
ANOXOMER
Explanation
This compound has not previously been reviewed by the Joint
FAO/WHO Expert Committee on Food Additives.
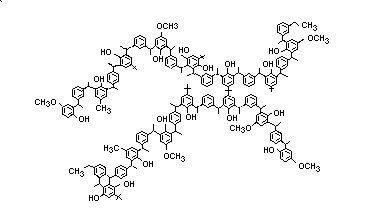
Anoxomer is a complex polymeric substance with an average
molecular weight of 4500-5000. No more than 5% of the polymer can be
greater than 50 000 daltons and no more than 1% of the polymer can be
less than 500 daltons. Anoxomer is to be used as an antioxidant at a
level no greater than 0.5% of the fat and oil content of foods. It is
an off-white powder with the following structure:
 BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of C14-anoxomer, 10 mg in Sprague-Dawley rats,
1.8 mg in IRC/SM mice, 0.5 mg in Hartley guinea-pigs and 0.6 mg in
Dutch-belted rabbits indicated that only 0.2-0.6% of the administered
compound is absorbed. The recovery of radioactivity in these studies
varied from 78 to 110% (Halladay et al., 1978; Parkinson et al., 1978;
Enderlin & Halladay, 1977a, 1977b). Absorption of a single dose of
C14-anoxomer was no greater in Sprague-Dawley rats fed 5% anoxomer
for 90 days than in rats given C14-anoxomer acutely (Enderlin &
Halladay, 1977c). Following oral dosing of 10 mg of C14-anoxomer, the
residual radioactivity was assessed in the mesenteric lymph nodes 30
days later. There was some indication from this study that anoxomer
was selectively partitioning in lipid stores (Enderlin & Halladay,
1977c).
Effects on enzymes and other biochemical parameters
2.8 and 5 g/kg of anoxomer was added to the basal diet of male
Sprague-Dawley rats for 90 and 60 days, respectively. The level of
cytochrome P-450, cytochrome C reductase activity and microsomal
mixed-function oxygenase (MFO) activity for aminopyrine demethylase
(APDM), p-nitroanisole demethylase (NADM), hexobarbital oxidase (HO)
and benzo[alpha]pyrene hydroxylase (BH) were measured in the hepatic
S-9 homogenates. There were no differences between control and
anoxomer-fed animals, with regard to liver weights and in hepatic
homogenates. There were no differences in the activity of cytochrome
P-450, cytochrome C reductase or any of the MFO enzymes (Ryerson et
al., 1977).
In another study, 2.8 and 5 g/kg of anoxomer was added to the
basal diet of male Sprague-Dawley rats for 90 and 60 days,
respectively. The levels of cytochrome P-450, cytochrome C reductase
activity and microsomal mixed-function oxygenase (MFO) activity for
the hydrolysis of indophenol acetate (IPA) and benzo[alpha]-pyrene
hydroxylase (BH) was measured in the intestinal S-9 homogenates.
Anoxomer caused no alterations in the concentration of cytochrome
P-450 and cytochrome C reductase activity over the duration of the
90-day study (Ryerson et al., 1978).
TOXICOLOGICAL STUDIES
Special studies on blood coagulation
During the conduct of the chronic rat feeding study, 4 high-dose
(2500 mg/kg bw) male rats suffered unexplained and lethal
haemorrhaging of the pelvic region. Thus, a study to assess the
influence of anoxomer on blood clotting factors in male rats was
conducted. Ten male rats in each of 2 groups received either 0 or
2500 mg/kg bw of anoxomer in basal lab diet for 21 days. There was no
statistically significant differences between treated and control
animals in prothrombin time, activated partial thromboplastin time,
fibrinogen concentration or bleeding time. There was a statistically
significant increase in platelet count in treated males (Anon.,
1981c). Based on these observations, anoxomer appears to be free of
any influence on blood clotting factors.
Special studies on dermal irritation
Six healthy, New Zealand white rabbits were used in this test.
Skin reactions were scored by the Draize system and, since the primary
irritation score was 0.2, anoxomer was not judged to be a primary
irritant (Anon., 1976b).
Special studies on eye irritation
In 6 healthy, New Zealand white rabbits, 63 mg of anoxomer was
instilled in the conjunctival sac of the left eye with the right eye
serving as control. Anoxomer was not judged to be an eye irritant,
since findings of irritation were present in only one of 6 test
subjects (Anon., 1976a).
Special studies on influence on lymphatic tissues
Female Sprague-Dawley rats were given 5% anoxomer in the diet for
28 weeks. No gross or microscopic alterations were noted in the
mesenteric lymph nodes of any control or high-dose animals that were
examined (Anon., 1978).
Eighteen female rats (Simonsen strain) were administered 10.74 mg
(39 µCi) of 14C-labelled anoxomer by gavage = 50 mg/kg bw. On each of
6 days (days 1, 3, 7, 14, 21 and 28) following dosing, 3 animals were
sacrificed and the level of radioactivity assessed in liver, spleen,
kidneys, stomach, small intestine, caecum, large intestine, intestinal
contents, lymph nodes (mesenteric, axillary, cervical and
inguinofemoral), fat (from mesenteric, peri-uterine and subcutaneous
regions) and muscle (gracilis). Specimens from blood, urine and faeces
were also collected and analysed for radioactivity. Control (undosed)
animals were used to determine the limits of detection of the
radiolabelled anoxomer. The levels initially detected were negligible
in all tissues but the gastrointestinal tract. Even the initially high
levels found in the stomach and large and small intestine declined
rapidly. This study gave no evidence of a selective tissue
accumulation of anoxomer (Enderlin, 1981). This contrasts with the
earlier metabolism studies (Enderlin & Halladay, 1977c) which
suggested anoxomer concentrations in fat tissue. This earlier
observation may have been due to measurement uncertainties (error)
related to counts per minute extrapolations from small tissue samples
(50 mg). At the many-fold higher doses used in the subchronic and
lifetime animal studies (10 g/kg bw), some abnormal or selective
accumulation in fat or lymphoid tissue may occur: however, at the
doses used in this study (about 50 mg/kg bw), there is no evidence of
selective accumulation of anoxomer in either fat or lymphoid tissues.
Special studies on mutagenicity
Anoxomer, in concentrations up to 5 mg/plate, was neither
mutagenic nor cytotoxic in the Ames test using 5 strains of his-
Salmonella typhimurium (strains used were sensitive for both base-
pair and frame-shift mutations) either with or without activation by
rat microsomal preparation. Four strains of trp-Escherichia coli
were employed to test anoxomer in concentrations up to 1 mg/plate with
and without metabolic activation. Anoxomer was nonmutagenic in this
bacterial test system (Dietrich et al., 1977).
Anoxomer was evaluated for its ability to induce mitotic
crossing over or intergenic recombination and reverse mutation of
Saccharomyces cerevisiae. Anoxomer was tested in concentrations up
to 5 mg/ml and at time periods up to 16 hours with and without
metabolic activation. No dose- or time-related effects were seen with
any of these endpoints (Dietrich et al., 1977).
The potential mutagenicity of anoxomer in mammalian cells was
tested in L5178Y mice lymphoma cells. There were no effects on forward
point mutations at the thymidine kinase locus, sister chromatid
exchanges or chromosome aberrations (Anon., 1977). The validity of
this particular study is questioned at doses above 0.32 mg/ml because
of precipitation of the test materials, and because the incidence of
chromosomal aberrations was not different between the positive and
solvent controls.
Special studies on reproduction
A 3-generation reproduction and teratology study was conducted
with anoxomer in Sprague-Dawley rats. Control animals received basal
diets while test animals received 0.5, 1.58 or 5% of anoxomer in basal
diet throughout the study. Each parental generation consisted of 30
males and 30 females.
There was a statistically significant decrease in neonatal
survival index in the 1.58 and 5% offspring and in the weaning
survival index of all 3 treated groups in the F1a litters. There was
a significant decrease in male birth weight and in male and female
weaning weights at the 5% level in the F1b litters. The neonatal and
weaning survival indices as well as weaning weights were significantly
reduced in the 5% groups of the F2a litters. Weaning survival was
decreased in the 0.5 and 5% offspring, and weaning weight of all males
in the same groups was decreased. In the F3a, F3b and F3c litters,
the pregnancy rate was low in all groups including the control
animals. However, none of these effects were observed consistently
during the successive generations, nor was there any indication that
these effects were more marked during the successive breedings. Based
on all the information from the study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet.
The F3d litters were utilized for the teratology phase of this
study. The test animals were sacrificed on day 19. There was no effect
on numbers of corpora lutea, implantations, resorptions, liver of dead
foetuses for any of the groups. In addition, there was no treatment-
related effects on body weight or size or on the incidences of
visceral or skeletal anomalies (Anon., 1979a).
Special studies on teratogenicity
Thirty female Sprague-Dawley rats, each of 4 treatment groups,
were fed anoxomer at 0, 250, 800 or 2500 mg/kg bw per day on days 6-15
of gestation. The test animals were sacrificed on gestation day 20. No
compound-related effects were found in maternal body weights, deaths
or pathology at necropsy. No compound-related differences were
observed in pregnancy rate, number of corpora lutea, implantations,
resorptions, live foetuses, foetal body weight or length (Anon.,
1981a). The mean number of visceral and skeletal variants increased in
the treated groups, but the increase did not appear to be dose
related.
In 2 studies, A and B, 20 or 25 female rabbits, respectively in
each of 4 treatment groups received anoxomer at 0, 250, 500 or
1000 mg/kg bw per day by oral intubation from day 6 through day 18 and
sacrifice was on gestation day 29. In both studies, maternal gross
pathology did not indicate any treatment-related effects. No effect
was apparent in the number of dead foetuses, live foetuses, or sex
ratio. There appeared to be no treatment-related increase in skeletal
problems in both studies. In study A, there was a dose-related
increase in dilated pelvises and ectopic kidneys in all groups. Study
B utilized the same protocol, but did not confirm the renal effects
(Anon., 1979, 1981a).
Acute toxicity
LD50 Reference
Animal Route (g/kg bw)
Mouse Oral 10 Anon., 1981c
i.p. 10 Jorgenson & Rushbrook, 1976
Rat (Sprague-Dawley) Oral 10 Jorgenson & Rushbrook, 1976
i.p. 10 Jorgenson & Rushbrook, 1976
Dog Oral 10 Jorgenson & Rushbrook, 1976
i.p. 5 Jorgenson & Rushbrook, 1976
Short-term studies
Rat
Fifty young, adult Sprague-Dawley rats (25 males and 25 females)
in 4 different groups were fed 0, 0.5, 1.58 or 5% anoxomer in rat diet
for 90 days. Water intake, activity and behaviour were normal and
comparable for all groups. Body weights and food consumption for males
and females in all groups remained in the normal ranges for the
duration of the study. The haematology, differential counts, platelet
counts and reticulocyte counts of all groups were also within normal
limits. The serum enzyme and biochemistry were within the expected
limits of biological variation. Gross necropsy revealed a 15-20%
incidence of emphysema, slight haemorrhaging and/or congestion of the
lungs in all groups, including controls. This was probably the result
of handling and CO2 asphyxiation and not the result of treatment. Two
control males and 2 0.5% dose level females had cataracts. No dose-
response effects were evident and the gross findings were within
normal ranges for this strain of rat. All organ weights and organ-to-
body weight ratios were comparable between groups. Slight to moderate
hyperplasia of the lymph node was seen in 2 high-dose rats and
haemorrhage of the lymph node was observed in another high-dose rat.
Slight focal haemorrhage and slight focal congestion was found in the
thymus, respectively, in 2 high-dose rats (Jorgenson & Rushbrook,
1977).
Long-term studies
Mouse
One hundred and seventy-five sexually mature HAM/ICR mice were
selected on the basis of good health and randomly assigned either to
one of two control groups or to treatment groups and fed 0, 0.5, 1.58
and 5% anoxomer for 104 weeks to assess the chronic toxicity and
carcinogenicity potential of this substance. After 1 week, animals
were mated. Female mice were maintained on control or test diet
throughout gestation, delivery and lactation. Fifty animals per sex
per dose were then randomly assigned to the chronic feeding phase of
the present study.
There were no compound-related effects indicated by the survival
data from the test and control groups. While the high-dose male and
female groups' mean weights were decreased during the first 6 months
of the study, by the end of the first year, there were no significant
differences in body weights of the offspring of the high-dose F0
groups. The early decreased body weight would appear to reflect the
test animals exposure in utero to the test compound. Thus, the
decreased body weights of the high-dose males and females in the early
phase were considered treatment related. This effect on offspring body
weight may have been due to simple dilution of the nutritive value of
the diet because of the presence of the diet compound. Problems of
food spillage, however, made food consumption data of questionable
reliability (Anon., 1980a).
At termination of the study at 104 weeks, gross pathology was
characterized in large measure as being comparable across treatment
and control groups. Certain lesions occurred with nearly equal
frequency across all treatment groups, e.g., discoloration of the
lungs, enlarged spleens and darkened areas or lobes of the liver.
The results of histopathological findings were essentially
comparable between the high-dose treatment group and control group 1.
The more common incidental findings observed were: chronic murine
pneumonia, hydronephrosis, focal ulceration of the gastric mucosa,
malignant lymphoma originating in the thymus, hepatocellular carcinoma
and chronic interstitial nephritis. None of these lesions occurred in
other than an apparently random distribution. Statistical analysis was
performed on the comparative incidences of malignant lymphoma (all
types combined alveolar/bronchiolar carcinoma and hepatocellular
carcinoma in control group 1 and the high-dose groups. These 3 tumour
categories were selected since they were representative of the tumour
types occurring most frequently. There was not a statistically
significant increase in incidence of these tumours in the treated
group compared to control group 1 (Anon., 1980a). An examination of
the pattern and occurrence of tumours in other organs and tissues did
not reveal a frequency of distribution across treatment groups which
is compound related.
Rat
Fifty animals (Fischer 344 strain) per sex per dose were fed 0,
0.5, 1.58 or 5% anoxomer for 130 weeks in this study to assess the
chronic toxicity and carcinogenicity potential of this substance. The
parents of these animals had also been fed 0, 0.5, 1.58 or 5%
anoxomer. Exposure to anoxomer continued during mating, gestation,
delivery and lactation.
Survival in all groups at 18 months exceeded the 55% protocol
requirement. The higher mortality experienced by the mid-dose males is
considered to be incidental and not compound related. Anoxomer
administration resulted in statistically significant decreases in body
weight gain (growth rates) through week 51 in low- and high-dose
males. In addition, during the chronic feeding phase of the study, the
mean body weights of the 3 treated male groups were consistently lower
than the 2 control groups. This finding is considered to be compound
related; however, the decreases were similar across groups and bore no
relationship to those observed among the female treatment groups. The
slightly lower mean body weight of certain of the treated groups
coupled with the increased levels of food consumption by some of the
treated groups suggests that the lower body weights were the result of
dilution of the nutritive value of the diet due to the presence of
anoxomer. Additional evidence of the non-specific rather than toxic
nature of the decreased weight was the fact that there were no
statistically significant differences in terminal organ or organ-to-
body weight ratios observed between treated and control groups.
During week 37, some rats exhibited clinical signs of
sialodacryoadenitis (SDA) virus. There was no treatment of this
normally self-limited disease and no effects were observed which were
interpreted as being detrimental to the conduct of the present study.
Gross eye findings included a slightly increased frequency of cloudy
eyes in high-dose animals during the latter part of the study, e.g.,
at week 99, the overall incidence ranged from about 2 to 9% for
control, low- and mid-dose groups and was 17% for the high-dose group.
The presumptive diagnosis of degenerative cataracts secondary to
probable senile retinal dystrophy was made. The findings did not
provide definite evidence of a compound-related effect in the high-
dose group; however, the slightly higher incidence suggested a
potential indirect effect on normal geriatric process. The frequency
of this finding across treatment groups became virtually the same by
the end of the study. Also, during week 37, 4 high-dose males were
found moribund, and 3 animals manifested posterior paresis. At
necropsy, suggestions of haemorrhage were present with dark red fluid
in the urinary bladder of 2 males, and large areas of diffuse redness
in the bladder wall of a third animal. A fourth animal found dead
during week 37 had haemorrhage and a large haematoma in the pelvic
area. Prothrombin determinations and platelet counts made at 55 weeks
were comparable to control animals. No additional animals or groups
suffered these lesions.
Other representative findings were: discoloration of pituitary,
lungs, liver, kidneys, adrenals and testes; enlargement, thickened
appearance or variations in size of pituitary, liver, spleen and
testes; ulcerated areas of the stomach; cloudy or opaque eyes; nodules
in various organs; masses in subcutaneous tissue, in body cavities and
associated with viscera and enlargement or discoloration of lymph
modes. A wide variety of spontaneous neoplasms and lesions were
observed. Among the commonly observed neoplasms, such as pituitary "C"
cell tumours of the thyroid, pheochromocytoma of the adrenals, mammary
fibroadenomas in females, monocytic leukaemia, testicular interstitial
cell tumours, and uterine endometrial stromal polyps. Statistical
analyses were conducted on the comparative incidences of monocytic
leukaemia, pituitary adenoma, adrenal pheochromocytoma and mammary
fibroadenoma in control group 1 and the high-dose group. The overall
incidence of these tumour types did not differ significantly between
the high-dose groups and their respective controls. An examination of
the pattern and occurrence of tumours in other organs and tissues
reveals no evidence of compound-related effects (Anon., 1980b).
Dog
Eight male and 8 female adult, pure-bred beagles were fed 0, 0.5,
1.58 or 5% anoxomer for 52 weeks.
No deaths occurred during the experimental period. Clinical
observations were occasional instances of emesis, soft stools and
diarrhoea, but these were observed in both treated and control groups.
The consistently lower weight gains of the high-dose animals are
considered to be treatment related. Food consumption of the treatment
groups did not vary significantly from controls; however, absolute
levels of food ingested were slightly depressed in the low- and mid-
dose males. Haematology and clinical chemistry evaluations were made
on weeks 4, 13, 26 and 52 and no findings suggesting a compound-
related effect were discovered in any test group. There were no
compound-related findings with regard to urinalysis data. There were
no treatment-related ocular changes in any of the test subjects.
At 13 weeks, 2 males and 2 females from each treatment group were
sacrificed and at 52 weeks 6 additional animals per test group were
terminated and subjected to gross and histopathological examination.
With respect to histomorphological alterations, there were only
occasional spontaneous disease lesions found and these were not
systematically associated with compound administration (Anon., 1979b).
OBSERVATIONS IN MAN
Six healthy, male human subjects received 13.8 mg or 50 µCi of
radiolabelled anoxomer orally. Urinary and faecal excretion were 0.02%
and 95%, respectively. Based upon data from blood and urine,
intestinal absorption was estimated to be less than 0.1% of the
administered dose.
Comments
Various metabolic studies show that no more than 0.2% of an
orally administered dose of anoxomer is absorbed in mice, rats,
rabbits, guinea-pigs and man. In spite of having a lipophilic
structure, anoxomer does not appear to selectively accumulate in
either lymph tissues or lipid-rich regions of the organism.
Acute oral toxicity studies in mice, rats and dogs with anoxomer
suggest that the LD50 for all these species is greater than 10 g/kg
bw. A multigeneration reproduction study in rats did not show any
consistent effects during the successive breedings, nor was there any
indication that the effects were more marked in successive generation.
Based on all the information from this study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet. The no-effect levels for teratogenicity studies in rats and
rabbits were, respectively, 2.5 g/kg and 1.0 g/kg bw per day. Anoxomer
was not mutagenic in a series of bacterial tests. It was not
carcinogenic in lifetime studies in both rats and mice. No other
significant compound-related effects were observed in these studies,
except in the rat study where there was an increase in the occurrence
of cloudy eyes, in the high-dose groups (5% of the diet) at week 99.
However, the frequency of the occurrence of this effect, which has
been tentatively diagnosed as degenerative cataracts secondary to
probable senile retinal dystrophy, became virtually the same in all
groups at termination of the study (week 104). Additional studies are
presently under way to determine if there is an anoxomer-related
increase in lenticular opacities.
A 1-year feeding study in dogs showed no compound-related
effects, except liver weight gains in dogs, maintained on diets
containing 5% anoxomer. No lenticular opacities were observed in any
of the test groups.
EVALUATION
Level causing no toxicological effect
15 800 ppm (1.58%) in the diet equivalent to 800 mg/kg bw.
Estimate of temporary acceptable daily intake for man
0-8 mg/kg bw.
FURTHER WORK OR INFORMATION
Required by 1984
Further information about the occurrence of senile cataract in
rats exposed to high levels of anoxomer in the diet.
REFERENCES
Anon. (1976a) Final report. Acute eye irritation potential study in
rabbits. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1976b) Final report. Primary skin irritation study in rabbits.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1977) Mutagenicity evaluation of D00079 (Lot No. 0314) in the
mouse lymphoma multiple endpoint test (MET). Unpublished study by
Dynapol, Inc., submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Anon. (1978) Gross and microscopic evaluation of mesenteric lymph
nodes in female rats. D00079. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979a) A three-generation reproduction and teratology study in
rats, D00079, final report. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979b) Final report. Chronic dietary administration in dogs.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1980a) Chronic dietary administration in mice, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1980b) Chronic toxicity study in mice, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981a) Teratology study in rats, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981b) Teratology study in rabbits, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981c) Hematological evaluations in male rats, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Dietrich, P. S., Brown, J. P. & Bakner, C. M. (1977) Phase II
mutagenicity testing of Poly AOTM-79: Microbial tests with
Salmonella/microsome assay, Escherichia coli and
Saccharomyces cerevisiae. Unpublished study by Dynapol, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977a) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in guinea pigs.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977b) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in rabbits.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977c) Intestinal absorption of
the polymeric antioxidant [14C]-D00079 in rat. V. Absorption in
rats previously maintained for 90 days on 5% D00079 in the diet.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. (1981) Distribution of radioactivity in internal
organs and tissues of the rat after oral administration of a high
activity dose of Poly AOTM79-[14C]. II. Determined at six
different times between 1 and 28 days after dosing. Unpublished
study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Halladay, S. C. et al. (1978) The bile duct ligated animal: A model
for determining total intestinal absorption, Drug Chem.
Toxicol., 1, 203-213
Jorgenson, T. A. & Rushbrook, C. J. (1976) (1) Acute oral toxicity
studies in rats, mice and dogs; (2) Acute intraperitoneal
toxicity in rats, mice and dogs; (3) Maximum tolerated dose study
in rats. Unpublished study by Stanford Research Institute,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Jorgenson, T. A. & Rushbrook, C. J. (1977) Toxicity study of D00079, A
ninety-day subacute study in rats. Unpublished study by Stanford
Research Institute, submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Parkinson, T. M., Honohan, T. & Enderlin, F. E. (1978) Intestinal
absorption, distribution and excretion of an orally administered
polymeric antioxidant in rats and mice, Fd. Cosmet. Toxicol.,
16, 321-330
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1977) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in hepatic tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1978) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in intestinal tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Walson, P. D. et al. (1979) Intestinal absorption of two potential
polymeric food additives in man, Fd. Cosmet Toxicol., 17,
201-203
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of C14-anoxomer, 10 mg in Sprague-Dawley rats,
1.8 mg in IRC/SM mice, 0.5 mg in Hartley guinea-pigs and 0.6 mg in
Dutch-belted rabbits indicated that only 0.2-0.6% of the administered
compound is absorbed. The recovery of radioactivity in these studies
varied from 78 to 110% (Halladay et al., 1978; Parkinson et al., 1978;
Enderlin & Halladay, 1977a, 1977b). Absorption of a single dose of
C14-anoxomer was no greater in Sprague-Dawley rats fed 5% anoxomer
for 90 days than in rats given C14-anoxomer acutely (Enderlin &
Halladay, 1977c). Following oral dosing of 10 mg of C14-anoxomer, the
residual radioactivity was assessed in the mesenteric lymph nodes 30
days later. There was some indication from this study that anoxomer
was selectively partitioning in lipid stores (Enderlin & Halladay,
1977c).
Effects on enzymes and other biochemical parameters
2.8 and 5 g/kg of anoxomer was added to the basal diet of male
Sprague-Dawley rats for 90 and 60 days, respectively. The level of
cytochrome P-450, cytochrome C reductase activity and microsomal
mixed-function oxygenase (MFO) activity for aminopyrine demethylase
(APDM), p-nitroanisole demethylase (NADM), hexobarbital oxidase (HO)
and benzo[alpha]pyrene hydroxylase (BH) were measured in the hepatic
S-9 homogenates. There were no differences between control and
anoxomer-fed animals, with regard to liver weights and in hepatic
homogenates. There were no differences in the activity of cytochrome
P-450, cytochrome C reductase or any of the MFO enzymes (Ryerson et
al., 1977).
In another study, 2.8 and 5 g/kg of anoxomer was added to the
basal diet of male Sprague-Dawley rats for 90 and 60 days,
respectively. The levels of cytochrome P-450, cytochrome C reductase
activity and microsomal mixed-function oxygenase (MFO) activity for
the hydrolysis of indophenol acetate (IPA) and benzo[alpha]-pyrene
hydroxylase (BH) was measured in the intestinal S-9 homogenates.
Anoxomer caused no alterations in the concentration of cytochrome
P-450 and cytochrome C reductase activity over the duration of the
90-day study (Ryerson et al., 1978).
TOXICOLOGICAL STUDIES
Special studies on blood coagulation
During the conduct of the chronic rat feeding study, 4 high-dose
(2500 mg/kg bw) male rats suffered unexplained and lethal
haemorrhaging of the pelvic region. Thus, a study to assess the
influence of anoxomer on blood clotting factors in male rats was
conducted. Ten male rats in each of 2 groups received either 0 or
2500 mg/kg bw of anoxomer in basal lab diet for 21 days. There was no
statistically significant differences between treated and control
animals in prothrombin time, activated partial thromboplastin time,
fibrinogen concentration or bleeding time. There was a statistically
significant increase in platelet count in treated males (Anon.,
1981c). Based on these observations, anoxomer appears to be free of
any influence on blood clotting factors.
Special studies on dermal irritation
Six healthy, New Zealand white rabbits were used in this test.
Skin reactions were scored by the Draize system and, since the primary
irritation score was 0.2, anoxomer was not judged to be a primary
irritant (Anon., 1976b).
Special studies on eye irritation
In 6 healthy, New Zealand white rabbits, 63 mg of anoxomer was
instilled in the conjunctival sac of the left eye with the right eye
serving as control. Anoxomer was not judged to be an eye irritant,
since findings of irritation were present in only one of 6 test
subjects (Anon., 1976a).
Special studies on influence on lymphatic tissues
Female Sprague-Dawley rats were given 5% anoxomer in the diet for
28 weeks. No gross or microscopic alterations were noted in the
mesenteric lymph nodes of any control or high-dose animals that were
examined (Anon., 1978).
Eighteen female rats (Simonsen strain) were administered 10.74 mg
(39 µCi) of 14C-labelled anoxomer by gavage = 50 mg/kg bw. On each of
6 days (days 1, 3, 7, 14, 21 and 28) following dosing, 3 animals were
sacrificed and the level of radioactivity assessed in liver, spleen,
kidneys, stomach, small intestine, caecum, large intestine, intestinal
contents, lymph nodes (mesenteric, axillary, cervical and
inguinofemoral), fat (from mesenteric, peri-uterine and subcutaneous
regions) and muscle (gracilis). Specimens from blood, urine and faeces
were also collected and analysed for radioactivity. Control (undosed)
animals were used to determine the limits of detection of the
radiolabelled anoxomer. The levels initially detected were negligible
in all tissues but the gastrointestinal tract. Even the initially high
levels found in the stomach and large and small intestine declined
rapidly. This study gave no evidence of a selective tissue
accumulation of anoxomer (Enderlin, 1981). This contrasts with the
earlier metabolism studies (Enderlin & Halladay, 1977c) which
suggested anoxomer concentrations in fat tissue. This earlier
observation may have been due to measurement uncertainties (error)
related to counts per minute extrapolations from small tissue samples
(50 mg). At the many-fold higher doses used in the subchronic and
lifetime animal studies (10 g/kg bw), some abnormal or selective
accumulation in fat or lymphoid tissue may occur: however, at the
doses used in this study (about 50 mg/kg bw), there is no evidence of
selective accumulation of anoxomer in either fat or lymphoid tissues.
Special studies on mutagenicity
Anoxomer, in concentrations up to 5 mg/plate, was neither
mutagenic nor cytotoxic in the Ames test using 5 strains of his-
Salmonella typhimurium (strains used were sensitive for both base-
pair and frame-shift mutations) either with or without activation by
rat microsomal preparation. Four strains of trp-Escherichia coli
were employed to test anoxomer in concentrations up to 1 mg/plate with
and without metabolic activation. Anoxomer was nonmutagenic in this
bacterial test system (Dietrich et al., 1977).
Anoxomer was evaluated for its ability to induce mitotic
crossing over or intergenic recombination and reverse mutation of
Saccharomyces cerevisiae. Anoxomer was tested in concentrations up
to 5 mg/ml and at time periods up to 16 hours with and without
metabolic activation. No dose- or time-related effects were seen with
any of these endpoints (Dietrich et al., 1977).
The potential mutagenicity of anoxomer in mammalian cells was
tested in L5178Y mice lymphoma cells. There were no effects on forward
point mutations at the thymidine kinase locus, sister chromatid
exchanges or chromosome aberrations (Anon., 1977). The validity of
this particular study is questioned at doses above 0.32 mg/ml because
of precipitation of the test materials, and because the incidence of
chromosomal aberrations was not different between the positive and
solvent controls.
Special studies on reproduction
A 3-generation reproduction and teratology study was conducted
with anoxomer in Sprague-Dawley rats. Control animals received basal
diets while test animals received 0.5, 1.58 or 5% of anoxomer in basal
diet throughout the study. Each parental generation consisted of 30
males and 30 females.
There was a statistically significant decrease in neonatal
survival index in the 1.58 and 5% offspring and in the weaning
survival index of all 3 treated groups in the F1a litters. There was
a significant decrease in male birth weight and in male and female
weaning weights at the 5% level in the F1b litters. The neonatal and
weaning survival indices as well as weaning weights were significantly
reduced in the 5% groups of the F2a litters. Weaning survival was
decreased in the 0.5 and 5% offspring, and weaning weight of all males
in the same groups was decreased. In the F3a, F3b and F3c litters,
the pregnancy rate was low in all groups including the control
animals. However, none of these effects were observed consistently
during the successive generations, nor was there any indication that
these effects were more marked during the successive breedings. Based
on all the information from the study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet.
The F3d litters were utilized for the teratology phase of this
study. The test animals were sacrificed on day 19. There was no effect
on numbers of corpora lutea, implantations, resorptions, liver of dead
foetuses for any of the groups. In addition, there was no treatment-
related effects on body weight or size or on the incidences of
visceral or skeletal anomalies (Anon., 1979a).
Special studies on teratogenicity
Thirty female Sprague-Dawley rats, each of 4 treatment groups,
were fed anoxomer at 0, 250, 800 or 2500 mg/kg bw per day on days 6-15
of gestation. The test animals were sacrificed on gestation day 20. No
compound-related effects were found in maternal body weights, deaths
or pathology at necropsy. No compound-related differences were
observed in pregnancy rate, number of corpora lutea, implantations,
resorptions, live foetuses, foetal body weight or length (Anon.,
1981a). The mean number of visceral and skeletal variants increased in
the treated groups, but the increase did not appear to be dose
related.
In 2 studies, A and B, 20 or 25 female rabbits, respectively in
each of 4 treatment groups received anoxomer at 0, 250, 500 or
1000 mg/kg bw per day by oral intubation from day 6 through day 18 and
sacrifice was on gestation day 29. In both studies, maternal gross
pathology did not indicate any treatment-related effects. No effect
was apparent in the number of dead foetuses, live foetuses, or sex
ratio. There appeared to be no treatment-related increase in skeletal
problems in both studies. In study A, there was a dose-related
increase in dilated pelvises and ectopic kidneys in all groups. Study
B utilized the same protocol, but did not confirm the renal effects
(Anon., 1979, 1981a).
Acute toxicity
LD50 Reference
Animal Route (g/kg bw)
Mouse Oral 10 Anon., 1981c
i.p. 10 Jorgenson & Rushbrook, 1976
Rat (Sprague-Dawley) Oral 10 Jorgenson & Rushbrook, 1976
i.p. 10 Jorgenson & Rushbrook, 1976
Dog Oral 10 Jorgenson & Rushbrook, 1976
i.p. 5 Jorgenson & Rushbrook, 1976
Short-term studies
Rat
Fifty young, adult Sprague-Dawley rats (25 males and 25 females)
in 4 different groups were fed 0, 0.5, 1.58 or 5% anoxomer in rat diet
for 90 days. Water intake, activity and behaviour were normal and
comparable for all groups. Body weights and food consumption for males
and females in all groups remained in the normal ranges for the
duration of the study. The haematology, differential counts, platelet
counts and reticulocyte counts of all groups were also within normal
limits. The serum enzyme and biochemistry were within the expected
limits of biological variation. Gross necropsy revealed a 15-20%
incidence of emphysema, slight haemorrhaging and/or congestion of the
lungs in all groups, including controls. This was probably the result
of handling and CO2 asphyxiation and not the result of treatment. Two
control males and 2 0.5% dose level females had cataracts. No dose-
response effects were evident and the gross findings were within
normal ranges for this strain of rat. All organ weights and organ-to-
body weight ratios were comparable between groups. Slight to moderate
hyperplasia of the lymph node was seen in 2 high-dose rats and
haemorrhage of the lymph node was observed in another high-dose rat.
Slight focal haemorrhage and slight focal congestion was found in the
thymus, respectively, in 2 high-dose rats (Jorgenson & Rushbrook,
1977).
Long-term studies
Mouse
One hundred and seventy-five sexually mature HAM/ICR mice were
selected on the basis of good health and randomly assigned either to
one of two control groups or to treatment groups and fed 0, 0.5, 1.58
and 5% anoxomer for 104 weeks to assess the chronic toxicity and
carcinogenicity potential of this substance. After 1 week, animals
were mated. Female mice were maintained on control or test diet
throughout gestation, delivery and lactation. Fifty animals per sex
per dose were then randomly assigned to the chronic feeding phase of
the present study.
There were no compound-related effects indicated by the survival
data from the test and control groups. While the high-dose male and
female groups' mean weights were decreased during the first 6 months
of the study, by the end of the first year, there were no significant
differences in body weights of the offspring of the high-dose F0
groups. The early decreased body weight would appear to reflect the
test animals exposure in utero to the test compound. Thus, the
decreased body weights of the high-dose males and females in the early
phase were considered treatment related. This effect on offspring body
weight may have been due to simple dilution of the nutritive value of
the diet because of the presence of the diet compound. Problems of
food spillage, however, made food consumption data of questionable
reliability (Anon., 1980a).
At termination of the study at 104 weeks, gross pathology was
characterized in large measure as being comparable across treatment
and control groups. Certain lesions occurred with nearly equal
frequency across all treatment groups, e.g., discoloration of the
lungs, enlarged spleens and darkened areas or lobes of the liver.
The results of histopathological findings were essentially
comparable between the high-dose treatment group and control group 1.
The more common incidental findings observed were: chronic murine
pneumonia, hydronephrosis, focal ulceration of the gastric mucosa,
malignant lymphoma originating in the thymus, hepatocellular carcinoma
and chronic interstitial nephritis. None of these lesions occurred in
other than an apparently random distribution. Statistical analysis was
performed on the comparative incidences of malignant lymphoma (all
types combined alveolar/bronchiolar carcinoma and hepatocellular
carcinoma in control group 1 and the high-dose groups. These 3 tumour
categories were selected since they were representative of the tumour
types occurring most frequently. There was not a statistically
significant increase in incidence of these tumours in the treated
group compared to control group 1 (Anon., 1980a). An examination of
the pattern and occurrence of tumours in other organs and tissues did
not reveal a frequency of distribution across treatment groups which
is compound related.
Rat
Fifty animals (Fischer 344 strain) per sex per dose were fed 0,
0.5, 1.58 or 5% anoxomer for 130 weeks in this study to assess the
chronic toxicity and carcinogenicity potential of this substance. The
parents of these animals had also been fed 0, 0.5, 1.58 or 5%
anoxomer. Exposure to anoxomer continued during mating, gestation,
delivery and lactation.
Survival in all groups at 18 months exceeded the 55% protocol
requirement. The higher mortality experienced by the mid-dose males is
considered to be incidental and not compound related. Anoxomer
administration resulted in statistically significant decreases in body
weight gain (growth rates) through week 51 in low- and high-dose
males. In addition, during the chronic feeding phase of the study, the
mean body weights of the 3 treated male groups were consistently lower
than the 2 control groups. This finding is considered to be compound
related; however, the decreases were similar across groups and bore no
relationship to those observed among the female treatment groups. The
slightly lower mean body weight of certain of the treated groups
coupled with the increased levels of food consumption by some of the
treated groups suggests that the lower body weights were the result of
dilution of the nutritive value of the diet due to the presence of
anoxomer. Additional evidence of the non-specific rather than toxic
nature of the decreased weight was the fact that there were no
statistically significant differences in terminal organ or organ-to-
body weight ratios observed between treated and control groups.
During week 37, some rats exhibited clinical signs of
sialodacryoadenitis (SDA) virus. There was no treatment of this
normally self-limited disease and no effects were observed which were
interpreted as being detrimental to the conduct of the present study.
Gross eye findings included a slightly increased frequency of cloudy
eyes in high-dose animals during the latter part of the study, e.g.,
at week 99, the overall incidence ranged from about 2 to 9% for
control, low- and mid-dose groups and was 17% for the high-dose group.
The presumptive diagnosis of degenerative cataracts secondary to
probable senile retinal dystrophy was made. The findings did not
provide definite evidence of a compound-related effect in the high-
dose group; however, the slightly higher incidence suggested a
potential indirect effect on normal geriatric process. The frequency
of this finding across treatment groups became virtually the same by
the end of the study. Also, during week 37, 4 high-dose males were
found moribund, and 3 animals manifested posterior paresis. At
necropsy, suggestions of haemorrhage were present with dark red fluid
in the urinary bladder of 2 males, and large areas of diffuse redness
in the bladder wall of a third animal. A fourth animal found dead
during week 37 had haemorrhage and a large haematoma in the pelvic
area. Prothrombin determinations and platelet counts made at 55 weeks
were comparable to control animals. No additional animals or groups
suffered these lesions.
Other representative findings were: discoloration of pituitary,
lungs, liver, kidneys, adrenals and testes; enlargement, thickened
appearance or variations in size of pituitary, liver, spleen and
testes; ulcerated areas of the stomach; cloudy or opaque eyes; nodules
in various organs; masses in subcutaneous tissue, in body cavities and
associated with viscera and enlargement or discoloration of lymph
modes. A wide variety of spontaneous neoplasms and lesions were
observed. Among the commonly observed neoplasms, such as pituitary "C"
cell tumours of the thyroid, pheochromocytoma of the adrenals, mammary
fibroadenomas in females, monocytic leukaemia, testicular interstitial
cell tumours, and uterine endometrial stromal polyps. Statistical
analyses were conducted on the comparative incidences of monocytic
leukaemia, pituitary adenoma, adrenal pheochromocytoma and mammary
fibroadenoma in control group 1 and the high-dose group. The overall
incidence of these tumour types did not differ significantly between
the high-dose groups and their respective controls. An examination of
the pattern and occurrence of tumours in other organs and tissues
reveals no evidence of compound-related effects (Anon., 1980b).
Dog
Eight male and 8 female adult, pure-bred beagles were fed 0, 0.5,
1.58 or 5% anoxomer for 52 weeks.
No deaths occurred during the experimental period. Clinical
observations were occasional instances of emesis, soft stools and
diarrhoea, but these were observed in both treated and control groups.
The consistently lower weight gains of the high-dose animals are
considered to be treatment related. Food consumption of the treatment
groups did not vary significantly from controls; however, absolute
levels of food ingested were slightly depressed in the low- and mid-
dose males. Haematology and clinical chemistry evaluations were made
on weeks 4, 13, 26 and 52 and no findings suggesting a compound-
related effect were discovered in any test group. There were no
compound-related findings with regard to urinalysis data. There were
no treatment-related ocular changes in any of the test subjects.
At 13 weeks, 2 males and 2 females from each treatment group were
sacrificed and at 52 weeks 6 additional animals per test group were
terminated and subjected to gross and histopathological examination.
With respect to histomorphological alterations, there were only
occasional spontaneous disease lesions found and these were not
systematically associated with compound administration (Anon., 1979b).
OBSERVATIONS IN MAN
Six healthy, male human subjects received 13.8 mg or 50 µCi of
radiolabelled anoxomer orally. Urinary and faecal excretion were 0.02%
and 95%, respectively. Based upon data from blood and urine,
intestinal absorption was estimated to be less than 0.1% of the
administered dose.
Comments
Various metabolic studies show that no more than 0.2% of an
orally administered dose of anoxomer is absorbed in mice, rats,
rabbits, guinea-pigs and man. In spite of having a lipophilic
structure, anoxomer does not appear to selectively accumulate in
either lymph tissues or lipid-rich regions of the organism.
Acute oral toxicity studies in mice, rats and dogs with anoxomer
suggest that the LD50 for all these species is greater than 10 g/kg
bw. A multigeneration reproduction study in rats did not show any
consistent effects during the successive breedings, nor was there any
indication that the effects were more marked in successive generation.
Based on all the information from this study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet. The no-effect levels for teratogenicity studies in rats and
rabbits were, respectively, 2.5 g/kg and 1.0 g/kg bw per day. Anoxomer
was not mutagenic in a series of bacterial tests. It was not
carcinogenic in lifetime studies in both rats and mice. No other
significant compound-related effects were observed in these studies,
except in the rat study where there was an increase in the occurrence
of cloudy eyes, in the high-dose groups (5% of the diet) at week 99.
However, the frequency of the occurrence of this effect, which has
been tentatively diagnosed as degenerative cataracts secondary to
probable senile retinal dystrophy, became virtually the same in all
groups at termination of the study (week 104). Additional studies are
presently under way to determine if there is an anoxomer-related
increase in lenticular opacities.
A 1-year feeding study in dogs showed no compound-related
effects, except liver weight gains in dogs, maintained on diets
containing 5% anoxomer. No lenticular opacities were observed in any
of the test groups.
EVALUATION
Level causing no toxicological effect
15 800 ppm (1.58%) in the diet equivalent to 800 mg/kg bw.
Estimate of temporary acceptable daily intake for man
0-8 mg/kg bw.
FURTHER WORK OR INFORMATION
Required by 1984
Further information about the occurrence of senile cataract in
rats exposed to high levels of anoxomer in the diet.
REFERENCES
Anon. (1976a) Final report. Acute eye irritation potential study in
rabbits. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1976b) Final report. Primary skin irritation study in rabbits.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1977) Mutagenicity evaluation of D00079 (Lot No. 0314) in the
mouse lymphoma multiple endpoint test (MET). Unpublished study by
Dynapol, Inc., submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Anon. (1978) Gross and microscopic evaluation of mesenteric lymph
nodes in female rats. D00079. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979a) A three-generation reproduction and teratology study in
rats, D00079, final report. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979b) Final report. Chronic dietary administration in dogs.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1980a) Chronic dietary administration in mice, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1980b) Chronic toxicity study in mice, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981a) Teratology study in rats, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981b) Teratology study in rabbits, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981c) Hematological evaluations in male rats, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Dietrich, P. S., Brown, J. P. & Bakner, C. M. (1977) Phase II
mutagenicity testing of Poly AOTM-79: Microbial tests with
Salmonella/microsome assay, Escherichia coli and
Saccharomyces cerevisiae. Unpublished study by Dynapol, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977a) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in guinea pigs.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977b) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in rabbits.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977c) Intestinal absorption of
the polymeric antioxidant [14C]-D00079 in rat. V. Absorption in
rats previously maintained for 90 days on 5% D00079 in the diet.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. (1981) Distribution of radioactivity in internal
organs and tissues of the rat after oral administration of a high
activity dose of Poly AOTM79-[14C]. II. Determined at six
different times between 1 and 28 days after dosing. Unpublished
study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Halladay, S. C. et al. (1978) The bile duct ligated animal: A model
for determining total intestinal absorption, Drug Chem.
Toxicol., 1, 203-213
Jorgenson, T. A. & Rushbrook, C. J. (1976) (1) Acute oral toxicity
studies in rats, mice and dogs; (2) Acute intraperitoneal
toxicity in rats, mice and dogs; (3) Maximum tolerated dose study
in rats. Unpublished study by Stanford Research Institute,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Jorgenson, T. A. & Rushbrook, C. J. (1977) Toxicity study of D00079, A
ninety-day subacute study in rats. Unpublished study by Stanford
Research Institute, submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Parkinson, T. M., Honohan, T. & Enderlin, F. E. (1978) Intestinal
absorption, distribution and excretion of an orally administered
polymeric antioxidant in rats and mice, Fd. Cosmet. Toxicol.,
16, 321-330
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1977) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in hepatic tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1978) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in intestinal tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Walson, P. D. et al. (1979) Intestinal absorption of two potential
polymeric food additives in man, Fd. Cosmet Toxicol., 17,
201-203
 BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of C14-anoxomer, 10 mg in Sprague-Dawley rats,
1.8 mg in IRC/SM mice, 0.5 mg in Hartley guinea-pigs and 0.6 mg in
Dutch-belted rabbits indicated that only 0.2-0.6% of the administered
compound is absorbed. The recovery of radioactivity in these studies
varied from 78 to 110% (Halladay et al., 1978; Parkinson et al., 1978;
Enderlin & Halladay, 1977a, 1977b). Absorption of a single dose of
C14-anoxomer was no greater in Sprague-Dawley rats fed 5% anoxomer
for 90 days than in rats given C14-anoxomer acutely (Enderlin &
Halladay, 1977c). Following oral dosing of 10 mg of C14-anoxomer, the
residual radioactivity was assessed in the mesenteric lymph nodes 30
days later. There was some indication from this study that anoxomer
was selectively partitioning in lipid stores (Enderlin & Halladay,
1977c).
Effects on enzymes and other biochemical parameters
2.8 and 5 g/kg of anoxomer was added to the basal diet of male
Sprague-Dawley rats for 90 and 60 days, respectively. The level of
cytochrome P-450, cytochrome C reductase activity and microsomal
mixed-function oxygenase (MFO) activity for aminopyrine demethylase
(APDM), p-nitroanisole demethylase (NADM), hexobarbital oxidase (HO)
and benzo[alpha]pyrene hydroxylase (BH) were measured in the hepatic
S-9 homogenates. There were no differences between control and
anoxomer-fed animals, with regard to liver weights and in hepatic
homogenates. There were no differences in the activity of cytochrome
P-450, cytochrome C reductase or any of the MFO enzymes (Ryerson et
al., 1977).
In another study, 2.8 and 5 g/kg of anoxomer was added to the
basal diet of male Sprague-Dawley rats for 90 and 60 days,
respectively. The levels of cytochrome P-450, cytochrome C reductase
activity and microsomal mixed-function oxygenase (MFO) activity for
the hydrolysis of indophenol acetate (IPA) and benzo[alpha]-pyrene
hydroxylase (BH) was measured in the intestinal S-9 homogenates.
Anoxomer caused no alterations in the concentration of cytochrome
P-450 and cytochrome C reductase activity over the duration of the
90-day study (Ryerson et al., 1978).
TOXICOLOGICAL STUDIES
Special studies on blood coagulation
During the conduct of the chronic rat feeding study, 4 high-dose
(2500 mg/kg bw) male rats suffered unexplained and lethal
haemorrhaging of the pelvic region. Thus, a study to assess the
influence of anoxomer on blood clotting factors in male rats was
conducted. Ten male rats in each of 2 groups received either 0 or
2500 mg/kg bw of anoxomer in basal lab diet for 21 days. There was no
statistically significant differences between treated and control
animals in prothrombin time, activated partial thromboplastin time,
fibrinogen concentration or bleeding time. There was a statistically
significant increase in platelet count in treated males (Anon.,
1981c). Based on these observations, anoxomer appears to be free of
any influence on blood clotting factors.
Special studies on dermal irritation
Six healthy, New Zealand white rabbits were used in this test.
Skin reactions were scored by the Draize system and, since the primary
irritation score was 0.2, anoxomer was not judged to be a primary
irritant (Anon., 1976b).
Special studies on eye irritation
In 6 healthy, New Zealand white rabbits, 63 mg of anoxomer was
instilled in the conjunctival sac of the left eye with the right eye
serving as control. Anoxomer was not judged to be an eye irritant,
since findings of irritation were present in only one of 6 test
subjects (Anon., 1976a).
Special studies on influence on lymphatic tissues
Female Sprague-Dawley rats were given 5% anoxomer in the diet for
28 weeks. No gross or microscopic alterations were noted in the
mesenteric lymph nodes of any control or high-dose animals that were
examined (Anon., 1978).
Eighteen female rats (Simonsen strain) were administered 10.74 mg
(39 µCi) of 14C-labelled anoxomer by gavage = 50 mg/kg bw. On each of
6 days (days 1, 3, 7, 14, 21 and 28) following dosing, 3 animals were
sacrificed and the level of radioactivity assessed in liver, spleen,
kidneys, stomach, small intestine, caecum, large intestine, intestinal
contents, lymph nodes (mesenteric, axillary, cervical and
inguinofemoral), fat (from mesenteric, peri-uterine and subcutaneous
regions) and muscle (gracilis). Specimens from blood, urine and faeces
were also collected and analysed for radioactivity. Control (undosed)
animals were used to determine the limits of detection of the
radiolabelled anoxomer. The levels initially detected were negligible
in all tissues but the gastrointestinal tract. Even the initially high
levels found in the stomach and large and small intestine declined
rapidly. This study gave no evidence of a selective tissue
accumulation of anoxomer (Enderlin, 1981). This contrasts with the
earlier metabolism studies (Enderlin & Halladay, 1977c) which
suggested anoxomer concentrations in fat tissue. This earlier
observation may have been due to measurement uncertainties (error)
related to counts per minute extrapolations from small tissue samples
(50 mg). At the many-fold higher doses used in the subchronic and
lifetime animal studies (10 g/kg bw), some abnormal or selective
accumulation in fat or lymphoid tissue may occur: however, at the
doses used in this study (about 50 mg/kg bw), there is no evidence of
selective accumulation of anoxomer in either fat or lymphoid tissues.
Special studies on mutagenicity
Anoxomer, in concentrations up to 5 mg/plate, was neither
mutagenic nor cytotoxic in the Ames test using 5 strains of his-
Salmonella typhimurium (strains used were sensitive for both base-
pair and frame-shift mutations) either with or without activation by
rat microsomal preparation. Four strains of trp-Escherichia coli
were employed to test anoxomer in concentrations up to 1 mg/plate with
and without metabolic activation. Anoxomer was nonmutagenic in this
bacterial test system (Dietrich et al., 1977).
Anoxomer was evaluated for its ability to induce mitotic
crossing over or intergenic recombination and reverse mutation of
Saccharomyces cerevisiae. Anoxomer was tested in concentrations up
to 5 mg/ml and at time periods up to 16 hours with and without
metabolic activation. No dose- or time-related effects were seen with
any of these endpoints (Dietrich et al., 1977).
The potential mutagenicity of anoxomer in mammalian cells was
tested in L5178Y mice lymphoma cells. There were no effects on forward
point mutations at the thymidine kinase locus, sister chromatid
exchanges or chromosome aberrations (Anon., 1977). The validity of
this particular study is questioned at doses above 0.32 mg/ml because
of precipitation of the test materials, and because the incidence of
chromosomal aberrations was not different between the positive and
solvent controls.
Special studies on reproduction
A 3-generation reproduction and teratology study was conducted
with anoxomer in Sprague-Dawley rats. Control animals received basal
diets while test animals received 0.5, 1.58 or 5% of anoxomer in basal
diet throughout the study. Each parental generation consisted of 30
males and 30 females.
There was a statistically significant decrease in neonatal
survival index in the 1.58 and 5% offspring and in the weaning
survival index of all 3 treated groups in the F1a litters. There was
a significant decrease in male birth weight and in male and female
weaning weights at the 5% level in the F1b litters. The neonatal and
weaning survival indices as well as weaning weights were significantly
reduced in the 5% groups of the F2a litters. Weaning survival was
decreased in the 0.5 and 5% offspring, and weaning weight of all males
in the same groups was decreased. In the F3a, F3b and F3c litters,
the pregnancy rate was low in all groups including the control
animals. However, none of these effects were observed consistently
during the successive generations, nor was there any indication that
these effects were more marked during the successive breedings. Based
on all the information from the study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet.
The F3d litters were utilized for the teratology phase of this
study. The test animals were sacrificed on day 19. There was no effect
on numbers of corpora lutea, implantations, resorptions, liver of dead
foetuses for any of the groups. In addition, there was no treatment-
related effects on body weight or size or on the incidences of
visceral or skeletal anomalies (Anon., 1979a).
Special studies on teratogenicity
Thirty female Sprague-Dawley rats, each of 4 treatment groups,
were fed anoxomer at 0, 250, 800 or 2500 mg/kg bw per day on days 6-15
of gestation. The test animals were sacrificed on gestation day 20. No
compound-related effects were found in maternal body weights, deaths
or pathology at necropsy. No compound-related differences were
observed in pregnancy rate, number of corpora lutea, implantations,
resorptions, live foetuses, foetal body weight or length (Anon.,
1981a). The mean number of visceral and skeletal variants increased in
the treated groups, but the increase did not appear to be dose
related.
In 2 studies, A and B, 20 or 25 female rabbits, respectively in
each of 4 treatment groups received anoxomer at 0, 250, 500 or
1000 mg/kg bw per day by oral intubation from day 6 through day 18 and
sacrifice was on gestation day 29. In both studies, maternal gross
pathology did not indicate any treatment-related effects. No effect
was apparent in the number of dead foetuses, live foetuses, or sex
ratio. There appeared to be no treatment-related increase in skeletal
problems in both studies. In study A, there was a dose-related
increase in dilated pelvises and ectopic kidneys in all groups. Study
B utilized the same protocol, but did not confirm the renal effects
(Anon., 1979, 1981a).
Acute toxicity
LD50 Reference
Animal Route (g/kg bw)
Mouse Oral 10 Anon., 1981c
i.p. 10 Jorgenson & Rushbrook, 1976
Rat (Sprague-Dawley) Oral 10 Jorgenson & Rushbrook, 1976
i.p. 10 Jorgenson & Rushbrook, 1976
Dog Oral 10 Jorgenson & Rushbrook, 1976
i.p. 5 Jorgenson & Rushbrook, 1976
Short-term studies
Rat
Fifty young, adult Sprague-Dawley rats (25 males and 25 females)
in 4 different groups were fed 0, 0.5, 1.58 or 5% anoxomer in rat diet
for 90 days. Water intake, activity and behaviour were normal and
comparable for all groups. Body weights and food consumption for males
and females in all groups remained in the normal ranges for the
duration of the study. The haematology, differential counts, platelet
counts and reticulocyte counts of all groups were also within normal
limits. The serum enzyme and biochemistry were within the expected
limits of biological variation. Gross necropsy revealed a 15-20%
incidence of emphysema, slight haemorrhaging and/or congestion of the
lungs in all groups, including controls. This was probably the result
of handling and CO2 asphyxiation and not the result of treatment. Two
control males and 2 0.5% dose level females had cataracts. No dose-
response effects were evident and the gross findings were within
normal ranges for this strain of rat. All organ weights and organ-to-
body weight ratios were comparable between groups. Slight to moderate
hyperplasia of the lymph node was seen in 2 high-dose rats and
haemorrhage of the lymph node was observed in another high-dose rat.
Slight focal haemorrhage and slight focal congestion was found in the
thymus, respectively, in 2 high-dose rats (Jorgenson & Rushbrook,
1977).
Long-term studies
Mouse
One hundred and seventy-five sexually mature HAM/ICR mice were
selected on the basis of good health and randomly assigned either to
one of two control groups or to treatment groups and fed 0, 0.5, 1.58
and 5% anoxomer for 104 weeks to assess the chronic toxicity and
carcinogenicity potential of this substance. After 1 week, animals
were mated. Female mice were maintained on control or test diet
throughout gestation, delivery and lactation. Fifty animals per sex
per dose were then randomly assigned to the chronic feeding phase of
the present study.
There were no compound-related effects indicated by the survival
data from the test and control groups. While the high-dose male and
female groups' mean weights were decreased during the first 6 months
of the study, by the end of the first year, there were no significant
differences in body weights of the offspring of the high-dose F0
groups. The early decreased body weight would appear to reflect the
test animals exposure in utero to the test compound. Thus, the
decreased body weights of the high-dose males and females in the early
phase were considered treatment related. This effect on offspring body
weight may have been due to simple dilution of the nutritive value of
the diet because of the presence of the diet compound. Problems of
food spillage, however, made food consumption data of questionable
reliability (Anon., 1980a).
At termination of the study at 104 weeks, gross pathology was
characterized in large measure as being comparable across treatment
and control groups. Certain lesions occurred with nearly equal
frequency across all treatment groups, e.g., discoloration of the
lungs, enlarged spleens and darkened areas or lobes of the liver.
The results of histopathological findings were essentially
comparable between the high-dose treatment group and control group 1.
The more common incidental findings observed were: chronic murine
pneumonia, hydronephrosis, focal ulceration of the gastric mucosa,
malignant lymphoma originating in the thymus, hepatocellular carcinoma
and chronic interstitial nephritis. None of these lesions occurred in
other than an apparently random distribution. Statistical analysis was
performed on the comparative incidences of malignant lymphoma (all
types combined alveolar/bronchiolar carcinoma and hepatocellular
carcinoma in control group 1 and the high-dose groups. These 3 tumour
categories were selected since they were representative of the tumour
types occurring most frequently. There was not a statistically
significant increase in incidence of these tumours in the treated
group compared to control group 1 (Anon., 1980a). An examination of
the pattern and occurrence of tumours in other organs and tissues did
not reveal a frequency of distribution across treatment groups which
is compound related.
Rat
Fifty animals (Fischer 344 strain) per sex per dose were fed 0,
0.5, 1.58 or 5% anoxomer for 130 weeks in this study to assess the
chronic toxicity and carcinogenicity potential of this substance. The
parents of these animals had also been fed 0, 0.5, 1.58 or 5%
anoxomer. Exposure to anoxomer continued during mating, gestation,
delivery and lactation.
Survival in all groups at 18 months exceeded the 55% protocol
requirement. The higher mortality experienced by the mid-dose males is
considered to be incidental and not compound related. Anoxomer
administration resulted in statistically significant decreases in body
weight gain (growth rates) through week 51 in low- and high-dose
males. In addition, during the chronic feeding phase of the study, the
mean body weights of the 3 treated male groups were consistently lower
than the 2 control groups. This finding is considered to be compound
related; however, the decreases were similar across groups and bore no
relationship to those observed among the female treatment groups. The
slightly lower mean body weight of certain of the treated groups
coupled with the increased levels of food consumption by some of the
treated groups suggests that the lower body weights were the result of
dilution of the nutritive value of the diet due to the presence of
anoxomer. Additional evidence of the non-specific rather than toxic
nature of the decreased weight was the fact that there were no
statistically significant differences in terminal organ or organ-to-
body weight ratios observed between treated and control groups.
During week 37, some rats exhibited clinical signs of
sialodacryoadenitis (SDA) virus. There was no treatment of this
normally self-limited disease and no effects were observed which were
interpreted as being detrimental to the conduct of the present study.
Gross eye findings included a slightly increased frequency of cloudy
eyes in high-dose animals during the latter part of the study, e.g.,
at week 99, the overall incidence ranged from about 2 to 9% for
control, low- and mid-dose groups and was 17% for the high-dose group.
The presumptive diagnosis of degenerative cataracts secondary to
probable senile retinal dystrophy was made. The findings did not
provide definite evidence of a compound-related effect in the high-
dose group; however, the slightly higher incidence suggested a
potential indirect effect on normal geriatric process. The frequency
of this finding across treatment groups became virtually the same by
the end of the study. Also, during week 37, 4 high-dose males were
found moribund, and 3 animals manifested posterior paresis. At
necropsy, suggestions of haemorrhage were present with dark red fluid
in the urinary bladder of 2 males, and large areas of diffuse redness
in the bladder wall of a third animal. A fourth animal found dead
during week 37 had haemorrhage and a large haematoma in the pelvic
area. Prothrombin determinations and platelet counts made at 55 weeks
were comparable to control animals. No additional animals or groups
suffered these lesions.
Other representative findings were: discoloration of pituitary,
lungs, liver, kidneys, adrenals and testes; enlargement, thickened
appearance or variations in size of pituitary, liver, spleen and
testes; ulcerated areas of the stomach; cloudy or opaque eyes; nodules
in various organs; masses in subcutaneous tissue, in body cavities and
associated with viscera and enlargement or discoloration of lymph
modes. A wide variety of spontaneous neoplasms and lesions were
observed. Among the commonly observed neoplasms, such as pituitary "C"
cell tumours of the thyroid, pheochromocytoma of the adrenals, mammary
fibroadenomas in females, monocytic leukaemia, testicular interstitial
cell tumours, and uterine endometrial stromal polyps. Statistical
analyses were conducted on the comparative incidences of monocytic
leukaemia, pituitary adenoma, adrenal pheochromocytoma and mammary
fibroadenoma in control group 1 and the high-dose group. The overall
incidence of these tumour types did not differ significantly between
the high-dose groups and their respective controls. An examination of
the pattern and occurrence of tumours in other organs and tissues
reveals no evidence of compound-related effects (Anon., 1980b).
Dog
Eight male and 8 female adult, pure-bred beagles were fed 0, 0.5,
1.58 or 5% anoxomer for 52 weeks.
No deaths occurred during the experimental period. Clinical
observations were occasional instances of emesis, soft stools and
diarrhoea, but these were observed in both treated and control groups.
The consistently lower weight gains of the high-dose animals are
considered to be treatment related. Food consumption of the treatment
groups did not vary significantly from controls; however, absolute
levels of food ingested were slightly depressed in the low- and mid-
dose males. Haematology and clinical chemistry evaluations were made
on weeks 4, 13, 26 and 52 and no findings suggesting a compound-
related effect were discovered in any test group. There were no
compound-related findings with regard to urinalysis data. There were
no treatment-related ocular changes in any of the test subjects.
At 13 weeks, 2 males and 2 females from each treatment group were
sacrificed and at 52 weeks 6 additional animals per test group were
terminated and subjected to gross and histopathological examination.
With respect to histomorphological alterations, there were only
occasional spontaneous disease lesions found and these were not
systematically associated with compound administration (Anon., 1979b).
OBSERVATIONS IN MAN
Six healthy, male human subjects received 13.8 mg or 50 µCi of
radiolabelled anoxomer orally. Urinary and faecal excretion were 0.02%
and 95%, respectively. Based upon data from blood and urine,
intestinal absorption was estimated to be less than 0.1% of the
administered dose.
Comments
Various metabolic studies show that no more than 0.2% of an
orally administered dose of anoxomer is absorbed in mice, rats,
rabbits, guinea-pigs and man. In spite of having a lipophilic
structure, anoxomer does not appear to selectively accumulate in
either lymph tissues or lipid-rich regions of the organism.
Acute oral toxicity studies in mice, rats and dogs with anoxomer
suggest that the LD50 for all these species is greater than 10 g/kg
bw. A multigeneration reproduction study in rats did not show any
consistent effects during the successive breedings, nor was there any
indication that the effects were more marked in successive generation.
Based on all the information from this study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet. The no-effect levels for teratogenicity studies in rats and
rabbits were, respectively, 2.5 g/kg and 1.0 g/kg bw per day. Anoxomer
was not mutagenic in a series of bacterial tests. It was not
carcinogenic in lifetime studies in both rats and mice. No other
significant compound-related effects were observed in these studies,
except in the rat study where there was an increase in the occurrence
of cloudy eyes, in the high-dose groups (5% of the diet) at week 99.
However, the frequency of the occurrence of this effect, which has
been tentatively diagnosed as degenerative cataracts secondary to
probable senile retinal dystrophy, became virtually the same in all
groups at termination of the study (week 104). Additional studies are
presently under way to determine if there is an anoxomer-related
increase in lenticular opacities.
A 1-year feeding study in dogs showed no compound-related
effects, except liver weight gains in dogs, maintained on diets
containing 5% anoxomer. No lenticular opacities were observed in any
of the test groups.
EVALUATION
Level causing no toxicological effect
15 800 ppm (1.58%) in the diet equivalent to 800 mg/kg bw.
Estimate of temporary acceptable daily intake for man
0-8 mg/kg bw.
FURTHER WORK OR INFORMATION
Required by 1984
Further information about the occurrence of senile cataract in
rats exposed to high levels of anoxomer in the diet.
REFERENCES
Anon. (1976a) Final report. Acute eye irritation potential study in
rabbits. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1976b) Final report. Primary skin irritation study in rabbits.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1977) Mutagenicity evaluation of D00079 (Lot No. 0314) in the
mouse lymphoma multiple endpoint test (MET). Unpublished study by
Dynapol, Inc., submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Anon. (1978) Gross and microscopic evaluation of mesenteric lymph
nodes in female rats. D00079. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979a) A three-generation reproduction and teratology study in
rats, D00079, final report. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979b) Final report. Chronic dietary administration in dogs.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1980a) Chronic dietary administration in mice, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1980b) Chronic toxicity study in mice, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981a) Teratology study in rats, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981b) Teratology study in rabbits, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981c) Hematological evaluations in male rats, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Dietrich, P. S., Brown, J. P. & Bakner, C. M. (1977) Phase II
mutagenicity testing of Poly AOTM-79: Microbial tests with
Salmonella/microsome assay, Escherichia coli and
Saccharomyces cerevisiae. Unpublished study by Dynapol, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977a) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in guinea pigs.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977b) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in rabbits.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977c) Intestinal absorption of
the polymeric antioxidant [14C]-D00079 in rat. V. Absorption in
rats previously maintained for 90 days on 5% D00079 in the diet.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. (1981) Distribution of radioactivity in internal
organs and tissues of the rat after oral administration of a high
activity dose of Poly AOTM79-[14C]. II. Determined at six
different times between 1 and 28 days after dosing. Unpublished
study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Halladay, S. C. et al. (1978) The bile duct ligated animal: A model
for determining total intestinal absorption, Drug Chem.
Toxicol., 1, 203-213
Jorgenson, T. A. & Rushbrook, C. J. (1976) (1) Acute oral toxicity
studies in rats, mice and dogs; (2) Acute intraperitoneal
toxicity in rats, mice and dogs; (3) Maximum tolerated dose study
in rats. Unpublished study by Stanford Research Institute,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Jorgenson, T. A. & Rushbrook, C. J. (1977) Toxicity study of D00079, A
ninety-day subacute study in rats. Unpublished study by Stanford
Research Institute, submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Parkinson, T. M., Honohan, T. & Enderlin, F. E. (1978) Intestinal
absorption, distribution and excretion of an orally administered
polymeric antioxidant in rats and mice, Fd. Cosmet. Toxicol.,
16, 321-330
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1977) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in hepatic tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1978) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in intestinal tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Walson, P. D. et al. (1979) Intestinal absorption of two potential
polymeric food additives in man, Fd. Cosmet Toxicol., 17,
201-203
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Absorption, distribution and excretion
Single oral doses of C14-anoxomer, 10 mg in Sprague-Dawley rats,
1.8 mg in IRC/SM mice, 0.5 mg in Hartley guinea-pigs and 0.6 mg in
Dutch-belted rabbits indicated that only 0.2-0.6% of the administered
compound is absorbed. The recovery of radioactivity in these studies
varied from 78 to 110% (Halladay et al., 1978; Parkinson et al., 1978;
Enderlin & Halladay, 1977a, 1977b). Absorption of a single dose of
C14-anoxomer was no greater in Sprague-Dawley rats fed 5% anoxomer
for 90 days than in rats given C14-anoxomer acutely (Enderlin &
Halladay, 1977c). Following oral dosing of 10 mg of C14-anoxomer, the
residual radioactivity was assessed in the mesenteric lymph nodes 30
days later. There was some indication from this study that anoxomer
was selectively partitioning in lipid stores (Enderlin & Halladay,
1977c).
Effects on enzymes and other biochemical parameters
2.8 and 5 g/kg of anoxomer was added to the basal diet of male
Sprague-Dawley rats for 90 and 60 days, respectively. The level of
cytochrome P-450, cytochrome C reductase activity and microsomal
mixed-function oxygenase (MFO) activity for aminopyrine demethylase
(APDM), p-nitroanisole demethylase (NADM), hexobarbital oxidase (HO)
and benzo[alpha]pyrene hydroxylase (BH) were measured in the hepatic
S-9 homogenates. There were no differences between control and
anoxomer-fed animals, with regard to liver weights and in hepatic
homogenates. There were no differences in the activity of cytochrome
P-450, cytochrome C reductase or any of the MFO enzymes (Ryerson et
al., 1977).
In another study, 2.8 and 5 g/kg of anoxomer was added to the
basal diet of male Sprague-Dawley rats for 90 and 60 days,
respectively. The levels of cytochrome P-450, cytochrome C reductase
activity and microsomal mixed-function oxygenase (MFO) activity for
the hydrolysis of indophenol acetate (IPA) and benzo[alpha]-pyrene
hydroxylase (BH) was measured in the intestinal S-9 homogenates.
Anoxomer caused no alterations in the concentration of cytochrome
P-450 and cytochrome C reductase activity over the duration of the
90-day study (Ryerson et al., 1978).
TOXICOLOGICAL STUDIES
Special studies on blood coagulation
During the conduct of the chronic rat feeding study, 4 high-dose
(2500 mg/kg bw) male rats suffered unexplained and lethal
haemorrhaging of the pelvic region. Thus, a study to assess the
influence of anoxomer on blood clotting factors in male rats was
conducted. Ten male rats in each of 2 groups received either 0 or
2500 mg/kg bw of anoxomer in basal lab diet for 21 days. There was no
statistically significant differences between treated and control
animals in prothrombin time, activated partial thromboplastin time,
fibrinogen concentration or bleeding time. There was a statistically
significant increase in platelet count in treated males (Anon.,
1981c). Based on these observations, anoxomer appears to be free of
any influence on blood clotting factors.
Special studies on dermal irritation
Six healthy, New Zealand white rabbits were used in this test.
Skin reactions were scored by the Draize system and, since the primary
irritation score was 0.2, anoxomer was not judged to be a primary
irritant (Anon., 1976b).
Special studies on eye irritation
In 6 healthy, New Zealand white rabbits, 63 mg of anoxomer was
instilled in the conjunctival sac of the left eye with the right eye
serving as control. Anoxomer was not judged to be an eye irritant,
since findings of irritation were present in only one of 6 test
subjects (Anon., 1976a).
Special studies on influence on lymphatic tissues
Female Sprague-Dawley rats were given 5% anoxomer in the diet for
28 weeks. No gross or microscopic alterations were noted in the
mesenteric lymph nodes of any control or high-dose animals that were
examined (Anon., 1978).
Eighteen female rats (Simonsen strain) were administered 10.74 mg
(39 µCi) of 14C-labelled anoxomer by gavage = 50 mg/kg bw. On each of
6 days (days 1, 3, 7, 14, 21 and 28) following dosing, 3 animals were
sacrificed and the level of radioactivity assessed in liver, spleen,
kidneys, stomach, small intestine, caecum, large intestine, intestinal
contents, lymph nodes (mesenteric, axillary, cervical and
inguinofemoral), fat (from mesenteric, peri-uterine and subcutaneous
regions) and muscle (gracilis). Specimens from blood, urine and faeces
were also collected and analysed for radioactivity. Control (undosed)
animals were used to determine the limits of detection of the
radiolabelled anoxomer. The levels initially detected were negligible
in all tissues but the gastrointestinal tract. Even the initially high
levels found in the stomach and large and small intestine declined
rapidly. This study gave no evidence of a selective tissue
accumulation of anoxomer (Enderlin, 1981). This contrasts with the
earlier metabolism studies (Enderlin & Halladay, 1977c) which
suggested anoxomer concentrations in fat tissue. This earlier
observation may have been due to measurement uncertainties (error)
related to counts per minute extrapolations from small tissue samples
(50 mg). At the many-fold higher doses used in the subchronic and
lifetime animal studies (10 g/kg bw), some abnormal or selective
accumulation in fat or lymphoid tissue may occur: however, at the
doses used in this study (about 50 mg/kg bw), there is no evidence of
selective accumulation of anoxomer in either fat or lymphoid tissues.
Special studies on mutagenicity
Anoxomer, in concentrations up to 5 mg/plate, was neither
mutagenic nor cytotoxic in the Ames test using 5 strains of his-
Salmonella typhimurium (strains used were sensitive for both base-
pair and frame-shift mutations) either with or without activation by
rat microsomal preparation. Four strains of trp-Escherichia coli
were employed to test anoxomer in concentrations up to 1 mg/plate with
and without metabolic activation. Anoxomer was nonmutagenic in this
bacterial test system (Dietrich et al., 1977).
Anoxomer was evaluated for its ability to induce mitotic
crossing over or intergenic recombination and reverse mutation of
Saccharomyces cerevisiae. Anoxomer was tested in concentrations up
to 5 mg/ml and at time periods up to 16 hours with and without
metabolic activation. No dose- or time-related effects were seen with
any of these endpoints (Dietrich et al., 1977).
The potential mutagenicity of anoxomer in mammalian cells was
tested in L5178Y mice lymphoma cells. There were no effects on forward
point mutations at the thymidine kinase locus, sister chromatid
exchanges or chromosome aberrations (Anon., 1977). The validity of
this particular study is questioned at doses above 0.32 mg/ml because
of precipitation of the test materials, and because the incidence of
chromosomal aberrations was not different between the positive and
solvent controls.
Special studies on reproduction
A 3-generation reproduction and teratology study was conducted
with anoxomer in Sprague-Dawley rats. Control animals received basal
diets while test animals received 0.5, 1.58 or 5% of anoxomer in basal
diet throughout the study. Each parental generation consisted of 30
males and 30 females.
There was a statistically significant decrease in neonatal
survival index in the 1.58 and 5% offspring and in the weaning
survival index of all 3 treated groups in the F1a litters. There was
a significant decrease in male birth weight and in male and female
weaning weights at the 5% level in the F1b litters. The neonatal and
weaning survival indices as well as weaning weights were significantly
reduced in the 5% groups of the F2a litters. Weaning survival was
decreased in the 0.5 and 5% offspring, and weaning weight of all males
in the same groups was decreased. In the F3a, F3b and F3c litters,
the pregnancy rate was low in all groups including the control
animals. However, none of these effects were observed consistently
during the successive generations, nor was there any indication that
these effects were more marked during the successive breedings. Based
on all the information from the study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet.
The F3d litters were utilized for the teratology phase of this
study. The test animals were sacrificed on day 19. There was no effect
on numbers of corpora lutea, implantations, resorptions, liver of dead
foetuses for any of the groups. In addition, there was no treatment-
related effects on body weight or size or on the incidences of
visceral or skeletal anomalies (Anon., 1979a).
Special studies on teratogenicity
Thirty female Sprague-Dawley rats, each of 4 treatment groups,
were fed anoxomer at 0, 250, 800 or 2500 mg/kg bw per day on days 6-15
of gestation. The test animals were sacrificed on gestation day 20. No
compound-related effects were found in maternal body weights, deaths
or pathology at necropsy. No compound-related differences were
observed in pregnancy rate, number of corpora lutea, implantations,
resorptions, live foetuses, foetal body weight or length (Anon.,
1981a). The mean number of visceral and skeletal variants increased in
the treated groups, but the increase did not appear to be dose
related.
In 2 studies, A and B, 20 or 25 female rabbits, respectively in
each of 4 treatment groups received anoxomer at 0, 250, 500 or
1000 mg/kg bw per day by oral intubation from day 6 through day 18 and
sacrifice was on gestation day 29. In both studies, maternal gross
pathology did not indicate any treatment-related effects. No effect
was apparent in the number of dead foetuses, live foetuses, or sex
ratio. There appeared to be no treatment-related increase in skeletal
problems in both studies. In study A, there was a dose-related
increase in dilated pelvises and ectopic kidneys in all groups. Study
B utilized the same protocol, but did not confirm the renal effects
(Anon., 1979, 1981a).
Acute toxicity
LD50 Reference
Animal Route (g/kg bw)
Mouse Oral 10 Anon., 1981c
i.p. 10 Jorgenson & Rushbrook, 1976
Rat (Sprague-Dawley) Oral 10 Jorgenson & Rushbrook, 1976
i.p. 10 Jorgenson & Rushbrook, 1976
Dog Oral 10 Jorgenson & Rushbrook, 1976
i.p. 5 Jorgenson & Rushbrook, 1976
Short-term studies
Rat
Fifty young, adult Sprague-Dawley rats (25 males and 25 females)
in 4 different groups were fed 0, 0.5, 1.58 or 5% anoxomer in rat diet
for 90 days. Water intake, activity and behaviour were normal and
comparable for all groups. Body weights and food consumption for males
and females in all groups remained in the normal ranges for the
duration of the study. The haematology, differential counts, platelet
counts and reticulocyte counts of all groups were also within normal
limits. The serum enzyme and biochemistry were within the expected
limits of biological variation. Gross necropsy revealed a 15-20%
incidence of emphysema, slight haemorrhaging and/or congestion of the
lungs in all groups, including controls. This was probably the result
of handling and CO2 asphyxiation and not the result of treatment. Two
control males and 2 0.5% dose level females had cataracts. No dose-
response effects were evident and the gross findings were within
normal ranges for this strain of rat. All organ weights and organ-to-
body weight ratios were comparable between groups. Slight to moderate
hyperplasia of the lymph node was seen in 2 high-dose rats and
haemorrhage of the lymph node was observed in another high-dose rat.
Slight focal haemorrhage and slight focal congestion was found in the
thymus, respectively, in 2 high-dose rats (Jorgenson & Rushbrook,
1977).
Long-term studies
Mouse
One hundred and seventy-five sexually mature HAM/ICR mice were
selected on the basis of good health and randomly assigned either to
one of two control groups or to treatment groups and fed 0, 0.5, 1.58
and 5% anoxomer for 104 weeks to assess the chronic toxicity and
carcinogenicity potential of this substance. After 1 week, animals
were mated. Female mice were maintained on control or test diet
throughout gestation, delivery and lactation. Fifty animals per sex
per dose were then randomly assigned to the chronic feeding phase of
the present study.
There were no compound-related effects indicated by the survival
data from the test and control groups. While the high-dose male and
female groups' mean weights were decreased during the first 6 months
of the study, by the end of the first year, there were no significant
differences in body weights of the offspring of the high-dose F0
groups. The early decreased body weight would appear to reflect the
test animals exposure in utero to the test compound. Thus, the
decreased body weights of the high-dose males and females in the early
phase were considered treatment related. This effect on offspring body
weight may have been due to simple dilution of the nutritive value of
the diet because of the presence of the diet compound. Problems of
food spillage, however, made food consumption data of questionable
reliability (Anon., 1980a).
At termination of the study at 104 weeks, gross pathology was
characterized in large measure as being comparable across treatment
and control groups. Certain lesions occurred with nearly equal
frequency across all treatment groups, e.g., discoloration of the
lungs, enlarged spleens and darkened areas or lobes of the liver.
The results of histopathological findings were essentially
comparable between the high-dose treatment group and control group 1.
The more common incidental findings observed were: chronic murine
pneumonia, hydronephrosis, focal ulceration of the gastric mucosa,
malignant lymphoma originating in the thymus, hepatocellular carcinoma
and chronic interstitial nephritis. None of these lesions occurred in
other than an apparently random distribution. Statistical analysis was
performed on the comparative incidences of malignant lymphoma (all
types combined alveolar/bronchiolar carcinoma and hepatocellular
carcinoma in control group 1 and the high-dose groups. These 3 tumour
categories were selected since they were representative of the tumour
types occurring most frequently. There was not a statistically
significant increase in incidence of these tumours in the treated
group compared to control group 1 (Anon., 1980a). An examination of
the pattern and occurrence of tumours in other organs and tissues did
not reveal a frequency of distribution across treatment groups which
is compound related.
Rat
Fifty animals (Fischer 344 strain) per sex per dose were fed 0,
0.5, 1.58 or 5% anoxomer for 130 weeks in this study to assess the
chronic toxicity and carcinogenicity potential of this substance. The
parents of these animals had also been fed 0, 0.5, 1.58 or 5%
anoxomer. Exposure to anoxomer continued during mating, gestation,
delivery and lactation.
Survival in all groups at 18 months exceeded the 55% protocol
requirement. The higher mortality experienced by the mid-dose males is
considered to be incidental and not compound related. Anoxomer
administration resulted in statistically significant decreases in body
weight gain (growth rates) through week 51 in low- and high-dose
males. In addition, during the chronic feeding phase of the study, the
mean body weights of the 3 treated male groups were consistently lower
than the 2 control groups. This finding is considered to be compound
related; however, the decreases were similar across groups and bore no
relationship to those observed among the female treatment groups. The
slightly lower mean body weight of certain of the treated groups
coupled with the increased levels of food consumption by some of the
treated groups suggests that the lower body weights were the result of
dilution of the nutritive value of the diet due to the presence of
anoxomer. Additional evidence of the non-specific rather than toxic
nature of the decreased weight was the fact that there were no
statistically significant differences in terminal organ or organ-to-
body weight ratios observed between treated and control groups.
During week 37, some rats exhibited clinical signs of
sialodacryoadenitis (SDA) virus. There was no treatment of this
normally self-limited disease and no effects were observed which were
interpreted as being detrimental to the conduct of the present study.
Gross eye findings included a slightly increased frequency of cloudy
eyes in high-dose animals during the latter part of the study, e.g.,
at week 99, the overall incidence ranged from about 2 to 9% for
control, low- and mid-dose groups and was 17% for the high-dose group.
The presumptive diagnosis of degenerative cataracts secondary to
probable senile retinal dystrophy was made. The findings did not
provide definite evidence of a compound-related effect in the high-
dose group; however, the slightly higher incidence suggested a
potential indirect effect on normal geriatric process. The frequency
of this finding across treatment groups became virtually the same by
the end of the study. Also, during week 37, 4 high-dose males were
found moribund, and 3 animals manifested posterior paresis. At
necropsy, suggestions of haemorrhage were present with dark red fluid
in the urinary bladder of 2 males, and large areas of diffuse redness
in the bladder wall of a third animal. A fourth animal found dead
during week 37 had haemorrhage and a large haematoma in the pelvic
area. Prothrombin determinations and platelet counts made at 55 weeks
were comparable to control animals. No additional animals or groups
suffered these lesions.
Other representative findings were: discoloration of pituitary,
lungs, liver, kidneys, adrenals and testes; enlargement, thickened
appearance or variations in size of pituitary, liver, spleen and
testes; ulcerated areas of the stomach; cloudy or opaque eyes; nodules
in various organs; masses in subcutaneous tissue, in body cavities and
associated with viscera and enlargement or discoloration of lymph
modes. A wide variety of spontaneous neoplasms and lesions were
observed. Among the commonly observed neoplasms, such as pituitary "C"
cell tumours of the thyroid, pheochromocytoma of the adrenals, mammary
fibroadenomas in females, monocytic leukaemia, testicular interstitial
cell tumours, and uterine endometrial stromal polyps. Statistical
analyses were conducted on the comparative incidences of monocytic
leukaemia, pituitary adenoma, adrenal pheochromocytoma and mammary
fibroadenoma in control group 1 and the high-dose group. The overall
incidence of these tumour types did not differ significantly between
the high-dose groups and their respective controls. An examination of
the pattern and occurrence of tumours in other organs and tissues
reveals no evidence of compound-related effects (Anon., 1980b).
Dog
Eight male and 8 female adult, pure-bred beagles were fed 0, 0.5,
1.58 or 5% anoxomer for 52 weeks.
No deaths occurred during the experimental period. Clinical
observations were occasional instances of emesis, soft stools and
diarrhoea, but these were observed in both treated and control groups.
The consistently lower weight gains of the high-dose animals are
considered to be treatment related. Food consumption of the treatment
groups did not vary significantly from controls; however, absolute
levels of food ingested were slightly depressed in the low- and mid-
dose males. Haematology and clinical chemistry evaluations were made
on weeks 4, 13, 26 and 52 and no findings suggesting a compound-
related effect were discovered in any test group. There were no
compound-related findings with regard to urinalysis data. There were
no treatment-related ocular changes in any of the test subjects.
At 13 weeks, 2 males and 2 females from each treatment group were
sacrificed and at 52 weeks 6 additional animals per test group were
terminated and subjected to gross and histopathological examination.
With respect to histomorphological alterations, there were only
occasional spontaneous disease lesions found and these were not
systematically associated with compound administration (Anon., 1979b).
OBSERVATIONS IN MAN
Six healthy, male human subjects received 13.8 mg or 50 µCi of
radiolabelled anoxomer orally. Urinary and faecal excretion were 0.02%
and 95%, respectively. Based upon data from blood and urine,
intestinal absorption was estimated to be less than 0.1% of the
administered dose.
Comments
Various metabolic studies show that no more than 0.2% of an
orally administered dose of anoxomer is absorbed in mice, rats,
rabbits, guinea-pigs and man. In spite of having a lipophilic
structure, anoxomer does not appear to selectively accumulate in
either lymph tissues or lipid-rich regions of the organism.
Acute oral toxicity studies in mice, rats and dogs with anoxomer
suggest that the LD50 for all these species is greater than 10 g/kg
bw. A multigeneration reproduction study in rats did not show any
consistent effects during the successive breedings, nor was there any
indication that the effects were more marked in successive generation.
Based on all the information from this study, the no-effect level on
reproductive performance and indices was determined to be 1.58% of the
diet. The no-effect levels for teratogenicity studies in rats and
rabbits were, respectively, 2.5 g/kg and 1.0 g/kg bw per day. Anoxomer
was not mutagenic in a series of bacterial tests. It was not
carcinogenic in lifetime studies in both rats and mice. No other
significant compound-related effects were observed in these studies,
except in the rat study where there was an increase in the occurrence
of cloudy eyes, in the high-dose groups (5% of the diet) at week 99.
However, the frequency of the occurrence of this effect, which has
been tentatively diagnosed as degenerative cataracts secondary to
probable senile retinal dystrophy, became virtually the same in all
groups at termination of the study (week 104). Additional studies are
presently under way to determine if there is an anoxomer-related
increase in lenticular opacities.
A 1-year feeding study in dogs showed no compound-related
effects, except liver weight gains in dogs, maintained on diets
containing 5% anoxomer. No lenticular opacities were observed in any
of the test groups.
EVALUATION
Level causing no toxicological effect
15 800 ppm (1.58%) in the diet equivalent to 800 mg/kg bw.
Estimate of temporary acceptable daily intake for man
0-8 mg/kg bw.
FURTHER WORK OR INFORMATION
Required by 1984
Further information about the occurrence of senile cataract in
rats exposed to high levels of anoxomer in the diet.
REFERENCES
Anon. (1976a) Final report. Acute eye irritation potential study in
rabbits. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1976b) Final report. Primary skin irritation study in rabbits.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1977) Mutagenicity evaluation of D00079 (Lot No. 0314) in the
mouse lymphoma multiple endpoint test (MET). Unpublished study by
Dynapol, Inc., submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Anon. (1978) Gross and microscopic evaluation of mesenteric lymph
nodes in female rats. D00079. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979a) A three-generation reproduction and teratology study in
rats, D00079, final report. Unpublished study by Hazelton
Laboratories of America, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Anon. (1979b) Final report. Chronic dietary administration in dogs.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1980a) Chronic dietary administration in mice, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Anon. (1980b) Chronic toxicity study in mice, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981a) Teratology study in rats, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981b) Teratology study in rabbits, D00079, final report.
Unpublished study by Hazelton Laboratories of America, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Anon. (1981c) Hematological evaluations in male rats, D00079, final
report. Unpublished study by Hazelton Laboratories of America,
Inc., submitted to the World Health Organization by Dynapol,
Inc., Palo Alto, California, USA
Dietrich, P. S., Brown, J. P. & Bakner, C. M. (1977) Phase II
mutagenicity testing of Poly AOTM-79: Microbial tests with
Salmonella/microsome assay, Escherichia coli and
Saccharomyces cerevisiae. Unpublished study by Dynapol, Inc.,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977a) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in guinea pigs.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977b) Urinary and fecal excretion
of the polymeric antioxidant [14C]-D00079 in rabbits.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. & Halladay, S. C. (1977c) Intestinal absorption of
the polymeric antioxidant [14C]-D00079 in rat. V. Absorption in
rats previously maintained for 90 days on 5% D00079 in the diet.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Enderlin, F. E. (1981) Distribution of radioactivity in internal
organs and tissues of the rat after oral administration of a high
activity dose of Poly AOTM79-[14C]. II. Determined at six
different times between 1 and 28 days after dosing. Unpublished
study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Halladay, S. C. et al. (1978) The bile duct ligated animal: A model
for determining total intestinal absorption, Drug Chem.
Toxicol., 1, 203-213
Jorgenson, T. A. & Rushbrook, C. J. (1976) (1) Acute oral toxicity
studies in rats, mice and dogs; (2) Acute intraperitoneal
toxicity in rats, mice and dogs; (3) Maximum tolerated dose study
in rats. Unpublished study by Stanford Research Institute,
submitted to the World Health Organization by Dynapol, Inc., Palo
Alto, California, USA
Jorgenson, T. A. & Rushbrook, C. J. (1977) Toxicity study of D00079, A
ninety-day subacute study in rats. Unpublished study by Stanford
Research Institute, submitted to the World Health Organization by
Dynapol, Inc., Palo Alto, California, USA
Parkinson, T. M., Honohan, T. & Enderlin, F. E. (1978) Intestinal
absorption, distribution and excretion of an orally administered
polymeric antioxidant in rats and mice, Fd. Cosmet. Toxicol.,
16, 321-330
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1977) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in hepatic tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Ryerson, B. A., Halladay, S. C. & Dollar, L. A. (1978) Effects of
orally administered D00079 and butylated hydroxytoluene on
microsomal mixed function oxygenase activity, cytochrome C
reductase and cytochrome P-450 in intestinal tissues of rats.
Unpublished study by Dynapol, Inc., submitted to the World Health
Organization by Dynapol, Inc., Palo Alto, California, USA
Walson, P. D. et al. (1979) Intestinal absorption of two potential
polymeric food additives in man, Fd. Cosmet Toxicol., 17,
201-203
