
CARAZOLOL
First draft prepared by
Dr. F.X.R. van Leeuwen
Toxicology Advisory Centre
National Institute of Public Health
and Environmental Protection
Bilthoven, The Netherlands
1. EXPLANATION
Carazolol is a mixed ß-adrenoceptor blocking agent primarily used
in pigs to prevent sudden death due to stress during transport.
Carazolol has not been previously evaluated by the Joint FAO/WHO
Expert Committee on Food Additives.
2. BIOLOGICAL DATA
2.1 Biochemical aspects
2.1.1 Absorption, distribution and excretion
Groups of female Sprague-Dawley rats (10-15 weeks) were given
0.5 mg/kg side-chain labelled 14C-carazolol intraperitoneally.
Carazolol was determined in plasma and tissue samples after 0.25,
0.5, 1, 2, 6, 24, and 48 hours. Peak plasma levels were observed
after 0.25 to 1 hour and the half-life was about 24 hours. Highest
tissue levels had been obtained by 0.25 hours in liver, kidneys and
lungs. (Less than 0.18% was expired to air within 24 hours). After
48 hours 93% of the dose was excreted, 45% in urine and 48% in
faeces.
Female non-pregnant Russian rabbits given a single oral dose of
10 mg side-chain labelled 14C-carazolol/kg excreted 61.6% of the
radioactivity in the urine within 24 hours (faecal excretion was not
determined). Serum levels peaked 1 hour after dosing and the
half-life was about 20 hours. Pregnant rabbits given 0, 10, or
100 mg/kg orally showed the same pattern in renal excretion and
plasma serum levels (Koch & Bartsch, 1977).
In dogs given side-chain labelled 14C-carazolol at doses of
5 mg/kg i.v. or 50 mg/kg orally a slow elimination with plasma
half-lives of 30 and 20 hours, respectively, was observed. After
intravenous administration 22% and 38% of the dose was excreted in
the urine and faeces, respectively, within 48 hours; whereas after
oral administration urinary excretion accounted for 13% and faecal
excretion for 45% (Koch & Bartsch, 1977).
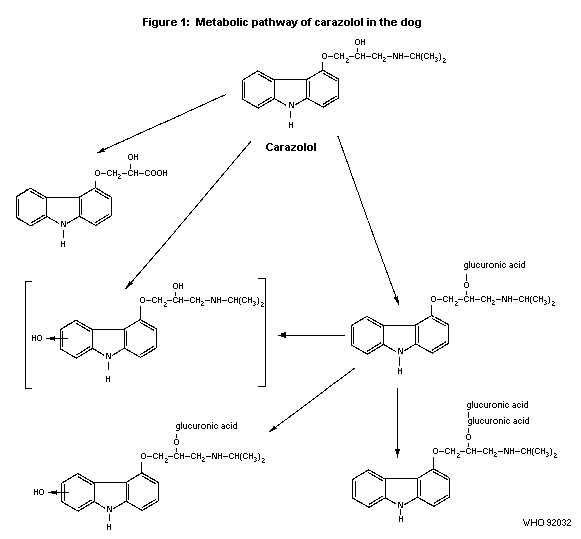
2.1.2 Biotransformation
One male beagle dog was given 10 mg side-chain labelled
14C-carazolol (purity 96%). Urine was collected for 24 hours and
used for metabolite investigations. Within 24 hours about 31% of the
dose was excreted in urine. Apart from carazolol 6 metabolites were
isolated and identified by mass spectrometry as carazolol with a
shortened side-chain, a diastereomer pair of
carazolol-O-glucuronides, an O-glucuronide of 6(7)-hydroxy carazolol
and a diastereomer pair of carazolol-bis-O-glucuronide (Koch, 1977).
Unchanged carazolol, carazolol mono-and bis-glucuronide and
carazolol lactate were identified in the urine of pigs
intramuscularly injected with 1 mg carazolol/100 kg b.w. Two human
volunteers each ingested 5 mg carazolol. Urine was collected during
the following 4.5 hours; the parent compound, carazolol
monoglucuronide, carazolol lactate and carazolol acetate were found
(Rudolph, 1988).
 2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of carazolol is given in Table 1. Deaths
shown were recorded within the first 24 hours. The animals exhibited
sedation, apathy, ventral position, slowed respiration and
convulsions shortly before death.
Table 1: The acute toxicity of carazolol
Species Route Sex LD50 (mg/kg b.w.) Reference
Mouse oral M 1321 Czerwek, 1978
F 1601
i.p. M 561
F 621
i.v. M&F 142
Rat oral M 881
F 801
i.p. M 621
F 591
i.v. M&F 10.43
Rabbit i.v. M&F 5.24
1. vehicle: tylose
2. vehicle: Dimethylformamide (DMF) in glucose
3. vehicle: trisodium citrate, citric acid and polyethylene glycol
in water
4. vehicle: DMF in NaCl
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of Sprague-Dawley rats (10/sex/group) were fed a diet
containing 0, 20, 70, or 200 mg/kg carazolol for 14 days. No
dose-related effects were observed on clinical signs, body weight,
food intake, food efficiency, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology. The NOAEL in
this study was 200 mg carazolol/kg food, equal to 18.9 and
18.3 mg/kg b.w. for males and females, respectively (Rebel et al.,
1974a).
Groups of Sprague-Dawley rats (10/sex/group) were given i.p.
injections once daily of 0, 1.0, 3.0, or 9.0 mg carazolol/kg b.w.
for 4 weeks. No treatment-related effects were observed on clinical
signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, macroscopy, or histopathology. The NOAEL in
this study was 9 mg/kg b.w. (Rebel et al., 1975a).
Groups of Sprague-Dawley rats (20/sex/group) were administered
0, 40, 120, or 400 mg carazolol/kg food (equal to 0, 2.7 and 3.2,
8.2 and 9.5, or 27.3 and 30.4 mg/kg b.w./day for males and females,
respectively) in the diet for 3 months. Rats (5/sex) of the control
and highest dose group were given a 4-week recovery period.
High-dose females exhibited a decreased body weight gain due to a
lower food intake from week 5 onwards. They had completely recovered
from this effect after 4 weeks. A significantly lower heart rate in
conscious rats was observed in all treated animals (males and
females combined), without any dose-response relationship. The
latter might be due to a (sub)maximal pharmacological dose. However,
interpretation of the results is hampered by a pre-existing
difference in initial heart rate between rats allocated to the
control and the treatment groups. No treatment-related effect was
observed on clinical signs, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology (Rebel
et al., 1976a).
Groups of Sprague-Dawley rats (30/sex/group) were fed a diet
containing 0, 150, 300, or 600-1800 mg carazolol/kg food (equal to 7
and 9, 14, and 17, or 33 - 69 and 36 - 86 mg/kg b.w./day for males
and females, respectively) for 12 months. The concentration of
carazolol in diet of the highest dose group was 600 mg/kg up to week
22, 1200 mg/kg from week 22 to week 40, and 1800 mg/kg from week 40
to the end of the experiment. Five male and 5 female rats per group
were given a 4-week recovery period at the end of the experiment.
Male as well as female rats at the highest dose group showed a rough
pelt on increasing the dose to 1200 mg/kg. High-dose rats showed a
decreased food intake (from week 40) and a decrease in body weight
gain from week 28 in males and from week 33 in females. At recovery
a higher food consumption was observed in the same dose group; in
males delayed body weight gain was reversible. In male as well as in
female rats relative heart weight showed a significant but not
dose-related increase at all dose levels. Relative kidney, ovary and
testes weights were increased in rats at the highest dose. No
treatment-related effects were observed on haematology, clinical
chemistry, urinalysis, macroscopy and microscopy (Hebold et al.,
1978a).
2.2.2.2. Dogs
Groups of beagle dogs (2/sex/group) were administered 0, 1, 3,
or 10 mg carazolol/kg b.w./day for 14 days. Observations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, heart rate, BSP retention, neurological
investigations, organ weight, macroscopy, and histopathology.
Treatment-related effects observed in high-dose females consisted of
decreased food consumption and body weight, decrease in Hb, Ht and
erythrocytes, a marked acceleration of the erythrocyte sedimentation
rate, an increase in glucose, cholesterol, AP and ALAT. At
histopathology, accumulation of mesenchyme cells was seen in one
high-dose female, the other one showed an increased activation of
Kupffer's cells. The NOAEL in this study was 3 mg/kg b.w./day (Rebel
et al., 1974b).
Groups of beagle dogs (2/sex/group) were given i.v. injections
once daily of 0, 0.5, 1.5, or 4.5 mg carazolol/kg b.w. for 4 weeks.
No treatment-related effects were observed on clinical signs, body
weight, food consumption, heart rate, neurological investigations,
haematology, clinical chemistry, urinalysis, organ weight,
macroscopy and histopathology (Rebel et al., 1975b).
Groups of beagle dogs (3/sex/group, 13 months old) were given
0, 2, 6, or 20 mg/kg b.w. carazolol in 2 daily doses for 3 months.
One male and one female dog from the control group and the high-dose
group remained on a 4-week recovery period. Observations included
clinical signs, food intake, body weight, heart rate, neurological
investigations, haematology, clinical chemistry, urinalysis, organ
weights, macroscopy and histopathology. High-dose females exhibited
an increase in erythrocyte sedimentation rate, AP and ALAT activity
and cholesterol concentration (Rebel et al., 1976b).
Groups of beagle dogs (5/sex/group) were administered 2 daily
doses of 0, 7, 15, or 30 (week 1-18) and 60 (week 18-52) mg
carazolol/kg b.w. for 12 months. Two male and 2 female dogs were
observed during a subsequent 5-week recovery period. No effects were
observed on clinical signs, food consumption, body weight, heart
rate, neurological investigations, haematology, clinical chemistry,
urinalysis, macroscopy, or microscopy. An increase in relative
weight of liver, kidney, and testes was apparent in male dogs at the
highest dose. The NOEL in this study was 15 mg/kg b.w. twice daily
(Hebold, et al., 1978b). Remark: no statistics were performed.
2.2.3 Long-term carcinogenicity study
2.2.3.1 Rats
Groups of Sprague-Dawley rats (72/sex/group) received a diet
containing 0, 100, 300, or 900 mg/kg food for 26 months.
Observations included clinical signs, food consumption, body weight,
haematology, clinical chemistry, macroscopy and microscopy. Survival
was increased in dosed rats (48/72 and 41/72, 44/72 and 47/72, 51/72
and 53/72, and 60/72 and 57/72 for males and females at 0, 100, 300,
and 900 ppm, respectively). A treatment-related significant decrease
in body weight gain was observed at 300 and 900 mg/kg in both sexes
and at 100 mg/kg food in female rats. Food consumption was
significantly decreased in high-dose male and female rats and in
females at 300 mg/kg from day 464 onwards. A decrease in neutrophils
and eosinophils (males only) was observed at 300 and 900 mg/kg food.
At the same dose levels glucose and protein levels were decreased in
both males and females. At 900 mg/kg food glucose was decreased in
females and creatinine in males. The incidence of all tumours
combined was not enhanced (Hartig, 1981). Remark: only summarized
data were available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Male rats were administered diets containing 0, 40, or
400 mg/kg food mg carazolol/kg food for 10 weeks. Rats were then
allowed to mate with untreated females. The newly-born were counted
and weighed. Body weight was slightly decreased in high-dosed males.
No treatment-related effects were observed on indices for fertility,
gestation, viability and lactation. No difference in offspring born
alive or dead, number of live fetuses, fetal weight or behaviour of
the offspring was observed. At 21 days of age the offspring were
killed and no malformations were observed (Hebold & Czerwek, 1978).
Remark: summary only available.
In a 3-generation reproduction study with a teratology phase
(see Special studies on embryotoxicity and teratogenicity, Section
2.2.5.1) female Sprague-Dawley rats (76/group) were orally
administered by gavage 0, 15, 30, or 60 mg/kg b.w./day carazolol
suspended in methyl cellulose. Administration started from 14 days
before the first mating with untreated males (for all females/group)
throughout the entire pregnancy (22-27 pregnant rats/group) until
day 21 of lactation. Eleven-12 dams/group were killed on day 21 of
pregnancy and their fetuses were delivered by caesarian section.
F1 animals (1/sex/litter) were mated and gave birth to the F2
generation. During the 14-day premating period a slight sedation was
observed from days 3-6 at the highest dose and a significantly
increased body weight as well as body weight gain were observed at
30 and 60 mg/kg b.w./day. No effects were observed on the fertility
index. At 60 mg/kg b.w./day the rearing performance of the F0
animals during the lactation period was impaired. At 60 mg/kg b.w.
post-implantation loss was significantly increased in the F0 dams
and the number of F1 pups alive at the end of the lactation period
was significantly decreased. At the highest dose a significantly
lower pup weight was observed in the F1 generation shortly after
delivery. At 30 and 60 mg/kg b.w./day post-implantation loss was
significantly increased in F1 dams and a significant decrease was
observed in the number of F2 young alive on the day after birth.
The NOAEL in this study was 15 mg carazolol/kg b.w./day (Sterz &
Vollmar, 1978).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 24 pregnant Sprague-Dawley rats were administered 0,
25, 50, or 100 mg carazolol/kg b.w/day from days 6-15 of pregnancy
by gavage. Dams were killed on gestation day 19. Observations
included clinical signs, condition of faeces, food and drinking
water consumption, and body weight. On day 19 of gestation the
females were sacrificed and the uteri and ovaries were examined.
Other organs were macroscopally examined. Fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
malformations. Maternal toxicity (ataxia and sedation) was observed
at 50 mg carazolol/kg b.w./day, and more marked at 100 mg/kg
b.w./day. At the highest dose an increase in post-implantation loss
was observed. There was no evidence of embryotoxicity or
irreversible structural effects (Leuschner, 1975).
A teratological examination was conducted during the course of
the 2-generation rat reproduction study (see Reproduction studies,
Section 2.2.4). No effects were observed on number of corpora lutea,
number of implantations, pre-implantation loss, resorptions, live
and dead fetuses, fetal weight, sex ratio, or external, visceral,
and skeletal malformations. From days 1-22 of pregnancy high-dose
dams exhibited a reduced body weight gain. Placental weight was
significantly increased at 30 and 60 mg/kg b.w./day (not
dose-related). A decrease in number of ossified cervical vertebrae
or tail vertebrae was observed in the mid- and high-dose groups. No
irreversible structural effects were observed (Sterz & Vollmar,
1978).
2.2.5.2 Rabbits
Groups of 17 pregnant Himalayan rabbits were orally
administered 0, 25, 50, or 100 mg carazolol/kg b.w/day from days
7-19 of gestation. The females were killed on gestation day 31 and
fetuses were delivered by caesarean section. During the
administration period food consumption was reduced in all groups,
significantly at 100 mg/kg b.w./day; a significant increase in food
consumption was observed in high-dose females the last 6 days of the
test. Weight gain of dams at the highest dose was significantly
increased from days 20 to 31 of pregnancy. Independent of the
dosage, 3 cases of arthrogryposis occurred in 30 litters (0, 1, 1,
and 1 at 0, 25, 50 and 100 mg/kg b.w./day, respectively). No
treatment-related effects were observed in number of corpora lutea,
implantation loss, resorptions, litter size, live and dead fetuses,
fetal weight, sex ratio, or internal and skeletal malformations
(Sterz & Glocke, 1977a).
Groups of pregnant Himalayan rabbits (13-14/group) received
orally 0, 6.26, 12.50, or 100 mg carazolol/kg b.w./day from days
7-19 of gestation. The females were killed on day 31 of gestation
and the fetuses were delivered by caesarean section. Food
consumption was decreased at the highest dose. In the mid-dose
group, one dead and one underdeveloped fetus were seen. In the
high-dose group, one dead and two underdeveloped fetuses were seen.
Placental weight was significantly decreased at the highest dose and
fetal weight was significantly and dose-relatedly decreased at 12.50
and 100 mg/kg b.w./day. One fetus with pin-head size meningocele on
the occiput was observed at 100 mg/kg b.w./day. Arthrogryposis was
observed in 0, 1, 1, and 1 fetus at 0, 6.25, 12.50, and 100 mg/kg
b.w./day, respectively. One fetus with spondylosis syndrome and one
fetus with scoliosis due to reduction and absence of parts of the
first two lumbar vertebrae were observed at the mid- and high-dose,
respectively. No treatment-related effects were observed on
fertility index, body weight, number of corpora lutea, implantation
loss, resorptions, litter size, or sex ratio (Sterz & Glocke,
1977b).
A third experiment (using the same experimental design) was
carried out in which particular attention was directed towards any
occurrence of deformities of the limbs (arthrogryposis). Twenty-nine
and 20 Himalayan pregnant rabbits were orally administered 0 and
100 mg carazolol/kg b.w./day from days 9-12 of gestation,
respectively. No treatment-related effects were observed on food
consumption or body weight (only recorded in 10 control and 4
experimental rabbits) or reproduction performance. No abnormalities
were observed after skeletal and visceral examinations, except for a
significantly increased number of extra ribs at the 1st lumbar
vertebra. The authors concluded that the observed cases of
arthrogrypsosis in the previous reported studies were in fact a
fortuitous increase in incidence of this spontaneous deformity of
the rabbits strain used (Sterz & Glocke, 1977c).
2.2.6 Special studies on pharmacology
2.2.6.1 Mice
Groups of female SPF mice fasted for 18 hours were given i.p.
doses of 0, 20, or 200 mg carazolol/kg b.w. or 200 mg propranolol/kg
b.w. Intestinal motility was significantly increased after both
carazolol and propranolol administration (Roesch, 1978). Barbiturate
sleep in groups of mice given s.c. 8, 16, 32, or 64 mg carazolol/kg
b.w. was not affected at doses < 16 mg/kg b.w.; all mice died at
64 mg/kg b.w. Induced fighting behaviour was dose-dependently
inhibited in mice given 0, 2, 4, 8 or 16 mg carazolol s.c./kg b.w.
at doses of 4-16 mg/kg. Electroshock-induced convulsions were
inhibited in 1/10 mice and 10/10 mice at 16 and 32 mg carazolol/kg
b.w., respectively. Metrazole-induced convulsions were
dose-dependently inhibited in mice given 4-32 mg carazolol/kg b.w.
In a traction test with mice the ability to cling to the wire was
blocked in 0/10, 1/10, and 10/10 mice at 8, 16, and 32 mg
carazolol/kg b.w., respectively (Kumada & Ohtsuka, 1978).
2.2.6.1 Rats
No indication of an effect on the central nervous system was
observed in male Sprague-Dawley rats given intraperitoneally
10 mg/kg b.w. carazolol (Roesch, 1973). No effects were observed on
the urine and electrolyte excretion in female SPF-Sprague-Dawley
rats after the oral or i.p. administration of 10 mg/kg b.w.
carazolol (Bartsch, 1974a).
2.2.6.3 Rabbits
1 mg carazolol/kg b.w. administered intravenously to conscious
rabbits did not cause an effect on body temperature; blood glucose
levels were slightly increased at this dose (Hebold, 1974).
Carazolol given at doses of 50 mg/kg b.w. orally, or at 5 or
10 mg/kg b.w i.v. to male and female mixed-breed rabbits inhibited
an isoprenaline-induced tachycardia; at 20 mg/kg b.w. orally no
inhibition was found. A dose of 5 mg carazolol/kg b.w. intravenously
administered to conscious rabbits inhibited isoprenaline-induced
tachycardia (Bartsch 1974b).
2.2.6.4 Dogs
No effects were observed on the urine and electrolyte excretion
in female beagle dogs given 5 mg carazolol/kg b.w. orally (Bartsch,
1974). Conscious dogs were intravenously administered 0.5, 1.0, 2.0,
or 5.0 mg carazolol/kg b.w. over a period of 15 seconds. At 1.0 and
2.0 mg/kg b.w. heart rate was increased without affecting the PQ
interval, but with slight changes in the ECG. At these doses the
behaviour of the dogs was normal. At the highest dose of 5.0 mg/kg
vomiting, tachypnoea, tachycardia and changes in the PQ-section of
the ECG were observed (Bartsch, 1977).
One, 10, or 100 mg carazolol/kg b.w. given i.v. to
anaesthetized mongrel dogs antagonized isoprenaline-induced
responses. In another experiment, 0.01, 0.1, 1.0, or 10 mg
carazolol/kg b.w. was intravenously given to anaesthetized mongrel
dogs. At doses of 1.0 mg/kg b.w. and higher, depressive effects on
the cardiovascular system which included decreases in ABP (arterial
blood pressure), LVP (left ventricular pressure) and dp/dt (cardiac
output), and an increase of IVEDP (left ventricular end diastolic
pressure) were observed (Satoh et al., 1980).
One, 4, 16, or 64 mg carazolol/kg b.w. was intravenously given
to mongrel dogs. A dose-dependent inhibition of
isoproterenol-induced tachycardia as well as isoprenaline-induced
increase of cardiac output was observed; at the highest dose an
almost complete blockade was observed. In mongrel dogs a
dose-dependent inhibition of tachycardia as well as increase in
cardiac output induced by sympathetic nerve stimulation was also
observed after the i.v. administration of 0.25 to 16 mg carazolol/kg
b.w. (Kumada & Ohtsuka, 1978).
2.2.7 Special studies on genotoxicity
Table 2: Results of genotoxicity assays on carazolol
Test system Test object Concentration Result Reference
In vitro S. typhimurium 0-5000 mg/pl1 negative Grafe, 1978
Ames test TA98, TA100 in DMF mg/pl1
TA1535, TA1537
TA1538
In vivo male NMRI single oral dose negative Grafe &
Dominant mice of 20 or 80 mg/kg b.w. Vollmar,
lethal assay or 13.5 mg/kg b.w. 1976
single i.p. dose
Dominant female NMRI single oral dose negative Grafe &
lethal assay Kisslegg mice of 50 mg/kg b.w. or Vollmar,
13.5 mg/kg b.w. single 1976
i.p. dose
Host-mediated NMRI/Kisslegg 80 mg/kg b.w. oral or negative Grafe &
assay mice with 13.6 mg/kg b.w. s.c. Vollmar,
S. typhimurium 1976
G46 S. marcescens
Cytogenicity Chinese hamster 2 x 400 mg/kg b.w. negative Grafe &
assay oral or 1 x 20 mg/kg Vollmar,
(spermatogonia b.w. i.p. or 3 x weekly 1976
as well as for 13 weeks a
bone marrow)2 treatment with 20 mg/kg
b.w. oral or 1 mg/kg
b.w. i.p.; all suspended
in CMC
1. both with and without rat liver S-9 fraction
2. only a negative control group (compound not indicated) was tested
2.3 Observations in humans
Twelve healthy male volunteers were given carazolol in a 3-way
crossover experiment at doses of 0.5 mg i.v., 5.0 mg oral or 7.5 mg
oral. Ergometric tests were carried out before and 1, 2, 3, 4, 8,
12, and 16 hours after carazolol administration. The subjects laid
down for about 20 minutes before each test. The heart rate, blood
pressure, the pressure-rate product, and the relative enteral
efficacy were determined. Heart rate was maximally inhibited by
1-2 hours after administration (15%, 13%, and 15% at 0.5 mg i.v.;
5.0 and 7.5 mg oral, respectively). During exercise the systolic
blood pressure was lowered by a maximum of 12% (oral dose) or 15%
(i.v. dose) at 1 hour. The maximal reduction of the pressure rate
product was 28%, 26%, and 25% at 0.5 mg i.v., 5.0 and 7.5 mg oral,
respectively. The mean duration of action of an oral dose of 5 mg
was estimated as 10 hours. The relative enteral efficacy calculated
by comparison of the inhibitory areas after both oral doses with
those of an i.v. dose did not differ significantly and was about
10%. By using the dose-reponse curve the authors extrapolated a
no-effect dose for the oral route of 10 mg/kg b.w. (Mollendorff
et al., 1979).
In a cross-over experiment 8 patients with chronic bronchitis
and partial reversibility of the obstruction were given a placebo or
carazolol randomly. Two patients received both 0.1 and 0.7 mg
carazolol, while the other 6 received either 0.1 or 0.7 mg. Vital
capacity (VC) and forced expiratory volume (FEV) were measured
before and 1, 2, and 3 hours after administration of the substances.
No statistics were carried out. At 0.7 mg carazolol a definite
increase in bronchial resistance was observed and dyspnoea was seen
in 3/5 patients leading to discontinuation of the test. At 0.1 mg
carazolol, the tests did not have to be discontinued in any of the
patients; only one patient showed slight dyspnoea with a decrease in
FEV . The author estimated that a dose of 0.03 mg carazolol no
longer induces any effect on the bronchial system (Huckauf,
undated).
3. COMMENTS
The Committee considered pharmacological and toxicological data
from pharmacodynamic, pharmacokinetic, metabolism, acute and
short-term toxicity, carcinogenicity, genotoxicity, reproduction and
teratology studies, as well as the results of clinical trials in
humans.
The distribution, excretion and biotransformation of
radiolabelled carazolol were studied in rats, rabbits and dogs.
After oral ingestion, carazolol was rapidly absorbed. In rats, the
radioactivity was widely distributed in the tissues, with the
highest levels in liver, kidneys, and lungs. Excretion was almost
complete within 48 hours, equally divided between the urine and
faeces. In rabbits, peak plasma levels were reached 1 hour after a
single oral dose of 10 mg/kg b.w., and about 60% of the dose was
excreted in urine. Following oral administration of carazolol in
dogs, 13% of the radioactivity was excreted in urine and 45% in
faeces within 48 hours. In the urine of a dog given 10 mg of [14C]
carazolol/kg body weight intravenously, the parent compound and six
metabolites were identified. Both carazolol and its glucuronide,
lactate, and acetate metabolites have been identified in the urine
of pigs and humans.
In a short-term study in rats, in which carazolol was
administered in the diet at levels up to 400 mg/kg of feed for 13
weeks, females exhibited a decrease in both body weight and food
intake at the highest dose. Heart rate was decreased even at the
lowest dose of 40 mg/kg of feed. When rats received carazolol in the
feed for 1 year a decrease in body weight due to decreased food
intake at the highest dose level was observed in both males and
females only after progessively increasing this dose to 1200 and
subsequently to 1800 mg/kg of feed. A statistically significant
increase in relative heart weight was observed in rats at all dose
levels, but this was not dose related.
In short-term studies in dogs dosed orally at up to 20 mg kg
b.w./day, blood chemistry and histopathological changes provided
evidence of hepatoxicity. The NOEL was 3 mg/kg b.w./day. In a 1-year
study, changes in relative organ weights (liver, kidney and testes)
were observed at the dose of 30-60 mg/kg b.w./day, with a NOEL of
15 mg/kg b.w./day.
In a long-term study with rats at dose levels of 100, 300 and
900 mg/kg of feed, body weight gain was reduced, even at the lowest
dose level in females, and haematological and biochemical changes
were found at the two highest dose levels. No increase in tumour
incidence was observed.
In teratogenicity studies in rats, no malformations were
observed at doses of up to 100 mg/kg b.w./day but embryotoxicity and
maternal toxicity were observed at doses of 30 mg/kg b.w./day and
above. The NOEL was 15 mg/kg b.w./day In one of three teratogenicity
studies in rabbits, one fetus with structural malformations was
observed in the mid-dose group and one in the high dose group,
giving a NOEL of 6.25 mg/kg b.w./day.
In a two-generation reproduction study in rats, in which
carazolol was administered by gavage at doses of up to 60 mg/kg
b.w./day, an increase in post implantation losses, and a decrease in
the number of live pups in the first and second generations were
observed in both the mid- and the high-dose groups. The NOEL in this
study was 15 mg/kg b.w./day.
Carazolol gave negative results in five in vitro and in vivo
genotoxicity tests.
The ß-adrenoceptor-blocking activity of carazolol was
established in specific function tests in mice, rats, rabbits, and
dogs. A NOEL of 0.02 mg/kg b.w. for the inhibition of
isoprenaline-induced tachycardia was observed in rabbits after
administration of a single oral dose.
In a clinical trial with healthy human volunteers, the effect
on cardiac function was determined following administration of a
single oral dose of 5 or 7 mg of carazolol/person. From the
dose-response curve, a dose without effect of about 0.01 mg/kg b.w.
was extrapolated. Patients suffering from chronic bronchitis showed
a clear effect on respiratory function after a single oral dose of
0.7 mg/person and a marginal effect at a dose of 0.1 mg/person.
Based on these results, a NOEL of about 0.03 mg/person, equivalent
to 0.5 µg/kg b.w., was extrapolated, but the Committee noted that no
information was provided about the procedures used in these
extrapolations.
4. EVALUATION
The Committee noted that most of the toxicological data were
available only in summary form and that only one long-term toxicity/
carcinogenicity study was available. Since the NOEL of 3 mg/kg
b.w./day, observed in short-term toxicity studies in dogs, was
several orders of magnitude higher than the NOELs for carazolol in
pharmacological function studies, the Committee concluded that the
pharmacological effect provided a more appropriate basis for the
safety evaluation of residues. Because the information required to
extrapolate to a dose without effect was lacking in the human
studies, the Committee was not able to derive a clear NOEL for
humans that would also cover specific groups who might be at risk,
such as people suffering from cardiac or respiratory disease,
particularly asthma. A temporary ADI of 0-0.1 µg/kg b.w. was
therefore established, based on a NOEL of 0.02 mg/kg b.w. for the
inhibition of isoprenaline-induced tachycardia in rabbits and the
application of a conventional safety factor of 200 for a temporary
ADI based on animal studies. The Committee noted that, if the
pharmacological NOELs extrapolated from the human studies were used,
an ADI of a similar order of magnitude would be obtained.
5. REFERENCES
BARTSCH, W. (1974a) General pharmacology of BM 51.052. Unpublished
Reports E 2, E 4, dated 2-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1974b) Special pharmacology of BM 51.052. Unpublished
Reports D1, D2, D4, dated 3-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1977) Tolerance testing on intravenous administration
of high doses of BM 51.052 in conscious dogs. Unpublished Report No.
E 6 dated 22-11-1977 from Boehringer, Mannheim. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
BARTSCH, W. (1978) Special pharmacology of BM 51.052. Unpublished
Reports D3 and D5 dated 7-1978 from Boehringer, Mannheim. Submitted
to WHO by Praemix Wirkstoff GMBH, Mannheim.
CZERWEK, H. (1978) Akute toxizitat BM 51.052. Unpublished Report
dated 1-9-1978 from Boehringer, Abt. fur Toxicologie, Mannheim.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE (1978) Mutagenicity BM 51.052 Ames-test. Unpublished Report
dated 23-10-1978 from Department of Genetics, Boehringer Mannheim
GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE & VOLLMAR, J. (1976) Mutagenicity BM 51.052. Unpublished
Report dated 30-03-1976 from Abteilung fur Genetik, Boehringer
Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HARTIG, F. (1981) Chronic oral carcinogenicity test of BM 51.052,
2-year experiment in rats. Unpublished Report 1-12/16/8/D-E/DrH/btza
dated 25-11-1981 submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
HEBOLD, G. (1974) Effect of BM 51.052 on the body temperature and
blood sugar of conscious rabbits. Unpublished Report E 5 dated
11-2-1974 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978a) Chronic oral toxicity test of BM 51.052 12 months'
experiment in rats. Unpublished Report 9-2/07/11/D-E/DrH/btza dated
23-06-1978 from Boehringer Mannheim GmbH. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978b) Pathology and toxicology BM 51.052 1 year experiment in
dogs. Unpublished Report no. DrH/elm 8-8/31/2 dated 19-7-1978 from
Boehringer Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
HEBOLD, G. & CZERWEK, H. (1978) Fertility tests on male rats given
BM 51.052. Unpublished report dated 2-08-1978 from Boehringer
Mannheim GmbH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HUCKAUF, H. (undated) Clinical trial of carazolol to determine a
threshold dose on the bronchial system. Report from Medizinische
Klinik und Poliklinik, Klinikum Steglitz, Freie Universitat Berlin.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
KOCH, K. (1977) Metabolism of BM 51.052. Unpublished report dated
09-08-1977 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
KOCH, K. & BARTSCH, W. (1977) Investigations on the pharmacokinetics
of BM 51.052 (carazolol). Unpublished report dated 20-02-1977 from
Boehringer, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
LEUSCHNER, F. (1975) The effect of BM 51.052 (micronized), Batch no
807035 A on the pregnant rat and foetus on administration by gavage.
Unpublished report dated 18-11-1975 from Mannheim Boehringer,
laboratorium fur farmakologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
MOLLENDORF, E.v., GLOCKE, M.H. & ABSHAGEN, U. (1979) Effect kinetics
of carazolol (BM 510952). Unpublished Report M5-01-78 dated
17-10-1979 from Department of Clinical Pharmacology, Boehringer,
Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974a) Pathology and
toxicology BM 51.052 14 day experiment in rats. Unpublished Report
7-1/1-/2 dated 26-7-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974b) Pathology and
toxicology BM 51.052 14-day experiment in dogs. Unpublished Report
7-1/11/1 dated 16-8-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975a) Pathology and
toxicology BM 51.052 4 week experiment in rats. Unpublished Report
6-12/15/2 dated 2-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975b) Pathology and
toxicology BM 51.052 4 week experiment in dogs. Unpublished Report
6-12/21/1 dated 31-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976a) Pathology and
Toxicology BM 51.052 3 month experiment in rats. Unpublished Report
6-12/27/2 dated 28-1-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976b) Pathology and
toxicology BM 51.052 3 month experiment in dogs. Unpublished Report
6-12/27/1 dated 2-2-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
ROESCH, A, (1973) Investigation of the action of BM 51.052 on the
central nervous system of the rat. Unpublished Report E.1 dated
21-08-1973 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
ROESCH, A. (1978) Transport function of the intestines-mouse.
Unpublished Report E 3 dated 26-06-1978 from Boehringer, Mannheim.
Submitted to WHO by Praemix Wirkstodd GMBH, Mannheim.
SATOH, H., MIURA, Y., INUI, J. & HASEGAWA, G. (1980) ß-adrenoceptor
blocking, cardiovascular and electrophysiological effects of
carazolol. Unpublished report from research laboratories, Yoshitomi
Pharmaceutical Industry Ltd. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977a) Teratological investigations with
BM 51052 in rabbits. Unpublished Report K2A dated 18-03-1977 from
Boehringer Mannheim GMBH, teratology department. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977b) Teratological investigations with
BM 51.052 in rabbits (1st repeat and extension). Unpublished Report
K2B dated 18-03-1977 from Boehringer Mannheim GMBH, teratological
department. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977c) Teratological investigations with
BM 51052 in rabbits (2nd repeat). Unpublished Report K2C dated
18-03-1977 from Boehringer Mannheim GMBH, Teratological Department.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & VOLLMAR, J. (1978) Reproduction toxicology
investigations phase I (Fertility) in female rats using BM 51.052
p.o. Unpublished Report K4 from Department of Reproduction
Toxicology/General Biometry, Boehringer Mannheim GmbH. Submitted to
WHO by Praemix Wirkstoff GMBH, Mannheim.
2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of carazolol is given in Table 1. Deaths
shown were recorded within the first 24 hours. The animals exhibited
sedation, apathy, ventral position, slowed respiration and
convulsions shortly before death.
Table 1: The acute toxicity of carazolol
Species Route Sex LD50 (mg/kg b.w.) Reference
Mouse oral M 1321 Czerwek, 1978
F 1601
i.p. M 561
F 621
i.v. M&F 142
Rat oral M 881
F 801
i.p. M 621
F 591
i.v. M&F 10.43
Rabbit i.v. M&F 5.24
1. vehicle: tylose
2. vehicle: Dimethylformamide (DMF) in glucose
3. vehicle: trisodium citrate, citric acid and polyethylene glycol
in water
4. vehicle: DMF in NaCl
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of Sprague-Dawley rats (10/sex/group) were fed a diet
containing 0, 20, 70, or 200 mg/kg carazolol for 14 days. No
dose-related effects were observed on clinical signs, body weight,
food intake, food efficiency, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology. The NOAEL in
this study was 200 mg carazolol/kg food, equal to 18.9 and
18.3 mg/kg b.w. for males and females, respectively (Rebel et al.,
1974a).
Groups of Sprague-Dawley rats (10/sex/group) were given i.p.
injections once daily of 0, 1.0, 3.0, or 9.0 mg carazolol/kg b.w.
for 4 weeks. No treatment-related effects were observed on clinical
signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, macroscopy, or histopathology. The NOAEL in
this study was 9 mg/kg b.w. (Rebel et al., 1975a).
Groups of Sprague-Dawley rats (20/sex/group) were administered
0, 40, 120, or 400 mg carazolol/kg food (equal to 0, 2.7 and 3.2,
8.2 and 9.5, or 27.3 and 30.4 mg/kg b.w./day for males and females,
respectively) in the diet for 3 months. Rats (5/sex) of the control
and highest dose group were given a 4-week recovery period.
High-dose females exhibited a decreased body weight gain due to a
lower food intake from week 5 onwards. They had completely recovered
from this effect after 4 weeks. A significantly lower heart rate in
conscious rats was observed in all treated animals (males and
females combined), without any dose-response relationship. The
latter might be due to a (sub)maximal pharmacological dose. However,
interpretation of the results is hampered by a pre-existing
difference in initial heart rate between rats allocated to the
control and the treatment groups. No treatment-related effect was
observed on clinical signs, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology (Rebel
et al., 1976a).
Groups of Sprague-Dawley rats (30/sex/group) were fed a diet
containing 0, 150, 300, or 600-1800 mg carazolol/kg food (equal to 7
and 9, 14, and 17, or 33 - 69 and 36 - 86 mg/kg b.w./day for males
and females, respectively) for 12 months. The concentration of
carazolol in diet of the highest dose group was 600 mg/kg up to week
22, 1200 mg/kg from week 22 to week 40, and 1800 mg/kg from week 40
to the end of the experiment. Five male and 5 female rats per group
were given a 4-week recovery period at the end of the experiment.
Male as well as female rats at the highest dose group showed a rough
pelt on increasing the dose to 1200 mg/kg. High-dose rats showed a
decreased food intake (from week 40) and a decrease in body weight
gain from week 28 in males and from week 33 in females. At recovery
a higher food consumption was observed in the same dose group; in
males delayed body weight gain was reversible. In male as well as in
female rats relative heart weight showed a significant but not
dose-related increase at all dose levels. Relative kidney, ovary and
testes weights were increased in rats at the highest dose. No
treatment-related effects were observed on haematology, clinical
chemistry, urinalysis, macroscopy and microscopy (Hebold et al.,
1978a).
2.2.2.2. Dogs
Groups of beagle dogs (2/sex/group) were administered 0, 1, 3,
or 10 mg carazolol/kg b.w./day for 14 days. Observations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, heart rate, BSP retention, neurological
investigations, organ weight, macroscopy, and histopathology.
Treatment-related effects observed in high-dose females consisted of
decreased food consumption and body weight, decrease in Hb, Ht and
erythrocytes, a marked acceleration of the erythrocyte sedimentation
rate, an increase in glucose, cholesterol, AP and ALAT. At
histopathology, accumulation of mesenchyme cells was seen in one
high-dose female, the other one showed an increased activation of
Kupffer's cells. The NOAEL in this study was 3 mg/kg b.w./day (Rebel
et al., 1974b).
Groups of beagle dogs (2/sex/group) were given i.v. injections
once daily of 0, 0.5, 1.5, or 4.5 mg carazolol/kg b.w. for 4 weeks.
No treatment-related effects were observed on clinical signs, body
weight, food consumption, heart rate, neurological investigations,
haematology, clinical chemistry, urinalysis, organ weight,
macroscopy and histopathology (Rebel et al., 1975b).
Groups of beagle dogs (3/sex/group, 13 months old) were given
0, 2, 6, or 20 mg/kg b.w. carazolol in 2 daily doses for 3 months.
One male and one female dog from the control group and the high-dose
group remained on a 4-week recovery period. Observations included
clinical signs, food intake, body weight, heart rate, neurological
investigations, haematology, clinical chemistry, urinalysis, organ
weights, macroscopy and histopathology. High-dose females exhibited
an increase in erythrocyte sedimentation rate, AP and ALAT activity
and cholesterol concentration (Rebel et al., 1976b).
Groups of beagle dogs (5/sex/group) were administered 2 daily
doses of 0, 7, 15, or 30 (week 1-18) and 60 (week 18-52) mg
carazolol/kg b.w. for 12 months. Two male and 2 female dogs were
observed during a subsequent 5-week recovery period. No effects were
observed on clinical signs, food consumption, body weight, heart
rate, neurological investigations, haematology, clinical chemistry,
urinalysis, macroscopy, or microscopy. An increase in relative
weight of liver, kidney, and testes was apparent in male dogs at the
highest dose. The NOEL in this study was 15 mg/kg b.w. twice daily
(Hebold, et al., 1978b). Remark: no statistics were performed.
2.2.3 Long-term carcinogenicity study
2.2.3.1 Rats
Groups of Sprague-Dawley rats (72/sex/group) received a diet
containing 0, 100, 300, or 900 mg/kg food for 26 months.
Observations included clinical signs, food consumption, body weight,
haematology, clinical chemistry, macroscopy and microscopy. Survival
was increased in dosed rats (48/72 and 41/72, 44/72 and 47/72, 51/72
and 53/72, and 60/72 and 57/72 for males and females at 0, 100, 300,
and 900 ppm, respectively). A treatment-related significant decrease
in body weight gain was observed at 300 and 900 mg/kg in both sexes
and at 100 mg/kg food in female rats. Food consumption was
significantly decreased in high-dose male and female rats and in
females at 300 mg/kg from day 464 onwards. A decrease in neutrophils
and eosinophils (males only) was observed at 300 and 900 mg/kg food.
At the same dose levels glucose and protein levels were decreased in
both males and females. At 900 mg/kg food glucose was decreased in
females and creatinine in males. The incidence of all tumours
combined was not enhanced (Hartig, 1981). Remark: only summarized
data were available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Male rats were administered diets containing 0, 40, or
400 mg/kg food mg carazolol/kg food for 10 weeks. Rats were then
allowed to mate with untreated females. The newly-born were counted
and weighed. Body weight was slightly decreased in high-dosed males.
No treatment-related effects were observed on indices for fertility,
gestation, viability and lactation. No difference in offspring born
alive or dead, number of live fetuses, fetal weight or behaviour of
the offspring was observed. At 21 days of age the offspring were
killed and no malformations were observed (Hebold & Czerwek, 1978).
Remark: summary only available.
In a 3-generation reproduction study with a teratology phase
(see Special studies on embryotoxicity and teratogenicity, Section
2.2.5.1) female Sprague-Dawley rats (76/group) were orally
administered by gavage 0, 15, 30, or 60 mg/kg b.w./day carazolol
suspended in methyl cellulose. Administration started from 14 days
before the first mating with untreated males (for all females/group)
throughout the entire pregnancy (22-27 pregnant rats/group) until
day 21 of lactation. Eleven-12 dams/group were killed on day 21 of
pregnancy and their fetuses were delivered by caesarian section.
F1 animals (1/sex/litter) were mated and gave birth to the F2
generation. During the 14-day premating period a slight sedation was
observed from days 3-6 at the highest dose and a significantly
increased body weight as well as body weight gain were observed at
30 and 60 mg/kg b.w./day. No effects were observed on the fertility
index. At 60 mg/kg b.w./day the rearing performance of the F0
animals during the lactation period was impaired. At 60 mg/kg b.w.
post-implantation loss was significantly increased in the F0 dams
and the number of F1 pups alive at the end of the lactation period
was significantly decreased. At the highest dose a significantly
lower pup weight was observed in the F1 generation shortly after
delivery. At 30 and 60 mg/kg b.w./day post-implantation loss was
significantly increased in F1 dams and a significant decrease was
observed in the number of F2 young alive on the day after birth.
The NOAEL in this study was 15 mg carazolol/kg b.w./day (Sterz &
Vollmar, 1978).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 24 pregnant Sprague-Dawley rats were administered 0,
25, 50, or 100 mg carazolol/kg b.w/day from days 6-15 of pregnancy
by gavage. Dams were killed on gestation day 19. Observations
included clinical signs, condition of faeces, food and drinking
water consumption, and body weight. On day 19 of gestation the
females were sacrificed and the uteri and ovaries were examined.
Other organs were macroscopally examined. Fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
malformations. Maternal toxicity (ataxia and sedation) was observed
at 50 mg carazolol/kg b.w./day, and more marked at 100 mg/kg
b.w./day. At the highest dose an increase in post-implantation loss
was observed. There was no evidence of embryotoxicity or
irreversible structural effects (Leuschner, 1975).
A teratological examination was conducted during the course of
the 2-generation rat reproduction study (see Reproduction studies,
Section 2.2.4). No effects were observed on number of corpora lutea,
number of implantations, pre-implantation loss, resorptions, live
and dead fetuses, fetal weight, sex ratio, or external, visceral,
and skeletal malformations. From days 1-22 of pregnancy high-dose
dams exhibited a reduced body weight gain. Placental weight was
significantly increased at 30 and 60 mg/kg b.w./day (not
dose-related). A decrease in number of ossified cervical vertebrae
or tail vertebrae was observed in the mid- and high-dose groups. No
irreversible structural effects were observed (Sterz & Vollmar,
1978).
2.2.5.2 Rabbits
Groups of 17 pregnant Himalayan rabbits were orally
administered 0, 25, 50, or 100 mg carazolol/kg b.w/day from days
7-19 of gestation. The females were killed on gestation day 31 and
fetuses were delivered by caesarean section. During the
administration period food consumption was reduced in all groups,
significantly at 100 mg/kg b.w./day; a significant increase in food
consumption was observed in high-dose females the last 6 days of the
test. Weight gain of dams at the highest dose was significantly
increased from days 20 to 31 of pregnancy. Independent of the
dosage, 3 cases of arthrogryposis occurred in 30 litters (0, 1, 1,
and 1 at 0, 25, 50 and 100 mg/kg b.w./day, respectively). No
treatment-related effects were observed in number of corpora lutea,
implantation loss, resorptions, litter size, live and dead fetuses,
fetal weight, sex ratio, or internal and skeletal malformations
(Sterz & Glocke, 1977a).
Groups of pregnant Himalayan rabbits (13-14/group) received
orally 0, 6.26, 12.50, or 100 mg carazolol/kg b.w./day from days
7-19 of gestation. The females were killed on day 31 of gestation
and the fetuses were delivered by caesarean section. Food
consumption was decreased at the highest dose. In the mid-dose
group, one dead and one underdeveloped fetus were seen. In the
high-dose group, one dead and two underdeveloped fetuses were seen.
Placental weight was significantly decreased at the highest dose and
fetal weight was significantly and dose-relatedly decreased at 12.50
and 100 mg/kg b.w./day. One fetus with pin-head size meningocele on
the occiput was observed at 100 mg/kg b.w./day. Arthrogryposis was
observed in 0, 1, 1, and 1 fetus at 0, 6.25, 12.50, and 100 mg/kg
b.w./day, respectively. One fetus with spondylosis syndrome and one
fetus with scoliosis due to reduction and absence of parts of the
first two lumbar vertebrae were observed at the mid- and high-dose,
respectively. No treatment-related effects were observed on
fertility index, body weight, number of corpora lutea, implantation
loss, resorptions, litter size, or sex ratio (Sterz & Glocke,
1977b).
A third experiment (using the same experimental design) was
carried out in which particular attention was directed towards any
occurrence of deformities of the limbs (arthrogryposis). Twenty-nine
and 20 Himalayan pregnant rabbits were orally administered 0 and
100 mg carazolol/kg b.w./day from days 9-12 of gestation,
respectively. No treatment-related effects were observed on food
consumption or body weight (only recorded in 10 control and 4
experimental rabbits) or reproduction performance. No abnormalities
were observed after skeletal and visceral examinations, except for a
significantly increased number of extra ribs at the 1st lumbar
vertebra. The authors concluded that the observed cases of
arthrogrypsosis in the previous reported studies were in fact a
fortuitous increase in incidence of this spontaneous deformity of
the rabbits strain used (Sterz & Glocke, 1977c).
2.2.6 Special studies on pharmacology
2.2.6.1 Mice
Groups of female SPF mice fasted for 18 hours were given i.p.
doses of 0, 20, or 200 mg carazolol/kg b.w. or 200 mg propranolol/kg
b.w. Intestinal motility was significantly increased after both
carazolol and propranolol administration (Roesch, 1978). Barbiturate
sleep in groups of mice given s.c. 8, 16, 32, or 64 mg carazolol/kg
b.w. was not affected at doses < 16 mg/kg b.w.; all mice died at
64 mg/kg b.w. Induced fighting behaviour was dose-dependently
inhibited in mice given 0, 2, 4, 8 or 16 mg carazolol s.c./kg b.w.
at doses of 4-16 mg/kg. Electroshock-induced convulsions were
inhibited in 1/10 mice and 10/10 mice at 16 and 32 mg carazolol/kg
b.w., respectively. Metrazole-induced convulsions were
dose-dependently inhibited in mice given 4-32 mg carazolol/kg b.w.
In a traction test with mice the ability to cling to the wire was
blocked in 0/10, 1/10, and 10/10 mice at 8, 16, and 32 mg
carazolol/kg b.w., respectively (Kumada & Ohtsuka, 1978).
2.2.6.1 Rats
No indication of an effect on the central nervous system was
observed in male Sprague-Dawley rats given intraperitoneally
10 mg/kg b.w. carazolol (Roesch, 1973). No effects were observed on
the urine and electrolyte excretion in female SPF-Sprague-Dawley
rats after the oral or i.p. administration of 10 mg/kg b.w.
carazolol (Bartsch, 1974a).
2.2.6.3 Rabbits
1 mg carazolol/kg b.w. administered intravenously to conscious
rabbits did not cause an effect on body temperature; blood glucose
levels were slightly increased at this dose (Hebold, 1974).
Carazolol given at doses of 50 mg/kg b.w. orally, or at 5 or
10 mg/kg b.w i.v. to male and female mixed-breed rabbits inhibited
an isoprenaline-induced tachycardia; at 20 mg/kg b.w. orally no
inhibition was found. A dose of 5 mg carazolol/kg b.w. intravenously
administered to conscious rabbits inhibited isoprenaline-induced
tachycardia (Bartsch 1974b).
2.2.6.4 Dogs
No effects were observed on the urine and electrolyte excretion
in female beagle dogs given 5 mg carazolol/kg b.w. orally (Bartsch,
1974). Conscious dogs were intravenously administered 0.5, 1.0, 2.0,
or 5.0 mg carazolol/kg b.w. over a period of 15 seconds. At 1.0 and
2.0 mg/kg b.w. heart rate was increased without affecting the PQ
interval, but with slight changes in the ECG. At these doses the
behaviour of the dogs was normal. At the highest dose of 5.0 mg/kg
vomiting, tachypnoea, tachycardia and changes in the PQ-section of
the ECG were observed (Bartsch, 1977).
One, 10, or 100 mg carazolol/kg b.w. given i.v. to
anaesthetized mongrel dogs antagonized isoprenaline-induced
responses. In another experiment, 0.01, 0.1, 1.0, or 10 mg
carazolol/kg b.w. was intravenously given to anaesthetized mongrel
dogs. At doses of 1.0 mg/kg b.w. and higher, depressive effects on
the cardiovascular system which included decreases in ABP (arterial
blood pressure), LVP (left ventricular pressure) and dp/dt (cardiac
output), and an increase of IVEDP (left ventricular end diastolic
pressure) were observed (Satoh et al., 1980).
One, 4, 16, or 64 mg carazolol/kg b.w. was intravenously given
to mongrel dogs. A dose-dependent inhibition of
isoproterenol-induced tachycardia as well as isoprenaline-induced
increase of cardiac output was observed; at the highest dose an
almost complete blockade was observed. In mongrel dogs a
dose-dependent inhibition of tachycardia as well as increase in
cardiac output induced by sympathetic nerve stimulation was also
observed after the i.v. administration of 0.25 to 16 mg carazolol/kg
b.w. (Kumada & Ohtsuka, 1978).
2.2.7 Special studies on genotoxicity
Table 2: Results of genotoxicity assays on carazolol
Test system Test object Concentration Result Reference
In vitro S. typhimurium 0-5000 mg/pl1 negative Grafe, 1978
Ames test TA98, TA100 in DMF mg/pl1
TA1535, TA1537
TA1538
In vivo male NMRI single oral dose negative Grafe &
Dominant mice of 20 or 80 mg/kg b.w. Vollmar,
lethal assay or 13.5 mg/kg b.w. 1976
single i.p. dose
Dominant female NMRI single oral dose negative Grafe &
lethal assay Kisslegg mice of 50 mg/kg b.w. or Vollmar,
13.5 mg/kg b.w. single 1976
i.p. dose
Host-mediated NMRI/Kisslegg 80 mg/kg b.w. oral or negative Grafe &
assay mice with 13.6 mg/kg b.w. s.c. Vollmar,
S. typhimurium 1976
G46 S. marcescens
Cytogenicity Chinese hamster 2 x 400 mg/kg b.w. negative Grafe &
assay oral or 1 x 20 mg/kg Vollmar,
(spermatogonia b.w. i.p. or 3 x weekly 1976
as well as for 13 weeks a
bone marrow)2 treatment with 20 mg/kg
b.w. oral or 1 mg/kg
b.w. i.p.; all suspended
in CMC
1. both with and without rat liver S-9 fraction
2. only a negative control group (compound not indicated) was tested
2.3 Observations in humans
Twelve healthy male volunteers were given carazolol in a 3-way
crossover experiment at doses of 0.5 mg i.v., 5.0 mg oral or 7.5 mg
oral. Ergometric tests were carried out before and 1, 2, 3, 4, 8,
12, and 16 hours after carazolol administration. The subjects laid
down for about 20 minutes before each test. The heart rate, blood
pressure, the pressure-rate product, and the relative enteral
efficacy were determined. Heart rate was maximally inhibited by
1-2 hours after administration (15%, 13%, and 15% at 0.5 mg i.v.;
5.0 and 7.5 mg oral, respectively). During exercise the systolic
blood pressure was lowered by a maximum of 12% (oral dose) or 15%
(i.v. dose) at 1 hour. The maximal reduction of the pressure rate
product was 28%, 26%, and 25% at 0.5 mg i.v., 5.0 and 7.5 mg oral,
respectively. The mean duration of action of an oral dose of 5 mg
was estimated as 10 hours. The relative enteral efficacy calculated
by comparison of the inhibitory areas after both oral doses with
those of an i.v. dose did not differ significantly and was about
10%. By using the dose-reponse curve the authors extrapolated a
no-effect dose for the oral route of 10 mg/kg b.w. (Mollendorff
et al., 1979).
In a cross-over experiment 8 patients with chronic bronchitis
and partial reversibility of the obstruction were given a placebo or
carazolol randomly. Two patients received both 0.1 and 0.7 mg
carazolol, while the other 6 received either 0.1 or 0.7 mg. Vital
capacity (VC) and forced expiratory volume (FEV) were measured
before and 1, 2, and 3 hours after administration of the substances.
No statistics were carried out. At 0.7 mg carazolol a definite
increase in bronchial resistance was observed and dyspnoea was seen
in 3/5 patients leading to discontinuation of the test. At 0.1 mg
carazolol, the tests did not have to be discontinued in any of the
patients; only one patient showed slight dyspnoea with a decrease in
FEV . The author estimated that a dose of 0.03 mg carazolol no
longer induces any effect on the bronchial system (Huckauf,
undated).
3. COMMENTS
The Committee considered pharmacological and toxicological data
from pharmacodynamic, pharmacokinetic, metabolism, acute and
short-term toxicity, carcinogenicity, genotoxicity, reproduction and
teratology studies, as well as the results of clinical trials in
humans.
The distribution, excretion and biotransformation of
radiolabelled carazolol were studied in rats, rabbits and dogs.
After oral ingestion, carazolol was rapidly absorbed. In rats, the
radioactivity was widely distributed in the tissues, with the
highest levels in liver, kidneys, and lungs. Excretion was almost
complete within 48 hours, equally divided between the urine and
faeces. In rabbits, peak plasma levels were reached 1 hour after a
single oral dose of 10 mg/kg b.w., and about 60% of the dose was
excreted in urine. Following oral administration of carazolol in
dogs, 13% of the radioactivity was excreted in urine and 45% in
faeces within 48 hours. In the urine of a dog given 10 mg of [14C]
carazolol/kg body weight intravenously, the parent compound and six
metabolites were identified. Both carazolol and its glucuronide,
lactate, and acetate metabolites have been identified in the urine
of pigs and humans.
In a short-term study in rats, in which carazolol was
administered in the diet at levels up to 400 mg/kg of feed for 13
weeks, females exhibited a decrease in both body weight and food
intake at the highest dose. Heart rate was decreased even at the
lowest dose of 40 mg/kg of feed. When rats received carazolol in the
feed for 1 year a decrease in body weight due to decreased food
intake at the highest dose level was observed in both males and
females only after progessively increasing this dose to 1200 and
subsequently to 1800 mg/kg of feed. A statistically significant
increase in relative heart weight was observed in rats at all dose
levels, but this was not dose related.
In short-term studies in dogs dosed orally at up to 20 mg kg
b.w./day, blood chemistry and histopathological changes provided
evidence of hepatoxicity. The NOEL was 3 mg/kg b.w./day. In a 1-year
study, changes in relative organ weights (liver, kidney and testes)
were observed at the dose of 30-60 mg/kg b.w./day, with a NOEL of
15 mg/kg b.w./day.
In a long-term study with rats at dose levels of 100, 300 and
900 mg/kg of feed, body weight gain was reduced, even at the lowest
dose level in females, and haematological and biochemical changes
were found at the two highest dose levels. No increase in tumour
incidence was observed.
In teratogenicity studies in rats, no malformations were
observed at doses of up to 100 mg/kg b.w./day but embryotoxicity and
maternal toxicity were observed at doses of 30 mg/kg b.w./day and
above. The NOEL was 15 mg/kg b.w./day In one of three teratogenicity
studies in rabbits, one fetus with structural malformations was
observed in the mid-dose group and one in the high dose group,
giving a NOEL of 6.25 mg/kg b.w./day.
In a two-generation reproduction study in rats, in which
carazolol was administered by gavage at doses of up to 60 mg/kg
b.w./day, an increase in post implantation losses, and a decrease in
the number of live pups in the first and second generations were
observed in both the mid- and the high-dose groups. The NOEL in this
study was 15 mg/kg b.w./day.
Carazolol gave negative results in five in vitro and in vivo
genotoxicity tests.
The ß-adrenoceptor-blocking activity of carazolol was
established in specific function tests in mice, rats, rabbits, and
dogs. A NOEL of 0.02 mg/kg b.w. for the inhibition of
isoprenaline-induced tachycardia was observed in rabbits after
administration of a single oral dose.
In a clinical trial with healthy human volunteers, the effect
on cardiac function was determined following administration of a
single oral dose of 5 or 7 mg of carazolol/person. From the
dose-response curve, a dose without effect of about 0.01 mg/kg b.w.
was extrapolated. Patients suffering from chronic bronchitis showed
a clear effect on respiratory function after a single oral dose of
0.7 mg/person and a marginal effect at a dose of 0.1 mg/person.
Based on these results, a NOEL of about 0.03 mg/person, equivalent
to 0.5 µg/kg b.w., was extrapolated, but the Committee noted that no
information was provided about the procedures used in these
extrapolations.
4. EVALUATION
The Committee noted that most of the toxicological data were
available only in summary form and that only one long-term toxicity/
carcinogenicity study was available. Since the NOEL of 3 mg/kg
b.w./day, observed in short-term toxicity studies in dogs, was
several orders of magnitude higher than the NOELs for carazolol in
pharmacological function studies, the Committee concluded that the
pharmacological effect provided a more appropriate basis for the
safety evaluation of residues. Because the information required to
extrapolate to a dose without effect was lacking in the human
studies, the Committee was not able to derive a clear NOEL for
humans that would also cover specific groups who might be at risk,
such as people suffering from cardiac or respiratory disease,
particularly asthma. A temporary ADI of 0-0.1 µg/kg b.w. was
therefore established, based on a NOEL of 0.02 mg/kg b.w. for the
inhibition of isoprenaline-induced tachycardia in rabbits and the
application of a conventional safety factor of 200 for a temporary
ADI based on animal studies. The Committee noted that, if the
pharmacological NOELs extrapolated from the human studies were used,
an ADI of a similar order of magnitude would be obtained.
5. REFERENCES
BARTSCH, W. (1974a) General pharmacology of BM 51.052. Unpublished
Reports E 2, E 4, dated 2-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1974b) Special pharmacology of BM 51.052. Unpublished
Reports D1, D2, D4, dated 3-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1977) Tolerance testing on intravenous administration
of high doses of BM 51.052 in conscious dogs. Unpublished Report No.
E 6 dated 22-11-1977 from Boehringer, Mannheim. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
BARTSCH, W. (1978) Special pharmacology of BM 51.052. Unpublished
Reports D3 and D5 dated 7-1978 from Boehringer, Mannheim. Submitted
to WHO by Praemix Wirkstoff GMBH, Mannheim.
CZERWEK, H. (1978) Akute toxizitat BM 51.052. Unpublished Report
dated 1-9-1978 from Boehringer, Abt. fur Toxicologie, Mannheim.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE (1978) Mutagenicity BM 51.052 Ames-test. Unpublished Report
dated 23-10-1978 from Department of Genetics, Boehringer Mannheim
GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE & VOLLMAR, J. (1976) Mutagenicity BM 51.052. Unpublished
Report dated 30-03-1976 from Abteilung fur Genetik, Boehringer
Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HARTIG, F. (1981) Chronic oral carcinogenicity test of BM 51.052,
2-year experiment in rats. Unpublished Report 1-12/16/8/D-E/DrH/btza
dated 25-11-1981 submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
HEBOLD, G. (1974) Effect of BM 51.052 on the body temperature and
blood sugar of conscious rabbits. Unpublished Report E 5 dated
11-2-1974 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978a) Chronic oral toxicity test of BM 51.052 12 months'
experiment in rats. Unpublished Report 9-2/07/11/D-E/DrH/btza dated
23-06-1978 from Boehringer Mannheim GmbH. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978b) Pathology and toxicology BM 51.052 1 year experiment in
dogs. Unpublished Report no. DrH/elm 8-8/31/2 dated 19-7-1978 from
Boehringer Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
HEBOLD, G. & CZERWEK, H. (1978) Fertility tests on male rats given
BM 51.052. Unpublished report dated 2-08-1978 from Boehringer
Mannheim GmbH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HUCKAUF, H. (undated) Clinical trial of carazolol to determine a
threshold dose on the bronchial system. Report from Medizinische
Klinik und Poliklinik, Klinikum Steglitz, Freie Universitat Berlin.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
KOCH, K. (1977) Metabolism of BM 51.052. Unpublished report dated
09-08-1977 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
KOCH, K. & BARTSCH, W. (1977) Investigations on the pharmacokinetics
of BM 51.052 (carazolol). Unpublished report dated 20-02-1977 from
Boehringer, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
LEUSCHNER, F. (1975) The effect of BM 51.052 (micronized), Batch no
807035 A on the pregnant rat and foetus on administration by gavage.
Unpublished report dated 18-11-1975 from Mannheim Boehringer,
laboratorium fur farmakologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
MOLLENDORF, E.v., GLOCKE, M.H. & ABSHAGEN, U. (1979) Effect kinetics
of carazolol (BM 510952). Unpublished Report M5-01-78 dated
17-10-1979 from Department of Clinical Pharmacology, Boehringer,
Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974a) Pathology and
toxicology BM 51.052 14 day experiment in rats. Unpublished Report
7-1/1-/2 dated 26-7-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974b) Pathology and
toxicology BM 51.052 14-day experiment in dogs. Unpublished Report
7-1/11/1 dated 16-8-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975a) Pathology and
toxicology BM 51.052 4 week experiment in rats. Unpublished Report
6-12/15/2 dated 2-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975b) Pathology and
toxicology BM 51.052 4 week experiment in dogs. Unpublished Report
6-12/21/1 dated 31-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976a) Pathology and
Toxicology BM 51.052 3 month experiment in rats. Unpublished Report
6-12/27/2 dated 28-1-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976b) Pathology and
toxicology BM 51.052 3 month experiment in dogs. Unpublished Report
6-12/27/1 dated 2-2-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
ROESCH, A, (1973) Investigation of the action of BM 51.052 on the
central nervous system of the rat. Unpublished Report E.1 dated
21-08-1973 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
ROESCH, A. (1978) Transport function of the intestines-mouse.
Unpublished Report E 3 dated 26-06-1978 from Boehringer, Mannheim.
Submitted to WHO by Praemix Wirkstodd GMBH, Mannheim.
SATOH, H., MIURA, Y., INUI, J. & HASEGAWA, G. (1980) ß-adrenoceptor
blocking, cardiovascular and electrophysiological effects of
carazolol. Unpublished report from research laboratories, Yoshitomi
Pharmaceutical Industry Ltd. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977a) Teratological investigations with
BM 51052 in rabbits. Unpublished Report K2A dated 18-03-1977 from
Boehringer Mannheim GMBH, teratology department. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977b) Teratological investigations with
BM 51.052 in rabbits (1st repeat and extension). Unpublished Report
K2B dated 18-03-1977 from Boehringer Mannheim GMBH, teratological
department. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977c) Teratological investigations with
BM 51052 in rabbits (2nd repeat). Unpublished Report K2C dated
18-03-1977 from Boehringer Mannheim GMBH, Teratological Department.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & VOLLMAR, J. (1978) Reproduction toxicology
investigations phase I (Fertility) in female rats using BM 51.052
p.o. Unpublished Report K4 from Department of Reproduction
Toxicology/General Biometry, Boehringer Mannheim GmbH. Submitted to
WHO by Praemix Wirkstoff GMBH, Mannheim.
 2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of carazolol is given in Table 1. Deaths
shown were recorded within the first 24 hours. The animals exhibited
sedation, apathy, ventral position, slowed respiration and
convulsions shortly before death.
Table 1: The acute toxicity of carazolol
Species Route Sex LD50 (mg/kg b.w.) Reference
Mouse oral M 1321 Czerwek, 1978
F 1601
i.p. M 561
F 621
i.v. M&F 142
Rat oral M 881
F 801
i.p. M 621
F 591
i.v. M&F 10.43
Rabbit i.v. M&F 5.24
1. vehicle: tylose
2. vehicle: Dimethylformamide (DMF) in glucose
3. vehicle: trisodium citrate, citric acid and polyethylene glycol
in water
4. vehicle: DMF in NaCl
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of Sprague-Dawley rats (10/sex/group) were fed a diet
containing 0, 20, 70, or 200 mg/kg carazolol for 14 days. No
dose-related effects were observed on clinical signs, body weight,
food intake, food efficiency, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology. The NOAEL in
this study was 200 mg carazolol/kg food, equal to 18.9 and
18.3 mg/kg b.w. for males and females, respectively (Rebel et al.,
1974a).
Groups of Sprague-Dawley rats (10/sex/group) were given i.p.
injections once daily of 0, 1.0, 3.0, or 9.0 mg carazolol/kg b.w.
for 4 weeks. No treatment-related effects were observed on clinical
signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, macroscopy, or histopathology. The NOAEL in
this study was 9 mg/kg b.w. (Rebel et al., 1975a).
Groups of Sprague-Dawley rats (20/sex/group) were administered
0, 40, 120, or 400 mg carazolol/kg food (equal to 0, 2.7 and 3.2,
8.2 and 9.5, or 27.3 and 30.4 mg/kg b.w./day for males and females,
respectively) in the diet for 3 months. Rats (5/sex) of the control
and highest dose group were given a 4-week recovery period.
High-dose females exhibited a decreased body weight gain due to a
lower food intake from week 5 onwards. They had completely recovered
from this effect after 4 weeks. A significantly lower heart rate in
conscious rats was observed in all treated animals (males and
females combined), without any dose-response relationship. The
latter might be due to a (sub)maximal pharmacological dose. However,
interpretation of the results is hampered by a pre-existing
difference in initial heart rate between rats allocated to the
control and the treatment groups. No treatment-related effect was
observed on clinical signs, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology (Rebel
et al., 1976a).
Groups of Sprague-Dawley rats (30/sex/group) were fed a diet
containing 0, 150, 300, or 600-1800 mg carazolol/kg food (equal to 7
and 9, 14, and 17, or 33 - 69 and 36 - 86 mg/kg b.w./day for males
and females, respectively) for 12 months. The concentration of
carazolol in diet of the highest dose group was 600 mg/kg up to week
22, 1200 mg/kg from week 22 to week 40, and 1800 mg/kg from week 40
to the end of the experiment. Five male and 5 female rats per group
were given a 4-week recovery period at the end of the experiment.
Male as well as female rats at the highest dose group showed a rough
pelt on increasing the dose to 1200 mg/kg. High-dose rats showed a
decreased food intake (from week 40) and a decrease in body weight
gain from week 28 in males and from week 33 in females. At recovery
a higher food consumption was observed in the same dose group; in
males delayed body weight gain was reversible. In male as well as in
female rats relative heart weight showed a significant but not
dose-related increase at all dose levels. Relative kidney, ovary and
testes weights were increased in rats at the highest dose. No
treatment-related effects were observed on haematology, clinical
chemistry, urinalysis, macroscopy and microscopy (Hebold et al.,
1978a).
2.2.2.2. Dogs
Groups of beagle dogs (2/sex/group) were administered 0, 1, 3,
or 10 mg carazolol/kg b.w./day for 14 days. Observations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, heart rate, BSP retention, neurological
investigations, organ weight, macroscopy, and histopathology.
Treatment-related effects observed in high-dose females consisted of
decreased food consumption and body weight, decrease in Hb, Ht and
erythrocytes, a marked acceleration of the erythrocyte sedimentation
rate, an increase in glucose, cholesterol, AP and ALAT. At
histopathology, accumulation of mesenchyme cells was seen in one
high-dose female, the other one showed an increased activation of
Kupffer's cells. The NOAEL in this study was 3 mg/kg b.w./day (Rebel
et al., 1974b).
Groups of beagle dogs (2/sex/group) were given i.v. injections
once daily of 0, 0.5, 1.5, or 4.5 mg carazolol/kg b.w. for 4 weeks.
No treatment-related effects were observed on clinical signs, body
weight, food consumption, heart rate, neurological investigations,
haematology, clinical chemistry, urinalysis, organ weight,
macroscopy and histopathology (Rebel et al., 1975b).
Groups of beagle dogs (3/sex/group, 13 months old) were given
0, 2, 6, or 20 mg/kg b.w. carazolol in 2 daily doses for 3 months.
One male and one female dog from the control group and the high-dose
group remained on a 4-week recovery period. Observations included
clinical signs, food intake, body weight, heart rate, neurological
investigations, haematology, clinical chemistry, urinalysis, organ
weights, macroscopy and histopathology. High-dose females exhibited
an increase in erythrocyte sedimentation rate, AP and ALAT activity
and cholesterol concentration (Rebel et al., 1976b).
Groups of beagle dogs (5/sex/group) were administered 2 daily
doses of 0, 7, 15, or 30 (week 1-18) and 60 (week 18-52) mg
carazolol/kg b.w. for 12 months. Two male and 2 female dogs were
observed during a subsequent 5-week recovery period. No effects were
observed on clinical signs, food consumption, body weight, heart
rate, neurological investigations, haematology, clinical chemistry,
urinalysis, macroscopy, or microscopy. An increase in relative
weight of liver, kidney, and testes was apparent in male dogs at the
highest dose. The NOEL in this study was 15 mg/kg b.w. twice daily
(Hebold, et al., 1978b). Remark: no statistics were performed.
2.2.3 Long-term carcinogenicity study
2.2.3.1 Rats
Groups of Sprague-Dawley rats (72/sex/group) received a diet
containing 0, 100, 300, or 900 mg/kg food for 26 months.
Observations included clinical signs, food consumption, body weight,
haematology, clinical chemistry, macroscopy and microscopy. Survival
was increased in dosed rats (48/72 and 41/72, 44/72 and 47/72, 51/72
and 53/72, and 60/72 and 57/72 for males and females at 0, 100, 300,
and 900 ppm, respectively). A treatment-related significant decrease
in body weight gain was observed at 300 and 900 mg/kg in both sexes
and at 100 mg/kg food in female rats. Food consumption was
significantly decreased in high-dose male and female rats and in
females at 300 mg/kg from day 464 onwards. A decrease in neutrophils
and eosinophils (males only) was observed at 300 and 900 mg/kg food.
At the same dose levels glucose and protein levels were decreased in
both males and females. At 900 mg/kg food glucose was decreased in
females and creatinine in males. The incidence of all tumours
combined was not enhanced (Hartig, 1981). Remark: only summarized
data were available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Male rats were administered diets containing 0, 40, or
400 mg/kg food mg carazolol/kg food for 10 weeks. Rats were then
allowed to mate with untreated females. The newly-born were counted
and weighed. Body weight was slightly decreased in high-dosed males.
No treatment-related effects were observed on indices for fertility,
gestation, viability and lactation. No difference in offspring born
alive or dead, number of live fetuses, fetal weight or behaviour of
the offspring was observed. At 21 days of age the offspring were
killed and no malformations were observed (Hebold & Czerwek, 1978).
Remark: summary only available.
In a 3-generation reproduction study with a teratology phase
(see Special studies on embryotoxicity and teratogenicity, Section
2.2.5.1) female Sprague-Dawley rats (76/group) were orally
administered by gavage 0, 15, 30, or 60 mg/kg b.w./day carazolol
suspended in methyl cellulose. Administration started from 14 days
before the first mating with untreated males (for all females/group)
throughout the entire pregnancy (22-27 pregnant rats/group) until
day 21 of lactation. Eleven-12 dams/group were killed on day 21 of
pregnancy and their fetuses were delivered by caesarian section.
F1 animals (1/sex/litter) were mated and gave birth to the F2
generation. During the 14-day premating period a slight sedation was
observed from days 3-6 at the highest dose and a significantly
increased body weight as well as body weight gain were observed at
30 and 60 mg/kg b.w./day. No effects were observed on the fertility
index. At 60 mg/kg b.w./day the rearing performance of the F0
animals during the lactation period was impaired. At 60 mg/kg b.w.
post-implantation loss was significantly increased in the F0 dams
and the number of F1 pups alive at the end of the lactation period
was significantly decreased. At the highest dose a significantly
lower pup weight was observed in the F1 generation shortly after
delivery. At 30 and 60 mg/kg b.w./day post-implantation loss was
significantly increased in F1 dams and a significant decrease was
observed in the number of F2 young alive on the day after birth.
The NOAEL in this study was 15 mg carazolol/kg b.w./day (Sterz &
Vollmar, 1978).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 24 pregnant Sprague-Dawley rats were administered 0,
25, 50, or 100 mg carazolol/kg b.w/day from days 6-15 of pregnancy
by gavage. Dams were killed on gestation day 19. Observations
included clinical signs, condition of faeces, food and drinking
water consumption, and body weight. On day 19 of gestation the
females were sacrificed and the uteri and ovaries were examined.
Other organs were macroscopally examined. Fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
malformations. Maternal toxicity (ataxia and sedation) was observed
at 50 mg carazolol/kg b.w./day, and more marked at 100 mg/kg
b.w./day. At the highest dose an increase in post-implantation loss
was observed. There was no evidence of embryotoxicity or
irreversible structural effects (Leuschner, 1975).
A teratological examination was conducted during the course of
the 2-generation rat reproduction study (see Reproduction studies,
Section 2.2.4). No effects were observed on number of corpora lutea,
number of implantations, pre-implantation loss, resorptions, live
and dead fetuses, fetal weight, sex ratio, or external, visceral,
and skeletal malformations. From days 1-22 of pregnancy high-dose
dams exhibited a reduced body weight gain. Placental weight was
significantly increased at 30 and 60 mg/kg b.w./day (not
dose-related). A decrease in number of ossified cervical vertebrae
or tail vertebrae was observed in the mid- and high-dose groups. No
irreversible structural effects were observed (Sterz & Vollmar,
1978).
2.2.5.2 Rabbits
Groups of 17 pregnant Himalayan rabbits were orally
administered 0, 25, 50, or 100 mg carazolol/kg b.w/day from days
7-19 of gestation. The females were killed on gestation day 31 and
fetuses were delivered by caesarean section. During the
administration period food consumption was reduced in all groups,
significantly at 100 mg/kg b.w./day; a significant increase in food
consumption was observed in high-dose females the last 6 days of the
test. Weight gain of dams at the highest dose was significantly
increased from days 20 to 31 of pregnancy. Independent of the
dosage, 3 cases of arthrogryposis occurred in 30 litters (0, 1, 1,
and 1 at 0, 25, 50 and 100 mg/kg b.w./day, respectively). No
treatment-related effects were observed in number of corpora lutea,
implantation loss, resorptions, litter size, live and dead fetuses,
fetal weight, sex ratio, or internal and skeletal malformations
(Sterz & Glocke, 1977a).
Groups of pregnant Himalayan rabbits (13-14/group) received
orally 0, 6.26, 12.50, or 100 mg carazolol/kg b.w./day from days
7-19 of gestation. The females were killed on day 31 of gestation
and the fetuses were delivered by caesarean section. Food
consumption was decreased at the highest dose. In the mid-dose
group, one dead and one underdeveloped fetus were seen. In the
high-dose group, one dead and two underdeveloped fetuses were seen.
Placental weight was significantly decreased at the highest dose and
fetal weight was significantly and dose-relatedly decreased at 12.50
and 100 mg/kg b.w./day. One fetus with pin-head size meningocele on
the occiput was observed at 100 mg/kg b.w./day. Arthrogryposis was
observed in 0, 1, 1, and 1 fetus at 0, 6.25, 12.50, and 100 mg/kg
b.w./day, respectively. One fetus with spondylosis syndrome and one
fetus with scoliosis due to reduction and absence of parts of the
first two lumbar vertebrae were observed at the mid- and high-dose,
respectively. No treatment-related effects were observed on
fertility index, body weight, number of corpora lutea, implantation
loss, resorptions, litter size, or sex ratio (Sterz & Glocke,
1977b).
A third experiment (using the same experimental design) was
carried out in which particular attention was directed towards any
occurrence of deformities of the limbs (arthrogryposis). Twenty-nine
and 20 Himalayan pregnant rabbits were orally administered 0 and
100 mg carazolol/kg b.w./day from days 9-12 of gestation,
respectively. No treatment-related effects were observed on food
consumption or body weight (only recorded in 10 control and 4
experimental rabbits) or reproduction performance. No abnormalities
were observed after skeletal and visceral examinations, except for a
significantly increased number of extra ribs at the 1st lumbar
vertebra. The authors concluded that the observed cases of
arthrogrypsosis in the previous reported studies were in fact a
fortuitous increase in incidence of this spontaneous deformity of
the rabbits strain used (Sterz & Glocke, 1977c).
2.2.6 Special studies on pharmacology
2.2.6.1 Mice
Groups of female SPF mice fasted for 18 hours were given i.p.
doses of 0, 20, or 200 mg carazolol/kg b.w. or 200 mg propranolol/kg
b.w. Intestinal motility was significantly increased after both
carazolol and propranolol administration (Roesch, 1978). Barbiturate
sleep in groups of mice given s.c. 8, 16, 32, or 64 mg carazolol/kg
b.w. was not affected at doses < 16 mg/kg b.w.; all mice died at
64 mg/kg b.w. Induced fighting behaviour was dose-dependently
inhibited in mice given 0, 2, 4, 8 or 16 mg carazolol s.c./kg b.w.
at doses of 4-16 mg/kg. Electroshock-induced convulsions were
inhibited in 1/10 mice and 10/10 mice at 16 and 32 mg carazolol/kg
b.w., respectively. Metrazole-induced convulsions were
dose-dependently inhibited in mice given 4-32 mg carazolol/kg b.w.
In a traction test with mice the ability to cling to the wire was
blocked in 0/10, 1/10, and 10/10 mice at 8, 16, and 32 mg
carazolol/kg b.w., respectively (Kumada & Ohtsuka, 1978).
2.2.6.1 Rats
No indication of an effect on the central nervous system was
observed in male Sprague-Dawley rats given intraperitoneally
10 mg/kg b.w. carazolol (Roesch, 1973). No effects were observed on
the urine and electrolyte excretion in female SPF-Sprague-Dawley
rats after the oral or i.p. administration of 10 mg/kg b.w.
carazolol (Bartsch, 1974a).
2.2.6.3 Rabbits
1 mg carazolol/kg b.w. administered intravenously to conscious
rabbits did not cause an effect on body temperature; blood glucose
levels were slightly increased at this dose (Hebold, 1974).
Carazolol given at doses of 50 mg/kg b.w. orally, or at 5 or
10 mg/kg b.w i.v. to male and female mixed-breed rabbits inhibited
an isoprenaline-induced tachycardia; at 20 mg/kg b.w. orally no
inhibition was found. A dose of 5 mg carazolol/kg b.w. intravenously
administered to conscious rabbits inhibited isoprenaline-induced
tachycardia (Bartsch 1974b).
2.2.6.4 Dogs
No effects were observed on the urine and electrolyte excretion
in female beagle dogs given 5 mg carazolol/kg b.w. orally (Bartsch,
1974). Conscious dogs were intravenously administered 0.5, 1.0, 2.0,
or 5.0 mg carazolol/kg b.w. over a period of 15 seconds. At 1.0 and
2.0 mg/kg b.w. heart rate was increased without affecting the PQ
interval, but with slight changes in the ECG. At these doses the
behaviour of the dogs was normal. At the highest dose of 5.0 mg/kg
vomiting, tachypnoea, tachycardia and changes in the PQ-section of
the ECG were observed (Bartsch, 1977).
One, 10, or 100 mg carazolol/kg b.w. given i.v. to
anaesthetized mongrel dogs antagonized isoprenaline-induced
responses. In another experiment, 0.01, 0.1, 1.0, or 10 mg
carazolol/kg b.w. was intravenously given to anaesthetized mongrel
dogs. At doses of 1.0 mg/kg b.w. and higher, depressive effects on
the cardiovascular system which included decreases in ABP (arterial
blood pressure), LVP (left ventricular pressure) and dp/dt (cardiac
output), and an increase of IVEDP (left ventricular end diastolic
pressure) were observed (Satoh et al., 1980).
One, 4, 16, or 64 mg carazolol/kg b.w. was intravenously given
to mongrel dogs. A dose-dependent inhibition of
isoproterenol-induced tachycardia as well as isoprenaline-induced
increase of cardiac output was observed; at the highest dose an
almost complete blockade was observed. In mongrel dogs a
dose-dependent inhibition of tachycardia as well as increase in
cardiac output induced by sympathetic nerve stimulation was also
observed after the i.v. administration of 0.25 to 16 mg carazolol/kg
b.w. (Kumada & Ohtsuka, 1978).
2.2.7 Special studies on genotoxicity
Table 2: Results of genotoxicity assays on carazolol
Test system Test object Concentration Result Reference
In vitro S. typhimurium 0-5000 mg/pl1 negative Grafe, 1978
Ames test TA98, TA100 in DMF mg/pl1
TA1535, TA1537
TA1538
In vivo male NMRI single oral dose negative Grafe &
Dominant mice of 20 or 80 mg/kg b.w. Vollmar,
lethal assay or 13.5 mg/kg b.w. 1976
single i.p. dose
Dominant female NMRI single oral dose negative Grafe &
lethal assay Kisslegg mice of 50 mg/kg b.w. or Vollmar,
13.5 mg/kg b.w. single 1976
i.p. dose
Host-mediated NMRI/Kisslegg 80 mg/kg b.w. oral or negative Grafe &
assay mice with 13.6 mg/kg b.w. s.c. Vollmar,
S. typhimurium 1976
G46 S. marcescens
Cytogenicity Chinese hamster 2 x 400 mg/kg b.w. negative Grafe &
assay oral or 1 x 20 mg/kg Vollmar,
(spermatogonia b.w. i.p. or 3 x weekly 1976
as well as for 13 weeks a
bone marrow)2 treatment with 20 mg/kg
b.w. oral or 1 mg/kg
b.w. i.p.; all suspended
in CMC
1. both with and without rat liver S-9 fraction
2. only a negative control group (compound not indicated) was tested
2.3 Observations in humans
Twelve healthy male volunteers were given carazolol in a 3-way
crossover experiment at doses of 0.5 mg i.v., 5.0 mg oral or 7.5 mg
oral. Ergometric tests were carried out before and 1, 2, 3, 4, 8,
12, and 16 hours after carazolol administration. The subjects laid
down for about 20 minutes before each test. The heart rate, blood
pressure, the pressure-rate product, and the relative enteral
efficacy were determined. Heart rate was maximally inhibited by
1-2 hours after administration (15%, 13%, and 15% at 0.5 mg i.v.;
5.0 and 7.5 mg oral, respectively). During exercise the systolic
blood pressure was lowered by a maximum of 12% (oral dose) or 15%
(i.v. dose) at 1 hour. The maximal reduction of the pressure rate
product was 28%, 26%, and 25% at 0.5 mg i.v., 5.0 and 7.5 mg oral,
respectively. The mean duration of action of an oral dose of 5 mg
was estimated as 10 hours. The relative enteral efficacy calculated
by comparison of the inhibitory areas after both oral doses with
those of an i.v. dose did not differ significantly and was about
10%. By using the dose-reponse curve the authors extrapolated a
no-effect dose for the oral route of 10 mg/kg b.w. (Mollendorff
et al., 1979).
In a cross-over experiment 8 patients with chronic bronchitis
and partial reversibility of the obstruction were given a placebo or
carazolol randomly. Two patients received both 0.1 and 0.7 mg
carazolol, while the other 6 received either 0.1 or 0.7 mg. Vital
capacity (VC) and forced expiratory volume (FEV) were measured
before and 1, 2, and 3 hours after administration of the substances.
No statistics were carried out. At 0.7 mg carazolol a definite
increase in bronchial resistance was observed and dyspnoea was seen
in 3/5 patients leading to discontinuation of the test. At 0.1 mg
carazolol, the tests did not have to be discontinued in any of the
patients; only one patient showed slight dyspnoea with a decrease in
FEV . The author estimated that a dose of 0.03 mg carazolol no
longer induces any effect on the bronchial system (Huckauf,
undated).
3. COMMENTS
The Committee considered pharmacological and toxicological data
from pharmacodynamic, pharmacokinetic, metabolism, acute and
short-term toxicity, carcinogenicity, genotoxicity, reproduction and
teratology studies, as well as the results of clinical trials in
humans.
The distribution, excretion and biotransformation of
radiolabelled carazolol were studied in rats, rabbits and dogs.
After oral ingestion, carazolol was rapidly absorbed. In rats, the
radioactivity was widely distributed in the tissues, with the
highest levels in liver, kidneys, and lungs. Excretion was almost
complete within 48 hours, equally divided between the urine and
faeces. In rabbits, peak plasma levels were reached 1 hour after a
single oral dose of 10 mg/kg b.w., and about 60% of the dose was
excreted in urine. Following oral administration of carazolol in
dogs, 13% of the radioactivity was excreted in urine and 45% in
faeces within 48 hours. In the urine of a dog given 10 mg of [14C]
carazolol/kg body weight intravenously, the parent compound and six
metabolites were identified. Both carazolol and its glucuronide,
lactate, and acetate metabolites have been identified in the urine
of pigs and humans.
In a short-term study in rats, in which carazolol was
administered in the diet at levels up to 400 mg/kg of feed for 13
weeks, females exhibited a decrease in both body weight and food
intake at the highest dose. Heart rate was decreased even at the
lowest dose of 40 mg/kg of feed. When rats received carazolol in the
feed for 1 year a decrease in body weight due to decreased food
intake at the highest dose level was observed in both males and
females only after progessively increasing this dose to 1200 and
subsequently to 1800 mg/kg of feed. A statistically significant
increase in relative heart weight was observed in rats at all dose
levels, but this was not dose related.
In short-term studies in dogs dosed orally at up to 20 mg kg
b.w./day, blood chemistry and histopathological changes provided
evidence of hepatoxicity. The NOEL was 3 mg/kg b.w./day. In a 1-year
study, changes in relative organ weights (liver, kidney and testes)
were observed at the dose of 30-60 mg/kg b.w./day, with a NOEL of
15 mg/kg b.w./day.
In a long-term study with rats at dose levels of 100, 300 and
900 mg/kg of feed, body weight gain was reduced, even at the lowest
dose level in females, and haematological and biochemical changes
were found at the two highest dose levels. No increase in tumour
incidence was observed.
In teratogenicity studies in rats, no malformations were
observed at doses of up to 100 mg/kg b.w./day but embryotoxicity and
maternal toxicity were observed at doses of 30 mg/kg b.w./day and
above. The NOEL was 15 mg/kg b.w./day In one of three teratogenicity
studies in rabbits, one fetus with structural malformations was
observed in the mid-dose group and one in the high dose group,
giving a NOEL of 6.25 mg/kg b.w./day.
In a two-generation reproduction study in rats, in which
carazolol was administered by gavage at doses of up to 60 mg/kg
b.w./day, an increase in post implantation losses, and a decrease in
the number of live pups in the first and second generations were
observed in both the mid- and the high-dose groups. The NOEL in this
study was 15 mg/kg b.w./day.
Carazolol gave negative results in five in vitro and in vivo
genotoxicity tests.
The ß-adrenoceptor-blocking activity of carazolol was
established in specific function tests in mice, rats, rabbits, and
dogs. A NOEL of 0.02 mg/kg b.w. for the inhibition of
isoprenaline-induced tachycardia was observed in rabbits after
administration of a single oral dose.
In a clinical trial with healthy human volunteers, the effect
on cardiac function was determined following administration of a
single oral dose of 5 or 7 mg of carazolol/person. From the
dose-response curve, a dose without effect of about 0.01 mg/kg b.w.
was extrapolated. Patients suffering from chronic bronchitis showed
a clear effect on respiratory function after a single oral dose of
0.7 mg/person and a marginal effect at a dose of 0.1 mg/person.
Based on these results, a NOEL of about 0.03 mg/person, equivalent
to 0.5 µg/kg b.w., was extrapolated, but the Committee noted that no
information was provided about the procedures used in these
extrapolations.
4. EVALUATION
The Committee noted that most of the toxicological data were
available only in summary form and that only one long-term toxicity/
carcinogenicity study was available. Since the NOEL of 3 mg/kg
b.w./day, observed in short-term toxicity studies in dogs, was
several orders of magnitude higher than the NOELs for carazolol in
pharmacological function studies, the Committee concluded that the
pharmacological effect provided a more appropriate basis for the
safety evaluation of residues. Because the information required to
extrapolate to a dose without effect was lacking in the human
studies, the Committee was not able to derive a clear NOEL for
humans that would also cover specific groups who might be at risk,
such as people suffering from cardiac or respiratory disease,
particularly asthma. A temporary ADI of 0-0.1 µg/kg b.w. was
therefore established, based on a NOEL of 0.02 mg/kg b.w. for the
inhibition of isoprenaline-induced tachycardia in rabbits and the
application of a conventional safety factor of 200 for a temporary
ADI based on animal studies. The Committee noted that, if the
pharmacological NOELs extrapolated from the human studies were used,
an ADI of a similar order of magnitude would be obtained.
5. REFERENCES
BARTSCH, W. (1974a) General pharmacology of BM 51.052. Unpublished
Reports E 2, E 4, dated 2-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1974b) Special pharmacology of BM 51.052. Unpublished
Reports D1, D2, D4, dated 3-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1977) Tolerance testing on intravenous administration
of high doses of BM 51.052 in conscious dogs. Unpublished Report No.
E 6 dated 22-11-1977 from Boehringer, Mannheim. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
BARTSCH, W. (1978) Special pharmacology of BM 51.052. Unpublished
Reports D3 and D5 dated 7-1978 from Boehringer, Mannheim. Submitted
to WHO by Praemix Wirkstoff GMBH, Mannheim.
CZERWEK, H. (1978) Akute toxizitat BM 51.052. Unpublished Report
dated 1-9-1978 from Boehringer, Abt. fur Toxicologie, Mannheim.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE (1978) Mutagenicity BM 51.052 Ames-test. Unpublished Report
dated 23-10-1978 from Department of Genetics, Boehringer Mannheim
GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE & VOLLMAR, J. (1976) Mutagenicity BM 51.052. Unpublished
Report dated 30-03-1976 from Abteilung fur Genetik, Boehringer
Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HARTIG, F. (1981) Chronic oral carcinogenicity test of BM 51.052,
2-year experiment in rats. Unpublished Report 1-12/16/8/D-E/DrH/btza
dated 25-11-1981 submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
HEBOLD, G. (1974) Effect of BM 51.052 on the body temperature and
blood sugar of conscious rabbits. Unpublished Report E 5 dated
11-2-1974 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978a) Chronic oral toxicity test of BM 51.052 12 months'
experiment in rats. Unpublished Report 9-2/07/11/D-E/DrH/btza dated
23-06-1978 from Boehringer Mannheim GmbH. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978b) Pathology and toxicology BM 51.052 1 year experiment in
dogs. Unpublished Report no. DrH/elm 8-8/31/2 dated 19-7-1978 from
Boehringer Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
HEBOLD, G. & CZERWEK, H. (1978) Fertility tests on male rats given
BM 51.052. Unpublished report dated 2-08-1978 from Boehringer
Mannheim GmbH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HUCKAUF, H. (undated) Clinical trial of carazolol to determine a
threshold dose on the bronchial system. Report from Medizinische
Klinik und Poliklinik, Klinikum Steglitz, Freie Universitat Berlin.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
KOCH, K. (1977) Metabolism of BM 51.052. Unpublished report dated
09-08-1977 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
KOCH, K. & BARTSCH, W. (1977) Investigations on the pharmacokinetics
of BM 51.052 (carazolol). Unpublished report dated 20-02-1977 from
Boehringer, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
LEUSCHNER, F. (1975) The effect of BM 51.052 (micronized), Batch no
807035 A on the pregnant rat and foetus on administration by gavage.
Unpublished report dated 18-11-1975 from Mannheim Boehringer,
laboratorium fur farmakologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
MOLLENDORF, E.v., GLOCKE, M.H. & ABSHAGEN, U. (1979) Effect kinetics
of carazolol (BM 510952). Unpublished Report M5-01-78 dated
17-10-1979 from Department of Clinical Pharmacology, Boehringer,
Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974a) Pathology and
toxicology BM 51.052 14 day experiment in rats. Unpublished Report
7-1/1-/2 dated 26-7-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974b) Pathology and
toxicology BM 51.052 14-day experiment in dogs. Unpublished Report
7-1/11/1 dated 16-8-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975a) Pathology and
toxicology BM 51.052 4 week experiment in rats. Unpublished Report
6-12/15/2 dated 2-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975b) Pathology and
toxicology BM 51.052 4 week experiment in dogs. Unpublished Report
6-12/21/1 dated 31-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976a) Pathology and
Toxicology BM 51.052 3 month experiment in rats. Unpublished Report
6-12/27/2 dated 28-1-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976b) Pathology and
toxicology BM 51.052 3 month experiment in dogs. Unpublished Report
6-12/27/1 dated 2-2-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
ROESCH, A, (1973) Investigation of the action of BM 51.052 on the
central nervous system of the rat. Unpublished Report E.1 dated
21-08-1973 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
ROESCH, A. (1978) Transport function of the intestines-mouse.
Unpublished Report E 3 dated 26-06-1978 from Boehringer, Mannheim.
Submitted to WHO by Praemix Wirkstodd GMBH, Mannheim.
SATOH, H., MIURA, Y., INUI, J. & HASEGAWA, G. (1980) ß-adrenoceptor
blocking, cardiovascular and electrophysiological effects of
carazolol. Unpublished report from research laboratories, Yoshitomi
Pharmaceutical Industry Ltd. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977a) Teratological investigations with
BM 51052 in rabbits. Unpublished Report K2A dated 18-03-1977 from
Boehringer Mannheim GMBH, teratology department. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977b) Teratological investigations with
BM 51.052 in rabbits (1st repeat and extension). Unpublished Report
K2B dated 18-03-1977 from Boehringer Mannheim GMBH, teratological
department. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977c) Teratological investigations with
BM 51052 in rabbits (2nd repeat). Unpublished Report K2C dated
18-03-1977 from Boehringer Mannheim GMBH, Teratological Department.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & VOLLMAR, J. (1978) Reproduction toxicology
investigations phase I (Fertility) in female rats using BM 51.052
p.o. Unpublished Report K4 from Department of Reproduction
Toxicology/General Biometry, Boehringer Mannheim GmbH. Submitted to
WHO by Praemix Wirkstoff GMBH, Mannheim.
2.2 Toxicological studies
2.2.1 Acute toxicity
The acute toxicity of carazolol is given in Table 1. Deaths
shown were recorded within the first 24 hours. The animals exhibited
sedation, apathy, ventral position, slowed respiration and
convulsions shortly before death.
Table 1: The acute toxicity of carazolol
Species Route Sex LD50 (mg/kg b.w.) Reference
Mouse oral M 1321 Czerwek, 1978
F 1601
i.p. M 561
F 621
i.v. M&F 142
Rat oral M 881
F 801
i.p. M 621
F 591
i.v. M&F 10.43
Rabbit i.v. M&F 5.24
1. vehicle: tylose
2. vehicle: Dimethylformamide (DMF) in glucose
3. vehicle: trisodium citrate, citric acid and polyethylene glycol
in water
4. vehicle: DMF in NaCl
2.2.2 Short-term studies
2.2.2.1 Rats
Groups of Sprague-Dawley rats (10/sex/group) were fed a diet
containing 0, 20, 70, or 200 mg/kg carazolol for 14 days. No
dose-related effects were observed on clinical signs, body weight,
food intake, food efficiency, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology. The NOAEL in
this study was 200 mg carazolol/kg food, equal to 18.9 and
18.3 mg/kg b.w. for males and females, respectively (Rebel et al.,
1974a).
Groups of Sprague-Dawley rats (10/sex/group) were given i.p.
injections once daily of 0, 1.0, 3.0, or 9.0 mg carazolol/kg b.w.
for 4 weeks. No treatment-related effects were observed on clinical
signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, macroscopy, or histopathology. The NOAEL in
this study was 9 mg/kg b.w. (Rebel et al., 1975a).
Groups of Sprague-Dawley rats (20/sex/group) were administered
0, 40, 120, or 400 mg carazolol/kg food (equal to 0, 2.7 and 3.2,
8.2 and 9.5, or 27.3 and 30.4 mg/kg b.w./day for males and females,
respectively) in the diet for 3 months. Rats (5/sex) of the control
and highest dose group were given a 4-week recovery period.
High-dose females exhibited a decreased body weight gain due to a
lower food intake from week 5 onwards. They had completely recovered
from this effect after 4 weeks. A significantly lower heart rate in
conscious rats was observed in all treated animals (males and
females combined), without any dose-response relationship. The
latter might be due to a (sub)maximal pharmacological dose. However,
interpretation of the results is hampered by a pre-existing
difference in initial heart rate between rats allocated to the
control and the treatment groups. No treatment-related effect was
observed on clinical signs, haematology, clinical chemistry,
urinalysis, organ weight, macroscopy or histopathology (Rebel
et al., 1976a).
Groups of Sprague-Dawley rats (30/sex/group) were fed a diet
containing 0, 150, 300, or 600-1800 mg carazolol/kg food (equal to 7
and 9, 14, and 17, or 33 - 69 and 36 - 86 mg/kg b.w./day for males
and females, respectively) for 12 months. The concentration of
carazolol in diet of the highest dose group was 600 mg/kg up to week
22, 1200 mg/kg from week 22 to week 40, and 1800 mg/kg from week 40
to the end of the experiment. Five male and 5 female rats per group
were given a 4-week recovery period at the end of the experiment.
Male as well as female rats at the highest dose group showed a rough
pelt on increasing the dose to 1200 mg/kg. High-dose rats showed a
decreased food intake (from week 40) and a decrease in body weight
gain from week 28 in males and from week 33 in females. At recovery
a higher food consumption was observed in the same dose group; in
males delayed body weight gain was reversible. In male as well as in
female rats relative heart weight showed a significant but not
dose-related increase at all dose levels. Relative kidney, ovary and
testes weights were increased in rats at the highest dose. No
treatment-related effects were observed on haematology, clinical
chemistry, urinalysis, macroscopy and microscopy (Hebold et al.,
1978a).
2.2.2.2. Dogs
Groups of beagle dogs (2/sex/group) were administered 0, 1, 3,
or 10 mg carazolol/kg b.w./day for 14 days. Observations included
clinical signs, body weight, food consumption, haematology, clinical
chemistry, urinalysis, heart rate, BSP retention, neurological
investigations, organ weight, macroscopy, and histopathology.
Treatment-related effects observed in high-dose females consisted of
decreased food consumption and body weight, decrease in Hb, Ht and
erythrocytes, a marked acceleration of the erythrocyte sedimentation
rate, an increase in glucose, cholesterol, AP and ALAT. At
histopathology, accumulation of mesenchyme cells was seen in one
high-dose female, the other one showed an increased activation of
Kupffer's cells. The NOAEL in this study was 3 mg/kg b.w./day (Rebel
et al., 1974b).
Groups of beagle dogs (2/sex/group) were given i.v. injections
once daily of 0, 0.5, 1.5, or 4.5 mg carazolol/kg b.w. for 4 weeks.
No treatment-related effects were observed on clinical signs, body
weight, food consumption, heart rate, neurological investigations,
haematology, clinical chemistry, urinalysis, organ weight,
macroscopy and histopathology (Rebel et al., 1975b).
Groups of beagle dogs (3/sex/group, 13 months old) were given
0, 2, 6, or 20 mg/kg b.w. carazolol in 2 daily doses for 3 months.
One male and one female dog from the control group and the high-dose
group remained on a 4-week recovery period. Observations included
clinical signs, food intake, body weight, heart rate, neurological
investigations, haematology, clinical chemistry, urinalysis, organ
weights, macroscopy and histopathology. High-dose females exhibited
an increase in erythrocyte sedimentation rate, AP and ALAT activity
and cholesterol concentration (Rebel et al., 1976b).
Groups of beagle dogs (5/sex/group) were administered 2 daily
doses of 0, 7, 15, or 30 (week 1-18) and 60 (week 18-52) mg
carazolol/kg b.w. for 12 months. Two male and 2 female dogs were
observed during a subsequent 5-week recovery period. No effects were
observed on clinical signs, food consumption, body weight, heart
rate, neurological investigations, haematology, clinical chemistry,
urinalysis, macroscopy, or microscopy. An increase in relative
weight of liver, kidney, and testes was apparent in male dogs at the
highest dose. The NOEL in this study was 15 mg/kg b.w. twice daily
(Hebold, et al., 1978b). Remark: no statistics were performed.
2.2.3 Long-term carcinogenicity study
2.2.3.1 Rats
Groups of Sprague-Dawley rats (72/sex/group) received a diet
containing 0, 100, 300, or 900 mg/kg food for 26 months.
Observations included clinical signs, food consumption, body weight,
haematology, clinical chemistry, macroscopy and microscopy. Survival
was increased in dosed rats (48/72 and 41/72, 44/72 and 47/72, 51/72
and 53/72, and 60/72 and 57/72 for males and females at 0, 100, 300,
and 900 ppm, respectively). A treatment-related significant decrease
in body weight gain was observed at 300 and 900 mg/kg in both sexes
and at 100 mg/kg food in female rats. Food consumption was
significantly decreased in high-dose male and female rats and in
females at 300 mg/kg from day 464 onwards. A decrease in neutrophils
and eosinophils (males only) was observed at 300 and 900 mg/kg food.
At the same dose levels glucose and protein levels were decreased in
both males and females. At 900 mg/kg food glucose was decreased in
females and creatinine in males. The incidence of all tumours
combined was not enhanced (Hartig, 1981). Remark: only summarized
data were available.
2.2.4 Reproduction studies
2.2.4.1 Rats
Male rats were administered diets containing 0, 40, or
400 mg/kg food mg carazolol/kg food for 10 weeks. Rats were then
allowed to mate with untreated females. The newly-born were counted
and weighed. Body weight was slightly decreased in high-dosed males.
No treatment-related effects were observed on indices for fertility,
gestation, viability and lactation. No difference in offspring born
alive or dead, number of live fetuses, fetal weight or behaviour of
the offspring was observed. At 21 days of age the offspring were
killed and no malformations were observed (Hebold & Czerwek, 1978).
Remark: summary only available.
In a 3-generation reproduction study with a teratology phase
(see Special studies on embryotoxicity and teratogenicity, Section
2.2.5.1) female Sprague-Dawley rats (76/group) were orally
administered by gavage 0, 15, 30, or 60 mg/kg b.w./day carazolol
suspended in methyl cellulose. Administration started from 14 days
before the first mating with untreated males (for all females/group)
throughout the entire pregnancy (22-27 pregnant rats/group) until
day 21 of lactation. Eleven-12 dams/group were killed on day 21 of
pregnancy and their fetuses were delivered by caesarian section.
F1 animals (1/sex/litter) were mated and gave birth to the F2
generation. During the 14-day premating period a slight sedation was
observed from days 3-6 at the highest dose and a significantly
increased body weight as well as body weight gain were observed at
30 and 60 mg/kg b.w./day. No effects were observed on the fertility
index. At 60 mg/kg b.w./day the rearing performance of the F0
animals during the lactation period was impaired. At 60 mg/kg b.w.
post-implantation loss was significantly increased in the F0 dams
and the number of F1 pups alive at the end of the lactation period
was significantly decreased. At the highest dose a significantly
lower pup weight was observed in the F1 generation shortly after
delivery. At 30 and 60 mg/kg b.w./day post-implantation loss was
significantly increased in F1 dams and a significant decrease was
observed in the number of F2 young alive on the day after birth.
The NOAEL in this study was 15 mg carazolol/kg b.w./day (Sterz &
Vollmar, 1978).
2.2.5 Special studies on embryotoxicity and teratogenicity
2.2.5.1 Rats
Groups of 24 pregnant Sprague-Dawley rats were administered 0,
25, 50, or 100 mg carazolol/kg b.w/day from days 6-15 of pregnancy
by gavage. Dams were killed on gestation day 19. Observations
included clinical signs, condition of faeces, food and drinking
water consumption, and body weight. On day 19 of gestation the
females were sacrificed and the uteri and ovaries were examined.
Other organs were macroscopally examined. Fetuses were weighed,
sexed, and examined for external, visceral, and skeletal
malformations. Maternal toxicity (ataxia and sedation) was observed
at 50 mg carazolol/kg b.w./day, and more marked at 100 mg/kg
b.w./day. At the highest dose an increase in post-implantation loss
was observed. There was no evidence of embryotoxicity or
irreversible structural effects (Leuschner, 1975).
A teratological examination was conducted during the course of
the 2-generation rat reproduction study (see Reproduction studies,
Section 2.2.4). No effects were observed on number of corpora lutea,
number of implantations, pre-implantation loss, resorptions, live
and dead fetuses, fetal weight, sex ratio, or external, visceral,
and skeletal malformations. From days 1-22 of pregnancy high-dose
dams exhibited a reduced body weight gain. Placental weight was
significantly increased at 30 and 60 mg/kg b.w./day (not
dose-related). A decrease in number of ossified cervical vertebrae
or tail vertebrae was observed in the mid- and high-dose groups. No
irreversible structural effects were observed (Sterz & Vollmar,
1978).
2.2.5.2 Rabbits
Groups of 17 pregnant Himalayan rabbits were orally
administered 0, 25, 50, or 100 mg carazolol/kg b.w/day from days
7-19 of gestation. The females were killed on gestation day 31 and
fetuses were delivered by caesarean section. During the
administration period food consumption was reduced in all groups,
significantly at 100 mg/kg b.w./day; a significant increase in food
consumption was observed in high-dose females the last 6 days of the
test. Weight gain of dams at the highest dose was significantly
increased from days 20 to 31 of pregnancy. Independent of the
dosage, 3 cases of arthrogryposis occurred in 30 litters (0, 1, 1,
and 1 at 0, 25, 50 and 100 mg/kg b.w./day, respectively). No
treatment-related effects were observed in number of corpora lutea,
implantation loss, resorptions, litter size, live and dead fetuses,
fetal weight, sex ratio, or internal and skeletal malformations
(Sterz & Glocke, 1977a).
Groups of pregnant Himalayan rabbits (13-14/group) received
orally 0, 6.26, 12.50, or 100 mg carazolol/kg b.w./day from days
7-19 of gestation. The females were killed on day 31 of gestation
and the fetuses were delivered by caesarean section. Food
consumption was decreased at the highest dose. In the mid-dose
group, one dead and one underdeveloped fetus were seen. In the
high-dose group, one dead and two underdeveloped fetuses were seen.
Placental weight was significantly decreased at the highest dose and
fetal weight was significantly and dose-relatedly decreased at 12.50
and 100 mg/kg b.w./day. One fetus with pin-head size meningocele on
the occiput was observed at 100 mg/kg b.w./day. Arthrogryposis was
observed in 0, 1, 1, and 1 fetus at 0, 6.25, 12.50, and 100 mg/kg
b.w./day, respectively. One fetus with spondylosis syndrome and one
fetus with scoliosis due to reduction and absence of parts of the
first two lumbar vertebrae were observed at the mid- and high-dose,
respectively. No treatment-related effects were observed on
fertility index, body weight, number of corpora lutea, implantation
loss, resorptions, litter size, or sex ratio (Sterz & Glocke,
1977b).
A third experiment (using the same experimental design) was
carried out in which particular attention was directed towards any
occurrence of deformities of the limbs (arthrogryposis). Twenty-nine
and 20 Himalayan pregnant rabbits were orally administered 0 and
100 mg carazolol/kg b.w./day from days 9-12 of gestation,
respectively. No treatment-related effects were observed on food
consumption or body weight (only recorded in 10 control and 4
experimental rabbits) or reproduction performance. No abnormalities
were observed after skeletal and visceral examinations, except for a
significantly increased number of extra ribs at the 1st lumbar
vertebra. The authors concluded that the observed cases of
arthrogrypsosis in the previous reported studies were in fact a
fortuitous increase in incidence of this spontaneous deformity of
the rabbits strain used (Sterz & Glocke, 1977c).
2.2.6 Special studies on pharmacology
2.2.6.1 Mice
Groups of female SPF mice fasted for 18 hours were given i.p.
doses of 0, 20, or 200 mg carazolol/kg b.w. or 200 mg propranolol/kg
b.w. Intestinal motility was significantly increased after both
carazolol and propranolol administration (Roesch, 1978). Barbiturate
sleep in groups of mice given s.c. 8, 16, 32, or 64 mg carazolol/kg
b.w. was not affected at doses < 16 mg/kg b.w.; all mice died at
64 mg/kg b.w. Induced fighting behaviour was dose-dependently
inhibited in mice given 0, 2, 4, 8 or 16 mg carazolol s.c./kg b.w.
at doses of 4-16 mg/kg. Electroshock-induced convulsions were
inhibited in 1/10 mice and 10/10 mice at 16 and 32 mg carazolol/kg
b.w., respectively. Metrazole-induced convulsions were
dose-dependently inhibited in mice given 4-32 mg carazolol/kg b.w.
In a traction test with mice the ability to cling to the wire was
blocked in 0/10, 1/10, and 10/10 mice at 8, 16, and 32 mg
carazolol/kg b.w., respectively (Kumada & Ohtsuka, 1978).
2.2.6.1 Rats
No indication of an effect on the central nervous system was
observed in male Sprague-Dawley rats given intraperitoneally
10 mg/kg b.w. carazolol (Roesch, 1973). No effects were observed on
the urine and electrolyte excretion in female SPF-Sprague-Dawley
rats after the oral or i.p. administration of 10 mg/kg b.w.
carazolol (Bartsch, 1974a).
2.2.6.3 Rabbits
1 mg carazolol/kg b.w. administered intravenously to conscious
rabbits did not cause an effect on body temperature; blood glucose
levels were slightly increased at this dose (Hebold, 1974).
Carazolol given at doses of 50 mg/kg b.w. orally, or at 5 or
10 mg/kg b.w i.v. to male and female mixed-breed rabbits inhibited
an isoprenaline-induced tachycardia; at 20 mg/kg b.w. orally no
inhibition was found. A dose of 5 mg carazolol/kg b.w. intravenously
administered to conscious rabbits inhibited isoprenaline-induced
tachycardia (Bartsch 1974b).
2.2.6.4 Dogs
No effects were observed on the urine and electrolyte excretion
in female beagle dogs given 5 mg carazolol/kg b.w. orally (Bartsch,
1974). Conscious dogs were intravenously administered 0.5, 1.0, 2.0,
or 5.0 mg carazolol/kg b.w. over a period of 15 seconds. At 1.0 and
2.0 mg/kg b.w. heart rate was increased without affecting the PQ
interval, but with slight changes in the ECG. At these doses the
behaviour of the dogs was normal. At the highest dose of 5.0 mg/kg
vomiting, tachypnoea, tachycardia and changes in the PQ-section of
the ECG were observed (Bartsch, 1977).
One, 10, or 100 mg carazolol/kg b.w. given i.v. to
anaesthetized mongrel dogs antagonized isoprenaline-induced
responses. In another experiment, 0.01, 0.1, 1.0, or 10 mg
carazolol/kg b.w. was intravenously given to anaesthetized mongrel
dogs. At doses of 1.0 mg/kg b.w. and higher, depressive effects on
the cardiovascular system which included decreases in ABP (arterial
blood pressure), LVP (left ventricular pressure) and dp/dt (cardiac
output), and an increase of IVEDP (left ventricular end diastolic
pressure) were observed (Satoh et al., 1980).
One, 4, 16, or 64 mg carazolol/kg b.w. was intravenously given
to mongrel dogs. A dose-dependent inhibition of
isoproterenol-induced tachycardia as well as isoprenaline-induced
increase of cardiac output was observed; at the highest dose an
almost complete blockade was observed. In mongrel dogs a
dose-dependent inhibition of tachycardia as well as increase in
cardiac output induced by sympathetic nerve stimulation was also
observed after the i.v. administration of 0.25 to 16 mg carazolol/kg
b.w. (Kumada & Ohtsuka, 1978).
2.2.7 Special studies on genotoxicity
Table 2: Results of genotoxicity assays on carazolol
Test system Test object Concentration Result Reference
In vitro S. typhimurium 0-5000 mg/pl1 negative Grafe, 1978
Ames test TA98, TA100 in DMF mg/pl1
TA1535, TA1537
TA1538
In vivo male NMRI single oral dose negative Grafe &
Dominant mice of 20 or 80 mg/kg b.w. Vollmar,
lethal assay or 13.5 mg/kg b.w. 1976
single i.p. dose
Dominant female NMRI single oral dose negative Grafe &
lethal assay Kisslegg mice of 50 mg/kg b.w. or Vollmar,
13.5 mg/kg b.w. single 1976
i.p. dose
Host-mediated NMRI/Kisslegg 80 mg/kg b.w. oral or negative Grafe &
assay mice with 13.6 mg/kg b.w. s.c. Vollmar,
S. typhimurium 1976
G46 S. marcescens
Cytogenicity Chinese hamster 2 x 400 mg/kg b.w. negative Grafe &
assay oral or 1 x 20 mg/kg Vollmar,
(spermatogonia b.w. i.p. or 3 x weekly 1976
as well as for 13 weeks a
bone marrow)2 treatment with 20 mg/kg
b.w. oral or 1 mg/kg
b.w. i.p.; all suspended
in CMC
1. both with and without rat liver S-9 fraction
2. only a negative control group (compound not indicated) was tested
2.3 Observations in humans
Twelve healthy male volunteers were given carazolol in a 3-way
crossover experiment at doses of 0.5 mg i.v., 5.0 mg oral or 7.5 mg
oral. Ergometric tests were carried out before and 1, 2, 3, 4, 8,
12, and 16 hours after carazolol administration. The subjects laid
down for about 20 minutes before each test. The heart rate, blood
pressure, the pressure-rate product, and the relative enteral
efficacy were determined. Heart rate was maximally inhibited by
1-2 hours after administration (15%, 13%, and 15% at 0.5 mg i.v.;
5.0 and 7.5 mg oral, respectively). During exercise the systolic
blood pressure was lowered by a maximum of 12% (oral dose) or 15%
(i.v. dose) at 1 hour. The maximal reduction of the pressure rate
product was 28%, 26%, and 25% at 0.5 mg i.v., 5.0 and 7.5 mg oral,
respectively. The mean duration of action of an oral dose of 5 mg
was estimated as 10 hours. The relative enteral efficacy calculated
by comparison of the inhibitory areas after both oral doses with
those of an i.v. dose did not differ significantly and was about
10%. By using the dose-reponse curve the authors extrapolated a
no-effect dose for the oral route of 10 mg/kg b.w. (Mollendorff
et al., 1979).
In a cross-over experiment 8 patients with chronic bronchitis
and partial reversibility of the obstruction were given a placebo or
carazolol randomly. Two patients received both 0.1 and 0.7 mg
carazolol, while the other 6 received either 0.1 or 0.7 mg. Vital
capacity (VC) and forced expiratory volume (FEV) were measured
before and 1, 2, and 3 hours after administration of the substances.
No statistics were carried out. At 0.7 mg carazolol a definite
increase in bronchial resistance was observed and dyspnoea was seen
in 3/5 patients leading to discontinuation of the test. At 0.1 mg
carazolol, the tests did not have to be discontinued in any of the
patients; only one patient showed slight dyspnoea with a decrease in
FEV . The author estimated that a dose of 0.03 mg carazolol no
longer induces any effect on the bronchial system (Huckauf,
undated).
3. COMMENTS
The Committee considered pharmacological and toxicological data
from pharmacodynamic, pharmacokinetic, metabolism, acute and
short-term toxicity, carcinogenicity, genotoxicity, reproduction and
teratology studies, as well as the results of clinical trials in
humans.
The distribution, excretion and biotransformation of
radiolabelled carazolol were studied in rats, rabbits and dogs.
After oral ingestion, carazolol was rapidly absorbed. In rats, the
radioactivity was widely distributed in the tissues, with the
highest levels in liver, kidneys, and lungs. Excretion was almost
complete within 48 hours, equally divided between the urine and
faeces. In rabbits, peak plasma levels were reached 1 hour after a
single oral dose of 10 mg/kg b.w., and about 60% of the dose was
excreted in urine. Following oral administration of carazolol in
dogs, 13% of the radioactivity was excreted in urine and 45% in
faeces within 48 hours. In the urine of a dog given 10 mg of [14C]
carazolol/kg body weight intravenously, the parent compound and six
metabolites were identified. Both carazolol and its glucuronide,
lactate, and acetate metabolites have been identified in the urine
of pigs and humans.
In a short-term study in rats, in which carazolol was
administered in the diet at levels up to 400 mg/kg of feed for 13
weeks, females exhibited a decrease in both body weight and food
intake at the highest dose. Heart rate was decreased even at the
lowest dose of 40 mg/kg of feed. When rats received carazolol in the
feed for 1 year a decrease in body weight due to decreased food
intake at the highest dose level was observed in both males and
females only after progessively increasing this dose to 1200 and
subsequently to 1800 mg/kg of feed. A statistically significant
increase in relative heart weight was observed in rats at all dose
levels, but this was not dose related.
In short-term studies in dogs dosed orally at up to 20 mg kg
b.w./day, blood chemistry and histopathological changes provided
evidence of hepatoxicity. The NOEL was 3 mg/kg b.w./day. In a 1-year
study, changes in relative organ weights (liver, kidney and testes)
were observed at the dose of 30-60 mg/kg b.w./day, with a NOEL of
15 mg/kg b.w./day.
In a long-term study with rats at dose levels of 100, 300 and
900 mg/kg of feed, body weight gain was reduced, even at the lowest
dose level in females, and haematological and biochemical changes
were found at the two highest dose levels. No increase in tumour
incidence was observed.
In teratogenicity studies in rats, no malformations were
observed at doses of up to 100 mg/kg b.w./day but embryotoxicity and
maternal toxicity were observed at doses of 30 mg/kg b.w./day and
above. The NOEL was 15 mg/kg b.w./day In one of three teratogenicity
studies in rabbits, one fetus with structural malformations was
observed in the mid-dose group and one in the high dose group,
giving a NOEL of 6.25 mg/kg b.w./day.
In a two-generation reproduction study in rats, in which
carazolol was administered by gavage at doses of up to 60 mg/kg
b.w./day, an increase in post implantation losses, and a decrease in
the number of live pups in the first and second generations were
observed in both the mid- and the high-dose groups. The NOEL in this
study was 15 mg/kg b.w./day.
Carazolol gave negative results in five in vitro and in vivo
genotoxicity tests.
The ß-adrenoceptor-blocking activity of carazolol was
established in specific function tests in mice, rats, rabbits, and
dogs. A NOEL of 0.02 mg/kg b.w. for the inhibition of
isoprenaline-induced tachycardia was observed in rabbits after
administration of a single oral dose.
In a clinical trial with healthy human volunteers, the effect
on cardiac function was determined following administration of a
single oral dose of 5 or 7 mg of carazolol/person. From the
dose-response curve, a dose without effect of about 0.01 mg/kg b.w.
was extrapolated. Patients suffering from chronic bronchitis showed
a clear effect on respiratory function after a single oral dose of
0.7 mg/person and a marginal effect at a dose of 0.1 mg/person.
Based on these results, a NOEL of about 0.03 mg/person, equivalent
to 0.5 µg/kg b.w., was extrapolated, but the Committee noted that no
information was provided about the procedures used in these
extrapolations.
4. EVALUATION
The Committee noted that most of the toxicological data were
available only in summary form and that only one long-term toxicity/
carcinogenicity study was available. Since the NOEL of 3 mg/kg
b.w./day, observed in short-term toxicity studies in dogs, was
several orders of magnitude higher than the NOELs for carazolol in
pharmacological function studies, the Committee concluded that the
pharmacological effect provided a more appropriate basis for the
safety evaluation of residues. Because the information required to
extrapolate to a dose without effect was lacking in the human
studies, the Committee was not able to derive a clear NOEL for
humans that would also cover specific groups who might be at risk,
such as people suffering from cardiac or respiratory disease,
particularly asthma. A temporary ADI of 0-0.1 µg/kg b.w. was
therefore established, based on a NOEL of 0.02 mg/kg b.w. for the
inhibition of isoprenaline-induced tachycardia in rabbits and the
application of a conventional safety factor of 200 for a temporary
ADI based on animal studies. The Committee noted that, if the
pharmacological NOELs extrapolated from the human studies were used,
an ADI of a similar order of magnitude would be obtained.
5. REFERENCES
BARTSCH, W. (1974a) General pharmacology of BM 51.052. Unpublished
Reports E 2, E 4, dated 2-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1974b) Special pharmacology of BM 51.052. Unpublished
Reports D1, D2, D4, dated 3-1974 from Boehringer, pharmakologisches
labor, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
BARTSCH, W. (1977) Tolerance testing on intravenous administration
of high doses of BM 51.052 in conscious dogs. Unpublished Report No.
E 6 dated 22-11-1977 from Boehringer, Mannheim. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
BARTSCH, W. (1978) Special pharmacology of BM 51.052. Unpublished
Reports D3 and D5 dated 7-1978 from Boehringer, Mannheim. Submitted
to WHO by Praemix Wirkstoff GMBH, Mannheim.
CZERWEK, H. (1978) Akute toxizitat BM 51.052. Unpublished Report
dated 1-9-1978 from Boehringer, Abt. fur Toxicologie, Mannheim.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE (1978) Mutagenicity BM 51.052 Ames-test. Unpublished Report
dated 23-10-1978 from Department of Genetics, Boehringer Mannheim
GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
GRAFE & VOLLMAR, J. (1976) Mutagenicity BM 51.052. Unpublished
Report dated 30-03-1976 from Abteilung fur Genetik, Boehringer
Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HARTIG, F. (1981) Chronic oral carcinogenicity test of BM 51.052,
2-year experiment in rats. Unpublished Report 1-12/16/8/D-E/DrH/btza
dated 25-11-1981 submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
HEBOLD, G. (1974) Effect of BM 51.052 on the body temperature and
blood sugar of conscious rabbits. Unpublished Report E 5 dated
11-2-1974 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978a) Chronic oral toxicity test of BM 51.052 12 months'
experiment in rats. Unpublished Report 9-2/07/11/D-E/DrH/btza dated
23-06-1978 from Boehringer Mannheim GmbH. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
HEBOLD, G., BLEUEL, H., CZERWEK, H., HARTIG, F. & TEUTE, H.W.
(1978b) Pathology and toxicology BM 51.052 1 year experiment in
dogs. Unpublished Report no. DrH/elm 8-8/31/2 dated 19-7-1978 from
Boehringer Mannheim GMBH. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
HEBOLD, G. & CZERWEK, H. (1978) Fertility tests on male rats given
BM 51.052. Unpublished report dated 2-08-1978 from Boehringer
Mannheim GmbH. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
HUCKAUF, H. (undated) Clinical trial of carazolol to determine a
threshold dose on the bronchial system. Report from Medizinische
Klinik und Poliklinik, Klinikum Steglitz, Freie Universitat Berlin.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
KOCH, K. (1977) Metabolism of BM 51.052. Unpublished report dated
09-08-1977 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
KOCH, K. & BARTSCH, W. (1977) Investigations on the pharmacokinetics
of BM 51.052 (carazolol). Unpublished report dated 20-02-1977 from
Boehringer, Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH,
Mannheim.
LEUSCHNER, F. (1975) The effect of BM 51.052 (micronized), Batch no
807035 A on the pregnant rat and foetus on administration by gavage.
Unpublished report dated 18-11-1975 from Mannheim Boehringer,
laboratorium fur farmakologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
MOLLENDORF, E.v., GLOCKE, M.H. & ABSHAGEN, U. (1979) Effect kinetics
of carazolol (BM 510952). Unpublished Report M5-01-78 dated
17-10-1979 from Department of Clinical Pharmacology, Boehringer,
Mannheim. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974a) Pathology and
toxicology BM 51.052 14 day experiment in rats. Unpublished Report
7-1/1-/2 dated 26-7-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1974b) Pathology and
toxicology BM 51.052 14-day experiment in dogs. Unpublished Report
7-1/11/1 dated 16-8-1974 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975a) Pathology and
toxicology BM 51.052 4 week experiment in rats. Unpublished Report
6-12/15/2 dated 2-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxikologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1975b) Pathology and
toxicology BM 51.052 4 week experiment in dogs. Unpublished Report
6-12/21/1 dated 31-10-1975 from Boehringer Mannheim GmbH, Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976a) Pathology and
Toxicology BM 51.052 3 month experiment in rats. Unpublished Report
6-12/27/2 dated 28-1-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
REBEL, CZERWEK, H., HARTIG, F. & TEUTE, H.W. (1976b) Pathology and
toxicology BM 51.052 3 month experiment in dogs. Unpublished Report
6-12/27/1 dated 2-2-1976 from Boehringer Mannheim GmbH. Abteilung
fur experimentelle pathologie und toxicologie. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
ROESCH, A, (1973) Investigation of the action of BM 51.052 on the
central nervous system of the rat. Unpublished Report E.1 dated
21-08-1973 from Boehringer, Mannheim. Submitted to WHO by Praemix
Wirkstoff GMBH, Mannheim.
ROESCH, A. (1978) Transport function of the intestines-mouse.
Unpublished Report E 3 dated 26-06-1978 from Boehringer, Mannheim.
Submitted to WHO by Praemix Wirkstodd GMBH, Mannheim.
SATOH, H., MIURA, Y., INUI, J. & HASEGAWA, G. (1980) ß-adrenoceptor
blocking, cardiovascular and electrophysiological effects of
carazolol. Unpublished report from research laboratories, Yoshitomi
Pharmaceutical Industry Ltd. Submitted to WHO by Praemix Wirkstoff
GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977a) Teratological investigations with
BM 51052 in rabbits. Unpublished Report K2A dated 18-03-1977 from
Boehringer Mannheim GMBH, teratology department. Submitted to WHO by
Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977b) Teratological investigations with
BM 51.052 in rabbits (1st repeat and extension). Unpublished Report
K2B dated 18-03-1977 from Boehringer Mannheim GMBH, teratological
department. Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & GLOCKE, M.H. (1977c) Teratological investigations with
BM 51052 in rabbits (2nd repeat). Unpublished Report K2C dated
18-03-1977 from Boehringer Mannheim GMBH, Teratological Department.
Submitted to WHO by Praemix Wirkstoff GMBH, Mannheim.
STERZ, H. & VOLLMAR, J. (1978) Reproduction toxicology
investigations phase I (Fertility) in female rats using BM 51.052
p.o. Unpublished Report K4 from Department of Reproduction
Toxicology/General Biometry, Boehringer Mannheim GmbH. Submitted to
WHO by Praemix Wirkstoff GMBH, Mannheim.
