
ANTHELMINTHIC AGENTS
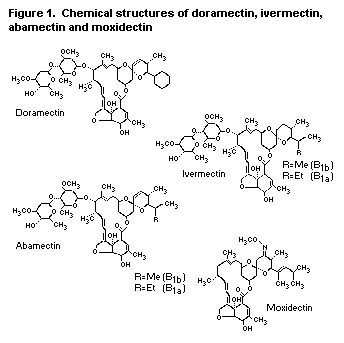
The Committee considered doramectin, which belongs to the
avermectin class of compounds, and moxidectin, which belongs to the
milbemycin class of compounds. Doramectin and moxidectin share a
number of structural similarities with each other and with abamectin
and ivermectin. Ivermectin was previously evaluated at the thirty-
sixth and fortieth meetings of the Committee (Annex 1, references 91
and 104). The structures of these compounds are shown in Fig. 1.
The Committee also considered the benzimidazoles febantel,
fenbendazole and oxfendazole, which were previously evaluated at the
thirty-eighth meeting (Annex 1, reference 97).
Figure 1. Chemical structures of doramectin, ivermectin,
abamectin and moxidectin
 DORAMECTIN
First draft prepared by
Dr G. Roberts
Toxicology Evaluation Section
Department of Human Services and Health
Canberra, Australia
Explanation
Biological data
Biochemical aspects
Absorption
Metabolism
Pharmacological effects
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Reproductive toxicity studies
Special studies on neonatal toxicity
Special studies on embryotoxicity and teratogenicity
Special studies on genotoxicity
Special studies on permeability of blood-brain barrier
Special studies on pharmacology
Comments
Evaluation
References
1. EXPLANATION
Doramectin is the fermentation product of a specific strain of
Streptomyces avermitilis that has close structural similarities with
abamectin and ivermectin. It is used as an endoparasitic agent in
non-lactating cattle. Doramectin had not been previously reviewed by
the Committee.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption
2.1.1.1 Rats
Groups of 5 Long-Evans rats were administered single doses of
doramectin as follows:
A - 3 mg/kg bw given over a 2-hour period in the diet
B - 5 mg/kg bw in methyl cellulose vehicle
C - 5 mg/kg bw in sesame oil vehicle
Peak drug levels were achieved in 3 h for groups A and B, and at
7 h for group C. Maximum levels were 0.06, 0.06 and 0.36 µg/ml,
respectively. The areas under the plasma concentration-time curves
(AUC) were 1.65, 1.71 and 9.69 µg-h/ml for groups A, B, and C,
respectively (Pfizer, 1987a).
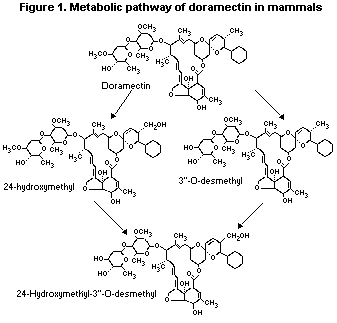
2.1.2 Metabolism
Doramectin labelled with tritium in the 5-position was
administered as a single dose to Sprague-Dawley rats (2 males given
5 mg/kg bw in propylene glycol:glycerol by gavage), a beagle dog
(1 female given 3.5 mg/kg bw in sesame oil by gavage) and cattle
(5 males given 0.2 mg/kg bw subcutaneously). The liver and faeces from
each species and the fat of cattle were sampled for characterization
of metabolites by liquid chromatography (Pfizer, 1992a).
In a second experiment, 2 male and 2 female cattle were given a
single s.c. injection of 0.2 mg/kg bw 3H-doramectin. Metabolites in
liver and fat were analyzed by liquid chromatography (Pfizer, 1993a).
As shown in Table 1, the metabolites detected in liver and faeces
from each species were similar in nature indicating comparable
degradation pathways in rats, dogs and cattle.
Table 1. Percent of tritium radiolabel recovered from sample.
24-hydroxymethyl-
Total Unchanged 3"-O-desmethyl 24-hydroxymethyl 3"-O-desmethyl
recovered doramectin doramectin doramectin doramectin
Sample (%) (%) (%) (%) (%)
Rat liver 37 18 12 3 2
Rat faeces na 22 19 14 16
Dog liver 51 28 12 nd nd
Dog faeces 46 6 8 5 4
Cattle liver 95 70 9 nd 7
Cattle faeces 75 24 14 5 4
Cattle fat * 79 68 nd nd nd
Cattle liver 82 49 5.7 3.6 6.6
Cattle fat # 82 75 nd nd nd
na not available
nd not detected
* 8% of radioactivity was associated with mixed fatty acid esters of 24-hydroxymethyl
doramectin but structural identification was not pursued.
# 6% of radioactivity was identified as the 2-epimer of doramectin.
The metabolism of doramectin in mammals is presented in Figure 1.
Figure 1. Metabolic pathway of doramectin in mammals
DORAMECTIN
First draft prepared by
Dr G. Roberts
Toxicology Evaluation Section
Department of Human Services and Health
Canberra, Australia
Explanation
Biological data
Biochemical aspects
Absorption
Metabolism
Pharmacological effects
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Reproductive toxicity studies
Special studies on neonatal toxicity
Special studies on embryotoxicity and teratogenicity
Special studies on genotoxicity
Special studies on permeability of blood-brain barrier
Special studies on pharmacology
Comments
Evaluation
References
1. EXPLANATION
Doramectin is the fermentation product of a specific strain of
Streptomyces avermitilis that has close structural similarities with
abamectin and ivermectin. It is used as an endoparasitic agent in
non-lactating cattle. Doramectin had not been previously reviewed by
the Committee.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption
2.1.1.1 Rats
Groups of 5 Long-Evans rats were administered single doses of
doramectin as follows:
A - 3 mg/kg bw given over a 2-hour period in the diet
B - 5 mg/kg bw in methyl cellulose vehicle
C - 5 mg/kg bw in sesame oil vehicle
Peak drug levels were achieved in 3 h for groups A and B, and at
7 h for group C. Maximum levels were 0.06, 0.06 and 0.36 µg/ml,
respectively. The areas under the plasma concentration-time curves
(AUC) were 1.65, 1.71 and 9.69 µg-h/ml for groups A, B, and C,
respectively (Pfizer, 1987a).
2.1.2 Metabolism
Doramectin labelled with tritium in the 5-position was
administered as a single dose to Sprague-Dawley rats (2 males given
5 mg/kg bw in propylene glycol:glycerol by gavage), a beagle dog
(1 female given 3.5 mg/kg bw in sesame oil by gavage) and cattle
(5 males given 0.2 mg/kg bw subcutaneously). The liver and faeces from
each species and the fat of cattle were sampled for characterization
of metabolites by liquid chromatography (Pfizer, 1992a).
In a second experiment, 2 male and 2 female cattle were given a
single s.c. injection of 0.2 mg/kg bw 3H-doramectin. Metabolites in
liver and fat were analyzed by liquid chromatography (Pfizer, 1993a).
As shown in Table 1, the metabolites detected in liver and faeces
from each species were similar in nature indicating comparable
degradation pathways in rats, dogs and cattle.
Table 1. Percent of tritium radiolabel recovered from sample.
24-hydroxymethyl-
Total Unchanged 3"-O-desmethyl 24-hydroxymethyl 3"-O-desmethyl
recovered doramectin doramectin doramectin doramectin
Sample (%) (%) (%) (%) (%)
Rat liver 37 18 12 3 2
Rat faeces na 22 19 14 16
Dog liver 51 28 12 nd nd
Dog faeces 46 6 8 5 4
Cattle liver 95 70 9 nd 7
Cattle faeces 75 24 14 5 4
Cattle fat * 79 68 nd nd nd
Cattle liver 82 49 5.7 3.6 6.6
Cattle fat # 82 75 nd nd nd
na not available
nd not detected
* 8% of radioactivity was associated with mixed fatty acid esters of 24-hydroxymethyl
doramectin but structural identification was not pursued.
# 6% of radioactivity was identified as the 2-epimer of doramectin.
The metabolism of doramectin in mammals is presented in Figure 1.
Figure 1. Metabolic pathway of doramectin in mammals
 2.1.3 Pharmacological effects
Avermectins induce rapid, non-spastic paralysis in nematodes and
arthropods. One common feature of avermectins appears to be the
modulation of trans-membrane chloride ion (Cl-) channel activity in
nematode nerve cells, and in both nerve and muscle cells of
arthropods. These Cl- channels may be gated by a variety of
neurotransmitter receptors including gamma-aminobutyric acid (GABA),
glutamate and acetylcholine (Kass et al, 1984; Duce & Scott, 1985;
Zufall et al, 1989). Activation of the Cl- channels by avermectins
leads to an increase in Cl- conductance which results in a changed
membrane potential and this causes inhibition of electrical activity
in the target nerve or muscle cell. GABA is also a major inhibitory
neurotransmitter in the mammalian CNS and avermectins do have
intrinsic activity on the mammalian GABA receptor/Cl- channel
complex.
Avermectins have been reported to bind to glycine receptor/Cl-
channel complexes which are restricted to the CNS in mammals
(Graham et al, 1982). Penetration of the blood brain barrier by
avermectins is extremely poor and this may account for the wide margin
of safety exhibited by these compounds following administration to
mammals.
2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with doramectin are
summarized in Table 2.
Clinical signs of toxicity were similar in both mice and rats and
by both routes of administration, the most common being decreased
activity and respiration, hunched position, shakiness, weakness,
tremors, ataxia and weight loss.
Doramectin was applied to normal and abraded skin of 3 New
Zealand white rabbits (0.5 g under an occlusive patch) for 24 h.
Slight erythema was observed 24 and 48 h post-dose at 1 of 3 intact
sites and 2 of 3 abraded sites. There was no edema at any site and all
sites appeared normal 72 h post-dose.
Table 2. Results of acute toxicity studies with doramectin.
Species
(strain) Route Vehicle1 Sex LD50 in mg/kg bw2 Reference
Mouse po aqueous M&F > 2000(0/3) Pfizer, 1988a
(CD-1)
po oil F 250(0/3)-500(3/3) Pfizer, 1992b
po oil F 75(0/5)-200(5/5) Pfizer, 1994a
ip aqueous M 700(0/3)-1000(2/3) Pfizer, 1988a
ip oil M 100(0/3)-250(3/3) Pfizer, 1992b
Rat po aqueous M 1000(0/3)-2000(3/3) Pfizer, 1988a
(Sprague-Dawley) F 500(0/3)-1000(2/3) Pfizer, 1988a
po oil M 50(0/3)-100(2/3) Pfizer, 1992b
F 100(1/3)-200(3/3) Pfizer, 1992b
ip aqueous M >300(1/3) Pfizer, 1988a
ip oil M 50(0/3)-100(3/3) Pfizer, 1992b
1 aqueous vehicle was 0.1% aqueous methylcellulose; oil vehicle was sesame oil.
2 figures in brackets represent mortality incidences.
Doramectin powder (18.8 mg) was instilled into the conjunctival
sac of one eye in each of 3 New Zealand white rabbits. Slight corneal
and conjunctival reddening, chemosis and iritis were seen within 1 h
of dosing. The changes subsided within 6 h and all eyes were normal by
48 h post-dose (Pfizer, 1991a).
A comparison of the acute toxicity of different ivermectins in
mice is given in Table 3.
Table 3. Acute oral toxicity of doramectin in comparison with abamectin, ivermectin
and moxidectin in female CD-1 mice (Pfizer, 1994a).
Dose in mg/kg bw1 Doramectin Abamectin Ivermectin Moxidectin
Maximum
"asymptomatic" dose 25 5 255
Minimum
"symptomatic" dose 75 25 5025
Minimum
lethal dose2 200(5/5) 75(5/5) 75(1/5) 75(4/5)
LD50 dose 75-200 25-75 >75 25-75
1 - vehicle was sesame oil
2 - figures in brackets represent mortality incidences.
Signs of CNS toxicity were similar after dosing with each
compound and included gait abnormalities with splayed hind limbs,
intermittent tremors, ataxia and decreased, irregular or laboured
respiration (Pfizer, 1994a).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of CD-1 mice (10/sex/dose) were administered doramectin
(purity 94.1%) in the diet for 43 days. Target doses were 0, 10, 20,
40, or 60 mg/kg bw/day on days 1 to 14; 0, 10, 20, 80 or 100 mg/kg
bw/day on days 15 to 28; and 0, 100, 200, 400 or 600 mg/kg bw/day
on days 29 to 43.
Toxic signs included lethargy, hunched posture and tremors at 400
and 600 mg/kg bw/day, and hunched and ungroomed appearance in some
mice at 100 and 200 mg/kg bw/day. A number of animals given 400 and
600 mg/kg bw/day were sacrificed moribund and the remaining mice in
these groups were killed on day 33. There were no effects on body
weight or food consumption. Relative liver weights were slightly
higher in most treated groups but the changes were minor and not
dose-related. Clinical laboratory parameters and pathological
examinations were not carried out.
Plasma drug levels were dose-related, although non-linear, up to
100 mg/kg bw/day, and reached a plateau at higher doses. Drug levels
ranged from 0.23 µg/ml at 10 mg/kg bw/day to 3.9 µg/ml at 600 mg/kg
bw/day. An unidentified degradation product, represented a
progressively greater fraction of drug-related material at doses of
100 mg/kg bw/day and above (Pfizer, 1994b).
Groups of CD-1 mice (10/sex/group) were fed doramectin (purity
94.1%) in the diet for 92 days. Target doses were 0, 100, 200 or
300 mg/kg bw/day. Actual doramectin intake was 83-121, 154-191 or
221-322 mg/kg bw/day. Tremors, hunched posture, unkempt appearance and
lethargy were observed at the mid- and high-dose groups and resulted
in death or moribund sacrifice of 9 mice at 300 mg/kg bw/day and 3
mice at 200 mg/kg bw/day. The remaining animals in these two groups
were killed on day 12 or 19, respectively. Body-weight gain was
depressed in association with reduced food consumption at 200 mg/kg
bw/day and above.
Serum creatinine and BUN were slightly increased at 100 mg/kg
bw/day with no effects at higher doses, presumably due to early
culling of mice in these groups. Haematological parameters were
unaffected. All treated groups had increased liver weights and
hypertrophy of centrilobular hepatocytes, with multinucleate
non-proliferative liver cells at 100 mg/kg bw/day only. Dead and
moribund animals showed lymphocyte lysis in lymphoid organs, cellular
depletion of bone marrow and necrosis of the adrenal cortex which may
have resulted from stress and weight loss.
Satellite groups of 3 mice/sex/dose were used to determine plasma
drug levels. In the 100 mg/kg bw/day group, concentrations reached a
plateau at 2.8 µg/ml by day 45. Doramectin levels were not markedly
different at higher doses with peaks of 3.6 µg/ml and 2.7 µg/ml at 200
and 300 mg/kg bw/day, respectively. An unidentified degradation
product was present at levels proportional to both dose and duration
of treatment (Pfizer, 1994c).
2.2.2.2 Rats
Groups of Long-Evans rats (3/sex/group) were given 0, 2.5, 5 or
10 mg/kg bw/day doramectin (purity unspecified) in the diet for 14
days. There were no overt signs of toxicity and no effects on body
weight, haematology, serum chemistry, urinalysis, liver or kidney
weight. Examination of a limited number of organs revealed no
pathological changes. Mean plasma drug levels at day 10 were
approximately 0.05, 0.06 and 0.17 µg/ml with increasing doses
(Pfizer, 1987b).
In a 1-month study, groups of Long-Evans rats (10/sex/group) were
fed doramectin (purity 95.3%) in the diet at dose levels of 0, 5, 10
or 20 mg/kg bw/day. There were no clinical signs of toxicity and no
effects on food intake or body-weight gain. Ophthalmology,
haematology, serum chemistry and urinalysis were unaffected. Liver
weight was slightly increased in males given 20 mg/kg bw/day but
pathological examination was unremarkable. Plasma drug levels were
proportional to the dose; maximum concentrations achieved were 0.28,
0.76 and 1.79 µg/ml, respectively. The NOEL in this study was 10 mg/kg
bw/day (Pfizer, 1988b).
Groups of Long-Evans rats (10/sex/group) were fed a diet
containing doramectin (purity 94.1%) at levels intended to give
target doses of 0, 20, 40, 60 or 80 mg/kg bw/day for 38 days.
Chromorhinorrhea, chromodacryorrhea and urogenital staining were seen
at 40 mg/kg bw/day and above. Whole body tremors were also present at
the two highest doses and resulted in the sacrifice of 2 rats at
60 mg/kg bw/day and all rats at 80 mg/kg bw/day. Body-weight gain and
food consumption were lower at 60 mg/kg bw/day and above. Serum
5'-nucleotidase was increased at 80 mg/kg bw/day with elevated levels
of BUN at 40 mg/kg bw/day and above. Liver weights were higher in the
40 and 60 mg/kg bw/day groups but with no dose relationship. Organs
were not examined for pathology.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin concentrations were progressively increased
throughout the study at each dose, and levels were dose-related up to
60 mg/kg bw/day with no further increase at 80 mg/kg bw/day. Highest
levels were 0.89, 3.52, 3.78 and 3.54 µg/ml at the 4 doses,
respectively (Pfizer, 1993b).
Groups of Long-Evans rats (5/sex/group) were administered by
gavage doses of 0, 2, 5 or 10 mg/kg/bw/day doramectin (purity 92.1%)
in sesame oil for 38 days. Three females in the 10 mg/kg bw/day group
were sacrificed after showing hunched appearance, ataxia, tremors and
urine stains. There were no effects on body weight, food consumption,
haematology, serum chemistry or urinalysis. Some rats given 5 and
10 mg/kg bw/day had increased liver weights but pathological
examination of a limited number of organs was unremarkable. Plasma
drug levels were similar on days 10 and 38 and were dose-related;
approximate levels were 0.5, 1.6 and 3.0 µg/ml in the low-, mid- and
high-dose groups, respectively (Pfizer, 1989a).
Groups of Long-Evans rats (10/sex/dose) were fed doramectin
(purity 89.7%-94.1%) in the diet at target doses of 0, 30, 40 or
50 mg/kg bw/day, for 92 days. Based on food consumption, actual
doramectin intake ranged from 16-32, 11-49 and 14-59 mg/kg bw/day,
respectively. Thus systemic exposure to doramectin was similar in each
treated group which was reflected in the toxicological findings.
Clinical signs, noted in all treated animals, included
chromorhinorrhea, chromodacryorrhea, urine stains and tremors. Seven
rats at 30 mg/kg bw/day, all rats at 40 mg/kg bw/day, and 19 rats at
50 mg/kg bw/day died or were sacrificed moribund. Body-weight gain was
markedly reduced in all treated groups in association with diminished
food intake. Ophthalmological examinations were unchanged. All treated
groups showed decreased leucocytes, erythrocytes, haemoglobin and
haematocrit, and increased BUN. Serum levels of ALAT and ASAT were
increased and protein decreased in some animals given 40 and 50 mg/kg
bw/day. Urinalysis was unaffected.
At necropsy, animals which failed to survive the treatment period
showed minimal body fat and a few had stomach erosions. Apparent
increases in relative kidney and testes weights in treated groups were
probably due to the lower body weights. Pathological changes, observed
in all treated groups, comprised kidney nephrosis with protein
accumulation in the corticomedullary junction, hepatocyte atrophy with
occasional necrotic cells and haemosiderosis, lymphoid depletion in
thymus, spleen and mesenteric lymph node, bone marrow atrophy, and
lipid depletion in the adrenal cortex.
Satellite groups of 5 rats/sex/dose were treated in order to
determine plasma drug levels. Doramectin plasma concentrations
increased during the course of the study in the 30 and 40 mg/kg bw/day
groups, attaining levels of 4.6 and 5.8 µg/ml, respectively. A maximum
level of 4.1 µg/ml was achieved by day 16 in rats given 50 mg/kg
bw/day (Pfizer, 1994d).
Groups of Long-Evans rats (20/sex/dose) were selected from the
F1 offspring in a multigeneration reproductive toxicity study in
which doramectin was administered by gavage at doses of 0, 0.1, 0.3 or
1.0 mg/kg bw/day. Thus the animals had received secondary exposure in
utero and during lactation, and direct exposure for 3 to 4 weeks
post-weaning. Subsequently, the respective groups were administered
gavage doses of 0, 0.5, 2 or 8 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil for 3 months.
There were no overt signs of toxicity, mortality or treatment-
related ocular lesions. Initial body weights of the 8 mg/kg bw/day
group were lower which may be attributed to the prior in utero
or neonatal exposure to doramectin. During the course of the study
proper, treated groups gained more weight than controls. Food
consumption, haematology, serum chemistry and urinalysis were
unaffected. Liver and kidney weights were increased at 8 mg/kg bw/day
but there were no concomitant pathological alterations.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin levels were dose-related with mean levels
of 0.07, 0.4 and 2.5 µg/ml on day 3, and 0.1, 0.7 and 3.2 µg/ml on day
87, in the low-, mid-, and high-dose groups, respectively. The NOEL in
this study was 2 mg/kg bw/day (Pfizer. 1990a).
2.2.2.3 Dogs
Groups of beagle dogs (1/sex/group) were fed doramectin (purity
unspecified) in the diet at dose levels of 0, 0.5, 1 or 2 mg/kg bw/day
for 14 days. There were no signs of toxicity or effects on body
weight, haematology, serum chemistry or urinalysis. Dogs were not
sacrificed. Plasma drug levels increased during the course of the
study attaining concentrations of approximately 0.045, 0.07 and
0.26 µg/ml, respectively on day 8 (Pfizer, 1987c).
Groups of beagle dogs (1/sex/group) were given gavage doses of
0, 1, 2 or 4 mg/kg bw/day doramectin (purity 92.1%-93.3%) in sesame
oil for 29 days. Body weight was depressed in both animals
administered 4 mg/kg bw/day and the male showed salivation, mydriasis,
tremors, ataxia and decreased heart rate prior to sacrifice on day 16.
All drug-treated dogs exhibited mydriasis. There were no effects on
haematology, serum chemistry, urinalysis, organ weight or pathological
examinations. Plasma drug levels were proportional to the dose,
highest concentrations achieved were approximately 0.4, 1.2 and
3.0 µg/ml, respectively (Pfizer, 1989b).
Groups of beagle dogs (3/sex/group) were administered doramectin
(purity 95.3%) in the diet at doses of 0, 1, 2 or 4 mg/kg bw/day for
36 days. Apart from mydriasis in two dogs given 4 mg/kg bw/day, there
were no clinical signs of toxicity. Body weight was depressed in the
4 mg/kg bw/day group during the first 3 weeks but returned to baseline
thereafter. Haematology, serum chemistry, urinalysis, organ weights
and pathology were unaffected. Plasma drug levels increased with
duration of dosing, reaching maximum concentrations of 0.21, 0.5 and
0.88 µg/ml, at the low-, mid- and high-dose groups, respectively. The
NOEL was 2 mg/kg bw/day. (Pfizer, 1988c).
Beagle dogs (4/sex/group) were given gavage doses of 0, 0.5, 1 or
2 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 91 days. A
slow pupillary light reflex was noted in all treated animals and
mydriasis was diagnosed in 1, 2, and 5 dogs given 0.5, 1, and 2 mg/kg
bw/day, respectively. One animal given 2 mg/kg bw/day showed anorexia,
tremors and ataxia and was sacrificed on day 23. No effects were
observed on body weight, haematology, serum chemistry, urinalysis,
organ weight or pathology. Plasma drug levels were dose-related but
were higher on day 30 than on day 90. The levels were approximately
0.29, 0.5 and 1.2 µg/ml on day 30 and 0.24, 0.3 and 0.6 µg/ml on day
90, at the 3 respective doses (Pfizer, 1989c).
Beagle dogs (3/sex/dose) were given gavage doses of 0, 0.1 or
0.3 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 92 days.
The only treatment-related finding was mild to moderate mydriasis
which was diagnosed in 1 female given 0.3 mg/kg bw/day. Plasma drug
levels were dose-related, with approximate mean concentrations of 0.1
and 0.28 µg/ml, respectively. The NOEL in this study was 0.1 mg/kg
bw/day (Pfizer, 1990b).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 1.5, 3 or
6 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Males were
dosed from 10 weeks before pairing and to the end of the mating
period. Females were dosed from 2 weeks prior to pairing until the end
of the study.
There were no effects on food consumption or body weight of F0
adults, and copulation, pregnancy rate and gestation length were
unaffected. Size and weight of F1 litters at birth were similar
between groups. During lactation, pup weights decreased in the 3 and
6 mg/kg bw/day groups and most of these pups died by day 7
post-partum. Survival was unaffected in the 1.5 mg/kg bw/day group but
body-weight gain was lower in these offspring and the study was
discontinued on lactation day 7.
Subsequently, a number of F0 dams with litters (culled to 8
pups/litter), were assigned to 6 groups consisting of 3 females each.
Gavage doses of 0, 0.25, 0.5, 1, 3 or 6 mg/kg bw/day were administered
on lactation days 12 to 21 to determine potential effects on older
pups. Pup survival was not affected but body-weight gain was reduced
at 6 mg/kg bw/day (Pfizer, 1990c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 0.1, 0.3
or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Treatment
of F0 males commenced 10 weeks prior to pairing, continuing until
the end of the mating period. F0 females were treated from 2 weeks
before pairing, through gestation until the end of lactation. Food
intake, body-weight gain, copulation, pregnancy rate and duration of
gestation were unaffected in F0 rats and gross examination of these
animals did not reveal any abnormalities.
F1 litter size, weight at birth, and post-natal survival were
similar in all groups. There was slight depression of body-weight gain
of the 1 mg/kg bw/day pups during lactation and the weight of these
animals remained below other groups for the remainder of the study.
Where necessary, F1 litters were reduced to 8 pups each on lactation
day 4. At weaning, 25 male and 25 female pups/group were randomly
selected to produce the F2 generation and were dosed until the end
of the study. On pairing of F1 rats, copulation incidences were
adversely affected leading to low pregnancy rates in all groups, most
notably in the control.
F2 litter sizes at birth were lower than normal in all groups
but subsequent survival was not affected. Body weights of the 1 mg/kg
bw/day offspring were lower throughout the lactation period. In
limited tests for post-natal development in 1 male and 1 female/litter,
there were no significant treatment-related deficits. Gross examination
of treated F1 rats and F2 pups showed no abnormalities.
Due to the adverse effects on conception described above, the
study was terminated, except for rats in the F1 control group which
were paired for a second mating. Again, pregnancy rates were very low,
and vaginal smears indicated irregular estrous cycling in many of the
females with some not cycling at all (Pfizer, 1992c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were administered gavage doses of
0, 0.1, 0.3 or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil.
F0 males were treated from 10 weeks prior to pairing until the end
of mating, and females were dosed from 2 weeks prior to pairing,
during mating, gestation, and lactation periods. There were no effects
on food consumption or body weight of F0 rats and copulation,
pregnancy rate and gestation length were unaffected. Gross examination
of these animals was unremarkable.
F1 litter size and weight at birth and subsequent survival and
growth were similar in all groups. Each F1 litter was culled to 8
pups, where necessary, on lactation day 4. At weaning, 30 male and 30
female pups/group were randomly selected to produce the F2
generation and were dosed directly until study termination. These F1
rats were paired on two occasions, at age 11 to 12 weeks and 22 to
23 weeks. On both occasions, copulation and duration of gestation were
unaffected. However, conception was impaired in all groups, the
pregnancy rate being approximately 70% for the F2a and 50% for the
2bF matings, respectively. Gross examinations of F1 rats showed no
internal or external abnormalities.
In both the F2a and F2b litters, the number and weight of
offspring at birth, and survival to the end of lactation were not
affected by treatment. Pup body-weight gain was depressed in the
1 mg/kg bw/day group. On lactation day 21, 1 male and 1 female
pup/F2a litter were tested for locomotor activity and auditory
function and were given an ophthalmoscopic examination. No
treatment-related effects were observed in these developmental tests
or on gross examination of all F2a and F2b pups. The NOEL in this
study was 0.3 mg/kg bw/day (Pfizer, 1991b).
2.2.4 Special studies on neonatal toxicity
2.2.4.1 Rats
Groups of 7 (2 in control group) presumed pregnant Long-Evans
rats were given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin
(purity 92.5%) in sesame oil from gestation day 2 to day 3 post-partum.
There was no effect on body weight of dams but 1 female given 6 mg/kg
bw/day was sacrificed moribund. Milk, blood and brain of dams were
collected 3-4 h after the last dose. Blood and brain from the pups
were collected 24 h after dosing of the dams.
Analysis of drug levels in plasma samples revealed higher levels
in dams than in pups. However, drug levels in the brain of dams were
generally lower than in pup brains. The plasma to brain ratio was
approximately 16 in dams and 2 in pups indicating that doramectin was
more accessible to the CNS of neonates. Milk to plasma ratios in dams
were about 2-3, indicating that the drug was readily excreted in milk
(Pfizer, 1990d).
Groups of 10 presumed pregnant Long-Evans rats were given gavage
doses of 0, 0.1, 0.2, 0.5 or 1 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil during gestation days 4 to lactation day 21. Litters
were culled to 8 pups each on lactation day 4, surviving pups and dams
were killed on lactation day 21. There was no effect on body weight of
dams. One dam given 1 mg/kg bw/day showed piloerection and lethargy
and died on day 13 of treatment. Litter size and weight at birth were
unaffected. At a dose of 1 mg/kg bw/day, pups body-weight gain was
reduced during lactation and 6 of 8 pups in a single litter died
(Pfizer, 1992d).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 9 presumed pregnant CD-1 mice were given gavage doses
of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin (purity 93.6%)
in sesame oil on gestation days 6 to 13. There were no effects on body
weight of dams, one female given 1.6 mg/kg bw/day had blood in the
urogenital area and was found dead on gestation day 12. The remaining
dams were killed on gestation day 18. Embryomortality and number of
live fetuses were similar between groups. Fetal weights were lower at
1.6 mg/kg bw/day. Fetuses were not examined (Pfizer, 1988d).
Groups of 7 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%) in sesame oil
on gestation days 6 to 13. One 6 mg/kg bw/day female showed
hypothermia, piloerection and blood on the vagina, but there were no
treatment-related deaths and no effects on body weight. Dams were
killed on gestation day 18. There were no effects on embryomortality,
number of live fetuses or fetal body weight. Fetuses were not examined
(Pfizer, 1988e).
Groups of 20 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%) in sesame oil
on gestation days 6 to 13. There were no drug-related deaths or
clinical signs in dams and body weight was unaffected. Dams were
killed on gestation day 18. Embryomortality was increased at 6 mg/kg
bw/day but did not attain statistical significance. Fetal body weight
and the incidence of fetal abnormalities were unchanged.
An additional group of 10 mice were similarly treated with
6 mg/kg bw/day for analysis of drug levels on gestation day 13. Plasma
drug levels in dams, 1 h after the last dose varied from 0.088 to
0.28 µg/ml. Six hours after dosing, plasma levels in dams were between
0.37 and 0.58 µg/ml, amniotic fluid levels varied from non-detectable
to 0.019 µg/ml and fetal levels varied from non-detectable to
0.12 µg/g. The NOEL in this study was 3 mg/kg bw/day (Pfizer, 1988f).
2.2.5.2 Rats
Groups of 6 or 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 6 to 15. Dams showed no
signs of toxicity or effects on body weight. Females were killed on
gestation day 20. Embryomortality, number of live fetuses and fetal
body weight were similar between groups. Fetuses were not examined
(Pfizer, 1988g).
Groups of 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. No toxic signs or adverse effects on body weight were noted in
dams. Embryotoxicity and numbers of live fetuses and fetal body weight
were unaffected. Fetuses were not examined (Pfizer, 1988h).
Groups of 20 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. There were no effects on body weight of dams. Embryomortality
was increased at 6 mg/kg bw/day but was not statistically significant
and was within the historical control range. Fetal examination
revealed slightly increased incidences of rudimentary ribs, wavy ribs,
unossified hyoid and 5th metacarpals, and dilatation of ureters and
kidney pelves, but the incidences were not dose-related and were
within the historical control range.
A satellite group of 10 rats were administered 6 mg/kg bw/day and
used for drug analysis on gestation day 15. Plasma drug levels in
dams, 1-5 h after dosing, varied from 0.41 to 1.27 µg/ml. Five hours
after the dose, amniotic fluid showed a mean level of 0.014 µg/ml and
fetal levels were between 0.27 and 1.1 µg/g. The NOEL in this study
was 6 mg/kg bw/day, the highest dose tested (Pfizer, 1988i).
2.2.5.3 Rabbits
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.2, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 7 to 18. Body-weight
gain was reduced in the 1.6 mg/kg bw/day group during gestation days 1
to 19. Does were killed on gestation day 28. Embryomortality was
slightly increased at 1.6 mg/kg bw/day but with no effect on fetal
weight. Fetuses were not examined. Plasma drug levels in dams given
1.6 mg/kg bw/day, 2 h after dosing on gestation day 17, ranged between
0.037 and 0.42 µg/ml (Pfizer, 1988j).
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity
92.1%) in sesame oil on gestation days 7 to 18. Body-weight gain was
significantly depressed at 6 mg/kg bw/day, in association with reduced
food consumption, on gestation days 7 to 28. Dams were killed on
gestation day 28. Embryonic death was increased and foetal weight was
slightly depressed in the 6 mg/kg bw/day group. Fetuses were not
examined (Pfizer, 1988k).
Groups of 20 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.75, 1.5 or 3 mg/kg bw/day doramectin
(purity 92.1%) in sesame oil on gestation days 7 to 18. There was no
drug-related mortality but during the treatment period, food intake
was reduced at 1.5 and 3 mg/kg bw/day with reduced body-weight gain at
3 mg/kg bw/day. Embryomortality and fetal weight were similar between
groups. Major malformations, occurring in the 3 mg/kg bw/day group
only, were cleft palate in 3 fetuses from 1 litter and 1 fetus which
exhibited phocomelia, syndactyly and coelosomia. The incidence of
cleft palate was within the historical control range whereas the other
fetal abnormalities had never been observed in the testing laboratory.
Ossification of pubic bones was delayed at 1.5 and 3 mg/kg bw/day.
A satellite group of 4 rabbits were dosed with 3 mg/kg bw/day and
sacrificed on gestation day 18 for analysis of drug levels. Maternal
plasma levels, measured 1, 3 and 5 h after dosing, ranged from 0.126
to 0.838 µg/ml. Doramectin was not detected in amniotic fluid or fetal
tissue, 5 h after the last dose, but detection limits were relatively
high due to interfering peaks. The NOEL in this study was 0.75 mg/kg
bw/day (Pfizer, 1988l).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with doramectin are given in
Table 4.
2.2.7 Special study on permeability of blood-brain barrier
2.2.7.1 Mice
Groups of 3 adult female CD-1 and CF-1 mice were given a single
gavage dose of 0.4 mg/kg bw ivermectin in sesame oil. Additional
groups received an i.p. injection of 50 mg/kg bw CP-100,356, an
inhibitor of p-glycoprotein activity, 1 h prior to ivermectin
administration. Animals were killed 24 h after drug dosing for removal
of blood and brain samples.
In CD-1 mice, ivermectin B1a could not be detected in plasma
(limit of quantitation 10 ng/ml), while brain levels were
1.38-1.98 ng/g. Pretreatment with CP-100,356 resulted in plasma levels
of 17.2-18.7 ng/ml and brain levels of 1.95-2.13 ng/g.
In CF-1 mice, ivermectin B1a levels in plasma were
14.9-21.0 ng/ml and brain levels were 109, 3.69 and 2.59 ng/g.
Pretreatment with CP-100,356 resulted in plasma levels of 30.1, 33.1
and 17.4 ng/ml and brain levels of 6.06, 8.97 and 1.98 ng/g.
Pretreatment with the p-glycoprotein inhibitor did not markedly affect
plasma or brain drug levels in either strain. Since the study used
very few animals and the treatment schedule and tissue sampling times
were not justified, no conclusion can be drawn from this study
(Pfizer, 1995).
2.2.8 Special studies on pharmacology
Table 5 summarizes the results of pharmacological studies with
doramectin.
Table 4. Results of genotoxicity assays on doramectin
Test system1 Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.02 to 10 mg/plate negative Pfizer, 1991c
TA98, TA100 (-S9)3
TA1535, 0.005 to 2 mg/plate negative
TA 1537 (+S9)3
Urine from mice negative
dosed with 0.2 to
4 mg/kg ip (-S9)3
Reverse mutation2 S. typhimurium 9.8 to 5000 µg/plate negative Pfizer, 1991d
TA98, TA100, (±S9)3
TA1535, TA1537
E. coli WP2 uvrA.
Forward mutation2 L5178Y mouse 8 to 35 µg/ml negative Pfizer, 1991c
lymphoma cells. (-S9)3
13 to 62 µg/ml negative
(+S9)3
UDS assay Primary culture 1.7 to 10 µg/ml3 negative Pfizer, 1991c
of rat hepatocytes.
Micronucleus Mouse bone 500 to 2000 mg/kg negative Pfizer, 1991e
test marrow bw/day for 3 days,
po.4
1. Positive controls used
2. Both with and without liver microsomal activation
3. Vehicle was dimethylsulfoxide
4. Vehicle was 0.5% methylcellulose
Table 5. Results of pharmacological assays with doramectin
Assay type Species Dose Result Reference
(mg/kg bw)
Safety study1 Collie dogs 0.062 negative Cruthers, 1991
(n = 2) 0.125 negative
0.25 negative
0.5 vomiting, moist
muzzle, enlarged
pupils, unsteady
legs
Diuretic assay SD rats(n= 10) 0.1 to 1.0 negative Gromelski et al, 1991a
Gastrointestinal CD-1 mice 0.1 to 1.0 negative Varner et al, 1991
motility assay (n = 10)
Arterial SD rats 0.1 to 1.0 negative Gromelski et al, 1991b
blood gas (n = 10)
1. Ivermectin was tested in this same experiment and elicited clinical effects
at doses as low as 0.125 mg/kg bw.
3. COMMENTS
The Committee considered data from a range of studies on
doramectin, including the results of studies on its metabolism, acute
and short-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The oral bioavailability of doramectin in rats, as measured by
the plasma concentration of the drug and the area under the plasma
concentration-time curve, was approximately six times greater when
doramectin was administered in sesame oil as compared to an aqueous
vehicle or in the diet. Metabolism studies in rats, dogs and cattle
revealed a similar spectrum of metabolites in the liver and faeces of
each species, suggesting that laboratory animals are suitable models
for testing the toxicity of doramectin.
Acute toxicity studies were carried out in rodents. When
doramectin was administered orally in an aqueous vehicle, the LD50
values were in the range 500-2000 mg/kg bw in rats and greater than
2000 mg/kg bw in mice. When the drug was given in a sesame oil
vehicle, the oral LD50s were 50-200 mg/kg bw in rats and
75-500 mg/kg bw in mice. These marked differences in acute toxicity
reflect the enhanced absorption of doramectin when administered as an
oil preparation. A further investigation in mice showed that the acute
toxicities of orally administered doramectin and ivermectin were
similar and that both compounds were less acutely toxic than abamectin
or moxidectin. Toxic signs were indicative of effects on the CNS,
since doramectin, like abamectin and other drugs in this class,
affects gamma-aminobutyric acid (GABA)-sensitive neurons, which can
lead to neurotoxicity, as shown by tremors, ataxia and gait
abnormalities.
Short-term administration of doramectin in the diet to mice was
associated with clinical signs of neurotoxicity, and there was
evidence of minor toxic effects on the liver and kidney at and above
doses of 100 mg/kg bw/day. Plasma drug levels were dose-related,
although the relationship was non-linear, up to 100 mg/kg bw/day and
reached a plateau at doses greater than 100 mg/kg bw/day, which
suggests that the absorption of doramectin from the feed reached
saturation. The highest plasma concentration was 3.9 µg/ml.
A number of short-term toxicity studies were carried out in rats
for periods of up to 3 months. Administration of doramectin in the
diet at doses of 30 mg/kg bw/day and above resulted in markedly
reduced food intake and body-weight gain, as well as severe
neurotoxicity, which necessitated the early sacrifice of some of the
animals. Other findings at the same doses were atrophic changes in
hepatocytes and lymphoid organs and nephrosis. Minor increases in
liver weight were seen at 20 mg/kg bw/day in the diet, but hepatic
morphology was unaffected at this dose. The maximum plasma drug level
attained in these studies was 5.8 µg/ml. In a 1-month study in which
doramectin was given in the diet, the NOEL in rats was
10 mg/kg bw/day.
In two studies, rats were given doses of doramectin by gavage for
up to 3 months. Signs of CNS toxicity were observed at the highest
dose of 10 mg/kg bw/day. At doses of 5 mg/kg bw/day and above, there
were increases in liver weight, while kidney weight was increased at
8 mg/kg bw/day. However, there were no concomitant pathological
changes and all other parameters were unaffected. Plasma drug levels
reached a peak of 3.2 µg/ml. The NOEL in gavage studies in rats was
2 mg per kg of body weight per day.
When dogs were fed doramectin in the diet for periods of up to 36
days, the only treatment-related findings were mydriasis and reduced
body weight at a dose of 4 mg/kg bw/day. The highest plasma drug level
was 0.88 µg/ml. The NOEL in this study was 2 mg/kg bw/day.
In three studies in dogs, which received doramectin by gavage in
a sesame oil vehicle for up to 92 days, body weights were depressed at
4 mg/kg bw/day and clinical signs of CNS toxicity were noted at
2 mg/kg bw/day and above. The most sensitive indication of a
drug-related effect was mydriasis, which was seen at doses as low as
0.3 mg/kg bw/day. No other toxic effects were observed. The maximum
plasma drug level in these studies was 3 µg/ml. The NOEL was 0.1 mg/kg
bw/day.
Reproductive toxicity studies in rats revealed treatment-related
deaths among pups during the early postnatal period and a reduction in
body-weight gain of pups throughout the lactation period. These
effects were observed at doses as low as 3 and 1 mg/kg bw/day,
respectively. Doramectin was shown to be readily excreted in the milk
of lactating rats following administration by gavage and, as compared
with adult animals, higher drug levels were attained in the brain of
neonates, suggesting greater penetration through the incompletely
formed blood-brain barrier in newborn rats. The NOEL was 0.3 mg/kg
bw/day, based on toxicity in neonatal animals.
The administration of doramectin by gavage to pregnant mice and
rats did not result in fetal abnormalities. The only drug-related
finding was a slight increase in embryo mortality in mice given
6 mg/kg bw/day. The NOEL in mice was 3 mg/kg bw/day, while in rats
there were no adverse effects at the highest dose of 6 mg/kg bw/day.
In rabbits given 3 mg/kg bw/day during pregnancy, there was
significant maternal toxicity, and cleft palate was observed in three
fetuses from one litter; one fetus also exhibited phocomelia,
syndactyly and coelosomia. Ossification of pubic bones was delayed at
doses of 1.5 and 3 mg/kg bw/day. In a dose-ranging study, a dose of
6 mg/kg bw/day caused severe maternal toxicity and embryotoxicity. The
NOEL for maternal toxicity in rabbits was 0.75 mg/kg bw/day.
In view of the negative results in a range of in vitro
genotoxicity assays and a micronucleus test in mouse bone marrow, the
Committee concluded that doramectin was not genotoxic.
The Committee noted the close structural similarities between
doramectin and abamectin, the only difference being the presence of a
cyclohexyl group at the C-25 position in the doramectin molecule
rather than an isopropyl or isobutyl group in the case of abamectin
B1a and abamectin B1b, respectively. The available metabolic data
suggest that the biotransformation of doramectin and abamectin follows
a similar pathway. Extensive toxicological tests have been conducted
on both compounds, and the Committee reviewed several aspects of their
comparative toxicology. Both compounds exert a pharmacological effect
on the CNS through effects on gamma-aminobutyric acid-sensitive
neurons, which results in a range of neurotoxic signs such as
mydriasis, tremors, ataxia and gait abnormalities. In multiple-dose
studies, the compounds have been associated with few adverse
toxicological effects, reduced body-weight gain and minor toxic
effects on the liver being the most common. Toxicity in neonates is
the most sensitive indicator in reproductive toxicity studies with
both compounds. Neither compound is considered to have any genotoxic
activity. Carcinogenicity studies with abamectin were negative at
maximum tolerated doses in mice and rats. In view of the chemical,
biochemical and toxicological similarities, the Committee concluded
that it was unnecessary to request data from long-term toxicity and
carcinogenicity studies on doramectin.
4. EVALUATION
The Committee considered that the most relevant effect for the
safety evaluation of residues of doramectin was the effect on the
mammalian nervous system. An ADI of 0-0.5 µg/kg bw was established,
based on a NOEL of 0.1 mg/kg bw/day for mydriasis in the 3-month
gavage study in dogs. A safety factor of 200 was applied because the
test systems used to assess the neurotoxicity of doramectin were of
uncertain sensitivity (see section 2.2 of the Report, Annex 1,
reference 119). The ADI provides an adequate margin of safety for
neonatal toxicity in rats and developmental toxicity in rabbits.
5. REFERENCES
Cruthers, L.R. (1991). Dose Escalation Safety Study in Ivermectin
Sensitive Collies. Unpublished study No. 9112 from Professional
Laboratory and Research Services Inc. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Duce, I. R. & Scott, R.H. (1985). Actions of dihydroavermectin B1a
on insect muscle. British J. Pharmacol. 85. 395-401.
Graham, D., Pfeiffer, F. & Betz, H. (1982). Avermectin B1a inhibits
the binding of strychnine to the glycine receptor of rat spinal cord.
Neuroscience Letters. 29. 173-176.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991a). Diuretic
Assay. Unpublished study No. PH209-PZ-001-91 from Pharmakon Labs.
Submitted to WHO by Pfizer, Central Research, Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991b). Arterial
Blood Gas Determinations in Rats. Unpublished study
No. PH1051-PZ-001-91 from Pharmakon Labs. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Kass, I.S., Stretton, A.O.W. & Wang, C.C. (1984). The effects of
avermectin and drugs related to acetylcholine and 4-aminobutyric acid
on neurotransmission in Ascaris suum. Molec. Biochem. Parasit.
13. 213-225.
Pfizer (1987a). Comparison of Dosing Vehicles for Oral Exposure of
Rats. Unpublished study No. 87-657-07 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987b). Two Week Oral Feed Study on Long-Evans Rats.
Unpublished study No 87-657-03 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987c). Two Week Toleration Study in Beagle Dogs. Unpublished
study No. 87-657-04 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988a). Acute Oral and Intraperitoneal Toxicity Studies in
Mice and Rats. Unpublished study No. 87-657-01 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988b). A One Month Feeding Study in Long-Evans Rats at Dose
Levels of 20, 10, 5 and 0 mg/kg/day. Unpublished study No. 87-657-06
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1988c). A One Month Feed Study in Beagle Dogs at Dose Levels
of 4,2,1 and 0 mg/kg/day. Unpublished study No. 87-657-05 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988d). Preliminary Fetotoxicity Study (Maternal Toxicity) in
Mice by the Oral Route. Unpublished study No. 87094 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988e). Maternal Toxicity Study in Mice by the Oral Route.
Unpublished study No. 88035 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988f). Fetotoxicity Study in Mice by the Oral Route.
Unpublished study No. 88091/88092 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988g). Maternal Toxicity Study in Rats by the Oral Route.
Unpublished study No. 87165 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988h). UK-67,994: Maternal Toxicity Study in Rats by the Oral
Route. Unpublished study No. 88034 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988i). Fetotoxicity Study in Rats By the Oral Route.
Unpublished study No. 88079/88080 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988j). Maternal Toxicity Study in Rabbits by the Oral Route.
Unpublished study No. 87161 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988k). UK-67,994: Maternal Toxicity Study in Rabbits by the
Oral Route. Unpublished study No. 88077 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988l). Fetotoxicity Study in Rabbits by the Oral Route.
Unpublished study No. 88106/88107 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989a). A Five-Week Range Finding Gavage Study in Long-Evans
Rats. Unpublished study No. 88-657-11 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989b). A One Month Range Finding Gavage Study in Beagle Dogs.
Unpublished study No. 88-657-10 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989c). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 88-657-13 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990a). A Three Month Oral Gavage Study in Long-Evans Rats
with In Utero Exposure. Unpublished study No. 89-657-17 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1990b). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 89-657-19 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990c). A Two-Generation Gavage Study in Long-Evans Rats
(Terminated after first generation). Unpublished study No. 88-657-12
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1990d). An Exploratory Lactation Study in Long-Evans Rats.
Unpublished study No. 89-657-18 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991a). Acute Dermal and Ocular Irritation Studies in Albino
Rabbits. Unpublished study No. 91-657-22 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991b). A Two-Generation Gavage Study in Long-Evans Rats.
Unpublished study No. 89-657-20 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991c). Genetic Toxicology Report. Unpublished study
No. 87-657-02 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1991d). Genetic Toxicology (Microbial) Assays. Unpublished
study No. 91-67-81 from Pfizer Central Research, Taketoyo, Japan.
Submitted to WHO by Pfizer, USA.
Pfizer (1991e). Genetic Toxicology Report. Unpublished study
No. 90-657-21 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1992a). The Comparative Metabolism of 3H-Doramectin in
Cattle, Dog and Rat. Unpublished study No. CM-92-01 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992b). Single Dose Oral and Intraperitoneal Toxicity Studies
in Mice and Rats. Unpublished study No. 91-657-23 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992c). Two Generation Study in Long-Evans Rats (Terminated
prior to completion). Unpublished study No. 88-657-15 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992d). Range Finding Fetal/Neonatal Toxicity Study in
Long-Evans Rats. Unpublished study No. 88-657-16 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993a). Identification of Residues of Doramectin in Liver and
Fat of Cattle Sacrificed 21-Days Following SC Administration of
[3H]-Doramectin at 0.2 mg/kg. Unpublished study No. CM-93-01 from
Pfizer Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993b). One Month Range Finding Feeding Study in Long-Evans
Rats. Unpublished study No. 92-657-24 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994a). Single Dose Oral Toxicity Study in Albino (CD-1) Mice.
Unpublished study No. 94-657-29 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994b). A Range Finding Feed Study in CD-1 Mice. Unpublished
study No. 92-675-25 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1994c). Three Month Range Finding Feeding Study in CD-1 Mice.
Unpublished study No. 92-657-27 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994d). Three Month in Feed Study in Long-Evans Rats.
Unpublished study No. 92-657-26 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1995). Comparison of Plasma/Brain Ivermectin (B1a) Ratios in
CD-1 and CF-1 Mice. Unpublished study No. AHDM-94-08 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1991). Gastro-
intestinal Propulsion Assay in Male Mice. Unpublished study
No. PH239-PZ-001-91 from Pharmakon Labs. Submitted to WHO by
Pfizer, USA.
Zufall, F., Franke, Ch. & Hatt, H. (1989). The insecticide avermectin
B1a activates a chloride channel in crayfish muscle membrane.
J. Exp. Biol. 142. 191-205.
2.1.3 Pharmacological effects
Avermectins induce rapid, non-spastic paralysis in nematodes and
arthropods. One common feature of avermectins appears to be the
modulation of trans-membrane chloride ion (Cl-) channel activity in
nematode nerve cells, and in both nerve and muscle cells of
arthropods. These Cl- channels may be gated by a variety of
neurotransmitter receptors including gamma-aminobutyric acid (GABA),
glutamate and acetylcholine (Kass et al, 1984; Duce & Scott, 1985;
Zufall et al, 1989). Activation of the Cl- channels by avermectins
leads to an increase in Cl- conductance which results in a changed
membrane potential and this causes inhibition of electrical activity
in the target nerve or muscle cell. GABA is also a major inhibitory
neurotransmitter in the mammalian CNS and avermectins do have
intrinsic activity on the mammalian GABA receptor/Cl- channel
complex.
Avermectins have been reported to bind to glycine receptor/Cl-
channel complexes which are restricted to the CNS in mammals
(Graham et al, 1982). Penetration of the blood brain barrier by
avermectins is extremely poor and this may account for the wide margin
of safety exhibited by these compounds following administration to
mammals.
2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with doramectin are
summarized in Table 2.
Clinical signs of toxicity were similar in both mice and rats and
by both routes of administration, the most common being decreased
activity and respiration, hunched position, shakiness, weakness,
tremors, ataxia and weight loss.
Doramectin was applied to normal and abraded skin of 3 New
Zealand white rabbits (0.5 g under an occlusive patch) for 24 h.
Slight erythema was observed 24 and 48 h post-dose at 1 of 3 intact
sites and 2 of 3 abraded sites. There was no edema at any site and all
sites appeared normal 72 h post-dose.
Table 2. Results of acute toxicity studies with doramectin.
Species
(strain) Route Vehicle1 Sex LD50 in mg/kg bw2 Reference
Mouse po aqueous M&F > 2000(0/3) Pfizer, 1988a
(CD-1)
po oil F 250(0/3)-500(3/3) Pfizer, 1992b
po oil F 75(0/5)-200(5/5) Pfizer, 1994a
ip aqueous M 700(0/3)-1000(2/3) Pfizer, 1988a
ip oil M 100(0/3)-250(3/3) Pfizer, 1992b
Rat po aqueous M 1000(0/3)-2000(3/3) Pfizer, 1988a
(Sprague-Dawley) F 500(0/3)-1000(2/3) Pfizer, 1988a
po oil M 50(0/3)-100(2/3) Pfizer, 1992b
F 100(1/3)-200(3/3) Pfizer, 1992b
ip aqueous M >300(1/3) Pfizer, 1988a
ip oil M 50(0/3)-100(3/3) Pfizer, 1992b
1 aqueous vehicle was 0.1% aqueous methylcellulose; oil vehicle was sesame oil.
2 figures in brackets represent mortality incidences.
Doramectin powder (18.8 mg) was instilled into the conjunctival
sac of one eye in each of 3 New Zealand white rabbits. Slight corneal
and conjunctival reddening, chemosis and iritis were seen within 1 h
of dosing. The changes subsided within 6 h and all eyes were normal by
48 h post-dose (Pfizer, 1991a).
A comparison of the acute toxicity of different ivermectins in
mice is given in Table 3.
Table 3. Acute oral toxicity of doramectin in comparison with abamectin, ivermectin
and moxidectin in female CD-1 mice (Pfizer, 1994a).
Dose in mg/kg bw1 Doramectin Abamectin Ivermectin Moxidectin
Maximum
"asymptomatic" dose 25 5 255
Minimum
"symptomatic" dose 75 25 5025
Minimum
lethal dose2 200(5/5) 75(5/5) 75(1/5) 75(4/5)
LD50 dose 75-200 25-75 >75 25-75
1 - vehicle was sesame oil
2 - figures in brackets represent mortality incidences.
Signs of CNS toxicity were similar after dosing with each
compound and included gait abnormalities with splayed hind limbs,
intermittent tremors, ataxia and decreased, irregular or laboured
respiration (Pfizer, 1994a).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of CD-1 mice (10/sex/dose) were administered doramectin
(purity 94.1%) in the diet for 43 days. Target doses were 0, 10, 20,
40, or 60 mg/kg bw/day on days 1 to 14; 0, 10, 20, 80 or 100 mg/kg
bw/day on days 15 to 28; and 0, 100, 200, 400 or 600 mg/kg bw/day
on days 29 to 43.
Toxic signs included lethargy, hunched posture and tremors at 400
and 600 mg/kg bw/day, and hunched and ungroomed appearance in some
mice at 100 and 200 mg/kg bw/day. A number of animals given 400 and
600 mg/kg bw/day were sacrificed moribund and the remaining mice in
these groups were killed on day 33. There were no effects on body
weight or food consumption. Relative liver weights were slightly
higher in most treated groups but the changes were minor and not
dose-related. Clinical laboratory parameters and pathological
examinations were not carried out.
Plasma drug levels were dose-related, although non-linear, up to
100 mg/kg bw/day, and reached a plateau at higher doses. Drug levels
ranged from 0.23 µg/ml at 10 mg/kg bw/day to 3.9 µg/ml at 600 mg/kg
bw/day. An unidentified degradation product, represented a
progressively greater fraction of drug-related material at doses of
100 mg/kg bw/day and above (Pfizer, 1994b).
Groups of CD-1 mice (10/sex/group) were fed doramectin (purity
94.1%) in the diet for 92 days. Target doses were 0, 100, 200 or
300 mg/kg bw/day. Actual doramectin intake was 83-121, 154-191 or
221-322 mg/kg bw/day. Tremors, hunched posture, unkempt appearance and
lethargy were observed at the mid- and high-dose groups and resulted
in death or moribund sacrifice of 9 mice at 300 mg/kg bw/day and 3
mice at 200 mg/kg bw/day. The remaining animals in these two groups
were killed on day 12 or 19, respectively. Body-weight gain was
depressed in association with reduced food consumption at 200 mg/kg
bw/day and above.
Serum creatinine and BUN were slightly increased at 100 mg/kg
bw/day with no effects at higher doses, presumably due to early
culling of mice in these groups. Haematological parameters were
unaffected. All treated groups had increased liver weights and
hypertrophy of centrilobular hepatocytes, with multinucleate
non-proliferative liver cells at 100 mg/kg bw/day only. Dead and
moribund animals showed lymphocyte lysis in lymphoid organs, cellular
depletion of bone marrow and necrosis of the adrenal cortex which may
have resulted from stress and weight loss.
Satellite groups of 3 mice/sex/dose were used to determine plasma
drug levels. In the 100 mg/kg bw/day group, concentrations reached a
plateau at 2.8 µg/ml by day 45. Doramectin levels were not markedly
different at higher doses with peaks of 3.6 µg/ml and 2.7 µg/ml at 200
and 300 mg/kg bw/day, respectively. An unidentified degradation
product was present at levels proportional to both dose and duration
of treatment (Pfizer, 1994c).
2.2.2.2 Rats
Groups of Long-Evans rats (3/sex/group) were given 0, 2.5, 5 or
10 mg/kg bw/day doramectin (purity unspecified) in the diet for 14
days. There were no overt signs of toxicity and no effects on body
weight, haematology, serum chemistry, urinalysis, liver or kidney
weight. Examination of a limited number of organs revealed no
pathological changes. Mean plasma drug levels at day 10 were
approximately 0.05, 0.06 and 0.17 µg/ml with increasing doses
(Pfizer, 1987b).
In a 1-month study, groups of Long-Evans rats (10/sex/group) were
fed doramectin (purity 95.3%) in the diet at dose levels of 0, 5, 10
or 20 mg/kg bw/day. There were no clinical signs of toxicity and no
effects on food intake or body-weight gain. Ophthalmology,
haematology, serum chemistry and urinalysis were unaffected. Liver
weight was slightly increased in males given 20 mg/kg bw/day but
pathological examination was unremarkable. Plasma drug levels were
proportional to the dose; maximum concentrations achieved were 0.28,
0.76 and 1.79 µg/ml, respectively. The NOEL in this study was 10 mg/kg
bw/day (Pfizer, 1988b).
Groups of Long-Evans rats (10/sex/group) were fed a diet
containing doramectin (purity 94.1%) at levels intended to give
target doses of 0, 20, 40, 60 or 80 mg/kg bw/day for 38 days.
Chromorhinorrhea, chromodacryorrhea and urogenital staining were seen
at 40 mg/kg bw/day and above. Whole body tremors were also present at
the two highest doses and resulted in the sacrifice of 2 rats at
60 mg/kg bw/day and all rats at 80 mg/kg bw/day. Body-weight gain and
food consumption were lower at 60 mg/kg bw/day and above. Serum
5'-nucleotidase was increased at 80 mg/kg bw/day with elevated levels
of BUN at 40 mg/kg bw/day and above. Liver weights were higher in the
40 and 60 mg/kg bw/day groups but with no dose relationship. Organs
were not examined for pathology.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin concentrations were progressively increased
throughout the study at each dose, and levels were dose-related up to
60 mg/kg bw/day with no further increase at 80 mg/kg bw/day. Highest
levels were 0.89, 3.52, 3.78 and 3.54 µg/ml at the 4 doses,
respectively (Pfizer, 1993b).
Groups of Long-Evans rats (5/sex/group) were administered by
gavage doses of 0, 2, 5 or 10 mg/kg/bw/day doramectin (purity 92.1%)
in sesame oil for 38 days. Three females in the 10 mg/kg bw/day group
were sacrificed after showing hunched appearance, ataxia, tremors and
urine stains. There were no effects on body weight, food consumption,
haematology, serum chemistry or urinalysis. Some rats given 5 and
10 mg/kg bw/day had increased liver weights but pathological
examination of a limited number of organs was unremarkable. Plasma
drug levels were similar on days 10 and 38 and were dose-related;
approximate levels were 0.5, 1.6 and 3.0 µg/ml in the low-, mid- and
high-dose groups, respectively (Pfizer, 1989a).
Groups of Long-Evans rats (10/sex/dose) were fed doramectin
(purity 89.7%-94.1%) in the diet at target doses of 0, 30, 40 or
50 mg/kg bw/day, for 92 days. Based on food consumption, actual
doramectin intake ranged from 16-32, 11-49 and 14-59 mg/kg bw/day,
respectively. Thus systemic exposure to doramectin was similar in each
treated group which was reflected in the toxicological findings.
Clinical signs, noted in all treated animals, included
chromorhinorrhea, chromodacryorrhea, urine stains and tremors. Seven
rats at 30 mg/kg bw/day, all rats at 40 mg/kg bw/day, and 19 rats at
50 mg/kg bw/day died or were sacrificed moribund. Body-weight gain was
markedly reduced in all treated groups in association with diminished
food intake. Ophthalmological examinations were unchanged. All treated
groups showed decreased leucocytes, erythrocytes, haemoglobin and
haematocrit, and increased BUN. Serum levels of ALAT and ASAT were
increased and protein decreased in some animals given 40 and 50 mg/kg
bw/day. Urinalysis was unaffected.
At necropsy, animals which failed to survive the treatment period
showed minimal body fat and a few had stomach erosions. Apparent
increases in relative kidney and testes weights in treated groups were
probably due to the lower body weights. Pathological changes, observed
in all treated groups, comprised kidney nephrosis with protein
accumulation in the corticomedullary junction, hepatocyte atrophy with
occasional necrotic cells and haemosiderosis, lymphoid depletion in
thymus, spleen and mesenteric lymph node, bone marrow atrophy, and
lipid depletion in the adrenal cortex.
Satellite groups of 5 rats/sex/dose were treated in order to
determine plasma drug levels. Doramectin plasma concentrations
increased during the course of the study in the 30 and 40 mg/kg bw/day
groups, attaining levels of 4.6 and 5.8 µg/ml, respectively. A maximum
level of 4.1 µg/ml was achieved by day 16 in rats given 50 mg/kg
bw/day (Pfizer, 1994d).
Groups of Long-Evans rats (20/sex/dose) were selected from the
F1 offspring in a multigeneration reproductive toxicity study in
which doramectin was administered by gavage at doses of 0, 0.1, 0.3 or
1.0 mg/kg bw/day. Thus the animals had received secondary exposure in
utero and during lactation, and direct exposure for 3 to 4 weeks
post-weaning. Subsequently, the respective groups were administered
gavage doses of 0, 0.5, 2 or 8 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil for 3 months.
There were no overt signs of toxicity, mortality or treatment-
related ocular lesions. Initial body weights of the 8 mg/kg bw/day
group were lower which may be attributed to the prior in utero
or neonatal exposure to doramectin. During the course of the study
proper, treated groups gained more weight than controls. Food
consumption, haematology, serum chemistry and urinalysis were
unaffected. Liver and kidney weights were increased at 8 mg/kg bw/day
but there were no concomitant pathological alterations.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin levels were dose-related with mean levels
of 0.07, 0.4 and 2.5 µg/ml on day 3, and 0.1, 0.7 and 3.2 µg/ml on day
87, in the low-, mid-, and high-dose groups, respectively. The NOEL in
this study was 2 mg/kg bw/day (Pfizer. 1990a).
2.2.2.3 Dogs
Groups of beagle dogs (1/sex/group) were fed doramectin (purity
unspecified) in the diet at dose levels of 0, 0.5, 1 or 2 mg/kg bw/day
for 14 days. There were no signs of toxicity or effects on body
weight, haematology, serum chemistry or urinalysis. Dogs were not
sacrificed. Plasma drug levels increased during the course of the
study attaining concentrations of approximately 0.045, 0.07 and
0.26 µg/ml, respectively on day 8 (Pfizer, 1987c).
Groups of beagle dogs (1/sex/group) were given gavage doses of
0, 1, 2 or 4 mg/kg bw/day doramectin (purity 92.1%-93.3%) in sesame
oil for 29 days. Body weight was depressed in both animals
administered 4 mg/kg bw/day and the male showed salivation, mydriasis,
tremors, ataxia and decreased heart rate prior to sacrifice on day 16.
All drug-treated dogs exhibited mydriasis. There were no effects on
haematology, serum chemistry, urinalysis, organ weight or pathological
examinations. Plasma drug levels were proportional to the dose,
highest concentrations achieved were approximately 0.4, 1.2 and
3.0 µg/ml, respectively (Pfizer, 1989b).
Groups of beagle dogs (3/sex/group) were administered doramectin
(purity 95.3%) in the diet at doses of 0, 1, 2 or 4 mg/kg bw/day for
36 days. Apart from mydriasis in two dogs given 4 mg/kg bw/day, there
were no clinical signs of toxicity. Body weight was depressed in the
4 mg/kg bw/day group during the first 3 weeks but returned to baseline
thereafter. Haematology, serum chemistry, urinalysis, organ weights
and pathology were unaffected. Plasma drug levels increased with
duration of dosing, reaching maximum concentrations of 0.21, 0.5 and
0.88 µg/ml, at the low-, mid- and high-dose groups, respectively. The
NOEL was 2 mg/kg bw/day. (Pfizer, 1988c).
Beagle dogs (4/sex/group) were given gavage doses of 0, 0.5, 1 or
2 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 91 days. A
slow pupillary light reflex was noted in all treated animals and
mydriasis was diagnosed in 1, 2, and 5 dogs given 0.5, 1, and 2 mg/kg
bw/day, respectively. One animal given 2 mg/kg bw/day showed anorexia,
tremors and ataxia and was sacrificed on day 23. No effects were
observed on body weight, haematology, serum chemistry, urinalysis,
organ weight or pathology. Plasma drug levels were dose-related but
were higher on day 30 than on day 90. The levels were approximately
0.29, 0.5 and 1.2 µg/ml on day 30 and 0.24, 0.3 and 0.6 µg/ml on day
90, at the 3 respective doses (Pfizer, 1989c).
Beagle dogs (3/sex/dose) were given gavage doses of 0, 0.1 or
0.3 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 92 days.
The only treatment-related finding was mild to moderate mydriasis
which was diagnosed in 1 female given 0.3 mg/kg bw/day. Plasma drug
levels were dose-related, with approximate mean concentrations of 0.1
and 0.28 µg/ml, respectively. The NOEL in this study was 0.1 mg/kg
bw/day (Pfizer, 1990b).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 1.5, 3 or
6 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Males were
dosed from 10 weeks before pairing and to the end of the mating
period. Females were dosed from 2 weeks prior to pairing until the end
of the study.
There were no effects on food consumption or body weight of F0
adults, and copulation, pregnancy rate and gestation length were
unaffected. Size and weight of F1 litters at birth were similar
between groups. During lactation, pup weights decreased in the 3 and
6 mg/kg bw/day groups and most of these pups died by day 7
post-partum. Survival was unaffected in the 1.5 mg/kg bw/day group but
body-weight gain was lower in these offspring and the study was
discontinued on lactation day 7.
Subsequently, a number of F0 dams with litters (culled to 8
pups/litter), were assigned to 6 groups consisting of 3 females each.
Gavage doses of 0, 0.25, 0.5, 1, 3 or 6 mg/kg bw/day were administered
on lactation days 12 to 21 to determine potential effects on older
pups. Pup survival was not affected but body-weight gain was reduced
at 6 mg/kg bw/day (Pfizer, 1990c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 0.1, 0.3
or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Treatment
of F0 males commenced 10 weeks prior to pairing, continuing until
the end of the mating period. F0 females were treated from 2 weeks
before pairing, through gestation until the end of lactation. Food
intake, body-weight gain, copulation, pregnancy rate and duration of
gestation were unaffected in F0 rats and gross examination of these
animals did not reveal any abnormalities.
F1 litter size, weight at birth, and post-natal survival were
similar in all groups. There was slight depression of body-weight gain
of the 1 mg/kg bw/day pups during lactation and the weight of these
animals remained below other groups for the remainder of the study.
Where necessary, F1 litters were reduced to 8 pups each on lactation
day 4. At weaning, 25 male and 25 female pups/group were randomly
selected to produce the F2 generation and were dosed until the end
of the study. On pairing of F1 rats, copulation incidences were
adversely affected leading to low pregnancy rates in all groups, most
notably in the control.
F2 litter sizes at birth were lower than normal in all groups
but subsequent survival was not affected. Body weights of the 1 mg/kg
bw/day offspring were lower throughout the lactation period. In
limited tests for post-natal development in 1 male and 1 female/litter,
there were no significant treatment-related deficits. Gross examination
of treated F1 rats and F2 pups showed no abnormalities.
Due to the adverse effects on conception described above, the
study was terminated, except for rats in the F1 control group which
were paired for a second mating. Again, pregnancy rates were very low,
and vaginal smears indicated irregular estrous cycling in many of the
females with some not cycling at all (Pfizer, 1992c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were administered gavage doses of
0, 0.1, 0.3 or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil.
F0 males were treated from 10 weeks prior to pairing until the end
of mating, and females were dosed from 2 weeks prior to pairing,
during mating, gestation, and lactation periods. There were no effects
on food consumption or body weight of F0 rats and copulation,
pregnancy rate and gestation length were unaffected. Gross examination
of these animals was unremarkable.
F1 litter size and weight at birth and subsequent survival and
growth were similar in all groups. Each F1 litter was culled to 8
pups, where necessary, on lactation day 4. At weaning, 30 male and 30
female pups/group were randomly selected to produce the F2
generation and were dosed directly until study termination. These F1
rats were paired on two occasions, at age 11 to 12 weeks and 22 to
23 weeks. On both occasions, copulation and duration of gestation were
unaffected. However, conception was impaired in all groups, the
pregnancy rate being approximately 70% for the F2a and 50% for the
2bF matings, respectively. Gross examinations of F1 rats showed no
internal or external abnormalities.
In both the F2a and F2b litters, the number and weight of
offspring at birth, and survival to the end of lactation were not
affected by treatment. Pup body-weight gain was depressed in the
1 mg/kg bw/day group. On lactation day 21, 1 male and 1 female
pup/F2a litter were tested for locomotor activity and auditory
function and were given an ophthalmoscopic examination. No
treatment-related effects were observed in these developmental tests
or on gross examination of all F2a and F2b pups. The NOEL in this
study was 0.3 mg/kg bw/day (Pfizer, 1991b).
2.2.4 Special studies on neonatal toxicity
2.2.4.1 Rats
Groups of 7 (2 in control group) presumed pregnant Long-Evans
rats were given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin
(purity 92.5%) in sesame oil from gestation day 2 to day 3 post-partum.
There was no effect on body weight of dams but 1 female given 6 mg/kg
bw/day was sacrificed moribund. Milk, blood and brain of dams were
collected 3-4 h after the last dose. Blood and brain from the pups
were collected 24 h after dosing of the dams.
Analysis of drug levels in plasma samples revealed higher levels
in dams than in pups. However, drug levels in the brain of dams were
generally lower than in pup brains. The plasma to brain ratio was
approximately 16 in dams and 2 in pups indicating that doramectin was
more accessible to the CNS of neonates. Milk to plasma ratios in dams
were about 2-3, indicating that the drug was readily excreted in milk
(Pfizer, 1990d).
Groups of 10 presumed pregnant Long-Evans rats were given gavage
doses of 0, 0.1, 0.2, 0.5 or 1 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil during gestation days 4 to lactation day 21. Litters
were culled to 8 pups each on lactation day 4, surviving pups and dams
were killed on lactation day 21. There was no effect on body weight of
dams. One dam given 1 mg/kg bw/day showed piloerection and lethargy
and died on day 13 of treatment. Litter size and weight at birth were
unaffected. At a dose of 1 mg/kg bw/day, pups body-weight gain was
reduced during lactation and 6 of 8 pups in a single litter died
(Pfizer, 1992d).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 9 presumed pregnant CD-1 mice were given gavage doses
of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin (purity 93.6%)
in sesame oil on gestation days 6 to 13. There were no effects on body
weight of dams, one female given 1.6 mg/kg bw/day had blood in the
urogenital area and was found dead on gestation day 12. The remaining
dams were killed on gestation day 18. Embryomortality and number of
live fetuses were similar between groups. Fetal weights were lower at
1.6 mg/kg bw/day. Fetuses were not examined (Pfizer, 1988d).
Groups of 7 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%) in sesame oil
on gestation days 6 to 13. One 6 mg/kg bw/day female showed
hypothermia, piloerection and blood on the vagina, but there were no
treatment-related deaths and no effects on body weight. Dams were
killed on gestation day 18. There were no effects on embryomortality,
number of live fetuses or fetal body weight. Fetuses were not examined
(Pfizer, 1988e).
Groups of 20 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%) in sesame oil
on gestation days 6 to 13. There were no drug-related deaths or
clinical signs in dams and body weight was unaffected. Dams were
killed on gestation day 18. Embryomortality was increased at 6 mg/kg
bw/day but did not attain statistical significance. Fetal body weight
and the incidence of fetal abnormalities were unchanged.
An additional group of 10 mice were similarly treated with
6 mg/kg bw/day for analysis of drug levels on gestation day 13. Plasma
drug levels in dams, 1 h after the last dose varied from 0.088 to
0.28 µg/ml. Six hours after dosing, plasma levels in dams were between
0.37 and 0.58 µg/ml, amniotic fluid levels varied from non-detectable
to 0.019 µg/ml and fetal levels varied from non-detectable to
0.12 µg/g. The NOEL in this study was 3 mg/kg bw/day (Pfizer, 1988f).
2.2.5.2 Rats
Groups of 6 or 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 6 to 15. Dams showed no
signs of toxicity or effects on body weight. Females were killed on
gestation day 20. Embryomortality, number of live fetuses and fetal
body weight were similar between groups. Fetuses were not examined
(Pfizer, 1988g).
Groups of 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. No toxic signs or adverse effects on body weight were noted in
dams. Embryotoxicity and numbers of live fetuses and fetal body weight
were unaffected. Fetuses were not examined (Pfizer, 1988h).
Groups of 20 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. There were no effects on body weight of dams. Embryomortality
was increased at 6 mg/kg bw/day but was not statistically significant
and was within the historical control range. Fetal examination
revealed slightly increased incidences of rudimentary ribs, wavy ribs,
unossified hyoid and 5th metacarpals, and dilatation of ureters and
kidney pelves, but the incidences were not dose-related and were
within the historical control range.
A satellite group of 10 rats were administered 6 mg/kg bw/day and
used for drug analysis on gestation day 15. Plasma drug levels in
dams, 1-5 h after dosing, varied from 0.41 to 1.27 µg/ml. Five hours
after the dose, amniotic fluid showed a mean level of 0.014 µg/ml and
fetal levels were between 0.27 and 1.1 µg/g. The NOEL in this study
was 6 mg/kg bw/day, the highest dose tested (Pfizer, 1988i).
2.2.5.3 Rabbits
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.2, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 7 to 18. Body-weight
gain was reduced in the 1.6 mg/kg bw/day group during gestation days 1
to 19. Does were killed on gestation day 28. Embryomortality was
slightly increased at 1.6 mg/kg bw/day but with no effect on fetal
weight. Fetuses were not examined. Plasma drug levels in dams given
1.6 mg/kg bw/day, 2 h after dosing on gestation day 17, ranged between
0.037 and 0.42 µg/ml (Pfizer, 1988j).
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity
92.1%) in sesame oil on gestation days 7 to 18. Body-weight gain was
significantly depressed at 6 mg/kg bw/day, in association with reduced
food consumption, on gestation days 7 to 28. Dams were killed on
gestation day 28. Embryonic death was increased and foetal weight was
slightly depressed in the 6 mg/kg bw/day group. Fetuses were not
examined (Pfizer, 1988k).
Groups of 20 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.75, 1.5 or 3 mg/kg bw/day doramectin
(purity 92.1%) in sesame oil on gestation days 7 to 18. There was no
drug-related mortality but during the treatment period, food intake
was reduced at 1.5 and 3 mg/kg bw/day with reduced body-weight gain at
3 mg/kg bw/day. Embryomortality and fetal weight were similar between
groups. Major malformations, occurring in the 3 mg/kg bw/day group
only, were cleft palate in 3 fetuses from 1 litter and 1 fetus which
exhibited phocomelia, syndactyly and coelosomia. The incidence of
cleft palate was within the historical control range whereas the other
fetal abnormalities had never been observed in the testing laboratory.
Ossification of pubic bones was delayed at 1.5 and 3 mg/kg bw/day.
A satellite group of 4 rabbits were dosed with 3 mg/kg bw/day and
sacrificed on gestation day 18 for analysis of drug levels. Maternal
plasma levels, measured 1, 3 and 5 h after dosing, ranged from 0.126
to 0.838 µg/ml. Doramectin was not detected in amniotic fluid or fetal
tissue, 5 h after the last dose, but detection limits were relatively
high due to interfering peaks. The NOEL in this study was 0.75 mg/kg
bw/day (Pfizer, 1988l).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with doramectin are given in
Table 4.
2.2.7 Special study on permeability of blood-brain barrier
2.2.7.1 Mice
Groups of 3 adult female CD-1 and CF-1 mice were given a single
gavage dose of 0.4 mg/kg bw ivermectin in sesame oil. Additional
groups received an i.p. injection of 50 mg/kg bw CP-100,356, an
inhibitor of p-glycoprotein activity, 1 h prior to ivermectin
administration. Animals were killed 24 h after drug dosing for removal
of blood and brain samples.
In CD-1 mice, ivermectin B1a could not be detected in plasma
(limit of quantitation 10 ng/ml), while brain levels were
1.38-1.98 ng/g. Pretreatment with CP-100,356 resulted in plasma levels
of 17.2-18.7 ng/ml and brain levels of 1.95-2.13 ng/g.
In CF-1 mice, ivermectin B1a levels in plasma were
14.9-21.0 ng/ml and brain levels were 109, 3.69 and 2.59 ng/g.
Pretreatment with CP-100,356 resulted in plasma levels of 30.1, 33.1
and 17.4 ng/ml and brain levels of 6.06, 8.97 and 1.98 ng/g.
Pretreatment with the p-glycoprotein inhibitor did not markedly affect
plasma or brain drug levels in either strain. Since the study used
very few animals and the treatment schedule and tissue sampling times
were not justified, no conclusion can be drawn from this study
(Pfizer, 1995).
2.2.8 Special studies on pharmacology
Table 5 summarizes the results of pharmacological studies with
doramectin.
Table 4. Results of genotoxicity assays on doramectin
Test system1 Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.02 to 10 mg/plate negative Pfizer, 1991c
TA98, TA100 (-S9)3
TA1535, 0.005 to 2 mg/plate negative
TA 1537 (+S9)3
Urine from mice negative
dosed with 0.2 to
4 mg/kg ip (-S9)3
Reverse mutation2 S. typhimurium 9.8 to 5000 µg/plate negative Pfizer, 1991d
TA98, TA100, (±S9)3
TA1535, TA1537
E. coli WP2 uvrA.
Forward mutation2 L5178Y mouse 8 to 35 µg/ml negative Pfizer, 1991c
lymphoma cells. (-S9)3
13 to 62 µg/ml negative
(+S9)3
UDS assay Primary culture 1.7 to 10 µg/ml3 negative Pfizer, 1991c
of rat hepatocytes.
Micronucleus Mouse bone 500 to 2000 mg/kg negative Pfizer, 1991e
test marrow bw/day for 3 days,
po.4
1. Positive controls used
2. Both with and without liver microsomal activation
3. Vehicle was dimethylsulfoxide
4. Vehicle was 0.5% methylcellulose
Table 5. Results of pharmacological assays with doramectin
Assay type Species Dose Result Reference
(mg/kg bw)
Safety study1 Collie dogs 0.062 negative Cruthers, 1991
(n = 2) 0.125 negative
0.25 negative
0.5 vomiting, moist
muzzle, enlarged
pupils, unsteady
legs
Diuretic assay SD rats(n= 10) 0.1 to 1.0 negative Gromelski et al, 1991a
Gastrointestinal CD-1 mice 0.1 to 1.0 negative Varner et al, 1991
motility assay (n = 10)
Arterial SD rats 0.1 to 1.0 negative Gromelski et al, 1991b
blood gas (n = 10)
1. Ivermectin was tested in this same experiment and elicited clinical effects
at doses as low as 0.125 mg/kg bw.
3. COMMENTS
The Committee considered data from a range of studies on
doramectin, including the results of studies on its metabolism, acute
and short-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The oral bioavailability of doramectin in rats, as measured by
the plasma concentration of the drug and the area under the plasma
concentration-time curve, was approximately six times greater when
doramectin was administered in sesame oil as compared to an aqueous
vehicle or in the diet. Metabolism studies in rats, dogs and cattle
revealed a similar spectrum of metabolites in the liver and faeces of
each species, suggesting that laboratory animals are suitable models
for testing the toxicity of doramectin.
Acute toxicity studies were carried out in rodents. When
doramectin was administered orally in an aqueous vehicle, the LD50
values were in the range 500-2000 mg/kg bw in rats and greater than
2000 mg/kg bw in mice. When the drug was given in a sesame oil
vehicle, the oral LD50s were 50-200 mg/kg bw in rats and
75-500 mg/kg bw in mice. These marked differences in acute toxicity
reflect the enhanced absorption of doramectin when administered as an
oil preparation. A further investigation in mice showed that the acute
toxicities of orally administered doramectin and ivermectin were
similar and that both compounds were less acutely toxic than abamectin
or moxidectin. Toxic signs were indicative of effects on the CNS,
since doramectin, like abamectin and other drugs in this class,
affects gamma-aminobutyric acid (GABA)-sensitive neurons, which can
lead to neurotoxicity, as shown by tremors, ataxia and gait
abnormalities.
Short-term administration of doramectin in the diet to mice was
associated with clinical signs of neurotoxicity, and there was
evidence of minor toxic effects on the liver and kidney at and above
doses of 100 mg/kg bw/day. Plasma drug levels were dose-related,
although the relationship was non-linear, up to 100 mg/kg bw/day and
reached a plateau at doses greater than 100 mg/kg bw/day, which
suggests that the absorption of doramectin from the feed reached
saturation. The highest plasma concentration was 3.9 µg/ml.
A number of short-term toxicity studies were carried out in rats
for periods of up to 3 months. Administration of doramectin in the
diet at doses of 30 mg/kg bw/day and above resulted in markedly
reduced food intake and body-weight gain, as well as severe
neurotoxicity, which necessitated the early sacrifice of some of the
animals. Other findings at the same doses were atrophic changes in
hepatocytes and lymphoid organs and nephrosis. Minor increases in
liver weight were seen at 20 mg/kg bw/day in the diet, but hepatic
morphology was unaffected at this dose. The maximum plasma drug level
attained in these studies was 5.8 µg/ml. In a 1-month study in which
doramectin was given in the diet, the NOEL in rats was
10 mg/kg bw/day.
In two studies, rats were given doses of doramectin by gavage for
up to 3 months. Signs of CNS toxicity were observed at the highest
dose of 10 mg/kg bw/day. At doses of 5 mg/kg bw/day and above, there
were increases in liver weight, while kidney weight was increased at
8 mg/kg bw/day. However, there were no concomitant pathological
changes and all other parameters were unaffected. Plasma drug levels
reached a peak of 3.2 µg/ml. The NOEL in gavage studies in rats was
2 mg per kg of body weight per day.
When dogs were fed doramectin in the diet for periods of up to 36
days, the only treatment-related findings were mydriasis and reduced
body weight at a dose of 4 mg/kg bw/day. The highest plasma drug level
was 0.88 µg/ml. The NOEL in this study was 2 mg/kg bw/day.
In three studies in dogs, which received doramectin by gavage in
a sesame oil vehicle for up to 92 days, body weights were depressed at
4 mg/kg bw/day and clinical signs of CNS toxicity were noted at
2 mg/kg bw/day and above. The most sensitive indication of a
drug-related effect was mydriasis, which was seen at doses as low as
0.3 mg/kg bw/day. No other toxic effects were observed. The maximum
plasma drug level in these studies was 3 µg/ml. The NOEL was 0.1 mg/kg
bw/day.
Reproductive toxicity studies in rats revealed treatment-related
deaths among pups during the early postnatal period and a reduction in
body-weight gain of pups throughout the lactation period. These
effects were observed at doses as low as 3 and 1 mg/kg bw/day,
respectively. Doramectin was shown to be readily excreted in the milk
of lactating rats following administration by gavage and, as compared
with adult animals, higher drug levels were attained in the brain of
neonates, suggesting greater penetration through the incompletely
formed blood-brain barrier in newborn rats. The NOEL was 0.3 mg/kg
bw/day, based on toxicity in neonatal animals.
The administration of doramectin by gavage to pregnant mice and
rats did not result in fetal abnormalities. The only drug-related
finding was a slight increase in embryo mortality in mice given
6 mg/kg bw/day. The NOEL in mice was 3 mg/kg bw/day, while in rats
there were no adverse effects at the highest dose of 6 mg/kg bw/day.
In rabbits given 3 mg/kg bw/day during pregnancy, there was
significant maternal toxicity, and cleft palate was observed in three
fetuses from one litter; one fetus also exhibited phocomelia,
syndactyly and coelosomia. Ossification of pubic bones was delayed at
doses of 1.5 and 3 mg/kg bw/day. In a dose-ranging study, a dose of
6 mg/kg bw/day caused severe maternal toxicity and embryotoxicity. The
NOEL for maternal toxicity in rabbits was 0.75 mg/kg bw/day.
In view of the negative results in a range of in vitro
genotoxicity assays and a micronucleus test in mouse bone marrow, the
Committee concluded that doramectin was not genotoxic.
The Committee noted the close structural similarities between
doramectin and abamectin, the only difference being the presence of a
cyclohexyl group at the C-25 position in the doramectin molecule
rather than an isopropyl or isobutyl group in the case of abamectin
B1a and abamectin B1b, respectively. The available metabolic data
suggest that the biotransformation of doramectin and abamectin follows
a similar pathway. Extensive toxicological tests have been conducted
on both compounds, and the Committee reviewed several aspects of their
comparative toxicology. Both compounds exert a pharmacological effect
on the CNS through effects on gamma-aminobutyric acid-sensitive
neurons, which results in a range of neurotoxic signs such as
mydriasis, tremors, ataxia and gait abnormalities. In multiple-dose
studies, the compounds have been associated with few adverse
toxicological effects, reduced body-weight gain and minor toxic
effects on the liver being the most common. Toxicity in neonates is
the most sensitive indicator in reproductive toxicity studies with
both compounds. Neither compound is considered to have any genotoxic
activity. Carcinogenicity studies with abamectin were negative at
maximum tolerated doses in mice and rats. In view of the chemical,
biochemical and toxicological similarities, the Committee concluded
that it was unnecessary to request data from long-term toxicity and
carcinogenicity studies on doramectin.
4. EVALUATION
The Committee considered that the most relevant effect for the
safety evaluation of residues of doramectin was the effect on the
mammalian nervous system. An ADI of 0-0.5 µg/kg bw was established,
based on a NOEL of 0.1 mg/kg bw/day for mydriasis in the 3-month
gavage study in dogs. A safety factor of 200 was applied because the
test systems used to assess the neurotoxicity of doramectin were of
uncertain sensitivity (see section 2.2 of the Report, Annex 1,
reference 119). The ADI provides an adequate margin of safety for
neonatal toxicity in rats and developmental toxicity in rabbits.
5. REFERENCES
Cruthers, L.R. (1991). Dose Escalation Safety Study in Ivermectin
Sensitive Collies. Unpublished study No. 9112 from Professional
Laboratory and Research Services Inc. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Duce, I. R. & Scott, R.H. (1985). Actions of dihydroavermectin B1a
on insect muscle. British J. Pharmacol. 85. 395-401.
Graham, D., Pfeiffer, F. & Betz, H. (1982). Avermectin B1a inhibits
the binding of strychnine to the glycine receptor of rat spinal cord.
Neuroscience Letters. 29. 173-176.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991a). Diuretic
Assay. Unpublished study No. PH209-PZ-001-91 from Pharmakon Labs.
Submitted to WHO by Pfizer, Central Research, Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991b). Arterial
Blood Gas Determinations in Rats. Unpublished study
No. PH1051-PZ-001-91 from Pharmakon Labs. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Kass, I.S., Stretton, A.O.W. & Wang, C.C. (1984). The effects of
avermectin and drugs related to acetylcholine and 4-aminobutyric acid
on neurotransmission in Ascaris suum. Molec. Biochem. Parasit.
13. 213-225.
Pfizer (1987a). Comparison of Dosing Vehicles for Oral Exposure of
Rats. Unpublished study No. 87-657-07 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987b). Two Week Oral Feed Study on Long-Evans Rats.
Unpublished study No 87-657-03 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987c). Two Week Toleration Study in Beagle Dogs. Unpublished
study No. 87-657-04 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988a). Acute Oral and Intraperitoneal Toxicity Studies in
Mice and Rats. Unpublished study No. 87-657-01 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988b). A One Month Feeding Study in Long-Evans Rats at Dose
Levels of 20, 10, 5 and 0 mg/kg/day. Unpublished study No. 87-657-06
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1988c). A One Month Feed Study in Beagle Dogs at Dose Levels
of 4,2,1 and 0 mg/kg/day. Unpublished study No. 87-657-05 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988d). Preliminary Fetotoxicity Study (Maternal Toxicity) in
Mice by the Oral Route. Unpublished study No. 87094 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988e). Maternal Toxicity Study in Mice by the Oral Route.
Unpublished study No. 88035 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988f). Fetotoxicity Study in Mice by the Oral Route.
Unpublished study No. 88091/88092 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988g). Maternal Toxicity Study in Rats by the Oral Route.
Unpublished study No. 87165 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988h). UK-67,994: Maternal Toxicity Study in Rats by the Oral
Route. Unpublished study No. 88034 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988i). Fetotoxicity Study in Rats By the Oral Route.
Unpublished study No. 88079/88080 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988j). Maternal Toxicity Study in Rabbits by the Oral Route.
Unpublished study No. 87161 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988k). UK-67,994: Maternal Toxicity Study in Rabbits by the
Oral Route. Unpublished study No. 88077 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988l). Fetotoxicity Study in Rabbits by the Oral Route.
Unpublished study No. 88106/88107 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989a). A Five-Week Range Finding Gavage Study in Long-Evans
Rats. Unpublished study No. 88-657-11 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989b). A One Month Range Finding Gavage Study in Beagle Dogs.
Unpublished study No. 88-657-10 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989c). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 88-657-13 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990a). A Three Month Oral Gavage Study in Long-Evans Rats
with In Utero Exposure. Unpublished study No. 89-657-17 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1990b). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 89-657-19 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990c). A Two-Generation Gavage Study in Long-Evans Rats
(Terminated after first generation). Unpublished study No. 88-657-12
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1990d). An Exploratory Lactation Study in Long-Evans Rats.
Unpublished study No. 89-657-18 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991a). Acute Dermal and Ocular Irritation Studies in Albino
Rabbits. Unpublished study No. 91-657-22 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991b). A Two-Generation Gavage Study in Long-Evans Rats.
Unpublished study No. 89-657-20 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991c). Genetic Toxicology Report. Unpublished study
No. 87-657-02 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1991d). Genetic Toxicology (Microbial) Assays. Unpublished
study No. 91-67-81 from Pfizer Central Research, Taketoyo, Japan.
Submitted to WHO by Pfizer, USA.
Pfizer (1991e). Genetic Toxicology Report. Unpublished study
No. 90-657-21 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1992a). The Comparative Metabolism of 3H-Doramectin in
Cattle, Dog and Rat. Unpublished study No. CM-92-01 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992b). Single Dose Oral and Intraperitoneal Toxicity Studies
in Mice and Rats. Unpublished study No. 91-657-23 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992c). Two Generation Study in Long-Evans Rats (Terminated
prior to completion). Unpublished study No. 88-657-15 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992d). Range Finding Fetal/Neonatal Toxicity Study in
Long-Evans Rats. Unpublished study No. 88-657-16 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993a). Identification of Residues of Doramectin in Liver and
Fat of Cattle Sacrificed 21-Days Following SC Administration of
[3H]-Doramectin at 0.2 mg/kg. Unpublished study No. CM-93-01 from
Pfizer Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993b). One Month Range Finding Feeding Study in Long-Evans
Rats. Unpublished study No. 92-657-24 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994a). Single Dose Oral Toxicity Study in Albino (CD-1) Mice.
Unpublished study No. 94-657-29 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994b). A Range Finding Feed Study in CD-1 Mice. Unpublished
study No. 92-675-25 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1994c). Three Month Range Finding Feeding Study in CD-1 Mice.
Unpublished study No. 92-657-27 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994d). Three Month in Feed Study in Long-Evans Rats.
Unpublished study No. 92-657-26 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1995). Comparison of Plasma/Brain Ivermectin (B1a) Ratios in
CD-1 and CF-1 Mice. Unpublished study No. AHDM-94-08 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1991). Gastro-
intestinal Propulsion Assay in Male Mice. Unpublished study
No. PH239-PZ-001-91 from Pharmakon Labs. Submitted to WHO by
Pfizer, USA.
Zufall, F., Franke, Ch. & Hatt, H. (1989). The insecticide avermectin
B1a activates a chloride channel in crayfish muscle membrane.
J. Exp. Biol. 142. 191-205.
 DORAMECTIN
First draft prepared by
Dr G. Roberts
Toxicology Evaluation Section
Department of Human Services and Health
Canberra, Australia
Explanation
Biological data
Biochemical aspects
Absorption
Metabolism
Pharmacological effects
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Reproductive toxicity studies
Special studies on neonatal toxicity
Special studies on embryotoxicity and teratogenicity
Special studies on genotoxicity
Special studies on permeability of blood-brain barrier
Special studies on pharmacology
Comments
Evaluation
References
1. EXPLANATION
Doramectin is the fermentation product of a specific strain of
Streptomyces avermitilis that has close structural similarities with
abamectin and ivermectin. It is used as an endoparasitic agent in
non-lactating cattle. Doramectin had not been previously reviewed by
the Committee.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption
2.1.1.1 Rats
Groups of 5 Long-Evans rats were administered single doses of
doramectin as follows:
A - 3 mg/kg bw given over a 2-hour period in the diet
B - 5 mg/kg bw in methyl cellulose vehicle
C - 5 mg/kg bw in sesame oil vehicle
Peak drug levels were achieved in 3 h for groups A and B, and at
7 h for group C. Maximum levels were 0.06, 0.06 and 0.36 µg/ml,
respectively. The areas under the plasma concentration-time curves
(AUC) were 1.65, 1.71 and 9.69 µg-h/ml for groups A, B, and C,
respectively (Pfizer, 1987a).
2.1.2 Metabolism
Doramectin labelled with tritium in the 5-position was
administered as a single dose to Sprague-Dawley rats (2 males given
5 mg/kg bw in propylene glycol:glycerol by gavage), a beagle dog
(1 female given 3.5 mg/kg bw in sesame oil by gavage) and cattle
(5 males given 0.2 mg/kg bw subcutaneously). The liver and faeces from
each species and the fat of cattle were sampled for characterization
of metabolites by liquid chromatography (Pfizer, 1992a).
In a second experiment, 2 male and 2 female cattle were given a
single s.c. injection of 0.2 mg/kg bw 3H-doramectin. Metabolites in
liver and fat were analyzed by liquid chromatography (Pfizer, 1993a).
As shown in Table 1, the metabolites detected in liver and faeces
from each species were similar in nature indicating comparable
degradation pathways in rats, dogs and cattle.
Table 1. Percent of tritium radiolabel recovered from sample.
24-hydroxymethyl-
Total Unchanged 3"-O-desmethyl 24-hydroxymethyl 3"-O-desmethyl
recovered doramectin doramectin doramectin doramectin
Sample (%) (%) (%) (%) (%)
Rat liver 37 18 12 3 2
Rat faeces na 22 19 14 16
Dog liver 51 28 12 nd nd
Dog faeces 46 6 8 5 4
Cattle liver 95 70 9 nd 7
Cattle faeces 75 24 14 5 4
Cattle fat * 79 68 nd nd nd
Cattle liver 82 49 5.7 3.6 6.6
Cattle fat # 82 75 nd nd nd
na not available
nd not detected
* 8% of radioactivity was associated with mixed fatty acid esters of 24-hydroxymethyl
doramectin but structural identification was not pursued.
# 6% of radioactivity was identified as the 2-epimer of doramectin.
The metabolism of doramectin in mammals is presented in Figure 1.
Figure 1. Metabolic pathway of doramectin in mammals
DORAMECTIN
First draft prepared by
Dr G. Roberts
Toxicology Evaluation Section
Department of Human Services and Health
Canberra, Australia
Explanation
Biological data
Biochemical aspects
Absorption
Metabolism
Pharmacological effects
Toxicological studies
Acute toxicity studies
Short-term toxicity studies
Reproductive toxicity studies
Special studies on neonatal toxicity
Special studies on embryotoxicity and teratogenicity
Special studies on genotoxicity
Special studies on permeability of blood-brain barrier
Special studies on pharmacology
Comments
Evaluation
References
1. EXPLANATION
Doramectin is the fermentation product of a specific strain of
Streptomyces avermitilis that has close structural similarities with
abamectin and ivermectin. It is used as an endoparasitic agent in
non-lactating cattle. Doramectin had not been previously reviewed by
the Committee.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption
2.1.1.1 Rats
Groups of 5 Long-Evans rats were administered single doses of
doramectin as follows:
A - 3 mg/kg bw given over a 2-hour period in the diet
B - 5 mg/kg bw in methyl cellulose vehicle
C - 5 mg/kg bw in sesame oil vehicle
Peak drug levels were achieved in 3 h for groups A and B, and at
7 h for group C. Maximum levels were 0.06, 0.06 and 0.36 µg/ml,
respectively. The areas under the plasma concentration-time curves
(AUC) were 1.65, 1.71 and 9.69 µg-h/ml for groups A, B, and C,
respectively (Pfizer, 1987a).
2.1.2 Metabolism
Doramectin labelled with tritium in the 5-position was
administered as a single dose to Sprague-Dawley rats (2 males given
5 mg/kg bw in propylene glycol:glycerol by gavage), a beagle dog
(1 female given 3.5 mg/kg bw in sesame oil by gavage) and cattle
(5 males given 0.2 mg/kg bw subcutaneously). The liver and faeces from
each species and the fat of cattle were sampled for characterization
of metabolites by liquid chromatography (Pfizer, 1992a).
In a second experiment, 2 male and 2 female cattle were given a
single s.c. injection of 0.2 mg/kg bw 3H-doramectin. Metabolites in
liver and fat were analyzed by liquid chromatography (Pfizer, 1993a).
As shown in Table 1, the metabolites detected in liver and faeces
from each species were similar in nature indicating comparable
degradation pathways in rats, dogs and cattle.
Table 1. Percent of tritium radiolabel recovered from sample.
24-hydroxymethyl-
Total Unchanged 3"-O-desmethyl 24-hydroxymethyl 3"-O-desmethyl
recovered doramectin doramectin doramectin doramectin
Sample (%) (%) (%) (%) (%)
Rat liver 37 18 12 3 2
Rat faeces na 22 19 14 16
Dog liver 51 28 12 nd nd
Dog faeces 46 6 8 5 4
Cattle liver 95 70 9 nd 7
Cattle faeces 75 24 14 5 4
Cattle fat * 79 68 nd nd nd
Cattle liver 82 49 5.7 3.6 6.6
Cattle fat # 82 75 nd nd nd
na not available
nd not detected
* 8% of radioactivity was associated with mixed fatty acid esters of 24-hydroxymethyl
doramectin but structural identification was not pursued.
# 6% of radioactivity was identified as the 2-epimer of doramectin.
The metabolism of doramectin in mammals is presented in Figure 1.
Figure 1. Metabolic pathway of doramectin in mammals
 2.1.3 Pharmacological effects
Avermectins induce rapid, non-spastic paralysis in nematodes and
arthropods. One common feature of avermectins appears to be the
modulation of trans-membrane chloride ion (Cl-) channel activity in
nematode nerve cells, and in both nerve and muscle cells of
arthropods. These Cl- channels may be gated by a variety of
neurotransmitter receptors including gamma-aminobutyric acid (GABA),
glutamate and acetylcholine (Kass et al, 1984; Duce & Scott, 1985;
Zufall et al, 1989). Activation of the Cl- channels by avermectins
leads to an increase in Cl- conductance which results in a changed
membrane potential and this causes inhibition of electrical activity
in the target nerve or muscle cell. GABA is also a major inhibitory
neurotransmitter in the mammalian CNS and avermectins do have
intrinsic activity on the mammalian GABA receptor/Cl- channel
complex.
Avermectins have been reported to bind to glycine receptor/Cl-
channel complexes which are restricted to the CNS in mammals
(Graham et al, 1982). Penetration of the blood brain barrier by
avermectins is extremely poor and this may account for the wide margin
of safety exhibited by these compounds following administration to
mammals.
2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with doramectin are
summarized in Table 2.
Clinical signs of toxicity were similar in both mice and rats and
by both routes of administration, the most common being decreased
activity and respiration, hunched position, shakiness, weakness,
tremors, ataxia and weight loss.
Doramectin was applied to normal and abraded skin of 3 New
Zealand white rabbits (0.5 g under an occlusive patch) for 24 h.
Slight erythema was observed 24 and 48 h post-dose at 1 of 3 intact
sites and 2 of 3 abraded sites. There was no edema at any site and all
sites appeared normal 72 h post-dose.
Table 2. Results of acute toxicity studies with doramectin.
Species
(strain) Route Vehicle1 Sex LD50 in mg/kg bw2 Reference
Mouse po aqueous M&F > 2000(0/3) Pfizer, 1988a
(CD-1)
po oil F 250(0/3)-500(3/3) Pfizer, 1992b
po oil F 75(0/5)-200(5/5) Pfizer, 1994a
ip aqueous M 700(0/3)-1000(2/3) Pfizer, 1988a
ip oil M 100(0/3)-250(3/3) Pfizer, 1992b
Rat po aqueous M 1000(0/3)-2000(3/3) Pfizer, 1988a
(Sprague-Dawley) F 500(0/3)-1000(2/3) Pfizer, 1988a
po oil M 50(0/3)-100(2/3) Pfizer, 1992b
F 100(1/3)-200(3/3) Pfizer, 1992b
ip aqueous M >300(1/3) Pfizer, 1988a
ip oil M 50(0/3)-100(3/3) Pfizer, 1992b
1 aqueous vehicle was 0.1% aqueous methylcellulose; oil vehicle was sesame oil.
2 figures in brackets represent mortality incidences.
Doramectin powder (18.8 mg) was instilled into the conjunctival
sac of one eye in each of 3 New Zealand white rabbits. Slight corneal
and conjunctival reddening, chemosis and iritis were seen within 1 h
of dosing. The changes subsided within 6 h and all eyes were normal by
48 h post-dose (Pfizer, 1991a).
A comparison of the acute toxicity of different ivermectins in
mice is given in Table 3.
Table 3. Acute oral toxicity of doramectin in comparison with abamectin, ivermectin
and moxidectin in female CD-1 mice (Pfizer, 1994a).
Dose in mg/kg bw1 Doramectin Abamectin Ivermectin Moxidectin
Maximum
"asymptomatic" dose 25 5 255
Minimum
"symptomatic" dose 75 25 5025
Minimum
lethal dose2 200(5/5) 75(5/5) 75(1/5) 75(4/5)
LD50 dose 75-200 25-75 >75 25-75
1 - vehicle was sesame oil
2 - figures in brackets represent mortality incidences.
Signs of CNS toxicity were similar after dosing with each
compound and included gait abnormalities with splayed hind limbs,
intermittent tremors, ataxia and decreased, irregular or laboured
respiration (Pfizer, 1994a).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of CD-1 mice (10/sex/dose) were administered doramectin
(purity 94.1%) in the diet for 43 days. Target doses were 0, 10, 20,
40, or 60 mg/kg bw/day on days 1 to 14; 0, 10, 20, 80 or 100 mg/kg
bw/day on days 15 to 28; and 0, 100, 200, 400 or 600 mg/kg bw/day
on days 29 to 43.
Toxic signs included lethargy, hunched posture and tremors at 400
and 600 mg/kg bw/day, and hunched and ungroomed appearance in some
mice at 100 and 200 mg/kg bw/day. A number of animals given 400 and
600 mg/kg bw/day were sacrificed moribund and the remaining mice in
these groups were killed on day 33. There were no effects on body
weight or food consumption. Relative liver weights were slightly
higher in most treated groups but the changes were minor and not
dose-related. Clinical laboratory parameters and pathological
examinations were not carried out.
Plasma drug levels were dose-related, although non-linear, up to
100 mg/kg bw/day, and reached a plateau at higher doses. Drug levels
ranged from 0.23 µg/ml at 10 mg/kg bw/day to 3.9 µg/ml at 600 mg/kg
bw/day. An unidentified degradation product, represented a
progressively greater fraction of drug-related material at doses of
100 mg/kg bw/day and above (Pfizer, 1994b).
Groups of CD-1 mice (10/sex/group) were fed doramectin (purity
94.1%) in the diet for 92 days. Target doses were 0, 100, 200 or
300 mg/kg bw/day. Actual doramectin intake was 83-121, 154-191 or
221-322 mg/kg bw/day. Tremors, hunched posture, unkempt appearance and
lethargy were observed at the mid- and high-dose groups and resulted
in death or moribund sacrifice of 9 mice at 300 mg/kg bw/day and 3
mice at 200 mg/kg bw/day. The remaining animals in these two groups
were killed on day 12 or 19, respectively. Body-weight gain was
depressed in association with reduced food consumption at 200 mg/kg
bw/day and above.
Serum creatinine and BUN were slightly increased at 100 mg/kg
bw/day with no effects at higher doses, presumably due to early
culling of mice in these groups. Haematological parameters were
unaffected. All treated groups had increased liver weights and
hypertrophy of centrilobular hepatocytes, with multinucleate
non-proliferative liver cells at 100 mg/kg bw/day only. Dead and
moribund animals showed lymphocyte lysis in lymphoid organs, cellular
depletion of bone marrow and necrosis of the adrenal cortex which may
have resulted from stress and weight loss.
Satellite groups of 3 mice/sex/dose were used to determine plasma
drug levels. In the 100 mg/kg bw/day group, concentrations reached a
plateau at 2.8 µg/ml by day 45. Doramectin levels were not markedly
different at higher doses with peaks of 3.6 µg/ml and 2.7 µg/ml at 200
and 300 mg/kg bw/day, respectively. An unidentified degradation
product was present at levels proportional to both dose and duration
of treatment (Pfizer, 1994c).
2.2.2.2 Rats
Groups of Long-Evans rats (3/sex/group) were given 0, 2.5, 5 or
10 mg/kg bw/day doramectin (purity unspecified) in the diet for 14
days. There were no overt signs of toxicity and no effects on body
weight, haematology, serum chemistry, urinalysis, liver or kidney
weight. Examination of a limited number of organs revealed no
pathological changes. Mean plasma drug levels at day 10 were
approximately 0.05, 0.06 and 0.17 µg/ml with increasing doses
(Pfizer, 1987b).
In a 1-month study, groups of Long-Evans rats (10/sex/group) were
fed doramectin (purity 95.3%) in the diet at dose levels of 0, 5, 10
or 20 mg/kg bw/day. There were no clinical signs of toxicity and no
effects on food intake or body-weight gain. Ophthalmology,
haematology, serum chemistry and urinalysis were unaffected. Liver
weight was slightly increased in males given 20 mg/kg bw/day but
pathological examination was unremarkable. Plasma drug levels were
proportional to the dose; maximum concentrations achieved were 0.28,
0.76 and 1.79 µg/ml, respectively. The NOEL in this study was 10 mg/kg
bw/day (Pfizer, 1988b).
Groups of Long-Evans rats (10/sex/group) were fed a diet
containing doramectin (purity 94.1%) at levels intended to give
target doses of 0, 20, 40, 60 or 80 mg/kg bw/day for 38 days.
Chromorhinorrhea, chromodacryorrhea and urogenital staining were seen
at 40 mg/kg bw/day and above. Whole body tremors were also present at
the two highest doses and resulted in the sacrifice of 2 rats at
60 mg/kg bw/day and all rats at 80 mg/kg bw/day. Body-weight gain and
food consumption were lower at 60 mg/kg bw/day and above. Serum
5'-nucleotidase was increased at 80 mg/kg bw/day with elevated levels
of BUN at 40 mg/kg bw/day and above. Liver weights were higher in the
40 and 60 mg/kg bw/day groups but with no dose relationship. Organs
were not examined for pathology.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin concentrations were progressively increased
throughout the study at each dose, and levels were dose-related up to
60 mg/kg bw/day with no further increase at 80 mg/kg bw/day. Highest
levels were 0.89, 3.52, 3.78 and 3.54 µg/ml at the 4 doses,
respectively (Pfizer, 1993b).
Groups of Long-Evans rats (5/sex/group) were administered by
gavage doses of 0, 2, 5 or 10 mg/kg/bw/day doramectin (purity 92.1%)
in sesame oil for 38 days. Three females in the 10 mg/kg bw/day group
were sacrificed after showing hunched appearance, ataxia, tremors and
urine stains. There were no effects on body weight, food consumption,
haematology, serum chemistry or urinalysis. Some rats given 5 and
10 mg/kg bw/day had increased liver weights but pathological
examination of a limited number of organs was unremarkable. Plasma
drug levels were similar on days 10 and 38 and were dose-related;
approximate levels were 0.5, 1.6 and 3.0 µg/ml in the low-, mid- and
high-dose groups, respectively (Pfizer, 1989a).
Groups of Long-Evans rats (10/sex/dose) were fed doramectin
(purity 89.7%-94.1%) in the diet at target doses of 0, 30, 40 or
50 mg/kg bw/day, for 92 days. Based on food consumption, actual
doramectin intake ranged from 16-32, 11-49 and 14-59 mg/kg bw/day,
respectively. Thus systemic exposure to doramectin was similar in each
treated group which was reflected in the toxicological findings.
Clinical signs, noted in all treated animals, included
chromorhinorrhea, chromodacryorrhea, urine stains and tremors. Seven
rats at 30 mg/kg bw/day, all rats at 40 mg/kg bw/day, and 19 rats at
50 mg/kg bw/day died or were sacrificed moribund. Body-weight gain was
markedly reduced in all treated groups in association with diminished
food intake. Ophthalmological examinations were unchanged. All treated
groups showed decreased leucocytes, erythrocytes, haemoglobin and
haematocrit, and increased BUN. Serum levels of ALAT and ASAT were
increased and protein decreased in some animals given 40 and 50 mg/kg
bw/day. Urinalysis was unaffected.
At necropsy, animals which failed to survive the treatment period
showed minimal body fat and a few had stomach erosions. Apparent
increases in relative kidney and testes weights in treated groups were
probably due to the lower body weights. Pathological changes, observed
in all treated groups, comprised kidney nephrosis with protein
accumulation in the corticomedullary junction, hepatocyte atrophy with
occasional necrotic cells and haemosiderosis, lymphoid depletion in
thymus, spleen and mesenteric lymph node, bone marrow atrophy, and
lipid depletion in the adrenal cortex.
Satellite groups of 5 rats/sex/dose were treated in order to
determine plasma drug levels. Doramectin plasma concentrations
increased during the course of the study in the 30 and 40 mg/kg bw/day
groups, attaining levels of 4.6 and 5.8 µg/ml, respectively. A maximum
level of 4.1 µg/ml was achieved by day 16 in rats given 50 mg/kg
bw/day (Pfizer, 1994d).
Groups of Long-Evans rats (20/sex/dose) were selected from the
F1 offspring in a multigeneration reproductive toxicity study in
which doramectin was administered by gavage at doses of 0, 0.1, 0.3 or
1.0 mg/kg bw/day. Thus the animals had received secondary exposure in
utero and during lactation, and direct exposure for 3 to 4 weeks
post-weaning. Subsequently, the respective groups were administered
gavage doses of 0, 0.5, 2 or 8 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil for 3 months.
There were no overt signs of toxicity, mortality or treatment-
related ocular lesions. Initial body weights of the 8 mg/kg bw/day
group were lower which may be attributed to the prior in utero
or neonatal exposure to doramectin. During the course of the study
proper, treated groups gained more weight than controls. Food
consumption, haematology, serum chemistry and urinalysis were
unaffected. Liver and kidney weights were increased at 8 mg/kg bw/day
but there were no concomitant pathological alterations.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin levels were dose-related with mean levels
of 0.07, 0.4 and 2.5 µg/ml on day 3, and 0.1, 0.7 and 3.2 µg/ml on day
87, in the low-, mid-, and high-dose groups, respectively. The NOEL in
this study was 2 mg/kg bw/day (Pfizer. 1990a).
2.2.2.3 Dogs
Groups of beagle dogs (1/sex/group) were fed doramectin (purity
unspecified) in the diet at dose levels of 0, 0.5, 1 or 2 mg/kg bw/day
for 14 days. There were no signs of toxicity or effects on body
weight, haematology, serum chemistry or urinalysis. Dogs were not
sacrificed. Plasma drug levels increased during the course of the
study attaining concentrations of approximately 0.045, 0.07 and
0.26 µg/ml, respectively on day 8 (Pfizer, 1987c).
Groups of beagle dogs (1/sex/group) were given gavage doses of
0, 1, 2 or 4 mg/kg bw/day doramectin (purity 92.1%-93.3%) in sesame
oil for 29 days. Body weight was depressed in both animals
administered 4 mg/kg bw/day and the male showed salivation, mydriasis,
tremors, ataxia and decreased heart rate prior to sacrifice on day 16.
All drug-treated dogs exhibited mydriasis. There were no effects on
haematology, serum chemistry, urinalysis, organ weight or pathological
examinations. Plasma drug levels were proportional to the dose,
highest concentrations achieved were approximately 0.4, 1.2 and
3.0 µg/ml, respectively (Pfizer, 1989b).
Groups of beagle dogs (3/sex/group) were administered doramectin
(purity 95.3%) in the diet at doses of 0, 1, 2 or 4 mg/kg bw/day for
36 days. Apart from mydriasis in two dogs given 4 mg/kg bw/day, there
were no clinical signs of toxicity. Body weight was depressed in the
4 mg/kg bw/day group during the first 3 weeks but returned to baseline
thereafter. Haematology, serum chemistry, urinalysis, organ weights
and pathology were unaffected. Plasma drug levels increased with
duration of dosing, reaching maximum concentrations of 0.21, 0.5 and
0.88 µg/ml, at the low-, mid- and high-dose groups, respectively. The
NOEL was 2 mg/kg bw/day. (Pfizer, 1988c).
Beagle dogs (4/sex/group) were given gavage doses of 0, 0.5, 1 or
2 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 91 days. A
slow pupillary light reflex was noted in all treated animals and
mydriasis was diagnosed in 1, 2, and 5 dogs given 0.5, 1, and 2 mg/kg
bw/day, respectively. One animal given 2 mg/kg bw/day showed anorexia,
tremors and ataxia and was sacrificed on day 23. No effects were
observed on body weight, haematology, serum chemistry, urinalysis,
organ weight or pathology. Plasma drug levels were dose-related but
were higher on day 30 than on day 90. The levels were approximately
0.29, 0.5 and 1.2 µg/ml on day 30 and 0.24, 0.3 and 0.6 µg/ml on day
90, at the 3 respective doses (Pfizer, 1989c).
Beagle dogs (3/sex/dose) were given gavage doses of 0, 0.1 or
0.3 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 92 days.
The only treatment-related finding was mild to moderate mydriasis
which was diagnosed in 1 female given 0.3 mg/kg bw/day. Plasma drug
levels were dose-related, with approximate mean concentrations of 0.1
and 0.28 µg/ml, respectively. The NOEL in this study was 0.1 mg/kg
bw/day (Pfizer, 1990b).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 1.5, 3 or
6 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Males were
dosed from 10 weeks before pairing and to the end of the mating
period. Females were dosed from 2 weeks prior to pairing until the end
of the study.
There were no effects on food consumption or body weight of F0
adults, and copulation, pregnancy rate and gestation length were
unaffected. Size and weight of F1 litters at birth were similar
between groups. During lactation, pup weights decreased in the 3 and
6 mg/kg bw/day groups and most of these pups died by day 7
post-partum. Survival was unaffected in the 1.5 mg/kg bw/day group but
body-weight gain was lower in these offspring and the study was
discontinued on lactation day 7.
Subsequently, a number of F0 dams with litters (culled to 8
pups/litter), were assigned to 6 groups consisting of 3 females each.
Gavage doses of 0, 0.25, 0.5, 1, 3 or 6 mg/kg bw/day were administered
on lactation days 12 to 21 to determine potential effects on older
pups. Pup survival was not affected but body-weight gain was reduced
at 6 mg/kg bw/day (Pfizer, 1990c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 0.1, 0.3
or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Treatment
of F0 males commenced 10 weeks prior to pairing, continuing until
the end of the mating period. F0 females were treated from 2 weeks
before pairing, through gestation until the end of lactation. Food
intake, body-weight gain, copulation, pregnancy rate and duration of
gestation were unaffected in F0 rats and gross examination of these
animals did not reveal any abnormalities.
F1 litter size, weight at birth, and post-natal survival were
similar in all groups. There was slight depression of body-weight gain
of the 1 mg/kg bw/day pups during lactation and the weight of these
animals remained below other groups for the remainder of the study.
Where necessary, F1 litters were reduced to 8 pups each on lactation
day 4. At weaning, 25 male and 25 female pups/group were randomly
selected to produce the F2 generation and were dosed until the end
of the study. On pairing of F1 rats, copulation incidences were
adversely affected leading to low pregnancy rates in all groups, most
notably in the control.
F2 litter sizes at birth were lower than normal in all groups
but subsequent survival was not affected. Body weights of the 1 mg/kg
bw/day offspring were lower throughout the lactation period. In
limited tests for post-natal development in 1 male and 1 female/litter,
there were no significant treatment-related deficits. Gross examination
of treated F1 rats and F2 pups showed no abnormalities.
Due to the adverse effects on conception described above, the
study was terminated, except for rats in the F1 control group which
were paired for a second mating. Again, pregnancy rates were very low,
and vaginal smears indicated irregular estrous cycling in many of the
females with some not cycling at all (Pfizer, 1992c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were administered gavage doses of
0, 0.1, 0.3 or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil.
F0 males were treated from 10 weeks prior to pairing until the end
of mating, and females were dosed from 2 weeks prior to pairing,
during mating, gestation, and lactation periods. There were no effects
on food consumption or body weight of F0 rats and copulation,
pregnancy rate and gestation length were unaffected. Gross examination
of these animals was unremarkable.
F1 litter size and weight at birth and subsequent survival and
growth were similar in all groups. Each F1 litter was culled to 8
pups, where necessary, on lactation day 4. At weaning, 30 male and 30
female pups/group were randomly selected to produce the F2
generation and were dosed directly until study termination. These F1
rats were paired on two occasions, at age 11 to 12 weeks and 22 to
23 weeks. On both occasions, copulation and duration of gestation were
unaffected. However, conception was impaired in all groups, the
pregnancy rate being approximately 70% for the F2a and 50% for the
2bF matings, respectively. Gross examinations of F1 rats showed no
internal or external abnormalities.
In both the F2a and F2b litters, the number and weight of
offspring at birth, and survival to the end of lactation were not
affected by treatment. Pup body-weight gain was depressed in the
1 mg/kg bw/day group. On lactation day 21, 1 male and 1 female
pup/F2a litter were tested for locomotor activity and auditory
function and were given an ophthalmoscopic examination. No
treatment-related effects were observed in these developmental tests
or on gross examination of all F2a and F2b pups. The NOEL in this
study was 0.3 mg/kg bw/day (Pfizer, 1991b).
2.2.4 Special studies on neonatal toxicity
2.2.4.1 Rats
Groups of 7 (2 in control group) presumed pregnant Long-Evans
rats were given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin
(purity 92.5%) in sesame oil from gestation day 2 to day 3 post-partum.
There was no effect on body weight of dams but 1 female given 6 mg/kg
bw/day was sacrificed moribund. Milk, blood and brain of dams were
collected 3-4 h after the last dose. Blood and brain from the pups
were collected 24 h after dosing of the dams.
Analysis of drug levels in plasma samples revealed higher levels
in dams than in pups. However, drug levels in the brain of dams were
generally lower than in pup brains. The plasma to brain ratio was
approximately 16 in dams and 2 in pups indicating that doramectin was
more accessible to the CNS of neonates. Milk to plasma ratios in dams
were about 2-3, indicating that the drug was readily excreted in milk
(Pfizer, 1990d).
Groups of 10 presumed pregnant Long-Evans rats were given gavage
doses of 0, 0.1, 0.2, 0.5 or 1 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil during gestation days 4 to lactation day 21. Litters
were culled to 8 pups each on lactation day 4, surviving pups and dams
were killed on lactation day 21. There was no effect on body weight of
dams. One dam given 1 mg/kg bw/day showed piloerection and lethargy
and died on day 13 of treatment. Litter size and weight at birth were
unaffected. At a dose of 1 mg/kg bw/day, pups body-weight gain was
reduced during lactation and 6 of 8 pups in a single litter died
(Pfizer, 1992d).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 9 presumed pregnant CD-1 mice were given gavage doses
of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin (purity 93.6%)
in sesame oil on gestation days 6 to 13. There were no effects on body
weight of dams, one female given 1.6 mg/kg bw/day had blood in the
urogenital area and was found dead on gestation day 12. The remaining
dams were killed on gestation day 18. Embryomortality and number of
live fetuses were similar between groups. Fetal weights were lower at
1.6 mg/kg bw/day. Fetuses were not examined (Pfizer, 1988d).
Groups of 7 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%) in sesame oil
on gestation days 6 to 13. One 6 mg/kg bw/day female showed
hypothermia, piloerection and blood on the vagina, but there were no
treatment-related deaths and no effects on body weight. Dams were
killed on gestation day 18. There were no effects on embryomortality,
number of live fetuses or fetal body weight. Fetuses were not examined
(Pfizer, 1988e).
Groups of 20 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%) in sesame oil
on gestation days 6 to 13. There were no drug-related deaths or
clinical signs in dams and body weight was unaffected. Dams were
killed on gestation day 18. Embryomortality was increased at 6 mg/kg
bw/day but did not attain statistical significance. Fetal body weight
and the incidence of fetal abnormalities were unchanged.
An additional group of 10 mice were similarly treated with
6 mg/kg bw/day for analysis of drug levels on gestation day 13. Plasma
drug levels in dams, 1 h after the last dose varied from 0.088 to
0.28 µg/ml. Six hours after dosing, plasma levels in dams were between
0.37 and 0.58 µg/ml, amniotic fluid levels varied from non-detectable
to 0.019 µg/ml and fetal levels varied from non-detectable to
0.12 µg/g. The NOEL in this study was 3 mg/kg bw/day (Pfizer, 1988f).
2.2.5.2 Rats
Groups of 6 or 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 6 to 15. Dams showed no
signs of toxicity or effects on body weight. Females were killed on
gestation day 20. Embryomortality, number of live fetuses and fetal
body weight were similar between groups. Fetuses were not examined
(Pfizer, 1988g).
Groups of 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. No toxic signs or adverse effects on body weight were noted in
dams. Embryotoxicity and numbers of live fetuses and fetal body weight
were unaffected. Fetuses were not examined (Pfizer, 1988h).
Groups of 20 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. There were no effects on body weight of dams. Embryomortality
was increased at 6 mg/kg bw/day but was not statistically significant
and was within the historical control range. Fetal examination
revealed slightly increased incidences of rudimentary ribs, wavy ribs,
unossified hyoid and 5th metacarpals, and dilatation of ureters and
kidney pelves, but the incidences were not dose-related and were
within the historical control range.
A satellite group of 10 rats were administered 6 mg/kg bw/day and
used for drug analysis on gestation day 15. Plasma drug levels in
dams, 1-5 h after dosing, varied from 0.41 to 1.27 µg/ml. Five hours
after the dose, amniotic fluid showed a mean level of 0.014 µg/ml and
fetal levels were between 0.27 and 1.1 µg/g. The NOEL in this study
was 6 mg/kg bw/day, the highest dose tested (Pfizer, 1988i).
2.2.5.3 Rabbits
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.2, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 7 to 18. Body-weight
gain was reduced in the 1.6 mg/kg bw/day group during gestation days 1
to 19. Does were killed on gestation day 28. Embryomortality was
slightly increased at 1.6 mg/kg bw/day but with no effect on fetal
weight. Fetuses were not examined. Plasma drug levels in dams given
1.6 mg/kg bw/day, 2 h after dosing on gestation day 17, ranged between
0.037 and 0.42 µg/ml (Pfizer, 1988j).
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity
92.1%) in sesame oil on gestation days 7 to 18. Body-weight gain was
significantly depressed at 6 mg/kg bw/day, in association with reduced
food consumption, on gestation days 7 to 28. Dams were killed on
gestation day 28. Embryonic death was increased and foetal weight was
slightly depressed in the 6 mg/kg bw/day group. Fetuses were not
examined (Pfizer, 1988k).
Groups of 20 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.75, 1.5 or 3 mg/kg bw/day doramectin
(purity 92.1%) in sesame oil on gestation days 7 to 18. There was no
drug-related mortality but during the treatment period, food intake
was reduced at 1.5 and 3 mg/kg bw/day with reduced body-weight gain at
3 mg/kg bw/day. Embryomortality and fetal weight were similar between
groups. Major malformations, occurring in the 3 mg/kg bw/day group
only, were cleft palate in 3 fetuses from 1 litter and 1 fetus which
exhibited phocomelia, syndactyly and coelosomia. The incidence of
cleft palate was within the historical control range whereas the other
fetal abnormalities had never been observed in the testing laboratory.
Ossification of pubic bones was delayed at 1.5 and 3 mg/kg bw/day.
A satellite group of 4 rabbits were dosed with 3 mg/kg bw/day and
sacrificed on gestation day 18 for analysis of drug levels. Maternal
plasma levels, measured 1, 3 and 5 h after dosing, ranged from 0.126
to 0.838 µg/ml. Doramectin was not detected in amniotic fluid or fetal
tissue, 5 h after the last dose, but detection limits were relatively
high due to interfering peaks. The NOEL in this study was 0.75 mg/kg
bw/day (Pfizer, 1988l).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with doramectin are given in
Table 4.
2.2.7 Special study on permeability of blood-brain barrier
2.2.7.1 Mice
Groups of 3 adult female CD-1 and CF-1 mice were given a single
gavage dose of 0.4 mg/kg bw ivermectin in sesame oil. Additional
groups received an i.p. injection of 50 mg/kg bw CP-100,356, an
inhibitor of p-glycoprotein activity, 1 h prior to ivermectin
administration. Animals were killed 24 h after drug dosing for removal
of blood and brain samples.
In CD-1 mice, ivermectin B1a could not be detected in plasma
(limit of quantitation 10 ng/ml), while brain levels were
1.38-1.98 ng/g. Pretreatment with CP-100,356 resulted in plasma levels
of 17.2-18.7 ng/ml and brain levels of 1.95-2.13 ng/g.
In CF-1 mice, ivermectin B1a levels in plasma were
14.9-21.0 ng/ml and brain levels were 109, 3.69 and 2.59 ng/g.
Pretreatment with CP-100,356 resulted in plasma levels of 30.1, 33.1
and 17.4 ng/ml and brain levels of 6.06, 8.97 and 1.98 ng/g.
Pretreatment with the p-glycoprotein inhibitor did not markedly affect
plasma or brain drug levels in either strain. Since the study used
very few animals and the treatment schedule and tissue sampling times
were not justified, no conclusion can be drawn from this study
(Pfizer, 1995).
2.2.8 Special studies on pharmacology
Table 5 summarizes the results of pharmacological studies with
doramectin.
Table 4. Results of genotoxicity assays on doramectin
Test system1 Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.02 to 10 mg/plate negative Pfizer, 1991c
TA98, TA100 (-S9)3
TA1535, 0.005 to 2 mg/plate negative
TA 1537 (+S9)3
Urine from mice negative
dosed with 0.2 to
4 mg/kg ip (-S9)3
Reverse mutation2 S. typhimurium 9.8 to 5000 µg/plate negative Pfizer, 1991d
TA98, TA100, (±S9)3
TA1535, TA1537
E. coli WP2 uvrA.
Forward mutation2 L5178Y mouse 8 to 35 µg/ml negative Pfizer, 1991c
lymphoma cells. (-S9)3
13 to 62 µg/ml negative
(+S9)3
UDS assay Primary culture 1.7 to 10 µg/ml3 negative Pfizer, 1991c
of rat hepatocytes.
Micronucleus Mouse bone 500 to 2000 mg/kg negative Pfizer, 1991e
test marrow bw/day for 3 days,
po.4
1. Positive controls used
2. Both with and without liver microsomal activation
3. Vehicle was dimethylsulfoxide
4. Vehicle was 0.5% methylcellulose
Table 5. Results of pharmacological assays with doramectin
Assay type Species Dose Result Reference
(mg/kg bw)
Safety study1 Collie dogs 0.062 negative Cruthers, 1991
(n = 2) 0.125 negative
0.25 negative
0.5 vomiting, moist
muzzle, enlarged
pupils, unsteady
legs
Diuretic assay SD rats(n= 10) 0.1 to 1.0 negative Gromelski et al, 1991a
Gastrointestinal CD-1 mice 0.1 to 1.0 negative Varner et al, 1991
motility assay (n = 10)
Arterial SD rats 0.1 to 1.0 negative Gromelski et al, 1991b
blood gas (n = 10)
1. Ivermectin was tested in this same experiment and elicited clinical effects
at doses as low as 0.125 mg/kg bw.
3. COMMENTS
The Committee considered data from a range of studies on
doramectin, including the results of studies on its metabolism, acute
and short-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The oral bioavailability of doramectin in rats, as measured by
the plasma concentration of the drug and the area under the plasma
concentration-time curve, was approximately six times greater when
doramectin was administered in sesame oil as compared to an aqueous
vehicle or in the diet. Metabolism studies in rats, dogs and cattle
revealed a similar spectrum of metabolites in the liver and faeces of
each species, suggesting that laboratory animals are suitable models
for testing the toxicity of doramectin.
Acute toxicity studies were carried out in rodents. When
doramectin was administered orally in an aqueous vehicle, the LD50
values were in the range 500-2000 mg/kg bw in rats and greater than
2000 mg/kg bw in mice. When the drug was given in a sesame oil
vehicle, the oral LD50s were 50-200 mg/kg bw in rats and
75-500 mg/kg bw in mice. These marked differences in acute toxicity
reflect the enhanced absorption of doramectin when administered as an
oil preparation. A further investigation in mice showed that the acute
toxicities of orally administered doramectin and ivermectin were
similar and that both compounds were less acutely toxic than abamectin
or moxidectin. Toxic signs were indicative of effects on the CNS,
since doramectin, like abamectin and other drugs in this class,
affects gamma-aminobutyric acid (GABA)-sensitive neurons, which can
lead to neurotoxicity, as shown by tremors, ataxia and gait
abnormalities.
Short-term administration of doramectin in the diet to mice was
associated with clinical signs of neurotoxicity, and there was
evidence of minor toxic effects on the liver and kidney at and above
doses of 100 mg/kg bw/day. Plasma drug levels were dose-related,
although the relationship was non-linear, up to 100 mg/kg bw/day and
reached a plateau at doses greater than 100 mg/kg bw/day, which
suggests that the absorption of doramectin from the feed reached
saturation. The highest plasma concentration was 3.9 µg/ml.
A number of short-term toxicity studies were carried out in rats
for periods of up to 3 months. Administration of doramectin in the
diet at doses of 30 mg/kg bw/day and above resulted in markedly
reduced food intake and body-weight gain, as well as severe
neurotoxicity, which necessitated the early sacrifice of some of the
animals. Other findings at the same doses were atrophic changes in
hepatocytes and lymphoid organs and nephrosis. Minor increases in
liver weight were seen at 20 mg/kg bw/day in the diet, but hepatic
morphology was unaffected at this dose. The maximum plasma drug level
attained in these studies was 5.8 µg/ml. In a 1-month study in which
doramectin was given in the diet, the NOEL in rats was
10 mg/kg bw/day.
In two studies, rats were given doses of doramectin by gavage for
up to 3 months. Signs of CNS toxicity were observed at the highest
dose of 10 mg/kg bw/day. At doses of 5 mg/kg bw/day and above, there
were increases in liver weight, while kidney weight was increased at
8 mg/kg bw/day. However, there were no concomitant pathological
changes and all other parameters were unaffected. Plasma drug levels
reached a peak of 3.2 µg/ml. The NOEL in gavage studies in rats was
2 mg per kg of body weight per day.
When dogs were fed doramectin in the diet for periods of up to 36
days, the only treatment-related findings were mydriasis and reduced
body weight at a dose of 4 mg/kg bw/day. The highest plasma drug level
was 0.88 µg/ml. The NOEL in this study was 2 mg/kg bw/day.
In three studies in dogs, which received doramectin by gavage in
a sesame oil vehicle for up to 92 days, body weights were depressed at
4 mg/kg bw/day and clinical signs of CNS toxicity were noted at
2 mg/kg bw/day and above. The most sensitive indication of a
drug-related effect was mydriasis, which was seen at doses as low as
0.3 mg/kg bw/day. No other toxic effects were observed. The maximum
plasma drug level in these studies was 3 µg/ml. The NOEL was 0.1 mg/kg
bw/day.
Reproductive toxicity studies in rats revealed treatment-related
deaths among pups during the early postnatal period and a reduction in
body-weight gain of pups throughout the lactation period. These
effects were observed at doses as low as 3 and 1 mg/kg bw/day,
respectively. Doramectin was shown to be readily excreted in the milk
of lactating rats following administration by gavage and, as compared
with adult animals, higher drug levels were attained in the brain of
neonates, suggesting greater penetration through the incompletely
formed blood-brain barrier in newborn rats. The NOEL was 0.3 mg/kg
bw/day, based on toxicity in neonatal animals.
The administration of doramectin by gavage to pregnant mice and
rats did not result in fetal abnormalities. The only drug-related
finding was a slight increase in embryo mortality in mice given
6 mg/kg bw/day. The NOEL in mice was 3 mg/kg bw/day, while in rats
there were no adverse effects at the highest dose of 6 mg/kg bw/day.
In rabbits given 3 mg/kg bw/day during pregnancy, there was
significant maternal toxicity, and cleft palate was observed in three
fetuses from one litter; one fetus also exhibited phocomelia,
syndactyly and coelosomia. Ossification of pubic bones was delayed at
doses of 1.5 and 3 mg/kg bw/day. In a dose-ranging study, a dose of
6 mg/kg bw/day caused severe maternal toxicity and embryotoxicity. The
NOEL for maternal toxicity in rabbits was 0.75 mg/kg bw/day.
In view of the negative results in a range of in vitro
genotoxicity assays and a micronucleus test in mouse bone marrow, the
Committee concluded that doramectin was not genotoxic.
The Committee noted the close structural similarities between
doramectin and abamectin, the only difference being the presence of a
cyclohexyl group at the C-25 position in the doramectin molecule
rather than an isopropyl or isobutyl group in the case of abamectin
B1a and abamectin B1b, respectively. The available metabolic data
suggest that the biotransformation of doramectin and abamectin follows
a similar pathway. Extensive toxicological tests have been conducted
on both compounds, and the Committee reviewed several aspects of their
comparative toxicology. Both compounds exert a pharmacological effect
on the CNS through effects on gamma-aminobutyric acid-sensitive
neurons, which results in a range of neurotoxic signs such as
mydriasis, tremors, ataxia and gait abnormalities. In multiple-dose
studies, the compounds have been associated with few adverse
toxicological effects, reduced body-weight gain and minor toxic
effects on the liver being the most common. Toxicity in neonates is
the most sensitive indicator in reproductive toxicity studies with
both compounds. Neither compound is considered to have any genotoxic
activity. Carcinogenicity studies with abamectin were negative at
maximum tolerated doses in mice and rats. In view of the chemical,
biochemical and toxicological similarities, the Committee concluded
that it was unnecessary to request data from long-term toxicity and
carcinogenicity studies on doramectin.
4. EVALUATION
The Committee considered that the most relevant effect for the
safety evaluation of residues of doramectin was the effect on the
mammalian nervous system. An ADI of 0-0.5 µg/kg bw was established,
based on a NOEL of 0.1 mg/kg bw/day for mydriasis in the 3-month
gavage study in dogs. A safety factor of 200 was applied because the
test systems used to assess the neurotoxicity of doramectin were of
uncertain sensitivity (see section 2.2 of the Report, Annex 1,
reference 119). The ADI provides an adequate margin of safety for
neonatal toxicity in rats and developmental toxicity in rabbits.
5. REFERENCES
Cruthers, L.R. (1991). Dose Escalation Safety Study in Ivermectin
Sensitive Collies. Unpublished study No. 9112 from Professional
Laboratory and Research Services Inc. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Duce, I. R. & Scott, R.H. (1985). Actions of dihydroavermectin B1a
on insect muscle. British J. Pharmacol. 85. 395-401.
Graham, D., Pfeiffer, F. & Betz, H. (1982). Avermectin B1a inhibits
the binding of strychnine to the glycine receptor of rat spinal cord.
Neuroscience Letters. 29. 173-176.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991a). Diuretic
Assay. Unpublished study No. PH209-PZ-001-91 from Pharmakon Labs.
Submitted to WHO by Pfizer, Central Research, Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991b). Arterial
Blood Gas Determinations in Rats. Unpublished study
No. PH1051-PZ-001-91 from Pharmakon Labs. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Kass, I.S., Stretton, A.O.W. & Wang, C.C. (1984). The effects of
avermectin and drugs related to acetylcholine and 4-aminobutyric acid
on neurotransmission in Ascaris suum. Molec. Biochem. Parasit.
13. 213-225.
Pfizer (1987a). Comparison of Dosing Vehicles for Oral Exposure of
Rats. Unpublished study No. 87-657-07 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987b). Two Week Oral Feed Study on Long-Evans Rats.
Unpublished study No 87-657-03 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987c). Two Week Toleration Study in Beagle Dogs. Unpublished
study No. 87-657-04 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988a). Acute Oral and Intraperitoneal Toxicity Studies in
Mice and Rats. Unpublished study No. 87-657-01 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988b). A One Month Feeding Study in Long-Evans Rats at Dose
Levels of 20, 10, 5 and 0 mg/kg/day. Unpublished study No. 87-657-06
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1988c). A One Month Feed Study in Beagle Dogs at Dose Levels
of 4,2,1 and 0 mg/kg/day. Unpublished study No. 87-657-05 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988d). Preliminary Fetotoxicity Study (Maternal Toxicity) in
Mice by the Oral Route. Unpublished study No. 87094 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988e). Maternal Toxicity Study in Mice by the Oral Route.
Unpublished study No. 88035 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988f). Fetotoxicity Study in Mice by the Oral Route.
Unpublished study No. 88091/88092 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988g). Maternal Toxicity Study in Rats by the Oral Route.
Unpublished study No. 87165 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988h). UK-67,994: Maternal Toxicity Study in Rats by the Oral
Route. Unpublished study No. 88034 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988i). Fetotoxicity Study in Rats By the Oral Route.
Unpublished study No. 88079/88080 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988j). Maternal Toxicity Study in Rabbits by the Oral Route.
Unpublished study No. 87161 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988k). UK-67,994: Maternal Toxicity Study in Rabbits by the
Oral Route. Unpublished study No. 88077 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988l). Fetotoxicity Study in Rabbits by the Oral Route.
Unpublished study No. 88106/88107 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989a). A Five-Week Range Finding Gavage Study in Long-Evans
Rats. Unpublished study No. 88-657-11 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989b). A One Month Range Finding Gavage Study in Beagle Dogs.
Unpublished study No. 88-657-10 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989c). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 88-657-13 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990a). A Three Month Oral Gavage Study in Long-Evans Rats
with In Utero Exposure. Unpublished study No. 89-657-17 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1990b). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 89-657-19 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990c). A Two-Generation Gavage Study in Long-Evans Rats
(Terminated after first generation). Unpublished study No. 88-657-12
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1990d). An Exploratory Lactation Study in Long-Evans Rats.
Unpublished study No. 89-657-18 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991a). Acute Dermal and Ocular Irritation Studies in Albino
Rabbits. Unpublished study No. 91-657-22 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991b). A Two-Generation Gavage Study in Long-Evans Rats.
Unpublished study No. 89-657-20 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991c). Genetic Toxicology Report. Unpublished study
No. 87-657-02 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1991d). Genetic Toxicology (Microbial) Assays. Unpublished
study No. 91-67-81 from Pfizer Central Research, Taketoyo, Japan.
Submitted to WHO by Pfizer, USA.
Pfizer (1991e). Genetic Toxicology Report. Unpublished study
No. 90-657-21 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1992a). The Comparative Metabolism of 3H-Doramectin in
Cattle, Dog and Rat. Unpublished study No. CM-92-01 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992b). Single Dose Oral and Intraperitoneal Toxicity Studies
in Mice and Rats. Unpublished study No. 91-657-23 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992c). Two Generation Study in Long-Evans Rats (Terminated
prior to completion). Unpublished study No. 88-657-15 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992d). Range Finding Fetal/Neonatal Toxicity Study in
Long-Evans Rats. Unpublished study No. 88-657-16 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993a). Identification of Residues of Doramectin in Liver and
Fat of Cattle Sacrificed 21-Days Following SC Administration of
[3H]-Doramectin at 0.2 mg/kg. Unpublished study No. CM-93-01 from
Pfizer Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993b). One Month Range Finding Feeding Study in Long-Evans
Rats. Unpublished study No. 92-657-24 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994a). Single Dose Oral Toxicity Study in Albino (CD-1) Mice.
Unpublished study No. 94-657-29 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994b). A Range Finding Feed Study in CD-1 Mice. Unpublished
study No. 92-675-25 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1994c). Three Month Range Finding Feeding Study in CD-1 Mice.
Unpublished study No. 92-657-27 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994d). Three Month in Feed Study in Long-Evans Rats.
Unpublished study No. 92-657-26 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1995). Comparison of Plasma/Brain Ivermectin (B1a) Ratios in
CD-1 and CF-1 Mice. Unpublished study No. AHDM-94-08 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1991). Gastro-
intestinal Propulsion Assay in Male Mice. Unpublished study
No. PH239-PZ-001-91 from Pharmakon Labs. Submitted to WHO by
Pfizer, USA.
Zufall, F., Franke, Ch. & Hatt, H. (1989). The insecticide avermectin
B1a activates a chloride channel in crayfish muscle membrane.
J. Exp. Biol. 142. 191-205.
2.1.3 Pharmacological effects
Avermectins induce rapid, non-spastic paralysis in nematodes and
arthropods. One common feature of avermectins appears to be the
modulation of trans-membrane chloride ion (Cl-) channel activity in
nematode nerve cells, and in both nerve and muscle cells of
arthropods. These Cl- channels may be gated by a variety of
neurotransmitter receptors including gamma-aminobutyric acid (GABA),
glutamate and acetylcholine (Kass et al, 1984; Duce & Scott, 1985;
Zufall et al, 1989). Activation of the Cl- channels by avermectins
leads to an increase in Cl- conductance which results in a changed
membrane potential and this causes inhibition of electrical activity
in the target nerve or muscle cell. GABA is also a major inhibitory
neurotransmitter in the mammalian CNS and avermectins do have
intrinsic activity on the mammalian GABA receptor/Cl- channel
complex.
Avermectins have been reported to bind to glycine receptor/Cl-
channel complexes which are restricted to the CNS in mammals
(Graham et al, 1982). Penetration of the blood brain barrier by
avermectins is extremely poor and this may account for the wide margin
of safety exhibited by these compounds following administration to
mammals.
2.2 Toxicological Studies
2.2.1 Acute toxicity studies
The results of acute toxicity studies with doramectin are
summarized in Table 2.
Clinical signs of toxicity were similar in both mice and rats and
by both routes of administration, the most common being decreased
activity and respiration, hunched position, shakiness, weakness,
tremors, ataxia and weight loss.
Doramectin was applied to normal and abraded skin of 3 New
Zealand white rabbits (0.5 g under an occlusive patch) for 24 h.
Slight erythema was observed 24 and 48 h post-dose at 1 of 3 intact
sites and 2 of 3 abraded sites. There was no edema at any site and all
sites appeared normal 72 h post-dose.
Table 2. Results of acute toxicity studies with doramectin.
Species
(strain) Route Vehicle1 Sex LD50 in mg/kg bw2 Reference
Mouse po aqueous M&F > 2000(0/3) Pfizer, 1988a
(CD-1)
po oil F 250(0/3)-500(3/3) Pfizer, 1992b
po oil F 75(0/5)-200(5/5) Pfizer, 1994a
ip aqueous M 700(0/3)-1000(2/3) Pfizer, 1988a
ip oil M 100(0/3)-250(3/3) Pfizer, 1992b
Rat po aqueous M 1000(0/3)-2000(3/3) Pfizer, 1988a
(Sprague-Dawley) F 500(0/3)-1000(2/3) Pfizer, 1988a
po oil M 50(0/3)-100(2/3) Pfizer, 1992b
F 100(1/3)-200(3/3) Pfizer, 1992b
ip aqueous M >300(1/3) Pfizer, 1988a
ip oil M 50(0/3)-100(3/3) Pfizer, 1992b
1 aqueous vehicle was 0.1% aqueous methylcellulose; oil vehicle was sesame oil.
2 figures in brackets represent mortality incidences.
Doramectin powder (18.8 mg) was instilled into the conjunctival
sac of one eye in each of 3 New Zealand white rabbits. Slight corneal
and conjunctival reddening, chemosis and iritis were seen within 1 h
of dosing. The changes subsided within 6 h and all eyes were normal by
48 h post-dose (Pfizer, 1991a).
A comparison of the acute toxicity of different ivermectins in
mice is given in Table 3.
Table 3. Acute oral toxicity of doramectin in comparison with abamectin, ivermectin
and moxidectin in female CD-1 mice (Pfizer, 1994a).
Dose in mg/kg bw1 Doramectin Abamectin Ivermectin Moxidectin
Maximum
"asymptomatic" dose 25 5 255
Minimum
"symptomatic" dose 75 25 5025
Minimum
lethal dose2 200(5/5) 75(5/5) 75(1/5) 75(4/5)
LD50 dose 75-200 25-75 >75 25-75
1 - vehicle was sesame oil
2 - figures in brackets represent mortality incidences.
Signs of CNS toxicity were similar after dosing with each
compound and included gait abnormalities with splayed hind limbs,
intermittent tremors, ataxia and decreased, irregular or laboured
respiration (Pfizer, 1994a).
2.2.2 Short-term toxicity studies
2.2.2.1 Mice
Groups of CD-1 mice (10/sex/dose) were administered doramectin
(purity 94.1%) in the diet for 43 days. Target doses were 0, 10, 20,
40, or 60 mg/kg bw/day on days 1 to 14; 0, 10, 20, 80 or 100 mg/kg
bw/day on days 15 to 28; and 0, 100, 200, 400 or 600 mg/kg bw/day
on days 29 to 43.
Toxic signs included lethargy, hunched posture and tremors at 400
and 600 mg/kg bw/day, and hunched and ungroomed appearance in some
mice at 100 and 200 mg/kg bw/day. A number of animals given 400 and
600 mg/kg bw/day were sacrificed moribund and the remaining mice in
these groups were killed on day 33. There were no effects on body
weight or food consumption. Relative liver weights were slightly
higher in most treated groups but the changes were minor and not
dose-related. Clinical laboratory parameters and pathological
examinations were not carried out.
Plasma drug levels were dose-related, although non-linear, up to
100 mg/kg bw/day, and reached a plateau at higher doses. Drug levels
ranged from 0.23 µg/ml at 10 mg/kg bw/day to 3.9 µg/ml at 600 mg/kg
bw/day. An unidentified degradation product, represented a
progressively greater fraction of drug-related material at doses of
100 mg/kg bw/day and above (Pfizer, 1994b).
Groups of CD-1 mice (10/sex/group) were fed doramectin (purity
94.1%) in the diet for 92 days. Target doses were 0, 100, 200 or
300 mg/kg bw/day. Actual doramectin intake was 83-121, 154-191 or
221-322 mg/kg bw/day. Tremors, hunched posture, unkempt appearance and
lethargy were observed at the mid- and high-dose groups and resulted
in death or moribund sacrifice of 9 mice at 300 mg/kg bw/day and 3
mice at 200 mg/kg bw/day. The remaining animals in these two groups
were killed on day 12 or 19, respectively. Body-weight gain was
depressed in association with reduced food consumption at 200 mg/kg
bw/day and above.
Serum creatinine and BUN were slightly increased at 100 mg/kg
bw/day with no effects at higher doses, presumably due to early
culling of mice in these groups. Haematological parameters were
unaffected. All treated groups had increased liver weights and
hypertrophy of centrilobular hepatocytes, with multinucleate
non-proliferative liver cells at 100 mg/kg bw/day only. Dead and
moribund animals showed lymphocyte lysis in lymphoid organs, cellular
depletion of bone marrow and necrosis of the adrenal cortex which may
have resulted from stress and weight loss.
Satellite groups of 3 mice/sex/dose were used to determine plasma
drug levels. In the 100 mg/kg bw/day group, concentrations reached a
plateau at 2.8 µg/ml by day 45. Doramectin levels were not markedly
different at higher doses with peaks of 3.6 µg/ml and 2.7 µg/ml at 200
and 300 mg/kg bw/day, respectively. An unidentified degradation
product was present at levels proportional to both dose and duration
of treatment (Pfizer, 1994c).
2.2.2.2 Rats
Groups of Long-Evans rats (3/sex/group) were given 0, 2.5, 5 or
10 mg/kg bw/day doramectin (purity unspecified) in the diet for 14
days. There were no overt signs of toxicity and no effects on body
weight, haematology, serum chemistry, urinalysis, liver or kidney
weight. Examination of a limited number of organs revealed no
pathological changes. Mean plasma drug levels at day 10 were
approximately 0.05, 0.06 and 0.17 µg/ml with increasing doses
(Pfizer, 1987b).
In a 1-month study, groups of Long-Evans rats (10/sex/group) were
fed doramectin (purity 95.3%) in the diet at dose levels of 0, 5, 10
or 20 mg/kg bw/day. There were no clinical signs of toxicity and no
effects on food intake or body-weight gain. Ophthalmology,
haematology, serum chemistry and urinalysis were unaffected. Liver
weight was slightly increased in males given 20 mg/kg bw/day but
pathological examination was unremarkable. Plasma drug levels were
proportional to the dose; maximum concentrations achieved were 0.28,
0.76 and 1.79 µg/ml, respectively. The NOEL in this study was 10 mg/kg
bw/day (Pfizer, 1988b).
Groups of Long-Evans rats (10/sex/group) were fed a diet
containing doramectin (purity 94.1%) at levels intended to give
target doses of 0, 20, 40, 60 or 80 mg/kg bw/day for 38 days.
Chromorhinorrhea, chromodacryorrhea and urogenital staining were seen
at 40 mg/kg bw/day and above. Whole body tremors were also present at
the two highest doses and resulted in the sacrifice of 2 rats at
60 mg/kg bw/day and all rats at 80 mg/kg bw/day. Body-weight gain and
food consumption were lower at 60 mg/kg bw/day and above. Serum
5'-nucleotidase was increased at 80 mg/kg bw/day with elevated levels
of BUN at 40 mg/kg bw/day and above. Liver weights were higher in the
40 and 60 mg/kg bw/day groups but with no dose relationship. Organs
were not examined for pathology.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin concentrations were progressively increased
throughout the study at each dose, and levels were dose-related up to
60 mg/kg bw/day with no further increase at 80 mg/kg bw/day. Highest
levels were 0.89, 3.52, 3.78 and 3.54 µg/ml at the 4 doses,
respectively (Pfizer, 1993b).
Groups of Long-Evans rats (5/sex/group) were administered by
gavage doses of 0, 2, 5 or 10 mg/kg/bw/day doramectin (purity 92.1%)
in sesame oil for 38 days. Three females in the 10 mg/kg bw/day group
were sacrificed after showing hunched appearance, ataxia, tremors and
urine stains. There were no effects on body weight, food consumption,
haematology, serum chemistry or urinalysis. Some rats given 5 and
10 mg/kg bw/day had increased liver weights but pathological
examination of a limited number of organs was unremarkable. Plasma
drug levels were similar on days 10 and 38 and were dose-related;
approximate levels were 0.5, 1.6 and 3.0 µg/ml in the low-, mid- and
high-dose groups, respectively (Pfizer, 1989a).
Groups of Long-Evans rats (10/sex/dose) were fed doramectin
(purity 89.7%-94.1%) in the diet at target doses of 0, 30, 40 or
50 mg/kg bw/day, for 92 days. Based on food consumption, actual
doramectin intake ranged from 16-32, 11-49 and 14-59 mg/kg bw/day,
respectively. Thus systemic exposure to doramectin was similar in each
treated group which was reflected in the toxicological findings.
Clinical signs, noted in all treated animals, included
chromorhinorrhea, chromodacryorrhea, urine stains and tremors. Seven
rats at 30 mg/kg bw/day, all rats at 40 mg/kg bw/day, and 19 rats at
50 mg/kg bw/day died or were sacrificed moribund. Body-weight gain was
markedly reduced in all treated groups in association with diminished
food intake. Ophthalmological examinations were unchanged. All treated
groups showed decreased leucocytes, erythrocytes, haemoglobin and
haematocrit, and increased BUN. Serum levels of ALAT and ASAT were
increased and protein decreased in some animals given 40 and 50 mg/kg
bw/day. Urinalysis was unaffected.
At necropsy, animals which failed to survive the treatment period
showed minimal body fat and a few had stomach erosions. Apparent
increases in relative kidney and testes weights in treated groups were
probably due to the lower body weights. Pathological changes, observed
in all treated groups, comprised kidney nephrosis with protein
accumulation in the corticomedullary junction, hepatocyte atrophy with
occasional necrotic cells and haemosiderosis, lymphoid depletion in
thymus, spleen and mesenteric lymph node, bone marrow atrophy, and
lipid depletion in the adrenal cortex.
Satellite groups of 5 rats/sex/dose were treated in order to
determine plasma drug levels. Doramectin plasma concentrations
increased during the course of the study in the 30 and 40 mg/kg bw/day
groups, attaining levels of 4.6 and 5.8 µg/ml, respectively. A maximum
level of 4.1 µg/ml was achieved by day 16 in rats given 50 mg/kg
bw/day (Pfizer, 1994d).
Groups of Long-Evans rats (20/sex/dose) were selected from the
F1 offspring in a multigeneration reproductive toxicity study in
which doramectin was administered by gavage at doses of 0, 0.1, 0.3 or
1.0 mg/kg bw/day. Thus the animals had received secondary exposure in
utero and during lactation, and direct exposure for 3 to 4 weeks
post-weaning. Subsequently, the respective groups were administered
gavage doses of 0, 0.5, 2 or 8 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil for 3 months.
There were no overt signs of toxicity, mortality or treatment-
related ocular lesions. Initial body weights of the 8 mg/kg bw/day
group were lower which may be attributed to the prior in utero
or neonatal exposure to doramectin. During the course of the study
proper, treated groups gained more weight than controls. Food
consumption, haematology, serum chemistry and urinalysis were
unaffected. Liver and kidney weights were increased at 8 mg/kg bw/day
but there were no concomitant pathological alterations.
Satellite groups of 3 rats/sex/dose were used for plasma drug
determinations. Doramectin levels were dose-related with mean levels
of 0.07, 0.4 and 2.5 µg/ml on day 3, and 0.1, 0.7 and 3.2 µg/ml on day
87, in the low-, mid-, and high-dose groups, respectively. The NOEL in
this study was 2 mg/kg bw/day (Pfizer. 1990a).
2.2.2.3 Dogs
Groups of beagle dogs (1/sex/group) were fed doramectin (purity
unspecified) in the diet at dose levels of 0, 0.5, 1 or 2 mg/kg bw/day
for 14 days. There were no signs of toxicity or effects on body
weight, haematology, serum chemistry or urinalysis. Dogs were not
sacrificed. Plasma drug levels increased during the course of the
study attaining concentrations of approximately 0.045, 0.07 and
0.26 µg/ml, respectively on day 8 (Pfizer, 1987c).
Groups of beagle dogs (1/sex/group) were given gavage doses of
0, 1, 2 or 4 mg/kg bw/day doramectin (purity 92.1%-93.3%) in sesame
oil for 29 days. Body weight was depressed in both animals
administered 4 mg/kg bw/day and the male showed salivation, mydriasis,
tremors, ataxia and decreased heart rate prior to sacrifice on day 16.
All drug-treated dogs exhibited mydriasis. There were no effects on
haematology, serum chemistry, urinalysis, organ weight or pathological
examinations. Plasma drug levels were proportional to the dose,
highest concentrations achieved were approximately 0.4, 1.2 and
3.0 µg/ml, respectively (Pfizer, 1989b).
Groups of beagle dogs (3/sex/group) were administered doramectin
(purity 95.3%) in the diet at doses of 0, 1, 2 or 4 mg/kg bw/day for
36 days. Apart from mydriasis in two dogs given 4 mg/kg bw/day, there
were no clinical signs of toxicity. Body weight was depressed in the
4 mg/kg bw/day group during the first 3 weeks but returned to baseline
thereafter. Haematology, serum chemistry, urinalysis, organ weights
and pathology were unaffected. Plasma drug levels increased with
duration of dosing, reaching maximum concentrations of 0.21, 0.5 and
0.88 µg/ml, at the low-, mid- and high-dose groups, respectively. The
NOEL was 2 mg/kg bw/day. (Pfizer, 1988c).
Beagle dogs (4/sex/group) were given gavage doses of 0, 0.5, 1 or
2 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 91 days. A
slow pupillary light reflex was noted in all treated animals and
mydriasis was diagnosed in 1, 2, and 5 dogs given 0.5, 1, and 2 mg/kg
bw/day, respectively. One animal given 2 mg/kg bw/day showed anorexia,
tremors and ataxia and was sacrificed on day 23. No effects were
observed on body weight, haematology, serum chemistry, urinalysis,
organ weight or pathology. Plasma drug levels were dose-related but
were higher on day 30 than on day 90. The levels were approximately
0.29, 0.5 and 1.2 µg/ml on day 30 and 0.24, 0.3 and 0.6 µg/ml on day
90, at the 3 respective doses (Pfizer, 1989c).
Beagle dogs (3/sex/dose) were given gavage doses of 0, 0.1 or
0.3 mg/kg bw/day doramectin (purity 92.5%) in sesame oil for 92 days.
The only treatment-related finding was mild to moderate mydriasis
which was diagnosed in 1 female given 0.3 mg/kg bw/day. Plasma drug
levels were dose-related, with approximate mean concentrations of 0.1
and 0.28 µg/ml, respectively. The NOEL in this study was 0.1 mg/kg
bw/day (Pfizer, 1990b).
2.2.3 Reproductive toxicity studies
2.2.3.1 Rats
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 1.5, 3 or
6 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Males were
dosed from 10 weeks before pairing and to the end of the mating
period. Females were dosed from 2 weeks prior to pairing until the end
of the study.
There were no effects on food consumption or body weight of F0
adults, and copulation, pregnancy rate and gestation length were
unaffected. Size and weight of F1 litters at birth were similar
between groups. During lactation, pup weights decreased in the 3 and
6 mg/kg bw/day groups and most of these pups died by day 7
post-partum. Survival was unaffected in the 1.5 mg/kg bw/day group but
body-weight gain was lower in these offspring and the study was
discontinued on lactation day 7.
Subsequently, a number of F0 dams with litters (culled to 8
pups/litter), were assigned to 6 groups consisting of 3 females each.
Gavage doses of 0, 0.25, 0.5, 1, 3 or 6 mg/kg bw/day were administered
on lactation days 12 to 21 to determine potential effects on older
pups. Pup survival was not affected but body-weight gain was reduced
at 6 mg/kg bw/day (Pfizer, 1990c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were given gavage doses of 0, 0.1, 0.3
or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil. Treatment
of F0 males commenced 10 weeks prior to pairing, continuing until
the end of the mating period. F0 females were treated from 2 weeks
before pairing, through gestation until the end of lactation. Food
intake, body-weight gain, copulation, pregnancy rate and duration of
gestation were unaffected in F0 rats and gross examination of these
animals did not reveal any abnormalities.
F1 litter size, weight at birth, and post-natal survival were
similar in all groups. There was slight depression of body-weight gain
of the 1 mg/kg bw/day pups during lactation and the weight of these
animals remained below other groups for the remainder of the study.
Where necessary, F1 litters were reduced to 8 pups each on lactation
day 4. At weaning, 25 male and 25 female pups/group were randomly
selected to produce the F2 generation and were dosed until the end
of the study. On pairing of F1 rats, copulation incidences were
adversely affected leading to low pregnancy rates in all groups, most
notably in the control.
F2 litter sizes at birth were lower than normal in all groups
but subsequent survival was not affected. Body weights of the 1 mg/kg
bw/day offspring were lower throughout the lactation period. In
limited tests for post-natal development in 1 male and 1 female/litter,
there were no significant treatment-related deficits. Gross examination
of treated F1 rats and F2 pups showed no abnormalities.
Due to the adverse effects on conception described above, the
study was terminated, except for rats in the F1 control group which
were paired for a second mating. Again, pregnancy rates were very low,
and vaginal smears indicated irregular estrous cycling in many of the
females with some not cycling at all (Pfizer, 1992c).
In a 2-generation reproductive toxicity study, groups of
Long-Evans rats (45/sex/group) were administered gavage doses of
0, 0.1, 0.3 or 1 mg/kg bw/day doramectin (purity 92.5%) in sesame oil.
F0 males were treated from 10 weeks prior to pairing until the end
of mating, and females were dosed from 2 weeks prior to pairing,
during mating, gestation, and lactation periods. There were no effects
on food consumption or body weight of F0 rats and copulation,
pregnancy rate and gestation length were unaffected. Gross examination
of these animals was unremarkable.
F1 litter size and weight at birth and subsequent survival and
growth were similar in all groups. Each F1 litter was culled to 8
pups, where necessary, on lactation day 4. At weaning, 30 male and 30
female pups/group were randomly selected to produce the F2
generation and were dosed directly until study termination. These F1
rats were paired on two occasions, at age 11 to 12 weeks and 22 to
23 weeks. On both occasions, copulation and duration of gestation were
unaffected. However, conception was impaired in all groups, the
pregnancy rate being approximately 70% for the F2a and 50% for the
2bF matings, respectively. Gross examinations of F1 rats showed no
internal or external abnormalities.
In both the F2a and F2b litters, the number and weight of
offspring at birth, and survival to the end of lactation were not
affected by treatment. Pup body-weight gain was depressed in the
1 mg/kg bw/day group. On lactation day 21, 1 male and 1 female
pup/F2a litter were tested for locomotor activity and auditory
function and were given an ophthalmoscopic examination. No
treatment-related effects were observed in these developmental tests
or on gross examination of all F2a and F2b pups. The NOEL in this
study was 0.3 mg/kg bw/day (Pfizer, 1991b).
2.2.4 Special studies on neonatal toxicity
2.2.4.1 Rats
Groups of 7 (2 in control group) presumed pregnant Long-Evans
rats were given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin
(purity 92.5%) in sesame oil from gestation day 2 to day 3 post-partum.
There was no effect on body weight of dams but 1 female given 6 mg/kg
bw/day was sacrificed moribund. Milk, blood and brain of dams were
collected 3-4 h after the last dose. Blood and brain from the pups
were collected 24 h after dosing of the dams.
Analysis of drug levels in plasma samples revealed higher levels
in dams than in pups. However, drug levels in the brain of dams were
generally lower than in pup brains. The plasma to brain ratio was
approximately 16 in dams and 2 in pups indicating that doramectin was
more accessible to the CNS of neonates. Milk to plasma ratios in dams
were about 2-3, indicating that the drug was readily excreted in milk
(Pfizer, 1990d).
Groups of 10 presumed pregnant Long-Evans rats were given gavage
doses of 0, 0.1, 0.2, 0.5 or 1 mg/kg bw/day doramectin (purity 92.5%)
in sesame oil during gestation days 4 to lactation day 21. Litters
were culled to 8 pups each on lactation day 4, surviving pups and dams
were killed on lactation day 21. There was no effect on body weight of
dams. One dam given 1 mg/kg bw/day showed piloerection and lethargy
and died on day 13 of treatment. Litter size and weight at birth were
unaffected. At a dose of 1 mg/kg bw/day, pups body-weight gain was
reduced during lactation and 6 of 8 pups in a single litter died
(Pfizer, 1992d).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Mice
Groups of 9 presumed pregnant CD-1 mice were given gavage doses
of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin (purity 93.6%)
in sesame oil on gestation days 6 to 13. There were no effects on body
weight of dams, one female given 1.6 mg/kg bw/day had blood in the
urogenital area and was found dead on gestation day 12. The remaining
dams were killed on gestation day 18. Embryomortality and number of
live fetuses were similar between groups. Fetal weights were lower at
1.6 mg/kg bw/day. Fetuses were not examined (Pfizer, 1988d).
Groups of 7 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%) in sesame oil
on gestation days 6 to 13. One 6 mg/kg bw/day female showed
hypothermia, piloerection and blood on the vagina, but there were no
treatment-related deaths and no effects on body weight. Dams were
killed on gestation day 18. There were no effects on embryomortality,
number of live fetuses or fetal body weight. Fetuses were not examined
(Pfizer, 1988e).
Groups of 20 presumed pregnant CD-1 mice were given gavage doses
of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%) in sesame oil
on gestation days 6 to 13. There were no drug-related deaths or
clinical signs in dams and body weight was unaffected. Dams were
killed on gestation day 18. Embryomortality was increased at 6 mg/kg
bw/day but did not attain statistical significance. Fetal body weight
and the incidence of fetal abnormalities were unchanged.
An additional group of 10 mice were similarly treated with
6 mg/kg bw/day for analysis of drug levels on gestation day 13. Plasma
drug levels in dams, 1 h after the last dose varied from 0.088 to
0.28 µg/ml. Six hours after dosing, plasma levels in dams were between
0.37 and 0.58 µg/ml, amniotic fluid levels varied from non-detectable
to 0.019 µg/ml and fetal levels varied from non-detectable to
0.12 µg/g. The NOEL in this study was 3 mg/kg bw/day (Pfizer, 1988f).
2.2.5.2 Rats
Groups of 6 or 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 0.1, 0.2, 0.4, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 6 to 15. Dams showed no
signs of toxicity or effects on body weight. Females were killed on
gestation day 20. Embryomortality, number of live fetuses and fetal
body weight were similar between groups. Fetuses were not examined
(Pfizer, 1988g).
Groups of 7 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.3%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. No toxic signs or adverse effects on body weight were noted in
dams. Embryotoxicity and numbers of live fetuses and fetal body weight
were unaffected. Fetuses were not examined (Pfizer, 1988h).
Groups of 20 presumed pregnant Sprague-Dawley rats were given
gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity 92.1%)
in sesame oil on gestation days 6 to 15. Dams were killed on gestation
day 20. There were no effects on body weight of dams. Embryomortality
was increased at 6 mg/kg bw/day but was not statistically significant
and was within the historical control range. Fetal examination
revealed slightly increased incidences of rudimentary ribs, wavy ribs,
unossified hyoid and 5th metacarpals, and dilatation of ureters and
kidney pelves, but the incidences were not dose-related and were
within the historical control range.
A satellite group of 10 rats were administered 6 mg/kg bw/day and
used for drug analysis on gestation day 15. Plasma drug levels in
dams, 1-5 h after dosing, varied from 0.41 to 1.27 µg/ml. Five hours
after the dose, amniotic fluid showed a mean level of 0.014 µg/ml and
fetal levels were between 0.27 and 1.1 µg/g. The NOEL in this study
was 6 mg/kg bw/day, the highest dose tested (Pfizer, 1988i).
2.2.5.3 Rabbits
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.2, 0.8 or 1.6 mg/kg bw/day doramectin
(purity 92.3%) in sesame oil on gestation days 7 to 18. Body-weight
gain was reduced in the 1.6 mg/kg bw/day group during gestation days 1
to 19. Does were killed on gestation day 28. Embryomortality was
slightly increased at 1.6 mg/kg bw/day but with no effect on fetal
weight. Fetuses were not examined. Plasma drug levels in dams given
1.6 mg/kg bw/day, 2 h after dosing on gestation day 17, ranged between
0.037 and 0.42 µg/ml (Pfizer, 1988j).
Groups of 7 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 1.5, 3 or 6 mg/kg bw/day doramectin (purity
92.1%) in sesame oil on gestation days 7 to 18. Body-weight gain was
significantly depressed at 6 mg/kg bw/day, in association with reduced
food consumption, on gestation days 7 to 28. Dams were killed on
gestation day 28. Embryonic death was increased and foetal weight was
slightly depressed in the 6 mg/kg bw/day group. Fetuses were not
examined (Pfizer, 1988k).
Groups of 20 presumed pregnant New Zealand white rabbits were
given gavage doses of 0, 0.75, 1.5 or 3 mg/kg bw/day doramectin
(purity 92.1%) in sesame oil on gestation days 7 to 18. There was no
drug-related mortality but during the treatment period, food intake
was reduced at 1.5 and 3 mg/kg bw/day with reduced body-weight gain at
3 mg/kg bw/day. Embryomortality and fetal weight were similar between
groups. Major malformations, occurring in the 3 mg/kg bw/day group
only, were cleft palate in 3 fetuses from 1 litter and 1 fetus which
exhibited phocomelia, syndactyly and coelosomia. The incidence of
cleft palate was within the historical control range whereas the other
fetal abnormalities had never been observed in the testing laboratory.
Ossification of pubic bones was delayed at 1.5 and 3 mg/kg bw/day.
A satellite group of 4 rabbits were dosed with 3 mg/kg bw/day and
sacrificed on gestation day 18 for analysis of drug levels. Maternal
plasma levels, measured 1, 3 and 5 h after dosing, ranged from 0.126
to 0.838 µg/ml. Doramectin was not detected in amniotic fluid or fetal
tissue, 5 h after the last dose, but detection limits were relatively
high due to interfering peaks. The NOEL in this study was 0.75 mg/kg
bw/day (Pfizer, 1988l).
2.2.6 Special studies on genotoxicity
The results of genotoxicity studies with doramectin are given in
Table 4.
2.2.7 Special study on permeability of blood-brain barrier
2.2.7.1 Mice
Groups of 3 adult female CD-1 and CF-1 mice were given a single
gavage dose of 0.4 mg/kg bw ivermectin in sesame oil. Additional
groups received an i.p. injection of 50 mg/kg bw CP-100,356, an
inhibitor of p-glycoprotein activity, 1 h prior to ivermectin
administration. Animals were killed 24 h after drug dosing for removal
of blood and brain samples.
In CD-1 mice, ivermectin B1a could not be detected in plasma
(limit of quantitation 10 ng/ml), while brain levels were
1.38-1.98 ng/g. Pretreatment with CP-100,356 resulted in plasma levels
of 17.2-18.7 ng/ml and brain levels of 1.95-2.13 ng/g.
In CF-1 mice, ivermectin B1a levels in plasma were
14.9-21.0 ng/ml and brain levels were 109, 3.69 and 2.59 ng/g.
Pretreatment with CP-100,356 resulted in plasma levels of 30.1, 33.1
and 17.4 ng/ml and brain levels of 6.06, 8.97 and 1.98 ng/g.
Pretreatment with the p-glycoprotein inhibitor did not markedly affect
plasma or brain drug levels in either strain. Since the study used
very few animals and the treatment schedule and tissue sampling times
were not justified, no conclusion can be drawn from this study
(Pfizer, 1995).
2.2.8 Special studies on pharmacology
Table 5 summarizes the results of pharmacological studies with
doramectin.
Table 4. Results of genotoxicity assays on doramectin
Test system1 Test object Concentration Results Reference
Reverse mutation2 S. typhimurium 0.02 to 10 mg/plate negative Pfizer, 1991c
TA98, TA100 (-S9)3
TA1535, 0.005 to 2 mg/plate negative
TA 1537 (+S9)3
Urine from mice negative
dosed with 0.2 to
4 mg/kg ip (-S9)3
Reverse mutation2 S. typhimurium 9.8 to 5000 µg/plate negative Pfizer, 1991d
TA98, TA100, (±S9)3
TA1535, TA1537
E. coli WP2 uvrA.
Forward mutation2 L5178Y mouse 8 to 35 µg/ml negative Pfizer, 1991c
lymphoma cells. (-S9)3
13 to 62 µg/ml negative
(+S9)3
UDS assay Primary culture 1.7 to 10 µg/ml3 negative Pfizer, 1991c
of rat hepatocytes.
Micronucleus Mouse bone 500 to 2000 mg/kg negative Pfizer, 1991e
test marrow bw/day for 3 days,
po.4
1. Positive controls used
2. Both with and without liver microsomal activation
3. Vehicle was dimethylsulfoxide
4. Vehicle was 0.5% methylcellulose
Table 5. Results of pharmacological assays with doramectin
Assay type Species Dose Result Reference
(mg/kg bw)
Safety study1 Collie dogs 0.062 negative Cruthers, 1991
(n = 2) 0.125 negative
0.25 negative
0.5 vomiting, moist
muzzle, enlarged
pupils, unsteady
legs
Diuretic assay SD rats(n= 10) 0.1 to 1.0 negative Gromelski et al, 1991a
Gastrointestinal CD-1 mice 0.1 to 1.0 negative Varner et al, 1991
motility assay (n = 10)
Arterial SD rats 0.1 to 1.0 negative Gromelski et al, 1991b
blood gas (n = 10)
1. Ivermectin was tested in this same experiment and elicited clinical effects
at doses as low as 0.125 mg/kg bw.
3. COMMENTS
The Committee considered data from a range of studies on
doramectin, including the results of studies on its metabolism, acute
and short-term toxicity, reproductive and developmental toxicity, and
genotoxicity.
The oral bioavailability of doramectin in rats, as measured by
the plasma concentration of the drug and the area under the plasma
concentration-time curve, was approximately six times greater when
doramectin was administered in sesame oil as compared to an aqueous
vehicle or in the diet. Metabolism studies in rats, dogs and cattle
revealed a similar spectrum of metabolites in the liver and faeces of
each species, suggesting that laboratory animals are suitable models
for testing the toxicity of doramectin.
Acute toxicity studies were carried out in rodents. When
doramectin was administered orally in an aqueous vehicle, the LD50
values were in the range 500-2000 mg/kg bw in rats and greater than
2000 mg/kg bw in mice. When the drug was given in a sesame oil
vehicle, the oral LD50s were 50-200 mg/kg bw in rats and
75-500 mg/kg bw in mice. These marked differences in acute toxicity
reflect the enhanced absorption of doramectin when administered as an
oil preparation. A further investigation in mice showed that the acute
toxicities of orally administered doramectin and ivermectin were
similar and that both compounds were less acutely toxic than abamectin
or moxidectin. Toxic signs were indicative of effects on the CNS,
since doramectin, like abamectin and other drugs in this class,
affects gamma-aminobutyric acid (GABA)-sensitive neurons, which can
lead to neurotoxicity, as shown by tremors, ataxia and gait
abnormalities.
Short-term administration of doramectin in the diet to mice was
associated with clinical signs of neurotoxicity, and there was
evidence of minor toxic effects on the liver and kidney at and above
doses of 100 mg/kg bw/day. Plasma drug levels were dose-related,
although the relationship was non-linear, up to 100 mg/kg bw/day and
reached a plateau at doses greater than 100 mg/kg bw/day, which
suggests that the absorption of doramectin from the feed reached
saturation. The highest plasma concentration was 3.9 µg/ml.
A number of short-term toxicity studies were carried out in rats
for periods of up to 3 months. Administration of doramectin in the
diet at doses of 30 mg/kg bw/day and above resulted in markedly
reduced food intake and body-weight gain, as well as severe
neurotoxicity, which necessitated the early sacrifice of some of the
animals. Other findings at the same doses were atrophic changes in
hepatocytes and lymphoid organs and nephrosis. Minor increases in
liver weight were seen at 20 mg/kg bw/day in the diet, but hepatic
morphology was unaffected at this dose. The maximum plasma drug level
attained in these studies was 5.8 µg/ml. In a 1-month study in which
doramectin was given in the diet, the NOEL in rats was
10 mg/kg bw/day.
In two studies, rats were given doses of doramectin by gavage for
up to 3 months. Signs of CNS toxicity were observed at the highest
dose of 10 mg/kg bw/day. At doses of 5 mg/kg bw/day and above, there
were increases in liver weight, while kidney weight was increased at
8 mg/kg bw/day. However, there were no concomitant pathological
changes and all other parameters were unaffected. Plasma drug levels
reached a peak of 3.2 µg/ml. The NOEL in gavage studies in rats was
2 mg per kg of body weight per day.
When dogs were fed doramectin in the diet for periods of up to 36
days, the only treatment-related findings were mydriasis and reduced
body weight at a dose of 4 mg/kg bw/day. The highest plasma drug level
was 0.88 µg/ml. The NOEL in this study was 2 mg/kg bw/day.
In three studies in dogs, which received doramectin by gavage in
a sesame oil vehicle for up to 92 days, body weights were depressed at
4 mg/kg bw/day and clinical signs of CNS toxicity were noted at
2 mg/kg bw/day and above. The most sensitive indication of a
drug-related effect was mydriasis, which was seen at doses as low as
0.3 mg/kg bw/day. No other toxic effects were observed. The maximum
plasma drug level in these studies was 3 µg/ml. The NOEL was 0.1 mg/kg
bw/day.
Reproductive toxicity studies in rats revealed treatment-related
deaths among pups during the early postnatal period and a reduction in
body-weight gain of pups throughout the lactation period. These
effects were observed at doses as low as 3 and 1 mg/kg bw/day,
respectively. Doramectin was shown to be readily excreted in the milk
of lactating rats following administration by gavage and, as compared
with adult animals, higher drug levels were attained in the brain of
neonates, suggesting greater penetration through the incompletely
formed blood-brain barrier in newborn rats. The NOEL was 0.3 mg/kg
bw/day, based on toxicity in neonatal animals.
The administration of doramectin by gavage to pregnant mice and
rats did not result in fetal abnormalities. The only drug-related
finding was a slight increase in embryo mortality in mice given
6 mg/kg bw/day. The NOEL in mice was 3 mg/kg bw/day, while in rats
there were no adverse effects at the highest dose of 6 mg/kg bw/day.
In rabbits given 3 mg/kg bw/day during pregnancy, there was
significant maternal toxicity, and cleft palate was observed in three
fetuses from one litter; one fetus also exhibited phocomelia,
syndactyly and coelosomia. Ossification of pubic bones was delayed at
doses of 1.5 and 3 mg/kg bw/day. In a dose-ranging study, a dose of
6 mg/kg bw/day caused severe maternal toxicity and embryotoxicity. The
NOEL for maternal toxicity in rabbits was 0.75 mg/kg bw/day.
In view of the negative results in a range of in vitro
genotoxicity assays and a micronucleus test in mouse bone marrow, the
Committee concluded that doramectin was not genotoxic.
The Committee noted the close structural similarities between
doramectin and abamectin, the only difference being the presence of a
cyclohexyl group at the C-25 position in the doramectin molecule
rather than an isopropyl or isobutyl group in the case of abamectin
B1a and abamectin B1b, respectively. The available metabolic data
suggest that the biotransformation of doramectin and abamectin follows
a similar pathway. Extensive toxicological tests have been conducted
on both compounds, and the Committee reviewed several aspects of their
comparative toxicology. Both compounds exert a pharmacological effect
on the CNS through effects on gamma-aminobutyric acid-sensitive
neurons, which results in a range of neurotoxic signs such as
mydriasis, tremors, ataxia and gait abnormalities. In multiple-dose
studies, the compounds have been associated with few adverse
toxicological effects, reduced body-weight gain and minor toxic
effects on the liver being the most common. Toxicity in neonates is
the most sensitive indicator in reproductive toxicity studies with
both compounds. Neither compound is considered to have any genotoxic
activity. Carcinogenicity studies with abamectin were negative at
maximum tolerated doses in mice and rats. In view of the chemical,
biochemical and toxicological similarities, the Committee concluded
that it was unnecessary to request data from long-term toxicity and
carcinogenicity studies on doramectin.
4. EVALUATION
The Committee considered that the most relevant effect for the
safety evaluation of residues of doramectin was the effect on the
mammalian nervous system. An ADI of 0-0.5 µg/kg bw was established,
based on a NOEL of 0.1 mg/kg bw/day for mydriasis in the 3-month
gavage study in dogs. A safety factor of 200 was applied because the
test systems used to assess the neurotoxicity of doramectin were of
uncertain sensitivity (see section 2.2 of the Report, Annex 1,
reference 119). The ADI provides an adequate margin of safety for
neonatal toxicity in rats and developmental toxicity in rabbits.
5. REFERENCES
Cruthers, L.R. (1991). Dose Escalation Safety Study in Ivermectin
Sensitive Collies. Unpublished study No. 9112 from Professional
Laboratory and Research Services Inc. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Duce, I. R. & Scott, R.H. (1985). Actions of dihydroavermectin B1a
on insect muscle. British J. Pharmacol. 85. 395-401.
Graham, D., Pfeiffer, F. & Betz, H. (1982). Avermectin B1a inhibits
the binding of strychnine to the glycine receptor of rat spinal cord.
Neuroscience Letters. 29. 173-176.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991a). Diuretic
Assay. Unpublished study No. PH209-PZ-001-91 from Pharmakon Labs.
Submitted to WHO by Pfizer, Central Research, Groton, CT, USA.
Gromelski, G.J., Panasevich, R.E. & Ciofalo, V.B. (1991b). Arterial
Blood Gas Determinations in Rats. Unpublished study
No. PH1051-PZ-001-91 from Pharmakon Labs. Submitted to WHO by Pfizer,
Central Research, Groton, CT, USA.
Kass, I.S., Stretton, A.O.W. & Wang, C.C. (1984). The effects of
avermectin and drugs related to acetylcholine and 4-aminobutyric acid
on neurotransmission in Ascaris suum. Molec. Biochem. Parasit.
13. 213-225.
Pfizer (1987a). Comparison of Dosing Vehicles for Oral Exposure of
Rats. Unpublished study No. 87-657-07 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987b). Two Week Oral Feed Study on Long-Evans Rats.
Unpublished study No 87-657-03 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1987c). Two Week Toleration Study in Beagle Dogs. Unpublished
study No. 87-657-04 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988a). Acute Oral and Intraperitoneal Toxicity Studies in
Mice and Rats. Unpublished study No. 87-657-01 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988b). A One Month Feeding Study in Long-Evans Rats at Dose
Levels of 20, 10, 5 and 0 mg/kg/day. Unpublished study No. 87-657-06
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1988c). A One Month Feed Study in Beagle Dogs at Dose Levels
of 4,2,1 and 0 mg/kg/day. Unpublished study No. 87-657-05 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988d). Preliminary Fetotoxicity Study (Maternal Toxicity) in
Mice by the Oral Route. Unpublished study No. 87094 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988e). Maternal Toxicity Study in Mice by the Oral Route.
Unpublished study No. 88035 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988f). Fetotoxicity Study in Mice by the Oral Route.
Unpublished study No. 88091/88092 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988g). Maternal Toxicity Study in Rats by the Oral Route.
Unpublished study No. 87165 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988h). UK-67,994: Maternal Toxicity Study in Rats by the Oral
Route. Unpublished study No. 88034 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988i). Fetotoxicity Study in Rats By the Oral Route.
Unpublished study No. 88079/88080 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988j). Maternal Toxicity Study in Rabbits by the Oral Route.
Unpublished study No. 87161 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1988k). UK-67,994: Maternal Toxicity Study in Rabbits by the
Oral Route. Unpublished study No. 88077 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1988l). Fetotoxicity Study in Rabbits by the Oral Route.
Unpublished study No. 88106/88107 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989a). A Five-Week Range Finding Gavage Study in Long-Evans
Rats. Unpublished study No. 88-657-11 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989b). A One Month Range Finding Gavage Study in Beagle Dogs.
Unpublished study No. 88-657-10 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1989c). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 88-657-13 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990a). A Three Month Oral Gavage Study in Long-Evans Rats
with In Utero Exposure. Unpublished study No. 89-657-17 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1990b). A Three Month Gavage Study in Beagle Dogs. Unpublished
study No. 89-657-19 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1990c). A Two-Generation Gavage Study in Long-Evans Rats
(Terminated after first generation). Unpublished study No. 88-657-12
from Pfizer Central Research, Groton, CT. Submitted to WHO by
Pfizer, USA.
Pfizer (1990d). An Exploratory Lactation Study in Long-Evans Rats.
Unpublished study No. 89-657-18 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991a). Acute Dermal and Ocular Irritation Studies in Albino
Rabbits. Unpublished study No. 91-657-22 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991b). A Two-Generation Gavage Study in Long-Evans Rats.
Unpublished study No. 89-657-20 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1991c). Genetic Toxicology Report. Unpublished study
No. 87-657-02 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1991d). Genetic Toxicology (Microbial) Assays. Unpublished
study No. 91-67-81 from Pfizer Central Research, Taketoyo, Japan.
Submitted to WHO by Pfizer, USA.
Pfizer (1991e). Genetic Toxicology Report. Unpublished study
No. 90-657-21 from Pfizer Central Research, Groton, CT. Submitted to
WHO by Pfizer, USA.
Pfizer (1992a). The Comparative Metabolism of 3H-Doramectin in
Cattle, Dog and Rat. Unpublished study No. CM-92-01 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992b). Single Dose Oral and Intraperitoneal Toxicity Studies
in Mice and Rats. Unpublished study No. 91-657-23 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992c). Two Generation Study in Long-Evans Rats (Terminated
prior to completion). Unpublished study No. 88-657-15 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1992d). Range Finding Fetal/Neonatal Toxicity Study in
Long-Evans Rats. Unpublished study No. 88-657-16 from Pfizer Central
Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993a). Identification of Residues of Doramectin in Liver and
Fat of Cattle Sacrificed 21-Days Following SC Administration of
[3H]-Doramectin at 0.2 mg/kg. Unpublished study No. CM-93-01 from
Pfizer Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1993b). One Month Range Finding Feeding Study in Long-Evans
Rats. Unpublished study No. 92-657-24 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994a). Single Dose Oral Toxicity Study in Albino (CD-1) Mice.
Unpublished study No. 94-657-29 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994b). A Range Finding Feed Study in CD-1 Mice. Unpublished
study No. 92-675-25 from Pfizer Central Research, Groton, CT.
Submitted to WHO by Pfizer, USA.
Pfizer (1994c). Three Month Range Finding Feeding Study in CD-1 Mice.
Unpublished study No. 92-657-27 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1994d). Three Month in Feed Study in Long-Evans Rats.
Unpublished study No. 92-657-26 from Pfizer Central Research,
Groton, CT. Submitted to WHO by Pfizer, USA.
Pfizer (1995). Comparison of Plasma/Brain Ivermectin (B1a) Ratios in
CD-1 and CF-1 Mice. Unpublished study No. AHDM-94-08 from Pfizer
Central Research, Groton, CT. Submitted to WHO by Pfizer, USA.
Varner, L.L., Panasevich, R.E. & Ciofalo, V.B. (1991). Gastro-
intestinal Propulsion Assay in Male Mice. Unpublished study
No. PH239-PZ-001-91 from Pharmakon Labs. Submitted to WHO by
Pfizer, USA.
Zufall, F., Franke, Ch. & Hatt, H. (1989). The insecticide avermectin
B1a activates a chloride channel in crayfish muscle membrane.
J. Exp. Biol. 142. 191-205.
