
SOLANINE AND CHACONINE
First draft prepared by
Dr T. Kuiper-Goodman and Dr P.S. Nawrot
Bureau of Chemical Safety
Health and Welfare Canada
Ottawa, Ontario, Canada
1. EXPLANATION
The common potato, Solanum tuberosum, contains toxic
steroidal glycoalkaloids derived biosynthetically from cholesterol
(Sharma & Salunkhe, 1989). In older literature (before 1954) these
have been referred to only as 'solanine' or as total glycoalkaloids
(TGA). The potato glycoalkaloids have not been evaluated previously
by the Joint FAO/WHO Expert Committee.
Potatoes that have been exposed to light in the field or during
storage may become green, due to an accumulation of chlorophyll.
This greening may affect only the surface (peel) or it may extend
into the flesh of the potato. Exposure to light is only one of the
stress factors affecting potatoes. Other pre- or post-harvest stress
factors are mechanical damage, improper storage conditions, either
as a tuber or after partial food processing, and sprouting (Sharma &
Salunkhe, 1989).
As a result of any of the above stress factors, there can be a
rapid increase in the concentration of TGA, notably, alpha-solanine
and alpha-chaconine, which gives the potatoes a bitter taste. These
natural toxicants (stress metabolites) have insecticidal and
fungicidal properties; each of the two major glycoalkaloids is
normally present in all tubers in small amounts (< 5 mg/100 g of
tuber fresh weight) (Table 1). The glycoalkaloids are formed in the
parenchyma cells of the periderm and cortex of tubers, and in areas
of high metabolic activity such as the eye regions. The
glycoalkaloids are unevenly distributed throughout the potato, with
a large part concentrated under the skin (Table 1). Some cultivars
are more prone to develop elevated levels of TGA than others.
Growing conditions may also affect the level of glycoalkaloids. None
of cooking, baking, frying nor microwaving destroys the
glycoalkaloids (Bushway & Ponnampalam, 1981).
Table 1. Normal Levels of TGA in various tuber tissues
TGA1
mg/100g FW
whole tuber 7.5 (4.3-9.7)
flesh 1.2-5
skin 2-3% of tuber 30-60
peel 10-15% of tuber 15-30
bitter tuber 25-80
peel from bitter tuber 150-220
1 Wood & Young, 1974
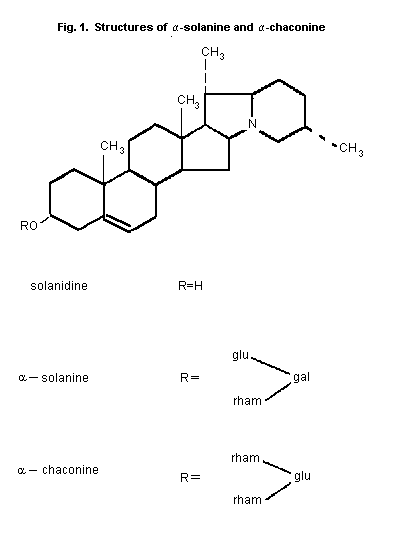
In commercially available potato tubers destined for human
consumption, as much as 95% of the TGA fraction consists of
alpha-solanine and alpha-chaconine (Fig. 1) There is usually
slightly more alpha-chaconine than alpha-solanine. These compounds
are derivatives of the aglycone solanidine, each containing three
sugar moieties. Solanidine itself may also be present in potato
tubers. The remainder of the TGA fraction may consist of other
glycoalkaloids or their aglycones (Sharma & Salunkhe, 1989). Other
aglycones include demissidine, tomatidenol and
5ß-solanidan-3alpha-ol. Alpha- And ß-solamarine are examples of
glycoalkaloids derived from tomatidenol found in potatoes. Through
plant breeding using wild potatoes other glycoalkaloids, such as
commersonine and demissine, both derived from demissidine, and
various leptines, derived from leptinidine, may be introduced
(Sharma & Salunkhe, 1989).
The most extensive review on Solanum and solanine is by
Jadhav et al. (1981); other reviews are by Maga (1980), Dalvi &
Bowie (1983), Morris & Lee, (1984), Morgan & Coxon (1987) and Sharma
& Salunkhe (1989). Most of the toxicity data deals with
alpha-chaconine and alpha-solanine. A Nordic view and assessment of
the health risks from glycoalkaloids in potatoes was recently
compiled (Slanina, 1990a,b).
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
Groups of 4 female Swiss-Webster mice, weighing 20 g, were
orally administered alpha-chaconine once at a dose of 10 mg/kg bw.
Animals were sacrificed by exsanguination at 3, 6, 14, 72 and 120 h
after dosing. Blood was obtained (0.1 ml/time point) by multiple
incisions into the tail vein. Absorption was slow, with peak values
in blood (0.82 µg/ml) obtained after 14 h. Decrease in blood levels
was slow, with 0.31 µg/ml present at 120 h. The peak level in the
liver (2.97 µg/g) was reached 6 h after dosing. A second, but lower,
peak of radioactivity was seen in the liver at 120 h, suggesting
enterohepatic recycling. Cell fractionation studies showed that
within liver cells, there was no preferential location for
alpha-chaconine, and there was no binding of alkaloid to isolated
RNA or DNA fractions (Sharma et al., 1983).
2.1.1.2 Rats
Male Fischer rats (180-250 g) were orally administered
alpha-solanine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). Radioactivity in
the gastrointestinal tract started to disappear from 3 to 6 h after
dosing. During the first 24 h interval the total urinary and faecal
excretion was 78% of the dose, with most in the faeces. Maximum
concentrations of radio-activity occurred near 12 h for all tissues,
with the largest concentration in the kidneys, spleen, liver, and
lungs, and the lowest concentration in the blood. By 24 h only 10%
of the administered dose remained, and 12% was unaccounted for
(presumed to be associated with other organs and the carcass). At
that time the amount of tritium in the liver represented 1.54% of
the dose, and for blood this was 0.375% (based on a total blood
volume of 64.1 ml/kg bw). By 4 days 84% of the dose had been
excreted by the faecal route, and urinary excretion accounted for
10% of the dose (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). alpha-Chaconine was
poorly absorbed since faecal elimination accounted for 60% of the
dose within 12 h, and 80% of the dose within 24 h. Urinary excretion
of tritium was 5% of the dose 3 to 6 h after dosing, and reached a
plateau of 10% of the dose between 12 and 24 h. Maximum
concentrations of radioactivity occurred between 6 to 12 h for all
tissues, with the largest concentration in the liver. Intermediate
concentrations were seen in the kidneys, spleen, and lungs, and the
lowest concentrations were seen in the blood, brain and abdominal
fat. At 24 h after dosing the amount of tritium associated with the
liver represented 1.29% of the dose, and that with the blood was
0.17% of the dose (based on a total blood volume of 64.1 ml/kg bw)
(Norred et al., 1976).
2.1.1.3 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h) hamsters (3 animals per time point)
were exsanguinated by cardiac puncture. (The reviewers noted an
error in reporting and have adjusted the results of the original
report by changing ng to µg). At 3 h after dosing, the highest
concentration of alpha-chaconine was seen in the intestines,
including intestinal contents (125 µg/g), and this represented 63%
of the administered dose. By 24 h these values were 75 µg/g or 44%
of the administered dose, and by 168 h they had declined to 1.73
µg/g or 0.92% of the administered dose. Peak blood (1.74 µg/ml) and
peak tissue levels (liver = 27.2 µg/g) of alpha-chaconine for most
tissues were seen by 12 h, and for the heart and kidneys by 24 h. By
168 h after dosing, blood levels had declined to 0.29 µg/ml. The
ratio of liver concentration to blood concentration at 72 h was
greater than at 24 h, indicating the possibility of enterohepatic
recycling. Only small amounts of radioactivity were recovered from
the faeces in the elimination phase (non-detectable at 3 h, 0.15% at
24 h, to 0.24% of the administered dose by 168 h). In the urine
these values increased from non-detectable at 3 h to 0.25% at 12 h,
and to 21% by 168 h. These results suggest that most of the
alpha-chaconine was absorbed, but that absorption from the
gastrointestinal tract was slow. Much of the radioactivity appeared
in various tissues in bound form (Alozie et al., 1979a).
2.1.1.4 Humans
Tritiated solanidine (dose not given, but expressed as
radioactivity) was administered to 3 human volunteers (2 males, 1
female) by iv injection. Blood and urine samples were collected at
various times up to 150 h. Ninety per cent of tritium had
disappeared from the blood within 20 minutes of injection. Presuming
that radioactivity represented solanidine or its metabolites, three
phases of elimination were identified in plasma with half-lives of 2
to 3.7 min, 2 to 5 h, and 72 to 104 h, respectively. Within minutes
of injection, the concentration of tritium in erythrocytes exceeded
that in plasma. Erythrocytes were found to be a mobile reserve of
solanidine, thereby delaying transfer of solanidine from vascular to
extravascular compartments. Low rates of excretion were seen in
urine and faeces, and together accounted for about 5% of the
administered dose during the first 24 h. Thus a fraction in excess
of 90% of the dose was sequestered somewhere in the body 24 h after
dosing. After this time, the rate of elimination from the body was
low, about 1-2% per day, corresponding to an overall half-life of 34
to 68 days. The authors calculated that if absorption of solanidine
were 1 mg/day, then with a fractional rate of excretion of 0.02, the
body burden would be 50 mg. The authors suggested that mobilization
from various storage loci could occur during times of 'metabolic
stress', including pregnancy (Claringbold et al., 1982).
Mean levels of 1.56 ± 1.17 (7 males) and 1.20 ± 0.93 (27
females) ng/ml solanidine were found, using radioimmunoassay, in
human plasma samples obtained by a hospital clinic in the UK,
collected in the morning before lunch (Matthew et al., 1983).
Thirty healthy males, aged 18-44 years, and 27 healthy females,
aged 16-62 years, participated in a study in the UK designed to
measure levels of serum solanidine in persons eating their usual
diet (during the winter). Intake of the type of potato product
(i.e., French fried, boiled or baked, and whether the skin was
included) was recorded daily for one month, with arbitrary units,
corresponding to approximate levels of TGA in those products,
assigned to each product; the weight of product ingested was not
measured. Serum samples were collected before the midday meal, and
were analyzed by radioimmunoassay (detection limit 0.5 ng/ml). In
males the mean level of solanidine was 10.8 ± 5.4 ng/ml (range
2.1-22.5 ng/ml), whereas in females the respective values were 7.9 ±
4.3 (range 1.6-18.5). For both genders there was a significant
correlation between serum solanidine levels and the alkaloid intake
(expressed in units as indicated above) during the month (R = 0.878
and R = 0.703, respectively). In two male subjects serum solanidine
levels dropped to 0.5 ng/ml 2 to 3 weeks after they had been on a
potato avoidance diet, indicating a relatively long serum half-life
for solanidine. It was suggested by the authors that solanidine may
be bound to blood constituents such as free sterols (Harvey et al.,
1985a).
Eighteen healthy males, aged 20-45 years, and 15 healthy
females, aged 19-63 years, from the London area in the UK,
participated in a study designed to measure levels of total serum
alkaloids (alpha-solanine + alpha-chaconine + solanidine) and
solanidine in persons eating their usual diet (during the summer).
For comparison, 5 males, aged 31-41 years, and 5 females, aged 31-67
years, from the Uppsala area in Sweden also participated in this
study. In Sweden, 2 of the males and 1 female consumed 200-300 g
potatoes of 2 varieties high in TGA, including the skin, for 1 week
(mean 24 mg TGA/100 g), giving an intake of approximately
60 mg/person or 1 mg/kg bw/day. Blood samples were collected before
the midday meal, and were analyzed by radio-immunoassay (detection
limits for total alkaloids and solanidine were 0.4 and 0.5 ng/ml
serum, respectively). The mean levels of serum solanidine were,
respectively, 3.5 and 4.0 ng/ml in the UK and Swedish subjects
eating their usual diets, whereas in those three Swedes consuming
potatoes with a higher TGA content the mean serum solanidine level
was 31 ng/ml (range 27.8-35.5). The respective serum total alkaloid
levels were 12.0, 16.9 and 50 ng/ml. The mean serum total alkaloid
concentration was about 2.7 times the solanidine concentration,
which, according to the authors, suggests considerable metabolism in
man of the glycoalkaloids alpha-chaconine and alpha-solanine (they
represent the major proportion of alkaloids in potatoes) through
hydrolysis of the sugar residues. It was suggested that hydrolysis
could take place in the acid medium of the stomach, or at the site
of absorption, or the ratio could reflect the preferential
absorption of the more lipophilic solanidine. Alternatively,
alpha-solanine and alpha-chaconine might be absorbed unchanged and
metabolized within the body (Harvey et al., 1985b).
Blood serum levels of alpha-solanine, alpha-chaconine, and
solanidine resulting from a single meal of mashed potatoes
(equivalent to 1 mg TGA/kg bw/day) were monitored in 8 healthy
subjects (HPLC, detection limit 1 ng/ml). Peak concentrations were
achieved after 4-8 h; these were 3-11 ng/ml for alpha-solanine and
6-21 ng/ml for alpha-chaconine. The 1:2 ratio was maintained for the
duration of the experiment. After longer time intervals the level of
solanidine was < 4 ng/ml. The serum half-lives for alpha-solanine
and alpha-chaconine were 11 and 19 h, respectively (unpublished data
by K.E. Hellenäs, cited by Slanina, 1990b).
2.1.2 Biotransformation
2.1.2.1 Rats
Male Fischer rats (180-250 g) were orally administered 5 mg/kg
bw solanine, tritiated at the carbon atoms adjacent to the nitrogen
atom and the double bond (Fig. 1). Approximately 65% of the
radioactivity in the faeces was identified as solanidine. In urine
72% of radioactivity was present as basic compounds of which 6% was
identified as solanidine. Two other compounds, present at 80% and
13%, possessed intermediate polarity with respect to solanine and
solanidine (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1) at a dose of 5 mg/kg bw.
Urine and faecal samples were collected 24 h later. The major
constituent in both faeces and urine was presumed to be solanidine
because it showed the same Rf. Similarly, 25% of the radioactivity
in the faeces was attributed to unchanged alpha-chaconine. In
addition, 2 minor compounds, possessing intermediate polarity
between solanidine and alpha-chaconine and representing 1-5% of
total activity, were found in faecal and urine extracts. The authors
concluded that the absorption and metabolism of alpha-chaconine was
similar to alpha-solanine (Norred et al., 1976).
2.1.2.2 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h), hamsters were exsanguinated by
cardiac puncture (3 animals per time point) (see above Alozie
et al., 1979a). Thin-layer chromatographic separation was
performed on the chloroform soluble fractions from urine and faeces
collected at various time intervals after dosing. In urine, over
half of the eliminated radioactivity during the initial 24 h was due
to unaltered alpha-chaconine. A major urinary metabolite was
solanidine, which was the major peak by 72 h. In addition, 4 other
unidentified metabolites were present at various concentrations. Two
of these were the major peaks by 168 h after dosing. In faeces, much
of the eliminated radioactivity was due to alpha-chaconine, and a
major metabolite was solanidine. There were 2 additional
unidentified metabolites present in about the same concentration as
solanidine (Alozie et al., 1979b).
2.1.3 Effects on enzymes and other biochemical parameters
Groups of male Sprague-Dawley rats (5/group) were fasted
overnight and then given alpha-solanine by gavage at 0 and 250 mg/kg
bw or i.p. at 0 and 20 mg/kg bw. In the orally dosed animals serum
glutamic oxalacetic transaminase (SGOT) and serum glutamic pyruvic
transaminase (SGPT) were increased, and cholinesterase activity was
decreased, but the differences were not statistically significant.
With the i.p.-dosed animals statistically significant increases of
29 and 63% in SGOT and SGPT, respectively, and a 27% decrease in
cholinesterase activity were observed. In addition, a significant
inhibition of liver benzphetamine N-demethylase activity and a
decrease in liver cytochrome P-450 were observed after i.p. dosing,
whereas after oral dosing these differences were statistically
insignificant (Dalvi, 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Oral LD50 values for solanine in rodents are considerably
higher than LD50 values determined after intraperitoneal
administration (Table 2), probably because these species do not
absorb much of the solanine. Post-mortem examination failed to
reveal the cause of death in rats that had been dosed orally
(stomach tube). The oral LD50 values in rodents were 300 to > 500
times the toxic dose of about 2 mg/kg bw and a lethal dose of 3 to
6 mg/kg bw estimated for humans (see Section 2.3.1).
Table 2. LD50 values in mg/kg bw
alpha-solanine alpha-chaconine solanidine
i.p. p.o. i.p. i.p.
Mice 32.31 >10002 19.25 >5002
42.02 27.56
32.35
30.06
Rat 753 5903
674
Rabbit <201,2 506
406
Rhesus <407
Monkey
1 Patil et al. (1972)
2 Nishie et al. (1971)
3 Gull et al. (1970)
4 Chaube & Swinyard (1976)
5 Sharma et al. (1979)
6 Nishie et al. (1975)
7 Swinyard & Chaube (1973)
Two rhesus monkeys died 48 h after an i.p. injection of
40 mg/kg bw of total glycoalkaloids; one other died 2 h after having
been dosed i.p. twice (24 h apart) with 20 mg solanine/kg bw
(Swinyard & Chaube, 1973).
2.2.2 Short term studies
2.2.2.1 Rabbits
One group of 4 rabbits, each weighing about 950 g (strain not
given), was fed normal potatoes (TGA content 7.5 mg/100 g) and 4
more rabbits were fed greened potatoes (TGA content 20.4 mg/100 g)
for a period of 20 days. After 4 to 6 days the latter group,
consuming 49-53 mg TGA/kg bw/day, became dull and inactive; after 10
days, diarrhoea, hair loss and weight loss occurred, which was
followed by watering eyes, body rigidity and dullness. Protein
digestibility (amount of protein from potatoes ingested less amount
of protein excreted expressed as a percentage of protein from
ingested potatoes) decreased by 45% from day 1. This was accompanied
by a significant decrease in body weight, resulting in an average
body weight of about 650 g for treated and 1150 g for 'control'
rabbits at 25 days after cessation of feeding the experimental
diets. One of the 4 treated rabbits died within 10 to 20 days. The
control animals which consumed 20 to 23 mg TGA/kg bw/day were
unaffected (Azim et al., 1983).
The same authors similarly fed 2 groups of 5 rabbits each
(weight and strain not given) a control potato diet (7.5 mg
TGA/100 g) and a high TGA potato diet (29.75 mg TGA/100 g) for 45
days. Daily intake of TGA was 16.8 to 17.9 mg/kg bw in the control
diet, and 73.9 to 75.0 mg/kg bw in the high TGA diet. Blood samples
were collected from the ear vein every 15 days. RBC counts and
haemoglobin concentrations were determined. A significant decrease
in RBC counts was seen throughout feeding the high TGA diet, and
this was 27.5% by 45 days. This compared to a decrease of 12.5% seen
by 45 days in the control diet. Decreases in haemoglobin
concentrations paralleled the findings with RBC counts. The authors
suggested that these results indicate that the rabbits developed
haemolytic anaemia. This could be explained by the metabolite
solanidine increasing the permeability and fragility of RBC
membranes (Azim et al., 1984).
2.2.2.2 Monkeys
Four time-mated rhesus monkeys were fed ad libitum a diet of
diced potatoes of the B5141-6 variety (since withdrawn from the
market), containing on average 26 mg solanine per 100 g tuber for 25
consecutive days during days 0-42 following mating. It subsequently
became apparent that the monkeys were not pregnant. The monkeys
ingested the equivalent of 0.77 to 1.02 mg/kg bw/day alpha-solanine
or 3.08 to 4.07 mg/kg bw/day TGA. No adverse effects were observed
(Swinyard & Chaube, 1973).
2.2.3 Long-term/carcinogenicity studies
No studies available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Holzman rats, approximately 4 months of age, were
mated, one male to 3 females. Increase in weight was taken as an
indication of pregnancy, and afterwards the females were
individually caged and given a basal diet of (I) ground lab chow;
(II) ground lab chow to which was added 10% ground frozen potato
sprouts; (III) 30 mg/kg diet solanine (commercial); (IV) 40 mg/kg
diet solanine (commercial); and (V) 30 mg/kg diet solanine that was
isolated from the frozen sprouts. The time on the test diets was
variable, since increase in weight is not a sensitive indicator of
pregnancy, and some dams dropped their litters within a few days on
the test diet. They were then kept on the test diet until they had a
second litter. No food consumption records were kept, but the rats
readily ate the diets. With diets II to V, many of the pups died
within 3 days of birth, evidently from starvation as indicated by an
absence of milk in their stomachs. The percentage of pups
successfully weaned was 82.6, 50.6, 31.0, 31.1, and 19.5 for diets I
to V, respectively. All of the pups in 18/33 litters born of the
rats eating the test diets died before reaching weaning age, whereas
only one of 11 control litters was lost. The authors concluded that
the toxicity of the potato sprout diet was due to 'solanine'. It was
speculated by the authors that solanine may exert an anti-hormonal
effect and prevent lactation in some sensitive dams. The reviewers
feel that further studies to examine these effects and other aspects
of reproduction are necessary (Kline et al., 1961).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Potential teratogenicity of alpha-solanine and alpha-chaconine
was investigated in four different experiments with Wistar rats
weighing 175-200 g. In the first experiment, three groups of rats
(9-10/group) were gavaged with alpha-solanine at dose levels of 0.3,
1.0 or 3.0 mg/kg bw/day, from days 6 to 15 of gestation. In the
second experiment, a group of 9 rats was given alpha-solanine by
gavage at a dose level of 6 mg/kg bw/day, from days 7 to 10 of
gestation. In the third experiment, groups of rats (3-4/group)
received alpha-solanine at dose levels of 2, 10 or 25 mg/kg bw/day
from days 8 to 11 of gestation. In the fourth experiment, a group of
4 rats received alpha-chaconine by gavage at a dose level of
1.5 mg/kg bw/day, from days 6 to 15 of gestation. In the first three
experiments, concurrent control groups composed of 2 to 10
rats/group were included. On day 22 of gestation, all females were
sacrificed and the following parameters were investigated: corpora
lutea, resorption sites, litter size, litter weight, and gross,
visceral and skeletal fetal anomalies. The only adverse effect was
observed in the first experiment. One fetus with craniorachischisis
and exopthalmos (1/117), from the group receiving 3 mg/kg bw/day,
and another with twisted pelvic limbs and absent tail (1/108), from
the 0.3 mg/kg bw/day group were observed. No maternal toxicity was
reported. The authors concluded that the observed effects were not
treatment-related (Ruddick et al., 1974).
A group of 14 Wistar rats, weighing 175-200 g, was fed a diet
containing about 73% of cooked and freeze-dried, visibly blighted
parts of potato tubers, from days 1 to 22 of gestation. The intake
of blighted potatoes was approximately 70 g/kg bw/day. The control
group of 13 females was fed a diet containing the same amount of
freeze-dried potatoes inoculated with heat-killed Phytophthora
infestans. The content of glycoalkaloids in the diets was not
determined. All dams were sacrificed on day 22 of gestation and the
standard parameters (corpora lutea, resorption sites, litter size,
litter weight, and gross, visceral and skeletal anomalies) were
investigated. There was no evidence of maternal toxicity, fetal
toxicity nor teratogenicity (Ruddick et al., 1974).
2.2.5.2 Hamsters
Groups of hamsters (12-15/group), weighing about 100 g, were
fed from days 5 to 10 of gestation diets of commercial hamster
ration containing 50% freeze-dried, unblighted potato concentrate
(group 1); 50% Phytophthora infestans infected freeze-dried,
blighted potato concentrate (group 2); or 50% Alternaria solani
infected freeze-dried, blighted potato concentrate (group 3). A
group of 13 hamsters was fed commercial hamster ration only,
throughout gestation (group 4). The content of glycoalkaloids in the
diets was not determined. Food and water were provided ad libitum.
On day 15 of gestation, the dams were sacrificed, and fetuses were
examined for gross, visceral and skeletal anomalies. Feed
consumption, maternal body weight gain, litter size, number of
resorptions and fetal weight were not affected by the treatment. The
most frequent gross anomaly was haemorrhagic necrosis of the central
nervous system, but the frequency of this effect was not
treatment-related (group 1 - 1/114; group 2 - 3/153; group 3 -
0/135; group 4 - 11/99) (Sharma et al., 1978).
Groups of Syrian hamsters (body weights and age not given) were
gavaged on day 8 of gestation with alpha-chaconine, isolated from
Arran Pilot potato sprouts, at levels of 165 mg/kg bw/day (23/group)
or 180 mg/kg bw/day (14/group) and alpha-solanine, isolated from
Arran Pilot potato sprouts, at a level of 200 mg/kg bw/day
(37/group). Females of the vehicle control group (37/group) received
vehicle material alone (2% ethanol at pH 5-6 in 1% carboxymethyl
cellulose or in water). Animals were individually caged in a room
maintained at 20-26 °C and received food and water ad libitum.
Maternal toxicity was monitored by daily weighing and clinical
observation. On day 15 of gestation, the dams were sacrificed and
necropsied. The uteri were exposed and the number of resorptions and
live fetuses were determined. The corpora lutea were counted and
all fetuses were examined for gross anomalies. Maternal mortality
was observed in all treated dose groups; 4 and 6 dams died in the
165 and 180 mg/kg bw/day alpha-chaconine dose groups, respectively,
and 3 dams died in the 200 mg/kg bw/day alpha-solanine dose group.
The days of gestation on which the dams had died were not indicated.
No maternal mortality was observed in the vehicle control group. A
high incidence of neural tube defects such as interparietal
encephalocoele (11-13%) and exencephaly (5-12%) was observed in
fetuses, of which the mothers were exposed to either alpha-chaconine
or alpha-solanine. In the vehicle control group only 1 fetus of 393
examined exhibited exencephaly. The authors concluded that the
observed teratogenic effects were treatment-related. They also
indicated that a number of fetuses exhibited CNS malformations
without apparent toxicity or weight loss in the dam, making it
unlikely that the malformations were secondary to maternal toxicity.
Occasional short tail and minor digital anomalies were noted in
fetuses from all experimental groups, but those effects were not
treatment-related (Renwick et al., 1984).
2.2.5.3 Rabbits
Groups of New Zealand rabbits (2-6/group), weighing on average
4 kg, were fed, throughout gestation, diets containing 50%
freeze-dried, unblighted, potato concentrate, 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate, or
50% Alternaria solani infected freeze-dried, blighted, potato
concentrate. The content of glycoalkaloids in the diets was not
determined. Prior to parturition (day not defined), all dams were
sacrificed and fetuses removed and examined for gross, visceral and
skeletal anomalies. During fetal examination particular attention
was paid to any malformations of brain and spinal cord. Among 21
fetuses examined in the Phytophthora infestans blighted potato
group, three fetuses (from three litters) exhibited incomplete
closure of the caudal vertebral column, and two other fetuses were
very small and had shortened appendages. Among 28 fetuses examined
in the Alternaria solani blighted potato group, two fetuses
exhibited incomplete closure of the caudal vertebral column, one
fetus had a very small brain (nearly half the normal size) and the
cranial cavity was filled with fluid, and two other fetuses were
abnormally small in size. All six abnormal fetuses were from
different litters. None of the nine fetuses (two litters) from the
unblighted potato group were affected. The authors concluded that
feeding pregnant rabbits potatoes blighted with either of the fungi,
at high concentrations in the diet, can produce a low incidence of
the caudal vertebral column malformation. This result must be
considered with caution since the small number of control litters
examined does not permit an adequate estimate of the spontaneous
incidence of this malformation in rabbits (Sharma et al., 1978).
2.2.5.4 Miniature swine
Groups of female miniature swine (2/group), weighing about
39 kg, were fed laboratory diets containing 50% freeze-dried,
unblighted, potato concentrate (group 1); 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate
(group 2); or 50% Alternaria solani infected freeze-dried,
blighted, potato concentrate (group 3), during the first half of
gestation (about the first 57 days of gestation). The content of
glycoalkaloids in the diets was not determined. At the end of
gestation (the day was not indicated), all dams were sacrificed and
necropsied. All fetuses were removed and examined for gross and
visceral malformations. Depressed weight gain was observed in sows
of group 3. One fetus of 15 examined from group 2 exhibited
anencephaly with extensive internal hydrocephaly. Other fetuses from
this and other groups were not affected. The authors concluded that
feeding potatoes blighted with Phytophthora infestans may be a
causative factor in the production of anencephaly in miniature
swine. However, the small sample size makes definite conclusions
difficult (Sharma et al., 1978).
2.2.5.5 Marmosets
A group of 6 female marmosets (Callithrix jacchus), five
years of age, weighing about 375 g, previously producing normal
offspring, was fed a diet containing freeze-dried concentrate of
blighted potatoes (Kerr's Pink variety), at a level of 4.7 g/kg
bw/day (equivalent to 0.9 mg/kg bw/day of glyco-alkaloids), for 50
days, during either days 0-50 or 20-70 of gestation. A control group
of 6 pregnant marmosets received a standard unsupplemented diet.
Marmosets were sacrificed between days 80-120 of gestation and
fetuses were examined for developmental anomalies. Four of 11
fetuses in the blighted potato groups exhibited gross abnormalities,
described as cranial osseous defects. Histological examination
revealed replacement of bone by a collagenous membrane in the
occipital area. Brain examination of affected fetuses revealed
enlargement of the lateral ventricle. Eleven fetuses of the control
group showed no gross abnormalities in any system. The investigators
indicated that, during the 2 years of existence of the marmoset
colony, similar defects had occurred once spontaneously in twin
fetuses (spontaneously aborted) among 104 live births. The authors
concluded that the occurrence of cranial dysplasia in 4 of 11
fetuses, in the experimental group, is suggestive of teratogenicity
of blighted potatoes in marmosets (Poswillo et al., 1972, 1973).
Three experiments were conducted to investigate possible
teratogenic effects of different varieties of unblemished or
blemished potatoes in 5 year-old, pregnant marmosets (Callithrix
jacchus), previously producing normal offspring. In the first
experiment, a group of 6 female marmosets were fed freeze-dried
concentrate of unblemished 'domestic potatoes' (Cornish White and
King Edward varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.56 mg/kg bw/day of glycoalkaloids. In the second
experiment a group of 7 female marmosets were fed freeze-dried
concentrate of blemished 'industry rejected potatoes' (King Edward,
Cornish White varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.78 mg/kg bw/day of glycoalkaloids. In the third
experiment a group of 5 female marmosets were fed freeze-dried
concentrate of King Edward variety potatoes, infected with Erwinia
carotovera (a bacterial pathogen responsible for 'blackleg'), at a
level of about 4.7 g/kg bw/day, equivalent to 0.07 mg/kg bw/day of
glycoalkaloids. Feeding trials were commenced 10 days postpartum of
the previous litter, to cover the period of the expected postpartum
oestrous and were carried throughout an undefined period of
gestation. Female marmosets fed both the 'domestic potatoes' and
'industry rejected potatoes' diets were allowed to proceed with
pregnancy to term, and the offspring was grossly examined at birth
and at regular intervals up to 6 months of age. Females fed
'infected potatoes' (with Erwinia carotovora) diet were sacrificed
between days 90 to 110 of gestation, and fetuses were examined
grossly and radiographically for abnormalities. Behavioural
anomalies such as continuous clinging to parents or siblings, and
prolonged weaning time, were observed in three sets of twins, born
to dams of the second experiment. No anatomical abnormalities were
observed in any experimental groups. The authors concluded that the
significance of the behavioural abnormalities observed in this study
cannot be determined at this stage but further observation of growth
and development to sexual maturity may throw more light on this
phenomenon (Poswillo et al., 1973).
2.2.5.6 Chicken embryos
Fertile chicken eggs (White Leghorn) were injected either with
pure solanine, mixed glycoalkaloids or an ethanol extract (obtained
from potatoes infected with Phytophthora infestans) into the yolk
sac, at levels ranging from 0.13 to 0.26 mg/egg, between 0 and 26 h
of incubation. A high incidence of embryo mortality (20-27%) and
increased incidence of abnormalities (16-25%) such as cranioschisis,
celosoma, cardiac septal defects, rumplessness (absence of tail) and
trunklessness (absence of trunk below the wing bud) were observed in
treated embryos. The most frequent defect was rumplessness and
trunklessness. In controls injected with chick Ringer or HCl
solvent, the percentage of abnormal embryos was 9-10% and the
mortality was 1-8% (Jelinek et al., 1976; Mun et al., 1975).
2.2.6 Special studies on cholinesterase inhibition
The inhibitory effect of alpha-solanine and solanidine, as well
as an extract from potatoes, were studied using a 1:100 dilution of
sera from 21 human individuals. These persons had been previously
phenotyped as 'usual' (95% of population in Great Britain),
'intermediate' (3-4% of the population), and 'atypical' (uncommon),
using the acetylcholinesterase inhibitor dibucaine. At a
concentration of 2.88 µM and 3.14 µM, respectively, alpha-solanine
and solanidine were about equally effective causing 86.2 ± 1.2 % and
80.0 ± 1.4 % inhibition in the 'usual' phenotype, and parallel
effects to dibucaine in the other two phenotypes. The results with
the potato extract were similar. The authors noted that it is not
clear to what extent the toxic effects of solanine can be attributed
to the inhibition of serum cholinesterase, but if it plays a role
then individuals with the 'atypical' phenotype, would presumably be
less susceptible (Harris & Whittaker, 1962).
Male and female New Zealand rabbits (2 of each sex) were given
a single i.p. dose of 20 or 30 mg solanine/kg body weight. These
doses resulted in severe depression, with difficult breathing and
prostration, and were lethal in 3 of the rabbits within 24 h. One
rabbit survived. Blood samples were obtained at 15 to 225 min
post-dosing; plasma and erythrocyte acetylcholinesterase activities
were measured and compared to control samples taken from the same
rabbit before dosing. Solanine was a weak to moderate inhibitor of
both specific and non-specific cholinesterase. Maximum inhibition of
plasma cholinesterase was seen at 80 min after injection, with the
activity decreasing to about 45% of the control value; inhibition of
erythrocyte cholinesterase was somewhat lower, and was maximally
reduced to 68.6% at 85 min after injection.
The same authors injected i.v. 5 doses of 6 mg/kg body weight,
10 min apart, in one anaesthetized male dog (15 kg bw). Quick
inhibition of serum cholinesterase was followed by rapid recovery.
Erythrocyte cholinesterase was not inhibited (Patil et al., 1972).
Male Sprague-Dawley rats (3 per group) were injected i.p. with
0, 10, 30 or 60 mg/kg bw of alpha-chaconine and sacrificed 3 h after
dosing. All rats administered alpha-chaconine showed initial signs
of depression, as well as other signs of poisoning by an
anticholinesterase agent, such as respiratory depression. Following
electrophoresis in acrylamide slabs, homogenates of brain (diluted
1:6) showed 3 zones of acetylcholinesterase isoenzyme activity with
a dose-related decrease in peak heights. Overall
acetylcholinesterase activity, using a colorimetric method, was
reduced to 79, 55, and 18% of the control value for the 3 respective
dose groups. Heart acetylcholinesterase activity was reduced to
about 40% of the control value in all treatment groups; plasma
cholinesterase activity in controls was about 30% of that seen in
brain homogenates, and was reduced to 50% in the 10 mg/kg bw dosage
group, with no further reduction in rats given 30 mg/kg bw. The
authors concluded that alpha-chaconine is a fairly potent inhibitor
of cholinesterases (Alozie et al., 1978).
In an in vitro assay the anticholinesterase activity of
several glycoalkaloids was compared, using highly purified acetyl
cholinesterase isolated from human and bovine erythrocytes (Sigma).
Alpha-solanine and alpha-chaconine were equally effective, 100 µM
caused about 80% inhibition of both human and bovine enzymes.
Tomatine was less effective, causing 40% and 50% inhibition of
bovine and human enzymes, respectively. Solasine, solamargine and
the aglycones solanidine, tomatidine and solasidine were
ineffective. Over a range of Ph 5 to pH 8, pH of the medium was not
very important. These results show that the nature of the aglycone
moiety is important (Roddick, 1989).
2.2.7 Special studies on genotoxicity
Pure alpha-solanine (Sigma) at 0.01 to 0.05 mg/plate and
extracts from potatoes were negative in the Ames test both with
strains TA98 and TA100, and in the presence or absence of activation
by S9 fraction from PCB-induced rat liver (Ness et al., 1984).
Alpha-solanine (25 and 250 µM) tested negative in a
DNA-cell-binding assay using Ehrlich ascites cells and Escherichia
coli cells mixed with 32P-labelled nucleic acids (Kubinski et
al., 1981).
2.2.8 Special studies on mitotic index
Cultured human fibroblasts were treated up to 40 h with 0, 4.1,
8.3, 16.6, 33.3, and 66.6 µg alpha-solanine/ml. At the highest dose
there was an inhibition of growth, whereas at lower dose levels
there was a stimulation of growth, as evidenced by an increase in
the mitotic index from 1.7% in controls to 2.4% at the 4.1 µg/ml
dose level, which according to the authors was similar to the
sex-hormonal type of effect exerted by estrogens on target tissues.
Using pulse labelling with tritiated thymidine, it was shown that at
5 µg alpha-solanine/ml the mean cell cycle time decreased from 42.5
± 1.87 h in controls to 28.5 ± 0.29 h in treated cells. This was
however accompanied by a 4 h increase in the period of DNA synthesis
(S phase) in treated cells, and a decrease to virtually zero for the
G1 phase. The authors concluded that if alpha-solanine reached the
fetus, the observed types of effects could be hazardous to it, and
could lead to malformations (Kirk & Mittwoch, 1975).
2.2.9 Special studies on calcium transport
Alpha-solanine (100 µM at pH 7.4) caused a 90% inhibition of
active calcium transport in rat duodenum when added to everted
intestinal sacs (8 replicates) in vitro. A Dixon plot revealed
that the inhibition by alpha-solanine was non-competitive, and the
inhibition constant was 25 µM. The inhibition of active calcium
transport was accompanied by a 40% decrease in oxygen consumption
(Michalska et al., 1985).
When alpha-solanine was given to 12 male and female Wistar
albino rats (5-6 weeks old) in their drinking water (5 mM, pH 6.4)
for 12 days, calcium transport in duodenal sacs was reduced to about
one-third of the control value, but oxygen consumption was not
significantly reduced (Michalska et al., 1985).
2.3 Observations in humans
2.3.1 Gastrointestinal and neurotoxic effects
There have been many reported cases of human poisonings
(sometimes fatal) due to the ingestion of greened or otherwise
damaged potatoes. The symptoms of low grade solanine poisoning are
acute gastrointestinal upset with diarrhoea, vomiting and severe
abdominal pain. In more severe cases, neurological symptoms,
including drowsiness and apathy, confusion, weakness, and vision
disturbances, followed by unconsciousness and, in some cases, death
have also been reported. The vital signs include fever, rapid and
weak pulse, low blood pressure and rapid respiration. Onset of
symptoms has ranged from minutes to 2 days after ingestion of toxic
potatoes, with longer incubation periods generally associated with
the more severe cases.
As is usual with case histories of this nature, the available
data are not complete. Over the years, various analytical methods or
assays have been used to determine the concentration of 'solanine'
in cases of suspected poisonings. With most of the older data, the
estimate for solanine included the other glycoalkaloids, such as
alpha-chaconine. McMillan and Thompson (1979) showed that
gravimetric methods gave higher values than colorimetric methods. A
few case reports, for which the reviewers have estimated the dose
ingested, are given below. Additional reports were compiled by
Morris and Lee (1984), who indicated that more than 2000 cases with
about 30 deaths have been reported in the literature. Not all of
these reports were available to the reviewers.
Fifty-six German soldiers suffered typical 'solanine' poisoning
after eating 1 to 1.5 kg cooked peeled potatoes containing 24 mg
TGA/100 g (whole uncooked tubers contained 38 mg TGA/100 g). In a
few cases jaundice and partial paralysis were also observed. If one
assumes a body weight of 70 kg, the intake of 'solanine' was 3.4 to
5.1 mg/kg bw (Pfuhl, 1899).
In 18 separate households in Scotland, 61 persons suffered
typical 'solanine' poisoning soon to several hours after eating
potatoes. Persons not eating potatoes were not ill. One 5-year old
died. The potatoes in that household contained 41 mg 'solanine'/100
g. Assuming the child ate 200 g potatoes and had a bw of 18 kg, the
lethal dose was estimated at 4.5 mg/kg bw. Assuming adults ate 500 g
potatoes and had a bw of 60 kg, their intake of 'solanine' would
have been 3.4 mg/kg bw (Harris & Cockburn, 1918).
A small outbreak of solanine poisoning affected a family of
four adults on three consecutive Sunday evenings in Great Britain,
about 8 h after they had eaten 1 to 3 baked potatoes in their
jackets (weight of potatoes not given). A 5th person who only ate
the flesh of the potatoes was not affected. The severity of symptoms
was related to the number of potatoes ingested, and consisted of
abdominal pain, diarrhoea, and general malaise. Patients recovered
within 24 h. The level of solanine was 50 mg/100 g tuber, as
determined chemically and by cholinesterase inhibition. Assuming a
weight of 150 g per potato, and body weights of 60 and 70 kg, the
dose was estimated at 1.25 to 3.2 mg 'solanine'/kg bw (Wilson,
1959).
Seventy-eight junior schoolboys in Great Britain became ill
from solanine poisoning 7 to 9 h after eating two small boiled
peeled potatoes each (weight of potatoes not given) as part of their
lunch, and 17 were admitted to hospital. Symptoms included vomiting,
diarrhoea, and general abdominal pain. Most of the boys developed a
fever, suffered from headache, dizziness, mental confusion,
hallucinations and their vision was affected. Three boys were
comatose and stuporose on admission, with peripheral circulatory
collapse. All were discharged 6-11 days following admission, and 4-5
weeks later there were no sequelae. Tests for the presence of
biocides, such as nicotine, organophosphorus or organochloride
pesticides were negative. Six days after eating the meal, plasma
pseudocholinesterase levels in 10 out of 17 schoolboys was subnormal
(about 25% below the normal range for this age group). Red blood
cell cholinesterase levels were normal. The source of toxic potatoes
was traced to a bag of old potatoes that had been condemned for
consumption because of their appearance, but that had inadvertently
been cooked (peeling of the potatoes had been done by an automatic
peeling machine). Insufficient potatoes were left over after the
meal for direct chemical analysis. Solanine levels in the boiled
peeled potatoes were therefore estimated from the in vitro
reduction in pseudocholinesterase activity in human plasma, using
acetylcholine as a substrate, and were equivalent to 25-30 mg/100 g
tuber of alpha-solanine. Assuming an intake of 200 g potatoes and a
bw of 40 kg (age = 11-14 years), the reviewers estimate that the
intake of 'solanine' by the schoolboys would therefore have been
approximately 1.4-1.6 mg/kg bw. Because of the small margin of
safety between normal potatoes and toxic potatoes, the authors
speculated that in toxic potatoes other toxic steroids besides
glycoalkaloids may be synthesized, such as sapogenins and saponins,
which might enhance the toxicity of solanine alkaloids by promoting
gastro-intestinal absorption or other means (McMillan & Thompson,
1979).
In a recent (1983) poisoning associated with a school lunch
programme, 61 of 109 school children and staff in Alberta, Canada,
became ill, most within 5 minutes, after eating baked potato (weight
of potato not given) containing 49.4 mg 'solanine' per 100 g
(analytical method not indicated). Test results showed that there
was no evidence that the illness occurred due to the presence of
viruses, bacteria, moulds, pesticides or other chemicals in the food
items or their containers. The potatoes had a slight tinge of green
and had a bitter or unusual taste (noted by 44% of those affected),
causing a burning sensation in the throat of 18% of those affected.
The predominant symptoms in order of frequency were nausea (69%),
abdominal cramps (43%), headache (33%), vomiting (11%), fever and
diarrhoea (8%). The children recovered in about 3 h. The reviewers
estimate that, assuming the children ingested 200 g, and had a bw of
40 kg, the dose was about 2.5 mg 'solanine'/kg bw (Anon, 1984).
Based on the available human data (Table 3), an intake of
3-6 mg TGA/kg bw is considered a potentially lethal dose for humans,
and >1 to 3 mg TGA/kg bw is considered a toxic dose for humans.
Children may be more sensitive than adults. Other factors may be
present in suspect potatoes and modulate the toxicity of the
steroidal glycoalkaloids.
No signs of acute toxicity were noted in 3 Swedish adult
volunteers who ingested for 1 week a diet estimated to give an
intake of 1 mg/kg bw TGA (Harvey et al., 1985b).
Table 3. Summary of published reports of solanine poisoning in humans
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
56 peeled, 1-1.5 kg 24 3.4-5.1 recovered Pfuhl,
(soldiers) cooked (38) 1899
(whole
uncooked)
60 adults potatoes 500 g ? 41 3.4 recovered Harris &
1 child 200 g ? 4.5 1 fatal Cockburn,
(lethal) (5 yr-old) 1918
7 (family) greened ? ? ? 2 fatal Hansen, 1925
potatoes
50-60 shoots, ? 27 ? 1 fatal Willimot,
(Cyprus) leaves 49 1933
Prisoners experimental ? ? 2.8 recovered Report cited
by Ruhl,
1951
Child potato ? ? ? 1 fatal Report cited
berries by Ruhl,
1951
4 (family baked 1-3 50 1.2-3.2 dose-related, Wilson, 1959
adults) potatoes potatoes recovered
with skin 150-450 g
Table 3 (continued)
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
78 old potatoes 2 small 25-30 1-4-1.6 3 comatose, McMillan &
(schoolboys) potatoes all Thompson,
200 g recovered; 1979
young
boys,
more affected
61 baked potato 200 g 49 2.5 recovered Anon. 1984
(school-children)
Alberta
? Data not available.
2.3.2 Teratogenic effects
In 1972, Renwick showed that areas with an increased incidence
of neural tube defects (NTD) (anencephaly and spina bifida) were
associated with areas where potato consumption was higher, and where
potato blight was more common. The worldwide incidence of NTD varies
from < 1 to about 7 per 1000 total births. He postulated that this
disease was due to toxic factors in potatoes, such as
alpha-chaconine and alpha-solanine. These antifungal compounds offer
resistance to potato blight and increase in amount in blighted
potatoes, infected with the fungus Phytophthora infestans. Several
studies have been conducted to prove or disprove this theory
(Renwick, 1972). The same author more recently suggested that a long
half-life of potato glycoalkaloids could lead to their retention in
the body, and possible release early during pregnancy (Renwick,
1982).
In a prospective study with women who had previously borne a
child with NTD, 27 women did not handle or eat potatoes or potato
containing foods after deciding on a future pregnancy, and
throughout gestation; another 61 women, attending the same clinic,
did not avoid potatoes. The allocation to the two groups was
non-random, but voluntary. The groups did not differ significantly
with respect to age distribution, social class, parity or history of
outcome of previous pregnancies. The incidence of NTD was 8.7% in
the group of women avoiding potatoes, and 3.6% in the group eating
potatoes (p=0.58). This study failed to support the Renwick
hypothesis, but the authors pointed out that the size of the groups
was small (Nevin & Merrett, 1975).
Although there is a geographical similarity between neural tube
defect occurrence and potato blight in Canada, no annual or seasonal
associations were demonstrated. The author concluded that
socioeconomic factors were probably more important as a risk factor
for NTD, but suggested that better exposure assessment to factors
present in potatoes, at the level of the individual, would be
necessary to resolve this question. Such prospective studies should
also assess the significance of other risk factors (Elwood, 1976).
An epidemiological study (prospective study) was conducted in
Great Britain, whereby human serum specimens from 380 patients, who
were being screened for NTD by measuring their serum
alpha-fetoprotein at 15-22 weeks of gestation (most at 16 weeks),
were also analyzed for potato glycoalkaloids, using a sensitive
radioimmunoassay. The samples were analyzed blind, regardless of the
outcome of pregnancy, which resulted in 210 NTD cases and 170 normal
offspring. In most of the 9 centres studied, serum TGA and serum
solanidine levels were higher (p <0.05 in 2 centres) in the women
with a normal fetus than in those with a fetus affected by NTD.
Although closure of the neural tube normally takes place at 4-5
weeks of gestation, the authors felt that measurements at the later
date might reflect glycoalkaloid exposure earlier during gestation.
The results of this study are therefore the opposite of what one
would expect if the ingestions of potatoes contributed to the
etiology of NTD. Instead the authors suggested that avoidance of
potatoes might contribute to a vitamin deficiency thereby increasing
rather than decreasing the incidence of NTD (Harvey et al., 1986).
3. COMMENTS
Numerous studies performed on a variety of experimental animal
species to elucidate the toxicological properties of glycoalkaloids,
including teratogenicity, have been evaluated.
Cranial abnormalities have been observed in some teratogenicity
studies with laboratory animals, particularly with the hamster at
levels of 165-200 mg glycoalkaloids/kg bw/day. However, the
suggested association of the consumption of blighted potatoes during
pregnancy with increased incidences of spina bifida and anencephaly
has not been substantiated.
In a limited study in humans, the daily consumption of potato
tubers containing approximately 24 mg glycolkaloids/100 g did not
result in any signs of acute toxicity. However, human poisonings
have been associated with the consumption of poor-quality potato
tubers with elevated levels of glycoalkaloids. The signs of
low-grade glycoalkaloid poisoning are acute gastrointestinal upset
with diarrhoea, vomiting, and severe abdominal pain. In more severe
cases, neurological symptoms, including drowsiness, apathy,
confusion, weakness, and vision disturbances followed by
unconsciousness, have also been reported.
4. EVALUATION
The Committee considered that, despite the long history of
human consumption of plants containing glycoalkaloids, the available
epidemiological and experimental data from human and laboratory
animal studies did not permit the determination of a safe level of
intake. The Committee recognized that the development of empirical
data to support such a level would require considerable effort.
Nevertheless, it felt that the large body of experience with the
consumption of potatoes, frequently on a daily basis, indicated that
normal glycoalkaloid levels (20-100 mg/kg) found in properly grown
and handled tubers were not of concern. To support the continued
safe use of potato tubers, those developing new cultivars, and
others growing, harvesting, storing, processing, and consuming
potatoes, should be aware of the possibility of inadvertently
increasing the content of glfycoalkaloids to potentially toxic
levels.
5. REFERENCES
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1978). Inhibition of
rat cholinesterase isoenzymes in vitro and in vivo by the potato
alkaloid, alpha-chaconine. J. Food Biochem., 2: 259-276.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979a). Physiological
disposition, subcellular distribution and tissue binding of
alpha-chaconine (3H). J. Food Safety, 1: 257-273.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979b). Excretion of
alpha-chaconine-3H, a steroidal glycoalkaloid from
Solanum-tuberosum L. and its metabolites in hamsters. Pharmacol.
Res. Commun., 11: 483-490.
ANON. (1979). Solanine poisoning [editorial]. Br. Med. J., 2:
1458-1459.
ANON. (1984). Solanine food poisoning associated with a school lunch
program - Alberta. Canada Diseases Weekly Report, Health and
Welfare Canada, 10-18: 71.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1983). Toxic effects of high
glycoalkaloid feeding on the protein digestibility and growth of
rabbits. J. Pharm. Univ. Karachi., 2: 15-24.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1984). Toxic effects of high
glycoalkaloid feeding on the red blood cell counts and haemoglobin
concentration of rabbit blood. J. Pharm. Univ. Karachi, 3: 43-49.
BÖMER, A. & MATTIS, H. (1924). [Solanine content of potatoes] Der
Solaningehalt der Kartoffeln. Z. Nahr. Genussm., 47: 97-127.
BUSHWAY, R.J. & PONNAMPALAM, R. (1981). alpha-chaconine and
alpha-solanine content of potato products and their stability during
several modes of cooking. J. Agric. Food Chem., 29: 814-817.
CHAUBE, S. & SWINYARD, C.A. (1976). Teratological and toxicological
studies of alkaloidal and phenolic compounds from Solanum tuberosum
L. Toxicol. Appl. Pharmacol., 36: 227-237.
CLARINGBOLD, W.D.B., FEW, J.D. & RENWICK, J.H. (1982). Kinetics and
retention of solanidine in man. Xenobiotica, 12: 293-302.
DALVI, R.R. & BOWIE, W.C. (1983). Toxicology of solanine: an
overview. Vet. Hum. Toxicol., 25: 13-15.
DALVI, R.R. (1985). Comparative assessment of the effect of solanine
administered orally and intraperitoneally on hepatic dysfunction in
male rats. Jpn. J. Vet. Sci., 47: 657-659.
ELWOOD, J.M. (1976). Anencephalus, spina bifida and potato blight in
Canada. Can. J. Public Health, 67: 122-126.
GULL, S.D., ISENBERG, F.M. & BRYAN, H.H. (1970). Alkaloid toxicology
of Solanum-tuberosum. Hort. Science, 5: 316.
HANSEN, A.A. (1925). Two fatal cases of potato poisoning. Science,
61: 340-341.
HARRIS, F.W. & COCKBURN, T. (1918). Alleged poisoning by potatoes.
Am. J. Pharm., 90: 722-726.
HARRIS, H. & WHITTAKER, M. (1962). Differential inhibition of the
serum cholinesterase phenotypes by solanine and solanidine. Ann.
Hum. Genet., 26: 71-76.
HARVEY, M.H., McMILLAN, M., MORGAN, M.R.A. & CHAN, H.W.-S. (1985a).
Solanidine is present in sera of healthy individuals and in amounts
dependent on their dietary potato consumption. Hum. Toxicol., 4:
187-194.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1985b).
Measurement of potato steroidal alkaloids in human serum and saliva
by radioimmunoassay. Hum. Toxicol., 4: 503-512.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1986). Potato
steroidal alkaloids and neural tube defects: serum concentrations
fail to demonstrate a causal relation. Hum. Toxicol., 5: 249-253.
JADHAV, S.J., SHARMA, R.P. & SALUNKHE, D.K. (1981). Naturally
occurring toxic alkaloids in foods. Crit. Rev. Toxicol., 9:
21-104.
JELINEK, R., KYZLINK, V. & BLATTNY, C., Jr. (1976). An evaluation of
the embryotoxic effects of blighted potatoes on chicken embryos.
Teratology, 14: 335-342.
KIRK, D. & MITTWOCH, U. (1975). Changes in the mitotic cycle induced
by alpha-solanine. Humangenetik, 26: 105-111.
KLINE, B.E., VON ELBE, H., DAHLE, N.A. & KUPCHAN, S.M. (1961). Toxic
effects of potato sprouts and of solanine fed to pregnant rats.
Proc. Soc. Exp. Biol. Med., 107: 807-809.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
MAGA, J.A. (1980). Potato glycoalkaloids. Crit. Rev. Food Sci.
Nutr., 12: 371-405.
MATTHEW, J.A., MORGAN, M.R.A., McNERNEY, R., CHAN, H.W.-S. & COXON,
D.T. (1983). Determination of solanidine in human plasma by
radioimmunoassay. Food Chem. Toxicol., 21: 637-640.
McMILLAN, M. & THOMPSON, J.C. (1979). An outbreak of suspected
solanine poisoning in schoolboys: examination of criteria of
solanine poisoning. Q. J. Med., 48: 227-243.
MICHALSKA, L., NAGEL, G., SWINIARSKI, E. & ZYDOWO, M.M. (1985). The
effect of alpha-solanine on the active calcium transport in rat
intestine. Gen. Pharmacol., 16: 69-70.
MORGAN, M.R.A. & COXON, D.T. (1987). Tolerances: glycoalkaloids in
potatoes. Ch. 7. In: Watson, D.H. (ed.). Ellis Horwood series in
food science and technology: Natural toxicants in food: progress
and prospects, Ellis Horwood, Chichester, England, pp. 221-230.
MORRIS, S.C. & LEE, T.H. (1984). The toxicity and teratogenicity of
Solanaceae glycoalkaloids particularly those of the potato
(Solanum tuberosum): a review. Food Technol. Aust., 36: 118-124.
MUN, A.M., BARDEN, E.S., WILSON, J.M. & HOGAN, J.M. (1975).
Teratogenic effects in early chick embryos of solanine and
glycoalkaloids from potatoes infected with late-blight,
Phytophthora infestans. Teratology, 11: 73-77.
NESS, E., JONER, P.E. & DAHLE, H.K. (1984). Alpha-solanine tested
for mutagenicity with the Ames test. Acta Vet. Scand., 25:
145-147.
NEVIN, N.C. & MERRETT, J.D. (1975) Potato avoidance during pregnancy
in women with a previous infant with either anencephaly and/or spina
bifida. Br. J. Prev. Soc. Med., 29: 111-115.
NISHIE, K., GUMBMANN, M.R. & KEYL, A.C. (1971). Pharmacology of
solanine. Toxicol. Appl. Pharmacol., 19: 81-92.
NISHIE, K., NORRED, W.P. & SWAIN, A.P. (1975). Pharmacology and
toxicology of chaconine and tomatine. Res. Commun. Chem. Pathol.
Pharmacol., 12: 657-668.
NORRED, W.P., NISHIE, K. & OSMAN, S.F. (1976). Excretion,
distribution and metabolic fate of 3H-alpha-chaconine. Res.
Commun. Chem. Pathol. Pharmacol., 13: 161-171.
PATIL, B.C., SHARMA, R.P., SALUNKHE, D.K. & SALUNKHE, K. (1972).
Evaluation of solanine toxicity. Food Cosmet. Toxicol., 10:
395-398.
PFUHL, E. (1899). [Regarding an outbreak of illness due to poisoning
by solanine in potatoes] Über eine Massenerkrankung durch Vergiftung
mit stark solaninhaltigen Kartoffeln. Deutsch. Med. Wochenschr.,
25: 753-754.
POSWILLO, D.E., SOPHER, D. & MITCHELL, S.J. (1972) Experimental
induction of fetal malformation with "blighted" potato: a
preliminary report. Nature, 239: 462-464.
POSWILLO, D.E., SOPHER, D., MITCHELL, S.J., COXON, D.T., CURTIS,
R.F. & PRICE, K.R. (1973). Investigations into the teratogenic
potential of imperfect potatoes. Teratology, 8: 339-347.
RENWICK, J.H. (1972). Hypothesis: anencephaly and spina bifida are
usually preventable by avoidance of a specific but unidentified
substance present in certain potato tubers. Br. J. Prev. Soc. Med.,
26: 67-88.
RENWICK, J.H. (1982). Food and malformation. Practitioner, 226:
1947-1953.
RENWICK, J.H., CLARINGBOLD, W.D.B., EARTHY, M.E., FEW, J.D. &
McLEAN, A.C.S. (1984). Neural-tube defects produced in Syrian
hamsters by potato glycoalkaloids. Teratology, 30: 371-381.
RODDICK, J.G. (1989). The acetylcholinesterase-inhibitory activity
of steroidal glycoalkaloids and their aglycones. Phytochemistry,
28: 2631-2634.
RUDDICK, J.A., HARWIG, J. & SCOTT, P.M. (1974). Nonteratogenicity in
rats of blighted potatoes and compounds contained in them.
Teratology, 9: 165-168.
RÜHL, R. (1951). [Contribution on the pathology and toxicology of
solanine] Beitrag zur Pathologie und Toxikologie des Solanins.
Arch. Pharm., 284: 67-74.
SHARMA, R.P., WILLHITE, C.C., WU, M.T. & SALUNKHE, D.K. (1978).
Teratogenic potential of blighted potato concentrate in rabbits,
hamsters, and miniature swine. Teratology, 18: 55-61.
SHARMA, R.P., WILLHITE, C.C., SHUPE, J.L. & SALUNKHE, D.K. (1979).
Acute toxicity and histopathological effects of certain
glycoalkaloids and extracts of Alternaria solani or Phytophthora
infestans in mice. Toxicol. Lett., 3: 349-355.
SHARMA, R.P., TAYLOR, M.J. & BOURCIER, D.R. (1983). Subcellular
distribution of alpha-chaconine in mouse hepatocytes. Drug Chem.
Toxicol., 6: 219-234.
SHARMA, R.P. & SALUNKHE, D.K. (1989). Solanum glycoalkaloids. In:
Cheeke, P. R. (ed.). Toxicants of Plant Origin, Vol. 1 Alkaloids,
CRC Press, Boca Raton, Florida. pp. 179-236.
SLANINA, P. (1990a). Assessment of health-risks related to
glycoalkaloids ("solanine") in potatoes: a Nordic view. Report from
the Nordic working group on food toxicology and risk assessment.
Vår Föda, 43: 1-14.
SLANINA, P. (1990b). Solanine (glycoalkaloids) in potatoes:
toxicological evaluation. Food Chem. Toxicol., 28: 759-761.
SWINYARD, C.A. & CHAUBE, S. (1973). Are potatoes teratogenic for
experimental animals? Teratology, 8: 349-357.
WILLIMOTT, S.G. (1933). An investigation of solanine poisoning.
Analyst, 58: 431.
WILSON, G.S. (1959). A small outbreak of solanine poisoning.
Monthly Bulletin, Ministry of Health (London), 18: 207-210.
WILSON, A.M., McGANN, D.F. & BUSHWAY, R.J. (1983). Effect of growth,
location and length of storage on glycoalkaloid content of roadside
stand potatoes as stored by consumers. J. Food Prot., 46: 119-121.
WOOD, F.A. & YOUNG, D.A. (1974). TGA in potatoes. Agric. Can. Publ.
1533: pp. 1-2.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
Groups of 4 female Swiss-Webster mice, weighing 20 g, were
orally administered alpha-chaconine once at a dose of 10 mg/kg bw.
Animals were sacrificed by exsanguination at 3, 6, 14, 72 and 120 h
after dosing. Blood was obtained (0.1 ml/time point) by multiple
incisions into the tail vein. Absorption was slow, with peak values
in blood (0.82 µg/ml) obtained after 14 h. Decrease in blood levels
was slow, with 0.31 µg/ml present at 120 h. The peak level in the
liver (2.97 µg/g) was reached 6 h after dosing. A second, but lower,
peak of radioactivity was seen in the liver at 120 h, suggesting
enterohepatic recycling. Cell fractionation studies showed that
within liver cells, there was no preferential location for
alpha-chaconine, and there was no binding of alkaloid to isolated
RNA or DNA fractions (Sharma et al., 1983).
2.1.1.2 Rats
Male Fischer rats (180-250 g) were orally administered
alpha-solanine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). Radioactivity in
the gastrointestinal tract started to disappear from 3 to 6 h after
dosing. During the first 24 h interval the total urinary and faecal
excretion was 78% of the dose, with most in the faeces. Maximum
concentrations of radio-activity occurred near 12 h for all tissues,
with the largest concentration in the kidneys, spleen, liver, and
lungs, and the lowest concentration in the blood. By 24 h only 10%
of the administered dose remained, and 12% was unaccounted for
(presumed to be associated with other organs and the carcass). At
that time the amount of tritium in the liver represented 1.54% of
the dose, and for blood this was 0.375% (based on a total blood
volume of 64.1 ml/kg bw). By 4 days 84% of the dose had been
excreted by the faecal route, and urinary excretion accounted for
10% of the dose (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). alpha-Chaconine was
poorly absorbed since faecal elimination accounted for 60% of the
dose within 12 h, and 80% of the dose within 24 h. Urinary excretion
of tritium was 5% of the dose 3 to 6 h after dosing, and reached a
plateau of 10% of the dose between 12 and 24 h. Maximum
concentrations of radioactivity occurred between 6 to 12 h for all
tissues, with the largest concentration in the liver. Intermediate
concentrations were seen in the kidneys, spleen, and lungs, and the
lowest concentrations were seen in the blood, brain and abdominal
fat. At 24 h after dosing the amount of tritium associated with the
liver represented 1.29% of the dose, and that with the blood was
0.17% of the dose (based on a total blood volume of 64.1 ml/kg bw)
(Norred et al., 1976).
2.1.1.3 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h) hamsters (3 animals per time point)
were exsanguinated by cardiac puncture. (The reviewers noted an
error in reporting and have adjusted the results of the original
report by changing ng to µg). At 3 h after dosing, the highest
concentration of alpha-chaconine was seen in the intestines,
including intestinal contents (125 µg/g), and this represented 63%
of the administered dose. By 24 h these values were 75 µg/g or 44%
of the administered dose, and by 168 h they had declined to 1.73
µg/g or 0.92% of the administered dose. Peak blood (1.74 µg/ml) and
peak tissue levels (liver = 27.2 µg/g) of alpha-chaconine for most
tissues were seen by 12 h, and for the heart and kidneys by 24 h. By
168 h after dosing, blood levels had declined to 0.29 µg/ml. The
ratio of liver concentration to blood concentration at 72 h was
greater than at 24 h, indicating the possibility of enterohepatic
recycling. Only small amounts of radioactivity were recovered from
the faeces in the elimination phase (non-detectable at 3 h, 0.15% at
24 h, to 0.24% of the administered dose by 168 h). In the urine
these values increased from non-detectable at 3 h to 0.25% at 12 h,
and to 21% by 168 h. These results suggest that most of the
alpha-chaconine was absorbed, but that absorption from the
gastrointestinal tract was slow. Much of the radioactivity appeared
in various tissues in bound form (Alozie et al., 1979a).
2.1.1.4 Humans
Tritiated solanidine (dose not given, but expressed as
radioactivity) was administered to 3 human volunteers (2 males, 1
female) by iv injection. Blood and urine samples were collected at
various times up to 150 h. Ninety per cent of tritium had
disappeared from the blood within 20 minutes of injection. Presuming
that radioactivity represented solanidine or its metabolites, three
phases of elimination were identified in plasma with half-lives of 2
to 3.7 min, 2 to 5 h, and 72 to 104 h, respectively. Within minutes
of injection, the concentration of tritium in erythrocytes exceeded
that in plasma. Erythrocytes were found to be a mobile reserve of
solanidine, thereby delaying transfer of solanidine from vascular to
extravascular compartments. Low rates of excretion were seen in
urine and faeces, and together accounted for about 5% of the
administered dose during the first 24 h. Thus a fraction in excess
of 90% of the dose was sequestered somewhere in the body 24 h after
dosing. After this time, the rate of elimination from the body was
low, about 1-2% per day, corresponding to an overall half-life of 34
to 68 days. The authors calculated that if absorption of solanidine
were 1 mg/day, then with a fractional rate of excretion of 0.02, the
body burden would be 50 mg. The authors suggested that mobilization
from various storage loci could occur during times of 'metabolic
stress', including pregnancy (Claringbold et al., 1982).
Mean levels of 1.56 ± 1.17 (7 males) and 1.20 ± 0.93 (27
females) ng/ml solanidine were found, using radioimmunoassay, in
human plasma samples obtained by a hospital clinic in the UK,
collected in the morning before lunch (Matthew et al., 1983).
Thirty healthy males, aged 18-44 years, and 27 healthy females,
aged 16-62 years, participated in a study in the UK designed to
measure levels of serum solanidine in persons eating their usual
diet (during the winter). Intake of the type of potato product
(i.e., French fried, boiled or baked, and whether the skin was
included) was recorded daily for one month, with arbitrary units,
corresponding to approximate levels of TGA in those products,
assigned to each product; the weight of product ingested was not
measured. Serum samples were collected before the midday meal, and
were analyzed by radioimmunoassay (detection limit 0.5 ng/ml). In
males the mean level of solanidine was 10.8 ± 5.4 ng/ml (range
2.1-22.5 ng/ml), whereas in females the respective values were 7.9 ±
4.3 (range 1.6-18.5). For both genders there was a significant
correlation between serum solanidine levels and the alkaloid intake
(expressed in units as indicated above) during the month (R = 0.878
and R = 0.703, respectively). In two male subjects serum solanidine
levels dropped to 0.5 ng/ml 2 to 3 weeks after they had been on a
potato avoidance diet, indicating a relatively long serum half-life
for solanidine. It was suggested by the authors that solanidine may
be bound to blood constituents such as free sterols (Harvey et al.,
1985a).
Eighteen healthy males, aged 20-45 years, and 15 healthy
females, aged 19-63 years, from the London area in the UK,
participated in a study designed to measure levels of total serum
alkaloids (alpha-solanine + alpha-chaconine + solanidine) and
solanidine in persons eating their usual diet (during the summer).
For comparison, 5 males, aged 31-41 years, and 5 females, aged 31-67
years, from the Uppsala area in Sweden also participated in this
study. In Sweden, 2 of the males and 1 female consumed 200-300 g
potatoes of 2 varieties high in TGA, including the skin, for 1 week
(mean 24 mg TGA/100 g), giving an intake of approximately
60 mg/person or 1 mg/kg bw/day. Blood samples were collected before
the midday meal, and were analyzed by radio-immunoassay (detection
limits for total alkaloids and solanidine were 0.4 and 0.5 ng/ml
serum, respectively). The mean levels of serum solanidine were,
respectively, 3.5 and 4.0 ng/ml in the UK and Swedish subjects
eating their usual diets, whereas in those three Swedes consuming
potatoes with a higher TGA content the mean serum solanidine level
was 31 ng/ml (range 27.8-35.5). The respective serum total alkaloid
levels were 12.0, 16.9 and 50 ng/ml. The mean serum total alkaloid
concentration was about 2.7 times the solanidine concentration,
which, according to the authors, suggests considerable metabolism in
man of the glycoalkaloids alpha-chaconine and alpha-solanine (they
represent the major proportion of alkaloids in potatoes) through
hydrolysis of the sugar residues. It was suggested that hydrolysis
could take place in the acid medium of the stomach, or at the site
of absorption, or the ratio could reflect the preferential
absorption of the more lipophilic solanidine. Alternatively,
alpha-solanine and alpha-chaconine might be absorbed unchanged and
metabolized within the body (Harvey et al., 1985b).
Blood serum levels of alpha-solanine, alpha-chaconine, and
solanidine resulting from a single meal of mashed potatoes
(equivalent to 1 mg TGA/kg bw/day) were monitored in 8 healthy
subjects (HPLC, detection limit 1 ng/ml). Peak concentrations were
achieved after 4-8 h; these were 3-11 ng/ml for alpha-solanine and
6-21 ng/ml for alpha-chaconine. The 1:2 ratio was maintained for the
duration of the experiment. After longer time intervals the level of
solanidine was < 4 ng/ml. The serum half-lives for alpha-solanine
and alpha-chaconine were 11 and 19 h, respectively (unpublished data
by K.E. Hellenäs, cited by Slanina, 1990b).
2.1.2 Biotransformation
2.1.2.1 Rats
Male Fischer rats (180-250 g) were orally administered 5 mg/kg
bw solanine, tritiated at the carbon atoms adjacent to the nitrogen
atom and the double bond (Fig. 1). Approximately 65% of the
radioactivity in the faeces was identified as solanidine. In urine
72% of radioactivity was present as basic compounds of which 6% was
identified as solanidine. Two other compounds, present at 80% and
13%, possessed intermediate polarity with respect to solanine and
solanidine (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1) at a dose of 5 mg/kg bw.
Urine and faecal samples were collected 24 h later. The major
constituent in both faeces and urine was presumed to be solanidine
because it showed the same Rf. Similarly, 25% of the radioactivity
in the faeces was attributed to unchanged alpha-chaconine. In
addition, 2 minor compounds, possessing intermediate polarity
between solanidine and alpha-chaconine and representing 1-5% of
total activity, were found in faecal and urine extracts. The authors
concluded that the absorption and metabolism of alpha-chaconine was
similar to alpha-solanine (Norred et al., 1976).
2.1.2.2 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h), hamsters were exsanguinated by
cardiac puncture (3 animals per time point) (see above Alozie
et al., 1979a). Thin-layer chromatographic separation was
performed on the chloroform soluble fractions from urine and faeces
collected at various time intervals after dosing. In urine, over
half of the eliminated radioactivity during the initial 24 h was due
to unaltered alpha-chaconine. A major urinary metabolite was
solanidine, which was the major peak by 72 h. In addition, 4 other
unidentified metabolites were present at various concentrations. Two
of these were the major peaks by 168 h after dosing. In faeces, much
of the eliminated radioactivity was due to alpha-chaconine, and a
major metabolite was solanidine. There were 2 additional
unidentified metabolites present in about the same concentration as
solanidine (Alozie et al., 1979b).
2.1.3 Effects on enzymes and other biochemical parameters
Groups of male Sprague-Dawley rats (5/group) were fasted
overnight and then given alpha-solanine by gavage at 0 and 250 mg/kg
bw or i.p. at 0 and 20 mg/kg bw. In the orally dosed animals serum
glutamic oxalacetic transaminase (SGOT) and serum glutamic pyruvic
transaminase (SGPT) were increased, and cholinesterase activity was
decreased, but the differences were not statistically significant.
With the i.p.-dosed animals statistically significant increases of
29 and 63% in SGOT and SGPT, respectively, and a 27% decrease in
cholinesterase activity were observed. In addition, a significant
inhibition of liver benzphetamine N-demethylase activity and a
decrease in liver cytochrome P-450 were observed after i.p. dosing,
whereas after oral dosing these differences were statistically
insignificant (Dalvi, 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Oral LD50 values for solanine in rodents are considerably
higher than LD50 values determined after intraperitoneal
administration (Table 2), probably because these species do not
absorb much of the solanine. Post-mortem examination failed to
reveal the cause of death in rats that had been dosed orally
(stomach tube). The oral LD50 values in rodents were 300 to > 500
times the toxic dose of about 2 mg/kg bw and a lethal dose of 3 to
6 mg/kg bw estimated for humans (see Section 2.3.1).
Table 2. LD50 values in mg/kg bw
alpha-solanine alpha-chaconine solanidine
i.p. p.o. i.p. i.p.
Mice 32.31 >10002 19.25 >5002
42.02 27.56
32.35
30.06
Rat 753 5903
674
Rabbit <201,2 506
406
Rhesus <407
Monkey
1 Patil et al. (1972)
2 Nishie et al. (1971)
3 Gull et al. (1970)
4 Chaube & Swinyard (1976)
5 Sharma et al. (1979)
6 Nishie et al. (1975)
7 Swinyard & Chaube (1973)
Two rhesus monkeys died 48 h after an i.p. injection of
40 mg/kg bw of total glycoalkaloids; one other died 2 h after having
been dosed i.p. twice (24 h apart) with 20 mg solanine/kg bw
(Swinyard & Chaube, 1973).
2.2.2 Short term studies
2.2.2.1 Rabbits
One group of 4 rabbits, each weighing about 950 g (strain not
given), was fed normal potatoes (TGA content 7.5 mg/100 g) and 4
more rabbits were fed greened potatoes (TGA content 20.4 mg/100 g)
for a period of 20 days. After 4 to 6 days the latter group,
consuming 49-53 mg TGA/kg bw/day, became dull and inactive; after 10
days, diarrhoea, hair loss and weight loss occurred, which was
followed by watering eyes, body rigidity and dullness. Protein
digestibility (amount of protein from potatoes ingested less amount
of protein excreted expressed as a percentage of protein from
ingested potatoes) decreased by 45% from day 1. This was accompanied
by a significant decrease in body weight, resulting in an average
body weight of about 650 g for treated and 1150 g for 'control'
rabbits at 25 days after cessation of feeding the experimental
diets. One of the 4 treated rabbits died within 10 to 20 days. The
control animals which consumed 20 to 23 mg TGA/kg bw/day were
unaffected (Azim et al., 1983).
The same authors similarly fed 2 groups of 5 rabbits each
(weight and strain not given) a control potato diet (7.5 mg
TGA/100 g) and a high TGA potato diet (29.75 mg TGA/100 g) for 45
days. Daily intake of TGA was 16.8 to 17.9 mg/kg bw in the control
diet, and 73.9 to 75.0 mg/kg bw in the high TGA diet. Blood samples
were collected from the ear vein every 15 days. RBC counts and
haemoglobin concentrations were determined. A significant decrease
in RBC counts was seen throughout feeding the high TGA diet, and
this was 27.5% by 45 days. This compared to a decrease of 12.5% seen
by 45 days in the control diet. Decreases in haemoglobin
concentrations paralleled the findings with RBC counts. The authors
suggested that these results indicate that the rabbits developed
haemolytic anaemia. This could be explained by the metabolite
solanidine increasing the permeability and fragility of RBC
membranes (Azim et al., 1984).
2.2.2.2 Monkeys
Four time-mated rhesus monkeys were fed ad libitum a diet of
diced potatoes of the B5141-6 variety (since withdrawn from the
market), containing on average 26 mg solanine per 100 g tuber for 25
consecutive days during days 0-42 following mating. It subsequently
became apparent that the monkeys were not pregnant. The monkeys
ingested the equivalent of 0.77 to 1.02 mg/kg bw/day alpha-solanine
or 3.08 to 4.07 mg/kg bw/day TGA. No adverse effects were observed
(Swinyard & Chaube, 1973).
2.2.3 Long-term/carcinogenicity studies
No studies available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Holzman rats, approximately 4 months of age, were
mated, one male to 3 females. Increase in weight was taken as an
indication of pregnancy, and afterwards the females were
individually caged and given a basal diet of (I) ground lab chow;
(II) ground lab chow to which was added 10% ground frozen potato
sprouts; (III) 30 mg/kg diet solanine (commercial); (IV) 40 mg/kg
diet solanine (commercial); and (V) 30 mg/kg diet solanine that was
isolated from the frozen sprouts. The time on the test diets was
variable, since increase in weight is not a sensitive indicator of
pregnancy, and some dams dropped their litters within a few days on
the test diet. They were then kept on the test diet until they had a
second litter. No food consumption records were kept, but the rats
readily ate the diets. With diets II to V, many of the pups died
within 3 days of birth, evidently from starvation as indicated by an
absence of milk in their stomachs. The percentage of pups
successfully weaned was 82.6, 50.6, 31.0, 31.1, and 19.5 for diets I
to V, respectively. All of the pups in 18/33 litters born of the
rats eating the test diets died before reaching weaning age, whereas
only one of 11 control litters was lost. The authors concluded that
the toxicity of the potato sprout diet was due to 'solanine'. It was
speculated by the authors that solanine may exert an anti-hormonal
effect and prevent lactation in some sensitive dams. The reviewers
feel that further studies to examine these effects and other aspects
of reproduction are necessary (Kline et al., 1961).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Potential teratogenicity of alpha-solanine and alpha-chaconine
was investigated in four different experiments with Wistar rats
weighing 175-200 g. In the first experiment, three groups of rats
(9-10/group) were gavaged with alpha-solanine at dose levels of 0.3,
1.0 or 3.0 mg/kg bw/day, from days 6 to 15 of gestation. In the
second experiment, a group of 9 rats was given alpha-solanine by
gavage at a dose level of 6 mg/kg bw/day, from days 7 to 10 of
gestation. In the third experiment, groups of rats (3-4/group)
received alpha-solanine at dose levels of 2, 10 or 25 mg/kg bw/day
from days 8 to 11 of gestation. In the fourth experiment, a group of
4 rats received alpha-chaconine by gavage at a dose level of
1.5 mg/kg bw/day, from days 6 to 15 of gestation. In the first three
experiments, concurrent control groups composed of 2 to 10
rats/group were included. On day 22 of gestation, all females were
sacrificed and the following parameters were investigated: corpora
lutea, resorption sites, litter size, litter weight, and gross,
visceral and skeletal fetal anomalies. The only adverse effect was
observed in the first experiment. One fetus with craniorachischisis
and exopthalmos (1/117), from the group receiving 3 mg/kg bw/day,
and another with twisted pelvic limbs and absent tail (1/108), from
the 0.3 mg/kg bw/day group were observed. No maternal toxicity was
reported. The authors concluded that the observed effects were not
treatment-related (Ruddick et al., 1974).
A group of 14 Wistar rats, weighing 175-200 g, was fed a diet
containing about 73% of cooked and freeze-dried, visibly blighted
parts of potato tubers, from days 1 to 22 of gestation. The intake
of blighted potatoes was approximately 70 g/kg bw/day. The control
group of 13 females was fed a diet containing the same amount of
freeze-dried potatoes inoculated with heat-killed Phytophthora
infestans. The content of glycoalkaloids in the diets was not
determined. All dams were sacrificed on day 22 of gestation and the
standard parameters (corpora lutea, resorption sites, litter size,
litter weight, and gross, visceral and skeletal anomalies) were
investigated. There was no evidence of maternal toxicity, fetal
toxicity nor teratogenicity (Ruddick et al., 1974).
2.2.5.2 Hamsters
Groups of hamsters (12-15/group), weighing about 100 g, were
fed from days 5 to 10 of gestation diets of commercial hamster
ration containing 50% freeze-dried, unblighted potato concentrate
(group 1); 50% Phytophthora infestans infected freeze-dried,
blighted potato concentrate (group 2); or 50% Alternaria solani
infected freeze-dried, blighted potato concentrate (group 3). A
group of 13 hamsters was fed commercial hamster ration only,
throughout gestation (group 4). The content of glycoalkaloids in the
diets was not determined. Food and water were provided ad libitum.
On day 15 of gestation, the dams were sacrificed, and fetuses were
examined for gross, visceral and skeletal anomalies. Feed
consumption, maternal body weight gain, litter size, number of
resorptions and fetal weight were not affected by the treatment. The
most frequent gross anomaly was haemorrhagic necrosis of the central
nervous system, but the frequency of this effect was not
treatment-related (group 1 - 1/114; group 2 - 3/153; group 3 -
0/135; group 4 - 11/99) (Sharma et al., 1978).
Groups of Syrian hamsters (body weights and age not given) were
gavaged on day 8 of gestation with alpha-chaconine, isolated from
Arran Pilot potato sprouts, at levels of 165 mg/kg bw/day (23/group)
or 180 mg/kg bw/day (14/group) and alpha-solanine, isolated from
Arran Pilot potato sprouts, at a level of 200 mg/kg bw/day
(37/group). Females of the vehicle control group (37/group) received
vehicle material alone (2% ethanol at pH 5-6 in 1% carboxymethyl
cellulose or in water). Animals were individually caged in a room
maintained at 20-26 °C and received food and water ad libitum.
Maternal toxicity was monitored by daily weighing and clinical
observation. On day 15 of gestation, the dams were sacrificed and
necropsied. The uteri were exposed and the number of resorptions and
live fetuses were determined. The corpora lutea were counted and
all fetuses were examined for gross anomalies. Maternal mortality
was observed in all treated dose groups; 4 and 6 dams died in the
165 and 180 mg/kg bw/day alpha-chaconine dose groups, respectively,
and 3 dams died in the 200 mg/kg bw/day alpha-solanine dose group.
The days of gestation on which the dams had died were not indicated.
No maternal mortality was observed in the vehicle control group. A
high incidence of neural tube defects such as interparietal
encephalocoele (11-13%) and exencephaly (5-12%) was observed in
fetuses, of which the mothers were exposed to either alpha-chaconine
or alpha-solanine. In the vehicle control group only 1 fetus of 393
examined exhibited exencephaly. The authors concluded that the
observed teratogenic effects were treatment-related. They also
indicated that a number of fetuses exhibited CNS malformations
without apparent toxicity or weight loss in the dam, making it
unlikely that the malformations were secondary to maternal toxicity.
Occasional short tail and minor digital anomalies were noted in
fetuses from all experimental groups, but those effects were not
treatment-related (Renwick et al., 1984).
2.2.5.3 Rabbits
Groups of New Zealand rabbits (2-6/group), weighing on average
4 kg, were fed, throughout gestation, diets containing 50%
freeze-dried, unblighted, potato concentrate, 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate, or
50% Alternaria solani infected freeze-dried, blighted, potato
concentrate. The content of glycoalkaloids in the diets was not
determined. Prior to parturition (day not defined), all dams were
sacrificed and fetuses removed and examined for gross, visceral and
skeletal anomalies. During fetal examination particular attention
was paid to any malformations of brain and spinal cord. Among 21
fetuses examined in the Phytophthora infestans blighted potato
group, three fetuses (from three litters) exhibited incomplete
closure of the caudal vertebral column, and two other fetuses were
very small and had shortened appendages. Among 28 fetuses examined
in the Alternaria solani blighted potato group, two fetuses
exhibited incomplete closure of the caudal vertebral column, one
fetus had a very small brain (nearly half the normal size) and the
cranial cavity was filled with fluid, and two other fetuses were
abnormally small in size. All six abnormal fetuses were from
different litters. None of the nine fetuses (two litters) from the
unblighted potato group were affected. The authors concluded that
feeding pregnant rabbits potatoes blighted with either of the fungi,
at high concentrations in the diet, can produce a low incidence of
the caudal vertebral column malformation. This result must be
considered with caution since the small number of control litters
examined does not permit an adequate estimate of the spontaneous
incidence of this malformation in rabbits (Sharma et al., 1978).
2.2.5.4 Miniature swine
Groups of female miniature swine (2/group), weighing about
39 kg, were fed laboratory diets containing 50% freeze-dried,
unblighted, potato concentrate (group 1); 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate
(group 2); or 50% Alternaria solani infected freeze-dried,
blighted, potato concentrate (group 3), during the first half of
gestation (about the first 57 days of gestation). The content of
glycoalkaloids in the diets was not determined. At the end of
gestation (the day was not indicated), all dams were sacrificed and
necropsied. All fetuses were removed and examined for gross and
visceral malformations. Depressed weight gain was observed in sows
of group 3. One fetus of 15 examined from group 2 exhibited
anencephaly with extensive internal hydrocephaly. Other fetuses from
this and other groups were not affected. The authors concluded that
feeding potatoes blighted with Phytophthora infestans may be a
causative factor in the production of anencephaly in miniature
swine. However, the small sample size makes definite conclusions
difficult (Sharma et al., 1978).
2.2.5.5 Marmosets
A group of 6 female marmosets (Callithrix jacchus), five
years of age, weighing about 375 g, previously producing normal
offspring, was fed a diet containing freeze-dried concentrate of
blighted potatoes (Kerr's Pink variety), at a level of 4.7 g/kg
bw/day (equivalent to 0.9 mg/kg bw/day of glyco-alkaloids), for 50
days, during either days 0-50 or 20-70 of gestation. A control group
of 6 pregnant marmosets received a standard unsupplemented diet.
Marmosets were sacrificed between days 80-120 of gestation and
fetuses were examined for developmental anomalies. Four of 11
fetuses in the blighted potato groups exhibited gross abnormalities,
described as cranial osseous defects. Histological examination
revealed replacement of bone by a collagenous membrane in the
occipital area. Brain examination of affected fetuses revealed
enlargement of the lateral ventricle. Eleven fetuses of the control
group showed no gross abnormalities in any system. The investigators
indicated that, during the 2 years of existence of the marmoset
colony, similar defects had occurred once spontaneously in twin
fetuses (spontaneously aborted) among 104 live births. The authors
concluded that the occurrence of cranial dysplasia in 4 of 11
fetuses, in the experimental group, is suggestive of teratogenicity
of blighted potatoes in marmosets (Poswillo et al., 1972, 1973).
Three experiments were conducted to investigate possible
teratogenic effects of different varieties of unblemished or
blemished potatoes in 5 year-old, pregnant marmosets (Callithrix
jacchus), previously producing normal offspring. In the first
experiment, a group of 6 female marmosets were fed freeze-dried
concentrate of unblemished 'domestic potatoes' (Cornish White and
King Edward varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.56 mg/kg bw/day of glycoalkaloids. In the second
experiment a group of 7 female marmosets were fed freeze-dried
concentrate of blemished 'industry rejected potatoes' (King Edward,
Cornish White varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.78 mg/kg bw/day of glycoalkaloids. In the third
experiment a group of 5 female marmosets were fed freeze-dried
concentrate of King Edward variety potatoes, infected with Erwinia
carotovera (a bacterial pathogen responsible for 'blackleg'), at a
level of about 4.7 g/kg bw/day, equivalent to 0.07 mg/kg bw/day of
glycoalkaloids. Feeding trials were commenced 10 days postpartum of
the previous litter, to cover the period of the expected postpartum
oestrous and were carried throughout an undefined period of
gestation. Female marmosets fed both the 'domestic potatoes' and
'industry rejected potatoes' diets were allowed to proceed with
pregnancy to term, and the offspring was grossly examined at birth
and at regular intervals up to 6 months of age. Females fed
'infected potatoes' (with Erwinia carotovora) diet were sacrificed
between days 90 to 110 of gestation, and fetuses were examined
grossly and radiographically for abnormalities. Behavioural
anomalies such as continuous clinging to parents or siblings, and
prolonged weaning time, were observed in three sets of twins, born
to dams of the second experiment. No anatomical abnormalities were
observed in any experimental groups. The authors concluded that the
significance of the behavioural abnormalities observed in this study
cannot be determined at this stage but further observation of growth
and development to sexual maturity may throw more light on this
phenomenon (Poswillo et al., 1973).
2.2.5.6 Chicken embryos
Fertile chicken eggs (White Leghorn) were injected either with
pure solanine, mixed glycoalkaloids or an ethanol extract (obtained
from potatoes infected with Phytophthora infestans) into the yolk
sac, at levels ranging from 0.13 to 0.26 mg/egg, between 0 and 26 h
of incubation. A high incidence of embryo mortality (20-27%) and
increased incidence of abnormalities (16-25%) such as cranioschisis,
celosoma, cardiac septal defects, rumplessness (absence of tail) and
trunklessness (absence of trunk below the wing bud) were observed in
treated embryos. The most frequent defect was rumplessness and
trunklessness. In controls injected with chick Ringer or HCl
solvent, the percentage of abnormal embryos was 9-10% and the
mortality was 1-8% (Jelinek et al., 1976; Mun et al., 1975).
2.2.6 Special studies on cholinesterase inhibition
The inhibitory effect of alpha-solanine and solanidine, as well
as an extract from potatoes, were studied using a 1:100 dilution of
sera from 21 human individuals. These persons had been previously
phenotyped as 'usual' (95% of population in Great Britain),
'intermediate' (3-4% of the population), and 'atypical' (uncommon),
using the acetylcholinesterase inhibitor dibucaine. At a
concentration of 2.88 µM and 3.14 µM, respectively, alpha-solanine
and solanidine were about equally effective causing 86.2 ± 1.2 % and
80.0 ± 1.4 % inhibition in the 'usual' phenotype, and parallel
effects to dibucaine in the other two phenotypes. The results with
the potato extract were similar. The authors noted that it is not
clear to what extent the toxic effects of solanine can be attributed
to the inhibition of serum cholinesterase, but if it plays a role
then individuals with the 'atypical' phenotype, would presumably be
less susceptible (Harris & Whittaker, 1962).
Male and female New Zealand rabbits (2 of each sex) were given
a single i.p. dose of 20 or 30 mg solanine/kg body weight. These
doses resulted in severe depression, with difficult breathing and
prostration, and were lethal in 3 of the rabbits within 24 h. One
rabbit survived. Blood samples were obtained at 15 to 225 min
post-dosing; plasma and erythrocyte acetylcholinesterase activities
were measured and compared to control samples taken from the same
rabbit before dosing. Solanine was a weak to moderate inhibitor of
both specific and non-specific cholinesterase. Maximum inhibition of
plasma cholinesterase was seen at 80 min after injection, with the
activity decreasing to about 45% of the control value; inhibition of
erythrocyte cholinesterase was somewhat lower, and was maximally
reduced to 68.6% at 85 min after injection.
The same authors injected i.v. 5 doses of 6 mg/kg body weight,
10 min apart, in one anaesthetized male dog (15 kg bw). Quick
inhibition of serum cholinesterase was followed by rapid recovery.
Erythrocyte cholinesterase was not inhibited (Patil et al., 1972).
Male Sprague-Dawley rats (3 per group) were injected i.p. with
0, 10, 30 or 60 mg/kg bw of alpha-chaconine and sacrificed 3 h after
dosing. All rats administered alpha-chaconine showed initial signs
of depression, as well as other signs of poisoning by an
anticholinesterase agent, such as respiratory depression. Following
electrophoresis in acrylamide slabs, homogenates of brain (diluted
1:6) showed 3 zones of acetylcholinesterase isoenzyme activity with
a dose-related decrease in peak heights. Overall
acetylcholinesterase activity, using a colorimetric method, was
reduced to 79, 55, and 18% of the control value for the 3 respective
dose groups. Heart acetylcholinesterase activity was reduced to
about 40% of the control value in all treatment groups; plasma
cholinesterase activity in controls was about 30% of that seen in
brain homogenates, and was reduced to 50% in the 10 mg/kg bw dosage
group, with no further reduction in rats given 30 mg/kg bw. The
authors concluded that alpha-chaconine is a fairly potent inhibitor
of cholinesterases (Alozie et al., 1978).
In an in vitro assay the anticholinesterase activity of
several glycoalkaloids was compared, using highly purified acetyl
cholinesterase isolated from human and bovine erythrocytes (Sigma).
Alpha-solanine and alpha-chaconine were equally effective, 100 µM
caused about 80% inhibition of both human and bovine enzymes.
Tomatine was less effective, causing 40% and 50% inhibition of
bovine and human enzymes, respectively. Solasine, solamargine and
the aglycones solanidine, tomatidine and solasidine were
ineffective. Over a range of Ph 5 to pH 8, pH of the medium was not
very important. These results show that the nature of the aglycone
moiety is important (Roddick, 1989).
2.2.7 Special studies on genotoxicity
Pure alpha-solanine (Sigma) at 0.01 to 0.05 mg/plate and
extracts from potatoes were negative in the Ames test both with
strains TA98 and TA100, and in the presence or absence of activation
by S9 fraction from PCB-induced rat liver (Ness et al., 1984).
Alpha-solanine (25 and 250 µM) tested negative in a
DNA-cell-binding assay using Ehrlich ascites cells and Escherichia
coli cells mixed with 32P-labelled nucleic acids (Kubinski et
al., 1981).
2.2.8 Special studies on mitotic index
Cultured human fibroblasts were treated up to 40 h with 0, 4.1,
8.3, 16.6, 33.3, and 66.6 µg alpha-solanine/ml. At the highest dose
there was an inhibition of growth, whereas at lower dose levels
there was a stimulation of growth, as evidenced by an increase in
the mitotic index from 1.7% in controls to 2.4% at the 4.1 µg/ml
dose level, which according to the authors was similar to the
sex-hormonal type of effect exerted by estrogens on target tissues.
Using pulse labelling with tritiated thymidine, it was shown that at
5 µg alpha-solanine/ml the mean cell cycle time decreased from 42.5
± 1.87 h in controls to 28.5 ± 0.29 h in treated cells. This was
however accompanied by a 4 h increase in the period of DNA synthesis
(S phase) in treated cells, and a decrease to virtually zero for the
G1 phase. The authors concluded that if alpha-solanine reached the
fetus, the observed types of effects could be hazardous to it, and
could lead to malformations (Kirk & Mittwoch, 1975).
2.2.9 Special studies on calcium transport
Alpha-solanine (100 µM at pH 7.4) caused a 90% inhibition of
active calcium transport in rat duodenum when added to everted
intestinal sacs (8 replicates) in vitro. A Dixon plot revealed
that the inhibition by alpha-solanine was non-competitive, and the
inhibition constant was 25 µM. The inhibition of active calcium
transport was accompanied by a 40% decrease in oxygen consumption
(Michalska et al., 1985).
When alpha-solanine was given to 12 male and female Wistar
albino rats (5-6 weeks old) in their drinking water (5 mM, pH 6.4)
for 12 days, calcium transport in duodenal sacs was reduced to about
one-third of the control value, but oxygen consumption was not
significantly reduced (Michalska et al., 1985).
2.3 Observations in humans
2.3.1 Gastrointestinal and neurotoxic effects
There have been many reported cases of human poisonings
(sometimes fatal) due to the ingestion of greened or otherwise
damaged potatoes. The symptoms of low grade solanine poisoning are
acute gastrointestinal upset with diarrhoea, vomiting and severe
abdominal pain. In more severe cases, neurological symptoms,
including drowsiness and apathy, confusion, weakness, and vision
disturbances, followed by unconsciousness and, in some cases, death
have also been reported. The vital signs include fever, rapid and
weak pulse, low blood pressure and rapid respiration. Onset of
symptoms has ranged from minutes to 2 days after ingestion of toxic
potatoes, with longer incubation periods generally associated with
the more severe cases.
As is usual with case histories of this nature, the available
data are not complete. Over the years, various analytical methods or
assays have been used to determine the concentration of 'solanine'
in cases of suspected poisonings. With most of the older data, the
estimate for solanine included the other glycoalkaloids, such as
alpha-chaconine. McMillan and Thompson (1979) showed that
gravimetric methods gave higher values than colorimetric methods. A
few case reports, for which the reviewers have estimated the dose
ingested, are given below. Additional reports were compiled by
Morris and Lee (1984), who indicated that more than 2000 cases with
about 30 deaths have been reported in the literature. Not all of
these reports were available to the reviewers.
Fifty-six German soldiers suffered typical 'solanine' poisoning
after eating 1 to 1.5 kg cooked peeled potatoes containing 24 mg
TGA/100 g (whole uncooked tubers contained 38 mg TGA/100 g). In a
few cases jaundice and partial paralysis were also observed. If one
assumes a body weight of 70 kg, the intake of 'solanine' was 3.4 to
5.1 mg/kg bw (Pfuhl, 1899).
In 18 separate households in Scotland, 61 persons suffered
typical 'solanine' poisoning soon to several hours after eating
potatoes. Persons not eating potatoes were not ill. One 5-year old
died. The potatoes in that household contained 41 mg 'solanine'/100
g. Assuming the child ate 200 g potatoes and had a bw of 18 kg, the
lethal dose was estimated at 4.5 mg/kg bw. Assuming adults ate 500 g
potatoes and had a bw of 60 kg, their intake of 'solanine' would
have been 3.4 mg/kg bw (Harris & Cockburn, 1918).
A small outbreak of solanine poisoning affected a family of
four adults on three consecutive Sunday evenings in Great Britain,
about 8 h after they had eaten 1 to 3 baked potatoes in their
jackets (weight of potatoes not given). A 5th person who only ate
the flesh of the potatoes was not affected. The severity of symptoms
was related to the number of potatoes ingested, and consisted of
abdominal pain, diarrhoea, and general malaise. Patients recovered
within 24 h. The level of solanine was 50 mg/100 g tuber, as
determined chemically and by cholinesterase inhibition. Assuming a
weight of 150 g per potato, and body weights of 60 and 70 kg, the
dose was estimated at 1.25 to 3.2 mg 'solanine'/kg bw (Wilson,
1959).
Seventy-eight junior schoolboys in Great Britain became ill
from solanine poisoning 7 to 9 h after eating two small boiled
peeled potatoes each (weight of potatoes not given) as part of their
lunch, and 17 were admitted to hospital. Symptoms included vomiting,
diarrhoea, and general abdominal pain. Most of the boys developed a
fever, suffered from headache, dizziness, mental confusion,
hallucinations and their vision was affected. Three boys were
comatose and stuporose on admission, with peripheral circulatory
collapse. All were discharged 6-11 days following admission, and 4-5
weeks later there were no sequelae. Tests for the presence of
biocides, such as nicotine, organophosphorus or organochloride
pesticides were negative. Six days after eating the meal, plasma
pseudocholinesterase levels in 10 out of 17 schoolboys was subnormal
(about 25% below the normal range for this age group). Red blood
cell cholinesterase levels were normal. The source of toxic potatoes
was traced to a bag of old potatoes that had been condemned for
consumption because of their appearance, but that had inadvertently
been cooked (peeling of the potatoes had been done by an automatic
peeling machine). Insufficient potatoes were left over after the
meal for direct chemical analysis. Solanine levels in the boiled
peeled potatoes were therefore estimated from the in vitro
reduction in pseudocholinesterase activity in human plasma, using
acetylcholine as a substrate, and were equivalent to 25-30 mg/100 g
tuber of alpha-solanine. Assuming an intake of 200 g potatoes and a
bw of 40 kg (age = 11-14 years), the reviewers estimate that the
intake of 'solanine' by the schoolboys would therefore have been
approximately 1.4-1.6 mg/kg bw. Because of the small margin of
safety between normal potatoes and toxic potatoes, the authors
speculated that in toxic potatoes other toxic steroids besides
glycoalkaloids may be synthesized, such as sapogenins and saponins,
which might enhance the toxicity of solanine alkaloids by promoting
gastro-intestinal absorption or other means (McMillan & Thompson,
1979).
In a recent (1983) poisoning associated with a school lunch
programme, 61 of 109 school children and staff in Alberta, Canada,
became ill, most within 5 minutes, after eating baked potato (weight
of potato not given) containing 49.4 mg 'solanine' per 100 g
(analytical method not indicated). Test results showed that there
was no evidence that the illness occurred due to the presence of
viruses, bacteria, moulds, pesticides or other chemicals in the food
items or their containers. The potatoes had a slight tinge of green
and had a bitter or unusual taste (noted by 44% of those affected),
causing a burning sensation in the throat of 18% of those affected.
The predominant symptoms in order of frequency were nausea (69%),
abdominal cramps (43%), headache (33%), vomiting (11%), fever and
diarrhoea (8%). The children recovered in about 3 h. The reviewers
estimate that, assuming the children ingested 200 g, and had a bw of
40 kg, the dose was about 2.5 mg 'solanine'/kg bw (Anon, 1984).
Based on the available human data (Table 3), an intake of
3-6 mg TGA/kg bw is considered a potentially lethal dose for humans,
and >1 to 3 mg TGA/kg bw is considered a toxic dose for humans.
Children may be more sensitive than adults. Other factors may be
present in suspect potatoes and modulate the toxicity of the
steroidal glycoalkaloids.
No signs of acute toxicity were noted in 3 Swedish adult
volunteers who ingested for 1 week a diet estimated to give an
intake of 1 mg/kg bw TGA (Harvey et al., 1985b).
Table 3. Summary of published reports of solanine poisoning in humans
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
56 peeled, 1-1.5 kg 24 3.4-5.1 recovered Pfuhl,
(soldiers) cooked (38) 1899
(whole
uncooked)
60 adults potatoes 500 g ? 41 3.4 recovered Harris &
1 child 200 g ? 4.5 1 fatal Cockburn,
(lethal) (5 yr-old) 1918
7 (family) greened ? ? ? 2 fatal Hansen, 1925
potatoes
50-60 shoots, ? 27 ? 1 fatal Willimot,
(Cyprus) leaves 49 1933
Prisoners experimental ? ? 2.8 recovered Report cited
by Ruhl,
1951
Child potato ? ? ? 1 fatal Report cited
berries by Ruhl,
1951
4 (family baked 1-3 50 1.2-3.2 dose-related, Wilson, 1959
adults) potatoes potatoes recovered
with skin 150-450 g
Table 3 (continued)
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
78 old potatoes 2 small 25-30 1-4-1.6 3 comatose, McMillan &
(schoolboys) potatoes all Thompson,
200 g recovered; 1979
young
boys,
more affected
61 baked potato 200 g 49 2.5 recovered Anon. 1984
(school-children)
Alberta
? Data not available.
2.3.2 Teratogenic effects
In 1972, Renwick showed that areas with an increased incidence
of neural tube defects (NTD) (anencephaly and spina bifida) were
associated with areas where potato consumption was higher, and where
potato blight was more common. The worldwide incidence of NTD varies
from < 1 to about 7 per 1000 total births. He postulated that this
disease was due to toxic factors in potatoes, such as
alpha-chaconine and alpha-solanine. These antifungal compounds offer
resistance to potato blight and increase in amount in blighted
potatoes, infected with the fungus Phytophthora infestans. Several
studies have been conducted to prove or disprove this theory
(Renwick, 1972). The same author more recently suggested that a long
half-life of potato glycoalkaloids could lead to their retention in
the body, and possible release early during pregnancy (Renwick,
1982).
In a prospective study with women who had previously borne a
child with NTD, 27 women did not handle or eat potatoes or potato
containing foods after deciding on a future pregnancy, and
throughout gestation; another 61 women, attending the same clinic,
did not avoid potatoes. The allocation to the two groups was
non-random, but voluntary. The groups did not differ significantly
with respect to age distribution, social class, parity or history of
outcome of previous pregnancies. The incidence of NTD was 8.7% in
the group of women avoiding potatoes, and 3.6% in the group eating
potatoes (p=0.58). This study failed to support the Renwick
hypothesis, but the authors pointed out that the size of the groups
was small (Nevin & Merrett, 1975).
Although there is a geographical similarity between neural tube
defect occurrence and potato blight in Canada, no annual or seasonal
associations were demonstrated. The author concluded that
socioeconomic factors were probably more important as a risk factor
for NTD, but suggested that better exposure assessment to factors
present in potatoes, at the level of the individual, would be
necessary to resolve this question. Such prospective studies should
also assess the significance of other risk factors (Elwood, 1976).
An epidemiological study (prospective study) was conducted in
Great Britain, whereby human serum specimens from 380 patients, who
were being screened for NTD by measuring their serum
alpha-fetoprotein at 15-22 weeks of gestation (most at 16 weeks),
were also analyzed for potato glycoalkaloids, using a sensitive
radioimmunoassay. The samples were analyzed blind, regardless of the
outcome of pregnancy, which resulted in 210 NTD cases and 170 normal
offspring. In most of the 9 centres studied, serum TGA and serum
solanidine levels were higher (p <0.05 in 2 centres) in the women
with a normal fetus than in those with a fetus affected by NTD.
Although closure of the neural tube normally takes place at 4-5
weeks of gestation, the authors felt that measurements at the later
date might reflect glycoalkaloid exposure earlier during gestation.
The results of this study are therefore the opposite of what one
would expect if the ingestions of potatoes contributed to the
etiology of NTD. Instead the authors suggested that avoidance of
potatoes might contribute to a vitamin deficiency thereby increasing
rather than decreasing the incidence of NTD (Harvey et al., 1986).
3. COMMENTS
Numerous studies performed on a variety of experimental animal
species to elucidate the toxicological properties of glycoalkaloids,
including teratogenicity, have been evaluated.
Cranial abnormalities have been observed in some teratogenicity
studies with laboratory animals, particularly with the hamster at
levels of 165-200 mg glycoalkaloids/kg bw/day. However, the
suggested association of the consumption of blighted potatoes during
pregnancy with increased incidences of spina bifida and anencephaly
has not been substantiated.
In a limited study in humans, the daily consumption of potato
tubers containing approximately 24 mg glycolkaloids/100 g did not
result in any signs of acute toxicity. However, human poisonings
have been associated with the consumption of poor-quality potato
tubers with elevated levels of glycoalkaloids. The signs of
low-grade glycoalkaloid poisoning are acute gastrointestinal upset
with diarrhoea, vomiting, and severe abdominal pain. In more severe
cases, neurological symptoms, including drowsiness, apathy,
confusion, weakness, and vision disturbances followed by
unconsciousness, have also been reported.
4. EVALUATION
The Committee considered that, despite the long history of
human consumption of plants containing glycoalkaloids, the available
epidemiological and experimental data from human and laboratory
animal studies did not permit the determination of a safe level of
intake. The Committee recognized that the development of empirical
data to support such a level would require considerable effort.
Nevertheless, it felt that the large body of experience with the
consumption of potatoes, frequently on a daily basis, indicated that
normal glycoalkaloid levels (20-100 mg/kg) found in properly grown
and handled tubers were not of concern. To support the continued
safe use of potato tubers, those developing new cultivars, and
others growing, harvesting, storing, processing, and consuming
potatoes, should be aware of the possibility of inadvertently
increasing the content of glfycoalkaloids to potentially toxic
levels.
5. REFERENCES
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1978). Inhibition of
rat cholinesterase isoenzymes in vitro and in vivo by the potato
alkaloid, alpha-chaconine. J. Food Biochem., 2: 259-276.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979a). Physiological
disposition, subcellular distribution and tissue binding of
alpha-chaconine (3H). J. Food Safety, 1: 257-273.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979b). Excretion of
alpha-chaconine-3H, a steroidal glycoalkaloid from
Solanum-tuberosum L. and its metabolites in hamsters. Pharmacol.
Res. Commun., 11: 483-490.
ANON. (1979). Solanine poisoning [editorial]. Br. Med. J., 2:
1458-1459.
ANON. (1984). Solanine food poisoning associated with a school lunch
program - Alberta. Canada Diseases Weekly Report, Health and
Welfare Canada, 10-18: 71.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1983). Toxic effects of high
glycoalkaloid feeding on the protein digestibility and growth of
rabbits. J. Pharm. Univ. Karachi., 2: 15-24.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1984). Toxic effects of high
glycoalkaloid feeding on the red blood cell counts and haemoglobin
concentration of rabbit blood. J. Pharm. Univ. Karachi, 3: 43-49.
BÖMER, A. & MATTIS, H. (1924). [Solanine content of potatoes] Der
Solaningehalt der Kartoffeln. Z. Nahr. Genussm., 47: 97-127.
BUSHWAY, R.J. & PONNAMPALAM, R. (1981). alpha-chaconine and
alpha-solanine content of potato products and their stability during
several modes of cooking. J. Agric. Food Chem., 29: 814-817.
CHAUBE, S. & SWINYARD, C.A. (1976). Teratological and toxicological
studies of alkaloidal and phenolic compounds from Solanum tuberosum
L. Toxicol. Appl. Pharmacol., 36: 227-237.
CLARINGBOLD, W.D.B., FEW, J.D. & RENWICK, J.H. (1982). Kinetics and
retention of solanidine in man. Xenobiotica, 12: 293-302.
DALVI, R.R. & BOWIE, W.C. (1983). Toxicology of solanine: an
overview. Vet. Hum. Toxicol., 25: 13-15.
DALVI, R.R. (1985). Comparative assessment of the effect of solanine
administered orally and intraperitoneally on hepatic dysfunction in
male rats. Jpn. J. Vet. Sci., 47: 657-659.
ELWOOD, J.M. (1976). Anencephalus, spina bifida and potato blight in
Canada. Can. J. Public Health, 67: 122-126.
GULL, S.D., ISENBERG, F.M. & BRYAN, H.H. (1970). Alkaloid toxicology
of Solanum-tuberosum. Hort. Science, 5: 316.
HANSEN, A.A. (1925). Two fatal cases of potato poisoning. Science,
61: 340-341.
HARRIS, F.W. & COCKBURN, T. (1918). Alleged poisoning by potatoes.
Am. J. Pharm., 90: 722-726.
HARRIS, H. & WHITTAKER, M. (1962). Differential inhibition of the
serum cholinesterase phenotypes by solanine and solanidine. Ann.
Hum. Genet., 26: 71-76.
HARVEY, M.H., McMILLAN, M., MORGAN, M.R.A. & CHAN, H.W.-S. (1985a).
Solanidine is present in sera of healthy individuals and in amounts
dependent on their dietary potato consumption. Hum. Toxicol., 4:
187-194.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1985b).
Measurement of potato steroidal alkaloids in human serum and saliva
by radioimmunoassay. Hum. Toxicol., 4: 503-512.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1986). Potato
steroidal alkaloids and neural tube defects: serum concentrations
fail to demonstrate a causal relation. Hum. Toxicol., 5: 249-253.
JADHAV, S.J., SHARMA, R.P. & SALUNKHE, D.K. (1981). Naturally
occurring toxic alkaloids in foods. Crit. Rev. Toxicol., 9:
21-104.
JELINEK, R., KYZLINK, V. & BLATTNY, C., Jr. (1976). An evaluation of
the embryotoxic effects of blighted potatoes on chicken embryos.
Teratology, 14: 335-342.
KIRK, D. & MITTWOCH, U. (1975). Changes in the mitotic cycle induced
by alpha-solanine. Humangenetik, 26: 105-111.
KLINE, B.E., VON ELBE, H., DAHLE, N.A. & KUPCHAN, S.M. (1961). Toxic
effects of potato sprouts and of solanine fed to pregnant rats.
Proc. Soc. Exp. Biol. Med., 107: 807-809.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
MAGA, J.A. (1980). Potato glycoalkaloids. Crit. Rev. Food Sci.
Nutr., 12: 371-405.
MATTHEW, J.A., MORGAN, M.R.A., McNERNEY, R., CHAN, H.W.-S. & COXON,
D.T. (1983). Determination of solanidine in human plasma by
radioimmunoassay. Food Chem. Toxicol., 21: 637-640.
McMILLAN, M. & THOMPSON, J.C. (1979). An outbreak of suspected
solanine poisoning in schoolboys: examination of criteria of
solanine poisoning. Q. J. Med., 48: 227-243.
MICHALSKA, L., NAGEL, G., SWINIARSKI, E. & ZYDOWO, M.M. (1985). The
effect of alpha-solanine on the active calcium transport in rat
intestine. Gen. Pharmacol., 16: 69-70.
MORGAN, M.R.A. & COXON, D.T. (1987). Tolerances: glycoalkaloids in
potatoes. Ch. 7. In: Watson, D.H. (ed.). Ellis Horwood series in
food science and technology: Natural toxicants in food: progress
and prospects, Ellis Horwood, Chichester, England, pp. 221-230.
MORRIS, S.C. & LEE, T.H. (1984). The toxicity and teratogenicity of
Solanaceae glycoalkaloids particularly those of the potato
(Solanum tuberosum): a review. Food Technol. Aust., 36: 118-124.
MUN, A.M., BARDEN, E.S., WILSON, J.M. & HOGAN, J.M. (1975).
Teratogenic effects in early chick embryos of solanine and
glycoalkaloids from potatoes infected with late-blight,
Phytophthora infestans. Teratology, 11: 73-77.
NESS, E., JONER, P.E. & DAHLE, H.K. (1984). Alpha-solanine tested
for mutagenicity with the Ames test. Acta Vet. Scand., 25:
145-147.
NEVIN, N.C. & MERRETT, J.D. (1975) Potato avoidance during pregnancy
in women with a previous infant with either anencephaly and/or spina
bifida. Br. J. Prev. Soc. Med., 29: 111-115.
NISHIE, K., GUMBMANN, M.R. & KEYL, A.C. (1971). Pharmacology of
solanine. Toxicol. Appl. Pharmacol., 19: 81-92.
NISHIE, K., NORRED, W.P. & SWAIN, A.P. (1975). Pharmacology and
toxicology of chaconine and tomatine. Res. Commun. Chem. Pathol.
Pharmacol., 12: 657-668.
NORRED, W.P., NISHIE, K. & OSMAN, S.F. (1976). Excretion,
distribution and metabolic fate of 3H-alpha-chaconine. Res.
Commun. Chem. Pathol. Pharmacol., 13: 161-171.
PATIL, B.C., SHARMA, R.P., SALUNKHE, D.K. & SALUNKHE, K. (1972).
Evaluation of solanine toxicity. Food Cosmet. Toxicol., 10:
395-398.
PFUHL, E. (1899). [Regarding an outbreak of illness due to poisoning
by solanine in potatoes] Über eine Massenerkrankung durch Vergiftung
mit stark solaninhaltigen Kartoffeln. Deutsch. Med. Wochenschr.,
25: 753-754.
POSWILLO, D.E., SOPHER, D. & MITCHELL, S.J. (1972) Experimental
induction of fetal malformation with "blighted" potato: a
preliminary report. Nature, 239: 462-464.
POSWILLO, D.E., SOPHER, D., MITCHELL, S.J., COXON, D.T., CURTIS,
R.F. & PRICE, K.R. (1973). Investigations into the teratogenic
potential of imperfect potatoes. Teratology, 8: 339-347.
RENWICK, J.H. (1972). Hypothesis: anencephaly and spina bifida are
usually preventable by avoidance of a specific but unidentified
substance present in certain potato tubers. Br. J. Prev. Soc. Med.,
26: 67-88.
RENWICK, J.H. (1982). Food and malformation. Practitioner, 226:
1947-1953.
RENWICK, J.H., CLARINGBOLD, W.D.B., EARTHY, M.E., FEW, J.D. &
McLEAN, A.C.S. (1984). Neural-tube defects produced in Syrian
hamsters by potato glycoalkaloids. Teratology, 30: 371-381.
RODDICK, J.G. (1989). The acetylcholinesterase-inhibitory activity
of steroidal glycoalkaloids and their aglycones. Phytochemistry,
28: 2631-2634.
RUDDICK, J.A., HARWIG, J. & SCOTT, P.M. (1974). Nonteratogenicity in
rats of blighted potatoes and compounds contained in them.
Teratology, 9: 165-168.
RÜHL, R. (1951). [Contribution on the pathology and toxicology of
solanine] Beitrag zur Pathologie und Toxikologie des Solanins.
Arch. Pharm., 284: 67-74.
SHARMA, R.P., WILLHITE, C.C., WU, M.T. & SALUNKHE, D.K. (1978).
Teratogenic potential of blighted potato concentrate in rabbits,
hamsters, and miniature swine. Teratology, 18: 55-61.
SHARMA, R.P., WILLHITE, C.C., SHUPE, J.L. & SALUNKHE, D.K. (1979).
Acute toxicity and histopathological effects of certain
glycoalkaloids and extracts of Alternaria solani or Phytophthora
infestans in mice. Toxicol. Lett., 3: 349-355.
SHARMA, R.P., TAYLOR, M.J. & BOURCIER, D.R. (1983). Subcellular
distribution of alpha-chaconine in mouse hepatocytes. Drug Chem.
Toxicol., 6: 219-234.
SHARMA, R.P. & SALUNKHE, D.K. (1989). Solanum glycoalkaloids. In:
Cheeke, P. R. (ed.). Toxicants of Plant Origin, Vol. 1 Alkaloids,
CRC Press, Boca Raton, Florida. pp. 179-236.
SLANINA, P. (1990a). Assessment of health-risks related to
glycoalkaloids ("solanine") in potatoes: a Nordic view. Report from
the Nordic working group on food toxicology and risk assessment.
Vår Föda, 43: 1-14.
SLANINA, P. (1990b). Solanine (glycoalkaloids) in potatoes:
toxicological evaluation. Food Chem. Toxicol., 28: 759-761.
SWINYARD, C.A. & CHAUBE, S. (1973). Are potatoes teratogenic for
experimental animals? Teratology, 8: 349-357.
WILLIMOTT, S.G. (1933). An investigation of solanine poisoning.
Analyst, 58: 431.
WILSON, G.S. (1959). A small outbreak of solanine poisoning.
Monthly Bulletin, Ministry of Health (London), 18: 207-210.
WILSON, A.M., McGANN, D.F. & BUSHWAY, R.J. (1983). Effect of growth,
location and length of storage on glycoalkaloid content of roadside
stand potatoes as stored by consumers. J. Food Prot., 46: 119-121.
WOOD, F.A. & YOUNG, D.A. (1974). TGA in potatoes. Agric. Can. Publ.
1533: pp. 1-2.
 2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
Groups of 4 female Swiss-Webster mice, weighing 20 g, were
orally administered alpha-chaconine once at a dose of 10 mg/kg bw.
Animals were sacrificed by exsanguination at 3, 6, 14, 72 and 120 h
after dosing. Blood was obtained (0.1 ml/time point) by multiple
incisions into the tail vein. Absorption was slow, with peak values
in blood (0.82 µg/ml) obtained after 14 h. Decrease in blood levels
was slow, with 0.31 µg/ml present at 120 h. The peak level in the
liver (2.97 µg/g) was reached 6 h after dosing. A second, but lower,
peak of radioactivity was seen in the liver at 120 h, suggesting
enterohepatic recycling. Cell fractionation studies showed that
within liver cells, there was no preferential location for
alpha-chaconine, and there was no binding of alkaloid to isolated
RNA or DNA fractions (Sharma et al., 1983).
2.1.1.2 Rats
Male Fischer rats (180-250 g) were orally administered
alpha-solanine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). Radioactivity in
the gastrointestinal tract started to disappear from 3 to 6 h after
dosing. During the first 24 h interval the total urinary and faecal
excretion was 78% of the dose, with most in the faeces. Maximum
concentrations of radio-activity occurred near 12 h for all tissues,
with the largest concentration in the kidneys, spleen, liver, and
lungs, and the lowest concentration in the blood. By 24 h only 10%
of the administered dose remained, and 12% was unaccounted for
(presumed to be associated with other organs and the carcass). At
that time the amount of tritium in the liver represented 1.54% of
the dose, and for blood this was 0.375% (based on a total blood
volume of 64.1 ml/kg bw). By 4 days 84% of the dose had been
excreted by the faecal route, and urinary excretion accounted for
10% of the dose (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). alpha-Chaconine was
poorly absorbed since faecal elimination accounted for 60% of the
dose within 12 h, and 80% of the dose within 24 h. Urinary excretion
of tritium was 5% of the dose 3 to 6 h after dosing, and reached a
plateau of 10% of the dose between 12 and 24 h. Maximum
concentrations of radioactivity occurred between 6 to 12 h for all
tissues, with the largest concentration in the liver. Intermediate
concentrations were seen in the kidneys, spleen, and lungs, and the
lowest concentrations were seen in the blood, brain and abdominal
fat. At 24 h after dosing the amount of tritium associated with the
liver represented 1.29% of the dose, and that with the blood was
0.17% of the dose (based on a total blood volume of 64.1 ml/kg bw)
(Norred et al., 1976).
2.1.1.3 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h) hamsters (3 animals per time point)
were exsanguinated by cardiac puncture. (The reviewers noted an
error in reporting and have adjusted the results of the original
report by changing ng to µg). At 3 h after dosing, the highest
concentration of alpha-chaconine was seen in the intestines,
including intestinal contents (125 µg/g), and this represented 63%
of the administered dose. By 24 h these values were 75 µg/g or 44%
of the administered dose, and by 168 h they had declined to 1.73
µg/g or 0.92% of the administered dose. Peak blood (1.74 µg/ml) and
peak tissue levels (liver = 27.2 µg/g) of alpha-chaconine for most
tissues were seen by 12 h, and for the heart and kidneys by 24 h. By
168 h after dosing, blood levels had declined to 0.29 µg/ml. The
ratio of liver concentration to blood concentration at 72 h was
greater than at 24 h, indicating the possibility of enterohepatic
recycling. Only small amounts of radioactivity were recovered from
the faeces in the elimination phase (non-detectable at 3 h, 0.15% at
24 h, to 0.24% of the administered dose by 168 h). In the urine
these values increased from non-detectable at 3 h to 0.25% at 12 h,
and to 21% by 168 h. These results suggest that most of the
alpha-chaconine was absorbed, but that absorption from the
gastrointestinal tract was slow. Much of the radioactivity appeared
in various tissues in bound form (Alozie et al., 1979a).
2.1.1.4 Humans
Tritiated solanidine (dose not given, but expressed as
radioactivity) was administered to 3 human volunteers (2 males, 1
female) by iv injection. Blood and urine samples were collected at
various times up to 150 h. Ninety per cent of tritium had
disappeared from the blood within 20 minutes of injection. Presuming
that radioactivity represented solanidine or its metabolites, three
phases of elimination were identified in plasma with half-lives of 2
to 3.7 min, 2 to 5 h, and 72 to 104 h, respectively. Within minutes
of injection, the concentration of tritium in erythrocytes exceeded
that in plasma. Erythrocytes were found to be a mobile reserve of
solanidine, thereby delaying transfer of solanidine from vascular to
extravascular compartments. Low rates of excretion were seen in
urine and faeces, and together accounted for about 5% of the
administered dose during the first 24 h. Thus a fraction in excess
of 90% of the dose was sequestered somewhere in the body 24 h after
dosing. After this time, the rate of elimination from the body was
low, about 1-2% per day, corresponding to an overall half-life of 34
to 68 days. The authors calculated that if absorption of solanidine
were 1 mg/day, then with a fractional rate of excretion of 0.02, the
body burden would be 50 mg. The authors suggested that mobilization
from various storage loci could occur during times of 'metabolic
stress', including pregnancy (Claringbold et al., 1982).
Mean levels of 1.56 ± 1.17 (7 males) and 1.20 ± 0.93 (27
females) ng/ml solanidine were found, using radioimmunoassay, in
human plasma samples obtained by a hospital clinic in the UK,
collected in the morning before lunch (Matthew et al., 1983).
Thirty healthy males, aged 18-44 years, and 27 healthy females,
aged 16-62 years, participated in a study in the UK designed to
measure levels of serum solanidine in persons eating their usual
diet (during the winter). Intake of the type of potato product
(i.e., French fried, boiled or baked, and whether the skin was
included) was recorded daily for one month, with arbitrary units,
corresponding to approximate levels of TGA in those products,
assigned to each product; the weight of product ingested was not
measured. Serum samples were collected before the midday meal, and
were analyzed by radioimmunoassay (detection limit 0.5 ng/ml). In
males the mean level of solanidine was 10.8 ± 5.4 ng/ml (range
2.1-22.5 ng/ml), whereas in females the respective values were 7.9 ±
4.3 (range 1.6-18.5). For both genders there was a significant
correlation between serum solanidine levels and the alkaloid intake
(expressed in units as indicated above) during the month (R = 0.878
and R = 0.703, respectively). In two male subjects serum solanidine
levels dropped to 0.5 ng/ml 2 to 3 weeks after they had been on a
potato avoidance diet, indicating a relatively long serum half-life
for solanidine. It was suggested by the authors that solanidine may
be bound to blood constituents such as free sterols (Harvey et al.,
1985a).
Eighteen healthy males, aged 20-45 years, and 15 healthy
females, aged 19-63 years, from the London area in the UK,
participated in a study designed to measure levels of total serum
alkaloids (alpha-solanine + alpha-chaconine + solanidine) and
solanidine in persons eating their usual diet (during the summer).
For comparison, 5 males, aged 31-41 years, and 5 females, aged 31-67
years, from the Uppsala area in Sweden also participated in this
study. In Sweden, 2 of the males and 1 female consumed 200-300 g
potatoes of 2 varieties high in TGA, including the skin, for 1 week
(mean 24 mg TGA/100 g), giving an intake of approximately
60 mg/person or 1 mg/kg bw/day. Blood samples were collected before
the midday meal, and were analyzed by radio-immunoassay (detection
limits for total alkaloids and solanidine were 0.4 and 0.5 ng/ml
serum, respectively). The mean levels of serum solanidine were,
respectively, 3.5 and 4.0 ng/ml in the UK and Swedish subjects
eating their usual diets, whereas in those three Swedes consuming
potatoes with a higher TGA content the mean serum solanidine level
was 31 ng/ml (range 27.8-35.5). The respective serum total alkaloid
levels were 12.0, 16.9 and 50 ng/ml. The mean serum total alkaloid
concentration was about 2.7 times the solanidine concentration,
which, according to the authors, suggests considerable metabolism in
man of the glycoalkaloids alpha-chaconine and alpha-solanine (they
represent the major proportion of alkaloids in potatoes) through
hydrolysis of the sugar residues. It was suggested that hydrolysis
could take place in the acid medium of the stomach, or at the site
of absorption, or the ratio could reflect the preferential
absorption of the more lipophilic solanidine. Alternatively,
alpha-solanine and alpha-chaconine might be absorbed unchanged and
metabolized within the body (Harvey et al., 1985b).
Blood serum levels of alpha-solanine, alpha-chaconine, and
solanidine resulting from a single meal of mashed potatoes
(equivalent to 1 mg TGA/kg bw/day) were monitored in 8 healthy
subjects (HPLC, detection limit 1 ng/ml). Peak concentrations were
achieved after 4-8 h; these were 3-11 ng/ml for alpha-solanine and
6-21 ng/ml for alpha-chaconine. The 1:2 ratio was maintained for the
duration of the experiment. After longer time intervals the level of
solanidine was < 4 ng/ml. The serum half-lives for alpha-solanine
and alpha-chaconine were 11 and 19 h, respectively (unpublished data
by K.E. Hellenäs, cited by Slanina, 1990b).
2.1.2 Biotransformation
2.1.2.1 Rats
Male Fischer rats (180-250 g) were orally administered 5 mg/kg
bw solanine, tritiated at the carbon atoms adjacent to the nitrogen
atom and the double bond (Fig. 1). Approximately 65% of the
radioactivity in the faeces was identified as solanidine. In urine
72% of radioactivity was present as basic compounds of which 6% was
identified as solanidine. Two other compounds, present at 80% and
13%, possessed intermediate polarity with respect to solanine and
solanidine (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1) at a dose of 5 mg/kg bw.
Urine and faecal samples were collected 24 h later. The major
constituent in both faeces and urine was presumed to be solanidine
because it showed the same Rf. Similarly, 25% of the radioactivity
in the faeces was attributed to unchanged alpha-chaconine. In
addition, 2 minor compounds, possessing intermediate polarity
between solanidine and alpha-chaconine and representing 1-5% of
total activity, were found in faecal and urine extracts. The authors
concluded that the absorption and metabolism of alpha-chaconine was
similar to alpha-solanine (Norred et al., 1976).
2.1.2.2 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h), hamsters were exsanguinated by
cardiac puncture (3 animals per time point) (see above Alozie
et al., 1979a). Thin-layer chromatographic separation was
performed on the chloroform soluble fractions from urine and faeces
collected at various time intervals after dosing. In urine, over
half of the eliminated radioactivity during the initial 24 h was due
to unaltered alpha-chaconine. A major urinary metabolite was
solanidine, which was the major peak by 72 h. In addition, 4 other
unidentified metabolites were present at various concentrations. Two
of these were the major peaks by 168 h after dosing. In faeces, much
of the eliminated radioactivity was due to alpha-chaconine, and a
major metabolite was solanidine. There were 2 additional
unidentified metabolites present in about the same concentration as
solanidine (Alozie et al., 1979b).
2.1.3 Effects on enzymes and other biochemical parameters
Groups of male Sprague-Dawley rats (5/group) were fasted
overnight and then given alpha-solanine by gavage at 0 and 250 mg/kg
bw or i.p. at 0 and 20 mg/kg bw. In the orally dosed animals serum
glutamic oxalacetic transaminase (SGOT) and serum glutamic pyruvic
transaminase (SGPT) were increased, and cholinesterase activity was
decreased, but the differences were not statistically significant.
With the i.p.-dosed animals statistically significant increases of
29 and 63% in SGOT and SGPT, respectively, and a 27% decrease in
cholinesterase activity were observed. In addition, a significant
inhibition of liver benzphetamine N-demethylase activity and a
decrease in liver cytochrome P-450 were observed after i.p. dosing,
whereas after oral dosing these differences were statistically
insignificant (Dalvi, 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Oral LD50 values for solanine in rodents are considerably
higher than LD50 values determined after intraperitoneal
administration (Table 2), probably because these species do not
absorb much of the solanine. Post-mortem examination failed to
reveal the cause of death in rats that had been dosed orally
(stomach tube). The oral LD50 values in rodents were 300 to > 500
times the toxic dose of about 2 mg/kg bw and a lethal dose of 3 to
6 mg/kg bw estimated for humans (see Section 2.3.1).
Table 2. LD50 values in mg/kg bw
alpha-solanine alpha-chaconine solanidine
i.p. p.o. i.p. i.p.
Mice 32.31 >10002 19.25 >5002
42.02 27.56
32.35
30.06
Rat 753 5903
674
Rabbit <201,2 506
406
Rhesus <407
Monkey
1 Patil et al. (1972)
2 Nishie et al. (1971)
3 Gull et al. (1970)
4 Chaube & Swinyard (1976)
5 Sharma et al. (1979)
6 Nishie et al. (1975)
7 Swinyard & Chaube (1973)
Two rhesus monkeys died 48 h after an i.p. injection of
40 mg/kg bw of total glycoalkaloids; one other died 2 h after having
been dosed i.p. twice (24 h apart) with 20 mg solanine/kg bw
(Swinyard & Chaube, 1973).
2.2.2 Short term studies
2.2.2.1 Rabbits
One group of 4 rabbits, each weighing about 950 g (strain not
given), was fed normal potatoes (TGA content 7.5 mg/100 g) and 4
more rabbits were fed greened potatoes (TGA content 20.4 mg/100 g)
for a period of 20 days. After 4 to 6 days the latter group,
consuming 49-53 mg TGA/kg bw/day, became dull and inactive; after 10
days, diarrhoea, hair loss and weight loss occurred, which was
followed by watering eyes, body rigidity and dullness. Protein
digestibility (amount of protein from potatoes ingested less amount
of protein excreted expressed as a percentage of protein from
ingested potatoes) decreased by 45% from day 1. This was accompanied
by a significant decrease in body weight, resulting in an average
body weight of about 650 g for treated and 1150 g for 'control'
rabbits at 25 days after cessation of feeding the experimental
diets. One of the 4 treated rabbits died within 10 to 20 days. The
control animals which consumed 20 to 23 mg TGA/kg bw/day were
unaffected (Azim et al., 1983).
The same authors similarly fed 2 groups of 5 rabbits each
(weight and strain not given) a control potato diet (7.5 mg
TGA/100 g) and a high TGA potato diet (29.75 mg TGA/100 g) for 45
days. Daily intake of TGA was 16.8 to 17.9 mg/kg bw in the control
diet, and 73.9 to 75.0 mg/kg bw in the high TGA diet. Blood samples
were collected from the ear vein every 15 days. RBC counts and
haemoglobin concentrations were determined. A significant decrease
in RBC counts was seen throughout feeding the high TGA diet, and
this was 27.5% by 45 days. This compared to a decrease of 12.5% seen
by 45 days in the control diet. Decreases in haemoglobin
concentrations paralleled the findings with RBC counts. The authors
suggested that these results indicate that the rabbits developed
haemolytic anaemia. This could be explained by the metabolite
solanidine increasing the permeability and fragility of RBC
membranes (Azim et al., 1984).
2.2.2.2 Monkeys
Four time-mated rhesus monkeys were fed ad libitum a diet of
diced potatoes of the B5141-6 variety (since withdrawn from the
market), containing on average 26 mg solanine per 100 g tuber for 25
consecutive days during days 0-42 following mating. It subsequently
became apparent that the monkeys were not pregnant. The monkeys
ingested the equivalent of 0.77 to 1.02 mg/kg bw/day alpha-solanine
or 3.08 to 4.07 mg/kg bw/day TGA. No adverse effects were observed
(Swinyard & Chaube, 1973).
2.2.3 Long-term/carcinogenicity studies
No studies available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Holzman rats, approximately 4 months of age, were
mated, one male to 3 females. Increase in weight was taken as an
indication of pregnancy, and afterwards the females were
individually caged and given a basal diet of (I) ground lab chow;
(II) ground lab chow to which was added 10% ground frozen potato
sprouts; (III) 30 mg/kg diet solanine (commercial); (IV) 40 mg/kg
diet solanine (commercial); and (V) 30 mg/kg diet solanine that was
isolated from the frozen sprouts. The time on the test diets was
variable, since increase in weight is not a sensitive indicator of
pregnancy, and some dams dropped their litters within a few days on
the test diet. They were then kept on the test diet until they had a
second litter. No food consumption records were kept, but the rats
readily ate the diets. With diets II to V, many of the pups died
within 3 days of birth, evidently from starvation as indicated by an
absence of milk in their stomachs. The percentage of pups
successfully weaned was 82.6, 50.6, 31.0, 31.1, and 19.5 for diets I
to V, respectively. All of the pups in 18/33 litters born of the
rats eating the test diets died before reaching weaning age, whereas
only one of 11 control litters was lost. The authors concluded that
the toxicity of the potato sprout diet was due to 'solanine'. It was
speculated by the authors that solanine may exert an anti-hormonal
effect and prevent lactation in some sensitive dams. The reviewers
feel that further studies to examine these effects and other aspects
of reproduction are necessary (Kline et al., 1961).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Potential teratogenicity of alpha-solanine and alpha-chaconine
was investigated in four different experiments with Wistar rats
weighing 175-200 g. In the first experiment, three groups of rats
(9-10/group) were gavaged with alpha-solanine at dose levels of 0.3,
1.0 or 3.0 mg/kg bw/day, from days 6 to 15 of gestation. In the
second experiment, a group of 9 rats was given alpha-solanine by
gavage at a dose level of 6 mg/kg bw/day, from days 7 to 10 of
gestation. In the third experiment, groups of rats (3-4/group)
received alpha-solanine at dose levels of 2, 10 or 25 mg/kg bw/day
from days 8 to 11 of gestation. In the fourth experiment, a group of
4 rats received alpha-chaconine by gavage at a dose level of
1.5 mg/kg bw/day, from days 6 to 15 of gestation. In the first three
experiments, concurrent control groups composed of 2 to 10
rats/group were included. On day 22 of gestation, all females were
sacrificed and the following parameters were investigated: corpora
lutea, resorption sites, litter size, litter weight, and gross,
visceral and skeletal fetal anomalies. The only adverse effect was
observed in the first experiment. One fetus with craniorachischisis
and exopthalmos (1/117), from the group receiving 3 mg/kg bw/day,
and another with twisted pelvic limbs and absent tail (1/108), from
the 0.3 mg/kg bw/day group were observed. No maternal toxicity was
reported. The authors concluded that the observed effects were not
treatment-related (Ruddick et al., 1974).
A group of 14 Wistar rats, weighing 175-200 g, was fed a diet
containing about 73% of cooked and freeze-dried, visibly blighted
parts of potato tubers, from days 1 to 22 of gestation. The intake
of blighted potatoes was approximately 70 g/kg bw/day. The control
group of 13 females was fed a diet containing the same amount of
freeze-dried potatoes inoculated with heat-killed Phytophthora
infestans. The content of glycoalkaloids in the diets was not
determined. All dams were sacrificed on day 22 of gestation and the
standard parameters (corpora lutea, resorption sites, litter size,
litter weight, and gross, visceral and skeletal anomalies) were
investigated. There was no evidence of maternal toxicity, fetal
toxicity nor teratogenicity (Ruddick et al., 1974).
2.2.5.2 Hamsters
Groups of hamsters (12-15/group), weighing about 100 g, were
fed from days 5 to 10 of gestation diets of commercial hamster
ration containing 50% freeze-dried, unblighted potato concentrate
(group 1); 50% Phytophthora infestans infected freeze-dried,
blighted potato concentrate (group 2); or 50% Alternaria solani
infected freeze-dried, blighted potato concentrate (group 3). A
group of 13 hamsters was fed commercial hamster ration only,
throughout gestation (group 4). The content of glycoalkaloids in the
diets was not determined. Food and water were provided ad libitum.
On day 15 of gestation, the dams were sacrificed, and fetuses were
examined for gross, visceral and skeletal anomalies. Feed
consumption, maternal body weight gain, litter size, number of
resorptions and fetal weight were not affected by the treatment. The
most frequent gross anomaly was haemorrhagic necrosis of the central
nervous system, but the frequency of this effect was not
treatment-related (group 1 - 1/114; group 2 - 3/153; group 3 -
0/135; group 4 - 11/99) (Sharma et al., 1978).
Groups of Syrian hamsters (body weights and age not given) were
gavaged on day 8 of gestation with alpha-chaconine, isolated from
Arran Pilot potato sprouts, at levels of 165 mg/kg bw/day (23/group)
or 180 mg/kg bw/day (14/group) and alpha-solanine, isolated from
Arran Pilot potato sprouts, at a level of 200 mg/kg bw/day
(37/group). Females of the vehicle control group (37/group) received
vehicle material alone (2% ethanol at pH 5-6 in 1% carboxymethyl
cellulose or in water). Animals were individually caged in a room
maintained at 20-26 °C and received food and water ad libitum.
Maternal toxicity was monitored by daily weighing and clinical
observation. On day 15 of gestation, the dams were sacrificed and
necropsied. The uteri were exposed and the number of resorptions and
live fetuses were determined. The corpora lutea were counted and
all fetuses were examined for gross anomalies. Maternal mortality
was observed in all treated dose groups; 4 and 6 dams died in the
165 and 180 mg/kg bw/day alpha-chaconine dose groups, respectively,
and 3 dams died in the 200 mg/kg bw/day alpha-solanine dose group.
The days of gestation on which the dams had died were not indicated.
No maternal mortality was observed in the vehicle control group. A
high incidence of neural tube defects such as interparietal
encephalocoele (11-13%) and exencephaly (5-12%) was observed in
fetuses, of which the mothers were exposed to either alpha-chaconine
or alpha-solanine. In the vehicle control group only 1 fetus of 393
examined exhibited exencephaly. The authors concluded that the
observed teratogenic effects were treatment-related. They also
indicated that a number of fetuses exhibited CNS malformations
without apparent toxicity or weight loss in the dam, making it
unlikely that the malformations were secondary to maternal toxicity.
Occasional short tail and minor digital anomalies were noted in
fetuses from all experimental groups, but those effects were not
treatment-related (Renwick et al., 1984).
2.2.5.3 Rabbits
Groups of New Zealand rabbits (2-6/group), weighing on average
4 kg, were fed, throughout gestation, diets containing 50%
freeze-dried, unblighted, potato concentrate, 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate, or
50% Alternaria solani infected freeze-dried, blighted, potato
concentrate. The content of glycoalkaloids in the diets was not
determined. Prior to parturition (day not defined), all dams were
sacrificed and fetuses removed and examined for gross, visceral and
skeletal anomalies. During fetal examination particular attention
was paid to any malformations of brain and spinal cord. Among 21
fetuses examined in the Phytophthora infestans blighted potato
group, three fetuses (from three litters) exhibited incomplete
closure of the caudal vertebral column, and two other fetuses were
very small and had shortened appendages. Among 28 fetuses examined
in the Alternaria solani blighted potato group, two fetuses
exhibited incomplete closure of the caudal vertebral column, one
fetus had a very small brain (nearly half the normal size) and the
cranial cavity was filled with fluid, and two other fetuses were
abnormally small in size. All six abnormal fetuses were from
different litters. None of the nine fetuses (two litters) from the
unblighted potato group were affected. The authors concluded that
feeding pregnant rabbits potatoes blighted with either of the fungi,
at high concentrations in the diet, can produce a low incidence of
the caudal vertebral column malformation. This result must be
considered with caution since the small number of control litters
examined does not permit an adequate estimate of the spontaneous
incidence of this malformation in rabbits (Sharma et al., 1978).
2.2.5.4 Miniature swine
Groups of female miniature swine (2/group), weighing about
39 kg, were fed laboratory diets containing 50% freeze-dried,
unblighted, potato concentrate (group 1); 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate
(group 2); or 50% Alternaria solani infected freeze-dried,
blighted, potato concentrate (group 3), during the first half of
gestation (about the first 57 days of gestation). The content of
glycoalkaloids in the diets was not determined. At the end of
gestation (the day was not indicated), all dams were sacrificed and
necropsied. All fetuses were removed and examined for gross and
visceral malformations. Depressed weight gain was observed in sows
of group 3. One fetus of 15 examined from group 2 exhibited
anencephaly with extensive internal hydrocephaly. Other fetuses from
this and other groups were not affected. The authors concluded that
feeding potatoes blighted with Phytophthora infestans may be a
causative factor in the production of anencephaly in miniature
swine. However, the small sample size makes definite conclusions
difficult (Sharma et al., 1978).
2.2.5.5 Marmosets
A group of 6 female marmosets (Callithrix jacchus), five
years of age, weighing about 375 g, previously producing normal
offspring, was fed a diet containing freeze-dried concentrate of
blighted potatoes (Kerr's Pink variety), at a level of 4.7 g/kg
bw/day (equivalent to 0.9 mg/kg bw/day of glyco-alkaloids), for 50
days, during either days 0-50 or 20-70 of gestation. A control group
of 6 pregnant marmosets received a standard unsupplemented diet.
Marmosets were sacrificed between days 80-120 of gestation and
fetuses were examined for developmental anomalies. Four of 11
fetuses in the blighted potato groups exhibited gross abnormalities,
described as cranial osseous defects. Histological examination
revealed replacement of bone by a collagenous membrane in the
occipital area. Brain examination of affected fetuses revealed
enlargement of the lateral ventricle. Eleven fetuses of the control
group showed no gross abnormalities in any system. The investigators
indicated that, during the 2 years of existence of the marmoset
colony, similar defects had occurred once spontaneously in twin
fetuses (spontaneously aborted) among 104 live births. The authors
concluded that the occurrence of cranial dysplasia in 4 of 11
fetuses, in the experimental group, is suggestive of teratogenicity
of blighted potatoes in marmosets (Poswillo et al., 1972, 1973).
Three experiments were conducted to investigate possible
teratogenic effects of different varieties of unblemished or
blemished potatoes in 5 year-old, pregnant marmosets (Callithrix
jacchus), previously producing normal offspring. In the first
experiment, a group of 6 female marmosets were fed freeze-dried
concentrate of unblemished 'domestic potatoes' (Cornish White and
King Edward varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.56 mg/kg bw/day of glycoalkaloids. In the second
experiment a group of 7 female marmosets were fed freeze-dried
concentrate of blemished 'industry rejected potatoes' (King Edward,
Cornish White varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.78 mg/kg bw/day of glycoalkaloids. In the third
experiment a group of 5 female marmosets were fed freeze-dried
concentrate of King Edward variety potatoes, infected with Erwinia
carotovera (a bacterial pathogen responsible for 'blackleg'), at a
level of about 4.7 g/kg bw/day, equivalent to 0.07 mg/kg bw/day of
glycoalkaloids. Feeding trials were commenced 10 days postpartum of
the previous litter, to cover the period of the expected postpartum
oestrous and were carried throughout an undefined period of
gestation. Female marmosets fed both the 'domestic potatoes' and
'industry rejected potatoes' diets were allowed to proceed with
pregnancy to term, and the offspring was grossly examined at birth
and at regular intervals up to 6 months of age. Females fed
'infected potatoes' (with Erwinia carotovora) diet were sacrificed
between days 90 to 110 of gestation, and fetuses were examined
grossly and radiographically for abnormalities. Behavioural
anomalies such as continuous clinging to parents or siblings, and
prolonged weaning time, were observed in three sets of twins, born
to dams of the second experiment. No anatomical abnormalities were
observed in any experimental groups. The authors concluded that the
significance of the behavioural abnormalities observed in this study
cannot be determined at this stage but further observation of growth
and development to sexual maturity may throw more light on this
phenomenon (Poswillo et al., 1973).
2.2.5.6 Chicken embryos
Fertile chicken eggs (White Leghorn) were injected either with
pure solanine, mixed glycoalkaloids or an ethanol extract (obtained
from potatoes infected with Phytophthora infestans) into the yolk
sac, at levels ranging from 0.13 to 0.26 mg/egg, between 0 and 26 h
of incubation. A high incidence of embryo mortality (20-27%) and
increased incidence of abnormalities (16-25%) such as cranioschisis,
celosoma, cardiac septal defects, rumplessness (absence of tail) and
trunklessness (absence of trunk below the wing bud) were observed in
treated embryos. The most frequent defect was rumplessness and
trunklessness. In controls injected with chick Ringer or HCl
solvent, the percentage of abnormal embryos was 9-10% and the
mortality was 1-8% (Jelinek et al., 1976; Mun et al., 1975).
2.2.6 Special studies on cholinesterase inhibition
The inhibitory effect of alpha-solanine and solanidine, as well
as an extract from potatoes, were studied using a 1:100 dilution of
sera from 21 human individuals. These persons had been previously
phenotyped as 'usual' (95% of population in Great Britain),
'intermediate' (3-4% of the population), and 'atypical' (uncommon),
using the acetylcholinesterase inhibitor dibucaine. At a
concentration of 2.88 µM and 3.14 µM, respectively, alpha-solanine
and solanidine were about equally effective causing 86.2 ± 1.2 % and
80.0 ± 1.4 % inhibition in the 'usual' phenotype, and parallel
effects to dibucaine in the other two phenotypes. The results with
the potato extract were similar. The authors noted that it is not
clear to what extent the toxic effects of solanine can be attributed
to the inhibition of serum cholinesterase, but if it plays a role
then individuals with the 'atypical' phenotype, would presumably be
less susceptible (Harris & Whittaker, 1962).
Male and female New Zealand rabbits (2 of each sex) were given
a single i.p. dose of 20 or 30 mg solanine/kg body weight. These
doses resulted in severe depression, with difficult breathing and
prostration, and were lethal in 3 of the rabbits within 24 h. One
rabbit survived. Blood samples were obtained at 15 to 225 min
post-dosing; plasma and erythrocyte acetylcholinesterase activities
were measured and compared to control samples taken from the same
rabbit before dosing. Solanine was a weak to moderate inhibitor of
both specific and non-specific cholinesterase. Maximum inhibition of
plasma cholinesterase was seen at 80 min after injection, with the
activity decreasing to about 45% of the control value; inhibition of
erythrocyte cholinesterase was somewhat lower, and was maximally
reduced to 68.6% at 85 min after injection.
The same authors injected i.v. 5 doses of 6 mg/kg body weight,
10 min apart, in one anaesthetized male dog (15 kg bw). Quick
inhibition of serum cholinesterase was followed by rapid recovery.
Erythrocyte cholinesterase was not inhibited (Patil et al., 1972).
Male Sprague-Dawley rats (3 per group) were injected i.p. with
0, 10, 30 or 60 mg/kg bw of alpha-chaconine and sacrificed 3 h after
dosing. All rats administered alpha-chaconine showed initial signs
of depression, as well as other signs of poisoning by an
anticholinesterase agent, such as respiratory depression. Following
electrophoresis in acrylamide slabs, homogenates of brain (diluted
1:6) showed 3 zones of acetylcholinesterase isoenzyme activity with
a dose-related decrease in peak heights. Overall
acetylcholinesterase activity, using a colorimetric method, was
reduced to 79, 55, and 18% of the control value for the 3 respective
dose groups. Heart acetylcholinesterase activity was reduced to
about 40% of the control value in all treatment groups; plasma
cholinesterase activity in controls was about 30% of that seen in
brain homogenates, and was reduced to 50% in the 10 mg/kg bw dosage
group, with no further reduction in rats given 30 mg/kg bw. The
authors concluded that alpha-chaconine is a fairly potent inhibitor
of cholinesterases (Alozie et al., 1978).
In an in vitro assay the anticholinesterase activity of
several glycoalkaloids was compared, using highly purified acetyl
cholinesterase isolated from human and bovine erythrocytes (Sigma).
Alpha-solanine and alpha-chaconine were equally effective, 100 µM
caused about 80% inhibition of both human and bovine enzymes.
Tomatine was less effective, causing 40% and 50% inhibition of
bovine and human enzymes, respectively. Solasine, solamargine and
the aglycones solanidine, tomatidine and solasidine were
ineffective. Over a range of Ph 5 to pH 8, pH of the medium was not
very important. These results show that the nature of the aglycone
moiety is important (Roddick, 1989).
2.2.7 Special studies on genotoxicity
Pure alpha-solanine (Sigma) at 0.01 to 0.05 mg/plate and
extracts from potatoes were negative in the Ames test both with
strains TA98 and TA100, and in the presence or absence of activation
by S9 fraction from PCB-induced rat liver (Ness et al., 1984).
Alpha-solanine (25 and 250 µM) tested negative in a
DNA-cell-binding assay using Ehrlich ascites cells and Escherichia
coli cells mixed with 32P-labelled nucleic acids (Kubinski et
al., 1981).
2.2.8 Special studies on mitotic index
Cultured human fibroblasts were treated up to 40 h with 0, 4.1,
8.3, 16.6, 33.3, and 66.6 µg alpha-solanine/ml. At the highest dose
there was an inhibition of growth, whereas at lower dose levels
there was a stimulation of growth, as evidenced by an increase in
the mitotic index from 1.7% in controls to 2.4% at the 4.1 µg/ml
dose level, which according to the authors was similar to the
sex-hormonal type of effect exerted by estrogens on target tissues.
Using pulse labelling with tritiated thymidine, it was shown that at
5 µg alpha-solanine/ml the mean cell cycle time decreased from 42.5
± 1.87 h in controls to 28.5 ± 0.29 h in treated cells. This was
however accompanied by a 4 h increase in the period of DNA synthesis
(S phase) in treated cells, and a decrease to virtually zero for the
G1 phase. The authors concluded that if alpha-solanine reached the
fetus, the observed types of effects could be hazardous to it, and
could lead to malformations (Kirk & Mittwoch, 1975).
2.2.9 Special studies on calcium transport
Alpha-solanine (100 µM at pH 7.4) caused a 90% inhibition of
active calcium transport in rat duodenum when added to everted
intestinal sacs (8 replicates) in vitro. A Dixon plot revealed
that the inhibition by alpha-solanine was non-competitive, and the
inhibition constant was 25 µM. The inhibition of active calcium
transport was accompanied by a 40% decrease in oxygen consumption
(Michalska et al., 1985).
When alpha-solanine was given to 12 male and female Wistar
albino rats (5-6 weeks old) in their drinking water (5 mM, pH 6.4)
for 12 days, calcium transport in duodenal sacs was reduced to about
one-third of the control value, but oxygen consumption was not
significantly reduced (Michalska et al., 1985).
2.3 Observations in humans
2.3.1 Gastrointestinal and neurotoxic effects
There have been many reported cases of human poisonings
(sometimes fatal) due to the ingestion of greened or otherwise
damaged potatoes. The symptoms of low grade solanine poisoning are
acute gastrointestinal upset with diarrhoea, vomiting and severe
abdominal pain. In more severe cases, neurological symptoms,
including drowsiness and apathy, confusion, weakness, and vision
disturbances, followed by unconsciousness and, in some cases, death
have also been reported. The vital signs include fever, rapid and
weak pulse, low blood pressure and rapid respiration. Onset of
symptoms has ranged from minutes to 2 days after ingestion of toxic
potatoes, with longer incubation periods generally associated with
the more severe cases.
As is usual with case histories of this nature, the available
data are not complete. Over the years, various analytical methods or
assays have been used to determine the concentration of 'solanine'
in cases of suspected poisonings. With most of the older data, the
estimate for solanine included the other glycoalkaloids, such as
alpha-chaconine. McMillan and Thompson (1979) showed that
gravimetric methods gave higher values than colorimetric methods. A
few case reports, for which the reviewers have estimated the dose
ingested, are given below. Additional reports were compiled by
Morris and Lee (1984), who indicated that more than 2000 cases with
about 30 deaths have been reported in the literature. Not all of
these reports were available to the reviewers.
Fifty-six German soldiers suffered typical 'solanine' poisoning
after eating 1 to 1.5 kg cooked peeled potatoes containing 24 mg
TGA/100 g (whole uncooked tubers contained 38 mg TGA/100 g). In a
few cases jaundice and partial paralysis were also observed. If one
assumes a body weight of 70 kg, the intake of 'solanine' was 3.4 to
5.1 mg/kg bw (Pfuhl, 1899).
In 18 separate households in Scotland, 61 persons suffered
typical 'solanine' poisoning soon to several hours after eating
potatoes. Persons not eating potatoes were not ill. One 5-year old
died. The potatoes in that household contained 41 mg 'solanine'/100
g. Assuming the child ate 200 g potatoes and had a bw of 18 kg, the
lethal dose was estimated at 4.5 mg/kg bw. Assuming adults ate 500 g
potatoes and had a bw of 60 kg, their intake of 'solanine' would
have been 3.4 mg/kg bw (Harris & Cockburn, 1918).
A small outbreak of solanine poisoning affected a family of
four adults on three consecutive Sunday evenings in Great Britain,
about 8 h after they had eaten 1 to 3 baked potatoes in their
jackets (weight of potatoes not given). A 5th person who only ate
the flesh of the potatoes was not affected. The severity of symptoms
was related to the number of potatoes ingested, and consisted of
abdominal pain, diarrhoea, and general malaise. Patients recovered
within 24 h. The level of solanine was 50 mg/100 g tuber, as
determined chemically and by cholinesterase inhibition. Assuming a
weight of 150 g per potato, and body weights of 60 and 70 kg, the
dose was estimated at 1.25 to 3.2 mg 'solanine'/kg bw (Wilson,
1959).
Seventy-eight junior schoolboys in Great Britain became ill
from solanine poisoning 7 to 9 h after eating two small boiled
peeled potatoes each (weight of potatoes not given) as part of their
lunch, and 17 were admitted to hospital. Symptoms included vomiting,
diarrhoea, and general abdominal pain. Most of the boys developed a
fever, suffered from headache, dizziness, mental confusion,
hallucinations and their vision was affected. Three boys were
comatose and stuporose on admission, with peripheral circulatory
collapse. All were discharged 6-11 days following admission, and 4-5
weeks later there were no sequelae. Tests for the presence of
biocides, such as nicotine, organophosphorus or organochloride
pesticides were negative. Six days after eating the meal, plasma
pseudocholinesterase levels in 10 out of 17 schoolboys was subnormal
(about 25% below the normal range for this age group). Red blood
cell cholinesterase levels were normal. The source of toxic potatoes
was traced to a bag of old potatoes that had been condemned for
consumption because of their appearance, but that had inadvertently
been cooked (peeling of the potatoes had been done by an automatic
peeling machine). Insufficient potatoes were left over after the
meal for direct chemical analysis. Solanine levels in the boiled
peeled potatoes were therefore estimated from the in vitro
reduction in pseudocholinesterase activity in human plasma, using
acetylcholine as a substrate, and were equivalent to 25-30 mg/100 g
tuber of alpha-solanine. Assuming an intake of 200 g potatoes and a
bw of 40 kg (age = 11-14 years), the reviewers estimate that the
intake of 'solanine' by the schoolboys would therefore have been
approximately 1.4-1.6 mg/kg bw. Because of the small margin of
safety between normal potatoes and toxic potatoes, the authors
speculated that in toxic potatoes other toxic steroids besides
glycoalkaloids may be synthesized, such as sapogenins and saponins,
which might enhance the toxicity of solanine alkaloids by promoting
gastro-intestinal absorption or other means (McMillan & Thompson,
1979).
In a recent (1983) poisoning associated with a school lunch
programme, 61 of 109 school children and staff in Alberta, Canada,
became ill, most within 5 minutes, after eating baked potato (weight
of potato not given) containing 49.4 mg 'solanine' per 100 g
(analytical method not indicated). Test results showed that there
was no evidence that the illness occurred due to the presence of
viruses, bacteria, moulds, pesticides or other chemicals in the food
items or their containers. The potatoes had a slight tinge of green
and had a bitter or unusual taste (noted by 44% of those affected),
causing a burning sensation in the throat of 18% of those affected.
The predominant symptoms in order of frequency were nausea (69%),
abdominal cramps (43%), headache (33%), vomiting (11%), fever and
diarrhoea (8%). The children recovered in about 3 h. The reviewers
estimate that, assuming the children ingested 200 g, and had a bw of
40 kg, the dose was about 2.5 mg 'solanine'/kg bw (Anon, 1984).
Based on the available human data (Table 3), an intake of
3-6 mg TGA/kg bw is considered a potentially lethal dose for humans,
and >1 to 3 mg TGA/kg bw is considered a toxic dose for humans.
Children may be more sensitive than adults. Other factors may be
present in suspect potatoes and modulate the toxicity of the
steroidal glycoalkaloids.
No signs of acute toxicity were noted in 3 Swedish adult
volunteers who ingested for 1 week a diet estimated to give an
intake of 1 mg/kg bw TGA (Harvey et al., 1985b).
Table 3. Summary of published reports of solanine poisoning in humans
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
56 peeled, 1-1.5 kg 24 3.4-5.1 recovered Pfuhl,
(soldiers) cooked (38) 1899
(whole
uncooked)
60 adults potatoes 500 g ? 41 3.4 recovered Harris &
1 child 200 g ? 4.5 1 fatal Cockburn,
(lethal) (5 yr-old) 1918
7 (family) greened ? ? ? 2 fatal Hansen, 1925
potatoes
50-60 shoots, ? 27 ? 1 fatal Willimot,
(Cyprus) leaves 49 1933
Prisoners experimental ? ? 2.8 recovered Report cited
by Ruhl,
1951
Child potato ? ? ? 1 fatal Report cited
berries by Ruhl,
1951
4 (family baked 1-3 50 1.2-3.2 dose-related, Wilson, 1959
adults) potatoes potatoes recovered
with skin 150-450 g
Table 3 (continued)
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
78 old potatoes 2 small 25-30 1-4-1.6 3 comatose, McMillan &
(schoolboys) potatoes all Thompson,
200 g recovered; 1979
young
boys,
more affected
61 baked potato 200 g 49 2.5 recovered Anon. 1984
(school-children)
Alberta
? Data not available.
2.3.2 Teratogenic effects
In 1972, Renwick showed that areas with an increased incidence
of neural tube defects (NTD) (anencephaly and spina bifida) were
associated with areas where potato consumption was higher, and where
potato blight was more common. The worldwide incidence of NTD varies
from < 1 to about 7 per 1000 total births. He postulated that this
disease was due to toxic factors in potatoes, such as
alpha-chaconine and alpha-solanine. These antifungal compounds offer
resistance to potato blight and increase in amount in blighted
potatoes, infected with the fungus Phytophthora infestans. Several
studies have been conducted to prove or disprove this theory
(Renwick, 1972). The same author more recently suggested that a long
half-life of potato glycoalkaloids could lead to their retention in
the body, and possible release early during pregnancy (Renwick,
1982).
In a prospective study with women who had previously borne a
child with NTD, 27 women did not handle or eat potatoes or potato
containing foods after deciding on a future pregnancy, and
throughout gestation; another 61 women, attending the same clinic,
did not avoid potatoes. The allocation to the two groups was
non-random, but voluntary. The groups did not differ significantly
with respect to age distribution, social class, parity or history of
outcome of previous pregnancies. The incidence of NTD was 8.7% in
the group of women avoiding potatoes, and 3.6% in the group eating
potatoes (p=0.58). This study failed to support the Renwick
hypothesis, but the authors pointed out that the size of the groups
was small (Nevin & Merrett, 1975).
Although there is a geographical similarity between neural tube
defect occurrence and potato blight in Canada, no annual or seasonal
associations were demonstrated. The author concluded that
socioeconomic factors were probably more important as a risk factor
for NTD, but suggested that better exposure assessment to factors
present in potatoes, at the level of the individual, would be
necessary to resolve this question. Such prospective studies should
also assess the significance of other risk factors (Elwood, 1976).
An epidemiological study (prospective study) was conducted in
Great Britain, whereby human serum specimens from 380 patients, who
were being screened for NTD by measuring their serum
alpha-fetoprotein at 15-22 weeks of gestation (most at 16 weeks),
were also analyzed for potato glycoalkaloids, using a sensitive
radioimmunoassay. The samples were analyzed blind, regardless of the
outcome of pregnancy, which resulted in 210 NTD cases and 170 normal
offspring. In most of the 9 centres studied, serum TGA and serum
solanidine levels were higher (p <0.05 in 2 centres) in the women
with a normal fetus than in those with a fetus affected by NTD.
Although closure of the neural tube normally takes place at 4-5
weeks of gestation, the authors felt that measurements at the later
date might reflect glycoalkaloid exposure earlier during gestation.
The results of this study are therefore the opposite of what one
would expect if the ingestions of potatoes contributed to the
etiology of NTD. Instead the authors suggested that avoidance of
potatoes might contribute to a vitamin deficiency thereby increasing
rather than decreasing the incidence of NTD (Harvey et al., 1986).
3. COMMENTS
Numerous studies performed on a variety of experimental animal
species to elucidate the toxicological properties of glycoalkaloids,
including teratogenicity, have been evaluated.
Cranial abnormalities have been observed in some teratogenicity
studies with laboratory animals, particularly with the hamster at
levels of 165-200 mg glycoalkaloids/kg bw/day. However, the
suggested association of the consumption of blighted potatoes during
pregnancy with increased incidences of spina bifida and anencephaly
has not been substantiated.
In a limited study in humans, the daily consumption of potato
tubers containing approximately 24 mg glycolkaloids/100 g did not
result in any signs of acute toxicity. However, human poisonings
have been associated with the consumption of poor-quality potato
tubers with elevated levels of glycoalkaloids. The signs of
low-grade glycoalkaloid poisoning are acute gastrointestinal upset
with diarrhoea, vomiting, and severe abdominal pain. In more severe
cases, neurological symptoms, including drowsiness, apathy,
confusion, weakness, and vision disturbances followed by
unconsciousness, have also been reported.
4. EVALUATION
The Committee considered that, despite the long history of
human consumption of plants containing glycoalkaloids, the available
epidemiological and experimental data from human and laboratory
animal studies did not permit the determination of a safe level of
intake. The Committee recognized that the development of empirical
data to support such a level would require considerable effort.
Nevertheless, it felt that the large body of experience with the
consumption of potatoes, frequently on a daily basis, indicated that
normal glycoalkaloid levels (20-100 mg/kg) found in properly grown
and handled tubers were not of concern. To support the continued
safe use of potato tubers, those developing new cultivars, and
others growing, harvesting, storing, processing, and consuming
potatoes, should be aware of the possibility of inadvertently
increasing the content of glfycoalkaloids to potentially toxic
levels.
5. REFERENCES
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1978). Inhibition of
rat cholinesterase isoenzymes in vitro and in vivo by the potato
alkaloid, alpha-chaconine. J. Food Biochem., 2: 259-276.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979a). Physiological
disposition, subcellular distribution and tissue binding of
alpha-chaconine (3H). J. Food Safety, 1: 257-273.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979b). Excretion of
alpha-chaconine-3H, a steroidal glycoalkaloid from
Solanum-tuberosum L. and its metabolites in hamsters. Pharmacol.
Res. Commun., 11: 483-490.
ANON. (1979). Solanine poisoning [editorial]. Br. Med. J., 2:
1458-1459.
ANON. (1984). Solanine food poisoning associated with a school lunch
program - Alberta. Canada Diseases Weekly Report, Health and
Welfare Canada, 10-18: 71.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1983). Toxic effects of high
glycoalkaloid feeding on the protein digestibility and growth of
rabbits. J. Pharm. Univ. Karachi., 2: 15-24.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1984). Toxic effects of high
glycoalkaloid feeding on the red blood cell counts and haemoglobin
concentration of rabbit blood. J. Pharm. Univ. Karachi, 3: 43-49.
BÖMER, A. & MATTIS, H. (1924). [Solanine content of potatoes] Der
Solaningehalt der Kartoffeln. Z. Nahr. Genussm., 47: 97-127.
BUSHWAY, R.J. & PONNAMPALAM, R. (1981). alpha-chaconine and
alpha-solanine content of potato products and their stability during
several modes of cooking. J. Agric. Food Chem., 29: 814-817.
CHAUBE, S. & SWINYARD, C.A. (1976). Teratological and toxicological
studies of alkaloidal and phenolic compounds from Solanum tuberosum
L. Toxicol. Appl. Pharmacol., 36: 227-237.
CLARINGBOLD, W.D.B., FEW, J.D. & RENWICK, J.H. (1982). Kinetics and
retention of solanidine in man. Xenobiotica, 12: 293-302.
DALVI, R.R. & BOWIE, W.C. (1983). Toxicology of solanine: an
overview. Vet. Hum. Toxicol., 25: 13-15.
DALVI, R.R. (1985). Comparative assessment of the effect of solanine
administered orally and intraperitoneally on hepatic dysfunction in
male rats. Jpn. J. Vet. Sci., 47: 657-659.
ELWOOD, J.M. (1976). Anencephalus, spina bifida and potato blight in
Canada. Can. J. Public Health, 67: 122-126.
GULL, S.D., ISENBERG, F.M. & BRYAN, H.H. (1970). Alkaloid toxicology
of Solanum-tuberosum. Hort. Science, 5: 316.
HANSEN, A.A. (1925). Two fatal cases of potato poisoning. Science,
61: 340-341.
HARRIS, F.W. & COCKBURN, T. (1918). Alleged poisoning by potatoes.
Am. J. Pharm., 90: 722-726.
HARRIS, H. & WHITTAKER, M. (1962). Differential inhibition of the
serum cholinesterase phenotypes by solanine and solanidine. Ann.
Hum. Genet., 26: 71-76.
HARVEY, M.H., McMILLAN, M., MORGAN, M.R.A. & CHAN, H.W.-S. (1985a).
Solanidine is present in sera of healthy individuals and in amounts
dependent on their dietary potato consumption. Hum. Toxicol., 4:
187-194.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1985b).
Measurement of potato steroidal alkaloids in human serum and saliva
by radioimmunoassay. Hum. Toxicol., 4: 503-512.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1986). Potato
steroidal alkaloids and neural tube defects: serum concentrations
fail to demonstrate a causal relation. Hum. Toxicol., 5: 249-253.
JADHAV, S.J., SHARMA, R.P. & SALUNKHE, D.K. (1981). Naturally
occurring toxic alkaloids in foods. Crit. Rev. Toxicol., 9:
21-104.
JELINEK, R., KYZLINK, V. & BLATTNY, C., Jr. (1976). An evaluation of
the embryotoxic effects of blighted potatoes on chicken embryos.
Teratology, 14: 335-342.
KIRK, D. & MITTWOCH, U. (1975). Changes in the mitotic cycle induced
by alpha-solanine. Humangenetik, 26: 105-111.
KLINE, B.E., VON ELBE, H., DAHLE, N.A. & KUPCHAN, S.M. (1961). Toxic
effects of potato sprouts and of solanine fed to pregnant rats.
Proc. Soc. Exp. Biol. Med., 107: 807-809.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
MAGA, J.A. (1980). Potato glycoalkaloids. Crit. Rev. Food Sci.
Nutr., 12: 371-405.
MATTHEW, J.A., MORGAN, M.R.A., McNERNEY, R., CHAN, H.W.-S. & COXON,
D.T. (1983). Determination of solanidine in human plasma by
radioimmunoassay. Food Chem. Toxicol., 21: 637-640.
McMILLAN, M. & THOMPSON, J.C. (1979). An outbreak of suspected
solanine poisoning in schoolboys: examination of criteria of
solanine poisoning. Q. J. Med., 48: 227-243.
MICHALSKA, L., NAGEL, G., SWINIARSKI, E. & ZYDOWO, M.M. (1985). The
effect of alpha-solanine on the active calcium transport in rat
intestine. Gen. Pharmacol., 16: 69-70.
MORGAN, M.R.A. & COXON, D.T. (1987). Tolerances: glycoalkaloids in
potatoes. Ch. 7. In: Watson, D.H. (ed.). Ellis Horwood series in
food science and technology: Natural toxicants in food: progress
and prospects, Ellis Horwood, Chichester, England, pp. 221-230.
MORRIS, S.C. & LEE, T.H. (1984). The toxicity and teratogenicity of
Solanaceae glycoalkaloids particularly those of the potato
(Solanum tuberosum): a review. Food Technol. Aust., 36: 118-124.
MUN, A.M., BARDEN, E.S., WILSON, J.M. & HOGAN, J.M. (1975).
Teratogenic effects in early chick embryos of solanine and
glycoalkaloids from potatoes infected with late-blight,
Phytophthora infestans. Teratology, 11: 73-77.
NESS, E., JONER, P.E. & DAHLE, H.K. (1984). Alpha-solanine tested
for mutagenicity with the Ames test. Acta Vet. Scand., 25:
145-147.
NEVIN, N.C. & MERRETT, J.D. (1975) Potato avoidance during pregnancy
in women with a previous infant with either anencephaly and/or spina
bifida. Br. J. Prev. Soc. Med., 29: 111-115.
NISHIE, K., GUMBMANN, M.R. & KEYL, A.C. (1971). Pharmacology of
solanine. Toxicol. Appl. Pharmacol., 19: 81-92.
NISHIE, K., NORRED, W.P. & SWAIN, A.P. (1975). Pharmacology and
toxicology of chaconine and tomatine. Res. Commun. Chem. Pathol.
Pharmacol., 12: 657-668.
NORRED, W.P., NISHIE, K. & OSMAN, S.F. (1976). Excretion,
distribution and metabolic fate of 3H-alpha-chaconine. Res.
Commun. Chem. Pathol. Pharmacol., 13: 161-171.
PATIL, B.C., SHARMA, R.P., SALUNKHE, D.K. & SALUNKHE, K. (1972).
Evaluation of solanine toxicity. Food Cosmet. Toxicol., 10:
395-398.
PFUHL, E. (1899). [Regarding an outbreak of illness due to poisoning
by solanine in potatoes] Über eine Massenerkrankung durch Vergiftung
mit stark solaninhaltigen Kartoffeln. Deutsch. Med. Wochenschr.,
25: 753-754.
POSWILLO, D.E., SOPHER, D. & MITCHELL, S.J. (1972) Experimental
induction of fetal malformation with "blighted" potato: a
preliminary report. Nature, 239: 462-464.
POSWILLO, D.E., SOPHER, D., MITCHELL, S.J., COXON, D.T., CURTIS,
R.F. & PRICE, K.R. (1973). Investigations into the teratogenic
potential of imperfect potatoes. Teratology, 8: 339-347.
RENWICK, J.H. (1972). Hypothesis: anencephaly and spina bifida are
usually preventable by avoidance of a specific but unidentified
substance present in certain potato tubers. Br. J. Prev. Soc. Med.,
26: 67-88.
RENWICK, J.H. (1982). Food and malformation. Practitioner, 226:
1947-1953.
RENWICK, J.H., CLARINGBOLD, W.D.B., EARTHY, M.E., FEW, J.D. &
McLEAN, A.C.S. (1984). Neural-tube defects produced in Syrian
hamsters by potato glycoalkaloids. Teratology, 30: 371-381.
RODDICK, J.G. (1989). The acetylcholinesterase-inhibitory activity
of steroidal glycoalkaloids and their aglycones. Phytochemistry,
28: 2631-2634.
RUDDICK, J.A., HARWIG, J. & SCOTT, P.M. (1974). Nonteratogenicity in
rats of blighted potatoes and compounds contained in them.
Teratology, 9: 165-168.
RÜHL, R. (1951). [Contribution on the pathology and toxicology of
solanine] Beitrag zur Pathologie und Toxikologie des Solanins.
Arch. Pharm., 284: 67-74.
SHARMA, R.P., WILLHITE, C.C., WU, M.T. & SALUNKHE, D.K. (1978).
Teratogenic potential of blighted potato concentrate in rabbits,
hamsters, and miniature swine. Teratology, 18: 55-61.
SHARMA, R.P., WILLHITE, C.C., SHUPE, J.L. & SALUNKHE, D.K. (1979).
Acute toxicity and histopathological effects of certain
glycoalkaloids and extracts of Alternaria solani or Phytophthora
infestans in mice. Toxicol. Lett., 3: 349-355.
SHARMA, R.P., TAYLOR, M.J. & BOURCIER, D.R. (1983). Subcellular
distribution of alpha-chaconine in mouse hepatocytes. Drug Chem.
Toxicol., 6: 219-234.
SHARMA, R.P. & SALUNKHE, D.K. (1989). Solanum glycoalkaloids. In:
Cheeke, P. R. (ed.). Toxicants of Plant Origin, Vol. 1 Alkaloids,
CRC Press, Boca Raton, Florida. pp. 179-236.
SLANINA, P. (1990a). Assessment of health-risks related to
glycoalkaloids ("solanine") in potatoes: a Nordic view. Report from
the Nordic working group on food toxicology and risk assessment.
Vår Föda, 43: 1-14.
SLANINA, P. (1990b). Solanine (glycoalkaloids) in potatoes:
toxicological evaluation. Food Chem. Toxicol., 28: 759-761.
SWINYARD, C.A. & CHAUBE, S. (1973). Are potatoes teratogenic for
experimental animals? Teratology, 8: 349-357.
WILLIMOTT, S.G. (1933). An investigation of solanine poisoning.
Analyst, 58: 431.
WILSON, G.S. (1959). A small outbreak of solanine poisoning.
Monthly Bulletin, Ministry of Health (London), 18: 207-210.
WILSON, A.M., McGANN, D.F. & BUSHWAY, R.J. (1983). Effect of growth,
location and length of storage on glycoalkaloid content of roadside
stand potatoes as stored by consumers. J. Food Prot., 46: 119-121.
WOOD, F.A. & YOUNG, D.A. (1974). TGA in potatoes. Agric. Can. Publ.
1533: pp. 1-2.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution, and excretion
2.1.1.1 Mice
Groups of 4 female Swiss-Webster mice, weighing 20 g, were
orally administered alpha-chaconine once at a dose of 10 mg/kg bw.
Animals were sacrificed by exsanguination at 3, 6, 14, 72 and 120 h
after dosing. Blood was obtained (0.1 ml/time point) by multiple
incisions into the tail vein. Absorption was slow, with peak values
in blood (0.82 µg/ml) obtained after 14 h. Decrease in blood levels
was slow, with 0.31 µg/ml present at 120 h. The peak level in the
liver (2.97 µg/g) was reached 6 h after dosing. A second, but lower,
peak of radioactivity was seen in the liver at 120 h, suggesting
enterohepatic recycling. Cell fractionation studies showed that
within liver cells, there was no preferential location for
alpha-chaconine, and there was no binding of alkaloid to isolated
RNA or DNA fractions (Sharma et al., 1983).
2.1.1.2 Rats
Male Fischer rats (180-250 g) were orally administered
alpha-solanine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). Radioactivity in
the gastrointestinal tract started to disappear from 3 to 6 h after
dosing. During the first 24 h interval the total urinary and faecal
excretion was 78% of the dose, with most in the faeces. Maximum
concentrations of radio-activity occurred near 12 h for all tissues,
with the largest concentration in the kidneys, spleen, liver, and
lungs, and the lowest concentration in the blood. By 24 h only 10%
of the administered dose remained, and 12% was unaccounted for
(presumed to be associated with other organs and the carcass). At
that time the amount of tritium in the liver represented 1.54% of
the dose, and for blood this was 0.375% (based on a total blood
volume of 64.1 ml/kg bw). By 4 days 84% of the dose had been
excreted by the faecal route, and urinary excretion accounted for
10% of the dose (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1), at a dose of 5 mg/kg bw.
Blood samples were taken from the abdominal aorta at 1, 3, 6, 12,
24, 48, 72, and 96 h (2 animals per time point). alpha-Chaconine was
poorly absorbed since faecal elimination accounted for 60% of the
dose within 12 h, and 80% of the dose within 24 h. Urinary excretion
of tritium was 5% of the dose 3 to 6 h after dosing, and reached a
plateau of 10% of the dose between 12 and 24 h. Maximum
concentrations of radioactivity occurred between 6 to 12 h for all
tissues, with the largest concentration in the liver. Intermediate
concentrations were seen in the kidneys, spleen, and lungs, and the
lowest concentrations were seen in the blood, brain and abdominal
fat. At 24 h after dosing the amount of tritium associated with the
liver represented 1.29% of the dose, and that with the blood was
0.17% of the dose (based on a total blood volume of 64.1 ml/kg bw)
(Norred et al., 1976).
2.1.1.3 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h) hamsters (3 animals per time point)
were exsanguinated by cardiac puncture. (The reviewers noted an
error in reporting and have adjusted the results of the original
report by changing ng to µg). At 3 h after dosing, the highest
concentration of alpha-chaconine was seen in the intestines,
including intestinal contents (125 µg/g), and this represented 63%
of the administered dose. By 24 h these values were 75 µg/g or 44%
of the administered dose, and by 168 h they had declined to 1.73
µg/g or 0.92% of the administered dose. Peak blood (1.74 µg/ml) and
peak tissue levels (liver = 27.2 µg/g) of alpha-chaconine for most
tissues were seen by 12 h, and for the heart and kidneys by 24 h. By
168 h after dosing, blood levels had declined to 0.29 µg/ml. The
ratio of liver concentration to blood concentration at 72 h was
greater than at 24 h, indicating the possibility of enterohepatic
recycling. Only small amounts of radioactivity were recovered from
the faeces in the elimination phase (non-detectable at 3 h, 0.15% at
24 h, to 0.24% of the administered dose by 168 h). In the urine
these values increased from non-detectable at 3 h to 0.25% at 12 h,
and to 21% by 168 h. These results suggest that most of the
alpha-chaconine was absorbed, but that absorption from the
gastrointestinal tract was slow. Much of the radioactivity appeared
in various tissues in bound form (Alozie et al., 1979a).
2.1.1.4 Humans
Tritiated solanidine (dose not given, but expressed as
radioactivity) was administered to 3 human volunteers (2 males, 1
female) by iv injection. Blood and urine samples were collected at
various times up to 150 h. Ninety per cent of tritium had
disappeared from the blood within 20 minutes of injection. Presuming
that radioactivity represented solanidine or its metabolites, three
phases of elimination were identified in plasma with half-lives of 2
to 3.7 min, 2 to 5 h, and 72 to 104 h, respectively. Within minutes
of injection, the concentration of tritium in erythrocytes exceeded
that in plasma. Erythrocytes were found to be a mobile reserve of
solanidine, thereby delaying transfer of solanidine from vascular to
extravascular compartments. Low rates of excretion were seen in
urine and faeces, and together accounted for about 5% of the
administered dose during the first 24 h. Thus a fraction in excess
of 90% of the dose was sequestered somewhere in the body 24 h after
dosing. After this time, the rate of elimination from the body was
low, about 1-2% per day, corresponding to an overall half-life of 34
to 68 days. The authors calculated that if absorption of solanidine
were 1 mg/day, then with a fractional rate of excretion of 0.02, the
body burden would be 50 mg. The authors suggested that mobilization
from various storage loci could occur during times of 'metabolic
stress', including pregnancy (Claringbold et al., 1982).
Mean levels of 1.56 ± 1.17 (7 males) and 1.20 ± 0.93 (27
females) ng/ml solanidine were found, using radioimmunoassay, in
human plasma samples obtained by a hospital clinic in the UK,
collected in the morning before lunch (Matthew et al., 1983).
Thirty healthy males, aged 18-44 years, and 27 healthy females,
aged 16-62 years, participated in a study in the UK designed to
measure levels of serum solanidine in persons eating their usual
diet (during the winter). Intake of the type of potato product
(i.e., French fried, boiled or baked, and whether the skin was
included) was recorded daily for one month, with arbitrary units,
corresponding to approximate levels of TGA in those products,
assigned to each product; the weight of product ingested was not
measured. Serum samples were collected before the midday meal, and
were analyzed by radioimmunoassay (detection limit 0.5 ng/ml). In
males the mean level of solanidine was 10.8 ± 5.4 ng/ml (range
2.1-22.5 ng/ml), whereas in females the respective values were 7.9 ±
4.3 (range 1.6-18.5). For both genders there was a significant
correlation between serum solanidine levels and the alkaloid intake
(expressed in units as indicated above) during the month (R = 0.878
and R = 0.703, respectively). In two male subjects serum solanidine
levels dropped to 0.5 ng/ml 2 to 3 weeks after they had been on a
potato avoidance diet, indicating a relatively long serum half-life
for solanidine. It was suggested by the authors that solanidine may
be bound to blood constituents such as free sterols (Harvey et al.,
1985a).
Eighteen healthy males, aged 20-45 years, and 15 healthy
females, aged 19-63 years, from the London area in the UK,
participated in a study designed to measure levels of total serum
alkaloids (alpha-solanine + alpha-chaconine + solanidine) and
solanidine in persons eating their usual diet (during the summer).
For comparison, 5 males, aged 31-41 years, and 5 females, aged 31-67
years, from the Uppsala area in Sweden also participated in this
study. In Sweden, 2 of the males and 1 female consumed 200-300 g
potatoes of 2 varieties high in TGA, including the skin, for 1 week
(mean 24 mg TGA/100 g), giving an intake of approximately
60 mg/person or 1 mg/kg bw/day. Blood samples were collected before
the midday meal, and were analyzed by radio-immunoassay (detection
limits for total alkaloids and solanidine were 0.4 and 0.5 ng/ml
serum, respectively). The mean levels of serum solanidine were,
respectively, 3.5 and 4.0 ng/ml in the UK and Swedish subjects
eating their usual diets, whereas in those three Swedes consuming
potatoes with a higher TGA content the mean serum solanidine level
was 31 ng/ml (range 27.8-35.5). The respective serum total alkaloid
levels were 12.0, 16.9 and 50 ng/ml. The mean serum total alkaloid
concentration was about 2.7 times the solanidine concentration,
which, according to the authors, suggests considerable metabolism in
man of the glycoalkaloids alpha-chaconine and alpha-solanine (they
represent the major proportion of alkaloids in potatoes) through
hydrolysis of the sugar residues. It was suggested that hydrolysis
could take place in the acid medium of the stomach, or at the site
of absorption, or the ratio could reflect the preferential
absorption of the more lipophilic solanidine. Alternatively,
alpha-solanine and alpha-chaconine might be absorbed unchanged and
metabolized within the body (Harvey et al., 1985b).
Blood serum levels of alpha-solanine, alpha-chaconine, and
solanidine resulting from a single meal of mashed potatoes
(equivalent to 1 mg TGA/kg bw/day) were monitored in 8 healthy
subjects (HPLC, detection limit 1 ng/ml). Peak concentrations were
achieved after 4-8 h; these were 3-11 ng/ml for alpha-solanine and
6-21 ng/ml for alpha-chaconine. The 1:2 ratio was maintained for the
duration of the experiment. After longer time intervals the level of
solanidine was < 4 ng/ml. The serum half-lives for alpha-solanine
and alpha-chaconine were 11 and 19 h, respectively (unpublished data
by K.E. Hellenäs, cited by Slanina, 1990b).
2.1.2 Biotransformation
2.1.2.1 Rats
Male Fischer rats (180-250 g) were orally administered 5 mg/kg
bw solanine, tritiated at the carbon atoms adjacent to the nitrogen
atom and the double bond (Fig. 1). Approximately 65% of the
radioactivity in the faeces was identified as solanidine. In urine
72% of radioactivity was present as basic compounds of which 6% was
identified as solanidine. Two other compounds, present at 80% and
13%, possessed intermediate polarity with respect to solanine and
solanidine (Nishie et al., 1971).
Male Sprague-Dawley rats (200-300 g) were orally administered
alpha-chaconine, tritiated at the carbon atoms adjacent to the
nitrogen atom and the double bond (Fig. 1) at a dose of 5 mg/kg bw.
Urine and faecal samples were collected 24 h later. The major
constituent in both faeces and urine was presumed to be solanidine
because it showed the same Rf. Similarly, 25% of the radioactivity
in the faeces was attributed to unchanged alpha-chaconine. In
addition, 2 minor compounds, possessing intermediate polarity
between solanidine and alpha-chaconine and representing 1-5% of
total activity, were found in faecal and urine extracts. The authors
concluded that the absorption and metabolism of alpha-chaconine was
similar to alpha-solanine (Norred et al., 1976).
2.1.2.2 Hamsters
Golden hamsters (130-150 g) were orally administered randomly
tritiated alpha-chaconine at a dose of 10 mg/kg bw. At specified
times (3, 12, 24, 72, and 168 h), hamsters were exsanguinated by
cardiac puncture (3 animals per time point) (see above Alozie
et al., 1979a). Thin-layer chromatographic separation was
performed on the chloroform soluble fractions from urine and faeces
collected at various time intervals after dosing. In urine, over
half of the eliminated radioactivity during the initial 24 h was due
to unaltered alpha-chaconine. A major urinary metabolite was
solanidine, which was the major peak by 72 h. In addition, 4 other
unidentified metabolites were present at various concentrations. Two
of these were the major peaks by 168 h after dosing. In faeces, much
of the eliminated radioactivity was due to alpha-chaconine, and a
major metabolite was solanidine. There were 2 additional
unidentified metabolites present in about the same concentration as
solanidine (Alozie et al., 1979b).
2.1.3 Effects on enzymes and other biochemical parameters
Groups of male Sprague-Dawley rats (5/group) were fasted
overnight and then given alpha-solanine by gavage at 0 and 250 mg/kg
bw or i.p. at 0 and 20 mg/kg bw. In the orally dosed animals serum
glutamic oxalacetic transaminase (SGOT) and serum glutamic pyruvic
transaminase (SGPT) were increased, and cholinesterase activity was
decreased, but the differences were not statistically significant.
With the i.p.-dosed animals statistically significant increases of
29 and 63% in SGOT and SGPT, respectively, and a 27% decrease in
cholinesterase activity were observed. In addition, a significant
inhibition of liver benzphetamine N-demethylase activity and a
decrease in liver cytochrome P-450 were observed after i.p. dosing,
whereas after oral dosing these differences were statistically
insignificant (Dalvi, 1985).
2.2 Toxicological studies
2.2.1 Acute toxicity studies
Oral LD50 values for solanine in rodents are considerably
higher than LD50 values determined after intraperitoneal
administration (Table 2), probably because these species do not
absorb much of the solanine. Post-mortem examination failed to
reveal the cause of death in rats that had been dosed orally
(stomach tube). The oral LD50 values in rodents were 300 to > 500
times the toxic dose of about 2 mg/kg bw and a lethal dose of 3 to
6 mg/kg bw estimated for humans (see Section 2.3.1).
Table 2. LD50 values in mg/kg bw
alpha-solanine alpha-chaconine solanidine
i.p. p.o. i.p. i.p.
Mice 32.31 >10002 19.25 >5002
42.02 27.56
32.35
30.06
Rat 753 5903
674
Rabbit <201,2 506
406
Rhesus <407
Monkey
1 Patil et al. (1972)
2 Nishie et al. (1971)
3 Gull et al. (1970)
4 Chaube & Swinyard (1976)
5 Sharma et al. (1979)
6 Nishie et al. (1975)
7 Swinyard & Chaube (1973)
Two rhesus monkeys died 48 h after an i.p. injection of
40 mg/kg bw of total glycoalkaloids; one other died 2 h after having
been dosed i.p. twice (24 h apart) with 20 mg solanine/kg bw
(Swinyard & Chaube, 1973).
2.2.2 Short term studies
2.2.2.1 Rabbits
One group of 4 rabbits, each weighing about 950 g (strain not
given), was fed normal potatoes (TGA content 7.5 mg/100 g) and 4
more rabbits were fed greened potatoes (TGA content 20.4 mg/100 g)
for a period of 20 days. After 4 to 6 days the latter group,
consuming 49-53 mg TGA/kg bw/day, became dull and inactive; after 10
days, diarrhoea, hair loss and weight loss occurred, which was
followed by watering eyes, body rigidity and dullness. Protein
digestibility (amount of protein from potatoes ingested less amount
of protein excreted expressed as a percentage of protein from
ingested potatoes) decreased by 45% from day 1. This was accompanied
by a significant decrease in body weight, resulting in an average
body weight of about 650 g for treated and 1150 g for 'control'
rabbits at 25 days after cessation of feeding the experimental
diets. One of the 4 treated rabbits died within 10 to 20 days. The
control animals which consumed 20 to 23 mg TGA/kg bw/day were
unaffected (Azim et al., 1983).
The same authors similarly fed 2 groups of 5 rabbits each
(weight and strain not given) a control potato diet (7.5 mg
TGA/100 g) and a high TGA potato diet (29.75 mg TGA/100 g) for 45
days. Daily intake of TGA was 16.8 to 17.9 mg/kg bw in the control
diet, and 73.9 to 75.0 mg/kg bw in the high TGA diet. Blood samples
were collected from the ear vein every 15 days. RBC counts and
haemoglobin concentrations were determined. A significant decrease
in RBC counts was seen throughout feeding the high TGA diet, and
this was 27.5% by 45 days. This compared to a decrease of 12.5% seen
by 45 days in the control diet. Decreases in haemoglobin
concentrations paralleled the findings with RBC counts. The authors
suggested that these results indicate that the rabbits developed
haemolytic anaemia. This could be explained by the metabolite
solanidine increasing the permeability and fragility of RBC
membranes (Azim et al., 1984).
2.2.2.2 Monkeys
Four time-mated rhesus monkeys were fed ad libitum a diet of
diced potatoes of the B5141-6 variety (since withdrawn from the
market), containing on average 26 mg solanine per 100 g tuber for 25
consecutive days during days 0-42 following mating. It subsequently
became apparent that the monkeys were not pregnant. The monkeys
ingested the equivalent of 0.77 to 1.02 mg/kg bw/day alpha-solanine
or 3.08 to 4.07 mg/kg bw/day TGA. No adverse effects were observed
(Swinyard & Chaube, 1973).
2.2.3 Long-term/carcinogenicity studies
No studies available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Groups of Holzman rats, approximately 4 months of age, were
mated, one male to 3 females. Increase in weight was taken as an
indication of pregnancy, and afterwards the females were
individually caged and given a basal diet of (I) ground lab chow;
(II) ground lab chow to which was added 10% ground frozen potato
sprouts; (III) 30 mg/kg diet solanine (commercial); (IV) 40 mg/kg
diet solanine (commercial); and (V) 30 mg/kg diet solanine that was
isolated from the frozen sprouts. The time on the test diets was
variable, since increase in weight is not a sensitive indicator of
pregnancy, and some dams dropped their litters within a few days on
the test diet. They were then kept on the test diet until they had a
second litter. No food consumption records were kept, but the rats
readily ate the diets. With diets II to V, many of the pups died
within 3 days of birth, evidently from starvation as indicated by an
absence of milk in their stomachs. The percentage of pups
successfully weaned was 82.6, 50.6, 31.0, 31.1, and 19.5 for diets I
to V, respectively. All of the pups in 18/33 litters born of the
rats eating the test diets died before reaching weaning age, whereas
only one of 11 control litters was lost. The authors concluded that
the toxicity of the potato sprout diet was due to 'solanine'. It was
speculated by the authors that solanine may exert an anti-hormonal
effect and prevent lactation in some sensitive dams. The reviewers
feel that further studies to examine these effects and other aspects
of reproduction are necessary (Kline et al., 1961).
2.2.5 Special studies on embryotoxicity/teratogenicity
2.2.5.1 Rats
Potential teratogenicity of alpha-solanine and alpha-chaconine
was investigated in four different experiments with Wistar rats
weighing 175-200 g. In the first experiment, three groups of rats
(9-10/group) were gavaged with alpha-solanine at dose levels of 0.3,
1.0 or 3.0 mg/kg bw/day, from days 6 to 15 of gestation. In the
second experiment, a group of 9 rats was given alpha-solanine by
gavage at a dose level of 6 mg/kg bw/day, from days 7 to 10 of
gestation. In the third experiment, groups of rats (3-4/group)
received alpha-solanine at dose levels of 2, 10 or 25 mg/kg bw/day
from days 8 to 11 of gestation. In the fourth experiment, a group of
4 rats received alpha-chaconine by gavage at a dose level of
1.5 mg/kg bw/day, from days 6 to 15 of gestation. In the first three
experiments, concurrent control groups composed of 2 to 10
rats/group were included. On day 22 of gestation, all females were
sacrificed and the following parameters were investigated: corpora
lutea, resorption sites, litter size, litter weight, and gross,
visceral and skeletal fetal anomalies. The only adverse effect was
observed in the first experiment. One fetus with craniorachischisis
and exopthalmos (1/117), from the group receiving 3 mg/kg bw/day,
and another with twisted pelvic limbs and absent tail (1/108), from
the 0.3 mg/kg bw/day group were observed. No maternal toxicity was
reported. The authors concluded that the observed effects were not
treatment-related (Ruddick et al., 1974).
A group of 14 Wistar rats, weighing 175-200 g, was fed a diet
containing about 73% of cooked and freeze-dried, visibly blighted
parts of potato tubers, from days 1 to 22 of gestation. The intake
of blighted potatoes was approximately 70 g/kg bw/day. The control
group of 13 females was fed a diet containing the same amount of
freeze-dried potatoes inoculated with heat-killed Phytophthora
infestans. The content of glycoalkaloids in the diets was not
determined. All dams were sacrificed on day 22 of gestation and the
standard parameters (corpora lutea, resorption sites, litter size,
litter weight, and gross, visceral and skeletal anomalies) were
investigated. There was no evidence of maternal toxicity, fetal
toxicity nor teratogenicity (Ruddick et al., 1974).
2.2.5.2 Hamsters
Groups of hamsters (12-15/group), weighing about 100 g, were
fed from days 5 to 10 of gestation diets of commercial hamster
ration containing 50% freeze-dried, unblighted potato concentrate
(group 1); 50% Phytophthora infestans infected freeze-dried,
blighted potato concentrate (group 2); or 50% Alternaria solani
infected freeze-dried, blighted potato concentrate (group 3). A
group of 13 hamsters was fed commercial hamster ration only,
throughout gestation (group 4). The content of glycoalkaloids in the
diets was not determined. Food and water were provided ad libitum.
On day 15 of gestation, the dams were sacrificed, and fetuses were
examined for gross, visceral and skeletal anomalies. Feed
consumption, maternal body weight gain, litter size, number of
resorptions and fetal weight were not affected by the treatment. The
most frequent gross anomaly was haemorrhagic necrosis of the central
nervous system, but the frequency of this effect was not
treatment-related (group 1 - 1/114; group 2 - 3/153; group 3 -
0/135; group 4 - 11/99) (Sharma et al., 1978).
Groups of Syrian hamsters (body weights and age not given) were
gavaged on day 8 of gestation with alpha-chaconine, isolated from
Arran Pilot potato sprouts, at levels of 165 mg/kg bw/day (23/group)
or 180 mg/kg bw/day (14/group) and alpha-solanine, isolated from
Arran Pilot potato sprouts, at a level of 200 mg/kg bw/day
(37/group). Females of the vehicle control group (37/group) received
vehicle material alone (2% ethanol at pH 5-6 in 1% carboxymethyl
cellulose or in water). Animals were individually caged in a room
maintained at 20-26 °C and received food and water ad libitum.
Maternal toxicity was monitored by daily weighing and clinical
observation. On day 15 of gestation, the dams were sacrificed and
necropsied. The uteri were exposed and the number of resorptions and
live fetuses were determined. The corpora lutea were counted and
all fetuses were examined for gross anomalies. Maternal mortality
was observed in all treated dose groups; 4 and 6 dams died in the
165 and 180 mg/kg bw/day alpha-chaconine dose groups, respectively,
and 3 dams died in the 200 mg/kg bw/day alpha-solanine dose group.
The days of gestation on which the dams had died were not indicated.
No maternal mortality was observed in the vehicle control group. A
high incidence of neural tube defects such as interparietal
encephalocoele (11-13%) and exencephaly (5-12%) was observed in
fetuses, of which the mothers were exposed to either alpha-chaconine
or alpha-solanine. In the vehicle control group only 1 fetus of 393
examined exhibited exencephaly. The authors concluded that the
observed teratogenic effects were treatment-related. They also
indicated that a number of fetuses exhibited CNS malformations
without apparent toxicity or weight loss in the dam, making it
unlikely that the malformations were secondary to maternal toxicity.
Occasional short tail and minor digital anomalies were noted in
fetuses from all experimental groups, but those effects were not
treatment-related (Renwick et al., 1984).
2.2.5.3 Rabbits
Groups of New Zealand rabbits (2-6/group), weighing on average
4 kg, were fed, throughout gestation, diets containing 50%
freeze-dried, unblighted, potato concentrate, 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate, or
50% Alternaria solani infected freeze-dried, blighted, potato
concentrate. The content of glycoalkaloids in the diets was not
determined. Prior to parturition (day not defined), all dams were
sacrificed and fetuses removed and examined for gross, visceral and
skeletal anomalies. During fetal examination particular attention
was paid to any malformations of brain and spinal cord. Among 21
fetuses examined in the Phytophthora infestans blighted potato
group, three fetuses (from three litters) exhibited incomplete
closure of the caudal vertebral column, and two other fetuses were
very small and had shortened appendages. Among 28 fetuses examined
in the Alternaria solani blighted potato group, two fetuses
exhibited incomplete closure of the caudal vertebral column, one
fetus had a very small brain (nearly half the normal size) and the
cranial cavity was filled with fluid, and two other fetuses were
abnormally small in size. All six abnormal fetuses were from
different litters. None of the nine fetuses (two litters) from the
unblighted potato group were affected. The authors concluded that
feeding pregnant rabbits potatoes blighted with either of the fungi,
at high concentrations in the diet, can produce a low incidence of
the caudal vertebral column malformation. This result must be
considered with caution since the small number of control litters
examined does not permit an adequate estimate of the spontaneous
incidence of this malformation in rabbits (Sharma et al., 1978).
2.2.5.4 Miniature swine
Groups of female miniature swine (2/group), weighing about
39 kg, were fed laboratory diets containing 50% freeze-dried,
unblighted, potato concentrate (group 1); 50% Phytophthora
infestans infected freeze-dried, blighted, potato concentrate
(group 2); or 50% Alternaria solani infected freeze-dried,
blighted, potato concentrate (group 3), during the first half of
gestation (about the first 57 days of gestation). The content of
glycoalkaloids in the diets was not determined. At the end of
gestation (the day was not indicated), all dams were sacrificed and
necropsied. All fetuses were removed and examined for gross and
visceral malformations. Depressed weight gain was observed in sows
of group 3. One fetus of 15 examined from group 2 exhibited
anencephaly with extensive internal hydrocephaly. Other fetuses from
this and other groups were not affected. The authors concluded that
feeding potatoes blighted with Phytophthora infestans may be a
causative factor in the production of anencephaly in miniature
swine. However, the small sample size makes definite conclusions
difficult (Sharma et al., 1978).
2.2.5.5 Marmosets
A group of 6 female marmosets (Callithrix jacchus), five
years of age, weighing about 375 g, previously producing normal
offspring, was fed a diet containing freeze-dried concentrate of
blighted potatoes (Kerr's Pink variety), at a level of 4.7 g/kg
bw/day (equivalent to 0.9 mg/kg bw/day of glyco-alkaloids), for 50
days, during either days 0-50 or 20-70 of gestation. A control group
of 6 pregnant marmosets received a standard unsupplemented diet.
Marmosets were sacrificed between days 80-120 of gestation and
fetuses were examined for developmental anomalies. Four of 11
fetuses in the blighted potato groups exhibited gross abnormalities,
described as cranial osseous defects. Histological examination
revealed replacement of bone by a collagenous membrane in the
occipital area. Brain examination of affected fetuses revealed
enlargement of the lateral ventricle. Eleven fetuses of the control
group showed no gross abnormalities in any system. The investigators
indicated that, during the 2 years of existence of the marmoset
colony, similar defects had occurred once spontaneously in twin
fetuses (spontaneously aborted) among 104 live births. The authors
concluded that the occurrence of cranial dysplasia in 4 of 11
fetuses, in the experimental group, is suggestive of teratogenicity
of blighted potatoes in marmosets (Poswillo et al., 1972, 1973).
Three experiments were conducted to investigate possible
teratogenic effects of different varieties of unblemished or
blemished potatoes in 5 year-old, pregnant marmosets (Callithrix
jacchus), previously producing normal offspring. In the first
experiment, a group of 6 female marmosets were fed freeze-dried
concentrate of unblemished 'domestic potatoes' (Cornish White and
King Edward varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.56 mg/kg bw/day of glycoalkaloids. In the second
experiment a group of 7 female marmosets were fed freeze-dried
concentrate of blemished 'industry rejected potatoes' (King Edward,
Cornish White varieties), at a level of about 4.7 g/kg bw/day,
equivalent to 0.78 mg/kg bw/day of glycoalkaloids. In the third
experiment a group of 5 female marmosets were fed freeze-dried
concentrate of King Edward variety potatoes, infected with Erwinia
carotovera (a bacterial pathogen responsible for 'blackleg'), at a
level of about 4.7 g/kg bw/day, equivalent to 0.07 mg/kg bw/day of
glycoalkaloids. Feeding trials were commenced 10 days postpartum of
the previous litter, to cover the period of the expected postpartum
oestrous and were carried throughout an undefined period of
gestation. Female marmosets fed both the 'domestic potatoes' and
'industry rejected potatoes' diets were allowed to proceed with
pregnancy to term, and the offspring was grossly examined at birth
and at regular intervals up to 6 months of age. Females fed
'infected potatoes' (with Erwinia carotovora) diet were sacrificed
between days 90 to 110 of gestation, and fetuses were examined
grossly and radiographically for abnormalities. Behavioural
anomalies such as continuous clinging to parents or siblings, and
prolonged weaning time, were observed in three sets of twins, born
to dams of the second experiment. No anatomical abnormalities were
observed in any experimental groups. The authors concluded that the
significance of the behavioural abnormalities observed in this study
cannot be determined at this stage but further observation of growth
and development to sexual maturity may throw more light on this
phenomenon (Poswillo et al., 1973).
2.2.5.6 Chicken embryos
Fertile chicken eggs (White Leghorn) were injected either with
pure solanine, mixed glycoalkaloids or an ethanol extract (obtained
from potatoes infected with Phytophthora infestans) into the yolk
sac, at levels ranging from 0.13 to 0.26 mg/egg, between 0 and 26 h
of incubation. A high incidence of embryo mortality (20-27%) and
increased incidence of abnormalities (16-25%) such as cranioschisis,
celosoma, cardiac septal defects, rumplessness (absence of tail) and
trunklessness (absence of trunk below the wing bud) were observed in
treated embryos. The most frequent defect was rumplessness and
trunklessness. In controls injected with chick Ringer or HCl
solvent, the percentage of abnormal embryos was 9-10% and the
mortality was 1-8% (Jelinek et al., 1976; Mun et al., 1975).
2.2.6 Special studies on cholinesterase inhibition
The inhibitory effect of alpha-solanine and solanidine, as well
as an extract from potatoes, were studied using a 1:100 dilution of
sera from 21 human individuals. These persons had been previously
phenotyped as 'usual' (95% of population in Great Britain),
'intermediate' (3-4% of the population), and 'atypical' (uncommon),
using the acetylcholinesterase inhibitor dibucaine. At a
concentration of 2.88 µM and 3.14 µM, respectively, alpha-solanine
and solanidine were about equally effective causing 86.2 ± 1.2 % and
80.0 ± 1.4 % inhibition in the 'usual' phenotype, and parallel
effects to dibucaine in the other two phenotypes. The results with
the potato extract were similar. The authors noted that it is not
clear to what extent the toxic effects of solanine can be attributed
to the inhibition of serum cholinesterase, but if it plays a role
then individuals with the 'atypical' phenotype, would presumably be
less susceptible (Harris & Whittaker, 1962).
Male and female New Zealand rabbits (2 of each sex) were given
a single i.p. dose of 20 or 30 mg solanine/kg body weight. These
doses resulted in severe depression, with difficult breathing and
prostration, and were lethal in 3 of the rabbits within 24 h. One
rabbit survived. Blood samples were obtained at 15 to 225 min
post-dosing; plasma and erythrocyte acetylcholinesterase activities
were measured and compared to control samples taken from the same
rabbit before dosing. Solanine was a weak to moderate inhibitor of
both specific and non-specific cholinesterase. Maximum inhibition of
plasma cholinesterase was seen at 80 min after injection, with the
activity decreasing to about 45% of the control value; inhibition of
erythrocyte cholinesterase was somewhat lower, and was maximally
reduced to 68.6% at 85 min after injection.
The same authors injected i.v. 5 doses of 6 mg/kg body weight,
10 min apart, in one anaesthetized male dog (15 kg bw). Quick
inhibition of serum cholinesterase was followed by rapid recovery.
Erythrocyte cholinesterase was not inhibited (Patil et al., 1972).
Male Sprague-Dawley rats (3 per group) were injected i.p. with
0, 10, 30 or 60 mg/kg bw of alpha-chaconine and sacrificed 3 h after
dosing. All rats administered alpha-chaconine showed initial signs
of depression, as well as other signs of poisoning by an
anticholinesterase agent, such as respiratory depression. Following
electrophoresis in acrylamide slabs, homogenates of brain (diluted
1:6) showed 3 zones of acetylcholinesterase isoenzyme activity with
a dose-related decrease in peak heights. Overall
acetylcholinesterase activity, using a colorimetric method, was
reduced to 79, 55, and 18% of the control value for the 3 respective
dose groups. Heart acetylcholinesterase activity was reduced to
about 40% of the control value in all treatment groups; plasma
cholinesterase activity in controls was about 30% of that seen in
brain homogenates, and was reduced to 50% in the 10 mg/kg bw dosage
group, with no further reduction in rats given 30 mg/kg bw. The
authors concluded that alpha-chaconine is a fairly potent inhibitor
of cholinesterases (Alozie et al., 1978).
In an in vitro assay the anticholinesterase activity of
several glycoalkaloids was compared, using highly purified acetyl
cholinesterase isolated from human and bovine erythrocytes (Sigma).
Alpha-solanine and alpha-chaconine were equally effective, 100 µM
caused about 80% inhibition of both human and bovine enzymes.
Tomatine was less effective, causing 40% and 50% inhibition of
bovine and human enzymes, respectively. Solasine, solamargine and
the aglycones solanidine, tomatidine and solasidine were
ineffective. Over a range of Ph 5 to pH 8, pH of the medium was not
very important. These results show that the nature of the aglycone
moiety is important (Roddick, 1989).
2.2.7 Special studies on genotoxicity
Pure alpha-solanine (Sigma) at 0.01 to 0.05 mg/plate and
extracts from potatoes were negative in the Ames test both with
strains TA98 and TA100, and in the presence or absence of activation
by S9 fraction from PCB-induced rat liver (Ness et al., 1984).
Alpha-solanine (25 and 250 µM) tested negative in a
DNA-cell-binding assay using Ehrlich ascites cells and Escherichia
coli cells mixed with 32P-labelled nucleic acids (Kubinski et
al., 1981).
2.2.8 Special studies on mitotic index
Cultured human fibroblasts were treated up to 40 h with 0, 4.1,
8.3, 16.6, 33.3, and 66.6 µg alpha-solanine/ml. At the highest dose
there was an inhibition of growth, whereas at lower dose levels
there was a stimulation of growth, as evidenced by an increase in
the mitotic index from 1.7% in controls to 2.4% at the 4.1 µg/ml
dose level, which according to the authors was similar to the
sex-hormonal type of effect exerted by estrogens on target tissues.
Using pulse labelling with tritiated thymidine, it was shown that at
5 µg alpha-solanine/ml the mean cell cycle time decreased from 42.5
± 1.87 h in controls to 28.5 ± 0.29 h in treated cells. This was
however accompanied by a 4 h increase in the period of DNA synthesis
(S phase) in treated cells, and a decrease to virtually zero for the
G1 phase. The authors concluded that if alpha-solanine reached the
fetus, the observed types of effects could be hazardous to it, and
could lead to malformations (Kirk & Mittwoch, 1975).
2.2.9 Special studies on calcium transport
Alpha-solanine (100 µM at pH 7.4) caused a 90% inhibition of
active calcium transport in rat duodenum when added to everted
intestinal sacs (8 replicates) in vitro. A Dixon plot revealed
that the inhibition by alpha-solanine was non-competitive, and the
inhibition constant was 25 µM. The inhibition of active calcium
transport was accompanied by a 40% decrease in oxygen consumption
(Michalska et al., 1985).
When alpha-solanine was given to 12 male and female Wistar
albino rats (5-6 weeks old) in their drinking water (5 mM, pH 6.4)
for 12 days, calcium transport in duodenal sacs was reduced to about
one-third of the control value, but oxygen consumption was not
significantly reduced (Michalska et al., 1985).
2.3 Observations in humans
2.3.1 Gastrointestinal and neurotoxic effects
There have been many reported cases of human poisonings
(sometimes fatal) due to the ingestion of greened or otherwise
damaged potatoes. The symptoms of low grade solanine poisoning are
acute gastrointestinal upset with diarrhoea, vomiting and severe
abdominal pain. In more severe cases, neurological symptoms,
including drowsiness and apathy, confusion, weakness, and vision
disturbances, followed by unconsciousness and, in some cases, death
have also been reported. The vital signs include fever, rapid and
weak pulse, low blood pressure and rapid respiration. Onset of
symptoms has ranged from minutes to 2 days after ingestion of toxic
potatoes, with longer incubation periods generally associated with
the more severe cases.
As is usual with case histories of this nature, the available
data are not complete. Over the years, various analytical methods or
assays have been used to determine the concentration of 'solanine'
in cases of suspected poisonings. With most of the older data, the
estimate for solanine included the other glycoalkaloids, such as
alpha-chaconine. McMillan and Thompson (1979) showed that
gravimetric methods gave higher values than colorimetric methods. A
few case reports, for which the reviewers have estimated the dose
ingested, are given below. Additional reports were compiled by
Morris and Lee (1984), who indicated that more than 2000 cases with
about 30 deaths have been reported in the literature. Not all of
these reports were available to the reviewers.
Fifty-six German soldiers suffered typical 'solanine' poisoning
after eating 1 to 1.5 kg cooked peeled potatoes containing 24 mg
TGA/100 g (whole uncooked tubers contained 38 mg TGA/100 g). In a
few cases jaundice and partial paralysis were also observed. If one
assumes a body weight of 70 kg, the intake of 'solanine' was 3.4 to
5.1 mg/kg bw (Pfuhl, 1899).
In 18 separate households in Scotland, 61 persons suffered
typical 'solanine' poisoning soon to several hours after eating
potatoes. Persons not eating potatoes were not ill. One 5-year old
died. The potatoes in that household contained 41 mg 'solanine'/100
g. Assuming the child ate 200 g potatoes and had a bw of 18 kg, the
lethal dose was estimated at 4.5 mg/kg bw. Assuming adults ate 500 g
potatoes and had a bw of 60 kg, their intake of 'solanine' would
have been 3.4 mg/kg bw (Harris & Cockburn, 1918).
A small outbreak of solanine poisoning affected a family of
four adults on three consecutive Sunday evenings in Great Britain,
about 8 h after they had eaten 1 to 3 baked potatoes in their
jackets (weight of potatoes not given). A 5th person who only ate
the flesh of the potatoes was not affected. The severity of symptoms
was related to the number of potatoes ingested, and consisted of
abdominal pain, diarrhoea, and general malaise. Patients recovered
within 24 h. The level of solanine was 50 mg/100 g tuber, as
determined chemically and by cholinesterase inhibition. Assuming a
weight of 150 g per potato, and body weights of 60 and 70 kg, the
dose was estimated at 1.25 to 3.2 mg 'solanine'/kg bw (Wilson,
1959).
Seventy-eight junior schoolboys in Great Britain became ill
from solanine poisoning 7 to 9 h after eating two small boiled
peeled potatoes each (weight of potatoes not given) as part of their
lunch, and 17 were admitted to hospital. Symptoms included vomiting,
diarrhoea, and general abdominal pain. Most of the boys developed a
fever, suffered from headache, dizziness, mental confusion,
hallucinations and their vision was affected. Three boys were
comatose and stuporose on admission, with peripheral circulatory
collapse. All were discharged 6-11 days following admission, and 4-5
weeks later there were no sequelae. Tests for the presence of
biocides, such as nicotine, organophosphorus or organochloride
pesticides were negative. Six days after eating the meal, plasma
pseudocholinesterase levels in 10 out of 17 schoolboys was subnormal
(about 25% below the normal range for this age group). Red blood
cell cholinesterase levels were normal. The source of toxic potatoes
was traced to a bag of old potatoes that had been condemned for
consumption because of their appearance, but that had inadvertently
been cooked (peeling of the potatoes had been done by an automatic
peeling machine). Insufficient potatoes were left over after the
meal for direct chemical analysis. Solanine levels in the boiled
peeled potatoes were therefore estimated from the in vitro
reduction in pseudocholinesterase activity in human plasma, using
acetylcholine as a substrate, and were equivalent to 25-30 mg/100 g
tuber of alpha-solanine. Assuming an intake of 200 g potatoes and a
bw of 40 kg (age = 11-14 years), the reviewers estimate that the
intake of 'solanine' by the schoolboys would therefore have been
approximately 1.4-1.6 mg/kg bw. Because of the small margin of
safety between normal potatoes and toxic potatoes, the authors
speculated that in toxic potatoes other toxic steroids besides
glycoalkaloids may be synthesized, such as sapogenins and saponins,
which might enhance the toxicity of solanine alkaloids by promoting
gastro-intestinal absorption or other means (McMillan & Thompson,
1979).
In a recent (1983) poisoning associated with a school lunch
programme, 61 of 109 school children and staff in Alberta, Canada,
became ill, most within 5 minutes, after eating baked potato (weight
of potato not given) containing 49.4 mg 'solanine' per 100 g
(analytical method not indicated). Test results showed that there
was no evidence that the illness occurred due to the presence of
viruses, bacteria, moulds, pesticides or other chemicals in the food
items or their containers. The potatoes had a slight tinge of green
and had a bitter or unusual taste (noted by 44% of those affected),
causing a burning sensation in the throat of 18% of those affected.
The predominant symptoms in order of frequency were nausea (69%),
abdominal cramps (43%), headache (33%), vomiting (11%), fever and
diarrhoea (8%). The children recovered in about 3 h. The reviewers
estimate that, assuming the children ingested 200 g, and had a bw of
40 kg, the dose was about 2.5 mg 'solanine'/kg bw (Anon, 1984).
Based on the available human data (Table 3), an intake of
3-6 mg TGA/kg bw is considered a potentially lethal dose for humans,
and >1 to 3 mg TGA/kg bw is considered a toxic dose for humans.
Children may be more sensitive than adults. Other factors may be
present in suspect potatoes and modulate the toxicity of the
steroidal glycoalkaloids.
No signs of acute toxicity were noted in 3 Swedish adult
volunteers who ingested for 1 week a diet estimated to give an
intake of 1 mg/kg bw TGA (Harvey et al., 1985b).
Table 3. Summary of published reports of solanine poisoning in humans
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
56 peeled, 1-1.5 kg 24 3.4-5.1 recovered Pfuhl,
(soldiers) cooked (38) 1899
(whole
uncooked)
60 adults potatoes 500 g ? 41 3.4 recovered Harris &
1 child 200 g ? 4.5 1 fatal Cockburn,
(lethal) (5 yr-old) 1918
7 (family) greened ? ? ? 2 fatal Hansen, 1925
potatoes
50-60 shoots, ? 27 ? 1 fatal Willimot,
(Cyprus) leaves 49 1933
Prisoners experimental ? ? 2.8 recovered Report cited
by Ruhl,
1951
Child potato ? ? ? 1 fatal Report cited
berries by Ruhl,
1951
4 (family baked 1-3 50 1.2-3.2 dose-related, Wilson, 1959
adults) potatoes potatoes recovered
with skin 150-450 g
Table 3 (continued)
Affected Potato type Quantity Concentration Estimated Outcome Reference
Consumed of TGA Toxic Dose
mg/kg bw mg/kg bw
78 old potatoes 2 small 25-30 1-4-1.6 3 comatose, McMillan &
(schoolboys) potatoes all Thompson,
200 g recovered; 1979
young
boys,
more affected
61 baked potato 200 g 49 2.5 recovered Anon. 1984
(school-children)
Alberta
? Data not available.
2.3.2 Teratogenic effects
In 1972, Renwick showed that areas with an increased incidence
of neural tube defects (NTD) (anencephaly and spina bifida) were
associated with areas where potato consumption was higher, and where
potato blight was more common. The worldwide incidence of NTD varies
from < 1 to about 7 per 1000 total births. He postulated that this
disease was due to toxic factors in potatoes, such as
alpha-chaconine and alpha-solanine. These antifungal compounds offer
resistance to potato blight and increase in amount in blighted
potatoes, infected with the fungus Phytophthora infestans. Several
studies have been conducted to prove or disprove this theory
(Renwick, 1972). The same author more recently suggested that a long
half-life of potato glycoalkaloids could lead to their retention in
the body, and possible release early during pregnancy (Renwick,
1982).
In a prospective study with women who had previously borne a
child with NTD, 27 women did not handle or eat potatoes or potato
containing foods after deciding on a future pregnancy, and
throughout gestation; another 61 women, attending the same clinic,
did not avoid potatoes. The allocation to the two groups was
non-random, but voluntary. The groups did not differ significantly
with respect to age distribution, social class, parity or history of
outcome of previous pregnancies. The incidence of NTD was 8.7% in
the group of women avoiding potatoes, and 3.6% in the group eating
potatoes (p=0.58). This study failed to support the Renwick
hypothesis, but the authors pointed out that the size of the groups
was small (Nevin & Merrett, 1975).
Although there is a geographical similarity between neural tube
defect occurrence and potato blight in Canada, no annual or seasonal
associations were demonstrated. The author concluded that
socioeconomic factors were probably more important as a risk factor
for NTD, but suggested that better exposure assessment to factors
present in potatoes, at the level of the individual, would be
necessary to resolve this question. Such prospective studies should
also assess the significance of other risk factors (Elwood, 1976).
An epidemiological study (prospective study) was conducted in
Great Britain, whereby human serum specimens from 380 patients, who
were being screened for NTD by measuring their serum
alpha-fetoprotein at 15-22 weeks of gestation (most at 16 weeks),
were also analyzed for potato glycoalkaloids, using a sensitive
radioimmunoassay. The samples were analyzed blind, regardless of the
outcome of pregnancy, which resulted in 210 NTD cases and 170 normal
offspring. In most of the 9 centres studied, serum TGA and serum
solanidine levels were higher (p <0.05 in 2 centres) in the women
with a normal fetus than in those with a fetus affected by NTD.
Although closure of the neural tube normally takes place at 4-5
weeks of gestation, the authors felt that measurements at the later
date might reflect glycoalkaloid exposure earlier during gestation.
The results of this study are therefore the opposite of what one
would expect if the ingestions of potatoes contributed to the
etiology of NTD. Instead the authors suggested that avoidance of
potatoes might contribute to a vitamin deficiency thereby increasing
rather than decreasing the incidence of NTD (Harvey et al., 1986).
3. COMMENTS
Numerous studies performed on a variety of experimental animal
species to elucidate the toxicological properties of glycoalkaloids,
including teratogenicity, have been evaluated.
Cranial abnormalities have been observed in some teratogenicity
studies with laboratory animals, particularly with the hamster at
levels of 165-200 mg glycoalkaloids/kg bw/day. However, the
suggested association of the consumption of blighted potatoes during
pregnancy with increased incidences of spina bifida and anencephaly
has not been substantiated.
In a limited study in humans, the daily consumption of potato
tubers containing approximately 24 mg glycolkaloids/100 g did not
result in any signs of acute toxicity. However, human poisonings
have been associated with the consumption of poor-quality potato
tubers with elevated levels of glycoalkaloids. The signs of
low-grade glycoalkaloid poisoning are acute gastrointestinal upset
with diarrhoea, vomiting, and severe abdominal pain. In more severe
cases, neurological symptoms, including drowsiness, apathy,
confusion, weakness, and vision disturbances followed by
unconsciousness, have also been reported.
4. EVALUATION
The Committee considered that, despite the long history of
human consumption of plants containing glycoalkaloids, the available
epidemiological and experimental data from human and laboratory
animal studies did not permit the determination of a safe level of
intake. The Committee recognized that the development of empirical
data to support such a level would require considerable effort.
Nevertheless, it felt that the large body of experience with the
consumption of potatoes, frequently on a daily basis, indicated that
normal glycoalkaloid levels (20-100 mg/kg) found in properly grown
and handled tubers were not of concern. To support the continued
safe use of potato tubers, those developing new cultivars, and
others growing, harvesting, storing, processing, and consuming
potatoes, should be aware of the possibility of inadvertently
increasing the content of glfycoalkaloids to potentially toxic
levels.
5. REFERENCES
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1978). Inhibition of
rat cholinesterase isoenzymes in vitro and in vivo by the potato
alkaloid, alpha-chaconine. J. Food Biochem., 2: 259-276.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979a). Physiological
disposition, subcellular distribution and tissue binding of
alpha-chaconine (3H). J. Food Safety, 1: 257-273.
ALOZIE, S.O., SHARMA, R.P. & SALUNKHE, D.K. (1979b). Excretion of
alpha-chaconine-3H, a steroidal glycoalkaloid from
Solanum-tuberosum L. and its metabolites in hamsters. Pharmacol.
Res. Commun., 11: 483-490.
ANON. (1979). Solanine poisoning [editorial]. Br. Med. J., 2:
1458-1459.
ANON. (1984). Solanine food poisoning associated with a school lunch
program - Alberta. Canada Diseases Weekly Report, Health and
Welfare Canada, 10-18: 71.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1983). Toxic effects of high
glycoalkaloid feeding on the protein digestibility and growth of
rabbits. J. Pharm. Univ. Karachi., 2: 15-24.
AZIM, A., SHAIKH, H.A. & AHMAD, R. (1984). Toxic effects of high
glycoalkaloid feeding on the red blood cell counts and haemoglobin
concentration of rabbit blood. J. Pharm. Univ. Karachi, 3: 43-49.
BÖMER, A. & MATTIS, H. (1924). [Solanine content of potatoes] Der
Solaningehalt der Kartoffeln. Z. Nahr. Genussm., 47: 97-127.
BUSHWAY, R.J. & PONNAMPALAM, R. (1981). alpha-chaconine and
alpha-solanine content of potato products and their stability during
several modes of cooking. J. Agric. Food Chem., 29: 814-817.
CHAUBE, S. & SWINYARD, C.A. (1976). Teratological and toxicological
studies of alkaloidal and phenolic compounds from Solanum tuberosum
L. Toxicol. Appl. Pharmacol., 36: 227-237.
CLARINGBOLD, W.D.B., FEW, J.D. & RENWICK, J.H. (1982). Kinetics and
retention of solanidine in man. Xenobiotica, 12: 293-302.
DALVI, R.R. & BOWIE, W.C. (1983). Toxicology of solanine: an
overview. Vet. Hum. Toxicol., 25: 13-15.
DALVI, R.R. (1985). Comparative assessment of the effect of solanine
administered orally and intraperitoneally on hepatic dysfunction in
male rats. Jpn. J. Vet. Sci., 47: 657-659.
ELWOOD, J.M. (1976). Anencephalus, spina bifida and potato blight in
Canada. Can. J. Public Health, 67: 122-126.
GULL, S.D., ISENBERG, F.M. & BRYAN, H.H. (1970). Alkaloid toxicology
of Solanum-tuberosum. Hort. Science, 5: 316.
HANSEN, A.A. (1925). Two fatal cases of potato poisoning. Science,
61: 340-341.
HARRIS, F.W. & COCKBURN, T. (1918). Alleged poisoning by potatoes.
Am. J. Pharm., 90: 722-726.
HARRIS, H. & WHITTAKER, M. (1962). Differential inhibition of the
serum cholinesterase phenotypes by solanine and solanidine. Ann.
Hum. Genet., 26: 71-76.
HARVEY, M.H., McMILLAN, M., MORGAN, M.R.A. & CHAN, H.W.-S. (1985a).
Solanidine is present in sera of healthy individuals and in amounts
dependent on their dietary potato consumption. Hum. Toxicol., 4:
187-194.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1985b).
Measurement of potato steroidal alkaloids in human serum and saliva
by radioimmunoassay. Hum. Toxicol., 4: 503-512.
HARVEY, M.H., MORRIS, B.A., McMILLAN, M. & MARKS, V. (1986). Potato
steroidal alkaloids and neural tube defects: serum concentrations
fail to demonstrate a causal relation. Hum. Toxicol., 5: 249-253.
JADHAV, S.J., SHARMA, R.P. & SALUNKHE, D.K. (1981). Naturally
occurring toxic alkaloids in foods. Crit. Rev. Toxicol., 9:
21-104.
JELINEK, R., KYZLINK, V. & BLATTNY, C., Jr. (1976). An evaluation of
the embryotoxic effects of blighted potatoes on chicken embryos.
Teratology, 14: 335-342.
KIRK, D. & MITTWOCH, U. (1975). Changes in the mitotic cycle induced
by alpha-solanine. Humangenetik, 26: 105-111.
KLINE, B.E., VON ELBE, H., DAHLE, N.A. & KUPCHAN, S.M. (1961). Toxic
effects of potato sprouts and of solanine fed to pregnant rats.
Proc. Soc. Exp. Biol. Med., 107: 807-809.
KUBINSKI, H., GUTZKE, G.E. & KUBINSKI, Z.O. (1981). DNA-cell-binding
(DCB) assay for suspected carcinogens and mutagens. Mutat. Res.,
89: 95-136.
MAGA, J.A. (1980). Potato glycoalkaloids. Crit. Rev. Food Sci.
Nutr., 12: 371-405.
MATTHEW, J.A., MORGAN, M.R.A., McNERNEY, R., CHAN, H.W.-S. & COXON,
D.T. (1983). Determination of solanidine in human plasma by
radioimmunoassay. Food Chem. Toxicol., 21: 637-640.
McMILLAN, M. & THOMPSON, J.C. (1979). An outbreak of suspected
solanine poisoning in schoolboys: examination of criteria of
solanine poisoning. Q. J. Med., 48: 227-243.
MICHALSKA, L., NAGEL, G., SWINIARSKI, E. & ZYDOWO, M.M. (1985). The
effect of alpha-solanine on the active calcium transport in rat
intestine. Gen. Pharmacol., 16: 69-70.
MORGAN, M.R.A. & COXON, D.T. (1987). Tolerances: glycoalkaloids in
potatoes. Ch. 7. In: Watson, D.H. (ed.). Ellis Horwood series in
food science and technology: Natural toxicants in food: progress
and prospects, Ellis Horwood, Chichester, England, pp. 221-230.
MORRIS, S.C. & LEE, T.H. (1984). The toxicity and teratogenicity of
Solanaceae glycoalkaloids particularly those of the potato
(Solanum tuberosum): a review. Food Technol. Aust., 36: 118-124.
MUN, A.M., BARDEN, E.S., WILSON, J.M. & HOGAN, J.M. (1975).
Teratogenic effects in early chick embryos of solanine and
glycoalkaloids from potatoes infected with late-blight,
Phytophthora infestans. Teratology, 11: 73-77.
NESS, E., JONER, P.E. & DAHLE, H.K. (1984). Alpha-solanine tested
for mutagenicity with the Ames test. Acta Vet. Scand., 25:
145-147.
NEVIN, N.C. & MERRETT, J.D. (1975) Potato avoidance during pregnancy
in women with a previous infant with either anencephaly and/or spina
bifida. Br. J. Prev. Soc. Med., 29: 111-115.
NISHIE, K., GUMBMANN, M.R. & KEYL, A.C. (1971). Pharmacology of
solanine. Toxicol. Appl. Pharmacol., 19: 81-92.
NISHIE, K., NORRED, W.P. & SWAIN, A.P. (1975). Pharmacology and
toxicology of chaconine and tomatine. Res. Commun. Chem. Pathol.
Pharmacol., 12: 657-668.
NORRED, W.P., NISHIE, K. & OSMAN, S.F. (1976). Excretion,
distribution and metabolic fate of 3H-alpha-chaconine. Res.
Commun. Chem. Pathol. Pharmacol., 13: 161-171.
PATIL, B.C., SHARMA, R.P., SALUNKHE, D.K. & SALUNKHE, K. (1972).
Evaluation of solanine toxicity. Food Cosmet. Toxicol., 10:
395-398.
PFUHL, E. (1899). [Regarding an outbreak of illness due to poisoning
by solanine in potatoes] Über eine Massenerkrankung durch Vergiftung
mit stark solaninhaltigen Kartoffeln. Deutsch. Med. Wochenschr.,
25: 753-754.
POSWILLO, D.E., SOPHER, D. & MITCHELL, S.J. (1972) Experimental
induction of fetal malformation with "blighted" potato: a
preliminary report. Nature, 239: 462-464.
POSWILLO, D.E., SOPHER, D., MITCHELL, S.J., COXON, D.T., CURTIS,
R.F. & PRICE, K.R. (1973). Investigations into the teratogenic
potential of imperfect potatoes. Teratology, 8: 339-347.
RENWICK, J.H. (1972). Hypothesis: anencephaly and spina bifida are
usually preventable by avoidance of a specific but unidentified
substance present in certain potato tubers. Br. J. Prev. Soc. Med.,
26: 67-88.
RENWICK, J.H. (1982). Food and malformation. Practitioner, 226:
1947-1953.
RENWICK, J.H., CLARINGBOLD, W.D.B., EARTHY, M.E., FEW, J.D. &
McLEAN, A.C.S. (1984). Neural-tube defects produced in Syrian
hamsters by potato glycoalkaloids. Teratology, 30: 371-381.
RODDICK, J.G. (1989). The acetylcholinesterase-inhibitory activity
of steroidal glycoalkaloids and their aglycones. Phytochemistry,
28: 2631-2634.
RUDDICK, J.A., HARWIG, J. & SCOTT, P.M. (1974). Nonteratogenicity in
rats of blighted potatoes and compounds contained in them.
Teratology, 9: 165-168.
RÜHL, R. (1951). [Contribution on the pathology and toxicology of
solanine] Beitrag zur Pathologie und Toxikologie des Solanins.
Arch. Pharm., 284: 67-74.
SHARMA, R.P., WILLHITE, C.C., WU, M.T. & SALUNKHE, D.K. (1978).
Teratogenic potential of blighted potato concentrate in rabbits,
hamsters, and miniature swine. Teratology, 18: 55-61.
SHARMA, R.P., WILLHITE, C.C., SHUPE, J.L. & SALUNKHE, D.K. (1979).
Acute toxicity and histopathological effects of certain
glycoalkaloids and extracts of Alternaria solani or Phytophthora
infestans in mice. Toxicol. Lett., 3: 349-355.
SHARMA, R.P., TAYLOR, M.J. & BOURCIER, D.R. (1983). Subcellular
distribution of alpha-chaconine in mouse hepatocytes. Drug Chem.
Toxicol., 6: 219-234.
SHARMA, R.P. & SALUNKHE, D.K. (1989). Solanum glycoalkaloids. In:
Cheeke, P. R. (ed.). Toxicants of Plant Origin, Vol. 1 Alkaloids,
CRC Press, Boca Raton, Florida. pp. 179-236.
SLANINA, P. (1990a). Assessment of health-risks related to
glycoalkaloids ("solanine") in potatoes: a Nordic view. Report from
the Nordic working group on food toxicology and risk assessment.
Vår Föda, 43: 1-14.
SLANINA, P. (1990b). Solanine (glycoalkaloids) in potatoes:
toxicological evaluation. Food Chem. Toxicol., 28: 759-761.
SWINYARD, C.A. & CHAUBE, S. (1973). Are potatoes teratogenic for
experimental animals? Teratology, 8: 349-357.
WILLIMOTT, S.G. (1933). An investigation of solanine poisoning.
Analyst, 58: 431.
WILSON, G.S. (1959). A small outbreak of solanine poisoning.
Monthly Bulletin, Ministry of Health (London), 18: 207-210.
WILSON, A.M., McGANN, D.F. & BUSHWAY, R.J. (1983). Effect of growth,
location and length of storage on glycoalkaloid content of roadside
stand potatoes as stored by consumers. J. Food Prot., 46: 119-121.
WOOD, F.A. & YOUNG, D.A. (1974). TGA in potatoes. Agric. Can. Publ.
1533: pp. 1-2.
