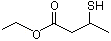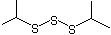WHO FOOD ADDITIVES SERIES: 52
SIMPLE ALIPHATIC AND AROMATIC SULFIDES AND THIOLS (addendum)
First draft prepared by
Dr A. Mattia
Division of Biotechnology and GRAS Notice Review, Office of Food Additive Safety, Center for Food Safety and Applied Nutrition, Food & Drug Administration, College Park, Maryland, USA
1. EVALUATION
1.1 Introduction
The Committee evaluated 12 flavouring agents that included simple aliphatic and aromatic sulfides and thiols (see Table 1) by the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1, Introduction).
Table 1. Summary of safety evaluations of simple aliphatic and aromatic sulfides and thiols used as flavouring agentsa,b,c
|
Flavouring agent |
No. |
CAS No. and structure |
Step B3 d Does intake exceed the threshold for human intake? |
Step B4 Adequate margin of safety for substances or related substances? |
Comments on predicted metabolism |
Conclusion based on current intake |
|
Subgroup ii—Acyclic sulfides with oxidized side chains Structural class I |
|
2-(Methylthio) ethanol |
1297 |
5271-38-5
 |
No
Europe: 1
USA: 0.9 |
Yes. The NOEL of 1.4 mg/kg bw per day (Cox et al., 1979) for the related substance 2-(methylthiomethyl)-3-phenylpropenal (No. 505) is >10 000 times the estimated daily intake of 2-(methylthio)ethanol when used as a flavouring agent |
See notes 7 and 2 |
No safety concern |
|
Ethyl 5-(methylthio)valerate |
1298 |
233665-98-0
 |
No
Europe: 2
USA: 2 |
Yes. The NOEL of 1.4 mg/kg bw per day (Cox et al., 1979) for the related substance 2-(methylthiomethyl)-3-phenylpropenal (No. 505) is >10 000 times the estimated daily intake of ethyl 5-(methylthio)valerate when used as a flavouring agent |
See notes 5 and 7 |
No safety concern |
|
Subgroup iii—Cyclic sulfides
Structural class III |
|
spiro(2,4-Dithia-1-methyl-8- oxabicyclo(3.3.0)octane-3,3’- (1’-oxa-2’-methyl)cyclopentane) |
1296 |
38325-25-6
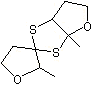 |
No
Europe: ND
USA: 2 |
Yes. The NOEL of 25 mg/kg bw per day for spiro(2,4-dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl)-cyclopentane) is >100 000 times the estimated daily intake when used as a flavouring agent |
See notes 10, 1 and 3 |
No safety concern |
|
Subgroup v—Thiols with oxidized side chains
Structural class I |
|
erythro- and threo-3-Mercapto-2-methylbutanol |
1289 |
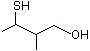 |
No
Europe: 1
USA: 2 |
Yes. The NOEL of 0.7 mg/kg bw per day (Cox et al., 1974) for the related substance 2-mercapto-3-butanol (No. 546) is >10 000 times the estimated daily intake of erythro- and threo-mercapto-2-methylbutan-1-ol when used as a flavouring agent |
See notes 1 and 2 |
No safety concern |
|
(±)2-Mercapto-2-methylpentan-1-ol |
1290 |
258823-39-1
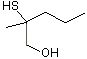 |
No
Europe: 3
USA: 4 |
Yes. The NOEL of 0.7 mg/kg bw per day (Cox et al., 1974) for the related substance 2-mercapto-3-butanol (No. 546) is >10 000 times the estimated daily intake of (±)2-mercapto-2-methylpentan-1-ol when used as a flavouring agent |
See notes 1 and 2 |
No safety concern |
|
3-Mercapto-2-methylpentan-1-ol (racemic) |
1291 |
227456-27-1
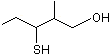 |
No
Europe: 1
USA: 0.7 |
Yes. The NOEL of 0.7 mg/kg bw per day (Cox et al., 1974) for the related substance 2-mercapto-3-butanol (No. 546) is >10 000 times the estimated daily intake of 3-mercapto-2-methylpentan-1-ol (racemic) when used as a flavouring agent |
See notes 1 and 2 |
No safety concern |
|
3-Mercapto-2-methylpentanal |
1292 |
227456-28-2
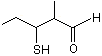 |
No
Europe: 3
USA: 4 |
Yes. The NOEL of 0.7 mg/kg bw per day (Cox et al., 1974) for the related substance 2-mercapto-3-butanol (No. 546) is >10 000 times the estimated daily intake of 3-mercapto-2-methylpentenal when used as a flavouring agent |
See notes 1 and 4 |
No safety concern |
|
4-Mercapto-4-methyl-2-pentanone |
1293 |
19872-52-7
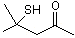 |
No
Europe: 0.01
USA: 0.02 |
Yes. The NOEL of 1.9 mg/kg bw per day (Morgareidge, 1971) for the related substance 3-mercapto-2-pentanone (No. 560) is >10 000 times the estimated daily intake of 4-mercapto-4-methyl-2-pentanone when used as a flavouring agent |
See notes 1 and 3 |
No safety concern |
|
(±)Ethyl 3-mercaptobutyrate |
1294 |
156472-94-5
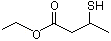 |
No
Europe: 4
USA: 4 |
Yes. The NOEL of 0.7 mg/kg bw per day (Cox et al., 1974) for the related substance 2-mercapto-3-butanol (No. 546) is >10 000 times the estimated daily intake of (±)ethyl 3-mercaptobutyrate when used as a flavouring agent |
See notes 1 and 5 |
No safety concern |
|
Subgroup vii—Simple disulfides
Structural class I |
|
2,3,5-Trithiahexane |
1299 |
42474-44-2
 |
No
Europe: 0.03
USA: 0.04 |
Yes. The NOEL of 0.3 mg/kg bw per day for the related substance3-methyl-1,2,4-trithiane (No. 574) (Mondino, 1981) is >10 000 times the estimated daily intake of 2,3,5-trithiahexane when used as a flavouring agent |
See notes 7, 8 and 9 |
No safety concern |
|
Subgroup ix—Trisulfides and polysulfides
Structural class I |
|
Diisopropyl trisulfide |
1300 |
5943-34-0
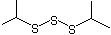 |
No
Europe: 0.006
USA: 0.007 |
Yes. The NOEL of 4.8 mg/kg bw per day (Morgareidge & Oser, 1970) for the related substance dipropyltrisulfide (No. 585) is >100 000 times the estimated daily intake of diisopropyl trisulfide when used as a flavouring agent |
See notes 7, 8 and 9 |
No safety concern |
|
Subgroup xi—Thioesters
Structural class I |
|
Ethyl 4-(acetylthio)butyrate |
1295 |
104228-51-5
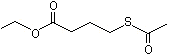 |
No
Europe: 4
USA: 4 |
Yes. The NOEL of 6.5 mg/kg bw per day (Shellenberger, 1970) for the related substance ethylthioacetate (No. 483) is >10 000 times the estimated daily intake of ethyl 4-(acetylthio)butyrate when used as a flavouring agent |
See notes 1, 5 and 6 |
No safety concern |
Notes to Table 1
|
CAS, Chemical Abstracts Service; ND, no data on intake reported |
|
a |
One hundred and thirty-seven (137) flavouring agents in this group were previously evaluated by the Committee (Annex 1, reference 144). To facilitate the evaluations, the group was divided into 12 subgroups based on the position of the sulfur atom. The subgroup designations are indicated in the table |
|
b |
Step 1: Eleven flavouring agents are in structural class I and one (No. 1296) is in structural class III |
|
c |
Step 2: All of the agents in this group cannot be predicted to be metabolized to innocuous products |
|
d |
The threshold for human intake for structural class I, II and III are 1800, 540 and 90 µg/day, respectively. All intake values are expressed in µg/day. The combined per capita intake of the 11 flavouring agents in structure class I is approximately 21 µg per day in Europe and 24 µg per day in the USA. The combined per capita intake of the remaining flavouring agent in structural class III is 2 µg per day in the USA. The cumulative per capita intake for the amended group as a whole including the 137 substances in the original evaluation and the 12 additional substances is 1181 and 1034 µg/person per day in Europe and the USA, respectively |
|
Notes: |
|
1. |
Sulfur is expected to be oxidized to sulfonic acid, undergo alkylation and conjugation followed by excretion |
|
2. |
The hydroxy group is expected to undergo oxidation to the carboxylic acid |
|
3. |
The ketone group is expected to be reduced to the alcohol, conjugated and subsequently excreted |
|
4. |
The aldehyde group is expected to be oxidized to the corresponding carboxylic acid, conjugated and subsequently excreted |
|
5. |
The ester is expected to undergo hydrolysis to the corresponding carboxylic acid and alcohol |
|
6. |
The thioester is expected to undergo hydrolysis to acetate and the corresponding thiol, which will be further oxidized |
|
7. |
The sulfur is expected to be oxidized to the sulfoxide and sulfone |
|
8. |
The di- or trisulfides are expected to be reduced to free thiols |
|
9. |
Free thiols may form mixed disulfides with glutathione or cysteine |
|
10. |
Thioketal will hydrolyse to liberate the corresponding ketone and dithiol |
At its fifty-third meeting, the Committee evaluated 137 other members of this chemical group of flavouring agents (Annex 1, reference 143). The group was divided into 12 subgroups on the basis of the position of the sulfur atom, in order to facilitate the assessment of the relevant data on metabolism and toxicity. All 137 substances in that group were concluded to be of no safety concern on the basis of currently estimated levels of intake.
Of the 12 additional flavouring agents considered in this monograph addendum, six agents are thiols with oxidized side chains (subgroup v) (Nos 1289–1294) and contain an additional alcohol, aldehyde, ketone, or ester functional group. Two agents are acyclic sulfides with oxidized side-chains (subgroup ii) (Nos 1297 and 1298) in which an alcohol or ester functional group is present. The remaining four substances are a thioester (subgroup xi) (No. 1295), a disulfide (subgroup vii) (No. 1299), a trisulfide (subgroup ix) (No. 1300) and a cyclic sulfide (subgroup iii) (No. 1296). None of these agents has been evaluated previously.
Seven of the 12 flavouring agents in this group are naturally occurring components of food (Nos 1291–1294, 1297, 1299, 1300) and have been detected in onions, fruits, broccoli, cabbage, cauliflower, hop oil, wine, fish and cheese (Darriet et al., 1995; Private communication to FEMA, 1996–2002; Maarse et al., 1999; Kendrick, 2000).
1.2 Estimated daily intake
The total annual volume of production of the 12 simple aliphatic and aromatic sulfides and thiols is approximately 150 kg in Europe and in the USA (Private communication to FEMA, 1996–2002; Lucas et al., 1999). The daily per capita intake of each agent is reported in Table 1. Annual volumes of production of this group of flavouring agents are summarized in Table 2.
Table 2. Annual volumes of production of simple aliphatic and aromatic sulfides and thiols used as flavouring agents
|
Agent (No.) |
Most recent annual volume (kg)a |
Intakeb ("eaters only") |
Intake of alcohol equivalents µg/kg bw per dayc |
Annual volume in naturally occurring foods (kg)d |
Consumption ratioe |
|
µg/day |
µg/kg bw per day |
|
erythro and threo-Mercapto-2-methylbutan-1-ol (1289) |
|
Europe |
10 |
1 |
0.02 |
|
|
|
|
USA |
10 |
2 |
0.03 |
|
- |
NA |
|
(±)2-Mercapto-2-methylpentanol (1290) |
|
Europe |
20 |
3 |
0.05 |
|
|
|
|
USA |
20 |
4 |
0.06 |
|
- |
NA |
|
3-Mercapto-2-methylpentan-1-ol (racemic) (1291) |
|
Europe |
10 |
1 |
0.02 |
|
|
|
|
USA |
4 |
0.7 |
0.01 |
|
+f |
NA |
|
3-Mercapto-2-methylpentanal (1292) |
|
Europe |
20 |
3 |
0.05 |
|
|
|
|
USA |
20 |
4 |
0.06 |
|
+f |
NA |
|
4-Mercapto-4-methyl-2-pentanone (1293) |
|
Europej |
0.1 |
0.01 |
0.0002 |
|
|
|
|
USAj |
0.1 |
0.02 |
0.0003 |
|
+g |
NA |
|
(±)Ethyl 3-mercaptobutyrate (1294) |
|
Europe |
25 |
4 |
0.06 |
0.02 |
|
|
|
USA |
25 |
4 |
0.07 |
0.02 |
+h |
NA |
|
Ethyl 4-(acetylthio)butyrate (1295) |
|
Europe |
25 |
4 |
0.06 |
0.01 |
|
|
|
USA |
25 |
4 |
0.07 |
0.02 |
- |
NA |
|
spiro(2,4-Dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl)cyclopentane (1296) |
|
Europe |
ND |
ND |
ND |
|
|
|
|
USAi |
14 |
2 |
0.04 |
|
- |
NA |
|
2-(Methylthio)ethanol (1297) |
|
Europe |
8 |
1 |
0.02 |
|
|
|
|
USA |
5 |
0.9 |
0.01 |
|
+ |
NA |
|
Ethyl 5-(methylthio)valerate (1298) |
|
Europe |
15 |
2 |
0.04 |
0.01 |
|
|
|
USA |
10 |
2 |
0.03 |
0.01 |
- |
NA |
|
2,3,5-Trithiahexane (1299) |
|
Europek |
0.2 |
0.03 |
0.0005 |
|
|
|
|
USAk |
0.2 |
0.04 |
0.001 |
|
+ |
NA |
|
Diisopropyl trisulfide (1300) |
|
Europel |
0.04 |
0.006 |
0.0001 |
|
|
|
|
USAl |
0.04 |
0.007 |
0.0001 |
|
+ |
NA |
|
Total Europe |
133 |
19 |
0.3 |
|
|
|
|
USA |
133 |
21 |
0.3 |
|
|
|
|
NA, not available; ND, no intake data reported; +, reported to occur naturally in foods (Maarse et al., 1999), but no quantitative data; -, not reported to occur naturally in foods |
|
a |
The volumes cited, unless otherwise indicated, are anticipated annual volumes (Private communication to FEMA 1996–2002), which were the maximum amount of the flavouring agent estimated to be used annually in both Europe and the USA by the manufacturer at the time the material was proposed for use as a flavouring agent |
|
b |
Intake (µg/person per day) was calculated as follows: |
| |
[(annual volume, kg) × (1 × 109 mg/kg)]/[population × survey correction factor × 365 days], |
| |
where population (10%, "eaters only") = 32 × 106 for Europe and 26 × 106 for the USA; |
| |
where correction factor = 0.6 for Europe and USA anticipated volumes and 0.8 for the Lucas et al. survey in the USA, representing the assumption that only 60% and 80% of the annual flavour volume, respectively, was reported in the poundage surveys (International Organization of the Flavor Industry, 1995; Lucas et al., 1999; Private communication to FEMA 1996–2002) |
| |
Intake (µg/kg bw/d) calculated as follows: |
| |
[(µg/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding |
|
c |
Calculated as follows: (molecular weight of alcohol/molecular weight of ester) × daily per capita intake ("eaters only") of ester |
|
d |
Quantitative data for the USA reported by Stofberg & Grundschober (1987) |
|
e |
The consumption ratio was calculated as follows: |
| |
(annual consumption via food, kg)/(most recent reported volume as a flavouring agent, kg) |
|
f |
Natural occurrence data reported in a private communication to FEMA (1996–2002) |
|
g |
Darriet et al., 1995 |
|
h |
Kendrick, 2000 |
|
i |
Annual volume reported in the 1995 FEMA Poundage Survey (Lucas et al., 1999) |
|
j |
Intake calculated based on 1% solution of 4-mercapto-4-methyl-2-pentanone in propylene glycol |
|
k |
Intake calculated based on a 10% solution of 2,3,5-trithiahexane in triacetin |
|
l |
Intake calculated based on a 2% solution of diisopropyl trisulfide in triacetin |
1.3 Absorption, distribution, metabolism and elimination
All of the sulfur-containing flavouring agents considered in this addendum are of low relative molecular mass and are sufficiently lipophilic to be absorbed. These flavouring agents are expected to be metabolized through various pathways, described below and in the previous evaluation by the Committee (Annex 1, reference 143).
Thiols with oxidized side-chains (Nos 1289–1294)
The metabolism of thiols with oxidized side-chains is predicted to involve a combination of pathways for simple thiols together with further oxidation or conjugation of the oxidized side-chain. Metabolic options for simple thiols include oxidation to form unstable sulfenic acids (RSOH) which are oxidized to sulfinic acids (RSO2H), undergo methylation to yield methyl sulfides which then form sulfoxides and sulfones, react with endogenous thiols to form mixed disulfides, are conjugated with glucuronic acid, or undergo oxidation of the alpha-carbon which results in desulfuration and the formation of an aldehyde (Dutton & Illing, 1972; McBain & Menn, 1969; Maiorino et al., 1988; Richardson et al., 1991).
Acyclic sulfides with oxidized side-chains (Nos 1297 and 1298)
The presence of oxygenated functional groups, such as an alcohol (No. 1297) or ester (No. 1298), provides additional sites for biotransformation of sulfides (thioethers), and the presence of these polar sites would result in increased renal excretion of these agents. The biotransformation of such oxygenated groups is well characterized and has been described for groups of flavouring agents evaluated previously by the Committee (Annex 1, references 131, 132, 138, 144). Simultaneous metabolism of sulfur and oxygenated functional groups has been reported for various substrates (Fatih et al., 1988; Gachon et al., 1988; Feng & Solsten, 1991; Wilson et al., 1991; Black et al., 1993). Sulfoxide formation is usually the predominant metabolic detoxication pathway.
Cyclic sulfides (No. 1296)
Cyclic sulfides can be expected to undergo extensive S-oxidation by the cytochrome P450 superfamily to produce the corresponding sulfoxides.
Simple disulfides (No. 1299)
The reduction of xenobiotic disulfides is believed to be extensive and can be catalysed enzymatically, by glutathione reductase (Waring, 1996) or thioltransferases (Wells et al., 1993), as well as chemically, by exchange with glutathione, thioredoxin, cysteine or other endogenous thiols. Reduction of non-cyclic disulfides (No. 1299) would result in the formation of thiols of low molecular mass, which are metabolized via the various pathways described above for simple thiols.
Trisulfides (No. 1300)
The trisulfide of glutathione is labile and readily converted to the disulfide, with the release of sulfur as hydrogen sulfide (Moutiez et al., 1994). Trisulfides are predicted to be converted rapidly to the corresponding disulfides with subsequent reduction to thiols, which would then be metabolized via the various pathways described above for simple thiols.
Thioesters (No. 1295)
Thioesters are hydrolysed by lipase and esterases (Kurooka et al., 1976); the rate of hydrolysis increases as the length of the carbon chain increases and decreases as the oxygenation of the carbon chain in the thiol moiety increases (Greenzaid & Jenks, 1971). After hydrolysis, the resulting alcohol and carboxylic acid would participate in the metabolic pathways described above for sulfides containing oxygenated functional groups.
1.4 Application of the procedure for the safety evaluation of flavouring agents
|
Step B1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents to these 12 flavouring agents, the Committee assigned 11 agents (Nos 1289–1295, 1297–1300) to structural class I. The remaining flavouring agent (No. 1296) was assigned to class III (Cramer et al., 1978). |
|
Step B2. |
At currently estimated levels of intake, none of the flavouring agents in this group is predicted to be metabolized to innocuous products. The evaluation of these substances therefore proceeded via the B-side of the decision-tree. |
|
Step B3. |
The estimated daily per capita intakes of the 11 flavouring agents in this group in structural class I are below the threshold of concern (i.e. 1800 µg). The estimated daily per capita intake for the one flavouring agent in structural class III is below the threshold of concern (i.e. 90 µg). Accordingly, the evaluation of all 12 agents in the group proceeded to step B4. |
|
Step B4. |
For erythro- and threo-3-mercapto-2-methylbutanol (No. 1289), the NOEL of 0.7 mg/kg body weight per day for the structurally related substance 2-mercapto-3-butanol (No. 546) from a 92-day study in rats fed by gavage (Cox et al., 1974) provides an adequate margin of safety (>10 000) in relation to known levels of intake of this agent. This NOEL is also appropriate for the structurally related agents (±)-2-mercapto-2-methylpentan-1-ol (No. 1290), 3-mercapto-2-methylpentan-1-ol (racemic) (No. 1291), 3-mercapto-2-methylpentanal (No. 1292), and (±)-ethyl 3-mercaptobutyrate (No. 1294), because they are all acyclic thiols with oxidized side-chains that are anticipated to undergo oxidation or hydrolysis and subsequent metabolism via similar metabolic pathways. |
| |
For 4-mercapto-4-methyl-2-pentanone (No. 1293), the NOEL of 1.9 mg/kg bw per day for the structurally related substance 3-mercapto-2-pentanone (No. 560) administered to rats by gavage in a 92-day study (Morgareidge, 1971) provides an adequate margin of safety (>10 000) in relation to known levels of intake of this agent. |
| |
For ethyl 4-(acetylthio)butyrate (1295), the NOEL of 6.5 mg/kg bw per day reported in a 13-week study in rats (Shellenberger, 1970) fed with the structurally related substance ethylthioacetate (No. 483) provides an adequate margin of safety (>10 000) in relation to known levels of intake of this agent. |
| |
For ethyl 2-(methylthio)ethanol (No. 1297), the NOEL of 1.4 mg/kg bw per day reported in a 13-week study in rats (Cox et al., 1979) fed by gavage with the structurally related substance 2-(methylthiomethyl)-3-phenylpropenal (No. 505) provides an adequate margin of safety (>10 000) in relation to known levels of intake of this agent. This NOEL is also appropriate for the structurally related agent ethyl 5-(methylthio)valerate (No. 1298), which is also an acyclic sulfide with an oxidized side-chain that is anticipated to undergo oxidation and subsequent metabolism via similar pathways. |
| |
For 2,3,5-trithiahexane (No. 1299), the NOEL of 0.3 mg/kg bw per day reported in a 13-week study (Mondino, 1981) in rats fed with the structurally related substance 3-methyl-1,2,4-trithiane (No. 574) provides an adequate margin of safety (>10 000) in relation to known levels of intake of this agent. |
| |
For diisopropyl trisulfide (No. 1300), the NOEL of 4.8 mg/kg bw per day reported in a 13-week study (Morgareidge & Oser, 1970) in rats fed by gavage with the structurally related substance dipropyltrisulfide (No. 585) provides an adequate margin of safety (>100 000) in relation to known levels of intake of this agent. |
| |
For spiro(2,4-dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl)-cyclopentane) (No. 1296), the NOEL of 25 mg/kg bw per day in the diet reported in a 13-week study in rats (Wheldon et al., 1970) provides an adequate margin of safety (>100 000) in relation to known levels of intake of this agent. |
Table 1 summarizes the evaluations of the 12 simple aliphatic and aromatic sulfides and thiols in this group.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that the 11 agents considered in this evaluation and the 97 agents considered previously in structural class I were to be consumed concurrently on a daily basis, the estimated combined intake would not exceed the daily per capita human intake threshold for class I (1800 µg). In the unlikely event that the one agent considered in this evaluation and the six agents considered previously in structural class III were to be consumed concurrently on a daily basis, the estimated combined daily per capita intake would not exceed the human intake threshold for class III (90 µg).
1.6 Consideration of secondary components
One member of this group of flavouring agents (No. 1293, 4-mercapto-4-methyl-2-pentanone) has a minimum assay value of <95%. Information on the safety of the secondary component of this compound is summarized in Annex 6 (Summary of the safety evaluation of secondary components of flavouring agents with minimum assay values of less than 95%). The secondary component (4-methyl-3-penten-2-one) was evaluated by the Committee at its fifty-ninth meeting, and was considered not to present a safety concern at current levels of intake.
1.7 Conclusion
The Committee concluded that these 12 flavouring agents, which are additions to the group of simple aliphatic and aromatic sulfides and thiols evaluated previously, would not give rise to safety concerns at the currently estimated levels of intake.
2. RELEVANT BACKGROUND INFORMATION
2.1 Additional considerations on intake
There is no additional information on intake.
2.2 Biological data
2.2.1 Biochemical data: absorption, distribution, metabolism and excretion
No significant changes in the absorption, distribution, metabolism, and excretion of these agents have been reported since the submission of the original monograph (Annex 1, reference 143). Some references not included in the original group monograph are cited in section 1.3.
2.2.2 Toxicological studies
(a) Acute toxicity
There are no additional studies of acute toxicity. No significant changes in the acute toxicity of flavouring agents in this group have been reported since the submission of the original monograph (Annex 1, reference 143).
(b) Short-term studies of toxicity
The results of one additional 90-day study are described below and are summarized in Table 3.
Table 3. Results of short-term studies of toxicity with simple aliphatic and aromatic sulfides and thiols
|
No. |
Flavouring agent |
Species; sex |
No. of test groupsa/no. per groupb |
Route |
Duration (days) |
NOEL (mg/kg bw per day) |
Reference |
|
1296 |
spiro(2,4-Dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl) cyclopentane) |
Rat; M |
3/15 |
Diet |
90 |
25 |
Wheldon et al. (1970) |
|
M, male |
|
a |
Total number of test groups does not include control animals |
|
b |
Total number per test group includes both male and female animals |
(i) Spiro(2,4-Dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl)cyclopentane) (No. 1296)
In a 90-day study, groups of 15 male Cfy Wistar rats were fed diets containing spiro(2,4 -dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl)cyclopentane) at a concentration of 0, 500, 5000 or 5000 ppm (raised to 10 000 ppm after week 1 and to 20 000 ppm at week 6) (Wheldon et al., 1970). The three lower concentrations correspond to estimated intakes of 0, 25 and 250 mg/kg bw per day, respectively (Food and Drug Administration, 1993). Rats in the group receiving the highest dose were initially given an estimated intake of 250 mg/kg bw per day, which was increased to 500 mg/kg bw per day after week 1, and finally to 1000 mg/kg bw per day at week 6. Clinical observations were performed daily. Animals were housed five per cage and had access to food and water ad libitum.
A decrease in food consumption, which was presumably a result of the unpalatability of the diet containing the malodorous sulfur-containing test agents, was reported in all treated groups throughout the study. Weekly measurement of body weights showed a decrease in body-weight gain at the two higher doses throughout the study, and at the lowest dose only after 8 weeks. The calculated food conversion efficiency was significantly reduced only at the two higher doses. Haematological examination revealed decreases in erythrocyte volume fraction (p <0.01), concentration of haemoglobin (p <0.001) and erythrocyte count (p <0.001), and increases in corpuscular volume (p <0.001), neutrophil (p <0.001) and lymphocyte counts (p <0.01) at week 13 in the group given the highest dose. Similar changes were also reported for the group given the intermediate dose, but the decreases in erythrocyte volume fraction and differential leukocyte counts were not statistically significant. The animals in the group given the highest dose also showed abnormal blood pigmentation. Haematological measurements for the group given the low dose and for the control group were comparable throughout the study. At necropsy, measurement of organ weights (adrenals, heart, kidneys, liver, lungs, spleen, testes, and thyroid) revealed increased absolute and relative spleen weights at the highest dose. Increases in relative (to body weights) but not absolute organ weights at the two higher doses were associated with the significant decrease in body weights reported for these two groups. Histopathological examination of 13 different tissues revealed pigmented macrophages in the liver and spleen of the animals in the group receiving the highest dose. The authors reported a NOEL of 250 mg/kg bw per day (Wheldon et al., 1970). Owing to the decreased body-weight gain and significantly reduced food conversion efficiency reported at this dose, however, a NOEL of 25 mg/kg bw per day is more conservative. The results of this study are summarized in Table 3.
(b) Genotoxicity
The results of one additional test for genotoxicity in vitro are described below and summarized in Table 4 (Annex 1, reference 144).
Table 4. Results of studies of genotoxicity with simple aliphatic and aromatic sulfides and thiols
|
No. |
Flavouring agent |
End-point |
Test system |
Concentration |
Results |
Reference |
|
In vitro |
|
|
|
|
|
|
|
1289 |
Erythro- and threo-3-mercapto- 2-methylbutanol |
Reverse mutation |
S. typhimurium TA1535, TA97, TA98, TA100, TA102 |
50–5000 µg/ plate |
Negativea |
Gocke (1997) |
a With and without metabolic activation from S9
Erythro- and threo-3-mercapto-2-methylbutanol (No. 1289) (50–5000 µg/plate) was evaluated for mutagenic activity in the modified Ames test with preincubation in the presence and absence of metabolic activation in Salmonella typhimurium strains TA1535, TA97, TA98, TA100 and TA102. No genotoxic effects were observed (Gocke, 1997).
3. REFERENCES
Black, R.M., Brewster, K., Clarke, R.J., Hambrook, J.L., Harrison, J.M. & Howells, D.J. (1993) Metabolism of thioglycol (2,2’-thiobis-ethanol): Isolation and identification of urinary metabolites following intraperitoneal administration to rats. Xenobiotica, 23, 473–481.
Cox, G.E., Bailey, D.E. & Morgareidge, K. (1974) 90-Day feeding studies in rats with compound 14935 (2-mecapto-3-butanol). Unpublished report by Food & Drug Research Laboratories, Inc. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States of America, Washington, DC, USA.
Cox, G.E., Rucci, G. & Babish, J.G. (1979) 90-Day subacute dietary toxicity study of 78-002-2 in Sprague-Dawley rats. Unpublished report by Food & Drug Research Laboratories, Inc. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States of America, Washington, DC, USA.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard—a decision tree approach. Food. Cosmet. Toxicol., 16, 255–276.
Darriet, P., Tominaga, T., Lavigne, V., Boidron, J.-N. & Dubourdieu, D. (1995) Identification of a powerful aromatic component of Vitis vinifera L. var. Sauvignon wines: 4-mercapto-4-methylpentan-2-one. Flavour and Fragrance Journal, 10, 385–392.
Dutton, G.J. & Illing, H.P.A. (1972) Mechanism of biosynthesis of thio-beta-D-glucosides. Biochem. J., 129, 539–550.
Fatih, E., Karim, I.A., Millership, J.S., Temple, D.J. & Woolfson, A.D. (1988) An investigation of the metabolism of S-carboxymethyl-L-cysteine in man using a novel HPLC-ECD method. Eur. J. Drug Metab. Pharmacokin., 13, 253.
Feng, P.C.C. & Solsten, R.T. (1991) In vitro transformation of dithiopyr by rat liver enzymes: Conversion of methylthioesters to acids by oxygenases. Xenobiotica, 21, 1265.
Gachon, F., Nicolas, C., Maurizis, C., Verny, M., Chabard, J.L., Faurie, M. & Gaillard, G. (1988) Disposition and metabolism of letosteine in rats. Drug Metab. Dispos., 253.
Gocke, E. (1997) Evaluation of the mutagenic potential of Ro 84-1446/000 in the Ames test (Study No.: 134M97). Private communication to the Flavor and Extract Manufacturers Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States of America, Washington, DC, USA.
Greenzaid, P. & Jenks, W.P. (1971) Pig liver esterase. Reactions with alcohols, structure-reactivity correlations, and the acyl-enzyme intermediate. Biochemistry, 10, 1210.
International Organization of the Flavor Industry (1995). European inquiry on volume use. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Kendrick, L. (2000) Identification of ethyl 3-mercaptobutyrate in mango. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Kurooka, S., Hashimoto, M., Tomita, M., Maki, A. & Yoshimura, Y. (1976) Relationship between the structure of S-acyl thiol compounds and their rates of hydrolysis by pancreatic lipase and hepatic carboxylic esterase. J. Biochem., 79, 533–541.
Lucas, C.D., Putnam, J.M. & Hallagan, J.B. (1999) Flavor and Extract Manufacturers Association of the United States 1995 Poundage and Technical Effects Update Survey, Washington DC: Flavor and Extract Manufacturers Association of the United States.
Maarse, H., Visscher, C.A., Willemsens, L.C. & Boelens, M.H. (1999) Volatile Components in Food Qualitative and Quantitative Data. Centraal Instituut Voor Voedingssonderzioek TNO., Zeist, The Netherlands.
Maiorino, R.M., Bruce D.C., & Aposhian H.V. (1988) Determination and metabolism of dithiol chelating agents VI. Isolation and identification of the mixed disulfides of meso-2,3-dimercaptosuccinic acid with l-cysteine in human urine. Toxicol. Appl. Pharmacol., 97, 338.
McBain, D.A. & Menn, J.J. (1969) S-methylation, oxidation, hydroxylation, and conjugation of thiophenol in the rat. Biochem. Pharmacol., 18, 2282–2285.
Mondino, A. (1981) Thirteen week repeated dose study of the test article TT191 (3-methyl-1,2,4-trithiane) orally administered to Sprague-Dawley Charles River CD(SD)BR rats. Unpublished report from Instituto di richierche biomedicine "Antoine Marxer" SpA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Morgareidge, K. (1971) 90-Day feeding study with 2-keto-3-pentanthiol in rats. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Morgareidge, K. & Oser, B.L. (1970) 90-Day feeding studies in rats with dipropyltrisulfide (30204). Unpublished report by Food & Drug Research Laboratories, Inc. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Moutiez, M., Aumercier, M., Tessier, E., Parmentier, B., Tartar, A. & Sergheraert, C. (1994) Reduction of a trisulfide derivative of glutathione by glutathione reductases. Biochem. Biophys. Res. Commun., 202, 1380–1386.
Private communication to the Flavor and Extract Manufacturers Association (FEMA). (1996–2002) Submitted to WHO by FEMA, Washington, DC, USA.
Richardson K.A., Edward V.T., Jones B.C., & Hutson D.H. (1991) Metabolism in the rat of a model xenobiotic plant metabolite S-benzyl-N-malonyl-l-cysteine. Xenobiotica, 21, 371.
Shellenberger, T.E. (1970) Subacute toxicity evaluation of ethyl thioacetate in rats: Final report. Unpublished report GSRI Project NC-373 from Gulf South Research Institute Association. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC, USA.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfumer Flavorist, 12, 27.
Waring, R.H. (1996) Sulfur–sulfur compounds. In: Mitchell, S.C., ed., Biological Interactions of Sulfur Compounds. London: Taylor & Francis, pp. 145–173.
Wells, W.W., Yang, Y., Diets, T.L. & Gan, Z.R. (1993) Thioltransferases. Adv. Enzymol. Relat. Areas Mol. Biol., 66, 149–201.
Wheldon, G.H., Amyes, S.J., Street, A.E., Hague, P.H. & Mawdesley-Thomas, L.E. (1970) Toxicity of Wa4295 [spiro(2,4-dithia-1-methyl-8-oxabicyclo(3.3.0)octane-3,3’-(1’-oxa-2’-methyl)-cyclopentane)], Sa927, Stl3048 and Wa3328. Unpublished report from Huntingdon Research Centre. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington, DC.
Wilson, J.E., Chissick, H., Fowler, A.M., Frearson, F.J., Gittins, M. & Swinbourne, F.J. (1991) Metabolism of benzothiazole I. Identification of ring-cleavage products. Xenobiotica, 21, 1179.