 (two animals) or 100 mg/kg bw (10 animals) of methyl 2-(dimethylamino)-N-hydroxy-2-oxo[1-14C]ethanimidothioate (14C-DMTO; purity, 97%), which is one of the main metabolites of oxamyl. Urine was collected at 8, 24 and 48 h, and faeces were collected at 24 and 48 h. Within 48 h, 100102% of the dose was excreted in urine, with 93100% within 24 h; 23% was excreted in faeces (Hawkins et al., 1990).
(a) In vitro
Mice
After incubation for 2 h of [14C]oxamyl with mouse liver homogenate or subcellular liver fractions, most of the radioactivity represented parent compound. Minor concentrations of DMTO, dimethylamino(oxo)acetic acid (DMOA), N,N-dimethyl-2-nitriloacetamide (DMCF), N-methyloxamic acid and methyl N-methyl-N-[(methylcarbamoyl)oxy]-1-thiooxamimidate were detected. Degradation of oxamyl was found in nuclear, mitochondrial and microsomal plus soluble liver fractions. No individual data were presented (Chang & Knowles, 1979).
Rats
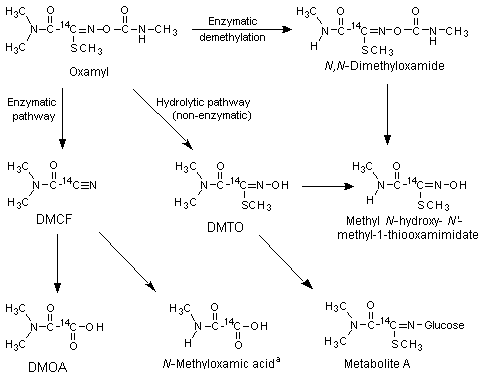
Freshly prepared ChR-CD rat (sex not specified) liver microsomes were incubated with 0.3, 1 or 2 mg of [14C]oxamyl or with 0.3, 1 or 5 mg of [14C]-labelled DMCF, DMTO or its glucose conjugate metabolite A (Figure 1) for 2 h at 37 ΊC, to determine the metabolic pathway of oxamyl in vitro. Aliquots of the incubations were extracted with ethyl acetate. The ethyl acetate and aqueous phases were radioassayed by liquid scintillation counting, and metabolites were identified by thin-layer chromatography, high-performance liquid chromatography and gas chromatography with mass spectrometry. After incubation of 1 or 2 mg of oxamyl with rat liver microsomal fractions, the parent compound was the major fraction found (5960%) followed by DMCF (1719%), DMTO (1013%) and DMOA (7%). Small amounts of methyl N-methyl-N-[(methylcarbamoyl)oxy]-1-thiooxamimidate (about 4%) and methyl N-hydroxy-N-methyl-1-thiooxamimidate (about 1%) were also found. A control incubation without microsomes produced only DMTO, at concentrations comparable to those found after incubation in the presence of liver microsomes, suggesting that the hydrolysis was not mediated by liver enzymes. About 0.3 mg of [14C]oxamyl was degraded to a slightly greater extent, with the same metabolite pattern but with a slightly larger fraction of DMCF. Incubation with 0.35 mg of DMCF resulted in the formation of DMOA (520%, depending on the amount incubated). DMTO was not metabolized in vitro, and metabolite A to only a limited extent (< 10%). The results of these experiments in vitro and those of the experiments in vivo (see below) are reflected in the metabolism scheme shown in Figure 1 (Harvey & Han, 1978).

Figure 1. Metabolic pathways of oxamyl in rats and mice
DMTO, methyl 2-(dimethylamino)-N-hydroxy-2-oxo-ethanimidothioate;
DMCF, N,N-dimethyl-2-nitriloacetamide; DMOA, dimethylamino(oxo)acetic acid;
Metabolite A, glucose conjugate of DMTO
a In rats, found only in vivo
(b) In vivo
Mice
Four groups of five male Swiss-Webster mice received an intraperitoneal injection of [14C]oxamyl (purity, > 97%; position of radiolabel not indicated) in saline at a dose of 1.2 mg/kg bw. Urine samples were collected from groups of five mice 6, 12, 24 , 48, 72 and 96 h after treatment. The urine samples were extracted with ethyl acetate to obtain an aqueous phase and compounds soluble in organic solvents. After ethyl acetate extraction, most of the radiolabel remained in the aqueous phase (ranging from 75% at 6 h to 92% at 96 h). The metabolites in the aqueous fraction were not identified, although about 7% of the radioactivity appeared to represent glucuronidated and sulfated metabolites. The concentration of radiolabel in the fraction soluble in organic solvents decreased from 25% at 6 h to 8% at 96 h. In this fraction, 16% of the radioactivity was associated with parent compound. Five metabolites were identified in this phase by thin-layer chromatography (for structures, see Figure 1). The main metabolite was DMTO (44%), and others were DMOA, DMCF, methyl N-hydroxy-N-methyl-1-thiooxamimidate, N-methyloxamic acid and several unknowns (percentages not indicated) (Chang & Knowles, 1979).
Rats
Experiments were conducted in ChR-CD rats to determine the chemobiokinetics of oxamyl in vivo (see Table 1). Excretion of oxamyl was investigated in glass metabolism cages. Urine, faeces and exhaled air were analysed for radiolabel by liquid scintillation counting, and metabolites were identified by thin-layer chromatography and gas chromatography with mass spectrometry, with or without extraction with ethyl acetate or methanol (faeces were extracted with distilled water before analysis). At sacrifice, organs and tissues were isolated, freeze-dried and combusted before quantification of radioactivity. The tissue samples were also analysed by combined high-performance liquid chromatography and gas chromatography with mass spectrometry (with or without acid hydrolysis or enzymatic degradation).
Table 1. Design of studies of the metabolism of [14C]oxamyl, [14C]metabolite A and [14C]N,N-dimethyl-2-nitriloacetamide (DMCF) in rats in vivo
|
Pretreatment |
Dose in mg/kg bw (purity, specific activity), vehicle, mode of administration |
|
50 mg/kg unlabelled oxamyl in food for 32 days |
2.5 mg/kg bw [14C]oxamyl (purity not given; 3.7 ΅Ci) in peanut oil, by gavage |
|
150 mg/kg unlabelled oxamyl in food for 18 days |
4.6 mg/kg bw [14C]oxamyl (purity not given; 5.6 ΅Ci) in peanut oil, by gavage |
|
1540 mg/l unlabelled metabolite A in drinking-water for 8 days |
3.6 mg/kg bw [14C]metabolite A (purity, > 99%; 3.8 ΅Ci) in water, by gavage |
|
450 mg/kg unlabelled DMCF in food for 7 days |
3.9 mg/kg bw [14C]DMCF (purity, > 99%; 11 ΅Ci) in water, by gavage |
From Harvey & Han (1978)
One male rat per treatment (sex of that given 150 mg/kg not stated but presumed to be male); single doses; urine,
exhaled air and faeces collected at 024, 2448 and 4872 h; sacrifice at 72 h
The experiments showed similar biokinetics, regardless of which 14C component was administered. Rats given diets containing 50 mg/kg of oxamyl, metabolite A or DMCF excreted about 60% of the administered radioactivity in urine and 56% in faeces, whereas the rat given the diet containing 150 mg/kg of oxamyl excreted 50% in urine and 20% in faeces. Of the radioactivity in urine and faeces of oxamyl-treated rats, 7080% was recovered as polar conjugates (not further identified) of DMTO, methyl N-hydroxy-N-methyl-1-thiooxamimidate, DMOA and N-methyloxamic acid. Of the radioactivity in the urine of the rat treated with metabolite A, 45% was accounted for by metabolite A itself, and 19% appeared to be conjugates of DMTO and methyl N-hydroxy-N-methyl-1-thiooxamimidate; the remaining radioactivity was associated with highly polar water-soluble conjugates, most of which were conjugates of DMOA and N-methyloxamic acid. In the urine of the DMCF-treated rat, 15% of the radioactivity was attributable to conjugates of DMOA and 7% to N-methyloxamic acid. In the rats given oxamyl, about 20% of the administered radioactivity was recovered from tissues, mainly in skin, hair, carcass, gastrointestinal tract, liver and blood. No non-polar metabolites of oxamyl, oxamyl itself or conjugates were found in the tissues of oxamyl-treated rats. About half of the radioactivity found in the skin, hair, blood and liver was incorporated into amino acids (Harvey & Han, 1978).
Only a summary of the results of this study was published, and the original data were not available. The purity of the unlabelled oxamyl, metabolite A and DMCF was not reported. Moreover, all results were obtained in single rats, and none of the data were obtained in naive rats. Because of these limitations, the results of this study were considered to be only qualitatively useful.
Five male and five female CD rats received [1-14C]oxamyl (purity, 96%) at a single oral dose of 1 mg/kg bw. Metabolites were analysed in urine collected over 024 h and 2448 h. An additional 10 male rats received DMTO (oxime), one of the main metabolites of oxamyl, at a dose of 100 mg/kg bw. Metabolites in urine were identified by thin-layer chromatography, high-performance liquid chromtography and mass spectrometry. The main urinary metabolite of oxamyl, representing about 35% of the administered dose in urine, was a glucuronide of DMTO. Oxamyl and DMTO represented 9% and 16% of the administered dose, respectively. Three other metabolites in urine, representing about 20% of the administered radioactivity, could not be identified. After administration of DMTO, a similar metabolite profile in urine was observed. No marked sex differences were observed in the relative proportions of metabolites formed (Hawkins et al., 1990).
Studies of biotransformation in vitro and in vivo thus showed that oxamyl is metabolized in rats and mice via two major pathways, hydrolysis to the oxime (DMTO) and enzymatic conversion to DMOA (see Figure 1).
The acute toxicity of oxamyl is summarized in Table 2.
Table 2. Acute toxicity of oxamyl
|
Species |
Strain |
Sex |
Route |
Purity (%) |
LD50/LC50 (mg/kg bw; mg/l) |
Reference |
|
Rat |
ChR-CD |
Male, female |
Oral |
97.1 |
2.5 |
Hinkle (1980) |
|
Rat |
Crl:CD |
Male, female |
Inhalation (4 h)a |
98 |
0.050.065 |
Kelly (2001) |
|
Rat |
ChR-CD |
Male |
Inhalation (4 h)b |
95 |
0.064 |
Tayfun (1969a) |
|
Rat |
ChR-CD |
Male, female |
Inhalation (1 h)b |
95 |
0.12 |
Tayfun (1969b) |
|
Rabbit |
New Zealand white |
Male, female |
Dermal |
97.1 |
> 2000 |
Brock (1988a) |
a Median mass aerodynamic diameter, 3.24.2 ΅m
b Median mass aerodynamic diameter, 3.5 ΅m
In a study performed according to guideline 40 CFR 163.81-1 of the Environmental Protection Agency (USA), oxamyl was administered by gavage to ChR-CD rats at doses of 15 mg/kg bw. Deaths occurred at doses >2.0 mg/kg bw in males and 2.4 mg/kg bw in females. The signs of toxicity included tremors, fasciculation, exophthalmus, salivation, chromo-dacryorrhoea, piloerection, lachrymation and body-weight loss (Hinckle, 1980).
Groups of five Crl:CD rats of each sex were exposed nose-only for 4 h to oxamyl at nominal concentrations of 0.050.12 mg/l. Deaths occurred at all concentrations. The clinical signs during exposure were a diminished response to an alerting stimulus, red nasal discharge, gasping and salivation. During the first week after exposure, lethargy, decreased muscle tone, tremors, spasms, fasciculation, abnormal posture and gait, abnormal hind-limb gait, high carriage, ataxia and (reversible) body-weight loss were observed. The study was performed according to guidelines OPPTS 870.1300 and OECD 403. Statements of adherence to GLP and QA were included (Kelly, 2001).
Groups of six male rats were exposed for 4 h head-only to oxamyl at actual concentrations of 0.020.09 mg/l. Deaths occurred at concentrations > 0.053 mg/l. The clinical signs during exposure were intense salivation, facial fasciculation, exophthalmos, lachrymation, red nasal discharge and difficulty in breathing. After exposure, minor body-weight loss and occasionally dilated pupils were observed (Tayfun, 1969a).
Groups of six rats of each sex were exposed for 1 h head-only to oxamyl at actual concentrations of 0.10.21 mg/l. Males died at concentrations > 0.16 mg/l, and females died at all doses. The clinical signs during exposure were facial fasciculation, exophthalmos, lachrymation, red discharge around the nose and eyes, salivation and gasping. Pallor and severe body-weight loss were observed on the first day after exposure (Tayfun, 1969b).
In a study performed according to Environmental Protection Agency guideline 81-2, rabbits received dermal applications of oxamyl at 2000, 3500 or 5000 mg/kg bw. The application site was occluded for 24 h. Two of five males at 3500 mg/kg bw, two of five males at 5000 mg/kg bw, one of five females at 2000 mg/kg bw and one of five females at 3500 mg/kg bw died. The clinical signs were slight (reversible) body-weight loss, erythema and oedema. The gross pathological findings in dead animals were consistent with inhibition of cholinesterase activity. Statements of adherence to GLP and QA were included (Brock, 1988a).
In a study of primary ocular irritation performed according to guidelines OPPTS 870.2400 and OECD 405, six adult male HM:(NZW)fBR New Zealand white rabbits received an instillation of 24 mg of technical-grade oxamyl (purity, 98%) into the conjunctival sac of the right eye. The eyes were examined at 1, 24, 48 and 72 h and scored for irritation according to the method of Draize (1977). Statements of adherence to GLP and QA were included. Slight irritation (score 1 for iritis in five animals, score 1 or 2 for conjunctival redness in four animals and score 1, 2 or 3 for discharge) was observed on the day of treatment. In all treated eyes, the pupil was constricted and did not react to light. Other clinical signs observed on the day of treatment were shivering, salivation, effects on balance, rapid and irregular breathing and lung noise. After 24 h, the treated eyes of five of the six animals were normal. By 48 h, the eyes of all animals were normal. The compound was considered not to be irritating to the eyes (Ladics, 2001a).
In a study of primary dermal irritation performed according to guidelines OPPTS 870.2500 and OECD 404, the shaved skin of six male New Zealand white rabbits was exposed to 0.5 g of technical-grade oxamyl (purity, 98 %), moistened with water and kept under semi-occlusion for 4 h. The skin was scored for dermal effects according to the method of Draize (1977) 1, 24, 48 and 72 h after exposure. Statements of adherence to GLP and QA were included. Erythema of score 1 was observed in one rabbit 1 and 24 h after exposure. No clinical signs were observed in any animal. Oxamyl was considered not to be irritating to the skin (Ladics, 2001b).
When oxamyl (purity, 96.9%) was administered to guinea-pigs according to the Magnusson-Kligman maximization and Buehler methods, all animals died after intradermal injection, while animals treated topically either died or had significant clinical signs of cholinesterase inhibition. The study was discontinued for humane reasons. The animals survived only when oxamyl was applied at one-half the maximum dose rate (0.5 ml of a 50% dilution in water) according to the Buehler method, although clinical signs of cholinesterase inhibition were still observed (Du Pont, 2002).
On the basis of these results, 0.4 ml of oxamyl (purity, 42%) was tested for skin sensitization in a standard Buehler assay, performed according to Environmental Protection Agency guideline 81-6, in groups of 10 male and 10 female Duncan Hartley guinea-pigs. Five animals of each sex served as vehicle controls, and dinitrochlorobenzene was used as a positive control in three males and two females. Under the conditions of the test, oxamyl did not induce skin sensitization. Statements of adherence to GLP and QA were included (Brock, 1988b).
(a) Oral administration
Rats
In a limited 10-day study of toxicity, six young adult male ChR-CD rats received oxamyl in 1% acetone and 99% peanut oil by gavage at a dose of 2.4 mg/kg bw per day, 5 days/week for 2 weeks. Three rats were killed 4 h after the last dose, and the other three were killed after a 14-day recovery period. Oxamyl caused body-weight loss during the first 2 days of treatment and on day 1 of the second week. The clinical signs were fasciculation, slight pallor and salivation. Mild inflammation of the stomach was observed in all animals at both sacrifices and also in control animals, but of lesser severity (Fretz & Sherman, 1968). The report provided only a summary of the observed effects, and no group or individual data were provided.
Groups of 16 Sprague-Dawley rats of each sex received diets containing oxamyl at a concentration of 0, 50, 100 or 150 ppm, equivalent to 0, 2.5, 5 and 7.5 mg/kg bw per day, for 9195 days. Rats at 150 ppm initially received a diet containing 500 ppm oxamyl for 4 days followed by 3 days of control diet. Blood samples were taken before the start of the study, after 1 and 2 months of treatment and at the end of the study for examination of a limited number of haematological and blood chemical parameters. After 9195 days of feeding, 10 males and 10 females at each dose were killed for pathological examination.
The animals given 500 ppm had fasciculation after 2 days, and by day 4 showed fasciculation, ruffled fur, mild diarrhoea, bulging eyes, lachrymation, food spillage and body-weight loss, and the dose was lowered to 150 ppm. One male rat at 500 ppm was found dead on day 5. Rats at 100 and 150 ppm had reduced body weight and body-weight gain. Food consumption was increased at 50 ppm and decreased at 150 ppm. Analysis of urine from rats at 150 ppm showed increased proteinuria and occult blood. Females at 100 ppm showed decreased absolute kidney, liver and lung weights, and males at 100 and 150 ppm had decreased absolute weights of kidney, heart, thymus and spleen. Males and females at 150 ppm had decreased liver weights, and the females also had an increased stomach weight. No toxicologically relevant effects were observed at 50 ppm, equivalent to 2.5 mg/kg bw per day (Snee, 1969). Because a number of results, such as relative organ weights, blood chemical and histopathological data (including cholinesterase activity), were omitted, a NOAEL could not be identified.
Dogs
Groups of four beagle dogs of each sex were fed diets containing oxamyl (purity unknown, assumed to be 100%) at a concentration of 50, 100 or 150 ppm, equal to 1.5, 2.8 and 5 mg/kg bw per day for males and 1.3, 2.6 and 4.2 mg/kg bw per day for females, for 13 weeks. Clinical observations were made daily, body weight and food consumption were measured weekly, and haematology, clinical chemistry and urine analysis were performed at initiation and at weeks 4 and 13. Cholinesterase activity was not measured. At the end of the study, the animals were killed and examined grossly and histopathologically. No treatment-related effects were observed. The NOAEL was 150 ppm, equal to 4.2 mg/kg bw per day (Holsing, 1969).
Groups of four beagle dogs of each sex received diets containing oxamyl (purity, 95%) at a concentration of 0, 50, 100 or 150 ppm, equivalent to 0, 1.3, 2.5 and 3.8 mg/kg bw per day, for 2 years. Body weight and food consumption were measured weekly, clinical signs were assessed daily, and haematological, clinical chemical and urine analyses were performed before and 1, 2, 3, 6, 9, 12, 15, 18, 21 and 24 months after the start of treatment. Cholinesterase and aliesterase activities were assessed in whole blood before and 1, 6, 12 and 24 months after the start of treatment. After 1 year of treatment, one dog of each sex in the control group and that at 150 ppm were killed for histopathological examination. The remaining animals were killed after 2 years. All animals were examined grossly. A complete range of organs and tissues from animals of the control and 150-ppm groups and the liver, kidneys and testes of animals at 50 and 100 ppm were examined microscopically.
No treatment-related effects on body weight, food consumption, clinical signs or cholinesterase or aliesterase activity were observed. Haemoglobin concentrations, erythrocyte volume fraction and erythrocyte count were reduced throughout treatment at most times in animals at 150 ppm. Increased cholesterol concentration and increased alkaline phosphatase activity were found in animals at 150 ppm, particularly in males. Increased cholesterol concentrations were also found in dogs at 50 and 100 ppm. Occasional differences in other haematological and biochemical parameters were observed. Differences from background values were seen in some haematological and urine chemistry parameters, but the significance of the differences could not be established as no statistical analysis was reported. The time between feeding and blood sampling for determination of cholinesterase and aliesterase activity was not indicated. The data for the control and 150-ppm groups after 1 year of treatment were based on only three animals per group. (Sherman, 1972). Although the JMPR in 1984 based the ADI on the results of this study, the present Meeting concluded that a NOAEL could not be identified.
In a study performed according to Environmental Protection Agency guideline 83-1, groups of five beagle dogs of each sex received diets containing oxamyl (purity, 97.1%) at a concentration of 0, 50, 150 or 250 ppm, equal to 1.6, 4.6 and 8 mg/kg bw per day for males and 1.5, 4.5 and 7.8 mg/kg bw per day for females, for 382386 days. The doses were chosen on the basis of two range-finding tests. Blood samples for haematological and blood chemical examination were taken before the experiment and on days 81, 181, 264 and 356 of treatment. For determination of cholinesterase activity in blood, samples were taken on days 7, 34, 88, 187, 270 and 363, 3 h after feeding. The dogs were killed on days 382386. Statements of adherence to GLP and QA were included.
One female at 250 ppm died on day 348; the cause of death could not be established. Body weight, body-weight gain and food use efficiency were dose-dependently decreased in males in all treated groups and in females at 150 and 250 ppm. Food consumption was decreased in males and females at 150 and 250 ppm. No effects on haematological or blood chemical end-points were observed. Increased relative brain and kidney weights were found in males at 250 ppm. Animals at 150 and 250 ppm showed tremors, salivation and increased incidences of vomiting, diarrhoea and mucoidal stool. Tremors were also observed in females at 50 ppm. The effects on cholinesterase activity are summarized in Table 3. Erythrocyte cholinesterase activity did not correlate well with plasma cholinesterase activity. A statistically significant decrease in erythrocyte cholinesterase activity was found in males at 250 ppm only after 6 months of treatment. The authors considered that all the decreases in cholinesterase activity in the brain in treated males and in the caudate nucleus in females at 150 and 250 ppm were biologically significant. As tremors occurred in females at 50 ppm and decreased body weight and decreased cholinesterase activity in the brain were found in males at 50 ppm, a NOAEL could not be identified. The LOAEL was 50 ppm, equal to 1.5 mg/kg bw per day (Mebus, 1990).
Table 3. Effects of dietary administration of oxamyl on cholinesterase activity in dogs
|
Substrate |
Cholinesterase activity (% change) |
|||||
|
50 ppm |
150 ppm |
250 ppm |
||||
|
Male |
Female |
Male |
Female |
Male |
Female |
|
|
Plasma (terminal) |
32* |
8 |
48* |
37 |
58* |
45* |
|
Erythrocyte (terminal) |
4 |
22 |
3 |
1 |
6 |
2 |
|
Brain |
||||||
|
Caudate nucleus |
31* |
0 |
47* |
24 |
30* |
30 |
|
Cerebellum/medulla |
17 |
3 |
38* |
22 |
28 |
14 |
|
Cerebrum |
23 |
10 |
46 |
16 |
34 |
15 |
From Mebus (1990)
* Significantly different from control
In a study performed according to Environmental Protection Agency guideline 83-1, groups of five male beagle dogs received diets containing oxamyl (purity, 97.1%) at a concentration of 0, 12.5, 20, 35 or 50 ppm, equal to 0, 0.37, 0.58, 0.93 and 1.4 mg/kg bw per day, for 52 weeks. The stated purpose of the study was to determine the NOAEL for inhibition of cholinesterase activity. Animals were observed twice daily for deaths, moribundity and clinical signs. Body weights and food consumption were measured weekly for the first 15 weeks and every fourth week thereafter. Ophthalmic examinations were performed before the start of treatment and at weeks 26, 39 and 53. Blood samples were taken for haematological and blood chemical examination, including erythrocyte and plasma cholinesterase activity, before the start of treatment, at weeks 13, 26 and 39 of the study and at termination. At termination, organs were weighed and examined macroscopically, and brain tissue was dissected for determination of cholinesterase activity. Statements of adherence to GLP and QA were included.
No deaths occurred, and no treatment-related effects were observed on body weight, food consumption, clinical signs, haematological, blood chemical or ophthalmic end-points, organ weights or macroscopic appearance or cholinesterase activity in brain (caudate nucleus, cerebellum/medulla, cerebrum) or plasma. In view of the lack of effects, the authors considered the NOAEL to be 50 ppm, equal to 1.4 mg/kg bw per day (Dickrell, 1991).
In dogs at the highest dose, erythrocyte cholinesterase activity was decreased by 21% 2 weeks before treatment, by 30% in week 13 and by 22% in week 53 of treatment. The toxicological relevance of the decreases during treatment was therefore unclear, and it was difficult to determine whether 50 ppm was the NOAEL or the LOAEL. The Meeting combined the results for the two 1-year studies and identified an overall NOAEL in dogs of 35 ppm, equal to 0.93 mg/kg bw per day.
(b) Dermal administration
Rabbits
In a study performed according to Environmental Protection Agency guideline 82-2, groups of five male and five female New Zealand white rabbits (10 males and 10 females in the control and highest-dose groups) received dermal applications of oxamyl (purity, 97.2%) in 5 ml of distilled water at a dose of 0, 2.5, 50 or 250 mg/kg bw per day on the clipped skin of the back; the sites were maintained under an occlusive wrapping for 22 days. The doses were based on the results of a range-finding study. Oxamyl was removed 6 h after application each day by washing the treated skin with warm water, and the application site was observed for dermal irritation and scored according to Draize (1977). The animals were observed for clinical signs or signs of dermal toxicity each morning. After 22 days, five male and five female animals per group were killed. The remaining rabbits in the control and highest-dose groups were allowed to recover for 14 days before sacrifice. Blood was sampled for haematology, clinical chemistry and cholinesterase determinations before the start of the study, 1 h after the end of treatment on day 22 and on day 36 (controls and animals at the highest dose only). Brain cholinesterase activity was determined on days 22 and 36. Statements of adherence to GLP and QA were included.
Three bucks at the highest dose died during the study; although the causes of death could not be established, they were considered not to be treatment-related. On day 22, a dose-related decrease in cholinesterase activity was found in plasma, erythrocyte and brain in animals at 50 and 250 mg/kg bw per day. There were no significant sex differences in cholinesterase activity. The decreases in cholinesterase activity in males and females at 50 mg/kg bw per day were 51% and 45% in plasma, 18% and 15% in erythrocyte and 31% and 25% in brain, respectively. In male and female rabbits at 250 mg/kg bw per day, the decreases in cholinesterase activity were 73% and 75% in plasma, 36% and 30% in erythrocytes and 63% and 66% in brain, respectively. After the recovery period, no significant decrease in cholinesterase activity was observed in animals at 250 mg/kg bw per day. An unidentified eosinophilic substance was found in the duodenal submucosa of five animals at 250 mg/kg bw per day. No treatment-related effects on body weight, food intake or clinical signs were observed. Occasional statistically significant effects on dermal irritation, haematology, clinical chemistry, organ weights, macroscopic and microscopic appearance were considered not to be toxicologically relevant. The Meeting endorsed that view. The NOAEL was 2.5 mg/kg bw per day, on the basis of decreased brain cholinesterase activity at 50 mg/kg bw per day (Brock, 1988c).
In a study of dermal toxicity performed according to guidelines OECD 410 and Environmental Protection Agency 82-2, oxamyl (purity, 96.9%) in deionized water was applied under a semi-occlusive dressing for 6 h/day for 21 consecutive days at a dose of 0, 25, 40, 50 or 75 mg/kg bw per day to the shaved intact skin of groups of six HM:(NZW)fBR rabbits of each sex. The animals were checked daily for clinical signs and skin irritation, twice a week for body weight and weekly for food consumption. Blood was sampled before treatment and 1 h after the end of the last exposure for measurement of plasma and erythrocyte cholinesterase activity. After blood sampling on day 21, the animals were killed and subjected to gross pathological examination. The brains were collected for assessment of cholinesterase activity. Statements of adherence to GLP and QA were included.
No compound-related effects on body weight, body-weight gain, food consumption or clinical signs were observed. Females at 75 mg/kg bw per day showed decreases in cholinesterase activity of 11% in brain, 24% in erythrocytes and 29% in plasma. The decrease in cholinesterase activity in erythrocytes did not reach statistical significance. In males, the decreases in cholinesterase activity were < 20% and did not reach statistical significance. In the absence of clinical signs and in view of the minor decreases in brain cholinesterase activity, the NOAEL was 75 mg/kg bw per day, the highest dose tested (Malley, 1999)
Mice
In a 2-year study of toxicity and carcinogenicity, groups of 80 CD-1 mice of each sex, aged 4.5 weeks, received diets containing oxamyl (purity, 97.1%) at a concentration of 0, 25, 50 or 75 ppm, equivalent to 0, 3.8, 7.5 and 11 mg/kg bw per day. The animals at the highest dose received a diet containing 100 ppm of oxamyl for the first 6 weeks, but because 13 males and 11 females died during this period the concentration was decreased to 75 ppm. Eight extra males and eight females from the same shipment were added to the group receiving the highest dietary concentration, one male and four females were added to the group receiving 50 ppm, and one female was added to the group receiving 25 ppm to replace animals found dead. The animals were observed twice daily for deaths, signs of toxicity and behavioural changes and palpated weekly for masses. Individual body weights and food consumption were assessed in weeks 129, every other week in weeks 2953 and monthly thereafter. Blood for haematological analysis was sampled from the orbital sinus at weeks 4, 13, 26, 52, 78 and 104 from 10 animals of each sex per dose that were randomly selected at each time. Surviving animals were killed by asphyxiation with CO2 and necropsied. Liver, kidney, testis, brain, brain stem and heart were weighed. Specimens of a full range of tissues were collected for microscopic examination. Cholinesterase activity was not determined. Statements of adherence to GLP and QA were included.
During the first 6 weeks, the mortality rates were high among males at 100 ppm and females at 50 (9/80) and 100 ppm. After reduction of the highest dose to 75 ppm, long-term survival was not changed by treatment. Males at 50 ppm had reduced body weight throughout the study; males at 75 ppm had reduced body weight during the first 28 weeks of the study and incidental decreases in body weight from week 29. Males at 50 and 75 ppm had consistently reduced food consumption throughout the study. Those at 25 ppm group showed sporadic reductions in food consumption during the first 37 weeks but a consistent reduction for the remainder of the study. Females at 50 and 75 ppm showed significant but sporadic decreases in body weight throughout the study, and significant reductions in food consumption were found sporadically in females at 25, 50 and 75 ppm, with no clear dose-related pattern.
There were no dose-related changes in haematological parameters, although significant decreases in erythrocyte count, haemoglobin concentration and erythrocyte volume fraction were observed at 4 weeks in males at the highest dietary concentration, which was 100 ppm oxamyl at that time. These parameters were not similarly affected at other times, when the animals had a lower dietary intake. No unusual cell types were seen in peripheral blood. Other significant differences were observed sporadically, but as they were not dose-dependent they were considered not to be treatment-related. At termination, the absolute weight of the liver was decreased in males at 50 ppm and the relative kidney weight was slightly increased in males at 75 ppm. Oxamyl was not carcinogenic in this mouse strain at the doses tested. The NOAEL was 25 ppm, equivalent to 3.8 mg/kg bw per day, on the basis of effects on body weight (Adamik, 1981).
Rats
In a 2-year study of toxicity, groups of 36 ChR-CD rats of each sex were given diets containing oxamyl at a concentration of 0 (two groups), 50, 100 or 150 ppm, equivalent to 0, 2.5, 5 and 7.5 mg/kg bw per day. Individual body weights and food consumption were measured weekly for the first 7 months, every 2 weeks during months 712 and monthly thereafter. Haematological and urine end-points and the activities of alkaline phosphatase and alanine aminotransferase were measured in whole blood from 10 animals per sex at 0, 100 and 150 ppm after 1, 3, 6, 9, 12, 18 and 24 months of feeding. Whole-blood cholinesterase activity was assessed at 4 and 8 days and at 1, 6, 12 and 24 months of feeding in 10 animals of each sex given 0, 100 (1 and 6 months only) and 150 ppm. Whole-blood aliesterase activity was measured in these animals at 1, 12 and 24 months. After 1 year, six rats of each sex per dose and after 2 years all surviving animals were killed for gross and microscopic examination. Histopathological data for controls and for animals at 150 ppm are presented below.
A dose-related decrease in body weight was observed in treated males and females at 100 and 150 ppm, and the food consumption of those at 150 ppm was slightly reduced. No effect on food use efficiency was observed. Decreased cholinesterase activity in whole blood was observed in the group at 150 ppm shortly after the start of treatment, in females at day 4 (19% inhibition) and in males at day 8 (33% inhibition). Aliesterase activity was not affected. No treatment-related clinical signs, deaths or effects on haematological end-points, alkaline phosphatase or alanine aminotransferase activity or histological appearance were observed. Treatment-related changes (> 10%) in relative organ weights were observed in males and females. After 1 year, increased relative weights of the brain, heart, lung and stomach were observed in female rats at all dietary concentrations, and decreased relative liver weights were found in males at 100 and 150 ppm and females at all concentrations. After 2 years, the relative weights of the brain, lungs, testes and adrenals were increased in males at 150 ppm, and in females the relative weights of the adrenals (at 150 ppm only, not dose-related), brain, heart, lungs, kidneys and stomach (at all dietary concentrations) were dose-dependently increased. The relative spleen weights were increased in females at 50 and 100 ppm. The increases in relative organ weights were considered to be secondary to the effects of oxamyl on body weight. No increase in tumour incidence was observed in treated groups. The results were not analysed statistically (Sherman, 1972). Although the 1984 JMPR based the ADI on the results of this study, the present Meeting concluded that a NOAEL could not be identified owing to the shortcomings of the study.
In a 2-year study of toxicity and carcinogenicity conducted according to Environmental Protection Agency guideline 83-5, groups of 62 Sprague-Dawley rats of each sex were given diets containing oxamyl (purity, 97.1%) at a concentration of 0, 25, 50, 100 or 150 ppm, equal to 0, 0.99, 2, 4.2 and 7 mg/kg bw per day for males and 0, 1.3, 2.7, 6.7 and 11 mg/kg bw per day for females. Urine and blood samples were taken from 710 rats of each sex per dose, the latter for haematological and blood chemical analysis at 1 (for plasma and erythrocyte cholinesterase activity only), 3, 6, 12, 18 and 24 months after the start of the study. Except at the 1-month measurement, the rats were fasted for at least 16 h before blood samples were collected. Urine samples were collected during the fasting period. Ophthalmic examinations were carried out before the start of the study and at 358 and 728 days of treatment. At 378 days, an interim sacrifice was performed on 10 rats of each sex per dose. All surviving rats were killed on day 728 of the study, for a full pathological examination. Portions (not specified) of the brains of 710 rats of each sex per dose selected for haematological examination were used for determination of brain cholinesterase activity. Statements of adherence to GLP and QA were included.
A dose-dependent reduction in mortality rate was observed. Body weight and body-weight gain were reduced for rats at 100 and 150 ppm. In these groups, food consumption was slightly decreased in males and slightly increased in females. These animals also showed increased incidences of hyperreactivity, alopecia, skin sores and swollen legs or paws. Slightly increased incidences of swollen legs or paws were also seen in males at 25 and 50 ppm. Males at 100 ppm had an increased incidence of masses. A treatment-related increase in the percentage of rats with pale ocular fundi was observed, but in the absence of treatment-related microscopic ocular lesions this increase was considered not toxicologically relevant. Female rats at 150 ppm had an increased incidence of photoreceptor cell atrophy, which the authors considered to be secondary to the nutritional status of this group. Rats at 50, 100 and 150 ppm showed small decreases in erythrocyte count and increases in Na, K and Cl concentrations; however, since the effects were small and within the range of biological variation, they were considered not to be toxicologically relevant. Rats at 100 and 150 ppm had increased absolute brain weights, which were considered to be related to the brain compression induced by the increased incidence of pituitary tumours in the control groups (a common finding in ageing rats). In the absence of treatment-related histopathological findings, other effects on absolute and relative organ weights were considered to be secondary to the effect of oxamyl on body weight. Males and females at 150 ppm had increased incidences of myeloid hyperplasia of bone marrow, and males showed extramedullary haematopoiesis in the spleen. No treatment-related increase in the incidence of any particular tumour was observed. One month after initiation of treatment, dose-related, statistically significant inhibition of plasma cholinesterase activity was observed in all treated groups. No other treatment-related effects on plasma, erythrocyte or brain cholinesterase activity were found. The author considered the NOAEL to be 50 ppm on the basis of decreased body weight and inhibition of plasma cholinesterase activity at 100 ppm (Malley, 1991).
The Meeting noted that clinical chemical analysis and determination of blood and brain cholinesterase activity were carried out after a fast of at least 16 h in only 710 rats of each sex per dose, except for the 1-month determination in plasma. As cholinesterase activity probably recovered during the fasting period, no conclusion about the effect of oxamyl on this parameter could be drawn, and the Meeting considered that this study could not be used to identify a NOAEL for inhibition of cholinesterase activity. The Meeting identified a NOAEL of 50 ppm, equal to 2 mg/kg bw per day, on the basis of the effects on body weight, body-weight gain and clinical signs at 100 ppm.
Oxamyl has been tested in a range of tests for genotoxicity in vitro (Table 4). No study of gene mutation in mammalian cells was available.
Table 4. Results of studies on the genotoxicity of oxamyl in vitro
|
End-point |
Test object |
Concentration |
Purity (%) |
Results |
Reference |
|
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
5010 000 ΅g/plate (± S9) |
97.1 |
Negative |
Arce (1987)a |
|
Reverse mutation |
S. typhimurium TA97a, TA98, TA100, TA1535; E. coli WP2 uvrA (pKM101) |
55000 ΅g/plate (± S9) |
96.9 |
Negative |
Gladnick (1999)a |
|
Chromosomal aberration |
Chinese hamster ovary cells |
0.7100 ΅g/ml for 8.510 h (S9)b |
NR |
Negative |
Galloway (1982) |
|
2.3700 ΅g/ml for 2 h (+S9)c |
Negative |
||||
|
Gene mutation |
Chinese hamster ovary cells |
50-1200 ΅mol/l for 1819 h (S9)b |
97.1 |
Negative |
Rickard (1987)a |
|
25700 ΅mol/l for 5 h (+S9)b |
Negative |
||||
|
Gene mutation |
Chinese hamster ovary cells |
50300 ΅g/ml for 5 h (S9)d |
96.9 |
Negative |
San & Clarke (2000)a |
|
150500 ΅g/ml for 5 h (+S9)d |
Negative |
||||
|
Chromosomal aberration |
Human lymphocytes |
1050 ΅g/ml for 4 h (S9) |
Negative |
96.9 |
Gudi & Schadly (2000)a |
|
50200 ΅g/ml for 20 h (S9) |
Negative |
||||
|
100300 ΅g/ml for 4 h (+S9) |
Negative |
||||
|
Unscheduled DNA synthesis |
Rat hepatocytes |
0.0110 000 ΅mol/l for 18 he |
97.1 |
Negative |
Vincent (1987)a |
|
Positive and negative (solvent) controls were included in all studies. NR, not reported |
|
|
a |
Statements of adherence to GLP and QA were included. |
|
b |
Concentration-related cytoxocity was observed in tests with (19% survival at 700 ΅mol/l) and without metabolic activation (17% survival at 1200 ΅mol/l). |
|
c |
Dose-related cytotoxicity was observed at concentrations 23 ΅g/ml without metabolic activation and at concentrations > 70 ΅g/ml with metabolic activation. QA statement included. |
|
d |
Cloning efficiency was < 50 % of solvent control at concentrations of 150 ΅g/ml and 250 ΅g/ml without and with metabolic activation, respectively. |
|
e |
Cytotoxicity was observed at concentrations of 5 and 10 mmol/l. |
Rats
In a three-generation study of reproductive toxicity, groups of 16 ChR-CD rats of each sex were fed diets containing oxamyl (purity, 95%) at a concentration of 0, 50, 100 or 150 ppm, equivalent to 0, 3.3, 6.7 and 10 mg/kg bw per day. Over a 15-day period, each female was mated for 5 days each with three males in the same treated group. One week after weaning of the first litters (F1a), the F0 females were again mated to produce the F1b litters. Animals in these litters remained on the diet and were mated at 110 days of age, as described above, to produce the F2a and F2b litters. Animals in the F2b litters were used to produce the F3a and F3b litters. Litters containing more than 10 pups were reduced to 10 on day 4 after birth. The number of pregnancies, litter size at birth and on days 4, 12 and 21, and pup body weights were assessed. Two rats of each sex from five litters of the control and 150-ppm group of the F3b generation were examined histologically. Ten randomly selected animals of each sex from the control and 150-ppm groups of the F3b generation remained on their respective diets for 8 weeks, while another 10 were transferred from the control group to 150 ppm or vice versa for the same duration.
Oxamyl had no effect on the number of pregnancies or on gestation or fertility indexes. Dose-dependent reductions in litter size and body weights of weanlings at 100 and 150 ppm were observed consistently throughout the study; the body weights of weanlings at 50 ppm were slightly reduced. Occasional reductions in viability and lactation indexes were observed in rats at 100 and 150 ppm. In the cross-feeding study with F3b weanlings, the reductions in body weight of weanlings at 150 ppm appeared to be reversible within 8 weeks, whereas control weanlings subsequently given 150 ppm showed reduced body-weight gain. Weanlings of the F3b generation had slightly increased relative kidney weights (at 150 ppm) and relative testis weights (at 100 and 150 ppm). No treatment-related gross or histopathological abnormalities were observed in the F3b animals at 150 ppm (Sherman, 1972). The Meeting noted that mating each female with three males might have obscured effects on male fertility. Furthermore, the description of the study was very brief, and no individual data were presented for most parameters; no statistical analysis of the data was performed.
In a two-generation study of reproductive toxicity performed according to FIFRA guideline 40 CFR 160, reference 83-4, groups of 30 male and 30 female Sprague-Dawley rats received diets containing oxamyl (purity, 97.1%) at a concentration of 0, 25, 75 or 150 ppm, equivalent to 0, 1.7, 5 and 10 mg/kg bw per day. F0 rats were mated in a 1:1 ratio 74 days after the beginning of treatment to produce the F1 generation. In this generation, 24 males and 24 females per dose were given the corresponding experimental diet for at least 105 days after weaning and subsequently mated in a 1:1 ratio to produce the F2 generation. Treatment of the F1 rats was continued until weaning of the F2 generation. Statements of adherence to GLP and QA were included.
Of the F1 parents, two females at 25 ppm and one female at 75 ppm died; these deaths were considered not to be related to treatment. Body weight, body-weight gain and food use efficiency were reduced in F0 and F1 parental animals at 75 and 150 ppm. Food consumption was decreased in parental males of the F0 and F1 generations at 75 and 150 ppm and increased in parental females at 150 ppm. F1 males at 150 ppm showed increased reactivity, and females had a higher incidence of alopecia. In F0 and F1 males at 75 and 150 ppm, the relative testis weight was increased. The number of pups per litter in the F1 and F2 generations was decreased at 150 ppm, and the viability of these pups and litter survival were decreased during the first 4 days after birth. The weights of pups at 75 and 150 ppm were decreased. The percentage of pups with small bodies was increased in animals at 150 ppm in the F1 generation and in those at 75 and 150 ppm in the F2 generation. The number of pups with no milk spot was increased in animals at 150 ppm group in the F1 generation.
The NOAEL for parental toxicity was 25 ppm, equivalent to 1.7 mg/kg bw per day, on the basis of decreases in body weight, body-weight gain, food consumption, food use efficiency and increased relative testis weight. The NOAEL for developmental toxicity was 25 ppm, equivalent to 1.7 mg/kg bw per day, on the basis of reduced pup weight. The NOAEL for reproductive toxicity was 75 ppm, equivalent to 5 mg/kg bw per day (Hurtt, 1990). Measurements of cholinesterase activity in brain, erythrocytes or plasma were not included in this study.
Rats
Groups of 2628 pregnant ChR-CD rats were given diets containing oxamyl at a concentration of 0, 50, 100, 150 or 300 ppm, equivalent to 0, 2.5, 5, 7.5 and 15 mg/kg bw per day, on days 615 of gestation. Dams at concentrations > 100 ppm had dose-related decreases in body weight and food consumption. At sacrifice on day 20 of gestation, no macroscopic changes in tissues or organs were observed. Oxamyl did not affect the numbers of implantation sites, resorptions or dead or live fetuses, nor did it affect embryo weight or crownrump length. No treatment-related increase in skeletal malformations and anomalies was observed. The description of the study was very brief, and no individual data were presented (Culik & Sherman, 1971).
In a study of developmental toxicity performed according to Environmental Protection Agency guideline 83-3, groups of 25 female Sprague-Dawley rats received oxamyl (purity, 97.2%) at a dose of 0, 0.2, 0.5, 0.8 or 1.5 mg/kg bw per day in distilled water by gavage on days 716 of gestation. The dams were killed on day 22 of gestation, the fetuses were removed, and the dams and fetuses were examined to determine maternal toxicity and reproductive and developmental effects. Statements of adherence to GLP and QA were included.
The body-weight gain of the dams was reduced dose-dependently (by 8.6%, 21% and 30% at 0.5, 0.8 and 1.5 mg/kg bw per day, respectively), and the food consumption of dams at 0.8 and 1.5 mg/kg bw per day was decreased. Dams at 1.5 mg/kg bw per day showed clinical signs of cholinesterase inhibition (tremors, salivation, ocular discharge, wet perineum, wet body and diarrhoea), and 4/25 females at 0.8 mg/kg bw per day had tremors. Small but significant reductions in fetal weight were observed (by 3.9%, 6.8% and 6.9% at 0.5, 0.8 and 1.5 mg/kg bw per day, respectively). Treatment with oxamyl caused no irreversible structural changes in the fetuses.
The NOAEL for maternal toxicity was 0.5 mg/kg bw per day on the basis of decreases in body-weight gain and food consumption and tremors at 0.8 mg/kg bw per day. The NOAEL for fetal toxicity was 0.5 mg/kg bw per day, on the basis of decreased fetal body weight at 0.8 mg/kg bw per day (Rickard, 1988; Munley, 1998). Cholinesterase activity in brain, erythrocytes and plasma was not measured in this study.
Rabbits
Groups of 17 artificially inseminated New Zealand white rabbits were given oxamyl (purity, 97.1%) at a dose of 0, 1, 2 or 4 mg/kg bw per day by gavage on days 619 of gestation. On day 29, all animals were killed and the fetuses were removed. The pregnancy rates were 17/17, 15/17, 15/17 and 13/17 at 0, 1, 2 and 4 mg/kg bw per day, respectively. One animal each at 1 and 4 mg/kg bw per day died, probably because of tracheal intubation. The body-weight gain of does at 1 mg/kg bw per day was slightly reduced and that of does at 2 and 4 mg/kg bw per day was significantly reduced during treatment. The ovarian and uterine weights with and without fetuses of does at 2 mg/kg bw per day were slightly reduced. Fetal viability was slightly reduced in does at the highest dose, and two had 100% resorption. One female at the intermediate dose had one dead fetus. No treatment-related developmental toxicity was observed, and no irreversible structural changes were found. The NOAEL for maternal toxicity was 1 mg/kg bw per day on the basis of decreased body-weight gain during treatment at 2 mg/kg bw per day. The NOAEL for fetal toxicity was 2 mg/kg bw per day on the basis of an increased percentage of resorptions at the highest dose (Hoberman, 1980). Cholinesterase activity in brain, erythrocyte or plasma was not measured in this study.
In a study of delayed neurotoxicity, groups of five 1-year-old white Leghorn hens received a single instillation of oxamyl (purity unknown) as a 1% suspension in water directly into the crop at a dose of 40 mg (the acute lethal dose, as determined in a pilot experiment) or 20 mg. A few minutes before dosing, the hens received atropine by intramuscular injection at a dose of 0.5 mg/kg bw. Treatment with oxamyl caused sudden depression, lethargy, slightly ruffled feathers, slight respiratory difficulty, ataxia and impaired coordination immediately after dosing. The respiratory signs disappeared within 30 min, but the depression and nervous signs continued for 12 h. All hens survived up to 28 days after treatment. In the pilot experiment, 40 mg without prior treatment with atropine caused the same clinical signs to a very severe degree and resulted in death of the hens within 5 min. Treatment with oxamyl (with or without atropine) did not cause any gross or histopathological changes (the sciatic nerve was included in the pathological examination) (Lee, 1970). The study was considered to be outdated and therefore of limited value. Owing to the poor description of the study design, its compliance with OECD 418 could not be ascertained.
Groups of 40 Crl:CD BR rats of each sex received technical-grade oxamyl (purity, 98.3%) in deionized water by gavage at a single dose of 1 mg/kg bw. All rats were examined for clinical signs at 30 min, and 10 rats of each sex per dose were examined 2, 3 and 4 h after dosing, examined for clinical signs and bled. The animals were then killed, and their brains were collected for measurement of cholinesterase activity. Statements of adherence to GLP and QA were included. No deaths occurred. Tremors were observed in 38/40 males and 36/40 females 30 min after treatment. Cholinesterase activity was reduced in males and females, by 57% and 50% in plasma, 58% and 61% in erythrocytes and 45% and 48% in whole brain, respectively, 30 min after dosing. By 2, 3 and 4 h after treatment, no clinical signs were observed, and the deviations from control values for cholinesterase activity were <13%. There were no obvious changes in cholinesterase activity over 24 h (Malley, 1997a).
In a study performed according to Environmental Protection Agency guideline 81-8, groups of 42 Crl:CD BR rats of each sex were given oxamyl (purity, 98.3%) by gavage in deionized water at a single dose of 0.1, 1 or 2 mg/kg bw for males and 0.1, 0.75 or 1.5 mg/kg bw for females. Clinical signs, body weight, body-weight gain and food consumption were assessed periodically in 12 animals of each sex per dose. These animals were also administered a functional observational battery (FOB) and motor activity tests before treatment and 3060 min and 7 and 14 days after treatment. Of these rats, six of each sex were killed on day 15 and examined grossly. Brain, spinal cord, nerve and muscular tissue from animals in the control group and at the highest dose were examined histologically for neuropathological effects. Cholinesterase activity was determined in brain tissue, plasma and erythrocytes from groups of 10 rats of each sex per dose 3060 min and 1 and 14 days after dosing. In addition, plasma and erythrocyte cholinesterase activity was assessed before dosing in the animals killed after 3060 min. Statements of adherence to GLP and QA were included.
During the first day after treatment, body-weight gain and food consumption were reduced in males at the two higher doses and body-weight gain was reduced in females at the highest dose on the first day after administration. One male at 2 mg/kg bw died on the day of administration. Virtually all animals at the two higher doses showed tremors, and dose-dependent increases in salivation, low posture and wet perineum (females only) were observed. Other effects related to cholinesterase inhibition in these groups were decreased grip strength and response to tail pinch, decreased coordination, urination and defaecation, decreased reaction to approach and touch, decreased vocalization during handling and increased foot splay; they also showed soiled fur, lachrymation, dilated pupils, slow righting reflex, abnormal gait, impaired locomotion, low arousal, splayed limbs and laboured breathing. Furthermore, decreases in palpebral closure and in the mean and total number and duration of movements and increases in curled-up posture and docile behaviour were observed at the two higher doses. Effects on clinical signs and the results of the FOB and motor activity tests were observed 3060 min after application. No clinical signs or behavioural effects in the two tests were seen with the lowest dose. Behavioural effects observed occasionally on days 7 and 14 after treatment and on cholinesterase activity on days 1 and 14 after treatment were considered to be incidental, as the study on reversibility showed that the effects of oxamyl were short-lasting.
The effects of oxamyl on cholinesterase activity are summarized in Table 5. Large reductions in cholinesterase activity were found in brain and blood 3060 min after administration in rats at the two higher doses. A significant reduction in cholinesterase activity of 25% found in the cerebellum of females at 0.1 mg/kg bw was considered to be of no toxicological significance, because significant inhibition of cholinesterase activity was not observed in other brain structures or in the half-brain preparation, nor in erythrocytes or plasma of males and females at this dose. Macroscopic and microscopic neuropathological examination revealed no treatment-related, toxicologically relevant effects. As different doses were used for males and females, it is difficult to assess whether there is a sex difference in sensitivity to the neurotoxic effects of oxamyl. The NOAEL was 0.1 mg/kg bw on the basis of inhibition of cholinesterase activity in brain, plasma and erythrocytes, a variety of clinical signs and effects in the FOB at 0.75 mg/kg bw (Malley, 1997b).
Table 5. Effects of oxamyl on cholinesterase activity (% change) in rats 3060 min afteradministration by gavage
|
Site |
Dose (mg/kg bw) |
|||||
|
Males |
Females |
|||||
|
0.1 |
1 |
2 |
0.1 |
0.75 |
1.5 |
|
|
Blood |
||||||
|
Plasma |
10 |
60* |
77* |
+9 |
38* |
72* |
|
Erythrocytes |
7 |
57* |
63* |
+13 |
55* |
70* |
|
Brain |
||||||
|
Cortex |
19 |
56* |
71* |
15 |
59* |
68* |
|
Hippocampus |
10 |
38* |
74* |
15 |
40* |
71* |
|
Midbrain |
+3 |
40* |
60* |
8 |
50* |
70* |
|
Cerebellum |
+4 |
26* |
70* |
25* |
51* |
74* |
|
Half brain |
2 |
47* |
66* |
1 |
46* |
67* |
From Malley (1997b)
* Significantly different from control
Groups of 42 Crl:CD rats of each sex recived diets containing oxamyl (purity, 98.3%) at a concentration of 0, 10, 30 or 250 ppm, equal to 0, 0.55, 1.7 and 15 mg/kg bw per day for males and 0, 0.67, 2 and 20 mg/kg bw per day for females, for 90 days in a study performed according to Environmental Protection Agency guideline 81-8. The two higher concentrations were initially 100 and 300 ppm but were reduced to 30 and 250 ppm after 7 days due to severe toxicity at the highest dose. Body weights and food consumption were measured weekly. Rats were checked for clinical signs daily for the first 2 weeks (the first 4 weeks for rats at the highest dose) and weekly thereafter. FOB and motor activity tests were given to 12 rats of each sex per dose before and 4, 8 and 13 weeks after the start of treatment. Cholinesterase activity in brain, plasma and erythrocytes was determined in 10 rats of each sex per dose at weeks 4, 8 and 13. Statements of adherence to GLP and QA were included.
Dose-related clinical signs including tremors, abnormal gait, hunched posture, exophthalmus, ptosis, hyperreactivity, piloerection, coloured discharge from the eyes, wet chin, stained perineum or inguen and lachrymation were observed at 100 and 300 ppm during the first week of treatment. After reduction of the concentrations, treatment-related clinical signs were observed in males and females at the highest concentration. Although the clinical signs were observed throughout treatment, the incidences were highest during the first 4 weeks of treatment. It is not clear whether this higher incidence was due to a higher intake of oxamyl during the first weeks of treatment or the result of an adaptive response to prolonged treatment, or both. Body weight and body-weight gain were reduced in males and females at 100 and 300 ppm during the first week. After reduction of the two higher dietary concentrations on day 7, the body weights and body-weight gain recovered completely in males at 30 ppm and females at 30 and 250 ppm and partially in males at 250 ppm. During the first week, food consumption and food use efficiency were decreased in animals at 100 and 300 ppm and thereafter in males at 250 ppm. In the FOB, hind-limb grip strength was reduced in males and females at 250 ppm in week 4 and in females in weeks 8 and 13. Hind-limb foot splay was decreased at week 8 (females only) and week 13 in animals at 250 ppm. This group showed increased incidences of ptosis, absent pupillary responses, piloerection, abnormal gait and absent defaecation throughout treatment. Although these increases were not always significant, they were associated with cholinesterase inhibition and were considered to be treatment-related. Motor activity (duration and number of movements) was slightly reduced in animals at 250 ppm, in weeks 4 and 8 in males and week 13 in males and females.
The effects of oxamyl at a dietary concentration of 250 ppm on cholinesterase activity are summarized in Table 6. The reduction in cholinesterase activity was constant over the measurement intervals. In general, cholinesterase activity was more severely inhibited in females than in males, perhap due to a slightly higher intake by females. Generally, the inhibition of cholinesterase activity was < 20% in the groups at 10 and 30 ppm and in only one instance reached statistical significance. No macroscopic or microscopic neuropathological changes were observed. The NOAEL was 30 ppm, equal to 1.7 mg/kg bw per day, on the basis of effects on body weight and body-weight gain, clinical effects, behavioural changes in the FOB and inhibition of cholinesterase activity in brain, erythrocytes and plasma (Malley, 1998).
Table 6. Effects of oxamyl at a dietary concentration of 250 ppm on cholinesterase activity (% change) in rats during 13 weeks
|
Site |
Dose (mg/kg bw) |
|||||
|
Males |
Females |
|||||
|
4 |
8 |
13 |
4 |
8 |
13 |
|
|
Blood |
||||||
|
Plasma |
34 |
28 |
24 |
67 |
56 |
60 |
|
Erythrocytes |
40 |
26 |
48 |
65 |
41 |
55 |
|
Brain |
||||||
|
Cortex |
46 |
35 |
40 |
55 |
36 |
51 |
|
Hippocampus |
31 |
34 |
27 |
60 |
40 |
42 |
|
Midbrain |
30 |
36 |
31 |
40 |
20 |
51 |
|
Cerebellum |
31 |
24 |
32 |
37 |
32 |
39 |
|
Half brain |
38 |
35 |
42 |
56 |
41 |
49 |
From Malley (1998)
The acute toxicity of metabolites of oxamyl was studied only in ChR-CD male rats treated orally.
Rats received DMCF (purity unknown) at single doses of 901000 mg/kg bw by gavage. Deaths occurred at doses > 450 mg/kg bw. The clinical signs were low posture, salivation, hyperresponsiveness to noise, weakness and body-weight loss (Ashley, 1974).
DMOA (purity unknown) was administered to groups of five rats by gavage at a dose of 2500 or 5000 mg/kg bw. All animals at the higher dose but none of those at the lower dose died. The clinical signs were irregular respiration, low posture, half-closed eyes, pallor, weakness and body-weight loss (Barbo, 1972).
Methyl N-hydroxy-N-methyl-1-thiooxamimidate (purity unknown) was administered to groups of 10 rats by gavage at doses of 40007000 mg/kg bw. Dose-related deaths occurred at doses > 6000 mg/kg bw. At 4500 and 5000 mg/kg bw, no deaths occurred; at 4000 mg/kg bw, one animal died. The clinical signs were lethargy, prostration, ruffled fur, half-closed eyes, polyuria, pallor and body-weight loss (Dale, 1973).
DMTO (purity unknown) was administered orally at doses of 9011 000 mg/kg bw. Deaths occurred at the highest dose. The clinical signs were discomfort, light-coloured faeces, half-closed eyes, salivation and body-weight loss (Fretz, 1968).
Rats
In a limited 10-day study, six young adult male ChR-CD rats received DMCF (purity unknown) orally at a dose of 90 mg/kg bw per day, 5 days/week for 2 weeks. Three rats were killed 4 h after the last dose, and the other three were killed after a 14-day recovery period. Treatment decreased body weight and body-weight gain, decreased the absolute weights of the liver and kidney and decreased the absolute and relative weights of the spleen and thymus. The weight of the testis showed an absolute decrease but a relative increase. All animals showed vacuolation of hepatocytes of the centrilobular area of the liver. At the end of treatment, slight atrophy of the spleen, thymus and bone marrow was observed, and one animal had acute pancreatitis. After the recovery period, no atrophy was observed. The animals were limp during treatment, and mild lethargy was reported during the recovery period. The study report was very brief, with no data on individuals or groups, and the observed effects were only summarized (Dashiell, 1976).
Groups of 16 ChR-CD rats of each sex received diets containing DMCF (purity, 100%) at a concentration of 50, 150 or 450 ppm, providing measured average daily intakes of active ingredient for the three respective groups of 7, 21 and 60 mg/kg bw per day at the start of the study and 2.4, 6.7 and 20 mg/kg bw per day at the end of the study. The parameters assessed were clinical signs (daily), food consumption and body weight (weekly) and haematological, clinical chemical and urine parameters (at 30, 60 and 90 days). After 90 days of feeding, six rats of each sex per group were selected for a one-generation study of reproductive toxicity, and the other rats were killed and subjected to gross and microscopic pathological examination.
There were no deaths. Body-weight gain and food consumption were decreased in rats at 450 ppm. Exophthalmus and alopecia were observed in two female rats at 450 ppm, while males had a decreased erythrocyte volume fraction and decreased erythrocyte counts after 30, 60 and 90 days; at day 90, the haemoglobin concentration was also decreased. Female rats in this group had lower erythrocyte counts and haemoglobin concentrations at 30, 60 and 90 days and lower erythrocyte volume fraction and leukocyte counts at 60 and 90 days. Males at 150 ppm had lower erythrocyte counts and erythrocyte volume fraction, and female rats had lower erythrocyte and leukocyte counts at day 90. No gross or micropathological treatment-related effects were observed.
The female rats selected for the study of reproductive toxicity were paired with three males of the same dietary group, each for a period of 5 days. In rats at 150 ppm, the percentage of matings resulting in pregnancy was decreased. At 450 ppm, the average body weight of pups at weaning was decreased (Kaplan, 1976).
Groups of six male ChR-CD rats received DMTO dissolved in corn oil by gavage at a dose of 0 or 1000 mg/kg bw per day, 5 days/week for 2 weeks or 2200 mg/kg bw per day for 5 days. Four of six animals at 2200 mg/kg bw per day died. The clinical signs were stained perineal area, chromodacryorrhoea, weakness, unkemptness and continued body-weight loss. The pathological changes were atrophy of the spleen and thymus, hypoplasia of bone marrow, centrilobular necrosis, congestion and haemorrhage of the liver. In the groups at 1000 mg/kg bw per day, the clinical signs were weakness, unkemptness and body-weight loss. In the three animals at 1000 mg/kg bw per day killed at the end of treatment, atrophy of the spleen and thymus, hypoplasia of bone marrow and depletion of hepatic glycogen were observed. In the three remaining animals, which were allowed to recover for 14 days, the body weight was partially regained. Gross and microscopic examination showed no pathological changes in these animals. The study report was very brief, and no group or individual data were reported (Wasileski, 1971).
DMCF (purity, 100%) was tested for reverse mutation at doses of 25010 000 ΅g/plate, in the absence and presence of an exogenous metabolic system in Salmonella typhimurium TA98, TA100, TA1535, TA1537 and TA1538. The result of the test was negative. Cytotoxicity occurred at > 3000 ΅g/plate (Sipple, 1978).
A number of toxicologically relevant effects of oxamyl (purity, 97.6%) were determined in male volunteers aged 1939 years given a capsule containing a single oral dose of 0.005, 0.015, 0.03, 0.06, 0.09 or 0.15 mg/kg bw, with 10 men in the control group and five at each dose. The capsule was administered 5 min after a standard breakfast. Blood samples for determination of erythrocyte and plasma cholinesterase activity were collected at screening, 2 days, 16 h and 30 min before dosing, every 15 min for the first 2 h after dosing and 3, 4, 6, 8, 12 and 24 h and 7 (± 2) days after dosing. The baseline was defined as the mean of all available values before dosing. At 16 h and 30 min before dosing and 1, 2, 3, 4, 8 and 24 h after dosing, initial pupil size, pupil reaction to and recovery from a light flash and the quantity of saliva secreted within 5 min were assessed. Haematological and clinical chemical examinations were performed on blood samples taken at screening, 30 min before dosing and 24 h after dosing. Urine was analysed at screening and 24 h after dosing. To ensure safety, physical examinations were conducted, and vital signs (blood pressure, heart rate, clinical signs), oral temperature and electroencephalographic trace were monitored. Statements of adherence to GLP and QA were included.
No treatment-related effects on the encephalogram, heart rate, pulse, blood pressure, respiratory rate, body temperature, haematological, clinical chemical (except cholinesterase activity) or urine parameters or on the pupils were observed. At doses up to 0.09 mg/kg bw, no effects on salivary secretion were observed. Occasional changes in erythrocyte or plasma cholinesterase activity observed at doses <0.06 mg/kg bw were considered incidental and not toxicologically relevant. Significant effects on cholinesterase activity in erythrocytes (Table 7) and plasma were observed in men given 0.09 and 0.15 mg/kg bw. At 0.09 mg/kg bw, small but statistically significant decreases in cholinesterase activity were observed in erythrocytes (7.3% at 30 min) and plasma (1012% from 75120 min), but these decreases were considered not to be adverse as they were < 20%, and similar changes in plasma and erythrocyte cholinesterase activity were found in men in the control group. At 0.15 mg/kg bw, plasma cholinesterase activity was decreased by 2143% between 30 min and 2 h after dosing, and cholinesterase activity in erythrocytes was decreased by 2328% between 30 and 60 min after dosing. These men also had a significant increase in saliva production (161%) 1 h after dosing. The NOAEL was 0.09 mg/kg bw, on the basis of inhibition of plasma and erythrocyte cholinesterase activity and increased salivation at 0.15 mg/kg bw (McFarlane & Freestone, 1999).
Table 7. Inhibition of erythrocyte cholinesterase activity (% change from baselinea) in male volunteers after a single oral dose of oxamyl in a capsule
|
Time (h) |
Dose (mg/kg bw) |
||||||||
|
0 |
0.09 |
0.15 |
|||||||
|
Mean |
Minimum |
Maximum |
Mean |
Minimum |
Maximum |
Mean |
Minimum |
Maximum |
|
|
0.25 |
4.9 |
8 |
26 |
1.0 |
12 |
12 |
2.3 |
25 |
12 |
|
0.5 |
7.8 |
5 |
27 |
7.3* |
18 |
5 |
23* |
33 |
6 |
|
0.75 |
6.5 |
9 |
22 |
0.2 |
17 |
13 |
28* |
38 |
6 |
|
1 |
9.4 |
4 |
28 |
0.3 |
9 |
8 |
27* |
43 |
17 |
|
1.25 |
5.5 |
11 |
28 |
0.7 |
9 |
10 |
17* |
30 |
9 |
|
1.5 |
11 |
0 |
29 |
1.8 |
5 |
10 |
16* |
28 |
3 |
|
1.75 |
4.5 |
17 |
25 |
1.1 |
9 |
6 |
9.2* |
19 |
1 |
|
2 |
7.9 |
2 |
34 |
2.1 |
15 |
6 |
8.0 |
24 |
3 |
|
3 |
10 |
4 |
23 |
0.2 |
14 |
15 |
0.4 |
7 |
10 |
|
4 |
12 |
2 |
31 |
3.0 |
16 |
16 |
3.7 |
14 |
15 |
|
6 |
3.5 |
20 |
24 |
21 |
3 |
38 |
11 |
4 |
22 |
From McFarlane & Freestone (1999)
a Baseline was defined as the mean of all available values before dosing, at screening, 2 days, 16 h, 30 min.
* Significantly different from baseline
Absorption of oxamyl was rapid and nearly complete after oral administration to rats and intraperitoneal administration to male mice. Elimination was rapid, urine being the main route of excretion (80% within 24 h and 95% within 168 h in rats; 89% within 96 h in mice). The tissue concentrations were low. Studies of biotransformation in vitro and in vivo indicated that oxamyl is metabolized in rats and mice via two major pathways: non-enzymatic hydrolysis to the oxime and enzymatic conversion to dimethyloxamic acid via dimethylcyanoformamide. These and other metabolites were present as polar conjugates in the urine of rats. No marked sex difference was observed in the excretion pattern, tissue distribution or metabolite profile in rats.
The oral LD50 in rats was 2.5 mg/kg bw; the inhalation LC50 (4-h, nose-only) in rats was 0.05 mg/l; and the dermal LD50 in rabbits was > 2000 mg/kg bw. The signs of acute intoxication with oxamyl were consistent with inhibition of cholinesterase activity. WHO has classified oxamyl as highly hazardous (WHO, 2000).
In studies in New Zealand white rabbits, oxamyl was not irritating to the eyes or skin; however, ocular treatment induced signs of acute intoxication consistent with inhibition of cholinesterase activity. Oxamyl did not sensitize the skin of guinea-pigs in the Buehler test.
The most sensitive effect of oxamyl was inhibition of cholinesterase activity, often accompanied at the same or higher doses by clinical signs. The effect of oxamyl on cholinesterase activity is rapid and transient. In rats given oxamyl at a single dose of 1 mg/kg bw by gavage, cholinesterase inhibition and clinical signs were observed within 0.5 h, and recovery was virtually complete within 2 h.
The NOAELs after dietary administration were higher than those after treatment by gavage. In a study of acute neurotoxicity in rats treated by gavage, inhibition of brain, erythrocyte and plasma cholinesterase activity, a variety of clinical signs and disturbances in a battery of functional tests indicative of cholinesterase inhibition were observed at doses of 0.75 and 1 mg/kg bw and above in females and males, respectively. A significant, 25% reduction in cholinesterase activity in the cerebellum of females at 0.1 mg/kg bw was considered not to be of toxicological significance because significant inhibition of cholinesterase activity was not observed in other brain structures or in a half-brain preparation or in erythrocytes or plasma of either sex at this dose. The NOAEL in this study was 0.1 mg/kg bw.
In a 90-day study in rats given oxamyl at a concentration of 10300 ppm in the diet, behavioural effects in a battery of functional tests and clinical signs typical of cholinesterase inhibition were observed at doses of 100 ppm and higher. In addition, reductions in body weight, body-weight gain, food consumption and feed use efficiency were seen at these doses. Reductions in brain, erythrocyte and plasma cholinesterase activity were observed at 4, 8 and 13 weeks of treatment and remained constant during these three periods. The NOAEL was 30 ppm, equal to 1.7 mg/kg bw per day. In a 1-year study in beagle dogs treated in the diet, inhibition of brain and plasma cholinesterase activity was observed in males at 50 ppm (equal to 1.5 mg/kg bw per day), the lowest dose tested. Tremors were observed in females at this dose. On the basis of this study and a second 1-year study in dogs that was performed to determine the NOAEL for cholinesterase inhibition in dogs, the NOAEL was 35 ppm, equal to 0.93 mg/kg bw per day.
A number of studies of toxicity in mice, rats and dogs given repeated doses showed not only inhibition of cholinesterase activity but also effects on body weight and body-weight gain and, to a lesser degree, on food consumption and feed use efficiency, sometimes accompanied by effects on organ weights. These results were seen in a 3-month study in rats, 2-year studies in mice and rats, a two-generation study of reproductive toxicity in rats and studies of developmental toxicity in rats and rabbits. The lowest NOAEL for these effects was 0.5 mg/kg bw per day in a study of developmental toxicity in rats treated by gavage.
In long-term studies in mice and rats, no carcinogenic effect of oxamyl was observed. The Meeting concluded that oxamyl is unlikely to pose a carcinogenic risk to humans.
The genotoxic potential of oxamyl in vitro was investigated in a number of assays for reverse mutation in bacteria, in tests for gene mutation and chromosomal aberration and in an assay for unscheduled DNA synthesis in mammalian cells. Negative results were obtained in all the studies. In view of the consistently negative results in a comprehensive range of well-conducted assays in vitro, the Meeting concluded that oxamyl is unlikely to be genotoxic. This conclusion was supported by the absence of other toxicological effects, such as carcinogenicity and reproductive toxicity, which could have a genotoxic mechanism.
In a two-generation study of reproductive toxicity in rats, oxamyl was administered in the feed at a concentration of 0, 25, 75 or 150 ppm. Parental animals showed reductions in body weight, body-weight gain, food consumption and feed use efficiency at concentrations of 75 ppm and above. Decreased pup body weight and an increase in the number of pups with low body weights were also seen at these concentrations. The NOAEL for parental and developmental toxicity (based on the oxamyl intake of the dams) was 25 ppm, equivalent to 1.7 mg/kg bw per day. At 150 ppm (equivalent to 10 mg/kg bw per day in dams), a reduction in number of pups per litter, indicative of reproductive toxicity, was observed. The NOAEL for reproductive toxicity was 75 ppm (equivalent to 5 mg/kg bw per day). Effects on pup weight were observed at similar doses in an older, three-generation study of reproductive toxicity. Cholinesterase activity in brain, erythrocytes or plasma was not measured in these studies.
A dose of 0.8 mg/kg bw per day given to rats by gavage caused tremors in the dams and reductions in weight gain and food consumption. A small (6.8%) but significant reduction in fetal body weight was also observed, which was considered to be related to the maternal toxicity. The NOAEL for maternal and fetotoxicity was 0.5 mg/kg bw per day. In a study in rabbits, decreased body-weight gain was observed in does at a dose of 2 mg/kg bw per day given by gavage. At 4 mg/kg bw per day, the percentage of resorptions was increased and fetal viability was lowered slightly. The NOAELs for maternal and fetal toxicity were 1 and 2 mg/kg bw per day, respectively. Oxamyl did not induce irreversible structural effects in either rats or rabbits, and the Meeting concluded that oxamyl has no teratogenic potential. Cholinesterase activity in brain, erythrocytes or plasma was not measured in these studies.
The Meeting concluded that the existing database was sufficient to characterize the potential hazard of oxamyl to fetuses, infants and children.
There was no evidence that a single dose of oxamyl to hens induced delayed polyneuropathy.
In general, the studies of biochemical effects and toxicity in animals did not reveal marked differences between males and females.
Male volunteers received a single gelatine capsule containing oxamyl at a dose of 0, 0.005, 0.015, 0.03, 0.06, 0.09 or 0.15 mg/kg bw. Cholinesterase activity was inhibited in plasma (within 0.52 h) and erythrocytes (> 20% inhibition within 0.51 h) by a dose of 0.15 mg/kg bw, and the effect was accompanied by increased production of saliva. Small (< 12 %) but significant reductions in plasma and erythrocyte cholinesterase activity observed with a dose of 0.09 mg/kg bw were considered not to be adverse since the magnitude of the decrease was < 20% and similar changes in plasma and erythrocyte cholinesterase activity were observed in individuals in the control group. The NOAEL was 0.09 mg/kg bw.
Acute and short-term studies in rats given the metabolites dimethyloxamic acid, methyl N-hydroxy-N-methyl-1-thiooxamimidate, dimethylcyanoformamide and the oxime metabolite orally suggested that they were less toxic than oxamyl. Dimethylcyanoformamide did not induce reverse mutation in bacteria.
The toxicological profile of oxamyl showed rapid restoration of cholinesterase activity after inhibition, and repeated administration did not change the character of the recovery. Moreover, no sex differences were found with respect to the effects of oxamyl in experimental animals. The Meeting established an ADI of 00.009 mg/kg bw on the basis of the NOAEL of 0.09 mg/kg bw per day in male volunteers, in whom increased salivation and decreased erythrocyte cholinesterase activity were observed at a higher dose, and a safety factor of 10.
The Meeting established an acute RfD of 0.009 mg/kg bw on the basis of the NOAEL of 0.09 mg/kg bw in the study with volunteers and a safety factor of 10. This acute RfD is supported by the NOAEL of 0.1 mg/kg bw in the study of acute neurotoxicity in rats.
|
Levels relevant for risk assessment |
||||
|
Species |
Study |
Effect |
NOAEL |
LOAEL |
|
Mouse |
2-year study of toxicity and carcinogenicitya,b |
Toxicity |
25 ppm, equivalent to |
50 ppm, equivalent to |
|
Rat |
2-year study of toxicity and carcinogenicitya,b |
Toxicity |
50 ppm, equal to |
100 ppm, equal to |
|
Two-generation study of reproductive toxicitya,b |
Parental and pup toxicity |
25 ppm, equivalent to |
75 ppm, equivalent to |
|
|
Reproductive toxicity |
75 ppm, equivalent to |
150 ppm, equivalent to |
||
|
Developmental toxicityb,c |
Maternal toxicity |
0.5 mg/kg bw per day |
0.8 mg/kg bw per day |
|
|
Fetotoxicity |
0.5 mg/kg bw per day |
0.8 mg/kg bw per day |
||
|
Acute neurotoxicityc |
Neurotoxicity |
0.1 mg/kg bw |
0.75 mg/kg bw |
|
|
90-day neurotoxicitya |
Neurotoxicity |
30 ppm, equal to |
250 ppm, equal to |
|
|
Rabbit |
Developmental toxicityb,c |
Maternal toxicity |
1 mg/kg bw per day |
2 mg/kg bw per day |
|
Embryo and fetotoxicity |
2 mg/kg bw per day |
4 mg/kg bw per day |
||
|
Dog |
1-year studies of toxicitya,d |
Toxicity |
35 ppm, equal to |
50 ppm, equal to |
|
Human |
Study in volunteers with single dosese |
Cholinesterase inhibition, salivation |
0.09 mg/kg bw |
0.15 mg/kg bw |
a Dietary administration
b (Adequate) measurements of cholinesterase activity not included
c Gavage
d Two studies combined
e Capsule
Estimate of acceptable daily intake for humans
00.009 mg/kg bw
Estimate of acute reference dose
0.009 mg/kg bw
Studies that would provide information useful for continued evaluation of the compound
Further observations in humans
List of end-points relevant for setting guidance values for dietary and non-dietary exposure
|
Absorption, distribution, excretion and metabolism in animals |
|
|
Rate and extent of absorption |
Rapid and extensive |
|
Dermal absorption |
No data (rabbit: systemic toxicity at > 50 mg/kg bw per day) |
|
Distribution |
Throughout body, highest concentrations in blood, heart, liver, kidney, lung, spleen and gastrointestinal tract |
|
Potential for accumulation |
Low |
|
Rate and extent of excretion |
Relatively rapid (mouse: 76% after 6 h, 89% after 24 h; rat: 81% after 24 h), mainly in urine |
|
Metabolism in animals |
Extensively metabolized, no parent compound found in urine |
|
Toxicologically significant compounds |
Oxamyl |
|
Acute toxicity |
|
|
Rat, LD50, oral |
2.5 mg/kg bw |
|
Rabbit, LD50, dermal |
> 2000 mg/kg bw |
|
Rat, LC50, inhalation |
0.05 mg/l (4 h, nose-only) |
|
Dermal irritation |
Not irritating, rabbit |
|
Ocular irritation |
Not irritating, rabbit |
|
Dermal sensitization |
Not sensitizing, guinea-pig (Buehler test) |
|
Short-term toxicity |
|
|
Target/critical effect |
Inhibition of cholinesterase activity in brain and erythrocytes, clinical and behavioural effects associated with cholinesterase inhibition, reduction in body weight and body-weight gain |
|
Lowest relevant oral NOAEL |
35 ppm, equal to 0.93 mg/kg bw per day, dogs |
|
Lowest relevant dermal NOAEL |
2.5 mg/kg bw per day, rabbits |
|
Long-term toxicity and carcinogenicity |
|
|
Target/critical effect |
Reduction in body weight and body-weight gain (cholinesterase activity not assessed) |
|
Lowest relevant NOAEL |
50 ppm, equivalent to 2 mg/kg bw per day, rats |
|
Carcinogenicity |
Not carcinogenic |
|
Genotoxicity |
No concern about genotoxicity |
|
Reproductive toxicity |
|
|
Target/critical effect for reproductive toxicity |
Reduction in number of pups per litter (in presence of parental toxicity) |
|
Lowest relevant NOAEL for reproductive toxicity |
75 ppm, equivalent to 5 mg/kg bw per day, rats |
|
Target/critical effect for developmental toxicity |
Reduction in body weight (in presence of maternal toxicity); not teratogenic |
|
Lowest relevant NOAEL for developmental toxicity |
0.5 mg/kg bw per day |
|
Neurotoxicity |
|
|
Neurotoxicity |
Inhibition of cholinesterase activity in brain, plasma and erythrocytes and clinical and behavioural effects associated with cholinesterase inhibition |
|
Lowest relevant oral NOAEL |
0.1 mg/kg bw, rats |
|
Delayed neurotoxicity |
No concern |
|
Medical data |
|
|
Single dose |
Inhibition of cholinesterase activity in plasma and erythrocytes and increased saliva production |
|
Lowest relevant oral NOAEL |
0.09 mg/kg bw |
|
Summary |
Value |
Study |
Safety factor |
|
ADI |
00.009 mg/kg bw |
Humans, single dose |
10 |
|
Acute RfD |
0.009 mg/kg bw |
Humans, single dose |
10 |
Adamik, E.R. (1981) Long term feeding study in mice with oxamyl. Unpublished report No. HLO 252-81 from WIL Research Laboratories, Inc., Ashland, Ohio, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Arce, G.T. (1987) Mutagenicity evaluation of IND1410-196 in Salmonella typhimurium. Unpublished report No. HLR 614-81, Revision 1, from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Ashley, W.E. (1974) Acute oral test. Unpublished report No. HLR 585-74 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Barbo, E.C. (1972) Oral LD50 test. Unpublished report No. HLR 399-72 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Brock, W.J. (1988a) Acute dermal toxicity study of IN D1410-196 in rabbits. Unpublished report No. HLR 114-88 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Brock, W.J. (1988b) Closed-patch repeated insult dermal sensitization study (Buehler method) with IN D1410-304 in guinea pigs. Unpublished report No. HLR 179-88 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Brock, W.J. (1988c) Repeated dose dermal toxicity: 21-day study with IN D1410-196 in rabbits. Unpublished report No. HLR 523-88 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Chang, K.M. & Knowles, C.O. (1979) Metabolism of oxamyl in mice and two-spotted spider mites. Arch. Environ. Contam. Toxicol., 8, 499508.
Culik, R. & Sherman, H. (1971) Teratogenic study in rats with S-methyl-1-dimethylcarbamoyl-N-[(methylcarbamoyl)oxy] thioformimidate (IND-1410). Unpublished report No. HLR 5-71 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Dale, N.C. (1973) Oral LD50 test. Unpublished report No. HLR 126-73 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Dashiell, O.L. (1976) 10 day subacute test. Unpublished report No. HLR 390-76 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Dickrell, L. (1991) 52-week dietary toxicity study with IND-1410 (oxamyl) in male dogs. Unpublished report No. HLO 555-90 from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Draize, J.H. (1977) Dermal and eye toxicity tests. In: Principles and Procedures for Evaluating the Toxicity of Household Substances, Washington DC: National Academy of Sciences, pp. 4849.
Fretz, S.B. (1968) Acute oral test. Unpublished report No. HLR 300-68 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Fretz, S.B. & Sherman, H. (1968) Ten dose subacute oral test. Unpublished report No. HLR 150-68 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Galloway, S.M. (1982) Mutagenicity evaluation of H#14190 in an in vitro cytogenetic assay measuring chromosome aberration frequencies in Chinese hamster ovary (CHO) cells. Unpublished report No. HLO 363-82, from Litton Bionetics, Kensington, Maryland, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Gladnick, N.L. (1999) Oxamyl technical: Bacterial reverse mutation test in Salmonella typhimurium and Escherichia coli. Unpublished report No. DuPont-3084 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Gudi, R & Schadly, E.H. (2000) Oxamyl technical: In vitro mammalian chromosome aberration test. Unpublished report No. Dupont-2936 from BioReliance, Rockville, Maryland, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Harvey, J. & Han, J., (1978) Metabolism of oxamyl and selected metabolites in the rat. J. Agric. Food. Chem., 26, 902910.
Hawkins, D.R., Mayo, B.C., Pollard, A.D., Haynes, L.M. & Donschak, W.W. (1990) Biokinetics and metabolism of 14C-oxamyl in rats. Unpublished report No. AMR 1226-88 from Huntingdon Research Centre, Ltd, Cambridgeshire, United Kingdom. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Hinckle, L. (1980) Oral LD50 test in ratsEnvironmental Protection Agency proposed guidelines. Unpublished report No. HLR 775-80 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Hoberman, A.M. (1980) Teratology study in rabbitsOxamyl. Unpublished report No. HLO 0801-80 from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Holsing, G.C. (1969) 13-week oral administrationDogs: Insecticide 1410. Unpublished report No. HLO 328-69 from Hazleton Laboratories America, Inc., Madison, Wisconsin, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Hurtt, M.E. (1990) Reproductive and fertility effects with oxamyl (IN D1410). Multigeneration reproduction study in rats. Unpublished report No. HLR 423-90 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Kaplan, A.M. (1976) Ninety-day feeding study in rats with 1-cyano-N,N-dimethylformamide (INN-79), metabolite of Vydate. Unpublished report No. HLR 630-76 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Kelly, D.P. (2001) Oxamyl (DPX-D1410) technical (98% w/w): Inhalation median lethal concentration (LC50) study in rats. Unpublished report No. DuPont-6331 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Ladics, G.S. (2001a) Oxamyl (DPX-D1410) technical (98% w/w): Primary eye irritation study in rabbits. Unpublished report No. DuPont-7059 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Ladics, G.S. (2001b) Oxamyl (DPX-D1410) technical (98% w/w): Primary dermal irritation study in rabbits. Unpublished report No. DuPont-7060 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Lee, K.P. (1970) Oral ALD and delayed leg paralysis test. Unpublished report No. HLR 234-70 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Malley, L.A. (1991) Combined chronic toxicity/oncogenicity study with oxamyl (IN D1410-196) long term feeding study in rats. Unpublished report No. HLR 278-91 (4 volumes) from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Malley, L.A. (1997a) Reversibility study with carbamate insecticides in rats. Unpublished report No. HL-1997-00641 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Malley, L.A. (1997b) Acute oral neurotoxicity study of oxamyl technical in rats. Unpublished report No. HLR 1118-96 (2 volumes) from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Malley, L.A. (1998) Oxamyl technical: Subchronic oral neurotoxicity study in rats. Unpublished report No. HL-1998-00708 (2 volumes) from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Malley, L.A. (1999) Oxamyl technical: 21-day repeated dose dermal toxicity study in rabbits. Unpublished report No. DuPont-1599 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
McFarlane, P. & Freestone, S. (1999) A randomised double blind ascending oral dose study with oxamyl. Unpublished report No. HLO-1998-01505 (2 volumes) from Inveresk Research International Ltd (Scotland), Tranent, Scotland. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Mebus, C.A. (1990) Chronic toxicity study with oxamyl (IN D1410-196) one-year feeding study in dogs. Unpublished report No. HLR 381-90 (2 volumes) from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Munley, S.M. 1998. DuPonts position on fetal weight changes in rats following developmental toxicity testing with oxamyl. Unpublished report No. DuPont-1954 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Rickard, L.B. (1987) Mutagenicity evaluation of IND1410-196 in the CHO/HPRT assay. Unpublished report No. HLR 265-82, Revision 1, from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Rickard, L.B. (1988) Teratology study of IN D1410-196 in the rat. Unpublished report No. HLR 473-88 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
San, R.H. & Clarke, J.J. (2000) Oxamyl technical: In vitro mammalian cell gene mutation (CHO/HGPRT) test with an independent repeat assay. Unpublished report No. DuPont-2937 from BioReliance, Rockville, Maryland, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Sherman, H. (1972) Long term feeding study in rats and dogs with 1-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)thioformimidic acid, methyl ester (IND-1410): Final report. Unpublished report No. HLR 37-72 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Sipple, M.E. (1978) Mutagenic activity of formamide, 1-cyano-N,N-dimethyl- in the Salmonella/microsome assay. Unpublished report No. HLR 284-78 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Snee, D.A. (1969) Ninety-day feeding study in rats with 1-(dimethylcarbamoyl)-N-(methylcarbamoyloxy)-thioformimidic acid, methyl ester (IND-1410). Unpublished report No. HLR 308-69 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Tayfun, F.O. (1969a) Acute dust inhalation toxicity (ChR-CD ratshead-onlyfour hours). Unpublished report No. HLR 280-69 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Tayfun, F.O. (1969b) One-hour inhalation toxicity (ChR-CD ratshead-only). Unpublished report No. HLR 281-69 from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Vincent, D.R. (1987) Assessment of IND1410-196 in the in vitro unscheduled DNA synthesis assay in rat primary hepatocytes. Unpublished report No. HLR 719-82, Revision 1 and Supplement 1, from DuPont Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
Wasileski, L.S. (1971) Ten-dose subacute oral tests. Unpublished report No. HLR 228-71 DuPont from Haskell Laboratory, Newark, Delaware, USA. Submitted to WHO by E.I. duPont de Nemours & Co., Wilmington, Delaware, USA.
WHO (2000) The WHO recommended classification of pesticides by hazard and guidelines to classification 20002202 (WHO/PCS/01.5). Available from the International Programme on Chemical Safety, World Health Organization, Geneva, Switzerland.
See Also:
Toxicological Abbreviations
Oxamyl (Pesticide residues in food: 1980 evaluations)
Oxamyl (Pesticide residues in food: 1983 evaluations)
Oxamyl (Pesticide residues in food: 1984 evaluations)
Oxamyl (Pesticide residues in food: 1985 evaluations Part II Toxicology)