
XYLAZINE
First draft prepared by
Dr Pamela L. Chamberlain
Center for Veterinary Medicine
Food and Drug Administration,
Rockville, Maryland, USA
1. Explanation
2. Biological data
2.1 Biochemical aspects
2.1.1 Pharmacodynamics
2.1.2 Absorption, distribution and excretion
2.1.3 Biotransformation
2.2 Toxicological studies
2.2.1 Acute toxicity studies of xylazine and 2,6-xylidine
2.2.2 Short-term toxicity studies
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.4 Special studies on teratogenicity
2.2.5 Special studies on genotoxicity
2.2.6 Special studies on methaemoglobin and haemoglobin
adduct formation with 2,6-xylidine
2.3 Observations in humans
3. Comments
4. Evaluation
5. Acknowledgements
6. References
1. EXPLANATION
Xylazine is a clonidine analogue that acts on presynaptic and
postsynaptic receptors of the central and peripheral nervous systems.
It is an alpha2-adrenergic agonist used in animals, including
cattle, horses, dogs, cats and deer, for its tranquillizing, muscle
relaxant and analgesic effects, but it has numerous other
pharmacological effects. It inhibits the effects of postganglionic
cholinergic nerve stimulation.
Xylazine is administered by the intramuscular, intravenous or
subcutaneous (in cats) routes, often in combination with other
anaesthetic agents, e.g., barbiturates, chloral hydrate, halothane and
ketamine.
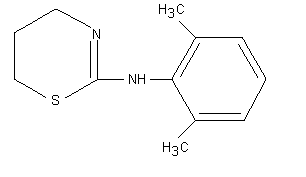
Xylazine had not been previously evaluated by the Committee. The
molecular structure of xylazine is shown below.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Pharmacodynamics
With respect to xylazine's sedative effect, there are marked
species differences in the dose rates required to achieve this state.
Table 1 illustrates dosages required for various animal species
(Gross & Tranquilli, 1989).
Table 1. Dosage of xylazine in various animal species
Xylazine (mg/kg bw)
Species Intravenous Intramuscular
Horses 0.5 to 1.1 1 to 2
Cattle 0.03 to 0.11 0.1 to 0.21
Sheep 0.05 to 0.11 0.1 to 0.31
Goats 0.01 to 0.51 0.05 to 0.51
Swine 2 to 3
Dogs 0.5 to 1 1 to 2
Cats 0.5 to 1 1 to 2
Birds 5 to 10
1 Lower end of dose range should be used if sedation without
recumbency is desired (Gross & Tranquilli, 1989).
Mydriasis is a feature of xylazine-induced sedation in the cat.
The mechanism has been determined as central inhibition of
parasympathetic tone in the iris due to xylazine's activation of
post-synaptic alpha-2 receptors (Hsu et al., 1981).
Thermoregulatory control is impaired in cats administered
xylazine. They become more susceptible to hyper- and hypothermia both
during and after recovery from the sedative effects of the drug. Foals
have demonstrated a hypothermic response to xylazine. Thermoregulatory
effects in cattle have been variable (Ponder & Clark, 1980;
Booth, 1988; Robertson et al., 1990).
Cardiovascular effects of xylazine include decreased heart rate
and variable effects on blood pressure. Xylazine-induced arrhythmia is
common in the horse due to sinoatrial and atrioventricular blocks.
Arrhythmias have also been recorded in dogs, but could not be induced
in sheep. The induction of cardiovascular effects may be influenced by
route of administration, e.g., xylazine administered epidurally to
horses produced no cardiovascular changes, whereas cattle injected by
this route experienced decreases in heart rate and arterial blood
pressure (Sagner et al., 1969; Holmes & Clark, 1977; Freire
et al., 1981; Hsu et al., 1981; Wasak, 1983; Singh et al., 1983;
Leblanc & Eberhart, 1990; Skarda et al., 1990).
The effects of xylazine on respiration, acid-base balance and
blood gas values vary according to species and anaesthetic
combination. In cattle, xylazine causes a slowing of the respiratory
rate. This is accompanied by an increase in pH and metabolic acidosis.
Respiratory rate is also slowed in dogs administered xylazine, but
arterial pH, pO2 or pCO2 are not significantly affected. The
literature contains conflicting reports on the effect of xylazine on
the respiratory rate of horses. Tachypnoea is characteristic of the
ovine response to xylazine. Hypoxaemia induced by xylazine in sheep
can be life-threatening (DeMoor & Desmet, 1971; Klide et al., 1975;
Holmes & Clark, 1977; Hsu et al., 1989; Carter et al., 1990;
Wagner et al., 1991).
Hyperglycaemia is induced by xylazine in adults of all target
species. Increased blood glucose concentrations are accompanied by a
decrease in insulin levels. In adult horses, hyperglycaemia is
accompanied by increased urine volume without glycosuria. Xylazine
administered to neonatal foals did not result in hyperglycaemia. The
hyperglycaemic effect of xylazine is thought to be due to its direct
effect on alpha-2-adrenoceptors of pancreatic islet beta cells
resulting in an inhibition of insulin release (Symonds, 1976; Feldberg
& Symonds, 1980; Hsu & Hummel, 1981; Thurmon et al., 1982, 1984;
Benson et al., 1984).
Serum chemistry and cerebral spinal fluid alterations were
observed in adult female goats administered intramuscularly with
0.2 mg xylazine/kg bw. Significant elevations of urea nitrogen, total
protein and total cholesterol were found in serum. Glucose and urea
nitrogen levels were significantly increased (P<0.01) and chloride
levels were significantly decreased (P<0.05) in the cerebral spinal
fluid (Amer & Misk, 1980).
Erythrocyte counts, haematocrit values and haemoglobin
concentrations in cattle and dogs have shown significant but
reversible decreases following xylazine administration
(Eichner et al., 1979; Wasak, 1983).
Gastrointestinal effects in ruminants include decreased gut
motility, prolongation of gastrointestinal transit time and inhibition
of reticulorumen contractions. Xylazine causes decreased muscle tone
of the colon and rectum which facilitates rectal examination. Xylazine
inhibition of rumen contractions can lead to tympany, which is a
potential cause of death in xylazine-sedated ruminants. Ruminants are
fasted prior to sedation and maintained in sternal recumbency during
sedation to reduce the risk of xylazine-induced tureen tympany.
Because xylazine also impairs deglutition, the head and neck of
xylazine-sedated ruminants are lowered to avoid aspiration of saliva
or ruminal fluid. Tolazoline (an alpha-2-adrenergic antagonist) has
shown effectiveness in reversing recumbency, gastric paresis and loss
of voluntary lingual control caused by xylazine in cattle
(Swift, 1977; Bolte & Stupariu, 1978; Ruckebusch & Toutain, 1984).
Gastrointestinal effects in dogs and cats include decreased
transit time and vomiting. The mechanism for induction of vomiting is
thought to involve the effect of xylazine on alpha-2-adrenoceptors in
the area postrema (the chemoreceptor trigger zone for vomiting) in the
medulla oblongata (Cullen & Jones, 1977; Colby et al., 1981; Hsu &
McNeel, 1983; Hikasa et al., 1987, 1989).
2.1.2 Absorption, distribution and excretion
2.1.2.1 Rats
Male Sprague-Dawley rats (170 g bw) were administered xylazine at
dosages of 0.02 to 10 mg/kg bw (i.v.) or 0.02 to 100 mg/kg bw (oral).
The drug was labelled with both 35S and 14C on the thiazine ring.
Following oral administration, absorption was > 95% with a half-life
of approximately 5 minutes. After i.v. administration, the drug was
distributed within a few minutes to almost all organs but primarily to
the kidneys and central nervous system. Relatively high activity
concentrations occurred in the pancreas, thyroid glands, liver and
cranial glands (e.g., extraorbital, sublingual). Several hours
following i.v. administration of 2 mg/kg bw, only small concentrations
(< 0.3 µg/g tissue) were present in the musculature. Following oral
or i.v. administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal elimination following
oral or i.v. administration was associated with a half-life of 2 to 3
hours. High oral doses (100 mg/kg bw) were associated with a delay in
renal elimination. Faecal elimination was comparable to biliary
elimination after oral or i.v. administration. Enterohepatic
circulation did not occur to a notable extent (Duhm et al., 1968,
1969).
2.1.2.2 Cattle
Three male calves (200-250 kg) and one dairy cow (450 kg) were
injected intramuscularly with a 0.33 mg/kg dose of 14C-xylazine
labelled in the thiazine ring. Radioactivity in blood plasma reached
its peak in the first 1.5 hours after injection. Total excretion of
radioactivity in urine and faeces was 68, 86, 83 and 100% at 10, 24,
48 and 72 hours, respectively (Murphy & Jacobs, 1975).
In another study, five 2-month old calves and four lactating cows
were administered a single intramuscular dose (0.3 or 0.6 mg/kg bw) of
xylazine hydrochloride. Maximum concentrations of xylazine were
achieved in blood 20 minutes after dosing. These were 0.04 mg/litre
for the 0.3 mg/kg bw dose and 0.06 mg/litre for the 0.6 mg/kg bw dose.
No xylazine was found in blood 8 hours after administration
(Takase et al., 1976).
Three lactating cows were administered an i.m. dose of 0.2 mg
xylazine/kg bw and two others were administered an i.m. dose of 0.4 mg
xylazine/kg bw. Milk was analysed for the presence of xylazine at 5
and 21 hours following administration. No xylazine was found at either
time point for either dose. The limit of detection was 0.06 mg/litre
(Pütter & Sagner, 1973).
Urinary excretion of xylazine was studied in three cows. Two were
administered an i.m. dose of 0.2 mg xylazine/kg bw and one was
administered an i.m. dose of 0.5 mg xylazine/kg bw. Less than 1% of
the dose was excreted unchanged in the urine. Unchanged xylazine was
no longer detectable 6 hours following administration. Metabolites
were no longer detected in urine 10 hours after administration. The
limit of detection for unchanged xylazine was 1-5 µg/litre (Pütter &
Sagner, 1973).
2.1.2.3 Comparative pharmacokinetics in dogs, sheep, cattle and
horses
The comparative pharmacokinetics of xylazine in dogs, sheep,
cattle and horses are summarized in Table 2.
Pharmacokinetic parameters do not vary greatly between species
following intravenous administration. The rapid elimination of
xylazine is attributed to extensive metabolism, and not to rapid renal
excretion of unchanged xylazine. Significant amounts of parent
xylazine were not found in the urine of sheep collected at 10-minute
intervals after dosing. The pharmacokinetics of xylazine were
unmodified when it was administered to rabbits with occluded renal
arteries. The lack of correlation between pharmacokinetic parameters
and clinical effects of xylazine in cattle suggests that clinical
effects in cattle are due to a rapidly produced long-acting
metabolite(s) and not due to an increased sensitivity to xylazine
(Garcia-Villar et al., 1981).
Table 2. Single-dose pharmacokinetics of xylazine in domestic species (Garcia-Villar et al., 1981)
Species Dog Sheep Cattle Horse
Body weight range (kg) 14-24 42-65 240-440 415-550
Dose rate (mg/kg bw)1 1.4 1.0 0.2 0.6
Number 4 6 4 4
Intravenous2
Distribution half-life (min) 2.57 1.89 1.21 5.97
Volume of distribution (l/kg) 2.52 2.74 1.94 2.46
Elimination half-life (min) 30.13 23.11 36.48 49.51
Body clearance (ml/min/kg) 81 83 42 21
Intramuscular2
Absorption half-life (min) 3.44 5.45 ND 2.72
Elimination half-life (min) 34.65 22.36 ND 57.7
Cmax (mg/ml) 0.43 0.13 ND 0.17
Tmax (min) 12.7 14.68 ND 12.92
Bioavailability:
mean (%) 73.9 40.8 ND 44.6
standard deviation (%) 17.89 23.81 4.16
range (%) 52-90 17-73 40-48
1 Dosage expressed as xylazine-base
2 Blood sampling times after injection: 1, 2, 4, 8, 16, 30 and 120 minutes.
ND = Not determined (assay was not sensitive enough to determine xylazine plasma
concentrations lower than 0.01 mg/litre)
2.1.3 Biotransformation
2.1.3.1 Rats
Studies were conducted with urine and bile of rats administered
2 mg xylazine (35S or 14C)/kg bw intravenously. Approximately 20
metabolites were detected and quantified as xylazine equivalents.
Approximately 8% of the dose was eliminated as unchanged compound in
the urine 24 hours after dosing. The major metabolite comprised 35% of
the administered dose. Final products of metabolism were inorganic
sulfate and carbon dioxide (Duhm et al., 1968).
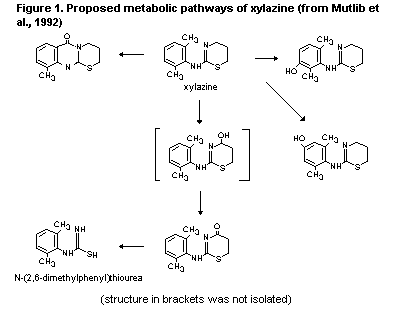
Specific metabolites of xylazine were identified following
incubation of xylazine with rat liver microsomes. Those metabolites
were 2-(4'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-1,3-
thiazine, 2-(3'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-
1,3-thiazine, N-(2,6-dimethylphenyl)thiourea and 2-(2',6'-
dimethylphenylamino)-4-oxo-5,6-dihydro-1,3-thiazine. N-(2,6-dime-
thylphenyl)thiourea was the major metabolite produced in vitro.
Figure 1 shows the proposed metabolic pathways of xylazine based on
these findings (Mutlib et al., 1992).
2.1.3.2 Horses
One mare was administered a 1 g dose of xylazine (route not
stated) and urine was collected over 24 hours. Metabolites were
recovered from horse urine only after the urine was hydrolysed with
beta-glucuronidase. The major urinary metabolites detected were the
same as those produced by incubating xylazine with rat liver
microsomes, described in section 2.1.3.1 (Mutlib et al., 1992).
2.1.3.3 Cattle
Urine from three cows administered an i.m. dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow) was
examined for metabolites. One urinary metabolite, identified as
2,6-xylidine1, was found in both free and conjugated forms. The
authors concluded that xylazine was essentially eliminated in cattle
by rapid biotransformation. Breakdown of the thiazine ring, resulting
in formation of 2,6-xylidine, was proposed as the primary
biotransformation pathway (Patter & Sagner, 1973).
1 2,6-xylidine is also known as 1-amino-2,6-dimethylbenzene and as
2,6-dimethylaniline
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Pharmacodynamics
With respect to xylazine's sedative effect, there are marked
species differences in the dose rates required to achieve this state.
Table 1 illustrates dosages required for various animal species
(Gross & Tranquilli, 1989).
Table 1. Dosage of xylazine in various animal species
Xylazine (mg/kg bw)
Species Intravenous Intramuscular
Horses 0.5 to 1.1 1 to 2
Cattle 0.03 to 0.11 0.1 to 0.21
Sheep 0.05 to 0.11 0.1 to 0.31
Goats 0.01 to 0.51 0.05 to 0.51
Swine 2 to 3
Dogs 0.5 to 1 1 to 2
Cats 0.5 to 1 1 to 2
Birds 5 to 10
1 Lower end of dose range should be used if sedation without
recumbency is desired (Gross & Tranquilli, 1989).
Mydriasis is a feature of xylazine-induced sedation in the cat.
The mechanism has been determined as central inhibition of
parasympathetic tone in the iris due to xylazine's activation of
post-synaptic alpha-2 receptors (Hsu et al., 1981).
Thermoregulatory control is impaired in cats administered
xylazine. They become more susceptible to hyper- and hypothermia both
during and after recovery from the sedative effects of the drug. Foals
have demonstrated a hypothermic response to xylazine. Thermoregulatory
effects in cattle have been variable (Ponder & Clark, 1980;
Booth, 1988; Robertson et al., 1990).
Cardiovascular effects of xylazine include decreased heart rate
and variable effects on blood pressure. Xylazine-induced arrhythmia is
common in the horse due to sinoatrial and atrioventricular blocks.
Arrhythmias have also been recorded in dogs, but could not be induced
in sheep. The induction of cardiovascular effects may be influenced by
route of administration, e.g., xylazine administered epidurally to
horses produced no cardiovascular changes, whereas cattle injected by
this route experienced decreases in heart rate and arterial blood
pressure (Sagner et al., 1969; Holmes & Clark, 1977; Freire
et al., 1981; Hsu et al., 1981; Wasak, 1983; Singh et al., 1983;
Leblanc & Eberhart, 1990; Skarda et al., 1990).
The effects of xylazine on respiration, acid-base balance and
blood gas values vary according to species and anaesthetic
combination. In cattle, xylazine causes a slowing of the respiratory
rate. This is accompanied by an increase in pH and metabolic acidosis.
Respiratory rate is also slowed in dogs administered xylazine, but
arterial pH, pO2 or pCO2 are not significantly affected. The
literature contains conflicting reports on the effect of xylazine on
the respiratory rate of horses. Tachypnoea is characteristic of the
ovine response to xylazine. Hypoxaemia induced by xylazine in sheep
can be life-threatening (DeMoor & Desmet, 1971; Klide et al., 1975;
Holmes & Clark, 1977; Hsu et al., 1989; Carter et al., 1990;
Wagner et al., 1991).
Hyperglycaemia is induced by xylazine in adults of all target
species. Increased blood glucose concentrations are accompanied by a
decrease in insulin levels. In adult horses, hyperglycaemia is
accompanied by increased urine volume without glycosuria. Xylazine
administered to neonatal foals did not result in hyperglycaemia. The
hyperglycaemic effect of xylazine is thought to be due to its direct
effect on alpha-2-adrenoceptors of pancreatic islet beta cells
resulting in an inhibition of insulin release (Symonds, 1976; Feldberg
& Symonds, 1980; Hsu & Hummel, 1981; Thurmon et al., 1982, 1984;
Benson et al., 1984).
Serum chemistry and cerebral spinal fluid alterations were
observed in adult female goats administered intramuscularly with
0.2 mg xylazine/kg bw. Significant elevations of urea nitrogen, total
protein and total cholesterol were found in serum. Glucose and urea
nitrogen levels were significantly increased (P<0.01) and chloride
levels were significantly decreased (P<0.05) in the cerebral spinal
fluid (Amer & Misk, 1980).
Erythrocyte counts, haematocrit values and haemoglobin
concentrations in cattle and dogs have shown significant but
reversible decreases following xylazine administration
(Eichner et al., 1979; Wasak, 1983).
Gastrointestinal effects in ruminants include decreased gut
motility, prolongation of gastrointestinal transit time and inhibition
of reticulorumen contractions. Xylazine causes decreased muscle tone
of the colon and rectum which facilitates rectal examination. Xylazine
inhibition of rumen contractions can lead to tympany, which is a
potential cause of death in xylazine-sedated ruminants. Ruminants are
fasted prior to sedation and maintained in sternal recumbency during
sedation to reduce the risk of xylazine-induced tureen tympany.
Because xylazine also impairs deglutition, the head and neck of
xylazine-sedated ruminants are lowered to avoid aspiration of saliva
or ruminal fluid. Tolazoline (an alpha-2-adrenergic antagonist) has
shown effectiveness in reversing recumbency, gastric paresis and loss
of voluntary lingual control caused by xylazine in cattle
(Swift, 1977; Bolte & Stupariu, 1978; Ruckebusch & Toutain, 1984).
Gastrointestinal effects in dogs and cats include decreased
transit time and vomiting. The mechanism for induction of vomiting is
thought to involve the effect of xylazine on alpha-2-adrenoceptors in
the area postrema (the chemoreceptor trigger zone for vomiting) in the
medulla oblongata (Cullen & Jones, 1977; Colby et al., 1981; Hsu &
McNeel, 1983; Hikasa et al., 1987, 1989).
2.1.2 Absorption, distribution and excretion
2.1.2.1 Rats
Male Sprague-Dawley rats (170 g bw) were administered xylazine at
dosages of 0.02 to 10 mg/kg bw (i.v.) or 0.02 to 100 mg/kg bw (oral).
The drug was labelled with both 35S and 14C on the thiazine ring.
Following oral administration, absorption was > 95% with a half-life
of approximately 5 minutes. After i.v. administration, the drug was
distributed within a few minutes to almost all organs but primarily to
the kidneys and central nervous system. Relatively high activity
concentrations occurred in the pancreas, thyroid glands, liver and
cranial glands (e.g., extraorbital, sublingual). Several hours
following i.v. administration of 2 mg/kg bw, only small concentrations
(< 0.3 µg/g tissue) were present in the musculature. Following oral
or i.v. administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal elimination following
oral or i.v. administration was associated with a half-life of 2 to 3
hours. High oral doses (100 mg/kg bw) were associated with a delay in
renal elimination. Faecal elimination was comparable to biliary
elimination after oral or i.v. administration. Enterohepatic
circulation did not occur to a notable extent (Duhm et al., 1968,
1969).
2.1.2.2 Cattle
Three male calves (200-250 kg) and one dairy cow (450 kg) were
injected intramuscularly with a 0.33 mg/kg dose of 14C-xylazine
labelled in the thiazine ring. Radioactivity in blood plasma reached
its peak in the first 1.5 hours after injection. Total excretion of
radioactivity in urine and faeces was 68, 86, 83 and 100% at 10, 24,
48 and 72 hours, respectively (Murphy & Jacobs, 1975).
In another study, five 2-month old calves and four lactating cows
were administered a single intramuscular dose (0.3 or 0.6 mg/kg bw) of
xylazine hydrochloride. Maximum concentrations of xylazine were
achieved in blood 20 minutes after dosing. These were 0.04 mg/litre
for the 0.3 mg/kg bw dose and 0.06 mg/litre for the 0.6 mg/kg bw dose.
No xylazine was found in blood 8 hours after administration
(Takase et al., 1976).
Three lactating cows were administered an i.m. dose of 0.2 mg
xylazine/kg bw and two others were administered an i.m. dose of 0.4 mg
xylazine/kg bw. Milk was analysed for the presence of xylazine at 5
and 21 hours following administration. No xylazine was found at either
time point for either dose. The limit of detection was 0.06 mg/litre
(Pütter & Sagner, 1973).
Urinary excretion of xylazine was studied in three cows. Two were
administered an i.m. dose of 0.2 mg xylazine/kg bw and one was
administered an i.m. dose of 0.5 mg xylazine/kg bw. Less than 1% of
the dose was excreted unchanged in the urine. Unchanged xylazine was
no longer detectable 6 hours following administration. Metabolites
were no longer detected in urine 10 hours after administration. The
limit of detection for unchanged xylazine was 1-5 µg/litre (Pütter &
Sagner, 1973).
2.1.2.3 Comparative pharmacokinetics in dogs, sheep, cattle and
horses
The comparative pharmacokinetics of xylazine in dogs, sheep,
cattle and horses are summarized in Table 2.
Pharmacokinetic parameters do not vary greatly between species
following intravenous administration. The rapid elimination of
xylazine is attributed to extensive metabolism, and not to rapid renal
excretion of unchanged xylazine. Significant amounts of parent
xylazine were not found in the urine of sheep collected at 10-minute
intervals after dosing. The pharmacokinetics of xylazine were
unmodified when it was administered to rabbits with occluded renal
arteries. The lack of correlation between pharmacokinetic parameters
and clinical effects of xylazine in cattle suggests that clinical
effects in cattle are due to a rapidly produced long-acting
metabolite(s) and not due to an increased sensitivity to xylazine
(Garcia-Villar et al., 1981).
Table 2. Single-dose pharmacokinetics of xylazine in domestic species (Garcia-Villar et al., 1981)
Species Dog Sheep Cattle Horse
Body weight range (kg) 14-24 42-65 240-440 415-550
Dose rate (mg/kg bw)1 1.4 1.0 0.2 0.6
Number 4 6 4 4
Intravenous2
Distribution half-life (min) 2.57 1.89 1.21 5.97
Volume of distribution (l/kg) 2.52 2.74 1.94 2.46
Elimination half-life (min) 30.13 23.11 36.48 49.51
Body clearance (ml/min/kg) 81 83 42 21
Intramuscular2
Absorption half-life (min) 3.44 5.45 ND 2.72
Elimination half-life (min) 34.65 22.36 ND 57.7
Cmax (mg/ml) 0.43 0.13 ND 0.17
Tmax (min) 12.7 14.68 ND 12.92
Bioavailability:
mean (%) 73.9 40.8 ND 44.6
standard deviation (%) 17.89 23.81 4.16
range (%) 52-90 17-73 40-48
1 Dosage expressed as xylazine-base
2 Blood sampling times after injection: 1, 2, 4, 8, 16, 30 and 120 minutes.
ND = Not determined (assay was not sensitive enough to determine xylazine plasma
concentrations lower than 0.01 mg/litre)
2.1.3 Biotransformation
2.1.3.1 Rats
Studies were conducted with urine and bile of rats administered
2 mg xylazine (35S or 14C)/kg bw intravenously. Approximately 20
metabolites were detected and quantified as xylazine equivalents.
Approximately 8% of the dose was eliminated as unchanged compound in
the urine 24 hours after dosing. The major metabolite comprised 35% of
the administered dose. Final products of metabolism were inorganic
sulfate and carbon dioxide (Duhm et al., 1968).
Specific metabolites of xylazine were identified following
incubation of xylazine with rat liver microsomes. Those metabolites
were 2-(4'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-1,3-
thiazine, 2-(3'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-
1,3-thiazine, N-(2,6-dimethylphenyl)thiourea and 2-(2',6'-
dimethylphenylamino)-4-oxo-5,6-dihydro-1,3-thiazine. N-(2,6-dime-
thylphenyl)thiourea was the major metabolite produced in vitro.
Figure 1 shows the proposed metabolic pathways of xylazine based on
these findings (Mutlib et al., 1992).
2.1.3.2 Horses
One mare was administered a 1 g dose of xylazine (route not
stated) and urine was collected over 24 hours. Metabolites were
recovered from horse urine only after the urine was hydrolysed with
beta-glucuronidase. The major urinary metabolites detected were the
same as those produced by incubating xylazine with rat liver
microsomes, described in section 2.1.3.1 (Mutlib et al., 1992).
2.1.3.3 Cattle
Urine from three cows administered an i.m. dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow) was
examined for metabolites. One urinary metabolite, identified as
2,6-xylidine1, was found in both free and conjugated forms. The
authors concluded that xylazine was essentially eliminated in cattle
by rapid biotransformation. Breakdown of the thiazine ring, resulting
in formation of 2,6-xylidine, was proposed as the primary
biotransformation pathway (Patter & Sagner, 1973).
1 2,6-xylidine is also known as 1-amino-2,6-dimethylbenzene and as
2,6-dimethylaniline
 2.2 Toxicological studies
Because 2,6-xylidine is also a chemical intermediate used in
dyes, a component of tobacco smoke and a degradation product of
aniline-based pesticides, its toxicology has been studied extensively.
Toxicological studies conducted with this compound were also reviewed
and will be presented in addition to the review and results of
toxicological studies of xylazine.
2.2.1 Acute toxicity studies of xylazine and 2,6-xylidine
The acute systemic toxicity of xylazine has been investigated in
both laboratory and domestic species. It is generally recognized that
ruminants are much more sensitive than most other species to the
pharmacological and toxicological effects of xylazine.
Results of LD50 studies of xylazine and 2,6-xylidine are
summarized in Table 3.
2.2.1.1 Acute toxicity of xylazine in dogs
Adult dogs (four males, four females) and cats (two males, four
females) were administered a single i.m. or i.v. dose of 22 mg
xylazine/kg bw (10 times the recommended therapeutic dose). One cat
out of three receiving the i.v. dose died, and two dogs out of four
receiving the i.m. dose died. All others recovered from convulsions,
unconsciousness and respiratory depression with no apparent
after-effects. The authors concluded that xylazine was slightly toxic
in this study (Crawford et al., 1970a).
Table 3. Results of acute toxicity studies on xylazine and 2,6-xylidine
Species Sex1 Route LD50 Reference
(mg/kg bw)
Xylazine
Rat NA p.o. 130 Sagner, 1967
Cat male & female2 s.c. 100-110 Bauman & Nelson, 1969
Dog male & female3 i.m 47 Nelson et al., 1968b
Dog 4 male & 3 female i.v 20-25 Nelson et al., 1968b
Horses NA4 i.m. 60-705 Nelson et al., 1968a
i.v. 15-285
2,6-Xylidine
Mouse male p.o. 710 Vernot et al., 1977
Rat p.o. 2042 Lindstrom et al., 1969
male p.o. 840 Jacobson, 1972
male p.o. 630 Short et al., 1983
male p.o. 1230 Vernot et al., 1977
female p.o. 1160 & 1270 US National Toxicology
Program, 1990
male p.o. 620-1250 &
1310
1 NA = Information not available
2 Number per sex not stated; 10 animals were used
3 Number per sex not stated; 17 animals were used
4 Sex of test animals was not stated; 5 animals were used
5 Minimum lethal dose
2.2.1.2 Acute toxicity of xylazine in horses
Adult horses were administered 11 mg xylazine/kg bw, i.v. (three
mares, one gelding) or 22 mg xylazine/kg bw, i.m. (two mares, two
geldings). One mare died following i.v. administration. All other test
animals recovered from treatment-related effects 24 hours following
i.v. administration and 48 hours following i.m. administration. The
authors concluded that the i.v. dose was slightly toxic and that the
i.m. dose produced no apparent toxicity in this study (Crawford
et al., 1970b).
2.2.2 Short-term toxicity studies
2.2.2.1 Xylazine
a) Rats
Xylazine was administered in the diet to Wistar rats (10/sex/
group) for 32 weeks. Dosages administered were 0, 50, 100, 250
or 500 mg/kg diet (equal to 0, 3, 6, 21 or 41 mg/kg bw per day for
males and 0, 4, 8, 19 or 45 mg/kg bw per day for females).
Haematology, urinalysis and gross and histopathological evaluations
were performed.
Decreases in body weight observed in females in the two
highest-dose groups (statistically significant (p<0.02) at 500 mg/kg
diet) were considered by the author to be treatment-related.
Microscopic examination of livers, lungs and kidneys revealed that
animals in all groups were diseased but no treatment-related pathology
was identified. Based on the dose-related decrease in weight gain
observed in females at 250 and 500 mg/kg diet, the NOEL in this study
was 100 mg/kg diet, equal to 6 mg/kg bw per day. The author regarded
the dose of 250 mg/kg diet as the non-toxic application rate. The
reliability of any NOEL derived from this study should be considered
questionable, owing to the presence of infection in all groups
(Tettenborn & Hobik, 1968a; Trossmann & Hobik, 1970).
b) Dogs
Dogs of undetermined breed or source were given xylazine orally
in gelatin capsules for 14-16 weeks at dose levels of 25, 50 or
100 mg/kg bw per day, 5 days/week. The low-dose group consisted of one
male and one female, the mid-dose group of two males and the high-dose
group of two males and two females. Haematology, clinical chemistry,
blood coagulation, urinalysis and postmortem gross and microscopic
evaluations were performed.
During week 8 of the test, one animal in the high-dose group died
and was replaced with a new animal. Postmortem gross findings in this
animal included diffuse reddening of the stomach and intestinal mucous
membrane.
Histopathological findings in livers (fatty degeneration and
necrosis) and kidneys (tubular epithelial necrosis and fat
accumulation) of the high-dose group were considered treatment-
related. Fatty deposits were noted in the liver and kidneys of the
low-dose female. These findings were attributed to parturition, which
occurred 3 weeks before the animal was killed. The author concluded
that the NOEL for this study was 50 mg/kg bw per day. The reliability
of any NOEL derived from this study should be considered questionable
due to the lack of a control group and small numbers of animals in
each test group (Tettenborn & Hobik, 1968b).
Beagle dogs (two/sex/group) were administered xylazine orally by
dietary admixture for 13 weeks. Dosages administered were 0, 10, 30 or
100 mg/kg diet (equal to 0, 0.3, 0.9 or 3 mg/kg bw per day). At the
beginning of the test the animals were approximately 8.5 months old
and weighed 7-10 kg. Parameters evaluated included general appearance,
ophthalmology, electrocardiography, haematology, clinical chemistry,
urinalyses and gross and microscopic pathology.
No treatment-related adverse effects were observed in any of the
parameters evaluated. The NOEL for this study was 3 mg/kg bw per day
(Tettenborn, 1969; Mawdesley-Thomas, 1970).
2.2.2.2 2,6-Xylidine
a) Rats
Three groups (nine or ten/group) of male Fischer-344 rats were
given oral (gavage) doses of 160 mg 2,6-xylidine/kg bw per day for 5,
10 or 20 days. The dosage administered was 25% of the estimated LD50
determined by the investigator. A significant increase in splenic
haemosiderosis (indicative of erythrocyte damage) after 20 days was
noted as a treatment-related effect in this study. Splenic congestion
and evidence for increased erythropoiesis were minimal
(Short et al., 1983).
Three groups of Sprague-Dawley rats (five/sex/group for controls,
low and mid dose; four/sex/group for high dose) were administered
0, 20, 100 or 500-700 mg 2,6-xylidine/kg bw per day by gavage for 4
weeks. Treatment-related effects included decreased weight gain,
decreased haemoglobin levels and hepatomegaly. In this study, the rat
appeared to be about 10 times less susceptible to hepatotoxicity of
2,6-xylidine than the dog (see section 2.2.2.2b) (Magnusson et al.,
1971; IARC, 1993).
Two groups of 8-week-old Sprague-Dawley rats (5/sex/group) were
orally administered (gastric intubation) a dose of 0 or 400 mg
2,6-xylidine per kg bw per day for 1 week immediately followed by a
daily dose of 0 or 500 mg/kg bw for 3 weeks. Decreased body weight
gain and hepatomegaly (most pronounced in centrilobular regions) were
noted as treatment-related effects. Electron microscopy of liver
tissue showed proliferation of hepatic smooth endoplasmic reticulum,
which was deemed responsible for the observed hepatomegaly in treated
rats. An increase in microsomal glucuronyltransferase was observed in
males while aniline hydroxylase levels were increased in females.
Decreases in liver glycogen and glucose-6-phosphatase activity were
also observed in the centrilobular regions of treated animals
(Magnusson et al., 1979).
Male Osborne-Mendel rats were administered up to 10 000 mg
2,6-xylidine per kg in the diet for 3-6 months. Treatment-related
effects included 25% weight reduction, anaemia, hepatomegaly with no
associated microscopic changes, splenic congestion and renal toxicity
(Lindstrom et al., 1963).
Groups of F-344/N rats (five/sex/group) were administered doses
of 0, 80, 160, 310, 620 or 1250 mg/kg bw of 2,6-xylidine in corn oil
by gavage 5 days/week for 2 weeks. Parameters evaluated included
clinical observations, body weight, urinalysis, haematology, blood pH
and carbon dioxide determinations, and gross postmortem findings.
Treatment-related deaths occurred at and above 620 mg/kg bw. All
animals in the highest dose group died before the end of the study. A
decrease of more than 10% in body weight was observed in males at and
above 310 mg/kg bw and in females at and above 160 mg/kg bw.
Generalized leukocytosis and an increase in the number of nucleated
red blood cells were observed in male rats administered 310 or
620 mg/kg bw. Slight anisocytosis, poikilocytosis and polychromasia of
the red blood cells occurred more frequently in dosed animals than in
vehicle control animals. Moderate poikilocytosis occurred at 310 mg/kg
bw and moderate polychromasia at 310 and 620 mg/kg bw. Slightly
macrocytic erythrocytes were observed at the two highest doses. Slight
anisocytosis, poikilocytosis and polychromasia were observed in female
rats at 310 and 620 mg/kg bw. The NOEL for this study was 80 mg/kg bw
per day (US National Toxicology Program, 1990).
Groups of F-344/N rats (10/sex/group) were given doses of 0, 20,
40, 80, 160 or 310 mg/kg bw of 2,6-xylidine in corn oil by gavage, 5
days/week for 13 weeks. Parameters evaluated included clinical
observations, haematology, urinalysis, serum chemistry and enzyme
analyses, gross and histopathological postmortem examinations.
A decrease in body weight gain of more than 10% occurred in males
and females in the highest dose group and in females at 40 and
160 mg/kg bw per day. In the highest dose group, relative liver
weights were significantly (P=0.003) increased for males and females.
Relative liver weight was also increased for males in the 160 mg/kg bw
group. The liver weight to brain weight and kidney weight to brain
weight ratios were significantly increased in females at 310 mg/kg bw
per day.
Treatment-related effects on haematology included significantly
decreased total leukocyte counts in males at doses of 40 mg/kg bw or
more. These were accompanied by decreases in the percentage of
lymphocytes and increases in the percentage of segmented neutrophils
at doses of 80 mg/kg bw or more. In males, haemoglobin levels were
significantly decreased at 160 and 310 mg/kg bw and erythrocyte and
haematocrit levels were decreased at 310 mg/kg bw. The NOEL for this
study was 20 mg/kg bw per day (US National Toxicology Program, 1990).
b) Dogs
Four groups of beagle dogs (one/sex/group) were given an oral
(gelatin capsule) dose of 0, 2, 10 or 50 mg 2,6-xylidine/kg bw per day
for 4 weeks. Treatment-related effects included vomiting (mid- and
high-dose groups), poor condition and decreased body weights
(high-dose group), hyperbilirubinaemia (mid- and high-dose groups),
hypoproteinaemia (mid-and high-dose groups) and fatty degenerative
changes in the liver that increased in severity with increasing dose
(Magnusson et al., 1971; IARC, 1993).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Xylazine
No carcinogenicity studies have been performed with xylazine
2.2.3.2 2,6-Xylidine
Four groups of Charles River CRL:COBS CD (SD) BR rats
(56/sex/group) were fed diets containing 2,6-xylidine (99.06% pure) at
concentrations of 0, 300, 1000 or 3000 mg/kg diet (equivalent to
0, 15, 50 or 150 mg/kg bw per day) for 102 weeks. The animals assigned
to this study were F1a, generation weanlings from a multigeneration
study in which animals were fed diets containing 0, 300, 1000 or
3000 mg/kg 2,6-xylidine beginning at 5 weeks of age. Parameters
evaluated in the carcinogenicity study included clinical observations,
haematology, blood urea nitrogen, glucose, SGOT, alkaline phosphatase
and gross and microscopic postmortem examinations.
Treatment-related clinical effects included a decrease in mean
body weight gain in high-dose males and females (>10%). Mortality was
significantly (P < 0.001) increased (relative to controls) in males
in the high-dose group. Mortality was also increased for mid-dose
males. Survival at 105 weeks was 43/56, 40/56, 33/56 and 14/56 for
males in the control, low-, mid- and high-dose groups, respectively.
For females, survival was 33/56, 25/56, 32/56 and 24/56 for the
controls, low-, mid- and high-dose groups, respectively.
Microscopically, a significant increase in carcinoma of the nasal
cavity was observed in high-dose males (26/56; P < 0.001, life table
test). For females, the incidence of carcinomas of the nasal cavity
were 0/56, 0/56, 1/56 and 24/56 in the low-, mid- and high-dose
groups, respectively (P<0.001, life table test). Two adenocarcinomas
were diagnosed in high-dose males. The incidence of papillary adenomas
in males was 0/56 in controls, 0/56 in low-dose, 2/56 in mid-dose and
10/56 in high-dose rats (P=0.001, incidental tumour test). For
females, nasal adenomas occurred in 0/56 in controls, 0/56 in low-
dose, 1/56 in mid-dose and 6/56 in high-dose rats (P=0.02, incidental
tumour test). Several unusual neoplasms of the nasal cavity were also
considered to be related to treatment. These included one undifferen-
tiated sarcoma identified in one high-dose female, rhabdomyosarcomas
which occurred in two high-dose male and two high-dose females and
malignant mixed tumours having features associated with both adenocar-
cinoma and rhabdomyosarcoma were observed in one high-dose male and
one high-dose female rat. Non-neoplastic nasal cavity lesions included
acute inflammation (rhinitis), epithelial hyperplasia and squamous
metaplasia. These occurred at increased incidence (relative to
controls) in high-dose male and female rats. The incidence of
subcutaneous fibromas and fibrosarcomas combined in males was 0/56,
2/56, 2/56 and 5/56 for the control, low-, mid- and high-dose groups,
respectively (P=0.001, life table test; P<0.001 life table trend
test). For females, the incidence of these tumours combined was 1/56,
2/56, 2/56 and 6/56 for controls, low-, mid- and high-dose groups,
respectively ((P=0.01, life table trend test). Neoplastic nodules occurred
in livers of female rats with a significant positive trend. The
incidence was 0/56, 1/56, 2/56 and 4/55 for the controls, low-, mid-,
and high-dose groups, respectively (P=0.03, incidental test; P=0.012,
incidental trend test).
Treatment-related effects on haematology included decreases in
erythrocyte counts and haemoglobin levels at 18 months in the
high-dose males. Decreases in these parameters were also observed in
the mid- and high-dose females at 12 months. The author remarked that
these changes were not severe enough to be considered indicative of
anaemia.
The author concluded that under the conditions of this study,
2,6-xylidine was dearly carcinogenic for male and female Charles River
CD rats. This was based on the observed significant increases in the
incidence of adenomas and carcinomas of the nasal cavity. Additionally
the author stated that the increased incidence of subcutaneous
fibromas and fibrosarcomas in male and female rats and increased
incidence of neoplastic nodules of the liver in female rats could have
been treatment-related (US National Toxicology Program, 1990).
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenic risk of 2,6-xylidine to humans. The Working
Group concluded that there was inadequate evidence in humans but
sufficient evidence in experimental animals for the carcinogenicity of
2,6-xylidine. The IARC classified 2,6-xylidine as Group 2B (possibly
carcinogenic to humans) (IARC, 1993).
2.2.4 Special studies on teratogenicity
Xylazine was administered by gavage to groups of pregnant rats
(22 animals/group) on gestation days 6 to 15, then killed on day 20
for examination of uterine contents. Dosages administered were 0, 1, 4
or 16 mg/kg bw per day. The study was conducted in accordance with the
principles of Good Laboratory Practice Standards and Guidelines of the
OECD, United Kingdom, FDA and Japan.
Treatment-related maternal effects included partial closing of
the eyelids, underactivity, ataxia, flat posture and slightly reduced
body weight gain in the high-dose group only. Fetal effects included a
decrease in mean fetal weight in the high-dose group only. A
teratogenic potential of xylazine was not evident at levels up to and
including 16 mg/kg bw per day. The NOEL in this study was 4 mg/kg bw
per day (Reynolds, 1994).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with xylazine and
2,6-xylidine are summarized in Table 4.
The results of bacterial mutagenicity testing of xylazine were
considered to be negative by the author. Reviewing the data, the
Committee concluded that a more than two-fold reproducible increase in
revertant colonies in tester strains TA1535 and TA1538 represents weak
mutagenic activity, even in the absence of a clear dose-response.
2.2.6 Special studies on methaemoglobin and haemoglobin adduct
formation with 2,6-xylidine
2.2.6.1 Cats and dogs
Cats and dogs (numbers not specified) were administered an i.v.
dose of 30 mg 2,6-xylidine/kg bw or an oral dose of 164 mg N-acetyl
2,6-xylidine/kg bw. 2,6-Xylidine induced a 10% methaemoglobinaemia in
cats, and N-acetyl 2,6-xylidine induced a 5% methaemoglobinaemia in
cats. Haemoglobin was unaffected in dogs in this study (McLean
et al., 1967).
Five adult cats (>24 months old) were administered an i.v. dose
of 30 mg 2,6-xylidine/kg bw. Blood samples were drawn at 1, 2, 3, 4
and 5 hours after dosing and analysed for methaemoglobin formation.
The mean methaemoglobin concentration determined from these sampling
intervals was 7% (range = 4.8% to 8.7%). Prior to treatment the mean
methaemoglobin concentration of the 152 cats used in this study was
approximately 1% (Mclean et al., 1969).
2.2.6.2 Humans
2,6-Xylidine-haemoglobin adduct levels have been found to be
elevated in human patients receiving lidocaine treatment for local
anaesthesia (1 mg/kg bw) or cardiac arrhythmias (up to 50 mg/kg bw,
i.v.). 2,6-Xylidine-haemoglobin adducts are also found in humans with
no known exposure to lidocaine. This is attributed to the 120-day
lifespan of the erythrocyte and chronic exposure to environmental or
iatrogenic sources of aromatic amines, e.g., cigarette smoke. The
levels of 2,6-xylidine-haemoglobin adducts found correspond to at an
estimated daily exposure (from iatrogenic and environmental sources)
of 23 µg (IARC, 1993; Bryant et al., 1994).
Methaemoglobinaemia induced by i.v. administration of lidocaine
was studied in 40 human cardiac patients. Treatment consisted of a
1 mg/kg bw i.v. bolus followed 15 minutes later with a 0.5 mg/kg bw
i.v. bolus. Patients were maintained between and after bolus doses
with an infusion rate of 1-4 mg lidocaine/min. Blood samples were
drawn before treatment and 1 and 6 hours after treatment. Although the
investigators found methaemoglobin levels in these patients to be
significantly elevated, the increase was not large enough to be of
clinical concern. The highest methaemoglobin level attained was 1.2%.
The author did not address the possible role of the 2,6-xylidine
metabolite in the observed increases in methaemoglobin levels in
treated patients (Weiss et al., 1987).
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine
Test system Test object Concentration Results Reference
Xylazine
In vitro
Reverse S. typhimurium
mutation1 TA1535, 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA1538 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA100, 0.4-12 mg/plate Negative Herbold, 1984
TA98, 0.4-12 mg/plate Negative Herbold, 1984
TA1537 0.4-12 mg/plate Negative Herbold, 1984
Mammalian V79/HGPRT 62-1250 µg/ml Negative Brendler-
cell forward (-S9) Schwaab,
mutation1 2-40 µg/ml (+S9) 1994
In vivo
Cytogenetic Mouse bone 50 mg/kg bw, Negative Herbold, 1995
assay marrow i.p.2
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
2,6-Xylidine
In vitro
Reverse S. typhimurium
mutation1 TA1535 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
3 µmol/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al., 1988
TA100 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin el al.,
19804
0.1-10 mg/plate Neg (-S9), Zeiger et al.,
Pos (+S9) 19883
480-4000 Negative Kugler-
µg/plate Steigmeier
et al., 1989
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
TA1537 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al.,
1988
TA98 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1 - 10 mg/plate Negative Zeiger et al.,
1988
Gene Mouse lymphoma Not given Positive Rudd et al.,
mutation1 L5178Y cells, 19837
tk locus
Sister Chinese 30-1500 µg/ml Positive Galloway et al.,
chromatid hamster 1987
exchange1 ovary cells
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
In vivo
Cytogenetic ICR mouse 350 mg/kg bw, Inconclusive6 Parton et al.,
assay bone marrow p.o. 1988
375 mg/kg bw, Inconclusive6 Parton et al.,
p.o. 1990
In vivo- Rat primary 40-850 mg/kg Negative Mirsails et al.,
in vitro DNA hepatocytes bw, p.o. 1989
repair assay
Covalent Rats 87.2 µCi Positive Short et al., 1989
DNA 14C-labelled
binding 2,6-xylidine/rat,
i.p5
1 Both with and without rat liver S9 fraction
2 Cyclophosphamide positive control
3 Weakly positive in two of three laboratories, negative in the third
4 Spot tests only
5 Pretreatment with unlabelled 262.5 mg/kg bw 2,6-xylidine daily for 9 days
6 Results suggest test article may not have reached target tissue (bone marrow)
7 Reference was an abstract and doses were not stated in that reference
2.3 Observations in humans
A 34-year-old man self-injected 10 ml of a 100 mg/ml solution of
xylazine intramuscularly. The estimated dose was 15 mg/kg bw. The
individual was discovered (30 minutes after retiring for bed) in a
deeply comatose, apnoeic and areflexic state. An empty Rompun
(xylazine) bottle (known to have contained 10 cc earlier) lay by his
side. He was immediately admitted to the hospital. Upon admission his
pupils were of moderate size and responded slowly to light. Other
findings included a blood pressure of 120/70 mmHg and heart rate of
60 bpm with stable sinus rhythm. Lactate dehydrogenase (LDH) activity
was elevated, with the LDH-1 isoenzyme predominating. Creatine
phosphokinase (CPK) activity was also elevated, particularly in the
CPK-3 and CPK-2 isoenzymes. These enzyme changes persisted for 5 to 7
days. Plasma glucose level was also elevated. Two days following
hospital admission sinus tachycardia developed, interspersed with runs
of multifocal premature ventricular contractions which were controlled
with lidocaine infusion. Blood pressure remained approximately 120/80
for the duration of hospitalization. Coma and respiratory depression
lasted 60 hours. The patient was discharged from the hospital 17 days
after admission.
The author noted that the patient might have died if he had not
been found shortly after the injection was administered, owing to the
marked respiratory depression that occurred. Hypotension, which has
been reported as an effect of xylazine in humans, did not occur in
this case. According to the author, the enzyme activity increases
indicated that myocardial muscle damage had occurred and an intrinsic
cardiotoxic effect of xylazine was suspected. Finally, in this case
the greatest threat to life was the CNS-depressant effect of xylazine
(Carruthers et al., 1979).
A 20-year-old woman ingested 400 mg of xylazine. Approximately 2
hours later she became drowsy, incontinent (urine), difficult to
arouse and occasionally unresponsive to verbal commands. She was
admitted to the hospital approximately 3 hours after the ingestion. In
a similar way to the case of human poisoning described by Carruthers
et al. (1979), she experienced a relatively low initial cardiac rate,
which later gradually increased, significant central nervous system
and respiratory depression, transient hyperglycaemia and ventricular
arrhythmias. However there was no evidence of myocardial damage. A
sample of this patient's urine was analysed using a gas chromatograph-
mass spectrometer computer system. Xylazine was found largely unchanged
in the urine as shown by a lack of any structurally related compounds
in the basic urine extract. No xylazine was found in a blood sample
taken at the time of admission. The author concluded that plasma
levels were below the limit of detection of the method (100 ng/ml).
The patient was discharged ambulatory and without apparent adverse
effects two days following admission to the hospital (Gallanosa
et al., 1981).
A 36-year-old man died following ingestion of alcohol and
clorazepate combined with an injection of approximately 40 ml of
xylazine (100 mg/ml). Xylazine was found in the decedent's blood,
brain, kidney, liver, lung, fat and urine at concentrations of 0.2,
0.4, 0.6, 0.9, 1.1, 0.05 and 7 ppm, respectively (Poklis et al.,
1985).
A 29-year-old woman self-injected 40 mg of xylazine
intramuscularly. The estimated dose was 0.73 mg/kg bw. Clinical
findings included disorientation, miosis, hypotension and bradycardia,
but no cardiac arrhythmias were noted. The abnormalities resolved
spontaneously (Spoerke et al., 1986).
A 37-year-old woman self-injected 24 ml (2400 mg) xylazine
intramuscularly. The estimated dose was 22 mg/kg bw. Twenty minutes
after the injection her blood pressure was 166/130 mmHg, heart rate
was 76 bpm and respirations 18 per minute. The serum glucose level was
175 mg/dl. Blood pressure later decreased to 130/90 mmHg and she
became apnoeic. No cardiac arrhythmias were observed during her 3 days
of hospitalization. Hypotension and bradycardia occurred two days
after the injection. The patient survived (Spoerke et al., 1986).
A 29-year-old woman self-injected an unknown amount of xylazine
intravenously. She became apnoeic and had an initial blood pressure of
130/90 mmHg with a pulse of 60 bpm. Serum glucose levels did not
exceed 90 mg/dl. Twenty-four hours after the injection the patient
experienced hypotension and bradycardia. Spontaneous respiration
resumed 18 hours after hospital admission. The patient recovered fully
(Spoerke et al., 1986).
A 19-year-old man accidentally injected himself subcutaneously
with 2 ml (100 mg/ml) of xylazine. The dose administered was 3 mg/kg
bw. Thirty minutes later he became difficult to rouse and was
hospitalized. Clinical findings included miosis, hyporeflexia,
hypotension, bradycardia, respiratory and central nervous system
depression and hyperglycaemia. He was treated with intravenous fluids
and assisted ventilation. Eight hours after hospitalization the
patient was alert and responsive. Twenty-four hours later he was
released (Samanta et al., 1990).
A 39-year-old woman was admitted to the hospital with symptoms of
tiredness, faintness and blurred vision. Clinical findings included
sinus bradycardia with a blood pressure of 130/90. Xylazine was found
in the urine and serum at concentrations of 1674 µg/litre and
30 µg/litre, respectively (Lewis et al., 1983).
3. COMMENTS
The Committee considered toxicological data on xylazine,
including the results of acute and short-term toxicity studies as well
as studies on pharmacodynamics, pharmacokinetics, reproductive and
developmental toxicity, genotoxicity and effects in humans. In
addition, toxicological studies on 2,6-xylidine, a metabolite of
xylazine, were reviewed; these included studies on acute and
short-term toxicity, carcinogenicity and genotoxicity.
Numerous pharmacological side-effects of xylazine have been
observed in treated animals, including mydriasis, impairment of
thermo-regulatory control, various effects on the cardiovascular
system, acid-base balance and respiration, hyperglycaemia, and
haematological and gastrointestinal effects. Cattle and sheep are
approximately 10 times more sensitive to xylazine than horses, dogs
and cats.
Rats were administered radiolabelled xylazine intravenously at
doses of 0.02 to 10 mg/kg bw or orally at doses of 0.02 to
100 mg/kg bw. More than 95% of the oral dose was absorbed, with a
half-life of approximately 5 minutes. Following oral or intravenous
administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal excretion following oral
or intravenous administration was associated with a half-life of 2 to
3 hours. Enterohepatic circulation did not occur to a notable extent.
In cattle administered an intramuscular dose of 0.2 or 0.5 mg
xylazine/kg bw, less than 1% of the dose was excreted unchanged in the
urine, and the parent compound was detected in the urine up to 6 hours
following administration. Metabolites of xylazine were detected in
urine from these cattle up to 10 hours following administration.
Pharmacokinetic parameters following intravenous administration
showed minor variations between species. Xylazine disappeared rapidly
from plasma following intravenous administration, with an elimination
half-life of approximately 40 minutes in cattle and approximately 20
minutes in sheep. Xylazine could not be detected in the plasma of
cattle following intramuscular administration of a single therapeutic
dose.
In rats administered an intravenous dose of 2 mg/kg bw radio-
labelled xylazine, approximately 20 metabolites were quantified as
xylazine equivalents in urine and bile. The major metabolite comprised
35% of the administered dose. Approximately 8% of the dose was
eliminated as unchanged xylazine 24 hours after dosing. In an
in vitro study, 4 metabolites were identified when xylazine was
incubated with rat liver microsomes. The same metabolites were
identified in the urine of horses treated with xylazine. The major
metabolite in both cases was identified as N-(2,6-dimethylphenyl)
thiourea. In cattle administered an intramuscular dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow),
2,6-xylidine was identified as a metabolite excreted in urine in both
conjugated and unconjugated forms.
The acute oral toxicities of xylazine and 2,6-xylidine were
tested in mice and rats. Xylazine was determined to be moderately
toxic (LD50 = 121-240 mg/kg bw) and 2,6-xylidine to be slightly
toxic (LD50 = 600-1000 mg/kg bw).
Three studies on the short-term toxicity of xylazine were
reviewed. A 32-week dietary study in rats and a 16-week oral
(capsules) study in dogs were considered inadequate for the
determination of the toxicity of xylazine owing to the use of
insufficient numbers, poor quality animals and inadequate study
design. The third was a 13-week oral study in beagle dogs fed
diets containing 0, 10, 30 or 100 mg/kg xylazine in the feed (equal
to 0.3, 0.9 or 3 mg/kg bw per day). No treatment-related effects
were observed in any of the treated groups.
In a two-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 80, 160, 310, 620 or 1250 mg
2,6-xylidine/kg bw per day, 5 days per week. Treatment-related effects
included increased mortality (all animals in the high-dose group
died), decreased body weight (males at 310 mg/kg bw per day and above
and females at 160 mg/kg bw per day and above) and various effects on
haematological parameters as indicated by leukocytosis and changes in
red blood cell parameters indicative of increased erythropoiesis
(males and females at 310 mg/kg bw per day and above). The NOEL in
this study was 80 mg/kg bw per day.
In a 13-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 20, 40, 80, 160 or 310 mg
2,6-xylidine/kg bw per day, 5 days per week for 13 weeks. Treatment-
related effects included decreased body weight gain (males at 310
mg/kg bw per day and females at 40 mg/kg bw per day and above),
increased absolute and relative liver weights (females at 160 mg/kg bw
per day and above; males at 310 mg/kg bw per day), leukopenia (males
at 40 mg/kg bw per day and above), haemoglobinaemia (males at
160 mg/kg bw per day and above) and anaemia (males at 310 mg/kg bw per
day). The NOEL was 20 mg/kg bw per day.
In a carcinogenicity study, male and female rats were fed diets
containing 2,6-xylidine at concentrations of 300, 1000 or 3000 mg/kg
food (equivalent to 15, 50 or 150 mg/kg bw per day). Significant
increases in the incidences of papillomas and carcinomas of the nasal
cavity were observed in high-dose males and females. There was a
significant dose-related increase in the incidence of adenomas in the
nasal cavity in both males and females. In addition, unusual rhabdo-
myosarcomas and malignant mixed tumours of the nasal cavity were
observed in the high-dose males and females. There was a dose-related
significant increase in the incidence of subcutaneous fibromas and
fibrosarcomas in both treated males and females. In females,
neoplastic nodules occurred in livers with a significant positive
trend and the increase was significant in the high-dose group by the
incidental tumour test. The Committee concluded that 2,6-xylidine was
carcinogenic in this study.
The International Agency for Research on Cancer has evaluated the
carcinogenic risk of 2,6-xylidine and has classified it as Group 2B
(possibly carcinogenic to humans).
In a teratogenicity study, xylazine was administered to pregnant
rats at doses of 1, 4 or 16 mg xylazine/kg bw per day on gestation
days 6 to 15. Treatment-related maternal effects included partial
closing of the eyelids, hypoactivity, ataxia, flat posture and
slightly reduced body weight gain in the high-dose group only. A
decrease in mean fetal weight was seen in the high-dose group. No
teratogenic effects were noted in this study. The NOEL for maternal
and fetal effects was 4 mg/kg bw per day.
Xylazine has been tested in reverse mutation assays in
Salmonella, a forward mutation assay in cultured mammalian cells and
in an in vivo cytogenetic assay. In Salmonella, weak positive
results were obtained. Negative results were observed in a forward
mutation assay on cultured mammalian cells and in a mouse bone marrow
micronucleus test. The Committee concluded that xylazine is weakly
mutagenic.
2,6-Xylidine was tested in a series of in vitro and in vivo
genotoxic assays. It was weakly positive for reverse mutation in
Salmonella. In mammalian cells, it induced forward mutation and was
positive in a sister chromatid exchange test. Inconclusive results
were obtained in a mouse bone marrow micronucleus test because there
was no assurance that the bone marrow had been adequately exposed.
2,6-Xylidine was found to be inactive in an in vivo-in vitro rat
hepatocyte unscheduled DNA synthesis assay. Covalent binding of the
compound to DNA was observed in rats. The Committee concluded that
2,6-xylidine is genotoxic.
The potential for 2,6-xylidine to induce methaemoglobinaemia was
reviewed by the Committee. Single doses of 30 mg 2,6-xylidine/kg bw
intravenously or 164 mg/kg bw N-acetyl-2,6-xylidine orally have
been shown to induce methaemoglobinaemia in cats but not in dogs.
2,6-Xylidine has also been shown to be a product of lidocaine
metabolism in humans. Methaemoglobin and 2,6-xylidine-haemoglobin
adduct levels have been shown to increase in human cardiac patients
receiving lidocaine treatment.
Effects of xylazine on humans poisoned following accidental or
intentional self-injection (0.7-15 mg/kg bw) or ingestion (7 mg/kg bw)
included symptoms of central nervous system depression, respiratory
depression, hypo- and hypertension, bradycardia, tachycardia,
ventricular arrhythmias, and transient hyperglycaemia.
4. EVALUATION
The Committee was unable to establish an ADI for xylazine because
it concluded that the 2,6-xylidine metabolite was genotoxic and
carcinogenic. Annex 4 lists the information that would be required for
further review.
5. ACKNOWLEDGMENTS
The preparer of the first draft would like to recognize the
following individuals for their assistance and contributions to the
preparation of the first draft:
Ms. Deborah Brooks, information specialist, Center for Veterinary
Medicine
Dr. Steve Brynes, residue chemist, Center for Veterinary Medicine
Dr. Jennifer Burris, veterinary pathologist, Center for Veterinary
Medicine
Dr. Robert Condon, biostatistician, Center for Veterinary Medicine
Dr. Haydee Fernandez, toxicologist, Center for Veterinary Medicine
Dr. Devaraya Jagannath, genetic toxicologist, Center for Veterinary
Medicine
Dr. Alan Pinter, toxicologist, National Institute of Public Health,
Budapest, Hungary
Dr. Leonard Schechtman, genetic toxicologist, Center for Veterinary
Medicine
6. REFERENCES
Amer, A.A. & Misk, N.A. (1980). Rompun in goats with special reference
to its effect on the cerebral spinal fluid (c.s.f.). Vet. Med. Rev.,
2, 168-174.
Bauman, E. K. & Nelson, D.L. (1969). Toxicity of BAY Va 1470 to cats.
Unpublished report No. 24208 from Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Benson, G.J., Thurmon, J.C., Neff-Davis, C.A., Corbin, J.E.,
Davis, L.E., Wilkinson, B., & Tranquilli, W.J. (1984). Effect of
xylazine hydrochloride upon plasma glucose concentrations in adult
pointer dogs. J. Am. Anita. Hosp. Assoc., 20, 791-794.
Bolte, S. & Stupariu, A (1978). Motility of the rumen in cattle and
sheep under the influence of neuroleptics and analgesics, particularly
xylazine, propionylpromazine and diazepam. Lucrari Stiintifice
Inatitutul Agron. Timisoara, Ser. Med. Vet., 15, 157-167.
Booth, N.H. (1988). Nonnarcotic analgesics. In: Booth N.H.&
McDonald E. (eds.), Veterinary Pharmacology and Therapeutics, 6th
edition, Iowa State University Press, Ames, Iowa, pp. 351-359.
Brendler-Schwaab, S. (1994). Rompun hydrochloride. Mutagenicity study
for the detection of induced forward mutations in the V79-HGPRT assay
in vitro. Unpublished report No. T9049349 from Bayer AG, Fachbereich
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bryant, M.S., Simmons, H.F., Harrell, R.E., & Hinson, J.A. (1994).
2,6-Dimethylaniline-hemoglobin adducts from lidocaine in humans.
Carcinogenesis, 15(10), 2287-2290.
Carruthers, S.G., Nelson, M., Wexler H.R., & Stiller, C.R. (1979).
Xylazine hydrochloride (Rompun) iverdise in man. Clin. Toxicol.,
15(3), 281-285.
Carter, S.W., Robertson, S.A., Steel, C.J., & Jourdenais, D.A. (1990).
Cardiopulmonary effects of xylazine sedation in the foal.
Equine Vet. J., 22(6), 384-388.
Colby, E.D., McCarthy, L.E., & Borison, H.L. (1981). Emetic action of
xylazine on the chemoreceptor trigger zone for vomiting in cats.
J. Vet. Pharmacol. Ther., 4, 93-96.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970a). The toxicity of
BAY Va 1470 2% injectable to dogs and cats given at ten times
recommended dose. Unpublished report No. 28139 from Research
Department, Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970b). The toxicity of
BAY Va 1470 10% injectable alone and in combination with (R)NEGUVON*
soluble powder (batch No. 0050337) to horses. Unpublished report
No. 28136 from Research Department, Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Cullen, L.K. & Jones, R.S. (1977). Clinical observations on
xylazine/ketamine anaesthesia in the cat. Vet. Rec., 101, 115-116.
Demoor, A. & Desmet, P. (1971). Effect of Rompun on acid-base
equilibrium and arterial oxygen pressure in cattle. Vet. Med. Rev.,
2-3, 163-169.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1968). Experimental tests on animals with radioactive labeled BAY Va
1470. Unpublished report No. 22874 from Bayer Isotope Institute,
Elberfeld Branch. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1969). Experiments using radioactively tagged BAY Va 1470 on rats.
Berl. Münch. Tierärztl. Wochenschr., 82, 104-109.
Eichner, R.D., Prior, R.L., & Kvasnicka, W.G (1979). Xylazine-induced
hyperglycemia in beef cattle. Am. J. Vet. Res., 40(1), 127-129.
Feldberg, W. & Symonds, H.W. (1980). Hyperglycemic effect of xylazine.
J. Vet. Pharmacol. Ther., 3, 197-202.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Freire, A.C.T, Gontijo, R.M., Pessoa, J.M., & Souza, R. (1981). Effect
of xylazine on the electrocardiogram of sheep. Br. Vet. J.,
137, 590-595.
Gallanosa, A.G., Spyker, D.A., Shipe, J.R., & Morris, D.L. (1981).
Human xylazine overdose: A comparative review with clonidine,
phenothiazines, and tricyclic antidepressants. Clin. Toxicol.,
18(6), 663-678.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon C., Bloom, A.D., Nakamura, F., Ahmed, N., Duk, S., Rimpo, J.,
Margolin, B.H., Resnick, M.A., Anderson, B., & Zeiger, E. (1987).
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10), 1-175
Garcia-Villar, R., Toutain, P.L., Alvinerie, M., & Ruckenbusch Y.
(1981). The pharmacokinetics of xylazine hydrochloride: an
interspecific study. J. Vet. Pharmacol. Ther., 4, 87-92.
Gross, M.E. & Tranquilli, W.J. (1989). Use of alpha-2-adrenergic
receptor antagonists. J. An. Vet. Med. Assoc., 195(3), 378-381.
Herbold, B. (1984). Salmonella/microsome test to evaluate for
point-mutagenic effects. Unpublished report No. T0016805 from Bayer
Sparte Pharma. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995). Rompun Hydrochloride. Micronucleus test on the
mouse. Unpublished report No. T9058169 + T2058171 from Bayer AG
Fachbereich Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hikasa, Y. Takase, K., & Ogasawara, S. (1989). Evidence for the
involvement of alpha-2-adrenoceptors in the emetic action of xylazine
in cats. Am. J. Vet. Res., 50, 1348-1351.
Hikasa, Y., Takase, K., Osada, T., Takamatsu, H., & Ogasawara, S.
(1987). Xylazine-induced vomiting in dogs: elimination by ablation of
the area postrema and blockade by yohimbine. Zent. bl. Vet. med.,
A34(2), 154-158.
Holmes, A.M. & Clark, W.T. (1977). Xylazine sedation of horses.
N.Z. Vet. J., 25, 159-161.
Hsu, W.H. & Hummel, S.K. (1981). Xylazine-induced hyperglycemia in
cattle: a possible involvement of alpha-2-adrenergic receptors
regulating insulin release. Endocrinology, 109, 825-829.
Hsu, W.H. & McNeel, S.V. (1983). Effect of yohimbine on
xylazine-induced prolongation of gastrointestinal transit in dogs.
J. Am. Vet. Med. Assoc., 183(3), 297-300.
Hsu, W.H., Betts, D.H., & Lee, P. (1981). Xylazine-induced mydriasis:
possible involvement of a central postsynaptic regulation of
parasympathetic tone. J. Vet. Pharmacol. Ther., 4, 209-214.
Hsu, W.H., Hanson, C.E., Hembrough, F.B., & Schaffer, D.D. (1989).
Effects of idazoxan, tolazoline and yohimbine on xylazine induced
respiratory changes and central nervous system depression in ewes.
Am. J. Vet. Res., 50(9), 1570-1573.
IARC (1993). 2,6-Dimethylaniline (2,6-xylidine). IARC Monograph on the
Evaluation of Carcinogenic Risks to Humans: Occupational exposures of
hairdressers and barbers and personal use of hair colourants; some
hair dyes, cosmetic colourants, industrial dyestuffs and aromatic
amines, 57, 323-335.
Jacobson, K.H. (1972). Short communication. Acute oral toxicity of
mono- and di-alkyl ring-substituted derivatives of aniline.
Toxicol. Appl. Pharmacol., 22, 153-154.
Klide, A.M., Calderwood, H.W., & Simen L.R. (1975). Cardio-pulmonary
effects of xylazine in dogs. Am. J. Vet. Res., 36(7), 931-935.
Kugler-Steigmeier, M.E., Frierich, U., Graf, U., Lutz, W.K.,
Maier, P., & Schlatter, C. (1989). Genotoxicity of aniline derivatives
in various short-term tests. Mutat. Res., 211, 279-289.
Leblanc, P.H. & Eberhart, S.W. (1990). Cardiopulmonary effects of
epidurally administered xylazine in the horse. Equine Vet. J.,
22(6), 389-391.
Lewis, S., O'Callaghan, C.L.P., & Toghill, P.J. (1983). Clinical
curio: self medication with xylazine. Br. Med. J., 287, 1369.
Lindstrom, H.V., Hansen, W.H., Nelson, A.A., & Fitzhugh, O.G. (1963).
The metabolism of FD&C Red No. 1. II. The fate of 2,5- para-xylidine
and 2,6- meta-xylidine in rats and observations of the toxicity of
xylidine isomers. J. Pharmacol. Exp. Ther., 142, 257-264.
Lindstrom, H.V., Bowie, W.C., Wallace, W.C., Nelson, A.A., &
Fitzhugh, O.G. (1969). The toxicity and metabolism of mesidine and
pseudocumidine in rats. J. Pharmacol. Exp. Ther., 167(2), 223-234.
McLean, S., Murphy, B.P., Starmer, G.A., & Thomas, J. (1967).
Methaemoglobin formation induced by aromatic amines and amides.
J. Pharm. Pharmacol., 19, 146-154.
McLean, S., Starmer, G.A., & Thomas, J. (1969). Methaemoglobin
formation by aromatic amines. J. Pharm. Pharmacol., 21, 441-450.
Magnusson, G., Bodin, N.-O., & Hansson, E. (1971). Hepatic changes in
dogs and rats induced by xylidine isomers. Acta Pathol. Microbiol.
Scand., 79, 639-648.
Magnusson, G., Majeed, S.K., Down, W.H., Sacharin, R.M., & Jorgeson,
W. (1979). Hepatic effects of xylidine isomers in rats. Toxicology,
12, 63-74.
Mawdesley-Thomas, L.E. (1970). Pathology report of the subchronic
toxicity for dogs by oral administration of compound BAY Va 1470.
Unpublished report No. 26692 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L, Loh, E.K., Hamilton,
C.M., Bakke, J.P., & Splading, J.W. (1989). Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: testing of 24 compounds. Environ. Mol. Mutagen.,
13, 155-164.
Murphy, J.J. & Jacobs, K. (1975). Residues of ROMPUN and its
metabolites in cattle. Unpublished report No. 43814 from Chemagro
Agricultural Division, Mobay Chemical Corporation. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mutlib, A.E., Chui, Y.C., Young, L.M., & Abbott, F.S. (1992).
Characterization of metabolites of xylazine produced in vivo and
in vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos.,
20(6), 840-848.
Nelson, D.L., Bauman, E.K., Mosier, J.O., & Allen, A.D. (1968a). A
study of the effect of large doses of BAY Va 1470 to horses.
Unpublished report No. 23405 from Research and Development Department,
Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Nelson, D.L., White, R.G. Bauman, E.K., & Allen, A.D. (1968b). The
effect of large injected doses of BAY Va 1470 to dogs. Unpublished
report No. 23679 from Research and Development Department, Chemagro
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Parton, J.W., Probst, G.S., & Garriott, M.L. (1988). The in vivo
effects of 2,6-xylidine on induction of micronuclei in mouse bone
marrow cells. Mutat. Res., 206, 281-283.
Parton, J.W., Beyers, J.E., Garriott, M.L., & Tamura, R.N. (1990). The
evaluation of multiple dosing protocol for the mouse bone-marrow
micronucleus assay using benzidine and 2,6-xylidine. Mutat. Res.,
234, 165-168.
Poklis, A., MacKell, M.A., & Case, M.E.S. (1985). Xylazine in human
tissues and fluids in a case of fatal drug abuse. J. Anal. Toxicol.,
9, 234-236.
Ponder, S.W. & Clark, W.G. (1980). Prolonged depression of
thermoregulation after xylazine administration to cats.
J. Vet. Pharmacol. Ther., 3, 203-207.
Pütter, J. & Sagner, G. (1973). Chemical studies to detect residues of
xylazine hydrochloride. Vet. Med. Rev., 2, 145-159.
Reynolds, S.M. (1994). Xylazine hydrochloride: teratology study in the
rat. Unpublished report No. 94/BAG240/0268 from Pharmaco LSR.
Submitted to WHO by Bayer AG, Leverkusen, Germany (GLP statement and
report summary, only).
Robertson, S.A., Carter, S.W., Donovan, M., & Steele, C. (1990).
Effects of intravenous xylazine hydrochloride on blood glucose, plasma
insulin and rectal temperature in neonatal foals. Equine Vet. J.,
22(1), 43-47.
Ruckebusch, Y. & Toutain, P.L. (1984). Specific antagonism of xylazine
effects on reticulo-rumen motor function in cattle. Vet. Med. Rev.,
1, 1-12.
Rudd, C.J., Mitchell, A.D., & Spalding, J. (1983). L5178Y Mouse
lymphoma cell mutagenesis assay of coded chemicals incorporating
analyses of the colony size distributions (Abstract No. Cd-19).
Environ. Mutagen., 5(3), 419.
Samanta, A., Roffe, C., & Woods, K.L. (1990). Accidental self
administration of xylazine in a veterinary nurse. Postgrad. Med. J.,
66, 244-245.
Sagner, G. (1967). Bay Va 1470, anaesthetic, analgesic and sedative
for veterinary medicine. Unpublished report No. 1470 from Bayer AG
Institute of Pharmacology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sagner, G., Hoffmeister, F., & Kroneberg, G. (1969). Pharmacological
principles of a new preparation for analgesia, sedation and relaxation
in veterinary medicine (Bay Ve 1470). Vet. Med. Rev., 3, 226-228.
Short, C.R., King, C., Sistrunk, P.W., & Kerr, K.M. (1983). Subacute
toxicity of several ring-substituted dialkylanilines in the rat.
Fundam. Appl. Toxicol. 3, 285-292.
Short, C.R., Joseph, M., & Hardy, M.L. (1989). Covalent binding of
[14C]-2,6-dimethylaniline to DNA of rat liver and ethmoid turbinate.
J. Toxicol. Environ. Health, 27, 85-89.
Singh, J., Peshin, P.K., Singh, A.P., & Nigam, J.M. (1983).
Haemo-dynamic, acid base and blood gas alterations after xylazine
administration in calves. Indian J. Vet. Surg., 4, 10-15.
Skarda, R.T., Jean, G., & Muir, W.W. (1990). Influence of tolazoline
on caudal epidural administration of xylazine in cattle. Am. J. Vet.
Res., 51(4), 556-560.
Spoerke, D.G., Hall, A.H., Grimes, M.J., Honea, B.N., & Rumack, B.H.
(1986). Human overdose with veterinary tranquilizer xylazine.
Am. J. Emerg. Med., 4, 222-224.
Swift, B.L. (1977). A technique for the surgical removal of the spleen
in calves. Vet. Med.-Small Anim. Clin., January, 77-79.
Symonds, W.H. (1976). The effect of xylazine on hepatic glucose
production and blood flow rate in the lactating dairy cow.
Vet. Rec., 99, 234-236.
Takase, I., Terada, H., & Fujii, T. (1976) Xylazine residues in organs
and tissues of calves and milk of cows. Unpublished report No. 76/
8278a from the Department of Veterinary Science, Faculty of
Agriculture, Tokyo University of Agriculture and Technology. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tettenborn, D. (1969). BAY Va 1470. Subchronic toxicity for dogs
during application as feed additive. Unpublished report No. 26692
(1731) from Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968a). BAY Va 1470. Chronic toxicity to
rats from oral administration. Unpublished report No. 23414 (956) from
Bayer AG, Institute of Pharmacology and Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968b). BAY Va 1470. Subchronic toxicity
to dogs from oral administration. Unpublished report No. 23415 (958)
from Bayer AG, Institute of Pharmacology and Toxicology. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thurmon, J.C., Neff-Davis, C., Davis, L.E., Stoker, R.A., Benson,
G.J., & Lock, T.F. (1982). Xylazine hydrochloride-induced
hyperglycemia and hypoinsulinemia in thoroughbred horses. J. Vet.
Pharmacol. Ther., 5, 241-245.
Thurmon, J.C., Steffey, E.P., Zinkl, J.G., Waliner, M., & Howland, D.
(1984). Xylazine causes transient dose-related hyperglycemia and
increased urine volume in mares. Am. J. Vet. Res., 45(2), 224-227.
Trossmann, G. & Hobik, H.P. (1970). BAY Va 1470. Histopathological
changes in organs of rats after feeding for 32 weeks. Unpublished
report No. 28576 (956) from Bayer AG, Institute of Pathology and
Histology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
US National Toxicology Program (1990). Toxicology and Carcinogenesis
Studies of 2,6-Xylidine (2,6-Dimethylaniline) (CAS No. 87-62-7) in
Charles River CD Rats (Feed Studies). US National Toxicology Program,
Research Triangle Park, NC, USA (NTP Technical Report No. 278;
NIH Publication No. 90-2534).
Vernot, E.H., Macewen, J.D., Haun, C.C., & Kinkead, E.R. (1977). Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol.,
42, 417-423.
Wagner, A.E., Muir, W.W., & Hinchcliff, K.W. (1991). Cardio-vascular
effects of xylazine and detomidine in horses. Am J. Vet. Res.,
52(5),:651-657.
Wasak, A. (1983). Haemetological and electrocardiographical changes in
dogs after xylazine. Med. Weter., 39, 235-237.
Weiss, L.D., Generalovich, T., Heller, M.B., Paris, P.M., Stewart,
R.D., Kaplan, R.M., & Thompson, D.R. (1987). Methemoglobin levels
following intravenous lidocaine administration. Ann. Emerg.
Med., 16(3), 323-325.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988). Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 11(12), 1-158.
2.2 Toxicological studies
Because 2,6-xylidine is also a chemical intermediate used in
dyes, a component of tobacco smoke and a degradation product of
aniline-based pesticides, its toxicology has been studied extensively.
Toxicological studies conducted with this compound were also reviewed
and will be presented in addition to the review and results of
toxicological studies of xylazine.
2.2.1 Acute toxicity studies of xylazine and 2,6-xylidine
The acute systemic toxicity of xylazine has been investigated in
both laboratory and domestic species. It is generally recognized that
ruminants are much more sensitive than most other species to the
pharmacological and toxicological effects of xylazine.
Results of LD50 studies of xylazine and 2,6-xylidine are
summarized in Table 3.
2.2.1.1 Acute toxicity of xylazine in dogs
Adult dogs (four males, four females) and cats (two males, four
females) were administered a single i.m. or i.v. dose of 22 mg
xylazine/kg bw (10 times the recommended therapeutic dose). One cat
out of three receiving the i.v. dose died, and two dogs out of four
receiving the i.m. dose died. All others recovered from convulsions,
unconsciousness and respiratory depression with no apparent
after-effects. The authors concluded that xylazine was slightly toxic
in this study (Crawford et al., 1970a).
Table 3. Results of acute toxicity studies on xylazine and 2,6-xylidine
Species Sex1 Route LD50 Reference
(mg/kg bw)
Xylazine
Rat NA p.o. 130 Sagner, 1967
Cat male & female2 s.c. 100-110 Bauman & Nelson, 1969
Dog male & female3 i.m 47 Nelson et al., 1968b
Dog 4 male & 3 female i.v 20-25 Nelson et al., 1968b
Horses NA4 i.m. 60-705 Nelson et al., 1968a
i.v. 15-285
2,6-Xylidine
Mouse male p.o. 710 Vernot et al., 1977
Rat p.o. 2042 Lindstrom et al., 1969
male p.o. 840 Jacobson, 1972
male p.o. 630 Short et al., 1983
male p.o. 1230 Vernot et al., 1977
female p.o. 1160 & 1270 US National Toxicology
Program, 1990
male p.o. 620-1250 &
1310
1 NA = Information not available
2 Number per sex not stated; 10 animals were used
3 Number per sex not stated; 17 animals were used
4 Sex of test animals was not stated; 5 animals were used
5 Minimum lethal dose
2.2.1.2 Acute toxicity of xylazine in horses
Adult horses were administered 11 mg xylazine/kg bw, i.v. (three
mares, one gelding) or 22 mg xylazine/kg bw, i.m. (two mares, two
geldings). One mare died following i.v. administration. All other test
animals recovered from treatment-related effects 24 hours following
i.v. administration and 48 hours following i.m. administration. The
authors concluded that the i.v. dose was slightly toxic and that the
i.m. dose produced no apparent toxicity in this study (Crawford
et al., 1970b).
2.2.2 Short-term toxicity studies
2.2.2.1 Xylazine
a) Rats
Xylazine was administered in the diet to Wistar rats (10/sex/
group) for 32 weeks. Dosages administered were 0, 50, 100, 250
or 500 mg/kg diet (equal to 0, 3, 6, 21 or 41 mg/kg bw per day for
males and 0, 4, 8, 19 or 45 mg/kg bw per day for females).
Haematology, urinalysis and gross and histopathological evaluations
were performed.
Decreases in body weight observed in females in the two
highest-dose groups (statistically significant (p<0.02) at 500 mg/kg
diet) were considered by the author to be treatment-related.
Microscopic examination of livers, lungs and kidneys revealed that
animals in all groups were diseased but no treatment-related pathology
was identified. Based on the dose-related decrease in weight gain
observed in females at 250 and 500 mg/kg diet, the NOEL in this study
was 100 mg/kg diet, equal to 6 mg/kg bw per day. The author regarded
the dose of 250 mg/kg diet as the non-toxic application rate. The
reliability of any NOEL derived from this study should be considered
questionable, owing to the presence of infection in all groups
(Tettenborn & Hobik, 1968a; Trossmann & Hobik, 1970).
b) Dogs
Dogs of undetermined breed or source were given xylazine orally
in gelatin capsules for 14-16 weeks at dose levels of 25, 50 or
100 mg/kg bw per day, 5 days/week. The low-dose group consisted of one
male and one female, the mid-dose group of two males and the high-dose
group of two males and two females. Haematology, clinical chemistry,
blood coagulation, urinalysis and postmortem gross and microscopic
evaluations were performed.
During week 8 of the test, one animal in the high-dose group died
and was replaced with a new animal. Postmortem gross findings in this
animal included diffuse reddening of the stomach and intestinal mucous
membrane.
Histopathological findings in livers (fatty degeneration and
necrosis) and kidneys (tubular epithelial necrosis and fat
accumulation) of the high-dose group were considered treatment-
related. Fatty deposits were noted in the liver and kidneys of the
low-dose female. These findings were attributed to parturition, which
occurred 3 weeks before the animal was killed. The author concluded
that the NOEL for this study was 50 mg/kg bw per day. The reliability
of any NOEL derived from this study should be considered questionable
due to the lack of a control group and small numbers of animals in
each test group (Tettenborn & Hobik, 1968b).
Beagle dogs (two/sex/group) were administered xylazine orally by
dietary admixture for 13 weeks. Dosages administered were 0, 10, 30 or
100 mg/kg diet (equal to 0, 0.3, 0.9 or 3 mg/kg bw per day). At the
beginning of the test the animals were approximately 8.5 months old
and weighed 7-10 kg. Parameters evaluated included general appearance,
ophthalmology, electrocardiography, haematology, clinical chemistry,
urinalyses and gross and microscopic pathology.
No treatment-related adverse effects were observed in any of the
parameters evaluated. The NOEL for this study was 3 mg/kg bw per day
(Tettenborn, 1969; Mawdesley-Thomas, 1970).
2.2.2.2 2,6-Xylidine
a) Rats
Three groups (nine or ten/group) of male Fischer-344 rats were
given oral (gavage) doses of 160 mg 2,6-xylidine/kg bw per day for 5,
10 or 20 days. The dosage administered was 25% of the estimated LD50
determined by the investigator. A significant increase in splenic
haemosiderosis (indicative of erythrocyte damage) after 20 days was
noted as a treatment-related effect in this study. Splenic congestion
and evidence for increased erythropoiesis were minimal
(Short et al., 1983).
Three groups of Sprague-Dawley rats (five/sex/group for controls,
low and mid dose; four/sex/group for high dose) were administered
0, 20, 100 or 500-700 mg 2,6-xylidine/kg bw per day by gavage for 4
weeks. Treatment-related effects included decreased weight gain,
decreased haemoglobin levels and hepatomegaly. In this study, the rat
appeared to be about 10 times less susceptible to hepatotoxicity of
2,6-xylidine than the dog (see section 2.2.2.2b) (Magnusson et al.,
1971; IARC, 1993).
Two groups of 8-week-old Sprague-Dawley rats (5/sex/group) were
orally administered (gastric intubation) a dose of 0 or 400 mg
2,6-xylidine per kg bw per day for 1 week immediately followed by a
daily dose of 0 or 500 mg/kg bw for 3 weeks. Decreased body weight
gain and hepatomegaly (most pronounced in centrilobular regions) were
noted as treatment-related effects. Electron microscopy of liver
tissue showed proliferation of hepatic smooth endoplasmic reticulum,
which was deemed responsible for the observed hepatomegaly in treated
rats. An increase in microsomal glucuronyltransferase was observed in
males while aniline hydroxylase levels were increased in females.
Decreases in liver glycogen and glucose-6-phosphatase activity were
also observed in the centrilobular regions of treated animals
(Magnusson et al., 1979).
Male Osborne-Mendel rats were administered up to 10 000 mg
2,6-xylidine per kg in the diet for 3-6 months. Treatment-related
effects included 25% weight reduction, anaemia, hepatomegaly with no
associated microscopic changes, splenic congestion and renal toxicity
(Lindstrom et al., 1963).
Groups of F-344/N rats (five/sex/group) were administered doses
of 0, 80, 160, 310, 620 or 1250 mg/kg bw of 2,6-xylidine in corn oil
by gavage 5 days/week for 2 weeks. Parameters evaluated included
clinical observations, body weight, urinalysis, haematology, blood pH
and carbon dioxide determinations, and gross postmortem findings.
Treatment-related deaths occurred at and above 620 mg/kg bw. All
animals in the highest dose group died before the end of the study. A
decrease of more than 10% in body weight was observed in males at and
above 310 mg/kg bw and in females at and above 160 mg/kg bw.
Generalized leukocytosis and an increase in the number of nucleated
red blood cells were observed in male rats administered 310 or
620 mg/kg bw. Slight anisocytosis, poikilocytosis and polychromasia of
the red blood cells occurred more frequently in dosed animals than in
vehicle control animals. Moderate poikilocytosis occurred at 310 mg/kg
bw and moderate polychromasia at 310 and 620 mg/kg bw. Slightly
macrocytic erythrocytes were observed at the two highest doses. Slight
anisocytosis, poikilocytosis and polychromasia were observed in female
rats at 310 and 620 mg/kg bw. The NOEL for this study was 80 mg/kg bw
per day (US National Toxicology Program, 1990).
Groups of F-344/N rats (10/sex/group) were given doses of 0, 20,
40, 80, 160 or 310 mg/kg bw of 2,6-xylidine in corn oil by gavage, 5
days/week for 13 weeks. Parameters evaluated included clinical
observations, haematology, urinalysis, serum chemistry and enzyme
analyses, gross and histopathological postmortem examinations.
A decrease in body weight gain of more than 10% occurred in males
and females in the highest dose group and in females at 40 and
160 mg/kg bw per day. In the highest dose group, relative liver
weights were significantly (P=0.003) increased for males and females.
Relative liver weight was also increased for males in the 160 mg/kg bw
group. The liver weight to brain weight and kidney weight to brain
weight ratios were significantly increased in females at 310 mg/kg bw
per day.
Treatment-related effects on haematology included significantly
decreased total leukocyte counts in males at doses of 40 mg/kg bw or
more. These were accompanied by decreases in the percentage of
lymphocytes and increases in the percentage of segmented neutrophils
at doses of 80 mg/kg bw or more. In males, haemoglobin levels were
significantly decreased at 160 and 310 mg/kg bw and erythrocyte and
haematocrit levels were decreased at 310 mg/kg bw. The NOEL for this
study was 20 mg/kg bw per day (US National Toxicology Program, 1990).
b) Dogs
Four groups of beagle dogs (one/sex/group) were given an oral
(gelatin capsule) dose of 0, 2, 10 or 50 mg 2,6-xylidine/kg bw per day
for 4 weeks. Treatment-related effects included vomiting (mid- and
high-dose groups), poor condition and decreased body weights
(high-dose group), hyperbilirubinaemia (mid- and high-dose groups),
hypoproteinaemia (mid-and high-dose groups) and fatty degenerative
changes in the liver that increased in severity with increasing dose
(Magnusson et al., 1971; IARC, 1993).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Xylazine
No carcinogenicity studies have been performed with xylazine
2.2.3.2 2,6-Xylidine
Four groups of Charles River CRL:COBS CD (SD) BR rats
(56/sex/group) were fed diets containing 2,6-xylidine (99.06% pure) at
concentrations of 0, 300, 1000 or 3000 mg/kg diet (equivalent to
0, 15, 50 or 150 mg/kg bw per day) for 102 weeks. The animals assigned
to this study were F1a, generation weanlings from a multigeneration
study in which animals were fed diets containing 0, 300, 1000 or
3000 mg/kg 2,6-xylidine beginning at 5 weeks of age. Parameters
evaluated in the carcinogenicity study included clinical observations,
haematology, blood urea nitrogen, glucose, SGOT, alkaline phosphatase
and gross and microscopic postmortem examinations.
Treatment-related clinical effects included a decrease in mean
body weight gain in high-dose males and females (>10%). Mortality was
significantly (P < 0.001) increased (relative to controls) in males
in the high-dose group. Mortality was also increased for mid-dose
males. Survival at 105 weeks was 43/56, 40/56, 33/56 and 14/56 for
males in the control, low-, mid- and high-dose groups, respectively.
For females, survival was 33/56, 25/56, 32/56 and 24/56 for the
controls, low-, mid- and high-dose groups, respectively.
Microscopically, a significant increase in carcinoma of the nasal
cavity was observed in high-dose males (26/56; P < 0.001, life table
test). For females, the incidence of carcinomas of the nasal cavity
were 0/56, 0/56, 1/56 and 24/56 in the low-, mid- and high-dose
groups, respectively (P<0.001, life table test). Two adenocarcinomas
were diagnosed in high-dose males. The incidence of papillary adenomas
in males was 0/56 in controls, 0/56 in low-dose, 2/56 in mid-dose and
10/56 in high-dose rats (P=0.001, incidental tumour test). For
females, nasal adenomas occurred in 0/56 in controls, 0/56 in low-
dose, 1/56 in mid-dose and 6/56 in high-dose rats (P=0.02, incidental
tumour test). Several unusual neoplasms of the nasal cavity were also
considered to be related to treatment. These included one undifferen-
tiated sarcoma identified in one high-dose female, rhabdomyosarcomas
which occurred in two high-dose male and two high-dose females and
malignant mixed tumours having features associated with both adenocar-
cinoma and rhabdomyosarcoma were observed in one high-dose male and
one high-dose female rat. Non-neoplastic nasal cavity lesions included
acute inflammation (rhinitis), epithelial hyperplasia and squamous
metaplasia. These occurred at increased incidence (relative to
controls) in high-dose male and female rats. The incidence of
subcutaneous fibromas and fibrosarcomas combined in males was 0/56,
2/56, 2/56 and 5/56 for the control, low-, mid- and high-dose groups,
respectively (P=0.001, life table test; P<0.001 life table trend
test). For females, the incidence of these tumours combined was 1/56,
2/56, 2/56 and 6/56 for controls, low-, mid- and high-dose groups,
respectively ((P=0.01, life table trend test). Neoplastic nodules occurred
in livers of female rats with a significant positive trend. The
incidence was 0/56, 1/56, 2/56 and 4/55 for the controls, low-, mid-,
and high-dose groups, respectively (P=0.03, incidental test; P=0.012,
incidental trend test).
Treatment-related effects on haematology included decreases in
erythrocyte counts and haemoglobin levels at 18 months in the
high-dose males. Decreases in these parameters were also observed in
the mid- and high-dose females at 12 months. The author remarked that
these changes were not severe enough to be considered indicative of
anaemia.
The author concluded that under the conditions of this study,
2,6-xylidine was dearly carcinogenic for male and female Charles River
CD rats. This was based on the observed significant increases in the
incidence of adenomas and carcinomas of the nasal cavity. Additionally
the author stated that the increased incidence of subcutaneous
fibromas and fibrosarcomas in male and female rats and increased
incidence of neoplastic nodules of the liver in female rats could have
been treatment-related (US National Toxicology Program, 1990).
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenic risk of 2,6-xylidine to humans. The Working
Group concluded that there was inadequate evidence in humans but
sufficient evidence in experimental animals for the carcinogenicity of
2,6-xylidine. The IARC classified 2,6-xylidine as Group 2B (possibly
carcinogenic to humans) (IARC, 1993).
2.2.4 Special studies on teratogenicity
Xylazine was administered by gavage to groups of pregnant rats
(22 animals/group) on gestation days 6 to 15, then killed on day 20
for examination of uterine contents. Dosages administered were 0, 1, 4
or 16 mg/kg bw per day. The study was conducted in accordance with the
principles of Good Laboratory Practice Standards and Guidelines of the
OECD, United Kingdom, FDA and Japan.
Treatment-related maternal effects included partial closing of
the eyelids, underactivity, ataxia, flat posture and slightly reduced
body weight gain in the high-dose group only. Fetal effects included a
decrease in mean fetal weight in the high-dose group only. A
teratogenic potential of xylazine was not evident at levels up to and
including 16 mg/kg bw per day. The NOEL in this study was 4 mg/kg bw
per day (Reynolds, 1994).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with xylazine and
2,6-xylidine are summarized in Table 4.
The results of bacterial mutagenicity testing of xylazine were
considered to be negative by the author. Reviewing the data, the
Committee concluded that a more than two-fold reproducible increase in
revertant colonies in tester strains TA1535 and TA1538 represents weak
mutagenic activity, even in the absence of a clear dose-response.
2.2.6 Special studies on methaemoglobin and haemoglobin adduct
formation with 2,6-xylidine
2.2.6.1 Cats and dogs
Cats and dogs (numbers not specified) were administered an i.v.
dose of 30 mg 2,6-xylidine/kg bw or an oral dose of 164 mg N-acetyl
2,6-xylidine/kg bw. 2,6-Xylidine induced a 10% methaemoglobinaemia in
cats, and N-acetyl 2,6-xylidine induced a 5% methaemoglobinaemia in
cats. Haemoglobin was unaffected in dogs in this study (McLean
et al., 1967).
Five adult cats (>24 months old) were administered an i.v. dose
of 30 mg 2,6-xylidine/kg bw. Blood samples were drawn at 1, 2, 3, 4
and 5 hours after dosing and analysed for methaemoglobin formation.
The mean methaemoglobin concentration determined from these sampling
intervals was 7% (range = 4.8% to 8.7%). Prior to treatment the mean
methaemoglobin concentration of the 152 cats used in this study was
approximately 1% (Mclean et al., 1969).
2.2.6.2 Humans
2,6-Xylidine-haemoglobin adduct levels have been found to be
elevated in human patients receiving lidocaine treatment for local
anaesthesia (1 mg/kg bw) or cardiac arrhythmias (up to 50 mg/kg bw,
i.v.). 2,6-Xylidine-haemoglobin adducts are also found in humans with
no known exposure to lidocaine. This is attributed to the 120-day
lifespan of the erythrocyte and chronic exposure to environmental or
iatrogenic sources of aromatic amines, e.g., cigarette smoke. The
levels of 2,6-xylidine-haemoglobin adducts found correspond to at an
estimated daily exposure (from iatrogenic and environmental sources)
of 23 µg (IARC, 1993; Bryant et al., 1994).
Methaemoglobinaemia induced by i.v. administration of lidocaine
was studied in 40 human cardiac patients. Treatment consisted of a
1 mg/kg bw i.v. bolus followed 15 minutes later with a 0.5 mg/kg bw
i.v. bolus. Patients were maintained between and after bolus doses
with an infusion rate of 1-4 mg lidocaine/min. Blood samples were
drawn before treatment and 1 and 6 hours after treatment. Although the
investigators found methaemoglobin levels in these patients to be
significantly elevated, the increase was not large enough to be of
clinical concern. The highest methaemoglobin level attained was 1.2%.
The author did not address the possible role of the 2,6-xylidine
metabolite in the observed increases in methaemoglobin levels in
treated patients (Weiss et al., 1987).
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine
Test system Test object Concentration Results Reference
Xylazine
In vitro
Reverse S. typhimurium
mutation1 TA1535, 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA1538 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA100, 0.4-12 mg/plate Negative Herbold, 1984
TA98, 0.4-12 mg/plate Negative Herbold, 1984
TA1537 0.4-12 mg/plate Negative Herbold, 1984
Mammalian V79/HGPRT 62-1250 µg/ml Negative Brendler-
cell forward (-S9) Schwaab,
mutation1 2-40 µg/ml (+S9) 1994
In vivo
Cytogenetic Mouse bone 50 mg/kg bw, Negative Herbold, 1995
assay marrow i.p.2
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
2,6-Xylidine
In vitro
Reverse S. typhimurium
mutation1 TA1535 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
3 µmol/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al., 1988
TA100 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin el al.,
19804
0.1-10 mg/plate Neg (-S9), Zeiger et al.,
Pos (+S9) 19883
480-4000 Negative Kugler-
µg/plate Steigmeier
et al., 1989
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
TA1537 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al.,
1988
TA98 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1 - 10 mg/plate Negative Zeiger et al.,
1988
Gene Mouse lymphoma Not given Positive Rudd et al.,
mutation1 L5178Y cells, 19837
tk locus
Sister Chinese 30-1500 µg/ml Positive Galloway et al.,
chromatid hamster 1987
exchange1 ovary cells
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
In vivo
Cytogenetic ICR mouse 350 mg/kg bw, Inconclusive6 Parton et al.,
assay bone marrow p.o. 1988
375 mg/kg bw, Inconclusive6 Parton et al.,
p.o. 1990
In vivo- Rat primary 40-850 mg/kg Negative Mirsails et al.,
in vitro DNA hepatocytes bw, p.o. 1989
repair assay
Covalent Rats 87.2 µCi Positive Short et al., 1989
DNA 14C-labelled
binding 2,6-xylidine/rat,
i.p5
1 Both with and without rat liver S9 fraction
2 Cyclophosphamide positive control
3 Weakly positive in two of three laboratories, negative in the third
4 Spot tests only
5 Pretreatment with unlabelled 262.5 mg/kg bw 2,6-xylidine daily for 9 days
6 Results suggest test article may not have reached target tissue (bone marrow)
7 Reference was an abstract and doses were not stated in that reference
2.3 Observations in humans
A 34-year-old man self-injected 10 ml of a 100 mg/ml solution of
xylazine intramuscularly. The estimated dose was 15 mg/kg bw. The
individual was discovered (30 minutes after retiring for bed) in a
deeply comatose, apnoeic and areflexic state. An empty Rompun
(xylazine) bottle (known to have contained 10 cc earlier) lay by his
side. He was immediately admitted to the hospital. Upon admission his
pupils were of moderate size and responded slowly to light. Other
findings included a blood pressure of 120/70 mmHg and heart rate of
60 bpm with stable sinus rhythm. Lactate dehydrogenase (LDH) activity
was elevated, with the LDH-1 isoenzyme predominating. Creatine
phosphokinase (CPK) activity was also elevated, particularly in the
CPK-3 and CPK-2 isoenzymes. These enzyme changes persisted for 5 to 7
days. Plasma glucose level was also elevated. Two days following
hospital admission sinus tachycardia developed, interspersed with runs
of multifocal premature ventricular contractions which were controlled
with lidocaine infusion. Blood pressure remained approximately 120/80
for the duration of hospitalization. Coma and respiratory depression
lasted 60 hours. The patient was discharged from the hospital 17 days
after admission.
The author noted that the patient might have died if he had not
been found shortly after the injection was administered, owing to the
marked respiratory depression that occurred. Hypotension, which has
been reported as an effect of xylazine in humans, did not occur in
this case. According to the author, the enzyme activity increases
indicated that myocardial muscle damage had occurred and an intrinsic
cardiotoxic effect of xylazine was suspected. Finally, in this case
the greatest threat to life was the CNS-depressant effect of xylazine
(Carruthers et al., 1979).
A 20-year-old woman ingested 400 mg of xylazine. Approximately 2
hours later she became drowsy, incontinent (urine), difficult to
arouse and occasionally unresponsive to verbal commands. She was
admitted to the hospital approximately 3 hours after the ingestion. In
a similar way to the case of human poisoning described by Carruthers
et al. (1979), she experienced a relatively low initial cardiac rate,
which later gradually increased, significant central nervous system
and respiratory depression, transient hyperglycaemia and ventricular
arrhythmias. However there was no evidence of myocardial damage. A
sample of this patient's urine was analysed using a gas chromatograph-
mass spectrometer computer system. Xylazine was found largely unchanged
in the urine as shown by a lack of any structurally related compounds
in the basic urine extract. No xylazine was found in a blood sample
taken at the time of admission. The author concluded that plasma
levels were below the limit of detection of the method (100 ng/ml).
The patient was discharged ambulatory and without apparent adverse
effects two days following admission to the hospital (Gallanosa
et al., 1981).
A 36-year-old man died following ingestion of alcohol and
clorazepate combined with an injection of approximately 40 ml of
xylazine (100 mg/ml). Xylazine was found in the decedent's blood,
brain, kidney, liver, lung, fat and urine at concentrations of 0.2,
0.4, 0.6, 0.9, 1.1, 0.05 and 7 ppm, respectively (Poklis et al.,
1985).
A 29-year-old woman self-injected 40 mg of xylazine
intramuscularly. The estimated dose was 0.73 mg/kg bw. Clinical
findings included disorientation, miosis, hypotension and bradycardia,
but no cardiac arrhythmias were noted. The abnormalities resolved
spontaneously (Spoerke et al., 1986).
A 37-year-old woman self-injected 24 ml (2400 mg) xylazine
intramuscularly. The estimated dose was 22 mg/kg bw. Twenty minutes
after the injection her blood pressure was 166/130 mmHg, heart rate
was 76 bpm and respirations 18 per minute. The serum glucose level was
175 mg/dl. Blood pressure later decreased to 130/90 mmHg and she
became apnoeic. No cardiac arrhythmias were observed during her 3 days
of hospitalization. Hypotension and bradycardia occurred two days
after the injection. The patient survived (Spoerke et al., 1986).
A 29-year-old woman self-injected an unknown amount of xylazine
intravenously. She became apnoeic and had an initial blood pressure of
130/90 mmHg with a pulse of 60 bpm. Serum glucose levels did not
exceed 90 mg/dl. Twenty-four hours after the injection the patient
experienced hypotension and bradycardia. Spontaneous respiration
resumed 18 hours after hospital admission. The patient recovered fully
(Spoerke et al., 1986).
A 19-year-old man accidentally injected himself subcutaneously
with 2 ml (100 mg/ml) of xylazine. The dose administered was 3 mg/kg
bw. Thirty minutes later he became difficult to rouse and was
hospitalized. Clinical findings included miosis, hyporeflexia,
hypotension, bradycardia, respiratory and central nervous system
depression and hyperglycaemia. He was treated with intravenous fluids
and assisted ventilation. Eight hours after hospitalization the
patient was alert and responsive. Twenty-four hours later he was
released (Samanta et al., 1990).
A 39-year-old woman was admitted to the hospital with symptoms of
tiredness, faintness and blurred vision. Clinical findings included
sinus bradycardia with a blood pressure of 130/90. Xylazine was found
in the urine and serum at concentrations of 1674 µg/litre and
30 µg/litre, respectively (Lewis et al., 1983).
3. COMMENTS
The Committee considered toxicological data on xylazine,
including the results of acute and short-term toxicity studies as well
as studies on pharmacodynamics, pharmacokinetics, reproductive and
developmental toxicity, genotoxicity and effects in humans. In
addition, toxicological studies on 2,6-xylidine, a metabolite of
xylazine, were reviewed; these included studies on acute and
short-term toxicity, carcinogenicity and genotoxicity.
Numerous pharmacological side-effects of xylazine have been
observed in treated animals, including mydriasis, impairment of
thermo-regulatory control, various effects on the cardiovascular
system, acid-base balance and respiration, hyperglycaemia, and
haematological and gastrointestinal effects. Cattle and sheep are
approximately 10 times more sensitive to xylazine than horses, dogs
and cats.
Rats were administered radiolabelled xylazine intravenously at
doses of 0.02 to 10 mg/kg bw or orally at doses of 0.02 to
100 mg/kg bw. More than 95% of the oral dose was absorbed, with a
half-life of approximately 5 minutes. Following oral or intravenous
administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal excretion following oral
or intravenous administration was associated with a half-life of 2 to
3 hours. Enterohepatic circulation did not occur to a notable extent.
In cattle administered an intramuscular dose of 0.2 or 0.5 mg
xylazine/kg bw, less than 1% of the dose was excreted unchanged in the
urine, and the parent compound was detected in the urine up to 6 hours
following administration. Metabolites of xylazine were detected in
urine from these cattle up to 10 hours following administration.
Pharmacokinetic parameters following intravenous administration
showed minor variations between species. Xylazine disappeared rapidly
from plasma following intravenous administration, with an elimination
half-life of approximately 40 minutes in cattle and approximately 20
minutes in sheep. Xylazine could not be detected in the plasma of
cattle following intramuscular administration of a single therapeutic
dose.
In rats administered an intravenous dose of 2 mg/kg bw radio-
labelled xylazine, approximately 20 metabolites were quantified as
xylazine equivalents in urine and bile. The major metabolite comprised
35% of the administered dose. Approximately 8% of the dose was
eliminated as unchanged xylazine 24 hours after dosing. In an
in vitro study, 4 metabolites were identified when xylazine was
incubated with rat liver microsomes. The same metabolites were
identified in the urine of horses treated with xylazine. The major
metabolite in both cases was identified as N-(2,6-dimethylphenyl)
thiourea. In cattle administered an intramuscular dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow),
2,6-xylidine was identified as a metabolite excreted in urine in both
conjugated and unconjugated forms.
The acute oral toxicities of xylazine and 2,6-xylidine were
tested in mice and rats. Xylazine was determined to be moderately
toxic (LD50 = 121-240 mg/kg bw) and 2,6-xylidine to be slightly
toxic (LD50 = 600-1000 mg/kg bw).
Three studies on the short-term toxicity of xylazine were
reviewed. A 32-week dietary study in rats and a 16-week oral
(capsules) study in dogs were considered inadequate for the
determination of the toxicity of xylazine owing to the use of
insufficient numbers, poor quality animals and inadequate study
design. The third was a 13-week oral study in beagle dogs fed
diets containing 0, 10, 30 or 100 mg/kg xylazine in the feed (equal
to 0.3, 0.9 or 3 mg/kg bw per day). No treatment-related effects
were observed in any of the treated groups.
In a two-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 80, 160, 310, 620 or 1250 mg
2,6-xylidine/kg bw per day, 5 days per week. Treatment-related effects
included increased mortality (all animals in the high-dose group
died), decreased body weight (males at 310 mg/kg bw per day and above
and females at 160 mg/kg bw per day and above) and various effects on
haematological parameters as indicated by leukocytosis and changes in
red blood cell parameters indicative of increased erythropoiesis
(males and females at 310 mg/kg bw per day and above). The NOEL in
this study was 80 mg/kg bw per day.
In a 13-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 20, 40, 80, 160 or 310 mg
2,6-xylidine/kg bw per day, 5 days per week for 13 weeks. Treatment-
related effects included decreased body weight gain (males at 310
mg/kg bw per day and females at 40 mg/kg bw per day and above),
increased absolute and relative liver weights (females at 160 mg/kg bw
per day and above; males at 310 mg/kg bw per day), leukopenia (males
at 40 mg/kg bw per day and above), haemoglobinaemia (males at
160 mg/kg bw per day and above) and anaemia (males at 310 mg/kg bw per
day). The NOEL was 20 mg/kg bw per day.
In a carcinogenicity study, male and female rats were fed diets
containing 2,6-xylidine at concentrations of 300, 1000 or 3000 mg/kg
food (equivalent to 15, 50 or 150 mg/kg bw per day). Significant
increases in the incidences of papillomas and carcinomas of the nasal
cavity were observed in high-dose males and females. There was a
significant dose-related increase in the incidence of adenomas in the
nasal cavity in both males and females. In addition, unusual rhabdo-
myosarcomas and malignant mixed tumours of the nasal cavity were
observed in the high-dose males and females. There was a dose-related
significant increase in the incidence of subcutaneous fibromas and
fibrosarcomas in both treated males and females. In females,
neoplastic nodules occurred in livers with a significant positive
trend and the increase was significant in the high-dose group by the
incidental tumour test. The Committee concluded that 2,6-xylidine was
carcinogenic in this study.
The International Agency for Research on Cancer has evaluated the
carcinogenic risk of 2,6-xylidine and has classified it as Group 2B
(possibly carcinogenic to humans).
In a teratogenicity study, xylazine was administered to pregnant
rats at doses of 1, 4 or 16 mg xylazine/kg bw per day on gestation
days 6 to 15. Treatment-related maternal effects included partial
closing of the eyelids, hypoactivity, ataxia, flat posture and
slightly reduced body weight gain in the high-dose group only. A
decrease in mean fetal weight was seen in the high-dose group. No
teratogenic effects were noted in this study. The NOEL for maternal
and fetal effects was 4 mg/kg bw per day.
Xylazine has been tested in reverse mutation assays in
Salmonella, a forward mutation assay in cultured mammalian cells and
in an in vivo cytogenetic assay. In Salmonella, weak positive
results were obtained. Negative results were observed in a forward
mutation assay on cultured mammalian cells and in a mouse bone marrow
micronucleus test. The Committee concluded that xylazine is weakly
mutagenic.
2,6-Xylidine was tested in a series of in vitro and in vivo
genotoxic assays. It was weakly positive for reverse mutation in
Salmonella. In mammalian cells, it induced forward mutation and was
positive in a sister chromatid exchange test. Inconclusive results
were obtained in a mouse bone marrow micronucleus test because there
was no assurance that the bone marrow had been adequately exposed.
2,6-Xylidine was found to be inactive in an in vivo-in vitro rat
hepatocyte unscheduled DNA synthesis assay. Covalent binding of the
compound to DNA was observed in rats. The Committee concluded that
2,6-xylidine is genotoxic.
The potential for 2,6-xylidine to induce methaemoglobinaemia was
reviewed by the Committee. Single doses of 30 mg 2,6-xylidine/kg bw
intravenously or 164 mg/kg bw N-acetyl-2,6-xylidine orally have
been shown to induce methaemoglobinaemia in cats but not in dogs.
2,6-Xylidine has also been shown to be a product of lidocaine
metabolism in humans. Methaemoglobin and 2,6-xylidine-haemoglobin
adduct levels have been shown to increase in human cardiac patients
receiving lidocaine treatment.
Effects of xylazine on humans poisoned following accidental or
intentional self-injection (0.7-15 mg/kg bw) or ingestion (7 mg/kg bw)
included symptoms of central nervous system depression, respiratory
depression, hypo- and hypertension, bradycardia, tachycardia,
ventricular arrhythmias, and transient hyperglycaemia.
4. EVALUATION
The Committee was unable to establish an ADI for xylazine because
it concluded that the 2,6-xylidine metabolite was genotoxic and
carcinogenic. Annex 4 lists the information that would be required for
further review.
5. ACKNOWLEDGMENTS
The preparer of the first draft would like to recognize the
following individuals for their assistance and contributions to the
preparation of the first draft:
Ms. Deborah Brooks, information specialist, Center for Veterinary
Medicine
Dr. Steve Brynes, residue chemist, Center for Veterinary Medicine
Dr. Jennifer Burris, veterinary pathologist, Center for Veterinary
Medicine
Dr. Robert Condon, biostatistician, Center for Veterinary Medicine
Dr. Haydee Fernandez, toxicologist, Center for Veterinary Medicine
Dr. Devaraya Jagannath, genetic toxicologist, Center for Veterinary
Medicine
Dr. Alan Pinter, toxicologist, National Institute of Public Health,
Budapest, Hungary
Dr. Leonard Schechtman, genetic toxicologist, Center for Veterinary
Medicine
6. REFERENCES
Amer, A.A. & Misk, N.A. (1980). Rompun in goats with special reference
to its effect on the cerebral spinal fluid (c.s.f.). Vet. Med. Rev.,
2, 168-174.
Bauman, E. K. & Nelson, D.L. (1969). Toxicity of BAY Va 1470 to cats.
Unpublished report No. 24208 from Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Benson, G.J., Thurmon, J.C., Neff-Davis, C.A., Corbin, J.E.,
Davis, L.E., Wilkinson, B., & Tranquilli, W.J. (1984). Effect of
xylazine hydrochloride upon plasma glucose concentrations in adult
pointer dogs. J. Am. Anita. Hosp. Assoc., 20, 791-794.
Bolte, S. & Stupariu, A (1978). Motility of the rumen in cattle and
sheep under the influence of neuroleptics and analgesics, particularly
xylazine, propionylpromazine and diazepam. Lucrari Stiintifice
Inatitutul Agron. Timisoara, Ser. Med. Vet., 15, 157-167.
Booth, N.H. (1988). Nonnarcotic analgesics. In: Booth N.H.&
McDonald E. (eds.), Veterinary Pharmacology and Therapeutics, 6th
edition, Iowa State University Press, Ames, Iowa, pp. 351-359.
Brendler-Schwaab, S. (1994). Rompun hydrochloride. Mutagenicity study
for the detection of induced forward mutations in the V79-HGPRT assay
in vitro. Unpublished report No. T9049349 from Bayer AG, Fachbereich
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bryant, M.S., Simmons, H.F., Harrell, R.E., & Hinson, J.A. (1994).
2,6-Dimethylaniline-hemoglobin adducts from lidocaine in humans.
Carcinogenesis, 15(10), 2287-2290.
Carruthers, S.G., Nelson, M., Wexler H.R., & Stiller, C.R. (1979).
Xylazine hydrochloride (Rompun) iverdise in man. Clin. Toxicol.,
15(3), 281-285.
Carter, S.W., Robertson, S.A., Steel, C.J., & Jourdenais, D.A. (1990).
Cardiopulmonary effects of xylazine sedation in the foal.
Equine Vet. J., 22(6), 384-388.
Colby, E.D., McCarthy, L.E., & Borison, H.L. (1981). Emetic action of
xylazine on the chemoreceptor trigger zone for vomiting in cats.
J. Vet. Pharmacol. Ther., 4, 93-96.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970a). The toxicity of
BAY Va 1470 2% injectable to dogs and cats given at ten times
recommended dose. Unpublished report No. 28139 from Research
Department, Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970b). The toxicity of
BAY Va 1470 10% injectable alone and in combination with (R)NEGUVON*
soluble powder (batch No. 0050337) to horses. Unpublished report
No. 28136 from Research Department, Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Cullen, L.K. & Jones, R.S. (1977). Clinical observations on
xylazine/ketamine anaesthesia in the cat. Vet. Rec., 101, 115-116.
Demoor, A. & Desmet, P. (1971). Effect of Rompun on acid-base
equilibrium and arterial oxygen pressure in cattle. Vet. Med. Rev.,
2-3, 163-169.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1968). Experimental tests on animals with radioactive labeled BAY Va
1470. Unpublished report No. 22874 from Bayer Isotope Institute,
Elberfeld Branch. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1969). Experiments using radioactively tagged BAY Va 1470 on rats.
Berl. Münch. Tierärztl. Wochenschr., 82, 104-109.
Eichner, R.D., Prior, R.L., & Kvasnicka, W.G (1979). Xylazine-induced
hyperglycemia in beef cattle. Am. J. Vet. Res., 40(1), 127-129.
Feldberg, W. & Symonds, H.W. (1980). Hyperglycemic effect of xylazine.
J. Vet. Pharmacol. Ther., 3, 197-202.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Freire, A.C.T, Gontijo, R.M., Pessoa, J.M., & Souza, R. (1981). Effect
of xylazine on the electrocardiogram of sheep. Br. Vet. J.,
137, 590-595.
Gallanosa, A.G., Spyker, D.A., Shipe, J.R., & Morris, D.L. (1981).
Human xylazine overdose: A comparative review with clonidine,
phenothiazines, and tricyclic antidepressants. Clin. Toxicol.,
18(6), 663-678.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon C., Bloom, A.D., Nakamura, F., Ahmed, N., Duk, S., Rimpo, J.,
Margolin, B.H., Resnick, M.A., Anderson, B., & Zeiger, E. (1987).
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10), 1-175
Garcia-Villar, R., Toutain, P.L., Alvinerie, M., & Ruckenbusch Y.
(1981). The pharmacokinetics of xylazine hydrochloride: an
interspecific study. J. Vet. Pharmacol. Ther., 4, 87-92.
Gross, M.E. & Tranquilli, W.J. (1989). Use of alpha-2-adrenergic
receptor antagonists. J. An. Vet. Med. Assoc., 195(3), 378-381.
Herbold, B. (1984). Salmonella/microsome test to evaluate for
point-mutagenic effects. Unpublished report No. T0016805 from Bayer
Sparte Pharma. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995). Rompun Hydrochloride. Micronucleus test on the
mouse. Unpublished report No. T9058169 + T2058171 from Bayer AG
Fachbereich Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hikasa, Y. Takase, K., & Ogasawara, S. (1989). Evidence for the
involvement of alpha-2-adrenoceptors in the emetic action of xylazine
in cats. Am. J. Vet. Res., 50, 1348-1351.
Hikasa, Y., Takase, K., Osada, T., Takamatsu, H., & Ogasawara, S.
(1987). Xylazine-induced vomiting in dogs: elimination by ablation of
the area postrema and blockade by yohimbine. Zent. bl. Vet. med.,
A34(2), 154-158.
Holmes, A.M. & Clark, W.T. (1977). Xylazine sedation of horses.
N.Z. Vet. J., 25, 159-161.
Hsu, W.H. & Hummel, S.K. (1981). Xylazine-induced hyperglycemia in
cattle: a possible involvement of alpha-2-adrenergic receptors
regulating insulin release. Endocrinology, 109, 825-829.
Hsu, W.H. & McNeel, S.V. (1983). Effect of yohimbine on
xylazine-induced prolongation of gastrointestinal transit in dogs.
J. Am. Vet. Med. Assoc., 183(3), 297-300.
Hsu, W.H., Betts, D.H., & Lee, P. (1981). Xylazine-induced mydriasis:
possible involvement of a central postsynaptic regulation of
parasympathetic tone. J. Vet. Pharmacol. Ther., 4, 209-214.
Hsu, W.H., Hanson, C.E., Hembrough, F.B., & Schaffer, D.D. (1989).
Effects of idazoxan, tolazoline and yohimbine on xylazine induced
respiratory changes and central nervous system depression in ewes.
Am. J. Vet. Res., 50(9), 1570-1573.
IARC (1993). 2,6-Dimethylaniline (2,6-xylidine). IARC Monograph on the
Evaluation of Carcinogenic Risks to Humans: Occupational exposures of
hairdressers and barbers and personal use of hair colourants; some
hair dyes, cosmetic colourants, industrial dyestuffs and aromatic
amines, 57, 323-335.
Jacobson, K.H. (1972). Short communication. Acute oral toxicity of
mono- and di-alkyl ring-substituted derivatives of aniline.
Toxicol. Appl. Pharmacol., 22, 153-154.
Klide, A.M., Calderwood, H.W., & Simen L.R. (1975). Cardio-pulmonary
effects of xylazine in dogs. Am. J. Vet. Res., 36(7), 931-935.
Kugler-Steigmeier, M.E., Frierich, U., Graf, U., Lutz, W.K.,
Maier, P., & Schlatter, C. (1989). Genotoxicity of aniline derivatives
in various short-term tests. Mutat. Res., 211, 279-289.
Leblanc, P.H. & Eberhart, S.W. (1990). Cardiopulmonary effects of
epidurally administered xylazine in the horse. Equine Vet. J.,
22(6), 389-391.
Lewis, S., O'Callaghan, C.L.P., & Toghill, P.J. (1983). Clinical
curio: self medication with xylazine. Br. Med. J., 287, 1369.
Lindstrom, H.V., Hansen, W.H., Nelson, A.A., & Fitzhugh, O.G. (1963).
The metabolism of FD&C Red No. 1. II. The fate of 2,5- para-xylidine
and 2,6- meta-xylidine in rats and observations of the toxicity of
xylidine isomers. J. Pharmacol. Exp. Ther., 142, 257-264.
Lindstrom, H.V., Bowie, W.C., Wallace, W.C., Nelson, A.A., &
Fitzhugh, O.G. (1969). The toxicity and metabolism of mesidine and
pseudocumidine in rats. J. Pharmacol. Exp. Ther., 167(2), 223-234.
McLean, S., Murphy, B.P., Starmer, G.A., & Thomas, J. (1967).
Methaemoglobin formation induced by aromatic amines and amides.
J. Pharm. Pharmacol., 19, 146-154.
McLean, S., Starmer, G.A., & Thomas, J. (1969). Methaemoglobin
formation by aromatic amines. J. Pharm. Pharmacol., 21, 441-450.
Magnusson, G., Bodin, N.-O., & Hansson, E. (1971). Hepatic changes in
dogs and rats induced by xylidine isomers. Acta Pathol. Microbiol.
Scand., 79, 639-648.
Magnusson, G., Majeed, S.K., Down, W.H., Sacharin, R.M., & Jorgeson,
W. (1979). Hepatic effects of xylidine isomers in rats. Toxicology,
12, 63-74.
Mawdesley-Thomas, L.E. (1970). Pathology report of the subchronic
toxicity for dogs by oral administration of compound BAY Va 1470.
Unpublished report No. 26692 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L, Loh, E.K., Hamilton,
C.M., Bakke, J.P., & Splading, J.W. (1989). Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: testing of 24 compounds. Environ. Mol. Mutagen.,
13, 155-164.
Murphy, J.J. & Jacobs, K. (1975). Residues of ROMPUN and its
metabolites in cattle. Unpublished report No. 43814 from Chemagro
Agricultural Division, Mobay Chemical Corporation. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mutlib, A.E., Chui, Y.C., Young, L.M., & Abbott, F.S. (1992).
Characterization of metabolites of xylazine produced in vivo and
in vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos.,
20(6), 840-848.
Nelson, D.L., Bauman, E.K., Mosier, J.O., & Allen, A.D. (1968a). A
study of the effect of large doses of BAY Va 1470 to horses.
Unpublished report No. 23405 from Research and Development Department,
Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Nelson, D.L., White, R.G. Bauman, E.K., & Allen, A.D. (1968b). The
effect of large injected doses of BAY Va 1470 to dogs. Unpublished
report No. 23679 from Research and Development Department, Chemagro
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Parton, J.W., Probst, G.S., & Garriott, M.L. (1988). The in vivo
effects of 2,6-xylidine on induction of micronuclei in mouse bone
marrow cells. Mutat. Res., 206, 281-283.
Parton, J.W., Beyers, J.E., Garriott, M.L., & Tamura, R.N. (1990). The
evaluation of multiple dosing protocol for the mouse bone-marrow
micronucleus assay using benzidine and 2,6-xylidine. Mutat. Res.,
234, 165-168.
Poklis, A., MacKell, M.A., & Case, M.E.S. (1985). Xylazine in human
tissues and fluids in a case of fatal drug abuse. J. Anal. Toxicol.,
9, 234-236.
Ponder, S.W. & Clark, W.G. (1980). Prolonged depression of
thermoregulation after xylazine administration to cats.
J. Vet. Pharmacol. Ther., 3, 203-207.
Pütter, J. & Sagner, G. (1973). Chemical studies to detect residues of
xylazine hydrochloride. Vet. Med. Rev., 2, 145-159.
Reynolds, S.M. (1994). Xylazine hydrochloride: teratology study in the
rat. Unpublished report No. 94/BAG240/0268 from Pharmaco LSR.
Submitted to WHO by Bayer AG, Leverkusen, Germany (GLP statement and
report summary, only).
Robertson, S.A., Carter, S.W., Donovan, M., & Steele, C. (1990).
Effects of intravenous xylazine hydrochloride on blood glucose, plasma
insulin and rectal temperature in neonatal foals. Equine Vet. J.,
22(1), 43-47.
Ruckebusch, Y. & Toutain, P.L. (1984). Specific antagonism of xylazine
effects on reticulo-rumen motor function in cattle. Vet. Med. Rev.,
1, 1-12.
Rudd, C.J., Mitchell, A.D., & Spalding, J. (1983). L5178Y Mouse
lymphoma cell mutagenesis assay of coded chemicals incorporating
analyses of the colony size distributions (Abstract No. Cd-19).
Environ. Mutagen., 5(3), 419.
Samanta, A., Roffe, C., & Woods, K.L. (1990). Accidental self
administration of xylazine in a veterinary nurse. Postgrad. Med. J.,
66, 244-245.
Sagner, G. (1967). Bay Va 1470, anaesthetic, analgesic and sedative
for veterinary medicine. Unpublished report No. 1470 from Bayer AG
Institute of Pharmacology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sagner, G., Hoffmeister, F., & Kroneberg, G. (1969). Pharmacological
principles of a new preparation for analgesia, sedation and relaxation
in veterinary medicine (Bay Ve 1470). Vet. Med. Rev., 3, 226-228.
Short, C.R., King, C., Sistrunk, P.W., & Kerr, K.M. (1983). Subacute
toxicity of several ring-substituted dialkylanilines in the rat.
Fundam. Appl. Toxicol. 3, 285-292.
Short, C.R., Joseph, M., & Hardy, M.L. (1989). Covalent binding of
[14C]-2,6-dimethylaniline to DNA of rat liver and ethmoid turbinate.
J. Toxicol. Environ. Health, 27, 85-89.
Singh, J., Peshin, P.K., Singh, A.P., & Nigam, J.M. (1983).
Haemo-dynamic, acid base and blood gas alterations after xylazine
administration in calves. Indian J. Vet. Surg., 4, 10-15.
Skarda, R.T., Jean, G., & Muir, W.W. (1990). Influence of tolazoline
on caudal epidural administration of xylazine in cattle. Am. J. Vet.
Res., 51(4), 556-560.
Spoerke, D.G., Hall, A.H., Grimes, M.J., Honea, B.N., & Rumack, B.H.
(1986). Human overdose with veterinary tranquilizer xylazine.
Am. J. Emerg. Med., 4, 222-224.
Swift, B.L. (1977). A technique for the surgical removal of the spleen
in calves. Vet. Med.-Small Anim. Clin., January, 77-79.
Symonds, W.H. (1976). The effect of xylazine on hepatic glucose
production and blood flow rate in the lactating dairy cow.
Vet. Rec., 99, 234-236.
Takase, I., Terada, H., & Fujii, T. (1976) Xylazine residues in organs
and tissues of calves and milk of cows. Unpublished report No. 76/
8278a from the Department of Veterinary Science, Faculty of
Agriculture, Tokyo University of Agriculture and Technology. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tettenborn, D. (1969). BAY Va 1470. Subchronic toxicity for dogs
during application as feed additive. Unpublished report No. 26692
(1731) from Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968a). BAY Va 1470. Chronic toxicity to
rats from oral administration. Unpublished report No. 23414 (956) from
Bayer AG, Institute of Pharmacology and Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968b). BAY Va 1470. Subchronic toxicity
to dogs from oral administration. Unpublished report No. 23415 (958)
from Bayer AG, Institute of Pharmacology and Toxicology. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thurmon, J.C., Neff-Davis, C., Davis, L.E., Stoker, R.A., Benson,
G.J., & Lock, T.F. (1982). Xylazine hydrochloride-induced
hyperglycemia and hypoinsulinemia in thoroughbred horses. J. Vet.
Pharmacol. Ther., 5, 241-245.
Thurmon, J.C., Steffey, E.P., Zinkl, J.G., Waliner, M., & Howland, D.
(1984). Xylazine causes transient dose-related hyperglycemia and
increased urine volume in mares. Am. J. Vet. Res., 45(2), 224-227.
Trossmann, G. & Hobik, H.P. (1970). BAY Va 1470. Histopathological
changes in organs of rats after feeding for 32 weeks. Unpublished
report No. 28576 (956) from Bayer AG, Institute of Pathology and
Histology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
US National Toxicology Program (1990). Toxicology and Carcinogenesis
Studies of 2,6-Xylidine (2,6-Dimethylaniline) (CAS No. 87-62-7) in
Charles River CD Rats (Feed Studies). US National Toxicology Program,
Research Triangle Park, NC, USA (NTP Technical Report No. 278;
NIH Publication No. 90-2534).
Vernot, E.H., Macewen, J.D., Haun, C.C., & Kinkead, E.R. (1977). Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol.,
42, 417-423.
Wagner, A.E., Muir, W.W., & Hinchcliff, K.W. (1991). Cardio-vascular
effects of xylazine and detomidine in horses. Am J. Vet. Res.,
52(5),:651-657.
Wasak, A. (1983). Haemetological and electrocardiographical changes in
dogs after xylazine. Med. Weter., 39, 235-237.
Weiss, L.D., Generalovich, T., Heller, M.B., Paris, P.M., Stewart,
R.D., Kaplan, R.M., & Thompson, D.R. (1987). Methemoglobin levels
following intravenous lidocaine administration. Ann. Emerg.
Med., 16(3), 323-325.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988). Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 11(12), 1-158.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Pharmacodynamics
With respect to xylazine's sedative effect, there are marked
species differences in the dose rates required to achieve this state.
Table 1 illustrates dosages required for various animal species
(Gross & Tranquilli, 1989).
Table 1. Dosage of xylazine in various animal species
Xylazine (mg/kg bw)
Species Intravenous Intramuscular
Horses 0.5 to 1.1 1 to 2
Cattle 0.03 to 0.11 0.1 to 0.21
Sheep 0.05 to 0.11 0.1 to 0.31
Goats 0.01 to 0.51 0.05 to 0.51
Swine 2 to 3
Dogs 0.5 to 1 1 to 2
Cats 0.5 to 1 1 to 2
Birds 5 to 10
1 Lower end of dose range should be used if sedation without
recumbency is desired (Gross & Tranquilli, 1989).
Mydriasis is a feature of xylazine-induced sedation in the cat.
The mechanism has been determined as central inhibition of
parasympathetic tone in the iris due to xylazine's activation of
post-synaptic alpha-2 receptors (Hsu et al., 1981).
Thermoregulatory control is impaired in cats administered
xylazine. They become more susceptible to hyper- and hypothermia both
during and after recovery from the sedative effects of the drug. Foals
have demonstrated a hypothermic response to xylazine. Thermoregulatory
effects in cattle have been variable (Ponder & Clark, 1980;
Booth, 1988; Robertson et al., 1990).
Cardiovascular effects of xylazine include decreased heart rate
and variable effects on blood pressure. Xylazine-induced arrhythmia is
common in the horse due to sinoatrial and atrioventricular blocks.
Arrhythmias have also been recorded in dogs, but could not be induced
in sheep. The induction of cardiovascular effects may be influenced by
route of administration, e.g., xylazine administered epidurally to
horses produced no cardiovascular changes, whereas cattle injected by
this route experienced decreases in heart rate and arterial blood
pressure (Sagner et al., 1969; Holmes & Clark, 1977; Freire
et al., 1981; Hsu et al., 1981; Wasak, 1983; Singh et al., 1983;
Leblanc & Eberhart, 1990; Skarda et al., 1990).
The effects of xylazine on respiration, acid-base balance and
blood gas values vary according to species and anaesthetic
combination. In cattle, xylazine causes a slowing of the respiratory
rate. This is accompanied by an increase in pH and metabolic acidosis.
Respiratory rate is also slowed in dogs administered xylazine, but
arterial pH, pO2 or pCO2 are not significantly affected. The
literature contains conflicting reports on the effect of xylazine on
the respiratory rate of horses. Tachypnoea is characteristic of the
ovine response to xylazine. Hypoxaemia induced by xylazine in sheep
can be life-threatening (DeMoor & Desmet, 1971; Klide et al., 1975;
Holmes & Clark, 1977; Hsu et al., 1989; Carter et al., 1990;
Wagner et al., 1991).
Hyperglycaemia is induced by xylazine in adults of all target
species. Increased blood glucose concentrations are accompanied by a
decrease in insulin levels. In adult horses, hyperglycaemia is
accompanied by increased urine volume without glycosuria. Xylazine
administered to neonatal foals did not result in hyperglycaemia. The
hyperglycaemic effect of xylazine is thought to be due to its direct
effect on alpha-2-adrenoceptors of pancreatic islet beta cells
resulting in an inhibition of insulin release (Symonds, 1976; Feldberg
& Symonds, 1980; Hsu & Hummel, 1981; Thurmon et al., 1982, 1984;
Benson et al., 1984).
Serum chemistry and cerebral spinal fluid alterations were
observed in adult female goats administered intramuscularly with
0.2 mg xylazine/kg bw. Significant elevations of urea nitrogen, total
protein and total cholesterol were found in serum. Glucose and urea
nitrogen levels were significantly increased (P<0.01) and chloride
levels were significantly decreased (P<0.05) in the cerebral spinal
fluid (Amer & Misk, 1980).
Erythrocyte counts, haematocrit values and haemoglobin
concentrations in cattle and dogs have shown significant but
reversible decreases following xylazine administration
(Eichner et al., 1979; Wasak, 1983).
Gastrointestinal effects in ruminants include decreased gut
motility, prolongation of gastrointestinal transit time and inhibition
of reticulorumen contractions. Xylazine causes decreased muscle tone
of the colon and rectum which facilitates rectal examination. Xylazine
inhibition of rumen contractions can lead to tympany, which is a
potential cause of death in xylazine-sedated ruminants. Ruminants are
fasted prior to sedation and maintained in sternal recumbency during
sedation to reduce the risk of xylazine-induced tureen tympany.
Because xylazine also impairs deglutition, the head and neck of
xylazine-sedated ruminants are lowered to avoid aspiration of saliva
or ruminal fluid. Tolazoline (an alpha-2-adrenergic antagonist) has
shown effectiveness in reversing recumbency, gastric paresis and loss
of voluntary lingual control caused by xylazine in cattle
(Swift, 1977; Bolte & Stupariu, 1978; Ruckebusch & Toutain, 1984).
Gastrointestinal effects in dogs and cats include decreased
transit time and vomiting. The mechanism for induction of vomiting is
thought to involve the effect of xylazine on alpha-2-adrenoceptors in
the area postrema (the chemoreceptor trigger zone for vomiting) in the
medulla oblongata (Cullen & Jones, 1977; Colby et al., 1981; Hsu &
McNeel, 1983; Hikasa et al., 1987, 1989).
2.1.2 Absorption, distribution and excretion
2.1.2.1 Rats
Male Sprague-Dawley rats (170 g bw) were administered xylazine at
dosages of 0.02 to 10 mg/kg bw (i.v.) or 0.02 to 100 mg/kg bw (oral).
The drug was labelled with both 35S and 14C on the thiazine ring.
Following oral administration, absorption was > 95% with a half-life
of approximately 5 minutes. After i.v. administration, the drug was
distributed within a few minutes to almost all organs but primarily to
the kidneys and central nervous system. Relatively high activity
concentrations occurred in the pancreas, thyroid glands, liver and
cranial glands (e.g., extraorbital, sublingual). Several hours
following i.v. administration of 2 mg/kg bw, only small concentrations
(< 0.3 µg/g tissue) were present in the musculature. Following oral
or i.v. administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal elimination following
oral or i.v. administration was associated with a half-life of 2 to 3
hours. High oral doses (100 mg/kg bw) were associated with a delay in
renal elimination. Faecal elimination was comparable to biliary
elimination after oral or i.v. administration. Enterohepatic
circulation did not occur to a notable extent (Duhm et al., 1968,
1969).
2.1.2.2 Cattle
Three male calves (200-250 kg) and one dairy cow (450 kg) were
injected intramuscularly with a 0.33 mg/kg dose of 14C-xylazine
labelled in the thiazine ring. Radioactivity in blood plasma reached
its peak in the first 1.5 hours after injection. Total excretion of
radioactivity in urine and faeces was 68, 86, 83 and 100% at 10, 24,
48 and 72 hours, respectively (Murphy & Jacobs, 1975).
In another study, five 2-month old calves and four lactating cows
were administered a single intramuscular dose (0.3 or 0.6 mg/kg bw) of
xylazine hydrochloride. Maximum concentrations of xylazine were
achieved in blood 20 minutes after dosing. These were 0.04 mg/litre
for the 0.3 mg/kg bw dose and 0.06 mg/litre for the 0.6 mg/kg bw dose.
No xylazine was found in blood 8 hours after administration
(Takase et al., 1976).
Three lactating cows were administered an i.m. dose of 0.2 mg
xylazine/kg bw and two others were administered an i.m. dose of 0.4 mg
xylazine/kg bw. Milk was analysed for the presence of xylazine at 5
and 21 hours following administration. No xylazine was found at either
time point for either dose. The limit of detection was 0.06 mg/litre
(Pütter & Sagner, 1973).
Urinary excretion of xylazine was studied in three cows. Two were
administered an i.m. dose of 0.2 mg xylazine/kg bw and one was
administered an i.m. dose of 0.5 mg xylazine/kg bw. Less than 1% of
the dose was excreted unchanged in the urine. Unchanged xylazine was
no longer detectable 6 hours following administration. Metabolites
were no longer detected in urine 10 hours after administration. The
limit of detection for unchanged xylazine was 1-5 µg/litre (Pütter &
Sagner, 1973).
2.1.2.3 Comparative pharmacokinetics in dogs, sheep, cattle and
horses
The comparative pharmacokinetics of xylazine in dogs, sheep,
cattle and horses are summarized in Table 2.
Pharmacokinetic parameters do not vary greatly between species
following intravenous administration. The rapid elimination of
xylazine is attributed to extensive metabolism, and not to rapid renal
excretion of unchanged xylazine. Significant amounts of parent
xylazine were not found in the urine of sheep collected at 10-minute
intervals after dosing. The pharmacokinetics of xylazine were
unmodified when it was administered to rabbits with occluded renal
arteries. The lack of correlation between pharmacokinetic parameters
and clinical effects of xylazine in cattle suggests that clinical
effects in cattle are due to a rapidly produced long-acting
metabolite(s) and not due to an increased sensitivity to xylazine
(Garcia-Villar et al., 1981).
Table 2. Single-dose pharmacokinetics of xylazine in domestic species (Garcia-Villar et al., 1981)
Species Dog Sheep Cattle Horse
Body weight range (kg) 14-24 42-65 240-440 415-550
Dose rate (mg/kg bw)1 1.4 1.0 0.2 0.6
Number 4 6 4 4
Intravenous2
Distribution half-life (min) 2.57 1.89 1.21 5.97
Volume of distribution (l/kg) 2.52 2.74 1.94 2.46
Elimination half-life (min) 30.13 23.11 36.48 49.51
Body clearance (ml/min/kg) 81 83 42 21
Intramuscular2
Absorption half-life (min) 3.44 5.45 ND 2.72
Elimination half-life (min) 34.65 22.36 ND 57.7
Cmax (mg/ml) 0.43 0.13 ND 0.17
Tmax (min) 12.7 14.68 ND 12.92
Bioavailability:
mean (%) 73.9 40.8 ND 44.6
standard deviation (%) 17.89 23.81 4.16
range (%) 52-90 17-73 40-48
1 Dosage expressed as xylazine-base
2 Blood sampling times after injection: 1, 2, 4, 8, 16, 30 and 120 minutes.
ND = Not determined (assay was not sensitive enough to determine xylazine plasma
concentrations lower than 0.01 mg/litre)
2.1.3 Biotransformation
2.1.3.1 Rats
Studies were conducted with urine and bile of rats administered
2 mg xylazine (35S or 14C)/kg bw intravenously. Approximately 20
metabolites were detected and quantified as xylazine equivalents.
Approximately 8% of the dose was eliminated as unchanged compound in
the urine 24 hours after dosing. The major metabolite comprised 35% of
the administered dose. Final products of metabolism were inorganic
sulfate and carbon dioxide (Duhm et al., 1968).
Specific metabolites of xylazine were identified following
incubation of xylazine with rat liver microsomes. Those metabolites
were 2-(4'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-1,3-
thiazine, 2-(3'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-
1,3-thiazine, N-(2,6-dimethylphenyl)thiourea and 2-(2',6'-
dimethylphenylamino)-4-oxo-5,6-dihydro-1,3-thiazine. N-(2,6-dime-
thylphenyl)thiourea was the major metabolite produced in vitro.
Figure 1 shows the proposed metabolic pathways of xylazine based on
these findings (Mutlib et al., 1992).
2.1.3.2 Horses
One mare was administered a 1 g dose of xylazine (route not
stated) and urine was collected over 24 hours. Metabolites were
recovered from horse urine only after the urine was hydrolysed with
beta-glucuronidase. The major urinary metabolites detected were the
same as those produced by incubating xylazine with rat liver
microsomes, described in section 2.1.3.1 (Mutlib et al., 1992).
2.1.3.3 Cattle
Urine from three cows administered an i.m. dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow) was
examined for metabolites. One urinary metabolite, identified as
2,6-xylidine1, was found in both free and conjugated forms. The
authors concluded that xylazine was essentially eliminated in cattle
by rapid biotransformation. Breakdown of the thiazine ring, resulting
in formation of 2,6-xylidine, was proposed as the primary
biotransformation pathway (Patter & Sagner, 1973).
1 2,6-xylidine is also known as 1-amino-2,6-dimethylbenzene and as
2,6-dimethylaniline
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Pharmacodynamics
With respect to xylazine's sedative effect, there are marked
species differences in the dose rates required to achieve this state.
Table 1 illustrates dosages required for various animal species
(Gross & Tranquilli, 1989).
Table 1. Dosage of xylazine in various animal species
Xylazine (mg/kg bw)
Species Intravenous Intramuscular
Horses 0.5 to 1.1 1 to 2
Cattle 0.03 to 0.11 0.1 to 0.21
Sheep 0.05 to 0.11 0.1 to 0.31
Goats 0.01 to 0.51 0.05 to 0.51
Swine 2 to 3
Dogs 0.5 to 1 1 to 2
Cats 0.5 to 1 1 to 2
Birds 5 to 10
1 Lower end of dose range should be used if sedation without
recumbency is desired (Gross & Tranquilli, 1989).
Mydriasis is a feature of xylazine-induced sedation in the cat.
The mechanism has been determined as central inhibition of
parasympathetic tone in the iris due to xylazine's activation of
post-synaptic alpha-2 receptors (Hsu et al., 1981).
Thermoregulatory control is impaired in cats administered
xylazine. They become more susceptible to hyper- and hypothermia both
during and after recovery from the sedative effects of the drug. Foals
have demonstrated a hypothermic response to xylazine. Thermoregulatory
effects in cattle have been variable (Ponder & Clark, 1980;
Booth, 1988; Robertson et al., 1990).
Cardiovascular effects of xylazine include decreased heart rate
and variable effects on blood pressure. Xylazine-induced arrhythmia is
common in the horse due to sinoatrial and atrioventricular blocks.
Arrhythmias have also been recorded in dogs, but could not be induced
in sheep. The induction of cardiovascular effects may be influenced by
route of administration, e.g., xylazine administered epidurally to
horses produced no cardiovascular changes, whereas cattle injected by
this route experienced decreases in heart rate and arterial blood
pressure (Sagner et al., 1969; Holmes & Clark, 1977; Freire
et al., 1981; Hsu et al., 1981; Wasak, 1983; Singh et al., 1983;
Leblanc & Eberhart, 1990; Skarda et al., 1990).
The effects of xylazine on respiration, acid-base balance and
blood gas values vary according to species and anaesthetic
combination. In cattle, xylazine causes a slowing of the respiratory
rate. This is accompanied by an increase in pH and metabolic acidosis.
Respiratory rate is also slowed in dogs administered xylazine, but
arterial pH, pO2 or pCO2 are not significantly affected. The
literature contains conflicting reports on the effect of xylazine on
the respiratory rate of horses. Tachypnoea is characteristic of the
ovine response to xylazine. Hypoxaemia induced by xylazine in sheep
can be life-threatening (DeMoor & Desmet, 1971; Klide et al., 1975;
Holmes & Clark, 1977; Hsu et al., 1989; Carter et al., 1990;
Wagner et al., 1991).
Hyperglycaemia is induced by xylazine in adults of all target
species. Increased blood glucose concentrations are accompanied by a
decrease in insulin levels. In adult horses, hyperglycaemia is
accompanied by increased urine volume without glycosuria. Xylazine
administered to neonatal foals did not result in hyperglycaemia. The
hyperglycaemic effect of xylazine is thought to be due to its direct
effect on alpha-2-adrenoceptors of pancreatic islet beta cells
resulting in an inhibition of insulin release (Symonds, 1976; Feldberg
& Symonds, 1980; Hsu & Hummel, 1981; Thurmon et al., 1982, 1984;
Benson et al., 1984).
Serum chemistry and cerebral spinal fluid alterations were
observed in adult female goats administered intramuscularly with
0.2 mg xylazine/kg bw. Significant elevations of urea nitrogen, total
protein and total cholesterol were found in serum. Glucose and urea
nitrogen levels were significantly increased (P<0.01) and chloride
levels were significantly decreased (P<0.05) in the cerebral spinal
fluid (Amer & Misk, 1980).
Erythrocyte counts, haematocrit values and haemoglobin
concentrations in cattle and dogs have shown significant but
reversible decreases following xylazine administration
(Eichner et al., 1979; Wasak, 1983).
Gastrointestinal effects in ruminants include decreased gut
motility, prolongation of gastrointestinal transit time and inhibition
of reticulorumen contractions. Xylazine causes decreased muscle tone
of the colon and rectum which facilitates rectal examination. Xylazine
inhibition of rumen contractions can lead to tympany, which is a
potential cause of death in xylazine-sedated ruminants. Ruminants are
fasted prior to sedation and maintained in sternal recumbency during
sedation to reduce the risk of xylazine-induced tureen tympany.
Because xylazine also impairs deglutition, the head and neck of
xylazine-sedated ruminants are lowered to avoid aspiration of saliva
or ruminal fluid. Tolazoline (an alpha-2-adrenergic antagonist) has
shown effectiveness in reversing recumbency, gastric paresis and loss
of voluntary lingual control caused by xylazine in cattle
(Swift, 1977; Bolte & Stupariu, 1978; Ruckebusch & Toutain, 1984).
Gastrointestinal effects in dogs and cats include decreased
transit time and vomiting. The mechanism for induction of vomiting is
thought to involve the effect of xylazine on alpha-2-adrenoceptors in
the area postrema (the chemoreceptor trigger zone for vomiting) in the
medulla oblongata (Cullen & Jones, 1977; Colby et al., 1981; Hsu &
McNeel, 1983; Hikasa et al., 1987, 1989).
2.1.2 Absorption, distribution and excretion
2.1.2.1 Rats
Male Sprague-Dawley rats (170 g bw) were administered xylazine at
dosages of 0.02 to 10 mg/kg bw (i.v.) or 0.02 to 100 mg/kg bw (oral).
The drug was labelled with both 35S and 14C on the thiazine ring.
Following oral administration, absorption was > 95% with a half-life
of approximately 5 minutes. After i.v. administration, the drug was
distributed within a few minutes to almost all organs but primarily to
the kidneys and central nervous system. Relatively high activity
concentrations occurred in the pancreas, thyroid glands, liver and
cranial glands (e.g., extraorbital, sublingual). Several hours
following i.v. administration of 2 mg/kg bw, only small concentrations
(< 0.3 µg/g tissue) were present in the musculature. Following oral
or i.v. administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal elimination following
oral or i.v. administration was associated with a half-life of 2 to 3
hours. High oral doses (100 mg/kg bw) were associated with a delay in
renal elimination. Faecal elimination was comparable to biliary
elimination after oral or i.v. administration. Enterohepatic
circulation did not occur to a notable extent (Duhm et al., 1968,
1969).
2.1.2.2 Cattle
Three male calves (200-250 kg) and one dairy cow (450 kg) were
injected intramuscularly with a 0.33 mg/kg dose of 14C-xylazine
labelled in the thiazine ring. Radioactivity in blood plasma reached
its peak in the first 1.5 hours after injection. Total excretion of
radioactivity in urine and faeces was 68, 86, 83 and 100% at 10, 24,
48 and 72 hours, respectively (Murphy & Jacobs, 1975).
In another study, five 2-month old calves and four lactating cows
were administered a single intramuscular dose (0.3 or 0.6 mg/kg bw) of
xylazine hydrochloride. Maximum concentrations of xylazine were
achieved in blood 20 minutes after dosing. These were 0.04 mg/litre
for the 0.3 mg/kg bw dose and 0.06 mg/litre for the 0.6 mg/kg bw dose.
No xylazine was found in blood 8 hours after administration
(Takase et al., 1976).
Three lactating cows were administered an i.m. dose of 0.2 mg
xylazine/kg bw and two others were administered an i.m. dose of 0.4 mg
xylazine/kg bw. Milk was analysed for the presence of xylazine at 5
and 21 hours following administration. No xylazine was found at either
time point for either dose. The limit of detection was 0.06 mg/litre
(Pütter & Sagner, 1973).
Urinary excretion of xylazine was studied in three cows. Two were
administered an i.m. dose of 0.2 mg xylazine/kg bw and one was
administered an i.m. dose of 0.5 mg xylazine/kg bw. Less than 1% of
the dose was excreted unchanged in the urine. Unchanged xylazine was
no longer detectable 6 hours following administration. Metabolites
were no longer detected in urine 10 hours after administration. The
limit of detection for unchanged xylazine was 1-5 µg/litre (Pütter &
Sagner, 1973).
2.1.2.3 Comparative pharmacokinetics in dogs, sheep, cattle and
horses
The comparative pharmacokinetics of xylazine in dogs, sheep,
cattle and horses are summarized in Table 2.
Pharmacokinetic parameters do not vary greatly between species
following intravenous administration. The rapid elimination of
xylazine is attributed to extensive metabolism, and not to rapid renal
excretion of unchanged xylazine. Significant amounts of parent
xylazine were not found in the urine of sheep collected at 10-minute
intervals after dosing. The pharmacokinetics of xylazine were
unmodified when it was administered to rabbits with occluded renal
arteries. The lack of correlation between pharmacokinetic parameters
and clinical effects of xylazine in cattle suggests that clinical
effects in cattle are due to a rapidly produced long-acting
metabolite(s) and not due to an increased sensitivity to xylazine
(Garcia-Villar et al., 1981).
Table 2. Single-dose pharmacokinetics of xylazine in domestic species (Garcia-Villar et al., 1981)
Species Dog Sheep Cattle Horse
Body weight range (kg) 14-24 42-65 240-440 415-550
Dose rate (mg/kg bw)1 1.4 1.0 0.2 0.6
Number 4 6 4 4
Intravenous2
Distribution half-life (min) 2.57 1.89 1.21 5.97
Volume of distribution (l/kg) 2.52 2.74 1.94 2.46
Elimination half-life (min) 30.13 23.11 36.48 49.51
Body clearance (ml/min/kg) 81 83 42 21
Intramuscular2
Absorption half-life (min) 3.44 5.45 ND 2.72
Elimination half-life (min) 34.65 22.36 ND 57.7
Cmax (mg/ml) 0.43 0.13 ND 0.17
Tmax (min) 12.7 14.68 ND 12.92
Bioavailability:
mean (%) 73.9 40.8 ND 44.6
standard deviation (%) 17.89 23.81 4.16
range (%) 52-90 17-73 40-48
1 Dosage expressed as xylazine-base
2 Blood sampling times after injection: 1, 2, 4, 8, 16, 30 and 120 minutes.
ND = Not determined (assay was not sensitive enough to determine xylazine plasma
concentrations lower than 0.01 mg/litre)
2.1.3 Biotransformation
2.1.3.1 Rats
Studies were conducted with urine and bile of rats administered
2 mg xylazine (35S or 14C)/kg bw intravenously. Approximately 20
metabolites were detected and quantified as xylazine equivalents.
Approximately 8% of the dose was eliminated as unchanged compound in
the urine 24 hours after dosing. The major metabolite comprised 35% of
the administered dose. Final products of metabolism were inorganic
sulfate and carbon dioxide (Duhm et al., 1968).
Specific metabolites of xylazine were identified following
incubation of xylazine with rat liver microsomes. Those metabolites
were 2-(4'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-1,3-
thiazine, 2-(3'-hydroxy-2',6'-dimethylphenylamino)-5,6-dihydro-4H-
1,3-thiazine, N-(2,6-dimethylphenyl)thiourea and 2-(2',6'-
dimethylphenylamino)-4-oxo-5,6-dihydro-1,3-thiazine. N-(2,6-dime-
thylphenyl)thiourea was the major metabolite produced in vitro.
Figure 1 shows the proposed metabolic pathways of xylazine based on
these findings (Mutlib et al., 1992).
2.1.3.2 Horses
One mare was administered a 1 g dose of xylazine (route not
stated) and urine was collected over 24 hours. Metabolites were
recovered from horse urine only after the urine was hydrolysed with
beta-glucuronidase. The major urinary metabolites detected were the
same as those produced by incubating xylazine with rat liver
microsomes, described in section 2.1.3.1 (Mutlib et al., 1992).
2.1.3.3 Cattle
Urine from three cows administered an i.m. dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow) was
examined for metabolites. One urinary metabolite, identified as
2,6-xylidine1, was found in both free and conjugated forms. The
authors concluded that xylazine was essentially eliminated in cattle
by rapid biotransformation. Breakdown of the thiazine ring, resulting
in formation of 2,6-xylidine, was proposed as the primary
biotransformation pathway (Patter & Sagner, 1973).
1 2,6-xylidine is also known as 1-amino-2,6-dimethylbenzene and as
2,6-dimethylaniline
 2.2 Toxicological studies
Because 2,6-xylidine is also a chemical intermediate used in
dyes, a component of tobacco smoke and a degradation product of
aniline-based pesticides, its toxicology has been studied extensively.
Toxicological studies conducted with this compound were also reviewed
and will be presented in addition to the review and results of
toxicological studies of xylazine.
2.2.1 Acute toxicity studies of xylazine and 2,6-xylidine
The acute systemic toxicity of xylazine has been investigated in
both laboratory and domestic species. It is generally recognized that
ruminants are much more sensitive than most other species to the
pharmacological and toxicological effects of xylazine.
Results of LD50 studies of xylazine and 2,6-xylidine are
summarized in Table 3.
2.2.1.1 Acute toxicity of xylazine in dogs
Adult dogs (four males, four females) and cats (two males, four
females) were administered a single i.m. or i.v. dose of 22 mg
xylazine/kg bw (10 times the recommended therapeutic dose). One cat
out of three receiving the i.v. dose died, and two dogs out of four
receiving the i.m. dose died. All others recovered from convulsions,
unconsciousness and respiratory depression with no apparent
after-effects. The authors concluded that xylazine was slightly toxic
in this study (Crawford et al., 1970a).
Table 3. Results of acute toxicity studies on xylazine and 2,6-xylidine
Species Sex1 Route LD50 Reference
(mg/kg bw)
Xylazine
Rat NA p.o. 130 Sagner, 1967
Cat male & female2 s.c. 100-110 Bauman & Nelson, 1969
Dog male & female3 i.m 47 Nelson et al., 1968b
Dog 4 male & 3 female i.v 20-25 Nelson et al., 1968b
Horses NA4 i.m. 60-705 Nelson et al., 1968a
i.v. 15-285
2,6-Xylidine
Mouse male p.o. 710 Vernot et al., 1977
Rat p.o. 2042 Lindstrom et al., 1969
male p.o. 840 Jacobson, 1972
male p.o. 630 Short et al., 1983
male p.o. 1230 Vernot et al., 1977
female p.o. 1160 & 1270 US National Toxicology
Program, 1990
male p.o. 620-1250 &
1310
1 NA = Information not available
2 Number per sex not stated; 10 animals were used
3 Number per sex not stated; 17 animals were used
4 Sex of test animals was not stated; 5 animals were used
5 Minimum lethal dose
2.2.1.2 Acute toxicity of xylazine in horses
Adult horses were administered 11 mg xylazine/kg bw, i.v. (three
mares, one gelding) or 22 mg xylazine/kg bw, i.m. (two mares, two
geldings). One mare died following i.v. administration. All other test
animals recovered from treatment-related effects 24 hours following
i.v. administration and 48 hours following i.m. administration. The
authors concluded that the i.v. dose was slightly toxic and that the
i.m. dose produced no apparent toxicity in this study (Crawford
et al., 1970b).
2.2.2 Short-term toxicity studies
2.2.2.1 Xylazine
a) Rats
Xylazine was administered in the diet to Wistar rats (10/sex/
group) for 32 weeks. Dosages administered were 0, 50, 100, 250
or 500 mg/kg diet (equal to 0, 3, 6, 21 or 41 mg/kg bw per day for
males and 0, 4, 8, 19 or 45 mg/kg bw per day for females).
Haematology, urinalysis and gross and histopathological evaluations
were performed.
Decreases in body weight observed in females in the two
highest-dose groups (statistically significant (p<0.02) at 500 mg/kg
diet) were considered by the author to be treatment-related.
Microscopic examination of livers, lungs and kidneys revealed that
animals in all groups were diseased but no treatment-related pathology
was identified. Based on the dose-related decrease in weight gain
observed in females at 250 and 500 mg/kg diet, the NOEL in this study
was 100 mg/kg diet, equal to 6 mg/kg bw per day. The author regarded
the dose of 250 mg/kg diet as the non-toxic application rate. The
reliability of any NOEL derived from this study should be considered
questionable, owing to the presence of infection in all groups
(Tettenborn & Hobik, 1968a; Trossmann & Hobik, 1970).
b) Dogs
Dogs of undetermined breed or source were given xylazine orally
in gelatin capsules for 14-16 weeks at dose levels of 25, 50 or
100 mg/kg bw per day, 5 days/week. The low-dose group consisted of one
male and one female, the mid-dose group of two males and the high-dose
group of two males and two females. Haematology, clinical chemistry,
blood coagulation, urinalysis and postmortem gross and microscopic
evaluations were performed.
During week 8 of the test, one animal in the high-dose group died
and was replaced with a new animal. Postmortem gross findings in this
animal included diffuse reddening of the stomach and intestinal mucous
membrane.
Histopathological findings in livers (fatty degeneration and
necrosis) and kidneys (tubular epithelial necrosis and fat
accumulation) of the high-dose group were considered treatment-
related. Fatty deposits were noted in the liver and kidneys of the
low-dose female. These findings were attributed to parturition, which
occurred 3 weeks before the animal was killed. The author concluded
that the NOEL for this study was 50 mg/kg bw per day. The reliability
of any NOEL derived from this study should be considered questionable
due to the lack of a control group and small numbers of animals in
each test group (Tettenborn & Hobik, 1968b).
Beagle dogs (two/sex/group) were administered xylazine orally by
dietary admixture for 13 weeks. Dosages administered were 0, 10, 30 or
100 mg/kg diet (equal to 0, 0.3, 0.9 or 3 mg/kg bw per day). At the
beginning of the test the animals were approximately 8.5 months old
and weighed 7-10 kg. Parameters evaluated included general appearance,
ophthalmology, electrocardiography, haematology, clinical chemistry,
urinalyses and gross and microscopic pathology.
No treatment-related adverse effects were observed in any of the
parameters evaluated. The NOEL for this study was 3 mg/kg bw per day
(Tettenborn, 1969; Mawdesley-Thomas, 1970).
2.2.2.2 2,6-Xylidine
a) Rats
Three groups (nine or ten/group) of male Fischer-344 rats were
given oral (gavage) doses of 160 mg 2,6-xylidine/kg bw per day for 5,
10 or 20 days. The dosage administered was 25% of the estimated LD50
determined by the investigator. A significant increase in splenic
haemosiderosis (indicative of erythrocyte damage) after 20 days was
noted as a treatment-related effect in this study. Splenic congestion
and evidence for increased erythropoiesis were minimal
(Short et al., 1983).
Three groups of Sprague-Dawley rats (five/sex/group for controls,
low and mid dose; four/sex/group for high dose) were administered
0, 20, 100 or 500-700 mg 2,6-xylidine/kg bw per day by gavage for 4
weeks. Treatment-related effects included decreased weight gain,
decreased haemoglobin levels and hepatomegaly. In this study, the rat
appeared to be about 10 times less susceptible to hepatotoxicity of
2,6-xylidine than the dog (see section 2.2.2.2b) (Magnusson et al.,
1971; IARC, 1993).
Two groups of 8-week-old Sprague-Dawley rats (5/sex/group) were
orally administered (gastric intubation) a dose of 0 or 400 mg
2,6-xylidine per kg bw per day for 1 week immediately followed by a
daily dose of 0 or 500 mg/kg bw for 3 weeks. Decreased body weight
gain and hepatomegaly (most pronounced in centrilobular regions) were
noted as treatment-related effects. Electron microscopy of liver
tissue showed proliferation of hepatic smooth endoplasmic reticulum,
which was deemed responsible for the observed hepatomegaly in treated
rats. An increase in microsomal glucuronyltransferase was observed in
males while aniline hydroxylase levels were increased in females.
Decreases in liver glycogen and glucose-6-phosphatase activity were
also observed in the centrilobular regions of treated animals
(Magnusson et al., 1979).
Male Osborne-Mendel rats were administered up to 10 000 mg
2,6-xylidine per kg in the diet for 3-6 months. Treatment-related
effects included 25% weight reduction, anaemia, hepatomegaly with no
associated microscopic changes, splenic congestion and renal toxicity
(Lindstrom et al., 1963).
Groups of F-344/N rats (five/sex/group) were administered doses
of 0, 80, 160, 310, 620 or 1250 mg/kg bw of 2,6-xylidine in corn oil
by gavage 5 days/week for 2 weeks. Parameters evaluated included
clinical observations, body weight, urinalysis, haematology, blood pH
and carbon dioxide determinations, and gross postmortem findings.
Treatment-related deaths occurred at and above 620 mg/kg bw. All
animals in the highest dose group died before the end of the study. A
decrease of more than 10% in body weight was observed in males at and
above 310 mg/kg bw and in females at and above 160 mg/kg bw.
Generalized leukocytosis and an increase in the number of nucleated
red blood cells were observed in male rats administered 310 or
620 mg/kg bw. Slight anisocytosis, poikilocytosis and polychromasia of
the red blood cells occurred more frequently in dosed animals than in
vehicle control animals. Moderate poikilocytosis occurred at 310 mg/kg
bw and moderate polychromasia at 310 and 620 mg/kg bw. Slightly
macrocytic erythrocytes were observed at the two highest doses. Slight
anisocytosis, poikilocytosis and polychromasia were observed in female
rats at 310 and 620 mg/kg bw. The NOEL for this study was 80 mg/kg bw
per day (US National Toxicology Program, 1990).
Groups of F-344/N rats (10/sex/group) were given doses of 0, 20,
40, 80, 160 or 310 mg/kg bw of 2,6-xylidine in corn oil by gavage, 5
days/week for 13 weeks. Parameters evaluated included clinical
observations, haematology, urinalysis, serum chemistry and enzyme
analyses, gross and histopathological postmortem examinations.
A decrease in body weight gain of more than 10% occurred in males
and females in the highest dose group and in females at 40 and
160 mg/kg bw per day. In the highest dose group, relative liver
weights were significantly (P=0.003) increased for males and females.
Relative liver weight was also increased for males in the 160 mg/kg bw
group. The liver weight to brain weight and kidney weight to brain
weight ratios were significantly increased in females at 310 mg/kg bw
per day.
Treatment-related effects on haematology included significantly
decreased total leukocyte counts in males at doses of 40 mg/kg bw or
more. These were accompanied by decreases in the percentage of
lymphocytes and increases in the percentage of segmented neutrophils
at doses of 80 mg/kg bw or more. In males, haemoglobin levels were
significantly decreased at 160 and 310 mg/kg bw and erythrocyte and
haematocrit levels were decreased at 310 mg/kg bw. The NOEL for this
study was 20 mg/kg bw per day (US National Toxicology Program, 1990).
b) Dogs
Four groups of beagle dogs (one/sex/group) were given an oral
(gelatin capsule) dose of 0, 2, 10 or 50 mg 2,6-xylidine/kg bw per day
for 4 weeks. Treatment-related effects included vomiting (mid- and
high-dose groups), poor condition and decreased body weights
(high-dose group), hyperbilirubinaemia (mid- and high-dose groups),
hypoproteinaemia (mid-and high-dose groups) and fatty degenerative
changes in the liver that increased in severity with increasing dose
(Magnusson et al., 1971; IARC, 1993).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Xylazine
No carcinogenicity studies have been performed with xylazine
2.2.3.2 2,6-Xylidine
Four groups of Charles River CRL:COBS CD (SD) BR rats
(56/sex/group) were fed diets containing 2,6-xylidine (99.06% pure) at
concentrations of 0, 300, 1000 or 3000 mg/kg diet (equivalent to
0, 15, 50 or 150 mg/kg bw per day) for 102 weeks. The animals assigned
to this study were F1a, generation weanlings from a multigeneration
study in which animals were fed diets containing 0, 300, 1000 or
3000 mg/kg 2,6-xylidine beginning at 5 weeks of age. Parameters
evaluated in the carcinogenicity study included clinical observations,
haematology, blood urea nitrogen, glucose, SGOT, alkaline phosphatase
and gross and microscopic postmortem examinations.
Treatment-related clinical effects included a decrease in mean
body weight gain in high-dose males and females (>10%). Mortality was
significantly (P < 0.001) increased (relative to controls) in males
in the high-dose group. Mortality was also increased for mid-dose
males. Survival at 105 weeks was 43/56, 40/56, 33/56 and 14/56 for
males in the control, low-, mid- and high-dose groups, respectively.
For females, survival was 33/56, 25/56, 32/56 and 24/56 for the
controls, low-, mid- and high-dose groups, respectively.
Microscopically, a significant increase in carcinoma of the nasal
cavity was observed in high-dose males (26/56; P < 0.001, life table
test). For females, the incidence of carcinomas of the nasal cavity
were 0/56, 0/56, 1/56 and 24/56 in the low-, mid- and high-dose
groups, respectively (P<0.001, life table test). Two adenocarcinomas
were diagnosed in high-dose males. The incidence of papillary adenomas
in males was 0/56 in controls, 0/56 in low-dose, 2/56 in mid-dose and
10/56 in high-dose rats (P=0.001, incidental tumour test). For
females, nasal adenomas occurred in 0/56 in controls, 0/56 in low-
dose, 1/56 in mid-dose and 6/56 in high-dose rats (P=0.02, incidental
tumour test). Several unusual neoplasms of the nasal cavity were also
considered to be related to treatment. These included one undifferen-
tiated sarcoma identified in one high-dose female, rhabdomyosarcomas
which occurred in two high-dose male and two high-dose females and
malignant mixed tumours having features associated with both adenocar-
cinoma and rhabdomyosarcoma were observed in one high-dose male and
one high-dose female rat. Non-neoplastic nasal cavity lesions included
acute inflammation (rhinitis), epithelial hyperplasia and squamous
metaplasia. These occurred at increased incidence (relative to
controls) in high-dose male and female rats. The incidence of
subcutaneous fibromas and fibrosarcomas combined in males was 0/56,
2/56, 2/56 and 5/56 for the control, low-, mid- and high-dose groups,
respectively (P=0.001, life table test; P<0.001 life table trend
test). For females, the incidence of these tumours combined was 1/56,
2/56, 2/56 and 6/56 for controls, low-, mid- and high-dose groups,
respectively ((P=0.01, life table trend test). Neoplastic nodules occurred
in livers of female rats with a significant positive trend. The
incidence was 0/56, 1/56, 2/56 and 4/55 for the controls, low-, mid-,
and high-dose groups, respectively (P=0.03, incidental test; P=0.012,
incidental trend test).
Treatment-related effects on haematology included decreases in
erythrocyte counts and haemoglobin levels at 18 months in the
high-dose males. Decreases in these parameters were also observed in
the mid- and high-dose females at 12 months. The author remarked that
these changes were not severe enough to be considered indicative of
anaemia.
The author concluded that under the conditions of this study,
2,6-xylidine was dearly carcinogenic for male and female Charles River
CD rats. This was based on the observed significant increases in the
incidence of adenomas and carcinomas of the nasal cavity. Additionally
the author stated that the increased incidence of subcutaneous
fibromas and fibrosarcomas in male and female rats and increased
incidence of neoplastic nodules of the liver in female rats could have
been treatment-related (US National Toxicology Program, 1990).
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenic risk of 2,6-xylidine to humans. The Working
Group concluded that there was inadequate evidence in humans but
sufficient evidence in experimental animals for the carcinogenicity of
2,6-xylidine. The IARC classified 2,6-xylidine as Group 2B (possibly
carcinogenic to humans) (IARC, 1993).
2.2.4 Special studies on teratogenicity
Xylazine was administered by gavage to groups of pregnant rats
(22 animals/group) on gestation days 6 to 15, then killed on day 20
for examination of uterine contents. Dosages administered were 0, 1, 4
or 16 mg/kg bw per day. The study was conducted in accordance with the
principles of Good Laboratory Practice Standards and Guidelines of the
OECD, United Kingdom, FDA and Japan.
Treatment-related maternal effects included partial closing of
the eyelids, underactivity, ataxia, flat posture and slightly reduced
body weight gain in the high-dose group only. Fetal effects included a
decrease in mean fetal weight in the high-dose group only. A
teratogenic potential of xylazine was not evident at levels up to and
including 16 mg/kg bw per day. The NOEL in this study was 4 mg/kg bw
per day (Reynolds, 1994).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with xylazine and
2,6-xylidine are summarized in Table 4.
The results of bacterial mutagenicity testing of xylazine were
considered to be negative by the author. Reviewing the data, the
Committee concluded that a more than two-fold reproducible increase in
revertant colonies in tester strains TA1535 and TA1538 represents weak
mutagenic activity, even in the absence of a clear dose-response.
2.2.6 Special studies on methaemoglobin and haemoglobin adduct
formation with 2,6-xylidine
2.2.6.1 Cats and dogs
Cats and dogs (numbers not specified) were administered an i.v.
dose of 30 mg 2,6-xylidine/kg bw or an oral dose of 164 mg N-acetyl
2,6-xylidine/kg bw. 2,6-Xylidine induced a 10% methaemoglobinaemia in
cats, and N-acetyl 2,6-xylidine induced a 5% methaemoglobinaemia in
cats. Haemoglobin was unaffected in dogs in this study (McLean
et al., 1967).
Five adult cats (>24 months old) were administered an i.v. dose
of 30 mg 2,6-xylidine/kg bw. Blood samples were drawn at 1, 2, 3, 4
and 5 hours after dosing and analysed for methaemoglobin formation.
The mean methaemoglobin concentration determined from these sampling
intervals was 7% (range = 4.8% to 8.7%). Prior to treatment the mean
methaemoglobin concentration of the 152 cats used in this study was
approximately 1% (Mclean et al., 1969).
2.2.6.2 Humans
2,6-Xylidine-haemoglobin adduct levels have been found to be
elevated in human patients receiving lidocaine treatment for local
anaesthesia (1 mg/kg bw) or cardiac arrhythmias (up to 50 mg/kg bw,
i.v.). 2,6-Xylidine-haemoglobin adducts are also found in humans with
no known exposure to lidocaine. This is attributed to the 120-day
lifespan of the erythrocyte and chronic exposure to environmental or
iatrogenic sources of aromatic amines, e.g., cigarette smoke. The
levels of 2,6-xylidine-haemoglobin adducts found correspond to at an
estimated daily exposure (from iatrogenic and environmental sources)
of 23 µg (IARC, 1993; Bryant et al., 1994).
Methaemoglobinaemia induced by i.v. administration of lidocaine
was studied in 40 human cardiac patients. Treatment consisted of a
1 mg/kg bw i.v. bolus followed 15 minutes later with a 0.5 mg/kg bw
i.v. bolus. Patients were maintained between and after bolus doses
with an infusion rate of 1-4 mg lidocaine/min. Blood samples were
drawn before treatment and 1 and 6 hours after treatment. Although the
investigators found methaemoglobin levels in these patients to be
significantly elevated, the increase was not large enough to be of
clinical concern. The highest methaemoglobin level attained was 1.2%.
The author did not address the possible role of the 2,6-xylidine
metabolite in the observed increases in methaemoglobin levels in
treated patients (Weiss et al., 1987).
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine
Test system Test object Concentration Results Reference
Xylazine
In vitro
Reverse S. typhimurium
mutation1 TA1535, 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA1538 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA100, 0.4-12 mg/plate Negative Herbold, 1984
TA98, 0.4-12 mg/plate Negative Herbold, 1984
TA1537 0.4-12 mg/plate Negative Herbold, 1984
Mammalian V79/HGPRT 62-1250 µg/ml Negative Brendler-
cell forward (-S9) Schwaab,
mutation1 2-40 µg/ml (+S9) 1994
In vivo
Cytogenetic Mouse bone 50 mg/kg bw, Negative Herbold, 1995
assay marrow i.p.2
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
2,6-Xylidine
In vitro
Reverse S. typhimurium
mutation1 TA1535 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
3 µmol/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al., 1988
TA100 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin el al.,
19804
0.1-10 mg/plate Neg (-S9), Zeiger et al.,
Pos (+S9) 19883
480-4000 Negative Kugler-
µg/plate Steigmeier
et al., 1989
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
TA1537 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al.,
1988
TA98 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1 - 10 mg/plate Negative Zeiger et al.,
1988
Gene Mouse lymphoma Not given Positive Rudd et al.,
mutation1 L5178Y cells, 19837
tk locus
Sister Chinese 30-1500 µg/ml Positive Galloway et al.,
chromatid hamster 1987
exchange1 ovary cells
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
In vivo
Cytogenetic ICR mouse 350 mg/kg bw, Inconclusive6 Parton et al.,
assay bone marrow p.o. 1988
375 mg/kg bw, Inconclusive6 Parton et al.,
p.o. 1990
In vivo- Rat primary 40-850 mg/kg Negative Mirsails et al.,
in vitro DNA hepatocytes bw, p.o. 1989
repair assay
Covalent Rats 87.2 µCi Positive Short et al., 1989
DNA 14C-labelled
binding 2,6-xylidine/rat,
i.p5
1 Both with and without rat liver S9 fraction
2 Cyclophosphamide positive control
3 Weakly positive in two of three laboratories, negative in the third
4 Spot tests only
5 Pretreatment with unlabelled 262.5 mg/kg bw 2,6-xylidine daily for 9 days
6 Results suggest test article may not have reached target tissue (bone marrow)
7 Reference was an abstract and doses were not stated in that reference
2.3 Observations in humans
A 34-year-old man self-injected 10 ml of a 100 mg/ml solution of
xylazine intramuscularly. The estimated dose was 15 mg/kg bw. The
individual was discovered (30 minutes after retiring for bed) in a
deeply comatose, apnoeic and areflexic state. An empty Rompun
(xylazine) bottle (known to have contained 10 cc earlier) lay by his
side. He was immediately admitted to the hospital. Upon admission his
pupils were of moderate size and responded slowly to light. Other
findings included a blood pressure of 120/70 mmHg and heart rate of
60 bpm with stable sinus rhythm. Lactate dehydrogenase (LDH) activity
was elevated, with the LDH-1 isoenzyme predominating. Creatine
phosphokinase (CPK) activity was also elevated, particularly in the
CPK-3 and CPK-2 isoenzymes. These enzyme changes persisted for 5 to 7
days. Plasma glucose level was also elevated. Two days following
hospital admission sinus tachycardia developed, interspersed with runs
of multifocal premature ventricular contractions which were controlled
with lidocaine infusion. Blood pressure remained approximately 120/80
for the duration of hospitalization. Coma and respiratory depression
lasted 60 hours. The patient was discharged from the hospital 17 days
after admission.
The author noted that the patient might have died if he had not
been found shortly after the injection was administered, owing to the
marked respiratory depression that occurred. Hypotension, which has
been reported as an effect of xylazine in humans, did not occur in
this case. According to the author, the enzyme activity increases
indicated that myocardial muscle damage had occurred and an intrinsic
cardiotoxic effect of xylazine was suspected. Finally, in this case
the greatest threat to life was the CNS-depressant effect of xylazine
(Carruthers et al., 1979).
A 20-year-old woman ingested 400 mg of xylazine. Approximately 2
hours later she became drowsy, incontinent (urine), difficult to
arouse and occasionally unresponsive to verbal commands. She was
admitted to the hospital approximately 3 hours after the ingestion. In
a similar way to the case of human poisoning described by Carruthers
et al. (1979), she experienced a relatively low initial cardiac rate,
which later gradually increased, significant central nervous system
and respiratory depression, transient hyperglycaemia and ventricular
arrhythmias. However there was no evidence of myocardial damage. A
sample of this patient's urine was analysed using a gas chromatograph-
mass spectrometer computer system. Xylazine was found largely unchanged
in the urine as shown by a lack of any structurally related compounds
in the basic urine extract. No xylazine was found in a blood sample
taken at the time of admission. The author concluded that plasma
levels were below the limit of detection of the method (100 ng/ml).
The patient was discharged ambulatory and without apparent adverse
effects two days following admission to the hospital (Gallanosa
et al., 1981).
A 36-year-old man died following ingestion of alcohol and
clorazepate combined with an injection of approximately 40 ml of
xylazine (100 mg/ml). Xylazine was found in the decedent's blood,
brain, kidney, liver, lung, fat and urine at concentrations of 0.2,
0.4, 0.6, 0.9, 1.1, 0.05 and 7 ppm, respectively (Poklis et al.,
1985).
A 29-year-old woman self-injected 40 mg of xylazine
intramuscularly. The estimated dose was 0.73 mg/kg bw. Clinical
findings included disorientation, miosis, hypotension and bradycardia,
but no cardiac arrhythmias were noted. The abnormalities resolved
spontaneously (Spoerke et al., 1986).
A 37-year-old woman self-injected 24 ml (2400 mg) xylazine
intramuscularly. The estimated dose was 22 mg/kg bw. Twenty minutes
after the injection her blood pressure was 166/130 mmHg, heart rate
was 76 bpm and respirations 18 per minute. The serum glucose level was
175 mg/dl. Blood pressure later decreased to 130/90 mmHg and she
became apnoeic. No cardiac arrhythmias were observed during her 3 days
of hospitalization. Hypotension and bradycardia occurred two days
after the injection. The patient survived (Spoerke et al., 1986).
A 29-year-old woman self-injected an unknown amount of xylazine
intravenously. She became apnoeic and had an initial blood pressure of
130/90 mmHg with a pulse of 60 bpm. Serum glucose levels did not
exceed 90 mg/dl. Twenty-four hours after the injection the patient
experienced hypotension and bradycardia. Spontaneous respiration
resumed 18 hours after hospital admission. The patient recovered fully
(Spoerke et al., 1986).
A 19-year-old man accidentally injected himself subcutaneously
with 2 ml (100 mg/ml) of xylazine. The dose administered was 3 mg/kg
bw. Thirty minutes later he became difficult to rouse and was
hospitalized. Clinical findings included miosis, hyporeflexia,
hypotension, bradycardia, respiratory and central nervous system
depression and hyperglycaemia. He was treated with intravenous fluids
and assisted ventilation. Eight hours after hospitalization the
patient was alert and responsive. Twenty-four hours later he was
released (Samanta et al., 1990).
A 39-year-old woman was admitted to the hospital with symptoms of
tiredness, faintness and blurred vision. Clinical findings included
sinus bradycardia with a blood pressure of 130/90. Xylazine was found
in the urine and serum at concentrations of 1674 µg/litre and
30 µg/litre, respectively (Lewis et al., 1983).
3. COMMENTS
The Committee considered toxicological data on xylazine,
including the results of acute and short-term toxicity studies as well
as studies on pharmacodynamics, pharmacokinetics, reproductive and
developmental toxicity, genotoxicity and effects in humans. In
addition, toxicological studies on 2,6-xylidine, a metabolite of
xylazine, were reviewed; these included studies on acute and
short-term toxicity, carcinogenicity and genotoxicity.
Numerous pharmacological side-effects of xylazine have been
observed in treated animals, including mydriasis, impairment of
thermo-regulatory control, various effects on the cardiovascular
system, acid-base balance and respiration, hyperglycaemia, and
haematological and gastrointestinal effects. Cattle and sheep are
approximately 10 times more sensitive to xylazine than horses, dogs
and cats.
Rats were administered radiolabelled xylazine intravenously at
doses of 0.02 to 10 mg/kg bw or orally at doses of 0.02 to
100 mg/kg bw. More than 95% of the oral dose was absorbed, with a
half-life of approximately 5 minutes. Following oral or intravenous
administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal excretion following oral
or intravenous administration was associated with a half-life of 2 to
3 hours. Enterohepatic circulation did not occur to a notable extent.
In cattle administered an intramuscular dose of 0.2 or 0.5 mg
xylazine/kg bw, less than 1% of the dose was excreted unchanged in the
urine, and the parent compound was detected in the urine up to 6 hours
following administration. Metabolites of xylazine were detected in
urine from these cattle up to 10 hours following administration.
Pharmacokinetic parameters following intravenous administration
showed minor variations between species. Xylazine disappeared rapidly
from plasma following intravenous administration, with an elimination
half-life of approximately 40 minutes in cattle and approximately 20
minutes in sheep. Xylazine could not be detected in the plasma of
cattle following intramuscular administration of a single therapeutic
dose.
In rats administered an intravenous dose of 2 mg/kg bw radio-
labelled xylazine, approximately 20 metabolites were quantified as
xylazine equivalents in urine and bile. The major metabolite comprised
35% of the administered dose. Approximately 8% of the dose was
eliminated as unchanged xylazine 24 hours after dosing. In an
in vitro study, 4 metabolites were identified when xylazine was
incubated with rat liver microsomes. The same metabolites were
identified in the urine of horses treated with xylazine. The major
metabolite in both cases was identified as N-(2,6-dimethylphenyl)
thiourea. In cattle administered an intramuscular dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow),
2,6-xylidine was identified as a metabolite excreted in urine in both
conjugated and unconjugated forms.
The acute oral toxicities of xylazine and 2,6-xylidine were
tested in mice and rats. Xylazine was determined to be moderately
toxic (LD50 = 121-240 mg/kg bw) and 2,6-xylidine to be slightly
toxic (LD50 = 600-1000 mg/kg bw).
Three studies on the short-term toxicity of xylazine were
reviewed. A 32-week dietary study in rats and a 16-week oral
(capsules) study in dogs were considered inadequate for the
determination of the toxicity of xylazine owing to the use of
insufficient numbers, poor quality animals and inadequate study
design. The third was a 13-week oral study in beagle dogs fed
diets containing 0, 10, 30 or 100 mg/kg xylazine in the feed (equal
to 0.3, 0.9 or 3 mg/kg bw per day). No treatment-related effects
were observed in any of the treated groups.
In a two-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 80, 160, 310, 620 or 1250 mg
2,6-xylidine/kg bw per day, 5 days per week. Treatment-related effects
included increased mortality (all animals in the high-dose group
died), decreased body weight (males at 310 mg/kg bw per day and above
and females at 160 mg/kg bw per day and above) and various effects on
haematological parameters as indicated by leukocytosis and changes in
red blood cell parameters indicative of increased erythropoiesis
(males and females at 310 mg/kg bw per day and above). The NOEL in
this study was 80 mg/kg bw per day.
In a 13-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 20, 40, 80, 160 or 310 mg
2,6-xylidine/kg bw per day, 5 days per week for 13 weeks. Treatment-
related effects included decreased body weight gain (males at 310
mg/kg bw per day and females at 40 mg/kg bw per day and above),
increased absolute and relative liver weights (females at 160 mg/kg bw
per day and above; males at 310 mg/kg bw per day), leukopenia (males
at 40 mg/kg bw per day and above), haemoglobinaemia (males at
160 mg/kg bw per day and above) and anaemia (males at 310 mg/kg bw per
day). The NOEL was 20 mg/kg bw per day.
In a carcinogenicity study, male and female rats were fed diets
containing 2,6-xylidine at concentrations of 300, 1000 or 3000 mg/kg
food (equivalent to 15, 50 or 150 mg/kg bw per day). Significant
increases in the incidences of papillomas and carcinomas of the nasal
cavity were observed in high-dose males and females. There was a
significant dose-related increase in the incidence of adenomas in the
nasal cavity in both males and females. In addition, unusual rhabdo-
myosarcomas and malignant mixed tumours of the nasal cavity were
observed in the high-dose males and females. There was a dose-related
significant increase in the incidence of subcutaneous fibromas and
fibrosarcomas in both treated males and females. In females,
neoplastic nodules occurred in livers with a significant positive
trend and the increase was significant in the high-dose group by the
incidental tumour test. The Committee concluded that 2,6-xylidine was
carcinogenic in this study.
The International Agency for Research on Cancer has evaluated the
carcinogenic risk of 2,6-xylidine and has classified it as Group 2B
(possibly carcinogenic to humans).
In a teratogenicity study, xylazine was administered to pregnant
rats at doses of 1, 4 or 16 mg xylazine/kg bw per day on gestation
days 6 to 15. Treatment-related maternal effects included partial
closing of the eyelids, hypoactivity, ataxia, flat posture and
slightly reduced body weight gain in the high-dose group only. A
decrease in mean fetal weight was seen in the high-dose group. No
teratogenic effects were noted in this study. The NOEL for maternal
and fetal effects was 4 mg/kg bw per day.
Xylazine has been tested in reverse mutation assays in
Salmonella, a forward mutation assay in cultured mammalian cells and
in an in vivo cytogenetic assay. In Salmonella, weak positive
results were obtained. Negative results were observed in a forward
mutation assay on cultured mammalian cells and in a mouse bone marrow
micronucleus test. The Committee concluded that xylazine is weakly
mutagenic.
2,6-Xylidine was tested in a series of in vitro and in vivo
genotoxic assays. It was weakly positive for reverse mutation in
Salmonella. In mammalian cells, it induced forward mutation and was
positive in a sister chromatid exchange test. Inconclusive results
were obtained in a mouse bone marrow micronucleus test because there
was no assurance that the bone marrow had been adequately exposed.
2,6-Xylidine was found to be inactive in an in vivo-in vitro rat
hepatocyte unscheduled DNA synthesis assay. Covalent binding of the
compound to DNA was observed in rats. The Committee concluded that
2,6-xylidine is genotoxic.
The potential for 2,6-xylidine to induce methaemoglobinaemia was
reviewed by the Committee. Single doses of 30 mg 2,6-xylidine/kg bw
intravenously or 164 mg/kg bw N-acetyl-2,6-xylidine orally have
been shown to induce methaemoglobinaemia in cats but not in dogs.
2,6-Xylidine has also been shown to be a product of lidocaine
metabolism in humans. Methaemoglobin and 2,6-xylidine-haemoglobin
adduct levels have been shown to increase in human cardiac patients
receiving lidocaine treatment.
Effects of xylazine on humans poisoned following accidental or
intentional self-injection (0.7-15 mg/kg bw) or ingestion (7 mg/kg bw)
included symptoms of central nervous system depression, respiratory
depression, hypo- and hypertension, bradycardia, tachycardia,
ventricular arrhythmias, and transient hyperglycaemia.
4. EVALUATION
The Committee was unable to establish an ADI for xylazine because
it concluded that the 2,6-xylidine metabolite was genotoxic and
carcinogenic. Annex 4 lists the information that would be required for
further review.
5. ACKNOWLEDGMENTS
The preparer of the first draft would like to recognize the
following individuals for their assistance and contributions to the
preparation of the first draft:
Ms. Deborah Brooks, information specialist, Center for Veterinary
Medicine
Dr. Steve Brynes, residue chemist, Center for Veterinary Medicine
Dr. Jennifer Burris, veterinary pathologist, Center for Veterinary
Medicine
Dr. Robert Condon, biostatistician, Center for Veterinary Medicine
Dr. Haydee Fernandez, toxicologist, Center for Veterinary Medicine
Dr. Devaraya Jagannath, genetic toxicologist, Center for Veterinary
Medicine
Dr. Alan Pinter, toxicologist, National Institute of Public Health,
Budapest, Hungary
Dr. Leonard Schechtman, genetic toxicologist, Center for Veterinary
Medicine
6. REFERENCES
Amer, A.A. & Misk, N.A. (1980). Rompun in goats with special reference
to its effect on the cerebral spinal fluid (c.s.f.). Vet. Med. Rev.,
2, 168-174.
Bauman, E. K. & Nelson, D.L. (1969). Toxicity of BAY Va 1470 to cats.
Unpublished report No. 24208 from Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Benson, G.J., Thurmon, J.C., Neff-Davis, C.A., Corbin, J.E.,
Davis, L.E., Wilkinson, B., & Tranquilli, W.J. (1984). Effect of
xylazine hydrochloride upon plasma glucose concentrations in adult
pointer dogs. J. Am. Anita. Hosp. Assoc., 20, 791-794.
Bolte, S. & Stupariu, A (1978). Motility of the rumen in cattle and
sheep under the influence of neuroleptics and analgesics, particularly
xylazine, propionylpromazine and diazepam. Lucrari Stiintifice
Inatitutul Agron. Timisoara, Ser. Med. Vet., 15, 157-167.
Booth, N.H. (1988). Nonnarcotic analgesics. In: Booth N.H.&
McDonald E. (eds.), Veterinary Pharmacology and Therapeutics, 6th
edition, Iowa State University Press, Ames, Iowa, pp. 351-359.
Brendler-Schwaab, S. (1994). Rompun hydrochloride. Mutagenicity study
for the detection of induced forward mutations in the V79-HGPRT assay
in vitro. Unpublished report No. T9049349 from Bayer AG, Fachbereich
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bryant, M.S., Simmons, H.F., Harrell, R.E., & Hinson, J.A. (1994).
2,6-Dimethylaniline-hemoglobin adducts from lidocaine in humans.
Carcinogenesis, 15(10), 2287-2290.
Carruthers, S.G., Nelson, M., Wexler H.R., & Stiller, C.R. (1979).
Xylazine hydrochloride (Rompun) iverdise in man. Clin. Toxicol.,
15(3), 281-285.
Carter, S.W., Robertson, S.A., Steel, C.J., & Jourdenais, D.A. (1990).
Cardiopulmonary effects of xylazine sedation in the foal.
Equine Vet. J., 22(6), 384-388.
Colby, E.D., McCarthy, L.E., & Borison, H.L. (1981). Emetic action of
xylazine on the chemoreceptor trigger zone for vomiting in cats.
J. Vet. Pharmacol. Ther., 4, 93-96.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970a). The toxicity of
BAY Va 1470 2% injectable to dogs and cats given at ten times
recommended dose. Unpublished report No. 28139 from Research
Department, Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970b). The toxicity of
BAY Va 1470 10% injectable alone and in combination with (R)NEGUVON*
soluble powder (batch No. 0050337) to horses. Unpublished report
No. 28136 from Research Department, Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Cullen, L.K. & Jones, R.S. (1977). Clinical observations on
xylazine/ketamine anaesthesia in the cat. Vet. Rec., 101, 115-116.
Demoor, A. & Desmet, P. (1971). Effect of Rompun on acid-base
equilibrium and arterial oxygen pressure in cattle. Vet. Med. Rev.,
2-3, 163-169.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1968). Experimental tests on animals with radioactive labeled BAY Va
1470. Unpublished report No. 22874 from Bayer Isotope Institute,
Elberfeld Branch. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1969). Experiments using radioactively tagged BAY Va 1470 on rats.
Berl. Münch. Tierärztl. Wochenschr., 82, 104-109.
Eichner, R.D., Prior, R.L., & Kvasnicka, W.G (1979). Xylazine-induced
hyperglycemia in beef cattle. Am. J. Vet. Res., 40(1), 127-129.
Feldberg, W. & Symonds, H.W. (1980). Hyperglycemic effect of xylazine.
J. Vet. Pharmacol. Ther., 3, 197-202.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Freire, A.C.T, Gontijo, R.M., Pessoa, J.M., & Souza, R. (1981). Effect
of xylazine on the electrocardiogram of sheep. Br. Vet. J.,
137, 590-595.
Gallanosa, A.G., Spyker, D.A., Shipe, J.R., & Morris, D.L. (1981).
Human xylazine overdose: A comparative review with clonidine,
phenothiazines, and tricyclic antidepressants. Clin. Toxicol.,
18(6), 663-678.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon C., Bloom, A.D., Nakamura, F., Ahmed, N., Duk, S., Rimpo, J.,
Margolin, B.H., Resnick, M.A., Anderson, B., & Zeiger, E. (1987).
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10), 1-175
Garcia-Villar, R., Toutain, P.L., Alvinerie, M., & Ruckenbusch Y.
(1981). The pharmacokinetics of xylazine hydrochloride: an
interspecific study. J. Vet. Pharmacol. Ther., 4, 87-92.
Gross, M.E. & Tranquilli, W.J. (1989). Use of alpha-2-adrenergic
receptor antagonists. J. An. Vet. Med. Assoc., 195(3), 378-381.
Herbold, B. (1984). Salmonella/microsome test to evaluate for
point-mutagenic effects. Unpublished report No. T0016805 from Bayer
Sparte Pharma. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995). Rompun Hydrochloride. Micronucleus test on the
mouse. Unpublished report No. T9058169 + T2058171 from Bayer AG
Fachbereich Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hikasa, Y. Takase, K., & Ogasawara, S. (1989). Evidence for the
involvement of alpha-2-adrenoceptors in the emetic action of xylazine
in cats. Am. J. Vet. Res., 50, 1348-1351.
Hikasa, Y., Takase, K., Osada, T., Takamatsu, H., & Ogasawara, S.
(1987). Xylazine-induced vomiting in dogs: elimination by ablation of
the area postrema and blockade by yohimbine. Zent. bl. Vet. med.,
A34(2), 154-158.
Holmes, A.M. & Clark, W.T. (1977). Xylazine sedation of horses.
N.Z. Vet. J., 25, 159-161.
Hsu, W.H. & Hummel, S.K. (1981). Xylazine-induced hyperglycemia in
cattle: a possible involvement of alpha-2-adrenergic receptors
regulating insulin release. Endocrinology, 109, 825-829.
Hsu, W.H. & McNeel, S.V. (1983). Effect of yohimbine on
xylazine-induced prolongation of gastrointestinal transit in dogs.
J. Am. Vet. Med. Assoc., 183(3), 297-300.
Hsu, W.H., Betts, D.H., & Lee, P. (1981). Xylazine-induced mydriasis:
possible involvement of a central postsynaptic regulation of
parasympathetic tone. J. Vet. Pharmacol. Ther., 4, 209-214.
Hsu, W.H., Hanson, C.E., Hembrough, F.B., & Schaffer, D.D. (1989).
Effects of idazoxan, tolazoline and yohimbine on xylazine induced
respiratory changes and central nervous system depression in ewes.
Am. J. Vet. Res., 50(9), 1570-1573.
IARC (1993). 2,6-Dimethylaniline (2,6-xylidine). IARC Monograph on the
Evaluation of Carcinogenic Risks to Humans: Occupational exposures of
hairdressers and barbers and personal use of hair colourants; some
hair dyes, cosmetic colourants, industrial dyestuffs and aromatic
amines, 57, 323-335.
Jacobson, K.H. (1972). Short communication. Acute oral toxicity of
mono- and di-alkyl ring-substituted derivatives of aniline.
Toxicol. Appl. Pharmacol., 22, 153-154.
Klide, A.M., Calderwood, H.W., & Simen L.R. (1975). Cardio-pulmonary
effects of xylazine in dogs. Am. J. Vet. Res., 36(7), 931-935.
Kugler-Steigmeier, M.E., Frierich, U., Graf, U., Lutz, W.K.,
Maier, P., & Schlatter, C. (1989). Genotoxicity of aniline derivatives
in various short-term tests. Mutat. Res., 211, 279-289.
Leblanc, P.H. & Eberhart, S.W. (1990). Cardiopulmonary effects of
epidurally administered xylazine in the horse. Equine Vet. J.,
22(6), 389-391.
Lewis, S., O'Callaghan, C.L.P., & Toghill, P.J. (1983). Clinical
curio: self medication with xylazine. Br. Med. J., 287, 1369.
Lindstrom, H.V., Hansen, W.H., Nelson, A.A., & Fitzhugh, O.G. (1963).
The metabolism of FD&C Red No. 1. II. The fate of 2,5- para-xylidine
and 2,6- meta-xylidine in rats and observations of the toxicity of
xylidine isomers. J. Pharmacol. Exp. Ther., 142, 257-264.
Lindstrom, H.V., Bowie, W.C., Wallace, W.C., Nelson, A.A., &
Fitzhugh, O.G. (1969). The toxicity and metabolism of mesidine and
pseudocumidine in rats. J. Pharmacol. Exp. Ther., 167(2), 223-234.
McLean, S., Murphy, B.P., Starmer, G.A., & Thomas, J. (1967).
Methaemoglobin formation induced by aromatic amines and amides.
J. Pharm. Pharmacol., 19, 146-154.
McLean, S., Starmer, G.A., & Thomas, J. (1969). Methaemoglobin
formation by aromatic amines. J. Pharm. Pharmacol., 21, 441-450.
Magnusson, G., Bodin, N.-O., & Hansson, E. (1971). Hepatic changes in
dogs and rats induced by xylidine isomers. Acta Pathol. Microbiol.
Scand., 79, 639-648.
Magnusson, G., Majeed, S.K., Down, W.H., Sacharin, R.M., & Jorgeson,
W. (1979). Hepatic effects of xylidine isomers in rats. Toxicology,
12, 63-74.
Mawdesley-Thomas, L.E. (1970). Pathology report of the subchronic
toxicity for dogs by oral administration of compound BAY Va 1470.
Unpublished report No. 26692 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L, Loh, E.K., Hamilton,
C.M., Bakke, J.P., & Splading, J.W. (1989). Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: testing of 24 compounds. Environ. Mol. Mutagen.,
13, 155-164.
Murphy, J.J. & Jacobs, K. (1975). Residues of ROMPUN and its
metabolites in cattle. Unpublished report No. 43814 from Chemagro
Agricultural Division, Mobay Chemical Corporation. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mutlib, A.E., Chui, Y.C., Young, L.M., & Abbott, F.S. (1992).
Characterization of metabolites of xylazine produced in vivo and
in vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos.,
20(6), 840-848.
Nelson, D.L., Bauman, E.K., Mosier, J.O., & Allen, A.D. (1968a). A
study of the effect of large doses of BAY Va 1470 to horses.
Unpublished report No. 23405 from Research and Development Department,
Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Nelson, D.L., White, R.G. Bauman, E.K., & Allen, A.D. (1968b). The
effect of large injected doses of BAY Va 1470 to dogs. Unpublished
report No. 23679 from Research and Development Department, Chemagro
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Parton, J.W., Probst, G.S., & Garriott, M.L. (1988). The in vivo
effects of 2,6-xylidine on induction of micronuclei in mouse bone
marrow cells. Mutat. Res., 206, 281-283.
Parton, J.W., Beyers, J.E., Garriott, M.L., & Tamura, R.N. (1990). The
evaluation of multiple dosing protocol for the mouse bone-marrow
micronucleus assay using benzidine and 2,6-xylidine. Mutat. Res.,
234, 165-168.
Poklis, A., MacKell, M.A., & Case, M.E.S. (1985). Xylazine in human
tissues and fluids in a case of fatal drug abuse. J. Anal. Toxicol.,
9, 234-236.
Ponder, S.W. & Clark, W.G. (1980). Prolonged depression of
thermoregulation after xylazine administration to cats.
J. Vet. Pharmacol. Ther., 3, 203-207.
Pütter, J. & Sagner, G. (1973). Chemical studies to detect residues of
xylazine hydrochloride. Vet. Med. Rev., 2, 145-159.
Reynolds, S.M. (1994). Xylazine hydrochloride: teratology study in the
rat. Unpublished report No. 94/BAG240/0268 from Pharmaco LSR.
Submitted to WHO by Bayer AG, Leverkusen, Germany (GLP statement and
report summary, only).
Robertson, S.A., Carter, S.W., Donovan, M., & Steele, C. (1990).
Effects of intravenous xylazine hydrochloride on blood glucose, plasma
insulin and rectal temperature in neonatal foals. Equine Vet. J.,
22(1), 43-47.
Ruckebusch, Y. & Toutain, P.L. (1984). Specific antagonism of xylazine
effects on reticulo-rumen motor function in cattle. Vet. Med. Rev.,
1, 1-12.
Rudd, C.J., Mitchell, A.D., & Spalding, J. (1983). L5178Y Mouse
lymphoma cell mutagenesis assay of coded chemicals incorporating
analyses of the colony size distributions (Abstract No. Cd-19).
Environ. Mutagen., 5(3), 419.
Samanta, A., Roffe, C., & Woods, K.L. (1990). Accidental self
administration of xylazine in a veterinary nurse. Postgrad. Med. J.,
66, 244-245.
Sagner, G. (1967). Bay Va 1470, anaesthetic, analgesic and sedative
for veterinary medicine. Unpublished report No. 1470 from Bayer AG
Institute of Pharmacology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sagner, G., Hoffmeister, F., & Kroneberg, G. (1969). Pharmacological
principles of a new preparation for analgesia, sedation and relaxation
in veterinary medicine (Bay Ve 1470). Vet. Med. Rev., 3, 226-228.
Short, C.R., King, C., Sistrunk, P.W., & Kerr, K.M. (1983). Subacute
toxicity of several ring-substituted dialkylanilines in the rat.
Fundam. Appl. Toxicol. 3, 285-292.
Short, C.R., Joseph, M., & Hardy, M.L. (1989). Covalent binding of
[14C]-2,6-dimethylaniline to DNA of rat liver and ethmoid turbinate.
J. Toxicol. Environ. Health, 27, 85-89.
Singh, J., Peshin, P.K., Singh, A.P., & Nigam, J.M. (1983).
Haemo-dynamic, acid base and blood gas alterations after xylazine
administration in calves. Indian J. Vet. Surg., 4, 10-15.
Skarda, R.T., Jean, G., & Muir, W.W. (1990). Influence of tolazoline
on caudal epidural administration of xylazine in cattle. Am. J. Vet.
Res., 51(4), 556-560.
Spoerke, D.G., Hall, A.H., Grimes, M.J., Honea, B.N., & Rumack, B.H.
(1986). Human overdose with veterinary tranquilizer xylazine.
Am. J. Emerg. Med., 4, 222-224.
Swift, B.L. (1977). A technique for the surgical removal of the spleen
in calves. Vet. Med.-Small Anim. Clin., January, 77-79.
Symonds, W.H. (1976). The effect of xylazine on hepatic glucose
production and blood flow rate in the lactating dairy cow.
Vet. Rec., 99, 234-236.
Takase, I., Terada, H., & Fujii, T. (1976) Xylazine residues in organs
and tissues of calves and milk of cows. Unpublished report No. 76/
8278a from the Department of Veterinary Science, Faculty of
Agriculture, Tokyo University of Agriculture and Technology. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tettenborn, D. (1969). BAY Va 1470. Subchronic toxicity for dogs
during application as feed additive. Unpublished report No. 26692
(1731) from Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968a). BAY Va 1470. Chronic toxicity to
rats from oral administration. Unpublished report No. 23414 (956) from
Bayer AG, Institute of Pharmacology and Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968b). BAY Va 1470. Subchronic toxicity
to dogs from oral administration. Unpublished report No. 23415 (958)
from Bayer AG, Institute of Pharmacology and Toxicology. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thurmon, J.C., Neff-Davis, C., Davis, L.E., Stoker, R.A., Benson,
G.J., & Lock, T.F. (1982). Xylazine hydrochloride-induced
hyperglycemia and hypoinsulinemia in thoroughbred horses. J. Vet.
Pharmacol. Ther., 5, 241-245.
Thurmon, J.C., Steffey, E.P., Zinkl, J.G., Waliner, M., & Howland, D.
(1984). Xylazine causes transient dose-related hyperglycemia and
increased urine volume in mares. Am. J. Vet. Res., 45(2), 224-227.
Trossmann, G. & Hobik, H.P. (1970). BAY Va 1470. Histopathological
changes in organs of rats after feeding for 32 weeks. Unpublished
report No. 28576 (956) from Bayer AG, Institute of Pathology and
Histology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
US National Toxicology Program (1990). Toxicology and Carcinogenesis
Studies of 2,6-Xylidine (2,6-Dimethylaniline) (CAS No. 87-62-7) in
Charles River CD Rats (Feed Studies). US National Toxicology Program,
Research Triangle Park, NC, USA (NTP Technical Report No. 278;
NIH Publication No. 90-2534).
Vernot, E.H., Macewen, J.D., Haun, C.C., & Kinkead, E.R. (1977). Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol.,
42, 417-423.
Wagner, A.E., Muir, W.W., & Hinchcliff, K.W. (1991). Cardio-vascular
effects of xylazine and detomidine in horses. Am J. Vet. Res.,
52(5),:651-657.
Wasak, A. (1983). Haemetological and electrocardiographical changes in
dogs after xylazine. Med. Weter., 39, 235-237.
Weiss, L.D., Generalovich, T., Heller, M.B., Paris, P.M., Stewart,
R.D., Kaplan, R.M., & Thompson, D.R. (1987). Methemoglobin levels
following intravenous lidocaine administration. Ann. Emerg.
Med., 16(3), 323-325.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988). Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 11(12), 1-158.
2.2 Toxicological studies
Because 2,6-xylidine is also a chemical intermediate used in
dyes, a component of tobacco smoke and a degradation product of
aniline-based pesticides, its toxicology has been studied extensively.
Toxicological studies conducted with this compound were also reviewed
and will be presented in addition to the review and results of
toxicological studies of xylazine.
2.2.1 Acute toxicity studies of xylazine and 2,6-xylidine
The acute systemic toxicity of xylazine has been investigated in
both laboratory and domestic species. It is generally recognized that
ruminants are much more sensitive than most other species to the
pharmacological and toxicological effects of xylazine.
Results of LD50 studies of xylazine and 2,6-xylidine are
summarized in Table 3.
2.2.1.1 Acute toxicity of xylazine in dogs
Adult dogs (four males, four females) and cats (two males, four
females) were administered a single i.m. or i.v. dose of 22 mg
xylazine/kg bw (10 times the recommended therapeutic dose). One cat
out of three receiving the i.v. dose died, and two dogs out of four
receiving the i.m. dose died. All others recovered from convulsions,
unconsciousness and respiratory depression with no apparent
after-effects. The authors concluded that xylazine was slightly toxic
in this study (Crawford et al., 1970a).
Table 3. Results of acute toxicity studies on xylazine and 2,6-xylidine
Species Sex1 Route LD50 Reference
(mg/kg bw)
Xylazine
Rat NA p.o. 130 Sagner, 1967
Cat male & female2 s.c. 100-110 Bauman & Nelson, 1969
Dog male & female3 i.m 47 Nelson et al., 1968b
Dog 4 male & 3 female i.v 20-25 Nelson et al., 1968b
Horses NA4 i.m. 60-705 Nelson et al., 1968a
i.v. 15-285
2,6-Xylidine
Mouse male p.o. 710 Vernot et al., 1977
Rat p.o. 2042 Lindstrom et al., 1969
male p.o. 840 Jacobson, 1972
male p.o. 630 Short et al., 1983
male p.o. 1230 Vernot et al., 1977
female p.o. 1160 & 1270 US National Toxicology
Program, 1990
male p.o. 620-1250 &
1310
1 NA = Information not available
2 Number per sex not stated; 10 animals were used
3 Number per sex not stated; 17 animals were used
4 Sex of test animals was not stated; 5 animals were used
5 Minimum lethal dose
2.2.1.2 Acute toxicity of xylazine in horses
Adult horses were administered 11 mg xylazine/kg bw, i.v. (three
mares, one gelding) or 22 mg xylazine/kg bw, i.m. (two mares, two
geldings). One mare died following i.v. administration. All other test
animals recovered from treatment-related effects 24 hours following
i.v. administration and 48 hours following i.m. administration. The
authors concluded that the i.v. dose was slightly toxic and that the
i.m. dose produced no apparent toxicity in this study (Crawford
et al., 1970b).
2.2.2 Short-term toxicity studies
2.2.2.1 Xylazine
a) Rats
Xylazine was administered in the diet to Wistar rats (10/sex/
group) for 32 weeks. Dosages administered were 0, 50, 100, 250
or 500 mg/kg diet (equal to 0, 3, 6, 21 or 41 mg/kg bw per day for
males and 0, 4, 8, 19 or 45 mg/kg bw per day for females).
Haematology, urinalysis and gross and histopathological evaluations
were performed.
Decreases in body weight observed in females in the two
highest-dose groups (statistically significant (p<0.02) at 500 mg/kg
diet) were considered by the author to be treatment-related.
Microscopic examination of livers, lungs and kidneys revealed that
animals in all groups were diseased but no treatment-related pathology
was identified. Based on the dose-related decrease in weight gain
observed in females at 250 and 500 mg/kg diet, the NOEL in this study
was 100 mg/kg diet, equal to 6 mg/kg bw per day. The author regarded
the dose of 250 mg/kg diet as the non-toxic application rate. The
reliability of any NOEL derived from this study should be considered
questionable, owing to the presence of infection in all groups
(Tettenborn & Hobik, 1968a; Trossmann & Hobik, 1970).
b) Dogs
Dogs of undetermined breed or source were given xylazine orally
in gelatin capsules for 14-16 weeks at dose levels of 25, 50 or
100 mg/kg bw per day, 5 days/week. The low-dose group consisted of one
male and one female, the mid-dose group of two males and the high-dose
group of two males and two females. Haematology, clinical chemistry,
blood coagulation, urinalysis and postmortem gross and microscopic
evaluations were performed.
During week 8 of the test, one animal in the high-dose group died
and was replaced with a new animal. Postmortem gross findings in this
animal included diffuse reddening of the stomach and intestinal mucous
membrane.
Histopathological findings in livers (fatty degeneration and
necrosis) and kidneys (tubular epithelial necrosis and fat
accumulation) of the high-dose group were considered treatment-
related. Fatty deposits were noted in the liver and kidneys of the
low-dose female. These findings were attributed to parturition, which
occurred 3 weeks before the animal was killed. The author concluded
that the NOEL for this study was 50 mg/kg bw per day. The reliability
of any NOEL derived from this study should be considered questionable
due to the lack of a control group and small numbers of animals in
each test group (Tettenborn & Hobik, 1968b).
Beagle dogs (two/sex/group) were administered xylazine orally by
dietary admixture for 13 weeks. Dosages administered were 0, 10, 30 or
100 mg/kg diet (equal to 0, 0.3, 0.9 or 3 mg/kg bw per day). At the
beginning of the test the animals were approximately 8.5 months old
and weighed 7-10 kg. Parameters evaluated included general appearance,
ophthalmology, electrocardiography, haematology, clinical chemistry,
urinalyses and gross and microscopic pathology.
No treatment-related adverse effects were observed in any of the
parameters evaluated. The NOEL for this study was 3 mg/kg bw per day
(Tettenborn, 1969; Mawdesley-Thomas, 1970).
2.2.2.2 2,6-Xylidine
a) Rats
Three groups (nine or ten/group) of male Fischer-344 rats were
given oral (gavage) doses of 160 mg 2,6-xylidine/kg bw per day for 5,
10 or 20 days. The dosage administered was 25% of the estimated LD50
determined by the investigator. A significant increase in splenic
haemosiderosis (indicative of erythrocyte damage) after 20 days was
noted as a treatment-related effect in this study. Splenic congestion
and evidence for increased erythropoiesis were minimal
(Short et al., 1983).
Three groups of Sprague-Dawley rats (five/sex/group for controls,
low and mid dose; four/sex/group for high dose) were administered
0, 20, 100 or 500-700 mg 2,6-xylidine/kg bw per day by gavage for 4
weeks. Treatment-related effects included decreased weight gain,
decreased haemoglobin levels and hepatomegaly. In this study, the rat
appeared to be about 10 times less susceptible to hepatotoxicity of
2,6-xylidine than the dog (see section 2.2.2.2b) (Magnusson et al.,
1971; IARC, 1993).
Two groups of 8-week-old Sprague-Dawley rats (5/sex/group) were
orally administered (gastric intubation) a dose of 0 or 400 mg
2,6-xylidine per kg bw per day for 1 week immediately followed by a
daily dose of 0 or 500 mg/kg bw for 3 weeks. Decreased body weight
gain and hepatomegaly (most pronounced in centrilobular regions) were
noted as treatment-related effects. Electron microscopy of liver
tissue showed proliferation of hepatic smooth endoplasmic reticulum,
which was deemed responsible for the observed hepatomegaly in treated
rats. An increase in microsomal glucuronyltransferase was observed in
males while aniline hydroxylase levels were increased in females.
Decreases in liver glycogen and glucose-6-phosphatase activity were
also observed in the centrilobular regions of treated animals
(Magnusson et al., 1979).
Male Osborne-Mendel rats were administered up to 10 000 mg
2,6-xylidine per kg in the diet for 3-6 months. Treatment-related
effects included 25% weight reduction, anaemia, hepatomegaly with no
associated microscopic changes, splenic congestion and renal toxicity
(Lindstrom et al., 1963).
Groups of F-344/N rats (five/sex/group) were administered doses
of 0, 80, 160, 310, 620 or 1250 mg/kg bw of 2,6-xylidine in corn oil
by gavage 5 days/week for 2 weeks. Parameters evaluated included
clinical observations, body weight, urinalysis, haematology, blood pH
and carbon dioxide determinations, and gross postmortem findings.
Treatment-related deaths occurred at and above 620 mg/kg bw. All
animals in the highest dose group died before the end of the study. A
decrease of more than 10% in body weight was observed in males at and
above 310 mg/kg bw and in females at and above 160 mg/kg bw.
Generalized leukocytosis and an increase in the number of nucleated
red blood cells were observed in male rats administered 310 or
620 mg/kg bw. Slight anisocytosis, poikilocytosis and polychromasia of
the red blood cells occurred more frequently in dosed animals than in
vehicle control animals. Moderate poikilocytosis occurred at 310 mg/kg
bw and moderate polychromasia at 310 and 620 mg/kg bw. Slightly
macrocytic erythrocytes were observed at the two highest doses. Slight
anisocytosis, poikilocytosis and polychromasia were observed in female
rats at 310 and 620 mg/kg bw. The NOEL for this study was 80 mg/kg bw
per day (US National Toxicology Program, 1990).
Groups of F-344/N rats (10/sex/group) were given doses of 0, 20,
40, 80, 160 or 310 mg/kg bw of 2,6-xylidine in corn oil by gavage, 5
days/week for 13 weeks. Parameters evaluated included clinical
observations, haematology, urinalysis, serum chemistry and enzyme
analyses, gross and histopathological postmortem examinations.
A decrease in body weight gain of more than 10% occurred in males
and females in the highest dose group and in females at 40 and
160 mg/kg bw per day. In the highest dose group, relative liver
weights were significantly (P=0.003) increased for males and females.
Relative liver weight was also increased for males in the 160 mg/kg bw
group. The liver weight to brain weight and kidney weight to brain
weight ratios were significantly increased in females at 310 mg/kg bw
per day.
Treatment-related effects on haematology included significantly
decreased total leukocyte counts in males at doses of 40 mg/kg bw or
more. These were accompanied by decreases in the percentage of
lymphocytes and increases in the percentage of segmented neutrophils
at doses of 80 mg/kg bw or more. In males, haemoglobin levels were
significantly decreased at 160 and 310 mg/kg bw and erythrocyte and
haematocrit levels were decreased at 310 mg/kg bw. The NOEL for this
study was 20 mg/kg bw per day (US National Toxicology Program, 1990).
b) Dogs
Four groups of beagle dogs (one/sex/group) were given an oral
(gelatin capsule) dose of 0, 2, 10 or 50 mg 2,6-xylidine/kg bw per day
for 4 weeks. Treatment-related effects included vomiting (mid- and
high-dose groups), poor condition and decreased body weights
(high-dose group), hyperbilirubinaemia (mid- and high-dose groups),
hypoproteinaemia (mid-and high-dose groups) and fatty degenerative
changes in the liver that increased in severity with increasing dose
(Magnusson et al., 1971; IARC, 1993).
2.2.3 Long-term toxicity/carcinogenicity studies
2.2.3.1 Xylazine
No carcinogenicity studies have been performed with xylazine
2.2.3.2 2,6-Xylidine
Four groups of Charles River CRL:COBS CD (SD) BR rats
(56/sex/group) were fed diets containing 2,6-xylidine (99.06% pure) at
concentrations of 0, 300, 1000 or 3000 mg/kg diet (equivalent to
0, 15, 50 or 150 mg/kg bw per day) for 102 weeks. The animals assigned
to this study were F1a, generation weanlings from a multigeneration
study in which animals were fed diets containing 0, 300, 1000 or
3000 mg/kg 2,6-xylidine beginning at 5 weeks of age. Parameters
evaluated in the carcinogenicity study included clinical observations,
haematology, blood urea nitrogen, glucose, SGOT, alkaline phosphatase
and gross and microscopic postmortem examinations.
Treatment-related clinical effects included a decrease in mean
body weight gain in high-dose males and females (>10%). Mortality was
significantly (P < 0.001) increased (relative to controls) in males
in the high-dose group. Mortality was also increased for mid-dose
males. Survival at 105 weeks was 43/56, 40/56, 33/56 and 14/56 for
males in the control, low-, mid- and high-dose groups, respectively.
For females, survival was 33/56, 25/56, 32/56 and 24/56 for the
controls, low-, mid- and high-dose groups, respectively.
Microscopically, a significant increase in carcinoma of the nasal
cavity was observed in high-dose males (26/56; P < 0.001, life table
test). For females, the incidence of carcinomas of the nasal cavity
were 0/56, 0/56, 1/56 and 24/56 in the low-, mid- and high-dose
groups, respectively (P<0.001, life table test). Two adenocarcinomas
were diagnosed in high-dose males. The incidence of papillary adenomas
in males was 0/56 in controls, 0/56 in low-dose, 2/56 in mid-dose and
10/56 in high-dose rats (P=0.001, incidental tumour test). For
females, nasal adenomas occurred in 0/56 in controls, 0/56 in low-
dose, 1/56 in mid-dose and 6/56 in high-dose rats (P=0.02, incidental
tumour test). Several unusual neoplasms of the nasal cavity were also
considered to be related to treatment. These included one undifferen-
tiated sarcoma identified in one high-dose female, rhabdomyosarcomas
which occurred in two high-dose male and two high-dose females and
malignant mixed tumours having features associated with both adenocar-
cinoma and rhabdomyosarcoma were observed in one high-dose male and
one high-dose female rat. Non-neoplastic nasal cavity lesions included
acute inflammation (rhinitis), epithelial hyperplasia and squamous
metaplasia. These occurred at increased incidence (relative to
controls) in high-dose male and female rats. The incidence of
subcutaneous fibromas and fibrosarcomas combined in males was 0/56,
2/56, 2/56 and 5/56 for the control, low-, mid- and high-dose groups,
respectively (P=0.001, life table test; P<0.001 life table trend
test). For females, the incidence of these tumours combined was 1/56,
2/56, 2/56 and 6/56 for controls, low-, mid- and high-dose groups,
respectively ((P=0.01, life table trend test). Neoplastic nodules occurred
in livers of female rats with a significant positive trend. The
incidence was 0/56, 1/56, 2/56 and 4/55 for the controls, low-, mid-,
and high-dose groups, respectively (P=0.03, incidental test; P=0.012,
incidental trend test).
Treatment-related effects on haematology included decreases in
erythrocyte counts and haemoglobin levels at 18 months in the
high-dose males. Decreases in these parameters were also observed in
the mid- and high-dose females at 12 months. The author remarked that
these changes were not severe enough to be considered indicative of
anaemia.
The author concluded that under the conditions of this study,
2,6-xylidine was dearly carcinogenic for male and female Charles River
CD rats. This was based on the observed significant increases in the
incidence of adenomas and carcinomas of the nasal cavity. Additionally
the author stated that the increased incidence of subcutaneous
fibromas and fibrosarcomas in male and female rats and increased
incidence of neoplastic nodules of the liver in female rats could have
been treatment-related (US National Toxicology Program, 1990).
The International Agency for Research on Cancer (IARC) has
evaluated the carcinogenic risk of 2,6-xylidine to humans. The Working
Group concluded that there was inadequate evidence in humans but
sufficient evidence in experimental animals for the carcinogenicity of
2,6-xylidine. The IARC classified 2,6-xylidine as Group 2B (possibly
carcinogenic to humans) (IARC, 1993).
2.2.4 Special studies on teratogenicity
Xylazine was administered by gavage to groups of pregnant rats
(22 animals/group) on gestation days 6 to 15, then killed on day 20
for examination of uterine contents. Dosages administered were 0, 1, 4
or 16 mg/kg bw per day. The study was conducted in accordance with the
principles of Good Laboratory Practice Standards and Guidelines of the
OECD, United Kingdom, FDA and Japan.
Treatment-related maternal effects included partial closing of
the eyelids, underactivity, ataxia, flat posture and slightly reduced
body weight gain in the high-dose group only. Fetal effects included a
decrease in mean fetal weight in the high-dose group only. A
teratogenic potential of xylazine was not evident at levels up to and
including 16 mg/kg bw per day. The NOEL in this study was 4 mg/kg bw
per day (Reynolds, 1994).
2.2.5 Special studies on genotoxicity
The results of genotoxicity studies with xylazine and
2,6-xylidine are summarized in Table 4.
The results of bacterial mutagenicity testing of xylazine were
considered to be negative by the author. Reviewing the data, the
Committee concluded that a more than two-fold reproducible increase in
revertant colonies in tester strains TA1535 and TA1538 represents weak
mutagenic activity, even in the absence of a clear dose-response.
2.2.6 Special studies on methaemoglobin and haemoglobin adduct
formation with 2,6-xylidine
2.2.6.1 Cats and dogs
Cats and dogs (numbers not specified) were administered an i.v.
dose of 30 mg 2,6-xylidine/kg bw or an oral dose of 164 mg N-acetyl
2,6-xylidine/kg bw. 2,6-Xylidine induced a 10% methaemoglobinaemia in
cats, and N-acetyl 2,6-xylidine induced a 5% methaemoglobinaemia in
cats. Haemoglobin was unaffected in dogs in this study (McLean
et al., 1967).
Five adult cats (>24 months old) were administered an i.v. dose
of 30 mg 2,6-xylidine/kg bw. Blood samples were drawn at 1, 2, 3, 4
and 5 hours after dosing and analysed for methaemoglobin formation.
The mean methaemoglobin concentration determined from these sampling
intervals was 7% (range = 4.8% to 8.7%). Prior to treatment the mean
methaemoglobin concentration of the 152 cats used in this study was
approximately 1% (Mclean et al., 1969).
2.2.6.2 Humans
2,6-Xylidine-haemoglobin adduct levels have been found to be
elevated in human patients receiving lidocaine treatment for local
anaesthesia (1 mg/kg bw) or cardiac arrhythmias (up to 50 mg/kg bw,
i.v.). 2,6-Xylidine-haemoglobin adducts are also found in humans with
no known exposure to lidocaine. This is attributed to the 120-day
lifespan of the erythrocyte and chronic exposure to environmental or
iatrogenic sources of aromatic amines, e.g., cigarette smoke. The
levels of 2,6-xylidine-haemoglobin adducts found correspond to at an
estimated daily exposure (from iatrogenic and environmental sources)
of 23 µg (IARC, 1993; Bryant et al., 1994).
Methaemoglobinaemia induced by i.v. administration of lidocaine
was studied in 40 human cardiac patients. Treatment consisted of a
1 mg/kg bw i.v. bolus followed 15 minutes later with a 0.5 mg/kg bw
i.v. bolus. Patients were maintained between and after bolus doses
with an infusion rate of 1-4 mg lidocaine/min. Blood samples were
drawn before treatment and 1 and 6 hours after treatment. Although the
investigators found methaemoglobin levels in these patients to be
significantly elevated, the increase was not large enough to be of
clinical concern. The highest methaemoglobin level attained was 1.2%.
The author did not address the possible role of the 2,6-xylidine
metabolite in the observed increases in methaemoglobin levels in
treated patients (Weiss et al., 1987).
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine
Test system Test object Concentration Results Reference
Xylazine
In vitro
Reverse S. typhimurium
mutation1 TA1535, 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA1538 0.4-12 mg/plate Weak positive Herbold, 1984
(-S9)
TA100, 0.4-12 mg/plate Negative Herbold, 1984
TA98, 0.4-12 mg/plate Negative Herbold, 1984
TA1537 0.4-12 mg/plate Negative Herbold, 1984
Mammalian V79/HGPRT 62-1250 µg/ml Negative Brendler-
cell forward (-S9) Schwaab,
mutation1 2-40 µg/ml (+S9) 1994
In vivo
Cytogenetic Mouse bone 50 mg/kg bw, Negative Herbold, 1995
assay marrow i.p.2
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
2,6-Xylidine
In vitro
Reverse S. typhimurium
mutation1 TA1535 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
3 µmol/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al., 1988
TA100 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin el al.,
19804
0.1-10 mg/plate Neg (-S9), Zeiger et al.,
Pos (+S9) 19883
480-4000 Negative Kugler-
µg/plate Steigmeier
et al., 1989
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
TA1537 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1-10 mg/plate Negative Zeiger et al.,
1988
TA98 100-9900 Negative US National
µg/plate Toxicology
Program, 1990
360 µg/plate Negative Florin et al.,
19804
0.1 - 10 mg/plate Negative Zeiger et al.,
1988
Gene Mouse lymphoma Not given Positive Rudd et al.,
mutation1 L5178Y cells, 19837
tk locus
Sister Chinese 30-1500 µg/ml Positive Galloway et al.,
chromatid hamster 1987
exchange1 ovary cells
Table 4. Genotoxicity assays with xylazine and 2,6-xylidine (cont'd).
Test system Test object Concentration Results Reference
In vivo
Cytogenetic ICR mouse 350 mg/kg bw, Inconclusive6 Parton et al.,
assay bone marrow p.o. 1988
375 mg/kg bw, Inconclusive6 Parton et al.,
p.o. 1990
In vivo- Rat primary 40-850 mg/kg Negative Mirsails et al.,
in vitro DNA hepatocytes bw, p.o. 1989
repair assay
Covalent Rats 87.2 µCi Positive Short et al., 1989
DNA 14C-labelled
binding 2,6-xylidine/rat,
i.p5
1 Both with and without rat liver S9 fraction
2 Cyclophosphamide positive control
3 Weakly positive in two of three laboratories, negative in the third
4 Spot tests only
5 Pretreatment with unlabelled 262.5 mg/kg bw 2,6-xylidine daily for 9 days
6 Results suggest test article may not have reached target tissue (bone marrow)
7 Reference was an abstract and doses were not stated in that reference
2.3 Observations in humans
A 34-year-old man self-injected 10 ml of a 100 mg/ml solution of
xylazine intramuscularly. The estimated dose was 15 mg/kg bw. The
individual was discovered (30 minutes after retiring for bed) in a
deeply comatose, apnoeic and areflexic state. An empty Rompun
(xylazine) bottle (known to have contained 10 cc earlier) lay by his
side. He was immediately admitted to the hospital. Upon admission his
pupils were of moderate size and responded slowly to light. Other
findings included a blood pressure of 120/70 mmHg and heart rate of
60 bpm with stable sinus rhythm. Lactate dehydrogenase (LDH) activity
was elevated, with the LDH-1 isoenzyme predominating. Creatine
phosphokinase (CPK) activity was also elevated, particularly in the
CPK-3 and CPK-2 isoenzymes. These enzyme changes persisted for 5 to 7
days. Plasma glucose level was also elevated. Two days following
hospital admission sinus tachycardia developed, interspersed with runs
of multifocal premature ventricular contractions which were controlled
with lidocaine infusion. Blood pressure remained approximately 120/80
for the duration of hospitalization. Coma and respiratory depression
lasted 60 hours. The patient was discharged from the hospital 17 days
after admission.
The author noted that the patient might have died if he had not
been found shortly after the injection was administered, owing to the
marked respiratory depression that occurred. Hypotension, which has
been reported as an effect of xylazine in humans, did not occur in
this case. According to the author, the enzyme activity increases
indicated that myocardial muscle damage had occurred and an intrinsic
cardiotoxic effect of xylazine was suspected. Finally, in this case
the greatest threat to life was the CNS-depressant effect of xylazine
(Carruthers et al., 1979).
A 20-year-old woman ingested 400 mg of xylazine. Approximately 2
hours later she became drowsy, incontinent (urine), difficult to
arouse and occasionally unresponsive to verbal commands. She was
admitted to the hospital approximately 3 hours after the ingestion. In
a similar way to the case of human poisoning described by Carruthers
et al. (1979), she experienced a relatively low initial cardiac rate,
which later gradually increased, significant central nervous system
and respiratory depression, transient hyperglycaemia and ventricular
arrhythmias. However there was no evidence of myocardial damage. A
sample of this patient's urine was analysed using a gas chromatograph-
mass spectrometer computer system. Xylazine was found largely unchanged
in the urine as shown by a lack of any structurally related compounds
in the basic urine extract. No xylazine was found in a blood sample
taken at the time of admission. The author concluded that plasma
levels were below the limit of detection of the method (100 ng/ml).
The patient was discharged ambulatory and without apparent adverse
effects two days following admission to the hospital (Gallanosa
et al., 1981).
A 36-year-old man died following ingestion of alcohol and
clorazepate combined with an injection of approximately 40 ml of
xylazine (100 mg/ml). Xylazine was found in the decedent's blood,
brain, kidney, liver, lung, fat and urine at concentrations of 0.2,
0.4, 0.6, 0.9, 1.1, 0.05 and 7 ppm, respectively (Poklis et al.,
1985).
A 29-year-old woman self-injected 40 mg of xylazine
intramuscularly. The estimated dose was 0.73 mg/kg bw. Clinical
findings included disorientation, miosis, hypotension and bradycardia,
but no cardiac arrhythmias were noted. The abnormalities resolved
spontaneously (Spoerke et al., 1986).
A 37-year-old woman self-injected 24 ml (2400 mg) xylazine
intramuscularly. The estimated dose was 22 mg/kg bw. Twenty minutes
after the injection her blood pressure was 166/130 mmHg, heart rate
was 76 bpm and respirations 18 per minute. The serum glucose level was
175 mg/dl. Blood pressure later decreased to 130/90 mmHg and she
became apnoeic. No cardiac arrhythmias were observed during her 3 days
of hospitalization. Hypotension and bradycardia occurred two days
after the injection. The patient survived (Spoerke et al., 1986).
A 29-year-old woman self-injected an unknown amount of xylazine
intravenously. She became apnoeic and had an initial blood pressure of
130/90 mmHg with a pulse of 60 bpm. Serum glucose levels did not
exceed 90 mg/dl. Twenty-four hours after the injection the patient
experienced hypotension and bradycardia. Spontaneous respiration
resumed 18 hours after hospital admission. The patient recovered fully
(Spoerke et al., 1986).
A 19-year-old man accidentally injected himself subcutaneously
with 2 ml (100 mg/ml) of xylazine. The dose administered was 3 mg/kg
bw. Thirty minutes later he became difficult to rouse and was
hospitalized. Clinical findings included miosis, hyporeflexia,
hypotension, bradycardia, respiratory and central nervous system
depression and hyperglycaemia. He was treated with intravenous fluids
and assisted ventilation. Eight hours after hospitalization the
patient was alert and responsive. Twenty-four hours later he was
released (Samanta et al., 1990).
A 39-year-old woman was admitted to the hospital with symptoms of
tiredness, faintness and blurred vision. Clinical findings included
sinus bradycardia with a blood pressure of 130/90. Xylazine was found
in the urine and serum at concentrations of 1674 µg/litre and
30 µg/litre, respectively (Lewis et al., 1983).
3. COMMENTS
The Committee considered toxicological data on xylazine,
including the results of acute and short-term toxicity studies as well
as studies on pharmacodynamics, pharmacokinetics, reproductive and
developmental toxicity, genotoxicity and effects in humans. In
addition, toxicological studies on 2,6-xylidine, a metabolite of
xylazine, were reviewed; these included studies on acute and
short-term toxicity, carcinogenicity and genotoxicity.
Numerous pharmacological side-effects of xylazine have been
observed in treated animals, including mydriasis, impairment of
thermo-regulatory control, various effects on the cardiovascular
system, acid-base balance and respiration, hyperglycaemia, and
haematological and gastrointestinal effects. Cattle and sheep are
approximately 10 times more sensitive to xylazine than horses, dogs
and cats.
Rats were administered radiolabelled xylazine intravenously at
doses of 0.02 to 10 mg/kg bw or orally at doses of 0.02 to
100 mg/kg bw. More than 95% of the oral dose was absorbed, with a
half-life of approximately 5 minutes. Following oral or intravenous
administration, approximately 70% of the administered dose was
eliminated in urine and 30% in faeces. Renal excretion following oral
or intravenous administration was associated with a half-life of 2 to
3 hours. Enterohepatic circulation did not occur to a notable extent.
In cattle administered an intramuscular dose of 0.2 or 0.5 mg
xylazine/kg bw, less than 1% of the dose was excreted unchanged in the
urine, and the parent compound was detected in the urine up to 6 hours
following administration. Metabolites of xylazine were detected in
urine from these cattle up to 10 hours following administration.
Pharmacokinetic parameters following intravenous administration
showed minor variations between species. Xylazine disappeared rapidly
from plasma following intravenous administration, with an elimination
half-life of approximately 40 minutes in cattle and approximately 20
minutes in sheep. Xylazine could not be detected in the plasma of
cattle following intramuscular administration of a single therapeutic
dose.
In rats administered an intravenous dose of 2 mg/kg bw radio-
labelled xylazine, approximately 20 metabolites were quantified as
xylazine equivalents in urine and bile. The major metabolite comprised
35% of the administered dose. Approximately 8% of the dose was
eliminated as unchanged xylazine 24 hours after dosing. In an
in vitro study, 4 metabolites were identified when xylazine was
incubated with rat liver microsomes. The same metabolites were
identified in the urine of horses treated with xylazine. The major
metabolite in both cases was identified as N-(2,6-dimethylphenyl)
thiourea. In cattle administered an intramuscular dose of 0.2 mg
xylazine/kg bw (two cows) or 0.5 mg xylazine/kg bw (one cow),
2,6-xylidine was identified as a metabolite excreted in urine in both
conjugated and unconjugated forms.
The acute oral toxicities of xylazine and 2,6-xylidine were
tested in mice and rats. Xylazine was determined to be moderately
toxic (LD50 = 121-240 mg/kg bw) and 2,6-xylidine to be slightly
toxic (LD50 = 600-1000 mg/kg bw).
Three studies on the short-term toxicity of xylazine were
reviewed. A 32-week dietary study in rats and a 16-week oral
(capsules) study in dogs were considered inadequate for the
determination of the toxicity of xylazine owing to the use of
insufficient numbers, poor quality animals and inadequate study
design. The third was a 13-week oral study in beagle dogs fed
diets containing 0, 10, 30 or 100 mg/kg xylazine in the feed (equal
to 0.3, 0.9 or 3 mg/kg bw per day). No treatment-related effects
were observed in any of the treated groups.
In a two-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 80, 160, 310, 620 or 1250 mg
2,6-xylidine/kg bw per day, 5 days per week. Treatment-related effects
included increased mortality (all animals in the high-dose group
died), decreased body weight (males at 310 mg/kg bw per day and above
and females at 160 mg/kg bw per day and above) and various effects on
haematological parameters as indicated by leukocytosis and changes in
red blood cell parameters indicative of increased erythropoiesis
(males and females at 310 mg/kg bw per day and above). The NOEL in
this study was 80 mg/kg bw per day.
In a 13-week oral (gavage) toxicity study in rats with
2,6-xylidine, rats were dosed with 20, 40, 80, 160 or 310 mg
2,6-xylidine/kg bw per day, 5 days per week for 13 weeks. Treatment-
related effects included decreased body weight gain (males at 310
mg/kg bw per day and females at 40 mg/kg bw per day and above),
increased absolute and relative liver weights (females at 160 mg/kg bw
per day and above; males at 310 mg/kg bw per day), leukopenia (males
at 40 mg/kg bw per day and above), haemoglobinaemia (males at
160 mg/kg bw per day and above) and anaemia (males at 310 mg/kg bw per
day). The NOEL was 20 mg/kg bw per day.
In a carcinogenicity study, male and female rats were fed diets
containing 2,6-xylidine at concentrations of 300, 1000 or 3000 mg/kg
food (equivalent to 15, 50 or 150 mg/kg bw per day). Significant
increases in the incidences of papillomas and carcinomas of the nasal
cavity were observed in high-dose males and females. There was a
significant dose-related increase in the incidence of adenomas in the
nasal cavity in both males and females. In addition, unusual rhabdo-
myosarcomas and malignant mixed tumours of the nasal cavity were
observed in the high-dose males and females. There was a dose-related
significant increase in the incidence of subcutaneous fibromas and
fibrosarcomas in both treated males and females. In females,
neoplastic nodules occurred in livers with a significant positive
trend and the increase was significant in the high-dose group by the
incidental tumour test. The Committee concluded that 2,6-xylidine was
carcinogenic in this study.
The International Agency for Research on Cancer has evaluated the
carcinogenic risk of 2,6-xylidine and has classified it as Group 2B
(possibly carcinogenic to humans).
In a teratogenicity study, xylazine was administered to pregnant
rats at doses of 1, 4 or 16 mg xylazine/kg bw per day on gestation
days 6 to 15. Treatment-related maternal effects included partial
closing of the eyelids, hypoactivity, ataxia, flat posture and
slightly reduced body weight gain in the high-dose group only. A
decrease in mean fetal weight was seen in the high-dose group. No
teratogenic effects were noted in this study. The NOEL for maternal
and fetal effects was 4 mg/kg bw per day.
Xylazine has been tested in reverse mutation assays in
Salmonella, a forward mutation assay in cultured mammalian cells and
in an in vivo cytogenetic assay. In Salmonella, weak positive
results were obtained. Negative results were observed in a forward
mutation assay on cultured mammalian cells and in a mouse bone marrow
micronucleus test. The Committee concluded that xylazine is weakly
mutagenic.
2,6-Xylidine was tested in a series of in vitro and in vivo
genotoxic assays. It was weakly positive for reverse mutation in
Salmonella. In mammalian cells, it induced forward mutation and was
positive in a sister chromatid exchange test. Inconclusive results
were obtained in a mouse bone marrow micronucleus test because there
was no assurance that the bone marrow had been adequately exposed.
2,6-Xylidine was found to be inactive in an in vivo-in vitro rat
hepatocyte unscheduled DNA synthesis assay. Covalent binding of the
compound to DNA was observed in rats. The Committee concluded that
2,6-xylidine is genotoxic.
The potential for 2,6-xylidine to induce methaemoglobinaemia was
reviewed by the Committee. Single doses of 30 mg 2,6-xylidine/kg bw
intravenously or 164 mg/kg bw N-acetyl-2,6-xylidine orally have
been shown to induce methaemoglobinaemia in cats but not in dogs.
2,6-Xylidine has also been shown to be a product of lidocaine
metabolism in humans. Methaemoglobin and 2,6-xylidine-haemoglobin
adduct levels have been shown to increase in human cardiac patients
receiving lidocaine treatment.
Effects of xylazine on humans poisoned following accidental or
intentional self-injection (0.7-15 mg/kg bw) or ingestion (7 mg/kg bw)
included symptoms of central nervous system depression, respiratory
depression, hypo- and hypertension, bradycardia, tachycardia,
ventricular arrhythmias, and transient hyperglycaemia.
4. EVALUATION
The Committee was unable to establish an ADI for xylazine because
it concluded that the 2,6-xylidine metabolite was genotoxic and
carcinogenic. Annex 4 lists the information that would be required for
further review.
5. ACKNOWLEDGMENTS
The preparer of the first draft would like to recognize the
following individuals for their assistance and contributions to the
preparation of the first draft:
Ms. Deborah Brooks, information specialist, Center for Veterinary
Medicine
Dr. Steve Brynes, residue chemist, Center for Veterinary Medicine
Dr. Jennifer Burris, veterinary pathologist, Center for Veterinary
Medicine
Dr. Robert Condon, biostatistician, Center for Veterinary Medicine
Dr. Haydee Fernandez, toxicologist, Center for Veterinary Medicine
Dr. Devaraya Jagannath, genetic toxicologist, Center for Veterinary
Medicine
Dr. Alan Pinter, toxicologist, National Institute of Public Health,
Budapest, Hungary
Dr. Leonard Schechtman, genetic toxicologist, Center for Veterinary
Medicine
6. REFERENCES
Amer, A.A. & Misk, N.A. (1980). Rompun in goats with special reference
to its effect on the cerebral spinal fluid (c.s.f.). Vet. Med. Rev.,
2, 168-174.
Bauman, E. K. & Nelson, D.L. (1969). Toxicity of BAY Va 1470 to cats.
Unpublished report No. 24208 from Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Benson, G.J., Thurmon, J.C., Neff-Davis, C.A., Corbin, J.E.,
Davis, L.E., Wilkinson, B., & Tranquilli, W.J. (1984). Effect of
xylazine hydrochloride upon plasma glucose concentrations in adult
pointer dogs. J. Am. Anita. Hosp. Assoc., 20, 791-794.
Bolte, S. & Stupariu, A (1978). Motility of the rumen in cattle and
sheep under the influence of neuroleptics and analgesics, particularly
xylazine, propionylpromazine and diazepam. Lucrari Stiintifice
Inatitutul Agron. Timisoara, Ser. Med. Vet., 15, 157-167.
Booth, N.H. (1988). Nonnarcotic analgesics. In: Booth N.H.&
McDonald E. (eds.), Veterinary Pharmacology and Therapeutics, 6th
edition, Iowa State University Press, Ames, Iowa, pp. 351-359.
Brendler-Schwaab, S. (1994). Rompun hydrochloride. Mutagenicity study
for the detection of induced forward mutations in the V79-HGPRT assay
in vitro. Unpublished report No. T9049349 from Bayer AG, Fachbereich
Toxicology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Bryant, M.S., Simmons, H.F., Harrell, R.E., & Hinson, J.A. (1994).
2,6-Dimethylaniline-hemoglobin adducts from lidocaine in humans.
Carcinogenesis, 15(10), 2287-2290.
Carruthers, S.G., Nelson, M., Wexler H.R., & Stiller, C.R. (1979).
Xylazine hydrochloride (Rompun) iverdise in man. Clin. Toxicol.,
15(3), 281-285.
Carter, S.W., Robertson, S.A., Steel, C.J., & Jourdenais, D.A. (1990).
Cardiopulmonary effects of xylazine sedation in the foal.
Equine Vet. J., 22(6), 384-388.
Colby, E.D., McCarthy, L.E., & Borison, H.L. (1981). Emetic action of
xylazine on the chemoreceptor trigger zone for vomiting in cats.
J. Vet. Pharmacol. Ther., 4, 93-96.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970a). The toxicity of
BAY Va 1470 2% injectable to dogs and cats given at ten times
recommended dose. Unpublished report No. 28139 from Research
Department, Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Crawford, C.R., Nelson, D.L., & Anderson, R.H (1970b). The toxicity of
BAY Va 1470 10% injectable alone and in combination with (R)NEGUVON*
soluble powder (batch No. 0050337) to horses. Unpublished report
No. 28136 from Research Department, Chemagro Corporation. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Cullen, L.K. & Jones, R.S. (1977). Clinical observations on
xylazine/ketamine anaesthesia in the cat. Vet. Rec., 101, 115-116.
Demoor, A. & Desmet, P. (1971). Effect of Rompun on acid-base
equilibrium and arterial oxygen pressure in cattle. Vet. Med. Rev.,
2-3, 163-169.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1968). Experimental tests on animals with radioactive labeled BAY Va
1470. Unpublished report No. 22874 from Bayer Isotope Institute,
Elberfeld Branch. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Duhm, B., Maul, W., Medenwald, H., Patzschke, K., & Wegner, L.A.
(1969). Experiments using radioactively tagged BAY Va 1470 on rats.
Berl. Münch. Tierärztl. Wochenschr., 82, 104-109.
Eichner, R.D., Prior, R.L., & Kvasnicka, W.G (1979). Xylazine-induced
hyperglycemia in beef cattle. Am. J. Vet. Res., 40(1), 127-129.
Feldberg, W. & Symonds, H.W. (1980). Hyperglycemic effect of xylazine.
J. Vet. Pharmacol. Ther., 3, 197-202.
Florin, I., Rutberg, L., Curvall, M., & Enzell, C.R. (1980). Screening
of tobacco smoke constituents for mutagenicity using the Ames' test.
Toxicology, 15, 219-232.
Freire, A.C.T, Gontijo, R.M., Pessoa, J.M., & Souza, R. (1981). Effect
of xylazine on the electrocardiogram of sheep. Br. Vet. J.,
137, 590-595.
Gallanosa, A.G., Spyker, D.A., Shipe, J.R., & Morris, D.L. (1981).
Human xylazine overdose: A comparative review with clonidine,
phenothiazines, and tricyclic antidepressants. Clin. Toxicol.,
18(6), 663-678.
Galloway, S.M., Armstrong, M.J., Reuben, C., Colman, S., Brown, B.,
Cannon C., Bloom, A.D., Nakamura, F., Ahmed, N., Duk, S., Rimpo, J.,
Margolin, B.H., Resnick, M.A., Anderson, B., & Zeiger, E. (1987).
Chromosome aberrations and sister chromatid exchanges in Chinese
hamster ovary cells: evaluations of 108 chemicals. Environ. Mol.
Mutagen., 10(10), 1-175
Garcia-Villar, R., Toutain, P.L., Alvinerie, M., & Ruckenbusch Y.
(1981). The pharmacokinetics of xylazine hydrochloride: an
interspecific study. J. Vet. Pharmacol. Ther., 4, 87-92.
Gross, M.E. & Tranquilli, W.J. (1989). Use of alpha-2-adrenergic
receptor antagonists. J. An. Vet. Med. Assoc., 195(3), 378-381.
Herbold, B. (1984). Salmonella/microsome test to evaluate for
point-mutagenic effects. Unpublished report No. T0016805 from Bayer
Sparte Pharma. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Herbold, B. (1995). Rompun Hydrochloride. Micronucleus test on the
mouse. Unpublished report No. T9058169 + T2058171 from Bayer AG
Fachbereich Toxicology. Submitted to WHO by Bayer AG, Leverkusen,
Germany.
Hikasa, Y. Takase, K., & Ogasawara, S. (1989). Evidence for the
involvement of alpha-2-adrenoceptors in the emetic action of xylazine
in cats. Am. J. Vet. Res., 50, 1348-1351.
Hikasa, Y., Takase, K., Osada, T., Takamatsu, H., & Ogasawara, S.
(1987). Xylazine-induced vomiting in dogs: elimination by ablation of
the area postrema and blockade by yohimbine. Zent. bl. Vet. med.,
A34(2), 154-158.
Holmes, A.M. & Clark, W.T. (1977). Xylazine sedation of horses.
N.Z. Vet. J., 25, 159-161.
Hsu, W.H. & Hummel, S.K. (1981). Xylazine-induced hyperglycemia in
cattle: a possible involvement of alpha-2-adrenergic receptors
regulating insulin release. Endocrinology, 109, 825-829.
Hsu, W.H. & McNeel, S.V. (1983). Effect of yohimbine on
xylazine-induced prolongation of gastrointestinal transit in dogs.
J. Am. Vet. Med. Assoc., 183(3), 297-300.
Hsu, W.H., Betts, D.H., & Lee, P. (1981). Xylazine-induced mydriasis:
possible involvement of a central postsynaptic regulation of
parasympathetic tone. J. Vet. Pharmacol. Ther., 4, 209-214.
Hsu, W.H., Hanson, C.E., Hembrough, F.B., & Schaffer, D.D. (1989).
Effects of idazoxan, tolazoline and yohimbine on xylazine induced
respiratory changes and central nervous system depression in ewes.
Am. J. Vet. Res., 50(9), 1570-1573.
IARC (1993). 2,6-Dimethylaniline (2,6-xylidine). IARC Monograph on the
Evaluation of Carcinogenic Risks to Humans: Occupational exposures of
hairdressers and barbers and personal use of hair colourants; some
hair dyes, cosmetic colourants, industrial dyestuffs and aromatic
amines, 57, 323-335.
Jacobson, K.H. (1972). Short communication. Acute oral toxicity of
mono- and di-alkyl ring-substituted derivatives of aniline.
Toxicol. Appl. Pharmacol., 22, 153-154.
Klide, A.M., Calderwood, H.W., & Simen L.R. (1975). Cardio-pulmonary
effects of xylazine in dogs. Am. J. Vet. Res., 36(7), 931-935.
Kugler-Steigmeier, M.E., Frierich, U., Graf, U., Lutz, W.K.,
Maier, P., & Schlatter, C. (1989). Genotoxicity of aniline derivatives
in various short-term tests. Mutat. Res., 211, 279-289.
Leblanc, P.H. & Eberhart, S.W. (1990). Cardiopulmonary effects of
epidurally administered xylazine in the horse. Equine Vet. J.,
22(6), 389-391.
Lewis, S., O'Callaghan, C.L.P., & Toghill, P.J. (1983). Clinical
curio: self medication with xylazine. Br. Med. J., 287, 1369.
Lindstrom, H.V., Hansen, W.H., Nelson, A.A., & Fitzhugh, O.G. (1963).
The metabolism of FD&C Red No. 1. II. The fate of 2,5- para-xylidine
and 2,6- meta-xylidine in rats and observations of the toxicity of
xylidine isomers. J. Pharmacol. Exp. Ther., 142, 257-264.
Lindstrom, H.V., Bowie, W.C., Wallace, W.C., Nelson, A.A., &
Fitzhugh, O.G. (1969). The toxicity and metabolism of mesidine and
pseudocumidine in rats. J. Pharmacol. Exp. Ther., 167(2), 223-234.
McLean, S., Murphy, B.P., Starmer, G.A., & Thomas, J. (1967).
Methaemoglobin formation induced by aromatic amines and amides.
J. Pharm. Pharmacol., 19, 146-154.
McLean, S., Starmer, G.A., & Thomas, J. (1969). Methaemoglobin
formation by aromatic amines. J. Pharm. Pharmacol., 21, 441-450.
Magnusson, G., Bodin, N.-O., & Hansson, E. (1971). Hepatic changes in
dogs and rats induced by xylidine isomers. Acta Pathol. Microbiol.
Scand., 79, 639-648.
Magnusson, G., Majeed, S.K., Down, W.H., Sacharin, R.M., & Jorgeson,
W. (1979). Hepatic effects of xylidine isomers in rats. Toxicology,
12, 63-74.
Mawdesley-Thomas, L.E. (1970). Pathology report of the subchronic
toxicity for dogs by oral administration of compound BAY Va 1470.
Unpublished report No. 26692 from Huntingdon Research Centre,
Huntingdon, England. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Mirsalis, J.C., Tyson, C.K., Steinmetz, K.L, Loh, E.K., Hamilton,
C.M., Bakke, J.P., & Splading, J.W. (1989). Measurement of unscheduled
DNA synthesis and S-phase synthesis in rodent hepatocytes following
in vivo treatment: testing of 24 compounds. Environ. Mol. Mutagen.,
13, 155-164.
Murphy, J.J. & Jacobs, K. (1975). Residues of ROMPUN and its
metabolites in cattle. Unpublished report No. 43814 from Chemagro
Agricultural Division, Mobay Chemical Corporation. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Mutlib, A.E., Chui, Y.C., Young, L.M., & Abbott, F.S. (1992).
Characterization of metabolites of xylazine produced in vivo and
in vitro by LC/MS/MS and by GC/MS. Drug Metab. Dispos.,
20(6), 840-848.
Nelson, D.L., Bauman, E.K., Mosier, J.O., & Allen, A.D. (1968a). A
study of the effect of large doses of BAY Va 1470 to horses.
Unpublished report No. 23405 from Research and Development Department,
Chemagro Corporation. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Nelson, D.L., White, R.G. Bauman, E.K., & Allen, A.D. (1968b). The
effect of large injected doses of BAY Va 1470 to dogs. Unpublished
report No. 23679 from Research and Development Department, Chemagro
Corporation. Submitted to WHO by Bayer AG, Leverkusen, Germany.
Parton, J.W., Probst, G.S., & Garriott, M.L. (1988). The in vivo
effects of 2,6-xylidine on induction of micronuclei in mouse bone
marrow cells. Mutat. Res., 206, 281-283.
Parton, J.W., Beyers, J.E., Garriott, M.L., & Tamura, R.N. (1990). The
evaluation of multiple dosing protocol for the mouse bone-marrow
micronucleus assay using benzidine and 2,6-xylidine. Mutat. Res.,
234, 165-168.
Poklis, A., MacKell, M.A., & Case, M.E.S. (1985). Xylazine in human
tissues and fluids in a case of fatal drug abuse. J. Anal. Toxicol.,
9, 234-236.
Ponder, S.W. & Clark, W.G. (1980). Prolonged depression of
thermoregulation after xylazine administration to cats.
J. Vet. Pharmacol. Ther., 3, 203-207.
Pütter, J. & Sagner, G. (1973). Chemical studies to detect residues of
xylazine hydrochloride. Vet. Med. Rev., 2, 145-159.
Reynolds, S.M. (1994). Xylazine hydrochloride: teratology study in the
rat. Unpublished report No. 94/BAG240/0268 from Pharmaco LSR.
Submitted to WHO by Bayer AG, Leverkusen, Germany (GLP statement and
report summary, only).
Robertson, S.A., Carter, S.W., Donovan, M., & Steele, C. (1990).
Effects of intravenous xylazine hydrochloride on blood glucose, plasma
insulin and rectal temperature in neonatal foals. Equine Vet. J.,
22(1), 43-47.
Ruckebusch, Y. & Toutain, P.L. (1984). Specific antagonism of xylazine
effects on reticulo-rumen motor function in cattle. Vet. Med. Rev.,
1, 1-12.
Rudd, C.J., Mitchell, A.D., & Spalding, J. (1983). L5178Y Mouse
lymphoma cell mutagenesis assay of coded chemicals incorporating
analyses of the colony size distributions (Abstract No. Cd-19).
Environ. Mutagen., 5(3), 419.
Samanta, A., Roffe, C., & Woods, K.L. (1990). Accidental self
administration of xylazine in a veterinary nurse. Postgrad. Med. J.,
66, 244-245.
Sagner, G. (1967). Bay Va 1470, anaesthetic, analgesic and sedative
for veterinary medicine. Unpublished report No. 1470 from Bayer AG
Institute of Pharmacology. Submitted to WHO by Bayer AG,
Leverkusen, Germany.
Sagner, G., Hoffmeister, F., & Kroneberg, G. (1969). Pharmacological
principles of a new preparation for analgesia, sedation and relaxation
in veterinary medicine (Bay Ve 1470). Vet. Med. Rev., 3, 226-228.
Short, C.R., King, C., Sistrunk, P.W., & Kerr, K.M. (1983). Subacute
toxicity of several ring-substituted dialkylanilines in the rat.
Fundam. Appl. Toxicol. 3, 285-292.
Short, C.R., Joseph, M., & Hardy, M.L. (1989). Covalent binding of
[14C]-2,6-dimethylaniline to DNA of rat liver and ethmoid turbinate.
J. Toxicol. Environ. Health, 27, 85-89.
Singh, J., Peshin, P.K., Singh, A.P., & Nigam, J.M. (1983).
Haemo-dynamic, acid base and blood gas alterations after xylazine
administration in calves. Indian J. Vet. Surg., 4, 10-15.
Skarda, R.T., Jean, G., & Muir, W.W. (1990). Influence of tolazoline
on caudal epidural administration of xylazine in cattle. Am. J. Vet.
Res., 51(4), 556-560.
Spoerke, D.G., Hall, A.H., Grimes, M.J., Honea, B.N., & Rumack, B.H.
(1986). Human overdose with veterinary tranquilizer xylazine.
Am. J. Emerg. Med., 4, 222-224.
Swift, B.L. (1977). A technique for the surgical removal of the spleen
in calves. Vet. Med.-Small Anim. Clin., January, 77-79.
Symonds, W.H. (1976). The effect of xylazine on hepatic glucose
production and blood flow rate in the lactating dairy cow.
Vet. Rec., 99, 234-236.
Takase, I., Terada, H., & Fujii, T. (1976) Xylazine residues in organs
and tissues of calves and milk of cows. Unpublished report No. 76/
8278a from the Department of Veterinary Science, Faculty of
Agriculture, Tokyo University of Agriculture and Technology. Submitted
to WHO by Bayer AG, Leverkusen, Germany.
Tettenborn, D. (1969). BAY Va 1470. Subchronic toxicity for dogs
during application as feed additive. Unpublished report No. 26692
(1731) from Bayer AG, Institute of Toxicology. Submitted to WHO by
Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968a). BAY Va 1470. Chronic toxicity to
rats from oral administration. Unpublished report No. 23414 (956) from
Bayer AG, Institute of Pharmacology and Toxicology. Submitted to WHO
by Bayer AG, Leverkusen, Germany.
Tettenborn, D. & Hobik, H.P. (1968b). BAY Va 1470. Subchronic toxicity
to dogs from oral administration. Unpublished report No. 23415 (958)
from Bayer AG, Institute of Pharmacology and Toxicology. Submitted to
WHO by Bayer AG, Leverkusen, Germany.
Thurmon, J.C., Neff-Davis, C., Davis, L.E., Stoker, R.A., Benson,
G.J., & Lock, T.F. (1982). Xylazine hydrochloride-induced
hyperglycemia and hypoinsulinemia in thoroughbred horses. J. Vet.
Pharmacol. Ther., 5, 241-245.
Thurmon, J.C., Steffey, E.P., Zinkl, J.G., Waliner, M., & Howland, D.
(1984). Xylazine causes transient dose-related hyperglycemia and
increased urine volume in mares. Am. J. Vet. Res., 45(2), 224-227.
Trossmann, G. & Hobik, H.P. (1970). BAY Va 1470. Histopathological
changes in organs of rats after feeding for 32 weeks. Unpublished
report No. 28576 (956) from Bayer AG, Institute of Pathology and
Histology. Submitted to WHO by Bayer AG, Leverkusen, Germany.
US National Toxicology Program (1990). Toxicology and Carcinogenesis
Studies of 2,6-Xylidine (2,6-Dimethylaniline) (CAS No. 87-62-7) in
Charles River CD Rats (Feed Studies). US National Toxicology Program,
Research Triangle Park, NC, USA (NTP Technical Report No. 278;
NIH Publication No. 90-2534).
Vernot, E.H., Macewen, J.D., Haun, C.C., & Kinkead, E.R. (1977). Acute
toxicity and skin corrosion data for some organic and inorganic
compounds and aqueous solutions. Toxicol. Appl. Pharmacol.,
42, 417-423.
Wagner, A.E., Muir, W.W., & Hinchcliff, K.W. (1991). Cardio-vascular
effects of xylazine and detomidine in horses. Am J. Vet. Res.,
52(5),:651-657.
Wasak, A. (1983). Haemetological and electrocardiographical changes in
dogs after xylazine. Med. Weter., 39, 235-237.
Weiss, L.D., Generalovich, T., Heller, M.B., Paris, P.M., Stewart,
R.D., Kaplan, R.M., & Thompson, D.R. (1987). Methemoglobin levels
following intravenous lidocaine administration. Ann. Emerg.
Med., 16(3), 323-325.
Zeiger, E., Anderson, B., Haworth, S., Lawlor, T., & Mortelmans, K.
(1988). Salmonella mutagenicity tests: IV. Results from the testing
of 300 chemicals. Environ. Mol. Mutagen., 11(12), 1-158.
