
TILMICOSIN
First draft prepared by
Dr G. Roberts
Commonwealth Department of Health and Family Services
Canberra, Australia
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Excretion
2.1.2 Biotransformation
2.2 Toxicological studies
2.2.1 Acute studies
2.2.2 Short-term toxicity studies
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.4 Reproductive toxicity studies
2.2.5 Special studies on embryotoxicity and
teratogenicity
2.2.6 Special studies on genotoxicity
2.2.7 Special study on the immune system
2.2.8 Special studies on microbiological activity
2.2.9 Special studies on pharmacology
2.3 Observations in humans
3. Comments
4. Evaluation
5. References
1. EXPLANATION
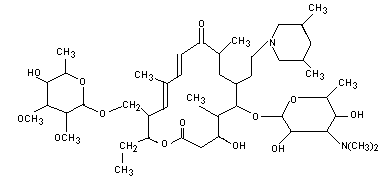
Tilmicosin is a macrolide antibiotic with the chemical name of
20-deoxo-20-(3,5-dimethyl piperidin-1-yl) desmycosin. It is
structurally similar to tylosin. Tilmicosin is a mixture of one cis
and two trans isomers in the approximate ratio 85:15.
Tilmicosin is available as an injectable formulation for the
treatment of respiratory diseases in cattle and sheep (10 mg/kg bw)
and as a feed premix for the treatment and control of respiratory
diseases in pigs (200 to 400 mg/kg in the feed). It had not been
previously evaluated by the Committee. The molecular structure of
tilmicosin is shown below.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Excretion
Pigs were administered a dose of 110 mg 14C-tilmicosin in the
diet over the course of one day. The recovery of radioactivity was 15%
in the urine and 80% in the faeces (Giera & Thomson, 1986).
In two studies, pigs were given a dose of 154 or 400 mg
14C-tilmicosin in the diet following a similar dose given for 5
days. The recovery of radioactivity was 4 to 6% in urine and 62 to 75%
in faeces. Radioactivity was detected in the bile but was not
quantified (Donoho & Thomson, 1988; Donoho et al., 1993).
2.1.2 Biotransformation
2.1.2.1 Rats
Tilmicosin, labelled with 14C in both the desmycosin macrolide
ring and the piperidine ring, was given orally to 15 male and 15
female Fischer-344 rats. The dosage was 20 mg/kg bw per day for 3
days. In the liver, radiolabel corresponded to tilmicosin and a
desmethyl derivative, T1(demethylated in the mycaminose ring). The
single radioactive substance identified in urine was unchanged
tilmicosin, while in faeces the major peak was parent compound with
lesser amounts of desmethyl tilmicosin and a high molecular weight
compound known to be present as an impurity in the administered
substance, T2 (consisting of two macrolide rings and one piperidine
ring) (Donoho, 1988).
Fischer-344 rats (10 males and 10 females) were given gavage
doses of 50 mg/kg bw per day 14C-tilmicosin for 5 days. An analysis
of faecal radioactivity for the presence of the sulfate metabolite
that was found in the faeces of pigs revealed the presence of a
similar compound, but quantification was not undertaken (Donoho &
Kennington, 1993).
2.1.2.2 Sheep
Beulah cross lambs were administered a single subcutaneous dose
of 20 mg/kg bw per day 14C-tilmicosin. The major radioactive
component in the liver, kidneys and urine was the parent drug,
together with lesser amounts of T1 and T2, and minor amounts of other
unidentified substances (Hawkins et al., 1993).
2.1.2.3 Pigs
Pigs were fed diets containing 14C-tilmicosin, which resulted
in daily doses of 400 mg, for 5 days. Of the radiolabel in liver and
kidney, approximately 60 to 70% was in the form of the parent drug and
there were small amounts of T1. Similarly in urine and faeces, there
were high levels of tilmicosin and low levels of T1. A further
metabolite was detected that comprised 14% of faecal and 25% of
urinary radioactivity and that was consistent with reduction of one
double bond in the macrolide ring followed by sulfation
(Donoho et al., 1993).
2.1.2.4 Cattle
In a summary of results obtained in cattle injected with
14C-tilmicosin, it was reported that the radioactivity profile in
the liver of treated rats was similar to that in the faeces. In
animals treated with a highly purified sample of tilmicosin,
metabolite T2 was not detected in the liver, suggesting that its
presence was a result of direct administration as a component of the
drug substance. Radioactivity in the kidneys was essentially in the
form of unchanged tilmicosin (Donoho, 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Major signs of toxicity in mice and rats were leg weakness,
hypoactivity, lethargy, ataxia, poor grooming and coma. Monkeys given
20 mg/kg vomited during the first day but were subsequently normal.
The single monkey given 30 mg/kg vomited, exhibited hypoactivity,
laboured respiration, vocalization and ataxia, and died within 2
hours.
New Zealand white rabbits (five males and five females) received
5000 mg/kg bw tilmicosin onto clipped skin for a period of 24 hours,
under a non-occlusive dressing. There were no deaths and no signs of
skin irritation (Jordan et al., 1987).
A volume of 0.1 ml (17 mg) of tilmicosin was instilled into one
eye of three male and three female New Zealand White rabbits.
Conjunctival hyperaemia and chemosis were noted for several days,
clearing by the end of one week (Jordan et al., 1987).
Hartley albino guinea-pigs (6 to 12 females) were given 10
intracutaneous induction doses of tilmicosin (0.05 to 0.1 ml of a
50 mg/ml solution). Fourteen days after the induction phase each
animal was injected intracutaneously with 0.05 ml tilmicosin. This
challenge dose did not result in an enhancement of skin reactions,
suggesting that a skin sensitization response had not been elicited
(Jordan et al., 1989b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Fischer-344 rats were given
gavage doses of 0, 50, 175 or 600 mg/kg bw per day tilmicosin
(purity 88%) in an aqueous vehicle. Drug administration was continued
for 2 weeks, during which there was no mortality or clinical signs of
toxicity. Food consumption was lower in males and females at 600 mg/kg
and body weight gain was depressed in males throughout the study and
in females during the first few days only.
Table 1. Results of acute toxicity studies with tilmicosin
Species Route Vehicle Sex LD503 Reference
(strain) (mg/kg bw)
Mouse sc aqueous M 97 Jordan
(ICR) F 109 et al., 1986a
Rat1 oral aqueous M 850 Jordan
(Sprague- F 800 et al., 1986b
Dawley)
Rat2 oral aqueous M & F > 2000 Piroozi
(Fischer-344) et al., 1993
Rat sc aqueous M 185 Jordan
(Fischer-344) F 440 et al., 1986c
Rat inhalation aerosol M & F > 494 (0/20) Jordan
(Fischer-344) < 4053 (14/20) et al., 1987
Rabbit dermal - M & F > 5000 Jordan
(NZ White) et al., 1987
Monkey im aqueous ? > 20 (0/2) Jordan &
(Rhesus) 30 (1/1) Shoufler, 1990
Sheep sc aqueous ? > 150 (0/5) Cochrane &
iv aqueous ? > 7.5 (1/5) Thomson, 1990
1 Animals were fasted
2 Animals were non-fasted
3 Figures in brackets represent the incidence of deaths
In the 600 mg/kg bw per day groups, there were increases in
haematocrit owing to an increase in corpuscular volume and in serum
levels of alanine aminotransferase and hepatic p-nitroanisole
O-demethylase activity. Thrombocyte counts were slightly decreased
at this dose. Urinalysis was unaffected. At the highest dose, there
were slight decreases in the weights of kidneys, spleen and ovaries,
increased adrenal weight and multifocal inflammation in the liver of
some animals. All treated animals exhibited caecal distension, which
is a typical response to high doses of an antibiotic compound
(Jordan, 1986).
Groups of 20 male and 20 female Sprague Dawley (Crl:CD) rats
received 0, 50, 250 or 1000 mg/kg bw per day tilmicosin (purity 88%)
in an aqueous vehicle, by gavage for 3 months. Signs of overt toxicity
were noted at 1000 mg/kg and included thinness, ventral soiling,
chromorhinorrhea, chromodacryorrhea, alopecia and poor grooming.
Mortality was increased at this highest dose and in females given
250 mg/kg bw per day.
Although food consumption was depressed in the 1000 mg/kg bw per
day group (males only), body weight gain was lower in males and
females at 1000 mg/kg bw per day and females at 250 mg/kg bw per day.
At 1000 mg/kg bw per day, serum alanine aminotransferase was increased
in males, blood urea nitrogen (BUN) was increased in males and
females, urinary pH was slightly lower in females and the presence of
occult blood was greater in males and females. There were no effects
on ophthalmology, haematology or hepatic p-nitroanisole
O-demethylase activity.
The weights of kidneys, liver and heart were increased in females
at 1000 mg/kg bw per day and adrenal weight was increased at 250 mg/kg
bw per day (females only) and 1000 mg/kg bw per day (males and
females). Gross pathology revealed caecal enlargement or distension at
250 mg/kg bw per day or more and small spleens in a few rats at
1000 mg/kg bw per day. Slight nephrosis was noted in two males in each
of the 250 and 1000 mg/kg bw per day groups. Other pathological
alterations were seen only in males and females of the 1000 mg/kg bw
per day group, i.e. hypertrophy of the zona fasciculata of the adrenal
cortex in most rats, necrosis of individual skeletal muscle fibres in
some rats; myocardial degeneration was increased and lymphoid
depletion was evident in the spleen and thymus of some animals. The
NOEL was 50 mg/kg bw per day (Jordan, 1988).
2.2.2.2 Dogs
Beagle dogs (two males and two females per group) were exposed by
inhalation to aerosols of 0, 12, 47 or 251 mg/m3 tilmicosin
(purity 83%). The treatment was administered for 4 hours per day on 12
days out of a 16-day period (excluding weekends). Particle median
equivalent aerodynamic diameters were between 1.2 and 1.5 µm. The mean
serum concentrations of tilmicosin were 0.23 to 0.33 and 1.67 to
2.46 µg/ml at 47 and 251 mg/m3 respectively. Drug levels at
12 mg/m3 were below the detection limit of 0.1 µg/ml.
One male given 251 mg/m3 died on the final day of exposure but
no other overt signs of toxicity were observed. Heart rates were
increased in dogs at 251 mg/m3. Food intake, body weight,
haematology and blood chemistry were unaffected. At autopsy, lung
weight was increased in females given 251 mg/m3 and inflammation was
noted in the respiratory tract at 47 mg/m3 (females only) and
251 mg/m3 (males and females) (Jordan et al., 1991).
Groups of four male and four female beagle dogs were given 0, 6,
20 or 70 mg/kg bw per day tilmicosin (purity 88%) in capsules for 3
months. The daily dose was administered in two equal amounts, 6 hours
apart. Serum concentrations of tilmicosin were measured 3 hours and 24
hours after the first of the two daily doses in weeks 2, 3, 5, 9 and
13. At increasing doses, the mean concentrations were 0.1 to 0.2, 0.74
to 1.49 and 3.24 to 6.05 µg/ml at 3 hours and 0, 0.26 to 0.59 and 1.72
to 3.96 µg/ml at 24 hours.
Half the 70 mg/kg bw per day males and females died during the
first month. Clinical signs prior to death were pale mucous membranes
in two dogs and ataxia in one dog. Food intake, body weight, haemato-
logy and urinalysis were unaffected. Serum alanine aminotransferase
activity was progressively increased at 70 mg/kg bw per day, while
hepatic p-nitroanisole O-demethylase activity was increased
in females at this level.
Two of the four surviving dogs at 70 mg/kg bw per day showed
bilateral multifocal areas of subretinal fluid concentrated along
arterioles in the tapetal region of the eye. The changes were claimed
to be consistent with those associated with systemic hypertension. One
of these dogs also had retinal degeneration and the other showed
miosis with normal pupillary light responses.
Heart rate increases were dose related, being marked at 70 mg/kg
bw per day and moderate to severe at 20 mg/kg bw per day. The increase
in males given 6 mg/kg bw per day was not significant. At the highest
dose, examination of the electrocardiogram revealed depression of the
ST segment.
At 70 mg/kg bw per day, heart weight was increased in males and
females, and liver and kidney weights were increased in females. Gross
necropsy showed small spleens in the females that died during the
study and an enlarged heart in a surviving male at 70 mg/kg bw per
day. Slight diffuse mucosal oedema was seen in the gall bladders of
two dogs at 70 mg/kg bw per day. Despite the physiological changes in
the eyes and heart, there were no associated pathological alterations.
The NOEL was 6 mg/kg bw per day (Jordan, 1987).
Groups of four male and four female beagle dogs were given 0, 4,
12 or 36 mg/kg bw per day tilmicosin (purity 86%) in capsules for 12
months. The daily dose was administered in two equal amounts, 6 hours
apart. There were no deaths. Peripheral redness was seen sporadically
in some animals of all groups, in particular at 12 and 36 mg/kg bw per
day.
Body weight gains were lower at > 12 mg/kg bw per day. There
were no effects on ophthalmology, haematology, blood chemistry and
urinalysis. Heart rates were markedly increased at 36 mg/kg bw per day
with sporadic depression of the ST segment in some dogs.
Heart weight was increased at 36 mg/kg bw per day and four males
and one female at this dose showed enlarged hearts. Mild chronic
dermatitis was noted in the external ears of dogs from all treated
groups. The findings included minimal thickening of the epidermis,
foci of acantholysis and inflammatory cell infiltration in the dermis.
These changes were only slight and non-dose-related, and therefore of
questionable relationship to treatment. The NOEL was 4 mg/kg bw per
day (Jordan & Bernhard, 1989).
2.2.3 Long-term toxicity/carcinogenicity studies
Tilmicosin has not been tested in toxicological studies longer
than 12 months in duration and hence the carcinogenic potential of
this drug has not been directly determined. There are a number of
observations which are relevant in assessing the carcinogenicity of
tilmicosin:
a) The results of available toxicological studies with tilmicosin
have not identified lesions or proliferative changes which could
be considered suggestive of potential neoplasia.
b) Tilmicosin has been tested in a wide range of genotoxicity
assays. All results were negative and it was concluded that the
compound has no genotoxic activity.
c) Tilmicosin is a macrolide antibiotic. This class of chemical has
been in widespread usage in humans for many years but there is no
evidence of carcinogenicity. Tylosin is the closest structural
analogue and this chemical was reviewed by the thirty-eighth
meeting of JECFA (Annex 1, reference 97).
2.2.3.1 Reconsideration of tylosin tumorigenicity
Tylosin was tested in 2-year feeding studies in rats. The
findings revealed an association between drug administration at doses
of 150 and 300 mg/kg bw per day in the diet and an increased incidence
of pituitary adenomas in males. While it was claimed that the increase
in the incidence of tumours was an indirect result of the ability of
tylosin to increase survival and weight gain, supporting data were not
available to the Committee and an ADI was not established.
New information, not considered at the thirty-eighth meeting,
indicates that spontaneous pituitary tumours occur in male rats at
variable rates with increased rates occurring in animals with higher
body weights (Gries & Young, 1982). In the tylosin studies, 12-month
body weights were somewhat higher in treated males rats and mortality
was increased in control animals due to respiratory infection. The
highest observed incidence of tumours in tylosin-treated groups was
comparable to the upper limit of the historical control range (23%) in
the test facility.
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A dose-ranging study was carried out in groups of 10 female
Sprague-Dawley (Crl:COBS CD) rats given gavage doses of 0, 50, 125,
250, 500 or 750 mg/kg bw per day tilmicosin (purity unknown). The
females were treated from 14 days before mating with untreated males
until the end of study on post-partum day 4.
Excess salivation was dose-related at > 250 mg/kg bw per day.
Chromodacryorrhea, urine-stained fur and alopecia were seen in some
rats at 750 mg/kg bw per day; the three most severely affected died
during the study. Prior to death, these animals exhibited reduced food
intake, lost weight and were emaciated. Body weight gain was depressed
in surviving rats at 750 mg/kg bw per day during the pre-mating period
while food intake was reduced at > 250 mg/kg bw per day in week 1
and at 750 mg/kg bw per day in week 2. The pregnancy rate was
decreased at > 500 mg/kg bw per day. Duration of gestation, litter
size and weight, pup survival and weight gain to post-partum day 4
were unaffected (Dearlove et al., 1987).
Groups of 30 male and 30 female Sprague Dawley (Crl: COBS CD BR)
rats were administered 0, 10, 45 or 200 mg/kg bw per day tilmicosin
(purity 87%) by gavage in an aqueous vehicle. Treatment commenced 70
days (males) and 14 days (females) before the first mating period and
was continued through two litters per generation for two generations.
F1 litters were culled to five pups per sex on post-partum day 4 and
F1b pups (40 males and 40 females per group) were bred to produce
the following generation. F2a and F2b pups were killed on
post-partum day 4.
In adult animals, salivation was noted in males and females at
200 mg/kg bw per day, but there were no other signs of toxicity. Body
weight gain and food consumption were depressed in females of the 45
and 200 mg/kg bw per day groups during the pre-mating period only.
During the production of the four litters in this study there were no
effects on mating performance, pregnancy rates, duration of gestation,
litter size and weight and the weight gain of offspring. In both F1
litters at 200 mg/kg bw per day, pup mortality was slightly increased
up to post-partum day 4, but the finding was not duplicated in either
F2 litter. The NOEL was 10 mg/kg bw per day in adult rats (Christian
& Hoberman, 1989).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 25 presumed pregnant female Sprague Dawley (Crl: CD)
rats were administered gavage doses of 0, 10, 70 or 500 mg/kg bw per
day tilmicosin (purity 86%) in an aqueous vehicle. The dams were
treated on gestation days 6 to 15 and were killed on gestation day 20.
Increased salivation was seen at 70 and 500 mg/kg bw per day and
alopecia at 500 mg/kg bw per day. There were no deaths or abortions.
Body weight gain was reduced at 70 and 500 mg/kg bw per day, and food
intake was reduced at 500 mg/kg bw per day, during gestation days 6 to
10.
The number of resorption or live fetuses, fetal weight, sex ratio
and the incidence of fetal malformations were similar between groups.
The incidences of total skeletal and visceral anomalies were increased
in treated groups, but there was no dose-response relationship and the
findings were within the historical control values for the laboratory.
The NOEL for maternotoxicity was 10 mg/kg bw per day (Jordan & Higdon,
1988).
2.2.5.2 Rabbits
Groups of 15 presumed pregnant female Japanese White-NIBS rabbits
were given gavage doses of 0, 8, 19 or 48 mg/kg bw per day tilmicosin
(purity unknown) in an aqueous vehicle. Does were treated on gestation
days 6 to 18 and were killed on gestation day 28. One female given
48 mg/kg bw per day aborted on gestation day 26 and died. Reduced
faeces were seen at 19 and 48 mg/kg bw per day with only a transient
effect at 8 mg/kg bw per day. During the treatment period food intake
was depressed in a dose-related manner and body weight loss was
observed at 19 and 48 mg/kg bw per day.
Fetal and placental weight tended to decrease at 19 and 48 mg/kg
bw per day but did not achieve statistical significance. There were no
meaningful effects on the incidence of resorptions or fetal deaths or
on sex ratio. Open eyelids were observed in 11/91 and 16/68 fetuses
from the 19 and 48 mg/kg bw per day groups, respectively, and some of
these fetuses showed cleft palate or club foot. The affected fetuses
had low body weights and the seven litters involved were derived from
does that had lost body weight during drug administration. Skeletal
examination revealed retardation of fetal development at 19 and
48 mg/kg bw per day. Similar effects have been seen in this laboratory
in dietary restricted rabbits, suggesting the effects on the fetus
were secondary to maternal malnutrition (Noda, 1993).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on tilmicosin
Test system1 Test object Concentration Results Reference
Reverse S. typhimurium 1 - 100 µg/plate negative Jordan
mutation2 TA98, TA100, (± S9) et al., 1986f
TA1535, TA1537,
TA1538
Reverse E. coli 1 - 100 µg/plate negative Garriott
mutation2 WP2uvrA (± S9) et al., 1993
Forward L5178Y mouse 100-900 µg/ml negative Jordan
mutation2 lymphoma cells (- S9) et al., 1986e
200-1000 µg/ml
(+ S9)
Forward HGPRT+ Chinese 25-250 µg/ml negative Jordan
mutation2 Hamster ovary (- S9) et al., 1989c
cells 50-300/ µg/ml
(+ S9)
Unscheduled primary cultures 0.5-10 µg/ml negative Jordan
DNA synthesis of rat hepatocytes et al., 1985
assay
Sister chromatid Chinese hamster 200-1800 × 1 negative Jordan et al.,
exchange bone marrow mg/kg bw oral 1986d, 1989a
assay
Chromosome rat bone marrow 180-1800 × 1 and negative Jordan &
aberrations 100-1000 × 5, Ivett, 1989
mg/kg bw per
day oral
1 Positive controls used
2 Both with and without liver microsomal activation
2.2.7 Special study on the immune system
Groups of eight male CD-1 mice were given gavage doses of 0,
10, 250, 500 or 1000 mg/kg bw per day tilmicosin (purity 87%) in
an aqueous vehicle. Drug treatment was continued for 10 days.
After the third dose mice were injected intravenously with sheep
red blood cells. At the end of the study, serum from each mouse
was assayed for antibodies (haemagglutinin). There was one death
at 500 mg/kg bw per day and six at 1000 mg/kg bw per day, but
antibody production was not affected at any level (McGrath
et al., 1988a).
2.2.8 Special studies on microbiological activity
2.2.8.1 In vitro
The antibacterial activity of tilmicosin was determined
against a range of organisms representative of human intestinal
flora. Cultures were initiated using inoculum sizes of between
3 × 105 and 1.6 × 106 cells per spot on blood agar plates and
incubated anaerobically at 37°C.
The median MIC values for the clinical isolates of
Bifidobacterium and Peptostreptococcus, respectively, were
0.015 and 0.5 µg/ml. The above results were obtained at pH 7.7.
At pH 6.6 the MIC values were one to two orders of magnitude
higher (Scott et al., 1993).
The antibacterial activity of tilmicosin was examined
against a number of organisms used in industrial food processing
and originally obtained from dairy products. Cultures were
initiated in blood agar plates using three strains of
Propionibacterium and four strains of Lactobacillus. Under
anaerobic conditions the MICs were between 8 and 64 µg/ml and
under microaerophilic conditions the values were between 4 and
32 µg/ml (McLaren, 1994).
2.2.8.2 Rats
In two separate studies, female germ-free rats were orally
dosed with 1 ml of 10% (w/v) pooled human faecal suspension.
Three weeks later groups of two males and two females were given
gavage doses of 0, 0.12 or 0.4 mg/kg bw per day tilmicosin
(purity 87%) for 5 days. Rat faecal samples were collected before
treatment, daily during treatment and once during each of the 2
weeks following cessation of dosing. Body weight gain remained
unaffected.
The animals in each study received faecal suspensions
obtained from different donors. Results from different studies
varied widely, possibly reflecting differences between the
bacterial populations used. Total anaerobes were not reduced by
tilmicosin treatment. Total enterobacterial counts and the
proportion of enterobacteria with respect to total anaerobe
counts were transiently increased at 0.4 mg/kg bw per day in one
study only. Tilmicosin-resistant enterobacteria were not
significantly increased in either study. Spiramycin, at a dose of
0.5 mg/kg bw per day, increased total enterobacterial counts and
the number of spiramycin-resistant enterobacteria; the latter
appeared to remain high after the cessation of treatment. The
NOEL for tilmicosin was 0.4 mg/kg bw per day (Rumney, 1993).
2.2.9 Special studies on pharmacology
Table 3. Results of pharmacological assays with tilmicosin
Test system Doses Results Reference
Isolated 0.0009 to No effect on non-stimulated organ. Williams
guinea-pig 90 µg/ml 90 µg/ml slightly inhibited acetylcholine- et al.,
ileum and angiotensin-induced contraction 1988
and significantly inhibited electrically
stimulated contraction
Isolated 0.0009 to No effect on non-stimulated tissue and Williams
rat uterus 90 µg/ml no change to oxytocin-induced et al.,
(estrogen contractions 1988
primed)
Isolated rat 0.0009 to No effect on non-stimulated tissue and Williams
vas deferens 90 µg/ml no change to noradrenaline-induced et al.,
contractions 1988
Isolated 0.0009 to In spontaneously beating tissue, > 90 Williams
guinea-pig 900 µg/ml µg/ml decreased force of contraction; et al.,
atria and 900 µg/ml increased rate of 1988;
contraction. 90 µg/ml inhibited Jordan
isoprenaline-, noradrenaline- and Bay et al.,
K8644 (calcium agonist)-induced force 1990
and rate of contraction, and
histamine-induced rate of contraction.
Table 3. Results of pharmacological assays with tilmicosin (cont'd).
Test system Doses Results Reference
Conscious 0.25, 1, 2.5 Heart rate increased at > 1 mg/kg. Left Jordan &
dogs or 5 mg/kg ventricular function decreased at > 0.25 Sarazan,
iv mg/kg. Aortic pulse pressure decreased 1991;
at > 1 mg/kg. ECG revealed "Ventricular Sarazan
Alternans" (ventricular contraction fails et al.,
to achieve adequate pressure to open 1993
aortic valve and cause an arterial pulse).
Anaesthetised 0.5, 1 or 5 Heart rate increased, stroke volume, Jordan
dogs mg/kg iv stroke work index and cardiac output et al.,
decreased at > 1 mg/kg. At 5 mg/kg 2/3 1988
dogs developed second degree heart block
CD-1 mice 10, 100, Piloerection and grasping loss at > 500 Jordan
500 or mg/kg. Ptosis at 1000 mg/kg. No effect et al.,
1000 mg/kg on electroshock- or pentylenetetrazol- 1989d
oral induced seizures or on body temperature.
Hexobarbital-induced sleep time
prolonged by 500 (x1) and 1000 (x3) mg/kg
Sprague- 10, 30, 90 Urine volume and creatinine reduced McGrath
Dawley or 270 at 270 mg/kg with increases in osmolality et al.,
rats mg/kg oral and electrolytes. 1988b
2.3 Observations in humans
A total of 241 human exposures to tilmicosin (Micotil) were
reported to the Rocky Mountain Poison Center from May 1992 to May
1993. Needlesticks and scrapes (n=112) and accidental injection
(n=43) caused either no effect or local reactions including
soreness, numbness, stinging, swelling, redness, burning and
stiffness. Some injected subjects experienced anxiety, sweating,
headache and lightheadedness. Skin exposures (n=50) resulted in
redness and tingling of the skin, and eye exposures (n=11)
resulted in stinging and swelling. Persons accidentally ingesting
the drug (n=39) reported either no symptoms or bitter taste,
nausea, numbness of lips and tongue, vomiting, thirst and
headache (Montanio & Dart, 1993).
Over a 30-month period, 36 cases of accidental exposure to
tilmicosin (Micotil) were reported to the Ontario Regional Poison
Information Centre. Percutaneous injection (n=26) always resulted
in pain at the site and seven subjects mentioned a variety of
local reactions consistent with an irritant action. One person
showed "peaked T waves" 30 minutes following the injection of
1 ml into his arm, but ECG changes were not noted in other
subjects. The remaining subjects received splashes into the mouth
or eyes or onto the skin. Unpleasant taste, a burning sensation
on the hard palate and ocular irritation were each recorded in
one person (McGuigan, 1994).
3. COMMENTS
The Committee considered toxicological data on tilmicosin,
including the results of studies on acute and short-term
toxicity, pharmacokinetics, metabolism, reproductive toxicity,
teratogenicity, genotoxicity, antimicrobial activity and
pharmacology. The Committee also considered observations in
humans accidentally exposed to tilmicosin.
Tilmicosin is absorbed from the gastrointestinal tract as
shown by the recovery of radiolabelled compound in urine and/or
bile of orally dosed pigs (110 to 400 mg per animal), and the
presence of dose-related serum concentrations of tilmicosin dosed
orally in dogs for 3 months. However, the results from available
studies did not allow any conclusion to be reached concerning the
extent of absorption. The metabolites detected in a range of farm
animals were also found in rats, which suggests that the rat is a
suitable model for determining the potential toxicological
hazards posed by tilmicosin.
The LD50 in fasted rats was 800 to 850 mg/kg bw, but
toxicity was substantially lower in non-fasted animals, among
which there were no deaths following administration of a single
dose of 2000 mg tilmicosin/kg bw. Studies in a range of
pharmacological models identified depression of cardiac muscle
contractility and reduced heart function as the main adverse
effects.
Rats were administered oral doses of 50, 250 or 1000 mg/kg
bw per day for 3 months. At 1000 mg/kg bw per day, animals
exhibited chromorhinorrhea, chromodacryorrhea, alopecia, poor
grooming, reduced food consumption and they appeared thin.
Body-weight gain was reduced and mortality was increased in
females at 250 mg/kg bw per day and in both sexes at 1000 mg/kg
bw per day. Toxic effects on the kidney and liver were indicated
by increased organ weights, increased serum levels of alanine
aminotransferase and blood urea nitrogen, and the presence of
blood in the urine. However, pathological examination of these
organs showed only slight nephrosis in two males in each of the
groups given 250 or 1000 mg/kg bw per day. At the highest dose,
hypertrophy of the zona fasciculata of the adrenal cortex was
noted in most rats, and myocardial degeneration, necrosis of
individual skeletal muscle fibres and lymphoid depletion in the
spleen and thymus were seen in some rats. The NOEL was 50 mg/kg
bw per day.
Dogs were given oral doses of 6, 20 or 70 mg/kg bw per day
for three months. Half of the animals given 70 mg/kg bw per day
died during the first month. Heart rate was increased at 20 and
70 mg/kg bw per day and at the higher dose there was depression
of the ST segment of the electrocardiogram. Ophthalmoscopic
examination of the eyes showed bilateral areas of subretinal
fluid concentrated along arterioles in the tapetal region of two
of the four surviving dogs at 70 mg/kg bw per day. The NOEL was
6 mg/kg bw per day.
Dogs were given oral doses of 4, 12 or 36 mg/kg bw per day
for twelve months. Body weight gain was depressed at 12 and
36 mg/kg bw per day. Heart rate was markedly increased at 36
mg/kg bw per day, with occasional depression of the ST segment
of the electrocardiogram. Hearts were enlarged at 36 mg/kg bw per
day, but there were no associated pathological changes. The NOEL
was 4 mg/kg bw per day.
In a two-generation reproductive toxicity study, rats were
administered oral doses of 10, 45 or 200 mg tilmicosin/kg bw per
day. Body weight gain and food consumption were depressed in
females given 45 or 200 mg/kg bw per day, but only during the
pre-mating period. Fertility and reproduction parameters were
unaffected at any dose level. At 200 mg/kg bw per day, mortality
in pups was slightly increased in both first-generation litters
up to post-partum day 4, but not in either second-generation
litter. The NOEL was 10 mg/kg bw per day.
Developmental toxicity studies were reported in rats and
rabbits. Rats were dosed with 10, 70 or 500 mg tilmicosin/kg bw
per day by gavage. Growth and development in rat fetuses were not
affected by treatment, but increased salivation and reduced body
weight gain of dams were observed at 70 and 500 mg/kg bw per day.
The NOEL for maternal toxicity in rats was 10 mg/kg bw per day.
In the study in rabbits, there were dose-related signs of
toxicity in the does at all doses (8, 19 and 48 mg/kg bw per
day). Severely affected members of the groups given 19 or
48 mg/kg bw per day produced offspring that had open eyelids and
low incidences of cleft palate or club foot. These fetuses had
low body weights and were clearly retarded in development. The
effects were attributed to treatment-related malnutrition in
maternal animals, which is commonly observed in rabbits dosed
with antibiotics, confirming that this species is an inappropriate
model for studying the teratogenic potential of antibiotics.
Tilmicosin has been tested for reverse mutation in bacteria,
forward mutation in cultured mammalian cells, unscheduled DNA
synthesis in primary cultures of rat hepatocytes, and in in vivo
cytogenetic assays. All results were negative, and the Committee
concluded that tilmicosin has no genotoxic potential.
The carcinogenic potential of tilmicosin has not been
tested. However, a number of factors are relevant in assessing
the carcinogenicity of this drug. Toxicity studies with
tilmicosin have not resulted in lesions or proliferative changes
suggestive of neoplasia, and tilmicosin was uniformly negative in
a wide range of genotoxicity assays. Tilmicosin is a macrolide
antibiotic, and this class of chemicals is not known to induce
neoplasia despite widespread use in humans over many years. The
closest structural analogue, tylosin, was reviewed at the
thirty-eighth meeting of the Committee (Annex 1, reference 97).
In a 2-year feeding study in rats, tylosin administration was
associated with an increased incidence of pituitary adenomas in
male animals when compared to concurrent controls. New evidence,
not considered at the thirty-eighth meeting, indicates that
spontaneous pituitary neoplasms occur in male rats at variable
rates. Additionally, increased rates occur in animals with higher
body weights. In tylosin-treated groups of male rats, the
12-month body weights were somewhat higher than in the control
groups and the incidences of pituitary adenomas were comparable
to the upper limit of the historical control range. The lower
incidence of pituitary neoplasms in the control animals may have
been due to the earlier mortality in this group caused by
respiratory disease. In light of this information the Committee
considered that the concerns of the thirty-eighth meeting over
the potential tumorigenicity of tylosin had been satisfactorily
addressed and that there was no concern for the carcinogenic
potential of tylosin. For the above reasons, the Committee
considered that carcinogenicity studies would not be required for
tilmicosin.
In assessing the microbiological activity of tilmicosin, the
results of a study using germ-free rats colonized with human
intestinal microflora was considered the most relevant. At the
highest dose administered (0.4 mg/kg bw per day for 5 days) there
were no significant alterations in numbers of total anaerobes or
enterobacteria in rat faeces.
Reports of accidental human exposure to tilmicosin have
identified local reactions indicative of an irritant action
following dermal and ocular exposure. Humans accidentally
injected with tilmicosin have reported anxiety, sweating,
headache and lightheadedness. Changes in the electrocardiogram
pattern were observed in only one person. Contact with the buccal
mucosa or ingestion has resulted in a range of symptoms including
nausea, vomiting, thirst, numbness or burning sensation in the
mouth, and headache.
4. EVALUATION
The NOEL from toxicological studies was 4 mg/kg bw per day
in a 12-month study in dogs. Treatment of rats colonized with
human intestinal microflora with 0.4 mg tilmicosin/kg bw per day
produced no significant microbiological effect. The Committee
established an ADI of 0-40 µg/kg bw, based on the toxicological
NOEL of 4 mg/kg bw per day and a safety factor of 100. An
identical ADI would have been established using the data from the
study on rats colonized with human intestinal microflora and a
safety factor of 10 to account for variability among humans.
5. REFERENCE
Christian, M.S. & Hoberman, A.M. (1989). Reproductive effects of
EL-870 administered orally via gavage to Crl:COBS CD(SD) BR rats
for two generations, with two litters per generation. Unpublished
study No. 112-001 from Argus Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Cochrane, R.L. & Thomson, T.D. (1990). Toxicology and pharmacology
of tilmicosin following administration of subcutaneous and intravenous
injections to sheep. Unpublished study No. T5C768908 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Dearlove, G.H., Hoberman, A.M., & Christian, M.S. (1987). Dosage-range
study of EL-870 administered orally via gavage to Crl:COBS CD(SD) BR
rats (Pilot study). Unpublished study No. 112-001P from Argus Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. (1988). Comparative metabolism of 14C-tilmicosin
in cattle and rats. Unpublished study No. ABC-0395 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. & Kennington, A.S. (1993). Tilmicosin metabolite
study with rat excreta. Unpublished study No. T5C759302 from
Lilly Research Laboratories. Submitted to WHO by Lilly, Basingstoke,
UK.
Donoho, A.L. & Thomson, T.D. (1988). 14C-Tilmicosin balance-
excretion study in swine. Unpublished study No. ABC-0409 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L., Darby, J.M., Helton, S.L., Sweeney, D.J.,
Occolowitz, J.L., & Dorman, D.E. (1993). Tilmicosin metabolism
study in tissues and excreta of pigs fed 400 ppm 14C-tilmicosin.
Unpublished study No. T5C759201 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Garriott, M.L., Rexroat, M.A., & Jordan, W.H. (1993). The effect
of tilmicosin on the induction of reverse mutations in
Escherichia coli using the Ames test. Unpublished study
No. 920825AMS2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Giera, D.D. & Thomson, T.D. (1986). Preliminary 14C EL-870
balance excretion and tissue residue in swine. Unpublished study
No. ABC-0305 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Gries, C.L. & Young, S.S. (1982). Positive correlation of body
weight with pituitary tumor incidence in rats. Fundam. Appl.
Toxicol., 2, 145-148.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A,. &
Cameron, D.M. (1993). The metabolism and residues of 14C-tilm-
icosin following subcutaneous administration to sheep. Unpublished
study No. HRC/LLY 36/930447 from Huntingdon Research Centre. Submitted
to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1986). The toxicity of compound 177370 (EL-870)
given by oral gavage to Fischer 344 rats for two weeks.
Unpublished study No. RI5585 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1987). The toxicity of EL-870 given orally to
Beagle dogs for three months. Unpublished study No. DO8286 from
Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. (1988). The toxicity of tilmicosin given orally to
Crl:CD(SD) rats for three months. Unpublished study No. RO9886
from Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. & Bernhard, N.R. (1989). A one year chronic toxicity
study in Beagle dogs given oral doses of tilmicosin. Unpublished
study No. DO7187 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Higdon, G.L. (1988). A teratology study of
tilmicosin (EL-870, compound 177370) administered orally to CD
rats. Unpublished study No. RI3387 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Ivett, J.L. (1989). Mutagenicity test on
tilmicosin in the rat bone marrow cytogenetic assay. Unpublished
study No. 10646-0-452 from Hazleton Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Sarazan, R.D. (1991). An acute study of the
cardiovascular effects of intravenous Micotil 300 in conscious
dogs. Unpublished study No. DV0890 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Shoufler, J.R. (1990). The acute toxicity of
Micotil 300 administered intramuscularly to Rhesus monkeys.
Unpublished studies Nos. PO4089 and PO7689 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Hill, L.E., & Probst, G.S. (1985). The effect of
compound 177370 (EL-870) on the induction of DNA repair synthesis
in primary cultures of adult rat hepatocytes. Unpublished studies
Nos. 851008UDS2449 and 851015UDS2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986a). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the ICR mouse. Unpublished studies
Nos. M-C-41-85 and M-C-40-85 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986b). The acute toxicity of compound 177370 (EL-870)
administered orally to the Sprague-Dawley rat. Unpublished
studies Nos. R-O-40-86 and R-O-39-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986c). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the Fischer 344 rat. Unpublished
studies Nos. R-C-02-86 and R-C-01-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Neal, S.B., & Probst, G.S. (1986d). The effect of
compound 177370 (EL-870) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters.
Unpublished study No. 851014SCE2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Oberly, T.J., Bewsey, B.J., & Probst, G.S. (1986e).
The effect of compound 177370 (EL-870) on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished studies Nos. 851106MLA2449 and
851113MLA2449 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Jordan, W.H., Rexroat, M.A., & Probst,G.S. (1986f). The effect of
compound 177370 (EL-870) on the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished studies
Nos. 850930AMS2449, 851028AMS2449 and 851111AMS2449 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Markey, T.F., Oakley, L.M., & Torrence, T.L.
(1987). The acute dermal and inhalation toxicity and ocular and
dermal irritation of tilmicosin technical. Unpublished studies
Nos. B-D-45-86, B-E-39-86 and R-H-037-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Turk, J.A. (1988). Cardiovascular
effects of tilmicosin administered intravenously in anaesthetised
beagle dogs. Unpublished study No. PC8806 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Brunny, J.D., & Garriott, M.L. (1989a). The effect
of tilmicosin on the in vivo induction of sister chromatid
exchange in bone marrow of Chinese hamsters. Unpublished study
No. 881114SCE2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Gardner, J.B., & Weaver, D.E. (1989b). An
intracutaneous sensitisation study in albino guinea pigs with
tilmicosin. Unpublished study No. GO1888 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Richardson, K.K., & Oberly, T.J. (1989c). The
effect of tilmicosin on the induction of forward mutation at the
HGPRT+ locus of Chinese hamster ovary cells. Unpublished
studies Nos. 881130CHT2449 and 890111CHO2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Helton, D.R. (1989d). CNS
behavioural effects of tilmicosin administered orally in the male
CD-1 mouse. Unpublished study No. PN8805 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Colbert, W.E. (1990). In vitro
studies of tilmicosin in the cardiac muscle of Hartley Albino
guinea pigs. Unpublished study No. PM8946 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Wolff, R.K., & Carlson, K.H. (1991). A subchronic
inhalation toxicity study of tilmicosin in Beagle dogs.
Unpublished study No. DO5589 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L., & Lochmueller, C.A. (1988a). A
study of the immune response in CD-1 mice treated orally with
tilmicosin. Unpublished study No. MI4288 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L,. & Stephenson, R.O. (1988b). A
study of the acute effects of urine and electrolyte excretion of
tilmicosin administered orally to female Crl:CD(SD) rats.
Unpublished study No. RI8688 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGuigan, M.A. (1994). Human exposures to tilmicosin (Micotil).
Vet. Hum. Toxicol., 36(4), 306-308.
McLaren, I.M. (1994). Determination of the minimum inhibitory
concentration of tilmicosin for Lactobacillus and
Propionibacterium Sp. Unpublished study from Central Veterinary
Laboratory, UK. Submitted to WHO by Lilly, Basingstoke, UK.
Montanio, C.D. & Dart, R.C. (1993). Micotil 300. Human exposures
May 1992-May 1993. Unpublished report. Submitted to WHO by Lilly,
Basingstoke, UK.
Noda, A. (1993). Teratogenicity study of EL-870 (tilmicosin
aqueous) in rabbits by gavage. Unpublished study No. 91-001 from
Research Institute for Animal Science in Biochemistry and
Toxicology, Japan. Submitted to WHO by Lilly, Basingstoke, UK.
Piroozi, K.S., Keaton, M.J., & Jordan, W.H. (1993). The acute
toxicity of tilmicosin administered orally to Fischer 344 rats.
Unpublished study No. R21493 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Rumney, C.J. (1993). Microbiological end-point determination for
two antibiotics. Unpublished study No. 1164/3/93 from Bibra
Toxicology International, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Sarazan, R.D., Main, B.W., & Jordan, W.H. (1993). Cardiovascular
effects of tilmicosin alone, or in combination with propranolol
or dobutamine, in conscious unrestrained dogs. Unpublished study
No. DV1591 from Lilly Research Laboratories. Submitted to WHO by
Lilly, Basingstoke, UK.
Scott, R.J.D., Morgan, J.M., Pether, J.V.S., & Gosling, P.
(1993). Determination of the minimum inhibitory concentrations of
tilmicosin for human isolates of Bifidobacterium and Pepto-
streptococcus. Unpublished study No. SMS/9301 from Southern
Microbiological Services, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Williams, P.D., Jordan, W.H., & Colbert, W.E. (1988). In vitro
studies of tilmicosin (EL-870, Compound 177370) in smooth and
cardiac muscle. Unpublished study No. PM8808 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Excretion
Pigs were administered a dose of 110 mg 14C-tilmicosin in the
diet over the course of one day. The recovery of radioactivity was 15%
in the urine and 80% in the faeces (Giera & Thomson, 1986).
In two studies, pigs were given a dose of 154 or 400 mg
14C-tilmicosin in the diet following a similar dose given for 5
days. The recovery of radioactivity was 4 to 6% in urine and 62 to 75%
in faeces. Radioactivity was detected in the bile but was not
quantified (Donoho & Thomson, 1988; Donoho et al., 1993).
2.1.2 Biotransformation
2.1.2.1 Rats
Tilmicosin, labelled with 14C in both the desmycosin macrolide
ring and the piperidine ring, was given orally to 15 male and 15
female Fischer-344 rats. The dosage was 20 mg/kg bw per day for 3
days. In the liver, radiolabel corresponded to tilmicosin and a
desmethyl derivative, T1(demethylated in the mycaminose ring). The
single radioactive substance identified in urine was unchanged
tilmicosin, while in faeces the major peak was parent compound with
lesser amounts of desmethyl tilmicosin and a high molecular weight
compound known to be present as an impurity in the administered
substance, T2 (consisting of two macrolide rings and one piperidine
ring) (Donoho, 1988).
Fischer-344 rats (10 males and 10 females) were given gavage
doses of 50 mg/kg bw per day 14C-tilmicosin for 5 days. An analysis
of faecal radioactivity for the presence of the sulfate metabolite
that was found in the faeces of pigs revealed the presence of a
similar compound, but quantification was not undertaken (Donoho &
Kennington, 1993).
2.1.2.2 Sheep
Beulah cross lambs were administered a single subcutaneous dose
of 20 mg/kg bw per day 14C-tilmicosin. The major radioactive
component in the liver, kidneys and urine was the parent drug,
together with lesser amounts of T1 and T2, and minor amounts of other
unidentified substances (Hawkins et al., 1993).
2.1.2.3 Pigs
Pigs were fed diets containing 14C-tilmicosin, which resulted
in daily doses of 400 mg, for 5 days. Of the radiolabel in liver and
kidney, approximately 60 to 70% was in the form of the parent drug and
there were small amounts of T1. Similarly in urine and faeces, there
were high levels of tilmicosin and low levels of T1. A further
metabolite was detected that comprised 14% of faecal and 25% of
urinary radioactivity and that was consistent with reduction of one
double bond in the macrolide ring followed by sulfation
(Donoho et al., 1993).
2.1.2.4 Cattle
In a summary of results obtained in cattle injected with
14C-tilmicosin, it was reported that the radioactivity profile in
the liver of treated rats was similar to that in the faeces. In
animals treated with a highly purified sample of tilmicosin,
metabolite T2 was not detected in the liver, suggesting that its
presence was a result of direct administration as a component of the
drug substance. Radioactivity in the kidneys was essentially in the
form of unchanged tilmicosin (Donoho, 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Major signs of toxicity in mice and rats were leg weakness,
hypoactivity, lethargy, ataxia, poor grooming and coma. Monkeys given
20 mg/kg vomited during the first day but were subsequently normal.
The single monkey given 30 mg/kg vomited, exhibited hypoactivity,
laboured respiration, vocalization and ataxia, and died within 2
hours.
New Zealand white rabbits (five males and five females) received
5000 mg/kg bw tilmicosin onto clipped skin for a period of 24 hours,
under a non-occlusive dressing. There were no deaths and no signs of
skin irritation (Jordan et al., 1987).
A volume of 0.1 ml (17 mg) of tilmicosin was instilled into one
eye of three male and three female New Zealand White rabbits.
Conjunctival hyperaemia and chemosis were noted for several days,
clearing by the end of one week (Jordan et al., 1987).
Hartley albino guinea-pigs (6 to 12 females) were given 10
intracutaneous induction doses of tilmicosin (0.05 to 0.1 ml of a
50 mg/ml solution). Fourteen days after the induction phase each
animal was injected intracutaneously with 0.05 ml tilmicosin. This
challenge dose did not result in an enhancement of skin reactions,
suggesting that a skin sensitization response had not been elicited
(Jordan et al., 1989b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Fischer-344 rats were given
gavage doses of 0, 50, 175 or 600 mg/kg bw per day tilmicosin
(purity 88%) in an aqueous vehicle. Drug administration was continued
for 2 weeks, during which there was no mortality or clinical signs of
toxicity. Food consumption was lower in males and females at 600 mg/kg
and body weight gain was depressed in males throughout the study and
in females during the first few days only.
Table 1. Results of acute toxicity studies with tilmicosin
Species Route Vehicle Sex LD503 Reference
(strain) (mg/kg bw)
Mouse sc aqueous M 97 Jordan
(ICR) F 109 et al., 1986a
Rat1 oral aqueous M 850 Jordan
(Sprague- F 800 et al., 1986b
Dawley)
Rat2 oral aqueous M & F > 2000 Piroozi
(Fischer-344) et al., 1993
Rat sc aqueous M 185 Jordan
(Fischer-344) F 440 et al., 1986c
Rat inhalation aerosol M & F > 494 (0/20) Jordan
(Fischer-344) < 4053 (14/20) et al., 1987
Rabbit dermal - M & F > 5000 Jordan
(NZ White) et al., 1987
Monkey im aqueous ? > 20 (0/2) Jordan &
(Rhesus) 30 (1/1) Shoufler, 1990
Sheep sc aqueous ? > 150 (0/5) Cochrane &
iv aqueous ? > 7.5 (1/5) Thomson, 1990
1 Animals were fasted
2 Animals were non-fasted
3 Figures in brackets represent the incidence of deaths
In the 600 mg/kg bw per day groups, there were increases in
haematocrit owing to an increase in corpuscular volume and in serum
levels of alanine aminotransferase and hepatic p-nitroanisole
O-demethylase activity. Thrombocyte counts were slightly decreased
at this dose. Urinalysis was unaffected. At the highest dose, there
were slight decreases in the weights of kidneys, spleen and ovaries,
increased adrenal weight and multifocal inflammation in the liver of
some animals. All treated animals exhibited caecal distension, which
is a typical response to high doses of an antibiotic compound
(Jordan, 1986).
Groups of 20 male and 20 female Sprague Dawley (Crl:CD) rats
received 0, 50, 250 or 1000 mg/kg bw per day tilmicosin (purity 88%)
in an aqueous vehicle, by gavage for 3 months. Signs of overt toxicity
were noted at 1000 mg/kg and included thinness, ventral soiling,
chromorhinorrhea, chromodacryorrhea, alopecia and poor grooming.
Mortality was increased at this highest dose and in females given
250 mg/kg bw per day.
Although food consumption was depressed in the 1000 mg/kg bw per
day group (males only), body weight gain was lower in males and
females at 1000 mg/kg bw per day and females at 250 mg/kg bw per day.
At 1000 mg/kg bw per day, serum alanine aminotransferase was increased
in males, blood urea nitrogen (BUN) was increased in males and
females, urinary pH was slightly lower in females and the presence of
occult blood was greater in males and females. There were no effects
on ophthalmology, haematology or hepatic p-nitroanisole
O-demethylase activity.
The weights of kidneys, liver and heart were increased in females
at 1000 mg/kg bw per day and adrenal weight was increased at 250 mg/kg
bw per day (females only) and 1000 mg/kg bw per day (males and
females). Gross pathology revealed caecal enlargement or distension at
250 mg/kg bw per day or more and small spleens in a few rats at
1000 mg/kg bw per day. Slight nephrosis was noted in two males in each
of the 250 and 1000 mg/kg bw per day groups. Other pathological
alterations were seen only in males and females of the 1000 mg/kg bw
per day group, i.e. hypertrophy of the zona fasciculata of the adrenal
cortex in most rats, necrosis of individual skeletal muscle fibres in
some rats; myocardial degeneration was increased and lymphoid
depletion was evident in the spleen and thymus of some animals. The
NOEL was 50 mg/kg bw per day (Jordan, 1988).
2.2.2.2 Dogs
Beagle dogs (two males and two females per group) were exposed by
inhalation to aerosols of 0, 12, 47 or 251 mg/m3 tilmicosin
(purity 83%). The treatment was administered for 4 hours per day on 12
days out of a 16-day period (excluding weekends). Particle median
equivalent aerodynamic diameters were between 1.2 and 1.5 µm. The mean
serum concentrations of tilmicosin were 0.23 to 0.33 and 1.67 to
2.46 µg/ml at 47 and 251 mg/m3 respectively. Drug levels at
12 mg/m3 were below the detection limit of 0.1 µg/ml.
One male given 251 mg/m3 died on the final day of exposure but
no other overt signs of toxicity were observed. Heart rates were
increased in dogs at 251 mg/m3. Food intake, body weight,
haematology and blood chemistry were unaffected. At autopsy, lung
weight was increased in females given 251 mg/m3 and inflammation was
noted in the respiratory tract at 47 mg/m3 (females only) and
251 mg/m3 (males and females) (Jordan et al., 1991).
Groups of four male and four female beagle dogs were given 0, 6,
20 or 70 mg/kg bw per day tilmicosin (purity 88%) in capsules for 3
months. The daily dose was administered in two equal amounts, 6 hours
apart. Serum concentrations of tilmicosin were measured 3 hours and 24
hours after the first of the two daily doses in weeks 2, 3, 5, 9 and
13. At increasing doses, the mean concentrations were 0.1 to 0.2, 0.74
to 1.49 and 3.24 to 6.05 µg/ml at 3 hours and 0, 0.26 to 0.59 and 1.72
to 3.96 µg/ml at 24 hours.
Half the 70 mg/kg bw per day males and females died during the
first month. Clinical signs prior to death were pale mucous membranes
in two dogs and ataxia in one dog. Food intake, body weight, haemato-
logy and urinalysis were unaffected. Serum alanine aminotransferase
activity was progressively increased at 70 mg/kg bw per day, while
hepatic p-nitroanisole O-demethylase activity was increased
in females at this level.
Two of the four surviving dogs at 70 mg/kg bw per day showed
bilateral multifocal areas of subretinal fluid concentrated along
arterioles in the tapetal region of the eye. The changes were claimed
to be consistent with those associated with systemic hypertension. One
of these dogs also had retinal degeneration and the other showed
miosis with normal pupillary light responses.
Heart rate increases were dose related, being marked at 70 mg/kg
bw per day and moderate to severe at 20 mg/kg bw per day. The increase
in males given 6 mg/kg bw per day was not significant. At the highest
dose, examination of the electrocardiogram revealed depression of the
ST segment.
At 70 mg/kg bw per day, heart weight was increased in males and
females, and liver and kidney weights were increased in females. Gross
necropsy showed small spleens in the females that died during the
study and an enlarged heart in a surviving male at 70 mg/kg bw per
day. Slight diffuse mucosal oedema was seen in the gall bladders of
two dogs at 70 mg/kg bw per day. Despite the physiological changes in
the eyes and heart, there were no associated pathological alterations.
The NOEL was 6 mg/kg bw per day (Jordan, 1987).
Groups of four male and four female beagle dogs were given 0, 4,
12 or 36 mg/kg bw per day tilmicosin (purity 86%) in capsules for 12
months. The daily dose was administered in two equal amounts, 6 hours
apart. There were no deaths. Peripheral redness was seen sporadically
in some animals of all groups, in particular at 12 and 36 mg/kg bw per
day.
Body weight gains were lower at > 12 mg/kg bw per day. There
were no effects on ophthalmology, haematology, blood chemistry and
urinalysis. Heart rates were markedly increased at 36 mg/kg bw per day
with sporadic depression of the ST segment in some dogs.
Heart weight was increased at 36 mg/kg bw per day and four males
and one female at this dose showed enlarged hearts. Mild chronic
dermatitis was noted in the external ears of dogs from all treated
groups. The findings included minimal thickening of the epidermis,
foci of acantholysis and inflammatory cell infiltration in the dermis.
These changes were only slight and non-dose-related, and therefore of
questionable relationship to treatment. The NOEL was 4 mg/kg bw per
day (Jordan & Bernhard, 1989).
2.2.3 Long-term toxicity/carcinogenicity studies
Tilmicosin has not been tested in toxicological studies longer
than 12 months in duration and hence the carcinogenic potential of
this drug has not been directly determined. There are a number of
observations which are relevant in assessing the carcinogenicity of
tilmicosin:
a) The results of available toxicological studies with tilmicosin
have not identified lesions or proliferative changes which could
be considered suggestive of potential neoplasia.
b) Tilmicosin has been tested in a wide range of genotoxicity
assays. All results were negative and it was concluded that the
compound has no genotoxic activity.
c) Tilmicosin is a macrolide antibiotic. This class of chemical has
been in widespread usage in humans for many years but there is no
evidence of carcinogenicity. Tylosin is the closest structural
analogue and this chemical was reviewed by the thirty-eighth
meeting of JECFA (Annex 1, reference 97).
2.2.3.1 Reconsideration of tylosin tumorigenicity
Tylosin was tested in 2-year feeding studies in rats. The
findings revealed an association between drug administration at doses
of 150 and 300 mg/kg bw per day in the diet and an increased incidence
of pituitary adenomas in males. While it was claimed that the increase
in the incidence of tumours was an indirect result of the ability of
tylosin to increase survival and weight gain, supporting data were not
available to the Committee and an ADI was not established.
New information, not considered at the thirty-eighth meeting,
indicates that spontaneous pituitary tumours occur in male rats at
variable rates with increased rates occurring in animals with higher
body weights (Gries & Young, 1982). In the tylosin studies, 12-month
body weights were somewhat higher in treated males rats and mortality
was increased in control animals due to respiratory infection. The
highest observed incidence of tumours in tylosin-treated groups was
comparable to the upper limit of the historical control range (23%) in
the test facility.
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A dose-ranging study was carried out in groups of 10 female
Sprague-Dawley (Crl:COBS CD) rats given gavage doses of 0, 50, 125,
250, 500 or 750 mg/kg bw per day tilmicosin (purity unknown). The
females were treated from 14 days before mating with untreated males
until the end of study on post-partum day 4.
Excess salivation was dose-related at > 250 mg/kg bw per day.
Chromodacryorrhea, urine-stained fur and alopecia were seen in some
rats at 750 mg/kg bw per day; the three most severely affected died
during the study. Prior to death, these animals exhibited reduced food
intake, lost weight and were emaciated. Body weight gain was depressed
in surviving rats at 750 mg/kg bw per day during the pre-mating period
while food intake was reduced at > 250 mg/kg bw per day in week 1
and at 750 mg/kg bw per day in week 2. The pregnancy rate was
decreased at > 500 mg/kg bw per day. Duration of gestation, litter
size and weight, pup survival and weight gain to post-partum day 4
were unaffected (Dearlove et al., 1987).
Groups of 30 male and 30 female Sprague Dawley (Crl: COBS CD BR)
rats were administered 0, 10, 45 or 200 mg/kg bw per day tilmicosin
(purity 87%) by gavage in an aqueous vehicle. Treatment commenced 70
days (males) and 14 days (females) before the first mating period and
was continued through two litters per generation for two generations.
F1 litters were culled to five pups per sex on post-partum day 4 and
F1b pups (40 males and 40 females per group) were bred to produce
the following generation. F2a and F2b pups were killed on
post-partum day 4.
In adult animals, salivation was noted in males and females at
200 mg/kg bw per day, but there were no other signs of toxicity. Body
weight gain and food consumption were depressed in females of the 45
and 200 mg/kg bw per day groups during the pre-mating period only.
During the production of the four litters in this study there were no
effects on mating performance, pregnancy rates, duration of gestation,
litter size and weight and the weight gain of offspring. In both F1
litters at 200 mg/kg bw per day, pup mortality was slightly increased
up to post-partum day 4, but the finding was not duplicated in either
F2 litter. The NOEL was 10 mg/kg bw per day in adult rats (Christian
& Hoberman, 1989).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 25 presumed pregnant female Sprague Dawley (Crl: CD)
rats were administered gavage doses of 0, 10, 70 or 500 mg/kg bw per
day tilmicosin (purity 86%) in an aqueous vehicle. The dams were
treated on gestation days 6 to 15 and were killed on gestation day 20.
Increased salivation was seen at 70 and 500 mg/kg bw per day and
alopecia at 500 mg/kg bw per day. There were no deaths or abortions.
Body weight gain was reduced at 70 and 500 mg/kg bw per day, and food
intake was reduced at 500 mg/kg bw per day, during gestation days 6 to
10.
The number of resorption or live fetuses, fetal weight, sex ratio
and the incidence of fetal malformations were similar between groups.
The incidences of total skeletal and visceral anomalies were increased
in treated groups, but there was no dose-response relationship and the
findings were within the historical control values for the laboratory.
The NOEL for maternotoxicity was 10 mg/kg bw per day (Jordan & Higdon,
1988).
2.2.5.2 Rabbits
Groups of 15 presumed pregnant female Japanese White-NIBS rabbits
were given gavage doses of 0, 8, 19 or 48 mg/kg bw per day tilmicosin
(purity unknown) in an aqueous vehicle. Does were treated on gestation
days 6 to 18 and were killed on gestation day 28. One female given
48 mg/kg bw per day aborted on gestation day 26 and died. Reduced
faeces were seen at 19 and 48 mg/kg bw per day with only a transient
effect at 8 mg/kg bw per day. During the treatment period food intake
was depressed in a dose-related manner and body weight loss was
observed at 19 and 48 mg/kg bw per day.
Fetal and placental weight tended to decrease at 19 and 48 mg/kg
bw per day but did not achieve statistical significance. There were no
meaningful effects on the incidence of resorptions or fetal deaths or
on sex ratio. Open eyelids were observed in 11/91 and 16/68 fetuses
from the 19 and 48 mg/kg bw per day groups, respectively, and some of
these fetuses showed cleft palate or club foot. The affected fetuses
had low body weights and the seven litters involved were derived from
does that had lost body weight during drug administration. Skeletal
examination revealed retardation of fetal development at 19 and
48 mg/kg bw per day. Similar effects have been seen in this laboratory
in dietary restricted rabbits, suggesting the effects on the fetus
were secondary to maternal malnutrition (Noda, 1993).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on tilmicosin
Test system1 Test object Concentration Results Reference
Reverse S. typhimurium 1 - 100 µg/plate negative Jordan
mutation2 TA98, TA100, (± S9) et al., 1986f
TA1535, TA1537,
TA1538
Reverse E. coli 1 - 100 µg/plate negative Garriott
mutation2 WP2uvrA (± S9) et al., 1993
Forward L5178Y mouse 100-900 µg/ml negative Jordan
mutation2 lymphoma cells (- S9) et al., 1986e
200-1000 µg/ml
(+ S9)
Forward HGPRT+ Chinese 25-250 µg/ml negative Jordan
mutation2 Hamster ovary (- S9) et al., 1989c
cells 50-300/ µg/ml
(+ S9)
Unscheduled primary cultures 0.5-10 µg/ml negative Jordan
DNA synthesis of rat hepatocytes et al., 1985
assay
Sister chromatid Chinese hamster 200-1800 × 1 negative Jordan et al.,
exchange bone marrow mg/kg bw oral 1986d, 1989a
assay
Chromosome rat bone marrow 180-1800 × 1 and negative Jordan &
aberrations 100-1000 × 5, Ivett, 1989
mg/kg bw per
day oral
1 Positive controls used
2 Both with and without liver microsomal activation
2.2.7 Special study on the immune system
Groups of eight male CD-1 mice were given gavage doses of 0,
10, 250, 500 or 1000 mg/kg bw per day tilmicosin (purity 87%) in
an aqueous vehicle. Drug treatment was continued for 10 days.
After the third dose mice were injected intravenously with sheep
red blood cells. At the end of the study, serum from each mouse
was assayed for antibodies (haemagglutinin). There was one death
at 500 mg/kg bw per day and six at 1000 mg/kg bw per day, but
antibody production was not affected at any level (McGrath
et al., 1988a).
2.2.8 Special studies on microbiological activity
2.2.8.1 In vitro
The antibacterial activity of tilmicosin was determined
against a range of organisms representative of human intestinal
flora. Cultures were initiated using inoculum sizes of between
3 × 105 and 1.6 × 106 cells per spot on blood agar plates and
incubated anaerobically at 37°C.
The median MIC values for the clinical isolates of
Bifidobacterium and Peptostreptococcus, respectively, were
0.015 and 0.5 µg/ml. The above results were obtained at pH 7.7.
At pH 6.6 the MIC values were one to two orders of magnitude
higher (Scott et al., 1993).
The antibacterial activity of tilmicosin was examined
against a number of organisms used in industrial food processing
and originally obtained from dairy products. Cultures were
initiated in blood agar plates using three strains of
Propionibacterium and four strains of Lactobacillus. Under
anaerobic conditions the MICs were between 8 and 64 µg/ml and
under microaerophilic conditions the values were between 4 and
32 µg/ml (McLaren, 1994).
2.2.8.2 Rats
In two separate studies, female germ-free rats were orally
dosed with 1 ml of 10% (w/v) pooled human faecal suspension.
Three weeks later groups of two males and two females were given
gavage doses of 0, 0.12 or 0.4 mg/kg bw per day tilmicosin
(purity 87%) for 5 days. Rat faecal samples were collected before
treatment, daily during treatment and once during each of the 2
weeks following cessation of dosing. Body weight gain remained
unaffected.
The animals in each study received faecal suspensions
obtained from different donors. Results from different studies
varied widely, possibly reflecting differences between the
bacterial populations used. Total anaerobes were not reduced by
tilmicosin treatment. Total enterobacterial counts and the
proportion of enterobacteria with respect to total anaerobe
counts were transiently increased at 0.4 mg/kg bw per day in one
study only. Tilmicosin-resistant enterobacteria were not
significantly increased in either study. Spiramycin, at a dose of
0.5 mg/kg bw per day, increased total enterobacterial counts and
the number of spiramycin-resistant enterobacteria; the latter
appeared to remain high after the cessation of treatment. The
NOEL for tilmicosin was 0.4 mg/kg bw per day (Rumney, 1993).
2.2.9 Special studies on pharmacology
Table 3. Results of pharmacological assays with tilmicosin
Test system Doses Results Reference
Isolated 0.0009 to No effect on non-stimulated organ. Williams
guinea-pig 90 µg/ml 90 µg/ml slightly inhibited acetylcholine- et al.,
ileum and angiotensin-induced contraction 1988
and significantly inhibited electrically
stimulated contraction
Isolated 0.0009 to No effect on non-stimulated tissue and Williams
rat uterus 90 µg/ml no change to oxytocin-induced et al.,
(estrogen contractions 1988
primed)
Isolated rat 0.0009 to No effect on non-stimulated tissue and Williams
vas deferens 90 µg/ml no change to noradrenaline-induced et al.,
contractions 1988
Isolated 0.0009 to In spontaneously beating tissue, > 90 Williams
guinea-pig 900 µg/ml µg/ml decreased force of contraction; et al.,
atria and 900 µg/ml increased rate of 1988;
contraction. 90 µg/ml inhibited Jordan
isoprenaline-, noradrenaline- and Bay et al.,
K8644 (calcium agonist)-induced force 1990
and rate of contraction, and
histamine-induced rate of contraction.
Table 3. Results of pharmacological assays with tilmicosin (cont'd).
Test system Doses Results Reference
Conscious 0.25, 1, 2.5 Heart rate increased at > 1 mg/kg. Left Jordan &
dogs or 5 mg/kg ventricular function decreased at > 0.25 Sarazan,
iv mg/kg. Aortic pulse pressure decreased 1991;
at > 1 mg/kg. ECG revealed "Ventricular Sarazan
Alternans" (ventricular contraction fails et al.,
to achieve adequate pressure to open 1993
aortic valve and cause an arterial pulse).
Anaesthetised 0.5, 1 or 5 Heart rate increased, stroke volume, Jordan
dogs mg/kg iv stroke work index and cardiac output et al.,
decreased at > 1 mg/kg. At 5 mg/kg 2/3 1988
dogs developed second degree heart block
CD-1 mice 10, 100, Piloerection and grasping loss at > 500 Jordan
500 or mg/kg. Ptosis at 1000 mg/kg. No effect et al.,
1000 mg/kg on electroshock- or pentylenetetrazol- 1989d
oral induced seizures or on body temperature.
Hexobarbital-induced sleep time
prolonged by 500 (x1) and 1000 (x3) mg/kg
Sprague- 10, 30, 90 Urine volume and creatinine reduced McGrath
Dawley or 270 at 270 mg/kg with increases in osmolality et al.,
rats mg/kg oral and electrolytes. 1988b
2.3 Observations in humans
A total of 241 human exposures to tilmicosin (Micotil) were
reported to the Rocky Mountain Poison Center from May 1992 to May
1993. Needlesticks and scrapes (n=112) and accidental injection
(n=43) caused either no effect or local reactions including
soreness, numbness, stinging, swelling, redness, burning and
stiffness. Some injected subjects experienced anxiety, sweating,
headache and lightheadedness. Skin exposures (n=50) resulted in
redness and tingling of the skin, and eye exposures (n=11)
resulted in stinging and swelling. Persons accidentally ingesting
the drug (n=39) reported either no symptoms or bitter taste,
nausea, numbness of lips and tongue, vomiting, thirst and
headache (Montanio & Dart, 1993).
Over a 30-month period, 36 cases of accidental exposure to
tilmicosin (Micotil) were reported to the Ontario Regional Poison
Information Centre. Percutaneous injection (n=26) always resulted
in pain at the site and seven subjects mentioned a variety of
local reactions consistent with an irritant action. One person
showed "peaked T waves" 30 minutes following the injection of
1 ml into his arm, but ECG changes were not noted in other
subjects. The remaining subjects received splashes into the mouth
or eyes or onto the skin. Unpleasant taste, a burning sensation
on the hard palate and ocular irritation were each recorded in
one person (McGuigan, 1994).
3. COMMENTS
The Committee considered toxicological data on tilmicosin,
including the results of studies on acute and short-term
toxicity, pharmacokinetics, metabolism, reproductive toxicity,
teratogenicity, genotoxicity, antimicrobial activity and
pharmacology. The Committee also considered observations in
humans accidentally exposed to tilmicosin.
Tilmicosin is absorbed from the gastrointestinal tract as
shown by the recovery of radiolabelled compound in urine and/or
bile of orally dosed pigs (110 to 400 mg per animal), and the
presence of dose-related serum concentrations of tilmicosin dosed
orally in dogs for 3 months. However, the results from available
studies did not allow any conclusion to be reached concerning the
extent of absorption. The metabolites detected in a range of farm
animals were also found in rats, which suggests that the rat is a
suitable model for determining the potential toxicological
hazards posed by tilmicosin.
The LD50 in fasted rats was 800 to 850 mg/kg bw, but
toxicity was substantially lower in non-fasted animals, among
which there were no deaths following administration of a single
dose of 2000 mg tilmicosin/kg bw. Studies in a range of
pharmacological models identified depression of cardiac muscle
contractility and reduced heart function as the main adverse
effects.
Rats were administered oral doses of 50, 250 or 1000 mg/kg
bw per day for 3 months. At 1000 mg/kg bw per day, animals
exhibited chromorhinorrhea, chromodacryorrhea, alopecia, poor
grooming, reduced food consumption and they appeared thin.
Body-weight gain was reduced and mortality was increased in
females at 250 mg/kg bw per day and in both sexes at 1000 mg/kg
bw per day. Toxic effects on the kidney and liver were indicated
by increased organ weights, increased serum levels of alanine
aminotransferase and blood urea nitrogen, and the presence of
blood in the urine. However, pathological examination of these
organs showed only slight nephrosis in two males in each of the
groups given 250 or 1000 mg/kg bw per day. At the highest dose,
hypertrophy of the zona fasciculata of the adrenal cortex was
noted in most rats, and myocardial degeneration, necrosis of
individual skeletal muscle fibres and lymphoid depletion in the
spleen and thymus were seen in some rats. The NOEL was 50 mg/kg
bw per day.
Dogs were given oral doses of 6, 20 or 70 mg/kg bw per day
for three months. Half of the animals given 70 mg/kg bw per day
died during the first month. Heart rate was increased at 20 and
70 mg/kg bw per day and at the higher dose there was depression
of the ST segment of the electrocardiogram. Ophthalmoscopic
examination of the eyes showed bilateral areas of subretinal
fluid concentrated along arterioles in the tapetal region of two
of the four surviving dogs at 70 mg/kg bw per day. The NOEL was
6 mg/kg bw per day.
Dogs were given oral doses of 4, 12 or 36 mg/kg bw per day
for twelve months. Body weight gain was depressed at 12 and
36 mg/kg bw per day. Heart rate was markedly increased at 36
mg/kg bw per day, with occasional depression of the ST segment
of the electrocardiogram. Hearts were enlarged at 36 mg/kg bw per
day, but there were no associated pathological changes. The NOEL
was 4 mg/kg bw per day.
In a two-generation reproductive toxicity study, rats were
administered oral doses of 10, 45 or 200 mg tilmicosin/kg bw per
day. Body weight gain and food consumption were depressed in
females given 45 or 200 mg/kg bw per day, but only during the
pre-mating period. Fertility and reproduction parameters were
unaffected at any dose level. At 200 mg/kg bw per day, mortality
in pups was slightly increased in both first-generation litters
up to post-partum day 4, but not in either second-generation
litter. The NOEL was 10 mg/kg bw per day.
Developmental toxicity studies were reported in rats and
rabbits. Rats were dosed with 10, 70 or 500 mg tilmicosin/kg bw
per day by gavage. Growth and development in rat fetuses were not
affected by treatment, but increased salivation and reduced body
weight gain of dams were observed at 70 and 500 mg/kg bw per day.
The NOEL for maternal toxicity in rats was 10 mg/kg bw per day.
In the study in rabbits, there were dose-related signs of
toxicity in the does at all doses (8, 19 and 48 mg/kg bw per
day). Severely affected members of the groups given 19 or
48 mg/kg bw per day produced offspring that had open eyelids and
low incidences of cleft palate or club foot. These fetuses had
low body weights and were clearly retarded in development. The
effects were attributed to treatment-related malnutrition in
maternal animals, which is commonly observed in rabbits dosed
with antibiotics, confirming that this species is an inappropriate
model for studying the teratogenic potential of antibiotics.
Tilmicosin has been tested for reverse mutation in bacteria,
forward mutation in cultured mammalian cells, unscheduled DNA
synthesis in primary cultures of rat hepatocytes, and in in vivo
cytogenetic assays. All results were negative, and the Committee
concluded that tilmicosin has no genotoxic potential.
The carcinogenic potential of tilmicosin has not been
tested. However, a number of factors are relevant in assessing
the carcinogenicity of this drug. Toxicity studies with
tilmicosin have not resulted in lesions or proliferative changes
suggestive of neoplasia, and tilmicosin was uniformly negative in
a wide range of genotoxicity assays. Tilmicosin is a macrolide
antibiotic, and this class of chemicals is not known to induce
neoplasia despite widespread use in humans over many years. The
closest structural analogue, tylosin, was reviewed at the
thirty-eighth meeting of the Committee (Annex 1, reference 97).
In a 2-year feeding study in rats, tylosin administration was
associated with an increased incidence of pituitary adenomas in
male animals when compared to concurrent controls. New evidence,
not considered at the thirty-eighth meeting, indicates that
spontaneous pituitary neoplasms occur in male rats at variable
rates. Additionally, increased rates occur in animals with higher
body weights. In tylosin-treated groups of male rats, the
12-month body weights were somewhat higher than in the control
groups and the incidences of pituitary adenomas were comparable
to the upper limit of the historical control range. The lower
incidence of pituitary neoplasms in the control animals may have
been due to the earlier mortality in this group caused by
respiratory disease. In light of this information the Committee
considered that the concerns of the thirty-eighth meeting over
the potential tumorigenicity of tylosin had been satisfactorily
addressed and that there was no concern for the carcinogenic
potential of tylosin. For the above reasons, the Committee
considered that carcinogenicity studies would not be required for
tilmicosin.
In assessing the microbiological activity of tilmicosin, the
results of a study using germ-free rats colonized with human
intestinal microflora was considered the most relevant. At the
highest dose administered (0.4 mg/kg bw per day for 5 days) there
were no significant alterations in numbers of total anaerobes or
enterobacteria in rat faeces.
Reports of accidental human exposure to tilmicosin have
identified local reactions indicative of an irritant action
following dermal and ocular exposure. Humans accidentally
injected with tilmicosin have reported anxiety, sweating,
headache and lightheadedness. Changes in the electrocardiogram
pattern were observed in only one person. Contact with the buccal
mucosa or ingestion has resulted in a range of symptoms including
nausea, vomiting, thirst, numbness or burning sensation in the
mouth, and headache.
4. EVALUATION
The NOEL from toxicological studies was 4 mg/kg bw per day
in a 12-month study in dogs. Treatment of rats colonized with
human intestinal microflora with 0.4 mg tilmicosin/kg bw per day
produced no significant microbiological effect. The Committee
established an ADI of 0-40 µg/kg bw, based on the toxicological
NOEL of 4 mg/kg bw per day and a safety factor of 100. An
identical ADI would have been established using the data from the
study on rats colonized with human intestinal microflora and a
safety factor of 10 to account for variability among humans.
5. REFERENCE
Christian, M.S. & Hoberman, A.M. (1989). Reproductive effects of
EL-870 administered orally via gavage to Crl:COBS CD(SD) BR rats
for two generations, with two litters per generation. Unpublished
study No. 112-001 from Argus Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Cochrane, R.L. & Thomson, T.D. (1990). Toxicology and pharmacology
of tilmicosin following administration of subcutaneous and intravenous
injections to sheep. Unpublished study No. T5C768908 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Dearlove, G.H., Hoberman, A.M., & Christian, M.S. (1987). Dosage-range
study of EL-870 administered orally via gavage to Crl:COBS CD(SD) BR
rats (Pilot study). Unpublished study No. 112-001P from Argus Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. (1988). Comparative metabolism of 14C-tilmicosin
in cattle and rats. Unpublished study No. ABC-0395 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. & Kennington, A.S. (1993). Tilmicosin metabolite
study with rat excreta. Unpublished study No. T5C759302 from
Lilly Research Laboratories. Submitted to WHO by Lilly, Basingstoke,
UK.
Donoho, A.L. & Thomson, T.D. (1988). 14C-Tilmicosin balance-
excretion study in swine. Unpublished study No. ABC-0409 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L., Darby, J.M., Helton, S.L., Sweeney, D.J.,
Occolowitz, J.L., & Dorman, D.E. (1993). Tilmicosin metabolism
study in tissues and excreta of pigs fed 400 ppm 14C-tilmicosin.
Unpublished study No. T5C759201 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Garriott, M.L., Rexroat, M.A., & Jordan, W.H. (1993). The effect
of tilmicosin on the induction of reverse mutations in
Escherichia coli using the Ames test. Unpublished study
No. 920825AMS2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Giera, D.D. & Thomson, T.D. (1986). Preliminary 14C EL-870
balance excretion and tissue residue in swine. Unpublished study
No. ABC-0305 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Gries, C.L. & Young, S.S. (1982). Positive correlation of body
weight with pituitary tumor incidence in rats. Fundam. Appl.
Toxicol., 2, 145-148.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A,. &
Cameron, D.M. (1993). The metabolism and residues of 14C-tilm-
icosin following subcutaneous administration to sheep. Unpublished
study No. HRC/LLY 36/930447 from Huntingdon Research Centre. Submitted
to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1986). The toxicity of compound 177370 (EL-870)
given by oral gavage to Fischer 344 rats for two weeks.
Unpublished study No. RI5585 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1987). The toxicity of EL-870 given orally to
Beagle dogs for three months. Unpublished study No. DO8286 from
Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. (1988). The toxicity of tilmicosin given orally to
Crl:CD(SD) rats for three months. Unpublished study No. RO9886
from Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. & Bernhard, N.R. (1989). A one year chronic toxicity
study in Beagle dogs given oral doses of tilmicosin. Unpublished
study No. DO7187 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Higdon, G.L. (1988). A teratology study of
tilmicosin (EL-870, compound 177370) administered orally to CD
rats. Unpublished study No. RI3387 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Ivett, J.L. (1989). Mutagenicity test on
tilmicosin in the rat bone marrow cytogenetic assay. Unpublished
study No. 10646-0-452 from Hazleton Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Sarazan, R.D. (1991). An acute study of the
cardiovascular effects of intravenous Micotil 300 in conscious
dogs. Unpublished study No. DV0890 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Shoufler, J.R. (1990). The acute toxicity of
Micotil 300 administered intramuscularly to Rhesus monkeys.
Unpublished studies Nos. PO4089 and PO7689 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Hill, L.E., & Probst, G.S. (1985). The effect of
compound 177370 (EL-870) on the induction of DNA repair synthesis
in primary cultures of adult rat hepatocytes. Unpublished studies
Nos. 851008UDS2449 and 851015UDS2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986a). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the ICR mouse. Unpublished studies
Nos. M-C-41-85 and M-C-40-85 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986b). The acute toxicity of compound 177370 (EL-870)
administered orally to the Sprague-Dawley rat. Unpublished
studies Nos. R-O-40-86 and R-O-39-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986c). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the Fischer 344 rat. Unpublished
studies Nos. R-C-02-86 and R-C-01-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Neal, S.B., & Probst, G.S. (1986d). The effect of
compound 177370 (EL-870) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters.
Unpublished study No. 851014SCE2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Oberly, T.J., Bewsey, B.J., & Probst, G.S. (1986e).
The effect of compound 177370 (EL-870) on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished studies Nos. 851106MLA2449 and
851113MLA2449 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Jordan, W.H., Rexroat, M.A., & Probst,G.S. (1986f). The effect of
compound 177370 (EL-870) on the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished studies
Nos. 850930AMS2449, 851028AMS2449 and 851111AMS2449 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Markey, T.F., Oakley, L.M., & Torrence, T.L.
(1987). The acute dermal and inhalation toxicity and ocular and
dermal irritation of tilmicosin technical. Unpublished studies
Nos. B-D-45-86, B-E-39-86 and R-H-037-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Turk, J.A. (1988). Cardiovascular
effects of tilmicosin administered intravenously in anaesthetised
beagle dogs. Unpublished study No. PC8806 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Brunny, J.D., & Garriott, M.L. (1989a). The effect
of tilmicosin on the in vivo induction of sister chromatid
exchange in bone marrow of Chinese hamsters. Unpublished study
No. 881114SCE2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Gardner, J.B., & Weaver, D.E. (1989b). An
intracutaneous sensitisation study in albino guinea pigs with
tilmicosin. Unpublished study No. GO1888 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Richardson, K.K., & Oberly, T.J. (1989c). The
effect of tilmicosin on the induction of forward mutation at the
HGPRT+ locus of Chinese hamster ovary cells. Unpublished
studies Nos. 881130CHT2449 and 890111CHO2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Helton, D.R. (1989d). CNS
behavioural effects of tilmicosin administered orally in the male
CD-1 mouse. Unpublished study No. PN8805 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Colbert, W.E. (1990). In vitro
studies of tilmicosin in the cardiac muscle of Hartley Albino
guinea pigs. Unpublished study No. PM8946 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Wolff, R.K., & Carlson, K.H. (1991). A subchronic
inhalation toxicity study of tilmicosin in Beagle dogs.
Unpublished study No. DO5589 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L., & Lochmueller, C.A. (1988a). A
study of the immune response in CD-1 mice treated orally with
tilmicosin. Unpublished study No. MI4288 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L,. & Stephenson, R.O. (1988b). A
study of the acute effects of urine and electrolyte excretion of
tilmicosin administered orally to female Crl:CD(SD) rats.
Unpublished study No. RI8688 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGuigan, M.A. (1994). Human exposures to tilmicosin (Micotil).
Vet. Hum. Toxicol., 36(4), 306-308.
McLaren, I.M. (1994). Determination of the minimum inhibitory
concentration of tilmicosin for Lactobacillus and
Propionibacterium Sp. Unpublished study from Central Veterinary
Laboratory, UK. Submitted to WHO by Lilly, Basingstoke, UK.
Montanio, C.D. & Dart, R.C. (1993). Micotil 300. Human exposures
May 1992-May 1993. Unpublished report. Submitted to WHO by Lilly,
Basingstoke, UK.
Noda, A. (1993). Teratogenicity study of EL-870 (tilmicosin
aqueous) in rabbits by gavage. Unpublished study No. 91-001 from
Research Institute for Animal Science in Biochemistry and
Toxicology, Japan. Submitted to WHO by Lilly, Basingstoke, UK.
Piroozi, K.S., Keaton, M.J., & Jordan, W.H. (1993). The acute
toxicity of tilmicosin administered orally to Fischer 344 rats.
Unpublished study No. R21493 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Rumney, C.J. (1993). Microbiological end-point determination for
two antibiotics. Unpublished study No. 1164/3/93 from Bibra
Toxicology International, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Sarazan, R.D., Main, B.W., & Jordan, W.H. (1993). Cardiovascular
effects of tilmicosin alone, or in combination with propranolol
or dobutamine, in conscious unrestrained dogs. Unpublished study
No. DV1591 from Lilly Research Laboratories. Submitted to WHO by
Lilly, Basingstoke, UK.
Scott, R.J.D., Morgan, J.M., Pether, J.V.S., & Gosling, P.
(1993). Determination of the minimum inhibitory concentrations of
tilmicosin for human isolates of Bifidobacterium and Pepto-
streptococcus. Unpublished study No. SMS/9301 from Southern
Microbiological Services, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Williams, P.D., Jordan, W.H., & Colbert, W.E. (1988). In vitro
studies of tilmicosin (EL-870, Compound 177370) in smooth and
cardiac muscle. Unpublished study No. PM8808 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Excretion
Pigs were administered a dose of 110 mg 14C-tilmicosin in the
diet over the course of one day. The recovery of radioactivity was 15%
in the urine and 80% in the faeces (Giera & Thomson, 1986).
In two studies, pigs were given a dose of 154 or 400 mg
14C-tilmicosin in the diet following a similar dose given for 5
days. The recovery of radioactivity was 4 to 6% in urine and 62 to 75%
in faeces. Radioactivity was detected in the bile but was not
quantified (Donoho & Thomson, 1988; Donoho et al., 1993).
2.1.2 Biotransformation
2.1.2.1 Rats
Tilmicosin, labelled with 14C in both the desmycosin macrolide
ring and the piperidine ring, was given orally to 15 male and 15
female Fischer-344 rats. The dosage was 20 mg/kg bw per day for 3
days. In the liver, radiolabel corresponded to tilmicosin and a
desmethyl derivative, T1(demethylated in the mycaminose ring). The
single radioactive substance identified in urine was unchanged
tilmicosin, while in faeces the major peak was parent compound with
lesser amounts of desmethyl tilmicosin and a high molecular weight
compound known to be present as an impurity in the administered
substance, T2 (consisting of two macrolide rings and one piperidine
ring) (Donoho, 1988).
Fischer-344 rats (10 males and 10 females) were given gavage
doses of 50 mg/kg bw per day 14C-tilmicosin for 5 days. An analysis
of faecal radioactivity for the presence of the sulfate metabolite
that was found in the faeces of pigs revealed the presence of a
similar compound, but quantification was not undertaken (Donoho &
Kennington, 1993).
2.1.2.2 Sheep
Beulah cross lambs were administered a single subcutaneous dose
of 20 mg/kg bw per day 14C-tilmicosin. The major radioactive
component in the liver, kidneys and urine was the parent drug,
together with lesser amounts of T1 and T2, and minor amounts of other
unidentified substances (Hawkins et al., 1993).
2.1.2.3 Pigs
Pigs were fed diets containing 14C-tilmicosin, which resulted
in daily doses of 400 mg, for 5 days. Of the radiolabel in liver and
kidney, approximately 60 to 70% was in the form of the parent drug and
there were small amounts of T1. Similarly in urine and faeces, there
were high levels of tilmicosin and low levels of T1. A further
metabolite was detected that comprised 14% of faecal and 25% of
urinary radioactivity and that was consistent with reduction of one
double bond in the macrolide ring followed by sulfation
(Donoho et al., 1993).
2.1.2.4 Cattle
In a summary of results obtained in cattle injected with
14C-tilmicosin, it was reported that the radioactivity profile in
the liver of treated rats was similar to that in the faeces. In
animals treated with a highly purified sample of tilmicosin,
metabolite T2 was not detected in the liver, suggesting that its
presence was a result of direct administration as a component of the
drug substance. Radioactivity in the kidneys was essentially in the
form of unchanged tilmicosin (Donoho, 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Major signs of toxicity in mice and rats were leg weakness,
hypoactivity, lethargy, ataxia, poor grooming and coma. Monkeys given
20 mg/kg vomited during the first day but were subsequently normal.
The single monkey given 30 mg/kg vomited, exhibited hypoactivity,
laboured respiration, vocalization and ataxia, and died within 2
hours.
New Zealand white rabbits (five males and five females) received
5000 mg/kg bw tilmicosin onto clipped skin for a period of 24 hours,
under a non-occlusive dressing. There were no deaths and no signs of
skin irritation (Jordan et al., 1987).
A volume of 0.1 ml (17 mg) of tilmicosin was instilled into one
eye of three male and three female New Zealand White rabbits.
Conjunctival hyperaemia and chemosis were noted for several days,
clearing by the end of one week (Jordan et al., 1987).
Hartley albino guinea-pigs (6 to 12 females) were given 10
intracutaneous induction doses of tilmicosin (0.05 to 0.1 ml of a
50 mg/ml solution). Fourteen days after the induction phase each
animal was injected intracutaneously with 0.05 ml tilmicosin. This
challenge dose did not result in an enhancement of skin reactions,
suggesting that a skin sensitization response had not been elicited
(Jordan et al., 1989b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Fischer-344 rats were given
gavage doses of 0, 50, 175 or 600 mg/kg bw per day tilmicosin
(purity 88%) in an aqueous vehicle. Drug administration was continued
for 2 weeks, during which there was no mortality or clinical signs of
toxicity. Food consumption was lower in males and females at 600 mg/kg
and body weight gain was depressed in males throughout the study and
in females during the first few days only.
Table 1. Results of acute toxicity studies with tilmicosin
Species Route Vehicle Sex LD503 Reference
(strain) (mg/kg bw)
Mouse sc aqueous M 97 Jordan
(ICR) F 109 et al., 1986a
Rat1 oral aqueous M 850 Jordan
(Sprague- F 800 et al., 1986b
Dawley)
Rat2 oral aqueous M & F > 2000 Piroozi
(Fischer-344) et al., 1993
Rat sc aqueous M 185 Jordan
(Fischer-344) F 440 et al., 1986c
Rat inhalation aerosol M & F > 494 (0/20) Jordan
(Fischer-344) < 4053 (14/20) et al., 1987
Rabbit dermal - M & F > 5000 Jordan
(NZ White) et al., 1987
Monkey im aqueous ? > 20 (0/2) Jordan &
(Rhesus) 30 (1/1) Shoufler, 1990
Sheep sc aqueous ? > 150 (0/5) Cochrane &
iv aqueous ? > 7.5 (1/5) Thomson, 1990
1 Animals were fasted
2 Animals were non-fasted
3 Figures in brackets represent the incidence of deaths
In the 600 mg/kg bw per day groups, there were increases in
haematocrit owing to an increase in corpuscular volume and in serum
levels of alanine aminotransferase and hepatic p-nitroanisole
O-demethylase activity. Thrombocyte counts were slightly decreased
at this dose. Urinalysis was unaffected. At the highest dose, there
were slight decreases in the weights of kidneys, spleen and ovaries,
increased adrenal weight and multifocal inflammation in the liver of
some animals. All treated animals exhibited caecal distension, which
is a typical response to high doses of an antibiotic compound
(Jordan, 1986).
Groups of 20 male and 20 female Sprague Dawley (Crl:CD) rats
received 0, 50, 250 or 1000 mg/kg bw per day tilmicosin (purity 88%)
in an aqueous vehicle, by gavage for 3 months. Signs of overt toxicity
were noted at 1000 mg/kg and included thinness, ventral soiling,
chromorhinorrhea, chromodacryorrhea, alopecia and poor grooming.
Mortality was increased at this highest dose and in females given
250 mg/kg bw per day.
Although food consumption was depressed in the 1000 mg/kg bw per
day group (males only), body weight gain was lower in males and
females at 1000 mg/kg bw per day and females at 250 mg/kg bw per day.
At 1000 mg/kg bw per day, serum alanine aminotransferase was increased
in males, blood urea nitrogen (BUN) was increased in males and
females, urinary pH was slightly lower in females and the presence of
occult blood was greater in males and females. There were no effects
on ophthalmology, haematology or hepatic p-nitroanisole
O-demethylase activity.
The weights of kidneys, liver and heart were increased in females
at 1000 mg/kg bw per day and adrenal weight was increased at 250 mg/kg
bw per day (females only) and 1000 mg/kg bw per day (males and
females). Gross pathology revealed caecal enlargement or distension at
250 mg/kg bw per day or more and small spleens in a few rats at
1000 mg/kg bw per day. Slight nephrosis was noted in two males in each
of the 250 and 1000 mg/kg bw per day groups. Other pathological
alterations were seen only in males and females of the 1000 mg/kg bw
per day group, i.e. hypertrophy of the zona fasciculata of the adrenal
cortex in most rats, necrosis of individual skeletal muscle fibres in
some rats; myocardial degeneration was increased and lymphoid
depletion was evident in the spleen and thymus of some animals. The
NOEL was 50 mg/kg bw per day (Jordan, 1988).
2.2.2.2 Dogs
Beagle dogs (two males and two females per group) were exposed by
inhalation to aerosols of 0, 12, 47 or 251 mg/m3 tilmicosin
(purity 83%). The treatment was administered for 4 hours per day on 12
days out of a 16-day period (excluding weekends). Particle median
equivalent aerodynamic diameters were between 1.2 and 1.5 µm. The mean
serum concentrations of tilmicosin were 0.23 to 0.33 and 1.67 to
2.46 µg/ml at 47 and 251 mg/m3 respectively. Drug levels at
12 mg/m3 were below the detection limit of 0.1 µg/ml.
One male given 251 mg/m3 died on the final day of exposure but
no other overt signs of toxicity were observed. Heart rates were
increased in dogs at 251 mg/m3. Food intake, body weight,
haematology and blood chemistry were unaffected. At autopsy, lung
weight was increased in females given 251 mg/m3 and inflammation was
noted in the respiratory tract at 47 mg/m3 (females only) and
251 mg/m3 (males and females) (Jordan et al., 1991).
Groups of four male and four female beagle dogs were given 0, 6,
20 or 70 mg/kg bw per day tilmicosin (purity 88%) in capsules for 3
months. The daily dose was administered in two equal amounts, 6 hours
apart. Serum concentrations of tilmicosin were measured 3 hours and 24
hours after the first of the two daily doses in weeks 2, 3, 5, 9 and
13. At increasing doses, the mean concentrations were 0.1 to 0.2, 0.74
to 1.49 and 3.24 to 6.05 µg/ml at 3 hours and 0, 0.26 to 0.59 and 1.72
to 3.96 µg/ml at 24 hours.
Half the 70 mg/kg bw per day males and females died during the
first month. Clinical signs prior to death were pale mucous membranes
in two dogs and ataxia in one dog. Food intake, body weight, haemato-
logy and urinalysis were unaffected. Serum alanine aminotransferase
activity was progressively increased at 70 mg/kg bw per day, while
hepatic p-nitroanisole O-demethylase activity was increased
in females at this level.
Two of the four surviving dogs at 70 mg/kg bw per day showed
bilateral multifocal areas of subretinal fluid concentrated along
arterioles in the tapetal region of the eye. The changes were claimed
to be consistent with those associated with systemic hypertension. One
of these dogs also had retinal degeneration and the other showed
miosis with normal pupillary light responses.
Heart rate increases were dose related, being marked at 70 mg/kg
bw per day and moderate to severe at 20 mg/kg bw per day. The increase
in males given 6 mg/kg bw per day was not significant. At the highest
dose, examination of the electrocardiogram revealed depression of the
ST segment.
At 70 mg/kg bw per day, heart weight was increased in males and
females, and liver and kidney weights were increased in females. Gross
necropsy showed small spleens in the females that died during the
study and an enlarged heart in a surviving male at 70 mg/kg bw per
day. Slight diffuse mucosal oedema was seen in the gall bladders of
two dogs at 70 mg/kg bw per day. Despite the physiological changes in
the eyes and heart, there were no associated pathological alterations.
The NOEL was 6 mg/kg bw per day (Jordan, 1987).
Groups of four male and four female beagle dogs were given 0, 4,
12 or 36 mg/kg bw per day tilmicosin (purity 86%) in capsules for 12
months. The daily dose was administered in two equal amounts, 6 hours
apart. There were no deaths. Peripheral redness was seen sporadically
in some animals of all groups, in particular at 12 and 36 mg/kg bw per
day.
Body weight gains were lower at > 12 mg/kg bw per day. There
were no effects on ophthalmology, haematology, blood chemistry and
urinalysis. Heart rates were markedly increased at 36 mg/kg bw per day
with sporadic depression of the ST segment in some dogs.
Heart weight was increased at 36 mg/kg bw per day and four males
and one female at this dose showed enlarged hearts. Mild chronic
dermatitis was noted in the external ears of dogs from all treated
groups. The findings included minimal thickening of the epidermis,
foci of acantholysis and inflammatory cell infiltration in the dermis.
These changes were only slight and non-dose-related, and therefore of
questionable relationship to treatment. The NOEL was 4 mg/kg bw per
day (Jordan & Bernhard, 1989).
2.2.3 Long-term toxicity/carcinogenicity studies
Tilmicosin has not been tested in toxicological studies longer
than 12 months in duration and hence the carcinogenic potential of
this drug has not been directly determined. There are a number of
observations which are relevant in assessing the carcinogenicity of
tilmicosin:
a) The results of available toxicological studies with tilmicosin
have not identified lesions or proliferative changes which could
be considered suggestive of potential neoplasia.
b) Tilmicosin has been tested in a wide range of genotoxicity
assays. All results were negative and it was concluded that the
compound has no genotoxic activity.
c) Tilmicosin is a macrolide antibiotic. This class of chemical has
been in widespread usage in humans for many years but there is no
evidence of carcinogenicity. Tylosin is the closest structural
analogue and this chemical was reviewed by the thirty-eighth
meeting of JECFA (Annex 1, reference 97).
2.2.3.1 Reconsideration of tylosin tumorigenicity
Tylosin was tested in 2-year feeding studies in rats. The
findings revealed an association between drug administration at doses
of 150 and 300 mg/kg bw per day in the diet and an increased incidence
of pituitary adenomas in males. While it was claimed that the increase
in the incidence of tumours was an indirect result of the ability of
tylosin to increase survival and weight gain, supporting data were not
available to the Committee and an ADI was not established.
New information, not considered at the thirty-eighth meeting,
indicates that spontaneous pituitary tumours occur in male rats at
variable rates with increased rates occurring in animals with higher
body weights (Gries & Young, 1982). In the tylosin studies, 12-month
body weights were somewhat higher in treated males rats and mortality
was increased in control animals due to respiratory infection. The
highest observed incidence of tumours in tylosin-treated groups was
comparable to the upper limit of the historical control range (23%) in
the test facility.
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A dose-ranging study was carried out in groups of 10 female
Sprague-Dawley (Crl:COBS CD) rats given gavage doses of 0, 50, 125,
250, 500 or 750 mg/kg bw per day tilmicosin (purity unknown). The
females were treated from 14 days before mating with untreated males
until the end of study on post-partum day 4.
Excess salivation was dose-related at > 250 mg/kg bw per day.
Chromodacryorrhea, urine-stained fur and alopecia were seen in some
rats at 750 mg/kg bw per day; the three most severely affected died
during the study. Prior to death, these animals exhibited reduced food
intake, lost weight and were emaciated. Body weight gain was depressed
in surviving rats at 750 mg/kg bw per day during the pre-mating period
while food intake was reduced at > 250 mg/kg bw per day in week 1
and at 750 mg/kg bw per day in week 2. The pregnancy rate was
decreased at > 500 mg/kg bw per day. Duration of gestation, litter
size and weight, pup survival and weight gain to post-partum day 4
were unaffected (Dearlove et al., 1987).
Groups of 30 male and 30 female Sprague Dawley (Crl: COBS CD BR)
rats were administered 0, 10, 45 or 200 mg/kg bw per day tilmicosin
(purity 87%) by gavage in an aqueous vehicle. Treatment commenced 70
days (males) and 14 days (females) before the first mating period and
was continued through two litters per generation for two generations.
F1 litters were culled to five pups per sex on post-partum day 4 and
F1b pups (40 males and 40 females per group) were bred to produce
the following generation. F2a and F2b pups were killed on
post-partum day 4.
In adult animals, salivation was noted in males and females at
200 mg/kg bw per day, but there were no other signs of toxicity. Body
weight gain and food consumption were depressed in females of the 45
and 200 mg/kg bw per day groups during the pre-mating period only.
During the production of the four litters in this study there were no
effects on mating performance, pregnancy rates, duration of gestation,
litter size and weight and the weight gain of offspring. In both F1
litters at 200 mg/kg bw per day, pup mortality was slightly increased
up to post-partum day 4, but the finding was not duplicated in either
F2 litter. The NOEL was 10 mg/kg bw per day in adult rats (Christian
& Hoberman, 1989).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 25 presumed pregnant female Sprague Dawley (Crl: CD)
rats were administered gavage doses of 0, 10, 70 or 500 mg/kg bw per
day tilmicosin (purity 86%) in an aqueous vehicle. The dams were
treated on gestation days 6 to 15 and were killed on gestation day 20.
Increased salivation was seen at 70 and 500 mg/kg bw per day and
alopecia at 500 mg/kg bw per day. There were no deaths or abortions.
Body weight gain was reduced at 70 and 500 mg/kg bw per day, and food
intake was reduced at 500 mg/kg bw per day, during gestation days 6 to
10.
The number of resorption or live fetuses, fetal weight, sex ratio
and the incidence of fetal malformations were similar between groups.
The incidences of total skeletal and visceral anomalies were increased
in treated groups, but there was no dose-response relationship and the
findings were within the historical control values for the laboratory.
The NOEL for maternotoxicity was 10 mg/kg bw per day (Jordan & Higdon,
1988).
2.2.5.2 Rabbits
Groups of 15 presumed pregnant female Japanese White-NIBS rabbits
were given gavage doses of 0, 8, 19 or 48 mg/kg bw per day tilmicosin
(purity unknown) in an aqueous vehicle. Does were treated on gestation
days 6 to 18 and were killed on gestation day 28. One female given
48 mg/kg bw per day aborted on gestation day 26 and died. Reduced
faeces were seen at 19 and 48 mg/kg bw per day with only a transient
effect at 8 mg/kg bw per day. During the treatment period food intake
was depressed in a dose-related manner and body weight loss was
observed at 19 and 48 mg/kg bw per day.
Fetal and placental weight tended to decrease at 19 and 48 mg/kg
bw per day but did not achieve statistical significance. There were no
meaningful effects on the incidence of resorptions or fetal deaths or
on sex ratio. Open eyelids were observed in 11/91 and 16/68 fetuses
from the 19 and 48 mg/kg bw per day groups, respectively, and some of
these fetuses showed cleft palate or club foot. The affected fetuses
had low body weights and the seven litters involved were derived from
does that had lost body weight during drug administration. Skeletal
examination revealed retardation of fetal development at 19 and
48 mg/kg bw per day. Similar effects have been seen in this laboratory
in dietary restricted rabbits, suggesting the effects on the fetus
were secondary to maternal malnutrition (Noda, 1993).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on tilmicosin
Test system1 Test object Concentration Results Reference
Reverse S. typhimurium 1 - 100 µg/plate negative Jordan
mutation2 TA98, TA100, (± S9) et al., 1986f
TA1535, TA1537,
TA1538
Reverse E. coli 1 - 100 µg/plate negative Garriott
mutation2 WP2uvrA (± S9) et al., 1993
Forward L5178Y mouse 100-900 µg/ml negative Jordan
mutation2 lymphoma cells (- S9) et al., 1986e
200-1000 µg/ml
(+ S9)
Forward HGPRT+ Chinese 25-250 µg/ml negative Jordan
mutation2 Hamster ovary (- S9) et al., 1989c
cells 50-300/ µg/ml
(+ S9)
Unscheduled primary cultures 0.5-10 µg/ml negative Jordan
DNA synthesis of rat hepatocytes et al., 1985
assay
Sister chromatid Chinese hamster 200-1800 × 1 negative Jordan et al.,
exchange bone marrow mg/kg bw oral 1986d, 1989a
assay
Chromosome rat bone marrow 180-1800 × 1 and negative Jordan &
aberrations 100-1000 × 5, Ivett, 1989
mg/kg bw per
day oral
1 Positive controls used
2 Both with and without liver microsomal activation
2.2.7 Special study on the immune system
Groups of eight male CD-1 mice were given gavage doses of 0,
10, 250, 500 or 1000 mg/kg bw per day tilmicosin (purity 87%) in
an aqueous vehicle. Drug treatment was continued for 10 days.
After the third dose mice were injected intravenously with sheep
red blood cells. At the end of the study, serum from each mouse
was assayed for antibodies (haemagglutinin). There was one death
at 500 mg/kg bw per day and six at 1000 mg/kg bw per day, but
antibody production was not affected at any level (McGrath
et al., 1988a).
2.2.8 Special studies on microbiological activity
2.2.8.1 In vitro
The antibacterial activity of tilmicosin was determined
against a range of organisms representative of human intestinal
flora. Cultures were initiated using inoculum sizes of between
3 × 105 and 1.6 × 106 cells per spot on blood agar plates and
incubated anaerobically at 37°C.
The median MIC values for the clinical isolates of
Bifidobacterium and Peptostreptococcus, respectively, were
0.015 and 0.5 µg/ml. The above results were obtained at pH 7.7.
At pH 6.6 the MIC values were one to two orders of magnitude
higher (Scott et al., 1993).
The antibacterial activity of tilmicosin was examined
against a number of organisms used in industrial food processing
and originally obtained from dairy products. Cultures were
initiated in blood agar plates using three strains of
Propionibacterium and four strains of Lactobacillus. Under
anaerobic conditions the MICs were between 8 and 64 µg/ml and
under microaerophilic conditions the values were between 4 and
32 µg/ml (McLaren, 1994).
2.2.8.2 Rats
In two separate studies, female germ-free rats were orally
dosed with 1 ml of 10% (w/v) pooled human faecal suspension.
Three weeks later groups of two males and two females were given
gavage doses of 0, 0.12 or 0.4 mg/kg bw per day tilmicosin
(purity 87%) for 5 days. Rat faecal samples were collected before
treatment, daily during treatment and once during each of the 2
weeks following cessation of dosing. Body weight gain remained
unaffected.
The animals in each study received faecal suspensions
obtained from different donors. Results from different studies
varied widely, possibly reflecting differences between the
bacterial populations used. Total anaerobes were not reduced by
tilmicosin treatment. Total enterobacterial counts and the
proportion of enterobacteria with respect to total anaerobe
counts were transiently increased at 0.4 mg/kg bw per day in one
study only. Tilmicosin-resistant enterobacteria were not
significantly increased in either study. Spiramycin, at a dose of
0.5 mg/kg bw per day, increased total enterobacterial counts and
the number of spiramycin-resistant enterobacteria; the latter
appeared to remain high after the cessation of treatment. The
NOEL for tilmicosin was 0.4 mg/kg bw per day (Rumney, 1993).
2.2.9 Special studies on pharmacology
Table 3. Results of pharmacological assays with tilmicosin
Test system Doses Results Reference
Isolated 0.0009 to No effect on non-stimulated organ. Williams
guinea-pig 90 µg/ml 90 µg/ml slightly inhibited acetylcholine- et al.,
ileum and angiotensin-induced contraction 1988
and significantly inhibited electrically
stimulated contraction
Isolated 0.0009 to No effect on non-stimulated tissue and Williams
rat uterus 90 µg/ml no change to oxytocin-induced et al.,
(estrogen contractions 1988
primed)
Isolated rat 0.0009 to No effect on non-stimulated tissue and Williams
vas deferens 90 µg/ml no change to noradrenaline-induced et al.,
contractions 1988
Isolated 0.0009 to In spontaneously beating tissue, > 90 Williams
guinea-pig 900 µg/ml µg/ml decreased force of contraction; et al.,
atria and 900 µg/ml increased rate of 1988;
contraction. 90 µg/ml inhibited Jordan
isoprenaline-, noradrenaline- and Bay et al.,
K8644 (calcium agonist)-induced force 1990
and rate of contraction, and
histamine-induced rate of contraction.
Table 3. Results of pharmacological assays with tilmicosin (cont'd).
Test system Doses Results Reference
Conscious 0.25, 1, 2.5 Heart rate increased at > 1 mg/kg. Left Jordan &
dogs or 5 mg/kg ventricular function decreased at > 0.25 Sarazan,
iv mg/kg. Aortic pulse pressure decreased 1991;
at > 1 mg/kg. ECG revealed "Ventricular Sarazan
Alternans" (ventricular contraction fails et al.,
to achieve adequate pressure to open 1993
aortic valve and cause an arterial pulse).
Anaesthetised 0.5, 1 or 5 Heart rate increased, stroke volume, Jordan
dogs mg/kg iv stroke work index and cardiac output et al.,
decreased at > 1 mg/kg. At 5 mg/kg 2/3 1988
dogs developed second degree heart block
CD-1 mice 10, 100, Piloerection and grasping loss at > 500 Jordan
500 or mg/kg. Ptosis at 1000 mg/kg. No effect et al.,
1000 mg/kg on electroshock- or pentylenetetrazol- 1989d
oral induced seizures or on body temperature.
Hexobarbital-induced sleep time
prolonged by 500 (x1) and 1000 (x3) mg/kg
Sprague- 10, 30, 90 Urine volume and creatinine reduced McGrath
Dawley or 270 at 270 mg/kg with increases in osmolality et al.,
rats mg/kg oral and electrolytes. 1988b
2.3 Observations in humans
A total of 241 human exposures to tilmicosin (Micotil) were
reported to the Rocky Mountain Poison Center from May 1992 to May
1993. Needlesticks and scrapes (n=112) and accidental injection
(n=43) caused either no effect or local reactions including
soreness, numbness, stinging, swelling, redness, burning and
stiffness. Some injected subjects experienced anxiety, sweating,
headache and lightheadedness. Skin exposures (n=50) resulted in
redness and tingling of the skin, and eye exposures (n=11)
resulted in stinging and swelling. Persons accidentally ingesting
the drug (n=39) reported either no symptoms or bitter taste,
nausea, numbness of lips and tongue, vomiting, thirst and
headache (Montanio & Dart, 1993).
Over a 30-month period, 36 cases of accidental exposure to
tilmicosin (Micotil) were reported to the Ontario Regional Poison
Information Centre. Percutaneous injection (n=26) always resulted
in pain at the site and seven subjects mentioned a variety of
local reactions consistent with an irritant action. One person
showed "peaked T waves" 30 minutes following the injection of
1 ml into his arm, but ECG changes were not noted in other
subjects. The remaining subjects received splashes into the mouth
or eyes or onto the skin. Unpleasant taste, a burning sensation
on the hard palate and ocular irritation were each recorded in
one person (McGuigan, 1994).
3. COMMENTS
The Committee considered toxicological data on tilmicosin,
including the results of studies on acute and short-term
toxicity, pharmacokinetics, metabolism, reproductive toxicity,
teratogenicity, genotoxicity, antimicrobial activity and
pharmacology. The Committee also considered observations in
humans accidentally exposed to tilmicosin.
Tilmicosin is absorbed from the gastrointestinal tract as
shown by the recovery of radiolabelled compound in urine and/or
bile of orally dosed pigs (110 to 400 mg per animal), and the
presence of dose-related serum concentrations of tilmicosin dosed
orally in dogs for 3 months. However, the results from available
studies did not allow any conclusion to be reached concerning the
extent of absorption. The metabolites detected in a range of farm
animals were also found in rats, which suggests that the rat is a
suitable model for determining the potential toxicological
hazards posed by tilmicosin.
The LD50 in fasted rats was 800 to 850 mg/kg bw, but
toxicity was substantially lower in non-fasted animals, among
which there were no deaths following administration of a single
dose of 2000 mg tilmicosin/kg bw. Studies in a range of
pharmacological models identified depression of cardiac muscle
contractility and reduced heart function as the main adverse
effects.
Rats were administered oral doses of 50, 250 or 1000 mg/kg
bw per day for 3 months. At 1000 mg/kg bw per day, animals
exhibited chromorhinorrhea, chromodacryorrhea, alopecia, poor
grooming, reduced food consumption and they appeared thin.
Body-weight gain was reduced and mortality was increased in
females at 250 mg/kg bw per day and in both sexes at 1000 mg/kg
bw per day. Toxic effects on the kidney and liver were indicated
by increased organ weights, increased serum levels of alanine
aminotransferase and blood urea nitrogen, and the presence of
blood in the urine. However, pathological examination of these
organs showed only slight nephrosis in two males in each of the
groups given 250 or 1000 mg/kg bw per day. At the highest dose,
hypertrophy of the zona fasciculata of the adrenal cortex was
noted in most rats, and myocardial degeneration, necrosis of
individual skeletal muscle fibres and lymphoid depletion in the
spleen and thymus were seen in some rats. The NOEL was 50 mg/kg
bw per day.
Dogs were given oral doses of 6, 20 or 70 mg/kg bw per day
for three months. Half of the animals given 70 mg/kg bw per day
died during the first month. Heart rate was increased at 20 and
70 mg/kg bw per day and at the higher dose there was depression
of the ST segment of the electrocardiogram. Ophthalmoscopic
examination of the eyes showed bilateral areas of subretinal
fluid concentrated along arterioles in the tapetal region of two
of the four surviving dogs at 70 mg/kg bw per day. The NOEL was
6 mg/kg bw per day.
Dogs were given oral doses of 4, 12 or 36 mg/kg bw per day
for twelve months. Body weight gain was depressed at 12 and
36 mg/kg bw per day. Heart rate was markedly increased at 36
mg/kg bw per day, with occasional depression of the ST segment
of the electrocardiogram. Hearts were enlarged at 36 mg/kg bw per
day, but there were no associated pathological changes. The NOEL
was 4 mg/kg bw per day.
In a two-generation reproductive toxicity study, rats were
administered oral doses of 10, 45 or 200 mg tilmicosin/kg bw per
day. Body weight gain and food consumption were depressed in
females given 45 or 200 mg/kg bw per day, but only during the
pre-mating period. Fertility and reproduction parameters were
unaffected at any dose level. At 200 mg/kg bw per day, mortality
in pups was slightly increased in both first-generation litters
up to post-partum day 4, but not in either second-generation
litter. The NOEL was 10 mg/kg bw per day.
Developmental toxicity studies were reported in rats and
rabbits. Rats were dosed with 10, 70 or 500 mg tilmicosin/kg bw
per day by gavage. Growth and development in rat fetuses were not
affected by treatment, but increased salivation and reduced body
weight gain of dams were observed at 70 and 500 mg/kg bw per day.
The NOEL for maternal toxicity in rats was 10 mg/kg bw per day.
In the study in rabbits, there were dose-related signs of
toxicity in the does at all doses (8, 19 and 48 mg/kg bw per
day). Severely affected members of the groups given 19 or
48 mg/kg bw per day produced offspring that had open eyelids and
low incidences of cleft palate or club foot. These fetuses had
low body weights and were clearly retarded in development. The
effects were attributed to treatment-related malnutrition in
maternal animals, which is commonly observed in rabbits dosed
with antibiotics, confirming that this species is an inappropriate
model for studying the teratogenic potential of antibiotics.
Tilmicosin has been tested for reverse mutation in bacteria,
forward mutation in cultured mammalian cells, unscheduled DNA
synthesis in primary cultures of rat hepatocytes, and in in vivo
cytogenetic assays. All results were negative, and the Committee
concluded that tilmicosin has no genotoxic potential.
The carcinogenic potential of tilmicosin has not been
tested. However, a number of factors are relevant in assessing
the carcinogenicity of this drug. Toxicity studies with
tilmicosin have not resulted in lesions or proliferative changes
suggestive of neoplasia, and tilmicosin was uniformly negative in
a wide range of genotoxicity assays. Tilmicosin is a macrolide
antibiotic, and this class of chemicals is not known to induce
neoplasia despite widespread use in humans over many years. The
closest structural analogue, tylosin, was reviewed at the
thirty-eighth meeting of the Committee (Annex 1, reference 97).
In a 2-year feeding study in rats, tylosin administration was
associated with an increased incidence of pituitary adenomas in
male animals when compared to concurrent controls. New evidence,
not considered at the thirty-eighth meeting, indicates that
spontaneous pituitary neoplasms occur in male rats at variable
rates. Additionally, increased rates occur in animals with higher
body weights. In tylosin-treated groups of male rats, the
12-month body weights were somewhat higher than in the control
groups and the incidences of pituitary adenomas were comparable
to the upper limit of the historical control range. The lower
incidence of pituitary neoplasms in the control animals may have
been due to the earlier mortality in this group caused by
respiratory disease. In light of this information the Committee
considered that the concerns of the thirty-eighth meeting over
the potential tumorigenicity of tylosin had been satisfactorily
addressed and that there was no concern for the carcinogenic
potential of tylosin. For the above reasons, the Committee
considered that carcinogenicity studies would not be required for
tilmicosin.
In assessing the microbiological activity of tilmicosin, the
results of a study using germ-free rats colonized with human
intestinal microflora was considered the most relevant. At the
highest dose administered (0.4 mg/kg bw per day for 5 days) there
were no significant alterations in numbers of total anaerobes or
enterobacteria in rat faeces.
Reports of accidental human exposure to tilmicosin have
identified local reactions indicative of an irritant action
following dermal and ocular exposure. Humans accidentally
injected with tilmicosin have reported anxiety, sweating,
headache and lightheadedness. Changes in the electrocardiogram
pattern were observed in only one person. Contact with the buccal
mucosa or ingestion has resulted in a range of symptoms including
nausea, vomiting, thirst, numbness or burning sensation in the
mouth, and headache.
4. EVALUATION
The NOEL from toxicological studies was 4 mg/kg bw per day
in a 12-month study in dogs. Treatment of rats colonized with
human intestinal microflora with 0.4 mg tilmicosin/kg bw per day
produced no significant microbiological effect. The Committee
established an ADI of 0-40 µg/kg bw, based on the toxicological
NOEL of 4 mg/kg bw per day and a safety factor of 100. An
identical ADI would have been established using the data from the
study on rats colonized with human intestinal microflora and a
safety factor of 10 to account for variability among humans.
5. REFERENCE
Christian, M.S. & Hoberman, A.M. (1989). Reproductive effects of
EL-870 administered orally via gavage to Crl:COBS CD(SD) BR rats
for two generations, with two litters per generation. Unpublished
study No. 112-001 from Argus Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Cochrane, R.L. & Thomson, T.D. (1990). Toxicology and pharmacology
of tilmicosin following administration of subcutaneous and intravenous
injections to sheep. Unpublished study No. T5C768908 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Dearlove, G.H., Hoberman, A.M., & Christian, M.S. (1987). Dosage-range
study of EL-870 administered orally via gavage to Crl:COBS CD(SD) BR
rats (Pilot study). Unpublished study No. 112-001P from Argus Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. (1988). Comparative metabolism of 14C-tilmicosin
in cattle and rats. Unpublished study No. ABC-0395 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. & Kennington, A.S. (1993). Tilmicosin metabolite
study with rat excreta. Unpublished study No. T5C759302 from
Lilly Research Laboratories. Submitted to WHO by Lilly, Basingstoke,
UK.
Donoho, A.L. & Thomson, T.D. (1988). 14C-Tilmicosin balance-
excretion study in swine. Unpublished study No. ABC-0409 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L., Darby, J.M., Helton, S.L., Sweeney, D.J.,
Occolowitz, J.L., & Dorman, D.E. (1993). Tilmicosin metabolism
study in tissues and excreta of pigs fed 400 ppm 14C-tilmicosin.
Unpublished study No. T5C759201 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Garriott, M.L., Rexroat, M.A., & Jordan, W.H. (1993). The effect
of tilmicosin on the induction of reverse mutations in
Escherichia coli using the Ames test. Unpublished study
No. 920825AMS2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Giera, D.D. & Thomson, T.D. (1986). Preliminary 14C EL-870
balance excretion and tissue residue in swine. Unpublished study
No. ABC-0305 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Gries, C.L. & Young, S.S. (1982). Positive correlation of body
weight with pituitary tumor incidence in rats. Fundam. Appl.
Toxicol., 2, 145-148.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A,. &
Cameron, D.M. (1993). The metabolism and residues of 14C-tilm-
icosin following subcutaneous administration to sheep. Unpublished
study No. HRC/LLY 36/930447 from Huntingdon Research Centre. Submitted
to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1986). The toxicity of compound 177370 (EL-870)
given by oral gavage to Fischer 344 rats for two weeks.
Unpublished study No. RI5585 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1987). The toxicity of EL-870 given orally to
Beagle dogs for three months. Unpublished study No. DO8286 from
Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. (1988). The toxicity of tilmicosin given orally to
Crl:CD(SD) rats for three months. Unpublished study No. RO9886
from Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. & Bernhard, N.R. (1989). A one year chronic toxicity
study in Beagle dogs given oral doses of tilmicosin. Unpublished
study No. DO7187 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Higdon, G.L. (1988). A teratology study of
tilmicosin (EL-870, compound 177370) administered orally to CD
rats. Unpublished study No. RI3387 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Ivett, J.L. (1989). Mutagenicity test on
tilmicosin in the rat bone marrow cytogenetic assay. Unpublished
study No. 10646-0-452 from Hazleton Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Sarazan, R.D. (1991). An acute study of the
cardiovascular effects of intravenous Micotil 300 in conscious
dogs. Unpublished study No. DV0890 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Shoufler, J.R. (1990). The acute toxicity of
Micotil 300 administered intramuscularly to Rhesus monkeys.
Unpublished studies Nos. PO4089 and PO7689 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Hill, L.E., & Probst, G.S. (1985). The effect of
compound 177370 (EL-870) on the induction of DNA repair synthesis
in primary cultures of adult rat hepatocytes. Unpublished studies
Nos. 851008UDS2449 and 851015UDS2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986a). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the ICR mouse. Unpublished studies
Nos. M-C-41-85 and M-C-40-85 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986b). The acute toxicity of compound 177370 (EL-870)
administered orally to the Sprague-Dawley rat. Unpublished
studies Nos. R-O-40-86 and R-O-39-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986c). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the Fischer 344 rat. Unpublished
studies Nos. R-C-02-86 and R-C-01-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Neal, S.B., & Probst, G.S. (1986d). The effect of
compound 177370 (EL-870) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters.
Unpublished study No. 851014SCE2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Oberly, T.J., Bewsey, B.J., & Probst, G.S. (1986e).
The effect of compound 177370 (EL-870) on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished studies Nos. 851106MLA2449 and
851113MLA2449 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Jordan, W.H., Rexroat, M.A., & Probst,G.S. (1986f). The effect of
compound 177370 (EL-870) on the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished studies
Nos. 850930AMS2449, 851028AMS2449 and 851111AMS2449 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Markey, T.F., Oakley, L.M., & Torrence, T.L.
(1987). The acute dermal and inhalation toxicity and ocular and
dermal irritation of tilmicosin technical. Unpublished studies
Nos. B-D-45-86, B-E-39-86 and R-H-037-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Turk, J.A. (1988). Cardiovascular
effects of tilmicosin administered intravenously in anaesthetised
beagle dogs. Unpublished study No. PC8806 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Brunny, J.D., & Garriott, M.L. (1989a). The effect
of tilmicosin on the in vivo induction of sister chromatid
exchange in bone marrow of Chinese hamsters. Unpublished study
No. 881114SCE2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Gardner, J.B., & Weaver, D.E. (1989b). An
intracutaneous sensitisation study in albino guinea pigs with
tilmicosin. Unpublished study No. GO1888 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Richardson, K.K., & Oberly, T.J. (1989c). The
effect of tilmicosin on the induction of forward mutation at the
HGPRT+ locus of Chinese hamster ovary cells. Unpublished
studies Nos. 881130CHT2449 and 890111CHO2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Helton, D.R. (1989d). CNS
behavioural effects of tilmicosin administered orally in the male
CD-1 mouse. Unpublished study No. PN8805 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Colbert, W.E. (1990). In vitro
studies of tilmicosin in the cardiac muscle of Hartley Albino
guinea pigs. Unpublished study No. PM8946 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Wolff, R.K., & Carlson, K.H. (1991). A subchronic
inhalation toxicity study of tilmicosin in Beagle dogs.
Unpublished study No. DO5589 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L., & Lochmueller, C.A. (1988a). A
study of the immune response in CD-1 mice treated orally with
tilmicosin. Unpublished study No. MI4288 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L,. & Stephenson, R.O. (1988b). A
study of the acute effects of urine and electrolyte excretion of
tilmicosin administered orally to female Crl:CD(SD) rats.
Unpublished study No. RI8688 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGuigan, M.A. (1994). Human exposures to tilmicosin (Micotil).
Vet. Hum. Toxicol., 36(4), 306-308.
McLaren, I.M. (1994). Determination of the minimum inhibitory
concentration of tilmicosin for Lactobacillus and
Propionibacterium Sp. Unpublished study from Central Veterinary
Laboratory, UK. Submitted to WHO by Lilly, Basingstoke, UK.
Montanio, C.D. & Dart, R.C. (1993). Micotil 300. Human exposures
May 1992-May 1993. Unpublished report. Submitted to WHO by Lilly,
Basingstoke, UK.
Noda, A. (1993). Teratogenicity study of EL-870 (tilmicosin
aqueous) in rabbits by gavage. Unpublished study No. 91-001 from
Research Institute for Animal Science in Biochemistry and
Toxicology, Japan. Submitted to WHO by Lilly, Basingstoke, UK.
Piroozi, K.S., Keaton, M.J., & Jordan, W.H. (1993). The acute
toxicity of tilmicosin administered orally to Fischer 344 rats.
Unpublished study No. R21493 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Rumney, C.J. (1993). Microbiological end-point determination for
two antibiotics. Unpublished study No. 1164/3/93 from Bibra
Toxicology International, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Sarazan, R.D., Main, B.W., & Jordan, W.H. (1993). Cardiovascular
effects of tilmicosin alone, or in combination with propranolol
or dobutamine, in conscious unrestrained dogs. Unpublished study
No. DV1591 from Lilly Research Laboratories. Submitted to WHO by
Lilly, Basingstoke, UK.
Scott, R.J.D., Morgan, J.M., Pether, J.V.S., & Gosling, P.
(1993). Determination of the minimum inhibitory concentrations of
tilmicosin for human isolates of Bifidobacterium and Pepto-
streptococcus. Unpublished study No. SMS/9301 from Southern
Microbiological Services, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Williams, P.D., Jordan, W.H., & Colbert, W.E. (1988). In vitro
studies of tilmicosin (EL-870, Compound 177370) in smooth and
cardiac muscle. Unpublished study No. PM8808 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Excretion
Pigs were administered a dose of 110 mg 14C-tilmicosin in the
diet over the course of one day. The recovery of radioactivity was 15%
in the urine and 80% in the faeces (Giera & Thomson, 1986).
In two studies, pigs were given a dose of 154 or 400 mg
14C-tilmicosin in the diet following a similar dose given for 5
days. The recovery of radioactivity was 4 to 6% in urine and 62 to 75%
in faeces. Radioactivity was detected in the bile but was not
quantified (Donoho & Thomson, 1988; Donoho et al., 1993).
2.1.2 Biotransformation
2.1.2.1 Rats
Tilmicosin, labelled with 14C in both the desmycosin macrolide
ring and the piperidine ring, was given orally to 15 male and 15
female Fischer-344 rats. The dosage was 20 mg/kg bw per day for 3
days. In the liver, radiolabel corresponded to tilmicosin and a
desmethyl derivative, T1(demethylated in the mycaminose ring). The
single radioactive substance identified in urine was unchanged
tilmicosin, while in faeces the major peak was parent compound with
lesser amounts of desmethyl tilmicosin and a high molecular weight
compound known to be present as an impurity in the administered
substance, T2 (consisting of two macrolide rings and one piperidine
ring) (Donoho, 1988).
Fischer-344 rats (10 males and 10 females) were given gavage
doses of 50 mg/kg bw per day 14C-tilmicosin for 5 days. An analysis
of faecal radioactivity for the presence of the sulfate metabolite
that was found in the faeces of pigs revealed the presence of a
similar compound, but quantification was not undertaken (Donoho &
Kennington, 1993).
2.1.2.2 Sheep
Beulah cross lambs were administered a single subcutaneous dose
of 20 mg/kg bw per day 14C-tilmicosin. The major radioactive
component in the liver, kidneys and urine was the parent drug,
together with lesser amounts of T1 and T2, and minor amounts of other
unidentified substances (Hawkins et al., 1993).
2.1.2.3 Pigs
Pigs were fed diets containing 14C-tilmicosin, which resulted
in daily doses of 400 mg, for 5 days. Of the radiolabel in liver and
kidney, approximately 60 to 70% was in the form of the parent drug and
there were small amounts of T1. Similarly in urine and faeces, there
were high levels of tilmicosin and low levels of T1. A further
metabolite was detected that comprised 14% of faecal and 25% of
urinary radioactivity and that was consistent with reduction of one
double bond in the macrolide ring followed by sulfation
(Donoho et al., 1993).
2.1.2.4 Cattle
In a summary of results obtained in cattle injected with
14C-tilmicosin, it was reported that the radioactivity profile in
the liver of treated rats was similar to that in the faeces. In
animals treated with a highly purified sample of tilmicosin,
metabolite T2 was not detected in the liver, suggesting that its
presence was a result of direct administration as a component of the
drug substance. Radioactivity in the kidneys was essentially in the
form of unchanged tilmicosin (Donoho, 1988).
2.2 Toxicological studies
2.2.1 Acute studies
Major signs of toxicity in mice and rats were leg weakness,
hypoactivity, lethargy, ataxia, poor grooming and coma. Monkeys given
20 mg/kg vomited during the first day but were subsequently normal.
The single monkey given 30 mg/kg vomited, exhibited hypoactivity,
laboured respiration, vocalization and ataxia, and died within 2
hours.
New Zealand white rabbits (five males and five females) received
5000 mg/kg bw tilmicosin onto clipped skin for a period of 24 hours,
under a non-occlusive dressing. There were no deaths and no signs of
skin irritation (Jordan et al., 1987).
A volume of 0.1 ml (17 mg) of tilmicosin was instilled into one
eye of three male and three female New Zealand White rabbits.
Conjunctival hyperaemia and chemosis were noted for several days,
clearing by the end of one week (Jordan et al., 1987).
Hartley albino guinea-pigs (6 to 12 females) were given 10
intracutaneous induction doses of tilmicosin (0.05 to 0.1 ml of a
50 mg/ml solution). Fourteen days after the induction phase each
animal was injected intracutaneously with 0.05 ml tilmicosin. This
challenge dose did not result in an enhancement of skin reactions,
suggesting that a skin sensitization response had not been elicited
(Jordan et al., 1989b).
2.2.2 Short-term toxicity studies
2.2.2.1 Rats
Groups of 20 male and 20 female Fischer-344 rats were given
gavage doses of 0, 50, 175 or 600 mg/kg bw per day tilmicosin
(purity 88%) in an aqueous vehicle. Drug administration was continued
for 2 weeks, during which there was no mortality or clinical signs of
toxicity. Food consumption was lower in males and females at 600 mg/kg
and body weight gain was depressed in males throughout the study and
in females during the first few days only.
Table 1. Results of acute toxicity studies with tilmicosin
Species Route Vehicle Sex LD503 Reference
(strain) (mg/kg bw)
Mouse sc aqueous M 97 Jordan
(ICR) F 109 et al., 1986a
Rat1 oral aqueous M 850 Jordan
(Sprague- F 800 et al., 1986b
Dawley)
Rat2 oral aqueous M & F > 2000 Piroozi
(Fischer-344) et al., 1993
Rat sc aqueous M 185 Jordan
(Fischer-344) F 440 et al., 1986c
Rat inhalation aerosol M & F > 494 (0/20) Jordan
(Fischer-344) < 4053 (14/20) et al., 1987
Rabbit dermal - M & F > 5000 Jordan
(NZ White) et al., 1987
Monkey im aqueous ? > 20 (0/2) Jordan &
(Rhesus) 30 (1/1) Shoufler, 1990
Sheep sc aqueous ? > 150 (0/5) Cochrane &
iv aqueous ? > 7.5 (1/5) Thomson, 1990
1 Animals were fasted
2 Animals were non-fasted
3 Figures in brackets represent the incidence of deaths
In the 600 mg/kg bw per day groups, there were increases in
haematocrit owing to an increase in corpuscular volume and in serum
levels of alanine aminotransferase and hepatic p-nitroanisole
O-demethylase activity. Thrombocyte counts were slightly decreased
at this dose. Urinalysis was unaffected. At the highest dose, there
were slight decreases in the weights of kidneys, spleen and ovaries,
increased adrenal weight and multifocal inflammation in the liver of
some animals. All treated animals exhibited caecal distension, which
is a typical response to high doses of an antibiotic compound
(Jordan, 1986).
Groups of 20 male and 20 female Sprague Dawley (Crl:CD) rats
received 0, 50, 250 or 1000 mg/kg bw per day tilmicosin (purity 88%)
in an aqueous vehicle, by gavage for 3 months. Signs of overt toxicity
were noted at 1000 mg/kg and included thinness, ventral soiling,
chromorhinorrhea, chromodacryorrhea, alopecia and poor grooming.
Mortality was increased at this highest dose and in females given
250 mg/kg bw per day.
Although food consumption was depressed in the 1000 mg/kg bw per
day group (males only), body weight gain was lower in males and
females at 1000 mg/kg bw per day and females at 250 mg/kg bw per day.
At 1000 mg/kg bw per day, serum alanine aminotransferase was increased
in males, blood urea nitrogen (BUN) was increased in males and
females, urinary pH was slightly lower in females and the presence of
occult blood was greater in males and females. There were no effects
on ophthalmology, haematology or hepatic p-nitroanisole
O-demethylase activity.
The weights of kidneys, liver and heart were increased in females
at 1000 mg/kg bw per day and adrenal weight was increased at 250 mg/kg
bw per day (females only) and 1000 mg/kg bw per day (males and
females). Gross pathology revealed caecal enlargement or distension at
250 mg/kg bw per day or more and small spleens in a few rats at
1000 mg/kg bw per day. Slight nephrosis was noted in two males in each
of the 250 and 1000 mg/kg bw per day groups. Other pathological
alterations were seen only in males and females of the 1000 mg/kg bw
per day group, i.e. hypertrophy of the zona fasciculata of the adrenal
cortex in most rats, necrosis of individual skeletal muscle fibres in
some rats; myocardial degeneration was increased and lymphoid
depletion was evident in the spleen and thymus of some animals. The
NOEL was 50 mg/kg bw per day (Jordan, 1988).
2.2.2.2 Dogs
Beagle dogs (two males and two females per group) were exposed by
inhalation to aerosols of 0, 12, 47 or 251 mg/m3 tilmicosin
(purity 83%). The treatment was administered for 4 hours per day on 12
days out of a 16-day period (excluding weekends). Particle median
equivalent aerodynamic diameters were between 1.2 and 1.5 µm. The mean
serum concentrations of tilmicosin were 0.23 to 0.33 and 1.67 to
2.46 µg/ml at 47 and 251 mg/m3 respectively. Drug levels at
12 mg/m3 were below the detection limit of 0.1 µg/ml.
One male given 251 mg/m3 died on the final day of exposure but
no other overt signs of toxicity were observed. Heart rates were
increased in dogs at 251 mg/m3. Food intake, body weight,
haematology and blood chemistry were unaffected. At autopsy, lung
weight was increased in females given 251 mg/m3 and inflammation was
noted in the respiratory tract at 47 mg/m3 (females only) and
251 mg/m3 (males and females) (Jordan et al., 1991).
Groups of four male and four female beagle dogs were given 0, 6,
20 or 70 mg/kg bw per day tilmicosin (purity 88%) in capsules for 3
months. The daily dose was administered in two equal amounts, 6 hours
apart. Serum concentrations of tilmicosin were measured 3 hours and 24
hours after the first of the two daily doses in weeks 2, 3, 5, 9 and
13. At increasing doses, the mean concentrations were 0.1 to 0.2, 0.74
to 1.49 and 3.24 to 6.05 µg/ml at 3 hours and 0, 0.26 to 0.59 and 1.72
to 3.96 µg/ml at 24 hours.
Half the 70 mg/kg bw per day males and females died during the
first month. Clinical signs prior to death were pale mucous membranes
in two dogs and ataxia in one dog. Food intake, body weight, haemato-
logy and urinalysis were unaffected. Serum alanine aminotransferase
activity was progressively increased at 70 mg/kg bw per day, while
hepatic p-nitroanisole O-demethylase activity was increased
in females at this level.
Two of the four surviving dogs at 70 mg/kg bw per day showed
bilateral multifocal areas of subretinal fluid concentrated along
arterioles in the tapetal region of the eye. The changes were claimed
to be consistent with those associated with systemic hypertension. One
of these dogs also had retinal degeneration and the other showed
miosis with normal pupillary light responses.
Heart rate increases were dose related, being marked at 70 mg/kg
bw per day and moderate to severe at 20 mg/kg bw per day. The increase
in males given 6 mg/kg bw per day was not significant. At the highest
dose, examination of the electrocardiogram revealed depression of the
ST segment.
At 70 mg/kg bw per day, heart weight was increased in males and
females, and liver and kidney weights were increased in females. Gross
necropsy showed small spleens in the females that died during the
study and an enlarged heart in a surviving male at 70 mg/kg bw per
day. Slight diffuse mucosal oedema was seen in the gall bladders of
two dogs at 70 mg/kg bw per day. Despite the physiological changes in
the eyes and heart, there were no associated pathological alterations.
The NOEL was 6 mg/kg bw per day (Jordan, 1987).
Groups of four male and four female beagle dogs were given 0, 4,
12 or 36 mg/kg bw per day tilmicosin (purity 86%) in capsules for 12
months. The daily dose was administered in two equal amounts, 6 hours
apart. There were no deaths. Peripheral redness was seen sporadically
in some animals of all groups, in particular at 12 and 36 mg/kg bw per
day.
Body weight gains were lower at > 12 mg/kg bw per day. There
were no effects on ophthalmology, haematology, blood chemistry and
urinalysis. Heart rates were markedly increased at 36 mg/kg bw per day
with sporadic depression of the ST segment in some dogs.
Heart weight was increased at 36 mg/kg bw per day and four males
and one female at this dose showed enlarged hearts. Mild chronic
dermatitis was noted in the external ears of dogs from all treated
groups. The findings included minimal thickening of the epidermis,
foci of acantholysis and inflammatory cell infiltration in the dermis.
These changes were only slight and non-dose-related, and therefore of
questionable relationship to treatment. The NOEL was 4 mg/kg bw per
day (Jordan & Bernhard, 1989).
2.2.3 Long-term toxicity/carcinogenicity studies
Tilmicosin has not been tested in toxicological studies longer
than 12 months in duration and hence the carcinogenic potential of
this drug has not been directly determined. There are a number of
observations which are relevant in assessing the carcinogenicity of
tilmicosin:
a) The results of available toxicological studies with tilmicosin
have not identified lesions or proliferative changes which could
be considered suggestive of potential neoplasia.
b) Tilmicosin has been tested in a wide range of genotoxicity
assays. All results were negative and it was concluded that the
compound has no genotoxic activity.
c) Tilmicosin is a macrolide antibiotic. This class of chemical has
been in widespread usage in humans for many years but there is no
evidence of carcinogenicity. Tylosin is the closest structural
analogue and this chemical was reviewed by the thirty-eighth
meeting of JECFA (Annex 1, reference 97).
2.2.3.1 Reconsideration of tylosin tumorigenicity
Tylosin was tested in 2-year feeding studies in rats. The
findings revealed an association between drug administration at doses
of 150 and 300 mg/kg bw per day in the diet and an increased incidence
of pituitary adenomas in males. While it was claimed that the increase
in the incidence of tumours was an indirect result of the ability of
tylosin to increase survival and weight gain, supporting data were not
available to the Committee and an ADI was not established.
New information, not considered at the thirty-eighth meeting,
indicates that spontaneous pituitary tumours occur in male rats at
variable rates with increased rates occurring in animals with higher
body weights (Gries & Young, 1982). In the tylosin studies, 12-month
body weights were somewhat higher in treated males rats and mortality
was increased in control animals due to respiratory infection. The
highest observed incidence of tumours in tylosin-treated groups was
comparable to the upper limit of the historical control range (23%) in
the test facility.
2.2.4 Reproductive toxicity studies
2.2.4.1 Rats
A dose-ranging study was carried out in groups of 10 female
Sprague-Dawley (Crl:COBS CD) rats given gavage doses of 0, 50, 125,
250, 500 or 750 mg/kg bw per day tilmicosin (purity unknown). The
females were treated from 14 days before mating with untreated males
until the end of study on post-partum day 4.
Excess salivation was dose-related at > 250 mg/kg bw per day.
Chromodacryorrhea, urine-stained fur and alopecia were seen in some
rats at 750 mg/kg bw per day; the three most severely affected died
during the study. Prior to death, these animals exhibited reduced food
intake, lost weight and were emaciated. Body weight gain was depressed
in surviving rats at 750 mg/kg bw per day during the pre-mating period
while food intake was reduced at > 250 mg/kg bw per day in week 1
and at 750 mg/kg bw per day in week 2. The pregnancy rate was
decreased at > 500 mg/kg bw per day. Duration of gestation, litter
size and weight, pup survival and weight gain to post-partum day 4
were unaffected (Dearlove et al., 1987).
Groups of 30 male and 30 female Sprague Dawley (Crl: COBS CD BR)
rats were administered 0, 10, 45 or 200 mg/kg bw per day tilmicosin
(purity 87%) by gavage in an aqueous vehicle. Treatment commenced 70
days (males) and 14 days (females) before the first mating period and
was continued through two litters per generation for two generations.
F1 litters were culled to five pups per sex on post-partum day 4 and
F1b pups (40 males and 40 females per group) were bred to produce
the following generation. F2a and F2b pups were killed on
post-partum day 4.
In adult animals, salivation was noted in males and females at
200 mg/kg bw per day, but there were no other signs of toxicity. Body
weight gain and food consumption were depressed in females of the 45
and 200 mg/kg bw per day groups during the pre-mating period only.
During the production of the four litters in this study there were no
effects on mating performance, pregnancy rates, duration of gestation,
litter size and weight and the weight gain of offspring. In both F1
litters at 200 mg/kg bw per day, pup mortality was slightly increased
up to post-partum day 4, but the finding was not duplicated in either
F2 litter. The NOEL was 10 mg/kg bw per day in adult rats (Christian
& Hoberman, 1989).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 25 presumed pregnant female Sprague Dawley (Crl: CD)
rats were administered gavage doses of 0, 10, 70 or 500 mg/kg bw per
day tilmicosin (purity 86%) in an aqueous vehicle. The dams were
treated on gestation days 6 to 15 and were killed on gestation day 20.
Increased salivation was seen at 70 and 500 mg/kg bw per day and
alopecia at 500 mg/kg bw per day. There were no deaths or abortions.
Body weight gain was reduced at 70 and 500 mg/kg bw per day, and food
intake was reduced at 500 mg/kg bw per day, during gestation days 6 to
10.
The number of resorption or live fetuses, fetal weight, sex ratio
and the incidence of fetal malformations were similar between groups.
The incidences of total skeletal and visceral anomalies were increased
in treated groups, but there was no dose-response relationship and the
findings were within the historical control values for the laboratory.
The NOEL for maternotoxicity was 10 mg/kg bw per day (Jordan & Higdon,
1988).
2.2.5.2 Rabbits
Groups of 15 presumed pregnant female Japanese White-NIBS rabbits
were given gavage doses of 0, 8, 19 or 48 mg/kg bw per day tilmicosin
(purity unknown) in an aqueous vehicle. Does were treated on gestation
days 6 to 18 and were killed on gestation day 28. One female given
48 mg/kg bw per day aborted on gestation day 26 and died. Reduced
faeces were seen at 19 and 48 mg/kg bw per day with only a transient
effect at 8 mg/kg bw per day. During the treatment period food intake
was depressed in a dose-related manner and body weight loss was
observed at 19 and 48 mg/kg bw per day.
Fetal and placental weight tended to decrease at 19 and 48 mg/kg
bw per day but did not achieve statistical significance. There were no
meaningful effects on the incidence of resorptions or fetal deaths or
on sex ratio. Open eyelids were observed in 11/91 and 16/68 fetuses
from the 19 and 48 mg/kg bw per day groups, respectively, and some of
these fetuses showed cleft palate or club foot. The affected fetuses
had low body weights and the seven litters involved were derived from
does that had lost body weight during drug administration. Skeletal
examination revealed retardation of fetal development at 19 and
48 mg/kg bw per day. Similar effects have been seen in this laboratory
in dietary restricted rabbits, suggesting the effects on the fetus
were secondary to maternal malnutrition (Noda, 1993).
2.2.6 Special studies on genotoxicity
Table 2. Results of genotoxicity studies on tilmicosin
Test system1 Test object Concentration Results Reference
Reverse S. typhimurium 1 - 100 µg/plate negative Jordan
mutation2 TA98, TA100, (± S9) et al., 1986f
TA1535, TA1537,
TA1538
Reverse E. coli 1 - 100 µg/plate negative Garriott
mutation2 WP2uvrA (± S9) et al., 1993
Forward L5178Y mouse 100-900 µg/ml negative Jordan
mutation2 lymphoma cells (- S9) et al., 1986e
200-1000 µg/ml
(+ S9)
Forward HGPRT+ Chinese 25-250 µg/ml negative Jordan
mutation2 Hamster ovary (- S9) et al., 1989c
cells 50-300/ µg/ml
(+ S9)
Unscheduled primary cultures 0.5-10 µg/ml negative Jordan
DNA synthesis of rat hepatocytes et al., 1985
assay
Sister chromatid Chinese hamster 200-1800 × 1 negative Jordan et al.,
exchange bone marrow mg/kg bw oral 1986d, 1989a
assay
Chromosome rat bone marrow 180-1800 × 1 and negative Jordan &
aberrations 100-1000 × 5, Ivett, 1989
mg/kg bw per
day oral
1 Positive controls used
2 Both with and without liver microsomal activation
2.2.7 Special study on the immune system
Groups of eight male CD-1 mice were given gavage doses of 0,
10, 250, 500 or 1000 mg/kg bw per day tilmicosin (purity 87%) in
an aqueous vehicle. Drug treatment was continued for 10 days.
After the third dose mice were injected intravenously with sheep
red blood cells. At the end of the study, serum from each mouse
was assayed for antibodies (haemagglutinin). There was one death
at 500 mg/kg bw per day and six at 1000 mg/kg bw per day, but
antibody production was not affected at any level (McGrath
et al., 1988a).
2.2.8 Special studies on microbiological activity
2.2.8.1 In vitro
The antibacterial activity of tilmicosin was determined
against a range of organisms representative of human intestinal
flora. Cultures were initiated using inoculum sizes of between
3 × 105 and 1.6 × 106 cells per spot on blood agar plates and
incubated anaerobically at 37°C.
The median MIC values for the clinical isolates of
Bifidobacterium and Peptostreptococcus, respectively, were
0.015 and 0.5 µg/ml. The above results were obtained at pH 7.7.
At pH 6.6 the MIC values were one to two orders of magnitude
higher (Scott et al., 1993).
The antibacterial activity of tilmicosin was examined
against a number of organisms used in industrial food processing
and originally obtained from dairy products. Cultures were
initiated in blood agar plates using three strains of
Propionibacterium and four strains of Lactobacillus. Under
anaerobic conditions the MICs were between 8 and 64 µg/ml and
under microaerophilic conditions the values were between 4 and
32 µg/ml (McLaren, 1994).
2.2.8.2 Rats
In two separate studies, female germ-free rats were orally
dosed with 1 ml of 10% (w/v) pooled human faecal suspension.
Three weeks later groups of two males and two females were given
gavage doses of 0, 0.12 or 0.4 mg/kg bw per day tilmicosin
(purity 87%) for 5 days. Rat faecal samples were collected before
treatment, daily during treatment and once during each of the 2
weeks following cessation of dosing. Body weight gain remained
unaffected.
The animals in each study received faecal suspensions
obtained from different donors. Results from different studies
varied widely, possibly reflecting differences between the
bacterial populations used. Total anaerobes were not reduced by
tilmicosin treatment. Total enterobacterial counts and the
proportion of enterobacteria with respect to total anaerobe
counts were transiently increased at 0.4 mg/kg bw per day in one
study only. Tilmicosin-resistant enterobacteria were not
significantly increased in either study. Spiramycin, at a dose of
0.5 mg/kg bw per day, increased total enterobacterial counts and
the number of spiramycin-resistant enterobacteria; the latter
appeared to remain high after the cessation of treatment. The
NOEL for tilmicosin was 0.4 mg/kg bw per day (Rumney, 1993).
2.2.9 Special studies on pharmacology
Table 3. Results of pharmacological assays with tilmicosin
Test system Doses Results Reference
Isolated 0.0009 to No effect on non-stimulated organ. Williams
guinea-pig 90 µg/ml 90 µg/ml slightly inhibited acetylcholine- et al.,
ileum and angiotensin-induced contraction 1988
and significantly inhibited electrically
stimulated contraction
Isolated 0.0009 to No effect on non-stimulated tissue and Williams
rat uterus 90 µg/ml no change to oxytocin-induced et al.,
(estrogen contractions 1988
primed)
Isolated rat 0.0009 to No effect on non-stimulated tissue and Williams
vas deferens 90 µg/ml no change to noradrenaline-induced et al.,
contractions 1988
Isolated 0.0009 to In spontaneously beating tissue, > 90 Williams
guinea-pig 900 µg/ml µg/ml decreased force of contraction; et al.,
atria and 900 µg/ml increased rate of 1988;
contraction. 90 µg/ml inhibited Jordan
isoprenaline-, noradrenaline- and Bay et al.,
K8644 (calcium agonist)-induced force 1990
and rate of contraction, and
histamine-induced rate of contraction.
Table 3. Results of pharmacological assays with tilmicosin (cont'd).
Test system Doses Results Reference
Conscious 0.25, 1, 2.5 Heart rate increased at > 1 mg/kg. Left Jordan &
dogs or 5 mg/kg ventricular function decreased at > 0.25 Sarazan,
iv mg/kg. Aortic pulse pressure decreased 1991;
at > 1 mg/kg. ECG revealed "Ventricular Sarazan
Alternans" (ventricular contraction fails et al.,
to achieve adequate pressure to open 1993
aortic valve and cause an arterial pulse).
Anaesthetised 0.5, 1 or 5 Heart rate increased, stroke volume, Jordan
dogs mg/kg iv stroke work index and cardiac output et al.,
decreased at > 1 mg/kg. At 5 mg/kg 2/3 1988
dogs developed second degree heart block
CD-1 mice 10, 100, Piloerection and grasping loss at > 500 Jordan
500 or mg/kg. Ptosis at 1000 mg/kg. No effect et al.,
1000 mg/kg on electroshock- or pentylenetetrazol- 1989d
oral induced seizures or on body temperature.
Hexobarbital-induced sleep time
prolonged by 500 (x1) and 1000 (x3) mg/kg
Sprague- 10, 30, 90 Urine volume and creatinine reduced McGrath
Dawley or 270 at 270 mg/kg with increases in osmolality et al.,
rats mg/kg oral and electrolytes. 1988b
2.3 Observations in humans
A total of 241 human exposures to tilmicosin (Micotil) were
reported to the Rocky Mountain Poison Center from May 1992 to May
1993. Needlesticks and scrapes (n=112) and accidental injection
(n=43) caused either no effect or local reactions including
soreness, numbness, stinging, swelling, redness, burning and
stiffness. Some injected subjects experienced anxiety, sweating,
headache and lightheadedness. Skin exposures (n=50) resulted in
redness and tingling of the skin, and eye exposures (n=11)
resulted in stinging and swelling. Persons accidentally ingesting
the drug (n=39) reported either no symptoms or bitter taste,
nausea, numbness of lips and tongue, vomiting, thirst and
headache (Montanio & Dart, 1993).
Over a 30-month period, 36 cases of accidental exposure to
tilmicosin (Micotil) were reported to the Ontario Regional Poison
Information Centre. Percutaneous injection (n=26) always resulted
in pain at the site and seven subjects mentioned a variety of
local reactions consistent with an irritant action. One person
showed "peaked T waves" 30 minutes following the injection of
1 ml into his arm, but ECG changes were not noted in other
subjects. The remaining subjects received splashes into the mouth
or eyes or onto the skin. Unpleasant taste, a burning sensation
on the hard palate and ocular irritation were each recorded in
one person (McGuigan, 1994).
3. COMMENTS
The Committee considered toxicological data on tilmicosin,
including the results of studies on acute and short-term
toxicity, pharmacokinetics, metabolism, reproductive toxicity,
teratogenicity, genotoxicity, antimicrobial activity and
pharmacology. The Committee also considered observations in
humans accidentally exposed to tilmicosin.
Tilmicosin is absorbed from the gastrointestinal tract as
shown by the recovery of radiolabelled compound in urine and/or
bile of orally dosed pigs (110 to 400 mg per animal), and the
presence of dose-related serum concentrations of tilmicosin dosed
orally in dogs for 3 months. However, the results from available
studies did not allow any conclusion to be reached concerning the
extent of absorption. The metabolites detected in a range of farm
animals were also found in rats, which suggests that the rat is a
suitable model for determining the potential toxicological
hazards posed by tilmicosin.
The LD50 in fasted rats was 800 to 850 mg/kg bw, but
toxicity was substantially lower in non-fasted animals, among
which there were no deaths following administration of a single
dose of 2000 mg tilmicosin/kg bw. Studies in a range of
pharmacological models identified depression of cardiac muscle
contractility and reduced heart function as the main adverse
effects.
Rats were administered oral doses of 50, 250 or 1000 mg/kg
bw per day for 3 months. At 1000 mg/kg bw per day, animals
exhibited chromorhinorrhea, chromodacryorrhea, alopecia, poor
grooming, reduced food consumption and they appeared thin.
Body-weight gain was reduced and mortality was increased in
females at 250 mg/kg bw per day and in both sexes at 1000 mg/kg
bw per day. Toxic effects on the kidney and liver were indicated
by increased organ weights, increased serum levels of alanine
aminotransferase and blood urea nitrogen, and the presence of
blood in the urine. However, pathological examination of these
organs showed only slight nephrosis in two males in each of the
groups given 250 or 1000 mg/kg bw per day. At the highest dose,
hypertrophy of the zona fasciculata of the adrenal cortex was
noted in most rats, and myocardial degeneration, necrosis of
individual skeletal muscle fibres and lymphoid depletion in the
spleen and thymus were seen in some rats. The NOEL was 50 mg/kg
bw per day.
Dogs were given oral doses of 6, 20 or 70 mg/kg bw per day
for three months. Half of the animals given 70 mg/kg bw per day
died during the first month. Heart rate was increased at 20 and
70 mg/kg bw per day and at the higher dose there was depression
of the ST segment of the electrocardiogram. Ophthalmoscopic
examination of the eyes showed bilateral areas of subretinal
fluid concentrated along arterioles in the tapetal region of two
of the four surviving dogs at 70 mg/kg bw per day. The NOEL was
6 mg/kg bw per day.
Dogs were given oral doses of 4, 12 or 36 mg/kg bw per day
for twelve months. Body weight gain was depressed at 12 and
36 mg/kg bw per day. Heart rate was markedly increased at 36
mg/kg bw per day, with occasional depression of the ST segment
of the electrocardiogram. Hearts were enlarged at 36 mg/kg bw per
day, but there were no associated pathological changes. The NOEL
was 4 mg/kg bw per day.
In a two-generation reproductive toxicity study, rats were
administered oral doses of 10, 45 or 200 mg tilmicosin/kg bw per
day. Body weight gain and food consumption were depressed in
females given 45 or 200 mg/kg bw per day, but only during the
pre-mating period. Fertility and reproduction parameters were
unaffected at any dose level. At 200 mg/kg bw per day, mortality
in pups was slightly increased in both first-generation litters
up to post-partum day 4, but not in either second-generation
litter. The NOEL was 10 mg/kg bw per day.
Developmental toxicity studies were reported in rats and
rabbits. Rats were dosed with 10, 70 or 500 mg tilmicosin/kg bw
per day by gavage. Growth and development in rat fetuses were not
affected by treatment, but increased salivation and reduced body
weight gain of dams were observed at 70 and 500 mg/kg bw per day.
The NOEL for maternal toxicity in rats was 10 mg/kg bw per day.
In the study in rabbits, there were dose-related signs of
toxicity in the does at all doses (8, 19 and 48 mg/kg bw per
day). Severely affected members of the groups given 19 or
48 mg/kg bw per day produced offspring that had open eyelids and
low incidences of cleft palate or club foot. These fetuses had
low body weights and were clearly retarded in development. The
effects were attributed to treatment-related malnutrition in
maternal animals, which is commonly observed in rabbits dosed
with antibiotics, confirming that this species is an inappropriate
model for studying the teratogenic potential of antibiotics.
Tilmicosin has been tested for reverse mutation in bacteria,
forward mutation in cultured mammalian cells, unscheduled DNA
synthesis in primary cultures of rat hepatocytes, and in in vivo
cytogenetic assays. All results were negative, and the Committee
concluded that tilmicosin has no genotoxic potential.
The carcinogenic potential of tilmicosin has not been
tested. However, a number of factors are relevant in assessing
the carcinogenicity of this drug. Toxicity studies with
tilmicosin have not resulted in lesions or proliferative changes
suggestive of neoplasia, and tilmicosin was uniformly negative in
a wide range of genotoxicity assays. Tilmicosin is a macrolide
antibiotic, and this class of chemicals is not known to induce
neoplasia despite widespread use in humans over many years. The
closest structural analogue, tylosin, was reviewed at the
thirty-eighth meeting of the Committee (Annex 1, reference 97).
In a 2-year feeding study in rats, tylosin administration was
associated with an increased incidence of pituitary adenomas in
male animals when compared to concurrent controls. New evidence,
not considered at the thirty-eighth meeting, indicates that
spontaneous pituitary neoplasms occur in male rats at variable
rates. Additionally, increased rates occur in animals with higher
body weights. In tylosin-treated groups of male rats, the
12-month body weights were somewhat higher than in the control
groups and the incidences of pituitary adenomas were comparable
to the upper limit of the historical control range. The lower
incidence of pituitary neoplasms in the control animals may have
been due to the earlier mortality in this group caused by
respiratory disease. In light of this information the Committee
considered that the concerns of the thirty-eighth meeting over
the potential tumorigenicity of tylosin had been satisfactorily
addressed and that there was no concern for the carcinogenic
potential of tylosin. For the above reasons, the Committee
considered that carcinogenicity studies would not be required for
tilmicosin.
In assessing the microbiological activity of tilmicosin, the
results of a study using germ-free rats colonized with human
intestinal microflora was considered the most relevant. At the
highest dose administered (0.4 mg/kg bw per day for 5 days) there
were no significant alterations in numbers of total anaerobes or
enterobacteria in rat faeces.
Reports of accidental human exposure to tilmicosin have
identified local reactions indicative of an irritant action
following dermal and ocular exposure. Humans accidentally
injected with tilmicosin have reported anxiety, sweating,
headache and lightheadedness. Changes in the electrocardiogram
pattern were observed in only one person. Contact with the buccal
mucosa or ingestion has resulted in a range of symptoms including
nausea, vomiting, thirst, numbness or burning sensation in the
mouth, and headache.
4. EVALUATION
The NOEL from toxicological studies was 4 mg/kg bw per day
in a 12-month study in dogs. Treatment of rats colonized with
human intestinal microflora with 0.4 mg tilmicosin/kg bw per day
produced no significant microbiological effect. The Committee
established an ADI of 0-40 µg/kg bw, based on the toxicological
NOEL of 4 mg/kg bw per day and a safety factor of 100. An
identical ADI would have been established using the data from the
study on rats colonized with human intestinal microflora and a
safety factor of 10 to account for variability among humans.
5. REFERENCE
Christian, M.S. & Hoberman, A.M. (1989). Reproductive effects of
EL-870 administered orally via gavage to Crl:COBS CD(SD) BR rats
for two generations, with two litters per generation. Unpublished
study No. 112-001 from Argus Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Cochrane, R.L. & Thomson, T.D. (1990). Toxicology and pharmacology
of tilmicosin following administration of subcutaneous and intravenous
injections to sheep. Unpublished study No. T5C768908 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Dearlove, G.H., Hoberman, A.M., & Christian, M.S. (1987). Dosage-range
study of EL-870 administered orally via gavage to Crl:COBS CD(SD) BR
rats (Pilot study). Unpublished study No. 112-001P from Argus Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. (1988). Comparative metabolism of 14C-tilmicosin
in cattle and rats. Unpublished study No. ABC-0395 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L. & Kennington, A.S. (1993). Tilmicosin metabolite
study with rat excreta. Unpublished study No. T5C759302 from
Lilly Research Laboratories. Submitted to WHO by Lilly, Basingstoke,
UK.
Donoho, A.L. & Thomson, T.D. (1988). 14C-Tilmicosin balance-
excretion study in swine. Unpublished study No. ABC-0409 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Donoho, A.L., Darby, J.M., Helton, S.L., Sweeney, D.J.,
Occolowitz, J.L., & Dorman, D.E. (1993). Tilmicosin metabolism
study in tissues and excreta of pigs fed 400 ppm 14C-tilmicosin.
Unpublished study No. T5C759201 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Garriott, M.L., Rexroat, M.A., & Jordan, W.H. (1993). The effect
of tilmicosin on the induction of reverse mutations in
Escherichia coli using the Ames test. Unpublished study
No. 920825AMS2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Giera, D.D. & Thomson, T.D. (1986). Preliminary 14C EL-870
balance excretion and tissue residue in swine. Unpublished study
No. ABC-0305 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Gries, C.L. & Young, S.S. (1982). Positive correlation of body
weight with pituitary tumor incidence in rats. Fundam. Appl.
Toxicol., 2, 145-148.
Hawkins, D.R., Elsom, L.F., Dighton, M.H., Kaur, A,. &
Cameron, D.M. (1993). The metabolism and residues of 14C-tilm-
icosin following subcutaneous administration to sheep. Unpublished
study No. HRC/LLY 36/930447 from Huntingdon Research Centre. Submitted
to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1986). The toxicity of compound 177370 (EL-870)
given by oral gavage to Fischer 344 rats for two weeks.
Unpublished study No. RI5585 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. (1987). The toxicity of EL-870 given orally to
Beagle dogs for three months. Unpublished study No. DO8286 from
Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. (1988). The toxicity of tilmicosin given orally to
Crl:CD(SD) rats for three months. Unpublished study No. RO9886
from Lilly Research Laboratories. Submitted to WHO by Lilly,
Basingstoke, UK.
Jordan, W.H. & Bernhard, N.R. (1989). A one year chronic toxicity
study in Beagle dogs given oral doses of tilmicosin. Unpublished
study No. DO7187 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Higdon, G.L. (1988). A teratology study of
tilmicosin (EL-870, compound 177370) administered orally to CD
rats. Unpublished study No. RI3387 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Ivett, J.L. (1989). Mutagenicity test on
tilmicosin in the rat bone marrow cytogenetic assay. Unpublished
study No. 10646-0-452 from Hazleton Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Sarazan, R.D. (1991). An acute study of the
cardiovascular effects of intravenous Micotil 300 in conscious
dogs. Unpublished study No. DV0890 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H. & Shoufler, J.R. (1990). The acute toxicity of
Micotil 300 administered intramuscularly to Rhesus monkeys.
Unpublished studies Nos. PO4089 and PO7689 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Hill, L.E., & Probst, G.S. (1985). The effect of
compound 177370 (EL-870) on the induction of DNA repair synthesis
in primary cultures of adult rat hepatocytes. Unpublished studies
Nos. 851008UDS2449 and 851015UDS2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986a). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the ICR mouse. Unpublished studies
Nos. M-C-41-85 and M-C-40-85 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986b). The acute toxicity of compound 177370 (EL-870)
administered orally to the Sprague-Dawley rat. Unpublished
studies Nos. R-O-40-86 and R-O-39-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., McKinley, E.R., Brown, G.E., & Hawkins, D.R.
(1986c). The acute toxicity of compound 177370 (EL-870)
administered subcutaneously to the Fischer 344 rat. Unpublished
studies Nos. R-C-02-86 and R-C-01-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Neal, S.B., & Probst, G.S. (1986d). The effect of
compound 177370 (EL-870) on the in vivo induction of sister
chromatid exchange in bone marrow of Chinese hamsters.
Unpublished study No. 851014SCE2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Oberly, T.J., Bewsey, B.J., & Probst, G.S. (1986e).
The effect of compound 177370 (EL-870) on the induction of
forward mutation at the thymidine kinase locus of L5178Y mouse
lymphoma cells. Unpublished studies Nos. 851106MLA2449 and
851113MLA2449 from Lilly Research Laboratories. Submitted to WHO
by Lilly, Basingstoke, UK.
Jordan, W.H., Rexroat, M.A., & Probst,G.S. (1986f). The effect of
compound 177370 (EL-870) on the induction of reverse mutations in
Salmonella typhimurium using the Ames test. Unpublished studies
Nos. 850930AMS2449, 851028AMS2449 and 851111AMS2449 from Lilly
Research Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Markey, T.F., Oakley, L.M., & Torrence, T.L.
(1987). The acute dermal and inhalation toxicity and ocular and
dermal irritation of tilmicosin technical. Unpublished studies
Nos. B-D-45-86, B-E-39-86 and R-H-037-86 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Turk, J.A. (1988). Cardiovascular
effects of tilmicosin administered intravenously in anaesthetised
beagle dogs. Unpublished study No. PC8806 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Brunny, J.D., & Garriott, M.L. (1989a). The effect
of tilmicosin on the in vivo induction of sister chromatid
exchange in bone marrow of Chinese hamsters. Unpublished study
No. 881114SCE2449 from Lilly Research Laboratories. Submitted to
WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Gardner, J.B., & Weaver, D.E. (1989b). An
intracutaneous sensitisation study in albino guinea pigs with
tilmicosin. Unpublished study No. GO1888 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Richardson, K.K., & Oberly, T.J. (1989c). The
effect of tilmicosin on the induction of forward mutation at the
HGPRT+ locus of Chinese hamster ovary cells. Unpublished
studies Nos. 881130CHT2449 and 890111CHO2449 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Helton, D.R. (1989d). CNS
behavioural effects of tilmicosin administered orally in the male
CD-1 mouse. Unpublished study No. PN8805 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Williams, P.D., & Colbert, W.E. (1990). In vitro
studies of tilmicosin in the cardiac muscle of Hartley Albino
guinea pigs. Unpublished study No. PM8946 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
Jordan, W.H., Wolff, R.K., & Carlson, K.H. (1991). A subchronic
inhalation toxicity study of tilmicosin in Beagle dogs.
Unpublished study No. DO5589 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L., & Lochmueller, C.A. (1988a). A
study of the immune response in CD-1 mice treated orally with
tilmicosin. Unpublished study No. MI4288 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
McGrath, J.P., Hamelink, J.L,. & Stephenson, R.O. (1988b). A
study of the acute effects of urine and electrolyte excretion of
tilmicosin administered orally to female Crl:CD(SD) rats.
Unpublished study No. RI8688 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
McGuigan, M.A. (1994). Human exposures to tilmicosin (Micotil).
Vet. Hum. Toxicol., 36(4), 306-308.
McLaren, I.M. (1994). Determination of the minimum inhibitory
concentration of tilmicosin for Lactobacillus and
Propionibacterium Sp. Unpublished study from Central Veterinary
Laboratory, UK. Submitted to WHO by Lilly, Basingstoke, UK.
Montanio, C.D. & Dart, R.C. (1993). Micotil 300. Human exposures
May 1992-May 1993. Unpublished report. Submitted to WHO by Lilly,
Basingstoke, UK.
Noda, A. (1993). Teratogenicity study of EL-870 (tilmicosin
aqueous) in rabbits by gavage. Unpublished study No. 91-001 from
Research Institute for Animal Science in Biochemistry and
Toxicology, Japan. Submitted to WHO by Lilly, Basingstoke, UK.
Piroozi, K.S., Keaton, M.J., & Jordan, W.H. (1993). The acute
toxicity of tilmicosin administered orally to Fischer 344 rats.
Unpublished study No. R21493 from Lilly Research Laboratories.
Submitted to WHO by Lilly, Basingstoke, UK.
Rumney, C.J. (1993). Microbiological end-point determination for
two antibiotics. Unpublished study No. 1164/3/93 from Bibra
Toxicology International, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Sarazan, R.D., Main, B.W., & Jordan, W.H. (1993). Cardiovascular
effects of tilmicosin alone, or in combination with propranolol
or dobutamine, in conscious unrestrained dogs. Unpublished study
No. DV1591 from Lilly Research Laboratories. Submitted to WHO by
Lilly, Basingstoke, UK.
Scott, R.J.D., Morgan, J.M., Pether, J.V.S., & Gosling, P.
(1993). Determination of the minimum inhibitory concentrations of
tilmicosin for human isolates of Bifidobacterium and Pepto-
streptococcus. Unpublished study No. SMS/9301 from Southern
Microbiological Services, UK. Submitted to WHO by Lilly,
Basingstoke, UK.
Williams, P.D., Jordan, W.H., & Colbert, W.E. (1988). In vitro
studies of tilmicosin (EL-870, Compound 177370) in smooth and
cardiac muscle. Unpublished study No. PM8808 from Lilly Research
Laboratories. Submitted to WHO by Lilly, Basingstoke, UK.
