
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY
WORLD HEALTH ORGANIZATION
Toxicological evaluation of certain veterinary drug
residues in food
WHO FOOD ADDITIVES SERIES 39
Prepared by:
The forty-eighth meeting of the Joint FAO/WHO Expert
Committee on Food Additives (JECFA)
World Health Organization, Geneva 1997
DANOFLOXACIN
First draft prepared by Dr E.A.M. Good
Veterinary Medicines Directorate
Addlestone, United Kingdom
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.2 Biotransformation
2.2 Toxicological studies
2.2.1 Acute toxicity
2.2.2 Short-term toxicity
2.2.3 Long-term toxicity and carcinogenicity
2.2.4 Genotoxicity
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
2.2.5.2 Developmental toxicity
2.2.6 Special study on delayed contact hypersensitization
2.2.7 Special studies on pharmacological activity
2.2.8 Special studies on microbiological effects
2.3 Observations in humans
3. Comments
4. Evaluation
5. References
1. EXPLANATION
Danofloxacin is a 'third-generation' fluoroquinolone antibiotic.
It is manufactured by a stereospecific synthesis, resulting in the
pure S (laevorotatory) form. Danofloxacin is used in veterinary
medicine as the mesylate salt for the treatment of respiratory
diseases in cattle, swine, and chickens.
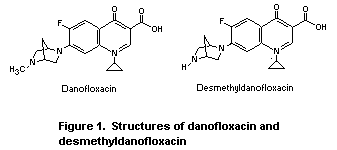
All of the studies of toxicity were carried out with the mesylate
salt of dano-floxacin. The doses are expressed in terms of the active
moiety, the zwitterion base. Desmethyldanofloxacin, which is the
primary metabolite of danofloxacin, has similar properties. The
toxicity and antimicrobial activity of desmethyl-danofloxacin were
also examined, again using the mesylate salt. The structures of
danofloxacin and desmethyldanofloxacin are shown in Figure 1.
Danofloxacin has not previously been reviewed by the Joint
FAO/WHO Expert Committee on Food Additives.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Chickens
Groups of male 18-day-old broiler chickens were given
drinking-water that had been medicated to provide 5 mg/kg bw per day
danofloxacin, for three days. Steady-state concentrations of 0.43 and
0.21 µg/ml were obtained in lung and plasma, respectively, during the
period of administration. The half-life of danofloxacin was 4.9 h in
plasma and 5.8 h in lung. No residues of desmethyldanofloxacin were
detected in any of the plasma or lung samples (Lynch et al., 1990a).
Groups of broiler chickens were given drinking-water that had
been medicated to provide 5 mg/kg bw per day 3H-danofloxacin for
five days. The test substance was tritiated in the N1-cyclopropyl
ring. The study complied with GLP guidelines. Six hours after
withdrawal of the treated water, unchanged danofloxacin accounted for
75% of the radiolabel in excreta, and desmethyl-danofloxacin accounted
for 5-7% (Lynch et al., 1990a).
Groups of 12 male and 12 female 22-day-old broiler chickens were
given drinking-water which had been medicated to provide 5 mg/kg bw
per day 3H-danofloxacin, for five days. The test substance was
tritiated in the N1-cyclo-propyl ring and had a specific activity of
12.1 µCi/mg (expressed as base). Excreta were collected every 24 h and
pooled by sex and day. The study complied with GLP guidelines. The
mean level of radiolabel in excreta attained a steady state in the
range 35-46 µg/g by the first day of treatment but declined to 20 and
6.6 µg/g on the first and second days after withdrawal, respectively.
About 85% of the radiolabel in faeces was identified as unmetabolized
danofloxacin. There was no difference between the sexes in the pattern
of excretion (Lynch et al., 1988).
Pigs
Castrated male and female pigs (mean body weight, 40 kg) were
given single intramuscular injections of 1.25 mg/kg bw of a prototype
2.5% formulation of danofloxacin. Absorption was rapid, with peak
concentrations of 0.40 µg/g in plasma and 1.68 µg/g in lung about 1 h
after dosing. The half-life of plasma elimination was approximately
7 h (Risk et al., 1991).
Six castrated male and six female pigs (mean body weight, 43.3
kg) were given five daily intramuscular injections of 1.25 mg/kg bw of
danofloxacin tritiated in the N1-cyclopropyl ring in a 2.5% aqueous
vehicle. The study complied with GLP guidelines. The mean radiolabel
concentration during the two days before and the day after the last of
the five doses was 7-8 µg/g in pooled faecal homogenates and 14-18
µg/g in pooled urine samples. During this same period, the total daily
recovery of radiolabel from urine and faeces accounted for 75-81% of
the daily dose. The mean radiolabel concentration in bile was 1.7 µg/g
12 h after the end of treatment and had declined to 0.21 µg/g by 48 h
(Lynch et al., 1989b).
In pigs given a single dose of 5 mg/kg bw of danofloxacin
intravenously, intramuscularly, or orally, detectable residues of
danofloxacin appeared rapidly in plasma. A peak plasma concentration
of 0.42 µg/ml was attained about 3 h after oral dosing. The
bioavailability was estimated to be 76% after intramuscular
administration and 89% after oral administration (Mann & Frame, 1992).
Cattle
A group of 36 male and female calves weighing about 250 kg were
given a single intramuscular injection of 1.25 mg/kg bw of a 2.5%
prototype formulation of danofloxacin, and the kinetics of the
compound were evaluated in plasma and lung tissues. The study complied
with GLP guidelines. Absorption was rapid, with peak plasma and lung
concentrations of 0.35 µg/ml and 1.44 µg/g attained 1 h after dosing.
The half-time for plasma elimination was 3.4 h (Lynch et al.,
1990b).
In a three-way cross-over design study, 12 male and female beef
calves (mean body weight, 112 kg) were given single intravenous doses
or five subcutaneous or intramuscular doses of 1.25 mg/kg bw per day
of a 2.5% prototype formulation of danofloxacin. The study complied
with GLP guidelines. Absorption was rapid: peak plasma concentrations
of 0.37 and 0.47 µg/ml were attained about 1 h after single
subcutaneous and intramuscular doses, respectively, and the
bioavailability was almost 100%. The values for the area under the
concentration-time curve indicated that the levels achieved after
intramuscular and subcutaneous administration were bioequivalent after
one, three, and five injections (Lynch et al., 1991a).
The pattern of excretion of 3H-danofloxacin (tritiated in the
N1-cyclopropyl ring) was studied in five castrated male and four
female calves (body weight, about 180 kg) after five daily
intramuscular injections of 1.25 mg/kg bw (in a 2.5% aqueous vehicle).
The study complied with GLP guidelines. The total concentration of
danofloxacin-related material in excreta reached a plateau by the
third day of treatment. Approximately equal amounts were excreted in
urine and faeces. Unchanged danofloxacin accounted for about 48% of
material excreted in faeces and 89% in urine. The desmethyl metabolite
accounted for 12% of the material in urine samples, but the
concentrations in faeces were too low for measurement (Lynch et al.,
1989a).
2.1.2 Biotransformation
The biotransformation of danofloxacin has been investigated in
rats, dogs, chickens, pigs, and cattle. In all of these species, the
main residue in the faeces was unmetabolized danofloxacin; smaller
amounts of desmethyldanofloxacin were also found (Lynch et al.,
1989a,b,c, 1991a,b,c,d). The main residue in the urine of cattle,
dogs, and rats was also danofloxacin. Desmethyldanofloxacin,
danofloxacin- N-oxide and the ß-glucuronide were identified in rat,
dog, and pig urine (Lynch et al., 1991d).
In the livers of most species, danofloxacin was the major
residue, but significant residues of the metabolite
desmethyldanofloxacin were also present (Table 1). A piperazine-ring
degradation product constituted 43 and 27% of the radiolabel in bile
from male and female cattle, respectively, but it was present in only
trace amounts in bile from rats and dogs. The bile of dogs contained
residues of danofloxacin- N-oxide, which was present in only trace
amounts in bile from the other species (Lynch et al., 1991d).
2.2 Toxicological data
2.2.1 Acute toxicity
The results of studies of the acute toxicity of danofloxacin and
desmethyl-danofloxacin are summarized in Table 2. All of the studies
complied with GLP guidelines.
In the studies by oral administration, signs of toxicity included
exophthalmia, decreased activity, tremors, and twitching, which
occurred within a few minutes of dosing. One of three mice given
2000 mg/kg bw died 29 min after dosing. Retching, clonic convulsions,
and gasping were observed before death. After intravenous dosing, all
three mice given 100 mg/kg bw and all three rats given 150 mg/kg bw
died within 3 min. The signs of toxicity were similar to those
produced after oral administration. Similar signs of toxicity were
observed after oral and intravenous dosing with desmethyldanofloxacin.
Table 1. Composition of residues in liver in five species given danofloxacin
Species Dose regimen Sex Total Danofloxacina Desmethyldanofloxacina Reference
residue
Rat 5 × 6.25 mg/kg bw M 2.3 ppm 67% 18% Lynch et al.
per day orally F 2.5 ppm 70% 11% (1991d)
Dog 5 × 2.4 mg/kg bw M 6.7 ppm 66% 28% Lynch et al.
per day orally F 5.4 ppm 58% 37% (1991d)
Chicken 5 × 5 mg/kg bw M NR 78% 16% Lynch et al.
per day orally F NR 74% 12% (1988)
Pig M 0.95 ppm 58% 23% Lynch et al.
F 0.96 ppm 68% 29% (1989c)
Cowb 5 × 1.25 mg/kg bw M 0.82 ppm 24% 43% Lynch et al.
intramuscularly F 1.1 ppm 29% 42% (1991d)
a Relative to the radiolabel recovered by high-performance liquid chromatography
b Killed 12 h after the last dose
Table 2. Results of studies of the acute toxicity of danofloxacin and desmethyldanofloxacin
Species Route Vehicle Sex LD50 Reference
(strain) (mg/kg bw)
Danofloxacin
Rat (Sprague- Oral Distilled M & F > 2000 Stadnicki et al. (1988a)
Dawley) water
Mouse (ICR) Oral Distilled water M & F > 2000 Stadnicki et al. (1988a)
Rat (Sprague- Intravenous Sterile water M 100-150 Stadnicki et al. (1988a)
Dawley)
Mouse (ICR) Intravenous Sterile water M 50-100 Stadnicki et al. (1988a)
Rabbit (New Dermal M & F > 299a Stadnicki et al. (1988b)
Zealand white)
Desmethyldanofloxacin
Rat (Sprague- Oral Deionized M & F > 2000 Stadnicki et al. (1989)
Dawley) water
Mouse (ICR) Oral Deionized M > 2000 Stadnicki et al. (1989)
water
Mouse (ICR) Oral Deionized F 1500-2000 Stadnicki et al. (1989)
water
Rat (Sprague- Intravenous Sterile water M 40-50 Stadnicki et al. (1989)
Dawley)
Mouse (ICR) Intravenous Sterile water M 7.5-10 Stadnicki et al. (1989)
a 0.5 g danofloxacin was applied to one site on intact skin and one on abraded skin of two male and one
female New Zealand white rabbits under an occlusive dressing for 24 h. There were no deaths or signs
of toxicity, except for mild erythema at all treated sites.
A dose of 26 mg danofloxacin (in a volume of 0.1 ml) was
instilled into the conjunctival sac of the left eyes of three New
Zealand white rabbits. The eyes were not rinsed after dosing. Mild
conjunctivitis and a colourless discharge were observed within 1 h of
treatment. All signs had cleared within 96 h (Stadnicki et al.,
1988b).
2.2.2 Short-term toxicity
Mice
In a study designed to identify suitable doses for a two-year
feeding study, groups of 15 male and 15 female Crl:CD-1 (ICR)BR
VAF/Plus mice were fed diets calculated to provide 0, 150, 300, or 600
mg/kg bw per day of danofloxacin (74% activity) for up to 102 days.
The study complied with GLP guidelines. One female given the high dose
was killed in a moribund condition. Body-weight gain was reduced in
males at the low and high doses in comparison with that of the
controls. Decreased erythrocytic parameters were seen occasionally in
males and females at the high dose. There were no consistent
dose-related changes in clinical chemistry. At termination, the
absolute mean group weight of the kidneys of females receiving the
high dose was significantly increased, but there were no corresponding
pathological changes. Microscopic examination revealed caecal
dilatation in six mice at the low dose, 23 at the intermediate dose,
and 27 at the high dose, but not in the controls. Inflammation of the
caecum was seen in two mice at the high dose, and Gram-positive
bacteria, tentatively identified as Clostridium difficile, were
found in the inflamed caeca of both animals. Thymic lymphocytosis was
seen in one of 15 females at the low dose, two of 15 at the
intermediate dose, and eight of 15 at the high dose and in three of 13
males at the intermediate dose and six of 14 at the high dose but in
no control. A NOEL was not identified (Stadnicki et al., 1994a).
Rats
A two-week exploratory study was carried out in which groups of
three male and three female Long-Evans rats received oral doses of 0,
25, 50, or 100 mg/kg bw per day of danofloxacin in an aqueous vehicle.
There were no significant effects on body-weight gain, food
consumption, haematological, clinical chemical, or urinary parameters,
or organ weights. At necropsy, treatment-related caecal enlargement
was observed in treated animals (Reynolds et al., 1987a).
Groups of 10 male and 10 female Long-Evans rats were given doses
of 0, 25, 75, or 150 mg/kg bw per day danofloxacin by gavage in an
aqueous vehicle for one month. Serum chemistry and haematology were
evaluated before treatment and on days 11-12 and 30-31. The study
complied with GLP guidelines. There were no signs of toxicity and no
effects on body-weight gain or food consumption. Alanine
aminotrasferase levels were significantly increased in treated females
receiving the high dose, and absolute liver and liver:body weight
ratios were significantly decreased in males at this dose. No
substance-related effects were seen in about 30 tissues from animals
at 0 and 150 mg/kg bw and macroscopic lesions from animals in the
other groups examined microscopically (Fisher et al., 1988a).
In a range-finding study designed to identify suitable doses for
a long-term study of toxicity, groups of 10 male and 10 female Fischer
rats were fed diets calculated to provide 0, 150, 300, 450, or 600
mg/kg bw per day of danofloxacin for 36-39 days. Satellite groups of
three animals of each sex were used to monitor plasma concentrations
of the drug. Five rats given 600 mg/kg bw per day died. Body-weight
gain and food consumption were reduced in all treated groups, and
there was an initial dose-related decrease in food consumption.
Changes were seen in serum chemistry, haematological parameters and
organ weights, due to the effects of starvation and dehydration.
Caecal dilatation was observed in all treated animals (Stadnicki
et al., 1994b).
In a subsequent exploratory study in groups of 10 Long-Evans rats
of each sex, doses intended to provide 0, 75, 150, or 300 mg/kg bw per
day of danofloxacin were administered in the feed for seven days; the
animals then received untreated feed for seven days and the treated
feed for a further 12 days. Body-weight gain and food consumption were
reduced in rats at the intermediate and high doses. Serum globulin
levels were reduced and leukopenia was observed in all treated groups.
Crystalluria was seen in most treated rats but in only two of 20
controls. There was no histopathological evidence of nephropathy
(Stadnicki et al., 1994c).
In a study designed to establish doses to be used in a two-year
feeding study, groups of 15 Long-Evans rats of each sex were fed diets
calculated to provide 0, 75, 150, or 300 mg/kg bw per day of
danofloxacin for about three months. The study complied with GLP
guidelines. Alopecia was seen in four females at the high dose and one
at the intermediate dose. Discoloured urine (especially in animals at
75 mg/kg bw) and crystalluria were observed in all treated groups, but
there were no corresponding pathological changes in the kidneys.
Body-weight gain was significantly reduced in a dose-related manner.
There were significant, dose-related increases in urinary and serum
magnesium concentrations, which were attributed to chelation of
magnesium ions by the test substance. Caecal dilatation was observed
in rats at 150 and 300 mg/kg bw. A range of degenerative changes were
observed in the testes of all males at the high dose and in one at 0,
one at 75, and one at 150 mg/kg bw per day. Degenerating germinal
cells were found in the epididymides of all males at the high dose and
nine at the middle dose (Stadnicki et al., 1994d).
Groups of 20 male and 20 female weanling Long-Evans rats were
selected from the F1 offspring of rats used in a multigeneration
study which had been exposed to danofloxacin both in utero and
during lactation. They were then given oral doses of 25, 75, or 150
mg/kg bw per day for up to three months, whereas 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58).
There were no substance-related deaths and no adverse effects on
food consumption or body-weight gain. There were no consistent
dose-related trends in haematological or clinical chemical values. A
dose-related increase in proteinuria was seen in females but not
males, which was correlated with the finding of tubular nephropathy in
individual animals. Kidney weights were unaffected by treatment. The
mean absolute and relative weights of the testes of rats at 75 and 150
mg/kg bw were about 10% lower than those of controls. Lesions of the
heart were found primarily in animals at these doses and consisted of
either multifocal myocardial degeneration and necrosis, or multifocal
fibrosis, or both. There was no NOEL, as renal tubular nephropathy was
observed in all treated females (Fisher et al., 1989a).
The study was repeated at lower doses in order to establish a
NOEL. Groups of 20 male and 20 female weanling Long-Evans rats were
again selected from the F1 offspring in the multigeneration study,
which had been exposed to danofloxacin both in utero and during
lactation. They were then given oral doses of 1, 2.5, or 6.25 mg/kg bw
per day for up to three months. Twenty controls of each sex were given
deionized water. The study was conducted in accordance with US FDA GLP
guidelines (21 CFR Part 58).
There were no treatment-related deaths or signs of toxicity.
Body-weight gain, food consumption, and haematological and clinical
chemical parameters were unaffected by treatment. Urinalysis revealed
a marginal increase in the incidence of haematuria in males given the
high dose and of proteinuria in a single female at this dose. The
absence of any corresponding pathological changes in the kidney
indicates that the findings reflected biological variability rather
than a substance-related effect. The NOEL was 6.25 mg/kg bw per day
(Fisher et al., 1990a).
Groups of 20 male and 20 female Long-Evans rats were given oral
doses of 1, 2.5, or 6.25 mg/kg bw per day of desmethyldanofloxacin for
up to three months. The rats were selected from the F1 offspring in
a multigeneration study and had been exposed to the test substance
both in utero and during lactation. Groups of 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58). There were no effects on
mortality, body-weight gain, food consumption, haematological or
clinical chemical parameters, organ weights, or gross or
histo-pathological findings. The NOEL was 6.25 mg/kg bw per day
(Stadnicki et al., 1990a).
Rabbits
In an exploratory study, groups of three female New Zealand white
rabbits were given oral doses of 0, 25, 50, or 100 mg/kg bw per day of
danofloxacin in an aqueous vehicle. Owing to significantly reduced
feed intake and decreased body weight, treatment of rabbits at the low
dose was stopped after 12 doses, that of animals at the intermediate
dose after seven doses, and that of rabbits at the high dose after
four doses. The groups were fed untreated diet for a further five to
eight days, then killed and necropsied. Pathological examination
revealed a dose-related incidence of enlarged caeca (Lundeen et al.,
1993).
Dogs
In an exploratory study, groups of one male and one female beagle
dogs were given oral doses of 0, 12.5, 25, or 50 mg/kg bw per day of
danofloxacin in gelatin capsules for two weeks. The female at the high
dose had reduced body-weight gain and food consumption and increased
serum blood urea nitrogen and creatinine levels. At necropsy, this dog
had acute segmental nephrosis and renal failure. The male at the high
dose had chronic focal necrosis without renal failure (Reynolds
et al., 1987b).
In a one-month exploratory study, groups of three male and three
female beagle dogs (10-12 months old) were given oral doses of 0, 5,
10, or 25 mg/kg bw per day of danofloxacin in gelatin capsules. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). There were no effects on behaviour, food consumption,
body-weight gain, blood pressure, electrocardiographic profile, or
ophthalmic, serum chemical, or haematological parameters. At necropsy,
a full range of tissues from all animals was examined, with particular
attention to the weight-bearing joints. Shallow erosions were found in
all dogs, including controls, although the grade of erosions was
slightly higher in those at the high dose. The lesions were typical of
those found in older dogs and did not resemble quinolone-induced
lesions (Fisher et al., 1988b).
Groups of four beagle dogs of each sex aged about six months were
given oral doses of 0, 5, 10, or 25 mg/kg bw of danofloxacin in
gelatin capsules. The substance was administered as two equally
divided doses per day for three months. At termination, all dogs were
subjected to a complete pathological examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
By day 7, reduced activity and signs of joint pain were seen in all
eight dogs given 25 mg/kg bw and in three dogs given 10 mg/kg bw. Most
of the affected animals showed signs of recovery by week 6, despite
continued treatment. Body-weight gain, food consumption, heart rate,
and respiratory rate were decreased in animals that showed clinical
signs. There were no effects on electrocardiographic profiles, blood
pressure, or ophthalmic, haematological, clinical chemical, or urinary
parameters. Gross pathological changes were observed in the articular
cartilage of the major joints of all treated animals, except for one
female receiving the low dose. The lesions were characterized by areas
of cartilage separation and cartilage loss (erosions). The severity of
the lesions was dose-related. Microscopic examination revealed further
changes, including the apparent alteration of collagen fibres when
viewed with polarized light (Fisher et al., 1989b).
A second three-month study, which complied with the same GLP
guidelines, was carried out at lower doses in order to establish a
NOEL. Groups of four male and four female beagle dogs, aged about five
months, were given oral doses of 0, 1, or 2.4 mg/kg bw per day of
danofloxacin in gelatin capsules as two equally divided doses, for 91
days. There were no signs of toxicity and no effects on body-weight
gain, food consumption, clinical chemical or urinary parameters, or
organ weights. There were no substance-related pathological findings.
The NOEL was 2.4 mg/kg bw per day (Fisher et al., 1989c).
Groups of three male and three female beagle dogs, four to six
months old, were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules, administered as two equally
divided doses, for about three months The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58). There were no
notable effects on body-weight gain, electrocardio-graphic profiles,
serum chemical or haematological values, or organ weights. One male
given the high dose showed signs of pain on day 65 when pressure was
applied to the left ankle joint. One female at the low dose showed
signs of pain on day 92 when pressure was applied to the inside of the
left upper hind limb. At termination, histopathological changes
typical of quinolone-induced lesions were found in the articular
cartilage of the right femoral condyle of one male given the high dose
(Stadnicki et al., 1990b).
Groups of three male and three female beagle dogs, five to six
months old, were given oral doses of 0, 0.25 or 0.5 mg/kg bw per day
of desmethyldanofloxacin in gelatin capsules, as two equally divided
doses, for about three months. Further groups of dogs were given 10
mg/kg bw per day of danofloxacin. The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58).
There were no signs of toxicity in the dogs treated with
desmethyldano-floxacin. The dogs given danofloxacin showed signs of
hind limb weakness, reduced activity, and stiff gait. Treatment had no
effect on electrocardiographic profiles, serum chemical or
haematological parameters, or organ weights. Pathological examination
revealed typical quinolone-induced arthropathy in all dogs treated
with danofloxacin. One male in the group given 0.5 mg/kg bw per day
desmethyldano-floxacin was found to have a single articular erosion in
the patellar groove of the right knee. Microscopically, the erosion
extended into zone 2 of the articular cartilage and resembled those
seen with other quinolones. The NOEL was 0.25 mg/kg bw per day
(Stadnicki et al., 1990c).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing the
equivalent of 10, 50, or 100 mg/kg bw per day of danofloxacin for up
to two years. Two groups of 50 controls of each sex were fed untreated
diets. The study complied with GLP guidelines. There were no adverse
dose-related effects on mortality rate, body weight, food consumption,
or haematological parameters. Females receiving 100 mg/kg bw per day
gained significantly more weight than animals in the other groups. At
termination, less than 50% of the animals in each group, except for
the males receiving 10 mg/kg bw per day, were still alive. The mean
absolute kidney weight (but not the relative kidney:body weight) was
significantly increased in females receiving 100 mg/kg bw per day,
which probably reflected the increased body weight of these mice.
There was no evidence of carcinogenicity (Takatsu et al., 1995).
Rats
Groups of 50 male and 50 female Long-Evans rats were fed diets
containing the equivalent of 10, 50, or 100 mg/kg bw per day of
danofloxacin for up to two years. Two groups of 50 controls of each
sex were fed untreated diets. The study complied with GLP guidelines.
There were no clinical signs of toxicity, and survival was
unaffected by treatment. At termination, however, less than 50% of
animals in all groups were alive, except for females receiving 100
mg/kg bw per day and one of the groups of female controls. Occasional
statistically significant reductions in body-weight gain were seen in
males at this dose, and females at 50 and 100 mg/kg bw per day had
significantly increased body-weight gain between three and 16 months
of treatment, which was correlated with increased food intake during
this period. Overall, the body-weight changes associated with
treatment were minimal. Ophthalmoscopic examinations during weeks 12,
18, and 23 revealed no differences between control rats and those
receiving 100 mg/kg bw per day. At termination, the mean leukocyte and
neutrophil counts were significantly reduced in males receiving 100
mg/kg bw per day; in females at this dose, the mean haemoglobin,
haematocrit, and lymphocyte counts were reduced. Males also had
significant increases in aspartate aminotrans-ferase and sorbitol
dehydrogenase values, and the mean globulin value was reduced, with a
corresponding increase in albumin:globulin ratio. The sorbitol
dehydrogenase level was also increased in males receiving 50 mg/kg bw
per day. The effects on clinical chemical values were not seen in
females. There were no substance-related effects on urinary values.
The mean testis:body weight ratio was significantly reduced in the
group receiving 100 mg/kg bw per day. There were no significant
effects on absolute organ weights. Gross pathological examination
revealed an increased incidence of caecal enlargement in treated
animals which was not correlated with any microscopic findings. (Table
3). The histopathological findings included an increased incidence of
renal papillary oedema, increased oligospermia, and abnormal content
of the epididymides in rats at 100 mg/kg bw per day (Table 4).
Table 3. Incidence of caecal enlargement in rats treated with
danofloxacin in the diet for up to two years
Sex Dose (mg/kg No. with caecal enlargement/
bw per day) no. examined
Male 0 0/50
0 0/50
10 5/50
50 3/50
100 6/50
Female 0 1/50
0 0/50
10 1/50
50 1/50
100 2/50
Table 4. Incidence of some non-neoplastic histopathological alterations in groups of
50 rats fed diets containing danofloxacin for up to two years
Sex Dose (mg/kg No. with papillary No. with No. with abnormal
bw per day) oedema oligospermia epidydimal content
Male 0 1 17 25
0 0 16 32
10 0 18 28
50 1 17 29
100 4 31 36
Female 0 0
0 2
10 2
50 2
100 4
There was a significant positive trend in the incidence of
granular-cell tumours of the uterus and vagina in treated female rats
(Table 5); however, the trend was not significant when corrected for
multiple comparisons. Granular-cell foci were distinguished from
tumours by their smaller size and the absence of compressed adjacent
tissue; nevertheless, the morphology of these lesions was essentially
the same. When the incidences of uterine and vaginal foci and tumours
were combined, there was no statistically significant trend in the
combined incidence across groups. There was a significant positive
trend in the incidence of pituitary adenomas in female rats (Table 6),
which was not significant when corrected for multiple comparisons. In
addition, the incidence of pituitary adenomas was within the range
seen in controls in the five previous studies at the same laboratory:
30/48 to 47/49 (Fisher et al., 1996).
2.2.4 Genotoxicity
The results of assays for genotoxicity with danofloxacin and
desmethyl-danofloxacin are summarized in Table 7. All of the studies
complied with GLP guidelines.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, groups of 45
male and 45 female Long-Evans rats were given oral doses of 0, 25, 75,
or 150 mg/kg bw per day of danofloxacin in an aqueous vehicle. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). Dams at 150 mg/kg bw per day showed reduced body-weight gain
during gestation and fewer implantation sites and produced fewer live
pups than those at other doses. The pup weights were significantly
reduced at birth and during lactation. Similar effects were observed
at the first F1 mating, which were more pronounced at the second
F1 mating, when the pregnancy rate was adversely affected in all
treated groups. The weights of all treated F2b pups were reduced in
a dose-related manner (Fisher et al., 1990b).
The F2b pups at 25 mg/kg bw per day were used in a new study
(complying with similar GLP guidelines) when they were two months old,
and treatment was continued. After mating to produce the F3
generation, the pregnancy rate was only 38%, whereas that in the
control group was 65%. Post-implantation losses were significantly
increased and pup body weights and survival were adversely affected
(Fisher et al., 1990c).
Table 5. Incidences of granular-cell proliferative lesions of the uterus
and vagina in female rats fed diets containing danofloxacin for up to
two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
Uterus
No. examined 48 50 50 50 50
Granular-cell tumour 1 3 6 5 6
Granular-cell foci 5 6 1 1 2
Vagina 48 49 49 49 49
Granular-cell tumour 3 2 2 3 7
Granular-cemm foci 0 0 1 0 0
Uterus plus vagina
Granular-cell tumour 4 5 8 8 13
Granular-cell foci5 5 6 2 1 2
Total 9 11 10 9 15
Table 6. Incidence of proliferative lesions of the pituitary gland in
female rats fed diets containing danofloxacin for up to two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
No. examined 49 50 50 50 50
No. with pituitary adenoma 32 32 39 39 40
No. with pituitary hyperplasia 13 11 5 6 5
No. of rats alive at 24 months 22 26 19 18 26
Table 7. Assays for genotoxicity with danofloxacin and desmethyldanofloxacin
End-point Test object Concentration S9 Results Reference
Danofloxacin
In vitro
Gene mutationa S. typhimurium 0.01-0.2 µg/platea - Negative Amacher et al.
TA98, TA100, 0.001-0.1 µg/plate + Negative (1988)
TA1535, TA1537 0.0005-0.2 µg/plate + Negative
Gene mutation L5178Y/tk+/- mouse 51-287 µg/ml - Negative Amacher et al.
lymphoma cellsc 16-215 µg/ml + Negative (1988)
Gene mutation hprt, Chinese 141-1070 µg/ml - Negative Guzzie et al.
hamster ovary 465-2500 µg/ml + Negative (1992)
cellsc
Unscheduled Primary rat 50-400 µg/ml Negative Amacher et al.
DNA synthesis hepatocytesc (1988)
Cytogenetic Human lymphocytes 25-70 µg/ml - Positived Amacher et al.
alterations 200-600 µg/ml + Positive (1990)
In vivo
Micronucleus ICR mouse bone 1000 mg/kg bw Negative Amacher et al.
formation marrow orally (1990)
Desmethyldanofloxacin
In vitro
Gene mutation S. typhimurium 0.001-0.5 µg/plate + Negative Amacher et al.
TA98, TA100, 0.01-5 µg/plate - Negative (1991a)
TA1535, TA1537
Gene mutation L5178Y mouse 90-388 µg/ml - Negative Holden et al.
lymphoma cells 63-269 µg/ml +e Negative (1988)
Unscheduled Primary rat 2.54-102 µg/ml and Positive Hazleton
DNA synthesis hepatocytes 5.02-100 µg/ml Positive Laboratories
America Inc.
(1991a)
Unscheduled Primary rat 62.5-250 µg/ml Positive Amacher et al.
DNA synthesis hepatocytes 62.5-500 µg/mlf Positive (1991b)
Table 7. (continued)
End-point Test object Concentration S9 Results Reference
In vivo
Unscheduled Fischer 344 rat 1 × 250-2000 mg/kg Negative Hazleton
DNA synthesis hepatocytes bw per day orally Laboratories
America Inc.
(1991b)
Micronucleus CD-1 mouse bone 3 × 250-1000 Negative Amacher et al.
formation marrow and peripheral mg/kg bw per day (1991c)
blood orally
S9, 9000 × g fraction of rat liver
a The same bacterial strains were incubated with urine collected from mice given danofloxacin
intraperitoneally at 5, 50, or 100 mg/kg bw per day, with no mutagenic response.
b Higher concentrations toxic to tester strains
c Not replicated independently
d Significant increase in abnormal cells (chromatid breaks). To check whether the clastogenicity
was due to chelation, the assay was repeated without S9 but with the addition of 400 µg/ml
magnesium sulfate; no increase in abnormal cells was observed. In the presence of S9, extra
washes to remove the test substance and the addition of magnesium sulfate diminished the
clastogenic response.
e From livers of uninduced rodents
f 1.62 mmol/litre magnesium ions (as magnesium sulfate heptahydrate) added to culture medium did
not prevent a similar dose-related increase in unscheduled DNA synthesis.
In a three-generation study of reproductive toxicity, groups of
30 male and 30 female Long-Evans rats were given oral doses of 1, 2.5,
6.25, or 150 mg/kg bw per day of danofloxacin. Two control groups
received the deionized water vehicle. Treatment was begun nine weeks
before cohabitation for males and two weeks previously for females
(1:1 mating) and was continued throughout gestation, parturition, and
weaning of the F1 offspring. Of these, groups of 25 of each sex were
selected randomly, and treatment was continued during breeding of the
F2a and F2b generations. The F2b offspring were mated in the
same way to produce the F3 litters. Litters were culled to eight
pups on day 4 post partum. Postnatal development of the F2b pups was
assessed at weaning by measuring locomotor activity, auditory
function, and ophthalmic parameters. All F0, F1, and F2 rats used
for breeding were necropsied and the reproductive organs and main
target organs (kidney, joints, brain, heart, and liver) were weighed
and preserved for possible microscopic examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
Adult animals showed no treatment-related effects on survival,
body-weight gain, food consumption, or clinical signs. In those at 150
mg/kg bw per day, copulatory rate and pregnancy rate were reduced and
the length of gestation was increased. (The copulatory rate was
defined as the percentage of those females which cohabited that had a
vaginal flush with sperm, or an internal copulatory plug, or which
delivered with no previous signs of mating.) Administration of 150
mg/kg bw per day also resulted in reduced litter size and pup weight
at birth, reduced weight gain of the neonates, and a reduction in the
number of pups surviving to day 4 post partum. The group receiving 150
mg/kg bw per day was terminated before the second F1 mating. The
NOEL was 6.25 mg/kg bw per day (Stadnicki et al., 1990d).
In a study of exactly the same design as that described above,
the animals received desmethyldanofloxacin instead of danofloxacin.
There were no treatment-related effects on parental body-weight gain
or food consumption, clinical signs, pregnancy rate, length of
gestation, number of implantation sites, or post-implantation loss.
Pup body-weight gain and survival were unaffected by treatment. There
were no grossly observed abnormalities in the dams or sires (Stadnicki
et al., 1991).
2.2.5.2 Developmental toxicity
Mice
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were given in an aqueous
vehicle to groups of seven mated female ICR mice on days 6-13 of
gestation. The study complied with GLP guidelines. The mean weights of
the male fetuses of animals at 200 mg/kg bw per day were reduced. No
effects were seen on dams (Kessedjian et al., 1988a).
In the main study, groups of 20 female Crl:COBS-CDI(ICR)BR mice
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-13 of gestation and were killed on gestation day 18.
A further group of 10 dams was given 200 mg/kg bw per day and used to
monitor drug concentrations in maternal plasma and amniotic fluid. The
study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were two to three time those in maternal plasma. The incidence of
non-gravid females was high: the total numbers of pregnant females
were only 13, 16, 11, and 13 in the control, low, intermediate, and
high dose groups, respectively. One dam given 200 mg/kg bw per day
showed piloerection and prostration on days 7-10 of gestation and was
found to have a cutaneous abscess at necropsy. The mean body-weight
gain of the dams at the high dose was significantly reduced during
treatment. There were no meaningful effects on the incidences of
resorptions or fetal deaths or on the sex ratio. The mean weights of
both male and female fetuses were significantly reduced at the high
dose, and the incidence of delayed ossification was increased. There
was no evidence of teratogenicity. The NOEL was 100 mg/kg bw per day,
on the basis of maternal and fetal toxicity (Kesseddjian et al.,
1989a).
Rats
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were administered in an
aqueous vehicle to groups of seven mated female Sprague-Dawley rats on
days 6-15 of gestation. The study complied with GLP guidelines.
Maternal body-weight gain was reduced in all treated groups, and the
mean number of viable fetuses was reduced at 200 mg/kg bw per day
(Kessedjian et al., 1988b).
In the main study, groups of 20 female Sprague-Dawley rats
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle; 19-20 dams in
each group were subsequently confirmed to be pregnant. The animals
were treated on days 6-15 of gestation and were killed on gestation
day 20. A further group of five dams was given 200 mg/kg bw per day
and used to monitor drug concentrations in maternal plasma and
amniotic fluid. The study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were about three time those in maternal plasma. There were significant
dose-related reductions in maternal body-weight gain in the rats at
100 and 200 mg/kg bw per day, and mean fetal weights were also
significantly reduced. The incidences of delayed ossification and
dilatation of the cerebral ventricles in the fetuses were
significantly increased at the intermediate and high doses. The NOEL
was 50 mg/kg bw per day, on the basis of maternal and fetal toxicity
(Kesseddjian et al., 1989b).
Rabbits
In a preliminary range-finding study, groups of 11 New Zealand
white rabbits presumed to be pregnant were given oral doses of 0, 5,
10, or 20 mg/kg bw per day of danofloxacin on days 6-20 of gestation.
The study complied with GLP guidelines. Inappetance was seen in all
groups, but maternal body weight was not affected. Animals at the high
dose had a decreased number of live fetuses and higher resorption
rates. The mean fetal weights were reduced at the intermediate and
high doses (Tassinari et al., 1995a).
In the main study, groups of 20 female New Zealand white rabbits
presumed to be pregnant were given oral doses of 0, 2.5, 7.5, or 15
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-20 of gestation and were killed on gestation day 28.
Because of a high incidence of non-gravid females, new animals were
randomized to the different doses so that the total numbers were 32
controls, 29 at the low dose, 33 at the intermediate dose, and 39 at
the high dose. The study complied with GLP guidelines.
Eleven does at the high dose showed loss of body weight and
reduced food consumption and then aborted their litters between days
22 and 28 of gestation. The mean food consumption of the does with
viable litters at 15 mg/kg bw per day was significantly reduced during
days gestation days 13-20. There were no treament-related effects on
litter size, sex ratio, fetal weight, or the incidences of
malformations or variations. The NOEL was 7.5 mg/kg bw per day, on the
basis of maternal toxicity (Tassinari et al., 1995b).
2.2.6 Special study on delayed contact hypersensitization
In a study using the Buehler closed patch technique, which
complied with GLP guidelines, danofloxacin mesylate did not cause
delayed contact hypersensitivity in the guinea-pig. A clear positive
result was obtained with the known sensitizer, dinitrochlorobenzene
(Beutler et al., 1992).
2.2.7 Special studies on pharmacological activity
A number of pharmacological studies of danofloxacin were
available that were not carried out in accordance with GLP guidelines
or with any national or international guidelines. The main features of
these studies are summarized in Table 8.
Table 8. Results of pharmacological assays with danofloxacin
Test system Doses Results Reference
Groups of six male 0, 5, 10, 20 mg/kg bw No significant diuretic Varner et al.
Sprague-Dawley rats per day orally in activity (1990a)
distilled water
Groups of two male 5 mg/kg bw per day Mild transient decreases Gromelski et al.
and two female intravenously in blood pressure, cardiac (1990)
beagle dogs output, left ventricular
pressure, and left ventricular
end diastolic pressure in two
dogs. No effects on electro-
cardiographic wave forms
Groups of three male 1, 10, 100, 1000 mg/kg No effect on central or Varner et al.
Sprague-Dawley rats bw per day orally peripheral nervous system (1990b)
at 100 mg/kg bw per day;
salivation and tremors at
1000 mg/kg bw per day
Groups of eight male 0, 5, 10, 20 mg/kg bw 18, 27, and 23% decreases Varner et al.
CD-1 mice per day orally in in gastrointestinal motility in (1990c)
distilled water; 4 mg/kg comparison with vehicle
morphine sulfate (positive control
control)
Groups of eight 0, 5, 10, 20 mg/kg bw Increased gastric fluid Varner et al.
pyloricligated male per day intraduodenally volume at all doses (1990d)
Sprague-Dawley rats in 0.25% methylcellulosea; (not dose-related);
10 mg/kg bw per day increased gastric acidity
cimetidine (positive at all doses
control)
a The solvent vehicle was reported to be 0.25% methylcellulose in some parts of the report and distilled water in others.
2.2.8 Special studies on microbiological effects
In a study carried out according to the methods described in
documents M11A (1979) and M11A2 (1990) of the National Committee for
Clinical Laboratory Standards, USA, the antibacterial activity of
danofloxacin and desmethyldano-floxacin was determined against 64
isolates of six genera of organisms representative of the human
intestinal anaerobic microflora. In order to assess the
reproducibility of the assay, four reference strains, Bacterides
fragilis ATCC 25285, Bacillus thetaiomicron ATCC 29741,
Clostridium perfringens ATCC 13124, and Eubacterium lentum ATCC
43055, were added to each batch of tests. In addition, data were
provided for the facultative anaerobes Lactobacillus spp (14
strains) and Proteus spp (11 strains) isolated from faeces of
patients in the hospital of Tourcoing, France. Escherichia coli ATCC
25922 and Enterococcus faecalis ATCC 29242 were added as reference
strains. The calculated MIC50 values are shown in Tables 9 and 10.
2.3 Observations in humans
No information was available. Danofloxacin is not authorized for
human use.
Table 9. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains of
bacteria representative of those found in the human gut
Species No. of Inoculum MIC50 (µg/ml)
strains density
(cfu/ml) Danofloxacin Desmethyldanofloxacin
Anaerobes
Bacillus fragilis group 12 107-108 4 128
Fusobacterium spp. 10 4 16
Clostridium spp. 10 0.5 0.5
Eubacterium spp. 10 0.5 1
Bifidobacterium spp. 10 2 8
Peptostreptococcus spp. 12 0.5 2
Facultative anaerobes
Lactobacillus spp. 14 106 16 > 128
Proteus spp. 11 106 0.25 0.06
Table 10. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains used for quality control
Species Anaerobic incubation Aerobic incubation
Danofloxacin Desmethyldanofloxacin Danofloxacin Desmethyldanofloxacin
Escherichia coli 0.06 0.06 0.03 0.015
ATCC 25922
Enterococcus faecalis 1 4 2 1
ATCC 29242a
a Shown as Enterococcus faecalis ATCC 29242 on p. 5 of the report but as Enterococcus faecalis ATCC 29212 in the
accompanying tables (Dubreuil, 1994)
3. COMMENTS
The Committee considered data from studies on the
pharmacokinetics, acute, short-term, and long-term toxicity,
carcinogenicity, reproductive toxicity, genotoxicity, and
antimicrobial activity of danofloxacin. The results of studies on the
acute and short-term toxicity, reproductive toxicity, genotoxicity,
and antimicrobial activity of the metabolite desmethyldanofloxacin
were also reviewed. Most of the studies critical for the evaluation
were carried out in accordance with appropriate standards for study
protocol and conduct.
Danofloxacin was rapidly absorbed after oral administration to
chickens and pigs and after intramuscular administration to pigs and
cattle. Only one study was carried out in which the oral
bioavailability of danofloxacin was calculated; in this study, the
bioavailability after oral administration of 5 mg/kg bw to pigs was
approximately 90%. The substance was well distributed to the tissues.
Urine and faeces contained approximately equal amounts of danofloxacin
and its metabolites.
In cattle, dogs, and rats, unchanged danofloxacin was the main
substance present in the faeces; smaller amounts of
desmethyldanofloxacin were found. Danofloxacin was also the main
component in urine. Desmethyldanofloxacin, danofloxacin- N-oxide, and
the ß-glucuronide were also found in urine. A piperazine-ring
degradation product was present in the bile of cattle but was found in
only trace amounts in the bile of rats and dogs. Residues of both
danofloxacin and desmethyldanofloxacin were found in liver samples
from rats, dogs, and the three target species. The Committee
considered that the metabolism of danofloxacin was very similar in
laboratory animals and in the three target species.
Single oral doses of both danofloxacin and desmethyldanofloxacin
were slightly toxic to rats and mice (acute oral LD50 values in the
range 1500 to > 2000 mg/kg bw). Signs of toxicity typical of
stimulation of the central nervous system were observed prior to
death.
Several exploratory studies were carried out in rats given
danofloxacin in the feed and by gavage and in rabbits given
danofloxacin by gavage. Caecal dilatation was seen at doses of 25
mg/kg bw per day and above in most of these studies. In rats,
increased incidences of crystals in the urine were observed at doses
of 75 mg/kg bw per day and above. There were no corresponding
pathological changes in the kidneys. In a three-month study in which
danofloxacin was administered to rats in the diet, degenerated
germinal cells were found in the epididymides of all males given 300
mg/kg bw per day and in 9 out of 15 males given 150 mg/kg bw per day.
In view of the high doses used, the results of these studies were not
useful in evaluating the safety of danofloxacin.
Rats were exposed to danofloxacin in utero and during lactation
by administration to the dams of doses of 0, 25, 75, or 150 mg/kg bw
per day. The rats were orally dosed at the same levels for an
additional three months after weaning. In females, there was a
dose-related increase in proteinuria which correlated with the finding
of tubular nephropathy in individual animals. In males at 75 and 150
mg/kg bw per day, both the mean absolute and relative testicular
weights were 10% lower than those of controls. In a follow-up
three-month study with lower doses, the highest dose, 6.25 mg/kg bw
per day, was the NOEL, on the basis of renal tubular nephropathy.
Rats were exposed to desmethyldanofloxacin in utero and during
lactation by administration to the dams of oral doses of 0, 1, 2.5, or
6.25 mg/kg bw per day. The rats were treated orally at the same levels
for an additional three months after weaning. No adverse effects were
observed at any dose.
Six-month-old dogs were given oral doses of 0, 5, 10, or 25 mg/kg
bw per day of danofloxacin in gelatin capsules for three months. Those
given the two higher doses showed signs of joint pain. Pathological
examination revealed arthropathy characterized by areas of cartilage
separation and erosion in all treated groups, and the severity of the
lesions was dose-related. A second three-month study was carried out
in immature dogs given oral doses of 0, 1, or 2.4 mg/kg bw per day
danofloxacin in gelatin capsules. There was no evidence of arthropathy
or any other treatment-related effect. On the basis of the results of
the two studies, the Committee concluded that 2.4 mg/kg bw per day was
the overall NOEL for arthropathy in dogs.
A further three-month study was carried out in which immature
dogs were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules. One male of three given the
highest dose and one female of three given the lowest dose showed
signs of pain on examination. In the male, morphological changes were
found in the articular cartilage of one joint. When the study was
repeated at doses of 0, 0.25, or 0.5 mg/kg bw per day
desmethyldanofloxacin, one male of three given 0.5 mg/kg bw per day
was found to have histopathological changes in the right knee typical
of quinolone-induced arthropathy. The NOEL for desmethyldanofloxacin
was 0.25 mg/kg bw per day, on the basis of arthropathy.
In a 102-day range-finding study, mice were fed diets containing
danofloxacin at concentrations equivalent to 0, 150, 300, or 600 mg/kg
bw per day. At the highest dose, the body-weight gain of males was
reduced, and decreased haematological parameters and increased kidney
weights were observed in females. Caecal dilatation was observed at
all doses, and two of 30 mice given the highest dose had inflammation
of the caecum.
In a two-year study of carcinogenicity, mice were fed diets
containing doses equal to 0, 10, 50, or 100 mg/kg bw per day. Females
at the highest dose gained more weight than the controls and showed
increased absolute kidney weights. There were no adverse effects on
haematological parameters, but clinical chemistry was not monitored.
No increase in tumour incidence was observed at any dose.
In a two-year study of carcinogenicity, rats were fed diets
providing doses of 0, 10, 50, or 100 mg/kg bw per day danofloxacin. At
the highest dose, mean haemoglobin, haematocrit, and lymphocyte counts
were decreased in females, and serum aspartate aminotransferase
activity was increased and serum globulin levels were reduced in
males. Serum sorbitol dehydrogenase activity was increased in males
given 50 or 100 mg/kg bw per day. The relative testicular weight was
reduced in rats at the highest dose, and increased oligospermia and
abnormal epididymal content were observed in this group. Caecal
enlargement was noted in all treated groups but was not correlated
with any microscopic findings. An increased incidence of papillary
oedema was seen in the kidneys of rats at 100 mg/kg bw per day. There
was a significant positive trend in the incidence of granular-cell
tumours of the uterus and vagina in treated female rats. Tumours were
distinguished from foci by their larger size and the compression of
adjacent tissue, but the morphology of these lesions was similar, and
the Committee considered that it was appropriate to combine the
incidences of these uterine and vaginal foci and tumours. There was no
significant trend in the combined incidence across groups. There was a
significant positive trend in the incidence of pituitary adenomas in
females, but the number of rats with these lesions was within the
range in historical controls. In addition, none of the trends in
tumour incidence was significant when corrected for multiple
comparisons. The Committee concluded that neither the granular-cell
lesions of the uterus and vagina nor the pituitary adenomas were
indicative of a carcinogenic response to treatment with danofloxacin.
The genotoxic properties of danofloxacin were investigated in
vitro in assays for gene mutation in bacteria and in mammalian
cells, unscheduled DNA synthesis, and cytogenetics alterations in
mammalian cells and for cytogenetic effects in vivo. All of the
tests gave negative results, except for an assay of cytogenetic
alterations in human lymphocytes in vitro. The clastogenicity
observed in vitro was reduced or abolished by addition of magnesium
sulfate to the culture medium and/or washing the cells after treatment
to remove danofloxacin, and appeared to be associated with the cation-
chelating properties of danofloxacin. There was no evidence of
clastogenicity in vivo. Desmethyldanofloxacin induced a significant
increase in unscheduled DNA synthesis in two independent assays in
primary rat hepatocytes in vitro; however, negative results were
obtained with desmethyldanofloxacin in an assay for unscheduled DNA
synthesis and in a test for micronucleus formation in vivo. Thus,
although desmethyldanofloxacin induced unscheduled DNA synthesis, this
genotoxic potential did not appear to be expressed in vivo.
In a two-generation study of reproductive toxicity, rats received
doses of 0, 25, 75, or 150 mg/kg bw per day of danofloxacin by gavage.
In the parental generation, maternal body-weight gain was reduced at
the highest dose and these dams had fewer implantation sites and
produced fewer viable pups. The effects were observed at lower doses
with subsequent matings. At the second mating of the first-generation
animals, the pregnancy rate was adversely affected in all treated
groups. No NOEL could be identified.
A three-generation study of reproductive toxicity was carried out
in which rats were given danofloxacin at doses of 0, 1, 2.5, 6.25, or
150 mg/kg bw per day by gavage. At 150 mg/kg bw per day, the number of
mated females and the pregnancy rate were reduced, the duration of
gestation was increased and the litter sizes and pup weights were
reduced; this group was terminated before the second mating of the
first generation. In this study, in which lower doses were used than
in the study described above, the NOEL for reproductive toxicity was
6.25 mg/kg bw per day.
No adverse effects were observed in a three-generation study of
reproductive toxicity in rats with desmethyldanofloxacin in which
doses of up to 6.25 mg/kg bw per day were administered by gavage.
In a study of developmental toxicity in mice, there was no
evidence of teratogenicity when danofloxacin was administered by
gavage at doses of up to 200 mg/kg bw per day on days 6-13 of
gestation. The dose of 200 mg/kg bw per day was toxic to the dams,
reducing body-weight gain, and was fetotoxic, producing a reduction in
mean fetal weight and an increased incidence of delayed ossification.
The study was compromised by the small numbers of gravid dams in all
groups, including the controls. The NOEL for both maternal and fetal
toxicity was 100 mg/kg bw per day.
Oral doses of 0, 50, 100, or 200 mg/kg bw per day were
administered to rats on days 6-15 of gestation in a study of
developmental toxicity. Maternal body-weight gain and food consumption
were reduced in animals at 100 or 200 mg/kg bw per day. At these
doses, the incidences of delayed ossification and dilatation of the
cerebral ventricles were significantly increased. The NOEL for both
maternal and fetal toxicity was 50 mg/kg bw per day.
In a study of developmental toxicity, oral doses of 0, 2.5, 7.5,
or 15 mg/kg bw per day danofloxacin were administered to rabbits on
days 6-20 of gestation. At a dose of 15 mg/kg bw per day, maternal
body-weight loss, reduced food consumption, and abortion were
observed. The NOEL for maternal toxicity was 7.5 mg/kg bw per day.
The minimum concentration of danofloxacin resulting in 50%
inhibition (MIC50) was determined for 64 isolates of the six
predominant genera of human intestinal anaerobic microflora
( Bacteroides, Fusobacterium, Clostridium, Eubacterium,
Bifidobacterium, and Peptostreptococcus). In addition, data were
provided for the facultative anaerobes Lactobacillus, Proteus, and
Escherichia coli. Although E. coli and Proteus were the most
sensitive organisms, the Committee agreed that they should not be
taken into account in the calculation of the MIC50 because they are
not predominant species in the human intestine. Instead, the Committee
derived the mean MIC50 from the data available on 32 strains of the
most sensitive relevant genera isolated from the human
gastrointestinal tract, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp. The mean MIC50
for these strains was 1 µg/ml. This figure was used in calculating the
upper limit of the ADI from the formula described on p. 12:
Upper limit 1 µg/ga × 220 g
=
of ADI 0.1b × 1c × 60 kg
= 37 µg/kg bw
a Mean MIC50 for the most sensitive relevant species in the
human intestine, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp.
b Bioavailability was determined to be about 90%, based on a
study in pigs given an oral dose of 5 mg/kg bw. The finding that
danofloxacin is strongly bound to cattle faeces was considered by
the Committee to add confidence to use of this value.
c A safety factor of 1 was used because sufficient relevant
microbiological data were provided.
The metabolite desmethyldanofloxacin was one-quarter to one-half
as active as danofloxacin against the same isolates.
4. EVALUATION
The Committee noted that danofloxacin belongs to a group of
fluoroquinolones that is active against aerobic gram-negative bacteria
and that the main components of the flora in the human
gastrointestinal tract are largely unaffected by these compounds.
Therefore, the Committee decided to base the ADI on the toxicity of
danofloxacin and not on its effects on the intestinal flora. Moreover,
the toxicological end-point resulted in a lower ADI.
The Committee established an ADI of 0-20 µg/kg bw per day on the
basis of the NOEL of 2.4 mg/kg bw per day for arthropathy in a
three-month study in immature dogs and a safety factor of 100. The ADI
was rounded to one significant figure, as is the standard practice
(Annex 1, reference 91, section 2.7).
The Committee noted that the NOEL for arthropathy in studies with
desmethyldanofloxacin was 0.25 mg/kg bw per day. The studies of
pharmacokinetics and metabolism showed that dogs that received oral
doses of danofloxacin were also exposed systemically to the major
metabolite desmethyldanofloxacin. Therefore the Committee concluded
that it was not necessary to calculate a separate ADI for
desmethyldanofloxacin. The Committee agreed, however, that the
approximately 10-fold higher toxicity of the metabolite should be
taken into account when recommending MRLs, as consumers may be
directly exposed to desmethyldano-floxacin in liver.
5. REFERENCES
Amacher, D.E., Turner, G.N., Ellis, J.H., Holden, H.E. & Kluwe, W.M.
(1988) Danofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Mammalian cell gene mutation assays. Unscheduled DNA
synthesis assays. Unpublished studies Nos. 86-607-01 and 87-607-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Muehlbauer, P.A., Wahrenburg M.G. &
Kluwe, W.M. (1990) Danofloxacin. Genetic toxicology report. Addendum.
In vitro cytogenetics assays. In vivo cytogenetics assays.
Unpublished study No. 89-607-18 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Ellis, J.H. & Kluwe, W.M. (1991a)
Desmethyldanofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Unpublished study No. 90-576-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Amacher, D.E., Holden, H. E., Muehlbauer, P.A. & Kluwe, W.M. (1991b)
Genetic toxicology report. Unscheduled DNA synthesis assay.
Unpublished study No. 90-576-12 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Zelljadt, I. & Kluwe, W.M. (1991c)
Desmethyldano-floxacin. Genetic toxicology report. In vivo
micronucleus assay. Unpublished study No. 90-576-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Beutler, N.J., Tinkel, J.H. & Knickerbocker, M. (1992) Delayed contact
hyper-sensitivity study in guinea pigs. Unpublished study No.
92-607-27 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Dubreuil, L. (1994) Antibacterial activity of a fluoroquinolone
against bacteria isolated from human gut flora: Danofloxacin and its
metabolite desmethyldano-floxacin (DMD). Unpublished study from
Microbiology Department, Faculty of Pharmacy, University of Lille,
France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Vernon, G.S., Mayne, J.T., Frame, G.M., Milisen, W.B.,
Estes, P.C. & Levinsky, H.V. (1988a) A one month oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-08 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Frame, G.M., Saunders,
W.J., Estes, P.C. & Levinsky, H.V. (1988b) Danofloxacin. One month
oral capsule study in beagle dogs. Unpublished study No. 87-607-07
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Milisen, W.B., Estes,
P.C. & Levinsky, H.V. (1989a) Danofloxacin. Three months oral gavage
study with in utero exposure in Long-Evans rats. Unpublished study
No. 88-607-12 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Soler, G.N., Mayne, J.T., Walsh, A.H., Levinsky, H.V. &
Estes, P.C. (1989b) Danofloxacin. Three month oral capsule study in
beagle dogs. Unpublished study No. 88-607-14 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Carson, D.L., Son, D.L., Walsh, A.H.,
Levinsky, H.V. & Estes, P.C (1989c) Danofloxacin. Three month oral
capsule study in beagle dogs. Unpublished study No. 88-607-15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Fisher, D.O., Hills-Perry, P.A., Rice, J., Coleman, G.L., Estes, P.C.
& Levinsky, H.V. (1990a) Danofloxacin. Three months oral gavage study
with in utero exposure in Long-Evans rats. Unpublished study No.
89-607-19 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Beierschmitt, W.P., Milisen, W.B., Estes, P.C. &
Levinsky, H.V. (1990b) A two-generation oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Powers, K.A., Beierschmitt, W.P. & Levinsky, H.V.
(1990c) An exploratory reproductive oral gavage study in Long-Evans
rats. Unpublished study No. 89-607-17 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Milisen, W.B., Kluwe, W.M. & Ochoa, R. (1996) Two year
oncogenicity study with dietary administration in Long-Evans rats.
Unpublished study No. 93-607-31 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1990) Acute
hemodynamic effects of an intravenous infusion of CP-76,136-27 in the
open-chest anesthetized dog. Unpublished study No. PH 247-PZ-001-90
from Pharmakon Laboratories, Waverly, PA, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, Groton, CT, USA.
Guzzie, P.J., Turner, G.N., Amacher, D.E. & Kluwe, W.M (1992) Genetic
toxicology report. Antimicrobial. Unpublished study No. 92-607-26 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991a) UDS assay in primary rat
hepatocytes. Unpublished study no. 12180-0-447R. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991b) In vivo/in vitro UDS
assay in primary rat hepatocytes. Unpublished study no. 12180-0-494.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Holden, H.E., Turner, G.N. & Ray, V.A. (1988) Genetic toxicology
report. Nalidixic acid analog. Unpublished study No. 86-576-01 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988a) Danofloxacin.
Maternal toxicity study in mice by the oral route. Unpublished study
No. 88028 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988b) Danofloxacin.
Maternal toxicity study in rats by the oral route. Unpublished study
No. 88059 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989a) Danofloxacin.
Fetotoxicity study in mice by the oral route. Segment II. Unpublished
studies No. 88115 and 88116 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989b) Danofloxacin.
Fetotoxicity study in rats by the oral route. Segment II. Unpublished
studies No. 88093 and 88094 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lundeen, G.R., Macaione, G.M., Santacroce, E.M. & Rice, J.R. (1993)
Two week exploratory toleration study in New Zealand white rabbits.
Unpublished study No. 92-607-30 from Pfizer Central Research, Terre
Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc., USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1988) Danofloxacin.
Nonclinical total residue depletion study in broiler chickens.
Unpublished study No. 1515N-60-88-006 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989a) Danofloxacin.
Nonclinical total residue excretion study in cattle. Unpublished study
No. 1535N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989b) Danofloxacin. A
nonclinical total residue excretion study in swine. Unpublished study
No. 1525C-60-88-009 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989c) Danofloxacin. A
nonclinical total residue depletion study in swine. Unpublished study
No. 1525N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1990a) Danofloxacin. Plasma and lung
pharmacokinetics study in broiler chickens. Unpublished Study No.
1514C-60-90-002 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Horan, M.R. & Gummerus, J.A. (1990b) Danofloxacin. Nonclinical plasma
and lung pharmacokinetics study in cattle. Unpublished study No.
1534N-60-89-003 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R. &
Harran, L.P. (1991a) Danofloxacin. Determination of plasma
pharmacokinetics and bioavailability in cattle. Unpublished study No.
1532N-60-89-005 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1991b) Danofloxacin.
Nonclinical total residue excretion study in broiler chickens.
Unpublished study No. 1515N-60-88-007 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991c) A plasma and lung pharmacokinetics
study in swine. Unpublished study No. 1524C-60-89-003 from Pfizer
Central Research, Terre Haute, IN, and Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ericson, J., Calcagni, A., Gummerus, J. & Nowakowski,
M.A. (1991d) The comparative metabolism of 3H-danofloxacin in
cattle, dog and rat species. Unpublished study No. CM-90-02 from
Pfizer Central Research, Terre Haute, IN, and Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Mann, D.D. & Frame, G.M (1992) Pharmacokinetic study of danofloxacin
in cattle and swine. Am. J. Vet. Res., 53, 1022-1026.
Reynolds, J.A., Saunders, W., Hatch, G.W. & Vessella, D.J. (1987a) 2
week oral gavage toleration study in Long-Evans rats. Unpublished
study No. 87-607-03 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Reynolds, J.A., Milisen, W.B. & Frame, G.M. (1987b) Two week oral
capsule toleration study in beagle dogs. Unpublished study No.
87-607-05 from Pfizer Central Research, Terre Haute, IN, and Groton,
CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Risk, J.E., Ronfeld, R.A., Magonigle, R.A., Lynch, M.J., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991) Danofloxacin. Plasma and lung
pharmacokinetics study in swine. Unpublished study No. 1524C-60-89-003
from Pfizer Central Research, Terre Haute, IN, and Groton, CT.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Stack, T.L., Knickerbocker, M. & Beutler, N.J.
(1988a) Danofloxacin. Acute oral and intravenous toxicity studies in
mice and rats. Unpublished study No. 87-607-10 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Nilson, A.L. Knickerbocker, M. & Beutler, N.J.
(1988b) Danofloxacin. Acute dermal and ocular irritation studies in
rabbits (FHSA testing). Unpublished study No. 88-607-11 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Stadnicki S.W., Rinaldi, M.T.S., Knickerbocker, M. & Beutler, N.J.
(1989) Desmethyldanofloxacin. Acute oral and intravenous toxicity
studies in albino mice and Sprague-Dawley rats. Unpublished study No.
89-576-05 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, B.A., Lundeen, G.R., Rice, J., Roesler,
A.R., Estes, P.C. & Levinsky, H.V. (1990a) Desmethyldanofloxacin.
Three month gavage study in Long-Evans rats with in utero exposure.
Unpublished study No. 89-576-06 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Walsh,
A.H., Estes, P.C. & Levinsky, H.V. (1990b) Desmethyldanofloxacin.
Three months oral capsule study in beagle dogs. Unpublished study No.
89-576-03 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Estes, P.C.
& Levinsky, H.V. (1990c) Desmethyldanofloxacin. Three months oral
capsule study in beagle dogs. Unpublished study No. 89-576-07 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Stadnicki, S.W., Hallam, J.A., Ball, D.J., Pettersen, B.A., Levinsky,
H.V., Milisen, W.B. & Estes, P.C. (1990d) Danofloxacin. A
three-generation oral gavage study in Long-Evans rats. Unpublished
study No. 89-607-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Santacroce, E.M., Lundeen, G.R., Coleman, G.L.,
Estes, P.C. & Levinsky, H.V. (1991) Desmethyldanofloxacin. Three
generation gavage study in Long-Evans rats. Unpublished study No.
89-576-04 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Walsh, A.H. & Rice, J.R. (1994a) Three month feeding study in CD-1
mice. Unpublished study No. 92-607-29 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Chatman, L.A. & Rice, J.R. (1994b) One month feeding study in Fischer
rats. Unpublished study No. 91-607-23 from Pfizer Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Cacciatore, A., Davenport, C.J., Coleman, G.L. &
Rice, J.R (1994c) Three week feeding study in Long-Evans rats.
Unpublished study no. 92-607-25 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Boldt, B.K., Rovetti, C.C., Davenport, C.J., Milisen,
W.B. & Rice, J.R. (1994d) Three month feeding study in Long-Evans
rats. Unpublished study No. 92-607-28 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Takatsu, S., Walsh, A.H., Shirai, N., Iidaka, T, Fukazawa, H., Iijima,
M. & Tachibana, M. (1995) Two year oncogenicity study with dietary
administration in ICR mice. Unpublished study No. 93-76-31 from Pfizer
Central Research, Chita-gun, Aichi, Japan. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Tassinari, M.S., Rizoli, A.M., Hills-Perry, P., Linhares, M. & Gupta,
U. (1995a) Maternal toxicity study by oral gavage in New Zealand white
rabbits. Unpublished study No. 93-607-33 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Tassinari, M.S., Laithwaite, H.T., Podurgiel, S.J. & Gupta, U. (1995b)
Danofloxacin. Reproductive study III (teratology) in New Zealand white
rabbits. Unpublished study No. 93-607-34 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990a) Diuretic assay.
Unpublished study No. PH 209-PZ-001-90 from Pharmakon Laboratories,
Waverly, PA, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990b)
Neuropharmacological profile (NPP) in rats. Unpublished study No. PH
200A-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990c)
Gastrointestinal propulsion assay in male mice. Unpublished study No.
PH 239-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990d) Secretory
screen in pyloric ligated Shay rats. Unpublished study No. PH
252-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Chickens
Groups of male 18-day-old broiler chickens were given
drinking-water that had been medicated to provide 5 mg/kg bw per day
danofloxacin, for three days. Steady-state concentrations of 0.43 and
0.21 µg/ml were obtained in lung and plasma, respectively, during the
period of administration. The half-life of danofloxacin was 4.9 h in
plasma and 5.8 h in lung. No residues of desmethyldanofloxacin were
detected in any of the plasma or lung samples (Lynch et al., 1990a).
Groups of broiler chickens were given drinking-water that had
been medicated to provide 5 mg/kg bw per day 3H-danofloxacin for
five days. The test substance was tritiated in the N1-cyclopropyl
ring. The study complied with GLP guidelines. Six hours after
withdrawal of the treated water, unchanged danofloxacin accounted for
75% of the radiolabel in excreta, and desmethyl-danofloxacin accounted
for 5-7% (Lynch et al., 1990a).
Groups of 12 male and 12 female 22-day-old broiler chickens were
given drinking-water which had been medicated to provide 5 mg/kg bw
per day 3H-danofloxacin, for five days. The test substance was
tritiated in the N1-cyclo-propyl ring and had a specific activity of
12.1 µCi/mg (expressed as base). Excreta were collected every 24 h and
pooled by sex and day. The study complied with GLP guidelines. The
mean level of radiolabel in excreta attained a steady state in the
range 35-46 µg/g by the first day of treatment but declined to 20 and
6.6 µg/g on the first and second days after withdrawal, respectively.
About 85% of the radiolabel in faeces was identified as unmetabolized
danofloxacin. There was no difference between the sexes in the pattern
of excretion (Lynch et al., 1988).
Pigs
Castrated male and female pigs (mean body weight, 40 kg) were
given single intramuscular injections of 1.25 mg/kg bw of a prototype
2.5% formulation of danofloxacin. Absorption was rapid, with peak
concentrations of 0.40 µg/g in plasma and 1.68 µg/g in lung about 1 h
after dosing. The half-life of plasma elimination was approximately
7 h (Risk et al., 1991).
Six castrated male and six female pigs (mean body weight, 43.3
kg) were given five daily intramuscular injections of 1.25 mg/kg bw of
danofloxacin tritiated in the N1-cyclopropyl ring in a 2.5% aqueous
vehicle. The study complied with GLP guidelines. The mean radiolabel
concentration during the two days before and the day after the last of
the five doses was 7-8 µg/g in pooled faecal homogenates and 14-18
µg/g in pooled urine samples. During this same period, the total daily
recovery of radiolabel from urine and faeces accounted for 75-81% of
the daily dose. The mean radiolabel concentration in bile was 1.7 µg/g
12 h after the end of treatment and had declined to 0.21 µg/g by 48 h
(Lynch et al., 1989b).
In pigs given a single dose of 5 mg/kg bw of danofloxacin
intravenously, intramuscularly, or orally, detectable residues of
danofloxacin appeared rapidly in plasma. A peak plasma concentration
of 0.42 µg/ml was attained about 3 h after oral dosing. The
bioavailability was estimated to be 76% after intramuscular
administration and 89% after oral administration (Mann & Frame, 1992).
Cattle
A group of 36 male and female calves weighing about 250 kg were
given a single intramuscular injection of 1.25 mg/kg bw of a 2.5%
prototype formulation of danofloxacin, and the kinetics of the
compound were evaluated in plasma and lung tissues. The study complied
with GLP guidelines. Absorption was rapid, with peak plasma and lung
concentrations of 0.35 µg/ml and 1.44 µg/g attained 1 h after dosing.
The half-time for plasma elimination was 3.4 h (Lynch et al.,
1990b).
In a three-way cross-over design study, 12 male and female beef
calves (mean body weight, 112 kg) were given single intravenous doses
or five subcutaneous or intramuscular doses of 1.25 mg/kg bw per day
of a 2.5% prototype formulation of danofloxacin. The study complied
with GLP guidelines. Absorption was rapid: peak plasma concentrations
of 0.37 and 0.47 µg/ml were attained about 1 h after single
subcutaneous and intramuscular doses, respectively, and the
bioavailability was almost 100%. The values for the area under the
concentration-time curve indicated that the levels achieved after
intramuscular and subcutaneous administration were bioequivalent after
one, three, and five injections (Lynch et al., 1991a).
The pattern of excretion of 3H-danofloxacin (tritiated in the
N1-cyclopropyl ring) was studied in five castrated male and four
female calves (body weight, about 180 kg) after five daily
intramuscular injections of 1.25 mg/kg bw (in a 2.5% aqueous vehicle).
The study complied with GLP guidelines. The total concentration of
danofloxacin-related material in excreta reached a plateau by the
third day of treatment. Approximately equal amounts were excreted in
urine and faeces. Unchanged danofloxacin accounted for about 48% of
material excreted in faeces and 89% in urine. The desmethyl metabolite
accounted for 12% of the material in urine samples, but the
concentrations in faeces were too low for measurement (Lynch et al.,
1989a).
2.1.2 Biotransformation
The biotransformation of danofloxacin has been investigated in
rats, dogs, chickens, pigs, and cattle. In all of these species, the
main residue in the faeces was unmetabolized danofloxacin; smaller
amounts of desmethyldanofloxacin were also found (Lynch et al.,
1989a,b,c, 1991a,b,c,d). The main residue in the urine of cattle,
dogs, and rats was also danofloxacin. Desmethyldanofloxacin,
danofloxacin- N-oxide and the ß-glucuronide were identified in rat,
dog, and pig urine (Lynch et al., 1991d).
In the livers of most species, danofloxacin was the major
residue, but significant residues of the metabolite
desmethyldanofloxacin were also present (Table 1). A piperazine-ring
degradation product constituted 43 and 27% of the radiolabel in bile
from male and female cattle, respectively, but it was present in only
trace amounts in bile from rats and dogs. The bile of dogs contained
residues of danofloxacin- N-oxide, which was present in only trace
amounts in bile from the other species (Lynch et al., 1991d).
2.2 Toxicological data
2.2.1 Acute toxicity
The results of studies of the acute toxicity of danofloxacin and
desmethyl-danofloxacin are summarized in Table 2. All of the studies
complied with GLP guidelines.
In the studies by oral administration, signs of toxicity included
exophthalmia, decreased activity, tremors, and twitching, which
occurred within a few minutes of dosing. One of three mice given
2000 mg/kg bw died 29 min after dosing. Retching, clonic convulsions,
and gasping were observed before death. After intravenous dosing, all
three mice given 100 mg/kg bw and all three rats given 150 mg/kg bw
died within 3 min. The signs of toxicity were similar to those
produced after oral administration. Similar signs of toxicity were
observed after oral and intravenous dosing with desmethyldanofloxacin.
Table 1. Composition of residues in liver in five species given danofloxacin
Species Dose regimen Sex Total Danofloxacina Desmethyldanofloxacina Reference
residue
Rat 5 × 6.25 mg/kg bw M 2.3 ppm 67% 18% Lynch et al.
per day orally F 2.5 ppm 70% 11% (1991d)
Dog 5 × 2.4 mg/kg bw M 6.7 ppm 66% 28% Lynch et al.
per day orally F 5.4 ppm 58% 37% (1991d)
Chicken 5 × 5 mg/kg bw M NR 78% 16% Lynch et al.
per day orally F NR 74% 12% (1988)
Pig M 0.95 ppm 58% 23% Lynch et al.
F 0.96 ppm 68% 29% (1989c)
Cowb 5 × 1.25 mg/kg bw M 0.82 ppm 24% 43% Lynch et al.
intramuscularly F 1.1 ppm 29% 42% (1991d)
a Relative to the radiolabel recovered by high-performance liquid chromatography
b Killed 12 h after the last dose
Table 2. Results of studies of the acute toxicity of danofloxacin and desmethyldanofloxacin
Species Route Vehicle Sex LD50 Reference
(strain) (mg/kg bw)
Danofloxacin
Rat (Sprague- Oral Distilled M & F > 2000 Stadnicki et al. (1988a)
Dawley) water
Mouse (ICR) Oral Distilled water M & F > 2000 Stadnicki et al. (1988a)
Rat (Sprague- Intravenous Sterile water M 100-150 Stadnicki et al. (1988a)
Dawley)
Mouse (ICR) Intravenous Sterile water M 50-100 Stadnicki et al. (1988a)
Rabbit (New Dermal M & F > 299a Stadnicki et al. (1988b)
Zealand white)
Desmethyldanofloxacin
Rat (Sprague- Oral Deionized M & F > 2000 Stadnicki et al. (1989)
Dawley) water
Mouse (ICR) Oral Deionized M > 2000 Stadnicki et al. (1989)
water
Mouse (ICR) Oral Deionized F 1500-2000 Stadnicki et al. (1989)
water
Rat (Sprague- Intravenous Sterile water M 40-50 Stadnicki et al. (1989)
Dawley)
Mouse (ICR) Intravenous Sterile water M 7.5-10 Stadnicki et al. (1989)
a 0.5 g danofloxacin was applied to one site on intact skin and one on abraded skin of two male and one
female New Zealand white rabbits under an occlusive dressing for 24 h. There were no deaths or signs
of toxicity, except for mild erythema at all treated sites.
A dose of 26 mg danofloxacin (in a volume of 0.1 ml) was
instilled into the conjunctival sac of the left eyes of three New
Zealand white rabbits. The eyes were not rinsed after dosing. Mild
conjunctivitis and a colourless discharge were observed within 1 h of
treatment. All signs had cleared within 96 h (Stadnicki et al.,
1988b).
2.2.2 Short-term toxicity
Mice
In a study designed to identify suitable doses for a two-year
feeding study, groups of 15 male and 15 female Crl:CD-1 (ICR)BR
VAF/Plus mice were fed diets calculated to provide 0, 150, 300, or 600
mg/kg bw per day of danofloxacin (74% activity) for up to 102 days.
The study complied with GLP guidelines. One female given the high dose
was killed in a moribund condition. Body-weight gain was reduced in
males at the low and high doses in comparison with that of the
controls. Decreased erythrocytic parameters were seen occasionally in
males and females at the high dose. There were no consistent
dose-related changes in clinical chemistry. At termination, the
absolute mean group weight of the kidneys of females receiving the
high dose was significantly increased, but there were no corresponding
pathological changes. Microscopic examination revealed caecal
dilatation in six mice at the low dose, 23 at the intermediate dose,
and 27 at the high dose, but not in the controls. Inflammation of the
caecum was seen in two mice at the high dose, and Gram-positive
bacteria, tentatively identified as Clostridium difficile, were
found in the inflamed caeca of both animals. Thymic lymphocytosis was
seen in one of 15 females at the low dose, two of 15 at the
intermediate dose, and eight of 15 at the high dose and in three of 13
males at the intermediate dose and six of 14 at the high dose but in
no control. A NOEL was not identified (Stadnicki et al., 1994a).
Rats
A two-week exploratory study was carried out in which groups of
three male and three female Long-Evans rats received oral doses of 0,
25, 50, or 100 mg/kg bw per day of danofloxacin in an aqueous vehicle.
There were no significant effects on body-weight gain, food
consumption, haematological, clinical chemical, or urinary parameters,
or organ weights. At necropsy, treatment-related caecal enlargement
was observed in treated animals (Reynolds et al., 1987a).
Groups of 10 male and 10 female Long-Evans rats were given doses
of 0, 25, 75, or 150 mg/kg bw per day danofloxacin by gavage in an
aqueous vehicle for one month. Serum chemistry and haematology were
evaluated before treatment and on days 11-12 and 30-31. The study
complied with GLP guidelines. There were no signs of toxicity and no
effects on body-weight gain or food consumption. Alanine
aminotrasferase levels were significantly increased in treated females
receiving the high dose, and absolute liver and liver:body weight
ratios were significantly decreased in males at this dose. No
substance-related effects were seen in about 30 tissues from animals
at 0 and 150 mg/kg bw and macroscopic lesions from animals in the
other groups examined microscopically (Fisher et al., 1988a).
In a range-finding study designed to identify suitable doses for
a long-term study of toxicity, groups of 10 male and 10 female Fischer
rats were fed diets calculated to provide 0, 150, 300, 450, or 600
mg/kg bw per day of danofloxacin for 36-39 days. Satellite groups of
three animals of each sex were used to monitor plasma concentrations
of the drug. Five rats given 600 mg/kg bw per day died. Body-weight
gain and food consumption were reduced in all treated groups, and
there was an initial dose-related decrease in food consumption.
Changes were seen in serum chemistry, haematological parameters and
organ weights, due to the effects of starvation and dehydration.
Caecal dilatation was observed in all treated animals (Stadnicki
et al., 1994b).
In a subsequent exploratory study in groups of 10 Long-Evans rats
of each sex, doses intended to provide 0, 75, 150, or 300 mg/kg bw per
day of danofloxacin were administered in the feed for seven days; the
animals then received untreated feed for seven days and the treated
feed for a further 12 days. Body-weight gain and food consumption were
reduced in rats at the intermediate and high doses. Serum globulin
levels were reduced and leukopenia was observed in all treated groups.
Crystalluria was seen in most treated rats but in only two of 20
controls. There was no histopathological evidence of nephropathy
(Stadnicki et al., 1994c).
In a study designed to establish doses to be used in a two-year
feeding study, groups of 15 Long-Evans rats of each sex were fed diets
calculated to provide 0, 75, 150, or 300 mg/kg bw per day of
danofloxacin for about three months. The study complied with GLP
guidelines. Alopecia was seen in four females at the high dose and one
at the intermediate dose. Discoloured urine (especially in animals at
75 mg/kg bw) and crystalluria were observed in all treated groups, but
there were no corresponding pathological changes in the kidneys.
Body-weight gain was significantly reduced in a dose-related manner.
There were significant, dose-related increases in urinary and serum
magnesium concentrations, which were attributed to chelation of
magnesium ions by the test substance. Caecal dilatation was observed
in rats at 150 and 300 mg/kg bw. A range of degenerative changes were
observed in the testes of all males at the high dose and in one at 0,
one at 75, and one at 150 mg/kg bw per day. Degenerating germinal
cells were found in the epididymides of all males at the high dose and
nine at the middle dose (Stadnicki et al., 1994d).
Groups of 20 male and 20 female weanling Long-Evans rats were
selected from the F1 offspring of rats used in a multigeneration
study which had been exposed to danofloxacin both in utero and
during lactation. They were then given oral doses of 25, 75, or 150
mg/kg bw per day for up to three months, whereas 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58).
There were no substance-related deaths and no adverse effects on
food consumption or body-weight gain. There were no consistent
dose-related trends in haematological or clinical chemical values. A
dose-related increase in proteinuria was seen in females but not
males, which was correlated with the finding of tubular nephropathy in
individual animals. Kidney weights were unaffected by treatment. The
mean absolute and relative weights of the testes of rats at 75 and 150
mg/kg bw were about 10% lower than those of controls. Lesions of the
heart were found primarily in animals at these doses and consisted of
either multifocal myocardial degeneration and necrosis, or multifocal
fibrosis, or both. There was no NOEL, as renal tubular nephropathy was
observed in all treated females (Fisher et al., 1989a).
The study was repeated at lower doses in order to establish a
NOEL. Groups of 20 male and 20 female weanling Long-Evans rats were
again selected from the F1 offspring in the multigeneration study,
which had been exposed to danofloxacin both in utero and during
lactation. They were then given oral doses of 1, 2.5, or 6.25 mg/kg bw
per day for up to three months. Twenty controls of each sex were given
deionized water. The study was conducted in accordance with US FDA GLP
guidelines (21 CFR Part 58).
There were no treatment-related deaths or signs of toxicity.
Body-weight gain, food consumption, and haematological and clinical
chemical parameters were unaffected by treatment. Urinalysis revealed
a marginal increase in the incidence of haematuria in males given the
high dose and of proteinuria in a single female at this dose. The
absence of any corresponding pathological changes in the kidney
indicates that the findings reflected biological variability rather
than a substance-related effect. The NOEL was 6.25 mg/kg bw per day
(Fisher et al., 1990a).
Groups of 20 male and 20 female Long-Evans rats were given oral
doses of 1, 2.5, or 6.25 mg/kg bw per day of desmethyldanofloxacin for
up to three months. The rats were selected from the F1 offspring in
a multigeneration study and had been exposed to the test substance
both in utero and during lactation. Groups of 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58). There were no effects on
mortality, body-weight gain, food consumption, haematological or
clinical chemical parameters, organ weights, or gross or
histo-pathological findings. The NOEL was 6.25 mg/kg bw per day
(Stadnicki et al., 1990a).
Rabbits
In an exploratory study, groups of three female New Zealand white
rabbits were given oral doses of 0, 25, 50, or 100 mg/kg bw per day of
danofloxacin in an aqueous vehicle. Owing to significantly reduced
feed intake and decreased body weight, treatment of rabbits at the low
dose was stopped after 12 doses, that of animals at the intermediate
dose after seven doses, and that of rabbits at the high dose after
four doses. The groups were fed untreated diet for a further five to
eight days, then killed and necropsied. Pathological examination
revealed a dose-related incidence of enlarged caeca (Lundeen et al.,
1993).
Dogs
In an exploratory study, groups of one male and one female beagle
dogs were given oral doses of 0, 12.5, 25, or 50 mg/kg bw per day of
danofloxacin in gelatin capsules for two weeks. The female at the high
dose had reduced body-weight gain and food consumption and increased
serum blood urea nitrogen and creatinine levels. At necropsy, this dog
had acute segmental nephrosis and renal failure. The male at the high
dose had chronic focal necrosis without renal failure (Reynolds
et al., 1987b).
In a one-month exploratory study, groups of three male and three
female beagle dogs (10-12 months old) were given oral doses of 0, 5,
10, or 25 mg/kg bw per day of danofloxacin in gelatin capsules. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). There were no effects on behaviour, food consumption,
body-weight gain, blood pressure, electrocardiographic profile, or
ophthalmic, serum chemical, or haematological parameters. At necropsy,
a full range of tissues from all animals was examined, with particular
attention to the weight-bearing joints. Shallow erosions were found in
all dogs, including controls, although the grade of erosions was
slightly higher in those at the high dose. The lesions were typical of
those found in older dogs and did not resemble quinolone-induced
lesions (Fisher et al., 1988b).
Groups of four beagle dogs of each sex aged about six months were
given oral doses of 0, 5, 10, or 25 mg/kg bw of danofloxacin in
gelatin capsules. The substance was administered as two equally
divided doses per day for three months. At termination, all dogs were
subjected to a complete pathological examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
By day 7, reduced activity and signs of joint pain were seen in all
eight dogs given 25 mg/kg bw and in three dogs given 10 mg/kg bw. Most
of the affected animals showed signs of recovery by week 6, despite
continued treatment. Body-weight gain, food consumption, heart rate,
and respiratory rate were decreased in animals that showed clinical
signs. There were no effects on electrocardiographic profiles, blood
pressure, or ophthalmic, haematological, clinical chemical, or urinary
parameters. Gross pathological changes were observed in the articular
cartilage of the major joints of all treated animals, except for one
female receiving the low dose. The lesions were characterized by areas
of cartilage separation and cartilage loss (erosions). The severity of
the lesions was dose-related. Microscopic examination revealed further
changes, including the apparent alteration of collagen fibres when
viewed with polarized light (Fisher et al., 1989b).
A second three-month study, which complied with the same GLP
guidelines, was carried out at lower doses in order to establish a
NOEL. Groups of four male and four female beagle dogs, aged about five
months, were given oral doses of 0, 1, or 2.4 mg/kg bw per day of
danofloxacin in gelatin capsules as two equally divided doses, for 91
days. There were no signs of toxicity and no effects on body-weight
gain, food consumption, clinical chemical or urinary parameters, or
organ weights. There were no substance-related pathological findings.
The NOEL was 2.4 mg/kg bw per day (Fisher et al., 1989c).
Groups of three male and three female beagle dogs, four to six
months old, were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules, administered as two equally
divided doses, for about three months The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58). There were no
notable effects on body-weight gain, electrocardio-graphic profiles,
serum chemical or haematological values, or organ weights. One male
given the high dose showed signs of pain on day 65 when pressure was
applied to the left ankle joint. One female at the low dose showed
signs of pain on day 92 when pressure was applied to the inside of the
left upper hind limb. At termination, histopathological changes
typical of quinolone-induced lesions were found in the articular
cartilage of the right femoral condyle of one male given the high dose
(Stadnicki et al., 1990b).
Groups of three male and three female beagle dogs, five to six
months old, were given oral doses of 0, 0.25 or 0.5 mg/kg bw per day
of desmethyldanofloxacin in gelatin capsules, as two equally divided
doses, for about three months. Further groups of dogs were given 10
mg/kg bw per day of danofloxacin. The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58).
There were no signs of toxicity in the dogs treated with
desmethyldano-floxacin. The dogs given danofloxacin showed signs of
hind limb weakness, reduced activity, and stiff gait. Treatment had no
effect on electrocardiographic profiles, serum chemical or
haematological parameters, or organ weights. Pathological examination
revealed typical quinolone-induced arthropathy in all dogs treated
with danofloxacin. One male in the group given 0.5 mg/kg bw per day
desmethyldano-floxacin was found to have a single articular erosion in
the patellar groove of the right knee. Microscopically, the erosion
extended into zone 2 of the articular cartilage and resembled those
seen with other quinolones. The NOEL was 0.25 mg/kg bw per day
(Stadnicki et al., 1990c).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing the
equivalent of 10, 50, or 100 mg/kg bw per day of danofloxacin for up
to two years. Two groups of 50 controls of each sex were fed untreated
diets. The study complied with GLP guidelines. There were no adverse
dose-related effects on mortality rate, body weight, food consumption,
or haematological parameters. Females receiving 100 mg/kg bw per day
gained significantly more weight than animals in the other groups. At
termination, less than 50% of the animals in each group, except for
the males receiving 10 mg/kg bw per day, were still alive. The mean
absolute kidney weight (but not the relative kidney:body weight) was
significantly increased in females receiving 100 mg/kg bw per day,
which probably reflected the increased body weight of these mice.
There was no evidence of carcinogenicity (Takatsu et al., 1995).
Rats
Groups of 50 male and 50 female Long-Evans rats were fed diets
containing the equivalent of 10, 50, or 100 mg/kg bw per day of
danofloxacin for up to two years. Two groups of 50 controls of each
sex were fed untreated diets. The study complied with GLP guidelines.
There were no clinical signs of toxicity, and survival was
unaffected by treatment. At termination, however, less than 50% of
animals in all groups were alive, except for females receiving 100
mg/kg bw per day and one of the groups of female controls. Occasional
statistically significant reductions in body-weight gain were seen in
males at this dose, and females at 50 and 100 mg/kg bw per day had
significantly increased body-weight gain between three and 16 months
of treatment, which was correlated with increased food intake during
this period. Overall, the body-weight changes associated with
treatment were minimal. Ophthalmoscopic examinations during weeks 12,
18, and 23 revealed no differences between control rats and those
receiving 100 mg/kg bw per day. At termination, the mean leukocyte and
neutrophil counts were significantly reduced in males receiving 100
mg/kg bw per day; in females at this dose, the mean haemoglobin,
haematocrit, and lymphocyte counts were reduced. Males also had
significant increases in aspartate aminotrans-ferase and sorbitol
dehydrogenase values, and the mean globulin value was reduced, with a
corresponding increase in albumin:globulin ratio. The sorbitol
dehydrogenase level was also increased in males receiving 50 mg/kg bw
per day. The effects on clinical chemical values were not seen in
females. There were no substance-related effects on urinary values.
The mean testis:body weight ratio was significantly reduced in the
group receiving 100 mg/kg bw per day. There were no significant
effects on absolute organ weights. Gross pathological examination
revealed an increased incidence of caecal enlargement in treated
animals which was not correlated with any microscopic findings. (Table
3). The histopathological findings included an increased incidence of
renal papillary oedema, increased oligospermia, and abnormal content
of the epididymides in rats at 100 mg/kg bw per day (Table 4).
Table 3. Incidence of caecal enlargement in rats treated with
danofloxacin in the diet for up to two years
Sex Dose (mg/kg No. with caecal enlargement/
bw per day) no. examined
Male 0 0/50
0 0/50
10 5/50
50 3/50
100 6/50
Female 0 1/50
0 0/50
10 1/50
50 1/50
100 2/50
Table 4. Incidence of some non-neoplastic histopathological alterations in groups of
50 rats fed diets containing danofloxacin for up to two years
Sex Dose (mg/kg No. with papillary No. with No. with abnormal
bw per day) oedema oligospermia epidydimal content
Male 0 1 17 25
0 0 16 32
10 0 18 28
50 1 17 29
100 4 31 36
Female 0 0
0 2
10 2
50 2
100 4
There was a significant positive trend in the incidence of
granular-cell tumours of the uterus and vagina in treated female rats
(Table 5); however, the trend was not significant when corrected for
multiple comparisons. Granular-cell foci were distinguished from
tumours by their smaller size and the absence of compressed adjacent
tissue; nevertheless, the morphology of these lesions was essentially
the same. When the incidences of uterine and vaginal foci and tumours
were combined, there was no statistically significant trend in the
combined incidence across groups. There was a significant positive
trend in the incidence of pituitary adenomas in female rats (Table 6),
which was not significant when corrected for multiple comparisons. In
addition, the incidence of pituitary adenomas was within the range
seen in controls in the five previous studies at the same laboratory:
30/48 to 47/49 (Fisher et al., 1996).
2.2.4 Genotoxicity
The results of assays for genotoxicity with danofloxacin and
desmethyl-danofloxacin are summarized in Table 7. All of the studies
complied with GLP guidelines.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, groups of 45
male and 45 female Long-Evans rats were given oral doses of 0, 25, 75,
or 150 mg/kg bw per day of danofloxacin in an aqueous vehicle. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). Dams at 150 mg/kg bw per day showed reduced body-weight gain
during gestation and fewer implantation sites and produced fewer live
pups than those at other doses. The pup weights were significantly
reduced at birth and during lactation. Similar effects were observed
at the first F1 mating, which were more pronounced at the second
F1 mating, when the pregnancy rate was adversely affected in all
treated groups. The weights of all treated F2b pups were reduced in
a dose-related manner (Fisher et al., 1990b).
The F2b pups at 25 mg/kg bw per day were used in a new study
(complying with similar GLP guidelines) when they were two months old,
and treatment was continued. After mating to produce the F3
generation, the pregnancy rate was only 38%, whereas that in the
control group was 65%. Post-implantation losses were significantly
increased and pup body weights and survival were adversely affected
(Fisher et al., 1990c).
Table 5. Incidences of granular-cell proliferative lesions of the uterus
and vagina in female rats fed diets containing danofloxacin for up to
two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
Uterus
No. examined 48 50 50 50 50
Granular-cell tumour 1 3 6 5 6
Granular-cell foci 5 6 1 1 2
Vagina 48 49 49 49 49
Granular-cell tumour 3 2 2 3 7
Granular-cemm foci 0 0 1 0 0
Uterus plus vagina
Granular-cell tumour 4 5 8 8 13
Granular-cell foci5 5 6 2 1 2
Total 9 11 10 9 15
Table 6. Incidence of proliferative lesions of the pituitary gland in
female rats fed diets containing danofloxacin for up to two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
No. examined 49 50 50 50 50
No. with pituitary adenoma 32 32 39 39 40
No. with pituitary hyperplasia 13 11 5 6 5
No. of rats alive at 24 months 22 26 19 18 26
Table 7. Assays for genotoxicity with danofloxacin and desmethyldanofloxacin
End-point Test object Concentration S9 Results Reference
Danofloxacin
In vitro
Gene mutationa S. typhimurium 0.01-0.2 µg/platea - Negative Amacher et al.
TA98, TA100, 0.001-0.1 µg/plate + Negative (1988)
TA1535, TA1537 0.0005-0.2 µg/plate + Negative
Gene mutation L5178Y/tk+/- mouse 51-287 µg/ml - Negative Amacher et al.
lymphoma cellsc 16-215 µg/ml + Negative (1988)
Gene mutation hprt, Chinese 141-1070 µg/ml - Negative Guzzie et al.
hamster ovary 465-2500 µg/ml + Negative (1992)
cellsc
Unscheduled Primary rat 50-400 µg/ml Negative Amacher et al.
DNA synthesis hepatocytesc (1988)
Cytogenetic Human lymphocytes 25-70 µg/ml - Positived Amacher et al.
alterations 200-600 µg/ml + Positive (1990)
In vivo
Micronucleus ICR mouse bone 1000 mg/kg bw Negative Amacher et al.
formation marrow orally (1990)
Desmethyldanofloxacin
In vitro
Gene mutation S. typhimurium 0.001-0.5 µg/plate + Negative Amacher et al.
TA98, TA100, 0.01-5 µg/plate - Negative (1991a)
TA1535, TA1537
Gene mutation L5178Y mouse 90-388 µg/ml - Negative Holden et al.
lymphoma cells 63-269 µg/ml +e Negative (1988)
Unscheduled Primary rat 2.54-102 µg/ml and Positive Hazleton
DNA synthesis hepatocytes 5.02-100 µg/ml Positive Laboratories
America Inc.
(1991a)
Unscheduled Primary rat 62.5-250 µg/ml Positive Amacher et al.
DNA synthesis hepatocytes 62.5-500 µg/mlf Positive (1991b)
Table 7. (continued)
End-point Test object Concentration S9 Results Reference
In vivo
Unscheduled Fischer 344 rat 1 × 250-2000 mg/kg Negative Hazleton
DNA synthesis hepatocytes bw per day orally Laboratories
America Inc.
(1991b)
Micronucleus CD-1 mouse bone 3 × 250-1000 Negative Amacher et al.
formation marrow and peripheral mg/kg bw per day (1991c)
blood orally
S9, 9000 × g fraction of rat liver
a The same bacterial strains were incubated with urine collected from mice given danofloxacin
intraperitoneally at 5, 50, or 100 mg/kg bw per day, with no mutagenic response.
b Higher concentrations toxic to tester strains
c Not replicated independently
d Significant increase in abnormal cells (chromatid breaks). To check whether the clastogenicity
was due to chelation, the assay was repeated without S9 but with the addition of 400 µg/ml
magnesium sulfate; no increase in abnormal cells was observed. In the presence of S9, extra
washes to remove the test substance and the addition of magnesium sulfate diminished the
clastogenic response.
e From livers of uninduced rodents
f 1.62 mmol/litre magnesium ions (as magnesium sulfate heptahydrate) added to culture medium did
not prevent a similar dose-related increase in unscheduled DNA synthesis.
In a three-generation study of reproductive toxicity, groups of
30 male and 30 female Long-Evans rats were given oral doses of 1, 2.5,
6.25, or 150 mg/kg bw per day of danofloxacin. Two control groups
received the deionized water vehicle. Treatment was begun nine weeks
before cohabitation for males and two weeks previously for females
(1:1 mating) and was continued throughout gestation, parturition, and
weaning of the F1 offspring. Of these, groups of 25 of each sex were
selected randomly, and treatment was continued during breeding of the
F2a and F2b generations. The F2b offspring were mated in the
same way to produce the F3 litters. Litters were culled to eight
pups on day 4 post partum. Postnatal development of the F2b pups was
assessed at weaning by measuring locomotor activity, auditory
function, and ophthalmic parameters. All F0, F1, and F2 rats used
for breeding were necropsied and the reproductive organs and main
target organs (kidney, joints, brain, heart, and liver) were weighed
and preserved for possible microscopic examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
Adult animals showed no treatment-related effects on survival,
body-weight gain, food consumption, or clinical signs. In those at 150
mg/kg bw per day, copulatory rate and pregnancy rate were reduced and
the length of gestation was increased. (The copulatory rate was
defined as the percentage of those females which cohabited that had a
vaginal flush with sperm, or an internal copulatory plug, or which
delivered with no previous signs of mating.) Administration of 150
mg/kg bw per day also resulted in reduced litter size and pup weight
at birth, reduced weight gain of the neonates, and a reduction in the
number of pups surviving to day 4 post partum. The group receiving 150
mg/kg bw per day was terminated before the second F1 mating. The
NOEL was 6.25 mg/kg bw per day (Stadnicki et al., 1990d).
In a study of exactly the same design as that described above,
the animals received desmethyldanofloxacin instead of danofloxacin.
There were no treatment-related effects on parental body-weight gain
or food consumption, clinical signs, pregnancy rate, length of
gestation, number of implantation sites, or post-implantation loss.
Pup body-weight gain and survival were unaffected by treatment. There
were no grossly observed abnormalities in the dams or sires (Stadnicki
et al., 1991).
2.2.5.2 Developmental toxicity
Mice
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were given in an aqueous
vehicle to groups of seven mated female ICR mice on days 6-13 of
gestation. The study complied with GLP guidelines. The mean weights of
the male fetuses of animals at 200 mg/kg bw per day were reduced. No
effects were seen on dams (Kessedjian et al., 1988a).
In the main study, groups of 20 female Crl:COBS-CDI(ICR)BR mice
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-13 of gestation and were killed on gestation day 18.
A further group of 10 dams was given 200 mg/kg bw per day and used to
monitor drug concentrations in maternal plasma and amniotic fluid. The
study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were two to three time those in maternal plasma. The incidence of
non-gravid females was high: the total numbers of pregnant females
were only 13, 16, 11, and 13 in the control, low, intermediate, and
high dose groups, respectively. One dam given 200 mg/kg bw per day
showed piloerection and prostration on days 7-10 of gestation and was
found to have a cutaneous abscess at necropsy. The mean body-weight
gain of the dams at the high dose was significantly reduced during
treatment. There were no meaningful effects on the incidences of
resorptions or fetal deaths or on the sex ratio. The mean weights of
both male and female fetuses were significantly reduced at the high
dose, and the incidence of delayed ossification was increased. There
was no evidence of teratogenicity. The NOEL was 100 mg/kg bw per day,
on the basis of maternal and fetal toxicity (Kesseddjian et al.,
1989a).
Rats
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were administered in an
aqueous vehicle to groups of seven mated female Sprague-Dawley rats on
days 6-15 of gestation. The study complied with GLP guidelines.
Maternal body-weight gain was reduced in all treated groups, and the
mean number of viable fetuses was reduced at 200 mg/kg bw per day
(Kessedjian et al., 1988b).
In the main study, groups of 20 female Sprague-Dawley rats
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle; 19-20 dams in
each group were subsequently confirmed to be pregnant. The animals
were treated on days 6-15 of gestation and were killed on gestation
day 20. A further group of five dams was given 200 mg/kg bw per day
and used to monitor drug concentrations in maternal plasma and
amniotic fluid. The study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were about three time those in maternal plasma. There were significant
dose-related reductions in maternal body-weight gain in the rats at
100 and 200 mg/kg bw per day, and mean fetal weights were also
significantly reduced. The incidences of delayed ossification and
dilatation of the cerebral ventricles in the fetuses were
significantly increased at the intermediate and high doses. The NOEL
was 50 mg/kg bw per day, on the basis of maternal and fetal toxicity
(Kesseddjian et al., 1989b).
Rabbits
In a preliminary range-finding study, groups of 11 New Zealand
white rabbits presumed to be pregnant were given oral doses of 0, 5,
10, or 20 mg/kg bw per day of danofloxacin on days 6-20 of gestation.
The study complied with GLP guidelines. Inappetance was seen in all
groups, but maternal body weight was not affected. Animals at the high
dose had a decreased number of live fetuses and higher resorption
rates. The mean fetal weights were reduced at the intermediate and
high doses (Tassinari et al., 1995a).
In the main study, groups of 20 female New Zealand white rabbits
presumed to be pregnant were given oral doses of 0, 2.5, 7.5, or 15
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-20 of gestation and were killed on gestation day 28.
Because of a high incidence of non-gravid females, new animals were
randomized to the different doses so that the total numbers were 32
controls, 29 at the low dose, 33 at the intermediate dose, and 39 at
the high dose. The study complied with GLP guidelines.
Eleven does at the high dose showed loss of body weight and
reduced food consumption and then aborted their litters between days
22 and 28 of gestation. The mean food consumption of the does with
viable litters at 15 mg/kg bw per day was significantly reduced during
days gestation days 13-20. There were no treament-related effects on
litter size, sex ratio, fetal weight, or the incidences of
malformations or variations. The NOEL was 7.5 mg/kg bw per day, on the
basis of maternal toxicity (Tassinari et al., 1995b).
2.2.6 Special study on delayed contact hypersensitization
In a study using the Buehler closed patch technique, which
complied with GLP guidelines, danofloxacin mesylate did not cause
delayed contact hypersensitivity in the guinea-pig. A clear positive
result was obtained with the known sensitizer, dinitrochlorobenzene
(Beutler et al., 1992).
2.2.7 Special studies on pharmacological activity
A number of pharmacological studies of danofloxacin were
available that were not carried out in accordance with GLP guidelines
or with any national or international guidelines. The main features of
these studies are summarized in Table 8.
Table 8. Results of pharmacological assays with danofloxacin
Test system Doses Results Reference
Groups of six male 0, 5, 10, 20 mg/kg bw No significant diuretic Varner et al.
Sprague-Dawley rats per day orally in activity (1990a)
distilled water
Groups of two male 5 mg/kg bw per day Mild transient decreases Gromelski et al.
and two female intravenously in blood pressure, cardiac (1990)
beagle dogs output, left ventricular
pressure, and left ventricular
end diastolic pressure in two
dogs. No effects on electro-
cardiographic wave forms
Groups of three male 1, 10, 100, 1000 mg/kg No effect on central or Varner et al.
Sprague-Dawley rats bw per day orally peripheral nervous system (1990b)
at 100 mg/kg bw per day;
salivation and tremors at
1000 mg/kg bw per day
Groups of eight male 0, 5, 10, 20 mg/kg bw 18, 27, and 23% decreases Varner et al.
CD-1 mice per day orally in in gastrointestinal motility in (1990c)
distilled water; 4 mg/kg comparison with vehicle
morphine sulfate (positive control
control)
Groups of eight 0, 5, 10, 20 mg/kg bw Increased gastric fluid Varner et al.
pyloricligated male per day intraduodenally volume at all doses (1990d)
Sprague-Dawley rats in 0.25% methylcellulosea; (not dose-related);
10 mg/kg bw per day increased gastric acidity
cimetidine (positive at all doses
control)
a The solvent vehicle was reported to be 0.25% methylcellulose in some parts of the report and distilled water in others.
2.2.8 Special studies on microbiological effects
In a study carried out according to the methods described in
documents M11A (1979) and M11A2 (1990) of the National Committee for
Clinical Laboratory Standards, USA, the antibacterial activity of
danofloxacin and desmethyldano-floxacin was determined against 64
isolates of six genera of organisms representative of the human
intestinal anaerobic microflora. In order to assess the
reproducibility of the assay, four reference strains, Bacterides
fragilis ATCC 25285, Bacillus thetaiomicron ATCC 29741,
Clostridium perfringens ATCC 13124, and Eubacterium lentum ATCC
43055, were added to each batch of tests. In addition, data were
provided for the facultative anaerobes Lactobacillus spp (14
strains) and Proteus spp (11 strains) isolated from faeces of
patients in the hospital of Tourcoing, France. Escherichia coli ATCC
25922 and Enterococcus faecalis ATCC 29242 were added as reference
strains. The calculated MIC50 values are shown in Tables 9 and 10.
2.3 Observations in humans
No information was available. Danofloxacin is not authorized for
human use.
Table 9. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains of
bacteria representative of those found in the human gut
Species No. of Inoculum MIC50 (µg/ml)
strains density
(cfu/ml) Danofloxacin Desmethyldanofloxacin
Anaerobes
Bacillus fragilis group 12 107-108 4 128
Fusobacterium spp. 10 4 16
Clostridium spp. 10 0.5 0.5
Eubacterium spp. 10 0.5 1
Bifidobacterium spp. 10 2 8
Peptostreptococcus spp. 12 0.5 2
Facultative anaerobes
Lactobacillus spp. 14 106 16 > 128
Proteus spp. 11 106 0.25 0.06
Table 10. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains used for quality control
Species Anaerobic incubation Aerobic incubation
Danofloxacin Desmethyldanofloxacin Danofloxacin Desmethyldanofloxacin
Escherichia coli 0.06 0.06 0.03 0.015
ATCC 25922
Enterococcus faecalis 1 4 2 1
ATCC 29242a
a Shown as Enterococcus faecalis ATCC 29242 on p. 5 of the report but as Enterococcus faecalis ATCC 29212 in the
accompanying tables (Dubreuil, 1994)
3. COMMENTS
The Committee considered data from studies on the
pharmacokinetics, acute, short-term, and long-term toxicity,
carcinogenicity, reproductive toxicity, genotoxicity, and
antimicrobial activity of danofloxacin. The results of studies on the
acute and short-term toxicity, reproductive toxicity, genotoxicity,
and antimicrobial activity of the metabolite desmethyldanofloxacin
were also reviewed. Most of the studies critical for the evaluation
were carried out in accordance with appropriate standards for study
protocol and conduct.
Danofloxacin was rapidly absorbed after oral administration to
chickens and pigs and after intramuscular administration to pigs and
cattle. Only one study was carried out in which the oral
bioavailability of danofloxacin was calculated; in this study, the
bioavailability after oral administration of 5 mg/kg bw to pigs was
approximately 90%. The substance was well distributed to the tissues.
Urine and faeces contained approximately equal amounts of danofloxacin
and its metabolites.
In cattle, dogs, and rats, unchanged danofloxacin was the main
substance present in the faeces; smaller amounts of
desmethyldanofloxacin were found. Danofloxacin was also the main
component in urine. Desmethyldanofloxacin, danofloxacin- N-oxide, and
the ß-glucuronide were also found in urine. A piperazine-ring
degradation product was present in the bile of cattle but was found in
only trace amounts in the bile of rats and dogs. Residues of both
danofloxacin and desmethyldanofloxacin were found in liver samples
from rats, dogs, and the three target species. The Committee
considered that the metabolism of danofloxacin was very similar in
laboratory animals and in the three target species.
Single oral doses of both danofloxacin and desmethyldanofloxacin
were slightly toxic to rats and mice (acute oral LD50 values in the
range 1500 to > 2000 mg/kg bw). Signs of toxicity typical of
stimulation of the central nervous system were observed prior to
death.
Several exploratory studies were carried out in rats given
danofloxacin in the feed and by gavage and in rabbits given
danofloxacin by gavage. Caecal dilatation was seen at doses of 25
mg/kg bw per day and above in most of these studies. In rats,
increased incidences of crystals in the urine were observed at doses
of 75 mg/kg bw per day and above. There were no corresponding
pathological changes in the kidneys. In a three-month study in which
danofloxacin was administered to rats in the diet, degenerated
germinal cells were found in the epididymides of all males given 300
mg/kg bw per day and in 9 out of 15 males given 150 mg/kg bw per day.
In view of the high doses used, the results of these studies were not
useful in evaluating the safety of danofloxacin.
Rats were exposed to danofloxacin in utero and during lactation
by administration to the dams of doses of 0, 25, 75, or 150 mg/kg bw
per day. The rats were orally dosed at the same levels for an
additional three months after weaning. In females, there was a
dose-related increase in proteinuria which correlated with the finding
of tubular nephropathy in individual animals. In males at 75 and 150
mg/kg bw per day, both the mean absolute and relative testicular
weights were 10% lower than those of controls. In a follow-up
three-month study with lower doses, the highest dose, 6.25 mg/kg bw
per day, was the NOEL, on the basis of renal tubular nephropathy.
Rats were exposed to desmethyldanofloxacin in utero and during
lactation by administration to the dams of oral doses of 0, 1, 2.5, or
6.25 mg/kg bw per day. The rats were treated orally at the same levels
for an additional three months after weaning. No adverse effects were
observed at any dose.
Six-month-old dogs were given oral doses of 0, 5, 10, or 25 mg/kg
bw per day of danofloxacin in gelatin capsules for three months. Those
given the two higher doses showed signs of joint pain. Pathological
examination revealed arthropathy characterized by areas of cartilage
separation and erosion in all treated groups, and the severity of the
lesions was dose-related. A second three-month study was carried out
in immature dogs given oral doses of 0, 1, or 2.4 mg/kg bw per day
danofloxacin in gelatin capsules. There was no evidence of arthropathy
or any other treatment-related effect. On the basis of the results of
the two studies, the Committee concluded that 2.4 mg/kg bw per day was
the overall NOEL for arthropathy in dogs.
A further three-month study was carried out in which immature
dogs were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules. One male of three given the
highest dose and one female of three given the lowest dose showed
signs of pain on examination. In the male, morphological changes were
found in the articular cartilage of one joint. When the study was
repeated at doses of 0, 0.25, or 0.5 mg/kg bw per day
desmethyldanofloxacin, one male of three given 0.5 mg/kg bw per day
was found to have histopathological changes in the right knee typical
of quinolone-induced arthropathy. The NOEL for desmethyldanofloxacin
was 0.25 mg/kg bw per day, on the basis of arthropathy.
In a 102-day range-finding study, mice were fed diets containing
danofloxacin at concentrations equivalent to 0, 150, 300, or 600 mg/kg
bw per day. At the highest dose, the body-weight gain of males was
reduced, and decreased haematological parameters and increased kidney
weights were observed in females. Caecal dilatation was observed at
all doses, and two of 30 mice given the highest dose had inflammation
of the caecum.
In a two-year study of carcinogenicity, mice were fed diets
containing doses equal to 0, 10, 50, or 100 mg/kg bw per day. Females
at the highest dose gained more weight than the controls and showed
increased absolute kidney weights. There were no adverse effects on
haematological parameters, but clinical chemistry was not monitored.
No increase in tumour incidence was observed at any dose.
In a two-year study of carcinogenicity, rats were fed diets
providing doses of 0, 10, 50, or 100 mg/kg bw per day danofloxacin. At
the highest dose, mean haemoglobin, haematocrit, and lymphocyte counts
were decreased in females, and serum aspartate aminotransferase
activity was increased and serum globulin levels were reduced in
males. Serum sorbitol dehydrogenase activity was increased in males
given 50 or 100 mg/kg bw per day. The relative testicular weight was
reduced in rats at the highest dose, and increased oligospermia and
abnormal epididymal content were observed in this group. Caecal
enlargement was noted in all treated groups but was not correlated
with any microscopic findings. An increased incidence of papillary
oedema was seen in the kidneys of rats at 100 mg/kg bw per day. There
was a significant positive trend in the incidence of granular-cell
tumours of the uterus and vagina in treated female rats. Tumours were
distinguished from foci by their larger size and the compression of
adjacent tissue, but the morphology of these lesions was similar, and
the Committee considered that it was appropriate to combine the
incidences of these uterine and vaginal foci and tumours. There was no
significant trend in the combined incidence across groups. There was a
significant positive trend in the incidence of pituitary adenomas in
females, but the number of rats with these lesions was within the
range in historical controls. In addition, none of the trends in
tumour incidence was significant when corrected for multiple
comparisons. The Committee concluded that neither the granular-cell
lesions of the uterus and vagina nor the pituitary adenomas were
indicative of a carcinogenic response to treatment with danofloxacin.
The genotoxic properties of danofloxacin were investigated in
vitro in assays for gene mutation in bacteria and in mammalian
cells, unscheduled DNA synthesis, and cytogenetics alterations in
mammalian cells and for cytogenetic effects in vivo. All of the
tests gave negative results, except for an assay of cytogenetic
alterations in human lymphocytes in vitro. The clastogenicity
observed in vitro was reduced or abolished by addition of magnesium
sulfate to the culture medium and/or washing the cells after treatment
to remove danofloxacin, and appeared to be associated with the cation-
chelating properties of danofloxacin. There was no evidence of
clastogenicity in vivo. Desmethyldanofloxacin induced a significant
increase in unscheduled DNA synthesis in two independent assays in
primary rat hepatocytes in vitro; however, negative results were
obtained with desmethyldanofloxacin in an assay for unscheduled DNA
synthesis and in a test for micronucleus formation in vivo. Thus,
although desmethyldanofloxacin induced unscheduled DNA synthesis, this
genotoxic potential did not appear to be expressed in vivo.
In a two-generation study of reproductive toxicity, rats received
doses of 0, 25, 75, or 150 mg/kg bw per day of danofloxacin by gavage.
In the parental generation, maternal body-weight gain was reduced at
the highest dose and these dams had fewer implantation sites and
produced fewer viable pups. The effects were observed at lower doses
with subsequent matings. At the second mating of the first-generation
animals, the pregnancy rate was adversely affected in all treated
groups. No NOEL could be identified.
A three-generation study of reproductive toxicity was carried out
in which rats were given danofloxacin at doses of 0, 1, 2.5, 6.25, or
150 mg/kg bw per day by gavage. At 150 mg/kg bw per day, the number of
mated females and the pregnancy rate were reduced, the duration of
gestation was increased and the litter sizes and pup weights were
reduced; this group was terminated before the second mating of the
first generation. In this study, in which lower doses were used than
in the study described above, the NOEL for reproductive toxicity was
6.25 mg/kg bw per day.
No adverse effects were observed in a three-generation study of
reproductive toxicity in rats with desmethyldanofloxacin in which
doses of up to 6.25 mg/kg bw per day were administered by gavage.
In a study of developmental toxicity in mice, there was no
evidence of teratogenicity when danofloxacin was administered by
gavage at doses of up to 200 mg/kg bw per day on days 6-13 of
gestation. The dose of 200 mg/kg bw per day was toxic to the dams,
reducing body-weight gain, and was fetotoxic, producing a reduction in
mean fetal weight and an increased incidence of delayed ossification.
The study was compromised by the small numbers of gravid dams in all
groups, including the controls. The NOEL for both maternal and fetal
toxicity was 100 mg/kg bw per day.
Oral doses of 0, 50, 100, or 200 mg/kg bw per day were
administered to rats on days 6-15 of gestation in a study of
developmental toxicity. Maternal body-weight gain and food consumption
were reduced in animals at 100 or 200 mg/kg bw per day. At these
doses, the incidences of delayed ossification and dilatation of the
cerebral ventricles were significantly increased. The NOEL for both
maternal and fetal toxicity was 50 mg/kg bw per day.
In a study of developmental toxicity, oral doses of 0, 2.5, 7.5,
or 15 mg/kg bw per day danofloxacin were administered to rabbits on
days 6-20 of gestation. At a dose of 15 mg/kg bw per day, maternal
body-weight loss, reduced food consumption, and abortion were
observed. The NOEL for maternal toxicity was 7.5 mg/kg bw per day.
The minimum concentration of danofloxacin resulting in 50%
inhibition (MIC50) was determined for 64 isolates of the six
predominant genera of human intestinal anaerobic microflora
( Bacteroides, Fusobacterium, Clostridium, Eubacterium,
Bifidobacterium, and Peptostreptococcus). In addition, data were
provided for the facultative anaerobes Lactobacillus, Proteus, and
Escherichia coli. Although E. coli and Proteus were the most
sensitive organisms, the Committee agreed that they should not be
taken into account in the calculation of the MIC50 because they are
not predominant species in the human intestine. Instead, the Committee
derived the mean MIC50 from the data available on 32 strains of the
most sensitive relevant genera isolated from the human
gastrointestinal tract, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp. The mean MIC50
for these strains was 1 µg/ml. This figure was used in calculating the
upper limit of the ADI from the formula described on p. 12:
Upper limit 1 µg/ga × 220 g
=
of ADI 0.1b × 1c × 60 kg
= 37 µg/kg bw
a Mean MIC50 for the most sensitive relevant species in the
human intestine, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp.
b Bioavailability was determined to be about 90%, based on a
study in pigs given an oral dose of 5 mg/kg bw. The finding that
danofloxacin is strongly bound to cattle faeces was considered by
the Committee to add confidence to use of this value.
c A safety factor of 1 was used because sufficient relevant
microbiological data were provided.
The metabolite desmethyldanofloxacin was one-quarter to one-half
as active as danofloxacin against the same isolates.
4. EVALUATION
The Committee noted that danofloxacin belongs to a group of
fluoroquinolones that is active against aerobic gram-negative bacteria
and that the main components of the flora in the human
gastrointestinal tract are largely unaffected by these compounds.
Therefore, the Committee decided to base the ADI on the toxicity of
danofloxacin and not on its effects on the intestinal flora. Moreover,
the toxicological end-point resulted in a lower ADI.
The Committee established an ADI of 0-20 µg/kg bw per day on the
basis of the NOEL of 2.4 mg/kg bw per day for arthropathy in a
three-month study in immature dogs and a safety factor of 100. The ADI
was rounded to one significant figure, as is the standard practice
(Annex 1, reference 91, section 2.7).
The Committee noted that the NOEL for arthropathy in studies with
desmethyldanofloxacin was 0.25 mg/kg bw per day. The studies of
pharmacokinetics and metabolism showed that dogs that received oral
doses of danofloxacin were also exposed systemically to the major
metabolite desmethyldanofloxacin. Therefore the Committee concluded
that it was not necessary to calculate a separate ADI for
desmethyldanofloxacin. The Committee agreed, however, that the
approximately 10-fold higher toxicity of the metabolite should be
taken into account when recommending MRLs, as consumers may be
directly exposed to desmethyldano-floxacin in liver.
5. REFERENCES
Amacher, D.E., Turner, G.N., Ellis, J.H., Holden, H.E. & Kluwe, W.M.
(1988) Danofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Mammalian cell gene mutation assays. Unscheduled DNA
synthesis assays. Unpublished studies Nos. 86-607-01 and 87-607-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Muehlbauer, P.A., Wahrenburg M.G. &
Kluwe, W.M. (1990) Danofloxacin. Genetic toxicology report. Addendum.
In vitro cytogenetics assays. In vivo cytogenetics assays.
Unpublished study No. 89-607-18 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Ellis, J.H. & Kluwe, W.M. (1991a)
Desmethyldanofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Unpublished study No. 90-576-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Amacher, D.E., Holden, H. E., Muehlbauer, P.A. & Kluwe, W.M. (1991b)
Genetic toxicology report. Unscheduled DNA synthesis assay.
Unpublished study No. 90-576-12 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Zelljadt, I. & Kluwe, W.M. (1991c)
Desmethyldano-floxacin. Genetic toxicology report. In vivo
micronucleus assay. Unpublished study No. 90-576-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Beutler, N.J., Tinkel, J.H. & Knickerbocker, M. (1992) Delayed contact
hyper-sensitivity study in guinea pigs. Unpublished study No.
92-607-27 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Dubreuil, L. (1994) Antibacterial activity of a fluoroquinolone
against bacteria isolated from human gut flora: Danofloxacin and its
metabolite desmethyldano-floxacin (DMD). Unpublished study from
Microbiology Department, Faculty of Pharmacy, University of Lille,
France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Vernon, G.S., Mayne, J.T., Frame, G.M., Milisen, W.B.,
Estes, P.C. & Levinsky, H.V. (1988a) A one month oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-08 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Frame, G.M., Saunders,
W.J., Estes, P.C. & Levinsky, H.V. (1988b) Danofloxacin. One month
oral capsule study in beagle dogs. Unpublished study No. 87-607-07
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Milisen, W.B., Estes,
P.C. & Levinsky, H.V. (1989a) Danofloxacin. Three months oral gavage
study with in utero exposure in Long-Evans rats. Unpublished study
No. 88-607-12 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Soler, G.N., Mayne, J.T., Walsh, A.H., Levinsky, H.V. &
Estes, P.C. (1989b) Danofloxacin. Three month oral capsule study in
beagle dogs. Unpublished study No. 88-607-14 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Carson, D.L., Son, D.L., Walsh, A.H.,
Levinsky, H.V. & Estes, P.C (1989c) Danofloxacin. Three month oral
capsule study in beagle dogs. Unpublished study No. 88-607-15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Fisher, D.O., Hills-Perry, P.A., Rice, J., Coleman, G.L., Estes, P.C.
& Levinsky, H.V. (1990a) Danofloxacin. Three months oral gavage study
with in utero exposure in Long-Evans rats. Unpublished study No.
89-607-19 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Beierschmitt, W.P., Milisen, W.B., Estes, P.C. &
Levinsky, H.V. (1990b) A two-generation oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Powers, K.A., Beierschmitt, W.P. & Levinsky, H.V.
(1990c) An exploratory reproductive oral gavage study in Long-Evans
rats. Unpublished study No. 89-607-17 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Milisen, W.B., Kluwe, W.M. & Ochoa, R. (1996) Two year
oncogenicity study with dietary administration in Long-Evans rats.
Unpublished study No. 93-607-31 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1990) Acute
hemodynamic effects of an intravenous infusion of CP-76,136-27 in the
open-chest anesthetized dog. Unpublished study No. PH 247-PZ-001-90
from Pharmakon Laboratories, Waverly, PA, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, Groton, CT, USA.
Guzzie, P.J., Turner, G.N., Amacher, D.E. & Kluwe, W.M (1992) Genetic
toxicology report. Antimicrobial. Unpublished study No. 92-607-26 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991a) UDS assay in primary rat
hepatocytes. Unpublished study no. 12180-0-447R. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991b) In vivo/in vitro UDS
assay in primary rat hepatocytes. Unpublished study no. 12180-0-494.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Holden, H.E., Turner, G.N. & Ray, V.A. (1988) Genetic toxicology
report. Nalidixic acid analog. Unpublished study No. 86-576-01 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988a) Danofloxacin.
Maternal toxicity study in mice by the oral route. Unpublished study
No. 88028 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988b) Danofloxacin.
Maternal toxicity study in rats by the oral route. Unpublished study
No. 88059 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989a) Danofloxacin.
Fetotoxicity study in mice by the oral route. Segment II. Unpublished
studies No. 88115 and 88116 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989b) Danofloxacin.
Fetotoxicity study in rats by the oral route. Segment II. Unpublished
studies No. 88093 and 88094 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lundeen, G.R., Macaione, G.M., Santacroce, E.M. & Rice, J.R. (1993)
Two week exploratory toleration study in New Zealand white rabbits.
Unpublished study No. 92-607-30 from Pfizer Central Research, Terre
Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc., USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1988) Danofloxacin.
Nonclinical total residue depletion study in broiler chickens.
Unpublished study No. 1515N-60-88-006 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989a) Danofloxacin.
Nonclinical total residue excretion study in cattle. Unpublished study
No. 1535N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989b) Danofloxacin. A
nonclinical total residue excretion study in swine. Unpublished study
No. 1525C-60-88-009 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989c) Danofloxacin. A
nonclinical total residue depletion study in swine. Unpublished study
No. 1525N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1990a) Danofloxacin. Plasma and lung
pharmacokinetics study in broiler chickens. Unpublished Study No.
1514C-60-90-002 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Horan, M.R. & Gummerus, J.A. (1990b) Danofloxacin. Nonclinical plasma
and lung pharmacokinetics study in cattle. Unpublished study No.
1534N-60-89-003 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R. &
Harran, L.P. (1991a) Danofloxacin. Determination of plasma
pharmacokinetics and bioavailability in cattle. Unpublished study No.
1532N-60-89-005 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1991b) Danofloxacin.
Nonclinical total residue excretion study in broiler chickens.
Unpublished study No. 1515N-60-88-007 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991c) A plasma and lung pharmacokinetics
study in swine. Unpublished study No. 1524C-60-89-003 from Pfizer
Central Research, Terre Haute, IN, and Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ericson, J., Calcagni, A., Gummerus, J. & Nowakowski,
M.A. (1991d) The comparative metabolism of 3H-danofloxacin in
cattle, dog and rat species. Unpublished study No. CM-90-02 from
Pfizer Central Research, Terre Haute, IN, and Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Mann, D.D. & Frame, G.M (1992) Pharmacokinetic study of danofloxacin
in cattle and swine. Am. J. Vet. Res., 53, 1022-1026.
Reynolds, J.A., Saunders, W., Hatch, G.W. & Vessella, D.J. (1987a) 2
week oral gavage toleration study in Long-Evans rats. Unpublished
study No. 87-607-03 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Reynolds, J.A., Milisen, W.B. & Frame, G.M. (1987b) Two week oral
capsule toleration study in beagle dogs. Unpublished study No.
87-607-05 from Pfizer Central Research, Terre Haute, IN, and Groton,
CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Risk, J.E., Ronfeld, R.A., Magonigle, R.A., Lynch, M.J., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991) Danofloxacin. Plasma and lung
pharmacokinetics study in swine. Unpublished study No. 1524C-60-89-003
from Pfizer Central Research, Terre Haute, IN, and Groton, CT.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Stack, T.L., Knickerbocker, M. & Beutler, N.J.
(1988a) Danofloxacin. Acute oral and intravenous toxicity studies in
mice and rats. Unpublished study No. 87-607-10 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Nilson, A.L. Knickerbocker, M. & Beutler, N.J.
(1988b) Danofloxacin. Acute dermal and ocular irritation studies in
rabbits (FHSA testing). Unpublished study No. 88-607-11 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Stadnicki S.W., Rinaldi, M.T.S., Knickerbocker, M. & Beutler, N.J.
(1989) Desmethyldanofloxacin. Acute oral and intravenous toxicity
studies in albino mice and Sprague-Dawley rats. Unpublished study No.
89-576-05 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, B.A., Lundeen, G.R., Rice, J., Roesler,
A.R., Estes, P.C. & Levinsky, H.V. (1990a) Desmethyldanofloxacin.
Three month gavage study in Long-Evans rats with in utero exposure.
Unpublished study No. 89-576-06 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Walsh,
A.H., Estes, P.C. & Levinsky, H.V. (1990b) Desmethyldanofloxacin.
Three months oral capsule study in beagle dogs. Unpublished study No.
89-576-03 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Estes, P.C.
& Levinsky, H.V. (1990c) Desmethyldanofloxacin. Three months oral
capsule study in beagle dogs. Unpublished study No. 89-576-07 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Stadnicki, S.W., Hallam, J.A., Ball, D.J., Pettersen, B.A., Levinsky,
H.V., Milisen, W.B. & Estes, P.C. (1990d) Danofloxacin. A
three-generation oral gavage study in Long-Evans rats. Unpublished
study No. 89-607-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Santacroce, E.M., Lundeen, G.R., Coleman, G.L.,
Estes, P.C. & Levinsky, H.V. (1991) Desmethyldanofloxacin. Three
generation gavage study in Long-Evans rats. Unpublished study No.
89-576-04 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Walsh, A.H. & Rice, J.R. (1994a) Three month feeding study in CD-1
mice. Unpublished study No. 92-607-29 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Chatman, L.A. & Rice, J.R. (1994b) One month feeding study in Fischer
rats. Unpublished study No. 91-607-23 from Pfizer Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Cacciatore, A., Davenport, C.J., Coleman, G.L. &
Rice, J.R (1994c) Three week feeding study in Long-Evans rats.
Unpublished study no. 92-607-25 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Boldt, B.K., Rovetti, C.C., Davenport, C.J., Milisen,
W.B. & Rice, J.R. (1994d) Three month feeding study in Long-Evans
rats. Unpublished study No. 92-607-28 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Takatsu, S., Walsh, A.H., Shirai, N., Iidaka, T, Fukazawa, H., Iijima,
M. & Tachibana, M. (1995) Two year oncogenicity study with dietary
administration in ICR mice. Unpublished study No. 93-76-31 from Pfizer
Central Research, Chita-gun, Aichi, Japan. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Tassinari, M.S., Rizoli, A.M., Hills-Perry, P., Linhares, M. & Gupta,
U. (1995a) Maternal toxicity study by oral gavage in New Zealand white
rabbits. Unpublished study No. 93-607-33 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Tassinari, M.S., Laithwaite, H.T., Podurgiel, S.J. & Gupta, U. (1995b)
Danofloxacin. Reproductive study III (teratology) in New Zealand white
rabbits. Unpublished study No. 93-607-34 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990a) Diuretic assay.
Unpublished study No. PH 209-PZ-001-90 from Pharmakon Laboratories,
Waverly, PA, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990b)
Neuropharmacological profile (NPP) in rats. Unpublished study No. PH
200A-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990c)
Gastrointestinal propulsion assay in male mice. Unpublished study No.
PH 239-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990d) Secretory
screen in pyloric ligated Shay rats. Unpublished study No. PH
252-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Chickens
Groups of male 18-day-old broiler chickens were given
drinking-water that had been medicated to provide 5 mg/kg bw per day
danofloxacin, for three days. Steady-state concentrations of 0.43 and
0.21 µg/ml were obtained in lung and plasma, respectively, during the
period of administration. The half-life of danofloxacin was 4.9 h in
plasma and 5.8 h in lung. No residues of desmethyldanofloxacin were
detected in any of the plasma or lung samples (Lynch et al., 1990a).
Groups of broiler chickens were given drinking-water that had
been medicated to provide 5 mg/kg bw per day 3H-danofloxacin for
five days. The test substance was tritiated in the N1-cyclopropyl
ring. The study complied with GLP guidelines. Six hours after
withdrawal of the treated water, unchanged danofloxacin accounted for
75% of the radiolabel in excreta, and desmethyl-danofloxacin accounted
for 5-7% (Lynch et al., 1990a).
Groups of 12 male and 12 female 22-day-old broiler chickens were
given drinking-water which had been medicated to provide 5 mg/kg bw
per day 3H-danofloxacin, for five days. The test substance was
tritiated in the N1-cyclo-propyl ring and had a specific activity of
12.1 µCi/mg (expressed as base). Excreta were collected every 24 h and
pooled by sex and day. The study complied with GLP guidelines. The
mean level of radiolabel in excreta attained a steady state in the
range 35-46 µg/g by the first day of treatment but declined to 20 and
6.6 µg/g on the first and second days after withdrawal, respectively.
About 85% of the radiolabel in faeces was identified as unmetabolized
danofloxacin. There was no difference between the sexes in the pattern
of excretion (Lynch et al., 1988).
Pigs
Castrated male and female pigs (mean body weight, 40 kg) were
given single intramuscular injections of 1.25 mg/kg bw of a prototype
2.5% formulation of danofloxacin. Absorption was rapid, with peak
concentrations of 0.40 µg/g in plasma and 1.68 µg/g in lung about 1 h
after dosing. The half-life of plasma elimination was approximately
7 h (Risk et al., 1991).
Six castrated male and six female pigs (mean body weight, 43.3
kg) were given five daily intramuscular injections of 1.25 mg/kg bw of
danofloxacin tritiated in the N1-cyclopropyl ring in a 2.5% aqueous
vehicle. The study complied with GLP guidelines. The mean radiolabel
concentration during the two days before and the day after the last of
the five doses was 7-8 µg/g in pooled faecal homogenates and 14-18
µg/g in pooled urine samples. During this same period, the total daily
recovery of radiolabel from urine and faeces accounted for 75-81% of
the daily dose. The mean radiolabel concentration in bile was 1.7 µg/g
12 h after the end of treatment and had declined to 0.21 µg/g by 48 h
(Lynch et al., 1989b).
In pigs given a single dose of 5 mg/kg bw of danofloxacin
intravenously, intramuscularly, or orally, detectable residues of
danofloxacin appeared rapidly in plasma. A peak plasma concentration
of 0.42 µg/ml was attained about 3 h after oral dosing. The
bioavailability was estimated to be 76% after intramuscular
administration and 89% after oral administration (Mann & Frame, 1992).
Cattle
A group of 36 male and female calves weighing about 250 kg were
given a single intramuscular injection of 1.25 mg/kg bw of a 2.5%
prototype formulation of danofloxacin, and the kinetics of the
compound were evaluated in plasma and lung tissues. The study complied
with GLP guidelines. Absorption was rapid, with peak plasma and lung
concentrations of 0.35 µg/ml and 1.44 µg/g attained 1 h after dosing.
The half-time for plasma elimination was 3.4 h (Lynch et al.,
1990b).
In a three-way cross-over design study, 12 male and female beef
calves (mean body weight, 112 kg) were given single intravenous doses
or five subcutaneous or intramuscular doses of 1.25 mg/kg bw per day
of a 2.5% prototype formulation of danofloxacin. The study complied
with GLP guidelines. Absorption was rapid: peak plasma concentrations
of 0.37 and 0.47 µg/ml were attained about 1 h after single
subcutaneous and intramuscular doses, respectively, and the
bioavailability was almost 100%. The values for the area under the
concentration-time curve indicated that the levels achieved after
intramuscular and subcutaneous administration were bioequivalent after
one, three, and five injections (Lynch et al., 1991a).
The pattern of excretion of 3H-danofloxacin (tritiated in the
N1-cyclopropyl ring) was studied in five castrated male and four
female calves (body weight, about 180 kg) after five daily
intramuscular injections of 1.25 mg/kg bw (in a 2.5% aqueous vehicle).
The study complied with GLP guidelines. The total concentration of
danofloxacin-related material in excreta reached a plateau by the
third day of treatment. Approximately equal amounts were excreted in
urine and faeces. Unchanged danofloxacin accounted for about 48% of
material excreted in faeces and 89% in urine. The desmethyl metabolite
accounted for 12% of the material in urine samples, but the
concentrations in faeces were too low for measurement (Lynch et al.,
1989a).
2.1.2 Biotransformation
The biotransformation of danofloxacin has been investigated in
rats, dogs, chickens, pigs, and cattle. In all of these species, the
main residue in the faeces was unmetabolized danofloxacin; smaller
amounts of desmethyldanofloxacin were also found (Lynch et al.,
1989a,b,c, 1991a,b,c,d). The main residue in the urine of cattle,
dogs, and rats was also danofloxacin. Desmethyldanofloxacin,
danofloxacin- N-oxide and the ß-glucuronide were identified in rat,
dog, and pig urine (Lynch et al., 1991d).
In the livers of most species, danofloxacin was the major
residue, but significant residues of the metabolite
desmethyldanofloxacin were also present (Table 1). A piperazine-ring
degradation product constituted 43 and 27% of the radiolabel in bile
from male and female cattle, respectively, but it was present in only
trace amounts in bile from rats and dogs. The bile of dogs contained
residues of danofloxacin- N-oxide, which was present in only trace
amounts in bile from the other species (Lynch et al., 1991d).
2.2 Toxicological data
2.2.1 Acute toxicity
The results of studies of the acute toxicity of danofloxacin and
desmethyl-danofloxacin are summarized in Table 2. All of the studies
complied with GLP guidelines.
In the studies by oral administration, signs of toxicity included
exophthalmia, decreased activity, tremors, and twitching, which
occurred within a few minutes of dosing. One of three mice given
2000 mg/kg bw died 29 min after dosing. Retching, clonic convulsions,
and gasping were observed before death. After intravenous dosing, all
three mice given 100 mg/kg bw and all three rats given 150 mg/kg bw
died within 3 min. The signs of toxicity were similar to those
produced after oral administration. Similar signs of toxicity were
observed after oral and intravenous dosing with desmethyldanofloxacin.
Table 1. Composition of residues in liver in five species given danofloxacin
Species Dose regimen Sex Total Danofloxacina Desmethyldanofloxacina Reference
residue
Rat 5 × 6.25 mg/kg bw M 2.3 ppm 67% 18% Lynch et al.
per day orally F 2.5 ppm 70% 11% (1991d)
Dog 5 × 2.4 mg/kg bw M 6.7 ppm 66% 28% Lynch et al.
per day orally F 5.4 ppm 58% 37% (1991d)
Chicken 5 × 5 mg/kg bw M NR 78% 16% Lynch et al.
per day orally F NR 74% 12% (1988)
Pig M 0.95 ppm 58% 23% Lynch et al.
F 0.96 ppm 68% 29% (1989c)
Cowb 5 × 1.25 mg/kg bw M 0.82 ppm 24% 43% Lynch et al.
intramuscularly F 1.1 ppm 29% 42% (1991d)
a Relative to the radiolabel recovered by high-performance liquid chromatography
b Killed 12 h after the last dose
Table 2. Results of studies of the acute toxicity of danofloxacin and desmethyldanofloxacin
Species Route Vehicle Sex LD50 Reference
(strain) (mg/kg bw)
Danofloxacin
Rat (Sprague- Oral Distilled M & F > 2000 Stadnicki et al. (1988a)
Dawley) water
Mouse (ICR) Oral Distilled water M & F > 2000 Stadnicki et al. (1988a)
Rat (Sprague- Intravenous Sterile water M 100-150 Stadnicki et al. (1988a)
Dawley)
Mouse (ICR) Intravenous Sterile water M 50-100 Stadnicki et al. (1988a)
Rabbit (New Dermal M & F > 299a Stadnicki et al. (1988b)
Zealand white)
Desmethyldanofloxacin
Rat (Sprague- Oral Deionized M & F > 2000 Stadnicki et al. (1989)
Dawley) water
Mouse (ICR) Oral Deionized M > 2000 Stadnicki et al. (1989)
water
Mouse (ICR) Oral Deionized F 1500-2000 Stadnicki et al. (1989)
water
Rat (Sprague- Intravenous Sterile water M 40-50 Stadnicki et al. (1989)
Dawley)
Mouse (ICR) Intravenous Sterile water M 7.5-10 Stadnicki et al. (1989)
a 0.5 g danofloxacin was applied to one site on intact skin and one on abraded skin of two male and one
female New Zealand white rabbits under an occlusive dressing for 24 h. There were no deaths or signs
of toxicity, except for mild erythema at all treated sites.
A dose of 26 mg danofloxacin (in a volume of 0.1 ml) was
instilled into the conjunctival sac of the left eyes of three New
Zealand white rabbits. The eyes were not rinsed after dosing. Mild
conjunctivitis and a colourless discharge were observed within 1 h of
treatment. All signs had cleared within 96 h (Stadnicki et al.,
1988b).
2.2.2 Short-term toxicity
Mice
In a study designed to identify suitable doses for a two-year
feeding study, groups of 15 male and 15 female Crl:CD-1 (ICR)BR
VAF/Plus mice were fed diets calculated to provide 0, 150, 300, or 600
mg/kg bw per day of danofloxacin (74% activity) for up to 102 days.
The study complied with GLP guidelines. One female given the high dose
was killed in a moribund condition. Body-weight gain was reduced in
males at the low and high doses in comparison with that of the
controls. Decreased erythrocytic parameters were seen occasionally in
males and females at the high dose. There were no consistent
dose-related changes in clinical chemistry. At termination, the
absolute mean group weight of the kidneys of females receiving the
high dose was significantly increased, but there were no corresponding
pathological changes. Microscopic examination revealed caecal
dilatation in six mice at the low dose, 23 at the intermediate dose,
and 27 at the high dose, but not in the controls. Inflammation of the
caecum was seen in two mice at the high dose, and Gram-positive
bacteria, tentatively identified as Clostridium difficile, were
found in the inflamed caeca of both animals. Thymic lymphocytosis was
seen in one of 15 females at the low dose, two of 15 at the
intermediate dose, and eight of 15 at the high dose and in three of 13
males at the intermediate dose and six of 14 at the high dose but in
no control. A NOEL was not identified (Stadnicki et al., 1994a).
Rats
A two-week exploratory study was carried out in which groups of
three male and three female Long-Evans rats received oral doses of 0,
25, 50, or 100 mg/kg bw per day of danofloxacin in an aqueous vehicle.
There were no significant effects on body-weight gain, food
consumption, haematological, clinical chemical, or urinary parameters,
or organ weights. At necropsy, treatment-related caecal enlargement
was observed in treated animals (Reynolds et al., 1987a).
Groups of 10 male and 10 female Long-Evans rats were given doses
of 0, 25, 75, or 150 mg/kg bw per day danofloxacin by gavage in an
aqueous vehicle for one month. Serum chemistry and haematology were
evaluated before treatment and on days 11-12 and 30-31. The study
complied with GLP guidelines. There were no signs of toxicity and no
effects on body-weight gain or food consumption. Alanine
aminotrasferase levels were significantly increased in treated females
receiving the high dose, and absolute liver and liver:body weight
ratios were significantly decreased in males at this dose. No
substance-related effects were seen in about 30 tissues from animals
at 0 and 150 mg/kg bw and macroscopic lesions from animals in the
other groups examined microscopically (Fisher et al., 1988a).
In a range-finding study designed to identify suitable doses for
a long-term study of toxicity, groups of 10 male and 10 female Fischer
rats were fed diets calculated to provide 0, 150, 300, 450, or 600
mg/kg bw per day of danofloxacin for 36-39 days. Satellite groups of
three animals of each sex were used to monitor plasma concentrations
of the drug. Five rats given 600 mg/kg bw per day died. Body-weight
gain and food consumption were reduced in all treated groups, and
there was an initial dose-related decrease in food consumption.
Changes were seen in serum chemistry, haematological parameters and
organ weights, due to the effects of starvation and dehydration.
Caecal dilatation was observed in all treated animals (Stadnicki
et al., 1994b).
In a subsequent exploratory study in groups of 10 Long-Evans rats
of each sex, doses intended to provide 0, 75, 150, or 300 mg/kg bw per
day of danofloxacin were administered in the feed for seven days; the
animals then received untreated feed for seven days and the treated
feed for a further 12 days. Body-weight gain and food consumption were
reduced in rats at the intermediate and high doses. Serum globulin
levels were reduced and leukopenia was observed in all treated groups.
Crystalluria was seen in most treated rats but in only two of 20
controls. There was no histopathological evidence of nephropathy
(Stadnicki et al., 1994c).
In a study designed to establish doses to be used in a two-year
feeding study, groups of 15 Long-Evans rats of each sex were fed diets
calculated to provide 0, 75, 150, or 300 mg/kg bw per day of
danofloxacin for about three months. The study complied with GLP
guidelines. Alopecia was seen in four females at the high dose and one
at the intermediate dose. Discoloured urine (especially in animals at
75 mg/kg bw) and crystalluria were observed in all treated groups, but
there were no corresponding pathological changes in the kidneys.
Body-weight gain was significantly reduced in a dose-related manner.
There were significant, dose-related increases in urinary and serum
magnesium concentrations, which were attributed to chelation of
magnesium ions by the test substance. Caecal dilatation was observed
in rats at 150 and 300 mg/kg bw. A range of degenerative changes were
observed in the testes of all males at the high dose and in one at 0,
one at 75, and one at 150 mg/kg bw per day. Degenerating germinal
cells were found in the epididymides of all males at the high dose and
nine at the middle dose (Stadnicki et al., 1994d).
Groups of 20 male and 20 female weanling Long-Evans rats were
selected from the F1 offspring of rats used in a multigeneration
study which had been exposed to danofloxacin both in utero and
during lactation. They were then given oral doses of 25, 75, or 150
mg/kg bw per day for up to three months, whereas 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58).
There were no substance-related deaths and no adverse effects on
food consumption or body-weight gain. There were no consistent
dose-related trends in haematological or clinical chemical values. A
dose-related increase in proteinuria was seen in females but not
males, which was correlated with the finding of tubular nephropathy in
individual animals. Kidney weights were unaffected by treatment. The
mean absolute and relative weights of the testes of rats at 75 and 150
mg/kg bw were about 10% lower than those of controls. Lesions of the
heart were found primarily in animals at these doses and consisted of
either multifocal myocardial degeneration and necrosis, or multifocal
fibrosis, or both. There was no NOEL, as renal tubular nephropathy was
observed in all treated females (Fisher et al., 1989a).
The study was repeated at lower doses in order to establish a
NOEL. Groups of 20 male and 20 female weanling Long-Evans rats were
again selected from the F1 offspring in the multigeneration study,
which had been exposed to danofloxacin both in utero and during
lactation. They were then given oral doses of 1, 2.5, or 6.25 mg/kg bw
per day for up to three months. Twenty controls of each sex were given
deionized water. The study was conducted in accordance with US FDA GLP
guidelines (21 CFR Part 58).
There were no treatment-related deaths or signs of toxicity.
Body-weight gain, food consumption, and haematological and clinical
chemical parameters were unaffected by treatment. Urinalysis revealed
a marginal increase in the incidence of haematuria in males given the
high dose and of proteinuria in a single female at this dose. The
absence of any corresponding pathological changes in the kidney
indicates that the findings reflected biological variability rather
than a substance-related effect. The NOEL was 6.25 mg/kg bw per day
(Fisher et al., 1990a).
Groups of 20 male and 20 female Long-Evans rats were given oral
doses of 1, 2.5, or 6.25 mg/kg bw per day of desmethyldanofloxacin for
up to three months. The rats were selected from the F1 offspring in
a multigeneration study and had been exposed to the test substance
both in utero and during lactation. Groups of 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58). There were no effects on
mortality, body-weight gain, food consumption, haematological or
clinical chemical parameters, organ weights, or gross or
histo-pathological findings. The NOEL was 6.25 mg/kg bw per day
(Stadnicki et al., 1990a).
Rabbits
In an exploratory study, groups of three female New Zealand white
rabbits were given oral doses of 0, 25, 50, or 100 mg/kg bw per day of
danofloxacin in an aqueous vehicle. Owing to significantly reduced
feed intake and decreased body weight, treatment of rabbits at the low
dose was stopped after 12 doses, that of animals at the intermediate
dose after seven doses, and that of rabbits at the high dose after
four doses. The groups were fed untreated diet for a further five to
eight days, then killed and necropsied. Pathological examination
revealed a dose-related incidence of enlarged caeca (Lundeen et al.,
1993).
Dogs
In an exploratory study, groups of one male and one female beagle
dogs were given oral doses of 0, 12.5, 25, or 50 mg/kg bw per day of
danofloxacin in gelatin capsules for two weeks. The female at the high
dose had reduced body-weight gain and food consumption and increased
serum blood urea nitrogen and creatinine levels. At necropsy, this dog
had acute segmental nephrosis and renal failure. The male at the high
dose had chronic focal necrosis without renal failure (Reynolds
et al., 1987b).
In a one-month exploratory study, groups of three male and three
female beagle dogs (10-12 months old) were given oral doses of 0, 5,
10, or 25 mg/kg bw per day of danofloxacin in gelatin capsules. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). There were no effects on behaviour, food consumption,
body-weight gain, blood pressure, electrocardiographic profile, or
ophthalmic, serum chemical, or haematological parameters. At necropsy,
a full range of tissues from all animals was examined, with particular
attention to the weight-bearing joints. Shallow erosions were found in
all dogs, including controls, although the grade of erosions was
slightly higher in those at the high dose. The lesions were typical of
those found in older dogs and did not resemble quinolone-induced
lesions (Fisher et al., 1988b).
Groups of four beagle dogs of each sex aged about six months were
given oral doses of 0, 5, 10, or 25 mg/kg bw of danofloxacin in
gelatin capsules. The substance was administered as two equally
divided doses per day for three months. At termination, all dogs were
subjected to a complete pathological examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
By day 7, reduced activity and signs of joint pain were seen in all
eight dogs given 25 mg/kg bw and in three dogs given 10 mg/kg bw. Most
of the affected animals showed signs of recovery by week 6, despite
continued treatment. Body-weight gain, food consumption, heart rate,
and respiratory rate were decreased in animals that showed clinical
signs. There were no effects on electrocardiographic profiles, blood
pressure, or ophthalmic, haematological, clinical chemical, or urinary
parameters. Gross pathological changes were observed in the articular
cartilage of the major joints of all treated animals, except for one
female receiving the low dose. The lesions were characterized by areas
of cartilage separation and cartilage loss (erosions). The severity of
the lesions was dose-related. Microscopic examination revealed further
changes, including the apparent alteration of collagen fibres when
viewed with polarized light (Fisher et al., 1989b).
A second three-month study, which complied with the same GLP
guidelines, was carried out at lower doses in order to establish a
NOEL. Groups of four male and four female beagle dogs, aged about five
months, were given oral doses of 0, 1, or 2.4 mg/kg bw per day of
danofloxacin in gelatin capsules as two equally divided doses, for 91
days. There were no signs of toxicity and no effects on body-weight
gain, food consumption, clinical chemical or urinary parameters, or
organ weights. There were no substance-related pathological findings.
The NOEL was 2.4 mg/kg bw per day (Fisher et al., 1989c).
Groups of three male and three female beagle dogs, four to six
months old, were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules, administered as two equally
divided doses, for about three months The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58). There were no
notable effects on body-weight gain, electrocardio-graphic profiles,
serum chemical or haematological values, or organ weights. One male
given the high dose showed signs of pain on day 65 when pressure was
applied to the left ankle joint. One female at the low dose showed
signs of pain on day 92 when pressure was applied to the inside of the
left upper hind limb. At termination, histopathological changes
typical of quinolone-induced lesions were found in the articular
cartilage of the right femoral condyle of one male given the high dose
(Stadnicki et al., 1990b).
Groups of three male and three female beagle dogs, five to six
months old, were given oral doses of 0, 0.25 or 0.5 mg/kg bw per day
of desmethyldanofloxacin in gelatin capsules, as two equally divided
doses, for about three months. Further groups of dogs were given 10
mg/kg bw per day of danofloxacin. The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58).
There were no signs of toxicity in the dogs treated with
desmethyldano-floxacin. The dogs given danofloxacin showed signs of
hind limb weakness, reduced activity, and stiff gait. Treatment had no
effect on electrocardiographic profiles, serum chemical or
haematological parameters, or organ weights. Pathological examination
revealed typical quinolone-induced arthropathy in all dogs treated
with danofloxacin. One male in the group given 0.5 mg/kg bw per day
desmethyldano-floxacin was found to have a single articular erosion in
the patellar groove of the right knee. Microscopically, the erosion
extended into zone 2 of the articular cartilage and resembled those
seen with other quinolones. The NOEL was 0.25 mg/kg bw per day
(Stadnicki et al., 1990c).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing the
equivalent of 10, 50, or 100 mg/kg bw per day of danofloxacin for up
to two years. Two groups of 50 controls of each sex were fed untreated
diets. The study complied with GLP guidelines. There were no adverse
dose-related effects on mortality rate, body weight, food consumption,
or haematological parameters. Females receiving 100 mg/kg bw per day
gained significantly more weight than animals in the other groups. At
termination, less than 50% of the animals in each group, except for
the males receiving 10 mg/kg bw per day, were still alive. The mean
absolute kidney weight (but not the relative kidney:body weight) was
significantly increased in females receiving 100 mg/kg bw per day,
which probably reflected the increased body weight of these mice.
There was no evidence of carcinogenicity (Takatsu et al., 1995).
Rats
Groups of 50 male and 50 female Long-Evans rats were fed diets
containing the equivalent of 10, 50, or 100 mg/kg bw per day of
danofloxacin for up to two years. Two groups of 50 controls of each
sex were fed untreated diets. The study complied with GLP guidelines.
There were no clinical signs of toxicity, and survival was
unaffected by treatment. At termination, however, less than 50% of
animals in all groups were alive, except for females receiving 100
mg/kg bw per day and one of the groups of female controls. Occasional
statistically significant reductions in body-weight gain were seen in
males at this dose, and females at 50 and 100 mg/kg bw per day had
significantly increased body-weight gain between three and 16 months
of treatment, which was correlated with increased food intake during
this period. Overall, the body-weight changes associated with
treatment were minimal. Ophthalmoscopic examinations during weeks 12,
18, and 23 revealed no differences between control rats and those
receiving 100 mg/kg bw per day. At termination, the mean leukocyte and
neutrophil counts were significantly reduced in males receiving 100
mg/kg bw per day; in females at this dose, the mean haemoglobin,
haematocrit, and lymphocyte counts were reduced. Males also had
significant increases in aspartate aminotrans-ferase and sorbitol
dehydrogenase values, and the mean globulin value was reduced, with a
corresponding increase in albumin:globulin ratio. The sorbitol
dehydrogenase level was also increased in males receiving 50 mg/kg bw
per day. The effects on clinical chemical values were not seen in
females. There were no substance-related effects on urinary values.
The mean testis:body weight ratio was significantly reduced in the
group receiving 100 mg/kg bw per day. There were no significant
effects on absolute organ weights. Gross pathological examination
revealed an increased incidence of caecal enlargement in treated
animals which was not correlated with any microscopic findings. (Table
3). The histopathological findings included an increased incidence of
renal papillary oedema, increased oligospermia, and abnormal content
of the epididymides in rats at 100 mg/kg bw per day (Table 4).
Table 3. Incidence of caecal enlargement in rats treated with
danofloxacin in the diet for up to two years
Sex Dose (mg/kg No. with caecal enlargement/
bw per day) no. examined
Male 0 0/50
0 0/50
10 5/50
50 3/50
100 6/50
Female 0 1/50
0 0/50
10 1/50
50 1/50
100 2/50
Table 4. Incidence of some non-neoplastic histopathological alterations in groups of
50 rats fed diets containing danofloxacin for up to two years
Sex Dose (mg/kg No. with papillary No. with No. with abnormal
bw per day) oedema oligospermia epidydimal content
Male 0 1 17 25
0 0 16 32
10 0 18 28
50 1 17 29
100 4 31 36
Female 0 0
0 2
10 2
50 2
100 4
There was a significant positive trend in the incidence of
granular-cell tumours of the uterus and vagina in treated female rats
(Table 5); however, the trend was not significant when corrected for
multiple comparisons. Granular-cell foci were distinguished from
tumours by their smaller size and the absence of compressed adjacent
tissue; nevertheless, the morphology of these lesions was essentially
the same. When the incidences of uterine and vaginal foci and tumours
were combined, there was no statistically significant trend in the
combined incidence across groups. There was a significant positive
trend in the incidence of pituitary adenomas in female rats (Table 6),
which was not significant when corrected for multiple comparisons. In
addition, the incidence of pituitary adenomas was within the range
seen in controls in the five previous studies at the same laboratory:
30/48 to 47/49 (Fisher et al., 1996).
2.2.4 Genotoxicity
The results of assays for genotoxicity with danofloxacin and
desmethyl-danofloxacin are summarized in Table 7. All of the studies
complied with GLP guidelines.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, groups of 45
male and 45 female Long-Evans rats were given oral doses of 0, 25, 75,
or 150 mg/kg bw per day of danofloxacin in an aqueous vehicle. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). Dams at 150 mg/kg bw per day showed reduced body-weight gain
during gestation and fewer implantation sites and produced fewer live
pups than those at other doses. The pup weights were significantly
reduced at birth and during lactation. Similar effects were observed
at the first F1 mating, which were more pronounced at the second
F1 mating, when the pregnancy rate was adversely affected in all
treated groups. The weights of all treated F2b pups were reduced in
a dose-related manner (Fisher et al., 1990b).
The F2b pups at 25 mg/kg bw per day were used in a new study
(complying with similar GLP guidelines) when they were two months old,
and treatment was continued. After mating to produce the F3
generation, the pregnancy rate was only 38%, whereas that in the
control group was 65%. Post-implantation losses were significantly
increased and pup body weights and survival were adversely affected
(Fisher et al., 1990c).
Table 5. Incidences of granular-cell proliferative lesions of the uterus
and vagina in female rats fed diets containing danofloxacin for up to
two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
Uterus
No. examined 48 50 50 50 50
Granular-cell tumour 1 3 6 5 6
Granular-cell foci 5 6 1 1 2
Vagina 48 49 49 49 49
Granular-cell tumour 3 2 2 3 7
Granular-cemm foci 0 0 1 0 0
Uterus plus vagina
Granular-cell tumour 4 5 8 8 13
Granular-cell foci5 5 6 2 1 2
Total 9 11 10 9 15
Table 6. Incidence of proliferative lesions of the pituitary gland in
female rats fed diets containing danofloxacin for up to two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
No. examined 49 50 50 50 50
No. with pituitary adenoma 32 32 39 39 40
No. with pituitary hyperplasia 13 11 5 6 5
No. of rats alive at 24 months 22 26 19 18 26
Table 7. Assays for genotoxicity with danofloxacin and desmethyldanofloxacin
End-point Test object Concentration S9 Results Reference
Danofloxacin
In vitro
Gene mutationa S. typhimurium 0.01-0.2 µg/platea - Negative Amacher et al.
TA98, TA100, 0.001-0.1 µg/plate + Negative (1988)
TA1535, TA1537 0.0005-0.2 µg/plate + Negative
Gene mutation L5178Y/tk+/- mouse 51-287 µg/ml - Negative Amacher et al.
lymphoma cellsc 16-215 µg/ml + Negative (1988)
Gene mutation hprt, Chinese 141-1070 µg/ml - Negative Guzzie et al.
hamster ovary 465-2500 µg/ml + Negative (1992)
cellsc
Unscheduled Primary rat 50-400 µg/ml Negative Amacher et al.
DNA synthesis hepatocytesc (1988)
Cytogenetic Human lymphocytes 25-70 µg/ml - Positived Amacher et al.
alterations 200-600 µg/ml + Positive (1990)
In vivo
Micronucleus ICR mouse bone 1000 mg/kg bw Negative Amacher et al.
formation marrow orally (1990)
Desmethyldanofloxacin
In vitro
Gene mutation S. typhimurium 0.001-0.5 µg/plate + Negative Amacher et al.
TA98, TA100, 0.01-5 µg/plate - Negative (1991a)
TA1535, TA1537
Gene mutation L5178Y mouse 90-388 µg/ml - Negative Holden et al.
lymphoma cells 63-269 µg/ml +e Negative (1988)
Unscheduled Primary rat 2.54-102 µg/ml and Positive Hazleton
DNA synthesis hepatocytes 5.02-100 µg/ml Positive Laboratories
America Inc.
(1991a)
Unscheduled Primary rat 62.5-250 µg/ml Positive Amacher et al.
DNA synthesis hepatocytes 62.5-500 µg/mlf Positive (1991b)
Table 7. (continued)
End-point Test object Concentration S9 Results Reference
In vivo
Unscheduled Fischer 344 rat 1 × 250-2000 mg/kg Negative Hazleton
DNA synthesis hepatocytes bw per day orally Laboratories
America Inc.
(1991b)
Micronucleus CD-1 mouse bone 3 × 250-1000 Negative Amacher et al.
formation marrow and peripheral mg/kg bw per day (1991c)
blood orally
S9, 9000 × g fraction of rat liver
a The same bacterial strains were incubated with urine collected from mice given danofloxacin
intraperitoneally at 5, 50, or 100 mg/kg bw per day, with no mutagenic response.
b Higher concentrations toxic to tester strains
c Not replicated independently
d Significant increase in abnormal cells (chromatid breaks). To check whether the clastogenicity
was due to chelation, the assay was repeated without S9 but with the addition of 400 µg/ml
magnesium sulfate; no increase in abnormal cells was observed. In the presence of S9, extra
washes to remove the test substance and the addition of magnesium sulfate diminished the
clastogenic response.
e From livers of uninduced rodents
f 1.62 mmol/litre magnesium ions (as magnesium sulfate heptahydrate) added to culture medium did
not prevent a similar dose-related increase in unscheduled DNA synthesis.
In a three-generation study of reproductive toxicity, groups of
30 male and 30 female Long-Evans rats were given oral doses of 1, 2.5,
6.25, or 150 mg/kg bw per day of danofloxacin. Two control groups
received the deionized water vehicle. Treatment was begun nine weeks
before cohabitation for males and two weeks previously for females
(1:1 mating) and was continued throughout gestation, parturition, and
weaning of the F1 offspring. Of these, groups of 25 of each sex were
selected randomly, and treatment was continued during breeding of the
F2a and F2b generations. The F2b offspring were mated in the
same way to produce the F3 litters. Litters were culled to eight
pups on day 4 post partum. Postnatal development of the F2b pups was
assessed at weaning by measuring locomotor activity, auditory
function, and ophthalmic parameters. All F0, F1, and F2 rats used
for breeding were necropsied and the reproductive organs and main
target organs (kidney, joints, brain, heart, and liver) were weighed
and preserved for possible microscopic examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
Adult animals showed no treatment-related effects on survival,
body-weight gain, food consumption, or clinical signs. In those at 150
mg/kg bw per day, copulatory rate and pregnancy rate were reduced and
the length of gestation was increased. (The copulatory rate was
defined as the percentage of those females which cohabited that had a
vaginal flush with sperm, or an internal copulatory plug, or which
delivered with no previous signs of mating.) Administration of 150
mg/kg bw per day also resulted in reduced litter size and pup weight
at birth, reduced weight gain of the neonates, and a reduction in the
number of pups surviving to day 4 post partum. The group receiving 150
mg/kg bw per day was terminated before the second F1 mating. The
NOEL was 6.25 mg/kg bw per day (Stadnicki et al., 1990d).
In a study of exactly the same design as that described above,
the animals received desmethyldanofloxacin instead of danofloxacin.
There were no treatment-related effects on parental body-weight gain
or food consumption, clinical signs, pregnancy rate, length of
gestation, number of implantation sites, or post-implantation loss.
Pup body-weight gain and survival were unaffected by treatment. There
were no grossly observed abnormalities in the dams or sires (Stadnicki
et al., 1991).
2.2.5.2 Developmental toxicity
Mice
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were given in an aqueous
vehicle to groups of seven mated female ICR mice on days 6-13 of
gestation. The study complied with GLP guidelines. The mean weights of
the male fetuses of animals at 200 mg/kg bw per day were reduced. No
effects were seen on dams (Kessedjian et al., 1988a).
In the main study, groups of 20 female Crl:COBS-CDI(ICR)BR mice
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-13 of gestation and were killed on gestation day 18.
A further group of 10 dams was given 200 mg/kg bw per day and used to
monitor drug concentrations in maternal plasma and amniotic fluid. The
study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were two to three time those in maternal plasma. The incidence of
non-gravid females was high: the total numbers of pregnant females
were only 13, 16, 11, and 13 in the control, low, intermediate, and
high dose groups, respectively. One dam given 200 mg/kg bw per day
showed piloerection and prostration on days 7-10 of gestation and was
found to have a cutaneous abscess at necropsy. The mean body-weight
gain of the dams at the high dose was significantly reduced during
treatment. There were no meaningful effects on the incidences of
resorptions or fetal deaths or on the sex ratio. The mean weights of
both male and female fetuses were significantly reduced at the high
dose, and the incidence of delayed ossification was increased. There
was no evidence of teratogenicity. The NOEL was 100 mg/kg bw per day,
on the basis of maternal and fetal toxicity (Kesseddjian et al.,
1989a).
Rats
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were administered in an
aqueous vehicle to groups of seven mated female Sprague-Dawley rats on
days 6-15 of gestation. The study complied with GLP guidelines.
Maternal body-weight gain was reduced in all treated groups, and the
mean number of viable fetuses was reduced at 200 mg/kg bw per day
(Kessedjian et al., 1988b).
In the main study, groups of 20 female Sprague-Dawley rats
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle; 19-20 dams in
each group were subsequently confirmed to be pregnant. The animals
were treated on days 6-15 of gestation and were killed on gestation
day 20. A further group of five dams was given 200 mg/kg bw per day
and used to monitor drug concentrations in maternal plasma and
amniotic fluid. The study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were about three time those in maternal plasma. There were significant
dose-related reductions in maternal body-weight gain in the rats at
100 and 200 mg/kg bw per day, and mean fetal weights were also
significantly reduced. The incidences of delayed ossification and
dilatation of the cerebral ventricles in the fetuses were
significantly increased at the intermediate and high doses. The NOEL
was 50 mg/kg bw per day, on the basis of maternal and fetal toxicity
(Kesseddjian et al., 1989b).
Rabbits
In a preliminary range-finding study, groups of 11 New Zealand
white rabbits presumed to be pregnant were given oral doses of 0, 5,
10, or 20 mg/kg bw per day of danofloxacin on days 6-20 of gestation.
The study complied with GLP guidelines. Inappetance was seen in all
groups, but maternal body weight was not affected. Animals at the high
dose had a decreased number of live fetuses and higher resorption
rates. The mean fetal weights were reduced at the intermediate and
high doses (Tassinari et al., 1995a).
In the main study, groups of 20 female New Zealand white rabbits
presumed to be pregnant were given oral doses of 0, 2.5, 7.5, or 15
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-20 of gestation and were killed on gestation day 28.
Because of a high incidence of non-gravid females, new animals were
randomized to the different doses so that the total numbers were 32
controls, 29 at the low dose, 33 at the intermediate dose, and 39 at
the high dose. The study complied with GLP guidelines.
Eleven does at the high dose showed loss of body weight and
reduced food consumption and then aborted their litters between days
22 and 28 of gestation. The mean food consumption of the does with
viable litters at 15 mg/kg bw per day was significantly reduced during
days gestation days 13-20. There were no treament-related effects on
litter size, sex ratio, fetal weight, or the incidences of
malformations or variations. The NOEL was 7.5 mg/kg bw per day, on the
basis of maternal toxicity (Tassinari et al., 1995b).
2.2.6 Special study on delayed contact hypersensitization
In a study using the Buehler closed patch technique, which
complied with GLP guidelines, danofloxacin mesylate did not cause
delayed contact hypersensitivity in the guinea-pig. A clear positive
result was obtained with the known sensitizer, dinitrochlorobenzene
(Beutler et al., 1992).
2.2.7 Special studies on pharmacological activity
A number of pharmacological studies of danofloxacin were
available that were not carried out in accordance with GLP guidelines
or with any national or international guidelines. The main features of
these studies are summarized in Table 8.
Table 8. Results of pharmacological assays with danofloxacin
Test system Doses Results Reference
Groups of six male 0, 5, 10, 20 mg/kg bw No significant diuretic Varner et al.
Sprague-Dawley rats per day orally in activity (1990a)
distilled water
Groups of two male 5 mg/kg bw per day Mild transient decreases Gromelski et al.
and two female intravenously in blood pressure, cardiac (1990)
beagle dogs output, left ventricular
pressure, and left ventricular
end diastolic pressure in two
dogs. No effects on electro-
cardiographic wave forms
Groups of three male 1, 10, 100, 1000 mg/kg No effect on central or Varner et al.
Sprague-Dawley rats bw per day orally peripheral nervous system (1990b)
at 100 mg/kg bw per day;
salivation and tremors at
1000 mg/kg bw per day
Groups of eight male 0, 5, 10, 20 mg/kg bw 18, 27, and 23% decreases Varner et al.
CD-1 mice per day orally in in gastrointestinal motility in (1990c)
distilled water; 4 mg/kg comparison with vehicle
morphine sulfate (positive control
control)
Groups of eight 0, 5, 10, 20 mg/kg bw Increased gastric fluid Varner et al.
pyloricligated male per day intraduodenally volume at all doses (1990d)
Sprague-Dawley rats in 0.25% methylcellulosea; (not dose-related);
10 mg/kg bw per day increased gastric acidity
cimetidine (positive at all doses
control)
a The solvent vehicle was reported to be 0.25% methylcellulose in some parts of the report and distilled water in others.
2.2.8 Special studies on microbiological effects
In a study carried out according to the methods described in
documents M11A (1979) and M11A2 (1990) of the National Committee for
Clinical Laboratory Standards, USA, the antibacterial activity of
danofloxacin and desmethyldano-floxacin was determined against 64
isolates of six genera of organisms representative of the human
intestinal anaerobic microflora. In order to assess the
reproducibility of the assay, four reference strains, Bacterides
fragilis ATCC 25285, Bacillus thetaiomicron ATCC 29741,
Clostridium perfringens ATCC 13124, and Eubacterium lentum ATCC
43055, were added to each batch of tests. In addition, data were
provided for the facultative anaerobes Lactobacillus spp (14
strains) and Proteus spp (11 strains) isolated from faeces of
patients in the hospital of Tourcoing, France. Escherichia coli ATCC
25922 and Enterococcus faecalis ATCC 29242 were added as reference
strains. The calculated MIC50 values are shown in Tables 9 and 10.
2.3 Observations in humans
No information was available. Danofloxacin is not authorized for
human use.
Table 9. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains of
bacteria representative of those found in the human gut
Species No. of Inoculum MIC50 (µg/ml)
strains density
(cfu/ml) Danofloxacin Desmethyldanofloxacin
Anaerobes
Bacillus fragilis group 12 107-108 4 128
Fusobacterium spp. 10 4 16
Clostridium spp. 10 0.5 0.5
Eubacterium spp. 10 0.5 1
Bifidobacterium spp. 10 2 8
Peptostreptococcus spp. 12 0.5 2
Facultative anaerobes
Lactobacillus spp. 14 106 16 > 128
Proteus spp. 11 106 0.25 0.06
Table 10. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains used for quality control
Species Anaerobic incubation Aerobic incubation
Danofloxacin Desmethyldanofloxacin Danofloxacin Desmethyldanofloxacin
Escherichia coli 0.06 0.06 0.03 0.015
ATCC 25922
Enterococcus faecalis 1 4 2 1
ATCC 29242a
a Shown as Enterococcus faecalis ATCC 29242 on p. 5 of the report but as Enterococcus faecalis ATCC 29212 in the
accompanying tables (Dubreuil, 1994)
3. COMMENTS
The Committee considered data from studies on the
pharmacokinetics, acute, short-term, and long-term toxicity,
carcinogenicity, reproductive toxicity, genotoxicity, and
antimicrobial activity of danofloxacin. The results of studies on the
acute and short-term toxicity, reproductive toxicity, genotoxicity,
and antimicrobial activity of the metabolite desmethyldanofloxacin
were also reviewed. Most of the studies critical for the evaluation
were carried out in accordance with appropriate standards for study
protocol and conduct.
Danofloxacin was rapidly absorbed after oral administration to
chickens and pigs and after intramuscular administration to pigs and
cattle. Only one study was carried out in which the oral
bioavailability of danofloxacin was calculated; in this study, the
bioavailability after oral administration of 5 mg/kg bw to pigs was
approximately 90%. The substance was well distributed to the tissues.
Urine and faeces contained approximately equal amounts of danofloxacin
and its metabolites.
In cattle, dogs, and rats, unchanged danofloxacin was the main
substance present in the faeces; smaller amounts of
desmethyldanofloxacin were found. Danofloxacin was also the main
component in urine. Desmethyldanofloxacin, danofloxacin- N-oxide, and
the ß-glucuronide were also found in urine. A piperazine-ring
degradation product was present in the bile of cattle but was found in
only trace amounts in the bile of rats and dogs. Residues of both
danofloxacin and desmethyldanofloxacin were found in liver samples
from rats, dogs, and the three target species. The Committee
considered that the metabolism of danofloxacin was very similar in
laboratory animals and in the three target species.
Single oral doses of both danofloxacin and desmethyldanofloxacin
were slightly toxic to rats and mice (acute oral LD50 values in the
range 1500 to > 2000 mg/kg bw). Signs of toxicity typical of
stimulation of the central nervous system were observed prior to
death.
Several exploratory studies were carried out in rats given
danofloxacin in the feed and by gavage and in rabbits given
danofloxacin by gavage. Caecal dilatation was seen at doses of 25
mg/kg bw per day and above in most of these studies. In rats,
increased incidences of crystals in the urine were observed at doses
of 75 mg/kg bw per day and above. There were no corresponding
pathological changes in the kidneys. In a three-month study in which
danofloxacin was administered to rats in the diet, degenerated
germinal cells were found in the epididymides of all males given 300
mg/kg bw per day and in 9 out of 15 males given 150 mg/kg bw per day.
In view of the high doses used, the results of these studies were not
useful in evaluating the safety of danofloxacin.
Rats were exposed to danofloxacin in utero and during lactation
by administration to the dams of doses of 0, 25, 75, or 150 mg/kg bw
per day. The rats were orally dosed at the same levels for an
additional three months after weaning. In females, there was a
dose-related increase in proteinuria which correlated with the finding
of tubular nephropathy in individual animals. In males at 75 and 150
mg/kg bw per day, both the mean absolute and relative testicular
weights were 10% lower than those of controls. In a follow-up
three-month study with lower doses, the highest dose, 6.25 mg/kg bw
per day, was the NOEL, on the basis of renal tubular nephropathy.
Rats were exposed to desmethyldanofloxacin in utero and during
lactation by administration to the dams of oral doses of 0, 1, 2.5, or
6.25 mg/kg bw per day. The rats were treated orally at the same levels
for an additional three months after weaning. No adverse effects were
observed at any dose.
Six-month-old dogs were given oral doses of 0, 5, 10, or 25 mg/kg
bw per day of danofloxacin in gelatin capsules for three months. Those
given the two higher doses showed signs of joint pain. Pathological
examination revealed arthropathy characterized by areas of cartilage
separation and erosion in all treated groups, and the severity of the
lesions was dose-related. A second three-month study was carried out
in immature dogs given oral doses of 0, 1, or 2.4 mg/kg bw per day
danofloxacin in gelatin capsules. There was no evidence of arthropathy
or any other treatment-related effect. On the basis of the results of
the two studies, the Committee concluded that 2.4 mg/kg bw per day was
the overall NOEL for arthropathy in dogs.
A further three-month study was carried out in which immature
dogs were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules. One male of three given the
highest dose and one female of three given the lowest dose showed
signs of pain on examination. In the male, morphological changes were
found in the articular cartilage of one joint. When the study was
repeated at doses of 0, 0.25, or 0.5 mg/kg bw per day
desmethyldanofloxacin, one male of three given 0.5 mg/kg bw per day
was found to have histopathological changes in the right knee typical
of quinolone-induced arthropathy. The NOEL for desmethyldanofloxacin
was 0.25 mg/kg bw per day, on the basis of arthropathy.
In a 102-day range-finding study, mice were fed diets containing
danofloxacin at concentrations equivalent to 0, 150, 300, or 600 mg/kg
bw per day. At the highest dose, the body-weight gain of males was
reduced, and decreased haematological parameters and increased kidney
weights were observed in females. Caecal dilatation was observed at
all doses, and two of 30 mice given the highest dose had inflammation
of the caecum.
In a two-year study of carcinogenicity, mice were fed diets
containing doses equal to 0, 10, 50, or 100 mg/kg bw per day. Females
at the highest dose gained more weight than the controls and showed
increased absolute kidney weights. There were no adverse effects on
haematological parameters, but clinical chemistry was not monitored.
No increase in tumour incidence was observed at any dose.
In a two-year study of carcinogenicity, rats were fed diets
providing doses of 0, 10, 50, or 100 mg/kg bw per day danofloxacin. At
the highest dose, mean haemoglobin, haematocrit, and lymphocyte counts
were decreased in females, and serum aspartate aminotransferase
activity was increased and serum globulin levels were reduced in
males. Serum sorbitol dehydrogenase activity was increased in males
given 50 or 100 mg/kg bw per day. The relative testicular weight was
reduced in rats at the highest dose, and increased oligospermia and
abnormal epididymal content were observed in this group. Caecal
enlargement was noted in all treated groups but was not correlated
with any microscopic findings. An increased incidence of papillary
oedema was seen in the kidneys of rats at 100 mg/kg bw per day. There
was a significant positive trend in the incidence of granular-cell
tumours of the uterus and vagina in treated female rats. Tumours were
distinguished from foci by their larger size and the compression of
adjacent tissue, but the morphology of these lesions was similar, and
the Committee considered that it was appropriate to combine the
incidences of these uterine and vaginal foci and tumours. There was no
significant trend in the combined incidence across groups. There was a
significant positive trend in the incidence of pituitary adenomas in
females, but the number of rats with these lesions was within the
range in historical controls. In addition, none of the trends in
tumour incidence was significant when corrected for multiple
comparisons. The Committee concluded that neither the granular-cell
lesions of the uterus and vagina nor the pituitary adenomas were
indicative of a carcinogenic response to treatment with danofloxacin.
The genotoxic properties of danofloxacin were investigated in
vitro in assays for gene mutation in bacteria and in mammalian
cells, unscheduled DNA synthesis, and cytogenetics alterations in
mammalian cells and for cytogenetic effects in vivo. All of the
tests gave negative results, except for an assay of cytogenetic
alterations in human lymphocytes in vitro. The clastogenicity
observed in vitro was reduced or abolished by addition of magnesium
sulfate to the culture medium and/or washing the cells after treatment
to remove danofloxacin, and appeared to be associated with the cation-
chelating properties of danofloxacin. There was no evidence of
clastogenicity in vivo. Desmethyldanofloxacin induced a significant
increase in unscheduled DNA synthesis in two independent assays in
primary rat hepatocytes in vitro; however, negative results were
obtained with desmethyldanofloxacin in an assay for unscheduled DNA
synthesis and in a test for micronucleus formation in vivo. Thus,
although desmethyldanofloxacin induced unscheduled DNA synthesis, this
genotoxic potential did not appear to be expressed in vivo.
In a two-generation study of reproductive toxicity, rats received
doses of 0, 25, 75, or 150 mg/kg bw per day of danofloxacin by gavage.
In the parental generation, maternal body-weight gain was reduced at
the highest dose and these dams had fewer implantation sites and
produced fewer viable pups. The effects were observed at lower doses
with subsequent matings. At the second mating of the first-generation
animals, the pregnancy rate was adversely affected in all treated
groups. No NOEL could be identified.
A three-generation study of reproductive toxicity was carried out
in which rats were given danofloxacin at doses of 0, 1, 2.5, 6.25, or
150 mg/kg bw per day by gavage. At 150 mg/kg bw per day, the number of
mated females and the pregnancy rate were reduced, the duration of
gestation was increased and the litter sizes and pup weights were
reduced; this group was terminated before the second mating of the
first generation. In this study, in which lower doses were used than
in the study described above, the NOEL for reproductive toxicity was
6.25 mg/kg bw per day.
No adverse effects were observed in a three-generation study of
reproductive toxicity in rats with desmethyldanofloxacin in which
doses of up to 6.25 mg/kg bw per day were administered by gavage.
In a study of developmental toxicity in mice, there was no
evidence of teratogenicity when danofloxacin was administered by
gavage at doses of up to 200 mg/kg bw per day on days 6-13 of
gestation. The dose of 200 mg/kg bw per day was toxic to the dams,
reducing body-weight gain, and was fetotoxic, producing a reduction in
mean fetal weight and an increased incidence of delayed ossification.
The study was compromised by the small numbers of gravid dams in all
groups, including the controls. The NOEL for both maternal and fetal
toxicity was 100 mg/kg bw per day.
Oral doses of 0, 50, 100, or 200 mg/kg bw per day were
administered to rats on days 6-15 of gestation in a study of
developmental toxicity. Maternal body-weight gain and food consumption
were reduced in animals at 100 or 200 mg/kg bw per day. At these
doses, the incidences of delayed ossification and dilatation of the
cerebral ventricles were significantly increased. The NOEL for both
maternal and fetal toxicity was 50 mg/kg bw per day.
In a study of developmental toxicity, oral doses of 0, 2.5, 7.5,
or 15 mg/kg bw per day danofloxacin were administered to rabbits on
days 6-20 of gestation. At a dose of 15 mg/kg bw per day, maternal
body-weight loss, reduced food consumption, and abortion were
observed. The NOEL for maternal toxicity was 7.5 mg/kg bw per day.
The minimum concentration of danofloxacin resulting in 50%
inhibition (MIC50) was determined for 64 isolates of the six
predominant genera of human intestinal anaerobic microflora
( Bacteroides, Fusobacterium, Clostridium, Eubacterium,
Bifidobacterium, and Peptostreptococcus). In addition, data were
provided for the facultative anaerobes Lactobacillus, Proteus, and
Escherichia coli. Although E. coli and Proteus were the most
sensitive organisms, the Committee agreed that they should not be
taken into account in the calculation of the MIC50 because they are
not predominant species in the human intestine. Instead, the Committee
derived the mean MIC50 from the data available on 32 strains of the
most sensitive relevant genera isolated from the human
gastrointestinal tract, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp. The mean MIC50
for these strains was 1 µg/ml. This figure was used in calculating the
upper limit of the ADI from the formula described on p. 12:
Upper limit 1 µg/ga × 220 g
=
of ADI 0.1b × 1c × 60 kg
= 37 µg/kg bw
a Mean MIC50 for the most sensitive relevant species in the
human intestine, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp.
b Bioavailability was determined to be about 90%, based on a
study in pigs given an oral dose of 5 mg/kg bw. The finding that
danofloxacin is strongly bound to cattle faeces was considered by
the Committee to add confidence to use of this value.
c A safety factor of 1 was used because sufficient relevant
microbiological data were provided.
The metabolite desmethyldanofloxacin was one-quarter to one-half
as active as danofloxacin against the same isolates.
4. EVALUATION
The Committee noted that danofloxacin belongs to a group of
fluoroquinolones that is active against aerobic gram-negative bacteria
and that the main components of the flora in the human
gastrointestinal tract are largely unaffected by these compounds.
Therefore, the Committee decided to base the ADI on the toxicity of
danofloxacin and not on its effects on the intestinal flora. Moreover,
the toxicological end-point resulted in a lower ADI.
The Committee established an ADI of 0-20 µg/kg bw per day on the
basis of the NOEL of 2.4 mg/kg bw per day for arthropathy in a
three-month study in immature dogs and a safety factor of 100. The ADI
was rounded to one significant figure, as is the standard practice
(Annex 1, reference 91, section 2.7).
The Committee noted that the NOEL for arthropathy in studies with
desmethyldanofloxacin was 0.25 mg/kg bw per day. The studies of
pharmacokinetics and metabolism showed that dogs that received oral
doses of danofloxacin were also exposed systemically to the major
metabolite desmethyldanofloxacin. Therefore the Committee concluded
that it was not necessary to calculate a separate ADI for
desmethyldanofloxacin. The Committee agreed, however, that the
approximately 10-fold higher toxicity of the metabolite should be
taken into account when recommending MRLs, as consumers may be
directly exposed to desmethyldano-floxacin in liver.
5. REFERENCES
Amacher, D.E., Turner, G.N., Ellis, J.H., Holden, H.E. & Kluwe, W.M.
(1988) Danofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Mammalian cell gene mutation assays. Unscheduled DNA
synthesis assays. Unpublished studies Nos. 86-607-01 and 87-607-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Muehlbauer, P.A., Wahrenburg M.G. &
Kluwe, W.M. (1990) Danofloxacin. Genetic toxicology report. Addendum.
In vitro cytogenetics assays. In vivo cytogenetics assays.
Unpublished study No. 89-607-18 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Ellis, J.H. & Kluwe, W.M. (1991a)
Desmethyldanofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Unpublished study No. 90-576-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Amacher, D.E., Holden, H. E., Muehlbauer, P.A. & Kluwe, W.M. (1991b)
Genetic toxicology report. Unscheduled DNA synthesis assay.
Unpublished study No. 90-576-12 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Zelljadt, I. & Kluwe, W.M. (1991c)
Desmethyldano-floxacin. Genetic toxicology report. In vivo
micronucleus assay. Unpublished study No. 90-576-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Beutler, N.J., Tinkel, J.H. & Knickerbocker, M. (1992) Delayed contact
hyper-sensitivity study in guinea pigs. Unpublished study No.
92-607-27 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Dubreuil, L. (1994) Antibacterial activity of a fluoroquinolone
against bacteria isolated from human gut flora: Danofloxacin and its
metabolite desmethyldano-floxacin (DMD). Unpublished study from
Microbiology Department, Faculty of Pharmacy, University of Lille,
France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Vernon, G.S., Mayne, J.T., Frame, G.M., Milisen, W.B.,
Estes, P.C. & Levinsky, H.V. (1988a) A one month oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-08 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Frame, G.M., Saunders,
W.J., Estes, P.C. & Levinsky, H.V. (1988b) Danofloxacin. One month
oral capsule study in beagle dogs. Unpublished study No. 87-607-07
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Milisen, W.B., Estes,
P.C. & Levinsky, H.V. (1989a) Danofloxacin. Three months oral gavage
study with in utero exposure in Long-Evans rats. Unpublished study
No. 88-607-12 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Soler, G.N., Mayne, J.T., Walsh, A.H., Levinsky, H.V. &
Estes, P.C. (1989b) Danofloxacin. Three month oral capsule study in
beagle dogs. Unpublished study No. 88-607-14 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Carson, D.L., Son, D.L., Walsh, A.H.,
Levinsky, H.V. & Estes, P.C (1989c) Danofloxacin. Three month oral
capsule study in beagle dogs. Unpublished study No. 88-607-15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Fisher, D.O., Hills-Perry, P.A., Rice, J., Coleman, G.L., Estes, P.C.
& Levinsky, H.V. (1990a) Danofloxacin. Three months oral gavage study
with in utero exposure in Long-Evans rats. Unpublished study No.
89-607-19 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Beierschmitt, W.P., Milisen, W.B., Estes, P.C. &
Levinsky, H.V. (1990b) A two-generation oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Powers, K.A., Beierschmitt, W.P. & Levinsky, H.V.
(1990c) An exploratory reproductive oral gavage study in Long-Evans
rats. Unpublished study No. 89-607-17 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Milisen, W.B., Kluwe, W.M. & Ochoa, R. (1996) Two year
oncogenicity study with dietary administration in Long-Evans rats.
Unpublished study No. 93-607-31 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1990) Acute
hemodynamic effects of an intravenous infusion of CP-76,136-27 in the
open-chest anesthetized dog. Unpublished study No. PH 247-PZ-001-90
from Pharmakon Laboratories, Waverly, PA, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, Groton, CT, USA.
Guzzie, P.J., Turner, G.N., Amacher, D.E. & Kluwe, W.M (1992) Genetic
toxicology report. Antimicrobial. Unpublished study No. 92-607-26 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991a) UDS assay in primary rat
hepatocytes. Unpublished study no. 12180-0-447R. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991b) In vivo/in vitro UDS
assay in primary rat hepatocytes. Unpublished study no. 12180-0-494.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Holden, H.E., Turner, G.N. & Ray, V.A. (1988) Genetic toxicology
report. Nalidixic acid analog. Unpublished study No. 86-576-01 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988a) Danofloxacin.
Maternal toxicity study in mice by the oral route. Unpublished study
No. 88028 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988b) Danofloxacin.
Maternal toxicity study in rats by the oral route. Unpublished study
No. 88059 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989a) Danofloxacin.
Fetotoxicity study in mice by the oral route. Segment II. Unpublished
studies No. 88115 and 88116 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989b) Danofloxacin.
Fetotoxicity study in rats by the oral route. Segment II. Unpublished
studies No. 88093 and 88094 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lundeen, G.R., Macaione, G.M., Santacroce, E.M. & Rice, J.R. (1993)
Two week exploratory toleration study in New Zealand white rabbits.
Unpublished study No. 92-607-30 from Pfizer Central Research, Terre
Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc., USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1988) Danofloxacin.
Nonclinical total residue depletion study in broiler chickens.
Unpublished study No. 1515N-60-88-006 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989a) Danofloxacin.
Nonclinical total residue excretion study in cattle. Unpublished study
No. 1535N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989b) Danofloxacin. A
nonclinical total residue excretion study in swine. Unpublished study
No. 1525C-60-88-009 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989c) Danofloxacin. A
nonclinical total residue depletion study in swine. Unpublished study
No. 1525N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1990a) Danofloxacin. Plasma and lung
pharmacokinetics study in broiler chickens. Unpublished Study No.
1514C-60-90-002 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Horan, M.R. & Gummerus, J.A. (1990b) Danofloxacin. Nonclinical plasma
and lung pharmacokinetics study in cattle. Unpublished study No.
1534N-60-89-003 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R. &
Harran, L.P. (1991a) Danofloxacin. Determination of plasma
pharmacokinetics and bioavailability in cattle. Unpublished study No.
1532N-60-89-005 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1991b) Danofloxacin.
Nonclinical total residue excretion study in broiler chickens.
Unpublished study No. 1515N-60-88-007 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991c) A plasma and lung pharmacokinetics
study in swine. Unpublished study No. 1524C-60-89-003 from Pfizer
Central Research, Terre Haute, IN, and Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ericson, J., Calcagni, A., Gummerus, J. & Nowakowski,
M.A. (1991d) The comparative metabolism of 3H-danofloxacin in
cattle, dog and rat species. Unpublished study No. CM-90-02 from
Pfizer Central Research, Terre Haute, IN, and Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Mann, D.D. & Frame, G.M (1992) Pharmacokinetic study of danofloxacin
in cattle and swine. Am. J. Vet. Res., 53, 1022-1026.
Reynolds, J.A., Saunders, W., Hatch, G.W. & Vessella, D.J. (1987a) 2
week oral gavage toleration study in Long-Evans rats. Unpublished
study No. 87-607-03 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Reynolds, J.A., Milisen, W.B. & Frame, G.M. (1987b) Two week oral
capsule toleration study in beagle dogs. Unpublished study No.
87-607-05 from Pfizer Central Research, Terre Haute, IN, and Groton,
CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Risk, J.E., Ronfeld, R.A., Magonigle, R.A., Lynch, M.J., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991) Danofloxacin. Plasma and lung
pharmacokinetics study in swine. Unpublished study No. 1524C-60-89-003
from Pfizer Central Research, Terre Haute, IN, and Groton, CT.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Stack, T.L., Knickerbocker, M. & Beutler, N.J.
(1988a) Danofloxacin. Acute oral and intravenous toxicity studies in
mice and rats. Unpublished study No. 87-607-10 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Nilson, A.L. Knickerbocker, M. & Beutler, N.J.
(1988b) Danofloxacin. Acute dermal and ocular irritation studies in
rabbits (FHSA testing). Unpublished study No. 88-607-11 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Stadnicki S.W., Rinaldi, M.T.S., Knickerbocker, M. & Beutler, N.J.
(1989) Desmethyldanofloxacin. Acute oral and intravenous toxicity
studies in albino mice and Sprague-Dawley rats. Unpublished study No.
89-576-05 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, B.A., Lundeen, G.R., Rice, J., Roesler,
A.R., Estes, P.C. & Levinsky, H.V. (1990a) Desmethyldanofloxacin.
Three month gavage study in Long-Evans rats with in utero exposure.
Unpublished study No. 89-576-06 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Walsh,
A.H., Estes, P.C. & Levinsky, H.V. (1990b) Desmethyldanofloxacin.
Three months oral capsule study in beagle dogs. Unpublished study No.
89-576-03 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Estes, P.C.
& Levinsky, H.V. (1990c) Desmethyldanofloxacin. Three months oral
capsule study in beagle dogs. Unpublished study No. 89-576-07 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Stadnicki, S.W., Hallam, J.A., Ball, D.J., Pettersen, B.A., Levinsky,
H.V., Milisen, W.B. & Estes, P.C. (1990d) Danofloxacin. A
three-generation oral gavage study in Long-Evans rats. Unpublished
study No. 89-607-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Santacroce, E.M., Lundeen, G.R., Coleman, G.L.,
Estes, P.C. & Levinsky, H.V. (1991) Desmethyldanofloxacin. Three
generation gavage study in Long-Evans rats. Unpublished study No.
89-576-04 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Walsh, A.H. & Rice, J.R. (1994a) Three month feeding study in CD-1
mice. Unpublished study No. 92-607-29 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Chatman, L.A. & Rice, J.R. (1994b) One month feeding study in Fischer
rats. Unpublished study No. 91-607-23 from Pfizer Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Cacciatore, A., Davenport, C.J., Coleman, G.L. &
Rice, J.R (1994c) Three week feeding study in Long-Evans rats.
Unpublished study no. 92-607-25 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Boldt, B.K., Rovetti, C.C., Davenport, C.J., Milisen,
W.B. & Rice, J.R. (1994d) Three month feeding study in Long-Evans
rats. Unpublished study No. 92-607-28 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Takatsu, S., Walsh, A.H., Shirai, N., Iidaka, T, Fukazawa, H., Iijima,
M. & Tachibana, M. (1995) Two year oncogenicity study with dietary
administration in ICR mice. Unpublished study No. 93-76-31 from Pfizer
Central Research, Chita-gun, Aichi, Japan. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Tassinari, M.S., Rizoli, A.M., Hills-Perry, P., Linhares, M. & Gupta,
U. (1995a) Maternal toxicity study by oral gavage in New Zealand white
rabbits. Unpublished study No. 93-607-33 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Tassinari, M.S., Laithwaite, H.T., Podurgiel, S.J. & Gupta, U. (1995b)
Danofloxacin. Reproductive study III (teratology) in New Zealand white
rabbits. Unpublished study No. 93-607-34 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990a) Diuretic assay.
Unpublished study No. PH 209-PZ-001-90 from Pharmakon Laboratories,
Waverly, PA, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990b)
Neuropharmacological profile (NPP) in rats. Unpublished study No. PH
200A-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990c)
Gastrointestinal propulsion assay in male mice. Unpublished study No.
PH 239-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990d) Secretory
screen in pyloric ligated Shay rats. Unpublished study No. PH
252-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
Chickens
Groups of male 18-day-old broiler chickens were given
drinking-water that had been medicated to provide 5 mg/kg bw per day
danofloxacin, for three days. Steady-state concentrations of 0.43 and
0.21 µg/ml were obtained in lung and plasma, respectively, during the
period of administration. The half-life of danofloxacin was 4.9 h in
plasma and 5.8 h in lung. No residues of desmethyldanofloxacin were
detected in any of the plasma or lung samples (Lynch et al., 1990a).
Groups of broiler chickens were given drinking-water that had
been medicated to provide 5 mg/kg bw per day 3H-danofloxacin for
five days. The test substance was tritiated in the N1-cyclopropyl
ring. The study complied with GLP guidelines. Six hours after
withdrawal of the treated water, unchanged danofloxacin accounted for
75% of the radiolabel in excreta, and desmethyl-danofloxacin accounted
for 5-7% (Lynch et al., 1990a).
Groups of 12 male and 12 female 22-day-old broiler chickens were
given drinking-water which had been medicated to provide 5 mg/kg bw
per day 3H-danofloxacin, for five days. The test substance was
tritiated in the N1-cyclo-propyl ring and had a specific activity of
12.1 µCi/mg (expressed as base). Excreta were collected every 24 h and
pooled by sex and day. The study complied with GLP guidelines. The
mean level of radiolabel in excreta attained a steady state in the
range 35-46 µg/g by the first day of treatment but declined to 20 and
6.6 µg/g on the first and second days after withdrawal, respectively.
About 85% of the radiolabel in faeces was identified as unmetabolized
danofloxacin. There was no difference between the sexes in the pattern
of excretion (Lynch et al., 1988).
Pigs
Castrated male and female pigs (mean body weight, 40 kg) were
given single intramuscular injections of 1.25 mg/kg bw of a prototype
2.5% formulation of danofloxacin. Absorption was rapid, with peak
concentrations of 0.40 µg/g in plasma and 1.68 µg/g in lung about 1 h
after dosing. The half-life of plasma elimination was approximately
7 h (Risk et al., 1991).
Six castrated male and six female pigs (mean body weight, 43.3
kg) were given five daily intramuscular injections of 1.25 mg/kg bw of
danofloxacin tritiated in the N1-cyclopropyl ring in a 2.5% aqueous
vehicle. The study complied with GLP guidelines. The mean radiolabel
concentration during the two days before and the day after the last of
the five doses was 7-8 µg/g in pooled faecal homogenates and 14-18
µg/g in pooled urine samples. During this same period, the total daily
recovery of radiolabel from urine and faeces accounted for 75-81% of
the daily dose. The mean radiolabel concentration in bile was 1.7 µg/g
12 h after the end of treatment and had declined to 0.21 µg/g by 48 h
(Lynch et al., 1989b).
In pigs given a single dose of 5 mg/kg bw of danofloxacin
intravenously, intramuscularly, or orally, detectable residues of
danofloxacin appeared rapidly in plasma. A peak plasma concentration
of 0.42 µg/ml was attained about 3 h after oral dosing. The
bioavailability was estimated to be 76% after intramuscular
administration and 89% after oral administration (Mann & Frame, 1992).
Cattle
A group of 36 male and female calves weighing about 250 kg were
given a single intramuscular injection of 1.25 mg/kg bw of a 2.5%
prototype formulation of danofloxacin, and the kinetics of the
compound were evaluated in plasma and lung tissues. The study complied
with GLP guidelines. Absorption was rapid, with peak plasma and lung
concentrations of 0.35 µg/ml and 1.44 µg/g attained 1 h after dosing.
The half-time for plasma elimination was 3.4 h (Lynch et al.,
1990b).
In a three-way cross-over design study, 12 male and female beef
calves (mean body weight, 112 kg) were given single intravenous doses
or five subcutaneous or intramuscular doses of 1.25 mg/kg bw per day
of a 2.5% prototype formulation of danofloxacin. The study complied
with GLP guidelines. Absorption was rapid: peak plasma concentrations
of 0.37 and 0.47 µg/ml were attained about 1 h after single
subcutaneous and intramuscular doses, respectively, and the
bioavailability was almost 100%. The values for the area under the
concentration-time curve indicated that the levels achieved after
intramuscular and subcutaneous administration were bioequivalent after
one, three, and five injections (Lynch et al., 1991a).
The pattern of excretion of 3H-danofloxacin (tritiated in the
N1-cyclopropyl ring) was studied in five castrated male and four
female calves (body weight, about 180 kg) after five daily
intramuscular injections of 1.25 mg/kg bw (in a 2.5% aqueous vehicle).
The study complied with GLP guidelines. The total concentration of
danofloxacin-related material in excreta reached a plateau by the
third day of treatment. Approximately equal amounts were excreted in
urine and faeces. Unchanged danofloxacin accounted for about 48% of
material excreted in faeces and 89% in urine. The desmethyl metabolite
accounted for 12% of the material in urine samples, but the
concentrations in faeces were too low for measurement (Lynch et al.,
1989a).
2.1.2 Biotransformation
The biotransformation of danofloxacin has been investigated in
rats, dogs, chickens, pigs, and cattle. In all of these species, the
main residue in the faeces was unmetabolized danofloxacin; smaller
amounts of desmethyldanofloxacin were also found (Lynch et al.,
1989a,b,c, 1991a,b,c,d). The main residue in the urine of cattle,
dogs, and rats was also danofloxacin. Desmethyldanofloxacin,
danofloxacin- N-oxide and the ß-glucuronide were identified in rat,
dog, and pig urine (Lynch et al., 1991d).
In the livers of most species, danofloxacin was the major
residue, but significant residues of the metabolite
desmethyldanofloxacin were also present (Table 1). A piperazine-ring
degradation product constituted 43 and 27% of the radiolabel in bile
from male and female cattle, respectively, but it was present in only
trace amounts in bile from rats and dogs. The bile of dogs contained
residues of danofloxacin- N-oxide, which was present in only trace
amounts in bile from the other species (Lynch et al., 1991d).
2.2 Toxicological data
2.2.1 Acute toxicity
The results of studies of the acute toxicity of danofloxacin and
desmethyl-danofloxacin are summarized in Table 2. All of the studies
complied with GLP guidelines.
In the studies by oral administration, signs of toxicity included
exophthalmia, decreased activity, tremors, and twitching, which
occurred within a few minutes of dosing. One of three mice given
2000 mg/kg bw died 29 min after dosing. Retching, clonic convulsions,
and gasping were observed before death. After intravenous dosing, all
three mice given 100 mg/kg bw and all three rats given 150 mg/kg bw
died within 3 min. The signs of toxicity were similar to those
produced after oral administration. Similar signs of toxicity were
observed after oral and intravenous dosing with desmethyldanofloxacin.
Table 1. Composition of residues in liver in five species given danofloxacin
Species Dose regimen Sex Total Danofloxacina Desmethyldanofloxacina Reference
residue
Rat 5 × 6.25 mg/kg bw M 2.3 ppm 67% 18% Lynch et al.
per day orally F 2.5 ppm 70% 11% (1991d)
Dog 5 × 2.4 mg/kg bw M 6.7 ppm 66% 28% Lynch et al.
per day orally F 5.4 ppm 58% 37% (1991d)
Chicken 5 × 5 mg/kg bw M NR 78% 16% Lynch et al.
per day orally F NR 74% 12% (1988)
Pig M 0.95 ppm 58% 23% Lynch et al.
F 0.96 ppm 68% 29% (1989c)
Cowb 5 × 1.25 mg/kg bw M 0.82 ppm 24% 43% Lynch et al.
intramuscularly F 1.1 ppm 29% 42% (1991d)
a Relative to the radiolabel recovered by high-performance liquid chromatography
b Killed 12 h after the last dose
Table 2. Results of studies of the acute toxicity of danofloxacin and desmethyldanofloxacin
Species Route Vehicle Sex LD50 Reference
(strain) (mg/kg bw)
Danofloxacin
Rat (Sprague- Oral Distilled M & F > 2000 Stadnicki et al. (1988a)
Dawley) water
Mouse (ICR) Oral Distilled water M & F > 2000 Stadnicki et al. (1988a)
Rat (Sprague- Intravenous Sterile water M 100-150 Stadnicki et al. (1988a)
Dawley)
Mouse (ICR) Intravenous Sterile water M 50-100 Stadnicki et al. (1988a)
Rabbit (New Dermal M & F > 299a Stadnicki et al. (1988b)
Zealand white)
Desmethyldanofloxacin
Rat (Sprague- Oral Deionized M & F > 2000 Stadnicki et al. (1989)
Dawley) water
Mouse (ICR) Oral Deionized M > 2000 Stadnicki et al. (1989)
water
Mouse (ICR) Oral Deionized F 1500-2000 Stadnicki et al. (1989)
water
Rat (Sprague- Intravenous Sterile water M 40-50 Stadnicki et al. (1989)
Dawley)
Mouse (ICR) Intravenous Sterile water M 7.5-10 Stadnicki et al. (1989)
a 0.5 g danofloxacin was applied to one site on intact skin and one on abraded skin of two male and one
female New Zealand white rabbits under an occlusive dressing for 24 h. There were no deaths or signs
of toxicity, except for mild erythema at all treated sites.
A dose of 26 mg danofloxacin (in a volume of 0.1 ml) was
instilled into the conjunctival sac of the left eyes of three New
Zealand white rabbits. The eyes were not rinsed after dosing. Mild
conjunctivitis and a colourless discharge were observed within 1 h of
treatment. All signs had cleared within 96 h (Stadnicki et al.,
1988b).
2.2.2 Short-term toxicity
Mice
In a study designed to identify suitable doses for a two-year
feeding study, groups of 15 male and 15 female Crl:CD-1 (ICR)BR
VAF/Plus mice were fed diets calculated to provide 0, 150, 300, or 600
mg/kg bw per day of danofloxacin (74% activity) for up to 102 days.
The study complied with GLP guidelines. One female given the high dose
was killed in a moribund condition. Body-weight gain was reduced in
males at the low and high doses in comparison with that of the
controls. Decreased erythrocytic parameters were seen occasionally in
males and females at the high dose. There were no consistent
dose-related changes in clinical chemistry. At termination, the
absolute mean group weight of the kidneys of females receiving the
high dose was significantly increased, but there were no corresponding
pathological changes. Microscopic examination revealed caecal
dilatation in six mice at the low dose, 23 at the intermediate dose,
and 27 at the high dose, but not in the controls. Inflammation of the
caecum was seen in two mice at the high dose, and Gram-positive
bacteria, tentatively identified as Clostridium difficile, were
found in the inflamed caeca of both animals. Thymic lymphocytosis was
seen in one of 15 females at the low dose, two of 15 at the
intermediate dose, and eight of 15 at the high dose and in three of 13
males at the intermediate dose and six of 14 at the high dose but in
no control. A NOEL was not identified (Stadnicki et al., 1994a).
Rats
A two-week exploratory study was carried out in which groups of
three male and three female Long-Evans rats received oral doses of 0,
25, 50, or 100 mg/kg bw per day of danofloxacin in an aqueous vehicle.
There were no significant effects on body-weight gain, food
consumption, haematological, clinical chemical, or urinary parameters,
or organ weights. At necropsy, treatment-related caecal enlargement
was observed in treated animals (Reynolds et al., 1987a).
Groups of 10 male and 10 female Long-Evans rats were given doses
of 0, 25, 75, or 150 mg/kg bw per day danofloxacin by gavage in an
aqueous vehicle for one month. Serum chemistry and haematology were
evaluated before treatment and on days 11-12 and 30-31. The study
complied with GLP guidelines. There were no signs of toxicity and no
effects on body-weight gain or food consumption. Alanine
aminotrasferase levels were significantly increased in treated females
receiving the high dose, and absolute liver and liver:body weight
ratios were significantly decreased in males at this dose. No
substance-related effects were seen in about 30 tissues from animals
at 0 and 150 mg/kg bw and macroscopic lesions from animals in the
other groups examined microscopically (Fisher et al., 1988a).
In a range-finding study designed to identify suitable doses for
a long-term study of toxicity, groups of 10 male and 10 female Fischer
rats were fed diets calculated to provide 0, 150, 300, 450, or 600
mg/kg bw per day of danofloxacin for 36-39 days. Satellite groups of
three animals of each sex were used to monitor plasma concentrations
of the drug. Five rats given 600 mg/kg bw per day died. Body-weight
gain and food consumption were reduced in all treated groups, and
there was an initial dose-related decrease in food consumption.
Changes were seen in serum chemistry, haematological parameters and
organ weights, due to the effects of starvation and dehydration.
Caecal dilatation was observed in all treated animals (Stadnicki
et al., 1994b).
In a subsequent exploratory study in groups of 10 Long-Evans rats
of each sex, doses intended to provide 0, 75, 150, or 300 mg/kg bw per
day of danofloxacin were administered in the feed for seven days; the
animals then received untreated feed for seven days and the treated
feed for a further 12 days. Body-weight gain and food consumption were
reduced in rats at the intermediate and high doses. Serum globulin
levels were reduced and leukopenia was observed in all treated groups.
Crystalluria was seen in most treated rats but in only two of 20
controls. There was no histopathological evidence of nephropathy
(Stadnicki et al., 1994c).
In a study designed to establish doses to be used in a two-year
feeding study, groups of 15 Long-Evans rats of each sex were fed diets
calculated to provide 0, 75, 150, or 300 mg/kg bw per day of
danofloxacin for about three months. The study complied with GLP
guidelines. Alopecia was seen in four females at the high dose and one
at the intermediate dose. Discoloured urine (especially in animals at
75 mg/kg bw) and crystalluria were observed in all treated groups, but
there were no corresponding pathological changes in the kidneys.
Body-weight gain was significantly reduced in a dose-related manner.
There were significant, dose-related increases in urinary and serum
magnesium concentrations, which were attributed to chelation of
magnesium ions by the test substance. Caecal dilatation was observed
in rats at 150 and 300 mg/kg bw. A range of degenerative changes were
observed in the testes of all males at the high dose and in one at 0,
one at 75, and one at 150 mg/kg bw per day. Degenerating germinal
cells were found in the epididymides of all males at the high dose and
nine at the middle dose (Stadnicki et al., 1994d).
Groups of 20 male and 20 female weanling Long-Evans rats were
selected from the F1 offspring of rats used in a multigeneration
study which had been exposed to danofloxacin both in utero and
during lactation. They were then given oral doses of 25, 75, or 150
mg/kg bw per day for up to three months, whereas 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58).
There were no substance-related deaths and no adverse effects on
food consumption or body-weight gain. There were no consistent
dose-related trends in haematological or clinical chemical values. A
dose-related increase in proteinuria was seen in females but not
males, which was correlated with the finding of tubular nephropathy in
individual animals. Kidney weights were unaffected by treatment. The
mean absolute and relative weights of the testes of rats at 75 and 150
mg/kg bw were about 10% lower than those of controls. Lesions of the
heart were found primarily in animals at these doses and consisted of
either multifocal myocardial degeneration and necrosis, or multifocal
fibrosis, or both. There was no NOEL, as renal tubular nephropathy was
observed in all treated females (Fisher et al., 1989a).
The study was repeated at lower doses in order to establish a
NOEL. Groups of 20 male and 20 female weanling Long-Evans rats were
again selected from the F1 offspring in the multigeneration study,
which had been exposed to danofloxacin both in utero and during
lactation. They were then given oral doses of 1, 2.5, or 6.25 mg/kg bw
per day for up to three months. Twenty controls of each sex were given
deionized water. The study was conducted in accordance with US FDA GLP
guidelines (21 CFR Part 58).
There were no treatment-related deaths or signs of toxicity.
Body-weight gain, food consumption, and haematological and clinical
chemical parameters were unaffected by treatment. Urinalysis revealed
a marginal increase in the incidence of haematuria in males given the
high dose and of proteinuria in a single female at this dose. The
absence of any corresponding pathological changes in the kidney
indicates that the findings reflected biological variability rather
than a substance-related effect. The NOEL was 6.25 mg/kg bw per day
(Fisher et al., 1990a).
Groups of 20 male and 20 female Long-Evans rats were given oral
doses of 1, 2.5, or 6.25 mg/kg bw per day of desmethyldanofloxacin for
up to three months. The rats were selected from the F1 offspring in
a multigeneration study and had been exposed to the test substance
both in utero and during lactation. Groups of 20 controls of each
sex received deionized water. The study was conducted in accordance
with US FDA GLP guidelines (21 CFR Part 58). There were no effects on
mortality, body-weight gain, food consumption, haematological or
clinical chemical parameters, organ weights, or gross or
histo-pathological findings. The NOEL was 6.25 mg/kg bw per day
(Stadnicki et al., 1990a).
Rabbits
In an exploratory study, groups of three female New Zealand white
rabbits were given oral doses of 0, 25, 50, or 100 mg/kg bw per day of
danofloxacin in an aqueous vehicle. Owing to significantly reduced
feed intake and decreased body weight, treatment of rabbits at the low
dose was stopped after 12 doses, that of animals at the intermediate
dose after seven doses, and that of rabbits at the high dose after
four doses. The groups were fed untreated diet for a further five to
eight days, then killed and necropsied. Pathological examination
revealed a dose-related incidence of enlarged caeca (Lundeen et al.,
1993).
Dogs
In an exploratory study, groups of one male and one female beagle
dogs were given oral doses of 0, 12.5, 25, or 50 mg/kg bw per day of
danofloxacin in gelatin capsules for two weeks. The female at the high
dose had reduced body-weight gain and food consumption and increased
serum blood urea nitrogen and creatinine levels. At necropsy, this dog
had acute segmental nephrosis and renal failure. The male at the high
dose had chronic focal necrosis without renal failure (Reynolds
et al., 1987b).
In a one-month exploratory study, groups of three male and three
female beagle dogs (10-12 months old) were given oral doses of 0, 5,
10, or 25 mg/kg bw per day of danofloxacin in gelatin capsules. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). There were no effects on behaviour, food consumption,
body-weight gain, blood pressure, electrocardiographic profile, or
ophthalmic, serum chemical, or haematological parameters. At necropsy,
a full range of tissues from all animals was examined, with particular
attention to the weight-bearing joints. Shallow erosions were found in
all dogs, including controls, although the grade of erosions was
slightly higher in those at the high dose. The lesions were typical of
those found in older dogs and did not resemble quinolone-induced
lesions (Fisher et al., 1988b).
Groups of four beagle dogs of each sex aged about six months were
given oral doses of 0, 5, 10, or 25 mg/kg bw of danofloxacin in
gelatin capsules. The substance was administered as two equally
divided doses per day for three months. At termination, all dogs were
subjected to a complete pathological examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
By day 7, reduced activity and signs of joint pain were seen in all
eight dogs given 25 mg/kg bw and in three dogs given 10 mg/kg bw. Most
of the affected animals showed signs of recovery by week 6, despite
continued treatment. Body-weight gain, food consumption, heart rate,
and respiratory rate were decreased in animals that showed clinical
signs. There were no effects on electrocardiographic profiles, blood
pressure, or ophthalmic, haematological, clinical chemical, or urinary
parameters. Gross pathological changes were observed in the articular
cartilage of the major joints of all treated animals, except for one
female receiving the low dose. The lesions were characterized by areas
of cartilage separation and cartilage loss (erosions). The severity of
the lesions was dose-related. Microscopic examination revealed further
changes, including the apparent alteration of collagen fibres when
viewed with polarized light (Fisher et al., 1989b).
A second three-month study, which complied with the same GLP
guidelines, was carried out at lower doses in order to establish a
NOEL. Groups of four male and four female beagle dogs, aged about five
months, were given oral doses of 0, 1, or 2.4 mg/kg bw per day of
danofloxacin in gelatin capsules as two equally divided doses, for 91
days. There were no signs of toxicity and no effects on body-weight
gain, food consumption, clinical chemical or urinary parameters, or
organ weights. There were no substance-related pathological findings.
The NOEL was 2.4 mg/kg bw per day (Fisher et al., 1989c).
Groups of three male and three female beagle dogs, four to six
months old, were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules, administered as two equally
divided doses, for about three months The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58). There were no
notable effects on body-weight gain, electrocardio-graphic profiles,
serum chemical or haematological values, or organ weights. One male
given the high dose showed signs of pain on day 65 when pressure was
applied to the left ankle joint. One female at the low dose showed
signs of pain on day 92 when pressure was applied to the inside of the
left upper hind limb. At termination, histopathological changes
typical of quinolone-induced lesions were found in the articular
cartilage of the right femoral condyle of one male given the high dose
(Stadnicki et al., 1990b).
Groups of three male and three female beagle dogs, five to six
months old, were given oral doses of 0, 0.25 or 0.5 mg/kg bw per day
of desmethyldanofloxacin in gelatin capsules, as two equally divided
doses, for about three months. Further groups of dogs were given 10
mg/kg bw per day of danofloxacin. The study was conducted in
accordance with US FDA GLP guidelines (21 CFR Part 58).
There were no signs of toxicity in the dogs treated with
desmethyldano-floxacin. The dogs given danofloxacin showed signs of
hind limb weakness, reduced activity, and stiff gait. Treatment had no
effect on electrocardiographic profiles, serum chemical or
haematological parameters, or organ weights. Pathological examination
revealed typical quinolone-induced arthropathy in all dogs treated
with danofloxacin. One male in the group given 0.5 mg/kg bw per day
desmethyldano-floxacin was found to have a single articular erosion in
the patellar groove of the right knee. Microscopically, the erosion
extended into zone 2 of the articular cartilage and resembled those
seen with other quinolones. The NOEL was 0.25 mg/kg bw per day
(Stadnicki et al., 1990c).
2.2.3 Long-term toxicity and carcinogenicity
Mice
Groups of 50 ICR mice of each sex were fed diets containing the
equivalent of 10, 50, or 100 mg/kg bw per day of danofloxacin for up
to two years. Two groups of 50 controls of each sex were fed untreated
diets. The study complied with GLP guidelines. There were no adverse
dose-related effects on mortality rate, body weight, food consumption,
or haematological parameters. Females receiving 100 mg/kg bw per day
gained significantly more weight than animals in the other groups. At
termination, less than 50% of the animals in each group, except for
the males receiving 10 mg/kg bw per day, were still alive. The mean
absolute kidney weight (but not the relative kidney:body weight) was
significantly increased in females receiving 100 mg/kg bw per day,
which probably reflected the increased body weight of these mice.
There was no evidence of carcinogenicity (Takatsu et al., 1995).
Rats
Groups of 50 male and 50 female Long-Evans rats were fed diets
containing the equivalent of 10, 50, or 100 mg/kg bw per day of
danofloxacin for up to two years. Two groups of 50 controls of each
sex were fed untreated diets. The study complied with GLP guidelines.
There were no clinical signs of toxicity, and survival was
unaffected by treatment. At termination, however, less than 50% of
animals in all groups were alive, except for females receiving 100
mg/kg bw per day and one of the groups of female controls. Occasional
statistically significant reductions in body-weight gain were seen in
males at this dose, and females at 50 and 100 mg/kg bw per day had
significantly increased body-weight gain between three and 16 months
of treatment, which was correlated with increased food intake during
this period. Overall, the body-weight changes associated with
treatment were minimal. Ophthalmoscopic examinations during weeks 12,
18, and 23 revealed no differences between control rats and those
receiving 100 mg/kg bw per day. At termination, the mean leukocyte and
neutrophil counts were significantly reduced in males receiving 100
mg/kg bw per day; in females at this dose, the mean haemoglobin,
haematocrit, and lymphocyte counts were reduced. Males also had
significant increases in aspartate aminotrans-ferase and sorbitol
dehydrogenase values, and the mean globulin value was reduced, with a
corresponding increase in albumin:globulin ratio. The sorbitol
dehydrogenase level was also increased in males receiving 50 mg/kg bw
per day. The effects on clinical chemical values were not seen in
females. There were no substance-related effects on urinary values.
The mean testis:body weight ratio was significantly reduced in the
group receiving 100 mg/kg bw per day. There were no significant
effects on absolute organ weights. Gross pathological examination
revealed an increased incidence of caecal enlargement in treated
animals which was not correlated with any microscopic findings. (Table
3). The histopathological findings included an increased incidence of
renal papillary oedema, increased oligospermia, and abnormal content
of the epididymides in rats at 100 mg/kg bw per day (Table 4).
Table 3. Incidence of caecal enlargement in rats treated with
danofloxacin in the diet for up to two years
Sex Dose (mg/kg No. with caecal enlargement/
bw per day) no. examined
Male 0 0/50
0 0/50
10 5/50
50 3/50
100 6/50
Female 0 1/50
0 0/50
10 1/50
50 1/50
100 2/50
Table 4. Incidence of some non-neoplastic histopathological alterations in groups of
50 rats fed diets containing danofloxacin for up to two years
Sex Dose (mg/kg No. with papillary No. with No. with abnormal
bw per day) oedema oligospermia epidydimal content
Male 0 1 17 25
0 0 16 32
10 0 18 28
50 1 17 29
100 4 31 36
Female 0 0
0 2
10 2
50 2
100 4
There was a significant positive trend in the incidence of
granular-cell tumours of the uterus and vagina in treated female rats
(Table 5); however, the trend was not significant when corrected for
multiple comparisons. Granular-cell foci were distinguished from
tumours by their smaller size and the absence of compressed adjacent
tissue; nevertheless, the morphology of these lesions was essentially
the same. When the incidences of uterine and vaginal foci and tumours
were combined, there was no statistically significant trend in the
combined incidence across groups. There was a significant positive
trend in the incidence of pituitary adenomas in female rats (Table 6),
which was not significant when corrected for multiple comparisons. In
addition, the incidence of pituitary adenomas was within the range
seen in controls in the five previous studies at the same laboratory:
30/48 to 47/49 (Fisher et al., 1996).
2.2.4 Genotoxicity
The results of assays for genotoxicity with danofloxacin and
desmethyl-danofloxacin are summarized in Table 7. All of the studies
complied with GLP guidelines.
2.2.5 Reproductive toxicity
2.2.5.1 Multigeneration reproductive toxicity
Rats
In a two-generation study of reproductive toxicity, groups of 45
male and 45 female Long-Evans rats were given oral doses of 0, 25, 75,
or 150 mg/kg bw per day of danofloxacin in an aqueous vehicle. The
study was conducted in accordance with US FDA GLP guidelines (21 CFR
Part 58). Dams at 150 mg/kg bw per day showed reduced body-weight gain
during gestation and fewer implantation sites and produced fewer live
pups than those at other doses. The pup weights were significantly
reduced at birth and during lactation. Similar effects were observed
at the first F1 mating, which were more pronounced at the second
F1 mating, when the pregnancy rate was adversely affected in all
treated groups. The weights of all treated F2b pups were reduced in
a dose-related manner (Fisher et al., 1990b).
The F2b pups at 25 mg/kg bw per day were used in a new study
(complying with similar GLP guidelines) when they were two months old,
and treatment was continued. After mating to produce the F3
generation, the pregnancy rate was only 38%, whereas that in the
control group was 65%. Post-implantation losses were significantly
increased and pup body weights and survival were adversely affected
(Fisher et al., 1990c).
Table 5. Incidences of granular-cell proliferative lesions of the uterus
and vagina in female rats fed diets containing danofloxacin for up to
two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
Uterus
No. examined 48 50 50 50 50
Granular-cell tumour 1 3 6 5 6
Granular-cell foci 5 6 1 1 2
Vagina 48 49 49 49 49
Granular-cell tumour 3 2 2 3 7
Granular-cemm foci 0 0 1 0 0
Uterus plus vagina
Granular-cell tumour 4 5 8 8 13
Granular-cell foci5 5 6 2 1 2
Total 9 11 10 9 15
Table 6. Incidence of proliferative lesions of the pituitary gland in
female rats fed diets containing danofloxacin for up to two years
Lesion Dose (mg/kg bw per day)
0 0 10 50 100
No. examined 49 50 50 50 50
No. with pituitary adenoma 32 32 39 39 40
No. with pituitary hyperplasia 13 11 5 6 5
No. of rats alive at 24 months 22 26 19 18 26
Table 7. Assays for genotoxicity with danofloxacin and desmethyldanofloxacin
End-point Test object Concentration S9 Results Reference
Danofloxacin
In vitro
Gene mutationa S. typhimurium 0.01-0.2 µg/platea - Negative Amacher et al.
TA98, TA100, 0.001-0.1 µg/plate + Negative (1988)
TA1535, TA1537 0.0005-0.2 µg/plate + Negative
Gene mutation L5178Y/tk+/- mouse 51-287 µg/ml - Negative Amacher et al.
lymphoma cellsc 16-215 µg/ml + Negative (1988)
Gene mutation hprt, Chinese 141-1070 µg/ml - Negative Guzzie et al.
hamster ovary 465-2500 µg/ml + Negative (1992)
cellsc
Unscheduled Primary rat 50-400 µg/ml Negative Amacher et al.
DNA synthesis hepatocytesc (1988)
Cytogenetic Human lymphocytes 25-70 µg/ml - Positived Amacher et al.
alterations 200-600 µg/ml + Positive (1990)
In vivo
Micronucleus ICR mouse bone 1000 mg/kg bw Negative Amacher et al.
formation marrow orally (1990)
Desmethyldanofloxacin
In vitro
Gene mutation S. typhimurium 0.001-0.5 µg/plate + Negative Amacher et al.
TA98, TA100, 0.01-5 µg/plate - Negative (1991a)
TA1535, TA1537
Gene mutation L5178Y mouse 90-388 µg/ml - Negative Holden et al.
lymphoma cells 63-269 µg/ml +e Negative (1988)
Unscheduled Primary rat 2.54-102 µg/ml and Positive Hazleton
DNA synthesis hepatocytes 5.02-100 µg/ml Positive Laboratories
America Inc.
(1991a)
Unscheduled Primary rat 62.5-250 µg/ml Positive Amacher et al.
DNA synthesis hepatocytes 62.5-500 µg/mlf Positive (1991b)
Table 7. (continued)
End-point Test object Concentration S9 Results Reference
In vivo
Unscheduled Fischer 344 rat 1 × 250-2000 mg/kg Negative Hazleton
DNA synthesis hepatocytes bw per day orally Laboratories
America Inc.
(1991b)
Micronucleus CD-1 mouse bone 3 × 250-1000 Negative Amacher et al.
formation marrow and peripheral mg/kg bw per day (1991c)
blood orally
S9, 9000 × g fraction of rat liver
a The same bacterial strains were incubated with urine collected from mice given danofloxacin
intraperitoneally at 5, 50, or 100 mg/kg bw per day, with no mutagenic response.
b Higher concentrations toxic to tester strains
c Not replicated independently
d Significant increase in abnormal cells (chromatid breaks). To check whether the clastogenicity
was due to chelation, the assay was repeated without S9 but with the addition of 400 µg/ml
magnesium sulfate; no increase in abnormal cells was observed. In the presence of S9, extra
washes to remove the test substance and the addition of magnesium sulfate diminished the
clastogenic response.
e From livers of uninduced rodents
f 1.62 mmol/litre magnesium ions (as magnesium sulfate heptahydrate) added to culture medium did
not prevent a similar dose-related increase in unscheduled DNA synthesis.
In a three-generation study of reproductive toxicity, groups of
30 male and 30 female Long-Evans rats were given oral doses of 1, 2.5,
6.25, or 150 mg/kg bw per day of danofloxacin. Two control groups
received the deionized water vehicle. Treatment was begun nine weeks
before cohabitation for males and two weeks previously for females
(1:1 mating) and was continued throughout gestation, parturition, and
weaning of the F1 offspring. Of these, groups of 25 of each sex were
selected randomly, and treatment was continued during breeding of the
F2a and F2b generations. The F2b offspring were mated in the
same way to produce the F3 litters. Litters were culled to eight
pups on day 4 post partum. Postnatal development of the F2b pups was
assessed at weaning by measuring locomotor activity, auditory
function, and ophthalmic parameters. All F0, F1, and F2 rats used
for breeding were necropsied and the reproductive organs and main
target organs (kidney, joints, brain, heart, and liver) were weighed
and preserved for possible microscopic examination. The study was
conducted in accordance with US FDA GLP guidelines (21 CFR Part 58).
Adult animals showed no treatment-related effects on survival,
body-weight gain, food consumption, or clinical signs. In those at 150
mg/kg bw per day, copulatory rate and pregnancy rate were reduced and
the length of gestation was increased. (The copulatory rate was
defined as the percentage of those females which cohabited that had a
vaginal flush with sperm, or an internal copulatory plug, or which
delivered with no previous signs of mating.) Administration of 150
mg/kg bw per day also resulted in reduced litter size and pup weight
at birth, reduced weight gain of the neonates, and a reduction in the
number of pups surviving to day 4 post partum. The group receiving 150
mg/kg bw per day was terminated before the second F1 mating. The
NOEL was 6.25 mg/kg bw per day (Stadnicki et al., 1990d).
In a study of exactly the same design as that described above,
the animals received desmethyldanofloxacin instead of danofloxacin.
There were no treatment-related effects on parental body-weight gain
or food consumption, clinical signs, pregnancy rate, length of
gestation, number of implantation sites, or post-implantation loss.
Pup body-weight gain and survival were unaffected by treatment. There
were no grossly observed abnormalities in the dams or sires (Stadnicki
et al., 1991).
2.2.5.2 Developmental toxicity
Mice
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were given in an aqueous
vehicle to groups of seven mated female ICR mice on days 6-13 of
gestation. The study complied with GLP guidelines. The mean weights of
the male fetuses of animals at 200 mg/kg bw per day were reduced. No
effects were seen on dams (Kessedjian et al., 1988a).
In the main study, groups of 20 female Crl:COBS-CDI(ICR)BR mice
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-13 of gestation and were killed on gestation day 18.
A further group of 10 dams was given 200 mg/kg bw per day and used to
monitor drug concentrations in maternal plasma and amniotic fluid. The
study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were two to three time those in maternal plasma. The incidence of
non-gravid females was high: the total numbers of pregnant females
were only 13, 16, 11, and 13 in the control, low, intermediate, and
high dose groups, respectively. One dam given 200 mg/kg bw per day
showed piloerection and prostration on days 7-10 of gestation and was
found to have a cutaneous abscess at necropsy. The mean body-weight
gain of the dams at the high dose was significantly reduced during
treatment. There were no meaningful effects on the incidences of
resorptions or fetal deaths or on the sex ratio. The mean weights of
both male and female fetuses were significantly reduced at the high
dose, and the incidence of delayed ossification was increased. There
was no evidence of teratogenicity. The NOEL was 100 mg/kg bw per day,
on the basis of maternal and fetal toxicity (Kesseddjian et al.,
1989a).
Rats
In a preliminary range-finding study, oral doses of 0, 50, 100,
or 200 mg/kg bw per day of danofloxacin were administered in an
aqueous vehicle to groups of seven mated female Sprague-Dawley rats on
days 6-15 of gestation. The study complied with GLP guidelines.
Maternal body-weight gain was reduced in all treated groups, and the
mean number of viable fetuses was reduced at 200 mg/kg bw per day
(Kessedjian et al., 1988b).
In the main study, groups of 20 female Sprague-Dawley rats
presumed to be pregnant were given oral doses of 0, 50, 100, or 200
mg/kg bw per day of danofloxacin in an aqueous vehicle; 19-20 dams in
each group were subsequently confirmed to be pregnant. The animals
were treated on days 6-15 of gestation and were killed on gestation
day 20. A further group of five dams was given 200 mg/kg bw per day
and used to monitor drug concentrations in maternal plasma and
amniotic fluid. The study complied with GLP guidelines.
The concentrations of danofloxacin in the amniotic fluid 5 h
after the last of eight consecutive doses of 200 mg/kg bw per day were
similar to those in maternal plasma, while those in fetal homogenates
were about three time those in maternal plasma. There were significant
dose-related reductions in maternal body-weight gain in the rats at
100 and 200 mg/kg bw per day, and mean fetal weights were also
significantly reduced. The incidences of delayed ossification and
dilatation of the cerebral ventricles in the fetuses were
significantly increased at the intermediate and high doses. The NOEL
was 50 mg/kg bw per day, on the basis of maternal and fetal toxicity
(Kesseddjian et al., 1989b).
Rabbits
In a preliminary range-finding study, groups of 11 New Zealand
white rabbits presumed to be pregnant were given oral doses of 0, 5,
10, or 20 mg/kg bw per day of danofloxacin on days 6-20 of gestation.
The study complied with GLP guidelines. Inappetance was seen in all
groups, but maternal body weight was not affected. Animals at the high
dose had a decreased number of live fetuses and higher resorption
rates. The mean fetal weights were reduced at the intermediate and
high doses (Tassinari et al., 1995a).
In the main study, groups of 20 female New Zealand white rabbits
presumed to be pregnant were given oral doses of 0, 2.5, 7.5, or 15
mg/kg bw per day of danofloxacin in an aqueous vehicle. The dams were
treated on days 6-20 of gestation and were killed on gestation day 28.
Because of a high incidence of non-gravid females, new animals were
randomized to the different doses so that the total numbers were 32
controls, 29 at the low dose, 33 at the intermediate dose, and 39 at
the high dose. The study complied with GLP guidelines.
Eleven does at the high dose showed loss of body weight and
reduced food consumption and then aborted their litters between days
22 and 28 of gestation. The mean food consumption of the does with
viable litters at 15 mg/kg bw per day was significantly reduced during
days gestation days 13-20. There were no treament-related effects on
litter size, sex ratio, fetal weight, or the incidences of
malformations or variations. The NOEL was 7.5 mg/kg bw per day, on the
basis of maternal toxicity (Tassinari et al., 1995b).
2.2.6 Special study on delayed contact hypersensitization
In a study using the Buehler closed patch technique, which
complied with GLP guidelines, danofloxacin mesylate did not cause
delayed contact hypersensitivity in the guinea-pig. A clear positive
result was obtained with the known sensitizer, dinitrochlorobenzene
(Beutler et al., 1992).
2.2.7 Special studies on pharmacological activity
A number of pharmacological studies of danofloxacin were
available that were not carried out in accordance with GLP guidelines
or with any national or international guidelines. The main features of
these studies are summarized in Table 8.
Table 8. Results of pharmacological assays with danofloxacin
Test system Doses Results Reference
Groups of six male 0, 5, 10, 20 mg/kg bw No significant diuretic Varner et al.
Sprague-Dawley rats per day orally in activity (1990a)
distilled water
Groups of two male 5 mg/kg bw per day Mild transient decreases Gromelski et al.
and two female intravenously in blood pressure, cardiac (1990)
beagle dogs output, left ventricular
pressure, and left ventricular
end diastolic pressure in two
dogs. No effects on electro-
cardiographic wave forms
Groups of three male 1, 10, 100, 1000 mg/kg No effect on central or Varner et al.
Sprague-Dawley rats bw per day orally peripheral nervous system (1990b)
at 100 mg/kg bw per day;
salivation and tremors at
1000 mg/kg bw per day
Groups of eight male 0, 5, 10, 20 mg/kg bw 18, 27, and 23% decreases Varner et al.
CD-1 mice per day orally in in gastrointestinal motility in (1990c)
distilled water; 4 mg/kg comparison with vehicle
morphine sulfate (positive control
control)
Groups of eight 0, 5, 10, 20 mg/kg bw Increased gastric fluid Varner et al.
pyloricligated male per day intraduodenally volume at all doses (1990d)
Sprague-Dawley rats in 0.25% methylcellulosea; (not dose-related);
10 mg/kg bw per day increased gastric acidity
cimetidine (positive at all doses
control)
a The solvent vehicle was reported to be 0.25% methylcellulose in some parts of the report and distilled water in others.
2.2.8 Special studies on microbiological effects
In a study carried out according to the methods described in
documents M11A (1979) and M11A2 (1990) of the National Committee for
Clinical Laboratory Standards, USA, the antibacterial activity of
danofloxacin and desmethyldano-floxacin was determined against 64
isolates of six genera of organisms representative of the human
intestinal anaerobic microflora. In order to assess the
reproducibility of the assay, four reference strains, Bacterides
fragilis ATCC 25285, Bacillus thetaiomicron ATCC 29741,
Clostridium perfringens ATCC 13124, and Eubacterium lentum ATCC
43055, were added to each batch of tests. In addition, data were
provided for the facultative anaerobes Lactobacillus spp (14
strains) and Proteus spp (11 strains) isolated from faeces of
patients in the hospital of Tourcoing, France. Escherichia coli ATCC
25922 and Enterococcus faecalis ATCC 29242 were added as reference
strains. The calculated MIC50 values are shown in Tables 9 and 10.
2.3 Observations in humans
No information was available. Danofloxacin is not authorized for
human use.
Table 9. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains of
bacteria representative of those found in the human gut
Species No. of Inoculum MIC50 (µg/ml)
strains density
(cfu/ml) Danofloxacin Desmethyldanofloxacin
Anaerobes
Bacillus fragilis group 12 107-108 4 128
Fusobacterium spp. 10 4 16
Clostridium spp. 10 0.5 0.5
Eubacterium spp. 10 0.5 1
Bifidobacterium spp. 10 2 8
Peptostreptococcus spp. 12 0.5 2
Facultative anaerobes
Lactobacillus spp. 14 106 16 > 128
Proteus spp. 11 106 0.25 0.06
Table 10. MIC50 values for danofloxacin and desmethyldanofloxacin in vitro against some strains used for quality control
Species Anaerobic incubation Aerobic incubation
Danofloxacin Desmethyldanofloxacin Danofloxacin Desmethyldanofloxacin
Escherichia coli 0.06 0.06 0.03 0.015
ATCC 25922
Enterococcus faecalis 1 4 2 1
ATCC 29242a
a Shown as Enterococcus faecalis ATCC 29242 on p. 5 of the report but as Enterococcus faecalis ATCC 29212 in the
accompanying tables (Dubreuil, 1994)
3. COMMENTS
The Committee considered data from studies on the
pharmacokinetics, acute, short-term, and long-term toxicity,
carcinogenicity, reproductive toxicity, genotoxicity, and
antimicrobial activity of danofloxacin. The results of studies on the
acute and short-term toxicity, reproductive toxicity, genotoxicity,
and antimicrobial activity of the metabolite desmethyldanofloxacin
were also reviewed. Most of the studies critical for the evaluation
were carried out in accordance with appropriate standards for study
protocol and conduct.
Danofloxacin was rapidly absorbed after oral administration to
chickens and pigs and after intramuscular administration to pigs and
cattle. Only one study was carried out in which the oral
bioavailability of danofloxacin was calculated; in this study, the
bioavailability after oral administration of 5 mg/kg bw to pigs was
approximately 90%. The substance was well distributed to the tissues.
Urine and faeces contained approximately equal amounts of danofloxacin
and its metabolites.
In cattle, dogs, and rats, unchanged danofloxacin was the main
substance present in the faeces; smaller amounts of
desmethyldanofloxacin were found. Danofloxacin was also the main
component in urine. Desmethyldanofloxacin, danofloxacin- N-oxide, and
the ß-glucuronide were also found in urine. A piperazine-ring
degradation product was present in the bile of cattle but was found in
only trace amounts in the bile of rats and dogs. Residues of both
danofloxacin and desmethyldanofloxacin were found in liver samples
from rats, dogs, and the three target species. The Committee
considered that the metabolism of danofloxacin was very similar in
laboratory animals and in the three target species.
Single oral doses of both danofloxacin and desmethyldanofloxacin
were slightly toxic to rats and mice (acute oral LD50 values in the
range 1500 to > 2000 mg/kg bw). Signs of toxicity typical of
stimulation of the central nervous system were observed prior to
death.
Several exploratory studies were carried out in rats given
danofloxacin in the feed and by gavage and in rabbits given
danofloxacin by gavage. Caecal dilatation was seen at doses of 25
mg/kg bw per day and above in most of these studies. In rats,
increased incidences of crystals in the urine were observed at doses
of 75 mg/kg bw per day and above. There were no corresponding
pathological changes in the kidneys. In a three-month study in which
danofloxacin was administered to rats in the diet, degenerated
germinal cells were found in the epididymides of all males given 300
mg/kg bw per day and in 9 out of 15 males given 150 mg/kg bw per day.
In view of the high doses used, the results of these studies were not
useful in evaluating the safety of danofloxacin.
Rats were exposed to danofloxacin in utero and during lactation
by administration to the dams of doses of 0, 25, 75, or 150 mg/kg bw
per day. The rats were orally dosed at the same levels for an
additional three months after weaning. In females, there was a
dose-related increase in proteinuria which correlated with the finding
of tubular nephropathy in individual animals. In males at 75 and 150
mg/kg bw per day, both the mean absolute and relative testicular
weights were 10% lower than those of controls. In a follow-up
three-month study with lower doses, the highest dose, 6.25 mg/kg bw
per day, was the NOEL, on the basis of renal tubular nephropathy.
Rats were exposed to desmethyldanofloxacin in utero and during
lactation by administration to the dams of oral doses of 0, 1, 2.5, or
6.25 mg/kg bw per day. The rats were treated orally at the same levels
for an additional three months after weaning. No adverse effects were
observed at any dose.
Six-month-old dogs were given oral doses of 0, 5, 10, or 25 mg/kg
bw per day of danofloxacin in gelatin capsules for three months. Those
given the two higher doses showed signs of joint pain. Pathological
examination revealed arthropathy characterized by areas of cartilage
separation and erosion in all treated groups, and the severity of the
lesions was dose-related. A second three-month study was carried out
in immature dogs given oral doses of 0, 1, or 2.4 mg/kg bw per day
danofloxacin in gelatin capsules. There was no evidence of arthropathy
or any other treatment-related effect. On the basis of the results of
the two studies, the Committee concluded that 2.4 mg/kg bw per day was
the overall NOEL for arthropathy in dogs.
A further three-month study was carried out in which immature
dogs were given oral doses of 0, 2.5, 5, or 10 mg/kg bw per day
desmethyldanofloxacin in gelatin capsules. One male of three given the
highest dose and one female of three given the lowest dose showed
signs of pain on examination. In the male, morphological changes were
found in the articular cartilage of one joint. When the study was
repeated at doses of 0, 0.25, or 0.5 mg/kg bw per day
desmethyldanofloxacin, one male of three given 0.5 mg/kg bw per day
was found to have histopathological changes in the right knee typical
of quinolone-induced arthropathy. The NOEL for desmethyldanofloxacin
was 0.25 mg/kg bw per day, on the basis of arthropathy.
In a 102-day range-finding study, mice were fed diets containing
danofloxacin at concentrations equivalent to 0, 150, 300, or 600 mg/kg
bw per day. At the highest dose, the body-weight gain of males was
reduced, and decreased haematological parameters and increased kidney
weights were observed in females. Caecal dilatation was observed at
all doses, and two of 30 mice given the highest dose had inflammation
of the caecum.
In a two-year study of carcinogenicity, mice were fed diets
containing doses equal to 0, 10, 50, or 100 mg/kg bw per day. Females
at the highest dose gained more weight than the controls and showed
increased absolute kidney weights. There were no adverse effects on
haematological parameters, but clinical chemistry was not monitored.
No increase in tumour incidence was observed at any dose.
In a two-year study of carcinogenicity, rats were fed diets
providing doses of 0, 10, 50, or 100 mg/kg bw per day danofloxacin. At
the highest dose, mean haemoglobin, haematocrit, and lymphocyte counts
were decreased in females, and serum aspartate aminotransferase
activity was increased and serum globulin levels were reduced in
males. Serum sorbitol dehydrogenase activity was increased in males
given 50 or 100 mg/kg bw per day. The relative testicular weight was
reduced in rats at the highest dose, and increased oligospermia and
abnormal epididymal content were observed in this group. Caecal
enlargement was noted in all treated groups but was not correlated
with any microscopic findings. An increased incidence of papillary
oedema was seen in the kidneys of rats at 100 mg/kg bw per day. There
was a significant positive trend in the incidence of granular-cell
tumours of the uterus and vagina in treated female rats. Tumours were
distinguished from foci by their larger size and the compression of
adjacent tissue, but the morphology of these lesions was similar, and
the Committee considered that it was appropriate to combine the
incidences of these uterine and vaginal foci and tumours. There was no
significant trend in the combined incidence across groups. There was a
significant positive trend in the incidence of pituitary adenomas in
females, but the number of rats with these lesions was within the
range in historical controls. In addition, none of the trends in
tumour incidence was significant when corrected for multiple
comparisons. The Committee concluded that neither the granular-cell
lesions of the uterus and vagina nor the pituitary adenomas were
indicative of a carcinogenic response to treatment with danofloxacin.
The genotoxic properties of danofloxacin were investigated in
vitro in assays for gene mutation in bacteria and in mammalian
cells, unscheduled DNA synthesis, and cytogenetics alterations in
mammalian cells and for cytogenetic effects in vivo. All of the
tests gave negative results, except for an assay of cytogenetic
alterations in human lymphocytes in vitro. The clastogenicity
observed in vitro was reduced or abolished by addition of magnesium
sulfate to the culture medium and/or washing the cells after treatment
to remove danofloxacin, and appeared to be associated with the cation-
chelating properties of danofloxacin. There was no evidence of
clastogenicity in vivo. Desmethyldanofloxacin induced a significant
increase in unscheduled DNA synthesis in two independent assays in
primary rat hepatocytes in vitro; however, negative results were
obtained with desmethyldanofloxacin in an assay for unscheduled DNA
synthesis and in a test for micronucleus formation in vivo. Thus,
although desmethyldanofloxacin induced unscheduled DNA synthesis, this
genotoxic potential did not appear to be expressed in vivo.
In a two-generation study of reproductive toxicity, rats received
doses of 0, 25, 75, or 150 mg/kg bw per day of danofloxacin by gavage.
In the parental generation, maternal body-weight gain was reduced at
the highest dose and these dams had fewer implantation sites and
produced fewer viable pups. The effects were observed at lower doses
with subsequent matings. At the second mating of the first-generation
animals, the pregnancy rate was adversely affected in all treated
groups. No NOEL could be identified.
A three-generation study of reproductive toxicity was carried out
in which rats were given danofloxacin at doses of 0, 1, 2.5, 6.25, or
150 mg/kg bw per day by gavage. At 150 mg/kg bw per day, the number of
mated females and the pregnancy rate were reduced, the duration of
gestation was increased and the litter sizes and pup weights were
reduced; this group was terminated before the second mating of the
first generation. In this study, in which lower doses were used than
in the study described above, the NOEL for reproductive toxicity was
6.25 mg/kg bw per day.
No adverse effects were observed in a three-generation study of
reproductive toxicity in rats with desmethyldanofloxacin in which
doses of up to 6.25 mg/kg bw per day were administered by gavage.
In a study of developmental toxicity in mice, there was no
evidence of teratogenicity when danofloxacin was administered by
gavage at doses of up to 200 mg/kg bw per day on days 6-13 of
gestation. The dose of 200 mg/kg bw per day was toxic to the dams,
reducing body-weight gain, and was fetotoxic, producing a reduction in
mean fetal weight and an increased incidence of delayed ossification.
The study was compromised by the small numbers of gravid dams in all
groups, including the controls. The NOEL for both maternal and fetal
toxicity was 100 mg/kg bw per day.
Oral doses of 0, 50, 100, or 200 mg/kg bw per day were
administered to rats on days 6-15 of gestation in a study of
developmental toxicity. Maternal body-weight gain and food consumption
were reduced in animals at 100 or 200 mg/kg bw per day. At these
doses, the incidences of delayed ossification and dilatation of the
cerebral ventricles were significantly increased. The NOEL for both
maternal and fetal toxicity was 50 mg/kg bw per day.
In a study of developmental toxicity, oral doses of 0, 2.5, 7.5,
or 15 mg/kg bw per day danofloxacin were administered to rabbits on
days 6-20 of gestation. At a dose of 15 mg/kg bw per day, maternal
body-weight loss, reduced food consumption, and abortion were
observed. The NOEL for maternal toxicity was 7.5 mg/kg bw per day.
The minimum concentration of danofloxacin resulting in 50%
inhibition (MIC50) was determined for 64 isolates of the six
predominant genera of human intestinal anaerobic microflora
( Bacteroides, Fusobacterium, Clostridium, Eubacterium,
Bifidobacterium, and Peptostreptococcus). In addition, data were
provided for the facultative anaerobes Lactobacillus, Proteus, and
Escherichia coli. Although E. coli and Proteus were the most
sensitive organisms, the Committee agreed that they should not be
taken into account in the calculation of the MIC50 because they are
not predominant species in the human intestine. Instead, the Committee
derived the mean MIC50 from the data available on 32 strains of the
most sensitive relevant genera isolated from the human
gastrointestinal tract, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp. The mean MIC50
for these strains was 1 µg/ml. This figure was used in calculating the
upper limit of the ADI from the formula described on p. 12:
Upper limit 1 µg/ga × 220 g
=
of ADI 0.1b × 1c × 60 kg
= 37 µg/kg bw
a Mean MIC50 for the most sensitive relevant species in the
human intestine, in this case Eubacterium spp.,
Bifidobacterium spp., and Peptostreptococcus spp.
b Bioavailability was determined to be about 90%, based on a
study in pigs given an oral dose of 5 mg/kg bw. The finding that
danofloxacin is strongly bound to cattle faeces was considered by
the Committee to add confidence to use of this value.
c A safety factor of 1 was used because sufficient relevant
microbiological data were provided.
The metabolite desmethyldanofloxacin was one-quarter to one-half
as active as danofloxacin against the same isolates.
4. EVALUATION
The Committee noted that danofloxacin belongs to a group of
fluoroquinolones that is active against aerobic gram-negative bacteria
and that the main components of the flora in the human
gastrointestinal tract are largely unaffected by these compounds.
Therefore, the Committee decided to base the ADI on the toxicity of
danofloxacin and not on its effects on the intestinal flora. Moreover,
the toxicological end-point resulted in a lower ADI.
The Committee established an ADI of 0-20 µg/kg bw per day on the
basis of the NOEL of 2.4 mg/kg bw per day for arthropathy in a
three-month study in immature dogs and a safety factor of 100. The ADI
was rounded to one significant figure, as is the standard practice
(Annex 1, reference 91, section 2.7).
The Committee noted that the NOEL for arthropathy in studies with
desmethyldanofloxacin was 0.25 mg/kg bw per day. The studies of
pharmacokinetics and metabolism showed that dogs that received oral
doses of danofloxacin were also exposed systemically to the major
metabolite desmethyldanofloxacin. Therefore the Committee concluded
that it was not necessary to calculate a separate ADI for
desmethyldanofloxacin. The Committee agreed, however, that the
approximately 10-fold higher toxicity of the metabolite should be
taken into account when recommending MRLs, as consumers may be
directly exposed to desmethyldano-floxacin in liver.
5. REFERENCES
Amacher, D.E., Turner, G.N., Ellis, J.H., Holden, H.E. & Kluwe, W.M.
(1988) Danofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Mammalian cell gene mutation assays. Unscheduled DNA
synthesis assays. Unpublished studies Nos. 86-607-01 and 87-607-02
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Muehlbauer, P.A., Wahrenburg M.G. &
Kluwe, W.M. (1990) Danofloxacin. Genetic toxicology report. Addendum.
In vitro cytogenetics assays. In vivo cytogenetics assays.
Unpublished study No. 89-607-18 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Ellis, J.H. & Kluwe, W.M. (1991a)
Desmethyldanofloxacin. Genetic toxicology report. Microbial reverse
mutation assays. Unpublished study No. 90-576-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Amacher, D.E., Holden, H. E., Muehlbauer, P.A. & Kluwe, W.M. (1991b)
Genetic toxicology report. Unscheduled DNA synthesis assay.
Unpublished study No. 90-576-12 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Amacher, D.E., Holden, H.E., Zelljadt, I. & Kluwe, W.M. (1991c)
Desmethyldano-floxacin. Genetic toxicology report. In vivo
micronucleus assay. Unpublished study No. 90-576-10 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Beutler, N.J., Tinkel, J.H. & Knickerbocker, M. (1992) Delayed contact
hyper-sensitivity study in guinea pigs. Unpublished study No.
92-607-27 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Dubreuil, L. (1994) Antibacterial activity of a fluoroquinolone
against bacteria isolated from human gut flora: Danofloxacin and its
metabolite desmethyldano-floxacin (DMD). Unpublished study from
Microbiology Department, Faculty of Pharmacy, University of Lille,
France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Vernon, G.S., Mayne, J.T., Frame, G.M., Milisen, W.B.,
Estes, P.C. & Levinsky, H.V. (1988a) A one month oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-08 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Frame, G.M., Saunders,
W.J., Estes, P.C. & Levinsky, H.V. (1988b) Danofloxacin. One month
oral capsule study in beagle dogs. Unpublished study No. 87-607-07
from Pfizer Central Research, Groton, CT, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Santacroce, C.E., Mayne, J.T., Milisen, W.B., Estes,
P.C. & Levinsky, H.V. (1989a) Danofloxacin. Three months oral gavage
study with in utero exposure in Long-Evans rats. Unpublished study
No. 88-607-12 from Pfizer Central Research, Groton, CT, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Soler, G.N., Mayne, J.T., Walsh, A.H., Levinsky, H.V. &
Estes, P.C. (1989b) Danofloxacin. Three month oral capsule study in
beagle dogs. Unpublished study No. 88-607-14 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Santacroce, C.E., Carson, D.L., Son, D.L., Walsh, A.H.,
Levinsky, H.V. & Estes, P.C (1989c) Danofloxacin. Three month oral
capsule study in beagle dogs. Unpublished study No. 88-607-15 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Fisher, D.O., Hills-Perry, P.A., Rice, J., Coleman, G.L., Estes, P.C.
& Levinsky, H.V. (1990a) Danofloxacin. Three months oral gavage study
with in utero exposure in Long-Evans rats. Unpublished study No.
89-607-19 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Beierschmitt, W.P., Milisen, W.B., Estes, P.C. &
Levinsky, H.V. (1990b) A two-generation oral gavage study in
Long-Evans rats. Unpublished study No. 87-607-09 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Fisher, D.O., Powers, K.A., Beierschmitt, W.P. & Levinsky, H.V.
(1990c) An exploratory reproductive oral gavage study in Long-Evans
rats. Unpublished study No. 89-607-17 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Fisher, D.O., Milisen, W.B., Kluwe, W.M. & Ochoa, R. (1996) Two year
oncogenicity study with dietary administration in Long-Evans rats.
Unpublished study No. 93-607-31 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1990) Acute
hemodynamic effects of an intravenous infusion of CP-76,136-27 in the
open-chest anesthetized dog. Unpublished study No. PH 247-PZ-001-90
from Pharmakon Laboratories, Waverly, PA, USA. Submitted to WHO by
Pfizer Inc., Groton, CT, Groton, CT, USA.
Guzzie, P.J., Turner, G.N., Amacher, D.E. & Kluwe, W.M (1992) Genetic
toxicology report. Antimicrobial. Unpublished study No. 92-607-26 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991a) UDS assay in primary rat
hepatocytes. Unpublished study no. 12180-0-447R. Submitted to WHO by
Pfizer Inc., Groton, CT, USA.
Hazleton Laboratories America Inc. (1991b) In vivo/in vitro UDS
assay in primary rat hepatocytes. Unpublished study no. 12180-0-494.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Holden, H.E., Turner, G.N. & Ray, V.A. (1988) Genetic toxicology
report. Nalidixic acid analog. Unpublished study No. 86-576-01 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988a) Danofloxacin.
Maternal toxicity study in mice by the oral route. Unpublished study
No. 88028 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1988b) Danofloxacin.
Maternal toxicity study in rats by the oral route. Unpublished study
No. 88059 from Pfizer Central Research, Amboise Cedex, France.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989a) Danofloxacin.
Fetotoxicity study in mice by the oral route. Segment II. Unpublished
studies No. 88115 and 88116 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Kesseddjian, M.J., Stadler, J. & Paulus, G. (1989b) Danofloxacin.
Fetotoxicity study in rats by the oral route. Segment II. Unpublished
studies No. 88093 and 88094 from Pfizer Central Research, Amboise
Cedex, France. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lundeen, G.R., Macaione, G.M., Santacroce, E.M. & Rice, J.R. (1993)
Two week exploratory toleration study in New Zealand white rabbits.
Unpublished study No. 92-607-30 from Pfizer Central Research, Terre
Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc., USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1988) Danofloxacin.
Nonclinical total residue depletion study in broiler chickens.
Unpublished study No. 1515N-60-88-006 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989a) Danofloxacin.
Nonclinical total residue excretion study in cattle. Unpublished study
No. 1535N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989b) Danofloxacin. A
nonclinical total residue excretion study in swine. Unpublished study
No. 1525C-60-88-009 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1989c) Danofloxacin. A
nonclinical total residue depletion study in swine. Unpublished study
No. 1525N-60-88-008 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1990a) Danofloxacin. Plasma and lung
pharmacokinetics study in broiler chickens. Unpublished Study No.
1514C-60-90-002 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Horan, M.R. & Gummerus, J.A. (1990b) Danofloxacin. Nonclinical plasma
and lung pharmacokinetics study in cattle. Unpublished study No.
1534N-60-89-003 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R. &
Harran, L.P. (1991a) Danofloxacin. Determination of plasma
pharmacokinetics and bioavailability in cattle. Unpublished study No.
1532N-60-89-005 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ronfield, R.A., Rainier, R.H., Magonigle, R.A., Frame,
G.M., Risk, J.E., Mosher, F.R. & Harran, L.P. (1991b) Danofloxacin.
Nonclinical total residue excretion study in broiler chickens.
Unpublished study No. 1515N-60-88-007 from Pfizer Central Research,
Terre Haute, IN, and Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
USA.
Lynch, M.J., Ronfield, R.A., Magonigle, R.A., Risk, J.E., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991c) A plasma and lung pharmacokinetics
study in swine. Unpublished study No. 1524C-60-89-003 from Pfizer
Central Research, Terre Haute, IN, and Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Lynch, M.J., Ericson, J., Calcagni, A., Gummerus, J. & Nowakowski,
M.A. (1991d) The comparative metabolism of 3H-danofloxacin in
cattle, dog and rat species. Unpublished study No. CM-90-02 from
Pfizer Central Research, Terre Haute, IN, and Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Mann, D.D. & Frame, G.M (1992) Pharmacokinetic study of danofloxacin
in cattle and swine. Am. J. Vet. Res., 53, 1022-1026.
Reynolds, J.A., Saunders, W., Hatch, G.W. & Vessella, D.J. (1987a) 2
week oral gavage toleration study in Long-Evans rats. Unpublished
study No. 87-607-03 from Pfizer Central Research, Terre Haute, IN, and
Groton, CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Reynolds, J.A., Milisen, W.B. & Frame, G.M. (1987b) Two week oral
capsule toleration study in beagle dogs. Unpublished study No.
87-607-05 from Pfizer Central Research, Terre Haute, IN, and Groton,
CT. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Risk, J.E., Ronfeld, R.A., Magonigle, R.A., Lynch, M.J., Rice, J.R.,
Millas, W.J. & Harran, L.P. (1991) Danofloxacin. Plasma and lung
pharmacokinetics study in swine. Unpublished study No. 1524C-60-89-003
from Pfizer Central Research, Terre Haute, IN, and Groton, CT.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Stack, T.L., Knickerbocker, M. & Beutler, N.J.
(1988a) Danofloxacin. Acute oral and intravenous toxicity studies in
mice and rats. Unpublished study No. 87-607-10 from Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Nilson, A.L. Knickerbocker, M. & Beutler, N.J.
(1988b) Danofloxacin. Acute dermal and ocular irritation studies in
rabbits (FHSA testing). Unpublished study No. 88-607-11 from Pfizer
Central Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc.,
Groton, CT, USA.
Stadnicki S.W., Rinaldi, M.T.S., Knickerbocker, M. & Beutler, N.J.
(1989) Desmethyldanofloxacin. Acute oral and intravenous toxicity
studies in albino mice and Sprague-Dawley rats. Unpublished study No.
89-576-05 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, B.A., Lundeen, G.R., Rice, J., Roesler,
A.R., Estes, P.C. & Levinsky, H.V. (1990a) Desmethyldanofloxacin.
Three month gavage study in Long-Evans rats with in utero exposure.
Unpublished study No. 89-576-06 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Walsh,
A.H., Estes, P.C. & Levinsky, H.V. (1990b) Desmethyldanofloxacin.
Three months oral capsule study in beagle dogs. Unpublished study No.
89-576-03 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Engel, S.M., Blackwell, D.K., Rice, J.R., Estes, P.C.
& Levinsky, H.V. (1990c) Desmethyldanofloxacin. Three months oral
capsule study in beagle dogs. Unpublished study No. 89-576-07 from
Pfizer Central Research, Groton, CT, USA. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Stadnicki, S.W., Hallam, J.A., Ball, D.J., Pettersen, B.A., Levinsky,
H.V., Milisen, W.B. & Estes, P.C. (1990d) Danofloxacin. A
three-generation oral gavage study in Long-Evans rats. Unpublished
study No. 89-607-16 from Pfizer Central Research, Groton, CT, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Santacroce, E.M., Lundeen, G.R., Coleman, G.L.,
Estes, P.C. & Levinsky, H.V. (1991) Desmethyldanofloxacin. Three
generation gavage study in Long-Evans rats. Unpublished study No.
89-576-04 from Pfizer Central Research, Groton, CT, USA. Submitted to
WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Walsh, A.H. & Rice, J.R. (1994a) Three month feeding study in CD-1
mice. Unpublished study No. 92-607-29 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Cacciatore, A., Rovetti, C.C., Davenport, C.J.,
Chatman, L.A. & Rice, J.R. (1994b) One month feeding study in Fischer
rats. Unpublished study No. 91-607-23 from Pfizer Pfizer Central
Research, Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton,
CT, USA.
Stadnicki, S.W., Cacciatore, A., Davenport, C.J., Coleman, G.L. &
Rice, J.R (1994c) Three week feeding study in Long-Evans rats.
Unpublished study no. 92-607-25 from Pfizer Central Research, Groton,
CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Stadnicki, S.W., Boldt, B.K., Rovetti, C.C., Davenport, C.J., Milisen,
W.B. & Rice, J.R. (1994d) Three month feeding study in Long-Evans
rats. Unpublished study No. 92-607-28 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Takatsu, S., Walsh, A.H., Shirai, N., Iidaka, T, Fukazawa, H., Iijima,
M. & Tachibana, M. (1995) Two year oncogenicity study with dietary
administration in ICR mice. Unpublished study No. 93-76-31 from Pfizer
Central Research, Chita-gun, Aichi, Japan. Submitted to WHO by Pfizer
Inc., Groton, CT, USA.
Tassinari, M.S., Rizoli, A.M., Hills-Perry, P., Linhares, M. & Gupta,
U. (1995a) Maternal toxicity study by oral gavage in New Zealand white
rabbits. Unpublished study No. 93-607-33 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Tassinari, M.S., Laithwaite, H.T., Podurgiel, S.J. & Gupta, U. (1995b)
Danofloxacin. Reproductive study III (teratology) in New Zealand white
rabbits. Unpublished study No. 93-607-34 from Pfizer Central Research,
Groton, CT, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990a) Diuretic assay.
Unpublished study No. PH 209-PZ-001-90 from Pharmakon Laboratories,
Waverly, PA, USA. Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990b)
Neuropharmacological profile (NPP) in rats. Unpublished study No. PH
200A-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990c)
Gastrointestinal propulsion assay in male mice. Unpublished study No.
PH 239-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA.
Submitted to WHO by Pfizer Inc., Groton, CT, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1990d) Secretory
screen in pyloric ligated Shay rats. Unpublished study No. PH
252-PZ-001-90 from Pharmakon Laboratories, Waverly, PA, USA. Submitted
to WHO by Pfizer Inc., Groton, CT, USA.
