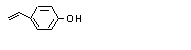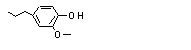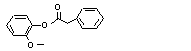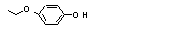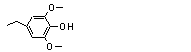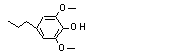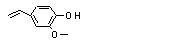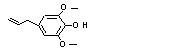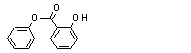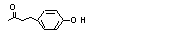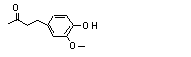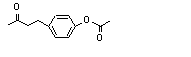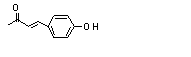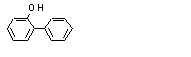WHO FOOD ADDITIVES SERIES 46:PHENOL AND PHENOL DERIVATIVES
First draft prepared by Dr A. Mattia
Division of Product Policy, Office of Premarket Approval, Center for Food Safety and Applied Nutrition, US Food and Drug Administration,
Washington DC, USA
and
Professor G.I. Sipes
Department of Pharmacology and Toxicology, College of Pharmacy, University of Arizona, Tucson, Arizona, USA
1. EVALUATION
1.1 Introduction
The Committee evaluated a group of 48 flavouring agents that includes phenol, an ester of phenol, and resorcinol; alkyl-, alkenyl-, or aryl-substituted phenols and their corresponding esters; alkoxy phenols; and phenol derivatives with alkyl side-chains containing a ketone function (see Table 1). The evaluations were conducted according to the Procedure for the Safety Assessment of Flavouring Agents.
2-Phenylphenol (No. 735) was evaluated by the Committee at its eighth meeting, when an ADI of 0-0.2 mg/kg bw was established (Annex 1, reference 8). The 1999
Table 1. Summary of the results of safety evaluations of phenol and 47 phenol derivatives used as flavouring agentsa
|
Flavouring agent |
No. |
CAS no. and structure |
Step A3b
Does intake exceed the threshold for human intake?b |
Step A4
Is the substance or are its metabolites endogenous? |
Step A5
Adequate NOEL for substance or related substance? |
Comments |
Conclusion based on current intake |
|
Structural class I
Phenol |
690 |
108-95-2
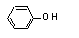
|
No
Europe: 6
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
ortho-Cresol |
691 |
95-48-7
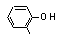
|
No
Europe: 290
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
meta-Cresol |
692 |
108-39-4
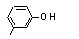
|
No
Europe: 0.1
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
para-Cresol |
693 |
106-44-5
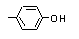
|
No
Europe: 1
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
para-Ethylphenol |
694 |
123-07-9
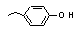
|
No
Europe: 4
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
ortho-Propylphenol |
695 |
644-35-9
|
No
Europe: 0.1
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
para-Propylphenol |
696 |
645-56-7
 |
No
Europe: 0.1
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2-Isopropylphenol |
697 |
88-69-7
 |
No
Europe: 16
USA: 0.3 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-(1,1-Dimethyl)ethyl-phenol |
733 |
98-54-4
 |
No
Europe: 0.01
USA: 0.001 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Phenyl acetate |
734 |
122-79-2
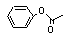 |
No
Europe: 0.07
USA: 0.01 |
N/R |
N/R |
See note 2. |
No safety concern |
|
ortho-Tolyl acetate |
698 |
533-18-6
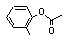 |
No
Europe: 0.1
USA: 40 |
N/R |
N/R |
See note 2. |
No safety concern |
|
para-Tolyl acetate |
699 |
140-39-6
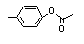 |
No
Europe: N/D
USA: 70 |
N/R |
N/R |
See note 2. |
No safety concern |
|
ortho-Tolyl isobutyrate |
700 |
36438-54-7
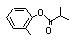 |
No
Europe: 0.03
USA: 0.1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
para-Tolyl isobutyrate |
701 |
103-93-5
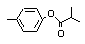 |
No
Europe: 0.04
USA: 0.01 |
N/R |
N/R |
See note 2. |
No safety concern |
|
para-Tolyl-3-methyl butyrate |
702 |
55066-56-3
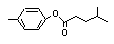 |
No
Europe: 0.4
USA: 0.1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
para-Tolyl octanoate |
703 |
59558-23-5
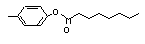 |
No
Europe: 0.03
USA: 1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
para-Tolyl laurate |
704 |
10024-57-4
 |
No
Europe: N/D
USA: 0.3 |
N/R |
N/R |
See note 2. |
No safety concern |
|
para-Tolyl phenyl-acetate |
705 |
101-94-0
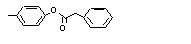 |
No
Europe: 0.7
USA: 0.1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
2,5-Xylenol |
706 |
95-87-4
 |
No
Europe: 1
USA: 0.03 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2,6-Xylenol |
707 |
576-26-1
 |
No
Europe: 2
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
3,4-Xylenol |
708 |
95-65-8
 |
No
Europe: 7
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2,3,6-Trimethylphenol |
737 |
2416-94-6
 |
No
Europe: 0.3
USA: 0.3 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Thymol |
709 |
89-83-8
 |
No
Europe: 59
USA: 160 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Carvacrol |
710 |
499-75-2
 |
No
Europe: 16
USA: 0.3 |
N/R |
N/R |
See note 1. |
No safety concern |
|
para-Vinylphenol |
711 |
2628-17-3
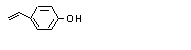 |
No
Europe: 0.1
USA: 6 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Resorcinol |
712 |
108-46-3
 |
No
Europe: 1
USA: 0.3 |
N/R |
N/R |
See notes 1. |
No safety concern |
|
Guaiacol |
713 |
90-05-1
 |
No
Europe: 51
USA: 16 |
N/R |
N/R |
See note 1. |
No safety concern |
|
ortho-(Ethoxymethyl)-phenol |
714 |
20920-83-6
 |
No
Europe: 2
USA: 0.01 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2-Methoxy-4-methyl-phenol |
715 |
93-51-6
 |
No
Europe: 37
USA: 3 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-Ethylguaiacol |
716 |
2785-89-9
 |
No
Europe: 8
USA: 0.4 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2-Methoxy-4-propyl-phenol |
717 |
6627-88-9
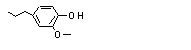 |
No
Europe: 210
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Guaiacyl phenylacetate |
718 |
613-70-7
 |
No
Europe: 0.01
USA: 0.1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Guaiacyl phenylacetate |
719 |
4112-89-4
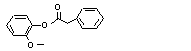 |
No
Europe: 0.4
USA: 2 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Hydroquinone mono-ethyl ether |
720 |
622-62-8
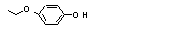 |
No
Europe: N/D
USA: 0.4 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2,6-Dimethoxyphenol |
721 |
91-10-1
 |
No
Europe: 6
USA: 12 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-Methyl-2,6-dimethoxy-phenol |
722 |
6638-05-7
 |
No
Europe: N/D
USA: 0.04 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-Ethyl-2,6-dimethoxy-phenol |
723 |
14059-92-8
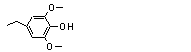 |
No
Europe: N/D
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-Propyl-2,6-dimethoxy-phenol |
724 |
6766-82-1
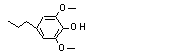 |
No
Europe: N/D
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2-Methoxy-4-vinyl-phenol |
725 |
7786-61-0
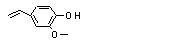 |
No
Europe: 3
USA: 1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-Allyl-2,6-dimethoxy-phenol |
726 |
6627-88-9
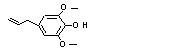 |
No
Europe: 0.01
USA: 6 |
N/R |
N/R |
See note 1. |
No safety concern |
|
2-Hydroxyaceto-phenone |
727 |
118-93-4
 |
No
Europe: 0.1
USA: 8 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Phenyl salicylate |
736 |
118-55-8
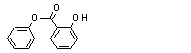 |
No
Europe: 9
USA: 8 |
N/R |
N/R |
See note 1 |
No safety concern |
|
4-(para-Hydroxy-phenyl)-2-butanone |
728 |
5471-51-2
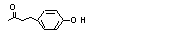 |
No
Europe: 2800
USA: 3800 |
N/R |
N/R |
Yes. The NOEL of 280 mg/kg bw per day in a 13-week study in rats given multiple doses (Gaunt et al., 1970) is > 1000 times the daily intakes of 46 µg/kg bw in Europe and 63 µg/kg bw in the USA. |
No safety concern |
|
Dihydroxyaceto-phenone |
729 |
28631-86 9
 |
No
Europe: 0.01
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Zingerone |
730 |
122-48-5
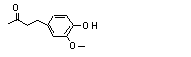 |
No
Europe: 40
USA: 83 |
N/R |
N/R |
See note 1. |
No safety concern |
|
4-(para-Acetoxyphenyl)-2-butanone |
731 |
3572-06-3
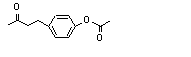 |
No
Europe: N/D
USA: 0.1 |
N/R |
N/R |
See note 2. |
No safety concern |
|
Vanillylidene acetone |
732 |
1080-12-2
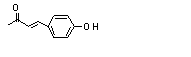 |
No
Europe: N/D
USA: 0.1 |
N/R |
N/R |
See note 1. |
No safety concern |
|
Structural class III
2-Phenylphenolc |
735 |
90-43-7
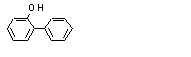 |
No
Europe: 0.01
USA: 0.01 |
N/R |
N/R |
See note 1. |
No safety concern |
CAS, Chemical Abstracts Service; N/D, no intake data reported; N/R, not required for evaluation because consumption of the substance was determined to be of no safety concern at Step 3 of the Procedure.
a
Step 2: All of the substances in this group are expected to be metabolized to innocuous products.
b
The thresholds for human intake are 1800 µg/day for structural class I and 90 µg/day for class III. All intake values are expressed in µg/day.
c
An ADI of 0-0.4 mg/kg bw has been established for 2-phenylphenol (FAO, 1999).
Notes:
1. Detoxication of phenol primarily involves conjugation of the hydroxyl group with sulfate and glucuronic acid.
2. Phenyl acetate undergoes rapid hzydrolysis followed by conjugation with sulfate and glucuronic acid.
Joint FAO/WHO Meeting on Pesticide Residues evaluated 2-phenylphenol and established an ADI of 0-0.4 mg/kg bw (FAO, 1999).
Thirty-two of the 48 flavouring agents in this group are natural components of foods. They have been detected in berries, coffee, and meat.
1.2 Estimated daily intake
The total annual volume of production of the 48 phenols considered here is approximately 25 t in Europe (International Organization of the Flavor Industry, 1995) and 32 t in the USA (National Academy of Sciences, 1987; Lucas et al., 1999; Table 2). About 78% of the total annual volume in Europe and 90% of that in the USA is accounted for by 4-(para-hydroxyphenyl)-2-butanone (raspberry ketone, No. 728 and the estimated intake of this substance is 2.8 mg/day in Europe and 3.8 mg/day in the USA.
Other flavouring agents for which the estimated intakes are in the range 37-300 µg/day include ortho-cresol (No. 691) (290 m g/day in Europe), ortho- and para-tolyl acetates (Nos 698 and 699) (40 µg/day and 70 µg/day, respectively, in the USA), 2-methoxy-4-methylphenol (No. 715) (37 µg/day in Europe), thymol (No. 709) (59 µg/day in Europe and 160 µg/day in the USA), guaiacol (No. 713) (51 µg/day in Europe), 2-methoxy-4-propylphenol (No. 717) (210 µg/day in Europe), and zingerone (No. 730) (40 µg/day in Europe and 83 µg/day in the USA). The intakes of all other flavouring agents in the group the intake are in the range 0.01-16 µg/day. For seven of the flavouring agents used in the USA, the annual production volumes in Europe were not reported.
1.3 Metabolic considerations
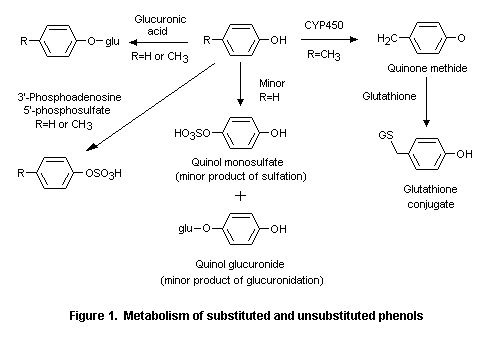
Phenol (No. 690) and its derivatives are rapidly absorbed from the gastrointestinal tract and share common pathways of metabolism (Hughes & Hall, 1995). Phenol (No. 690), phenyl acetate (No. 734), resorcinol (No. 712), and alkyl-, alkenyl-, and aryl-substituted phenols and their corresponding esters (Nos 691-697, 698-711, 733, 735, 737) are conjugated with sulfate and glucuronic acid after hydrolysis of the esters and excreted primarily in the urine. Other metabolic pathways, observed mainly at high doses, include ring hydroxylation and side-chain oxidation. Phenols containing alkoxy groups (Nos 713-717, 720-726) and those that contain a ketone function on an alkyl side-chain (Nos 727-732, 736) are also metabolized mainly by conjugation.
Alternative metabolic pathways include dealkylation of alkoxyphenols, reduction of side-chain ketones, side-chain oxidation, and ring hydroxylation. At very high doses (> 500 mg/kg bw), small amounts of para-cresol (No. 693), para-ethylphenol (No. 694), 2-methoxy-4-methyl phenol (No. 715), 2-methoxy-4-propylphenol (No. 717), 2-methoxy-4-vinylphenol (No. 725), and 4-allyl-2,6-dimethoxyphenol (No. 726) are oxidized to reactive quinone methide intermediates (Thompson et al., 1994, 1995a,b; Figure 1). Given the presence of a detoxication pathway (glutathione conjugation) for such quinone methides, the safety of the intermediates potentially formed after high doses of the specified phenol derivatives would not be a concern under the conditions of their use as flavouring agents.
Table 2. Annual volume and intake of phenol and phenol derivatives used as flavouring substances in Europe and the USA
|
Substance (No.) |
Most recent annual volume (kg)a |
Intakeb |
Annual volume in naturally occurring foods (kg)d |
Consumption ratioe |
|
µg/day |
µg/kg bw per day |
para-Cresol equivalents (mg/kg bw per day)c |
|
Phenol (690) |
|
Europe |
43 |
6 |
0.1 |
|
41 193 |
960 |
|
USA |
5 |
1 |
0.01 |
|
41 193 |
8 200 |
|
ortho-Cresol (691) |
|
Europe |
2039 |
290 |
5 |
|
7 451 |
3.6 |
|
USA |
0.5 |
0.1 |
0.001 |
|
7 451 |
15 000 |
|
meta-Cresol (692) |
|
Europe |
1 |
0.1 |
0.002 |
|
4 534 |
4 500 |
|
USA |
0.5 |
0.1 |
0.001 |
|
4 534 |
9 100 |
|
para-Cresol (693) |
|
Europe |
8 |
1 |
0.02 |
|
7 995 |
1 000 |
|
USA |
8 |
1 |
0.02 |
|
7 995 |
1 000 |
|
para-Ethylphenol (694) |
|
Europe |
29 |
4 |
0.1 |
|
10 235 |
350 |
|
USA |
1 |
0.1 |
0.002 |
|
10 235 |
10 000 |
|
ortho-Propylphenol (695) |
|
Europe |
1 |
0.1 |
0.002 |
|
+ |
NA |
|
USA |
10 |
1 |
0.02 |
|
+ |
NA |
|
para-Propylphenol (696) |
|
Europe |
0.4 |
0.1 |
0.001 |
|
+ |
NA |
|
USA |
0.5 |
0.1 |
0.001 |
|
+ |
NA |
|
2-Isopropylphenol (697) |
|
Europe |
112 |
16 |
0.3 |
|
+ |
NA |
|
USA |
2 |
0.3 |
0.004 |
|
+ |
NA |
|
4-(1,1-Dimethyl)ethyl phenol (733) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
Phenyl acetate (734) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
ortho-Tolyl acetate (698) |
|
Europe |
1 |
0.1 |
0.002 |
|
- |
NA |
|
USA |
300 |
40 |
1 |
|
- |
NA |
|
para-Tolyl acetate (699) |
|
Europe |
N/D |
N/D |
N/D |
N/D |
- |
NA |
|
USA |
530 |
70 |
1 |
0.7 |
- |
NA |
|
ortho-Tolyl isobutyrate (700) |
|
Europe |
0.2 |
0.03 |
0.0005 |
|
- |
NA |
|
USA |
1 |
0.1 |
0.002 |
|
- |
NA |
|
para-Tolyl isobutyrate (701) |
|
Europe |
0.3 |
0.04 |
0.0007 |
0.0006 |
- |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
0.0001 |
- |
NA |
|
para-Tolyl 3-methylbutyrate (702) |
|
Europe |
3 |
0.4 |
0.007 |
0.003 |
- |
NA |
|
USA |
0.9 |
0.1 |
0.002 |
0.001 |
- |
NA |
|
para-Tolyl octanoate (703) |
|
Europe |
0.2 |
0.03 |
0.0005 |
|
- |
NA |
|
USA |
7 |
1 |
0.02 |
|
- |
NA |
|
para-Tolyl laurate (704) |
|
Europe |
N/D |
N/D |
N/D |
|
- |
NA |
|
USA |
2 |
0.3 |
0.004 |
|
- |
NA |
|
para-Tolyl phenylacetate (705) |
|
Europe |
5 |
0.7 |
0.01 |
0.006 |
- |
NA |
|
USA |
0.9 |
0.1 |
0.002 |
0.001 |
- |
NA |
|
2,5-Xylenol (706) |
|
Europe |
4 |
1 |
0.009 |
|
1 673 |
420 |
|
USA |
0.2 |
0.03 |
0.0004 |
|
1 673 |
8 400 |
|
2,6-Xylenol (707) |
|
Europe |
14 |
2 |
0.03 |
|
558 |
40 |
|
USA |
4 |
1 |
0.009 |
|
558 |
140 |
|
3,4-Xylenol (708) |
|
Europe |
47 |
7 |
0.1 |
|
892 |
19 |
|
USA |
7 |
1 |
0.02 |
|
892 |
130 |
|
2,3,6-Trimethylphenol (737) |
|
Europe |
2 |
0.3 |
0.005 |
|
+ |
NA |
|
USA |
2 |
0.3 |
0.004 |
|
+ |
NA |
|
Thymol (709) |
|
Europe |
415 |
59 |
1 |
|
25 767 |
62 |
|
USA |
1 240 |
160 |
3 |
|
25 767 |
21 |
|
Carvacrol (710) |
|
Europe |
114 |
16 |
0.3 |
|
80 203 |
700 |
|
USA |
2 |
0.3 |
0.004 |
|
80 203 |
40 000 |
|
para-Vinylphenol (711) |
|
Europe |
1 |
0.1 |
0.002 |
|
17 745 |
18 000 |
|
USA |
43 |
6 |
0.1 |
|
17 745 |
410 |
|
Resorcinol (712) |
|
Europe |
10 |
1 |
000 |
|
+ |
NA |
|
USA |
2 |
0.3 |
0.004 |
|
+ |
NA |
|
Guaiacol (713) |
|
Europe |
358 |
51 |
1 |
|
8 184 |
44 |
|
USA |
120 |
16 |
0.3 |
|
8 184 |
68 |
|
ortho-(Ethoxymethyl)phenol (714) |
|
Europe |
12 |
2 |
0.03 |
|
- |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
|
- |
NA |
|
2-Methoxy-4-methylphenol (715) |
|
Europe |
257 |
37 |
1 |
|
226 |
0.9 |
|
USA |
25 |
3 |
0.1 |
|
226 |
9 |
|
4-Ethylguaiacol (716) |
|
Europe |
57 |
8 |
0.1 |
|
625 035 |
11 000 |
|
USA |
3 |
0.4 |
0.007 |
|
625 035 |
210 000 |
|
2-Methoxy-4-propylphenol (717) |
|
Europe |
1 484 |
210 |
4 |
|
38 |
0 |
|
USA |
0.9 |
0.1 |
0.002 |
|
38 |
42 |
|
Guaiacyl acetate (718) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
- |
NA |
|
USA |
0.9 |
0.1 |
0.002 |
|
- |
NA |
|
Guaiacyl phenylacetate (719) |
|
Europe |
3 |
0.4 |
0.007 |
|
- |
NA |
|
USA |
15 |
2 |
0.03 |
- |
|
NA |
|
Hydroquinone monoethyl ether (720) |
|
Europe |
N/D |
N/D |
N/D |
|
- |
NA |
|
USA |
3 |
0.4 |
0.007 |
|
- |
NA |
|
2,6-Dimethoxyphenol (721) |
|
Europe |
44 |
6 |
0.1 |
|
+ |
NA |
|
USA |
89 |
12 |
0.2 |
|
+ |
NA |
|
4-Methyl-2,6-dimethoxyphenol (722) |
|
Europe |
N/D |
N/D |
N/D |
|
+ |
NA |
|
USA |
0.3 |
0.04 |
0.0007 |
|
+ |
NA |
|
4-Ethyl-2,6-dimethoxyphenol(723) |
|
Europe |
N/D |
N/D |
N/D |
|
108 |
NA |
|
USA |
11 |
1 |
0.02 |
|
108 |
0.1 |
|
4-Propyl-2,6-dimethoxyphenol (724) |
|
Europe |
N/D |
N/D |
N/D |
|
+ |
NA |
|
USA |
0.5 |
0.1 |
0.001 |
|
+ |
NA |
|
2-Methoxy-4-vinylphenol (725) |
|
Europe |
21 |
3 |
0.05 |
|
38 066 |
1 800 |
|
USA |
11 |
1 |
0.02 |
|
38 066 |
3 500 |
|
4-Allyl-2,6-dimethoxyphenol (726) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
USA |
44 |
6 |
0.1 |
|
+ |
NA |
|
2-Hydroxyacetophenone (727) |
|
Europe |
1 |
0.1 |
0.002 |
|
1 912 |
1 900 |
|
USA |
0.1 |
0.01 |
0.0002 |
|
1 912 |
19 000 |
|
Phenyl salicylate (736) |
|
Europe |
60 |
9 |
0.1 |
|
- |
NA |
|
USA |
60 |
8 |
0.1 |
|
- |
NA |
|
4-(para-Hydroxyphenyl)-2-butanone (728) |
|
Europe |
19 494 |
2800 |
46 |
|
111 |
0.006 |
|
USA |
28 900 |
3800 |
63 |
|
111 |
0.004 |
|
Dihydroxyacetophenone (729) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
- |
NA |
|
USA |
1 |
0.1 |
0.002 |
|
- |
NA |
|
Zingerone (730) |
|
Europe |
278 |
40 |
1 |
|
20 |
0.072 |
|
USA |
630 |
83 |
1 |
|
20 |
0.032 |
|
4-(para-Acetoxyphenyl)-2-butanone (731) |
|
Europe |
N/D |
N/D |
N/D |
|
- |
NA |
|
USA |
0.5 |
0.1 |
0.001 |
|
- |
NA |
|
Vanillylidene acetone (732) |
|
Europe |
N/D |
N/D |
N/D |
|
- |
NA |
|
USA |
0.9 |
0.1 |
0.002 |
|
- |
NA |
|
2-Phenylphenol (735) |
|
Europe |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
USA |
0.1 |
0.01 |
0.0002 |
|
+ |
NA |
|
Total |
|
Europe |
25 000 |
|
|
|
|
|
|
USA |
32 000 |
|
|
|
|
|
NA, not available; N/D, no intake data reported; +, reported to occur naturally in foods (Maarse et al., 1996), but no quantitative data; -, not reported to occur naturally in foods
a
From International Organization of the Flavour Industry (1995) and Lucas (1999)
b
Intake (µg/person per day) calculated as follows: ((annual volume, kg) x (1 x 109 µg/kg) / (population x survey correction factor x 365 days)], where population (10%, 'eaters only') = 32 x 106 for Europe and 26 x 106 for the USA. The correction factor = 0.6 for Europe and 0.8 for the USA, representing the assumption that only 60% and 80% of the annual volume of the flavour, respectively, was reported in the poundage surveys (International Organization of the Flavor Industry, 1995; Lucas et al., 1999). Intake (µg/kg bw per day) calculated as follows: [(µ/person per day)/body weight], where body weight = 60 kg. Slight variations may occur from rounding.
c
Calculated as follows: (relative molecular mass alcohol/relative molecular mass ester) x daily per capita intake ('eaters only') ester
d
Quantitative data from Stofberg & Grundschober (1987)
e
Calculated as follows: (annual consumption in food, kg)/(most recently reported volume as a flavouring agent, kg).
1.4 Application of the Procedure for the Safety Evaluation of Flavouring Agents
|
Step 1. |
In applying the Procedure for the Safety Evaluation of Flavouring Agents (see Figure 1) to the above-mentioned substances, the Committee assigned 47 of the 48 flavouring agents with the lowest toxic potential to structural class I (Cramer et al., 1978). The remaining flavouring agent, 2-phenylphenol (No. 735), was assigned to structural class III. |
|
Step 2. |
At current levels of intake, the flavouring agents can tie predicted to be metabolized to innocuous products, and the pathways involved would not be expected to be saturated. The evaluation of these substances therefore proceeded via the left-hand side of the decision-tree. |
|
Step A3. |
The estimated daily per capita intake of 46 of the flavouring agents in structural class I is below the threshold for human intake for the class (i.e. 1800 m g) and that of the one agent in structural class III is below the threshold for human intake for that class (i.e. 90 m g). The Committee concluded that the safety of these 47 flavouring agents poses no concern when they are used at their currently estimated levels of intake. The intakes of 4-(para-hydroxyphenyl)-2-butanone (No. 728) are 2800 µg/person per day in Europe (International Organization of the Flavor Industry, 1995) and 3800 µg/person per day in the USA (Lucas et al., 1999). These levels exceed the threshold for human intake for class I (1800 µg/person per day). Accordingly, evaluation of this substance proceeded to step A4. |
|
Step A4. |
4-(para-Hydroxyphenyl)-2-butanone (No. 728) does not occur endogenously in humans. Evaluation of this substance therefore proceeded to step A5. |
|
Step A5. |
The NOEL of 280 mg/kg bw per day for 4-(para-hydroxyphenyl)-2-butanone (No. 728) in a 13-week study in rats (Gaunt et al., 1970) provides an adequate margin of safety (i.e. > 10 000) at current levels of intake in Europe (46 µg/kg bw per day) and in the USA (63 µg/kg bw per day). |
Table 1 summarizes the evaluation of phenol and 47 phenol derivatives used as flavouring agents.
1.5 Consideration of combined intakes from use as flavouring agents
In the unlikely event that all foods containing the six esters of para-cresol (Nos 699, 701-705) were consumed concurrently with para-cresol (No. 693) on a daily basis, the combined intake of para-cresol equivalents (see Table 2) would not exceed the threshold for human intake for structural class I (1800 µg/person per day). In the unlikely event that all foods containing all 48 substances were consumed daily, the estimated combined intake would exceed the human intake threshold for class I but would not saturate the available high-capacity, conjugation pathways involved in the metabolism of these substances (Hagan et al., 1967; Posternak & Linder, 1969; Gaunt et al., 1970; Maasik, 1970; Kociba et al., 1976; National Toxicology Program, 1980; Hirose et al., 1986; Environmental Protection Agency, 1988a,b; Hirose et al., 1989; Maruyama et al., 1991; National Toxicology Program, 1992a,b). Moreover, approximately 78% of the annual volume consumed in Europe and approximately 90% of that consumed in the USA is accounted for by 4-(para-hydroxyphenyl)-2-butanone (No. 728), for which a NOEL providing an adequate margin of safety was available.
1.6 Conclusions
The Committee concluded that the safety of phenol and the 47 derivatives of phenol in this group would not raise concern at the currently estimated levels of intake. Other data on the toxicity of phenol and its derivatives were consistent with the results of the safety evaluation.
The Committee took note of the ADI of 0-0.4 mg/kg bw for 2-phenylphenol (No. 735) established by the 1999 Joint FAO/WHO Meeting on Pesticide Residues (FAO, 1999).
2. RELEVANT BACKGROUND INFORMATION
2.1 Explanation
This monograph summarizes data pertinent to evaluating the safety of phenol and 47 phenol derivatives used as flavouring substances (see Table 1). The group of substances was selected on the basis of the structural criteria that all members possess an aromatic ring containing one (e.g. phenol, No. 690) or more (e.g. resorcinol, No. 712) free hydroxyl groups or are the esters of phenol derivatives (e.g. phenyl acetate, No. 734). The available chemical, metabolic, and toxicological data indicate that phenol and its derivatives fit into four structural categories: phenol, two esters of phenol, and resorcinol (Nos 690, 734, 712, 736); alkyl-, alkenyl-, or aryl-substituted phenols and their corresponding esters (Nos 691-711, 733, 735, and 737); alkoxy phenols (Nos 713-726); and phenol derivatives that contain alkyl side-chains containing a ketone function (Nos 727-732). Substances with multiple functional groups were categorized according to their most reactive functional group.
2.2 Additional considerations on intake
Thirty-two of the phenol derivatives are natural components of foods and are found predominantly in berries, coffee, and meat (Maarse et al., 1996). Quantitative data on natural occurrence and consumption ratios, which have been reported for 20 of the substances in the group, indicate that they are consumed primarily as natural components of food (i.e. consumption ratios > 1) (Stofberg & Kirschman, 1985; Stofberg & Grundschober, 1987; see Table 2). Consumption ratios of < 1 were calculated for 2-methoxy-4-methylphenol (No. 715), 4-(para-hydroxyphenyl)2-butanone (No. 728), and zingerone (No. 730), as 0.9, 0.004, and 0.03, respectively.
Phenol (No. 690) has a sharp, burning flavour and a distinct sweet, acrid odour that is discernable at a concentration of 3.8 mg/m3 (National Institute for Occupational Safety and Health, 1976).
2.3 Biological data
2.3.1 Absorption, distribution, and excretion
Phenol (No. 690) is a normal constituent of human and animal tissues, blood, urine, faeces, and sweat. Most endogenous phenol arises from the degradation of tyrosine by Escherichia coli in the intestines. Diets with a high protein content promote phenol production (Babich & Davis, 1981). It is thought that para-cresol (No.693), found in the serum of healthy humans at a concentration of 0.23 ± 0.17 mg/100 ml, is formed by the same bacterial mechanisms (Wengle & Hellström, 1972).
When phenol and its derivatives are ingested as natural or-added components of food, they are rapidly absorbed from the gastrointestinal tract and participate in common pathways of metabolism (Hughes & Hall, 1995). Phenol (No. 690), phenyl acetate (No. 734), and resorcinol (No. 712), alkyl-, alkenyl-, and aryl-substituted phenols and their corresponding esters (Nos 691-712, 718, 719, 733, 735, and 737) are conjugated with sulfate or glucuronic acid and excreted primarily in the urine. The simple esters of substituted phenols undergo hydrolysis prior to conjugation. Other metabolic pathways, observed mainly at high doses, include ring hydroxylation and side-chain oxidation. Phenols containing alkoxy groups (Nos 713-717 and 720-726) and those that contain an alkyl side-chain with a ketone function (Nos 727-732 and 736) are also metabolized mainly by conjugation. Alkoxy phenols may undergo para-hydroxylation before conjugation. Minor metabolites are formed through alternative pathways that include dealkylation of alkoxyphenols, reduction of side-chain ketones, side-chain oxidation, and ring hydroxylation.
At high doses, para-cresol (No. 693), para-ethylphenol (No. 694), 2-methoxy-4-methylphenol (No. 715), 2-methoxy-4-propylphenol (No. 717), 2-methoxy-4-vinylphenol (No. 725), and 4-allyl-2,6-dimethoxyphenol (No. 726) are oxidized to reactive quinone methide intermediates. Quinone methides are detoxified by glutathione; however, at very high concentrations this detoxication pathway may be saturated. Low concentrations (< 0.25 mal/L) of quinone methide intermediates undergo detoxication via glutathione (Thompson et al., 1994, 1995a,b) (see Figure 1).
Phenol (No. 690), phenyl acetate (734), phenyl salicylate (No. 736), and resorcinol (No. 712)
The primary route of metabolism of phenol (No. 690) involves rapid absorption and conjugation of the hydroxyl group with sulfate or glucuronic acid, primarily in the liver (see Figure 1) (Balling et al., 1995). Ring hydroxylation to yield para-hydroxyphenol (i.e. quinol) has also been reported. In human subjects, in whom > 95% of a single oral dose of 0.01 mg/kg bw of phenol was excreted in the urine within 24 h. The principal urinary metabolites included the sulfate (77%) and glucuronic acid (16%) conjugates of phenol. Quinol monosulfate (the sulfate of 1,4-benzenediol) and quinol monoglucuronide were the only minor metabolites present in detectable amounts (Capel et al., 1972). Sulfation also occurs in the human metabolism of related substances: when seven human tissues were incubated with ortho-, meta-, and para-hydroxybiphenyl, the rate of sulfation of each isomer was highest in the liver and lowest in the brain (Pacifici et al., 1991).
Small amounts of ingested phenol (No. 690) are excreted in the free form. A man ingested one lozenge containing 32.5 mg of phenol per lozenge every 2 h for a total of eight doses in one day, and his urine was collected over 72 h after the first dose, fractionated into 8-h collection periods. The concentration of phenol based on the total volume of urine peaked at 270 mg/L during the third collection period, when the concentration of free phenol peaked at 10 mg/L. The concentrations of both total and free urinary phenols returned to baseline levels (7 and 1 mg/L, respectively) 48 h after the final dose (Fishbeck et al., 1975).
The absorption and metabolism of phenol are comparable in rats. Approximately 70-85% of an oral dose of 0.031 mg/kg bw of phenol (No. 690) was excreted in the urine of female rats within 4 h of administration, and only 1-5% remained in the body 72 h after exposure (Hughes & Hall, 1995). Male and female rats given single intravenous injections of phenol at133 µmol/kg bw excreted 53% and 44% as phenyl sulfate, respectively, within 3 h of dosing. The difference in sulfate excretion by males and females was thought to result from a slower rate of excretion of phenyl sulfate by females rather than from a difference in the degree of sulfate conjugation. Approximately 42% of the administered dose was excreted in the urine of both males and females within 3 h as phenyl glucuronide (Meerman et al., 1987).
Glucuronic acid conjugation of phenol (No. 690) is thought to be proportional to the dose, while sulfate conjugation depends on the availability of sulfate (Bray et al., 1952). In rats, increasing an intravenous dose of phenol from 0.013 to 0.266 mg/kg bw resulted in an increase in glucuronidation from 28 to 60% of the dose and a decrease in sulfation from 72 to 40% (Weitering et al., 1979). These observations suggest saturation of the sulfation pathway, which would leave more free phenol available to form the glucuronic acid conjugate and undergo ring hydroxylation to yield polyphenols (quinol) (Williams, 1959; Weitering et al., 1979; Kenyon et al., 1995). In another study, 75 mg/kg bw per day of phenol were administered to rats by intraperitoneal injection. Urinary analysis revealed small amounts of N-acetyl-S(2,5-dihydroxyphenyl)-L-cysteine, a product of glutathione conjugation of benzoquinone intermediates (Nerland & Pierce, 1990).-In rats; also, phenol was excreted in the bile, mostly as the glucuronide conjugate. Approximately 5% of-an intraperitoneal dose of 50 mg/kg bw of phenol was excreted in the bile of female Wistar albino rats, phenyl glucuronide predominating (Abou-el-Makarem et al., 1967). In male rats with ligated kidneys, the biliary excretion of-phenol conjugates was three tines that of rats with intact kidneys (Weitering et al., 1979).
A comparable pattern of metabolism is observed in other animal species. Rabbits given a single lethal dose of 500 mg/kg bw of phenol (No. 690) died or were killed 3, 15, 82, 120, 150, or 360 min later. Phenol was found after 3 min in all tissues tested. Conjugated phenol was present mostly in the liver and kidneys, indicating that detoxication had begun. After 15 min, the liver contained the highest concentration of free (64 mg/100 g) and conjugated phenol (0.9 mg/100 g). After 82 min, total (free and conjugated) phenol was approximately equally distributed in the blood, liver, kidneys, and lungs. After 360 min, approximately 65% of the total phenol was present in the conjugated form (Deichmann, 1944). Also in rabbits, phenol given as single doses of 125 and 250 mg/kg bw was excreted in the urine as phenyl glucuronide (70%) and phenyl sulfate (19%), with small amounts of quinol (1,4-benzenediol; 10%), catechol, and hydroxyquinol (4-hydroxyresorcinol) within 24 h (Porteous & Williams, 1949).
Sheep given a single oral dose of 25 mg/kg bw of phenol (No. 690) excreted 49% as phenylglucuronides, 32% as sulfate conjugates, and 12% as phosphate conjugates. Pigs given the same dose of phenol excreted 83% as glucuronic acid conjugates and only 1 % as sulfate conjugates. Rats receiving the same dose excreted 42% as glucuronic acid conjugates and 55% as sulfate conjugates. Phosphate conjugates were absent from the urine of phenol-treated pigs and rats. Sheep, pigs, and rats excreted 80-90% of the dose within 8 h, indicating rapid absorption and urinary elimination in all species (Kao et al., 1979).
Like phenols, polyphenols are conjugated primarily with sulfate or glucuronic acid and excreted. Three rabbits fed single doses of 100 mg/kg bw of resorcinol (No. 712) through a stomach tube excreted 52% of the dose as glucuronic acid conjugates, 14% as sulfate conjugates, and 11 % as free resorcinol (Garton & Williams, 1949). Male and female Fischer 344 rats that had been given resorcinol as a single oral dose of 112 mg/kg bw excreted about 70% in the urine as glucuronic acid conjugates within 24 h. The urine of females contained a greater portion of sulfate conjugates, while males excreted a larger percentage of a sulfate and glucuronide diconjugate. Less than 3% was excreted in the faeces of both males and females. The ratio of metabolic products was not significantly altered by administration of oral doses of 225 mg/kg-bw per day of resorcinol for 5 consecutive days (Kim & Matthews, 1987).
Phenyl salicylate (No. 736) would be expected to be hydrolysed to salicylic acid and phenol. A man was given one 90-mg capsule of phenyl salicylate each hour for 8 h, and his urine was collected for 72 h after the first dose, fractionated into 8-h collection periods. Total urinary phenol showed a peak of 470 mg/kg during the second collection period, when the concentration of free urinary phenol peaked at 25 mg/kg. Approximately 60 h after the first dose, the concentrations of total and free-urinary phenol had returned to-baseline levels,(7 and 1 mg/kg, respectively) (Fishbeck et al., 1975). Methyl salicylate, a related substance; was hydrolysed in rats, dogs; and humans in vivo (Davison et al., 1961).
Alkyl- and alkenyl-substituted phenols and their corresponding esters (Nos 691-711, 733, 735, and 737)
Monoalkyl-substituted phenols are conjugated mainly with sulfate or glucuronic acid in a manner similar to phenol (No. 690). They undergo a small degree of side-chain oxidation or ring hydroxylation, which results in a second hydroxyl group ortho or para to the existing hydroxyl group (Williams, 1959). In rabbits and dogs, a single oral dose of 200 mg/kg bw of ortho- or meta-cresol (Nos 691 and 692) was hydroxylated to a very minor extent (< 1 %), while up to 10% of para-cresol (No. 693) was oxidized on the side-chain and excreted as para-hydroxybenzoic acid (Bray et al., 1950a). In rats, < 0.2% of an oral dose of para-cresol of 100 mg/kg bw was ring hydroxylated and ortho-methylated to yield 4- and 5-methylguaiacol (2-methoxy-4 or 5-methylphenol) (Bakke, 1970; Bakke & Scheline, 1970).
Studies of metabolism in vitro provide evidence that a reactive metabolite of para-substituted alkylphenols is generated at high doses. The cytotoxicity of para-cresol (No. 693) and para-ethylphenol (No. 694) arises from the oxidative formation of reactive quinone methide intermediates (see Figure 1). At doses < 0.25 mmol/L, this reactive intermediate is detoxicated with glutathione, whereas at high concentrations (> 0.25 mmol/L), the quantity of quinone methides exceeds the availability of glutathione (Thompson et al., 1994).
Groups of 10 male B6C3F1 mice were given single doses of 2-phenylphenol (No. 735) at 15 or 800 mg/kg bw by oral gavage. Within 48 h, 84-98% of the administered dose was recovered in the urine of both groups, and 6-11% was recovered in the faeces; less than 1% was found in the tissues and carcass at necropsy. As part of the same study, two male and two female Fischer 344 rats received a single dose of 28 mg/kg bw by oral gavage, and 81 % of the administered dose was recovered in the urine within 12 h. In both species, most of the 2-phenylphenol that was recovered was in the form of glucuronic acid and sulfate conjugates. Glucuronic acid and sulfate conjugates of the para-hydroxylated metabolite, 4-hydroxy-2-phenylphenol, represented minor components of the recovered dose in both species (Bartels et al., 1998). In rats given a single oral dose of 1000 mg/kg bw of 2-phenylphenol, the glutathione conjugate of 4-hydroxy-2phenylphenol (at 4% of the dose) was detected in bile after 6 h. Conversion of 2-phenylphenol to 4-hydroxy-2-phenylphenol was shown to occur via the microsomal mono-oxygenase system in vitro (Nakagawa & Tayama, 1989).
Incorporation of a second alkyl substituent does not significantly alter the sulfate and glucuronic acid conjugation pathway. Doses of 1 g of 2,5-xylenol, 2,6-xylenol, or 3,4-xylenol (2;5-methylphenol, 2,6-methylphenol, and 3,4-methylphenol; Nos 706-708) given to rabbits were excreted as xylenyl sulfates (8-16%) and glucuronides (50-72%); only 1-3% of the dose was excreted unconjugated. Ring hydroxylation was evident in only a small portion of the metabolites of the 2,6-xylenol isomer (Bray et al., 1950b). Thymol (5-methyl-2-isopropylphenol; No. 23) (No. 709) and carvacrol (2-methyl-5-isopropylphenol) (No. 710) are also excreted after conjugation with sulfates and glucuronic acid. Thymoquinol (2-isopropyl-5-methyl-1,4-benzenediol)has been detected as a minor oxidation product of thymol (Williams, 1959). The period over which metabolites were collected was not specified in either study.
The simple esters of these alkyl-substituted phenols (Nos. 698-705) are expected to undergo hydrolysis in vivo, followed by conjugation and excretion as sulfate and glucuronic acid conjugates. ortho-Tolyl acetate (No. 698) was 60% hydrolysed in vitro after incubation with pancreatin for 2 h at 37 °C (Grundschober, 1977). para-Vinylphenol (No. 711), a metabolite of styrene, was excreted as the glucuronic acid conjugate of para-vinylphenol or as a mandelic acid derivative formed by oxidation of the vinyl group (Pfäffli et al., 1981).
Alkoxyphenols
(Nos 713-726)
Alkoxyphenols are excreted mainly as glucuronic acid or sulfate conjugates. The presence of both methoxy and hydroxy functions activates the ring towards further substitution, which may lead to the formation of polyphenols. In humans, 73% of a 50-mg dose of guaiacol (2-methoxyphenol; No. 713) was excreted as the sulfate and glucuronic acid conjugates within 14 h (Sedivec & Flek, 1970); however, the period of urine collection was not specified. In cats, a single oral dose of 2,6-dimethoxyphenol (No. 721) at 20 or 40 mg/kg bw was excreted mainly as conjugated 2,6-dimethoxy-4-hydroxyphenol after ortho demethylation (90% as disulfate) and as unconjugated (3%) 2,6-dimethoxy-4-hydroxyphenol within 24 h of dosing. Free (5%) and conjugated (2% with glucuronic acid) forms of the parent phenol were also detected (Miller et al., 1976).
Like the para-alkylphenols, 2-methoxy-4-alkylphenols can form electrophilic quinone methide intermediates at high cellular concentrations. 2-Methoxy-4-methylphenol (No. 715), 2-methoxy-4-propylphenol (No. 717), 2-methoxy-4-vinylphenol (No. 725), and 4-allyl-2,6-dimethoxyphenol (No. 726) were oxidized to quinone methides in rat liver slices in vitro. The half-lives of the quinone methides formed from these substances were 1, 6, 410, and 4300 s, respectively. Hepatocellular toxicity, measured as intracellular potassium leakage, was detected in rat liver slices incubated with each phenol for 6 h. These findings suggest that only quinone methides with half-lives of 10 s to 10 min are reactive enough to be cytotoxic at high concentrations (Thompson et al., 1995a).
Phenol derivatives with an alkyl side-chain containing a ketone function (Nos 727-732)
The presence of a ketone functiona confers the possibility of rapid metabolism like that of related phenols. Zingerone (3-methoxy-4-hydroxybenzylacetone; No. 730) given to rats as a single oral or intraperitoneal dose of 100 mg/kg bw was excreted in the urine mainly as glucuronic acid and sulfate conjugates. Reduction of the ketone to zingerol (12%) was also observed, with lesser ring hydroxylation to 4(3,4-dihydroxyphenyl)-2-butanone (6%) and side-chain oxidation to 4-hydroxy-3-methoxyphenylacetic acid (8%) (Monge et al., 1976).
The closely related 4-(para-hydroxyphenyl)-2-butanone (No. 728), the phenol derivative produced in the highest volume, would be expected to be metabolized by essentially the same pathways.
2.3.2 Toxicological studies
Studies-lasting 90 days and longer-term studies of toxicity and carcinogenicity were available for 12 of the 48 substances in this group and addressed-all four structural categories of phenol derivatives. Five concerned phenol or resorcinol (National Toxicology Program, 1980; Hirose et al., 1986, 1989; Maruyama et al., 1991; National Toxicology Program, 1992b), 12 were conducted with alkyl- or aryl-substituted phenols, and their corresponding esters (Hodge et al., 1952; Hagan et al., 1967; Posternak & Linder, 1969; Maasik, 1970; Hagiwara et al., 1984; Hiraga & Fujii, 1984; Hirose et al., 1986; Environmental Protection Agency, 1988a,b; National Toxicology Program, 1992a; Quast et al., 1997; Wahle et al., 1997), one was carried out with alkoxy phenols (Posternak & Linder, 1969), and two addressed phenol derivatives with an alkyl side-chain containing a ketone function (Gaunt et al., 1970; Kociba et al., 1976).
2-Phenylphenol
(No. 735)
2-Phenylphenol (No. 735) was evaluated by the Committee at its eighth meeting, when an ADI of 0-0.2 mg/kg bw was established (Annex 1, reference 7). The 1999 Joint FAO/WHO Meeting on Pesticide Residues evaluated the toxicity of 2-phenylphenol and established an ADI of 0-0.4 mg/kg bw (FAO, 1999). As the available studies on the toxicology of 2-phenylphenol are summarized in the monograph (WHO, 2000), the data on this substance and its sodium salt (sodium ortho-phenylphenate) are not presented here. However, some consideration was given to studies of the genotoxicity of these substances, because the Joint Meeting found that there were unresolved questions with regard to the genotoxic potential of 2-phenylphenol.
2.3.2.1 Acute toxicity
LD50 values in rats treated orally were available for 29 of the 48 substances in this. group. The values ranged from 300 to 5700 mg/kg (Deichmann & Witherup, 1944; Hodge et al., 1952; Jenner et al., 1964; Taylor et al., 1964; Rinehart et al., 1967; Smyth et al., 1969; Gaunt et al., 1970; Denine & Palanker, 1973; Moreno, 1973; Levenstein,1974; McGee, 1974; Levenstein, 1975; Moreno, 1975, 1977, 1978; Sauer & Salem, 1980; Piccirillo et al. 1981; Piccirillo & Hartman, 1982; Klonne et al., 1988; Van den Heuvel, 1990; Schlicht et al., 1992; Berman et al., 1995). LD50 values for mice treated orally were available for 12 substances and ranged from 120 to 3100 mg/kg bw (MacIntosh, 1945; McOmie et al., 1949; Pellmont, 1973; Uzhdavini et al., 1974; Cioli et al., 1980; Moran et al., 1980; Schafer & Bowles, 1985; Hasegawa et al., 1989). Over half of the LD50 values reported are > 1000 mg/kg bw.
2.3.2.2 Short-term studies of toxicity
The usefulness of a number of the short-term studies of toxicity in mice and rats for evaluating the safety of the substances in this group of flavouring agents was
Table 3. Results of short-term studies of toxicity and long-term studies of toxicity and carcinogenicity on phenol and phenol derivatives used as flavouring agents administered orally
|
No. |
Substance |
Species; sex |
No. test groupsa/ no. per groupb |
Route |
Duration |
NOEL (mg/kg bw per day) |
Reference |
|
690 |
Phenol |
Mice; M, F |
5/20 |
Drinking-water |
13 weeks |
750 |
National Toxicology Program (1980) |
|
690 |
Phenol |
Mice; M, F |
2/100 |
Drinking-water |
103 weeks |
< 620 |
National Toxicology Program (1980) |
|
690 |
Phenol |
Rats; M, F |
5/20 |
Drinking-water |
13 weeks |
300 |
National Toxicology Program (1980) |
|
690 |
Phenol |
Rats; M, F |
2/100 |
Drinking-water |
103 weeks |
250 |
National Toxicology Program (1980) |
|
691 |
ortho-Cresol |
Mice; M, F |
5/40 |
Food |
13 weeks |
< 220 (M)
450 (F) |
National Toxicology Program (1992a) |
|
691 |
ortho-Cresol |
Rats; M, F |
5/40 |
Food |
13 weeks |
250 |
National Toxicology Program (1992a) |
|
691 |
ortho-Cresol |
Rats; M, F |
3/60 |
Gavage |
13 weeks |
180 |
Environmental Protection Agency (1988a) |
|
692, 693 |
meta-Cresol, para-cresolc |
Mice; M, F |
5/40 |
Food |
13 weeks |
440 (M)
220 (F) |
National Toxicology Program (1992a) |
|
692, 693 |
meta-Cresol, para-cresolc |
Rats; M, F |
5/40 |
Food |
13 weeks |
250 |
National Toxicology Program (1992a) |
|
693 |
para-Cresol |
Rats; M, F |
3/60 |
Gavage |
13 weeks |
50 |
Environmental Protection Agency (1988b) |
|
693 |
para-Cresol |
Hamsters, M |
1/15 |
Food |
20 weeks |
< 1800 |
Hirose et al. (1986) |
|
694 |
para-Ethylphenol |
Rats; M, F |
1/20-32 |
Food |
90 days |
0.20c (M)
0.22c (F) |
Posternak & Linder (1969) |
|
733 |
4-(1,1-Dimethyl)ethyl phenol |
Hamsters, M |
1/15 |
Food |
20 weeks |
< 1800 |
Hirose et al. (1986) |
|
707 |
2,6-Xylenol |
Rats |
2/13 |
Gavage |
8 months |
0.06 |
Maasik (1970) |
|
708 |
3,4-Xylenol |
Rats |
2/13 |
Gavage |
8 months |
0.14 |
Maasik (1970) |
|
709 |
Thymol |
Rats; M, F |
2/10 |
Food |
19 weeks |
1000d |
Hagan et al. (1967) |
|
735 |
2-Phenylphenol |
Mice; M, F |
3/100 |
Food |
96 weeks |
1300 (M)
<750 (F) |
Hagiwara et al. (1984) |
|
735 |
2-Phenylphenol |
Mice; M, F |
3/50 |
Food |
2 years |
< 250 |
Quest et al. (1997) |
|
735 |
2-Phenylphenol |
Rats; M, F |
5/22-24 |
Food |
13 weeks |
410 (M)
430 (F) |
Hiraga & Fujii (1984) |
|
735 |
2-Phenylphenol |
Rats, M |
3/20-24 |
Food |
91 weeks |
270 |
Hiraga & Fujii (1984) |
|
735 |
2-Phenylphenol |
Rats; M, F |
3/50 |
Food |
2 years |
100 |
(Hodge et al. (1952) |
|
735 |
2-Phenylphenol |
Rats; M, F |
3/NR |
Food |
2 years |
39 |
Wahle et al. (1997) |
|
712 |
Resorcinol |
Mice; M, F |
5/20 |
Gavage |
13 weeks |
<28 (M)
220 (F). |
National Toxicology Program (1992b) |
|
712 |
Resorcinol |
Mice; M, F |
2/120 |
Gavage |
104 weeks |
220d (M)
110 (F) |
National Toxicology Program (1992b) |
|
712 |
Resorcinol |
Rats; M, F |
5/20 |
Gavage |
13 weeks |
< 32 |
National Toxicology Program (1992b) |
|
712 |
Resorcinol |
Rats, M |
1/16 |
Food |
51 weeks |
800d |
Hirose et al. (1989) |
|
712 |
Resorcinol |
Rats, F |
3/60 |
Gavage |
104 weeks |
100 |
National Toxicology Program (1992b) |
|
712 |
Resorcinol |
Rats, M |
2/60 |
Gavage |
104 weeks |
110 |
National Toxicology Program (1992b) |
|
712 |
Resorcinol |
Hamsters, M |
1/15 |
Food |
20 weeks |
< 300 |
Hirose et al. (1986) |
|
712 |
Resorcinol |
Hamsters, F |
1/15 |
Food |
16 weeks |
< 1800 |
Maruyama et al. (1991) |
|
721 |
2,6-Dimethoxyphenol |
Rats; M, F |
1/20-32 |
Food |
90 days |
6.0d (M)
6.9 d (F) |
Posternak & Linder (1969) |
|
728 |
4-(para-Hydroxyphenyl)-2-butanone |
Rats; M, F |
4/30 |
Food |
13 weeks |
280 (M)
700 (F) |
Gaunt et al. (1970) |
M, male; F, female; if not listed, sex was not specified in the report; NR, not reported
a
Total number of test groups does not include control animals.
b
Total number per test group includes both male and female animals.
c
Administered as a mixture: meta-cresol, 60%; para-cresol, 40%
d
Study performed with either a single dose or multiple doses that had no adverse effect; the value is therefore the highest dose tested.
limited by their short duration; they are summarized briefly below. The results of. studies that were 90 days or longer in duration and in which NOELS were identified are described in greater detail and are shown in Table 3.
Phenol
(No. 690)
Mice
A study to determine the doses for a subsequent 2-year study was conducted, in which groups of 10 male and 10-female B6C3F1 mice were provided with tap-water containing phenol (No. 690) at a concentration of 0, 100, 300, 1000, 3000,.or 10 000 mg/L for 13 weeks. The concentrations were calculated (Food & Drug Administration, 1993) to provide average intakes of 0, 25, 75, 250, 750, and 2500 mg/kg bw per day. All the mice survived to the end of the study: The mean body-weight gain of animals at the highest dose was depressed; animals in this group rejected the treated water. Microscopic examination revealed no treatment-related differences between treated and control mice. The NOELwas 750 mg/kg bw per day (National Toxicology Program, 1980).
Rats
A study to determine the doses for a subsequent 2-year study was conducted, in which groups of 10 male and 10 female Fischer 344 rats were provided with tapwater containing phenol (No. 690) at a concentration of 0, 100, 300, 1000, 3000, or 10 000 mg/L for 13 weeks. The concentrations were calculated (Food & Drug Administration, 1993) to provide average intakes of 0, 10, 30, 100, 300, or.1000 mg/ kg bw per day,. All the rats lived to the end of the study. Statistically significant decreases in mean body-weight gain were observed in animals of each sex at the highest dose; animals in this group rejected the treated water. No changes attributable to treatment were observed in tissues or organs. The NOEL was 300 mg/kg bw per day (National Toxicology Program, 1980).
ortho-Cresol (No. 691), meta-cresol (No. 692), and para-cresol (No. 693)
Mice
In a 28-day study of toxicity, groups of five male and five female B6C3F1 mice were fed diets containing ortho-cresol (No. 691), meta-cresol (No. 692), para-cresol (No. 693), or meta/para-cresol (60%/40%) at a concentration of 0, 300, 1000, 3000, 10 000, or 30 000 mg/kg, providing average intakes of 0, 62, 200, 580, 1700, or 4700 mg /kg bw per day. Nineteen animals died or were killed when moribund during the 28 days: two males and one female at the highest dose of ortho-cresol, one female at the second highest dose of meta-cresol, two males and two females at the highest dose of meta-cresol, one male at the second highest dose of para-cresol, and all animals at the highest dose of para-cresol; all animals treated with meta/para-cresol lived to the end of the study. Hunched posture, lethargy, and hypothermia were observed in the groups receiving the highest dose of any substance. At the end of the study, significantly decreased body-weight gains were reported for males or females receiving the two higher doses of any substance. A significantly increased relative liver weight over that of controls was reported in females given meta-cresol at the lowest dose, and this effect was observed consistently in animals at the three higher doses of any substance. Significantly increased relative weights of the brain and kidney were also found in groups receiving the two higher doses of any substance, but no microscopic changes were associated with these changes in organ weights. Uterine and ovarian atrophy were seen in several females mainly at the highest doses of ortho-, meta-, or meta/para-cresol. No gross lesions were seen in the organs of treated animals, but renal and hepatic necroses were prevalent in animals receiving the highest dose of para-cresol, all of which died early in the study. Bone-marrow hypocellularity was found in some mice at the highest doses of para- and meta/para-cresol, and mild nasal and respiratory epithelial hyperplasia was found in most of these animals-presumably due to irritation caused by the test substance. Minimal hyperplasia of the oesophagus, forestomach, and/or respiratory squamous-cell epithelium was also reported among animals in these groups (National Toxicology Program, 1992a).
In a 13-week study of toxicity, groups of 10 male and 10 female B6C3F1.mice were fed diets containing ortho-cresol (No. 691) at a concentration of 0, 1250, 2500, 5000, 10 000, or 20 000 mg/kg or meta/para-cresol (60%/40%) at concentrations of 0, 625, 1250, 2500, 5000, or 10 000 mg/kg. The average intakes were reported to have been 0, 220, 450, 860, 2200, and 6000 mg/kg bw per day of ortho-cresol and 0, 110, 220, 440, 850, and 3200 mg/kg bw per day of meta/para-cresol. All mice lived to the end of the study, when the body weights of males and females receiving 20 000 mg/kg of diet of ortho-cresol were reduced by about 16% and 21%, respectively, and those of males and females receiving 10 000 mg/kg of diet of meta/para-cresol were reduced by about 9% and 7%, respectively, when compared with controls. Females receiving 10 000 mg/kg of diet of ortho-cresol also showed statistically significant decreases in weight gain (9% reduction compared with controls). Necropsy revealed significant increases in the relative weights of the liver in all groups of males given ortho-cresol and in males receiving 5000 or 10 000 mg/ kg of diet of meta/para-cresol. These responses are consistent with the known hepatocellular sensitivity of male B6C3F1 mice (Maronpot & Boorman, 1982; Maronpot et al., 1987). The relative liver weights were also significantly increased in female mice receiving the three higher doses of ortho-cresol or the highest dose of meta/para-cresol. Males given the highest dose of ortho-cresol had significantly increased relative testicular weights and males and females at this dose had increased relative thymus weights. Haematology, clinical chemistry, and urinary analysis revealed normal values. Microscopic examination of liver tissue showed no remarkable changes. Minimal epithelial hyperplasia was seen in the forestomach in 4 of 10 males and 3 of 10 females receiving the highest dose of ortho-cresol. An increased incidence of hyperplasia of the nasal respiratory epithelium was found in males receiving the two higher doses of meta/para-cresol (4/10 at 5000 mg/kg of diet and 10/10 at 10 000 mg/kg of diet) and in females receiving the highest dose of this substance (7/10). Females given 20 000 mg/kg of diet of ortho-cresol had lengthened estrus cycles. No biologically significant changes in reproductive function were noted in animals given meta/para-cresol at any dose (National Toxicology Program, 1992a).
Rats
A 28-day range-finding study was conducted in which groups of five male and five female Fischer 344 rats were fed diets containing ortho-cresol (No. 691), meta-cresol (No: 692), para-cresol (No. 693), or meta/para-cresol (60%/40%) at a concentration of 0, 300, 1000, 3000, 10 000, or 30 000 mg/kg, providing average intakes of each substance of 0; 26, 87, 260, 860, and 2400 mg/kg bw per day. All rats lived to the end of the study. No clinical signs of toxicity were observed in rats given ortho- or meta-cresol, but male and female rats given the highest dose of para- or meta/para-cresol appeared thin, and animals at the highest dose of each cresol isomer had significantly reduced body-weight gain and decreased food consumption. Significantly increased relative liver weights were observed consistently in groups given the two highest doses of each isomer; a similar effect was seen in males given ortho- or meta/para-cresol at 3000 mg/kg of diet and in females given meta/para-cresol at 1000 or 3000 mg/kg of diet or para-cresol at 3000 mg/kg of diet. Significantly increased relative kidney weights were also observed generally at the two highest doses. The relative weights of the brain and testes were increased in animals at the highest doses; probably due to reduced body-weight gain. No gross or microscopic lesions were recorded in rats treated with ortho-cresol. Mild to moderate uterine atrophy was reported in females given the highest dose of meta- or para-cresol. para-Cresol induced bone-marrow hypocellularity in males at the three higher doses and in females at the two higher doses. This effect was also observed in rats given the highest dose of meta/para-cresol. Male and female rats at the three higher doses of this substance had mild epithelial hyperplasia of the nasal cavity, oesophagus, and forestomach. No gross lesions were seen in the organs of any treated animal (National Toxicology Program, 1992a).
A 13-week study was conducted in which ortho-cresol (No. 691) was given to groups of 30 male and 30 female Sprague-Dawley rats at a dose of 0, 50, 180, or 600 mg/kg bw per day by oral gavage in corn oil. Of the animals at the high dose, 19 females and nine males died during the study; no deaths occurred in the other groups. At the end of the study, males at the high dose showed a 10% reduction in body-weight gain when compared with controls. Clinical chemistry, haematology, urinary analyses, and measurements of organ weights revealed no significant changes in treated rats, No gross or histological lesions and no opthalmological changes were found. Congestion, oedema, and oil droplets were found in the lungs of the 28 animals at the high dose that died during the study, but the authors suggested that these findings were related to terminal stress and aspiration of the mixture of ortho-cresol and corn oil during convulsive seizures. The NOEL was 180 mg/kg bw per day (Environmental Protection Agency, 1988a).
Groups of 20 Fischer 344 rats of each sex were fed diets containing ortho-cresol or meta/para-cresol (60%/40%) at a concentration of 0, 1900, 3800, 7500, 15 000, or 30 000 mg/kg for 13 weeks, providing average intakes of 0, 130, 250, 500, 1000, and 2000 mg/kg bw per day. All rats lived to the end of the study except for one female receiving the highest dose of ortho-cresol. At the end of the study, the body weights of males and females at the two higher doses of both substances were significantly lower than those of. controls, except for males given 15 000 mg/kg of diet of ortho-cresol. The relative weights of the liver were increased in males and females at the three higher doses of both isomers. The relative weights of the kidney were increased in animals at the two higher doses of ortho-cresol and in males at the three higher doses and females at the highest dose of meta/para-cresol. The relative weights of the testes were increased in males at the highest dose of ortho-cresol and at the two higherdoses of meta/para-cresol. Substantially increased concentrations of serum bile salts were reported in male and female rats given either isomer, particularly at the two highest doses, throughout the study. Consistent decreases in the activity of 5'-nucleotidase in serum were seen in rats given meta/para-cresol. Mild uterine atrophy was observed at the two higher doses of meta/para-cresol. Minimal to mild hypocellularity of the bone marrow was reported in males. and females at the two higher doses of both isomers, except in males at 15 000 mg/kg of diet of ortho-cresol. All animals receiving meta/para-cresol showed hyperplasia of the olfactory and respiratory epithelium. Both isomers lengthened the estrus cycle of females treated at 7500 or 30 000 mg/kg of diet. The NOEL for both ortho-cresol and meta/para-cresol was 250 mg/kg bw per day (National Toxicology Program, 1992a).
A 13-week study was conducted in which groups of 30 male and 30 female Sprague-Dawley rats were given para-cresol at a dose of 0, 50, 175, or 600 mg/kg bw per day by oral gavage. At the end of the study, the mean body weights of males and females at the highest dose were significantly lower than those of controls. Females at the intermediate and high doses showed statistically significant reductions in erythrocyte count, haemoglobin, and haematocrit, but compensatory responses to the anaemic state were not observed. Females at the highest dose also had elevated liver transaminase activity, but this finding was attributed to large increases in 4/10 animals, including two with chronic hepatic inflammation. Statistically significant increases in serum cholesterol were reported in females at the highest dose and in total protein in males at the two higher doses. Necropsy revealed significantly increased relative kidney weights in males at the intermediate dose and in males and females at the highest dose. Significantly increased relative weights of the liver, heart, testis, and brain were noted in males at the highest dose, while females at the lowest dose showed significantly increased relative spleen weights. Gross examination showed no treatment-related lesions. Histological evaluation revealed a significantly increased incidence of chronic nephropathy in males at the lowest and highest doses. A significantly increased incidence of epithelial metaplasia of the trachea was observed in animals at the highest dose than in controls, but no other treatment-related histological findings were reported. The NOEL was 50 mg/kg bw per day (Environmental Protection Agency, 1988b).
para-Ethylphenol (No. 694)
Groups of 20-32 male and female Charles River CD rats were fed a basal diet supplemented with para-ethylphenol (No. 694) for 90 days, giving average intakes of 0.20 mg/kg bw per day for males and 0.22 mg/kg bw per day for females. A control group was fed the basal diet alone. Weekly measurements of body weight, food consumption, and the calculated efficiency of food use showed no significant differences from controls. Haematological examinations and clinical chemistry performed at weeks 7 and 13 showed no effects. At necropsy, there were no significant changes in liver or kidney weights. Gross and histological examination of a wide range of organs (unspecified in the report) revealed no lesions related to treatment with either substance. The NOEL was 0.20 mg/kg bw per day (Posternak & Linder, 1969).
2,6-Xylenol
(No. 707)
Groups of 13 white rats (sex not specified) were given 2,6-xylenol (No. 707) at a dose of 0.06 or 6.0 mg/kg bw per day by stomach tube for 8 months. No effects on weight gain, haemoglobin concentration, or erythrocyte count were seen, but the higher dose had adverse effects on the cells of the liver, spleen, kidneys, and heart. No pathological or histological changes were seen at the lower dose. The NOEL was 0.06 mg/kg bw per day (Maasik, 1970).
3,4-Xylenol
(No. 708)
In the study described above, groups of 13 white rats were given 3,4-xylenol (No. 1708) at a dose of 0.14 or 14 mg/kg bw per day by stomach tube for 8 months. Animals given the higher dose showed delayed weight gain and significantly decreased haemoglobin concentration and erythrocyte count. Adrenaline injections were used to study the effects of 3,4-xylenol on the capacity to regulate blood pressure. This capacity was impaired at the higher dose, but it is unclear whether the effect was statistically significant. The high dose had adverse effects on the cells of the liver, spleen, kidneys, and heart, but no pathological or histological changes were seen at the lower dose. The NOEL was 0.14 mg/kg bw per day (Maasik, 1970).
Thymol
(No. 709)
Two groups of five male and five female weanling Osborne-Mendel rats received a diet containing thymol (2-isopropyl-5-methylphenol, No. 709) at a concentration of 1000 or 10 000 mg/kg for 19 weeks, calculated (Food & Drug Administration, 1993) to provide average intakes of 50 or 500 mg/kg bw per day. Body weights, food intake, and the general condition of each animal were recorded weekly. At the end of the study, haematological parameters were measured in both groups and animals at the higher dose were necropsied. No effects on growth or haematological parameters were reported, and no macroscopic or microscopic changes were observed in tissues from rats at the higher dose. The NOEL was 1000 mg/kg bw per day (Hagan et al., 1967).
Resorcinol
(No. 712)
Mice
Groups of five male and five female B6C3F1 mice were given resorcinol (No. 712) at a dose of 0, 37.5, 75, 150, 300, or 600 mg/kg bw per day by oral gavage on 5 days/week for 17 days. The clinical findings at the three higher doses included prostration and tremors lasting up to 2 h. One male at 300 mg/kg bw per day and five females and four males at 600 mg/kg bw per day died during the study. At necropsy, the organ weights were found to be similar to those of control animals. Microscopic examination of tissues revealed no abnormalities attributable to treatment (National Toxicology Program, 1992b).
Groups of 10 male and 10 female B6C3F1 mice were given resorcinol (No. 712) at a dose 0, 28, 56, 112, 220, or 420 mg/kg bw per day by oral gavage for 13 weeks. Deaths during the study occurred only among animals at the highest dose (seven males and seven females during the first week, one male at week 4, and one female at week 12). Dypnoea, prostration, and tremors were observed in males at the highest dose-within 30 min after treatment. At the end of the study, the two surviving male; at this dose showed a statistically significant decrease in mean body weight. No other significant changes in body weight were observed. Significant decreases in the absolute and relative weights of the adrenal gland were observed in all groups of treated males. Haematology and clinical chemistry showed no effects, and gross and microscopic examinations revealed no lesions related to treatment. The NOEL was 220 mg/kg, bw per day (National Toxicology Program, 1992b).
Rats
Groups of five male and five female Fischer 344/N rats were given resorcinol (No. 712) at a dose of 0, 27.5, 55, 110, 220, or 450 mg/kg bw per day by oral gavage on 5 days/week for 17 days. Males at the two higher doses and females at the four higher doses showed clinical signs including hyperexcitability and tachypnoea lasting up to 2 h. None of the rats died. The final mean body weights of the treated animals were comparable to those of controls. Females at the highest dose had significantly decreased absolute and relative thymus weights, but no other significant changes in organ weights were reported. Microscopic examination of the tissues revealed no abnormalities attributable to treatment (National Toxicology Program, 1992b).
Groups of 10 male and 10 female Fischer 344/N rats were given resorcinol (No 712) at a dose 0, 32, 65, 130, 260, or 520 mg/kg bw per day by oral gavage for 13 weeks. Eight males and 10 females at the highest dose died within the first 4 weeks; the deaths of two males and four females in the group at 260 mg/kg by per day dose during the first week were attributed to accidental administration o 520 mg/kg bw per day. The final mean body weights of the treated animals were comparable to those of controls. At necropsy, statistically significant increases it the absolute and relative weights of the liver were reported for males receiving 130 or 260 mg/kg bw per day and females receiving 65, 130, or 260 mg/kg bw per day of resorcinol. Significantly increased absolute and relative adrenal gland weights were also reported in all surviving treated males. Haematology, clinical chemistry, and gross and microscopic examination revealed no findings related to treatment (National Toxicology Program, 1992b).
2,6-Dimethoxyphenol
(No. 721)
Groups of 20-32 male and female Charles River CD rats were fed a basal diet supplemented with 2,6-dimethoxyphenol (No. 721) for 90 days, providing average intakes of 6.0 mg/kg bw per day for males and 6.8 mg/kg bw per day for females. A control group was fed the basal diet alone. Weekly measurements of body weight, food consumption, and calculated efficiency of food use showed no significant differences from controls. Haematological and clinical chemical examinations at weeks 7 and 13 showed no effects. At necropsy, there were no significant changes in liver or kidney weights. Gross and histological examination of a wide range of organs (unspecified in the report) revealed no lesions related to treatment with either substance. The NOEL was 6.0 mg/kg bw per day (Posternak & Linder, 1969).
4-(
para-Hydroxyphenyl)-2-butanone (No. 728)
Groups of 15 male and 15 female SPF-derived CFE rats were given diet; containing 4-(para-hydroxyphenyl)-2-butanone (No. 728) at a concentration of 0, 0.1, 0.2, 0.4, or 1.0% for 13 weeks. The average intakes, calculated on the basis of data on body weights and food consumption, were 0, 70, 140, 280, and 700 mg/kg bw per day. No changes in appearance, behaviour, or food intake were noted at any time during the study. A slight but statistically significant (p < 0.05) reduction in weight gain was observed in male rats at the highest dose from week 5 onwards, but the decrease in weight was not associated with decreased food consumption. No significant differences were found in absolute organ weights. In males, the relative weights of the liver and kidney were increased at the two higher doses and those of the adrenal gland at the highest dose. The increases in relative organ weights may have been due to the decrease in body weight. Urinary analyses showed ketones in the urine of all treated animals at 7 and 13 weeks. The authors reported that this effect appeared within 12 h in rats fed a diet containing 1% 4-(para-hydroxyphenyl)-2-butanone for-7 days, and disappeared within 9 h when the rats were returned to normal diet. The ketonuria, which was possibly due to metabolites present in the urine, was considered not to be a toxic effect. Histopathological examination of rats at the highest dose showed no effect of treatment on organs of the digestive, reproductive, circulatory, or central nervous systems (Gaunt et al.,1970). The NOEL of 280 mg/kg bw per day is 10 000 times greater than the estimated daily per capita intake of 4-(para-hydroxyphenyl)-2-butanone in Europe (46 µg/kg bw per day) and the USA (63 µg/kg bw per day) from use as a flavouring substance.
Phenyl salicylate
(No. 736)
A study in which three beagle dogs (sex not specified) were given phenyl salicylate (No. 736) at a dose of 250 or 500 mg/kg bw per day in a capsule for 51 days was reported as an abstract. All the treated animals showed decreased appetite, body weight, and activity and dark urine and faeces. The serum activities of alanine and aspartate aminotransferases were elevated. In a subsequent study, three beagle dogs were given phenyl salicylate at a dose of 125 mg/kg bw per day in a capsule for 51 days. At this dose, phenol excretion was markedly elevated. Haematology and tests for liver and kidney function, performed regularly, showed no effects. At necropsy, no gross or microscopic abnormalities were observed, but the usefulness of this study is limited by deficiencies in reporting (Kociba et al., 1976).
2.3.2.4 Long-term studies of toxicity and carcinogenicity
Phenol
(No. 690)
Mice
Groups of 50 male and 50 female B6C3F1 mice were given tap-water containing phenol (No. 690) at a concentration of 0, 2500, or 5000 mg/L for 103 weeks, calculated (Food & Drug Administration, 1993) to provide average intakes of 0, 620, or 1200 mg/kg bw per day. The animals were observed twice daily and weighed every 2 weeks during the first 12 weeks, monthly thereafter, and at the end of the study. Water consumption was recorded weekly. Gross and histological examinations were performed on all major organs and tissues, including those of animals found dead or killed moribund during the study. No clinical signs of toxicity were seen. Although the mean body weights were significantly reduced at most times in all treated groups, the food consumption did not differ significantly from that of controls. Water consumption was depressed by 25% in the low-dose group and 40-50% in the high-dose group compared with controls. Neoplasms were observed in both control and treated animals but were of similar number and type to those observed in ageing control B6C3F1 mice in previous studies. Phenol was considered not to be carcinogenic, and there was no histological evidence of toxicity (National Toxicology Program; 1980).
Rats
Groups of 50 male and 50 female Fischer 344 rats were given tap-water containing phenol (No. 690) at a concentration of 0; 2500; or 5000 mg/L for 103 weeks calculated (Food & Drug Administration, 1993) to provide average intakes of 0, 250, or 500 mg/kg bw per day. The same protocol of observations and necropsy as used in the study in mice described above was followed. There was no significant difference in the rate of survival between treated and control rats. Animals at the high dose had lower mean body weights than controls, although the food consumption was comparable. The water consumption of rats at the low and high doses was depressed by 20% and 10% of that of controls, respectively. No clinical signs of toxicity were observed. Significantly increased incidences of leukaemias, lymphomas, and phaeochromocytomas of the adrenal medulla were observed only in males at the low dose. However, none of the tumours were clearly associated with exposure to phenol. The NOEL was 250 mg/kg bw per day (National Toxicology Program, 1980).
Resorcinol
(No. 712)
Mice
Groups of 60 male and 60 female B6C3F1 mice were given resorcinol (No. 712) at a dose of 0, 110, or 220 mg/kg bw per day in deionized water by oral gavage on 5 days/week for 104 weeks. All animals were observed twice daily, and clinical signs were noted every 4 weeks. Body weights were measured weekly through week 13 and every 4 weeks thereafter until the last 3 months, when they were recorded every 2 weeks. Ten mice of each sex per dose were selected for interim evaluations of organ weights, haematological and clinical chemical parameters, and histology. Necropsies were performed on all remaining animals at the end of the study. Complete histological examinations were conducted on all control and high-dose mice. For animals at the low dose, only tissues containing gross lesions were examined microscopically at both the interim and terminal evaluations.
The mean body weights of all treated males and females at the low dose were comparable to those of controls throughout the study; however, the mean body weights of females at the high dose were 10-15% lower than those of controls from week 85 through week 104. Recumbence and tremors were common among all treated mice for a short period after dosing. No treatment-related effects were reported at the 15-month interim evaluation. The survival rate of treated mice at termination was similar to that of controls. Some animals examined at 6, 12, 18, and 24 months had mouse hepatitis virus, but no clinical or histological evidence of disease was detected. The incidences of neoplastic and non-neoplastic lesions were not statistically or biologically increased in any group of mice. The clinical signs (ataxia, recumbency and tremors) observed in all treated mice suggest an effect on the central nervous system; however, there were no associated morphological findings. Similar clinical signs were observed in rats in the bioassay described below (National Toxicology Program, 1992a).
Rats
Groups of 60 male and 60 female Fischer 344/N rats were given resorcinol (No. 712) at a dose of 0, 110, or 220 mg/kg bw per day in deionized water by oral gavage on 5 days/week for 103 weeks. Some rats were given one or two extra doses during week 104. As 16 females at the high dose died after 22 weeks of treatment, four further groups of 60 females were given doses of 0, 50, 100, or 150 mg/kg bw per day. The same protocol of observations and evaluations as used in the study in mice described above was followed. Ten rats of each sex per dose were used for interim evaluations of organ weights, haematology, clinical chemistry, and histology at 15 months. As several males at the high dose died prematurely, the remaining animals in the interim group of males at this dose were not killed as originally planned. Instead, 10 males at the high dose in the 2-year study group that either died or were killed when moribund during weeks 62 and 67 were used for the interim histological evaluation. Organ weighings, haematology, and clinical chemistry were not performed in this group of male rats.
At the 15-month interim evaluation, significantly reduced terminal body weights were found for high-dose males (220 mg/kg bw per day) and females (150 mg/kg bw per day). Significantly increased relative weights of the brain were found in males at the low dose and of the liver in females at the high dose, which were associated with decreased body weights. Haematology and clinical chemistry revealed no treatment-related effects (assessed in female rats only). No treatment-related neoplastic or non-neoplastic lesions were found in any group.
The mean body weights of males given 220 mg/kg bw per day and females given 150 mg/kg bw per day were significantly lower than those of controls throughout the 2-year study, whereas the mean body weights of animals at the low dose were comparable to those of controls. Haematology and clinical chemistry revealed normal values. Ataxia, prostration, salivation, and tremors were observed shortly after treatment in all groups of treated males and in females receiving 100 or 150 mg/kg bw per day. These effects lasted for 0.5-1 h and became more pronounced at the end of each 5-day dosing period. The rate of survival of males and females at the high dose was significantly lower than that of controls. At the end of the study, 57% of control males, 15% of high-dose males, 68% of control females, and 50% of high-dose females were still alive. Some animals examined at 6,12, 18, and 24 months were found to be infected with Sendai virus and rat sialodacryoadenitis virus (which is a type of rat coronavirus); however, no clinical or histological evidence of disease was found. Complete histological examination on all remaining animals at the end of the study revealed no pathological lesions. The incidence of neoplastic or non-neoplastic lesions was not statistically or biologically significantly increased in any treated group. A statistically significant decrease in the incidence of mammary gland fibroadenomas was observed in all three groups of treated females, the lesions occurring in 18% of high-dose females, 24% of mid-dose females, 28% of low-dose females, and 50% of control females. In all groups, the incidences were within the range of historical controls (16-53%). Although clinical signs (ataxia, prostration, salivation, and tremors) suggesting an effect of resorcinol on the central nervous system were observed in male rats at both doses and females at the two higher doses, there were no related morphological findings. Similar clinical signs were observed in mice in the bioassay described above (National Toxicology Program, 1992b).
2.3.2.5 Genotoxicity
In vitro
Negative results were reported in the standard assay for reverse mutation in Salmonella typhimurium strains TA98, TA100, TA1535, and TA1537 incubated with phenol at concentrations up to 9400 µg/plate (Florin et al., 1980; Gocke et al., 1981; Pool & Lin, 1982; Haworth et-al., 1983; Aeschbacher et al.; 1989; Massey et al., 1994); ortho-cresol at up to 5000 µg/plate (Douglas et a1., 1980; Florin et al., 1980 Nestmann et al., 1980; Pool & Lin, 1982; Haworth et al., 1983; Massey et al., 1994); meta-cresol and para-cresol at up to 5000 µg/plate (Douglas et a1., 1980; Florin et al., 1980; Nestmann et al., 1980; Pool & Lin,.1982;Haworth et al., 1983); para-ethylphenol, 2,5-xylenol, 2,6-xylenol, and 3,4-xylenol at 367 µg/plate (Florin et al., 1980); 4-(1,1-dimethyl)ethyl phenol at up to 2000 µg/plate (Dean et al., 1985); thymol at up to 1000 µg/plate (Florin et al., 1980; Azizan & Blevins, 1995); 2-phenylphenol at up to 200 µg/plate (National Toxicology Program, 1986); resorcinol at up to 7700 µg/plate (Gocke et al., 1981; Haworth et al., 1983); guaiacol at up to 111 726 µg/plate (Douglas et al., 1980; Nestmann et al., 1980; Pool & Lin, 1982; Haworth et al., 1983; Aeschbacher et al., 1989); 2,6-dimethoxyphenol at up to 16 000 µg/plate (McMahon et al., 1979; Douglas et al., 1980; Florin et al., 1980; Pool & Lin, 1982); 2-hydroxyacetophenone at 408 µg/plate (Florin et al., 1980); and phenyl salicylate at up to 333 µg (Zeiger et al., 1987), with and without metabolic activation (see Table 4). However, in an assay with a modified minimal ZLM medium for Escherichia coli, the results varied by bacterial strain (Gocke et al., 1981). At concentrations of 470-9400 µg/plate, phenol caused reverse mutation only in strain TA98 in the presence of metabolic activation. Resorcinol was mutagenic at doses of 550-7700 µg/plate only in TA1535 without metabolic activation and in TA100 with metabolic activation. The same authors reported negative results in all five S. typhimurium strains with and without metabolic activation in the standard Vogel-Bonner medium, which contains a concentration of citrate and other ions that is two to four times higher (Gocke et al., 1981). Negative results with phenol and resorcinol at doses up to 3333 µg/plate were reported in another study when Vogel-Bonner medium was used (Haworth et al., 1983). No mutagenicity was found in E. coli exposed to 2,6-dimethoxyphenol at concentrations up to 1000 µg/ml (McMahon et al., 1979).
Forward mutation was not induced in mouse lymphoma L5178YTk+/- cells by phenol at concentrations of 100-3200 µg/ml or resorcinol at 125-2000 µg/ml without metabolic activation (McGregor et al., 1988). However, positive results were reported with phenol at concentrations of 5-42 µg/ml with activation and 178-887 µg/ml without activation (Wangenheim & Bolcsfoldi, 1988). Positive results in this assay were also reported with 2-phenylphenol at doses of 0.32-60 µg/ml without metabolic activation and 0.32-5 µg/ml with activation, but these doses were cytotoxic (National Toxicology Program, 1986).
Sister chromatid exchange was not induced in human lymphocytes by phenol at concentrations up to 188 µg/ml, ortho-cresol at up to 54 µg/ml, meta-cresol at up to 108.µg/ml, para-cresol at up to 54 µg/ml, para-ethyl phenol at up to 27 µg/ml, 2,6-xylenol at up to 31 µg/ml, resorcinol at up to 28 µg/ml, 2,6-dimethoxyphenol at up to 77 µg/ml (Jansson et al., 1986, 1988) or para-vinylphenol at up to 12 µg/ml (Jansson et al., 1988) (see Table 4). Phenol at 19 µg/ml also did not induce sister chromatid exchange in human lymphocytes, but positive results were reported when the
Table 4. Results of studies of genotoxicity with phenol and phenol derivatives used as flavouring substances
|
No. |
Substance |
End-point |
Test system |
Concentration |
Result |
Reference |
|
690 |
Phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA102 |
6.59-6587 µg/platea,b |
Negative |
Aeschbacher et al. (1989) |
|
690 |
Phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
3 µg/platea,b |
Negative |
Florin et al. (1980) |
|
690 |
Phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
0-9400 µg/platea,b |
Negative |
Gocke et al. (1981) |
|
690 |
Phenol |
Reverse mutationc |
S. typhimurium TA98 |
0-9400 µg/platea
470-9400 µg/plateb |
Negative
Positive |
Gocke et al. (1981) |
|
690 |
Phenol |
Reverse mutationc |
S. typhimurium TA100, TA1535, TA1537, TA1538 |
0-9400 µg/platea,b |
Negative |
Gocke et al. (1981) |
|
690 |
Phenol |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA98, TA100 |
33-3333 µg/platea,b |
Negative |
Haworth et al. (1983) |
|
690 |
Phenol |
Reverse mutation |
S. typhimurium TA98, TA100 |
5 µg/platea,b |
Negative |
Massey et al. (1994) |
|
690 |
Phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
5000 µg/platea,b |
Negative |
Pool & Lin (1982) |
|
690 |
Phenol |
Forward mutation |
Mouse lymphoma cells |
100-3200 µg/mla |
Negative |
McGregor et al. (1988) |
|
690 |
Phenol |
Forward mutation |
Mouse lymphoma cells |
178-887 µg/mla
5-42 µg/mlb |
Positive |
Wangenheim & Bolcsfoldi (1988) |
|
690 |
Phenol |
Sister chromatid exchange |
Human lymphocytes |
0-188 µg/ml |
Negative |
" Jansson et al. (1986) |
|
690 |
Phenol |
Sister chromatid exchange |
Human lymphocytes |
19 µg/ml
94 µg/ml |
Negative
Positive |
Morimoto & Wolff (1980) |
|
690 |
Phenol |
Sister chromatid exchange |
Chinese hamster ovary cells |
300-400 µg/mla
1670-3000 µg/mlb |
Positive
Weakly positive |
Ivett et al. (1989) |
|
690 |
Phenol |
Chromosomal aberration |
Chinese hamster ovary cells |
600-800 µg/mla
2000-3000 µg/mlb |
Negative
Positive |
Ivett et al. (1989) |
|
690 |
Phenol |
DNA strand breaks |
Chinese hamster ovary cells |
94, 110 µg/ml |
Negative |
Sze et al. (1996) |
|
690 |
Phenol |
Micronucleus formation |
Human lymphocytes |
94 µg/mla |
Negative |
Robertson et al. (1991) |
|
691 |
ortho-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
2.5 µl/platea,b |
Negative |
Douglas et al. (1980) |
|
691 |
ortho-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
324 µg/platea,b |
Negative |
Florin et al. (1980) |
|
691 |
ortho-Cresol |
Reverse mutation |
S. typhimurium TA 1535, TA1537, TA98, TA100 |
1-100 µg/platea,b |
Negative |
Haworth et al. (1983) |
|
691 |
ortho-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100 |
5 µg/platea,b |
Negative |
Massey et al. (1994) |
|
691 |
ortho-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA7535, TA1537 TA1538 |
2600 µg/platea,b |
Negativeb |
Nestmann et al. (1980) |
|
691 |
ortho-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
5000 µg/platea,b |
Negative |
Pool & Lin (1982) |
|
691 |
ortho-Cresol |
Sister chromatid exchange |
Human fibroblasts |
86.5-433 µg/ml
865 µg/ml |
Negative
Weakly positive |
(Cheng & Kligerman (1984) |
|
691 |
ortho-Cresol |
Sister chromatid exchange |
Human lymphocytes |
0-54 µg/ml |
Negative |
Jansson et al. (1986) |
|
691 |
ortho-Cresol |
Sister chromatid exchange |
Human lymphocytes |
0-54 µg/ml |
Negative |
Jansson et al. (1988) |
|
692 |
meta-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
2000 µg/platea,b |
Negative |
Douglas et al. (1980) |
|
692 |
meta-Cresol |
Reverse mutation |
S. typhimurium TA98, A100, TA1535, TA1537, TA1538 |
324 µg/platea,b |
Negative |
Florin et al. (1980) |
|
692 |
meta-Cresol |
Reverse mutation |
S. typhimurium TA 1535, TA1537, TA98, TA100 |
3.3-333 µg/platea,b |
Negatived |
Haworth et al. (1983) |
|
692 |
meta-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 TA1538 |
2000 µg/platea,b |
Negatived |
Nestmann et al. (1980) |
|
692 |
mete-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
5000 µg/platea,b |
Negative |
Pool & Lin (1982) |
|
692 |
meta-Cresol |
Sister chromatid exchange |
Human fibroblasts |
86.5-865 µg/ml |
Negative |
Cheng & Kligerman (1984) |
|
692 |
meta-Cresol |
Sister chromatid exchange |
Human lymphocytes |
0-108 µg/ml |
Negative |
Jansson et al. (1986) |
|
692 |
meta-Cresol |
Sister chromatid exchange |
Human lymphocytes |
0-108 µg/ml |
Negative |
Jansson et al. (1988) |
|
693 |
para-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
1000 µg/platea,b |
Negative |
Douglas et al. (1980) |
|
693 |
para-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
324 µg/platea,b |
Negative |
Florin et al. (1980) |
|
693 |
para-Cresol |
Reverse mutation |
S. typhimurium TA 1535, TA1537, TA98, TA100 |
3.3-333 µg/platea,b |
Negative |
Haworth et al. (1983) |
|
693 |
para-Cresol |
Reverse mutation |
S. typhimurium TA98, TA 100 |
5 µg/platea,b |
Negative |
Massey et al. (1994) |
|
693 |
para-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
1000 µg/platea,b |
Negatived |
Nestmann et al. (1980) |
|
693 |
para-Cresol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
5000 µg/platea,b |
Negative |
Pool and Lin (1982) |
|
693 |
para-Cresol |
Sister chromatid exchange |
Human fibroblasts |
86.5-865 µg/ml |
Negative |
Cheng & Kligerman (1984) |
|
693 |
para-Cresol |
Sister chromatid exchange |
Human lymphocytes |
0-54 µg/ml |
Negative |
Jansson et al. (1986) |
|
693 |
para-Cresol |
Sister chromatid exchange |
Human lymphocytes |
0-54 µg/ml |
Negative |
Jansson et al. (1988) |
|
694 |
para-Ethylphenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
367 µg/platea,b |
Negative |
Florin et al. (1980) |
|
694 |
para-Ethylphenol |
Sister chromatid exchange |
Human lymphocytes |
0-27 µg/ml |
Negative |
Jansson et al. (1986) |
|
694 |
para-Ethylphenol |
Sister chromatid exchange |
Human lymphocytes |
0-2.7 µ/ml |
Negative |
Jansson et al. (1988) |
|
733 |
4-(1,1-Dimethyl)-ethyl phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
0.2-2000 µg/platea,b |
Negative |
Dean et al. (1985) |
|
733 |
4-(1,1-Dimethyl)-ethyl phenol |
Reverse mutation |
E. coli. WP2 and WP2 uvrA |
0.2-2000 µg/platea,b |
Negative |
Dean et al. (1985) |
|
733 |
4-(1,1-Dimethyl)-ethyl phenol |
Mitotic gene conversion |
S. cerevisiae JD1 |
0.2-2000 µg/platea,b |
Negative |
Dean et al. (1985) |
|
733 |
4-(1,1-Dimethyl)-ethyl phenol |
Chromosomal aberration |
Rat liver cell lines RL1, RL2 |
Not specifiede |
Negative |
Dean et al. (1985) |
|
706 |
2,5-Xylenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
367 µg/platea,b |
Negative |
Florin et al. (1980) |
|
707 |
2,6-Xylenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
367 µg/platea,b |
Negative |
Florin et al. (1980) |
|
707 |
2,6-Xylenol |
Sister chromatid exchange |
Human lymphocytes |
0-31 µg/ml |
Negative |
Jansson et al. (1986) |
|
707 |
2,6-Xylenol |
Sister chromatid exchange |
Human lymphocytes |
0-31 µg/ml |
Negative |
Jansson et al. (1988) |
|
708 |
3,4-Xylenolv |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
367 µg/platea,b |
Negative |
Florin et al. (1980) |
|
709 |
Thymol |
Reverse mutation |
S. typhimurium TA97, TA98, TA100 |
1000 µg/mla,b |
Negative |
Azizan & Blevins (1995 |
|
709 |
Thymol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA7537, TA1538 |
451 µg/platea,b |
Negative |
Florin et al. (1980) |
|
709 |
Thymol |
Sister chromatid |
Syrian hamster embryo cells |
0.3-30 µg/ml |
Positive |
Fukuda (1987) |
|
709 |
Thymol |
Unscheduled DNA synthesis |
Syrian hamster embryo cells |
0.3-10 µg/mla
1-10 µg/mlb |
Negative
Positive |
Fukuda (1987) |
|
735 |
2-Phenyl-phenol |
Reverse mutation |
S. typhimurium TA98, TA700, TA1537 |
3.3-200 µg/platea,b |
Negative |
National Toxicology Program (1986) |
|
735 |
2-Phenyl-phenol |
Reverse mutation |
S. typhimurium TA1535 |
3.3-200 µg/platea
3.3-200 µg/plateb |
Positive
Negative |
National Toxicology Program (1986) |
|
735 |
2-Phenyl-phenol |
Forward mutation |
Mouse lymphoma cells |
20-60 µg/mla
0.32-5 µg/mlb |
Positive
|
National Toxicology Program (1986) |
|
735 |
2-Phenyl-phenol |
Sister chromatid exchange |
Chinese hamster embryo cells |
14.9-20 µg/mla
29.9 µg/mla
14.9-29.9 µg/mb |
Negative
Positive
Negative |
National Toxicology Program (1986) |
|
735 |
2-Phenyl-phenol |
Chromosomal aberrations |
Chinese hamster embryo cells |
60-80 µg/mla,b |
Negative |
National Toxicology Program (1986) |
|
711 |
para-Vinyl-phenol |
Sister chromatid exchange |
Human lymphocytes |
0-12 µg/ml |
Negative |
Jansson et al. (1988) |
|
712 |
Resorcinol |
Reverse mutationc |
S. typhimurium TA1535 |
550-7700 µg/platea
0-7700 µg/plateb |
Positive
Negative |
Gocke et al. (1981) |
|
712 |
Resorcinol |
Reverse mutationc |
S. typhimurium TA100 |
550-7700 µg/plateb
0-7700 µg/platea |
Positive
Negative |
Gocke et al. (1981) |
|
712 |
Resorcinol |
Reverse mutations |
S. typhimurium TA98, TA1537, TA1538 |
0-7700 µg/platea,b |
Negative |
Gocke et al. (1981) |
|
712 |
Resorcinol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
0-7700 µg/platea,b |
Negative |
Gocke et al. (1981) |
|
712 |
Resorcinol |
Reverse mutation |
S. typhimurium TA 1535, TA1537, TA98, TA100 |
33-3333 µg/platea,b |
Negative |
Haworth et al. (1983) |
|
712 |
Resorcinol |
Forward mutation |
Mouse lymphoma cells |
125-2000 µg/mla |
Positive |
McGregor et al. (1988) |
|
712 |
Resorcinol |
Sister chromatid exchange |
Human lymphocytes |
0-28 µg/ml |
Negative |
Jansson et al. (1986) |
|
712 |
Resorcinol |
Sister chromatid exchange |
Human lymphocytes |
0-28 µg/ml |
Negative |
Jansson et al. (1988) |
|
712 |
Resorcinol |
Sister chromatid exchange |
Chinese hamster embryo cells |
0.6-2.2 µg/ml |
Negative |
Wild et al. (1981) |
|
713 |
Guaiacol |
Reverse mutation |
S. typhimurium TA98, TA100, TA102 |
1-111 726 µg/platea,b |
Negative |
Aeschbacher et al. (1989) |
|
713 |
Guaiacol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
16 000 µg/platea,b |
Negative |
Douglas et al. (1980) |
|
713 |
Guaiacol |
Reverse mutation |
S. typhimurium TA1535, TA1537, TA98, TA100 |
33-10 000 µg/platea,b |
Negative |
Haworth et al. (1983) |
|
713 |
Guaiacol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
16 000 µg/platea,b |
Negative |
Nestmann et al. (1980) |
|
713 |
Guaiacol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
5000 µg/platea,b |
Negative |
Pool & Lin (1982) |
|
713 |
Guaiacol |
Sister chromatid exchange |
Human lymphocytes |
£ 31 µg/ml |
Positive |
Jansson et al. (1988) |
|
715 |
2-Methoxy-4-methyl-phenol |
Sister chromatid exchange |
Human lymphocytes |
£ 138 µg/ml |
Positive |
Jansson et al. (1988) |
|
716 |
4-Ethylguaiacol |
Sister chromatid exchange |
Human lymphocytes |
0-152 µg/ml |
Negative |
Jansson et al. (1988) |
|
721 |
2,6-Dimethoxy-phenol |
Reverse mutation |
S. typhimurium TA98, TA700, TA1535, TA1537, TA1538 |
16 000 µg/platea,b |
Negative |
Douglas et al. (1980) |
|
721 |
2,6-Dimethoxy-phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
463 µg/platea,b |
Negative |
Florin et al. (1980) |
|
721 |
2,6-Dimethoxy-phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537 |
0.1-1000 µg/mla,b |
Negative |
McMahon et al. (1979) |
|
721 |
2,6-Dimethoxy-phenol |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
5000 µg/platea,b |
Negative |
Pool & Lin (1982) |
|
721 |
2,6-Dimethoxy-phenol |
Reverse mutation |
E. coli |
1-1000 µg/mla,b |
Negative |
McMahon et al. (1979) |
|
721 |
2,6-Dimethoxy-phenol |
Sister chromatid exchange |
Human lymphocytes |
0-77 µg/ml |
Negative |
Jansson et al. (1986) |
|
721 |
2,6-Diinethoxy-phenol |
Sister chromatid exchange |
Human lymphocytes |
0-77 µg/ml |
Negative |
Jansson et al. (1988) |
|
725 |
2-Methoxy-4-vinyl-phenol |
Sister chromatid exchange |
Human lymphocytes |
£ 75 µg/ml |
Positive |
Jansson et al. (1988) |
|
727 |
2-Hydroxyacetophenone |
Reverse mutation |
S. typhimurium TA98, TA100, TA1535, TA1537, TA1538 |
408 µg/platea,b |
Negative |
Florin et al. (1980) |
|
736 |
Phenyl salicylate |
Reverse mutation |
S. typhimurium TA97, TA98, TA100, TA1535, TA1537 |
3-333 µg/platea,b |
Negative |
Zeiger et al. (1987) |
a
Without metabolic activation
b
With metabolic activation
c
ZLM medium used in place of Vogel-Bonner medium
d
Presumably non-mutagenic, but solubility did not allow testing in amounts that result in lethality
e
Concentrations selected corresponded to 0.5, 0.25, and 0.125 of the concentration that caused 50% growth, inhibition (not specified) as determined in an assay for cytotoxicity.
concentration was increased to 94 µg/ml (Morimoto & Wolff, 1980). This concentration greatly exceeds that which induces cytotoxicity (47 µg/ml), as estimated by the authors. Genotoxic effects observed in conjunction with cytotoxicity may be artefacts of lysosome breakdown and release of DNase. Such action leads to DNA single- and double-strand breaks, sister chromatid exchange, and chromosomal aberration (Bradley et al., 1987). Sister chromatid exchange was also induced in human lymphocytes incubated with guaiacol at doses up to 31 µg/ml or with 2-methoxy-4-methy1phenol (No. 715) at doses up to 138 µg (Jansson et al., 1988); however, the cytotoxic concentration was. not established for either substance. No evidence of sister chromatid exchange was found in human fibroblasts exposed to ortho-cresol at concentrations up to 433 µg/ml or to meta- or para-cresol at 865 µg/ml (Cheng & Kligerman, 1984), or in Chinese hamster ovary cells exposed to resorcinol at 0.6-2 µg/ml (Wild et al., 1981). Weakly positive results were reported with ortho-cresol at a concentration of 865 µg/ml (Cheng & Kligerman, 1984).
Sister chromatid exchange was induced by phenol at doses of 300-400 µg/ml in Chinese hamster ovary cells without metabolic activation, and weakly positive results were reported at 1670-3000 µg/ml with metabolic activation. Cytotoxic effects, including cell cycle delay, were also observed at these doses (Ivett et al., 1989). After a 27-h expression period, sister chromatid exchange was induced in Chinese hamster ovary cells incubated with 2-phenylphenol at 15-30 µg/ml without metabolic activation. When the treated cells were allowed a 42-h expression period, however, the frequency of sister chromatid exchange was reduced to the control level. The authors suggested that sister chromatid exchange is induced by 2-phenylphenol in the first and second S-phases, but that the DNA damage caused by the substance is eliminated after a subsequent round of DNA synthesis. There was no evidence of sister chromatid exchange in the presence of metabolic activation (National Toxicology Program, 1986). In an assay to determine the ability of thymol to induce sister chromatid exchange in Siberian hamster embryo cells, positive results were reported with concentrations of 0.3-30 µg/ml, but negative results were reported in the absence of metabolic activation (Fukuda, 1987).
No chromosomal aberrations were induced when Chinese hamster ovary cells were incubated with phenol at 600-800 µg/ml without metabolic activation (Ivett et al., 1989) or 2-phenylphenol at 60-80 µg/ml with and without metabolic activation (National Toxicology Program, 1986). However, positive results were reported when a metabolic activation system was added and the phenol concentration was increased to 2000-3000 µg/ml (Ivett et al., 1989). The authors did not determine the cytotoxic threshold or the pH of the test media. Mammalian cells in situ rely on complex regulatory mechanisms to maintain homeostatic conditions, and those in culture are not equipped to respond to environmental changes. The pH of the culture media used in mammalian cell assays must be maintained at approximately 6.8-7.5, because lower values or changes in osmolality due to acidic or ionizing test substances (e.g. phenol) may result in false-positive results, especially with metabolic activation systems. Acidity facilitates the breakdown of the components of such systems into mutagenic agents (Brusick, 1986). Micronuclei were not induced in human lymphocytes exposed to phenol at a concentration of 94 µg/ml without metabolic activation (Robertson et al., 1991).
No evidence of DNA strand breaks was found in Chinese hamster ovary cells incubated with phenol at a dose of 94 110 µg/ml (Sze et al., 1996). Unscheduled DNA synthesis did not occur in Syrian hamster embryo cells incubated with thymol at 1-10 µg/ml without the addition of metabolic activation; however, positive results were reported at the same concentrations when an activation system was added (Fukuda, 1987).
In vivo
The results of assays for genotoxicity in vivo were predominantly negative. The frequency of micronucleated polychromatic erythrocytes was not increased in mice after intraperitoneal injections of phenol of doses of 40-188 mg/kg bw (Barale et al., 1990; Shelby et al., 1993; Marrazzini et a1.; 1994; Chen & Eastmond, 1995 [three doses of 50-100 mg/bw administered 24 h apart]). Negative results were obtained in the same assay after intraperitoneal injections of resorcinol at doses of 55-220 mg/kg bw (Gocke et al., 1981 [two doses of 220 mg/kg bw administered 24 h apart]; Wild et al.; 1981). Increased frequencies of micronuclei were reported in mice given intraperitoneal injections of phenol at 120-180 mg/kg bw (Shelby et al., 1993; Marrazzini et al., 1994; Chen & Eastmond, 1995 [three doses of 160 mg/kg bw administered 24 h apart]) or a single oral dose of 250 mg/kg bw (Karim et al., 1986).
The ability of ortho-cresol (No. 691), meta-cresol (No. 692), and para-cresol (No. 693) to induce sister chromatid exchange was also studied in vivo (Cheng & Kligerman, 1984). Mouse bone-marrow cells, alveolar macrophages, and regenerating liver cells were examined after intraperitoneal administration of ortho- or meta-cresol at 200 mg/kg bw or para-cresol at 75 mg/kg bw. The results were negative.
2-Phenylphenol has been tested in a variety of tests for genetic toxicity, both in vitro and in vivo. Scattered positive results were observed in vitro, primarily in tests for chromosomal aberrations. In vivo, 2-phenylphenol did not induce chromosomal aberrations an rat bone marrow or dominant lethal mutations in mice. No DNA damage or DNA adduct formation was observed in the urinary bladder of male mice treated with [14C]2-phenyl phenol. DNA adducts were found by 32P-postlabelling in the urinary bladder, but only at high doses. This observation does not indicate covalent binding of 2-phenylphenol to DNA. The sodium salt of 2-phenylphenol (sodium ortho-phenylphenate) and its metabolites phenyl hydroquinone and phenylbenzoquinone, although less extensively. tested, show the same profile of responses in tests for genetic toxicity as 2-phenylphenol (WHO, 2000).
Overall, the 48 phenol derivatives in this group of flavouring substances are unlikely to be genotoxic in vivo.
2.3.2.6 Reproductive toxicity
Phenol (No. 690), para-cresol (No. 693), resorcinol (No. 712), and hydroquinone monoethyl ether (No. 720)
In a study to develop a model for quantitative assessment of structure-activity relationships for maternal and developmental toxicity, the activity of 27 substituted phenols, including phenol (No. 690), para-cresol (No. 693), and resorcinol (No. 712), was addressed. Groups of 12-15 timed-pregnant Sprague-Dawley rats were given phenol (No. 690), para-cresol (No. 693), resorcinol (No. 712), or hydroquinone monoethyl ether (No. 720) at a dose of 0, 100, 330, 670, or 1000 mg/kg bw per day by oral intubation on day 11 of gestation. The pregnant animals were observed for clinical signs of toxicity for several hours after dosing, and all were weighed on days 10, 11, 12, 14, 17, and 21 of gestation. The weights and viability of the pups were assessed on days 1, 3 and 6 post partum. Any overt malformations were noted, and dead and moribund pups were necropsied. After weaning of each litter, the dam was killed and implantation scars in the uterus were counted. Perinatal loss of offspring was calculated as the difference between the number of uterine implantation sites and the number of live pups on postnatal day 6. The pups were necropsied after weaning and examined for any previously unnoticed external abnormalities.
Hind limb paralysis or tail malformations were found in 21% and 27% of the offspring of dams given phenol at 670 and 1000 mg/kg bw per day, respectively. Significantly decreased maternal weight was also noted in these groups. Pregnant animals that received doses ³ 330 mg/kg bw per day of para-cresol and ³ 670 mg/kg bw per day of resorcinol had decreased body weights. Decreased maternal and pup weights were observed in the groups given hydroquinone monoethyl ether at doses ³ 670 mg/kg bw per day. No statistically significant perinatal loss was observed at any dose of these substances. (Kavlock, 1990).
Thymol
(No. 709)
In a study published in 1933, thymol (No. 709) was extracted from the ether oil of Thimus vulgaris and administered to three pregnant rabbits in gelatinous capsules containing 294-299 mg/kg bw once a day for 7 days, beginning on approximately day 19 of gestation. At the end of the study, all the adult animals were necropsied. Macroscopic evaluations were performed on all organs of the chest and abdomen. Microscopic evaluations were performed on tissues from the liver, kidney, ovary, uterus, and placenta. On day 6 of dosing, one rabbit aborted five dead fetuses, however, this rabbit still received the final dose of thymol on the last day of the study. The fetuses carried by the other two rabbits were all found alive at necropsy. No major lesions were found in the uterus or placenta. The fetal liver and kidney appeared normal. No remarkable changes were found on examination of the livers of the adults, except for some nuclei of parvicellular infiltration in the interlobular zones. The kidneys showed enlargement of the capsule and parvicellular infiltrations and tubular degeneration toward the medullary. A few atresic follicles and enhanced interstitial gland development were found in the ovaries (Savignoni & de Maria, 1933).
2.3.2.7 Special studies: Effects on forestomach and glandular stomach epithelium
para-Cresol (No. 693) and 4-(1,1-dimethyl)ethylphenol (No. 733)
A group of 15 male Syrian hamsters were fed a diet containing 1.5% para-cresol (No. 693) or 4-(1,1-dimethyl)ethyl phenol (No. 733) for 20 weeks, calculated (Food & Drug Administration, 1993) to provide an average intake of 1800 mg/kg bw per day. A control group was fed the basal diet alone. At necropsy, the body and organ weights were measured, and the forestomach, glandular stomach, and urinary bladder were evaluated for hyperplasia and papillomatous lesions. The average body weight of the test animals did not differ significantly from that of the controls. A slight, nonsignificant increase in liver weight was reported in hamsters given 4-(1,1-dimethyl)ethyl phenol, and those given para-cresol had mild to moderate (< 0.5 mm) hyperplasia in the forestomach. A thickening of the epithelium that appeared as a white, keratin-like substance was reported in the anterior and posterior walls along the lesser curvature of and adjacent to the oesophagus. Prominent thickening of the forestomach epithelium, severe (> 0.5 mm) hyperplasia and papillomatous lesions of the forestomach and a significant increase in the labelling index in forestomach epithelium were observed in hamsters given 4-(1,1-dimethyl)ethyl phenol. Marked or significant changes were not reported in the stomach, and no changes were observed in the urinary bladder (Hirose et al., 1986).
Resorcinol
(No. 712)
Five young male Fischer 344 rats were fed a basal diet containing 0.8% resorcinol for 8 weeks, calculated (Food & Drug Administration, 1993) to provide an average intake of 800 mg/kg bw per day. Bromodeoxyuridine (BrdU) staining was used to assess cell proliferation and was coupled with histological examination to evaluate the effects of resorcinol on the forestomach and glandular stomach epithelium. No difference was found between treated and control animals (Shibata et al., 1990).
Resorcinol was tested in a study of the effects of dihydroxybenzene derivatives on the induction of tumours of the forestomach and glandular stomach in rats by N-methyl-N'-nitro-N-nitrosoguanidine. Two groups of 16 male Fischer 344 rats, with and without pretreatment with the nitrosamine, were given a diet containing 0.8% resorcinol for 51 weeks. This concentration was calculated (Food & Drug Administration, 1993) to provide an average intake of 800 mg/kg bw per day. A control group of rats was fed the basal diet only. Mild to moderate hyperplasia of the glandular forestomach epithelium was seen in two rats given resorcinol alone, but this incidence was not significantly different from that of the controls. Resorcinol did not induce papillomas, carcinomas, or squamous-cell carcinomas of the forestomach and did not promote tumours induced by N-methyl-N'-nitro-N-nitrosoguanidine (Hirose et al., 1989).
In a study to assess the effects of 13 phenolic compounds, including para-cresol (No. 693) and 4-(1,1-dimethyl)ethyl phenol (No. 733), on the induction of proliferative lesions in the forestomach, glandular stomach, and urinary bladder, 15 male Syrian golden hamsters were given a diet containing 0.25% resorcinol for 20 weeks. This dose was calculated (Food & Drug Administration, 1993) to provide an average intake of 300 mg/kg bw per day. Control animals were fed the basal diet alone. At necropsy, the body and liver weights of the treated animals did not differ significantly from those of controls. Twelve of the 15 hamsters given resorcinol showed mild to moderate hyperplasia of the forestomach mucosa, but the incidence of these effects did not differ significantly from that of the controls (7/12). There was no evidence of neoplastic lesions in the forestomach of hamsters given resorcinol (Hirose et al., 1986).
In a study to assess the effects of catechol antixoxidants on pancreatic carcinogenesis initiated by N-nitrosobis(2-oxopropyl)amine, a diet containing 1.5% resorcinol was fed to 15 female Syrian hamsters for 16 weeks, calculated (Food & Drug Administration, 1993) to provide an intake of 1800 mg/kg bw per day. Control animals were fed the basal diet alone. At necropsy, the pancreas, liver, and stomach were removed for evaluation. Resorcinol weakly inhibited the effects of the nitrosamine. The livers of hamsters given resorcinol alone weighed significantly less than those of controls. Epithelial hyperplasia was seen in the forestomach and glandular, stomach of resorcinol-treated animals, but there was no evidence of neoplastic lesions in the stomach, liver, or pancreas (Maruyama et al.,1991).
3. REFERENCES
Abou-el-Makarem, M. M., Millburn, P., Smith, R.L. & Williams, R.T. (1967) Biliary excretion of foreign compounds. Biochem. J., 105, 1269-1274.
Aeschbacher, H.U., Wolleb, U., Loliger, J., Spadone; J.C. & Liardon, R. (1989) Contribution of coffee aroma constituents to the mutagenicity of coffee. Food Chem. Toxicol., 27, 227-232.
Azizan, A. & Blevins, R.D. (1995) Mutagenicity and antimutagenicity testing of six chemicals associated with the pungent properties of specific spices as revealed by the Ames Salmonella microsomal assay. Arch. Environ. Contam. Toxicol., 28, 248-258.
Babich, H. & Davis, D.L. (1981) Phenol: A review of environmental and health risks. Regul. Toxicol. Pharmacol., 1, 90-109.
Bakke, O.M. (1970) o-Methylation of simple phenols in the rat. Acta Pharmacol. Toxicol., 28, 28-38.
Bakke, O.M. & Scheline, R.R. (1970) Hydroxylation of aromatic hydrocarbons in the rat. Toxicol. Appl. Pharmacol., 16, 691-700.
Ballinger, L.N., Cross, S.E. & Roberts, M.S. (1995) Availability and mean transit times of phenol and its metabolites in the isolated perfused rat liver: Normal and retrograde studies using tracer concentrations of phenol. J. Pharm. Pharmacol., 47, 949-956.
Barale, R., Marrazzini, A., Betti, C., Vangelisti, V., Loprieno, N. & Barrai, I. (1990) Genotoxicity of two metabolites of benzene: Phenol and hydroquinone show strong synergistic effects in vivo. Mutat. Res., 244, 15-20.
Bartels, M.J., McNett, D.A., Timchalki, C., Mendrala, A.L., Christenson, W.R., Sangha, G.K., Brzak, K.A. & Shabrang, S.N. (1998) Comparative metabolism of ortho-phenylphenol in mouse, rat, and man. Xenobiotica, 28, 579-594.
Berman, E., Schlicht, M., Moser, V.C. & MacPhail, R.C. (1995) A multidisciplinary approach to toxicological screening: I. Systemic toxicity. J. Toxicol. Environ. Health, 45, 127-143.
Bradley, M.D., Taylor, V.I., Armstrong, M.J. & Galloway, S.M. (1987) Relationships among cytotoxicity, lysosomal breakdown, chromosome aberrations, and DNA double-strand breaks. Mutat. Res., 189, 69-79.
Bray, H.G., Thorpe, W.V. & White, K. (1950a) Metabolism of derivatives of toluene. 4. Cresols. Biochem. J., 46, 275-278.
Bray, H.G., Thorpe, W.V. & White, K. (1950b) Metabolism of derivatives of toluene. 5. The fate of xylenols in the rabbit with further observations on the metabolism of the xylenes. Biochem. J., 47, 395-399.'
Bray, H.G., Thorpe, W.V. & White, K. (1952) Kinetic studies of the metabolism of foreign organic compounds. 4. The conjugation of phenols with sulfuric acid. Biochem. J., 52, 419-423.
Brusick, D. (1986) Genotoxic effects in cultured mammalian cells produced by low pH treatment conditions and increased ion concentrations. Environ. Mutag., 8, 879-886.
Capel, I.D., French, M.R., Millburn, P., Smith, R.L. & Williams, R.T. (1972) The fate of [(14)C]phenol in various species. Xenobiotica, 2, 25-34.
Chen, H.W. & Eastmond, D.A. (1995) Synergistic increase in chromosomal breakage within the euchromatin induced by an interaction of the benzene metabolites phenol and hydroquinone in mice. Carcinogenesis, 16, 1963-1969.
Cheng, M. & Kligerman, A.D. (1984) Evaluation of the genotoxicity of cresols using sister-chromatid exchange (SCE). Mutat. Res., 137, 51-55.
Cioli, V., Putzolu, S., Rossi, V. & Corradino, C. (1980) A toxicological and pharmacological study of ibuprofen guaiacol ester (AF 2259) in the rat. Toxicol. Appl. Pharmacol., 54, 332-339.
Cramer, G.M., Ford, R.A. & Hall, R.L. (1978) Estimation of toxic hazard — A decision tree approach. Food Cosmet. Toxicol., 16, 255-276.
Davidson, C., Zimmerman, E.F. & Smith, P.K. (1961) On the metabolism and toxicity of methyl salicylate. J. Pharmacol. Exp. Ther., 132, 207-211.
Dean, B.J., Brooks, T.M., Hodson-Walker, G. & Hutson, D.H. (1985) Genetic toxicology testing of 41 industrial chemicals. Mutat. Res., 153, 57-77.
Deichmann, E.B. (1944) Phenol studies. V. The distribution, detoxification, and excretion of phenol in the mammalian body. Arch. Biochem. Biophys., 3, 345-355.
Deichmann, W.B. & Witherup, S. (1944) Phenol studies. VI. The acute and comparative toxicity of phenol and o-, m- and p-cresols for experimental animals. J. Pharmacol. Exp. Ther., 80, 233-240.
Denine, E.P. & Palanker, A.L. (1973) Acute oral and dermal toxicity studies. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Douglas, G.R., Nestmann, E.R., Betts, J.L., Mueller, J.C., Lee, E.G.H., Stich, H.F., San, R.H.C., Brouzes, R.J.P., Chmelauskas, A., Paavila, H.D. & Walden; C.C. (1980) Mutagenic activity in pulp mill effluents. Water Chlorination Environ. Impact Health Effects, 3, 865-880.
Environmental Protection Agency (1988a) Subchronic Toxicity of ortho-Cresol in Sprague-Dawley Rats, EPA/PB88-197496. From US Department of Commerce, National Technical Information Service, Springfield, Virginia, USA.
Environmental Protection Agency (1988b) Subchronic Toxicity of para-Cresol in Sprague-Dawley rats: MBA Chemical No. 25, EPA/PB88-195292. From US Department of Commerce, National Technical Information Service, Springfield, Virginia, USA.
FAO (1999) Pesticide residues in food — 1999. Report of the Joint Meeting of the FAO Panel of Experts on Pesticide Residues in Food and the Environment and the WHO Core Assessment Group on Pesticide Residues, Rome, Italy, 20-29 September 1999 (Plant Production and Protection Paper 153). Rome, Food and Agriculture Organization of the United Nations
Fishbeck, W.A., Langner, R. R. & Kociba R.J. (1975) Elevated urinary phenol levels not related to benzene exposure. Am. Ind. Hyg. Assoc. J., 36, 820-824.
Florin, I., Rutberg, L., Curvall, M. & Enzell, C.R. (1980) Screening of tobacco smoke constituents for mutagenicity using the Ames' test. Toxicology, 15, 219-232.
Food & Drug Administration (1993) Priority-based Assessment of Food Additives (PAFA) Database, Center for Food Safety and Applied Nutrition, Washington DC, p. 58.
Fukuda, S. (1987) Assessment of the carcinogenic hazard of 6 substances used in dental practices. I. Morphological transformations, DNA damage and SCE in cultured Syrian hamster embryo cells induced by camphor, eugenol, thymol, EDTA, benzalkonium chloride and benzethonium chloride. Shigaku, 74, 1365-1384.
Garton, G.A. & Williams, R.T. (1949) Studies in detoxication. 21. The fates of quinol and resorcinol in the rabbit in relation to the metabolism of benzene. Biochem. J., 44, 234-238.
Gaunt, I.F., Sharratt, M., Colley, J., Lansdown, A.B.G. & Grasso, P. (1970) Acute and short-term toxicity of p-hydroxybenzyl acetone in rats. Food Cosmet. Toxicol., 8, 349-358.
Gocke, E., King, M.-T., Eckhardt, K. & Wild, D. (1981) Mutagenicity of cosmetics ingredients licensed by the European communities. Mutat. Res., 90, 91-109.
Grundschober, F. (1977) Toxicological assessment of flavouring esters. Toxicology, 8, 387-390.
Hagan, E.C., Hansen, W.H., Fitzhugh, O.G., Jenner, P.M., Jones, W.I., Taylor, J.M., Long, E.L., Nelson. A.M. & Brouwer, J.B. (1967) Food flavorings and compounds of related structure. II. Subacute and chronic toxicity. Food Cosmet. Toxicol., 5, 141-157.
Hagiwara, A., Shibata, M., Hirose, M., Fukushima, S. & Ito, N. (1984) Long-term toxicity and carcinogenicity study of sodium o-phenylphenate in B6C3F1 mice. Food Chem. Toxicol., 22, 809-814.
Hasegawa, R., Nakaji, Y., Kurokawa, Y. & Tobe, M. (1989) Acute toxicity tests on 113 environmental chemicals. Sci. Rep. Res. Inst. Tokoku Univ. Ser. C Med., 36, 10-16.
Haworth, S., Lawlor, T., Mortelmans, K., Speck, W. & Zeiger, E. (1983) Salmonella mutagenicity test results for 250 chemicals. Environ. Mutag., 5 (Suppl. 1), 3-142.
Hiraga, K. & Fujii, T. (.1984) Induction of tumours of the urinary bladder in F344 rats by dietary administration of o-phenyphenol. Foos Che. Toxicol., 22, 865-870.
Hirose, M., Inoue, T., Asamoto, M. Tagawa, Y. & Ito, N. (,1986) Comparison of effects of 13 phenolic compounds in induction of proliferative lesions of forestomach and increase in labeling indices of glandular stomach and urinary bladder epithelium of Syrian golden hamsters. Carcinogenesis, 7, 1285-1289.
Hirose, M., Yamaguchi, S., Fukushima, Hasegawa, R., Takahashi, S. & Ito, N. (1989) Promotion by dihydroxybenzene derivatives of N-methyl-N'-nitro-N-nitrosoguanidine-induced F344 rat forestomach and glandular stomach carcinogenesis. Cancer Res., 49, 5143-5147.
Hodge, H.C., Maynard, E.A., Blanchett, H.J., Spencer, H.C. & Rowe, V.K. (1952) Toxicological studies of orthophenylphenol (Dowicide® 1). J. Parmacol. Exp. Ther., 104, 202-210.
Hughes, M.F. & Hall, L.L. (1995) Disposition of phenol in rat after oral, dermal, intravenous, and intratracheal administration. Xenobiotica, 25, 873-883.
International Organization of the Flavour Industry (1995) European Inquiry on Volume of Use. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA..
Ivett, J.L., Brown, B.M., Rodgers, C., Anderson, B.E., Resnick, M.A. & Zeiger, E. (1989) Chromosomal aberrations and sister chromatid exchange tests in Chinese hamster ovary cells in vitro. IV. Results with 15 chemicals. Environ. Mol. Mutag., 14, 165-187.
Jansson, T., Curvall, M., Hedin, A. & Enzell, C.R. (1986) In vitro studies of biological effects of cigarette smoke condensate. II. Induction of sister-chromatid exchanges in human lymphocytes by weakly acidic, semivolatile constituents. Mutat. Res., 169, 129-139.
Jansson, T., Curvall, M., Hedin, A. & Enzell, C.R. (1988) In vitro studies of the biological effects of cigarette smoke condensate. III. Induction of SCE by some phenolic and related constituents derived from cigarette smoke — A study of structure-activity relationships. Mutat. Res., 206, 17-24.
Jenner, P.M., Hagan, E.C., Taylor, J.M., Cook, E.L. & Fitzhugh, O.G. (1964) Food flavorings and compounds of related structure. I. Acute oral toxicity. Food Cosmet Toxicol., 2, 327-343.
Kao, J., Bridges, J.W. & Faulkner, J.K. (1979) Metabolism of [(14)C]phenol by sheep, pig and rat. Xenobiotica, 9, 141-147.
Karim, M.M.G., Ramanujam, V.M.S. & Legator, M.S. (1986) Correlation between the induction of micronuclei in bone marrow by benzene exposure and the excretion of metabolites in urine of CD-1 mice. Toxicol. Appl. Pharmacol., 85, 464-477.
Kavlock, R.J. (1990) Structure-activity relationships in the developmental toxicity of substituted phenols: In vivo effects. Teratology, 41, 43-59.
Kenyon, E.M., Seeley, M.E., Janszen, D. & Medinsky, M.A. (1995) Dose-, route-, and sex-dependent urinary excretion of phenol metabolites in B6C3F1 mice. J. Toxicol. Environ. Health, 44, 219-233.
Kim, YC. & Matthews, H.B. (1987) Comparative metabolism and excretion of resorcinol in male and female F344 rats. Fundam. Appl. Toxicol., 9, 409-414.
Klonne, D.R., Myers, R.C. & Nachreiner, D.J. (1988) Acute toxicity and primary irritation of para-tertiary butylphenol. Drug Chem. Toxicol., 11, 43-54.
Kociba, R.J., Kalnins, R.V., Wade, C.E., Garfield, E.L. & Fishbeck, W.A. (1976) Elevated urinary phenol levels in dogs ingesting salol. Toxicol. Appl. Pharmacol., 37, 121.
Levenstein, I. (1974) Acute toxicity studies in rats, mice, and rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the F1avor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Levenstein, I. (1975) Acute toxicity studies in rats, mice and rabbits. Unpublished report to Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers Association of the United States, Washington DC, USA.
Lucas, C.D., Putnam, J.M., Hallagan, J.B. & the FEMA Flavor Ingredients Committee (1999) 1995 Poundage and Technical Effects Update Survey. Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Maarse, H., Visscher, C.A., Willemsens, L.C., Nijssen, L.M. & Boelens, M.H., eds (1996) Volatile Components in Food, Suppl. 5 to the 6th Edition, Zeist, TNO Nutrition and Food Research.
Maasik, I. (1970) The toxicity of small doses of dimethyl-phenol in chronic experiments. Vopr. Gig. Tr. Profzabol. Mater. Nauch. Konf., 2, 171-176.
MacIntosh, F.C. (1945) The toxicity of diphenyl and o-phenyl-phenol. Analyst, 70, 334-335.
Maronpot, R.R. & Boorman, G.A. (1982) Interpretation of rodent hepatocellular proliferative alterations and hepatocellular tumors in chemical safety assessment. Toxicol. Pathol., 10, 72-80.
Maronpot, R.R., Haseman, J.K., Boorman, G.A., Eustis, S.E., Rao, G.N. & Huff, J.E. (1987) Liver lesions in B6C3F1 mice: The National Toxicology Program, experience and position. Arch. Toxicol., Suppl. 10, 10-26.
Marrazzini, A., Chelotti, L., Barrai, I., Loprieno, N. & Barale, R. (1994) In vivo genotoxic interactions among three phenolic benzene metabolites. Mutat. Res., 341, 29-46.
Maruyama, H., Amanuma, T., Nakae, D., Tsutsumi, M., Kondo, S., Tsujiuchi, T., Denda, A. & Konishi, Y. (1991) Effects of catechol and its analogs on pancreatic carcinogenesis initiated by N-nitrosobis(2-oxopropyl)amine in Syrian hamsters. Carcinogenesis, 12, 1331-1334.
Massey, I.J., Aitken, M.D., Ball, L.M. & Heck, P.E. (1994) Mutagenicity screening of reaction products from the enzyme-catalyzed oxidation of phenolic pollutants. Environ. Toxicol. Chem., 13, 1743-1752.
McGee, G. (1974) Acute toxicity studies in rats and rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
McGregor, D.B., Riach, C.G., Brown, A., Edwards, I., Reynolds, D., West, K. & Willington, S. (1988) Reactivity of catecholamines and related substances in the mouse lymphoma L5178Y cell assay for mutagens. Environ. Mol. Mutag., 11, 523-544.
McMahon, R.E., Cline, J.C. & Thompson, C.Z. (1979) Assay of 855 test chemicals in ten tester strains using a new modification of the Ames test for bacterial mutagens. Cancer Res., 39, 682-693.
McOmie, W.A., Anderson, H.E. & Estess, F.M. (1949) Comparative toxicity of certain t-butyl substituted cresols and xylenols. J. Am. Pharm. Assoc., 38, 366-369.
Meerman, J.H., Nijlan, C. & Mulder, G.J. (1987) Sex differences in sulfation and glucuronidation of phenol, 4-nitrophenol, and N-hydroxy-2-acetalaminofluorene in the rat in vivo. Biochem. Pharmacol., 36, 2605-2608.
Miller, J.J., Powell, G.M., Olavesen, A.H. & Curtis, C.G. (1976) The toxicity of dimethoxyphenol and related compounds in the cat. Toxicol. Appl. Pharmacol., 38, 47-57.
Monge, P., Scheline, R. & Solheim, E. (1976) The metabolism of zingerone, a pungent principle of ginger. Xenobiotica, 6, 411-423.
Moran, E.J., Easterday, O.D. & Oser, B.L. (1980) Acute oral toxicity of selected flavor chemicals. Drug Chem. Toxicol., 3, 249-258.
Moreno, O.M. (1973) Acute toxicity studies on rats and rabbits. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington, DC, USA.
Moreno, O.M. (1975) Acute toxicity studies in rats, mice, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Moreno, O.M. (1977) Acute toxicity study in rats, rabbits and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Moreno, O.M. (1978) Acute toxicity studies in rats, mice, rabbits, and guinea pigs. Unpublished report to the Research Institute for Fragrance Materials, Inc., Englewood Cliffs, New Jersey, USA. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Morimoto, K. & Wolff, S. (1980) Increase of sister chromatid exchanges and perturbations of cell division kinetics in human lymphocytes by benzene metabolites. Cancer Res., 40, 1189-1193.
Nakagawa, Y & Tayama, S. (1989) Formation of ortho-phenylphenol glutathione conjugates in the rat liver. Xenobiotica, 19, 499-507.
National Academy of Sciences (1987) Evaluating the Safety of Food Chemicals, Washington DC.
National Institute for Occupational Safety and Health (1976) Criteria for Recommended Standard for Phenol, NIOSH Publication No. 76-196. US Department of Health, Education, and Welfare, Public Health Service, Center for Disease Control, Atlanta, Georgia, USA.
National Toxicology Program (1980) Bioassay of Phenol for Possible Carcinogenicity. NCI-CG-TR-203; NTP-80-15; PB-80-1759. US Department of Health and Human Services, Research Triangle Park, North Carolina, USA.
National Toxicology Program (1986) Toxicology and Carcinogenesis Studies of ortho-Phenylphenol Alone and with 7,12-Dimethylbenz(a)anthracene in Swiss CD-1 Mice (Dermal Studies). NTP-TR-301; PB-86-2557. US Department of Health and Human Services, Research Triangle Park, North Carolina, USA.
National Toxicology Program (1992a) Toxicity Studies of Cresols in F344/N Rats and B6C3F1 Mice (Feed Studies). NTP-TOX 9. US Department of Health and Human Services, Research Triangle Park, North Carolina, USA.
National Toxicology Program (1992b) Toxicology and Carcinogenesis Studies of Resorcinol in F344/N Rats and B6C3F1 Mice (Gavage Studies). NTP-TR-403; PB-92-2858. US Department of Health and Human Services, Research Triangle Park, North Carolina, USA.
Nerland, D.E. & Pierce, W.M. (1990) Identification of N-acetyl-S-(2,5-hydroxyphenyl)-L-cysteine as a urinary metabolite of benzene, phenol, and hydroquinone. Drug Metab. Disposition, 18, 958-961.
Nestmann, E.R., Lee, E.G.H., Matula, T.I., Douglas, G.R. & Mueller, J.C. (1980) Mutagenicity of constituents identified in pulp and paper mill effluents using the Salmonella/mammalian-microsome assay. Mutat. Res., 79, 203-212.
Pacifici, G.M., Vannucci, L., Bencini, C., Tusini, G. & Mosca, F. (1991) Sulfation of hydroxybiphenyls in human tissues. Xenobiotica, 21, 1113-1118.
Pellmont, B. (1973) Acute oral toxicity in mice with p-propylphenol. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Pfäffli, P., Hesso, A., Vainio, H. & Hyvonen, M. (1981) 4-Vinylphenol excretion suggestive of arene oxide formation in workers occupationally exposed to. styrene. J. Toxicol. Appl. Pharmacol., 60, 85-90.
Piccirillo, V.J. & Hartman, W.C. (1982) Acute oral toxicity (LD50) study with 4-methyl-2,6-dimethoxy phenol in rats. Unpublished report. Submitted to WHO by-the Flavor and Extract Manufacturers' Association of the United States, Washington DC; USA.
Piccirillo,V.J.; Hartman, W.C. & Swidersky, P. (1981) Acute oral LD50 determination in rats with p-vinylphenol. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Pool, B.L. & Lin, P.Z. (1982) Mutagenicity testing in the Salmonella typhimurium assay of phenolic compounds and phenolic fractions obtained from smokehouse smoke condensates. Food Chem. Toxicol., 20, 383-391.
Porteous, J.W. & Williams, R.T (1949) Studies in detoxication. 19. The metabolism of benzene. I. The determination of phenol in urine with 2,6-dichloroquinonechloroimide; the excretion of phenol, glucuronic acid and ethereal sulphate by rabbits receiving benzene and phenol; observations on the determination of catechol, quinol, and muconic acid in urine. Biochem. J., 44, 46-55.
Posternak, J.M. & Linder, A. (1969) Summaries of toxicological data., Toxicological tests on flavoring matters. Food Cosmet. Toxicol., 7, 405-407.
Quast, J.F., McGuirk, R.J. & Kociba, R.J. (1997) Results of a two-year dietary toxicity on cogenicity study of ortho-phenylphenol (OPP) in mice. Toxicologist, 36, 341.
Rinehart, W.E., Kaschak, M. & Pfitzer, E.A. (1967) Range-finding toxicity data for 43 compounds. Ind. Hyg. Found. Am. Chem. Toxicol. Ser. Bull., 6, 1-8.
Robertson, M.L., Eastmond, D.A. & Smith, M.T. (1991) Two benzene metabolites, catechol and hydroquinone, produce a synergistic induction of micronuclei and toxicity in cultured human lymphocytes. Mutat. Res., 249, 201-209.
Sauer, C. & Salem, H. (1980) Acute oral toxicity test in rats with 4-(p-acetoxyphenyl)-2-butanone. Unpublished report. Submitted to WHO by the Flavor and Extract Manufacturers' Association of the United States, Washington DC, USA.
Savignoni, F. & de Maria, G. (1933) The influence of some anthelminthic preparations on mother and fetus. Sperimentale, 87, 557-584.
Schafer, E.W., Jr & Bowles, W.A., Jr (1985) Acute oral toxicity and repellency of 933 chemicals to house and deer mice. Arch. Environ. Contam. Toxicol., 14, 111-129.
Schlicht, M.P., Moser, V.C., Sumrell, B.M., Berman, E. & MacPhail, R.C. (1992) Systemic and neurotoxic effects of acute and repeated phenol administration. Toxicologist, 12, 274.
Sedivec, V. & Flek, J. (1970) Estimation of toxic substances and their metabolites in biological solutions by gas-chromatography. III. Occurrence and estimation of guajacol in urine. Prac Lek., 22, 176-181.
Shelby, M.D., Erexson, G.L., Hook, G.L. & Tice, R.R. (1993) Evaluation of a three-exposure mouse bone marrow micronucleus protocol: Results with 49 chemicals. Environ. Mol. Mutag., 21, 160-179
Shibata, M.A., Yamada, M., Hirose, M., Asakawa, E., Tatematsu, M. & Ito, N. (1990) Early proliferative responses of forestomach and glandular stomach of rats treated with five different phenolic antioxidants. Carcinogenesis, 11, 425-429.
Smyth, H.F., Jr, Weil, C.S., West, J.S. & Carpenter, C.P. (1969) An exploration of joint toxic action: Twenty-seven industrial chemicals intubated in rats in all possible pairs. Toxicol. Appl. Pharmacol., 14, 340-347.
Stofberg, J. & Grundschober, F. (1987) Consumption ratio and food predominance of flavoring materials. Perfum. Flavorist, 12, 27-68.
Stofberg, J. & Kirschman, J.C. (1985) The consumption ratio of flavoring materials: A mechanism for setting priorities for safety evaluation. Food Chem. Toxicol., 23, 857-860.
Sze, C.C., Shi, C.Y & Ong, C.N. (1996) Cytotoxicity and DNA strand breaks induced by benzene and its metabolites in Chinese hamster ovary cells. J. Appl. Toxicol., 16, 259-264.
Taylor, J.M., Jenner, P.M. & Jones, W.I. (1964) A comparison of the toxicity of some allyl, propenyl, and propyl compounds in the rat. Toxicol. Appl. Pharmacol., 6, 378-387.
Thompson, D.C., Perera, K., Fisher, R. & Brendel, K. (1994) Cresol isomers: Comparison of toxic potency in rat liver slices. Toxicol. Appl. Pharmacol., 125; 51-58.
Thompson, D.C., Perera, K. & London, R. (1995a) Quinone methide formation from para isomers of methylphenol (cresol), ethylphenol, and isopropylphenol: Relationship to toxicity. Chem. Res. Toxicol., 8, 55-60.
Thompson, D:C., Perera, K., Krol, E.S. & Bolton, J.L. (1995b) o-Methoxy-4-alkylphenols that form quinone methides of intermediate reactivity are the most toxic in rat liver slices. Chem. Res. Toxicol., 8, 323-327.
Uzhdavini, R.R., Astafyeva, I.K. & Mamayeva, A.A. (1974) Acute toxicity of lower phenols. Gig. Tr. Prof. Zabol., 2, 58-59.
Van den Heuvel, M.J., Clark, D.G., Fielder, R.J., Koundakjian, P.P., Oliver, G.J.A., Pelling, D., Tomlinson, N.J. & Walker, A.P. (1990) The international validation of a fixed-dose procedure as an alternative to the classical LD50 tests. Food Chem. Toxicol., 28, 469-482.
Wahle, B.S., Christenson, W.R., Lake, S.G., Elcock, L.E., Moore, K.D., Sangha, G.K. & Thyssen, J.H. (1997) Technical grade ortho-phenylphenol: A combined chronic toxicity/oncogenicity testing study in the rat. Toxicologist, 36, 341.
Wangenheim, J. & Bolcsfoldi, G. (1988) Mouse lymphoma L5178Y thymidine kinase locus assay of 50 compounds. Mutagenesis, 3, 193-205.
Weitering, J.G., Krijgsheld, K.R. & Mulder, G.J. (1979) The availability of inorganic sulfate as a rate limiting factor in the sulfate conjugation of xenobiotics in the rat? Biochem. Pharmacol., 28, 757-762.
Wengle, B. & Hellström, K. (1972) Volatile phenols in serum of uraemic patients. Clin. Sci., 43, 493-498.
WHO (2000) Pesticide Residues in Food — 1999. Evaluations. Part II — Toxicological (WHO/ PCS/004), Geneva
Wild, D., King, M.T., Eckhardt, K. & Gocke, E. (1981) Mutagenic activity of aminophenols, and diphenols, and relations with chemical structure. Mutat. Res., 85, 456.
Williams, R.T (1959) Detoxication Mechanisms, London: Chapman & Hall.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., Mortelmans, K. & Speck, W. (1987) Salmonella mutagenicity tests: III. Results from the testing of 255 chemicals. Environ. Mutag., 9, 1-109.
See Also:
Toxicological Abbreviations
![]()
![]()
![]()
![]()
![]()
![]()
![]()