
FAO Nutrition Meetings
Report Series No. 40A,B,C
WHO/Food Add./67.29
TOXICOLOGICAL EVALUATION OF SOME
ANTIMICROBIALS, ANTIOXIDANTS, EMULSIFIERS,
STABILIZERS, FLOUR-TREATMENT AGENTS, ACIDS AND BASES
The content of this document is the result of the deliberations of the
Joint FAO/WHO Expert Committee on Food Additives which met at Rome,
13-20 December, 19651 Geneva, 11-18 October, 19662
1 Ninth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1966 No. 40;
Wld Hlth Org. techn. Rep. Ser., 1966, 339
2 Tenth Report of the Joint FAO/WHO Expert Committee on Food
Additives, FAO Nutrition Meetings Report Series, 1967, in press;
Food and Agriculture Organization of the United Nations
World Health Organization
1967
DISODIUM ETHYLENEDIAMINETETRAACETATE
Synonyms Disodium EDTA; Disodium Edetate
Chemical names Disodium dihydrogen
ethylenediaminetetraacetate
Disodium dihydrogen (ethylenedinitrilo)
tetraacetate
Empirical formula C10H14N2Na2O8.2H2O
Structural formula
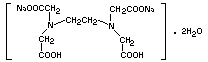 Molecular weight 372.24
Definition Disodium ethylenediaminetetraacetate
contains not less than 99.0 per cent. of
C10H14N2Na2O8.2H2O.
Description Disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder.
Use As a sequestrant.
Biological Data
Biochemical aspects
See calcium disodium ethylenediaminetetraacetate.
Special studies
Disodium EDTA injected at levels of 3.4, 1.7 and 0.35 mg,/egg,
gave 40, 50 and 85 per cent. hatch, respectively. At the highest
level, some embryos which failed to hatch showed anomalies (McLaughlin
& Scott, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 2 000-2 200 Yang, 1964
Rabbit oral 2 300 Shibata, 1956
471 Shibate, 1956
1 Dose depending on the rate of infusion.
Short-term studies
Rat. Rats were fed for 44-52 weeks on a diet containing 0.5 per
cent. disodium EDTA without any deleterious effect on weight gain,
appetite, activity and appearance (Krum, 1948). In another experiment
3 groups of 10-13 males and females were fed a low-mineral diet (0.54
per cent. Ca and 0.013 per cent. Fe) with the addition of 0, 0.5 and 1
per cent. disodium EDTA for 205 days. At the 1 per cent. level some
abnormal symptoms were observed: growth retardation of the males,
lowered erythrocyte and leucocyte counts, a prolonged blood
coagulation time, slightly but significantly raised blood calcium
level, a significantly lower ash content of the bone, considerable
erosion of the molars and diarrhoea. Gross and histological
examination of the major organs revealed nothing abnormal. Rats fed
for 220 days on an adequate mineral diet containing 1 per cent.
disodium EDTA showed no evidence of dental erosion (Chan, 1964).
Groups of 6 rats ware maintained for 12 weeks on diets containing
0.5, 1 and 5 per cent. disodium EDTA. No deaths occurred and there
were no toxic symptoms except diarrhoea and lowered food consumption
at the 5 per cent. level. Mating in each group was carried out when
the animals were 100 days old. Mating was repeated 10 days after
weaning the first litters. Parent generation rats of 0, 0.5 and 1 per
cent. levels gave birth to normal first and second litters. The
animals given 5 per cent. failed to produce litters (Yang, 1964). To
elucidate possible teratogenic effects, daily doses of 20-40 mg/rat
EDTA were injected intramuscularly into pregnant rats at days 6-9,
10-15 and 16 to the end of pregnancy. A dose of 40 mg was lethal
within 4 days but 20 mg was well tolerated, allowing normal foetal
development; 40 mg, injected during days 6-8 or 10-15 produced some
dead or malformed foetuses, especially polydactyl, double tail,
generalised oedema or circumscribed head oedema (Tuchmann-Duplessis &
Mercier-Parot, 1956).
Groups of 5 male rats were given 250, 400 or 500 mg/kg
body-weight disodium EDTA i.p. daily for 3-31 days; some groups were
observed for another 2 weeks. At the 500 mg level all rats became
lethargic and died within 9 days, the kidneys being pale and swollen,
with moderate dilatation of bowl and subserosal haemorrhages.
Histological examination of a number of organs showed lesions only in
the kidneys. Animals at the 400 mg level died within 14 days, kidney
and bowel symptoms being similar to the 500 mg level. One rat at the
250 mg dose level showed haemorrhage of the thymus. All 3 groups
showed varying degrees of hydropic necrosis of the renal proximal
convoluted tubules with epithelial sloughing: recovery occurred in all
groups after withdrawal of disodium EDTA (Reuber & Schmieler, 1962).
Rabbit. Eight groups of 3 rabbits ware given either 0.1, 1, 10
or 20 mg/kg body-weight disodium EDTA i.v., or 50, 100, 500 or 1000
mg/kg body-weight orally for 1 month. All animals on the highest oral
test level exhibited severe diarrhoea and died. In the other groups
body-weight, haemogram, urinary nitrogen and urobilinogen were
unaffected. Histopathological examination of a number of organs showed
degenerative changes in the liver, kidney, parathyroid and endocrine
organs and oedema in muscle, brain and heart at all levels of
treatment (Shibata, 1956).
Long-term studies
Rat. In a 2-year study 5 groups totalling 33 rats were fed 0,
0.5, 1 and 5 per cent. disodium EDTA. The 5 per cent. group showed
diarrhoea and consumed less food than the rats in other groups. No
significant effects on weight gain ware noted nor were blood
coagulation time, red blood cell counts or bone ash adversely
affected. The mortality of the animals could not be correlated with
the level of disodium EDTA. The highest mortality rate occurred in the
control group. Gross and microscopic examination of various organs
revealed no significant differences between the groups (Yang, 1964).
Comments
The long-term studies on rats are difficult to assess because of
the small number of animals and the high mortality rate even in the
control group. Metabolic studies and feeding, experiments demonstrate
that the use of calcium disodium EDTA is preferable to that of
disodium EDTA.
Evaluation
Because of its effect on calcium, the use of disodium EDTA as a
food additive is not recommended. Under certain circumstances,
necessitating an accurate complexing of calcium, it may be used
provided no excess of disodium EDTA remains and the only compound
finally present is calcium disodium EDTA.
REFERENCES
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763
Krum J. K. (1948) Thesis University, of Massachusetts
McLaughlin, J. jr, & Scott, W. F. (1964) Fed. Proc., 23, 406
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Shibata, S. (1956) Folio pharmacol. Jap., 52, 113
Tuchmann-Duplessis, H. & Mercier-Parot L. (1956) C.R. Acad.
Sci.,243, 1064
Yang, Shou-Shih, (1964) Food Cosmet. Toxicol., 2, 763
Molecular weight 372.24
Definition Disodium ethylenediaminetetraacetate
contains not less than 99.0 per cent. of
C10H14N2Na2O8.2H2O.
Description Disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder.
Use As a sequestrant.
Biological Data
Biochemical aspects
See calcium disodium ethylenediaminetetraacetate.
Special studies
Disodium EDTA injected at levels of 3.4, 1.7 and 0.35 mg,/egg,
gave 40, 50 and 85 per cent. hatch, respectively. At the highest
level, some embryos which failed to hatch showed anomalies (McLaughlin
& Scott, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 2 000-2 200 Yang, 1964
Rabbit oral 2 300 Shibata, 1956
471 Shibate, 1956
1 Dose depending on the rate of infusion.
Short-term studies
Rat. Rats were fed for 44-52 weeks on a diet containing 0.5 per
cent. disodium EDTA without any deleterious effect on weight gain,
appetite, activity and appearance (Krum, 1948). In another experiment
3 groups of 10-13 males and females were fed a low-mineral diet (0.54
per cent. Ca and 0.013 per cent. Fe) with the addition of 0, 0.5 and 1
per cent. disodium EDTA for 205 days. At the 1 per cent. level some
abnormal symptoms were observed: growth retardation of the males,
lowered erythrocyte and leucocyte counts, a prolonged blood
coagulation time, slightly but significantly raised blood calcium
level, a significantly lower ash content of the bone, considerable
erosion of the molars and diarrhoea. Gross and histological
examination of the major organs revealed nothing abnormal. Rats fed
for 220 days on an adequate mineral diet containing 1 per cent.
disodium EDTA showed no evidence of dental erosion (Chan, 1964).
Groups of 6 rats ware maintained for 12 weeks on diets containing
0.5, 1 and 5 per cent. disodium EDTA. No deaths occurred and there
were no toxic symptoms except diarrhoea and lowered food consumption
at the 5 per cent. level. Mating in each group was carried out when
the animals were 100 days old. Mating was repeated 10 days after
weaning the first litters. Parent generation rats of 0, 0.5 and 1 per
cent. levels gave birth to normal first and second litters. The
animals given 5 per cent. failed to produce litters (Yang, 1964). To
elucidate possible teratogenic effects, daily doses of 20-40 mg/rat
EDTA were injected intramuscularly into pregnant rats at days 6-9,
10-15 and 16 to the end of pregnancy. A dose of 40 mg was lethal
within 4 days but 20 mg was well tolerated, allowing normal foetal
development; 40 mg, injected during days 6-8 or 10-15 produced some
dead or malformed foetuses, especially polydactyl, double tail,
generalised oedema or circumscribed head oedema (Tuchmann-Duplessis &
Mercier-Parot, 1956).
Groups of 5 male rats were given 250, 400 or 500 mg/kg
body-weight disodium EDTA i.p. daily for 3-31 days; some groups were
observed for another 2 weeks. At the 500 mg level all rats became
lethargic and died within 9 days, the kidneys being pale and swollen,
with moderate dilatation of bowl and subserosal haemorrhages.
Histological examination of a number of organs showed lesions only in
the kidneys. Animals at the 400 mg level died within 14 days, kidney
and bowel symptoms being similar to the 500 mg level. One rat at the
250 mg dose level showed haemorrhage of the thymus. All 3 groups
showed varying degrees of hydropic necrosis of the renal proximal
convoluted tubules with epithelial sloughing: recovery occurred in all
groups after withdrawal of disodium EDTA (Reuber & Schmieler, 1962).
Rabbit. Eight groups of 3 rabbits ware given either 0.1, 1, 10
or 20 mg/kg body-weight disodium EDTA i.v., or 50, 100, 500 or 1000
mg/kg body-weight orally for 1 month. All animals on the highest oral
test level exhibited severe diarrhoea and died. In the other groups
body-weight, haemogram, urinary nitrogen and urobilinogen were
unaffected. Histopathological examination of a number of organs showed
degenerative changes in the liver, kidney, parathyroid and endocrine
organs and oedema in muscle, brain and heart at all levels of
treatment (Shibata, 1956).
Long-term studies
Rat. In a 2-year study 5 groups totalling 33 rats were fed 0,
0.5, 1 and 5 per cent. disodium EDTA. The 5 per cent. group showed
diarrhoea and consumed less food than the rats in other groups. No
significant effects on weight gain ware noted nor were blood
coagulation time, red blood cell counts or bone ash adversely
affected. The mortality of the animals could not be correlated with
the level of disodium EDTA. The highest mortality rate occurred in the
control group. Gross and microscopic examination of various organs
revealed no significant differences between the groups (Yang, 1964).
Comments
The long-term studies on rats are difficult to assess because of
the small number of animals and the high mortality rate even in the
control group. Metabolic studies and feeding, experiments demonstrate
that the use of calcium disodium EDTA is preferable to that of
disodium EDTA.
Evaluation
Because of its effect on calcium, the use of disodium EDTA as a
food additive is not recommended. Under certain circumstances,
necessitating an accurate complexing of calcium, it may be used
provided no excess of disodium EDTA remains and the only compound
finally present is calcium disodium EDTA.
REFERENCES
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763
Krum J. K. (1948) Thesis University, of Massachusetts
McLaughlin, J. jr, & Scott, W. F. (1964) Fed. Proc., 23, 406
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Shibata, S. (1956) Folio pharmacol. Jap., 52, 113
Tuchmann-Duplessis, H. & Mercier-Parot L. (1956) C.R. Acad.
Sci.,243, 1064
Yang, Shou-Shih, (1964) Food Cosmet. Toxicol., 2, 763
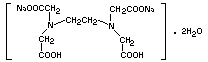 Molecular weight 372.24
Definition Disodium ethylenediaminetetraacetate
contains not less than 99.0 per cent. of
C10H14N2Na2O8.2H2O.
Description Disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder.
Use As a sequestrant.
Biological Data
Biochemical aspects
See calcium disodium ethylenediaminetetraacetate.
Special studies
Disodium EDTA injected at levels of 3.4, 1.7 and 0.35 mg,/egg,
gave 40, 50 and 85 per cent. hatch, respectively. At the highest
level, some embryos which failed to hatch showed anomalies (McLaughlin
& Scott, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 2 000-2 200 Yang, 1964
Rabbit oral 2 300 Shibata, 1956
471 Shibate, 1956
1 Dose depending on the rate of infusion.
Short-term studies
Rat. Rats were fed for 44-52 weeks on a diet containing 0.5 per
cent. disodium EDTA without any deleterious effect on weight gain,
appetite, activity and appearance (Krum, 1948). In another experiment
3 groups of 10-13 males and females were fed a low-mineral diet (0.54
per cent. Ca and 0.013 per cent. Fe) with the addition of 0, 0.5 and 1
per cent. disodium EDTA for 205 days. At the 1 per cent. level some
abnormal symptoms were observed: growth retardation of the males,
lowered erythrocyte and leucocyte counts, a prolonged blood
coagulation time, slightly but significantly raised blood calcium
level, a significantly lower ash content of the bone, considerable
erosion of the molars and diarrhoea. Gross and histological
examination of the major organs revealed nothing abnormal. Rats fed
for 220 days on an adequate mineral diet containing 1 per cent.
disodium EDTA showed no evidence of dental erosion (Chan, 1964).
Groups of 6 rats ware maintained for 12 weeks on diets containing
0.5, 1 and 5 per cent. disodium EDTA. No deaths occurred and there
were no toxic symptoms except diarrhoea and lowered food consumption
at the 5 per cent. level. Mating in each group was carried out when
the animals were 100 days old. Mating was repeated 10 days after
weaning the first litters. Parent generation rats of 0, 0.5 and 1 per
cent. levels gave birth to normal first and second litters. The
animals given 5 per cent. failed to produce litters (Yang, 1964). To
elucidate possible teratogenic effects, daily doses of 20-40 mg/rat
EDTA were injected intramuscularly into pregnant rats at days 6-9,
10-15 and 16 to the end of pregnancy. A dose of 40 mg was lethal
within 4 days but 20 mg was well tolerated, allowing normal foetal
development; 40 mg, injected during days 6-8 or 10-15 produced some
dead or malformed foetuses, especially polydactyl, double tail,
generalised oedema or circumscribed head oedema (Tuchmann-Duplessis &
Mercier-Parot, 1956).
Groups of 5 male rats were given 250, 400 or 500 mg/kg
body-weight disodium EDTA i.p. daily for 3-31 days; some groups were
observed for another 2 weeks. At the 500 mg level all rats became
lethargic and died within 9 days, the kidneys being pale and swollen,
with moderate dilatation of bowl and subserosal haemorrhages.
Histological examination of a number of organs showed lesions only in
the kidneys. Animals at the 400 mg level died within 14 days, kidney
and bowel symptoms being similar to the 500 mg level. One rat at the
250 mg dose level showed haemorrhage of the thymus. All 3 groups
showed varying degrees of hydropic necrosis of the renal proximal
convoluted tubules with epithelial sloughing: recovery occurred in all
groups after withdrawal of disodium EDTA (Reuber & Schmieler, 1962).
Rabbit. Eight groups of 3 rabbits ware given either 0.1, 1, 10
or 20 mg/kg body-weight disodium EDTA i.v., or 50, 100, 500 or 1000
mg/kg body-weight orally for 1 month. All animals on the highest oral
test level exhibited severe diarrhoea and died. In the other groups
body-weight, haemogram, urinary nitrogen and urobilinogen were
unaffected. Histopathological examination of a number of organs showed
degenerative changes in the liver, kidney, parathyroid and endocrine
organs and oedema in muscle, brain and heart at all levels of
treatment (Shibata, 1956).
Long-term studies
Rat. In a 2-year study 5 groups totalling 33 rats were fed 0,
0.5, 1 and 5 per cent. disodium EDTA. The 5 per cent. group showed
diarrhoea and consumed less food than the rats in other groups. No
significant effects on weight gain ware noted nor were blood
coagulation time, red blood cell counts or bone ash adversely
affected. The mortality of the animals could not be correlated with
the level of disodium EDTA. The highest mortality rate occurred in the
control group. Gross and microscopic examination of various organs
revealed no significant differences between the groups (Yang, 1964).
Comments
The long-term studies on rats are difficult to assess because of
the small number of animals and the high mortality rate even in the
control group. Metabolic studies and feeding, experiments demonstrate
that the use of calcium disodium EDTA is preferable to that of
disodium EDTA.
Evaluation
Because of its effect on calcium, the use of disodium EDTA as a
food additive is not recommended. Under certain circumstances,
necessitating an accurate complexing of calcium, it may be used
provided no excess of disodium EDTA remains and the only compound
finally present is calcium disodium EDTA.
REFERENCES
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763
Krum J. K. (1948) Thesis University, of Massachusetts
McLaughlin, J. jr, & Scott, W. F. (1964) Fed. Proc., 23, 406
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Shibata, S. (1956) Folio pharmacol. Jap., 52, 113
Tuchmann-Duplessis, H. & Mercier-Parot L. (1956) C.R. Acad.
Sci.,243, 1064
Yang, Shou-Shih, (1964) Food Cosmet. Toxicol., 2, 763
Molecular weight 372.24
Definition Disodium ethylenediaminetetraacetate
contains not less than 99.0 per cent. of
C10H14N2Na2O8.2H2O.
Description Disodium ethylenediaminetetraacetate
occurs as white, odourless crystalline
granules or as a white to nearly white
powder.
Use As a sequestrant.
Biological Data
Biochemical aspects
See calcium disodium ethylenediaminetetraacetate.
Special studies
Disodium EDTA injected at levels of 3.4, 1.7 and 0.35 mg,/egg,
gave 40, 50 and 85 per cent. hatch, respectively. At the highest
level, some embryos which failed to hatch showed anomalies (McLaughlin
& Scott, 1964).
Acute toxicity
Animal Route LD50 References
(mg/kg
body-weight)
Rat oral 2 000-2 200 Yang, 1964
Rabbit oral 2 300 Shibata, 1956
471 Shibate, 1956
1 Dose depending on the rate of infusion.
Short-term studies
Rat. Rats were fed for 44-52 weeks on a diet containing 0.5 per
cent. disodium EDTA without any deleterious effect on weight gain,
appetite, activity and appearance (Krum, 1948). In another experiment
3 groups of 10-13 males and females were fed a low-mineral diet (0.54
per cent. Ca and 0.013 per cent. Fe) with the addition of 0, 0.5 and 1
per cent. disodium EDTA for 205 days. At the 1 per cent. level some
abnormal symptoms were observed: growth retardation of the males,
lowered erythrocyte and leucocyte counts, a prolonged blood
coagulation time, slightly but significantly raised blood calcium
level, a significantly lower ash content of the bone, considerable
erosion of the molars and diarrhoea. Gross and histological
examination of the major organs revealed nothing abnormal. Rats fed
for 220 days on an adequate mineral diet containing 1 per cent.
disodium EDTA showed no evidence of dental erosion (Chan, 1964).
Groups of 6 rats ware maintained for 12 weeks on diets containing
0.5, 1 and 5 per cent. disodium EDTA. No deaths occurred and there
were no toxic symptoms except diarrhoea and lowered food consumption
at the 5 per cent. level. Mating in each group was carried out when
the animals were 100 days old. Mating was repeated 10 days after
weaning the first litters. Parent generation rats of 0, 0.5 and 1 per
cent. levels gave birth to normal first and second litters. The
animals given 5 per cent. failed to produce litters (Yang, 1964). To
elucidate possible teratogenic effects, daily doses of 20-40 mg/rat
EDTA were injected intramuscularly into pregnant rats at days 6-9,
10-15 and 16 to the end of pregnancy. A dose of 40 mg was lethal
within 4 days but 20 mg was well tolerated, allowing normal foetal
development; 40 mg, injected during days 6-8 or 10-15 produced some
dead or malformed foetuses, especially polydactyl, double tail,
generalised oedema or circumscribed head oedema (Tuchmann-Duplessis &
Mercier-Parot, 1956).
Groups of 5 male rats were given 250, 400 or 500 mg/kg
body-weight disodium EDTA i.p. daily for 3-31 days; some groups were
observed for another 2 weeks. At the 500 mg level all rats became
lethargic and died within 9 days, the kidneys being pale and swollen,
with moderate dilatation of bowl and subserosal haemorrhages.
Histological examination of a number of organs showed lesions only in
the kidneys. Animals at the 400 mg level died within 14 days, kidney
and bowel symptoms being similar to the 500 mg level. One rat at the
250 mg dose level showed haemorrhage of the thymus. All 3 groups
showed varying degrees of hydropic necrosis of the renal proximal
convoluted tubules with epithelial sloughing: recovery occurred in all
groups after withdrawal of disodium EDTA (Reuber & Schmieler, 1962).
Rabbit. Eight groups of 3 rabbits ware given either 0.1, 1, 10
or 20 mg/kg body-weight disodium EDTA i.v., or 50, 100, 500 or 1000
mg/kg body-weight orally for 1 month. All animals on the highest oral
test level exhibited severe diarrhoea and died. In the other groups
body-weight, haemogram, urinary nitrogen and urobilinogen were
unaffected. Histopathological examination of a number of organs showed
degenerative changes in the liver, kidney, parathyroid and endocrine
organs and oedema in muscle, brain and heart at all levels of
treatment (Shibata, 1956).
Long-term studies
Rat. In a 2-year study 5 groups totalling 33 rats were fed 0,
0.5, 1 and 5 per cent. disodium EDTA. The 5 per cent. group showed
diarrhoea and consumed less food than the rats in other groups. No
significant effects on weight gain ware noted nor were blood
coagulation time, red blood cell counts or bone ash adversely
affected. The mortality of the animals could not be correlated with
the level of disodium EDTA. The highest mortality rate occurred in the
control group. Gross and microscopic examination of various organs
revealed no significant differences between the groups (Yang, 1964).
Comments
The long-term studies on rats are difficult to assess because of
the small number of animals and the high mortality rate even in the
control group. Metabolic studies and feeding, experiments demonstrate
that the use of calcium disodium EDTA is preferable to that of
disodium EDTA.
Evaluation
Because of its effect on calcium, the use of disodium EDTA as a
food additive is not recommended. Under certain circumstances,
necessitating an accurate complexing of calcium, it may be used
provided no excess of disodium EDTA remains and the only compound
finally present is calcium disodium EDTA.
REFERENCES
Chan, M. S. (1964) Food Cosmet. Toxicol., 2, 763
Krum J. K. (1948) Thesis University, of Massachusetts
McLaughlin, J. jr, & Scott, W. F. (1964) Fed. Proc., 23, 406
Reuber, M. D. & Schmieller, G. C. (1962) Arch. environ. Health, 5,
430
Shibata, S. (1956) Folio pharmacol. Jap., 52, 113
Tuchmann-Duplessis, H. & Mercier-Parot L. (1956) C.R. Acad.
Sci.,243, 1064
Yang, Shou-Shih, (1964) Food Cosmet. Toxicol., 2, 763
