
ALLURA RED AC
Explanation
This food colorant was first evaluated by JECFA in 1974 (see
Annex I, Ref. 34). At that time the Committee decided not to set an
ADI for this substance because of a lack of metabolism studies and the
unsatisfactory nature of the only long-term study in rats available
for evaluation; too few animals remained at the end of the study to
allow a satisfactory assessment to be made. Additional studies in
these areas were found essential to be carried out before a definite
evaluation could be made. A short monograph was published (see Annex
I, Ref. 35). The substance was re-evaluated subsequently when some
metabolic studies using radio-labelled materials were made available
(see Annex I, Ref. 43). These studies demonstrated that the metabolism
of this colour was similar to that of other members of the azo dye
class. No monograph was prepared and no acceptable daily intake (ADI)
was allocated. JECFA in 1979 had again re-evaluated this compound (see
Annex I, Ref. 51).
Since the previous evaluations additional data have become
available and are summarized and discussed in the following monograph.
The previous monograph has been expanded and is reproduced in its
entirety below.
BIOLOGICAL DATA
BIOCHEMICAL ASPECTS
Rats were fed a diet containing 5.19% of Allura Red (White,
1970). It was observed that 0.1% and 29% of the intact dye was
excreted in the urine and faeces respectively. In later studies, rats
and dogs were pretreated daily with nonradioactive Allura Red.
Subsequently, the animals were dosed with the 35S labelled compound
and studied for up to 72 hours for excretion and distribution patterns
of the colour. Both species showed limited absorption of the compound
with the major route of excretion being via the faeces. In the dog 92
to 95% of the recovered radioactivity appeared in the faeces within 72
hours while in the rat 76 to 92% of the recovered radioactivity
appeared in the faeces within this time period. Urinary recoveries of
the colour in rats and dogs, respectively varied between 5.7 and
19.8% and 2.7 and 3.6%. After sacrifice, significant retention of
radioactivity was located in the intestinal contents of both species
and in the washed intestines of the rats. This was thought to be due
to adhesion of the compound to the intestinal wall, since the total
carcass and viscera of these animals contained less than 0.4% of the
administered dose (Guyton & Reno, 1975).
Cresidinesulfonic acid was found to be the major metabolite of
Allura Red in the urine of these two species, whereas the parent
compound was not measurable. In addition, two other unidentifiable
metabolites were found in the urine of the rats. In the rat faecal
extracts, cresidinesulfonic acid was a major metabolite along with two
unknowns and the parent compound. The dog faecal sample revealed an
identical metabolite pattern as seen in the rat, and in addition, a
third unknown was discovered. One of the urinary unknowns demonstrated
an Rf value which was identical to that of the one of the faecal
unknowns suggesting that they were one and the same. The other
unknowns exhibited distinctive Rf values which indicated that these
metabolites were different (Guyton & Stanovick, 1975).
It has been postulated that azo reduction by gut flora of the dye
will yield the two components of the parent compound:
2-methoxy-5-methyl-aniline-4-sulfonic acid (cresidine-4-sulfonic acid)
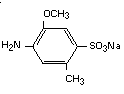 and 1-amino-2-naphthol-6-sulfonic acid
and 1-amino-2-naphthol-6-sulfonic acid
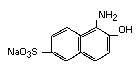 (White, 1970)
It appears that negligible quantities of intact Red are absorbed
and excreted in the urine, and that the major portion of the colour is
excreted as metabolites in the faeces.
TOXICOLOGICAL STUDIES
Reproduction
Rat
Groups of 10 male and 20 female rats received 0, 0.37, 1.39 or
5.19% of Allura Red in their diet through two parental (F1A was the
P2 generation) and two filial generations. Mating occurred after 27
weeks on either the control or test diets for both the P1 and P2
generation. The fertility indices were low for the controls and test
animals in the F1A and F1B generation as well as in the low test
level F2A and all test levels of F2B generation. Growth was
suppressed slightly for the lowest test level in F1B and high test
levels in F1A and F1B as well as for the high test levels in F2A
and F2B pups. Other indices, litter size and pup weight at 24 hours,
were comparable in each group in each generation. No consistent
pathological changes were noted in the P1, F1A, F1B and F2B
generations. No evidence was seen of teratogenic or embryotoxic
effects regarding implantation sites, resorption sites and live
foetuses indices. No difference from controls was noted with regard to
appearance, anatomy and structure of test foetuses (Blackmore et al.,
1969).
Teratogenicity
Rat
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of foetuses per litter and average
foetus weight (Collins, 1974).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of compound-related effects with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal dose. No adverse effects on implantation and litter
data were noted nor were any foetal abnormalities observed (Reno,
1974).
Mutagenicity studies
Microbial assay systems (plate and suspension tests) with and
without the addition of mammalian metabolic activation enzymes were
used to determine the mutagenic potential of Allura Red. One strain of
yeast, Saccharomyces cerevisiae, and five strains of the bacteria
Salmonella typhimurium were used in the study which employed
negative and positive controls. Preliminary toxicity studies were
conducted and the compound was found to be nontoxic at the 5%
concentration. Allura Red did not exhibit genetic activity in any of
the assay systems employed (Brusick, 1976).
Genetic tests were also conducted using three strains of
Saccharomyces cerevisiae with and without liver enzyme induction
(Anonymous, 1977a). The colour also proved to be negative in these
systems.
The Salmonella/microsome system was used to test a number of azo
dyes including FD&C Red No. 40. The dye, as well as its chemically
reduced component amines, were tested with five tester strains
(TA 1535, TA 100, TA 1537, TA 1538 and TA 98). In addition, the
effects of the colour were analysed in the same five tester strains
with microsomal activation (S-9) and in an aerobic liquid test with
microsomal activation. None of these test systems demonstrated
mutagenic activity of FD&C Red No. 40 (Brown et al., 1978).
In a second Salmonella/mammalian-microsome study, the colour was
tested for mutagenic potential in two frame-shift histidine (TA 1537
and TA 98) and two base-pair substituted histidine mutants (TA 1535
and TA 100). Both the spot test and the plate incorporation assay,
with and without the S-9 mix, were employed in these experiments. As
before, Allura Red was found to have no mutagenic effects in these
assay systems (Muzzall & Cook, 1979).
The same four strains of Salmonella typhimurium (TA 1535,
TA 1538, TA 100 and TA 98) with and without microsomal activation were
used in the spot and plate tests to study the effects of Allura Red.
None of the tests performed demonstrated either a mutagenic or
cytotoxic activity of this compound (Viola & Nosotti, 1978).
A rat hepatoma cell culture system was utilized to determine if
the colour had enzyme induction capabilities, and it was found that
the colour could not induce aryl hydrocarbon hydroxylase activity
(Bradlaw, 1979).
Allura Red was tested in the genetic analysis for recessive
lethal effects by the oral feeding to Drosophila melanogaster at the
LD50 dose for 24 days. The following genetic tests were conducted:
loss of X or Y chromosome, visible mutation at specific loci and
sex-linked recessive lethal damage in both mature and immature
spermatozoa, chromosomal translocation, sex-linked lethal damage of
aged-in-the female spermatozoa and sex-linked mosaic recessive lethal
damage in mature spermatozoa. There was no significant increase in the
proportion of mutation in any category when comparisons were made with
the individual controls. However, when the controls were combined with
the controls used in two other studies, a significant increase was
seen for the category of sex-linked mosaic recessive lethal damage
(Anonymous, 1977b, 1978).
The heritable translocation potential of Allura Red was analysed
in eight to 10-week-old male mice who were fed diets containing the
colour at dose levels of 4000 and 20 000 ppm for eight weeks. Each
male was mated with two females to produce an F1 generation. The
males in later generations were mated and their reproductive
performance was analysed. The administration of the colour did not
affect the fertility of the treated mice. Although several of the
animals receiving the colour were potential translocation
heterozygotes, cytogenetic analysis showed that these animals were
normal. It was concluded that the compound was negative with respect
to induction of inheritable translocation in mice (Jorgenson et al.,
1978).
Acute toxicity studies
LD50
Animal Route (mg/kg bw) Reference
Rat Oral (gavage) 10 000 Weir, 1965a
Rabbit Dermal 10 000 Weir, 1967
Dog Oral (gavage) 5 000 Weir, 1965b
Rat
The colour was administered to six groups (five animal/sex) at
doses varying from 215 to 10 000 mg/kg. The only compound-related
effect of note was the red coloration of the urine and faeces (Weir,
1965a).
Skin irritation and sensitization studies
Rabbit
Tests for dermal irritation on intact and abraded skin showed no
gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg bw of
Allura Red; none the less a persistent skin staining did occur (Weir,
1967).
Daily application of the colour at rates of 0.5 g/kg, five days a
week for 15 applications on abraded and 65 applications on intack skin
of rabbits, of 0.1 and 1% solutions in water or as a hydrophilic
ointment revealed no compound-related effect on general appearance,
body weight, clinical laboratory studies and gross and microscopic
pathology. No dermal irritation was noted, except that produced by the
hydrophilic ointment base (Blackmore, 1968).
Human
Prophetic patch test
Allura Red was applied either as a neat or as a 25% aqueous
solution to the skin of 200 human subjects. The initial exposure to
the compound was for 72 hours, and this was followed by a 24-hour
application 10 to 14 days later. None of the subjects exhibited
compound induced irritation or sensitization (Osbourn, 1972).
Draize-Shelanski repeated insult patch test
In this study, the colour and its alumina lake were applied to
the subjects volvar forearms (200 subjects) as an aqueous solution for
10 alternate days, for 24-hour periods, followed by a 14-day rest
period. Challenge batches were then applied under occlusion to fresh
skin sites on the subjects scapular backs for 24 hours. The colour did
not produce either irritation or allergic responses during the
induction phase nor contact dermatitis in the challenge period (Jolly,
1973).
Photosensitization potential test
As in the previous study, Allura Red and its lake were evaluated
on sites under occlusion for five 48-hour, alternate-day periods.
These sites had been previously irradiated for five minutes with Xenon
light which had been filtered through a window-glass equivalent to
limit the exposure to non-erythema-producing, long-wave radiation. A
10-day rest period followed this induction exposure, and then the
colour was applied to fresh skin sites, irradiated for five minutes
with Xenon and subsequently removed and the sites were evaluated.
Allura Red was shown not to produce photosensitization on the 25
subjects studied (Jolly, 1973).
Hypersensitivity reactions
In 52 patients who were suffering from urticaria or angioedema
for more than four weeks were placed on an elimination diet for at
least three weeks. All non-vital drugs were suspended during the study
as were any food ingredients which were known to cause urticaria. In
most patients, challenge with Allura Red was performed when the
patients were free of symptoms. Reactions were considered positive
only when the flare-up of symptoms was reproducible within the same
time from intake to skin reaction. Allura Red was administered via the
oral route in either a dose of 1 or 10 mg, and induced a positive
reaction in 15% of the 52 patients challenged (Mikkelsen et al.,
1978).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% of Allure Red for six weeks. No
evidence of compound-related effects were noted as regards body
weight, food consumption, survival, organ weights, gross and
microscopic pathology. Haematology and urinalysis were normal and no
evidence of Heinz body formation was noted (Weir & Crews, 1966a).
Dog
Four groups of one male and one female beagle dogs received
Allure Red daily in capsule form at the following doses, 0, 125, 250
and 500 mg/kg bw. No adverse effects were noted on body weight, food
consumption, survival, organ weights, gross and histopathology,
haematology and clinical chemistry. At the highest level, there were
slight ill-defined hepatic parenchymal changes in both sexes (Weir &
Crews, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% of Allure Red in their diet for 104 weeks. All animals
remained normal regarding appearance, behaviour, haematology and
clinical chemistry findings and gross and histopathology. Both faeces
and urine were coloured at all test levels. At 52 weeks, an interim
sacrifice was performed and revealed that the adrenal cortical cells
of the high level groups showed some vacuolation. There was brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Olson et al., 1970).
Pig
SPF pigs of Danish Landrace were equilibrated for a period of 21
days, whereupon they were distributed according to body weight into
nine groups which consisted of two males and two females. The compound
was administered by gavage three hours after feeding in the morning.
The dye was administered at a dose of 1000 mg/kg/day for the first 21
days, thereafter the dose was increased to 1500 mg/kg/day and this
dosage was given for an additional 54 days. Body weight was recorded
weekly and food intake daily. Blood samples were collected two days
prior to and five, 19 and 68 days following the initiation of colorant
administration. The haematological and clinical parameters measured
were haemoglobin, packed cell volume, total erythrocyte count, counts
of Heinz bodies and reticulocytes and serum activities of LDH and
isoenzymes. Autopsy and histological examination of kidneys, spleen,
liver, hepatic and renal lymph nodes and bone marrow were performed.
The colour produced no significant effects on clinical and
haematological parameters nor did it elicit any observable
pathological changes (Sondergaard et al., 1977).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female and a test group of 50 male and 50
female mice were treated dermally with 0.1 ml of either distilled
water, 10 µg 3,4-benzypyrene in acetone or a 5% Allura Red test
solution twice weekly for 20 months. The results in the positive
control group showed the mouse strain used to be sensitive to
benzypyrene carcinogenesis. Survival, gross and histopathology of
major organs were comparable in the negative controls and animals
treated with Allura Red. Histology of the skin revealed comparable
incidence and degree of severity of acanthosis, hyperkeratosis and
dermatitis for the negative control and the Allura Red groups
(Voelker, 1970).
Groups of 50 CD(R)-1 albino mice/sex received Allura Red in
their diets at levels of 0.37, 1.39 and 5.19%. These animals were the
offspring of parental mice treated at identical levels for one week
prior to breeding and through gestation and lactation. An additional
group of 50 animals/sex served as a negative control group. An interim
sacrifice was performed at 42 weeks which resulted in reducing the
number of animals in each group and sex to 30. This was done to
determine compound effects upon the early onset of tumours of the
lymphatic system. Prior to this, neoplasms which were histologically
diagnosed as lymphomas were found in several animals which received
the colour. Statistical analysis of the incidence of tumours occurring
throughout the study did not reveal any increase in tumour incidence
due to compound administration. No treatment-related effects were
noted in the survival, clinical signs and clinical laboratory data.
The animals on the highest dosage level demonstrated lower body
weights and effects on organ weight data and organ/body weight ratios,
however, the meaning of these observations ware considered to be
inconsequential. Gross and microscopic examination of all the animals
revealed an absence of treatment-related effects and spontaneous
disease-related lesions were considered to be within acceptable limits
(Serota et al., 1977a).
A second lifetime dietary mouse study was performed using a
protocol identical to the one described above, with the exception that
100 animals/sex/group and two negative control groups were used. As
before, statistical evaluation of neoplasms of the reticuloendothelial
system failed to show a significant correlation between tumour
response and increasing dosage. Sporadic changes were noted in the
clinical signs, body weight, food consumption and survival rates; none
the less, these could not be related to the administration of the
colour. Alterations of the absolute and relative weights of the
adrenals and thyroids were noted in several of the treatment groups.
Histological examination of these tissues in the animals in the
highest dose group at the completion of the study demonstrated no
abnormalities. Therefore, the significance of weight gains in these
two organs could not be established. Generally, gross pathological
findings were similar in the control and treated animals. There was,
however, a higher incidence of dark areas in the lungs of those
animals in the high dose group which died or were sacrificed
in extremis and of cystic foci areas in the kidneys of the high dose
males. No distinct treatment-related effects could be ascribed to
these findings. Histopathological evaluation did not show effects
which could be attributed to compound administration, nor was there
evidence for a dose-related increase in the incidence of spontaneously
occurring tumours (Reno et al., 1978).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% of Allura Red for 92 weeks. Moderate growth
depression occurred at the 5.19% level in both sexes. No other
compound-related effects were noted as regards appearance, behaviour,
survival, organ weights, clinical laboratory studies or gross and
histopathology. No evidence of Heinz body formation was noted apart
from a slight tendency to anaemia (Olson & Voelker, 1970).
50 CD(R)-1 albino rats/sex were placed in a negative control
group and three test groups which contained varying percentages (0.37,
1.39 and 5.19) of Allura Red. The males were administered the diets
for 118 weeks, and the females were fed for 121 weeks. All of these
animals were the F1a offspring of parental rats which were treated at
identical levels for one week prior to breeding, through a three-week
breeding period and during the gestation and lactation periods. No
treatment-related effects were noted in comparisons of survival,
clinical signs, clinical laboratory data and organ weight data. The
mean body weights and growth rates of the animals in the high-dose
group were lower than those of control animals. However, the low dose
group exhibited greater mean food consumption values than the control
groups. Gross pathological and histopathological findings were
essentially comparable between the control and treated groups.
However, increased incidences of kidney discoloration and firm
granular material in the pelvis of the kidney were noted in the males
of the colour treated groups. A slightly higher incidence of granules
in the pelvis was also noted in the high-dose males sacrificed at
termination. However, no significant dose-related trend was noted in
the statistical analysis of these data. An increased incidence of
renal calculi and focal epithelial proliferation was noted in the
high-dose rats, but additional examination of the other groups
indicated that this toxic effect could not be attributed to the
administration of the compound (Serota et al., 1977b).
Comments
Allura Red when administered orally undergoes partial azo
reduction prior to absorption. Metabolic studies indicate that the
colour is poorly absorbed in the body, and the major route of
excretion is via the faeces. In a multigeneration reproductive study
on rats it was shown that the progeny of the parents who were fed
51.9 g of the colour per kg of food demonstrated a slight growth
depression. The "no effect" level on reproductive physiology of this
colour in the rat is 13.9 g/kg of food. Teratogenicity studies in rats
and rabbits failed to show any compound-related embryotoxic effects. A
variety of mutagenicity studies carried out with Allura Red indicated
that there were no mutagenic effects. Another study on acute and
short-term oral toxicity of Allura Red in several species revealed
that apart from the coloration of the urine and faeces, there were no
other compound-related responses. Dermal studies (both short- and
long-term) also indicated an absence of colour-induced toxic
responses. In long-term feeding studies on mice and rats, the most
consistent observation was that the animals that received the greatest
amount of colour (51.9 g/kg of food) exhibited lower body weights
compared to control animals. One study suggested that mice that were
fed on this colour demonstrated an earlier onset of tumours of the
lymphatic system compared to control mice.1 However, a second more
extensive mouse study has not borne this out. The long-term study and
the mutagenicity studies suggest that Allura Red does not possess
carcinogenic potential.
The data were sufficient to establish a temporary acceptable
daily intake for man pending the availability of the statistical
analysis of the long-term mouse study.
EVALUATION
Level causing no toxicological effect
Rat: 1.39% in the diet corresponding to 695 mg/kg bw.
Estimate of acceptable daily intake for man
0-7 mg/kg bw.*
FURTHER WORK OR INFORMATION
Required by 1981
(1) Adequate information of statistical analysis of the long-term
mouse studies.
* Temporary.
REFERENCES
Anonymous. Mutagenicity investigations with allura red. Sex-linked
recessive lethals, Drosophila melanogaster, Bowling Green
University, Ohio, USA. Interim report of the Working Group on FD
& C Red, No. 40, 19 January 1977b, submitted to WHO by the US
Food and Drug Administration
Anonymous. Mutagenicity investigations with allura red. Saccharomyces
cerevisiae. Genetic Toxicology Branch of the FDA. Interim
report of the Working Group on FD & C Red No. 40, 19 January
1977a
Anonymous. Evaluation of substances of interest for genetic damage
using Drosophila melanogaster. Unpublished report submitted to
WHO by the United States Food and Drug Administration, 31 March
1978
Blackmore, R. H. Repeated dermal irritation study-rabbits, nontoxic
Red Z4576. Unpublished report 165-120 by Hazelton Laboratories,
Inc., submitted to WHO by Allied Chemical Corporation, 1968
Blackmore, R. H., Olson, W. A. & Voelker, R. W. Two-generation
reproduction study in rats, Red Z-4576. Unpublished report
165-133 by Hazelton Laboratories, Inc., submitted by Allied
Chemical Corporation, 1969
Bradlaw, J. Enzyme induction screen of seven color and cosmetic
ingredients. FDA Toxicology Laboratory, United States Food and
Drug Administration, manuscript in preparation, 1979
Brown, J., Roehm, G. & Brown, R. Mutagenicity testing of certified
food colors and related azo, xanthene and triphenylmethane dyes
with the Salmonella/microsome system, Mut. Res., 56: 249-271,
1978
Brusick, D. Mutagenicity evaluation NTRZ-4576 (Allura Red AC).
Unpublished report number 2547 by Litton Bionetics, Inc.,
submitted to WHO by Allied Chemical Corporation, 1976
Collins, T. F. X. Preliminary results of teratological studies on
Osborne-Mendel rats with FDC Red No. 40. Unpublished report by
Food and Drug Administration, USA, submitted to WHO, 1974
Guyton, C. L. & Reno, F. E. Metabolic disposition of 35S Allura Red AC
in the dog and rat. Unpublished report 165-146 by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Guyton, C. L. & Stanovick, R. P. Determination of the metabolites of
Allura Red AC in the rat and dog. Unpublished report by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Jolly, R. E. An assessment of irritation and sensitization, potential
of Allura Red AC utilizing the Draize-Shelanski reported
insult patch test and photosensitization potential evaluation.
Unpublished report by Biometric Testing, Inc., submitted to WHO
by Allied Chemical Corporation, 1973
Jorgenson, T. A., Rushbrook, C. J., Newell, G. W. & Skinner, W. A.
Study of the mutagenic effects of FD & C Red 40 (75-60) by the
heritable translocation study in mice. Unpublished report
LSU-5588 by SRI International, supplied by the United States
Food and Drug Administration, 1978
Mikkelsen, H., Larsen, J. & Tarding, F. Hypersensitivity reactions to
food colours with special reference to the natural colour annatto
extract (butter colour). Toxicological Aspects of Food Safety,
Arch. Tox. Suppl., 1:141-143, 1978
Muzzall, J. & Cook, W. Mutagenicity test of dyes used in cosmetics
with Salmonella/mammalian-microsome test, Mut. Res., 67:1-8,
1979
Olson, W. A. & Voelker, R. W. Twenty-one month dietary administration
albino rats, Red Z-4576. Unpublished report 165-121 by Hazelton
Laboratories, Inc., submitted to WHO by Allied Chemical
Corporation, 1970
Olson, W. A., Voelker, R. W. & Shott, L. D. One hundred and four-week
dietary administration-dogs, Red Z-4576. Unpublished report
165-132 by Hazelton Laboratories, Inc., submitted to WHO by
Allied Chemical Corporation, 1970
Osbourn, R. A. Report of human patch tests on FD & C Red No. 40.
Unpublished report 165-141 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1972
Reno, F. E. Teratology study in rabbits FD & C Red No. 40. Unpublished
report 165-142 by Hazelton Laboratories, Inc., submitted to WHO
by Allied Chemical Corporation, 1974
Reno, F. E., Mossburg, P. A., Serota, D. G., Tiede, J. J. & Voelker,
R. W. Carcinogenic bioassay and life-time dietary study in mice.
Unpublished report 165-174 by Hazelton Laboratories America,
Inc., submitted to WHO by Allied Chemical/Buffalo Color
Corporations, 1978
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the ICR Swiss mouse. Unpublished report
number 165-150 by Hazelton Laboratories America, Inc., submitted
to WHO by Allied Chemical/Buffalo Color Corporations, 1977a
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the albino rat. Unpublished report number
165-149 by Hazelton Laboratories America, Inc., submitted to WHO
by Allied Chemical/Buffalo Color Corporations, 1977b
Sondergaard, D., Hansen, E. & Wurtzen, H. A. A short-term study in the
pig of the effects on the liver and on the blood of eight azo
dyes, Toxicology, 8:381-386, 1977
Viola, M. & Nosotti, A. Application of the Ames test to certain color
additives, Boll. Chim. Farm., 117:402-415, 1978
Voelker, R. W. Repeated dermal application - Swiss mice, Red Z-4576.
Unpublished report 165-123 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1970
Weir, R. J. Acute oral administration - rats, five experimental
non-toxic red colors. Unpublished report 165-114 by Hazelton
Laboratories, Inc., submitted by Allied Chemical Corporation,
1965a
Weir, R. J. Acute oral toxicity - dogs, five experimental non-toxic
red colors. Unpublished report by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1965b
Weir, R. J. Acute dermal application - rabbits, Red Z-4576.
Unpublished report 165-119 by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1967
Weir, R. J. & Crews, L. M. Six-week dietary feeding study - rats,
experimental non-toxic Red Z-4576, Red Z-4578, Red Z-4598 and Red
Z-4808. Unpublished report 165-112 Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966a
Weir, R. J. & Crews, L. M. Six-week oral administration - dogs,
experimental non-toxic Red Z-4576, Z-4578, Z-4598 and Z-4808.
Unpublished report 165-116 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966b
White, R. G. Metabolic fate of orally ingested non-toxic Red Z-4576.
Unpublished report number 21855 by Buffalo Research Laboratory,
submitted to WHO by Allied Chemical Corporation, 1970
(White, 1970)
It appears that negligible quantities of intact Red are absorbed
and excreted in the urine, and that the major portion of the colour is
excreted as metabolites in the faeces.
TOXICOLOGICAL STUDIES
Reproduction
Rat
Groups of 10 male and 20 female rats received 0, 0.37, 1.39 or
5.19% of Allura Red in their diet through two parental (F1A was the
P2 generation) and two filial generations. Mating occurred after 27
weeks on either the control or test diets for both the P1 and P2
generation. The fertility indices were low for the controls and test
animals in the F1A and F1B generation as well as in the low test
level F2A and all test levels of F2B generation. Growth was
suppressed slightly for the lowest test level in F1B and high test
levels in F1A and F1B as well as for the high test levels in F2A
and F2B pups. Other indices, litter size and pup weight at 24 hours,
were comparable in each group in each generation. No consistent
pathological changes were noted in the P1, F1A, F1B and F2B
generations. No evidence was seen of teratogenic or embryotoxic
effects regarding implantation sites, resorption sites and live
foetuses indices. No difference from controls was noted with regard to
appearance, anatomy and structure of test foetuses (Blackmore et al.,
1969).
Teratogenicity
Rat
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of foetuses per litter and average
foetus weight (Collins, 1974).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of compound-related effects with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal dose. No adverse effects on implantation and litter
data were noted nor were any foetal abnormalities observed (Reno,
1974).
Mutagenicity studies
Microbial assay systems (plate and suspension tests) with and
without the addition of mammalian metabolic activation enzymes were
used to determine the mutagenic potential of Allura Red. One strain of
yeast, Saccharomyces cerevisiae, and five strains of the bacteria
Salmonella typhimurium were used in the study which employed
negative and positive controls. Preliminary toxicity studies were
conducted and the compound was found to be nontoxic at the 5%
concentration. Allura Red did not exhibit genetic activity in any of
the assay systems employed (Brusick, 1976).
Genetic tests were also conducted using three strains of
Saccharomyces cerevisiae with and without liver enzyme induction
(Anonymous, 1977a). The colour also proved to be negative in these
systems.
The Salmonella/microsome system was used to test a number of azo
dyes including FD&C Red No. 40. The dye, as well as its chemically
reduced component amines, were tested with five tester strains
(TA 1535, TA 100, TA 1537, TA 1538 and TA 98). In addition, the
effects of the colour were analysed in the same five tester strains
with microsomal activation (S-9) and in an aerobic liquid test with
microsomal activation. None of these test systems demonstrated
mutagenic activity of FD&C Red No. 40 (Brown et al., 1978).
In a second Salmonella/mammalian-microsome study, the colour was
tested for mutagenic potential in two frame-shift histidine (TA 1537
and TA 98) and two base-pair substituted histidine mutants (TA 1535
and TA 100). Both the spot test and the plate incorporation assay,
with and without the S-9 mix, were employed in these experiments. As
before, Allura Red was found to have no mutagenic effects in these
assay systems (Muzzall & Cook, 1979).
The same four strains of Salmonella typhimurium (TA 1535,
TA 1538, TA 100 and TA 98) with and without microsomal activation were
used in the spot and plate tests to study the effects of Allura Red.
None of the tests performed demonstrated either a mutagenic or
cytotoxic activity of this compound (Viola & Nosotti, 1978).
A rat hepatoma cell culture system was utilized to determine if
the colour had enzyme induction capabilities, and it was found that
the colour could not induce aryl hydrocarbon hydroxylase activity
(Bradlaw, 1979).
Allura Red was tested in the genetic analysis for recessive
lethal effects by the oral feeding to Drosophila melanogaster at the
LD50 dose for 24 days. The following genetic tests were conducted:
loss of X or Y chromosome, visible mutation at specific loci and
sex-linked recessive lethal damage in both mature and immature
spermatozoa, chromosomal translocation, sex-linked lethal damage of
aged-in-the female spermatozoa and sex-linked mosaic recessive lethal
damage in mature spermatozoa. There was no significant increase in the
proportion of mutation in any category when comparisons were made with
the individual controls. However, when the controls were combined with
the controls used in two other studies, a significant increase was
seen for the category of sex-linked mosaic recessive lethal damage
(Anonymous, 1977b, 1978).
The heritable translocation potential of Allura Red was analysed
in eight to 10-week-old male mice who were fed diets containing the
colour at dose levels of 4000 and 20 000 ppm for eight weeks. Each
male was mated with two females to produce an F1 generation. The
males in later generations were mated and their reproductive
performance was analysed. The administration of the colour did not
affect the fertility of the treated mice. Although several of the
animals receiving the colour were potential translocation
heterozygotes, cytogenetic analysis showed that these animals were
normal. It was concluded that the compound was negative with respect
to induction of inheritable translocation in mice (Jorgenson et al.,
1978).
Acute toxicity studies
LD50
Animal Route (mg/kg bw) Reference
Rat Oral (gavage) 10 000 Weir, 1965a
Rabbit Dermal 10 000 Weir, 1967
Dog Oral (gavage) 5 000 Weir, 1965b
Rat
The colour was administered to six groups (five animal/sex) at
doses varying from 215 to 10 000 mg/kg. The only compound-related
effect of note was the red coloration of the urine and faeces (Weir,
1965a).
Skin irritation and sensitization studies
Rabbit
Tests for dermal irritation on intact and abraded skin showed no
gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg bw of
Allura Red; none the less a persistent skin staining did occur (Weir,
1967).
Daily application of the colour at rates of 0.5 g/kg, five days a
week for 15 applications on abraded and 65 applications on intack skin
of rabbits, of 0.1 and 1% solutions in water or as a hydrophilic
ointment revealed no compound-related effect on general appearance,
body weight, clinical laboratory studies and gross and microscopic
pathology. No dermal irritation was noted, except that produced by the
hydrophilic ointment base (Blackmore, 1968).
Human
Prophetic patch test
Allura Red was applied either as a neat or as a 25% aqueous
solution to the skin of 200 human subjects. The initial exposure to
the compound was for 72 hours, and this was followed by a 24-hour
application 10 to 14 days later. None of the subjects exhibited
compound induced irritation or sensitization (Osbourn, 1972).
Draize-Shelanski repeated insult patch test
In this study, the colour and its alumina lake were applied to
the subjects volvar forearms (200 subjects) as an aqueous solution for
10 alternate days, for 24-hour periods, followed by a 14-day rest
period. Challenge batches were then applied under occlusion to fresh
skin sites on the subjects scapular backs for 24 hours. The colour did
not produce either irritation or allergic responses during the
induction phase nor contact dermatitis in the challenge period (Jolly,
1973).
Photosensitization potential test
As in the previous study, Allura Red and its lake were evaluated
on sites under occlusion for five 48-hour, alternate-day periods.
These sites had been previously irradiated for five minutes with Xenon
light which had been filtered through a window-glass equivalent to
limit the exposure to non-erythema-producing, long-wave radiation. A
10-day rest period followed this induction exposure, and then the
colour was applied to fresh skin sites, irradiated for five minutes
with Xenon and subsequently removed and the sites were evaluated.
Allura Red was shown not to produce photosensitization on the 25
subjects studied (Jolly, 1973).
Hypersensitivity reactions
In 52 patients who were suffering from urticaria or angioedema
for more than four weeks were placed on an elimination diet for at
least three weeks. All non-vital drugs were suspended during the study
as were any food ingredients which were known to cause urticaria. In
most patients, challenge with Allura Red was performed when the
patients were free of symptoms. Reactions were considered positive
only when the flare-up of symptoms was reproducible within the same
time from intake to skin reaction. Allura Red was administered via the
oral route in either a dose of 1 or 10 mg, and induced a positive
reaction in 15% of the 52 patients challenged (Mikkelsen et al.,
1978).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% of Allure Red for six weeks. No
evidence of compound-related effects were noted as regards body
weight, food consumption, survival, organ weights, gross and
microscopic pathology. Haematology and urinalysis were normal and no
evidence of Heinz body formation was noted (Weir & Crews, 1966a).
Dog
Four groups of one male and one female beagle dogs received
Allure Red daily in capsule form at the following doses, 0, 125, 250
and 500 mg/kg bw. No adverse effects were noted on body weight, food
consumption, survival, organ weights, gross and histopathology,
haematology and clinical chemistry. At the highest level, there were
slight ill-defined hepatic parenchymal changes in both sexes (Weir &
Crews, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% of Allure Red in their diet for 104 weeks. All animals
remained normal regarding appearance, behaviour, haematology and
clinical chemistry findings and gross and histopathology. Both faeces
and urine were coloured at all test levels. At 52 weeks, an interim
sacrifice was performed and revealed that the adrenal cortical cells
of the high level groups showed some vacuolation. There was brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Olson et al., 1970).
Pig
SPF pigs of Danish Landrace were equilibrated for a period of 21
days, whereupon they were distributed according to body weight into
nine groups which consisted of two males and two females. The compound
was administered by gavage three hours after feeding in the morning.
The dye was administered at a dose of 1000 mg/kg/day for the first 21
days, thereafter the dose was increased to 1500 mg/kg/day and this
dosage was given for an additional 54 days. Body weight was recorded
weekly and food intake daily. Blood samples were collected two days
prior to and five, 19 and 68 days following the initiation of colorant
administration. The haematological and clinical parameters measured
were haemoglobin, packed cell volume, total erythrocyte count, counts
of Heinz bodies and reticulocytes and serum activities of LDH and
isoenzymes. Autopsy and histological examination of kidneys, spleen,
liver, hepatic and renal lymph nodes and bone marrow were performed.
The colour produced no significant effects on clinical and
haematological parameters nor did it elicit any observable
pathological changes (Sondergaard et al., 1977).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female and a test group of 50 male and 50
female mice were treated dermally with 0.1 ml of either distilled
water, 10 µg 3,4-benzypyrene in acetone or a 5% Allura Red test
solution twice weekly for 20 months. The results in the positive
control group showed the mouse strain used to be sensitive to
benzypyrene carcinogenesis. Survival, gross and histopathology of
major organs were comparable in the negative controls and animals
treated with Allura Red. Histology of the skin revealed comparable
incidence and degree of severity of acanthosis, hyperkeratosis and
dermatitis for the negative control and the Allura Red groups
(Voelker, 1970).
Groups of 50 CD(R)-1 albino mice/sex received Allura Red in
their diets at levels of 0.37, 1.39 and 5.19%. These animals were the
offspring of parental mice treated at identical levels for one week
prior to breeding and through gestation and lactation. An additional
group of 50 animals/sex served as a negative control group. An interim
sacrifice was performed at 42 weeks which resulted in reducing the
number of animals in each group and sex to 30. This was done to
determine compound effects upon the early onset of tumours of the
lymphatic system. Prior to this, neoplasms which were histologically
diagnosed as lymphomas were found in several animals which received
the colour. Statistical analysis of the incidence of tumours occurring
throughout the study did not reveal any increase in tumour incidence
due to compound administration. No treatment-related effects were
noted in the survival, clinical signs and clinical laboratory data.
The animals on the highest dosage level demonstrated lower body
weights and effects on organ weight data and organ/body weight ratios,
however, the meaning of these observations ware considered to be
inconsequential. Gross and microscopic examination of all the animals
revealed an absence of treatment-related effects and spontaneous
disease-related lesions were considered to be within acceptable limits
(Serota et al., 1977a).
A second lifetime dietary mouse study was performed using a
protocol identical to the one described above, with the exception that
100 animals/sex/group and two negative control groups were used. As
before, statistical evaluation of neoplasms of the reticuloendothelial
system failed to show a significant correlation between tumour
response and increasing dosage. Sporadic changes were noted in the
clinical signs, body weight, food consumption and survival rates; none
the less, these could not be related to the administration of the
colour. Alterations of the absolute and relative weights of the
adrenals and thyroids were noted in several of the treatment groups.
Histological examination of these tissues in the animals in the
highest dose group at the completion of the study demonstrated no
abnormalities. Therefore, the significance of weight gains in these
two organs could not be established. Generally, gross pathological
findings were similar in the control and treated animals. There was,
however, a higher incidence of dark areas in the lungs of those
animals in the high dose group which died or were sacrificed
in extremis and of cystic foci areas in the kidneys of the high dose
males. No distinct treatment-related effects could be ascribed to
these findings. Histopathological evaluation did not show effects
which could be attributed to compound administration, nor was there
evidence for a dose-related increase in the incidence of spontaneously
occurring tumours (Reno et al., 1978).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% of Allura Red for 92 weeks. Moderate growth
depression occurred at the 5.19% level in both sexes. No other
compound-related effects were noted as regards appearance, behaviour,
survival, organ weights, clinical laboratory studies or gross and
histopathology. No evidence of Heinz body formation was noted apart
from a slight tendency to anaemia (Olson & Voelker, 1970).
50 CD(R)-1 albino rats/sex were placed in a negative control
group and three test groups which contained varying percentages (0.37,
1.39 and 5.19) of Allura Red. The males were administered the diets
for 118 weeks, and the females were fed for 121 weeks. All of these
animals were the F1a offspring of parental rats which were treated at
identical levels for one week prior to breeding, through a three-week
breeding period and during the gestation and lactation periods. No
treatment-related effects were noted in comparisons of survival,
clinical signs, clinical laboratory data and organ weight data. The
mean body weights and growth rates of the animals in the high-dose
group were lower than those of control animals. However, the low dose
group exhibited greater mean food consumption values than the control
groups. Gross pathological and histopathological findings were
essentially comparable between the control and treated groups.
However, increased incidences of kidney discoloration and firm
granular material in the pelvis of the kidney were noted in the males
of the colour treated groups. A slightly higher incidence of granules
in the pelvis was also noted in the high-dose males sacrificed at
termination. However, no significant dose-related trend was noted in
the statistical analysis of these data. An increased incidence of
renal calculi and focal epithelial proliferation was noted in the
high-dose rats, but additional examination of the other groups
indicated that this toxic effect could not be attributed to the
administration of the compound (Serota et al., 1977b).
Comments
Allura Red when administered orally undergoes partial azo
reduction prior to absorption. Metabolic studies indicate that the
colour is poorly absorbed in the body, and the major route of
excretion is via the faeces. In a multigeneration reproductive study
on rats it was shown that the progeny of the parents who were fed
51.9 g of the colour per kg of food demonstrated a slight growth
depression. The "no effect" level on reproductive physiology of this
colour in the rat is 13.9 g/kg of food. Teratogenicity studies in rats
and rabbits failed to show any compound-related embryotoxic effects. A
variety of mutagenicity studies carried out with Allura Red indicated
that there were no mutagenic effects. Another study on acute and
short-term oral toxicity of Allura Red in several species revealed
that apart from the coloration of the urine and faeces, there were no
other compound-related responses. Dermal studies (both short- and
long-term) also indicated an absence of colour-induced toxic
responses. In long-term feeding studies on mice and rats, the most
consistent observation was that the animals that received the greatest
amount of colour (51.9 g/kg of food) exhibited lower body weights
compared to control animals. One study suggested that mice that were
fed on this colour demonstrated an earlier onset of tumours of the
lymphatic system compared to control mice.1 However, a second more
extensive mouse study has not borne this out. The long-term study and
the mutagenicity studies suggest that Allura Red does not possess
carcinogenic potential.
The data were sufficient to establish a temporary acceptable
daily intake for man pending the availability of the statistical
analysis of the long-term mouse study.
EVALUATION
Level causing no toxicological effect
Rat: 1.39% in the diet corresponding to 695 mg/kg bw.
Estimate of acceptable daily intake for man
0-7 mg/kg bw.*
FURTHER WORK OR INFORMATION
Required by 1981
(1) Adequate information of statistical analysis of the long-term
mouse studies.
* Temporary.
REFERENCES
Anonymous. Mutagenicity investigations with allura red. Sex-linked
recessive lethals, Drosophila melanogaster, Bowling Green
University, Ohio, USA. Interim report of the Working Group on FD
& C Red, No. 40, 19 January 1977b, submitted to WHO by the US
Food and Drug Administration
Anonymous. Mutagenicity investigations with allura red. Saccharomyces
cerevisiae. Genetic Toxicology Branch of the FDA. Interim
report of the Working Group on FD & C Red No. 40, 19 January
1977a
Anonymous. Evaluation of substances of interest for genetic damage
using Drosophila melanogaster. Unpublished report submitted to
WHO by the United States Food and Drug Administration, 31 March
1978
Blackmore, R. H. Repeated dermal irritation study-rabbits, nontoxic
Red Z4576. Unpublished report 165-120 by Hazelton Laboratories,
Inc., submitted to WHO by Allied Chemical Corporation, 1968
Blackmore, R. H., Olson, W. A. & Voelker, R. W. Two-generation
reproduction study in rats, Red Z-4576. Unpublished report
165-133 by Hazelton Laboratories, Inc., submitted by Allied
Chemical Corporation, 1969
Bradlaw, J. Enzyme induction screen of seven color and cosmetic
ingredients. FDA Toxicology Laboratory, United States Food and
Drug Administration, manuscript in preparation, 1979
Brown, J., Roehm, G. & Brown, R. Mutagenicity testing of certified
food colors and related azo, xanthene and triphenylmethane dyes
with the Salmonella/microsome system, Mut. Res., 56: 249-271,
1978
Brusick, D. Mutagenicity evaluation NTRZ-4576 (Allura Red AC).
Unpublished report number 2547 by Litton Bionetics, Inc.,
submitted to WHO by Allied Chemical Corporation, 1976
Collins, T. F. X. Preliminary results of teratological studies on
Osborne-Mendel rats with FDC Red No. 40. Unpublished report by
Food and Drug Administration, USA, submitted to WHO, 1974
Guyton, C. L. & Reno, F. E. Metabolic disposition of 35S Allura Red AC
in the dog and rat. Unpublished report 165-146 by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Guyton, C. L. & Stanovick, R. P. Determination of the metabolites of
Allura Red AC in the rat and dog. Unpublished report by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Jolly, R. E. An assessment of irritation and sensitization, potential
of Allura Red AC utilizing the Draize-Shelanski reported
insult patch test and photosensitization potential evaluation.
Unpublished report by Biometric Testing, Inc., submitted to WHO
by Allied Chemical Corporation, 1973
Jorgenson, T. A., Rushbrook, C. J., Newell, G. W. & Skinner, W. A.
Study of the mutagenic effects of FD & C Red 40 (75-60) by the
heritable translocation study in mice. Unpublished report
LSU-5588 by SRI International, supplied by the United States
Food and Drug Administration, 1978
Mikkelsen, H., Larsen, J. & Tarding, F. Hypersensitivity reactions to
food colours with special reference to the natural colour annatto
extract (butter colour). Toxicological Aspects of Food Safety,
Arch. Tox. Suppl., 1:141-143, 1978
Muzzall, J. & Cook, W. Mutagenicity test of dyes used in cosmetics
with Salmonella/mammalian-microsome test, Mut. Res., 67:1-8,
1979
Olson, W. A. & Voelker, R. W. Twenty-one month dietary administration
albino rats, Red Z-4576. Unpublished report 165-121 by Hazelton
Laboratories, Inc., submitted to WHO by Allied Chemical
Corporation, 1970
Olson, W. A., Voelker, R. W. & Shott, L. D. One hundred and four-week
dietary administration-dogs, Red Z-4576. Unpublished report
165-132 by Hazelton Laboratories, Inc., submitted to WHO by
Allied Chemical Corporation, 1970
Osbourn, R. A. Report of human patch tests on FD & C Red No. 40.
Unpublished report 165-141 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1972
Reno, F. E. Teratology study in rabbits FD & C Red No. 40. Unpublished
report 165-142 by Hazelton Laboratories, Inc., submitted to WHO
by Allied Chemical Corporation, 1974
Reno, F. E., Mossburg, P. A., Serota, D. G., Tiede, J. J. & Voelker,
R. W. Carcinogenic bioassay and life-time dietary study in mice.
Unpublished report 165-174 by Hazelton Laboratories America,
Inc., submitted to WHO by Allied Chemical/Buffalo Color
Corporations, 1978
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the ICR Swiss mouse. Unpublished report
number 165-150 by Hazelton Laboratories America, Inc., submitted
to WHO by Allied Chemical/Buffalo Color Corporations, 1977a
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the albino rat. Unpublished report number
165-149 by Hazelton Laboratories America, Inc., submitted to WHO
by Allied Chemical/Buffalo Color Corporations, 1977b
Sondergaard, D., Hansen, E. & Wurtzen, H. A. A short-term study in the
pig of the effects on the liver and on the blood of eight azo
dyes, Toxicology, 8:381-386, 1977
Viola, M. & Nosotti, A. Application of the Ames test to certain color
additives, Boll. Chim. Farm., 117:402-415, 1978
Voelker, R. W. Repeated dermal application - Swiss mice, Red Z-4576.
Unpublished report 165-123 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1970
Weir, R. J. Acute oral administration - rats, five experimental
non-toxic red colors. Unpublished report 165-114 by Hazelton
Laboratories, Inc., submitted by Allied Chemical Corporation,
1965a
Weir, R. J. Acute oral toxicity - dogs, five experimental non-toxic
red colors. Unpublished report by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1965b
Weir, R. J. Acute dermal application - rabbits, Red Z-4576.
Unpublished report 165-119 by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1967
Weir, R. J. & Crews, L. M. Six-week dietary feeding study - rats,
experimental non-toxic Red Z-4576, Red Z-4578, Red Z-4598 and Red
Z-4808. Unpublished report 165-112 Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966a
Weir, R. J. & Crews, L. M. Six-week oral administration - dogs,
experimental non-toxic Red Z-4576, Z-4578, Z-4598 and Z-4808.
Unpublished report 165-116 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966b
White, R. G. Metabolic fate of orally ingested non-toxic Red Z-4576.
Unpublished report number 21855 by Buffalo Research Laboratory,
submitted to WHO by Allied Chemical Corporation, 1970
 and 1-amino-2-naphthol-6-sulfonic acid
and 1-amino-2-naphthol-6-sulfonic acid
 (White, 1970)
It appears that negligible quantities of intact Red are absorbed
and excreted in the urine, and that the major portion of the colour is
excreted as metabolites in the faeces.
TOXICOLOGICAL STUDIES
Reproduction
Rat
Groups of 10 male and 20 female rats received 0, 0.37, 1.39 or
5.19% of Allura Red in their diet through two parental (F1A was the
P2 generation) and two filial generations. Mating occurred after 27
weeks on either the control or test diets for both the P1 and P2
generation. The fertility indices were low for the controls and test
animals in the F1A and F1B generation as well as in the low test
level F2A and all test levels of F2B generation. Growth was
suppressed slightly for the lowest test level in F1B and high test
levels in F1A and F1B as well as for the high test levels in F2A
and F2B pups. Other indices, litter size and pup weight at 24 hours,
were comparable in each group in each generation. No consistent
pathological changes were noted in the P1, F1A, F1B and F2B
generations. No evidence was seen of teratogenic or embryotoxic
effects regarding implantation sites, resorption sites and live
foetuses indices. No difference from controls was noted with regard to
appearance, anatomy and structure of test foetuses (Blackmore et al.,
1969).
Teratogenicity
Rat
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of foetuses per litter and average
foetus weight (Collins, 1974).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of compound-related effects with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal dose. No adverse effects on implantation and litter
data were noted nor were any foetal abnormalities observed (Reno,
1974).
Mutagenicity studies
Microbial assay systems (plate and suspension tests) with and
without the addition of mammalian metabolic activation enzymes were
used to determine the mutagenic potential of Allura Red. One strain of
yeast, Saccharomyces cerevisiae, and five strains of the bacteria
Salmonella typhimurium were used in the study which employed
negative and positive controls. Preliminary toxicity studies were
conducted and the compound was found to be nontoxic at the 5%
concentration. Allura Red did not exhibit genetic activity in any of
the assay systems employed (Brusick, 1976).
Genetic tests were also conducted using three strains of
Saccharomyces cerevisiae with and without liver enzyme induction
(Anonymous, 1977a). The colour also proved to be negative in these
systems.
The Salmonella/microsome system was used to test a number of azo
dyes including FD&C Red No. 40. The dye, as well as its chemically
reduced component amines, were tested with five tester strains
(TA 1535, TA 100, TA 1537, TA 1538 and TA 98). In addition, the
effects of the colour were analysed in the same five tester strains
with microsomal activation (S-9) and in an aerobic liquid test with
microsomal activation. None of these test systems demonstrated
mutagenic activity of FD&C Red No. 40 (Brown et al., 1978).
In a second Salmonella/mammalian-microsome study, the colour was
tested for mutagenic potential in two frame-shift histidine (TA 1537
and TA 98) and two base-pair substituted histidine mutants (TA 1535
and TA 100). Both the spot test and the plate incorporation assay,
with and without the S-9 mix, were employed in these experiments. As
before, Allura Red was found to have no mutagenic effects in these
assay systems (Muzzall & Cook, 1979).
The same four strains of Salmonella typhimurium (TA 1535,
TA 1538, TA 100 and TA 98) with and without microsomal activation were
used in the spot and plate tests to study the effects of Allura Red.
None of the tests performed demonstrated either a mutagenic or
cytotoxic activity of this compound (Viola & Nosotti, 1978).
A rat hepatoma cell culture system was utilized to determine if
the colour had enzyme induction capabilities, and it was found that
the colour could not induce aryl hydrocarbon hydroxylase activity
(Bradlaw, 1979).
Allura Red was tested in the genetic analysis for recessive
lethal effects by the oral feeding to Drosophila melanogaster at the
LD50 dose for 24 days. The following genetic tests were conducted:
loss of X or Y chromosome, visible mutation at specific loci and
sex-linked recessive lethal damage in both mature and immature
spermatozoa, chromosomal translocation, sex-linked lethal damage of
aged-in-the female spermatozoa and sex-linked mosaic recessive lethal
damage in mature spermatozoa. There was no significant increase in the
proportion of mutation in any category when comparisons were made with
the individual controls. However, when the controls were combined with
the controls used in two other studies, a significant increase was
seen for the category of sex-linked mosaic recessive lethal damage
(Anonymous, 1977b, 1978).
The heritable translocation potential of Allura Red was analysed
in eight to 10-week-old male mice who were fed diets containing the
colour at dose levels of 4000 and 20 000 ppm for eight weeks. Each
male was mated with two females to produce an F1 generation. The
males in later generations were mated and their reproductive
performance was analysed. The administration of the colour did not
affect the fertility of the treated mice. Although several of the
animals receiving the colour were potential translocation
heterozygotes, cytogenetic analysis showed that these animals were
normal. It was concluded that the compound was negative with respect
to induction of inheritable translocation in mice (Jorgenson et al.,
1978).
Acute toxicity studies
LD50
Animal Route (mg/kg bw) Reference
Rat Oral (gavage) 10 000 Weir, 1965a
Rabbit Dermal 10 000 Weir, 1967
Dog Oral (gavage) 5 000 Weir, 1965b
Rat
The colour was administered to six groups (five animal/sex) at
doses varying from 215 to 10 000 mg/kg. The only compound-related
effect of note was the red coloration of the urine and faeces (Weir,
1965a).
Skin irritation and sensitization studies
Rabbit
Tests for dermal irritation on intact and abraded skin showed no
gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg bw of
Allura Red; none the less a persistent skin staining did occur (Weir,
1967).
Daily application of the colour at rates of 0.5 g/kg, five days a
week for 15 applications on abraded and 65 applications on intack skin
of rabbits, of 0.1 and 1% solutions in water or as a hydrophilic
ointment revealed no compound-related effect on general appearance,
body weight, clinical laboratory studies and gross and microscopic
pathology. No dermal irritation was noted, except that produced by the
hydrophilic ointment base (Blackmore, 1968).
Human
Prophetic patch test
Allura Red was applied either as a neat or as a 25% aqueous
solution to the skin of 200 human subjects. The initial exposure to
the compound was for 72 hours, and this was followed by a 24-hour
application 10 to 14 days later. None of the subjects exhibited
compound induced irritation or sensitization (Osbourn, 1972).
Draize-Shelanski repeated insult patch test
In this study, the colour and its alumina lake were applied to
the subjects volvar forearms (200 subjects) as an aqueous solution for
10 alternate days, for 24-hour periods, followed by a 14-day rest
period. Challenge batches were then applied under occlusion to fresh
skin sites on the subjects scapular backs for 24 hours. The colour did
not produce either irritation or allergic responses during the
induction phase nor contact dermatitis in the challenge period (Jolly,
1973).
Photosensitization potential test
As in the previous study, Allura Red and its lake were evaluated
on sites under occlusion for five 48-hour, alternate-day periods.
These sites had been previously irradiated for five minutes with Xenon
light which had been filtered through a window-glass equivalent to
limit the exposure to non-erythema-producing, long-wave radiation. A
10-day rest period followed this induction exposure, and then the
colour was applied to fresh skin sites, irradiated for five minutes
with Xenon and subsequently removed and the sites were evaluated.
Allura Red was shown not to produce photosensitization on the 25
subjects studied (Jolly, 1973).
Hypersensitivity reactions
In 52 patients who were suffering from urticaria or angioedema
for more than four weeks were placed on an elimination diet for at
least three weeks. All non-vital drugs were suspended during the study
as were any food ingredients which were known to cause urticaria. In
most patients, challenge with Allura Red was performed when the
patients were free of symptoms. Reactions were considered positive
only when the flare-up of symptoms was reproducible within the same
time from intake to skin reaction. Allura Red was administered via the
oral route in either a dose of 1 or 10 mg, and induced a positive
reaction in 15% of the 52 patients challenged (Mikkelsen et al.,
1978).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% of Allure Red for six weeks. No
evidence of compound-related effects were noted as regards body
weight, food consumption, survival, organ weights, gross and
microscopic pathology. Haematology and urinalysis were normal and no
evidence of Heinz body formation was noted (Weir & Crews, 1966a).
Dog
Four groups of one male and one female beagle dogs received
Allure Red daily in capsule form at the following doses, 0, 125, 250
and 500 mg/kg bw. No adverse effects were noted on body weight, food
consumption, survival, organ weights, gross and histopathology,
haematology and clinical chemistry. At the highest level, there were
slight ill-defined hepatic parenchymal changes in both sexes (Weir &
Crews, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% of Allure Red in their diet for 104 weeks. All animals
remained normal regarding appearance, behaviour, haematology and
clinical chemistry findings and gross and histopathology. Both faeces
and urine were coloured at all test levels. At 52 weeks, an interim
sacrifice was performed and revealed that the adrenal cortical cells
of the high level groups showed some vacuolation. There was brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Olson et al., 1970).
Pig
SPF pigs of Danish Landrace were equilibrated for a period of 21
days, whereupon they were distributed according to body weight into
nine groups which consisted of two males and two females. The compound
was administered by gavage three hours after feeding in the morning.
The dye was administered at a dose of 1000 mg/kg/day for the first 21
days, thereafter the dose was increased to 1500 mg/kg/day and this
dosage was given for an additional 54 days. Body weight was recorded
weekly and food intake daily. Blood samples were collected two days
prior to and five, 19 and 68 days following the initiation of colorant
administration. The haematological and clinical parameters measured
were haemoglobin, packed cell volume, total erythrocyte count, counts
of Heinz bodies and reticulocytes and serum activities of LDH and
isoenzymes. Autopsy and histological examination of kidneys, spleen,
liver, hepatic and renal lymph nodes and bone marrow were performed.
The colour produced no significant effects on clinical and
haematological parameters nor did it elicit any observable
pathological changes (Sondergaard et al., 1977).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female and a test group of 50 male and 50
female mice were treated dermally with 0.1 ml of either distilled
water, 10 µg 3,4-benzypyrene in acetone or a 5% Allura Red test
solution twice weekly for 20 months. The results in the positive
control group showed the mouse strain used to be sensitive to
benzypyrene carcinogenesis. Survival, gross and histopathology of
major organs were comparable in the negative controls and animals
treated with Allura Red. Histology of the skin revealed comparable
incidence and degree of severity of acanthosis, hyperkeratosis and
dermatitis for the negative control and the Allura Red groups
(Voelker, 1970).
Groups of 50 CD(R)-1 albino mice/sex received Allura Red in
their diets at levels of 0.37, 1.39 and 5.19%. These animals were the
offspring of parental mice treated at identical levels for one week
prior to breeding and through gestation and lactation. An additional
group of 50 animals/sex served as a negative control group. An interim
sacrifice was performed at 42 weeks which resulted in reducing the
number of animals in each group and sex to 30. This was done to
determine compound effects upon the early onset of tumours of the
lymphatic system. Prior to this, neoplasms which were histologically
diagnosed as lymphomas were found in several animals which received
the colour. Statistical analysis of the incidence of tumours occurring
throughout the study did not reveal any increase in tumour incidence
due to compound administration. No treatment-related effects were
noted in the survival, clinical signs and clinical laboratory data.
The animals on the highest dosage level demonstrated lower body
weights and effects on organ weight data and organ/body weight ratios,
however, the meaning of these observations ware considered to be
inconsequential. Gross and microscopic examination of all the animals
revealed an absence of treatment-related effects and spontaneous
disease-related lesions were considered to be within acceptable limits
(Serota et al., 1977a).
A second lifetime dietary mouse study was performed using a
protocol identical to the one described above, with the exception that
100 animals/sex/group and two negative control groups were used. As
before, statistical evaluation of neoplasms of the reticuloendothelial
system failed to show a significant correlation between tumour
response and increasing dosage. Sporadic changes were noted in the
clinical signs, body weight, food consumption and survival rates; none
the less, these could not be related to the administration of the
colour. Alterations of the absolute and relative weights of the
adrenals and thyroids were noted in several of the treatment groups.
Histological examination of these tissues in the animals in the
highest dose group at the completion of the study demonstrated no
abnormalities. Therefore, the significance of weight gains in these
two organs could not be established. Generally, gross pathological
findings were similar in the control and treated animals. There was,
however, a higher incidence of dark areas in the lungs of those
animals in the high dose group which died or were sacrificed
in extremis and of cystic foci areas in the kidneys of the high dose
males. No distinct treatment-related effects could be ascribed to
these findings. Histopathological evaluation did not show effects
which could be attributed to compound administration, nor was there
evidence for a dose-related increase in the incidence of spontaneously
occurring tumours (Reno et al., 1978).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% of Allura Red for 92 weeks. Moderate growth
depression occurred at the 5.19% level in both sexes. No other
compound-related effects were noted as regards appearance, behaviour,
survival, organ weights, clinical laboratory studies or gross and
histopathology. No evidence of Heinz body formation was noted apart
from a slight tendency to anaemia (Olson & Voelker, 1970).
50 CD(R)-1 albino rats/sex were placed in a negative control
group and three test groups which contained varying percentages (0.37,
1.39 and 5.19) of Allura Red. The males were administered the diets
for 118 weeks, and the females were fed for 121 weeks. All of these
animals were the F1a offspring of parental rats which were treated at
identical levels for one week prior to breeding, through a three-week
breeding period and during the gestation and lactation periods. No
treatment-related effects were noted in comparisons of survival,
clinical signs, clinical laboratory data and organ weight data. The
mean body weights and growth rates of the animals in the high-dose
group were lower than those of control animals. However, the low dose
group exhibited greater mean food consumption values than the control
groups. Gross pathological and histopathological findings were
essentially comparable between the control and treated groups.
However, increased incidences of kidney discoloration and firm
granular material in the pelvis of the kidney were noted in the males
of the colour treated groups. A slightly higher incidence of granules
in the pelvis was also noted in the high-dose males sacrificed at
termination. However, no significant dose-related trend was noted in
the statistical analysis of these data. An increased incidence of
renal calculi and focal epithelial proliferation was noted in the
high-dose rats, but additional examination of the other groups
indicated that this toxic effect could not be attributed to the
administration of the compound (Serota et al., 1977b).
Comments
Allura Red when administered orally undergoes partial azo
reduction prior to absorption. Metabolic studies indicate that the
colour is poorly absorbed in the body, and the major route of
excretion is via the faeces. In a multigeneration reproductive study
on rats it was shown that the progeny of the parents who were fed
51.9 g of the colour per kg of food demonstrated a slight growth
depression. The "no effect" level on reproductive physiology of this
colour in the rat is 13.9 g/kg of food. Teratogenicity studies in rats
and rabbits failed to show any compound-related embryotoxic effects. A
variety of mutagenicity studies carried out with Allura Red indicated
that there were no mutagenic effects. Another study on acute and
short-term oral toxicity of Allura Red in several species revealed
that apart from the coloration of the urine and faeces, there were no
other compound-related responses. Dermal studies (both short- and
long-term) also indicated an absence of colour-induced toxic
responses. In long-term feeding studies on mice and rats, the most
consistent observation was that the animals that received the greatest
amount of colour (51.9 g/kg of food) exhibited lower body weights
compared to control animals. One study suggested that mice that were
fed on this colour demonstrated an earlier onset of tumours of the
lymphatic system compared to control mice.1 However, a second more
extensive mouse study has not borne this out. The long-term study and
the mutagenicity studies suggest that Allura Red does not possess
carcinogenic potential.
The data were sufficient to establish a temporary acceptable
daily intake for man pending the availability of the statistical
analysis of the long-term mouse study.
EVALUATION
Level causing no toxicological effect
Rat: 1.39% in the diet corresponding to 695 mg/kg bw.
Estimate of acceptable daily intake for man
0-7 mg/kg bw.*
FURTHER WORK OR INFORMATION
Required by 1981
(1) Adequate information of statistical analysis of the long-term
mouse studies.
* Temporary.
REFERENCES
Anonymous. Mutagenicity investigations with allura red. Sex-linked
recessive lethals, Drosophila melanogaster, Bowling Green
University, Ohio, USA. Interim report of the Working Group on FD
& C Red, No. 40, 19 January 1977b, submitted to WHO by the US
Food and Drug Administration
Anonymous. Mutagenicity investigations with allura red. Saccharomyces
cerevisiae. Genetic Toxicology Branch of the FDA. Interim
report of the Working Group on FD & C Red No. 40, 19 January
1977a
Anonymous. Evaluation of substances of interest for genetic damage
using Drosophila melanogaster. Unpublished report submitted to
WHO by the United States Food and Drug Administration, 31 March
1978
Blackmore, R. H. Repeated dermal irritation study-rabbits, nontoxic
Red Z4576. Unpublished report 165-120 by Hazelton Laboratories,
Inc., submitted to WHO by Allied Chemical Corporation, 1968
Blackmore, R. H., Olson, W. A. & Voelker, R. W. Two-generation
reproduction study in rats, Red Z-4576. Unpublished report
165-133 by Hazelton Laboratories, Inc., submitted by Allied
Chemical Corporation, 1969
Bradlaw, J. Enzyme induction screen of seven color and cosmetic
ingredients. FDA Toxicology Laboratory, United States Food and
Drug Administration, manuscript in preparation, 1979
Brown, J., Roehm, G. & Brown, R. Mutagenicity testing of certified
food colors and related azo, xanthene and triphenylmethane dyes
with the Salmonella/microsome system, Mut. Res., 56: 249-271,
1978
Brusick, D. Mutagenicity evaluation NTRZ-4576 (Allura Red AC).
Unpublished report number 2547 by Litton Bionetics, Inc.,
submitted to WHO by Allied Chemical Corporation, 1976
Collins, T. F. X. Preliminary results of teratological studies on
Osborne-Mendel rats with FDC Red No. 40. Unpublished report by
Food and Drug Administration, USA, submitted to WHO, 1974
Guyton, C. L. & Reno, F. E. Metabolic disposition of 35S Allura Red AC
in the dog and rat. Unpublished report 165-146 by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Guyton, C. L. & Stanovick, R. P. Determination of the metabolites of
Allura Red AC in the rat and dog. Unpublished report by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Jolly, R. E. An assessment of irritation and sensitization, potential
of Allura Red AC utilizing the Draize-Shelanski reported
insult patch test and photosensitization potential evaluation.
Unpublished report by Biometric Testing, Inc., submitted to WHO
by Allied Chemical Corporation, 1973
Jorgenson, T. A., Rushbrook, C. J., Newell, G. W. & Skinner, W. A.
Study of the mutagenic effects of FD & C Red 40 (75-60) by the
heritable translocation study in mice. Unpublished report
LSU-5588 by SRI International, supplied by the United States
Food and Drug Administration, 1978
Mikkelsen, H., Larsen, J. & Tarding, F. Hypersensitivity reactions to
food colours with special reference to the natural colour annatto
extract (butter colour). Toxicological Aspects of Food Safety,
Arch. Tox. Suppl., 1:141-143, 1978
Muzzall, J. & Cook, W. Mutagenicity test of dyes used in cosmetics
with Salmonella/mammalian-microsome test, Mut. Res., 67:1-8,
1979
Olson, W. A. & Voelker, R. W. Twenty-one month dietary administration
albino rats, Red Z-4576. Unpublished report 165-121 by Hazelton
Laboratories, Inc., submitted to WHO by Allied Chemical
Corporation, 1970
Olson, W. A., Voelker, R. W. & Shott, L. D. One hundred and four-week
dietary administration-dogs, Red Z-4576. Unpublished report
165-132 by Hazelton Laboratories, Inc., submitted to WHO by
Allied Chemical Corporation, 1970
Osbourn, R. A. Report of human patch tests on FD & C Red No. 40.
Unpublished report 165-141 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1972
Reno, F. E. Teratology study in rabbits FD & C Red No. 40. Unpublished
report 165-142 by Hazelton Laboratories, Inc., submitted to WHO
by Allied Chemical Corporation, 1974
Reno, F. E., Mossburg, P. A., Serota, D. G., Tiede, J. J. & Voelker,
R. W. Carcinogenic bioassay and life-time dietary study in mice.
Unpublished report 165-174 by Hazelton Laboratories America,
Inc., submitted to WHO by Allied Chemical/Buffalo Color
Corporations, 1978
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the ICR Swiss mouse. Unpublished report
number 165-150 by Hazelton Laboratories America, Inc., submitted
to WHO by Allied Chemical/Buffalo Color Corporations, 1977a
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the albino rat. Unpublished report number
165-149 by Hazelton Laboratories America, Inc., submitted to WHO
by Allied Chemical/Buffalo Color Corporations, 1977b
Sondergaard, D., Hansen, E. & Wurtzen, H. A. A short-term study in the
pig of the effects on the liver and on the blood of eight azo
dyes, Toxicology, 8:381-386, 1977
Viola, M. & Nosotti, A. Application of the Ames test to certain color
additives, Boll. Chim. Farm., 117:402-415, 1978
Voelker, R. W. Repeated dermal application - Swiss mice, Red Z-4576.
Unpublished report 165-123 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1970
Weir, R. J. Acute oral administration - rats, five experimental
non-toxic red colors. Unpublished report 165-114 by Hazelton
Laboratories, Inc., submitted by Allied Chemical Corporation,
1965a
Weir, R. J. Acute oral toxicity - dogs, five experimental non-toxic
red colors. Unpublished report by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1965b
Weir, R. J. Acute dermal application - rabbits, Red Z-4576.
Unpublished report 165-119 by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1967
Weir, R. J. & Crews, L. M. Six-week dietary feeding study - rats,
experimental non-toxic Red Z-4576, Red Z-4578, Red Z-4598 and Red
Z-4808. Unpublished report 165-112 Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966a
Weir, R. J. & Crews, L. M. Six-week oral administration - dogs,
experimental non-toxic Red Z-4576, Z-4578, Z-4598 and Z-4808.
Unpublished report 165-116 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966b
White, R. G. Metabolic fate of orally ingested non-toxic Red Z-4576.
Unpublished report number 21855 by Buffalo Research Laboratory,
submitted to WHO by Allied Chemical Corporation, 1970
(White, 1970)
It appears that negligible quantities of intact Red are absorbed
and excreted in the urine, and that the major portion of the colour is
excreted as metabolites in the faeces.
TOXICOLOGICAL STUDIES
Reproduction
Rat
Groups of 10 male and 20 female rats received 0, 0.37, 1.39 or
5.19% of Allura Red in their diet through two parental (F1A was the
P2 generation) and two filial generations. Mating occurred after 27
weeks on either the control or test diets for both the P1 and P2
generation. The fertility indices were low for the controls and test
animals in the F1A and F1B generation as well as in the low test
level F2A and all test levels of F2B generation. Growth was
suppressed slightly for the lowest test level in F1B and high test
levels in F1A and F1B as well as for the high test levels in F2A
and F2B pups. Other indices, litter size and pup weight at 24 hours,
were comparable in each group in each generation. No consistent
pathological changes were noted in the P1, F1A, F1B and F2B
generations. No evidence was seen of teratogenic or embryotoxic
effects regarding implantation sites, resorption sites and live
foetuses indices. No difference from controls was noted with regard to
appearance, anatomy and structure of test foetuses (Blackmore et al.,
1969).
Teratogenicity
Rat
Groups of 24, 19, 20, 21 and 16 pregnant rats received
respectively 0, 15, 30, 100 and 200 mg/kg of the dye by gavage daily
during pregnancy days 0-19. Gross observations indicate no dye-induced
effects in terms of early or late deaths, resorptions per litter,
pre-implantation loss, number of foetuses per litter and average
foetus weight (Collins, 1974).
Rabbit
In three groups of 14 rabbits, Allura Red was given in doses of
0, 200 and 700 mg/kg bw by gavage from day 6 to 18 of pregnancy. There
were no indications of compound-related effects with regard to
appearance and behaviour, body weight or in gross necropsy findings
for the maternal dose. No adverse effects on implantation and litter
data were noted nor were any foetal abnormalities observed (Reno,
1974).
Mutagenicity studies
Microbial assay systems (plate and suspension tests) with and
without the addition of mammalian metabolic activation enzymes were
used to determine the mutagenic potential of Allura Red. One strain of
yeast, Saccharomyces cerevisiae, and five strains of the bacteria
Salmonella typhimurium were used in the study which employed
negative and positive controls. Preliminary toxicity studies were
conducted and the compound was found to be nontoxic at the 5%
concentration. Allura Red did not exhibit genetic activity in any of
the assay systems employed (Brusick, 1976).
Genetic tests were also conducted using three strains of
Saccharomyces cerevisiae with and without liver enzyme induction
(Anonymous, 1977a). The colour also proved to be negative in these
systems.
The Salmonella/microsome system was used to test a number of azo
dyes including FD&C Red No. 40. The dye, as well as its chemically
reduced component amines, were tested with five tester strains
(TA 1535, TA 100, TA 1537, TA 1538 and TA 98). In addition, the
effects of the colour were analysed in the same five tester strains
with microsomal activation (S-9) and in an aerobic liquid test with
microsomal activation. None of these test systems demonstrated
mutagenic activity of FD&C Red No. 40 (Brown et al., 1978).
In a second Salmonella/mammalian-microsome study, the colour was
tested for mutagenic potential in two frame-shift histidine (TA 1537
and TA 98) and two base-pair substituted histidine mutants (TA 1535
and TA 100). Both the spot test and the plate incorporation assay,
with and without the S-9 mix, were employed in these experiments. As
before, Allura Red was found to have no mutagenic effects in these
assay systems (Muzzall & Cook, 1979).
The same four strains of Salmonella typhimurium (TA 1535,
TA 1538, TA 100 and TA 98) with and without microsomal activation were
used in the spot and plate tests to study the effects of Allura Red.
None of the tests performed demonstrated either a mutagenic or
cytotoxic activity of this compound (Viola & Nosotti, 1978).
A rat hepatoma cell culture system was utilized to determine if
the colour had enzyme induction capabilities, and it was found that
the colour could not induce aryl hydrocarbon hydroxylase activity
(Bradlaw, 1979).
Allura Red was tested in the genetic analysis for recessive
lethal effects by the oral feeding to Drosophila melanogaster at the
LD50 dose for 24 days. The following genetic tests were conducted:
loss of X or Y chromosome, visible mutation at specific loci and
sex-linked recessive lethal damage in both mature and immature
spermatozoa, chromosomal translocation, sex-linked lethal damage of
aged-in-the female spermatozoa and sex-linked mosaic recessive lethal
damage in mature spermatozoa. There was no significant increase in the
proportion of mutation in any category when comparisons were made with
the individual controls. However, when the controls were combined with
the controls used in two other studies, a significant increase was
seen for the category of sex-linked mosaic recessive lethal damage
(Anonymous, 1977b, 1978).
The heritable translocation potential of Allura Red was analysed
in eight to 10-week-old male mice who were fed diets containing the
colour at dose levels of 4000 and 20 000 ppm for eight weeks. Each
male was mated with two females to produce an F1 generation. The
males in later generations were mated and their reproductive
performance was analysed. The administration of the colour did not
affect the fertility of the treated mice. Although several of the
animals receiving the colour were potential translocation
heterozygotes, cytogenetic analysis showed that these animals were
normal. It was concluded that the compound was negative with respect
to induction of inheritable translocation in mice (Jorgenson et al.,
1978).
Acute toxicity studies
LD50
Animal Route (mg/kg bw) Reference
Rat Oral (gavage) 10 000 Weir, 1965a
Rabbit Dermal 10 000 Weir, 1967
Dog Oral (gavage) 5 000 Weir, 1965b
Rat
The colour was administered to six groups (five animal/sex) at
doses varying from 215 to 10 000 mg/kg. The only compound-related
effect of note was the red coloration of the urine and faeces (Weir,
1965a).
Skin irritation and sensitization studies
Rabbit
Tests for dermal irritation on intact and abraded skin showed no
gross irritation at levels of 0.316, 1.0, 3.16 and 10 g/kg bw of
Allura Red; none the less a persistent skin staining did occur (Weir,
1967).
Daily application of the colour at rates of 0.5 g/kg, five days a
week for 15 applications on abraded and 65 applications on intack skin
of rabbits, of 0.1 and 1% solutions in water or as a hydrophilic
ointment revealed no compound-related effect on general appearance,
body weight, clinical laboratory studies and gross and microscopic
pathology. No dermal irritation was noted, except that produced by the
hydrophilic ointment base (Blackmore, 1968).
Human
Prophetic patch test
Allura Red was applied either as a neat or as a 25% aqueous
solution to the skin of 200 human subjects. The initial exposure to
the compound was for 72 hours, and this was followed by a 24-hour
application 10 to 14 days later. None of the subjects exhibited
compound induced irritation or sensitization (Osbourn, 1972).
Draize-Shelanski repeated insult patch test
In this study, the colour and its alumina lake were applied to
the subjects volvar forearms (200 subjects) as an aqueous solution for
10 alternate days, for 24-hour periods, followed by a 14-day rest
period. Challenge batches were then applied under occlusion to fresh
skin sites on the subjects scapular backs for 24 hours. The colour did
not produce either irritation or allergic responses during the
induction phase nor contact dermatitis in the challenge period (Jolly,
1973).
Photosensitization potential test
As in the previous study, Allura Red and its lake were evaluated
on sites under occlusion for five 48-hour, alternate-day periods.
These sites had been previously irradiated for five minutes with Xenon
light which had been filtered through a window-glass equivalent to
limit the exposure to non-erythema-producing, long-wave radiation. A
10-day rest period followed this induction exposure, and then the
colour was applied to fresh skin sites, irradiated for five minutes
with Xenon and subsequently removed and the sites were evaluated.
Allura Red was shown not to produce photosensitization on the 25
subjects studied (Jolly, 1973).
Hypersensitivity reactions
In 52 patients who were suffering from urticaria or angioedema
for more than four weeks were placed on an elimination diet for at
least three weeks. All non-vital drugs were suspended during the study
as were any food ingredients which were known to cause urticaria. In
most patients, challenge with Allura Red was performed when the
patients were free of symptoms. Reactions were considered positive
only when the flare-up of symptoms was reproducible within the same
time from intake to skin reaction. Allura Red was administered via the
oral route in either a dose of 1 or 10 mg, and induced a positive
reaction in 15% of the 52 patients challenged (Mikkelsen et al.,
1978).
Short-term studies
Rat
Groups of 10 male and 10 female rats were fed diets containing 0,
0.37, 0.72, 1.39, 2.69 and 5.19% of Allure Red for six weeks. No
evidence of compound-related effects were noted as regards body
weight, food consumption, survival, organ weights, gross and
microscopic pathology. Haematology and urinalysis were normal and no
evidence of Heinz body formation was noted (Weir & Crews, 1966a).
Dog
Four groups of one male and one female beagle dogs received
Allure Red daily in capsule form at the following doses, 0, 125, 250
and 500 mg/kg bw. No adverse effects were noted on body weight, food
consumption, survival, organ weights, gross and histopathology,
haematology and clinical chemistry. At the highest level, there were
slight ill-defined hepatic parenchymal changes in both sexes (Weir &
Crews, 1966b).
Groups of four male and four female beagle dogs received 0, 0.37,
1.39 and 5.19% of Allure Red in their diet for 104 weeks. All animals
remained normal regarding appearance, behaviour, haematology and
clinical chemistry findings and gross and histopathology. Both faeces
and urine were coloured at all test levels. At 52 weeks, an interim
sacrifice was performed and revealed that the adrenal cortical cells
of the high level groups showed some vacuolation. There was brown
pigment deposition in the Kupffer cells of females at the two lower
test levels. These changes had disappeared by 104 weeks and special
histological examination of the eyes revealed no adverse changes
(Olson et al., 1970).
Pig
SPF pigs of Danish Landrace were equilibrated for a period of 21
days, whereupon they were distributed according to body weight into
nine groups which consisted of two males and two females. The compound
was administered by gavage three hours after feeding in the morning.
The dye was administered at a dose of 1000 mg/kg/day for the first 21
days, thereafter the dose was increased to 1500 mg/kg/day and this
dosage was given for an additional 54 days. Body weight was recorded
weekly and food intake daily. Blood samples were collected two days
prior to and five, 19 and 68 days following the initiation of colorant
administration. The haematological and clinical parameters measured
were haemoglobin, packed cell volume, total erythrocyte count, counts
of Heinz bodies and reticulocytes and serum activities of LDH and
isoenzymes. Autopsy and histological examination of kidneys, spleen,
liver, hepatic and renal lymph nodes and bone marrow were performed.
The colour produced no significant effects on clinical and
haematological parameters nor did it elicit any observable
pathological changes (Sondergaard et al., 1977).
Long-term studies
Mouse
A control group of 50 male and 50 female mice, a positive control
group of 25 male and 25 female and a test group of 50 male and 50
female mice were treated dermally with 0.1 ml of either distilled
water, 10 µg 3,4-benzypyrene in acetone or a 5% Allura Red test
solution twice weekly for 20 months. The results in the positive
control group showed the mouse strain used to be sensitive to
benzypyrene carcinogenesis. Survival, gross and histopathology of
major organs were comparable in the negative controls and animals
treated with Allura Red. Histology of the skin revealed comparable
incidence and degree of severity of acanthosis, hyperkeratosis and
dermatitis for the negative control and the Allura Red groups
(Voelker, 1970).
Groups of 50 CD(R)-1 albino mice/sex received Allura Red in
their diets at levels of 0.37, 1.39 and 5.19%. These animals were the
offspring of parental mice treated at identical levels for one week
prior to breeding and through gestation and lactation. An additional
group of 50 animals/sex served as a negative control group. An interim
sacrifice was performed at 42 weeks which resulted in reducing the
number of animals in each group and sex to 30. This was done to
determine compound effects upon the early onset of tumours of the
lymphatic system. Prior to this, neoplasms which were histologically
diagnosed as lymphomas were found in several animals which received
the colour. Statistical analysis of the incidence of tumours occurring
throughout the study did not reveal any increase in tumour incidence
due to compound administration. No treatment-related effects were
noted in the survival, clinical signs and clinical laboratory data.
The animals on the highest dosage level demonstrated lower body
weights and effects on organ weight data and organ/body weight ratios,
however, the meaning of these observations ware considered to be
inconsequential. Gross and microscopic examination of all the animals
revealed an absence of treatment-related effects and spontaneous
disease-related lesions were considered to be within acceptable limits
(Serota et al., 1977a).
A second lifetime dietary mouse study was performed using a
protocol identical to the one described above, with the exception that
100 animals/sex/group and two negative control groups were used. As
before, statistical evaluation of neoplasms of the reticuloendothelial
system failed to show a significant correlation between tumour
response and increasing dosage. Sporadic changes were noted in the
clinical signs, body weight, food consumption and survival rates; none
the less, these could not be related to the administration of the
colour. Alterations of the absolute and relative weights of the
adrenals and thyroids were noted in several of the treatment groups.
Histological examination of these tissues in the animals in the
highest dose group at the completion of the study demonstrated no
abnormalities. Therefore, the significance of weight gains in these
two organs could not be established. Generally, gross pathological
findings were similar in the control and treated animals. There was,
however, a higher incidence of dark areas in the lungs of those
animals in the high dose group which died or were sacrificed
in extremis and of cystic foci areas in the kidneys of the high dose
males. No distinct treatment-related effects could be ascribed to
these findings. Histopathological evaluation did not show effects
which could be attributed to compound administration, nor was there
evidence for a dose-related increase in the incidence of spontaneously
occurring tumours (Reno et al., 1978).
Rat
Groups of 30 male and 30 female rats received in their diet 0,
0.37, 1.39 and 5.19% of Allura Red for 92 weeks. Moderate growth
depression occurred at the 5.19% level in both sexes. No other
compound-related effects were noted as regards appearance, behaviour,
survival, organ weights, clinical laboratory studies or gross and
histopathology. No evidence of Heinz body formation was noted apart
from a slight tendency to anaemia (Olson & Voelker, 1970).
50 CD(R)-1 albino rats/sex were placed in a negative control
group and three test groups which contained varying percentages (0.37,
1.39 and 5.19) of Allura Red. The males were administered the diets
for 118 weeks, and the females were fed for 121 weeks. All of these
animals were the F1a offspring of parental rats which were treated at
identical levels for one week prior to breeding, through a three-week
breeding period and during the gestation and lactation periods. No
treatment-related effects were noted in comparisons of survival,
clinical signs, clinical laboratory data and organ weight data. The
mean body weights and growth rates of the animals in the high-dose
group were lower than those of control animals. However, the low dose
group exhibited greater mean food consumption values than the control
groups. Gross pathological and histopathological findings were
essentially comparable between the control and treated groups.
However, increased incidences of kidney discoloration and firm
granular material in the pelvis of the kidney were noted in the males
of the colour treated groups. A slightly higher incidence of granules
in the pelvis was also noted in the high-dose males sacrificed at
termination. However, no significant dose-related trend was noted in
the statistical analysis of these data. An increased incidence of
renal calculi and focal epithelial proliferation was noted in the
high-dose rats, but additional examination of the other groups
indicated that this toxic effect could not be attributed to the
administration of the compound (Serota et al., 1977b).
Comments
Allura Red when administered orally undergoes partial azo
reduction prior to absorption. Metabolic studies indicate that the
colour is poorly absorbed in the body, and the major route of
excretion is via the faeces. In a multigeneration reproductive study
on rats it was shown that the progeny of the parents who were fed
51.9 g of the colour per kg of food demonstrated a slight growth
depression. The "no effect" level on reproductive physiology of this
colour in the rat is 13.9 g/kg of food. Teratogenicity studies in rats
and rabbits failed to show any compound-related embryotoxic effects. A
variety of mutagenicity studies carried out with Allura Red indicated
that there were no mutagenic effects. Another study on acute and
short-term oral toxicity of Allura Red in several species revealed
that apart from the coloration of the urine and faeces, there were no
other compound-related responses. Dermal studies (both short- and
long-term) also indicated an absence of colour-induced toxic
responses. In long-term feeding studies on mice and rats, the most
consistent observation was that the animals that received the greatest
amount of colour (51.9 g/kg of food) exhibited lower body weights
compared to control animals. One study suggested that mice that were
fed on this colour demonstrated an earlier onset of tumours of the
lymphatic system compared to control mice.1 However, a second more
extensive mouse study has not borne this out. The long-term study and
the mutagenicity studies suggest that Allura Red does not possess
carcinogenic potential.
The data were sufficient to establish a temporary acceptable
daily intake for man pending the availability of the statistical
analysis of the long-term mouse study.
EVALUATION
Level causing no toxicological effect
Rat: 1.39% in the diet corresponding to 695 mg/kg bw.
Estimate of acceptable daily intake for man
0-7 mg/kg bw.*
FURTHER WORK OR INFORMATION
Required by 1981
(1) Adequate information of statistical analysis of the long-term
mouse studies.
* Temporary.
REFERENCES
Anonymous. Mutagenicity investigations with allura red. Sex-linked
recessive lethals, Drosophila melanogaster, Bowling Green
University, Ohio, USA. Interim report of the Working Group on FD
& C Red, No. 40, 19 January 1977b, submitted to WHO by the US
Food and Drug Administration
Anonymous. Mutagenicity investigations with allura red. Saccharomyces
cerevisiae. Genetic Toxicology Branch of the FDA. Interim
report of the Working Group on FD & C Red No. 40, 19 January
1977a
Anonymous. Evaluation of substances of interest for genetic damage
using Drosophila melanogaster. Unpublished report submitted to
WHO by the United States Food and Drug Administration, 31 March
1978
Blackmore, R. H. Repeated dermal irritation study-rabbits, nontoxic
Red Z4576. Unpublished report 165-120 by Hazelton Laboratories,
Inc., submitted to WHO by Allied Chemical Corporation, 1968
Blackmore, R. H., Olson, W. A. & Voelker, R. W. Two-generation
reproduction study in rats, Red Z-4576. Unpublished report
165-133 by Hazelton Laboratories, Inc., submitted by Allied
Chemical Corporation, 1969
Bradlaw, J. Enzyme induction screen of seven color and cosmetic
ingredients. FDA Toxicology Laboratory, United States Food and
Drug Administration, manuscript in preparation, 1979
Brown, J., Roehm, G. & Brown, R. Mutagenicity testing of certified
food colors and related azo, xanthene and triphenylmethane dyes
with the Salmonella/microsome system, Mut. Res., 56: 249-271,
1978
Brusick, D. Mutagenicity evaluation NTRZ-4576 (Allura Red AC).
Unpublished report number 2547 by Litton Bionetics, Inc.,
submitted to WHO by Allied Chemical Corporation, 1976
Collins, T. F. X. Preliminary results of teratological studies on
Osborne-Mendel rats with FDC Red No. 40. Unpublished report by
Food and Drug Administration, USA, submitted to WHO, 1974
Guyton, C. L. & Reno, F. E. Metabolic disposition of 35S Allura Red AC
in the dog and rat. Unpublished report 165-146 by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Guyton, C. L. & Stanovick, R. P. Determination of the metabolites of
Allura Red AC in the rat and dog. Unpublished report by Hazelton
Laboratories America, Inc., submitted to WHO by Allied Chemical
Corporation, 1975
Jolly, R. E. An assessment of irritation and sensitization, potential
of Allura Red AC utilizing the Draize-Shelanski reported
insult patch test and photosensitization potential evaluation.
Unpublished report by Biometric Testing, Inc., submitted to WHO
by Allied Chemical Corporation, 1973
Jorgenson, T. A., Rushbrook, C. J., Newell, G. W. & Skinner, W. A.
Study of the mutagenic effects of FD & C Red 40 (75-60) by the
heritable translocation study in mice. Unpublished report
LSU-5588 by SRI International, supplied by the United States
Food and Drug Administration, 1978
Mikkelsen, H., Larsen, J. & Tarding, F. Hypersensitivity reactions to
food colours with special reference to the natural colour annatto
extract (butter colour). Toxicological Aspects of Food Safety,
Arch. Tox. Suppl., 1:141-143, 1978
Muzzall, J. & Cook, W. Mutagenicity test of dyes used in cosmetics
with Salmonella/mammalian-microsome test, Mut. Res., 67:1-8,
1979
Olson, W. A. & Voelker, R. W. Twenty-one month dietary administration
albino rats, Red Z-4576. Unpublished report 165-121 by Hazelton
Laboratories, Inc., submitted to WHO by Allied Chemical
Corporation, 1970
Olson, W. A., Voelker, R. W. & Shott, L. D. One hundred and four-week
dietary administration-dogs, Red Z-4576. Unpublished report
165-132 by Hazelton Laboratories, Inc., submitted to WHO by
Allied Chemical Corporation, 1970
Osbourn, R. A. Report of human patch tests on FD & C Red No. 40.
Unpublished report 165-141 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1972
Reno, F. E. Teratology study in rabbits FD & C Red No. 40. Unpublished
report 165-142 by Hazelton Laboratories, Inc., submitted to WHO
by Allied Chemical Corporation, 1974
Reno, F. E., Mossburg, P. A., Serota, D. G., Tiede, J. J. & Voelker,
R. W. Carcinogenic bioassay and life-time dietary study in mice.
Unpublished report 165-174 by Hazelton Laboratories America,
Inc., submitted to WHO by Allied Chemical/Buffalo Color
Corporations, 1978
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the ICR Swiss mouse. Unpublished report
number 165-150 by Hazelton Laboratories America, Inc., submitted
to WHO by Allied Chemical/Buffalo Color Corporations, 1977a
Serota, D. G., Voelker, R. W., Reno, F. E. & Tiede, J. J. Lifetime
carcinogenic study in the albino rat. Unpublished report number
165-149 by Hazelton Laboratories America, Inc., submitted to WHO
by Allied Chemical/Buffalo Color Corporations, 1977b
Sondergaard, D., Hansen, E. & Wurtzen, H. A. A short-term study in the
pig of the effects on the liver and on the blood of eight azo
dyes, Toxicology, 8:381-386, 1977
Viola, M. & Nosotti, A. Application of the Ames test to certain color
additives, Boll. Chim. Farm., 117:402-415, 1978
Voelker, R. W. Repeated dermal application - Swiss mice, Red Z-4576.
Unpublished report 165-123 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1970
Weir, R. J. Acute oral administration - rats, five experimental
non-toxic red colors. Unpublished report 165-114 by Hazelton
Laboratories, Inc., submitted by Allied Chemical Corporation,
1965a
Weir, R. J. Acute oral toxicity - dogs, five experimental non-toxic
red colors. Unpublished report by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1965b
Weir, R. J. Acute dermal application - rabbits, Red Z-4576.
Unpublished report 165-119 by Hazelton Laboratories, Inc.,
submitted by Allied Chemical Corporation, 1967
Weir, R. J. & Crews, L. M. Six-week dietary feeding study - rats,
experimental non-toxic Red Z-4576, Red Z-4578, Red Z-4598 and Red
Z-4808. Unpublished report 165-112 Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966a
Weir, R. J. & Crews, L. M. Six-week oral administration - dogs,
experimental non-toxic Red Z-4576, Z-4578, Z-4598 and Z-4808.
Unpublished report 165-116 by Hazelton Laboratories, Inc.,
submitted to WHO by Allied Chemical Corporation, 1966b
White, R. G. Metabolic fate of orally ingested non-toxic Red Z-4576.
Unpublished report number 21855 by Buffalo Research Laboratory,
submitted to WHO by Allied Chemical Corporation, 1970
